EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; origin of epidermis with no clear pattern [probably from inner layer of root cap], trichoblasts [differentiated root R-Put-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen 3-colpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [≤8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID I / LAMIIDAE / [CARDIOPTERIDALES [GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]]: ?
[GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]: G [2], superposed; loss of introns 18-23 in RPB2 d copy. - check
[ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]: vessel elements with with simple perforation plates; nodes 1:1.
[[GENTIANALES + BORAGINALES] VAHLIALES, SOLANALES, LAMIALES] / CORE LAMIIDS: herbaceous habit widespread; (8-ring deoxyflavonols +); C forming a distinct tube, initiation late [sampling!]; A epipetalous; (vascularized) nectary at base of G; style long; several ovules/carpel; fruit a septicidal capsule, K persistent.
Evolution: Divergence & Distribution. The complex patterns of variation in a number of characters in this part of the tree is discussed on the Gentianales page.
Phylogeny. For the relationships of Lamiales, see discussion under Gentianales.
LAMIALES Bromhead - Main Tree.
Cornoside, verbascosides [caffeoyl phenylethanoid glucosides (CPGs), caffeic acid esters, = acteosides], methyl- and oxygenated flavones +, iridoids 0; eglandular hairs multicellular; leaves opposite; inflorescence cymose/determinate/closed; K connate; A adnate to C, anther sacs with placentoids; fruit capsular [?level]; chalazal endosperm haustorium +, cotyledons incumbent; protein bodies in nuclei; mitochondrial coxII.i3 intron 0. - 24/25 families, 1,059 genera, 23,755 species.
Includes Acanthaceae, Bignoniaceae, Byblidaceae, Calceolariaceae, Carlemanniaceae, Gesneriaceae, Lamiaceae, Lentibulariaceae, Linderniaceae, Martyniaceae, Mazaceae, Oleaceae, Orobanchaceae, Paulowniaceae, Pedaliaceae, Peltantheraceae, Phrymaceae, Plantaginaceae, Plocospermataceae, Schlegeliaceae, Scrophulariaceae, Stilbaceae, Tetrachondraceae, Thomandersiaceae, Verbenaceae, Wightiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Estimates of the age of crown-group Lamiales are about 61.5 Ma (Tank et al. 2015: Table S2), 77 Ma (Magallón et al. (2015), (96-)87(-77) Ma (Wikström et al. 2015), (105.3-)93(-80.5) Ma (Tank & Olmstead pers. comm.), ca 97 Ma (K. Bremer et al. 2004a), 100.6-97.5 Ma (Nylinder et al. 2012: suppl.), (104-)101.6(-98.2) Ma (Roalson & Roberts 2016), ca 94.9 Ma (C. Zhang et al. 2020) and ca 91.8 Ma (Fonseca 2021).
Evolution: Divergence & Distribution. The trees in Magallón et al. (2015) and Fonseca (2021) have numerous ages for nodes in Lamiales, but their topologies (see below) differ from that used here and for the most part only crown-group family ages have been added..
Lamiales contain ca 12.3% eudicot diversity. Most of this diversity is concentrated in families whose members are herbaceous to shrubby and have rather large, monosymmetric flowers; about half the species have fruits with many rather small seeds (Sims 2013), and although about half the species have only eight or fewer seeds per fruit, but they are not very big. Fonseca (2021) suggested that rather unusually shifts from the extratropics to the tropics preponderated in Lamiales; normally, movement is in the other direction.
For a useful general discussion, including suggestions of apomorphies for some clades, see Soltis et al. (2005b); Kadereit (2004b) provided a summary of the order and its evolution. Endress (2011a) suggested that a key innovation somewhere in Lamiales was tenuinucellate ovules. For the evolution of features of pollen morphology, see L.-E Yang et al. (2020), but c.f. topology, etc.. Ovule number is notably variable in the basal clades and will be difficult to optimize, but carpels with two ovules are common in the three basalmost clades. The four clades that are successively sister to other Lamiales either lack iridoids or have iridoids distinctively different (Oleaceae) from those in the other members of the clade, so iridoid (re)aquisition is pegged well within Lamiales; whether or not Carlemanniaceae have iridoids is unknown.
Taxa with 4-merous or predominantly 4-merous flowers are common in the basal pectinations of the Lamiales tree (see also Mayr & Weber 2006). Carlemanniaceae have both 4- and 5-merous flowers, while Calceolariaceae, in core Lamiales, have 4-merous flowers, each lip representing two completely connate petals, although some have interpreted their flowers as being 5-merous (Mayr & Weber 2006 and references). Monosymmetry is unlikely to be plesiomorphic in the order (c.f. Ronse de Craene 2010 and references), but it is very interesting that the evolution of corolla monosymmetry has happened in a stepwise fashion, and may be evident as early (branching-wise) as in Oleaceae (Zhong & Kellogg 2015). Floral evolution in basal Lamiales is not simple, and where changes in floral merosity and floral symmetry are to be placed on the tree is unclear.
Endress (2001b) suggested that families such as Orobanchaceae, Lamiaceae and Acanthaceae formed a clade with strongly monosymmetric flowers that mostly lack a staminode, but such a grouping is not obviously consistent with the relationships currently being recovered.
Confirmation of the phylogenetic positions of Carlemanniaceae, placed sister to Oleaceae, and of Plocospermataceae, as well as studies of their anatomy, chemistry, floral development, etc., are important for understanding the evolution of the chemistry and floral morphology in particular of Lamiales as a whole (c.f. Endress 2001). Oleaceae, also in this area, have recently been studied in some detail by Dupin et al. (2022) and W. Dong et al. (2022). Thus, given their position, one might expect Carlemanniaceae to lack iridoids - at least, to lack route II decarboxylated iridoids - and to have only a single (micropylar) endosperm haustorium. As might be anticipated, there is little morphological support for internal nodes in much of Lamiales and also for several of the families, and this is likely to be true whatever the relationships in the order.
Ecology & Physiology. The recent discoveries of Pereira et al. (2012) have added another phylogenetically isolated carnivorous clade (Philcoxia, in Plantaginaceae), so carnivory, direct or indirect, has arisen three times in Lamiales (Lentibulariaceae, Byblidaceae, Plantaginaceae); Martyniaceae may also be carnivorous - insects stick to the glandular hairs, and they are eaten by other insects...
Genes & Genomes. The pattern of duplication of the FLO=LFY and DEF=AP3 genes within Lamiales is largely congruent with the relationships discussed above; duplication occurred in the representatives of Phrymaceae, Verbenaceae, Paulowniaceae and Orobanchaceae examined, but not in those of Plantaginaceae or Oleaceae (Aagard et al. 2005).
Chemistry, Morphology, etc.. A great deal of work on characterising iridoids and understanding their distribution in Lamiales has been carried out by S. R. Jensen and collaborators. The presence of cornosides and iridoids in Lamiales is largely mutually exclusive, except in Martynia louisiana (Jensen 1992, 2000a, 2000b). Verbascoside, a disaccharide derivative of the hydroxycinnamic acid, caffeic acid (= caffeoyl phenylethanoid glycoside), is common. It and trisaccharide derivatives (over 325 structures altogether - S. R. Jensen, pers. comm.) are phenylpropanoid glycosides, a class of compounds usually with a central glucose, a C6C2 unit, commonly dihydroxyphenyl-ß-ethanol, and a C6C3 unit, hydroxycinnamic acid (Mølgaard & Ravn 1988). Such compounds are very rarely found elsewhere; an exception is Cassinopsis (Cometa et al. 1993), which is sister to all other Icacinaceae (Byng et al. 2014; Stull et al. 2015).
Nodal anatomy needs study. Neubauer (e.g. 1977, 1978) suggested that the single trace often divided immediately into three or more, and this nodal type is indeed common in the order. Bailey (1956) recorded 2:2 nodes in Lamiaceae, and other nodal morphologies occur, e.g. in Gesneriaceae, Bignoniaceae, etc. Intermediary cells with distinctive plasmodesmata associated with the ultimate leaf veins may be plesiomorphic in Lamiales; their presence is linked with the transport of raffinose and stachyose, oligosaccharides commonly found in phloem exudate in the order (Turgeon et al. 2001; Turgeon 2010a). Leaf teeth have a glandular apex, with one accessory vein proceeding into the tooth, the other going above it.
Taxa with tricellular pollen grains are scattered throughout the order. For integument thickness, for which I have no generally comparable information but which may be of systematic importance, see also Hjertsen (1997) and Fischer (2004b). A chalazal hypostase is common - e.g. Buddleja, some "scrophs" - but the level of this feature is unknown. Oleaceae seem to have a rather diferent embryo development from that of other Lamiales studied (Yamazaki 1974). A long, narrow suspensor may be common in Lamiales (di Fulvio 1979; Maldonado de Magnano 1987), but I do not know the general distribution of this character - it is certainly not found in Loganiaceae. Details of endosperm development and of endosperm haustoria are variable, but there is little obvious phylogenetic signal in the former. Thus endosperm development in Orobanchaceae is overall rather similar - four cells or nuclei at the micropylar end, two at the chalazal - but in Bignoniaceae, Incarvillea differs greatly from the rest, as does Gratiola (Plantaginaceae) from other ex-Scrophulariaceae s.l. (see e.g. Mauritzon 1935a; Krishna Iyengar 1940a, 1942 and references). The seed is ruminate in various ways (Hartl 1959, 1965-1974; Hilliard 1994). Seed pedestals, developed from the funicle and/or placenta, are scattered, being known from e.g. Tetrachondraceae, Calceolariaceae, Orobanchaceae and Paulowniaceae (Rebernig & Weber 2007; Hilliard 1994).
For general information, see Kadereit (2004a), for chemistry, see Harborne and Williams (1971: scutellarein, etc.), Zindler-Frank (1978: oxalate accumulation), Young and Siegler (1981: anthraquinones), Mølgaard and Ravn (1988: caffeoyl esters), Tomás Barberán et al. (1988: flavone glycosides), Scogin (1992: acteoside), Jensen (1992), and Grayer et al. (1999: general). For proteinaceous nuclear inclusions, see Bigazzi (1984, 1989a, 1989b, 1993, 1995) and Speta (1977, 1979). Information on a number of families recognised here is to be found under Scrophulariaceae in the old sense - see e.g. Schmid (1906: ovules), Hartl (1956: placentation), and Hartl (1965-1974), and Fischer (2004b), all general. For a sumary of inflorescence morphology, see Weber (2013), for some gynoecial variation, see Shamrov (2014b).
Phylogeny. Oxelman et al. (1999a), Mueller et al. (2001) and Hilu et al. (2001) among others suggested that Plocospermataceae were sister to all other Lamiales. Savolainen et al. (2000b, rbcL data alone; see also H.-L. Lee et al. 2007, Plocospermataceae not included) placed Carlemannia as sister to Oleaceae (only 1 species in analysis) with moderate support, and Bremer et al. (2001) found that the two genera formed a sister group that was part of a trichotomy at the base of Lamiales; Oleaceae (Ligustrum only included) and [all other Lamiales] completed the trichotomy, while Plocospermataceae again were not studied. A sister relationship [Carlemanniaceae + Oleaceae] is also supported by Yang et al. (2007: 1.0 p.p., Plocosperma included, but sampling still very poor; Refulio-Rodriguez & Olmstead 2014), and that seems the best place to put the family. The peltate, glandular hairs with unicellular stalks and flowers with two stamens (their position is not entirely certain) of Carlemanniaceae also suggest Lamiales, and anatomical features (see below) are consistent with this relationship.
S. Andersson (2006, two genes, sampling poor) found 75% jacknife support for the clade [Calceolariaceae + Gesneriaceae], and 100% support for that clade as sister to remaining Lamiales, even though Mayr and Weber (2006) did not think that the two families were particularly near each other. However, chemistry and morphology also suggest a close relationship between the two, and their position as sister to the remaining Lamiales. Qiu et al. (2010), Soltis et al. (2011) and Refulio-Rodriguez and Olmstead (2014) suggest that Peltanthera may fall outide the [Calceolariaceae + Gesneriaceae] clade (see below, but c.f. Perret et al. 2012).
Relationships in the "Scrophulariaceae" - Acanthaceae - Bignoniaceae - Lamiaceae area have been uncertain for some time, see e.g. Wagstaff and Olmstead (1997), Olmstead et al. (2001), and Xia et al. (2009). B. Bremer et al. (2002) analysed variation in three coding and three non-coding regions of the chloroplast genome; their sampling was sketchy, so the support for some family groupings is difficult to evaluate; Freeman and Scogin (1999) focused on the old Scrophulariaceae, but the pattern of relationships they found was unclear. A tree in K. Müller et al. (2004) suggested that at least a partial resolution of relationships was in sight, although sampling was again poor (this study focused on Lentibulariaceae); the three families then known or suspected to be carnivorous (Byblidaceae, Lentibulariaceae and Martyniaceae) were not immediately related. Rahmanzadeh et al. (2004), Albach et al. (2005) and Oxelman et al. (2005) began to clarify the contents of the separate clades that used to be subsumed in Scrophulariceae s. l. (see also Tank et al. 2006 for a summary). Thomandersia, from tropical Africa and previously usually included in Acanthaceae, appeared to go near Schlegeliaceae, from tropical America and previously usually included in Bignoniaceae, however, support for this association was weak (Wortley et al. 2005a and especially 2007a). Characters like the vasculature of the floral nectary and petiole, also the nectaries on the outside of the calyx, might link the two.
Lamiaceae and Verbenaceae were initially separated on two main distinctions, plant more or less herbaceous vs more or less woody, and style (often) gynobasic vs style terminal. However, details of gynoecial morphology had long suggested (Junell 1934) a separation along the lines of those followed today: inflorescence branches cymose vs inflorescence racemose and stigma bifid vs. more or less capitate. Many Lamiaceae have a single layer of sclerenchymatous, bone-shaped cells on the inside of the mesocarp, others have thicker pericarp walls, and the cells are often crystalliferous, while the pericarp anatomy of Verbenaceae is more complex (Ryding 1995). There may be differences in seed coat anatomy: the testa of at least some Verbenaceae has the hypodermal mesotestal layer(s) thickened, while in Lamiaceae it is the exotestal cells that are thickened, particularly on their inner periclinal and anticlinal walls (Rohwer 1994a). The molecular relationships of Verbenaceae s. str. and Lamiaceae were for a time unclear (e.g. Olmstead et al. 2001, only one member of each sampled); see Wagstaff & Olmstead (1997) for more information. Petraea (Verbenaceae) was sister to Bignoniaceae in some early molecular phylogenies (Wagstaff & Olmstead 1997), similarly, Nie et al. (2006) linked Verbenaceae with Bignoniaceae, and Phrymaceae went with Paulowniaceae. Phrymaceae have remained cladistically close to Paulowniaceae (see below ), although they were included in Verbenaceae by Cronquist (1981) and others in the past, in part because they have a similar racemose inflorescence and gynoecium (see Cantino 2004). Indeed, the gynobasic style and four nutlets that were supposed to characterize Lamiaceae may have evolved more than once (Cantino 1992a), and a considerable number of ex-Verbenaceae are now included in Lamiaceae (Cantino et al. 1992a, b). The monophyly of the recircumscribed families has rarely been called into question, even if their relationships are unclear (see below). Note that phenetic analyses early Dplaced verbenaceous genera in two groups within Lamiaceae, in one mixed with other Lamiaceae (El Gazzar & Watson 1970), while recently Oyebanji et al. (2020) using two chloroplast genes recovered a paraphyletic Verbenaceae in which Lamiaceae were embedded ("two large monophyletic clades": ibid., p. 9), but no other Lamiales were included and the outgroups were rosids.
Amyloid is found in both Pedaliaceae and Acanthaceae, two families that have sometimes been weakly associated in molecular analyses (Soltis et al. 2005b and references), and both Martyniaceae and Pedaliaceae, perhaps not immediately related, have 10-hydroxylated carboxylic iridoids. Refulio-Rodriguez and Olmstead (2014) linked all three families, although support was weak. Byblidaceae may be sister to Lentibulariaceae (e.g. Albert et al. 1992), although K. Müller et al. (2004) found no association between the two, nor of either with any Lamiales with viscid indumentum like that of Martyniaceae and Pedaliaceae, a feature which could perhaps be considered to be "precursory" to insectivory. On the other hand, Müller et al. (2004) found a weak association of Lentibulariaceae and Bignoniaceae. Soltis et al. (2007a) found few strongly supported relationships in the bulk of the order; Wortley et al. (2005b), who had sequenced over 4600 bp, estimated that at least 10000 bp more would need to be added to resolve relationships within the clade.
Although focusing on Triaenophora (ex "scroph", now Orobanchaceae s.l.), the relationships that Albach et al. (2009) found are broadly consistent with those suggested by others and the phylogeny of Schäferhoff et al. (2010), the latter in part followed here. The bulk of the free-living Scrophulariaceae are paraphyletic at the base of the iridoid-containing clade of Lamiales, Lamiaceae and Verbenaceae are not sister taxa, the insectivorous and putatively insectivorous clades in Lamiales are unrelated, etc. (Schäferhoff et al. 2010). However, the tree still lacked resolution, especially around the Bignoniaceae-Verbenaceae area.
Findings by McDade et al. (2012: focus on Acanthaceae) apparently contradict part of the tree found by Schäferhoff et al. (2010). In particular, Byblidaceae, Stilbaceae and, surprisingly, Thomandersiaceae all occur on the tree between Plantaginaceae and Scrophulariaceae (support is strong), and Linderniaceae are sister to Scrophulariaceae (support is weak), however, these relationships have not been recovered elsewhere. A clade including Lamiaceae, Orobanchaceae, etc., was retrieved by McDade et al. (2012), although many of the internal support values were low; this clade has received support since. Although Bell et al. (2010) recovered a clade [Oleaceae [Byblidaceae, Plantaginaceae, The Rest (including Gesneriaceae)]], support along the spine was rather weak for the most part. On the other hand, Perret et al. (2012: focus on Gesneriaceae) found relationships more similar to those in Schäferhoff et al. (2010), although the two carnivorous clades Byblidaceae and Lentibulariaceae were sister taxa.
Refulio-Rodriguez and Olmstead (2008: summary) suggested that substantial progress in disentangling relationships around Lamiacae-Verbenaceae and Scrophulariaceae s.l. might be on the horizon (see also Xia et al. 2009). Details of their findings (Refulio-Rodriguez & Olmstead 2014) largely agree with those of Schäferhoff et al. (2010), although more genes were analyzed and support values are usually higher. In particular, nodes along the back-bone of the tree up to [Stilbaceae + The rest] all have very strong support. Resolution along much of the rest of the backbone remains weak, and family groupings also have little support (see also Z.-D. Chen et al. 2016). The family groupings in the part of the tree [Byblidaceae + The Rest] are those of Refulio-Rodriguez and Olmstead (2014), but for the most part they await confirmation; relationships suggested by Wikström et al. (2015) are rather different - and see also below. However, the Lamiaceae-Orobanchaceae clade had moderate-good support in Refulio-Rodriguez and Olmstead (2014; see also Schäferhoff et al. 2010; Z.-D. Chen et al. 2016; W.-Q. Xu et al. 2018: only four families examined), and with rather higher internal support values. Genera are still being moved around in this clade, and some relationships within it are still not entirely clear. For further discussion of relationships around here, see below.
W.-Q. Xu et al. (2018) looked at chloroplast genomes in a number of Lamiales and suggested some relationships in the middle of the tree (relationships elsewhere were similar to those adopted below). In particular, they found two "strongly supported" clades, [Pedaliaceae* [Bignoniaceae + Verbenaceae]] and [Acanthaceae* + Lentibulariaceae], although in fact maximum likelihood support for the relationships of the taxa with asterisks were low to very low (Xu et al. 2018). Sampling may also be an issue here, since Xu et al. (2018) included only five of the eleven families in this area in their analyses. However, families beyond Stilbaceae formed two major clades, albeit one poorly supported, as Schäferhoff et al. (2010) had earlier found, and two groups also appeared in Z.-D. Chu et al. (2016), although Martynia was sister to the Lamiaceae-Orobanchaceae clade. Similar changes also are suggested by H.-T. Li et al. (2019) who recovered the relationships [[(poor support) Byblidaceae + Linderniaceae] [Stilbaceae [group 1 = Schlegeliaceae [Pedaliaceae [[Verbenaceae + Bignoniaceae] [Lentibulariaceae + Acanthaceae]]]] [group 2 = Lamiaceae-Orobanchaceae clade], groups 1 and 2 being strongly supported. In the more comprehensive plastome analysis of Li et al. (2021) relationships were [[(poor) Byblidaceae + Linderniaceae] [Stilbaceae [Schlegeliaceae [(poor)[(poor) Martyniaceae [(poor)Lentibulariaceae + Thomandersiaceae]] [(poor)[Bignoniaceae + Verbenaceae] [(very poor) Pedaliaceae + Acanthaceae]]]] [Lamiaceae [Mazaceae [[Phrymaceae + Wightiaceae] [Paulowniaceae + Orobanchaceae]]]]]]]. Xia et al. (2019: chloroplast protein coding genes) found that Pedaliaceae associated with the [Verbenaceae + Bignoniaceae] clade, and in their nine-plastid-marker tree, Martyniaceae did too, and Lentibulariaceae grouped with Pedaliaceae, albeit with very poor support, while Thomandersiaceae and Schlegeliaceae linked with Acanthaceae, but again with very poor support. B. Liu et al. (2019) also recovered these two groups, which they called the LAVB and LMPO clades respectively. In Luna et al. (2019) the former clade had the relationships [[Acanthaceae [Thomandersiaceae + Schlegeliaceae]] [[Pedaliaceae + Lentibulariaceae] [Verbenaceae [Martyniaceae + Bignoniaceae]]]], and although the families were individually strongly supported (Bignoniaceae rather less so) deeper relationships were generally very weak. Although Sarzi et al. (2019: plastid analyses) recovered a set of relationships that are very much scrambled compared with those being discussed, there may well be sampling issues. Similarly, relationships are very different in Deng et al. (2020), Schlegeliaceae and Lamiaceae are close to switching positions and a [Lentibulariaceae + Acanthaceae] clade are sister to the remainder or the [group 1 + 2] clade. In another plastome analysis, Xia et al. (2020) found the relationships [Scrophulariaceae [[[Lentibulariaceae + Acanthaceae] [Pedaliaceae [Verbenaceae + Bignoniaceae]]] [[Mazaceae + Lamiaceae] [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]], mostly well supported; Orobanchaceae were the focus of this study. Results of analyses of variation in nuclear genomes by C. Zhang et al. (2020) are in part well supported and suggest relationships that are rather different again in much of the Core Lamiales - [Plantaginaceae [Byblidaceae, Linderniaceae, Scrophulariaceae [Stilbaceae [Lentibulariaceae, Pedaliaceae, Acanthaceae [[Schlegiaceae [Verbenaceae [Martyniaceae + Bignoniaceae]]] [Lamiaceae [[Mazaceae + Phrymaceae] [Paulowniaceae + Orobanchaceae]]]]]]]]. Note, however, S. A. Smith (2013) had earlier suggested that there were problems with the relationships of a number of Lamiales, while relationships recovered by W. J. Baker et al. (2021a: see Seed Plant Tree, Angiosperms353 nuclear gene data set) above Stilbaceae in the sequence here show a number of differences - thus relationships in Lamiales finish [... [Mazaceae [Lamiaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]]. A number of the relationships recovered by Fonseca (2021: 6910 spp., 842 genera, 9 chloroplast loci + ITS) had poor support (80>% bootstrap), although the families themselves were well supported; 130 genera were poly- or paraphyletic, but in some of the others only a single species was sampled. Relationships here are [Plocospermaceae [[Carlemanniaceae + Oleaceae] [Tetrachondraceae [[Gesneriaceae [Peltantheraceae + Calceolariaceae]] [Plantaginaceae *[Scrophulariaceae *[Byblidaceae *[Linderniaceae *[Stilbaceae [*[Pedaliaceae *[[Lentibulariaceae + Acanthaceae] *[Thomandersiaceae *[Schlegeliaceae *[Martyniaceae *[Bignoniaceae + Verbenaceae]]]]]] [Lamiaceae [Mazaceae *[Phrymaceae *[Orobanchaceae [Paulowniaceae + Wightiaceae]]]]]]. Finally, X. Wang et al. (2023) looked at the plastomes of 107 genera - the focus was on relationships of the old Scrophulariaceae - recovering the following set of relationships, nearly all well supported - [Oleaceae [Gesneriaceae [Plantaginaceae [Scrophulariaceae [Linderniaceae [[*[Lentibulariaceae + Acanthaceae]*[Pedaliaceae [Verbenaceae + Bignoniaceae]]] [Lamiaceae [Mazaceae [[Wightiaceae + Phrymaceae] [Paulowniaceae + Orobanchaceae]]]]]]]]]] - indeed, the old Scrophulariaceae are polyphyletic, appearing in no fewer than seven families.
Further developments in this area, especially those based on variation in the nuclear genome, are of interest. Recent trees (Zuntini et al. 2024) based on such data showed relationships part familiar, part unfamiliar: Vahliaceae are sister to the whole order, and there relationships are [Plocospermaceae [[Carlemanniaceae + Oleaceae] [Tetrachondraceae* [[Peltantheraceae [Calceolariaceae + Gesneriaceae]] [Plantaginaceae *[*[Linderniaceae + Scrophulariaceae] [*[Stilbaceae + Byblidaceae] [*[Thomandsersiaceae *[Pedaliaceae + Acanthaceae]] [*[Tetrachondraceae [Schlegeliaceae [Verbenaceae *[Lentibulariaceae [Martyniaceae + Bignoniaceae]]]]] [Lamiaceae [[Mazaceae + Phrymaceae] *[Wightiaceae *[Paulowniaceae + Orobanchaceae]]]]]]]]]]] - "*" means support could be stronger. Note that in the plastome analysis of H.-T. Li et al. (2021) relationships were similar, although Tetrachodraceae were by themselves, Vahliaceae were associated with Solanales, and so on, while in the plastome study of X. Dong et al. (2022; Fig. 6) Mazus, Lancea and Dodartia ended up sister to Lamiaceae - Mazaceae do seem to move around a bit. Note, however, that the tree in Dong et al. (2022: Fig. 8), which apparently is based on the same data as that in Fig. 6, relationships are very substantially different.
Two other genera have sometimes been associated with Lamiales. Lens et al. (2008a) and Weigend et al. (2013b) suggested that Vahlia/Vahliaceae was sister to all other Lamiales, although support was weak. However, Vahlia (now Vahliales) is perhaps more likely to be sister to Solanales (Refulio-Rodriguez & Olmstead 2014; see also H.-T. Li et al. 2019, 2021), although its final resting place in core Lamiales is unclear (see above, Phylogeny. The position of Hydrostachys/Hydrostachyaceae within the asterids has also not been easy to determine. There is quite good evidence for its inclusion in Cornales, and there is a discussion of its relationships there. Burleigh et al. (2009) had suggested that it was a member of Lamiales, and its morphology is in general agreement with such a position - if indeed it should end up in Lamiales, it would be likely to be towards the basal part of the tree. Interestingly, the positions of both genera, especially that of the latter, are rather different in the preliminary nuclear phylogeny of W. J. Baker et al. (2021a), but neither is close to Lamiales.
Classification. R. Olmstead (pers. comm.) has been compiling a synoptical classification of Lamiales from which some of the numbers of taxa included in the families below are taken. The limits of families like Scrophulariaceae have long been problematic (Thieret 1967 for a summary), and Olmstead (2002) provided a readable account of some of the changes in our ideas of relationships in the Scrophulariaceae s.l. in particular. Family limits immediately below Orobanchaceae on the tree have been narrowly drawn, the result of keeping Orobanchaceae themselves, nearly all hemi- or holoparasitic, separate. However, although family limits need not be changed because of the findings of C. Zhang et al. (2020: see above), it is clear from the discussion above that family groupings in the Core Lamiales may turn out to be rather different from those sketched out below.
Despite the lack of morphological support for some of the families, little is to be gained and more lost if their limits are much expanded.
PLOCOSPERMATACEAE Hutchinson - Plocosperma buxifolia Bentham - Back to Lamiales
Subdeciduous shrubs or trees; vessels in radial multiples; large groups of fibres in outer cortex at nodal region; petiole bundle annular; styloids +; hairs unicellular, calcified and/or with cystoliths, also bicellular, club-shaped, glandular; cuticle wax crystalloids 0; short shoots +; petiole articulated near base; plant cryptically dioecious; inflorescences axillary; bracteoles 0; flowers 5-6-merous; staminate flowers: anthers extrorse, versatile, with largely separate thecae; nectary ?0; pistillode +; carpelate flower: staminodes +; nectary +; stylar fusion postgenital, placentation parietal, style twice divided, stigmas not expanded; ovules 2/carpel; seeds with tuft of hairs at chalazal end, hairs multicellular; coat ?; endosperm ?development, slight; n = x = ?, protein bodies in nucleus?
1 [list]/1. Central America. Map: from Leeuwenberg (1967).
Evolution: Divergence & Distribution. Diversification in Plocospermataceae seems to have slowed down (Magallón et al. 2018).
Chemistry, Morphology, etc.. Plocospermataceae are poorly known. Jensen (1992) recorded verbascosides and cornoside from Plocosperma, but not iridoids.
Struwe and Jensen (2004) described the inflorescence as being a congested raceme or dichasium, and this, and the apparent absence of nectaries in the staminate flowers, should be confirmed. Carpel number is given as (2-)4 in neotropikey. For ovule position, see Leeuwenberg (1967).
See also D'Arcy and Keating (1973: as Lithophytum, esp. anatomy), M. Endress et al. (1996), and Struwe and Jensen (2004), both general, for information.
Previous Relationships. Plocospermataceae were included in Gentianales by Takhtajan (1997), probably because Plocosperma had long been associated with Loganiaceae. Cronquist (1981) included the genus in his Apocynaceae, probably because of the hairs on its seeds.
[[Carlemanniaceae + Oleaceae] [Tetrachondraceae [[Peltantheraceae [Calceolariaceae + Gesneriaceae]] [Plantaginaceae [Scrophulariaceae [Stilbaceae [[Byblidaceae + Linderniaceae] [[Pedaliaceae, Martyniaceae, Acanthaceae] [Bignoniaceae [[[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]]] [Lamiaceae [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]]]]]]]]]: A-type gibberellin receptor lost; phloem loading via intermediary cells [specialized companion cells with numerous plasmodesmata, raffinose etc. involved]; cells in heads of glandular hairs with radial vertical walls; flowers 4-merous [?: reverses to 5-merous....]; placentation axile.
Age. Janssens et al. (2009) give a date of 95±11.9 Ma for this node, Magallón et al. (2015) an age of ca 71 Ma, Bell et al. (2010) an age of ca (78-)74, 69(-61) Ma, while Magallón and Castillo (2009) offer estimates of ca 62.8 Ma, Nylinder et al. (2012: suppl., c.f. topology) of about 97.1-74 Ma and Wikström et al. (2015) an age of (89-)79(-69) My; ca 56.2 Ma is the age in Tank et al. (2015: Table S2), (99.8-)85.1(-70.6) Ma in W.-Q. Xu (2018) and ca 88.7 Ma in Y. Xu et al. (2022), while at around 53.9 Ma, the estimate by Naumann et al. (2013) is the youngest.
Evolution: Divergence & Distribution. For the loss of a giberellin receptor, see Yoshida et al. (2018).
There may be low-level and symmetrical expression of CYCLOIDEA2-like genes in Oleaceae (Zhong & Kellogg 2015), perhaps the beginning of changes that led to the development of monosymmetric flowers, and this novelty could perhaps be placed at this node. Hileman (2014; also Hileman & Cubas 2009) discusses the evolution of monosymmetry in Lamiales; there disymmetry is included in monosymmetry (see Hileman 2014: c.f. Fig. 2b).
Genes & Genomes. Edger et al. (2017) put the Mimulus-α duplication event at this node.
Chemistry, Morphology, etc.. Note that the arrangement of the sepals (and petals) is orthogonal in Oleaceae and Calceolariaceae, while that of Tetrachondraceae (and of 4-merous Veronica) is diagonal (Mayr & Weber 2006). For phloem loading, see Turgeon et al. (1993), Rennie and Turgeon (2009) and Davidson et al. 2010).
[Carlemanniaceae + Oleaceae]: C valvate; A 2; pollen 3-colpate; stigma ± clavate; exotestal cells ± palisade, endothelium persistent.
Age. The age of this clade is estimated to be (83.7-)69.4(-54.7) Ma (Tank & Olmstead pers. comm.), (64.5-)62.6(-60.6) Ma (W. Dong et al. 2022) or ca 69.2 Ma (Fonseca 2021).
CARLEMANNIACEAE Airy Shaw - Back to Lamiales
Perennial herbs or shrubs; chemistry?; pericyclic fibers few; nodes?; cuticle waxes 0; lamina margins toothed; inflorescences terminal and axillary; flowers weakly obliquely monosymmetric, 4- or 5-merous, (heterostylous - Silvianthus); K members unequal or not, ± linear, aestivation open?, C aestivation induplicate-valvate; anthers connivent in pairs around the style, latrorse, ?attachment; ovary inferior, nectary on top, style clavate; ovules many/carpel, integument ?5-7 cells across [Silvianthus]; fruit fleshy-capsular, loculicidal, or 5-valved [valves correspond to calyx segments], opening widely, exposing the placentae - Silvianthus, K persistent; exotestal cells with radial walls thickened, interior cells unthickened, or polygonal, all walls thickened [Carlemannia], (endothelium persistent - Silvianthus); endosperm +, ruminate [Silvianthus], embryo small; n = 15, 19, x = ?, protein bodies in nucleus?
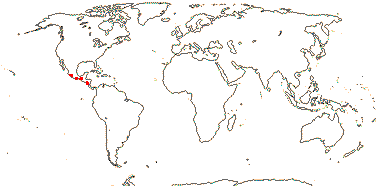
2 [list]/5: Carlemannia (3). E. Nepal to Myanmar, Thailand, Laos, Vietnam, S.W. China, Sumatra. Map: Fl. China vol. 19 (2011).
Age. Crown-group Carlemanniaceae are ca 25.3 Ma (Fonseca 2021).
Evolution: Divergence & Distribution. Carlemanniaceae have a ca 44 My fuse (Fonseca 2021).
Chemistry, Morphology, etc.. Tange (1998a) noted that the pollen grains of Silvianthus were tricolporate and that the stamens were neither on the radius of the sepals or that of the petals.
Some information is taken from Tange (1998a); Thiv (2004) provides a general account, and X. Yang et al. (2007) information on chromosome numbers, etc..
Carlemanniaceae are not well known.
Previous Relationships. Carlemanniaceae have usually been associated with Caprifoliaceae or Rubiaceae in the past; they were included in Caprifoliaceae by Cronquist (1981) and in Rubiales by Takhtajan (1997). However, characters such as superficial cork, two stamens with connivent anthers and two carpels each with many ovules would remove Carlemanniaceae from Caprifoliaceae, and the toothed, exstipulate leaves, 2 stamens, anomocytic stomata, and absence of raphides from Rubiaceae (Solereder 1893; Airy Shaw 1965).
OLEACEAE Hoffmannsegg & Link, nom. cons. - Back to Lamiales
Woody; flavonols, route I iridoids [deoxyloganic acid, loganin, etc. precursors; carbocyclic iridoids], verbascoside or variants, myricetin, orobanchin, sugar alcohol mannitol +; wood with minute calcium oxalate crystals; vessels single, (vessel elements with scalariform perforation plates); fibre tracheids +; sclereids + (0), various; foliar calcium oxalate crystals as styloids, raphides (0; druses +); petiole bundle arcuate; cuticle deeply furrowed (waxes ribbons, platelets); (serial [superposed] axillary buds +); branching from previous innovation; lamina margins entire to toothed, (secondary veins palmate); flowers 4-merous; K valvate, initiation orthogonal; anther thecae ± back-to-back; tapetal cells often 3≤ nucleate; pollen (grains tricellular), intine thickening at the apertures [= oncus] [?first two tribes?]; (style short), stigma dry; ovules apical, (hemitropous), epitropous or apotropous, integument 7-8 cells across, (postament +), hypostase +; testa often vascularized, exotesta moderately and evenly thickened (endotesta fibrous), (endothelium ?not persistent); endosperm +/0, first division asymmetrical; x = 13, nuclear protein bodies crystalline-globular, nuclear genome [1 C] (0.067-)1.237(-22.832) pg; plastome 9 bp deletion in ndhF gene.
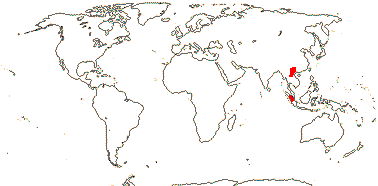
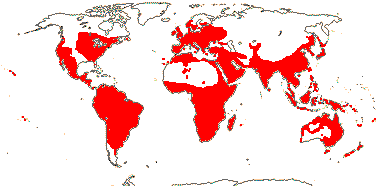
23/620 (790): [list - to subtribes] - five tribes below, one with four subtribes. More or less worldwide, especially East Asia. Map: from Meusel et al. (1975) and Australia's Flora Online (consulted xii.2012).
Age. The age of crown-group Oleaceae is (73-)60.1(-49.2) Ma (Tank & Olmstead, pers. comm.), ca 58.2 Ma (Fonseca 2021), (64.1-)60.5(-56.0) Ma (W. Dong et al. 2022) or 87.2-)85.9(-83.7 Ma (Dupin et al. 2024).
1. Myxopyreae Boerlage —— Synonymy: Nyctanthaceae J. Agardh
Woody, ± scandent (perennial herb); (flavones + [different from Oleeae]), myxopyroside iridoids [carbocyclic]; inverted cortical bundles in corners of angled stem (Dimetra not); petiole bundles three, arcuate; leaf blades ± triplinerved; K initiation diagonal, C aestivation various, (early tube formation - Nyctanthes); (G collateral); ovules 1(-3)/carpel, ± basal, integument to 20 cells across [Nyctanthes]; (megaspore mother cells several, embryo sac bisporic, 8-nucleate [Allium type]); fruit a berry/schizocarp/deeply lobed drupe; endosperm cellular [Nyctanthes]; n = 11, 12 (16, 22, 23); germination epigeal.
3/7: Myxopyrum (4). Indo-Malesia.
Age. Crown group Myxopyreae are around 29.5 Ma (W. Dong 2022) or (75.3-)68.0(-60.2) Ma (Dupin et al. 2024).
[Fontanesieae [Forsythieae [Jasmineae + Oleeae]]: ?
Age. The crown-group age of this clade is some 83.3-)80.0(-76.5) Ma (Dupin et al. 2024).
2. Fontanesieae L. Johnson - Fontanesia phillyraeoides Labillardière
Plant deciduous; route 1b iridoids; pits ± vestured; petiole bundle annular; C free, imbricate; ovule 1/carpel; fruit a samara; testa crushed; n = 13.
1/1-2. Sicily, Middle East (Turkey, Syria), China.
Age. This clade is only (1.5-)0.3(-0.0) Ma (Dupin et al. 2024).
[Forsythieae [Jasmineae + Oleeae]]: ?
Age. This clade may be (52-)44.9(-39.4) Ma (W.-Q. Xu et al. 2018) or (81.4-)78.0(-74.3) ma (Dupin et al. 2024).
3. Forsythieae L. Johnson
Plant deciduous; cornosides, route 1b iridoids [forsythide]; pith chambered; plant heterostylous; anther wall devlopment basic type [epidermis persistent]; tapetal cells binucleate; ovules 1-many/carpel, micropyle long, integument 5-15 cells across, nucellar cap ca 2 cells across, hypostase +, funicular obturator +; archesporial cells several; fruit a samara [Abeliophyllum]/loculicidal capsule, exotestal [A.]; n = 14.
2/10: Forsythia (9). S.E. Europe, East Asia.
Age. The age of crown-group Forsythieae is about 19.2 Ma (W. Dong et al. 2022) or (17.7-)13.6(-10.1) Ma (Dupin et al. 2024).
[Jasmineae + Oleeae]: route 1b/1c iridoids [secoiridoids - oleoside]; leaves odd-pinnate to simple; suspensor very long.
Age. The age of this node may be (52-)48, 39(-35) Ma (Wikström et al. 2001), ca 78.3 Ma (H.-L. Lee et al. 2007), (62-)45, 41(-24) Ma (Bell et al. 2010), (45.7-)41.6(-38.4) my (W.-Q. Xu et al. 2018) or (77.5-)73.9(-69.9) Ma (Dupin et al. 2024).
4. Jasmineae Lamarck & de Candolle —— Synonymy: Bolivaraceae Grisebach, Jasminaceae Jussieu
(Herbs), shrubs, climbers, usu. evergreen; oleoside +; (also libriform fibres); leaves (spiral), simple to pinnate; (K, C to 14 or more), first 4 K initiation diagonal, C quincuncial-imbricate, tube formation early; endothelium 0; ovules/carpel (1, 4), ± horizontal,; (megaspore mother cells several, several elongated embryo sacs developing); fruit deeply bilobed, style basal, indehiscent (loculicidally dehiscent)/dry, each lobe circumscissile [Menodora]; seed coat multilayered, sarcoexotesta + (0 - sect. Alternifolia), mesotesta/1 layer of mesotestal cells with wholly thickened or band-thickened anticlinal walls (0), vascular bundles 1-several; endosperm free-nuclear, (0), (cotyledons with starch); n = 11-13; 21 kb chloroplast inversion, serial loss of accD gene.
3/225-450: Jasminum (ca 200). Tropical to warm temperate Old World, some in America. Photo: Flower.
Age. Crown group Jasmineae are estimated to be ca 37.8 Ma (W. Dong et al. 2022), about 42.1 Ma (H.-L. Lee et al. 2007) or (57.4-)52.3(-46.6) Ma (Dupin et al. 2024).
5. Oleeae Dumortier
Flavone glycosides, flavanones, etc., oleoside +; vessel elements in multiples, libriform fibres +; (pits vestured), (torus-margo pits +), libriform fibres + (0), fibre tracheids 0 (+); petiole bundle also annular (medullary bundles +), (1-3 pairs of wing bundles); marginal parenchyma +/0; flowers heterostylous [?]; K (diagonal), (open), (with one trace), C (induplicate-)valvate, tube formation late; A (1-4 or more); ovules pendulous; (archesporial cells several), (embryo sac bisporic [the chalazal dyad] and 8-celled [Allium type]); fruit a drupe/schizocarp/loculicidal capsule; testal cells (compressed, walls thickened); n = 23 (20, 22); germination epigeal or hypogeal; plastid transmission biparental.
16/420. Tropical and subtropical.
Age. Crown group Oleeae may be around 46.6 Ma (W. Dong et al. 2022) or (59.9-)56.7(-54.1) Ma (Dupin et al. 2024).
[Schreberinae [Ligustrinae [Fraxininae + Oleinae]]]: ?
5A. Schreberinae E. Wallander & V. Albert - Schrebera Roxburgh —— Synonymy: Schreberaceae Schnizlein
Leaves simple to imparipinnate, lamina glandular-punctate; (flowers homostylous); C (-7 merous), imbricate; ovules 4/carpel; fruit a loculicidal capsule; seeds winged.
1/16. Tropical, largely Old World, esp. Madagascar, not East Malesia, 1 sp. Peru.
Age. The age of Schreberinae is some 22.5 Ma (W. Dong et al. 2022), (37.1-)34.4(-32.) Ma (Hong-Ma et al. 2023) or (44.8-)35.1-(26.1) Ma (Dupin et al. 2024).
[Ligustrinae [Fraxininae + Oleinae]: ovules 2/carpel, pendulous.
Age. The age of this clade is some (55.7-)53.0(-50.7) Ma (Dupin et al. 2024).
5B. Ligustrinae Koehne - Ligustrum L. —— Synonymy: Ligustraceae G. Meyer, Syringaceae Horaninow
(± Deciduous) shrubs to small trees; lamina with flat abaxial glands; C tube formation early; fruit a drupe/loculicidal capsule.
1/60. Europe (1 sp. North Africa) to East Asia, 1 sp. to Australia.
Age. Crown-group Ligustrinae are estimated to be ca 34.1 Ma (W. Dong et al(. 2022) or (42.9-)32.5(-23.6) Ma (Dupin et al. 2024).
Age. This clade is estimated to be around 39.4 Ma (W. Dong et al. 2022) or (49.7-)48.5(47.9) Ma (Dupin et al. 2024).
5C. Fraxininae E. Wallander & V. A. Albert - Fraxinus L. —— Synonymy: Fraxinaceae Vest
Deciduous (evergreen); plant dioecious/androdioecious/etc.; K + C 0/C 0, 2, 4 (connate)+; (A with apiculate connective - F. pennsylvanica); fruit a samara.
1/47(?-65). North temperate (subtropical).
(Age. Crown group Fraxininae are estimated to be (47.2-)45.4(-43.6) Ma (Hinsinger et al. 2013), around 27.7 Ma (W. Dong et al. 2022) or (45.5-)41.0(-37.1) Ma (Dupin et al. 2024).
Hinsinger et al. (2013) discuss fossils from the early Tertiary that have been attributed to Fraxinus, but question their identity. Samaras of F. eoemarginata, from Quilchena in the Okanagan Highlands, British Columbia, are dated to the early Eocene ca 51.5 Ma (Mathewes et al. 2021), and samaras are also reported from mid-Eocene deposits in Tennessee that are some 44 Ma (Call & Dilcher 1992). Migration to Asia had occurred by the early Eocene.
5D. Oleinae E. Wallander & V. A. Albert —— Synonymy: Forestieraceae Meisner
(Indumentum of peltate scales), lamina vernation conduplicate [Chionanthus], (bracteoles adnate to C - Olea); C (free: Chionanthus); fruit a drupe.
/290: Noronhia (105), Chionanthus (60-120), Olea (33), Notelaea (23). Widespread, tropical to temperate, inc. Macaronesia, Hawaii, etc..
Age. Crown-group Oleinae are estimated to be ca 33.8 Ma (W. Dong et al. 2022) or (37.8-)33.7(-30.1) Ma (Dupin et al. 2024).
Wood identified as Oleinae (= Oleeae above) has been found in the Deccan Traps 67-65 Ma (Srivastava et al. 2015), however, Rhamnaceae and Rutaceae are also possibilities (see also S. Y. Smith et al. 2015; Wheeler et al. 2017).
Evolution: Divergence & Distribution. Some molecular dates are incompatible with the fossil record (see above). Furthermore, ages for the same event can vary considerably - for example, the [Madagascan + African] clade circumscribed by Schrebera alata and S. madagascariensis was thought to be (28.4-)25.6(-22.9) Ma by Hong-Wa et al. (2023) but was estimated to be a mere ca 7.4 Ma by S. A. Smith and Brown (2018).
W. Dong et al. (2022: Figs 8, esp. 9) describe a rather complex history of hybridization and introgression, not to mention incomplete lineage sorting as well, in Oleaceae. That paper should be read for details, and here I mention only that Oleeae seemed to be the result of a hybridization event between stem Forsythieae (the staminate parent) and a ghost lineage of stem Jasmineae (the carpelate parent), and this resulted in a tetraploid Oleeae. Within Oleeae there appeared to have been hybridization between stem Ligustrinae and stem [Fraxininae + Oleinae], and also hybridization/introgression from stem Fraxininae to stem Oleinae (Dong et al. 2022) - and here the clades that hybridized have remained diploid. Of course, in all cases ghost lineages are likely to have been involved. However, Dupin et al. (2024) found no evidence for this hybridization.
Much diversification within Oleaceae occurred during the Caenozoic (Besnard et al. 2009a). Hinsinger et al. (2013: q.v. for numerous dates) discuss diversification in Oleeae-Fraxininae-Fraxinus, a genus that they suggest originated in Eocene North America, but with subsequent migrations back and forth across the Beringian land bridge and associated vicariance/geographic speciation events, hybridization also being involved (see also above; M.-X. Wu et al. 2021, etc., for the fossil history of Fraxinus). Oleinae-Hesperelaea, from Guadalupe Island, west of Baja California, but now extinct, was probably older than Guadalupe Island itself - (27.5-)19.7(-12.5) versus (9-)7(-5) Ma - and is likely to have moved there from the mainland (Zedane et al. 2015). Picconia excelsa, from the Madeira Islands, may be a component of the old laurel forests there. However, Palaeo-Macaronesia is likely to be 60 Ma or more old (Gelmacher et al. 2005; Fernández-Palacios et al. 2011), while Picconia is related to Notelaea s.l., together making a clade scattered from Macaronesia to Hawaii and (23.8-)23.2(-14.7) Ma, while Notelaea itself, (20.7-)15.3(-10.5) or ca 18.9 Ma, is widely dispersed in the Pacific, being known from New Zealand, New Caledonia, Norfolk Island, and Australia to Hawaii, such distributions as always raising questions of how and when the plant got to where it lives now (Dupin et al. 2022 - see also Nothofagaceae, Amborella, etc.). Although details of the basal phylogeography of Schreberinae are unknown, its wide distribution has probably been achieved by long distance dispersal events (Hong-Wa et al. 2023).
In their extensive study of Oleaceae, Dupin et al. (2024) suggested that Asia, both temperate and tropical, is likely to have been central to the early evolution of the family, although for Fontanesieae Europe may also have been involved. Subsequently frequent dispersal events facilitated the current distribution of the family, and here Asia was central. Perhaps 45/88 dispersal events were from tropical Asia, 24 from temperate Asia - and most of the former were to temperate Asia, while most of the latter were to tropical Asia (Dupin et al. 2024).
Breeding systems in Fraxinus are diverse, and a number of species are androdioecious, uncommon in angiosperms; basic floral morphology and pollination are also variable (Wallander 2013). Although heterstyly is known from a number of Oleeae, details of its evolution are unclear (Hong-Wa et al. 2023).
Wallander and Albert (2000: esp. Fig. 3) looked at the evolution of a number of characters in the family, and this is accompanied by a detailed discussion (e.g. see Myxopyreae); their work is followed here, slightly modified. Oleoside, (4S,5E,6S)-4-(carboxymethyl)-5-ethylidene-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylic acid, is found in both Jasmineae and Oleeae, perhaps an apomorphy for the two even allowing for the hybrid origin of the latter given the parental status of the former.
Faster substitution rates in Jasmineae that have long been evident, as in Wallander and Albert (2000), etc., may be linked to the shorter generation times there, Jasmineae including herbs and small shrubs, etc., but not trees (W. Dong et al. 2022). Jasmineae are, however, not notably speciose when compared with Oleeae, their sister group (c.f. Dong et al. 2022).
Ecology & Physiology. Jasminum is one of the ten globally most species-rich genera of climbers (Sperotto et al. 2023).
Pollination Biology & Seed Dispersal. For the evolution of wind pollination, dioecy, etc., in Fraxinus, see Wallander (2008, 2013).
Plant-Animal Interactions. Caterpillars of some Sphinginae are quite common on Oleaceae (and the same genera may also be found on Solanaceae: Forbes 1958).
Larvae of the emerald ash borer, the buprestid Agrilus planipennis, burrow under the bark of ash trees (Fraxinus - Oleeae-Fraxininae) and kill them; recently (late 1980s) introduced into eastern North America from China, the insect is a major threat to ash on the North American continent. Semizer-Cuming et al. (2019) discuss the threats posed to ash worldwide (see also below).
Plant-Bacterial/Fungal Associations. In Europe, a comparable threat to Fraxinus excelsior is posed by the ascomycete Hymenoscyphus fraxineus (its correct name is unclear) which causes ash dieback; it was introduced there in the early 1990s. This fungus has recently been found in England growing on two genera of cultivated Oleeae-Oleinae, a subtribe that includes olives, in England, although neither Jasminum, Syringa nor Ligustrum is known to be affected (Forest Research 2018: https://www.forestresearch.gov.uk/news/chalara-ash-dieback-different-ash-species-and-non-ash-hosts/). Interestingly, iridoid glycoside abundance was higher in trees of F. excelsior susceptible to H. fraxineus, but such trees may also be less susceptible to herbivores (Sollars et al. 2017).
Genes & Genomes. It has been suggested that there have been genome duplications in the clade leading to Olea europea 28 and 59 Ma (Turgay Unver et al. 2017), the older one being quite deep in the family. Sollars et al. (2017) and Edger et al. (2017) found a duplication in the [Fraxinus + Olea] clade; its place in the family could not be established given the sampling. Hybridization of Fraxinae with stem-group Oleinae - hence the polyploid nature of that subtribe - is a possibility (W. Dong et al. 2022). However, Dupin et al. (2024) did not recover evidence of hybridization here.
For the extensive reorganization of the plastid genome in Jasmineae that is caused by overlapping inversions and with a reduction in size of the small single copy portion (H.-L. Lee et al. 2007 - their work should be followed up); this reorganization is not unexpected given that plastid transmission is biparental here (W. Dong et al. 2022). The chloroplast gene accD (= ORF512, zpfA) has been lost (Doyle et al. 1995 and references) in at least some Oleaceae - again, see Jasminum s.l. (Lee et al. 2007).
Economic Importance. For olive oil, see papers in Vollmann and Rajcan (2009).
Chemistry, Morphology, etc.. The route I secoiridoids are unlike other route I secoiridoids, e.g. those in Gentianaceae (Jensen 1992; Jensen et al. 2002; see also Gousiadou et al. 2015). Damtoft et al. (1995) noted that the secoiridoids of Fontanesia (loganic acid, etc., and 5-hydroxylated derivates like swertiamarin) were produced by a somewhat different biosynthetic pathway than the oleoside-type secoiridoids common elsewhere in the family. Abeliophyllum, in a clade possibly sister to rest of Oleaceae (but see Forsythieae above), has cornosides and verbascosides, and some lack iridoids; these features may be plesiomorphies - but certainly not if Myxopyreae are sister to the rest of the family (see below); absence (= loss) or iridoids occurs elewhere in the family (Jensen et al. 2002). Franzyk et al. (2001) noted that Myxopyrum and Nyctanthes, both in Myxopyreae, had similar iridoids. There is a single report of cornosides from the [Jasmineae + Oleeae] clade (Jensen et al. 2002).
At least some species of Osmanthus (and the related Picconia/Chionanthus retusus) have lignified, torus-bearing, intervascular pit membranes (Coleman et al. 2004: Dute et al. 2010b; Dute 2015; Nguyen et al. 2017). There are peltate-glandular hairs in e.g. Chionanthus (Kiew & Ibrahim 1982; it is unclear how these relate to the hairs with radial vertical walls mentioned above as a synapomorphy for the majority of Lamiales. Govil (1973) showed how the lateral bundles of the petiole in Nyctanthes were derived from the cortical vascular system. The diversity of crystal types in the vegetative plant (other than the wood) is very great, but druses, the crystal form in which calcium oxalate commonly occurs elsewhere, are uncommon here (Lersten & Horner 2008a, 2009a, esp. b). Crystals are often clustered in epidermal cells at the bases of trichomes, an unusual distribution pattern (Lersten & Horner 2009b). Groups of few-celled secretory hairs may form extrafloral nectaries (Zimmermann 1932).
The calyx is sometimes diagonally oriented (Sehr & Weber 2009), and there is variation in corolla tube initiation, both early and late initiation being known in the family (Sehr & Weber 2009). Nectar is reported to be secreted from the ovary in Syringa and Ligustrum (Weberling 1989). Osmophores are common and their absence from the anthers may be of systematic interest (Nilson 2000: sampling?); orbicules may be absent (Vinckier & Smets 2002a). There is infrageneric variation in the orientation of the two carpels; however, the two stamens are always borne in the plane of the ovary septum (Eichler 1874). Baillon (1891) illustrated both epitropous and apotropous ovules. Ghimire and Heo (2014a, see also Ghimire et al. 2020) suggested that the integument of Abeliophyllum was 5-7 cells across, but from their Fig. 3d (for example), it is at least 10 cells across; they also describe the integument of the whole family as being multiplicative (but c.f. Ahn et al. 2023). Indeed, the seed coat may be 14-20 or so cells across, the outer epidermis being enlarged (Patel 1963). Exotestal testal cells of Fraxinus are in short longitudinal rows separated by small underlying mesotestal cells (Ghimire et al. 2018). The endocarp s. str. of Olea is little thickened (King 1938).
For more information, see Green (2004: general), Jensen et al. (2002 and references: iridoids), Baas et al. (1988: wood anatomy), Song and Hog (2012: some petiole anatomy, Naghiloo et al. (2013: inflorescence morphology), Sehr and Weber (2009) and Dadpour et al. (2011a), both floral ontogeny, the latter also some inflorescence morphology. Bigazzi (1989a: protein nuclear inclusions), Kiew and Baas (1984) and Rohwer (1994b), both Nyctanthes, King (1938: Olea), Andersson (1931), Kapil and Vani (1966), Maheswari Devi (1975) and Ghimire et al. (2020: Abeliophyllum), all embryology, and Rohwer (1993b, 1995, 1996: fruit and seed).
Phylogeny. Wallander and Albert (2000: some morphology also) found a [Jasmineae + Oleeae] clade, the two tribes having a fair bit of internal resolution; relationships of the other three tribes were unresolved. H.-L. Lee et al. (2007), however, found Myxopyreae to be sister to the rest of the family (100% bootstrap support), with Fontanesieae, Forsythieae and [Jasmineae + Oleeae] forming a tritomy. Kim and Kim (2011) suggested a quite well supported set of groupings [[Fontanesieae + Jasmineae] [Oleeae + Forsythieae]]; unfortunately, they did not sample other members of the family. The position of Jasmineae and Forsythieae is also switched from that followed here in Z.-D. Chen et al. (2016), but support there was not strong. Dupin et al. (2020: 80 plastid and 37 mitochondrial markers, nuclear ribosomal cluster, phyB and phyE) carried out a variety of analyses on 61 taxa that represented all genera in the family. Although the arrangement of tribes and subtribes in the combined analysis and the plastome analysis is that shown above, the chondrome analysis started off [Forsythieae [Fontanesieae [Myxopyreae ...]]], and the nuclear analyses were problematic, the topologies they yielded being rather different. The phylogeny suggested by W. Dong et al. (2022) that involved extensive hybridization is discussed above. Dong et al. (2022) caried out a variety of analyses using plastomes, nuclear SNPs, and many nuclear genes, and recovered quite extensive discordance when the various topologies were compared; see also Olofsson et al. (2019: 86 spp.) for plastome—nuclear conflict, but little conflict was found comparing SNP analyses using two different reference nuclear genomes. Dupin et al. (2024: 36 plastid and low copy nuclear markers) returned to the fray, incorporating 298 species, around 2/5 of the family, in their study.
Jasmineae. Jasminum was paraphyletic if Menodora was recognized (e.g. Jeyarani et al. 2018), Jasminum section Alternifolia (now = Chrysojasminum) being sister to [Menodora + the rest of Jasminum].
Relationships recovered in Oleeae by Dupin et al. (2020, 2024) were [Schreberinae [Ligustrinae [Fraxininae + Oleinae]]], rather than the reverse order for the first two. Besnard et al. (2009a) and Guo et al. (2011) examined relationships in some Oleeae, while Hong-Wa and Besnard (2013, also 2014; see also Dupin et al. 2022) found considerable geographical signal in the clades they obtained in studies of relationships around Noronhia and in other Oleinae - although polyploidy presented a problem in some of their analyses. The recently extinct genus Hesperelaea was placed in a clade with Forestiera and Priogymnanthus by Zedane et al. (2015; see also Oloffsen et al. 2019; Dupin et al. 2022, 2024). Polyphyly in Chionanthus was found to be extreme, Tetrapilus was biphyletic (Dupin et al. 2024). Wallander (2008, 2013), Hinsinger et al. (2013: 2 chloroplast markers, nuclear ITS, ETS, phantastica) and others discuss the phylogeny of Fraxinus. Relationships in Schreberinae were clarified by Hong-Wa et al. (2023: mostly plastid genes).
Classification. The tribes and subtribes recognised above are those of Wallander and Albert (2000). Generic limits in Oleeae in particular need much attention, thus Olea itself, Osmanthus, Nestegis and Chionanthus (an utter mess) are all polyphyletic (Besnard et al. 2009a; Guo et al. 2011; Olofsson et al. 2019; Dupin et al. 2020); although Chionanthus had included Linociera, this is questionable. Hong-Wa and Besnard (2013) have begun the necessary process of generic realignment in this area while Dupin et al. (2022) expand the limits of Notelaea-Oleinae, i.a. clarifying the limits of Osmanthus.
Wallander (2013) provides a sectional classification for Fraxinus; three species were unplaced.
Previous Relationships. The position of Nyctanthes has been uncertain, and it was often included in Verbenaceae in the old sense; Filonenko et al. (2010) considered the genus to be separate from both families.
[Tetrachondraceae [[Peltantheraceae [Calceolariaceae + Gesneriaceae]] [Plantaginaceae [Scrophulariaceae [Stilbaceae [[Byblidaceae + Linderniaceae] [[Pedaliaceae, Martyniaceae, Acanthaceae] [Bignoniaceae [[[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]]] [Lamiaceae [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]]]]]]]]: plants ± herbaceous; mycorrhizae uncommon; enhanced but symmetrical expression of CYCLOIDEA2-like genes; C and A initiated simultaneously, or A before C; seeds ≤4 mm long; endosperm also with chalazal haustoria; deletion in the matK gene.
Age. The age of this node is around 87 Ma (K. Bremer et al. 2004a), (94.5-)81.6(-70.5) Ma (Tank & Olmstead pers. comm.), (84-)74(-64) Ma (Wikström et al. 2015), ca 66.7 Ma (Magallón et al. 2015), ca 81 Ma (Fonseca 2021) or as little as ca 53.5 Ma in Tank et al. (2015: Table S1, S2).
Evolution: Genes & Genomes. A genome duplication, the ANMAα event, ca 56.2 Ma, would be placed here, based on the list of genera that have it (Landis et al. 2018).
Chemistry, Morphology, etc.. For information on the matK deletion, see Hilu et al. (2000); the sampling needs to be improved.
TETRACHONDRACEAE Wettstein - Back to Lamiales
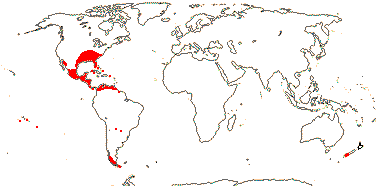
Herb; sugar alcohol sorbitol + [carbohydrate transport]; cork?; nodes with split laterals; leaves amphistomatic; flowers small [≤5 mm across], 4-merous; C with short tube, mouth occupied by hairs; A adnate to C, anthers free; pollen in tetrads, 6-sulcate, smooth; nectary 0; G transverse, slightly inferior, stigma small, capitate/not; fruit with persistent green K; seed pedestals +; testa thin, endothelial cells with persistent thickened inner walls; endosperm copious; x = 9 (?6, ?10), n = 10, 11, x = 9 (?6), protein bodies in nucleus?
2 [list]/3. New Zealand, S.E. U.S.A. to South America. Map: from Fl. Neotrop vol. 81. (2000). Photo: Polypremum flower.
Age. Crown-group Tetrachondraceae are ca 46 Ma (K. Bremer et al. 2004a), (68.4-)39.7(-14.2) Ma (Tank & Olmstead pers. comm.), (61-)39(-18) Ma (Wikström et al. 2015) or ca 47.2 Ma (Fonseca 2021).
1. Polypremum procumbens L. —— Synonymy: Polypremaceae Reveal
± Erect (annual) herb; hairs moniliform; leaves joined by line across node, 1-veined; infloresecence a leafy cyme; placentae peltate, style short; ovules many/carpel, integument 3-4 cells across; fruit a loculicidal (+ septicidal) capsule,
1/1. S.E. U.S.A. to South America.
2. Tetrachondra Oliver
Prostrate, succulent herb, ± aquatic; leaves joined by basal sheath; flowers 1(-2 together), axillary; K initiation diagonal; G with false septum, style gynobasic; ovules 2/carpel, basal; fruit a schizocarp.
1/2. Patagonia, New Zealand.
Evolution: Divergence & Distribution. Wagstaff et al. (2000) found that the sequences of the two species of Tetrachondra, from the Antipodes and S. South America, were almost identical - the distribution is probably recent.
Genes & Genomes. The POPRα genome duplication event, ca 41.2 Ma, occurred here (Landis et al. 2018).
Chemistry, Morphology, etc.. The leaves of both genera are described as having an "interstipular sheath" (Wagstaff (2004a), but from the illustration it would seem that what is meant is that the leaf bases are connate.
Polypremum has both micropylar and chalazal endosperm haustoria; this should be checked in Tetrachondra, a very poorly known genus. The embryo sac of Polypremum protrudes through the nucellar epidermis (Moore 1948).
For general information, see Wagstaff (2004a), some additional information is taken from Mayr & Weber (2006), and Sehr and Weber (2009), also chemistry (Harborne & Williams 1971 - scutellarein +, c.f. Gelsemium!; Jensen 2000a), endothelium presence (absent in Loganiaceae), endosperm type, etc., of Polypremum are right for a position in Lamiales.
Phylogeny. The [Polypremum + Tetrachondra] clade is strongly supported (Oxelman et al. 1999a); see also Wagstaff et al. (2000).
Previous Relationships. Tetrachondra was placed in Boraginales by Takhtajan (1997: two ovules/carpel, gynobasic style in common) and in Lamiaceae by Cronquist (1981: ditto). Polypremum has always been associated with Loganiaceae s.l.; Takhtajan (1997) included it in his Buddlejaceae.
[[Peltantheraceae [Calceolariaceae + Gesneriaceae]] [Plantaginaceae [Scrophulariaceae [Stilbaceae [[Byblidaceae + Linderniaceae] [[Pedaliaceae, Martyniaceae, Acanthaceae] [Bignoniaceae [[[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]]] [Lamiaceae [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]]]]]]] / Core Lamiales: shikimic-acid derived anthraquinones, 6- and/or 8- hydroxylated flavone glycosides + [? Tetrachondraceae], storage substances stachyose and other oligosaccarides; leaf teeth cunonioid; inflorescence indeterminate/open; flowers vertically monosymmetric, 5-merous; K ± asymmetric, C usu. ± bilabiate, 2-lobed upper lip, 3-lobed lower lip [= 2:3], asymmetrical expression of CYCLOIDEA2-like genes, adaxial lobes outside the others in bud [ascending cochleate], tube formation late; A 4, didynamous, placentoids +; pollen tubes lacking callose [?level]; ovules many/carpel; fruit capsular; second division of the endosperm longitudinal, suspensor large.
Age. Bell et al. (2010: note topology) estimated an age of (72-)64, 61(-56) Ma for this node and Magallón et al. (2015) an age of ca 58.1 My; the age in Bremer et al. (2004) is around 78 Ma, in Wikström et al. (2015) it is (76-)65(-56) Ma, and in Wikström et al. (2001: note topology) it is (60-)56, 45(-41) Ma. However, Tank et al. (2015: Table S1) suggest that this node is only ca 43.9 Ma, while ca 73.2 Ma is the age in W.-Q. Xu et al. (2018) and ca 67.7 Ma that in Fonseca (2021).
Martínez-Millán (2010), using a rather scanty fossil record, suggested an Oligocene age for diversification within Lamiales - this would be at this node, since fossil Oleaceae are known from the Eocene.
Evolution: Divergence & Distribution. Many core Lamiales are herbaceous or shrubby and have often quite large monosymmetric, bilabiate flowers, and about equal numbers of species have fruits with many small seeds or with about eight or fewer (but still not very big) seeds. This clade includes the bulk of the diversity within Lamiales, and overall diversification rates are high (Magallón & Sanderson 2001; Magallón & Castillo 2009; Tank et al. 2015), although rates and patterns of diversification of clades in core Lamiales vary considerably. High speciation rates are common in other non-mycorrhizal groups, many of which are herbaceous (Maherali et al. 2016), and also in herbaceous clades per se (e.g. Eriksson & Bremer 1992; S. A. Smith & Donoghue 2008). Monosymmetric flowers may be a key innovation in Lamiales (Endress 2011) and would be pegged to this level, so several things could be driving diversification here.
Monosymmetry may be associated with a shift from determinate paniculate to indeterminate racemose or thyrsoid inflorescences, monosymmetric flowers being lateral on the inflorescence (Degtjareva and Sokoloff 2012). A didynamous androecium, with stamens in two pairs of unequal lengths, is common; the fusion, or at least close attachment, of the paired anthers may improve pollen removal from the flower (Ren & Tang 2010). Endress (1992) discussed floral diversity in the Cronquistian Scrophulariales, i.e., a paraphyletic group that includes Oleaceae (which he did not mention) but excludes Lamiaceae and Verbenaceae, emphasizing parallel evolution in the context of pollination biology. Endress (1997a) looked at the posession of staminodes in core Lamiales, suggesting i.a. that Gesneriaceae showed the weakest expression on monosymmetry in the group, quite often having an androecium with four stamens and a staminode, not just four stamens, and there was reversion to polysymmetric flowers with five stamens in 10 genera or parts thereof. As Endress (1998) noted, monosymmetry in core Lamiales in particular is expressed in the corolla, but one stamen (sometimes more) is commonly reduced or lost, while in Asterales monosymmetry is expressed mainly in the corolla, the number of stamens being equal to the corolla lobes. Zhong and Kellogg (2015) discussed the stepwise evolution of the expression patterns in CYC and RAD genes, involved in the expression of monosymmetry but also expressed in more basal polysymmetric clades here. If the position of Peltanthera, with five stamens and a more or less polysymmetric flower, is confirmed (see below), optimisation of monosymmetry on the tree becomes interesting; understanding the floral development of the genus is important.
Ecology & Physiology. The establishment of arbuscular mycorrhizal associations has a considerable effect on plant defences, upregulating some and downregulating others (e.g. Jung et al. 2012). It is not surprising that both parasitic and insectivorous members of Lamiales are largely non-mycorrhizal (Brundrett 2004 and references), but the absence of mycorrhizae is common in general here (Brundrett 2008, 2017b; Maherali 2016). There is a connection between the herbaceous habit, thin roots, high specific root lengths (i.e. long roots per unit of biomass), and the absence of mycorrhizae (Ma et al. 2018).
Pollination Biology. The evolution of monosymmetric flowers may be connected with the evolution of bee clades like euglossine and bumble bees (ages: 71-40 Ma for plants, 75-65 Ma for bees), derived and generalist bees that can handle complex flowers (Westerkamp & Claßen-Bockhoff 2007; Zhong & Kellogg 2014 and references).
Genes & Genomes. It appears that CYC genes have duplicated independently within the clade and become separately involved in the development of the strongly monosymmetric flowers of Antirrhinum, Mimulus and Gesneriaceae (e.g. Damerval & Manuel 2003; Gübitz et al. 2003; Preston et al. 2011b). However, recent work suggests that recurrent duplications may not play a direct role, but CYC2-like and RAD-like genes have very asymmetric patterns of expression in the corolla of members of core Lamiales with monosymmetric flowers, although not in the more basal clades examined (Zhong & Kellogg 2014, 2015).
Chemistry, Morphology, etc.. For callose, see Prospéri and Cocucci (1979: Oleaceae, etc., not sampled, ?Gesneriaceae). For the distribution of various flavone glycosides, see Tomás-Barberán et al. (1988): Mimulus and Orobanche lack the glycosides of Lamiaceae, Verbenaceae, Scrophulariaceae and Plantaginaceae, while those of Lentibulariaceae are somewhat different, variation broadly consistent with phylogenetic relationships here.
Westerkamp and Claßen-Bockhoff (2007) outline the morphological variation of the corolla. Monosymmetry of the 2:3 type is common and there are four stamens which are often didynamous and sometimes with connivent anthers; for staminodes and stamen reduction in general, see Endress (1998) and Song et al. (2009: molecular mechanisms). Nectary vascularization varies. Nectaries may be vascularized by branches from the main carpellary vascular traces, as in Schlegeliaceae, some Pedaliaceae, Verbenaceae, or separately from the gynoecium, as in Bignoniaceae, Acanthaceae, and other Pedaliaceae. This suggests that either the distinction between gynoecial and receptacular nectaries (Smets 1988; Smets et al. 2003) is overly simplistic and/or there is homoplasy in this feature. There are septal vascular bundles, the gynoecial vascular system forming a sort of figure of 8 in transverse section, in Bignoniaceae and Schlegeliaceae, while in taxa like Acanthaceae there are no septal bundles, the gynoecial vasculature being almost in a circle (there are of course placental bundles: see Wortley et al. 2005a for details). Knowledge of the distribution of this character needs to be extended. The level at which to peg a character "embryo suspensor large" [sic] is unclear. There is much about ovules, seeds, etc., for various taxa, esp. Scrophulariaceae sensu latissimo and Lentibulariaceae, in Takhtajan (2013).
[Peltantheraceae [Calceolariaceae + Gesneriaceae]]: flowers in groups of four.
Age. The age for this node is ca 71 Ma (K. Bremer et al. 2004a), (80.2-)65.5(-48.8) Ma (Tank & Olmstead pers. comm.), or (68-)52(-32) Ma (Wikström et al. 2015).
Phylogeny. Peltanthera has been placed with Gesneriaceae (e.g. Oxelman et al. 1999a; see also Clark et al. 2010), and there have been suggestions that it was perhaps to be included in the family, but c.f. Soltis et al. (2011). More recently Perret et al. (2012), in a study focusing on Gesnerioideae, found the well supported clade [Peltanthera [Sanango + Gesneriaceae s. str.]] and Luna et al. (2019) recovered the relationships [Calceolariaceae [Peltanthera + Gesneriaceae]], but support for the position of Peltanthera was weak. However, Refulio-Rodriguez and Olmstead (2014; see also Ogutcen et al. 2021: nuclear genome) found strong support for the position of Peltanthera as sister to the whole group, a relationship that is followed here.
Chemistry, Morphology, etc.. See Weber (2013) for inflorescence morphology; the ultimate units of the inflorescence of Peltanthera with their four flowers are not so very different from the pair-flowered cymes that characterize the rest of the clade...
PELTANTHERACEAE Molinari - Peltanthera floribunda Bentham - Back to Lamiales
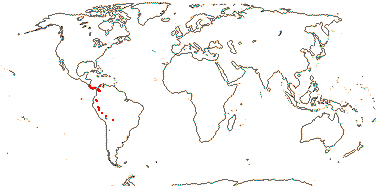
Small tree; cornoside derivatives +; nodes 3:3; petiole bundle ± flattened-annular, with (medullary and) wing bundles; lamina bundle sclerenchyma slight; hairs branched-moniliform; leaves not joined at the base; lamina vernation involute; inflorescence axillary, much branched, flowers in groups of five, ± polysymmetric, "small" [<5 mm long], bracteoles 0; K ± free, C valvate; A 5, anther thecae confluent, appearing to be peltate; nectary small; stigma capitate; capsule loculicidal; seeds dust-like, ridged [walls of adjacent cells in longitudinal files]; n = x = ?
1 [list]/1. Costa Rica to Bolivia (Map: from Fl. Neotrop. v. 81. 2000; TROPICOS ii.2013).
Evolution: Divergence & Distribution. Given the sister-group relationships in this part of the tree, Peltanthera has diversified very little...
Chemistry, Morphology, etc.. Peltanthera is very similar in wood anatomy to Buddleja; both genera are of course woody (Carlquist 1997c).
See Hunziker and di Fulvio (1957) and Norman (2000) for general information, Carlquist (1997c) for wood anatomy, and Jensen (2000a) for chemistry. Peltanthera floribunda is poorly known.
[Calceolariaceae + Gesneriaceae]: leaves rather soft, leaf bases joined by a slight ridge, lamina margin serrate; inflorescence units pair-flowered cymes; endothelial cells in longitudinal rows; endosperm longitudinally furrowed [aulacospermous].
Age. Ages for this node are (108.6-)87.7, 46.7(-26.2) Ma (Nylinder et al. 2012), (74.6-)58.5(-41.7) Ma (Tank & Olmstead pers. comm.), ca 48.6 Ma (Magallón et al. 2015) or a mere 39.6 Ma (Tank et al. 2015: Table S2).
Chemistry, Morphology, etc.. In paired-flower cymes the two flowers of the flower-pair have the same orientation. Since the flower in front of the terminal flower is sometimes subtended by a "bracteole" that can be interpreted as the bract of that flower, the flower opposite it being totally suppressed, what appears to be a rather strange dichasial cyme is then a modified "panicle" (Weber 1973, 2013; Haston & Ronse De Craene 2007). Both Calceolariaceae and Gesneriaceae have at least some taxa with septicidal capsule dehiscence, but how the distribution of this character might appear on a combined tree of the two is unclear.
CALCEOLARIACEAE Olmstead - Back to Lamiales
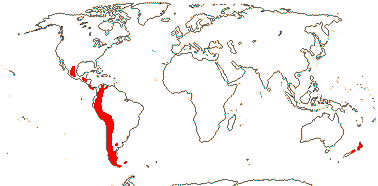
Herbs (annuals) to shrubs; napthoquinones +; cork?; wood rayless; nodes 1:1; pericyclic fibres 0; petiole bundle(s) arcuate; (lamina margins entire); flowers ?4-merous, strongly monosymmetric; K orthogonal, valvate, C bilabiate, abaxial lobe saccate, (adaxial "lip" strongly bilobed - Calceolaria triandra), elaiophores on inside of abaxial lip [pads of multicellular hairs] (0); A 2 [lateral pair] (3 [inc. abaxial member - C. triandra]), thecae (parallel) divergent, confluent on dehiscence or not, (theca 1), staminodes 0; nectary 0; (G semi-inferior), stigma small or capitate or obscurely bilobed; ovules with integument 3-4 cells across; capsule both septicidal and loculicidal; seed pedestals +; testa with anticlinal walls sinuous (straight); endosperm +; n = (8) 9, x = 9.
2 [list]/260: Calceolaria (240-270). Upland tropical and W. temperate South America, Brasil, Jovellana New Zealand (2 species) and Chile. Map: from Sérsic (2004). Photo: Habit, Flower.
Age. Crown group diversification began (27-)15(-4) Ma (Renner & Schaefer 2010), (37.6-)21(-6.8) Ma (Tank & Olmstead pers. comm.), (51.3-)30.8, 12.9(-5.1) Ma (Nylinder et al. (2012) or ca 21.8 Ma (Fonseca 2021).
Evolution: Divergence & Distribution. Note that from the age estimates above the phylogenetic fuse for Calceolariaceae may be 50-30 million years. The bulk of the diversity in the family is included in Calceolaria, common along the Andes. Its crown group age may be as recent as (6-)5(-1) Ma (Renner & Schaefer 2010), which suggests rapid diversification within the genus, while the estimate in Frankel et al. (2022) is (14.4-)13.95(-13.5) Ma (see also Cosacov et al. 2009; Madriñán et al. 2013). Calceolaria may have originated in southern Chile and then moved north along the Andes; the Huancabamba Deflection has been important, with a number of species found only to the north (Cosacov et al. 2009). Woodiness is probably plesiomiorphic, and Cosacov et al. (2009) estimated that there had been at lest ten shifts to herbaceousness and then three or more to the annual habit.
Mean ages for the split between the South American and New Zealand clades of Jovellana range from 9.3-5.3 Ma - probably long distance dispersal was involved (Nylinder et al. 2012).
Perhaps the development of nototribe pollination mechanisms was a key innovation here (Cosacov et al. 2009).
Pollination Biology & Seed Dispersal. Pollination in Calceolaria has been studied in detail, in particular by Sérsic (2004). Oil from elaiophores, pads of specialised multicellular hairs, is a common reward in the genus (e.g. Tölke et al. 2019), and sternotribic flowers of Calceolaria that are pollinated by Apinae-Centridini-Centris bees seem to be plesiomorphous; species with such flowers are diploid and are basically Chilean (Cosacov et al. 2009). Smaller Chalepogenus bees are the other main pollinators (both are anthophorids). Flowers with a closed mouth are visited by larger bees, those with an open mouth by smaller bees (see Murúa & Espíndola 2015 for morphometric analyses; also Rasmussen & Olesen 2000; Possobom & Machado 2017a and references). All told, about 4/5 of the genus have oil flowers (Sérsic 2004), and the ability to produce oil was gained once but it has been lost several times (Renner & Schaefer 2010; see also Cosacov et al. 2009). Oil glands were probably acquired after the split of Calceolaria from Jovellana, which lacks oil glands (Cosacov et al. 2009; Renner & Schaefer 2010), and crown-group Calceolaria has been variously dated: (14.4-)13.95(-13.5) or (6-)5(-1) Ma (see above). Bombus and Xylocopa visit flowers that lack oil; pollen is their reward. Remarkable food bodies on the lower lip are the reward provided by species like C. uniflora that are pollinated by non-nectarivorous birds like the fruit- and seed-eating charadriform Thinocorus rumicivorous (Vogel 1974; Sérsic & Cocucci 1996; Rasmussen & Olesen 2000).
Chemistry, Morphology, etc.. There has been much discussion over the basic floral merosity, but flowers in the family seem to be best interpreted as being 4- rather than modified 5-merous (Mayr & Weber 2006: superb micrographs; c.f. e.g. Sérsic 2004). From vasculature, etc., each lip of the flower seems to be formed from two petals; these primordium pairs may become connate only rather late in floral development (Mayr & Weber 2006). For floral development, see also Endress (1999).
Some information is taken from Weber (1973: inflorescence) and Molau (1988), Ehrhart (2000), and Fischer (2004b), all general, in Scrophulariaceae; see also Tank et al. (2006).
Phylogeny. For a phylogeny of the family, see S. Andersson (2006) and especially Cosakov et al. (2009). Frankel et al. (2022) carried out a plastome analysis, although sampling, at 13 species, was exiguous; the topology they obtained is rather different than that of Cosacov et al. (2009), the latter being based on 103 species, 2 genes and 36 morphological characters.
Classification. Porodittia, with three stamens, is a synonym of Stemotria, but neither name is needed as Stemotria is clearly derived from within Calceolaria, thus P. triandra = C. triandra (S. Andersson 2006). The limits of the subgenera and sections need adjusting (Frankel et al. 2022).
Thanks. I am grateful to Pamela Puppo for comments.
GESNERIACEAE Richard & Jussieu, nom. cons. - Back to Lamiales
Distinctive verbascosides [e.g. sanangoside]; petiole bundle annular; stomata anisocytic; A 4 + staminode; (dust seeds +); x = 10 (?9), nuclear genome [1 C) (0.042-)0.713(-12.243) pg.
147(+) [list]/4,116 - three subfamilies below. Largely tropical.
Age. Estimated ages for crown-group Gesneriaceae are (67.6-)49.2(-30.6) Ma (Tank & Olmstead pers. comm.), (68.1-)57.5(-45.1) Ma (Perret et al. 2012), ca 71.9 Ma (Petrova et al. 2015, (81.3-)73.1(-51.9) Ma (Roalson and Roberts 2016) and ca 61.5 Ma (Fonseca 2021).
1. Sanangoideae A. Weber, J. L. Clark & Mich. Möller - Sanango racemosum (Ruíz & Pavón) Barringer
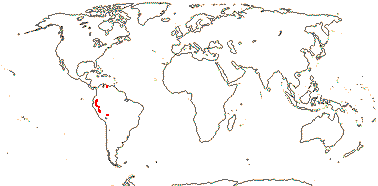
Shrub or small tree; vessel elements with scalariform perforation plates; nodes 7:7 + split laterals; stem with cortical bundles; petiole bundle with inverted adaxial bundles; lamina bundle with sheathing sclerenchyma; stomata in groups; lamina quite coriaceous; flower weakly monosymmetric [C tube curved]; K ± free; anther thecae confluent; G semi-inferior, placentation axile, style short, stigma capitate-lobed; capsule loculicidal + septicidal; n = 8.
1/1. Ecuador to Bolivia, Venezuela. Map: from Norman (1994) and TROPICOS (consulted ii.2013).
[Gesnerioideae + Didymocarpoideae]
Usu. herbs or weak-stemmed trees (trees), often epiphytes [ca 700 spp.]; (cambium storied); (vessel elements with scalariform perforation plates); nodes 1:1 (+ split laterals), 3 or more:3 or more + split laterals); hairs often dense, soft, also stalked glands, or with thickened terminal cells; petiole bundle(s) also arcuate; lamina bundle lacking sheathing sclerenchyma; (stomata in groups); lamina vernation involute, (margins entire); inflorescence axillary (terminal); flowers strongly monosymmetric (polysymmetric); K connate, C with abaxial lobe(s) outside others in bud [= descending cochleate] or quincuncial, (C spurred); A adnate to C, (5, 2, staminode 0, 3), anthers connivent in pairs, (thecae apically confluent); nectary vascularized; placentation intrusive parietal, placentae ± bilobed, triangular, usu. covered by ovules, stigma broadly bilobed to trumpet-shaped, wet or dry; integument 3-5 cells across; fruit a septicidal capsule; exotestal cells variously elongated and thickened, endotestal cells at most simply persisting; nuclear genome [1C] (251-)1118(-4220) Mb; GCyc duplication.
147/4,115. Largely tropical.
Age. Crown-group core Gesneriaceae may be a mere (47-)44, 34(-31) Ma (Wikström et al. 2001) or (60.5-)44.7(-37.1) Ma (Perret et al. 2012); however, Bell et al. (2010: note relationships) give an age of (66-)56, 52(-44) Ma, Petrova et al. (2015) ages of (67-)65.5(-55) Ma, and Roalson and Roberts (2016) ages of (77.1-)69.7(-48.2) Ma for this node, all rather older.
2. Gesnerioideae Link
3-desoxyanthocyanins +, chalcones, aurones 0; seeds without surface ornamentation, cells much elongated, spirally arranged (ornamented; shorter; not spirally arranged); endosperm conspicuous; GCyc2 gene lost.
75/1,830 - five tribes below. Predominantly Neotropical, a few spp. S.W. Pacific, 1 sp. East Asia. [Photo - Flower.]
Age. Crown-group Gesnerioideae can be dated to (48.7-)36.2(-32.3) Ma (Perret et al. 2012), (66.2-)46.8(-40.3) Ma (Roalson & Roberts 2016) or ca 41.9 Ma (Petrova et al. 2015). Note that the split of Beslerieae from other Gesneriaceae - Titanotrichum probably sister - is estimated to be around (74.9-)60 Ma (G. E. Ferreira et al. 2014).
2A. Coronanthereae Fritsch
Trees to ± shrubby-herbaceous, (rooting from the nodes); stomata anomocytic (paracytic); inflorescence (racemose - Pagothyra), (flowers axillary); (flowers polysymmetric); (C fringed); (A 2 [adaxial pair]; 5); nectary embedded in G wall, vascularized from A traces; capsules septicidal (and loculicidal), (placentae fleshy), (fruit a berry); n = 37(-45); gcyc duplication.
9/23: Coronanthera (11). Solomon Islands, Antilles, New Caledonia, S. South America (map: red, from Burtt 1998).
Age. Crown-group Coronanthereae are (32.2-)9.5(-7.6) Ma (Perret et al. 2012) or as much as (40.3-)23.2(-19.3) Ma (Roalson & Roberts 2016).
2B. Titanotricheae W. T. Wang - Titanotrichum oldhamii (Hemsley) Solereder
Scaly rhizomes +; (stomata anomocytic); leaves alternate/opposite/± anisophyllous; inflorescence racemose, branched, with bulbils; seed with membranous appendages at both ends, testa striate-reticulate; n = 20.
1/1. China, Japan, Taiwan. Map above: green, from Fl. China vol. 18. (1998), also known from Sumatra and Borneo?
2C. Gesnerieae Dumortier (inc. Episcieae) —— Synonymy: Belloniaceae Martynov
(small trees, climbers), (with scaly rhizomes; tubers; moniliform root tubers); (CAM photosynthesis - Codonopsis); (raphides, styloids +); (nodes 3:3, split-laterals); (petiole bundles deeply arcuate to annular (medullary bundles +)); (stomata on raised mounds, usually single [= stomatal turrets]); leaves (spiral), (blade peltate); (inflorescences long-lived, on leafless stem near ground - Dry.); (flowers resupinate); (K ± free), (C margins fimbriate - Dry.); A (2 + 3 staminodes), (anthers with basal pores - Dry.); ovary superior to inferior, nectary vascularized from numerous vascular bundles in wall, (dorsal lobe of stigma aborted); fruit various [berry, loculicidal or septicidal + loculicidal capsule, with fleshy placentae or funicles ["display capsule"] or not]; n = 8-14, polyploidy rare.
53/1400: Columnea (s.l. = 210+, s. str., 75+, + 4 genera, inc. Dalbergaria [90], Tricantha [75+]), Drymonia (91), Alloplectus (75+), Nautilocalyx (70+), Paradrymonia (70+), Gesneria (60), Sinningia (60), Gesneria (50). New World (map: from Brummitt 2007, in part). [Photo - Leaves, Flower.]
Age. The crown-group age of Gesnerieae is (36.9-)31.7(-24.8) Ma (Perret et al. 2012), ca 22.5 Ma (Petrova et al. 2015), or (29.9-)27.3(-25.2) Ma (Roalson & Roberts 2016).
[Beslerieae + Napeantheae]: storage organs 0; nodes 1:1.
2D. Beslerieae Bartling —— Synonymy: Besleriaceae Rafinesque
(Small tree to shrub); (indumentum lepidote); leaves ("alternate"); inflorescences condensed (not), (flowers axillary), bracteoles 0 (+); fruit (berry), (display capsule), (septicidal capsule); testa often striate; n = 16.
7/295: Besleria (>175). Tropical America, esp. northern Andes.
Age. The age of crown-group Beslerieae is ca 14 Ma (Perret et al. 2012), ca 16 Ma (Petrova et al. 2015), or (28.8-)22.6(-18.2) Ma (Roalson & Roberts 2016).
2E. Napeantheae Wiehler - Napeanthus Gardner
Stomata in groups; flower ± polysymmetrical; A 5/4 + staminode, free, nectary 0; n = ?
1/25. Central and tropical South America.
Age. Crown-group Napeantheae are (12.7-)10(-7.1) Ma (Roalson & Roberts 2016) or ca 5.5 Ma (Perret et al. 2013).
3. Didymocarpoideae Arnott (= the old Cyrtandroideae)
3-desoxyanthocyanins 0, chalcones, aurones +; ?stomata; ovary wall not richly vascularized, nectary vascularized from A traces; testa cells little elongated; endosperm slight, cotyledons unequal, one accrescent.
71/2,315 - two tribes below. Predominantly Old World, esp. South East Asia-Malesia and the Pacific (map: from van Steenis & van Balgooy 1966 [Malesia and Pacific]; Hilliard & Burtt 1971 [Africa].)
Age. The crown-group age of this clade is (54-)42(-28) Ma (Perret et al. 2012), ca 61.2 Ma (Petrova et al. 2015), or (75-)67.4(-46.6) Ma (Roalson & Roberts 2016).
3A. Epithemateae C. B. Clarke
Dihydroxyphenolics [e.g. acteoside] 0; secretory canals + [?resin]; (medullary bundles + - Rhynchoglossum); (vegetative plant body a single cotyledon), (anisophylly extreme), lamina asymmetrical; bracteoles 0; K usu. valvate; (abaxial C lobe inside others in bud); (A 2 [adaxial pair]); (nectary variously vascularized); (placentation axile), ovary short, abruptly narrowed into the style; integument 2-3 cells across [Platystemma]; endosperm ?0; n = (8-)10(-12); (seedling primary root not developed).
6/80: Monophyllaea (38). Predominantly Indo-Malesian, 1 sp. West Africa, 1 sp. (Rhynchoglossum azureum) southern Mexico to Peru.
Age. The age of crown-group Epithemateae is (74.1-)64.7(-48.5) Ma (Roalson & Roberts 2016).
3B. Trichosporeae Nees —— Synonymy: Cyrtandraceae Jack, Didymocarpaceae D. Don, Ramondaceae Godron
(Plant woody); (CAM photosynthesis - Haberlea, Ramonda); (nodes 1:1 with split laterals; 3:3 with split laterals; 5:5); (sclereids +); (anisophylly extreme), (plant body a single cotyledon); (flowers reciprocal or non-reciprocal mirror images); A (2 [abaxial pair, Streptocarpus]), staminodes 0-3, (anthers not coherent); placentae lamelliform-recurved, ovules restricted to distal end, ovary gradually narrowed into the style; (ovules hemitropous); capsule (dehiscence septicidal (and loculicidal)), (± elongated, twisted), (circumscissile), (a berry); (testa cells with (extremely long) hairs); n = (4, 8) 9-11 (12, 13) 14-17, etc., polyploidy not uncommon.
82/2,280: Cyrtandra (749), Primulina (175), Streptocarpus (175), Aeschynanthus (160), Paraboea (130), Oreocharis (126), Codonoboea (125), Didymocarpus (110), Agalmyla (100), Henckelia (70), Petrocosmea (60), Petrocodon (51), Microchirita (36). S. Europe (scattered), Old World, mostly Sri Lanka to Malesia (especially southern China) and the Pacific to Hawaii.
Age. Crown-group Trichosporeae are (72.8-)63.6(-43.6) Ma (Roalson & Roberts 2016) or ca 35.4 Ma (W.-Q. Xu et al. 2018: Haberlea-Primulina).
Evolution: Divergence & Distribution. Roalson and Roberts (2016) give dates for numerous clades in the family in the course of their discussion about diversification within it; Perret et al. (2012) give ages for tribes in Gesnerioideae and Petrova et al. (2015) dates throughout the family, mostly rather younger than those in Roalson and Roberts (2016).
Within Gesnerioideae-Coronanthereae there seems to have been one (J. F. Smith et al. 2006) or two (Woo et al. 2011) E. to W. dispersal events across the Pacific. Diversification in the New World Gloxinieae occurred some 30-20 Ma (Roalson et al. 2008b: see also biogeographic relationships; estimate in Perret et al. 2012 somewhat younger at (25.0-)21.7(-14.8) My). Didymocarpoideae-Didymocarpeae: Möller and Cronk (2002) discussed biogeographic relationships within the large African genus Streptocarpus.
Cyrtandra, with baccate fruits, is a very speciose genus found throughout Malesia and the Pacific. Interestingly, all species are diploid, 2n = 34, there has been extensive homoplasy, and nearly all of sections recognized by C. B. Clarke have turned out to be polyphyletic - of course, he was working some 140 years ago (Atkins et al. 2921). Diversification may have begun ca 48 Ma, although at ca 17.3 and 11.1 Ma, other estimates are very much younger (M. A. Johnson et al. 2017; Roalson & Roberts 2016). The genus is particularly speciose in places like New Guinea (Clark et al. 2009), and it is also widely distributed throughout the Pacific, the ca 175 species species there forming a single clade, and it has been called a "supertramp" genus (Cronk et al. 2005); surprisingly, perhaps, it is absent from the New Caledonian mainland (c.f. Psychotria s.l.). This Pacific clade has a long branch, and within this clade Hawaiian species are monophyletic and possibly sister to the rest (Cronk et al. 2005; J. R. Clark et al. 2009; Atkins et al. 2013; Johnson et al. 2019); the long branch has been broken up by a clade of species from the Solomon Islands, and other clades of Solomon Islands species are in both the Pacific and Malesian parts of the tree (J. R. Clark et al. 2013). The Fiji-Samoa area may have been where the Pacific was initially colonized (from the west) 11.4-8.9 Ma (Clark et al. 2009), and the orange-fruited C. taviunensis is sister to the rest of the Pacific species (Johnson et al. 2017). There are several (5-11) Fijian clades, and as relationships have become clarified it is clear that there were numerous dispersal events in the Pacific and within the Hawai'ian archipelago, the crown-group age of the Hawaiian species being somewhat over 5 Ma (Clark et al. 2008: a long stem substantially over 12 My), and most of these are west to east (Johnson et al. 2017, 2019). In general, species that can grow at low altitudes near the coast may be important in range extensions, with subsequent diversification occurring in upland forest habitats (Johnson et al. 2017) - shades of E. O. Wilson's taxon cycles in Melanesian ants (Wilson 1961).
On the Hawai'ian islands alone there are ca 60 endemic species of Cyrtandra, many very localized and kept apart via post-zygotic barriers such as reduced survivorship of hybrid seedlings; the species may have evolved in allopatry (Johnson et al. 2015; Lim & Marshall 2017: pattern of diversification on the islands). Along with incomplete lineage sorting, there has been much hybridization and introgression (Johnson et al. 2019; Kleinkopf et al. 2019). The Hawai'ian species result from a single colonization event from the west (Fiji), probably initially of Kaua'i (Johnson et al. 2019 and references), colonization being independent of that of the other Pacific islands. Radiation there has happened within the last 5 Ma or so (Clark et al. 2009, 2019; see also Roalson & Clark 2016 and Johnson et al. 2017 for more ages). Within the Hawai'ian archipelago, movement has been from the oldest (Kaua'i) to the youngest (Hawai'i) islands (Johnson et al. 2019). In both Hawai'i and Fiji up to eight species may grow in sympatry. All told some 327 woody species of Gesneriaceae grow on islands (woodiness has evolved ca 5 times); these are mostly members of Cyrtandra and have evolved on islands like New Guinea rather than on oceanic islands (Zizka et al. 2022). See also the silversword alliance, etc., Cyanea and relatives, Schiedea, Myrtaceae, see Diversity and Distribution for Metrosideros, early stages, and the Stachys area, etc., for other major Hawai'ian clades.
Indeed, hybridization is very common in Gesneriaceae in general (Whitney et al. 2010).For example L.-H. Yang et al. (2023a, b) found extensive hybridization/gene flow (and incomplete lineage sorting) in Didymocarpoideae-Trichosporeae, including Henckelia, and an allopolypoidy event that characterized core Didymocarpinae as a whole and that appeared to be associated with rapid radiation there.
Roalson and Roberts (2016) noted five major increases in diversification rates in the family, including Pacific Cyrtandra; the others include Beslerieae, core Nematanthus, core Columneinae and core Streptocarpus. G. E. Ferreira et al. (2024) suggested that diversification in Besleria may have begun in the Northern Andes, with more or less independant radiations in Central America and South Andes and S.E. Brazil. In core Streptocarpus (African) the distinctive unifoliate growth habit, or perhaps more general variation in growth form, seems to have spurred diversification (Roalson & Roberts 2016). There is extensive diversification in both flower and fruit in the speciose Episcieae (= Gesnerieae) (Clark et al. 2011, 2012). In the New World clades pollination by hummingbirds, accompanied by shifts in corolla colour to red, have been important, and Columneinae are epiphytic, although how that might interact with diversification is unclear; in the Old World bird pollination is less important and shifts in corolla colour have been back and forth, particularly between white and purple. Serrano-Serrano et al. (2015, 2017) discussed factors like the adoption of the epiphytic habit that, along with the adoption of ornithophily, may also have increased speciation in New World Gesneriaceae. The rate of diversification in Oreocharis, crown age ca 12.3 Ma and from mountains in the south of China, including the Hengduan Mountains, was initially very high, but then it tailed off; the drivers of diversification here are unclear, although climate may have been involved (Kong et al. 2021). Primulina, a related genus in which a stream of new species is being described, is common in the karst country of southern China; temperature and rainfall probably helped drive diversification here (Kong et al. 2017).
Thinking of hummingbird pollination in particular, centres of diversity of both Neotropical Gesneriaceae and of hummingbirds are in the Colombia-Ecuador region (Weber 2011; Ericaceae-Vaccinieae are also abundant there, Bromeliaceae and Campanulaceae-centropogonids rather more broadly down the Andes), but hummingbird-pollinated Gesneriaceae are spread throughout Central and South America. Roalson and Roberts (2016: Fig. 1 for other estimates) dated crown-group diversification in three major clades dominated by hummingbird pollination to ca 22.6 (Beslerieae), ca 22.4 (Columneinae) and ca 15.2 Ma (Ligeriinae), while Serrano-Serrano et al. (2017) noted that diversification in Gesnerioideae increased (25.5-)18.5(-5) Ma and that hummingbirds may have arrived in South America 25-20.3 Ma ((24.7-)22.4(-20.3) Ma - McGuire et al. 2014, see also Roalson & Roberts 2016), so the birds may have spurred this diversification. Serrano-Serrano et al. (2017) estimated that there have been (41.5-)31.5(-21.5) shifts to hummingbird from insect (bee) pollination, bee pollination probably being plesiomorphic in Gesnerioideae, and these shifts were often near the base of large clades that subsequently had very high subsequent diversification rates. The shifts had occurred throughout the range of the subfamily and in different biomes; overall ca 60% of Gesnerioideae they studied (351/590) were bird-pollinated (Serrano-Serrano et al. 2017), so Wiehler's (1978) estimate of some 600 bird-pollinated species in the subfamily seems to be on the mark. Zingiberales-Heliconiaceae, also commonly pollinated by hummingbirds and around 29-22 Ma is another hummingbird-plant association that is quite old, as are bird-pollinated Bromeliaceae, ca 14 My; Lamiaceae, in Nepetoideae-Salviinae, ca 22 Ma, while Ericaceae-Vaccinieae may be a fourth case. For the diversity of bird-pollinated taxa of Gondwanan origin in tropical and premontane parts of the northern Andes, see Gentry (1982), and for hummingbird pollination in general, see below.
Ecology & Physiology. Although many Gesneriaceae are almost succulent or quite delicate herbs, a surprising number grow on exposed rocks (Haberlea rhodopensis and Boea hygrometrica are examples) and are homoiochlorophyllous (their chloroplasts do not break down) resurrection plants tolerating extreme dessication (Burtt 1998; Bogacheva et al. 2013; Gaff & Oliver 2013: plants in 9 genera); dessication tolerance is when the plant dehydrates to less than 0.1 g H2O g-1 - and recovers. Along with sucrose, involved in stabilizing phospholipid bilayers in such situations (e.g. Proctor & Tuba 2002; Gaff & Oliver 2013), galactose oligosaccharides are quite abundant in the dried leaf (Albini et al. 1999; see also Navari et al. 1995). In a genome-level analysis of Boea hygrometrica the resurrection syndrome includes protection of the photosynthetic apparatus during drying and the rapid resumption of protein synthesis upon wetting, in part achieved by regulatory changes (Xiao et al. 2015; see also L. Wang et al. 2009); the authors note that both seeds and pollen tend to be dessication tolerant (the "source" of genes involved in dessication tolerance in other taxa - e.g. Oliver et al. 2005; Gaff & Oliver 2013; Costa et al. 2017). The genome is not particularly small, unlike those of at least some other extremophiles (Baniaga et al. 2016), although it may also be quite large in other angiosperm xerophytes (e.g. Farrant et al. 2015). Recent studies on the shrub Paraboea rufescens, a plant of karst habitats - at up to 40 cm tall, it is tall for a dessication-tolerant plant - shows root pressure rapidly recovering. For dessication tolerance in Haberlea rhodopensis, see Gechev et al. (2013).
Given the relative abundance of epilithic taxa, it is not surprising that epiphytes are also common in Gesneriaceae, with well over 400 epiphytic species in Neotropical Episcieae/Gesnerieae alone (Madison 1977; Weber 1978; Gentry & Dodson 1987), all told, there are perhaps 644 species of epiphytes, 5th in angiosperms; Svahnström et al. 2025, q.v. for commoness of epiphytic relative to non-epiphytic taxa). For gesneriaceous epiphytes, see Zotz et al. (2021b: list), and Hietz et al. (2021) and Zotz et al. (2021a) for the ecophysiological features of epiphytes. The adoption of the epiphytic habitat seems not to be associated with the evolution of extreme dessication tolerance, although the epiphytic habitat is associated with water stress, as in Orchidaceae. Although Zotz (2013) estimated that there were only 570 epiphytic species in the whole family, of which he noted that ca 275 were in the Old World Aeschynanthus and Agalmyla, a figure of ca 700 species seems likely (Melastomataceae, Piperaceae and Ericaceae are the other big epiphytic families of broad-leaved angiosperms, see also Bromeliaceae, Orchidaceae and ferns). The evolution of epiphytism within Coronanthereae is described by Salinas et al. (2010).
The monophylly of some Didymocarpoideae and some forms of anisophylly (see also Vegetative Variation below) seem to be adaptations for life on rocks and/or shady conditions, and Gesneriaceae can be common there. Monophyllous Gesneriaceae often grow on rocks, sometimes on the mossy surfaces if conditions are humid, or in cracks, and the single leaf hangs down and covers the rock surface quite efficiently; that the better lighted cotyledon of Monophyllaea is the one that develops makes sense in this context (see also Streptocarpus, i.e. taxa in both tribes of Didymocarpoideae have this habit). Plants whose stems seem to consist of sprays of alternating leaves are also common in such situations; again, a photosynthetic surface covering the rock is deployed. As in Pilea (Urticaceae) the leaves are opposite, but there is strong anisophylly, and extreme anisophylly also occurs in the spreading branches of Columnea (Gesnerioideae). Burtt (1970) and Saueregger and Weber (2004) discuss anisocotyly in general - more accurately, one cotyledon is accrescent - and its development in Didymocarpoideae.
Species of Gesnerioideae in particular have a variety of underground perennating structures (Weber 2004a), while Titanotrichum produces clusters of bulbils in its leaf axil, each bulbil consisting of a short piece of stem, two small leaves, and a terminal bud (C.-N. Wang & Cronk 2003).
Pollination Biology & Seed Dispersal. Considerable work has been carried out on pollination in Gesneriaceae - birds and bees are the major pollinators here. Wiehler (1978) estimated that perhaps 60% of Neotropical Gesnerioideae - some 600 species - were pollinated by hummingbirds, and this includes large genera like Columnea s.l. with well over 200 species. Wiehler divided the floral morphologies involved into three common and one less common "types" - rather narrowly tubular; strongly and broadly bilabiate; with a narrow mouth and an asymmetrically swollen tube; and tubular, with the limb more or less rotate. Perret et al. (2007; see also Sanmartin-Gajado & Sazima 2005) found that hummingbirds pollinated perhaps 2/3 of the ca 80 species of Sinningieae (= Ligeriinae), a group centred in the Atlantic Forest of Brazil, while Serrano-Serrano et al. (2015) looked at the complex interaction of floral traits associated with pollination (including flower size and resupination) and climate of some Gesnerioideae there. Self compatability is quite high in herbaceous, bird-pollinated Gesnerioideae, higher than in other bird-pollinated taxa considered (Wolowski et al. 2013). Diversification in bird-pollinated Gesneriaceae-Gesnerioideae increased (25.5-)18.5(-5) Ma (Serrano-Serrano et al. 2017), and Roalson and Roberts (2016) suggested that hummingbird-pollinated clades in Columneinae were ca 22.4 Ma old. For other major clades of hummingbird-pollinated species, see Gesneriaceae-Gesnerioideae, Bromeliaceae, Ericaceae-Vaccinieae, Heliconiaceae, and for a more general discussion on hummingbird pollination, see elsewhere.
Bird pollination is relatively less common in Old World Gesneriaceae, but it is likely to predominate in Aeschynanthus, which has some 185 species.
Another ca 30% of Gesnerioideae (ca 300 spp.) may be pollinated by euglossine bees of both sexes (c.f. Orchidaceae where male bees seeking scents are involved), and in these flowers the spreading corolla lobes sometimes have long-fimbriate margins (Wiehler 1978); divergence within euglossine bees began 42-27 Ma (Ramírez et al. 2010) or (38-)26, 25(-17) Ma (Cardinal & Danforth 2011; Martins et al. 2014a). Bee pollination is probably the original condition for Gesnerioideae, and Serrano-Serrano et al. (2017) estimated that there were later (95-)76(-58) switches from hummingbird back to bee pollination starting ca 5 Ma after the evolution of bird-pollinated flowers, however, diversification in those clades was not great; overall ca 40% of Gesnerioideae (231/590) were insect pollinated, and the great majority of these are likely to be bee pollinated. Clark et al. (2015) described the floral morphologies associated with particular pollinators in Drymonia. Here the plesiomorphic condition is to have campanulate corollas and anthers with poricidal dehiscence, the visitors being euglossine bees (note, however, there seems not to be buzz pollination here). In a number of taxa the corolla was constricted in various ways, a condition that had arisen independently eight or so times, and the anthers opened by longitudinal slits - the normal condition for the family, but here derived - and the visitors were hummingbirds. Roalson et al. (2003) explored the diversity of floral morphology in Achimenes, while Alexandre et al. (2015) looked at the genetic background of changes of the elements of pollination syndromes in Rhytidophyllum - unlinked genes with at least moderate effects were involved.
Martén-Rodríguez et al. (2015, see also 2009, 2010; Fleming & Kress 2013) discussed pollination in Antillean Gesneriaceae-Gesneriinae, comparing it with that of mainland taxa (belonging to other subtribes), noting that generalized and bat-pollinated taxa were proportionally more common on the islands (see also Alexandre et al. 2015; Serrano-Serrano et al. 2017; etc.).
Petrocosmea (Didymocarpoideae) has both strongly monosymmetric (specialization, coevolution[?]) and almost polysymmetric (generalist) flowers, and also flowers with relatively long (reversal: C.-Q. Li et al. 2019) and short tubes, unfortunately, nothing seems to be known about their pollinators, although buzz pollination for at least some seems likely (Z.-J. Qiu et al. 2015).
Harrison et al. (1999) discuss floral diversification in Streptocarpus, which includes species with strongly monosymmetric flowers as well as Saintpaulia, with almost polysymmetric flowers, so including very different floral morphologies - and pollinators. Indeed, more or less polysymmetric flowers - the corolla is radial and rotate, although the androecium is often technically monosymmetric - have arisen independently several times in the family, the ten or so genera involved not being immediately related (e.g. Burtt 1970; J. F. Smith et al. 2004a), indeed, polysymmetric flowers are notably abundant here compared with some other core lamialean families (Endress 1997a). The flowers of Bournea are initially monosymmetric but then become polysymmetric, floral symmetry genes being expressed early and down-regulated later (Zhong & Kellogg 2015). In gloxinia (Sinningia speciosa) at least, both monosymmetry and floral orientation are under the pleiotropic control of a single gene, SsCYC (Y. Dong et al. 2018). Relatively little is known about the pollination of such flowers, although the almost polysymmetric Saintpaulia-type flowers of Streptocarpus have the buzz pollination syndrome (Clark et al. 2011). For more on molecular details of floral development, see Citerne et al. (2000) and Zhou et al. (2008).
Flowers with inverted orientation are known from some Gesnerieae (Clark & Zimmer 2003); they seem to have evolved ca 3 times. This inverted orientation is evident from the very earliest stages of the ontogeny of the flower, and since there is no twisting of the pedicel (Clark et al. 2006), they are not resupinate by some definitions (see also Serrano-Serrano et al. 2015 for diversification in resupinate clades).
Synchronized flowering in Monophyllaea glabra is rather odd, all the plants in a population starting to flower simultaneously, whatever their size (Ayano et al. 2005).
Gesneriaceae commonly have capsular fruits with wind dispersed seeds. However, splash-cup dispersal is quite common, occurring in around 190 or more species from the New World alone, and the species involved grow in damp, forest floor/stream side type conditions; a persistent calyx or the valve walls of the capsule form the cup (Clark et al. 2012; Ertelt 2013). Birds and perhaps other animals may disperse fruits of Gesneriaceae, eating fleshy baccate fruits in their entirety, as in the Old World Cyrtandra and New World Columnea. There is also a variety of other presumably animal-dispersed fruit types, particularly in the New World, and the colours can be striking. There the glistening red or black seeds may be variously exposed on a fleshy placenta that is in turn displayed against the coloured inside of the capsule wall, and the open capsule is sometimes surrounded by a coloured calyx; here capsule dehiscence is normal. Other variants of fleshy capsule/drupe fruit types are quite common (Weber 2004a, b; Clark et al. 2006, esp. 2012). In some indehiscent berry-type fruits there has been a reversal to dehiscence, albeit irregular, as in Besleria, where the placenta + seeds are exposed against a background of the recurved carpel walls, the overall effect being similar to a berry with more or less recurved sepals of other taxa in the genus (Berger et al. 2015).
Plant-Animal Interactions. Gesneriaceae are not often eaten by lepidopteran caterpillars (Ehrlich & Raven 1964). In both the Old and New World tropics, some epiphytic Gesneriaceae may live in ant gardens (Orivel & Leroy 2011).
Vegetative Variation. Variation in growth patterns in Gesneriaceae is very considerable (see Weber 2004 for a useful survey). The architecture of some Didymocarpoideae (= the old Cyrtandroideae) is particularly diverse and distinctive. Streptocarpus (Didymocarpeae) shows much variation in growth patterns, and this is summarized by Nishii et al. (2020), with an emphasis on the interactions between plant hormones and developmental genes. Nishii et al. (2012) had found that in S. rexii heterocotyly developed only if the seedings were illuminated, and not under red light; both cotyledons initially have a basal meristem (Nishii et al. 2020). Adults of some species have only a single, huge, ever-growing cotyledon, although an abscission zone forms when growing conditions become unfavourable, a part of the leaf being lost. Other species are shrubs over 1 m tall (e.g. Hilliard & Burtt 1971; Jong & Burtt 1975; Nishii et al. 2015). The evolution of growth form here has many parallelisms - thus the unifoliate growth form has evolved more than once - and reversals, as well as being linked with other life history variables, such as age to flowering and flowering periodicity (Möller & Cronk 2001), and there are also connections with the development of compound leaves (Nishii et al. 2010), not to mention the leaves of Welwitschia (Wan et al. 2021). Jong and Burtt (1975) thought that the ever-growing cotyledon of Streptocarpus, which they called a phyllomorph, was an example of the evolution of morphological novelty. However, Kaplan (1997, 1: ch. 6) suggested that such cotyledons were an extreme example of the dominance of the leaf in development, the apical meristem effectively having been evicted - perhaps the same story from a different perspective. Phyllomorphs have three meristems: the basal meristem, from which the lamina develops, the petiolode meristem, and the groove meristem, from which new phyllomorphs develp (Nishii et al. 2020). Harrison et al. (2005a) found that genes involved in shoot development were now expressed on the petiole (= petiolode) in rosulate species of the genus, plants producing leaves, etc., from the petiole, but these genes were not expressed in strictly unifoliate species. Mantegazza et al. (2009) also suggested that the developmental pathways controlling meristem development appear to have become relocalised (see also Nishii & Nagata 2007). Beaufort-Murphy (1984: sample size small) found that Didymocarpoideae were more responsive to growth hormones, shoots developing from all over the explants, than were Gesnerioideae, which at most produced plantlets from the main veins adaxially; some Didymocarpoideae (e.g. Streptocarpus) at least are notably easy to propagate vegetatively. Foliar meristem activity ceases on flowering, so unifoliate species are monocarpic (Jong 1978: Hilliard & Burtt 1971). The petiolode of Streptocarpus, at least, is unifacial, although not at the seedling stage, when it is bifacial (Tononi et al. 2010). Interestingly, caulescent species, although looking rather ordinary vegetatively, initially lack an embryonic shoot apical meristem (SAM) just like the unifoliate and rosulate species, and their SAM develops post-embryogenically (Hilliard & Burtt 1971; Imaichi et al. 2007); leaves of these caulescent species also show prolonged basal meristem activity (Nishii et al. 2010). This prolonged foliar meristem activity involves the expression of KNOX1 genes, so maintaining an undifferentiated state in the leaves, effectively a shift from apical to lateral dominance (Nishii et al. 2010, 2020). Jong et al. (2012) discussed the morphology and anatomy of two woody Madagascan species of the genus. Interestingly, Microchirita initially develops a single large cotyledonary leaf, and flowers may develop at the apex of its petiole, but the normal plant body here is a herb to shrublet with opposite leaves that have flowers in their axils or along the petioles; there can be much variation in growth pattern within a single population (Puglisi & Middleton 2017). The plant body of many species of Monophyllaea is rather like that of Streptocarpus, consisting of a single, ever-growing structure that is derived from a single cotyledon. A meristem develops at the base of the cotyledon blade, and inflorescences later develop at the base of the phyllomorph; in some species (= the old genus Moultonia) the flowers arise along the midrib of the phyllomorph blade rather than from separate inflorescences (Burtt 1978; Imaichi et al. 2001; see also Tsukaya 2005; Ishikawa et al. 2017). The cotyledon that keeps on growing is the one that is exposed to more light (Saueregger & Weber 2005); The radicle of the seedling may not develop in Monophyllaea, and this has also been noted in other Epithemateae (Imaichi et al. 2001). In some species of Monophyllaea the plant body becomes more complex by repetition of the cotyledonary unit. Ishikawa et al. (2017) discuss differences in the development of the phyllomorphs in Streptocarpus and Monophyllaea, and there is also variation in whether the first root is exogenous or endogenous (Ayano et al. 2005; Imaichi et al. 2007).
Taxa like Rhynchoglossum ave two-ranked leaves with very aymmetrical blades; vegetatively they look rather like Begonia or Pentaphragma, which grow in similar habitats in the same general area. Interestingly, in Haberlea and Ramonda (inc. Jancaea), which may be sister to the rest of the tribe, there is development of anisophylly, albeit very weak (B.-H. Huang et al. 2019). Within Epithemateae, too, anisophylly is common, as is asymmetry of the lamina. For discussion of conditions favouring heterophylly, albeit in Melastomataceae, see Samra et al. (2025).
Finally, there is an amusing case of convergence in Besleria macropoda, which apparently has epiphyllous inflorescences - this is odd, because it is a member of Gesnerioideae. However, here the slender peduncle grows "inside" the petiole, which is in fact deeply and narrowly channelled, and the midrib also is channelled, the peduncle becoming apparent only in the middle of the lamina (Berger et al. 2015) - pseudoepiphylly.
Genes & Genomes. Skog (1984), Kiehn and Weber (1997), Möller and Kiehn (2004) and Christie et al. (2012) give chromosome numbers for the family; there is considerable infra-generic variation.
Chemistry, Morphology, etc.. Secondary metabolites (lack of iridoids, presence of the caffeoyl phenylethanoid glycoside, sanangoside) seem to suggest an association between Sanango and Gesnerioideae in particular (Jensen 1996).
There is quite a lot of anatomical variation to be integrated with the clades above; this would surely repay the effort involved. For example, stem sclereids are common; Aeschynanthus has strongly U-thickened sclereids in the pericycle, other taxa lack fibres or sclereids in the pericyclic position; some taxa have lignified hairs; and Gesneria has a U-shaped petiole bundle cradling a unmedullated circle of vascular tissue and there are also two wing bundles, while in other taxa the petiole bundle may be annular, with adaxial bundles - and so on. Nodal anatomy is quite variable (see also Howard 1970; Jong et al. 2012). (In addition to its distinctive anatomy, Gesneria has spirally-inserted serrate leaves with an almost coriaceous texture - it looks quite ungesneriaceous.) For leaf teeth, which may be hydathodal, see Rios et al.(2020).
Weber (e.g. 1973, 1978b, 1995) has published extensively on the paired-flower inflorescences of Gesneriaceae. Along these lines, T. Lu et al. (2019) note that flowers of some Didymocarpoideae-Trichosporeae might be asymmetrical, but paired and mirror images (reciprocal mirror image flowers - Didymocarpus itself), or although clearly asymmetrical, they were not paired (non-reciprocal - Streptocarpus-Saintpaulia). Song et al. (2009) found that CYC2 genes were involved in repression of the growth of both the single adaxial stamen and the abaxial stamen pair in Opithandra, so resulting in a flower with but two functional stamens, the adaxial stamen pair - c.f. Lentibulariaceae, where it is the abaxial stamen pair that remains fertile.
For more information, see Weber (1978a and references: Klugieae, Loxonieae), the many papers published by B. L. Burtt, including Burtt (1963), the beginning of the reworking of generic limits in the Old World Gesneriaceae, see also Wiehler (1982) for New World Gesneriaceae, Burtt and Wiehler (1995), Wiehler (1983), Weber (2004a: excellent account, 2004b: history of classification), Skog (1976: Gesnerieae s. str.) and J. L. Clark (2025: Drymonia), all general, Kvist and Pedersen (1986: phenolics), Wiehler (1970: vegetative anatomy, esp. Gesnerioideae), Trapp (1956b: androecium), C. L. Wilson (1974a, b: nectary vascularization), Erbar (2014: nectaries), Hildebrand (1872: seed hairs - chalazal prolongation, also micropylar - in Aeschynanthus) and Beaufort-Murphy (1983: seeds under the S.E.M.). Pan et al. (2002) discussed the floral development of Titanotrichum (see below for phylogeny). Pollen variation is either uninformative or suggests problems in everything from species delimitation on up - e.g. N. H. Williams (1978: Gesnerioideae), Schlag-Edler and Kien (2001) and Gasparino et al. (2020: Gesnerieae-Ligeriinae).
Dickison (1994), Jensen (1994, 1996), Norman (1994), and Wiehler (1994) all deal with Sanango.
Phylogeny. For an extensive summary, see Möller and Clark (2013). The relationships of Peltanthera are dealt with above; it has support as sister to [Calceolariaceae + Gesneriaceae]. Sanango is sister to [Gesnerioideae + Didymocarpoideae] (e.g. Perret et al. 2012; Refulio-Rodriguez & Olmstead 2014). See L. Yang et al. (2023a: 134 transcriptomes) for a phylogeny of the family; there is conflict between nuclear and plastome data, and within nuclear data are common, i.a. hybridization being common.
Didymocarpoideae (Cyrtandroideae that was). For the phylogeny of Didymocarpoideae, see also Möller et al. (2011a). Epithemateae are sister to Trichosporeae, and the monophyly of the former is well established - see J. F. Smith (1996), Smith et al. (1997a, b), Y.-Z. Wang et al. (2010), etc., and especially Mayer at al. (2003). Within Trichosporeae, Haberlea and Ramonda, temperate, European, and with polysymmetric flowers and five stamens, may be sister to the rest (e.g. Mayer et al. 2003), or more likely near the base of the clade (Wei et al. 2010; Y. Z. Wang et al. 2010); they have dihydrocaffeoyl ester that is found nowhere else in flowering plants (Jensen 1996). Indeed, Möller et al. (2009) placed a number of small Asian and European clades all with four or five, rarely two, stamens as basal in Didymocarpoideae. Of these, the odd Jerdonia, from the Western Ghats, India, has pollen in tetrads, four parietal placentae, large seeds with alveolate endosperm, and n = 14 (Burtt 1977b); it may be sister to the rest of the subfamily (Möller et al. 2009). Wang et al. (2010: Jerdonia not included) found that Corallodiscus, the Ramonda clade, and Streptocarpus are successive branches in the phylogeny; taxa with radially symmetric flowers are scattered through the tree, while Z.-D. Chen et al. (2016: Chinese taxa) found a similar position for Corallodiscus. Relationships in Luna et al. (2019) are [[Epithema + Rhynchoglossum] [Haberlea [Streptocarpus...]]].
Didymocarpus itself has been dismembered (Weber & Burtt 1998) and many species placed in Henckelia, although the limits of the latter were unclear (Möller et al. 2009; see also Middleton et al. 2013). For the diverse Cyrtandra, see Divergence & Distribution above. For relationships in Streptocarpus, which now includes i.a. Saintpaulia and all other African Trichosporeae, see Möller and Cronk (2001) and in particular the comprehensive analysis by Nishii et al. (2015). For a study of Aeschynanthus linking seed morphology and geography, see Denduangboripant et al. (2001). For a phylogeny of Chirita (= Henceklia) and relatives, see Y.-Z. Wang et al. (2011) and in particular Weber et al. (2011), and for relationships in an expanded Oreocharis, see Möller et al. (2011b) and especially Kong et al. (2021: transcriptomic data, 547 orthologous loci), the latter recovering two main clades, and they also noted that incomplete lineage sorting seems to have been pervasive there. Kong et al. (2017: 9 chloroplast, 11 nuclear markers) looked at the phylogeny of the related Primulina. Puglisi et al. (2016) examined relationships in Loxocarpinae. For the phylogeny of Petrocosmea, see Z.-J. Qiu et al. (2015) and C.-Q. Li et al. (2019).
It was earlier thought that Epithemateae were to include Cyrtandromoea (J. F. Smith et al. 1997a, b: long branch lengths, but c.f. B. Liu et al. 2019). However, that genus was sometimes placed in "Scrophulariaceae", as by Burtt (1965, q.v. for a revision), who linked it with Leucocarpus, now in Phrymaceae, and that is where Cyrtandromoea is to go: It has iridoids, axile placentation, isocotylous embryos, and so on. Recent molecular work also supports this position (Luna et al. 2019), and for more details see below. In chemistry Napeanthus (Gesnerioideae) is also similar.
Gesnerioideae. Kotarski et al. (2007) found 80% bootstrap support for the position of Coronanthereae as sister to other Gesnerioideae, and Titanotrichum was sister to the remainder. Other studies also placed the Old World but more or less isocotylar Titanotrichum basal in Gesnerioideae (C.-N. Wang et al. 2004: substantial amount of molecular data; c.f. D. Soltis et al. 2000; Albach et al. 2001), although that genus has also sometimes been placed in "Scrophulariaceae". Besleria and Napeanthus (n = 16) may also be near the base of the Gesnerioideae. In general agreement with these earlier studies, Perret et al. (2012) found basal relationships in Gesnerioideae to be [[Napeantheae + Beslerieae] [Coronathereae [Sinningieae + the rest (= Gessnerieae)]]]; Titanotrichum was sister to Napeanthus, and although Shuaria (see below) was not included in this study, there seemed to be little doubt about the placement of the former genus. See also de Araujo et al. (2016) and G. E. Ferreira et al. (2024) for relationships in this area. Serrano-Serrano et al. (2017) recovered the relationships [[Napeantheae + Beslerieae] [Titanotricheae [Coronathereae + Gesnerieae]]], while those in Luna et al. (2019) are [Napeanthus [[Titanotrichum + Besleria] [Coronathera etc.]]], although support at the base of the tree is weak. In a targeted-capture nuclear gene analysis Ogutcen et al. (2021) found the basic relationships [Napeantheae [Beslerieae [Coronantheae + Gesnerieae]]], with Titanotricheae sister to either Beslerieae or [Coronantheae + Gesnerieae], depending on the analysis.
For a phylogeny of Coronanthereae, see J. F. Smith et al. (2006) and Woo et al. (2011).
Shuaria, a woody plant superficially similar to Sanango that sometimes also has "alternate" leaves, was placed firmly in Beslerieae (J. L. Clark et al. 2010; Serrano-Serrano et al. 2017). For diversification in Beslerieae, see Roalson and Clark (2006). G. E. Ferreira et al. (2024: 80 spp., 3 chloroplaST and 1 nuclear markers) found 6 main clades inBesleria; they were not congruent with previously-described infrageneric taxa there.
Relationships along the spine of Gesnerieae remain only weakly supported (e.g. Woo et al. 2011; Perret et al. 2012; de Araujo et al. 2016). However, there seem to be five well supported clades, Ligeriinae (= the old Sinningieae), Sphaerorrhizinae, Gesneriinae, Gloxiniinae, and Columneinae (= the old Episcieae) (Perret et al. 2012). For other relationships in Gesnerieae, see Smith (2001), Zimmer et al. (2002) and Smith et al. (2004a, b). See Skog (1976) for a revision of Gesneria and relationships in Gesneriinae. For the phylogeny and biogeographic relationships of Gloxiniinae, see Roalson et al. (2005 a, b; 2008b: relationships in Central America and the Antilles). For diversification in Ligeriinae, see Perret et al. (2003, 2006: the limits of Sinningia need adjusting). For relationships within Columneinae, see Clark and Smith (2009) and in Columnea itself, see J. F. Smith et al. (2013) and Schulte et al. (2014). For relationships around Alloplectus, now much reduced in size, see Clark and Zimmer (2003). For a preliminary study of relationships in the complex Episcieae/Columneinae area, see Clark et al. (2012); Smith and Clark (2014) found that a number of species were placed outside recognized genera.
Classification. Perret et al. (2012) were undecided as to the circumscription of the family, sometimes suggesting that it be broadened to include Peltanthera and Sanango, sometimes suggesting that those genera might be excluded. However, Peltanthera seems not to be immediately associated with Gesneriaceae (Refulio-Rodriguez & Olmstead 2014: see Peltantheraceae), while Sanango is. Weber et al. (2013) include the latter in the family, for which they provide a formal classification down to the subtribal level, which is being followed here (I stop at tribes); the old tribes of Didymocarpoideae were decidedly unsatisfactory (Möller et al. 2009). See also the World Checklist and Bibliography of Gesneriaceae (Skog & Boggan 2005 a, b), The Genera of Gesneriaceae (Weber & Skog 2007), and in particular Weber et al. (2020), which includes keys down to tribes and genera, etc..
Genera in Gesneriaceae, perhaps particularly in Gesnerioideae, have long been noted as being difficult, as might be expected of a family in which there are conspicuous flowers and much obvious adaptation to pollinators (Wiehler 1982); many old genera that were based on such floral characters have turned out to be unsatisfactory (and intergeneric hybrids are common). Thus Clark et al. (2012) found that six of fifteen genera of Episcieae/Gesnerieae for which they sampled two or more species were para- or polyphyletic and Möller et al. (2011a) found that only 12/29 genera for which they sampled more than one species were monophyletic. However, much-needed changes in generic limits are underway, and some of these changes in New World Gesneriaceae are explained in a series of articles in Gesneriads 56(3). 2006, and all generic changes as of 2020 are conveniently summarized by Weber et al. (2020).
Some genera like Chirita were very much polyphyletic, and Primulina, originally monotypic, has been greatly expanded to ca 180≤ species in the course of understanding the limits of Chirita (Weber et al. 2011). For generic limits around Paradrymonia, see Mora and Clark (2016). There are also many monotypic genera, some of which are needed (J. F. Smith & Clark 2013), but others are not, thus Oreocharis has been expanded to include eleven mostly very small Chinese genera (Möller et al. 2011b). On the other hand, the huge Didymocarpus has been dismembered (Weber & Burtt 1998), species that had been included there being assigned to 27 genera (including two in Plantaginaceae)... Many species were initially placed in Henckelia, although this turned out not to be monophyletic, and it is now considerably restricted (Middleton et al. 2013). The limits of Paraboea have been adjusted (Puglisi et al. 2011), while Puglisi et al. (2016) revised generic limits in Loxocarpinae as a whole. Streptocarpus has been expanded and now includes Saintpaulia, etc., i.e. all African-Madagascan Trichosporeae (Nishii et al. 2015); chromosome numbers characterize the two subgenera recognized, and these are divided into sections.
The large genus Cyrtandra has been broken up into some 40 sections, although the form any final classification here will take is unclear (Atkins 2013).
Previous Relationships. The limits of Gesneriaceae have by and large been quite stable, although Sanango has previously been placed in Loganiaceae or Buddlejaceae and it was for some time unclear if a few other genera belonged here or elsewhere in Lamiales (see above).
[Plantaginaceae [Scrophulariaceae [Stilbaceae [[Byblidaceae + Linderniaceae] [[Pedaliaceae, Martyniaceae, Acanthaceae] [Bignoniaceae [[[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]]] [Lamiaceae [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]]]]]]]: route II decarboxylated iridoids as glucosides [aucubin, catalpol widespread], 6- or 8-hydroxyflavones or 6 methoxyflavones +, cornosides 0; inflorescence racemose [lateral and main axes of inflorescence indeterminate]; A not adnate to C; (embryo sac haustoria +).
Age. Bremer et al. (2004) suggested an age of ca 76 Ma for this node, Tank and Olmstead (2017) an age of (81.3-)70.2(-60.1) Ma, Cusimano and Wicke (2016) an age of (71.5-)65.9, 60.3(-56.7) Ma, Wikström et al. (2015) an age of (72-)62(-53) Ma, and Magallón et al. (2015: note topology) an age of around 52.9 My; the age of (51.7-)31.5(-12.8) Ma suggested by Naumann et al. (2013) is substantially younger, less than half the ca 69.2 Ma estimate in W.-Q. Xu et al. (2018) and ca 71.8 Ma by Fonseca (2021).
Evolution: Divergence & Distribution. Node ages in Tank et al. (2015: Table S2) within this large clade are all less than 40 Ma, even if details of the tree topology there differ from those here.
Plant-Animal Interactions. Caterpillars of Nymphalinae-Melitaeini and -"Kallimini" (Junoniini, Victorinini) butterflies are quite common on plants in this group (see also Plantaginaceae, Acanthaceae and Orobanchaceae below, literature of larval uptake of defensive compounds in Opitz & Müller 2009). They may have moved here from the Urticaceae group of families (Rosales) around about the K/P boundary, a shift that may have been followed by an increased diversification rate (Fordyce 2010). Some Melitaeini in turn adopted members of Asteraceae as food plants (Nylin & Wahlberg 2008; Wahlberg et al. 2009; Nylin et al. 2014).
Chemistry, Morphology, etc.. For the synthetic pathway of route II iridoids, see Jensen et al. (2002); 8-epi-irodial, 8-epi-iridotrial and 8-epi-deoxyloganic acid are the precursors. Iridioid acquisition seems best placed here, with an independent origin in Oleaceae (modified route I iridoids - Jensen et al. 2002). Flavonoid 7-O-glucosides and glucuroides are scattered in Lamiaceae, Pedaliaceae, and Plantaginaceae (Noguchi et al. 2009).
Extrafloral nectaries in this clade commonly are made up of rather scattered multicellular trichomes (Zimmermann 1932).
PLANTAGINACEAE Jussieu, nom. cons. - Back to Lamiales
Herbs; phloem loading not via intermediary cells, raffinose etc. not involved, mannitol +, (iridoids 0), little oxalate accumulation; cork initiation various; (wood rayless); hairs with gland head not vertically divided, (with cystolith); leaves also spiral, simple to compound; C (descending cochleate); stamens (2; 5-8), thecae parallel/end-to-end/sagittate/on connective arms, connective well developed, placentoids usu. 0, staminode usu. 0; pollen exine tectate and reticulate; (placentation intrusive parietal), stigma (slightly) capitate or bilobed, dry (wet); ovules (campylotropous?), integument 3-22 cells across; fruit a septicidal capsule (circumscissile); seeds (1-)many, (pedestals +), (variously sculpted/winged), exotestal cells with inner walls ± thickened, when winged, cells with reticulate thickenings, (mesotesta to 4 cells across); endosperm +/0, mannose-rich polysaccharides + [?distribution], (embryo chlorophyllous), (short), (curved), (suspensor long); n = 6-10+, x = 9, protein bodies in nucleus amorphous, nuclear genome [1 C] (0.12-)0.837(-5.863) pg/(313-)947(-4523) Mb.
Ca 90 [list, ± to tribes]/1,900. Mostly temperate. Map: from van Steenis and van Balgooy (1966), Hultén (1971), Meusel et al. (1978), Frankenberg and Klaus (1980), Hong (1983) and Heide-Jørgensen (2008).
Age. Bell et al. (2010: note sampling) suggested an age of (57-)46, 42(-34) Ma for crown Plantaginaceae; an age of ca 66 Ma was suggested by Bremer et al. (2004), (79.2-)66.5(-53.1) Ma by Tank and Olmstead (2017), an age of (62-)50(-37) Ma by Wikström et al. (2015) and an age of 70.7 Ma by Fonseca (2021); Meudt et al. (2015b) dated a clade [Globularia + Veronica] to ca 28 Ma, while the age of a clade [Globularia [Plantago + Digitalis]] was estimated to be ca 27.5 Ma (Affenzeller et al. 2018) and of a clade including Penstemon, Bacopa, Plantago, Gratiola and Antirrhinum around 22 Ma (Wolfe et al. 2021).
[Digitalideae, Veroniceae, Plantagineae, Globularieae, Hemiphragmeae]: ?
1. Digitalideae Dumortier —— Synonymy: Digitalidaceae Martynov, Erinaceae Pfeiffer
Biennial herbs to shrubby; cardenolides, steroidal saponins +; (xylem rays 0).
2/24: Digitalis (22). Europe and North Africa to Central Asia.
Veroniceae + Plantagineae] [if this clade exists]: xylem rays 0 (+), axial parenchyma usu. 0.
2. Veroniceae Duby —— Synonymy: Veronicaceae Cassel
Perennial (annual) herbs, (shrubs); ?foliar endodermis +; capsule loculicidal [Veronica].
10/525: Veronica (ca 460),Lagotis (30), Veronicastrum (19). Esp. Northern Hemisphere (Australasia - inc. 6 genera placed in Veronica). Photo: Veronica Flower.
3. Plantagineae Dumortier —— Synonymy: Aragoaceae D. Don, Littorellaceae Gray, Psylliaceae Horaninow
Herb, (stoloniferous), (annual), (ericoid shrub); (CAM photosynthesis +); rehmannioside [caffeoyl phenylethanoid glucoside], sugar alcohol sorbitol [carbohydrate transport] +; (CAM photosynthesis +); primary vascular system without bundles, xylem rayless; foliar endodermis + [Plantago]; leaves spiral, (whorled), (bases broad), blade (linear), (venation parallel); (plant monoecious - Littorella); inflorescence scapose/not; flowers ± polysymmetric, 4-merous; pollen polyporate; style (± 0), stigma long-papillate; fruit capsular, loculicidal + septicidal/circumscissile, (indehiscent, nut-like); ovules 1 basal/many axile per carpel; seed winged/coat often mucilaginous/neither; n = 4-6.
2/290 - or 3 genera: Plantago (270). ± Cosmopolitan, inc. oceanic islands.
Age. Crown-group Plantagineae are ca 7.1 or 2.8 Ma (Iwanycki Ahlstrand 2019 for references, also other estimates).
4. Globularieae Reichenbach —— Synonymy: Globulariaceae Candolle, nom. cons.
Herbs to shrublets; inflorescence more or less capitate (not); only the abaxial carpel developed [G.]; 1 pendulous ovule/carpel [G.]; fruit nutlike, surrounded by the calyx; n = 8-10, 19.
3/42: Globularia (22), Campylanthus (18). Macaronesia, Central and South Europe, North Africa to Pakistan.
5. Hemiphragmateae Rouy - Hemiphragma heterophyllum Wallich
Stoloniferous herb; axillary branches short shoots, fascicles of short, needle-like leaves; flowers axillary, single;, polysymmetric; subsessile; A 4, thecae confluent apically; submature fruit shiny red, berry-like, finally a loculicidal capsule; n = ?
1/1. Himalayas to China, Formosa, Philippines, Celebes.
6. Angelonieae Pennell —— Synonymy: Angeloniaceae V. C. Souza, P. Dias & Udulutsch, Oxycladaceae Schnizlein
Protein bodies in nucleus not amorphous; flowers oil-producing (not).
6/57: Angelonia (25), Ourisia (18). New World, Mexico southwards, New Zealand (some Ourisia).
7. Gratioleae Bentham —— Synonymy: Caprariaceae Martynov, Gratiolaceae Martynov, Scopariaceae Trinius, Trapellaceae Honda & Sakisaka
Prostrate to erect herbs, suffrutescent, (aquatic), (annual), (carnivorous - Philcoxia); root cortical phi [φ] cell wall thickenings radially oriented [Bacopa], stem with endodermis [B.]; (leaves whorled, linear); flowers (1(2)/axil), (sessile), (bracteoles 0); flowers (± polysymmetric), (resupinate); K ± equal (not), free to connate; A (2-)4(-5), (staminode +), (2, unithecate)/ etc.), (anthers stipitate); (pollen tricolpate); integument 3-6 cells across; protein bodies in nucleus not amorphous.
30/>300:Bacopa (55), Stemodia (25), Adenosma (22).
8. Antirrhineae Bentham —— Synonymy: Antirrhinaceae Persoon, Linariaceae Berchtold & J. Presl
Annual to perennial herbs (petiole climbers); sieve tubes with tubular protein bodies; bracteoles 0; C with spur/not, personate/not; staminode +; nectary on G, with nectarostomata; capsule poricidal; seeds winged/longitudinally-reticately ridged.
Ca 29/357 (297-501): Linaria (194), Nanorrhinum (29), Chaenorhinum (35), Kickxia (24), Antirrhinum (23). Pretty much global, few Africa.
Age. Crown-group Antirrhineae are (52-)ca 48(-36) Ma (Fernández-Mazuecos et al. 2019).
9. Callitricheae Dumortier —— Synonymy: Callitrichaceae Link, nom. cons., Hippuridaceae Vest, nom. cons.
Aquatics; stem with endodermis; hairs stalked, cells of head ± radially arranged; plant monoecious; K rim-like [2-4-lobed, entire/0, C 0; staminate flowers: A 1(-3); pollen grains tricellular, surface very various; carpelate flowers: G (1, inferior - Hippuris); ovules 1-2/carpel, apical; integument large/massive; fruit indehiscent/schizocarp; testa thin, undistinguished; n = 3-20.
2/76: Callitriche (ca 75). Close to cosmopolitan. Photos: Callitriche habit, Hippuris habit.
10. Sibthorpieae Bentham —— Synonymy: Ellisophyllaceae Honda, Sibthorpiaceae D. Don
Prostrate ± stoloniferous herbs; flowers axillary, single or fasciculate, 4-8-merous, polysymmetric; C rotate; pollen 3-colporate (-porate), rugulate (perforate) to (micro)reticulate, nanoechinate or not; n = 9, 10.
2/6: Sibthorpia (5). Mexico and the Caribbean, ± western South America south to Argentina, and Africa, where scattered and at higher elevations, also coastal Western Europe, eastern Himalayas to Japan and Malesia.
11. Cheloneae Bentham —— Synonymy: Chelonaceae Martynov
Pair-flowered cymes +; anther thecae confluent, staminode +; nectary at abaxial base of adaxial-lateral A, with nectar-secreting trichomes [Penstemon].
5/294: Penstemon (285). North America, esp. the West, Mexico.
Age. Crown-group Cheloeae are thought to be (19.0-)10.9 [or 14.9](-4.4) Ma (Wolfe et al. 2021).
12. Russelieae Pennell
(Arching subshrub): (leaves reduced); pair-flowered cymes +.
2/54: Russelia (52). Mexico to Colombia, Cuba.
Evolution: Divergence & Distribution. Clades of Plantaginaceae such as Hebe (deeply embedded in Veronica), Ourisia, Penstemon and Globularia have radiated, sometimes very extensively, in alpine habitats in various parts of the world (Madriñán et al. 2013; Hughes & Atchison 2015; Affenzeller et al. 2018). Indeed, the highly polyploid (6-18x) Hebe (= Veronica sect. Hebe), with some 130 species centred in New Zealand, some also in New Guinea but absent from New Caledonia, is the largest lineage of woody angiosperms there, and the variation in growth form is remarkable (Wagstaff & Garnock Jones 1998; Wagstaff et al. 2002; A. E. Thomas 2021; Zizka et al. 2022). Of course, the distributions of the segregates of Veronica involved are unlikely to have anything to do with Gondwanan vicariance, and the microphylly of some of the species is unlikely to have been inherited from gymnosperm ancestors (c.f. Heads 1994 and references), rather, diversification of section Hebe probably occured 10-5 Ma (Thomas et al. 2021.) In Veronica s.l. no correlation was initially found between speciation rates and rate of molecular evolution (K. Müller & Albach 2010), however, in a comprehensive study Meudt et al. (2015b, q.v. for dates, etc.) found that decrease in genome size, which is notably smaller in annuals, was linked with increased diversification rates. For more on the evolution of Veronica, see also Rojas-Andrés et al. (2015) and J.-C. Wang et al. (2016), the latter group focusing on the evolution of the annual habit which has occurred up to ten times here alone, perhaps in response to progressive aridification (V. filiformis is secondarily perennial).
Passive uplift of plants as the Andes formed may have been involved in the diversification of both Ourisia and Aragoa; the latter is sister to Plantago, which itself has some species growing in the páramo (Heads 2019b; c.f. Sklenár et al. 2011). The Plantago clade is 5-17 Ma old (Cho et al. 2004; Rønsted et al. 2002b) or ca 18.5 Ma (Iwanycki Ahlstrand et al. 2019: huge error bars) The flowers of Plantago, sister to Aragoa (e.g. Bello et al. 2002b), an ericoid shrub that grows in the Páramo, are small, polysymmetric (as are those of Aragoa, which has 4-merous flowers, but with five sepals), and are borne in dense spikes; they have four sepals, petals and stamens, and they are wind pollinated. Their evolution is connected with the degeneration of some floral symmetry genes, e.g. Cycloidea (Preston et al. 2011a). Bello et al. (2004) discuss floral evolution in the Plantago area, also emphasizing the evolution of polysymmetry; flowers of Aragoa are indeed monosymmetric - but only early in development; Reardon et al. (2014) suggested that a loss of the RAD gene in P. lanceolata results in polysymmetry there. The mucilaginous seed coats (hemicellulosic mucilage) of Plantago may have facilitated the three dispersals of this genus from Australia to New Zealand (Tay et al. 2010), and there have been several other long distance dispersal events to oceanic islands, mostly originating from the nearest mainlands, but these have been followed by diversification only on the Hawai'ian Islands and Rapa (Bass/Austral islands) (Iwanycki Ahlstrand et al. 2019; see also Schenk 2021). Kreitschitz et al. (2020) note that Plantago seeds germinate quite well even after passage through the gut of a pigeon, so perhaps this might enable long distance dispersal. However, ages are a little confusing around here.
Within Antirrhineae there are perhaps four independent connections between Californian and Mediterranean members of the tribe that have been dated to some time in the Miocene around (30-)21-19(-4) Ma, mostly well before the origin of the Mediterranean climates that the plants now prefer (Vargas et al. 2014). For the biogeography of Antirrhineae, world-wide but mostly Mediterranean, see also Ogutcen and Vamosi (2016); long distance dispersal accompanied by polyploidy may be involved. Most Antirrhineae have corolla spurs (but note that they are not an apomorphy for the tribe, but have been acquired four times or so), and the acquisition of these spurs is more or less associated with an increased rate of diversification, if with a lag period of 5-15 MA (Fernández-Mazuecos et al. 2019: lumpers and splitters accomodated).
The diversification rate of Mediterranean species of Linaria ser. Supinae has been notably fast (ca 2 Ma: Blanco-Pastor et al. 2012; see also Martín-Hernanz et al. 2019: Table 4). — Scatigna et al. (2022: Figs 4, 5) noted that there were ten distinct floral variants in the Gratioleae that they examined (Trapella not included), however, none of the characters involved (floral resupination, stipitate and/or monothecal anthers, etc.) were apomorphies for the tribe. — Bacopa is likely to have originated in tropical America; there have been perhaps four transitions to the fully aquatic habit and three shifts to Africa (Tippery et al. 2024). — Shehata et al. (2023) analyzed variation in fruit and seed of a number of Plantaginaceae phenetically, finding that Plantago was distinct from the rest and so was to be put in Plantaginaceae s. str., within these other genera Veronica seemed to be most similar; within the Plantago examined, P. major was quite distinctive.
Penstemon is a very speciose North American genus with some 285 species; the flowers often have a prominent bearded staminode, hence the name of the plant. Wolfe et al. (2021) noted that the origin of the genus seems to have been in the Eastern Cordillera of North America, and much speciation seems to have been in the Plesitocene via founder events as the genus moved on to newly-available ecological niches during the interglacial periods; the crown-group age of the genus is perhaps 3.7-3.6 Ma. Its overall diversification rate has been about as high as that of any other genus in a continental setting (Wolfe et al. 2021). P. Wilson et al. (2006, 2007; see also Wessinger et al. 2016, 2019) discussed the 17-21 shifts from bee to bird pollination in Penstemon, noting morphological changes that both facilitate hummingbirds and prevent bees pollinating the flowers (see also Castellanos et al. 2004: results not always straightforward). Interestingly, adoption of bird pollination has not been a notable stimulus to speciation (Abrahamczyk & Renner 2015: ten shifts; Wessinger et al. 2019: 17 shifts; Wessinger & Hileman 2020). For more on pollination, see Pollination & Seed Dispersal below.
There has been much work on molecular aspects of floral development in Plantaginaceae (e.g. Hileman & Cubas 2009; Hileman 2014; references in Reardon et al. 2016). Antirrhinum majus is a model organism used for understanding the development of monosymmetric flowers and the involvement of the CYC gene in this (e.g. Rosin & Kramer 2009; Preston et al. 2011 for references); there is duplication of the gene in Antirrhineae, but not in Digitalis (Gübitz et al. 2003). Although similar genes are involved in the development of monosymmetric flowers in Senecio vulgaris (Asteraceae), they are expressed differently (see also discussion under Euasterids). Floral evolution in the Veronica/Plantago clade is becoming better understood. Veronica has an open, 4-lobed corolla, but only two stamens; some species have two main veins in the adaxial corolla lobe, perhaps suggesting that it is formed by the fusion of the two adaxial lobes of other members of the family (Mair 1977 for development). Wulfenia, sister to Veronica, has tubular and rather weakly lipped (2 + 3) flowers. For more on Plantago, see above. Polysymmetric flowers have been derived from monosymmetric flowers several times in this family, thus Sibthorpia has 5-8-merous, polysymmetric flowers. The flowers of Linaria have a single well-developed abaxial spur (see Wessinger & Hileman 2020), and here and in Kickxia there seems to be movement of nectar to those spurs via nectar ducts/channels (Vogel 1998a). The polysymmetric Peloria mutant of Linaria vulgaris with five spurs is the result of epigenetic inactivation by methylation of the cycloidea gene which controls monosymmetry in Antirrhinum (Cubas et al. 1999). Variation in length of the spur in Linaria is linked to changes in cell number (Cullen et al. 2018: c.f. Delphinium, changes in cell expansion).
Details of characters like the distribution of R-Put morphologies and the timing of androecium initiation remain to be clarified, and morphological/developmental synapomorphies for Plantaginaceae may yet be found.
Ecology & Physiology. Philcoxia, a recently (2000) described white sand endemic with seven species from Brazil, was suspected of being carnivorous (e.g. Fritsch et al. 2007). This has been confirmed by Pereira et al. (2012): Nematodes stick to the mucilaginous secretions of the capitate glands on the leaves, which may be under the soil surface, and are then digested by the plant, and phosphatase activity has been detected in the hairs. The plants lack mycorrhizae, as is common when there is carnivory - but absence of mycorrhizae is a feature of core Lamiales in general... (see also papers in Ellison & Adamec 2018). Sister to Philcoxia is Lapaea, with four species and described still more recently in 2020 and from the Espinhaço Range, Minas Geraes, where Philcoxia is also to be found (Scatigna & Fleischmann 2021 and references).
Extreme dessication tolerance is known from several species in a few genera in the Linderniaceae area (Gaff & Oliver 2013), of which Craterostigma, whose walls reversibly collapse as the plant dries, has been quite extensively studied (e.g. Vicré et al. 2004a; Hilbricht et al. 2008). The plants are homoiochlorophyllous, retaining their photosynthetic apparatus and chlorophyll during the drying process, and as in other dessication-tolerant angiosperms, late embryogenesis-abundant (LEA) proteins have been coopted into the process, and water-stress-related genes found in dessication-sensitive plants are also involved, but they are expressed differently here (Rodriguez et al. 2010). Chamaegigas intrepidus is a dessication-tolerant aquatic(!) in seasonal pools on African inselbergs (Porembski & Barthlott 2000).
The submersed (at least for part of its life) aquatic Littorella uniflora, (the genus is sister to Plantago - Hoggard et al. 2003), is also a CAM plant (Keeley 1998), and its plastome also lacks functional ndh genes, consistent with its ecology (Mower et al. 2021, q.v. for possible connections between various distinctive life styles, including CAM/the aquatic habitat, that might affect the photosynthetic process and result in the loss of such genes; Sabater 2021; Lin et al. 2017 for heterotrophic taxa in particular).
Species of Callitriche are often aquatics and heterophyllous, although some produce only one leaf type (for phylogeny and movement back and forth between habitats, see Ito et al. 2017b). Details of the molecular control of heterophylly are unclear, although hormones appplied to aerial shoots that may lead to heterophylly in other aquatic plants seem not to be effective here (Koga et al. 2021). For stomatal development, see Doll et al. (2021); stomata are more or less absent in submerged leaves, as in Trapella.
Pollination Biology & Seed Dispersal. Floral morphology is very variable (see Reeves & Olmstead 1998), but Plantaginaceae are pollinated mainly by large insects and birds. Some kind of spur has evolved two to three times in Antirrhineae (Glover et al. 2015), and Vogel (1998b) discussed how nectar gets in to the spur. Guzmán et al. (2015) thought that the tribe as a whole could be characterized by a personate corolla, with its mask-like face, and in a number of species the pollinators (bees) have to exert substantial force to open the tube. Collinsia has remarkable papilionoid flowers. The distinctively bicolored and erect standard is formed from the two adaxial petals, while the three other petals are flat-coloured, the median abaxial petal forming a keel that encloses the stamens. Indeed, the overall colour scheme and functional floral morphology is very like that of some species of Lupinus. For pollination - in New World taxa, hummingbirds are involved again - in Antirrhineae, see Ogutcen et al. (2017) and Guzmán et al. (2017). See also Armbruster et al. (2009a), Baldwin et al. (2011) and Armbruster (2014) for floral evolution and pollination, and Kampny (1995, as Scrophulariaceae) for pollination.
Secretion of oil by multicellular hairs aggregated in elaiophores is quite commen in Plantaginaceae (Renner & Schaefer 2010; Tölke et al. 2019). The South American Monttea and Angelonia, genera that diverged (34-)13(-7) Ma, have weakly bisaccate oil-producing corollas and are visited by several genera of bees (Renner & Schaefer 2010); for oil secretion in Monttea, see Simpson et al. (1990), in the latter the visiting bees have either their front (Centris) or middle (Tapinotaspis) legs elongated (Sérsic & Cocucci 1999; Machado et al. 2002; Martins et al. 2013). At least 30 species in Angelonieae produce oil from hairs inside the corolla, although the bees may sometimes also pick up nectar or pollen; there may have been four or five gains of oil flowers (Renner & Schaefer 2010; Martins & Alves-dos-Santos 2013; Martins et al. 2014b, see also 2013; Possobom & Machado 2017a and references).
Perhaps 40 species of Penstemon are pollinated by hummingbirds (Abrahamczyk & Renner 2015: ten shifts; Wessinger et al. 2019: 17 shiftss; Wessinger & Hileman 2020). Bird pollination has evolved from bee pollination, and of course the morphologies of flowers with the two kinds of pollination are quite distinct (Wilson et al. 2004; see also Thomson et al. 2000), and there have apparently been no reversals from bird to bee pollination (see also Barrett 2013; Thomson & Wilson 2008). Despite the dissimilarity in the two kinds of flowers, in P. barbatus and its relatives at least most of the genome is similar, with species-diagnostic loci close to QTLs with major effects on pollination syndromes like flower colour, nectar volume and flower width that are spread over the genome (Wessinger et al. 2023). Depatie and Wessinger (2025) looked at the evolution of personate flowers in Penstemon; these are flowers in which the tube is more or less occluded by the development of deep abaxial pleats in the corolla. The focus was on species in subsection Penstemon, however, little definite is known about the evolution (although personate flowers appear to have evolved more than once here) and function of such corollas, and the rather different personate corollas found in Antirrhinum and other species of Antirrhineae will need to be included in any study.
Pollination in the aquatic Callitriche may be by wind, or on or under water (= hypohydrophily), or by selfing (Martinsson 1993); the latter two mechanisms evolved once in the genus. In self pollination the pollen grains germinate in the anthers of a staminate flower and the pollen tubes grow through the stem, etc., to the ovules of an adjacent carpelate flower (Osborn & Philbrick 1994; Philbrick & Les 2000; X.-F. Wang et al. 2011). The pollen of C. hermaphroditica, which is hypohyrophilous, may entirely lack exine, and the grains germinate in the anthers (Osborn & Philbrick 1994) - pollen grain + tube makes a better search vehicle? The pollen of C. truncata also lacks exine (Osborn et al. 2001); normal apertures have been lost, the exine may be crotonoid, and so on (for pollination, see also Franchi et al. 2011). There have been several transitions from the aquatic to the terrestrial habitat in the genus (Ito et al. 2017b).
Gametophytic self incompatability occurs in Antirrhinum, at least (Zhang & Xue 2008; McClure 2008).
Muñoz-Centeno et al. (2006) discuss seed morphology in the context of the phylogeny of Plantago; the seeds have mucilaginous coats (myxospermy) which may be involved in both exo- and endozoochory (Iwanycki Ahlstrand et al. 2019). Teixeira et al. (2020) found that a greater amount of the mucilage was associated with higher seed germination and seedling survival under very dry conditions. See also Western (2011) for myxospermy in the family.
Plant-Animal Interactions. For feeding preferences of a variety of insect groups that might suggest that the erstwhile Plantaginaceae s. str. and Scrophulariaceae s.l. are close, see Airy Shaw (1958), Allen (1960, 1961) and Tempère (1969). Allen (1960) found different insects eating Plantaginaceae s. str. and Scrophulariaceae s. str. (see also below). Larvae of Nymphalinae-Melitaeini butterflies are commonly found here and on Orobanchaceae, but not on Scrophulariaceae s. str. (Wahlberg 2001), and their ancestral host plant may have been Plantaginaceae (see also Beran & Petschenka 2022), For more on sequestration of plant metabolites, particularly iridoids, by caterpillars, but also by some other herbivores, see e.g. Bowers (1993), Opitz and Müller (2009), etc.. Dipteran agromyzid leaf miners have diversified on Plantaginaceae (Winkler et al. 2009).
Genes & Genomes. Meudt et al. (2015b) found genome size in Veronica to be reduced in polyploid taxa and there were smaller genomes in annuals than perennials. Prancl et al. (2020) looked at genome size in Callitriche; for more on genome size and evolution in Plantaginaceae, see above.
Mower et al. (2021) and J. Wang et al. (2024) examined variation in the plastome of Plantago, noting i.a. extensive inverted repeat expansions and contractions, increase of repeats (often over 200 or even 500 bp), and a variety of indels that were phylogenetically informative. Furthermore, in subgenus Coronopus in particular there are extensive intron losses that are notable even when looking at angiosperms as a whole; most plastome variation was in subgenera Plantago and Coronopus. Zhu et al. (2015) had noticed that genes in the inverted repeat might have very high synonymous substitution rates, but this occurs only in some genes; possibly replication, recombination and repair mechanisms are in some state of disarray (Wang et al. 2024). Interestingly, there are rather high levels of both plastome and chondrome variation in both subgenera Plantago and Coronopus, and also in the genera mentioned immediately below, but this correlation is not universal (Mower et al. 2021).
Bakker et al. (2006a) found considerable increases in the rate of evolution of the mitochondrial gene nad1 in Plantago and Littorella; Plantago has substitution rates at synonymous sites in the chondrome that are 3,000-4,000 times those of nearly all other angiosperm clades other than axa like Pelargonium, Ajuga and Silene (Cho et al. 2004; Mower et al. 2007; see also Zwonitzer et al. 2024, also Characters). At least three mitochondrial genes have recently been transferred from Cuscuta to species of the P. coronopus group, although for the most part they do not seem to be functional there (Mower et al. 2010). The cox1 intron is common in the family, and the cox1 gene itself has been been lost twice in Plantago, a loss not recorded in any other angiosperm (Sanchez-Puerta et al. 2008).
Economic Importance. For Digitalis, a source of important drugs, see Luckner and Wichtl (2000).
Chemistry, Morphology, etc.. Both Digitalis (and its synonym, Isoplexis) have cornosides. Iridoids with an 8,9 double bond - rather uncommon - occur in a number of genera (Jensen et al. 2007); aragoside, found in Arargoa, is rather like Veronica iridoids (Rønsted et al. 2003). At what level this character might be an apomorphy is unclear; they are to be found in both Veronica and Plantago (Rønsted et al. 2000). Veronica has mannitol (Taskova et al. 2012), while Plantago and Aragoa have sorbitol (Rønsted et al. 2003). P. Pedersen et al. (2007), Jensen et al. (2008a) and Maggi et al. (2009) report on some chemistry of ex-Hebe/Hebe s.l..
Penstemon is reported () to have a storied cambium. Veronica lyallii has successive subhypodermal phellogens (Gray 1937), while Besseya (= Veronica) and Plantago have a foliar endodermis (Lersten 1997). Buds or branches may develop from the petioles of Philcoxia (Scatigna et al. 2015). The cell walls in the heads of the glandular hairs are variously oriented. Lindernieae were until very recently included in Plantaginaceae (now see Linderniaceae) but the heads of their glandular hairs are divided by vertical partitions. However, Russelia and some species of Penstemon, still in Plantaginaceae, also have such hairs (Raman 1991 and references). Doll et al. (2021) looked at stomatal development in Callitriche noting that meristemoids tended not to form in amphibious species (see also Rudall & Knowles 2013 - Nymphaeaceae, etc.).
Penstemon and a few other genera have paired-flower cymes (Weber 2013). The development of the petaloid calyx of Rhodochiton is not connected with the expression of B-class genes (Landis et al. 2012).
In a number of taxa in Plantaginaceae the androecium is initiated before the corolla, but other patterns also occur, so the timing of androecium initiation is perhaps unlikely to be a synapomorphy for the family (Bello et al. 2004, c.f. Judd et al. 2002). Veronica and Plantago, as well as Digitalis, are members of a clade that has descending-cochleate aestivation (Bello et al. 2004), i.e. in bud the abaxial corolla lobes are outide the others. Petals have sometimes been lost in Synthyris (Hufford 1992b). Floral nectary development may repay further investigation. Katzer et al. (2025) recently noted that nectaries in Penstemon, on the abaxial bases of the two adaxial-lateral stamens lacked stomata (the CRABS CLAW gene was not involved), rather, nectar was secreted by glandular hairs with a single stalk cell and two head cells. Note that such hairs in Antirrhinum have a multicellular stalk and a single-celled head, while perhaps comparable hairs in Solanum have a single stalk cell and a four-celled head - Solanaceae-Solaneae have no nectaries; MIXTA-like genes are involved in gland-head develkopment here (Katzer et al. 2025). Illustrations in Chatin (1874) suggest that the ovules of Veronica may be crassinucellate. The large, transversely elongated endothelial cells in vertical rows in Gratioleae cause their seeds to have longitudinal ridges, and the extotestal cells have hook-like thickenings.
For general information, see Rahn (1996: Plantago), Sutton (1988: Antirrhineae), Leins and Erbar (2004a: Hippuridaceae), Erbar and Leins (2004b: Callitrichaceae), Schwarzbach (2004: Plantaginaceae), Ihlenfeldt (2004) and Takhtajan (2013), both Trapellaceae, Fischer (2004b: Scrophulariaceae p. pte), Wagenitz (2004: Globulariaceae) and Albach et al. (2021: Sibthorpeae, esp. pollen). For chemistry, see Jensen (2005), Taskova et al. (2006), and Jensen et al. (2009c), for Trapella, see Oliver (1888), and for a general survey, see Thieret (1967). Additional information is provided by Oskolski et al. (2021: esp. anatomy of Aragoa), Trapp (1933: foliar endodermis), Kampny et al. (1993), Schrock and Palser (1967), Leins and Erbar (1988, 2010), and Endress (1999), all floral development, De-yuan (1984: Veroniceae), Tsymbalyuk and Mosyakin (2013) and Sosa et al. (2025: Bacopa, all pollen, Schmid (1906: ovules, Scrophulariaceae s.l.), Elisens (1985: seeds, considerable variation in Antirrhineae) and Ahedor and Elisens (2015: seeds, Gratiolinae).
Phylogeny. Plantaginaceae as here circumscribed initially had only rather weak support, e.g. Olmstead et al. (2001, as Veronicaceae: inclusion of Cheloneae and Hemimerideae may be the problem; for the latter, see Scrophulariaceae below), but see Oxelman et al. (2005: support stronger), and Tank et al. (2006, summary, as Veronicaceae), Z.-D. Chen et al. (2016), Chinese taxa, fair support, also Olmstead and Reeves (1995) and Reeves and Olmstead (1998).
Gratiolaceae were recognised as a distinct family by Rahmanzadeh et al. (2004), although only three species were examined; Rahmanzadeh et al. (2004) did not characterise the family, and in it they included the widespread Limosella (here Scrophulariaceae) and, somewhat hesitantly, Lindenbergia (here Orobanchaceae) along with 30 other genera. Albach et al. (2005a) found relationships in a combined molecular tree to be [[Gratioleae + Angelonieae] [Cheloneae [Antirrhineae + The Rest]]]. However, Estes and Small (2008) placed Antirrhinum, along with members of Cheloneae and other tribes, in a clade sister to [Angelonieae + Gratioleae]; Limnophila was part of Gratioleae (1.0 p.p.), Limosella was not sampled. Kornhall and Bremer (2004) placed Limosella in Scrophulariaceae, but they did not look at other members of Gratiolaceae. Gratioleae, in which Trapella was embedded ([Bacopa [Trapella [2 spp. Gratiola]]] - strong support for this position), and Angelonieae formed a clade sister to all other Plantaginaceae examined (see Gormley et al. 2015; Fonseca 2021).
For the phylogeny of Antirrhineae, see Ghebrehiwit et al. (2003), Vargas et al. (2004), Guzmán et al. (2015) and Ogutcen and Vamosi (2016); some genera are not monophyletic, and both major patterns and details of relationships seemed not to be stable. Fernândez-Mazuecos et al. (2013) discuss relatonships within Linaria (which is monophyletic). The focus in Blanco-Pastor et al. (2012) was on relationships within the Mediterranean series Supinae, and they examined the nuclear ITS and AGT1 and two plastid regions; there was conflict between the analyses of the three sets of data, and they suggested that both hybridization and incomplete lineage sorting were likely to be involved, as in other groups from this area/time, i.e. within the last 2 million years or so. Bräuchler et al. (2004) discussed the phylogeny of the cardenolide-rich Digitalis (to include the bird-pollinated Isoplexis).
Callitricheae. Ito et al. (2017b: worldwide) and Franci et al. (2020) looked at the phylogeny of Callitriche which shows considerable variation in cytology and genome size; there has also been quite extensive hybridization.
Cheloneae. Wolfe et al. (2006) outline phylogenetic relationships in Penstemon (see also P. Wilson et al. 2007; Wessinger et al. 2016); section Dasanthera may be sister to the rest of the genus. Wolfe et al. (2021) looked at the variation in 43 nuclear gene in 239/285 spp; their work should be consulted for details of relationships here. For the phylogeny of Collinsia and the related Tonella, see Baldwin et al. (2011).
Globularieae. Affenzeller et al. (2018: 9.6 Ma - Poskea) examined the phylogeny of the ca 47 species of [Globularieae + Campylanthus], some 17 Ma; Campylanthus itelf has flowers that seem to be held upside down, at least sometimes, and are more or less polysymmetric when viewed face-on, and it has two stamens; sorbitol and similar compounds are found both here and in Globularia itself (Rønsted et al. 2003).
Gratioleae. Oxelman et al. (2005) located the majority of Gratiolaceae in Plantaginaceae, although Limosella remained in Scrophulariaceae (see also Schäferhoff et al. 2010). Stemodia is polyphyletic, Bacopa paraphyletic, and other genera likewise (Scatigna et al. 2018, 2020, 2022). Relationships in Scatigna et al. (2022: 88 spp., Trapella not included, 3 plastid and 1 nuclear markers) are [[Darcya + Mecardonia] [[Chodaphyton + Bacopa s.l.] ...]]. Relationships in Bacopa were clarified by Tippery et al. (2024: ca 33 spp, two chloroplast markers + nuclear ribosomal ITS); the genus is monophyletic if Conobea is included.
Plantagineae. For relationships in Plantago, see Rahn (1996: a morphological analysis), Rønsted et al. (2002b), Hoggard et al. (2003) and in particular Iwanycki Ahlstrand et al. (2019), the latter group finding that previous morphology-based sections, including those of Rahn (1996), were usually not supported, relationships more reflected geography (see also Hassemer et al. 2019). Hassemer et al. (2019: plastome data) examined relationships within subgenus Plantago, almost 3/5ths of the genus; three small clades from the northern hemisphere were successively sister to the rest of the subgenus. Mower et al. (2021) looked at plastome variation in 36 species of Plantago (24 plastomes complete). The phylogeny of these species was for the most part well supported and was consistent with the recognition of four subgenera; sections were also for the most part monophyletic (Mower et al. 2021). Interestingly, relationships found by Ishikawa et al. (2009), who used a nuclear marker, were rather different from others obtained; they thought that hybridization had been involved. The [Plantago + Littorella] clade is sister to the shrublet Aragoa (Bello et al. 2002b; Hoggard et al. 2003; Mower et al. 2021).
For a phylogeny of Veroniceae, see Albach et al. (2004a, c, 2005c), Taskova et al. (2004, 2006), and Albach and Meudt (2010); the "new" molecular relationships are at least sometimes supported by other data such as chromosome number and iridoid type (Albach et al. 2004b, 2005c; Albach & Meudt 2010). Albach (2008) discussed the limits of Veronica s.l., which is to include Hebe, etc.; for its chemistry, see Maggi et al. (2009 and references) and for its pollen, quite distinctive, see Tsymbaluk and Mosyakin (2013). The ca 130 species of the Hebe complex are found in New Zealand, except for a few from New Guinea (Albach et al. 2005b); the genus is polyphyletic. The Angiosperms353 sequences of section Hebe analyzed by A. E. Thomas et al. (2021) provided better phylogenetic resolution at shallower nodes. Wulfenia is sister to the expanded Veronica (see also Bello et al. 2002b; Meudt et al. 2015b). General. For more on relationships here, see also Estes and Small (2008) and Scatigna et al. (2018: Philcoxia definitely to be included).
Classification. The circumscription of Plantaginaceae adopted here is broad on the one hand (it incorporates several highly divergent but small clades previously recognized as families - see above), but narrow on the other (it includes but part of the old Scrophulariaceae). These small but florally very distinctive potential segregate families are derived members of a clade that also includes numerous species with relatively large but undistinguished monosymmetric flowers. Maintaining these families as distinct would entail the recognition of a number of other families that would be poorly characterised. Rahmanzadeh et al. (2004) included about 32 genera in their Gratiolaceae, Souza and Lorenzi (2012) included ca 20 genera and 250 species, among them the carnivore Philcoxia. Rahmanzadeh et al. (2004) thought that Angelonieae might also be part of their Gratiolaceae, but Souza and Lorenzi (2012) recognized an Angeloniaceae, often with oil flowers, that have a spurred corolla (5 genera, with 30 species, were mentioned); Ourisia (28) seems not to have been accounted for. Campylanthaceae nom. nud. (in Globularieae above) are mentioned in a flora of the Canary Islands (Muer et al. 2016). Generic limits here can be tricky - e.g. Antirrhineae.
Even accepting the circumscription of Plantaginaceae above, there is some debate as to what to call the clade. Some prefer Veronicaceae (see e.g. Hind & King 2020) because Plantago is hardly charismatic, its pollen is an allergen, the plant is a common weed, and as to its flowers - well, they barely exist...
For a subgeneric classification of Plantago, see Rønsted et al. (2002b), and for a sectional classification of Plantago subgenus Plantago, see Hassemer et al. (2019), and for more on a developing infrageneric classification there, see Mower et al. (2021). Veronica is to be expanded to include Hebe, Parahebe, Synthyris, etc.; recognizing them would entail the recognition of ca 9 genera in the complex (e.g. Albach et al. 2004; Meudt et al. 2015b and references). Tippery et al. (2024) recognised four sections within Bacopa. In general, generic limits continue (as of iii.2024) to need much attention. Various infrageneric classifications have been proposed for Penstemon, but that in Wolfe et al. (2021) should be followed.
Previous Relationships. Both Cronquist (1891) and Takhtajan (1997) recognise several of the smaller families just mentioned, but they are in the same general part of their sequences; Cronquist had a notably broad circumscription of Globulariaceae and included a number of genera here placed in Scrophulariaceae. Trapella has been included in Pedaliaceae (e.g. Cronquist 1981), in part because its stoutly-spiny fruits appear to be so similar to those of that family - but c.f. .
Botanical Trivia. Linnaeus was initially so impressed with the distinctive morphology of what he called the Peloria mutant, differing as it did so strikingly in floral characters (these would be generic characters for him) from Linaris vulgaris - as he wrote, "This is certainly no less remarkable than if a cow were to give birth to a calf with a wolf's head", but J. E. Smith sourly observed.
Thanks. I thank Dirk Albach for comments.
Age. Bremer et al. (2004) suggested an age of ca 75 Ma for this node, Tank and Olmstead (2017) an age of (76.8-)66.6(-57.4) Ma, W.-Q. Xu et al. (2018) an age of (75.6-)65.6(-49.1) Ma, Wikström et al. (2015) an age of (67-)58(-49) Ma and Fonseca (2021) an age of ca 70.3 Ma.
SCROPHULARIACEAE Jussieu, nom. cons. - Back to Lamiales
Herbs to shrubs, (vines); harpagide, harpagioside [8ß-8α-methyl substituted iridoids] +, (secoiridoids +), little oxalate accumulation; nodes also 1:3 + girdling bundle; leaves (spiral above), (basally connate), lamina vernation flat, (± foliaceous, stipuliform structures +); inflorescence branches cymose; K unequal or not; anthers synthecous, staminodia +/0; tapetal cells binucleate; nectary small or 0; stigma capitate (lingulate), dry or wet; ovules many/carpel, campylotropous, integument 5-11(-12) cells across; fruit a capsule, septicidal (and apically loculicidal); seeds many, to 1.5 mm long; exotestal and endotestal cells with thickened inner walls; endosperm moderate; x = 9, (protein crystal stacks in nucleus), nuclear genome [1 C] (0.089-)0.939(-9.686) pg/(349-)943(-1900) Mb.
59 [list]/2,245 - 10 groups below. World wide, but esp. southern Africa. Map: from Hultén (1958, 1971), van Steenis and van Balgooy (1966), Meusel et al. 1978), Leeuwenberg (1979), Hong (1983), Hilliard (1994), Norman (2000) and Lebrun (1977, 1979 [Sahara]).
Age. Bremer et al. (2004: Buddleja included) suggested an age of ca 68 Ma, Tank and Olmstead (2017) an age of (76.8-)66.6(-57.4) Ma, Wikström et al. (2015) an age of (61-)50(-38) Ma and Fonseca (2021) an age of ca 64.7 Ma for this clade; (80.5-)66.7(–53.3) Ma is the age suggested by Villaverde et al. (2023). Note that ages in X. Dong et al. (2022) are based on their Fig. 8 in which the topology is very different from that in their Fig. 6 and from trees recovered in other work on the order; these ages are not mentioned below.
[Aptosimeae [Androyeae [Myoporeae + Leucophylleae]]]: ?
Age. Bell et al. (2010) estimated an age of (58-)53, 51(-45) Ma for this clade (Buddleja was widely separated and sister to Paulowniaceae), and Villaverde et al. (2023: Fig. 3, S4, S5, c.f. Fig. 4) suggested an age of (75.1-)61.7(-47.7) Ma.
1. Aptosimeae (Bentham) Bentham
(Annual) herbs to shrubs; (C4 photosynthesis - Anticharis); flowers monosymmetric; staminode 0; pollen syncolporate, prolate, exine striate (not); stigma capitate-clavate to slightly bilobed; seeds rugose; endothelium with isodiamtric cube-shaped cells, walls equally thickened; n = ?
3/38: Aptosimum (23). Africa, drier areas, to India, Cape Verde Islands.
Age. This clade is (47.1-)31.3(-15.9) Ma (Villaverde et al. 2023: Figs S4, S5).
[Androyeae [Myoporeae + Leucophylleae]]: plant woody.
Age. Divergence here occurred (59.7-)41.4(26.5) Ma (Villaverde et al. 2023: c.f. Figs 3, 4, S4, S5).
2. Androyeae Olmstead - Androya decaryi Perrier
Small tree; leaves opposite; flowers 4-merous, polysymmetric; K (almost) free, C subrotate, tube short; A equal, thecae confluent at apex; pollen surface smooth; stigma large, clavate; ovules 2/carpel; capsule loculicidal; seeds two, winged.
1/1. Southern Madagascar.
[Myoporeae + Leucophylleae]: plant shrubby; lamina isobifacial, dorsiventrally flattened, pellucid gland dots +; pollen 3-colpate, colpi diorate, exine reticulate (rugulate, etc.); style somewhat impressed.
Age. These two diverged (36.4-)24.9(-14.8) Ma (Figs 3, S4, p. 1610) or close to 20 Ma (Fig. 4: Villaverde et al. 2023).
3. Myoporeae Reichenbach - Eremophila R. Brown —— Synonymy: Bontiaceae Horaninow, Myoporaceae R. Brown, nom. cons.
Prostrate shrub to small tree; schizogenous secretory cavities + [producing resin]; (hairs stellate/uniseriate/glandular/etc.); inflorescence various, bracts/bracteoles 0; flowers strongly monosymmetric to ± polysymmetric, (4:1); ovary to 12-locular [loculi secondarily divided], stigmatic surface in a notch at tip of slender style (capitate); ovules 1-4/loculus, collateral to superposed, pendulous, anatropous, epitropous; fruit a drupe/schizocarp; seeds 2-3 mm long; endosperm slight; n = 18.
1/270. Esp. Australia, also the Caribbean (1 sp.), scattered from Mauritius/Reunion (1 sp.), S. China and Japan to New Zealand, Hawaii. Map: Chinnock (2007: Map. 1). Photo: Myoporaceae s. str. flower, also Myoporeae.
4. Leucophylleae Miers
Vascular tissue in a continuous ring; hairs usu. branched/stellate; stomata anisocytic; leaves (bifacial), (pellucid gland dots 0; foliar cavities +); flowers usu. weakly monosymmetric to polysymmetric; K divided to near base; (A 2, 5), anthers synthecous; style tips expanded and flattened, stigma along the margins; ovules (1/carpel); (seeds to 2 mm long); n = 17.
3/17: Leucophyllum (12). S.W. U.S.A. and Mexico to tropical America.
[Hemimerideae [[Buddlejeae + Teedieae] [Camptolomeae [Limoselleae + Scrophularieae]]]]: ?
Age. This clade is ca 63.2 Ma (Villaverde et al. 2023: Fig. S4).
5. Hemimerideae Bentham —— Synonymy: Hemimeridaceae Doweld
Annual to perennial herbs (shrublets); flowers strongly monosymmetric, (resupinate - Alonsoa); C spurs 0, 1, 2; staminodia 0; pollen 3-, 5-8-colporate or 6-8-colpate, prolate, spheroidal; (oil flowers); stigma capitate to weakly bilobed; seeds winged, testa alveolate; n = 7, 9.
6/150: Diascia (73), Nemesia (65). Africa-Madagascar, esp. South Africa, few tropical America.
Age. Crown-group Hemimerideae are estimated to be ca 58.3 Ma (Fig. S4) or (70.0-)56.9(–44.7) Ma (Villaverde et al. 2023). The Alonsoa-Nemesia split (most of the tribe) is estimated to be 62-59 Ma (more likely, calibration of K. Bremer et al. 2004a), ca 52.1 (Villareal et al. 2023: Fig. S4) or 47.5-42 Ma (calibration of Wikström et al. 2001); see Datson et al. (2008).
[[Buddlejeae + Teedieae] [Camptolomeae [Limoselleae + Scrophularieae]]]: ?
Age. This clade is some (64.5-)52.4(-39.9) Ma or 41 Ma (Villaverde et al. 2023: Fig. 3, S4, S5, c.f. Fig. 4).
[Buddlejeae + Teedieae]: plant woody; bark smooth, lenticillate, cork (sub)epidermal, phelloderm + [Buddleja sect. Gomphostigma, Freylinia], moderate/slight phloem lignification.
Age. These tribes diverged (58.8-)47.3(-35.3) Ma (Villaverde et al. 2023: Fig. S4, 5).
6. Buddlejeae Bartling - Buddleja L. —— Synonymy: Buddlejaceae K. Wilhelm, nom. cons.
Shrubs to trees; vessel elements with helical thickenings; cork usu. inner cortical/pericyclic/outer phloem, phelloderm 0, massive phloem lignification); (?plant dioecious); flowers polysymmetric (monosymmetric), 4-merous; A (thecae separate), pollen 3-colporate, (4-colpate), surface smooth, orbicules 0; (G 4-locular), stigma globose to ± bifid; hypostase +; (fruit a berry), seeds winged or not, (alveolate); exotestal cells ± longitudinally elongated, inner walls thickened; endosperm +, chalazal haustorium single large cell; n = 14, 15, 19, etc..
1/108. The Americas, Asia, Africa, also Madagascar, Fiji, the Greater Antilles.
7. Teedieae Bentham —— Synonymy: Oftiaceae Takhtajan & Reveal
(Epiphytic) shrubs; inflorescences leafy; anther thecae separate, equal, often parallel; pollen (4-colporate), (macroreticulate).
5/17: Freylinia (8). Africa, mostly South Africa, also Madagascar (1 sp.).
Age. Crown-group Teedieae are (50.3-)36.8(25.5) Ma (Villaverde et al. 2023: Fig. S4, 5).
[Camptolomeae [Limoselleae + Scrophularieae]]: ?
Age. This clade is some (61.9-)50.1(-38.4) Ma (Villaverde et al. 2023: Fig. S4).
8. Camptolomeae Olmstead - Camptoloma Bentham
Perennial herbs or small shrubs; (flowers solitary); K deeply 5-lobed, obscurely 2-lipped; anther thecae confluent; seeds longitudinally ribbed.
1/3. N.E. and S.W. Africa, Arabia, the Canary Islands.
Age. Divergence here is estimated to have happened (21.1-)12.8(6.2) Ma (Villaverde et al. 2023: Fig. S4, 5).
Antherothamnus, unplaced, comes out around here in the tree used by Villaverde et al. (2023: Figs S4, S5) for dating, but it is included in Scrophularieae elsewhere (ibid.: Table S1).
[Limoselleae + Scrophularieae]: pollen ?apertures; testa alveolate.
Age. The age of this clade is around (56.0-)45.1(-34.3) Ma (Villaverde et al. 2023: Figs S4, S5).
9. Limoselleae Dumortier (inc. Manuleeae and Selagineae) —— Synonymy: Hebenstretiaceae Horaninow, Limosellaceae J. Agardh, Selaginaceae Choisy, nom. cons.
± Ericoid shrubs to (annual) herbs, (aquatics); (secoiridoids +); (bract adnate to C - Microdon); (K 3-8), deeply lobed to connate; C often ± polysymmetric, adaxial lobes external in bud, (4:0 - Hebenstretia); A (2, 5), filaments (broadened upwards); pollen surface reticulate; nectary asymmetrical, adaxial (0); (adaxial G aborted), stigma lingulate, with marginal papillae (bifid), (punctate with terminal papillae); (ovules 1-)many/carpel, apotropous or epitropous, obturator +; fruit often a 2-seeded schizocarp (indehiscent), pedestals/cushion-shaped scars on the placentae, (funicles massive - Selago, etc.); seeds small, (not alveolate); testa often rather thin, inner cuticle massively developed; n = 6-8(-10).
27/640: Selago (190), Jamesbrittenia (85), Manulea (75), Zaluzianskya (57), Sutera (49), Chaenostoma (46). Especially southern Africa, also temperate northern hemisphere (Limosella).
Age. Limoselleae (Jamesbrittenia + the rest) are (53.7-)42.8(-32.4) Ma (Villaverde et al. 2023: Fig. S4).
10. Scrophularieae Dumortier —— Synonymy: Verbascaceae Berchtold & J. Presl
Annual herbs to shrublets; (flowers weakly monosymmetric); (A 5 - Verbascum), staminodes often well developed, (orbicules 0 - Verbascum); stigma ± capitate; seeds longitudinally/transversely corrugated [aulacospermous/bohrospermous]; n = 9, 13, 15-18.
6/940: Verbascum (720 - or ca 360, or >450...), Scrophularia (200). North Temperate, to the Caribbean, few Africa. Photo - Flower.
Evolution: Divergence & Distribution. Scrophulariaceae are very diverse in southern Africa, having some 700 species there (Johnson 2010). The ancestral area for the family may have been Africa + South America + Madagascar + Australia, but diversification of the speciose Hemimerideae —— Scrophularieae clade seems to have taken place in southern Africa through much of the Paleogene; Villaverde et al. (2023) discuss details of the evolution and geography of the family. Scheunert and Heubl (2017) suggested that Scrophularia originated during the Miocene in Southwestern Asia whence it spread throughout North Temperate regions, etc.; there was much speciation in montane habitats.
Endress (1992) looked at floral diversity in the old Scrophulariales, i.e., very much a paraphyletic group, emphasizing parallel evolution in the context of pollination biology.
Ecology & Physiology. C4 photosynthesis, with Atriplicoid anatomy and the NAD-ME biochemical subtype, is known only from a clade made up of ca 4 annual species of Anticharis, the other species of the genus (including another annual) being more or less C3-C4 intermediates, and the other species of the tribe (Aptosimeae) having C3 photosynthesis. Interestingly, all taxa had rather dense foliar venation (Khoshravesh et al. 2012).
The corms of Limosella grandiflora perennate in a state of extreme dessication (Gaff & Oliver 2013). Annual species are quite common in southern Africa, the ca 50 species of annuals in Nemesia representing 3-4 evolutionary origins of the habit, and they grow in winter rainfall areas, the perennials preferring summer rainfall conditions (Datson et al. 2008). There are a number of vines in the family (see Sousa-Baena et al. 2018b and references).
Pollination Biology & Seed Dispersal. Scrophulariaceae include a number of species that have oil-flowers with oil-secreting hairs (Vogel 1974; Vogel & Cocucci 1995 for a list; Renner & Schaefer 2010; Martins et al. 2013; Possobom & Machado 2017a and references). Details of the pollination of the remarkable two-spurred oil-flowers of the southern African Diascia are quite well known. Several species of the melittid bee Rediviva collect oil from the oil-secreting hairs in the spurs using their sometimes remarkably elongated front pair of legs which have special hairs that absorb oils (Vogel 1984; Steiner & Whitehead 1988; Steiner 1990; Rasmussen & Olesen 2000; Steiner & Whitehead 1990, 1991; S. D. Johnson 2010; Renner & Schaefer 2010; Kahnt et al. 2017). However, these long legs, which may have evolved some five times, seem not to be immediately associated with the spectrum of Diascia plants the bees visited for oils (Kahnt et al. 2017). Thus Hollens et al. (2017) found both a long- and short-legged Rediviva visiting - and pollinating - four species of Diascia with tubes of varying lengths, however, leg and spur length matched the frequency of visits by these bees (see also Pauw et al. 2017). Note that the bees are not specialised for nectar or pollen acquisition, just oil - oil is what they use to provision their larvae. Phylogenetic relatedness in Diascia is connected with the spectrum of Rediviva species visiting the flowers, and overall there seems to be phylogenetic tracking by Rediviva of Diascia (Kahnt et al. 2019), while Steiner and Whitehead (1990, 1991) argue for the pollinator adapting to the plant - which would be very unusual if confirmed (see also Armbruster 2017 for comments on this system). Interestingly, flowers of some Orchidaceae-Orchidoideae-Coryciinae (e.g. Disperis) from the same area are rather similar (mimics) and are also pollinated by the bees (Pauw 2006), as are some other species of oil flowers, including Alonsoa and Hemimeris, also Scrophulariaceae (Renner & Schaefer 2010).
Flowers of several species of Scrophularia are pollinated by wasps (see Kampny 1995: also pollination elsewhere in the family), and evolution of pollinator preferences has been studied in detail there (Navarro-Pérez et al. 2013).
Myxospermy is reported from the old Scrophulariaceae (Grubert 1974).
Plant-Animal Interactions. Mohrbutter (1937) noted both fungi and leaf miners that attacked members of the old Buddlejaceae and Scrophulariaceae placed here. For example, the dipteran agromyzid miner Amauromyza verbasci has been found on Verbascum, Scrophularia and Buddleja (Spencer 1990) nand the aphid Aphis verbasci has similar preferences (Hille Ris Lambers 1979). Other insect herbivores also distinguish between Plantaginaceae and Scrophulariaceae (e.g. Allen 1960; Tempère 1969).
Plant-Bacterial/Fungal Associations. Arbuscular mycorrhizae are reported from Buddleja (Dickie et al. 2007).
Genes & Genomes. For the chloroplast genome of Scrophularia, see W.-Q. Xu et al. (2018).
Chemistry, Morphology, etc.. The iridoids harpagide and harpagioside, found quite commonly in Scrophulariaceae, are also scattered elsewhere in Lamiales, in Lamiaceae (inc. Caryopteris) and Pedaliaceae (Hegnauer & Kooiman 1978; Nicoletti et al. 1988; Georgiev et al. 2013); Soltis et al. (2005b) suggest that such acylated rhamnosyl iridoids characterise Scrophulariaceae. Secoiridoids are known from Manulea, which also has the more conventional verbascoside (Gousiadou et al. 2014). The chemistry of Buddlejaceae (see Jensen 2000b) and other Scrophulariaceae is similar (Houghton et al. 2003); for the chemistry of Myoporaceae s. str., see Ghisalberti (1994), and for that of Verbascum, see Georgiev et al. (2011). Nicodemia (= Buddleja) is reported to have tannin (Bate-Smith & Metcalfe 1957).
The wood anatomy of Buddleja is similar to that of Nuxia (Stilbaceae) and Peltanthera (Gesneriaceae), etc. (Carlquist 1997c), i.e. to taxa that are not immediately related. Frankiewicz et al. (2021b, 2023) looked at the bark anatomy of Buddleja, etc.; the bark of species in section Gomphostigma and that of Freylinia (Teedieae) may be plesiomorphic in the family. Some Scrophulariaceae have opposite leaves, an angled stem, and 1:3 nodes, however, I have not seen the little bundles of fibres that run along the ridges of otherwise similar stems in Linderniaceae (for variation in the number of bundles in the base of the petiole of Eremophila, see Chinnock 2007: Fig. 2). There are glands in the leaves of Leucophyllum and Capraria, c.f. those of Myoporeae s. str. (Lersten & Beaman 1998; Lersten & Curtis 2001). Scrophularia and Verbascum also have distinctive cells (idioblasts) in their leaves (Lersten & Curtis 1997) perhaps similar to these glands, although the clades to which they belong are not close.
Taxa with more or less polysymmetric flowers - sometimes rather like those of Silene, which some South African species may mimic (ref.?) - are common in almost all tribes. There are also taxa with five rotate corolla lobes and five stamens (Capraria) and four lobes and stamens (some species of Buddleja), and in both cases the flowers are fully polysymmetric. Flowers of Verbascum s. str. have five stamens, but those of Celsia, embedded in Verbascum (e.g. Ghahremaninejad et al. 2015), have only four. Hemimeris may have inverted (and inversostylous!) flowers, but the originally adaxial lobe of the corolla is patterned, i.e. it is not functionally different from the normal condition with patterning on the abaxial lobe and adjacent lateral abaxial lobes. The anthers may be straight or U-shaped, but they are not sagittate, and in taxa with confluent dehiscence the two thecae may be end-to-end after dehiscence. The pollen of Aptosimeae was described as being diporate (Oxelman et al. 2005), but Mosyakin and Tsymbalyuk (2015b) mention only a single pore. The number of carpels and the arrangement of the ovules in Eremophila is unclear (Chinnock 2007). The micropylar haustorium of Buddleja ramifies through th integument, almost reaching the chalaza (Maldonado de Magnano 1986b). The alveolate testa can be either bothrospermous or aulacospermous, and some taxa have cushion-shaped scars on the placenta, often with a central umbo or pedestal (e.g. Hilliard 1994). These mark the place where the seeds fell off. Limoselleae with single ovules have much-thickened funicles.
Additional general information is taken from Rogers (1986) and Norman (2000), both Loganiaceae, Hilliard (1994, 1998: nearly all Limoselleae), Oxelman et al. (2004a: Buddlejaceae s. str., 2005), Theisen and Fischer (2004: Myoporaceae), Fischer (2004b: Scrophulariaceae p. pte); see also Jansen (1999), Harborne and Williams (1971), Gousiadou et al. (2019: Limoselleae iridoids), all chemistry, Carlquist and Hoekman (1986) and Carlquist (1997c), both wood anatomy, Rodríguez-Riaño et al. (2015: vascular anatomy of Scrophularia flowers), Mair (1977: development of monosymmetry), Tsymbalyuk and Mosyakin (2013) and Mosyakin and Tsymbalyuk (2015a, b, 2017), all pollen, Vinckier and Smets (2002a: orbicules), Junell (1961: gynoecium of Selagineae), Bendre (1975), Maheswari Devi and Lakshminarayana (1980) and Maldonado de Magnano (1987), all embryology, and Hartl (1959: seed coat/rumination); for floral development, see Armstrong and Douglas (1989) and Endress (1999).
Phylogeny. For early stabs at phylogenetic relationships, see B. Bremer et al. (1994) and Nickrent et al. (1998). The main issues are the relationships between the old Selaginaceae, Buddlejaceae and Myoporaceae and the Scrophulariceae that make up the rest of the clade.
The old Selaginaceae/Selagineae with but a single apical ovule per loculus link with Scrophulariaceae-Manuleéae, and although the latter have more ovules, these are very variable in both number and orientation (see also Junell 1961; Hilliard & Burtt 1977; Hilliard 1994). Kornhall et al. (2001: see also character optimisations, sampling good), found that Selagineae were embedded in Manuleéae, while Kornhall and Bremer (2004) found that the cosmopolitan aquatic Limosella was also to be placed with these often quite xeromorphic and largely southern African taxa.
Buddleja, ex Loganiaceae, is very much paraphyletic and includes Nicodemia, Emorya, and Gomphostigma (see especially Chau et al. 2017: basal relationships around ex-Gomphostigma, B. salviifolia, etc., unclear); several lines of evidence place it in Scrophulariaceae (e.g. Maldonado de Magnano 1986b). Oftia has a racemose inflorescence, only four ovules/carpel and a drupaceous fruit, and the seeds have a very hard testa and copious endosperm (some information from Dahlgren & Rao 1971); it also has 4-colpate pollen (Niezgoda & Tomb 1975) and intraxylary phloem, not known from Teedia, its close relative. Teedia and Oftia have strong support as the sister group to Buddleja s.l. (Wallick et al. 2001, 2002).
The association of Leucophyllum with the old Myoporaceae is well established (e.g. Schwarzbach & McDade 2002; Gándara & Sosa 2013), and both have distinctive pollen - tricolpate, with each colpus diorate (Niezgoda & Tomb 1975; Mosyakin & Tsymbalyuk 2015b; c.f. Argue 1980). Within Leucophylleae, Leucophyllum is strongly paraphyletic, including Eremogeton and Capraria (Gándara & Sosa 2013: support poor to strong). Leucophyllum has only a single pellucid gland at the apex of the lamina and Eremogeton has none; Capraria also has leaf glands and pollen like that of other ex-Myoporaceae and it fits nicely here (for leaf glands, see Lersten & Beaman 1998; c.f. also Henrickson & Flyr 1985; Lersten & Curtis 2001; Henrickson 2004). Androya (ex-Buddlejaceae) and Aptosimum may be around here. The tricolporate pollen of the former has been compared with that of Nicodemia (Loganiaceae s. str.), and it was a member of Loganiaceae s.l.. However, Kornhall et al. (2001) found that Androya was sister to Myoporum, and it was well supported as sister to other Myoporeae in Oxelman et al. (2005) and Gándara and Sosa (2013). Originally placed in Oleaceae, its position here causes problems with character optimisations that I am ignoring for a while. Aptosimum and relatives, long considered a distinct little group, are sister to Myoporeae.
As the scope of phylogenetic studies in Scrophulariaceae has expanded, the broader picture of relationships has become clarified, fortunately, the topologies of the various studies are largely congruent. Kornhall et al. (2001) found that most other Scrophulariaceae were in a clade sister to Buddleja and immediate relatives, although Myoporum was outside this group. The more general relationships in Kornhall and Bremer (2004) are [Myoporum, etc. [[Buddleja, etc.] [[Scrophularia, etc.] + [Limoselleae, inc. Manuleéae, etc.]]]]. Oxelman et al. (2005) found the relationships [Hemimerideae (inc. Diascia) [[Myoporeae + Aptosimeae] [[Buddleja, etc.] [Limoselleae + Scrophularieae]]]]. Above I follow the relationships obtained in the most recent study, that by Villaverde et al. (2023: 49/56 genera, 849 nuclear loci) where quartet support values were over 50% for the majority of the nodes involved in the topology above and slightly undr 50% for the remainder. Santos et al. (2023) recently found that Ameroglossum is to be placed in Linderniaceae.
Buddlejeae. Chau et al. (2017) looked at relationships here; their sampling was pretty good.
Relationships in Hemimerideae are discussed by Oxelman et al. (1999b; see also 2004). [Diascia + Nemesia] may be sister to the rest of the tribe, but the position of the Alonsoa is unclear. For relationships within the South African Nemesia, see Datson et al. (2008: unreversed shift woody → herbs).
Limoselleae. Archibald et al. (2017) examined relationships in the African Zaluzianskya and relatives.
Myoporeae: Fowler et al. (2020) looked at relationships in the tribe based on plastome analyses and recovered an Australian and an extra-Australian clade; two species of Eremophia, both Australian, were included, and one was in each clade... Indeed, the sections of Eremophila recognized by Chinnock (2007) in Eremophila s. str. are not holding up in the much more extensive phylogeny using data from the nuclear ribosomal cistron in Fowler et al. (2021: Fig. 2), and this aside from the fact that Myoporum is well embedded in Eremophila, the extra-Australian clade being embedded in the rest of the genus.
Scrophularieae: For relationships within Scrophularia and Verbascum, see Scheneurt and Heubl (2014, 2017) and Ghahremaninejad et al. (2015: nrITS and 3 plastid markers); the latter group obtained rather poor resolution within Verbascum. Scheunert and Heubl (2014, esp. 2017: 147 spp., nuclear ribosomal ITS and two plastid markers) found substantial plastid—nuclear incongruence, intra-individual ribosomal incongruence, etc., in Scrophularia. X. Dong et al. (2022) looked at a few plastome-based relationships in Verbascum - c.f. their Figs 6 and 8 for broader relationships in the family and order.
Classification. Olmstead et al. (2001) suggested that the recognition of Myoporaceae might make Scrophulariaceae paraphyletic; Chinnock (2007), monographing Myoporaceae s. str., suggested that they could well be included in Scrophulariaceae, as here. Fowler et al. (2021) propose expanding Eremophila to include all of Myoporeae, which seems reasonable, although Eremophila will need to be conserved over some earlier names; whatever the outcome, the sections of Eremophila proposed by Chinnock (2007) will have to be reworked.
I initially largely followed Oxelman et al. (2005) for tribes; Myoporeae and Leucophylleae were kept separate, although they are clearly sister taxa and the combined clade has synapomorphies - except there was a problem with Androya. Villaverde et al. (2023: esp. Table S1) has provided an updated classification; Antherothamnus is included in Scrophularieae (c.f. above).
Generic limits in Leucophylleae will need to be redrawn; a single genus for the tribe would work, but Gándara and Sosa (2013) propose the recognition of five. Chau et al. (2017) justify a broader circumscription of Buddleja (now ≡ Buddlejeae) and its division into seven sections. Previous infrageneric groupings in Verbascum, e.g., those based on seed morphology, did not hold up in the study of Ghahremaninejad et al. (2015).
Previous Relationships. The limits of Scrophulariaceae have long been problematic (Thieret 1967 for a summary; Olmstead 2002 for a readable account of the implications of the findings of molecular data). Albach et al. (2005a) and Oxelman et al. (2005) clarifyied the contents of the separate clades that used to be subsumed in Scrophulariaceae s. l. (see also B. Bremer et al. 2002; Tank et al. 2006); for further details see the introduction to Lamiales above.
Members of the Scrophulariaceae of a generation ago are now also to be found in Plantaginaceae and Orobanchaceae (these include most of the taxa that have moved), as well as in Stilbaceae, Phrymaceae, Mazaceae, and Linderniaceae. Other genera previously associated with Scrophulariaceae and thought to be links with yet other families include Nelsonia and its relatives (see Acanthaceae) and Paulownia (see Paulowniaceae). On the other hand, Buddleja (et al.) were included in Loganiaceae or placed in their own family, and although Takhtajan (1997) placed it in its own family, which, he thought, was related to Myoporaceae and Scrophulariaceae, they are all together above.
Thanks. To F. Zapata, for useful comments on the family.
Age. This clade was estimated to be (70-)61, 54(-51)Ma by Bell et al. (2010) and (64-)55(-47)Ma by Wikström et al. (2015).
STILBACEAE Kunth, nom. cons. - Back to Lamiales —— Synonymy: Halleriaceae Trinius, Retziaceae Choisy
Ericoid shrubs, ordinary shrubs, or herbs; (iridoids from deoxyloganic acid - C-8 iridoid glucosides), (cornosides +); cork just outside pericycle; vessel elements also with scalariform perforation plates; nodes ?; petiole bundle?; stomata?, cuticle waxes as rods or threads; lamina vernation revolute or not, (margins minutely toothed); inflorescence branches cymose, (plant cauliflorous), (flowers axillary); bracteoles as long as K; flowers often polysymmetric, (4-)5(-7)-merous; K bilobed or not (free), C lobes equal to unequal; A = K, (one fewer; staminode +), anthers connivent in pairs, thecae confluent apically, or with separate parallel slits; oil flowers, ?nectary; ovary (apically) unilocular [1 G infertile, or septum 0], or bilocular, stigma slightly bifid or punctate; ovules 1-2/carpel, ascending and/or descending, apo/epitropous, or many [Nuxia]; micropyle long, integument several [ca 15?] cells across, hypostase +; embryo sac very long; fruit a loculicidal (and septicidal) capsule, (indehiscent), K and C persistent; (seeds with pedestals - Charadrophila); endosperm +, embryo cylindrical [always?]; n = 10, 12, 19, x = 13 (?12), protein bodies in nucleus crystalline [Halleria].
11[list]/39: Nuxia (15). Most South Africa, esp. the Cape Floristic Region, also tropical Africa, Madagascar, the Mascarenes and Arabia. Map: from Leeuwenberg (1975). [Photo - Nuxia Inflorescence, Halleria Flower.]
Age. Tank and Olmstead (2017) suggested an age of (60-)37.1(-13.3) Ma for this clade.
Evolution: Pollination Biology. Oil flowers are quite common in the family, and the oil is produced by multicellular trichomes aggregated on the inside of the corolla tube; Rediviva is recorded as being the pollinator, as is common in African oil flowers (Vogel 1974; Renner & Schaefer 2010 and references; Possobom & Machado 2017a; Tölke et al. 2019 and references). Renner and Schaefer (2010) suggest a stem age for Stilbaceae of about 48 Ma; it would be good to get estimates of the age of the evolution of oil flowers here.
Chemistry, Morphology, etc.. The C-8 iridoid glucosides common in Stilbaceae are extremely uncommon elsewhere (Frederiksen et al. 1999); for unedoside, present in at least some genera of Stilbaceae, see Oxelman et al. (2004a). Indeed, some iridoids in Stilbaceae are like those of Loasaceae and Hydrangeaceae; all three have unedoside (Jensen et al. 1998).
By and large, the gynoecium is reminiscent of that of Scrophulariaceae-Manueleae. Thesmophora appears to have two collateral carpels, each with one descending ovule (Rourke 1993) - perhaps an abaxial carpel divided by a false septum.
For general information, see Dahlgren (in Dahlgren & van Wyk 1988,) Weber (1989: Charadrophila), Fischer (2004b: Bowkerieae) and Linder (2004: narrow circumscription), for anatomy and morphology, see Carlquist (1986) and Dahgren et al. (1979), and for embryology, see Junell (1934).
Phylogeny. Retziaceae and Stilbaceae come out together in rbcL trees (Wagstaff & Olmstead 1997); for another early study, see B. Bremer et al. (1994). Nuxia (ex Loganiaceae) is also placed here in molecular phylogenies (Backlund et al. 2000; Wallick et al. 2002), and this makes phytochemical sense (Frederiksen et al. 1999). The cauliflorous Halleria is also to be included in Stilbaceae (Olmstead et al. 2001). Genera like the gesneriad-like Charadrophila (the common name for this plant is "Cape gloxinia") and Scrophulariaceae-Bowkerieae (Bowkeria, Anastrebe and Ixianthes) are now members of the family. Charadrophila and Halleria may form a clade - but little support yet - that is sister to well-supported clades that includes the rest of the family (Oxelman et al. 2005). Thesmophora has not been included in these studies.
Classification. Rourke (2000) recognised two subfamilies, Retzioideae and Stilboideae, in Retziaceae, while Kornhall (2004) recognized three tribes. However, the circumscription of the family has greatly changed from what it was (see also Tank et al. 2006).
Age. This node is around 48 Ma (Magallón et al. 2015) or ca 69.8 Ma (Fonseca 2021), in both cases note topology.
Evolution: Divergence & Distribution. Sensitive stigmatic lobes occur sporadically in this part of the tree (see also Endress 1994b).
[Byblidaceae + Linderniaceae]: bracteoles 0; x = 8, 9.
Chemistry, Morphology, etc.. Flowers are either axillary or the inflorescence is racemose in this clade - not too much difference between the two!
BYBLIDACEAE Domin, nom. cons. - Back to Lamiales
Rhizomatous and woody, or ephemeral herbs; cork?; young stem with separate bundles; nodes 1:1 or 1:3; stomata paracytic; leaves spiral, lamina linear, abaxially circinate or straight, with long-stalked mucilaginous glands and sessile digestive glands, veins parallel; flowers single, axillary; flowers subpolysymmetric; K connate only basally, C contorted, connate only basally, margins toothed/entire; A ± monosymmetric, stamens 5, shortly epipetalous, anthers connivent in pairs, dehiscing by short slits or pores, epidermal cells ephemeral; nectary 0; stigma punctate to capitate (slightly bilobed); ovules 2-several/carpel, ± apical; exotestal cells tangentially somewhat elongated, anticlinal walls not uniformly thickened, mesotesta sclerenchymatous; endosperm starchy, copious; x = 9, n = 8, 9, protein inclusions in the nucleus?, nuclear genome [1 C] (0.163-)0.804(-3.962) pg / 495-884 Mbp.
1 [list]/8. S.W. and N. Australia, S. New Guinea. Map: from van Steenis (1971) and FloraBase (consulted 2004). Photos: Collection.
Age. The age of crown-group Byblidaceae may be ca 37.2 Ma (Fonseca 2021).
A single seed, now destroyed, from the Middle Eocene of South Australia might have been assignable to this family (Conran & Christophel 2004).
Evolution: Ecology & Physiology. There is some discussion over whether or not carnivory occurs here. There seemed to be no evidence that the plant absorbs nutrients from the insects that often stick to it (Hartmeyer 1997, 1998; Mueller et al. 2001), but Conran and Carolin (2004) noted that mirid bugs (e.g. Setocoris bybliphilus) were associated with the genus, and so there might be a relationship similar to that in Roridula (Ericales) where the mirid eats insects stuck to the plant and the plant absorbs nutrients from the excreta of the bug (see Wheeler & Krimmel 2015 for mirids). Plachno et al. (2025) noted that in Byblis linifolia the sessile glands had two basal cells, an endodermoid cell, and 8 terminal secretory cells that produced digestive enzymes. but only once, while the long-stalked glands had a long, one-celled stalk, and the head was made up of an endodermoid cell connecting 16-32 glandular cells that were permanently mucilaginous. Movement of material in these hairs was via plasomodesmata, symplastic, because of the wall thickenings of the endodermoid cells (Plachno et al. 2025), and these authors noted cuticular holes in the secretory cell walls in the glandular hairs - similar structures are known from some other carnivorous plants - here perhaps involved in the "facilitation of both secretion and absorption of water-soluble substances" (ibid. p. 475).
Allan (2019a) suggested that the hairs of Byblis spp. were sensitive, and could distinguish between styrafoam and paper (no response) and cheese and fish (response); digestive secretions were produced at night, at least in the perennial Byblis gigantea (Allan 2019b). Poppinga et al. (2022) noted the long stalk cell of stalked, "glue"-producing B. gigantea hairs twisting and kinking as water moved from that cell into the basal cells, the prey would be brought closer to the leaf, i.e. to the digestive glands; reaction and movement times depended on the temperature, but were never fast, and even when conditions were favourable reaction times alone were (24-)37.6(-50) minutes. Interestingly, although the stalk cells could rehydrate and regain their initial shape, they did not produce "glue" again (Poppinga et al. 2022). Frenzke et al. (2016a) noted that the sticky substances produced by the stalked glands of R. gorgonias appeared to be pressure sensitive.
Pollination Biology & Seed Dispersal. The flowers are basically polysymmetric, but the stamens are held to one side of the flower, the style to the other; buzz pollination is likely.
Chemistry, Morphology, etc.. Byblis linifolia has leaves that are sometimes abaxially curled in bud and so are like those of Drosophyllum (Drosophyllaceae, Caryophyllales). However, the glandular hairs of Byblidaceae have the typical structure of those of core Lamiales and look like little parasols; those of Drosophyllum are vascularized and have irregularly arranged cells in the head.
Diels (1930b) drew the flower of Byblis with the odd sepal abaxial. Byblidaceae are often described as being bitegmic, but c.f. Diels (1930b) and Vani-Hardev (1972).
See Lloyd (1942), Juniper et al. (1989), Conran and Carolin (2004), McPherson (2008, 2010), Lowrie (2013: vol. 1), papers in Ellison and Adamec (2018), and the Carnivorous Plants Database for general information, also Wilkinson (1998: anatomy), Takahashi and Sohma (1981: pollen), Conran (1996: embryology), and Conran et al. (2002: chromosome numbers).
Previous Relationships. Roridula (see Roridulaceae - Ericales) has hitherto often been placed in the same family as Byblis (= Byblidaceae s.l.) and then included in Rosales, as by Cronquist (1981), or the two kept separate, but both placed in Byblidales (in Aralianae), as by Takhtajan (1997).
LINDERNIACEAE Borsch, K. Müller, & Eb. Fischer - Back to Lamiales
Ephemerals to suffruticose perennials; iridoids 0; cork?; nodes 1:3; stems angled; leaves (basally connate), lamina venation also palmate, margins entire or serrate, (glands in mesophyll); (flowers single, axillary, bracteoles +/0); K (± free); C with glandular hairs on the inside; A curved, 4 or 2 [the adaxial pair], anthers connivent, thecae parallel to ± head to head, staminodes +/0 [A 4], if A 2, 2 large abaxial ±Z-shaped staminodes with an appendage/with yellowish granular surface; pollen (blue), 3(-5)-colpate; stigma bilamellate/spatulate, lobes sensitive; embryo sac spathulate, protruding through the micropyle, integument 3-4 cells across; capsule septicidal or -fragal; exotestal cells in longitudinal files [?all], seeds with ruminate endosperm [surface alveolate - bothrospermous - or furrowed - aulacospermous] (smooth); n 7-9, 12-14, etc., x = 9, nuclear genome [1 C] (0.037))0.495(-6.583) pg.
18 [list]/270 (220): Vandella (55), Torenia (51), Crepidorhopalon (35), Lindernia (30). Pantropical to warm temperate. Map: based on Fischer (1992) and Lewis (2000).
Age. Linderniaceae are estimated to be ca 62.4 Ma (Fonseca 2021) and the clade [Torenia + Craterostigma] was dated to (45.6-)27.4(-10.2) Ma (Tank & Olmstead pers. comm.).
Evolution: Divergence & Distribution. Santos et al. (2023) suggest that the ancestor of Ameroglossum moved from the Old to the New World via long distance dispersal ca 15 Ma.
Ecology & Physiology. Although many Linderniaceae seem to be rather delicate little herbs, a number of taxa are in fact dessication tolerant (poikilohydric). These include the remarkable Chamaegigas intrepidus, an aquatic resurrection plant - close to an oxymoron - growing in transient pools on inselbergs, which probably uses glycine and serine as nitrogen sources (Heilmeier & Hartung 2011). Fresh leaves of Craterostigma plantagineum store large amounts of the unusual sugar, 2-octulose, which is converted into sucrose as the leaf dries (Bianchi et al. 1993; Farrant 2000). More shrubby Linderniaceae also grow in dry rocky areas of these inselbergs (Almeida et al. 2019). VanBuren et al. (2018b) found that dessication tolerance in Lindernia brevidens, quite close to Crat. plantagineum but growing in drought-free montane rainforest, has been achieved in part through rewiring parts of the network of genes involved in seed development - dessication tolerance of course occurs there, too - and coöpting some of the genes, and the two species of Lindernia that they studied share an old genome duplication (L. subracemosa, which they also studied, is drought sensitive). The taxa just mentioned are all from the Old World; for more on dessication tolerance in the family, see Dinakar and Bartels (2012 and references) and in angiosperms in general, see Artur et al. (2018).
The vegetative parts and calyx of the recently-described Crepidorhopalon droseroides, a tiny annual ca 1.5 cm tall from Mozambique, are densely covered by long-stalked glandular hairs to which dead insects are attached. Carnivory is suspected, as in similar plants elsewhere (Fischer et al. 2023).
Pollination Biology & Seed Dispersal. In some species the anthers of the abaxial stamens are yellow and lie against the abaxial lip; they appear to contribute to the attractive aspect of the lip. In other species the long, curved abaxial filaments, joined by the connate anthers, form a sort of balustrade across the mouth of the corolla. Various hairs develop on the knees of the abaxial anthers and inside the corolla, and the corolla may have projections, flanges, etc.; all in all, a complex little flower (see e.g. Magin et al. 1989; Rahmanzadeh et al. 2004 for photographs). It would be interesting to know details of pollination mechanisms for such flowers; small bees have been recorded as visitors (Magin et al. 1989). In Torenia fournieri, which has a less obviously distinctive floral morphology, the adaxial stamen pair elongate quickly and then more or less protrude from the mouth of the corolla, while the abaxial pair has anthers which, when touched on lever-like lateral flanges, forcibly extrude their pollen (Armstrong 1992). Tnere is hummingbird pollination in the N.E. Brazilian Ameroglossum (Santos et al. 2023).
Chemistry, Morphology, etc.. The nodes appear to be 1:3, rather than 3:3 as I originally thought. Small strands of lignified tissue are associated with the sharp ridges of the stems in the couple of species that I have seen. The glandular heads of the hairs on the corolla and the vegetative plant have vertical partitions, as is common in Lamiales.
For the floral development of Torenia, see Armstrong (1988). Lewis (2002) suggests that the anthers are extrorse and the ovules are straight; Fischer (1992), however, gives a floral diagram showing introrse anthers and describes the ovules as being anatropous to hemitropous. The embryo sac protrudes beyond the micropyle in some species of both Torenia and Lindernia, at least (Wardlaw 1955; Yamazaki 1955); the synergids can then be ablated easily in studies of fertilization (Higashiyama et al. 2006). The rumination of the endosperm is caused by inpushings of endothelial cells (alveoli); these can become confluent and the seeds then have longitudinal ridges.
For more information, see Fischer (1989, 1992, 2004b - the latter Scrophulariaceae pro parte) and Barker (2018), general, and Takhtajan (2013: ovule and seed).
Phylogeny. Rahmanzadeh et al. (2004) recovered this clade with 100% bootstrap support. Albach et al. (2005a) analysed four genes, separate analyses of three of which and the joint analysis suggested that Linderniaceae were distinct from Plantaginaceae. Micranthemum, with only two stamens, was sister to Lindernieae, whose members made up the rest of this clade (Albach et al. 2005a). Oxelman et al. (2005) found that Micranthemum was sister to Torenia, the two in turn were sister to Stemodiopsis, the only three Linderniaceae they examined. In a more extensive analysis the relationships [Stemodiopsis [Lindernia, etc.] [Torenia, Craterostigma, Vandella, etc.], all with strong support, were obtained (Fischer et al. 2013; Biffin et al. 2018: spine with quite good support). See also Tank et al. (2006) for a summary of our ideas of relationships within this clade, and for the inclusion of the Brazilian Cubitanthus, ex-Gesneriaceae, see Perret et al. (2012; not in the study of Fischer et al. 2013) - it was placed as sister to the African Stemodiopsis (see also Biffin et al. 2018). Ameroglossum has recently been moved from Scrophulariaceae to Linderniaceae (Santos et al. 2023).
Classification. See Rahmanzadeh et al. (2004), Tank et al. (2006) and especially Fischer et al. (2013) and Biffin et al. (2018) for the composition of Linderniaceae; the generic list here is rather notional, and there have been repeated movements of genera in and out of Lindernia, for example (Barker 2018).
Previous Relationships. Linderniaceae include taxa that used to be placed in Scrophulariaceae and also a few that were in Gesneriaceae (Cubitanthus).
Age. This age of this clade was estimated to be (60-)51(-44)Ma by Wikström et al. (2015: note topology) and (64.6-)57.1, 52.1(-48.9) Ma by Cusimano and Wicke (2016: Sesamum).
Age. The age of this clade [SesBigAcan] is (55.6-)52.0(-49.1) Ma (Zhigila & Musaya 2023) or 67.7 Ma (Fonseca 2021).
Phylogeny. Relationships within this clade are unclear; for further discussion, see above.
[Pedaliaceae [Martyniaceae + Acanthaceae]]: ?
Previous Relationships. Martyniaceae and Pedaliaceae have often been combined (as Pedaliaceae, e.g. Cronquist 1981), but there is no current evidence that they form a monophyletic group. Differences in pollen (inaperturate and with platelets vs several colpi) and placentation (parietal vs axile) clearly separate the two morphologically. In addition, the remarkable branched spines, etc., on the fruits of Pedaliaceae develop as such while those of Martyniaceae become evident only as the outer layers of the fruit rot away.
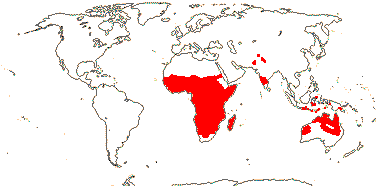
PEDALIACEAE R. Brown, nom. cons. - Back to Lamiales
10-hydroxylated carboxylic iridoids [harpagoside], orobanchin, amyloid +; (cambium storied); pericycle also with sclereids (fibers few); petiole bundle interrupted-annular; lamina bifacial, amphistomatic; hairs broadly capitate-stellate, heads 4-celled, outer walls very thick, mucilaginous; lamina margins toothed, lobed or entire, (venation palmate); base of pedicel with paired nectaries [modified flowers]; C ± spurred; A (5), staminode + (0), usu. dark-coloured gland at apex of connective (0); pollen 5-13 stephanocolpate; G [2(-4)], with false septa, stigma lobes broad, often sensitive, wet; ovules 2-many/carpel, integument 7-20 cells across, hypostase +; fruit with hooks or glochidiate spines, etc., (heterocarpic), (schizocarp; nut; variously winged); seeds winged or not, surface often sculpted, testa multiplicative, crystalliferous, exotestal cells (tanniniferous), (palisade or otherwise thickened); endosperm slight, cotyledons with fat and cell walls with xyloglucans [thick, pitted - amyloid]; x = 9, protein bodies in nucleus?, nuclear genome [1 C] (0.282-)0,878(-2.736) pg/(337-)1145(-1648) Mb.
15 [list, to tribes]/70: Sesamum (19), Pterodiscus (13). Mostly Old World tropical, in coastal or arid habitats. Map: from Ihlenfeldt and Grabow-Seidensticker (1979), FloraBase (2005), Australia's Virtual Herbarium (consulted xii.2012) and Ihlenfeldt (1994b, 2010).
Age. The age of Pedaliaceae is estimated to be (39-)20.1(-6) Ma (Tank & Olmstead pers. comm.), (51.3-)48.2(-43.2) Ma (Zhigila & Musaya 2023) or ca 24.8 Ma (Fonseca et al. 2021).
1. Pedalieae Dumortier
Herbs, annual or perennial, to shrubs or smallish trees, deciduous (base of stem swollen/root tubers); (leaves spiral), lamina subsucculent (not - Uncarinia); (flowers axillary); (pedicellar nectaries 0 - Uncarinia); anther thecae ovate, ± confluent, at right angles to filaments; tapetal nuclei polyploid; fruits with spines and/or wings; n = 8.
Ca 7/35: Pterodiscus (13), Rogeria (13). Africa south of the Sahara, also N.E. Africa; although Rogeria has been reported from Brazil, this seems to be a mistake (Volker Bittrich, pers. comm.).
[Sesamothamneae + Sesameae]: leaf margins entire; anthers basifixed, thecae oblong, not divergent; seeds winged.
Age. This clade is estimated to be (40.4-)38.4(-35.8) Ma (Zhigila & Musaya 2023).
2. Sesamothamneae Ihlenfeldt - Sesamothamnus Welwitsch
Shrubs to small trees, deciduous; short shoots +; leaves spiral, petiolar spines; C rotate, contorted, (lobes fimbriate), spur long; pollen in tetrads; fruit indehiscent, laterally compressed, emergences 0; n = ?
1/6. N.E. and southern Africa.
3. Sesameae Dumortier - Sesamum L. —— Synonymy: Sesamaceae Berchtold & J. Presl
Herb, annual to perennial, ± shrubby; Leaves (compound), (lobed), (margins dentate); flowers axillary; tapetal cells binucleate; (ovary 8-locular, 1 ovule/loculus - sect. Josephinia), (with false septa); fruit (indehiscent), (lacking spines); (seeds wingless); n = 13, 16.
1 (4)/31. Africa south of the Sahara, also N.E. Africa, Madagascar, India, Sri Lanka, ?Pakistan, eastern Malesia inc. Java and Australia (not the South).
Age. The age of crown-group Seameae is some (40.6-)35.7(-29.6) Ma (Zhigila & Musaya 2023).
Evolution: Pollination Biology & Seed Dispersal. The diversity of fruit morphology and dispersal "strategies" in this small family is remarkable, as is their variation in growth form (Ihlenfeldt 2010). At least Sesamum and Dewinteria are myxospermous (Grubert 1974).
Genes & Genomes. For genome size, see Lyu et al. (2017: c.f. Tables S3 and S5).
Chemistry, Morphology, etc.. The iridoid glycoside harpagoside, known from Harpagophytum and Rogeria, is scattered in other Lamiales such as Lamiaceae, Plantaginaceae, and quite commonly in Scrophulariaceae s. str. (Georgiev et al. 2013). The mucilage glands that are often so conspicuous here normally have four apical cells.
The apparently single axillary flowers of some taxa appear to be reduced cymes, the paired nectaries at the base of the pedicel representing modified flowers (Manning 1991). Josephinia s. str. may have four carpels, each loculus being divided - an unusual combination for a gentianid.
Some information is taken from Stapf (1895), Carlquist (1987b: wood anatomy), S. D. Manning (1991: U.S.A., general), and Ihlenfeldt (1967, 2004, 2010: general), also Jordaan (2011: seed coat of Harpagophytum - complex).
Phylogeny. Gormley et al. (2015) found good support for the relationships [Pedalieae [Sesamothamneae (monotypic) + Sesameae]] in a chloroplast analysis. However, the first tribe was paraphyletic in an analysis using the external transcribed spacer, with Rogeria sister to the rest of the family, albeit with little support; in Sesameae, Sesamum was paraphyletic in both analyses, although more so in the first. The tree in Zhigila and Muasya (2023: ITS plus 3 plastid markers), focused on Sesamum s.l., is largely compatible with those found earlier.
Classification. I have tentatively adopted the tribes recognized in the chloroplast analysis of Gormley et al. (2015); see also Ihlenfeldt (2004). For the expansion of the limits of Sesamum, also its included sections, see Gormley et al. (2015), Manning and Magee (2018) and Zhigila and Muasya (2023).
[Martyniaceae + Acanthaceae]: ?
MARTYNIACEAE Horaninow, nom. cons. - Back to Lamiales
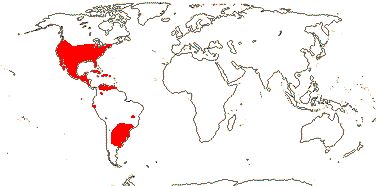
Annual herbs, roots often tuberous (perennials; woody); petiole bundle deeply arcuate, also adaxial cortical and medullary bundles; plant long sticky-hairy; leaves also spiral, lamina margins toothed; (K free); A (2 + 2 staminodes - Martynia), anther thecae in line [attached apically], staminode(s) +; pollen grain tricellular, inaperturate, exine made up of 20-40 platelets, adjacent or somewhat separate, with reticulate sculpture (smooth raised rings, surface inside smooth - Craniolaria); G paracarpous [attached by margins only], placentation parietal, placentae T-shaped, ovules at the end of the cross bar, stigma lobes sensitive; 2-many ovules/carpel; capsule with paired apical spurs or hooks [developing from sterile upper part of ovary], (± smooth), outer mesocarp ± fleshy, caducous, inner mesocarp woody, with crests and spines; seeds large [>10 mm long], (ca 3 mm long); testa ca 5 layers thick, exotesta subgelatinous, or inner and radial walls with cellulosic bands, inner layers lignified [Proboscidea], or exotesta only persistent, ?bands of thickening below [Martynia, no other cellular details], or lignified exotesta; endosperm at most thin; n = 15 (16, 18), x = 15/16, nuclear genome [1 C] (0.108-)0.677(-4.251) pg/ca 0.49 pg.
5 [list]/16: Proboscidea (10). Tropical and subtropical America, rather scattered. For map, see Gutierrez (2011).
Age. The age of crown-group Martyniaceae is estimated to be (54.3-)51.5(-49.3) Ma (Tank & Olmstead pers. comm.) or ca 16.2 Ma (Fonseca 2021).
Evolution: Divergence & Distribution. The primary division in the family is between North American and South American taxa (Gutierrez 2011; Gormley et al. 2015).
Ecology & Physiology. Insects may stick to the very viscid indumentum of Martyniaceae, although there is no evidence that the plants are carnivorous - certainly there is no evidence of the production of digestive enzymes (see Rice 2008; Plachno et al. 2009; Hartmeyer & Hartmeyer 2022). Stylidiaceae (Asterales) also have sticky hairs that trap insects, and they may indeed be carnivorous (Darnowski et al. 2006).
Seed Dispersal. The outer part of the pericarp rots away to expose the distinctive spiny/thorny fruits that are quintessential trample burrs; the anatomy of the fruit wall is complex (Horbens et al. 2014). The seeds of several Martyniaceae are very large compared with those of other core Lamiales.
Chemistry, Morphology, etc.. Prieu et al. (2017) record Craniolaria and Martynia as having pantoporate pollen, while Passarelli and González (2020) describe the pollen tubes as coming out between the platelets/insulae.
General information is taken from Stapf (1895), Ihlenfeldt (2004) and McPherson (2010, vol. 2, esp. photographs) and especially Gutierrez (2011); see also Carlquist (1987b: wood anatomy), Bretting and Nilsson (1988), pollen morphology, and S. P. Singh (1970: embryology, etc.). For the seed coat, see Ricketson & Schmidt 4981 (Proboscidea areania), Gentry & Zardini 48864 (Martynia annua).
Phylogeny. For relationships within Martyniaceae, see Gutierrez (2008, esp. 2011); the northern taxa Proboscidea and Martynia form a clade sister to southern taxa in the family (see also Gormley et al. 2015: support moderate for monophyly of the southern taxa).
ACANTHACEAE Jussieu, nom. cons. - Back to Lamiales
Quaternary methylammonium compounds, amyloid +; (cork cambium deep seated); stomata diacytic; nodes swollen; lamina margins entire to toothed; (inflorescence branches cymose), bracts large, conspicuous; K free or connate, often sharply pointed, (C lobes narrow); A usu. 4, staminode +/0; G synacidiate + symplicate, lacking septal bundles; ovule with "thin" integument [?]; embryo sac long, curved, (apex of 4-nucleate sac growing out of the micropyle and eventually into the placenta); (zygote pushed back into the ovule by a long suspensor); capsule dehiscence explosive, walls cartilaginous, K persistent; testa with hygroscopic trichomes; endosperm development highly asymmetric, the two haustoria lying close to each other, second division of the endosperm transverse, primary endosperm 3-celled, linear, embryo often ± curved; x = 9, nuclear genome [1 C] (0.103-)0.667(-4.325) pg.
191 [list, to tribes]/4,605 - four subfamilies and ten tribes below. Mostly tropical.
Age. Crown-group Acanthaceae may be slightly over 90 Ma (Tripp et al. 2013b), (92.3-)81.9(-71.7) Ma (Tripp & McDade 2014b), around 57 Ma (Tripp & McDade 2014a), (57.3-)49.3(-41.1) Ma (Tank & Olmstead pers. comm.) or ca 64.5 Ma (Fonseca 2021).
1. Nelsonioideae Pfeiffer —— Synonymy: Nelsoniaceae Sreemadhavan
Herbs; gland-headed hairs with 2-celled heads (0); (leaves spiral); inflorescence terminal/axillary, bracts spiral, (bracteoles 0 - Nelsonia); C (weakly monosymmetric), descending cochleate aestivation [adaxial lobes of C outside others]; A (2), anthers variable (e.g. thecae ± separate); pollen (colpate); ovary (with parietal placentation - Elytraria); stigma lobes broad (unequal/1), large, sensitive [Elytraria]; ovules (4-)many/carpel, campylotropous, endothelium +; antipodal cells persistent; funicular obturator +; seeds 6-68, ruminate, testa ± disorganised (± visible0, (hygroscopic trichomes 0 - Elytraria, Anisosepalaum); chalazal endosperm haustorium degenerates early, endosperm +, oily; n = 9.
6/175: Staurogyne (145). Tropical (warm temperate).
Age. Crown-group Nelsonioideae are estimated to be (81.5-)67.7(-53.8) Ma (Tripp & McDade 2014b).
[Acanthoideae [Thunbergioideae + Avicennioideae]]: (wood rayless); (inverted vascular bundles in the pith); acicular fibres +; pollen usu. other than 3-colpate or -colporate; ovules 2/carpel; endothelium 0, funicular obturator 0; endosperm 0 (+), (amyloid [xyloglucans] in cotyledons +).
Age. Estimates of the age for this node are (50-)41, 38(-29) Ma (Bell et al. 2010), (38-)35, 27(-24) Ma (Wikström et al. 2001), (42-)32(-17) Ma (Wikström et al. 2015), ca 54 Ma (K. Bremer et al. 2004a) and (80.7-)70.9(-61.4) Ma (Tripp & McDade 2014b).
2. Acanthoideae Eaton
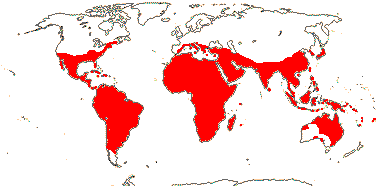
Herbs (to shrubs); (benzoxazinones +); petiole bundles arcuate, arranged in a circle, (annular); (leaf margins spiny); C often with abaxial lobe outside others in bud [= ascending cochleate aestivation], (slit-monosymmetric - rare); anthers sagittate, or thecae displaced and not opposite, (one theca ± reduced); stigma dry, usu. bifid; ?funicular obturator; capsules obovoid, explosive; seeds flattened, usu. 4, (hairy), borne on hook-like hardened funicles [= jaculators, retinacula]; exotesta palisade, (hypodermal cells thickened); both chalazal and micropylar haustoria +; cytologically very variable, x = ?7; nuclear genome [1 C] ca (416-)1462(-2841) Mb.
217/4,733 - seven tribes below. World-wide, most species Neotropical. Map: from Brummitt (2007). [Photo - Habit, Flower.]
Age. The age of crown-group Acanthoideae was estimated to be (102-)79(-65) Ma (Tripp et al. 2013b) or (80.1-)71.1(-61.9) Ma (Tripp & McDade 2014b).
2A. Acantheae Dumortier [parent of ×Physacantheae]
(C4 photosynthesis + - Blepharis); bracts with extra-floral nectaries - Aphelandra); A 4, anthers monothecate; pollen 3-colpate, colpus often narrow (broad, endexine with extexine granules - Acanthopsis); testa with hygroscopic trichomes +/0.
21/550: Aphelandra (170), Blepharis (130). Pantropical.
Age. Crown-group Acantheae are 54-24 Ma (Tripp et al. 2013b).
2B. ×Physacantheae Tripp & I. Darbyshire [= Acantheae x Ruellieae] - × Physacanthus Bentham
Herb; cystoliths 0"; lamina often variegated; K inflated; C left-contorted*, twisted in bud; A 4, monothecal", 1 staminode; pollen tricolporate* [with compound apertures], also tripseudocolpate*; stigma clavate, 2-lobed; seeds (4-)6-9; testa papillate. [" - characters of Acantheae; * - characters of Ruellieae.]
1/3. Tropical Africa.
[[Ruellieae + Justicieae] [BAWN clade]]: cystoliths +; nodes swollen; pollen (col)porate, hideously variable.
[Ruellieae + Justicieae]: (leaf/stem endodermis +); (anther endothecium 0); pollen with pseudocolpi.
2C. Ruellieae Dumortier [parent of ×Physacantheae]
(Trees); (flowers resupinate); C left-contorted; A (2 + 2 staminodes), anthers (with appendages), filament curtain + [= C tube compartmentalized by ridges where filaments are adnate to C]/0; pollen 3-polyporate/2-3-(col)porate (to multi-pseudocolpate), (pores with adjacent areas of raised reticulum [= sexine lips]), surface very coarsely reticulate [= Wabenpollen/honeycomb pollen] (less coarsely so), (spiny); stigma lobes filiform, adaxial shorter than abaxial lobe or 0; ovules 2 (>3)/carpel; integument massive [?>10 cell layers across]; testa with hygroscopic trichomes with annular thickenings, (on seed margin only/0), walls with spiral hickenings [?level], 2 [?mesotestal] layers sclerified [Dipteracanthus]; n = 6-68 [≡ variation in whole family], 15-17 common, x = 8?
37/1,200: Ruellia (375), Strobilanthes (350), Hygrophila (100), Dyschoriste (80), Asystasia (70), Hemigraphis (60), Sanchezia (60), Dyschoriste (60). Largely tropical, most genera Old World, esp. Africa.
Age. The age of crown-group Ruellieae is 55-31 Ma (Tripp et al. 2013b).
2D. Justicieae Dumortier —— Synonymy: Justiciaceae Rafinesque
Pyrroloquinazoline alkaloids +; stem with endodermis [Justicia]; (flowers resupinate, C twisted - Diclipterinae); C ascending cochleate, with parallel ridges on upper lip [rugula] holding style/0; A 2, thecae displaced, not opposite, connective expanded, (with appendages, etc.), (A 4 (2 monothecate)/A 2 + 2 staminodes - Pseuderanthemum lineage); pollen 3-colpate-hexapseudocolpate (biporate, tetrapseudocolpate, colpi equatorially fused in pairs), (interaperturate girdles of tectate exine [= Gürtelpollen, Isoglossiinae]); (filiform stigma lobes); 2-4 seeds/fruit; embryo cell walls with xyloglucans [thick, pitted - amyloid]; n = (7, 9-13)14(15-18, 20 ...40); 3 indels in trnl-trnK region.
Ca 96/2,473: Justicia (983), Dicliptera (220), Hypoestes (130-150), Pseuderanthemum (100), Isoglossa (81), Rungia (80), Stenostephanus (80), Anisostachya (60-100), Asystasia (64), Monechma (43). Tropical to warm temperate, but neither Mediterranean climates nor high latitudes.
Age. The age of crown-group Justicieae is ca 35.5 Ma (McDade et al. 2020).
[Neuracantheae [Whitfieldieae [Barlerieae + Andrographideae]]] / BAWN clade: testal hygroscopic trichomes + (0).
2E. Neuracantheae Reveal - Neuracanthus Nees
Flowers ± polysymmetric; K connate, 3 + 2, C ?induplicate, 2 adaxial connate; A 4, 2 abaxial monothecous; pollen tricolporate, intercolpal regions psilate/foveolate; capsule not stipitate; seeds 2, 4; n = 20.
1/30. Africa, Madagascar, Arabia to Vietnam.
[Whitfieldieae [Barlerieae + Andrographideae]]: ?
2F. Whitfieldieae Reveal
Inflorescence (raceme/spike); bracteoles conspicuous, ± enclosing K; C left-contorted; A 4 (2 + 2 staminodes); pollen bipororate, lenticular, granular around apertures, (pantoporate, triporate), interapertural regions smooth to faintly scabrate [c.f. Gürtelpollen]; stigma 2-lobed capitate; seeds with interrupted ridges forming concentric rings; n = ca 21.
8/33: Whitfieldia (13). Africa, Madagascar.[Barlerieae + Andrographideae]: ?
Age. The age of this clade is 44-30 Ma (Tripp & McDade 2014).
2G. Barlerieae Nees
(To small trees); (included or intraxylary phloem + - Barleria, Lepidogathis); double cystoliths common; (axillary spines/thorns +); K (2 abaxial ± completely connate), C 2 + 3/4 + 1/± polysymmetric, quincuncial, abaxial C internal; A = 2 + 0, 2, 3 staminodes/4 (2 postichous/abaxial monothecous) + 0/4 + 1 staminode; (endothecium 0); pollen 3-colporate, 3-, 6- porate, exine reticulate, with granules/not / gemmate; G symplicate, septum develops late, stigma with filiform lobes or capitate (1 lobe, linear to flattened); ovule integument 10-15 cells across, obturator +; capsule not stipitate; seeds 2, 4(+), disciform/not; n = 8-12, >15.
13?/520 (3 subtribes): Barleria (285 + 40-50 undescribed), Lepidagathis (117 - to be divided), Petalidium (40). Pantropical.
2H. Andrographideae Endlicher
C ascending cochleate; pollen colporate (pororate), surface smooth to hairy-echinate, aperture margins thickened or not, variously ornamented, often with comical spines, (whole aperture ornamented) [= Daubenpollen], microperforate [≡ to inner layer of bireticulate grain]; ovules 4+/carpel; seeds 6-20/fruit; testa (brown, surface cerebellar/deeply pitted); endosperm ruminate [?all]; n = 21, 25.
4/122: Gymnostachyum (50), Phlogacanthus (42), Andrographis (26). Sri Lanka and India to southern China and western Malesia.
[Thunbergioideae + Avicennioideae]: C left-contorted; filament bases thickened; ovules 2/carpel, collateral, apotropous, apex of nucellus exposed, surrounded by short integumentary rim, embryo sac ± on surface of nucellus; cotyledons folded.
Age. The age of this node was estimated to be ca 86 Ma (Tripp et al. 2013b) or (80.7-)70.9(-61.4) Ma (Tripp & McDade 2014b).
3. Thunbergioideae T. Anderson
Twining vines (erect); (iridoids from deoxyloganic acid - C-8 iridoid glucosides, unedoside); (intraxylary phloem/bicollateral vascular bundles +); petiole bundles arcuate or annular with wing bundles; lamina vernation strongly curved; inflorescence with axillary flowers, or fasciculate, 2 or more flowers in the median plane of the leaf/inflorescence bract, adaxial flowers opening first; bracts 0, bracteoles very large, connate or not; K a rim, (with up to 16 linear lobes), C (not contorted); (staminode +), anthers with lignified unicellular hairs (multicellular awns), sagittate, (thecae slightly displaced), dehiscing by (elongated) pores (slits), connective elongated; endothecium 0;stigma wet, small and sub-bilobed to trumpet-shaped and with broad and often unequal papillate lobes; capsule also septifragal; chalazal endosperm haustorium 0, secondary haustoria develop.
5/260. Tropical America, Africa and Madagascar, fewer in South East Asia—Malesia. Photo: Flowers.
Age. Crown-group Thunbergioideae are estimated to be (59.5-)47.2(-34.5) Ma (Tripp & McDade 2014b).
3A. Thunbergieae Dumortier —— Synonymy: Meyeniaceae Sreemadhavan, Thunbergiaceae Lilja
Wood rays 0; release of pollen content surrounded by intine; pollen 7-9-lobate and -colpate [Meyenia] or spiraperturate; capsule ovoid, prominently beaked; seeds 2, 4, with C18 petroselinic acid [CH3-[CH2]10-CH=CH-[CH2]4-COOH, i.e. cis-6-octadecanoic acid], embryo cell walls with xyloglucans [thick, pitted, amyloid]; n = 9, 28.
3/160: Thunbergia (150), Pseudocalyx (6). Palaeotropics and subtropics. [Photo - Flowers.]
3B. Mendoncieae Meisner —— Synonymy: Mendonciaceae Bremekamp
Adaxial carpel aborting; fruit a drupe; seeds 1, 2; cotyledons twice folded; n = 19.
2/100: Mendoncia (90). Africa, Madagascar/Mayotte, the Neotropics. Photo: Flowers.
>4. Avicennioideae Miers - Avicennia L. —— Synonymy: Avicenniaceae Miquel, nom. cons.
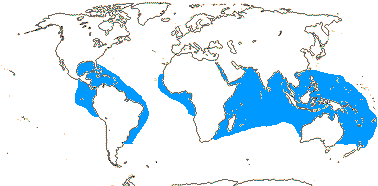
Trees, pneumatophores, (stilt roots) +; betaines +, plant tanniniferous; root cortical phi [φ] cell wall thickenings radially oriented; wood with successive cambia, phloem islands occurring in bands of conjunctive tissue, vessels in radial multiples; nodes 3:3; petiole bundle annular; sclereids +; twigs articulated at nodes; buds naked, lamina thick, with salt glands on both sides, abaxially dense club-shaped hairs, colleters +; inflorescence in dense thyrsoid spicate units[!]; flowers (polysymmetric), 4(-6)-merous; K ± free, quincuncial, C 4, nectar glands on inside of tube; stamens = and alternating with C; pollen 3-colporate; G (with false septa), loculi apically confluent, stigma with 2 blunt lobes; placentation becoming free central, ovules apical, 4, ± straight; fruit a sort of achene, K persistent, green; seeds large; chalazal endosperm haustorium degenerates early, micropylar haustoria aggressive; embryo chlorophyllous, cotyledons induplicate-reduplicate; n = 18, 32; nuclear genome [1C] ca 509 Mb; seeds ± viviparous [embryo breaking the seed coat before the seed falls from the tree].
1/8 (species limits need attention). Mangroves throughout the tropics, but also warm temperate. Map: from Moldenke (1960) and Tomlinson (1986). [Photo - Flower.]
Age. Ricklefs et al. (2006) dated ?crown-group Avicennia to ca 42 My; (39.3-)38.7(-38.4) Ma is the estimate in Tripp and McDade (2014b).
Avicennia is very common both as leaves (but no salt glands were seen) and wood in Late Middle Eocene deposits ca 39 Ma on the Pacific side of Peru (Woodcock et al. 2017) and it is also known from the early Eocene in Siberia (Suan et al. 2017).
Evolution: Divergence & Distribution. For a careful discussion of dating in the family, and also dates for nodes other than those given above, see Tripp and McDade (2014b). Depending on the calibration, dates varied by a factor of about two; the dates here are those preferred by Tripp and McDade (2014b: several fossil calibrations, none far from the in-group, no secondary calibrations, etc.). Tripp and McDade (2014b) validated the identity of a surprisingly large number of fossils that had been attributed to the family. Although the family is quite old (see estimates above, ca 90-47 My) much speciation is quite recent, thus the 350+ species of Ruellia seem to have diversified within the last 3.5 Ma or so (Tripp & Darbyshire 2017).
There are more species of Acanthoideae in the New World, more genera in the Old World, but that is probably an artefact of taxonomists' minds (Tripp et al. 2013a) - and of course genera don't mean very much. However, in six clade pairs listed by Tripp and Tsai (2017) the overall disparity New World:Old World in terms of species numbers was 1,340:389. Nearly all intercontinental (11/13) movements seem to have been from the Old to the New World; they are prominent in Acanthoideae - probably long distance dispersal - and have occurred within the last 20 Ma or so (Tripp & McDade 2014b; see also Kiel et al. 2017). For the biogeography and ecology of the Justicieae-Tetramerium group, also with an Old World origin, especially the many species adapted to drier conditions, see Daniel (2008) and Côtes et al. (2015); McDade et al. (2020) emphasize the importance of long distance dispersal in shaping the biogeography of Justicieae as a whole.
Physacanthus is apparently the product of an ancient hybridization event between Acantheae and Ruellieae and has characters of both; for example, it lacks cystoliths, as do the former, but it has colporate pollen, as do the latter (Tripp et al. 2011, esp. 2013b). There may have been back-crossing to Ruellieae, and, remarkably, Physacanthus has remained heteroplasmic, perhaps for some 65 Ma. The plants may be variegated, maybe because of incompatibilities developing between organelles from plants with different genomes (Tripp et al. 2013b).
Tripp et al. (2013c) noted that diversification in two New World bird-pollinated groups, the justicioids and Ruellia, was after diversification of their pollinating birds, suggesting that diffuse co-evolution was unlikely. In a study with a focus on Ruellia, of which 100-130 species may be bird pollinated (46/146 species were examined), it was noted that hummingbirds diversified considerably in the mid to late Miocene, but diversification of Ruellia began only (13.5-)9.0(-8.3) Ma, i.e. most diversification there is decidedly younger (Tripp & McDade 2014a; Tripp & Tsai 2017). Speciation rates in bird-pollinated clades was higher than in clades pollinated by other animals, moths, bees and bats, although pollinator reversals in the former were also more frequent (Tripp & Tsai 2017; see also Fleming & Kress 2013 for a summary). Z. Chen et al. (2020) looked at the spectral properties of red flowers in the family. Some red-flowered species in the Old World in particular were pollinated by bees and had a high secondary reflectance peak, while they found that in bird-pollinated red flowered species in the New World there were notably lower secondary reflectance peaks - such flowers were less apparent to the bees. Daniel et al. (2008, see also McDade et al. 2018) suggested that hummingbird pollination has evolved some eight times in the Tetramerium (Justicieae) area alone, while for hummingbird pollination in Aphelandra, see McDade (1992). Kiel et al. (2023) found that corolla morphology of some New World species of Justiciawas associated with pollinator type and also stamen morphology - flowers pollinated by hummingbirds (and perhaps butterflies) had anthers with parallel thecae, while the anthers of those pollinated by bees and flies had more or less asymmetrical, divergent thecae.
The seeds of Avicennia are readily dispersed by sea water, floating even when rotten (Mori et al. 2015), and A. germinans has moved across the Atlantic (?W → E). However, it does not disperse as readily as, say, Rhizophora, and thus its populations, e.g. on the Arabian Peninsula, tend to show more differentiation (Nettel & Dodd 2007; Nettel et al. (2008); Maguire et al. 2009; Lo et al. 2014; Mori et al. 2015; van der Stocken et al. (2018).
Borg et al. (2006) discuss the biogeography of Thunbergioideae and the evolution of some characters there, while Borg and Schönenberger (2011) mention possible floral/developmental apomorphies of Thunbergioideae and Avicennioideae. At least some of the features characterizing Nelsonioideae mentioned by Scotland and Vollesen (2000) - no retinacula or cystoliths, descending cochleate aestivation (i.e. the adaxial petals overlapping the abaxial petals in bud) - are likely to be plesiomorphies (see Eichler 1875; c.f. McDade et al. 2012), as is their sometimes rather undistinguished tricolpate or tricolporate pollen. Comito et al. (2025: Figs 6-9) thought about the variation in a number of floral characters in Barlerieae in the context of the phylogeny of the tribe that they had produced.
Ecology & Physiology. Many of the distinctive morphological features of Avicennia, a mangrove tree, are common in other plants in the mangrove habitat (for which, see Rhizophoraceae) in which it grows. These include the large, green, more or less viviparous embryos that are the units of dispersal, pneumatophores (Tomlinson 1986, 2017), and there are also salt glands on both sides of the leaf, although this last feature is perhaps more widespread in non-mangrove halophytes. These salt glands have largely radially-arranged cells in their heads (Fahn 1979), and appear to be variants of the common glandular hair type in Lamiales. Robert et al. (2009, 2011) discuss the hydraulic architecture of the wood of Avicennia in which both xylem and phloem are organized in a three-dimensional network. For salt and water balance, see Reef and Lovelock (2015) and other papers in Ann. Bot. 115(3). 2015 and Nguyen et al. (2017). It is the most widely distributed mangrove genus with perhaps the most species - 8. The shrub Acanthus ilicifolius is another member of the Acanthaceae often found in mangroves.
C4 photosynthesis is reported from a number of species of Blepharis section Acanthodium (Acantheae) (Sage 2004), and the other species of the section are C3 plants or intermediates - there may be as many as five origins of C4 photosynthesis (Fisher et al. 2015; Starr et al. 2025 and references). Heat and aridity seem to have promoted the evolution of C4 photosynthesis here within the last 10 Ma, and C4 plants grow predominantly in the Saharo-Sind and especially the southwest African areas (Fisher et al. 2015; Starr et al. 2025).
In some species of Strobilanthes (Ruellieae) all the individuals flower and fruit in synchrony and then die; this happens in a regular cycle every few years and can occur over very large areas (Janzen 1976; Tripp et al. 2013a, Manzitto-Tripp et al. 2021 for other examples in the family, most also Ruellieae). Large numbers of both pollinators and seed dispersers (the seeds are rich in oils) are attracted to the plants. See Fagaceae for more on masting.
Namibia and adjacent southern Angola are a centre for genera like Barleria, Blepharis, Monechma and Petalidium (Barlerieae) (Tripp & Tsai 2017), Petalidium in particular having diversified quite extensively in the Namib Desert within the last 4.8-1.4 Ma (37/40 species grow there). Acanthaceae are diverse in this area, have numerous endemic species, and are ecologically dominant; such diversification is rather unusual in hyperarid climates elsewhere - and note that this is a summer rainfall area (Tripp et al. 2017b). Most Blepharis grow in dry habitats, and some are very small, very spiny, and have remarkable growth forms. Very fast germination (within 24 hours after imbibition) is reported from Blepharis persica, the radicle of which can reach 5 cm in length within 24 hours of wetting of the seed - perhaps a record, and this may be connected with the habitat ion which it grows (Gutterman 2000 and references; Parsons 2012).
There are a number of taxa that accumulate (or hyperaccumulate) nickel (Reeves et al. 2007: three genera in three tribes).
In Mendoncia substantial amounts of fluid accumulate inside the bracteoles, i.e., it has water calyces (Magnaghi & Daniel 2017).
Pollination Biology & Seed Dispersal. Some 500-600 species of Acanthaceae are hummingbird pollinated (E. A. Tripp and L. McDade, pers. comm.; Tripp & Manos 2008). Tripp and Manos (2008) studied the pollination systems in the speciose Ruellia. They found that although flowers specialised for bird or bee pollination might reverse pollinators (bee to bird transitions are usually decidedly uncommon - Barrett 2013), sphingid-adapted flowers did not reverse, perhaps because they had entirely lost their floral pigments. A number of taxa of Ruellia are cleistogamous (Tripp & Manos 2006; Tripp 2007).
Full (180o) or partial resupination has evolved several times in Acanthoideae, and this is sometimes caused by the twisting of the corolla tube rather late in development (Daniel & McDade 2005), a rather unusual mechanism. Elytraria (Nelsonioideae) may also have inverted flowers, as may some species of Thunbergia with pendulous inflorescences (Dworaczek & Claßen Bockhoff 2016). The filament curtain, formed from decurrent filament ridges in the corolla tube and more or less connate filaments immediately above the adnate portion of the filaments, is found in Ruellieae and perhaps other taxa, too (Tripp et al. 2013a). The curtain divides the corolla tube vertically into compartments, and there may be transverse ridges on the corolla tube near the base, the nectar then becoming enclosed in a separate chamber (Mantkelow 2000; see also Moylan et al. 2004b). In some species of Barleria, two of the stamens may be considerably reduced, but their anthers still enclose a few pollen grains; they are called antherodes (Darbyshire et al. 2019a). The stigmas may be thigmotropic in Ruellieae (see Tripp et al. 2013a).
Capsules open explosively in all taxa except Avicennioideae and some Thunbergioideae. Witztum and Schulgasser (1995) discuss in detail capsule dehiscence in Acanthoideae with their distinctive jaculators (= retinacula); capsules may be hygrochastic (as in Ruellia, for example) or xerochastic (e.g. Sell 1969). Dehiscence was described in detail for the hygrochastic Ruellia ciliatiflora. When the capsule opens explosively very high backspin (ca 1660 Hz - revolutions/second) is imparted to the flattened seeds because they sit on the jaculator in a way that results in force from the opening capsule being applied below the centre of mass, and this backspin also gives the seeds some lift, and they can be dispersed up to 7 m (Cooper et al. 2018). Some seeds, "floppers", wobble when launched, and they do not travel so far, while more wobblers/seeds with much less backspin are found in other Acanthoideae (Cooper et al. 2018). There is some dispute as to whether Nelsonioideae have jaculators, but even if present, they are not functional, and although "rudimentary" jaculators are reported from the subfamily (Johri & Singh 1959; Roham Ram & Masand 1963), they are unlike jaculators in Acanthoideae (Daniel & McDade 2014). In a number of taxa the testa is mucilaginous, and the mucilage can form a layer impermeable to oxygen so inhibiting germination when there is too much water around because of flooding (Western 2011 for references), although mucilage may also facilitate seed dispersal after discharge. Overall, seed morphology shows considerable variation (Al-Hakimi et al. 2017). For both synchronized flowering over large areas and ultra-fast germination, see above under Ecology & Physiology.
Plant-Animal Interactions. Gall-forming fruit flies of the Tephretidae-Tephrellini are found here (and on Verbenaceae and Lamiaceae: Korneyev 2005). Neotropical species of Avicennia host a diversity of gall morphotypes, although the plant itself seems to be little affected (L. L. Silva et al. 2017a).
Larvae of Nymphalinae-Melitaeini butterflies commonly feed on Acanthaceae (Wahlberg 2001; Nylin & Wahlberg 2008). Mass defoliation of Avicennia by lepidopteran larvae seems to be not uncommon (Fernandes et al. 2009).
Genes & Genomes. Chromosome numbers are very variable - see Daniel (2018 and references); McDade et al. (2020) summarize numbers in Justiceae; suggested base numbers of groups within this tribe are quite high - x = 16, 18, 21, 40. Daniel (2000) thought that the base number for Acanthaceae, excluding Nelsonioideae, was x = 7. Lyu et al. (2017) give measurements of some genome sizes. For Physacanthus, probably the result of an ancient wide cross, see Divergence & Distribution above.
Chemistry, Morphology, etc.. Mendoncia lacks iridoids.
For cystoliths in Acanthaceae, see Inamdar et al. (1990 and references); in "double" or "triple" cystoliths, two or three cells are involved in the construction of what appears to be a single cystolith. Inverted vascular bundles in the pith, or anomalous secondary thickening where an internal and inverted cambium develops, or there are successive cambia, are scattered in the family (Roulet 1893; Mullenders 1947; Philipson et al. 1971; Schwarzbach & McDade 2002). Neither have yet been found in Nelsonioideae, although some species have odd vascular anatomy in the stem and even the root. Mendoncia belizensis has rather boraginaceous-looking hair bases. Anisophylly is known from Acanthaceae (Samra et al. 2025).
The inflorescence of Mendoncia is described as being pedunculate and dichasial (Magnaghi & Daniel 2017), the pedicels proper being short to absent, although in overall appearance it is fasciculate and with long pedicels; inflorescence morphology here needs to be confirmed, and whatever the outcome, what are described as peduncles are in fact pedicels. McDade and Turner(1997) describe extrafloral nectaries on the bracts of Aphelandra (Acantheae), and Thunbergia has similar nectaries on the calyx as well as nectaries inside the corolla tube, while in Avicennia nectar is secreted from glands on the inside of the corolla tube (for details, see Tomlinson 1986). In Avicennia there may be fewer corolla than sepal lobes ("connation" of a pair of the former?). Bravaisia (Acanthoideae) is distinctive in that it has small bracteoles and rounded calyx and corolla lobes (the former are more or less scarious); the anthers have short basal appendages. There is discussion as to the nature of corolla tube initiation, which is probably usually more or less late, rarely early (c.f. Leins & Erbar 1997; Schönenberger & Endress 1998; see also Endress 1999 for floral development). Anther morphology is particularly variable in Neotropical Justicia (Kiel et al. 2013, 2017). The diversity of pollen morphology in most of the family (not Nelsonioideae) is spectacular, and it also shows extensive homoplasy (e.g. Kiel et al. 2006). For variation within Strobilanthes s.l., see Carine and Scotland (2000) and Wang and Blackmore (2003), for that within Acanthoideae as a whole, see Raj (1961), Muller et al. (1989), Furness (1996 and references: Acantheae), Scotland (1992) and Dalawai and Murphy (2021), both Andrographideae, Scotland (1993) and references in Tripp et al. (2013a), both Ruellieae, Daniel (1998, 2010), House and Bakewill (2016), Al-Hakimi et al. (2017) and many other papers. Many Isoglossinae (Justicieae) have distinctive "Gürtelpollen" (Kiel et al. 2006), that is, lenticular biporate pollen with a prominent circumferential band (see also Whitfieldieae), but any functional significance of this is unclear. The [Acanthoideae [Thunbergioideae + Avicennioideae]] clade appears to lack a funicular obturator, but I am uncertain as to the polarity of this feature. The fruit of Avicennia is a capsule, according to Takhtajan (1997) and Schatz (2001), but it may open only as the seed germinates. For cotyledon folding, see Schwarzbach and McDade (2002).
Embryo sac development in some/most Acanthaceae is very distinctive. The tip of the embryo sac grows through the micropyle and eventually may lodge in the placenta, and this where the egg apparatus is formed (the movements of the polar nuclei are unclear). As the embryo develops, a very long suspensor forms and the embryo is pushed back into the endosperm - and so into the ovule and the developing seed (e.g. Mohan Ram & Masand 1963 and references; Nagl 1962). This is rather similar to comparable, but more extreme, behaviour in Loranthaceae. The ovule of Avicennia is reported to be straight; the embryo sac is extra-ovular, and the micropylar endosperm haustorium at least is also extra-ovular, being very much branched and reaching the placenta (Mauritzon 1934a; Padmanabhan 1964, 1970).
In Acanthoideae other than Acantheae, there is considerable variation in the details of endosperm development. There is often a central area in which divisions are free nuclear, walls being laid down subsequently, but in some taxa there is what is known as a "basal apparatus", an area in which walls are not laid down; this pattern of endosperm development occurs in no other angiosperms (Mohan Ram & Wadhi 1964; Johri et al. 1992 and references). In Nelsonioideae the central area is entirely cellular, but other details of endosperm and embryo sac development are like those just described (Johri & Singh 1959; Moham Ram & Masand 1963). This distinctive asymmetric endosperm development is also found in Lamiaceae-Nepetoideae (a parallelism). Seed oils of many Thunbergia species contain up to 92% of the monounsaturated petroselinic acid (18:1Δ6) - remarkably high amounts - but in other species there are quite large amounts of sapienic acid (16:1Δ6); petroselinic acid, also known from a number of Apiales, is synthesized in different ways in the two groups (Gan et al. 2022).
For an entry into the literature, keys, classification, and much else, see Manzitto-Tripp et al. (2021), for general anatomy, capsule dehiscence, etc., see van Tieghem (1908), for embryology, etc., see Mauritzon (1934a), and Wadhi (1970), for aspects of stem anatomy, see Patil et al. (2024), and for stomata, see Rohweder et al. (1971). Nelsonioideae: some information is taken from Bremekamp (1955) and especially Daniel and McDade (2014). Thunbergioideae: for Thunbergia, etc., see Schönenberger (1999). Avicennioideae: for embryology, etc., of Avicennia, see Padmanabhan (1970, as Verbenaceae) and also Borg and Schönenberger (2011) and for wood anatomy, see Carlquist (1990b), for general information, see Tomlinson (2016) and Sanders (1997). Acanthoideae: for Whitfieldieae, see Manktelow et al. (2009) and Grall and Darbyshire (2021), for chemistry, see H. F. W. Jensen et al. (1988) and Sicker et el. (2000: benzoxazinones), for information on acicular fibres, see Bremekamp (1965: "raphidines") and on foliar/cauline endodermes, see Lersten (1997), for corolla aestivation, which shows interesting variation, see Scotland et al. (1994), for floral morphology, see Endress (1994b), for floral development, see Hirao et al. (2019), for nectaries, see Vogel (1998c), and for some embryology, see Maheshwari and Negi (1955).
Phylogeny. The erstwhile Nelsoniaceae were placed sister to other Acanthaceae in Hedren et al. (1995), and this position seems quite firm (see esp. McDade et al. 2012). The general position of Avicennia (Avicenniaceae) within Acanthaceae s.l. is also well established, if its exact position is less certain; all have the same distinctive endosperm development and swollen nodes. Avicennia has a rather weakly supported sister group relationship with Thunbergioideae (Schwarzbach & McDade 2002; Hilu et al. 2003), support coming mostly from the chloroplast genes (McDade et al. 2008). However, relationships in Tripp and McDade (2014a) were [Nelsonioideae [Thunbergioideae [Avicennioideae + Acanthoideae]]], while in Z.-D. Chen et al. (2016) the positions of Avicennioideae and Thunbergioideae were reversed, although in neither was the position of Avicennioideae well supported. For more on early work on phylogenetic relationships, see Scotland et al. (1995), Scotland and Vollesen (2000) for other suggestions of relationships, palynology prominent. Morais et al. (2019) usher in transcriptome analyses; Arias et al. (2022) recovered expected relationships in their preliminary genome skimming/transcriptome analyses except for the position of Crabbea.
Within Nelsonioideae, Nelsonia and Elytraria are successively sister to the rest of the subfamily, within which there is quite a lot more structure (McDade et al. 2012, see also 2009; Daniel & McDade 2014; Wenk & Daniel 2009: position of Nelsonia uncertain).
For the phylogeny of Acanthoideae, see McDade et al. (2008, also McDade & Moody 1999; McDade et al. 2000a; McDade et al. 2006; Tripp & McDade 2014a): Acantheae are sister to the rest. In Acantheae, McDade et al. (2005) found that taxa with one- and two-lipped corollas formed separate clades, Old World and largely New World respectively.
Barlerieae: Comito et al. (2022) looked at relationships in Barleria. The Indian Petalidium barlerioides is probably sister to the rest of Petalidium, which is mostly from the Namib Desert area (Tripp et al. 2017b); for further relationships, see Darbyshire et al. (2019a). The focus in Comito et al. (2025: 117 taxa, nuclear data) was on Lepidagathis, but all genera were included; three main clades, to be subtribes, were recovered, the Crabbea clade being sister to the other two and Lepidagathis probably needing to be split into three genera.
For Justicieae, see McDade et al. (2000b, especially c); in the latter the relationships [Pseuderanthemum, etc. [Isoglossinae [Tetramerium, etc. [paraphyletic Old World justicioids [New World justicioids + Diclipterinae]]]]], mostly with a fair amount of support, were recovered. These relationships - [Pseuderanthemum lineage [[Isoglossinae + Ptyssiglottis lineage] [Tetramerium lineage + Justicioid lineage]]] - were largely confirmed by McDade et al. (2020); taxa like Pseuderanthemum, Graptophyllum and Oplonia are not monophyletic. For relationships within Isoglossinae, see Kiel et al. (2006), while for extensive studies of the justicioid groups, see Kiel et al. (2009, esp. 2017, 2018). In the latter, it was found that support along the backbone of the phylogeny was mostly strong, although the old sections were rarely recovered. Fonseca (2021) found 16 species from other genera inside Justicia... For relationships in the Tetramerium group, see Daniel et al. (2008), Côrtes et al. (2015) and especially McDade et al. (2018).
Ruellieae: see the very useful treatment by Tripp et al. (2013a). Ruellia is defined largely by pollen morphology, and it includes genera like Blechum, etc. (Tripp et al. 2013a). For more on relationships in Ruellia itself, see Tripp and Tsai (2017), for those in the Old World taxa, not many species but forming a basal grade in the genus, see Tripp and Darbyshire (2017) and for relationships in the 275 or so New World species (10% undescribed), see Manzitto-Tripp and Daniel (2023: 187 spp., ddRAD data). Brillantaisia may well be embedded in Hygrophila (Tripp et al. 2013a), and pollen morphology is largely consistent with this position (Furness 2013).
Whitfieldieae. Relationships here are those in McDade et al. (2008; Grall & Darbyshire 2021); generic limits in the Madagascan taxa are difficult. Finally, Physacanthus is probably an ancient inter-tribal (Ruellieae x Acantheae) hybrid (Tripp et al. 2013b, see also Divergence & Distribution above).
Borg et al. (2006) provide a phylogeny for Thunbergioideae.
Classification. A fairly recent reclassification of Acanthaceae is that by Scotland and Vollesen (2000), and the tribal classification of Acanthoideae here largely follows that in McDade et al. (2008). For the current classification of Acanthaceae, including subtribes, and with keys, some geographically-circumscribed, to genera, see Manzitto-Tripp et al. (2021).
Generic limits pretty much throughout the family are difficult, as in other groups where genera in the past have been based on variations in corolla morphology that represent adaptations to particular classes of pollinators that do not indicate broader relationhips (e.g. Daniel et al. 2008 and references; see also Côrtes et al. 2015). The very extensive variation in pollen morphology in the family has also led some to dismember genera, thus Bremekamp (1944) broke up Strobilanthes into some 54 genera most of which have been returned to whence they came. Floral variation in some Justicieae may be less reliable in marking genera than variation in e.g. seed and pollen (Kiel & McDade 2014). Recent work emphasizes the problems that have to be faced if broad or narrow generic circumscriptions are adopted (Kiel et al. 2017, 2018), and Darbyshire et al. (2019) incline towards a narrower circumscription of genera. Indeed, McDade et al. (2018: p. 97) talk about there being a "clade complex" (analogous to a species complex) in the Tetramerium area (also Justicieae) in which clades that are very distinctive in molecular analyses show no particular structural/morphological differences. Genera in Ruellieae are being recircumscribed (Tripp et al. 2013a; Tripp & Darbyshire 2020); see Tripp et al. (2013a) for a detailed discussion of subtribes here. For genera in Nelsonioideae, see Daniel and McDade (2014).
For a sectional classification of Old World Ruellia (at least 15 well-supported sections), see Tripp and Darbyshire (2017) and for that in the more speciose New World taxa, see Manzitto-Tripp and Daniel (2023: 15 sections, 205 species included). For an infrageneric classification of Barleria, see Darbyshire et al. (2019a), but understanding relationships there is very much a work in progress (Comito et al. 2022).
Species numbers seem particularly uncertain in Acanthaceae, as Tripp et al. (2013a) suggest.
Previous Relationships. Nelsonioideae have often been placed in Scrophulariaceae s.l. or considered "intermediate" between Scrophulariaceae and Acanthaceae while Cronquist (1981) was uncertain of the relationships of his Mendonciaceae. Avicennia was often included in Verbenaceae, largely because it is woody, has a more or less cymose inflorescence, and a gynoecium with two ovules per carpel. Thomandersia has seeds with a structure described as a retinaculum, although capsule dehiscence is not explosive (Manzitto-Trip 2021: see Thomandersiaceae).
Botanical Trivia. The pollen grains of Crossandra stenostachya are over 5 mm long (Furness 1990).
[Bignoniaceae [[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]: ?
BIGNONIACEAE Jussieu - Back to Lamiales
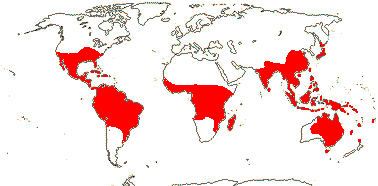
C-4 carboxyl and ecarboxylated iridoids +; phloem stratified; cork also cortical; paratracheal-aliform parenchyma + (0); nodes 1:1-9; petiole bundles annular (also rib or adaxial bundles); stomata helicocytic [?level]; leaves twice-compound, lamina vernation conduplicate, margins entire (toothed); inflorescence branches cymose; flowers large; K often with nectaries, A (5, 2), thecae sagittate or head-to-head, usu. not confluent, tapetum amoeboid; pollen 3-colpate, psilate/finely reticulate [?], nonperforate; post-zygotic incompatibility system [?all]; ovary wall with vascular bundles, these also opposite septa, ovules in two groups in each loculus, (placentae lobed), stigma lobes broad, sensitive, wet; integument 4-10 cells across; fruit often with epidermal extrafloral nectaries; seeds many, winged, relatively large; wings 1-2 cells thick, cells with helical/annular(none/reticulate) thickenings; endosperm 3-chambered, developing from cemtral chamber, micropylar haustoria 0, 0 in mature seed, resting zygote +; n = 20, x = 9, nuclear genome [1 C] (0.104-)0.771(-5.69) pg/(592-)1112(-1697) Mb; germination epigeal, phanerocotylar (cryptocotylar), cotyledons persistent, ± cordate basally, apex lobed; (plastid transmission biparental).
110 [list]/790 - eight groups and unassigned genera below. Mainly tropical, esp. South America (map: from van Steenis 1977).
Age. Crown-group Bignoniaceae are estimated to be (54.3-)51.5(-49.3) Ma (Tank & Olmstead pers. comm.), (51.6-)49.7(-47.7) Ma (Ragsac et al. 2021) or ca 61.1 Ma (Fonseca 2021).
Fossil seeds and fruit of Bignoniaceae are known from the Eocene of Washington State and are ca 49.4 Ma (Pigg & Wehr 2002).
1. Jacarandeae Fenzl - Jacaranda de Jussieu
Trees to shrubs; aliform parenchyma +, rays not storied; leaves simple to bicompound, K ± free; anthers unithecate, staminode large, with capitate-glandular hairs [bearded], (divided); G with parietal placentation; fruit orbicular, angustiseptate; n = 18.
1/50. Southern Mexico to northern Argentina.
[Tourrettieae [Tecomeae [Bignonieae [[Catalpeae + Oroxyleae] [Crescentieae + Coleeae]]]]]: (vessel elements with reticulate and/or foraminate perforation plates), rays storied.
Age. The age of this clade is some 45.6 Ma (Ragsac et al. 2021).
2. Tourrettieae G. Don
Vines, climbing by leaf tendrils; (leaves pedate);inflorescence racemose, bracteate; staminode 0.
2/6: Eccremocarpus (5). Mexico, Cenral America, and Andes in South America. Photo - Eccremocarpus Flower.\
Age. The age of crown-group Tourrettieae is ca 23.4 Ma (Ragsac et al. 2021).
[Tecomeae [Bignonieae [[Catalpeae + Oroxyleae] [Crescentieae + Coleeae]]]]: leaves once compound; (staminodes +, simple).
3. Tecomeae Endlicher
(Plants herbaceous, with strong taproot (annuals) - Incarvillea), shrubs to small trees; distinctive C-4 formyl iridoids, (dimeric flavonoids); (perforated ray cells); leaves (simple); capsule (septifragal).
12/55: Incarvillea (16), Tecoma (14). South eastern North America to western South America, eastern Africa, Central Asia and China to Malesia and eastern Australia.
Age. Ragsac et al. (2021) suggested a crown-group age of (41.8-)37.1(-31.6) Ma for this group.
[Bignonieae [[Catalpeae + Oroxyleae] [Crescentieae + Coleeae]]]: ?
Age. An estimate of the age for the clade [Campsis, Catalpa] is (32-)25(-17) Ma (Bell et al. 2010).
4. Bignonieae Dumortier
Lianes (shrubs), climbing by leaf tendrils; (monofluoracetates +); (cambium storied); vascular pit borders usu. 0-3.9μm across, aliform-confluent xylem parenchyma +, rays tall [usu. >1 mm tall], cells perforated; anomalous secondary thickening + [morphologically variant phloem in deep wedges, the xylem cylinder 4- or more lobed], (normal); (stratified phloem 0); (raphides/styloids +); (prophyllar pseudostipules +); (leaves once-compound, ternate, (with 3 [1, 2, several] petiolular tendrils) (lamina vernation involute - Pyrostegia); flower Anemopaegma-type [bee-pollinated, yellow, purple, etc., infundibular, tube straight, odoriferous and nectariferous]; fruit septifragal, with persistent septum and separate whip-like strands of woody tissue [= vascular bundles opposite septum], (indehiscent).
21/385: Adenocalymma (76), Fridericia (67), Amphilophium (47), Anemopaegma (45). America, largely tropical. Photo - Distictella Flower.
Age. Crown-group Bignonieae have been dated to (54.2-)49.8(-45.7) Ma, the crown clade that includes the tribe minus the monotypic Perianthomega being (52.2-)48.0(-43.9) Ma old (Lohmann et al. 2012; see also Alcantara et al. 2013).
[[Catalpeae + Oroxyleae] [Crescentieae + Coleeae]]: (vasicentic or aliform parenchyma +).
5. Catalpeae Meisner
Leaves (spiral), simple; (A 2, staminodes 3).
2-3/11: Catalpa (8). North America, the Greater Antilles, East Asia.
6. Oroxyleae A. H. Gentry
(Flowers polysymmetric); (A 5); fruits septicidal.
4/6. Indo-Malesian.
[Crescentieae + Coleeae]: fruit indehiscent.
7. Crescentieae G. Don —— Synonymy: Crescentiaceae Dumortier
Cambium storied; (short shoots +); leaves spiral, palmately compound, (unifoliolate), (simple), (phyllodinous); (flowers bat-pollinated, ± cauliflorous); (placentation parietal); ovules with hypostase; (fruits ± indehiscent, and seeds not or barely winged).
12/147: Tabebuia (70). Central and South America and the Greater Antilles. [Photo: Amphitecna Flower.]
[Crescentieae G. Don, s. str. —— Synonymy: Crescentiaceae Dumortier
Small (to large) trees; (short shoots +); leaves opposite/spiral, palmately compound, (unifoliolate), (simple), (phyllodinous); flowers bat-pollinated, ± cauliflorous; (placentation parietal); ovules with hypostase; fruits indehiscent, ± baccate, placentae fleshy/not; seeds (barely) winged [check].
3/37: Amphitecna (21). Central America, also the Antilles and N. South America, Photo: Amphitecna Flower.
]8. Coleeae Bojer
(Prophyllar pseudostipules +); leaves often whorled, (simple), (phyllodinous, articulated); flowers bat-pollinated, ± cauliflorous; (placentation parietal); fruits ± indehiscent; seeds ?not winged.
4/69 + 28 undescribed (Callmander et al. 2015): Colea (35). Madagascar and surrounding islands.
Evolution: Divergence & Distribution. Olmstead et al. (2009; see also Olmstead 2013) suggested that the family was probably New World in origin, with five or six subsequent shifts to the Old World and one back to the New World. Lohmann et al. (2012, q.v. for many dates) thought that the ancestors of fossils assignable to Bignonieae from Central and North American (Panama, Washington State) as well as of North American Bignonia itself might have arrived there by long distance dispersal. Interestingly, members of three clades which are surmised to have been involved in long distance dispersal are currently dispersed by animals; Olmstead (2013) thought that adaptation to animal dispersal had occurred after wind-assisted dispersal events. Within Bignonieae, areas of endemism and overall species richness seem to be concentrated on Amazonia and the Atlantic Forest areas (Narváez-Gómez et al. 2021). Thode et al. (2018) looked at diversification within the widely distributed and speciose Amphilophium (Bignonieae), where diversification in a clade particularly associated with Amazonian white sands began ca 7.2 Ma, while that in its sister clade, the rest of the genus, can be dated at ca 25.8 Ma with an initial focus on the Atlantic forest but with some subsequent wide dispersals. Ragsac et al. (2021a) explained the circumglobal distribution of the quite small (now a mere 56 species) and young (ca 37 Ma) Tecomeae by a mixture of long distance dispersal events, possibly aided by emergent oceanic ridges, etc., and migration, and this culminated in the movement of the ancestor of the South American Campsidium probably from Australia; it is embedded in the Australasian clade, and it diverged from its sister species there, Pandorea jasminoides, only ca 13 Ma.
Within Bignonieae, all New World, associations between different traits varies (Alcantara et al. 2013). Abiotic specialization seems to have been recent here, there is no correlation between phylogeny and habitat, and there is no evidence of competition between species for pollinators (bees are the main pollinators), although floral diversity was greatest in the Atlantic Tropical Forest, perhaps the original area for the tribe (Alcantara et al. 2014). For diversification in Tynanthus and Anemopaegma, both Bignonieae, see Hoorn et al. (2023); speciation in the former has been flat over the late Pliocene onwards.
Variation in wood anatomy in the family is optimised on a phylogenetic tree by Pace and Angyalossy (2013; see also Pace et al. 2015a) and the extensive variation of the distinctive variant phloem found in deep wedges in the secondary xylem in Bignonieae is placed in a phylogenetic context by Pace et al. (2015b; see also below). Bignoniaceae have the highest minimum diploid chromosome number - ca 36 - in seed plants, and Bromeliaceae are next at ca 32 (Elliott et al. 2022b: Fig. S6).
Ecology & Physiology. Bignoniaceae-Bignonieae, with almost 400 speciesz, are, along with Sapindaceae, perhaps the third most ecologically important group of Neotropical lianes (e.g. Gentry 1991); the family is, of course, notably more common in the New World. Bignoniaceae are the second most speciose family in seasonally drier tropical forest types in America (Gentry 1988, 1991), and drier forests and the liane habit may be connected, having in common toleration/prevention of water stress - the whole family is perhaps adapted to drought (Punyasena et al. 2008). Sousa-Baena et al. (2014a) discuss the evolution of tendrils in Bignonieae. The plants climb using foliar tendrils, which can be grapnel-like as in the appropriately-named Dolichandra unguis-cati, and attachment may be aided by glue-like exudates, as in Bignonia capreolata, or by tissue ingrowth into irregularities of the support, as in Amphilophium crucigerum (Seidelmann et al. 2012). The phloem of the lianes is particularly distinctive and is of two types. More ordinary phloem is found on the periphery of the vascular cylinder, and it has narrow sieve tubes, more parenchyma, broad rays, etc., and is perhaps involved in storage. The variant phloem, found in the phloem wedges, has prominent fibres and very wide sieve tubes, and is probably more involved in translocation of the photosynthesate (Pace et al. 2011). There are a number of differences between the wood of lianes, shrubs and trees in Bignoniaceae, as in other lianescent groups, lianes having i.a. a larger area of vessels, these vessels showing a great variation in size (= dimorphism); perhaps the narrower vessels remain functional when the wider vessels become cavitated (Gerolamo & Angyalossy 2017). Lianes in general tend to have extensive leaf areas considering the width of their stems, hence perhaps the importance of the variant phloem (e.g. Isnard & Feild 2015 and references). Overall, lianes use water very efficiently (van der Sande et al. 2019; Schnitzer et al. 2019; Dias et al. 2019 and references) and grow remarkably well in the dry season/dry conditions and proportionally much more than the trees there. Lianes are also abundant in forest edges, treefall gaps, and similar habitats where their growth habits will also be advantageous, and may have negative effects on the growth of co-occurring trees (Schnitzer 2018). Gerolamo et al. (2025) compared the anatomy of congeneric vines in Bignoniaceae growing in Amazonian rainforest and seasonally dry Atlantic Forest in Brazil; variation seemed to be unrelated to hydraulic safety but had some connection with hydraulic efficiency. For additional information about lianes and Bignoniaceae, see Angyalossy et al. (2015) and other references in Schnitzer et al. (2015); see also Sperotto et al. 2023).
Some Bignoniaceae are shrubs, and there are also a few herbs; the latter are discussed under Vegetative Variation below.
Pollination Biology & Seed Dispersal. The large flowers of Bignoniaceae are animal pollinated, and the considerable variation in floral morphology, including nectary type, and in flowering phenology is associated with the behaviour and type of visitor (Gentry 1974a, b, 1990; Alcantara & Lohmann 2010a, b; Alcantara et al. 2013) - in the New World, up to 20 species of Bignoniaceae may be found in the same community (Gentry 1974a, b, 1990). One of the commonest flower types here is the Anemopaegma type, visited by euglossine bees (along with anthophorids); this may be ancestral in Bignonieae, and has an infundibular, straight corolla tube, nectar, and is magenta, yellow or white in colour (Alcantara & Lohmann 2010a, 2014). The bees also visit Jacaranda, and here the prominent staminode may be involved - ?secretions from staminodial hairs (Ragsac et al. 2019 and references). The nectarless Cydista type, otherwise rather similar florally, is also visited by euglossines. (Note that euglossine bees began diversifying some 42-27 Ma - Ramírez et al. 2010.) Indeed, it has been suggested that nectarless Bignonieae have deceit pollination, however, in Zeyheria the small nectary was functional, in adddition, there were glandular hairs on the corolla secreting terpene/resin-type substances (Machado et al. 2017). Oroxylon is bat pollinated, and its flowers are almost polysymmetric and have five stamens; Fleming et al. (2009) list the species in the family that are known to be pollinated by bats; see also Marshall (1985: Old World taxa). There is sequential flowering of different species of Bignoniaceae at the one locality both in Madagascar and in the New World (Hosaka et al. 2016 for references, and for such sequential flowering in general). Alcantara and Lohmann (2010a, b) found that, in general, flower size in the lianescent Bignonieae was larger in the past than it is is in extant species. Although post-zygotic incompatibility systems are very common in Bignoniaceae (Gibbs 2014), the family seems to have few other features that have been associated with such systems (Wyatt & Lipow 2021 and references).
Dispersal syndromes of fruits are also quite diverse (Gentry 1983; 1990) but they are not particularly correlated with pollination syndromes. Thus Kigelia africana is bat-pollinated and has massive, sausage-shaped, indehiscent fruits that are eaten by everything from monkeys to elephants. Oroxylon is also pollinated by bats, but it has capsules and wind-dispersed seeds. Wind dispersal is common, and the seeds often have broad, papery wings. A number of taxa have seeds dispersed by water, including Dolichandrone, a mangrove plant; here the modified seed wing is corky and serves as a flotation device. In Crescentieae, Amphitecna and Crescentia (calabash) have spherical indehiscent fruits, Parmentiera has elongated fleshy fruits, although its seeds still have a small wing, and Spirotecoma and Tabebuia and relatives have elongated, dehiscent fruits and winged seeds.
Plant-Animal Interactions. Extrafloral nectaries are extremely common in Bignoniaceae, and they may be on reduced prophylls, and/or on the tips of young leaflets, at the nodes, on the outer surface of the calyx and on the ovary (Seibert 1948; ants are attracted (e.g. Gonzalez 2011; Weber & Keeler 2013; Gama et al. 2016b: patelliform structures, K and prophyll). Domatia are also common.
For iridoids and plant defence, see Bowers (1993: Catalpa).
Vegetative Variation. Bignonieae are nearly all lianes with branched tendrils and distinctively rayed xylem (Lohmann 2006 for a phylogeny). Within the tribe, variation in the details of the ray-like fluting of the xylem which becomes channeled or lobed can be interpreted as complexity increasing by terminal addition and is mirrored by ontogenetic increases in the numbers of channels as the individual grows; the simple pattern in shrubby members, a polyphyletic group, results from a heterochronic reversal (Pace et al. 2009). Pace et al. (2011) note that the variant phloem that causes the fluting of the vascular cylinder has large-diameter sieve tubes and numerous fibres, hence contributing substantially to both translocation and stem support; regular phloem has much narrower sieve tubes. (For wood anatomy in Campsis (Tecomeae), see Rajput et al. 2018.) Herbaceous species are known from Incarvillea and Argylia alone, and in both cases the plants grow at high elevations/in cold conditions, have large flowers and robust taproots, and have evolved from suffrutescent ancestors (Ragsac et al. 2021a). Perianthomega has biternate leaves, it also has robust unbranched twining petioles, the three small scars at their ends representing leaflets. Elsewhere in Bignonieae the tendrils are variously-branched terminal or lateral petiolules of the compound leaves so common in the family, and Sousa-Baena et al. (2014a, also 2018b) discussed tendril morphology and evolution. As for the genetic control of tendril development here, Sousa-Baena et al. (2014b, 2018a) examined this in some detail, noting i.a. the activity of SHOOTMERISTEMLESS (a KNOX1 gene), PHANTASTICA and LEAFY/FLORICAULA (c.f. the IRL clade in Fabaceae; the latter gene is most important). There is a considerable amount of variation in the epidermis of Bignonieae - thus there were 10 different types of stomata, 4 of cuticular waxes, etc., in 13 species (9 genera) of the tribe from Brazil examined (Lopes-Silva et al. 2020).
Palmate leaves have arisen more than once within Bignoniaceae, but are known only in New World taxa. The New World Tabebuia s.l., which has opposite, palmate leaves, is polyphyletic (Grose & Olmstead 2007b); a number of taxa - some apparently very different vegetatively - are derived from it. These include Amphitecna, with spiral, simple leaves like those of Crescentia. The petioles are short and the lamina has distinctive, widely spreading venation; they are phyllodinous, and in some species of Crescentia palmate leaflets are borne on the end of a lamina-like petiole confirming the morphological nature of the latter. Parmentiera and Spirotecoma, both with more ordinary opposite palmately-compound leaves, are also close; all four genera have bat-pollinated flowers. They are part of a clade of palmately-leaved taxa (Grose & Olmstead 2002, 2007a; see expanded Crescentieae above).
The simple and clearly petiolate leaves of Catalpa (opposite or whorled) and Chilopsis (spiral: the two genera hybridise), have a very different morphology from those of Crescentia, etc.; they appear to be conventionally simple, lacking articulations between petiole and lamins.
Chemistry, Morphology, etc.. Pace et al. (2016) summarize variation in wood anatomy in Bignoniaceae. For leaf teeth, see Seibert (1948) and Rios et al. (2020), and for the variety of extrafloral nectar glands in the family, see Seibert (1948).
There are four main carpel bundles, but only two in "Scrophulariaceae" (Armstrong 1985), Gesneriaceae, etc.. In Tourrettieae, Tourrettia has sub four-locular ovaries, each loculus having a single rank of ovules, while Eccremocarpus has parietal placentation. Ovule shape varies considerably, some species having a long chalazal beak; Pereira and Bittencourt (2016) note that details of the deposition of callose around the megaspores and also nucellar protrusion may be of systematic interest. Endosperm development also varies, thus Incarvillea has a huge micropylar endospermal cell (Mauritzon 1935). A number of Bignonieae with septifragal dehiscence also have cracks in the loculicidal position along the backs of the valves.
For general information, see Manning (2000) and Fischer et al. (2004a: the classification is very "classical", c.f. e.g. Lohmann 2006 and esp. Lohmann & Taylor 2014). For toxic monofluoracetates, see Lee et al. (2012), for iridoids, von Poser et al. (2000), for the chemistry of Fridericia, see Carvalho et al. (2022), for wood anatomy, see Rogers (1984: comparison with Rubiaceae), Gasson and Dobbins (1991: lianes and the rest compared) and especially Pace and Angyalossy (2013) and Pace et al. (2015a, b), and for nodal anatomy, see Trivedi et al. (1976). For information on pollen, which is very variable, see Buurman (1977), Gentry and Tomb (1979) and Burelo-Ramos et al. (2009: Pithecocteniinae), for tapetum, Huysmans et al. (1998), for some embryology, see Bittencourt and Mariath (2002), for seed anatomy, including that of Schlegliaceae and Paulowniaceae, see Lersten et al. (2002), and for protein bodies in the nucleus, see Bigazzi (1995).
Phylogeny. The basic phylogenetic structure within the family is [Jacarandeae [Tourrettieae [Tecomeae [Bignonieae + the rest]]] (Olmstead et al. 2002). This has been further amplified by Olmstead et al. (2009: ca 3/4 of the genera sampled, three genes; see also Ragsac et al. 2021a), although some relationships of major groups like Tecomeae remain poorly supported. Bignonieae may be close to Oroxylum and relatives - which have bicompound leaves and septicidal capsules - and Catalpa - which has only two stamens (Olmstead et al. 2002). Delostoma may be sister to the [Bignonieae [[Catalpeae + Oroxyleae] [Crescentieae + Coleeae]]] clade (Pace et al. 2015a). Ragsac et al. (2021a) noted that Argylia, which was weakly supported as sister to Tecomeae, was sister to Incarvillea, definitely a member of that tribe, in some nuclear analyses, and although geography did not make any sense there, morphology might (see Vegetative Variation above). Fonseca et al. (2023; 677 nuclear genes, 6/8 tribes) recovered a fair amount of structure in the family, although support along the spine of Bignonieae was weak and there was topological conflict in that tribe, also Nyctocalos (Oroxlyeae) was rather migratory.
Ragsac et al. (2019) found some conflict between topologies resulting from different analyses of Jacaranda (Jacarandeae). For a comprehensive (2-gene + morphology) phylogeny of Bignonieae, see Lohmann (2006a, 2012); [Perianthomega [[Adenocalymma + Neojobertia + The Rest]] is the basic phylogenetic structure. Major clades are supported by a mixture of floral and vegetative characters (Lohmann 2002, esp. 2006); the limits of Adenocalymma are being reworked (Fonseca & Lohmann 2018). Thode et al. (2018) examined relationships in Amphilophium, while Kaehler et al. (2019) looked at the Arrabidaea & Allies clade with a focus on the limits of and groupings in Fridericia. Coleeae as narrowly delimited here are restricted to Madagascar, and their phylogeny and fruit evolution has been examined by Zjhra et al. (2004) and especially by Callmander et al. (2015); they are part of a larger and well supported clade that includes Kigelia, Spathodea, etc.. Although Coleeae and Crescentieae have taxa with similar flowers and fruits and "simple" leaves, the latter differ morphologically. Relationships between the New World Tabebuia, with opposite, palmately-compound leaves and its relatives have been clarified. Crescentieae: Amphitecna and Crescentia, both with spiral, simple leaves, are probably derived (Grose & Olmstead 2007a, b); with Parmentiera, they make up Crescentieae s. s.tr. (Ragsac et al. 2021b). Relationships within Tecomeae have been examined by Ragsac et al. (2021a), and those they recovered were [[Campsis + Astianthus] [Tecoma [African clade, Australasian Clade, Incarvillea]]]. Generic groupings in this last clade had little support, while Incarvillea itself was on a long branch and showed much internal molecular divergence (Ragsac et al. 2021a); for relationships in that genus, see S. Chen et al. (2004).
Classification. The tribes above are in part those recognised by Olmstead et al. (2009). Note, however, that the tribal classification in that publication is not exhaustive in that not all genera are assigned to tribes, partly because their phylogenetic position was and still is ambiguous (e.g. Argylia, Delostoma). Crescentieae might be expanded to include Tabebuia, etc. (the Tabebeuia alliance of Grose & Olmstead 2007a, b), although Ragsac et al. (2021b ) circumscribe the tribe narrowly - but with unplaced genera. Coleeae, too, could well be expanded to include genera like Kigelia, Spathodea, etc. (but see Callmander et al. 2015). For a sectional classification of Jacaranda, see Ragsac et al. (2019).
Over-reliance on characters associated with pollination and dispersal syndromes as markers of generic distinctness has caused serious problems with generic limits (see Lohmann 2003, 2006), however, genera in Bignonieae, close to half the family, have now been reworked (Lohmann & Taylor 2014); further adjustments are ongoing.
There is a species level checklist for the family (Lohmann & Ulloa 2007).
Thanks. I am grateful to L. Lohmann for comments.
>[[Schlegeliaceae + Lentibulariaceae] [Thomandersiaceae + Verbenaceae]]: ?
[Schlegeliaceae + Lentibulariaceae]: ?
Phylogeny. For sampling, and support for this odd couple, see Refulio-Rodriguez and Olmstead (2014).
SCHLEGELIACEAE Reveal - Back to Lamiales
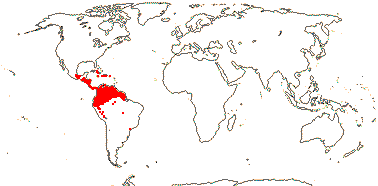
Woody shrubs or root-climbing vines, often epiphytes, (large trees); pericyclic sheath sclereidal; nodes 1:3; petiole bundle solid or (almost) annular, with wing bundles, pericyclic lignification 0; sclereids +; stomata variable; lamina (glands on the ad/abaxialsurface), margins entire or serrate/spiny, (pseudostipules [= prophylls] +); (inflorescence branched); flowers quite large; nectaries on outside of K; staminode +/0; nectary vascularized from carpellary bundles/0; placentae swollen, (placentation intrusive parietal, placentae uni- or bilobed); fruit a berry, K persistent to ± accrescent; seeds compressed or angular; exotestal cells with scalariform thickenings on the inner periclinal wall or mucilaginous with outer periclinal wall absent; endosperm +/0, cotyledons slightly over half the length of the embryo; n = 20, x = 10 (?9); seedlings epigeal and phanerocotylar, cotyledons lobed.
4 [list]/37: Schlegelia (24), Gibsoniothamnus (11). Mexico to tropical, esp. N.W., South America, the Antilles. Map: from Gentry (1980), Tropicos (consulted iii.2014). Photo Flower, Flower, Fruit.
Chemistry, Morphology, etc.. For wood anatomy, see Gasson and Dobbins (1991); there are no obvious differences in wood anatomy between Schlegeliaceae and Bignoniaceae. Schlegelia may have anomocytic or paracytic stomata, while those of Gibsoniothamnus are anisocytic or cyclocytic. There are often quite conspicuous "glands" on the lower surface of the lamina - these are hairs with the normal lamialean structure of radially-arranged cells in the head. Gibsoniothamnus may be anisophyllous (c.f. Thomandersia).
Winged seeds have been reported for e.g. Schlegelia (Fischer 2004b), but the combination of winged seeds and baccate fruits seems rather improbable; Gentry (1973) described the seeds of Schlegelia as being angular. I have not seen the Cuban Synapsis.
Some information is taken from Burger and Barringer (2000), Barringer (2004: Gibsoniothamnus), and Fischer (2004b: under Scrophulariaceae), all general, Leinfellner (1973: gynoecium of Schlegelia), and Armstrong (1985: floral anatomy). Gibsoniothamnus parvifolius: Herrera 672, - leaf, stem, seed; G. allenii: McPherson 11069 - leaf, seed; Schleglia darienensis: Neill et al. 11411 - seed.
Previous Relationships. Schlegelia and relatives have usually been included in Bignoniaceae or in Scrophulariaceae s.l., being "transitional" between the two (e.g. Cronquist 1981).
LENTIBULARIACEAE Richard, nom. cons. - Back to Lamiales
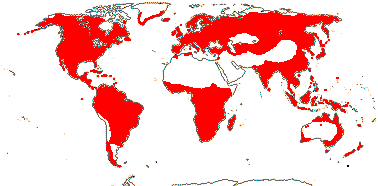
Plant herbs, rosette-forming; carnivorous [insectivorous]; plants often Al-accumulators, little oxalate; ?cork; vessel elements?; stem with endodermis; stomata also anisocytic; long-stalked gland hairs secreting mucilage, short-staled gland hairs secreting digestive enzymes, pores in cuticle of cells of gland hairs; leaves spiral, lamina margins entire; K 2, 4, 5, C quincuncial [abaxial lobe outside the others in bud - ascending cochlear], etc., with an abaxial spur, nectar produced by glandular hairs [cells of head with radial walls]; A 2 [the abaxial pair], filaments stout [always?], thecae confluent, (superposed), epidermal cells ephemeral; tapetal cells 2(-3)-nucleate; staminode 0; pollen (grains tricellular); G placentation free-central or basal, style hollow, short [up to as long as the ovary, often 0], stigma lobes broad, (sensitive), (one lobe only), wet; ovules (>1-)many/carpel, integument 2-6 cells across; (antipodal cells persisting); fruit a capsule of various types, (seeds with pedestals); exotestal cells variously thickened, elongated, and/or protruding; embryo chlorophyllous; n = 6-12+, x = 9, chromosomes 0.2-2.3µm long, nuclear genome [1 C] (0.078-)0.417(-2.231) pg / (61-)476(-1722) Mbp.
3 [list]/390. World-wide, introduced into Hawaii? Map: from Hultén (1958, 1962, 1971) and Taylor (1989). [Photo - Flower.]
Age. Crown-group Lentibulariaceae have been dated to (41-)38, 28(-25) Ma (Wikström et al. 2001), ca (54-)42, 37(-28) Ma (Bell et al. 2010), ca 40 Ma (Ibarra-Laclette et al. 2013; see also W.-Q. Xu et al. 2018, spread 55.5-24.6 Ma), ca 47 Ma (S. R. Silva et al. 2017a) and ca 58.8 Ma (Fonseca 2021).
1. Pinguicula L. —— Synonymy: Pinguiculaceae Dumortier
Verbascosides 0; primary and other roots poorly developed, root cap 0; plant (heterophyllous [forming hibernacula or winter rosettes]); lamina with adaxial surface sticky (not in all leaves); vernation circinate [P. heterophylla]; flowers single; C tube formation early; A ± free; pollen 4-10-zonocolporate, etc.; cotyledons (1); germination (hypogeal).
1/119. North temperate, but esp. Central and South America.
Genlisea + Utricularia: roots 0.
Age. The age of this clade is perhaps 39 Ma (Silva et al. 2017a).
2. Genlisea A. Sainte-Hilaire
Plant with elongated subterranean ± tubular trap-like structures [modified leaves].
1/30. Africa, Madagascar, Central and South America.
3. Utricularia L. —— Synonymy: Utriculariaceae Hoffmannsegg & Link
Plant (annual), with sensitive bladder traps; velum + [cuticle membranes covered by mucilage]; leaves (highly divided), (apparently 0); (embryo sac protrudes beyond the micropyle, haustorial); (embryo syncytia + [from cells of micropylar endosperm haustoria + adjacent nucellar tissue]); embryo often undifferentiated, (to 15 cotyledon-like structures).
1/240. World-wide.
Age. The age of crown-group Utricularia is somewhere around 31 Ma (Fleischmann et al. 2018; Jobson et al. 2018).
Evolution: Divergence & Distribution. South America and the Antipodes harbour most diversity within Utricularia, and S. R. Silva et al. (2017a) fleshed out possible scenarios for diversification following a suggested South American origin. Utricularia is also diverse in Australia, and species distributions/relationships - there are almost 50 species in northern Australia alone - seem to fit the peripheral vicariance pattern. The genus now grows mostly around the periphery of the continent after the drying out of the centre, a process that began in the Eocene (Nge et al. 2021c).
Shimai et al. (2021) emphasized the strong geographic component to species relationships in Pinguicula in trees based on analyses of nuclear data, Domínguez et al. (2024) noting that there were notable areas of endemism in Mexico (27 of the 52 Mexican species are known from the Sierra Madre Oriental) and eastern Cuba. These relationships also largely agreed both with chromosome number (x = 8 is likely to be basal, x = 11 derived) and with hibernacula and winter rosette formation (see below), but not with floral morphology. There was range contraction of temperate taxa and range expansion of tropical taxa (but not of coastal species) during the last glacial maximum (Domínguez et al. 2024).
Fleischmann et al. (2010) examined character evolution in Genlisea.
Ecology & Physiology. As befits their carnivorous proclivities, Lentibulariaceae are notably prominent in wet, acid habitats, Pinguicula, for instance, commonly growing in acid bogs. The ancestral habitat of Utricularia is terrestrial, although the fully aquatic lifestyle has evolved there more than once, some species living happily as members of the ephemeral flora of ponds on African inselbergs (Seine et al. 1996) or in bromeliad tanks (embryos may be viviparous in Utricularia living there - Plachno & Swiatek 2010a), and there are also a few rheophytes and epiphytes (S. R. Silva et al. 2017a). Tubers, apparently modified rhizomes, store water in a few American species of Utricularia (Rodrigues et al. 2017). Some species of Pinguicula are heterophyllous, the overwintering leaves being almost scale-like and protecting the plant against the cold (= hibernaculum), or almost fleshy, forming a rosette, and affording protection against dry conditions (= winter rosette), in neither case being involved in carnivory (Fleischmann 2021). In some species there is vegetative reproduction by bulbils and/or epiphyllous buds (Heslop-Harrison 2004).
Pinguicula has fly-paper traps, and the plants may smell, so perhaps attracting potential prey (Fleischmann 2016 and references; Klink et al. 2019: N and even some C uptake), and even pollen landing on the leaves may be digested (Rice 2011), According to the english summary of Titova (2012: p. 1162) the cotyledons of P. vulgaris have the "ability to flap and digest insects". Mirid bugs have been found on Pinguicula, and this may have implications for plant protection and/or nitrogen uptake (see Wheeler & Krimmel 2015 for mirids). Genlisea has long, spirally-twisted structures (= modified leaves, see below) that trap prey whose progress along the traps is guided by backwardly-pointing hairs (Martín-Roca et al. 2024), interestingly, a few species of Utricularia have passive traps rather like those of Genlisea (Westermeier et al. 2017).
How the traps of Genlisea function is being gradually clarified. Carmesin et al. (2021) looked at fluid flow in the tubular traps and the distribution, etc., of different hair types here. Fluid flowed more easily up the traps, and retrorse hairs, found along the traps, were longest at the mouth, perhaps to ensure that prey would not escape. However, Martín-Roca et al. (2024) suggest that these hairs "rectify" the movement of bacteria, etc., ensuring that they move towards the base of the traps, that is, towards the plant’s digestion vesicle. The products of digestion diffuse away from this vesicle, drawing larger microorganisms, e.g. ciliates, further inside the traps (Martín-Roca et al. 2024). Four-celled digestive glands were found in or near the digestion vesicle or chamber, but were replaced by bifid hairs of uncertain function near the mouth (Carmesin et al. 2021).
Nearly all species of Utricularia, whether growing in water or moist soil, have suction traps (for their morphology, see Merl 1915; Franck 1976; Reifenrath et al. 2006; Rutishauser 2016a; Westermeier et al. 2017). Fewer studies have beeen carried on how these traps work in terrestrial Utricularia, although Westermeier et al. (2017) is a notable exception. When water is pumped out of the bladder, the traps are under negative pressure (e.g. Adamec & Poppinga 2016). The trapdoor itself may be convex, i.e. it bulges outwards, and the entrance is short, or the trap-door forms a ± acute angle between the upper and lower sides of the entrance, and the entrance tube is long (Westermeier et al. 2017). In aquatic species, the stimulation of sensitive hairs at the mouth of the trap leads to the rapid inversion of curvature of the door (to concave) and its opening in a mere 300-700 micro seconds, water being sucked in the short mouth at a rate of around 2.7 m s-1 (Singh et al. 2011). There is also negative pressure generated by the trap walls. Some kind of velum is common in the genus - this consists of, loosely, "cuticle membranes covered by mucilage", although this rather bland description belies the complexity of the structures involved, since there may be an inner and outer velum, a threshold, a variable number of types of pavement epithelium, and the velum itself is made up of cuticle, it and the associated mucilage being produced by the glandular pavement epithelium trichomes (Plachno et al. 2019b) - so the velum is "cuticle membranes covered by mucilage from the pavement epithelium trichomes touching the surface of the door in set position and [it] also seals the trap door in resting reset state and, thus, it maintains the produced negative pressure necessary for trap firing and functioning" (ibid. p. 11). The overall result of stimulation of the trap is fast, "undeveloped" flow of incoming water - that is, there is no slowing of the flow close to the walls, as Müller et al. (2020) emphasized, comparing it with the much slower flow of the mouths of just-hatched fish which also have a suction mechanism, but which function in the intermediate or viscous-flow regime. Recovery/resetting of the trap may take up to half an hour, but is often much faster (see Westermeier et al. 2017 for details of the timing of the various phases of trap activity, videos, etc.). Jobson et al. (2004) and Laakkonen et al. (2006) suggest a possibly associated change in cytochrome c oxidase that may increase respiratory capacity so providing the energy needed for the rapid movements of the traps. Vincent et al. (2011; see also Singh et al. 2011) distinguish between a slow, energy-dependent phase in which water is pumped out of the trap, the trap becoming deformed and elastic energy being stored in the trap body, and a fast but passive phase in which the trap door opens and closes in less than a millisecond, with water and contained prey rushing inside. Bifid glands are commonest near the mouth are are involved in the expulsion of water from the trap (Adamec 2011c). Westermeier et al. (2017) describe a variety of trap morphologies (obliquity of the trapdoor, length of the entrance tube, R-Put position) and behaviours (does the trap deform before opening?) in the genus, and plot the complex variation that they describe on a tree. Spontaneous opening of traps without stimulation by prey is common (Adamec 2011a; Adamec & Poppinga 2016). Plachno et al. (2017) discuss the vascularization of the traps of Genlisea and Utricularia; it is unclear how the vascular system functions. Inside the trap are 2-armed hairs near the mouth and X- or H-shaped 4-armed hairs further in, although there are also 1- and 3-armed hairs (e.g. Taylor 1989).
What do the traps do? One thinks of small water animals swimming around, triggering the opening of the traps, getting sucked inside, and meeting their ends. Indeed, I have even seen a small ant that had somehow got trapped in a bladder - its head and thorax were inside the trap, and the animal did not seem too happy. However, the exact role of the traps in the life of the plant has been questioned. Sirová et al. (2017, see also 2018) observed that in their experience traps rarely caught anything, but all of them had microbial commensals inside them, and they found 4,500 microbial taxa in the traps and their periphyton. Indeed, the relationships between plant and algae - and potential animal prey, too - have been difficult to elucidate, and diatoms and other algae may be common in the bladders (Jobson & Morris 2001; Alkhalaf et al. 2011; Adamec 2012; Plachno et al. 2014; Klink et al. 2019). Some species of Utricularia may ingest aquatic algae, especially if the water is very acid, and algae may predominate in traps in such environments (Peroutka et al. 2008a). Nitrogen from 15N-labelled phytoplankton may move into the plant (Alkhalaf et al. 2009). Some bacteria in the traps are able to fix nitrogen, even if the high nitrogen concentration in the traps represses this activity (Sirová et al. 2014). The diverse microbial community in the traps can also aid in the uptake of phosphorus by the plant (Sirová et al. 2009). The plant may support the microbial community nutritionally when there is no prey in the traps (Adamec 2011a), carbon recently fixed by the plant ending up in the young traps and in the microorganisms there (Sirová et al. 2010). Sirová et al. (2017) described bacteria that could variously break down protein, amino acids, cellulose, etc., and methanotrophs that seemed to keep the traps free of methane. A protozoan community made up of euglenids, Tetrahymena, etc., species-poor but often with very high numbers of individuals, along with predatory bacteria (Bacteriovoraceae) kept the numbers of bacteria down. Ceschin et al. (2021) described the prey captured by Utricularia australis - they had to be the right size and moving about the right speed, and from the contents of the traps a number of chitinous arthropods had been caught, although soft-bodied organisms like rotifers and coelenterates may also have been prey, but if so and presumably being digested very quickly, that could not be confirmed. Juang et al. (2011) describe a symplastic pathway from the four cells of a quadrifid gland via the stalk, etc., to the stem. However, if there is much ammonium and dissolved organic C in the water that may be taken up directly, and carnivory, enegetically expensive, may be reduced (Kurosawa et al. 2024).
For further details of the morphology of Lentibulariaceae as it relates to their carnivorous proclivities, see Lloyd (1942) and Juniper et al. (1989) in particular; note that secretory glands throughout] the family are attached to single epidermal cells and have no contact with vessels. Peroutka et al. (2008b) discuss aspects of the functional biology of Lentibulariaceae (see also Rice 2011; Adamec 2011a, 2011b: ecophysiological perspective, and for recent developments in carnivory in general, see Hatcher et al. 2020 and Adamec et al. 2021).
Pollination & Seed Dispersal. Lustofin et al. (2020) suggest that some species of Pinguicula have non-glandular trichomes on their corollas that are eaten by their pollinators, largely bees. There are some very distinctive flower types among the 63 species of Australian Utricularia (Lowrie 2013: vol. 3). For nectar secretion and composition and pollination in Utricularia, see Plachno et al. (2019a and references; Lustofin et al. 2019); the nectar-secreting hairs are modified lamialean hairs with their typical radial anticlinal walls.
Utricularia may be myxospermous (Grubert 1974). The diversity of capsule type and especially testa morphology within Utricularia in particular is staggering (Taylor 1989).
Plant-Bacterial/Fungal Associations. Utricularia gibba has lost its endomycorrhiza-specific genes (Ibarra-Laclette et al. 2013; Delaux et al. 2014).
Vegetative Variation. Pinguicula, alone among Lentibulariaceae, has roots, if often rather poorly developed (Adlassnig et al. 2005; Whigham et al. 2008); ordinary roots do not occur in either Genlisea and Utricularia, even in the seedling (Ibarra-Laclette et al. 2013 for gene control). The distinctive overwintering leaves in some species of Pinguicula are mentioned under Ecology & Physiology above; if there is a flower coming from the middle of a winter rosette of small leaves, the plant looks very odd. The spiralling, positively geotropic passive traps of Genlisea are borne in the same phyllotactic sequence as its leaves, and the prolonged apical growth of these traps is like that of the leaves of some species of the morphologically much less problematic Pinguicula.
The vegetative morphology of some species of Utricularia in particular can be difficult to interpret in conventional terms (e.g. Arber; Rutishauser & Sattler 1989; Rutishauser 2016: summary). Here the embryo is usually undifferentiated, although some exceptions are mentioned by Plachno and Swiatek (2010a). Its early development can seem almost disorganized, with no obvious primordia evident (Kondo et al. 1978); cotyledons and radicle are not apparent, or there can be up to 15 cotyledon-like structures. Small fragments of the "leaves" or even the cut peduncle can regenerate the whole plant (Merl 1915). Chormanski and Richards (2012) describe the construction of U. gibba in detail; the plant is made up of stolons and dichotomously-branching leaf-like structures that bear the traps. These suction bladder-traps in Utricularia have no parallel in other flowering plants (see e.g. Sattler & Rutishauser 1990; Plachno & Swiatek 2010a for development), although Kaplan (1997, vol. 2: chap. 14, 17; e.g. also Lloyd 1942) suggested that the various structures bearing traps in Lentibulariaceae are all basically foliar in nature (see also Whitewoods et al. 2019). Some species of Utricularia have single traps on the ends of elongated leaf-like structures, and these may be positively geotropic (Taylor 1989, c.f. Genlisea). In U. gibba leaves are quite long and somewhat ab-/adaxially flattened, and their traps are adaxially curved structures that differ i.a. in how genes normally expressed on the adaxial and abaxial surfaces are expressed. In traps the expression of "adaxial" genes is restricted to the inside of the trap, a gene normally expressed abaxially even being expressed on the subapical-adaxial trap door and the opposing lip of the trap, and the whole structure becomes spherical because of the relative extent of expression of the ad- and abaxial genes (Whitewoods et al. 2019, c.f. Chomicki et al. 2024). K. J. I. Lee et al. (2019) suggested that a proximo-distal polarity field rather like that involved in Arabidopsis leaf development was involved in trap development, the main difference being that in Utricularia the tissue sheet was curved, not flat, and growth/cell expansion caused the various trap types, cell division simply maintaining the properties of the growing cell-wall mesh that made up the bladder (c.f. pitcher growth in Sarracenia, q.v.. Differently-shaped traps result from varying the relative growth rates along the dorsal and and ventral midlines. There are also quite commonly much more conventional leaf-like structures in Utricularia, and U. kuhlmannii (= U. trichophylla) was even described as having odd pinnate leaves by Merl (1915; see also Troll & Dietz 1953), while other taxa may have deeply cordate leaf blades, and so on. Rhizome- and tuber-like structures occur in some taxa (Rodrigues et al. 2017), while some nine different kinds of stolons have recently been described in species of Utricularia subgenus Polypompholyx (Reut & Plachno 2023).
Genes & Genomes. There was evidence of three (?two - Lan et al. 2017) rounds of genome duplications (with n initially = 6, or 8?) beyond the palaeohexaploidy event of the core eudicots and since the divergence of Solanales (Ibarra-Laclette et al. 2013).
Species of Genlisea like G. tuberosa have at 1C = 61 Mbp the smallest genomes known from angiosperms, the chromosomes of that species being bacterium-sized, only ca 0.2 µm long and so close to the resolution limit of light microscopes. However, there is substantial variation in genome size - 60Mb—1.5Gb/63.6-1722 Mbp - within the family, genomes of Pinguicula in particular being larger, although within Genlisea alone there is the whole range of variation in the family (Greilhuber et al. 2006; Fleischmann et al. 2014; S. R. Silva et al. 2017a). Interestingly, it is the diploids in Genlisea that have the larger genomes (Fleischmann et al. 2014). It has been suggested that both nitrogen availability and genome size tend to be low in carnivorous plants (Vesely et al. 2013), c.f. parasites (e.g. Orobanchaceae), but the genome sizes of carnivorous plants and their noncarnivorous relatives are similar even if genome sizes in some Lentibulariaceae may be very small (Veleba et al. 2020).
Ibarra-Laclette et al. (2013, see also 2011; Leushkin et al. 2013; Carretero-Paulet et al. 2015a, b) found that almost all the non-genic DNA in the tiny genome of U. gibba had been lost. However, gene number and overall functionality was similar to that in genomically more obese taxa, although overall gene loss and gain was very high (see also G. Sun et al. 2018: somewhat more gene families gained than lost, c.f.Cuscuta; Palfalvi et al. 2020: Droseraceae etc.). Wheeler and Carstens (2018) looked at changes in gene expression categories in G. aurea and U. gibba, finding notably more in the latter than in the former. Leushkin et al. (2013) noted that G. aurea had only 1/2-2/3 the genes of two other lamiids with which they compared it, and around two thirds of its genes in were the only members of their family, while in the three other rosids compared over 50% of the genes had two or more members per family (Leushkin et al. 2013). Indeed, G. aurea was found to have lost a notably large number of gene families (J. Zhang et al. 2020 - also Zostera). A mutation in cytochrome c oxidase (COX), a gene that may increase respiratory capacity (see above), has been suspected of being involved in the miniaturization of the genome, perhaps the consequence of an increase in reactive oxygen species also associated with the COX gene mutant, but any connection between genome miniaturization, the possible role of this gene in it, carnivory and the general evolutionary success of Lentibulariaceae is unclear (Veleba et al. 2020; Zedek et al. 2024). Indeed, some members of Genislea sect. Recurvatae that have this COX mutation also have the largest genomes known in Lentibulariaceae; these species are sister to the remainder of the genus species of which also have COX mutations (Zedek et al. 2024: Fig. 1). Tandem repeats may also have played an important role in the evolution of the U. gibba genome and of numerous genes involved in carnivory there (Lan et al. 2017). There is extensive variation in the GC content of the genome (34.0-45.1%), the lower values being found in some taxa with smaller genomes, although coding DNA, which dominates in such genomes, normally tends to have a high GC content (Veleba et al. 2014).
Genlisea and Utricularia in particular have a very high rate of nucleotide substitution in all three gene compartments (e.g. Jobson & Albert 2002; Jobson et al. 2004; S. R. Silva et al. 2018). There is a rather low number of Benchmarking Universal Single-Copy Orthologs, near-universal single-copy orthologues, here, rather similar to the situation in groups like Cuscuta (Convolvulaceae) and and Wolffia (Araceae), also lacking much in the way of conventional leaves and roots (Michael et al. 2020).
S. R. Silva et al. (2016) discuss the evolution of the plastome in Utriculariaceae. Mutation rates in the matK gene in Genlisea in particular, and also Utricularia, are about the highest in all angiosperms (K. Müller et al. 2004), and that of other genes is also high (Jobson et al. 2003). Genes in the plastid ndh gene complex (NAD(P)H-dehydrogenase) show complex patterns of presence and absence, being mostly absent from/truncated in the terrestrial members of the family examined (from all three genera), but present in the aquatic members, perhaps suggesting that the aquatic environment is more stressful for the plant (Silva et al. 2016, 2018, 2023: simple parsimony might suggest that functionality has been regained...; see also Wicke et al. 2014; Mower et al. 2014: Sabater 2021 for distinctive life styles like carnivory that might affect the photosynthetic process and result in the loss of ndh genes). Reduction in size of the small single copy portion of the genome shows a similar pattern.
For the chondrome of Utricularia reniformis, rather large, see S. R. Silva et al. (2017b, 2023: Utricularia and Genlisea).
Chemistry, Morphology, etc.. In Pinguicula a single antipodal cell may persist, enlarge, and divide (Kopczynska 1964). The integument may be multiplicative in Genlisea (see Merl 1915); testa morphology in Utricularia is very variable.
For early corolla tube formation in Utricularia, see Degtjareva and Sokoloff (2012); the stamens are initiated before the corolla. "Nutritive tissue" is described from the chalazal end of the ovule, the funicle, and the placenta, i.e., at both ends of the developing embryo, but it is not recorded from Pinguicula. In some taxa of Utricularia, at least, the embryo sac more or less escapes from the ovule and apparently takes nutrients from the placenta, and nuclei from the placenta have been found in the aggressive micropylar endosperm haustorium (Farooq 1966; Khan 1970 and references). This system is not mentioned by Fischer et al. (2004b), however, it appears that in the seeds of some species of Utricularia section Utricularia, at least, syncytia develop, and these are formed by fusion of cells from the placental nutritive tissue and the micropylar endosperm haustoria and dissolution of their walls, the endospern nuclei are very large and become amoeboid, the whole being surrounded by a cell wall with transfer cell morphology (Plachno & Swiatek 2010b; Plachno et al. 2023; see also Nothapodytes-Icacinaceae). The nuclei from the two tissues are of different constitutions and ploidy levels; such syncytia are unique in the angiosperms (Plachno & Swiatek 2010b). For vivipary and cryptocotylar germination in two Caribbean species of Pinguicula, see Temple et al. (2020).
There is additional general information in Goebel (1891, 1893), McPherson (2008: Pinguicula, 2010), the papers in Ellison and Adamec (2018), esp. Jobson et al (2018: Utricularia), Fleischmann and Roccia (2018: Pinguicula), Fleischmann (2012, 2018: Genlisea) and for a magnificent classic revision of Utricularia, see Taylor (1989), for some chemistry, see Damtoft et al. (1994), for growth and vegetative morphology, see Brugger and Rutishauser (1989), Rutishauser and Isler (2001) and Grob et al. (2007a: Pinguicula sympodial), for floral morphology see Gross et al. (2007a) and in particular Degtjareva and Sokoloff (2012), for pollen, see Rodondi et al. (2010: Pinguicula), for ovule morphology, see Plachno and Swiatek (2009), and for seeds and embryos, see Stolt (1936), Kausik (1938), Farooq (1965), Farooq and Bilquis (1966 and references), Degtjareva et al. (2004a) and Takhtajan (2013: also seedling and young plant).
Phylogeny. For the phylogeny of Lentibulariaceae, see Jobson et al. (2003), K. Müller et al. (2004, 2006b) and K. Müller and Borsch (2005a); relationships there are [Pinguicula [Utricularia + Genlisea]]. Pinguicula is on a long branch (Refulio-Rodriguez & Olmstead 2014). Similar relationships in Utricularia and Genlisea were retrieved in analyses of both plastome and chondrome data (S. R. Silva et al. 2023), so adding nuclear data will be interesting.
Cieslak et al. (2005) and Degtjareva et al. (2006a) discuss the phylogeny and evolution of Pinguicula. Shimai et al. (2021: see corrected version of Fig. 5) noted extensive incongruence between plastome and nuclear relationships, P. dertosensis and P. crenatiloba in particular differing substantially in their positions using the two kinds of data. Shimai et al. (2021) found that analyses of Pinguicula plastome data suggested that there were three main groups, while there were ten groups in nuclear analyses (four species were unplaced); the classic infrageneric classification, based on corolla morphology, did not hold up (see also Fleischmann & Roccia 2018; Fleischmann 2021). Zamora and Fleischmann (2024: ITS + plastome markers) found that sections Micranthus (P. alpina) and Nana (P. ramosa, P. variegata, P. villosa: ?ancient hybrids) in particular moved around the tree.
Fleischmann et al. (2010) looked at relationships in Genlisea.
Reut and Jobson (2010) and Jobson et al. (2017) focused on the phylogeny of Utricularia subgenus Polypompholyx in particular; this is sister to the rest of the genus (S. R. Silva et al. 2017a). For more about relationships in Utricularia, see Rodrigues et al. (2017); note that Westermeier et al. (2017) found that the relationships apparent between the subgenera in particular depended on the particular analysis being carried out. Silva et al. (2017a) examined relationships within Utricularia - 78 spp., 6 plastid loci and nuclear ITS were the weapons of choice.
Classification. Taylor's (1989) 33 sections in Utricularia subgenus Utricularia are holding up quite well, although very distinctive species like U. resupinata, U. pubescens and U. nana, all of which he placed in monotypic sections, are likely to be derived from within other sections. For the sectional classification of subgenus Polypompholyx, see Jobson et al. (2017). Fleischmann and Roccia (2018) divided Pinguicula into three subgenera and nine sections, but later, in response to the findings of Shimai et al. (2021) and also to Shimai's earlier thesis (n.v.) Fleischmann (2021) recognised three subgenera and 12 sections - section Pinguicula may be redundant, and I am not sure why P. crenatiloba ended up where it did in some studies. See also Zamora and Fleischmann (2024) for infrageneric nomenclature in Pinguicula - sections Micranthus and Nana (see above) unplaced.
Age. This node is ca 39.8 Ma (Magallón et al. 2015).
THOMANDERSIACEAE Sreemadhavan - Thomandersia Baillon - Back to Lamiales
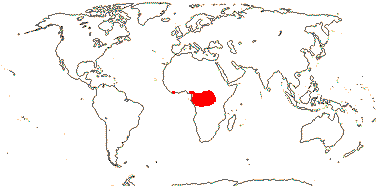
Shrub or small tree; 2-indolinone alkaloids +; phloem stratified; pericyclic fibres massively thickened, ?short; nodes 1:3; petiole bundles forming a ring or incurved C-shaped; stomata anisocytic; leaves ± heterophyllous, lamina with flat glands abaxially, (margins deeply lobed), petiole swollen at apex and base; K with nectaries on the outside; pollen 5-6-colpate; nectary vascularized by carpellary traces; gynoecial vasculature 8-shaped; ovules 1-3/carpel, hemianatropous; capsule loculicidal, K accrescent; seed with hook-shaped funicle, hilum rather large; testa with ascending-imbricate scales or warts, exotesta palisade, not lignified, (to 6 layers of cells in the warts); embryo strongly curved, cotyledons complexly folded, thin-foliaceous; n = x = ?
1 [list]/6. W. and C. Africa. Map: from Wortley et al. (2007a).
Chemistry, Morphology, etc.. The flat glands mentioned above are dark-drying and up to 3 mm across, and are quite different from the lamialean glandular hairs with their radially-segmented heads which also often occur on the abaxial surface of the lamina here.
Despite the presence of structures sometimes described as jaculators, fruit dehiscence is not explosive, unlike Acanthaceae. The seed, with its prominent hilum, sits in a thin, cup-like expansion of the funicle. Inside the seed coat described above is a layer of much crushed cells, in turn above a layer of a few less crushed cells; the outer layer of the endosperm has a distinct outer periclinal cell wall. I am not sure exactly how the cotyledons are folded.
Study of the development of the ovule, embryo, and endosperm and of seed anatomy might well be profitable.
For alkaloids, see Ngadjul et al. (1995), and for general details, see Wortley et al. (2005a and especially 2007a). Thomandersia hensii: de Wilde & Jongkind 9400, seed, stem; Ngok Bamak et al. 1263, leaf; T. laurifolia: Dibata 30, seed; Thomandersia sp.: Reitsma et al. 1819, leaf, stem.
Previous Relationships. Thomandersia was previously usually included in Acanthaceae; aside from its rather different fruits, it does not have swollen nodes, cystoliths, etc.
VERBENACEAE Jaume Saint-Hilaire, nom. cons. - Back to Lamiales
Ethereal oils +, plant often aromatic; sesquiterpene lactones, 4-carboxy-iridoids +; (pits vestured); petiole bundles arcuate (also medullary, associated with median bundle); needle crystals common; stomata diacytic, (anomocytic); stems often square; eglandular hairs unicellular; (flowers sessile); flower often rather weakly monosymmetric; space between K and C [water calyx]; (A of two lengths, but free), ± sessile [so usu. included], (filaments +), (staminode 0); tapetal cells 2-4-nucleate; pollen (colpate, por[or]ate), exine thickened near apertures; G also 1 (4), collateral, placenta on the margin of the carpel, style short [to 1/2 length of corolla tube], (long), stigma capitate (bilobed, oblique), with conspicuous stigmatoid tissue, wet; ovules 2/carpel, apotropous, integument 5-9 cells across, obturator +; (antipodal cells multinuclear); K persistent, enclosing fruit; seeds not dispersed separately; testa thin-walled; cotyledons spatulate; n = 5-12+, x = 8, nuclear genome [1 C] (0.113-)1.002(-8.876) pg.
32 [list, to tribes]/775: ten groups below. Pantropical (to warm temperate), but mostly New World, esp. S. South America. In Europe, Verbena officinalis may be native only from S. Europe and eastwards. Map: from van Steenis and van Balgooy (1966), Hultén (1971), Lebrun (1977), Meusel et al. (1978), Brummitt (2007) and Australia's Virtual Herbarium (consulted 12.2012).
Age. Crown-group Verbenaceae are some (55-)42.6(-23.4) Ma (Tank & Olmstead pers. comm.).
1. Petreeae Briquet Petrea L. —— Synonymy: Petreaceae J. Agardh
Shrubs and vines; lamina entire; flowers ± polysymmetric; K much enlarged, petal-like (not Petrea brevicalyx); G 1; fruit indehiscent, fleshy; n = 17.
1/12. Mexico to the Amazon Basin.
[Duranteae [[Casselieae + Citharexyleae] [Priveae [Neospartoneae [Verbeneae + Lantaneae]]]]]: fruit ?septicidal, two-parted.
Age. The age for this node may be at least 42 Ma (Nie et al. 2006).
2. Duranteae Bentham —— Synonymy: Durantaceae J. Agardh
Trees to herbs, (thorns +); (multiple axillary buds); eglandular hairs multicellular; (flowers sessile), (inverted - Durantha), (± polysymmetric); (A 2 + 2 staminodes); G 1 ([4]); K (accrescent in fruit); n = 17.
6/193: Stachytarpheta (120), Duranta (31), Chascanum (27). S. U.S.A. to Argentina, (Africa to India - Chascanum).
[[Casselieae + Citharexyleae] [Priveae [Neospartoneae [Verbeneae + Lantaneae]]]]: ?
[Casselieae + Citharexyleae]: ?
3. Casselieae Troncoso
Inflorescences axillary; (staminode 0); (G 1 [adaxial carpel]).
3/14: Casselia (6). Mexico and the Caribbean to Argentina.
4. Citharexyleae Briquet
Shrubs to trees; stomata anomocytic; (axillary (branched) thorns +); extrafloral nectaries +; (cryptic dioecy +); pedicels often short; staminode +/0; (G 1); fruit drupe; seeds 2/pyrene; n = 38.
2/63: Citharexylum (60). S. U.S.A. to Argentina, Caribbean. Photo Flower.
[Priveae [Neospartoneae [Verbeneae + Lantaneae]]]: flowers ± sessile; stigma bilobed (oblique, capitate).
5. Priveae Briquet
?
2/21 Priva (20). Pantropical-warm Temperate.
[Neospartoneae [Verbeneae + Lantaneae]]: flowers sessile.
6. Neospartoneae Olmstead & N. O'Leary
Ephedroid shrubs [leaves reduced, stem photosynthetic] (not); plant glabrous; inflorescences axillary (terminal) (inflorescences 0); flowers sessile; C tube curved (not); (staminode 0); stigma (unlobed); fruit unicarpellate, (fleshy - Neosparton); n = ?
3/6. Argentina, also Bolivia, Chile, S. to Patagonia.
[Verbeneae + Lantaneae]: staminode 0.
Age. An estimate of the age of this node is (40-)30, 29(-18) Ma (Bell et al. 2010); another is (31-)28, 20(-17) Ma (Wikström et al. 2001).
7. Verbeneae Dumortier
(Iridoids from deoxyloganic acid); lamina margin often serrate; (inflorescence unbranched); (A 5 - Verbena, 2, staminodes 0); fruits also septicidal [four pyrenes]; ksuspensor many-celled uniseriate; n = 5, 7, 10.
3/195: Glandularia (88), Verbena (57), Junellia (38). Mostly American, also Eurasia to Africa.
8. Lantaneae Endlicher —— Synonymy: Lantanaceae Martynov
Stomata anisocytic; inflorescence often ± capitate (rarely terminal); flowers sessile; K (4), 2, 0; C usu. 4-lobed, w/"unique upper lobe"; (adaxial 2A with long glandular connective appendages); G 1 ([2] - Coelocarpum), 2-locular, "style short"[?], stigma entire; (ovule 1/carpel - Lantana); (endosperm + - Lantana); n = 11.
11/297: Lippia (140), Lantana (100); Aloysia (36). Mostly New World, also Africa and Madagascar.
Evolution: Divergence & Distribution. The family appears to be of tropical South American origin (Olmstead 2013). A number of species grow in arid conditions in North America, and some have arrived from South America, but in various ways, while others (Citharexylum) seem to have come from mesic habitats in Central America (Frost et al. 2017).
O'Leary et al. (2012; Thode et al. 2013: characters useful below tribal level) reconstructed the evolution of characters and fruit. Both the tritomy [Lantaneae + Dipyrena + Verbeneae] and the uncertainty where Rhaphithamnus and Coelocarpum will end up on the tree affect our understanding of character evolution.
Ecology & Physiology. Junellia and other Verbeneae andAloysia (Lantaneae) often are dominants in communities in arid habitats in South America (Frost et al. 2017).
Pollination Biology & Seed Dispersal. Lu-Irving and Olmstead (2013) estimated that fleshy fruits had been derived from dry fruits at least five times in Lantaneae alone.
Plant-Animal Interactions. Gall-forming fruit flies of the Tephretidae-Tephrellini are found here (and on Acanthaceae and Lamiaceae: Korneyev 2005).
Genes & Genomes. A genome duplication has been reported in the Verbena-Glandularia area (Yuan et al. 2010b).
Economic Importance. Lantana camara is one of the most invasive naturalised plants (Pysek et al. 2017).
Chemistry, Morphology, etc.. Atkins (2004) listed a number of genera, all in Lantaneae that had essential oils. However, Josh Grosse (pers. comm. v.2022) pointed out that such oils - monoterpenes, sesquiterpenes - occur elsewhere in the family (e.g. El-Hela et al. 2009; Martino et al. 2009; El Ayeb-Zakhama et al. 2017; Pérez Zamora et al. 2018), and it is unclear where, if anywhere, this feature (now placed at the family level) might be an apomnorphy. For spines versus thorns versus prickles in Duranta, see Moroni and O'Leary (2020: Fig. 4B) and O'Leary et al. (2021: Figs 2, 3, spiny structure subtending leaf?).
0'leary et al. (2023) suggested that Petraea (and Nashia) have polysymmetric flowers (Jabbour et al. 2008); development of corolla lobes is variable in Lantaneae (O'Leary et al. 2023a)'. An endothelium is only poorly developed (Johri et al. 1992). For the position of the carpels, see Sattler (1973). The ovules are described as being attached to the margins of the carpel (Junell 1934). Both chambers of two-chambered mericarps or stones may contain an ovule/seed from each carpel (Sanders 2001), and indehiscent fruits may be fleshy (O'Leary et al. 2012), overall, the variation in the gynoecium (1-carpellate in many Lantaneae) and fruit is considerable (O'Leary et al. 2023a, esp. Fig. 5). Pericarp anatomy is complex (Ryding 1995). The testa of at least some Verbenaceae has the hypodermal layer(s) thickened (Rohwer 1994a).
For general information, see El-Gazzar and Watson (1970), Sanders (2001), Atkins (2004), Brummitt (2007) and O'Leary et al. (2023b: Lantaneae), for iridoids, see von Poser et al. (1997 - also Soltis et al. 2005b), for hairs and stomata, see Cantino (1990), for leaf teeth, sometimes hydathodal, see Rios et al. (2020), for pollen, see Raj (1983), for the megagametophyte, see Rudall and Clark (1992), for the suspensor, see Nagl (1962) and for exine thickening, see Chadwell et al. (1992).
Phylogeny. Marx and Olmstead (2007) found that Petraea and Duranta, both woody, were successively sister to the rest of the family. Marx et al. (2010) presented a comprehensive phylogeny of the family, although, as they noted, sampling within the big genera needed to be improved. A couple of genera have remained unplaced: Dipyrena may be close to Verbeneae while Rhaphithamnus may be close to Priveae, although branches within the latter are rather long (see also Cardoso et al. 2020). TYhe position within Lantaneae of Coelocarpum, morphologically plesiomorphic, was also uncertain (Marx et al. 2010). The topology above was also recovered by Yuan et al. (2010b), but the position of Rhaphithamnus was unclear, Dipyrena, however, was consistently sister to the [Verbeneae + Lantaneae] clade, although in Thode et al. (2013) it was sister to Verbeneae.
Citharexyleae. Frost et al. (2021) found that the Mexican Citharexylum altirameum was sister to the rest of the genus, which fell into two clades: A well-supported Mexican-Central American clade, and a poorly-supported largely South American clade. Neospartoneae. Relationships here are [Neosparton [Diostea + Lampayo (M. Lu et al. 2019). Verbeneae. For relationships around Verbena, see Yuan and Olmstead (2008), however, chloroplast and nuclear markers give substantially different topologies (Frost et al. 2017). Within the Lantana-Lippia complex, Aloysia formed a basal grade and the animal-dispersed Lantana with its pyrene-type fruits was polyphyletic (Lu-Irving et al. 2009, esp. 2014; Lu-Irving & Olmstead 2013; Lu-Irving et al. 2021: 7 nuclear loci).
Classification. For the circumscription of the family, see especially Cantino (1992a, b), and for a tribal classification, see Marx et al. (2010) and Cardoso et al. (2020). Marx et al. (2010) divided the family into eight tribes, but two genera, Dipyrena and Rhaphithamnus, were unplaced.
The whole Lantana-Lippia complex (= Lantaneae), speciose although it may be, could perhaps be reduced to a single genus, the larger genera within it that are currently recognised being para- or polyphyletic (Lu-Irving et al. 2009, see also 2014). On the other hand, Marx et al. (2010) suggested that nine genera could be recognised, but whatever Linnaean-type taxonomic solution is adopted major generic adjustments in this area will be needed. Earlier taxonomists had used fruit characteristics to delimit genera, but fruit evolution has turned out to be highly homoplasious (Lu-Irving & Olmstead 2013). Lu-Irving et al. (2021) revisited this problem, noting that the type species of Lantana and Lippia were in small clades close to one another, necessitating major taxonomic changes in any Linnaean-type system... O'Leary et al. (2023a) provide a Phylocode-take on the whole clade, their /Lantaneae; they used 28 clade names, and considered the recognition of a single large genus not an option (see also O'Leary 2023b: two new mnotypic genera...). The basic (Linnean) structure of Lantaneae is [Aloysia [4 small genera forming a paraphyletic complex [mostly Lippia [two clades, mostly Lippia basal, Lantana embedded]]]] (O'Leary et al. 2023a).
Elsewhere, the limits of genera around Junellia have been redrawn (O'Leary et al. 2009); for a revision of Junellia in the old sense, see Peralta et al. (2008). Frost et al. (2021) recognized three subgenera and six sections within Citharexylum.
Previous Relationships. Verbenaceae have often been considered very close to Lamiaceae, q.v. for differences. For Avicennia, also once included in Verbenaceae, see Acanthaceae; Phryma (Phrymaceae) is also separate; see below.
[Lamiaceae [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]] / the LMPO group: ?
Age. The crown-group age of this clade is about 40.3 Ma (Magallón et al. 2015), (62.4-)55.7, 52.1(-45) Ma (Cusimano & Wicke 2016), (72.6-)59.4(-47.4) Ma (W.-Q. Xu et al. 2018), ca 63.9 Ma (Fonseca 2021) or (78.9-)74.4(-69.8) Ma (Rose et al. 2022).
LAMIACEAE Martynov, nom. cons. / LABIATAE Jussieu, nom. cons. et nom alt. - Back to Lamiales
Herbs (trees, vines); diterpenoids, sequiterpene lactones, betaines, C4-decarboxylated iridoids +, (neo-clerodane diterpenoids +), (β-hydroxy-(3,4-dihyroxyphenyl)-ethanoid glycoside +); cork also deep-seated; (pits vestured); (nodes 1:2, leaving central stele two nodes before departure to leaf - ?level); petiole bundles arcuate (annular); stomata diacytic (anomocytic); stem often square; (eglandular hairs unicellular; stellate); lamina vernation variable, margins toothed; inflorescence branches cymose; staminode 0 (+); tapetal cells multinucleate; pollen 3-colpate, exine not thickened near apertures, orbicules 0; style (unequally) bifid, stigma inconspicuous, not expanded, dry (wet); ovules 2/carpel, borne on inner side of carpel margin, apotropous, subbasal, ascending, integument 5-9 cells across; fruit a schizocarp, nutlets 4; K persistent; testa usu. thin, exotestal cells elongated or not, thickened on radial and often inner walls, (hypodermal cells sclerenchymatous); x = 8 (?9), nuclear genome [1 C] (0.143-)0.892(-5.571) pg.
236 [list: to tribes]/7,280 - 12 groups below. World-wide. Map: from Vester (1940), Hultén (1971), Van Balgooy (1975), Lebrun (1979) and Frankenberg and Klaus (1980). Photos: Collection, Fleshy fruit.
Age. Crown-group Lamiaceae are estimated to be (71.3-)68.4(-65.6) Ma (Rose et al. 2022) or ca 59 Ma (Fonseca 2021).
The age of a [Viticoideae [Ajugoideae [Prostantheroideae [Nepetoideae [Scutellarioideae + Lamioideae]]]]] clade may be (54.6-)43.1(-28.3) Ma (Tank & Olmstead pers. comm.: note topology), The suggested age of an [Ajugoideae [Prostantheroideae [Nepetoideae [Scutellarioideae + Lamioideae]]] clade is (30-)28, 17(-15) Ma (Wikström et al. 2001) or ca 23.8 Ma (Salmaki et al. 2016), again, c.f. topology.
[Prostantheroideae + Callicarpoideae]: ?
The age of this clade is (70.3-)63.8(-55.8) Ma (Rose et al. 2022).
1. Prostantheroideae Luersson
(Plant aromatic); (leaves small - microphyllous); (nectary ± 0); endosperm +; n = ?
18/317. Australia.
A (2; staminodes 2), (anthers with appendages); G 4-lobed; genome duplication [?level].
5/>210: Prostanthera (100), Hemigenia (50). Australia.
Age. Westringeae are ca 36.6 Ma (Godden et al. 2019).
Indumentum usu, tomentose, hairs complex, dendritic; (flowers (-10-merous, polysymmetric; G unlobed; fruit dry, indehiscent, 1(-2)-seeded.
13/100: Pityrodia (45). Australia.
2. Callicarpoideae Bo Li & Olmstead - Callicarpa L.
Shrubs to trees (lianes); hairs branched/stellate (simple, hooked); inflorescences axillary; (plant dioecious); flowers polysymmetric, 4(-5)-merous; (A 5-7), (anthers porose); G incompletely 2-locular, stigma peltate or capitate; fruit a drupe, with 4 1-seeded pyrenes; endosperm 0; n = 8, 9.
1/170. Mainly tropical to subtropical.
Age. This clade is ca 68.1 Ma (Godden et al. 2019).
There are fossils from the Deccan Traps (K-P boundary) that show similarities with Vitex (Viticoideae) and Gmelina (Premnoideae) (Wheeler et al. 2017).
[Viticoideae + Symphorematoideae]: nectary 0; G without a false septum; endosperm 0.
Age. The age of this clade is some (61.5-)46.3(-29.4) Ma (Rose et al. 2022).
3. Viticoideae Briquet —— Synonymy: Viticaceae Jussieu
Often woody; chlorogenic acid +, verbascoside 0, (protocatechuic acid, a-tocopherolhydroquione +); (hairs branched); leaves palmately compound (unifoliate); flowers ± poly- or bisymmetric; nectary (+, poorly developed); fruit a drupe, (1-seeded), (capsular); n = 6, 8, ... 16, 17.
3/283: Vitex (250). Tropical (subtropical).
Age. The age of crown-group Viticoideae is (26.3-)16.7(-8.3) Ma (Rose et al. 2022).
4. Symphorematoideae Briquet —— Synonymy: Symphoremataceae Wight
Lianes; ?chemistry; hairs branched; inflorescences capitate, of 3-7-flowered cymes, involucrate [involucre ?= bracteoles]; flowers polysymmetric, 5-16-merous, (monosymmetric, 5-merous - Congea), ± sessile; G incompletely 2-locular; ovules apical, pendulous, straight, funicle 0; embryo sac ± on surface of ovule; fruit dry or subdrupaceous, (capsular); seeds 1(-2); n = 12, 14, 17, 18.
3/30: Sphenodesme (15), Congea (12). India, Sri Lanka, South East Asia, West Malesia.
Age. This clade is (61.3-)50.3(-39.9) Ma (W.-Q. Xu et al. 2018), or, with [Viticoideae + Symphorematoideae] above Nepetoideae, (70.3-)66.2(-62.8) Ma (Rose et al. 2022).
5. Nepetoideae (Dumortier) Luersson
Plant usu. herb; commonly aromatic [volatile terpenoids], rosmarinic acid and nepetoidin A and B [all caffeic acid esters] +, verbascoside 0; (distinctive seed fatty acids) +, betaine concentration low, iridoid glycosides 0 (+); stem endodermis +; flowers monosymmetric; K with epidermal prismatic calcium oxalate crystals; pollen tricellular, hexacolpate; style gynobasic; exocarp with mucilaginous cells producing hygroscopic spiral fibrils [i.e. myxospermy]; endosperm development highly asymmetric, the two haustoria lying close to each other, 1-layered [0?], cotyledons investing rest of embryo; genome duplication +; frequently attacked by Puccinia menthae.
105/3,675. World-wide, but esp. (warm) temperate.
Age. Diversification in the Nepetoideae is estimated to have begun (63.7-)57.6, 52.3(-42.3) Ma (Drew & Systma 2012a), ca 63.4 Ma (P. Li et al. 2017), ca 46.7 Ma, i.e. [Lavandula + Thymus] (Godden et al. 2019) or (59.7-)55.3(-51.3) Ma (Rose et al. 2022).
5A. Mentheae Dumortier —— Synonymy: Glechomaceae Martynov, Melissaceae Berchtold & J. Presl, Menthaceae Burnett, Monardaceae Döll, Nepetaceae Berchtold & J. Presl, Salviaceae Berchtold & J. Presl, Saturejaceae Döll
K 11-nerved, C distinctly 2-lipped (weakly so); disc symmetric (asymmetric and anterior lobe elongate; nutlets with an areolate abscission scar; n = >6.
60?/>2,100.
Age. Crown-group Mentheae are estimated to be (52.6-)45.8(-38.9) Ma (Rose et al. 2022), while the clade [Prunella + Salvia] is ca 41.4 Ma (P. Li et al. 2017).
Lycopinae - Lycopus L.
1/21. Temperate Northern Hemisphere, Australia.
MenthinaeStem endodermis with Casparian strip [Mentha].
16/: Thymus (220), Clinopodium (100), Micromeria (55), Hedeoma (40), Origanum (40), Satureja (38).
Prunellinae
3/15: Prunella (13, inc. 5 hybrids). Mostly Europe, the Near East, North Africa, also Japan, P. vulgaris throughout northern hemisphere.
Salviinae
(A 2, abaxial pair, unithecate, connective forming long lever arm - Salvia); nutlets mucilaginous/not.
3/1,100: Salvia (1,049), Lepechinia (47). Northern Hemisphere (to West Malesia), the Americas.
Nepetineae
(flowers resupinate); K 15-nerved, C strongly bilabiate; A adaxial pair longer than abaxial; disc 4-lobed or horned; stigma lobes subulate.
12/: Nepeta (200+), Clinopodium (100), Dracocephalum (70), Lophanthus (40)
Age. Crown group Nepetinae are ca 38.4 Ma (Moazzeni et al. 2025: large error bars).
Age. This node is ca 61.8 Ma (P. Li et al. 2017) or (59.2-)53.0(-47.4) Ma (Rose et al. 2022).
5B. Elsholtzieae Burnett
(Plant woody); (inflorescence spike, raceme or umbel); disc asymmetric, lower lobe elongated; n = (7)8(9)10...
7/75: Elsholtzia (43). Central Asia to India, W. Malesia and Japan, E. North America (Collinsonia).
Age. Crown-group Elsholtzieae are ca 50.1 Ma (P. Li et al. 2017). (49.1-)39.0(-28.5) Ma (Rose et al. 2022) or ca 41.8 Ma (Jin et al. 2025).
5C. Ocimeae Dumortier
(Plant tuberous), (annual); (CAM photosynthesis +); A declinate, anthers synthecous, dorsifixed, (C lip concave-compressed, pollen release explosive).
43/>1,235: Coleus (294), Hyptis (280), Isodon (140), Plectranthus (84), Ocimum (65), Syncolostemon (49), Platostoma (45), Aeollanthus (40), Lavandula (39). Mostly tropics and subtropics.
Age. The age of crown-group Ocimeae ia some (52.8-)42.2(-31.0) Ma (Rose et al. 2022). Crown-group [Lavandula + Hyptis] is ca 48.2 Ma (P. Li et al. 2017).
Age. The age of this node is some (67.6-)62.8(-58.1) Ma (Rose et al. 2022).
6. Tectonoideae Bo Li & Olmstead - Tectona L. f.
Large trees, deciduous; iridoids ?0; hairs stellate; inflorescences terminal and/or axillary; flowers polysymmetric, 5-7-merous; C infundibular, tube short; style terminal; fruit a drupe, ± surrounded by inflated calyx, stone 4-celled, with central cavity; endosperm 0; n = 12, 18.
1/4. India, Southeast Asia.
[Premnoideae [Ajugoideae [Peronematoideae [Scutellarioideae [Cymarioideae + Lamioideae]]]]]: ?
Age. Ca (66.0-)60.9(-55.8) Ma (Rose et al. 2022: inc. Premnoideae) is an estimated age for this clade.
7. Premnoideae Bo Li, Olmstead & Cantino
Shrubs to trees, (lianes), (geoxylic), aromatic; (leaves palmately compound); (flowers polysymmetric); (A 2+2 staminodes); (pollen 4-5-colpate - Cornutia); nectary 0 (+ - Cornutia); fruit a drupe, stone 4-celled, 4-seeded (1-seeded); endosperm +/0; n = 19.
3/ca 150: Premna (50-200), Gmelina (35). Palaeotropical to West Pacific, few Mexico to north South America, the Antilles.
[Ajugoideae [Peronematoideae [Scutellarioideae [Cymarioideae + Lamioideae]]]]: ?
Age. W.-Q. Xu et al. (2018) suggested an age of ca 43.5 Ma for this clade.
8. Ajugoideae Kosteletzky —— Synonymy: Aegiphilaceae Rafinesque, Ajugaceae Döll, Siphonanthaceae Rafinesque
Annual herbs to shrubs, (aromatic); (nodes 1:2); flowers (4-merous - Aegiphila), 1-lipped [0:5], (abaxial member toothed), (polysymmetric); pollen grains with supratectal spines to verrucae, tectum perforate or not, exine with branched (simple, granular, etc.) columellae; nectary slight-0 (+); (antipodal cells numerous); nutlets reticulate, (fruit a drupe, K coloured, accrescent); endosperm several-layered/0, cotyledons investing embryo [?common]; n = 7, 10, ?12, 13-16+.
23/770 (1,115?). Cosmopolitan, esp. South East Asia to Australia.
Age. Crown-group Ajugoideae are (61.8-)55.2(-48.0) Ma (Rose et al. 2022); the clade [Teu. Clero. Ajug.] is very approximately 16.3. Ma (Salmaki et al. 2016).
8A. Rotheceae C.-L. Xiang, Bo Li & Olmstead
Herbs to shrubs; ovary unlobed in flower, becoming imperfectly 4-lobed, style terminal; drupes (2-)4-lobed, mesocarp ± fleshy, endocarp separates into 4 stones or 2 pairs of stones.
4/83: Rotheca (60). Subsaharan Africa, tropical southern Asia to Malesia and Australia (Queensland).
[Teucrieae [Ajugeae + Clerodendreae]]: ?
8B. Teucrieae Dumortier
anther thecae confluent at anthesis.
3/260: Teucrium (250). ±Cosmopolitan.
8C. Ajugeae Bentham
anther thecae confluent at anthesis (not in Trichostema.
6/79: Ajuga (50), Trichostema (17). Eurasia, Trichostema Noarth America.
8D. Clerodendreae Briquet
Woody (liane, monocaul); petiole bundle arcuate or (much interrupted-)annular, (wing bundles +); flowers (polysymmetrical); A 5, 4, 2 + 2 staminodes; fruit drupaceous, with 4 1-seeded pyrenes, (a schizocarp, with 4 fleshy 1-seeded units); germination hypogeal [Clerodendrum].
9/450: Clerodendrum (250), Aegiphila (150), Oxera (37). Pantropical/subtropical.
[Peronematoideae [Scutellarioideae [Cymarioideae + Lamioideae]]]: ?
Age. This clade is some (61.7-)55.6(-49.3) Ma (Rose et al. 2022)
9. Peronematoideae Bo Li, Olmstead & Cantino
Shrubs to large trees (lianes); (verbascoside 0); (leaves pinnate/(bi)ternately compound); (bracts, etc., petal-like), flowers (± polysymmetric); C white to yellow; (A 2); nectary 0 or slight; G unlobed; fruit dry, indehiscent or with 2 or 4 nutlets, abscission scar as long as mericarp, K usu. accrescent (not - Peronema); endosperm 0; n = ?
4/17: Petraeovitex (8). India to southern China, Malesia and Melanesia.
Age. Crown-group Peronematoideae are around (58.2-)46.7(-32.1) Ma (Rose et al. 2022)
Age. Estimates of the age of this clade are ca 33.9 Ma (W.-Q. Xu et al. 2018) and (57.6-)51.0(-44.2) Ma (Rose et al. 2022)
10. Scutellarioideae (Dumortier) Caruel —— Synonymy: Salazariaceae F. A. Barkley, Scutellariaceae Döll
Annual herbs to shrubs, (aromatic); (leaves spiral); thyrses with single-flowered cymes, inflorescences raceme-like (cymose inflorescences - Tinnea and Holmskioldia); K strongly two-lipped, (rotate, coloured - Holmskioldia), lobes rounded, xylem fibres abundant; (C 0:5); (style ± terminal - Wenchengia); pericarp with tuberculate or elongate processes; seeds tuberculate; endosperm various; n = 12+.
5/382: Scutellaria (360-?500). ± Cosmopolitan.
Age. Estimates of the age of this clade are ca 33.9 Ma (W.-Q. Xu et al. 2018) and (57.6-)51.0(-44.2) Ma (Rose et al. 2022: no Wengchengia)
[Cymarioideae + Lamioideae]: endosperm +.
Age. Estimates of the age of this clade are (52.0-)45.1(-38.2) Ma (Rose et al. 2022).
11. Cymarioideae Bo Li, Olmstead & Cantino
Shrubs to subshrubs; ?chemistry; cymes long-pedunculate, branches monochasial; anther thecae divaricate, becoming confluent; nectary 0; abscission scar ca 1/2 length of mericarp; n = ?
2/3: Cymaria (2). Hainan to Malesia.
12. Lamioideae Harley —— Synonymy: Mellitidaceae Martynov, Stachydaceae Döll
Plant (aromatic), often herbaceous, (annual); laballenic fatty acid and related compounds [l. acid = CH3(CH2)10CH=C=CH(CH2)3COOH], 3,4-dihydroxyphenylethanoid glycosides and related compounds; (calyx mesophyll with narrow prismatic calcium oxalate crystals); , (anther thecae divaricate, becoming confluent); embryo sac with micropylar lobe longer and broader than chalazal lobe; nutlets hardly reticulate; endosperm several-layered, embryo spatulate; n = 6+.
62/1260. Esp. Europe and Africa to Asia, some cosmopolitan, but v. few Antipodean.
Age. Diversification in crown-group Lamioideae is estimated to have begun (26.1-)23.9(-19.9) Ma (Roy & Lindqvist 2015: both analyses, see below), (47.6-)41.0(-34.4) Ma (Rose et al. 2022) or ca 44.1 Ma (Y. Chen & Wang 2024).
12A. Pogostemoneae Briquet
Shrubs to subshrubs; A about the same length; nutlets dull, glandular/hairy/glabrous, shiny.
11/161: Pogostemon (80), Anisomeles (26), Achyrospermum (25). East to Southeast Asia, Malesia, few N.W. Australia and tropical Africa.
12B. Gomphostemmateae Scheen & Lindqvist
pollen with branched columellae; mesocarp with fibres.
2/54: Gomphostemma (36). India and China to West Malesia.
12C. Colquhounieae C. L. Xiang, Bo Li & Olmstead - Colquhounia Wallich
Shrubs; corolla 1:3; nutlets winged at apex.
1/5. Himalayas, inc. Nepal, N. India, S.W. China, to Thailand.
12D. Synandreae Rafinesque
Inflorescence spike-like, flowers sessile or very shortly pedicellate; filaments villous.
5/19: Physostegia (12). North America.
[Stachydeae [Galeopseae + Betoniceae]]: ?
12E. Stachydeae Dumortier
K lobes often spiny, throat often hairy; A with basalr pair bending outwards after pollination; nutlet apex usually rounded
12/470: Stachys (ca 400).
12F. Galeopseae (Dumortier) Visiani - Galeopsis L.
Annual herbs; C lower lip with two basal conical protuberances; anthers dehiscing by two valves, the upper fimbriate; x = 8.
1/10. Temperate Eurasia, esp. Europe.
12G. Betoniceae Bendiksby & Salmaki - Betonica L.
Leaves deeply crenate dentate; inflorescence unbranched, ±condensed; flowers sessile, two conical protuberancesbracteoles broadly based, spinescent; x = 8.
1/10. Western Eurasia.
[Paraphlomideae [Phlomideae [Leonureae [Roylea [Marrubieae [Lamieae + Leucadeae]]]]]]: ?
12H. Paraphlomideae Bendiksby
C 1/3, hairy upper lip, scarcely bearded along the margin; A included; G apically truncate.
3/31: Paraphlomis (25). China, Japan, some S. E. Asia to West Malesia (inc. Sulawesi).
[Phlomideae [Leonureae [Roylea [Marrubieae [Lamieae + Leucadeae]]]]]: ?
12I. Phlomideae Mathiesen
Herbs (root tuberous) to shrubs; (leaves spiral), (lamina pinnatisect); K lobes abruptly narrowed to narrow apex, C margins expanded, bearded, with dense branched hairs outside.
2/230: Phlomoides (150-170), Phlomis (50-90). Circum-Mediterranean to China.
[Leonureae [Roylea [Marrubieae [Lamieae + Leucadeae]]]]: ?
12J. Leonureae Dumortier
("Spines" in leaf axils); lamina with palmate venation and lobing/pinnatisect; K apex spiny; A included in C tube [not Loxocalyx]; ovule with glandular hairs [Leonurus].
6/80: Lagochilus (45). Eurasia, esp. Central Asia.
Age. The crown-group age of Leonureae is ca 23.7 Ma (Y. Chen & Wang 2024).
[Roylea [Marrubieae [Lamieae + Leucadeae]]: ?
12K. Roylea cinerea (D. Don) Baillon
1/1: Western Himalayas.
[Marrubieae [Lamieae + Leucadeae]]: ?
12L. Marrubieae Visiani
K widely campanulate to rotate, secondary K lobes common; A included or shortly exserted.
5/91: Marrubium (50), Pseudodictamnus (28). Macaronesia, Europe to Asia (1 sp. W. China), North Africa.
12M. Lamieae Cosson & Germain de Saint-Pierre
Plants annual to rhizomatous/stoloniferous perennial; (plant glabrous); (lamina subreniform, lobed, with hydathodes - Petrolamium); flowers ± sessile (pedicellate - Pet.); anthers exserted (included), hairy (not); nutlet triangular, apex subtruncate or truncate, (elaiosome +); n = 8, 9, (polyploid).
5/39: Lamium (25). Temperate and subtropical Old World, inc. North Africa.
12N. Leucadeae Scheen & Ryding
K distinctly monosymmetric, with secondary lobes, C with upper lip margin bearded.
6/134: Leucas (ca 100). Africa, Asia inc. China to Malesia, some in Australia (Queensland) andstevens islands of Pacific and Indian oceans.
Evolution: Divergence & Distribution. The oldest fossils identified as Lamiaceae are ca 28.4 Ma (Martínez-Millán 2010).
Crown Lamiaceae were perhaps Australian—S.E. Asian in origin, with clades like Callicarpoideae and Prostantheroideae Australian; several other subfamilies are S.E. Asian, although Nepetoideae, for example, may be S.W. Asian in origin (Rose et al. 2022). Although details of the subfamilial and tribal phylogeny are still suspect (see below), one can perhaps begin to get an idea of character variation in the family...
Nepetoideae include ca 2,300 species, about a third of the family, and this may be connected with the numerous genome duplications identified within it (Godden et al. 2019) - or not.
Diversification within Mentheae, a tribe that includes Salvia (Salviinae), a genus that has around 1,050 species (González-Gallegos et al. 2020, for more details see the end of this section), is estimated to have begun ca 46 Ma, perhaps in the Europe/Mediterranean area (Drew & Systma 2012a). Menthinae are probably West Asian/Mediterranean in origin but are diverse in the New World, moving there perhaps via the North Atlantic land bridge ca 10 Ma and with subsequent dispersal events onwards to South America; there has been adoption of hummming birds as pollinators in more arid areas in particular (Drew et al. 2017b).
- There was some diversification within Elsholtzieae ca 43-41 Ma (5 clades then), but there was only one more clade 10 Ma later, indeed, the clades resulting from this early burst had stems of 8-49 Ma - the latter is the age of the stem of the [Ombrocharis + Perillula] clade (P. Li et al. 2017). However, the pattern of diversification in Jin et al. (2025) is rather different, with a crown of ca 41.8 Ma, ca 7 clades evident by 26.6 Ma, and with subsequent diversification proceeding apace - although the stem of the [Ombrocharis + Perillula] clade is ca 31 Ma. Jin et al. (2025) suggested that diversification here was connected with the uplift of the Hengduan Mountains and the Himalayas and associated with intensification of the monsoon; they also discussed the extensive evolution of the inflorescence in this tribe.
- The major division within Clerodendron follows geography - Asia-Australian vs Africa-Madagascar (Satthaphorn et al. 2023).
- There has been quite a bit of work on Isodon (Ocimeae), common in the deep dry valleys of southwest China. Diversification here occurred around (19.4-)13.6(-10.4) Ma, and it is associated with, but probably not triggered by, the evolution of the shrubby habit in the genus and changes in the monsoon; these deep valleys are in the rainshadows of the surrounding mountaains. Speciation/net diversification rates increased as the intensity of the East Asian monsoon decreased, especially around 5-3 Ma, dcreasing after then (Y.-P. Chen et al. 2025); X.-Q. Yu et al. (2014) also drew attention to climatic and geological events and diversification here.
For divergence times and biogeographic scenarios in Lamioideae, see Roy and Lindqvist (2015). The ca 60 species of Lamiaceae endemic to Hawai'i represent a major radiation there. They are currently placed in three separate genera, Stenogyne, Haplostachys and Phyllostegia, and they are all polyploids with fleshy fruits. They are derived from within Stachys and they may be the descendants of a hybrid between temperate North American and Meso/South America taxa (Lindqvist & Albert 2002). They probably represent but a single introduction to the islands that has been dated to (7.4-)5(-3.6) Ma (Lindqvist & Albert 2002; Lindqvist et al. 2003; Roy et al. 2013, esp. 2015 and references; Welch et al. 2016; Lim & Marshall 2017; see also the silversword alliance (hybridization involved here, too), Cyrtandra, Cyanea and relatives, Myrtaceae, see Diversity and Distribution for Metrosideros, early stages, and Schiedea, etc., for other major Hawaiian clades. Sideritis subgenus Marrubiastrum has diversified extensively in Macaronesia within the last ca 4.2-3.3 Ma (S.-C. Kim et al. 2008). Indeed, Lamiaceae has a relatively high proportion of single-island endemics on oceanic islands on which they are found (Lenzner et al. 2017).
Zhong et al. (2017) showed that radial symmetry in the corolla of Callicarpa and in two cases in Menthoideae involved different expression patterns of the genes. For a useful discussion of apomorphies within Lamiaceae, see B. Li et al. (2016), while Junell (1934) noted that the distinctive placentation in Symphorematoideae could easily be derived from the placentation found in many Viticoideae. However, the positions of some subfamilies, etc., on the tree remain unclear because of problems with character state delimitation, phylogeny (further complicated by the findings of the Mint Evolutionary Genomics Consortium 2018), and/or variation within the terminals. Rose et al. (2022) look at the evolution of stamen number, herbaceousness and fruit type in the family. Although Nepetoideae express genes involved in iridoid biosynthesis, iridoids are largely absent from the subfamily, and those that are present, e.g. nepetalactone in Nepeta, are distinctive, i.a. being volatile - there are glycosylated iridoids in the rest of the family (see also Lichmann et al. 2020). The Mint Evolutionary Genomics Consortium (2018) discuss many other aspects of the evolution of the diversity of secondary chemistry, particularly terpenoid synthesis, in Lamiaceae.
Thinking about Salvia (Nepetoideae-Mentheae-Salvineae) in particular and its some 1,050 species, the limits of clades within the genus are becoming clearer (e.g. see Drew et al. 2017a; G.-X. Hu et al. 2018; Kriebel et al. 2019; Moein et al. 2021; Rose et al. 2021); the genus is particularly speciose in the New World. With a possible origin in southwest Asia, there have been several independent movements to Africa and to the New World and elsewhere in the Old World, and the genus probably began diversifying somewhat over 30 Ma in the earlier part of the Oligocene (Will & Claßssen-Bockhoff 2014, 2017; Moein et al. 2021, q.v. for ages or other clades) or (34.1-)27.8(-22.2) Ma (G.-X. Hu et al. 2018). Kriebel et al. (2019) estimate a similar crown-group age of ca 32-31 Ma, diversification beginning in southwest Asia in the western part of the Irano-Turanian area, rather than the Mediterranean (for the latter, see e.g. Will & Claßssen-Bockhoff 2017), and there have been two shifts to the New World. Fragoso-Martínez et al. (2017) suggested that Salvia> subgenus Calosphace moved some 12 times from Mexico-Central America, its home, into South America, and perhaps once from the Andes to the Antilles. Despite all this moving around, fruits in the genus do not have particularly distinctive dispersal mechanisms (Zona 2017). Pollination in Salvia has been much studied - see Pollination & Seed Dispersal below.
Ecology & Physiology. A number of Lamiaceae are gypsophiles, growing on soils high in gypsum, hydrous calcium sulphate (Escudero et al. 2014; Palacio et al. 2014). Prostantheroideae are an Australian radiation of often shrubby and small-leaved plants growing in dry conditions.
Pollination Biology & Seed Dispersal. Pollination in Salvia. Pollination in the large, primarily New World-Mediterranean genus Salvia has been much studied, birds and large insects, bees, being the main pollinators (e.g. Claßen-Bockhoff et al. 2003: early summary, 2004b). Small clades of Salvia are sister to the three major clades that make up the bulk of the genus, each including species with lever arms - subgenus Calosphace and the S. officinalis and S. glutinosa clades (Drew et al. 2017). There have been perhaps four main bursts of diversification, none obviously associated with either biome or pollinator shifts (Kriebel et al. 2020). The corolla tube varies from 4-51 mm long in Salvia, the ancestral length perhaps being 15-18 mm long (Moein et al. 2021). Species of Salvia often have only two unithecate anthers, while in the genus as a whole the connective is expanded and forms a distinctive pivoting lever arm which, when one end is hit by the pollinator, makes the other end, with its single theca, pivot and come down on the head, back or side of the pollinator (for androecial development, see Claßen-Bockhoff et al. 2004a); overall, there are over a dozen variants of this lever-arm pollination mechanism (G.-X. Hu et al. 2018 and references), and the biomechanics of the process have been studied in some detil. Little force is needed to make the system work, and variation in how it functions can lead to pollinator specifity (Speck et al. 2003). A lever arm is perhaps ancestral for the genus, or it may have evolved three times or so in both the Old and New Worlds via a bithecate condition with the thecae at opposite ends of an extended connective (see Walker & Systma 2007), but its evolution seems not to have affected diversification in any simple way (Moein et al. 2021). However, no other angiosperm has such a lever-arm pollination mechanism (Walker & Sytsma 2007; Drew et al. 2017a). Connective teeth, outgrowths of the lever and perhaps ancestral in subgenus Calosphace, are associated with the lever mechanism, indeed, a variety of barriers that more or less close the flower entrance have evolved here, and their arrangement is such that the bee's head, pushing past them, lowers the pollen sacs (Kriebel et al. 2022). A number of taxa have a stylar brush that is involved in secondary pollen presentation (Kriebel et al. 2022). G.-X. Hu et al. (2018) looked at pollination in the Old World subgenus Glutinaria, noting that in some taxa with a much-modified androecium there was passive unidirectional movement of flower parts. Overall, New and Old World bee flowers in the genus have rather different morphologies, the former perhaps being derived from bird-pollinated flowers (Kriebel et al. 2020; c.f. Thomson & Wilson 2008). There is more on pollination in Salvia below.Reith et al. (2007) described details of pollination by bees in Salvia pratensis, while Celep et al. (2020) looked at pollination of 12 species of Turkish Salvia. Note, however, that a number of Salvia spp. are pollinated at least sometimes by "secondary pollinators", pollinators other than those that would be expected from the morphology of the flower, even if the flowers are often quite specialized. Such secondary pollinators are perhaps particularly active in those species that have "mixed" floral phenotypes, i.e., the flowers do not obviously "fit" one particular floral syndrome, as was observed in S. mexicana var. minor (Tavera et al. 2025), although the situation gets complicated here, with different pollinators being recorded at different localities, furthermore, is one looking at a stable intermediate phenotype or a transition between different pollinator morphologies? Note that there can also be different pollination ecotypes within the one species, e.g. in S. stachydifolia (Izquierdo et al. 2023). Indeed, if one distinguishes between pollinator efficiency, as measured by the number of seeds that result from a single visit of a pollinator to a plant, and pollinator effectiveness, how many seeds overall result from vists of a particular species of pollinator to a plant, the most efficient polinator may not be the most effective pollnator - as in S. lavanduloides, where the most efficient pollinator is a wasp and the most effective pollinator is a bee, a far more frequent visitor (Tavera et al. 2025).
Wester and Claßen-Bockhoff (2006, 2007, 2011) and Barreto et al. (2024) focus on pollination by birds. There are over 300 bird-pollinated species of Salvia, and these are largely restricted to the New World; flowers with an ornithophilous syndrome neverthless include a considerable amount of variation (Wester & Claßen-Bockhoff 2011; Tavera et al. 2025). . Kriebel et al. (2020, 2021) and others have noted the distinctive morphology of the flowers pollinated by hummingbirds in the New World: The corolla is weakly bilabiate, the style and anther connective are more or less straight, the connective arms being largely fused and very close to the corolla (i.e. the lever arm as such has been lost), the upper stigmatic lobe is larger and there is a stigma brush, the latter perhaps involved in secondary pollen presentation (see also Wester & Claßen-Bockoff 2006b, 2007, 2011). A number of species of subgenus Calosphace have blue flowers but are pollinated by hummingbirds; here the corolla tube tends to be long and there is no landing platform or honey guide, so shape seems to be more important than colour (Wester et al. 2020); interestingly, colour changes here can be mediated by the pH of the cell - if the pH is less than 7 the anthocyanidins are red, if it is greater than 7 they are blue (Wester et al. 2020; c.f. Hydrangea).
The adoption of hummingbirds as pollinators in the large New World subgenus Calosphace is perhaps an exception to the quite common absence of an association between diversification and pollinator shifts, although even here things are not that simple. In the New World ca 180 or more species of subgenus Calosphace are pollinated by hummingbirds (Kriebel et al. 2020, 2021; Wester et al. 2020); this burst of diversification within subgenus Calosphace - at 550-580 species it includes over half the genus - occurred in Mexico 17.8-14.1 Ma. Hummingbird pollination evolved from a bee-pollinated type of flower, melittophily being plesiomorphic in the subgenus, and a number of floral changes are associated with this shift - straight anther connectives and style, fusion of the adjacent connective arms (which may be very close to the corolla tube), upper lobe of the stigma larger than lower lobe, presence of a stigma brush, changes in corolla tube and lobing (e.g. Walker & Systma 2007; Kriebel et al. 2020, 2021). Stem and crown group ages for this clade are ca 22 and 20 Ma respectively, and these ages are similar to the crown age of hummingbirds, ca 22 Ma (Kriebel et al. 2019; McGuire et al. 2014), while bumblebees arrived in the Neotropics some 15 Ma (Hines 2008). Interestingly, species of subgenus Calosphace with these distinctive bird-pollinated flowers are in a clade sister to a clade with bird- and bee-pollinated flowers that do not show these changes and is also not very diverse (Kriebel et al. 2020).
Details of the evolution of floral morphology in subgenus Calospace depend on the author. Thus Kriebel et al. (2019) suggested that there had been 9-11 shifts from bee to hummingbird pollination, perhaps 53-58 changes from bird to bee pollination (this is normally a rather unusual direction), and then a few shifts back to birds. Fragoso-Martinéz et al. (2017) estimated there had been ca 17 shifts to bird pollination, and one back to bees. On the other hand, Kriebel et al. (2019) suggested that there had been only a single origin of bird pollination, some 56(!) shifts back to bees (this latter is normally an unusual direction - J. D. Thomson & Wilson 2008), and a few from bees back to birds. Kriebel et al. (2021) proposed that bird preferences had driven the changes in floral morphology noted above, and in species that reverted to bee pollination features like stigma asymmetry and the stigma brush were retained - evolutionary constraint, perhaps, although such features have indeed on occasion been lost and even regained. Wester and Claßen-Bockhoff (2007) suggested that heterobathmy best described the evolution of these floral features, but Kriebel et al. (2021) thought that some degree of synchrony in evolution might be more likely. See also Gesneriaceae-Gesnerioideae which are also commonly pollinated by hummingbirds, and the association there, beginning ca 22.4 (Roalson & Roberts 2016) or (25.5-)18.5(-5) (Serrano-Serrano et al. 2017) Ma, is also quite old, as is that in Heliconiaceae in particular, are ca 87 or 32-28 Ma, (depends on the method used - Kress & Specht 2006), ca 32 Ma (McKenna & Farrell 2006), or (47-)39(-32) Ma (Iles et al. 2016), while that in Bromeliaceae is ca 14 Ma (Givnish et al. 2014a), Bird Pollination in the little-known Neotropical Ericaceae-Vaccinieae may be another example. For more on hummingbird pollination in general, see elsewhere.
The common ancestor of all unithecate clades had two more "ordinary" stamens (see also Himmelbaur & Stibal: 1932-1934: early account, stem Salvia gave rise to Monardeae and Meriandeae as well; Walker et al. 2015; Drew et al. 2017a). Taxa like Melissa and Lepechinia, both with 4 stamens, are at the base of that part of the tree that includes Salvia, and there are other taxa with two stamens immediately below the clades containing Salvia (Walker & Sytsma 2007), a number of other Mentheae, mostly New World, also having two stamens (Drew & Sytsma 2012a). Heterostyly is known from Salvia, e.g. S. brandegeei (Cohen 2019). For more on Salvia, see J. D. Thomson and Wilson (2008), Internat. J. Plant Sci. 181(8). 2020.
Wilson et al. (2017) looked at pollination in Prostanthera (Prostantheroideae), which have a variety of pollinators. The anthers may have appendages functionally rather similar to the lever arms of Salvia. Pollination in Nepetoideae-Ocimeae-Hyptidinae is often explosive, the resupinate stamens being held inside the concave-compressed lip and being explosively released when triggered by the pollinator (Pastore et al. 2021). Bee pollination is common, and in low Mediterranean shrublands, megachilid bees in particular are important pollinators of Lamiaceae (Petanidou & Ellis 1996), butterflies, etc.. are also involved (e.g. Kumar 2024 and literature). Pseudanthia are reported - Congea, Symphorema (Baczynski & Claßen-Bockhoff 2023: Table 1).
Drew and Sytsma (2012b) discussed the evolution of dioecy within the New World Lepechinia; it seems to have evolved at least three times there.
The calyx is an integral part of the dispersal mechanism of the propagules, whether being brightly coloured and helping to attract frugivores, as in Clerodendrum, having hooked hairs or being itself hooked (Priva and some species of Salvia respectively), or forming a kind of catapult mechanism (Scutellaria) or a wing. Various kinds of calcium oxalate crystals are found in the sepals, perhaps protecting the nutlets against insect predators (Ryding 2010b). Myxocarpy, the nutlets producing mucilage and so adhering to their disperser or anchoring the nutlet in the ground, is common in Nepetoideae (Pammel 1892; Ryding 1992, 2001; Western 2011; Ferreira et al. 2020; see also X. Yang et al. 2012); moreover, sand sticking to the nutlets may prevent their being eaten, granivores being fastidious about dirty food (Western 2011 and references). Genera like Lamium (Lamioideae) and Teucrium (Ajugoideae) have myrmecochorous nutlets (Lengyel et al. 2010). while myxospermy is common in Nepetoideae, including some species of Salvia (see Zona 2017 for dispersal mechanisms here - quite a variety).
Plant-Animal Interactions. The leaf beetle Phyllobrotica (Chrysomelidae) eats plants from Scutellarioideae, Lamioideae and Viticoideae, but not members of Nepetoideae - or Verbenaceae (Farrell & Mitter 1990). Larvae eat the roots, adults the above-ground parts, and they can decimate the plants. Gall-forming midges of the Tephretidae-Tephrellini are found on Lamiaceae (and on Acanthaceae and Verbenaceae: Korneyev 2005), as are gall-forming wasps of the Cynipidae-Cynipinae (Redfern 2011) and agromyzid dipteran leaf miners (Winkler et al. 2009). Turnip sawfly larvae (a tenthredine, Athalia rosae) sequester neo-clerodane diterpenoids, and those sequestering individuals help protect the whole group of larvae against carnivory (P. Singh et al. 2022). Clerodanes are notably widespread in Lamiaceae, there are some in Asteraceae, scattered elsewhere - there are none in other Lamiales, for example; those from Scutellaria, etc., act as insect antifeedants, and some are fungicidal (R.-T. Li et al. 2016). Ceutorhynchinae seed weevils are quite commonly found on Lamiaceae; the weevils have moved on to the family perhaps twice, and there have been movements on to other hosts (Letsch et al. 2018).
The distinctive volatile nepetalactone iridoids in Nepeta are effective against herbivores. They are also the basis of catnip, which in a number of felids elicits distinctive playful behaviour - perhaps a pheromone mimic there (Lichman et al. 2020).
Although the monoterpenoid 1-8-cineole, antimicrobial and an insecticide, is apparently a major component of the essential oil of the very successful Salvia (Pichersky & Raguso 2018), this is a somewhat isolated factoid at present...
A number of Lamiaceae are quite densely and viscidly hairy (Glas et al. 2012). Nevertheless, mirid bugs - often sap-suckers, but some are carnivores - of subtribe Dicyphini in particular are able to walk easily in such conditions (Wheeler & Krimmel 2015; LoPresti et al. 2015), and nitrogen from the excreta of the bugs may be taken up by the leaf (Spomer 1999).
Plant-Bacterial/Fungal Associations. Thymol, the essential oil 2-isopropyl-5-methylphenol and known from some Nepetoideae, at least, not only is bactericidal but it also seems to encourage nodulation of legume seedlings, which has implications for communty development (McKenna et al. 2013).
Genes & Genomes. See Gill et al. (1983) for chromosome numbers of woody Lamiaceae; for chromosome numbers in Teucrium, see Salmaki et al. (2016). Godden et al. (2019) looked at gene/genome duplications in the family, which are numerous. There were 8, perhaps up to 15, genome duplications in Nepetoideae alone, including along its stem, and also at various levels elsewhere in the family, and they have been added to the character hierarchy where possible; the duplications are dated (Godden et al. 2019).
F. Zhao et al. (2020) discuss plastome variation in Lamiaceae with a focus on Scutellaria; as with a number of other taxa the mitochondrial genome shows little important variation, although it can be useful in phylogenetic analyses.
Mitochondrial genomes of Ajuga have a very much increased rate of nucleotide substitution (A. Zhu et al. 2014; Zwonitzer et al. 2024: see also characters), see also Pelargonium, Plantago, Silene, etc..
Economic Importance. Chia, Salvia hispanica, has nutritious seeds very high in alpha-linolenic acid; it was a major crop in the Aztec empire (Ayerza & Coates 2005).
Chemistry, Morphology, etc.. Trisaccharide esters of verbascoside are found in Lamiaceae alone, and disaccharides are also found there, as well as in Verbenaceae, Oleaceae and Orobanchaceae in particular (Mølgaard & Ravn 1988). Clerodane diterpenoids are scattered throughout the family, and although I have seen no records from Prostantheroideae, there are some from Callicarpoideae, and those Verbenaceae in which it had been found are now in Lamiaceae; there are no records from other Lamiales (R.-T. Li et al. 2016); a possible apomorphy for the family. For the distinctive allenic fatty acids, see Aitzetmüller et al. (1997). Some Labiatae have tanning compounds, "Labiatengerbstoffe". Rosmarinic acid, a polyphenol ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, seems to be restricted to Nepetoideae, and although it is scattered in land plants, it is common elsewhere only in Boraginales (Petersen et al. 2009). For the chemistry of Lamiaceae, see also Barbero and Maffei (2017) and the Mint Evolutionary Genomics Consortium (2018).
Bailey (1956) and Balfour and Philipson (1962: variant of an Ascarina-type node) noted that the vegetative nodes of Lamiaceae and "Verbenaceae" were basically one gap, two trace, the latter finding that in Coleus blumei small bundles departed from these paired bundles and then fused, the fused bundle running between the larger bundles for the two internodes between origin of the foliar bundles and their departure into the petiole. Marsden and Bailey (1955) described 1:2 nodes in Clerodendron trichotomum in considerable detail, but the distribution of such nodes needs to be clarified. Species of Lamioideae and Scutellarioideae, but not Nepetoideae, tend to have relatively massive amounts of fibrous tissue associated with the veins in the calyx (Ryding 2007, 2010b).
Naghiloo et al. (2014b) discuss floral development in four genera of Nepetoideae. They noted substantial variation in patterns of initiation of corolla and androecium in particular, degree of gynobasy of the style, corolla aestivation, etc., and although all showed late corolla tube development, how early in development monosymmetry became evident varied considerably (Salvia - very early; Nepeta - late). There is further discussion of other asterids with polymerous flowers like those of Symphorematoideae elsewhere (see euasterids).
The pollen grains of at least some Lamiaceae become very much flattened as they dry out (Halbritter & Hesse 2004). The ovules are described as being attached (just) to the false septa (Junell 1934); there is variation in ovule attachment within the family. Many Lamiaceae have a single layer of sclerenchymatous, bone-shaped cells on the inside of the mesocarp, others have thicker pericarp walls, and the cells are often crystalliferous (Ryding 1995). The exotestal cells of the seed are thickened, particularly on their inner periclinal and anticlinal walls (Rohwer 1994a). Some Lamiaceae have asymmetric development of the endosperm such that the two haustoria come to lie very close to each other (Ram & Wadhi 1964 for references). This distinctive development may be restricted to Nepetoideae (further studies are needed), but it is also to be found in many Acanthaceae. Wunderlich (1967b) suggests that there is no endosperm in mature seeds of Nepetoideae.
For a comprehensive treatment of Lamiaceae, see Harley et al. (2004) and F. Zhao et al. (2021: subfamilies and tribes), and there is also much information in El-Gazzar and Watson (1970), although the groups there may have little to do with those recognized here - thus Verbenaceae and Stilbaceae are included. For phenolics, see Pedersen (2000: more to add), fatty acids in the seed, see Badami and Patil (1981), for betaine distribution, see Blunden et al. (1996: widespread, but Verbenaceae, other Lamiales?), for rosmarinic acid, see Petersen and Simmonds (2003) and Petersen et al. (2009), for flavonoids, see Frezza et al. (2021), for secondary metabolite evolution, see Grayer et al (2003) and Wink (2003), for hairs and stomata, see Cantino (1990), for leaf anatomy in Mentheae, see Moon et al. (2009a) and petiole anatomy in Clerodendreae, see Barrabé et al. (2015), for some floral development, see Mair (1977), Endress (1999) and Naghiloo et al. (2014a), for pollen of ex-Verbenaceae, see Raj (1983), for that of Clerodendrum, see X. Huang et al. (2023), for ovules, which have a vascular supply, see Guignard (1893), for gynoecial morphology and embryology, see Junell (1934), for seedlings, see Vassilczenko (1947: cotyledons in Lamiaceae s. str. usu. cordate to hastate), for pollen, ovules and seeds, see Wunderlich (1967b), for the megagametophyte, see Rudall and Clark (1992), for nutlet micromorphology, see Moon et al. (2009b: Mentheae) and especially Ryding (2010a and references), for nutlets in Stachys, see Salmaki et al. (2009), and for proteinaceous inclusions in the nucleus, see Speta (1979). Moon et al. (2008a, b) surveyed pollen morphology especially of Salviinae and other Mentheae.
Phylogeny. Bootstrap support for the family was early found to be good - 100% (Wagstaff et al. 1998). However, major relationships within the family still remain in part unclear. Bendiksby et al. (2011) recovered the relationships [Callicarpa [Prostantheroideae [[Symphorematoideae + Viticoideae] [[Premnoideae, Gmelina, Tectona] [Nepetoideae [Garrettia (= Peronematoideae below) [Scutellarioideae + Lamioideae]]]]]]], mostly with strong support, although sampling was mediocre and analyses of individual markers gave different topologies. These relationships differ little from those in the treatment above (see B. Li et al. 2016; c.f. Bramley et al. 2009). Relationships along the spine of the family are largely unresolved in the two-gene analysis of Y.-P. Chen et al. (2016), indeed, a [Nepetoideae [Scutellarioideae + Lamioideae]] clade was not recovered, while in the study of Chinese taxa by Z.-D. Chen et al. (2016) there was some support for the relationships [Nepetoideae [Callicarpa [Viticoideae [Tectona [Premna + The Rest]]]]]. In the five-gene (chloroplast) anaysis of B. Li et al. (2016) overall resolution was considerably improved although individual genes might provide little support. I have perhaps been overly cautious, and relationships may end up being [[Prostantheroideae + Callicarpa] [[Viticoideae + Symphorematoideae], Nepetoideae [Tectona [Premnoideae, Ajugoideae [Peronematoideae [Scutellarioideae [Cymarioideae + Lamioideae]]]]]]] (B. Li et al. 2016: Fig. 1). However, in analyses of the foliar transcriptomes of 48 species from 12 major clades in the family (Mint Evolutionary Genomics Consortium 2018; see also Godden et al. 2019) the relationships [[Prostantheroideae + Callicarpoideae] [Nepetoideae [Tectonoideae [[Viticoideae + Symphorematoideae] [Premnoideae (paraphyletic), Scutellarioideae [Peronematoideae [Ajugoideae + Lamioideae]]]]]]] were commonly obtained, so there is clearly more to do. Indeed, W.-Q. Xu (2018), looking at chloroplast genomes of Lamiales, included 18 members of Lamiaceae; these belonged to six subfamilies, and their relationships were largely as shown above, although there was a very weakly supported [Premnoideae + Tectonoideae] clade. F. Zhao et al. (2021) examined the plastomes of 79 genera in all subfamilies, while Rose et al. (2022) carried out quite extensive analyses of the family and its relatives using 4 or 5 plastome genes (depending on the analysis), Rose et al. (2022) ultimately returning to the issue of differences in the topologies obtained using plastome and nuclear genes (see esp. their Fig. 6). B. Li et al. (2016 and references) discuss previous ideas of relationships within the subfamilies in some detail; see also F. Zhao et al. (2021). In the plastome analysis of Xiu et al. (2024: focus on Vitex) relationships are very much in the tree above, but there reltionships in the Nepetoideae area differ somewhat. It will be interesting to see what future well-sampled nuclear analyses show.
For the phylogeny of the Australian-centred Chloantheae (Prostantheroideae), see Conn et al. (2009); Brachysola is sister to the rest of the tribe. For relationships in Prostanthera itself, with the classical (Bentham!) morphologically-circumscribed infrageneric taxa not standing up too well, see Wilson et al. (2012).
For relationships in Viticoideae, see Bramley et al. (2009); Vitex itself is paraphyletic. Nakashima et al. (2016) looked at relationships in Bornean Callicarpa with a focus on species thought to be myrmecophilous.
Within Nepetoideae, basal relationships were explored by Y.-P. Chen et al. (2016) in the course of their placement of Ombrocharis.
Mentheae. Moon et al. (2010) and Drew and Systma (2012a) began to circumscribe major clades within this huge tribe. Mentheae-Salviinae include the large New World-centred Salvia, with well over 900 species. Rosmarinus and some other mostly quite small genera are also involved (Walker et al. 2004, but c.f. Walker & Sytsma 2007; Moon et al. 2010; Drew et al. 2017a; Kriebel et al. 2019), so alas for "Scarborough Fair". Jenks et al. (2013) looked at relationships within the speciose (500-600 species) New World Salvia subg. Calosphace and found that many sections were not monophyletic, relationships following geography rather than morphology (there is substantial geographical signal in the Salvia area - see also Will & Claßssen-Bockhoff 2017) - hence much parallel evolution. The study by Fragoso-Martínez et al. (2017) extended that by Jenks et al. (2013); they found that only 12/42 of Carl Epling's sections for which they had data were monophyletic, and of these monophyly of 7 was apparent only in nuclear ribosomal analyses. Olvera-Mendoza et al. (2020: plastome and nuclear ribosomal cistron) in a small study that centred on Mexican sections found four sections to be embedded in section Scorodonieae (subgenus Calosphace). Lara-Cabrera et al. (2021), looking at 96 nuclear genes of 72 species, found 8/13 of Epling's sections to be monophyletic, although some were represented by only two species; there was little conflict with plastome trees (114 loci). G.-X. Hu et al. (2018) examined relationships in East Asian Salvia which they found to be monophyletic, but there was a certain amount of conflict between chloroplast and nuclear data; Moein et al. (2021) looked at relationships in the whole genus, although the focus was on Iranian taxa; they recovered the same major groupings as earlier workers, and found little chloroplast/nuclear conflict. Rose et al. (2021) looked at 114 nuclear loci from 179 terminals from across the genus; the backbone/subgeneric topology that they recovered was quite well supported and largely in agreement with plastome trees. At the same time, there was at times quite extensive gene tree discordance, and Rose et al. (2021) discussed this in the context of hybridization/introgression and incomplete lineage sorting.
Major changes in our ideas of relationships within Mentheae-Menthinae (for which, see Drew et al. 2017b) and in the limits of the subtribe are needed; species of Clinopodium, for example, are scattered through much of the tree (Bräuchler et al. 2010; Drew & Sytsma 2011; Drew et al. 2017b; Fonseca 2021).
Mentheae-Nepetinae are monophyletic, although Nepeta itself is polyphyletic (Serpooshan et al. 2018). Moazzeni et al. (2025: ITS, ETS, 3 chloroplast markers) focused on relationships in the Lophanthus area, Lophanthus s.l. being monophyletic, although within in species that used to be in Hymenocrater move around depending on the analysis and species represented by several accessions tend not to be monophyletric. Only some 10% the species of Nepeta were included; the genus was monophyletic in analyses of plastid data and polyphyletic when using nuclear data. For relationships in Dracocephalum and relatives, primarily temperate Eurasian, see Y.-P. Chen et al. (2022b: nuclear ITS and ETS plus 4 chloroplast markers; see also H. Liu et al. 2024); they found that Dracocephalum was paraphyletic and includied both Lallemantia and Hyssopus, however, support for some nodes along the backbone of the tree could be stronger. For Micromeria, from the Canary Islands, see Puppo et al. (2015). Drew and Sytsma (2011, 2012b) explored the limits and relationships of Lepechinia.
Nepetoideae also include the large tribe Ocimeae (see Paton et al. 2004 for relationships) which in turn include Hyptineae and the speciose genus Hyptis; the other genera of Hyptinae are embedded in a paraphyletic Hyptis (Pastore et al. 2011), however, there was little resolution along the backbone of the tree, so clade limits are unclear. Sampling of Hyptinae was improved by Pastore et al. (2021), but backbone support remained weak. The focus of Y.-P. Chen et al. (2022a: 80/105 species) was mostly on plastome relationships in Isodon, the data being analyzed in twelve ways. They concluded "Phylogenetic relationships within Clade IV [over 4/5ths of the genus] are much better resolved in the plastid tree than that in previous studies, but highly incongruent with the nrDNA tree and morphology and distribution" (ibid. p. 13) - hybridization/plastome capture had taken place, and more extensive analyses of the nuclear genome were in order. Chen et al. (2025: transcriptome sequencing, 7,204 orthologous genes) returned to the problem, sampling 126/140 species of Isodon; the relationships they obtained largely had good support - there were four main clades, two very small, one medium-sized, and one very large. Isodon also has two species in Africa, and their relationships - and how they might have got there - were clarified by X.-Q. Yu et al. (2014).
In Elsholtzieae [Perillula + Ombrocharis] (both monotypic; congeneric?) are sister to the rest of the tribe and Elsholtzia is polyphyletic (P. Li et al. 2017). Problems with relationships here were confirmed by Y. Wang et al. (2024: 99 samples, 503 nulear genomes + plastomes), who found that neither Elsholtzia nor Keiskea were monophyletic, and that there seemed to have been - they fingered the normal suspects, hybridization and ILS. Probable hybridization was confirmed by Jin et al. (2025: 56 spp., 279 orthologous nuclear loci) - relationships in the bofy of the tree are [Elsholzia [Elsholzia [Collinsonia [Keiskea (inc. Perilla frutescens) + Mosla]]]].
Generic relationships in Ajugoideae were examined by B. Li et al. (2016), who found that a small clade made up of Karomia, Rotheca, Discretitheca and Glossocarya was sister to the rest of the subfamily (= Rotheceae). C.-L. Xiang et al. (2018) looked at relationships here; species sampling was slight, but there was a fair bit of phylogenetic structure. The limits of Teucrium were slightly expanded by Salmaki et al. (2016).
Clerodendreae: Steane et al. (1999, 2004; see also Yuan et al. 2010a) looked at the relationships around the para/polyphyletic Clerodendrum; Satthaphorn et al. (2023: 75 spp., 337 loci from the Angiosperms 353 dataset) found the genus to be largely monophyletic apart from a couple of species to be placed in Volkameria. Barrabé et al. (2015) re-evaluated the limits of Oxera.
Scutellarioideae. Wenchengia has spiral leaves and a more or less terminal style, and it was initially unclear where it should be placed (Cantino & Abu-Asab 1993). However, a position sister to all other Scutellarioideae is strongly supported (B. Li et al. 2012) or, rather poorly supported, after Hymenopyramis (Z.-D. Chen et al. 2016).
Scutellarieae. Relationships in Scutellaria itself are still poorly understood, but even focusing on Iranian species alone, subgenus Scutellaria is strongly para-/polyphyletic as is subgenus Apeltanthus section Lupulinaria (Seyedipour et al. 2017, see also Safikhani et al. 2018; F. Zhao et al. 2020: plastome analyses). Salimov et al. (2021) found three main clades within the genus, but working out an infrageneric classification is premature.
Wagstaff et al. (1995) discussed phylogenetic relationships in Lamioideae. Scheen et al. (2010) found that Cymaria might be sister to the rest of the subfamily and Bendiksby et al. (2011) added Acrymia to Cymaria, although support for the sister group position of the combined clade was low, while Y.-P. Chen et al. (2014) preferred to exclude the two from the subfamily on morphological grounds (see also B. Li et al. 2016), although they included the odd genus Holocheila (see also Z.-D. Chen et al. 2016: Chinese taxa). A poorly-supported [Acrymia + Cymaria] clade was sister to the rest of the subfamily and Holocheila was in a strongly-supported Pogostemoneae in the chloroplast analyses of Roy and Lindqvist (2015). These odd genera were not included in their analyses using the nuclear pentatricopeptide repeat, and although many tribes in the two analyses were recovered in both, there were differences in their immediate relationships, and some tribes, like Pogostemoneae themselves, were paraphyletic in the second analysis, even if both the clades in which they appeared there had substantial morphological support (Roy & Lindqvist 2015).
Lamieae. Surina et al. (2025: plastid data, confirmed by nuclear analyses) looked at relationships in Lamioideae, but with a focus on Lamieae, and found that Petrolamium, recently discovered in the Dinaric karst country of southern Montenegro, was sister to the rest of the tribe.
Leonureae. Y. Chen and Wang (2024) discuss relationships here; Lagopsis and Leonurus appear not to be monophyletic.
Marrubieae. Siadati et al. (2018) clarify relationships in this small tribe.
Stachydeae. For relationships of the ca 60 species of lamioid mints endemic to Hawai'i, see Lindqvist and Albert (2002) and Lindqvist et al. (2003); recognition of the three genera in which they are placed makes Stachys paraphyletic (see also Roy & Lindqvist 2012; Roy et al. 2013), but this aside, the limits of Stachys are difficult to determine (see also Scheen et al. 2010; Bendiksby et al. 2011), and there may have been ancient hybridization here (Salmaki et al. (2013). Salmaki et al. (2019) confirmed that Stachys was strongly paraphyletic, but that twelve well supported clades could be recognized within Stachydeae. Leucas is also highly paraphyletic (e.g. Scheen & Albert 2009).
Phlomideae. Relationships in this tribe have been evaluated by Mathiesen et al. (2011) and Salmaki et al. (2012); relationships/infrageneric classifications in the Phlomis-Phlomoides area seem to be something of a mess - hybridization there, too (Y. Zhao et al. 2023, 2024). Zhao et al. (2024: 84 species, 9 plastid and 2 nuclear markers) looked at relationships within Phlomopsis. Here plastid data gave more resolution, but these data had far more variable sites; there was some conflict between the data sets. Six main clades were recovered, and of the eight morphological characters whose variation was mapped on to the plastid tree, five more or less correlated with major groupings (including one with Phlomis - Zhao et al. 2024).
Pogostemoneae. Pogostemon is common in southwest China, and there are also a few species in Africa (Yao et al. 2016).
Synandreae. See Scheen et al. (2007) for relationships around Physostegia.
Classification. The subfamilial classification here is based on that of B. Li et al. (2016) and Li and Omstead (2017); see Cantino and Sanders (1986) for the distinctions between the two biggest subfamilies, Lamioideae and Nepetoideae. Note that the circumscription of Viticoideae is more narrowly drawn than in Cantino et al. (1992). For tribal, etc., limits, see also Harley et al. (2004), for those in Lamioideae, see Scheen et al. (2010) and Bendiksby et al. (2011), while F. Zhao et al. (2021) discuss the limits of the 12 subfamilies and 22 tribes in the family, three of which are newly described (based on a plastome tree); they also recognize 3 subtribes in Nepetoideae-Ocimeae and five in -Mentheae.
In general, generic limits in Lamiaceae need attention (e.g. Kadereit 2016). In both Stachys and Leucas characters associated with pollination prove unreliable indicators of clades (Scheen et al. 2010). The former genus in particular may have to be considerably expanded or pulverised (Salmaki et al. 2013, 2019), and although there seem to be a number of sizeable clades there, they are not easy to recognize (F. Zhao et al. 2021). Clerodendrum has been dismembered (Steane & Mabberley 1998; Yuan et al. 2010a); Harley and Pastore (2012) reworked generic limits in Hyptidinae (see also Pastore et al. 2021 for discussion about generic limits here; some have suggested a Hyptidinae = Hyptis). Paton et al. (2018) reconfigured genera around Coleus/Plectranthus (Plectranthinae), the genera they recognized being more or less readily identifiable. How Salvia is to be treated presents a challenge; it seemed that perhaps Rosmarinus, Thymus, Mentha, and Origanum would have to be included (Walker et al. 2004, 2006; Walker & Sytsma 2007). Some of these genera turned out to have been misplaced, and a monophyletic somewhat expanded Salvia has now been attained with minimum pain - just fifteen new combinations (Drew et al. 2017a); this circumscription is followed above. (Note that Will et al. (2015) and Will and Claßssen-Bockhoff (2017) had proposed the dismemberment of Salvia s.l. into seven or more genera - hundreds of combinations would have been needed if this approach had been adopted.) Mentheae as a whole are large and sampling will have to be improved to evaluate generic limits, but clearly there are major problems (e.g. Drew & Sytsma 2011; Drew et al. 2017b; Serpooshan et al. 2018). Just about all the floral characters used to distinguish genera in Menthineae turn out to be homoplastic, and Clinopodium in particular is polyphyletic (Bräuchler et al. 2010). There are many other places where generic/clade rearrangements are to be expected, as in Chloantheae (Prostantheroideae: Conn et al. 2009).
Dracocephalum is to include Hyssopus, and Y.-P. Chen et al. (2022b) recognized 9 sections there, while Satthaphorn et al. (2023) recognized two subgenera and 13 sections within Clerodendrum. As to an infrageneric classification in Salvia, Epling (see Epling & Játiva 1963) recognized 102 sections within subgenus Calosphace alone, admittedly a large group (G.-X. Hu et al. 2018; Kriebel et al. 2019; Lara-Cabrera et al. 2021). For comments on Salvia and its classification, see Muñoz-Rodríguez et al. (2023); as with some other large genera, there is no formal infrageneric classification for the whole genus.
Previous Relationships. Lamiaceae and Boraginaceae have always been considered distinct, but their similar gynobasic styles and fruits with four separate nutlets (and also some chemistry) have invited comparisons between the two, and they have often been placed fairly close to each other in the system, as by Cronquist (1981) where both are in Lamiales. However, there are also numerous differences (chemistry, leaf insertion, floral symmetry, ovule morphology, etc.) between the two, and the radicle in Boraginaceae points upwards in fruit while in Lamiaceae it points downwards.
As their alternative name Labiatae implies, Lamiaceae have always been considered as an "eminently natural" family, being immediately recognisable because of their herbaceous habit, opposite serrate leaves, square stems, monosymmetric flowers, gynobasic style, and four nutlets. However, the gynobasic style and the four nutlets may have evolved more than once (Cantino 1992a), and a considerable number of ex-Verbenaceae must now be included in Lamiaceae (see Junell 1934 for important early work on the gynoecium; Cantino et al. 1992a, b). Those two families, previously considered close but separate, are no longer so close and are now more easily distinguishable morphologically than before.
[Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]]: inflorescence racemose; (protein crystal stacks in nucleus).
Age. The age of this node is estimated to be (60.9-)50.5(-37.6) Ma (Tank & Olmstead pers. comm.) or ca 63.3 Ma (Fonseca 2021).
Evolution: Genes & Genomes. There may have been a genome duplication/gene duplications around here, some of the gene copies later becoming involved in haustorium development in Orobanchaceae (Z. Yang et al. 2014).
Chemistry, Morphology, etc.. Note that there may be chemical differences within Phrymaceae s.l., thus Mazus has iridoids while Mimulus does not (Hegnauer & Kooiman 1978). However, sampling is poor, and needs to be expanded. For nuclear protein crystals, see Albach et al. (2009).
Argue (e.g. 1983, 1985) found that the pollen of genera like Monttea and Melosperma (Plantaginaceeae), tricolpate, the colpi ruptured at the equator, and the tectum (micro)reticulate, was similar to that of Mazaceae and some Phrymaceae.
Phylogeny. There have been problems with details of relationships in this clade, in particular, whether genera like Mazus are to be included in a broadly-circumscribed Phrymaceae, or not (Oxelman et al. 2005; Tank et al. 2006). Thus Albach et al. (2009) cast doubt on the monophyly of Phrymaceae (see also Schäferhoff et al. 2010). Phrymaceae were sister to Orobanchaceae while Mazaceae were part of a large polytomy (ITS), or all were members of polytomies (chloroplast), or relationships were [Mazaceae [[Rehmannia, etc. [Phrymaceae + Paulownia etc.]] remaining Orobanchaceae]] (combined) (Q.-M. Zhou et al. 2014). Xia et al. (2009), Albach et al. (2009), Schäferhoff et al. (2010) and Fischer et al. (2012) have all found support for the paraphyly of Phrymaceae s.l., with Mazus and Lancea (= Mazaceae) forming a clade separate from that containing the rest of the family, = Phrymacaeae s. str. (see also Nie et al. 2006; Luna et al. 2019), so dismemberment is in order. But although Xia et al. (2019) found Mazaceae to be in the position below, that is, sister to the [Phrymaceae [Paulowniaceae + Orobanchaceae]] clade, although bootstrap support could have been stronger, in their analysis of nine plastid markers, in their analysis using chloroplast protein coding genes, Mazaceae were sister to Lamiaceae, albeit the sampling was poor and the support not strong (and see also Xia et al. 2021), and Xia et al. (2019) also noted that other studies that had recovered the first position in fact had little support... Mazaceae and Phrymaceae are sister taxa in the Seed Plants Tree of Life as of ix.2024.
The woody Paulownia, Brandisia and Wightia have been variously associated with Bignoniaceae and/or Scrophulariaceae in the past, but there is now an agreement that they are to be placed somewhere around here. The question is, exactly where? Brandisia is perhaps best included in Orobanchaceae (Bennett & Mathews 2006; esp. McNeal et al. 2013; Q.-M. Zhou et al. 2014: Xia et al. 2019), although its exact position there is uncertain. There is some support for the placement of Paulownia either as sister to Lamiaceae (Olmstead et al. 2000) or with Phrymaceae interpolated between it and Orobanchaceae (some analyses in Albach et al. 2009), but a position sister to Orobanchaceae is on balance more likely (Olmstead et al. 2001; Mueller et al. 2001; Hilu et al. 2003; K. Müller et al. 2004; Wortley et al. 2005a; Xia et al. 2019: chloroplast genes and genomes). Zhou et al. (2014) drew attention to the fact that Paulownia and Hemichaena (Phrymaceae) both had the iridoid tomentoside, known from nowhere else (other Phrymaceae did not have this iridoid). Wightia continues to present problems. Chloroplast data did not link it with Paulownia, rather, with Phrymaceae, but ITS and combined analyses did (Q.-M. Zhou et al. 2014); other relationships in this area were labile. Xia et al. (2019: 9 chloroplast, 1 mitochondrial genes/chloroplast protein-coding genes) found that Wightia was well supported as being sister to Phrymaceae, and some e.g. pollen similarities might support such a position. However, they noted that ITS data had suggested a relationship with Paulownia and that there were some morphological characters in common between the two, and they thought that Wightia might represent the descendents of a hybrid between early members of the Phrymaceae and Paulowniaceae lineages - they thought it was best left unattached pending more detailed studies (Xia et al. 2019), a course that is followed here (see below). B. Liu et al. (2019) also noted that the position of Wightia shifted depending on whether chloroplast or nuclear genes were analyzed, but preferred to describe a new family, Wightiaceae. Of these genera, only Paulowina is included in the Seed Plant Tree of Life as of end of 2022, where it is sister to Orobanchaceae and with strong support, and together the pair are sister to [Mazaceae + Phrymaceae], albeit with rather moderate support.
MAZACEAE Reveal - Back to Lamiales
Annuals, perennial rhizomatous (suffrutescent) herbs, (shrubs); iridoids +; cork?; vessel elements?; pericyclic fibres 0; leaves initially opposite, becoming spiral, margins toothed; inflorescence a raceme, bracts (foliaceous), bracteoles +/0; K terete, C with very well developed lower lip; anther thecae divergent, staminode 0; stigma lobes sensitive; integument 5-6 cells across; (fruit indehiscent); n = 10 (19, 20), x = ?, nuclear genome [1 C] ca 362 Mb.
4 [list]/44: Mazus (39). Turkey and Central Asia and North China to the Antipodes, rather scattered, but esp. China. Map: from Barker (1991) and AgroAtlas (consulted viii.2012). India, Malesia esp. vague - see also Deng et al. (2020: Fig. 1, overly generalized).
Age. Crown-group Mazaceae are ca 32.8 Ma (Fonseca 2021).
Chemistry, Morphology, etc.. Mazus has 1:1 nodes and lacks a pericyclic sheath.
For general information, see Fischer (2004b: as Scrophulariaceae-Mimuleae), and for floral development in Mazus, see Rawat et al. (1988).
Little is known about this clade.
Phylogeny. Fischer et al. (2012) found that the monotypic Central Asian Dodartia, previously included in Phrymaceae, was sister to Mazus, while in Deng et al. (2020) relationships were [Mazus [Dodartia + Lancea]], and in both support was good. Within Mazus, the Antipodean species are sister to the others (Deng et al. 2020), but for possible problems with this analysis, see Xiang et al. (2021), while W.-B. Ju et al. (2023) found that M. motuoensis, which they had just described, was sister to the rest. Puchiumazus, described in C.-L. Xiang et al. (2021), is sister to the rest of the family. (However, note that in Ju et al. (2023), for example, no outgroup to the family is included.)
[Phrymaceae [Paulowniaceae + Orobanchaceae]]: ?
Age. The age of this node is around 67 Ma (Wikström et al. 2001), ca 57.5 Ma (W.-Q. Xu et al. 2018), (61.9-)54.2, 51.9(-43) Ma (Cusimano & Wicke 2016), (56-)45.4(-33.9) Ma (Tank & Olmstead pers. comm.), (54-)44(-33) Ma (Wikström et al. 2015), ca 32.2 Ma (Tank et al. 2015: Table S2, Paulowniaceae outside this clade and slightly older) or ca 56.4 Ma (Y. Xu et al. 2022).
Evolution: Genes & Genomes. There are gene duplications in common between Mimulus and Orobanchaceae (Z. Wang et al. 2014), but a duplication here (Mimulus =Erythranthe) was not recovered by Edger et al. (2017). A gene duplication in common between Mimulus and Orobanchaceae, the βL event, was dated to 73.5±6.6 Ma (Y. Xu et al. 2022), and that puts it very deep in Lamiales, perhaps even at the core Lamiales node.
PHRYMACEAE Schauer, nom. cons. - Back to Lamiales
Herbs; (iridoids 0), (tomentoside +); cork?; vessel elements?; lamina margin toothed (entire); inflorescence axillary, cymes/flowers axillary, or spike/raceme; K tubular, toothed, subplicate-ribbed (not); anthers subreniform; pollen grains (tricellular), surface (micro)reticulate; nectary +/0, stigma broadly 2-lamellate, sensitive; ovules many/carpel, integument 3-7 cells across; K persistent in fruit; (seeds with pedestals); endosperm +/almost 0, cotyledons (convolute = S-shaped in t.s.); x = 9, nuclear genome [ 1 C] (0.104-)0.972(-9.047) pg.
15 [list], to tribes/217. ± World-wide, esp. temperate and W. North America and Australia, but few humid tropics. Map: from Meusel et al. (1978), Barker (1982) and Hong (1983, 1993); India-Southeast Asia-Antipodes very inaccurate. [Photos: Collection, Mimulus Flower.]
Age. The crown age may be ca 40 Ma (Nie et al. 2006: Fig. 2 - ?), ehile (43.9-)29.5(-14.6) Ma is the estimate in Tank and Olmstead (2017)
[Phrymeae [Diplaceae, Leucocarpeae]]: ?
Age. The age of this clade is estimated to be (37.5-)32.9, 32.2(-27.8) Ma (Nie et al. 2006)?.
1. Diplaceae Bo Li, B. Liu, S. Liu & Y. H. Tan / Clade C (for A, B, C, D - see Barker et al. 2012)
Annual to (rhizomatous) perennial herbs; flowers sessile to long-pedicillate; =C (subpolysymmetric), (lobes toothed); pollen tricolp(or)ate/5-7 stephanocolpate [most Diplacus]; placentation parietal (placentae ± connate basally - Hemichaena); fruits apically attenuate; n = 8-10.
3/61: Diplacus (46). North America (commonest in the west) to Central America.
2. Phrymeae Hogg / Clade B - Phryma leptostachya L,
Perennial herb; stem endodermis +; venation mixed-craspedodromous; inflorescences terminal, ± spicate/racemose, (bracteoles 0); K monosymmetric; thecae separate, connective U-shaped; pollen 3-colpate; stigma lobes asymmetrical, not lamellate; ovule single, basal becoming lateral, placentae inconspicuous; integument ca 4 cells across; fruit secund, K accrescent, three adaxial teeth as indurated recurved spines, achene; testa poorly developed; endosperm slight, cotyledons convolute; n = 7; germination cryptocotylar, hypogeal.
1/1. Eastern Asia and E. North America.
3. Leucocarpeae Conzatti / Clade D
Annual to (rhizomatous) perennial herbs (shrubs); pollen tricolporate (5-7-stephanocolpate/spiraperturate); fruit (a berry); n = 13-16, 24, etc.
2/121: Erythranthe (120). North America, especially the west, South America, eastern Asia.
[Mimuleae + Cyrtandromoeeae]: ?
4. Mimuleae Dumortier / Clade A
Annual to (rhizomatous) perennial herbs, (submerged aquatics); (lamina glandular-punctate); (flowers ± polysymmetric); C blue to purple (yellow, white); (A 2 (+ 2 staminodes)), (anthers 1-celled); pollen tricolporate; stigma (1-lamellate, sensitive), (lobes linear, ?not sensitive - Elacholoma); n = 8, 10-12.
9/24: Mimulus (7). Australia to India-Southeast Asia-Malesia (also to Africa-Madagascar, in North America only Mimulus).
5. Cyrtandromoeeae Bo Li, B. Liu, S. Liu & Y. H. Tan - Cyrtandromoea Zollinger
Suffrutescent; inflorescences axillary (flowers single); K polysymmetric; anther thecae divergent, apices confluent; ?pollen; placentae stipitate-strongly C-shaped in t.s.; K accrescent, surrounding loculicidal capsule; exotesta with laminated, U-shaped thickenings in T.S.; endosperm +; n = ?
1/11. S.W. China to Malesia.
Evolution: Divergence & Distribution. Although Phryma is an old clade, its well-known East Asian - E. North American disjunction was established a mere ca 6-2 Ma (Nie et al. 2006, q.v. for other estimates, including 25-12.3 Ma - N. S. Lee et al. 1996, but see calibration).
Mimulus s. str., although a small genus, has species in (i.a.) east North America, Madagascar, and Australia - rather odd.
Ecology & Physiology. Members of the family are common in everything from very dry to more or less permanently inundated habitats. Species like the Erythranthe guttatus complex are noted for their tolerance of a variety of extreme conditions, including growing on serpentine soil (Selby et al. 2016 and Pennisi 2019 for literature and general account respectively, as Mimulus guttatus); parallel mutations have occurred in E. guttatus (Selby & Willis 2019).
Pollination Biology & Seed Dispersal. Much work has been carried out on pollination in Erythranthe in particular, also in Diplacus, and will usually be found under Mimulus in the literature (e.g. Schemske & Bradshaw 1999; Beardsley et al. 2003; Thomson & Wilson 2008; Twyford et al. 2015b). Despite all this work, it has only recently been found that what was thought to be parallel evolution of the hummingbird-pollination syndrome (E. cardinalis, E. verbenacea) represents but a single acquisition of the feature that has been obscured by extensive hybridization (Nelson et al. 2020). Note that in these plants traits associated with floral adaptation are tightly linked - c.f. Penstemon and to a lesser extent Petunia(Wassinger et al. 2023). Friedman et al. (2017) discuss sensitive stigmas and their loss in inbreeding taxa.
Genes & Genomes. There is extensive polyploidy and aneuploidy (both happening 10+ times) in North American Mimulus (= Erythranthe), but neither is associated with the evolution of major clades (Beardsley et al. 2004). Freyman and Höhna (2017) suggested that x = 8 for Mimulus s.l..
Morales-Briones et al. (2022) found evidence neither of a whole genome duplication here nor of any obvious reticulation event.
Chemistry Morphology, etc.. Whipple (1972) described the nodes of Phryma as having three traces coming from a single gap. Barker et al. (2012) decribed lamina venation as usually being more or less brochidromous, acrodromous and variants, or eucamptodromous, noting that the mixed-craspedodromous venation of Phryma seemed to be unique around here.
Argue (1983, also 1981) was unclear whether the equatorially ruptured colpus membrane of the pollen in some members of this clade might suggest that the grains were basically tricolporate, and whether or not some polycolpate grains might be polycolporate. The ovules were described as being apotropous and hemitropous, but epitropous in Cantino (2004). There has been no fundamental shift in the position of capsule dehiscence here - c.f. Barker et al. (2012).
For general information, see Fischer (2004b: Scrophulariaceae p. pte) and in particular Barker et al. (2012), for some chemistry, see Q.-M. Zhou et al. (2014), for pollen, see Argue (1980; Mimulus s.l., 1981) and Chadwell et al. (1992); for Phryma, see Cooper (1941), Whipple (1972), and Venkata Ramana et al. (2000), all embryology, seed development, etc., and Cantino (2004: general), for Cyrtandromoea, see also Singh and Jain (1978) and Burtt (1965: revision). For a monograph of Mimulus old style, species of which have been the subjects of many evolutionary studies, see Grant (1924) and Thompson (2005).
Phylogeny. Phryma and Mimulus and its relatives - and recently Cyrtandromoea and Mimulicalyx have been added - make up this rather unexpected clade. Four main clades were initially apparent in Phrymaceae, Phryma, the North American [Erythranthe + Leucarpon] clade, the largely Australian [Mimulus s. str., Glossostigma, Peplidium, etc.] clade, and the North American Diplacus et al. clade, but their interrelationships were unclear (Beardsley & Olmstead 2000, esp. 2002; Beardsley et al. 2001, 2004; Beardsley & Barker 2004; Barker et al. 2012). B. Liu et al. (2019: chloroplast data, sampling a bit exiguous; Luna et al. 2019) added Cyrtandromoea, which they found was sister to Mimulus and relatives (although with only moderate support), and they picked up the other three clades just mentioned; in both cases support was in general quite strong. However, ITS relationships suggested a very different topology (Liu et al. 2019), i.a. that Phryma was sister to the rest of the family, although support tended to be poor. In analyses of Morales-Briones et al. (2022: 24 genomes/transcriptomes, 4/5 tribes included) the monophyly of the family and tribes were usually recovered, but within [Mimuleae [Phrymeae, Diplaceae, Leucocarpeae]] there was extensive gene tree discordance in the latter clade. In the course of their placement of Mimulicalyx in the family (in Mimuleae), F. Zhao et al. (2022) found intratribal discordance in the relationships that they recovered using six chloroplast and nrITS plus ETS genes.
Classification. For a conspectus of the family, see Barker et al. (2012) where Mimulus was found to go in three separate clades (later = tribes), most species being placed in Erythranthe and Diplacus; Barker et al. (2012) also provide a sectional classification for these two genera, and B. Liu et al. (2019) provide an updated classification, including descriptions of two new tribes. Lowry et al. (2019), however, make an argument for continuing to use the name Mimulus in its old, broad sense (actually, it would be even broader, including the whole family in the one genus), to which Nesom et al. (2019) reply.
Previous Relationships. Phryma was previously placed in a monotypic family on account of its distinctive morphology, or allied with Verbenaceae, as in Cronquist (1981). Mimulus and other genera were included in Scrophulariaceae s.l.. Cyrtandromoea has been included in Gesneriaceae-Didymocarpoideae-Epithemateae, although Burtt (1965) linked it with Leucocarpus, which he thought belonged to Scrophulariaceae, but has since moved here.
Botanical Trivia. The mostly Australian Glossostigma is scarcely bigger than Lemna, while small plants of Mimulus jepsonii may be just a pair of cotyledons, a pair of foliage leaves, and a flower (T. Livschultz, pers. comm.).
[Paulowniaceae + Orobanchaceae]: ?
Age. An estimate of the age of this node (Paulownia sister to Buddleja!) is (58-)48, 38(-26) Ma (Bell et al. 2010); Bremer et al. (2004) suggest an age of ca 64 Ma, W.-Q. Xu et al. (2018) an age of ca 55.4 Ma, while Ma is the age in Wikström et al. (2001), (51-)40(-28) Ma in Wikström et al. (2015), (50.9-)39.8(-27.6) Ma in Tank and Olmstead (2017) and ca 38 Ma in Mortimer et al. (2022).
If Wightia and Paulownia are sister taxa: Plant woody, deciduous; iridoids +; hairs uniseriate-branched to stellate; inflorescence branched, ultimate units cymose; K ± valvate, leathery; staminode 0; pollen 3-colpate; placentae massive, stigma at most barely expanded; in fruit placentae like a subquadrangular seed cake.
Stem age of the two, ca 61.6 Ma, crown age 59.9 Ma (Fonseca 2021).
PAULOWNIACEAE Nakai - Paulownia Siebold & Zuccarini - Back to Lamiales
Trees, evergreen/deciduous; iridoids, tomentoside +; cork cambium outer cortical; nodes 1:1; petiolar bundle annular; twigs lenticillate; hairs uniseriate-branched to stellate; lamina margin entire (lobed), often serrate when young; inflorescence terminal, branched, ultimate branches cymose; K deeply lobed, valvate, leathery, space between K and C [water calyx], C with adaxial-lateral lobes outside others [quincuncial, ascending cochlear]; anther thecae head-to-head, divergent, staminode 0; endothecium massive, extending across connective; pollen 3-colpate; nectary vascularized; G fully 2-locular, placentae sessile-circular in t.s., style hollow, head expanded or not, stigma punctate, hollow; fruit loculicidal, placentae detaching from septum; seeds with pedestals, wings several, largest lateral, margins ± sinuous, cells elongated anticlinally; exotesta cells broad, with complex reticulate thickenings; endosperm +, sparse; n = 19, 20, x = 9, nuclear genome [1 C] ca 0.6 pg.
1 [list]/6. Laos, Vietnam, mostly China (map: from Hu 1959 - in Korea and Japan introduced). [Photo: Flower.]
Age. An estimate of the age of crown-group Paulowniaceae is ca 16 Ma (Fonseca 2021).
Chemistry, Morphology, etc.. The combination of cornoside with iridoids is unusual in Lamiales (Q.-M. Zhou et al. 2014).
Erbar and Gülden (2011) noted that the terminal flowers in an inflorescence of Paulownia might have five stamens - peloria. The ad- → abaxial direction of development of members of the calyx and the corolla whorls is unusual in Lamiales (Erbar & Gülden 2011), although observations are limited.
For additional information, see van Steenis (1949e) and Fischer (2004b: as Scrophulariaceae), both general, Schilling et al (1982: verbascoside, etc.), and Dos Santos and Miller (1993: wood anatomy).
Phylogeny. For a discussion on the relationships of Paulownia, see above.
Previous Relationships. Paulownia is superficially like Catalpa (Bignoniaceae) and the two used to shuttle back and forth between "Scrophulariaceae" and Bignoniaceae. Paulownia has endosperm and lacks the distinctive ovary and seed anatomy of Bignoniaceae (Armstrong 1985; Manning 2000; Lersten et al. 2002); on the other hand, Catalpa is definitely to be included in Bignoniaceae.
Wightia Wallich / WIGHTIACEAE Bo Li, B. Liu, S. Liu & Y. H. Tan - to go where?
Woody epiphytes turning stranglers, deciduous; iridoids, cornosides +; ?nodes; twigs lenticillate; hairs stellate/dendritic; lamina margins entire; inflorescence axillary, branched/unbranched, ultimate units cymose; K ± entire to irregularly lobed, ± valvate, leathery; C ?aestivation; anthers basifixed, thecae parallel and apically confluent, staminode 0; placentae stipitate-weakly C-shaped in t.s., stigma minutely bilobed; capsule septicidal, placentae like a subquadrangular seed cake; seeds elongated, wing lateral, all around; endosperm 0; n/x = ?; seedling with tubers.
1/2. Nepal to China (Yunnan) to west Malesia. Distribution: see Q.-M. Zhou et al. (2014).
Chemistry, Morphology, etc.. The co-occurrence of cornoside and iridoids is unusual in Lamiales (Q.-M. Zhou et al. 2014).
Zhou et al. (2014: p. 2010) show seedlings of Wightia as having "inflated tubers".
For additional information, see van Steenis (1949e) and Fischer (2004b: as Scrophulariaceae), both general, and Schilling et al. (1982).
Wightia is poorly known.
Phylogeny. For a discussion on the relationships of Wightia, see above.
Previous Relationships. Wightia was often associated with Paulownia in the past (Q.-M. Zhou et al. 2014 and references).
OROBANCHACEAE Ventenat, nom. cons. - Back to Lamiales
Herbs; plant turning black on drying (not); cork?; glandular hairs with head lacking vertical partitions; lamina margins often toothed to deeply lobed; inflorescence racemose; C with abaxial-median or abaxial-lateral lobes outside others [quincuncial, ascending cochlear - rhinanthoid]; staminode 0; placentation axile basally, parietal apically; ovules many/carpel; seed with exotestal cells variously thickened on the inner walls.
104 [list]/2,565. World wide, but especially North (warm) Temperate and Africa-Madagascar.
Age. Crown-group Orobanchaceae are estimated to be (63.5-)50.4(-37.6) Ma by W.-Q. Xu et al. (2018), (47.1-)35.7(-24.3) Ma by Tank and Olmstead (2017), (36-)30.2(-25.6) Ma by A. C. Schneider and Moore (2017: ?too young, but c.f. topology used as a calibration in Magallón et al. 2015), (52.7-)44.5(-36.8) Ma by Xia et al. (2021), (41.4-)35.7, 30.2(-25.6) Ma by Mortimer et al. (2022: Tables S6, S7; note calibrations, higher values usually based on Fu et al. 2017 and lower values on Schneider et al. 2017), ca 59.1 Ma (Fonseca 2021) and ca 46.5 Ma (Y. Xu et al. 2022).
1. Rehmannieae Rouy —— Synonymy: Rehmanniaceae Reveal
Rhizomatous; leaves spiral; bracts ± foliaceous, (bracteoles 0); C also descending cochlear [antirrhinoid]; (staminode +); stigma lobes sensitive; n = ?
2/7: Rehmannia (5). China. Map: from Flora of China vol. 18 (1998: green = R. glutinosa, also cultivated; Xia et al. 2021: Fig. 6).
Age. Crown-group Rehmannieae are estimated to be (30.9-)16.6(-4.9) Ma (Xia et al. 2021) or ca 15.5 Ma (Mortimer et al. 2022).
[Lindenbergieae [Cymbarieae [Orobancheae [Brandisia [Rhinantheae [Buchnereae + Pedicularideae]]]]]]: stomata do not close (usually...); x = 9, nuclear genome [1 C] (0.151-)1.105(-8-083) pg.
Age. Bremer et al. (2004) suggested that the age of this node could be put at around 48 Ma, the age in Wolfe et al. (2005) was ca 52.2 Ma, in H.-J. Wang et al. (2015) it was (96.3-)74.5(-52.3) Ma, while in Wikström et al. (2015) it was about a third of this, a mere (38-)26(-13) Ma. (56.5-)50, 44(-38.3) Ma (Cusimano & Wicke 2016), ca 43.1 Ma (W.-Q. Xu et al. 2018) and (38.5-)27.8(-17.3) Ma (Tank & Olmstead pers. comm.) are other estimates. X. Liu et al. (2019) suggested that the age of this node was ca 24.9 Ma while ca 42.1 Ma was the age in Y. Xu et al. (2022).
2. Lindenbergieae T. Yamazaki / Clade I - Lindenbergia Lehmann —— Synonymy: Lindenbergiaceae Doweld
Bracts ± leaf-like, bracteoles 0 (1); C also descending cochlear - antirrhinoid; A thecae on connective arms; testa usu. with hook-shaped thickenings adnate to surface; n = 16.
1/12. N.E. Africa to N. Philippines. Map: see Hjertson (1995).
[Cymbarieae [Orobancheae [Brandisia [Rhinantheae [Buchnereae + Pedicularideae]]]]] / Hemiparasitic clade: hemiparasites, on roots, haustoria from lateral roots; orobanchin +, little oxalate accumulation, 6- and/or 8-hydroxylated flavone glycosides 0; leaves spiral to opposite; (K ± free), C (aestivation imbricate); staminode 0, anther thecae parallel or ± synthecous, sagittate to inverted U-shaped, often hairy, with tails or basal awns, (tapetum amoeboid); pollen often starchy, commonly 3-colpate, surface retipilate, (polyporate); (placentation axile), placentae often 2, bilobed/4, stigma clavate to capitate; ovule (1/carpel), unvascularized or not, variants of anatropous, integument (2-)4-7(-12 ) cells across, (embryo sac protrudes beyond the micropyle); (antipodal cells persistent); capsule loculicidal to septicidal, (indehiscent); (seed pedestals +); (seed with elaiosomes), (cells of seed wings with reticulate thickenings on anticlinal walls), (cell layers other than exotesta thickened and lignified); endosperm +, (walls thickened; reserves starch, mannose-rich polysaccharides); embryo often small; nuclear genome [1C] (553-)3374(-8729) Mb; (germination via germination tube).
96/2040. Worldwide, but especially N. (warm) temperate and Africa-Madagascar. Map: from van Steenis and van Balgooy (1966), Hultén (1971), Meusel et al. (1978) and Hong (1983). [Photo - Plant, Collection.]
Age. The age of the hemiparasitic clade is estimated at (55.2-)49.5, 41.3(-35.7) Ma (Cusimano & Wicke 2016), ca 47.7 Ma (Wolfe et al. 2005), (33.1-)27.7(-23.5) Ma (A. C. Schneider & Moore 2017: ?too young), ca 30 Ma (Mortimer et al. 2022) or ca 38.6 Ma (Y. Xu et al. 2022).
3. Cymbarieae D. Don / Clade II
Anther thecae equal, ± rounded; seeds few to manyn = 18; plastome phyA with intron.
6/14: Cymbaria (4), Monochasma (4). E. North America (1 sp.), Eurasia.
Age. The age of this clade is ca 16.8 Ma (Y. Xu et al. 2022: Cym. Mon.).
[Orobancheae [Brandisia [Rhinantheae [Buchnereae + Pedicularideae]]]]: mannitol + [?all]; endodermis 0.
Age. This clade is ca 44.4 Ma (Wolfe et al. 2005), (31.9-)26.6(-22.6) Ma (A. C. Schneider & Moore 2017) or ca 37.5 Ma (Y. Xu et al. 2022).
(42.5-)35.8(29.0) - (Strig. Aeg. - 24.9) (Cist. Oro. - 34.0) — 32.0 (Ped. Aur. - 19.2) (Melam. Bart. - 17.1), all Y. Xu et al. 2022)
4. Orobancheae Lamarck & de Candolle / Clade III —— Synonymy: Aeginetiaceae Livera, Phelypaeaceae Horaninow
Holoparasitic, (annual); haustoria from radicle/primary root, root cap, quiescent centre 0; (leaves compound), (flowers single - Phelypaea); (bracteoles 0); C tube development early-late intermediate; A (free from C - Eremitilla), anthers (with sterile loculus that has a large spur - Christisonia); microsporogenesis successive [?level], pollen (inaperturate/trisyncolpate), (heteromorphic); G ([3]), placentation parietal, placentae 2-6(-10), stigma large, peltate/2-4-lobed; ovules many carpel; integumentary endothelium + [?level]; endosperm slight-copious, perisperm +; embryo undifferentiated, 50-100 cells, unsaturated oleic and linoleic acids +; n = 12, 18-20, ...
12/230: Orobanche (100), Christisonia (22), Aphyllon (21). North temperate, North Africa, Arabian Peninsula.
Age. The age of the holoparasitic clade [Epifagus + Orobanche] is estimated to be (56.4-)49.1, 39.7(-33.3) Ma (Cusimano & Wicke 2016), ca 39.4 Ma (Wolfe et al. 2005), a mere (19.7-)16.5(-13.7) Ma (A. C. Schneider & Moore 2017) or (Mortimer et al. 2022).
[Brandisia [Rhinantheae [Buchnereae + Pedicularideae]]]: ?
Age. This clade is around 27 Ma (Mortimer et al. 2022).
5. Brandisia J. D. Hooker & Thomson
Shrubs to lianas, "occasionally parasitic" [FoC]; hairs stellate, dense; leaves opposite; flowers single/paired, axillary, or racemose; anther thecae long-ciliate; n = ?
1/13. Myanmar to China.
[Rhinantheae [Buchnereae + Pedicularideae]]: ?
Age. This clade is estimated to be ca 33.9 Ma (Wolfe et al. 2005), a little over 24 Ma (A. C. Schneider & Moore 2017) or ca 25.5 Ma (Mortimer et al. 2022).
6. Rhinantheae Lamarck & de Candolle / Clade V —— Synonymy: Euphrasiaceae Martynov, Melampyraceae Hooker & Lindley, Rhinanthaceae Ventenat
(Annuals), (holoparasites - Lathraea [Lat.]); (leaves opposite), (lamina folded ?abaxially, with gland-lined cavities radiating from central "vestibule" on abaxial surface - Lat.); bracteoles 0; anther thecae separate; G 1-2-locular (Lat.), ovules 2[Lath.]-many carpel; capsule (explosive - Lat.); seeds 1-many/fruit, (dust-like); (endosperm starchy); n = 8-14...
18/540: Euphrasia (170-350), Neobartsia (47), Rhinanthus (45), Melampyrum (35). ± Worldwide, but esp. Eurasia.
Age. The age of this clade is (38.8-)30.8(-25.5) Ma (Uribe-Convers & Tank 2015) or (Mortimer et al. 2022).
[Buchnereae + Pedicularideae]: seeds minute, dust-like [?level].
Age. The crown-group age of this clade is around 23 Ma (Mortimer et al. 2022).
7. Buchnereae Bentham / Clade VI —— Synonymy: Buchneraceae Lilja, Cyclocheilaceae Marais, Nesogenaceae Marais
(Shrubs [Cyclocheila] Cy.], ?life style - Cy., Nesogenes [Ne.]), (holoparasites); (hairs stellate - Ne.); inflorescence (flowers single, axillary - Cy. / axillary 3-flowered cymes); (bracteoles enlarged, "bracts" paired, often opposite them, K ± 0 - Cy.), (C ± polysymmetric - Buchnera), (tube development early-late intermediate - Aeginetia); A (5), (thecae basally caudate - Ne.), (monothecous / one theca sterile); (tectum perforate, 5-7 elements ± regularly surrounding perforation - Ne., etc.); stigma (small, capitate - Ne.; ± long-linulate - Cy.); (ovules 1-5/carpel - Cy., Ne.); dust seeds + (0 - Cy., Ne.); endosperm copious (0 - Cy.), embryo undifferentiated, 40-100 cells; n = 9, 12, 14-16 ...
16/390: Buchnera (140), Alectra (40), Sopubia (40), Striga (33), Harveya (29). Tropics, inc. Australia.
Age. (Mortimer et al. 2022).
8. Pedicularideae Duby / Clade IV —— Synonymy: Pedicularidaceae Jussieu
(Annuals), (shrubs); (wood rayless); (flower asymmetrical); anther (thecae unequal / separate / single); (pollen crotonoid); n = 6-8, 10-16...
14/1,025: Pedicularis (711), Castilleja (160-200), Agalinis (70), Lamourouxia (30). Mostly northern hemisphere, some to South America and the Caribbean.
Age. This clade is about 33.3 Ma (Wolfe et al. 2005), (68.1-)51.1(-34.9) Ma (H.-J. Wang et al. 2015) or (Mortimer et al. 2022).
Evolution: Divergence & Distribution. Orobanchaceae may have diversified north of the Tethys Sea, perhaps in eastern Asia (Wolfe et al. 2005). The evolution of holoparasites with minute dust seeds - which may have happened three times or so - may have been driven by the expansion of grasslands in the middle of the Caenozoic (Eriksson & Kainulainen 2011; see also McNeal et al. 2013). The age of around 31.5 Ma for the adoption of the holoparasitic habit in Epifagus may not be too terribly far off the mark (see Naum,ann et al. 2013), but the sister taxon used in the estimation (Digitalis) is a very distant outgroup so the agreement of 31.5 Ma with anything is likely to be coincidental.
Orobanchaceae s. str. are somewhat unusual in that the non-parasitic Lindenbergia, with 12 species, is much less diverse than its sister group, which is hemi/holoparasitic (although phylogenetic relationships at the base of Orobanchaceae are not entirely clear, the three non-parasitic clades immediately basal to the parasitic clade have 12 (Lindenbergia), 7 (Rehmannieae) and 6 (Paulowniaceae) species respectively), and this is the reverse of the size imbalance common when comparing non-parasitic and parasitic sister clades, however, most Orobanchaceae are hemiparasitic (Hardy & Cook 2012). There are about 21 genera with 308 species of holoparasites here. and they grow on some 62 host families (Hatt et al. 2024c) and there are around 2,200 species of hemiparasites. Interestingly, the largely hemiparasitic Santalales also include substantial diversity, and rather like Orobanchaceae the successive sister taxa to the hemi/holoparasitic clade that makes up the bulk of the order have rather few species - 3, 18 and 40 in this case (see also below).
Pedicularis is particularly common in montane-alpine areas in the Northern Hemisphere although actual species numbers are uncertain (Mill 2001); some 400 of the estimayed 600 species total are found in the Himalaya-Hengduan Mountains region alone (R. Liu et al. 2024). There may have been two movements of Pedicularis from somewhere in eastern Asia to North America, the few European species being independently derived from within the North American clades; patterns of movement in the genus are complex (Robart et al. 2015). The some 25 species of Pedicularis found in the Arctic represent about 13 separate colonizations from mountainous regions at lower latitudes (this S→N movement seems to be common for Arctic taxa, see also Y. Hou et al. 2016), and they include the only polyploid species in the genus (Tkach et al. 2014). Pedicularis, as well as three of the six other orobanchaceous genera growing at high latitudes, include annual species (Tkach et al. 2014). In the Hengduan area, centre of diversity of the genus, up to nine species commonly flower together at the one locality, bumblebees, which behave as generalist pollinators, being the pollinators. The floral dissimilarities of these co-occurring species are notably greater than chance, so reducing competition between species; this local diversity is not linked to the phylogenetic relationships of the taxa concerned (Eaton et al. 2012, q.v. for more on the evolution of the genus) - see also Stylidium and Burmeisteria for similar patterns of local diversity. There has been extensive parallel evolution in the flowers in the genus, with a beaked gales being gained about3 times - and lost more than 20 times, and a long corolla tube gained 20 times or more (Liu et al. 2024). These authors looked at diversification of a major clade of the genus, clade 3, with around 90 species; diversification rates were not associated with corolla type, rather, clade 3 diversified around 20 Ma, its major subclades from ca 12.6 Ma on, and Mid-Miocene orogeny, intensification of the Asian monsoon, movement of species into the long, narrow, river valleys of the region and subsequent geographical speciation and ecological adaptation drove speciation (Liu et al. 2024). The beginning of diversification in Pedicularis has been dated to (52.2-)38.6(-26.2) Ma (H. J. Wang et al. 2015).
The pollination of the remarkable flowers of Pedicularis has been much studied. The flowers show considerable variation, especially in corolla tube length and in galea (the hood-shaped structure formed by the fused apices of the two adaxial petals) morphology (e.g. Li 1951 and references; W.-B. Yu 2013: galea development). Some species have a corolla tube ca 10 cm long or more, although this seems to have nothing to do with nectar production/pollination (Eaton et al. 2012) and/or there may be an asymmetric, almost proboscis-like extension of the galea, the beak (e.g. Li 1951 and references). There are around 400 species of Pedicularis in the Sino-Himalayan region (the HHM region) and they are likely to be pollinated by bumble bees - there are over fifty species of the bees, there, almost a quarter of the genus (P. Williams et al. 2009), and 217-270 species of Pedicularis (H. Yang et al. 1998; Boufford 2014) are known from the Hengduan region alone. About two thirds of the species lack nectar, and their flowers typically have a beak, and there have been more acquisitions of nectar production than losses, species with nectar typically lack a beak; overall, pollination mechanisms are remarkably labile. Thus flowers of P. dichotoma only sometimes had nectar, but if sugar solution (30% sucrose) was placed in the flowers, the bees changed from pollen to nectar foraging and pollen deposition changed from sternotribe to nototribe (Tong et al. 2018). The correlation between nectar presence and bee behaviour is quite good (Eaton et al. 2012). Species with red, long-tubed flowers and growing at higher elevations may lack nectar and be pollinated by pollen-collecting bumblebees, which raises the question of the function of these very long tubes - effectively they are pedicels, the plants not having long inflorescence axes (S.-Q. Huang & Fenster 2007); coiling of the style also occurs here (see also B. Wang et al. 2023). Character displacement, in which sympatric taxa differ more than would be expected, so reducing the chances of pollen being deposited on the wrong stigma (pollen interference), seems to be one component in the generation of the exceptional diversity of the genus in the Hengduan Mountains (Eaton et al. 2012). Sympatric species of Pedicularis sharing the same pollinating bee tend to deposit and pick up pollen from different parts of the bee's body, but if one species of Pedicularis is particularly common, this will help ensure pollinator constancy (S.-Q. Huang & Shi 2013), however, other studies suggest reproductive isolation caused by strongly differing floral morphologies is not that great (Armbruster et al. 2013a). Eaton and Ree (2013) noted introgrssion between Pedicularis taxa growing at lower altitudes, and it was quite widespread, but there floral diversity was low. Introgression was less widespread between taxa growing at higher altitudes, but floral diversity there was higher. For additional comments on the floral evolution of the genus, see also Macior (1971, 1984), Ree (2005a), W.-B. Yu et al. (2015), etc.; pollen morphology - there is quite extensive variation - has been linked with corolla morphology and pollinator type (H. Wang et al. 2009a). Corbett and Huang (2014; see also Buchmann & Hurley 1978) examined how pollen discharge might occur in buzz-pollinated Pedicularis, whether mediated by triboelectric/static charges and/or breaking of fluid bridges between pollen grains (see also Vaknin et al. 2020).
Within Rhinantheae, Euphrasia has a North Temperate - circum-Pacific distribution and is basically bipolar, much dispersal seeming to have been involved in attaining this range (Gussarova et al. 2008); at a little more than 10 Ma (Mortimer et al. 2022), it is hardly an old genus. Diversification within the large genus Castilleja (Pedicularidae) is becoming better understood. There is a speciose West North American/Central/South American perennial clade - some 160 species in North America alone - derived apparently quite recently from an annual ancestor; polyploidy is common in the perennials, but not in the annuals (Tank & Olmstead 2008, 2009; see also Hughes & Atchison 2015). Annuals have dispersed more than once to South America (Tank & Olmstead 2009). Agalinis is another member of this clade that shows a North → South American distribution pattern (Latvis et al. 2024).
Neobartsia showed increased diversification when it moved to South America (4.1-)2.6(-1.5) Ma, although exactly how it got there from Europe is unclear, whether by long distance dispersal or migration via North America, etc., and subsequent extinction throughout the path it took (Uribe-Convers & Tank 2015, 2016). Species of Neobartsia grow at high elevations (2800-4500 m), and a number have an erect, not recurved lower lip and are pollinated by hummingbirds.
Several stems of speciose clades in holoparasitic Orobancheae are long, up to ca 7 Ma (the whole clade is only ca 16.5 My), perhaps because extinction has been very common (A. C. Schneider & Moore 2017), and there has been much diversification of this group in West Asia, especially the Caucasus region, within the last 3 Ma, and more recent diversification both there and in the Mediterranean region (Piwowarczyk et al. 2021). For more suggestions about changes in diversification rates in the family, see Mortimer et al. (2022).
There are three amphitropical disjunctions in the family, all involving holoparasitic taxa - which might seem a little odd given the at least moderate host specificity shown by some of these plants. Aphyllon has moved to South America twice within the last 1.9 Ma, and both North American taxa close to the South American migrants and the South American species are parasitic on Grindelia (Asteraceae-Asteroideae-Astereae), while other species of Aphyllon also parasitize Asteraceae (A. C. Schneider & Moore 2017, q.v. for dates of both parties involved). Relatives of Orobanche cernua var. australiana from South Australia, are also from the Northern Hemisphere, and the move happened within the last 0.46 Ma (Schneider & Moore 2017).
Holoparasitism has evolved perhaps three times from hemiparasitism in Orobanchaceae; there have been no reversals, nor from hemiparasitism to autotrophy. Mortimer et al. (2022) suggested that there was an unobserved precursor to the origin of holoparasitism (see also nitrogen fixation in the N-fixing clade/fabids). In parasites here, haustoria on each other, while there are a number of cases where seeds feon one individual may germinate on another, autoparasitism (Krasylenko et al. 2021). Cai (2023) discussed the general issue of the evolution of parasitism, and of holoparasitism in particular, noting that holoparasitic Orobanchaceae were somewhat unusual in that they synthesized carotenoids - most holoparasites have anthocyanins. Indeed, flowers of holoparasitic Orobanchaceae are fairly conventional compared to those of other such plants, the whole family quite often being included in Scrophulariaceae, as by Fischer (2004b).
Inclusion of Nesogenes, and in particular Cyclocheilon and Asepalum - theae taxa are included in Buchnereae above - considerably increases the morphological diversity of Orobanchaceae; whether or not these taxa are parasites is unknown. Cyclocheilon and Asepalum (= Cyclocheilaceae) lack much in the way of a calyx, it being at most a minute rim, but have large bracteoles enveloping the flower bud (c.f. Acanthaceae-Nelsonioideae), and paired "bracts" often opposite the bracteoles. They are small shrubs with red roots [?always]; the flowers are single in the leaf axils; the exine is thickened near the apertures; the placentation is axile or parietal, with 1-5 apotropous ovules/carpel, endothelium?, the funicles are long and the stigma is lingulate. The fruit is a capsule or schizocarp and the seeds lack endosperm. Nesogenes (Nesogenaceae) has anthers that are basally caudate, there is a small, capitate stigma, only a single ovule per carpel and the seed has endosperm. Placentation and fruit type vary (see Marais 1981 for details). Harley (2004) noted similarities between the pollen of Cyclocheilaceae, Nesogenaceae (both have tricolpate pollen, that of Nesogenes is perhaps also pilate) and Orobanchaceae. I do not know about stomatal closure and parasitism in these plants. Morawetz et al. (2010) found that the genera were sister to Buchnereae - but clearly, the three genera need more work.
Ecology & Physiology. Note: there is facultative and obligate hemiparasitism and also holoparasitism in Orobanchaceae s. str. (and also Orchidaceae), and in both groups such terms can be applied to a species, or to particular stages in the life history of a species.
Hemiparasitism appears to have evolved once (e.g. McNeal et al. 2013) in Orobanchaceae, while holoparasites have evolved from hemiparasites perhaps three times (dePamphilis et al. 1997; Nickrent et al. 1998; Young et al. 1999; Schneeweiss et al. 2004a; Bennett & Mathews 2006; esp. McNeal et al. 2013; X. Li et al. 2019). The hemiparasitic Harveya obtusifolia is well embedded in a holoparasitic clade of the genus; whether there has been a reversion in life style here - this would be very unusual - or there have been yet more independent acquisitions of the holoparasitic habit in that part of the family is unclear (Morawetz & Randle 2009; species not included by McNeal et al. 2013); Morawetz et al. (2014) incline to the latter position. Indeed, the distinction between hemi- and holoparasitism is not sharp. Species like Striga linearifolia and Alectra sessiliflora are close to being holoparasitic, while Tozzia alpina may live underground for a decade or so as a holoparasite before producing photosynthetic, above-ground, fertile shoots (McNeal et al. 2013). And of course genera to concentrate on in this context are Nesogenes, Brandisia, Cyclocheilon and Asepalum.
Seeds may be relatively larger in hemi- than in holoparasitic species. Eriksson and Kainulainen (2011) discuss the distinctive dust seeds of many holoparasitic Orobanchaceae (also in Ericaceae-Monotropoideae and mycoheterotrophic taxa in general), indeed, single plants of Striga may produce up to 500,000 seeds that are only 200-300 µm in size (Spallek et al. 2013). Some holoparasitic taxa have seeds that can lie dormant in the soil for a decade or so (Runo & Kuria 2018; Bouwmeester et al. 2020) - interestingly, seeds of hemiparasites do not have the same longevity (Scholes & Press 2008; see also Mohamed et al. 2001: African species; Ejeta & Gressel 2007; Yoshida & Shirasu 2009; Irving & Cameron 2009; Joel 2013b; Parker 2013; Runo & Kuria 2018).The establishment of parasitism involves two stages, prehaustorium formation and haustorium formation/maturation. The former involves host-derived haustorium-inducing factors that induce processes that occur prior to the attachment of the parasite to the host (e.g. Govet et al. 2019), the latter what goes on during and immediately after the attachment of parasite to host. Indeed, one can even think of there being three stages in the establishment of parasitism, the first being seed conditioning. Here the seed responds to various cues normally involved in germination in other plants, although that does not immediately - maybe even for years - occur here, and there is a kind of secondary dormancy (e.g. Plakhine et al. 2009; Sallek et al. 2013). Haustorial development in the parasite - and also its control by the host - in Orobanchaceae is detailed in Musselman and Dickison (1975), Thorogood and Hiscock (2010), papers in Joel et al. (2013), Furuta et al. (2021), and so on; for early work on germination, see Vaucher (1823) and on haustorial anatomy, see Solms-Laubach (1867).
In the actual process of germination the seed responds to a variety of haustorium-inducing factors produced by the host (Westwood et al. 2010 and references) in forming prehaustoria (Timko & Scholes 2013; Yoneyama et al. 2013; Furuta et al. 2021), although Plakhine et al. (2003) suggest a somewhat different sequence of events. In species of Striga the production of ethylene and cytokinins obviate the need for germination-inducing factors (Govet et al. 2019). The germinating seedling is part of the prehaustorial phase; seedling morphology itself seems to be little reported, although the embryos of broomrapes (Orobanche, Phelipanche), for example, have no obvious cotyledons and there is no apical meristem, and the root of the seedling lacks a cap and procambium, etc. (Fernández Aparico et al. 2016).
Prehaustorium formation involves extensive signaling, particularly from the host to the parasite. Host-derived cell wall-related phenolics and quinones, cytokinins, etc., may be involved (Govet et al. 2019). Strigolactones, commonly exuded from plant roots, stimulate seed germination in a number of holoparasitic Orobanchaceae (Tsuchiya & McCourt 2009; Akiyama et al. 2010; Westwood et al. 2010; Cardoso et al. 2011; Conn et al. 2015; Mutuku et al. 2020; Bouwmeester et al. 2020); Uraguchi et al. (2018) discuss the development of a strigolactone derivative that induces germination of Striga hermonthica seeds. Yoshida et al. (2019) observed that there had been extensive evolution of the genes involved in strigolactone/host recognition, perhaps connected with the diversity of plants that Striga parasitizes; the particular gene involved was originally a karrikin (smoke) detector. Arellano-Saab et al. (2021) found that changes of the amino acids in only three key positions in KAI2, responsive to smoke-derived butenolides (karrikins), were sufficient to induce strigolactone sensitivity; karrikinin receptivity persisted. Surprisingly, although both Striga (and Phelipanche/Orobanche) require external strigolactones to germinate, they also synthesize them themselves (Das et al. 2015); endogenous production may be essential for the growth of the parasite since, as mentioned above, strigolactones also have a variety of functions associated with plant growth. Interestingly, the parasite does not respond to all the strigolactones produced by the host, thus Sorghum resistant to Striga produces the strigolactone orobanchol rather than the strigolactone 5-deoxystrigol which has the opposite stereochemistry and to which the parasite does respond (Gobena et al. 2017). The mechanism involved is similar to some specific germination responses in Arabidopsis thaliana mediated by the KAI2 gene, but evolved after an ancient - as old as the common ancestor of [Solanales + Lamiales] - duplication of the KAI gene. That gene controls the response to karrikin, a substance found in smoke that is i.a. responsible for the positive germination response of a number of plants to fires (e.g. Flematti et al. 2013), although karrikin receptors are found throughout embryophytes and are involved in the establishment of arbuscular mycorrhizal associations (Gutjahr et al. 2015 and references). (Both karrikins and strigolactones have a distinctive element, a butenolide four-carbon heterocyclic ring, e.g. -C=C-C[=O]-O-C-.) Orobanchaceae, including the hemiparasitic species, usually have more copies of the KAI2 paralogs than other Lamiales, and the orobanchaceous KAI2d genes respond to strigolactones. There are also suggestions that germination is in part controlled by maternal genes in persistent maternal (nucellar) tissue in the seed of the parasite (Plakhine et al. 2012). Although over 35 strigolactones may stimulate the germination of Orobanchaceae, at least some facultative root parasites such as Rhinanthus seem not to need any stimulants to germinate - as with parasites like mistletoes and Cuscuta (Bouwmeester et al. 2020); Clark and Bennett (2023) could find no particular reasons for this diversity in these exuded strigolactones (see also Bouwmeester et al. 2020). As in plants other than those with holomycoheterotrophic associations, giberellic acid had a positive effect on germination (references in Miura et al. 2023). Tesitel (2011, 2016 and references), Murdoch and Kebreab (2013), Spallek et al. (2013) and others also discuss germination.
There are more general issues to bear in mind when thinking of strigolactones. This class of compound is also important in the plant/fungus signaling involved in the establishment of arbuscular mycorrhizal associations, and this may well have been their original function (Kodama et al. 2022), and they are related to plant hormones that control branching, in general being essential for the host (e.g. X. Xie et al. 2010; J. Clark & Bennett 2023). The story of strigolactones as both plant hormones and rhizosphere signaling agents is complicated (see Kodama et al. 2022; Clark & Bennett 2023 - both emphasize the latter aspect). A word on terms. Strigolactones used to be divided into canonical and non-canonical types, the former consisting "of a tricyclic lactone ABC-ring system connected via an enol–ether bridge to a methylbutenolide D-ring", while the latter "lack the characteristic ABC-ring. The enol—ether-linked D-ring is present in both types, and is essential for biological activity in plants" (Clark & Bennett 2023: p. 1164). However, those authors thought that a better division might be into hormonal - active internally - and exuded strigolactones (ibid. p. 1167), so reflecting directly the distinction between strigolactones that are plant hormones and those that are involved in establishing associations with arbuscular mycorrhizae; the latter group included both canonical and non-canonical strigolactones and are what are under discussion here. All in all, a complex area, and D. M. Watson et al. (2022) even suggest that being root parasites with host-induced germination are key innovations driving diversity here.
There is also the perhaps related issue of how parasites avoid parasitiizing themselves. L. Xian et al. (2025) recently found that that there are endogenous haustorium-inducing factors (HIFs) like lignin monomers and associated phenolics in the hemiparasite Phtheirospermum japonicum, but the plant produces an enzyme that inactivates them - it responds to such HIFs produced by its hosts - a variety of plants. An interesting question is then why susceptible plants should produce these HIFs in the first place, and again lignins seem to be very important...
In holoparasitic Orobanchaceae in particular with tiny seeds that have little in the way of reserves, haustoria develop quickly at the end of the radicle within some four days or so of the start of germination, that is, the emergence of the radicle, and they terminate its elongation growth. However, in hemiparasites, whether obligate or facultative, and later in holoparasites, too, haustoria develop on lateral/adventitious roots of the host (Westwood et al. 2010; Joel 2013; Cui et al. 2015). There are commonly hairs on the haustorial surface that secrete mucilage and so ensure a close attachment between host and parasite, but papillae are poroduced by the holoparasites Phelipanche and Orobanche (Mutuku et al. 2020). As already mentioned, haustorial formation is induced by the host, and in the hemiparasite Phtheirospermum japonicum 2,6-dimethoxy-p-benzoquinone (DMBO or DMBQ) is one of the host products involved in hair formation in the parasite haustorium - and also in normal root hair formation in the host; mutants with fewer hairs form fewer haustoria (Cui et al. 2015). Note that the elongated intrusive cells found at the apex of the developing haustorium in both wild-type and mutant haustoria have nothing to do with root hairs. This whole process of seed conditioning, prehaustorium and early haustorium formation in particular, varies considerably within Orobanchaceae and can be mind-bogglingly complex, as in the holoparasite Citsanche armena (Krasylenko et al. 2023).
Haustoria are produced by both hemi- and holoparasitic Orobanchaceae, and probably have but a single origin within the family (Fischer 2004b: summary; Z. Yang et al. 2014). The immediate stimuli for haustorial induction are methoxyquinones coming from the host; hairs that bind to the host root are produced (Mutuku et al. 2020). In holoparasitic taxa the initial haustorium forms on the end of the radicle, the growth of which stops, while in hemiparasitic taxa haustoria are lateral, forming along roots whose future growth in not impaired; there are similar lateral haustoria on later-formed adventitious roots of holoparasites (Furuta et al. 2021). Yang et al. (2014) found that genes involved in root and also flower development were implicated in haustorium formation, while Noshida et al. (2018) found that in Striga lateral root genes were involved. Interestingly, there is intracellular penetration of the host root epidermal and cortical cells, and ultimately, pericyclic cells, etc., as well, by Orobanche cumana haustoria, unlike several other Orobanchaceae where penetration is usually intercellular; in O. cumana the parasite breaks down the host cell wall, however, the plasmalemma remains intact and the host cells often remain alive (Auriac et al. 2024). Recent work suggests that it was the hemiparasites Rhinanthus minor and Odontites vernus that produced the so-called "lignin-rich interfacial deposits" between parasite and host, not the hosts, and such deposits, quite commonly found in parasites both here and in other families, may be involved in sealing the connection between host and poarasite, strengthening it, in parasite protection and the like; these deposits may well not be a host product that is involved in its protection (Pielach et al. 2025). For Orobanchaceae cell wall adhesives, see also Takagawa and Yokoyama (2025: attachment of Cuscuta to host), and arabinogalactan proteins and pectic polysaccharides found in haustoria are also among the adhesives used by climbers to attach to their supports.
Furuta et al. (2021) describe various later stages of haustorial development, which include the development of procambium-type cells in the centre of the haustorium and of intrusive cells, anticlinally-elongated epidermal cells, on the surface which grow into the host and establish connections with the host xylem in a process that is perhaps akin to grafting (Mutuku et al. 2020). With the differentiation of tracheary elements in host and parasite, a xylem connection is established between the two, a xylem bridge forming (e.g. Heide-Jørgensen & Kuijt 1995; Furuta et al. 2021; Masumoto et al. 2021, q.v. for much detail). Pits between adjacent xylem cells (host/parasite), or oscula, tubular structures of the parasite that directly penetrate the host xylem, may form; the nature of the connection is unconnected with the style of parasitism (Masumoto et al. 2021), Batashev et al. (2013) discuss the distinctive morphology of the phloem companion cell morphology of some hemiparasites - there are large wall ingrowths and plasmodesmatal fields with branched plasmodesmata. For the distinctive anatomy and cell wall composition (arabino-galactan proteins are common in the cell walls - and also in Thesium - Santalaceae) of the hyaline body, parenchymatous tissue surrounding the xylem of the xylem bridge, see Pielach et al. (2014: presence/absence seems to vary within Striga). In other taxa paratracheal parenchyma surrounds the xylem bridge, and both of these may be involved in transfer of material from host to parasite, although the function of the parenchyma in particular is poorly understood (Mutuku et al. 2020; Masumoto et al. 2021). The perennial haustorium of the holoparasite Boschniaka hookeri - it has only xylem connections - and its host (Gaultheria, Ericaceae) forms a wood rose rather like those in the hosts of some hemiparasitic Santalaceae and Loranthaceae (Kuijt & Toth 1985).
Haustoria function in different ways. Taxa in the [Rhinantheae [Buchnereae + Pedicularideae]] clade may be obligate or facultative hemiparasites; the latter have chlorophyll and take up largely water and mineral nutrients from their hosts, the haustoria tapping the xylem alone (e.g. Joel 2013a; Westwood 2013). The lumina of the xylem cells in the host and parasite are in direct contact as haustorial cells (= oscula) punch through the walls of host xylem and form tubular openings of various shapes before themselves differentiating into water-conducting cells (Dörr 1997; Cameron et al. 2006). In these hemiparasites, carbon may also be obtained from the host, the parasite maximizing its water loss to maximize C gain from its host (Press et al. 1988) - indeed, sometimes most of the parasite's C needs may be supplied this way - and it moves through the xylem of the parasite (Tesitel et al. 2010b, 2010c, 2011 and references). Giesemann and Gebauer (2021) looked at the movement of C from host to parasite in a number of Central European hemiparasites that potentially accessed host C through the xylem - amounts taken up ranged from 0-51%, higher values being in taxa with more complex haustor1a. The holoparasite Lathraea lacks haustorial phloem connections, but host sap is taken up by the xylem of the parasite (Ziegler 1955; Dörr 1990). The holoparasite Orobanche also takes up organic materials, but here the haustoria also tap the phloem (Irving & Cameron 2009; Joel 2013; Tesitel 2016). Ordinary-looking sieve plates form between host and parasite phloem elements, although the latter are not associated with companion cells (Dörr & Kollmann 1995). Some holoparasites, particularly annuals, perhaps, store starch in thinnish, radiating, root-like structures (Thorogood & Rumsey 2021). Westwood (2013; see also D. M. Watson et al. 2022) summarizes what is known (relatively little) about the movement of various nutrients from host to parasite (and sometimes there is a reverse flow). Hemiparasiic Santalaceae (q.v.) behave similarly to hemiparasitic Orobanchaceae. Finally, it should not be forgotten that in plants like Castilleja the endophyte is endogenous, arising from from subdermal and cortical cells of the parasite, the host epidermis having broken down, while in Triphysaria, for example, the endophyte is exogenous, the host epidermis remaining intact (Heide-Jørgensen & Kuijt 1995).
It is not only water and nutrients that move. Iridoid glucosides as well as pyridine, pyrrolizidine and quinolizidine alkaloids, etc., may also move from host to parasite (e.g. Adler & Wink 2001; Hibberd & Jaeschke 2001; Shen et al. 2005 [also host selection]; Rasmussen et al. 2006; Scharenberg et al. 2019), and also from parasite to host. Thus some of the severe effects on the host caused by the parasite may be due in part to the breakdown of the iridoid glucosides of the parasite and the release of the cytotoxic iridoid aglucones, perhaps caused by the host's ß-glucosidases, themselves common because they are involved in the host's cyanogenic defence pathway (Rank et al. 2004). Cytokinins, specifically t-Z-type cytokinins, also move from the parasite Phtheirospermum to the host where they are involved in hypertrophic root growth of the nodule, the overall growth of the host being negatively affected (Spallek et al. 2017). For other information on parasitism in the family, see Irving and Cameron (2009) and Tesitel (2016) and references.
Host specificity, races or species of parasite found only on particular host species, is well-known in holoparasites like Orobanche in particular, also in the obligate hemiparasite Striga, and may be involved in speciation (see Thorogood et al. 2009; Westwood et al. 2010; Parker 2013; J. Zhang et al. 2023). On the other hand, some Orobanchaceae are associated with a wide variety of hosts - "hundreds of species" from a number of families being hosts for the annual O. minor (Rumsey & Jury 1991: p. 269; Manen et al. 2004; Cameron & Phoenix 2013), while Gibson and Watkinson (1982) noted that the annual Rhinanthus minor might parasitize some 50 species or so - species of Poaceae and Fabaceae were common hosts - but individual plants showed some selectivity, commonly only 3-4 species being parasitized by a single individual. Indeed, hemiparasitic Orobanchaceae in Europe parasitize mostly Fabaceae and Poaceae (Gibson & Watkinson 1982). Zhang et al. (2023) found that although Boschniakia himalaica was a parasite of Ericaceae and B. rossica of Betulaceae, the common ancestor of the two probably parasitized Rosaceae-Rosideae. Christisonia species are commonly found on Strobilanthes (Acanthaceae), perhaps also on bamboos, but C. tubulosa has a broad host range (Thazhe Arunraj et al. 2024); all but one of the ca 30 species of Striga parasitize a variety of grasses, both C3 and C4, the morphologically distinct S. gesnerioides being the exception (Spallek et al. 2013) - it is found on Fabaceae (cow pea) and Solanaceae (tobacco). Overall, perennial species tend to have narrower host ranges than annuals, and it is annuals that tend to be weedy (Schneeweiss 2008), while hemiparasites tend to have broader host ranges than holoparasites (Estabrook & Yoder 1998). However, even in Central Asian near-deserts dominated by the amaranth shrub Haloxylon ammodendron, the holoparasite Cistanche deserticola finds a congenial host. Sánchez Pedraja et al. (2016) provide an annotated list of the host plants of Orobanchaceae, while for Orobanchaceae parasitizing crops, see Economic Importance below.
Random variants. Some plants may not be parasitized because of allelopathic relationships between host and parasite, e.g. between Desmodium uncinatum and Striga hermonthica, the former not being parasitized even though the Striga germinates (such interactions can also be the basis of protection of susceptible crops growing with the pair, the Striga being effectively neutralised), the jasmonic and salicylic acid signalling pathways being important in plant defence (Timko & Scholes 2013; Pickett et al. 2013). Adler (2000, 2002, 2003; Adler & Wink 2001) found a particularly complex relationship between hosts and parasite, the annual Castilleja indivisa. Association of C. indivisa with Lupinus in particular led to fewer herbivores eating the parasite (sometimes), more visitors by pollinators, increased seed set, etc., when compared with other hosts. These effects were mediated by the movement both of alkaloids (defence) and nitrogenous compounds (increase in growth s.l.) from the lupin to the parasite; alkaloids acted as a deterrent to herbivores of the parasite and indirectly increased visits by aesthetically sensitive pollinators who were no longer put off by half-eaten inflorescences (Adler 2000). Iridoid glycosides can move from hemiparasite to the caterpillar eating it, the amount of a particular iridoid in the caterpillar not necessarily reflecting that in the hemiparasite (Haan et al. 2018).
The pattern of vascular tissue in the stem of Cistanche shows considerable variation (Lei et al. 2021).
A number of upregulated genes that may be involved in the evolution of the parasitic habit have been identified. Many are derived from genes that act in the root (e.g. LATERAL ORGANS BOUNDARIES DOMAIN 25), but also in pollen, the latter perhaps because, as with the intrusive cells above, pollen tube growth is intrusive and also involves interactions between organisms, i.e. the pollen tube and stylar tissue. Many of these genes in Orobanchaceae seem to have been repurposed following a genome duplication in the common ancestor of [Mazaceae ... Orobanchaceae] (Thorogood & Hiscock 2010; Z. Wang et al. 2014). Overall, there are also substantial similarities in haustorium initiation and development in the stem parasite Cuscuta (Convolvulaceae) and root parasites in Thesium (Santalaceae) (e.g. Jhu et al. 2021). Other parasite genes are related to those that have been involved in various kinds of horizontal gene transfer, and in Orobanchaceae, as in other parasitic groups, there has been transfer of a number of genes from host to parasite, but not in the other direction (see Genes & Genomes below).
Some angiosperms can recognize Striga and Orobanche as pathogenic and can resist colonization by them (references in Mutuku et al. 2020; Hegenauer et al. 2020); incompatability between the host and orobancheaceous root parasite is first evident in the endodermal region of the possible host, the endodermis becoming much lignified, at least in Orobanche (Thorogood & Hiscock 2010; see also Cameron & Phoenix 2013; Jhu et al. 2022b). Both in Vicia species that are resistant to infection by root-parasitic Orobanchaceae and also in Cuscuta (Convolvulaceae) that are similarly resistant to stem parasites it is the development of lignification in various places that seems to prevent haustorial penetration (references in Jhu et al. 2022b). Thus host genes associated with lignification and secondary cell wall formation, as well as the phytohormone jasmonic acid, etc., are all involved in defence against the parasite (Mutuku et al. 2020). Papers in Joel et al. (2013), Front. Plant Sci. 7. 2016, etc., provide more information on parasitism in Orobanchaceae.
Stomata in Orobanchaceae are often, but not always, perpetually open (Stewart & Press 1990; S. Smith & Stewart 1990), and stomata in the apparently autotrophic Lindenbergia, sister to most other Orobanchaceae, behave similarly, but the situation in Rehmannia and relatives. immediately outside Orobanchaceae s.l. (see below), is unknown. The stomata remain open despite the presence of large amounts of abscisic acid, which normally would be expected to result in their closure (e.g. Jiang et al. 2010). Amphistomaty as well as perpetually-open stomata and/or stomata open during dry periods are common in hemiparasitic plants in general because they increase the transpiration flow in the parasite so facilitating movement of water, nutrients, etc., from the host (e.g. Phoenix & Press 2005; Krasylenko et al. 2021) - in some cases the parasite can cause the host stomata to remain open (Landi et al. 2022), although I do not know if this happens in Orobanchaceae.
The effects of hemiparasitic Orobanchaceae on the general community in which they live can be considerable, affecting both overall species richness (perhaps, certainly not always) and evenness, the failure of individual species to dominate the community (e.g. Gibson & Watkinson 1992; McKibben & Henning 2018; D. M. Watson et al. 2022); see also Santalales-Mistletoes and Convolvulaceae-Cuscuta. Thus they may increase overall diversity if, like Rhinanthus minor, they parasitize dominant species like grasses in grasslands (they can colonize closed grassland communities), the growth of associated legumes and forbs then tending to increase (Pywell et al. 2004; Bardgett et al. 2006; Cameron et al. 2007; Irving & Cameron 2009). The result may be a mobile mosaic of areas dominated either by grasses or by forbs, etc. (Cameron & Phoenix 2013). Indeed, Rhinanthus may be used in grassland restoration, i.e., developing/restoring the diversity in grasslands (Caudron et al. 2021). This increase in diversity may be facilitated by light increasing in the grassland understory, as with grazing (Eskelinen et al. 2022), including that by megafauna (Lundgren et al. 2024). McKibben and Henning (2018) found that the presence of Castilleja reduced mycorrhizal - arbuscular, ericoid - colonization by ca 20% in a variety of dominant species at different elevations in the the Rocky Mountains in Colorado, species richness and evenness also increasing (details of how this might happen were not the focus here); c.f. also mistletoes (particularly Loranthaceae and Santalaceae-Visceae). Tesitel et al. (2010c) noted that hemiparasites reduced biomass production of their host, and since it was not fully compensated for by that of the parasite, overall community productivity decreased; at the same time, parasitism by Orobanchaceae may lead to a redistribution of nutrients in the community (see also mistletoes, Watson et al. 2022). Along similar lines, at 31% Rhinanthus alectorolophus density, overall yield decreased 26%, nevertheless, species diversity increased 12%, smaller species being favoured (Heer et al. 2018). Indeed, it has even been suggested that such hemiparasites could be used to help restore diverse grassland (e.g. Bullock & Pywell 2005; Heer et al. 2018). The hemiparasites, especially the annuals, increase the rate of community cycling of nutrients such as nitrogen, perhaps particularly in the Arctic. Their litter can be relatively rich in nutrients, nutrients not being resorbed by the plant as it senesces, that aside, it often decomposes more rapidly than that of other species in the community; overall, the positive ecological effects of the litter can counteract the negative effects of parasitism (Quested et al. 2003; Phoenix & Press 2005; Bardgett et al. 2006; Watson 2009; Tesitel et al. 2010c; Fisher et al. 2013; c.f. in part Cameron & Phoenix 2013). Note also that in this context the relationship between annual and perennial parasites and their hosts may differ; if the former kill their hosts, it doesn't really matter, but if the latter do, then it may well be to their disadvantage (Hodzic et al. 2022). Note also that their study of some North American hempiarasites led Hodzic et al. (2022) to question the effect of parasites on community richness, but not evenness, although they noted that this needed to be confirmed for both annual and perennial parasites; of the four common genera in their study, three (Comandra, Krameria, Pedicularis) were perennials, one (Castilleja) was mixed. However, the effect of parasite on the host is complex and is mediated by other microbial associates of the host (see below). For more on parasitism in Orobanchaceae, see other articles in Joel et al. (2013). Hemiparasitic Orobanchaceae prefer nutrient-poor environments, and elevated nutrients in general lead to the plants performing poorly (Cameron & Phoenix 2013). Other groups with root hemiparasites include Krameriaceae and Santalaceae and some other Santalales; leaf parasites may also affect community composition by their indirect effect on dominant grazers (T. Li et al. 2023).
Pollination Biology & Seed Dispersal. The evolution of the remarkable flowers of Pedicularis are discussed under Diversity & Distribution above. Flowers of Pedicularis are pollinated by bumblebees, and these collect nectar or pollen (buzz pollination) depending on the flower type. Buzz pollination is also known from Melampyrum (Teppner 2018 and references), Agalinis (Dieringer & Cabrera R. 2022), etc.. For more on buzz pollination, see elsewhere.
Hairs on the anthers are common in Orobanchaceae, and in Esterhazya in particular trichomes of the epidermal layer of the thecal wall form a pollination basket in which the pollen is held, perhaps preventing all the pollen from being dispersed too soon (Hesse et al. 2000). For other literature on pollination, see Kampny (1995: as Scrophulariaceae).
Melampyrum and Pedicularis in particular have myrmecochorus seeds (Lengyel et al. 2009, 2010), while Eriksson and Kainulainen (2011) discuss the distinctive dust seeds of many parasitic Orobanchaceae (see also above). The fleshy fruits of Phacellanthus tubiflorus, held at ground level, are dispersed by camel crickets (Rhaphidophoridae: e.g. Tachycines elegantissima), another "strategy" of mycoheterotrophs (Suestsugu 2017). However, Lathraea squamaria, parasitic on Corylus, has few and relatively large seeds (its fruits are capsules), perhaps connected with the need of the seedling radicle to reach the relatively deep roots of its future host.
Plant-Animal Interactions. Agromyzid dipteran leaf miners have diversified on hemiparasitic Orobanchaceae (Winkler et al. 2009), and larvae of Nymphalinae-Melitaeini butterflies are also commonly found on these plants (also on Plantaginaceae, but not on Scrophulariaceae: Wahlberg 2001).
Plant-Bacterial/Fungal Associations. The effect of species of Pedicularis that had differing nutrient requirements on their host, Trifolium repens, depended on the association of the latter with AM fungi and rhizobia, and in some combinations inoculation with the fungus might benefit both host and parasite, in the former alleviating damage caused by the parasite (Sui et al. 2018). Interestingly, Striga hermonthica and Orobanche aegyptiaca, at least, but not Pedicularis, are unable to form associations with AM fungi since they have lost their symbiosis-specific genes; Lindenbergia also does not have some of these genes (see Delaux et al. 2014: Fig. 3).
Genes & Genomes. There may have been a genome duplication in obligate hemiparasitic and holoparasitic taxa (Wickett et al. 2011). The αB duplication event seems to have occurred in Buchnereae soon after the Buchnereae-Orobancheae split (Y. Xu et al. 2022). Lyko and Wicke (2021) suggest that there have been other duplications, but these are questioned by Xu et al. (2022). For the evolution of nuclear genome size in the family, see Weiss-Schneeweiss et al. (2005); genome size is reduced after polyploidization (also see Lyo et al. 2017 for overall variation in the family). Unlike chloroplast genomes, the nuclear genomes of holoparasitic taxa are much larger - to almost 10x - than that of the free-living Lindenbergia, and although the nuclear genomes of the hemiparasites Schwalbea and Odontites are only slightly larger than that of Lindenbergia, that of the hemiparasite Melampyrum is bigger than most of those of other parasitic taxa (Wicke 2013). Orobanche has many more repetitive DNA clusters contributing to genome size increase (Piednoël et al. 2012; see also Westwood et al. 2010; Xu et al. 2022). Gruner et al. (2010) suggested that a facilitating factor for this increase was rootlessness; root growth in taxa with large nuclear genomes is in general reduced, but of course root growth in holoparasitic taxa is minimal, and so this constraint is relaxed. It has also been suggested that both nitrogen availability and genome size tend to be higher in parasites (Vesely et al. 2013), c.f. carnivorous plants, e.g. Lentibulariaceae. Although some gene families have contracted in the parasitic taxa, others have become larger, furthermore, some 11% of the Haustorial Highly Expressed Genes can be traced back to the βL event (Xu et al. 2022). Yoshida et al. (2019) note that there has been around 9% gene loss in the nuclear genome of Striga.
Horizontal nuclear gene transfer from Sorghum bicolor to Striga hermonthica (but not Orobanche) has been demonstrated, the transferred host gene functioning in the nucleus of the parasite (Yoshida et al. 2010), the nuclear-encoded SSL (strictosidine synthase-like) gene has moved from Brassicales to Orobanche aegyptica (D. Zhang et al. 2014), and the distinctive albumen-1 gene from Fabaceae-Faboideae (perhaps from a species close to Onobrychis) to Orobanche s.l. ca 16 Ma (Y. Zhang et al. 2013). Similarly, T. Sun et al. (2016) found that transposable elements had moved to the Phelipanche area from their brassicaceous hosts (perhaps Sisymbrieae or relatives), and were in fact expressed at higher levels in their new hosts in Orobancheae than in their original hosts; the move is dated to 33-16 Ma. Z. Yang et al. (2016) found horizontal transfer of a single nuclear gene in Lindenbergia, two in the facultatively hemiparasitic Triphysaria, ten in the obligately hemiparasitic Striga, and 34 in the holoparasite Aeginetia. Similarly, Kado and Innan (2018) catalogued the movement of quite a number of nuclear genes from Fabaceae and Poaceae to holoparasitic Orobanchaceae - some 84 genes in Aeginetia indica for example - but none to any of the three hemiparasitic taxa that they examined, and at least some of these genes may have been functional in their new home. The genes had introns, and DNA-mediated gene transfer is likely in Orobanchaceae (Kado & Innan 2018). Z. Yang et al. (2019, see also 2016) noted the transfer of numerous functional genes to Phelipanche, half of which came from Rosaceae alone, and many of these transferred genes are involved in haustorial development, as in Cuscuta (stem haustoria there!), however, none of these transfers were ancestral in non-parasitic Orobanchaceae. Organellar genes have made similar moves. The chloroplast rpoC2 gene has moved from Haloxylon ammodendron (Amaranthaceae-chenopod) to Cistanche deserticola (X. Li et al. 2013, q.v. for HGT of chloroplast genes in other Orobanchaceae). The mitochondrial atp6 gene has moved to Orobanche coerulescens probably from its host, Artemisia (Asteraceae) (Kwolek et al. 2017), and plastome genes from Galium to the mitochondrion of Aphyllon epigalium (A. C. Schneider et al. 2018a). For more possible horizontal transfers of chloroplast genes, see Cusimano and Wicke (2016). In general, gene transfer is more likely in holoparasitic species, the whole plant body developing from the terminal haustorium of the seedling (there may be no root cap), while the lateral haustoria prevalent in hemiparasitic Orobanchaceae have nothing immediately to do with tissues that contribute to the flowering shoots (Whigham et al. 2008; Kado & Innan 2018; see also Sanchez-Puerta et al. 2023). There may have been movements of fragments of plastome genes from Phelipanche to Orobanche (Park et al. 2006), although details of this have been questioned (Wicke 2013). See also C. C. Davis and Xi (2015), Cuscuta and Rafflesiaceae for movements of genes in various compartments.
Schneeweiss et al. (2004c) discuss chromosome numbers and karyotype evolution in Orobanche and relatives, and for genome evolution and chromosome numbers in the family as a whole, see Wicke (2013) and Wicke et al. (2013). Edger et al. (2017) suggested that there had been a genome duplication in a clade that contained Striga and Orobanche but not Mimulus (= Erythranthe).
Wicke et al. (2016; see also Wolfe et al. 1992: Epifagus; Wicke 2013; Gruzdev et al. 2019; X. Liu et al. 2019) discuss the evolution of the plastome in the hemi/holoparasitic members of the family. The plastome may be as small as 45 kb in holoparasitic taxa like Conopholis americana (Wicke et al. 2013) and be extensively rearranged. However, a few chlorophyll synthesis genes often remain functional, even in holoparasitic taxa, although overall such genes are much more affected by the adoption of parasitism than housekeeping genes (Wicke et al. 2016), and rbcL genes are also often conserved (Wickett et al. 2011; Wicke et al. 2013); rather unusually Cistanche deserticola retains all its 30 tRNA genes, also its rRNA genes (X. Li et al. 2013). The IR has been lost more than once (Wolfe et al. 1992; Jansen & Ruhlman 2012), thus C. americana has lost its IRa copy, while Phelipanche ramosa has lost its IRb copy (X. Liu et et al. 2019). Indeed, Liu et al. (2019: p. 13) suggested that the "whole Orobanchaceae" lacked the plastid genes psbH and ndhA, with gene losses like that of rpoC1 also being ancient (ca 19.1 Ma). For the loss of ndh genes, see Wicke et al. (2013); Mower et al. (2021) and Sabater (2021) discuss the general ecological context in which such changes are likely to occur. As the plastome changes, the rate at which it evolves changes, both speeding up and slowing down (Wicke et al. 2016; also Liu et al. 2019), but there is no correlation of rate of change with generation time (Cusimano & Wicke 2016). Interestingly, Frailey et al. (2018) found that the plastome in the hemiparasitic Buchnera and Striga in particular had increased greatly in size (25,812 bp in Lindenbergia philippensis, 63,240 bp in Striga forbesii, the inverted repeat expanding into the single copy regions - but in different ways in the five species examined; there was some gene loss, but that, too, varied between the species (Frailey et al. 2018). In Aphyllon sect. Aphyllon loss of photosynthesis genes was not associated with changes in plastome length, either with the loss or subsequently (A. C. Schneider et al. 2013a). Genes or gene fragments have moved (several times each) from the chloroplast to the mitochondrion and in particular the nucleus (Cusimano & Wicke 2016; X. Liu et al. 2019). Indeed, X. Liu et al. (2019) suggested that the atpI gene had been transferred into the nucleus or mitochondrion in some species of Cistanche yet was absent from the larger clade to which those species belong (it includes Conopholis and Epifagus), but since, they thought, there had been similar movement within those genera - both monotypic - this would suggest a complex evolutionary history for that gene. For plastome evolution in parasitic flowering plants in general, see also Wicke and Naumann (2018).
For variation and evolution of the mitochondrial genome, about which little is known, see Wicke (2013).
Economic Importance. A number of Orobanchaceae are very serious agricultural pests. For instance, they are found mainly on legume and grain crops in warmer and drier areas and especially in sub-Saharan Africa where they are still spreading (Parker 2013). These weedy species are for the most part annuals (Schneeweiss 2006), and may be hemi- or holoparasites, species of Striga being particularly serious obligate hemiparasites that affect a variety of monocot crops and also some legumes. Striga hermonthica causes the most damage; different strains grow on different crops and specialize on individual crops when the latter are abundant, depending on the particular environment where they are growing (Bellis et al. 2020. 2021). Striga affects ca 40% of the cereal-producing areas in Africa in particular and it causes average losses in yield of 30-90%, especially when the soils are N- or P-poor and so often in situations where farmers can least cope with the losses. Striga gesnerioides, however, attacks eudicots, and one strain can decimate cowpea (Vigna unguiculata), an important crop in drier (and again poorer) areas of West Africa. Low P in soil there and elsewhere increases Striga germination, strigolactone production increasing, while high P in agricultural soils of the developed world is associated with the Orobanchaceae there like the holoparasites Orobanche cumana (it grows on sunflowers) that are responding to stimulants other than strigolactones and Phelipanche ramosa with its tiny, long-lived seeds that grows on rapeseed, Brassica napus (Bouwmeester et al. 2020). These Orobanchaceae parasitize eudicot crops in particular (there is a fair degree of species specificity) in more or less temperate parts of the world, again with very serious results (Westwood et al. 2010; Fernández-Aparicio et al. 2016). Note that O. cumana seeds are also sensitive to strigolactones (see Bouwmeester et al. 2020 for strigol- versus orobanchol-type strigolactones stimulating he germination of Striga and Orobanche respectively). However, although losses are in the billions of dollars, accurate information about the extent and actual economic losses caused by Orobanche and Striga infestations is hard to obtain (Parker 2009, 2013). The hemiparasite Alectra vogelii may cause the complete loss of legume crops it infects (Morawetz & Wolfe 2009). For more information on these pests, see the various entries in the Invasive Species Compendium (CAB International), and for more on Orobanchaceae seeds and germination, see above.
Uraguchi et al. (2018) have synthesized a strigolactone derivative that even in extremely low concentrations induces germination of Striga hermonthica seeds, and since it seemed to have little effect on AM fungi it could perhaps clear the soil of S. hermonthica seeds, effectively sterilizing it.
It is estimated that the value of dried roots of Cistanche deserticola, of value medicinally in China, will soon reach 20 billion yuan (= >2 billion $ U.S.) (Lei et al. 2021).
Chemistry, Morphology, etc.. Orobanchaceae have orobanchin, a phenylpropanoid ester of caffeic acid, and silicic acid, and their iridoids are produced via the aucubin pathway (Thieret 1971; Rank et al. 2004); c.f. Gesneriaceae. For fatty acids in the seeds of Orobanche and relatives, see Velasco et al. (2000) and Ruraz et al. (2020).
Renaudin (1966) described the remarkable leaves of Lathraea clandestina with their cavities radiating from a central point on the lower surface of the leaf; it has more or less sessile shield glands ("glandes en boucliers") with the gland cells in a linear series and also long-stalked glands with a head like that of typical lamialean glands.
The collar-like base of the corolla tube persists after the rest has fallen off (Fischer 2004b) - is this a family character? Corolla aestivation is interesting in this clade. The abaxial-lateral pair of corolla lobes commonly envelops the adaxial-lateral lobes, while in Euphrasia and its relatives the abaxial lobe also envelops this latter pair of lobes - both forms of quincuncial aestivation; in other Orobanchaceae, the abaxial lobe envelops all other lobes, i.e. ascending cochleate aestivation (Armstrong & Douglas 1989; see also Xia et al. 2021). For floral development, see e.g. Armstrong and Douglas (1989), Endress (1999) and Xia et al. (2021); Erbar and Leins (1996b) noted that corolla initiation in Orobancheae was to a certain extent intermediate between "early" and "late" sympetaly. Greilhuber (1974) observed endomitotic polyploidization in the cells of the inner tapetum in some genera - but not in Pedicularis, Melampyrum, and Plantaginaceae. Pollen in Pedicularidae like Agalinis and Esterhazya, and to a certain extent Physocalyx and Melasma, is crotonoid (Santos & Melhem 2000). The chalazal haustorium of Melampyrum is massive and binucleate (Takhtajan 2013). Perisperm is prominent, even if only a singlr layer across, in holoparasitic taxa, but the endosperm there, although present, is less than in the hemiparasites (Joel & Bar 2013).
For general information, see Terekhin and Nikitcheva (1981), Fischer (2004b: Scrophulariaceae p. pte), Demissew (2004: Cyclocheilaceae), Harley (2004: Nesogenaceae), papers in Folia Geobot. 40(2-3). 2005, the Parasitic Plants website (Nickrent 1998 onwards), Heide-Jørgensen (2008), Schneeweiss (2013) and Nickrent (2020), see also Hjertsen (1995: Lindenbergia); for floral development, see also Canne-Hilliker (1987), Erbar and Leins (1996b) and Xia et al. (2021: Rehmannieae), for corolla aestivation, see Eichler (1875) and Armstrong and Douglas (1989), for the development of the upper lip/galea of the corolla in Pedicularis, see W.-B. Yu et al. (2013), for pollen, see Raj (1985: Nesogenaceae, Cyclocheilaceae - ?crotonoid), Minkin and Eshbaugh (1989), Lu et al. (2007: Rhinantheae), Zare et al. (2014: heteromorphism in Orobancheae), Piwowarczyk et al. (2015: C. European Orobancheae), and Coutinho et al. (2019) and Tsymbalyuk et al. (2023), both Orobancheae, for stigma morphology in Orobancheae, see Ruraz and Piwowarczyk (2022), for ovules and seeds, see Takhtajan (2013), for ovules of Cyclocheilon, etc., see Junell (1934), for embryology, see Krishna Iyengar (1940b), Tiagi (1963) and Arekal (1963) and references, for embryo and endosperm, see Crété (1955), Nagl (1962) and Baskin and Baskin (2021), and for seed morphology, see Musselman and Mann (1976), Joel et al. (2012), M.-L. Liu et al. (2013: Pedicularis), L.-N. Dong et al. (2015: substantial variation) and Piwowarczyk (2015: Boschniaka).
Phylogeny. For the delimitation and composition of Orobanchaceae, see dePamphilis (1995), Olmstead and Reeves (1995), Young et al. (1999), Wolfe et al. (2005), Bennett and Mathews (2006), Latvis et al. (2017) and Nickrent (2020: note i.a. positions of Cymbarieae and Orobancheae reversed). Mortimer et al. (2022) constructed a supermatrix using 12 gene regions that included over 900 species in the family - some 2/5 of the total; note also their Table 2 with ten non-monophyletic genera.
In a rather restricted phylogenetic analysis, Rehmannia (previously in Gesneriaceae) was associated with Oreosolen (Albach et al. 2007), in Scrophulariaceae s. str. (Oxelman et al. 2005), but this may be a rooting problem. In a rather more extended study, Rehmannia was sister to Orobanchaceae, while Oreosolen indeed linked with Verbascum and relatives, forming part of a north temperate group in Scrophulariaceae (Jensen et al. 2008b). In a tree found by Oxelman et al. (2005), Rehmannia linked very weakly with Phryma, Paulownia, Mazus and Lancea, as well as with genera of Orobanchaceae. McNeal et al (2013: both nuclear and plastid genes) included ca 2/3 the genera of Orobanchaceae and obtained a rather strongly supported phylogeny that is the basis for the groupings above, although the position of Brandisia was unclear. Recent work suggests that Rehmannia and Trianeophora, both East Asian, form a strongly supported clade that is sister to the rest of the family - i.e. Lindenbergia and the (hemi)parasitic taxa (Xia et al. 2009, 2019, 2021; see also Albach et al. 2009; Fischer et al. 2012). Albach et al. (2007) had recorded the presence of iridoids in Rehmannia, although these are at best very uncommon in Gesneriaceae, a previous resting place, and also at least some mannitol, a polyol not occurring in Scrophulariaceae s. str. but found i.a. in some Orobanchaceae. However, [Rehmannia + Trianeophora] did not immediately link with other Orobanchaceae in some analyses in Q.-M. Zhou et al. (2014). Rehmannia is not known to be hemiparasitic; it has a racemose inflorescence, its flowers lack bracteoles, the two abaxial-lateral corolla lobes are outside the others (as is common in Orobanchaceae), and its stigma lobes are sensitive. Trianeophora has bracteoles, it may have a staminode, but its floral aestivation is similar, if quite variable (Wang & Wang 2005: close to Digitalis). Phytochemistry also links Triaenophora closely with Rehmannia (Jensen et al. 2008b). Rehmannia has 1:3 nodes and petioles with arcuate + wing bundles, both very common in Lamiales (pers. obs.).
Lindenbergia may be sister to the remainder of the family (e.g. Wolfe et al. 2005; Albach et al. 2009, but sampling limited; Fischer et al. 2012) or it may link more particularly with a small group of parasitic taxa (Bennett & Mathews 2006: support weak). Recent work continues to place it sister to the rest of Orobanchaceae with overall rather strong support (McNeal et al. 2013: not basal in the PHYB + PHYA analyses; some analyses in Q.-M. Zhou et al. 2014; X. Li et al. 2019; Mortimer et al. 2022).
Relationships between clades in the rest of the family are somewhat unclear, however, the contents of those clades seem to be less problematical. Bennett and Mathews (2006) found the relationships [holoparasitic clade (O) [[Castilleja, Pedicularis, etc. (C-P)] [[Euphrasia, Rhinanthus, etc. (E-R)] + [tropical clade (P)]]]]. McNeal et al. (2013) used five genes, three nuclear and two plastid, and obtained a variety of topologies - even the nuclear genes PHYA and PHYB gave different topologies - but using all data their tree can be summarized as [C-S [O [Brandisia [[E-R + P] [S-A + C-P]]]]]. X. Li et al. (2019) analysed the variation in five different nuclear markers and obtained the relationships [O [Brandisia [C-P [C-S [P [S-A + E-R]]]]]], however, when they included the five genes from McNeal et al. (2013) they obtained another topology, [O [C-S [Brandisia [[C-P + A-S] [E-R + P]]]]] and with quite strong support.
R. G. Olmstead (pers. comm. 2003) noted that the inclusion in the tropical clade of Nesogenes (Nesogenaceae) and Cyclocheilon and Asepalum (Cyclocheilaceae), all poorly known, was likely (see also B. Bremer et al. 2002 for Cyclocheilon). There was strong support for Nesogenes (the only taxon of this group included) being sister to the shrubby Radamea (Bennett & Mathews 2006; McNeal et al. 2013), the two genera belonging to the strongly supported tropical clade (Bennett & Mathews 2006). In a comprehensive analysis, ex Cyclocheilaceae and Nesogenaceae are sister to this tropical clade, within which there was some resolution of relationships (Morawetz & Randle 2009, esp. Morawetz et al. 2010); Nesogenes was sister to Graderia and in a clade that includes Striga (Morawetz et al. 2010; see also Fischer et al. 2012). Relationships found by Z.-D. Chen et al. (2016) in Chinese Orobanchaceae largely are consistent with those above. In a study focusing on Acanthaceae, McDade et al. (2012) found a fascinating set of relationships, [Rehmannia [Lindenbergia [Cyclocheilon + the rest]]], although support for the position of Cyclocheilon was not that strong. More recently Xia et al. (2021: chloroplast genomes) recovered the relationships in the (hemi)parasitic part of the family [[Cymbarieae + Buchnereae] [Orobancheae [Rhinantheae + Pedicularideae]]], albeit sampling was poor - no Cyclocheilon, etc.. Y. Xu et al. (2022) looked at relationships between members of the family based on analyses of 907 nuclear orthogroups, and although the first branches in the family are unchanged, readjustments may be needed elsewhere.
The position of Brandisia, a woody liane, is unstable; certainly, the genus is isolated (Bennett & Mathews 2006; esp. McNeal et al. 2013; see also Z.-D. Chen et al. 2016). However, Wightia, with which it has been linked in the past, is not immediately related, but it does belong somewhere in the general area [Mazaceae [Phrymaceae [Paulowniaceae + Orobanchaceae]]] - see above). McNeal et al. (2013) had found that the positions of both Pterygiella and Brandisia varied depending on the datasets used, however, the two were close using the nrITS and the plastid datasets; Brandisia was close to the [Pedicularideae + Buchnereae + Rhinantheae] clade, the latter including Pterygiella, in the PHYB dataset. In another study, the position of Brandisia varied in nrITS and plastid DNA analyses - hard incongruence (W.-B. Yu et al. 2018). Brandisia was sister to Pterygiella, the two in turn sister to Rhinantheae, in the analysis by Xia et al. (2019: chloroplast data), and characters from fruit, seed, and pollen can be adduced to support this set of relationships. Only Wightia of the three genera just mentioned is included in the Seed Plant Tree of Life as of ix.2024, and it is weakly supported as sister to the whole Mazaceae—Orobanchaceae clade.
Orobancheae: For relationships around Orobanche, see Manen et al. (2004: rbcL), Schneeweiss et al. (2004a, c), Park et al. (2006, 2008), X. Li et al. (2017), A. C. Schneider and Moore (2017; focus on Aphyllon) and Gruzdev et al. (2019). Piwowarczyk et al. (2021) recovered the relationships [Cistanche [C. sinensis [[Aphyton + Phelipanche] [Pheleypaea [Bouchardia (a very long branch) + Orobanche]]]]], the whole clade being slightly under 12 Ma.
Pedicularideae: For a phylogeny of Pedicularis, see Ree (2005) and Eaton et al. (2012), while Z.-D. Chen et al. (2016) looked at relationships in ca 50 species of Chinese Pedicularis - P. lachnoglossa was weakly supported as being sister to the other species, while in Eaton et al. (2012) P. muscoides was in that position (support strong). R. Liu et al. (2024: plastome data, 60 spp.) clarified relationships in a major clade of the genus from the Himalaya-Hengduan Mountain region. Gussarova et al. (2008) examined relationships in Euphrasia. Latvis et al. (2024: 7 chloroplast, 4 nuclear markers, 51 spp.) looked at relationships in Agalinis and its relatives; there was some conflict between nuclear and plastid data, and North American species tended to be monophyletic, South American species polyphyletic.
For a re-evaluation of relationships of genera in the old Rhinantheae, of which the core, still substantial, remains, see Tesitel et al. (2010a); Scheunert et al. (2012) suggest that Rhinanthus itself is not monophyletic (see also Bennett & Mathews 2006). Pinto-Carrasco et al. (2017) focused on Odontites, and clearly the pattern of relationships within the tribe has been very fluid, depending in part on the compartment of the genome sampled. Scheunert et al. (2012) and in particular Uribe-Convers and Tank (2015: nuclear ribosomal ITS and ETS, plastid genes) and Uribe-Convers et al. (2016a) examined relationships around Bartsia, which has turned out to be polyphyletic; most species are from the New World and are now in Neobartsia, while Bartsia s. str. (B. alpina, from N.E. Canada to Europe) is not immediately related.
The recently-described Eremitilla is very distinctive morphologically, i.a. the stamens are free from the corolla tube, the anther thecae are more or less embedded in the expanded filament apex, and the ovary is 5-ribbed (Yatskievych & Contreras Jiménez 2009).
Classification. See McNeal et al. (2013) for the tribal classification that is followed here - Cyclocheilon and Asepalum were absentees. Although a number of genera were not sampled, they are small and will have only a marginal effect on species numbers of the clades. Thus 12 genera from the old Buchnereae (Fischer 2003) were not examined, but they include a mere 25 species.
Tank et al. (2009) provide a phylogenetic classification of Castillejinae; Uribe-Convers and Tank (2016) rearranged generic limits around Bartsia, A. C. Schneider (2016) those around Orobanche, and for genera in the Rhinantheae as a whole, see Pinto-Carrasco et al. (2017) - one hopes limits are now fixed, however, the poorly-known Lathraea purpurea currently (2024) resides uncomfortably in that genus (Hatt et al. 2024b).
Previous Relationships. Hemiparasitic genera like Euphrasia and Pedicularis used to be considered intermediates between holoparasitic Orobanchaceae and Scrophulariaceae s.l. (e.g. Boeshore 1920; Cronquist 1981). Rehmannia has often been linked with Titanotrichum and included in Gesneriaceae (Xia et al. 2009 for references).
Botanical Trivia. The purple-flowered Lathraea clandestina is one of the few parasitic plants cultivated for its horticultural merit.
Thanks. To David Tank for useful comments; Robert Mill also caught a number of mistakes around here.