EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm desiccation tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; plastome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm desiccation tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, paleo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and PHYA/PHYCgene pairs.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
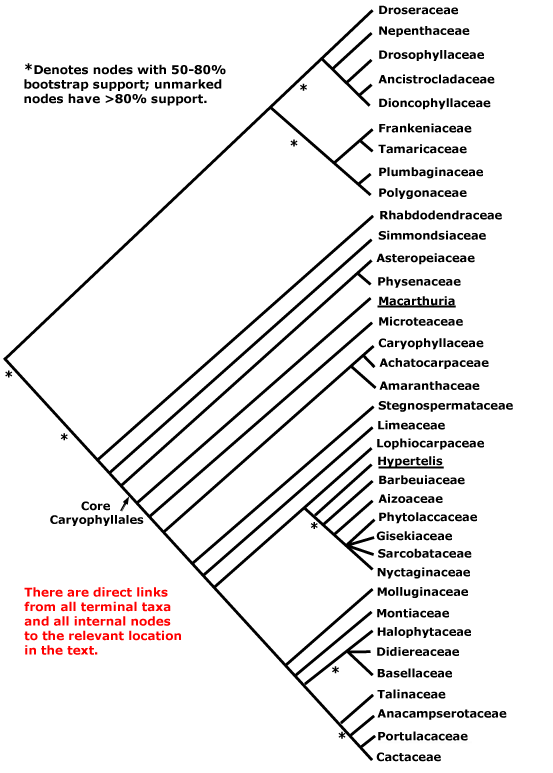
CARYOPHYLLALES Berchtold & J. Presl - Main Tree.
(Odd ecology and/or physiology); plant often not mycorrhizal; (successive cambia +), (medullary vascular bundles +); root hair cells in vertical files [sampling!]; (tracheids +); (cork pericyclic); perforation plates not bordered; only alternate vascular pitting; scanty vasicentric parenchyma; rays both uni- and multiseriate; ?nodes; reaction wood uncommon; (stomata transversely oriented); lamina margins entire; anther wall with outer secondary parietal cell layer developing directly into the endothecium, inner secondary parietal layer dividing, [i.e. the wall is 4 cells across]; pollen colpate, tectum spinulose; G [3], when G = K or P, opposite them, style branches long; ovules with outer integument 2-3(-4) cells across, inner integument 2(-3) cells across; fruit a loculicidal capsule; seed exotestal; embryo long; plastome rpl23 pseudogenized. - 37 families, 749 genera, 11,620 species.
Includes Achatocarpaceae, the ACPT clade, Aizoaceae, Amaranthaceae, Anacampserotaceae, Ancistrocladaceae, Asteropeiaceae, Barbeuiaceae, Basellaceae, Cactaceae, Caryophyllaceae, Chenopodiaceae, see Amaranthaceae, Core Caryophyllales, Didiereaceae, Dioncophyllaceae, Droseraceae, Drosophyllaceae, Frankeniaceae, Gisekiaceae, Halophytaceae, Kewaceae, Limeaceae, Lophiocarpaceae, Macarthuriaceae, Microteaceae, Molluginaceae, Montiaceae, Nepenthaceae, Nyctaginaceae, Petiveriaceae, Physenaceae, Phytolaccaceae, Plumbaginaceae, Polygonaceae, Portulacaceae, the Portullugo Clade, the Raphide Clade, Rhabdodendraceae, Sarcobataceae, Simmondsiaceae, Stegnospermataceae, Talinaceae, Tamaricaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Caryophyllales have been dated to 90-83 Ma (Wikström et al. 2001: see position of Rhabdodendraceae); Anderson et al. (2005) suggests figures of 102-99 Ma, while M. J. Moore et al. (2010: 95% HPD, based on only two taxa) estimated a mere (71-)67(-63) Ma and Xue et al. (2012) the still younger ages of ca 64.4 or 50.4 (the lowest estimate) Ma, Magallón and Castillo (2009) ca 94.35 Ma, Bell et al. (2010: Rhabdodendraceae sister to the rest) an age of (115-)106, 99(-91) Ma, and Naumann et al. (2013) an age or around 78.7 Ma. Sun et al. (2013) thought that the crown-group age was only 76-60 Ma, Z. Wu[?] et al. (2014) suggest an age of around 119 Ma, ca 107.1 Ma is the age suggested by Magallón et al. (2015), and ca 101 or 103 Ma the ages in Hernández-Hernández et al. (2014) and ca 98.7 Ma the age in Fu et al. (2019). Yao et al. (2019) estimate that the age of this node is (115.8-)114.4(-113.2) Ma; this date, and other recorded below for ages in Caryophyllales from this paper, exclude the [Hypertelis + Pharnaceum] clade (Molluginaceae) because its long branch substantially confused age estimates. Note also that some estimates of ages of nodes within Polygonaceae are more than 110 Ma (Schuster et al. 2013), so some ages above and elsewhere in the order may be called into question if these are confirmed.
Evolution: Divergence & Distribution. Caryophyllales contain ca 6.3% of eudicot diversity (Magallón et al. 1999). Maherali et al. (2016) note that the clade - or at least parts of it - have a high speciation rate, perhaps associated with the herbaceous habit that is common here. Just about all the families in Caryophyllales had diverged by the beginning of the Palaeogene ca 66 Ma (Yao et al. 2019). Diversification rate increases in the order are little nested - there are only 2/8 such events (Zuntini et al. 2024).
The fossil record of just about the whole of Caryophyllales is poor. However, X. Wang (2010a: p. 22) was inclined to the idea that Caryophyllales were a very ancient clade: "Caryophyllales should represent, or at least is close to, the most primitive angiosperms". He compared their flowers to the reproductive structures of the coniferous Voltziales. Less cosmically, Doyle (2012) suggested that tricolpate pollen was "retained" in Caryophyllales.
Cuénoud (2002a, b) summarized variation in Caryophyllales. There are many unusual characters scattered in the order, but their phylogenetic significance is unclear, partly because of sampling problems; e.g. sampling for anther wall development is not good (Dahlgren & Clifford 1982). Furthermore, members of the basal pectinations in the core Caryophyllales are particularly poorly known. Similarities in sieve tube plastids between Triplarieae (Polygonaceae) and centrosperms are here treated as parallelisms (c.f. Judd & Olmstead 2004). Ronse de Craene (2013: see Table 1) looked at aspects of perianth and androecium morphology and development in the order, optimizing a number of characters on the tree. Pantoporate pollen is scattered throughout the order, having evolved at least 14 times here, but only (in this context!) 66 times in all angiosperms (Prieu et al. 2017). Given the variation in carpel number in the clade, it is with some hesitation that three carpels is suggested as the plesiomorphic condition. For the loss of the chloroplast rpl23 gene, see Yao et al. (2019) and de Almeida et al. (2021).
S. A. Smith et al. (2017) found that the 13 genome duplications (see below) on which they focussed were quite often associated with shifts in climatic preferences, but there was no tight link between these duplications and increases in diversification rates. Parins-Fukuchi et al. (2021) saw a relationship between periods of morphological innovation and those of phylogenomic conflict, a period when successive cambia, various embryological features, betalains, etc., evolved. They dated this period to (101-)77(-59) Ma, although note ages around here tend to be rather conflicting. Cunha Neto et al. (2024: see Fig. 6) looked at diversification across the order and with a focus on any relationship it might have with the evolution of medullary vascular bundles.
Ecology & Physiology. Caryophyllales - especially Chenopodiaceae s. str. but also the [[Frankeniaceae + Tamaricaceae] [Plumbaginaceae + Polygonaceae]] clade - are notable for the number of taxa that are halophytes, tolerating salt concentrations of 200mM (Flowers & Colmer 2008; Flowers et al. 2010; Bromham 2015; Saslis-Lagoudakis et al. 2016; see also articles in Ann. Bot. 115(3). 2015; White et al. 2017: Na accumulation in both saline and non-saline environments). It is noteworthy that sulphated phenolic compounds occur in the [[Frankeniaceae + Tamaricaceae] [Plumbaginaceae + Polygonaceae]] part of the tree in particular, plants with such compounds often being halophytic - and this combination also includes seagrasses (McMillan et al. 1980). Furthermore, families scattered through this clade - Plumbaginaceae, Caryophyllaceae, Chenopodiaceae s. str., Tamaricaceae and Nyctaginaceae are examples (see also Douglas & Manos 2007) - include a number of species that are gypsophiles (gypsum = hydrous calcium sulphate) and so have to be able to handle sulphur (Escudero et al. 2014; M. J. Moore et al. 2016; C. T. Muller et al. 2017). Perhaps associated with this, Lee et al. (2011) found that genes involved in metabolic processes involving sulphur compounds clustered at this node. Interestingly, Caryophyllales are often not associated with arbuscular mycorrhizal (AM) fungi (but see Newman & Reddell 1987), and they quite commonly grow on soils with high phosphorus (P), as in weedy Amaranthaceae, or with low P or P that is not readily available, both situations in which other non-AM plants are common (Lambers et al. 2015c). Interestingly, gypsum-derived soils may have distinctive AM fungi (Torricellas et al. 2014); there is perhaps a parallel here with Brassicales, also often non-AM plants...
Cornwell et al. (2014) found that plants in Caryophyllales were characterized by being relatively small and having relatively high leaf nitrogen. S. A. Smith et al. (2017) noted that relatively very few Caryophyllales were to be found in wet ecosystems, the majority prefering (much) drier conditions. Taxa with other distinctive habits (e.g. grapnel climbers) or physiology (carnivory, salt tolerance, C4 pathway, CAM) or both are also common in this clade. Perhaps associated with this ecological diversity are various distinctive anatomical features that are notably common here (see also Carlquist 2010), these include anomalous secondary thickening (Carlquist 2013) and rayless wood (Carlquist 2015b).
Plant-Animal Interactions. Some chrysomelid beetles - Alticinae, Cassidinae-Hispinae - seem notably more common here than elsewhere (Jolivet & Hawkeswood 1995), and some insects eat taxa in both the main groups of this clade (Tempère 1969).
Plant-Bacterial/Fungal Associations. Landis et al. (2002) and Trappe (1987) suggested that both Polygonales and Caryophyllales (here just the one order) commonly lacked arbuscular mycorrhizal (AM) fungal associates, although there are exceptions, e.g. some Nyctaginaceae and Amaranthaceae (e.g. Maherali et al. 2016; Brundrett 2017b; also Soudzilovskaia et al. 2020). Indeed, the distinction between the non-mycorrhizal and AM conditions may not be that clear cut here, as elsewhere. Thus Dianthus deltoides may have AM fungi, vesicles sometimes being produced but arbuscules only rarely, and there may be some movement of carbon from plant to fungus (Lekberg et al. 2015). Kamel et al. (2016) discuss the possibility that resistance to oomycete infections is associated with the loss of ability to form mycorrhizae (see also Brassicales).
Purple-spored smuts and Uromyces rusts parasitize several families, including Plumbaginaceae, Polygonaceae and centrosperms (Savile 1979b); he considered this to be a strong sign that the groups were close.
Vegetative Variation. The placement of several features of wood anatomy on the tree needs checking, although Carlquist (e.g. 2002b, 2003a, 2010) has provided a vast amount of detail (see also the centrosperms). Non-bordered perforation plates may be a synapomorphy for Caryophyllales or Caryophyllales and Santalales, depending on where the two go (Carlquist 2000a; see also Carlquist 2010). Anomalous secondary thickening by successive cambia is widespread - it often occurs in lianes in general, but also elsewhere here - and there is considerable variation in the morphology of these cambia (Carlquist 2010, much discussion), and such cambia may also occur in the root (see also Bailey 1980). Maximally biseriate rays are also widespread, including in Asteropeiaceae, but not in centrosperms (Nandi et al. 1998). Reaction wood is at best uncommon (Höster & Liese 1966). Medullary vascular bundles are scattered throughout the order in 9 families (6 groups) - what is remarkable is that overall there have been 36.7 ± 5.3 gains of his character and 65.1 ± 11.2 losses, these changes sometimes happening in closely related taxa and mostly within the last 10 My or so (Cunha Neto et al. 2024). Trying to do much in the way of optimising this character on the tree would seem to be a largely academic exercise; see Cunha Neo et al. (2024: Fig. 4) for the complexity of the evolution of this feature. Cunha Neto et al. (2024) found that the diversification rate seemed to be higher in clades with medullary bundles, and also having them seemed to entail physiological consequences - near the stem apex the potential vascular conductivity of these bundles was 96% of the total, and it remained over 50% in older stems of herbaceous taxa, although it was less in taxa that were lianescent.
Genes & Genomes. For possible genome duplications here, see Y. Yang et al. (2015, 2017) and S. A. Smith et al. (2015, 2017). Of the 26 duplications found by Yang et al. (2017), double the number found by Yang et al. (2012), almost half were quite shallow in the tree, characterising species or small groups of species; Yang et al. (2017) suggested that two of these duplications were allopolyploidy events. Some duplications are placed uin the hierarchy below, but these papers should be consulted for the full picture; see also Stull et al. (2023).
Yao et al. (2019: esp. Fig. 3) discuss aspects of the evolution of the plastome throughout the order; for the loss/pseudogenization of some or all of the ndh genes, see also Mower et al. (2021) and de Almeida et al. (2021). The rpl23 gene is a pseudogene in the few Caryophyllales examined (Logacheva et al. 2008).
Chemistry, Morphology, etc.. Isoflavonoids are scattered in the group (Mackova et al. 2006), perhaps especially in the centrosperms. Flavonol sulphates occur in Plumbaginaceae, Polygonaceae, and Amaranthaceae (Chenopodiaceae s. str.), and sulphated betalains in Phytolaccaceae.
For root hair development, see Dolan and Costa (2001). Carlquist (2010) suggests that few families in Caryophyllales have "truly adult" wood patterns. For the leaf and stem anatomy of a number of halophytes of this clade, see Grigore et al. (2014), for general discussion on wood anatomy, see Schwallier et al. (2017). Taxa with transversely-oriented stomata are known from ten families or so scattered throughout Caryophyllales (Rudall et al. 2023b: p. 1044).
The outer stamens are often initiated in pairs, especially in centrosperms, but also elsewhere in the order (Ronse Decraene & Smets 1993). A petal and adjacent (antepetalous) stamen are developmental units in Plumbaginaceae and Caryophyllaceae (Friedrich 1956; Ronse Decraene et al. 1998). Tricellular pollen grains are common; for pollen evolution, see Y. Yu et al. (2018). Long style branches, or separate styles more or less joining at the apex of the ovary, are widespread. Carpels that are open in development are known both from Polygonaceae and some centrosperms (Tucker & Kantz 2001).
It is unclear where the character of starchy endosperm is to be put on the tree. The condition is unfortunately not known for taxa in the pectinations just below core Caryophyllales. Netolitzky (1926), however, noted that taxa that he knew about (and here included in the centrosperms) lacked starchy endosperm, and starch was not recorded from the thin endosperm found in the seeds e.g. of some Amaranthaceae (Rocén 1927; Shepherd et al. 2005b and references), while the absence of starch in the endosperm of Phytolacca (Woodford 1924) and Trianthema (Aizoaceae: Cocucci 1961) was also specifically noted. However, Bhargava (1935) recorded starch in the endosperm of Trianthema (Aizoaceae), Narayana and Lodha (1963) reported starch in the young endosperm of Orygia (and Corbichonia, both Lophiocarpaceae), Kajale (1940b) noted dense starch grains in the mature endosperm of Amaranthaceae, and Kajale (1954) starch in the endosperm of Rivina humilis (Phytolaccaceae). Although several centrosperm families are reported to have starchy endosperm in the Flora of China (e.g. Dequan & Gilbert 2003), this is likely to reflect confusion with the starchy perisperm. Those reports aside, the nature of any endosperm reserves in the Rhabdodendraceae to Cactaceae clade remains an open issue, and starchy endosperm is provisionally placed as an apomorphy for the Droseraceae to Polygonaceae clade alone.
Phylogeny. Hilu et al. (2003: matK analysis alone) suggest that Caryophyllales are sister to Asterids, a relationship that has been found in some other studies (e.g. Soltis et al. 1997, c.f. also Nandi et al. 1998). A relationship between Caryophyllales and Dilleniales has also been suggested (D. Soltis et al. 2003a). However, Caryophyllales alone (or perhaps with Santalales) now seem to be sister to the asterids, although the support is still only moderate; see the Pentapetalae page for further discussion.
There are two main clades within Caryophyllales, one includes the core Caryophyllales, which in turn includes the old Centrospermae, and the other includes Polygonaceae, etc., and a number of carnivorous taxa like Nepenthaceae and Droseraceae. This latter clade is well-supported (Morton et al. 1997b; Soltis et al. 2011), although it was not recovered in the mitochondrial analysis of Qiu et al. (2010) and relationships within it are scrambled in Bell et al. (2010). Indeed, although relationships around Polygonaceae seem stable, those in the rest of this clade do not. There are four carnivorous families here that have attracted a lot of attention (see also Albert et al. 1992; Meimberg et al. 2000; Cuénoud et al. 2002; Cameron et al. 2002; Renner & Specht 2010) and other families with distinctive vegetative morphologies (see also Heubl et al. 2006). Metcalfe (1952a) suggested relationships between members of this group based on anatomical similarities. S. Williams et al. (1994) drew atttention to connections between Dioncophyllaceae and Drosophyllum in particular, and Drosophyllum and Nepenthaceae have also been found to be weakly associated (Morton et al. 1997b). Soltis et al. (2011) found Drosera and Nepenthes to be sister taxa, but the support was only moderate and sampling not extensive. For detailed relationships, see Meimberg et al. (2000) and Cameron et al. (2002); Drosophyllaceae are sister to Dioncophyllaceae + Ancistrocladaceae, with good support, in an analysis of matK sequences, the position of Nepenthaceae being uncertain (Cuénoud et al. 2002). Indeed, Crawley and Hilu (2013: two genes) found either a weakly supported [Nepenthaceae + Droseraceae] clade, or Nepenthaceae were sister to the rest of the group, depending on the method of analysis, and the latter position was also recovered by Brockington et al. (2015). The position of Drosophyllaceae was particularly unstable in the 10-transcriptome study of J. F. Walker et al. (2017), while Y. Yang et al. (2017) recovered the relationships [Drosophyllaceae [Nepenthaceae + Droseraceae]]. Interestingly, the well supported relationships [[Droseraceae + Nepenthaceae] [Drosophyllaceae [Dioncophyllaceae + Ancistrocladaceae]]] were recovered in a phylogenomic analysis of the plastomes of 85 Caryophyllales by Yao et al. (2019) — "only" 91% BS support for the position of Nepenthaceae, although this depended on the analysis; some pollen characters may support the [Droseraceae + Nepenthaceae] grouping. The same relationships were found by H.-T. Li et al. (2019) in their plastome analysis. However, in the phylogenomic analysis of Murphy et al. (2019/2020) and in the recent study by W. J. Baker et al. (2021a: see the Seed Plant Tree Version 1) the relationships [Droseraceae [Nepenthaceae [Drosophyllaceae [Ancistrocladaceae + Dioncophyllaceae]]]] were obtained, and with strong support, and also by Scharmann et al. (2021), although the latter found that fewer than half of the gene trees agreed with this placement; this topology was used by Palfalvi et al. (2020). In the Seed Plant Tree ii.2022 version basal relationships are [[Droseraceae + Nepenthaceae] [Drosophyllaceae ...]] - the support was quite strong.
Indeed, the monophyly of this whole clade, carnivorous taxa plus Polygonaceae, etc., is not always evident (Walker et al. 2018a: transcriptome analyses, checking of signals responsible for discordant topologies, b). Walker et al. (2017) had recovered the relationships [Chenopodiaceae [[Frankeniaceae + Tamaricaceae] [[Plumbaginaceae + Polygonaceae] [the carnivorous clade]]]] in some analyses, although a monophyletic group including the first five families was also obtained, as were yet other topologies. Although the focus of this study was on the carnivorous taxa, other sampling being exiguous in the extreme, relationships in the Droseraceae-Plumbaginaceae area clearly need clarification, and so the basic topology of Caryophyllales used here is somewhat questionable (see also Walker et al. 2018a, b; Sheehan et al. 2019).
Within the other major clade, Rhabdodendraceae were sister to the other members in an early rbcL analysis of Fay et al. (1997b), in the Bayesian analysis of Soltis et al. (2007a) and also in Bell et al. (2010); see also Murphy et al. (2019/2020), etc.. Cuénoud et al. (2002) found that Simmondsia was grouped with Rhabdodendron in a matK analysis, but with only weak support, but in two- and four-gene analyses (with poorer sampling) it was associated with core Caryophyllales; in trees shown by Drysdale et al. (2007) and Brockington et al. (2007, esp. 2009, 2015) a position of Rhabdodendron as sister to all other core Caryophyllales was again found in most analyses, and is adopted here (see also Hilu et al. 2003: matK analysis; Soltis et al. 2011). Relationships around Rhabdodendraceae, etc., are somewhat jumbled in the tree presented by Qiu et al. (2010). However, Asteropeiaceae and Physenaceae often form a well supported pair, in turn showing a well-supported sister group relationship to the centrosperms (e.g. Källersjö et al. 1998; Brockington et al. 2015). Similarly, Asteropeiaceae and Simmondsiaceae, the only two taxa from this part of the order that were included, were successively sister groups to the core (D. Soltis et al. 2000). However, Rhabdodendraceae have sometimes been found to be sister to all Caryophyllales, albeit with weak support (Cuénoud 2006). The tree below is based largely on those presented by Meimberg et al. (2000), Cameron et al. (2002: 4 genes), Cuénoud et al. (2002: matK alone) and Yao et al. (2019: 85 plastomes). For details of relationships within the centrosperms, see below.
Classification. For a classification of Caryophyllales at the family level, including a complete generic-level synonymy, see Hernández-Ledesma et al. (2015). The main difference there from the classification here is that Amaranthaceae and Chenopodiaceae were kept separate and a broad concept of Phytolaccaceae was accepted. For some families the work of Hernández-Ledesma et al. has been substantially developed - see the Caryophyllales portal.
Previous Relationships. Takhtajan's (1997) Plumbaginanae are monotypic; Nepenthanae included Droseraceae and some other Caryophyllales, but also families now in Ericales, etc.. Many of the families in Caryophyllales were included in Cronquist's (1981) Caryophyllidae. Plumbaginaceae are rather similar in a few respects to Primulaceae and relatives (Ericales) and the two have been considered close in the past (see Cronquist 1981 for discussion); Friedrich (1956) had effectively discounted such ideas.
Synonymy: Aizoineae Doweld, Basellineae Doweld, Cactineae Bessey, Caryophyllineae Bessey, Chenopodiineae Engler, Nyctaginineae Doweld, Phytolaccineae Engler, Portulacineae Doweld, Simmondsiineae Reveal, Stegnospermatineae Doweld - Aizoales Boerlage, Alsinales J. Presl, Amaranthales Berchtold & J. Presl, Ancistrocladales Reveal, Atriplicales Horaninow, Cactales Berchtold & J. Presl, Chenopodiales Berchtold & J. Presl, Dioncophyllales Reveal, Droserales Berchtold & J. Presl, Frankeniales Link, Illecebrales Berchtold & J. Presl, Mesembryanthemales Link, Nepenthales Dumortier, Nyctaginales Berchtold & J. Presl, Opuntiales Willkom, Paronychiales Link, Petiveriales Link, Physenales Takhtajan, Phytolaccales Link, Plumbaginales Berchtold & J. Presl, Polygonales Berchtold & J. Presl, Portulacales Berchtold & J. Presl, Reaumuriales Martius, Rhabdodendrales Doweld, Riviniales Martius, Scleranthales Link, Silenales Lindley, Simmondsiales Reveal, Staticales Link, Stellariales Dumortier, Tamaricales Link, Telephiales Link - Caryophyllanae Takhtajan, Nepenthanae Reveal, Plumbaginanae Reveal, Polygonanae Reveal, Rhabdodendranae Doweld, Simmondsianae Doweld - Caryophyllidae Takhtajan, Plumbaginidae C. Y. Wu, Polygonidae C. Y. Wu - Amaranthopsida Horaninov, Cactopsida Brogniart, Caryophyllopsida Bartling, Opuntiopsida Endlicher, Polygonopsida Brongniart, Plumbaginopsida Endlicher
[[[Droseraceae + Nepenthaceae] [Drosophyllaceae [Ancistrocladaceae + Dioncophyllaceae]]] [[Frankeniaceae + Tamaricaceae] [Plumbaginaceae + Polygonaceae]]]: acetogenic naphthoquinones + [inc. plumbagin, 7-methyljugone]; endosperm starchy.
Age. The age of this clade is perhaps 75-67 Ma (Wikström et al. 2001); the crown group age in Bell et al. (2010) is (100-)91, 86(-77) Ma and ca 93.3 Ma in Veleba et al. (2017), in both [Frankenia + Tamarix] sister to the rest, The latter suggesting that [Polygonaceae + Plumbaginaceae] diverging from this whole group ca 98.7 Ma, while ca 99.3 Ma is the age in Magallón et al.( 2015).
Evolution: Divergence & Distribution. For gland morphology and vascularization in this part of the tree, see T. Renner and Specht (2011); optimisation is not easy. Although sessile, stalked and pit glands are found in the [Plumbaginaceae + Polygonaceae] clade, how similar they are to stalked glands found in some members of the carnivorous clade, and also to the sessile to depressed salt glands in the [Frankeniaceae + Tamaricaceae] clade is unclear; T. Renner and Specht (2011) did not include the latter clade in their study. Conran et al. (2007) noted that Frankeniaceae, Tamaricaceae and Plumbaginaceae all have flat, multicellular glands of subepidermal origin. This is perhaps an apomorphy there (or still higher), with a loss in Polygonaceae.
Genes & Genomes. J. F. Walker et al. (2017; see also S. A. Smith et al. 2017) found seven genome duplications in this clade, six being at the family level or within families; they also mention a genome duplication shared by the entire clade, but this seems to refer to the γ triplication event of the core eudicots.
Chemistry, Morphology, etc.. The acetogenic naphthoquinone plumbagin is known from Plumbaginaceae, Droseraceae, Nepenthaceae, and Dioncophyllaceae, and related compounds are found in Polygonaceae (Culham & Gornall 1994; Kovácik & Repcák 2006).
[[Droseraceae + Nepenthaceae] [Drosophyllaceae [Ancistrocladaceae + Dioncophyllaceae]]] / The Carnivorous Clade: plants carnivorous [insectivorous], forming a rosette at least when young; inflorescence ± cymose; C contorted; anthers extrorse; ovary unilocular.
Age. The age of this node in Bell et al. (2010) is (90-)77, 72(-60) Ma, but note the topology there; ca 83 Ma is the age suggested by Magallón et al. (2015), 83-68 Ma the age in J. F. Walker et al. (2017) and ca 74.5 Ma in Veleba et al. (2017). A number of ages for clades around here are suggested by Biswal et al. (e.g. 2017) in a paper that can easily be found on the internet, however, the rooting of the trees is sometimes wrong, with the result, for example, that the split between Sarraceniaceae and Nepenthaceae is estimated to be a mere 19.1 Ma (Biswal et al. 2017)...
Evolution: Divergence & Distribution. For a synapomorphy scheme for the whole group, see in part Albert and Stevenson (1996), Meimberg et al. (2000: the floral characters listed are mostly plesiomorphies), but especially Heubl et al. (2006).
Heubl et al. (2006) suggest that fly-paper traps are the plesiomorphic condition for the group, but where features like this or the possession of circinate leaves and pollen tetrads are placed on the tree will depend on the model of character optimisation used.
+Ecology & Physiology. The acquisition of carnivory may have happened more than once here, or it occurred once and then was lost, perhaps more likely given the topologies found (Meimberg et al. 2000; Cameron et al. 2002: see also Schlauer 1997). Interestingly, extrafloral nectar in at least Droseraceae, Drosophyllaceae and Nepenthaceae contains (+)-isoshinanolone, an acetylcholinesterase inhibitor that hinders neuronal activity of insects attracted to the traps, i.a. by the sugar in the nectar (Lathika et al. 2025)... T. Renner and Specht (2011) suggest scenarios for the evolution of digestive glands, and find that novel chitinase genes - otherwise involved in anti-fungal activities - have become involved in the extracellular degradation of the chitin of arthropods in this clade (Rottloff et al. 2011; Renner & Specht 2012). Other digestive enzymes involved seem to be coopted stress-responsive proteins, as is the case in other carnivorous plants like Sarraceniaceae and Cephalotaceae (Fukushima et al. 2017 and references). For much on carnivory, see papers in Ellison and Adamec (2018), and for recent developments, see Hatcher et al. (2020) and Adamec et al. (2021). Lloyd (1942) and Juniper et al. (1989) discuss carnivory in general.
Chemistry, Morphology, etc.. For the vegetative morphology of carnivorous members of this clade, see Kaplan (1997, vol. 2: chap. 18). For roots in carnivorous taxa, see Adlassnig et al. 2005).
For general information, especially photographs, see McPherson (2010), for information on acetogenic quinones and alkaloids, see Hegnauer (1986), Bringmann and Pokorny (1995) and Bentley (1998).
Phylogeny. For relationships in this part of the tree, see above.
[[Droseraceae + Nepenthaceae] - if this clade exists: endomycorrhizae +; flavonols +; (medullary vascular bundles +); large spirally-thickened fibre-sclereids [in pith, pericycle, etc.]; pollen in tetrads; endosperm +, nuclear.
Age. Ca 73.9 Ma is the age of this node (Tank et al. 2015: Table S2).
Evolution: Divergence & Distribution. Palfalvi et al. (2020) noted that there was some similarity in the genes involved in carnivory in Droseraceae and Nepenthaceae, although "sophisticated mechanisms of carnivory" had apparently evolved independently in the two.
Genes & Genomes. Heubl and Wistuba (1997) suggested that both Droseraceae and Nepenthaceae had ploidy levels of 8 or 16, based on x = 5 or thereabouts.
DROSERACEAE Salisbury, nom. cons. —— Synonymy: Aldrovandaceae Nakai, Dionaeaceae Rafinesque - Back to Caryophyllales
Rosette herbs, (woody), (cormose), (vines); ellagic acid +; cork?; vascular bundles initially separate, (in two rings), medullary rays broad; cambium 0; nodes 1:1; stem with endodermis; petiole bundles various; stomata also tetracytic or actinocytic; stalked glands with phloem only [Drosera], sessile glands +; leaves adaxially circinate, long-stalked glands or trigger hairs adaxial, leaf moves, stipules +, intrapetiolar, ± fimbriate, or 0; inflorescence terminal, cyme monochasial, (bracts/bracteoles 0); K often connate at base, C ± marcescent; stamens = and opposite K (-15 - Dionaea), anthers ?dorsifixed, (introrse), (connective expanded); (tapetum amoeboid); pollen bi- or tricellular, 3-multiporate, pores equatorial [stephanoporate], apertures large and complex, protrusions along the borders of adjoining grains, operculate or not, (with orbicules); G [3(-5)], median member abaxial, styles connate (Di.), if only ± basally (± free - Aldrovanda), often bifid to multifid, stigmas papillate; placentation parietal (basal - Di.); ovules 3-many/carpel, parietal tissue often absent, nucellar endothelium +, column of cells in suprachalazal zone; (fruit indehiscent); exotesta palisade or not, (endotesta with U thickenings), endotegmic cells small, ± sclerotic, or mucilaginous; (embryo short), (cotyledons 0); nuclear genome duplication [NCORE5]; n = 5, 5-17, 19, x = 7 (?8, ?6), chromosomes <1.5µm (<6µm - Di.), holocentric, [Cx] = (244-)509(-3745) Mbp; plastid transmission biparental, plastome rpl2 intron, trn K intron 0, new, intron-less trnK gene, ndh gene complex 0 [missing/non-functional]; (germination cryptocotylar), seedling roots poorly developed.
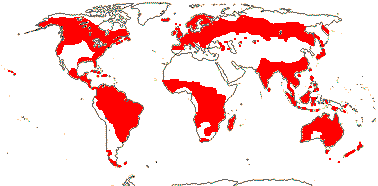
3/205: [list], Drosera (200). World-wide. Map: from Hultén (1971), Fl. Austral. vol. 8 (1982), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Correa A. and Silva (2005). [Photos - Collection, Collection.]
Age. Crown-group Droseraceae are estimated to be ca 54.7 Ma (Veleba et al. 2017). Biswal et al. (e.g. 2017) offered a stem age for Droseraceae of ca 84.1 Ma and crown ages around 65-55 Ma.
Paleoaldrovandra splendens was described by Knobloch and Mai (1984) from seeds found in rocks ca 90 Ma in what was then Czechoslovakia. However Hermanová and Kvacek (2010) suggest that the fossils differ in both gross morphology and details of cellular anatomy and are more likely to be the eggs of an insect - but, unfortunately, they could not be more precise. Palaeocene pollen identified as Drosera has been reported from Meghalaya, India (Kumar 1995).
Evolution: Divergence & Distribution. The beginning of diversification within Drosera may date to ca 42 Ma. However, a pre-continental drift time has also been suggested (Yesson & Culham 2006 and references), although Rivadavia et al. (2003) had suggested that Droseraceae are not Gondwanan. Drosera is exceptionally diverse in S.W. Australia, where about one third of the species in the genus grow; diversification may be linked to the onset of the Mediterranean climate there some 15-10 Ma - another case of diversification in "long-term, stable but heterogeneous habitats occurring on ancient, highly infertile soils" (Wilkinson et al. 2025), sections Bryastrum and Ergaleium being particularly diverse. All told, there are 163 Australian species described by Lowrie (2013: vols 1, 2). Rather surprisingly, the otherwise Australian pygmy sundew clade includes D. meristocaulis, a plant from Guyana (Rivadavia et al. 2012). Wilkinson et al. (2025) found relationships bwteeen the South African D. regia, the south-eastern Australian D. arcturi and D. murfetii and the New Zealand D. arcturi, albeit the support was not very strong, and this group also discussed other patterns of relationships in the genus.
There is a great deal of vegetative variation associated with different means of vegetative reproduction in Australian species of Drosera (Wilkinson et al. 2025).
For gland morphology (rather typological), germination and phylogeny, see Conran et al. (2007 and references). Interestingly, the calyx glands of noncarnivorous Plumbago (Plumbaginaceae) are anatomically similar to the mucilage glands of carnivorous genera Drosera and Drosophyllum; are they a synapomorphy for this part of Caryophyllales - = "a common ancestral gland structure" (Thorogood et al. 2018; Caperta et al. 2020: p. 3)?
Ecology & Physiology. For a review of the ecophysiology of Droseraceae, see Adamec (2011b) and references. Aldrovandra and Dionaea both have snap-traps with multicellular trigger hairs; more is known about the functioning of the trap of D. muscipula, Venus's flytrap, which can catch spiders, beetles and ants (Younstaedt et al. 2018). Gibson and Waller (2009; see also Williams 2002) discuss the evolution of the these traps in Dionaea, which are unique in angiosperms; they perhaps allow the plant to capture larger prey than it could otherwise utilize, but moderate-sized crickets seem to be the largest size prey the plant can handle (A. L. Davis et al. 2018). Kreuzwieser et al. (2014) noted that the leaves have a sweet scent, perhaps mimicking the smell of fruit that would attract fruit flies, although Williams and Hartmeyer (2017, see also above) found that in the wild its prey is largely other than flies, 70% consisting of spiders, ants and beetles. Hartmeyer et al. (2019) later noted that small ants were unlikely prey, since they could probably see (and avoid) the trigger hairs and could escape from the trap even after it was triggered since full closure was not immediate; in any case two stimuli were needed for the mechanism to work. Ants like Lasius neglectus tended to visit the marginal dry "alluring glands" rather than the centre of the leaf where the trigger hairs were (Hartmeyer et al. 2019).
Stuhlman (1948), Forterre et al. (2005), Sachse et al. (2020) and others have studied the mechanics of trap closure in Dionaea, while Tian et al. (2021) and W. Li et al. (2021: soft actuator) describe some applications of the physical principles involved. The two halves of the leaf go from being longitudinally concave to longitudinally convex as the upper epidermal cells lose fluids and the lower epidernal cells gain fluids, but less, the status of the mesophyll cells not changing. The traps close in 100-300 ms after the trigger hairs have been stimulated twice, i.e. after the leaf senses two action potentials resulting from stimulation of the hairs by the prey; leaf movement is speeded by the changes in shape of the leaf blade just mentioned. The trap finally becomes hermetically sealed and the prey is then bathed in digestive enzymes after five such action potentials are sensed (Forterre et al. 2005; Volkov et al. 2008; Böhm et al. 2016a, b; Hedrich & Neher 2018: general summary). Escalante-Pérez et al. (2011), Volkov and Markin (2014), Böhm et al. (2016a, b) and Bemm et al. (2016) provide details of the physiological changes that occur during closing and the post-closing digestive processes. Scherzer et al. (2017) noted that the contents of the secretory vesicles released follow a sequence that optimizes digestion of the animal - the first vesicles have H+ and Cl-, so the contents of the trap become acid, and later enzymes, etc., are secreted in a process that takes hours or days. Uptake of nutrients by the leaves stimulates uptake of nutrients by the roots (Adamec 2011b), as in other carnivorous plants.
The response is limited to a single leaf, as in Drosera (c.f. Mimosa pudica), and is identical to that caused by wounding with a needle - perhaps not surprising since carnivory is thought to have evolved from plant defence mechanisms that were activated by, for instance, damage/chewing by a herbivore (Pavolvic et al. 2017; Hedrich & Neher 2018). In particular, the touch hormone jasmonic acid that is involved in defence responses of the plant, e.g. response to herbivory, has been coopted into the process of carnivory in Dionaea. Normally it is involved in signalling cascades that can result in cell death, the production of toxins, etc., while in carnivory it is involved in responses that result in the digestion of the prey (Bemm et al. 2016).
How the traps of Aldrovanda vesiculosa work has been clarified recently. Smaller than the the traps of Dionaea, they also move perhaps up to 10 times as fast, trapping happening in 10-20 ms (Westermeier et al. 2020). Rather than the leaf blades deforming during closure, as in Dionaea, stress accumulates along the midrib (Poppinga & Joyeux 2011; Volkov et al. 2008; see also Adamec 2011b) and there are changes in cell turgor, altogether rather different than what goes on in Dionaea (Westermeier et al. 2018). After the prey is captured, the leaf encloses the prey more tightly and it is digested (Westermeier et al. 2020).
There is quite a variety of hair types in Drosera, and some of these may be similar to the marginal spines of Dionaea (Hartmeyer & Hartmeyer 2010; Hartmeyer et al. 2013). Thus the leaves of D. glanduligera have rapidly-moving eglandular marginal hairs that can snap tight in as little at 75 ms and that pin the prey against glandular hairs in the centre of the blade (Poppinga et al. 2012). These glandular hairs of Drosera can move, sometimes quite quickly, and the whole leaf may then bend and envelop the prey, although rather more slowly. This bending process in D. capensis is the result of the leaf architecture being asymmetrical; the auxin stimulus itself is homogeneously distributed in the leaf (La Porta et al. 2019). Some species of Drosera are sweetly scented (c.f. Dionaea above!), perhaps thereby attracting their prey, indeed, as many as 35 butterflies and moths have been found stuck to a single plant of the large, sprawling D. finlaysoniana (Fleischmann 2016). Dicyphine mirid bugs are able to negotiate the sticky leaf surfaces of Drosera, and this may have implications for nitrogen uptake and plant defence, as in other carnivorous plants like Roridulaceae, since the bugs may eat the trapped insects, nitrogen from bug excreta rather that directly from the prey moving into the plant - and digestive enzymes produced by the plant may not be needed (see Wheeler & Krimmel 2015 for mirids). The glands of at least some species of Drosera produce a ribonuclease which may aid the plant in obtaining phosphorus from its prey, and perhaps also in defence against viruses (Okabe et al. 2005), and they also secrete a chitinase on stimulation that is involved in insect digestion (Jopcik et al. 2017 and references). However, Wheeler and Carstens (2018) found few changes in gene expression categories in D. capensis compared with non-carnivorous plants, while the proteases in D. capensis that Butts et al. (2016) examined, while homologous to those in other plants, differed in ways that suggested that they might have new functionalities - perhaps new substrate recognition patterns. For further literature, see Peroutka et al. (2008b).
There has been extensive gene loss in all three genera, although somewhat less in Aldrovandra vesiculosa, and the genomes are rather small, for example, some 293 Mbp in Drosera spatulata, 509 Mbp in A. vesiculosa - but ca 3,187 Mbp in Dionaea muscipula (Palfalvi et al. 2020). (Similarly, some other carnivorous plants have small genomes, although again there is considerable variation within taxa like Cuscuta and Lentibulariaceae.) In Dionaea, at least, genes active in the digestive glands have been recruited from the root, while in Aldrovandra important regulatory genes involved in root development have been lost; gene families involved in different aspects of carnivory have become enriched (Palfalvi et al. 2020).
Interestingly, Schulze et al. (1991) found little evidence of nitrogen uptake from insects in rosette-forming apecies of Drosera from S.W. Australia, however, up to 64% of plant N came from insects in some climbing species. Phosphorus is also likely to be in short supply in the habitats in which Drosera grows.
Note that the smaller leaves of young Dionaea do not have a snap-buckling closure mechanism, there is a diversity of hair morphology and movement within Drosera itself, and the relationships between Drosera, Dionaea and Aldrovandra are uncertain. Overall, tere is still much to do to clarify the evolutionary contexts of the various trap mechanisms that have evolved in the family (see also Gibson & Waller 2009).
Perhaps rather surprisingly, some species of Drosera grow in seasonally-dry habitats and can tolerate burning (Lamont et al. 2018). Furthermore, when temperatures get cool species of the Australian section Bryastrum produce gemmae in splash cups formed by the stipules, although dispersal is over rather short distances, at most a few tens of centimetres (Bourke 2021).
Pollination Biology & Seed Dispersal. Dionaea muscipula, at least, rarely eats its pollinators, which are mostly bees and beetles (Youngstaedt et al. 2018).
Plant-Animal Interactions. The syrphid Toxomerus basalis lives almost its entire life on/associated with species of Drosera, apparently of section Brasilianae only (Fleischmann et al. 2022). The adults may eat pollen/pollinate the flowers, while the larvae eat insects that have been recently captured by the plant; they do not trigger the tentacles as they move around the leaf, perhaps because of the mucilage/slime (unfortunately of unknown composition) that they secrete and that covers their bodies (Fleischmann et al. 2022).
Plant-Bacterial/Fungal Associations. Endomycorrhizae have been reported from Drosera (Fuchs & Haselwandter 2004).
Genes & Genomes. There is a nuclear genome duplication, with an additional triplication in Aldrovandra vesiculosa (Y. Yang et al. 2017: NCORE5; S. A. Smith et al. 2017; Palfalvi et al. 2020). Schlauer et al. (2022) suggested that the base chromosome number of Droseraceae might be 10. Veleba et al. (2017) found no correlation between genome size or GC content with centromere type or the carnivorous habit in this clade, although there was a correlation between GC content and genome size. Genome size tended to increase in members of the Australian clade but decrease in those of the Cosmopolitan clade (Veleba et al. 2017). For holocentric chromosomes, see Cuacos et al. (2015) and especially Kolodin et al. (2018); Hoshi and Kondo (1998) give chromosome numbers found in the family.
Nevill et al. (2019) discuss the evolution of the plastome in Droseracaee (all three genera examined, Fagopyrum the outgroup), finding quite extensive changes, indeed, more apomorphies could have been added above, and making the point that there were similarities in the evolution of the plastome in both carnivorous and echlorophyllous mycoheterotropic plants (see also Mower et al. 2021 and references). Both groups of plants have more or less switched from a fully autotrophic mode of nutrition (Nevill et al. 2019).
Chemistry, Morphology, etc.. The root hairs of Drosera are up to 15 mm long (Adlassnig et al. 2005). Metcalfe and Chalk (1950) describe distinctive vascular patterns in the inflorescence axis and petiole here. In Drosera aliciae young inflorescences (before flower buds are evident) appear to be abaxially circinate, but this is probably a reflection of the way the monochasial cyme is developing.
Kubitzki (2002b) descrided the anthers as being 2-celled. The pollen is in tetrads, and has been described in detail by Takahashi and Sohma (1981). When the pollen of Drosera and Dionaea is hydrated, protrusions develop along the borders of adjoining grains of the tetrad, and in Dionaea and Drosera regia these protrusions persist in the dehydrated state and are operculate (Halbritter et al. 2012).
See Le Maout and Decaisne (1868), Baillon (1887), Kubitzki (2002d), McPherson (2008), papers in Ellison and Adamec (2018), esp. Fleischmann et al. (2018), and the Carnivorous Plants Database for general information, Hegnauer (1966, 1989) for chemistry, Schlauer et al. (2018) and Schlauer and Fleischmann (2022) for naphthoquinones and hairs, Gregory (1998) for general anatomy, Pace (1912), Venkatasubban (1950) and Boesewinkel (1989) for ovule and seed anatomy and Whigham et al. (2008) for seedlings.
Phylogeny. Aldrovanda and Dionaea may be sister taxa; both have snap-traps, although they are rather different in how they function, n = 6, etc. (Cameron et al. 2002; Rivadavia et al. 2003: little support; Veleba et al. 2017; Nevill et al. 2019: plastomes); see also Williams et al. (1994) for phylogeny.
Rivadavia et al. (2003) discussed the phylogeny of Drosera, in which the position of D. regia was unclear. In both chromosome number and pollen morphology (it has operculate protrusions in the pollen, rather like Dionaea) it is rather different from other species of Drosera. It may be sister to the rest of the genus, or even closer to the other genera in the family (Rivadavia et al. 2003). Veleba et al. (2017: 3 markers) recovered the relationships [D. arcturi [D. regia [Australian clade + Cosmopolitan clade]]]. Williamson et al. (2025: 331 Angiosperms353 and 61 OzBaits loci, plastomes, 85 terminals) examined relationships in Australian species. There was some hybridization between species in subgenus Ergaleium sections Bryastrum and Lasiocephala, also section Drosera.
Classification. Fleischmann et al. (2018b, see also comments in Williamson et al. 2025) provide an infrageneric classification for Drosera which seems to be holding up fairly well.
NEPENTHACEAE Dumortier, nom. cons. - Nepenthes L. - Back to Caryophyllales
Plant a liane, climbing by twining portion of leaves, (± a rosette plant); ellagic acid 0; cork pericyclic; (vessel elements with scalariform perforation plates); true tracheids +, rays?; SiO2 bodies +; pith lignified; stem with endodermis; nodes 5-9:5-9; cortical bundles in stem; petiole bundle arcuate; peltate glands +; leaves sessile, abaxially circinate, base broad, blade in lower half, vernation involute, then narrow twining portion (0), pitcher +, terminal [epiascidiate], (some pitchers, etc., subterranean - N. pudica); plant dioecious; inflorescence a raceme, bracts and bracteoles 0; P +, uniseriate, (3-)4, decussate, large flat nectariferous glands adaxially; staminate flowers: A connate into a central column, (4-)8-25; pollen tricellular, apertures 0/indistinct; pistillode 0; carpelate flowers: staminodes?; G [(3-)4(-6)], placentation axile, style short, stigma broad, papillate; ovules many/carpel, bistomal, outer integument becoming very long, parietal tissue 1 cell across, chalazal projection +; seeds numerous, spindle-shaped, minute; chalaza with hair-pin bundle, exotesta with much thickened inner walls; n = 40, x = ?10, nuclear genome [1 C] (0.034-)1.267(-46-752) pg.
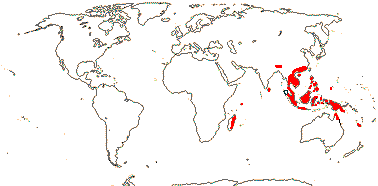
1 [list]/176. Madagascar to New Caledonia. Map: see Meimberg and Heubl (2006). Photo - Leaf; Collection.
Age. The clade [Nepenthes khasiana (the only non-Malesian species included) + Malesian species] is estimated to be (35-)20(-9) Ma (Nauheimer et al. 2019, q.v. for other estimates) while Scharmann et al. (2021) dated the whole clade to a mere (18.2-)12.3(-6.4) Ma, the age of [N. khasiana + the rest] being (11.9-)7.6(-3.3) Ma.
Fossil pollen of Nepenthes is known from Europe in the Eocene (Krutzsch 1989) and from Meghalaya, India, in the Palaeocene (Kumar 1995).
Evolution: Divergence & Distribution. For the biogeography of Nepenthes, see Meimberg and Heubl (2006); some analyses suggest that Malesian Nepenthes (including species from New Caledonia and Australia) are derived from a stock represented by the extant taxa found to the west of Malesia, but different relationships were suggested by different genes. Indeed, recent genome analyses by Murphy et al. (2019/2020: Angiosperms353 baits) showed that [Seychelles [Madagascar [N. India/Sri Lana [Halamahera and Waigeo islands [South China to Malesia and New Caledonia]]]]] represents the geography of the genus (see also Scharmann et al. 2021), and within the crown clade there is also a fair bit of geographic structure; there have been seven or so dispersal events across Wallace's Line in central Malesia (Murphy et al. 2019/2020).
Speciation in Nepenthes is discussed e.g. by Clarke and Moran (2016) who noted that climate was an important variable, species might be restricted to different soil types, e.g. limestone or ultramafic soils; vicariance seemed also to be involved since suitable habitats were decidedly patchy. Note that there may be a very long stem/phylogenetic fuse, over 60 Ma or so given the ages in Scharmann et al. (2021), in the evolution of Nepenthaceae. Scharmann et al. (2021) also noticed that the topology suggested by plastid data differed from that based on nuclear data, differences being evident even in the positions of the two Indian taxa (near basal); note, however, that there was variation in the topologies produced by different nuclear analyses, although differences were evident only above the Indian taxa in the tree. They emphasized the importance of incomplete lineage sorting and extensive introgression in the history of the genus, noting that the parentage of the speciose eastern clade (mostly Malesian) appears to include N. danseri, from Waigeo Island to the immediate west of the Bird's Head peninsula, New Guinea, and also a now-extinct taxon from the stem of the genus, an "archaic ghost" (Scharmann et al. 2021). Certainly a tree does not capture the complexitiies of evolution in this genus, and the current biogeography of the genus does not reflect that of the past.
Ecology & Physiology. The pitchers of Nepenthes are basically pitfall traps, however, how insects become trapped in the pitchers was for long unclear. Nectar is produced by the pitchers and attracts insects and other animals, indeed, Di Giusto et al. (2011) even thought that some kind of floral mimicry was involved in this attraction - see also Joel (1988), but c.f. Ruxton and Schaefer (2011). Recent work suggests that the rim (peristome) of the pitcher is extremely wettable (Chen et al. 2016 give a description of how this happens at the nanoscale level), and insects may aquaplane when they step on it, falling into the pitcher below where they die and get digested; when the rim is dry insects can walk on it easily, but they still may get trapped if the plant has wax-covered inner pitcher walls (Bohn & Federle 2004). The main capture mechanisms correlate with climate, species with distinctive epicuticular wax morphologies growing in drier climates (Moran et al. 2013). Moulton et al. (2023) linked the various peristome shapes and projections, how the peristome is held, etc., to the physics of prey capture. Interestingly, N. pervillei, sister to the rest of the genus (see Phylogeny below), has a very simple peristome, narrow and unornamented (the "base" type); the three other major variants are the flared, flat and toothed types - and the teeth can be quite remarkable (Block 2025). Based on what little is known, it seems that Nepenthes growing at lower altitudes may be more likely to capture ants, etc., while species at higher altitudes are more likely to capture winged insects (literature in Moulton et al. 2022). In another variant of insect capture, insects on the underside of the lid of N. gracilis are catapulted at high velocity into the pitcher when raindrops fall on the lid; this is because the lids are stiff, but thin (hey "act as torsion springs that generate high jerk forces" (Chomicki et al. 2024: p. 110); the lids are held horizontally, and the cuticle waxes on the lower surface pf the lid do not provide a good hold for the insects (Bauer et al. 2015; Chomicki et al. 2024). Pitchers of the basal N. pervillei also have similar springboard trapping; these last two species are the only species with this mechanism, and the association of three elements that make up the trap mechanism here represent "spontaneous coincidence" (Chomicki et al. 2024). Pavlovic et al. (2007) discuss the physiology of the pitcher trap (see also Mithöfer 2011 and Adamec 2011b and references). Lathika et al. (2025) suggest that the extrafloral nectar secreted by the pitchers of Nepenthes khasiana may not act as a simple insect attractant, rather, it contains substances like (+)-isoshinanolone that are acetylcholinesterase inhibitors, hindering neuronal activity of these insects indeed, (+)-isoshinanolone is produced by other species of Nepenthes and carnivorous Caryophyllales - the nectar may make the insects intoxicated (Block 2025). Termites, Hospitalitermes bicolor are attracted to the pitchers of N. albomarginata, the "albomarginata" refering to a white band of hairs around the mouth of the pitcher that the termites eat - and fall into the pitchers in their hundreds (Merbach et al. 2002). The nectar of N. inermis with small and rather unremarkable pitchers is extremely viscous, insects contacting it being unable to escape (Block 2025). The liquid in the tank tends to be acid, and contains enzymes from the plant (Peroutka et al. 2008b; Adlassnig et al. 2011); chitinases involved in the digestion of the insect exoskeleton have also been isolated (Rottloff et al. 2011 and references).
On the whole, however, the pitchers seem not to be very efficient at capturing insects (Joel 1988), and the plants may acquire nitrogen in other ways (Moran et al. 2018 for a summary). Some montane species of Nepenthes with particularly large pitchers collect the faeces of tree shrews (Tupaia montana) and other animals as they feed from carbohydrate-rich secretions produced by glands on the inner surfaces of the lids (the pitchers look "akin to a free-hanging aerial toilet bowl"), and substantial amounts of nitrogen may be taken up by the plant (Chin et al. 2010; Rice 2011; Cross et al. 2022: p. 936). The pitchers may also serve as bat roosts, and N. rafflesiana var. elongata gets about one third of its nitrogen from the faeces of Hardwicke’s woolly bats (Kerivoula hardwickii hardwickii), the pitcher being modified so the bat "fits" properly when it roosts (Grafe et al. 2011); here the upper part of the pitcher wall reflects bat calls and so orients the animal coming in to the pitcher (e.g. Lim et al. 2015; Schöner et al. 2015). Plant-ant relationships in pitcher plants can be very complex, thus Camponotus schmitzi lives in the petioles of N. bicalcarata; it hides under the pitcher rim and sallies forth to collect ants that have fallen in to the pitcher - it can both run across the wetted rim and swim in the fluid in the pitcher; it eats the ants it collects, their remains (and dead Camponotus, and its faeces) falling in to the pitcher whence nutrients ultimately make their way into the plant (Bonhomme et al. 2010; Bazile et al. 2012). Indeed, Schulze et al. (1997) estimated that (53.9-)61.5(-69.1)% of the nitrogen in N. mirabilis came from such associated insects. Finally, nutrients from litter that falls into the trap may be taken up by the plant (Adlassnig et al. 2011; Thorogood et al. 2017 and references). Capó-Baunçà et al. (2020) discuss the various elements - presence of prey, uptake of nutrients by the roots - affecting the rate of photosynthesis in Nepenthes x ventrata.
Most of the pitchers of the recently-described Nepethes pudica are subterranean, whether in soil, moss, etc., and are more or less echlorophyllous; remains of mites, beetles and especially ants, probably Crematogaster, that have been broken down in the pitcher, along with a variety of animals like mosquito larvae, nematodes and annelids that actually live in the pitcher, are found there (Dancák et al. 2022: Tables 2, 3). Nepenthes rhombicaulis also produces subterranean pitchers, as has been known for quite some time, although nothing is known about their biology (Golos & Leach 2025).
Plant-Animal Interactions. For the invertebrates, etc., living in the pitchers and their interactions, see Beaver (1983), also above; mites, flagellates, ciliates as well as bacteria are to be found there; for other examples of the fauna that live in the liquid in the pitchers, see Kitching (2000), Bittleson (2018). and Grothjan and Young (2019).
Plant-Bacterial/Fungal Associations. Endomycorrhizae have been reported from Nepenthes (Séjalon-Delmas in Delaux et al. 2014).
Vegetative Variation. The expanded part of the leaf is developed from the leaf base, as in many monocots, and the twining petiole and the pitcher from the rest (e.g. Troll 1932). The leaf is epiascidiate, that is, the inside of the pitcher of Nepenthes is developmentally equivalent to the adaxial surface of the lamina, the outside to the abaxial surface, but despite the remarkable appearance of such leaves compared with the ordinary flattened leaves common in angiosperms, relatively simple shifts in gene expression mediate between the two (see also Franck 1976; Whitewoods et al. 2019). Lessware et al. (2024) examined the development of the pitcher rim of N. × hookeriana, distinctive in its radially arranged rows of cells that formed ridges with retrorse papillae on the cells, very difficult for trapped insects to negotiate as they tried to esceps; Whitewoods (2024) emphasized how simple - if sometimes somewhat unexpected - patterns of cell division were involved in the process, the cells initially being unoriented, then becoming radially oriented around the peristome, the ultimate cell division leading to a reduction in cell size - i.e. cell division is unaccompanied by cell enlargement.
Genes & Genomes. Reports of a whole nuclear genome duplication in Nepenthes are disputed (see Y. Yang 2017; Murphy et al. 2019/2020). For hybridization/introgression, etc., see Divergence & Distribution above.
Chemistry, Morphology, etc.. The outer integument develops greatly after fertilization and forms an exostome (Goebel 1933). There is a hair-pin bundle in the testa (Takhtajan 1988).
For general information, see Cheek and Jebb (2001: almost a monograph), Kubitzki (2002d), McPherson (2008), papers in Ellison and Adamec (2018), esp. Clarke et al. (2018), Berendsohn et al. (2018), the Caryophyllales portal and the Carnivorous Plants Database, for chemistry, see Hegnauer (1966, 1990), for naphthoquinones, see Schlauer et al. (2022), for anatomy, Metcalfe (1952a), Pant and Bhatnagar (1977), Schwallier et al. (2016, esp. 2017) and Ghazalli et al. (2019) and for pollen, see Takahashi and Sohma (1981).
Phylogeny. For some relationships within Nepenthes, see Meimberg and Heubl (2006). Alamsyah and Ito (2013) recovered N. pervillei, from the Seychelles, and N. madagascariensis as successively sister to the rest of the genus, although support was weak, while Nauheimer et al. (2019) found N. khasiana to be sister to the rest of the genus (however, it was the only non-Malesian species that they included), and using genome skimming, they clarified other relationships - there has been hybridization. Murphy et al. (2019/2020) in a genome analysis with goood sampling found the well-supported relationships [N. pervillei [[N. madagascariensis + N. masaolensis] [[N. khasiana + N. distillatoria] [N. danseri [two major clades]]]]]; within the two major clades some relationships were fluid. These are largely the relationships recovered by Scharmann et al. (2021) - the emphasis there is on introgression, etc..
Classification. For a summary of the various classifications of Nepenthes, see Murphy et al. (2019/2020). A fully elaborated infrageneric classification seems premature, but major clades are becoming evident. The number of species included in the genus has about doubled since 2000 (Borsch et al. 2020).
[Drosophyllaceae [Ancistrocladaceae + Dioncophyllaceae]]: fibriform vessel elements +; rays 1-2 cells wide; petiole bundle(s) surrounded by massive sclerenchymatous ring with embedded vascular bundles, wing bundles +.
Age. The age of this node is estimated to be around 57.9 Ma (Magallón et al. 2015) or (44-)ca 31.5(-19) Ma (Martín-Rodríguez et al. 2020).
DROSOPHYLLACEAE Chrtek, Slavíkovà & Studnicka - Drosophyllum lusitanicum (L.) Link - Back to Caryophyllales
Plant woody, small; mycorrhizae?; chemistry?; cortical bundles in stem +, inverted; ?nodes; ?stomata; petiole bundles inverted, three, arcuate, sclerenchyma ring?; stalked and sessile glands with xylem and phloem; leaves linear, stalked glands abaxial, in lines, abaxially circinate, vernation revolute; flowers large, (C contorted), ± marcescent; A 10, attachment?; pollen grains tricellular, tectate, pantoporate, not spiny; G [5], opposite the K, placentation basal, styles separate, stigmas capitate; ovules several/carpel, parietal tissue ca 1 cell across, funicle long; fruit a septicidal capsule; seeds operculate, few; exotesta not palisade, endotesta crystalliferous, with U thickenings, exotegmen thick-walled; endosperm ?, embryo short; n = 6, x = 6 (?7), chromosomes ³15 µm long, nuclear genome [1 C] = 10,417 Mbp; chloroplast ndh gene complex 0 [missing/non-functional]; germination epigeal, ± cryptocotylar.
1 [list]/1. Southern Iberian Peninsula, Morocco (map: from Ortega et al. 1995). [Photos - Collection.]
Evolution: Divergence & Distribution. Although Drosophyllum lusitanicum grows on both sides of the Straits of Gibraltar, its distribution seems to have been achieved by a number of dispersal events across the Straits, probably from the south, after their opening ca 5.3 Ma rather than by vicariance (Martín-Rodríguez et al. 2020).
Ecology & Physiology. Drosophyllum grows in seasonally-dry habitats and is able to acquire an appreciable proportion of its nitrogen through carnivory (Skates et al. 2019). The leaf produces a sweet (?attractive to fruit flies and their like) scent, and catches insects even at night, and digestive secretions appear to be produced then (Allan 2019b). For more on carnivory, see Plachno et al. (2009) and Bertol et al. (2015).
Although Drosophyllum looks quite delicate, it grows in very dry conditions yet does not dry out fast. The mucilage on the tentacles is hygroscopic and may help the plant maintain a positive water balance (Adamec 2009).
Genes & Genomes. Mower et al. (2021) discuss possible connections between various distinctive life styles that might affect the photosynthetic process and result in the loss of ndh genes.
Chemistry, Morphology, etc.. Stem/leaf anatomy would repay investigation; both the cortical and petiole bundles appear to be inverted (Metcalfe & Chalk 1950, as Droseraceae).
The flowers are relatively large; the stamens opposite the calyx are longest. Dehiscence of the fruit is down the ribs of the capsule and the valves are opposite the calyx.
For some anatomy, see Metcalfe (1952a), for pollen, see Takahashi and Sohma (1981), for ovule and seed, see Boesewinkel (1989), and for general information, see Kubitzki (2002d), McPherson (2008), papers in Ellison and Adamec (2018), the Caryophyllales portal and the Carnivorous Plants Database.
[Ancistrocladaceae + Dioncophyllaceae]: plants woody, lianes; ?mycorrhizae; (acetogenic naphthylisoquinoline alkaloids +); cork deep seated; petiole with inverted bundles in sclerenchyma ring; stomata actinocyclic; A introrse; pollen grain nuclei?; embryo short; germination epigeal, cryptocotylar.
Age. Bell et al. (2010) suggested an age for this clade of (61-)41, 37(-20) Ma, Wikström et al. (2001) an age of 47-29 My; around 36.2 Ma is the age in Magallón et al. (2015) and ca 49.5 Ma in Tank et al. (2015: Table S2).
Chemistry, Morphology, etc.. The cotyledons of Ancistrocladaceae are shown as being recurved structures about the length of the stout hypocotyl/radicle (Gilg 1925), but they have also been described as being "remarkably folded" (Porembski 2002; c.f. Keng 1967). Indeed, the cotyledons as shown in Gilg (1925) are not that dissimilar from those of the three genera in Dioncophyllaceae (Airy Shaw 1952).
For general information, see Airy Shaw (1951), for the distinctive naphthyl isoquinoline alkaloids of the clade, see Bringmann (1986), Bringmann and Pokorny (1995), and Bringmann et al. (2008, and references) - since they are synthesised from polyketide precursors, not from aromatic amino acids, so are barely alkaloids in the strict sense, for growth patterns see Cremers (1974) and for anatomy, see Gottwald and Parameswaran (1968) and Metcalfe.
ANCISTROCLADACEAE Walpers, nom. cons. - Ancistrocladus Wallich - Back to Caryophyllales
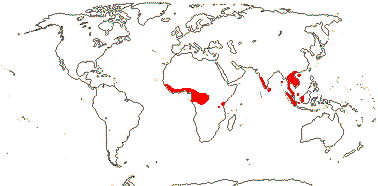
Climber, sympodially constructed, stem hooks borne in series, not carnivorous, ?rosette forming; myricetin +, ellagic acid?; spirally-thickened cells in axial parenchyma; nodes 3:3; SiO2 bodies in ray cells [African taxa]; xylem parenchyma apotracheal, banded; cortex with with elongated pitted sclereids, sclereid band indistinct; petiole bundle annular; lamina surface with wax-secreting pit glands [lepidote hairs in crypts], vernation supervolute, ?stipules; pedicels articulated; K quincuncial, unequal in size, (with abaxial glands), C basally connate or not, (imbricate); A (5) 10, whorl opposite petals larger, filaments widened and ± connate basally and adnate to C; nectary narrow raised ring; G [3(-4)], half or more inferior, style short, branches long, stigmas capitate-hippocrepiform or pinnatifid, ?type; ovule single [per flower], basal, hemitropous, outer integument "thick", ?nucellus; fruit a nut, K much enlarged; seed ruminate; exotesta "thin membranaceous"; endosperm cellular, hypocotyl/radicle stout, cotyledons ± recurved; n/x = ?
1 [list]/12 (21). Tropical Africa to W. Borneo and Formosa. Map: from van Steenis (1949a), Freson (1967) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and 6 (2011). [Photo - Fruits, Grapnels.]
Age. Ali et al. (2024) named fossil fruits in the Palana formation, Rajasthan, from some 55-52 Ma as Ancistrocladus eocenicus, the age rather greater than ages for the [Ancistrocladaceae + Dioncophyllaceae] clade suggested above.
Chemistry, Morphology, etc.. 1/3 species tested had fluorescing wood.
Both the branching and inflorescence structure of Ancistrocladaceae are at first sight difficult to understand, however, Ancistrocladus robertsoniorum was flowering at MoBot iv.2017. It appeared that neither inflorescences nor the complex series of hooks were immediately axillary to leaves, and the individual hooks themselves were also not subtended by leaves, although there were what are apparently very reduced leaves considerably below the hooks and the hooks may subtend buds. Massart (1896) early clarified the vegetative architecture of Ancistrocladus, noting appropriately that "few plants have a vegetative body of which the morphology is as complicated" (ibid.: p. 133). The climbing stem is built up of units consisting of two leaves (= prophylls), the axis of the unit then bending laterally and terminating in a hook, a series of sympodial hook units are then produced along this axis, the leaves on them being small. The growth of the climber continues via an orthotropic axillary shoot, and the whole process repeats itself. Cremers (1974) suggested that the upper leaf on the orthotropic module may have as many as five axillary buds, which end up in a variety of positions because of the strange growth of the plant. Most photosynthetic leaves are borne along short shoots. Whether or not the plant has stipules, and what these might be, is unclear (e.g. Taylor et al. 2005).
Porembski (2002: p. 25) described the inflorescences as being "racemes, spikes or dichasially branched panicles". The inflorescence is terminal and it may have some branches that are hooks (c.f. "mixed" inflorescences in some Vitaceae). The flowers in the branched inflorescences of Ancistrocladus robertsoniorum are not immediately subtended by bracts, although there are small, bract-like structures along the branches of the inflorescences. Interestingly, here it is the stamens opposite the sepals that are longer. The pollen is like that of Dioncophyllaceae (Cronquist 1981).
For for general information, see Keng (1967a), Porembski (2002), Taylor et al. (2005), Heubl et al. (2010), the Caryophyllales portal and papers in Ellison and Adamec (2018), for anatomy, see Metcalfe (1952a) and van Tieghem (1903b), and for chemistry, see Hegnauer (1989).
Previous Relationships. In the past Ancistrocladaceae have often been included in Violales or in Theales or Theanae (Cronquist 1981; Takhtajan 1997).
DIONCOPHYLLACEAE Airy Shaw, nom. cons. - Back to Caryophyllales
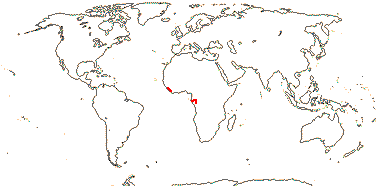
Climbers, (facultatively carnivorous), (shrubs); axially chiral naphthylisoquinoline alkaloids, cyclopentenoid cyanogenic glycosides +, ellagic acid?; successive cambia +; xylem with included phloem; true tracheids +; wood parenchyma vasicentric or apotracheal-diffuse; nodes ?; cortex with massive band of fibrous tissue; petiole bundles 1-3, arcuate; (stalked and sessile vascularized glands + - Triphyophyllum [T.]); (first leaves linear, adaxially circinate, lamina with parallel venation - T.), lamina apex with paired recurved hooks; C surrounding bud [?level]; K valvate or open; A 10-30; pollen not spiny; G [2, 5], placentation parietal, (connate style short), stigmas punctate, capitate (feathery - T.); ovules several/carpel, ?morphology, funicles long; capsule opening before maturity; seeds flattened, broadly winged, green when young; seed coat thick; endosperm ?nuclear, embryo with spreading semicircular cotyledons; n = 12, 18 [both T.], nuclear genome [Cx] = 584 Mbp [T.], x = 6 (?7).
3 [list]/3. Tropical West Africa. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
Evolution: Ecology & Physiology. Some leaves in young plants of Triphyophyllum peltatum have a short blade and glandular hairs on the abaxial surface of the midrib, which is prolonged beyond the blade (Green et al. 1979) - and they are abaxially circinate when young, just like the leaves of Drosophyllum. Such hairs develop only under conditions of phosphorus deficiency (not N or K deficiency) (Winkelmann et al. 2023: greenhouse conditions), and the plant is a facultative carnivore; it is at this stage that the plant may capture insects (Bringmann et al. 2001). The paired hooks at the ends of the leaves are apparently touch-sensitive and can be thought of as a kind of tendril (Sousa-Baena et al. 2018b).
Chemistry, Morphology, etc.. The report of cyclopentenoid cyanogenic glycosides from Dioncophyllum (Spencer & Seigler 1985b) should be confirmed for the family as a whole. Isoquinolines here are synthesized by a pathway that differs from that found in other plants (Bringmann et al. 2016).
Androecial variation in Dioncophyllum is considerable - there may be five stamens opposite the petals, ten stamens, or ca 27 stamens... (Airy Shaw 1952).
For anatomy, see Metcalfe (1952a) and Miller (1975), for growth and seedlings, see Cremers (1974), for chemistry, Hegnauer (1966, as Flacourtiaceae, 1989), Spencer and Siegler (1985) and Bringmann et al. (2016: napthylisoquinoline alkaloids, see also 2008), and for general information, see Airy Shaw (1952), Schmid (1964), Porembski and Barthlott (2002), McPherson (2008: excellent photographs), the Caryophyllales portal, and the Carnivorous Plants Database.
Previous Relationships. See Airy Shaw (1952) for a summary - the families with which Dioncophyllaceae have been associated are here placed in seven orders from Magnoliales to Asterales... Dioncophyllales were included in Theanae by Takhtajan (1997) and in Violales by Cronquist (1981).
[[Frankeniaceae + Tamaricaceae] [Plumbaginaceae + Polygonaceae]] / the salt gland clade: vessel elements with minute lateral wall pits +; sulphated flavonols, ellagic acid +; salt-excreting glands +; leaf base broad; pollen not spiny; ovary 1-locular; outer and inner integuments 2-3 cells across; seed exotestal.
Age. This node is about 93.3 Ma (Magallón et al. 2015).
Evolution: Ecology & Physiology. Evolution of the halophytic habit (e.g. Liphschitz & Waisel 1982; Saslis-Lagoudakis et al. 2016), tolerance of dry conditions, etc., would repay attention (see also articles in Rajakaruna et al. 2016). This whole clade has been called the salt-gland clade (e.g. Dörken et al. 2017), although there are both morphological and functional differences between the glands in [Frankeniaceae + Tamaricaceae] and Plumbaginaceae: Radially arranged cells, ions move to the apoplast via a transmembrane pathway - Plumbaginaceae; movement via vesicles - [Frankeniaceae + Tamaricaceae] (Faraday & Thomson 1986 a, b). Of course, Polygonaceae has no salt glands at all, and Caperta et al. (2020) suggested that salt glands evolved independently in the two groups. Sulphated phenolic compounds are common; plants with such compounds are often halophytic.
Plant-Animal Interactions. There are about 1,000 species in the straight-snouted weevil clade Brentidae-Apioninae-Apionini most of which are to be found on Fabaceae-Faboideae, however, their ancestral aniosperm hosts may have been in this clade (Winter et al. 2016).
Chemistry, Morphology, etc.. Whether or not this whole clade is rayless is unclear. There are few records from Polygonaceae, and in Frankenia there may be rays (Carlquist 2015b).
[Frankeniaceae + Tamaricaceae]: halophytic; bisulphated flavonols +, myricetin 0; wood storied; vessel elements with simple perforation plates; (rhomboidal crystals +); salt glands + [8-celled]; (stomatal orientation transverse); leaves small [≤1 cm long]; flowers small, 4-6-merous, C with adaxial appendages, nectariferous; G with median member abaxial, placentation (intruded) parietal, (basal), style +, branched, stigmas capitate-clavate; fruit a loculicidal capsule; exotestal cells bulging or as hairs; endosperm +.
Age. This node is dated to 43-30 Ma (Wikström et al. 2001), ca 49.7 Ma (Tank et al. 2015: Table S2), or 53.8 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. It is equally parsimonious to assume that petal appendages are apomorphies for the family pair as it is to assume that they have evolved independently. They are found in Frankeniaceae, and in Tamaricaceae, Reamuria, sister to the rest of the family, also has them; nectary is secreted by the petals (?these appendages). Seeds with copious endosperm have the same distribution.
Ecology & Physiology. For salt glands - in fact, they may secrete calcium carbonate, calcium sulphate, etc. - see Fahn (1979), Caperta et al. (2020) and especially Dörken et al. (2017 and references) and the discussion above.
Chemistry, Morphology, etc.. For ovules, etc., see Mauritzon (1936b).
Phylogeny. The monophyly of the two families and their sister-group relationship were confirmed by Gaskin et al. (2004).
Previous Relationships. Both Frankeniaceae and Tamaricaceae were placed in Violales by Cronquist (1981) and in Violanae by Takhtajan (1997), probably because of their parietal placentation.
FRANKENIACEAE Desvaux, nom. cons. - Frankenia L. - Back to Caryophyllales
Herbs to shrubs; root cork cambium superficial; stem (wood storeyed), vessels tiny [ca 88.4 µm long], fibriform vessel elements +, wood rayless; (secondary growth anomalous); nodes 1:1; cuticle wax crystalloids 0; leaves opposite, often ericoid, fasciculate; flowers solitary, terminal/inflorescence cymose; flowers also 7-merous; K connate, lobes induplicate-valvate, C clawed; A (3-)6(-24), (inner whorl staminodial), slightly connate at the base or not, extrorse, versatile; tapetal cells binucleate; ?nectary; pollen grains tricellular; G [(2-)3(-4)], style +, branches well developed, (stigmas capitate); ovules (1/ovary)2-6(-many)/carpel, parietal tissue 0, nucellar cap +, funicles long; seeds myxospermous; exotestal cells large, papillate, papillae with terminal nail-like thickenings, endotestal cells thin-walled [?fibers], endotegmen with thick cuticle, tanniniferous; (polyembryony +), coenocytic micropylar endosperm haustorium +; n = ?5, 10, 15, x = 5.
1/90: [list]. ± World-wide in warm, dry areas, but scattered. Map: from Fl. Austral. vol. 8 (1982), Whalen (1987), Jäger (1992), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and FloraBase (consulted 2004). [Photos - Collection]
Chemistry, Morphology, etc.. Vessels in the family are ca 88.4 x 12.7 μm (Olsen et al. 2003), which is tiny, only 5% of broad-leaved angiosperms having vessels ≤200 μm long, only 10% vessels less than 40 μm across. The cork cambium in the stem is probably superficial, although the sheathing leaf bases may make it appear more deeply-seated (Ragonese et al. 1966; Olson et al. 2003). Leinfellner (1959) described the ericoid leaves of Frankeniaceae which, like those of Erica, Empetrum, etc., are not revolute, rather, the revolute appearance is the result of outgrowths from the abaxial side of the lamina.
Some information is taken from Walia and Kapil (1965), Whalen (1987: taxonomy of Old World Frankenia) and Ronse de Craene (2018: floral morphology); for general accounts, see Surgis (1921) and and Kubitzki (2002d).
Phylogeny. For relationships within Frankenia, see Gaskin et al. (2004).
TAMARICACEAE Link, nom. cons. - Back to Caryophyllales —— Synonymy: Reaumuriaceae Lindley
Woody, also xeromorphic; (gypsum crystals +); ?nodes; cuticle waxes as tubes or curled rodlets; leaves small/scale-like, (main photosynthesizing organ = the stem); inflorescence racemose, (flowers solitary, terminal), bracteoles 0; K basally connate or not, C usu. lacking appendages; stamens = or 2 x C or more, free/connate at base/fasciculate, development centrifugal, anthers extrorse to introrse, variously attached; nectary (± disciform, with C and A on top, or inside or outside A, or ?0); G [(2-)3-4(-5)], opposite petals, styluli long/short, stigmas (± capitate), wet; ovules 2-many/carpel, parietal tissue 1-2 cells across; embryo sac tetrasporic [a variety of types, even in one species, often 16-nucleate bipolar]; seed with hairs, (at chalazal end only - on chalazal prolongation); exotestal cells periclinally elongated and thick-walled, endotestal cells thin-walled, crystalliferous; endosperm usu. scanty, perisperm +, thin, (0); n = (11) 12, x = 12, nuclear genome duplication + [NCORE3], [Cx] 1412-1436 Mbp.
3/90: [list]/90, Tamarix (55). Eurasia and Africa, esp. Mediterranean to Central Asia (map: from Hultén & Fries 1986; Meusel et al. 1978; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003), commonly naturalised in North America and elsewhere. [Photos - Collection]
Evolution: Ecology & Physiology. Halophytes are common in Tamaricaceae, perhaps evolving only once here (Moray et al. 2015); see also articles in Ann. Bot. 115(3). 2015. In common with some other groups inhabiting saline conditions (Amaranthaceae, Cactaceae), a number of taxa have very fast germination, i.e. they germinate within one day of the beginning of imbibition - Salix and Populus also do this, and some are pioneers in the same habitats as Tamarix, for example (Parsons 2012; Parsons et al. 2014). Tamarix can have very deep roots and so can survive even as the habitats in which it is living dry out. Sodium chloride is usually secreted by its salt glands, and this can result in the suppression of most understory plants (this is partly why Tamarix is such a noxious weed in the southwestern U.S.A.), but in some species, e.g. Myricaria germanica, calcium/magnesium carbonate/sulphate may be secreted by the salt glands (Dörken et al. 2017). Indeed, in some taxa, at least, the composition of the secretion depends on the substrate on which the plant is growing (e.g. Thomson et al. 1969, q.v. for details of the process of salt secretion).
Genes & Genomes. A genome duplication is reported here (Y. Yang et al. 2017: NCORE3; see also S. A. Smith et al. 2017); S. A. Smith et al. (2017) discuss the evolutionary context of.
Chemistry, Morphology, etc.. Reaumuria is distinctive in having single terminal flowers, a contorted corolla, and basal adaxial scales on the petals, c.f. Frankeniaceae. It also has many centrifugal stamens arising from 10 primordia, it lacks a nectary, and its seeds have endosperm (Ronse Decraene 1990).
See Frisendahl (1912), Joshi and Kajale (1936) and Johri and Kak (1954) for embryology, Hegnauer (1973, 1990) for chemistry, Czaja (1978) for seed storage, Zhang et al. (2001) for pollen and Gaskin (2002) for a general account.
Phylogeny. Relationships within the family are [Reaumuria [Myricaria + Tamarix]] (Gaskin et al. 2004). For a phylogeny of Myricaria, see Y. Wang et al. (2009), and for that of Tamarix, in which reticulation seems to have been involved, see Villar et al. (2018).
[Plumbaginaceae + Polygonaceae]: plants herbaceous; O-methylated flavonols, myricetin, quinones +; (SiO2 bodies +); (wood storeyed); cortical and/or medullary vascular bundles +; nodes 3:3; colleters + [?here]; inflorescence racemose; pollen usu. starchy; G with median member adaxial; stalked, sessile and pit glands +; ovule single [per flower], basal; fruit surrounded by accrescent calyx [usually part of the dispersal unit]; exotesta ± persistent, seed coat otherwise undistinguished; embryo straight; mitochondrial coxII.i3 intron 0.
Age. This node is dated variously at (125-)118.7, 110.9(-90.7) Ma (Schuster et al. 2013), ca 67.9 Ma (Magallón et al. 2015), (72-)60, 58(-44) Ma (Bell et al. 2010), ca 59.5 Ma (Tank et al. 2015: Table S1, S2), or as little as 52-37 Ma (Wikström et al. 2001).
Evolution: Divergence & Distribution. This clade may show an increase in diversification rate some (93.3-)76.9(-67.9) Ma (Magallón et al. 2018). It seems to be most diverse immediately away from the tropics (see Kostikova et al. 2014b for Polygonaceae).
Plant-Animal Interactions. Lycaeninae caterpillars are quite commonly found on this family pair, probably because of the polyphenolics in their leaves (Fiedler 1995).
Chemistry, Morphology, etc.. For sterol composition in comparison to that of centrosperms, see Wolfe et al. (1989).
There are early reports that both families have perisperm (see Rocén 1927: p. 169).
For anatomy, see Carlquist and Boggs (1996), for pollen, see Nowicke and Skvarla (1977, 1979), and for seed hairs, see Hildebrand (1872).
PLUMBAGINACEAE Jussieu, nom. cons. - Back to Caryophyllales
Choline-O-sulphate, quaternary ammonium compounds +, little oxalate accumulation; cork subepidermal or cortical; secondary thickening odd; rays multiseriate; petiole bundles arcuate; vascularized mucilage glands, salt glands + [usu. 16-celled]; cuticle wax crystalloids 0 (+, irregular platelets); stomata also paracytic; K connate, ribbed, C contorted; stamens = and opposite petals; pollen with irregular columellae, tectum continuous, itself with columellae, with rather coarse blunt spines [Plumbago-type]; nectary on adaxial side of filament bases (elsewhere); G [5], stigma monomorphic, capitate; ovule with parietal tissue 1-3 cells across, funicle long and curled, obturator from wall at apex of ovary; embryo sac tetrasporic; K green in fruit; endotegmen persistent; endosperm 4n or 5n, little persisting, embryo green; nuclear genome duplication + [NCORE2], x = 7, 8, nuclear genome [Cx] 335-4332 Mbp; (plastid transmission biparental).
26 [list]/1179 836 (725) - three groups below. Predominantly Mediterranean to Central Asia, scattered elsewhere. [Photos - Collection.]
Age. Crown-group Plumbaginaceae are dated to 40-27 Ma (Wikström et al. 2001) or 19-17 Ma (Lledó et al. 2005); Bell et al. (2010) suggest a crown-group age of (57-)43(-27) Ma, Costa et al. (2019) an age of ca 40 Ma and Koutourompa et al. (2021) an age of(77-)65.7(-52) Ma.
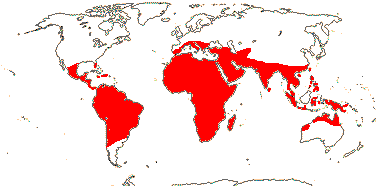
1. Plumbaginoideae Burnett / Plumbagineae Dumortier
Perennial herbs or (scrambling) shrubs; glycine betaine + [plumbaginin]; axillary bud vascular tissue derived from several leaf gaps [?level]; stems angled and striate; lamina (deeply lobed), petioles often short, (cauline stipules - Plumbago); K herbaceous, glandular, C (connate), lobes truncate-emarginate and then apiculate; distyly associated with herkogamy [different positions of anthers and stigmas in different morphs]; style single, stigmatic receptive areas in bouquet-like aggregations along branch; (nucellar cap ca 2 cells across - Plumbagella); embryo sac unimitotic; fruit a basally circumscissile capsule; n = 6, 7.
4/36. East Asia and Africa, Plumbago pantropical. Map: from Baker (1948), probably over-optimistic, Plumbago commonly cultivated.
Age. Crown-group Plumbaginoideae are estimated to be (43-)29.5(-18) Ma (Koutourompa et al. 2021: HPD).
2. Limonioideae Reveal
plant often salt tolerant; lamina cartilaginous, with 5-10 marginal rows of whitish cells; C connate; stamens basally adnate to corolla; styles separate.
Age. Crown-group Limonioideae are estimated to be some 17-16 Ma (Lledó et al. 2005), around 38 Ma (Costa et al. 2019) or (71-)57(-43) Ma (Koutourompa et al. 2021: HPD).
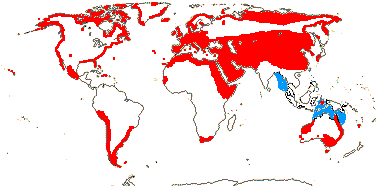
2A. Aegialitideae (Linczevski) T. H. Peng - Aegialitis R. Brown —— Synonymy: Aegialitidaceae Linczewski
Shrublet; ellagic acid +; successive cambia +; cortical vascular bundles +; branched sclereids +; leaf base surrounding stem, lamina vernation involute; ?embryo sac, etc.; fruit much elongated [>7 cm long], slender, pentagonal, longitudinally dehiscent [along angles, ?type]; n = ?
1/2. Indo-Malesia, N. Australia, in mangroves. Map: blue, from van Steenis (1949d).
2B. Limonieae Reveal —— Synonymy: Armeriaceae Horaninow, Limoniaceae Seringe, nom. cons., Staticaceae Cassel
Perennial (annual) herbs or shrubs (small tree - Limonium dendroides); glycine betaines usu. 0, then beta-alanine betaines (and other quaternary ammonium compounds) +; (interfascicular area parenchymatous - Acanth.; petiole bundles arcuate/scattered/± annular + scattered, (sclereids +); leaves ± basal, (isobifacial), (lamina margin deeply lobed), petioles often short; inflorescence capitate or branched, cymose, axis channelled, inflorescence scapose, or leaves reduced; heterostyly evident as differences in pollen morphology and stigma surface, (plant with herkogamy); K scarious (also C-like); (A adnate to C the length of the tube); pollen exine dimorphic [type A - coarsely reticulate/type B - finely reticulate], (monomorphic), columellae regular, tectum incomplete, reticulate; style +, branched, (styluli), stigma (filiform), dimorphic [cob/papillate]; embryo sac bimitotic; fruit an achene or circumscissile capsule; n = 8, 9, 17, 18, etc.; rpl23 a pseudogene.
14/?800: Limonium (350-600 - for the latter figure, see Koutourompa et al. 2021), Acantholimon (165), Armeria (100). Mostly Irano-Turanian (Mediterranean), but also S. Africa, S. South America, and W. Australia. Map above, red: from Hultén; Baker (1948), FloraBase (2004) and Australia's Virtual Herbarium (consulted xii.2012).
Age. Limonieae diversification is dated to about 30 Ma (Costa et al. 2019).
Evolution: Divergence & Distribution. Lledó et al. (2005, see also 2011) suggest a number of ages for nodes in Limonioideae; calibration was on the age of the island on which the endemic Limonium endroides grew - used as a maximum age.
Plumbaginoideae and Limonioideae-Aegialitideae are predominantly tropical, while Limonieae are most diverse in the area from the western Mediterranean to Central Asia. About half the 90 species of Armeria are from the Iberian Peninsula alone. In the Near East to Central Asia Acantholimon (which includes several Linczevskian segregates) in particular is common, and there are 164 species of that genus recognized in the Flora iranica (Lledó et al. 2005, 2011; Moharrek et al. 2017).
In Limonium there is hybridization, polyploidy (some species are triploids), and hundreds of microspecies, some apomictic; Brullo and Erben (2016) recently described 39 of the 98 species they recognized from Greece as new. Limonium may originally have been from the Macaronesian-Mediterranean area and it is now most speciose in Eurasia in particular, but odd species are also to be found in Australia, South Africa, Brazil, etc. (Malekmohammadi et al. 2017). Koutourompa et al. (2021: variation in tree topologies taken into account) discuss the evolution and diversification of the genus in some detail. They noted that around 70% of Limonium species were to be found in the Mediterranean-European area - quite a small area, since it does not include the Atlantic islands, North Africa, Turkey or even much of Europe. The hyperdiverse Mediterranean lineage is made up of apomicts, and diversification here is estimated to have occurred within the last (9-)6, 5.7(-3.4) Ma (Koutourompa et al. 2021: HPD). A combination of biological and environmental factors like the evolution of apomixis and the Messinian Salinity Crisis respectively helped drive diversification.
Ecology & Physiology. Members of Plumbaginaceae prefer saline and sometimes rather dry conditions. For some literature on the halophytic species, see Liphschitz and Waisel (1982), Faraday and Thomson (1986 a, b: both morphology and physiology, all three groups above) and articles in Ann. Bot. 115(3). 2015. Species of Limonium and some other Limonieae are succulent halophytes and may grow in salt marshes (Hanson et al. 1994; Flowers & Colmer 2008; Ogburn & Edwards 2010). The quaternary ammonium compounds of one sort or another that have been found in practically all members of the family examined are involved in salt excretion, while choline O-sulphate may be involved in sulphate detoxification (Hanson et al. 1994). As with some Tamaricaceae, calcium carbonate is secreted by the salt glands in some species that grow e.g. on gypsum-rich soils (Sakai 1974; Caperta et al. 2020). For the morphology of salt glands, see Ruhland (1915), Caperta et al. (2020) and Bordbar et al. (2022), In Limonium spp. growing in coastal areas of the Balearic Islands, there are groups of species that prefer different soil conditions, i.e. different amounts/proportions of features such as sand, Na+, Ca++ and Mg++ and CO3-- and SO4-- ions (Llorens et al. 2018). Taxa growing in conditions et are not saline, etc., may still have glandular structures on their leaves (Caperta et al. 2020).
A number of species (e.g. Armeria, Acantholimon) are cushion plants, which tend to prefer colder and drier climates (Boucher et al. 2016b). Thus Acantholimon often grows in dry conditions in the mountains of the Irano-Turanian area and typically is shrubby, more or less tussock-forming, with narrow, rigid, isobifacial leaves and a short inflorescence; interestingly, many of the small segregate genera recently included in Acantholimon look more like Limonium with their broad leaf blades and elongated inflorescences (Moharrek et al. 2017).
Aegialitis is a shrubby plant growing in the mangroves in the eastern Bay of Bengal and between New Guinea and Australia; its seeds tend to sink quite quickly (Clarke et al. 2001). Findlay et al. (1967) discussed osmoregulation here. For the mangrove habitat, see Rhizophoraceae
Pollination Biology & Seed Dispersal. Distyly in Plumbaginaceae does reduce selfing (see Costa et al. 2017, especially 2019 and references; Barrett & Shore 2008 and Cohen 2019: general), and this has been a matter of interest since Darwin's time (Darwin 1877). Heterostyly is varied in expression, for instance, in Plumbago the two floral morphs are quite similar, although the stamens and stigmas differ in positions, while in Armeria the pollen and stigmas of different plants differ in morphology, although there is no positional heterostyly (e.g. Baker 1953). Costa et al. (2019) discuss the evolution of heterostyly - and its loss - in detail, and they note the major differences in the expression of heterostyly between Plumbaginoideae and Limonioideae-Limonieae, parallels in the evolution of herkogamy, etc.. For apomixis in Limonium, which has a geographical component, see D'Amato (1949), Róis et al. (2016), Caperta et al. (2018: also other papers in Taxon 67(6). 2019) and Koutroumpa et al. (2021). Here triploidy is common, and as with other apomictic groups, there are large number of microspecies, for instance, there are ca 53 species of Limonium on the Balearic Islands alone (Llorens et al. 2018 and references).
In a number of Limonieae the calyx becomes scarious in fruit and helps in wind dispersal; in Plumbago the calyx with its sticky glands persists in fruit and attaches to a passing animal.
Genes & Genomes. For a genome duplication, see Y. Yang et al. (2017: NCORE2), S. A. Smith et al. (2017) and Landis et al. (2018: the LISBα event); the latter date it to to ca 40.6 Ma.
Darshetkar et al. (2021a) outline plastome variation; in Limonium there have been changes in the boundaries of the inverted repeat, the rpl23 gene is a pseudogene, the rpl16 intron may have been deleted, etc., although few taxa have been studied. The literature on plastid transmission is somewhat confusing (Q. Zhang et al. 2003).
Chemistry, Morphology, etc.. Glycine betaine is known from only a very few species of Limonium (and from Plumbago, etc.), but not from Aegialitis and Armeria and other Limonieae (Rhodes & Hanson 1993; Hanson et al. 1994); see Hanson et al. (1994) for choline-O-sulphate distribution.
For wood anatomy, which may be paedomorphic, the family perhaps having a more or less herbaceous ancestry, see Carlquist and Boggs (1996). Resin ducts are reported (Prado & Demarco 2018). There is extensive gross anatomical variation that probably can be integrated with the tribes/subfamilies - for example, there is a continuous ring of sclerenchyma outside the phloem in Plumbaginoideae, separate fascicles in Limonioideae, etc. (see Maury 1886); Bokhari (1973) outlined quite extensive anatomical variation in Limonium. Williams et al. (1994) suggested that it was not known if the mucilage glands were vascularized, although in their data matrix the family was scored as having vascularized glands (see also the discussion above). Leaf vernation is variable, being flat, convolute or involute.
The style branches of Armeria are papillate all around for their entire lengths. Many Plumbaginoideae seem to lack a protruding obturator (Dahlgren 1916). According to Dahlgren (1916), the embryo sac is tetrasporic but eight-nucleate, but Maheshwari (1947) suggested it was tetrasporic and four-celled, three of the megaspores fused and the mature embryo sac consisted of an egg cell, a single synergid, a tetraploid polar nucleus and a three-nucleate antipodal cell... Haig (2020) notes that there are two kinds of tetrasporic embryo sacs in the family (see also Boyes & Battaglia 1951a, b), although there is interesting variation in both types.
Aegalitis is little known.
There is much general information in Kubitzki (1993b); see also the Caryophyllales portal, Hegnauer (1969, 1990) for chemistry, Bordbar et al. (2022) for foliar anatomy of Acantholimon, Baker (1948, 1953) variation in floral morphology (pollen, stigmas, etc.), de Laet et al. (1995) floral development, and Dahlgren (1937), Fagerlind (e.g. 1938b) and D'Amato (1940) for embryo sac development.
Phylogeny. Lledó et al. (1998, 2001) suggest phylogenetic relationships within the group, however, Aegialitis is placed as sister to all the rest of the family in some analyses (Savolainen et al. 2000: rbcL only).
For the phylogeny of Limonium, see Lledó et al. (2005: relatives unclear), Akhani et al. (2013: Irano-Turanian taxa, also anatomy) and especially Malekmohammadi et al. (2017) and Koutroumpa et al. (2018). The latter group, sampling about a third of the genus, found that it divided into two clades, in which one (subgenus Pterocaulon), L. anthericoides, from the western Cape, South Africa, was sister to the rest of the subgenus, while in the other (subgenus Limonium) L. dendroides, from Gomera, the Canary Islands, was sister to the rest. Analysis of plastome and nuclear ITS genes in the Mediterranean Lineage suggested rather different relationships, with those using ITS providing more details (Koutroumpa et al. 2018). Koutroumpa et al. (2021: 1 nuclear and 3 plastid markers), sampling over one half the genus, extended these findings; basic relationships remained unchanged, and again there were some differences in the topologies of the plastid and mitochondrial analyses. Moharrek et al. (2014) found the relationships [Acantholimon [Limonium + Armeria]]; focussing on Acantholimon, they found that the old sections of Acantholimon were pulverized. Moharrek et al. (2017) confirmed these earlier findings and noted that there were two major clades within the genus.
Classification. The classification here is based in part on the phylogeny in Lledó et al. (1998, 2001); see also Hernández-Ledesma et al. (2015). There are a number of monotypic genera in Limonieae and generic limits need attention (Hernández-Ledesma et al. 2015); Moharrek et al. (2017) synonymized eight of these small genera in Acantholimon. Koutroumpa et al. (2018) suggest additional synonymy in the family and divide Limonium into two subgenera and seventeen sections which largely held in Koutroumpa et al. (2021). Malekmohammadi et al. (2024) noted that the position of Aegialitis depended on whether plastid (sister to Limonieae) or nuclear (sister to Plumbagineae) data were being studied - a three-tribal classification seems best.
Previous Relationships. Plumbaginaceae used to be associated with Primulaceae-Primuloideae. Both have stamens opposite the petals, common petal-stamen primordia, and a ± connate corolla (the latter especially in Limonioideae), but the two are not close - for Primulaceae, see Ericales.
POLYGONACEAE Jussieu, nom. cons. - Back to Caryophyllales
Growth monopodial, branching from previous flush; cork subepidermal (pericyclic); dark-staining deposits, esp. in rays; stem bundles distinct; pits vestured; nodes also 5 or more:5 or more; petiole with a (D-shaped) ring of bundles, (wing bundles +); mucilage cells common; soluble calcium oxalate accumulation; glandular hairs +, ± sessile [?level], cuticle waxes as platelets or rodlets; (stomata dia- aniso- or paracytic); lamina vernation revolute, (margins lobed), secondary veins also palmate, colleters +; inflorescences with flowers in fascicles; flowers small, pedicels articulated; hypanthium ± developed; P +, uniseriate, basally ± connate; stamens = to and alternate with P to 3 x P; pollen tricolporate to pantoporate; nectary various; G [3], (common style short), stigma ± penicillate or capitate; ovule straight, (unitegmic), nucellar beak +, hypostase +, funicle short; (megaspore mother cells several); fruit an achene, trigonous (lenticular); embryo straight, lateral; x = 7, nuclear genome [1 C] (0.088-)1.458(-24.221) pg; plastome inverted repeat expansion.
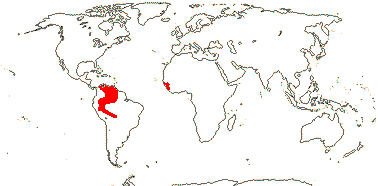
55 [list: to tribes]/1,115 - fifteen groups below. World-wide. [Photos - Collection]
Age. This age of this node has been estimated at (122.5-)105.5, 97.8(-78.2) Ma (Schuster et al. 2013), and (97.3-)90(-73.7) Ma (Kostikova et al. 2014b: p. 1862, it "split[] from Plumbaginaceae") - or 41-34 Ma (Forest & Chase, see Doorenweerd et al. 2016).
Includes Afrobrunnichia, Brunnichieae, Calligoneae, Coccolobeae, Eriogoneae, Eriogonoideae, Fagopyreae, Gymnopodieae, Leptogoneae, Oxygoneae, Persicarieae, Polygoneae, Polygonoideae, Pteroxygoneae, Rumiceae, Symmerioideae, Triplarideae.
1. Symmerioideae Meisner - Symmeria paniculata Bentham
Tree; petiole winged, wing ± surrounding stem; plant dioecious; inflorescence axillary; ?nectary; staminate flowers: A 20+; carpelate flowers: ovary with basal septum; achenes pyramidal, 3 P adnate to wall; ?endosperm, ?embryo; n = ?
1/1. N. South America, West Africa. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Tropicos (consulted i.2013).
[Afrobrunnichia [Polygonoideae + Eriogonoideae]]: stipule +, adaxial, tubular, ensheathing stem [= ochrea].
2. Afrobrunnichia Hutchinson & Dalziel
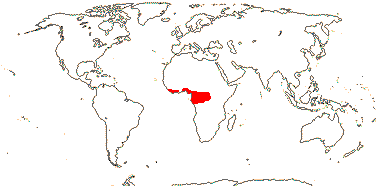
Liane, climbing with bifid, axillary branch tendrils; pedicel winged on both sides; inflorescence?; P basally connate; A usu. 8; ?nectary; funicle large; fruit a ?drupe ["turgid"], P lobes accrescent; seed deeply longitudinally 3-sulcate, irregularly ruminate; ?embryo; n = ?; chloroplast inverted repeat much expanded; germination epigeal, phanerocotylar
1/2. Tropical West Africa, Liberia to the Congo. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
[Polygonoideae + Eriogonoideae]: stigma capitate; cotyledons ± spathulate; genome duplication + [NCORE1].
Map: from Hultén (1971), Frankenberg and Klaus (1980), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), FloraBase and Australia's Virtual herbarium (consulted i.2013), and Tanya Schuster, pers. comm.; Indo-Malesia incomplete.
Age. This node has been dated to 32-28 Ma (Wikström et al. 2001), (122.5-)105.5, 97.8(-78.2) Ma (Schuster et al. 2013), and (90.5-)83(-65.7) Ma (Kostikova et al. 2014b) - c.f. details of tree.
3. Polygonoideae Arnott
(Chrysophanol + [anthroquinone]); stipule ± scarious; P with 2-5 primary veins; funicle long or short.
15/595. Especially north temperate.
3A. Oxygoneae T. M. Schuster & Reveal - Oxygonum Campderá
Annual to perennial herbs (shrubs); P and receptacle fused; A 8, nectariferous trichomes at base of inner whorl; achenes spiny/longitudinally ribbed; cotyledons flat; n = 13.
1/22. Africa, Madagascar.
[Persicarieae [Fagopyreae [Pteroxygoneae [Calligoneae [Rumiceae + Polygoneae]]]]]: ?
Age. This clade has been dated to (111.3-)93.5, 85.9(-68.4) Ma (Schuster et al. 2013: Persicarieae + The Rest) and ca 72.1 Ma (D.-L. Cao et al. 2022: Rumex + The Rest).
3B. Persicarieae Eaton —— Synonymy: Persicariaceae Martinov
Annual to perennial herbs; inflorescence racemose (capitate); P (3-)5; nectaries as hairs at base of A/disciform; A 5-8; pollen (colpi or pores scattered); G [(2-)3]; embryo slightly curved, cotyledons linear to orbicular; n = 8, 10-12, nuclear genome [Cx] = 683-1339 Mbp.
3/260: Persicaria (150), Koenigia (60), Bistorta (50). ± Cosmopolitan, esp. North Temperate to Arctic.
Age. Qu et al. (2022) suggest that crown-group Persicarieae are slightly over 77 Ma, while D.-L. Cao et al. (2022) suggest an age of ca 55.8 Ma.
[Fagopyreae [Pteroxygoneae [Calligoneae [Rumiceae + Polygoneae]]]]: ?genome duplication.
3C. Fagopyreae Eaton - Fagopyrum Miller
Annual to perennial, often twining vines; P 5, (with 1 primary vein), quincuncial; A 8; nectaries basal, globose, often stalked; embryo central in seed, cotyledons folded [condupolicate], foliaceous; n = 8, 10, 12, 14.
1/25. Eurasia, esp. China-Tibet, 1 sp. East Africa.
Age. Crown-group Fagopyreae (F. esculentum + F. urophyllum) may be ca 41.6 Ma (D.-L. Cao et al. 2022).
[Pteroxygoneae [Calligoneae [Rumiceae + Polygoneae]]]: ?
3D. Pteroxygoneae T. M. Schuster & Reveal - Pteroxygonum Dammer & Diels
Twining vines, tuber +, large, woody; lamina with red blotch, venation palmate; P 5; A 8, nectariferous near base; styles basally connate; P (not accrescent); embryo with folded cotyledons; n = ?
1/2. Central and southwest China.
[Calligoneae [Rumiceae + Polygoneae]]: ?
Age. This node is estimated to be (72-)70(-68) Ma (M.-L. Zhang et al. 2014).
3E. Calligoneae Eaton —— Synonymy: Calligonaceae Khalkuziew
Age. This clade is estimated to be (67.3-)50.6(-37.4) Ma (M.-L. Zhang et al. 2014).
Shrubs; C4 photosynthesis + [Calligonum]/0; stem photosynthetic, (phylloclades) [Calligonum]; leaves fasciculate, flat to terete, or small/rudimentary, (soon) deciduous; A 8, (10-)12-15(-18); filaments with long, basal filamentous nectarifeous hairs; G [(3-)4]; P not accrescent; achene winged, (wings bilobed)/unwinged, (inflated), with branched bristles; cotyledons linear to lanceolate; n = 9.
2/42-130: Calligonum (35-123). Mediterranean to India, North and North East Africa, most Middle East.
3F. Rumiceae Eaton —— Synonymy: Rumicaceae Martynov
Perennial or annual herbs; lamina (venation palmate); P (4) 6, whorls dimorphic; A (4); nectary as scales/glands/disc; stigma often plumose/penicillate; inner P usu. with spines/tubercles; embryo (curved), cotyledons linear to orbicular; n = 7-11, nuclear genome [Cx] = 434-3052 Mbp.
3/231: Rumex (200), Rheum (30). ± Cosmopolitan, esp. North Temperate
3G. Polygoneae Eaton
Annual to perennial herbs to shrubs (vines); stomata various; lamina convolute [Muhlenbeckia], petiole (with basal pit nectaries); P with 1 primary vein; embryo (curved), cotyledons linear to lanceolate; n = 7-16, nuclear genome [Cx] = 361-1035 Mbp.
6/135: Polygonum (29). Northern Hemisphere, inc. North Africa.Age. Polygoneae are estimated to be (64.9-)47.5(-35) Ma (M.-L. Zhang et al. 2014) or ca 53.2 Ma (Polygonum + Knorringia: D. L. Cao et al. 2022).
4. Eriogonoideae Arnott
(plant dioecious); P 5; nectary on P; A 8-9.
28/520. North and South America, the Antilles (West Africa).
Age. Crown Eriogonoideae have been dated to (104.7-)76.4, 69.1(-43.0) Ma (Schuster et al. 2013).
4A. Brunnichieae C. A. Mey. - Brunnichia Gartner
Lianescent, with branched branch/inflorescence tendrils; lamina base ± cordate, (venation palmate); placentation free-central; ovules anatropous, funicle long; fruit wings from ridges on floral tube and pedicel; endosperm (ruminate); n = 10, 12.
1/5. Southeast U.S.A. and Mexico to Costa Rica.
[Coccolobeae [Leptogoneae [Triplarideae [Gymnopodieae + Eriogoneae]]]: ?
4B. Coccolobeae Dumortier
Shrubs to (unbranched) trees or lianas; peltate glandular hairs +; petiole bundles in a D-shaped ring, medullary bundles ± inverted, vertically arranged wing bundles (outer ring of bundles +), (bundles scattered); epidermis mucilaginous, stomata para-/anisocytic; stipules notably variable; P 5; n = 11.
3/140: Coccoloba (130). Mexico to South America, the Antilles.
[Leptogoneae [Triplarideae [Gymnopodieae + Eriogoneae]]]: P 6.
4C. Leptogoneae Burke & Adr. Sanchez - Leptogonum domingense Bentham
Small tree; leaves at ends of branches; A 3; P not accrescent; n = ?
1/1. Hispaniola.
[Triplarideae [Gymnopodieae + Eriogoneae]]:
4D. Triplarideae C. A. Meyer
Trees (lianas); (internodes hollow, inhabited by ants); plant dioecious; staminate flowers: A adnate to P; carpellate flowers: P connate at least basally; stigma (peltate, decurrent); fruit samaroid, outer P accrescent, forming wings; endosperm (ruminate); n = 7, 11.
4/58: Ruprechtia (37). Southern Mexico, Central and South America.
4E. Gymnopodieae Burke & Adr. Sanchez - Gymnopodium Rolfe
Shrub to small tree; leaves 2-ranked; P very dimorphic; A = 9; ?endosperm; n = ?
1/1-3. Mexico (Isthmus of Tehuantupec) to Guatemala.
4F. Eriogoneae Dumortier —— Synonymy: Eriogonaceae G. Don
Herbs, commonly annual, to shrubs; (stems hollow); "growth sympodial"; leaves often basal; stipules usu. 0; inflorescence ± cymose, involucre + [= bracts, free or connate, (awned)]; A (3, 6); n = 12, 14, 17, 19-23.
17?/325: Eriogonum (240), Chorizanthe (50). North America, esp. the west, to Central Mexico, Chile and W. Argentina.
Evolution: Divergence & Distribution. For fin-wing fruited angiosperms in particular, see Manchester and O'Leary (2010), they described fossils from the late Maastrichtian of North Dakota ca 68 Ma like Polygonocarpum johnsonii (unplaced in family), and rather younger, from the Palaeocene but also from North Dakota, fruits that are placed in the extant genus Podopterus, P. antiqua (?Coccolobeae). For the fossil record in general, see Manchester et al. (2015).
If Oxygonum is sister to [Polygonaceae + Eriogonoideae], it may suggest that Polygonaceae were originally African/Gondwanan, and if age estimates here are accurate, then continental drift can be implicated in some distributions (Schuster et al. 2011b, see also 2013).
Schuster et al. (2013: additional dates) provide a detailed discussion on the biogeography of [Polygonoideae + Eriogonoideae], especially Muehlenbeckia, with additional ages. Diversity within the family as a whole peaks in more temperate regions rather than increasing towards the tropics; reduced extinction rates in temperate clades may be an explanation (Kostikova et al. 2014b). Diversification within Rheum occurred along with the uplift of the Tibetan Plateau in the last (16.1)-12.0, 9.9(-6.8) Ma, most species living in this area (Sun et al. 2012; see also Hughes & Atchison 2015). D.-L. Cao et al. (2022) discuss morphological evolution and biogeography of Persicarieae, the centre of distribution of which they suggest is in southwest China. For the evolution of extra-floral nectaries in some Polygoneae - no real effect on diversification rates - see Weber and Agrawal (2014).
Within Eriogonoideae, Eriogonum and relatives are very diverse in the drier regions of southwest North America (and there are also some species in southern South America), and it may represent a relatively recent radiation (Sanchez & Kron 2008). Koenemann and Burke (2020) thought that Coccoloba might have had a Mesoamerican origin, the Central American-Caribbean area being where members of the basal grade they recovered were to be found.
Ecology & Physiology. Basal Polygonaceae tend to grow in wetter (tropical) environments, although the family as a whole includes numerous taxa that prefer cooler conditions, a shift to such conditions being associated with the whole genome duplication found in most of the family (S. A. Smith et al. 2017). Calligonum can grow to 8 m tall and is particularly to be found in deserts in the Near to the Middle East (Winter 1981), indeed, woodiness seems to have evolved several times from the herbaceous habit (Lamb Frye & Kron 2003; Tian et al. 2011). Another genus of shrubs, Atraphaxis, may dominate in deserts, especially in Central Asia (M.-L. Zhang et al. 2014). Fallopia japonica produces resveratrol ((3,5,4’-trihydroxy-trans-stilbene), an allelopathic chemical that can inhibit seed germination and seedling growth of various species, hence accentuating the invasive potential of this noxious weed (Abgrall et al. 2018).
Calligonum includes perhaps up to 80 C4 species that grow in the halophytic vegetation in Turanian deserts otherwise dominated by C4 Chenopodiaceae s. str. (Winter 1981; Sage et al. 2011; Christin et al. 2011b for dates). There is a peripheral layer of small vascular bundles in the stems of at least some species of Calligonum, the xylem being adjacent to the ring of Kranz cells, and in both leaves and stems this Kranz ring surrounds all the central tissue, not individual vascular bundles; seedlings were also C4 (Doostmohammadi et al. 2020). Calligonum comosum, at least, has the NAD-ME subtype of C4 photosynthesis (Muhaidat et al. 2024). Terete leaves of Pteropyrum, the other genus in Calligoneae, have similar peripheral bundles (Doostmohammadi et al. 2020), and the bundle sheath cells of Pteropyrum are enlarged, although the plant is C3, and there is also water-storage tissue as in Calligonum (Doostmohammadi et al. 2020).
The speciose Eriogonum is a feature of drier areas of western North America, and perennial species may have broader niches than annual species, despite a contrary expectation based on the shorter generation times of the latter (Kostikova et al. 2013: focus on Californian species; see also Kostikova et al. 2016: evolution of climatic tolerances). However, the perennial species have to be able to put up with fluctuating environmental conditions over the course of their lives, while an annual can live its entire life under suitable conditions. For adaptive evolution and parallelism/convergence in Californian Eriogonum and relatives, see Kostikova et al. (2014a).
Polygonum (Bistorta) viviparum is a common perennial herbaceous ECM plant of the tundra, both as a pioneer and as a component of more established vegatation (e.g. Gardes & Dahlberg 1996; Michelsen et al. 1998; Brevik et al. 2010). Another ECM plant, Coccoloba uvifera, may form monodominant stands in Antillean coastal forests (Séne et al. (2017).
Desert rhubarb (Rheum palaestinum) may survive in the arid conditions in which it grows in part by condensing soil moisture on the underside of its very wrinkled leaf blades. These form a tight seal with the ground (Lev-Yadun et al. 2017) - does this happen in other species of the genus?
Pollination Biology & Seed Dispersal. Remarkable "glasshouse bract" inflorescences with large white recurved inflorescence bracts covering the flowers have evolved twice within Rheum growing at high altitudes in Southeast Asia (Sun et al. 2012), a parallelism evident at the molecular level (Liu et al. 2015). In R. nobile, about the largest plant in the placs where it grows, the sciarid fungus gnat Bradysia pollinates the flowers, also laying eggs at the same time; the association is mutualistic - pollination is effected, but some of the developing fruits are eaten by the developing larvae (B. Song et al. 2014), and since some flowers are pollinated, the developing parasitized fruits are not aborted (Song et al. 2016); the inside of the inflorescence is warmed and there is possible protection against ultraviolet radiation. A similar set of plant-insect interactions was also discovered in R. alexandrae, from the same habitat (Song et al. 2015); see also Yucca, Glochidion.
There is heterostyly in Fagopyrum (a supergene may be involved: Barrett & Shore 2008; Kappel et al. 2017; Gutiérrez-Valencia et al. 2021), and also in some Polygonum. It is uncommon to have heterostyly in taxa with bowl-shaped flowers (Cohen 2019).
Plant-Animal Interactions. Lycaena and Heliophorus (Lycaenini) are found on Polygonaceae throughout their extratropical range (Ehrlich & Raven 1964), and caterpillars of the lycaenid Euphilotes eat a number of species of Eriogonum (Shields & Reveal 1988). Enteucha, leaf miners belonging to the monotrysian Nepticulidae, are known only from here, and their crown age (84-51 My) is encompassed by the suggestions above (Doorenweerd et al. 2016: stem 135-102 My). Ceutorhynch seed weevils are quite commonly found on Polygonaceae; the weevils have moved on to the family about three times, and there have been movements on to other hosts (Letsch et al. 2018).
For insect vein cutting (trenching) in Rumex crispus and its effect of the photosynthesis of the leaf, see Delaney and Higley (2006).
Triplaris has an association with the ant Pseudomyrmex, but if the ant does not keep neighbouring vegetation away more aggressive ants may invade and usurp the association (see Davidson & McKey 1993). The Pseudomyrmex species involved form a small clade unrelated to other myrmecophytic species in the genus, and there it little specificity in the plant-ant interactions (Davidson & McKey 1993; Sanchez 2015); ascomycete fungi, Chaetothyriales, are also involved in the association (Vasse et al. 2017). The ants eat pearl bodies produced by the plants (Davidson & McKey 1993). The ant clade is significantly younger that that of the clade of Triplaris it inhabits, the latter being dated to ca 13.6 Ma (Chomicki et al. 2015; Chomicki & Renner 2015: ?sampling). The ant initially associated with the plant was perhaps Azteca (Chomicki et al. 2015), although this cannot be discerned in the more extensive study of Sanchez (2015).
Plant-Bacterial/Fungal Associations. Interestingly, in view of the general paucity of mycorrhizae in Caryophyllales, endomycorrhizae are reported from Eriogonum and ectomycorrhizae from both the tropical Coccoloba (Malloch et al. 1980; Tedersoo et al. 2010a) and the tundra-dwelling Polygonum (Bistorta) viviparum (e.g. Gardes & Dahlberg 1996; Brevik et al. 2010), and perhaps also Gymnopodium (see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, ages, etc.). ECM fungi on Coccoloba form a very distinct group (Tedersoo et al. 2014a). Newsham et al. (2009) also noted the frequency of arbuscular mycorrhizae in polar Polygonaceae.
Genes & Genomes. A genome duplication characterises much of this clade (Y. Yang et al. 2017: NCORE1; S. A. Smith et al. 2017), the POCNα event of some 54 Ma (Landis et al. 2018). Hibbins et al. (2025) suggest that there is a duplication that includes Fagopyrum and Rumex, while L. Zhang et al. (2017) date a duplication at 70.8-66.4 Ma (see also Fawcett et al. 2022).
Depending on the method of analysis, there may be confusion for some 61 taxa of Rumex as to whether thay are diploid or polyploid - is x = 5 or 10 here (Halabi et al. 2023)? Some species of Rumex subgenera Acetosa and Acetosella have XX/XY or XX/XY1Y2 systems determining the 'sex' of the plant (Westergaard 1958; Parker & Clark 1991; Hough et al. 2014: R. hastulus); polyploids may have only a single Y chromosome (Cuñado et al. 2007). See also Grant et al. (2022) and Hibbins et al. (2025) for sex system/dioecy evolution. The latter group note that there have been successive reductions in chromosome number in Rumex and a high rate of chromosome evolution across the genomes of species with sex chromosomes. There are taxa with XYY systems and extensive variation in such systems within R. hastatulus, and different ghost genomes may have introgressed into the XY and XYY clades. There have probably been two separate evolutions of dioecy, with R. bucephalophorus sister to the XY clade (R. hastatulus, R. acetosella and R. paucifolius are in the XY clade), while the XYY R. rothschildianus and R. thyrsiflorus are in a separate clade (Hibbins et al. 2025).
See Logacheva et al. (2008) for the expansion of the chloroplast inverted repeat. Koenigia delicatula has lost all 11 of its ndh genes, and its plastome is about 11% smaller than that of the rest of the genus; substitution rates in plastome and chondrome are correlated in Persicarieae (Qu et al. 2022).
Economic Importance. Polygonaceae include a disproportionally high number of notably serious and widespread weeds (Daehler 1997); the rhizomatous Reynoutria japonica and R. sachalinensis (Japanese knotweed) are particularly noxious weeds, although bee-keepers like the former, at least, for its flowers.
For the genome of the Tartary buckwheat, Fagopyrum tataricum, see L. Zhang et al. (2017) and for heterostyly, etc., in the commion buckwheat, see Fawcett et al. (2022).
Chemistry, Morphology, etc.. Williams et al. (1994) noted that although no plumbagin had been reported from the family, other quinones were known there.
Sieve tube plastids with protein fibres are reported from Triplarideae (Behnke 1999). The climber Antigonon has leaf tendrils and successive cambia (Carlquist 2003a; Rajput 2015; Sousa-Baena et al. 2018b: inflorescence tendrils, also see other taxa). There are often subepidermal strands of collenchyma or sclerenchyma in the stem in Polygonaceae (see also Plumbaginaceae). Guédèes (1968) described the sheathing stipules in plants like Polygonum bistorta as being ligular stipules (see also Caryophyllaceae).
There have been suggestions that the perianth of Polygonaceae is basically 3-merous and two-whorled; the carpels are opposite the outer perianth whorl (e.g. Galle 1977 for floral diagrams, etc.; see also Laubengayer 1937; Ronse Decraene 1989a; Vautier 1949: comprehensive survey of floral vasculature). Flowers with five tepals would then be derived from those with six, perhaps by fusion of two of the members. Recent work, however, suggests that the basic condition for the family is to have five perianth members (Lamb Frye & Kron 2003; Burke et al. 2009, esp. 2010; Ronse de Craene & Brockington 2013). However, the extensive earlier work on floral vascularization is not integrated into this scenario, and the floral vascularization of Symmeria, Afrobrunnichia and Oxygonum, phylogenetically critical taxa (see below), is unknown.
Stamens in Fagopyrum are both introrse and extrorse (Le Maout & Decaisne 1868), while in Persicaria campanulata five stamens are adnate to the perianth and three are completely free (Ronse Decraene & Akeroyd 1988). Since a nucellar beak is usually (?always) developed, there are several (4+) layers of parietal tissue. The chalazal end of the seed, i.e. the part below the point of junction of the integuments, can be quite massive (Cocucci 1957). The exact nature of the funicle is unclear; it might be a reduced basal placenta.
For more information, see Haraldson (1978) and Brandbyge (1993), both general, Hegnauer (1969, 1990: chemistry), Carlquist (2003a: wood anatomy), Lersten and Curtis (1992: Polygonum s.l.) and Pereira et al. (2020: Coccoloba), both leaf anatomy, Ronse Decraene and Smets (1991c: nectaries), Hong and Hedberg (1990: pollen, very variable), Ronse Decraene et al. (2000c: fruits in some Polygonoideae), Woodcock (1914: seeds), and Hutchinson and Dalziel (1928: general) and Cremers (1974: growth), both Afrobrunnichia.
Phylogeny. In the past, the largely herbaceous Eriogonoideae s. str., i.e. Eriogonum and its immediate relatives, were separated from Polygonoideae, variable in habit. The former lack a sheathing stipule, their inflorescence is cymose and involucrate, while the latter have a sheathing stipule and a racemose inflorescence that lacks an involucre. However, recent work suggests that there are two moderately/well supported major clades in the family, one largely woody, Eriogonoideae s.l., Eriogonoideae s. str. being derived within that clade, and the other, Polygonoideae, largely herbaceous (Cuénoud et al. 2002; Lamb Frye & Kron 2003; Burke et al. 2010; Sanchez et al. 2011).
Some genera are basal to these two clades. Symmeria and Afrobrunnichia - the position of latter is not so clear - may be immediately below the [Antigonon + Brunnichia] clade in Eriogonoideae (Sanchez et al. 2009a; Sanchez & Kron 2009; see also Burke et al. 2009; Schuster et al. 2015). However, Sanchez et al. (2009b) using chloroplast data placed Symmeria as sister to the whole of the rest of the family and Afrobrunnichia sister to Eriogonoideae, while ITS data suggested that Afrobrunnichia was sister to Polygonoideae and Symmeria sister to Eriogonoideae; in a combined analysis the relationships were [Symmeria [Afrobrunnichia [the rest of the family]]]. Not all support values were high, but this set of relationships was found by Schuster et al. (2011b), who also noted that the position of Oxygonum was uncertain, although it, too, might be part of a basal pectination (see also Burke et al. 2010 and Sanchez et al. 2011 for the position of Symmeria).
Eriogonoideae s. l. include the woody "Coccolobeae" which are both basal and paraphyletic (e.g. Cuénoud et al. 2002; Lamb Frye & Kron 2003); [Antigonon + Brunnichia] (Brunnichieae), both lianes, are sister to the rest of the woody clade (Sanchez & Kron 2008; Burke et al. 2010; Schuster et al. 2013: Symmeria etc. not included).
Coccolobeae. Koenemann and Burke (2020) looked at relationships within Coccoloba, where they found a basal grade and a South American clade; nodes tended to have little support. Within the rest of Eriogonoideae are clades with 5 and 6 perianth members, although the 5-membered Podopterus may be sister to the 6-membered clade (Burke et al. 2010).
Eriogoneae. Eriogonum is paraphyletic and includes taxa like Chorizanthe and Dedeckera (Sanchez & Kron 2006, 2008; Kempton 2012; Kostikova et al. 2016); Pearman et al. (2021: SNPs) obtained similar results.
Triplarideae. For relationships in the Neotropical Triplaris and Ruprechtia which have attracted attention because several species are ant plants, see Chomicki and Renner (2015) and Sanchez (2015). Basal relationships within Triplarideae are [Salta [Magoniella ...]] (Sanchez & Kron 2011).
In Polygonoideae Schuster et al. (2015) found that the African Oxygonum was sister to all the rest of the subfamily. Genera like Persicaria and Bistorta then make up Persicarieae, and they are sister to the remainder of the subfamily (Schuster et al. 2013, 2015). Schuster et al. (2015) discuss relationships between the other tribes here; the addition of taxa necessitated the description of two more clades as tribes, and Fallopia was highly polyphyletic. The mostly viney Muehlenbeckia is to be included in Polygonoideae; most species are sister to Fallopia s. str. (Schuster & Kron 2008; Schuster et al. 2011a, esp. 2013, 2015). For relationships among Chinese Polygonoideae, see Z.-D. Chen et al. (2016). The focus of D.-L. Cao et al. (2022: 74 plastomes) was on Persicarieae, but broader relationships were [Rumiceae [Polygoneae [Fagopyreae + Persicarieae]]].
Fagopyreae. Min et al. (2023: 3 or 5 chloroplast genes, 33 taxa) found three main clades with good support in the genus, although relationships along the spine of one of them, sect. Urophyllum, tended to be less well supported.
Persicarieae. Relationships around Koenigia and Aconogonon have been in a state of flux, and plastome and chondrome trees in Koenigia s.l. differ; Koenigia delicatula may be sister to the rest of the genus (Qu et al. 2022; Cao et al. 2022) while Acocnogonum is to be included in Koenigia (Cao et al. 2022).
Polygoneae. Schuster et al. (2011b, 2015) provide a molecular phylogeny of Polygoneae. For relationships within Atraphaxis, see M.-L. Zhang et al. (2014); relationships within the Asian Polygoneae are somewhat in a state of flux and hybridization may be causing problems (Yurtseva et al. 2016; Yurtseva & Mavrodiev 2019 and references). Desjardins et al. (2023) looked at relationships within Reynoutriinae.
Rumiceae. Rheum shows substantial morphological but little molecular variation, at least in the markers analysed (Wang et al. 2005). Grant et al. (2022: 1/3 species examined, 3 plastid markers) found that Emex was probably within Rumex (the subbasal clade), and there subgenus Rumex formed a basal polytomy, a 39-tomy, to be precise, probably because of the extensive hybridization in that group; all told, tey found that there were six major clades (including Emex) in Rumex. Hibbins et al. (2025) also found introgression - ghost lineages might be involved - in their whole transcriptome study of Rumex.
Classification. See Hernández-Ledesma et al. (2015). For a tribal classification of Polygonoideae, see Sanchez et al. (2011) and Schuster et al. (2015), for that of Eriogonoideae (and a subfamilial classification, too), see Burke and Sanchez (2011); although tribal limits in the old Eriogonoideae were holding up, subtribes and below were in somewhat of a state of disrepair (Kempton 2012). Generic limits around Polygonum are difficult, and now the tendency is to split the genus into an increasing number of pieces (e.g. Ronse Decraene & Akeroyd 1988; Brandbyge 1993; Ronse de Craene et al. 2004; Kim & Donoghue 2008; Kim et al. 2008; Galasso et al. 2009: comprehensive treatment; Yurtseva et al. 2010; D.-M. Fan et al. 2013; Yurtseva & Mavrodiev 2019: generic concepts). Desjardins et al. (2023) discuss generic limits around Fallopia.
Pearman et al. (2021) noted that just about all subgenera in Eriogonum that were not monotypic were polyphyletic, and reasonably suggested that a new classification was needed. Min et al. (2023) provide a sectional classification (three sections) for Fagopyrum. Grant et al. (2022) noted that Rechinger's subgenera in Rumex were not faring too badly.
Thanks. I thank Adriana Sanchez for comments.
[Rhabdodendraceae [Simmondsiaceae [[Asteropeiaceae + Physenaceae] [centrosperms, = Caryophyllaceae, Nyctaginaceae, Cactaceae, etc.]]]] / Core Caryophyllales: anthers much longer than filaments, basifixed; styles stigmatic their entire length; ovules 1-2/carpel; fruit 1-seeded; endosperm slight.
Age. Rhabdodendron may have diverged from all other Caryophyllales 90-83 Ma (Wikström et al. 2001), ca 87.4 Ma (Tank et al. 2015: Table S2) or ca 108.9 Mya (Magallón et al. 2015).
Evolution: Divergence & Distribution. Few (1-2) ovules per carpel may be an apomorphy for the whole clade (see also Sukhorukov et al. 2015 and Ronse De Craene 2020: Tables 2, 3 for other distinctive especially floral characters to be placed at this or the next four nodes), but the position of these ovules varies - apical in Simmondsiaceae, and basal in Rhabdodendraceae, just for starters. Basifixed anthers and stamens with very short filaments are common in these clades outide the centrosperms, and their optimisation on the tree is also difficult; taxa with such stamens also lack floral nectaries, perhaps being wind-pollinated. These taxa also lack a compitum, as far as is known (Armbruster et al. 2002).
Genes & Genomes. Y. Yang et al. (2015) found 10 or so genome duplications in this clade, including in Simmondsia and Physena. At least three gene duplications resulting in neofunctionalization of genes that are now integral to the betalain pathway may have occurred well before the evolution of betalains and the loss of abilty to produce anthocyanins. The loss of anthocyanins is here placed at a node further up the tree that is some 20 Ma or more younger than the oldest of these duplications that resulted in the ADHα gene; this gene facilitated the increased production of tyrosine, a critical precondition for the whole operation of betalain synthesis (Timoneda et al. 2019).
Chemistry, Morphology, etc.. For successive cambia, see Robert et al. (2011), and for the anatomy, etc., of single-seeded fruits, see Sukhorukov et al. (2015).
The morphology, embryology and in particular chemistry (see Fig. 4 in Brockington et al. 2015) of the basal members of this clade are rather poorly known, but the plants are rather different from the centrosperms.
Phylogeny. For relationships in this part of the tree, see above.
RHABDODENDRACEAE Prance - Rhabdodendron Gilg & Pilger - Back to Caryophyllales
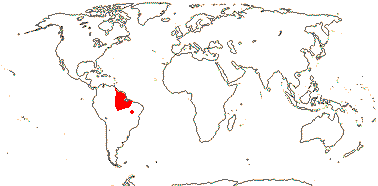
Woody; ellagic acid +, betalains?; cork?; successive cambia + (0); true tracheids +; dark-staining deposits esp. in rays; SiO2 bodies +; pits vestured; sieve tube plastids with protein crystalloids and starch; nodes 5:5 or 7:7; (cortical bundles +); ?pericycle; secretory cavities with resin; sclereids +; petiole bundle annular, bundles separate or not, (medullary vascular bundles +), wing bundles +; foliar (branched) fibre-like sclereids +; hairs peltate, cells with SiO2 bodies; lamina punctate, vernation revolute, secondary veins looping close to margin, petiole base rather broad-cordate; inflorescence axillary, branched, ?with a terminal flower; hypanthium +, short; K ± connate, short, C rather thick; A many, development ± simultaneous, anthers articulated with filament, anther with outer parietal layer of wall producing endothecium only, the inner the middle layer and tapetum [monocotyledonous type], exodermis tanniniferous; nectary 0; G 1, ?development, stylulus basal, compitum necessarily 0, stigma much elongated, ?type; ovules 1-2/carpel, basal, parallel, campylotropous, bitegmic zone short, outer integument 4-5 cells across, inner integument 2(-5) cells across, parietal tissue 10+ cells across, nucellar cap 0; megaspore mother cells several; fruit a drupe, basally surrounded by K/swollen receptacular area, filaments persistent, pedicel swollen; seed single, exotestal cells tangentially elongated, underlying cells short-tracheidal [with bar thickenings]; perisperm +, slight, embryo annular, chlorophyllous, with large cotyledons; n = 10, x = 6 (?7).
1 [list]/3. Tropical South America. Map: see Prance (1972c) and Aymard et al. (2016). Photo: Flower.
Chemistry, Morphology, etc.. The leaves are often rather congested and may grade into much smaller undifferentiated leaves at the beginning of each innovation. I have not seen stipules (see also Puff & Weber 1976; c.f. Prance 1972c), but the rather broad petiole base can be confused with them.
The ovule is often described as being unitegmic (e.g. Nandi et al. 1998, following Puff & Weber 1976), but see Tobe and Raven (1989). The stylulus may be stigmatic for only part of its length. The embryo is surrounded largely by testa that develops from the unitegmic part of the ovule, and the description above refers to this (Tobe & Raven 1989).
For general information, see Prance (2002), for some chemistry, see Wolter-Filho et al. (1989).
Previous relationships. The position of Rhabdodendraceae has long been uncertain. Thus they were placed in Rutales by Takhtajan (1997), although Prance (1968) had much earlier suggested a position around Caryophyllales; Cronquist (1983) placed them at the end of his Rosales after Surianaceae.
[Simmondsiaceae [[Asteropeiaceae + Physenaceae] [centrosperms = Caryophyllaceae, Nyctaginaceae, Cactaceae, etc.]]]: nodes 1:1; P +, uniseriate, 5.
Age. The age of this node may be around 100.9 Ma (Magallón et al. 2015), about 97 Ma (Hernández-Hernández et al. 2014), (102-)93, 88(-79) Ma (Bell et al. 2010), ca 83.8 Ma (Tank et al. 2015: Table S2) or 72-67 Ma (Wikström et al. 2001).
Evolution: Divergence & Distribution. A uniseriate perianth is tentatively pegged to this node, the implication being that petal-like structures common in centrosperms are in fact staminodial or calycine in origin (Ronse de Craene 2007); see also Brockington et al. (2009), Ronse de Craene and Brockington (2013), and the discussion below under the centrosperms.
Chemistry, Morphology, etc.. For variation in seed size, see Moles et al. (2005a).
SIMMONDSIACEAE van Tieghem - Simmondsia chinensis (Link) C. K. Schneider (!: note epithet) - Back to Caryophyllales
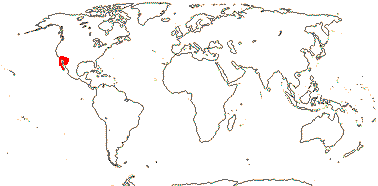
Evergreen shrubs; ellagic acid 0, seeds with C36-C46 long straight-chain wax ester [jojoba oil - a wax]; successive cambia +; pericyclic fibres +; cork pericyclic; true tracheids +; vascular tissue with phloem islands; petiole bundle ± C-shaped; stomata anomocytic, cyclocytic and laterocytic; hairs uniseriate; leaves opposite, articulated near stem, lamina vernation flat, secondary veins ascending from near base; plant dioecious; flowers small, (4, 6-merous); nectary 0; staminate plant: inflorescence usu. terminal, cymose; A 2 x P, extrorse; pollen ± porate, central part operculoid, spinules minute; carpelate plant: flowers single, axillary; G [3(-4)], carpels initiate separately around flat ± persistent floral apex [= cup-shaped], styluli +, papillate all around; ovule 1/carpel [= 2,1,0 per placenta], subapical, pendulous, apotropous, outer integument 6-10 cells across, inner integument 3-5 cells across, parietal tissue to 10 cells across, (nucellar cap to 2 cells across), obturator 0; fruit a 1(-3)-seeded capsule, exocarp of radially elongated sclereids, columella persistent, K accrescent, spreading; testa multiplicative, vascularized, with unicellular hairs, exotestal cells palisade, walls except inner periclinal thickened, mesotesta aerenchymatous, rest collapsed, cells tanniniferous; endosperm nuclear, reserve?, cotyledons incumbent; chloroplast ndh gene complex 0 [missing/non-functional]; germination hypogeal; n = 13, x = ?
1/1: [list]. S.W. North America, the Sonoran Desert. Map: see Sherbrooke and Haase (1974). [Photos - Collection.]
Evolution: Pollination Biology & Seed Dispersal. Wind eddies around the leaves seem to deflect pollin towards the stigmas, although whether or not the adoption of wind pollination here is recent is yet another matter (Niklas & Buchmann 1985).
Genes & Genomes. A genome duplication has been reported from Simmondsia chinensis (Y. Yang et al. 2017).
Chemistry, Morphology, etc.. Thulin et al. (2016) detected anthocyanins but not betalains here. The vessels have simple apertures and the root has anomalous secondary thickening (Bailey 1980). Ashow et al. (2013) provide a detailed description of the anatomy of the plant, i.a. they note that the petiole has ad- and abaxial C-shaped and perhaps also wing bundles.
The stamens are described as being latrorse (Takhtajan 1997). Ashow et al. (2013) describe the testa as having unicellular hairs. The large embryo contains liquid wax made up of esters of high molecular weight, mono-ethylenic acids.
For general information, see van Tieghem (1897), Mathou (1939) and Köhler (2002), for chemistry, see Hegnauer (1989, as Buxaceae), for wood anatomy, see Carlquist (2002b), for gynoecial morphology, see Ronse de Craene (2020: esp. Figs 2, 6), for embryology, see Wiger (1935), and for testa anatomy, etc., see Tobe et al. (1992b) and Sukhorukov et al. (2015).
Previous Relationships. Simmondsiaceae have usually been included in Buxaceae or placed in a separate family, but close to Buxaceae. However, a monotypic Simmondsiales have been included in Hamamelididae (Takhtajan 1997).
[[Asteropeiaceae + Physenaceae] [centrosperms = Caryophyllaceae, Nyctaginaceae, Cactaceae, etc.]]: chloroplast rpl2 intron 0.
Age. The age of this node is estimated at 61-52 Ma (Wikström et al. 2001), (95-)83, 79(-68) Ma (Bell et al. 2010) or about 96.2 Ma (Magallón et al. 2015).
[Asteropeiaceae + Physenaceae]: ?betalains; young stem with vascular cylinder; wood parenchyma aliform-confluent; vasicentric tracheids +, fibre tracheids +; rays 1-2 cells wide; successive cambia ?0; A latrorse; exocarp of radially elongated sclereids; cells of seed coat tanniniferous; endosperm at most slight.
Age. This node is estimated to be about 61.7 Ma (Magallón et al. 2015) or about 34.1 Ma (Tank et al. 2015: Table S1).
Evolution: Genes & Genomes. There are quite extensive changes in plastomes around here, especially in that of Physena (Yao et al. 2019).
Chemistry, Morphology, etc.. Some information on the general anatomy of these two families is taken from Harms (1893) and on fruit and seed anatomy from Sukhorukov et al. (2015); Carlquist (2006) compared their wood anatomy.
ASTEROPEIACEAE Reveal & Hoogland - Asteropeia Thouars - Back to Caryophyllales
Evergreen trees or scrambling shrubs; plant ectomycorrhizal; ellagic acid?; rays uniseriate; pericyclic fibres +; petiole bundle annular; cortical and mesophyll sclereids +; hypodermis several layered; ?stomata; inflorescence terminal, branched, pedicels with many bracteoles; C +, deciduous; A 9-15, ?obdiplostemonous, anthers dorsifixed, filaments basally connate; (pollen 6-rugate); G [(2) 3], ?development, gynophore +, short, compitum?, (style short, with lobed stigma), stigma continuous across G; ovules 2-several/carpel, ± apical, ?embryology; fruit nutlike, (several-seeded), K accrescent, spreading, forming wings, A persistent; seed coat 2-5 cells across; endosperm reserve?, embryo curved, cotyledons spirally coiled; n/x = ?
1/8: [list]. Madagascar. [Photos - Collection.]
Evolution: Plant-Bacterial/Fungal Associations. Asteropeia has both ecto- and arbuscular mycorrhizae (Bâ et al. 2011a, b: Henry et al. 2016, being dual mycorrhizal plants (Teste et al. 2019: Table S2); see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, ages, etc.).
Chemistry, Morphology, etc.. Some information is taken from Morton et al. (1997b), Schatz et al. (1999), and Kubitzki (2002d), all general, Beauvisage (1920: anatomy), Miller (1975: wood anatomy) and Ronse De Craene (2020: Fig. 6, gynoecium).
Previous Relationships. Asteropeiaceae were previously often included in Theaceae or Theales (Cronquist 1981; Takhtajan 1997), but are very different in wood anatomy (Baretta-Kuipers 1976).
PHYSENACEAE Takhtajan - Physena Thouars - Back to Caryophyllales
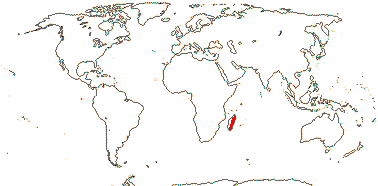
Shrub or tree; triterpene glycosides, oxohexadecanoic acid + [keto fatty acid]; ellagic acid?; pericyclic sclereids +; cuticle wax crystalloids?; ?petiole bundle; leaves two-ranked; plant dioecious; inflorescence axillary, racemose, pedicels long; P 5-9; staminate flowers: A (8-)10-14(-25); carpelate flowers: G [2], apical septae + [= invagination of apex], ?gynoecial development, ?compitum; ovules 2/carpel, ± subbasal, campylotropous, outer integument 6 calls across, inner integument ca 4 cells across, funicle long; fruit subdrupaceous?; seed single, large, (with hair-like outgrowths), coat vascularized, 16-20 layers thick, cell walls not notably thickened; endosperm reserve?, perisperm 0, embryo straight, cotyledons unequal; n/x = ?
1/2: [list]. Madagascar. [Photos - Collection.]
Evolution: Genes & Genomes. Y. Yang (2017) reported a genome duplication in Physena madagascariensis.
Chemistry, Morphology, etc.. The petiole is often described as being articulated; it commonly breaks transversely above the base, but there is no evidence that the leaf is derived from a compound leaf. The vascular bundles in the lamina are completely surrounded by mechanical tissue. There are brachysclereids in the secondary phloem and the placental bundles are inverted (Dickison & Miller 1993).
General information is taken from Morton et al. (1997b) and Dickison (2002); for triterpene glycosides, see Inoue et al. (2009), for fruit anatomy, see Sukhorukov et al. (2015).
Previous Relationships. Physenaceae were included in Urticales by Cronquist (1981) and placed in a monotypic Physenales in Dilleniidae by Takhtajan (1997).
[Macarthuriaceae [Microteaceae [[Caryophyllaceae [Achatocarpaceae + Amaranthaceae]] [Stegnospermataceae [Limeaceae [[Lophiocarpaceae [Kewaceae [Barbeuiaceae + the Raphide clade]]]] [Molluginaceae [Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] + the ACPT clade]]]]]]]]] (The Raphide clade = [Aizoaceae [Gisekiaceae [[Sarcobataceae + Phytolaccaceae] [Petiveriaceae + Nyctaginaceae]]]], the ACPT clade = [Talinaceae [Portulacaceae [Anacampserotaceae + Cactaceae]]]) / Centrospermae / Curvembryeae: plant herbaceous; (CAM [especially pervasive in succulents] +), (C4 photosynthesis +); unlignified cell walls with >3.5 mg g-1 ferulate [ester-linked to side chain arabinans and galactans of pectic polysaccharide rhamnogalacturonan-1; unlignified cell walls fluorescing blue under UV, green with NH3], (O-methylated) flavonols, quinones, triterpenoid saponins +, tannins, myricetin 0 or slight (phytoferritin +); sieve tube plastids with a ring of proteinaceous filaments and a central angular crystalloid (also with starch); pericyclic fibres 0, phloem-derived fibres quite widespread; (mucilage cells +); (stomatal orientation transverse); lamina often succulent; inflorescence cymose; pollen grains tricellular, (polyaperturate), foot layer thin; nectary on adaxial bases of stamens; G opposite P [so the median member adaxial], placentation free-central or basal, stigmas papillate, little expanded; ovules campylotropous, (space between the bases of the inner and outer integuments), (placental obturator +, papillate/with short hairs), (funicles long); fruits with more than one seed, seeds black; both exotestal and endotegmic cells with bar-like thickenings or latter with fine radial striations; endosperm 0, perisperm +, starchy, starch grains clustered, embryo curved, peripheral, cotyledons incumbent; germinal epigeal, phanerocotylar; no dark reversal Pfr → Pr; intron in chloroplast rpl2 gene 0 [+ in some Portulaca]; mitochondrial rps10 gene lost.
Age. Molecular estimates of the crown age of this clade in Bell et al. (2010) are (83-)71, 66(-57) Ma [actual node?].
Evolution: Divergence & Distribution. For ages of various clades in centrosperms, see Hernández-Hernández et al. (2014); not all have been added below.
Centrosperms contain ca 5.3% of eudicot diversity (Magallón et al. 1999).
The evolution of petals, betalains and anomalous secondary thickening and gynoecial morphology, etc., in this group has long been of interest, but our understanding of phylogenetic relationships particularly of clades towards the base of this part of the tree is still unclear. Of course, this then affects the evolutionary stories we can tell, furthermore, clarifying the basic phytochemical, morphological, anatomical and embryological variation in a number of centrosperm clades is essential. Thus Sukhorukov et al. (2015: p. 2) suggest a number of features common to "most representatives" of the core Caryophyllales, a clade that includes everything from Rhabdodendraceae on up, although note that a few families are not accounted for (Sukhorukov et al. 2015: Fig. 1). They optimise characters like embryo orientation (not applicable if there are 2 or more seeds/fruit or loculus), fruit succulence and pericarp layering on to a tree, and we will have to return to such optimisations when/if centrosperm phyogeny is more settled - see "Phylogeny" below.
Most of this clade have lost the ability to synthesize anthocyanins, instead synthesizing betalains, distinctively coloured pigments that replace them; Timoneda et al. (2019) have recently written a comprehensive synthesis of the evolution of betalain biosynthesis and the interested reader is recommended to consult this (see also S. O. Smith et al. 2019; Sakuta et al. 2022). Betalains may have been acquired once in this clade and then been lost four times, or gained twice and lost three times, or... (Cuénoud et al. 2002; Cuénoud 2002a; Brockington et al. 2015, c.f. 2011; Thulin et al. 2016; see also Clement & Mabry 1996), although data on betalain presence is lacking for a few families including Microteaceae (see Thulin et al. 2016; Lopez-Nieves et al. 2017; Timoneda et al. 2019) that some analyses are placing basal in the centrosperms (J. F. Walker et al. 2018a: see below). Betalain acquisition seems to have been preceded by duplications of some of the genes involved (Lopez-Nieves et al. 2017; Timoneda et al. 2019), which are organised as/in an operon (Brockington et al. 2015). A crucial step in the evolution of the betalain pathway is the evolution of the arogenate dehydrogenase (ADH) gene involved in the synthessis pf L-tyrosine. There has been duplication of this gene, and ADHα insensitive to high tyrosine levels, while ADHβ, like the ADH genes of other plants, is sensitive to tyrosine (Timoneda et al. 2019), so tyrosine accumulates (Lopez-Nieves et al. 2017). Tyrosine is then hydrolysed to L-3,4-dehydroxyphenyl alanine, and this is converted to betalamic acid, the central chromophore of all betalain pigments. The gene involved, DODA, also comes in two flavours, α and β, and the former, L-DOPA 4,5-deoxygenase, is part of the betalamic acid synthesis pathway (Sheehan et al. 2019; see also Brockington et al. 2015), amd the divergence of these genes happened after to divergence of the [Asteropeiaceae + Physenaceae] clade. Subsequently there have been substantial duplications, especially in the α lineage, although even in betalain-positive clades at least one α paralogue showed marginal activity, and marginal activity was evident across the whole DODA gene lineage. Although Brock et al. (2015) thought that there had been only a single origin of high-level betalamic acid production, but with subsequent losses, Sheehan et al. (2019) suggested that there had been four separate origins of betalamic acid — and no subsequent losses. Tyrosine is an integral component of betalains, and is part of the chromophore of both betacyanins and betaxanthins, reddish and yellowish pigments respectively, the former acquiring another modified tyrosine unit (Tanaka et al. 2008; Pichersky & Lewinsohn 2011; Gandía-Herrero & García-Carmona 2013; Brockington et al. 2015; Khan & Giridhar 2015; Timoneda et al. 2019). Interestingly, the ADHα enzyme is known from Simmondsiaceae (but there are no betalains there), but not from Physenaceae, while Asteropeiaceae and Rhabdodendraceae have not been tested; the duplication that gave rise to it may be somewhere along stem Caryophyllales or perhaps even earlier (Lopez-Nieves et al. 2017; but c.f. Timoneda et al. 2019).
Although the differences between betalain and anthocyanin (phenyalanine is a major component of the latter) synthesis pathways may not be that great (Strack et al. 2003; Shimada et al. 2007; Khan & Giridhar 2015), the two have never been found in the same organism (references in Lopez-Nieves et al. 2017). Shimada et al. (2005) found that anthocyanidin synthase genes were expressed in the seeds of both Phytolacca and Spinacia, proanthocyanidins accumulating in the cells of the seed coat (see also Timoneda et al. 2019). The blockage in the anthocyanin pathway is late, being just before the production of anthocyanins (Sakuta et al. 2022). Ectopic anthocyanin production can be induced in the petals of Astrophytum myriostigma (Cacteae: Sakuta et al. 2022).
That betalains have replaced anthocyanins in most centrosperms is clear, but why? Although other angiosperms have high levels of tyrosine, betalains have not evolved elsewhere (Timoneda et al. 2019). Jain and Gould (2015) suggest that the physiological properties of betalains and the particular ecological proclivities of centrosperms - CAM and C4 photosynthesis and the halophytic habitat are common - interact. For betalains as a possible defence against herbivory, etc., see Berardi et al. (2013).
Other features with rather spotty distributions are anatomical. These include the presence of wide-band tracheids. Here the cells have narrow lumina because of the height of the wall thickenings, and such tracheids are found in more or less desert-dwelling members of this clade (see below. The vascular anatomy of the lamina is quite often three-dimensional, with a ring of vascular bundles (peripheral vascular bundles) surrounding the midrib that have the xylem either internal (endoscopic) or external (exoscopic) (Ogburn & Edwards 2013; Melo-de-Pinna et al. 2014). These features are connected with the succulence, shape and photosynthetic pathway of the leaf and are particularly common in Chenopodioideae, Aizoaceae and Portulacineae (the two latter are CAM groups). In other taxa the lamina is more or less flattened and the orientation of the vascular bundles is normal.
In a number of taxa, including Dianthus, there appear to be two or more pairs of bracteoles below the flower - these have also been described as "epicalyx scales" (see also Fassou et al. 2022 for a discussion). The evolution of corolla-like structures, whether petals, staminodes, or floral bracts, is not simple (see Ronse de Craene 2010 for numerous floral diagrams of this group, esp. 2012 and 2013). Any "corolla" present, as in Caryophyllaceae, is often described as being of staminal origin (e.g. Ronse Decraene & Smets 1993; Leins et al. 2001). However, Wei and Ronse de Craene (2019), focussing on Caryophyllaceae, no longer think this, noting rather that nearly all taxa in the globular inclusion clade (and some others) have lost petals. Indeed, Greenberg and Donoghue (2011) had noted that it was perhaps surprising that such apparently staminodial petals in Caryophyllaceae are found in a clade in which stamen number is supposed to have increased from 5 to 10 irrespective of the presence/absence of these staminodes; Caryophyllaceae with 5 stamens only rarely have petals (but c.f. Ronse de Craene 2013). When there is a single perianth whorl, perhaps equivalent to the calyx of some Caryophyllaceae, this is quite often attractive and corolline, bracteoles then often being functionally calycine and borne immediately under the perianth. For literature on the calycine nature of the single perianth whori, see Borsch et al. (2018). Overall, "petals" may have evolved perhaps nine times or so in the clade and in a variety of ways (Brockington et al. 2009; see also Ronse de Craene & Brockington 2013; Ronse de Craene 2013; Glover et al. 2015). Brockington et al. (2012) showed that the numerous "petals" of Aizoaceae-Ruschioideae and -Mesembryanthemoideae were of probable staminal origin and B-class genes were expressed during their development. The sepals of Sesuvioideae, for example, look like petals when viewed adaxially, although B-class genes (AP3, PI) are not involved in their development (c.f. Ronse de Craene 2013), and Brockington et al. (2012) suggested that this might be the case throughout the centrosperms. A definitive synthesis of the evolution of calyx- and corolla-like structures in this part of Caryophyllales seems still to await.
Details of timing/pattern of intiation of both the perianth and androecial members vary, and this variation may be of phylogenetic interest (Ronse de Craene 2013: much information). The development of the androecium and its arrangement in the mature flower vary considerably. When there are ca 8 anthers, usually one or more pairs develop opposite the perianth members (Ronse Decraene et al. 1999), and when the stamens are equal in number to the perianth members they are usually opposite to them. In polystamineous taxa the initial primordia may alternate with the perianth members (Aizoaceae) or continue the spiral of the perianth (Pereskia - Cactaceae, Leins & Erbar 1994a, b: this may lack a perianth - see below); development is often centrifugal (see also Ronse de Craene 2013).
Gynoecial morphology and development in centrosperms was recently summarized by Ronse De Craene (2020), who tabulated the variation shown by 19 gynoecial characters in Caryophyllales and optimized them on a composite tree of centrosperm relationships - he used the topology [Microtea [Stegnosperma [[Macarthuria [amaranths and caryophylls, etc.]] [Limeum [[Lophiocarpus [Barbeuia [Kewa [the raphide clade below]]]] [Mollugo [Portulacineae below]]]]]]] for the core Caryophyllales - note that the limits of Portulacineae and the raphide clade there differ from those used below. In any event, integrating all the information in Ronse De Craene with centrosperm relationships awaits further work on the latter. His focus on gynoecial development has also allowed him to clarify features like ovule number per carpel. Several aspects of gynoecial development are incorporated in three groups that he recognized (Ronse De Craene 2020: esp. pp. 443-446, Fig. 1; see also Hofmann 1994): The carpels may initiate separately around a flat ± persistent floral apex [= cup-shaped type], both placentation and ovule and carpel number being notably variable, and the septae obliquely descending adaxially; or septae are at the same level as the floral apex and carpel lobes, all growing at about the same rate, and there are many ovules [= salt-shaker/Lychnis type]; or the floral apex is massive and enlarged when many ovules develop although less so when there is only a single ovule, peripheral tissue initiation being delayed, initially being a low rim around the floral apex, and septae are not prominent as they break down during development [= club-shaped/Cerastium type]. Ronse De Craene (2020) notes intermediates and/or infrafamilial variation in these last two types which he also suggested were often indistinguishable in later stages of development.
The evolution of gynoecial morphology and development is clearly difficult to ascertain. A single ovule/gynoecium could be a synapomorphy for all centrosperms minus Macarthuria (assuming it is basal here), but then two or more ovules/gynoecium would have evolved more than once, and the single ovule condition regained. Sukhorukov et al. (2015) suggested that the basal condition for core Caryophyllales was to have single-seeded fruits, but Ronse De Craene (2020) thought that the basal morphology was more probably a 5-carpellate gynoecium with two (or more?) ovules per carpel, single-seeded fruits being repeatedly derived. However, there seems little evidence of 5-carpellate gynoecia being basal from Ronse De Craene's own study (ibid. 2020: esp. Fig. 11), indeed, the situation becomes more complicated because there Geranium, admittedly 5-merous but surely not to be included in this clade, is sister to the [Drosera + Frankenia] clade, while the basal members of the clade [Rhabdodendraceae + The Rest] are not scored as having 5-carpellate gynoecia. This latter clade could be characterized by its low ovule number, and although Rhabdodendraceae and members of the next four pectinations are mostly poorly known, Ronse De Craene (2020) has provided some details of their gynoecial morphology. See Phylogeny below for relationships.
Seed morphology is overall rather similar, the embryo being curved and peripheral (hence the old name Curvembryae) (Martin 1946; see also Baskin & Baskin 2019). Starch-containing perisperm is the seed reserve, although endosperm may persist for quite some time during development; in Silene alba, at least, persisting endosperm cells, although alive, lacked reserves (Mohana Rao et al. 1988). Zheng et al. (2010) noted that the starchy perisperm tissue is formed not from the parietal tissue surrounding the embryo sac, but from tissue immediately below the embryo sac, i.e., the seed is technically chalazospermous. Note the complex variation patterns involving embryo shape and relative size of the cotyledons and nature of the seed reserve, if any, in Amaranthaceae (Vandelook et al. 2021).
Ecology & Physiology. 1. Succulents, both of leaf and/or stem, are common and many taxa have CAM photosynthesis or its variants or precursors; expression of CAM may depend on the developmental stage the plant is at and it may also be facultative and depend on environmental conditions (Ehleringer et al. 1997; Sage et al. 2012, 2014; Winter & Holtum 2014; Christin et al. 2015; Winter et al. 2015; esp Bräutigam et al. 2017; see also Ocampo & Colombus 2010; Christin et al. 2011b for dates); the PEPC enzymes involved in both C4 and CAM photosynthesis are members of the ppc-1E1 family (Christin et al. 2014b). See also Bräutigam et al. (2017) for the evolution of CAM photosynthesis and Males and Griffiths (2017) for the stomatal biology of CAM plants.
2. Other than Poales, species with C4 photosynthesis are most numerous in the centrosperms, indeed, there are about one third (21/61) of all origins of lineages with the syndrome in angiosperms here (Sage 2016). See also Sage et al. (1999), Muhaidat et al. (2007 and references), Christin and Osborne (2014). Schlüter and Weber (2020), etc., for the C4 pathway, its variants, etc., also papers in Ann. Bot. 115(3). 2015, also N. Wang et al. (2018) (focus on Portulacineae) for the ability of many centrosperms to handle harsh environmental conditions.
Robert et al. (2011) noted that woody taxa with successive cambia often (86% of cases, lianes/vines not included) grow in conditions in which there is some kind of water stress, and in at least some centrosperms both xylem and phloem form a three-dimensional network.
Plant-Animal Interactions. Most centrosperms are little liked by butterfly caterpillars (Ehrlich & Raven 1964), however the largely leaf mining yponomeutoid moth Heliodinidae are found here, especially on Nyctaginaceae (Sohn et al. 2013). Tempère (1969) discussed herbivory patterns that involve different members of this clade.
Plant-Bacterial/Fungal Associations. The oomycetous white blister rust, Wilsoniana, is parasitic on taxa scattered throughout this clade (Thines & Voglmayr 2009 and references).
Genes & Genomes. In comparisons between herbaceous and woody clades, substitution rates at all sites in protein-coding genes were much higher in herbaceous lineages than in their woody relatives (Y. Yang et al. 2015).
For the mitochondrial rps10 gene, see Adams et al. (2002b); eact position of loss? For the loss of the intron of the rpl2 gene, see Logacheva et al. (2008).
Chemistry, Morphology, etc.. For tannin (both hydrolysable and non-hydrolysable) distribution, see Mole (1993). Sterol composition may be of systematic interest (Wolfe et al. 1989; Patterson & Xu 1990), with distinctive sterols common or dominant in Caryophyllaceae, Phytolaccaceae, Amaranthaceae, and "Portulacaceae". Isoflavonoids (Reynaud et al. 2005), sometimes quite diverse, and phytoecdysones are scattered in the centrosperms, but perhaps not in the Cactaceae area. For unlignified cell wall fluorescence, see Hartley and Harris (1981) and Harris and Trethewey (2009). The latter note that ferulic acid is ester-linked to the side chain arabinans and galactans of the pectic polysaccharide rhamnogalacturonan-1, and diferulic and p-coumaric acids are less commonly involved than in monocots; although sampling was quite extensive, neither Macarthuriaceae, Microteaceae, nor members of the three clades basal to them were investigated so, like betalains, presence of ferulic acid may not characterize the clade.
Stomatal morphology is variable, but anomocytic stomata are common in nearly all families. However, in Cactaceae and relatives, parallelocytic and other kinds of stomata are found; some families in this area have predominantly paracytic stomata. Stomatal orientation on stem and/or leaf is commonly transverse throughout the order (Butterfass 1987, Amaranthaceae s. str.?), however, it is unlear which taxa have vertically or which unoriented stomata. Variation in structures associated with the leaf base, whether hairs/colleters or "stipules", is considerable (Rutishauser 1981) and would repay further study; note that the basic nodal anatomy of the clade is one trace-one gap, unusual for plants with stipules as commonly accepted.
For a good general survey of floral morphology, see Hofmann (1994). Sepals with an abaxial crest are described from Caryophyllaceae, Amaranthaceae, Aizoaceae, and Portulacaceae (Ronse de Craene & Brockington 2013). If there is a "corolla", it develops at the same time or after the androecium, not before it, and the "petals" and stamen(s) opposite them may form a developmental unit (e.g. Eichler 1875; Wagner & Harris 2000). The corona - in Lychnis viscaria, at least - arises from two bulges on the adaxial side of the "corolla", perhaps representing anther thecae.
Ronse De Craene (2020) provides a general summary of gynoecial morphology with a focus on core Caryophyllales. The carpels are quite commonly open in development, as in Polygonaceae (Tucker & Kantz 2001). Placentation is quite variable, although one commonly thinks of this group as typically having free-central placentation or its variants. A subepidermal layer of cells in the inside of the ovary wall may have calcium oxalate sand, as in some Amaranthaceae and Polygonaceae, while in Nyctaginaceae a ring of cells immediately below the ovary have conspicuous raphides (Guéguen 1901); there is little information on this feature. The integuments are often separated by a small space at their bases, but this seems to vary within Portulacaceae and Caryophyllaceae, and the space may be absent in Phytolacca and Amaranthaceae (e.g. Meunier 1890; Hakki 1973; c.f. Bittrich 1993). The apical cells of the nucellus are commonly elongated radially, as in Cactaceae, "Portulacaceae", Aizoaceae, Didiereaceae, Phytolaccaceae, and Amaranthaceae (see Johri et al. 1992; also Narayana 1962: e.g. Aizoaceae, Gisekiaceae, Molluginaceae), i.e., they form a nucellar pad, but it is unclear if this feature is of systematic significance. It seems to vary within Portulaca and Mesembryanthemum, and there may be confusion with radially elongated and periclinally divided nucellar epidermal cells, which would represent a nucellar cap, and which are also found here, e.g. in Cactaceae (Meunier 1890; Chopra 1950). There are often short hairs on the funicle that are directed towards the micropyle (Neumann 1935).
Seeds of a number of taxa have an operculum, although not necessarily identical in morphology (Bittrich & Ihlenfeldt 1984). Exotestal cells may have tanniniferous "stalactites" (Sukhorukov et al. 2018b), and there are commonly bar-like thickenings or fine radial striations on the walls of the endotegmic cells (e.g. Netolitsky 1926; Rauh & Schölch 1965; Bittrich 1993a; perhaps shown in Narayana 1962a; Sukhorukov et al. 2015). Variation in seed colour is correlated with the thickness of the seed coat - if the coat is 20-25μm or more across, it is usually black, if is less than 15μm across, it is often yellow (Sukhorukov et al. 2018b).
There is additional information in Tikhomirov and Sladkov (1990) Bittrich (1993a: useful summary) and Cuénoud (2006), also, see Hegnauer (1989: chemistry), Wolfe et al. (1989) and Patterson & Zu (1990), both sterols, Steglich and Strack (1990) and Strack et al. (2003), both betalains, Shimida et al. (2007: control of anthocyanin/betalain production), Barthlott (1994: waxes), Behnke et al. (1983a: sieve tube plastids), Behnke (1994a: sieve tube plastids, phytoferritin), Gibson (1994), Jansen et al. (2000c) and Timonin (2011), anatomy, esp. successive cambia, Rutishauser (1981: "stipules" and similar structures), Kendrick and Hillman (1971: ?sampling, dark reversal Pfr → Pr), Zandonella (1972, 1977) and Erbar (2014), both nectaries, the latter, no distinction between androecial and receptacular nectaries, Nowicke and Skvarla (1979) and Nowicke (1994a: pollen), Rocén (1927: embryology), Meunier (1890: ovules and testa), Werker (1997: seed coat) and Sukhorukov et al. (2015: seed and fruit anatomy, also embryo orientation, etc.).
Phylogeny. For general relationships in Caryophyllales, see above. Understanding where taxa from the old Phytolaccaceae and Molluginaceae, both polyphyletic, are to be placed in the tree has been critical for our understanding of relationships and evolution in centrosperms. [Amaranthaceae [Achatocarpaceae + Caryophyllaceae]] were early found to form a moderately well supported clade, the rest of the core Caryophyllales another (Källersjö et al. 1998), however, although 13 families were included in this study, sampling within them was poor. Similar relationships were suggested by Savolainen et al. (2000a). D. Soltis et al. (2000) found that Phytolaccaceae, Nyctaginaceae and Delosperma (Aizoaceae) formed a group, also [Amaranthaceae + Caryophyllaceae], but again the sampling was very sparse; for the position of Achatocarpaceae, see also K. Müller and Borsch (2005b). For other ideas of relationships, see Rodman (1994) and Downie and Palmer (1994: structural variation in chloroplast DNA).
Many of the relationships in the tree here are similar to those shown by Cuénoud et al. (2002: the Delosperma sequence was excluded, sampling still a bit sketchy), and these in turn are largely similar to relationships found by Källersjö et al. (1998) and other workers. Cuénoud et al. (2002) found two quite well supported clades within the centrosperms. There have been some recent improvements in our understanding of relationships along the backbone here, thus Schäferhoff et al. (2009) found that the poorly-known Microtea, one of whose previous resting places was Phytolaccaceae, was sister to the rest of the centrosperms (see also Y. Yang et al. 2015: Microtea the only problem genus included). In another study, Macarthuria, previously included in Limeaceae (and before that in Molluginaceae), occupied that position, and with strong support (Christin et al. 2011a), but Microtea was not included, and Limeum itself (as the monogeneric Limeaceae) remained in its old position quite well embedded in the centrosperms.
Other relationships have been suggested, but sampling is usually poor, or support is poor, or the markers are unreliable. Thus Harbaugh et al. (2010) found that Molluginaceae were sister to Caryophyllaceae, rather than Amaranthaceae, although two taxa from both families were all that were included in their study, which focussed on Caryophyllaceae. Stegnospermataceae were sister to all other centrosperms (support quite strong) and Limeum was placed with Amaranthaceae (support also quite strong) in a mitochondrial analysis by Qiu et al. (2010); however, Caryophyllaceae were not included. Support for the grouping [Stegnospermataceae [Caryophyllaceae + Amaranthaceae]] was found by M. J. Moore et al. (2011; see also Y. Yang et al. 2017: six small families along the spine of the tree around here not included). Hilu (2012) examined the effect of missing data and missing taxa on phylogenetic reconstructions; later they obtained trees that differ in several details from the one here, but support values were mostly low (see Crawley & Hilu 2013): Thus there were clades [Achatocarpaceae + Amaranthaceae] and [Stegnospermataceae + Limeaceae] or Stegnospermataceae were sister to all other centrosperms examined and Limeaceae were in about the same position as in the tree above, etc..
In the phylogenomic analysis of Murphy et al. (2019/2020), the relationships [Stegnospermataceae [Achatocarpaceae [Caryophyllaceae + Amaranthaceae]] [Limeaceae + Macarthuriaceae] [other centrosperms]]]]] were obtained, although support was not always strong and sampling poor. A clade [Microteaceae + Stegnospermaceae] was recovered by H.-T. Li et al. (2019), but with very low support and poor sampling. The summary tree in Hernández-Ledesma et al. (2015) also suggests some rather different relationships, perhaps most notably that Microteaceae and Macarthuriaceae are outside the [[Asteropeiaceae + Physenaceae] etc.] clade. In the phylotranscriptomic study of J. F. Walker et al. (2018a) relationships at the base of the centrosperms are [Microtea [[Macarthuria + Stegnosperma] ...]], although Limeum was the second clade in some analyses. Yao et al. (2019) in their extensive plastome analysis found little support for the positions of Microteaceae and Stegnospermataceae as successively sister to the rest of the centrosperms, and 91% bootstrap support for the clade [Macarthuriaceae [Caryophyllaceae [Achatocarpaceae + Amaranthaceae]]]; most nodes in this analysis were very strongly supported. Recently the relationships [Macarthuriaceae [Halophytaceae (!!) [Kewaceae [Microteaceae [Stegnospermaceae...]]]] have been recovered at the base of this part of the tree (W. J. Baker et al. 2021a: see Seed Plant Tree Version 1), but in the ii.2022 version basal relationships were [Macarthuria keigheryi [Microtea [Stegnosperma [[Macarthuria australis [caryophs & amaranths]]...]]]] and overall quite similar to the topology below apart from the position of Stegnosperma and the polyphyly of Macarthuria. Although some of these analyses have problems, relationships along the basal part of the centrosperm spine are decidedly uncertain, and of course this affects our understanding of character evolution - see the attempt by Ronse De Craene (2020) to understand gynoecial evolution in core Caryophyllales.
Cuénoud et al. (2002) recovered a monophyletic Aizoaceae, albeit with only slightly better than marginal (52% bootstrap) support, in an analysis of matK sequences, the only gene for which they had moderately good sampling; Gisekia moved position in analyses of rbcL sequences; and Sarcobatus was sister to Nyctaginaceae, albeit with only weak support, in matK analyses, while in a rbcL analysis it grouped with Agdestis. Corbichonia (Lophiocarpaceae) and most of Hypertelis (now Kewa, the type species remains in Molluginaceae) were well supported as successive sister clades at the base of the [Aizoaceae [Gisekiaceae [Sarcobataceae, Phytolaccaceae, Nyctaginaceae]]] clade (Christin et al. 2011a); Hypertelis was also found to be in this general area of the tree in Schäferhoff et al. (2009: included only in their petD analysis). Here both Kewa and Hypertelis are placed separately on the tree (see also Brockington et al. 2011), although it is not clear exactly what the support values for these positions are; Thulin et al. (2018) found the relationships [Corbichonia [Kewa + ± a polytomy of the remaining Aizoaceae-Nyctaginaceae clade]]. Arakaki et al. (2011) found that Gisekiaceae and Aizoaceae reversed positions, but with little support; that area was not the focus of their study. There is further discussion on relationships in the Gisekiaceae to Nyctaginaceae part of the tree below.
Portulacineae, which include Cactaceae, have been associated with Molluginaceae (e.g. Nyffeler & Eggli 2010; Arakaki et al. 2011). However, in a recent transcriptome study support for this clade was weaker (Y. Yang et al. 2017). Relationships around Cactaceae, themselves a monophyletic group, remain difficult, and although progress has been made here (Brockington et al. 2009; Nyffeler & Eggli 2009; Ocampo & Columbus 2010; Soltis et al. 2011), some relationships are still uncertain. Arakaki et al. (2011: see below) produced a largely resolved tree for that area, but there are still clades of uncertain position (see e.g. J. F. Walker et al. 2018b).
Classification. For generic synonymies of the families of this group, see Hernández-Ledesma et al. (2015), and for taxonomic problems in their German representatives, see Kadereit et al. (2016).
Previous Relationships. Most of this group was included in the old Centrospermae (so named because of the basal or free-central placentation that is common in the clade) or Caryophyllales in the strict sense. The shikimic acid pathway, particularly phenylalanine, is a starting point for the synthesis of nitrogen-containing benzylisoquinoline alkaloids and the betalains of the centrosperms, and Kubitzki (1994) suggested a relationship between centrosperms, Magnoliidae and monocots because all contained such compounds.
MACARTHURIACEAE Christenhusz - Macarthuria Endlicher - Back to Caryophyllales
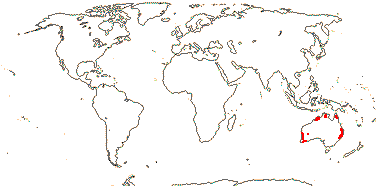
Herbs to ±rigid, rush-like shrubs; O-glycosylflavonoids; cork?; secondary growth abnormal; ?wood rays +; sieve tube plastids with cubic central crystalloid and starch grains; nodes?; leaves spiral, usu. ± reduced to scales, basal leaves undistinguished, (persistent), stipules 0; flowers small [to 5(-8) mm across]; K quincuncial, inner two usu. differentiated [e.g. C-like adaxially], C 5, clawed, linear-broadly obovate, same size or smaller than K, adnate to base of staminal tube, (-0), 3 A-C primordia [M. australis]; A 8, obdiplostemonous, only 3 A of outer whorl, connate basally, anthers basifixed; pollen grains finely punctate; G [3(-7)], odd member adaxial, placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker], becoming more the cup-shaped type], styluli +, impressed, stigma punctate; ovules 1-2(-3)/carpel, ± basal, collateral when 2, embryology?, obturator of hairs; fruit a leathery loculicidal capsule; seeds with funicular aril; n/x = ?
1 [list]/10. Australia, the periphery, esp. S.W. Australia (map: from Lepschi 1996; Australia's Virtual Herbarium xi.2013).
Evolution: Divergence & Distribution. Sheehan et al. (2019) suggested that betalain production was an apomorphy of the [Macarthuriaceae + Stegnospermataceae] clade.
Pollination Biology & Seed Dispersal. The seeds of Macarthuria are myrmecochorous (Lengyel et al. 2010).
Chemistry, Morphology, etc.. The first sepal to be initiated is in the abaxial-lateral position, although the overall floral orientation is "normal" for Pentapetalae (Ronse De Craene & Wei 2019, q.v. for details of floral morphology).
For further information, see M. Endress and Bittrich (1993: as Molluginaceae), Lepschi (1996) and Christenhusz et al. (2014), all general, Hofmann (1973: flower, growth), Ronse De Craene (2020: Fig. 3: floral development), Behnke et al. (1983b: pollen, sieve tube plastids, etc.), and Sukhorukov et al. (2015: fruit anatomy).
The genus is poorly known.
Previous Relationships. In earlier versions of this site (pre vi.2011) Macarthuria was included in Limeaceae; M. Endress and Bittrich (1993) placed it in Molluginaceae.
Evolution: Divergence & Distribution. It is unclear whether the acquisition of betalains is to be placed at this or a deeper node (see also Brockington et al. 2011, 2015).
MICROTEACEAE Schäferhoff & Borsch - Microtea Swartz - Back to Caryophyllales
(Annual) herbs (subshrubs, vines); betalain production?; cork?; secondary growth normal; sieve tube plastids lacking protein crystalloid, with a central starch grain; nodes?; calcium oxalate crystals 0; leaves spiral; inflorescence branched-racemose, flowers in groups of up to 3, (bracteoles 0); P (4), basally connate; A (2-)5(6-9), when 4, 5, alternating with P; anthers globose; pollen pantoporate; G [2-5], orientation variable, floral apex enlarged, peripheral tissue initiation delayed/low rim [= club-shaped], unilocular, style short, styluli linear, diverging, to triangular, ± erect; ovule 1 per flower, basal, ?embryology, funicle quite long; fruit a nut,surface ± raised reticulate, muricate to glochidate (not); seed single, shiny; ?exotestal cells quite large, ?tegmen ± flattened; n/x = ?
1 [list]/10. Antilles, Central and South America, Uruguay northwards, not western Peru, etc.. Map: from TROPICOS, vi.2011 - fill in the white space!; see also maps in Sukhorukov et al. (2019).
Chemistry, Morphology, etc.. The arrangement of the androecium in particular would repay attention.
Some information is taken from Rohwer (1993a; as Phytolaccaceae) and especially Sukhorukov et al. (2019); for sieve tube plastids, see Behnke (1993), for floral development, see Ronse De Craene (2020: Fig. 4), for pollen, etc., see Nowicke (1969: as Phytolaccaceae), and for fruit and seed, see Sukhorukov et al. (2015).
Microtea is very poorly known.
Phylogeny. See Schäferhoff et al. (2009) and Sukhorukov et al. (2019), the clade [Microtea debilis [ + ]] is sister to the rest of the genus.
Previous Relationships. In Amaranthaceae (early versions of this site), as Phytolaccaceae-Microteoideae, sometimes with Lophiocarpus in Phytolaccaceae (Rohwer 1993a) or as separate Lophiocarpaceae.
[[Caryophyllaceae [Achatocarpaceae + Amaranthaceae]] [Stegnospermataceae [Limeaceae [[Lophiocarpaceae [Kewaceae [Barbeuiaceae + the Raphide clade]]]] [Molluginaceae [Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] + the ACPT clade]]]]]]: anthocyanins 0, betalains + [chromoalkaloids]; sieve tube plastids with polygonal crystalloid; anther with outer parietal layer of wall producing endothecium only, the inner the middle layer and tapetum [monocotyledonous type].
Age. The age of this node has been estimated at 47-39 Ma (Wikström et al. 2001), ca 62.2 Ma (Tank et al. 2015: Table S2), about 89.5 Ma (Magallón et al. 2015), or about 88 Ma (Hernández-Hernández et al. 2014).
Evolution: Divergence & Distribution. There is a fair bit of diversification here.
Anther wall development of the monocot type has been placed as an apomorphy at this level; Veselova et al. (2016) discuss its distribution, and it is also known from Rhabdodendraceae. What goes on in the smaller families is mostly unknown, and the basic type of development is known from at least some of the raphide clade, but not Phytolaccaaceae s.l. (Veselova et al. 2016).
[Caryophyllaceae [Achatocarpaceae + Amaranthaceae]]: (phytoecdysteroids +); cork cambium deep-seated; stamens = and opposite P/K; G with floral apex enlarged, peripheral tissue initiation delayed/low rim [= club shaped]; ovule single [per flower], parietal tissue ca 4 cells across, nucellar cap 2-4 cells across; exotesta ?tanniniferous, outer wall thickened, with stalactite-like projections; embryo annular; mitochondrial rps1 and 19 genes lost.
Age. The crown age of this clade is estimated at 38-29 Ma (Wikström et al. 2001), (72-)59, 55(-44) Ma (Bell et al. 2010), ca 57.6/52.6 Ma (Tank et al. 2015: Table S2), or about 70.1 Ma (Magallón et al. 2015).
Chemistry, Morphology, etc.. For phytoecdysteroids, see Báthori et al. (1987), Dinan et al. (1998), and Zibareva et al. (2003). Mickesell (1990) listed both Amaranthaceae and Caryophyllaceae as having endosperm haustoria. Sukhorukov (2007) described the exotegmic cells of Chenopodiaceae s. str. as often having tannin deposits in the outer walls of the exotegmic cells that projected into the cell lumen (see also Kadereit et al. 2010; Sukhorukov et al. 2015: fruit and seed).
Since Achatocarpaceae are poorly known, most of the features mentioned above as possibly characterising this clade need to be confirmed.
CARYOPHYLLACEAE Jussieu, nom. cons. - Back to Caryophyllales
Herbs, annual to perennial, (tussock formrming / shrubs / lianes); cyclopeptides, glycoflavones, anthraquinones, anthocyanins +, betalains 0; true tracheids, fibres +; (wood rayless); sieve element plastid with polygonal central crystalloid; pericyclic fibres +; stomata often diacytic; (cuticle waxes as rodlets); lamina vernation conduplicate or ± flat, stipules +, ± scarious; flowers 4-5-merous; hypanthium +; P = K, quincuncial, + C; common A-C primordia [?level]; A 10; outer secondary parietal cell of anther wall dividing, tapetal cells 2-nucleate; (pollen 6(+) pantoporate); (nectary +, surrounding or abaxial to [esp. antesepalous] A on receptacle); G esp. [3, 5], when 5 alternate with/opposite to P/K, when 3 odd member adaxial, placentation ± axile, styles impressed [distribution?]; ovules with funicle longish to short, parietal tissue 3-10(-30) cells across, (in radial rows), (nucellar cap 2 cells across), (nucellar epidermis dividing along sides of ovules), obturator +, placental, hairy; fruit an utricle/achene/nutlet; (endotesta thickened; endotegmen ± thickened), ?tegmen bar thickenings; post-fertilization embryo sac with (chalazal) haustorium/diverticulum during early development, (suspensor massive), embryogeny solanad[!]; x = 9, nuclear genome [1 C] (0.092-)1.009(-11.079) pg; protein bodies in nuclei; mitochondrial coxII.i3 intron 0; sporophytic self-incompatibility system present.
101 [list]/2,285 (2,625) 3,300 - 11 tribes below. Mostly temperate, esp. Eurasian. Map: from Vester (1940), Frankenberg and Klaus (1980), Hultén (1971), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), FloraBase (consulted i.2013) and Australia's Virtual Herbarium (consulted i.2013). Photos: Collection, Minuartia Habit, Microphyes Flower.]
Age. The age of crown-group Caryophyllaceae is estimated to be ca 88.6 Ma (K. Feng et al. 2024).
1. Corrigioleae Dumortier —— Synonymy: Corrigiolaceae Dumortier, Telephiaceae Martynov
[Stipules auriculate]; leaves spiral; P with scarious margins; G with incomplete septae; fruit utricle/nutlet/many-seeded capsule; endotegmic cells lacking bar-like thickenings; genome size [1 C] ca 0.49 pg.
2/16. Mediterranean to Pakistan, Africa, Chile; Corrigiola litoralis widely distributed.
[Paronychieae [Polycarpeae [Sperguleae [[Sclerantheae + Sagineae] [[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogoneae]]]]]]]: nodes often swollen; leaves opposite: seeds tuberculate/not.
2. Paronychieae Dumortier —— Synonymy: Herniariaceae Martynov, Illecebraceae R. Brown, nom. cons., Paronychiaceae Jussieu
Stipules paired, subadaxial to petiole/paired, connate, adaxial/single, concave, adaxial or interpetiolar; P hooded, with subapical abaxial awn, (with scarious margins), (C +, filiform); (staminodes + - Paronychia); (pollen pantoporate); fruit a nutlet.
15/190: Paronychia (110), Herniaria (45). Worldwide, esp. Paronychia, many genera Mediterranean to Middle Eastern.
[Polycarpeae [Sperguleae [[Sclerantheae + Sagineae] [[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogoneae]]]]]: anthocyanins +, betalains 0 [?here, not in "Paronychioideae"]; C +, clawed, margin often ± deeply lobed/fimbriate; (placentation axile, septae at same level as floral apex and carpel lobes [salt-shaker type]).
3. Polycarpeae B. L. Robinson —— Synonymy: Ortegaceae Martynov, Polycarpaeaceae Martius
(C4 photosynthesis +); stipules + [interpetiolar fimbriae, from common primordium]; flowers ± perigynous; K usu. hooded, (awned), (with scarious margins), (pollen pantoporate); gynophore +, short; styles basally connate; (fruit a capsule, seeds ³2/fruit).
16/241: Polycarpaea (79), Spergularia (60), Drymaria (57). Almost worldwide.
[Sperguleae [[Sclerantheae + Sagineae] [[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogoneae]]]]] / Plurcaryophyllaceae [sic]: wood rayless [?here]; hypanthium 0; A 10(+), (staminodes +, alternating with P); (placentation axile at least basally when ovary young); fruit a septicidal and loculicidal capsule, ³2 seeds/fruit.
4. Sperguleae Dumortier —— Synonymy: Spergulaceae Bartling
Stipules +, interpetiolar, connate and encircling stem below leaves; K with scarious margin.
Spergula (60). ± Worldwide.
[[Sclerantheae + Sagineae] [[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogoneae]]]]: stipules 0; nuclear genome duplication [= CARY1/WGD1].
Age. This clade is ca 34.8 Ma (B. Xu et al. 2020); K. Feng et al. (2024) date CARY1/WGD1 to 64.4-56.7 Ma.
5. Sclerantheae de Candolle —— Synonymy: Scleranthaceae Berchtold & J. S. Presl
Plant (shrubby); (dioecious); (hypanthium +); (K with membranous margins); (C reduced-bifid, 0); (nectary); A 1-10, (5 staminodes); (fruit a nut, 1-seeded).
Schiedea (36), Scleranthus (11). Northern Hemisphere, Australasia and Ethiopia; also Hawai'i (Schiedea).
6. Sagineae J. Presl —— Synonymy: Minuartiaceae Martinov, Saginaceae Berchtold & Presl, Sabulinaceae Döll
(Hypanthium +); K connate, (lobes awned; with scarious margins); C entire, venation ± closed, adaxial appendages + [= ligules]; placentation free central; capsule valves = G, (nut, 1-seeded); seeds (with ridge of flat hairs on one side - Mcneillia).
Sabulina (65), Minuartia (55), Sagina (ca 30). Northern hemisphere, tropical montane, temperate southern hemisphere.
[[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogoneae]]]: stipules 0 [?next node]; capsule teeth often 2X styles/G; embryogeny caryophyllad.
7. Arenarieae Kittel
C margins often entire; (styles 2); seed ± globose, smooth, shiny, (aril/strophiole funicular, oily - Moehringia); cotyledons incumbent.
Arenaria (135). Northern hemisphere, Central and west South America.
8. Alsineae Lamarck & de Candolle —— Synonymy: Alsinaceae Bartling, nom. cons., Cerastiaceae Vest, Stellariaceae Berchtold & J. Presl
Annuals (biennials), perennials, (tuberous; climbers; cushion plants); (plant dioecious)(hypanthium +); (K with scarious margins), C ± strongly bilobed (0/entire/fimbriate); A (4-9, 11); G [(2)3(4)5]; styles free/connate; ovules several; micropyle endostomal, parietal tissue ca 3 cells across; (capsule valves = G - Lepyrodiclis), (nut, 1-seeded); seed black, shiny, to brown, rugose to tuberculate (-echinate), (winged), exotestal + endotegmic or testal; n = (6, 7) 10 (13, 15-19, etc.).
16/545: Cerastium (214), Stellaria (150-220), Odontostemma (65). Northern Hemisphere, esp. Eurasian, to the Andes, some cosmopolitan.
[Sileneae [Caryophylleae + Eremogoneae]]: veins at apex of lamina intramarginal; K ± connate; (anthophore [prolongation between K and the rest of the flower] +); C clawed, contorted, apex retuse or not, venation closed, coronal scale +/0; A and C primordia separate [?all].
9. Sileneae de Candolle —— Synonymy: Lychnidaceae Döll, Ortegaceae Martynov, Silenaceae Bartling
Annual to perennial herbs; (plant dioecious); K with commissural veins, C (imbricate), ?direction contorted; (placentation axile basally); capsule (loculicidal - Viscaria); seeds (with crown of flat hairs on one side); exotestal cells with anticlinal walls very deeply sinuous to ; n = ?
?6/738 or 888: Silene (486). North Temperate, African montane.
[Caryophylleae + Eremogoneae]: ?
10. Caryophylleae Lamarck & de Candolle —— Synonymy: Dianthaceae Vest
(Annual) perennial herbs, (subshrubs); leaves (spiny); ("epicalyx" +); K (with membranous commissures), commissural veins 0 (scarcely evident); C right-contorted (imbricate), (ligulate); G [2 (3)]; 2-many ovules/carpel; capsule valves 4 (6); (seeds peltate, embryo straight); n = 6, 10, 12-15, 17-18.
14/729: Dianthus (384), Gypsophila (150), Acanthophyllum (105), Jordania (14). Eurasia, N. Africa, few North America, Pacific Islands, Gypsophila australis Australia and New Zealand.
11. Eremogoneae Rabeler & W. L. Wagner
Plant ± rosette-forming; leaves linear to graminoid; hypanthium poorly (well) developed; K not connate, often with scarious margins, often hardened at base, C not clawed, ?contorted, coronal scale 0; capsule valves 2 x G; seeds large; cotyledons accumbent; n = 11.
2/97: Eremogone (90). (Eur)Asia, W. North America.
Age. This clade is ca 16.9 Ma (B. Xu et al. 2020).
Age. The earliest fossils associated with Caryophyllaceae seem to be of the pollen Periporopollenites polyoratus, from the Late Campanian ca 73 Ma. This has been linked with the macrofossil Caryophylliflora paleogenica from the Eocene of Tasmania, but these fossils cannot be identified as any known member of the family (Jordan & McPhail 2003).
Evolution: Divergence & Distribution. The diversification rate in European Dianthus, with (as of 2018) some 200 species (384 species - Fassou et al. 2022), is surprisingly high, 2.2-7.6 species/Ma, and (at the time) the highest rate recorded for either plants or terrestrial vertebrates (Valente et al. 2010a; also Martín-Hernanz et al. 2019: Table 4); see also Espeletia, Knope et al. (2012) for other rapid radiations. The genus is summer-flowering (i.e. it flowers during the dry season) and contains many narrow endemics (Valente et al. 2010a); some taxa moved south on to the African mountains, as in Ranunculus and Carex (Fassou et al. 2022, see also Gehrke & Linder 2009).
Schiedea forms a substantial radiation on Hawai'i, the ca 27 species being herbs with swollen roots to shrubs or large vines, and although the petals are lost or vestigial, the nectaries in some species with perfect flowers are remarkably elaborated, being long, curved and tubular, developing on the abaxial bases of the stamens opposite the sepals, the nectar itself being black in a small clade (Wagner & Harris 2000; Hansen et al. 2007; esp. Harris et al. 2012); some species have become wind pollinated and dioecious (Wagner et al. 1995; Sakai et al. 1997). There seems to have been an ancestral allopolyploidy event in the ancestor of Schiedea (Yang et al. 2018). For other major Hawai'an radiations, see the silversword alliance, etc., Cyrtandra, Cyanea and relatives, Myrtaceae, see Diversity and Distribution for Metrosideros, early stages, and the Stachys area, etc.. Relatives of Schiedia appear to include Honckenya and co., from the Arctic and Subarctic (Harbaugh et al. 2009a; see also Shaw & Gillespie 2016).
Alban et al. (2022) looked at the biogeographical relationships of the sister taxa Sagina and Colobanthus (Sagineae), both well represented in the Southern Hemisphere; their biogeographical histories are different. Silene (it includes the erstwhile Uebelinia and Lychnis) is a well-known component of the Afroalpine flora (Brochmann et al. 2021; see also Kandziora et al. 2022). Cerastium, perhaps 13.5 Ma, seems to have originated in Europe, and then moved around much of the world via a complex series of dispersal events (C. Liu et al. 2023). Smissen and Garnock Jones (2002: morphological analysis) emphasized the distinctive disjunct distribution of Northern and Southern Hemisphere (New Guinea, the Antipodes) groups in Scleranthus.
The phylogenetic structure now becoming evident in the family has considerable implications for character evolution. For optimisation of characters in the context of a well-sampled phylogeny, see Greenberg and Donoghue (2011, but c.f. Fig. 5c). Wei and Ronse De Craene (2019) optimised corolla evolution on a tree of the family and Smissen and Garnock Jones (2002) looked at the evolution of morphological characters, which they described in some detail, in Scleranthus.
Staminode development is of some interest in Paronychia (Paronychieae). Here the perfect flowers have five stemens and five staminodes; the latter, developing opposite the petals, are modified filaments. However, in dioecious taxa with female flowers, although the oppositipetalous staminodes are similar to those just described, the oppositsepalous staminodes consist of much-reduced, non-functional antherodes (Schenk & Appleton 2023).
Ecology & Physiology. Caryophyllaceae include numerous species that prefer cool/cold environments, movement to areas with such conditions perhaps being associated with the genome duplication recorded in the family (S. A. Smith et al. 2017; K. Feng et al. 2024). There are around 164 species that are cushion plants adapted to cold, dry environments and often growing at higher altitudes, and they are known from 20 genera, having evolved some 16 times here alone (Boucher et al. 2016b; Feng et al. 2024); individual cushions may be over 300 years old (literature in Kleier et al. 2015). Feng et al. (2024) suggested that whole genome duplication 1 was associated with a shift of the family into colder climates, certainly commoner in tribes with that duplication, howeever, similar proportions of tussock-forming species are to be found in tribes with and without that duplication. B. Xu et al. (2020) examine the evolution of two clades of such plants ([Thylacospermum + Arenaria subgenus Dolophragma], A. subgenus Eremeogoneastrum), the former maybe ca 11.7 Ma, the latter much younger, ca 1.85 Ma, now growing mostly at 3500-5300 m on the Qinghai-Tibet Plateau. The Plettkea lineage (placed in Stellaria) includes a number of cushion plants growing in the central Andes (see also Lupinus, Gomphrena); some are apetalous, some dioecious (Montesinos-Tubée & Borsch 2023).
In a number of species of Dianthus the stomata are blocked by wax deposits (Wulff 1898).
Pollination Biology & Seed Dispersal. Bird pollination is known from a few North American species of Silene (Fenster et al. 2015). There is an association between some species of Silene and its relatives, mostly from the Mediterranean region, and Hadena, a largely Eurasian noctuid moth. Attracted by lilac aldehydes (Döterl et al. 2008), Hadena lays eggs on the ovary/calyx, and the larvae eat at least some of the seeds, but the moth (both sexes) is also a pollinator; this is an obligate relationship for Hadena, rather like the yucca-yucca moth association (Kephart et al. 2006 for literature, also other papers in the New Phytologist 169(4). 2006; Prieto-Benítez et al. 2017). Some 70 species of Caryophyllaceae in over 10 genera and some 22 species of Hadena are involved (Prieto-Benítez et al. 2017), but these figures are likely to be underestimates. The flowers of Silene are pollinated by other moths whose caterpillars do not eat ovules or seeds (Kula et al. 2013). See also Asparagaceae-Agavoideae, Ranunculaceae, Phyllanthaceae, Saxifragaceae and Moraceae for similar interactions; Hembry and Althoff (2016) and Kawakita and Kato (2017f) review diversification and coevolution in these groups. Apetaly is quite common throughout the family, Sharples et al. (2021) finding that what amounts to functional apetaly (apetaly = taxa with petals less than half the length of the calyx) had evolved 19 times in Stellaria alone - petals were regained perhaps 5 times (apetaly was not associated with notable diversification, this seems to have occurred in petalous clades)
Plant-Animal Interactions. A number of Caryophyllaceae have glandular hairs, and dead insects trapped by these hairs may be eaten by otherinsects that also protect the plant (LoPresti et al. 2015; see also elsewhere).
Plant-Bacterial/Fungal Associations. Ectomycorrhizae have been reported from the family (Wang & Qiu 2006), while Newsham et al. (2009) noted the frequency of arbuscular mycorrhizae in polar Caryophyllaceae and Lekberg et al. (2015) found that Dianthus deltoides might be associated with AM fungi, there being some movement of carbon from plant to fungus.
For anther smut fungi, see Ngugi and Scherm (2006); pollen is replaced by teliospores of Microbotryum violaceum s.l. (Uredinomycota - see also Montiaceae) and tranported by would-be pollinators to other plants. Microbotryum is common on the family, especially on perennial Sileneae (ca 80% of the species), but not so much on the annuals or on members of the old Paronychioideae; strict cospeciation is not involved (Refrégier et al. 2008; Mena-Ali et al. 2009; Hood et al. 2010).
Genes & Genomes. K. Feng et al. (2024) give chromosome numbers for a number of taxa; these range from n = 5 (Illecebrum verticillatum) to 9-20, 22, etc..
Genome duplications in the family are common, and many of them are quite recent (Y. Yang et al. 2017: CARY1 is rather older; S. A. Smith et al. 2017). K. Feng et al. (2024) found 15 duplications, eight of which were new and some of which they aged, for a total of 19 duplications; they emphasized the importance of the CARY1/WGD1 duplication.
For the evolution of dioecy in Silene, which has happened three times or so, see Westergaard (1958), Desfeux et al. (1996), Zluvova et al. (2008) and Goldberg et al. (2017). Species of Silene subgenus Elisanthe have an X-Y sex determination system (Lebel-Hardenack et al. 2002; Charlesworth 2008), see also Sansome (1938: classic account), Papadopulos et al. (2015: rapid change in the Y chromosome of S. latifolia), Mrackova et al. (2018: independent origin of XY system in S. colpophylla, different chromosomes involved), H. Martin et al. (2019: S. otites, XY, S. pseudotites, ZW [female the heterogametic sex], again different chromosomes involved) and Hu and Filatov (2016) and references. Muyle et al. (2020) compare various population genetic parameters of dioecious taxa with those of other species of the genus with different breeding systems and found that the former did not seem to be disadvantaged. Akagi et al. (2025) confirmed the rapid change, noting the massive size of the Y chromosome, slightly under 500 Mb, and that transposable element insertions and rearrangements had accumulated in the male specific region of the chromosome.
Mutation rates of some chloroplast genes, even in the inverted repeat region, have greatly accelerated in some species, furthermore, there is variation in the position of the IR boundary and other structural changes in the plastome of some species of Silene with similar changes seeming to have occurred in parallel (Sloan et al. 2014; Zhu et al. 2015). Mutation rate increase may be linked to the presence of repeats, although they are not very large here (see also J. Wang et al. 2024). For the chloroplast clpP1 gene with high positive selection rates, etc., see Erixon and Oxelman (2008b: changes occurring in parallel).
The mitochondrial genome of Silene conica, at around 11.9 mb, is the largest of any embryophyte and bigger than the whole nuclear genome of some eukaryotes, but S. latfolia has quite a small chondrome of only 0.25 mb (Sloan et al. 2012a, b); overall, the range of mitochondrial genome size here is greater than that of any other comparable group of land plants. Species of Silene with these large mitochondrial genomes may have over a hundred micro-chromosomes, as least from the evidence provided by the mapping procedures used (Sloan et al. 2012a, b). At around 7 Mb in size (over 7 x 106 base pairs) the genome of S. noctiflora is partitioned among over 50 chromosomes, not all of which have functional genes and which are then easily lost (Z. Wu et al. 2015). Chromosome numbers may show considerable infraspecific variation, yet at the same time there is little variation in overall gene sequence (Wu et al. 2015; see also Ramsey & Mandel 2019). There has been sexual recombination in the mitochondrial genome of S. noctiflora, at least, although this can be difficult to detect because the sequences involved are almost identical (Wu & Sloan 2018).
There has been a massive increase in the rate of synonymous substitutions in the chondrome of species of Silene like S. noctiflora, but not in that of the plastome, nor in the substitution rates of its immediate relatives (Mower et al. 2007). Although Sloan et al. (2012b) noted that species with fast-evolving chondromes where change was genome wide and mutationally-driven might also have high mutation rates in the plastomes, however, in the latter change was at nonsynonymous sites and perhaps driven by selection. Mutation rates in the chondrome here are very high (Sloan et al. 2009), although they are not as high as in Plantago, while Pelargonium and Ajuga and some other genera also have very high substitution rates (see also Zwonitzer et al. 2024, also Characters).
Chemistry, Morphology, etc.. Anomalous ectopic/secondary thickening (successive cambia) occurs in the roots of some Caryophyllaceae (Krumbiegel & Kástner 1993). Variation in stipule morphology in Caryophyllaceae is considerable (see Guédèes 1968 for "ligular stipules") and even occurs in the course of development of a single plant, as in Paronychia argentea (Rutishauser 1981).
There has been some discussion over the nature of the perianth in Caryophyllaceae. It has been suggested that the plesiomorphic condition is for there to be both a calyx and corolla, although the C whorl tends to develop late and in association with a staminal whorl, and it may also be lost (see Wei & Ronse De Craene 2019, c.f. Ronse de Craene 2007, 2008), although Greenberg and Donoghue (2011) thought that initially there were no petals. Thus in Pseudostellaria the stamens are initiated before the corolla (Luo et al. 2012). In Sagina, Silene and Saponaria there is no common primordium, although the development of the C whorl is delayed in Sagina (Grant et al. 1994; Wei & Ronse De Craene 2019). Appleton and Schenk (2021) noted that staminodial structures in Paronychia developed inside the staminal whorl and alternating with the stamens, and although they varied in morphology, they lacked a vascular trace. In those taxa that have five carpels (not that common in Caryophyllaceae) and ten stamens, the carpels nay be opposite the sepals (the normal position) or opposite the petals. In the latter case the carpels are responding to space constraints developing because the sepals can be large, and together with the developing antisepalaous stamens the carpels are as it were pushed aside; there seems to be no systematic signal in this feature (Wei & Ronse De Craene 2020). Weberling (1989 and references, see also Endress 2019; esp. Thomson 1942) discuss placentation, which varies from axile, as in some species of Silene, perhaps the common condition in the family, to free central to the single, basal ovule of Uebelinia (this latter looks rather like a circinotropous basal ovule). Carpel morphology and development, ovule number, etc., is discussed further elsewhere. A "chalazal" haustorium or diverticulum develops on the inside of the curved embryo sac in many species and the nucellus in Agrostemma is massively thick (Rocén 1927). For fruit morphology (dehiscent?) and anatomy of Corrigioleae, see Sukhorukov et al. (2015). Martín-Gómez et al. (2024) looked at details of the morphology of seed tubercles, their size, shape, etc., in Silene in order to better compare them, and they also note their occurrence elsewhere in the family and order.
General information is taken from Bittrich (1993b) and McNeill (1962: the old Alsinoideae, also maps), for Sileneae, see Oxelman et al. (2013 onwards), for chemistry, see Hegnauer (1964, 1989), for the distribution of phytoecdysteroids, see Zibareva et al. (2003) and Zibareva (2009) and for that of cyclopeptides, see Jia et al. (2004), for stomata, see Rohweder et al. (1971: correlation between stomatal apparatus and leaf width), for stem anatomy, see Schweingruber (2007), for floral morphology, anatomy, etc., see Thompon (1942), Rohweder (1967b, 1970a), Rohweder and Urmi-König (1975, 1978) and Ronse De Craene (2020: Figs 3, 4, 6), for stamens or nectaries as corolla, see Mattfeld (1938) and Leins et al. (2001), for pericarp anatomy, see Kravtsova and Bolotova (2019: Sileneae), for much information on ovules and embryogenesis, etc., see Rocén (1927), also Cook (1909), Perotti (1913), Dahlgren (1940a), Nagl (1962) and L. Wang et al. (2017), and for seeds, see Meunier (1890), Mostafavi (2013: Minuartia), Sadeghian et al. (2014: Arenaria, etc.), Arabi et al. (2017: Alsineae, c.f. with recent generic limits), and Kravtsova and Romanova (2021: Sileneae) , all except the first external morphology.
Phylogeny. Of the old subfamilies, Paronychioideae - classically defined by the presence of stipules, lack of a corolla, and utricular fruit - form a basal grade, with Corrigioleae (Telephium, Corrigola) sister to all the rest of the family. Dicheranthus, Polycarpon, etc., may form the next clade, Paronychia, etc., the next. Drymaria and Pycnophyllum, both morphologically distinctive taxa, may be sister (Smissen et al. 2002 - they noted that Pycnophyllum [and Pentastemonodiscus] were not to be included in Caryophyllaceae-Alsinoideae, but they did not suggest where they should go; Fior et al. 2006). In the erstwhile Alsinoideae the calyx is free and the corolla has ± open venation. The tribes Sileneae and Caryophylleae are perhaps monophyletic, and together are sister to or form a polytomy with part of Arenaria (Nepokroeff et al. 2002; Fior et al. 2006). Relationships in Harbaugh et al. (2010) - on the whole well supported - from a three-gene analysis are [Corrigolieae [Paronychieae [Polycarpeae [Sperguleae [[Sclerantheae + Sagineae] [[Arenarieae + Alsineae] [Sileneae [Caryophylleae + Eremogeneae]]]]]]]]. Greenberg and Donoghue (2011) sampled more extensively but found a largely similar topology; a novel clade that they found, [[Sclerantheae + Sagineae] [[Arenarieae + Alsineae]], did not have much support. The position of the newly described Eremegoneae was uncertain in Harbaugh et al. (2010), but support was stronger in Greenberg and Donoghue (2011); in its current position its apomorphies are losses of the apomorphies of the whole [Sileneae [Caryophylleae + Eremogoneae]] clade, or, alternatively, these features arise independently in Sileneae and Caryophylleae... K. Feng et al. (2024: 59 transcriptomes, 11 tribes, ca 1/3 genera) also recovered the relationships above, although support for the nodes along the upper part of the tree could have been stronger. B. Xu et al. (2020) note a novel clade [Thylacospermum + Arenaria subgenus Dolophragma] ca 11.7 Ma, although its tribal status is questionable (Feng et al. 2024). Several genera have turned out to be highly polyphyletic. For example, members of the old Arenaria have ended up in tribes like Arenarieae, Alsineae and Eremogeneae (Sadeghian et al. 2015: B. Xu et al. 2020). For relationships among Chineae taxa, see Z.-D. Chen et al. (2016). The old Alsinoideae for the most part break down into two groups, A and B below.
Arenarieae. Fior and Karis (2007 and references) looked at relationships in Moehringia and its allies and the evolution of its strophiole.
Group A - Alsineae. Here are included Cerastium, Stellaria, etc.. Stellaria is strongly para-/polyphyletic, e.g. Fior et al. (2006), Greenberg and Donoghue (2011), M.-L. Zhang et al. (2017), etc.. Sharples and Tripp (2019: sampling good) using restriction site associated DNA sequencing delimited a somewhat slimmed-down Stellaria (but expanding since), and relationships in general had strong support. However, within Stellaria few previously delimited species groupings were supported, the key characters that had been used to delimit them showing extensive parallel evolution (Sharples 2019; see also Sharples et al. 2021 for a phylogeny of the genus with more extensive sampling). Arabi et al. (2022) found some differences in the topologies of the ITS and rps16 plastome trees in their analysis of the tribe; they preferred the former. Montesinos-Tubée and Borsch (2023: 2 chloroplast, 1 nuclear markers) looked at relationships within Andean taxa, and placed species from several genera in a clade of Stellaria; within that clade nuclear and chloroplast markers had yielded the same topology, but immediately outside there were differences. Liu et al. (2023) looked at relatiosnhips in Cerastium (75 spp.; 1 nuclear and 5 plastid markers); in some analyses C. fragillimum was sister to the rest of the genus, within which there were otherwise three main clades.
Within Caryophylleae, Pirani et al. (2014, 2020) discuss the phylogeny of Acanthophyllum, a large genus from the Irano-Turanian region, Valente et al. (2010a) the phylogeny of Dianthus in Eurasia, while Madhani et al. (2018) provide a comprehensive phylogeny of the tribe. A recent study of some 200 samples of Dianthus by Fassou et al. (2022: four plastid markers) found three large moderately well supported clades forming a tritomy, basal to these were a few small clades, and well supported as sister to the rest of the genus was the annual D. strictiformis (= D. recticaulis) with solitary flowers; previous infrageneric groupings were not supported.
Group B - Sagineae. Here are included much of Minuartia, Sagina, etc., and the tribe is holding up better phylogenetically. Alban et al. (2022: ITS, 2 plastome markers) looked at relationships around Sagina and Colobanthus, those in the latter groups being less well supported. Drypis, previously placed in Caryophylloideae because of its connate calyx, etc., is in the same immediate clade as Habrosia (ex Alsinoideae), so the variation there is considerable (Harbaugh et al. 2010; see also Greenberg & Donoghue 2011). In a comprehensive study, Dillenberger and Kadereit (2014) demonstrate the extensive polyphyly of Minuartia.
Sileneae For the phylogeny of Silene and its relatives - but Sileneae are perhaps not monophyletic - see Desfeux and Lejeune (1996), Erixon and Oxelman (2008a), Rautenberg et al. (2008: reticulation, 2012: the Californian S. multinervia isolated), Naciri et al. (2017) and especially Jafari et al. (2020: nuclear ITS and chloroplast rps16), although some basal relationships in the latter were unclear. For a phylogeny of Viscaria, etc., see Frajman et al. (2009), and for relationships around Pseudostellaria, see M.-L. Zhang et al. (2017).
Classification. The old tripartite division of the family into Silenoideae, Alsinoideae and Paronychioideae was based on the presence of a hypanthium, whether or not the petals were emarginate, whether or not the calyx was fused, etc., and is not confirmed by recent work. Here I follow the tribal classification of Harbaugh et al. (2010, see also 2012: ?Drypidae Fenzl?). These authors did not sample a number of genera, so tribal compositions were uncertain, but the situation was considerably improved by Greenberg and Donoghue (2011). The tribal implications of the study by Dillenberger and Kadereit (2014) are unclear.
Features like number of styles and whether there is obviously a common stylar region were often used to provide generic characters in the past, yet they have turned out to be of little value (e.g. Dillenberger & Kadereit 2014). Thus the limits of Silene, historically characterised by having three styles, need to be expanded to include some taxa with five styles (Desfeux & Lejeune 1996). Genera like Arenaria and Minuartia are polphyletic (Harbaugh et al. 2009); indeed, it was obvious that many generic limits needed attention (Greenberg & Donoghue 2011). Sadeghian et al. (2015) made some nomenclatural adjustments because of the polyphyly of Arenaria, while Dillenberger and Kadereit (2014) have dismembered Minuartia, necessary because the genus in its old, broad circumscription was hopelessly polyphyletic, and they noted that several other genera are para- or polyphyletic - yet more nomenclatural changes are needed (see also Kadereit et al. 2016; Arabi et al. 2022, etc.). See Madhani et al. (2018) for genera in Caryophylleae, Arabi et al. (2022) for a discussion justifying the generic limits that they adopted in Alsineae and Montesinos-Tubée and Borsch (2023 and references) clarify the limits of Stellaria. Naciri et al. (2017) provide a sectional classification of Silene, grouping the species into 44 sections, while Jafari et al. (2020) recognised 3 subgenera and 34 sections (one of the latter was unplaced); Pirani et al. (2020) suggest a sectional classification for Acanthophyllum.
Botanical Trivia. There are reports of placental tissue from 30,000 year old material of Silene stenophylla (or perhaps from another species of the genus) trapped in permafrost that have been persuaded to form whole plants (Yashina et al. 2012a, b).
Members of the old Paronychioideae in particular have solanad rather than caryophyllad embryo development...
[Achatocarpaceae + Amaranthaceae]: pollen pantoporate; P 5; style very short; ovule 1 per flower, basal; fruit indehiscent, 1-seeded.
Age. The age of this node may be around (94-)67.2(-46) Ma (Masson & Kadereit 2013), ca 64.2 Ma (Magallón et al. 2015), or ca 52.6 Ma (Tank et al. 2015: Table S2).
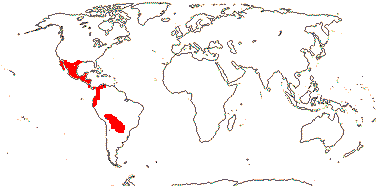
ACHATOCARPACEAE Heimerl, nom. cons. - Back to Caryophyllales
Woody; C-glycosylflavonoids +, betalain production?; secondary growth normal; sieve element plastid with polygonal central crystalloid; cork cambium?; nodes?; cuticle waxes as ± lobed platelets in clusters; P (4); A 10-20, basally connate or not; G [2], collateral or superposed, ?development, style branches very long; ovules (2), details?; fruit a berry; seeds with small aril; n/x = ?
2/10: [list], Achatocarpus (9). S.W. USA to South America. Map: from Fl. N. Am. vol. 4 (2003) and GBIF (consulted 2008). [Photo © C.E. Hughes - Fruits, Fruiting branch.]
Evolution: Plant-Bacterial/Fungal Associations. Achatocarpus may be ectomycorrhizal (see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, ages, etc.).
Genes & Genomes. Y. Yang et al. (2017) found a genome duplication in Achatocarpus.
Chemistry, Morphology, etc.. Some information is taken from Bittrich (1993b); for wood anatomy, see Carlquist (2000c), Sukhorukov et al. (2015: p. 13) describe the perisperm as having distinctive "starch-granule conglomerates".
AMARANTHACEAE Jussieu, nom. cons. - Back to Caryophyllales
Succulent, herbaceous or shrubby (lianes), often in saline conditions; anthraquinones, (isoquiniline alkaloids), 6-7-methylene-dioxyflavonols, isoflavonoids, betalain production +, soluble calcium oxalate accumulation; various-arranged medullary bundles innervating leaves, surrounded by ring of vascular bundles derived from continous procambium, successive cambia +, inc. roots; wood storied, rayless, esp. when young; vessels in multiples; pericyclic fibres few (0); cork cambium pericyclic [esp. chenopods; and elsewhere]; crystal sand + [less common in chenopods]; cortical and/or medullary vascular bundles + [less common in amaranths s. str.]; (stem with endodermis); sieve tube plastids lacking protein crystalloids, (starch grain +); nodes often swollen, (1:3, 1:5); petiole bundles ± annular; stomata also paracytic (dia- and anisocytic); hairs often uniseriate; leaves (opposite), lamina margins entire (toothed); A 5, opposite P; tapetal cells 2(-5)-nucleate, (amoeboid); pollen often starchy, foot layer well developed; G annular and open early in development, (median member abaxial); ovule also amphitropous, etc., parietal tissue 2-9 cells across, in radial rows or not, nucellar cap 2-4 cells across; embryo sac ± digesting chalazal region, (antipodal cells persist); endotegmen ± thickened and lignified, tanniniferous; endosperm +, at tip of radicle, (embryo sac haustorium during early development), embryo chlorophyllous or not; cotyledons longer than radicle [?level]; x = 9, nuclear genome [1 C] (0.124-)1.321(-14.052) pg.
180 [list]/2,050-2,500. ± World-wide. Map: from Hultén and Fries (1986), Jalas et al. (1999) and Culham (2007). [Photo - flowers, fruits, Collection.]
Age. Ages for the clade are 87-47 Ma (Kadereit et al. 2012: note topology) or ca 69.9 Ma (J. X. Huang et al. 2020).
Friis et al. (2011) thought that seeds possibly of Amaranthaceae from the late Cretaceous of southern Sweden do not in fact belong here.
1. Amaranthoideae Burnett
Cuticle waxes lacking platelets; hairs uniseriate; inflorescence branched or not, usu. spike-like or capitate; bracts disarticulating, bracteoles ± papyraceous/scarious; P scarious, variously pigmented; A 5, basally connate, [pseudo]staminodes +; pollen dodecahedral, exine metareticulate, tectum anulopunctate, pores (with hook-shaped stellate processes), borders distinct; ovules (circinotropous), integuments separated by basal air space [?level]; archesporium unicellular; seeds erect, radicle directed downwards [Ama. Bos. Char.]; embryogeny chenopodiad[!].
70/750. Tropical to subtropical, relatively few warm temperate or cooler.
Age. The age of this clade is estimated to be ca 59.5 Ma (J. X. Huang et al. 2020).
1A. Boseaeae Baillon - Bosea L.
Often much-branched shrubs with arching stems; pseudostaminodes short, fleshy; style ± 0, stigmas 2-3; fruit a berry; seeds black (spherical); n = 18.
1/3. Canary Islands, Cyprus, western Himalayas (Pakistan).
[Charpentiereae [Amarantheae [Aerveae [Achyrantheae [Iresineae + Gomphreneae]]]]]: ?
1B. Charpentiereae Z. H. Feng - Charpentiera Gaudichaud
Smallish trees; plant gynodioecious; flowers single; A basally connate, pseudostaminodes +; stye short-0, stigmas 2, capitate; fruit an utricle; seeds black; n = 26.
1/6. Hawai'i, Cook and Austral Islands.
[Amarantheae [Aerveae [Achyrantheae [Iresineae + Gomphreneae]]]]: ?
1C. Amarantheae Reichenbach —— Synonymy: Celosiaceae Martynov, Deeringiaceae J. Agardh
Annual or perennial herbs to small trees (climbers); leaves (opposite); plant monoecious/dioecious; inflorescence with flowers in groups of three (two sterile) / flowers single; bracts persistent, A (1-8), free (filaments ± connate); (pistillode +, staminodes 0); pseudostaminodes 0; (pollen pores with rounded protrusions); nectary + [?all]; G [2(-3-6)], style (long), stigma bilobed / trilobed / capitate / linear; ovules 1-many/ovary; fruit (achenial), (berry (splitting)), (dehiscence irregular), (circumscissile [= pyxidium]); seeds (arillate - Chamissoa); n = 9, 16-18; nuclear genome duplication [?here].
12/ :Amaranthus (74), Celosia (45). Tropical to warm temperate. 75 old Celosieae
The next two genera are of uncertain position
1/18. Old World, especially Africa.
Allmaniopsis fruticulosa Süssenguth
1/1. Kenya.
[Aerveae [Achyrantheae [Iresineae + Gomphreneae]]]: (pseudostaminodes 0); fruit thin-walled, (bursting).
1D. Aerveae Borsch, Kai M¨ller & Flores Olvera
(Annual) perennial herbs to shrublets; (successive cambial rings - A.); leaves (opposite); (plant dioecious); flowers single; P 5, quincuncial (4); A (1-)2(-)5, staminodes (1-4, (conspicuous)), appendages of the staminal cup 0-5 [?= pseudostaminodes]; style (long), (excentric - Ptilotus), stigma capitate, bilobed, or 2, filiform, shortly papillate; seeds pendulous, radicle directed upwards; n = 8, 9 (16, 17) 27 (29).
5-6/130: Ptilotus (120). Old World tropics, esp. Australia (all Ptilotus spp.).
Age. Crown-group Aerveae are some 27 Ma (Hammer et al. 2019).
[Achyrantheae [Iresineae + Gomphreneae]]: leaves opposite; genome duplication [allopolyploidy].
Age. This clade may be ca 28 Ma (Di Vincenzo et al. 2018).
1E. Achyrantheae Fenzl —— Synonymy: Achyranthaceae Rafinesque
Annual or perennial herbs to shrubs; leaves (spiral); inflorescence often spiciform/capitate, (flowers in threes, two lateral sterile), (2 extra bracteoles - Wadithamnus); P (hairs branched); A (4>/1 + 4 staminodes), (alternating with P - Wadithamnus), basally connate; pollen (pores with ± stellate-uncinate protrusions - subclade II), (metareticulate - Psilotrichum); style (excentric, curved), usu. long, stigma capitate (bilobed); (sterile flowers modified as hooks/spines/(hairs)); seeds pendulous, exotesta tanniniferous; radicle directed upwards; n = 12...21...26.
31/350: Cyathula (25). Largely African, but Cyathula pantropical, Achyranthes clade Old World, inc. Hawai'i.
Age. Crown-group Achyrantheae may be ca 22 Ma (Di Vincenzo et al. 2018).
y[Iresineae + Gomphreneae]: anthers bisporangiate, monothecal; style branches 2, filiform.
Iresineae Borsch & Flores Olvera - Iresine P. Browne
Herbs to trees; plant dioecious (gynodioecious); staminate flowers: (A 1 - Woehleria s. str.), pollen mesoporia with evenly spread microspines and punctae; pistillode +; carpelate flowers: P [also of perfect flowers] with long hairs; staminodes +; G unilocular.
1/ca 35. America: ± tropical, southern U.S.A. and the Caribbean southwards.
Age. An estimate for the age of crown-group Iresine is (36.4-)26.1(-16.0) Ma (Ortuño Limarino & Borsch 2020).
Gomphreneae Fenzl / "core Gomphrenoideae" —— Synonymy: Gomphrenaceae Rafinesque
Annual to perennial herbs, subshrubs (lianes); inflorescence (capitate), (pseudanthial); P free to connate, (two lateral wings developing - Froelichia); A 2-5, filaments ± connate, from ring meristem, appendages paired, lateral / single, from tube / 0; pollen with orbicules, pores (12-)20-150, deeply recessed, mesosporia narrow, higher than wide, punctae and microspines distal, reduction in the diameter of mesoporia [= metareticulate], (cuboid, pores with ± stellate-uncinate protrusions - Pseudoplantago [Pseu.]); G (stipitate), stigmas also broadly triangularc, bilobate; (fruit corky) (sterile flowers modified as hooks - Pseu.); n = 13, 22, nuclear genome duplicaton.
14/390: Gomphrena (139), Alternanthera (100), Pfaffia (33). Predominantly New World, tropics and subtropics, scattered elsewhere (Australia (especially), West Africa, Japan).
Age. An estimate for the age of this clade is ca 30.9 Ma (Ortuño Limarino & Borsch 2020).
Gomphrenoids: (flowers to >4.5 cm long - Gomphrena pulchella); pollen , tectum strongly reduced / covering the mesoporia and with only small perforations;. Ca 27.7 Ma.
Betoideae-Hablitzia + Polycnemoideae ca 57.1 Ma, Amaranthoideae-Aerva + Polycnemoideae ca 48.6 Ma (Masson & Kadereit 2013), Poly., Bet, [Chen. Corisp.]]] ca 65.3 Ma, this lot + Amaranthoideae ca 67.3 Ma (J. X. Huang et al. 2020).
2. Polycnemoideae Ulbrich —— Synonymy: Polycnemaceae Menge
Annual to perennial herbs or small shrubs; habitat often ± saline; leaves (opposite), needle-like/succulent; flowers axillary, bracts disarticulating, bracteoles large; P petaloid; A basally connate, (3, 2), anthers bisporangiate [?unithecous] (unisporangiate), (pseudostaminodes +); anther with outer parietal layer of wall producing endothecium and middle layer, the inner producing middle layer and tapetum [basic type]; epidermis with fibrous thickening on longitudinal anticlinal walls, endothecium with such thickening on transverse anticlinal walls; pollen smooth, (with microspines - Surreya), (polyhedral, metareticulate); archesporium unicellular; stigma ± capitate, papillate; G [2]; ovule circino-±campylotropous; integuments with space at base; outer periclinal wall of exotesta massive, with stalactites; n = 9.
4/13: Polycnemum (6). Widely scattered, ± temperate, but not East Asia, E. North America or southern Africa. Map: see Masson and Kadereit (2013: Fig. 1) and Australia's Virtual Herbarium (consulted vii.2013).
Age. Crown-group Polycnemoideae are dated to (54.0-)35.6(-19.5) Ma (Masson & Kadereit 2013).
[Betoideae [Salicornioideae + Chenopodioideae.]]: ?
Age. An estimate of the age of this clade is 65.0-56.5 Ma (G. Kadereit et al. 2005).
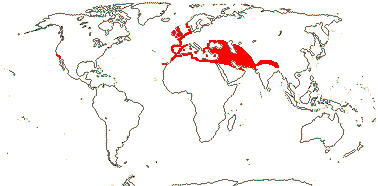
3. Betoideae Ulbrich —— Synonymy: Betaceae Burnett
Annual to perennial herbs (subshrub; vine); bracteoles +[?]; P (3) 5; A 5 (1); (G semi-inferior); (P persistent, accrescent - Beta); fruit circumscissile [= a pyxidium].
5/13: Beta (9). N. India to maritime Europe, California. Map: from Hohmann et al. (2006).
Age. The age of crown-group Betoideae is about 48.6-35.4 (including Acroglochin) or 38.4-27.5 (excluding it) Ma (Hohmann et al. 2006).
[Salicornioideae + Chenopodioideae] [if this clade exists]: isoflavonoids common; cuticle waxes as platelets; stomata oriented at right angles to the long axis of the leaf [?extent]; bracts persistent, bracteoles 0, bracts and P ± fleshy/herbaceous, red to ± green; P quincuncial, ± connate; carpelate flowers: P unequal, 3 smaller; disseminule an anthocarp, bract and perianth variously accrescent, (with spines, hooks, etc.); archesporium multicellular [?level]; (embryo chlorophyllous); x = 9; 300 bp deletion in chloroplast DNA inverted repeat.
104/1,400. Worldwide, especially deserts.
THIS CLADE IS BEING REWORKED.
Age. It has been suggested that polyporate pollen identified as Polyporina cribraria, of Maastrichtian age and from Canada, belongs to Chenopodiaceae (Srivastava 1969).
4. Salicornioideae Dumortier / chenopod II
(C4 photosynthesis +); plant glabrous.
Age. The age of this node is around 41.3-37.6 Ma (G. Kadereit et al. 2006); other estimates are 55.5-46.8 Ma (Kadereit & Freitag 2011) and ca 59.2 Ma (J. X. Huang et al. 2020).
[Suaedeae + Salicornieae]: plants often of saltmarshes; lamina not developed; P basally connate.
Age. This node has been dated to 38.2-28.7 Ma (Kadereit et al. 2006), ca 39 Ma (Piirainen et al. (2017) and ca 43.6 Ma (J. X. Huang et al. 2020).
4A. Suaedeae Moquin Tandon
Annual to perennial herbs to shrubs; lamina usually terete; inflorescence spicate, loose, leafy, bracteoles +; G [2-3], (inferior), (styles impressed), (connate and ± infundibuliform), (capitate), filiform, papillate all around; P ± enlarged/winged in fruit, diaspores commonly heteromorphic; perisperm 0, embryo spiral.
2/83: Suaeda (82). ± worldwide, especially Central to East Asia, not forests.
4B. Salicornieae Dumortier —— Synonymy: Salicorniaceae Martynov
Annual or perennial herbs to low shrubs; stem usu. articulated, sclereids +/(0 - Tec.); leaves usu. opposite, ± terete or scaly, (reduced to a rim, etc.), base (semi-)amplexicaul, (decurrent, connate); inflorescence dense, spike-like, leafless; P (2-)3-4(-5), [if 3, #3 abaxial], ± connate; A 1 [abaxial - Tec.], 2-3(-4); G [2-3]; perisperm + (0, endosperm copious), embryo spiral/curved or bent, radicle longer than cotyledons.
12/120: Tecticornia (68), Sarcocornia (30), Salicornia (>15; ca ?30). ± Worldwide, esp. temperate and tropics, not humid tropics, most in the Australian region.
Age. Crown-group Salicornieae are 35-25.3 Ma (G. Kadereit et al. 2006) or ca 29.1 Ma (Piirainen et al. 2017).
?
5. Salsoleae Dumortier s.l. —— Synonymy: Salsolaceae Menge
Leaves terete; bracteoles +; P separate, quincuncial; stigmas flattened, papillae adaxial; P developing abaxial wings, (capsule circumscissile); diaspores commonly heteromorphic; seed compressed, coat often thin; perisperm 0, embryo spiral, (cotyledons chlorophyllous).
33/300. Europe and the Mediterranean, esp. central to southwest Asia, northern and southern Africa, Australia.
Age. The age of this clade is some 43.6-36.1 Ma (G. Kadereit & Freitag 2011).

5. Camphorosmeae Moquin-Tandon
Often shrubby (herbaceous, annual); (C4 photosynthesis +); hairs with swollen bases, ("prickles" +); leaves terete; (flowers unisexual); pollen usu. with >70 pores; styles filiform, with papillae all around; fruiting perianth winged, fleshy, or spiny.
22/180: Sclerolaena (64), Maireana (57: paraphyletic). Northern Temperate to subtropical, to the Sahara, W. North America, southern Africa, esp. Australia. Map: from Kadereit and Freitag (2011).
Age. The age of crown-group Camphorosmeae has been estimated to be 27.0-17.3 Ma (G. Kadereit & Freitag 2011).
[Salsoleae + Caroxyleae]: P in fruit abaxially winged.
5. Salsoleae Dumortier / Salsoleae I —— Synonymy: Salsolaceae Menge
Subshrubs to shrubs (trees, annuals); C4 NAD malic subtype (C3/(C3-C4 intermediates); hairs adaxial to leaf base, multicellular, otherwise ± papillose; leaves (opposite), apex usu. mucronate to spinose; anther appendages 0 (small, non-vesiculose); cotyledons terete, filiform (flat).
18/ Anabasis (29), Salsola (25). Central and S.W. Asia, N. Africa.
5. Caroxylae Akhani & Roalson ("Caroxyloneae") / Salsoleae II
Annuals (subshrubs, hemicryptophytes); C4 NADP malic subtype ; hairs various, inc. long, multicellular, (mesifixed); anther appendages coloured, vesiculose (scabrous, smooth); (P in fruit not winged. scarious or cells inflated); cotyledons flat to linear.
Caroxylon (43), Climacoptera (42), Central and S.W. Asia, N. and S. Africa.
6. Chenopodioideae Burnett / chenopod I.
.
6A. Corispermeae Moquin Tandon —— Synonymy: Corispermaceae Link
Annual herbs; C4 photosynthesis 0; hairs stellate or branched; inflorescence spicate; (flowers unisexual), bracteoles 0; P 0-5, vascular bundles 0; fruits/seeds monomorphic; pericarp parenchymatous outside ["uppermost"], sclerenchymatous internally ["below"], no crystal layer; testa thin, tanniniferous, exotestal stalactite thickenings 0; perisperm 0, embryo horseshoe-shaped.
3/78: Corispermum (70). Eurasia and North America.
6B. Chenopodieae Burnett —— Synonymy: Atriplicaceae Jussieu, Blitaceae Kuntze, Chenopodiaceae Ventenat, nom. cons., Spinaciaceae Menge
Annual to perennial herbs (shrubs); (C4 photosynthesis - esp. Atriplex); root and stem cork cambium superficial (both: ?sampling); (cuticle waxes 0); flowers (unisexual), bracts disarticulating; staminate flowers: A basally connate; carpellode 0; carpelate flowers: P (0 - Atriplex); staminodes 0; fruit (circumscissile), (bracteoles enlarged); (diaspores heteromorphic); pericarp usu. thin, evascularized; exotesta much enlarged, with tanniniferous stalactites; n = (6, 8) 9 (10).
10/460: Atriplex (260), Chenopodium (150). More or less world-wide.
Age. The crown-group age of Atriplicieae is ca 28.9 Ma (Brignone et al. 2022).
6C. Axyrideae Burnett
.
3/10: Axyris (5). Europe to Korea, W. Nroth America.
6D. Dysphanieae Burnett —— Synonymy: Dysphaniaceae Pax, nom. cons.
Annual to short-lived perennial herbs; plant often aromatic; gland or bladder hairs, simple multicellular hairs +; (pericarp with long simple hairs); exotestal tanniniferous stalactites usu. 0; perisperm copious, farinose; embryo annular to ± straight; n = 8, 9.
4/43(-46): Dysphania (40). More or less world-wide, rather scattered, but not northern or Indian-East Asia-Malesia. Map: see Uotila et al. (2021: Fig. 1).
Evolution: Divergence & Distribution. For dates in Australian chenopods, see G. Kadereit (2005), Cabrera et al. (2012), etc., for those in Atripliceae, especially Atriplex, see Brignone et al. (2022), and for those in Gomphrena and relatives, see Ortuño Limarino and; Borsch (2020).
There may have been an increase in the diversifictaion rate in Amaranthaceae that is dated to (64.1-)51.0(-43.7) Ma (Magallón et al. 2018).
[Salicornioideae + Chenopodioideae], Chenopodioideae or just "chenopods" in the discussion below (Amaranthoideae are "amaranths"), probably originated in Eurasia, perhaps in environments close to the shore, with subsequent movement around the northern hemisphere and then into the southern hemisphere (e.g. Zhu 1996: maps; Hohmann et al. 2006; G. Kadereit et al. 2010, 2012). Within Betoideae, California-Mediterranean disjunctions have been dated to 15.4-8.1 Ma, the plants perhaps moving via Beringia (Hohmann et al. 2006). Chenopods are notably diverse in Australia, and there have been some nine invasions there, mostly since the late Miocene and thus within the last 10 Ma or so (G. Kadereit et al. 2005). Chenopods are very diverse in dry and/or saline conditions, especially in the centre of the country, and include 285 endemic species (as of 2004) (Kadereit et al. 2004). Sclerobliton may be the oldest, some 42-26 Ma, but with no subsequent diversification, while the Chenopodium section Orthospermum/Dysphania clade may be 16.1-9.9 Ma. Adaptations to salt tolerance like succulence are also adaptations to drought tolerance, and may have first appeared in coastal plants of Eurasia in the Eocene - thus Camphorosomeae have diversified in Australia, mainly in the central arid region, since around 8.3 Ma, but its relatives in Europe-Asia had also become adapted to drier/saline conditions (Hühn et al. 2024). Atriplex may have originated somewhere in the Middle East - Turkestan is likely - in the Oligocene-early Miocene, and species could probably deal with salinity, the presence of heavy metals, etc., and their diaspores were heteromorphic; long distance dispersal has probably been involved in achieving the broad range that they now have, and they radiated during the Pleistocene in places like Australia, the Aralo-Caspian and Pontic floristic provinces, more southern South America, North America including California and Baja California, etc., and the Mediterranean and adjacent regions (Zerdoner Calasan et al. 2022: e.g. Fig. 4E). Such adaptations may also have facilitated the subsequent adoption of C4 photosynthesis; C4 species developed in lineages that were already adapted to drought and could tolerate salt, etc., and they moved in to yet more arid environments (Kadereit et al. 2012).
Aridification in Australia had begun early in the Miocene ca 22 Ma, and pollen records show chenopods becoming more abundant from the late Miocene onwards (Kershaw et al. 1994). There are some 300 species of Australian chenopods, of which almost 150 species of shrubby drought- and salt-tolerant - and C3 - Camphorosmeae that split from their relatives around (16.0-)13.0(-10.0) Ma but began diversifying rather later, around (10.4-)8.3(-6.5) Ma (Hühn et al. 2024). These Australian Camphorosmeae have been placed in 12 genera, of which ten have 5 or fewer species; the genera are based on variation in the fruiting perianth (see below for generic limits). These Australian Camphorosmeae are sister to the Central Asian Grubovia, also C3 and including a mere three species; all told there are ca 30 spp. of Camphorosmeae found from the Canary Islands to Asia, also in South Africa (Shepherd et al. 2004; Kadereit et al. 2004; Kadereit & Freitag 2011; Cabrera et al. 2012; Freitag & Kadereit 2014; Mosyakin & Iamonico 2017). The diversity in Australian Salicornieae is also great (Wilson 1980; Piirainen et al. 2017). Cousins-Westerberg et al. (2023) looked at the whole tribe, noted for its general ability to withstand abiotic stresses, and found 2-4 origins of the evolution of cold tolerance in the Northern Hemisphere. Atriplex (Chenopodieae) has been quite peripatetic; now pretty much global, it probably originated in Continental Asia in the Early Miocene, and there seems to have been substantial subsequent long distance dispersal (Zerdoner Calasan et al. 2022). Thus A. chilensis, a C3 species fom South America, is embedded in a clade otherwise from Eurasia (Brignone et al. 2022). Atriplex has moved from Australia to South America, thence to North America, and then back to South America (Brignone et al. 2022); there may have been two invasions of Australia (Prideaux et al. 2009; Kadereit et al. 2010). C4 taxa in Atriplex have also diversified extensively in Australia, mostly after 6.3-4.8 Ma (Kadereit et al. 2010), and the genus is also diverse in the Americas.
Ortuño Limarino and Borsch (2020) discuss the evolution of Amaranthoideae-Gomphrena in some detail. The Australian C4 taxa form a clade, and Gomphrena s.l. immediately basal to this clade are plants of the sea shore, perhaps suggesting that this genus got to Australia via sea currents (see Ortuño Limarino & Borsch 2020: Fig. 8). For the evolution of some 24 characters, mostly floral, in the achyranthoid subclade II, see di Vincenzo et al. (2024: Figs 6-8).
Sheahan et al. (2020) suggested that betalain production might be an apomorphy for Amaranthaceae. Ortuño Limarino and Borsch (2020) discuss the evolution of a number of characters in Gomphrena are relatives, and these include root characters and various other vegetative and floral features. In Achyrantheae (amaranths) in particular there are sterile flowers associated with the fertile flower that have become modified as spines or hooks, presumably being involved in epizoochory, and it is estimated that these have evolved at least 14 times (Di Vincenzo et al. 2018). Although there is frequent mention of the two main pollen types in Amaranthoideae, theAmaranthus and Gomphrena types, it has proved remarkably difficult to find these types clearly distinguished/characterized in the literature. For the evolution of pollen morphology in Iresine and Gomphreneae, see Borsch et al. (2018).
Ecology & Physiology. Chenopods alone include some 558 species in about 40 genera that have the C4 photosynthetic syndrome (Freitag & Stichler 2000), fully one third of all BLA C4 species (Osmond et al. 1980; Sage et al. 2012; Sage 2016; Bena et al. 2017; Edwards 2019). There have been 9-13 or more independent acquistions of the C4 pathway, perhaps with reversals (e.g. Pyankov et al. 2001; G. Kadereit et al. 2012), including three acquisitions in Suaeda (Suaedeae) alone (Kapralov et al. 2006; Schütze et al. 2013). The first acquisition of C4 photosynthesis in Chenopodioideae (Salsoleae, Caroxyleae are a little younger) can be dated to the early Miocene ca 24 Ma, however, Kadereit et al. (2012) estimated that the first acquisition of C4 photosynthesis was a little earlier (47-22 Ma) at the Eocene/Oligocene boundary, perhaps in the [Salsoleae + Camphorosmeae] clade. There is a major C4 clade of some 180 species within Atriplex that may be 14.1-10.9 My (basal members C3, a single C4 origin somewhat under 9 Ma - Zerdoner Calasan et al. 2022); the C4 pathway in other groups may be a quarter of that age or less (Kadereit et al. 2003; Kadereit et al. 2010; Zacharias & Baldwin 2010; Kadereit & Freitag 2011; Brignone et al. 2022: Christin et al. 2011b for many dates). Suaedeae may be 25-20 Ma, or much younger, the C4 species being (16.9-)13.9(-11.2) Ma ().
Around 255 species of Amaranthoideae are C4 fixers, with some 5 origins that have been dated to (15.6-)8.8 Ma or less (Akhani et al. 1997; Pyankov et al. 2001; Kadereit et al. 2003, 2012; Sage et al. 2007, 2011; Kadereit & Freitag 2011; Sage 2016). In Amaranthoideae-Gomphreneae C4 photosynthesis has arisen three times or so, probably in species growing in warm and more or less humid environments, C4 species tending to occur in drier and winter-cool/cold habitats (Bena et al. 2017). The major clade of C4 plants here is in Gomphrena itself, all or nearly all the genus being involved (depending on the basal topology of the genus and its limits), some of these species growing at extremely high elevations (ca 4,700 m) in the Andes (Ortuño Limarino & Borsch 2020).
There are several types of C4 photosynthesis and ca 17 different kinds of C4 leaf anatomy in the family, particularly in the chenopods (e.g. Edwards & Voznesenskaya 2011; Freitag & Kadereit 2014; Ortuño Limarino & Borsch 2020); G. Kadereit et al. (2014) discussed parallel evolution of C4 leaf types in Camphorosmeae. For summary comparisons of the chloroplast types of C3 and C4 taxa, see Koteyeva et al. (2011b), and for comparisons of two C4 species of Suaeda, see Koteyeva et al. (2011c). Voznesenskaya et. al. (2013) discuss in detail transitions between photosynthetic types in the predominantly C4 Salsoleae. Rosnow et al. (2014b) noted that different amino acids were to be found in Suaedeae in a position thought to be critical in determining affinity for phospoenolpyruvate in the carboxylase enzyme. For the evolution of enzymes involved in C4 photosynthesis in Alternanthera (Gomphreneae), where there are also C2 intermediates, see Gowik et al. (2006); C4 photosynthesis seems to have originated once here (Sánchez-del Pino et al. 2012).
In at least four species of Suaedeae the various elements of C4 photosynthesis are all to be found within a single cell. Thus there is no conventional Kranz anatomy, but the chloroplasts involved in different parts of the carbon fixation process are distinct and spatially segregated; this condition has evolved independently at least twice (Voznesenskaya et al. 2002; Edwards et al. 2003;Kapralov et al. 2006: Bienertia, Suaeda). Partitioning of the plastids within the cell is maintained by the distinctive organization of the cytoskeleton (Chuong et al. 2006), although plasticity is induced by the light environment (Lara et al. 2008). The different plastids in Beinertia may be either proximal and distal (with respect to adjacent veins) in elongated cells, or peripheral and central, the latter domain including most chloroplasts and being where the C3 part of the pathway occurs (Offerman et al. 2011); Rosnow et al. (2014a) explore how the chloroplasts differentiate. Perhaps particularly interesting is Borszczowia (= Suaeda s.l.) in which there is just a single ring of palisade cells, but there is differentiation within these cells such that the outer part has relatively few chloroplasts and no starch is synthesized, but the inner part has numerous chloroplasts and starch is synthesized, etc., and so the latter part functions like the bundle sheath cells of other C4 plants (Freitag & Stichler 2000; Voznesenskaya et al. 2001, 2003). In this and some other anatomical variants that carry out C4 photosynthesis there is effectively a single ring of photosynthesising cells that surround a single ring of bundle sheath cells that surrounding a ring of vascular bundles (they vary in their orientation) - in some ways functionally equivalent to a single vascular bundle in a C4 grass.
Chenopods in general are most diverse in deserts from Sahara to Central Asia. Many succulent chenopod C4 halophytes grow in the Irano-Turanian region (Ogburn & Edwards 2010) and they make up a major element of the vegetation there. In the rather cold Gobi deserts 15-17% of the species are C4 plants (they are only 3.5% of the total Mongolian flora), and they contribute 30-90% of the biomass (Vostokova et al. 1995; Pyankov et al. 2000a). Over 50% of the total C4 flora in the Gobi Desert is made up of fast-growing C4 chenopods (there are also some Polygonaceae), some of which are arborescent. A similar combination of plants also dominates the halophytic vegetation of the Central Asian Turanian deserts (Winter 1981); these are somewhat warmer than the Gobi deserts. Some of these C4 plants get quite large, Haloxylon aphyllum (Salsoleae) reaching 10 m in height and with a trunk to 1 m across (Winter 1981). Succulent C3 chenopods are common in the Gobi in true desert conditions, and also in moist, saline soils (Pyankov et al. 2000a). As mentioned, chenopods are very diverse in Australia, chenopod shrublands dominating in the southern part of the continent where they grow in arid, saine conditions (Leigh 1994).
There are ontogenetic changes in photosynthetic mechanisms in some taxa like Atriplex, "Maireana" and Tecticornia (e.g. Pyankov et al. 2000b; Muhaidat et al. 2018 and references), and Akhani and Ghasemkhani (2007) looked at a number of chenopods from N.E. Iran and suggested that species with C3 cotyledons, the other leaves being C4, preferred the temperate or cold temperate conditions of the Irano-Turanian region, while those species all of the leaves of which were C4 preferred warmer areas or open, disturbed conditions. C4 + CAM has been reported in five succulent members of Amaranthaceae-Chenopodioideae- which, if confirmed, would be the first example of C4 photosynthesis evolving in a CAM lineage (Gilman et al. 2022).
The largest concentration of halophytes - plants that can tolerate conditions in which the electrical conductivity of the soil solution is equivalent to ca 80 mM NaCL or more (Bromham & Bennett 2014) - in flowering plants occurs in this clade, with ca 510 halophytic species, mostly chenopods (381 spp.) (see also Saslis-Lagoudakis et al. 2016: also [Cyperaceae + Juncaceae] - Poales). These halophytes show "extreme conservation of salt tolerance" (Bromham 2015: pp. 334-335), salt tolerance having arisen only once or twice and being a retained feature (G. Kadereit et al. 2012; Moray et al. 2015). This is unlike the "tippy" (highly polyphyletic) distribution in other families that have a substantial number of halophytes, as for instance in Poaceae, where there has also been frequent loss of this feature (Kadereit et al. 2012; Bennett et al. 2013; Bromham & Bennett 2014; Bromham 2015; Piirainen et al. 2017). A number of halophytes, perhaps ca 43%, are also C4 plants, and there is a connection between these two features (Sage & Monson 1999; Jacobs 2001; Sage 2002; Flowers & Colmer 2008; Kadereit et al. 2012; Bromham & Bennett 2014), and also with heavy metal tolerance (Lutts & Lefève 2015), as in other families like Poaceae (see Rajakaruna et al. 2016 for extreme physiologies in general). Interestingly, the bulk of the diversity in Camphorosmeae (14 genera and ca 147 species out of 22 genera, 180 species), is Australian, and are C3 plants that favour dry and often saline conditions (Freitag & Kadereit 2014; see also Leigh 1994: chenopod shrublands).
Most work on salt tolerance has been carried out on a few species of Atriplex (Chenopodieae: see Osmond et al. 1980), there are some studies on genera like Suaeda (Suaedeae) and Chenopodium, but knowledge of salt tolerance in other genera is sketchy. The salt glands are epidermal hairs consisting of a stalk one to a few cells long and a head. Chloroplasts are reported as occuring in the stalk cells, at least (Kelley et al. 1962), and the plant gets rid of the accumulated salt when the heads break off or abscise (Schirmer & Breckle 1982). The hairs are commonest on younger parts of the plant where the problem of salt accumulation is most severe, although a number of other functions have also been ascribed to these salt glands (Karimi & Ungar 1989). For additional information on salt tolerance in chenopods, see articles in Ann. Bot. 115(3). 2015.
Furthermore, in common with some other groups inhabiting dry and/or saline habitats (including Tamaricaceae and Cactaceae in Caryophyllales), a number of Amaranthaceae, especially chenopods, have very fast germination, the remarkable Saharan shrub Anabasis aretioides being perhaps the all-time fastest, the embryo breaking through the seed coat about ten minutes after imbibition begins. In such plants the seed coats are usually thin and embryos long - and quite often spirally twisted - and after imbibition starts, the seeds germinate within a single day (Zerdoner Calasan & Kadereit 2023 for references). This may begin when rain temporarily decreases salinity, temperatures are appropriate, etc. - quick establishment is of the essence in the conditions in which these plants live (Parsons 2012; Parsons et al. 2014; esp. G. Kadereit et al. 2017). However, Salicornieae and Salsoleae in particular can tolerate very high salt concentrations at germination, even saltier than seawater, and they may not germinate so quickly, while in taxa with heteromorphic diaspores (see also below), diaspores of one morph may show fast germination while those of the other morph enter the seed bank (Parsons 2012; Kadereit et al. 2017 for many details). C4 taxa may tolerate higher temperatures at germination, while in Australian Camphorosmeae in particular, many of which are C3 plants, the perianth part of the anthocarp is persistent and causes the seed to remain dormant (Kadereit et al. 2017). Fast germination is connected with the absence of perisperm, so increase in relative embryo size - importantly, no reserves have to be transported from the perisperm or endosperm to the embryo - in species with C4 photosynthesis and/or growing in saline habitats (Vandelook et al. 2021).
Fahn and Schori (1968) emphasized that there were anastomoses between xylem and phloem strands in different rings of secondary thickening in taxa from Atriplicoideae, Salsoloideae and Camphorosmoideae. This was perhaps important for facilitating the functioning of this tissue in desert plants; they also noted that the phloem, at least, remained functional for seven years or more.
Ptilotus (Aerveae) diversified in the central arid part of Australian (the Eremean) within the last ca 21.5 Ma (Hammer et al. 2020). Taxa of Ptilotus, with around 120 species in Australia, can accumulate phosphorus to levels at which other amaranths are harmed, and they can also grow in soils that are low in phosphorus (Suriyagoda et al. 2015).
Almost a hundred species of amaranths are to be found in the Brazilian Cerrado, and Campos et al. (2021) note that a variety of carbohydrates - sucrose and different kinds of fructans - were stored in the underground parts of different species.
Pollination Biology & Seed Dispersal. Ortuño Limarino and Borsch (2020) emphasize that the flowers of some Gomphrena are pseudanthial, being aggregated into heads and some having brightly-coloured inflorescence bracts. For the evolution of breeding systems in Atriplex, see Goldberg et al. (2017).
In chenopods in particular the perianth may become accrescent and envelop the fruit, being variously fleshy, winged or spiny and involved in dispersal, that is, the fruits are anthocarps (e.g. see illustrations in von Mueller 1889-1891; Fl. Austral. 4. 1984; Cabrera et al. 2009). In Achyrantheae (amaranths) in particular the two sterile flowers associated with the fertile flower are quite often modified as spines or hooks, sometimes hairs, in the former case presumably being involved in epizoochory (Di Vincenzo et al. 2018). There are a substantial number of taxa - again, mostly chenopods - with heteromorphic diaspores of one sort or another, and this heteromorphism may be at the level of the whole disseminule, fruit proper, or seed (e.g. size, testa colour and anatomy), and there is also variation in how far the disseminule is dispersed, the ability of the seed to enter dormancy, and in the conditions under which germination finally occurs, e.g. salt concentration, light requirements, etc. - indeed, substantial variation in requirements for the germination of the seed may be unaccompanied by any obvious variation in its morphology (Song & Wang 2015; see also Imbert 2002; L. Wang et al. 2010; Gul et al. 2013; Kadereit et al. 2017; esp. Zerdoner Calasan & Kadereit 2023, also above). Heteromorphy in disseminules may be evident at the level of the population, or between or within plants (in the latter, changing as the plant ages, or differing depending on the vigour of the plant), and currently it is difficult to see correlations in all the variation that is evident. Heteromorphy is relatively more common here than in any other group of comparable size/age, although it is also quite common in Asteraceae.
Plant/Animal Interactions. Cecidomyiid midges (Asphondylia) form galls on chenopods like Sarcocornia and Tecticornia in Australia; fungi also live in the galls, although the relationship between the fungi and the midge larvae — the former seem to be eaten by the latter — is unclear (Teresa Lebel, pers. comm.); this trophic relationship is also likely in the so-called ambrosia gallers (for fungi and cecidomyiids, see Dorchin et al. 2019 and references). Dorkin et al. (2019) noted that around 60% of the cecidomyiid Lasiopterini - there are perhaps some 5,000 species in the group, although most are undescribed - were to be found on chenopods.
Atriplex was probably a major item in the diet of the extinct giant (ca 230 kg) kangaroo Procoptodon goliah, and most of these species of Atriplex are in a clade that has diversified within the last 6.3-4.6 Ma (Prideaux et al. 2009; Kadereit et al. 2010). Indeed, this and other browsing sthenurine kangaroos represent a diversification that went on through the later Miocene into the Pliocene despite increasing aridity; chenopod biomass was, however, increasing (Couzens & Prideaux 2015).
Plant-Bacterial/Fungal Associations. Although the family is apparently largely without mycorrhizae (see Delaux et al. 2014 for Beta and Spinacea), vesicular-arbuscular mycorrhizae have been reported from chenopods in the Red Desert of Wyoming - but only on native taxa and under undisturbed conditions (Miller 1979); c.f. also Zygophyllaceae.
Vegetative Variation. Beta is perhaps the only plant in which the swollen stem develops from the hypocotyl alone (Tribble et al. 2021). The nodes in Amaranthaceae are nearly always unilacunar, but there is considerable variation in the number of traces that enter the leaf (e.g. Wilson 1924; Fahn & Brodo 1963: Salsola, Suaeda; Balfour & Philipson 1962). Some Salicornieae have 1:1 nodes in which two lateral branches diverge, descend, and enter the stem cortex (Wilson 1980), indeed, to say that there is basically 1:1 nodal anatomy here masks a considerable amount of variation (e.g. Bisalputra 1962: chenopods; Costea & de Mason 2001: Amaranthus). For a discussion about the cortical vascular system and leaves of Salicornia and relatives, see Fahn and Arzee (1959), James and Kyhos (1961) and Beck et al. (1982), etc. (more literature in Piirainen et al. 2017); Joshi (1931) and Costea and De Mason (2001) discuss medullary bundles. A question: Is the fleshy cortex of the stem of foliar or cauline origin? Fahn and Schori (1968) and others have looked at details of the architecture of secondary thickening here.
Variation in leaf anatomy in the chenopods is very considerable and is close to unmatched in other BLA families (Freitag & Stichler 2000). Tissue serving as water reserves may be central or peripheral; the position and development of palisade photosynthetic tissue varies greatly; vascular bundles may be in a plane or circle, complete or not, and peripheral vascular bundles may have xylem on the outside (with respect to the leaf centre) or the inside; a midrib vascular bundle may be evident or not, and so on (e.g. Freitag & Stichler 2000; Freitag & Kadereit 2014). Variants occur in taxa with C3 and C4 photosynthesis, thus in Camphorosmeae alone, of the 10 anatomical "types" there, four carry out C3 photosynthesis, five C4 photosynthesis, and one is intermediate, interestingly, the great majority of the group, all Australian, are C3 plants (Freitag & Kadereit 2014). All told, there are some 17 C4 anatomical variants (G. Kadereit et al. 2003; Schültze et al. 2003; for surveys, etc., see e.g. Carolin et al. 1975; Jacobs 2001); see also above. Majeed et al. (2022) looked at petiole anatomy in 14 species
Genes & Genomes. For genome size - small in the family as a whole - and evolution in Chenopodium s.l., see Kolano et al. (2015) and Mandák et al. (2016: ?sampling), and for that in Amaranthus see Stetter and Schmid (2017). Maiwald et al. (2020) discuss variation and distribution of Cassandra terminal repeats in the family. For a genome duplication at the Aerva/Alternanthera node (Aerveae-Gomphreneae), see Y. Yang et al. (2015, 2017; S. A. Smith et al. 2017); there is also a duplication pegged to Amaranthus (S. A. Smith et al. 2017; Yang et al. 2017: three duplications in Amaranthoideae).
Ptilotus and its sister taxon, Ouret, are polyploids on x = 27 (for some chromosome numbers, see Stewart & Barlow 1976), while there has been extensive hybridization in Chenopodium, C. quinoa itself being a tetraploid (Kolano et al. 2019).
Economic Importance. For the American grain amaranths, see Clouse et al. (2016) and Stetter and Schmid (2017) and references. There are also some nasty glyphosate-resistent weedy amaranths, the pigweeds, and Kreiner et al. (2022) discuss the very strong selection that has been going on in one of these, Amaranthus tuberculatus, in North America. Indeed, Amaranthaceae include a disproportionally large number of notably serious and widespread weeds (Daehler 1997) and are well represented among naturalized/invasive taxa (Pysek et al. 2017).
Chemistry, Morphology, etc.. Triterpenoid saponins are common enough in flowering plants, but 30-noroleanane triterpenoids are distinctly less common, but they have been found in two amaranth and four chenopod genera (Lyu et al. 2018); for saponins in general here, see Mroczek (2015).
Fron (1899) looked at the anatomy of stem and root in young chenopods, i.a. noting that the arrangement of the vascular tissue could be spiral, although there could be infrageneric variation in this. Polycnemum and Nitrophila have been reported to have ordinary secondary thickening, but c.f. Heklau et al. (2012) and Masson and Kadereit (2013); noye the rings in the swollen roots of beetroots and see Krumbiegel and Kástner (1993) for Chenopodium. The absence of rays in the wood is pervasive in the chenopods, with a few exceptions, and Amaranthoideae also lack them (Carlquist 2015b; see also Carlquist 2003c). Stem collenchyma is well developed; there are nucleated xylem fibres (Rajput 2002). Stem-borne roots of Polycnemum seem to have a superficial cork cambium (Heklau et al. 2012; see also Fron 1899).
Chenopod flowers show a considerable amount of variation, partly because of the involvement of the perianth in fruit dispersal, and partly because the flowers may be quite reduced; it can be difficult to interpret what one is looking at. In the reduced perianth of the Australian Tecticornia (Salicornieae) the odd member is abaxial (for floral development, see Shepherd et al. 2005b). The "bracteoles" enveloping the flower and fruit in some species of Atriplex (Chenopodieae) are modified perianth members (Flores-Olvera et al. 2011). Whether or not the flower parts of Chenopodium are spiral or whorled has occasioned much discussion (Sokoloff et al. 2018 and references).
Vrijdaghs et al. (2014), Borsch et al. (2018) and Sánchez-del Pino et al. (2020) suggest that the lateral appendages on either side of the filament in Gomphreneae, at least, cannot be called pseudostaminodes, and the latter suggest that the paired appendages on the filaments and the single structures coming from the filament tube between the filaments are not homologous (see also Ortuño Limarino & Borsch 2020). The arrangement of the androecium during development and in the mature flower of Pleuropetalum (Celosieae) is different (Ronse Decraene et al. 1999).
The ovule primordium, central on the receptacle, is initially surrounded by but detached from if later attached to the annular ovary wall, and so the gynoecium has been described as being "(ontogenetically) acarpellate" (Sánchez-del Pino et al. 2020: p. 315).
Pollen is mostly fairly homogeneous (Nowicke 1975; Skvarla & Nowicke 1976), having a similarly thickened tectum, apertures (pores) with reduced pointed flecks of exine underlain by lamellar plates, and a thickened endexine. K.-Q. Lu et al. (2018) divided the pollen of a number of eastern Asian chenopods into six types based on details of the variation in pore size and the ornamentation of the pore membranes; it will be interesting to see how these "types" hold up as sampling is improved. Pollen variation in part of Amaranthoideae is more extensive, the metareticulate pollen of Celosieae being particularly distinctive (e.g. Borsch 1998; Borsch & Barthlott 1998; Sánchez-del Pino et al. 2016). Livingstone et al. (1973), and especially K. Müller and Borsch (2006c) discuss the evolution of the distinctive stellate pore ornamentation of the pollen of Achyrantheae subclade II. The morphology of such pores is complex, but it can be decomposed into a number of variables which occur individually outside of this clade (Müller & Borsch 2006c); As Townsend (1993: p. 73) described such pores, they "rather resemble a hermit crab emerging from its shell" see also Borsch et al. (2018) for palynological variation within Iresine and Gomphreneae.
2-carpelate members of the family usually have collateral carpels, but they are sometimes superposed. The chalazal region of the ovule is more or less digested by the embryo sac in at least some Amaranthaceae - and this is also once recorded from Nyctaginaceae (Maheshwari 1950). For fruit wall and seed coat anatomy in chenopods, see Sukhorukov and Zhang (2013); heteromorphism of diaspore type may be accompanied by variation in whether the embryo is chlorophyllous or not (Imbert 2002). There are also complex variation patterns involving embryo shape, the relative size of the cotyledons and the nature of the seed reserve, if any (Vandelook et al. 2021); I certainly have not done them justice above.
Additional general information - "Am" = Amaranthoideae, "Chen" = Chenopodioideae + Salicornioideae, "Po" = Polycnemoideae - can be found in Eliasson (1988: Am), Robertson (1981: Am), Kühn (1993: Chen), Townsend (1993: Am), Judd and Ferguson (1999: Chen), Sukhorukov et al. (2014: Corispermeae) and Zhu and Sanderson (2017: Chen). See also Blunden et al. (1999: betaine distribution), Hegnauer (1964, 1989: chemistry), Shelke et al. (2019: secondary thickening, Suaeda), Hu and Yang (1994), Rajput (2002), and Grigore et al. (2014), various aspects of anatomy, Zumaya-Mendoza et al. (2019: Iresine stem anatomy), Bisalputra (1961: seedling, 1962: stem/node anatomy, both Chen), Carolin (1983: Am, Chen, indumentum), Acosta et al. (2009: Am) and Urmi-König (1981: Chen), both inflorescence morphology, Payer (1857), Sattler (1973), Choob and Yurtseva (2007) and Flores Olvera et al. (2008, 2011), all Chen floral morphology, Bakshi (1952: Am) and Hakki (1972, 1973: Chen), both floral morphology and embryology, Flores Olvera et al. (2006), Tsymbalyuk (2008) and Zhu and Sanderson (2017), all pollen, Ronse De Craene (2020: Am), gynoecium, Meunier (1890), Kajale (1940b: extensive, Am), Wilms (1980) and Naidu (1984), ovules and seeds, Veselova et al. (2016: Po, extensive discussion), and Shepherd et al. (2005b), Sukhorukov (2007, 2008) and Sukhorukov et al. (2015, 2018c), Chen fruits and seeds.
Phylogeny. Amaranthus was sister to Beta and other chenopods in an ORF 2280 phylogeny, and this group was in turn sister to a [Celosia (Amarantheae) + Froelichia (Gomphreneae)] clade (Downie et al. 1997). Cuénoud et al. (2002) found Amaranthaceae s. str. (= Amaranthoideae above) to be monophyletic, with very strong (97%) support, and the old Chenopodiaceae (evrything else) were perhaps monophyletic, but their subtending branch collapsed in a strict consensus tree; the sampling was moderately good, but only the matK gene was analysed. Such findings set the scene for subsequent results (Morales-Briones et al. 2019/2020: Fig. 1 is informative from this point of view). In an extensive rbcL analysis, much of the Chenopodiaceae were again monophyletic, but with little bootstrap support, ditto the Amaranthaceae (incl. Polycnemoideae), while Betoideae were paraphyletic (G. Kadereit et al. 2003). Other studies had suggested that Chenopodiaceae were paraphyletic and perhaps even that Amaranthaceae were polyphyletic (Pratt 2003; Pratt et al. 2001). In an analysis of matK/trnK sequences, K. Müller and Borsch (2005b, c) found that Polycnemum and Nitrophila (100% support) were sister to the rest. In G. Kadereit et al. (2005) relationships were [chenopods [Corispermum + the rest]]. Masson and Kadereit (2013: ?wrong reference) found a clade [other Amaranthaceae + Chenopodiaceae] had 70>% bootstrap support and still lower PP values, while Amaranthaceae s. str. had 100% support and Chenopodiaceae s. str. again 70>% bootstrap support yet 1.0 PP. Amaranthaceae s. str. and Chenopodiaceae s. str. had similar support values in Kadereit et al. (2017), Polycnemoideae being placed sister to Chenopodiaceae, but support for this position was not strong. See also Z.-D. Chen et al. (2016) for relationships between Chinese taxa, where these two main clades were not very well supported. J. F. Walker et al. (2018) also found that [Polycnemum + Nitrophila] might be sister to the chenopods, or might even form a clade with Beta, while [Polycnemum + Nitrophila] was sister to [chenopods + amaranths] in Di Vincenzo et al. (2018)... Yao et al. (2019) found that a well supported [Spinacia + Beta] clade was sister to their Amaranthaceeae s.str., although that position had little support and they noted that their sampling was exiguous.
In a complex series of analyses of nuclear (92 transcriptomes/reference genomes) and plastome data with pretty good overall sampling in the family, Morales-Briones et al. (2019/20) found gene-tree discordance, perhaps caused by hybridization between the main clades, incomplete lineage sorting, etc.; most genes were in fact phylogenetically uninformative. Basic relationships from nuclear data, well supported apart from the position of Polycnemoideae, were [amaranths [Polycnemoideae [Betoideae + chenopods]]], while chloroplast data suggested [chenopod II [[chenopod I + Betoideae] [amaranths + Polycnemoideae]]]. A number of relationships in the latter analysis were supported by a very low proportion of informative genes, with alternative positions about as likely (Morales-Briones et al. 2019/2020). J. X. Huang et al. (2020: 8 plastid markers, sampling quite good, but no Axyris, Psilotrichum or Allmaniopsis) found relationships almost a hybrid of those in Morales-Briones et al. (2019/20), with a clade [amaranths [Polycnemoideae [Betoideae + chenopod I]]] being sister to chenopod II.
In the rest of this section I shall use the names in the five-subfamily classification suggested by Morales-Briones et aL. (2019/2020) as far as possible:
Within Amaranthoideae Ogundipe and Chase (2009) found that Bosea and Charpentiera were successively sister to the rest (see also Borsch et al. 2026), but Amaranthoideae, Amarantheae and Amarathineae were paraphyletic. [[Amarantheae + Celosieae] [Psilotrichum ferrugineum [Aerveae [Gomphreneae + Achyrantheae]]]] were the core relationships recovered by Di Vincenzo et al. (2018). However, at the generic level there was extensive para/polyphyly, thus although Psilotrichum ferrugineum occupied an isolated position along the spine of the tree (which should be confirmed), other species of the genus were in Achyrantheae subclade II (Di Vincenzo et al. 2018). Relationships between Aerveae, Gomphreneae and Achyrantheae were unclear in Hammer et al. (2017). Di Vincenzo et al. (2024: 5 plastome genes plus nrITS) looked at relationships in this same subclade. Borsch et al. (2026: 3 plastid markers plus ITS, nearly all genera), which should be consulted, also looked at relationships throughout the subfamily.
Amarantheae: For relationships in Amaranthus itself see Stetter and Schmid (2017) and in particular Waselkov et al. (2018).
Gomphreneae. There is a [gomphrenoid (Gomphrena is polyphyletic) + alternantheroid (Alternanthera is monophyletic)] clade, = Gomphreneae (Sánchez-del Pino 2007; Sánchez-del Pino et al. 2009; see also Bena et al. 2017). The monophyly of Alternanthera has been confirmed (Sánchez-del Pino et al. 2012), while Ortuño Limarino and Borsch (2020) found genera like Philoxerus, Lithophila and Gossypianthus were embedded in Gomphrena (and some Gomphrena were close to genera like Pfaffia that were thought to be unrelated...). Depending on the analysis, a geographically heterogeneous [Guilleminea + Gomphrena prostrata] clade was sister to the rest of Gomphrena, or a small group of Gomphrena from Bahia, Brazil, occupied that position (Ortuño Limarino & Borsch 2020); Tidestromia lanuginosa was sister to the rest of the gomphrenoid group. Aerveae: Hammer et al. (2015, 2019: also generic limits, esp. 2021) discuss relationships in the Australian Ptilotus, where West Australian species are sister to the rest.
Iresineae. Iresine should be circumscribed broadly; see Borsch et al. (2018: 3 plastid markers, ca 23 spp.), for a phylogeny, and these authors recovered two main clades, well supported, and there was further structure within both those clades. Species limits needed work!J. F. Walker et al. (2018a) discussed the morphology and relationships of Polycnemoideae in some detail - morphology more = Amaranthaceae, habitat, = Chenopodiaceae. See also Masson and Kadereit (2013) for relationships within Polycnemoideae.
As to Betoideae, Hohmann et al. (2006) found that Acroglochin, with circumscissile capsules like other members of the subfamily, tended to wander around the tree; they did not place it.
Relationships within the old Chenopodiaceae are having to be reworked because the often highly reduced and modified flowers and fruits have been difficult to understand and interpret and previous taxon delimitations are unsatisfactory. Thus in Camphorosmeae and Salicornieae, for example, there is much variation in fruit and seed, the former in particular involving apparent adaptations for dispersal, and genera based on this variation are not holding up, even though some have only recently been described (Shepherd & Wilson 2007, c.f. Wilson 1980; G. Kadereit & Freitag 2011). Relationships between Dysphanieae (plant aromatic, with stalked or subsessile glands), Atripliceae (inc. Chenopodieae), Axyrideae and Anserineae (inc. Spinacieae) are unclear, although the groups seem to be monophyletic (Fuentes-Bazan et al. 2012b for a summary). G. Kadereit et al. (2005) discuss relationships in Australian chenopods - Australia is a centre of diversity for the group.
Chenopodioideae/Chenopod I. Chenopodieae: G. Kadereit et al. (2010) examined relationships in the old Atripliceae, and Chenopodium as included there turned out to be polyphyletic; Fuentes-Bazan et al. (2012a) found that Atriplex and other genera were nested within Chenopodium s.l. - in fact, members of four tribes of the old Chenopodioideae were intermingled (see also Kolano et al. 2015; Mandák et al. 2016 for Chenopodium s.l.). Sukhorkov et al. (2018) looked at relationships around Blitum. For Atriplex and relatives, see also Zacharias and Baldwin (2010: North American taxa) and especially Zerdoner Calasan et al. (2022: 208/260 spp., nuclear ITS and ETS markers).
For relationships in Dysphanieae, see Uotila et al. (2021), who found conflict between nuclear and chloroplast data in the basal relationships.
Salicornioideae/chenopod II. Camphorosmeae. Cabrera et al. (2009) looked at relationships in the Australian Camphorosmeae, a monophyletic group, and G. Kadereit and Freitag (2011) at those in Camphorosmeae as a whole. Like earlier authors, Hühn et al. (2024: customised RADseq, 103/147 spp examined) found that Maireana formed a grade but Sclerolaena. Branches in the basal grade were long, and Maireana and the small genera in the Camphorosmeae were mixed up while there had been extensive divergence within Sclerolaena; all told Hühn et al. (2024) found some 17 clades.
Salicornieae: see Kadereit et al. (2006). For relationships in the Australian Tecticornia and its relatives, see Shepherd et al. (2004, 2005a) and for the relationships between the paraphyletic Sarcocornia and Salicornia, see Steffen et al. (2015). Species limits in the ecologically important Salicornia are difficult, and there is hybridization (Chatrenoor & Akhani 2021). For a general phylogeny of the tribe, see Piirainen et al. (2017) and Cousins-Westerberg et al. (2023: nuclear ribosomal and plastid markers, sampling good).
Salsoleae: Wen et al. (2010) found that Salsoleae s.l. were monophyletic, Akhani et al. (2007) looked at relationships in Old World and other Salsoleae. Suaedeae: See Schütze et al. (2003) for relationships.
Classification. J. F. Walker et al. (2018a) inclined towards recognition of Chenopodiaceae and Amaranthaceae s. str., although Polycnemoideae were to a certain extent intermediate. Chenopodiaceae s. str. are recognised by Cronquist, and more recently by Hernández-Ledesma et al. (2015) and Zhu and Sanderson (2017). Indeed, in the past the two families have been recognised as being separate, if close, but given the pattern of relationships suggested e.g. by Morales-Briones et al. (2019/2020) a single family with five subfamilies does seem to be more sensible (see also Huang et al. 2020; Brignone et al. 2020, c.f. Brignone & Denham 2021).
The phylogeny of Amaranthoideae suggested by Borsch et al. (2026) is followed above; some genera are still unplaced. For a classification of the old Suaedoideae, see Schütze et al. (2003), of Salsoloideae, see Akhani et al. (2007), of Chenopodioideae, see Fuentes-Bazan et al. (2012b), of Camphorosmoideae, Kadereit and Freitag (2011), of Salicornioideae, see Piirainen et al. (2017 - a new genus still to be described), and of Betoideae, see Iamonico (2019). Apart from Betoideae, these classifications can largely be followed, but with subfamilies equivalent to tribes, etc..
Cabrera et al. (2009) found generic problems in the Australian Camphorosmeae, the more basal paraphyletic Maireana being in a particular mess (see also G. Kadereit & Freitag 2011; Hühn et al. 2024). A reclassification of this group is promised (as of 2024). Within Chenopodieae, Zacharias and Baldwin (2010) divided the C3 North American Atriplex and relatives, which are quite variable, into a number of genera, while Fuentes-Bazan et al. (2012b) made the needed nomenclatural changes for the dismemberment of Chenopodium s.l. into seven genera (see also Kadereit et al. 2016), however, the limits of Chenopodium in Australia have been expanded (Mosyakin & Iamonico 2017). Within Gomphreneae, Iresine should be circumscribed broadly and the limits of the polyphyletic Gomphrena (Sánchez-del Pino 2007; Sánchez-del Pino et al. 2009) are being adjusted (see Ortuño Limarino & Borsch 2020). Some of the extreme halophytic genera are morphologically much modified, and generic limits are difficult. For generic limits in Aerveae, see Hammer et al. (2019), while di Vincenzo et al. (2024) adjusted the limits of genera (two new) in the achyranthoid subclade II.
Given the obvious general similarities between the old Chenopodiaceae and Amaranthaceae and the complexity of the details of the relationships between them, a classification of Amaranthaceae s.l., albeit incomplete, is suggested above, however, what the future holds in terms of ideas of relationships will determine its fate...
Botanical Trivia. Amaranthus palmeri, a ruderal based in the south of the U.S., is noted for its high rate of photosynthesis (Sage 2016).
Age. The age of this node may be around 86.8 Ma (Magallón et al. 2015) or 60.2 Ma (Tank et al. 2015: Table S2).
STEGNOSPERMATACEAE Nakai - Stegnosperma Bentham - Back to Caryophyllales
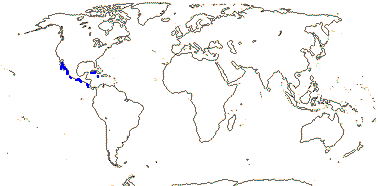
Woody, ± scandent or not; successive cambia +; true tracheids +; sieve element plastid with polygonal central crystalloid; plant glabrous; leaves fleshy; inflorescence racemose; C (2-)5; A (5) 8-10, connate basally; nectaries in depressions at base of G; G [2-5], alternate with P, placentation becoming free-central, apical septae + [= outgrowth between stylar lobes], ?gynoecial development, stigma/styles ± spreading; ovule 1/carpel, basal, epitropous, amphitropous, obturator +; fruit a capsule; seeds arillate; exotesta ± palisade, unlignified, endotegmen enlarged, persistent; n/x = ?
1 [list]/3. Central America, the Antilles. Map: from Bedell (1980). [Photo - Fruit]
Chemistry, Morphology, etc.. Like Caryophyllaceae, there are special cells in the wood that contain sphaerites; there is only diffuse axial xylem parenchyma. There is no nucellar cap. Are the seeds endospermic?
For more information, see Hofmann (1977), Bedell (1980) and Rohwer (1993a), all general, Horak (1981) and Carlquist (2012c), secondary thickening, Friedrich (1956: c.f. carpel position), Narayana and Narayana (1986: embryology) and Sukhorukov et al. (2015: fruit and seed, sometimes 2 seeds/loculus).
Previous Relationships. Stegnospermataceae have often been included in Phytolaccaceae. The two look rather similar, and have a somewhat similar gynoecium, but they are most obviously distinguishable by their flowers which have petals; Phytolaccaceae lack them. They also have pollen with a prominent foot layer and massive endexine - this is thin in Phytolaccaceae. The ovules are epitropous, while in pluricarpelate Phytolaccaceae they are apotropous (Rogers 1985).
[Limeaceae [[Lophiocarpaceae [Kewaceae [Barbeuiaceae + the Raphide clade]]]] [Molluginaceae [Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] + the ACPT clade]]]]]: ovules apotropous.
Age. This node is around 85 Ma (Magallón et al. 2015) or 58.3 Ma (Tank et al. 2015: Table S2).
LIMEACEAE Reveal - Limeum L. - Back to Caryophyllales
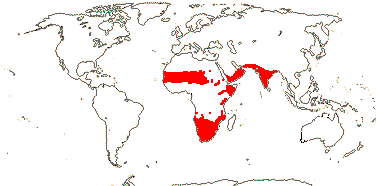
Herbs or subshrubs; anthocyanins +, betalains 0; cork?; (secondary thickening normal); sieve tube plastids with cubic central crystalloid; nodes?; leaves spiral; inflorescence leaf-opposed or not; K quincuncial, C +, small, clawed (0); A 5(-7), basally connate; G [2], abaxial member alone fertile, carpels initiate separately around flat ± persistent floral apex [= cup-shaped], apical septae + [= invagination of apex], styles 2; one G fertile, ovules 2, pendulous, obturator +; antipodal cells persist; fruit a schizocarp; seeds pale yellow/brown; exotesta with anticlinal walls strongly raised/laciniate [wax deposits]; n = 9, x = ?
1 [list]/21. Southern Africa (most species), to Ethiopia, S. Asia. Map: from Culham (2007) and esp. Trop. Afr. Fl. Pl. Ecol. Distr. 1 (2003), 6 (2011).
Chemistry, Morphology, etc.. The "petals"/petaloid staminodes are described as coming from the base of the outer stamens (Ronse de Craene 2013). The gynoecium is odd. There are certainly two stigmas and sometimes (at least) clearly two styles that are very close together at the base (e.g. Jeffrey 1961); Hoffmann (1973) interpreted the two ovules separated by a septum as coming from a single carpel, and this was confirmed by Ronse de Craene (2020).
For further information, see M. Endress and Bittrich (1993: general, as Molluginaceae), Behnke (1976) and Behnke et al. (1983a), both plastid morphology, Sharma (1963) and Ronse De Craene (2020: Figs 6, 8), both floral morphology, and Hassan et al. (2005a: seed).
Previous Relationships. Limeum is another member of the old Molluginaceae (M. Endress & Bittrich 1993).
[[Lophiocarpaceae [Kewaceae [Barbeuiaceae + the Raphide clade]]] [Molluginaceae [Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] + the ACPT clade]]]] / the globular inclusion clade: (wide-band tracheids +); sieve tube plastids with globular crystalloids; P ± uniseriate, not K + C.
Age. The age of this node is variously estimated at 40-30 Ma (Wikström et al. 2001), (74-)61, 58(-47) Ma (Bell et al. 2010: note position of Mollugo), 55-53 Ma (Arakaki et al. 2011), and 83 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. There may have been an increase in diversification at this node (S. A. Smith et al. 2017).
Physiology & Ecology. Wide-band tracheid pith cells are scattered in succulent members of this clade, e.g. Aizoaceae, Cactaceae, and Portulacaceae. They are also found in the leaf away from the midrib in Aizoaceae; bands are narrow but very tall (= "wide"), so the cell lumen is locally very narrow (Mauseth et al. 1995: similar in Hectorella [Montiaceae]; Carlquist 1998b). In a recent study of Ariocarpus fissuratus (Cactaceae), it was found that as the rays expanded these tracheids could contract, so allowing the whole root to contract, and the plant remained closer to the rocky ground where the temperatures were cooler (Garrett et al. 2010).
Chemistry, Morphology, etc.. Limeaceae, Cactaceae and "Portulacaceae" have cells in rows along the dorsal junction of the seed.
[Lophiocarpaceae [Kewaceae [Barbeuiaceae + the Raphide clade]]]: ?
Age. This node is about 79.6 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. Sheehan et al. (2019) suggest that betalain production is an apomorphy here.
Phylogeny. Corbichonia (Lophiocarpaceae) and most of Hypertelis (= Kewa; the type species of Hypertelis itself is in Molluginaceae) were well supported as successive sister clades at the base of this clade (Christin et al. 2011a). However, relationships along the backbone of the clade need confirmation.

LOPHIOCARPACEAE Doweld & Reveal Back to Caryophyllales
Herbs or succulent subshrubs; etalain production?; lamina not bifacial; K/P quincuncial; pollen trinucleate; placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker]; obturator of hairs; outer wall of exotesta with tanniniferous stalactites; x = 8.
2 [list]/6. Africa to western India. Map: approximate, from floras and florulas, Jeffrey (1961) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
Lamina ± terete; inflorescence indeterminate, spike-like, but with 3-flowered cymules; flowers sessile; C 0; A 4; tapetal cells 4-5-nucleate [2-nucleate by fusion]; G [2], oblique, 1-locular, stigmas very strongly bilobed; ovule single [from abaxial carpel], basal, obturator funicular; fruit achenial, surface verrucose to ribbed; exotestal cells much elongated radially; n = ?
1/4. Southern Africa.
2. Corbichonia Scopoli —— Synonymy: Corbichoniaceae Thulin
Inflorescence a leaf-opposed cyme [terminal inflorescence evicted]; "C" staminodial, many, ± connate; A many, centrifugal; tapetal cells 2-3-nucleate; G [5], opposite P, placentation axile; ovules many/carpel, obturator placental; fruit a loculicidal capsule, surface smooth; seeds arillate; exotestal cells papillate, pores on anticlinal walls; n = 9.
1/2. Africa to tropical Asia.
Chemistry, Morphology, etc.. The gynoecium of Corbichonia has 8 vascular bundles in its walls, with four that are diagonal to the carpels being notably larger than the others (Eckardt 1974). Its exotestal cells are described as being strongly elongated radially (Hakki 2013), which does not seem to be obviously consistent with the image in Sukhorukov et al. (2015: see fig. 8B), where they are described as being "alveolate", compared to "sinus-like, triangular in cross-sections" for Corbichonia, while other differences between the two such as the orientation of stalactites in the exotestal cells are mentioned.
For general information, see Adamson (1958), Hofmann (1973), Rohwer (1993a: Lophiocarpus) and M. Endress and Bittrich (1993: Corbichonia), for Corbichonia flowers, see also Ronse de Craene (2007, 2020: Figs 3, 8), for embryology, see Narayana (1962a) and Narayana and Lodha (1963: as Orygia, ovules shown as almost anatropous), and seeds, see Hassan et al. (2005a).
Classification. Corbichoniaceae are not recognized, pending development of a consensus; two families for two genera seems a bit much, despite their differences (c.f. Thulin et al. 2016).
Previous Relationships. The two genera were previously included in Phytolaccaceae (Lophiocarpus: Rohwer 1993a) and Molluginaceae (Corbichonia: M. Endress & Bittrich 1993).
[Kewaceae [Barbeuiaceae + the Raphide clade]]: ?
Age. The age of this node is around (57-)45(-37.5) Ma (Thulin et al. 2018: note topology).
Phylogeny. Yao et al. (2019) have the relationships [Barbeuiaceae [Kewaceae ...]] here, but this topology has very little support.
KEWACEAE Christenhusz - Kewa Christenhusz - Back to Caryophyllales
(Annual) herbs or subshrubs; anthocyanins +, betalains 0, oxalic acid +; cork?; secondary thickening?; ?sieve tube plastids; stout glandular hairs on conical epidermal protrusions, (0); leaves ± fasciculate, linear, ± terete, stipules adnate to base, scarious, ± sheathing; inflorescence pseudo-umbellate, pedunculate (peduncle 0); P 5, quincuncial, outer 2 ± calycine, inner 3 ± petal-like; A (3-)5-15, developmental centrifugal, shortly connate basally; nectary 0; G [(3-)5], opposite K, placentation axile, septae at same level as floral apex and carpel lobes [= saltshaker], style 0, stigmas quite short, spreading/as crests on G; ovules many/carpel; fruit a membranous loculicidal capsule; seeds brown or black, operculate, strophiole +, minute; testa with tanniniferous stalactites; embryo straight; n = 8, x = ?
1 [list]/6. South Africa (most), to Ethiopia, Madagascar, and St Helena. Map: from Adamson (1958a; Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and 6 (2011); esp. Thulin et al. (2018).
Age. Crown-group Kewaceae are (7.4-)3.9(-3.0) Ma (Thulin et al. 2018).
Evolution: Divergence & Distribution. Sheehan et al. (2019) agree that anthocyanin production is an apomorphy here.
Chemistry, Morphology, etc.. The inflorescence is interpreted as being terminal and cymose (Hofmann 1973). In bud, the perianth members enclose the rest of the flower and are sepal-like; in the open flower three or four expand and are petal-like, and the anthers and stigmas are also brightly colored.
For further information, see M. Endress and Bittrich (1993: as Molluginaceae), Adamson (1958a: South African species) and especially Thulin et al. (2018), all general, Behnke et al. (1983a: sieve tube plastids), and Ronse de Craene (2013, 2018: floral morphology). In older literature Kewa is very largely equivalent to Hypertelis, see Christenhusz et al. (2014).
Phylogeny. Relaationships within the genus have little support, but Kewa acida, from Saint Helena, may be sister to the rest of the genus (Thulin et al. 2018).
Classification. For the classification of this little family, see Christenhusz et al. (2014).
Previous Relationships. Another member of the old Molluginaceae (M. Endress & Bittrich 1993). The exclusion of Hypertelis spergulacea, the type of Hypertelis, the genus in which species of Kewa were formerly included (see Molluginaceae), makes morphological sense.
[Barbeuiaceae + the Raphide clade]: successive cambia +.
Age. This node is around 77.8 Ma (Magallón et al. 2015) or ca 52/48.1 Ma (Tank et al. 2015: Table S1, S2).
BARBEUIACEAE Nakai - Barbeuia madagascariensis Steudel - Back to Caryophyllales
Lianes; betalain production?; libriform fibers, diffuse axial parenchyma, true tracheids +; sieve tube plastids with polygonal crystalloids; cortical fibres +; druses +; leaves spiral; infloresecence axillary, fasciculate; A many; pollen tricolporoidate; G [2], septate, ?development; ovule 1/carpel; fruit a loculicidal capsule; seeds 1 or 2, arillate; testa cells elongated, with sinuous anticlinal walls, ?tegmen with bar thickenings; n/x = ?
1/1: [list]. E. Madagascar. Map: from Culham (2007).
Chemistry, Morphology, etc.. The plant dries black.
See Hofmann (1977: general), Rohwer (1993a: general, under Phytolaccaceae) and Carlquist and Schneider (2000: anatomy).
Previous Relationships. This is a refugee from the old Phytolaccaceae (Cronquist 1981).
[Aizoaceae [Gisekiaceae [[Sarcobataceae + Phytolaccaceae] [Petiveriaceae + Nyctaginaceae]]]] / the Raphide Clade: soluble oxalate accumulation; raphides +; A = P, A alternating with P, or A many, primordia alternating with P; anther wall from both secondary parietal layers; carpels initiate separately around flat ± persistent floral apex [= cup-shaped], gynoecium with apical septae + [= invagination of G apices].
Age. The crown age of this clade is estimated at (47-)38, 36(-27) Ma (Bell et al. 2010) or about twice that, ca 74.9 Ma (Magallón et al. 2015); ca 45.1 Ma is the figure in Tank et al. (2015: Table S2) while ca 13.57 Ma would seem to be the estimate in Valente et al. (2014: c.f. rooting in Fig 2).
Evolution: Divergence & Distribution. It may be that stamens alternating with the perianth, or in a polystaminate androecium, fascicle bundles alternating with the perianth, is a feature best placed at this node, although much remains to be found out about androecial development.
Genes & Genomes. Christin et al. (2014b) noted that ppc-1E1 genes in some Aizoaceae and Nyctaginaceae C4 plants did not have a Ser780, as is usual in such cases.
Chemistry & Morphology. For soluble oxalate accumulation, see Zindler-Frank (1976); Hartmann (2017) suggested that Aizoaceae-Sesuvioideae and -Aizooideae both had druses, which if true, might confuse the optimization of this feature.
AIZOACEAE Martynov, nom. cons. - Back to Caryophyllales
Leaf succulents; growth sympodial; CAM photosynthesis common; C-glycosylflavonoids -; cork from inner cortex or endodermis; wood storied, rayless; fibres ± in bands; cuticular waxes as ribbons or rodlets; stomata also para- and anisocytic; leaf trace bundles forming reticulum in cortex; leaves opposite, lamina with epidermal bladder-like cells ± developed [also elsewhere on plant], leaf base broad, margins membranous; inflorescence with well-developed bracts/bracteoles; hypanthium +; P coloured adaxially, with subapical abaxial appendage [= "horn"]; nectary annular, on hypanthium; A many, centrifugal, primordia 5, wall 5 cells across; tapetal cells 2-nucleate; pollen tricolp(oroid)ate; G septate; ovules apical to median, parietal tissue 1-3 cells across, epidermal cells radially elongated; exotesta tanniniferous, ± palisade, or tangentially elongated, inner periclinal walls not/less thickened; suspensor often massive, bi or multiseriate; x = 9, nuclear genome [1 C] (0.054-)1.175(-25.56) pg.
Ca 124 [list: subfamilies and some tribes assigned]/1,722 - five subfamilies, some tribes below. Esp. southern Africa, also Australia, etc., tropical and subtropical, arid. [Photos - Collection.]
Age. Aizoaceae are (56.4-)41.5(-38.7) Ma (Klak et al. 2016).
+1. Sesuvioideae Lindley —— Synonymy: Sesuviaceae Horaninow
(C4 photosynthesis +); (nodes 3:3); (lamina with peripheral vascular bundles, xylem external [exoscopic]); prophylls often prominent; (leaves spiral - Tribulocarpus - T.); petiolar "stipules" +; flowers with two small pairs of bracts; A 1-5, alternating with P/-many, primordia opposite P/development rather chaotic; anther with outer parietal layer of wall producing endothecium and middle layer, the inner producing middle layer and tapetum [basic type, ?level]; nectary plane; G (1-)[2(-5]); 2-many ovules/carpel, (nucellar cap +); capsule circumscissile [= pyxidium], (indehiscent, winged/compound, fused with spiny bracts - T.); seeds arillate (not); (n = 7).
4/65: Trianthema (32), Sesuvium (14). Tropics and Subtropics; Sesuvium portulacastrum is pantropical on beaches. Map: see Fl. Austral. vol. 4 (1984), Hartmann (2001a, b), Hartmann et al. (2011), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003; Fl. N. Am. vol. 4 (2003). [Photos - Habit, Flower.]
Age. Crown-group Sesuvioideae are around (40.7-)29.6(-17.9) Ma (Klak et al. 2016).
[Aizooideae [Acrosanthoideae [Mesembryanthemoideae + Ruschioideae]]]: (leaves spiral); inflorescence often not distinct from vegetative plant, bracteoles foliaceous; A fascicle bundles alternating with P; G opposite P; fruit loculicidal, a hygrochastic capsule, with expanding keel; seeds brown; genome size (1C) ca 1.53 pg [1 species].
Age. This clade is (14.93-)7.88(-3.01) m.y. (Valente et al. 2014) or (43.6-)35(-26.1) Ma (Klak et al. 2016).
2. Aizooideae Arnott —— Synonymy: Galeniaceae Rafinesque, Tetragoniaceae Lindley
Annual to perennial herbs or shrubs; leaves with mesomorphic epidermis [outer periclinal walls little thickened, cuticle not thickened, stomata not sunk]; accessory lateral branches + [?]; lamina (terete); P (basally connate); A (5, 10); nectary plane; G [2-10], to inferior; ovule 1/carpel, apical, apotropous, or many, (nucellar cap +), funicular obturator +, of glandular hairs; fruit (septicidal - Gunniopsis), (expanding keel 0), (nut-like - Tetragonia/drupe/winged); (cell walls of seed coat little thickened), (endotegmen palisade - Tetragonia).
5/116: Tetragonia (51), Aizoon (45). Drier parts of S. Africa, also Australia (Gunniopsis), few N. Africa and Asia Minor, N. America, etc. (Aizoon). Map: see Frankenberg and Klaus (1980), Fl. Austral. vol. 4 (1984), Hartmann (2001a, b); also Klak et al. (2015), but c.f. Klak et al. (2017) - to the Caspian Sea, not in China. [Photo - Flower.]
Age. This node is estimated to be (15.8-)9.5, 7.9(-3.0) Ma (Valente et al. 2014) or (43.6-)35(-26.1) Ma (Klak et al. 2016).
[Acrosanthoideae [Mesembryanthemoideae + Ruschioideae]]: lamina trigonous or terete, base expanded, connate, forming a sheath.
Age. This node is (45.4-)36.4(-23.8) Ma (Klak et al. 2016).
3. Acrosanthoideae Klak - Acrosanthes Ecklon & Zeyher
Shrublets; CAM photosynthesis 0; ?anatomy; leaves linear to spathulate; flowers single, terminal (pseudoaxillary); A 8-many, in groups/pairs or not; nectary ?0; G [2], ovary incompletely septate; ovules 1(-2)/carpel, basal, funicle short; capsule with parchment-like walls, expanding keel 0, ?loculicidally dehiscent; seeds black to dark brown, ± rugulose.
1/7. Fynbos, Western Cape, South Africa. Map: see Klak et al. (2017, 2019).
Age. Crown-group Acrosanthes is (10.7-)5.3(-1.9) Ma (Klak et al. 2016).
[Mesembryanthemoideae + Ruschioideae]: leaves very succulent, with peripheral vascular bundles, xylem internal [= endoscopic]; hypanthium 0; P green, sepal-like, horn 0; "C" = staminodia, many, linear; G more or less inferior, nectary interrupted [= meronectary]; x = 9; (plastid transmission biparental).
Age. This node has been dated to (21.6-)17.1(-12.6) Ma (Arakaki et al. 2011: check), (11.82-)6.02(-2.18) Ma (Valente et al. 2014) or (37.2-)29(-19.4) Ma (Klak et al. 2016).
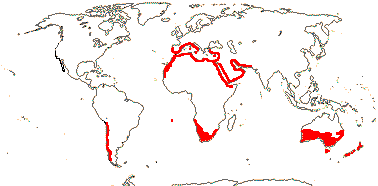
4. Mesembryanthemoideae Ihlenfeldt, Schwantes & Straka —— Synonymy: Mesembryaceae Dumortier, Mesembryanthemaceae Philibert, nom. cons.
(Annuals); distinctive alkaloids +; cortical bundles +; (stem succulents; succulent persistent green cortex in stem); leaves with mesomorphic epidermis, stomata on both stem and leaf transversely (vertically) oriented, spidermis; leaves flattened to cylindric; flowers 4-5-merous; staminodes ± petal-like (connate), (also filamentous); nectary hollow and ± shell-shaped [= koilomorphic], interrupted or not, (tubular/flat); G [(3-)4-5(-6)], placentation axile, styluli +, stigma wet [Aptenia s. str.]; parietal tissue 7-9 cells across, in radial rows or not; expanding keels of fruit purely septal; n = (18, 27); genome size (1 C) 0.47-3.83 pg.
1/105: Mesembryanthemum, or -320 spp., Phyllobolus s. str. (150), Psilocaulon s. str. (65), etc.. S. Africa, esp. succulent Karoo, a few species also W. South America, Australia, N. Africa, the Mediterranean and the Near East. Map: see Fl. Austral. vol. 4 (1984) and Pascale Chesselet (pers. comm. 2004), also Klak et al. (2015).
5. Ruschioideae Schwantes
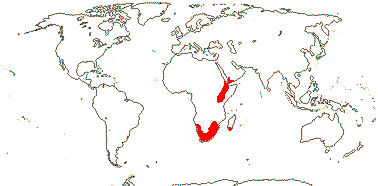
Lamina vernation flat to curved; (inflorescence distinct); "C" free; filaments papillate basally; G [(3-)5-15(-25)], placentation basal or parietal; expanding keels of fruit largely valvar, not reaching centre of fruit, with covering membranes [= inner part of fruit wall]; genome size (1C) 0.27-317 pg/0.26-3.1/Gbp.
111 or 146/1,595 - four groups below. Southern and eastern Africa, esp. Karoo, south Madagascar and southwest Arabia. Map: Pascale Chesselet (pers. comm. 2004).
Age. Crown-group Ruschioideae are about 4.49 Ma (Valente et al. 2014), perhaps a little more, 8.7-3.8 Ma (Klak et al. 2004) - or (37.5-)33.0(-28.5) Ma (Arakaki et al. 2011).
5A. Apatesieae Schwantes
Annuals to perennials; leaves flat, with mesomorphic epidermis, bladder cells on leaf margins/0, (central chlorophyll-free water-storing tissue); A primordia 5, development centrifugal, nectary annular, flat, broad, continuous; G [8-22], style-stigma funnel-shaped to pointed; fruit with expanding tissue slight or 0, septae longitudinally split, seeds in seed pockets formed by placental false septae [= paraspermy]/schizocarpic.
7/11[!]: Apatesia (3). South Africa, mostly southwest (see Klak et al. 2015).
[Dorotheantheae [Delospermeae + Ruschieae]]: epidermal bladder cells 0 [?position]; A ?from ring primordium; fruit with covering membranes.
Age. This node is estimated to be (6.49-)3.31(-1.13) My (Valente et al. 2014) or (12.6-)7(-3.4) Ma (Klak et al. 2016).
5B. Dorotheantheae Chesselet, G. F. Smith & A. E. van Wyk - Cleretrum N. E. Brown (inc. Dorotheanthus)
Annuals; vascular anatomy anomalous; leaves (spiral - extent?), flat, with papillate mesomorphic epidermis, peripheral vascular bundles 0; nectary plane, segmented or not.
1/13. Southwest South Africa.
Age. Crown-group Dorotheantheae can be dated to (6.6-)3.9, 3.3(-1.1) Ma (Valente et al. 2014).
[Delospermeae + Ruschieae] / Core Ruschioideae: perennials; wide-band tracheids + (0); vernation curved to flat, (apex/margin with teeth), base expanded, ± connate, central chlorophyll-free water-storing tissue +; (pollen tri(syn)colpate); nectaries annular, raised, ± crest-like [= lophomorphic, bulging], interrupted or not; embryo sacs often other than monosporic, 8-nucleate; (ordinary capsule = no closing bodies or covering membranes); (chloroplast PEP subunit β' rpoC1 intron lost), ARP gene duplicated.
Ca 96/ca 1,565. Africa, mostly the S.W., Madagascar and the Arabian Peninsula. Photos: Flower; Flower.
Age. Core Ruschioideae are about 17 Ma (Arakaki et al. 2011) about (3.14-)1.5(-0.35) Ma or (3.8-)2.0, 1.5(-0.35) Ma (Valente et al. 2014: check) or ca 8 Ma (Klak et al. 2016).
5C. Delospermeae Chesselet, G. F. Smith & A. E. van Wyk
(inner staminodes filamentous, ± erect); fruit (schizocarpic, expanding keels reduced).
27/524: Delosperma (140-165), Drosanthemum (100-110). Southern Africa, especially the summer rainfall area, also to Saudi Arabia, Yemen, Madagascar, Reunion (Delosperma: map in Hartmann 2008).
5D. Ruschieae Schwantes
(Lamina ± hemispherical) (connate) [plant stone-like]; calcium oxalate crystals in epidermis, hypodermal tanniniferous cells +; stomata in depressions (hidden by parastomatal cells); (thorns + [= dichasial inflorescence]); flowers (4-8-merous); filaments (basally connate - Conophytum); nectary continuous; G (to 12-locular); (funicular hairs +); fruit (persistent), often a multilocular capsule, (berry).
71/1,013 [numbers notional]: Ruschia (290-350), Conophytum (110), Lampranthus (85), Antimima (6-100), Lithops (38-50+), Cheiridopsis (38). Southern Africa, esp. the western coastal Succulent Karoo.
Evolution: Divergence & Distribution. There are more ages in Klak et al. (2016), where the focus is on Aizooideae.
The "meganiche" dominated by Aizoaceae in south western Africa — rather arid winter-rainfall areas with moderate temperatures — may be only some 5 Ma (Ihlenfeldt 1994a). Interestingly, neither Apatesieae and Dorotheantheae, mostly annuals and successively sister to the remainder of Ruschioideae, which are very largely perennials, are very speciose (c.f. a similar pattern in Crassula), while Delospermeae, alone in Ruschioideae, are quite widespread in the African-Arabian region. Klak et al. (2004) suggested that the radiation in Ruschieae in S.W. Africa, at least, was both recent (3.8-8.7 Ma) and very fast, splash dispersal of the seeds leading to relatively limited dispersal and so facilitating geographical isolation and subsequent speciation (see also Ihlenfeldt 1994a and Powell et al. 2022; Knope et al. 2012 for other rapid radiations); it was about this time (8 Ma) that changes in the ocean currents off Southwest Africa led to climatic changes inland, and pollen of Aizoaceae appeared in ocean sediments then (Dupont et al. 2011). Note that age estimates in Arakaki et al. (2011) are about twice as old, while ages in Valente et al. (2014) are somewhat younger. Valente et al. (2014) suggest that the diversification of core Ruschioideae in particular was after the origin of the Greater Cape Floristic Region in which they are now so common; Lampranthus is the only clade of any size with a substantial number of endemics in the Cape Floristic Region itself (Linder 2003). Long distance dispersal seems to have been quite common in the family (Holtum 2023)
Aizoaceae are the largest family of leaf succulents (Klak et al. 2003), furthermore, some 98% of the family, i.e. excluding Sesuvioideae alone, have hydrochastic capsules (Parolin 2006). Klak et al. (2004; see also above, Parolin 2006) suggested that the radiation in Ruschieae in S.W. Africa was very fast, splash dispersal of the seeds leading to relatively limited dispersal and so facilitating geographical isolation and subsequent speciation. These distinctive hygrochastic, and sometimes long-persistent (and few-seeded!), capsules have a complex anatomy, and in many of them seeds are ejected in jets of water, although there are other mechanisms involved, too (see e.g. Hartmann 1991; Parolin 2001, 2006; Kurzweil 2006), and opening and closing of the capsule of Delosperma nakurense (Ruschieae), powered by the expansion and contraction of cells that have layers of wettable cellulose, has been compared with the folding and unfolding of origami (Harrington et al. 2011). Core Ruschioideae, which include over 1,500 species, often have crest-like (lophomorphic) nectaries; other features are mentioned above. Klak et al. (2013, 2015) discussed the phylogeny of Ruschieae in particular, and of the Aizoaceae as a whole, in the context of adaptations to different rainfall regimes - there is much diversity in winter-rainfall areas - and geography.
Arakaki et al. (2011) suggested that succulents in general radiated/diversified in the mid to late Miocene to Pliocene, the climate drying and cooling, even if the clades involved originated substantially earlier, and they also mentioned radiations in succulent Euphorbia, also Cactaceae-Opuntioideae and Cactoideae, and Agavoideae in this context (in all, see Ecology & Physiology). Indeed, radiations in succulent groups in general seem to have occurred quite recently, around 10 Ma or so - and add Aloe, Crassula, etc. (M. Lu et al. 2021).
Kellner et al. (2011) looked at genetic differentiation in Lithops in the context of morphology and geography. Jainta and Vandenschrick (2024) discuss the biogeography of the genus, noting its preference for mineral soils derived from old, hard rocks rather than clayey soils derived from younger, softer rocks.
Powell et al. (2022) examined diversification in Conophytum (Ruschieae), a genus of 100≤ species from the Succulent Karoo (all told there may be 650 species of Aizoaceae from 80 genera in that area). They noted the distinctive features of a tubular flower (the staminodes are connate), the autumn/winter flowering in this clade (see also Liede & Hammer 1990), and there is also a fair amount of variation in flower colour and anther position; some species flower in the evening or night (unusual in the family - these species have distinctive pollen), etc. (see also Young 2023). Powell et al. (2022) suggested that diversification here, as in some Iridaceae from the same general area, might be driven by pollinators (there is also substantial vegetative variation). Species in genera like Crepsia and Lampranthus, also Ruschieae, tend to appear in abundance after fires, then become progressively less common and disappear - but reappear after the next fire, having persisted as seeds in the soil bank (Klak et al. 2024).
Bittrich and Hartmann (1988) started the process of refining the delimitation of the family and assigning apomorphies to the main groups. See also Chesselet et al. (2002) and Klak et al. (2015, 2016) for some apomorphies.
Ecology & Physiology. Aizoaceae, in particular Mesembryanthemoideae and Ruschioideae-Ruschieae, dominate much of the Succulent Karoo of southwestern Africa, making up to an astounding 90% of the biomass and up to more than 50% of the species, a number of which are narrow endemics (Crassula and some other Crassulaceae are also important). Members of these groups may be either salt-tolerant (see articles in Ann. Bot. 115(3). 2015) or drought-avoiders, and such variation in quite closely related species is unusual (Ogburn & Edwards 2010). Edaphic specialization - soils can vary considerably locally - also seems to be involved in the diversification of the family (Ellis & Weis 2006), core Ruschioideae in particular flourishing under such conditions (Valente et al. 2014). Although some work has been carried out on details of anatomy and cell micromorphology and possible links to ecophysiology (see Vegetative Variation below; Melo-de-Pinna et al. 2014), much more needs to be done. Large amounts of Na+ and Cl- are accumulated in the large epidermal bladder cells of Mesembryanthemum crystallinum that may have a volume of up to 5μl, bladder cells in total making up around 25% of the volume of the aerial part of the plant; although they are epidermal in origin, they have functional chloroplasts (Oh et al. 2015; Barkla et al. 2016; Adams et al. 1998: detailed account of the species; White et al. 2016: Na accumulation even in non-saline conditions). Bladder cells may also be involved in protection against UV and overheating (e.g. Oh et al. 2015). Hartmann (1993) suggested that such hairs were involved in water storage, and/or they may be involved with water uptake from dew or mist (Ihlenfeldt & Hartmann 1982). There are no bladder cells in the great majority of the succulent Ruschioideae (see Hartmann 1993 for possible evolutionary sequences).
C4 photosynthesis occurs in around 30 species in Sesuvioideae (Sage et al. 1999; Sage 2016); some origins there (perhaps up to six) may have occurred as much as (27-)22.1(-17.2) Ma, others are much younger (Christin et al. 2011b), and there also appear to have been reversals from C4 to C3 photosynthesis (Bohley et al. 2015, 2017 - Sesuvium). There are also C3/CAM intermediates, as in Mesembryanthemum crystallinum, while in Trianthema portulacastrum both C4 and low-level CAM photosynthesis occur in the same plant, the latter especially in the stems (see also Sesuvium-Portulacaceae: Winter & Holtum 2014; Winter et al. 2021; see also Mioto et al. 2014). CAM is common in the other subfamilies (?Acrosanthoideae: Bohley et al. 2015), and annual species of Mesembryanthemum switch from C3 to CAM photosynthesis under conditions of water stress (G. E. Edwards 1996; Keeley & Rundel 2003). For more on the evolution of CAM photosynthesis here, see Holtum (2023) and Gilman et al. (2023)
A duplication of the ARP genes (a group of genes i.a. perhaps involved in leaf determinancy - e.g. Tomescu 2008), involved elsewhere in leaf development, is correlated with the diversification of core Ruschioideae, and it may be involved in the evolution of the diverse leaf morphologies of this group (see below), although there is currently no more than a simple correlation on which to go (Illing et al. 2011, c.f. the phylogenetic interpretation there).
Germination in areas with reliable winter rainfall in the Cape region seems to be rapid, occurring within 2-3 days, elsewhere it is slower; if germination does not occur, the seeds may remain viable for decades (Hartmann 1991). Along these lines, Klak et al. (2024) noted that genera like Lampranthus germinated after fires, the plants then being very abundant, but they became less common, however, the numerous seeds in the soil would germinate after the next fire. For possible connections between smoke(karrikinin)-induced germination and the evolution of Ruschioideae, see Lamont et al. (2018b).
Pollination Biology & Seed Dispersal. Aizoaceae in the drier areas of southwestern Africa, including Namibia, are much visited by non-Apis bees, which also visit Asteraceae there (Kuhlmann & Eardley 2011) - the two do have grossly similar flowers; the bees are also important visitors on Zygophyllaceae and Fabaceae. Conophytum, also from this area, is much visited by the pollen-collecting wasp Quartinia (and the night-flowering species by moths - see Jürgens), although details of pollination here are largely unknown (Powell et al. 2022). Genera like Carpobrotus from the winter rainfall area of the Cape may be pollinated by monkey scarabs, Hopliini (Goldblatt & Manning 2011b).
Straka (1955), Ihlenfeldt (1983) and Hartmann (1988) among others have described the intricate morphology of the capsules of the [Aizooideae [Mesembryanthemoideae + Ruschioideae]] clade, which are often hydrochastic, i.e. they open when wetted. There are septal keels that reach from the central axis to the valve tips that expand and recurve when they absorb water. Seed dispersal is by "jet action" using the kinetic energy of falling raindrops (= ombro[hydro]chory: Parolin 2006; see also Kurzweil 2006). How far the seeds are dispersed depends on the details of the capsule morphology, the ease of ejection of the seeds being inversely correlated with the distance the seed travels - if easily ejected, the seeds are not propelled far, and in general dispersal distances are low; in Ruschioideae only a few seeds at a time leave the capsule. In a few taxa mericarps are the units of dispersal. There is also considerable variation in the establishment "strategies" of the seeds. Many Sesuvioideae, with more conventional fruits, have arillate seeds and are myrmecochorous (Lengyel et al. 2009).
Vegetative Variation. Variation in features such as leaf size and shape and internode elongation is considerable (Ihlenfeldt 1994a). Although species with foliaceous bracts/bracteoles in which the inflorescence is not distinct from the rest of the plant are sometimes distinguished from those with smaller bracts and distinct inflorescences (e.g. Hartmann 1993), it is unclear to me what the real growth characters are and where they go on the tree; in many Ruschioideae in particular the whole plant can be thought of as a long-lived inflorescence. The leaves of many core Ruschioideae, i.e., not including Drosanthemeae and Ruschieae, are more or less flush with the surface of the ground; they can be almost invisible in the stony habitats in which they grow, being greyish or brownish and looking like pebbles except when they flower - hence "flowering stones". These leaves are prophylls or bracteoles, the flower is terminal, and renewal shoots, i. e. the next flowering units, develop in the axils of the prophylls (Hartmann 2004, 2006 for a summary). In some species of Conophytum the leaves are almost completely connate except for a slit across the top out of which the flower and next pair(s) of leaves appear.
Details of the initial growth and subsequent inflorescence development along with the switch from C3 to CAM photosynthesis are fascinating (for Mesembryanthemum crystallinum, see Adams et al. 1998) and would repay synthesis (for switches to/from CAM photosynthesis, see Bräutigam et al. 2017, also above).
Aside from the taxa with bladder-like cells on the leaf surface (see above, sometimes called idioblasts), other taxa have an epidermis with massively-thickened outer cell walls that contain layers of calcium oxalate crystals (e.g. Ihlenfeldt & Hartmann 1982; see also Hartmann 1993). In addition, the epidermal surface may be sculpted and/or with epicuticular waxes, the stomatal openings may be deeply sunken, etc. (e.g. Ihlenfeldt & Hartmann 1982; Hartmann 2002; Opel 2005a). This syndrome of characters may be compared with the mesomorphic epidermis found in some taxa, where the outer epidermis walls and cuticle are not thickened and the stomata are not sunken (e.g. Jürgens et al. 1986; Klak et al. 2015). In core Ruschioideae the leaves lack the bladder-like epidermal cells of the rest of the family, although the epidermal cells may be papillate or with hairs (Powell et al. 2017: the two intergrade). The exposed surfaces of the leaves sometimes have distinctive "windows", and in Lithops this window patterning may reflect venation reticulation or the position of huge, tannin-containing, subepidermal cells (Korn 2011). Ihlenfeldt and Hartmann (1983) discuss the leaf surface here in some detail.
The leaves, or more specifically the lamina, of Mesembryathemoideae and core Ruschioideae are generally cylindrical or trigonous, not more or less flattened (Klak et al. 2004; Chesselet et al. 2004; Melo-de-Pinna et al. 2014; Ogura et al. 2018), and there is a system of peripheral vascular bundles surrounding the more or less arcuate midrib (3D vascular tissue - see Ogburn & Edwards 2013). Melo-de-Pinna et al. (2014) note a possible correlation between expanded, more or less connate leaf bases and a system of peripheral vascular bundles in the blade which have internal xylem, i.e. they are endoscopic - it is as if the lamina had been abaxialized (see also Ogura et al. 2018). Bohley et al. (2017) drew attention to the thin, sometimes laciunate margins to the leaf bases in Sesuvium - pseudostipules.
Genes & Genomes. There is a genome duplication event (DLECα) including Aizooideae and Ruschioideae that is dated to ca 42.8 Ma (Landis et al. 2018). For genome sizes in the family, see Powell et al. (2020).
Genome size in Mesembryanthemoideae and particularly Ruschioideae (Conophytum examined in some detail) are quite low, perhaps reflecting the fact that diversification is recent here and that the plants prefer arid regions - genome sizes in plants from such regions tend to be low (Powell et al. 2020).
Chemistry, Morphology, etc.. Studies of the wood anatomy of Aizooideae and Sesuvioideae are needed to clarify wood evolution there, and to confirm the extent of rayless wood in the family (Carlquist 2007a); see Rajput and Patil (2008) for a study of vascular development in Sesuvium portulacastrum.
The petal-like basal part of the perianth (= sepals) in Sesuvioideae is equivalent to the sheathing vegetative leaf base while the apical "horn" represents the rest of the leaf, rather as in monocot leaf development (c.f. Vorlaüferspitze). B-class floral genes were expressed neither in the petaloid basal part nor in the petal-like staminodes of Aizooideae and Ruschioideae (Brockington et al. 2012; for the latter c.f. in part Frohlich et al. 2007). The androecium may arise as a ring meristem or as five separate primordia. Veselova et al. (2016) note that
Smets (1986) recorded the presence of a receptacular nectary disc; much work has been carried out on nectaries here, e.g. Ihlenfeldt (1960), Zandonella (1972, 1977), Chesselet et al. (2000), Hartmann and Niesler (2009), etc.. Niesler and Hartmann (2007) suggested that the correlation of nectary morphology with major clades was not that strong, noting that the nectaries in Glottiphyllum (Ruschioideae) were more or less plane. Such nectaries also occur in the two basal clades in the Aizoaceae; crest-like nectaries occur in Mesembryanthemoideae. The terms used get complicated, and authors may not agree on how to describe the nectaries of the one species: "plain" nectaries in Niesler and Hartmann (2007) are "plane" above, not being at all raised, the flat nectary described there (ibid. Fig. 3) can also be called raised-annular, "flat" referring to the fact that the margin is not incised in any way. Ihlenfeldt and Gerbaulet (1990: p.) noted that the nectaries in the Apatesieae they examined were "weakly lophomorphic" and "almost flat", which suggests there may be a continuum of variation. Nectaries may be interrupted ("meronecataries") or continuous ("holonectaries"), and so on.
The nature of the inferior ovary may repay investigation. In Tetragonia, at least, flowers may develop in the axils of bracts on the outside of the ovary (Prakash 1967) - i.e. the ovary is enveloped by axial tissue, rather like the situation in some Cactaceae. Hartmann (1993) recorded a nucellar cap in Aizoaceae, but by this she meant the radially elongated cells of the nucellar epidermis; Cocucci (1961) noted that these cells divide anticlinally, heightening their palisade appearance. Prakash (1967) perhaps implies there is a nucellar cap in Tetragonia. Fruit morphology and anatomy in Mesembryanthemoideae and Ruschioideae in particular is very complex (e.g. Ihlenfeldt & Gerbaulet 1990). Sesuvioideae often have arillate seeds, although Tribulocarpus has an indehiscent fruit and so hardly surprisingly it lacks such seeds. In Delosperma heidihartmanniae (Ruschioideae-Delospermeae), at least, the seeds are exposed apparently embedded in reddish material (Liede-Schumann & Newton 2019: Fig. 10, seeds "light brown"), while Shamrov and Anisimova (2023) describe the complex pseudo-10-locular gynoecium of D. tradescantioides.
For general information, see Ihlenfeldt (1960), Bittrich (1986: esp. Mesembryanthemoideae), Hartmann 1991; Chesselet et al. (1995, 2002) and Frandsen (2017: photographs), esp. Mesembryanthemoideae and Ruschioideae), Hartmann (1993, 2001a, b, 2017: thousands of photographs, enumeration of taxa), Klak (2010, 2019) and Interactive Mesembs, also Adamson (1959: Acrosanthes, also other revisions, Cole and Cole (2005) and Piccione (anatomy, morphology), both Lithops, Hammer (2002, 2013: Conophytum) ; see also Hegnauer (1964, 1989: chemistry), Klak and Linder (1998) and Klak et al. (2006: esp. stomata), Jürgens (1986) and Ihlenfeldt and Gerbaulet (1990), all general anatomy, Landrum (2001: wide-band tracheids), Bhambie et al. (1977: nodal anatomy in Sesuvioideae - confirm), Opel (2005a: leaf anatomy of Conophytum), Niesler and Hartmann (2004: some leaf morphology), Hernandes-Lopes et al. (2015) and Melo-de-Pinna (2016), some leaf development, Hofmann (1973: morphology), Haas (1976: esp. flower and fruit), Sharma (1962b), Leins and Erbar (1993) and Ronse De Craene (2020: Fig. 2), floral development, Dupont (1977: family) and Young (2023: Conophytum, 6 pollen types), both pollen, Meunier (1890), Schmidt (1925), Raghavan and Srinivasan (1940b) and Kajale (1940c), all ovules and seeds, Schwantes (1957: esp. fruit dehiscence), Hassan et al. (2005a) and Earlé and Young (2020), both seed morphology, and Dupont (1968: seedlings, also stomata).
Acrosanthes is especially poorly known.
Phylogeny. I follow Klak et al. (2003) for basic groupings in the family; Aizoaceae s. str. (e.g. Chesselet et al. 1995) would seem to be paraphyletic. Acrosanthes has been included in Aizooideae, however, Klak et al. (2016, 2017) found that it was sister to [Mesembryanthemoideae + Ruschioideae], and with quite good support.
Sesuvioideae. For a phylogeny of Sesuvioideae, see Hassan et al. (2005b). Tribulocarpus, which used to be in Tetragonioideae (for which, see Aizooideae), is sister to other Sesuvioideae (Klak et al. 2003; Thulin et al. 2012a). Relationships are [Tribulocarpus [Trianthema + The Rest]] (Bohley et al. 2015), and Sesuvium is paraphyletic. Sesuvium has separate African and American clades (Bohley et al. 2017; Sukhorukov et al. 2018a). Tetragonia is embedded in Aizooideae (Klak et al. 2003), however, it has wood rays, it lacks the bands of xylem fibres of other Aizoaceae, and there is vasicentric parenchyma adjacent to these fibres (Carlquist 2007).
Ruschioideae. Within Ruschioideae, Apatesieae and Dorotheantheae are successively sister to the remainder (see above for morphology, etc.; Klak & Bruyns 2012 for a phylogeny of Dorotheantheae). The remainder, core Ruschioideae, have also lost the chloroplast PEP subunit β' rpoC1 intron (Thiede et al. 2007) - c.f. Cactoideae. There is a detailed phylogeny of Ruschieae in Klak et al. (2013), while Powell et al. (2016) also look at relationships in the clade. Opel (2005b) provided a morphological phylogenetic analysis of Conophytum, while Powell et al. (2017) looked at relationships around there and particularly at the circumscription of Cheiridopsis. In a more extensive analysis of Conophytum using six plastid markers, Powell et al. (2022) found overall rather little variation and poor resolution, although it was clear that earlier infrageneric classifications were not holding up well. For relationships in Delosperma, particularly among species outside of South Africa, see Liede-Schumann and Newton (2018) - here Trichodiadema is to be found in five clades within and immediately outside Delosperma. Relationships in Lampranthus were examined by Klak et al. (2024: 61 accessions, 9 plastome markers, quite extensive sampling in the tribe); the genus was largely monophyletic, although a few species were in two small clades unresolved elsewhere in Ruschieae - but note that support along the spine of the phylogeny outside Lampranthus is largely non-existent.
Classification. Nectary morphology has been much used in classification at various levels in Aizoaceae (e.g. Chesselet et al. 2000; Niesler & Hartmann 2007 and references).
Generic boundaries are somewhat in flux. For example, in the early twentieth century Mesembryanthemum included the whole of the Ruschioideae and Mesembryanthemoideae, and until recently Mesembryanthemoideae s. str., much the smaller of these two subfamilies, was divided into several genera. However, Klak et al. (2007) in a comprehensive study of the subfamily, obtained quite detailed phylogenetic resolution within it. Mesembryanthemum s. str., although quite a small genus, was polyphyletic, and any attempt to maintain current genera would, Klak et al. thought, have entailed the recognition of numerous and often poorly characterised taxa. As a result, Klak et al. (2007) recognized only the one genus, as here (see Klak & Bruyns 2013: infrageneric classification, 5 subgenera and 13 sections; Hernández-Ledesma et al. 2015), although others think that clades there can be characterised (V. Bittrich, pers. comm.; Liede-Schumann & Hartmann 2009). Similarly, Klak et al. (2013, see also 2024) note that there are problems with generic limits in Ruschieae, while Hernández-Ledesma et al. (2015) discussed the extent of generic problems in Ruschioideae as a whole. There are also problems with generic limits in Aizooideae (Klak et al. 2016). For an infrageneric classification of Drosanthemum (Ruschioideae-Delospermeae), see Hartmann (2007) and of Lampranthus, see Klak et al. (2024).
There are also major problems with species limits here. Hammer in 1993 observed that there were then about 1,800 known populations of Conophytum (Ruschioideae) and for which there were 450 names; current estimates of species numbers for this genus range from 87 to 290 (around 108 is the estimate in Powell et al. 2022), and indeed, morphological variation here is quite extensive.
[Gisekiaceae [[Sarcobataceae + Phytolaccaceae] [Petiveriaceae + Nyctaginaceae]]]: ovule 1/carpel, basal, funicle short; K persistent to ± accrescent in fruit, filaments also persistent; seeds not the dispersal units; ORF 2280 sequence similarity, 210 bp deletion in plastome.
Age. This node is estimated to be ca 73.7 Ma (Magallón et al. 2015) or about 43.2 My(Tank et al. 2015: Table S2).
Phylogeny. Relationships in this area are settling down. Brockington et al. (2009) found Gisekia to be strongly supported as sister to the whole clade (see also Bissinger et al. 2014; J. F. Walker 2018a; Yao et al. 2019), although the genus has been thought to be "discordant" wherever it is put, and in some phylogenies it has come out in or near Phytolaccaceae-Rivinioideae (see Cuénoud et al. 2002; Christin et al. 2011b) - a somewhat unlikely position.
Douglas and Manos (2007) found only moderate support for the monophyly of Nyctaginaceae and vanishing little support for that of Phytolaccaceae (including Sarcobataceae); relationships were [Nyctaginaceae [Sarcobataceae [Phytolaccaceae + Petiveriaceae]]. Relationships [Phytolaccaceae [Nyctaginaceae [Sarcobataceae + Petiveriaceae]]] were recovered by Brockington et al. (2009: support generally weak; Crawley & Hilu 2013). Ronse de Craene (2013) suggested that relationships in this area were [[Petiveriaceae + Nyctaginaceae] [Phytolaccaceae s. str. + Sarcobataceae]], and these relationships were also found by Y. Yang et al. (2015), although the position of Sarcobatus, which had a very long branch, was unclear. Some relationships in Sukhorukov et al. (2015) were poorly supported, but overall the topology was [Phytolaccaceae [Agdestidaceae + Sarcobataceae] [Nyctaginaceae [Seguieraceae, Galesia [Petiveriaceae + Rivinaceae]]]]. Y. Yang et al. (2017: Gisekiaceae not sampled) found the relationships [Sarcobataceae [Phytolaccaceae [Petiveriaceae + Nyctaginaceae]]].Indeed, the clade [Petiveriaceae + Nyctaginaceae] is commonly recovered (see also J. F. Walker et al. 2018a), and both have gynoecia with just a single carpel (Cuénoud et al. 2002); the carpels of Mirabilis and Rivinia do look remarkably similar to each other (Leins & Erbar 1994). If Sarcobataceae are placed somewhere around here their carpel number is a reversal. Indeed, Brockington et al. (2011) found some support for an [Agdestis + Sarcobatus] clade, in turn sister to Phytolaccaceae s. str., but with little support, relationships also found by Walker et al. (2018a) and Yao et al. (2019). In the latter, support for the [Agdestis + Sarcobatus] clade was strong, but that for its sister position to Phytolaccaceae less so.
GISEKIACEAE Nakai - Gisekia L. - Back to Caryophyllales
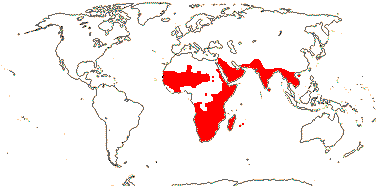
Prostrate annual (short-lived perennial) herbs; successive cambia 0; C4 photosynthesis +; leaves opposite; inflorescence dichasial to subumbellate; P quincuncial; A 5 or 10-15, alternating with P; G (3-)5(-15), pseudapocarpous, opposite P, ?development, styluli +; ovule with parietal tissue 2-3 cells across, nucellar cap 2-3 cells across, cells of nucellus expanded radially, funicle short; antipodal cells ± persistent; plant heterocarpic [?always], mericarps smooth to ± muricate or winged, K ± accrescent; exotestal cells tangentially elongated, exotegmic cells also thickened; n = 9, x = ?
1 [list]/1-7. Africa, southern Asia. Map: from Frankenberg and Klaus (1980), Flora Ethiopia Eritrea vol. 2(1) (2000), Trop. Afr. Fl. Pl. Ecol. Distr. vols 1 (2003), 6 (2011), Flora of China vol. 5 and Bissinger et al. (2014).
Age. Crown Gisekiaceae are (8.4-)4.8(-1.2) Ma (Christin et al. 2011b: c.f. outgroups) or (25.7-)14.9(-6.20 Ma (Bissinger et al. 2014).
Evolution: Divergence & Distribution. The origin of Gisekia may be in southern Africa (Bissinger et al. 2014).
Ecology & Physiology. Bissinger et al. (2014) provide details of C4 photosynthesis in the family; the pathway may not be fully optimized.
Chemistry, Morphology, etc.. See Gilbert (1993) for a review, Narayana and Narayana (1988) for a little chemistry, Behnke (1976) and Behnke et al. (1983a) for sieve tube plastids,, Hofmann (1973) for floral morphology, growth, Joshi and Rao (1936) for embryology, the suspensor apically curved and beak-like, and Narayana (1962a) and Hassan et al. (2005a) for seed morphology.
Phylogeny. For relationships in this clade as shown by a well sampled study with quite a lot of resolution, see Bissinger et al. (2014); species as currently recognised are not monophyletic.
[[Sarcobataceae + Phytolaccaceae] [Petiveriaceae + Nyctaginaceae]]: medullary bundles +, variously-arranged, successive cambia +; cork subepidermal; protein bodies in nuclei; fruit indehiscent; nuclear genome duplication.
Age. The age of this clade is estimated to be 28-21 Ma (Wikström et al. 2001: Delosperma included), ca 37.1 Ma (Tank et al. 2015: Table S2) or about 71.4 Ma (Magallón et al. 2015).
Phylogeny. See above.
Genes & Genomes. A gene duplication may have occurred at this node (Y. Yang et al. 2015, 2017: PHYT2; S. A. Smith et al. 2017; Landis et al. 2018: the ALINβ event), although what is going on in Gisekia seems to be unknown. For the rate of molecular evolution and woody/herbaceous clade comparisons, see Yang et al. (2015); three of the four (within Nyctaginaceae, Phytolaccaceae and Petiveriaceae) showed increases associated with the adoption of the herbaceous habit, the fourth (Sarcobatus) did not.
Chemistry, Morphology, etc.. Da Cunha Neto et al. (2020) suggested that the presence of medullary bundles - variously arranged - was best put at this node. These medullary bundles were surrounded by ring of vascular bundles derived from a continous procambium. For successive cambia, see Carlquist (2004, see also 2007b, 2013). Note, however, that in Cunha Neto et al. (2021) suggest that in many Nyctaginaceae the vascular tissue is at least latterly produced by the activity of a single cambium.
Classification. Family limits in this area may need adjusting. However, if there is a [[Agdestis + Sarcobatus] Phytolaccaceae s. str.] clade (Brockington et al. 2011), one might as well include Sarcobataceae in Phytolaccaceae.
[Sarcobataceae + Phytolaccaceae]: inflorescence terminal, racemose.
Age. This node is about 70.3 Ma (Magallón et al. 2015) or very much younger, ca 36 Ma (Tank et al. 2015: Table S2).
SARCOBATACEAE Behnke - Sarcobatus Nees - Back to Caryophyllales
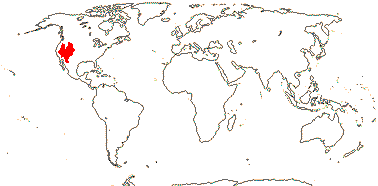
Shrub, with short shoots, thorns +; much Na and K oxalate; cork etc.?; wood rayless; ?stomata; leaves fleshy, sessile; plant monoecious; bracteoles 0; P 0; staminate plant: inflorescence densely spicate; flowers with peltate ± scaly "bracts"; A 1-4, anthers much longer than filaments; pollen pantoporate, pore margins raised; carpelate plant: flowers single, axillary, below staminate inflorescence; ?bracteoles connate, tubular, bilobed; G [2], ?development, style bilobed; ovule 1 per flower, funicle?; fruit an achene, bracteoles accresent, winged; ?tegmen bar thickenings; perisperm ± 0, embryo flattened, spiral, green; n = 18, 36, 54, x = 9 (?18), ?protein bodies in nuclei.
1 [list]/2. S.W. North America. Map: from Fl. N. Am. vol. 4 (2003). [Photos - Collection.]
Evolution: Physiology & Ecology. Sarcobatus is an important component of the vegetation of saline areas in southwest North America; sodium and potassium oxalate concentrations can reach 20% dry weight.
Genes & Genomes. Despite its woody habit, Sarcobatus is on a notably long branch and has a high substitution rate (Y. Yang et al. 2015: protein-coding genes).
Chemistry, Morphology, etc.. Some information is taken from Carlquist (2000a); for fruit, see Sukhorukov et al. (2015).
Previous Relationships. Sarcobatus used to be included in Chenopodiaceae, but sieve tube plastids with globular inclusions, etc., suggest that it goes somewhere around here (Behnke 1997).
Classification. Is Sarcobatus really worth placing in a separate family (c.f. Behnke 1997)? However, if there is a [Sarcobatus + Agdestis] clade (see above), J. F. Walker et al. (2018a) recommend placing the two genera in monotypic families given how different they are.
PHYTOLACCACEAE R. Brown, nom. cons. - Back to Caryophyllales
Cuticular waxes as platelets; lamina vernation conduplicate; (inflorescence with cymose clusters of flowers); pollen tricolpate; funicular hair-type [?always] obturator +; ?tegmen bar thickenings; x = 9, nuclear genome [1 C] (0.077-)1.228(-19.679) pg.
4 [list]/32 - two groups below. Tropical and warm temperate, esp. America. [Photos - Collection.]
Age. The age of this clade (inc. [Gisekia + Agdestis]) is (54.0-)40.9(-26.4) Ma (Cunha Neto et al. 2024).
1. Phytolaccoideae Arnott
Herbs, vines or soft-wooded trees; saponins +; fibers vasicentric; nodes 1:3; inflorescence a raceme (cymes at the base - Phytolacca), (leaf-opposed); P (4-)5; A 5-30, development centrifugal; tapetal cells multi-nucleate; G [3-17], (opposite P), free to laterally connate [forming a beautiful scalloped ring early in development], often pseudapocarpous, styluli broadly separate [basally connate?], stigma punctate/along the style; ovules apotropous, (outer integument ca 5 cells across), nucellar epidermal cells palisade, parietal tissue ca 2 cells across, nucellar cap 3-14 cells across, hypostase +, funicle short; fruit a berry; embryo white; n = 9, 18, etc.
3/31: Phytolacca (25). Chile, Mexico, few in Old World, also weedy (Phytolacca). Map: from Fl. N. Am. vol. 4 (2003) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
2. Agdestidoideae Nowicke - Agdestis clematidea de Candolle —— Synonymy: Agdestidaceae Nakai
Liane, root massively swollen; diffuse axial parenchyma, true tracheids +; wood rayless; Ca oxalate crystals 0; cuticle waxes with ± rounded platelets; lamina venation palmate; inflorescence branches cymose; P 4 (5); A 12-30, in groups alternating with P; nectary +; G [(3-)4], semi-inferior, septate, ?development, style branches becoming broadly recurved; ?ovules; fruit 1-seeded, achene, P forming wings; n = ?
1/1. South U.S.A. to Nicaragua. Map: from Fl. N. Am. vol. 4. (2003) and Culham (2007).
Evolution: Divergence & Distribution. Fossil fruits from the Upper Cretaceous (late Campanian) of Mexico are similar to those of Phytolacca, Cevallos-Ferriz et al. (2008) noting a palisade exotesta and also a palisade layer in the tegmen.
Pollination Biology & Seed Dispersal. The styluli of Phytolacca are quite separate so there is no stylar compitum. However, pollen tubes may exit the carpel via an adaxial hole at the base and travel across the papillate apex of the floral axis and fertilize the ovules in other carpels, so there is a distinctive extragynoecial compitum (Q. Zhang et al. 2018). The carpels of Phytolacca are initiated as a ring around the apex of the axis and are more or less connate laterally but not adaxially (Zheng et al. 2004, 2015; Zhang et al. 2018). However, Endress (2019, see also Rohweder 1965) prefer to interpret floral development here differently, and they suggest that the apparent apex of the floral axis more probably represents carpel margins.
Chemistry, Morphology, etc.. Phytolacca has been reported, probably incorrectly, to have glucosinolates (Fahey et al. 2001 for literature). Balfour and Philipson (1962) looked at the nodes of Phytolacca dioica and found that the leaf trace moved adaxially initially before swinging outwards; it finally exited the stem four or more nodes above the point of its origination in the central stele.
See also Rohwer (1993a: general), Hegnauer (1969, 1990: chemistry), Carlquist (2000b: anatomy), Eckardt (1957) and Ronse De Craene (2020: Fig. 6), both gynoecium, Meunier (1890: ovules and seeds), Mauritzon (1934c) and Kajale (1954), both embryology, Nowicke (1969) and Bortenschlager (1973), pollen, Hofmann (1977, 1994), Leins and Erbar (1993), Zheng et al. (2010, 2018) and Ronse de Craene (2013), all floral morphology/development.
[Petiveriaceae + Nyctaginaceae]: stomata also paracytic; G 1, stigma expanded, capitate.
P + aliis 57.1 PHO Gis Ag Nc 48 PhGisAg 40.9 GisAg 30.9PETIVERIACEAE Meissner - Back to Caryophyllales —— Synonymy: Hilleriaceae Nakai, Rivinaceae C. Agardh, Seguieriaceae Nakai
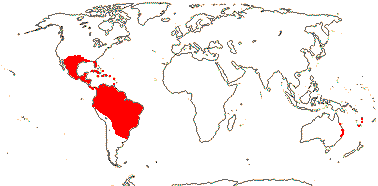
Herbs to trees or lianas; saponins 0, (plant smelling of garlic); stem anatomy normal [regular eustele], (medullary bundles +, amphivasal - Gallesia); (spines +, prophyllar); styloids, elongate crystals +; inflorescence raceme or spike, branched or not, (flowers terminal - Seguieria); (bracteoles slightly abaxial); (flowers weakly monosymmetric); P 4, (diagonal), (5 - Seguieria); A (-many, whorls centrifugal), (anthers extrorse - Hilleria), anthers H-shaped; pollen tricolpate, also 6-12 colpate or 7-many pantoporate; nectary 0; style ± 0, stigma basal/lateral, penicillate, fimbriate; outer integument 3-4 cells across [thicker towards chalaza], inner integument ca 2 cells across, parietal tissue 2-18 cells across, nucellar cap 2-9 cells across, placental obturator +; fruit winged [wings = P], utricle, or drupe; exotestal cells radially elongated, lumen ± obscure to obvious; perisperm +/± 0, micropylar endosperm haustorium [Petiveria alliacea], suspensor massive, (embryo straight - Petiveria), cotyledons (complexly folded), (convolute - Petiveria); n = 18, 54, x = ?; seedling epigeal, phanerocotylar.
9 [list]/13. Southern U.S.A. to South America, the Antilles, Australia, New Hebrides and New Caledonia (Monococcus), Map: from Rohwer (1982), Fl. Austral. vol. 4 (1984), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Fl. N. Am. vol. 4 (2003).
Chemistry, Morphology, etc.. Petiveria and Gallesia smell of onions. Nowicke (1969) described some Petiveriaceae as having "stipular thorns" up to 5 cm long; these are probably prophyllar.
Both Monococcus and Petiveria have four perianth parts that are diagonally arranged but their bracteoles are strictly lateral, while the perianth of the other genera is orthogonally arranged and the bracteoles are slightly adaxial (e.g. Vanvinckenroye et al. 1997). I am unclear about the stigma morphology here. Ovules of Petiveria have a nucellar beak.
See also Rohwer (1982, 1993a: general), Hegnauer (1969, 1990: chemistry), Carlquist (2000b, c) and Jansen et al. (2000c), both wood anatomy,Hofmann (1977), Ronse De Craene and Smets (1991d), Leins and Erbar (1993) and Sokoloff et al. (2017), all floral morphology/development, Nowicke (1969: general, also pollen), Bortenschlager (1973: pollen), Eckardt (1957) and Rohweder (1965), both gynoecium, Mauritzon (1934c: embryology), Rocén (1927: endosperm, in discussion), and Sukhorukov et al. (2015: fruit and seed).
Classification. Petiveriaceae are recognised because it has turned out that Phytolaccaceae, in which they were included, are not monophyletic; for discussion, see above.
NYCTAGINACEAE Jussieu, nom. cons. - Back to Caryophyllales
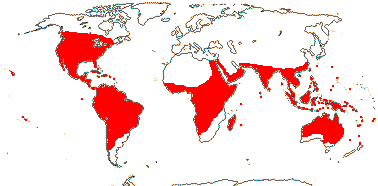
P connate, petal-like, lobes induplicate-valvate or -contorted; (nectary on receptacle); style long, slender, stigma asymmetric, also fimbriate; fruit surrounded by P [= anthocarp], fruit proper achene or nutlet, pericarp usu. thin.
32/405: [list], to tribes, seven groups below. Tropical to warm temperate. Map: see Stemmerik (1964), Fl. Austral. vol. 4 (1984), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Fl. N. Am. vol. 4 (2003) and Culham (2007). [Photo - Fruit, Collection.]
1. Caribeeae Douglas & Spellenberg - Caribea litoralis Alain
Perennial, with taproot; ?anatomy; leaves opposite, connate by expanded bases, blade ≤3 mm long; flower single, terminal, "bracteoles" 3-6; P constricted above the ovary, apically suburceolate; A 2, exserted, basally adnate to P; stigma capitate; ?embryology; "fruit" smooth; ?testa; ?embryo; n = ?
1/1. E. Cuba.
[Leucastereae [Boldoeae [Colignonieae, Nyctagineae [Bougainvilleeae + Pisonieae]]]]: (isoflavonoids +); (cork cortical); wood storied, often rayless; leaf wax crystalloids 0; (vessel elements with reticulate perforations); flowers in cymose clusters; A often connate at the very base; anther with outer parietal layer of wall producing endothecium and middle layer, the inner producing middle layer and tapetum [basic type = ?level], tapetal cells multinucleate, (massive); ovule terminating axis [?level], basal, outer integument ?4 cells across, inner integument ?2 cells across, parietal tissue 2-7 cells across, (nucellar cap 2 cells across); antipodal cells enlarged, >3; embryo sac haustorium +; ?tegmen bar thickenings; perisperm [in t.s.] two lobed or two-partite, embryo chlorophyllous; cotyledons much expanded, thin, enveloping perisperm; x = 9 (?13, ?12), nuclear genome [1 C] (0.047-)1.565(-52-547) pg.
Age. The age of this clade is estimated to be around (47.6-)42.3(-32.8) Ma (Cunha Neto et al. 2024).
2. Leucastereae Bentham & Hooker
Trees; stem anatomy normal [regular eustele]; styloids, etc.; indumentum ± stellate; A 2, 3 (10-20); style thick/0, stigma crest-like; (P accrescent in fruit - Ramisia), anthocarp 0, idioblasts with various kinds of crystals; (pericarp well developed); exotestal cells large; embryo hooked; n = ?
4/4. S.E. South America, esp. Brasil.
[Boldoeae [Colignonieae, Nyctagineae, [Bougainvilleeae + Pisonieae]]]: intraxylary phloem +; cambium from coninuous cylindrical primordium; idioblasts in anthocarps with raphides.
Age. The age of this clade is ca 35.3 Ma (Cunha Neto et al. 2024).
3. Boldoeae Heimerl
Bracteoles 0; A free; stigma inconspicuous (style 0, stigma fimbriate); anthocarp with layers of cells containing tannin globules; outer wall of exotesta with stalactite-like projections; perisperm entire; n = ?
3/4: Boldoa (?2). Mexico to Bolivia, the West Indies.
[Colignonieae [Nyctagineae [Bougainvilleeae + Pisonieae]]: (gypsophily); leaves opposite; (involucre +); P bipartite, tube stout, limb thin; A 1-many, of varying lengths; pollen pantoporate, also tricolpate, etc.; (ovule unitegmnic); basal part of P tube accrescent, often mucilaginous, rest withering, anthocarp with well developed sclerenchyma, the cells elongated parallel to the long axis of the fruit; cotyledons unequal; n = (8-)11(-13+).
Age. This clade is some 30.6 Ma (Cunha Neto et al. 2024).
4. Colignonieae Heimerl - Colignonia Endlicher
Stem becomes hollow; P connate only basally; anthocarp with layers of cells containing tannin globules; exotestal cells large; n = 16, 17.
1/6. Andean South America, to N. Argentina.
Age. The age of this clade is ca 28.6 Ma (Cunha Neto et al. 2024).
5. Nyctagineae Horaninow —— Synonymy: Allioniaceae Horaninow, Mirabilidaceae W. Oliver
(Plants annual); (C4 photosynthesis +); (stems with internodal epidermal sticky rings); pollen pantoporate; (enantiostyly + - Allionia), stigmatic transmitting tissue compact, enclosed by a ring of cells, in two tracts; (outer integument 5-7 cells thick - Mirabilis), obturator +; anthocarps various: (winged), (with gkandular emergences), (with mucilage cells), (sclerenchymatous cells elongated ar right angles to the long axis of the anthocarp), (tannins +), (epidermal cells thick-walled); (endotesta thickened [Mirabilis]); suspensor uniseriate, embryo hooked, (cotyledon 1 - Abronia); nuclear genome duplication [PHYT1]; n = 13, 20, 21, 22, 26, 27...; germination hypogeal, cryptocotylar/epigeal, phanerocotylar, hypocotyl with peg.
11/194: Boerhavia (50), Mirabilis (55), Abronia (33). Tropical to warm temperate, esp. herbs and shrubs in arid southwestern North America.
Age. Crown-group Nyctagineae are ca 18.1 Ma (Cunha Neto et al. 2024).
[Bougainvilleeae + Pisonieae]: pollen grains tricolpate; stigmatic transmitting tissue diffuse; outer integument 4-6 cells across, (integument single - 3-6 cells across), parietal tissue ca 4 cells across, obturator 0.
Age. The crown age of a clade including Bougainvillea and Mirabilis is estimated at 19-13 Ma (Wikström et al. 2001), (32-)23, 22(-13) Ma (Bell et al. 2010) or ca 24.8 Ma (Cunha Neto et al. 2024).
6. Bougainvilleeae Choisy —— Synonymy: Bougainvilleaceae J. Agardh
(Lianes, climbing by branch hooks); nodes 1:3; leaves spiral; pollen reserve lipids; style lateral; carpel also with median lateral vascular bundles; ovule sub-basal [Bougainvillea]; (pericarp well developed); n = 10, 17.
3/16: Bougainvillea (14-18). Central and tropical South America; southwest Africa.
Age. The age of this clade is around 20.8 Ma (Cunha Neto et al. 2024).
Bougainvillea-type pollen is reported from late Campanian deposits ca 70 Ma in Sakhalin (Manchester et al. 2015 for references).
7. Pisonieae Meisner —— Synonymy: Pisoniaceae J. Agardh
Plant ectomycorrhizal; (thorns +); (bracteoles 0); A 5-13(-many); pollen grains prolate, (pantocolpate); anthocarp dry, (with glands)/fleshy, (tannin-containing cells +); (testa multiplicative, unstructured [Pisonia]); embryo straight; n = 68 [two counts].
9/200: Neea (85), Guapira (70), Ceodes (20), Pisonia (20). Pantropical, but especially New World.
Evolution: Divergence & Distribution. Unfortunately, Caribea, sister to the rest of the family, is very poorly known. However, that genus aside, Sukhorukov et al. (2021) looked at the evolution of a number of characters of anthocarp anatomy. They noted that the fruit proper was little differentiated, the functions of the fruit having been taken over by the persistent perianth that surrounds it.
Ecology & Physiology. A xerophytic clade in S.W. North America is noted for its abundance in dry or desert conditions, diversification beginning in the Oligocene or Miocene. A number of species also tolerate gypsum-rich soils (Escudero et al. 2014; for Abronia, see Saunders & Sipes 2011), gypsum tolerance having evolved perhaps four times in the (Pliocene and) Pleistocene (Drummond et al. 2012). See also S. A. Smith et al. (2017) for a genome duplication here (in the ancestor of Nyctagineae) and its possible effects on diversification and association with a shift of the plants to drier, cooler conditions. The diversification rate seems to be higher in taxa wih medullary bundles (Cunha Neto et al. 2024) and also in those with polycyclic cambia (Cunha Neto et al. 2021).
The origin of C4 photosynthesis in Boerhavia and Allionia, the former with ca 42 C4 species, has been dated to within the last 7 Ma (Christin et al. 2011b).
Pollination Biology & Seed Dispersal. The single-flowered inflorescences of some species of Mirabilis can look remarkably like individual flowers: The green inflorescence bracts appear to be a calyx, and the brightly-coloured connate perianth looks like a sympetalous corolla. Taxa that flower in the evening or night (hence the name, the "four o'clock family") are quite common (Douglas & Manos 2007); Nores et al. (2013) summarize what is known about pollination biology in the family.
Sukhorukov et al. (2021) have examined the anatomy of the anthocarp wall, the anthocarp being the fruit proper closely invested by the perianth, which together form the dispersal unit. Elongated subepidermal cells on the wings/ribs of the perianth produce mucilage when the fruit is wetted, and this is especially notable in disseminules of the xerophytic North American clade (see also J. Wilson & Spellenberg 1977); such mucilage cells are quite common in Nyctagineae, where they have evolved more than once (Sukhorukov et al. 2021). In species like Pisonia the hypanthium on the long fruits becomes viscid and very sticky indeed; it is used as bird lime to catch birds (Western 2012).
Plant-Animal Interactions. Leaf mining or webbing caterpillars of the yponomeutoid Heliodinidae moths are notably common here (Sohn et al. 2013).
In Anulocaulis and some other Nyctagineae a ring of epidermal cells encircling the stem becomes hypertophied; the cell contents are very sticky, and it is thought that these sticky rings may help limit the activities of herbivores, for instance, perhaps reducing ant-aphid associations (da Cunha Neto et al. 2019, see also McClellan & Boecklen 1993).
Plant-Bacterial/Fungal Associations. Pisonieae, including Pisonia, are recorded as forming ectomycorrhizal associations with various basidiomycetes perhaps especially Thelephoraceae; individual ECM plants are often quite dispersed in the vegetation and only a single species of fungus may be involved in the association. ECM associations are known from Neea and Guapira growing in montane rainforest, being imperfectly developed in species that have only long roots, not long + short roots, and root hairs may be present (Haug et al. 2005; Kottke et al. 2008b; Põlme 2018), and in Pisonia the epidermal cells have what appear to be multicellular protrusions (Tedersoo et al. 2010a). The specificity of the association between fungus and plant is quite high (Tedersoo et al. 2010a). In some Pisonia, at least, although a fungal sheath is formed, there is no Hartig net, but the outer and radial walls of the plant epidermal cells develop transfer cell morphology (Cairney et al. 1994: see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, dates, etc.).
Plant-Animal Interactions. Nyctaginaceae with glandular hairs may trap insects on those hairs - see LoPresti et al. (2015), also elsewhere.
Genes & Genomes. For a gene duplication in Nyctagineae, see Y. Yang et al. (2015: PHYT1) and Landis et al. (2018: the ALINα event); the latter date it at ca 16.3 Ma. Cytologically Nyctaginaceae are poorly known.
Vegetative Variation. It was early realised that the primary stem anatomy could be complex. There are medullary vascular bundles (e.g. Sharma 1962; Pant & Mehra 1963), and Pant and Mehra (1962) described two rings of vascular bundles, the innermost perhaps made up of two large arcs of vascular tissue, the next consisting of a number of small bundles, while another ring, the outermost, which they called the belated ring, consisted of vascular bundles embedded in a more or less complete ring of sclerified tissue. Despite this unusual anatomy, Pant and Mehra (1962) suggested that the nodal anatomy was one gap, one trace, as in related groups with more conventional anatomy, however, Balfour and Philipson (1962) described a rather more complex system in Bougainvillea, a sort of 1:3 node. Carlquist (2004) examined secondary thickening in Nyctaginaceae in detail: There is a lateral meristem that produces secondary cortex to the outside, and to the inside rays, conjunctive tissue, and a succession of vascular cambia, from which the more or less isolated areas of vascular tissue (but not rays) are derived. Recent work by Da Cunha Neto et al. (2020, see also Cunha Neto 2023; Cunha Neto et al. 2021, 2022, 2024) is helping to clarify the situation. They looked at the distribution of medullary bundles and cambial development in some detail, noting that the medullary bundles varied in number, arrangement and size, and they might divide and anastomose at the nodes, so forming a kind of nodal plexus. Traces to leaves, etc., departed from these medullary bundles, not from the surrounding ring of procambium/vascular bundles (the "belated ring"). Cunha Neto et al. (2021) suggested that there may be a polycyclic eusteles initially; in many taxa various amounts of intraxylary phloem were produced by the activity of but a single cambium. Hernández-Ledesma et al. (2011) looked at anatomical variation within Mirabilis in some detail, and Cunha Neto et al. (2020) that of Allionia, where the cork cambium in the root is apparently subepidermal. Secondary thickening developed in all vascular bundles irrespective of their positions (Da Cunha Neto et al. 2020). There seemed to be intermediates between successive cambia and intraxlary phloem, and Cunha Neto et al. (2022) suggested that perhaps process or continuum morphology might be the best way to understand the complex morphology here. In Nyctaginaceae polycyclic eusteles were replaced by a single cambium, the continuous cylindrical procambium; there the rates of tissue production varies, hence the intraxylary phloem, common throughout the family; successive cambia do not occur in most Nyctaginaceae (Cunha Neto et al. 2021). The presence of cauline medullary bundles ("vascular fascicles" when secondary thickening develops) seems to be quite a high-level apomorphy in this part of the raphide clade, but stem anatomy is normal in Nyctaginaceae-Leucastereae (and unknown in -Caribeeae) and Petiveriaceae, for example.
Chemistry, Morphology, etc.. Some Nyctagineae have pollen grains ca 200 µm long, about the largest in angiosperms outside the aquatic Cymodoceaceae (Alismatales). For nectaries in the family, see Nores et al. (2013). The single ovule seems to terminate the apex of the floral axis (Sattler & Perlin 1982); exactly where this character should be placed on the tree is unclear.
See Rohweder and Huber (1974) and Bittrich and Kühn (1993) for general information, Hegnauer (1968, 1990) for chemistry, Sharma (1963a) for floral anatomy, Vanvinckenroye et al. (1993) and Ronse De Craene (2020: Oxybaphus) for floral development, Nores et al. (2015) for information about pollen and gynoecium, Rocén (1927) and Woodcock (1929) for ovules, and Sukhorukov et al. (2015) for fruit and seed.
Phylogeny. The South American Leucastereae and Mexican-Central American Boldoeae are successively sister taxa to the remainder of the family, positions that have moderate to strong support. Within the remainder of the family a North American xerophytic clade has very strong support. Here Bougainvilleeae and Pisonieae (with minor additions) form a clade, while Abronieae are embedded in a highly paraphyletic Nyctagineae plus Boerhavieae complex, all three included in Nyctagineae above (Douglas & Manos 2007; see also Levin 2000 for a more limited study; Y. Yang et al. 2017). For relationships in Pisonieae, where Pisonia has turned out to be paraphyletic, see Rossetto et al. (2019) and Rossetto and Caraballo-Ortiz (2020).
Classification. For a tribal classification, see Douglas and Spellenberg (2010); they also recognised a monotypic Caribeeae, but this was unplaced in the phylogeny. Rossetto et al. (2019) and Rossetto and Caraballo-Ortiz (2020) have straightened up generic limits in Pisonieae; Guapira and Neea are both recognized, c.f. Rossetto et al. (2019).
[Molluginaceae [Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] + the ACPT clade]]]] / the Portullugo clade: fruit a loculicidal capsule [?level].
Age. This node is estimated to be 35-24 Ma (Wikström et al. 2001), about 50.2 Ma (Tank et al. 2015: Table S2), ca 51.9 (± 4.7) or 56.1 (± 5.8) Ma (Christin et al. 2011a), (55.4-)53.3(-51.2) Ma (Arakaki et al. 2011), or as much as around 73.1 Ma (Magallón et al. 2015).
Evolution: Ecology & Physiology. There is extensive discussion about the evolution of CAM and C4 photosynthetic syndromes in this clade (Edwards & Ogburn 2012).
Nyffeler and Eggli (2010b) offer estimates of the numbers of succulent species in different families. Taxa with fleshy roots are scattered throughout the clade, being found in all families (except the monotypic Halophytaceae) as well as in all subfamilies of Cactaceae (e.g. Nyffeler et al. 2008). Ocampo and Columbus (2010) discuss the evolution of different photosynthetic pathways in this clade, which they reconstruct as being plesiomorphically C3. For CAM in the old Portulacaceae s.l., now scattered through this clade, see Guralnick and Jackson (2001) and especially Ocampo and Columbus (2010). CAM cycling is common; this occurs when plants do not completely shut their stomata during the day, and carbon is fixed at night from respiratory, not atmospheric, CO2.
Chemistry, Morphology, etc.. Variation within this clade is complex (see also Nyffeler 2007, especially Ogburn 2007; Nyffeler et al. 2008; Ogburn & Edwards 2009; Nyffeler & Eggli 2010b; Ocampo & Columbus 2010). For the absence of anomalous thickening in Portulaca, see Krumbiegel and Kástner (1993). Most taxa have mucilage cells, but there may be interesting variation within the group as to exactly where such cells occur in the plant (Ogburn & Edwards 2009). For the distribution of peripheral vascular bundles in the leaf, i.e., the leaf venation in three dimensions, see Ogburn and Edwards (2013).
Interpretation of the parts surrounding the flowers is complicated by how they have been described. Often there are paired structures, frequently more than a single pair, borne immediately below the flower and more or less completely surrounding it. Called bracteoles here, they have also often been called sepals. The inner/upper pair of bracteoles is in the median plane (e.g. Eichler 1878), as is the sole pair of bracteoles in Montia (Ronse de Craene 2010) and Halophyton (Pozner & Cocucci 2006), although they do not comment on the orientation. The transverse outer bracteoles may have flowers in their axils, the inner median bracteoles always lack them. In at least some species of Anacampseros the inner bracteoles are in the same plane as the bud-subtending bracteoles (Vanvinckenroye & Smets 1999), in species of Portulaca such as P. oligosperma there are two quite large bracteoles immediately underneath the flower and then four smaller bracteoles - ?= perianth - in a whorl separated from the first pair by a short internode (Geesink 1969), while in Lewisia there are lateral bracteoles and inner median bracteoles forming the involucel (Dos Santos & Ronse De Craene 2016). The whorl inside the bracteoles, usually 4- or 5-parted - called a perianth here - is like that of other centrosperms. Its members are often more or less brightly coloured and have been described as petals or petal-like. In Claytonia their development is much retarded relative to that of the androecium, and a theory is that the perianth proper has been lost and the apparent perianth is a modified part of the androecium (Dos Santos et al. 2012). The lateral members of the perianth in Montia develop first, and the median members, sometimes as many as 11, develop after the androecium (Dos Santos et al. 2016). Finally. we will see that in Cactaceae a case can be made for the absence of a conventional perianth.
Portulaca has an androecial ring primordium, as in some Cactaceae and in species of Anacampseros, sometimes also with centrifugal initiation of stamens; other species have fewer stamens, which may be initiated in pairs (facing each other!) opposite the perianth members, or as single stamens alternating with them (Vanvinckenroye & Smets 1999). When there is the same number of stamens as perianth members, their position relative to the carpels varies. Nowicke (1996) summarized a number of pollen characters that are shared in the group (her Portulacineae), although they might also occur outside it: Columellae either narrowed towards the middle or expanded towards the base, sometimes fused; pollen with granular internal surfaces; perforated foot layer; non-apertural endexine "thread-like" - the latter term unclear from the descriptions provided.
For general information, see Carolin (1987 [also a phylogenetic analysis], 1993), for chemistry, see Hegnauer (1969, 1990), for the vegetative plant, see Nyffeler et al. (2008), for anatomical information about the old Portulacaceae, see Becker (1895), and for pollen, see Nilsson (1967).
Phylogeny. It was clear over twenty years ago that relationships in this clade were not reflected by then current classifications. Thus Hershkovitz and Zimmer (1997) realized that if Cactaceae were recognised, Portulacaceae in the old sense would be paraphyletic (see also Appelquist & Wallace 1999, 2001). Later they found little major phylogenetic structure in a study of American Portulacaceae (Hershkovitz & Zimmer 2000: ribosomal DNA, Cactaceae not included). Hershkovitz (2006) found the same general pattern as he focussed on W. American "Portulacaceae" from the Andean region - there were perhaps half a dozen clades in that region, but no major groupings beyond that. Cactaceae, Didiereaceae and Portulacaceae remained a closely entwined complex (Appelquist & Wallace 2000), indeed, they can all be intergrafted (Anderson 1997). Cactaceae + Talinum + Portulaca + Anacampseros, etc., were found to make up a major and rather well supported clade (Hershkovitz & Zimmer 1997; Appelquist & Wallace 2001; Ocampo & Columbus 2010; etc.). See also Cuénoud et al. (2002) for relationships in this area, e.g. of Halophytum.
A number of studies since 2007 are helping clarify relationships around here, although they still remain rather uncertain in places. Many of the relationships found by Ocampo and Columbus (2010) were poorly supported, and Halophytaceae wandered around the tree; they noticed substantial conflict in relationships suggested by individual gene trees. Brockington et al. (2009; large amounts of data, rather skimpy sampling) found a clade [Portulacaceae + Talinaceae] with 98% boostrap support, and Claytonia (Montiaceae) was sister to the whole clade, which included Halophytaceae.
Using the nuclear PHYC and chloroplast trnK/matK genes and ca 250 species of this clade, Arakaki et al. (2011) confirmed with strong support the position of Molluginaceae as the sister taxon to Portulacineae (see also Soltis et al. 2011: support only moderate; Ogburn & Edwards 2015). There was strong support for most relationships along the spine of this clade, and Support for the monophyly of nearly all families was strong. The only exception is the [Halophytaceae [Didiereaceae + Basellaceae]] clade, thus support for the monophyly of Didieraceae (and Montiaceae) in Nyffeler and Eggli (2010a) was not strong. Arakaki et al. (2011) could not recover a monophyletic Didiereaceae, Basellaceae being sister to the Portulacaria group, and Halophytaceae were only weakly associated with the other two families. Soltis et al. (2011) found only weak support for the [Didiereaceae + Basellaceae] clade, but Anton et al. (2013) found some support for a [Didiereaceae [Halophytaceae + Basellaceae]] clade. There was weak support for a [Halophytaceae + Basellaceae] clade but stronger support for a [Didiereaceae [Talinaceae + the rest]] clade (A. J. Moore et al. 2017). Problems were emphasized by N. Wang et al. (2018) who found substantial conflict between relationships suggested by different gene trees in the Basellaceae-Didiereaceae area, and there was little support either for a clade that included those two families alone, or for a topology that placed the two families as successive sister taxa to remaining Portulacineae (Halophytaceae were not included). Yao et al. (2019) found a quite well supported [Didiereaceae [Halophytaceae + Basellaceae]] clade.
Details of relationships immediately around Cactaceae, i.e. in the ACPT clade, made up of Anacampserotaceae, Cactaceae, Portulacaceae and Talinaceae, have been unstable, so telling the story of the evolution of Cactaceae has been difficult. Nyffeler (2007: three genes, two compartments) found some support for a topology [Talinum and relatives [Portulaca [Anacampseros and relatives + Cactaceae]]], although the topology was different when the mitochondrial nad1 data were analyzed alone. Support for the [Anacampseros and relatives + Cactaceae] clade was appreciable in the combined analysis (78% bootstrap), where the chloroplast signal predominated. Nyffeler and Eggli (2010a) found few resolved relationships except in the Talinaceae-Cactaceae area, while Butterworth and Edwards (2008) found the relationships [Anacampserotaceae [Talinacaeae [Portulacaceae + Cactaceae (weak support)]]], although there was no outgroup, so Anacampserotaceae appeared to be paraphyletic. Portulaca and Pereskia (but not Claytonia) share a 500 bp chloroplast DNA deletion in the rbcL gene (Wallace & Gibson 2002 for details and references), a potentially informative molecular marker. There was "only" 78% likelihood bootstrap support for the [Anacampserotaceae [Portulacaceae + Cactaceae]] clade in Arakaki et al. (2011), although that also has some morphological support. However, Crawley and Hilu (2013) recovered the clade [Portulacaceae [Anacampserotaceae + Cactaceae]], as did Hernández-Hernández et al. (2014), while Y. Yang et al. (2017) found that Portulacaceae and Anacampserotaceae were sister taxa (see also J. F. Walker et al. 2018a). Ogburn and Edwards (2015) also recovered this latter clade, and it was also recovered - although not always - by A. J. Moore et al. (2017) using a targeted enrichment approach, and this latter work should be consulted for a discussion on the conflicting signals found here and elsewhere in this clade. N. Wang et al. (2018) also recovered a [Portulacaceae + Anacampserotaceae] clade sister to Cactaceae, other topologies had less support. However, Yao et al. (2019) recently recovered a [Portulacaceae [Anacampserotaceae (one species) + Cactaceae]] clade that had strong support in their plastome analysis, there was also strong support for these relationships in H.-T. Li et al. (2019), however, support was weak in Version 2 of the 352gene analysis (nuclear genome) in the Seed Plant Tree.
Classification. Basellaceae and Didiereaceae are kept separate. There are a few African genera that used to be included in Portulacaceae that have moved to the Didiereaceae, so making them less distinct, although morphology is largely consistent with their new position. Portulacaceae s.l. are strongly paraphyletic, their erstwhile members being placed in Portulacaceae s. str. (now a small group), Talinaceae, Anacampserotaceae and Montiaceae below. The morphologically rather distinctive and Antipodean Hectorellaceae are included in Montiaceae.
MOLLUGINACEAE Bartling, nom. cons. - Back to Caryophyllales ¯— Synonymy: Adenogrammaceae Nakai, Glinaceae Martius, Pharnaceaceae Martynov
Barely succulent (annual) herbs (shrubs), growth sympodial, modules with definite numbers of leaves; (C4 photosynthesis +); hopane saponins, C-glycosylflavonoids, anthocyanins +, betalains 0; wood rayless; (C4 photosynthesis +); cork?; (secondary growth normal); (sieve tube plastids with starch grains); pericyclic fibres +; (raphides +); (also rhomboidal crystals +); plant glabrous (hairs glandular or stellate); cuticle waxes as platelets or rodlets; stomata anomocytic; prophylls prominent; leaves often pseudoverticillate, opposite or spiral, stipules membranaceous (0); P quincuncial, ± K-like at base/down middle, ± C-like at apex/down sides, "C" (bifid to laciniate), (-20 - Glinus); A (2-)5-10(-30), alternate with P, (centrifugal), (filaments ± connate basally); (pollen pantoporate); G (1) [2-5(more)], opposite K/P or the median member adaxial, placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker], style (divided)/styluli +, stigmas linear (capitate); ovules 1[basal]-many/carpel, parietal tissue 1(-2) cells across, epidermal cells radially elongated, nucellar cap to 2 cells across, obturator +, funicles short to long; fruit (dehiscing by transverse slits), (nutlet); seeds (arillate - Glinus), (operculate), (ridged), (colliculate), (black); exotestal cells (with stalactite-like projections); embryo (± straight - ?Glinus); x = 9, nuclear genome [1 C] (0.048-)1.243(-32.205) pg.
11 [list]/87: Mollugo (35), Pharnaceum (25). Tropical, especially southern Africa, to warm temperate, some weedy. Map: from Frankenberg and Klaus (1980), Jalas and Suominen (1980), Fl. N. Am. vol. 4 (2003), Trop. Afr. Fl. Pl. Ecol. Distr. vols 1 (2003), 6, (2011), Australia's Virtual Herbarium (consulted vii.2013) - incomplete, and South America rather notional. [Photos - Habit & Flower.]
Age. Crown group Molluginaceae are estimated to be ca 46.7 (± 4.8) or 50.3 (± 5.8) Ma (Christin et al. 2011a) or (76.0-)58.9(-41.9) Ma (Sukhorukov et al. 2021).
Evolution: Divergence & Distribution. Dates obtained from nuclear and chloroplast gene trees were dramatically different, e.g. ages for crown-group Glinus were 21.5 and 6 Ma respectively (Sukhorukov et al. 2021). The rate of diversification of the Adenogramma-Pharnaceum clade is notably less than many others in this general area of Caryophyllales (Arakaki et al. 2011).
Sukhorukov et al. (2018b: beautiful images, see also supplementary material) optimized a number of seed characters on a tree for Molluginaceae, unfortunately, some character states were not clearly justified and why Kewaceae should be sister to Molluginaceae was unclear.
Ecology & Physiology. C4 photosynthesis probably has arisen more than once here (Christin et al. 2010b, 2011, q.v. for dates). There are also a few C3/C4 intermediates with C2 photosynthesis in Mollugo, and species such as Mollugo verticillata that photosynthesize like this may be some 10-20 Ma old (Christin et al. 2011a), a considerable age for such plants (Brätigam & Gowik 2016). Adoption of the new photosynthetic pathway is accompanied by an increase of tolerance of drier conditions (Christin & Osborne 2014).
Pollination Biology & Seed Dispersal. The loculicidal capsules of Glinus are hydrochastic (Sukhorukov et al. 2021).
Genes & Genomes. There is a genome duplication event (MOPEα) ca 49.4 Ma associated with this family (Landis et al. 2018); see also N. Wang et al. (2018) for a duplication associated with Mollugo verticillata in particular.
There are quite extensive changes in the plastomes of Hypertelis and Pharnaceum (Yao et al. 2019).
Chemistry, Morphology, etc.. For pigments, see Thulin et al. (2016).
The apparently anomalous occurrence of vascular rays in genera like Macarthuria (M. Endress & Bittrich 1993) is less anomalous when these genera are removed from the family; there has been a similar clarification of apparent variation in sieve tube plastid morphology. Para- dia- and anisocytic stomata are all recorded here; stomatal type should be checked against the new circumscription of the family. The stipule-like structures need examination.
There may be petals in Glinus (Wei & Ronse De Craene 2019). The androecium may be fasciculate; Adamson (1958a) noted that the 20-30 stamens of Hypertelis spergulacea, which belongs here (the rest of the genus is in Kewaceae), are in groups. For aril morpohology, see Sukhorukov et al. (2018b).
Some information is taken from Adamson (1960), Bogle (1970), M. Endress and Bittrich (1993) and Thulin et al. (2013), all general, Richardson (1981: flavonoids, but c.f. Behnke et al. 1983b), Vincken et al. (2007: saponins), Hegnauer (1964, 1989, as Aizoaceae: chemistry), Behnke (1976) and Behnke et al. (1983a), both sieve tube plastids, Payne (1933, 1935: Mollugo), Sharma (1963: floral morphology, includes segregates), Ronse De Craene (2020), Hofmann (1973: flower, growth), and Raghavan and Srinivasan (1940), Narayana and Lodha (1972), Bhargava (1934), Narayana (1962a) and Hassan et al. (2005a) all embryology, seeds and ovules.
Phylogeny. Nepokroeff et al. (2002) found that Mollugo and relatives and Pharnaceum and relatives each formed a well-supported clade, but the two were only weakly linked. However, support for a monophyletic Molluginaceae was strong both in Christin et al. (2011a) and Arakaki et al. (2011), and resolution of relationships within the clade was also good; branches in the Adenogramma-Pharnaceum clade were notably long (Arakaki et al. 2011). Thulin et al. (2016) provide a pretty comprehensive tree for the family, with all the major banches well supported. Mollugo is strongly para/polyphyletic (Christin et al. 2010b, 2011a; Arakaki et al. 2011; Thulin et al. 2016; N. Wang et al. 2018). Sukhorukov et al. (2018b) obtained the same tree with [Triconotheca [Mollugo + Glinus]] sister to the rest of the family; in a later study in which the focus was on Glinus Sukhorukov et al. (2021) obtained different topologies from nuclear and chloroplast genes.
Classification. Thulin et al. (2016) provide a generic level classification for the family.
Previous Relationships. The limits of Molluginaceae have long been unclear. Most Molluginaceae as circumscribed in M. Endress and Bittrich (1993) are included here, but Limeum, Corbichonia (Lophiocarpaceae), and Macarthuria, are elsewhere in the centrosperms as three separate mono- or digeneric families, the latter towards the base of the whole group. Kewa is another monogeneric family that includes species that used to be in Hypertelis, but the rest of that genus remains here. Polpoda ( Polpodaceae Nakai) is not incorporated in any description. It has P 4, A alternating with the perianth, G [2], basally connate styles, and scarious stipules (Hoffman 1994). There have been suggestions that Gisekia might be included in Phytolaccaceae-Rivinioideae (see Cuénoud et al. 2002), although here it is in its own family (Brockington et al. 2009).
[Montiaceae [[Halophytaceae [Didiereaceae + Basellaceae]] [the ACPT clade]]] / Cactineae Bessey (1895) = Portulacineae Engler (1898): (plants with tuberous roots) [at least some species in all families]; (CAM photosynthesis +); phloem parenchyma cells with phytoferritin [crystalline iron-protein complex in plastids]; Ca oxalate crystals in stem epidermis; mucilage cells +; stomata paracytic; (lamina with peripheral vascular bundles, phloem internal [orientation normal]); lamina ± succulent, amphistomatic [?Basellaceae]; two pairs of bracteoles, inner pair in the median plane, lacking subtending buds, immediately below and ± enclosing the flower; P petal-like, (-13), first two developing in the transverse plane; A (3) 5(opposite P)-8(-many); pollen pantocolpate; G ([2] - lateral), [3] [2 abaxial], style +, branches spreading; ovule lacking funicular/placental obturator; nuclear genome duplication, plastome 6-bp deletion in ndhf.
Age. An estimate of the age for this clade is (33.7-)18.8(-6.7) Ma (!: Ocampo & Columbus 2010), around 36.2 Ma (Tank et al. 2015: Table S2), (47.6-)44.9(-42.2) Ma (Arakaki et al. 2011), or about 42.6 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. This clade seems to be New World in origin (Ocampo & Columbus 2010). Hershkovitz and Zimmer (2000) suggested that there must have been a number of major dispersal/colonization events.
If the evolution of CAM photosynthesis can be pegged to this node, the ages of around 40 Ma suggested were at a time when atmospheric CO2 concentrations were ca 1,000 ppm (Gilman et al. 2023). Sheehan et al. (2019) think that betalain production was an apomorphy for this node.
Ecology & Physiology. A number of gene families probably involved in various aspects of stress tolerance, including CAM and C4 photosynthesis, have expanded considerably in this area (N. Wang et al. 2018; see also Christin et al. 2014b), although how such expansions might be related to genome duplication still needs clarification. For the evolution of CAM photosynthesis in this group (note - it is an apomorphy for it), see Holtum (2023); there are very few purely C3 species around here outside Montiaceae, and in Montiaceae such species seem to be reversals (Gilman et al. 2023).
Genes & Genomes. For a genome duplication in this area, see S. A. Smith et al. (2017), Y. Yang et al. (2017: PORT1), N. Wang et al. (2018) and perhaps X. Wang et al. (2023: P-β, dated at ca 66.3 Ma); the sister clade (Molluginaceae) is hardly very diverse.
Classification. For family limits and characterisations, see Nyffeler and Eggli (2010b).
MONTIACEAE Rafinesque - Back to Caryophyllales —— Synonymy: Hectorellaceae Philipson & Skipworth
Annual to perennial herbs, often with swollen roots and basal rosette, internodes short (subshrubs); ?betalains; (CAM photosynthesis); cork cambium initiation delayed; secondary growth little; vessel elements?; plant glabrous; stomata longitudinally oriented; cuticle waxes as procumbent platelets; leaves often with broad clasping bases, flat to terete with an adaxial impressed line (not succulent); inflorescences terminal or axillary, (monochasial) cymose, or single (axillary) flower; (transverse bracteoles absent, or -9 "sepaloids"); P 4-5(-19), (basally connate); equal and opposite perianth members, (or 1 fewer, alternating with P - Hectorella, Lyallia), (-100, development centrifugal), basally connate or not; tapetal cells multinucleate; pollen also tricolpate, pantoporate; G [2-8], placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker], (carpels initiate separately around flat ± persistent floral apex [= cup-shaped] - Lewisia), apical septae + [= invagination of apex], (placentation free central, with 4-7 ovules), style ± developed, branches diverging, stigma papillate; ovules (1/carpel [= 2,1,0/placenta]), with medium funicle, inner integument protruding considerably, parietal tissue 1-5 cells across, in radial rows; fruit also circumscissile, (1-seeded, indehiscent); seed ("arillate"), outer wall of exotesta with stalactite-like projections (not), ?tegmen bar thickenings; n = 5-13, etc., x = 12, nuclear genome [1 C] (0.182-)1.677(-15.434) pg.
14 [list]/225: Parakeelya/Rumicastrum (74), Montiopsis (40), Claytonia (27), Phemeranthus (25-30). Especially western North and South America, also the Antilles and the Subantarctic Islands (map: approximate, from Hultén & Fries 1986; Fl. N. Am. 4: 2003; Miller & Chambers 2006; M. Ogburn, pers. comm. ix.2012; Australia's Virtual Herbarium i.2013 - much naturalization, so not easy). [Photo - Collection, but not all.]
Age. The age for crown-group Montiaceae is (25.4-)13(-3.4) Ma (Ocampo & Columbus 2010: 95% highest posterior density) or (43.0-)39.9(-36.8) Ma (Arakaki et al. 2011); the latter age is more in line with that suggested for the Calandrinia s.l. clade (see below) that is estimated to be 30 ± 2.34 Ma (Hancock et al. 2018) and even the Antipodean Hectorella/Lyallia clade, which is perhaps (29.9-)22.1(-11.4) Ma (Wallis & Trewick 2009).
Evolution: Ecology & Physiology. Within Cactineae, Montiaceae are noted for their ecological expansion into both colder and more seasonally variable habitats, and there have been several habit/habitat shifts, many being between the annual and perennial habit, the latter occurring in taxa that had moved into cooler environments - hardly notable niche conservatism (Ogburn & Edwards 2012, 2014; S. A. Smith et al. 2017). On the other hand, Montiaceae are not very speciose and are sister to a clade with almost ten times the number of species - most being in Cactaceae, of course.
Some short-lived members of the Australian Parakeelya/Rumicastrum are facultative CAM plants (Winter & Holtum 2014, see also 2011; Holtum et al. 2017).
Pollination Biology & Seed Dispersal. The seeds may be forcibly ejected as the margins of the valves incurve during capsule dehiscence (Carolin 1993). The seeds of some Montiaceae are myrmecophytic (Lengyel et al. 2010).
Plant-Bacterial/Fungal Associations. The South American Calandrinia is a host of the anther smut Microbotryum (Uredinomycota), also found on Silene, etc. (Hood et al. 2010).
Genes & Genomes. There can be considerable infraspecific variation in chromosome numbers, for example, the diploid number in Claytonia virginica varies between 12 and 191 (e.g. Lewis et al. 1967; Bogle 1969; see also McIntyre & Strauss 2017). There are suggestions that there has been a genome duplication in the ancestor of Claytonia (S. A. Smith et al. 2017; Y. Yang et al. 2017), while N. Wang et al. (2018) note two duplications in the family.
Chemistry, Morphology, etc.. Hectorella has both spiral phyllotaxis and a closed vascular system, an unusual combination (Beck et al. 1982). The lamina of Phemeranthus has peripheral vascular bundles with the xylem external (Ocampo & Columbus 2010; Ogburn & Edwards 2013), also odd.
The inflorescence of Hectorella and Lyallia may be a reduced cyme; there are alternate/2-ranked bracts below the flower, and the latter genus may have more than one flower per axil (Skipworth 1961; Wagstaff & Hennion 2007). The paired bracteoles below the flower in these two genera are clearly described and illustrated as being transverse (lateral) by Skipworth (1961), but later described as being ad/abaxial (median) by Philipson and Skipworth (1961). Cave et al. (2010) described the lower two bracteoles of Calandrinia as developing successively, the upper pair being lateral(-abaxial). Montiopsis can have trilobed bracteoles. Nyffeler and Eggli (2010b) described the flower of Lewisia as having up to 9 "sepaloids" (= perianth members). Dos Santos et al. (2012) noted that the petaloids in Claytonia appeared well after the androecium was initiated; however, they thought that they were calycine, but with very delayed growth, rather than outgrowths of the filaments.
Schnizlein (1843-1870: fam. 206) showed carpels alternating with the perianth members, or the median member in the abaxial position, as in Claytonia. Taxa like Claytonia and Montia have only a single ovule per carpel, but in terms of the three placentae it works out at 2,1,0/placenta (Ronse De Craene 2020). Seed coat anatomy needs more study. Rocén (1927) thought that the endotegmen of Calandrinia (it looks like the exotegmen) had rod-like rhickenings; the tegmen was multiplicative.
Some additional information is taken from Carolin (1987, 1993), Philipson (1993: as Hectorellaceae), Lourteig (1994), Eggli (2002), Nyffeler and Eggli (2010b) and Hershkovitz (2019), all general, for Claytonia see Miller and Chambers (2006); see Ronse De Craene (2020: Fig. 8), ovary, Meunier (1890) for ovules and seeds, for pollen, see Nilsson (1967).
Phylogeny. For the circumscription of Montiaceae, see above. West American members of the old Portulacaceae to be included in Montiaceae include Montia, Lewisia, Phemeranthus (this used to be included in Talinum - Talinaceae here), etc. (e.g. Hershkovitz 1993, 2006; Hershkovitz & Zimmer 2000). The whole clade has strong support, as does the sister group relationship between Phemeranthus, with pantoporate pollen, and the rest of the clade (Applequist et al. 2006; see also Ocampo & Columbus 2010; Ogburn & Edwards 2015; Hancock et al. (2018: no Hectorelleae). Ogburn and Edwards (2015) found two main clades apart from Phemeranthus, one largely South American and the other, within which relationships between Parakeelya/Rumicastrum and Calandrinia s. str. (also pantoporate pollen) were unclear, was largely North American, although relationships were clarified in the topology appearing in most analyses of Hancock et al. (2018) where the two are quite distinct if successively sister taxa in a clade that also includes largely North American taxa like Montia and Lewisia.
Applequist et al. (2006: ndhf gene, see also e.g. Nepokroeff et al. 2002; Ogburn & Edwards 2015) also included the New Zealand-Antarctic Hectorellaceae (Hectorella, Lyallia), previously of uncertain relationships, as a new tribe of Portulacaceae. Although flower position (axillary) and bracteole and stamen position of Hectorellaceae differ from that of other Montiaceae and the gynoecium is unilocular, the anatomy of the two is very similar (Carlquist 1998b).
O'Quinn and Hufford (2005) outlined the phylogeny of Claytonia (tricolpate pollen) and its sister taxon, Montia (pantocolpate).
Classification. See Nyffeler and Eggli (2010b) for included genera. The Australian Parakeelya/Rumicastrum is quite separate from the New World Calandrinia (Hancock et al. 2018).
[[Halophytaceae [Didiereaceae + Basellaceae]] [the ACPT clade]]: plant mucilaginous; carpels initiate separately around flat ± persistent floral apex [= cup-shaped].
Age. Ocampo and Columbus (2010) suggested an age for this clade of (31.9-)17.6(-6.5) Ma, Arakaki et al. (2011) suggested an age of (45.3-)42.5(-39.7) Ma, and Magallón et al. (2015) an age of about 39.5 Ma.
Evolution: Genes & Genomes. There may have been a genome duplication (BAALβ) around here ca 36.4 Ma (Landis et al. 2018).
[Halophytaceae [Didiereaceae + Basellaceae]]: ovule 1 per flower; fruit indehiscent, single-seeded.
Age. This node is around 38.1 Ma (Magallón et al. 2015) or 34.6 Ma (Tank et al. 2015: Table S2).
Evolution: Divergence & Distribution. At what level around here pantoporate pollen is an apomorphy is unclear (c.f. Prieu et al. 2017).
Phylogeny. For relationships in this little clade, still somewhat uncertain, see above.
[Halophytaceae + Basellaceae] [if this clade exists]: stomata paracytic; x = 12.
Evolution: Divergence & Distribution. For possible apomorphies, some of which depend on the outcome of optimisation procedures, see Anton et al. (2014).
HALOPHYTACEAE A. Soriano - Halophytum ameghinoi (Spegazzini) Spegazzini - Back to Caryophyllales
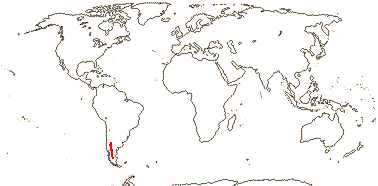
Annual herb, swollen roots 0; successive cambia +; wood rayless; ?mucilage; stomata paracytic (cyclocytic, parallelocytic); lamina with peripheral vascular bundles, phloem internal; plant monoecious; flowers sessile; nectary 0; staminate inflorescence: densely spicate; transverse bracteoles absent; P 4, barely petal-like, valvate-decussate; A alternate with P, anthers extrorse, dehiscing by pores by contraction of the connective, endothecium with frame-shaped thickening on anticlinal walls; pollen cuboid, hexapantoporate; pistillode 0; carpelate inflorescence: fasciculate, several flowers embedded in swollen leaf-bearing axis; P 0; staminodes 0; G [3], only adaxial carpel fertile, ?development, style +, short, stigmas spreading; ovule ?morphology; fruit a nutlet, swollen axis becoming hard, breaking up; ?tegmen bar thickenings; n/x = 12.
1 [list]/1. Argentina, Patagonia. Map: from Zuloaga and Morrone (1999).
Evolution: Ecology & Physiology. CAM photosynthesis is likely here, the plant having fleshy leaves and growing in dry to very dry habitats - and its δ13C values agree (Holtum et al. 2018).
Chemistry, Morphology, etc.. There are no endothecial thickenings at all on cells adjacent to the openings of the anthers (Pozner & Cocucci 2006).
Some general information is taken from Bittrich (1993b) and Nyffeler and Eggli (2010b); for stomata, see Di Fulvio (1975); Pozner and Cocucci (2006) describe the staminate flower in considerable detail, including the distinctive endothecial thickenings and anther dehiscence.
Details of embryology and female flower and fruit development are poorly understood.
Previous Relationships. Halophytaceae were included in Chenopodiaceae (Cronquist 1981). Relationships with Aizoaceae - also with rayless wood - have also been suggested (Gibson 1978).
[Didiereaceae + Basellaceae]: ?
Age. The age of this node is (28.5-)14.9(-3.9) Ma (Ocampo & Columbus 2010: 95% HPD), ca 33.9 Ma (Tank et al. 2015: Table S2) or about 36.8 Ma (Magallón et al. 2015).
DIDIEREACEAE Radlkofer, nom. cons. - Back to Caryophyllales
Plant woody; methylated flavonoids +; (wide-band tracheids +); cork cambium initiation precocious; tanniniferous cells +; mucilage ducts +; leaf stomata parallelocytic, transversely oriented; cuticular waxes as ribbons or rodlets; leaf vernation flat, lamina (terete, vascular bundles lateral); (transverse bracteoles absent); P 4-5; A with adaxial nectaries at base; pollen ± spinulate; G [(2-4)]; ovules 1(-2)/carpel; fruit 1-seeded (2-3); perisperm ± absent; x = 12 (?24).
6 [list: to subfamilies]/20 - three groups below. Madagascar, Southern and eastern Africa (Map: from Coates Palgrave 2002; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Bruyns et al. 2014b).
Age. Diversification began (24.4-)12.1(-2.4) Ma (Ocampo & Columbus 2010).
1. Portulacarioideae Applequist & R. S. Wallace - Portulacaria Jacquin —— Synonymy: Portulacariaceae Doweld
Shrubs to small trees; glabrous; (short shoots +); leaves opposite; plant (dioecious); infloresecence ± fasciculate, with involucral bracts; bracteoles connate; (P connate); A 5/4-10, (adnate to C); pollen tricolpate; fruit indehiscent; strophiole/aril 0; n = 22, 24, 36.
1/7. Southern Africa, epecially the southern part of the Namib, Kenya.
[Calyptrothecoideae + Didiereoideae]: seed with funicular strophiole or aril.
2. Calyptrothecoideae Pax & Gilg - Calyptrotheca Gilg
Shrubs to small trees/climbers; A many; pollen pantoporate; capsule dehiscing by six valves [septi- and loculicidally/], from the base, K strongly accrescent; n = ?
1/2. E. and N.E. Africa.
3. Didiereoideae Applequist & R. S. Wallace
Plant woody/succulent, (little branched), (deciduous); spiny; (facultative) CAM photosynthesis +; pith septate; stomata at bottom of tubular cavities in the cork (not, but lenticels - Alluaudiopsis); (phelloderm photosynthetic); short shoots +; plant (gyno)dioecious; inflorescence (fasciculate); A (6-)8 (9) 10(-12), in one whorl, basally connate; pollen 5-7-zonocolpate; stigma peltate/3 flattened lobes; fruit indehiscent; funicular papillae/mucilaginous hairs [= obturator]; cotyledons somewhat obliquely accumbent, somewhat longer than radicle; n = 24, often high-polyploid.
4/11. Southwest Madagascar. [Photos - Collection.]
Age. Diversification in Didiereoideae is estimated to have begun ca 17 Ma (Arakaki et al. 2011).
Age. The age for crown-group Didiereaceae is estimated to be (24.4-)12.1(-2.4) Ma (Ocampo & Columbus 2010).
Evolution: Divergence & Distribution. The distinctive 5-7-zonocolpate pollen of Didiereoideae is not an apomorphy for Didiereaceae (c.f. Nyffeler & Eggli 2010a, b).
Ecology & Physiology. CAM or facultative CAM occurs here (Winter 1979).
Plant-Animal Interactions. The spines om the branches of Didiereoideae may be protection against herbivory, especially by lemurs, including the extinct Hadropithecus and the extant Lepilemur, although the latter may have begun eating Alluaudia, currently an important food plant, only recently (Crowley & Godfrey 2012).
Genes & Genomes. N. Wang et al. (2018) found a genome duplication that characterized Didiereoideae; Calyptrotheca was not examined. Schill et al. (1974) provide some information on cytology.
Chemistry, Morphology, etc.. Rauh (1983) calls the spiky structures of Didiereaceae s. str. spines, being either leaves on short shoots or paired and stipular. Alluaudia has leaves subtending an axillary spiky structure, and later paired and apparently prophyllar leaves develop from an axillary bud immediately below it. This might suggest that the first spiky structure is a modified axillary shoot, i.e. = a thorn - or it could be a prophyll in a strange position...
The bracteoles immediately associated with each flower are in the median plane, and large bracteoles ("large bracts") may be obvious, as in Portulacaria.
Rauh and his collaborators have provided a great deal of information on Didiereaceae, see Rauh (1956: shoots, 1961: growth forms), Rauh and Schölch (1965: flowers and embryology) and Rauh and Dittmar (1970: ), see also Kubitzki (1993b), Schatz (2001) and Nyffeler and Eggli (2010b), all general, Hegnauer (1966, 1968, 1989: chemistry), Erbar and Leins (2006: floral ontogeny), and Sukhorukov et al. (2015: fruit and seed).
Phylogeny. This clade includes a morphologically distinctive monophyletic group of four Madagascan genera, Didiereaceae in the old sense, and immediately basal to them are some African ex-Portulacaceae. Of these, Ocampo and Columbus (2010) found a clade [Portulacaria + Ceraria] but Bruyns et al. (2014b), for example, found that the latter genus was embedded in the former. Relationships are [Portulacaria s.l. [Calyptrotheca + Didiereaceae s. str.]], and within the last group, Alluaudiopsis is sister to the rest, while the position of Decarya has only weak support (see e.g. Appelquist & Wallace 2000; Bruyns et al. 2014b).
Classification. Didiereaceae are expanded to include two genera of ex-Portulacaceae (see Appelquist & Wallace 2000, 2003); Appelquist and Wallace (2003) provide a subfamilial classification. See also Bruyns et al. (2014b) for genera.
Previous Relationships. Didiereaceae-Didieroideae are a morphologically odd group, and in the past they have even been included in Sapindaceae (e.g. van der Pijl 1957).
BASELLACEAE Rafinesque, nom. cons. —— Synonymy: Anrederaceae J. Agardh, Ullucaceae Nakai - Back to Caryophyllales
Vines/lianes, with swollen rhizomes or tubers; successive cambia +; cork cambium initiation timing?, in outer cortex; vascular bundles separate, bicollateral; leaf stomata paracytic, ?oriented; cuticle wax crystalloids 0; leaves (also opposite), vernation curved to ± conduplicate, (lamina margins serrate, with glands - Tournonia); inflorescence racemose, (cymose - Tournonia); flowers small; P (4-)5(-13), ± connate; (nectary +) [also inside A]; A 4-9, often equal and opposite P, adnate to them, basally connate, (reflexed in bud); tapetal cells multi-nucleate; pollen hexapericolpate/porate, (cuboid), (pantoporate - 3 spp.); G [3], (style single, short, branches short), stigma ± capitate or lobed; ovule single [per ovary], terminating axis [= basal], funicle stout, shortish; outer integument ca 2 cells across, inner integument 2-4 cells across, parietal tissue 2-8(?-16) cells across, nucellar beak +, no space between the integuments, placental obturator +; fruit an utricle, P persistent, (inner bracteoles surrounding fruit, fleshy/dry, winged), (fleshy annulus at style base - Anredera); testa in particular multiplicative, also tegmen, exotesta tanniniferous; perisperm scanty, starch grains clustered, embryo (spirally twisted), green; n = 12, 22, x = 12, nuclear genome [1C] (0.126-)1.617(-20.787) pg.
4/19: [list], Anredera (12). Africa, New World, apparently introduced into India-East Asia. Map: from Sperling (1987), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Fl. N. Am. vol. 4 (2003) and Moreira-Muñoz and Muñoz-Schick (2018). Photos: Collection.
Evolution: Ecology & Physiology. For CAM photosynthesis in Anredera baselloides, which occurs only when the plant is water stressed, see Holtum et al. (2018).
Chemistry, Morphology, etc.. Sperling (1987) reports both bracteoles and large, paired structures immediately surrounding the perianth (see also Eriksson 2007). How to interpret floral morphology varies - c.f. Friedrich (1956), LaCroix and Sattler (1988) and Sperling and Bittrich (1993), but the single ovule does seem to form on the floral apex (Sattler & LaCroix 1988).
For general information, see Bogle (1969), Sperling (1987), Eriksson (2007), and Nyffeler and Eggli (2010b). For chemistry, see Hegnauer (1964, 1989), for wood anatomy, see Carlquist (1999), for successive cambia, see Jansen et al. (2000c), for leaf development, see Hernandes-Lopes et al. (2015), for ovules, see Rocén (1927: outer integument "viel" cells across).
Classification. Eriksson (2007) includes a synopsis of species included in the family.
[Talinaceae [Portulacaceae [Anacampserotaceae + Cactaceae]]] / the ACPT clade: plant mucilaginous; secondary growth normal; pericyclic fibres 0; stomata parallelocytic; A development from a ring primordium, many; ovary septate, style branched; ovules ?several/carpel; fruit covered by dried P, pericarp 2-layered, exocarp ± caducous.
Age. For this node, Ocampo and Columbus (2010) suggested an age of (27.8-)15.2(-5.4) Ma, Arakaki et al. (2011) an age of (42.1-)39.4(-36.7) Ma, Magallón et al. (2015) an age of about 33.3 Ma, and Tank et al. (2015: Table S2) an age of around 30.8 Ma.
Chemistry, Morphology, etc.. Ex-Portulacaceae in the pectinations basal to Cactaceae have the pericarp strongly differentiated into two layers.
For anatomy, see Ogburn (2007) and Ogburn and Edwards (2009).
TALINACEAE Doweld - Back to Caryophyllales
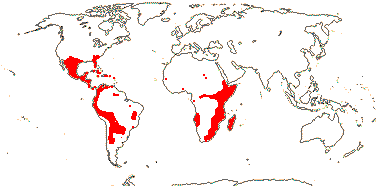
Herbs to (lianescent) shrubs, underground parts often tuberous; cork cambium initiation timing variable, (cortical); tanniniferous cells +; C3/CAM cycling; petiole bundle arcuate, with wing bundles; stem stomata parallel to stem axis, leaf stomata un- or weakly transversely oriented, morphology variable; leaf vernation revolute; (plant dioecious - some Talinum [T.]); P quincuncial; A ca 15 to many; archesporial cells uniseriate; pollen pantoporate [T.]; G [3], placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker]/carpels initiate separately around flat ± persistent floral apex [= cup-shaped]; ovules with parietal tissue 1-2 cells across, epidermal cells radially elongate, nucellar cap to 4 cells across; fruit (mucilaginous berry - some T.), epidermis papillate; seed black, strophiolate [= funicle?], exotesta well developed, with tanniniferous "stalactites", endotegmen thickening slight; n = 8, x = 12.
2 [list]/27: Talinum (26). America and Africa, including Madagascar. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. Vol. 1 (2003), Fl. N. Am. Vol. 4 (2003) and Tropicos (consulted iii.2014). Photo: Collection, but not all images.
Age. The age of crown-group Talinaceae is estimated at (18.3-)9.1(-2) Ma (Ocampo & Columbus 2010: see sampling) or the very different (33.2-)29.9(-26.3) Ma (Arakaki et al. 2011).
Evolution: Ecology & Physiology. Talinum triangulare is a facultative CAM plant, and CAM recycling may be induced by increased salinity in the environment (Winter & Holtum 2014; Montero et al. 2018).
Chemistry, Morphology, etc.. The roots are apparently polyarch (von Poellnitz 1934). Young leaves may have paired, axillary scales; these are the very tips of the prophylls.
See von Poellnitz (1934) and Nyffeler and Eggli (2010b), general, also Hernandes-Lopes et al. (2015: leaf development), Vanvinckenroye and Smets (1996) and Ronse De Craene (2020), both floral development, Meunier (1890: ovules and seeds), and Maheswari Devi and Pulliah (1975) and Veselova et al. (2012), both embryology.
Phylogeny. Talinella is nested within Talinum (Nyffeler 2007; Nyffeler & Eggli 2010b), within which it is now included.
Classification. See Nyffeler and Eggli (2010b). Some species once placed in Talinum are now in three separate genera in Montiaceae.
[Portulacaceae [Anacampserotaceae + Cactaceae]]: (sclereids in stem cortex); stem stomata 0[?]; leaves with axillary bi- or multiseriate hairs/scales; A development centrifugal; style lobed [?level]; outer integument ca 2 cells across, inner integument 2(-3) cells across.
Age. The age for this clade is some (26.6-)14.3(-5.1) Ma (Ocampo & Columbus 2010) or (39.6-)37(-34.4) Ma (Arakaki et al. 2011).
Evolution: Facultative CAM is notably common here?
Genes & Genomes. There is variation in the chloroplast infA gene in this clade, with both insertions and duplications occuring (Ocampo 2009).
aceae are uniseriate, although those of Pereskiopsis are biseriate at the base. Chorinsky (1931) remains a useful early study of these structures, which are never vascularized (see also Rutishauser 1981). There are scattered stomata on the peduncle of Portulaca and on the stem of some Anacampseros (pers. obs.), but they are by and large uncommon in the basal clades here (e.g. Ogburn & Edwards 2009).Phylogeny. For relationships in this area, for some time unclear, see above.
PORTULACACEAE Jussieu, nom. cons. - Portulaca L. - Back to Caryophyllales
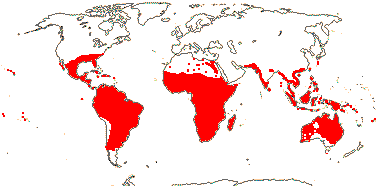
Succulent (annual) herbs; (C4 photosynthesis +); cork cambium initiation delayed; (wood rayless); (internodes short); ?stomata; leaves (opposite), blade (terete, vascular bundles peripheral), vernation flat to revolute, (axillary hairs 0); inflorescences terminal, ± capitate (cymose), (flowers single), with involucre; (transverse bracteoles absent); (P 4-8), (connate), with a single trace; tapetal cells multi-nucleate; G [(2-)5(-8)], semi-inferior, placentation also parietal/basal; ovules with parietal tissue ca 5 cells across, in radial rows, or ca 8 cells across, not in rows [P. pilosa], (nucellar cap 2 cells across); embryo sac with chalazal haustorium; bracts and K deciduous in fruit, capsule circumscissile, pericarp not 2-layered; seed with hilar aril; testa (smooth, featureless), cells often variously tuberculate (projections long, almost hair-like), anticlinal walls often sinuous; n = (8-)10, x = 12; (plastid transmission biparental); five copies of the ppc-1E1 gene lineage [?or whole Portulacineae].
1/107: [list]. Worldwide, but especially tropical and subtropical North and South America, (weedy). Map: approximate, from Legrand (1962), Geesink (1969), Frankenberg and Klaus (1980), Gilbert and Phillips (2000), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and FloraBase (consulted ii.2010). [Photo - Collection, but not all.]
Age. Ages suggested for crown-group Portulaca are (18.5-)9.6(-2.0) Ma (Ocampo & Columbus 2010) and (43-)23(-6.9) Ma (Ocampo & Columbus 2012: improved sampling, calibration on Hawaiian islands), or (21-)17.6(-14.3) Ma (Arakaki et al. 2011).
Evolution: Ecology & Physiology. There have been several switches to C4 photosynthesis dated from ca (33.8-)28.8(-23.8) Ma (Ocampo & Columbus 2009) to about one third this age (Christin et al. 2011b) here. Ocampo et al. (2013) subsequently suggested perhaps a single origin that could be dated to ca 23 Ma; C3-C4 intermediates may be derived. They noted that there were two C4 subtypes and three anatomical variants in the genus (see also Voznesenskaya et al. 2010), and there are also facultative CAM/C4 plants (Winter & Holtum 2014, see also 2017). However, Christin et al. (2014b, q.v. for ppc-1E1 duplication) thought that CAM evolved before C4 photosynthesis here, and Winter et al. (2019b, also a; Gilman et al. 2022) suggested that facultative CAM was ancestral to C4 photosynthesis, being found in all the species of Portulaca examined, and they noted that CAM could be found in leaves with any of the other photosynthetic types, C3, C4 and C3-4 intermediates. Moreno-Villena et al. (2022; see also Gilman et al. 2022; X. Wang et al. 2023) have found that in P. oleracea, at least, C4 and CAM metabolism are fully integrated and elements of both pathways take place within the same cell under drought conditions (when well-watered, C4 photosynthesis predominates) - genes related to C4 and CAM phosphoenolpyruvate carboxylase activity are more abundant in mesophyll cells, while decarboxylation and Calvin cycle genes are largely restricted to bundle sheath cells - indeed, in taxa like P. oleracea and P. grandiflora there may be C4 + CAM in the leaves and C3 + CAM in the stems (references in Holtum 2023), and in Trianthema portulacastrum-Aizoaceae these photosynthetic pathways may also co-occur in the one plant. Wang et al. (2023) discuss gene evolution in P. oleracea in the context of the CAM and C4 pathways and of the two gene duplications that they think have occured here, with there being PEPC duplicates associated with the Po-α duplication in both pathways and CAM-specific beta carbonic anhydrases associated with the earlier P-β event.
Cushion plants are disproportionately common in Portulaca (Boucher et al. 2016b).
Genes & Genomes. x = 9 was suggested by Oliveira Marinho et al. (2019). For the increased rate of molecular evolution in the herbaceous Portulacaceae compared to the woody Cactaceae, see Y. Yang et al. (2015).
X. Wang et al. (2023) suggested that there were two duplications in Portulaca oleracea. One, P-β, they thought was also to be found in Cactaceae, but they dated it to ca 66.3 Ma, far older than age estimates for the Cactaceae-Portulacaceae node (see above). This duplication may be the same as the PORT1 duplication of Yang et al. (2017) which the latter group placed at the Montiaceae to Cactaceae node, although even this larger clade is also substantally younger than 66.3 Ma. The other duplication, the Po-α clade, Wang et al. (2023) dated to ca 7.7 Ma, but they could not find it in P. amilis, and this is a member of the other major clade in the genus recovered by Yang et al. (2017) and also a CAM-C4 plant.
Chemistry, Morphology, etc.. For leaf anatomy and development, where there is consderable variation only in part connected with photosynthetic pathways, see Voznesenskaya et al. (2010), Hernandes-Lopes et al. (2015) and Melo-de-Pinna (2016).
The development of the perianth is much retarded relative to that of the androecium (dos Santos et al. 2012).
See Sharma (1954) for floral anatomy (the single traces to the P members divide into three), Ronse De Craene (2020: Figs 2, 6) for floral development, Meunier (1890), Rocén (1927), Kajale (1940c) and Ocampo (2013) for ovules and seeds, the last in particular for details of the seed surface, and Nyffeler and Eggli (2010b) for general information.
Phylogeny. Relationships within Portulaca are discussed by Ocampo and Columbus (2009). The genus consists of a clade with opposite leaves, in turn made up of Australian and African-Asian clades, and a clade with spiral leaves (Ocampo & Columbus 2012; Ocampo et al. 2013; Ocampo 2018).
Previous Relationships. For taxa included in earlier circumscriptions of Portulacaceae, see e.g. Carolin (1993) and Nyffeler and Eggli (2010b).
[Anacampserotaceae + Cactaceae]: (cork cambium initiation precocious).
ANACAMPSEROTACEAE Eggli & Nyffeler - Back to Caryophyllales
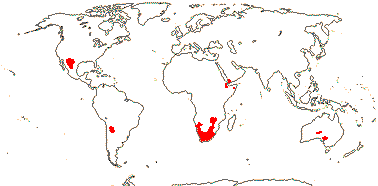
Subshrubs with ± tuberous roots, (stems fleshy), (rosette plants), internodes short; cork cambium (cortical); (wood rayless); (wide-band tracheids +); (sclereids +); (spirally-thickened fiber-sclereids in rays); (facultative CAM/?C4 photosynthesis); (blade ± terete, with peripheral vascular bundles), leaf stomata transversely oriented; leaves (opposite), lamina ± terete, (axillary hairs 0, but concave adaxial scale + - Avonia); upper and lower bracteoles in same plane; A 5-many, (outer 5 A alternating with P members), basally connate; G [(2) 3], placentation axile, septae at same level as floral apex and carpel lobes [= salt-shaker]/carpels initiate separately around flat ± persistent floral apex [= cup-shaped], style solid, stigmas papillate, receptive on both surfaces; parietal tissue ca 2 cells across, nucellar cap 0; (exocarp and endocarp not separating - Grahamia); seeds pale-coloured, (winged), exotesta ± separating from endotesta, cells large, thin walled, unlignified, (bullate to long-papillate); embryo only slightly (much) curved, not surrounding the poorly developed perisperm; x = 9 (?10); chloroplast ndh gene complex 0 [missing/non-functional: 1 record].
3 [list]/36 (61): Anacampseros (34/59). Very scattered: C. and S. Australia, Somalia to South Africa (most species), S. South America, N. Mexico and S.W. U.S.A.. Map: from Gerbaulet (1992a, 1993) and Fl. N. Am. vol. 4 (2003).
Age. The age of crown-group Anacampserotaceae is (22.6-)11.4(-3.2) Ma (Ocampo & Columbus 2010: Talinopsis sister to Grahamia, etc.) or (34.2-)31(-27.8) Ma (Arakaki et al. 2011).
Evolution: Divergence & Distribution. Anacampserotaceae probably arose in the New World; for more on the biogeography of this widely scattered clade, see Gerbaulet (1992b) and Holtum (2023).
Ecology & Physiology. Gerbaulet (1993) discussed the ecology of African Anacampserotaceae. Facultative CAM photosynthesis occurs here (Winter & Holtum 2017; see also Holtum 2023; Gilman et al. 2023).
The seeds have very thin testas, so it would be no surprise if at least some of the family have very fast germination (1> day: Kadereit et al. 2017 and references).
Genes & Genomes. For the loss of plastid ndh genes, see Mower et al. (2021).
Chemistry, Morphology, etc.. For the flat scale at the adaxial base of the leaf, see Melo-de Pinna et al. (2016: Anacampseros).
See also von Poellnitz (1933), Gerbaulet (1992a), Rowley (1994), Eggli (2002), Nyffeler and Eggli (2010a, b) and Frandsen (2017: images) for general information; for anatomy, etc., see von Poellnitz (1933), for floral development, see Vanvickenroye and Smets (1999), and for some ovule morphology, see Rocén (1927).
Phylogeny. Nyffeler (1997) included six species of Grahamia (old style) in his study, and they formed a perfect pectination; at least some of the nodes had good support. Talinopsis frutescens is sister to the rest of the family (Nyffeler & Eggli 2010b; Ocampo & Columbus 2010
Classification. For genera, see Nyffeler and Eggli (2010a, b).
*CACTACEAE Jussieu, nom. cons. - Back to Caryophyllales
?Habit; (Alkaloids +); C3/CAM cycling; rays wide and tall; calcium oxalate as whewellite [CaC2O4.H2O]; extensive primary expansion of the pith; xylem fibres nucleate [?level: remain alive, also pith and ray cells]; wood with vessels, fibers, scanty vasicentric parenchyma, (bands of nonlignified parenchyma) [= fibrous wood], (sclereids in phloem); nodes often 1:2-10; epidermis thick-walled, hypodermis with druses, numerous (0); leaf stomata unoriented; cuticular waxes as ribbons or rodlets, also thick prostrate plates; uni- or biseriate hairs +; axillary short shoots + [= areoles], with spines [?= leaves], (areoles long-lived, spines etc. continuing to be produced), surface smooth, epidermal cells undifferentiated, also photosynthetic leaves, stipules +; long shoot leaves large, flattened, petiolate; median bracteoles 0; cauline hypanthium ± +; "P" several-numerous [modified bracts], spiral, outer sepaline and inner petaline; A development initially from five separate primordia; pollen perforate, ± spinulose; nectary rather disc-like; G [5-many], ±surrounded by stem tissue [pericarpel; with sessile, flattened leaves, areole axillary, with some spines, etc.], (inflorescence proliferating from leaves on ovary); G incompletely septate, placentation ± basal/parietal [see below], stigmatic lobes 7-21, stigma ?wet, papillae +, uni- or multiseriate; ovules in three ranks, many/carpel, parietal tissue 1-2 cells across, nucellar cap +, nucellar epidermal cells radially elongated, lateral epidermal cells anticlinally divided [?all], funicle rather short and stout, obturator of short hairs; (archesporium multicellular); fruit baccate, pericarp not 2-layered; seed ?colour; testa multiplicative, to ca 4 cells across, exotesta thick-walled, with tanniniferous "stalactites" [?level], endotegmen ± crushed; suspensor uniseriate; x = 11, chromosomes 3> µm long, nuclear genome [1 C] (0.243-)1.806(-13.428) pg; plastome with 6 kb inversion in large single copy region.
139/1,866 (124-150/1,438-1,882): [list] - five groups below. Nearly all New World, esp. S.W. U. S. A., arid Mexico, Andean Peru and Bolivia, eastern Brazil, usu. arid conditions. [Photos - Collection.]
Age. Diversification in Cactaceae is thought to have begun in the mid-Caenozoic ca 30 Ma (Hershkovitz & Zimmer 1997, q.v. for other estimates) or ca 29.1 Ma (Tank et al. 2015: Table S1). Ocampo and Columbus (2010) suggest very much younger ages of (19.1-)10(-3.1) Ma while Arakaki et al. (2011) estimated an age of (30.5-)28.6(-26.7) Ma; (37.1-)26.9(-16.7) Ma is the estimate in Hernández-Hernández et al. (2014).
Note: See de Vos et al. (2025) for details of the groupings below; "*" means ± weak support for the monophyly of the taxon/node so signified, "" that the group so signified is paraphyletic (a temporary measure).
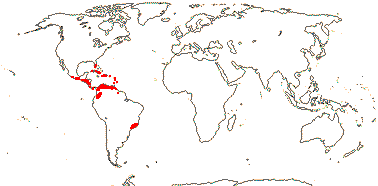
1. Leuenbergerioideae Mayta & Molinari - Leuenbergeria Lodé
± Lianescent; cork cambium initiation precocious, cortical, epidermis soon disappearing; sclereids isodiametric; stem stomata 0; plant deciduous, leaves well developed, venation pinnate; (P laciniate); pollen colpate; G semi-inferior (inferior); nucellar cap 0, "funicular protuberance" +; cotyledon:hypocotyl s.l. = ca 2:1; plastid transmission biparental.
1/8. Mexico and the Caribbean, Leuenbergeria aureiflora Brazil. Map: from Leuenberger (1986, 2008) and Edwards et al. (2005). Photo: Leaf, Flower, Fruit.
Age. The crown-group age may be (28.1-)25.8(-23.5) Ma (Arakaki et al. 2011).
[Pereskioideae [Opuntioideae [Maihuenioideae [Blossfeldioideae + Cactoideae]]]] / caulocacti: cork cambium initiation delayed, stem epidermis persistent, cuticle often thick, stomata numerous, parallel.
Age. Arakaki et al. (2011) suggested an age of (28.7-)27(-25.3) Ma and Hernández-Hernández et al. (2014) an age of (28.5-)20.5(-16.5) Ma for this node.
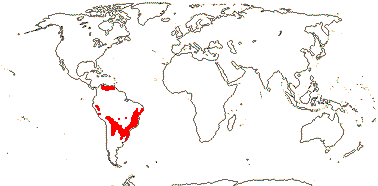
2. *Pereskioideae Engelmann - Pereskia Miller
± Lianescent to tree-like, (roots tuberous); sclereids (aggregated) fusiform; (?stipular spines + - P. aculeata), leaves well developed, lamina supervolute, venation pinnate; A centrifugal, from 5 primordia; pollen (tri-) 6-15-colpate; G with apical septae [= invagination of apex, ?level]; ovules (2/carpel - P. aculeata) [2/3/2]; "berry" with gelatinous pericarp and columella; cotyledon:hypocotyl s.l. = ca 6:4.
1/9. Andean, S. South America. Map: from Leuenberger (1986, 2008) and Edwards et al. (2005).
Age. Arakaki et al. (2011) suggest an age of around 27-25 Ma for the crown group.
[Opuntioideae [Maihuenioideae [Blossfeldioideae + Cactoideae]]] / caulocacti: stems succulent; CAM or facultative CAM +; (primary root growth determinate); wood also with wide-band tracheids [at least when plant is young]/vessels and abundant nonlignified parenchyma [= parenchymatous wood], hypodermal collenchyma +; cortical chlorenchyma forming mesophyllar tissue with intercellular spaces; stem ± fleshy, rounded in t.s., mucilaginous, internodes short; stomata opuntioid [subsidiary cells distinct, but apart from the innermost pair of cells more or less randomly arranged - ?here]; leaves rudimentary, terete, soon deciduous, stipules 0; inflorescences axillary [in areoles], flowers "solitary"; A from ring primordium; stigma commissural [?Maihuenioideae]; ovary inferior, placentation ± parietal; perisperm ± 0 [?here].
Age. Arakaki et al. (2011) suggest an age of around (26.5-)25.3(-24.1) Ma for this node while the age is around 7.7 Ma in Ocampo and Columbus (2010) - and if there is a clade [Maihuenioideae + Opuntioideae] it may be ca 5.7 Ma...
3. Opuntioideae Burnett
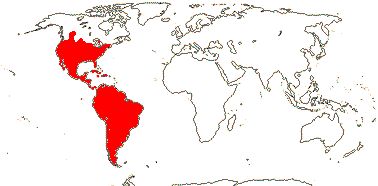
Full CAM photosynthesis +; stems articulated, sympodial; wide-band tracheids only in rays; vascular bundles lacking cap of phloem fibres; calcium oxalate in stem hypodermis, as druses or spherical clusters; (cork cambium initiation precocious); cortex not thick, cells not collapsible; (no stem stomata); leaf stomata parallel; spines retrorsely barbed, inc. minute bristle-spines [= glochids]; (hypanthium +, short); pollen (pantoporate), exine perforate or not, ribbed/reticulate/smooth; ovule with massive suprachalazal zone, funicle papillate [?obturator], seeds white, ± covered by bony funicle ["aril"], (hairy); testa lignin with guaiacyl/syringyl units [= normal], outer periclinal wall of testa little thickened; (cotyledons are storage organs of seed); n = >11, much polyploidy, deletion of the chloroplast accD gene.
15/220-349. From almost the Arctic Circle to Argentinian Patagonia. Map: see Thorne (1973) and Fl. N. Am. vol. 4 (2003). [Photo - Flower, Flower.]
Age. Crown-group Opuntioideae have been dated to (18-)15.1(-12.1) Ma (Arakaki et al. 2011), (13.8-)9.3(-5.9) Ma (Hernández-Hernández et al. 2014) and ca. 11.1 Ma (Las Peñas & Bernadello 2021).
3A. Opuntieae de Candolle —— Synonymy: Nopaleaceae Schmid & Curtman, Opuntiaceae Desvaux
Up to small trees; stem segmented (not), segments flattened (terete.
7/: Opuntia (200). Southern Canada to South America, the Caribbean.
*[Cylindropuntieae + Pterocacteae]: ?
3B. Cylindropuntieae Doweld
(leaves large, with lamina, venation parallel [modified rudimentary leaves], subpersistent or not); fruit (dry, burr-like); some chloroplast ndh genes 0.
5/79: Cylindropuntia (40). Esp. Western North America to northern Central America, Quiabentia Argentina and Paraguay.
Age. Crown-group Cylindropuntieae are ca 9.1 Ma (Hernández-Hernández et al. 2014) or (20.4-)17.9(-14.8) Ma (Majure et al. 2019).
3C. Pterocacteae Kuntze (inc. Tephrocacteae)
Shrubby geophytes, cushion plants, (small trees); roots tuberous; stems globose to cylindrical (not segmented); (central spine of areole flattened); (flowers apical); (seeds flattened, winged - Pterocactus); n = 11 (-77), Cx = 0.90-2.94 pg.
5/33: Maihueniopsis (15). Southern Andes/adjacent lowlands, S. to Patagonian Argentina.
Age. Crown-group Pterocacteae are perhaps some 9.4 Ma (Las Peñas & Bernadello 2021).
[Maihuenioideae [Blossfeldioideae + Cactoideae]]: (wood with wide-band tracheids and parenchyma in secondary xylem [= wide-band tracheid wood]); inflorescence not proliferating; testa interstitially pitted or cratered, exotestal cells with outer periclinal walls much thickened.
Age. An age for this node is some (25.4-)24.4(-23.4) Ma (Arakaki et al. 2011).
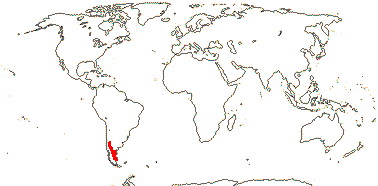
4. Maihuenioideae P. Fearn - Maihuenia F. A. C. Weber
Plant densely caespitose; full CAM photosynthesis 0; taproot fleshy; cork cambium initiation precocious; large mucilage reservoirs in stem medulla and cortex; phloem and cortical sclerenchyma 0, collenchyma, hypodermis 0; tangential walls of epidermis thickened, hypodermis and collenchyma 0; stem ± segmented, terete, branched, areolate; areolar crypts + [containing stomata, photosynthetic parenchyma at base]; leaves to ca 1 cm long, ± persistent, with peripheral reticulum of bundles, xylem external, central mucilage reservoir +, stomata transverse, (areoles producing leaves); pollen tricolpate; funicles in fruit long, mucilaginous.
1/2. S. Argentina and Chile. Map: from Leuenberger (1997, 2008).
Age. The two species of Maihuenia diverged (2-8-)1.4(-0.3) Ma (Hernández-Hernández et al. 2014).
[Blossfeldioideae + Cactoideae]: Full CAM photosynthesis +; plant essentially leafless [leaves up to 1.5(-2.5) mm long when mature]; calcium oxalate often as weddellite [CaC2O4.2H2O]; cortex massive, chlorophyllous in part; stem stomata unoriented; spine epidermal cells un- or variously differentiated; pollen 3-pantocolpate(-porate), (in tetrads); funicle length?; seeds with a conspicuous spongy hilum-micropyle region; (plastid transmission biparental), ndh gene complex 0 [missing/non-functional]t, rpl23 pseudogene/lost, ropC1 intron 0, ribosomal RNA genes in SSC, not in IR.
5. Blossfeldioideae Crozier - Blossfeldia liliputiana Werdermann
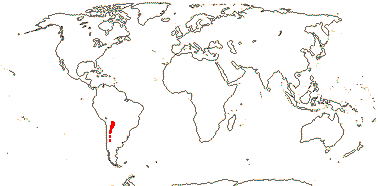
Plant small, (hummock-forming); tuberous roots 0; cortical bundles 0, other vascular bundles lacking cap of phloem fibres; calcium oxalate druses 0; hypodermal collenchyma 0; stems subspherical, small [12> mm across], areoles ± flat, spiral, leaves minute, areolar spines 0, short hairs +; epidermis (inc. cuticle) thin-walled, soon replaced by cork cambium; stem stomata few, in areolar crypts, photosynthetic parenchyma at base of crypts; ?pollen; fruits with fleshy scales overtopping the rim of the umbilicus [clarify]; seeds with a funicular aril [strophiolate]; testa with one short narrow projection per cell ["hairy"]; n = 33 [hexaploid], rpoC1 intron +.
1/?1. Bolivia to Argentina, eastern Andes. Map: from Leuenberger (2008).
6. *Cactoideae Eaton
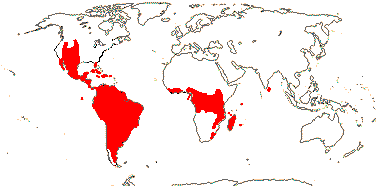
Cortical and medullary vascular bundles +, some cortical cells collapsible; stem ribbed; seed coat mucilaginous [?extent].
To be reduced... ( 1,2,3,4-tetrahydroisoquinoline alkaloids +); primary root growth determinate (not); (stem dichotomising); (raphides +); cortex broad, succulent; (areoles dimorphic); tapetal cells binucleate; pollen 3-pantocolpate(-porate), (in tetrads); ovules (circinotropous); parietal tissue 1-4 cells across; seeds black to brown, (arillate), funicular obturator +; testa ?operculate, lignin usu. with catechyl units (guaiacyl/syringyl; 5-hydroxyguaiacyl [5H] units); (perisperm and esp. endosperm slight - ?level), hypocotyl massive [storage - ?level];
112/1,498. New World, v. few Old World. Map: see Thorne (1973), Barthlott (1983), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1. 2003 [Rhipsalis], and Fl. N. Am. vol. 4. (2003). Photo: Plant, Flower.
Age. Crown-group Cactoideae are (23.5-)21.8(-20.1) Ma (Arakaki et al. (2011) or (24.5-)17.1(-12.7) Ma (Hernández-Hernández et al. 2014) - check Bloss both.
/--->*[Lymanbensonieae + Copiapoeae]: ?
6A. Lymanbensonieae N. Koroktova & W. A. Barthlott
Plant tree-like / epiphyte; (flower initially enclosed in pedicel - Calymmanthium).
2/6: Lymanbensonia (5). Bolivia, Ecuador, Peru.
6B. Copiapoeae Doweld - Copiapoa Britton & Rose
Plant much-branched and forming large cushions to stems single; (stem not ribbed - C. humilis>); pericarpels smooth, scales at apex, no spines; fruit opening apically.
1/32. Central and north Chile, the western Atacama Desert.
[Cacteae [Phyllocacteae [[Fraileeae + Rhipsalidae] [Notocacteae + Cereae]]]]:
6C. Cacteae Reichenbach
Medullary vascular bundles 0, vascular bundles lacking cap of phloem fibres; hypodermis poorly developed; (tubercle axils with glands); spines (hooked - Cochemiea) pericarpel naked; seed with hilum and micropyle separated; exotestatal cells with outer periclinal walls entire to pitted [whole wall thin]; chloroplast PEP subunit β' rpoC1 intron lost.
S. Canada southwards, esp. Mexico, Brazil, Peru-Bolivia.
Age. Arakaki et al. (2011) suggest an age of (21.7-)19.7(-17.7) Ma and Hernández-Hernández et al. (2014) an age of 21.9-)15.3(-10.9) Ma for this node.
6C1
(Massive) barrel cacti; (stem not ribbed), (tubercles much elongated - Astrophytum caput-meduae), (covered in hairs), areoles with nectaries - Sclerocactus); fruit dry.
5/29: Sclerocactus (15), Astrophytum (6). Mexico, Southwest U. S. A..
6C2 Ferocactinae Buxbaum
Plant forming large cushions to single-stemmed, barrel/columnar; stem (not ribbed/unclear); (tubercles very long - Leuctenbergia), (spines hooked), (nectaries above areoles)pericarpels smooth; (fruit opening through basal pore - Thelocactus).
6/60: Ferocactus (30), Thelocactus (13). Mexico, southwest U. S. A..
6C3. Cactinae Britton & Rose / inc. Mammilloid clade
Plant globular or columnar; stems (spirally) prominently areolate, (ribbed), (areoles with spines and flowers spatially separated, with connecting groove or not); (resin +, white, Mammillaria).
20/345: Mammillaria (135-200), Corypantha (56), Cochemeia (48), Pelycyphora (21),Turbinicarpus (15). North and Central America, Caribbean.
*[Phyllocacteae [[Fraileeae + Rhipsalidae] [Notocacteae + Cereae]]]:
6D. Phyllocacteae Salm-Dyk (inc. Hylocereae)
Plants epiphytes, stem (flattened); medullary vascular bundles 0 [pith narrow]; calcium oxalate as weddellite and whewellite; raphides +; pollen 3-colpate.
8/73. .
6D1. "Corrycactinae" Buxbaum
Small shrubs to tree-lie, (epiphytic); stem usu. segmented, (as flat, rather thin cladodes).
7/; . Chilean coast, Patagonian steppes to Amazonian forest.
[Leptocereinae [Hylocereinae + Echinocereiunae]:
6D2. Leptocereinae Eggli, Nyffeler & J. M. de Vos
Shrubs to trees; stem usu. segmented, (areoles with hairs alone); flowering nocturnal, flowers white (not); (pericarpel spineless - Castellanosia).
3/28: Leptocereus (20), Armatocereus (7). The Caribbean, Ecuador, Bolivia, Peru.
[Hylocereinae + Echinocereinae]:
6D3. Hylocereinae Britton & Rose
Plant columnar (climbers - Selenicereus), (semi-)epiphytic); stem columnar, segmented or not, (stem flattened, spines +/0); flowering nocturnal, flowers very large, white, sphingophilous.
9/: Selenicereus (31). Esp. tropical semi-humid habitats in Mesoamerica, the Caribbean and north South America.
6D4. Echinocereinae Britton & Rose
Terrestrial dwarf to medium-sized shrubs to large candelabriform branched trees or solitary columns ((semi-) epiphytic); stems usu. unsegmented, (few-ribbed, rooting along their length - Deamia); flowers often large, nocturnal, often chiropterophilous.
15/. North to Central America, esp. Mexico.
[[Fraileeae + Rhipsalidae] [Notocacteae + Cereae]]:
6E. Fraileeae B. P. R. Chéron - Frailea Britton & Rose
Plant small; spherical (short-columnar), branched; stem (spirally) ribbed or not, ± smooth to areolate; (flowers cleistogamous).
1/19. Central and east South America.
6F. Rhipsalideae de Candolle
Plant epiphytic (epilithic / climber); stem often articulated, terete / flattened (and lobed like a fern frond) / (ribbed); wide-band tracheids 0; wood parenchymatous, dimorphic; medullary vascular bundles 0 [pith narrow]; (flowers monosymmetric); pericarpel naked, (immersed - some Rhipsalis); seeds with hilum and micropyle fused, mucilaginous sheath +.
4/58: Rhipsalis (37-44), Schlumbergera (8). South America, esp. Bolivia, a few Central America, Rhipsalis to North America, Africa, Madagascar, Sri Lanka.
6G. Notocacteae Buxbaum
Plant globose, cylindrical, branched or not; stem (spirally) ribbed, (strongly areolate); pericarpels variously opening, (in central depression of stem - Yavia).
4/137: Eriosyce (73), Parodia (62). Central and southern South America.
6H. Cereeae Salm-Dyck / BCT Clade / [Browningieae + Cereae + Trichocereeae] —— Synonymy: Cereaceae de Candolle & Sprengel
Pollen 3-colpate; pericarpel, hypanthial tube, pericarp of unripe fruit lacking areoles and stiff spines.
35/445 - 7 subtribes. Mexico, Central America, South America, the Caribbean.
6H1. Uebelmanniinae N. P. Taylor - Uebelmannia Buining
Plant unbranched, spherical to shortly columnar; stem (not ribbed, with prominent areoles); pericarpel areole wooly, with a few bristles: fruits dry.
1/4. Brazil, Central-North Minas Gerais.
[Aylosterinae [Rebutiinae [[Gymnocalyciinae + Cercinae] [Reicheocactinae + Trichoereinae]]]]: ?
6H2. Aylosterinae N. P. Taylor - Aylostera Spegazzini
Plant small, ± globular; stems with ribs or not; pollen 6-8 colpate; pericarpels hairy.
1/26. E. Andes - central Bolivia and northwest Argentina.
[Rebutiinae [Gymnocalyciinae + Cereinae] [Reicheocactinae + Trichocereinae]]]: ?
6H3. *Rebutiinae Donald (inc. Browningieae Buxbaum)
Plant small, spherical (large trees - Browningia); stem (spirally) ribbed/n0t; pollen 3-colpate; pericarpels of scales, glabrous.
3/47: Weigartia (33), Browningia (11). Ecuador to Chile, Argentina, Paraguay, the Galapagos Islands.
[[Gymnocalyciinae + Cereinae] [Reicheocactinae + Trichocereinae]]: ?
*[Gymnocalyciinae + Cereinae]: ?
6H4. Gymnocalyciinae N. P. Taylor - Gymnocalycium Mittler
Plant small, solitary or cushion-forming; (areoles very prominent); flower buds with broad scales, lacking hair or spines; long polysaccharidic threads at margin of stomium and septum.
1/65. Plains and Andean slopes of eastern South America from southern Brazil and Uruguay to Argentina (well into Patagonia).
6H5. Cereinae Britton & Rose
cephalia common, terminal and lateral
20/: Pilosocereus (60), Melocactus (40), Cereus (33). Mexico to the eastern Andes and S.E. South America, the Caribbean.
[Reicheocactinae + Trichocereinae]: ?
6H6. Reicheocactinae Eggli, Nyffeler & J. M. de Vos - Reicheocactus famatimensis (Spegazzini) R. Schlechter
Plant small, single-stemmed (cushion-forming), main root tuberous; stems spherical-short columnar, apex strongly sunken, spines pectinate, weak and hardly pungent; pericarpel scales fleshy, with abundant wool and hairs in their axils, overtopping the rim of the umbilicus.
1/1. East Andean slopes, N.W. Argentina
6H7. Trichocereinae F. Buxbaum (Trichocereeae)
Plants small, globose, to tree-like, columnar; stem (areoles prominent); (cephalia +); (floral tube long); fruits with fleshy scales overtopping the rim of the umbilicus.
23/: Cleistocactus (28), Echinopsis (20). South American, Harrisia to the Caribbean and and Florida.
Evolution: Divergence & Distribution. Arakaki et al. (2011) suggested a number of clade ages; see also Nyffeler and Eggli (2010a) for dates.
The diversification rate may have increased around here some (33.3-)30.1(-28.8) Ma - or maybe in a clade including Portulacaceae (Magallón et al. 2018); Tank et al. (2015: Table S1) also recorded a notable increase in diversification in Cactaceae. Branch lenths are notably short - and relationships unclear - in parts of the family (de Vos et al. 2025). The evolution of a sort of hypanthium and the development of a long floral tube may have been a key innovation for Cactoideae allowing a greater diversity of pollinators for the flowers; Cactoideae are much more speciose than other clades in this area of the phylogeny (Schlumpberger 2012). Hernández-Hernández et al. (2014) thought that initial adaptation to dry conditions, probably in the central Andean region around 30 Ma, was important in the evolution of the family, subsequent diversification occurred rather later, 15-10 Ma, and a substantial component of this was in clades that had moved to North America. In Cactoideae in particular, the evolution of a diversity of growth forms and adaptations to various pollinators, particularly bats and birds, also played major roles (Hernández-Hernández et al. 2014: more on ages, diversification rates, etc.); homoplasy is common (deVos et al. 2025). For wood evolution, etc., in Cacteae, see Vásquez-Sánchez et al. (2017).
Many diversification rates within Cactaceae are quite high, with significant radiations occuring in the late Miocene-Pliocene, ca 8-3 Ma (Arakaki et al. (2011). Hardly surprisingly, the monotypic Blossfeldia, sister to all other Cactoidaeae (see below), represents a lineage with notably lowered diversification rates of 0 or 2.27 x 10-17/ma, depending on the particular measure used (Arakaki et al. 2011). Pachycereeae, which include the North American columnar cacti, also began diversifying in the Late Miocene ca 8.5 Ma (Barba Montoya et al. 2011), although it is difficult to understand details of evolution here because of gene/species tree discordance - independent lineage sorting (ILS) is coupled with very long generation times, and hemiplasy connected with this ILS accounts for about 60% of the apparent homoplasy (Copetti et al. 2017). Cereeae began diversifying ca 3 Ma or more recently, the central Brazilian Cerrado being the center for the group (Franco et al. 2017). Romeiro-Brito et al. (2023a) looked at the evolution of the largely Brazilian Pilosocereus associated with Caatinga and campo rupestre vegetation; diversification here is mostly Pleistocene. Crown group Opuntia in the narrow sense, with 150-180 species, may be (7.5-)5.6(-3.6) Ma old (Araki et al. 2011). Perhaps originating in southwest South America, it may have moved to North America by long-distance dispersal, subsequently diversifying there considerably (Majure et al. 2012); about half of its species are to be found in Mexico. Diversification in the chollas, [Grusonia + Cylindropuntia], may have been facilitated by desertification, climate shifts, and hybridization (Mayer & Rebman 2021). Breslin et al. (2022) discuss diversification in the mammilloid clade, especially Cochemiea, from Baja California, which is close to Mammillaria; 93/120 taxa (72%) from that area are endemic, well over twice the general level of endemicity there. Melocactus is diverse on Cuba and elsewhere in the Antilles, in part the result of several dispersals from South America (Majure et al. 2022b).
Cactaceae are an iconic family of the New World; for distribution maps of all the genera then recognised, see Barthlott et al. (2015b). However, Rhipsalis, epiphytic and bird-dispersed, has a few species growing in Africa, Madagascar, and Sri Lanka. Whether or not these Old World species are native has been questioned (Barthlott 1983). Thus Korotkova et al. (2011) emphasized the differences found by Barthlott (1981) between the Old World R. baccifera and other taxa and suggested that the genus has had a long history in the Old World, while Grosse-Veldmann et al. (2016a) thought that the evolution of autogamy in the genus might have facilitated its wide distribution.
Hybridization is quite common in Cactaceae, for instance, within Opuntia (Majure et al. 2012), while genera like Discocactus are of possible hybrid origin. However, it is not recorded from members of the mammillarioid clade in Baja California (Breslin et al. 2022), for example, and how important hybridization has been in the diversification of the family as a whole is unclear (Machado 2008, but see Capetti et al. 2017; Mayer & Rebman 2021). Van de Meer (2022) reported a number of deep hybrids, including between extant members of Leuenbergerioideae and Pereskioideae - their common ancestor is the age of the family.
There was little resolution of relationships in a phylogenetic analysis of Cactoideae using characters from stem anatomy (Terrazas & Arias 2003). Sánchez et al. (2022: Fig. A1) optimized a number of characters on a tree of Cacteae that had a focus on Coryphantha.
Ecology & Physiology. Cactaceae are an important component of the Succulent Biome in the New World. Thus some 80% or so of the aboveground plant biomass in the Sonoran Desert may be made up of Cactaceae, and other major groups in the Succulent Biome include Bursera and co. and Fabaceae. This biome also occurs in the Old World where some clades of Euphorbia, Didiereaceae, etc., are conspicuous succulents (Gagnon et al. 2018 and references). Arakaki et al. (2011) suggest that succulents in general radiated/diversified in the mid to late Miocene to Pliocene, even if the clades involved originated substantially earlier, and they also mentioned several radiations in Euphorbia, also core Ruschioideae and Agavoideae in this context (in all these groups, see Ecology & Physiology). Indeed, radiations in succulent groups in general seem to have occurred quite recently - and add Aloe, Crassula, etc. (M. Lu et al. 2021).
E. J. Edwards and Donoghue (2006; see also Mauseth 2006a; Edwards 2006; Edwards & Diaz 2006; Ogburn & Edwards 2010) discuss the eco-physiological evolution of Cactaceae (for which, also see Nobel 1982, 1988 and references). They emphasize that the leafy Pereskia and Rhodocactus (= Leuenbergeria) clades have high photosynthetic water use efficiency, very high minimum leaf water potentials (water movement is easy), and conservative stomatal behaviour, the stomata opening only when there is available water, i.e. at night or after rain. Nobel et al. (1992) looked at the water capacitance - storage - of some cacti growing in the Sonoroan Desert and found interesting variation. Two species of Opuntia and a species of Echinocereus contained substantial amounts of mucilage in their stems, but it was extracellular, so providing a passive apoplastic capacitor helping control their ability to store and manage water flow - indeed, the composition of the mucilage, the presence of solutes, etc., also play a part here. Interestingly, the barrel cactus Ferocactus acanthodes growing in the same area completely lacked such mucilage, but the stem contained large amounts of water storage tissue (Nobel et al. 1992). Other features of potential functional interest include the production of large amounts of water conducting tissue relative to leaf area, and also CAM-type photosynthesis. This latter is poorly developed in Pereskia, etc., but is well developed in succulent cacti (C. E. Martin & Wallace 2000; Edwards & Donoghue 2006), as it is in a number of other xerophytic plants. Cactus seedlings may switch from C3 to CAM photosynthesis during development (Keeley & Rundel 2003). For more on CAM photosynthesis and Cactaceae, see Holtum (2023), Gilman et al. (2023).
Calcium oxalate metabolism in Cactaceae and relatives is potentially interesting. There is variation in the degree of hydration of calcium oxalate, with two crystal forms, weddellite (CaC2O4.2H2O) and whewellite (CaC2O4.H2O); Cactoideae alone have weddellite (Rivera and Smith 1979: only druses were examined; Monje & Baran 2002; esp. Hartl et al. 2007). Some Cactaceae accumulate positively massive amounts of calcium oxalate crystals, for example, they make up ca 85% of the dry weight of Cactus senilis. The recent finding (Tooulakou et al. 2016) that in the eudicots studied (no Cactaceae, but two other Caryophyllales were) such crystals are broken down to produce CO2 during the day when the plant is stressed, reforming at night, so providing a reserve of CO2 for the plant - it is a kind of CAM-cycling, which could be relevant for Cactaceae given their preferred habitats. Indeed, Karabourniotis et al. (2020) noted that carbon dioxide and water produced on the breakdown of calcium oxalate would b and particularly important under drought conditions. Oxalate also chelates toxic metals like aluminium, and it is abundant in root-produced carboxylates (Abrahão et al. 2019; see also Kuo-Huang et al. 2007 and Karabourniotis et al. 2020 for other functions of calcium oxalate). When the cactus dies, the calcium oxalate in the plant returns to the soil as calcite, resulting in appreciable amounts of carbon being stored in the soil (?: Garvie 2006).
Most Cactaceae have a widely spreading and shallow rooting system that allows quick uptake of water after rain. In a number of members of the family, especially Cactoideae (but not Pereskia s.l.) the primary root is determinate in growth (Dubrovsky & North 2002; Shishkova et al. 2013 for the rather erratic distribution of this feature), perhaps facilitating the rapid development of lateral roots (Rodríguez-Rodríguez et al. 2003). "Rain roots", water-absorbing roots, develop quickly after rains and die when the soil subsequently dries up (e.g. Nobel 1988). Here, too, the main root usually aborts (Shishkova et al. 2008; Ogburn & Edwards 2010), but a skeletal root system of perennial, cork-covered roots persists (Gibson & Nobel 1986). Contraction of the roots, so keeping the plant close to the ground surface, is also known or suspected for some Cactoideae (Garrett et al. 2010). Roots in at least some species have rhizosheaths surrounding and adherent to the root and perhaps protecting it against desiccation (North & Nobel 1992); these rhizosheaths are made up of mucilage from the root, soil grains, etc. (Huang et al. 1993; Dubrovsky & North 2002). Abrahão et al. (2019) found that Discocactus placentiformis, common in the phosphorus-poor soils of campos rupestris vegetation in Brazil, has long, dense root hairs on its young roots, and these formed a rhizosheath (see also North & Nobel 1992); carboxylates, predominantly oxalates (see above), were produced, and these facilitated the uptake of phosphorus by the plant. Fleshy, water-storing roots are scattered in Cactaceae, including Pereskia (e.g. Rauh 1979); the taxa with such roots are usually small plants, and although the tissue involved is not always the same, suggesting the independent origin of fleshy roots, it is some kind of modified secondary vascular tissue (Stone-Palmquist & Mauseth 2002). These swollen roots seem to be particularly common in the taxa of the basal pectinations of Opuntioideae (Griffith & Porter 2009), and they are also scattered in families in the clades immediately basal to Cactaceae (see also Griffith 2004).
Diversification of the leafless Cactaceae is also connected with the development of a cauline water storage and uptake system as well as with the evolution of the other ecophysiological features just mentioned (and of course one would like to know much more about the physiology and anatomy of the clades immediately basal to Cactaceae). The ribbed and/or tuberculate stems of most Cactoideae allow the loss and gain of large amounts of water as the stem can easily contract or expand (see also Mauseth 2006a); some cells in the stem have mucilage that can take up water exceeding its own weight, although most water is stored in large parenchymatous cells (Nobel 1988). Few cacti are really desiccation tolerant, i.e. withstanding drying out to less than 0.1 g H2O g-1, although the diminutive Blossfeldia is one exception (Barthlott & Porembski 1996; Griffith 2009). Of course, Cactaceae are pre-eminently a group common in drier climates in the New World and are a notable component of the rather open conditions of seasonally dry tropical forests (Pennington et al. 2009), but a number of Cactoideae grow in more or less humid forest as lianes and epiphytes, several having flattened and leaf-like stems; the epiphytic habit may have evolved four times or so here (Korotkova et al. 2010). Some species of cacti can stand extreme cold (to -200C), cold-hardening occurring remarkably quickly in a matter of a very few days (Nobel 1982). However, unlike several other groups that grow in drier habitats, salt tolerance is uncommon in Cactaceae (Flowers et al. 2010).
Cactus spines are commonly thought of as a defence against herbivores, although other functions have been suggested (see Aliscioni et al. 2021 for a summary). There is substantial variation in the macro- and micromorphology of cactus spines, which can be variously hooked, barbed and channelled (e.g. Schill et al. 1973; Robinson 1974; Mosco 2009; Schlegel 2009; Repka & Gebauer 2012); for the mechanical properties of spines, see also F. Huang and Guo (2013). Opuntioideae have spines with retrorse barbs, the glochids - "a special type of spine that can be described as pure misery" (Mauseth 2017b: p. 223), and these abscise readily at the base and are particularly difficult to remove once embedded (c.f. porcupine quills) and so are likely to be an effective defence mechanism (Crofts & Anderson 2018).
Spines and the ribbing of the stem in Cactoideae often have ecological implications beyond protection against animals and water storage (Menezes et al. 2015; Aliscioni et al. 2021 and references). Thus the cylindrical cactus pads of the jumping cholla, Cylindropuntia fulgida, and of other members of the genus, can become attached to and dispersed by animals. The shading of the stem by spines may also afford protection against extreme heat (e.g. Nobel 1983) or cold, but at the same time the photosynthesis of the plant may be reduced (Aliscioni et al,. 2021). Sometimes, at least, cactus areoles, the spines and their associated uniseriate multicellular hairs, are involved in water uptake from transient fogs forming in the desert (c.f. Nobel 1988), and details of spine micromorphology play a central role here. Thus water moves down the glochids of Opuntia microdasys whether they are upside down or vertical; the glochids are smoother towards the base, and are variously channelled, which helps in the movement of water (Ju et al. 2012). Once the droplets reach the base they encounter the uniseriate multicellular trichomes (the "belt-structured trichomes" of Ju et al.). These have even finer groves than the spines and the water apparently finally moves through a pore in the centre of the tuft of hairs into mucilage cells below, and this last step is surprisingly rapid (K. Kim et al. 2017). C. Liu et al. (2015) observed a similar movement of water droplets down the spines of Gymnocalycium baldianum, i.e. Cactoideae, however, here the ornamentataion of the spines is almost the reverse of that of Opuntia glochids, the spines having small, antrorsely-projecting barbs; the first droplet down the spine leaves a thin film of water behind enabling subsequent droplets to move far faster. Spines may be recurved, or densely plumose, as in Mammillaria plumosa and Turbinicactus beguinii, and areoles of such plants also deserve investigation from the point of view of water uptake. Kundunati et al. (2022) describe how separate droplets of water coalesce alomg the channelled spines of Oreocereus trolli ("distant coalescence"), and here the droplets fall to the ground, the water then presumably beong absorbed by the roots. Nutrients in the water may also be taken up by the plant (Mauseth 2006c).
Rhipsalideae and Hylocereeae are smallish tribes that include ecologically distinctive taxa - epiphytic and epilithic plants and also a few climbers, and they are quite common in rainforest habitats.
Perhaps surprisingly, extrafloral nectaries are associated with areoles in a number of taxa, and these are often interpreted as being modified evascularized spines or leaves subtending areoles (Sandoval-Molina et al. 2018; Aliscioni et al. 2021). Nectaries may also be highly vascularized and stipitate, as in Ancistrocactus scheeri (Mauseth 1982), and they may also occur on the stem near the areoles (Mauseth et al. 2016). Little is known about the composition of the nectar, but that secreted by Opuntia robusta (Sandoval-Molina et al. 2018), Brasiliopuntia brasilensis and Neopalea cochenillifera (Silva et al. 2020), all Opuntioideae, and Ferocactus acanthoides (Cactoideae: Ruffner & Clark 1996), for instance, is collected by ants (see also Oliveira 1999; Aliscioni 2021). Secretions may differ considerably in the amount of amino acids they contain, those of B. brasilensis having over ten times the amino acid concentrations as those of N. cochenillifera, the former producing especially large amounts of arginine (Silva et al. 2020).
In common with some groups inhabiting saline/dry conditions (including Tamaricaceae, Amaranthaceae), some Cactaceae germinate very quickly, i.e. within one day of the start of imbibition (Parsons 2012; Parsons et al. 2014).
For details of the ecology of columnar cacti, including drought tolerance, photosynthetic rates, germination and seedling establishment, see papers in Fleming and Valiente-Banuet (2002) and Williams et al. (2014). Mauseth (2019) described how some Core Cactoideae II ensured the correct orientation of the bases of their branches by the development of what he called "reaction cortex", distinctive non-collapsing cortical cells that developed on the abaxial side of the stem; cortical cells otherwise had collapsing cells the walls of which became concertina-like; there was little wood in the stems of these plants, but there was more of it on the abaxial side of the stem. Interestingly, taxa like Haageocereus pacalaensis have prostrate stems that are upcurved only near the apex, but a zone of reaction cortex as it were moves along the stem, being found only in the zone of curvature of the stem - other species of the genus with upright stems had normal reaction cortex (Mauseth 2019). Hultine et al. (2023) described the ecology of columnar cacti in particular in the context of conservation concerns. Topics included the consequences of the often shallow root system for the plant, the amount of water stored in the stem (there is great variation in volume:surface area in the family, reflecting the trade-off between photosynthetic capacity and resource storage, not much of a link between stem storage capacity and aridity), the use of nurse plants by some cacti, and changing fire regimes and the response of cacti to fires. J. R. Moore (2025) looked at how the stems of saguaro cacti (Carnegiea gigantea) - they can be over 20 m tall - functioned. Here internal wooden ribs provide strength while the cortex and inner pith store water. He noted that taller plants were more rigid (higher Young's modulus), but in such plants in particular stiffness decreased vertically.
Some Cactaceae have substantial effects on the community as a whole. Thus the tussock-forming Maihueniopsis camachoi is common at around 3,000 m in the Atacama Desert, and Díaz er al. (2023) found that it was an important nurse plant, facilitating the growth of other plants found there (the C4 Atriplex was a subject of study), whether by ameliorating temperature, affecting water supply, or even providing nutrients (as the host plant decomposed).
Pollination Biology & Seed Dispersal. Bee pollination is probably plesiomorphic in Cactaceae, and there have been perhaps ten bee→hummingbird pollinator shifts and half as many bee→sphingid moth shifts, mostly in Cactoideae (Schlumpberger 2012). A variety of other pollinators also visit cacti flowers. Thus about 200 species in 51 genera are pollinated by bats (Dobat & Peikert-Holle 1985); Fleming et al. (2009) conservatively list 42 species in 26 genera. For bat pollination in columnar cacti, see Arizmendi et al. (2002 and references), also other papers in Fleming and Valiente-Banuet (2002). In hairy cephalia like those of Espostoa frutescens, bat pollinated, the hairs absorb ultrasound from the bat, but the flowers reflect it, so making the flowers more conspicuous to the echo-locating bat (Simon et al. 2019/2020). Some 390 species have reddish flowers, and these flowers are notably often tubular (ca 20%); there are perhaps 120 (Gorostiague & Ortega-Baes 2015: cautions should be heeded!) or 187 (Mutke et al. 2015) species with flowers that might be considered to be bird-pollinated, and many are found in the east Brazilian caatinga. Thigmonastic (touch-sensitive) flowers are reported from both Opuntioideae (e.g. Opuntia) and Cactoideae (Notocactus, = Parodia), but are best understood in Opuntia s. str. where the stamens move adaxially when stimulated and the flowers are pollinated by oligolectic bees (Schlindwein & Wittmann 1997; Cota-Sanchez et al. 2013). Almeida et al. (2013) looked at nectary morphology and nectar concentration in some Cactoideae with very different floral morphologies; species with more exposed nectaries had greater sugar concentrations, perhaps being bee-pollinated, while Grosse-Veldmann et al. (2016a) examined various aspects of pollination in Rhipsalis. The senita cactus, Lophocereus/Pachycereus schottii, is actively pollinated by a pyralid moth Upiga virescens that lays eggs in some flowers leading to the loss of the fruit; other animals that are potential pollinators also visit the flowers (Fleming & Holland 1998; Holland & Fleming 1999).
Animal - mostly bird - dispersal of the fruits is very common in the family; Rhipsalis (see above for its distribution) has mistletoe-like fruits and in South America Cactaceae with such fruits are dispersed by the mistletoe specialist friar birds (Euphoniinae, near Fringillidae: Snow 1981; Restrepo 1987). Cochemiea, mostly from Baja California, similarly has seeds embedded in sugary, mucilaginous, sticky pith (Breslin et al. 2022). Dispersal may also result from the attachment of cactus pads to passing animals (references in Crofts & Anderson 2018), while a number of chollas ([Grusonia + Cylindropuntia]) have burr-like indehiscent fruits that are similarly dispersed (Mayer & Rebman 2021). For seed dispersal, see also Callejas-Chavero et al. (2020).
The seeds may germinate while still in the fruit (a form of vivipary) in some Cactoideae in particular (Cota-Sánchez et al. 2007; Cota-Sánchez 2022: 77 staxa involved, scattered throughout the family). Mascot-Gómez et al. (2019) looked at the germination of five Cactoideae growing in the Chihuahuan desert; the seeds had a mucilaginous testa, removal of which increased the time to germination in two species and reduced the percentage of seeds germinating in three species; one species was unaffected (see also seed dispersal above).
Plant/Animal Interactions. The chemistry of cacti is complex, and they contain numerous potentially toxic compounds which the insects have to tolerate (López-Olmos et al. 2017 and references).
The cactus-feeding habit may have evolved only once in the pyralid phycitine moths, although support is weak (Simonsen 2008: morphology only). The phycitines include the famous/infamous (it depends on where you live) Cactoblastis cactorum. Introduced into Australia, it managed to control introduced Opuntia that had rendered extensive areas unfit for grazing animals, but unfortunately it has recently been introduced into the U.S.A. where it bids fair to decimate endemic - and ecologically important - species of the genus, nevertheless, even in the U.S.A. it is encouraged in places (see also Nobel 1988; Ervin 2012).
The Drosophila repleta species group, with over 100 species, has radiated on Cactaceae, the larvae growing on necrotic cacti fermenting cactus tissues, whether cactus pads or the stems of columnar cacti. The ability to do this may have arisen independently at least twice and allowed the spread and diversification of the repleta group (Oliveira et al. 2012). They moved on to necrotic Opuntia from fermenting fruits perhaps 16-12 Ma and from there they moved on to columnar cacti several times (López-Olmos et al. 2017; Moreyra et al. 2022). Columnar cacti are chemically more complex that Opuntia species and may grow in more extreme environments, and Moreyra et al. (2022) examined the changes that accompanied the host shift, noting i.a. that stress response genes were involved. Some Drosophila will grow on only a single host species since the latter contains sterols that can stand in for essential sterols missing from the ecdysone pathway of the insect (Lang et al. 2012). Rotting cacti in general provide habitats for numerous insects in the Sonoran desert region (Pfeiler et al. 2013). And of course Opuntia species were used as hosts of the cochineal scale insect Dactylopius coccus, a sternorrhynchine scale insect, from which the bright red carmine dye can be obtained (e.g. Nobel 1988).
Callejas-Chavero et al. (2020) summarize herbivory in Cactaceae - a number of vertebrates eat them, also invertebrates. There are complex interactions between a cactus (Myrtillocactus gometrizans), the host; two species of sap-sucking scale insects, herbivores; an ant, which may protect the scale insects and so increase herbivory; and various parasitoids of the scale insects, which may reduce herbivory (Callejas-Chavero et al. 2020). For more on cacti and ants, see Ecology & Physiology above.
Plant-Bacterial/Fungal Associations. Endophytic bacteria have been isolated from Cactoideae growing in the Sonora desert. These may help the cacti grow on rocks, and in vito fixation of nirtogen has also been observed (Puente et al. 2009; Lopez et al. 2011).
Vegetative Variation. There is considerable variation in growth form in "leafless" Cactaceae, which range from often bizarrely-branched to tall and unbranched trees to flat-discoid stem structures growing at ground surface, to tussock-forming to stoloniferous ("Wandersprosse", Creeping Devils) and occasionally even rhizomatous plants (see e.g. Rauh 1979), and this is discussed in a phylogenetic context by Hernández-Hernández et al. (2011). The stout, more or less succulent stems that characterise members of Cactaceae - even Pereskia has quite thick stems - are largely the result of primary or secondary thickening/expansion in the cortex, less often in the pith (for which, see Troll & Rauh 1950; Boke 1954, 1980). A number of cacti. including Opuntia, etc., have articulated stems, the articulations marking periods when growth of the stem stops.
There is potentially interesting variation within the parallelocytic stomata "type" so common here. Wallace and Dickie (2002) thought that the stomata of Opuntioideae were unique; in both Pereskia and Opuntioideae the subsidiary cells do not, or only barely, overlap the ends of the guard cells, the "opuntioid" stomatal type (it could be called brachyparallelocytic), while in other Cactaceae subsidiary cells successively more broadly invest the poles of the whole stomatal apparatus. There is also variation in stomatal orientation: The stomata on the stems of Pereskia and Opuntioideae are oriented parallel to the long axis of the stem, while in Cactoideae they tend to be unoriented (Eggli 1984). There are cuticle waxes in the form of spiral rodlets in Cereeae, at least.
The rather ordinary-looking leaves of Leuenbergioideae and Pereskioideae may represent the plesiomorphic condition for the family (but c.f. Griffith 2004, 2008). In Opuntioideae, the leaves of Pereskiopsis are rather similar, albeit with parallel, not pinnate venation, while those of Quiabentia (the two may be sister taxa - e.g. Butterworth & Evans 2008) are terete, unifacial, but are also persistent; such leaves that carry out appreciable photosynthesis have probably been derived more than once in Opuntioideae (Griffith 2009; Griffith & Porter 2009; Ritz et al. 2012). Stomata are restricted to leaves and the stem adjacent to areoles in leafy Opuntioideae (Griffith 2008), while in most other members of the subfamily the leaves are small, terete and deciduous. These leaves lack a blade meristem but have an hypodermal meristem around their periphery, and Boke (1944) rather hesitantly reported stipules associated with them. Although one commonly thinks of Cactoideae in particular as being leafless, Mauseth (2007) showed that most do have leaves, although they may be up to a mere 1.5(-2.5) mm long when mature and so are mostly shorter even than the small, terete leaves of Opuntioideae (see also Boke 1951: Echinocereus). Despite their small size, Cactoideae leaves may have a rudimentary lamina with vascular tissue, stomata, etc.. The leaf base is early distinguishable from the rest of the leaf, and its subsequent development results in the ribs and tubercles along the stem that are characteristic of so many Cactaceae (Boke 1954). Opuntioideae-Opuntieae have distinctively flattened stems (= pads), interestingly, in Consolea, sister to the rest of the tribe, the main stem may be terete (see also Cylindropuntieae).
Areoles in Cactaceae are often borne in little pits, and spines, hairs and flowers are restricted to these areoles and together they make up a short shoot; hairs in Cactaceae are very largely restricted to these areoles (e.g. Mauseth 2017a, b). Areoles have a complex vasculature (see e.g Boke 1951) and may keep on growing and adding spines, and also photosynthetic leaves as in Pereskia, and in the latter this is true even of the areoles on the fruit, although in some taxa the areoles fall off the fruits. The spines - also the glochids, in Opuntioideae - are modified leaves, alhough Miravel-Gabriel et al. (2024), looking at seedlings of Carnegiea gigantea, floated the possibility that spines might be modified stems. In Opuntioideae there are intermediates between spines and glochids, and both are initiated in spirals surrounding the areole apex - areoles represent aggregations of leaves, variously modified. Both spines and glochids completely lack vascular bundles (vascular traces may approch the spine base) and stomata and usually consist simply of a lignified epidermis that surrounds central fibres (Boke 1944, 1980; Repka & Gebauer 2012; de Arruda & Melo-de-Pinna 2016; Mauseth 2017a, b). Areoles bear flowers, sometimes over a number of seasons (Neoraimonda is an example), and branches can also develop from areoles (e.g. Rauh 1979; Leuenberger 2008; de Almeida et al. 2018). Mammillaria appears to have dimorphic areoles: There are normal spiny areoles borne on tubercules (hence the generic name) and spineless areoles that bear flowers that are found in the axils of the tubercules, and these two types of areoles are the result of dichotomous division of the original meristem (see also Rauh 1979 and Boke 1980 for inflorescence development; the flowers have also been described as coming from serial buds - Mauseth 2017a, b). In Echinocereus the areole meristem, whether vegetative or floral, becomes enclosed, but later breaks through the stem when it develops (Sánchez et al. 2015). And of course there are cacti like Lophophora and Astrophytum that lack spines, although there are hairs associated with their areoles. For water uptake and nectar production by spines, see Ecology & Physiology above.
Wide-band tracheids, a kind of tracheid in which the wall thickenings are very tall, so partly occluding the lumen, are common in Cactaceae. They are most frequently present in the seedlings, but they may persist in the adult plant if it is small and globular; if the plant tilts they are no longer produced, the plant needing stronger support tissue (e.g. Mauseth 2004a, 2006a, 2025). Onyenedum and Pace (2021) summarize the literature on cauline vascular anatomy in the family, which they place in three types of which the wide-band tracheid type is one, and they suggest developmental mechanisms leading to changes in vascular anatomy; indeed, as already suggested, vascular anatomy type may change as the plant grows, and this change may be quite abrupt (ibid.: Fig. 9). Landrum (2025) refers to his life-time observations on the vascular anatomy of Cactaceae, i.a. noting that parenchymatous wood, lacking fibres, including fibre caps, has much axial parenchyma and is found in smaller/globular cacti (also in some species of Crassula), while columnar cacti in particular have fibrous wood. This latter may be diffuse-porous or ring-porous, but the production of wood with annual rings may vary within a genus and even during the course of the lifetime of the plant (Mauseth 2025). Branched columnar cacti (and Opuntioideae) have constrictions where the branch joins the stem, which would seem to be rather hazardous biomechanically. However, Schwager et al. (2013) show how details of thickening pattern, fibre orientation, etc., make such constrictions in the three Cactoideae that they studied biomechanically not too risky. Interestingly, these species may not have tension wood.
Some species of Mammillaria branch dichotomously (Craig 1945); see also Schwager and Neinhuis (2015, 2016) for branching. Given the width of their stems, it is not surprising that many Cactaceae have very broad apical meristems 400-2565 µm across, rather broader than those of other flowering plants (Gifford 1954; Clowes 1961: sampling poor; Boke 1980; Mauseth 2006a), although they are only 80-329 µm across in Pereskia (Boke 1954). Many Cactoideae have ribbed stems, and Mauseth (2021a) discusses how the number of ribs can increase as the apical meristem increases in size; phyllotaxy here may not follow the Fibonacci series (Mauseth 2020). The cortex is particularly variable in Cactoideae.
Resin ducts - the resin is white and looks like latex - are reported from Mammillaria (Prado & Demarco 2018).
Mauseth and Landrum (1997) commented on the apparently very long-lived epidermis in many Cactaceae, which may remain functional for hundreds of years (see also Mauseth 2006a). A cork cambium may eventually develop, nearly always in the epidermis and nowhere else; the structure of the cambium is simple - there is no rhytidome, etc. (Evans & Cooney 2015). The development of such a hypodermal cork cambium may be a defence against attack by the loranthaceous Ligaria in Corryocactus (Mauseth et al. 2015). However, in some cases the plant may die if this cambium is extensively developed (L. S. Evans & Cooney 2015). Such bark has been found on 23 species of cacti in the Americas, and bark first develops on south-facing surfaces of the saguaro cactus (Carnegeia gigantea) in response to ultraviolet B (UV-B) radiation, and it spreads all around the stem by 45 years; once 80% of north-facing stem troughs are covered by bark, the plant will die within 8 years (Evans 2025). Interestingly, Evans was able to show that in the Tucson area, at least, the saguaro cactus did not develop such bark before 1956, UV-B levels having increased over the last decades. For the development of collenchymatous hypodermes, see de Almeida et al. (2018) and references.
Cortical and medullary vascular bundles are quite common in Cactoideae, and they develop secondary thickening, i.e. they become vascular fascicles, and may become amphivasal (Mauseth 2006a; Cunha Neto et al. 2024), and in the saguaro cactus they are estimated to remain functional for ca 150 years, the life span of the plant (references in Cunha Neto et al. 2024); see also Mauseth and Sajeva (1982), and Mauseth (1993).
Cephalia develop in a number of Cactoideae. Cephalia are confluent areoles associated with a very thick periderm; stems with this periderm do not carry out photosynthesis and there are often no stomata. Cephalia may be restricted to one side of the stem, with flowers being borne in the cephalia but nowhere else on the plant (Mauseth 2006a; Gorelick 2016); stem with such cephalia may look rather like a toothbrush.
Genes & Genomes. Although there is a fair amount (7.3%) of gene duplication at the origin of Cactaceae, this is apparently not accompanied by a genome duplication (N. Wang et al. 2018). Castro et al. (2016) discuss variation in the heterochromatic banding of the chromosomes, which may be of some systematic interest. For chromosome counts in the family, see Baker and Pinkava (2018) and references; chromosome numbers in Tephrocactus range from 2n = 22 to 2n = 319 (Las Peñas et al. 2019).
Intergeneric hybridization is quite common in the family and may have been important in its evolution (Machado 2008). There is likely to have been ancient hybridization in Opuntia (Majure et al. 2012; see also Granados Aguilar et al. 2020 and references), and tardy coalescence can cause major problems when inferring relationships (Copetti et al. 2017).
There is a substantial amount of variation in the plastome within Cactaceae and at various levels in the phylogeny, although understanding this awaits better sampling. There has been considerable restructuring of the plastome, especially in Cactoideae, with 66≤ repeats in Mammillaria solisioides, for example (de Almeida et al. 2021), and aside from rpl23, more rpl genes have been lost in Cactoideae, but not in Opuntioideae, other genes have been lost or pseudogenized, introns lost, etc.. For the loss of the inverted repeat (IR) and the chloroplast ndh genes in Carnegiea gigantea, see Sanderson et al. (2015). There the ndh function may be taken over by a nuclear gene, while in Melocactus glaucescens the ndh complex is nonfunctional (it represents 11/15 genes lost there - Costa et al. 2022), and similarly, in Discocactus bahiensis and Mel. ernestii five ndh genes have been lost and two much reduced in sixe, and these two plus four more have been pseudogenized (de Almeida et al. 2021). Mower et al. (2021) discuss possible connections between various distinctive life styles that might affect the photosynthetic process and result in the loss of such genes. Most of the LSC region of Opuntia quimilo is inverted and genes have moved around the genome (Köhler et al. 2020), the SSC is much reduced, and a number of genes have become pseudogenes. Recent studies on the plastomes of Opuntioideae (43 species were examined) document extensive variation in the subfamily, including variation in the size and content of the IR - it is even lost in a few taxa (Köhler et al. 2023). Pachycereus schottii also lacks an IR, while within Mammillaria the IR varies from 1>-14 kb long (Solórzano et al. 2020). Details of gene arrangements in the small single copy (SSC) of Cactoideae may distinguish that subfamily from the others (Solórzano et al. 2020; Köler et al. 2020). In Melo. glaucescens there has been the translocation and inversion of a large block of the large single copy (LSC), expansion of the IRs, and while tRNAs have been lost, they may have been replaced by genes moving into the plasmids from the cytosol (Costa et al. 2022; see also G. M. Silva et al. 2021).
Economic Importance. The cochineal insect, the sternorrhynchid Dactylopius, grows on species of Opuntia. The genus also provides food, fodder for livestock, and includes some seriously invasive species (e.g. Ervin 2012 for references). Mescaline. a protoalkaloid, is produced by Astrophytum and Lophophora, peyote (see Cassels et al. 2019 for literature). Goettsch et al. (2015) found that about 30% of all cacti, a very high percentage, were threatened because their habitats were being converted for other uses and the plants themselves collected by cactus-fanciers.
Chemistry, Morphology, etc.. Lignin in the testa of Cactaceae-Cactoideae is v coccusariable in composition, many members hav; the insect produces large amounts of carminic acid which deters possible predators - and which can easily be converted to the brilliant red cochinealing otplantse rare catechyl units (F. Chen et al. 2013: othez lignin units as well). The amount of lignin there is moderate, taxa with "norma," guaiacyl/syringyl units either have substantially more (Opuntioideae) or sometimes much less (some Cactoideae) (Chen et al. 2013), and there is also a correlation with seed colour. The survey of lignin composition could usefully be extended.
The inferior ovary of Cactaceae is a text-book example of cauline epigyny, the tissue investing the ovary being of axial origin (Boke 1964; see also Puri 1951, 1952; Tiagi 1955, 1963a and references). Thus in genera like Opuntia areoles arranged in spirals cover the inferior ovary; it is as if the ovary had sunk into the stem. In Pereskia nemorosa and a few other Cactaceae additional flowers may arise from the axils of the leaves (Pereskia) or from areoles on the ovary (I have seen two orders of floral branching from these ovarian areoles); these are the proliferating inflorescences in the characterization above (Tiagi 1955; Rauh 1979; Leuenberger 2008; de Almeida et al. 2018 - see also Tetragonia, = Aizooideae-Aizoaceae). Thus the "hypanthium" so conspicuous in some Cactoideae in particular is an elaboration of this axial tissue, and the "petals" may intergrade with the areole-subtending leaves on the axial tissue surrounding the ovary. Mauseth (2021b) drew attention to such flower-associated leaves in Cactoideae, noting that they intergraded with what are called petals and that there was the possibility that they carried out appreciable amounts of photosynthesis. The evolution of such leaves, petals and inferior ovary needs to be re-examined given the paraphyly of the old Pereskia and the immediate relationships of Cactaceae; some species of Pereskia s. str. have superior ovaries (see Buxbaum 1953; Tiagi 1955; Rauh 1979; Boke 1980; Edwards et al. 2005). As Edwards et al. (2005) note, the anatomy of the outgroups to Cactaceae is poorly known, as is the occurrence of proliferating inflorescences in Portulaca, also with a more or less inferior ovary and now quite often thought to be sister to Cactaceae (c.f. Edwards et al. 2005). Tiagi (1963a) noticed that in P. aculeata and P. sacharosa the course of the vascular tissue in the hypanthium was S-shaped, while in P. [= Leuenbergeria] bleo and P. grandifolia it took the course of an inverted U, however, the significance of this is unclear - indeed, the vascularization of "prophylls", bracts and "perianth" members of the flowers varies (Tiagi 1963a). De Almeida et al. (2018: but c.f. terms used) describe the development of the fruit in some detail, emphasizing the distinctive cauline origin of the outer part.
The initial stages of androecial development may be as either separate, more or less spirally-arranged primordia, or as a single ring primordium (Leins & Erbar 1994b); Tiagi (1955) interpreted the androecium as being fasciculate. There is discussion as to whether the stigma is wet or dry - it is certainly the latter in some cases - and over the nature of the stylar canal, often at least semi-closed and with several layers of pollen-tube transmitting tissue beneath the epidermis lining the canal (Vanesa González et al. 2023). Stigmatic papillae are uniseriate in Pereskioideae, Opuntioideae and Cactoideae-Phyllocacteae, otherwise they are multiseriate (Vanesa González et al. 2023). Tiagi (1955) suggested that the stylar bundles were carpellary dorsals and the stimatic lobes were carinal, whereas the stigmatic lobes in Opuntioideae and Cactoideae seemed to be commissural. Ovary placentation is variable: Placentae may alternate with septae, being involute septal margins (Vanesa González et al. 2023, see also Boke 1964, 1980) and/or be more or less basal, and there is also discussion over the homology of these ovary septae (see also Tiagi 1955). Leins and Schwitalla (1988) interpret the condition in which ovules are associated with incomplete septae proceeding from the ovary wall as the plesiomorphic condition for Cactaceae (see also Leins & Schwitalla 1986).
The funicle is more or less curved, and in some taxa it is branched, and funicular hairs may function as obturators (Vanesa González et al. 2023). The nucellus in Parodia may protrude through the micropyle (Rauh 1979), as may that in some species of Echinopsis, the embryo sac even being outside the ovule (Vanesa González et al. 2023). Cisneros et al. (2011) suggest that the inner integument of species of Hylocereus may be 4-5 cells across, but this is not readily to be seen in the images they provide. For discussion on embryo sac development, especially in Opuntia, see Chopra (1958).
For general information, see Boke (1980), Barthlott and Hunt (1993), Anderson (2001), Nobel (2002) and Schumannia 7. 2015 (= Barthlott et al. 2015a); Hunt et al. (2006) provides an excellent summary of the family, including a volume of superb photographs of nearly all species taken mostly in the wild (see also Lodé 2015a, b). For chemistry, see Hegnauer (1964, 1989) and Gibson et al. (1986: alkaloids), for general anatomy, see Gibson (1973: Cactoideae, 1978 and references: Opuntioideae), Terrazas and Arias (2003: esp. Cactoideae), Terrazas Salgado and Mauseth (2002) and Martínez-Quezada et al. (2020: Hylocereeae), for vascular organization, see Gibson (1976), for nodes, see Bailey (1960), and for wide-band tracheids in particular, see Mauseth (2004a), Godofredo and Melo-de-Pinna (2008) and Arruda and Melo-de-Pinna (2010). For Pereskia s.l., see Boke (1968 and references), Leuenberger (1986: general), Bailey (1966 and references, also inc. Pereskiopsis: vegetative anatomy), Mauseth and Landrum (1997: "relictual" anatomical characters), Neumann (1935: pollen, etc., development), Jiménez-Duran et al. (2016: embryology, Leuenbergia) and da Rosa and de Souza (2003: fruit and seed, Pereskia s. str.). For Opuntioideae, see Hunt and Taylor (2002) and papers in Succul. Plant Research 8. 2014, both general, and Stuppy (2002: morphology); for leaf anatomy, see Boke (1944), for general anatomy see Mauseth (2005), for wood anatomy, see Mauseth (2006c). For Maiheunia some information is taken from Gibson (1977) and Mauseth (1999), anatomy, and Leuenberger (1997), general; Taylor (2005) is a good introduction. For Blossfeldia, see Barthlott and Porembski (1996). For floral morphology, see Ross (1982), for pollen, see Leuenberger (1976a, b: general), dos Santos et al. (1997), Halbritter et al. (1997: Gymnocalycium), Garalla and Cuadrado (2007: Opuntioideae), Cuadrado and Garalla (2009) and Ruiz-Domínguez et al. (2020: Hylocereeae), for ovules, etc., see Mauritzon (1934d), Maheshwari & Chopra (1955), Sánchez and Vásquez-Santana (2018), Camacho-Velázquez et al. (2018) and Gentz et al. (2023: Parodia), for seed morphology, see Barthlott and Voigt (1979), Bregman (1992), and Barthlott and Hunt (2000: Cactoideae).
For revisions of critical taxa, see in particular work by Leuenberger, e.g. Leuenberger and Eggli (1999: Blossfeldia) and Leuenberger (1986: Pereskia and Leuenbergeria (Rhodocactus), 1997: Maiheunia, 2008: update on the literature of all three). Calvente (2012) enumerated the taxa in Rhipsalis. Rowley (2004) listed names of nothogenera, and these had doubled in number in the preceding ten years.
Phylogeny. Metzing and Kiesling (2008) summarize early (pre-DNA) studies in the family, and include reproductions of some remarkable evolutionary trees. The basic phylogenetic relationships within Cactaceae are still rather uncertain, and chloroplast and nuclear genes can suggest different major clades (Butterworth 2006a and Nyffeler & Eggli 2010a for early summaries; A. J. Moore et al. 2017; J. F. Walker et al. 2018a). A study by Nyffeler (2002) found rather weak support for the subfamilies and that perhaps rather distressingly Pereskia old style was not clearly monophyletic. Edwards et al. (2005) confirmed that Pereskia s.l. was paraphyletic, which allowed them to shed new light on the evolution of the cactus habit (c.f. Butterworth & Wallace 2005 - topology different). However, Walker et al. (2018a) found that Pereskia s.l. might be monophyletic, and relationships were unclear in N. Wang et al. (2018)... For more details on the relationships between the major clades in Cactaceae, now all individually quite well supported, see Butterworth and Edwards (2008), Hernández-Hernández et al. (2011: position of Maihuenioideae unclear, 2014: [Maihuenioideae [Opuntioideae + Cactoideae]]), Arakaki et al. (2011), Walker et al. (2018a) and N. Wang et al. (2018: Blossfeldia not sampled); details of relationships in Bárcenas et al. (2011) were less clear, but only the trnK-matK region was examined. Arakaki et al. (2011) obtained dates for Caryophyllales as a whole from an analysis using 83 chloroplast genes, but details of relationships in Cactaceae came from an analysis using far more species, but only the nuclear PHYC and again the chloroplast trnK and matK genes. Finally, a more recent study using plastomes found the quite well supported relationships [Opuntioideae [Pereskia sacharosa (= Pereskia s. str.) [Maihuenioideae + Cactoideae]]] (Yao et al. 2019). The distinctive Blossfeldia liliputana is sister to Cactoideae (Crozier 2004), and despite some initial controversy over this position, it has been confirmed (e.g. Gorelick 2004; Mauseth 2006b; Butterworth 2006b; Arakaki et al. 2011). De Vos et al. (2025) used Angiosperm353 data from 175 taxa - their focus was on commonly accepted genera, of which they included some 87%. They assembled four data sets on which they carried out a variety of analyses; they used ASTRAL analyses of a data set in which samples and loci for which sequence reconstruction was poor had been excluded (de Vos et al. 2025, q.v. for details, also many comments on previous work - there polyphyly, etc., was a major problem).
Opuntioideae. For relationships within Opuntioideae, see Griffith (2002), Wallace and Dickie (2002), Butterworth and Edwards (2008), Hernández-Hernández et al. (2011, 2014) and Griffith and Porter (2009). The latter found the well-supported set of relationships [Maihueniopsis et al. [Pterocactus [terete-stemmed species + flat-stemmed species]]]; the leafy Pereskiopsis is in a derived position in the clade (c.f. e.g. Mauseth 2005 on its apparently plesiomorphous features). Köhler et al. (2020) used plastome data to-clarify relationships in the subfamily, and their work is largely followed here (see also Majure et al. 2019). They noted that the topologies of single-gene trees usually differed from that of the concatenated tree...
- Cylindropuntieae. Bárcenas (2016) examined relationships in the Cylindropuntia area; Mayer and Rebman (2021) noted that there was a tritomy involving Grusonia and two clades of Cylindropuntia.
- Opuntieae. Majure and Puente (2014) looked at morphology and relationships around Opuntia, Ritz et al. (2012) examined the phylogeny and evolution of Andean species of Opuntia with terete stems, while Granados Aguilar et al. (2020) looked at hybridization in some Mexican species of Opuntia. Hybridization was also the focus in Martínez-González (2025: 7 markers, quite extensive analysis, see other articles by this author). Within the Opuntieae recovered by Köhler et al. (2020), the largest clade, Consolea, was sister to the rest. Köhler et al. (2023) looked at the plastomes of 43 taxa here (all the genera), and came up with largely consistent topologies in different analyses, although two major clades, one in Opuntia and one outside, did not have stable positions, there were problems with high bootstrap support not converting into much else, etc..
- Pterocacteae. Las Peñas et al. (2019) looked at the phylogeny and morphology of Tephrocactus.
Cactoideae. For the phylogeny and evolution of columnar Cactoideae, see Wallace & Gibson (2002) - Calymmanthium is odd. Hernández-Hernández et al. (2011, see also 2014) provide a quite detailed phylogeny of Cactoideae, although for the most part maximum likelihood bootstraps were rather low and maximum parsimony support still lower; earlier studies of Cacteae (Butterworth et al. 2002) and Mammillaria (Butterworth & Wallace 2004) faced the same problem. Disentangling relationships among North American columnar cacti presents major problems because of the consequences of slow coalescence (Copetti et al. 2017, also S. Hartmann et al. 2002). See Wallace and Cota (1996) for the PEP subunit β rpoCI intron. For relationships within South American mountain cacti, the BCT clade (Browningieae, Cereeae and Trichocereeae), see Ritz et al. (2007).
- Cacteae. Vázquez-Sánchez et al. (2013, 2017) and Vargas-Luna et al. (2018) discussed various aspects of the phylogeny of Cacteae, while Vásquez-Sánchez et al. (2019) found that Turbinicarpus - a "remarkable" genus - with some 20 species was polyphyletic, and they gave the three clades they found separate names. Breslin et al. (2021, 2022) looked at relationships around Mammillaria and divided the genus into Mammillaria s. str., Coryphantha and Cochemiea; to the extent that those genera do have distinctive characters, the distributions are rather erratic and they do not seem to be apomorphies. Sánchez et al. (2022), with a focus on Coryphantha and immediate relatives, found i.a. that C. macromeris was in the Excobaria clade and they moved M. mazatlanensis to Cochemiea.
- Cereeae. For Gymnocalycium, see Meregalli et al. (2010) and Demaio et al. (2011). Franco et al. (2017: plastome data) studied relationships in Cereeae, but c.f. in part Bombonata et al. (2020: RAD sequences). For Echinopsis (Trichocereinae) see Schlumpberger and Renner (2012). Majure et al. (2022b) looked at Melocactus (Cereinae), diverse in the Caribbean and with a number of cryptic species; relationships in its immediate outgroups were well supported, but sampling was poor. Fantinati et al. (2021: 1 nuclear and 4 chloroplast markers) found that Cereinae were monophyletic. Taylor et al. (2023: Cactaceae591 probe set, 18 plastid genes) found that there was some conflict between the topologies produced by the ontarget and supercontig data sets, and in particular in the former Cipocereus was inside Cereus, probably rapid radiation, and there had also been a deep introgression event between Cereus clades A and [C + D]. Romeiro-Brito et al. (2023a: 28 nuclear, plastid, mitochondrial loci, 31 spp.) examined the phylogeny of Pilosocereus; P. aureispinus was sister to rest of the genus - however, support along the spine of the tree could be better, agreement with earlier infrageneric groupings was poor, and there was conflict between analyses. Romeiro-Brito et al. (2023b) also looked at relationships - various analyses - within Cereeae as a whole using the Angiosperm353 and Cactaceae591 probe sets and sampling 43 and 29 respectively of the 48 recognized genera.
- In Echinocereeae, the focus in Tapia et al. (2017) was on relationships around Cephalocereus; for Echinocereus itself, see Sánchez et al. (2014).
- Korotkova et al. (2018) looked at relationships in Hylocereeae (doubtfully separate from the preceding), as did Cruz et al. (2016), several species being epiphytic and having flattened stems. Recent work has been quite extensive.
- In Notocacteae, the large genus Eriosyce turned out to be monophyletic, and major relationships within the genus were for the most part well supported (Guerrero et al. 2019).
- For relationships in Rhipsalideae, see Calvente et al. (2011a, b). Calvente et al. (2011a) found that Hatiora was polyphyletic, augmenting Schlumbergia and providing a key to the four genera of the Rhipsalideae that they recognized. The focus in Calvente et al. (2011b) was on relationships in Rhipsalis itself - 33/37 spp. examined, 3 plastid and 2 nuclear markers - and biogeography (origin in coastal Brazil), ecology (transition from the epiphytic habit to (epiphytic-)rupicolous) and character evolution. Korotkova et al. (2010) found that Pfeiffera - relationships unclear, perhaps Rhipsalidae - was polyphyletic, and they reinstated Lymanbensonia, suggesting that it should go in a separate tribe.
Classification. For a recent classification of the whole family, genera and tribes being listed, see Nyffeler and Eggli (2010a); Lodé (2015a, b) presented a new classification.
Over the years, there have been major disagreements over generic limits (e.g. Gibson et al. 1986), and depending on the author, the number of genera in the family varies by a factor of ten, and of the species by a factor of two. For example, there were a mere sixteen genera in Cactoideae in 1903, but now as many as 116 genera may be recognized there (Hunt 2002). Overall, generic limits tend to be drawn rather narrowly. Bárcenas et al. (2011) sampled quite extensively in the family and found that many tribes and genera in both the major subfamilies were not monophyletic: Only 4/6 and 14/36 genera of Opuntioideae and Cactoideae respectively for which two or more species were sampled turned out to be monophyletic, that is, only half the genera that had more than a single species were monophyletic. For a discussion on generic limits in the whole family, confirming that the situation is indeed chaotic at all levels, see Hernández-Ledesma et al. (2015). As elsewhere, floral traits often reflect pollinator preferences rather than clades, and growth habit is also labile (Schlumpberger & Renner 2012: Echinopsis area). Much phylogenetic work explicitly or implicitly has taxonomic implications (e.g. Korotkova et al. 2010; Calvente et al. 2011; especially Bárcenas et al. 2011), although the latter in particular were appropriately taxonomically conservative. For generic limits in Cacteae, see also Vázquez-Sánchez et al. (2013), Vargas-Luna et al. (2018) and Sánchez et al. (2022), and for those in Hylocereeae, see Korotkova et al. (2018). Opuntia has been broadly delimited, but Wallace and Dickie (2002) suggested that it should be dismembered, Opuntioideae then including sixteen genera. The situation in Opuntioideae is a mess, as is clear from the study by Griffith and Porter (2009). Hunt (1999, 2002) had earlier proposed the recognition of about eight broadly-delimited genera, roughly equivalent to tribes of other workers, which certainly made sense pending sorting out the phylogeny of the group as a whole - and might also be a sensible final solution. Whether or not the stakeholders (Griffith & Porter 2009) would agree would be another matter.
The classification above follows that proposed by de Vos et al. (2025), although a number of nodes there are poorly supported and two subtribes are paraphyletic - not, the authors note, because they support the use of paraphyletic taxa, but because relationships were unclear. There are a number of similarities with a classification proposed earlier by Nyffler and Eggli (2010a), however, it is clear that we can expect changes. De Vos et al. (2025) also include a useful generic synonymy.
For a checklist of Opuntioideae, see Hunt (2014), Hernández et alin Cactaceae . (2014) and Köhler et al. (2020: 17 genera recognized), and for an infrageneric classification of Coryphantha, see Sánchez et al. (2022). Romeiro-Brito et al. (2023b) provide a subtribal and generic classification for Cereeae, the erstwhile BCT clade. Calvente (2012) enumerated the taxa in Rhipsalis, recognising three subgenera. Rowley (2004) listed names of nothogenera, and these had doubled in number in the preceding ten years.
Previous Relationships. Despite the distinctive appearance of the "leafless" cacti, the relationships of the family with other Caryophyllales has generally been recognized.