EMBRYOPSIDA Pirani & Prado
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE // ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID I / LAMIIDAE / [CARDIOPTERIDALES [GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]]: ?
[GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]: G [2], superposed; loss of introns 18-23 in RPB2 d copy. - check
[ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]: vessel elements with with simple perforation plates; nodes 1:1.
Evolution: Divergence & Distribution. For apomorphies, see Stull et al. (2020a).
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
ICACINALES van Tieghem - Back to Main Tree.
Just the one family, 22 genera, 195 species.
Age. Wikström et al. (2015: note topology) estimate the age for this clade to be (117-)110(-100) Ma.
Synonymy: Icacinanae Doweld
ICACINACEAE Miers, nom. cons. - Back to Icacinales
Evergreen trees; ?iridoids, monoterpene indole/quinoline alkaloid camptothecin and derivatives +; vessel elements with simple perforation plates, ± in tangential multiples, 175-860(-1,200) µm long, fibres 650-1,630(-2,300) µm long; (vasicentric axial parenchyma +); laticifers + [?which]; nodes 1:1; stomata cyclocytic (anomocytic); lamina (margin serrate); pedicels articulated; K free to connate basally, C ± free, valvate, (with an adaxial ridge), apically incurved (flat, recurved), hairs ad- and abaxially (not); A free, filaments ?shorter than anthers; pollen (echinate; 1 G fertile; ovule collateral-superposed intermediate; lacking parietal tissue; (fruit ± compressed, ridged); seed coat?, testal bundles 0, endosperm copious, embryo long; n = ?
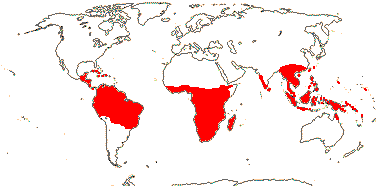
23 [list]/190 (165). Pantropical, inc. W. Pacific, to China and Japan. Map: Sleumer (1971a), Utteridge and Brummitt (2007), Trop. Afr. Fl. Pl. Ecol. Distr. 5. (2010) and Rozefelds et al. (2020: Figs 1, 4).
Age. K. Bremer et al. (2004a) suggested an age of around 115 Ma for this clade and Wikström et al. (2015) an age of (115-)102(-74) Ma (inc. Cassinopsis?).
1. Mappieae Baillon
Inflorescence cymose; nectary 0; style +, stigma capitate to punctate; chalazal endosperm haustorium +, forming syncytium with ovule cells [Nothapodytes]; n = ?
2/9. Tropical America, Sri Lanka, India, East Asia, Malesia.
[Icacineae [Iodeae + Phytocreneae]]: (lianes, secondary thickening anomalous).
2. Icacineae Engler
(Tuberous); lamina (margin dentate); (nectary +); (styles 2-3 - Casimirella); endosperm starchy ["seed starchy": Merrilliodendron]; n = 24.
7/35. Tropical Africa and America, Merrilliodendron east Malesia and west Pacific.
3. Iodeae Engler —— Synonymy: Iodaceae van Tieghem
Lianes, climbing by subaxillary shortly bifid branch tendrils or twining; secondary thickening often atypical [included phloem +, etc.]; medullary bundles + [Iodes]; (plant dioecious); nectary 0; style short/0, stigma broad; n = 10.
2/31: Iodes (29). Tropical Africa, Madagascar, south China to New Guinea and the Solomon Islands.
Age. There are fossil endocarps of Iodes ca 95 Ma in Cenomanian deposits from Europe (Knobloch & Mai 1986) and they were common in southern France 57-56 Ma - the latter fruits show some specific similarities with extant Asian taxa (Del Rio et al. 2019).
4. Phytocreneae Bentham & J. D. Hooker —— Synonymy: Phytocrenaceae R. Brown, Pleurisanthaceae van Tieghem, Sarcostigmataceae van Tieghem
(Lianes), (stem base massively swollen), (roots swollen); lamina (palmately lobed/veined), (margins toothed); dioecy common; inflorescences racemose, spicate, capitate; K ± free, (0 – Pyrenacantha - Pyr.), C flat; (filaments weakly adnate to C - Vadensea); nectary +/(glands between stamens - Hosiea)/0; style +, stigma capitate (linear) (with several lobes - Py.)/(style 0, stigma capitate - Sarcostigma); integument 7-10 cells across, (ovule apically bitegmic - Phytocrene); (C accrescent, surrounding fruit - Py.); stone rather flattened, pock-marked, outer cells of sclereidal layer with sinuous anticlinal walls [Stachyanthus, Miquelia, Phytocrene, Pyr.]; endosperm ruminate, (± 0 - Sarcostigma); n = 11, 20.
11/88: Pyrenacantha (30<). Africa-Madagascar, Southeast Asia and Indo Malesia to northeastern Australia.
Age. Fossil flowers (Icacinanthium tainiaphorum) have been found in basal Eocene Le Quesnoy amber in France ca 56 Ma; they linked with Phytocreneae in an analysis that included Icacinaceae and a number of other asterids (Del Rio et al. 2017b). Fruits from the early Palaeocene ca 63 Ma (Palaeophytocrene) are known from the far south of Argentina (Poore et al. 2023, q.v. for other literature of fossil fruits of Phytocreneae).
To integrate: petiole bundles arcuate and with wing bundles, or annular (and with medullary bundles); leaves conduplicate(-plicate); ovule (1/carpel), integument vascularized/not, funicular obturator +; (embryo short); seedling with hypocotyl, phanerocotylar.
Evolution: Divergence & Distribution. For a comprehensive review of the fossil record of Icacinaceae, see del Rio & de Franceschi (2020). The family was widespread and diverse in the Northern Hemisphere during the Palaeocene/Eocene, with genera now restricted to Southeast Asia-Malesia then growing in North America and Europe, Iodes being a good example (Knobloch & Mai 1986; Rankin et al. 2008; Stull et al. 2011, 2016; Del Rio et al. 2018). All told, there are 7 icacinaceous genera and 21 species from the London Clay flora alone (Stull et al. 2016). However, it is evident that Icacinaceae were even more widespread early in the Tertiary, with Middle to Late Eocene fossils of Phytocreneae from South Australia and Tasmania (Rozefelds et al. 2020), Early Oligocene fossils from North Africa (Stull et al. 2020b), while endocarps identifiable as Old World Phytocreneae are known from both North and South America, including the very far south of Arentinian Patagonia (Palaeophytocrene ga: Poore et al. 2023), in Palaeocene deposits about 63-58 Ma (see also Stull et al. 2012a). The family could quite possibly have moved between the southern continents via high southern latitudes, although no icacinaceous fossils are yet known from Antarctica (Rozefelds et al. 2020; Poore et al. 2023). Palaeophytocrene chicoensis, from the Upper Cretaceous of western North America, has recently been described by Atkinson (2022), and Rozefelds et al. (2020: Figs 1, 4) provide maps of fossil and extant Icacinaceae. For more on fossils of Iodeeae, see Pigg et al. (2008a) and Del Rio et al. (2019), for fossil icacinaceous pollen in particular, see Manchester et al. (2015).
Ecology & Physiology. There are about 70 species of lianes in Icacinaceae. These often have vessels with simple perforation plates, and a correlation between the liane habit and simple vessels has been noted here and elsewhere (Carlquist 1991b; Isnard & Feild 2015).
For the indole alkaloid camptothecin, found in a number of genera as well as in Nyssaceae, see Lorence and Nessler (2004). Camptothecin targets the eukaryotic topoisomerase 1 which regulates the winding of DNA and may prevent insect herbivory, however, the plants are probably protected by changes in the amino acid sequence of the enzyme, and at least one of these changes is identical to changes in camptothecin-resistant human leukemia cells (Sirikantaramas et al. 2009).
Plant-Bacterial/Fungal Associations. Camptothecin may be derived from secologanin, and ultimately synthesized by an endophytic fungus related to something like Rhizopus oryzae or the glomeromcyete Entrophosphora (Puri et al. 2005; Wink 2008; Shweta et al. 2010).
Chemistry, Morphology, etc.. Hosiea, with its long-petiolate leaves that have palmate venation and long-dentate margins, is particularly distinctive vegetatively. The leaf teeth of Pyrenacantha are well-developed, robust, almost tubular affairs; leaf tooth morphology would repay investigation.
Both sympetaly and the extensive adnation of the filaments to the corolla in Vadensia are only weakly developed (Jongkind & Lachenaud 2019).
Invaginations of the stone in Phytocreneae often signal invaginations of the endosperm, i.e. ruminate endosperm (Stull et al. 2012a and references). Swamy and Ganapathy (1958; see also Plachno & Swiatek 2010b) described the endosperm haustorium of Nothapodytes foetida as initially consisting of a single large cell that made contact with adjacent chalazal cells by making holes in their walls, the contents of those cells then moving into the endosperm cell, so forming a syncytium involving cells of different types (endosperm + maternal cells - c.f. Utricularia); that cell eventually died, but adjacent endosperm cells were also quite large.
For additional information, see Sleumer (1942a, 1971a), Howard (1942a, b), Kårehed (2001, 2002b), Utteridge et al. (2005) and Potgieter and Duno (2016), all general, also Kaplan et al. (1991: chemistry) and Guo et al. (2015: camptothecin and derivatives), Cremers (1973, 1974: growth patterns), Bailey and Howard (1941a-d) and Lens et al. (2008a: measurements here = range of means and upper end of variation, immature specimens excluded), all vascular anatomy, Heintzelmann and Howard (1948: crystals and indumentum), van Staveren and Baas (1973: epidermis), Baas (1973a: epidermis, 1974: stomata), Teo and Haron (1999: anatomy), Lobreau-Callen (1972, 1973, 1977, 1980), pollen, Mauritzon (1936c), Fagerlind (1945a), and Mauritzon in Sleumer (1942: Pyrenacantha), embryology, and Baillon (1874), Rankin et al. (2008) and Pigg et al. (2008a), all fruit anatomy, the two latter with a focus on fossils.
The family is very poorly known embryologically.
Phylogeny. Relationships in Lens et al. (2008a) were [[Nothapodytes + Mappia] [Natsiatum [[Iodes, Pyrenacantha, etc.] [Icacina, Alsodeiopsis]]]], although the last pair of sister taxa were not recovered in Bayesian analyses. There were similar relationships in Angulo et al. (2013: one gene, moderate to good support): [[Nothapodytes + Mappia] [[Iodes, Pyrenacantha, etc.] [Icacina, Leretia etc.]]]. Trees with this basic topology were also recovered by Stull et al. (2015), and the position of the [Nothapodytes + Mappia] clade as sister to the rest and also many other groupings had strong support; although Cassinopsis was sister to all other Icacinaceae, this position had only moderate support - and Cassinopsis has been found to be sister to Metteniusaceae (q.v.) included in the nuclear analysis of R. Zhang et al. 2020).
Classification. For some generic limits, see Byng et al. (2014).
Previous Relationships. The genera that until a few years ago made up Icacinaceae are now included in Icacinaceae s. str. (Icacinales) and as Cardiopteridales, Metteniusales, or as sister to all other core asterids as Oncothecales, and also in campanulids in Apiales (as Pennantiaceae, but position still has problems).