EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins uncommon; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; ="apo">seed single; duplication of the PI gene.
ASTERID II / CAMPANULIDAE / [[METTENIUSALES + ONCOTHECALES] [BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]]]: ?iridoids +.
8 orders, 25 families, genera, species.
Age. C. Zhang et al. (2020) suggested an age for this clade of ca 118.1 Ma.
The oldest fossils that have been associated with this clade are ca 83.5 Ma, from the Late Santonian-Early Campanian, and have been assigned to Paracryphiales (q.v.), perhaps the youngest campanulid order, although their identity is perhaps suspect (see also Cornales - Beaulieu et al. 2013a).
Evolution: Divergence & Distribution. Diversification at this node may have occurred in the southern hemisphere (Beaulieu et al. 2013a). Beaulieu et al. (2013a) and Beaulieu and; O'Meara (2018) suggested that crown group ages of all the Campanulid orders except Paracryphiales were Cenomanian, ca 100-94 Ma, while the crown-group ages of all families were Caenozoic.
Pollination Biology & Seed Dispersal. In the campanulids the fruits often have few (commonly only 1-2) seeds, although families like Campanulaceae and Goodeniaceae are exceptions. Even when each flower has only one or two seeds, these are generally small, indeed, gentianids as a whole have rather small seeds (Linkies et al. 2010). Beaulieu and Donoghue (2013; see Beaulieu & O'Meara 2016) examined fruit evolution in campanulids from an ecological point of view and concluded that the plesiomorphic fruit type for the whole group was likely to be dry, dehiscent, and with two or more seeds. Clades with achenes, dry, indehiscent, single-seeded fruits (they include Apiaceae, although there the disseminules are individual mericarps that result from the septicidal separation of the two-seeded fruits) were often associated with increased diversification rates. However, Beaulieu and Donoghue (2013) were unclear as to any particular causal connections between fruit types and diversification rates. These results may have to be modified somewhat when outgroups are included (e.g., question: What are the likely states for the gentianids as a whole?), if fruit types are redefined, and as different methods of analysis are used (Beaulieu & O'Meara 2016); it is possible that the plesiomorphic fruit morphology of the campanulids (and lamiids) was quite large, fleshy, indehiscent and single-seeded (see also elsewhere).
Plant-Animal Interactions. Clades of the dipteran agromyzid leaf miner Phytomyza diversified considerably in the campanulids; they moved there from Ranunculaceae (Winkler et al. 2009: >700 species in the genus).
Chemistry, Morphology, etc.. For more on the inferior/superior ovary distinction in the campanulids, see the Asterales page.
Phylogeny. There has been moderate to quite strong support for Aquifoliales as sister to all other campanulids (e.g. Olmstead et al. 2000; Soltis et al. 2000; B. Bremer et al. 2002; Janssens et al. 2009; Beaulieu et al. 2013b; Sun et al. 2014; Barba-Montoya et al. 2018; Beaulieu & O'Meara 2018; Stull et al. 2018; H.-T. Li et al. 2021; Y.-L. Qiu et al. 2024) - much of this work used chloroplast genes/genomes to estabish relationships. However, a position of Aquifoliales in the lamiids was then preferred; for further discussion about the position of Aquifoliaceae, the circumscription of Aquifoliales and other woody lineages (many ex-Icacinaceae s.l.) that may be at the base of the lamiid and campanulid (see also Kew Tree of Life ix.2024) clades, all related issues, see the euasterid page.
There are still question marks about the relationships of some of the major clades within the campanulids. The position of Dipsacales within this large clade in early phylogenetic analyses was unclear. Downie and Palmer (1992) associated Adoxaceae with Asterales, while they were sister to Apiales in some studies (Backlund & Bremer 1997). The clade [Asterales [Apiales + Dipsacales]] (e.g. Nandi et al. 1998; Olmstead et al. 2000; Lundberg 2001c; Lens et al. 2008a; see also B. Bremer et al. 2002; Winkworth et al. 2008a; Beaulieu et al. 2013b) is generally supported. On the other hand, Janssens et al. (2009: two genes) found weak support for the clade [Dipsacales [Asterales + Apiales]], Qiu et al. (2010) for [Apiales [Dipsacales + Asterales]], and Soltis et al. (2011) in their 17-gene study found little support for any relationships other than strong support for Aquifoliales as sister to the rest of the clade. Relationships towards the base of asterids are also different from those shown here - there is a clade [Escalloniales ["Bruniales" + Asterales]] - in Wikström et al. (2015: Bruniales here are not monophyletic). Beaulieu and O'Meara (2018) found Bruniales to be sister to [Dipsacales + Paracryphiales] in some analyses, but the position of Bruniaceae s. str. somewhere near the base of the campanulids has little support in the Kew Tree of Life ix.2024 and the position of Pennantia, previously in Icacinaceae and then placed in Apiales, is also uncertain, but it is possibly not a member of Apiales.
Various small families have been placed around Escalloniaceae in the campanulids, but initially with uncertain support; recent work may be clarifying their relationships (Winkworth et al. 2008a; especially Tank & Donoghue 2010; Beaulieu et al. 2013b; Wikström et al. 2015). For Polyosmaceae, Eremosynaceae, etc., see Escalloniaceae. However, where Escalloniales themselves are to go is somewhat unclear (see Beaulieu & O'Meara 2018 for literature). Thus Barba-Montoya et al. (2018) recovered a topology [Asterales [Escalloniales [Bruniales...]]], and in some analyses in Beaulieu and O'Meara (2018) a topology [Escalloniales [Asterales [Bruniales [Apiales...]]]] was recovered. Tank and Olmstead (pers. comm.) even recovered Escallonia as sister to Desfontainia (Bruniales), while Polyosma was well separated. Focussing on relationships along the spine of the Pentapetalae, Zeng et al. (2017) found the relationships [Apiales [Dipsacales + Asterales]], but no Escalloniales, Bruniales or Paracryphiales were included, only Asteraceae of Asterales, etc., so no sleep need be lost here. Magallón et al. (2015) recovered the relationships [[Escalloniales + Asterales] [Bruniales...]] and Stull et al. (2018) also recovered an [Asterales + Escalloniales] [Bruniales...]] grouping, and these relationships were strongly supported (95% bootstrap) by H.-T. Li et al. (2019) in their plastome analysis, while in Li et al. (2021) [Columelliaceae + Bruniaceae] were sister to [Apiales + Dipsacales], Escalloniaceae being sister to Asterales. However, in Zuntini et al. (2024) the relationships [Bruniaceae [[Columelliaceae, Escalloniaceae in Dipsacales] [Apiales]] [Asterales]]] were recovered. (Note that here Bruniales are divided into Bruniales s. str. (= Bruniaceae alone) plus Desfontaineales (= Columelliaceae alone).) Relationships in Y.-L. Qiu et al. (2024) are [Bruniales [Asterales [[Paracryphiales + Escalloniales] [Apiales [Dipsacales + Desfontaineales]]]]].
The relationships of Paracryphiaceae have also been causing some problems. They contain only the three genera Paracryphia, Quintinia and Sphenostemon, and Baker et al. (2021: see Seed Plant Tree) also found that the latter two genera were placed togther (Paracryphia itself was not sampled). Paracryphiaceae may be sister to Dipsacales (e.g. Stull et al. 2018). However, in an early study Paracryphia was linked quite strongly with Rutaceae + Meliaceae + Simaroubaceae (Källersjö et al. 1998, but c.f. Savolainen et al. 2000a). Similarly, Soltis et al. (2011: 17 genes) found that Quintinia linked with Polyosma (Escalloniales here), support for this position coming from the mitochondrial component of the analysis; Soltis et al. (2011) were inclined to think that horizontal gene transfer of the mitochondrial genes might be involved since the two genera sometimes grow together. Paracryphiaceae form a clade in Lundberg's three-gene Bayesian analysis (Lundberg 2001e); Cameron (2001, 2003) also suggested an association between Paracryphia and Sphenostemon. The clade [Paracryphia + Quintinia] was found to be sister to Dipsacales, although not in analyses that included coding chloroplast genes (Winkworth et al. 2008a); see also especially Tank and Donoghue (2010), but support for a [Paracryphiaceae + Dipsacales] clade was only slight in the full analysis of Soltis et al. (2011). The position of Quintinia remains unsatisfactory for some (Friis et al. 2013b).
Within Bruniales in the old (broad) sense, Bruniaceae were sister (1.0 Bayesian p.p.) to Columelliaceae in many analyses in Winkworth et al. (2008a), although not when coding chloroplast sequences were used alone, while Soltis et al. (2011) found strong support for this clade only when mitochondrial genes were removed from the analysis. The old Bruniales, Columelliaceae s.l. plus Bruniaceae, were an unexpected grouping. In the pre-April 2008 versions of this site [Columelliaceae + Desfontainiaceae] (= Columelliaceae s.l.) were placed sister to Dipsacales; the position of Columelliaceae s.l. in that area had early been suggested by Bremer et al. (2001) and especially by Lundberg (2001e; see also Backlund 1996), although support was at best moderate. Indeed, both Columelliaceae s.l. and Dipsacales have opposite leaves, and Columellia, like Dipsacales, has amoeboid tapetum (c.f. Bremer et al. 2001), although Desfontainia does not (Maldonado de Magnano 1986a). The similarities that the pair has with Dipsacales seemed to indicate either substantial homoplasy or (less likely) a suite of rather basal synapomorphies in the campanulids of which there is currently no indication. Other relationships have been suggested (Gustafsson et al. 1996; Backlund & Bremer 1997; Pyck & Smets 2000; Bell et al. 2001); for a possible association of Bruniaceae with Asterales, see Lundberg (2001e) and Soltis et al. (2011).
However, changes are underway. Relationships that have been discussed up to now have been based largely on analyses of chloroplast data, and recent analyses of the nuclear genome are suggesting a rather different story (C. Zhang et al. 2020). Metteniusales, previously included in the Asterid I/Lamiid clade, were found to be sister to all other Asterid II/Campanulid taxa, and Bruniales were found to be polyphyletic, Bruniaceae alone (= Bruniales s. str.) being sister to the remainder of the Campanulid group, while Columelliaceae are quite separate (see Columelliales); nearly all these relationships - less so the position of Metteniusales and of Bruniaceae - have strong support (C. Zhang et al. 2020). After Bruniales come Asterales, while most other campanulids can be represented as a polytomy for now, i.e. [Columelliales, Escalloniales, Paracryphiales, Dipsacales]. Zuntini et al. (2024) in their Angiosperms353 nuclear probe analysis found the following relationships (* = maximum likelihood value less than 0.95): [[Metteniusales + Oncothecales] [Bruniales *[Asterales [Apiales *[Columelliales *[Dipsacales [Escalloniales + Paracryphiales]]]]]]].
To summarize: The overall topology [Bruniales s.l. [Apiales [Paracryphiales + Dipsacales]]] seemed to be fairly well established (Winkworth et al. 2008a; Tank & Donoghue 2010; Beaulieu et al. 2013a; Barba-Montoya et al. 2018; Stull et al. 2018; c.f. Stull et al. 2020), with [Escalloniales + Asterales] sister to them. These relationships are breaking down, furthermore, Aquifoliales, which as mentioned were previously thought to be sister to all campanulids, are apparently polyphyletic and are now divided into Aquifoliales s. str. and Cardiopteridales that are (perhaps) both to be placed at/near the base of the Asterid I/lamiid clade, while Metteniusales, previously near-basal in the lamiids, now take the position of Aquifoliales as sister to all other members of the Asterid II/campanulid clade (C. Zhang et al. 2020).
In addition to Metteniusales below, the genera that until a few years ago made up Icacinaceae have been separated and are now included in the lamiids, i.e. as Icacinaceae s. str. (Icacinales) and as Cardiopteridales, as well as elsewhere in the campanulids in Apiales as Pennantiaceae and as sister to all other core asterids as Oncothecales.
METTENIUSALES Takhtajan - Main Tree.
Just the one family, 11 genera, 55 species.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Synonymy: Emmotales Doweld
METTENIUSACEAE Schnizlein —— Synonymy: Emmotaceae van Tieghem - Back to Metteniusales
Evergreen trees; carboxycyclic[?] iridoids +, (verbascosides + - Cassinopsis - C.], tanniniferous cells +; cork?; vessel elements (with simple perforation plates - Raphiostylis - R.), (680 [R.])- 860-2,230(-2,900) µm long, fibres [1,150-]1,680-4,240(-4,900) µm long; xylem parenchyma various; nodes (1:1 - R.; 5:5 - Metteniusa - M.); (axillary thorns + - C.); petiole bundle V-shaped to annular with up to three pairs of wing bundles, or annular, lacking pericyclic fibres; (epidermis mucilaginous - Apoodytes), (hairs T-shaped/stellate); stomata anomocytic to cyclocytic; mesophyll fibres/sclereids +; leaves spiral or 2-ranked (opposite - C), lamina vernation conduplicate, margins entire (toothed); (plant dioecious); inflorescence cymose/thyrsiform, pedicels articulated; K short, basally connate or not, quincuncial, C (0 - pistillate flowers of Platea), valvate (imbricate - C.), basally connate or not, (connate, tube formation late - M.), adaxially keeled (not - C.), hairs along keel, (secretory - M.); A adnate to base of C tube, (± latrorse - Emmotum [E.]/extrorse), (thecae 1-sporangiate - E.), loculi dehiscing individually, (anthers long, polysporangiate, pollen sacs separate, locelli dehiscing individually, connective massive - M.), (endothecial thickenings 0 - M., Pittosporopsis [P.]), tanniniferous cells +, (bulliform cells +), filament stout, constricted below anther [P], (connective apically prolonged/with appendages); nectary 0/+; [G 5], opposite C, 2 smallest each with 1 ovule - M./3 abaxial with 1-2 ovules, 2 adaxial 0 ovules - E./unilocular, 1 ovule - Dendrobangia/unilocular, 2 ovules [Oecopetalum, P.], style very short to long, slender (basally stout), stigma punctate; ovules superposed, apotropous, funicle massive [M.] and with ca 9 vascular bundles [P.], integument ca 6 cells across, parietal tissue 0 [Apodytes], or 20+ cells across, vascularized [M.], or partly bitegmic, integuments to 10 cells across [C.], or bitegmic, micropyle endostomal, outer integument 3-5 cells across, inner integument 3-4 cells across [E.], combined ca 15 cells across [P.], parietal tissue 0-3 cells across, endothelium + [E.]/0 [P.], (hypostase + - P.); fruit (very asymmetric), (pubescent inside - Ottoschulzia); seed (ruminate), coat thin, vascularized [M., E.]; embryo very short (long), (curved); n = 20, ?22; seedlings with hypocotyl, phanerocotylar.
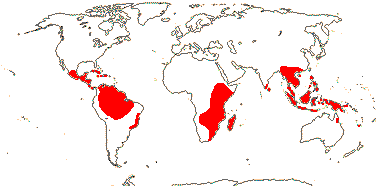
13 [list]/57: Emmotum (12), Raphiostylis (12), Calatola (9), Cassinopsis (8). Tropical. Map: from Sleumer (1971a) and Lozano C. and Lozano (1988). Photo Flower.
Evolution: Divergence & Distribution. The distinctive crotonoid pollen of the Malesian Platea is known from the Palaeocene of both west central Greenland (Agatdalen, the Nuussuaq Peninsula) and Arkansas (Manchester et al. 2015; Lobreau-Callen & Srivastava 1974).
Metteniusa is florally very autapomorphic (González & Rudall 2010), and in general there is a lot of variation in the androecium and gynoecium here, hence the length of the characterization above. The distinctive nodal and wood anatomy of Raphiostylis seem to be derived (see above: Lens et al. 2008a).
Chemistry, Morphology, etc.. Note that much of the older literature about Metteniusaceae is under Icacinaceae.
Calatola has the Cordia growth pattern in which the apex of the stem aborts after producing a whorl of plagiotropic branches, most branches remain plagiotropic while one becomes secondarily orthotropic and forms the renewal shoot.
Del Rio et al. (2020) discuss corolla hairs. There is considerable variation in anther and gynoecial morphology in Mettiusiaceae (see also D.-R. Kong et al. 2018, 2022). Metteniusa, very distinctive with its long, locellate anthers, is in a clade whose other members also tend to have long anthers (Sleumer 1942a). The description of the ovule of Pittosporopsis above is that of the fertile ovule; the other ovule has a much longer but more slender funicle with but a single bundle (Kong et al. 2022). The two integuments are closely adpressed, and Kong et al. (2022) give their combined thickness as ca 15 cells across; it may be that inner integument is quite thin (see their Fig. 4d). For the floral morphology of Metteniusa, see González and Rudall (2010), for that of Emmotum see Endress and Rapini (2014), also the discussion on the Icacinales page. The anthers ofOttoschulzia are adherent apically, forming a little basket-like structure over the gynoecium (Santiago-Valentín & Viruet-Oquendo 2013). The fruits can be very asymmetric, as in Raphiostylis and still more in Apodytes. In southern African species of the latter there is a large fleshy, aril-like structure, apparently developing from a reduced carpel, on one side of the fruit (Potgieter and van Wyk 1994a), on the other hand, the fruit morphology of Cassinopsis, from the same area, is relatively undistinguished (Potgieter & van Wyk 1994a).
For additional information, see Sleumer (1942a, 1971a), Howard (1942a, b), Kårehed (2001, 2002b), Utteridge et al. (2005), Dickison and Bittrich (2016) and Potgieter and Duno (2016), all general, also Cremers (1973, 1974: growth patterns), Kaplan et al. (1991: chemistry), Lens et al. (2008a: measurements = range of means and upper end of variation, immature specimens excluded) and Bailey and Howard (1941a-d) all vascular anatomy, Heintzelmann and Howard (1948: crystals and indumentum), van Staveren and Baas (1973: epidermis), Baas (1973a: epidermis, 1974: stomata); for floral morphology, Endress and Rapini (2014), pollen, see Lobreau-Callen (1972, 1973, 1977, 1980), embryology, Mauritzon (1936c) and Fagerlind (1945a), and fruit, Potgieter and van Wyk (1994b). For general information about Metteniusa, see Lozano C. and Lozano (1988), and for anatomy, etc., see Reed (1955).
Phylogeny. Stull et al. (2015) recovered a well-supported [Calatola + Platea] group as sister to the rest of Metteniusaceae, which is made up of the Apodytes and Emmotum groups; Metteniusa is well embedded in the latter, where it is sister to Ottoschulzia (see also Argulo et al. 2013), general relationships are [[Emmotum + Poraqueiba] [Metteniusa et al.]]. Relationships in the Apodytes group are [Apodytes [Raphiostylis + Dendrobangia]] (Stull et al. 2015). On the other hand, Hernández-Urban et al. (2019) found the relationships [Apodytes group [Platea group + Emmotum group]], and in the latter Metteniusa was sister to [Oecopetalum + Pittosporopsis], although with rather poor support.
Cassinopsis, previously thought to be sister to all other Icacinaceae, was found to be sister to Metteniusaceae by Stull et al. (2020) in their analysis of asterid nuclear genomes; Metteniusaceae there were sister to the lamiid clade, but that may be a sampling problem. C. Zhang et al. (2020) carried out a variety of analyses on the genomes/transcriptomes of 365 species of asterids and found some support for the Metteniusaceae they sampled (also including Cassinopsis) as being sister to everything in campanulids of pre-November 2020 versions of this site apart from Aquifoliales, that order now being in two pieces (Aquifoliales, Cardiopteridales) in the lamiids. Relationships in Metteniusaceae were [Cassinopsis [Apodytes [Pittosporopsis + Platea]]] (Zhang et al. 2019). Relationships in the Kew Tree of Life version ix.2024 are [Oncotheca humboldtiana [Metteniusa sp. [Cassinopsis ilicifolia [Apodytes dimidiata [Platea latifolia + P. parvifolia]]]]]. Thus in some analyses Cassinopsis is sister to all other Metteniusaceae; support values for the relationships are strong. For more on the circumscription and relationships of Metteniusales, campanulids and lamiids, see the euasterid clade.
Classification. Metteniusaceae are best expanded to include Cassinopsis, and although it does have some fairly distinctive features (see above) in vascular tissue, nodal anatomy, etc., it seems at home here.
Previous Relationships. A monotypic Metteniusales were placed immediately after a highly heterogeneous Icacinales by Takhtajan (1997); Metteniusa was not mentioned by Cronquist (1981).