EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID II / MALVIDAE / [[GERANIALES + MYRTALES] [CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]]: ?
[CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]: ?
[PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]: ovules 2/carpel, apical.
[SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]][: flavonols +; vessel elements with simple perforation plates; (cambium storied); petiole bundle(s) annular; style +; inner integument thicker than outer; endosperm at most scanty.
[HUERTEALES [MALVALES + BRASSICALES]]: ?
Age. The age of this node was estimated to be (94-)89(-85) or (80-)74(-68) Ma to around 96 Ma (Hengchang Wang et al. 2009), while Argout et al. (2010) give a date for this clade (or that of a bigger clade, one perhaps including the common ancestor of all malvids) of only ca 59 Ma (see also Xue et al. 2012), which has to be a major underestimate. A suggestion by Zhang et al. (2012) is for an age of (82-)73(-60) Ma, Foster et al. (2016a: q.v. for details) an age of ca 103 Ma, Magallón and Castillo (2009) estimated an age of ca 92 Ma, Naumann et al. (2013) ages of around 89.2 Ma, Hohmann et al. (2015) an age of ca 92.7 Ma, Tank et al. (2015: Table S1) an age of around 81 Ma, and the age in Muellner-Riehl et al. (2016) is (108.3-)88.1(-64.3) Ma.
Phylogeny. For discussion see the Sapindales page.
MALVALES Berchtold & J. Presl - Main Tree.
(Cyclopropenoid fatty acids +), flavones, myricetin +; mucilage cells + (0); C contorted [direction not fixed]; nectary 0; ovules 1/carpel; exotegmen palisade, endotegmen radial and/or inner periclinal walls thickened; embryo long, cotyledons thin, radicle short. - 10 families, 360 genera, 6,100 species.
Includes Bixaceae, Cistaceae, Cytinaceae, Dipterocarpaceae, Malvaceae, Muntingiaceae, Neuradaceae, Sarcolaenaceae, Sphaerosepalaceae, Thymelaeaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) estimate crown Malvales to be (75-)71, 67(-63) My; other suggestions are (80-)78(-76) and (76-)74(-72) Ma (H. Wang et al. 2009), ca 78.3 Ma (Tank et al. 2015: Table S2), 93.3-62.9 Ma (Magallón et al. 2015) and ca 102.4 Ma (Hernández-Gutiérrez & Magallón 2019).
Florissantia quilchenensis, from Eocene British Columbia, may be placed somewhere around here (Manchester 1992; López-Martínez et al. 2023a).
Evolution: Divergence & Distribution. Dating the separation of clades within this group is of considerable interest given the distributions of many of the families. Hernández-Gutiérrez and Magallón (2019) provide a careful study of ages throughout the order, paying attention to how the numbers of fossils used in calibrations, the accuracy of identification of those fossils, the use of Fossilized Birth-Death versus Node Dating methods, etc., all affected the ages; this paper should be consulted for more details. Ages aside, as Table 1 in Kubitzki and Chase (2002) suggests, details of the evolution of cyclopropenoid fatty acids (these have a 3-C ring in the middle of the C chain of a fatty acid) and many other features common in the order is difficult to understand.
Malvales contain ca 3.2% eudicot diversity (Magallón 1989) and show moderately high diversification rates (Magallón & Castillo 2009).
Plant-Bacterial/Fungal Associations. Taxa with ectomycorrhizal associations are common in this order, e.g. Malvaceae-Tilioideae and the Dipterocarpaceae/Cistaceae clade (Smith and Read 1997; Ducousso et al. 2004).
Chemistry, Morphology, etc.. For the distribution of cyclopropenoid fatty acids, which are also scattered outside Malvales, see Badami and Patil (1981), Gaydou and Ramanoelina (1983) and Bayer et al. (1999); these compounds are toxic to many insects, even vertebrates. Although taxa with geniculate petioles are quite common in Malvales, but this may not mentioned under individual families, however, this does not mean that those families lack pulvini, although they may (e.g. Cistaceae).
The androecium is possibly basically oligomerous, and the earliest initiated or innermost members are oppositisepalous with centrifugal or lateral polyandry. Starchy endosperm may be an apomorphy for the group. The exotesta appears to be palisade, but in at least some (basal) taxa the cells are also tangentially elongated (Nandi 1998a).
Some general information, esp. carpel orientation, is taken from from Nandi (1998a, b) and Kubitzki and Chase (2002); for wood anatomy, see i.a. den Outer and Vooren (1980) and den Outer and Schütz (1981).
Phylogeny Le Péchon and Gigord (2014) summarize phylogenetic studies in Malvales. Some clades within Malvales are quite well established, but relationships between them, as well as the position of one or two families, remain unclear (see also M. Sun et al. 2016). Fay et al. (1998a) and Bayer et al. (1999) discuss general relationships. These may be represented as [Muntingiaceae [[Bixaceae + Malvaceae] [[Thymelaeaceae + Sphaerosepalaceae] [Neuradaceae [Sarcolaenaceae [Dipterocarpaceae + Cistaceae]]]]]], but few of these relationships have much support, even after successive weighting (Bayer et al. 1999). Neuradaceae are likely to be sister to the rest of the order (see also Soltis et al. 2007a; Horn et al. 2016). Molecular data (Fay et al. 1998a) place Diegodendron close to Bixa in particular. Although Bixaceae are expanded here, it has also been suggested that Cochlospermum and relatives are close to Sphaerosepalaceae, less to Bixa (Johnson-Fulton & Watson 2008). The relationships of Sphaerosepalaceae within Malvales are unclear; in Bayer et al. (1999) they are weakly associated with Thymelaeaceae and in Alverson et al. (1998) with Bixaceae and Cochlospermaceae. H.-T. Li et al. (2019] recovered the relationships [Thymeleaceae [Malvaceae [Muntingiaceae [Bixaceae ...]]]], although support for the position of Malvaceae was not strong and some families were not sampled.
The clade [[Pakaraimaea + Cistaceae] [Sarcolaenaceae + Dipterocarpaceae]] is strongly supported in many molecular studies, including Li et al. (2019), Colli-Silva et al. (2025), etc.. Pakaraimaea in particular is sister to Cistaceae (Alverson et al. 1998; Kubitzki & Chase 2002; Ducousso et al. 2004; Horn et al. 2016). Indeed, evidence that Pakaraimaea has nothing immediately to do with Dipterocarpaceae, rather, it is sister to Cistaceae, now seems quite strong (Horn et al. 2016; see also Aubriot et al. 2016 if the tree in Fig. 2 is rooted between the outgroups and Sarcolaenaceae). Z.-D. Chen et al. (2016) found Helianthemum scopulicola to be embedded in Dipterocarpaceae-Dipterocarpoideae, but support for that position was only moderate. See also Soltis et al. (2011) for relationships, and although these are somewhat different from those discussed above, sampling is poor. Nandi (1998b) noted several similarities between Cistaceae and Sarcolaenaceae (hollow style, stigma morphology, carpel number and indumentum). Future morphological studies may well strengthen the characterisation of the whole clade, but relationships within it need to be clarified.
Hernández-Gutiérrez and Magallón (2019) recently studied relationships throughout the order. The positions of Neuradaceae, Cytinaceae and Muntingiaceae were unclear, and although relationships in their Bayesian analysis are most similar to those below, the topology of that tree differs from the tree that they used to discuss their dating analyses. [[Malvaceae + Muntingiaceae] [[Thymeleaceae [Cytinaceae + Neuradaceae]] [[Sphaerosepalaceae + Bixaceae] [Cistaceae [Sarcolaenaceae + Dipterocarpaceae]]]]]. Many of the relationships suggested in the comprehensive plastome analysis of H.-T. Li et al. (2021) did no+ have that strong support. Although with a focus on the Aquilaria area (Thymeleaceae), broader relationships in S. Y. Lee et al. (2022b) are [Dipterocarpaceae [Bixaceae + Thymeleaceae]] - Malvaceae are the outgroup. The recent study by Colli-Silva et al. (2025: 194 genera [just over half in the order], 309 genes of the Angiosperms353 data set) provides the basis for the family sequence and infrafamilial taxa recognized below, although support for the positions of Sphaerosepalaceae, the [Cytinaceae + Muntingiaceae] clade, for some subfamilies and tribes in Malvaceae, etc. are weak, Pakairaimea and members of Dipterocarpaceae-Dryobalanopseae were not included, and about half a dozen genera of Malvaceae that were included were left unplaced.
A relationship beween Cytinaceae and Malvales had early been suggested (Nickrent 2002), and this appeared in all analyses in Nickrent et al. (2004), although since the only Malvales included in the latter study were Cistaceae and Malvaceae, the placement of Cytinaceae remained somewhat provisional. However, Nickrent (2007: nuclear small-subunit [SSU] r-DNA was the nuclear gene used) found with much better sampling that Cytinaceae were sister to the poorly-known Muntingiaceae with moderate (maximum likelihood) to strong (maximum parsimony) support (see also H.-T. Li et al. 2021) - indeed, both Cytinaceae and other Malvales have exotegmic seeds, and aspects of the perianth of Cytinaceae and Malvaceae are perhaps similar. Naumann et al. (2013) recovered the relationships [Cytinaceae [Malvaceae [Bixaceae + Cistaceae]]], but no other Malvales were included, and M. Sun et al. (2016) also found it to be sister to the rest of the order, but with little support. Roquet et al. (2016) suggested that the relationships of Cytinaceae might be [Cytinaceae [Malvaceae + Thymelaeaceae]], but this is not shown in their Fig. 2. (Apodanthaceae, previously included in Cucurbitales but now included in Malpighiales sister to Rafflesiaceae (see Alzate et al. 2024), are also somewhat similar morphologically to Malvales (Nickrent et al. 2004).) However, overall relationships in Malvales were rather poorly supported, and those coming from a recent (vi.2023) analysis of an Angiosperms353 data set were [Neuradaceae [Cytinaceae [Thymelaeaceae [Malvaceae [Muntingiaceae [Sphaerosepalaceae [Bixaceae [Cistaceae [Sarcolaenaceae + Dipterocarpaceae]]]]]]]]] - no Pakaraimea, etc., included. Colli-Silva et al. (2025), using 309 genes of the Angiosperms353 data set, recovered a [Cytinaceae + Muntingiaceae] clade, and this is here quite deep in the phylogeny, not sister to Malvaceae, as I had placed it for quite some time on this site - however, as mentioned, support for this position is rather weak.
Previous Relationships. Elaeocarpaceae, previously usually included in (Cronquist 1981) or near Malvales, are here placed unambiguously - if somewhat unexpectedly - in Oxalidales. The enigmatic Huaceae, apparently now to be placed in a separate order, has a number of anatomical similarities that led Baas (e.g. Table II) to include them in Malvales, albeit tentatively. Most Malvales as delimited here are included in Takhtajan's (1997) Malvanae; the core Malvales in the past included just the families that are included in Malvaceae below - Malvaceae, Tiliaceae, Bombacaceae.
Synonymy: Aquilariales Link, Bixales Lindley, Bombacales Link, Byttneriales link, Cistales Berchtold & J. Presl, Cytinales Dumortier, Daphnales Lindley, Dipterocarpales Martius, Neuradales Martius, Sterculiales Berchtold & J. Presl, Thymelaeales Berchtold & J. Presl, Tiliales Berchtold & J. Presl - Malvanae Takhtajan - Cistopsida Bartling, Daphnopsida Meisner, Malvopsida R. Brown, Thymelaeopsida Endlicher - Malvidae Thorne & Reveal
NEURADACEAE Kostelvsky, nom. cons. - Back to Malvales —— Synonymy: Grielaceae Martynov
Annual (perennial) ± prostrate herbs/subshrubs; cyclopropenoid fatty acids +, ellagic acid?, tannins?; cork?; cambium storying?; pits not bordered; sieve tube plastids with protein crystalloids and starch; nodes ?; mucilage ducts +; petiole anatomy simple; cuticle waxes 0; hairs unicellular; leaves amphistomatic, spiral, lamina margins toothed to pinnatifid, secondary veins subpalmate, ?stipules; inflorescence cymose, ?cincinnus; hypanthium +, short; K valvate, C (imbricate), distinctively coloured when dry; A 10; pollen grains oblate, with 3 syncolpate at each pole, each colpus with a pore, [modified tri(tetra)colpo-diporate], tricellular; G [10], ± inferior, opposite sepals, ascidiate when young, (2-4 abaxial carpels infertile), styluli +, quite short, ± marginal, stigmas capitate; ovule (2/carpel), apotropous, lying horizontally, micropyle bistomal, outer integument ca 4 cells across, inner integument ca 4 cells across, parietal tissue ca 2 cells across, embryo sac podium +; fruit dry, indehiscent, (K accrescent, styles persistent, (forming spines); testa ± crushed, endotestal cells small, tegmen multiplicative, exotegmic cells also tangentially elongated, crystalliferous, other tegmic cells persistent; endosperm ?development, 0, embryo bent; n = 7, x = 7 (?8).
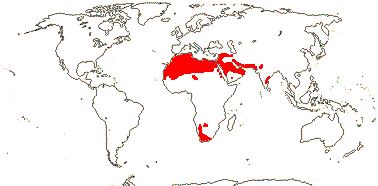
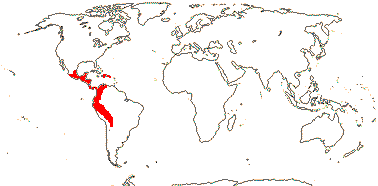
3/10: [list] - Grielum (5). Africa to India, dry or desert areas. Map: from Heywood (2007), also floras, esp. Miller and Cope (1996) and Trop. Afr. Fl. Pl. Ecol. Distr. 2 (2006). Photo: Flower.
Age. Neuradaceae are ca 26.3 Ma (Hernández-Gutiérrez & Magallón 2019).
Chemistry, Morphology, etc.. The vascular bundles have a mucilaginous sheath. The plant may lacks stipules, the stipule-like structures that are sometimes seen being prophylls (Bayer 2002); the presence of stipules should be confirmed. The basically cymose inflorescence/plant construction with paired but unequal leaves at the nodes makes things complicated.
The flowers of Grielum are obliquely monosymmetric, some of the carpels on one side of the flower being reduced and non-functional (Murbeck 1916). There appears to be no epicalyx (c.f. Remizowa 2019), but the outside of the ovary may have spines which become conspicuous in fruit; these develop centrifugally (Ronse DeCraene & Smets 1995d). The corolla of members of Neuradaceae changes colour on drying, as in some Malvaceae (Airy Shaw 1966) - see Huber (1993a). The pollen is odd; it normally has a triradiate colpar structure at each pole and with a porus in each branch - possibly a modified trisyncolpate, each colpus with two orae (see Bayer 2002; Polevova et al. 2010). There is sometimes a second, reduced ovule in the carpels (Murbeck 1916). The seed begins to germinate while still inside the fruit (e.g. Murbeck 1916), hence reports (Goldberg 1986; Takhtajan 1997) that the carpels dehisce ventrally.
For general information, see Murbeck (1916) and Bayer (2002); the family needs work.
Previous Relationships. Neuradaceae have previously been placed in Rosales (Cronquist 1981; Takhtajan 1997), or even within Rosaceae (Hutchinson 1973; Corner 1976), and the floral anatomy of the two is similar (Ronse Decraene & Smets 1995d), although Corner (1976) did note that the seed coat anatomy of the two appeared to be different.
Thanks. I am grateful to Z. Rogers for comments.
[Thymelaeaceae [[Muntingiaceae + Cytinaceae] [[Sphaerosepalaceae [Bixaceae [[Pakaraimaea + Cistaceae] [Sarcolaenaceae + Dipterocarpaceae]]]] Malvaceae]]]: pits vestured; phloem stratified, phloem rays wedge-shaped; style long; exotegmen much thickened and lignified, palisade.
Age. The age of this node is around (81-)75, 72(-65) Ma (Bell et al. 2010: note topology) or 80.8 to 78.3 Ma (Tank et al. 2015: Table S1, S2). On the other hand, if there is a clade [Malvaceae [Thymelaeaceae + Dipterocarpaceae]] - unlikely - the age of a [Thymelaeaceae + Dipterocarpaceae] clade is estimated to be 97.3/94.2/92.4 Ma and the age of the larger clade is around 102.7-102.4 Ma (Cvetkovic et al. (2022) while S. Y. Lee et al. (2022b) suggested that the age of a Bixaceae-Thymelaeaceae clade was ca 75.4 Ma and of a Dipterocarpaceae-Thymelaeaceae clade was ca 85.3 Ma...
Chemistry, Morphology, etc.. Species with stratified phloem always have wedge-shaped phloem rays, Sarcolaenaceae perhaps excepted (Kubitzki & Chase 2002).
THYMELAEACEAE Jussieu, nom. cons. - Back to Malvales
Wood often fluoresces; vascular tracheids +; secondary phloem fibres unlignified; nodes 1:1; crystal sand +; petiole bundle arcuate; epidermal cells (massively) mucilaginous; stomata cyclocytic; hairs simple; leaves spiral, lamina venation usu. pinnate, vernation supervolute (conduplicate), stipules 0 or minute; inflorescence cymose; flowers (3-)4-5(-6)-merous; K and C imbricate, C usu. connate, at least at the base; (filaments short); tapetal cells uninucleate; pollen grains tricellular; ovule epitropous, micropyle endostomal, (zig-zag), outer integument 3-6 cells across, inner integument 3-6 cells across, parietal tissue 3-7 cells across, nucellar cap 2-4 cells across, (± pachychalazal), hypostase + [cone of cells], obturator from near base of stylar canal; (testa fleshy), (tegmen multiplicative), exotegmen with brown contents, endotegmen with brown contents, reticulately thickened and lignified; embryo (suspensor 0), chlorophyll 0, cotyledons large; x = 9, nuclear genome [1 C] (0.14-)1.659(-19.721) pg.
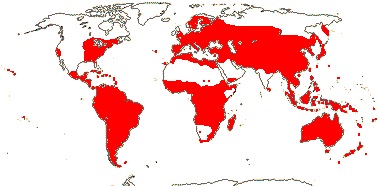
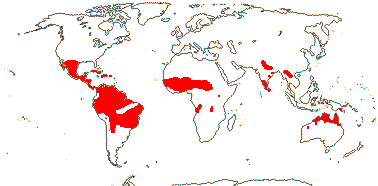
axillaryphloem not present; corolla usually gamopetalous, form-ing a floral tube, cupular or short; carpels 3–548/941: [list] - 3 groups below. World-wide, esp. trop. Africa and Australia. Map: from Domke (1934), Meusel et al. (1978), Coates Palgrave (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. 1 (2003). [Photo - Flower Flower, Fruit.]
1. Tepuianthoideae Reveal - Tepuianthus Maguire & Steyermark —— Synonymy: Tepuianthaceae Maguire & Steyermark
Trees or shrubs, bark bitter, ?chemistry; phloem rays narrow; pericyclic fibres 0; resin cells +; lamina vernation conduplicate, base continuous across adaxial petiole; plant androdioecious [?dioecious]; flowers small [10> mm across], K and C free, C ± clawed, ± = in length to K; nectary of 5-10 glandular scales ["exrastaminal disc"]; A 5, opposite K, or 12-22, in groups opposite C, connective produced or not; pollen 3-6 colp(or)ate; G [(2-)3], styluli +, bifid; ovular vascular bundles?; fruit a loculicidal capsule; seed 1/loculus, with a prominent angled raphe; testa ca 6 cells across, unlignified, exotegmen as lignified palisade cells, then a layer of low, lignified cells; endosperm +; n/x = ?
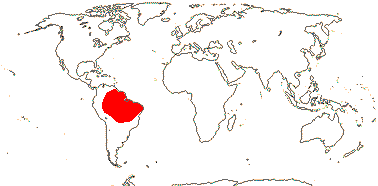
1/7. Guayana highlands, northeast South America.
[Gonystyloideae [Octolepidoideae [Aquilarioideae + Thymelaeoideae]]: C 0; cyclopropenoid fatty acids +; pollen oligo- to poly- pantoporate, minutely spinulose; style single, stigma ± capitate, dry; endotegmen with stripes on the inner surface; fruit a berry.
Age. This clade is ca 74.5 Ma (Hernández-Gutiérrez & Magallón 2019: + Cyt. Neurad 95.1) or ca 47.1 Ma (S. Y. Lee et al. 2022b).
2. Gonystyloideae Gilg —— Synonymy: Gonystylaceae van Tieghem, nom. cons.
Trees, shrubs or lianes; ?pericyclic fibres; lamina punctate [secretory cavities +]; ); hypanthium +, at most short, or 0; K (3-)5(6), also valvate, petaloid appendages deltoid to linear-subulate, 8-40; A 8-80, (connate), anther thecae apically connate, connective well developed, (basal layer with pendulous internal processes); pollen distinction between triangular subunits of exine ± lost; nectary 0; G (2-)3-5(-8), (clavate or subglobose parastyles +), (style 0), contorted, (stigma punctate); fruit a capsule; seed with a raphal aril, or angled at the raphe, or funicle swollen, nucellar tracheids +; tegmen (multiplicative), exotegmic cells also tangentially elongated [?all]; endosperm copious (or not?), cotyledons incumbent; n/x = ?
7/56: Gonystylus (32). Tropical Africa, Madagascar, Malesia to Australia, New Caledonia, Fiji.
[Octolepidoideae [Aquilarioideae + Thymelaeoideae]]: ?
2A. Octolepidoideae Thonner - Octolepis Oliver
Stomata anomocytic; leaf vernation conduplicate; plant monoecious or dioecious; bracts 0, pedicels articulated; floral tube 0; K 4-6, imbricate, C scale-like, 4 or 5 (10), strongly bifid, adnate to receptacle; staminate flower: A = 2 x C, anthers oblong; pollen 3-4-porate; pistillode +; pistillate flower: staminodes +; G [3-6], some usu. aborting, style straight, stigma lobed; ovules 1/carpel, minute, anatropous, pendulous, with one vascular bundle; fruit a loculicidal capsule, K, C, etc. persistent; seeds pubescent, palisade exotegmen incurved in chalazal region?, no stripes on the inner surface, "inner epidermis" finely striate, aril ?0; endosperm copious, cotyledons longitudinally curved, thin; n/x = ?
1/7. Ivory Coast, west-central tropical Africa, most spp. Madagascar.
Anthers hippocrepiform; tegmen vascularized [Gonystylus].
3/33:. Malesia, the Nicobar Islands,
[Aquilarioideae + Thymelaeoideae]: ovule with two vascular bundles.
Age. The Daphne-Aquilaria clade is ca 44.2 Ma (S. Y. Lee et al. 2022b).
3. Aquilarioideae Meisner
Heartwood infected by fungus produces resin-suffused agarwood; C tube conspicuous, cylindrical, petaloid appendages scale-like; G 2-locular; fruit a capsule.
2/30: Aquilaria (21), probably to include Gyrinops (9). Indo-S.E. Asia-Malesia.
Age. Crown-group Aquilaria is ca 1.2 Ma (S. Y. Lee et al. 2022b).
4. Thymelaeoideae Burnett —— Synonymy: Daphnaceae Ventenat, Gnidiaceae Berchtold & J. Presl, Phaleriaceae Meisner
Trees, shrubs, lianes or herbs; phorbol ester diterpenes [largely orthoesters and 1-alkyldaphnane derivates], chelidonic acid +, myricetin, tannins 0; (interxylary phloem +), internal phloem +; (torus-margo pits +); (styloids +); vascular bundles bicollateral; pericyclic fibres 0 - Edgeworthia; (stomata anomocytic); leaves often opposite; inflorescence often capitate; flowers 4-5-merous; hypanthium long, (0 - Synandrodaphne), funneliform or cylindric, (articulated in the middle), petaloid appendages ridge-like, "C" to 2 x K or 0; A 2-5, opposite (alternate with) P, (basally connate), or 10; anther wall monocot type, tapetal cells binucleate; pollen crotonoid, distinction between triangular subunits ± obvious; nectary +, morphology various (long-tubular), or 0; G 2, one locule often not developed and style then excentric/subterminal; inner integument to 10 cells across [Daphne], nucellus protrudes into micropyle, funicular obturator +; (antipodal cells persist, many); fruit a loculicidal capsule, drupe or achene; (seed with a chalazal fold and/or caruncle); (nucellar tracheids +), (testal cells enlarged), (palisade exotegmen 0), (tegmen multiplicative); endosperm 0 (quite copious - e.g. Daphne, Lachnaea); n = (7-)9(10), much polyploidy, nuclear genome [1 C] ca 0.75 pg.
38/690: Gnidia (140), Pimelea (110), Daphne (ca 100), Daphnopsis (55), Lachnaea (40); Phaleria (25). World-wide, esp. Australia and tropical Africa.
Evolution: Divergence & Distribution. The 40 or so species of Lachnaea are all restricted to the Cape Floristic Region (Linder 2003).
The inclusion of Tepuianthus in Thymelaeaceae makes eminent morphological sense (Wurdack & Horn 2001; Horn & Wurdack, ms.). It shares a number of apomorphies with other Thymelaeaceae, while the features in which it differs from them are mostly plesiomorphies, i.e., they are similarities to other Malvales. Tepuianthus has both a well-developed calyx and corolla and also scales outside the androecium (are the corolla scales of Gonystylus, and perhaps those of the rest of the family, homologous with these glandular scales?). Distinctive epidermal columns in the palisade mesophyll of the leaf of Tepuianthus are found in other Thymelaeaceae such as Solmsia, and its resin cavities may be compared with the secretory cells of Gonystyloideae. The bark of Tepuianthus is described as being bitter, while Thymelaeoideae are well known for often being rather poisonous, unfortunately, the chemistry of Octolepidoideae is poorly known. Finally, the well developed parallel venation of Tepuianthus is very like that of other Thymelaeaceae, and Solmsia (New Caledonia: Gonostyloideae) is vegetatively remarkably similar to Tepuianthus down to details of the leaf base and mucronate apex of the lamina.
The floral morphology of the family is poorly understood, especially the nature of the petals and parastyles. As Heinig (1951: p. 113, q.v. for references) summarized the issue:
"The petal-like organs found in this family have been variously interpreted: as petaloid glands (Payer, 1857) ; as scales by Meisner (1856), Baillon (1880), Bentham and Hooker (1883), and Bessey (1897); as mere outgrowths of the perigyn (Velenovsky, 1910); as aborted stamens by Endlicher (1836-1840) and Gerber (1899); and as squamellae by Moore (1919) who thinks that morphologically these might either be new structures of uncertain origin or modified parts of the androecium. Heintze (1925) suggests that they might be stipules. Eichler (1878), Gilg (1895), Hallier (1922), Hutchinson (1926), Domke (1934), Wettstein (1935), and Rendle (1938) consider these structures petals which they acknowledge might be reduced or otherwise greatly modified in form."
And 75 or so years later we still lack much in the way of comparative developmental studies on the flowers of Thymelaeaceae. The vasculature of the perianth here was studied by Heinig (1951). The vascular bundles supplying the structures inside the calyx, whether paired and more or less opposite the sepals or single and in the petaline position, came from lateral branches of the sepal vasculature, so equating these structures with stipules seems unlikely. Illustrations in Maguire and Steyermark (1981) suggest that Tepuianthus has colleters at the base of the calyx. Dicranolepis (Thymelaeoideae) has large "petals" that are variable in number but paired and opposite the petals, and they are sometimes serrate or laciniate, or they are paired and opposite the sepals. Other taxa have single structures alternating with the lobes of the perianth tube, while in Lachnaea (also Thymelaeoideae) there are paired structures in the perianth tube borne below the insertion of the two whorls of stamens (Herber 2002b).
Pollination & Seed Dispersal. Pimelia and Gnidia have pseudanthia (Baczynski & Claßen-Bockhoff 2023).
Wasps disperse the seeds of Aquilaria malaccensis in India (Manohara 2013), interestingly, the colours of the wasp and of the Aquilaria seeds are similar.
Genes & Genomes. S. Y. Lee et al. (2022a) noted that the plastome of Daphne genkwa was rather different from that of the rest of the genus: It had 20, not 2, genes in the SSC region, and this SSC region was ca 12 times longer than in the other species, the IR was ca 4.5 times shorter, and all told the plastome contained 106 genes, not 135-141, as in other species of the genus (and also in those few other species in the family examined).
Economic Importance. A number of taxa scattered throughout the family, but especially in Aquilaria (probably to include Gyrinops), A. malaccensis in particular, produce gaharu or agarwood. This is developed from the heartwood, whether of trunk or root, often after wounding and perhaps also after infection by fungi. For instance, an Ambrosia beetle Dinoplatypus chevrolati may bore into the wood, the ascomcete mould Phaeoacremonium parasitica then infecting the plant, and his in turn secretes a resin to combat the fungus. Gaharu has long been much esteemed as a source of incense and medicines, and some species that produce it have been decimated in the wild (Eurlings & Gravendeel 2005). The price of top-quality agarwood is reported to be ca $100,000/kg (Wikipedia xii.2017), a considerable increase from 2000 when the figure was ca $15,000/kg (Barden et al. 2000).
Chemistry, Morphology, etc.. Microsemma (= Lethedon-Gonystyloideae) has cyclopentenoid cyanogenic glycosides (Spencer & Seigler 1985).
Carlquist (2013) recorded interxylary phloem (IP) from several members of the family, and in Aquilaria sinensis, at least, cambium redifferentiates in the IP if the stem has been girdled (B. Luo et al. 2021). Luo et al. (2018, see also 2019) described the development of the IP in A. sinensis in some detail: It is diffuse, more or less scattered through the xylem, and interestingly, sieve tubes and pores were found in it but were not detected in the external phloem, which thus seems not to be involved in the transport of photosynthesates - very odd. The petiole anatomy of Gonystylus needs to be confirmed; the bundle is perhaps unlikely to be arcuate. For the lamina vernation of Tepuianthus, see Davidse et al. 17304. The base of the lamina joins the petiole on the adaxial side, and so the lamina is almost peltate.
I am agnostic about the occurrence of petals in Thymelaeoideae, but Heinig's work (Heinig 1951) is still useful. Note that Rogers (2005) described Octolepis as having oblong anthers and a straight style, Colli-Silva et al. (2025) as having horseshoe-shaped anthers and a contorted style (c.f. Herber 2002b in part); I follow the former.
The pollen of many Thymelaeoideae is similar to that of Euphorbiaceae-Crotonoideae, while that of Octolepidoideae is not really that dissimilar, although in the latter the distinction betwee the outer triangular subunits that make an often hexagonal pattern is lost. Nowicke et al. (1994) discuss this in detail, noting also the network of horizontal rods to which these subunits are attached that is in turn itself attached by columellae to the footlayer of the exine. The position of crotonoid pollen as an apomorphy could move deeper... The micropyle of Gnidia is zig-zag. Eckardt (1937) discussed gynoecial variation in members of Thymelaeoideae. Spichiger et al. (2004) showed Daphne alpina as having a straight (and sessile) ovule. Reports of a small embryo in Tepuianthus (Maguire & Steyermark 1981) need to be confirmed.
Some information is taken from Domke (1934), Ding Hou (1960), Herber (2002b), Rogers (2005: Octolepis), Rogers and Fuentes-Soriano (2021: Octolepideae), Colli-Silva et al. (2025: descriptions of subfamilies) and Horn and Wurdack (ms.), Kubitzki (2002: Tepuianthaceae) and Zachary Rogers's A World Checklist of Thymelaeaceae (2009 onwards). Evans and Taylor (1983: phorbol esters), H.-B. Wang et al. (2015) and Otsuki and Li (2023), tigliane diterpenoids, etc., Coleman et al. (2004), Dute et al. (2011) and Dute (2015), bordered pits, Luo et al. (2019: inter- and intraxylary phloem), Roth and Lindorf (1990: Tepuianthus anatomy), Weberling and Herkommer (1989: inflorescence morphology), Qi and Wu (2002: floral development in Wikstroemia), Rosello and Melhem (1998) and Herber (2002a), both pollen, Joshi (1936: nectary and gynoecium), and Guérin (1916), Fuchs (1938), Mauritzon (1939a), Kausik (1940b), Venkateswarlu (1947) and Manohara (2016: Aquilaria), all ovule and seed.
Phylogeny. For the phylogeny of the family, see van der Bank et al. (2002). They found the following set of relationships [Gilgiodaphne (= Synandrodaphne), Gonystyloideae [Aquilarioideae + Thymelaeoideae]]. These relationships, other than the position of Gilgiodaphne, were well supported, however, genera like Tepuia and Octolepis were not included, while Gnidia was highly polyphyletic. Two main groups, Gonystyloideae + the rest, were evident in the study by M. Sun et al. (2016), although support was weak. S. Y. Lee et al. (2022a) found much more pectinate basal relationships in a ML analysis of nrITS data (ca 58 taxa) than in a MP analysis, and there were other differences between the two. Colli-Silva et al. (2025) obtained quite good support at the subfamilial level in Thymelaeaceae in their nuclear analysis of Malvales.
Aquilarioideae. For relationships around Aquilaria, see Eurlings and Gravendeel (2005), indeed, Aquilaria and Gyrinops are intermixed on the tree resulting from the plastome analysis by S. Y. Lee et al. (2022b).
Thymelaeoideae. Motsi et al. (2010) and Foster et al. (2016b) looked at relationships around Pimelea, which should include Thecanthes. Beaumont et al. (2009) looked at relationships around Gnidia, clarifying the limits of that genus. Support for several of the nodes along the backbone of the phylogeny in the area Colli-Silva et al. (2025) was poor, although support for the subfamily as a whole - and indeed, for the rest of the subfamily - was better.
Classification. This is still somewhat unclear. The old Aquilarioideae are monogeneric and where the monogeneric Gilgiodaphnoideae (van der Bank et al. 2002) are to go is unclear. Herber (2002b) recognised an Octolepidoideae and Thymelaeaoiideae (Gonystyloideae were in a separate family), the former including two tribes (and Aquilaria, etc.), the latter three (one made up of Gilgiodaphne [= Synandrodaphne]), and and there were various species groups. Daphne is probably to include Wikstroemia (Halda 2001, but c.f. S. Y. Lee et al. 2022a); Daphne is polyphyletic, one species being removed to Wikstroemia, which is maintained, etc. (Lee et al. 2022a: c.f. topologies in different analyses). Many generic limits will need reconsideration because of the fragmentation of Gnidia, yet Gnidia s.l. is not necessarily "maximally stable" given the poor support for the clade it represents (c.f. Beaumont et al. 2009: p. 413). The classification here is that proposed by Colli-Silva et al. (2025).
Previous Relationships. The pollen of many Thymelaeaceae-Thymelaeoideae is similar to that of Euphorbiaceae-Crotonoideae, and the chemistry, including the presence of phorbol ester diterpenes, is also similar to that of Euphorbiaceae (Seigler 1994); not surprisingly, Takhtajan (1997) placed Thymelaeales immediately after Euphorbiales. Because Microsemma (= Lethedon) has cyclopentenoid cyanogenic glycosides, Spencer and Seigler (1985) suggested that it should be placed in Flacourtiaceae (see Achariaceae here). Thymelaeaceae were included in Myrtales by Cronquist (1981).
Thanks. I am grateful to Z. Rogers for comments.
[[Muntingiaceae + Cytinaceae] [[Sphaerosepalaceae [Bixaceae [[Pakaraimaea + Cistaceae] [Sarcolaenaceae + Dipterocarpaceae]]]] Malvaceae]]: ellagic acid +; hairs stellate/fasciculate; lamina venation ± palmate, vernation conduplicate(-plicate), stipules well developed; A many, developing centrifugally, from 5 or 10 (15) bundles [fasciculate], when 5 often opposite the C; ovules several [³6]/carpel, micropyle bistomal; exotegmen conspicuously incurved on either side of hypostase/chalaza.
Age. Magallón and Castillo (2009) estimated an age of a mere 33.9 Ma for this node; the stem age of Bixaceae was estimated at 73.2 Ma by Tank et al. (2015: Table S2, ?topology). The age for a clade [Cytinaceae [Malvaceae [Cistaceae + Bixaceae]]] is around (92.5-)72.1(-51.9) Ma (see Naumann et al. 2013).
[Cytinaceae + Muntingiaceae]: stipules 0; pollen with smooth exine; ovules numerous, outer integument 2-3 cells across, inner integument 2-3 cells across; fruit a berry; seeds numerous, mucilaginous, mucilage derived from funicle.
Chemistry, Morphology, etc.. For general information about the Cytinaceae + Muntingiaceae family pair, see Nickrent (2007).
CYTINACEAE A. Richard - Back to Malvales
Root parasites, herbs, plant endophytic; ellagitannins + [isoterchebin]; vessels 0; sieve tube plastids without starch or protein inclusions; stomata?; stems glandular-pubescent [San.]; leaves scale-lke, spiral; plant usu. monoecious or dioecious (andromonoecious; flowers perfect); inflorescence racemose, capitate or spicate; P +, uniseriate, imbricate [?all], C-like, 4-11, basally connate, (initially ± monosymmetric - Bd.); nectary at base of staminal column [nectariferous cavities between A]/lower part of P/on sigmoid-shaped abaxial T [San.]/0; (androgynophore +); staminate flowers: A 1-20+, connate, adnate to style [San.], extrorse, monothecal, connective massive, vascularized, (bundles branched), (0); (P joined by dissepiment to both A and stylodium); microsporogenesis successive/simultaneous; pollen 2-4-porate/3-5-colpate, rug(ul)ose, (in tetrads), 3-celled [?all]; stylodium +; carpelate flowers: staminodes 0; nectariferous cavities near base of style (perfect flowers with gynostemium - B.); G [3-14], inferior, placentation intrusive parietal, placentae branched, style +, hollow [Bd.], swollen towards the apex, stigma capitate-radiate, commissural, papillae uniseriate [Bd.]; ovules unvascularized, straight, uni- [Bd.] or bitegmic, micropyle endostomal, parietal tissue 0, (nucellar cap + - Bd.), covered in mucilage [Bd.], nucellar epidermis persists; antipodal cells persist; fruit ± baccate; seeds minute, ≤1.4 mm long, with projections at both ends [Bd., San.], embedded in mucilaginous pulp; exotegmic cells thickened all around [?all]; endosperm +, ca 1-layered; embryo undifferentiated, ca 10-celled; n = 12, 16, x = ?
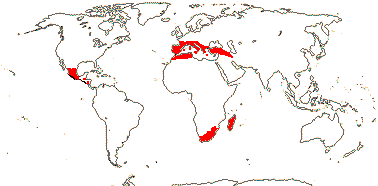
2 (?3)/11(?15): [list] - Cytinus (8). Mexico to Costa Rica, northern Colombia, the Mediterranean, Southern Africa and Madagascar. Map: from Jalas & Suominen (1976), Alvarado-Cárdenas (2009) and Fernández-Alonso and Cuadros-Villalobos (2012: fig. 7A). Photo: Collection of Cytinus, Bdallophytum - Staminate Flower, Carpellate Flower.
Age. Crown-group Cytinaceae are ca 60.9 Ma (Hernández-Gutiérrez & Magallón 2019); Neuradaceae are sister and ca 69.7 Ma is the age of the two clades combined.
Evolution: Divergence & Distribution. Species of Cytinus from the Mediterranean region, Southern Africa and Madagascar respectively are most closely related to each other (De Vega et al. 2023).
Ecology & Physiology. Cytinus is quite often parasitic on Cistaceae (same order), notably Cistus and the closely related Halimium, in the Mediterranean region, but it is also found on a variety of non-Malvalean families, perhaps especially Asteraceae, in Africa (Smythies & Burgoyne 2010). The endophytic system has been described in detail in the Mediterranean species (de Vega et al. 2007), endomycorrhizae from the hosts may also be found in tissues of the parasite, and although the physiological significance of this is unclear, there may be a tritrophic interaction (de Vega et al. 2010, c.f. Brundrett 2011 and de Vega et al. 2011a).
The American Bdallophytum is most commonly found on species of Bursera, the same host recorded for the recently-described Sanguisugu (Alvarado-Cárdenas 2009; Fernández-Alonso & Cuadros-Villalobos 2012: status unclear). García-Franco et al. (1997) discussed the population genetics of Bd. bambusarum.
Pollination Biology & Seed Dispersal. Pollination of Cytinus hypocistus is by ants, while that of other members of the genus is by other insects, mammals like rodents and shrews, and birds (de Vega et al. 2009, 2023; Smythies & Burgoyne 2010; Johnson et al. 2011b). De Vega and Herrera (2013) suggested that yeasts transported from flower to flower by ants increased the fructose and decreased the sucrose concentrations of the nectar, but the effect of this on pollination is unclear; there is secondary pollination presentation by way of attachment of the pollen to the perianth in Cytinus (El Ottra et al. 2023). Species of Bdallophytum are variously pollinated by flies (including carrion flies, sarcophagid dipterans, also the carrion-visiting syrphid Copestylum, which lands on the visually conspicuous apical anther connective) or stingless bees (Rios-Carrasco et al. 2023: see corrected Fig. 4). It is unclear whether or not there is nectar in the flowers of Bd. americanum, but nectar production is quite common in the family, coming from a modified perianth, trichomes at the base of the staminal column, and so on (Fernández-Alonso & Cuadros-Villalobos 2012); Harms (1935a) reported a nectary at the base of the style and the staminal tube of Cytinus. In a recent study of Bd. andrieuxii it was found that boh staminate and carpelate flowers were thermogenic; pollination was by the satyrid butterfly Cissia which laid eggs on the inflorescences, although apparently not affecting seed set; the flowers smell of rotting fruit but also produce nectar, altogether a rather unusual combination of floral features and pollinator (Rios-Carrasco et al. 2022).
The seeds become embedded in mucilaginous material derived from the placentae and funicles (Nickrent 2007; see also Rios-Carrasco & Vásquez-Santana 2021). Seeds of Cytinus hypocistis are ingested and dispersed by the tenebrionid beetle Pimelia costata (de Vega et al. 2011b) and of other species of the genus by ants and small mammals (de Vega et al. 2023) and those of Bdallophytum bambusarum by rodents, and also ants (Rios-Carrasco & Vásquez-Santana 2021).
Genes and Genomes. The chloroplast genome of Cytinus is very much reduced (Roquet 2016; Barrett & Kennedy 2018: Fig. 2).
The mitochondrial genes cox1 and matR in Cytinaceae showed considerable divergence, but not the atp1 gene (Barkman et al. 2007).
Chemistry, Morphology, etc.. The female flowers are at the base of the spike in Cytinus hypocistis and the male flowers are towards the top (de Vega et al. 2015); overall, breeding systems vary. Rios-Carrasco and Vásquez-Santana (2020: 3 spp. from Mexico) describe the flower types found in Bdallophytum. Anther development in Cytinus and Bdallophytum differs considerably (Rios-Carrasco & Vásquez-Santana 2021: table 2). The outer integument, when present, is much reduced. The seeds have blunt projections at both ends (Alvarado-Cárdenas 2009; de Vega et al. 2011). The fruits of Sanguisuga are described as being fleshy/bacciform, the inflorescence axis itself is also fleshy, the whole forming a coenocarp, and the seeds are mucilaginous (Fernández-Alonso & Cuadros-Villalobos 2012).
For general information see the Parasitic Plants website (Nickrent 1998 onwards), Heide-Jørgensen (2008) and Nickrent (2020), for a monograph of Bdallophytum, see Alvarado-Cárdenas (2009) and for Sanguisuga, see Fernández-Alonso and Cuadros-Villalobos (2012); see also Hegnauer in Meijer (1997) for some chemistry, Solms-Laubach (1867) and de Vega et al. (2007) for anatomy, including the endophytic portion in the host, Guzowska (1964) for ovules, embryology, etc., Baskin and Baskin (2021) for seeds, etc., and de Vega et al. (2008) for population differentiation in the western Mediterranean.
Classification. Sanguisuga caesarea (Fernández-Alonso & Cuadros-Villalobos 2012) is probably a species of Bdallophytum.
MUNTINGIACEAE C. Bayer, M. W. Chase & M. F. Fay - Back to Malvales
Trees; vessels single; pits not vestured; non-septate tracheids +; parenchyma storied; non-dispersive protein bodies?; mucilage canals 0; petiole bundle annular, lacking pericyclic fibres; stomata ?; hairs stellate or tufted; leaves two-ranked, lamina margins toothed, secondary veins pinnate to palmate, base obliquely cordate; prophylls basal, heteromorphic; inflorescence fasciculate, extra-axillary; flowers (4-)5-merous; K valvate, basally connate, C imbricate, shortly clawed, crumpled in bud; tapetal cells binucleate; pollen (in tetrads), small, ca 10 µm; nectary on (?inside of) broad disc [not Dicraspidia]; G [5(-7)], or inferior, opposite petals, placentation axile-laminar, septae numerous, or placentation axile-pendulous, placentae massive, bilobed, style stout, (± 0), stigma conical, 5-ridged, or ± capitate; ovules with micropyle exostomal, zig-zag, parietal tissue 5-6 cells across, nucellar cap 0, hypostase +, funicle long [Muntingia]; megaspore mother cells several, megaspore micropylar, embryo sac monosporic, tetranucleate [M.]; K persistent or deciduous; tegmen multiplicative, exotesta also mucilaginous, endotesta crystalliferous, cells of exotegmen shortly elongated; endosperm +, slight, ?diploid, starchy [details of seed from M.]; n = 15, x = 7 (?9, ?8).
3/3: [list]. Tropical America. Photo Flower.
Evolution: Divergence & Distribution. Some characters of Muntingiaceae (lack of stipules; pits not vestured) might suggest that the family may be rather basal in Malvales; characters of the young secondary tissue of Muntingia, such as widely flaring rays, stratified phloem, etc., are like those of most other Malvales.
Pollination Biology. Fertilization of Muntingia occurs 12-15 days after pollination (Corner 1976).
Chemistry, Morphology, etc.. Muntingia has erect uniseriate hairs in addition to its tufted hairs. Although Muntingiaceae appear to have stipules, this may not to be the case. Dicraspidia has strikingly asymmetric prophylls; on the adaxial side of the branch they are orbicular, foliaceous and persistent, while on the abaxial side they are linear, thin and caducous. In Muntingia only an adaxial prophyll is present, and it is narrow (Karima Gaafar, pers. comm.), while the situation in Neotessmannia is unknown. Sensarma (1957) suggested that the nodes of Muntingia are trilacunar, he interpreted the prophyll as a stipule, nevertheless, nodes indeed appear to be trilacunar. Given that stipules are common in Malvales, their apparent absence in Muntingiaceae needs to be clarified.
Muntingia has a superior ovary, caducous calyx, and pendulous placentae, the two other genera have inferior ovaries, laminar placentation, and a persistent calyx (?: Neotessmannia). Stamens, etc., are borne on a massive, almost disc-like structure towards the inside of which is a ring of dense hairs; the inner side of this disc as it faces the ovary seems to be nectariferous. Venkata Rao (1952a) suggested that there were glandular, nectar-secreting hairs in Muntingia, but the hairs seem eglandular to me.
See Benn and Lemke (1991) and Bayer (2002) for general information; for wood anatomy, see Gasson (1996) and Carlquist (2005a), and for carpel orientation, see Ronse Decraene (1992).
Thanks. I am grateful to Lucia Lohmann for sending me material of Muntingia.
Phylogeny. For relationships, see Bayer et al. (1998c).
Previous relationships. Muntingia was placed in Flacourtiaceae and Neotessmannia in Tiliaceae by Cronquist (1981) and both are to be found in Tiliaceae-Neotessmannioideae in Takhtajan (1997).
Morphology, Chemistry, etc.. For palmate venation and conduplicate-plicate vernation, see Couturier et al. (2009, 2011).
The micropyle is described as being "formed by the outer integument" in Nandi (1998a: 257). A water gap in the seed has been described from representatives of almost all families in this clade (Gama-Arachchige et al. 2013). Stomata in the exotesta seem to be quite common here (references in Jernstedt & Clark 1979).
[Sphaerosepalaceae [Bixaceae [[Cistaceae + Pakaraimaea] [Sarcolaenaceae + Dipterocarpaceae]]]]: ?
Age. The age of this clade is ca 96 Ma (Hernández-Gutiérrez & Magallón 2019).
Evolution: Divergence & Distribution. Lamont et al. (2022) discuss the distributions of Dipterocarpaceae—Sarcolaenaceae, Cistaceae—Pakaraimaea and Bixaceae—Cochlospermaceae—Sphaerosepalaceae clades in some detail and very largely in terms of continental drift-type events.
SPHAEROSEPALACEAE van Tieghem —— Synonymy: Rhopalocarpaceae Takhtajan - Back to Malvales
Deciduous trees; cambium storying ?0; pits not vestured; true tracheids +; rays uniseriate; secretory canals +; resin-filled cells outside veins; calcium oxalate crystals +; petiole bundles cylindrical, (with adaxial plate), (medullary bundles +); (stomata cyclocytic); hairs simple; leaves spiral or two-ranked, lamina (with secondary veins subpinnate), (fine venation closely raised), stipule intrapetiolar, , coonate, ± encircling stem, petiole pulvinate; inflorescences with subumbelliform cymules; flowers usu. 4-merous, K usu. 2 + 2, caducous, outer median, inner larger, C (3-)4(-9), clawed, aestivation various, with many short resin lines, caducous; A with broad connective; pollen grains 3-6 ?colpate, usu. ± spinose, endoaperurate [endoapertures larger than ectoapertures]; gynophore +, short, nectary on top; G [2(-5)], separate, or G on one side not developed, placentation basal, style continuous to gynobasic, stigma punctate or obscurely lobed; ovules 2-9/carpel, epitropous, micropyle endostomal; fruit ± baccate, capsular-indehiscent or schizocarp, muricate to finely verrucose, ± deeply lobed, 1 seed/carpel, (outer K persistent); aril funicular/0, seed ruminate or not, testa 6-20 cells across, exotesta mucilaginous, (exotegmen not incurved), operculum +; endosperm ?development, moderate, starchy, cotyledons cordate, bilobed apically, (much but irregularly divided); n = ?19, x = ?
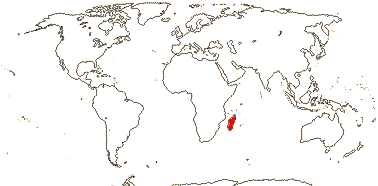
2/18: [list] - Rhophalocarpus (17). Madagascar. [Photos - Collection]
Age. Crown-group Sphaerosepalaceae are ca 23.3 Ma (Hernández-Gutiérrez & Magallón 2019).
Evolution: Divergence & Distribution. The family has a phylogenetic fuse of ca 50 Ma (Hernández-Gutiérrez & Magallón 2019).
Chemistry, Morphology, etc.. Secretory cavities are abundant, and the carpels produce an exudate when cut. The rays are not storied (den Outer & Schütz 1981), and Jansen et al. (2000a) did not find vestured pits (c.f. den Outer & Schütz 1981). Takhtajan (1997) described the stipules as being extrapetiolar and the endosperm as being copious.
The lateral sepal bundles are commissural, as in Thymelaeaceae. There are androecial trunk bundles opposite the petals. The apparently terminal style may be modified from the gynobasic condition (Horn 2004).
For more information, see Capuron (1962), Bayer (2002) and Horn (2004), all general, and Huard (1965), anatomy.
[Bixaceae [[Cistaceae + Pakaraimaea] [Sarcolaenaceae + Dipterocarpaceae]]]: (plant with secretory canals); K imbricate; hypostase chalazal plug with core and annulus [bixoid chalazal plug, ?level]; endosperm starchy, embryo chlorophyll 0.
Age. The age for a clade [Cistaceae + Bixaceae] is some 52.4 Ma (Naumann et al. 2013: note topology); see also Tedersoo & Brundrett (2017). See also Heckenhauer et al. (2017: c.f. Figs 1 and 5) for ages, but for a rather unlikely topology - [Dipterocarpoideae + Sarcolaenaceae] are sister to the rest of the clade - and calibration (split of Seychelles from India) is the node where Dipterocarpoideae and Sarcolaenaceae separate.
Evolution: Ecology & Physiology. The bixoid chalazal plug forms a water gap through which water enters the hard seeds, so breaking their physical dormancy (Baskin et al. 2000).
Phylogeny. The clade above is characterised by its distinctive seed anatomy (Nandi 1998a, q.v. for much else). In Bixaceae and Cistaceae the leaf teeth have a single vein proceeding to an opaque deciduous apex and the cotyledons are curved or folded.
BIXACEAE Kunth, nom. cons. - Back to Malvales
Plant with secretory canals; resin glands outside veins; hairs glandular, also unicellular but not tufted or stellate; leaves spiral; inflorescence terminal; flowers large [>2.5 cm across]; A with ringwall primordium, development centrifugal, 5 or 10 fascicular traces, anthers with apical pores; stigma at most slightly lobed; ovules many/carpel, micropyle zig-zag, funicles long; starchy perisperm + cotyledons spatulate, curved or folded; x = 7 (?8, ?9), nuclear genome [1 C] (0.035-)0.919(-24.44) pg.
3/21: [list] - three genera below. Pantropical.
Age. The age of this clade (?content) is ca 34.3 Ma (Hernández-Gutiérrez & Magallón 2019).
1. Cochlospermum Kunth (Cochlospermeae Endlicher) —— Synonymy: Cochlospermaceae Planchon, nom. cons.
Trees, geoxylic shrubs [esp. Old World] (perennial herbs); gums +; sieve tube plastids with protein crystalloids and starch; (cork in the pith); lamina (palmate), margin serrate (entire), a single vein proceeding to opaque deciduous apex of tooth, stipules narrow; bracteoles 0; flowers ± monosymmetric; K 5, (imbricate); (A dimorphic [outer larger, spreading]), [2 (1)]; post-zygotic incompatibility system [?all]; G [3-5], opposite C or odd member adaxial, (placentation parietal); ovule ± campylotropous, outer integument 3-4 cells across, inner integument 3-7 cells across, parietal tissue ca 6 cells across, nucellar cap 2-4 cells thick; fruit with woody loculicidal exocarp, largely septicidal membranous endocarp, the two separating; seeds hairy [fringed; long-pilose], (glabrous), cochleate-reniform (globose); tegmen thick, bony, outer hypodermal cells enlarged; endosperm copius, oily, starch 0, embryo curved (straight); n = 6.
1/15 (inc. Amoreuxia). Pantropical, Cochlospermum religiosum widely naturalised from Java to India. Map: from Poppendieck (1980, 1981), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Australia's Virtual Herbarium (consulted ii.2014); see also Johnson-Fulton and Watson (2017). [Photo - Habit, Flower.]
Age. Crown-group Cochlospermum is ca 79.5 Ma (Johnson-Fulton 2014).
[Bixa + Diegodendron]: glandular hairs peltate; petiole bundle cylindrical, with medullary strands; lamina margins entire, stipules ensheathing bud; G [2-4]; fruit muricate.
2. Bixa L.
Trees; flavones, flavonols, flavonoid sulphates +, non-hydrolysable tannins 0; pits vestured; pigment glands + [= syncytial carotenoid-, esp. bixin, filled gland, ≡ anastomising articulating laticifers]; petiole also with medullary bundle; (flowers vertically monosymmetric); K with paired basal abaxial glands, C imbricate; anthers inverted U-shaped, with 1-2 median pores/slits [morphologically subapical]; G [2-4], opposite K, individual carpels initially not visible, placentation parietal; ovule outer integument 3-6 cells across, inner integument 4-5 cells across, parietal tissue ca 2 cells across, nucellar cap ca 4 cells thick, postament +; fruit softly prickly; testa pulpy, endotegmen with ± thickened cells, hypodermal layer of hour-glass cells; n = 7.
1/5. Tropical America. [Photo - Flower, Fruit, Fruits.]
3. Diegodendron humbertii Capuron —— Synonymy: Diegodendraceae Capuron
Evergreen tree; wood aromatic, ?chemistry; cork?; wood weakly storied; nodes?; ?stratified phloem; mucilage cells?; (stomata cyclocytic); leaves two-ranked, lamina punctate, venation pinnate, ?vernation, stipules intrapetiolar, ± encircling stem; K unequal, C caducous; A ?development, anthers with slits; disc ?inconspicuous; G [2(-4)], orientation?, style gynobasic; ovules 2/carpel, basal, apotropous, otherwise unknown; fruit warty, with small glands, indehiscent; seed with a glutinous outer layer, coat thin [no palisade layer, bixoid plug, etc.]; endosperm 0; n = ?
1/1. Madagascar.
Evolution: Divergence & Distribution. For the evolution of some floral characters in Cochlospermeae, see Johnson-Fulton (2014). The absence of a bixoid chalazal plug in Diegodendron is probably because the fruit is indehiscent and the seed coat thin and poorly developed.
The family may have a phylogenetic fuse over 40 Ma (Hernández-Gutiérrez & Magallón 2019).
There are four distinctively-marked adaxial petals in monosymmetric flowers of Amoreuxia, rather unusual for such flowers - images sometimes suggest that the flowers are held transversely.
Economic Importance. The orange colouring of Bixa orellana, annatto, comes from exudate/latex obtained from pigment glands in the seeds which contains large amounts of carotenoids (Bouvier et al. 2003; Almeida et al. 2021) and is used as a food colouring, e.g. for margarine.
Chemistry, Morphology, etc.. The gums of Cochlospermum and those of Sterculia (Malvaceae-Sterculioideae) are similar, both containing acetylated acidic polysaccharides. Bixaceae are reported to have laticifers (Prado & Demarco 2018). The pigment glands of Bixa orellana are described by Almeida et al. (2021) as anastomosing articulated laticifers, although in the primary roots this pigment is produced in the exodermal cells.
In Cochlospermum vitiifolium the median sepal is abaxial, there are no bracteoles, and the sepals are of unequal size (or three "true" sepals + two bracteoles?). Flowers of Cochlospermum are monosymmetric in bud, and the floral vasculature is monosymmetric. The androecium has five or six bundles, and development is centrifugal. Carpel orientation needs to be checked if the flower is inverted. There is no obvious nectary. Amoreuxia has obliquely (?tranversely) monosymmetric flowers, the positionally "upper" stamens being much shorter than the lower ones and differently coloured; the four "upper" petals are bicolored.
Kubitzki and Chase (2002) mention the starchy perisperm of Bixaceae and Cochlospermaceae; the former family is scored as having starchy endosperm in their table 1.
See Keating (1970, 1972) and Poppendieck (1980, 2002) for general details and Venkatesh (1956), Dathan and Singh (1972) and Ronse Decraene (1989b) for embryology and floral development of Bixa and Cochlospermum and Johnson-Fulton (2014) for information about Cochlospermeae.
For general information on Bixa, see Poppendieck (2002). The wood anatomy of Diegodendron is very like that of Sphaerosepalaceae (Dickison 1988), but the genus is otherwise poorly known (see also Bayer 2002).
Phylogeny. For phylogenetic relationships in Cochlospermeae, see Johnson-Fulton (2014) and especially Johnson-Fulton and Watson (2017: ITS + 2 chloroplast markers). In the latter paper, Cochlospermum is paraphyletic, and, depending on the analysis, C. orinocense may be sister to the rest of the tribe and C. tetraporum sister to Amoreuxia, which is monophyletic.
Classification. Although Diegodendron does seem morphologically rather different from the other two genera (Kubitzki & Chase 2002: Table 1), nevertheless, all three have much in common. Cochlospermaceae and Diegodendraceae were provisionally placed in Bixaceae s.l. (see A.P.G. II 2003) and later combined (APG III 2009). In POWO (ix.2023) the limits of Cochlospermum are expanded to include Amoreuxia, which solves the problem of the two migratory Cochlospermum species mentioned above.
Previous Relationships. Diegodendron was included in Ochnaceae by Cronquist (1981), but excluded by Amaral (1991; see also Takhtajan (1997).
[[Cistaceae + Pakaraimaea] [Sarcolaenaceae + Dipterocarpaceae]]: plant ecto- (arbuscular) mycorrhizal; tracheids +; K with the two outer members often different from the rest, quincuncial; anthers basifixed; G [3], style single, hollow [?level].
Age. Ages for this node offered by Wikström et al. (2001) are (41-)39(-37) or (25-)23(-21) Ma, in Bell et al. (2010) are (56-)46, 42(-28) Ma, and in Tank et al. (2015) ca 49.2 My; the ages in Guzmán and Vargas (2009) are (27.6-)24(-23.0) Ma and in Aparicio et al. (2017) ca 35 Ma (see also Soulebeau 2015; Tedersoo & Brundrett 2017: more ages). Ca 83.2 Ma is the age suggested by Hernández-Gutiérrez and Magallón (2019). Note ages of Dipterocarpaceae and [Sarcolaenaceae + Dipterocarpaceae] below; there is more conflict.
Evolution: Ecology & Physiology. Ectomycorrhizae (ECM) are common in this clade (e.g. Appanah 1998; Ducoussu et al. 2004), and with some 915 species it may be the second largest ECM clade in angiosperms. The association has been dated to Gondwanan times (Moyersoen 2006; Alexander 2006; see also Sato et al. 2016). Note, however, that endomycorrhizal associations are also mentioned quite commonly in the literature for members of this family group, and in at least some Cistaceae the association is ectendomycorrhizal, the one fungus switching from ecto- to endomycorrhizal depending on soil water and nutrient status (Marquéz-Gálvez et al. 2020).
Plant-Bacterial/Fungal Associations. Cistaceae are reported to lack root hairs (Arrington & Kubitzki 2002), and they are likely to be absent/poorly developed elsewhere around here, as in Dipterocarpaceae, because of the prevalence of ectomycorrhizal associations. Hoewever, at least some Cistaceae, Sarcolaenaceae and Dipterocarpaceae are dual mycorrhizal plants (Teste et al. 2019: Table S2).
Chemistry, Morphology, etc.. For some morphological variation in this clade, see Heckenhauer et al. (2017).
CISTACEAE Jussieu, nom. cons. - Back to Malvales
Herbs to shrubs; (flavonoid sulphates +); cambium storying?; paratracheal parenchyma 0, rays uni(bi-, tri-)seriate; phloem not stratified; nodes 1:1 [confirm]; mucilage cells 0?; petiole bundles arcuate; cuticle waxes 0 (platelets, annular rodlets); hairs also glandular, simple, or stellate, each cell with a basal internal compartment; leaves spiral (opposite), ± linear, lamina vernation ± conduplicate-curved, margin toothed, venation pinnate, secondary veins also pinnate, stipules 0; K ± opposite C, K 5, 2 outer smaller than the others, C 5, (imbricate), crumpled in bud; A developing from ringwall primordium, centrifugal, (sensitive to touch); pollen often starchy, (surface striate-reticulate); G opposite petals or median member abaxial, placentation parietal, placentae filiform, stigmas dry, with multicellular multiseriate papillae; ovules straight, (micropyle exostomal), outer integument ca 2 cells across, inner integument 2-4 cells across, parietal tissue ca 2 cells across, nucellar cap ca 2 cells across, hypostase +; (megaspore mother cells several); testa often mucilaginous; embryo ± strongly curved, (chlorophyllous); x = 10 (?9, ?7), nuclear genome [1 C] (0.102-)1.879(-34.446) pg/(856-)2479(-4401) Mb.
8/207: [list] - three groups below. Eurasia, esp. the Mediterranean region, North Africa, North America, S. South America (map: from Meusel et al. 1978; Frankenberg & Klaus 1980; Flora of China 13. 2007). [Photos - Collection.]
Age. The age of crown-group Cistaceae is around (18.5-)14.2(-10.2) Ma (Guzmán & Vargas 2009; see also Vargas et al. 2014), ca 22.6 Ma (Aparicio et al. 2017) or ca 56.1 Ma (Hernández-Gutiérrez & Magallón 2019).
1. Lecheeae Spach - Lechea L.
Perennial, woody rootstock; stipules 0; C 3; A 3-25, primordia both single and compound; G [3], placentae shield-like, style 0, stigmas broad, papillate-plumose; ovules 2/carpel; embryo ± linear; n = ?
1/17. North and Central America, the West Indies.
Age. The age of the node [Lecheeae + Cisteae] was estimated to be (14.7-)11.8(-8.4) Ma (Guzmán & Vargas 2009; see also Vargas et al. 2014).
[Fumaneae + Cisteae]: embryo ± curved.
2. Fumaneae Wilkomm - Fumana (Dunal) Spach
Shrubby; (stipules +); outer A staminodial; stigma expanded; ovules anatropous, exostome prolonged [beaked], funicle short; n = 16.
1/9. Europe, the Mediterranean region.
Age. The age of a node [Fum. + Lec.] is some 15.6 Ma (Aparicio et al. 2017).
3. Cisteae Reichenbach —— Synonymy: Helianthemaceae G. Meyer
Plants ± herbaceous/subshrubby, (annual); (pits vestured - Cistus); leaves usu. opposite, also broad, (stipules +); K (3); (A 3≤); G ([5(-10] - Cistus), (placentation axile), (style 0), stigma capitate to punctate; ovules (1-)2-many/carpel; funicles long; embryo circinate to J-shaped, (cotyledons plicate); n = 5-10, etc..
6/181: Helianthemum (136), Crocanthemum (24), Cistus (18). Eurasia, esp. the Mediterranean region, also North Africa (inc. the Horn of Africa), North America, Crocanthemum also in the far S. of Brazil, Uruguay.
Age. The age of crown-group Cisteae was estimated to be (23.9-)14.1(-7.1) Ma (Aparicio et al. 2017).
Evolution: Divergence & Distribution. For additional dates, see Aparicio et al. (2017). The family has a ca 25 million year phylogenetic fuse (Hernández-Gutiérrez & Magallón 2019).
It has been suggested that the family has moved from the Old to the New World and back within the last 12 Ma (Vargas et al. 2014). Furthermore, much diversification may have occurred within the time that Mediterranean vegetation has become established, which is within the last 7 Ma or so; Martín-Hernanz et al. (2019) found notably fast diversification rates within each of the three major clades they recovered within Helianthemum, and these had also been found within white-flowered species of Cistus (Guzmán et al. 2009) - ages ca 2.9, 1.9, 1.7 and (Cistus1.0 Ma.
Ecology & Physiology. Cistus in particular dominates in the shrubby Maquis vegetation in the Mediterranean; Maquis may be transitional to Quercus- and Pinus-dominated vegetation (Comandini et al. 2006), but all are ECM communities. Lamont et al. (2018) suggested that hard-seeded Cistaceae arose ca 42 Ma - the hard seeds being associated with fire-prone environments - with diversification starting ca 28 Ma. In the Mediterranean, at least, Cistaceae are noted for including a number of gypsophiles, plants that can live on gypsum-rich soils, soils that are high in calcium sulphtae (Escudero et al. 2014).
Pollination Biology & Seed Dispersal. Many Cistaceae have stamens that are sensitive to touch, moving outwards and dusting the visiting insect with pollen when it disturbs them.
For mucilages in the seeds of Cistaceae and their possible functions, see Western (2012), Yang et al. (2012) and Engelbrecht et al. (2014).
Plant-Bacterial/Fungal Associations. Endomycorrhizae as well as ECM have been reported from Cistaceae (Comandini 2006; de Vega et al. 2010, 2011). Dickie et al. (2004) also mention ECM in Helianthemum; see also Arrington and Kubitzki (2002), and Kapil and Maheshwari (1965) note fungal hyphae in the ovules which, however, do not infect the seed. On the other hand, Marquéz-Gálvez et al. (2020) describe the association between the pezizomycete associates ("desert truffles") of Helianthemum as being ectendomycorrhizal - the association is more ectomycorrhizal in nature when nutrient concentrations are high and there is plenty of water, and more endomycorrhizal when conditions are the reverse. Hudsonia from eastern Canada, ECM plants, have very thin lateral roots only ca 59 µm across, comparable to the hair roots of Ericaceae with their ericoid mycorrhizae - modified ECM (Massicotte et al. 2010). Mycorrhizae are also to be found in the tissue of Cytinus (Cytinaceae) a parasite of Cistaceae in the Mediterranean region (de Vega et al. 2010, 2011, c.f. Brundrett 2011).
Genes & Genomes. For a nuclear genome duplication, the CIINα event that occured ca 36.9 Ma, see Landis et al. (2018).
Chemistry, Morphology, etc.. Wood rays are uniseriate and xylem parenchyma is almost absent (Keating 1966).
Corolla initiation in Cistaceae tends to be retarded (Nandi 1998b), although it is not in Dipterocarpaceae (Kocyan 2005). The androecium has ten vascular bundles, each bundle of the oppositisepalous whorl supplies a group of stamens while the traces of the inner whorl usually supply a single stamen only; Saunders (1936) suggested that in Cistus there are five oppositipetalous groups and also discussed calyx and corolla development and orientation. The pollen surface is variable, and the retipilate exine of some Fumana and Cistus (Sáenz de Rivas 1979) looks somewhat similar to a crotonoid exine... At least some Cistaceae have starchy pollen grains. The embryo is green (1 record) or white (Nandi 1998a). Fumana has n = 16 (Guzmán & Vargas 2009).
For general information on Cistaceae, see Arrington and Kubitzki (2002, revised in Arrington 2004), for floral diagrams, see Eichler (1878), for floral development, see Nandi (1998b), for pollen morphology, see Ukraintseva (1993), for ovule and seed anatomy, Nandi (1998a) and Gama-Arachchige et al. (2013: esp. water gap), for seedlings, see Gaume (2012). For more information on the web, see R. Page's Cistus and Halimium website.
Phylogeny. Fumana and Lechea are successively sister to the remainder of Cistaceae with 100% posterior probabilities but mediocre maximum parsimony support (Guzmán & Vargas 2009); the former in particular has a number of features that are plesiomorphic in the family (Arrington 2004), and Fumana, alone in the family, has staminodes. However, the relative positions of these two genera are reversed elsewhere, e.g. Collli-Silva e al. 2025). For relationships in Cistaceae, see also M. Sun et al. (2016) and Colli-Silva et al. (2025), note that Aparicio et al. (2017) obtained a weakly supported [Fumana + Lechea] clade, although relationships outside Helianthemum were not the focus of their study.
Cisteae For the phylogeny of Cistus, see Guzmán and Vargas (2005); the relationships of Halimium and Cistus are intertwined (Civeyrel et al. 2011). For a study of Helianthemum, see Aparicio et al. (2017); the genus broke into three well-supported clades that represent the two subgenera, but there was little support for other relationship except for some at the base of subgenus Plectolobum and in one of the two clades that included subgenus Helianthemum. Helianthemum itself was (almost) well supported as sister to the remainder of the family, which was made up of an Old World and a New World clade. The same three clades wihin Helianthemum were recovered by Martín-Hernanz et al. (2019: 73 spp. and 25 sspp., nuclear data); they noted that the taxonomically complex species that they included in their study were often not monophyletic.
Previous relationships. Takhtajan's Cistales included Cistaceae, Bixaceae and Cochlospermaceae. Corner (1976 1: 97) described Cistaceae as being "little more than variations on a single generic theme", and noted similarities between the three families mentioned.
Pakaraimaea Maguire & P. S. Ashton - Pakaraimaea dipterocarpacea (Maguire) P. S. Ashton - ?to be included in Cistaceae?
Trees, ectomycorrhizae +; triterpenoid dipterocarpol +; pits vestured; rays mostly biseriate, paratracheal parenchyma +, included phloem +; cortical sclereids +, grouped; elongate medullary mucilage cells; petiole bundle deeply arcuate, enclosing lobed vascular cylinder; ?stomata; leaves 2-ranked, venation pinnate, stipules +, ± encircling stem; K equal, C shorter than K; anthers short, subversatile, connective broad, apically prolonged; exine 4-layered; G [(4-)5], opposite C, stigma punctate, with short bristles; ovules (2-)4/carpel, both integuments prolonged [beaked], outer integument ca 4 cells across, inner integument ca 4 cells across; fruit a loculicidal capsule, seed single; ?endotegmic cells 2-layered, flattened, thick-walled; endosperm +; cotyledons grow after germination; x = ?
1/1. The Guaianan Highlands. Map: see two maps below, area in blue.
Age. The age of the [Pakaraimaea + Cistaceae] clade is 105≤ Ma (Lamont et al. 2022).
Plant-Bacterial/Fungal Associations. For ECM, see Moyersoen (2006) and M. E. Smith et al. (2013).
Chemistry, Morphology, etc.. It is difficult to understand details of seed anatomy in Maguire and Ashton (1980), but see Nandi (1998a).
For general information, see especially Maguire et al. (1977).
[Sarcolaenaceae + Dipterocarpaceae]: plant with secretory canals; petiole bundle with medullary strand; lamina venation pinnate, stipules usu. well developed; ?androecium development; capsule loculicidally dehiscent, K persistent; x = 7.
Age. Ducousso et al. (2004) suggest that Dipterocarpaceae and Sarcolaenaceae had a common ancestor some 88 Ma, that is, prior to the split of India and Madagascar, and the age in Hernández-Gutiérrez and Magallón (2019) is ca 74.8 Ma; ages suggested by Wikström et al. (2001) are only (30-)28(-26) or (16-)14(-12) Ma and the latter age is also that in Soulebeau (2015). Lamont et al. (2022: Sarcolaenaceae embedded in Dipterocarpaceae) suggested that the age of that clade was 115≤ Ma.
Evolution: Divergence & Distribution. Depending on the age given to this clade, continental drift may be involved in its distribution (e.g. Lamont et al. 2022).
SARCOLAENACEAE Caruel, nom. cons. - Back to Malvales —— Synonymy: Rhodolaenaceae Bullock, Schizolaenaceae Barnhart
Woody, usu. evergreen; cyclopropenoid fatty acids +; cork?; wood not storied, rays uniseriate, paratracheal parenchyma 0; phloem not stratified, cortical sclereids +; pith, etc., with mucilage cells; stomata?; hairs also stellate or glandular; leaves two-ranked, lamina vernation involute [Keller 1996]; inflorescence various, involucre of varying morphology subtending 1 or 2 flowers, (bract [largely two stipules] enclosing flower); K 3(-5), when 5, outer 2 smaller, C 5 (6); nectary disc +; A (10 [Leptolaena]-)many, ± connate into 5-10 bundles or not, of 2 or 3 lengths, anthers also dorsifixed; pollen in tetrads, usu. parasyncolpate, (colpus edges raised, variously ornamented); G (1) [2-4(-5)], densely hairy, placentation basically axile, stigma capitate and/or ± lobed, with multicellular papillae; ovules (1-)2-6(-many)/carpel, micropyle zig-zag, outer integument ca 2 cells across, inner integument ca 3 cells across; fruit (indehiscent), with dry to fleshy persistent/accrescent bracts or cupule, endocarp hairy; seed hairy or not, often ruminate; endosperm copious (0), cotyledons cordate; x = 7.
10/80: [list] - Schizolaena (22). Madagascar, mostly E. and C.. Photos: Collection.
Age. Diversification in Sarcolaenaceae may have begun as little as 4.5 Ma (Soulebeau 2015) or ca 21.3 Ma (Hernández-Gutiérrez & Magallón 2019).
Evolution: Divergence & Distribution. Fossil pollen of Sarcolaenaceae is known from the Caenozoic of south South Africa perhaps 15 Ma (Coetzee & Muller 1984; Nilsson et al. 1996).
Perhaps rather surprisingly, crown-group Sarcolaeaneae had the highest diversification rate of any family in Malvales, and it also has a phylogenetic fuse of over 50 Ma (Hernández-Gutiérrez & Magallón 2019).
Plant-Bacterial/Fungal Associations. Sarcolaenaceae are dual mycorrhizal plants, having both ecto- and arbuscular mycorrhizae (Bâ et al. 2011a, b; Teste et al. 2019: Table S3).
Chemistry, Morphology, etc.. For general information see Bayer (2002), for details of cyclopropenoid fatty acid distribution, see Gaydou and Ramanoelina (1983), for ECM, see Ducousso et al. (2004), for the remarkable pollen, see Nilsson et al. (1996) and Polevova et al. (2010) and references, for seed anatomy, see Nandi (1998a).
Phylogeny. For a morphological phylogeny of Sarcolaenaceae, with Schizolaena sister to the rest of the family, see Haevermans (1999). However, in a recent molecular phylogeny (Aubriot et al. 2016b) relationships were very poorly resolved, and there was no support for the monophyly of Rhodolaena, although it was not positively paraphyletic, either.
DIPTEROCARPACEAE Blume, nom. cons. - Back to Malvales
Trees; triterpenoid dipterocarpol, sesquiterpene oleoresins + [?here]; cork also outer cortical; cambium storied; (vessel elements with scalariform perforation plates); tyloses +; cortical bundles +; stomata various; hairs also peltate, or multicellular-stalked, glandular capitate; leaves spiral and two-ranked, petiole geniculate; inflorescence axillary, often branched; K (slightly connate); A fasciculate, anthers ± versatile, connective apically prolonged; exine "tilioid" [?level]; median G abaxial, style ?hollow, stigma slightly lobed or not; ovules 2/carpel, apical; K ± accrescent in fruit; seed usu. 1; cotyledons often folded, enclosing radicle; x = 7.
22/680: [list, to tribes] - two subfamilies, four tribes. More or less Pantropical, but overwhelmingly West Malesian in diversity. Map: from Gottwald and Parameswaran (1966) and Ashton (1982).
Age. Bansal et al. (2022a) thought that the crown-group age of Dipterocarpaceae was (112.2-)102.9(-93.5) Ma, Sanil et al. (2022) suggested an age of (83.6-)72.7(61.3) Ma.
Moyersoen (2006) suggested that Diperocarpaceae grew on Gondwana some 135 Ma (unlikely [before ii.2022!]: see below), ca 69 Ma is another suggestion (Hernández-Gutiérrez & Magallón 2019)..
1. Monotoideae Thonner —— Synonymy: Monotaceae Kostermans
Radicle (not medullated - Monotes); rays usu. uniseriate; paratracheal parenchyma +; pith/petiole with mucilage canals; petiole bundle C-shaped/incurved, medullary strand 0; (hairs simple/fasciculate); adaxial gland at base of lamina; (androgynophore +); anthers subversatile, (apical appendage 0); pollen tricolporate, exine 4-layered; G [(2-)3-5]; ovules (1/carpel), (straight), exostome prolonged [beaked], outer integument ca 3 cells across, inner integument 3-4 cells across; fruiting K subequal, thinnish; endosperm +, ?starchy; seeds 2-4/loculus; germination cryptocotylar, cotyledons grow after germination; n = ?
3/24: Monotes (20). Africa, Madagascar, South America (Colombian Amazon: Pseudomonotes). Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) - see above, area in green.
Age. Crown-group Monotoideae are estimated to be (84.5-)72.1(-63) Ma (Bansal et al. 2022a).
2. Dipterocarpoideae Burnett
Rays usu. multiseriate, paratracheal parenchyma ?0, pits ?vestured; vessels solitary; fibres with bordered pits; resin ducts +, pervasive in wood/petiole, scattered; nodes also 5:5, with at least lateral bundles leaving central cylinder well before they enter the leaf; K valvate (imbricate / open), unequal, (small and pointing backwards/deciduous), C (not contorted), basally connate; A (= and opposite K-)15-many, anthers ± basifixed, (connective not prolonged); pollen tricolpate, colpi long, sometimes almost reaching the poles, exine 2- or 3-layered, latter not tilioid; G [(2) 3]; ovules 2(-3)/carpel, epitropous, micropyle endo- or bistomal, outer integument 2-5 cells across, inner integument 2-9 cells across, parietal tissue ca 3 cells across; fruit a nut, endocarp hairy; testa vascularized [?all], (palisade exotegmen 0); endosperm 0, starch 0; n = (10) 11; nuclear genome [1 C] 0.326-0.672 pg; germination epigeal, phanerocotylar (cryptocotylar - cotyledons "prisonniers ou semi-prisonniers").
19/650: the Seychelles, Sri Lanka, India, South East Asia to New Guinea, but mostly West Malesian, often dominating in mixed-species stands. Map: above, area in red. Photo: Flower, Fruit.
Age. One estimate of the age of this clade is (104.3-)94.6(-85) Ma (Bansal et al. 2022a), and others range from ca 52.1/51.7/44.3 Ma (Cvetkovic et al. 2022) or ca 52.1 Ma (Sanil et al. 2022).
2A. Vaterieae Miquel
Vessels solitary; K (free); G (partly inferior); (micropyle bistomal, chalaza large, well vascularized - Vateriopsis; fruit a (loculicidal capsule - e.g. Upuna), K valvate, unthickened; seed (thin-arillate - Upuna).
7/123: Vatica (77), Stemonoporus (26). The Seychelles, Sri Lanka, India and Southeast Asia (inc. China) to East Malesia.
Age. Estimates of the age of crown-group Vaterieae are 52.1, 44.3 or 26.9 Ma (Cvetkovic et al. 2022), ca 35.1 Ma (Sanil et al. 2022) or ca 82.7 Ma (Bansal et al. 2022a).
[Dipterocarpeae [Dryobalanopseae + Shoreeae]]: ?
Age. The age of this node is around 70.2/55.9/46.9 Ma (Cvetkovic et al. 2022).
2B. Dipterocarpeae Reichenbach - Dipterocarpus C. F. Gaertner
Petiole bundles in one or more complex rings; lamina vernation plicate; K 2, valvate, winged; nectary +, at base of C; pollen grains large, gaping-tricolporate, exine tilioid; G basally inferior; micropyle bistomal, outer integument -10 cells across, chalaza large, well vascularized; K remaining valvate; endosperm +.
1/70. Sri Lanka, India, Southeast Asia (China) to Malesia.
Age. Crown-group Dipterocarpeae are estimated to be 37.2/19.2/9.3 Ma (Cvetkovic et al. 2022), ca 22.3 Ma (Sanil et al. 2022) or ca 72.6 Ma (Bansal et al. 2022b).
Pollen grains of Periretisyncolpites phosphaticus, a Dipterocarpus-type pollen, have been found in Maastrichtian deposits ca 68.5 Ma in South Sudan (Cole et al. 2017). Dipterocarpus dindoriensis, a fossil leaf was described recently from the Deccan Intertrappean deposits in the Mandla Subprovince of the Western Ghats from around the K-Pg transition ca 66 Ma; it was compared with D. alatus in particular (Khan et al. 2020a).
[Dryobalanopseae + Shoreeae]: Vessels in groups; ?fibres; K expanded, concave, thickened at case, C imbricate, forming a vase-like chanber and contorted at base [?]; x = 7.
Age. The age of this clade is ca 62.0/47.6/40.8 Ma (Cvetkovic et al. 2022) or ca 47.7 Ma (Sanil et al. 2022).
2C. Dryobalanopseae Baillon - Dryobalanops C. F. Gaertner
Vessels solitary; stomata cyclocytic, grouped in areoles, with trichome bases rounded in clusters on the rims, giant/D-type stomata on secondary-intersecondary veins; venation fine-parallel secondaries-intersecondaries; K in fruit ± fleshy, thickened, imbricate, becoming valvate as fruit develops.
1/7. West Malesia, not the Philippines.
Age. The crown-group age of Dryobalanopseae is 17.3/16.6/7.8 Ma (Cvetkovic et al. 2022), ca 13.0 Ma (Sanil et al. 2022) or ca 64 Ma (Bansal et al. 2022b).
2D. Shoreeae Miquel
Resin canals in tangential bands; K in fruit always imbricate.
10/322: Hopea (113), Rubroshorea (71), Shorea (47), Richetia (33), Anthoshorea (23). Sri Lanka, India, Southeast Asia (inc. China) to East Malesia.
Age. The age of this clade is around 55.76/41.6/33.4 Ma (Cvetkovic et al. 2022), ca 42.5 Ma (Sanil et al. 2022) or ca 75.8 Ma (Bansal et al. 2022a).
Evolution: Divergence & Distribution. For ages in Dipterocarpaceae, see Heckenhauer et al. (2017). However, since the topology of the tree in their Fig. 3 differs from the tree that they used when discussing relationships, and their Fig. 5, with ages, has a very different topology from the others, these ages are largely ignored below. There are some differences in topology between all three trees in Heckenhauer et al. (2017: Figs 1, 2, 5) and that in Bansal et al. (2022a: Fig. 3B). A number of clade ages suggested by Cvetkovic et al. (2022) are difficult to reconcile with ages discussed later in this section; Sanil et al. (2022: Fig. 2) estimate ages for all the nodes in their tree as do Bansal et al. (2022a).
Wood of fossil Dipterocarpoideae has been reproted from Tertiary deposits in north-east Africa (Mt Elgon) and the Horn of Africa (Lakhanpal 1970 for references; Ashton 1982); no Dipterocarpoideae grow on the continent today. The specimens from Mt Elgon have secretory canals containing "highly refractive globules suggesting oil drops" (Bancroft 1935: p. 170), which, on further analysis, were thought to be some kind of soft resin. For the fossil distribution of Dryobalanops, found in southernmost India in the past, see T.-X. Wang et al. (2025: Fig. 1).
Substantial deposits of amber (resin) in Gujarat, western India, may be from Dipterocarpoideae; these have been dated to the Ypresian (Early Eocene), some 52-50 Ma, quite soon after India docked with Asia; interestingly, insects in this amber do not suggest any particular isolation of the Indian continent (Rust 2010). These deposits contain bicadinanes, the breakdown products of the dammar resin of dipterocarps, and a melanin-forming ascomycete ECM fungus is associated with some roots found in the amber (Beimforde et al. 2011). However, bicadinanes are also formed from sources other than dipterocarps so they perhaps cannot be used as a "marker" for the family (Kooyman et al. 2019, but c.f. Bansal et al. 2022a). Resin deposits are also found in West Malesia-South East Asia rather later in the Caenozoic (for ambers, see also Seyfullah et al. 2018). Overall, ages for ambers may suggest the origin or early occurrence of the family in India and its later dispersal to South East Asia-Malesia after contact of the two was established ca 50 Ma (Dutta et al. 2011: Shukla et al. 2013); this idea is supported by the fossil Dipterocarpus dindoriensis leaf described from the Deccan Intertrappean Beds in India some 66 Ma (Khan et al. 2020a). A similar scenario was suggested i.a. by Ashton et al. (1988) and Morley (2018). However, Srivastava (2011; see also Kapgate 2013) emphasized that there was no evidence of Dipterocarpaceae in the rich fossil flora in the rocks of the Western Ghats laid down around the K/P boundary or in rocks elsewhere in India at this time; Datta-Roy and Karath (2008) are also agnostic as are Kooyman et al. (2019) after a careful review of all the evidence.
The monotypic Vateriopsis is currently restricted to the Seychelles, and it has been suggested that it diverged from other Dipterocarpeae ca 63 Ma (Ashton 2014); ocean crust separating India and the Seychelles dates to ca 63.4 Ma (Collier et al. 2008). Bansal et al. (2022a) date Vateriopsis to ca 68 Ma, although the stem-group age of the Vateriopsis clade is given there as 82.7 Ma. However, Heckenhauer et al. (2017) date the split between Vateriopsis and a clade containing the same taxa as in Bansal et al. (2022a) to ca 34.5 Ma and a mere 35.1 Ma is the age of this node in Sanil et al. (2022: Fig. 2, 33.6 Ma p. 1223) - so if these latter ages are confirmed a very different evolutionary scenario will be needed. Note that the genus has no obvious mechanisms for long distance dispersal and the fruits are not salt-tolerant. The monotypic Upuna, ca 62.5 Ma and currently restricted to Borneo, is given as another example of a palaeoendemic by Bansal et al. (2022a); ca 15.4 Ma is the age for this genus that was suggested by Heckenhauer et al. (2017) while ca 19.9 Ma is its age in Sanil et al. (2022). Some of these dates need to be reconciled with the 41 Ma (Eocene-Bartonian) or much younger ages proposed above for various branches in the [[Pakaraimaea + Cistaceae] [Sarcolaenaceae + Dipterocarpaceae]] clade. That Dipterocarpaceae grew on Gondwana some 135 Ma (Moyersoen 2006) seemed unlikely, and in any event the distinctive South American Pakaraimaea is now thought to be closer to Cistaceae than Dipterocarpaceae (see above), although Bansal et al. (2022a: p. 455) include it as a subfamily of Dipterocarpaceae. Pakaraimaea aside, Bansal et al. (2022a: Fig. 4) invoked both drift and LDD events to explain aspects of the current distribution of the family. In the course of their reconstruction of the phylogeny of Dipterocarpoideae, Sanil et al. (2022) suggested that 13 global vicariance events and 32 dispersals were needed to explain the distributions of the taxa involved. They thought that Dipterocarpaceae originated on Madagascar and thence moved to India-Seychelles, and then to Asia, and there was evidence of the divergence of major lineages in Dipterocarpoideae as the plants drifted north on the Indian plate 56-48 Ma, and again later as the plate collided with Asia 35-25 Ma. (Taxa like Euphorbiaceae-Crotonoideae, some Ebenaceae, and Arecaceae (q.v.) are also thought by some to have originated in Africa, then moved to India, etc..)
All this being said, the substantially greater ages suggested for Dipterocarpaceae by Bansal et al. (2022a) depend largely on fossil evidence. Thus the palynomorph Periretisyncolpites phosphaticus, a Dipterocarpus-type pollen, is recorded from Early Campanian (somewhat over 80 Ma) to latest Eocene deposits in the South Sudan and another Dipterocarpus-type pollen is known from somewhat younger Maastrichtian deposits there (Cole et al. 2017; Bansal et al. 2022a). Leaves identified as Dipterocarpus have been recorded from the end-Cretaceous Deccan Traps, while pollen from lignite mines in N.W. India from deposits dated to the early Eocene have been assigned to six more genera, and the result is that stem groups of just about all genera in the family were evident by around 50 Ma (Bansal et al. 2022a: Fig. 3B). This suggested an African origin for Dipterocarpaceae, clades adapting to tropical dry seasonal (Monotoideae) and wet seasonal/perhumid conditions (Dipterocarpoideae) and moving to India, and often ultimately to Malesia. The early pollen records suggested to Bansal et al. (2022a: p. 456) that dipterocarps were "one of the first obligate megathermal eudicot clades to originate in the mid-Cretaceous of Africa", where they may have been components of the earliest multistoried rainforests. Tedersoo (2017b) mentioned a massive end-Eocene extinction of Dipterocarpaceae in Central Europe, North America and East Asia while Bansal et al. (2022a) thought that Monotoideae had become extinct in India. Wood in Late Middle Eocene deposits ca 39 Ma on the Pacific side of Peru has been identified as that of Pseudomonotes, and this Peruvian locality is quite distant from where the genus currently grows (Woodcock et al. 2017). Complicated.
For features characterising Dipterocarpoideae and Monotoideae, see Bansal et al. (2022a). Heckenhauer et al. (2018) discussed floral evolution in Shoreeae, and they thought that flowers with 15 or fewer stamens and large oblong anthers with short appendages were likely to be basal in that clade (see Pollination Biology & Seed Dispersal below).
Ecology & Physiology. ECM Dipterocarpaceae (they are Dipterocarpoideae) are large trees that are often dominant in l.t.r.f. from India and Sri Lanka to West Malesia (Alexander 1989a); they are the major ECM family in that part of the world (Corrales et al.2018). They are the most diverse tree family in the West Malesian l.t.r.f. (Gentry 1988), and Shorea is about the most diverse genus there (Davies et al. 2005). In the West Malesian forests Dryobalanops aromatica and Shorea albida in particular may form very extensive and close to monodominant stands (e.g. Connell & Lowman 1989). In the Lambir forest, Sarawak, Dipterocarpaceae make up only 7.4% of the species but 41.6% of the basal area (918.4 m2); the figures for Shorea alone are 4.7%, 21%, and 467.8 m2, Dryobalanops aromatica and Dipterocarpus globosus between them accounted for 13.2% of the basal area, and seven dipterocarps (out of the ten most dominant species) accounting for 23.1% (H. S. Lee et al. 2002; Davies et al. 2005; also Alexander 1989). The trees are larger than the average in the forest, yet only 16% of the trees at least 1 cm d.b.h. are dipterocarps (Lee et al. 2002). Bansal et al. (2022a) observed that Dipterocarpoideae initially have plagiotropic branching, and this is followed by orthotropic branching, the leaves are both resinous and not very palatable to many herbivores, etc.. Different dipterocarps show overall similarity in several ecological traits (Fukami et al. 2017).
Like other ECM plants, large amounts of carbon accumulate in dipterocarp forests. In the lowland wet tropical West Malesian forests there are huge peat lenses on which dipterocarps dominate, although not in all communities (Anderson 1964, 1983; Richards 1996; van Schöll et al. 2008). It has been estimated that Southeast Asian tropical peatlands (mostly Malesian, in fact most Indonesian, all largely made up of dipterocarp peats) occupy about 3/5 of the tropical peatland area, close to 250,000 km2, and about 6.2% of the global peatland area (Page et al. 2011). This peat contains some 68.5 Gt carbon, 77% of the tropical and 11-14% of the global totals for peatlands (Page et al. 2011: estimates of above-ground dipterocarp biomass not included). See also Brown et al. (1993: soil to 1 m only) for biomass estimates, both actual (as impacted by human activities) and potential; other estimates are 84 Gt C in tropical peat, 16 Gt C in southern peats, and as much as 621 Gt C in northern peats (Rieley & Page 2016), corresponding figures in Z. Yu et al. (2010) are around 50, 15, and 550 Pg C - see Rydin and Jeglum (2013) and Rieley et al. (1997) for other estimates. Compared with temperate, usually moss-dominated peats, productivity and biomass accumulation in these tropical peats are high - and they have lost about 41% of their area over recent centuries (Temmink et al. 2022).
The formation of some peat deposits may have started in the late Pleistocene 40,000 y.a. and the peat is up to 25 m deep (Page et al. 2004, 2012). Carbon storage in this peat is long term and may have been affected by the indirect effects of climate change over the Pleistocene. During glacial periods sea levels tend to fall, in the case of the Sunda Shelf potentially exposing new areas for dipterocarp colonisation, while in the warmer interglacials sea levels rise, perhaps leading to the burial of coastal peat deposits (Treat et al. 2019). Note that in intact forests carbon loss is from recently fixed carbon, while in disturbed forest much older - hundreds to thousands (ca 4,180) of years old - carbon is lost (Moore et al. 2013). In a single near-surface Bornean peat examined carbohydrate concentrations were lower and aromatic (lignins, tannins, etc.) concentrations were higher than comparable more temperate (northern hemisphere) peats examined, perhaps contributing to the relative recalcitrance of dipterocarp peat (Hodgkins et al. 2018); note that Sphagnum peat is carbohydrate-rich and also decay-resistant, so lignin concentration is not the only indicator of decay resistance (Hájek et al. 2011 and references). Of the ten most important species (in terms of basal area) Dryobalanops aromatica and Dipterocarpus globosus grew on the more humic soils, along with two other dipterocarps; the three non-dipterocarps on this list also grew on the same more humic soils (Davies et al. 2005: read again).
Recent work has emphasized the great above-ground wood productivity of north Bornean dipterocarp forests, about half as much again when compared with non-dipterocarp forests in the western Amazon (Ecuador, Peru) that have comparable soils, precipitation, etc. (Banin et al. 2014). This high productivity was despite a much lower amount of P in the soil in the dipterocarp forests and a C:N ratio about 50% higher; overall, dipterocarps are taller trees that increase in diameter faster, and solar radiation is also higher in Borneo, the end result being a ca 40% greater above-ground wood production (Banin et al. 2014). It has recently been noted that dipterocarp woods (from Sabah) were notably low in nutrients like nitrogen, P and potassium, these nutrients being resorbed as the sapwood senesced - there was up to over 60% resorbtion of P, for example - and this was unlike the behaviour of other trees from the same area (Inagawa et al. 2023; see also Brearley et al. 2023). D. Johnson et al. (2023a) thought that an important aspect of at least dipterocarp- and Fagaceae-dominated ECM forests in Southeast Asia-Malesia was their ability to take up P as well as N, both perhaps in an organic form; the interaction of the common mycorrhizal network with seedlings was also important (Johnson et al. 2023a). However, Brearley et al. (2023) suggested that the story was unlikely to be so simple, noting i.a. that dipterocarp density in Malesia was unaffected by the concentration of P in the soil, and these authors thought that how dipterocarps were able to grow so fast was unclear. Johnson et al. (2023b) maintained that P acquision by dipterocarps was probably important. Prohaska et al. (2023) analyzed deposits up to 1,400 years old in a volcanic lake in N.E. Luzon, and found that pollen production of dipterocarps, and also palms and Pometia, was positively correlated with sedimentary P. Corrales et al. (2018) had noted that in some situations where there was evidence that tropical ECM plants might increase their nutrient uptake, new growth was not enhanced; increased uptake might be to deal with the nutrient demands of masting (see below). Intriguing findings, even if how they might relate to underground carbon storage and to ECM activities is to be established. And somewhat on a tangent, dipterocarp trees have notably large and tough leaves, and this perhaps explains some of the leaf morphological differences between Asian and African and American tropical forests (Aguirre-Gutiérrez et al. 2015). For further discussion on some differences between lowland tropical rainforests on different continents, see elsewhere.
Although West Malesian forests may be dominated by dipterocarps, these forests are often very diverse in terms of overall species numbers (e.g. Ashton & Hall 1992; H. S. Lee et al. 2002), and in this respect are rather unlike temperate/boreal ECM communities where diversity is frequently lower. Moreover, compared with some other very diverse forests in the New World, there are relatively few understory specialists (LaFrankie et al. 2006; Banin et al. 2014). Dipterocarpaceae are interesting in that they are tropical ECM plants sometimes with low population densities, they have animal pollination and wind dispersal of their seeds, a somehwat unusual combination of features - see also Yamawo and Ohno (2024), also elsewhere.
Shorea robusta (sal) is a gregarious tree that grows in monsoon areas from Pakistan to China, especially in the India-Assam-Myanmar area. Sal forests occupy 115,000 to 120,000 km2 (11.5x106 ha) and make up ca 15% of Indian forests (Tewari 1995). In Africa Monotes is found mostly in areas where ECM Detarieae are common, especially in the Zambezian region and also in Sudanian Brachystegia savanna, and it is oten described as being locally abundant (White 1983: see map above).
Masting. Dipterocarps are noted for being mast fruiters, and in the l.t.r.f. of S.W. Sri Lanka and in West Malesia all members of the family tend to flower and especially fruit together, but at irregular intervals, species reproducing less than one to several years apart, apparently in response to climatic changes induced by El Niño events; this masting behaviour is very uncommon in the tropics (e.g. Sakai et al. 2005, 2006). Dipterocarp flowering and fruiting is a more or less annual event outside the everwet l.t.r.f. today, indeed, earlier in the evolution of the family masting/fruiting may have been a response to dry/cool conditions in the more seasonal Gondwanan climates in which dipterocarps initially evolved, but it became a more intermittent yet still synchronized affair and responding to the same environmental cues in the everwet climates of later Caenozoic Malesian rainforests (Ashton et al. 1988; Ashton 2014; Kurten et al. 2017; Flenley 2018; Ascoli et al. 2021 and references). Yeoh et al. (2017; see also Y. Y. Chen et al. 2018) suggested that signals from drought/low temperature accumulated over 2-3 months and activated flowering genes, short droughts or cold snaps being inadequate, and it was then another 1-2 months until flowering; details of the signal needed to trigger flowering varied between species. Prohaska et al. (2023) looking at flowering (as evident in the pollen record) in Philippine dipterocarps over the last 1,400 years and noticed a corrleation with reduced rainfail associated with El Niño events; there was also increased mortality of dipterocarps associated with droughts to be taken into consideration... Interestingly, the flowering times of different species of Shorea, for example, tend to be staggered (noted also in Bignoniaceae, Melastomataceae, Heliconiaceae, Acacia where several species were growing together), while it is fruiting that tends to be synchronous (Hosaka et al. 2016) - and the earlier flowering species have time to develop larger fruits. Satake et al. (2020) found that a signal from drought and in particular drought plus cooling triggered the flowering/fruiting episodes, and it was noteworthy that subsequent seedling growth/establishment occurred when conditions were wet. Satake and Kelly (2021) looked at the interaction of delayed fertilization and variation in flowering time (the focus was on Fagaceae, but on entomophilous taxa), and suggested that there was a correlation. But not only dipterocarps are involved in these flowering events, and Appanah (1985) estimated that species belonging to around 41 families were involved in mast flowering in the Malay Peninsular (also e.g. Sakai 2002). Pigs and other animals, which may be migratory, following the food around, eat practically all the fallen fruits of some dipterocarp populations, yet leave others untouched, and this kind of predator satiation, leading to enhanced recruitment of seedlings in untouched populations, may be an advantage conferred by masting behaviour (e.g. Janzen 1974a; Curran & Leighton 2000; Hosaka et al. 2017), if not its cause. There are also invertebrate seed predators, including the ca 9 species of the nanophyid weevil genus Damnux whose larvae eat only dipterocarp seeds, and these can destroy 60-100% of the seed crop (Lyal & Curran 2003); Hosaka et al. (2016) noted there was only enough time in a masting event for a single generation of the weevils they studied to develop, i.e. its population could not explode. Visser et al. (2011) also suggest situations in which masting behaviour could evolve, and the ECM habit of the family has also been implicated in its phenological behaviour and/or predator satiation (Ashton 1982, 2002, esp. 2014). The complexity of the Malay archipelago may result in masting events happening at different times in different parts of the archipelago, although the events are more widespread if the ENSO event is strong (see also Ascoli et al. 2021). The series of events associated with masting may also include facilitation of germination and seedling establishment. See also Fagaceae for temperate masting, and Pesendorfer et al. (2021a) and other papers in "The ecology and evolution of synchronous seed production in plants" (Phil. Trans. Royal Soc. B, 376:1869. 2021) for mast fruiting in general.
A few Dipterocarpaceae, along with some species of Eucalyptus, Pinales and Cupressales, are so-called giant trees (>70 m tall: Tng et al. 2012).
There are more figures on dipterocarp productivity, etc., and further discussion under Clade Asymmetries, much information about Malesian dipterocarps in particular in papers in Osaki and Tsuji (2016), and see also Sasaki (2006), Corlett and Primack (2011), etc.. Finally, other major peat-producing ecosystems include Sphagnum bogs, mangroves and seagrasses.
Pollination Biology & Seed Dispersal. Pollination of species of Shorea section Mutica and of other dipterocarps in Sarawak is by chrysomelid beetles and especially curculionid weevils; in Peninsula Malaysia species of section Mutica are apparently pollinated by thrips (Appanah & Chan 1981; Sakai et al. 1999a; Corlett 2004 for a summary). However, in at least some cases it is a bug that feeds on the flower thrips that is most effective in cross pollination, while stipule-dwelling thrips maintain the population of the bug in between flowering events (Kondo et al. 2016 and references). Larger-flowered dipterocarps are pollinated by Apis and Trigona bees (Ashton et al. 2021). The pollinators are affected by the synchronized flowering common in the family (see Ecology & Physiology above), and Apis dorsata in particular moves its nest (these bees have large nests) long distances to take advantage of these mass-flowering events; it pollinates ca 10% of the trees, mostly large canopy trees (Sakai et al. 2005; Corlett & Primack 2011: see above). Heckenshauer et al. (2018) outline three pollination syndromes, the first two in Shoreeae, the last in Dipterocarpus, at least: 1) Many stamens, large anthers with short appendages, large pollen grains, perhaps large pollinators, relatively slow breeders, and annual flowering: 2) small flowers, ≤15 stamens, usu. slender ciliate appendages, pollinators especially thrips and flies with short life cycles so populations build up fast, masting; and 3) Many stamens, large yellow anthers, prominent stoutly acicular appendages, large pollen grains, pollinated by nectarivorous moths (other species lack nectar and are pollinated by large Apis dorsata bees - Harrison et al. 2005), masting.
Fruit dispersal is predominantly by wind; it is the fallen fruits that are eaten by mammalian seed predators.
Plant-Bacterial/Fungal Associations. For ECM, see Smits (1994), Appanah (1998), Tedersoo et al. (2007: dipterocarps on the Seychelles), Brearley (2012: summary), Phosri et al. (2012: dry dipterocarp forest, the ascomycete Cenococcum geophilum common) and Sato et al. (2015: wet dipterocarp forests, C. geophilum rare). Brearley et al. (2016) found no evidence for a common ectomycorrhizal network that might enhance dipterocarp seedling growth and establishment, at least in the Bornean forests that they studied.
Vesicular-arbuscular mycorrhizae have also been reported from dipterocarps (Brearley 2012). Sato et al. (2015) surveyed endophyte diversity, which was very high - perhaps in the region of 1,200 species, largely ascomycetes, from 442 root samples, although most species were very rare.
Parasitic rust fungi (Uredinales) are apparently unknown on Dipterocarpaceae, unlike many other ECM groups (Malloch et al. 1980); this observation should be confirmed, and extended to other members of this clade.
Plant-Animal Interactions. Up to 63% of the seeds of some Malesian Shorea are killed by weevils that eat developing seeds, and their effect on seed production is discussed by Hosaka et al. (2016). Hemipteran coccoid Beesonidae form distinctive galls on Dipterocarpoideae, although details of the association are poorly known (Gullan et al. 2005).
Genes & Genomes. Genomes of Dipterocarpaceae-Dipterocarpoideae are very small compared with those of other angiosperms, Ng et al. (2016) estimating that the ancestral size (1Cx) was (0.43-)0.48(-0.53) pg, with subsequent reduction in size to around 1C = (0.33-)0.42(-0.49) pg. Heckenhauer et al. (2017) also give genome sizes for some Dipterocarpoideae. The suggestion of base chromosome number in Carta et al. (2020) agrees with that in Raven (1975).
The cytology of Dipterocarpaceae is somewhat irregular, with triploid (and higher ploidy level) species known and apomixis and associated polyembryony of the seeds recorded (e.g. Kaur et al. 1986; Ghazoul 2016).
Economic Importance. Dipterocarpoideae, with their long, straight and clean boles and gregarious habits, are - "have been" might unfortunately be more accurate - a major source of commercial hardwood. In the mid 1980s they comprised 25% of the world trade in tropical hardwoods, and 80% of that was made up by timber from Shorea. Furthermore, both oleoresins and hard resins (dammar) are collected from a number of Dipterocarpoideae, while oil deposits in Malesia have been formed from dammar (Rust et al. 2010 for references). Dipterocarpoideae are also a source of lac (exudate produced by Coccoidea), butter fat from the fruits, etc. (Ashton 1982; Smits 1994 and references; Lambert et al. 2013 for the resins). Hardly surprisingly, dipterocarp forests have been particularly severely negatively impacted by human activities (Ashton et al. 2021).
Chemistry, Morphology, etc.. Bergenin, a derivative of gallic acid, is widespread. Stilbenoids, resveratrol and relatives, are common in Dipterocarpaceae (Wibowo et al. 2011), but I do not know if there is any systematic significance in this.
Maury (1978) provided a detailed discussion of nodal and petiole anatomy following changes from the cotyledons to leaves of the adult tree; she found considerable variation. The stipules of Stemonoporus are extremely caducous.
The calyx in many Shoreeae is imbricate in fruit, while the corolla is basally connate in many Dipterocarpeae. Dipterocarpus, at least has vascular bundles in the inner integument.
For general information, see Maury (1978: quite some thesis!), Ashton (1982, 2002), Maury-Lechon and Curtet (1998) and Appanah and Turnbull (1998) and other papers in these volumes, Ghazoul (2016), Ashton et al. (2021) and Colli-Silva et al. (2025), also Whitmore (1962: bark), Gottwald and Parameswaran (1966: wood anatomy), Talip et al. (2017: Hopea petiole anatomy), T.-X. Wang et al. (2025: Dryobalanops leaves), Kocyan (2005: floral development) and Maury et al. (1975: pollen). For additional information on Monotoideae see Catalina Londoño et al. (1995) and Morton (1995).
Phylogeny. See also Kajita et al. (1998), Morton et al. (1999), Dayanandan et al. (1999), Tsumura et al. (2011), M. Sun et al. (2016) and Heckenshauer et al. (2017, 2018, 2019) for relationships. Unfortunately, these remain poorly understood, although there are developing patterns, at least in analyses using chloroplast data. Gamage et al. (2006) found the relationships [[Vateriopsis + most Dipterocarpeae] [Dipterocarpus [Dryobalanops [Shorea with Hopea and Neobalanocarpus embedded]]]]. However, in Heckenshauer et al. (2017) Dipterocarpus is poorly supported as sister to [Dryobalanops + Shoreeae], while in the BEAST analysis it has rejoined the other Dipterocarpeae; again, they found that Shorea is paraphyletic, Hopea being embedded in it, while topologies in subsequent analyses are not totally consistent. In an analysis using plastome and nuclear ribosomal cistron data, Cvetkovic et al. (2022) recovered the topology they converted to the classification above, and although there were substantial differences between the chloroplast and nuclear topologies they obtained in Shoreeae, in terms of classifications the overall topology there does not differ from those in Sanil et al. (2022) and Ashton and Heckenhauer (2022). Note, however, that Sanil et al. (2022: 3 plastid markers, 146 dipterocarps) found the Vaterieae to be sister to Dipterocarpeae, although support was poor. The topology recovered by Sanil et al. (2022) showed that within Dipterocarpus, D. retusus was sister to the rest; Dipterocarpeae had a stem of around 27 Ma, and after the divergence of D. retusus there was a further 11 My before anything else happened with respect to extant species. And some suggest that Dipterocarpaceae are paraphyletic, the clade including Sarcolaenaceae (Lamont et al. 2022)...
Neobalanocarpus may be an intergeneric hybrid, Shorea/Anthoshorea sp. x Hopea sp. (Kamiya et al. 2005; Gamage et al. 2006; see also Heckenhauer et al. 2019; Cvetkovic et al. 2022: two hybridization events with Hopea?). Parashorea may be another hybrid (Heckenhauer et al. 2019).
Classification. Tribes still need attention. A three-tribe classification might make phylogenetic sense, however, in 2022 three different tribal classifications appeared; Sanil et al. (2022) recognised just Dipterocarpeae and Shoreeae, Ashton et al. (2022, see also Ashton & Heckenhauer 2022) three tribes, adding Vaterieae, while Cvetokovic et al. (2022) recognized four tribes, Dryobalanopsideae being split from Shoreeae (note that Sanil et al. 2022, unlike the other two, had recovered a [Vatica etc. + Dipterocarpus] clade), while if the paraphyly of Dipterocarpaceae (Lamont et al. 2022) is confirmed, this will entail yet another classification. However, as elsewhere, we also await the results of nuclear phylogenies.
Generic limits within Dipterocarpaceae-Dipterocarpoideae also need attention (e.g. Yulita et al. 2005). Thus Heckenhauer et al. (2018) noted that Shorea could be expanded to include Hopea etc., but since those two genera were very distinct they hesitated to take that step... (see also Heckenhauer et al. 2019); on the other hand, Ashton and Heckenhauer (2022) recognized Hopea and split Shorea, so getting rid of any paraphyly problems...
See Ashton and Heckenhauer (2022) for sectional classifications of Shorea and Rubroshorea.
MALVACEAE Jussieu, nom. cons. - Back to Malvales
Shrubs to trees (herbs); cyclopropenoid fatty acids, terpenoid-based quinones +, gums common; (cork cambium outer cortical); wood commonly fluoresces; pits not vestured; tile cells common; sieve tubes with non-dispersive protein bodies; hairs stellate/lepidote; leaves spiral or two-ranked, lamina margins entire or toothed, single vein running to the non-glandular apex, secondary venation palmate; inflorescence made up of modified cymose units ["bicolor units"]; (epicalyx +), K valvate, nectary a carpet of uni-/biseriate multicellular glandular hairs on basal-adaxial K, (C imbricate); (androgynophore +); A (5-)many, in five groups opposite the C, but fundamentally obdiplostemonous, at least basally connate, extrorse; tapetum amoeboid, cells 2(-4)-nucleate; post-zygotic incompatibility system [?all]; G [(3-)5(-many)], variable in orientation, style often hollow, usu. 5-branched apically, stigma usu. dry; ovules 1-many carpel; micropyle zig-zag (endo- or exostomal), outer integument develops first, 2-4(-7) cells across, inner integument 2-7(-10) cells across, parietal tissue 3-8 cells across, nucellar cap 0-5 cells across, obturator +/0 [often of placental hairs]; (megaspore mother cells several), inverse postament +; fruit a capsule (berry, schizocarp, etc.; muricate); testa (mucilaginous), multiplicative, vascularized, endotesta crystalliferous, tegmen multiplicative, endotegmic cells ± thickened; endosperm often starchy, resting zygote +, mature embryo often green; x = 10, 11 (?9), nuclear genome [1 C] (0.051-)0.743(-10.752) pg/(188-)1404(-4010) Mb; plastid rpl22 gene lost [?level].
243/4,225< [list - genera assigned to tribes] - 9 subfamilies, some tribes, below. Largely tropical, also temperate. Photos: Collection.
Age. Estimates of the age of crown Malvaceae are 24-21 Ma (X. Wang et al. 2016), (47-)44, 31(-27) Ma (Wikström et al. 2001), (78-)66(-64) and (44-)39(-22) Ma (Bell et al. 2010), ca 70 Ma (R.-G. Zhang et al. 20250, (78.6-)70.7(-63.4) Ma (Richardson et al. 2015), ca 90 Ma (Hernández-Gutiérrez & Magallón 2019) or (143-)110(-86) Ma (Cvetkovic et al. 2021: Dipt + Malv = (208-)153(-95) Ma). On the other hand, an estimate for the age of the node [Byttnerioideae + Malvoideae], effectively the age of the family, was around 19.0 or 18.2 Ma... (Xue et al. 2012).
Some fossils placed in the family are quite old, some being from the Cretaceous. Thus wood attributable to Malvaceae is known from the late Maastrichtian ca 68 Ma; it has simple perforation plates in radial multiples and storied wood, but tile cells were not reported (Wheeler et al. 1994). Still older malvaceous wood (Bombacoxylon) is reported from Campanian sediments in Texas ca 75 Ma (Wheeler & Lehman 2000) while Shukla et al. (2025) estimate the stem-group age of the Eriolaena clade (Dombeyoideae) to be around (105-)88(-72) Ma - this estimate is based on early Paleogene fossil leaves from Rajasthan, India.
[Grewioideae + Byttnerioideae] / Byttnerina clade: x = ll.
Age. Estimates for the age of this node are (37-)33, 37(-23) Ma (Wikström et al. 2001), (44-)32, 30(-19) Ma (Bell et al. (2010), ca 82.3 Ma (Hernández-Gutiérrez & Magallón 2019) or ca 65.8 Ma (Cvetkovic et al. 2021).
1. Grewioideae Dippel
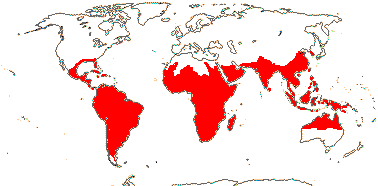
(Inflorescence leaf-opposed); K ± free, C usu. clawed, adaxially with various epidermal modifications; A (5-)many, usu. free, (bundles antesepalous), (ringwall primordium), development centrifugal, (staminodes many); pollen prolate; G [2-10], hypostase +; fruit fleshy, or capsular; (seeds winged); n = 7-9(-10).
24/770. Pantropical (warm temperate). Map: based on Cheek (2007, modified by Lebrun 1977), Frankenberg and Klaus (1980), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Fl. China vol. 12 (2007) and Australia's Virtual Herbarium (consulted xii.2012).
Age. Crown-group Grewioideae are estimated to be (60.6-)42.2(-25.6) Ma (Richardson et al. 2015) or ca 64.4 Ma (Hernández-Gutiérrez & Magallón 2019).
Woods of Grewinium, from Late Cretaceous sediments 67-65 Ma in the Deccan Traps, belong to Grewioideae (Wheeler et al. 2017).
1A. Apeibeae Bentham —— Synonymy: Sparrmanniaceae J. Agardh, nom. cons.
Hairs unicellular, with multicellular base (stellate); (extrafloral nectaries +); inflorescence usu. 2-3-flowered cymes; K apex with horn-like appendage (0); A developing in whorls, (anthers elongate, with sterile tip); androgynophore 0 (+, with nectariferous hairs opposite C); vascular bundles in carpel wall separate; fruit usu. spiny.
11/255: Triumfetta (150), Corchorus (40-100). Tropical, many spp. Australia, to New Zealand.
1B. Grewieae Endlicher —— Synonymy: Grewiaceae Doweld & Reveal
Hairs stellate (with multicellular base); inflorescence 4- or more-flowered panicles; C basally laterally constricted, nectariferous hairs carpet at base (0); A primordia complex at first, antesepalous primordia more pronounced, (fasciculate); androgynophore + [no nectaries] (0), (staminodes +, abaxial); stigma capitate; vascular bundles in carpel wall embedded in sclerechymatous sheath; plastid transmission biparental - Grewia.
13/515: Grewia (290), Microcos (60). Pantropical, some warm temperate.
2. Byttnerioideae Burnett
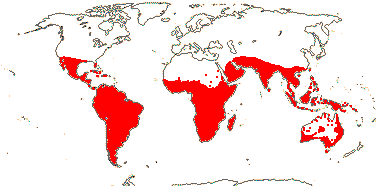
Usu. shrubs; distinctive 4-pyridones and 4-quinolines +; petiole bundle ± incurved-arcuate/subannular; secretory cavities/ducts + [Theobroma - mucilage]; (extrafloral nectaries +); leaves spiral or 2-ranked; K usu. connate, (nectariferous hairs 0), C develops last, broad towards the base, hooded, with inrolled margins, limb clawed, spathulate, linear, bifid, etc. (0); A epipetalous, usu. isostemonous to diplostemonous (-30), antepetalous, fasciculate, (obdiplostemonous, obhaplostemonous), staminodes + (0), antesepalous, linear, petal-like, forming a tube, etc.; (extragynoecial tapetum + [Seringia], tapetum false amoeboid [contents of cells resorbed]; (pollen 4≤ zono(col)porate); style apically ± branched; fruit usu. capsule, septicidal, (mericarps), (dehiscence explosive); strophiole common; (testa not multiplicative); n = (5-7) 10(-13).
23/650. Pantropical, esp. South America, also Australia. Map: from Cheek (2007), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Australia's Virtual Herbarium (consulted i.2013). [Photo - Flower, Flower, Fruit] - also Google Ayenia, etc..
Age. The age of crown-group Byttnerioideae is around (68-)53.4(-33.9) Ma (Richardson et al. 2015) or ca 70.2 Ma (Hernández-Gutiérrez & Magallón 2019).
2A. Byttnerieae de Candolle [Herminieae de Candolle] —— Synonymy: Byttneriaceae R. Brown, nom. cons.
(Climbers); K rotate, C claw erect, limbs concave, connate, appendage linear/0; A = C (15), forming a staminal tube, staminodes +; fruit a capsule, 1 seed per loculus.
6/280: Ayenia (216, inc. Byttneria), Leptonychia (38), Scaphopetalum (20). Pantropical.
[Theobromateae [Lasiopetaleae + Hermannieae]]: ?
2B. Theobromateae Stahl —— Synonymy: Cacaoaceae T. Post & Kuntze, nom. illeg., Theobromataceae J. Agardh
Trees (herbs - Glossostemon); braches mono-/sympodial (Theo.; (leaves palmately compound - Herrania s. str); (ramiflory/cauliflory); C = claw cucullate, basal + linear to filiform ligule, (nectarostomata + - Theo.); A 2-4/fascicle, staminodes ± petal-like; placentation axile; fruit a berry, longitudinally ridged or not, usu. whitish pulp =; seeds purplish, brown, cotyledons folded and corrugated; n = 10.
3/44: Theobroma (40: inc. Herrania). American tropics, Glossostemon Middle East.
[Lasiopetaleae + Hermannieae]: ?
2C. Lasiopetaleae de Candolle —— Synonymy: Lasiopetalaceae Reichenbach, Melochiaceae J. Agardh
(Stipules 0); (epicalyx bract petaloid), K petalois, vaariously ribbed, C 0/scale-like, bases concave; A = C (2 x C - Maxwellia - Max), anther thecae often confluent and ± porose; staminodes subulate; style (hairy), (apically divided), (placentation parietal - Max.); seeds arillate (not - Max.); testa ± developed, (hairy), meso- and endotegmen +; endosperm copious.
9/191: Lasiopetalum (55), Androcalva (33), Thomasia (32), Commersonia (25) - N.B., generic limits to change. Mostly Australia, few Madagascar, Southeast Asia and New Caledonia.
2D. Hermannieae de Candolle —— Synonymy: Hermanniaceae Marquis
Inflorescence subtended by 4 bracts, (flowers heterostylous - Waltheria); C flat; A = C, connate into a tube, (filaments carnose); fruit a capsule, (1 - W.) 2 (+) seeds/locule.
5/176: Hermannia (65+), Waltheria (55), Melochia (54). Pantropical.
[Helicteroideae, Durionoideae [Brownlowioideae, Tilioideae, Dombeyoideae, Sterculioideae] [Freemontodendreae [Bombacoideae [Matisioideae + Malvoideae]]]] / Malvadendrina clade: Mal-β genome duplication, x = 10, 21 bp deletion in ndhF gene.
Age. Possible ages for this node are (34-)31, 28(-25) Ma (Wikström et al. 2001), (36-)29, 27(-20) Ma (Bell et al. 2010) - note sampling in both, ca 81.8 Ma (Hernández-Gutiérrez & Magallón 2019), (112-)93(-79) Ma (Cvetkovic et al. 2021: note topology, split of Helicteroideae) and (77-)63(-48) Ma (Skema et al. 2022: Til. + Dombey).
Neesia unplaced
3. Helicteroideae Meisner —— Synonymy: Helicteraceae J. Agardh, Mansoniaceae A. Chevalier, Triplochitonaceae Schumann, nom. nud.
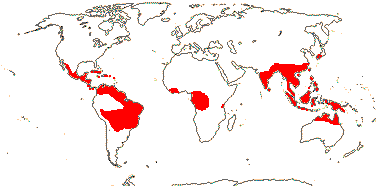
(Rays broad); (indumentum lepidote); petiole pulvinate; K connate, C clawed, often with lateral constrictions; (androgynophore +); A 10-many, usu. with short tube and/or fascicles, (anthers monothecate), (thecae end-to-end); pollen baculate or microverrucate to suprareticulate; outer integument ca 2 cells across, inner integument ca 2 cells across; (seeds winged), (arillate); n = 9, 14, 20, 25, etc.
8-12/95: Helicteres (40). Tropical, esp. Southeast Asia and W. Malesia. Map: from Cheek (2007). [Photo - Fruit.]
Age. Helicteroideae are around (50.4-)30(-11.7) Ma (Richardson et al. 2015), 66.3 Ma (Hernández-Gutiérrez & Magallón 2019) or ca 69 Ma (Cvetkovic et al. 2021: Dur, Reev).
3. Durionoideae R. G. Zhang —— Synonymy: Durionaceae Cheek
(Large) trees; indumentum lepidote or stellate; leaves simple, secondary veins pinnate; epicalyx +, initially fused; K free to variously connate; (C 0); A various; fruit large, muricate or generally spiny, capsular; seeds large, ± covered by aril, or funicle apex with fleshy outgrowth; cotyledons flat, fleshy or foliaceous; AABBCC genomes, x = 13.
5/39 Durio (26), Coelostegia (6). Southeast Asia to West Malesia.
[Brownlowioideae, Tilioideae, Dombeyoideae, Sterculioideae] [Freemontodendreae [Bombacoideae [Matisioideae + Malvoideae]]]] / Malvadendrina clade: ?
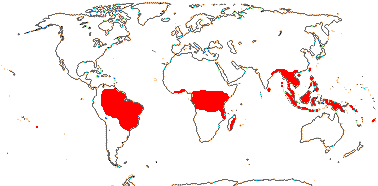
4. Brownlowioideae Burret
Trees; indumentum often lepidote; leaf blades cordate, lobed; inflorescences axillary; K connate, campanulate to urceolate, splitting irregularly into 2-3 lobes; A ca 30, in bundles, at most slightly fused, anther thecae sagittate, often broadly so, staminodia +, antesepalous, ± petaloid;; pollen 3 brevicolporate, exine reticulate; style or styles +; ovules 2/carpel; fruit (winged), (spiny), K persistent; (seeds hairy/peltate scales); AABB genomes, n = ?
10/98. Tropical, esp. Old World. Map: from Cheek (2007) and Fl. China vol. 12 (2007).
Age. Crown-group Brownlowioideae are some (37-)20.5(-5) Ma (Richardson et al. 2015) or ca 30.1 Ma (Hernández-Gutiérrez & Magallón 2019).
4A. Berryeae Burret —— Synonymy: Berryaceae Doweld
Staminodes quite often 0; (ovules to 6/carpel).
5/19: Berrya (6), Christiana (6). Sri Lanka, Tropical Asia to N.E. Australia and the S.W. Pacific, some Africa and America (Cuba, Surinam).
4B. Brownlowieae Bentham —— Synonymy: Brownlowiaceae Cheek
(pollen in tetrads - Somnuekia); (ovules to 10/loculus).
5/79: Pentace (30), Brownlowia (29), Diplodiscus (11). Sri Lanka, China to Malesia.
5. Tilioideae Arnott —— Synonymy: Tiliaceae Jussieu, nom. cons.
(Plant ectomycorrhizal); stachyose, raffinose + [phloem exudate - Tilia]; (leaves siliceous); petiole bundle with medullary phloem strands and inverted bundles; leaves 2-ranked, horizontally conduplicate [?always], cordate, (stipules 0 - Mortoniodendron); K free, C slightly clawed (nectariferous hairs on C/androgynophore); androgynophore 0; (A free), staminodia also opposite C, (0), antesepalous sector empty; pollen exine tilioid-like?; G opposite K; (postament +); fruits to 5(-15)-seeded, (splitting down sutures - T. endochrysea); (seeds arillate); cotyledons foliaceous, folded (flat); AABB genomes, n = 41 [2n = 82-328]; plastid transmission biparental [Tilia].
3/50: Tilia (23). N. temperate, to Vietname, Central America and northern South America. Map: from Meusel et al. (1978), Hultén & Fries (1986), Fl. China vol. 12 (2007) and Cheek (2007 - Central America).
Age. The age of crown-group Tilioideae is about (33.2-)17.1(-2.2) Ma (Richardson et al. 2015) or ca 72.7 Ma (Hernández-Gutiérrez & Magallón 2019).
6. Dombeyoideae Beilschmied —— Synonymy: Dombeyaceae Desfontaines, Pentapetaceae Berchtold & J. Presl
(Trees), (plant deciduous), (herbs - Pentapetes); leaves spiral, cordate; epicalyx +, 3 (spathiform), (0); K connate basally to free, C ± clawed (0); A uniseriate, basally connate (free), (5-)10-20(-30), elongated antesepalous staminodes + (0), forming a short tube, (secondary pollen presentation +); (anther wall 5 cells across [the basic type), tapetum glandular, cells 4-19-nucleate; pollen spherical, often tri- to pantoporate, spinulose (not Nesogordonia - N.); (nectar coloured), G [(2-)5(-10)], style branched from base, or branches apical; (ovule unitegmic); fruit (K accrescent), (C persistent, scarious), (winged), (explosively dehiscent - Eriolaena), endocarp epidermis often pubescent; seed (with umbonate sarcotestal projections), (unilaterally winged, from much-elongated chalaza [Pterospermum]/apically and dorsally winged [E.]/micropylar sarcotestal tissue [N.]); (exotesta thick-walled); cotyledons bifid, folded, (entire - N.); AABB genomes, n = 19, 20, 30, etc.
15/337: Dombeya (175[-225?]), Melhania (72), Eriolaena/"winged seed clade" (27), Pterospermum (25). Old World tropics, Australia, St Helena, esp. Madagascar and th]e Mascarenes. Map: partly from Cheek (2007), see also Wood (1997), Arbonnier (2002), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Fl. China vol. 12 (2007), FloraBase (consulted i.2013), Australia's Virtual Herbarium (consulted 1.2013), etc..
Age. The age of crown-group Dombeyoideae is estimated to be (41.8-)25.1(-11.2) Ma (Richardson et al. 2015), 49.3 Ma (Hernández-Gutiérrez & Magallón 2019) or (67-)53(-38 Ma (Skema et al. 2022).
Leaf fossils from Rajasthan, India, have recently been identified by as Eriolaena by Shukla et al. (2025), and these authors suggest that the stem-group age of the Eriolaena clade is (105.4-)88(-72.4) Ma, diversification occurring around (88.6-)74.2(-60.8) Ma.
7. Sterculioideae Beilschmied —— Synonymy: Sterculiaceae R. Salisbury, nom. cons., Triplobaceae Rafinesque, nom. illeg.
(Polyacetylenes +); petiole with medullary bundle; leaves spiral, usu. lobed and/or cordate / palmately compound; plant monoecious; inflorescence axillary, paniculate, obvious bicolor units absent, epicalyx 0; K petal-like, basally connate, C 0; androgynophore +, (nectariferous hairs at the base); staminate flowers: filaments connate, anther wall 5-6 cells across [basic type], staminodia 0; tapetal cells 4-19 nucleate; pistillodes 0; carpelate flowers: staminodes 0; G largely free, styluli +; integument lobed [Sterculia], outer integument ca 3 cells across, inner integument 4-5 cells across, parietal tissue 4-5 cells across, nucellar cap ca 10 cells across [check]; fruit a follicle (indehiscent), G separating, (endocarp pubescent); AABB genomes, n = (15, 16, 18, 19) 20 (21, etc.).
13/430: Sterculia (150), Cola (125). Pantropical. Map: from Cheek (2007), see also Wickens (1976), Arbonnier (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photo - Staminate flowers, Fruit.]
Age. Crown-group Sterculioideae are some (46-)28(-13.8) Ma (Richardson et al. 2015) or ca 41.9 Ma (Hernández-Gutiérrez & Magallón 2019).
Woods of Sterculinium from late Cretaceous/early Palaeocene sediments in India from the Deccan Traps and aged around 67-65 Ma, are probably to be assigned to Sterculioideae (Wheeler et al. 2017).
[Freemontodendreae [Bombacoideae [Matisioideae + Malvoideae]]]: ?
8. Fremontodendreae Airy Shaw - ex Bombacoideae. ?Subfamily? —— Synonymy: Chiranthodendraceae A. Gray
Shrub to tree; lamina variously palmately lobed; K imbricate, petaloid, esp. internally; C 0/?much reduced - Chiranthodendron; A 5, extrose, opposite C, (strongly monosymmetric - Ch.), 3-4 vascular bundles/stamen; K persisent; seeds 2-3 or 10(+)/carpel, ?aril/elaiosome +/0.
2/4: Fremontodendron (3). N.W. and S. Mexico, Guatemala, S.W. U.S.A. (California).
[Bombacoideae [Matisioideae + Malvoideae] / Malvatheca clade: root lacking hypodermis [?level]; leaves spiral; K connate; C adnate to base of A, the two falling off together; fascicles ?contorted, filaments forming a tube, anthers monothecal, (locellate [= "polysporangiate": some basal members]), (thecae sessile); G with unbranched synlateral [± oppositisepalous] vascular bundle; (inner integument -15 cells across); embryo curved, cotyledons conduplicate, not enclosing hypocotyl; decaploidy - AABBCCDDEE genomes [MAL-α event], 6 bp insertion in matK gene.
Age. Possible ages for this node are (24-)21, 17(-14) Ma (Wikström et al. 2001) or (26-)20, 29(-12) Ma (Bell et al. 2010) - note topology in both, ca 74.9 Ma (Hernández-Gutiérrez & Magallón 2019) or ca 71.7 Ma (Cvetkovic et al. 2021).
N.B.: Unplaced genera in this area include Ochroma, n = 42; nuclear genome [1 C] ca 2.15 pg; Patinoa; Septotheca.
9. Bombacoideae Burnett
Trunk often ± swollen, with parenchymatous water-storage tissue, so soon becomes punky when cut, bark thin, often green; leaves peltately-palmate, petioles pulvinate; K connate, ± lobed; anther wall 5-7 cells across, staminodes usu. 0; pollen ± flattened, triangular in polar view; (hypostase +); seeds large [2< cm long]; x = 44, n = to 138, 1 C = 1.13-4.77 pg.
17/164 - three groups below. Tropical, esp. America. Map: based on Aubréville (1974), Wickens (1976), Coates Palgrave (2002), Trop. Afr. Fl. Pl. Ecol. Distr. 1. (2003) and Australia's Virtual Herbarium (consulted i.2013).
Age. The age of crown-group Bombacoideae is estimated to be (47-)26(-12.2) Ma (Richardson et al. 2015), ca 59.6 Ma (Hernández-Gutiérrez & Magallón 2019) or ca 61.6 Ma (Cvetkovic et al. 2021: Bomb + Cei).
Pollen attributed to Bombacoideae (Bombacacidites) has been found in deposits as much as 69-65 Ma in both Hemispheres (Askin 1989; Krutzsch 1989; Pfeil et al. 2002 for references).
9A. Bernoullieae Carvalho-Sobrinho
Trees often huge, buttressed; inflorescences terminal, scorpioid; A 5-20, fused into a complete tube except for short lobes, often divided down one side, thecae sessile, (spirally twisted); G [5]; ovules ca 10/carpel; endocarp papyraceous; seeds winged; n = ?
3/9. Tropical America.
[Adansonieae + Bombaceae]: x = 44, chromosomes minute.
9B. Adansonieae Horaninow
Leaves often simple/unifoliolate; flowers often solitary; K rarely lobed; filaments often apically dilated; G [2-9]; ovules 1-2/carpel (many - Adansonia); fruit samara/woody berry/± dehiscent, endocarp spongy; (testa 6-8 cells across - Adansonia); (n = 80); nuclear genome [1 C] 1.67-3.35 pg.
5/30: Catostemma (12). Mostly tropical America, also Africa, the Arabian Peninsula, Madagascar and northern Australia.
9C. Bombaceae Kunth —— Synonymy: Bombacaceae Kunth, nom. cons.
Cyanidin-3,5-diglucoside +; stems with stout prickles (0); K usu. truncate; androecium often phalangiate, anthers 1-4-thecate; G [5-8]; ovules many/carpel; fruit capsules, endocarp woolly [= kapok]; seeds small; (n = 43, 46, 97, 138); nuclear genome [1 C] 1.13-2.28(-4.77 - Eriotheca) pg.
9/125: Pachira (45). Mostly tropical America, also Africa, Australia. [Photo - Flower], Seeds.]
[Matisioideae + Malvoideae]: ?
10. Matisioideae C. D. M. Ferreira
lamina base cordate or asymmetricall subcordae; flowers monosymmetric (polysymmetric - Quararibea); A tube long; pollen 3-porate, spherical, surface reticulate; G usu. semi-inferior (superior - Phragmotheca); fruit drupaceous, (1–)2–5-seeded.
3/26. Central America, the Caribbean, N. South America, to Peru.
11. Malvoideae Burnett
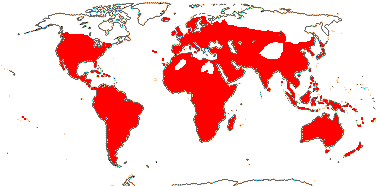
Petiole bundle annular; epicalyx +/0, median K often abaxial, C basally connate to free; A centrifugal, tube often with 5 apical teeth, (staminodes in fascicles, ?= antesepalous members), (synlateral bundle 0); pollen often echinate, 7+ pantoporate (tricolporate); G (1[2-)3-many], styles often separate, stigmas decurrent to capitate, hairy; ovules 1-many/carpel, campylotropous (anatropous), outer integument 5-10 cells across, inner integument 7-11 cells across, parietal tissue to 18 cells across; (fruit schizocarpic); (seeds hairy).
78/1,800. Temperate to tropical. Map: from Hultén and Fries (1986), Meusel et al. (1978), Frankenberg and Klaus (1980), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Australia's Virtual Herbarium (consulted xii.2012), New World: Cheek (2007) and Skoggan Fl. Canada vol. 3 (1978). [Photo - Flower.]
Age. The age of this clade, or more particularly, a major part of this clade (core Malvoideae, inc. Marcanodendron), has been estimated at (58-)47, 45(-43) Ma (Koopman & Baum 2008) and (both inc. Radyera) some (62.9-)61.2(-60.1) Ma (Richardson et al. 2015) or as much as ca 83.5 Ma (Areces-Berazain & Ackerman 2016: ca 81.5 Ma - How.-Lag.); the three tribes below have a crown age of ca 74.8 Ma (Areces-Berazain & Ackerman 2017). An estimate from X. Wang et al. (2016: Durio, Gossypium) is 22-20 Ma, while ca 71.3 Ma is the age in Hernández-Gutiérrez and Magallón (2019).
Leaves, Malvaciphyllum macondicus, from sediments 60-58 Ma in Cérrejon, Colombia, have been placed in Malvoideae (Carvalho et al. 2011). No mention is made of hairs of this fossil, but pollen of Bombacacidites was also common in the rocks (Carvalho et al. 2011). 67-65 Ma fruits from from Late Cretaceous sediments in the Deccan Traps have been placed in Malvoideae (e.g. Chitaley & Nambudiri 1973; Chitaley & Sheikh 1973). Manchester et al. (2022) in their review of fossils note that both fruits (Daberocarpon, Harrisocarpon) and pollen (Malvacipolloides) from rocks of this age from Central India are the earliest known for Malvoideae anywhere (they also mention early records of other subfamilies).
11A. Pentaplareae C. D. M. Ferreira - Pentaplaris L. O. Williams & Standley
Large trees, with buttresses; stipules sheathing; inflorescence a panicle, terminal; K basally connate, slightly quincuncial-imbricate; A fascicles opposite C; pollen ?3 brevi-colporate; G 2-celled, styles connate (not at apex), stigmas ± lobed, discoid; ovules 2/carpel, basal-axile; fruit indehiscent, accrescent K forming wings, 1-seeded; seed coat thin; cotyledons thick to thin, folded; n/x = ?
1/3. Peru, Bolivia, Ecuador, Costa Rica. Map: see Bayer and Dorr (1999: Fig. 1).
Radula, unplaced.
[Camptostemon, etc. [Hibisceae [[Alyogyne + Gossypieae] + Malveae]]]: ?
Age. The age of this clade is ca 74.8/77.2 Ma (Areces-Berazain & Ackerman 2016/2017) and ca 61.6 Ma in Cvetkovic et al. (2021: Hib. + Goss).
Shrubs to trees; indumentum lepidote/stellate; leaf margins entire to crenate or shallowly lobed; epicalyx +/0; pollen polyporate; 1-several ovules/carpel; seeds pubescent or not.
4/7: Camptostemon (3). Venezuela, Central Malesia and Australia (north, east and south, Norfolk and Lord Howe Islands). (Also Marcanodendron (was Uladendron), Lagunaria + Howittia.)
11B. Hibisceae Reichenbach (inc. Kydieae) —— Synonymy: Hibiscaceae J. Agardh
(Annual) herbs to trees (climbers); G [5](-[10]), styles usu. separate, (2 x G); fruit a capsule or schizocarp; endosperm copious, embryo small, bent, cotyledons partially conduplicate; n = 11-12, 14-20, 32-33, etc..
33/857: Pavonia (287), Hibiscus (220), Sabdariffa (120). Pantropical.
Age. The age of crown-group Hibisceae is (52-)41.4(-31) (Urena lobata sister)/66.4 (Jumelleanthus sister) Ma (Areces-Berazain & Ackerman 2016/2017).
[[Alyogyne + Gossypieae] Malveae]: ?
Age. This age of this clade is 72.8/76.2 Ma (Areces-Berazain & Ackerman 2016/2017)..
Age. This clade is some 64.9/67.2 Ma (Areces-Berazain & Ackerman 2016/2017).
Shrub; leaves deeply palmately divided, (segments linear); epicalyx basally connate; style undivided.
1/6. West (to Central) Australia, also Southeast.
11D. Gossypieae Alefeld
Perennial herbs [Cienfuegosia] to shrubs and trees; pigment glands +, synthesizing gossypol + [terpenoid aldehyde, C10H16 units]; leaves ± punctate; (pedicel with "bracteate articulation" - Thespesia); epicalyx +,nectaries 3, abaxial to epicalyx [CRABS CLAW...]; G [3(-5)], styles usu, connate; fruit a capsule; seeds arillate / hairy / neither; seed coat thick, with columnar layer; endosperm slight to ± absent, embryo with pigment glands, folding of cotyledons complex, enclosing mesocotyl + hypocotyl (not Cephalohibiscus); n/x = 10-13.
Ca 8/126: Gossypium (50). Tropical, esp. Australia.
Age. The age of crown-group Gossypieae is (50-)41.2(-32)/ca 44.9 Ma (Areces-Berazain & Ackerman 2016/2017).
11E. Malveae J. Presl
Plants ± herbaceous, (annuals, trees); A column basally adnate to C, with 5 apical teeth; G [(5-)7-10(-many)], stigma capitate; fruit a schizocarp, endoglossum +/- [= subbasal ingrowth of endocarp and adjacent mesocarp], 1-2+ seeds/carpel; endosperm copious, embryo small, ?cotyledons; n = 5-10, 14-17, 21, 22, etc..
73/626-1040. Pantropical to warm temperate.
Age. The age of crown-group Malveae is (66-)55.3(45)/ca 58.6 Ma (Areces-Berazain & Ackerman 2016/2017).
11E1. Abutilinae ?paraphyletic —— Synonymy: Philippodendraceae A. Jussieu, Plagianthaceae J. Agardh, Sidaceae Berchtold & J. Presl
pedicels articulated, breaking at articulation, apex of the part remaining nectariferous; epicalyx 0.
Sida (200), Abutilon (100), Cristaria (75), ?Callianthe (50), Gaya (39). Pantropical.
(tussock plants); (inflorescences epiphyllous - Nototriche); stigma decurrent [Malva]; tapetum non-syncytial invasive [Modiolastrum].
Nototriche (>100), Malva (61, inc. hybrids), Tarasa (29).
Camptostemon (lepidote; 1 ovule/carpel; seeds pubescent), Lagunaria, Howittia - unplaced.
Evolution: Divergence & Distribution. For the early Caenozoic fossil history of Craigia (Tilioideae), see Manchester et al. (2009); Reevesia (Helicteroideae), now East Asian, is known from throughout the Northern Hemisphere in the early Caenozoic (Ferguson et al. 1997); c.f. dates above. For morphotaxa of post-Oligocene (Neogene) European Malvaceae, see Worobiec et al. (2010).
Magallón et al. (2018) suggested that diversification increased around here as recently as (59.7-)39.5(-33.3) Ma as well as at a much deeper node at the Campanian-Maastrichtian boundary ca 72 Ma - the latter age could include most of or all of Malvales. Richardson et al. (2015) thought that the diversification rate of Tilioideae, a temperate clade, was similar to that of other Malvaceae (but Tilioideae are a small subfamily), while the diversification rate of Theobroma s.l. (another quite small clade) was relatively very high, perhaps because it was responding to factors associated with the Andean uplift. For more on diversification rates, see Hernández-Gutiérrez and Magallón (2019); they show both Sterculioideae and Brownlowioideae with phylogenetic fuses of some 50 million years, although in both fossils have been associated with the stem group. In any event, the evolution of Malvaceae has to be understood in the context of the extensive deep hybridization in the family; this is discussed under Genes & Genomes below.
Le Péchon et al. (2010) found that Dombeyoideae had colonized the Mascarenes more than once, indeed, a clade made up of species from Mauritius and Réunion was dated to (43.3-)34.6, 24.6(-11.8) Ma, well before those islands were above the sea, so presumably they were hopping around on now-submerged islands (Le Péchon et al. 2015) or are extinct in the place whence they came. Skema et al. (2022) elaborated on movements here, suggesting that there had been five shifts from Madagascar to Africa and six to the Mascarenes, and in the Cheirolaena alliance movements from Africa to St Helena and to Australia... Shukla et al. (2025) estimate the stem-group age of the Eriolaena clade (Dombeyoideae) with its apically and dorsally winged seeds to be around (105-)88(-72) Ma; they thought that the clade originated in Madagascar (two thirds of its species are endemic there), and from there it moved to Africa and India, and from Idnia to Southeast Asia in the Miocene. Overall, 17/19 of the dispersal events suggested by Skema et al. (2022) seem to have occurred within the last 10 My, and 14/19 within the last 5 My; interestingly, fossil pollen of Dombeyoideae 9-8 Ma is reported from St Helena, but how that might relate to Trochetiopsis, the St Helena endemic and with an age of less than 4 Ma, is unclear (Skema et al. 2022: esp. Fig. 4). The island of Madagascar is a notable jump-off point for movements of Dombeyoideae, about five times as many dispersal events being Madagascar → Africa as vice versa; overall, islands as sources of biota are unusual, but as Skema et al. (2022) note, Madagascar is a continental island.
Around 207 species of Grewia grow in Africa, although few in the Sahara or North Africa, and of these 93 species, 80 endemic, are known from Madagascar, especially the N, S.W. and S.E. - the topography and enviroment are complex (Karimi & Hanes 2024).
Taxa at the nodes at the base of the [Malvoideae + Bombacoideae] clade are likely to have been Neotropical (Baum et al. 2004; Nyffeler et al. 2005). Adansonia, from Madagascar, mainland Africa, Arabia, and Australia and 38-32 Ma, is likely to have achieved its current range via dispersal (Baum et al. 1998a; Sanmartín & Ronquist 2004). Diversification rates in Malvoideae-Malveae may be quite high, perhaps connected with the schizocarpous fruits and numerous carpels (usually 10 or more) in this clade (Areces-Berazain & Ackerman 2017); see Takeuchi et al. (2018) for fruit evolution in Gaya and related genera. Diversification and molecular evolution in Hibisceae pick up almost together (Baum et al. 2002, 2004). Baum et al. (2008: q.v. for dates), discuss the biogeography of Malveae. Within Gossypieae, Kokia, endemic to Hawai'i,is sister to the African Gossypioides; the two diverged ca 12 Ma (Seelanan et al. 1997; see Keeley & Funk 2011 for a list of Hawaiian endemics) or ca 5.3 Ma (or 3 Ma?: Grover et al. 2017), but how their ancestors navigated the 17,500 km that now separates them is unclear. There has also been extensive and recent long distance dispesal within Gossypium (e.g. Grover et al. 2018). For hybridization/polyploidy here, much studied in part because it is connected with the domestication story of cotton, see Wendel and Grover (2015) and Soltis et al. (2016b and references).
For the evolution of extra-floral nectaries in Byttneria, see Weber and Agrawal (2014); they have had little effect on diversification rates.
Corner (1976) discussed the seeds and fruits of Malvaceae in some detail, Malvaceae being an integral part of his "durian theory" (e.g. Corner 1953) concerning the evolution of tropical rainforests and their denizens. Pachycauly (stout stems), large flowers, armoured fruits, arillate seeds, etc., were all part of what he thought was a syndrome of primitive characters posessed by the early denizens of rainforests.
Ecology & Physiology. Bombacoideae are an important component of Neotropical seasonally dry tropical forest (and Malvoideae are a notable element in the ground cover) and Amazonian forests in general, being both speciose and having a disproportionally high number of common species among individuals with stems at least 10 cm across (Pennington et al. 2006b and papers in this volume, 2009; ter Steege et al. 2013).
Luna-Márquez et al. (2021) discussed climbing in theByttneria/Ayenia area. In the former, anomalous secondary thickening in the form of lobed vascular tissue/stems and stem prickles are to be found in climbers, while those species in the latter genus that are climbers climb by twining - secondary thickening is normal (Luna-Márquez et al. 2021).
Large calcium oxalate druses in the palisade mesophyll and epidermal silica deposits together are involved in enhancing photosynthesis in okra, Hibiscus esculentus. The former scatter incoming light into the spongy mesophyll and the latter reduce damage caused by UV radiation; interestingly, in surface views, their distribution in the lamina is mutually exclusive (Pierantoni et al. 2017).
There are a number of cushion plants in Malvoideae in particular (Boucher et al. 2016b), e.g. Nototriche (Malvoideae), a remarkable genus of dwarfed plants growing in the high Andes; the flowers are epiphyllous and there is considerable variation in leaf shape. At the same time Malvaceae include perhaps the oldest angiosperm tree, the baobab, Adansonia digitata, which may live for some 2,000 years (Patrut 2018 - note that this is far older than ages for most other seed plant trees, but c.f. Piovesan & Bondi 2021).
The genome sizes of the mangrove plants Hibiscus tiliaceus and Heritiera littoralis are quite similar to those of their non-mangrove relatives (Lyu et al. 2017).
Pollination Biology & Seed Dispersal. In most Malvaceae the nectary consists of carpets of multicellular glandular hairs; the same morphology even occurs in the foliar extrafloral nectaries in Triumfetta, etc. (for nectary physiology, etc., see Sawidis et al. 1989; Vogel 2000 and references; Leitão et al. 2005). The nectaries that provide rewards for pollinators are commonly found on the inside of the calyx near the base and the corolla is fused at the base, however, since the broad petal lobes are basally more or less clawed, the pollinator can get at the nectar between the petal claws. Thus access to the nectar is permitted to a pollinator probing the center of the flower while simultaneously pollinating the flower (Vogel 2000) - of course, if the corolla were completely connate there would be no easy way for the pollinator to reach the nectar.
The flowers of Helicteres isora (Helicteroideae), some species of Pavonia (Malvoideae), and the remarkable Chiranthodendron pentadactylon (the aptly-named devil's hand, this refers to the five-branched androecium), are monosymmetric, but I do not know the plane of symmetry. Pseudanthia are reported from Lasiopetalum (Baczynski & Claßen-Bockhoff 2023). Young et al. (1984, 1987, 1989) suggested somewhat hesitantly that there might be nectar-secreting stomata on the limb and the adaxial base of the petal in Theobromateae, but this report should be confirmed. Overall there is considerable variation in nectary - and staminodium - position and type in the family. Thus nectaries are borne on the petals in e.g. Grewia (see also Lattar et al. 2018) and Luehea (bat pollinated: Sazima et al. 1982: no staminodes, but c.f. Costa Rican plants) and on the androgynophore (and leaf!) in e.g. Triumfetta (Leitão et al. 2005) - and these plants are from Grewioideae alone. Nectar may also be produced on the outside of the calyx (Gossypieae) and from the apex of the pedicel after the flower bud has been abscised (Malveae).
Byttnerioideae, especially Byttnerieae and Theobromateae, have remarkably complex if sometimes quite tiny "basket" flowers that are pollinated by small flies and the like (Vogel 2000; Westerkamp et al. 2006; Whitlock & Hale 2011). The petal often has a concave basal portion, sometimes on the end of a long claw (as in Ayenia) or not (Theobroma) that more or less encloses the rest of the flower, the anthers in particular, the limb of the petal being represented by a small, discoloured projection (Ayenia), an elliptical petal-like structure that twists in the wind (Abroma), a filiform ligule (Theobroma) or a long (>20 cm long), linear, dangling structure (Herrania). The staminodia may be prominent linear structures opposite the sepals (Theobroma) or spreading and petal-like (Herrania s. str., = Theobroma; see also Bossa-Castro et al. 2024 for optimization of these and other characters on a tree). Midges are likely to be pollinators in a number of Malvaceae, ceratopogonids or perhaps cecidomyids in Theobroma (Wolcott et al. 2023), phorids in Herrania, while there is buzz pollination in Byttnerioideae-Lasiopetaleae (Vogel 2000) and also bird and bat pollination (Lattar et al. 2018 for literature).
For pollination in Bombacoideae, see Janka et al. (2008) and references. Bat pollination is very common (see also Marshall 1985), although some reconstructions suggest that bat pollination evolved before bats... (Fleming et al. 2009; Hernandéz & Magallón 2015).
It has been suggested that both the spines and pollenkitt of pollen, common in Malvoideae, for instance, may aid in pollination by making it more difficult for the visiting bee to store the pollen in its corbiculae; if the pollenkitt is removed or the spines are bent, the pollen gets tranferred to the corbiculae, so making it inaccessible to the plant (Lunau et al. 2015).
One of the most spectacular examples of secondary pollen presentation I have seen was in a species of Dombeya (Dombeyoideae), a genus in which I never expected to see such a pollination mechanism; the pollen was attached to the staminodes, where it made a striking colour contrast with the rest of the flower. The pollen can also be presented on the tips of the petals in Dombeya (Prenner 2002), and coloured nectar is known from the genus; Melhania (= Ruizia) also includes species that have coloured nectar (Hansen et al. 2007). Further studies in pollination around here would certainly be interesting.
The pattern of evolution of dioecy in Dombeya and its dispersal between Madagascar, the Mascarenes and Africa is complex (Le Péchon et al. 2009). There seem to have been four colonizations of the Mascarenes, with at least three acquisitions of dioecy there alone (Le Péchon et al. 2010; see also Skema 2012).
A sporophytic self-incompatibility system is reported [?where] from "Sterculiaceae".
Some Sterculioideae have myxospermous seeds (Western 2012).
Plant-Animal Interactions. Caterpillars of the nymphalid Acraea are quite commonly found on members of Malvaceae, as are members of Lycaeninae (Fielder 1995) and the skipper group Pyrginae-Pyrgini (Warren et al. 2009). Tilia is quite an important host plant for lepidopteran larvae in the contiguous United States of America, being included in the top 15 such plants (Narango et al. 2020).
Acanthoscelides and Spermophagus are bruchid beetles (Chrysomeloidea-Bruchinae) whose larvae eat seeds that have diversified on New World and Old World Malvaceae, respectively, esp. on Malvoideae; their primary hosts are members of Fabaceae (Kergoat et al. 2005b); diversification of Spermophagus, at least, may have occurred after the diversification of its Malvoideae hosts (Kergoat et al. 2015: c.f. Convolvulaceae hosts). Seed-eating bugs of the Hemiptera-Lygaeidae-Oxycareninae are also concentrated on Malvoideae (Slater 1976) while most of the ca 300-plus species of -Heteroptera-Pyrrhocoridae feed largely on seeds or fruits of Malvaceae (in older literature Malvales) - and also Welwitschia (Martinez et al. 2019).
Extrafloral nectaries are notably common here (Weber & Keeler 2013).
Plant-Bacterial/-Fungal Associations. Tilia is ectomycorrhizal, and some species are associated with truffles, the ascomycete Tuber (see Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, dates - crown-group Tilia 32-8 Ma - etc.).
Genes & Genomes. In addition to general genome duplication events, e.g. the genome triplication of the core eudicots (Vekemans et al. 2012), there has been extensive deep hybridization and genome duplication in Malvaceae. Sun et al. (2024), R. G. Zhang et al. (2025) and others have described the complexity of genome evolution here. Sun et al. (2024) found further complexities at deeper levels in the Malvadendrina group, the tetraploid clade. They suggested that an allotetraploid member of Sterculioideae hybridized with an unknown diploid to produce an allohexaploid Helicteroideae, and after hybridization of this latter with another unknown plant the result was an allodecaploid Bombacoideae, the allodecaploids baking up the Malvatheca group. Extensive reductions in chromosome number have taken place, for instance, a Durio-type genome with a scaffolding of 30 different chromosomes was involved in the last of these hybridizations, while Herrania umbratica (Byttnerioideae) has a scaffolding made up of 46 chromosomes; Bombacoideae tend to have high chromosome numbers, n = (28-)36-46(-75) (Baker & Baker 1968; see also Costa et al. 2017). The age of this decaploidization event, MAL-α, has been variously estimated to be 14-13 Ma (J. Wang et al. 2019a) and ca 61-57 Ma (Zhang et al. 2025), and the HICAα event, ca 44.1 Ma, was also detected in Dombeya (Landis et al. 2017: c.f. ages above). Conover et al. (2018) detected evidence of genome duplications in much of the family, although details of what had happened were unclear - thus the clades [Malvoideae + Bombacoideae] and [Sterculioideae + Tilioideae] both had duplications, but whether they were the same or nor was not known. Byttnerioideae, Grewioideae and Dombeyoideae that they examined did not have genome duplications (Conover et al. 2018). An hexaploidization event dated to 21-19 Ma has been reported from the durian, Durio zibethinus (J. Wang et al. 2019a). For genome evolution in Bombacoideae, see Casta et al. (2017). For chromosome numbers, see also Baker and Baker (1968: Bombacoideae), Marinho et al. (2014: focus on Bombacoideae, n = 164 in Tilioideae), P. Sun et al. (2024, c.f. Carta et al. 2020). etc..
Thus in the lineage leading to Gossypium (Malvoideae) the genome duplicated, and then triplicated after its divergence from Cacao (Byttnerioideae), resulting in a 30-36-fold duplication just within angiosperms and a ca 144 genome multiplication overall (Paterson et al. 2012; Wendel 2015); X. Wang et al. (2016) thought that there had been a local decaploidization in the ancestor of Gossypium, but the 210 chromosomes that resulted had been reduced to 26...; extensive end-to-end joining of the chromosomes has caused this post-polyploid diploidizatiom (R. G. Zhang et al. 2025). Subsequent gene loss has occurred preferentially in particular genomes, biased fractionation (Renny-Byfield et al. 2015), and looking at gene retention in the five genomes in the decaploid clade, Zhang et al. (2025) found the numbers to follow the overall sequence A
For plastome evolution, see Z. Chen et al. (2017), and for the loss of the chloroplast rpl22 gene, see Su et al. (2014); sampling for it needs to be improved.
Andreasen and Baldwin (2001) noted that the rate of molecular evolution of 18S–26S nuclear ribosomal DNA in annual species of Sidalcea was faster than that in the perennials.
Economic Importance. Gossypium barbadense, Pima cotton and with particularly long fibres, and G. hirsutum, upland cotton and by far the most widely cultivated cotton, are the two independently-derived tetraploid species of cultivated cotton, both with A (from Africa) and D (from America) genomes. The New World G. raimondii is the source of the D genome in both, while G. arboreum is the source of the A genome in G. barbadense and G. herbaceum is the source in G. hirsutum; interestingly, G. gossypioides, in the clade of American taxa immediately related to G. raimondii, also has an Old World x New World ancestry (Wendel & Grover 2015; Grover et al. 2018, also Dillehay et al. 2007; Wendel et al. 2010). The two diploid African taxa just mentioned, G. herbaceum and G. arboreum, the sources of the fibres in tetraploid cotton, are themselves long-cultivated, and they became independently cultivated (Renny-Byfield et al. 2016; Grover et al. 2022). For more on the evolution of cotton fibres, see e.g. Paterson et al. (2012), X. Liu et al. (2015), H. Kim et al. (2015) and references, anf for oils from cotton, see papers in Vollmann and Rajcan (2009).
Theobroma cacao is of course the source of chocolate, and Bossa-Castro et al. (2024: esp Fig. 2) optimize a number of characters, vegetative and floral, on their tree of Theobroma s.l. (inc. Herrania). Cacao has an extensive pre-Colombian history going back some 5,300 years; 11 genetic groups are recognised, and domestication began in Amazonia, moving to western and northwestern South America and thence to Central America and Mexico (Lanaud et al. 2024).
Chemistry, Morphology, etc.. The pentacyclic systems in the 4-pyridones and 4-quinolines "originate from a polyunsaturated sphingolipid-like compound with a benzoic acid starter unit" (Erwin et al. 2014, p. 361). Tile cells are best observed in radial section; they are of two or three main types (Manchester & Miller 1978; Carlquist 1988b; Tang et al. 2005b). Any correlation of tile cell "type" with phylogeny awaits a more completely resolved tree, thus the Durio type occurs in both Malvoideae and Byttnerioideae. Vestured pitting is reported, but perhaps incorrectly, from Schoutenia and Ochroma (Jansen et al. 2000a). Mucilage ducts are common in Malvaceae, and those in Theobroma, at least, are produced by the coalescence of irregularly-shaped cavities (Garcia et al. 2020). Indeed, mucilage is produced by structures with a variety of morphologies. For example, in Peltaea polymorpha (= Pavonia, Hibisceae) idioblasts divide transversely, in longitudinal section looking like a rather demented caterpillar; some cells collapse, but the cell walls do not break down, even if there are cytoplasmic connections between the cells (Rodrigues et al. 2025).
Carvalho et al. (2011) discuss leaf venation in Malvaceae in considerable detail; additional apomorphies for Malvoideae, at least, may result from such studies. Several taxa have palmate leaves. For leaf teeth, see Rios et al. (2020). In Brachychiton and Adansonia there is considerable variation in leaf morphology within a single flush - the first leaf/leaves have a very short petiole and long, narrow ?phyllode, while later leaves are palmate; any intermediates have winged petioles and a few leaflets. Kim et al. (2003) described the leaf of Pachira aquatica as being peltately palmate, and leaves of other Malvaceae with strong palmate venation may be basically the same, and in such cases the petiole has a unifacial construction. Leaf development in the whole family would repay more detailed investigation.
Janda (1937) describes extrafloral nectaries in Malvoideae - these are aggregations of glandular hairs (similar hairs are found singly throughout the subfamily), and are borne either abaxially on foliage leaves or the sepals, or at the apex of the pedicels - i.e., they may sometimes be nuptial nectaries... There are also various kinds of extrafloral nectaries in Triumfetta (Letãio et al. 2005).
The inflorescence in most Malvaceae is made up of "bicolor units" - a terminal flower with three "bracts", two of which may subtend flowers or cymose part inflorescences with normal bracteole number and arrangement while the third subtends nothing. The epicalyx, which has evolved several times in the family, may be made up of these three bracts, and so a flower with an epicalyx represents a highly reduced "bicolor unit" (Bayer 1999). However, 3-11 or so elements may make up the epicalyx, and in Hibiscus, for example, these elements may be free, connate, adnate to the calyx, or both connate and adnate (Ayanbamiji et al. 2012). Although Bello et al. (2016) interpreted the tripartite epicalyx of Lavatera trimestris as being the lamina plus two stipules of a bract, this would seem unlikely. Freire et al. (2024) noted that the pedicel of Malveae was articulated, and it sometimes broke at the articulation; a nectary then developed at the end of the part of the pedicel that remained - it is interesting that the taxa involved did not have an epicalyx. Although inflorescences in Sterculioideae seem to be very differently constructed from those of other Malvaceae, they, too, are composed of bicolor units, albeit much modified (Bayer 1999). (Malvoideae) has epiphyllous inflorescences.
For androecial development in Malvaceae s.l. compared to that of other Malvales, see Nandi (1998b) and von Balthazar et al. (2006). Von Balthazar et al. (2006) suggest an interpretation of the androecium of [Malvoideae + Bombacoideae] - and extend their findings to determine the basic androecial structure for Malvaceae as a whole. They propose that the basic androecial structure in Malvaceae is obdiplostemony, with stamens developing in one or both whorls; anther dehiscence is extrorse. In [Malvoideae + Bombacoideae] the androecial units consist of an antesepalous primordium with its own vascular supply and which remains sterile (usually), and this is flanked on both sides by a single primordium, each derived from a separate antepetalous primordium and that gives rise to a single sessile, elongated theca. The thecae are supplied by a branch from an antepetalous vascular bundle. The androecial unit thus consists of [half anther + sterile primordium + half anther]. Further details are given by Janka et al. (2008), with a focus on Adansonia (Bombacoideae) and relatives. Ceiba pentandra has only five alternisepalous stamens, and these are supplied by branches of the oppositipetalous traces, and these and other taxa like Fremontodendron are described as having bithecal anthers (Bayer & Kubitzki 2002), and the latter, at least, has ca 4 vascular bundles (Carlquist 1970). For additional details of androecial development in Malvoideae, see Janka (2003) and von Balthazar et al. (2004), and for its vascular supply, see Rohweder (1972). For floral morphology and development in Dombeyoideae, see Tang (1998) and Tang et al. (2006). In Grewioideae the stamens may arise from ten or five (if five, then oppositisepalous) primordia, or from ringwall primordia, and the vascular supply to the stamens is variable in origin (Brunken & Bayer 2005); for more, see Brunken (2003; Brunken & Muellner 2012). Van Heel (1966, 1967b, c) and Schönenberger and von Balthazar (2006) also discuss androecial development in Malvaceae s.l.. The androecia with numerous stalked, unithecate, staminal units that are so common in this clade are independently derived in Bombacoideae and Malvoideae (von Balthazar et al. 2006).
Pollen variation in the family is considerable. Thus Christensen (1986), describing pollen in Malvoideae, shows pollen that is tricolporate to polyporate, and the pores in the latter grains may be in beautiful spirals. Although starchy pollen is common in Malvaceae in the old sense, it is not reported from the old Sterculiaceae. Similarly, starch grains in the embryo sac are common in Malvoideae and Bombacoideae, but are not reported from the old Sterculiaceae (e.g. Venkata Rao 1950). Details of the variation of these features in the clades recognised above are unclear.
Not only are the carpels of Sterculioideae secondarily free, but in Firmiana they open early in development, exposing the developing seeds on the carpel margins; the carpels with their exposed mature seeds attached are dispersed by wind. However, there is a compitum even in these apocarpous Sterculioideae that is formed by the post-genital connation of the apical parts of the styles (Jenny 1988). The carpels are usually opposite the corolla, although not infrequently (e.g. Hibiscus, Fremontodendron, Sterculia) they are opposite the calyx; when there are three carpels, the median member may be either ad- or abaxial (reports of carpel orientation in individual taxa may conflict - e.g. Eichler 1878; Ronse Decraene 1992).
Although zig-zag micropyles are common, some taxa have an exostomal micropyle, but the micropyle is clearly off centre (i.e., there is a zig, but not a zag), while in taxa like Helicteres the apex of the nucellus is initially exposed and a bistomal, and a zig-zag micropyle becomes apparent only after fertilization; such variation is connected with the timing of development of the outer integument. Ovules of Pterospermum suberifolium are described as lacking parietal tissue but with a nucellar cap up to 16 cells across (Venkata Rao 1953a). For more details of embryology, which shows quite a bit of variation that needs to be integrated into the phylogeny - perhaps when it is better understood - see e.g. Venkata Rao (1951, 1952b, 1953c, 1954), Venkata Rao and Sambasiva Rao (1952), and for the embryology of Eriolaena in the context of embryological variation in Dombeyoideae as a whole, see Tang et al. (2009). Leptonychia has parietal placentation, short fibres in the exotegmen, but starchy endosperm, while Helicteres is described as having exotestal fibres (González & Cristóbal 1997). The endoglossum found in some Malveae is an ingrowth of the endocarp and adjacent mesocarp; initially basal-adaxial, its final position depends on the growth of the fruit, and at maturity it it is anything from a simple ingrowth to an elaborate structure projecting into the locule with aerenchyma, fibre strands, etc. (Masullo et al. 2019).
For general information, see the Malvaceae Pages website (Hinsley 2002), Colli-Silva et al. (2025: descriptions of tribes, etc.), Fryxell (1968: esp. Gossypieae, Hibisceae, 1997: New World Malvaceae), Robyns et al. (1977: Lasiopetaleae), Bayer and Dorr (1999: Pentaplareae), Cheek (2007), Wilkins and Chappill (2002: Lasiopetaleae), Brunken and Muellner (2012: Grewioideae tribes) and especially Bayer and Kubitzki (2002: whole family). For wood anatomy, see Chattaway (1933b, 1937), Webber (1934), Manchester and Miller (1978), Carlquist (1988b) and Tang et al. (2005a, b), for gums, see Lambert et al. (2013), for the chemistry of Bombacoideae, see Refaat et al. (2013), for inflorescence structure, see Bayer (1994, 1999), for floral development, see Bello et al. (2016), for petal development in Byttnerioideae, see Leinfellner (1960), for nectaries, see Vogel (2000) and Sawidis et al. (1989 and references), for the androecium of Byttneria, see Ronse De Craene and Bull-Hereñu (2016), for pollen in Grewioideae, Tilioideae and Brownlowioideae, Perveen et al. (2004), in some Helicteroideae, Bombacoideae, and unplaced Bombacoideae, Nilsson and Robyns (1986) and in heterostylous Waltheria, Saba and dos Santos (2015), for some gynoecial morphology, see Endress et al. (1983), for ovules of Gossypium, see Lintilhac (1974), for embryology, see Stenar (1925: mostly Malvoideae, some Tilioideae in the old sense), Venkata Rao (1949), and Lattar et al. (2016: Grewioideae), and for seed coat anatomy, (etc.) see Mohana Rao (1977), Serrato-Valenti et al. (1992), Marzinek and Mourão (2003: Chorisia, = Ceiba) and Gama-Arachchige et al. (2013 and references), esp. germination and the water gap.
Phylogeny. The old Malvales were a very distinct group, but apart from Malvaceae (= Malvoideae here), all the other families that were in it have turned out to be para- or polyphyletic. For the relationships in the extended family, see Alverson et al. (1998, esp. 1999) Bayer et al. (1999), and Nyffeler et al. (2005). [Grewioideae + Byttnerioideae] are probably sister to the rest of the family (see also Soltis et al. 2007a; Richardson et al. 2015; Z.-D. Chen et al. 2016), while Sterculioideae are perhaps sister to the well supported [Malvoideae + Bombacoideae] (Nyffeler et al. 2005; Chen et al. 2016). Other relationships between the subfamilies are unclear. However, Dombeyoideae and Tilioideae are sometimes weakly associated (Alverson et al. 1999; Richardson et al. 2015), but the former may rather be sister to all other taxa in the major polytomy (Nyffeler et al. 2005). Relationships between subfamilies were again poorly supported in M. Sun et al. (2016, q.v. for more detailed relationships, too), and Byttnerioideae and Grewioideae as well as Bombacoideae and Malvoideae were not separated. Conover et al. (2018) in their plastid analyses recovered relationships in the Malvadendrina area of [Helicteroideae [Dombeyoideae [[Tilioideae + Sterculioideae] [Malvoideae + Bombacoideae]]]], albeit they were poorly supported, and nuclear data placed Dombeyoideae as sister to the rest; they suggested hybridization might be involved - Helicterioideae x Dombeyoideae? Hernández-Gutiérrez and Magallón (2019) found the same subfamilies, but recovered the relationships ...[Helicteroideae [[Brownlowioideae + Dombeyoideae] [Tilioideae... and ...[Tilioideae [Helicteroideae [Brownlowioideae + Dombeyoideae]]]... depending on the particular analysis. Hernández-Gutiérrez et al. (2021: 25 taxa, 4 single-copy nuclear genes the focus) founf Byttnerioideae and Grewioideae were basal in the family, the clade [Malvoideae + Bombacoideae] was consistently recovered, but, depending on the analysis, the topology varied, and there was conflict even among these four nclear genes. Pending analyses of nuclear genomes and grappling with potential issues of hybridization, etc., relationships are treated as ...Helicteroideae, Tilioideae [Brownlowioideae + Dombeyoideae]... above. Recent work analysing plastomes of 48 Malvaceae recovered the largely well supported relationships [[Grewioideae + Byttnerioideae] [Helicteroideae [Sterculioideae [[Brownlowioideae [Tilioideae + Dombeyoideae]] [Malvoideae + Bombacoideae]]]]], although the position of Sterculioideae in particular was rather weakly supported (Cvetkovic et al. 2021). The focus in the nuclear analysis of Malvales by Colli-Silva et al. (2025) was on Malvaceae, but as is clear from the classification above, a number of problems remain.
In particular, relationships at the base of Malvoideae and Bombacoideae are unclear. Ochroma may be sister to other Bombacoideae; its filaments are connate into a tube. However, it and Patinoa formed a clade with no obvious immediate link with Bombacoideae in some analyses (Alverson et al. 1999; see also Baum et al. 2002, 2004) and the two genera may have to be excluded. Sister to the rest - or almost so - in this whole [Malvoideae + Bombacoideae] area may also be genera like he remarkable Fremontodendron, which hybridizes with Chiranthodendron (both ex Sterculiaceae - C 0), etc., and Quararibea, etc. (ex Bombacaceae), support was weak (Alverson et al. 1999; c.f. Bayer et al. 1999; Baum et al. 2002). Quararibea, etc., may be best placed in Malvoideae, while [Ochroma + Patinoa] and Septotheca are unplaced (Baum et al. 2004). Baum et al. (2004) suggest that [Fremontodendron + Chiranthodendron] may be sister to the rest of the [Malvoideae + Bombacoideae] since they lack a 6 bp deletion in a conserved region of the matK gene found in all other members of this clade, although there is little other evidence for this position. Carvalho-Sobrinho et al. (2016) and Areces-Berazain and Ackerman (2016) also note a number of displaced genera in this area; most are unplaced above. Richardson et al. (2015) found Quararibea and friends loosely linked with Bombacoideae, as are the other genera mentioned, while M. Sun et al. (2016) also found a number of Bombacoideae scattered in Malvoideae. Although Colli-Silva et al. (2025: Angiosperms353 data set) clarified a number of problems in Malvaceae, a number remain, as can be seen above - for example, relationships at the base of Malvoideae-Bombacoideae.
Bombacoideae. Carvalho-Sobrinho et al. (2016) discuss relationships within core Bombacoideae; Adansonia may be sister to the rest of its clade (Adansonieae). Karimi et al. (2020) suggested that there has been introgression involving the movement of traits involved in pollination syndromes within Madagascan species of Adansonia; the story is complex.
Byttnerioideae. Many taxa in Byttnerioideae have only five stamens, a derived condition, even although developmental work might suggest that the higher numbers may be produced by doubling (Whitlock et al. 2001b; Whitlock & Hale 2011).
Byttnerieae. Whitlock and Hale (2011) found that Byttneria was paraphyletic, with Ayenia being embedded in it; growth form was a fairly good indicator of relationships here.
Lasiopetaleae. Whitlock et al. (2011) evaluated relationships around Commersonia. The fucus in B. M. Anderson et al. (2025: 127 spp., 383 nuclear loci from the Angiosperms353 and Oz Baits probe sets) looked at relationships in the Lasiopetalum clade, i.e. [Guichenotia + Lasiopetalum + Thomasia + Lysiosepalum], where their samplinge was very extensive. Poly- and paraphyly were common there, and also extensive hybridization, some perhaps quite ancient (Anderson et al. 2025)
Theobromateae. Richardson et al. (2015) looked at relationships here. Bossa-Castro et al. (2024: 36 spp. Theobromateae, 5 regions of the WRKY loci, also 56 morphological characters (often divisions of continua), = 60 characters in the analysis) carried out analyses with and without the morphological characters. They found that Herrania was embedded in Theobroma (they reduced the former to synonymy of the latter), support along the backbone of their tree for the most part being strong (Bossa-Castro et al. 2024).
Brownlowioideae. For relationships here, see Hernández-Gutiérrez and Magallón (2019), Colli-Silva et al. (2025) and from the point of view of sampling especially Chalermwong et al. (2025: 3 plastid markers); the latter group included all genera, and Berrya seems to be poluphyletic.
Dombeyoideae. Here Dombeya is certainly paraphyletic (Le Péchon et al. 2009, 2010, 2014; Le Péchon & Gigord 2014: focus on Mascarene taxa; Won 2009: to include Corchoropsis; Skema 2012: focus on Malagasy taxa). Nesogordonia is morphologically rather out of place here, for instance, its pollen lacks spines and is flattened-triangular, however, Dorr and Wurdack (2020) found that it is sister to the rest of the subfamily, Burretiodendron being sister to the remainder. Skema et al. (2022: nuclear ITS plus 5 chloroplast markers) looked at 128 speces of Dombeyoideae, about 1/3 of the subfamily, including members of all twenty genera, and consistently recovered 10 main clades. Again Nesogordonia and Burretiodendron are basal clades, and Melhania, Eriolena and in particular Dombeya are para/polyphyletic (Skema et al. 2022).
Grewioideae. Brunken and Muellner (2012: ndhF, 17 genera) suggest a phylogeny for this subfamily; some characters are placed on the tree.
Helicteroideae. Helictereae (ex Sterculiaceae) are sister to Durioneae (ex Bombacaceae), the latter being from Sri Lanka, Burma to West Malesia and having lepidote indumentum and an initially connate epicalyx. The anthers of many Durioneae are polylocular - see also Nyffeler and Baum (2000).
Malvoideae. For groupings within Malvoideae, see La Duke and Doebley (1995: restriction site analysis), Judd et al. (2002) and Areces-Berazain and Ackerman (2017).
For relationships in Gossypieae, complicated by hybridization, see Seelana et al. (1997); Alyogyne, unplaced, was placed sister to the tribe, and with good support (Areces-Berazain & Ackerman 2017: some other genera included but not placed in the three subtribes).
For relationships within Hibisceae, see e.g. Pfeil et al. (2002), Pfeil and Crisp (2005) and Koopman and Baum (2008: Malagasy taxa); generic limits around Hibiscus are especially difficult - s. str. or s. lato?, but Hibiscus should probably include Pavonia and other genera. Sida is polyphyletic, as is Abutilon (Donnell et al. 2012). Hanes et al. (2024: 4 plastome markers, 200 Hibisceae, 21 genera, 118 spp. Hibiscus) confirmed extensive polyphyly in Hibiscus in particular and Pavonia; H. clypeatus was found to be sister to the rest of the tribe (and described as Blanchardia).
Within Malveae, Tate et al. (2005) found that presence or absence of an epicalyx correlated very well with two major clades recognizable on analysis of ITS sequence data; Jumelleanthus may be sister to the rest of the tribe (Areces-Berazain & Ackerman 2017). A subsequent study using ITS and four other genes found that Malva, at least, was polyphyletic (Escobar García et al. 2009).
Sterculioideae. Wilkie et al. (2006) suggest relationships within Sterculioideae and discuss evolution of fruit types, dispersal mechanisms, etc.; leathery follicles seem to be the basal fruit type of the group.
Tilioideae. Mortoniodendron is to be included in Tilioideae (Nyffeler et al. 2005). For relationships in Tilia, see J. Xie et al. (2022), but note the incongruence in relationships between nuclear and plastid trees.
Classification. For a discussion of groupings in the extended family, Robert Brown's comments over 200 years ago (Brown 1814) on family limits in the Malvales (= Malvaceae here) are a good starting point. Malvaceae + Bombacaceae + Sterculiaceae + Tiliaceae make a readily recognized and well circumscribed group, yet the clades within it are mostly difficult to distinguish, even with flowers, so combination seems sensible (Judd & Manchester 1997; Baum et al. 1998b; Alverson et al. 1999; Bayer et al. 1999), although Cheek (2007) opted for dismemberment of Malvaceae s.l. into ten families, his suggestions have not been followed. Note that the limits of both Malvoideae and Bombacoideae above are still (as of 2025) unclear, there being about a dozen unplaced genera in this area. And given the extensive hybridization in the group (see Genes & Genomes above) there is no simple link between the classification here and phylogeny...
Within Byttnerioideae-Lasiopetaleae Whitlock et al. (2011) and associated papers looked at generic limits around Commersonia. B. M. Anderson et al. (2025) discuss generic limits aroundLasiopetalum - here the choice is between expanding Guichenotia, which will need 100+ new combinations, or to resurrect two names and to describe two new genera, only ca 27 name changes needed - and there will still be intergeneric hybridization! They prefer the latter option; it seems reasonable. Colli-Silva et al. (2024) provide an infrageneric classification for the expanded Theobroma (Theobromateae) - 6 sections, one of which (the old genus Herrania) is divided into three subsections.
-For a tribal classification of Grewioideae, see Brunken and Muellner (2012) and for that of Bombacoideae s. str. , see Carvalho-Sobrinho et al. (2016). Xie et al. (2022) provide an infrageneric classification for Tilia: two sections and three subsections.
-In Dombeyoideae, Skema (2012) and Le Péchon and Gigord (2014) clarified generic changes around Dombeya, while Dorr and Wurdack (2020) looked at generic limits in the rest of the subfamily, in particular circumscribing Eriolaena broadly; Eriolaena was also mixed up with Helmiopsis and Helmiopsiella in Skema et al. (2022), and further generic adjustments in Dombeyoideae are needed.
-Generic limits in Malvoideae-Malveae had in the past been based mainly on the number of parts of the epicalyx and their fusion, but fruit characters seem to be more useful features for characterising clades (Escobar García et al. 2009); for generic limits around Abutilon, see Donnell et al. (2012). For suggestions as to the limits of Hibiscus and Pavonia, notoriously difficult, see Pfeil and Crisp (2005) and Koopman and Baum (2008). Finally, for the beginning of a reclassification of the whole Hibisceae, see Hanes et al. (2024) and R. L. Barrett et al. (2025: recognition of Sabdariffa).