EVOLUTION OF LAND PLANTS (UNDER CONSTRUCTION)
Background. As little as thirty years ago our understanding of the evolution of land plants was very different from what it is now. Then it was usually thought that bryophytes, i.e. mosses, liverworts and hornworts, represented the earliest land plants. Relationships between the bryophytes was unclear, but they seemed to form a group from within which other extant land plants evolved, while the aquatic Characeae (inc. Nitella) with their quite complex haploid plant bodies were thought to be the algal group most closely related to land plants (e.g. Graham 1993). Psilotum was thought to represent a very ancient group, often being compared with plants from the Devonian Rhynie Chert; horsetails were also thought to be very ancient, but not immediately related, rather being linked to the fossil Sphenophyllum and its relatives. Lycopodium, Selaginella and their relatives formed a group, after which came ferns and seed plants.
Before going any further, note that when describing relationships in the context of a branching phylogenetic tree, the use of terms like "high", "low", "basal", etc., to describe aspects of the branching pattern may seem perfectly appropriate, but at one level, even talking about branching points of this tree makes little sense; there is no trunk, and of any "branch" (one of a pair of sister clades), one cannot be basal to the other. Although I sometimes use the term "basal", this is in the context of a ladderized tree and refers to the branch in which there has subsequently been less diversification or to a branch below the one about which I am talking. Thus Takakia, Equisetum, Amborella, Acorus, Enkianthus, Humbertia, and many other clades can all be called "basal" from the first point of view, while branches on the seed plant tree that occur before angiosperms appear can be called "basal" with respect to angiosperms. To emphasize the point: "Basal" has only the topological connotations that I have just mentioned and there is certainly no implication that basal taxa necessarily lack apomorphies or are "primitive" in any way. Indeed, the terms "primitive" and "advanced" are heavily loaded and I have tried not to use them; "plesiomorphic" and its opposite, "derived" and "apomorphic", are the terms to use when talking about individual characters.
Relatives of Land Plants. Given the relationships just described, evolution seemed to have resulted in a fairly straightforward increase in complexity from "lower" to "higher" plants, but this comforting sequence has been severely challenged over the last twenty years or so. Land plants or Embryopsida, which include everything from mosses to monocots and more, are members of a clade embedded in a largely aquatic paraphyletic group, the "green algae" (paraphyletic groups will be denoted by scare quotes); the two together make up Chloroplastida (previously known as Viridiplantae). Chloroplastida can be divided into two main clades, Chlorophyta s. str. and Streptophyta, the latter including "Charophyta s.l." within which the monophyletic Embryopsida are embedded. For general information on the evolution of Chlorophyta and Streptophyta, see e.g. Lemieux et al. (2000), Turmel et al. (2002), Sanders et al. (2003), Waters (2003), Delwiche et al. (2004), Lewis and McCourt (2004), Becker et al. (2009), Leliaert et al. (2012, a great deal of information, 2016), Rieseberg et al. (2023, 2024/2025) and Shuai et al. (2025). Lewis and McCourt (2004) emphasise that many clades of "green algae" have terrestrial representatives, of which Embryopsida are merely the most prominent. Leliaert et al. (2016) note a number of similarities in the chloroplast genome between their Palmophyllophyceae, perhaps sister to other chlorophyta, and the basal streptophyte Mesostigma.
Within Chlorophyta s. str. there are a number of algae that are involved in lichen formation (Trebouxia and its relatives) as well as several ecologically very important marine algae. Volvox, Caulerpa, Ulva and Acetabularia are other Chlorophyta.
STREPTOPHYTA
Streptophytes include land plants (= embryophytes or Embryopsida) and a subset of largely freshwater green algae that form a grade immediately basal to them, i.e. taxa like Mesostigma viride (biciliate), Chlorokybus, Klebsormidium, Spirogyra, Coleochaete and Chara.
Age. Stem streptophytes have been dated to 1200-725 Ma (Yoon et al. 2004: Chaetosphaeridium vs. the rest), Zimmer et al. (2007) suggest an age of (963-)725(-587) Ma for the divergence of Chlamydomonas from the streptophytes and Morris et al. (2018a) HPD ages of 891-629 Ma (Mesostigma vs. the rest).
Evolution: Divergence & Distribution. Rieseberg et al. (2022: Box 1) summarized relationships in basal streptophytes; they are [Mesostigmatophyceae [Chlorokybophyceae [Klebsormidiophyceae [Charophyceae [Coleochaetophyceae [Zygnematophyceae + Embryophyta]]]]]]; [Charophyceae [Coleochaetophyceae [Zygnematophyceae + Embryophyta]]] make up Phragmoplastophyta. Of these clades, aside from Embryophyta, only Zygnematophyceae include many species, somewhat over 4,200. Note that recently L. Liu et al. (2025), using data from morphology, anatomy, physiology and the presence of key genes, recovered rather different relationships - [Zygnematophyceae [Coleochaetophyceae [Charophyceae + Embryophyta]]], and a charophycean fossil, Tarimochloa miraclensis, that they found in N.W. China in deposits 453-449 Ma lived in stenohaline marine conditions with a shallow carbonate facies.
Maybe the immediate relatives of embryophytes are not fully understood, but an ever-growing number of features formerly thought to be restricted to embryophytes are also to be found in streptophytes, particularly those that are immediately basal to embryophytes on the tree, e.g. Zygnematophyceae-Zygnematales (but note that there has been extensive gene loss there - see B. J. Harris et al. 2021/2022), Coleochaete/Chara et al. (Charales), if in a somewhat different form/context (e.g. Becker & Marin 2009; Popper & Tuohy 2010; Wodniok et al. 2011; C. Wang et al. 2015 and Schmidt et aL. 2023: hormones and hormone signaling pathways; de Vries et al. 2016, 2017b: phenylpropanoid biosynthesis, 2018: stress-signaling genes; Morozov et al. 2018: evolution of trans-acting small interfering RNAs; Gao et al. 2018: R - disease resistance genes; Schreiber et al. 2022). Rieseberg et al. (2022, see also 2024/2025) provide a comprehensive review of metabolic pathways, noting that "precursors" of embryophyte-type metabolic features are quite common in basal streptophytes, historical contingency helping to shape features of the embryophyte metabolome (see also Jacob 1977; Maeda & Fernie 2021, etc.). Thus genes involved in stress responses in embryophytes may have homologues in streptophytes, even if details of the pathways in which they are involved differ. For example, the major hubs in the vgenetic networks underpinning stress response and acclimation (especially temperature and light) are similar in both Mesotaenium endlicherianum (Zygnaemat0phyceae) and embryophytes and there also seems to be a homologous programme for stress-dependent lipid droplets (Rieseberg et al. 2022; Dadras et al. 2023). Bowles et al. (2022) look at the evolution of stomata, vascular tissue and roots in particular. In this context, thinking about some of these streptophyte/embryophyte versions in terms of preadaptations/exadaptations (Gould & Vrba 1982; see Becker & Marin 2009; Delaux et al. 2015; de Vries et al. 2018) may be helpful. Lang et al. (2010) noted that a considerable number of transcription-associated protein families evolved in the basal land plants or their immediate aquatic ancestors, many more than subsequently, but exactly where the later changes occur is unclear since nothing between Selaginella and angiosperms was included in this study. Boot et al. (2012) found that there was polar auxin transport in Chara; it is known from the sporophytic generation in mosses, but not liverworts, and it is poorly developed in hornworts (e.g. Poli et al. 2003; Sakakibara et al. 2008; Fujita et al. 2008; Fujita & 2009; Harrison 2017b). The evolution of gibberellin receptors is of considerable interest, with major groups differing in receptor type (Yoshida et al. 2018), while the process of hydrolysis of inactive auxin conjugates (e.g. with amino acids) may be particularly distinctive in tracheophytes, but again there are links to what goes on in bryophytes and yet more basal streptophytes (Campanella et al. 2018). S. Cheng et al. (2019) and others have noted that the molecular tool kit for life in a terrestrial environment evolved in streptophyte algae, indeed, early-diverging Zygnematophyceae (this is a class) like Spirogloea (spiral chloroplast, plastome IR) and Mesotaenium (2 chloroplasts) are subaerial or terrestrial. They lack flagellae/basal bodies, unlike most other nearby clades, and genes associated with them have been lost. However, they have a number of genes that are involved in responses to biotic and abiotic stresses, and these are also found in embryophytes. Furthermore, genes of the GRAS family that are “essential regulators of plant growth and development but also have functions in response to biotic and abiotic stresses and in symbiosis” (Cheng et al. 2019: p. 1061) are known from embryophytes and Zygnematophyceae and seem to have moved here by horizontal gene transfer from soil bacteria by around 580 Ma - note, however, that as mentioned elsewhere the rather simple plant body of Zygnemataceae seems to represent secondary simplification... For the Chara genome and what it has to say about terrestrialization (perhaps more than genomes of Zgynematophyceae), see e.g. Delwiche (2016) and Nishiyama et al. (2018: Fig. 1) - and even if the diploid plant body of Chara is restricted to the zygote, it may yet have embryophyte-like storage and stress-protection proteins there. For a more extensive discussion about many of these features, see also below.
There have been extensive changes to features involved in cell division, both mitosis and meiosis, in the basal streptophytes and also embryophytes. The cytological variation that was being discovered some 50 or more years ago had two major implications, first, that the old group of green algae should be broken up, and second, that a subset of these algae, the charophytes, had a number of similarities with land plants (Stewart & Mattox 1975). Thus the male gametes of bryophytes had asymmetrical sperm, while the insertion of the flagellae onto the basal multilayered band of microtubules was slightly lateral, a number of streptophytes have phragmoplasts, and so on (Stewart & Mattox 1975; see also Mattox & Stewart 1984; Simon et al. 2006; R. C. Brown & Lemmon 2007, etc.) - Southworth and Cresti (1997) compare flagellated and non-flagellated sperm in plants in general. Stewart and Mattox (1975) noted that the result of their synthesis was almost "a cytological classification", and its outlines have held. For characters of streptophytes and some of their subgroups, see e.g. Stewart and Mattox (1975) and Leliaert et al. (2012: esp. Fig 4). Buschmann and Zachgo (2016) discuss the evolution of cell division. Callose is much involved here - as it is in various aspects of development in embryophytes, including pollen/spore development (e.g. the inner spore wall in hornworts contains callose - Renzaglia et al. 2020a) and in pollen tube growth, plasmodesmata formation, the formation of sieve tube pores and, most importantly for the present discussion, cytokinesis (Ušák et al. 2023). In basal streptophytes there is a cleavage furrow and the new transverse wall develops centripetally, while in embryophytes the cell plate develops centrifugally where the preprophase band (p.p.b.) of microtubules had been - but more than that, the cell plate is made up of callose. The plate is a membranous structure formed by the fusion of vesicles mediated by phragmoplast microtubules. Note, however, that Klebsormidium, with centripetal cell division, also has callose in its centripetally-expanding wall, and it has three groups of callose synthase genes involved in cell division, as in all taxa higher up in the tree (Ušák et al. 2023). However, it is at the next node up - charophytes plus the rest - that cell division via cells walls developing cenrifugally is to be found, hence the name that has been applied to the clade [Charophyceae [Coleochaetophyceae [Zygnematophyceae + Embryophyta]]], Phragmoplastophyta (Ušák et al. 2023). Note, however, that in Zygnematophyceae Spirogyra and Penium have mixed wall development, and it is apparently centripetal in others - ?secondarily (Hess et al. 2022). In addition, basal streptophytes have a centrosome from which radiating microtubules develop and these are involved in pulling the dividing chromosomes apart, while in most embryophytes neither centrosome nor radiating microtubules develop. Thus changes in cell division are complex, and many are evident in various groups of streptophytes close to the embryophytes, although some show parallelism and even loss there (Buschmann & Zachgo 2016). Thus Mougeotia, in the clade sister to Embryopsida, lacks centrioles and has a p.p.b., while Coleochaete (same clade, or next clade down) has monoplastidic meiosis and centrioles (e.g. R. C. Brown & Lemmon 1993, 2011b, also 1990, 2013, etc.).
Needs work: The photorespiratory glycolate pathway occurs in peroxisomes rather than in mitochondria (Stabenau & Winkler 2005). The BIP multigene family is prominent (Friedl & Rybalka 2012). The duplication yielding the important GAPA/B gene pair occurred around here, and streptophytes have a particular isoform of glyceraldehyde-3-phosphate dehydogenase, GAPDH B (Peterson et al. 2006).
As will be clear from the discussion on phylogenetic relationships below, clarifying the relationships of and within Zygnematales is clearly critical if we are to understand the evolution of land plants - note that Zygnematales are now part of/have been placed in five orders that make up Zygnematophyceae (Hess et al. 2022). However, Zygnematophyceae do seem to be sister to Embryophyta, and they also appear to have lost close to 1,500 gene families, including those involved in multicellularity, although there seems subsequently to have been a burst of gene innovation in stem-group embryophytes, mostly duplications and fewer transfers and losses (B. J. Harris et al. 2021/2022). Indeed, Graham et al. (2000) quite early noted that most of the genes important in the colonization of land were homologous with genes in Coleochaete and Spirogyra or had solid hits there. Taxa like Coleochaete, Chaetospiridium and Spirogyra do seem to be cladistically closer to embryophytes than Chara et al., although this may not convert to much in the way of characters linking the first two groups mentioned. Nevertheless, these relationships and the suggestion that Bryophyta s.l. may be monophyletic and perhaps reduced in some of their attributes (e.g. Harris et al. 2021/2022), questions the old scenario in which land plants evolved ever more complex plant bodies culminating in those of angiosperms - things are rather more complicated than that.
Ecology & Physiology. Stebbins and Hill (1980) suggested some time ago that embryophytes were unlikely to have been the first streptophyte clade to live on land. They even thought that Charophyceae s. str. might have moved back to water (see also Harholt et al. 2016; Nishiyama et al. 2018: e.g. Fig. 5; Rensing 2018; S. Wang et al. 2020), thus the morphology of the charophytes is unlikely to represent that of the immediate ancestor of land plants, as had been thought in the preceding century. Indeed, the ability to live in a terrestrial habitat likely goes back to the very base of the Streptophyta, where Mesostiga viride, an aquatic unicell and the only streptophyte with flagellated vegetative cells, is sister to Chlorokybus atmophyticus, terrestrial and multicellular. The next clade, Klebsormidiophyceae, sister to Phragmoplastophyta, can also be terrestrial, and there are surely parallels between the problems they face and those faced by early terrestrial embryophytes - these centre on desiccation, acquisition of nutrients, movement of water through the plant once the plant body reached any size, protection from ultraviolet (UV-B) radiation, photosythesis under high CO2 concentrations, and so on (Hori et al. 2014: e.g. several hormones and their receptors there, protection against light). Thus Pierangelini et al. (2018, see also 2017) noted that some of the Klebsormidiophyceae they studied were desiccation tolerant (see also Holzinger et al. 2014) and avoided direct insolation by growing in low-light regimes, others could acclimate to high-light regimes but were not desiccation tolerant (some of the responses/behaviours they studied were facultative). Moreover, desiccation tolerance and photoacclimation were linked to the ability of taxa like Streptosarcina arenaria to form clumps ("packets") of cells, and in general cell aggregation, whether cell clumps or even biofilms, helped prevent dehydration (Pierangelini et al. 2018), indeed, Stebbins and Hill (1980) had noted that such three-dimensional growth forms would be resistant to desiccation. Similarly, Coleochaete orbicularis, normally aquatic and with an orbicular, unistratose plant body, can grow on agar or quartz sand, and then it produces clumps of cells, some with thickened, layered walls, and with least some desiccation tolerance (Graham et al. 2012) For divergence within Coleochaete and its morphological variation, see Delwiche et al. (2002) and for Coleochaete and the movement of plants on to land, see Graham et al. (2012). In Zygnematophyceae-Zygnematales-Zygnema desiccation tolerance was evident in lipid-rich and thick-walled cells (Herberger et al. 2015: the pre-akinete stage, cells still in filaments). Dadras et al. (2023) noted similarities in the genetic network involved in stress response and acclimation between Mesotaenium endlicheranum (Zygnematophyceae-Serritaeniales) and embryophytes. Response to temperature and irradiation were evident, and there was extensive chloroplast-nuclear communication; there was also stress-dependent formation of lipid droplets, as in embryophytes (Dadras et al. 2023). Moody et al. (2020) examined the transition from 2- to 3-dimensional growth in the moss Physcomitrium patens, from 2-D protonema to 3-D gametophore, and with a focus on the NO GAMETOPHORES 2 gene; they suggested that this gene functioned in the ascorbic acid pathway, this and/or related genes being involved in both cuticle formation and the synthesis of lignin, the latter after the divergence of bryophytes and vascular plants.
For the synthesis of the cell wall polysaccharides so important in land plants, but also found in more basal streptophytes, see Mikkelsen et al. (2014). Del-Bem (2018) noted that genes involved in xyloglucan metabolism are found in many streptophytes, although there are only a few in Chlorkybus and Mesostigma. These xyloglucans are the most important component of the non-cellulosic non-pectic matrix of the primary cell wall. The hemicellulose polysaccharide mannans are important components of the cell walls of embryophytes, and Zhong et al. (2019) looked at glucomannan acetylation there - although members of the enzyme family involved were to be found in more basal streptophytes, they had different functions. The genes particularly involved, mannan O-acetyl transferases, are found throughout the embryophytes. Galactoglucomannans are quite abundant and widely distributed in most embryophytes, but mannans in angiosperm hardwoods are only a minor component of of xylem cell walls (Zhong et al. 2019).
Xyloglucan-producing streptophytes may have been components of early (pre-embryophyte) soil crusts, perhaps also helping in the aggregation of soil particles around algal cells (Del-Bem 2018). Raven (2018) discussed early soil formation; he thought that this might (p. 1139, his emphasis) have occurred in the Proterozoic, yielding deeply entrenched river channels, however, mudstones, reflecting deep silicate weathering by roots, was evident only in the Late Ordovician. In fact, although the cell wall of embryophytes is a distinctive structure, Harholt et al. (2016) noted that few of its components were unique to embryophytes; it may reflect the adaptation of earlier streptophytes to life on land and its presence in Characeae s. str. represents the retention of ancestral (terrestrial) features. Taxa like Zygonema, Klebsormidium, etc., do not produce zoospores, again, reflecting a possible adaptation of their ancestors to life on land.
Interestingly, mosses and liverworts as well as at least some streptophytes immediately basal to embryophytes can take up mono- and/or disaccharides - glucose, fructose and sucrose - and thus mixotrophy could be the basal condition for embryophytes (Graham et al. 2010a, 2010b).
Genes & Genomes. Overall the transcriptomes of Coleochaete and Spirogyra are quite similar, but rather different from that of Arabidopsis, the latter being proportionately richer in genes involved in cellular metabolic processes, in transcription factor activity and membrane formation, the former richer in hydrolases, transferases and in biosynthetic processes (Timme & Delwiche 2010). However, 26 out of 36 transcription-associated protein (TAPs) family gains in embryophytes can in fact be traced back to their streptophyte ancestors (Wilhemsson et al. 2017).
Leliaert et al. (2012) summarize variation in the plastid and mitochondrial genomes in streptophytes and compare it with that in embryophytes, and some of this may convert to apomorphies when comparative data improve. For the evolution of the chloroplast genome, see de Vries et al. (2016); de Vries et al. (2017a) found an ultraconserved motif of the YCF1 gene in the higher streptophytes that is found in most land plants (see also Nakai 2015). The tufA gene has moved to the nucleus, although it is present, if very odd, in Coleochaete chloroplasts (e.g. Baldauf et al. 1990; de Vries et al. 2016). For the chloroplasts of Anthoceros and cycads, see C.-S. Wu and Chaw (2015); the chloroplast ultrastructure of Coleochaete is in some ways similar to that of hornworts (Vaughn et al. 1992). A possible synapomorphy for the node that includes Mesostigma viride and the rest is the presence of the ndh and rps15 and the loss of the rps9 chloroplast genes, although Chara has the rps9 but not the rps15 genes (Martín & Sabater 2010); the presence of introns in the ndhA and ndhB genes may also be apomorphies around here, and Ruhlman et al. (2015) summarize the increase in complexity of the NADH dehydrogenase-like (NDH) complex in these streptophytes. Both Zygnema and the desmid Staurastrum secondarily lack the chloroplast inverted repeat, present in other streptophytes (Turmel et al. 2007), indeed, evidence now suggests that the IR may have been lost several times in the [Zygnematales + Desmidales] clade as well as in Coleochaete (Civán et al. 2014; Lemieux et al. 2016;Mower & Vickery 2018). In general the chloroplast genome in the [Zygnematales + Desmidales] is very labile, group II introns frequently being lost, and some phage/viral genes have been gained, although overall the genes it contains are much less labile (see esp. Lemieux et al. 2016). For similarities in the organisation of the chloroplast genome in particular between basal streptophytes and basal green algae, see Leliaert et al. (2016). See also discussion under Embryopsida below.
Phylogeny. Resolving the relationship of the polyphyletic prasinophytes, mostly Chlorophyta, has been important (e.g. Lewis & McCourt 2004; Niklas & Kutschera 2010; esp. Leliaert et al. 2016 and references). Mesostigma viride, the only streptophyte with an eye spot, used to be included in that group, but along with Chlorokybus it is sister to the other streptophytes, as is indicated by nuclear and some, but by no means all, organellar genes (E. Kim et al. 2006: isoprenoid synthesis pathways and glycolate oxidizing enzymes agree). Its zoospores have scales but lack a cellulose cell wall (J. Petersen et al. 2006) and it links with the non-motile Chlorkybus (Simon et al. 2006); these two genera are placed outside a [Chlorophyta + Streptophyta] clade by Gitzendanner et al. (2018a).
It was often thought that Characeae/Charales s. str. (inc. Chara, Nitella, but not much else) were the immediate sister group of land plants (e.g. Graham 1993: still useful and readable; Karol et al. 2001; Turmel et al. 2003; Delwiche et al. 2004; Qiu et al. 2007: quite strong support), partly because they seemed to be intermediate in a progression between simple "algal"-like morphologies and the more complex land plant morphologies - they are filamentous, growth is by an apical cell, and they are oogamous. However, for more than twenty years molecular evidence has suggested otherwise (e.g. Hedderson et al. 1998: small subunit rRNA). Turmel et al. (2006, 2007) found Zygnematales (now part of Zygnematophyceae) to be sister to embryophytes in a number of analyses based on complete chloroplast genome sequences - a commonly-found set of relationships is [Chara [Chaetosphaeridium [[Zygnema + Staurastrum] + Embryopsida]]] (see also Chang & Graham 2011: Staurastrum not included). Similar relationships are commonly recovered, e.g. [Nitella [[Spirogyra, Closterium, Chaetosphaeridium, etc.] [Coleochaete + Embryopsida]]] (Finet et al. 2010), while B. Zhong et al. (2013a, b; Zhong et al. 2014a) found the variant relationships [Charales [Coleochaetales (inc. Chaetosphaeridium) [[Zygnematales + Desmidales] + Embryopsida]]] or [[Coleochaetales + Zygnematales] Embryopsida]; see also the relationships in Simon et al. (2006) and Sayou et al. (2014). Springer and Gatesy (2014) reanalysed the data of Zhong et al. (2013a) and found different topologies depend on how the data were analysed, but Charales were never sister to embryophytes. Overall, the relationships recovered around here are most often [[Chlorokybus + Mesostigma] [Klebsormidium [Nitella [[Coleochaete + Chaetosphaeridium] [[Penium + Spirogyra] + embryophytes]]]]], e.g. as found by Laurin-Lemay et al. (2012: q.v. for details) in a reanalysis of the data used by Finet et al. (2010), i.a. excluding contaminated data such as rotifer and diatom sequences; see also Cooper (2014), X.-X. Shen et al. (2017: evaluation of support) and O.T.P.T.I. (2019).
Thus evidence for the sister group relationship of embryophytes and Spirogyra and its relatives is now quite strong (Leliaert et al. 2012: literature; Timme et al. 2012; see also Wodniok et al. 2011; Ruhfel et al. 2014; Wickett et al. 2014; Puttick et al. 2018: two transcriptome analyses; C. C. Davis et al. 2014a; Civán et al. 2014; Lemieux et al. 2016 - all chloroplast genomes; O.T.P.T.I. 2019: nuclear genomes, good sampling; Donoghue et al. 2021: Fig. 2). The [Penium + Spirogyra] clade has a number of apomorphies, including the loss of cilia and also the loss of morphological complexity (Wodniok et al. 2011; Timme et al. 2012). Many genera of Desmidiaceae are polyphyletic (Friedl & Rybalka 2012 and references; Hess et al. 2022, they have recently placed members of Zygnematophyceae into five orders). Again the suggestion is that unicellularity is plesiomorphic for the whole clade, although perhaps derived; there have been some five origins of true filamentous growth here, plus two origins of chain-like growth in Desmidales. Hardly suprisingly, plasmodesmata are absent in the whole group, although more basally in streptophytes some taxa have plasmodesmata of sorts (see Brunkard & Zambryski 2017), and there is often just a single chloroplast - the shape varies considerably - per cell. Mougeotopsis calospora (Serritaeniales), apparently alone in the clade, lacks pyrenoids (Hess et al. 2022).
Classification. For a classification of life, see Ruggiero et al. (2015).
EMBRYOPSIDA Pirani & Prado / Embryophytes
Gametophyte independent, multicellular, initially ± globular, not motile, filamentous, branched; core symbiosis genetic program [involved in the establishment of arbuscular mycorrhizal associations]; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase [PAL], abscisic acid, jasmonic acid, salicylic acid, phytochrome, strigolactones, flavonoid lignin precursors, sporopollenin, cuticle +, canonical phytochrome + [Mesostigma, etc.], microbial terpene synthase-like genes +, also typical plant terpene synthases, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], cytokinins, xyloglucans in primary cell wall, side chains charged; plant poikilohydrous, ± desiccation tolerant, ectohydrous [free water outside plant physiologically important]; thalloid, development 3-D, leafy, with single-celled apical meristem, VNS-basd gene regulatory system underpinning development of water and supporting tissue, rhizoids +, unicellular; cells polyplastiidic, pyrenoids +; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], branching plasmodesmata +; antheridia and archegonia +, jacketed*, epidermal in origin, archegonia embedded/sunken [only neck protruding], neck canal cells +; meiotic phases coupled [DNA duplication, karyokinesis, cytokinesis in synchrony], male gametes [spermatozoids]: cellular architecture spiral, with left-handed coil; naked, complex cellulose wall 0; cilia 2, lateral, asymmetrical, little staggered [ca 1.5 µm distant]; plastid 1, mitochondria 2, one anterior, elongated, lying against the spine, one basal; MTOC [= MicroTubule Organizing Centre] +, associated with plastid, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [MLS: 4 layers: L1; L4, tubules; L2; L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules at basal end lacking symmetry, axonemal cap 0 [microtubules disorganized at apex of cilium]; nuclear chromatin condensed, transcriptional activity suppressed; protamines + [= sperm nuclear basal proteins]; oogamy; sporophyte + multicellular, initially surrounded by and growth dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; plane of first division of zygote transverse [with respect to long axis of archegonium/embryo sac], growth 3-dimensional*, no resting period, apical cell +, [at least transient] [?level], suspensor/foot +, cells at foot tip somewhat haustorial, cuticle +, stomata +, sporangium +, terminal, dehiscence longitudinal; meiosis sporic, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, monoplastidic, quadripolar microtubule system + [aligns plastids at poles, responsible for spindle development]; >1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae], naringenin +; plastid transmission maternal; nuclear genome [1 C] 1.4> pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; plastome with introns (not: Mesostigma), close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; chondrome with gene clusters, group I and II introns,42 protein genes; trnS(gcu) and trnN(guu) genes +. Extant: discussed under 6 main clades below, 403,911 spp. (Nic Lughadha et al. 2016).
Includes the following five major clades - bryophytes s. l. (= mosses, liverworts and hornworts) and four clades of vascular plants with extant taxa: angiosperms, ferns/monilophytes, gymnosperms, lycophytes.
Note: All groups in this site are extant crown groups unless specified otherwise. In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above). Finally, at this node in particular many of the bolded characters in the characterization are in fact apomorphies of clades of the type [subsets of streptophytes + embryophytes], not apomorphies of crown-group embryophytes per se.
Age. Clarke et al. (2011: other estimates, c.f. topology) suggested an age for crown Embryopsida of (815-)670(-568) Ma, Zimmer et al. (2007) and age of (580-)496(-412) Ma, Cooper et al. (2012) estimated its age at (519-)493(-469) Ma, and Magallón et al. (2013: including temporal constraints) an age of around (480.4-)475.3-474.6(-471) Ma (see the constraint age in Heinrichs et al. 2007) and a stem age of around (962.5-)913, 911(-870) Ma; the lowest crown date is around 439 Ma (Magallón & Hilu 2009). An age of (629.5-)530(-449) Ma is suggested in B. Zhong et al. (2014b: fossil calibration), ca 487 Ma in Evkaikina et al. (2017), 515-473.6 Ma in Morris et al. (2018a: HPD, other dates and extensive discussion), (485-)482(-473) Ma (Lutzoni et al. 2018: N.B., the stem could be up to 727 Ma) and 515-493 Ma, stem embryophytes being ca 630 Ma (B. J. Harris et al. 2021/2022: Fig. 2); crown embryophytes are estimated to be ca 467 Ma by Y.-L. Qiu et al. (2024), the stem involving [Chara + Nitella], i.e. several branches missing, being ca 538.9 Ma. P. Soltis et al. (2002), Guindon (2018) and Strother and Foster (2021) suggest a variety of ages that depend on calibrations, an issue that is also discusssed by Harris et al. (2021/2022), Qiu et al. (2024) and others.
A variety of spore types around 480 Ma seem to link older Cambrian cryptospores from Laurentia and younger Ordovician spores from Gondwana (Donoghue et al. 2021: dyads and tetrads ca 469 Ma; Strother & Foster 2021). There are 455-454 Ma fossils of leaves from Ordovician deposits in Wisconsin with distinctive cells that are remarkably similar to those of extant Sphagnum and probably belonging to a member of Spohagnopsida, and these are the earliest known vegetative remains of an embryophyte (Cardona-Correa et al. 2016; Turetsky et al. 2025). Libertín et al. (2018) record quite large Cooksonia-like fossils from deposits ca 432 Ma in the Czech Republic.
Evolution: Divergence & Distribution. Laenen et al. (2014) give ages and diversification rates for well over 150 major clades throughout land plants. Y.-L. Qiu et al. (2024) suggest over 600 ages for branches on their embryophyte tree, but notice its topology; only a few of their ages are mentioned below (also time constraints...).
To set the scene: "The origin of land plants is as much associated with gene co-option, exaptation and even horizontal gene transfer (HGT) as it is with fundamental innovation" (Donoghue et al. 2021: p. R1285), although a notable number of novelties are to be found both here and at the origin of streptophytes (ibid.). Similar gene co-option is quite common in other stages of land plant evolution, e.g. in the evolution of seeds (Pennisi 2022 and references). Bowles et al. (2020) also noted that there were novelties that could be associated especially with the origin of streptophytes (including gene groups involved in multicellularity) and with the ancestor of land plants (perhaps problems associated with terrestrialization were important here), but that there had also been substantial losses of homology groups of genes at various times (see also Timme et al. 2012: immediate relatives of embryophytes; Bowman et al. 2017 for apomorphies; Wilhelmsson et al. 2017; B. J. Harris et al. 2021/2022). C. Wang et al. (2015) suggest that several elements of hormone signaling arose around at about the evolution of embryophytes (abscisic and salicylic acids, jasmonate) or in more basal (auxin, cytokinin, strigolactone) streptophytes (however, strigolactones may initially have been involved in fungal signaling - Kodama et al. 2022). Note that substantial time may elapse between the origin of a gene and of the important phenotype with which it becomes associated (Bowles et al. 2020; Donoghue et al. et al. 2021). HGT - the genes may be of fungal, viral or bacterial origin - has been ongoing (J. Ma et al. 2022), and seems to have been particularly common around the time of divergence of the streptophyte Klebsormidium, ca 177 gene families being involved, around 58% being retained in seed plants (some with numerous duplicates/functions), and again somewhat later in ancestral land plants (107 gene families, 71% retained) (Ma et al. 2022), although Bowles et al. (2020) suggest a rather older early episode of gene movement. Indeed, HGT is involved in major evolutionary transitions like the origin of primary plastids (Van Etten & Bhattacharya 2020), while for HGT in Zygnematophyceae and the origin of land plants, see Cheng et al. (2019), Van Etten and Bhattacharya (2020), etc.. For more on HGT, see elsewhere.
J. W. Clark et al. (2023) looked at the overall evolution of land plants from the point of view of changes in phenotypic diversity≡disparity; this measure does not take phylogeny into account and is the difference in character states between two taxa (see e.g. Herting et al. 2023), being a property of groups, and phenotypic complexity, a property of individuals; they accumulated data on 548 characters and 248 extant taxa. They found notable changes in disparity between the major groups (the three bryophyte groups are quite similar and make up one major group), major differences being the presence of vascular tissue, seeds, fruits, etc., interestingly, the disparity included within the gymnosperms examined was quite extensive, the cycad, Ginkgo and the gnetophyte (Gnetum) examined forming a subgroup quite different from the rest, while there was relatively little disparity within the angiosperms, furthermore, phenotypic complexity was not a necessary prerequisite for disparity (Clark et al. 2023). Genome duplication may have been involved in the changes that resulted in extant angiosperms and gymnosperms, but pulses of gene family innovation followed by neofunctionalization, etc., may have been involved in the evolution of the other groups (Bowles et alg. 2020; Harris et al. 2022); changes in ploidy correlated with phenotypic complexity, not with disparity (Clark et al. 2023). Bryophytes s.l. have quite slow rates of speciation and of genome size evolution, as do pteridophytes and gymnosperms (particularly the latter), a situation that does not really change until after the ANA grade of angiosperms (Laenen et al. 2014; Puttick et al. 2015). Note, however, that overall variation in genome size itself is quite considerable (see below).
All embryophytic plants have alternation of generations. Here a sporophytic generation has been as it were interpolated into a life cycle that, with the exception of the diploid zygote, was entirely haploid/gametophytic, i.e. similar to the life cycle of some of the more basal aquatic streptophyes, plants like Chara, etc. - this is the antithetic theory of the origin of alternation of generations. It is unlikely that these alternating generations are the result of the divergence of initially morphologically similar diploid sporophytic and haploid gametophytic generations, i.e. the homologous theory (e.g. Haig 2008, 2015; Gerrienne & Gonez 2011; Strother 2015; Strother & Taylor 2018; etc.). The BELL1 homeobox gene may be involved in the interpolation of mitotic cell divisions between zygote formation and meiosis in early land plants (Horst & Reski 2016), although the gene is also known from Chlamydomonas, not at all closely related... The bigger and more complex sporophytic plant body would allow the production of meiospores, the immediate products of meiosis, in large numbers (e.g. R. C. Brown & Lemmon 2011a; Qiu et al. 2012; Edwards et al. 2014). This change may have been driven by the unpredictability of fertilization (Haig 2015). (Interestingly, in Coleochaete the large, maternally-provisioned zygote may produce up to 32 zoospores, although exactly when meiosis occurs is unclear - Haig 2015.) However, we lack reliable knowledge of life cycles in most charophyte algae, and this hampers our understanding of the events that led to the development of the alternation of generations in land plants (Haig 2010, 2015), but the evolution of alternation of generations is unikely to have had anything directly to do with the colonization of terrestrial habitats by streptophytes (Harholt et al. 2016).
Thus there may have been an initial development of a diploid spore-producing sporangium (= sporogonium) borne on the gametophyte, with the later elaboration of a full-blown free-living sporophyte in the polysporangiophyta (e.g. Kaplan 1997: chap. 19; Kato & Akiyama 2005; Qiu et al. 2012: extensive discussion; Ligrone et al. 2012b), the sporophyte eventually becoming independent of the parental gametophyte (Haig 2015; Qiu et al. 2012). Interestingly, the expression of genes in the two generations is similar, in ferns at least (Marchant et al. 2022; Pelosi et al. 2023). Some basal streptophytes and nearly all embryophytes have placental transfer cells or their equivalents on the sporophytic and/or gametophytic sides of where the two generations join; these cells have labyrinthine wall ingrowths that mediate an initial transfer of nutrients from the gametophyte to the sporophyte (see Gunning & Pate 1969b; Ligrone et al. 1993: much detail; Hilger et al. 2002; Carapa et al. 2003; Vaughn & Bowling 2008; Renzaglia & Whittier 2013 for a tree).
In Bryophyta s.l. the gametophyte remains free-living and quite elaborate, while in ferns, etc., the gametophyte, initially free-living, ultimately becomes dependent on the sporophyte, although both are complex, multicellular plant bodies. Root hairs develop from the sporophyte, rhizoids from the gametophyte, but both are apically-growing cells involved i.a. in water uptake and they are under similar developmental control (V. A. S. Jones & Dolan 2012). In flowering plants the gametophyte is wholly dependent on the sporophyte and is reduced to eight or fewer cells - the relationships of the two generations in streptophytes like Chara have become completely reversed in seed plants, only the pollen grains/tube and the embryo sac of flowering plants in particular remaining of the once-dominant gametophytic generation. Plasmodesmata are integral to transport and communication between the cells of the embryophyte plant body, activities that may be modulated by callose that can become associated with the plasmodesmata; although plasmodesmata of sorts occur in charophytes and some other basal multicellular streptophytes (there is also variation in e.g. some grasses, but on an embryophyte theme), they are probably an apomorphy of embryophytes (Brunkard & Zambryski 2017 for a summary; Johnston et al. 2023).
MADS-box transcription factors are involved in various aspects of the development of the gametophyte and sporophyte in embryophytes. In charophytes such genes are involved in the development of the gametophyte but are not expressed in the zygote, however, in embryophytes the situation is more complicated (Y. Qiu et al. 2024). After an early (basal embryophyte or thereabouts) MADS-box transcription factor gene duplication, one duplicate gave rise to the MIKCC-type genes which play an important role in the morphology and development of the embryophyte sporophyte, and there are high copy numbers across embryophytes. The other duplicate itself duplicated, the MIKC*-type factors becoming involved in male gametophyte development and the M-type factors being involved in female gametophyte and endosperm development; copy numbers are not so high, but those of the MIKC*-type are rather higher in mosses and hornworts (Qiu et al. 2024).
In the sperm, protamines are widespread in eukaryotes and are involved in chromosomal compaction (Buttress et al. 2022: see seed plants and angiosperms for changes). Sokoloff and Remizova (2020) discuss the complex history of the evolution of archegonia (see esp. their Fig. 13), establishing homologies between the cells of flowering plant embryo sacs and the various cells of archegonia elsewhere in land plants. In particular, the egg cell in flowering plants may not be homologous with the egg cell of other embryophytes, but with the primary ventral cell, the original egg cell having become a polar nucleus (Sokoloff & Remizova 2020), and the "typical" 8-celled embryo sac representing two 4-celled units (Porsch 1907; Friedman & Ryerson 2009). Howver, future work may add yet more characters of archegonial development to the tree.
Yue et al. (2012) suggested that numerous genes, including those involved in many embryophyte functions like cuticle and lateral root formation, had been acquired by HGT from fungi and bacteria (see also Emiliani et al. 2009; R. Chen et al. 2019: glycoside hydrolase subfamily GH5_11, from fungi, esp. cellulose degradation) - HGT is “the movement of genetic material across branches of the tree of life” (Van Etten and Bhattacharya 2020: p. 915). Along the same lines, an actinoporin in Physcomitrium patens, Tortula ruralis and Selaginella lepidophylla is involved in water stress responses, overexpression in the first species making the gametophore tolerant to dehydration (Hoang et al. 2009). Similar actinoporins are known from sea anemones, and Hoang et al. (2009) suggested that there had been HGT from sea anemones to land plants, and that bryoporin, the actinoporin in land plants, was involved in the response to water stress in the common ancestor of the three plants mentioned above, i.e. in the the common ancestor of extant land plants. HGT is reported to be sporadic in Bryophyta s.l., etc., and some genes from fungi, algae, etc., have moved to these plants. Van Etten and Bhattacharya (2020) found that only a small portion of the genome was involved, perhaps 0.04-2.97(-6.49)%, although these figures may be underestimates. The genes transferred may be involved in extremophily, colonization of the land, etc., however, HGT in also known in mesophytic plants, parasites, etc., and the genes transferred there have a variety of metabolic functions.
In mosses, at least, the great majority - ca 95% - of genes are expressed in both sporophyte and gametophyte generations (Szövényi et al. 2010; c.f. in part O'Donoghue et al. 2013), and in the fern Polypodium the situation is similar, with ca 97% of the genes being expressed in both generations, only some 10% of the genes differing in transcription levels between the two (Gigel et al. 2016). However, there are often substantial differences between the two generations in the expression of genes controlling apical meristem growth and auxin polarity (e.g. Fujita et al. 2008; Sakakibara et al. 2008; Harrison 2017a, b for literature), and other studies found that over 12% of the transcriptome of the moss Physcomitrium patens switched in the transition from gametophyte to sporophyte, especially those genes involved in carbohydrate and energy metabolism (O'Donoghue et al. 2013; see also Szovenyi et al. 2010). It is only in angiosperms that a substantial proportion (ca 25%) of genes are expressed in the sporophyte alone (Szövényi et al. 2010). Interestingly, in both angiosperms and Physcomitrium genes expressed in the gametophyte were younger than those expressed in the sporophyte (O'Donoghue et al. 2013; Cui et al. 2015; Gossmann et al. 2016), which is perhaps the opposite that might be expected given that the sporophyte is an evolutionary novelty in the embryophytes. Floyd and Bowman (2007) discuss developmental changes possibly occurring at this node, and Friedman et al. (2004) the evolution of plant development. There have indeed been dramatic changes in the expressions of some genes during land plant evolution (e.g. Banks et al. 2011), but where on the tree some of these changes occurred is still unknown. Thus Szövényi et al. (2010: ca 30% of the genome mapped) noted that a total of only ca 5% of the genes in Funaria hygrometrica were expressed uniquely in the sporophytic and gametophytic generations, but in Arabidopsis ca 5% of the genes were differentially expressed in the gametophyte alone and ca 25% in the sporophyte alone. Gene expression in neither bryophyte generation was like that in the Arabidopsis gametophytes, a shift that took place somewhere between Funaria and Arabidopsis... For other such studies, see Lang et al. (2010) and T. Zhu et al. (2012).
Three stories. With a monophyletic Bryophyta s.l. how we think of land plant evolution will change, and as Puttick et al. (2018: p. 10) noted, "Marchantia and other liverworts might not serve as an appropriate model for anything other than liverworts themselves." (c.f. e.g. Shimamura 2016; Bowman et al. 2017; Rensing et al. 2020). Lichens - algal-fungal associations that may grow in extreme terrestrial conditions today - seem not to be involved in the initial movement of embryophytes on to the land (see below). In any event, thinking of evolution, homology, etc., of embryophytes and their streptophyte relatives is complicated, as the following three stories suggest.
1. Rutaceae are well known for their pellucid foliar oil glands, and in a study involving Citrus and related/segregate genera and hybrids, H. Wang et al. (2024) elaborated on a number of connections that details of the development of these glands suggested. They noticed that the nucleus-localized class I homeo-domain leucine-zipper family transcription factor (HD-ZIP I) CsLMI1 was centrally involved in the development of these oil glands both here and also elsewhere in the family (the gland secretions include monoterpenes, flavonoids, etc.), as well as in the development of crenations of the lamina there (they are associated with oil glands). Furthermore, CsLMI1 was a homologue of HD-ZIP I genes from Arabidopsis and Cardamine that were key regulators of lamina serration and dissection respectively in those taxa. Finally, the only HD-ZIP I family member in Marchantia polymorpha was involved in oil body formation (Wang et al. 2024), the oil bodies being in distinctive cells that are found in both the sporophyte and gametophyte, the oils including a variety of terpenes, etc. - interestingly, liverworts have by far the greatest abundance of terpenes - ca 1,600 kinds - of the three bryophyte groups, but like other non-seed plants they have both general plant terpenes (also known from seed plants) and microbial terpene synthase-like genes (Jia et al. 2016; F. Chen et al. 2018; El Mahboubi et al. 2026). Teasing apart what, if any, connections there might be between terpenoid synthesis and the development of glandular structures in general, including glandular hairs in vascular plants, is clearly of interest (see also Lange 2015; H. Wang et al. 2024).
2. B. Xu et al. (2014) found that similar NAC transcription factor family genes were expressed during the development of both the hydroids of mosses and the xylem of vascular plants, despite the difference in the generation in which they were expressed and in the morphology of the cells concerned (hydroids have neither pitting nor lignification), i.a. inducing cell death in both. Such NAC genes are uncommon in liverworts. Similarly, the formation of both sporophytic root hairs in Arabidopsis and gametophytic rhizoids and caulonemal cells of the protonema of Physcomitrium patens, all cells in which there is tip growth, involves the same regulatory gene network, perhaps independent recruitment and/or some kind of heterochrony/topy (e.g. V. A. S. Jones & Dolan 2012, p. 205: "transference of gene function"; Menand et al. 2007; Pires & Dolan 2010; Pires et al. 2013; see also Szövényi et al. 2010; Salazar-Henao et al. 2016: ROOT HAIR DEFECTIVE SIX-LIKE (RSL) genes involved; L. Huang et al. 2017). Menand et al. (2007), Jones and Dolan (2012), Kenrick and Strullu-Derrien (2014), Tam et al. (2015) and Hwang et al. (2017) also discuss the evolution of root hairs and rhizoids; the development of "tip-growing" cells like rhizoids, caulonema cells and root hairs is controlled by a similar auxin-regulated network - even fungal hyphae show similarities (Rounds & Bezanilla 2013, L. Huang et al. 2017 and references). In Physcomitrium caulonemal and rhizoid formation are inhibited by the same mutations (Rounds & Bezanilla 2013 and references). Sakakibara (2016) summarizes such findings; their extension to liverworts would be of considerable interest. Rhizoids are involved in water, perhaps also nutrient, uptake and plant attachment, root haits especially in water and nutrient uptake (Jones& Dolan 2012).
3. An argument can be made for the independent origins of PIN(= auxin efflux facilitators)-mediated auxin transport polarity in the sporophytes of mosses and vascular plants (Harrison 2017b: Fig. 1, Table 1), although there is also auxin transport in the gametophyte of bryophytes s.l. (apolar, plasmodesmatal) and polar transport of sorts in Klebsormidium and Chara, at least (references in de Vries & Archibald 2018). PIN plays a substantial role in the development of the Physcomitrium gametophyte, and its activity is evident even in apically-growing cells of the protonema (Bennett et al. 2014; Viaene et al. 2014: not in root hairs or pollen tubes in angiosperms). Interestingly, disruption of PIN in the sporophyte can lead to its branching (e.g. Bennett et al. 2014), and fossils like Partitatheca which lack vascular tissue but have stomata may represent a branched bryophyte sporophyte (see Harrison 2017 and literature).
The evolution of transcription factors (TFs) is discussed by J. Zhang et al. (2020), who looked at some TF families and clades containing them that were involved in the development of the land plant body plan. 9/33 of the clades listed there (ibid. Fig. 2a), including all members of three families, seem to be associated with the evolution of embryophytes, i.e. the movement of plants on to land. Physcomitrium patens, in which there has been a genome duplication, showed a notable increase in the number of members of TF clades compared with the two other bryophyes examined. Five clades (two families) were specifically associated with the evolution of angiosperms, at least (gymnosperms were not included), while 19 or so of these clades, which include at least some members of 9 families, although found in most embryophytes and sometimes abundant there, particularly in Arabidopsis thaliana compared with Amborella trichopoda, are also to be found in at least some other streptophytes (Zhang et al. 2020).
The exact relationship between the sporophytes of polysporangiophytes and those of bryophytes s.l. is unclear (e.g. Shaw et al. 2011). The stalk of a moss capsule may not be strictly homologous to the branching sporophyte axis of the polysporangiophytes (Kato & Akiyama 2005; Qiu et al. 2012). Sterilization of the sporangial axis or simple elaboration of a bryophyte sporangium leading to the evolution of a branched sporophyte are ideas that have been suggested relating the sporophyte of bryophytes and polysporangiophytes, but prolongation of embryonic growth is perhaps more likely (Tomescu et al. 2014). Frank and Scanlon (2014) found that there was expression of meiosis gene families early in the development of both Physcomitrium patens (see also O'Donoghue et al. 2013: carbohydrate metabolism genes) and Marchantia sporophytes, which would perhaps make their extended growth unlikely, however, in the stem apex of maize and in the gametophyte apex of mosses, but not liverworts, similar patterning gene families were upregulated (see also Fujita et al. 2008; Sakakibara et al. 2008). A scenario for the evolution of a complex sporophyte may involve delayed expression of genes involved in meiosis, which would allow for indeterminate development of a sporophyte in which these patterning genes were expressed (Frank & Scanlon 2014). Genes expressed in the gametophyte may be essential for the subsequent elaboration of the sporophyte. Indeed, Cammarata et al. (2022) found that CLAVATA1 and RECEPTOR-LIKE PROTEIN KINASE2 orthologs regulated stem cell abundance in both the gametophyte of the moss P. patens (a single cell at the apex of the gametophore) and the sporophyte of Arabidopsis thaliana (several cells at the apex of the stem), although they thought that it was possible that independant evolution may have been involved. Interestingly, disruption of PIN, an auxin efflux facilitator, in e.g. Physcomitrium can lead to branching of the sporophyte (e.g. Bennett et al. 2014). Marchant and Walbot (2022) note that both P. patens and angiosperms have bHLH - basic helix-loop-helix - transcription factors, these being involved in tapetal development and fertility.
The early polysporangiophytes were often very small plants, as Edwards (1996) observed, they lived in a Lilliputian world. The sporophytes of the Silurian cooksonioids in particular are 1 mm or substantially less across and were probably dependent physiologically on the gametophyte (Rothwell 1995; Boyce 2008b; see also Edwards et al. 2014). However the ca 432 Ma Cooksonia barrandei from the early Silurian of the Czech Republic has a dichotomously-branched plant body over 5 cm long and 1-2.5 mm or more across and is likely to have been photosynthetic (Libertín et al. 2018), although Cooksonia-type fossils from Ireland around 427 Ma are notably smaller (see D. Edwards et al. 1983) and perhaps unable to photosynthesize. Conversely, some early gametophytes were quite elaborate structures, although different in morphology from the sporophytes, and they even had conducting tissue, stomata, a cuticle, and were apparently homoiohydric - i.e. they had many features now associated with the sporophyte (Remy & Haas 1991; D. Edwards 1993; Edwards et al. 1998; Edwards & Richardson 2004; Taylor et al. 2005; Gerrienne & Gonez 2011; Ligrone et al. 2012a; Cox 2018). (Cooksonia does have vascular tissue and stomata in the sporophyte, although apparently the former is not found in some species - see Edwards et al. 1992; Harrison 2017a, b and literature.) A stage in the evolution of vascular plants may involve an ancestor in which the generations were pretty much isomorphic, perhaps similar to some of the plants from the Lower Devonian Rhynie Chert of some 407 Ma, and they figure largely in attempts to understand the evolution of land plant life cycles (Niklas & Kutschera 2009, 2010). A modified homologous theory of alternation of generations in which the two generations were at one stage initially more or less similar can be envisaged, and here "the gametophyte body plan was substantially expressed in the sporophyte" (Kenrick 2017: p. 8; see also Libertín et al. 2018). The gametophytic generation became transformed - either ultimately much reduced, as in extant vascular plants, or remaining/becoming quite elaborate, as in extant bryophytes s.l. (Kenrick 2017; Libertín et al. 2018). The sequence may be as follows: Gametophyte only free living, sporophyte unbranched, dependent on the gametophyte → gametophyte only free living, sporophyte branched, dependent on the gametophyte → generations ± isomorphic, both free living → sporophyte dominant, gametophyte free-living → sporophyte dominant, gametophyte dependent on sporophyte (see also Tomescu et al. 2014; c.f. Qiu et al. 2012). Some early vascular plants had multicellular stem apices (Hueber 1992).
One thinks of DNA replication and sporogenesis during meiosis in embryophytes/land plants as being intimately connected, however, this may well not be the case in basal embryophytes. Details of chromosome formation, etc., in charophytes s.l. are poorly understood (Haig 2010, 2015), and Strother and Taylor (2018) also talk about the evolution of meiosis. Karyokinesis and cytokinessis were initially temporally decoupled, with rounds of DNA duplication occurring before nucleation and cytokinesis. A variety of spores, including trilete spores in permanent tetrads, may have been produced by protoembryophytic plants whose whole sporophyte was little more that these spores (see Graham et al. 2012, 2014: early movements of streptophytes on to land, Coleochaete the model). Cryptospores, initially largely defined by what they were not (Strother 1991; Volkova et al. 2016 for meiosis; esp. Strother & Taylor 2018), are known from the mid-Cambrian onwards, and cryptospores that are like bryophyte spores appear in the middle Ordovician, about 40 Ma later and ca 476 Ma (e.g. Gray 1993; Wellman 2004a; Rubinstein et al. 2010: 473-471 Ma spores from Argentina; R. C. Brown & Lemmon 2011a; Gerrienne et al. 2016 and references); for the evolution of the spore walls of land plants, see also Blackmore and Barnes (1987). Strother and Foster (2021) record a variety of spore types - tetrads, dyads, etc. - from Lower Ordovician deposits ca 480 Ma in N.W. Australia, and they suggested that "These pre-Darriwilian [mid-Ordovician] cryptospores are direct fossil evidence of charophyte [sic] adaptation to terrestrial setting." (ibid, p. 796). Such spores seem to have been produced by stem-group embryophytes, and there may be evidence that the various parts of meiosis were not as synchronized as they are in extant embryophytes (Strother et al. 2014).
Fragments of plant bodies of very early land plants appear in Late Ordovician rocks from Oman (Wellman et al. 2003). Edwards et al. (2014) discuss what they call cryptophytes, plants that cannot be confidently assigned to any extant group, from this period, and the cryptospores they produced, which were hilate monads, dyads, and permanent tetrads. Most of the earliest fossils seem to have been bryophytes of some sort, probably liverworts, vascular plants being thought of as being souped-up bryophytes (e.g. Kenrick 2000; Wellman et al. 2003; Rubinstein et al. 2010). Fossils very like Sphagnum and belonging to Sphagnopsida have been found in Ordovician rocks ca 455 Ma in North America (Graham et al. 2013, esp. Cardona-Correa et al. 2016; Turetsky et al. 2025). However, there are various tellings of the tale, and evidence (see below) currently suggests that extant vascular plants are sister to bryophytes sensu lato, not that vascular plants are derived from bryophytes. Edwards et al. (2021b) have recently described Eophytidae, a group that includes cryptospores and minute often dichotomizing axes with terminal sporangia that produce such spores.
EOPHYTIDAE Edwards, Morris, Axe, Duckett, Pressel & Kenrick / Cryptospores, cryptosporophytes
Gametophyte: rather like that of a bryophyte; sporophyte: plants probably 2≥ cm tall; axes 2≥ mm across, branching dichotomous (unbranched); leaves 0; axis surface plicate; water-conducting cells ?0, food-conducting cells +, cell walls minutely pitted, globules on surface, plasmodesmata not perforating walls; capsules terminal, extensions of axes, fusiform/infundibular/globose, usually valvate; stomata +, uncommon; spores≡cryptospores [permanent dyads or tetrads].
Edwards et al. (2021bt, see also Tomescu 2022) discuss the various morphologies that they would include - or exclude - in these eophytes. They emphasize that spore dyads with scars suggesting that they were initially tetrads, dyads that readily separated, and hilate monads (and other kinds of monads, including trilete monads) would not be included, nor would taxa with tracheids. Note that the spores of tetrads may be of different sizes, suggesting that cell formation had not been synchronized, and dyads may also show evidence of asynchronous sporogenesis (Stotler et al. 2014).
Salamon et al. (2018) describe possible polysporangiate fossils in Late Ordovician rocks ca 445 Ma from Poland. Spores there have irregular trilete markings, and hilate/trilete spores that do not remain as tetrads appear in the Early Silurian ca 443 Ma and may mark the origin of vascular plants (Steemans et al. 2009; Kenrick et al. 2012; Graham et al. 2012; Gerrienne et al. 2016 and references: trilete spores develop from tetrahedral tetrads), although not only vascular plants have such spores (Salamon et al. 2018 and references). A better understanding of early fossil polysporangiophytes, including the Early Devonian Rhynie Chert fossils, along with that of the eophytes, is particularly important; this includes understanding their food- amd water-conducting cells (Edwards et al. 2021b) and fungal associates (Strullu-Derrien et al. 2025 and references), and Edwards et al. (2003) had earlier documented the great diversity of cell wall morphologies in fossils from the slightly older Lochkovian Shropshire site 419-410 Ma.
For apomorphies of bryophyte groups and the early polysporangiophytes, see e.g. Garbary and Renzaglia (1998), Ligrone et al. (2012a). Gerrienne et al. (2016) characterize many embryophyte clades up to and including spermatophytes (but note that details of the topologies of both trees in their Fig. 2 differ from the topology of the relationships accepted here). Characters are placed on a land-plant tree in Z.-H. Chen et al. (2017: Fig. 1), but several are in odd places, c.f. also the tree topology. These, too, all have to be worked through.
There is a useful summary of the evolution of cell division in Buschmann and Zachgo (2016). R. C. Brown and Lemmon (e.g. 1990, 2007; Brown et al. 2010) and others have unravelled some of the complexity of both mitotic and meiotic cell divisions in land plants, particularly extreme in liverworts; for meiosis, see also Renzaglia and co-workers (e.g. Renzaglia et al. 2000a, Renzaglia & Garbary 2001). De Vries and Gould (2017) discuss the evolution of polyplastidy. Tracheophytes are consistently polyplastidic, although there is variation in bryophytes s.l. (monoplastidy in hornworts) - and movement of the minD and minE genes out of the chloroplast, movement that is thought to be necessary for the evolution of this condition, occurred in the basal part of the streptophyte clade where there is also much variation in chloroplast number (Chara braunii, at least, is monoplastidic - MacLeod et al. 2022). This may be correlated with patterns of microspore division (Rudall & Bateman 2007). For gene loss and monoplastidy in hornworts, see MacLeod et al. (2022).
Members of Klebsormidiales, Coleochaetales and Zygnematophyceae have pyrenoids, a biophysicasl carbon-concentrating mechanism (CCM), and the loss of pyrenoids may be an apomorphy for embryophytes (Cook 2004a, b), or they have been lost twice or more there. Thus there are suggestions that the presence of pyrenoids is ancestral in hornworts, and that they have since been lost here and elsewhere in land plants (see F.-W. Li et al. 2020). Since there are similarities in some genes expressed in the pyrenoid CCM in hornworts and in Chlamydomonas (C. reinhardtii is the main source of our information on pyrenoids - S. He et al. 2023), etc., this allows the possibility that the ability to produce pyrenoids is ancestral in embryophytes, but has subsequently been lost several times (Li et al. 2020; J. Zhang et al. 2020)... Overall, however, there seems to have been repeated gains and losses of pyrenoids in green algae, although overall they vastly preponderate in marine green algae, for example (He et al. 2023). Absence of pyrenoids is tentatively scored as an apomorphy for embryophytes above.
Hodges et al. (2012) survey the evolution of land plant cilia, in part with a focus on the ciliome and the role it continues to play in land plant gametes even after the cilia themselves are lost.
For studies on the early evolution of land plants with apomorphies at all levels and incorporating fossil members, see e.g. Kenrick and Crane (1997; also Kenrick et al. 2004; Doyle 2013; etc.). See Mishler and Churchill (1984, 1985) for important early morphological phylogenetic analyses, if now dated; Graham (1993) and Finet et al. (2010), both general; Stabenau & Winkler (2005), glycolate metabolism, from mitochondrial to cytoplasmic; Becker & Marin 2009, Domozych et al. 2010, Popper & Tuohy 2010, Sørensen et al. (2010), distinctive cell wall polysaccharide polymers evolved in charophyceans or before; Comparot-Moss and Denyer (2009), evolution of starch synthesis. For other possible apomorphies, see e.g. Goffinet (2000), Renzaglia et al. (2000), H. Schneider et al. (2002: much useful information), Rensing et al. (2007a), Johnson and Renzaglia (2009: embryology), Doyle (2013) and Bowman et al. (2017). Carothers and Duckett (1982: bryophytes) and Renzaglia and Garbary (2001) discussed the evolution of the male gametes in land plants in some detail. For information on bryophyte s.l. body form easily placed in a phylogenetic context, see Goffinet and Buck (2013). Nearly all these studies need to be re-evaluated and characters pegged to their appropriate nodes given the possibility that bryophytes s.l. are monophyletic and sister to vascular plants.
Ecology & Physiology. Although some of the problems of life on land faced by embryophytes may have been largely solved by their streptophyte ancestors (see above), such problems probably continued to shape the evolution of both embryophyte generations (e.g. Watkins et al. 2007a, b; Del Bem & Vincentz 2010; McAdam & Brodribb 2011; Willis & McElwain 2014; Graham et al. 2014; Proctor 2014; Raven & Edwards 2014; Robinson & Waterman 2014). Bateman et al. (1998), Hemsley and Poole (2004) and others discuss the physiology and ecology of early land plants. Below I treat fungi, lignins, etc. and stomata separately.
Prokaryotic blue-green and eukaryotic green algae may have formed algal mats, simple soil ecosystems developing even in the Proterozoic, but that was the extent of early land colonization by "plants" (references in Nelsen et al. 2019). Nelsen et al. (2019) suggested that there was no evidence that early embryophytes interacted with lichens or that lichens were the earliest colonizers of the land, the latter despite their position early in the succession in some extreme environments today. Ages of the fungal associates of lichens are younger than that of tracheophytes (estimated as Early Silurian, ca 426 Ma), as are the 95% HPD of their algal associates, although groups like Trentepohliales, for an example, are exceptions - but barely (Nelsen et al. 2019). Morphologically quite complex thalloid lichens (e.g. the photosynthesizing associates are in a layer under the cortex) are first known from Lower Devonian (ca 415 Ma) deposits in the Welsh Borderlands; the fungal associate is an ascomycete, and both blue-green and green are the "algal" associates (Honegger et al. 2013). Indeed, Honegger et al. (2013) did allow the possibility that lichens were early components of soil crust communities or grew on rock surfaces.
Mitchell et al. (2016) suggested that soil formation in current lichen-liverwort communities in Iceland with their associated mycorrhizal fungi, cyanobacteria, etc., might be an analogue for conditions in Rhynie chert communities; the soil formed was shallow, ca 1.5 cm deep, but it included smectite-type clay minerals, unlike the deeper (ca 3.5 cm) soils largely made up of mineral grains that developed in moss communities (the Yellowstone hot springs are also commonly thought of as being an analogue of the Rhynie community). Raven (2018) noted the role that glomalins, proteins in the walls of the glomeromycetes that are involved in arbuscular mycorrhizal symbioses, might play both in soil aggregation and carbon sequestration. There are similarities in the microbiomes of streptophytes and bryophytes, in particular, similar nitrogen-fixing and methanotrophic organisms occur in both, and this has implications both for the evolution of the atmosphere and for the organisms involved (Knack et al. 2015).
Fungi. The common ancestor of extant land plants is likely to have had genes that are now involved in the establishment of mycorrhizal associations, for example, such genes in liverworts and hornworts are able to rescue mutants in Medicago truncatula so enabling mycorrhiza formation in the latter (B. Wang et al. 2009: tree rooted on liverworts, more basal streptophytes not included). At least three genes involved in the establishment of mycorrhizae are likely to have been found in this common ancestor, and although the DM13 gene in particular seems to have other functions in many mosses, which are very largely non-mycorrhizal, this gene from hornworts can rescue the effect of dmi3 mutants in Medicago truncatula (B. Wang et al. 2010, commentary by Bonfante & Selosse 2010; see also Sgroi & Paszkowski 2020; F.-W. Li et al. 2020; Sgroi et al. 2024). Strigolactones are involved in the establishment of arbuscular mycorrhizal (AM) and some ectomycorrhizal (ECM) associations and in bacterial associations such as those involving the nitrogen fixing Rhizobium and Frankia, that is, they are part of the common signaling pathway (CSP) in embryophytes, and in Marchantia paleacea they are involved in the establishment of AM associations, but they have not evolved any hormonal functions (Kodama et al. 2022; Vernié et al. 2025). Strigolactones are known from streptophytes such as Charales and there they are implicated in the control of rhizoid elongation (Delaux et al. 2012; Martin et al. 2017), and a number of other genes involved in mycorrhizal establishment have a similar ancestry, although hardly surprisingly there have been elaborations in embryophytes (Delaux et al. 2015; see also Field & Pressel 2018). A karrikin receptor complex is involved in the initial interaction between plant and fungus (karrikin and strigolactone both have a butenolide element), and this complex is found widely throughout embryophytes (Delaux et al. 2012; Gutjahr et al. 2015). However, the relationships between fungi, strigolactones and plant in bryophytes s.l. may be somewhat different from those in vascular plants, especially spermatophytes, and not simply because the main plant body in bryophytes is a gametophyte and in vascular plants it is a sporophyte. Species of both mosses and liverworts are known to be associated wih strigolactones (J. Clark & Bennett 2023: Fig. 1: remember, among bryophytes s.l. it is liverworts that are much more commonly associated with fungi, both AM and other types). However, it has been suggested that mosses use their strigolactones as hormonal signals (they also function as hormones in vascular plants), while the strigolactones made by liverworts are perceived only by fungi (T. A. Bennett, pers. comm. 11.viii.2024; see Kodama et al. 2022). Vernié et al. (2025) discuss the three main genes of the CSP, SYMRK, CCaMK, and CYCLOPS that are found in the hepatic Marchantia paleacea; in neither that liverwort nor in flowering plants did AM symbioses develop if any of these genes was knocked out.
There are several scenarios for the origin and evolution of mycorrhizal associations (Field & Pressel 2018 for a summary) depending i.a. on the ages assigned to the various protagonists. For more discussion, and also suggested ages of this association, see also elsewhere. Field et al. (2015c, 2019) looked at the changing interactions between extant liverworts (as proxies for early land plants) and both glomeromycotes and mucoromycotes in the context of experiments that simulated the almost four-fold decrease in atmospheric CO2 concentrations from the Paleozoic to the present. Interestingly, if mucoromycotina were the only associates it is notable that the efficiency of acquisition of both phosphorus (from the organic nutrients supplied) and nitrogen by the liverwort from the fungus slightly inreased with decreasing CO2 concentrations, the amount of carbon going to the fungus decreased, and overall plant growth increased. If glomeromycotina were the only associates the efficiency of P transfer to the plant increased at higher atmospheric [CO2] (see also Rimington et al. 2016). However, when the liverwort was associated with both kinds of fungi, the efficiency of acquisition of both N and P by the liverwort from the fungus decreased with decreasing [CO2], although the plants benefited from both N and P transfer, although at an overall high metabolic cost.
It has been suggested that symbiotic AM—plant associations are basal in land plants. Sgroi et al. (2024; see also Sgroi & Paszkowski 2020) recovered ARBUSCULAR RECEPTOR-LIKE KINASE, found throughout AM embryophytes, along with some 56 other genes that were all part of a core symbiotic genetic program (involved in things like hormonal signaling pathways, aspects of symbiont nutrient metabolism, etc.), in the AM liverwrt Marchantia paleacea and AM angiosperms - even giberellin-like diterpenoids may be involved (bryophytes lack giberellin). Both glomeromycotes and mucoromycotes are associated with liverworts (Read et al. 2000; Duckett et al. 2006b; Pressel et al. 2010; Bidartondo et al. 2011; Field et al. 2015c, d; Weiß et al. 2016). Bidartondo et al. (2011; see also Pressel et al. 2010) found that Endogone-like fungi (Mucoromycotina) formed ECM associations with Treubia, Haplomitrium, some hornworts, etc., and surmised that this might represent the original land plant-fungus association (see also Rimington et al. 2014; Field et al. 2015d); Chang et al. (2018) dated the formation of this association to ca 420 Ma, mid-late Silurian. Both glomeromycotes and mucoromycotes might have been involved (Field et al. 2015c, d), or just glomeromycotes - mostly members of "basal" clades, far fewer Glomeraceae - may have been the early associates (Rimington et al. 2018: morphology of AM fungi various, but obviously there is little possibility of hyphae growing in intercellular spaces in these bryophytes - see also F. A. Smith & Smith 1997 for variation in AM types), and similar associations were also found in hornworts. Interestingly, such associations tended to be specific in liverworts, but not in hornworts (Rimington et al. 2018). For more literature on the possibility that AM Glomeromycota were associated with the earliest land plants, see van der Heijden et al. (2015) - the recently-described Archaeosporites rhyniensis, from the Rhynie chert, would have been placed in the extant glomeromycete genus Archaeosporium using morphology alone, but molecular data are needed to confirm relationships here (Harper et al. 2020). A diversity of glomeromycetes have been found in the Rhynie fossils, including taxa from several major glomeromycete lineages (Harper et al. 2020 and references), and Strullu-Derrien et al. (2025) recently described yet another glomeromycete from the Windyfield Chert, of about the same age. Brundrett et al. (2018) and Walker et al. (2018) also discuss mycorrhizae and early land plants.
However, both the nature and patterns of association of fungi even with extant bryophytes s.l. are complex. Recent work emphasises the importance of the mucoromycote fine root endophytes, previously confused with glomeromycotes. Although Feijen et al. (2017; see also Schreiber et al. 2022) suggest that an association with Mucoromycotina might be the basic condition for extant land plants, this may depend both on the method of ancestral state reconstruction used (Bayesian) and patterns of relationships - here there was a tritomy made up of vascular plants, [mosses + liverworts], and hornworts, and in the latter two of these non-mycorrhizal groups were sister to clades in which no evidence was given that Mucoromycotina were basal (Feijen et al. 2017). Even if Mucoromycotina were the first fungal associates of liverworts, the establishment of plant-fungus relationships there may be independent of those in other land plants. Both Glomeromycota and, perhaps, Mucoromycotina, have been associated with the ca 407 Ma Horneophyton ligneri from the Rhynie Chert (Strullu-Derrien et al. 2014b, c.f. Harper et al. 2020), and such co-infections still occur (Field & Pressel 2018). ECM associations between liverworts and basidiomycetes (especially Serendipitaceae) and ascomycetes are likely to be secondary (Bidartondo & Duckett 2009; Weiß et al. 2016; Field et al. 2015d). There are further complications. In angiosperms with Paris-type AM there is movement of nutrients from one angiosperm to another (see Giesemann et al. (2019, 2021); such fungi have intracellular hyphal coils and sparsely-branched intercellular hyphae. On the other hand, in Arum-type AM there are intracellular hyphal coils and at most few intercellular hyphae. Since bryophytes s.l. tend to lack intracellular spaces, by the same token they will lack Arum-type AM. In addition, fungi can move to a liverwort, for example, from a tracheophyte (Ligrone et al. 2007) or in the opposite direction (Pressel et al. 2010: see also Bidartondo & Duckett 2009). For the (mostly ascomycetous, but some Sebacinales-Serendipitaceae) endophytic fungi also to be found in mosses and liverworts, see e.g. Stenroos et al. (2010), Pressel et al. (2010) and Weiß et al. (2016), and in particular below.
To summarize: Mycorrhizal associations of embryophytes with fungi, whether glomeromycotes or perhaps Mucoromycotina, may have made it possible for early plants to establish themselves on land (e.g. B. Wang et al. 2010; Selosse et al. 2015; Feijen et al. 2017; Kamel et al. 2018). Mycorrhizal associations are known from some Rhynie Chert plants, but, as mentioned, they are common only in hepatics among extant bryophytes. Pirozynski and Malloch (1975: p. 162) emphasized the great importance of a long and close association between fungi and early land plants, and indeed throughout land plant history, noting that "land plants never had any independence, for if they had, they could never have colonized the land".
Phenylpropanoids, Lignins, etc.. Many changes evident in land plants are connected with the development of a distinctive and complex phenolic metabolism, the phenylpropanoid pathway (Vogt 2010; de Vries et al. 2017b; see also Berland et al. 2019) or phenolic network (Renault et al. 2017). The products of this network include lignins, central to support and water-conducting facilities of vascular plants in particular, sporopollenin, integral to spore production across land plants in general, flavonoids, etc..
Thinking about lignin in particular, phenylalanine ammonia lyase (PAL) is the first step in phenylpropanoid pathway, and Emiliani et al. (2009) suggested that embryophytes acquired this gene via lateral transfer from a bacterium; they found that it was also present in fungi, but absent from Nostoc. The second step in the synthesis of phenolic compounds is mediated by the cytochrome P450, the CYP73 gene, a cinnamate hydroxylase, and this is absent in streptophytes but duplicated in seed plants/?angiosperms in particular (Renault et al. 2017); for cytochrome P450 and the early steps of its evolution in charophytes - the ancient cytochrome b5 protein CB5D was coopted - see X. Zhao et al. (2024). The evolution of the cinnamyl/sinapyl alcoholase gene family involved in the synthesis of the hydroxycinnamyl alcohol monomers that constitute lignins, particularly guaiacyl (G) lignins, can perhaps be pegged to the vascular plant node (Guo et al. 2010; c.f. Gómez-Ros et al. 2006a; Zu et al. 2009). When these monomers are in lignin they are called monolignols, and the nomenclature is as follows: p-coumaryl [hydroxycinnamyl alcohol monomer]/p-hydroxyphenyl [monolignol] (H-lignin), coniferyl/guaiacyl (G-lignin), and sinapyl/syringyl (S-lignin) units, and Maüle staining targets the S-units; these three units ultimately constitute the main kinds of lignin. Overall S and particularly G units preponderate, less so the H units, but S lignin with its lower degradability is commonest in angiosperms (P. J. Harris 2005; Novo-Uzal et al. 2012; Ayuso-Fernández et al. 2019). Looking at the general synthetic pathway, which is now quite well understood, we see that p-coumaroyl CoA is the last step common to all lignins, while coniferyl aldehyde is the last step common to S- and G-lignins (e.g. Peter & Neale 2004; see also Lewis & Yamamoto 1990; Humphreys & Chapple 2002; Boerjan et al. 2003; Weng & Chapple 2010; Vanholme et al. 2010; Novo-Uzal et al. 2012; Ayuso-Fernández et al. 2019).
Delwiche et al. (1989) recorded lignin-like compounds (positive Mäule reaction, test for syringyl lignins) from Coleochaete and Ligrone et al. (2008) lignin-related epitopes from Nitella and the cell walls of various bryophytes, although the epitopes were not tissue-specific, unlike lignin in tracheophytes (see below, also Sørensen et al. 2011), so exactly where phenylalanine ammonia-lyase (PAL) moved into the streptophyte clade, if it did, is uncertain. L-phenylalanine is the major substrate for lignin formation, being converted to trans-cinnamic acid by PAL, and hence to diverse phenylpropanoids, including lignin monomers (X. Zhang & Liu 2015). Some of these lignin-related compounds are synthesised by other streptophytes, including by Mesostigma viride, but not by clades immediately above it on the tree (de Vries et al. 2017b). Much of the pathway by which lignin units are synthesized in vascular plants is also found in mosses (Gómez Ros et al. 2006; Z. Xu et al. 2009, see also Guo et al. 2010), but in some cases pathways in mosses and liverworts may differ (see below). Marchantia, which lacks vascular tissue, is quite rich in lignin monomers (Espiñeira et al. 2011; Ligrone et al. 2006). Physcomitrium has all the enzymes needed to synthesize at least the first two lignin groups above (Labeeuw et al. 2015), even if "real lignin" may not be found there (Vanholme et al. 2010). Certainly bryophytes produce a variety of compounds that are associated with the synthesis of lignins, but other than a possible lignin in which G units (see below) predominate that has been found in Marchantia, full-blown lignins seem not to occur, although other phenolics are to be found in the cell wall (Erickson & Miksche 1974; Novo-Uzal et al. 2012). Some xylans may be restricted to the sporophyte (Eeckhout et al. 2014).
As vascular plants became larger the need for water transport within the plant developed, and here lignin, being hydrophobic and impermeable to water, was important; another early function of lignin may have been in keeping tracheary elements open. Support also became important, and here lignin cross-linked carbohydrate polymers in the cell wall and imparted rigidity to them, and the support of the plant body changed from being hydrostatic to lignin-based. These needs thus became apparent as plant size increased, and in both lignin had a central role to play (e.g. Peter & Neale 2004). The formation of borate cross-linked rhamnogalactan II, at most sparse in bryophytes but present throughout extant vascular plants where it cross-links primary cell wall pectins, seems to have been an important early step in the development of lignified cell walls, and such rhamnogalactans are found in both fern sporophytes and gametophytes (Matsunaga et al. 2004). Initially it may have been lignin in the sclerenchyma, the stereome, towards the outside of the stem or petiole of the sporophyte and surrounding the vascular tissue, that may have provided support, not lignin in the vascular tissue itself, lignin in the vascular tissue rather facilitating water movement (Friedman & Cook 2000; Boyce et al. 2003; see also Espiñeira et al. 2010; Novo-Uzal et al. 2012). Indeed, lignins may initially have helped provide protection against pathogens and UV light (Raven 1984; Novo-Uzal et al. 2012). In genera like Aglaophyton, Rhynia and Asteroxylon lignin appears to have been deposited in the cortex only; the protracheophyte Aglaophyton has no thickenings on what appear to be its water-conducting cells, the rhyniophyte Rhynia has S-type thickenings, but these appear not to be lignified, while the thickenings on the walls of the water-conducting cells of the tracheophyte Asteroxylon appear to have been lignified (Boyce et al 2003; Novo-Uzal et al. 2012; see also Edwards et al. 1998). The tracheids in extant monilophytes are relatively thin-walled and they can be quite wide, which may improve their ability to transport water, but this would not be to the detriment of any plant support that they provide. Indeed, the initial function of secondary growth may have been to improve water transport in the small Early Devonian land plants in which it has first been found, only later did secondary tissue become involved in support, so helping enable the evolution of large sporophytes (e.g. Gerrienne et al. 2011; Strullu-Derrien et al. 2014a; Tomescu & Groover 2018; Decombeix et al. 2019). However, the amount and kind of secondary tissue produced by early arboreal vascular plants varied. Lycophytes were sometimes very large trees, but produced little in the way of secondary vascular tissue; there support may have been provided by the thick cortex and the secondary tissue produced by the periderm (Groover 2016). For more on fossils of the Rhynie Chert, see Kerp and Hass (2009).
However, discussing a generic "lignin" obscures the interesting complexities of patterns of deposition and decomposition of different types of lignin in vascular plants. There are four related sets of issues here: What are the major kinds of lignins, where in the phylogeny and where in the plant are these different types of lignin to be found, and what breaks these lignins down? S-lignin, made up of syringyl units (gives a positive = red Mäule reaction), has been found in some liverworts and is scattered elsewhere in vascular plants, especially Selaginella, and it is found in (practically) all angiosperms (e.g. Gibbs 1958). Both its presence and distribution in the plant is of interest (Z. Xu et al. 2008; Li & Chapple 2010; Espiñeira et al. 2010; see also Gómez Ros et al. 2006). But is not that straightforward to determine what is meant by "presence". Thus Gómez Ros et al. (2006) note that peroxidases of gymnosperms that have no syringyl lignin nevertheless may have structural motifs of peroxidases that can oxidize sinapyl alcohol, and they think that such peroxidases antedate crown-group vascular plants, and may be an apomorphy for them; they also note that similar perodixases are also to be found in at least some bryophytes s.l.. Syringyl lignins of one sort or another are also reported from some Huperzia and particularly some Isoëtes (Espiñeira et al. 2010; see Novo-Uzal et al. 2012), and they are also found in a few monilophytes, e.g. Equisetum sylvatica, Pteridium, etc., cycads like Stangeria eriopus, etc., Cupressales (Tetraclinis articulata), all three genera of Gnetales, and so on (Gibbs 1958; Gross 1989; Gómez Ros et al. 2006). S-lignin can also be produced, or be made to be produced, in other gymnosperms. Thus S-lignin produced by Ephedra viridis had a S:G ratio similar to that of the angiosperms examined (Gómez Ros et al. 2007). Although S-lignin is synthesized in suspension cell cultures of Ginkgo biloba, it is not a component of Ginkgo wood (Novo-Uzal et al. 2014 and references), and similarly, tracheary element cultures in Pinus radiata can be transformed to produce S-lignins (A. Wagner et al. 2015). This leads to the idea that the ability to produce S-lignin evolved pretty basally in the land-plant tree but was subsequently lost several times (e.g. Novo-Uzal et al. 2012; Espiñeira et al. 2010). However, details of the synthetic pathway for syringyl lignin are quite different in Selaginella, at least, and angiosperms (e.g. Weng et al. 2008, 2011; Weng & Chapple 2010; also Harholt et al. 2016) which suggests independent gains. Also, although Gnetales and angiosperms have both S-lignins and vessels, Jin et al. (2007) and others have noted that not all angiosperms with S-type lignin have vessels, and Gibbs (1958) observed that protoxylem did not stain for the Mäule reaction - no S-lignin, no fibres also? An additional complication is that in Arabidopsis thaliana the requirements for lignin polymerization varies according to cell type - whether laccases or peroxidases are needed (Rojas-Murcia et al. 2020). For further discussion about the distribution of lignin precursors, see Labeeuw et al. (2015) and Chao et al. (2017).
In ferns like Pteridium aquilinum (but not some other ferns) and quillworts like Isoetes fluitans (but not I. hystrix) that have S-type lignins there is also an outer lignified sheath where S-lignin is deposited as well as lignified vascular tissue with G-type lignin (Espiñeira et al. 2010). Overall lignin type in ferns in particular is variable, but G-type lignin is common. Gibbs (1958) also noted taxa with positive Mäule reactions in Zamiaceae (mostly in the stomata), Podocarpaceae (mostly in fibres or sclereids), Cupressaceae (Tetraclinis), etc..
G-lignins preponderate in the xylem of Selaginella, while S-lignin is found in more peripheral epidermal and cortical regions (Novo-Uzal et al. 2012: see Fig. 6). It was mentioned above that support for the trunks of fossil lycophytes may well have come from tissues in the periphery of those trunks, i.e. in a similar position to the S-lignified tissue of Selaginella, but the lignification of these tissues has been little discussed. Interestingly, aspects of lignin distribution in Selaginella and angiosperms is rather similar. In angiosperms S-lignin tends to be found in vascular fibres/libriform fibres and G-lignin in vessels/tracheids, both tissues providing support, even if both occur in vascular tissue - of course only the latter are involved in water transport (Anderson et al. 1973; Gross 1989; Yoshinaga et al. 1997; Vasquez-Cooz & Meyer 2006; Novo-Uzal et al. 2012). However, there has been no fundamental change in the tissue types in which the different types of lignin are laid down - G-type lignin is primarily associated with conducting tissue, S-type lignin with support tissue (e.g. Rowe et al. 2004; Pitterman et al. 2011; Novo-Uzal et al. 2012; Klepsch et al. 2015; c.f. in part Friedman & Cook 2000; Espiñeira et al. 2010). Gibbs (1958) notes a number of cases where S-type lignin is not in the vascular tissue per se in angiosperms - for instance, it is quite often in the fibrous sheath in monocots (and it is also may be outside the vascular tissue in those podocarps and cycads that Gibbs records as having S-lignin). Of course, where in the stem the strengthening tissue is located does change, and this may well have biomechanical implications. In gymnosperms thick-walled tracheids with G-type lignin provide both support for the plant and transport of water. The amount of lignin deposited may also vary; overall lignin content in angiosperms is lower than that in gymnosperms (Jin et al. 2007); there is less S-lignin in monocots, particularly in aquatic monocots (but also in some aquatic core eudicots), and less lignin in herbaceous eudicots than in other angiosperms (Gibbs 1958; Gross 1989). Yoshinaga et al. (1997) examined fine-scale variation of S-lignin deposition over the course of a single annual ring in Quercus mongolica and found that the proportion of G units decreased and that of syringyl units increased in vessels over the course of the season (noted also in other woods), furthermore, S-lignins increased in the sequence vessels→vasicentric tracheid→fibre tracheids→fibres, more or less paralleling the distance of the cells from the vessels - again, the association of S-lignin with fibres. However, J. S. Kim and Daniel (2016), looking at Q. robur did not find such a tidy story. They found higher G-type lignin in early-wood vessels, higher S-type lignin in late-wood vessels and in parenchyma, and differences in hemicelluloses, e.g. xylans did not have a water-conducting role and were present in similar amounts in early and late woods, while mannans were higher in late woods, especially in libriform fibres.
Blaschek et al. (2022: Arabidopsis thaliana, see also Meents et al. 2018) emphasized the role that laccase paralogs, embedded in the polysaccharide cell wall matrix, played in lignin deposition; combinations of these paralogs had specific activities and they controlled the polymerization of lignin units in different cell wall layers of the various xylem cell types so supporting their different functions - down to plant biomechanics and coping with environmental changes (Ménard et al. 2022). Phenylpropanoid biosynthesis genes were not involved in this part of the process, and the individual lignin units can move freely through the apoplast (Blaschek et al. 2022). Furthermore, Ménard et al. (2022) and other have noted that although tracheary elements become functional only when they die, their cell walls may be modified after their death, lignin continuing to be deposited, furthermore, variation in the composition of the lignin affects both its mechanical and water-transmitting properties. Thus post-mortem lignification may increase the resistance of tracheary elements to the negative pressures involved in water conduction, which is also affected by the surrounding unlignified xylem parenchyma and lignified xylary fibers (Ménard et al. 2022). Finally, a diversity of compounds can get added to lignins and they may affect their properties, although little is know about this (see e.g. Mottier et al. 2022). Tricin is a lignin monomer, but, unusually, it is not produced by the monolignol biosynthetic pathway; it is also known from Cyperaceae, for instance, a few core eudicots and lycophytes (Lam et al. 2021). Irrespective of lignin type, a massively lignified plant body cannot readily bend, and the reaction wood commonly developed in seed plant trunks and branches is one response to this problem, as is discussed elsewhere. Overall, it is clear that combining variation in cell type with the amount and kind of lignin deposition, the overall process of lignification of cell walls is a complex and still poorly understood process.
And finally there is the issue of lignin breakdown, the different types of lignins being susceptible to different types of ligninolytic enzymes (Ayuso-Fernández et al. 2019). Lignin in conifer wood is very largely G-type, and is attacked by brown-rot fungi (a variety of fungi, including basidiomycetes and some ascomycetes) with their manganese peroxidases that can break down phenolic compounds in these lignins; they digest cellulose and hemicellulose but not so much lignin itself, although it may get demethylated. As mentioned, lignin from pteridophytes, other gymnosperms, etc., often includes appreciable amounts of both S[syringyl]-type lignins, more complex, with a lower phenolic content and less degradable, and H-type lignins, with p-parahydroxyphenyl units, although G-type lignins are still normally the major component. Lignin peroxidases with catalytic tryptophan and latterly versatile peroxidases that combine properties of the other enzyme classes, are also to be found in white-rot fungi such as Polyporales and other agarics, fungi that can decompose angiosperm wood, and here cellulose, hemicellulose and lignins are all broken down leaving a whitish residue, hence "white rot fungi" (Ayuso-Fernández et al. 2019).
Sporopollenin. One of the major changes, or better, complex of changes, that facilitated the spread of land plants may have been the evolution of sporopollenin-covered spores. Sporopollenin is known from several streptophytes including Coleochaete (Delwiche et al. 1989), and there it is associated with the inner wall of the zygote (Gray 1993; Blackmore & Barnes 1987; see also Wallace et al. 2011; de Vries & Archibald 2018, etc.). In land plants it is associated with the walls of the haploid spores, perhaps by a complex process involving precocious initiation of cytokinesis (hence the often quadrilobed, quadripolar microtubule system), acceleration of meiosis, delay in wall deposition, etc., a mixture of heterochrony and heterotopy (R. C. Brown & Lemmon 2011a; Strother & Taylor 2018). Sporopollenin composition is remarkably stable in vascular plants, at least, the sporopollenin of the walls of lycophyte fossil spores ca 310 Ma was remarkably similar to that of extant lycophytes, ferns, angiosperms, etc. (Fraser et al. 2012), and it is similar to that in the walls of the embryos in some Charales. The phenylpropanoid pathway is involved in sporopollenin synthesis and it helps provide protection against UV light for the spores (J.-S. Xue et al. 2020). However, the sporopollenin of some mosses, at least, seems to be different, the spores there not being degraded by thioacidolysis, unlike the spores of vascular plants (Xue et al. 2020). The story is complicated, however, Steemans et al. (2010) finding that the walls of two species Of cryptospore tetrads they examined were made up of sporopollenin, as in trilete spores. Differences between the sporopollenin of conifers and lycopods, extant and fossil taxa of both being examined (Hemsley 1995) seem to be connected to the differing light regimes under which the plants involved grew (Fraser et al. 2012). Wellman (2004b) noted that sporopollenin deposition was associated with white line-centred lamellae, and such lamellae (or laminae) are to be found in cryptospores. Mature spores that lack these laminae - i.e. the common condition in extant land plants - are in plants that have active tapetal tissue surrounding the developing spores (Strother & Taylor 2018). Wallace et al. (2011) found homologues of just about all genes involved in pollen wall development in Arabidopsis and in Selaginella, and also in the moss Physcomitrium, emphasising the fundamental similarity of pollen and spores in extant embryophytes. F.-S. Li et al. (2018) was able to work out the structure of Pinus rigida sporopollenin using thioacidolysis techniques.
Working out the composition of sporopollenin has been difficult enough, but there are other formidable problems when thinking about the development of spore/pollen walls, well known for their complex structure and composition that quite often have systematic implications - for some literature, see elsewhere.
Flavonoids, which are antioxidants, pigments, etc., are also involved in UV screens (Renault et al. 2017). Here naringenin helps protect against UV-B absorbtion, and it is found in the spores of all land plants, although in relatively low amounts outside seed plants (J.-S. Xue et al. 2023). Chromosomes can be damaged by UV-B radiation, and it has even been suggested that early land plants had holocentric chromosomes, less susceptible to damage than monocentric chromosomes because fragmented chromosomes in the former might still be able to move to the poles. Zygnematophyceae appear to be the only streptophyte clade below the embryophytes with holocentric chromosomes (Zedek et al. 2020), furthermore, extant basal embryophytes do not have such chromosomes, so this seems unlikely. A variety of flavonols scavenge for reactive oxygen species in the pollen, but apparently only in euphyllophytes, and these are stored in an inactive glycosylated form in vacuoles (Xue et al. 2023). There may not have been flavonoid biosynthesis in the aquatic progenitor of embryophytes, although flavonoids are not found in hornworts, either (Xue et al. 2023), and although flavonoids are found in some other algal s.l. groups (Yonekura-Sakakibara et al. 2019: liquid chromatography/tandem mass spectography approach) it is unclear how these occurrences relate to flavonoids and embryophyte evolution.
Stomata, And The Role(s) They Play. Water transport in vascular plants is part of the whole photosynthetic process, open stomata allowing CO2 to enter the mesophyll, where it is fixed by photosynthesis, and although at the same time water from the mesophyll is lost through those same stomata it is replaced by water from the transpiration stream moving through the vascular tissue. However, stomata are also to be found on the sporophytes of most other land plants, but not on those of liverworts, some hornworts, for which, see e.g. F.-W. Li et al. (2020) and many mosses (Renzaglia et al. 2020b). They are unknown from gametophytes of extant embryophytes, although in the Rhynie Chert flora they are found on the gametophytes of Lyonophyton and Langiophyton - the corresponding sporophytes are Aglaophyton and Nothia (Edwards et al. 1998).
The function of stomata in extant vascular plants seems to be clearcut, even if details of stomatal control can be complex: Stomata are involved in gas exchange. However, this may be a derived feature that developed somewhere along the tracheophyte stem, hence the placement of stomata on the tree. However, little is known about the functions of stomata in early vascular plants, or in mosses. Stomata in fossils of early vascular plants, including Zosterophyllum, Rhynia and Astroxylon, are often large, being in the 50-140 μm length range, while in some Silurian plants the outer periclinal wall may be incomplete and in Devonian examples it is often thin, furthermore, stomata are not always found on parts of the plant where they are expected to be, i.e., in areas where there is likely to be much chlorophyllous tissue (Edwards et al. 1998; for stomatal pore length, see also Beerling & Woodward 1997). The discussion of the function of these stomata - some of the plants had vascular tissue of one sort or another - focuses on photosynthesis, but also on generation of a transpiration stream so improving the supply of nutrients to the sporangium (e.g. Edwards et al. 1998; Haig 2013a).
The earliest polysporangiophytes known from the Rhynie Chert lacked leaves, and the distribution of the stomata on the stems varied; they were sometimes associated with rhizoids. The stomata are often quite large, ranging from around 60-180µm long; they are anomocytic, not very dense, and there are often/usually? substomatal cavities of some sort, although they are not very extensive. Edwards et al. (1998) found the precise functions of these early stomata to be unclear, partly because the distribution of chlorophyllous tissue in the stem was uncertain and the atmospheric CO2 concentration was very much higher than it is now, but they may have enhanced water flow and hence the delivery of nutrients throughout the plant, they may have been involved in CO2 uptake and so in photosynthesis (see also Haig 2013a). Assuming the function of the stomata on Rhynie gametophytes (see above) was the same as that in the sporophyte, sporangial drying cannot be their basic function. However, Renzaglia et al. (2017) note that the Lower Devonian fossils Sporogonites and Tortilicaulis (relationships uncertain, perhaps polysporangiophytes) seem to have collapsing stomata similar to those in hornworts where such stomata are involved in the drying out of the capsule and spore discharge. If sporangial drying is indeed the original function of stomata (McAdam & Brodribb 2012a, b), then the central role that stomata now play in photosynthesis in vascular plants becomes a spectacular example of an exaption. For early stomata, see also Edwards and Axe (1992).
When bryophytes were considered to be paraphyletic, one hypothesis was that hornworts were most likely to be sister to vascular plants. Indeed, hornworts, with their relatively long-lived stomata-bearing sporophyte, could be considered an "intermediate" stage in the evolution both of stomata and of the sporophytic plant body in general. Other bryophytes often lack stomata and their sporophytes are short-lived, while sporophytes in the vascular plants are longer-lived and all (apart from a couple of aquatics and parasites) have stomata. However, given that bryophyte monophyly is likely, relationships being [hornworts [mosses + liverworts], if hornworts are still to be considered intermediates, they are perhaps an intermediate stage in the loss of stomata, and conducting tissue has also been lost (Cox 2018) - the world has been turned upside down. Indeed, stomatal differentiation in hornworts and vascular plants shows many points of similarity, similarities reaching back to before embryophyte origin, although recognizable stomata may well have had a common origin in embryophytes (B. J. Harris et al. 2020; F.-W. Li et al. 2020, but c.f. Kubásek et al. 2021). However, we need to understand more about stomata in stem-group vascular plants, since even if "presence of stomata" is an apomorphy for all extant embryophytes (the old Stomatophytes included all extant vascular plants except liverworts), we are not necessarily any closer to understanding the original ecological/functional context for their evolution (Puttick et al. 2018); they may not originally have been involved in photosynthesis. It certainly does seem unlikely that stomata were involved in photosynthesis when they first evolved in the same way that stomata of vascular plants are today (c.f. in part Harris et al. 2020).
Thinking about extant bryophytes s.l. in particular, there are not very many stomata on the capsules of either mosses or hornworts; they are found at the base of the capsule in mosses and along the capsule in hornworts. They develop directly from epidermal cells, but meristemoids are not involved, although they are in most vascular plants (references in Doll et al. 2021). Stomata in bryophytes may not be involved in photosynthetic gas exchange, the plants sometimes lacking substomatal cavities, Sphagnum being an example, rather, they may facilitate the drying out of the capsule and hence aid in spore dispersal (Duckett et al. 2009; Pressel et al. 2011; Merced & Renzaglia 2013; Merced 2015; Chater et al. 2016, 2017; Brodribb & McAdam 2017; Merced & Renzaglia 2018). Renzaglia et al. (2017) and Pressel et al. (2018) discuss the role that stomata in hornworts play in the drying out of the capsule, etc., noting that the outer periclinal walls of the guard cells are pectic and very thin. The stomata, larger than epidermal cells here (they are smaller, both in mosses and vascular plants) open just once as the guard cells become more turgid (as with stomata in general, they have chloroplasts) and wall thickenings are laid down, and then they die and collapse, remaining open and allowing the drying of the contents of the sporangium (the intercellular spaces may be filled with liquid) (Merced & Renzaglia 2018). The stomata - sometimes called pseudostomata - of Sphagnum may also be involved in the drying out of the sporangium, opening as they lose turgor (Duckett et al. 2009). Although the guard cells to be found elsewhere in mosses are thickened and do not normally collapse, a function for stomata in the maturation of the capsule has been suggested in Physcomitrium patens, while stomata are probably involved in the drying and dehiscence of the capsule there and in other mosses (e.g. Renzaglia et al. 2017; Moriya et al. 2022/2023). Certainly, any movement of such stomata is one way - if they open, they will not close again (McAdam et al. 2021). Interestingly, under humid conditions the stomata ofP. patens remain occluded by mucilage (Caine et al. 2020) - one wonders about capsule dehiscence in such situations. (It should not be forgotten that loss of water through stomata may be an important part of anther dehiscence in Arabidopsis - see Wei et al. 2018.) Kubásek et al. (2021) discuss the role of moss stomata in the sporophyte physiology, noting that the absence of intercellular spaces in some species probably limited any role they might play in CO2 diffusion/uptake, but this was not necessarily true of all species, although they concluded by saying "the bulk of the biomass of moss sporophytes derives from the parental gametophyte through the placenta and that C gained via the stomata is less significant" (ibid., pp. 1825-1826). The stomata showed no response to light or CO2 concentrations (Kubásek et al. 2021). Indeed, given that stomata have been lost over 60 times in mosses alone (Renzaglia et al. 2020b), this questions any fundamental importance of the functions that they might have (see also Edwards et al. 2021b).
Important elements of stress tolerance and chloroplast retrograde signalling may be similar in both streptophyte algae and in stomatal regulation in embryophytes in general, "suggesting that intricate cellular communication networks were already in place to prime stomatal regulation" (Chao et al. 2019, p. 5019; see also Schreiber et al. 2022). Indeed, C. Zhao et al. (2019) saw the adoption of such signalling in Sphagnum and its involvement in stomatal behaviour as an example of an underlying commonality in stomatal function. However, comments by McAdam et al. (2021) suggest that this is unlikely, and they summarize data including that in the preceding paragraph - and the frequent loss of stomata in bryophytes and their almost universal prevalence in the sporophytes of vascular plants might lead one to think that stomata vary in function across land plants.
Stomata in bryophytes and vascular plants may appear to be rather different, but similar genes are involved in stomatal development in both vascular plants and at least some mosses (see also O'Donoghue et al. 2013; Vatén & Bergmann 2012; Sakakibara 2016; Chater et al. 2013, 2016, 2017; Merced & Renzaglia 2018); for the molecular basis of stomatal development, see also Peterson et al. (2010). However, whether the stomata of Sphagnum are like those of other mosses in such respects is unclear (Merced 2015). Stomata are separated by one or more epidermal cells, and for the literature on stomatal patterning, i.e. the development of stomata and their associated/surrounding cells, of both extant and extinct embryophytes, see Rudall et al. (2013a), Moriya et al. (2022/2023), etc.. Some of the genes involved in stomatal development in Physcomitrium patens retain their function when moved to Arabidopsis (Caine et al. 2016). However, as is clear from work on features like C4 photosynthesis, independent acquisitions of the "same" feature can involve remarkable similarities at the molecular level, too.
Control of stomatal behaviour in vascular plants and bryophytes s.l. may differ (McAdam & Brodribb 2011, esp. Fig. 4), for instance, abscisic acid plays a crucial role in control of stomatal opening only in seed plants, even if similar genes involved in abscisic acid metabolism are found throughout land plants (McAdam & Brodribb 2012; McAdam et al. 2016; c.f. Hõrak et al. 2017) and stomata in hornworts behave quite differently (Renzaglia et al. 2017, see above). Chater et al. (2011) suggested that stomata of the moss Physcomitrium responded to at least some environmental stimuli rather like those of flowering plants (O'Donoghue et al. 2013), abscisic acid being involved in both (see also Beerling & Franks 2009; Chater et al. 2014; references in Raven & Edwards 2014; Lind et al. 2015). Genes involved in the signalling pathway in guard cell opening/closing are known from liverworts (Chater et al. 2014; Lind et al. 2015), but McAdam et al. (2016) suggest that in bryophytes such genes are primarily involved in spore dormancy, and it does seem that bryophyte stomata function rather differently than those in vascular plants (Brodribb & McAdam 2017); for stomatal control in early land plants, see also Ruszala et al. (2011). Field et al. (2015b) found that stomatal density and aperture size in taxa from mosses and hornworts were largely unresponsive to changing CO2 concentrations. Fortin and Friedman (2024; see also J. W. Clark 2024) review the distribution, morphology of stomata and stomatal look-alikes - are the pores of the gametophytes of hornworts some kind of stomata, or not?; they also note that there are stomata on the gametophytic plants found in Rhynie Chert. Given the uncertainty of relationships within bryophytes and between bryophytes and other embryophytes and what are to be called stomata it seems that practically any scenario involving stomatal evolution is possible (Fortin & Friedman 2024). There is further discussion about stomata under extant tracheophytes, but more work is clearly needed here.
Aspects of Stress and the Terrestrial Environment.Male Gametes.Details of the development and of the ultrastructure of the mature male gametes vary considerably between and to a certain extent within the major groups of plants, however, it is difficult to incorporate them in to the phylogeny - and not only because the terms used to describe the variation need a separate glossary... The angle of the spline with respect to the lamellar strip (= microtubule organizing centre, MTOC) is not mentioned since variation both within an individual gamete and within groups can be quite considerable (Renzaglia et al. 1999).
Although centrosomes and centrioles, which show vertical inheritance, are lost (Murata et al. 2007), similar structures (but they are not inherited) may develop from the blepharoplast during meiosis from microtubule organizing centres (MTOCs), and these vary considerably (Southworth & Cresti 1998; R. C. Brown & Lemmon 2007). Indeed, in Marchantia polymorpha the nature of the MTOC changes during meiosis (R. C. Brown et al. 2007). In general, γtubulin, involved in the nucleation of microtubules, is highly migratory. R. C. Brown and Lemmon (1997, see also 2008) reviewed the distribution of the quadripolar microtubule system in land plants; it occurs in all three bryophyte groups, in lycophytes, and in Marattiales, at least. This system is linked with monoplastidy, the MTOC being associated with the plastid. In streptophytes as a whole basal bodies develop de novo immediately prior to the formation of motile sperm cells (Wastenays 2002). Interestingly, the preprophasic band (p.p.b.) is sometimes absent, as in the protonema of at least some mosses, and even in megaspore development and in the endosperm of flowering plants, although elements of the p.p.b. apparatus may still be present (Webb & Gunning 1991; Buschmann & Zachgo 2016 and references).
Aspects of Stress and the Terrestrial Environment. A cuticle, made up in part of a cutin (polymerized esters of fatty acids, C16 and C18 hydroxy acids) scaffold and covered by various cuticular waxes, is a feature of all embryophytes, but it is not to be found in more basal clades, even if a number of the elements that were later to be involved in the synthesis of a fully-fledged cuticle are to be found there (Kong et al. 2020). Bryophytes in general have a poorly developed cuticle; interestingly, those hornworts and liverworts whose cuticle has been studied have substantial amounts of phenolic compounds in the cuticle, and such compouds absorb UV radiation, so protecting the plant, while the cutins of mosses and vascular plants have larger amounts of C16 and C18 fatty acids. A cuticle would interfere with the diffusion of CO2, etc., into the leaf of an aquatic streptophyte, and so could have been selected against there (Niklas et al. 2017).
Isoprenoids (xanthophylls and tocopherols) that protect against photo-oxidation and other insults of a dry, high-light environment are widely distributed in land plants and in some of their aquatic relatives, although some carotenoids involved in these functions may have evolved in land plants (Esteban et al. 2009).
The ability to tolerate dry conditions is obviously very important for land plants. Genes involved in desiccation tolerance in the spores of the earliest land plants may have been co-opted into desiccation tolerance in gametophytes (Oliver et al. 2005); spores and seeds in general are desiccation tolerant (Marks et al. 2021). Plastids are important here, since the plant's perception of stress is associated with cellular cross-talk from the plastid to the nucleus (retrograde signalling) and from the nucleus to the plastid (prograde signaling), the plastid being the site of many stress responses. It has been shown that Coleochaete and in particular Zygnema invest heavily in plastid-targeted proteins, while abscisic acid, central to stress responses in embryophytes, has also been found in Zygonema (de Vries et al. 2018; also Rippin et al. 2017; see Jarvis & López-Juez 2013 for plastids). Tolerance of extreme desiccation is quite widespread in both mosses and liverworts (Proctor & Pence 2002), and even their antheridia are notably desiccation-tolerant (Stark et al. 2016 and references). Desiccation tolerance may even be the ancestral condition for land plants (Oliver et al. 2000), being lost e.g. in some mosses and liverworts, perhaps several times, and perhaps also in stem hornworts (and since regained several times: Oliver et al. 2005); it can be induced in some mosses and liverworts (Oliver et al. 2005; see also Proctor & Tuba 2002). It is quie common in lycophytes and ferns as well, but rare in spermatophytes, being known from only 10 families of angiosperms (Marks et al. 2021). Homoiochlorophylly characterises all desiccation tolerant embyrophytes (apart from some monocots); here chlorophyll is retained and the photosynthetic apparatus maintained and protected during desiccation (Marks et al. 2021). The advantage of desiccation tolerance in early embryophytes would seem self-evident, but given what we know of relationships and the vagaries of ancestral state reconstruction, pinning down exactly where changes in this feature occurred is difficult. Interestingly, fern gametophytes are in part desiccation-tolerant (see Pinzón‐Camacho et al. 2025 and references), and as Watkins et al. (2007a: p. 716) observed, "fern gametophytes are, for all intents and purposes, bryophytes". Late Embryogenesis Abundant (LEA) genes expressed in response to water stress - desiccation in particular - are known from the gametophyte of the moss Physcomitrium patens (Rensing et al. 2007a), sporophytes of Selaginella (Klaus et al. 2017; VanBuren et al. 2018a), Picea, and throughout flowering plants (Artur et al. 2018), and their progenitors are to be found in aquatic streptophytes (e.g. Wodniok et al. 2011; Artur et al. 2018 and references). Thus the oospores of Chara are desiccation-tolerant (Oliver et al. 2005; Gaff & Oliver 2013) and desiccation tolerance in Klebsormidium is similar at the genic level to that in land plants (Holzinger et al. 2014) - see also above.
Banks et al. (2011) noted that some of the genes involved in the patterning and differentiation of vascular tissue are likely to have been present in the ancestral (gametophyte dominant) land plant and were recruited by the sporophytic generation, and conservation of regulatory networks is also involved (Pires et al. 2013; Rensing 2017). Thus the control of gametophytic rhizoids - and a variety of other epidermis-derived features such as gemmae, axillary hairs in both liverworts and mosses and the sporophytic roots hairs in Arabidopsis - is via (bHLH) root factors (Proust et al 2015; Rensing 2018), the Marchantia gene rescuing Arabidopsis mutants that could not produce root hairs. K.-J. Lu et al. (2019, see also Hofland et al. 2019) noted that transcription factors involved in the differentiation of water-conducting cells in the gametophyte of Physcomitrium patens are orthologous to those in Arabidopsis thaliana, but in the latter a particular heterodimer regulated the cell divisions involved in the development of the vascular system. The components of the heterodimer were also found in Klebsormidium, one in particular being like that found in land plants (Lu et al. 2020). Frank and Scanlon (2014) proposed a model for sporophyte evolution that is similar to that for vascular tissue evolution.
Recent ideas of relationships (bryophytes s.l. monophyletic, sister to vascular plants) and dates (crown-group land plants 515-473.6 Ma) need to be taken into account as one thinks of the evolution of the biosphere (Puttick et al. 2018; Morris et al. 2018a). Raven and Edwards (2014) list estimates of the net photosynthetic rates for bryophytes and other early land plants. Back in the Late Ordovician ca 450 Ma and with an atmospheric CO2 concentration about eight times today's levels, land plants along with lichens may have supported a level of chemical weathering two to three times that of today's vegetation (Porada et al. 2016). Such high levels are likely to have been temporary as embryophytes spread, nutrients became limited and nutrient recycling in the developing soil/humus layers increased, but they may have helped precipitate the later Permo-Carboniferous glaciation (Porada et al. 2016). For photosynthesis in bryophytes and early embryophytes, see Graham et al. (2014) and other articles in Hanson and Rice (2014).
Y. Zhang et al. (2019) looked at gravitropism in land plant roots and rhizoids, and although sampling was skimpy, the results suggested strongly that the rate of response to gravity changes in roots and rhizoids increased considerably at the extant seed plant node.
Fertilization and Spore Dispersal. D'Ario et al. (2023) modelled spore dispersal on fossils from the Lower Devonian-Lochkovian of 419-410 Ma and found a variety of probable dispersal mechanisms, some explosive. The results ranged from sedentary (spores very numerous, dispersal local: cooksonioid taxa) to opportunistic (above average spore number, local to relatively long distance: Partitatheca) to pioneer (long distance dispersal: Tortilicaulis) (D'Ario et al. 2023). Cascales-Miñana et al. (2021) document the remarkable variation of spore morphology in taxa from the Lower Devonian Rhynie chert (and some other mostly homosporous plants); basically, different spore morphologies may be produced by the one species, and may even be found in the same sporangium, but apparently not because of ontogenetic variation, and the same spore morphology can be found in quite unrelated plants. Linking differences in spore morphology to spore dispersal and plant diversity is not straightforward here!
Plant-Animal Interactions. It is difficult to imagine land plants without insects, and the reverse is very much true, too; as B. Wang et al. (2022) noted, insects had coevolved (sic) with land plants. However, little has been written about insects and early land plant evolution. Some caterpillars of Micropterigoidea, a jawed, lepidopteran clade that is perhaps Jurassic in age and sister to all other lepidoptera, are detritivores, but others eat mosses (e.g. Atrichum) and especially liverworts (Imada et al. 2011; Regier et al. 2015 and references; see also Hosts, consulted iii.2014), although they also may eat angiosperms (Davis & Landry 2012 and references); note that crown-group Lepidoptera have also been dated to Late Carboniferous (312.4-)299.5(-276.4) Ma (Kawahara et al. 2019). On balance, evidence suggests that the Araucaria-eating jawed moths, Agathiphagoidea, are sister to all other Lepidoptera (Heikkilä et al. 2015; Mitter et al. 2016; esp. Kawahara 2019; c.f. Regier et al. 2015; Kristiansen et al. 2015). For the host plants of other jawed moths, see also Nothofagaceae, Primulaceae (Cyclamen), etc.. Caterpillars of Mnesarchaeidae, members of a basal glossatan (= all other lepidoptera) clade, live in silk tunnels and also eat mosses and liverworts, as well as fern sporangia, etc., their sister taxon, Hepialoidea, with many more species, are also concealed feeders, but they eat just about everything (Regier et al. 2015). Indeed, moths in the Late Permian may have been internal tissue feeders (Kawahara et al. 2019).
Genes involved in the relationship between salicylic acid and jasmonic acid appear to be found in all land plants (Thaler et al. 2012). Salicylic acid, involved i.a. in resistance against pathogens that get their nutrition from living cells of the host and also against some phloem feeding insects, and jasmonic acid, involved i.a. in resistance against parasites that get their nutrition from dead cells of their host and against chewing herbivores, have an antagonistic relationship in many seed plants (Thaler et al. 2012).
Plant-Bacterial/Fungal Associations. For general information about non-pathogenic plant-fungal relationships, see elsewhere. Associations between embryophytes and fungi, initially with the gametophytes of the former, were established very early in the Silurian/Devonian (Selosse & Tacon 1998; Redecker et al. 2000b; Nebel et al. 2004; Köttke & Nebel 2005; see also Strullu-Derrien et al. 2014b, 2017; Selosse et al. 2015; Rimington et al. 2017, etc.). Four main fungal groups, Glomeromycotina, Mucoromycotina, Ascomycota and Basidiomycota, are associated with embryophytes, the first two forming arbuscular mycorrhizal (AM) and the last two ectomycorrhizal (ECM) associations - mucoromycotes are also sometimes involved in the latter. Relationships between these fungi are probably [[Glomeromycotina [Mortierellomycotina + Mucoromycotina]] [Ascomycota + Basidiomycota]] (Spatafora et al. 2016).
Lutzoni et al. (2018: bryophytes s.l. paraphyletic) suggest that the origin of glomeromycotes may have been (715-)659(-606) Ma, their diversification beginning (529-)484(-437) Ma, roughly contemporaneous with the early diversification of embryophytes (485-)482(-473) Ma; mucoromycotes, the sister group of glomeromycotes and possibly also involved in early AM symbioses, are ca 406 Ma, based on the divergence of Endogone. There are a number of other evolutionary events in fungi that may also be implicated in early embryophyte evolution, including specificially Tracheophyta, the stem age of which Lutzoni et al. (2018) estimate to be ca (466-)452(-438) Ma, but since tracheophytes may be sister to bryophytes s.l., or extant land plants may be derived from a tracheophyte-type ancestor...
Pattern-recognition receptor-triggered immunity (PTI) enables the recognition of components of bacterial (peptidogylycans) and fungal (chitin) extracts by the liverwort Marchantia polymorpha (Yotsui et al. 2023). PTI enables the development of appropriate immune responses and so resistance to microbial infection and it is important in protecting angiosperms against a diversity of pathogenic microbes; Yotsui et al. (2023) found that there are PTI components common to angiosperms and to the liverwort Marchantia polymorpha - PTI is perhaps a feature of embryophytes as a whole.
Genes & Genomes. For the evolution of the land plant genome taking into account what is known of green algal streptophytes, see Rensing et al. (2007a) and especially Bowman et al. (2017). Renzaglia et al. (1995) discuss genome size. See Wicke et al. (2011) for nuclear ribosomal DNA organization and Leitch and Leitch (2013) and Szövényi (2016) for the small nuclear genomes of bryophytes s.l.. Banks et al. (2011) and Jiao and Paterson (2014) suggest a gradual increase in genome size from algae → bryophytes → vascular plants. Lang et al. (2017) suggested that Physcomitrium patens there are syntenic blocks in some chromosomes in which the genes are colinear across all embryophytes; the genes there are involved in land plant-specific cell growth and tissue organization However, F.-W. Li et al. (2020) found little collinearity between the genomes of bryophytes s.l. and vascular plants.
Overall, the numbers of gene families increased substantially in land plants, and it continued to do so up the mesnagiosperms (J. Zhang et al. 2020: ?vascular plant node), although sampling in the study is very poor. Pires and Dolan (2010) found that basic helix-loop-helix proteins, a class of transcription factors, diversified very early, while Volokita et al. (2010) discuss the GDSL-lipase gene family and its evolution. RNA editing, in which the organelle-targeted pentatricopeptide repeat proteins play an important role, is restricted to Embryopsida (Rüdinger et al. 2008). Sayou et al. (2014, see also the subsequent discussion) looked at the evolution of the LEAFY gene, usually in a single copy in embryophytes (but two in gymnosperms), yet variously involved in cell division and specification of floral identity. Its binding specificity is notably variable (promiscuous) in the hornworts, and although the evolutionary scenario suggested by Sayou et al. (2014) is based on the rather unlikely topology [hornworts [mosses [liverworts + vascular plants]]] (see also below), they suggest that whatever the topology, this promiscuity hypothesis is likely (see their Fig. S9). Sakakibara (2016) summarized studies suggesting substantial similarities in transcriptional regulation and cellular function in water-conducting cells in Physcomitrium and Arabidopsis thaliana, in regulatory mechanisms of stomata, and in rhizoid/root hair differentiation. Indeed, hairs on Coleochaete have similarities with the rhizoids of bryophytes (Graham et al. 2010a), while Mello et al. (2018) suggest possible connections between auxin response and roots or root-like structures across land plants in general. For more on the evolution of rhizoids and rhizoid-like structures, see Duckett et al. (2014).
Much of interest is coming from the study of the evolution of individual metabolic pathways. Thus important signaling intermediates, the G-protein complex, are known from Chara and land plants (but not the green alga Micromeris), although elements of the pathway seem to be missing in some mosses and liverworts (Hackenberg et al. 2013). C3HDZ (class III homeodomain leucine zipper) genes, very important in development in euphyllophytes, etc., are also found in Chara (Prigge & Clark 2006; Floyd et al. 2006; Vasco et al. 2016). All land plants have some similar terpene synthase genes, but in addition there are microbial-type terpene synthase-like genes in all embryophytes except seed plants (at 3/7, hornworts have the lowest proportion of such genes), and these latter genes do not occur in streptophytes (Jia et al. 2016). Some of these microbial terpene synthase genes are similar to those of fungi and others to those of bacteria, and they were probably acquired by embryophytes through lateral transport (Jia et al. 2016, 2018), but other than the distinctive distribution of these genes, little is known about their evolution. Furthermore, triterpenoids are produced by CYP716 enzymes in all embryophytes except monocots (Miettinen et al. 2017). Various classes of phosphoprotein phosphatases also have interesting distributions, with the ApaH phosphatases currently being known from streptophytes only, while the ALPH class is absent from land plants (Uhrig et al. 2012). Several copies of LATERAL ORGANS BOUNDARIES DOMAIN genes are to be found in bryophytes, and they are also also known from Coleochaete and Spirogyra (Chanderbali et al. 2015). KNOX1 and KNOX2 genes occur in embryophytes, also in Klebsormidium, and KNOX1 regulates sporophytic meristems while KNOX2 suppresses the gametophytic developmental program in the sporophyte (Sakakibara et al. 2013, 2016). Two copies each of the plant homeobox KNOX genes, involved in meristem activity in vascular plants, and the MADS-box MIKC genes, i.a. floral identity genes, are found in mosses; subsequent duplication generated the diversity of these gene classes found in flowering plants in particular (Theißen et al. 2001). Embryophytes in general respond to ethylene, but its synthetic pathway in seed plants is not the same as that in other embryophytes, although details in the latter are unclear (F.-W. Li et al. 2018). Mosses, liverworts as well as lycophytes/ferns have a squalene-hopene cyclase gene that seems to have come from cyanobacteria by lateral gene transfer - and independently in the three groups; the gene is perhaps involved in stress tolerance (Li et al. 2018; Wickell & Li 2019).
Plastids. Relatively little is known about plastid and other organelle inheritance in homosporous land plants in particular. Although placing "maternal inheritance" at this node may seem somewhat notional, organelle inheritance is usually uniparental (Wicke et al. 2011a; Barnard Kubow et al. 2016); it shifts at the gymnosperm node.
Details of gene and genome evolution in embryophyte plastids are given by Jansen et al. (2007), A. M. Magee et al. (2010), Wicke et al. (2011a), Civán et al. (2014), Lemieux et al. (2016) and Mower and Vickrey (2018). Compared with their sister group, the [Zygnematales + Desmidales] clade, the chloroplast genome in basal land plants, especially in bryophytes s.l., is relatively stable (e.g. Turmel et al. 2003; Lemieux et al. 2016; Mower & Vickrey 2018). For the evolution of polyplastidy, see de Vries and Gould (2017) - it seems to be associated with the loss/movement to the nucleus of minD, minE genes, which happened quite deep in the streptophyte clade. The chloroplast genes trnLUAA and trnFGAA are not associated in other green plants (Quandt et al. 2004). Ruhlman et al. (2015) outline the increase in complexity of the NADH dehydrogenase (NDH) complex in embryophytes; little of this been incorporated into the tree here. Details of the evolution of the chloroplast trnS and trnN genes in embryophytes are complex (Knie et al. 2014); trnS(gcu) and trnN(guu) genes occur in the plastids of many streptophytes and also in liverworts; the former gene is also found in the plasids of the [monilophyte + lignophyte] clade, but not in Gnetales.
Knoop (2013) discussed the evolution of the chondrome in basal land plants. Overall, land plants have chondromes with 30-42 protein genes, and these genes are involved in oxidative phosphorylation, the translation, maturation and transport of proteins etc.; chondromes here have notably more genes than in most other organisms (Mower 2020). Most bryophytes s.l. have small chondromes and there is little variation in gene order compared with mitochondria in vascular plants (Y. Liu et al. 2014a), however, Fig. 1 in Mower (2020) suggests a more complex story, with the hornworts having somewhat larger chondromes than those of other bryophytes, but there is much more size variation in vascular plants (no data from pteridophytes), and absolute size is also sometimes very large (but also sometimes very small) there. Mower (2020: Fig. 2) also looked at the distribution of protein-coding genes across land plants, and there seems to be some phylogenetic signal here, although it is not yet incorporated here: Liverworts and mosses are similar (apart from two genes), there seems to be some similarity between hornworts and lycophytes and also between angiosperms and gymnosperms. For intron distributions in mitochondrial genes, see Dombrovska and Qiu (1994), Qiu et al. (1998), Regina et al. (2005). In land plants, group II introns are commoner in bryophytes s.l., especially liverworts, although not at all common even there, while group I introns are much more abundant, but they show little inter-group variation, especially in the three groups of bryophytes s.l. (see Mower 2020 for details). Individual stories can be fascinating - thus a number of angiosperms, and practically all parasitic angiosperms studied, have a cox1i729 intron acquired via HGT from fungi, and while liverworts also have this intron, it has only slight similarity to the angiosperm intron and was probably acquired by independent HGT (Mower 2020).
Chemistry, Morphology, etc.. For the hemicellulosic polysaccharide xyloglucans, see Del Bem and Vincentz (2010), Scheller and Ulvskov (2010) and Zabotina (2012) and especially Del-Bem (2018). Komatsu et al. (2014; see also Doi et al. 2006) discuss chloroplast movement, mediated by a single copy of the blue-light sensitive phototropin gene, in land plants. For the synthesis of pigmented flavonoids, the anthocyanins, involved in protection against abiotic stress, for instance protection against U.V. radiation damage, see e.g. Campanella et al. (2014), the cyanidin and pelargonidin (but not delphinidin) synthetic pathways having evolved very early although what had been thought to be anthocyanins in some liverworts have turned out to be different flavonoids, the pigmented auronidins (Berland et al. 2019). Flavones and flavonols, colourless flavonoids that can absorb U.V.-B radiation, are found in all major groups of land plants (Berland et al. 2019 and references).
See also Kenrick (2000) and Ligrone et al. (2012a), both morphology, O'Donoghue et al. (2013: xyloglucans), O'Rorke et al. (2015: sugars of pectic polysaccharides, interpretation of change depends very much on phylogeny), Taylor et al. (2009: fossils, inc. those of fungi associated with plants), Blackmore and Crane (1998: spore/pollen apertures), R. C. Brown and Lemmon (1990: mono/polyplastidy, 2013 and references: sporogenesis), R. C. Brown et al. (2015: spore walls of basal members of the bryophyte clades), Wallace et al. (2011: spore and pollen wall development in land plants), Renzaglia and Garbary (2001: male gametes), Wastenays (2002: microtubules), Tomescu et al. (2014: apical cells of sporophyte), Waters (2003: molecular adaptation), Hedges et al. (2004: timing), Renzagia et al. (1995) and Hodges et al. (2012) cilia, Doyle (2013: reproductive features) and de Vries et al. (2016: plastid evolution).
Previous Relationships. In the old telling of the tale when there was a basal paraphyletic Bryophyta s.l., plants were often lined up in a simple to complex sequence and evolution was read off accordingly. The emphasis was on the progressive acquisition of the features of vascular plants, e.g. of stomatal functions (Field et al. 2015b, but c.f. Renzaglia et al. 2017; Brodribb & McAdam 2017), aspects of sporophyte development, the vascular system, etc.. Indeed, prior to iii.2018 on this site there was a group, the Stomatophytes, that were characterized as follows:
Abscisic acid, L- and D-methionine distinguished metabolically; pro- and metaphase spindles acentric; class 1 KNOX genes expressed in sporangium alone; sporangium wall 4≤ cells across [≡ eusporangium], tapetum +, secreting sporopollenin, which obscures outer white-line centred lamellae, columella +, developing from endothecial cells; stomata +, on sporangium, anomocytic, cell lineage that produces them with symmetric divisions [perigenous]; underlying similarities in the development of conducting tissue and of rhizoids/root hairs; spores trilete; shoot meristem patterning gene families expressed; MIKC, MI*K*C* genes, post-transcriptional editing of chloroplast genes; gain of three group II mitochondrial introns, mitochondrial trnS(gcu) and trnN(guu) genes 0.
These stomatophytes included all embryophytes except the liverworts, and within them was an [Anthocerophyta + Polysporangiophyta] clade that was characterized as follows:
Gametophyte leafless; archegonia embedded/sunken [only neck protruding]; sporophyte long-lived, chlorophyllous; cell walls with xylans.
The task is now to see which of these features is best placed at the embryophyte node - "presence of stomata", for example, is placed there, "loss of stomata in sporophyte" then being an apomorphy for the liverwort clade (e.g. Puttick et al. 2018; B. J. Harris et al. 2020), although note that genes involved in stomatal development in other embryophytes are expressed in the sporangial seta there (Moriya et al. 2022/2023).
Gametophyte: (plants dioicious - UV sex chromosomes), with unicellular rhizoids, stomata 0; sporophyte-gametophyte junction with dead gametophytic cells, spaces containing mucilage; sporophyte: embryo shoot apex exoscopic [developing towards micropyle/archegonial neck, from epibasal cell]; shoot apex monoplex-type [with single tetrahedral apical cell in surface layer]; sporangium single, terminal [= capsulee,seta, foot]; stomatal guard mother cells developing directly from epidermal cells [perigenous, no meristemoid, susidiary cells not derived from guard cell lineage], entry division symmetrical; basal body with a proximal extension, basal body length, basal bodies dimorphic, etc., stellate array of filaments in transition zone in flagellar shaft, (for details, see e.g. Renzaglia et al. 1999; Hodges et al. 2012, esp. Table 1), lamellar strip [part of MLS] 0 at maturity, first disappearing at posterior, spline widest at the cell anterior; genome made up of euchromatin units interspersed within facultative and constitutive heterochromatin, tandem repeats of typical centromeres 0; transcription factor genes moderate; mitochondrial genome mostly collinear.
Age. Around 506.5-460.5 Ma is the spread of ages for crown-group bryophytes s.l. suggested by Morris et al. (2018a) and 500-473 Ma ("crown bryophytes") the ages suggested by B. J. Harris et al. (2021/2022); Harris et al. (ibid. p. [3]) also estimated the "divergence between Setaphytes (mosses + liverworts) and hornworts" as being 479-450 Ma, but c.f. their Fig. 2.
Evolution: Divergence & Distribution. When mosses were thought to be sister to vascular plants the conducting tissue in the centre of the stem in some moss gametophytes was homologized with the vascular tissue in the sporophytes of vascular plants (e.g. Mishler & Churchill 1984, 1985; Mishler et al. 1994). However, this seems in part to be due to the way in which characters describing such tissue were conceptualized; there seems to be little reason to consider the conductive tissues of mosses and those of polysporangiophytes as having much similarity other than that due to their similar functions (e.g. Ligrone et al. 2000, 2002). Ligrone et al. (2002) found no great similarity between the water conducting cells of Takakia, the hydroids of other mosses, and the conducting tissues in Haplomitrium and metzgerialean liverworts (see also Doyle 2013).
If the three groups of bryophytes form a clade (see below), then the interpretation of the evolution of such features as stomatal presence, cell division and the sporophyte shoot meristem, etc., differs from that advanced if they are thought to be paraphyletic. This will be particularly true if liverworts are sister to the other bryophytes (e.g. Frank & Scanlon 2014; Lind et al. 2015), although as mentioned elsewhere, this seems rather unlikely. It may be that many of the features in some bryophytes s.l. are apomorphies for extant land plants, being found either in the gametophyte (ancestral conducting tissue), the sporangium (stomata) or basic chromosomal construction, The sporophytic generation then became more elaborate in vascular plants, with rhizoids, stomata and vascular tissue forming a functional whole (Kenrick 2017). In the bryophytes s.l., however, features like "stomata in sporophytes" and "conductive tissues in gametophytes" may have been lost in liverworts and hornworts respectively, although in recent work it was found that genes involved in stomatal development in flowering plants are active in the seta of Marchantia polymorpha, at least (Moriya et aL. 2022/2023. Optimization of such distinctive and important features as the presence of a sporangium with a seta and columella, stomata, trilete spores, polarity of transport of auxin in the sporophyte, etc., is not easy whether bryophytes are paraphyletic or monophyletic. The morphology and function of stomata can be quite distinctive (see stomatophytes); Merced & Renzaglia 2013; Haig 2013a; Brodribb & McAdam 2017; Merced & Renzaglia 2018), perhaps even suggesting their independent origin within mosses (for recent literature, see Moriya et al. (2022/2023). Furthermore, although a group Setaphyta is recognized below, development of the seta in mosses and liverworts may be very different.
Qiu et al. (2006a, 2007 and references) suggested that a number of features of hornworts, particularly of the sporophyte, might be synapomorphies of [vascular plants + hornworts], however, it is possible such features are apomorphies of extant land plants that were subsequently lost in other bryophytes s.l.. For the evolution of trilete spores, see e.g. J. A. Doyle (2012b) and R. C. Brown et al. (2015). I had "primary cell walls with xylans (xyloglucans with fucosylated subunits)" as an apomorphy of [Anthocerophyta + Polysporangiophyta] prior to July 2016, but xylans seem to be an apomorphy of land plants, although the nature of their side chains (charged vs uncharged) varies (Scheller & Ulvskov 2010; see also Sarkar et al. 2009; Zabotina 2012 and references). There is little borate cross-linked rhamnogalactan II in bryophytes (or in Chara), but there is much more in vascular plants where it cross-links primary cell wall pectins and seems to have been an important early step in the development of lignified cell walls (Matsunaga et al. 2004). Finally, although there are some indications that the distinctive construction of the chromosomes in bryophytes with ancestral genome of bryophytes with units of euchromatin interspersed within facultative and constitutive heterochromatin may be plesiomorphic for embryophytes as a whwole, sampling in both basal vascular plants and in streptophytes is currently too poor to tell (Hisanaga et al. 2023).
Thinking about diversification in bryophytes s.l. is difficult. Laenen et al. (2014) suggested that overall diversification rates in bryophytes s.l. (paraphyletic) were lower than in angiosperms in particular, but also lower than in ferns. This was despite species-level diversification rates of a number of extant groups being comparable to those in angiosperms, and they suggested that there had been "massive extinctions" (or numerous smaller extinctions?) in the past, as with gymnosperms, which would explain the overall low level of bryophyte s.l. diversity (Laenen et al. 2014). However, some comparisons are tricky here. Laenen et al. (2014) found liverworts and mosses first diversified in the mid-Jurassic and mid-Cretaceous respectively, and rightly note that although Fiz-Palacios et al. (2011) found declining diversification rates for the former in the mid-Cretaceous, this may well have been because they used families as their unit of investigation, however, comparison of genus ages across bryophytes s.l. and angiosperms by Laenen et al. (2014) would seem to face similar problems.
Vanderpoorten et al. (2019) analyse patterns of dispersal in bryophytes. Commonly there is efficent short distance dispersal and also random long distance dispersal, the result being that the genetic diversity of colonizing propagules increases with increasing isolation, the inverse isolation hypothesis.
J. Zhang et al. (2020) discuss lateral gene transfer in bryophytes, especially Anthoceros.
Ecology & Physiology. Epiphytic bryophytes tend to be endosporic and have precocious germination, i.e. the spores are multinucleate, and sometimes they are green/chlorophyllous (Schuette & Renzaglia 2010; see also Nehira 1987). Taxa that are epiphytic or growing in permanently wet habitats may well lack associations with fungi (Pressel et al. 2021) Lloyd and Klekowski (1970) noted that some bryophytes s.l. had chlorophyllous spores; in ferns such spores are also common in epiphytes, and well as in taxa growing in wet conditions - see elsewhere for further details.
Fertilization & Spore Dispersal. For a discussion about various aspects of sexual and asexual reproduction in the gametophytic generation of bryophytes, asexual reproduction being very common and occurring in a variety of ways, see Maciel-Silva and Pôrto (2014). Interestingly, dwarf males that are epiphytic on the females have evolved in a number of taxa (Haigh 2016), and this might seem to compound the problem. However, Muggoch and Walton (1942) had noted that in some species of all three groups of bryophytes there is lipid-aided spread of the spermatozoids over the surface of the water which they think is more efficient in facilitating fertilization that brute-force swimming (as in the sperm of ferns), and Pressel and Duckett (2019) have recently provided a nice example in Marchantia of sperm moving almost 20 m in this way. Ekwealor and Fisher (2024) review reproduction and population dynamica in bryophytes a.l. with their autonomous gametophytes, and i.a. they suggest that phorogamy - the involvement of microarthropods like mites and springtails in fertilization - might be commoner than expected; this review should be consulted for details.
When gametophytes spread vegetatively, it can cause complications for sporophyte formation if the gametophytes are monoicous; if selfing takes place, the sporophyte is homozygous. Given this vegetative spread, dioicy is only a partial solution since although sperm swim in water (von Aderkas et al. 2022), they cannot swim very far. Indeed, at first sight it might seem that gametophytes that produce both antheridia and archegonia - these are widespread in bryophytes s.l. - might self. The result might be homozygous offspring since mitosis, not meiosis, is involved in the production of gametes, however, such gametophytic selfing seems to be uncommon (Haufler et al. 2016; Kinosian et al. 2022). Dioicy - a UV system - occurs in 1/3-1/2 or more of the three major bryophyte groups, but little is known about its evolution (Charlesworth 2022).
Work on wind-dispersed spores in all groups is relevant to bryophyte distribution - so see pollen/spore dispersal under the individual bryophyte groups, also lycophytes, Gnetales, pines etc. (for which, see below), leptosporangiate ferns and even wind-pollinated angiosperms under Pollination & Seed Dispersal/Fertilization & Spore Dispersal. Lewis et al. (2014) noted that some bryophytes had bipolar distributions, and that this was unlikely to be caused by wind dispersal. However, they found that bryophyte diaspores (plant fragments; also fungal spores, etc.) were to be found in the breast plumage of birds in their Arctic breeding grounds suggesting the possibility of long distance N→S dispersal by these birds when they migrated (Lewis et al. 2014).
Plant-Animal Relationships. There are not many insect larvae that eat bryophytes, but Kato et al. (2023) summarize those that do. These authors recently found that larvae of dipteran agromyzid flies of the Liriomyza group are leaf miners on liverworts and hornworts, with many host shits there since the Oligocene - roughly the same time as agromyzids diversified on angiosperms (Kato et al. 2023). The sister group to these bryophyte miners eats ferns; the bryophyte miners moved on to bryophytes from tracheophytes; agromyzids are ca 65 Ma, origuinating in the Palaeocene. However, the association of some insects with bryophytesis apparently much older. Thus Micropterigidae are sister to all other lepidoptera and may have been eating bryophytes since the Triassic, while Litoleptis, a dipteran rhagionid fly is a thallus-miner of complex thalloid liverworts and is Jurassic in age (Kato et al. 2023).
Plant-Bacterial/Fungal Associations. Pressel et al. (2021) provide a comprehensive summary of bryophyte-fungal relationships and this should be consulted for the numerous details discussed.
Genes & Genomes. Bryophytes s.l. seem to lack typical centromeric structures, lacking tandem repeats (Lang et al. 2018: F-W. Li et al. 2020). There are no collinear regions common to all three bryophyte groups; bryophytes s.str. had the most such blocks collinear with at least one other major land plant group. There was an overall loss of gene families throughout this clade and in the three bryophytes examined, one from each main group, the moss Physcomitrium being the exception, but in all cases some new families were acquired (J. Zhang et al. 2021).
For post-transcriptional editing of the chloroplast genes, see Martín and Sabater (2010).
Knie et al. (2015) note that the cis- state of the nad2i542g2 intron in the chondrome is likely to be the ancestral condition for land plants. For the loss of the loss of the mitochondrial trnS(gcu) and trnN(guu) genes at this node, see Knie et al. (2014), and for the gain of three group II mitochondrial introns, see Qiu et al. (1998b).
Genes & Genomes. For genomes, etc., in bryophytes s.l, see Szövényi et al. (2021).
Includes Hornworts, Liverworts, Mosses
Evolution:
Chemistry, Morphology, etc.. Phytolith/silica deposition is quite widely spread, and although quite common in complex thalloid liverworts and the mosses Bryales and Polytrichales, phytolith/silica presence is unlikely to be the ancestral condition for any larger group in bryophytes s.l. (Thummel et al. 2019). F. Chen et al. (2028) terpenes and related compounds in bryophytes.
Details of the sporophyte-gametophyte boundary are discussed by Duckett and Ligrone (2003). The sporangium develops from tiers of four cells (quadrants) which divide periclinally producing an outer amphithecium and an inner endothecium; the major groups of bryophytes differ in how the capsule wall and spores develop from these cells (Ligrone et al. 2012a for a summary). For spore morphology, see Qiu et al. (2012) and Blackmore et al. (2012) and references (correlations: monolete spores, successive sporogenesis, tetrads tetragonal/decussate, simultaneous sporogenesis).
Crum (2001), Goffinet and Shaw (2009) and Shaw et al. (2011) provide much general information about the "bryophytes" as a whole; see Harrison and Morris (2017) for their embryonic development in particular.
Phylogeny. Kenrick and Crane (1997), Nishiyama and Kato (1999) and Shaw and Renzaglia (2004) make early suggestions about bryophyte relationships. Although Garbary et al. (1993) in their analysis of characters taken from male gametogenesis found the relationships among the three bryophyte groups followed here, other relationships were more or less scrambled; neither lycophytes nor pteridophytes were monophyletic, Takakia was well embedded in the mosses, etc.. Indeed, the three groups of bryophytes, mosses, liverworts and hornworts, have for some time now nearly always been found to be individually monophyletic, although this has sometimes not been so for liverworts (Bopp & Capesius 1998: no vascular plants included; Quandt & Stech 2003, and references), and in some analyses Nickrent (2000) found that mosses were paraphyletic, although there was little support for this. There have been various hypotheses of relationships among/between the three groups of bryophytes and vascular plants (e.g. Nickrent et al. 2000; Puttick et al. 2018; Donoghue et al. 2021: Fig. 1; see also Cooper 2014), however, recent work by e.g. Wickett et al. (2014), Puttick et al. (2018) and Morris et al. (2018a) in particular is (perhaps) clarifying relationships in this area. Qiu and Mishler (2024: esp. Fig. 2 and the related T. 1) provide a detailed analysis of publications dealing with relationships between these taxa. Some of these papers are mentioned here.
1. The results of many earlier studies supported the set of relationships [liverworts + the rest], the rest = [mosses [hornworts + vascular plants]] (e.g. Kenrick & Crane 1997a, b: long branch subtending the rest; Goffinet & Shaw 2009; Shaw et al. 2011 for literature; X.-X. Shen et al. 2017: evaluation of support for two hypotheses; Evkaikina et al. 2017); Qiu et al. (2006) confirmed these relationships using three different data sets. This may be the most frequently supported hypothesis of relationships up to late 2017 (e.g. Lewis et al. 1997; Kelch et al. 2004; Wolf et al. 2006: many analyses, whole chloroplast genomes; Qiu et al. 2007; S. Li et al. 2013; Ruhfel et al. 2014: whole chloroplast genomes; Y. Liu et al. 2014b: mitochondrial nucleotide and amino acid data, other relationships very poorly supported; Magallón et al. 2013, 2016; Shimamura 2016 for literature). Dombrovska and Qiu (1994) had earlier outlined several lines of evidence such as the content of the inverted repeat and intron distributions that were consistent with the idea that liverworts were sister to all other land plants (see also Qiu et al. 1998b: mitochondrial introns; Antonov et al. 2000: cp rDNA ITS). This position is also favoured by an analysis of cpITS spacer sequences (Samigullin et al. 2002) and a complete plastome analysis (Karol et al. 2010). Kelch et al. (2004), using structural characters of the plastome, and Groth-Malonek et al. (2004, not all analyses; see also Knoop 2005), looking at trans-splicing mitochondrial introns, also suggested the same position for liverworts (see also Rydin & Källersjö 2002 and Karol et al. 2010, but neither in all analyses). Similarly, the distribution of group II nad4 mitochondrial introns suggests bryophyte s.l. paraphyly (Volkmar et al. 2012: Fig. 1 and references). Consistent with this hypothesis, the distribution of an extension of the chloroplast inverted repeat placed hornworts as sister to tracheophytes, as did the distribution of cell wall xylans (Carafa et al. 2005), and the distributions of some gene families also favoured this position (Szövényi 2016). However, things have changed - maybe - see 5 below. Indeed, Y.-L. Qiu et al. (2024: 2 plastid, 2 mitochondrial, 1 nuclear markers; 633 taxa) recently recovered a paraphyletic bryophytes s.l. in their comprehensive phylogeny of embryophytes as a whole, noting that there may be methodological problems in analyses using large nuclear data sets (see also Qiu & Mishler 2024).
2. A [liverwort + moss] basal clade has sometimes been found (Ruhfel et al. 2014); see also Finet et al. (2010: hornworts not sampled), some analyses in Karol et al. (2010) and in B. Zhong et al. (2013b: Anthoceros sometimes sister to tracheophyta, sometimes sister to Lycopodiales), u.s.w.. In an extensive analysis of chloroplast genomes, Lemieux et al. (2016) recovered the relationships [[mosses + liverworts] [hornworts + the rest]], although support for the position of the sole hornwort included, Anthoceros, was weak. This topology has some support in Puttick et al. (2018). Group 1 introns in the mitochondrial cox1 and nad5 suggest a [mosses + liverworts] clade - or they were an embryophyte apomorphy that was later lost (Volkmar et al. 2012 and references).
3. Mitochondrial sequence data have sometimes placed hornworts as sister to all other land plants (Sayou et al. 2014), and also in the analyses of Rai and Graham (2010), but there may be a rooting issue here. This position was also recovered by Hedderson et al. (1998: small subunit rRNA), with [mosses + liverworts] the next clade up, and then vascular plants, a topology that had some support in Puttick et al. (2018). Renzaglia and Garbary (2010) considered that the evidence for the same hornwort basal hypothesis was compelling, and they found this position to be quite strongly supported in their analysis of 123 morphological characters (Garbary & Renzaglia 1998), and several other studies have recovered/used this topology (Renzaglia et al. 2000a; Stech et al. 2003; Shanker et al. 2011 for references); relationships found by Nickrent et al. (2000) when 3rd codon position transitions were excluded were [hornworts [[mosses + liverworts] [vascular plants]]].
4. Mosses are sister to all other land plants in the analyses of Floyd et al. (2006, 2014) and Vasco et al. (2016), with either liverworts (Floyd et al. 2006) or hornworts (Vasco et al. 2016) sister to vascular plants, although basal land plant relationships were not the focus of these studies. There was no support for such topologies in Puttick et al. (2018).
5. Bryophytes s.l. are monophyletic. In the chloroplast proteome analysis of Shanker et al. (2011: c.f. position of Huperzia) bryophytes were monophyletic. Nishiyama et al. (2004) had also proposed that the three bryophyte groups formed a single clade; 51 genes from the entire chloroplast sequence were included, but taxon sampling was poor, e.g., no lycophytes were included. A similar grouping was also found in an analysis of the trnL intron (Quandt et al. 2004) and in another study that looked at many genes but with very skimpy sampling, that of Goremykin and Hellwig (2005). In the chloroplast genome Bayesian MCMC analysis of Civán et al. (2014) relationships were [[hornworts [mosses + liverworts]] [clubmosses, etc. + the rest]], although in a concensus tree [liverworts + hornworts] were sister to other embryophytes, within which relationships were pretty much scrambled. Cox et al. (2014) noticed that trees based on protein coding sequences and trees based on the proteins they coded differed in their topologies, and they suggested that there may have been synonymous substitutions in the sequences; they, too, argued strongly for the monophyly of bryophytes. A [liverwort + moss] clade was also recovered by Wickett et al. (2014) in transcriptome analyses, and in many analyses there, too, bryophytes (as [hornworts [mosses + liverworts]]) were monophyletic. In another comprehensive set of transcriptome analyses, this again was the predominant topology recovered (Puttick et al. 2018, see also Morris et al. 2018a; Gitzendanner et al. 2018a: chloroplast data; Cheng et al. 2018). Puttick et al. (2018) found that hypotheses that did not have a [moss + liverwort] clade could be rejected, and in none of these last three studies did hypothesis 1 above have significant support. Furthermore, a [hornwort [liverwort + moss]] clade sister to vascular plants was also recovered in the transcriptome analysis of O.T.P.T.I. (2019), and work using nuclear genomes by J. Zhang et al. (2020), B. J. Harris et al. (2020: 151 single copy genes), F.-W. Li et al. (2020: 742 nuclear genes, relative quartet frequency for this topology twice that of the others) and Breinholt et al. (2021: GoFlag451 probe set).
The [[hornwort [liverwort + moss]] vascular plants] topology - and its consequences in terms of character evolution - is followed here, see elsewhere. A recent study by Bechteler et al. (2023: 228 nuclear genes, 531 spp., 52/54 orders) provides a general framework for bryophyte phylogeny/evolution.
I. ANTHOCEROPHYTA Stotler & Crandall-Stotler / HORNWORTS
Flavonoids 0; YABBY TF gene family + [dorsiventrality]; Gametophyte: thalloid, (leafy), closely associated with N-fixing Nostoc; flavonoids 0; apical cell +, wedge-shaped, with four cutting faces; branching truly dichotomous; ventral mucilage clefts/cavities +, opening by stoma-like pore; vegetative cells monoplastidic, pyrenoids +/0, microtubules axial; antheridia in chambers [= endogenous]; bicentriole pair formed in cell generation before spermatogenous cells; stellate array in basal body of cilium absent; antheridia sunken, in mucilage-filled chambers [developed from subepidermal cell - Anth. - ?all], in groups, male gametes bilaterally symmetrical, with a right-handed coil; archegonia sunken; sporophyte-gametophyte junction with sporophytic haustorial cells [uniseriate/branched], transfer cells in gametophyte; sporophyte: embryo long-lived, chlorophyllous, with plasmodesmatal auxin transport, develops deep within the thallus, first cell division of zygote longitudinal, second transverse, foot ovoid-bulbous, apical cell 0 [initially + - Physcomitrium], basal meristem +, active for an extended period, seta short; stomata 51-81 μm long; sporangium dehiscing by 2 longitudinal slits; archesporial tissue from amphithecium, endothecium columellar tissue; meiosis not simultaneous, spores maturing from base of capsule to top, archesporium continuous over tope of columella; elaters +, spirally thickened, multicellular; xylans in walls of spores and elaters; n = 5, nuclear genome [1 C] 0.085-0.28 pg/(156-)244(-714) Mb; plastome 152-161 Kb; chondrome 185-242 kb, extensive gene loss/pseudogenization, mitochondrial rpl2 gene 0.
11/300. World-wide.
Age. Estimates for the age of hornworts are around 290 Ma (Villarreal & Renner 2012), ca 170 Ma, the overall variation being (245.2-)228.9, 160.2(-107.1) Ma (Villarreal et al. 2015a) or (294-)ca 250(-214) Ma (B. J. Harris et al. 2021/2022) despite lack of fossils, age is constrained by horizontal gene transfer to polypods). The age of this clade is estimated to be (348.6-)337.6 {L.}, 322.5(-316.8) Ma by Peñaloza-Bojacá et al. (2025: see Fig 2).
Leiosporocerataceae / Leiosporocerateles Hässel - Leiosporoceros dussii (Stephani) Hässel
Gametophyte: Nostoc in branching schizogenous strands in the centre of the thallus, mucilage clefts only in young uninfected plants, mycorrhizae 0; pyrenoids 0; antheridia up to 70/chamber; sporophyte: spore tetrads bilateral alterno-opposite, elaters thick-walled, spores "minute", monolete, surface smooth.
1/1. The Antilles, Panama
Age. The following two clades diverged (198.6-)190.5, 182.1 {L.}(-170.7) Ma (Peñaloza-Bojacá et al. 2025).
Anthocerotaceae / Anthoceratales Limpricht
Glomeromycete and mucoromycete symbionts + (0), rhizoids lack fungal hyphae; neochrome +; chloroplast grana lack highly curved end membranes, channel thylakoids connect adjacent grana stacks; gametophyte: plastids (>1/cell, but mitosis still monoplastidic), pyrenoids + (0); Nostoc in spherical colonies, mucilage clefts persist; antheridia 1-6/chamber; sporophyte: (first division of zygote vertical - Anthoceros); (stomata 0 if sporophytes more or less enclosed); 1-many chloroplasts/cell; white line-centred lamellae 0?; spores ornamented, (multicellular, chlorophyllous); phototropins lack introns; elevated rate of RNA editing.
2 Phaeoceros (), . World-wide.
Age. The age of this clade may be (399-)306, 248(-173) Ma (Villarreal & Renner 2012), 261-186 Ma (Laenen et al. 2014) or ca 288.3 Ma (Anthoceratales including Leiosporoceros, but c.f. figures given - Peñaloza-Bojacá et al. 2025: Fig. 2B) or (307.5-)299.4 {L.}, 288.3(-280.3) Ma (Peñaloza-Bojacá et al. 2025, Leiosporoceros sister to all hornworts).
Phymatocerotaceae / Phymatocerotales R. J. Duff
5/ Dendroceros (62)
Age. The crown-group age of this clade is estimated to be (139.5-)129.3 {L.}, 129.2(-119.4) Ma (Peñaloza-Bojacá et al. 2025).
Notothylacaceae / Notothyladales Hyvönen & Piippo
3/
Age. The crown-group age of this clade is estimated to be (198.6-)190.5, 182.1(-170.7) Ma (Peñaloza-Bojacá et al. 2025).
Evolution: Divergence and Distribution. For a discussion on the general fossil record of bryophytes s.l., see e.g. Tomescu et al. (2018).
Most diversification within Anthocerotopsida has taken place within the last 100 Ma or so, or even within the Cenozoic (Villarreal & Renner 2012; Villarreal et al. 2015a).
F.-Y. Li et al. (2020) found that homologues of some genes expressed in the shoot apical meristem of vascular plants are also to be found in Anthoceros where they are expressed mostly in its sporophyte. This is despite the apparently rather different patterns of growth in the two; it is possible that the sporophytes of other bryophytes are derived.
It has been suggested that pyrenoids (for which, see Hanson et al. 2014; S. He et al. 2023) have evolved five times or more in the hornworts between 101 and 18 Ma (and also subsequently been lost several times); they vary considerably in morphology. This repeated evolution of pyrenoids in Anthocerotopsida (they are not known from Leiosporoceros) may be an example of a "tendency", however, what is causing their gains and losses is unclear (Villarreal & Renner 2012). The alternative topologies for the position of Leiosporoceros recovered by Peñaloza-Bojacá et al. (2025: see Fig 2A, B) complicates this issue, making the evolution of pyrenoids unclear; note that in the ages taken from Peñaloza-Bojacá et al. (2025) and given above, {L.} refers to ages based on the topology when Leiosporoceros is sister to all other hornworts.
Macleod et al. (2022) discuss the basis of monoplastidy in horworts. A number of genes involved in plastid development and functioning have been lost sporadically in hornwors, but all hornworts have lost four genes involved in plastid division and two genes involved in liquid-liquid phase transitions (MacLeod et al. 2022: esp Fig 1A). These latter are in the chloroplast twin-arginine translocation (cpTat) pathway which would normally faciltate the movement of folded proteins into the chloroplast. De Vries and Gould (2017) note variation in the number of plastids per cell in streptophytes as a whole.
Ecology & Physiology. Neochromes, chimaeric photoreceptors in which red-sensing phytochrome and blue-sensing phototropin are fused into a single molecule, have been found in all hornworts sampled (Leiosporoceros not studied: Suetsugu et al. 2005; F.-W. Li et al. 2014, 2015), they subsequently moved to ferns ca 179 Ma from the stem [Phymatoceros [Nothoceros + Megaceros]] clade, probably by lateral gene transfer (see also Wickell & Li 2019).
Dendroceros is one of the few desiccation-tolerant hornworts, it is also epiphytic and has green, multicellular spores, all features tending to be associated with the epiphytic habitat (Schuette & Renzaglia 2010; see also green-spored ferns and the epiphytic habitat).
For details of stomatal opening, see Renzaglia et al. (2017; also Merced & Renzaglia 2018; Pressel et al. 2014, 2018). As Renzaglia et al. (2017) note, the stomata open once, remain open, and appear to assist in the drying of the capsule contents, indeed, the sporogenous tissue is initially bathed in mucilage, and intercellular spaces in the sporophyte are filled with liquid, not gas. Consistent with this function, neither stomatal size nor number respond to variations in CO2 concentration and stomata do not respond to CO2 or water availability (Field et al. 2015b; Pressel et al. 2018). Some taxa lack stomata on their sporangia, but in that case the sporangia are more or less enclosed by the gametophytic involucre (Renzaglia et al. 2017).
Pyrenoids, quite common in hornworts, may increase the concentration of CO2 around the chloroplasts and so increase the efficiency of photosynthesis (E. Smith & Griffiths 1996). See above for the evolution of pyrenoids in hornworts - now unclear - and land plants.
For the association of hornworts with the nitrogen-fixing Nostoc (polyphyletic), see Rai et al. (2000), and for what is known about Nostoc and nitrogen fixation, see Santi et al. (2013). F.-W. Li et al. (2020) found a number of genes that were induced in Anthoceros when cyanobionts were present, and although a couple of these genes were also involved in mycorrhizal and other microbial symbioses elsewhere, the genes involved were not orthologous.
Fertilization & Spore Dispersal. For literature on spermatozoids and fertilization, see Muggoch and Walton (1942: Anthoceros laevis) and Pressel and Duckett (2019).
Plant-Bacterial/Fungal Associations. Endogone-like fungi (Mucoromycotina) are associated with some hornworts (Bidartondo et al. 2011; Rimington et al. 2014, 2019), and are quite common there, as are Glomeromycota (Pressel et al. 2010). The two may form mycorrhizal associations with the same species, but whether either or both mycorrhizal association was ancestral in the group is unclear (Desirò et al. 2013); glomeromycote associations, at least, seem rather casual (Pressel et al. 2010; Rimington et al. 2018).
In most taxa Nostoc enters the gametophyte through mucilaginous clefts (Adams 2002; Adams & Duggan 2008).
Genes & Genomes. The extensive RNA editing in Anthoceros and its relatives is at codon positions that are otherwise universally conserved in land plants (Duff et al. 2007). Transcripion-associated proteins are uncommon here, nevertheless a few, e.g. YABBY, involved in specifying leaf adaxial-abaxial identity, are found mainly only here and in seed plants (F.-Y. Li et al. 2020; C. Li et al. 2023/2024). There seems to have been between 1-6 duplications in each of the three groups of GDSL lipases somewhere around here (Volokita et al. 2010). Hornworts may have sex chromosomes, and so, as might be expected, polyploidy is uncommon (Bowman et al. 2017 and references). There has been loss of genes from the nuclear genome in hornworts (MacLeod et al. 2022 and references).
Anthoceros has lost the chloroplast rps15 gene (Martín & Sabater 2010) and the IR has expanded somewhat (Villarreal et al. 2013).
There has been quite extensive loss of protein-coding genes in the mitochondrion, i.e. ca 1/2, versus less than 1/3 in other land plants (Y. Liu et al. 2014b). Leiosporoceros has an intron in the mitochondrial nad5 gene, as do Anthoceros and immediate relatives (Villarreal et al. 2013); there over 40 introns in the chondrome of Anthoceros (Mower 2020).
Chemistry, Morphology, etc.. The cell walls of spores and their elaters contain xylans, an important polysaccharide found in the secondary cell walls of vascular plants, but unknown in other bryophytes (Carafa et al. 2005). The elaters are multicellular structures of tapetal origin, quite unlike those of Equisetum (Pacini & Franchi 1991).
The pores of the mucilage clefts of hornwort gametophytes are probably not homologous with stomata, which are not known from gametophytes in extant land plants (Adams 2002; Adams & Duggan 2008; McAdam et al. 2021).
See also Ligrone et al. (2000) and especially Frangedakis et al. (2020) for general information, Villarreal et al. (2010) for a summary of our understanding of hornworts, Vaughn et al. (1992) for the distinctive and variable chloroplasts, R. C. Brown and Lemmon (2013) and Schuette and Renzaglia (2010: Dendroceras) for sporogenesis, and for antheridia, see Duff et al. (2004) and Cargill et al. (2005).
Phylogeny. Relationships within hornworts are still unclear in part. Leiosporoceros may be sister to all other hornworts, although the extensive RNA editing in other members of the clade obscures this position in some analyses (e.g. Duff et al. 2007); it has many distinctive features (see above). See also Stech et al. (2003), Duff et al. (2004) and Villarreal and Renner (2012) for relationships. Anthoceros and its possible segregate Folioceros are sister to remaining hornworts, and they have black or dark spores, etc. (in this they are like Leiosporoceros). Note, however, that a recent study by Peñaloza-Bojacá et al. (2025; 79 specimens, all genera) suggested that Leiosporoceros was sister to [Folioceros + Anthoceros] in multispecies coalescent analyses, but sister to the whole of the rest of the family in ML concatenation analyses; there was evidence of ancient hybridization.
Villarreal and Renner (2014) discussed the limits of and relationships around Nothoceros. Peñaloza-Bojacá et al. (2025) found that Folioceros was embedded in Anthoceros,
Classification. Several classifications of this clade have appeared recently (Frey & Stech 2005a; Stotler & Crandall-Stotler 2005; Duff et al. 2007); they tend to be rather elaborate. Söderström et al. (2016) provide a classification down to the level of species.
[MARCHANTIOPHYTA + BRYOPHYTA s.str.] / SETAPHYTA
Gametophyte: stem usu. with conducting tissue; perichaetial leaves +; archegonium exposed [venter free; sporophyte: short-lived, initial division of zygote transverse; capsule with stalk/seta.
Age. The age of this node is estimated at ca 473 Ma (Healey et al. 2023).
Evolution: Divergence and Distribution. There are interesting connections between stomatal and setal development, although the full story is not yet clear. Genes that are involved in stomatal development in other plants control the formation of the seta of the sporophyte in Marchantia polymorpha - MpSETA (a class Ia HelixLoopHelix gene) and MpICE2 (a class IIIb bHLH gene) transcription factors being involved; if the seta does not elongate, the sporangium does not dehisce (Moriya et al. 2022/2023). In Arabidopsis thaliana AtICE1 controls stomatal development on the anthers, so affecting anther dehydration which in turn affects i.a. pollen grain maturation, filament elongation and anther dehiscence - AtFAMA is also involved (Wei et al. 2018). Note that although the sporangia of most mosses and liverworts do have setae, they develop in rather different ways in the two. Thus the seta in mosses develops from an intercalary meristem while in liverworts all parts of the sporangium develop together (note that groups like Ricciaceae have no seta). Moriya et al. (2022/2023) suggest that the behaviour of the ICE-type genes in Marchantia is derived, posession of stomata being ancestral. At one level, in both liverworts, other bryophytes, and flowering plants stomatal genes are expressed in tissues involved in spore dispersal... Note, however, that what goes on both in liverworts that lack a seta and in mosses is not known.
A few mosses (e.g. Sphagnum) and liverworts have red anthocyanin pigments (Campanella et al. 2014; Piatkowski et al. 2020).
II. MARCHANTIOPHYTA / HEPATICS / LIVERWORTS
Prenylation of flavonoids +; gametophyte: fungal entry via rhizoids; thallus simple; lunularic acid +; rhizoids smooth, living/(pegged, dead); perforate water conducting cells +; membrane-surrounded oil bodies + (0); antheridia spherical, long-stalked; microtubule organizing centres + [MTOCs polar organizers]; cell walls with relatively little cellulose; sporophyte: second embryonic division vertical, no growth by apical cell; stomata 0; plasmodesmatal auxin transport apolar; foot ± conical to spheroidal, cell divisions uniform, sporangium initially enclosed, seta [stalk] evanescent, forming by cell elongation after the sporangium develops; sporangium columella 0, wall 1(2-4) cell layers across, opening by four slits; endothecial cells producing archesporial tissue; meiosis usu. polyplastidic; elaters +, unicellular; mitosis with polar MTOCs; active tapetum 0, spore walls lamellate; nuclear genome [1C] (0.21-)1.2-9.44(-20.46) pg/(206-)1,844(-20,010) Mb; plastome ca 119 Kb; chondrome 143-189 kb, group I cox1i729 intron +.
Ca 4,000-7,486 spp. (see Söderström et al. 2016).
Age. Crown group Marchantiophyta are dated to (509-)484(-452) Ma by Cooper et al. (2012) and 509-486 Ma by Laenen et al. (2014), although Heinrichs et al. (2007) suggested an age of (410.5-)407.6(-404.7) Ma, B. J. Harris et al. (2021/2022) an age of 440-412 Ma, rather older, and Y.-L. Qiu et al. (2024: c.f. topology) an age of (489.6-)456.0(-424.3) Ma.
From fossil evidence, liverworts or liverwort-like plants were present by the Ordovician, and liverworts may have been common through the Silurian and Devonian (Graham et al. 2011), e.g. see the Middle Devonian Metzgeriothallus sharonae from the eastern U. S. A. - Jungermanniopsida and Marchantiopsida and Metzgeriidae and Jungermanniidae had separated by then (Hernick et al. 2007). Heinrichs et al. (2007) evaluated the identity of fossils purporting to be liverworts.
1. Haplomitriopsida Stotler & Crandall-Stotler
Gametophyte: Endogonales (Mucoromycotina) +; plant body axial; thallus apical cell tetrahedral, stem anatomy complex [esp. Treubia], mucilage copious [stalked slime papillae]; stem erect, leaves spiral, (unlobed) [Haplomitrium - H.] or ± prostrate, leaves two-ranked, lobed; (rhizoids 0 - H.); plant dioicious; bracts surrounding sporophyte 0; meiosis monoplastidic [?all]; (spores in dyads); embryo haustorium?; distinctive blepharoplast; nuclear genome [1C] 4.35-10(-10.05) pg/4254-9794 Mbp; plastome with somewhat epanded IR.
3/16. More or less world-wide, scattered.
Age. Crown-group Haplomitriopsida are dated to (396-)353(-316) Ma (Newton et al. 2007) or (469-)414(-352) Ma (Cooper et al. 2012).
[Marchantiopsida + Jungermanniopsida]: gametophyte dorsiventral; fungal symbiont also Glomeromycotina; leaves 0; late development of placental wall ingrowths; (meiosis monoplastidic), (spore development endosporic).
Age. This node is dated to before the Middle Devonian (475-)442(-408) Ma (Cooper et al. 2012), while Heinrichs et al. (2007) suggest an age of (382.8-)372.6(-362.4) Ma (similar ages also in Newton et al. 2007) and B. Zhong et al. (2014b) an age of (569.7-)375.8(-172.5) Ma, which pretty much covers the waterfront.
2. Marchantiopsida Cronquist
Thallus branching truly dichotomous, ventral scales in two rows; gemmae in receptacles [gemma cups]; paraphyses +; oil bodies only in specialized cells; sporocytes often lacking lobing, (MTOCs at nuclear envelope); placental transfer cells variable; no RNA editing in organellar genomes.
Ca 340 spp.
Age. Crown-group Marchantiopsida are estimated to be (322-)284(-251) Ma (Cooper et al. 2012), (268-)248(-231) Ma (Newton et al. 2007), 269-207 Ma (Laenen et al. 2014) or (365-)295(-250) Ma (Villareal A. et al. 2015b).
There are fossils of complex thalloid liverworts from the Triassic and mid-Cretaceous (references in Villareal A. et al. 2015b).
2a. Blasiidae He-Nygrén
Fungal symbiont 0, association with Nostoc; thallus simple, margins lobed; plant dioicious; capsules ellipsoid, walls multistratose; meiosis monoplastidic; nuclear genome [1 C] 0.5 pg/488 Mbp.
2/2: North Temperate.
2b. Marchantiidae Engler
Thallus complex, with air chambers, air pores to the outside; transfer cells in both sporophyte and gametophyte; apical cells of sporophyte 0; sporocyte meiosis monoplastidic.
Age. The age of this clade is (328-)263(-226) Ma (Villareal A. et al. 2015b).
2b1. Neohodgsoniales D. G. Long
Thallus with compound air pores; rhizoids dimorphic, some dead; archegoniophore/carpocephalum +, branched; ?nuclear genome.
1/1: Neohodgsonia mirabilis: New Zealand and Tristan da Cunha.
2b2. Sphaerocarpales Caveo
Fungal symbiont 0; (thallus lacking air chambers/pores), (oil bodies 0), pegged rhizoids 0, ?= smooth, (carpocephala +); sporangium wall breaks down at maturity, elaters 0; ?nuclear genome.
4/20-30. Europe, North America, Australia, South Africa (!once found - Monocarpus).
2b3. The Rest: ?
Lunularia
(Plant annual); Mucoromycotina 0 (+), (Glomeromycotina 0); thallus differentiated (not), photosynthetic filaments + (0); air pores simple or compound; rhizoids dimorphic, some pegged, dead; archegoniophore/carpocephalum +, simple, with furrows in which there are pegged rhizoids.
Marchantia
Dumorttiera
(Plant epiphytic); (Mucoromycotina 0)/(dikaryon + [basidiomycete])/(Glomeromycotina 0)/(fungal symbiont 0); fructan sugars accumulated; thallus +, simple/leaves +, (2-)3-ranked, lobed, costa 0; ?rhizoids; oil bodies in ± all cells; placental fungal haustoria +, transfer cells only in sporophyte (0).
Ca 4,000 spp. or more.Age. Differences in suggested ages for crown-group Jungermanniopsida are rather great, almost 100 Ma: e.g. (425-)390(-353) Ma (Cooper et al. 2012), (335-)328.5(-322) Ma (Heinrichs et al. 2007) or (331-)292(-262) Ma (Newton et al. 2007).
Plant a simple thallus; nuclear genome [1C] 0.55-9.44(-20.46 - Phyllothallia) pg/894-9237(-20006) Mbp.
Metzgeriidae Bartholomew-Began
Plant a simple thallus; nuclear genome [1C] 0.74-9.24 pg/724-9037 Mbp.
Jungermanniidae Engler
Fungal associates ascomyetes or basidiomycetes; plant radial, leafy, cutting faces of apical cell at 120o, leaves succubous (incubous/transverse/plant a simple thallus); perigynium around sporangium; second embryonic division transverse [?level].
Evolution: Divergence and Distribution. Heinrichs et al. (2007) proposed possible divergence times for leafy liverwort clades (see also Newton et al. 2007; Coooper et al. 2012), while Villarreal A. et al. (2015b) provide dates in the complex-thalloid clade, i.e. Marchantiopsoda.
Prototaxites, a trunk-like structure up to 8.8 m long and 1.37 m diameter, although often much smaller, apparently is the largest land organism of the Late Silurian to Late Devonian 420-370 Ma. Its identity has been the subject of much discussion. Some evidence suggests that it is neither a fungus nor a lichen, but is made up largely of rhizoids of marchantioid liverworts and of the remains of their fungal associates, all rolled together (Graham et al. 2010a). Suggestions that it was a fungus persist, thus the Lower Devonian Nematasketum diversiforme, thought to be close to Prototaxites, was compared with fungal rhizomorphs, although neither fossil group has clamp connections or septae (Edwards & Axe 2012). Nelsen and Boyce (2022) suggested that it was some kind of filamentous heterotroph, perhaps an extinct fungus.
Overall, the fossil record of liverworts is poor, although they have been found in amber; for literature, see Heinrichs et al. (2018), Tomescu et al. (2018) and Bippus et al. (2019).
Evolution in the complex-thalloid clade was examined by Villarreal A. et al. (2015b), and they found that overall molecular evolution (plastid and mitochondria) was slow compared to that of other hepatics, except in Cyathodium, an annual plant (a reversal), but morphological evolution was less so. Given the topology of the tree (e.g. the position of Neohodgsonia is important), where features of the carpocephalum (= the archegonial receptacle after fertilization) and those involved in the differentiation of the thallus - the variation is extensive - are to be placed on the tree is unclear, but the pattern of gains and losses will be complex (Villareal A. et al. 2015b).
Heinrichs et al. (2007) discussed the evolution of the ca 4,500 species of leafy liverworts, and Wilson et al. (2007a, b) examined the diversification of Lejeuneaceae in particular. Although many liverwort families had diverged by the end of the Cretaceous, they have diversified considerably during the Tertiary (Cooper et al. 2012). Note that although ages in Newton et al. (2007), Heinrichs et al. (2007) and Cooper et al. (2012) can differ greatly, the overall conclusions of the authors differ less. Much of the diversification of Porellales and Jungermanniales (Jungermanniidae), both leafy liverworts epiphytic on bark and leaves of flowering plants, has occurred since the evolution of angiosperms (Ahonen et al. 2003; Forrest & Crandall-Stotler 2004), although the clades involved are (much) older (Cooper et al. 2012). Similarly, the nutritional mutualisms that are suggested to occur between vascular plants and liverworts via the fungi that they have in common (Hoysted et al. 2019) raise issues about the age of the particular association and of such associations in general.
Ecology & Physiology. Understanding the ecophysiology of the first liverworts is a challenge. Field et al. (2012, 2014 and references) discuss details of the nature of the mutualistic association between fungus and plant in some liverworts, including Haplomitriopsida (see also Ligrone et al. 2007; Field et al. 2014; Rimington et al. 2019; Pressel et al. 2021). The association of Mucoromycotina with Haplomitrium benefits both partners (N, P to the liverwort, C to the fungus), and since this liverwort lacks rhizoids the fungus is particularly important in nutrient uptake (Bidartondo et al. 2011; Field et al. 2014). Associations with glomeromycotes are known from complex thalloid liverworts (Pressel et al. 2010), while yet other liverworts are associated with both kinds of fungi, this double association being stable and with considerable implications for biosphere evolution (Field et al. 2015c; Rimington et al. 2019: some Marchantiopsida but few Jungermanniopsida included; see also elsewhere). Extant Marchantia, at least, are mixotrophic (Hata et al. 2000; Graham et al. 2010a, b).
The echlorophyllous Cryptothallus [= Aneura] mirabilis is the only mycoheterotrophic liverwort, indeed, it is the only mycoheterotrophic bryophyte s.l.. It can grow up to 20 cm below the surface of Sphagnum-dominated peat bogs and is associated with hyphae of the ectomycorrhizal basidiomycete Tulasnella. The latter is simultaneously associated with Betula or Pinus from which the liverwort indirectly obtains its carbon (Wickett et al. 2008 and references; Merckx et al. 2013a).
Fungi associated with liverworts, along with cyanobacteria, etc., are involved in soil formation, and although soil accumulation in less than in moss communities, at least in Iceland (ca 1.5 vs 3.5 cm), and clay minerals like smectite also accumulate (Mitchell et al. 2016).
Quite a number of liverworts, including epiphytic species, tolerate extreme desiccation, although most lack internal water-conducting cells (Ligrone et al. 2000). Desiccation tolerance here can be constitutive or induced (Oliver et al. 2005; Gaff & Oliver 2013).
In Marchantia polymorpha, at least, riccionidins, which had been thought to be anthocyanins, turn out to be a different group of flavonoids, the pigmented auronidins. Like anthocyanins, they also fluoresce under U.V., and they are thought to be involved in abiotic stress tolerance (Berland et al. 2019); little otherwise (function, distribution) is known about them. For prenylation of flavonoids in liverworts (and in angiosperms), see Yonekura-Sakakibara et al. (2019).
A squalene-hopane cyclase gene apparently moved from cyanobacteria via lateral gene transfer to liverworts, and also independently to mosses (F.-W. Li et al. 2018; Wickell & Li 2019). The L and D isomers of methionine are treated identically metabolically in liverworts, almost alone in land plants (Kenrick & Crane 1997).
Terpenoids, and probably other metabolites, are both synthesized and sequestered in the membrane-bounded oil bodies that characterise liverworts; these terpenoids may be toxic to the plant. The oil bodies are found in both sporophyte and gametophyte, and, depending on the species, they may be found in idioblastic cells, or scattered in all cells; the plesiomorphic condition for the whole group may be the presence of idioblasts (Romani et al. 2022). The oil bodies probably help deter herbivores, and they also may be toxic to microbes (references in Romani et al. 2022). Thus horizontally transferred microbial-like terpene synthase genes in Marchantia polymorpha may contribute to the exceptional terpene diversity of liverworts, bacterium-derived core immune components as well as specialized metabolic pathways may be involved in pathogen resistance (Colletotrichum nymphaeae was the test fungus - El Mahboubi et al. 2026). Among bryophytes s.l., liverworts are particularly noted for the diversity of their terpenoids - 1,600 or so different kinds being known here, around 16 times the number in mosses - and the genes involved may have come from microbes by HGT, microbial terpene synthase-like genes having been found both in liverworts (Romani et al. 2022) and in other embryophytes apart from seed plants (Jia et al. 2016; F. Chen et al. 2018; El Mahboubi et al. 2026). For more on oil glands, see elsewhere.
For the loss of stomata in liverworts, see F.-W. Li et al. (2020), the air pores here having nothing to do with stomata (e.g. B. J. Harris et al. 2020). For the loss of genes controlling stomatal development in liverworts, see F.-W. Li et al. (2020) and Harris et al. (2020).
Fertilization & Spore Dispersal. For fertilization, see Muggoch and Walton (1942), Pressel and Duckett (2019), etc.. The dead, pegged rhizoids to be found in marchantialean liverworts with complex thalli may help ensure the water supply to the stalked carpocephala (Duckett et al. 2014), and also faciltate fertilization. In a number of marchantialean liverworts, including Conocephalum (Fegatella) conicum, there is explosive antheridial dehiscence - basically wind dispersal of spermatozoids, at least initially, and depending on conditions, the spermatozoids may be dispersed anything from 1> cm to several metres (references in Pressel & Duckett 2019). However, the particular focus of the study by Pressel and Duckett (2019) was on fertilization in the dioicious pioneer Marchantia polymorpha ssp. ruderalis. Here, as in many other bryophytes in general (but apparently not ferns) spermatozoids move along air-water interfaces, their movement being facilitated by lipids liberated during antheridial dehiscence (see also Muggoch & Walton 1942). Flooding of the plants recurs throughout the season, and in any one flooding event, a single male Marchantia plant can release over 50 x 106 spermatozoids, and these spermatozoids, which can live for several hours, move along the air-water interface and fertilize female plants up to 19 m away - and perhaps even further, chemoattraction guiding the ultimate movements of the spermatozoids (Pressel & Duckett 2019, q.v. for details about other liverworts).
Spores and elaters are intermixed in liverwort capsules. In some species the twisting of the elaters as the humidity changes leads to the ejection of the spores, not very violently. In other species like Cephalozia bicuspidata the elaters are attached to the capsule at one end, they lose water, twist and contract - and then cavitation occurs, the elaters rapidly returning to their original shape, breaking free from the capsule, and slinging the spores into the air, quite a violent discharge. In another variant of this mechanism in Frullania and relatives, each elater is attached to the top and bottom of the capsule; as the capsule opens, the elaters are stretched, and then they break free at the bottom, contracting and throwing the spores into the air as they do so (Ingold 1939, 1974).
Plant-Animal Interactions. For a summary of herbivory and galling, including examples from the Middle Devonian where some cells of the fossils perhaps contained oil and represent defence against herbivory, see Labandeira et al. (2013). Caterpillars of some 20 or so species of Micropterigidae, a basal, jawed, lepidopteran clade, are common on Conocephalum conicum in Japan, diversifying so 20 Ma, and this group also includes species eating other liverworts elsewhere, also angiosperms, detritus, etc. (Imada et al. 2011; Ragier et al. 2015 and references).
Plant-Bacterial/Fungal Associations. All the major groups of fungi that form mycorrhizal associations with embryophytes as a whole are associated with liverworts (Read et al. 2000; Duckett et al. 2006b; Pressel et al. 2008c, 2010, 2021; Bidartondo et al. 2011; Field et al. 2014, 2015c; Rimington et al. 2019; Sgroi & Paszkowski 2020). Rimington et al. (2020) map the distribution of the different kinds on fungi on to a phylogeny of liverworts, and patterns of gains and losses (epiphytic taxa are in the latter) are very complex; interestingly, associations with ascomycetes and basidiomycetes are largely restricted to Jungermanniidae. The fungus may move from these liverworts to seed plants, or vice versa (Pressel et al. 2008c, 2010: see also Bidartondo & Duckett 2009). Thus the ascomycete Rhizoscyphus [= Hymenoscyphus] ericae is very commonly an associate of the hair roots of North Temperate Ericaceae (see elsewhere), and it also forms mycorrhizal associations with Jungermanniidae-Schistochilaceae and other leafy liverworts; the fungus colonizes the liverwort rhizoids, often inducing branching/septation (Duckett & Read 1995; Upson et al. 2007; Pressel et al. 2008b, c). Interestingly, reciprocal fungal associations form between the ascomycete fungi in Pachyschistochila, from the extreme southern end of the world, and a variety of other leafy liverworts from Britain, and also Calluna vulgaris; Pachyschistochila, but not the others, even forms associations with the basidiomycete associate of Lophozia-Lophoziaceae, also Jungermaniidae (Pressel et al. 2008c). Of course, indirect associations with seed plants have little necessarily to do with early liverwort-fungus associations (e.g. Duckett & Read 1995; Pressel et al. 2008c), but the antiquity of Schistochilaceae (>250 Ma?) and the commoness of ascomycetes in austral liverworts in general make one wonder exactly what associations formed when (Duckett et al. 2008c). Jungermanniopsida-Jungermanniales may have become associated with ascomycetes more than 250 Ma (Pressel et al. 2008b), but Ericaceae with ericoid hair roots and their distinctive fungi are well under 100 Ma (see above). Members of a clade of Sebacinales-Serendipitaceae are particularly associated with liverworts, again Jungermanniidae, (Weiß et al. 2016), and this is also likely to be a relatively recent interaction. Here colonization of the plant is of the Paris AM type, with extensive intracellular hyphal growth producing coils, but sometimes neither arbuscules nor vesicles (Field et al. 2015d). Given the movement of C, etc., between different angiosperms via common Paris AM type mycorrhizae (), Indeed, Pressel et al. (2021) describe such liverwort-fungus associations as mycorrhizal in nature.
Russell and Bulman (2005) found different phylotypes of Glomus group A fungi consistently associated wih Marchantia foliosa in New Zealand, and two of the main fungal clades are also to be found in podocarp roots.
Mucoromycotina-Endogonales commonly form associations with liverworts other than Jungermanniidae in particular, and such associations may even be ancestral; these associations are not nested (Rimington et al. 2019; see also Kottke & Nebel 2005; Duckett et al. 2006b; Field et al. 2014; Rimington et al. 2014, 2020). The basal Haplomitriopsida form associations with Mucooromycotina-Endogonales alone (Rimington et al. 2020; Pressel et al. 2021), and again such associations show complex patterns of gains and losses in other liverworts (c.f. glomalean fungi here - once the AM association is lost, it is not regained); the two kinds of fungi quite often co-occur, and in associations that appear to be stable; such fungal networks are nested (Rimington et al. 2019). Interestingly, mucoromycote-liverwort associations are regained only in liverworts that have maintained their AM associations (Rimington et al. 2019).
Epiphytic Porellales lack fungus associations, and in general epiphytic or epilithic liverworts are often not associated with fungi (Pressel et al. 2010, 2021), a tendency also evident in hornworts and angiosperms. Similarly, liverworts in more or less transiently wet and nutrient-rich habitats also lack mycorrhizae, as do hornworts, ferns like Equisetum and Salviniales (Pressel et al. 2016), and also aquatic angiosperms.
A number of endophytic fungi grow in Marchantia polymorpha, a species that rarely has mycorrhizae. Nelson et al. (2018) detail the variety of positive and negative effects these fungi have on the plant, effects that partly depend on the identity of other fungi in the plant, and partly even on the age of the association - and the effects may also be quite different on other plants with which the fungi are associated.
The morphology of the gametophyte of Treubia depends on whether or not it has associated fungi (Field et al. 2015a; Rimington et al. 2017).
The mycoheterotrophic Cryptothallus [= Aneura] mirabilis is associated with the ectomycorrhizal basidiomycete Tulasnella (Cantherellales) (Kottke & Nebel 2005; Wickett & Goffinet 2008; Wickett et al. 2008; Imhof et al. 2013; for Tulasnella and liverworts, see Oberwinkler et al. 2017). Aneura is also associated with basidiomycetes (Pressel et al. 2010).
Blasia (Marchantiopsida) fixes nitrogen by virtue of of its association with Nostoc (Rai et al. 2000; Warshan et al. 2018 for the relationships of N-fixing Nostoc), but it does not form any associations with fungi (Field et al. 2015c).
Genes & Genomes. Endopolyploidy has rarely been detected in liverwort nuclei (Bainard & Newmaster 2010a, b), although exceptions are the highly endopolyploid nuclei of the smooth rhizoids of at least some species (Duckett et al. 2014). Variation in genome size, even at the infraspecific level, is quite considerable, but there seems to be no correlation with chromosome number (Bainard et al. 2013). There is also little evidence of genome duplications in the Marchantia polymorpha genome, and Bowman et al. (2017) suggest that this is because of the early evolution of sex chromosomes here; presence of sex chromosomes is associated with genome stability (see also hornworts). If polyploidy does occur, the species usually becomes monoecious (Bowman et al. 2017 and references). There is substantial variation in the size of the nuclear genome, that of Marchantiopsida being particularly small, but few species have been examined (Villareal A. et al. 2015b).
Details of meiosis, whether there is one or more chloroplasts, etc., vary considerably in liverworts, and some derived taxa are quite like vascular plants in these respects (R. C. Brown & Lemmon 2008, 2013). There is monoplastidic meiosis in Monoclea, Haplomitrium and Blasia (Renzaglia et al. 1994a; R. C. Brown & Lemmon 2011a), but there is also polyplastidic meiosis as well as intermediate forms. Buschmann et al. (2016) discuss microtubles and cell division in Marchantia.
Silent substitution rates in the three genomic compartments vary considerably, the overall ratio being 1(chondrome):15(plastome):10(nucleome), and dN/dS ratio varies similarly, indicating stronger purifying selection in the plastome (Dong et al. 2021). Marchantiopsida have lost to ability to carry out RNA editing of the organellar genes (Rüdinger et al. 2008).
Plastomes in liverworts generally seem to be conserved and are rather similar to those in their streptophyte ancestors, if often somewhat smaller, while their GC contents are rather variable, those of Haplomitrium, Aneura and some other taxa being notably high (Y. Yu et al. 2019b, c). This overall stability - there seem to be no large or systematically very important changes in the plastome - was confirmed by Dong et al. (2021). Aneura and Treubia (the latter had the smallest plastome) had most pseudogenes (Dong et al. 2021). Aneura mirabilis is a mycoheterotroph , interestingly, the plastome is more or less normal in size, although it has functionally lost around 25 genes, including ndh genes, much in line with gene losses in parasitic, carnivorous, etc., angiosperms (see also Wickett et al. 2008; Bellot & Renner 2015; Mower et al. 2021).
Chemistry, Morphology, etc.. For features of Haplomitriopsida, see Duckett et al. (2006a), for those of Marchantia, see Shimamura (2016: also much else), and for Marchantiidae, see Flores et al. (2017). For apical cell division, see Piatkowski et al. (2013), for the development of the unicellular elaters, see Renzaglia et al. (1997) and Crandall-Stotler and Stotler (2000), for spore walls, see Wellman et al. (2003) and Strother and Taylor (2018), and for rhizoids, see Duckett et al. (2014).
Phylogeny. Morphological studies indicated that Sphaerocarpos might be sister to all other liverworts (Crandall-Stotler & Stotler 2000). However, molecular data suggest rather different relationships, and that genus is now included in Marchantiopsida (see also Forrest & Crandall-Stotler 2004, 2005; He-Nygrén et al. 2004; Qiu et al. 2006); the long branches associated with Haplomitrium and Treubia cause their position in the tree to be somewhat migratory. He-Nygrén et al. (2006: 3 chloroplast and 1 nuclear genes, morphology) outline the phylogeny and classification of liverworts, finding a basic structure [Treubiopsida [Marchantiopsida + Jungermanniopsida]] (N.B.: In older literature, Treubiopsida = Haplomitriopsida.) This basic topology is confirmed by Forrest et al. (2006: five genes, all three compartments, good sampling, esp. of thalloid liverworts, 2015), Volkmar and Knoop (2010) and Cooper et al. (2012). Dong et al. (2021) in a plastome analysis also found the same phylogenetic structure, relationships in the two latter being [Marchantiales [[Pelliales [Fossombroniales + Pallaviciniales]] [[Pleuroziales + Metzgeriales] [Jungermanniales [Philborneales + Porellales]]]]] - support was strong.
Marchantiopsida include Blasia, Sphaerocarpos, etc., although support for the inclusion of the former in this clade could be improved (but c.f. some analyses in Forrest & Crandall-Stotler 2004, esp. 2005; Qiu et al. 2007: Blasia sister to other Marchantiopsida; see also He-Nygrén et al. 2004; Volkmar et al. 2011 ). Cooper et al. (2012, see also Forrest et al. 2015) also found Blasia - with Cavicularia - to be sister to the other Marchantiopsida. Flores et al. (2020a) incorporated morphological data into their analysis of relationships in liverworts, and fossils into their study of complex thalloid liverworts (Marchantiidae: Flores et al. 2020b). Flores et al. (2020b) found that these fossils caused some changes in the relationships and apomorphies proposed by earlier studies (Villarreal et al. 2016; Flores et al. 2017), although basal relationships (up to Dumortiera) were the same in all three.
Within Jungermanniopsida, simple-thallus groups are paraphyletic with respect to the speciose and monophyletic leafy liverworts, within which Pleurozia is sister to the rest or near-basal (e.g. He-Nygrén et al. 2004; Davis 2004: P. grouped with some simple-thalloid genera; Masuzaki et al. 2010; Volkmar et al. 2011; Cooper et al. 2012: extensive study). These general relationships were also recovered by Qiu et al. (2007). Leafy liverworts have been studied by Cooper et al. (2011: the speciose Lepidoziaceae), and Gradstein et al. (2003) and Y. Yu et al. (2013), both Lejeunaceae. Yu et al. (2019a: 84 protein-coding genes, 35 taxa) found that Ptilidiales were sister to Jungermanniales.
Classification. See Frey and Stech (2005b) and Crandall-Stotler et al. (2009) for phylogeny-based classifications and also Söderström et al. (2016) for a classification (followed above) that goes down to species. Flores et al. (2020b) noted the number of monogeneric families in Marchantiales and suggested that some could be merged, which seems reasonable.
Gametophyte: several developing from a single spore; radial, axial; cells ± differentiated, rhizoids multicellular [?all], leaves +, unistratose, costa +; perichaetium +; sporophyte: early embryo spindle-shaped, foot ± elongate-tapering-pointed, seta developing from basal meristem [between epibasal and hypobasal cells], indurated, conducting tissue common; PIN[auxin efflux facilitators]-mediated polar auxin transport, calyptra +, persistent; endothecium also producing archesporial tissue; placenta with transfer cells in sporophyte alone; MTOC becoming diffuse, perinuclear; perine + [?level]; endopolyploidy widespread [at least Polytrichum + the rest]; x = 7, nuclear genome [1 C] (0.17-)0.38-0.92(-2.05) pg/(170-)504(-2,004) Mb; transcription factor genes numerous; plastome ca 127 Kb; chondrome 100-141 kb. 31 orders.
Age. Crown-group mosses may be (400-)379(-362) Ma (Newton et al. 2009), 420-364 Ma (B. J. Harris et al. 2021/2022) or (416.4-)367.7(-333.7) Ma (Y.-L. Qiu et al. 2024: c.f. topology).
The separation of Takakia and Sphagnum is dated to 319-129 Ma (Shaw et al. 2010a) and of Sphagnum from Ceratodon to ca 391 Ma (Healey et al. 2023).
1. Takakiopsida Stech & W. Frey / Takakiophytina - Takakia S. Hattori & Inoue
Gametophyte: initially ?thalloid, ?mycorrhizal; oil bodies + [?membrane-surrounded], rhizoids 0, vegetative cells monoplastidic [mitosis, too, monoplastidic]; cutting faces of apical cell at 120o; leaves forked, ± 3-ranked; perforate water conducting cells +; plant acrocarpous; perichaetial leaves 0; sporophtyte: capsule dehiscence spiral; stomata 0; sporocytes unlobed, spores not trilete; n = 4, ?genome.
1/2. East Asia, west North America.
[Sphagnopsida [Andreaeopsida + The Rest]]: gametophyte: initially a filamentous persistent protonema, cutting faces of apical cell at 136o [most]; rhizoids branched, multicellular [septate, branches very fine, 0.3> mm?]; mycorrhizae 0; leaves spiral, unlobed; cp ITS3 differences.
Age. The crown-group age of this clade is (360-)352(-344) Ma (Y. Liu et al. 2014a), 448.5-344.5 Ma (Morris et al. 2018a) or ca 330 ma (Y.-L. Qiu et al. 2024).
2. Sphagnopsida Ochyra / Sphagnophytina
Gametophyte: dioicious (monoicious); fructan sugars accumulated; protonema thalloid, rhizoids +, in mature gametophyte 0; branches in axillary fascicles, spreading and pendant; leaf cells dimorphic [groups of empty and hyaline cells surrounded by strands of chloroplast-containing cells]; antheridia borne singly in the axils of essentially undifferentiated leaves, long-staled, spherical; sporophyte: capsule sessile, borne on gametophytic pseudopodium; second embryonic division transverse; foot from hypobasal cell, bulbous, placental transfer tissue 0; stomata + [= pseudostomata], stomium 0, intercellular cavities beneath 0; capsule dehiscence subapical and transverse [= operculate], explosive; columella massive, overarched by spores; placental transfer tissue 0; amphithecium alone producing archesporial tissue, endothecium converted into columellar tissue, archesporium continuous over top of columella; sporocytes unlobed, spore wall multilayered; n = 38, 42.
4/355: Sphagnum (350). World-wide.
Age. Crown-group Sphagnopsida are around 104-30 Ma (Shaw et al. 2010a), a mere ca 25 or even 14 Ma (Shaw & Devos 2014) or ca 68.5 Ma (Y.-L. Qiu et al. 2024).
455-454 Ma fossils of leaves with a distinctive cell structure similar to that of extant Sphagnum have been described from Ordovician deposits in Wisconsin and placed in Sphagnopsida - no midrib could be found in these fossils, so identification did not proceed further (Cardona-Correa et al. 2016; see also Turetsky et al. 2025). Ivanov et al. (2018) discuss the detailed morphology of Palaeozoic protosphagnalean mosses, and although there are some similarities with the cell arrangement of Sphagnum, the similarities between the two have different origins, cell expansion versus cell division. The earliest reliable fossil record of Sphagnum may be that in Reissinger (1950) - Jurassic in age (see also Bomfleur et al. 2023), but there are fossils of Sphagnum-like plants from Germany that are some 330 Ma (Hübers et al. 2013).
[Andreaeopsida + The Rest]: second embryonic division oblique.
3. Andreaeopsida J. H. Schnaffner / Andraeophytina
Gametophyte: plant autoicous; protonata multiseriate-thallose; plant initially thalloid; acrocarpous; leaf costa +/0; sporophyte: capsule sessile, borne on gametophytic pseudopodium; stomata 0; columella overarched by spores/dome-shaped archesporial tissue; calyptra 0; dehiscence down four (eight) vertical slits, (slits apical - Acroschisma); spores not trilete, exine initiated as globules, white-line centred lamellae 0, germination endosporic.
2/111: Andreaea (110). World-wide, rather scattered, esp. cool southern/circum-Antarctic.
4. Andreaeobryopsida Goffinet & W. R. Buck - Andreaeobryum macrosporum Steere & B. M. Murray
Gametophyte: plant dioicious; calyptra +.
1/1. Alaska, Western Canada.
4. Bryophytina
Gametophyte: (L and D isomers of methionine identical metabolically - Mnium); plant acrocarpous (pleurocarpous); (rhizoids 0 - Haplomitrium); hydroids + [cells dead, no contents], leptoids containing refractive spherules; paraphyses + [multicellular chlorenchymatous hairs mixed with gametangia] (Buxbaumia 0, also plant dioicious; antheridia spherical, long-stalked); sporophyte: (); stomata + [Oedidpodium up] (0); capsule dehiscence transverse, peristome +; spores hilate; PEP α subunit rpoA gene 0 (+).
Age. The crown-group age of this clade is perhaps 602-488 Ma (Laenen et al. 2014: none of the three clades above was included).
[Oedopodipsida [Tetraphidopsida + Polytrichopsia]]: nematodontous
Oedipodium - ?genome
Tetraphis - multiseriate/squamulose thallose protonemata; apical gemma cups; peristome teeth nematodontous, 4, formed of whole cells, cells vertically elongated
Polytrichales: peristome nematodontous, teeth from undulation of basal rim of cells.
Polytrichastrum incurvum has been described from the Early Cretaceous Catefica flora from Portugal (Bomfleur et al. 2023).
Late Apt. early Alb.Dicranodontium minutum and Campylopus lusitanicus, both Dicranales-Leucobryaceae, have been described from the Early Cretaceous Catefica flora from Portugal (Bomfleur et al. 2023).
A spore capsule like those of DirichaceaeBRYIDAE Engler / Bryopsida
Peristome double, teeth alternating [arthrodontous].
BRYANAE
FUNARIALES
first division of zygote longitudinal - but c.f. Harrison & Morris 2017; guard cell single, binucleate.
HYPNANAE
plant pleurocarpous [female gametangia on short lateral ± leafless branches].
[Hypnodendrales [Ptychomniales [Hypopteygiales [Hookeriales + Hypnales]
[Ptychomniales [Hypopteygiales [Hookeriales + Hypnales]
[Hypopteygiales [Hookeriales + Hypnales]
[Hookeriales + Hypnales]
HOOKERIALES
HYPNALES
42 families, 430 genera, ca 4,000 species.
Evolution: Divergence & Distribution. For literature on the fossil record of mosses, see Tomescu et al. (2018).
Mosses in China showed weaker latitudinal diversity gradients than did liverworts, perhaps because the former can handle a wider diversity of environments than the latter, a number of which are epiphyllous and need humid conditions; moss diversity was linked in part to local habitat heterogeneity (S.-B. Chen et al. 2015).
At least sometimes capsule shape changes are associated with changed speciation rates, as in the adoption of the pleurocarpous habit; there is an association of sporangial morphology with habitat (Rose et al. 2016).
Dicranidae/haplolepidious taxa are a diverse but species-poor group compared to the ca 12,000 species of Hypnanae/hypnalian pleurocarpous/arthrodontous mosses (Cox et al. 2010); Newton et al. (2007, 2009; see also B. Zhong et al. 2014b) give dates for many clades.
Within the speciose pleurocarpous mosses - about 40% of all mosses - diversification seems to have been early and rapid, clades diverging in the early Cretaceous, but since then there has been semi-stasis (Kürschner & Parolly 1999; Shaw et al. 2003b; Newton et al. 2006, 2007), although there may also have been more recent ([post-]Cretaceous) diversification as well. The diversification of Hypnales, with ca 1/3 of all mosses, may be associated with a genome duplication - or several small duplications (M. G. Johnson et al. 2016).
There is strong geographical signal in the phylogeny of Polytrichopsida, clades being largely south or north temperate (Bell & Hyvönen 2010: intergeneric hybridization?). The closest relative of the South American endemic dung moss Tetraplodon fuegianus is a population from West North America (Washington), divergence occurring ca 8.6 Ma, interestingly, overall geographical relationships in Tetraplodon were [Papua* [Nepal [Alaska [[Washington + South America*] [other N. Hemisphere samples]]]]], asterisks representing clades other than T. mnioides (Lewis et al. 2017).
Since some combination of Andreaea, Takakia and Sphagnum and immediate relatives are at the base of the moss phylogenetic tree and all have distinctive morphologies, apomorphies for mosses as a whole have been unclear. However, Y. Liu et al. (2019) found quite strong support for the topology followed here ([Takakia [Sphagnum [Andreaea ...]]], and they mention several characters linked to the tree. For distinctive features of Sphagnales, see Shaw et al. (2016b). Huttunen et al. (2013: focus on Plagiothecaceae) optimize the evolution of a number of features in both Hypnales and Hookeriales. The occurrence of stomata is rather sporadic in mosses, Renzaglia et al. (2020) noting that stomata have been lost over sixty times here... Coudert et al. (2017) looked at the evolution of branching patterns in the gametophytes in a study that focussed on Bryidae and Hypnanae in particular. i.e. on pleurocarpous mosses, although a number of acrocarps, including a few Dicranidae, were also examined. Interestingly, the genome of Sphagnum showed no collinearity with that of any other bryophyte known (Healey et al. 2023).
Ecology & Physiology. Despite their lack of an epidermis and of much in the way of a cuticle - and note that the composition of this cuticle, such as it is, is more like that of the lycophytes, etc., than that of hornworts and liverworts (Kong et al. 2020) - desiccation tolerance is common in mosses, even in taxa like Sphagnum, however, neither Sphagnum nor Takakia shows the extreme desiccation tolerance that is quite common in other mosses, where it can be either constitutive or inducable (Oliver et al. 2005; Gaff & Oliver 2013; see also papers in Plant Ecol. 151(1). 2000). The antheridia remain functional if they dry slowly and then are subsequently rehydrated (Stark et al. 2016 and references). Although one tends to associate mosses with damp conditions, if the plant is covered in water by rain, etc., this presents a barrier to diffusion of CO2 into the plants and photosynthesis may decrease (Royles et al. 2022). Interestingly, Slate et al. (2019) suggest that desiccation-rehydration cycles may have an appreciable effect on soil nutrient balances since both carbon and nitrogen are lost from rehydrating mosses in appreciable quantities in the water used for rehydration (the equivalent of rainfall) so boosting their amounts in the soil; mosses that were continuously hydrated did not show this effect. In addition to their tolerance of desiccation, some mosses are also known for their tolerance of sometimes quite high NaCl and bisulphite (SO2), etc., concentrations (Rensing et al. 2007a and references; Hoang et al. 2009).
The absence of arbuscular mycorrhizae in mosses has occasioned comment, and it has been suggested that their finely branched rhizoids, as little as 2 µm across, match the nutrient-scavenging capabilities of fungal hyphae (Field et al. 2015d; Pressel et al. 2021). Such fungi are involved in soil formation in liverwort communities, but obviously not in mosses, which develop a thicker layer of soil (mineral) grains trapped by the plants, but less in the way of clay minerals, etc. (Mitchell et al. 2016). Mosses may dominate in some biological soil crusts, more so than liverworts or hornworts; the taxa involved are relatively young (Nelsen et al. 2019) so the formation of such crusts may be a fairly recent phenomenon.
Mosses are important components of tundra and boreal forest biomes (for the bryosphere, see Lindo & Gonzalez 2010) and can make up a substantial proportion of the biomass in boreal forests (Wardle et al. 2013). Hummock-forming Sphagnum in particular is often dominant in communities from poor fens to the forest floor in these tundra and boreal biomes. Indeed, rich fens in such areas commonly progress from so-called brown mosses like Drepanocladus and Calliergon and a pH of ca 6 to Sphagnum-dominated bogs with a pH of 4 or a little more (and low HCO3-). This oligotrophification and acidification is mediated by Sphagnum in a process that proceeds fastest in cool, wet conditions (Kuhry et al. 1993).
Alder, Alnus, is the only N-fixing tree in these biomes, but a number of species of moss are associated with cyanobacteria and fix N (Leppänen et al. 2013). Thus in northern ecosystems a few species of feather mosses, pleurocarpous Hypnales like Hylocomium splendens and Pleurozium schreberi, form close associations with the cyanobacterium Nostoc, N moving from the latter into the former (Bay et al. 2013); sufficient phosphorus and in particular molybdenum is essential (Rousk et al. 2016). Indeed, DeLuca et al. (2002) found that 1.5-2 kg N ha-1yr-1 was produced in late successional forests in northern Scandinavia, about 1/2-2/3 the 3 kg N added to the soil annually, while Stuiver et al. (2016) found that around 22% of the N accrued in Pinus sylvestris forests came from feather mosses - interestingly, high Empetrum hermaphroditicum biomass seemed to increase N fixation. Although the further movement of N in the ecosystem seemed rather unclear (Rousk et al. 2013, 2016; Lindo et al. 2013), rceent work suggests that breakdown of moss tissue in which fixed N had been incorporated was a route via which the N moved on, albeit slowly, there was also a fast pathway where N was incorporated into bacteria, fungi, and micro-algae of the feather moss microbiome, and thence onwards (Arróniz-Crespo et al. 2022). X. Liu and Rousk (2021) noted that free-living diazotrophic cyanobacteria tended to colonize mosses with short shoots and dense leaves and a good hydration rate, and these epiphytic bacteria were notably abundant on the filamentous paraphyllia on Hylocomium splendens.
Sphagnum is a major component of these (and other) biomes and a N-fixer. Its ecophysiology has been studied in some detail, and as Healey et al. (2023: p. 238) note, "Sphagnum (peat moss) is both an individual genus and an entire ecosystem"; moreover, since different species, even individuals of different sexes, may behave quite differently ecologically, things quickly get very complex (Turetsky et al. 2025 - I have drawn on this paper quite heavily below). Different species of Sphagnum tend to grow either on the hummocks or in the hollows of peat bogs, and these preferences correlate with different growth rates, rates of plant breakdown, phylogeny, and perhaps remarkably, to a certain extent even whether the plant is male or female, in the latter case pH stress being dependent on epistatic interactions between sex chromosomes and autosomal loci (e.g. Healey et al. 2023: two QTL peaks differ in the sexes; Ekwealor & Fisher 2024), and so on. Perhaps up to 20 species or so of Sphagnum may grow together (see e.g. Vitt & Slack 1984; Turetsky et al. 2008; Piatkowski et al. 2021; Shaw et al. 2022), and some species are highly cryptic (Nieto‐Lugilde et al. 2024; Turetsky et al. 2025). There is extensive pH variation in these bogs, which greatly affects gene regulation, and different species prefer habitats with different pHs, but here there is no large-scale correlation with phylogeny (M. G. Johnson et al. 2014). There is considerable variation between species, and sometimes in species in different parts of their ranges, or in areas where introduced; the N:K ratio is usually less than 3, the N:P ratio less than 30, but this in part depends on the species (Prager et al. 2023; Käärmelahti et al. 2023 and references). Sphagnum-dominated fens in northern Alberta may not be very productive in terms of gross primary productivity, however, the plants start photosynthesizing early in the year, etc., so overall net carbon productivity may be higher than in, for example, Carex-dominated rich fens (Flanagan 2014; see also Ragoebarsing et al. 2005). Particularly in exposed areas, damage from high-light conditions becomes important, as with other mosses, and drying out may also occur - but water reduces CO2 diffusion by a factor of 104, so potentially affecting plant productivity. However, there are methanotropic bacteria that live in the hyaline cells in Sphagnum leaves; these bacteria prefer damper conditions, oxidizing methane from decomposing peat and producing CO2 which is then utilized by the plant. This can provide up to a third of the CO2 supply for the moss (in Scorpidium scorpioides, which grows in similar habitats, the figure is up to 70%), and all 23 of the species of Sphagnum at a site in Finland could convert methane into a source of CO2, so this ability may be ubiquitous in the genus (Larmola et al. 2010; Hájek 2014; see also Turetsky et al. 2025).
The various microbiomes associated with different Sphagnum spp. are of considerable complexity, even if not much seems to be known about associations of fungi and protists - some of the latter are mixotrophs - with the plant. In Sphagnum the hyaline cells, dead at maturity, are reservoirs for water, but they also provide a stable environment for the members of the microbiome (Kostka et al. 2016; Turetsky et al. 2025). A variety of genera of N-fixing bacteria and algae are epiphytic on Sphagnum, and N-fixing bacteria like the cyanobacterium Nostoc are also to be found in the hyaline cells. N moves from Nostoc to the moss both as ammonium and as amino acids. A variety of Alphaproteobacteria are also found in Sphagnum, and here N-fixation is coupled with the oxidation of methane to CO2, sometimes supplying up to 1/5th of the plant"s needs (Turetsky et al. 2025). Overall N fixation rates depend on the species of Sphagnum, pH, and fixation also tends to be higher when decomposition rates and phosphorus levels are higher (Van den Elzen et al. 2020), and N fixation in Sphagnum bogs is about six times the rate than that in the Hylocomium splendens just mentioned (Rousk et al. 2015) - this seems a rather remarkable figure. Sphagnum can also take up mono- and disaccharides - the monosaccharide glucose is preferred - and so is mixotrophic, considerably enhancing the biomass of the plant and perhaps particularly important at times of carbon and/or light limitations, but at least some other mosses and liverworts can also do this (Graham et al. 2010b). For more on the microbiome of Sphagnum, integral to the functioning of the organism and its ecosystem, see Bragina et al. (2014), Graham et al. (2017) and especially Turetsky et al. (2025).
As mentioned, Sphagnum bogs tend to be notably acidic, and this makes bacterial breakdown of the organic material that is accumulating more difficult (Healey et al. 2023). In any event, breakdown of moss peat in general and Sphagnum peat in particular tends to be slow, and differences in the rate of peat breakdown can be linked to variation in carbohydrate metabolism within the genus (Turetsky et al. 2008), whether ther plant forms tussocks (growth and degradation slow) or grows in hollows (growth and degradation relatively fast), etc. (Turetsky et al. 2025). Prager et al. (2023) found that dry mass production was up to 4200kg.ha-1.year-1 in the establishment phase of Sphagnum bogs, the amount depending on the species. The balance between peat formation and sequestration is complex. As mentioned, the tissues of most of the species that grow on tussocks decompose only slowly, their litter taking over a year longer to lose half of its initial biomass than that of species growing in hollows (Piatkowski et al. 2021), however, the tropical hummock-forming S. portoricense has relatively quickly decomposing litter (Piatkowski et al. 2021). Cell wall pectin-like polysaccharides and glycuronoglycones (their dispersed form = sphagnan) have antimicrobial and tanning activity (see the Tollund Man!), while in their cell-wall bound form they acquire nutrients efficiently because of their high cation exchange capacity. Thus in peat they sequester nitrogen, for example, making it unavailable to microorganisms, so contributing to the resistance to decay of Sphagnum remains; microorganisms grow in it poorly, and overall their respiration rate is low (Painter 1991; Hájek et al. 2011). From this point of view, sphagnans have something of the ecological role of lignin, indeed, removing lignins affects the rate of Sphagnum breakdown very little, but it considerably increases it in Leucobryum and Polytrichum, mosses that behave more conventionally (Hájek et al. 2011).
Overall, about one quarter or a little more of terrestrial C is to be found in peatlands, i.e. 500±100 gigatons C from ca 3.7 x 106 km2 (average value) or perhaps 4 x 106/km2 peatlands, more C being found in living and dead shoots of Sphagnum than in any other genus (Z. C. Yu 2012; Piatkowski et al. 2021). Another way of looking at this are the estimates that "Sphagnum peatlands occupy some 3-5% of the Northern Hemisphere boreal zone, yet store some 30% of the total global terrestrial C pool" (Healey et al. 2023: p. 236; Z. Yu et al. 2010). (Note that different estimates of the amount of C in peat can be difficult to compare, sometimes only the top 1 m being taken into account. Furthermore, moss/Sphagnum peat is just one kind of peat, peats of various origins, even including large dipterocarp trees, being scattered throughout the tropics (Austin et al. 2025 for a map). However, the subpolar-temperate peatlands where Sphagnum is now so common seem to have developed within the last 17,000 years, largely as temperatures warmed after the last glacial maximum, although in the case of peats in the West Siberian lowlands, the triggering factor seems to have been increased precipitation (Morris et al. 2018a). As Treat et al. (2019) note, over the last 130,000 years northern peatlands in general have higher peat production in interglacials, however, peatlands near the coast may be buried as sea levels rise (and so the C is sequestered), while during colder periods it is mineral deposition, as by glaciers, that may result in the burial and sequestration of recently formed peat, while the fall in sea levels may expose new areas suitable for peat formation - so exactly when C sequestration developed is unclear, perhaps >270 gt were sequestered before 7,000 ya, or... (Yu 2012). Shaw et al. (2010a) dated Sphagnum diversification to within the last 20 Ma or so (estimates of crown-group diversification range from 104-15 Ma), and suggest a connection with the Miocene climatic cooling; Healey et al. (2023) suggested that hummock and hollow species diversified 20-7 Ma. Finally, a genome duplication in Sphagnum dated to (221-)197(-173) Ma may have been involved is its rise to dominance in peatlands (Devos et al. 2016) - and this gets us back to the dating of crown-group Sphagnum diversification, the fossil record of the genus, and where it might have grown when the earth was much warmer than it is now. Altogether a bit complicated.
Royles et al. (2022) note that Sphagnum and Polytrichum-type mosses dominate peat formation, but i.a. vary seasonally in C assimilation. Thus plants of Sphagnum maintain a very high water content despite there being seasonal variation in precipitation, while Polytrichum, apparently more poikilohydric, is affected by evaporation and there is associated reduction in photosynthesis. Looking at C in wetlands from a global perspective, C density [C stock/area] is highest in peatlands (1000-2000 Mg C ha-1), up to twice that of mangroves and still more for that of other wetlands (Temmink et al. 2022); C sequestration is high in tropical peatlands, but lower in peatlands elsewhere, and unlike mangroves and saltmarshes, peatlands do not trap C. Note that tropical peatlands form in association with trees like dipterocarps and so are quite different from the (cold) temperate Sphagnum-dominated peats. These peats have lost ca 57% of their area in recent centuries, a notably greater loss than that of any other wetland biome (see Temmink et al. 2022). Comparisons with these other major peat-producing ecosystems like mangroves, seagrasses and dipterocarp forests are instructive.
The discovery of Sphagnum-like fossils in Ordovician rocks ca 455 Ma suggests that the ecological equivalents of modern Sphagnum peatlands may have been around for a long time, and this could have considerable implications for C fixation and sequestration in general, the evolution of Sphagnum-associated biota in particular, and so on (Graham et al. 2013, 2017, esp. Cardona-Correa et al. 2016); note ages for Sphagnum above. Carpenter et al. (2015) found spores of Stereisporites - linked to Sphagnum - to be very common in fire-prone heathlands in Central Australia 75-65.5 Ma, while Daly et al. (2011) suggested that Sphagnum-type mosses were components of the peats produced by mire vegetation in northern Alaska ca 60 Ma that later were converted to coal. However, the beginning of the diversification that gave rise to extant species of peat-forming Sphagnum has been dated to as late as the mid-Miocene 16-14 Ma (Shaw et al. 2010a; Shaw & Devos 2014; Healey et al. 2023; Turetsky et al. 2025), the species that are major accumulators of peat today are species of subgenera Acutifolia, Sphagnum (hummock-forming species predominate in these two), Cuspidata and Subsecunda (hollow-forming species predominate), all of Northern Hemisphere origin (except perhaps not subgenus Sphagnum); there are also small segregate genera largely from the Southern Hemisphere that grow on moist to wet rock or soil but are not major peat accumulators (Shaw et al. 2016a; Piatkowski et al. 2021; Turetsky et al. 2025). For peatlands in general, see also Rydin and Jeglum (2013). However, given the great contemporary importance of Sphagnum in C sequestration, etc., a more detailed understanding about the ecological role that the genus and family have played in this over time is much to be desired.
Spores of Sphagnum ca 680 years old are able to germinate (Bu et al. 2017). Gametophytes of Chorisodontium aciphyllum, a moss from Antarctic islands, grew successfully after being frozen in moss banks for ca 1,600 years (Roads et al 2014), and even older ages seem quite likely.
Gametophyte vascularization is particularly well developed in Polytrichopsida, which may grow to over 30 cm tall, and the leptoids apparently transport organic molecules (Ligrone et al. 2000). Recent work on Polytrichum commune by Brodribb et al. (2020) showed that the hydroids moved water quite effectively - the ratio of water transport efficiency to photosynthetic capacity was similar to that in vascular plants, even if the anatomy of the tissues involved was quite different (the two also had similar cavitation patterns). Overall, however, the water was not used very efficiently, the exchange ratio of water vapour for photosynthetic CO2 being "profoundly inefficient" (ibid., p. 276), the leaf diffusive conductance of water being much higher than that of lycophytes and ferns, for instance.
This raises the issue of the role of stomata in mosses. They are restricted to the sporophyte, and in Sphagnum they may be involved in spore discharge (see immediately below), In the stomata of Funaria, at least, the stomium is encircled by a single cell that has two nuclei (Sack & Paolillo 1983; Merced & Renzaglia 2016: stomatal spacing), however, in other mosses the stomata have two guard cells (Merced & Renzaglia 2013, 2018). Although Kubásek et al. (2021) suggest that stomata may enhance photosynthesis in some sporophytes, they hardly give this idea a ringing endorsement. Indeed, stomata have been lost over 60 times in mosses (Renzaglia et al. 2020b).
Fertilization & Spore Dispersal. For the recurrent evolution of dioicy in mosses - at least 133 times - see McDaniel et al. (2013); dioicy may be accompanied by sexual dimorphism of the plants, and in Sphagnum - and elsewhere - there may be sex-associated ecological differences (Healey et al. 2023: in Spagnum no additive sex-biased phenotypic differences, but some associated with two QTLs). Reversal to hermaphroditism is less common, but diversification may be higher in hermaphroditic clades (McDaniel et al. 2013).
When the antheridium dehisces, the contents are released as a single sperm mass, and subsequently the individual sperm disperse (Stark et al. 2016 and references), one might think by swimming. However, very small arthropods like oribatid mites and collembolan springtails are attracted to volatile compounds produced by Ceratodon purpureus and are involved in the transfer of sperm to the archegonia, although details of what actually is going on are unclear (Rosenstiel et al. 2012; see also Cronberg et al. 2006). In a seminatural experiment, these arthropods increased the numbers of both male and female genotypes that reproduced, affecting the fitness of particular genotypes, and overall greatly increasing sporophyte production (Shortlidge et al. 2021). Pressel and Duckett (2019) also summarize information on fertilisation; in some taxa (but not Sphagnum) spermatozoids move along the water-air interface aided by lipids released by the dehiscing antheridia (see also Muggoch & Walton 1942). Splash-cup dispersal of spermatozoids is common.
Most mosses have capsules with peristomes. The opening of the capsule of Physcomitrium (= Physcomitrella in older literature - see Rensing et al. 2020) may be aided by stomata, opening under drier conditions, and mucilage on the cell walls in the substomatal cavity also drying, however, under wetter conditions the stomatal aperture remains occluded by mucilage... (Chater et al. 2016; Caine et al. 2020). The peristome may be one- or two-layered (haplolepideous versus diplolepideous), and the peristome teeth may be orthodontous or nematodontous, made up of parts of cell walls or dead cells. As the humidity changes the peristome teeth curl and uncurl, move from being erect to reflexed, etc., and, depending on the species, the capsule may end up being open or closed when conditions are humid (Gallenmüller et al. 2017). In any event, the changing configuration of the peristome allows the spores to escape, and in some species (e.g. Brachythecium populeum) rather rapid bending movements of the outer peristome lead to the spores being scooped up and flicked out as the teeth dry (Gallenmüller et al. 2017; see also Ingold 1939, 1974). For a comprehensive study of the evolution of capsule shape in mosses, see Rose et al. (2016). Wind dispersal of the spores is the norm, and Zanatta et al. (2016) discussed the settling rate of moss spores. They found that size was important, although noting that density might also play a role, furthermore, a number of species of mosses have asymmetrical spores, sometimes with dramatically different ornamentation on the two sides (Zanatta et al. 2022).
A few mosses have very different dispersal mechanisms. Sphagnum is one example. The capsules have stomata, but these lack both pores and air spaces immediately under the guard cells. The stomata open as they lose turgor and apparently help the capsule to dry out. As the capsule of Sphagnum dries, its walls contract and buckle and the water pressure inside increases considerably, and eventually the stomium pops off (the pop is audible) and the spores are shot into the air (e.g. Ingold 1939, 1974; Duckett et al. 2009; see also Merced & Renzaglia 2018). Another example is Splachnum which grows on dung (vernacular names, courtesy Wikipedia, "pinkstink dung moss", "petticoat moss"); the spores are sticky, and are dispersed by flies that land on the capsule attracted by the smell it emits (Cameron & Wyatt 1986).
Plant-Animal Interactions. Larvae of the cylindrotomine craneflies (Diptera) have a lobed integument, they may be green and very like the mosses on which many of them feed. However, predators are unlikely to be common in such habitats, and details of this mimicry, if that is what it is, are not well understood (Imada 2020).
Plant-Bacterial/Fungal Associations. Bay et al. (2013) discuss associations between Nostoc and some pleurocarpous mosses in boreal forests; other blue-green algae are also involved (Rousk et al. 2013 for a review). Warshan et al. (2018) discuss the relationships of N-fixing members of Nostoc; their ability to form such associations may be older than the age of mosses.
Functional mycorrhizal associations, i.e. associations that are involved in the exchange of nutrients, are very rare/absent in mosses, and most moss—fungus associations involve parasitic fungi (Read et al. 2000; Davey & Currah 2006). Fot the possibility that Buxbaumia is mycoheterotrophic, see Imhof et al. (2013). Other endophytic fungi may also affect moss growth and ecology (Read et al. 2000; Davey & Currah 2006).
Genes & Genomes. The base chromosome number for mosses may be x = 7 (Rensing et al. 2012), but note that x was ancestrally 5 in Sphagnum (Healey et al. 2023: n = (4 X 5) - 1, x = n = 19; x = 7 in Physcomitrium). Lang et al. (2018) noted that the chromosomes of P. patens were rather differently organized than those of seed plants, for instance, eu- and heterochromatin were fairly evenly distributed on the chromosomes, unlike the common condition in flowering plants with their repeat-rich pericentromeres. Centromeres were not so obvious as in vascular plants, although Copia-type transposable elements were concentrated there, however, the chromosomes did appear to be monocentric; for more on centromeres, those of Sphagnum having long terminal repeat retrotransposons (RLC5), as in Physcomitrium and other "bryophytes", see also Healey et al. (2023) and Hisanaga et al. (2023).
Genomes in mosses are small, 1 C values being less than 1.61 pg (Bennett & Leitch 2005; Bainard et al. 2019: Hookeria lucens largest). Endopolyploidy is widespread, although not in Sphagnum, and this is unlike other land plants except for angiosperms (Bainard & Newmaster 2010a, b; Bainard et al. 2019). There seems to be no correlation between stomatal and genome size (e.g. Duckett et al. 2020). Genome duplications are reported from Sphagnum (Devos et al. 2016; Healey et al. 2023), and overall, genome duplications were detected in 3/5 of the mosses examined by Lang et al. (2018); they are not known in other bryophytes s.l.. Two duplications may have been involved in the rise of Sphagnum to dominance in peatlands (see above), Healey et al. (2023) dating them to 247-189 Ma and 122-102 Ma respectively; the genes retained after these events are similar to those retained after such events in other plants, i.e. those involved in ion channels or signal transduction pathways. Of course, the ages are far older that those in the literature on Cretaceous and more recent Sphagnum bogs... Physcomitrium patens differs somewhat in this respect (Szövényi et al. 2015; Devos et al. 2016, and references), but there is evidence of two fairly recent duplications there (Rensing et al. 2007b; Lang et al. 2018). The species showed a notable increase in the number of members of transcription factor clades compared with the two other bryophytes examined (J. Zhang et al. 2020).
For the genome of Sphagnum magellanicum (= S. divinum), see Shaw et al. (2022).
Yue et al. (2012) suggested that a number of genes in Physcomitrium had come from fungi and bacteria by lateral transfer; how widely they might be distributed in other mosses is unknown. A squalene-hopane cyclase gene apparently moved from cyanobacteria via lateral gene transfer both to mosses and independently to liverworts (F.-W. Li et al. 2018; Wickell & Li 2019). J. Zhang et al. (2020: Fig. 4) also discuss lateral gene transfer in bryophytes, especially in Anthoceros.
The rate of molecular evolution is slow in the three genes from all three genomic compartments examined (Stenøien 2008: situation in liverworts, hornworts, and lycophytes?), however, Healey et al. (2023) estimate that the recombination rate of the moss genome is around an order of magnitude higher than that of most vascular plants. For the loss of the PEP α subunit-encoding rpoA gene (DNA-dependent RNA polymerase) in many mosses, see Goffinet et al. (2005); it is absent in Tetraphis and Diphyscium, present in Buxbaumia and Polytrichum.
There is a very large (ca 71 kb) inversion in the plastome in Funariaceae (which includes Physcomitrium), Disceliaceae, and Encalyptaceae, all Funariidae, although Gigaspermaceae lack this inversion (Goffinet et al. 2007).
For variation in the mitochondrial nad2 and -5 genes, see Beckert et al. (1999, 2001).
Chemistry & Morphology. Leaf waxes in Sphagnum are distinctive compared with those of vascular plants in that n-alkanes of chain length C23 and C25 predominate, rather than longer chains (Bush & McInerney 2013: ?other bryophytes).
As moss rhizoids branch, they become progressively narrower, about as wide as a fungal hypha; for the distinctive nature of their cytoplasm, see Pressel et al. (2008a) and Field et al. (2015d). Moody et al. (2020) examined the transition from 2- to 3-dimensional growth in Physcomitrium patens, from 2-D protonema to 3-D gametophore, and focusing on the NO GAMETOPHORES 2 gene. Phyllotaxis of the moss gametophore can be 2- or 3-ranked or spiral, and Véron et al. (2021; see also Moody et al. 2020) describe how the 3-ranked phyllotaxis of P. patens results from unequal divisions of the apical cell of the shoots (= gametophore), and how these apical cells develop on the gametophore; there are similarities - auxin-based patterning - between leaf initiation here and in Arabidopsis.
R. C. Brown et al. (1982) describe the spore wall morphology of Sphagnum, where some layers, e.g. the translucent layer, may be unique.
Goffinet et al. (2004) provide a general entry into the literature, and for information about pleurocarp mosses, see Newton and Tangney (2007); for growth forms and branching patterns, see La Farge-England (1996), for the operculum, see Robinson snd Shaw (1984), for sporogenesis, see R. C. Brown and Lemmon (1984: Andreaea, 2013), for apical cell division, see Piatkowski et al. (2013), for sieve elements/leptoids, see Scheirer (1990), for placental tissue, see Carapa et al. (2003), and for peristome development, see Ignatov (2019).
Phylogeny. Sphagnum, Andreaea and Takakia, the latter initially thought to be a liverwort (see Renzaglia et al. 1997), are all at the base of the moss tree, Sphagnum and Takakia perhaps being sister taxa and Andreaea sister to remaining mosses (e.g. Cox et al. 2004; Qiu et al. 2006, 2007: rather strong support; Volkmar & Knoop 2010; Shaw et al. 2010b [perhaps]; S. Li et al. 2013: 9 loci; Rose et al. 2016; Evkaikina et al. 2017: Andreaea not sampled). However, Takakia has a region in the cpITS3 sequence that is very like that of all other land plants but is deleted in other mosses, from this evidence alone, Takakia would be sister to all other mosses (Samigullin et al. 2002), and Y. Liu et al. (2019) found the same topology and with quite strong support. Beckert et al. (1999: Takakia not included) found that Polytrichum was interpolated between Sphagnum and Andreaea. Recent work suggests relationships may be [Takakia [Sphagnum [[Andreaea + Andreaeobryum] [Oedopodium [[Polytrichopsida + Tetraphidopsida] Bryopsida]]]]] (Chang & Graham 2009, esp. 2011, 2014; Rose et al. 2016: but c.f. in part above); a [Takakia + Sphagnum] clade was recovered only in some reconstructions. The odd, almost leafless Buxbaumia may be sister to all other Bryopsida.
See Shaw et al. (2010b; also Shaw et al. 2003a for morphology, 2010a, 2016a) for relationships in the Sphagnum et al. clade. The very distinctive S. leucobryoides (= Ambuchanania) was described only some twenty five years ago (Yamaguchi et al. 1990), and it and a very few other species of Sphagnum (placed in genera like Flatbergia) are outside of Sphagnum s. str., which makes up the bulk of the clade. Piatkowski et al. (2021) report the following relationships between the subgenera: [Rigida (ecology unclear) [[Sphagnum + Acutifolia (usu. grow on hummocks)] [Cuspidata + Subsecunda (usu. grow in depressions)]]. Meleshko et al. (2021) suggested that ancestrally there may have been introgression followed by ILS, more recently gene flow between species has been slight despite widespread interspecific hybridization. Shaw et al. (2022) note extensive cytonuclear discordance in the S. magellanicum complex (see also Healey et al. 2023: species formation here may be very recent).
Within the remaining mosses, Chang and Graham (2009, esp. 2011) and S. Li et al. (2013) found Oedopodium to be sister to the others. See Cox et al. (2010) for a phylogenetic study of mosses focusing on genera and families. Using variation in mitochondrial genes, Beckert et al. (2001) found Buxbaumiales to be paraphyletic immediately above the basal grade of mosses and below Bryopsida. Wahrmund et al. (2010) used a new mitochondrial locus to investigate relationships; the position of Timmia was particularly unclear. Most of the tree that Y. Liu et al. (2019) produced, based on the analysis of a large number of genes from all three compartments in 142 species from all bar one of the moss orders, had quite good support.
For relationships in Dicranidae (haplolepidious mosses), see Stech et al. (2012). Bell et al. (2007) discuss the phylogeny of the early diverging pleurocarp clades, and adjust their taxonomy accordingly, while Buck et al. (2005) discuss the phylogeny of Hookeriales. Most branch lengths in the speciose Hypnales are short (Huttunen et al. 2012).
Classification. For a classification of mosses based on phylogeny, see Shaw and Goffinet (2000, also Goffinet & Buck 2004), and for a classification of Sphagnum s.l., and recognition of five subgenera in Sphagnum itself, see Shaw et al. (2010b, 2016).
Sporophyte well developed, chlorophyllous, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata anomocytic, on stem; sporangia several, terminal.
Evolution: Divergence & Distribution. Note that the number of spores produced per sporangium is roughly the same here as in bryophytes s.l. (Qiu et al. 2012).
Plant-Bacterial/Fungal Associations. Vesicular arbuscular mycorrhizae (Glomites rhyniensis) have been found in the axes of Aglaophyton major from the early Devonian ca 400 Ma (Remy et al. 1994). Indeed, a variety of fungal associations have been recorded from fossils of early polysporangiophytes, whether the gametophyte or sporophyte (T. N. Taylor et al. 2004), maybe three species of endophytic fungus from Nothia aphylla alone (Krings et al. 2007); sometimes the fungi pervade the whole plant (e.g. Strullu-Derrien et al. 2014b). Reference may be made to Paris- and Arum-type arbuscular mycorrhizae (see Giesemann et al. 2009, 2011) in plants from the Rhynie Chert deposits (T. N. Taylor et al. 2004), but what these two types might have been doing ecologically and physiologically 400 Ma is unclear. Indeed, because true roots had not yet evolved, these fungal associations are perhaps best called paramycorrhizal (Kenrick & Strullu-Derrien 2014; for early fungal associations, see also Rimington et al. 2014).
Classification. Polysporangiophyta initially lacked vascular tissue of any sort, and tracheophytes are a subgroup; for the two broken out, and protracheophytes, with hydroid and leptoid conducting cells, paratracheophytes and eutracheophytes also separated, see Gerrienne et al. (2016).
IV-VIII. TRACHEOPHYTA / EXTANT VASCULAR PLANTS
Sporophyte long lived; cell walls with guaiacyl and parahydroxyphenyl [G + H] lignin units [sinapyl units uncommon, no Mäule reaction]; cells polyplastidic, pyrenoids 0,photosynthetic red light response; simplex-type apical meristem [with several apical initials]; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level] in secondary walls of vascular and mechanical tissue; guaiacyl (G) lignins + in xylem; roots +, often ≤1 mm across, root hairs +, distribution random [Type 1 - throughout vascular plants] or developing from the short cell of long cell-short cell pairs [Type 2 - lycophytes to magnoliids and monocots], root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types [lignified, with pores]; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, also on leaves, involved in gas exchange, close on drying, substomatal cavity air-filled, open in response to blue light; leaves +, vascularized, spirally arranged, mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, adaxial, lateral, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; spores trilete/hilate; basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets?, at maturity with lamellar strip [part of MLS], spline 150-200 microtubules across [in homosporous species], widest at the cell posterior; embryo shoot apex endoscopic [developing away from micropyle/archegonial neck, from hypobasal cell], root initiated from the stem, lateral [plant homorhizic]; centromeres with pericentromeric heterochromatin tandem repeats; transcription factor genes numerous; chondrome with group II introns only, synteny poor.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Clarke et al. (2011: many other estimates) suggested an age for vascular plants of (456-)446(-425) Ma, similar to the estimate of 451-431 Ma of Morris et al. (2018a: stem 25-60 Ma, other dates also suggested). There are somewhat younger ages, (434.3-)424-421.6(-416.2) Ma, in Magallón et al. (2013), while Larsén and Rydin (2015) suggest ages of ca 432 Ma, largely in line with fossil-based ages (Kenrick et al. 2012) which were used in the calibrations (a similar age in Villarreal & Renner 2014); ca 419 Ma is the estimate in Evkaikina et al. (2017). However, P. Soltis et al. (2002: variety of estimates) suggested an older crown age of (813-)603(-393) Ma. Silvestro et al. (2015) estimated that vascular plants were (449-)433.5(-424) Ma, and other estimates are broadly similar, e.g. (463.5-)429(-400) Ma (B. Zhong et al. 2013b), 458-442 Ma (Barba-Montoya et al. 2018), (449-)435(-426) Ma (Lutzoni et al. 2018: note topology, tracheophyte "differentiation" (467-)452(-438) Ma), (440-)431.5(-426) Ma (Testo et al. 2018b), ca 452.2 Ma (D. Wood et al. 2019/2020), 452-447 Ma (B. J. Harris et al. 2021/2022), (433.2-)405.0(-375.6) Ma (Y.-L. Qiu et al. 2024) and (426.8-)425(-323.6) Ma (sic: W. Sun et al. 2024).
Steemans et al. (2009) date vascular plants to the end-Ordovician (Ashgill series) ca 445 Ma, basing this on the dominance of hilate/trilete spores as evidence of their presence.
Evolution: Divergence & Distribution. Pryer et al. (2004b) provide a useful summary of the evolution of vascular plants; see also e.g. Kenrick and Crane (1997), Doyle (2013), etc.. J. W. Clark and Donoghue (2018) emphasise the number of characters that can be associated with this node, yet there is no evidence of whole genome duplication or its like (see Banks et al. 2011). For giberellin signaling, see C. Wang et al. (2015). The overall level of complexity, the number of distinctive plant parts, of free-sporing plants, i.e. lycophytes and monilophytes, has remained very much the same since the Devonian; plant complexity increased with the evolution of gymnosperms (Leslie et al. 2021).
There are examples of quite recent hybridizations between members of pteridophyte clades in particular that have been separated for some time and that are now placed in separate genera, for example, some members of Gymnocarpium and Cystidium can hybridize, and these clades are thought to have separated ca 58 Ma (Rothfels et al. 2015a). There is similar hybridization within Osmundaceae between clades that separated rather earlier (Bomfleur et al. 2014b/2015), thus Osmundastrum cinnamomea is able to hybridize with some species of Osmunda, but not with other Osmundaceae. Given an estimated date of the split of the first two of (264-)238(-233) Ma, only a little less than the age of crown-group Osmundaceae as a whole (Grimm et al. 2015), they have been separated for far longer than any other vascular plants that currently hybridize. All these are all examples of deep hybridization (Tseng et al. 2024). H.-M. Liu et al. (2020) thought that such long-separated clades in ferns, etc., can still hybridize perhaps because chromosome number and structure are similar (see also H. Schneider et al. 2015). Liu et al. (2020) evaluated the list of apparent nothotaxa in ferns, keeping only those that were necessary, i.e. referring to crosses between genera as recognised in the PPG [Pteridophyte Phylogeny Group] 1 classification of 2016, around 15 all told. These are all mentioned in the family accounts, and there are three in Dryopteridaceae and three in Thelypteridaceae alone. (Of course, such lists depend on generic concepts and correct identifications - see L. Zhang & Zhang 2020...) When it comes to nothospecies, these are found mostly in Aspleniaceae, Athyriaceae, Cystopteridaceae, Dryopteridaceae subfam. Dryopteridoideae and Equisetaceae, i.e. in temperate families (Liu et al. 2020).
Rothfels et al. (2015a) thought that lycopods, ferns and other plants with abiotically-mediated fertilization evolved reproductive incompatability only slowly, hence they have an intrinsically slow rate of speciation. At the small scale allopatric speciation is likely and outcrossing is the norm, indeed, speciation mechanisms/patterns here and in flowering plants seem to be similar, and speciation in the Andes, at least, can be quite rapid (Testo et al. 2018b, 2019a and literature). It has been suggested that the relatively few species of ferns (or gymnosperms, or mosses, etc.) compared to angiosperms reflects a low rate of speciation that in turn is connected with the dispersal of spores by wind that is in its turn linked to large population size/long distance dispersal of spores (for speciation in angiosperms, see Benton et al. 2021). Isolation of populations is more difficult to achieve, and so genetic barriers will not develop and there will be fewer species (A. R. Smith 1972; Rothfels et al. 2015a; Ranker & Sundue 2015). However, it was recently found that the overall genetic structure of Amazonian bryophytes (species in four genera of liverworts and four of mosses were examined) was similar to that of angiosperms, with isolation by distance being evident beyond distances of 1 km, and this despite the fact that the diaspores of the latter are orders of magnitude larger than those of bryophytes (Ledent, Gauthier et al. 2020). Indeed, there is no evidence that the small spores of homosporous lycopods and ferns are particularly associated with long distance dispersal, Gondwanan vicariance patterns being evident in some of their distributions. Pre-mating barriers are not developed in taxa like monilophytes and lycophytes, and hence it takes longer for isolation to develop (e.g. Kay et al. 2006b; Rothfels et al. 2015a). The eusporangiate Marattia and Angiopteris and the leptosporangiate royal and tree ferns (Osmundaceae, Cyatheaceae area) are almost living fossils and show little molecular and even morphological evolution (P. Soltis et al. 2002; B. Zhong et al. 2014b; Rothfels et al. 2015b; Bomfleur et al. 2014a, c.f. H. Schneider et al. 2015).
Aside from the evolution of euphylls (see below), several features characterizing various tracheophyte clades have evolved more than once, and these include heterospory, secondary thickening and various stelar morphologies (see Ecology & Physiology) and the presence of an initial free nuclear/coenocytic phase in the development of the megagametophyte. 1. Heterospory has evolved several times in land plants (e.g. Bateman & DiMichele 1994; Qiu et al. 2012). Seed plants are heterosporous, and spore-bearing and photosynthetic leaves seem to be quite different, while in other heterosporous vascular plants there is no fundamental dissimilarity between the two (e.g. Kaplan 1997, vol. 3: chap. 19; Boyce 2005b). Haig and Westoby (1988, 1989) outline the conditions conducive for the evolution of heterospory; it may first have appeared in Devonian vegetation which was becoming denser and casting more shade - it helped in the establishment of the germinating plants (J. Petersen & Burd 2018 and references). In ferns, at least, Duckett and Pang (1984) suggested that temporal dioicy in the gametophyte, e.g. archegonia being produced first, antheridia later, might be a prelude to heterospory. For more on heterospory, see Gene and Genome Duplication, and Genome Size, especially towards the end. 2. The pattern of secondary growth varies considerably. The vascular cambium is often unifacial, producing xylem internally only, and there may not be any anticlinal divisions of the cambial cells (see Tomescu & Groover 2018 for the diversity of cambia in vascular plants). Sphenophyllales alone outside the seed plant lineage developed a bifacial vascular cambium (e.g. Rothwell et al. 2008b; Spicer & Groover 2010; Hoffman & Tomescu 2011). 3. Rudall and Bateman (2019b) discuss the cellularization of the megagametophyte, noting that an initial free-nuclear phase is found only in heterosporous lycophytes and in seed plants (including some fossils), also heterosporous, but it appears not to occur in the few species known of heterosporous ferns.
On The Evolution of the Vascular Pant Body. The sporophytes of the earliest vascular plants were very small, although the patterns of vascular tissue in the stems of Early Devonian (400-395 Ma) plants from the Gaspé Peninsula of Canada are really quite complex. The shape of the central stele was thought to reflect the exigencies of plant size and branching, but Bouda et al. (2022) suggest that it more likely reflects increasing drought resistance, as for instance in the Devonian when atmospheric CO2 concentration decreased, necessitating a reliable supply of water to the photosynthetic area. Cavitation of the water-conducting tracheids could easily spread from cavitated cells to their neighbouring tracheids if the tracheary tissue was in the form of a solid terete haplostele; the spread of such cavitated tissue would affect photosynthesis and could ultimately also cause the death of the tissues involved (Bouda et al. 2022). The fewer the tracheids in immediate contact, the less the danger of the spread of cavitated tissue and of hydraulic failure; ways in which this seem to have occurred include various forms of medullation and/or lobing of the stele. In some Early Devonian plants there is evidence of secondary thickening (otherwise known in Middle to Late Devonian plants), yet at the same time they have P-type tracheids with scalariform bordered pits with multiaperturate pit membranes (otherwise earlier Devonian) (Bickner & Tomescu 2019). (Lalica and Tomescu (2023) discuss the evolution of periderm; perhaps initially it developed in association with wounds, but the initial trigger to the development of "canonical periderm" may have been the extension of the stem as the vascular cambium developed - this extension would be accompanied by tearing of the outer tissues, ≡ wounding (Lalica & Tomescu 2023) - one might these tissues to be more or less superficial, hence initially superficial cork cambium?) A variety of tracheid morphologies is evident early, apparently involved in the improvement of water conduction, and somewhat later a variety of cambia developed, initially facilitating increased water conduction, but latterly support and strengthening became an important role (Weng et al. 2010; Decombeix et al. 2019). Indeed, how the need for increased support might interact with the need to reduce the danger of cavitation is unclear (Bouda et al. 2022).
Archegonium development is discussed by Sokoloff and Remizowa (2020), and it shoulkd be borne in mind that it becomes difficult to establish homologies between the archegonia of gymnosperms and especially angiosperms and those of other land plants. The basic pattern of early development of the sporophyte, exoscopic or endoscopic, is relative to the gametophyte and is outlined by Niklas and Kutschera (2009); their scenario involved both gains and losses of endoscopic development. If bryophytes s.l. are monophyletic, then they and vascular plants may well develop differently and establishing ancestral conditions for embryophytes as a whole may become unclear, although both are denoted as apomorphies here (with some reversals in monilophytes)... For sporopollenin in land plants, see above. Baucher et al. (2007) discuss vascular evolution, G. P. Johnson and Renzaglia (2009) that of the embryo, while Arens et al. (1998: Virtual paleobotany laboratory) is a valuable web resource.
Chomicki et al. (2017b) looked at early tracheophytes in particular from the point of view of architectural models. They proposed twelve new models for plants with some kind of dichotomous branching, particularly common in early tracheophytes. They note the steady reduction in the frequency of plants with such branching from 100% to 42% over the period 429-280 Ma, although interestingly there seems to have been an uptick to 2-40% 120-70 Ma (Chomicki et al. 2017b).
Nearly all tracheophytes have some kind of root. Roots, and so root caps, apical meristems, radially-arranged cells, are thought to have evolved once, or two or more times (but with considerable convergence), in vascular plants (e.g. Raven & Edwards 2001; V. A. S. Jones & Dolan 2012; Pires & Dolan 2012; Kenrick & Strullu-Derrien 2014; L. Huang & Schiefelbein 2015; Hetherington et al. 2016b: Fujinami et al. 2017; Hetherington & Dolan 2018; Ferrari et al. 2020), although details are still not well understood. Kenrick and Strullu-Derrien (2014) distinguished between rhizoid-based rooting systems (RBRS) in which branching of the axes was exogenous and dichotomous/bifurcating, and roots proper, in which branching was endogenous. Fujinami et al. (2017) described four distinct variants of the apical meristems of extant lycophyte roots, including one with an apical meristem (Selaginella) approaching the common type of organization in monilophytes - this and two other variants are closed meristems, and some kind of closed meristem could be the ancestral condition for extant vascular plants. The fourth variant is a quiescent centre (= a common initial zone) or something similar approaching one kind of root apex organization found in seed plants, basically an open meristem; such structures have evolved more than once (Fujinami et al. 2017). Hetherington et al. (2020) discuss the evolution of dichotomous branching in roots and lateral root origin, while Vanneste and Beechman (2020) looked at the position of initiation of lateral roots (see also van Tieghem 1870). I have tentatively put the acquisition of roots with a root cap and root hairs at this node; note that seed plant roots/root caps sense and respond to gravity in a different way (via statocysts, far faster) that do the roots of other vascular plants (Y. Zhang et al. 2019); root hairs are apically-growing cells involved in water and nutrient uptaken that develop rather similarly to rhizoids (from the gametophyte) and are variously arranged along the root (e.g. Datta et al. 2011; V. A. S. Jones & Dolan 2012; K. Lee et al. 2024). Little is said about the presence of cuticle on roots (e.g. Kenrick 2013; Kenrick & Strullu-Derrien 2014), and although Matsunaga and Tomescu (2016) were unable to detect a cuticle in fossils of early vascular plant that they examined, they thought that it could have been there.
Within angiosperms, gene families involved in root development are conserved, the great majority being found in all six angiosperms studied, and this is true of genes involved in root development in Arabidopsis in particular (L. Huang & Schiefelbein 2015). Remarkably, around 82% of the gene families that are expressed in angiosperm roots are also expressed in roots of Selaginella, even root cap-associated genes. Although details of the functional similarities of these genes is unknown, there may have been parallel evolution of root-associated genes in lycophytes and other vascular plants, or the dissimilarity of roots of lycophytes and other vascular plants was, at one time at least, not that great, some sort of root occurring in the ancestor of extant vascular plants (Huang & Schiefelbein 2015). Note, however, that roots in lycophytes and pteridophytes are homorhizic, that is, roots are initiated separately from the stem, often in leaf axils, any radicle of the seedling certainly not developing into the main root-bearing structure of the plant, as in many seed plants (Kaplan 2022 for literature, etc.). Similarities in the acropetal flow of auxin in the roots of lycophytes and seed plants are probably the result of independent evolution (Sanders et al. 2011). Furthermore, at least some details of the molecular development of root hairs and rhizoids are similar across all vascular plants, with ROOT HAIR SPECIFIC genes interacting with others to stimulate root hair development (Hwang et al. 2017; see also L. Huang et al. 2017), and some similarities extend still more broadly into the bryophytes (see above). Root hairs may initially have been able to differentiate from almost any cell, i.e., there were no trichoblasts, visibly distinguishable precursors of root hairs (Clowes 2000), and although such root hair development is widespread, being scattered in many ferns, conifers and angiosperms, another developmental pattern, long-short cell alternation, is also widespread, being found in basal vascular plants including basal ferns, many monocots, etc. (D. W. Kim et al. 2006), while there is a third pattern scattered in core eudicots (K. Lee et al. 2024). Interestingly, in flowering plants at least, whatever the root hair distribution pattern, there is specificity of gene expression because of cis conserved root hair element motifs that occur in a variety of root hair specific genes, and it is upstream fate-determining components that are divergent and lead to the development of these different types (Kim et al. 2006).
Sporophyte root morphology and mycorrhizal relationships are connected. Pressel et al. (2016; see also Rimington et al. 2017) suggest that fungal associations have been progressively lost in monilophytes, perhaps because the epiphytic habit is common in Polypodiales (mycorrhizae tend to be less common in such habitats) and they have thin, wiry roots ≤1 mm across in which mycorrhizal associations are uncommon, unlike the thicker, more fleshy roots that may harbour mycorrhizae found in some other monilophytes. However, where the feature "wiry roots" is to be placed on the tree is unclear. I have provisionally put it at the level of vascular plants as a whole, since thin roots are common in Selaginellaceae, Lycopodiaceae and Equisetaceae, fleshy roots/rhizomes are found in some basal fern clades, but thin roots are found in most of the rest (Pressel et al. 2016). Any glomeromycotes associated with basal clades of vascular plants seem to be Glomeraceae, a derived group, compared with the associations of more basal glomeromycotes that are found in liverworts and hornworts (Rimington et al. 2018).
Relatively little is known about the patterns of development of root hairs, but Type 1, with hairs distributed randomly on the root, is scattered throughout vascular plants, and also occurs in the same orders as do root hairs of the other two types, one restricted to rosids and some asterids (K. Lee et al. 2024); root hairs seem to occur in some gymnosperms, e.g. in Pinaceae, but not in cycads, etc., and I know nothing about their development (see also K. Lee et al. 2024). It has been unclear exactly when/in what clade an endodermis, characterized by the suberized Casparian strip, evolved (Raven & Edwards 2001: p. 385). However, Seago et al. (2022: q.v. for detailed discussion) recently detected an endodermis in Lycopodiaceae-Huperzioideae (but not in Lycopodioideae, which seem to lack Casparian bands) and in the roots of Selaginella, at least, and of course they are well known in ferns and seed plants. There seem to be two stages in suberin deposition, the first, anchored in the primary cell wall, consists of the deposition of polymerized phenolic compounds, while the second stage is made up of fatty acid-derived aliphatic compounds, suberin, that are associated with phenolics, and these are laid down in layers on top; the aggregation of Casparian strip membrane domain proteins marks where on the cell wall the Casparian strip will form, i.e. the place where the poly(phenolic) element is laid down (Woolfson et al. 2022). Possession of an endodermis is provisionally placed as an apomorphy of extant vascular plants.
There has been considerable discussion over the origin of the apical meristem of polysporangiophyte shoots and the evolution of shoot development. Ambrose and Vasco (2015) suggested that the apical meristems of shoots of vascular plants could best be thought of as multicellular structures with cytohistochemical zonation (for plasmodesmata, see Imaichi & Hiratsuka 2007, c.f. interpretation of apical meristems there; Evkaikina et al. 2017). It is common to distinguish between tracheophytes with apical meristems of a single cell and those with meristems of several cells (Kato & Akiyama 2005; Imaichi 2008; Evkaikina et al. 2017 and Romanova et al. 2022: monoplex vs simplex/duplex; Maksimova et al. 2020). Multicellular meristems are evident in some fossils (Kidston & Lang 1920; Hueber 1992; D. Edwards 1993). Interestingly, the gene expression patterns in the single apical cells of Selaginella and Equisetum are quite different (Frank et al. 2015). This may suggest that the apical cells in ferns s.l. and Selaginella - and perhaps seed plants, too - evolved independently - or that the differences between the two reflect the long period they have been separated, some 400 Ma or more (see above for ages; Frank et al. 2015; Evkaikina et al. 2017). Evkaikina et al. (2017) suggest that having a single, apical cell is plesiomorphic for land plants as a whole. For further discussion, see elsewhere.
Floyd and Bowman (2006), Boyce (2008a), Boyce and Leslie (2012) and others emphasise the diversity of leaf morphologies, growth forms, etc., to be found in non-angiospermous plants in general. However, all leaves, whether megaphylls or microphylls, can be thought of as structures with "a determinate growth programme [added] to the indeterminate apical growth programme" (Harrison et al. 2005b: p. 509). Similar but independently recruited developmental mechanisms may be involved in the evolution of microphylls and of megaphylls in particular (Boyce & Knoll 2002; Harrison et al. 2005b), as in just about all other aspects of evolution in land plants (Pires & Dolan 2012).
Much has been written about the evolution of leaves. Zimmermann's (1930, see also 1952, 1965) dynamic telome theory suggested that euphylls were the result of overtopping, planation and webbing of a stem/branch system, and this theory has been influential, and if in detail it is no longer tenable, it is still cited (Kenrick 2002; Beerling & Fleming 2007; Chomicki et al. 2017b); seed plant leaves seem radically different from stems (Harrison et al. 2002). However, details of the evolution of the euphylls that are supposed to characterise the Euphyllophyta/[Monilophyta + Lignophyta] clade are unclear. H. Schneider et al. (2009: p. 461 and references) suggest that mega/euphylls arose once, and can be characterized by apical/marginal growth, apical origin of the venation, determinate growth, etc.. However, Floyd and Bowman (2007) suggested that they evolved independently in seed plants and monilophytes - an estimated 3-6 times in the latter alone - and perhaps elsewhere as well, while D.-M. Wang et al. (2015) proposed that there had been four independent origins of megaphylls in ferns/monilophytes, progymnosperms, and seed plants by the end of the Devonian (see also Kenrick & Crane 1997; Boyce & Knoll 2002: 4 origins; Osborne et al. 2004; Gensel & Kenrick 2007; Tomescu 2009: 3-6 origins; Niklas & Kutschera 2009; Sanders et al. 2009; Galtier 2010; Corvez et al. 2012: at least two origins; Sack & Scoffoni 2013; Doyle 2013; Tomescu et al. 2014; Chomicki et al. 2017b; Scarpella 2024; Romanova et al. 2021, 2023a: possible origin of microphylls; leaves arise by sterilisation of sporangia). Harrison and Morris (2017) also emphasise the likelihood of the independent evolution of megaphylls, and they note variation in how the microphylls of extant Lycophyta are put together (see also Wagner et al. 1982; Langdale et al. 2002; Tomescu & Whitewoods 2024). Overall, it is fair to say that megaphylls have evolved several times from microphylls, they are an apomorphy of the seed plants, for instancex, but have evolved independantly from the megaphylla of ferns/monilophytes.
The development of euphylls or megaphylls is indeed quite different from that of microphylls (e.g. Bower 1935; Floyd & Bowman 2006). Thus the leaves of lycophytes are characterized by having an intercalary meristem, development is marginal and the leaves are supplied by a single vein that does not leave a gap in the central stele when it departs (see Kaplan 1997, vol. 2: chap. 16, vol. 3: chap. 19, 2001 for leaf morphology), while megaphylls are determinate organs with ad/abaxial identities, development patterns are various, the vascular bundles (or bundle) supplying them leaving gaps in the central stele when they depart (see e.g. Sporne 1965, but c.f. Kaplan 1997, vol. 3: chap. 19, 2001 in particular) - see also Harrison et al. (2005, 2007), Boyce (2005a: summary of earlier literature, 2008), Tomescu (2008, 2009), Sanders et al. (2007, 2009), Corvez et al. (2012). An extended quotation from Boyce and Knoll (2002: p. 71) helps set the context: "By definition (Gifford and Foster 1989), megaphylls are associated with leaf gaps in the stele of the supporting stem; they can be frondose or entire, and typically are laminate and contain more than one vein (unless secondarily reduced as in most conifers). Although widely applied, this megaphyll typology is an artifact of the depauperate living flora. Once fossils are included, no component of the megaphyll concept emerges as a synapomorphy uniting these lineages. In particular, the central tenet of associated leaf gaps is not relevant to the earliest fossil representatives of these lineages, all of which are protostelic (Taylor and Taylor 1993)." Megaphylls may have evolved independently some four times, but in each case at least the early stages of their evolution were similar (Boyce &am; Knoll 2002).
On the other hand, Langdale et al (2002) thought that microphylls and megaphylls could perhaps have arisen by modifications of the one developmental pathway. Indeed, there may be more commonality between microphylls and megaphylls than might appear, and Vasco et al. (2016) suggest that C3HDZ (class III homeodomain leucine zipper) genes may have been coopted in both megaphyll and microphyll development. Following Zimmermann, both megaphylls and microphylls can be thought of as modified sporangia. In lycophytes one of a pair of sporangia became a microphyll (see also Crane & Kenrick 1997) with C3HDZ genes being expressed in their development, while in the [Monilophyta + Lignophyta] clade more complex telome systems make up the leaf and C3HDZ genes are initially expressed throughout the young primordium, but later on only on the adaxial surface and then they are involved in the development of dorsiventrality (there has been neofunctionalization - Vasco et al. 2016; see also Scarpella & Meijer 2004 for the radial/dorsiventral sequence). Some genes are involved both in meristem growth and acquisition of leaf identity (Tsiantis et al. 2002). Sanders et al. (2009: p. 867) suggested that "leaves in seed plants and ferns evolved through parallel modifications of the same developmental pathway but through a different sequence of events." In ferns evolution of bilateral symmetry and pinnate architecture preceded the evolution of determinancy, while in seed plant determinancy was first to appear, the two other features appearing later (Sanders et al. 2009). Overall, there are very complex patterns of gains, modifications, and losses of genes (Vasco et al. 2016; see also Evkaikina et al. 2017).
Papers in Cronk et al. (2002), see also Harrison et al. (2005b), Floyd et al. (2014), Vasco et al. (2016), Tomescu and Whitewoods (2024), etc., are helping to develop an understanding of the developmental background of euphylls or megaphylls or whatever they are to be called. Some of the genes involved in megaphyll formation may have evolved long before megaphylls appeared and they had different functions then (e.g. Beerling 2005a, b; Floyd 2006; Vasco et al. 2016 and references), repeating a pattern that we have already seen more than once in the evolution of streptophytes and embryophytes. LITTLE ZIPPER (ZPR) proteins are post-translational negative regulators of apical meristem and leaf development and are part of the C3HDZ stable of genes, which may initially have been expressed in sporangia throughout embryophytes (Vasco et al. 2016 and references). After gene duplication of the C3HDZ gene in the [Monilophyta + Lignophyta] clade, one of the copies degenerated and became the ZPR gene, and it and C3HDZ genes together were involved in the evolution of euphylls. However, Floyd et al. (2014) placed the origin of ZPR proteins at the [Monilophyta + Lignophyta] node; they could not find them in lycophytes. Along the same lines, Zumajo-Cardona (et al. 2019) found that KANADI genes involved in abaxial leaf identity were expressed in ferns (Equisetum), gymnosperms and angiosperms, but not in lycophytes (Selaginella). The angiosperm ARP (ASYMMETRICLEAVES/ROUGHSHEATH2/PHANTASTICA) genes, involved in leaf development, are not found in Huperzia and ferns, but they are known from Selaginella (Hernández-Hernández et al. 2020).
Edwards (1993; Edwards & Richardson 2004; also Niklas 2015 for some cautionary comments) reviewed the anatomy of the vegetative parts of sporophytes of early land plants; Crepet and Niklas (2019) suggest that early Palaeozoic taxa were rhizomatous herbs. Proctor (2014: p. 66) emphasised that endohydrous and homoiohydrous vascular plants clearly did not evolve as such (c.f. the birth of Athena), but that elements of the "vascular-plant package" were to be found in ecto- and poikilohydrous plants. Indeed, the distinction between these two "strategies" is not that sharp, and some ferns, for instance, may be homoiohydrous for only part of the year (e.g. Lösch et al. 2007), or gametophytes may be poikilohydric, sporophytes homoiohydrous (e.g. Kubásek et al. 2021), while whether or not a plant is dessication tolerant is yet another matter (Aros-Mualin & Kessler (2024).
Support for the stem in monilophytes is provided largely by the lignified stereome in the outer cortex, while the tracheids are relatively thin walled, and they can be quite wide, which may improve their ability to transport water, but this would not be to the detriment of plant support. However, in gymnosperms thick-walled tracheids provide much of this support, and in angiosperms, too, it is various elements of the vascular tissue that provide support (e.g. Rowe et al. 2004; Pitterman et al. 2011; Klepsch et al. 2015). Tomescu et al. (2014) and Proctor (2014) suggest that roots would allow plant size to increase both by providing stability and by facilitating increased water uptake. For the evolution of root hairs, see the discussion above.
Edwards (2003) and Edwards et al. (2003) examined conducting cells of early tracheophytes and compared the morphologies of the cells involved with those of the bryophytes s.l.. For a general discussion on the evolution of water-conducting cells, with particular attention to wall sculpturing and its nature, see especially Kenrick and Crane (1991, 1997), Cook and Friedman (1997), Friedman and Cook (2000) and Edwards et al. (2006). However, understanding the fossil record is difficult in part because our knowledge of the development and nature of the wall thickening even of extant vascular plants is surprisingly poor, and exactly where in the wall lignin is deposited affects water conductance (Sperry 2003). Programmed cell death in vascular plants is involved in tracheid development, for instance (see van Hautegem et al. 2015). Interestingly, the developmental control of conducting tissue in moss gametophytes and vascular plant sporophytes suggests that at one level they may not be that much different. For a general comparison of tracheary cells, see Bailey and Tupper (1918).
Cichan (1986) and Wilson (2015) and references relate details of xylem anatomy and water transport, linking extinct and extant plants; woods of early vascular plants may have been quite efficient in conducting water, although susceptible to embolism/cavitation (see Schenk et al. 2017 for the role of lipid surfactants in the xylem in preventing embolisms). Sperry (2003 and references) provides a general review on the evolution of xylem, connections between details of cell anatomy, cell wall chemistry and water transport, plant support, etc.. Stomata and the vascular system together - the depth of plant rooting, etc. - may have allowed the development of the homoiohydric habit, and as we shall see, transpiration through the stomata affects the global climate (Berry et al. 2010).
Ecology & Physiology.In seed plants, stomata open in response to both red and blue light. It initially seemed that stomata of those few ferns examined, all leptosporangiates (Pteris, Adiantum, Asplenium and Nephrolepis), lacked a blue light-specific opening response, although elements of the response, including the relevant phototropin, a blue light receptor protein kinase, were present (Doi et al. 2006). In the shaded understory environments that many ferns prefer, blue (and red) light has been preferentially absorbed by the canopy above; stomatal closure is mediated by red light, and response of fern stomata to red light was rapid (Doi et al. 2006, 2015). Doi et al. (2015) later found that there was a blue-light response in other ferns, lycophytes, cycads, etc., indeed, stomata of a few of these taxa, including Equisetum, did not respond to red light. Kawai et al. (2003; see also Suetsugu et al. 2005) noted that in some Polypodiales there was a distinctive chimaeric red/far red light photoreceptor (phy 3, = neochrome) in which red-sensing phytochrome and blue-sensing phototropin are fused into a single molecule (F.-W. Li et al. 2014), possibly aiding in phototropic responses in the shaded conditions in which ferns have diversified; neochrome is very sensitive to white light (Wada 2008). Neochrome seems to have been acquired by polypod ferns from hornworts via lateral gene transfer around 179 Ma, which, perhaps not so coincidentally, is about the age of Polypodiales (H. Schneider et al. 2004a), and it may even have been acquired more than once (F.-W. Li et al. 2014); it is also known from Mougeotia (Zygnemataceae) and related genera (Suetsugu et al. 2005; Li et al. 2014). Veins in fern leaves are not the same distance from the epidermis as they are from each other, i.e., they are not hydraulically optimised. However, photosynthesis may proceed best in the low levels of diffuse light in understory vegetation and so this hydraulic optimisation may not matter so much (Zwieniecki & Boyce 2014). (Note that in seed plants the effectiveness of the stomatal response is white/blue + red > blue > red > green light (Willmer & Fricker 1996) and that in many flowering plants leaves are hydraulically optimised - Zwieniecki & Boyce 2014.) For iridescence in fern leaves, and different kinds of chloroplasts in epidermal cells, see R. M. Graham et al. (1993) and Nasrulhaq-Boyce et al. (1991); being shade plants, they may have distinctively coloured/patterned fronds, show iridescence, and the like.
This somewhat confusing story has begun to be clarified by recent findings on the stomatal response of leptosporangiate Polypodiales, the Eupolypods in particular, in which duplicate copies of cryptochromes have evolved, and these cryptochromes facilitate the very speedy opening of the stomata - and so the facilitation of photosynthesis - in response to the blue light wavelengths that predominate in light that has passed through the canopy foliage, other wavelengths having been preferentially absorbed (Cai et al. 2020). Note that other vascular plants are responsive to blue light, but in different ways (cryptochromes, although universally present, as in some streptophyte algae, are not central); that Arabidopsis stomata have a rather slow response to blue-light, not unsurprising since it is hardly a plant of the forest floor; and finally that in terms of sheer number of cryptochromes, Alsophila (Cyatheaceae) has more, but all in cryptochrome types found in other vascular plants (c.f. Cai et al. 2020 in part). Two gene duplications were perhaps involved in the genesis of the unique Eupolypod cryptochromes (Cai et al. 2020). It may also be mentioned that Grunwald et al. (2022) found that the bundle sheath of Arabidopsis thaliana (and at least some other angiosperms) is sensitive to blue light, more so than the stomata (although the two systems have similar photoreceptors, etc,), enabling the maximum photosynthetic rate to be reached in about ten minutes after the beginning of irradiation, one third the time it takes for the stomata to fully open. There is a rapid increase in leaf radial hydraulic conductance, and dissolved CO2 in the water that moves through the bundle sheath into the mesophyll may be involved in photosynthesis (Grunwald et al. 2022).
Stomatal responses in non-flowering vascular plants have been characterized as being passive as the hydration of leaf tissues changes, the stomatal aperture changing as surrounding tissues flex, while those of flowering plants are called active as they respond to abscisic acid (ABA) and its activation of ion channels in the guard cells (Sussmilch et al. 2017). ABA synthesis is not triggered by changes in leaf turgor in non-flowering plants, but it is in flowering plants, although not all of the latter show the same sensitivity to turgor changes (McAdam & Brodribb 2016). Stomatal opening and closure here is in fact a complex affair, and it depends on the species involved, local conditions, etc., and any response to ABA in particular is conditional (Meigas et al. 2024). Ferns – including lycophytes - have vessel elements with lower hydraulic efficiency than angiosperms, leaf vein density is lower, and leaf hydraulic resistance is higher, and so stomatal conductance tends to be constrained (Y.-J. Yang et al. 2021). Indeed, seed plants as a whole have higher photosynthesis and transpiration rates and a higher stomatal conductance than do ferns and lycophytes. Campany et al. (2018) compared ferns (6 species) with Selaginella (7 species) growing in similar habitats on the forest floor in Costa Rica, and found that the two groups had similar net photosynthesis and water use efficiency, but Selaginella had a lower leaf mass/area ratio and nitrogen use efficiency in photosynthesis, and longer, less dense stomata. Interestingly, water use efficiency in seed plants is upregulated as conditions get drier – ABA increases, excessive stomatal opening is reduced - while under severe drought, water use efficiency is unchanged – in ferns water use efficiency decreases gradually, and leaf water potential decreases (Yang et al. 2021). It has been suggested that fern stomata do not respond to increased CO2 concentrations in the atmosphere (McAdam & Brodribb 2012a), although this has been questioned, such a response apparently being present in the common ancestor of extant vascular plants (Franks & Britton-Harper 2016, see also Horak et al. 2017 for fern stomatal responses to ABA and CO2). Furthermore, ABA is involved in many aspects of plant growth, including spore dormancy, the determination of sex in gametophytes, etc. (McAdam et al. 2016). However, as is obvious, details of the physiology of stomatal functioning may be unclear and in part conflicting (see also Lind et al. 2015; Roelfsema & Hedrich 2016 and references). Interestingly, the stomata of Equisetum seem to be (or they come to be) permanently closed, as perhaps also in Psilotum (Cullen & Rudall 2016).
Initially it seemed that the response of photosynthesis to red light and passive stomatal control of leaf hydration were best tagged to this node (see above: McAdam & Brodribb 2011). It was thought that the mechanism of stomatal closure in ferns was like that of lycophytes rather than that of seed plants (McAdam & Brodribb 2011, 2012, 2013; see also Haworth et al. 2011, 2013; McAdam et al. 2016); it is passive, and abscisic acid is not immediately involved. However, some recent work suggests that in some ferns, at least, there is an active response to both abscisic acid and CO2, the nature of the response depending both on the species involved and growth conditions, e.g., humidity (Hõrak et al. 2017; Meigas et al. 2024; c.f. Brodribb & McAdam 2017). It has been suggested that stomatal apertures in Equisetum are permanently closed - perhaps this is true of older stomata (see also Chater 2024) - and this may also be the case in Psilotum, but there is certainly movement in Lycopodiales (Cullen & Rudall 2016; Roelfsema & Hedrich 2016), and what goes on in Equisetum is not simple - there is certainly movement (Meigas et al. 2024). Indeed, in ferns, at least, part of the pathway involved in stomatal closing in seed plants is involved in spore dormancy (as perhaps in bryophytes) and in the determination of the sex of the gametophyte, and there, too, there is antagonism between gibberellic acid and abscisic acid (McAdam et al. 2016; see Cullen & Rudall 2016; Chater et al. 2017 and references for the evolution of stomatal patterning and development). In any event, stomata in extant vascular plants are clearly involved in gas exchange - for further discussion see above.
Kenrick et al. (2012) discussed the effect of vascular plants on the global carbon cycle. There are broad correlations between atmospheric CO2 concentration and stomatal size that have important implications for plant productivity, transpiration, and rock weathering. When the CO2 concentration of the atmosphere is low, leaves tend to have higher densities of smaller stomata, allowing more CO2 to diffuse into the leaf, when concentrations increase, relationships are the reverse (e.g. Franks & Beerling 2009). It has also been suggested that CO2 concentration, guard cell size, and genome size can be linked, the first and last being broadly correlated over the last 400 Ma (Haworth et al. 2011), although genome size reconstructions (see their Fig. 4) and some other aspects of this story (see also Franks et al. 2012) are difficult to understand. Within vascular plants there is a great range of relative pore area (pore area/total guard cell + pore area), that of Huperzia and Nephrolepis being much lower than that of flowering plants, and there is even greater variation in the rate of stomatal opening; in the two plants mentioned there was no movement of K+ ions that is an integral part of the whole process in flowering plants (Franks & Farquhar 2006). For water use efficiency throughout the Phanerozoic, see Franks and Beerling (2009a) and Assouline and Or (2013: a different interpretation for higher CO2 concentrations). The carbon cycle was further affected by the evolution of vascular plants with well developed secondary thickening, whether producing mostly bark or both bark and wood; much carbon could be sequestered in these tissues (see elsewhere).
The epiphytic habit may have become established quite early on in some clades of vascular plants. Thus part of Trichomanes, commonly epiphytic on tree ferns, a relatively old clade (Schuettpelz 2007; Hennequin et al. 2008; see also Schuettpelz & Pryer 2009), diversified some time between the early Middle Jurassic and the Late Cretaceous. Interestingly, epiphytes in Hymenophyllaceae are often low epiphytes, that is, they grow on the trunks and lower branches of trees, and this is perhaps an older habitat that that of crown epiphytes, which grow higher up in the tree and on branches (see also Lehnert et al. 2017; Lehnert & Krug 2019). About half - 190/380 species - of clubmosses, Lycopodiaceae, are epiphytic, and their diversification may have begun in the Late Cretaceous (Wikström & Kenrick 1997, 2001; Wikström 2001), although Testo et al. (2018b) suggest that Phlegmariurus, a major genus of Lycopodiaceae, was ancestrally epiphytic (see also Field et al. 2016), and was perhaps growing in this habitat as early as the middle Jurassic (an estimate of the age of Huperzioideae, of which Phlegmariurus makes up the major part, is (223-)199.5(-175) Ma - Testo et al. 2018b). Botryopteris (Ophioglossaceae) may have been another early epiphyte, apparently growing on the extinct marattialean tree fern Psaronius (Rothwell 1991). There is little direct fossil evidence of the epiphytic habit, although Psaronius, a tree fern whose stems were enclosed by a mantle of adventitious roots, was widespread in early Pennsylvanian to Triassic deposits some 320-225 Ma, and a variety of plants have been found associated with it, including climbers and (?rooted hemi)epiphytes (Rößler 2000). For plants that may be epiphytic on osmundaceous rhizomes, see Bippus et al. (2019 and references).
One of the ways in which ferns and lycophytes, whether the gametophytic or sporophytic generation, can grow in drier conditions is by being dessiccation tolerant, cutting down water loss, etc., even if the mechanism of stomatal control is overall similar to that in ferns growing in more mesic conditions (McAdam & Brodribb 2013).
Ferns, gymnosperms and lycophytes tolerate nutrient-poor (but sometimes rich in non-essential minerals) conditions, perhaps the ancestral condition for many of them (Page 2004). The evolution of some genes in plants involved in mycorrhizal associations that facilitate phosphorus uptake by the plant may be pegged to this node (Delaux et al. 2015), although any advantages to the plants in which mycorrrhizae were first established may differ from those to extant plants (Maherali et al. 2016).
Plant-Animal Interactions. Kato (2017) summarises a number of plant-animal interactions.
Plant-Bacterial/Fungal Associations. Early vascular plants are likely to have had a variety of associations with fungi, glomerimycete AM fungi long being known from plants of the Rhynie Chert some 407 Ma (e.g. Kidston & Lang 1921), indeed, the recently-described Archaeosporites rhyniensis perhaps could have been placed in the extant genus Archaeosporium if nucleic acid data had been available - morphologically the two are very similar (Harper et al. 2020), and they have well-preserved arbuscules (Strullu-Derrien et al. 2025). Mycorrhizal associations with AM Glomus (Glomeromycota) are common in extant vascular plants. The commonest mycorrhizal association seems to be the Paris type where the hyphae are intracellular, forming coiled structures between the plant cells (F. A. Smith & Smith 1997; Winther & Friedman 2008), although the Arum type, with extensive intercellular hyphal growth and intracellular arbuscules and vesicles is also frequent (Field et al. 2015d) and that is the association best understood at the molecular level (Cosme et al. 2018). Of course, although we talk about mycorrhizal "types", there is more a continuum of variation (Dickson 2004; Dickson et al. 2007). For further discussion about plant-fungal associations, see elsewhere
In Ophioglossum and Botrychium, some Lycopodiaceae, Psilotum, and a few ferns and angiosperms, etc., fungal associations are variously with echlorophyllous gametophytic and/or sporophytic stages (Winther & Friedman 2007, 2008, 2009; Leake et al. 2008; Hynson et al. 2013). Around 10% of all vascular plants are mycoheterotrophic for all or part of their life cycles (Leake & Cameron 2010), a number driven by the 26,000 or so species of Orchidaceae all, or nearly all, of which have such associations when they are germinating; there are perhaps 1,000 mycoheterotrophic species in the Lycopodiales and Monilophytes combined (Winther & Friedman 200). Full mycoheterotrophy, known from ca 880 species (this number includes ferns, etc., with echlorophyllous gametophytes), has evolved 46 or more times, including in liverworts (Merckx et al. 2013a). For "ancestral" AM genes in Selaginella, see Bravo et al. (2016).
Lutzoni et al. (2018) think about the possible link between diversification in the species-rich ascomycete group Leotiomyceta (inoperculate ascomycetes) and the early diversification of vascular plants, including the origin of spermatophytes.
Romanova et al. (2022) discuss variation of the stem apex and its possible evolution: The monoplex shoot apical meristem has a single apical cell and the simplex-type meristem has several apical initials. There is also a correlation with plasmodesmata type, monoplex plants being unable to establish intercellular communication between cells after their division by the development of so-called secondary plasmodesmata while simplex-type plants can. The simplex-type initials are known also in Rhynia, from the Rhynie chert, and may be plesiomorphic (Romanova et al. 2021).
Genes & Genomes. For the genomes, etc., of non-seed plants, see Szövényi et al. (2021) and for those of vascular plants, see Kinosian et al. (2022), and for basic chromosome construction, perhaps of all vascular plants, see Hisanaga et al. (2023). Nakazato et al. (2008) discuss the evolution of the nuclear genomes of lycophytes and ferns. For meiosis, MTOCs, etc., see R. C. Brown and Lemmon (2008); meiosis is polyplastidic and anastral [??]. There cannot be a genome duplication common to all tracheophytes since there is no evidence of genome duplication in Selaginellaceae (e.g. Banks et al. 2011; Baniaga et al. 2016; recent work suggests that there may be some hybridization here - Kang et al. 2024). Leitch and Leitch (2013) found that Lycopodiopsida had rather small nuclear 1 C genome sizes - (0.086-)1.7(11.96) pg - but sampling was poor; the difference was not evident in F.-G. Wang et al. (2022). Banks et al. (2011) and Jiao and Paterson (2014) offer suggestions for the genome size of the ancestor of vascular plants - 7,247 or 7,790 gene families respectively. Genome sizes increase (no figures for ferns), and in angiosperms like Populus trichocarpa and Sorghum bicolor the figures are in excess of 14,300 and 16,700 gene families respectively. A PCA analysis of functional protein domains suggested that Physcomitrella [= Physcomitrium], Selaginella and Gnetum were quite close, and rather different from but more or less linking with the three other gymnosperms in the study, only then with associations to the angiosperms (Wan et al. 2018: no ferns included).
Sessa and Der (2016) discussed genome size in the context of homo-/heterospory. In pteridophytes (including lycophytes) n = 57 on average, while in homosporous Lycopodium chromosome numbers are higher than in heterosporous Isoëtes and Selaginella. C. Li et al. (2023/2024) found that although Huperzia asiatica and Diphasiastrum complanatum (Lycopodiaceae) had diverged ca 350 Ma, about 30% of the genes remained in syntenic blocks, however, comparing the genomes of the heterosporous I. sinensis and S. kraussiana which diverged at around the same time, synteny levels were only some 4.4-7.2% (Li et al. 2023: see also other duplication events in homo- and heterosporous ferns and lycophytes). Overall, genomes in Lycopodiaceae are large compared with those of other Lycopodiopsida. The bottom line is that genome evolution in Lycopodiaceae is slow, contrasting with the situation in Selaginellaceae and Isoetaceae. For more on heterospory, see Gene and Genome Duplication, and Genome Size, especially towards the end.
Within ferns, there are similar correlations. Polyploidy is common there, but the amount of DNA per chromosome tends to be conserved, so fern genomes are large, but not very variable in size. Overall, homosporous ferns tend to have high base chromosome numbers and genome sizes (J. Clark et al. 2016; Kinosian et al. 2022), even if there has been less polyploidy than in angiosperms (Haufler 1987; Barker 2009, 2013; Sessa & Der 2016). Heterosporous ferns tend to have lower chromosome numbers and smaller genomes (Sessa & Der 2016; F.-W. Li et al. 2018), thus Wang et al. (2022) noted that the aquatic heterosporic Salviniales had notably small genomes; n = ca 14 here. Overall, diploidization in ferns involves extensive gene silencing and fractionation of individual genes, not large-scale structural changes and the loss of DNA or nucleotide substitutions (e.g. Clark et al. 2016; Y. Liu et al. 2019; C. Li et al. 2024). Clark et al. (2016) suggested that the epiphytic habit and increased genome size in eupolypods may be connected (see also F. G. Wang et al. 2022), if so, this is the reverse of the situation in orchids. For genome sizes in monilophytes and lycophytes, see also Nakazato et al. (2008) and Leitch and Leitch (2013).
In angiosperms chromosome numbers are relatively low - n = 16 on overage - and chromosome numbers in gymnosperms are on average also low (Barker 2013; see also Sessa & Der 2016), and although some flowering plants in particular do have very high chromosome numbers, the average is low. Polyploidization in gymnosperms is low, ca 5.7%, polyploidization is much higher in angiosperms, being over 30% (Halabi et al. 2023). Angiosperm genomes tend to be smaller than those in homosporous embryophytes, but again, they are much more variable in size and can be huge (see also Pellicer et al. 2018; C.-H. Huang et al. 2019; F.-G. Wang et al. 2022); genomic change is extensive and downsizing relatively fast (Ickert-Bond et al. 2020), there is extensive gene and genome remodelling and frequent and sometimes very pronounced genome downsizing after genome duplication/polyploidization events (see also elsewhere).
Baniaga et al. (2016) looked at the rate of genome size evolution in various clades of vascular plants - they thought that it was often low, but very low in Selaginella, quite high in ferns and still higher in many angiosperms. Kinosian et al. (2022) thought that genome size differences might reflect different histories of the transposable elements in the genomes. In homosporous plants the insertion of transposable elements (TEs) was relatively ancient which gave them more time to increase in copy number and so increase genome size, while in heterosporous plants, including heterosporous ferns, TEs are much younger, so genome size is less affected (see also Baniaga & Barker 2019). (In homosporous plants TEs are common in the pericentromeric and telomeric regions; such localization is uncommon elsewhere.) Kinosian et al. (2022) also observed that there was around 100 times more variation in the sizes of the chromosomes of flowering plants compared with those of ferns and lycophytes. For genome sizes in ferns and lycophytes, see also Nakazato et al. (2008) and Leitch and Leitch (2013). Amborella and other angiosperms have numerous Long Terminal Repeats in the pericentromeric regions of its chromosomes and the genes on its chromosomes are densely arranged; in other land plants these features are more evenly distributed along the chromosomes (Carey et al. 2024).
However, some recent work leads one to think that things are rather more complex. Fang et al. (2022) suggested that homosporous ferns had much larger genomes than heterosporous ferns because of the development of Long Terminal Repeat retrotransposons and Long Interspersed Nuclear Elements (85.25% of the genome), despite their fairly rapid elimination (comparison: Adiantum capillus-veneris (Pteridaceae) with Azolla and Salvinia) - i.e., a different way of getting large genomes. However, even in the small sample of land plants that they showed (Fang et al. 2022: Fig. 2) there was no obvious general correlation, Adiantum being bracketed by three heterosporous seed plants, and with the homosporous Marchantia having a very small genome. Fang et al. (2022) found little in the way of syntenic blocks of genes in Adiantum capillus-veneris, also Pteridaceae. Although there had been suggestions that the PTERa duplication was ancestral here (O.T.P.T.I. 2017), Fang et al. (2022) could find no evidence for this, rather, Adiantum might have been involved in a WGD event shared by all core leptosporangiate ferns (see also F.-W. Li et al. 2018) - either way, the duplication was not recent. Marchant et al. (2022) looked at Ceratopteris richardii (a third member of Pteridaceae, and a homosporous aquatic at that) and also found a much more dynamic genome than in other homosporous plants. Again, syntenic blocks were little in evidence (duplication ca 60 Ma), and there had been considerable gene loss, tandem duplication and multiple horizontal gene transfers from bacteria. Transposable elements had been inserted in introns, hence the large size of genes and the genome (Marchant et al. 2022). Thus, although in general heterosporous vascular plants do tend to have lower chromosome numbers and smaller genomes than do homosporous vascular plants (see also Pelosi et al. 2022: ferns), there are exceptions, and sampling needs to be improved (C. Li et al. 2023).
Three new families of transcription-associated proteins may have evolved in this general area (Lang et al. 2010: hornworts not included; see also Zhu et al. 2012, Lang et al. not cited). There is a squalene-hopane cyclase gene that apparently came from cyanobacteria via lateral gene transfer in lycophytes/ferns (F.-W. Li et al. 2018; Wickell & Li 2019). This is an odd distribution - has it been lost in seed plants, or did it move independently to ferns and lycophytes?
The plastome of vascular plants exists in three forms that differ in the orientation of the small single copy and one of the copies of the inverted repeat; one of these forms, haplotype C, is very uncommon (see Selaginella: W. Wang & Lanfear 2019). Most vascular plants have the two other forms, although if the inverted repeat has been lost or it is much reduced there may be only a single form (Wang & Lanfear 2019). For the evolution of the plastome in other than seed plants, see Wolf and Karol (2012). Chloroplast genomes are particularly labile in many taxa ouside the angiosperms (Guisinger et al. 2011 for references), although less so in Lycopodiaceae where they seem to be highly conserved (Mower et al. 2018).
The order of genes in the mitochondrial genome is relatively invariable in bryophytes, but it is much more variable in vascular plants (Y. Liu et al. 2014a). For more on the ancestral genome here, which may have had 41 protein-coding genes, see W. Guo et al. (2016b).
Chemistry, Morphology, etc.. Lignocellulose biosynthesis seems to have evolved in the ancestor of vascular plants (Ferrari et al. 2020). Condensed tannins are polymerized in a chloroplast thylakoid-derived tannosome (Brillouet et al. 2013).
For details of the sporophyte-gametophyte boundary, see Duckett and Ligrone (2003).
Kaplan (1997; vol. 3, 2001) provides an extensive discussion and analysis of the basic morphology of lycophytes and monilophytes in particular. See also Eames (1936) for the morphology and anatomy of the "lower groups" of vascular plants.
Phylogeny. In molecular phylogenies the relationships [lycophytes [monilophytes + lignophytes/seed plants]] are very commonly found, although not in some plastome analyses in Ruhfel et al. (2014) and in the mostly plastome analysis of Z.-D. Chen et al. (2016). In De la Torre-Bárcena et al. (2009: expressed sequence tags) a clade [monilophytes [lycophytes [bryophytes s.l.]]] was shown as sister to seed plants, although the major focus there was relationships among seed plants.
Gómez‐Noguez (et al. 2022) noted that the terminal velocity of pteridophyte (= Selaginella plus a number of ferns) spores was more affected by spore mass than size; "ornamental" appendages also reduced the terminal velocity. Cascales-Miñana et al. (2021) document the remarkable variation of spore morphology in taxa from the Rhynie chert (and some other mostly homosporous plants); basically, different spore morphologies may be produced by the one species, and may even be found in the same sporangium, but apparently not because of ontogenetic variation, and the same spore morphology can be found in quite unrelated plants. Linking differences in spore morphology to plant diversity is not straightforward.
The challenge becomes to integrate the fossil record, especially that of the oldest vascular plants, with the phylogeny of extant vascular plants, and of course the reliability of phylogenies based on plastomes (or mitochondrial) genomes or on morphological data alone immediately become issues. Crepet and Niklas (2018, 2019) examined the evolution of basal vascular plants using 54 characters scored for 37 fossils, largely Devonian, plus Equisetum. Aglaophyton, Cooksonia [Rhynia, Horneophyton, Nothia] formed a basal grade; there were then two main clades, in one [Asteroxylon + Baragwanathia] were sister to a clade that included Zosterophyllum and Lepidodendron, the lycophyte clade, while the other clade included Psilophyton, Aneurophyton, [[Archaeopteris + Lyginopteris] [Equisetum [Archaeocalamites + Calamites]]], a trimerophyte/euphyllophyte clade (Crepet & Niklas 2019). Reproductive and anatomical characters analysed alone gave very little resolution, morphological and reproductive characters together yielded a zosterophyllophyte + lycophyte grade, not clade, and so on (Crepet & Niklas 2018). Unreversed apomorphies for the first clade may include sporangia lateral and stem with ridges and furrows, but labeling is confused (Crepet & Niklas 2019). In a variant of this approach, Niklas and Crepet (2022) looked at the position of four taxa placed in Psilophyton in analyses that included a number of fossil taxa and also Equisetum, Archaeocalamites and Calamites, and in some analyses also Psilotum and Tmesipteris. Psilophyton grouped with trimerophytes and euphyllophytes, but the species of Psilophyton did not all group together, Psilotaceae grouped with a [zosterophyllophyte + lycophyte] clade, while Equisetum grouped with the sphenopsids Archaeocalamites and Calamites which together were embedded in the trimerophyte/euphyllophyte clade; all in all, it was unclear to Niklas and Crepet (2022) that morphological s.l. data could disentangle relationships between such ancient plants.
Classification. Lycophytes and monilophytes/ferns have traditionally been included in a broadly-circumscribed and paraphyletic "Pteridophyta".
IV. LYCOPODIOPSIDA Bartling / LYCOPHYTES
Sporophyte axes rhizoidal; branched rooting system; mitosis and meiosis monoplastidic; shoots arising by the bifurcation of the apical meristem, stem with protostele [= actinostele], protoxylem exarch; leaves ≡ microphylls, with a single vein, phloem surrounding xylem; sporangia lateral, 1/leaf, often heart-shaped, dorsiventrally flattened, dehiscence transverse [along line of conspicuously thickened cells]; stellate array of filaments in transition zone at base of flagellar shaft; zygote with variable plane of first cell division, elongating, with quadrant/octant formation, embryonic axis reorients during development; nuclear genome [1 C] (78-)1,165(-11,704) Mb; chondrome with 6 genes lost/pseudogenised [inc. all 4 ccm genes], introns numerous [but only 16 in common], some group II introns lost. - 3 families, 17 genera, 1,340 species.
Includes (extant taxa) Isoëtaceae, Lycopodiaceae, Selaginellaceae.
Age. Larsén and Rydin (2015) estimated an age of about 407 Ma and Magallón et al. (2013) an age of around 383.7 Ma for crown-group Lycopodiopsida see also Laenen et al. (2014), an age of ca 303.1 Ma, B. Zhong et al. (2014b), an age of (403-)386.3(-377.4) Ma, Villarreal and Renner (2014), an age of only around 270 Ma but Evkaikina et al. (2017) an age of ca 376 Ma. Other estimates include Testo et al. (2018b), an age of (414-)403.3(-394) Ma, D. Wood et al. (2019/2020) ca 415.1 Ma, B. J. Harris et a]l. (2021/2022) an age of 431-411 Ma, Xia et al. (2022) an age of (353-)326(-299) Ma and Y.-L. Qiu et al. (2024) an age of (400.3-)330.5(-251.9) Ma; see also P. Soltis et al. (2002).
Evolution: Divergence & Distribution. For the early evolution of Lycopodiopsida, which have a rich fossil record, see Gensel and Berry (2001), Wellman et al. (2009), Ambrose (2013), Gerrienne et al. (2016 and references) and Xue et al. (2016: Drepanophycus rhizomes ca 410 Ma). Lycophytes include the extinct zosterophylls, which have circinately-coiled stems and sporangia in two rows (see also Gensel 1992; Kenrick & Crane 1997; Gonez & Gerrienne 2010).
Much work has been carried out on fossil lycopsids, known from the Devonian onwards. DiMichele and Bateman (2020) discussed the phylogeny of Carboniferous lycopsids in particular, emphasizing phylogenies based on whole-plant reconstructions. Heterospory developed early (see Phillips 1979 for a good example), and heterosporous trees growing up to some 50 m tall are in the same immediate clade as today's lowly Isoëtes. Zosterophylls and some other genera may form a clade with Lycopodiaceae (Gonez & Gerrienne 2010). For more on zosterophyll relationships, see Nibbelink and Tomescu (2022), but there is not much yet in the way of well-supported relationships, phenetic and phylogenetic analyses and those of morphological and anatomical data sets telling different stories.
Laenen et al. (2014) give some crown-group ages for genera. Renzaglia et al. (1999) detailed variation in a number of characters of possible phylogenetic interest in this clade.
Ecology & Physiology. Tree lycopsids dominated in late Carboniferous peat swamps, but are known from well before. Thus there is evidence of forests in early Late Devonian deposits ca 380 Ma in Svalbard that were made up of Protolepidendropsis puchra with trunks up to 4 m tall and 9 cm across, although the flared base was up to 20 cm across (Berry & Marshall 2015: evidence for secondary thickening indirect).
Weng et al. (2008, 2010, 2011), Weng and Chapple (2010) and others have discussed the synthesis of syringyl lignin in Selaginella. Syringyl lignins are also found throughout angiosperms, but details of the synthetic pathways are quite different in the two (see also Harholt et al. 2016). Prior to xii.2020 I had this feature as an apomorphy for Selaginellaceae, and Gibbs (1958) had reported a positive Mäule reaction from nearly all Selaginella he examined. Note that syringyl lignin here is found in the cortical cells, while guaicyl lignin, the normal lignin type in lycophytes, is associated with the xylem. However, the situation is rather more complicated. Syringyl lignins of one sort or another are also reported from Huperzia and particularly Isoëtes, although in the latter they were not to be found in all species, being absent from I. fluitans, for example, but present in I. hystrix (Espiñeira et al. 2010; also Novo-Uzal et al. 2012). For further details of lignins and their evolution, see also above.
Green (2010) described photosynthesis in this clade, focussing on the tree-like extinct lycopsids and the extant Isoëtaceae. When tree lycopsids were flourishing, from the end of the Carboniferous to the beginning of the Permian, a period of about 100 Ma, it was a time when atmospheric CO2 concentrations were low and oxygen concentrations were high. Air canals/aerenchyma permeated both above- and below-ground parts of the plants, i.e. they had a parichnos system. CO2 produced by the plant remained in the plant and CO2 also moved into the plant from the anoxic CO2-rich soil, ultimately going to the leaves, where it was used in photosynthesis; this is the lycopsid photosynthetic pathway, LPP (Green 2010, esp. p. 2258). Oxygen may have moved to the roots in these canals, being used up in respiration. Although movement of oxygen within the plant may have been facilitated by the canal system, a pad of tissue at the base of the rootlets seems to cut off their air spaces from those of the stigmarian roots, yet overall CO2 movement and photosynthesis in the plant may have been little affected (Boyce & DiMichele 2015). Of course, such air spaces may provide support for the stem while at the same time economizing on tissue expense...
Some lycopsids reached perhaps 50 m tall and 2 m d.b.h., either branching along the trunk as they grew (Lepidophlois), or only when they reached more or less mature height (Lepidodendron, Sigillaria); the plants were either polycarpic or monocarpic. The trunks had little secondary or even primary phloem, and cork and perhaps a little xylem were all that was produced by any cambia (e.g. DiMichele & Bateman 2020). Indeed, D'Antonio and Boyce (2020) recently suggested that the periderm was a mere ca 2 cm - perhaps up to 8.5 or rarely 15 cm - thick, which makes thinking about tree support and stability difficult (see also Speck 1994). The plants seem to have undergone a period of establishment growth when the stigmarian root system developed and the apical meristem of the stem enlarged, the result being that when the stem finally began to elongate it was of the "adult" thickness (e.g. Phillips & DiMichele 1992; DiMichele & Bateman 2020), so from this point of view it grew rather like a palm; the very ealiest stages of the sporophyte known are thread-like structures (Phillips 1979; Stubblefield & Rothwell 1981). There has been considerable discussion on topics like cladoptosis, the evergreen habit and how fast the plants grew (DiMichele & Bateman 2020 for references). The phloem, in which sieve cell nuclei may have degenerated, is likely to have been very long lived (Boyce & DiMichele 2015), again, c.f. palms.
The rootlets on the Carboniferous stigmarian roots of tree lycopsids have the same branching pattern as in extant Isoëtes; branching is dichotomous, the roots becoming narrower at each dichotomy, but there is otherwise no secondary thickening/tapering, and there are root hairs - add similarities in anatomy, and Isoëtes is practically identical (Hetherington et al. 2016a). From the analysis of vascular tissue, it seems that auxin travelled from the base to the apex of the stigmarian root, the "wrong" direction for a stem - which it is morphologically - but the "right" direction for a root - which it is functionally (Sanders et al. 2011; see also Menand et al. 2007 for a similar example); the bipolar roots of seed plants show a similar pattern of auxin flow, but roots there are independently evolved (Kenrick 2013). Hetherington and Dolan (2017) observed that roots of all three extant groups of lycopsids - and their fossil relatives, where known - were morphologically very similar, having root hairs and root caps (see Friedman et al. 2004 for caveats over roots in tracheophytes in general, as in Hetherington et al. 2016b; Hetherington & Dolan 2018), but were borne on a great variety of structures on extinct and even extant lycopsids. Indeed, these roots may have evolved independently in Isoëtaceae (see below) and the other lycopsids. DiMichele et al. (2022) review various aspects of the anatomy, development and functional morphology of stigmarian roots.
Kong et al. (2020) discuss the evolution of cuticle composition of land plants; there may have been changes around here. Sampling is very limited, but some features (e.g. low amounts of phenolic compounds) of the cuticle of mosses may be more similar to those of lycophytes and other vascular plants than to bryophytes and hornworts.
Fertilization & Spore Dispersal. Sperm swim in water (von Aderkas et al. 2022).
Plant-Bacterial/Fungal Associations. There are various associations of fungi with the sporophytes of lycophytes, although such associations may quite often be absent (Rimington et al. 2014, 2017; Kenrick & Strullu-Derrien 2014; c.f. Winther & Friedman 2008). This absence may be connected with the thinness of the roots, which are often less than 1 mm across in lycopods and Selaginella, and mycorrhizae are uncommon in such roots (Pressel et al. 2016). Furthermore, the swampy habitats in which some lycophytes grew in the past may not have been ideal for the establishment of mycorrhizal associations, judging by extant plants.
Vegetative Variation. It has been suggested that root-bearing axes of many taxa in this group are modified dichotomizing branches (stigmarian roots), the rootlets/ultimate roots being modified leaves (Pigg 1992; Rothwell & Erwin 1985; Rothwell 1995; Kenrick 2013) or organs sui generis (Matsunaga & Tomescu 2016; see also Tomescu et al. 2014). Crane and Kenrick (1997) thought that all appendicular structures in Lycopodiopsida - microphylls, ligules, sporangia, and stigmarian roots - were equivalent, i.e., perhaps consistent with the former hypothesis. In Isoëtes it has been suggested that the roots are exogenous (Rothwell & Erwin 1985) and this is also true of the K-branching that produces root-bearing axes in other Lycopodiopsida, as in Drepanophycales (i.e. sister to extant lycophytes); in such K-branching, one branch of a dichotomy becomes a root-bearing axis, although it may have a few leaves along its upper part, the other becomes an erect leafy stem (Matsunaga & Tomescu 2016). Rothwell and Erwin (1965) described the roots of Lycopodium and Selaginella as being "adventitious", i.e. growing out from along the stem, interestingly, those of Isoëtes abscise in much the same way as microphylls (Hetherington & Dolan 2017 for references). The small, much-dichotomising ultimate roots (Matsunaga & Tomescu 2016) may have a root cap. However, Hetherington and Dolan (2018) have recently described the apices of rooting axes of Asteroxylon mackiei, a ca 407 Ma lycopsid from the Rhynie chert, and no root cap was evident there, just a continuous epidermis. Thus root caps may have evolved at least twice, once here and once in the seed plant (?or euphyllophyte) clade (see also Hetherington et al. 2016b). Here I largely follow Hetherington et al. (2021) in their careful reconstruction of the branching (shoots, roots) of A. mackiei and its implications for the evolution of roots in lycophytes as a whole. Rooting axes in Drepanophycales are like roots in extant lycophytes, both lacking cuticle, stomata, leaves, etc., but the former have neither root hairs nor a root cap and originate in an anisotomous rather than endogenous branching event (Hetherington et al. 2021).
Details of the construction of the apical meristem vary within extant lycophytes and they correlate with the richness of plasmodesmatal connections between the cells: many plasmodesmata - meristem a single cell, few plasmodesmata - meristem a group of cells (Imaichi & Hiratsuka 2007; see also discussion in Evkaikina et al. 2017: Fig. 1). Seago et al. (2022: not Isoetaceae) discussed root anatomy, especially details of that of the endodermis.
Genes & Genomes. Leitch and Leitch (2013) found that Lycopodiopsida had rather small nuclear 1 C genome sizes - (0.086-)1.7(11.96) pg - but sampling was poor; the difference was not evident in F.-G. Wang et al. (2022). For meiosis, MTOCs, etc., see R. C. Brown and Lemmon (2008); meiosis is polyplastidic and anastral [??]. General aspects of nuclear genome evolution in vascular plants are discussed elsewhere.
The plastomes of Lycopodiaceae and Isoëtaceae have 118-122 genes and 21-22 introns (Mower et al. 2018).
There is extensive variation in the mitochondrial genome in this clade, although details are known from only a few taxa (Knoop 2012 and literature). Thus introns vary considerably, unlike in the three groups of bryophytes (Y. Liu et al. 2012). Various mitochondrial genes have been lost, while ca 11 cis-splicing introns seem to have been gained (W. Guo et al. 2016b); the ccm genes that have been lost represent the loss of the mitochondrial cytochrome c maturation pathway.
Chemistry, Morphology, etc.. Selaginellales in particular, but also Lycopodiales, may contain fair amounts of silica (Trembath-Reichert et al. 2015).
For general information, see also Kenrick and Crane (1997), Boyce (2005a), Ranker and Haufler (2008 - in which esp. Imaichi 2008) and Ambrose (2013), for fossil members, see Gensel et al. (2013), for shoot development, see Harrison and Morris (2017), for details of phloem, see Evert (1990a), for sporophytes and their placentae, see Hilger et al. (2002), for spores, see Tryon and Lugardon (1991) and for embryogenesis in fossil lycophytes, see Phillips (1979) and Stubblefield and Rothwell (1981).
Garbary et al. (1993) in their analysis of characters taken from male gamertogenesis did not recover a monophyletic lycophyte group; Selaginella was sister to the three bryophyte groups...
Classification. Lycophytina include the three extant families below and their immediate fossil relatives, and their more distant fossil relatives, [zosterophylls [Nothia aphylla [Drepanophycales...]]], that are their successive sister taxa (e.g. Gensel 1992; Kenrick & Crane 1997; Gonez & Gerrienne 2010; Gerrienne et al. 2016; esp. Hetherington et al. 2021). See The Pteridophyte Phylogeny Group (2016) for a classification, also Christenhusz et al. (2011a) and H.-M. Liu (2016). The three extant families may be put in monofamilial orders, reasonable in their fossil relatives are included, but the fossil record is poorly treated here. Note the distinction between leafy shoots, root-bearing organs, and rooting axes/roots.
Zosterophylls†
Zosterophylls have been dated to 420.7≤ Ma (Donoghue et al. 2021).
Rooting system evolving independently.
[Nothia [Drepanophycales + Lycopodiaceae, etc.]]:
Nothia aphylla†
The age of Nothia fossils, from the Rhynie Chert, is ca 400 Ma (Krings et al. 2007).
[Drepanophycales + Lycopodiaceae, etc.] / Lycopsids: rooting axes originate by anisotomy, branching isotomous, cuticle 0, stomata 0, microphylls 0
Drepanophycales†
Root bearing organs strongly gravitropic
Lycopodiaceae, etc.: root branching endogenous, root hairs +, root cap +, endodermis + [= true roots].
LYCOPODIACEAE P. Beauvois - Back to Lycopodiopsida
Plant terrestrial or epiphytic; Al-accumulating, phytochrome duplicated; roots produced endogenously from stems, (with glomeromycotes and/or mucoromycotes); root apical meristem 3-4-tiered, open [with common initial zone/quiescent centre], or closed [with protoderm, root cap and ground meristem initials all separate], diarch; shoot apex simplex-type [several apical cells in surface layer], apical meristem often dichotomous, complex, plasmodesmatal density in whole SAM 0.4-4.2[mean]/μm2; phyllotaxis often of the n:(n + 1) type; tapetum 0; sporangium anticlinal epidermal walls sinuous; (meiosis polyplastidic); spores non-reticulate, germination may take time; gametophyte mycoheterotrophic [basidiomycetes, glomeromycotes], subterranean; male gametes: cellular architecture slightly elongated to ovoid [?Iso.]; cilia initially in cytoplasmic pockets adjacent to nucleus, (anterior mitochondria several); placenta transfer cells sporophytic and gametophytic; embryo ?suspensor; n = 34 [quite often; lots of other numbers], nuclear genome [1 C] ca 3.76 pg, [2 C] 14.1-31.7 pg; plastome 149,711-160,877 bp; chondrome with U→C RNA editing, 350> editing sites, recombination ± 0, nad7 gene 0.
17/395: [list: to subfamilies]. ± World-wide, esp. South America.
Age. The age of crown-group Lycopodiaceae is about 265 Ma (Laenen et al. 2014: crown age of Huperzia), 167 Ma (Larsén & Rydin 2015), (383-)368.4(-325 Ma (Testo et al. 2018b), ca 202.6 Ma (Xia et al. 2022: huge error bars) or ca 358.9 Ma (C. Li et al. 2023/2024).
1. Huperzioideae B. Ølgaard —— Synonymy: Huperziaceae Rothmaler, Phylloglossaceae Kunze
Plants erect, (epiphytic), (stem tuber +, plant rosette-forming, leaves deciduous - Phyll.); roots usu. basal, endodermis + (also elsewhere), "adventitious" roots grow through cortex ± parallel to stele, emergence cortical; branching isotomous, (heteroblastic); sporophylls ± leaflike, persistent; sporangia axillary, epidermal cells thick-walled, but not outer periclinal walls; spores foveate-fossulate; gametophyte: (gametophyte mycorrhizal → photosynthetic - Phyll.); (paraphyses + - Phyll., Hup.); male gametes (with ca 20 cilia - Phyll.); x = 67.
3/280: Phlegmariurus (250 - or more). Tropics and temperate regions ± worldwide.
Age. Crown-group Huperzioideae are some (223-)199.5(-175) Ma (Testo et al. 2018b), ca 73.2 Ma (Xia et al. 2022: Hup. + Phleg) or ca 126.5 Ma (C. Li et al. 2023/2024)
[Lycopodielloideae + Lycopodioideae]: plants ± creeping; branching anisotomous [= pseudomonopodial]; roots from along stem; sporangia in terminal strobili, sporophylls much modified, ± peltate, ephemeral, sporangia axillary/on sporophyll bases; x = 23, 24.
Age. The age of this clade has been estimated to be (320-)293.6(-262) Ma (Testo et al. 2018b), ca 145.4 Ma (Xia et al. 2022) or ca 286.5 Ma (C. Li et al. 2023/2024).
2. Lycopodielloideae Gilg
?Anatomy; branching flabellate [horizontal shoots] or dorsal [aerial shoots]; sporangium anticlinal epidermal walls straight, thin, unlignified, but with ± annular thickenings; spores rugulate, (± reticulate - Lateristachys); spore germination speedy; gametophyte: surface-living, upper part photosynthetic.
5/55: Palhinhaea (25). Tropics and subtropics, Lycopodiella North Temperate.
Age. Crown group Lycopodielloideae are around 118.8 Ma (Testo et al. 2018b).
3. Lycopodioideae Eaton
Roots etc., with endodermoid, "adventitious" roots grow through cortex perpendicular to stele; stem branching dorsilateral; sporangium anticlinal epidermal walls sinuous, thin, lignified throughout; spores reticulate (scabrate- Lycopodiastrum).
9/60: Diphasiastrum (20), Lycopodium (15). Widely distributed, in Africa mostly montane.
Age. The age of this clade is (236-)210.4(-202) Ma (Testo et al. 2018b).
Evolution: Divergence & Distribution. The initial distribution patterns developed in Lycopodiaceae are thought to reflect vicariance events associated with the break-up of Pangaea (Testo et al. 2018b). Within epiphytic Huperzia s.l. an Old World and New World clade are sister taxa; dated to 80≤ Ma, this split is also pre-drift, and it is perhaps associated with the evolution of broad-leaved forests - epiphytic Huperzia are no longer shaded out (Wikström et al. 1999).
Wikström and Kenrick (1997, 2001) and Wikström (2001) discuss diversification and phylogeny of extant Lycopodium s.l. and relatives. Diversification in Lycopodium s.l. may have begun some 200 or more Ma, i.e. the age of fossils with the distinctive plectostele that characterises Lycopodium s. str..
Ecology & Physiology. About two thirds of the species of Huperzia and a number of species of Phlegmariurus - all told around 200 species in the family - are epiphytic (Wikström et al. 1999; Schuettpelz & Pryer 2009; Zotz 2013). Páramo species of Phlegmariurus, ca 60/150 of the Neotropical species, are terrestrial in Alpine grassland, but are probably derived from epiphytic species within the last 10-15 Ma (Wikström et al. 1999; Sklenár et al. 2011; Testo et al. 2019a), and this genus shows extreme ecological flexibility (Testo et al. 2018a).
Renzaglia and Whittier (2013) discussed interactions between the gametophyte and young sporophyte when the former is subterranean and mycoheterotrophic. N was the main nutrient moving from the fungus to the plant in Lycopodiella inundata associated with mucoromycotes (Hoysted et al. 2019); C moves from the plant to the fungus. A member of Sebacinales-Sebacinaceae from Diphasiastrum alpinum was also found on Calluna vulgaris growing nearby, and nutrients moved from Calluna to the echlorophyllous lycopod gametophyte (Horn et al. 2013; c.f. Rimington et al. 2016).
9/10 species of Lycopodiaceae studied were aluminium accumulators containing ≥1000 mg Al kg-1 (Schmitt et al. 2017).
Plant-Bacterial/Fungal Associations. Glomales are associated with the echlorophyllous gametophytic and also the subterranean sporophytic stages (Merckx et al. 2013a; Imhof et al. 2013), and these fungi may also be associated with the autotrophic sporophytes, although overall a fairly low proportion of species in the sporophytic phase are associated with fungi (Winther & Friedman 2008; Lehnert et al. 2016). Although Pressel et al. (2016) noted that different major clades of mucoromycotes may be found in the one plant in some Lycopodiaceae, Winther and Friedman (2008) found that members of a single subclade of the Glomus A clade were involved with some six species of Lycopodium, which together formed a clade, while Perez-Lamarque et al. (2019, 2020) found that the mycorrhizal associations in the species they studied, all members of the Glomus A clade, were exclusive, the fungi involved not interacting with other plants. For ages suggested for this association, see Perez-Lamarque et al. (2020): that of Lycopodiaceae was ca 250 Ma, that of the Lycopodiaceae-associated fungi ca 49 Ma, and the latter had diverged 78 Ma from the other Glomus fungi. Leake et al. (2008) thought that although there was probably no direct intergenerational transfer of fungi, there was some similarity in the fungi to be found on the different generations. For other fungal associates of Lycopodiaceae, see Rimington et al. (2014), Lehnert et al. (2016) and Pressel et al. (2016). Note that the Glomus phyloytpes involved in the mycoheterotrophic association form a clade with fungi involved in similar associations in Arachnitis (Corsiaceae), Psilotum (Psilotaceae) and Botrychium (Ophioglossaceae) (Winther & Friedman 2007, 2008); all together some four subclades, all part of the Glomus A group, are involved (Schußler et al. 2001; Winther & Friedman 2008).
Vegetative Variation. Seago et al. (2022) noted interesting variation in the development of adventitious roots here - these grow vertically through the cortex at right angles to the stele (= epidermal emergence) or more or less parallel to the stele (= cortical emergence).
Turner et al. (2023) note that the phyllotaxis of leaf spirals in Lycopodiaceae, including fossil members like the Devonian Asteroxylon mackiei (Drepanophycales), often does not follow the Fibonacci series, rather, it is often of the n:(n + 1) type. Leaves and sporangia in A. mackiei are in the same phyllotactic spiral, so from that point of view they are similar sensu Remane; leaves ≡ sterilized sporophylls, furthermore, in Lycopodiales, at least, there is a link between dichotomous branching and this distinctive phyllotaxis (Turner et al. 2023).
Genes & Genomes. Xia et al. (2022) suggest that there had been a genome duplication in the common ancestor of Lycopodiaceae 214-206 Ma (and in that of Phlegmariurus ca 23-22 Ma).
C. Li et al. (2023/2024) noted the presence of the YABBY transcription factor gene family in Diphasiastrum complanatum; it is known from Huperzia selago although Li et al. could not find it in other species of the latter genus, and it has not been found in setaphytes nor in other non-seed vascular plants. In Huperzia, at least, it is expressed throughout the leaf and also in the sporangial primordia (Romanova et al. 2021). There is persistent (ca 350 Ma) synteny in Diphasiastrum and Huperzia (li et al. 2024); for more on some general aspects of nuclear genome evolution in vascular plants, see also above.
Knoop (2012) observed that the mitochondrial genome of Huperzia squarrosa was quite similar to that of setaphytes, although it had been quite extensively rearranged. Y. Liu et al. (2012) found that a gene cluster in the mitochondrial genome of that species with some 10 ribosomal protein genes (there was no recombination in the cluster) was likely to represent a polycistronic operon similar to those found in bryophytes, but at best uncommon in other vascular plants. The trnL and trnS genes in the mitochondrial genome of Huperzia, but not in those of other Lycopodiaceae, may have come from chlamydial bacteria by lateral gene transfer (Knie et al. 2015).
Chemistry, Morphology, etc.. Whether or not squalene cyclases occur in lycophytes is unclear (Shinozaki et al. 2020). Fujinami et al. (2020b) suggest that the organization of the root and shoot meristems in Lycopodium clavatum, at least, was similar, the former with something like a quiescent centre (plus root cap), the latter with something like an organizing centre.
For general information, see Øllgaard (1990) and Øllgaard and Windisch (2014), for branching patterns, see Øllgaard (1979), for the sporangium wall, see Øllgaard (1975), for spore morphology, see Wilce (1972) and Ramos Giacosa et al. (2016) and references, for the gametophyte, etc., see Bruchmann (1910 and references) and Wikström (2011: Phylloglossum), for spermatogenesis, see Renzaglia et al. (1994b), for the spline and microtubules, see Maden et al. (1996), and for phytochrome duplication, see F.-W. Li et al. (2015).
Phylogeny. Field et al. (2016) discuss relationships in Lycopodiaceae; Phlegmariurus, along with Phylloglossum and Huperzia, form a clade sister to the rest of the family; within the rest, Burnard et al. (2016) found that Lycopodielloideae were embedded in Lycopodioideae, although support for this position was not strong. The bulbiferous Huperzia selago group, also with distinctive spores, is sister to the rest of the genus (s.l., 400≤ spp.), which is mostly epiphytic; there are New and Old World clades (Wikström et al. 1999); the same position of the H. selago group was recovered by D.-K. Chen et al. (2021) in a slimmed-down version of Huperzia while there are Old and New World clades in Phlegmariurus. Testo et al. (2018a) discussed relationships within the morphologically and ecologically variable New World species of Phlegmariurus.
D.-K. Chen et al. (2021) provide a recent phylogeny checking on monophyly and relationships throughout the family; they looked at ca 133 species using seven plastid markers. Three main clades (= subfamilies) were recovered; members of Lycopodielloideae have rather long branches, as does Phylloglossum, embedded in Huperzioideae. The position of Phylloglossum has been unclear; here it was sister to Phlegmariurus, support being moderate. As Chen et al. (2021) note, the next step will involve nuclear analyses.
Classification. There have been major changes in classification in Lycopodiaceae, with subgenera in Lycopodium (e.g. Wilce 1973) being converted into subfamilies; two genera have morphed into seventeen (D.-K. Chen et al. 2021 for a convenient summary). Thus a recent classification of the group (The Pteridophyte Phylogeny Group 2016) is very different from that in the account by Øllgaard (1987), but c.f. e.g. Øllgaard and Windisch (2014) and Øllgaard (2016).
[Isoëtaceae + Selaginellaceae] / Isoëtopsida: root-producing organs exogenous, no cap-like structure; leaves spiral, with adaxial ligule; plant heterosporous; megaspore wall with much silica, outer and inner exine separated by discontinuity; female gametophyte development endosporic, with initial free-nuclear phase, sporophyte — gametophyte boundary smooth, intraplacental space + [= collapsed gametophytic tissue], transfer cells 0 [cells with wall ingrowths 0]; nuclear genome [1 C] ca 0.27 pg; chondrome with loss of 15 genes, ≥1,700 RNA editing sites.
Age. The age of this node is around 209 Ma (Laenen et al. 2014), (386-)375(-360) Ma (Pereira et al. 2017), 381 Ma (Larsén & Rydin 2015), (393-)383(-374) Ma (Klaus et al. 2017) or ca 358 Ma (D. Wood et al. 2019/2020).
Evolution: Divergence & Distribution. Blackmore et al. (2000) score the cells of this pair as not being monoplastidic, but c.f. R. C. Brown and Lemmon (1990), Schuette and Renzaglia (2010), etc.. For spore wall ultrastructure in fossil members of this clade (homosporous!), see e.g. Wellman et al. (2009). For more on heterospory, see Gene and Genome Duplication, and Genome Size, especially towards the end.
Genes & Genomes. The synteny levels between the genomes of Isoetes sinensis and Selaginella kraussiana are only some 4.4-7.2% (C. Li et al. 2023).
W. Guo et al. (2016b) found that a total of 21/46 mitochondrial genes were absent from or had become pseudogenised in Isoëtes and Selaginella.
Chemistry, Morphology, etc.. For cellularization in the early development of the gametophyte, see Rudall and Bateman (2019b). Renzaglia et al. (1999) discuss the ultrastructure of the male gamete. For embryo development, see Renzaglia and Whittier (2013).
ISOETACEAE Dumortier - Isoëtes L. - Back to Lycopodiopsida
Plant herbaceous, terrestrial or aquatic, evergreen or variously deciduous, (annual), rhizomorph/corm +, unbranched, 3(-2)-lobed, (elongated, dichotomously branching - I. andicola); mycorrhizae at most uncommon; CAM photosynthesis +/(0), plastids not starch-filled; meristem elongate groove, rootlets endogenous [but see below], arising from beneath the rhizomorph, 2-3-tiered, protoderm and root cap arise from common initials, with a central air space, vascular bundle single, excentric, xylem exarch; shoot apex simplex-type [several apical cells in surface layer], SAM with mean plasmodesmatal density 2.2-4.1/μm2 [cell interface-specific plasmodesmatal network]; xylem mesarch; distinctive vascular cambium + [cortical parenchyma developing abaxially, xylem and phloem adaxially]; stomata 0/+; leaves awl-shaped (flattened, with rounded point - e.g. I. wormaldii), with 4 (2) air chambers; megasporangia indehiscent, decaying, trabeculate; tapetum glandular; megaspores 200-830 µm, 50-300/megasporangium, spherical, surface tuberculate, etc., microsporogenesis successive, tetrads decussate, microspores 10-40 µm, monolete, segment-shaped, blepharoplast branched; megagametophyte lacking rhizoids [?all]; sporophyte — gametophyte junction with dead gametophytic cells; male gametes with many cilia [10-20]; embryo lacking suspensor; n = (10) 11; plastome 144,912-145,132 bp, chloroplast IR involved in gymnastics; mitochondrial genome with U→C RNA editing, with inserts from nuclear genome and plastome, 3 group I introns, one [cox1i1305] trans-spliced; leaves initially 2-ranked.
1/250-300 [list]. More or less world-wide.
Age. Suggestions for the age of crown-group Isoëtes are all over the map - (154-)147(-145) Ma (Pereira et al. 2017), (235-)165, 147(-96) Ma (Larsén & Rydin 2015), ca 251 Ma (C. Kim & Choi 2016), or (85.8-)46.4(-16.1) Ma (D. Wood et al. 2019/2020: nuclear genomes; see other dates, chloroplast ages about half this).
For fossils identified as Isoëtes, which go back to the Triassic or thereabouts, see Retallack (1997c) and D. Wood et al. (2019/2020).
Evolution: Divergence & Distribution. For ages within Isoëtes, see Larsén and Rydin (2015) and Pereira et al. (2017). However, dating diversification within the genus presents major problems, as the dates above suggest - and D. Wood et al. (2019/2020) found ages based on plastomes to be as little as (51.7-)28.5, 24.2(-5.5) Ma. Part of the problem is caused by the quite extensive fossil record of the genus, and where these fossils are to be placed in the phylogeny. Thus the older dates were in part the result of considering Triassic fossils to be crown-group Isoëtes, while Wood et al. (2019/2020) could not unambiguously link these fossils to the crown group.
The evolution of the whole group, including the arborescent fossils that have been linked with Isoëtes, has been discussed by Retallack (1997c), Grauvogel-Stamm and Lugardon (2001), Pigg (1992, 2001), DiMichele et al. (2022) and others; see also above. The fossil Pleuromeia, although reduced (it is unbranched and has no secondary tissue), may not have been on the stem lineage of Isoëtes.
Within Isoëtes, a clade widely distributed on Gondwanan continents is sister to the rest (or [African taxa [Australian, S.E. Asian, Central American taxa + the rest]] - Choi et al. 2018), and overall there is a fair bit of geographical signal in the relationships (Larsén & Rydin 2015), although it is clear that the distributions of the major groups do tend to be rather heterogeneous (Larsén et al. 2022). Dioecy might seem to preclude long distance dispersal events, however, if microspores are commonly dispersed along with megapores - the arguments go back and forth (Larsén et al. 2022). D. Wood et al. (2019/2020) think that distributions here are not determined by vicariance events - which will certainly be true if the age of the group is as young as they suggest.
Indeed. Wikström et al. (2022) looked at ages for Isoëtes, a genus for which recent work had suggested crown-group ages quite late in the Tertiary (e.g. D. Wood et al. 2019/2020; Pereira et al. 2021). Using chloroplast and nuclear data and three clock models, Wikström et al. (2022) obtained estimated ages for the plastid analyses of 216-27, 282-54, and 318-99 Ma, the upper, median and lower 95% HPD confidence intervals respectively, while the corresponding figures for the nuclear analyses were 208-59, 251-134 and 294-250 Ma (I. wormaldii was included in these analyses, see below). The plastid data here, as in other studies of Isoëtes, tended to give younger ages.
As always, the implications of this age variation for our understanding of evolution are considerable – as is our incomplete knowledge of the phylogeny here. Thus the amount of variation in the plastid genomes found by Pereira et al. (2021), for example, was roughly proportional to the crown-group ages suggested there for the three lycophyte groups, being Isoëtes < Lycopodium < Selaginella. However, if the older ages above are accurate, then there will have been remarkably little change in the plastomes of Isoëtes. But recently Larsén et al. (2022), found that the very rare I. wormaldii, from the eastern Cape, South Africa, previously unsequenced, was sister to the rest of the genus and its ITS sequences were very different from those of the other species, so there may be more molecular variation in the genus than was thought. Not surprisingly, Pereira et al. (2021), for example, suggested that long distance dispersal (LDD) might help explain the distribution patterns in the genus, while Larsén et al. (2022), although perhaps inclining to a late Palaeozoic to Mesozoic age when continental drift might be a factor to invoke and recognizing LDD might also have been involved, elected not to discuss distributions in detail pending clarification of minor factors like age...
Allopolyploidy is common in Isoëtes, around 60% of the species being polyploids (Troia et al. 2016); the parents of some species are unknown, a single allopolyploid species may have more than one origin, and so on, all in all making a very complex story (see e.g. Hoot et al. 2004; Larsén & Rydin 2016; Suissa et al. 2020). Pereira et al. (2017, 2019) dealt with relationships in the Neotropical species, and like the other studies there are problems caused by reticulating relationships. And the story will get still more complex if the older age estimates mentioned above are correct.
Ecology & Physiology. Some species of Isoëtes take up CO2 from the mud in which they grow via their very well developed roots, Stylites (= I. andicola) and submerged individuals of some other species of Isoëtes even lacking stomata, or lacking functional stomata (Bristow 1975; Keeley 1998, 2014; Raven et al. 1998 and references). Photosynthesis is by a sort of modified CAM pathway, the lycopsid photosynthetic pathway (see above), and this may well have a substantially more ancient origin than do many of the origins of the CAM pathway in flowering plants, and would be at a time when atmospheric concentrations of CO2 were quite high (Edwards & Ogburn 2012, see also Keeley & Rundell 2003; DiMichele et al. 2022; Holtum 2023; Gilman et al. 2023). The impetus for the development of this pathway is perhaps the low diffusivity of CO2 in water (Monson 1989; Hultine et al. 2019), or, more broadly, CO2 starvation, and this whether the plant is terrestrial or submerged (Suissa & Green 2021). Plants with stigmarian roots, probable ancestors of Isoëtes, are likely to have lived in swampy conditions, and DiMichele et al. (2022) discuss various aspects of the evolution and function/physiology of such roots. There may be bacterial-type PEPC genes in I. taiwanensis and the circadian cis-regulatory element system there seems a little odd (Wickell et al. 2021). Interestingly, "corms" of some species of Isoëtes can tolerate extreme desiccation (Proctor & Tuba 2002; Gaff & Oliver 2013; for Late Embryogenesis Abundant (LEA) genes and gene families, involved in desiccation tolerance in land plants in general, see Artur et al. 2018). A few species of Isoëtes seem always to be C3 plants (Keeley 2014).
Fertilization & Spore Dispersal. As mentioned above, it has been suggested that LDD occurs here, but this might seem to be complicated by the heterospory of Isoëtes. However, Troia et al. (2016) thought that the megaspores might be transported by birds, whether internally or externally, and microspores could travel along at the same time, nestling in the reticulations, etc., of the megaspores (see also Musselman 2002: esp. Figs 51-54; Larsén et al. 2022) - synaptospory. Microspores are about 1/20th of the size of the megaspores – around 10-40 versus 200-830 µm long.
Plant-Bacterial/Fungal Associations. Mycorrhizae would not be expected in a largely aquatic group, but arbuscular mycorrhizae (and dark septate hyphae) were found in some roots of two species of Isoëtes growing in a lake in the Czech Republic, although their identity and what they might have been doing in the plant is unclear (Sudová et al. 2011; see also Lehnert et al. 2016).
Genes & Genomes. Allopolyploidy is very common in Isoëtes; for further details, see Diversity & Distribution above.
The chloroplast inverted repeat seems to have been converted into a direct repeat, but then it became inverted again (Mower et al. 2018).
Grewe et al. (2009) provide an analysis of the mitochondrial genome of Isoëtes engelmannii emphasizing i.a. the amount of recombination and RNA editing in both mRNA and tRNA (see also Knoop 2008); overall this genome is very rich in introns but has the fewest genes of any mitochondrial genome known (as of 2011: Hecht et al. 2011). The mitochondrial genes have very small introns compared with those of other vascular plants (W. Guo et al. 2016b).
Chemistry, Morphology, etc.. The growth and anatomy of Isoëtes is poorly understood (e.g. Gifford & Foster 1988). Doyle (2013) described the vascular cambium as producing both xylem and phloem to the inside and parenchyma to the outside (c.f. Kaplan 1997, vol. 3: chap. 19). Note that Isoëtes rootlets do not force their way out through the tissues that surround them, but rather are carried out passively as secondary cortical tissues are produced. They have been homologized with leaves, i.a. showing no positive geotropism, but perhaps are better thought of as being a structure sui generis (DeMichele et al. 2022).
Strobili are not obvious; all leaves may be fertile. Isoëtes has placental cells with thickened, nacreous walls.
For general information, see also Jermy (1990); for megaspore morphology, quite diverse, see Hickey (1986), for corm growth, see Tribble et al. (2021), for roots, see Yi and Kato (2001).
Phylogeny. Rydin and Wikström (2002), Hoot et al. (2006: ?rooting), Choi et al. (2018: East Asian taxa), Larsén and Rydin (2015) and Pereira et al. (2017, 2019) discuss relationships within Isoëtes. Recently Larsén et al. (2022) found that the poorly-known I. wormaldii was sister to the rest of the genus; its nrITS sequences were so different from those of other species that these authors initially hesitated to include it in their ITS analyses. There was some topological conflict is the various trees produced by Larsén et al. (2022).
Classification. Troia et al. (2016) provide a checklist of the genus.
SELAGINELLACEAE Willkommen - Back to Lycopodiopsida
Terrestrial, prostrate to erect (epiphytic/climbing); syringyl (S) lignin in cortex; (roots with mucoromycotina); SiO2 accumulation common; rhizophores +, produce roots, growing in length via intercalary meristems, closed [protoderm and root cap separate], mono-/polystelic, monarch, hypodermis suberized/with Casparian strip; cauline stele in central cavity surrounded by trabeculate endodermal cells; (vessels +); shoot apex monoplex-type [with single tetrahedral apical cell in surface layer], mono-(-4-)stelic, (± actinostelic), plasmodesmatal density in whole SAM 27-44[mean]/μm2 [cell lineage-specific plasmodesmatal network], shoots arising from two short-lived epidermal cells; leaves (other than 1 vascular strand); megasporophylls several in strobilus; sporangia ± spherical, dehiscence explosive, spores ± spherical, megaspores 4/megasporangium, 155-825(-1500) µm, surface with solitary protrusions (scabrate, reticulate); microspores (in tetrads), 17-52 µm, trilete, often echinate; lamellar strip [part of MLS] 0 at maturity, spline ca 18 microtubules across at the posterior end, flagellae staggered [ca 11 µm]; megagametophyte with rhizoids; embryo with suspensor; n = (7-)9(-10, 12), x = 8, 9, nuclear genome [1 C] 0.08-0.19 pg, [2 C] ca 19.08 pg; plastomes 78,492-187,632 bp, with 68-93 genes and 7-11 introns, (very high GC content); chondrome with massive gene loss, C→U RNA editing.
19/ca 750: [list]. World-wide.
Age. The crown-group age of this clade is (352-)322, 312(-310) Ma (Arrigo et al. 2013), ca 328 Ma (Larsén & Rydin 2015), (387-)373(-354) Ma (Klaus et al. 2017) or ca 309.0 Ma (Kang et al. 2024).
Fossils (Selaginellites resimus) from the Lower Carboniferous-Visean ca 350-333 Ma are the earliest Selaginellaceae (Rowe 1988).
1. Selaginoidoideae Li Bing Zhang & X. M. Zhou (subg. Selaginella) - Selaginoides SéguierRhizophores 0; sterile leaves monomorphic, inc. at base of main stem; megaspores blunt echinate-tuberculate, microspores echinate; n = 9.
1/2. Circumboreal, Hawaii.
Age. The crown-group age of Selaginoidoideae is (135-)70(-39) Ma (Klaus et al. 2017).
[Boreoselaginelloideae [Mexiselaginelloideae [Gymnogynoideae [Sinoselaginelloideae [Pulvinielloideae [Lycopodioideae + Selaginelloideae]]]]]]: rhizophores +; sterile leaves ± dimorphic, in 4 ranks.Age. The age of this node is (348-)339(-329) Ma (Klaus et al. 2017).
The age of the hybridization event (Superclade A x Superclade C) that gave rise to the xBoreoselaginella clade is estimated to be (269-)248.1(-215) Ma (Kang et al. 2024).
2. Boreoselaginelloideae Li Bing Zhang & X. M. Zhou (subg. Boreoselaginella) - xBoreoselaginella (Warburg) Li Bing Zhang & X. M. Zhou
Rhizophores dorsal; sterile leaves ± monomorphic; sporophylls monomorphic; macrospores densely contiguous tuberculate, microspores rugose to verrucate; n = 15.
1/6. Eastern to western Asia, Russia
Age. The crown group age of this clade is ca 17.9 Ma (Kang et al. 2024).
[Mexiselaginelloideae [Gymnogynoideae [Sinoselaginelloideae [Pulvinielloideae [Lycopodioideae + Selaginelloideae]]]]]: ?Age. The clade is some (341-)318(-287) Ma (Klaus et al. 2017).
3. Mexiselaginelloideae Li Bing Zhang & X. M. Zhou - Mexiselaginella schaffneri Li Bing Zhang & X. M. Zhou
Rhizophores on tip of stem; sporophylls with forked (anastomosing) veins.
1/1. Mexico.
[Gymnogynoideae [Sinoselaginelloideae [Pulvinielloideae [Lycopodioideae + Selaginelloideae]]]]: ?4. Gymnogynoideae Li Bing Zhang & X. M. Zhou (subg. Gymnogynium/"Ericetorum") - Gymnogynum Palisot de Beauvois
Rhizophores dorsal; sterile leaves monomorphic or not, at base of stem monomorphic, spiral/decussate, (cells monoplastidic); (megasporophylls 1 (2) at base of strobilus); megaspores reticulate, muri high or wing-like, microspores verrucate to rugate or echinate; n = 9-10.
6/130: Bryodesma (60), Gymnogynium (50). America, inc. the Caribbean, Africa-Madagascar, Australasia, few Asia.
Age. Crown-group Gymnogynoideae are around (294-)262(-220) Ma (Klaus et al. 2017).
[Sinoselaginelloideae [Pulvinielloideae [Lycopodioideae + Selaginelloideae]]]: rhizophores ventral.Age. The age of this clade is (293-)250(-199) Ma (Klaus et al. 2017).
5. Sinoselaginelloideae Li Bing Zhang & X. M. ZhouMegasporophylls 1(2) at base of strobilus; megaspores usu. >600 μm across, verrucate to tuberculate, microspores rugate; n = ?
3/24: Korallia (15) E. Africa to China, Madagascar to the Seychelles, Australia (Queensland), plants of dry habitats.
[Pulvinielloideae [Lycopodioideae + Selaginelloideae]]: ? 6. Pulvinielloideae Li Bing Zhang & X. M. Zhou (subg. Pulviniella) - Pulviniella Li Bing Zhang & X. M. ZhouMegaspores not obviously ornamented, microspores globose, variously verrucate or granular; n = 10
1/17. Africa, Asia, North and Central America, plants of dry habitats.
[Lycopodioideae + Selaginelloideae]: sporophylls monomorphic or dimorphic, resupinate or not.Age. The age of this node is (258-)211(-163) Ma (Klaus et al. 2017).
7. Lycopodioideae Li Bing Zhang & X. M. Zhou (subg. Heterostachys)Megaspores verrucate to tuberculate, (reticulate, smooth), microspores various, n = 8-10.
4/263. Hypopterygiopsis (170), Didiclis (80). Asia, Africa, Europe, 2 spp. North America.
Age. Crown-group Lycopodioideae may be (213-)156(-125) Ma (Klaus et al. 2017).
8. Selaginelloideae Li Bing Zhang & X. M. Zhou (subg. Stachygynandrium)(Cells monoplastidic, bizonoplasts); egaspores reticulate, muri low and open muri (verrucate) microspores baculate (verrucate to blunt echinate); n = 9-11, etc..
3/330: Selaginella s. str. (230), Chuselaginella (70). ± Worldwide.
Age. The age of crown-group Selaginelloideae may be (169-)123(-82) Ma (Klaus et al. 2017).
Evolution: Divergence & Distribution. For ages of clades within Selaginellaceae and an evaluation of its fossil record, see Klaus et al. (2017: as Selaginella). Schmidt et al. (2022) described 20 new species of Selaginella from ca 100 Ma amber from Burma; half of them belong to subgenus Stachygynandrum (= subfamily Selaginelloideae) with its anisophyllous strobili.
Klaus et al. (2017) provide details of the evolution and distribution of Selaginella s.l.; species here are fairly old compared with those in Cycadales and Pinales. J. Petersen and Burd (2017b) noted that sex allocation in Selaginella was usually heavily male biased, unlike the situation in angiosperms (although in the latter there is allocation to maternal tissues like endosperm and fruit that are of course not found here). They thought that heterospory in Selaginella might be a particular advantage for species growing in more shaded habitats; such species tended to have larger megaspores than those growing in open habitats (Petersen & Burd 2018).
The small subgenus Selaginella (= Selaginoidoideae), perhaps sister to the rest of the genus (but see below for relationships), is relatively undistinguished vegetatively, including in its plastids, having monomorphic sterile leaves and lacking rhizophores, and subgenus Boreoselaginella (= Boreoselaginelloideae), sister to the remainder, also has more or less monomorphic sterile leaves. Details of their morphology, especially those of subgenus Selaginella, will affect character polarization. Rhizophore-like organs are found in fossils (e.g. Matsunaga et al. 2017), but the earliest fossils cannot be included in subgenus Selaginella (Klaus et al. 2017).
At (269-)248(-215) Ma the allopolyploidy hybridization event that resulted in the Boreoselaginella clade (see Genes & Genomes below) seems to be the earliest known in vascular plants, the exact nature of the earlier genome duplications like the ε and ζ events being unknown (Kang et al. 2024).
There is syringyl (S) lignin in cortical cells of Selaginella moellendorffii, at least (Weng et al. 2008, 2011); it is synthesised by a different pathway to S lignin elsewhere.
Ecology & Physiology. Selaginella grows in habitats varying from very humid and with considerable shade to open and xeric; plants in the latter habitats reconstitute quite nicely when water is added (Pampurova & van Dijck 2014). Desiccation tolerance has evolved several times (Korall & Kenrick 2004), a niche shift to drier conditions perhaps characterizing subgenus Tetragonostachys (= subgenus Rupestrae) as a whole (Arrigo et al. 2013). VanBuren et al. (2018) found great haplotype variation in the desiccation-tolerant S. lepidophylla, and it has a small genome - around 109 Mb - that contrasts with the larger genomes of desiccation-tolerant angiosperms (Farrant et al. 2015). However, in both groups Late Embryogenesis Abundant (LEA) genes are prominent (see also Z. Xu et al. 2018; Artur et al. 2018), and there are also interesting tandem duplications of genes, also apparently involved in desiccation tolerance (VanBuren et al. 2018a). Interestingly, S. tamariscina, also desiccation tolerant, has a genome 301 Mb in size, at the other extreme for Selaginella (Z. Xu et al. 2018). Stem ages of clades now growing in more or less xeric habitats are late Permian/early Triassic, around 260-252 Ma, one of the clades involved, subgenus Pulviniella, being called an example of extreme niche conservatism lasting hundreds of millions of year (Klaus et al. 2017).
Ferns (6 species) and Selaginella (7 species) growing in similar habitats on the forest floor in Costa Rica had similar net photosynthesis and water use efficiency, but the latter had a higher leaf mass/area and nitrogen use efficiency in photosynthesis, and shorter, more dense stomata (Campany et al. 2018). J.-W. Liu et al. (2020) noted that shade-dwelling Selaginella were often monoplastidic, and some of these species, but only in subgenus Stachygynandrum (= subfam. Selaginelloideae), had giant, often cup-shaped or deeply bilobed bizonoplasts, in which there were numerous parallel layers of stacked thylakoid membranes in the upper part of the chloroplast, perhaps leading to an increase in the efficiency of photosynthesis, while in the lower part the thylakoids were unorientated and there was more normal grana + stroma structure. All in all, there is remarkable variation in chloroplast morphology in Selaginellaceae (see also Sheue et al. 2007, 2015).
Photonic structures, helicoid structures in the cell wall, are known from a number of ferns; they produce a distinctive blue iridescence of the leaves of the plant (Lundquist et al. 2024). Iridescence has also been recorded in some taxa with bizonoplasts (Liu et al. 2020; see also Sheue et al. 2007, 2015)
The ratio of the surface area of the mesophyll to its volume in the only lycophyte examined, Selaginella kraussiana, is ca 0.09, a high ratio suggesting efficient movement of CO2 into the cell and as high as that of quite a number of eudicots and monocots (Théroux-Rancourt, Roddy et al. 2020/2021).
The stomata of Selaginella have been found to respond to abscisic acid (ABA), rather like those of seed plants, but the response is only slight, and even then it happens only at a very high concentration of ABA (Ruszala et al. 2010; see Sussmilch et al. 2017 and literature).
Fertilization & Spore Dispersal. Both micro- and megaspores are actively ejected from their sporangia, and although sporangium opening was found to be slow, ejection occurred as the sporangium snapped shut when drying, the cells cavitating. Megaspores, being larger, tended to travel further, up to 120 cm horizontally and 76 cm vertically upwards in Selaginella selaginoides; interestingly, the microspores may travel with the megaspores, being electrostatically attached to them - synaptospory (Schneller & Kessler 2020). Microspores when travelling singly do not travel so far on immediate ejection, but add assistance by wind, and the two travel similar distances. In S. martensii the megaspores initially move at a rate of ca 4.5 m/second (Schneller et al. 2008). Microspores are not explosively dispersed in all species, and in those that are, the spores may be dispersed as the sporangium itself is thrown from the plant; the megaspores are usually dispersed consecutively in opposite pairs, the uppermost pair first (Schneller et al. 2008). Gómez-Noguez et al. (2022) discuss the terminal velocity (Vt) of Selaginella spores, which depends in part whether they remain in tetrads or not; Vt of pteridophyte (= Selaginella plus a number of fern species) spores was more affected by spore mass than size; "ornamental" appendages might increase aerodynamical drag and reduced the Tv. Synaptospory, the transport of micro- and megaspores together, as perhaps occurs in Isoëtes, has not been recorded here here. The microspores are relatively somewhat larger than in Isoëtes, indeed, Selaginella pulvinata has both the largest microspores, some 45 µm across, and the smallest megaspores, ca 155 µm across, in the genus - at least among the Chinese species (X.-M. Zhou et al. 2015).
Plant-Animal Interactions. Selaginella s.l. is the only lycophyte known to produce galls (G. Santos et al. 2027).
Plant-Bacterial/Fungal Associations. For fungal associates of Selaginellaceae - arbuscular mycorrhizae are relatively uncommon - see Rimington et al. (2014) and Lehnert et al. (2016).
Vegetative Variation. Although ahll Selaginella have microphylls, there is in fact quite a bit of variation in leaf morphology and vasculature here (Wagner et al. 1982; Langdale et al. 2002). Sheue et al. (2007) and J.-W. Liu et al. (2020) discuss variation in chloroplast morphology (see above). Some species og Selaginalla s.l. have iridiscent leaves, the iridescence being because there are two transparent layers with different indices of refraction in the cell walls (Lee 1996).
Angle meristems (dorsal and ventral) in Selaginella form in the axil of the bifurcation of the shoot apex and develop into rhizophores (Imaichi & Kato 1989, 1991; Jernstedt et al. 1992). The rhizophore initially has a group of apical cells and is positively geotropic, but at the level of transcriptome analyses it differs from both shoot and root; genes associated with both, perhaps especially the root, are expressed, but also a set of unique genes (Mello et al. 2018). When the rhizophore divides it forms roots, the meristem soon comes to consist of a single cell, and root hairs develop and a root cap forms when the root becomes subterranean (Lu & Jernstedt 1996); auxin transport is acropetal (c.f. seed plants: Matsunaga et al. 2017). Root elongation is distinctive, not proceeding by divisions of the immediate derivates of the apical cell but by the activity of an intercalary meristem that is some way behind the apex (Otreba & Gola 2011). See Mello et al. (2018) for the connection between auxin response and roots or root-like structures across land plants in general, and Damus et al. (1997) for the root hypodermis.
Harrison et al. (2007) described the distinctive origin and development of shoots in Selaginella kraussiana via two short-lived apical initials; this appears to be the only member of the family in which leaf development is known. For the shoot apical cell of Selaginella, see Imaichi and Kato (1989); the shoot-patterning genes expressed in the apical cells of Selaginella and Equisteum are largely non-homologous (Frank et al. 2015).
Silica bodies varying both in morphology and distribution patterns, but of uncertain function, hve recently been described from the microphylls of several species of Selaginella (Lopes & Feio 2020). There is infraspecific variation in both morphology and distribution of these silica bodies in S. erythropus (Sheue et al. 2020).
Genes & Genomes. There is no evidence of genome duplication in Selaginella although there are different base numbers and ploidy levels there (e.g. Banks et al. 2011; VanBuren et al. 2018a). However, Kang et al. (2024) recently suggested that there had been hybridization between stem members of superclades A and B in the early Triassic, the result being (subgenus) Boreoselaginella which appeared in three places in phylogneetic analyses. Genome size has long been small in the clade, as with other heterosporous groups, the range being around 100-144 Mb, and rather larger genomes above 1.3 pg seem to be derived; overall, the rate of genome size evolution is very low indeed, unlike the situation in groups like Brassicales, also with small genomes (Baniaga et al. 2016). Indeed, extremophiles - and several species of Selaginella qualify - in general (but not all - see Boea-Gesneriaceae) tend to have small genomes (Baniaga et al. 2016). For the likely hybrid origin of Boreoselaginella, see Divergence & Distribution above.
Selaginella has a very variable and highly reorganized plastome (Tsuji et al. 2007; Z. Xu et al. 2018; see esp. Mower et al. 2018, Q.-P. Xiang et al. (2022) and X.-M. Zhou et al. 2022). There has been much recombination and there are trans-spliced introns otherwise known from spermatophytes only (Hecht et al. 2011; W. Guo et al. 2020); in some species the inverted repeat has been converted by an inversion into a direct repeat (Mower et al. 2018). The overall size of the plastome varies from 78492-187632 bp, and although the Sinensis group consistently has small genomes, these are sometimes found in other species, but at 78492 bp, the plastome of an accession of Selaginella fruticulosa (the Sinensis group) is the smallest known of that of any non-parasitic land plant (Zhou et al. 2022).
Xu et al. (2018) found that ndh genes, and some others, of Selaginalla tamariscina had been lost, although some of these other genes had been transferred to the nucleus; Mower et al. (2021) and Zhou et al. (2022) discuss possible connections between various distinctive life styles, including dealing with dry conditions, that might affect the photosynthetic process and result in the loss of such genes. Indeed, S. tamariscina has the fewest genes of any photosynthetic land plant, having 68-93 genes and 7-11 introns versus 118-122 genes and 21-22 introns in other lycophytes (Mower et al. 2018). Furthermore, the plastomes of Selaginella have the highest GC content of those of any land plant (D. R. Smith 2009; Y. Yu et al. 2019b), although in some species of the Sinensis group may be lower and similar to that of other other land plants (Zhou et al. 2022). There has also been a very large amount - if varying considerably between the species - of RNA editing, C → U changes having been tracked (D. R. Smith 2020). There are no t-RNAs in the Sinensis group (Zhou et al. 2022). The IR is sometimes converted into a direct repeat (but it can become inverted again), and so on (Mower et al. 2018; Zhou et al. 2022). There is only a single form of the plastome in S. tamariscina, and it is at best very uncommon in other vascular plants, which anyway usually have two forms (W. Wang & Lanfear 2019); I do not know of the situation in other lycophytes.
The chondrome of Selaginella is also very distinctive. It is small, but has the highest GC content of any land plant, 60.8-70.6% (Mower et al. 2018; J.-Y. Tang et al. 2022). It has only 17-21 genes, only 1/3 the number of other vascular plants (but 1/2 the number of Welwitschia, which also has quite a small chondrome), although they are intron-rich, and it totally lacks tRNA genes (W. Guo et al. 2016b; Tang et al. 2022).
Chemistry, Morphology, etc.. For the synthesis of syringyl lignin in Selaginella, see above. Harholt et al. (2012) looked at the cell wall polysaccharides of S. moellendorfii, comparing them with those of other land plants. López et al. (2024) looked at the extensive variation in ligule shape in Selaginella - much infraspecific - in the context of the phylogeny provided by Weststrand and Korall (2016a).
Korall and Taylor (2006: discussion about problematic quantitative characters, etc.), X.-M. Zhou et al. (2015b) and Zhou and Zhang (2015 and references) illustrate the sometimes baroque morphology of the micro- and megaspores of Selaginella; Zhou et al. (2015b) recognized 15 types of megaspores of which four were divided into 2-4 subtypes in Chinese species of Selaginella alone. For microspore development, see Wallace et al. (2011). Hemsley et al. (1998 and references) describe how self-assembly is involved in putting together the exine. Bruchmann (1912 and references) discusses details of the megagametophyte, etc.; Renzaglia et al. (1999) provide details of spermatogenesis in S. kraussiana.
For general information, see also Jermy (1990).
Phylogeny. Korall and Kenrick (2004), X.-M. Zhou et al. (2015a) and especially Weststrand and Korall (2016a) and Klaus et al. (2017) provide a comprehensive phylogeny of the genus. The Selaginella selaginoides clade (subgenus Selaginella) consistently comes out as sister to the rest of the genus, and the S. sanguinolenta clade (subgenus Boreoselaginella: Zhou & Zhang 2015: Boreoselaginella/Boreoselaginelloideae above) may be sister to the remainder (Zhou et al. 2015a; Klaus et al. 2017), although Weststrand and Korall (2016a) found that its position was uncertain and they included it in their large subgenus Stachygynandrum (Weststrand & Korall 2016b). However, although subgenera were generally recovered in the plastome analysis of X.-M. Zhou et al. (2022), relationships between the subgenera were unclear. In particular, although they found that the Sinensis group, on a long branch, was sister to the rest of the genus in a number of analyses, it switched positions with subgenus Selaginella or the two together formed a clade sister to the rest of the genus in other analyses (Zhou et al. 2022). Relationships of the S. sanguinolenta group have been particularly problematical (see also H.-R. Zhang et al. 2020; M.-H. Zhang et al. 2022). However, Tang et al. (2022) looked at the chondromes of 34 species, with a focus on the relationships of the S. sinensis group with its long plastome branch, and the S. sanguinolenta group; they recovered the relationships [subgenus Selaginella [the sanguinolenta group [subgenus Stachygynandrum (inc. the sinensis group) + the other subgenera]]], but they noted that the substitution rates in the S. sanguinolenta group were low... The focus in Zhou et al. (2025: 338 spp., 1 plastid, 5 nuclear markers) was on New World taxa (160 of the species they examined), and the subfamilies were somewhat rearranged.
Classification. See X.-M. Zhou and Zhang (2015) and Weststrand and Korall (2016b) for infrageneric classifications of Selaginella. Zhou and Zhang (2023) divide the family into 7 subfamilies and 19 genera - see above, a number of the old subgenera are included, while Zhou et al. (2025) added a subfamily.
[MONILOPHYTA/FERNS/POLYPODIOPSIDA + LIGNOPHYTA] / EUPHYLLOPHYTASporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [Glomeromycota], branched rooting system, lateral roots endogenous [origin in the pericycle]; ?endodermis +; G-type tracheids +, with scalariform-bordered pits; nodal vascular gaps in stem; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a longitudinal slit; spores with flavonols in vacuoles; meiosis polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate [>30 cilia, in spirals], stellate array of filaments in transition zone inside the cell; nuclear genome [1 C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; chondrome with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins +.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The divergence of the monilophytes and lignophytes may date to 401-380 Ma (Leebens-Mack et al. 2005) or ca 380 Ma (Hennequin et al. 2008); Theißen et al. (2001) suggested an age of ca 400 Ma, Clarke et al. (2011: also other estimates) an age of (452-)434(-410) Ma and Magallón et al. (2013) estimated an age of around (422-)411-404(-394) Ma; (463.5-)428.9(-400.1) Ma are the ages in B. Zhong et al. (2014b), (478.4-)447.4(-415.2) Ma in Rothfels et al. (2015b), 455-427 Ma in Barba-Montoya et al. (2018), 437.5-402 Ma in Morris et al. (2018a) and 432-414 Ma in B. J. Harris et al. (2021/2022). See also Pryer et al. (1995, 2000, 2001a, 2004), P. Soltis et al. (2002: a variety of ages, some very old), H. Schneider et al. (2002), Stein et al. (2012); Larsén and Rydin (2015) and D. Wood et al. (2019/2020).
Evolution: Divergence & Distribution. For possible apomorphies of crown members of Euphyllophyta, see Raubeson and Jansen (1992b), Kenrick and Crane (1997), and H. Schneider et al. (2009). Kenrick (2013) suggested that the development of an endodermis and the endogenous origin of root meristems might be associated with this node.
Extra-floral nectaries of one sort or another are scattered throughout [Monilophyta + Lignophyta], and Weber and Agrawal (2014) found that their evolution was often - but not always - associated with an increase in diversification of the clades in which they occurred; they indirectly facilitated diversification. Cyanogenic glycosides, α-hydroxynitrile glucosides that release hydrogen cyanide when acted upon by plant β-glucosides, are similarly scattered, and Bak et al. (2006) suggest they may be an apomorphy at this level. They note that ferns and gymnosperms have aromatic cyanogenic glucosides (derived from tyrosine or phenylalanine) and angiosperms have both aliphatic and aromatic glucosides (derived from leucine, isoleucine or valine), the latter group being derived - parallel evolution at different levels perhaps seems as likely.
The leaves of this clade, often called megaphylls or euphylls, vary considerably. The foliar vascular supply in monilophytes seems to have evolved by dissection of an amphiphloic siphonostele, although the vascular system of the rhizome in some true ferns consists of sympodia (Karafit et al. 2005), while "leaf gaps" in seed plants are rather different and are associated with a stele that consists of a series of sympodia of collateral vascular strands (see Namboodiri & Beck 1968c; Slade 1971). Gunning et al. (1970) noted that xylem transfer cells were associated with leaf gaps, Equisetum, which had such cells but lacked leaf gaps[?], seeming to be an exception, but it does belong here; xylem transfer cells may be an apomorphy at this level, although transfer cells of one sort or another are quite widely distributed in land plants and some aquatic streptophytes. However, since leaves with large blades in ferns and seed plants may be parallelisms rather than a synapomorphy, leaf gaps in the two may not be directly comparable; the term "megaphyll" can thus be used in a descriptive sense only (Namboodiri & Beck 1968c; Beck et al. 1982). Furthermore, the growth of fern leaves is accompanied by proliferation at the apex of the blade and that of angiosperms by proliferation at the base (Nardmann & Werr 2013), while Boyce (2005b, 2008a) noted the prevalence of marginal leaf growth in non-angiospermous leaf blades and of diffuse growth in angiosperm leaf blades - these may be oversimplifications, but again, they suggest differences between the two. Relating such differences to a common pattern of leaf development that also includes that of the Lycopodiales/lycophytes is a challenge. For further details of the development/evolution of euphylls, see above.
Vanneste and Beeckman (2020) discuss the origin of lateral roots; in seed plants, with few exceptions (Poaceae, Apiales) they arise opposite the xylem poles. Fang et al. (2022) noted that there was a similarity at the genetic level between the spores of ferns and the microspores/pollen of seed plants.
Ecology & Physiology. Osborne et al. (2004) suggest an ecological explanation for the origin of mega/euphylls based on falling CO2 levels in the latter part of the Devonian. Indeed, there seem to have been complex interactions between temperature and CO2 and O2 concentrations with photosynthetic rates and efficiency of water and photosynthetic nitrogen use over time, and these may help us understand the changing fates of monilophyte-, gymnosperm- and angiosperm-dominated vegetation (Yiotis & McElwain 2019).
Genes & Genomes. For the absence of numerous group II introns at this node, see W. Guo et al. (2016b); depending of how characters are optimized, 12-21 introns may have been lost here. For high-level variation in transcription factors and transcription regulators, see F.-W. Li et al. (2018).
Literature on the substantial inversion of the chloroplast genome that characterizes this clade is summarized by Szövényi (2016).
Chemistry, Morphology, etc.. For microtubules, see Schmit (2002). Pfeiler and Tomescu (2023 - see also Rothwell et al. 2014; Tomescu & Groover 2018; etc.) looked at the evolution of secondary growth in the Early Devonian. Their focus was on Perplexa praestigians, some 402–394 Ma and which they had just described, also on Armoricaphyton, Franhueberia and Gmujij. They thought of the evolution of wood in terms of more or less independent regulatory modules, which, variously combined, led to different developmental trajectories for vascular cambial growth. Thus the diagnostic anatomical features of vascular tisssue in living plants include radially aligned files of cells, anticlinal divisions of cambial initials that generate new radial files, and a combination of axially (conducting cells) and radially (rays) oriented components. Kenrick and Strullu-Derrien (2014), Hetherington et al. (2016b), Hetherington and Dolan (2018) and others discuss roots and their evolution, which quite possibly happened independently in lycophytes. For lamina morphology and venation development, see Boyce (2005b).
Noetinger et al. (2018) described the spore wall ultrastructure in Psilophyton dawsonii, noting that the wall consisted of inner and outer parts. The inner, probably lamellate but appearing homogeneous, was laid down by the spore, while the outer, consisting of a foveate basal part on which progressively smaller plates of sporopollenin were stacked, the tapering stacks ultimately forming spines, was of tapetal origin. For flavonols in the pollen/spores of this group, see J.-S. Xue et al. (2023).
Phylogeny. Ferns and their relatives, the monilophytes or Polypodiopsida, and lignophytes, the extant members of which are seed plants or spermatophytes, are both well supported clades.
Classification. The clade [monilophytes + lignophytes] is sometimes called Euphyllophyta/the euphyllophytes.
V. POLYPODIOPSIDA Cronquist, Takhtajan & Zimmermann / MONILOPHYTA - Back to Main Tree
Squalene cyclases + [synthesis of triterpenoids], phytochrome duplication; roots originating from the pericycle, lateral roots from the endodermis; apical meristem closed [protoderm and cap separate], with a single apical cell; shoot apex monoplex-type [with single tetrahedral apical cell], plasmodesmatal density in whole SAM 19-56[mean]/μm2 [lineage-specific mitochondrial network]; stereome = continuous hypodermal and outer-cortical band of fibres; siphonostele +, amphiphloic, discontinuities in stele in t.s. caused by frond gaps; protoxylem restricted to lobes of central xylem strand [giving a beaded appearance, hence monilophytes], xylem mesarch, tracheids with scalariform pits, G-type tracheids in protoxylem; phloem with refractive spherules in sieve tubes, phloem fibres rare; stem endodermis and pericycle +; leaves megaphyllous [ad/abaxial symmetry evolved first, then determinancy], development acropetal; petiole with multiple leaf traces coming from a U-shaped bundle; frond development by marginal meristems, veins not anastomosing, ending at leaf margin; sporangia grouped in sori, each sporangium developing from a group of epidermal cells, sporangium stalk 6< cells across, walls two cells across, dehiscence by exothecium, tapetum ± amoeboid; spores/sporangium 1000<, white, globose-tetrahedral, with orbicules, wall development centrifugal, exospore 3-layered, pseudoendospore +; gametophyte thalloid; antheridium wall ³5 cells thick, male gametes with 30-150 cilia, with numerous plastids and mitochondria; x = 22, nuclear genome [1 C] ca 14.3 pg/(0.25-)12(-148) Gb/14320 Mb [or rather larger], plastome LSC ca 4.5 kb inversion from trnT-GGU to trnG-GCC, small inversion covering trnD-GUC and its flanks, rps4 gene with nine-nucleotide insertion; loss of one group II mitochondrial intron.
Includes Anemiaceae, Aspleniaceae, Athyriaceae, Blechnaceae, Cibotiaceae, Culcitaceae, Cyatheaceae, Cystodiaceae, Cystopteridaceae, Davalliaceae, Dennstaedtiaceae, Desmophlebiaceae, Dicksoniaceae, Didymochlaenaceae, Diplaziopsidaceae, Dipteridaceae, Dryopteridaceae, Equisetaceae, Eupolypods, Eupolypod I, Eupolypod II, Eupolypods, Gleicheniaceae, Hemidictyaceae, Hymenophyllaceae, Hypodemataceae, Lindsaeaceae, Lomariopsidaceae, Lonchitidaceae, Loxosomataceae, Lygodiaceae, Marattiaceae, Marsileaceae, Matoniaceae, Metaxyaceae, Nephrolepidaceae, Oleandraceae, Onocleaceae, Ophioglossaceae, Osmundaceae, Plagiogyraceae, Polypodiaceae, Psilotaceae, Pteridaceae, Rhacidosoraceae, Saccolomataceae, Salviniaceae, Schizaeaceae, Tectariaceae, Thelypteridaceae, Thyrsopteridaceae, Woodsiaceae
Age. The oldest, at (482-)431(-420.6) Ma, is the estimate in Testo and Sundue (2016), but at (467.0-)421.3(-379.2) and (424.5-)411.1(-392.9) Ma the estimates in Lehtonen et al. (2017: molecules and fossils respectively) are quite close. Morris et al. (2018a) estimated a crown-group age of 411.5-385 Ma for this clade, Magallón et al. (2013) estimated an age of around (404-)394.3-389.9(-382) Ma, ca 360 and ca 364 Ma are the ages in Schuettpelz and Pryer (2007) and Y.-L. Qiu et al. (2007) respectively, (390.7-)368.5(-354) Ma in B. Zhong et al. (2014b), around 330 Ma in Villarreal and Renner (2014), a Devonian age of around 370 Ma or considerably earlier is also consistent with the findings of Elgorriaga et al. (2018), there is an estimate of ca 342.2 Ma in Christenhusz et al. (2021) and of ca 372.2 Ma in C.-H. Huang et al. (2019); see also P. Soltis et al. (2002) for suggestions. Pelosi et al. (2022) estimated that ferns originated around (347.0-)346(-341.5) Ma (= stem Equisetaceae) and Y.-L. Qiu et al. (2024) (314.6-)279.2(-245.2) Ma.
Evolution: Divergence & Distribution. Schuettpelz and Pryer (2009, esp. Supplement Tables 2, 3), Rothfels et al. (2015b: Appendix S4), Testo and Sundue (2016: e.g. Fig. S1) and C.-H. Huang et al. (2020: Fig. S8 and Table S5) provide numerous ages for monilophyte clades, and for more ages, see also Y.-L. Qiu et al. (2007), H. Schneider et al. (2004a), Lehtonen et al. (2017), Qi et al. (2018), Y.-L. Qiu et al. (2024) and especially Nitta et al. (2022). By no means all these ages, especially those in the last two publications, have been added here - note that ages in Nitta et al. (2022) are being updated annually.
Fossils associated with monilophytes very much expand the idea of what a fern might be, even if one includes extant horsetails in that concept. Some of these early fossils are placed in Cladoxylopsida. Of these, the middle Devonian pseudosporochnalean Calamophyton, was a small, branched tree to 2.5(-4) m tall; the primary stem increased in width for up to 2 m height (to 20 cm diameter) and there was secondary growth towards the base of the stem/trunk. The primary stem, at up to 10 cm across, was very stout; the branches themselves branched dichotomously and bore small appendages/leaves and were shed as units (= cladoptosis) (Giesen & Berry 2013). Eospermatopteris was ca 1 m across at the base (Stein et al. 2007), while an individual of the cladoxylopsid Xinicaulis lignescens some 374 Ma (Devonian: Frasnian) ca 70 cm across showed diffuse secondary growth of the ground tissue and secondary thickening of the individual xylem strands (H.-H. Xu et al. 2017: secondary thickening compared to that of palms; independent evolution!). Schuettpelz and Pryer (2009) list a number of fossil records (see also Rothwell & Stockey 2008).
Ferns as a whole show little variation in estimates of disparity - the amount/extent of morphological variation in a sample of taxa - over time (Oyston et al. 2016), although in leptosporangiate ferns in particular there has been a gradual increase. However, Suissa and Friedman (2022) suggested that the main diversification of stelar architecture in ferns occurred in the Carboniferous, not in the Cretaceous-Paleogene, when ferns diversified (in terms of species numbers). For instance, the protostele became medullated early in fern evolution, hence changing to a soleno-/siphonostele, with variants of this developing subsequently. This change seems to have happened when the stem diameter increased to above 5 mm or so, and there was a concomitant increase in leaf area (Suissa & Friedman 2022, q.v. for literature bearing on size—morphology relationships).
Testo et al. (2022 and literature) note that vicariance-type events, including climate-mediated vicariance, were perhaps surprisingly common in ferns, particularly in the Northern Hemisphere, while long distance dispersal was especially common in the Southern Hemispherte.
There have been several radiations of homosporous leptosporangiate ferns, the first in the Palaeozoic, giving rise to lineages that have since become extinct, and again in the Jurassic and the Cretaceous (Rothwell & Stockey 2008). There was a notable extinction of ferns ca 251 Ma (the Permo-Triassic boundary), but this was followed by accelerated origination rates and massive generic-level turnover, diversity not collapsing then - there was an increase in ecospace availability (Lehtonen et al. 2017; see also below). General fern diversity decreased (along with that of the cycads) through the Cretaceous (Wing & Boucher 1998). However, ferns appear to have temporarily dominated at least locally after the end-Cretaceous bolide impact/Deccan Traps eruptions (H. Schneider et al. 2004a). Indeed, some 98% of living ferns are leptosporangiates, and their main radiation may have have been after the diversification of the angiosperms in the late Cretaceous-early Caenozoic; initially it was a largely terrestrial radiation (Lovis 1977; H. Schneider et al. 2004a; Rothwell & Stockey 2008; Schuettpelz & Pryer 2009; H.-M. Liu et al. 2014). However, X.-Y. Du et al. (2021) suggest that Polypodiales diversified during the middle to late Cretaceous along with - or even somewhat before - angiosperms, and also insects, birds, etc.. The early history of polypod ferns, they think, goes back to the Triassic, and is rather like that of angiosperms (they use H.-T. Li et al. 2019 for the age of angiosperms), and their Fig. 5 shows a steady log-scale percentage increase in lineage numbers of ferns and angiosperms (the latter rather less steady) from some time from the early Triassic to early Jurassic (depending on the analysis) onwards (Du et al. 2021: note curves g and h in Fig. 5 appear to be switched; there are few suggestions that angiosperms originated as early as ca 350 Ma, certainly not in Li et al. 2019). Pelosi et al. (2022: single copy genes, 211 species, 43/48 families included) suggest a number of ages here. They provided two sets of estimates that were based on analyses including slightly different numbers both of taxa and of calibration points; the analysis with the larger numbers yielded similar ages for that of ferns as a whole, but its ages became progressively younger than those of the other analysis, that of the eupolypods, for example, being almost 20 million years younger (Pelosi et al. 2022: e.g. p. 6); the estimates here are based on analyses using the smaller numbers, i.e., they are the older ages.
For connections between genome size, polyploidy, diploidization, heterospory, etc., see below, i.e. Gene and Genome Duplication, and Genome Size, especially towards the end. Epiphytic ferns have rather large genomes (for 2 C values, see F.-G. Wang et al. 2022). C.-H. Huang et al. (2019) looked at fern diversification in relation to genome duplications; there seemed to be links at various stages during fern evolution, and Pelosi et al. (2022) also thought there might be connections between polyploidy events and diversification, although this needed to be confirmed. Looking at rates of molecular evolution, those in Cyatheales, Marattiales and Osmundales are decelerated, while those in groups like Aspleniaceae and Hymenophyllaceae-Trichomanoideae are relatively high - this may be connected with the increased generation time in the first group, although there are also ecological differences, etc., to take into account (Pelosi et al. 2022: see also comparable variation in angiosperms.
Within the leptosporangiate ferns, well over 70% are Eupolypods; about one third of these are epiphytes and they make up ca 10% of all vascular epiphytes (see Zotz et al. 2021b for a checklist of vascular epiphytes). There are several issues here, one being the evolution of the epiphytic habit, and another a change in the rate of stomatal response to blue light discussed in Ecology & Physiology below. It has been suggested that the diversification of epiphytic ferns in particular occurred during the Palaeogene, perhaps linked with the Palaeocene-Eocene thermal maximum (Schneider et al. 2004a, b; Schuettpelz 2007; esp. Schuettpelz & Pryer 2009: Supplemental Tables 2, 3; Watkins et al. 2010; c.f. Dubuisson et al. 2009 in part, also the preceding paragraph). One exception is Trichomanes and relatives (but not Hymenophyllum and its relatives, for which, see Dubuisson et al. 2009) which diversified in the early Cretaceous; they are commonly epiphytic on tree ferns, which themselves had begun diversifying in the Jurassic (Schuettpelz 2007; also Schuettpelz & Pryer 2009; Rothwell & Stockey 2008 for early radiations of leptosporangiate ferns). Testo and Sundue (2014) found that both terrestrial species and hemiepiphytes (where the plant is initially a climbing epiphyte, but roots from the climbing plant establish a connection with the soil; there are then roots primarily involved in attachment to the host, and roots involved in nutrient uptake from the soil) were derived within a clade of epiphytic Polypodiaceae; the plants often have two-ranked leaves, and in some cases the frond morphology changes dramatically when the roots first establish a connection with the ground (Sundue & Maraia 2014: several examples of hemiepiphytism in ferns in forests of New Britain). Carmona-Higuita et al. (2025) found that in the Neotropics most endemic Polypodiaceae were to be found in the Paramo (12.1%), Guianan (9.9%) and Yungas (8.8%) biogeographic areas, and despite the relatively few species of epiphytic ferns three (out of seven such species of vascular plants) were to be found almost throughout the Neotropics. Note, however, that the idea of a direct connection between fern diversification and the adoption of the epiphytic habit has been questioned (e.g. Sundue et al. 2015; Lehtonen et al. 2017). For ferns and the ecology of light conditions/epiphytism, see elsewhere. Suissa and Friedman (2022) found no connection between the radiation of eupolypods and rates of evolution of major stelar patterning; eupolypods are overwhelmingly dictyostelic even if in the Polypodiaceae area the dictyostele is much dissected.
Parins-Fukuchi et al. (2021) saw a relationship that was qualitatively apparent, if not statistically evident, between periods of morphological innovation and those of phylogenomic conflict, e.g. between gene trees and species trees, when looking at the evolution of monilophytes. This may have happened around 395-359 Ma, in the Devonian, and perhaps again at ca 251 Ma, aound the Permo-Triassic boundary.
The richness of ferns in Australia is correlated with precipitation, and depending on what aspect of diversity is emphasised, precipitation may be linked to climate equability, etc.; bryophytes, but not flowering plants, show rather similar patterns (Nagalingum et al. 2015).
The hierarchy of characters below is partly taken from A. R. Smith et al. (2006, 2008), Pryer et al. (1996) and Rothfels et al. (2012b). The variation in stem apex morphology is difficult to understand - see, for example, the complex discussion of the apex in Botrychium multifidum in Stevenson (1976). Romanova et al. (2023a) suggest that in both Osmundales and Marattiales there is variation in this feature, with some taxa having both monoplex and simplex-type initials, the former having a single apical cell and the latter having several apical apical initials in the surface layer. For a first stab at apomorphies for members of the polypod II clade, see Sundue and Rothfels (2013); their suggestions are largely followed here, and problems with sampling, character state delimitation, and different apomorphies produced by different methods of character optimization are taken into account as far as possible. Only unambiguous synapomorphies (Sundue & Rothfels 2013) are flagged (see also Pittermann et al. 2015 for petiolar vascular anatomy). Kaplan (1997, vol. 3: chap. 19) summarized the sporangium wall morphology of monilophyta; the annulus is an apomorphy of Polypodiidae, the leptosporangiate ferns, q.v. (for annulus evolution, see H. Shen et al. 2017). For other possible apomorphies, see e.g. H. Schneider et al. (2009). More features may need to be added, e.g. if the megaphyllous leaves of most ferns and those of seed plants have evolved independently, which seems likely (see above); D. M. Wang et al. (2015) suggest that leaves with laminas had evolved here by the Late Devonian (Famennian).
Ecology & Physiology. The evolutionary physiology of ferns is repaying examination. As mentioned already, a considerable number of leptosporangiate ferns in particular are epiphytes (25% of ferns are epiphytic, but only 9% of angiosperms: Zotz et al. 2021b; Aroz-Mualin & Kessler 2024), and ferns, along with bromeliads and orchids, are one of the three major epiphytic groups of vascular plants (Hietz et al. 2021). Here epiphytes are concentrated in Hymenophyllaceae, Polypodiaceae (the grammitids), Pteridaceae (e.g. the morphologically-distinctive vittarioids and relatives) and Dryopteridaceae (e.g. Elaphoglossum) (Schuettpelz & Pryer 2009; Watkins & Cardelús 2012; Kato & Tsutsumi 2013; Rothfels & Schuettpelz 2014; see also above). Epiphytic ferns differ from bromeliads in having thinner leaves/fronds, higher nutrient concentrations, and lower water content and water use efficiency (Hietz et al. 2021); see also Benzing (1990) and Zotz et al. (2021) for the ecophysiological features of epiphytes - like these other epiphytes, they are on the slow end of the Leaf Economic Spectrum.
There is an association between ferns with chlorophyllous spores and the epiphytic habitat in which many of these ferns live (and also associations with swampy habitats, the ability to colonize islands, and the lack of mycorrhizal associations - see below). Indeed, although only 14% of ferns have such spores, 70% of these are epiphytes, making up some 37% of all epiphytic ferns (Mellado-Mansilla et al. 2022). Lloyd and Klekowski (1970) had shown that spores with chlorophyll germinated faster and had reduced viability when compared with echlorophyllous spores, and associated with this they tend to be actively respiring, no time is spent in the development of chloroplasts from proplastids, etc. The mean time to germination of chlorophyllous spores was 1.46 days, that of of echlorophyllous spores over six times longer, and the mean viability of chlorophyllous spores was ca 48 days, and that of echlorophyllous spores about 22 times longer (Lloyd & Klekowski 1970). Gametophyte development of green-spored species appears to be more rapid in part because of a shorter period between spore germination and its first cell division (Lloyd &am; Klekowski 1970).
The gametophytes of epiphytic ferns are often strap-shaped or filamentous, not cordate, and are long-lived, and, even more than other fern gametophytes, they are desiccation- but not disturbance-tolerant (Watkins et al. 2007a; see also Nayar & Kaur 1971: survey of gametophyte diversity; Dassler & Farrar 1997, 2001; Farrar et al. 2008; Watkins et al. 2007; Ebihara et al. 2013; Rothfels & Schuettpelz 2014; Farrar 2016; Pryer et al. 2016). In general, gametophytes of epiphytic ferns can deal with low water availability and high irradiance (Pinzón‐Camacho et al. 2025 and references). Moreover, these gametophytes (e.g. in Grammitidaceae (= Polypodiaceae-Grammitidoideae), Hymenophyllaceae, Vittariaceae (= Pteridaceae)) may produce gemmae (e.g. Pryer et al. 2016: epiphytic vittarioids; Farrar 1974, 1990, 2016). This increases the longevity of the genotypes, and they may persist in sites that are hundreds of miles from the nearest sporophytes, Farrar (1990) estimating that gametophytes may persist reproducing vegetative for perhaps 10 million year os even more - epilithic gametophytes are also involved (e.g. Farrar 1967; Ebihara et al. 2013). Indeed, such long-lived gametophytes may never produce sporophytes, or produce sporophytes only in parts of their ranges (e.g. Farrar 1990; Pinson et al. 2016; Yoneoka et al. 2023: Haplopteris; Pelosi et al. 2023: Vittaria appalachiana - see also below). Consequently, there may in places be a mismatch between altitude and the general diversity of fern sporophytes and gametophytes, sporophytes being most diverse at ca 1,000 m, gametophyte diversity being largely independent of altitude (Nitta et al. 2017: Polynesia). Since gametophytes can now be identified directly by molecular sequencing, rather than waiting for them to produce sporophytes, such phenomena are turning out to be remarkably common (Ebihara et al. 2013), and it has interesting implications for the evolution of ferns (see e.g. Ebihara et al. 2009). Overall, clades with non-cordiform gametophytes seem to diversify quite rapidly (Sundue et al. 2015). (As a side issue, perhaps, Nitta et al. (2021) found that gametophytes of Polynesian Hymenophyllaceae were not necessarily more desiccation tolerant than their associated sporophytes even if the gametophytes might grow outside the ranges of these sporophytes.)
Mycorrhizae are less frequent in epiphytic than in terrestrial ferns (e.g. B. Wang & Qiu 2006; Kato & Tsutsumi 2013), possibly because of the difficulty of dipspersal of the spores of such fungi on to branches (Mellado-Mansilla et al. 2022). Mycorrhizae are uncommon in the strap-shaped gametophytes so common in these epiphytes (Pressel et al. 2016), although in the sporophytes of epiphytic Hymenophyllaceae dark septate endophytes are commonly to be found (Lehnert & Krug 2019). However, the sporophytes of epiphytic grammitid ferns have an apparently secondary association with ascomycetes; these ferns tend to be small and are twig epiphytes and they seem to be dependent on this association (Lehnert et al. 2016). Mycorrhizae are also uncommon in ferns growing in wet habitats, and on islands (Mellado-Mansilla et al. 2022).
Associated with the epiphytic habit is the ability of some of these plants to trap litter (e.g. Zona & Christenhusz 2015). Thus epiphytic ferns like Asplenium nidus (Aspleniaceae), Platycerium spp. (Polypodiaceae) and some Dryopteridaceae collect humus and the plants are home to large numbers of arthropods, etc. ("trash-basket epiphytes" - Ortega-Solis et al. 2021; see also some orchids and Araceae in particular). Thus Ellwood et al. (2002; see also Ellwood & Foster 2004) looked at Asplenium nidus growing in Bornean dipterocarp forests. Individuals could weigh as much as one ton dry mass (soil and plant material included), with as many as 41,000 invertebrates living in them. Interestingly, species of both other species of Asplenium and of Hymenophyllum are quite commonly associated with such trash-basket epiphytes which provide them with a suitable habitat (Ortega-Solis et al. 2021).
Of epiphytic ferns, Hymenophyllaceae are poikilohydric, that is, they cannot maintain and/or regulate their water content, the leaves perhaps being one cell thick and lacking cuticle and stomata, and the plants may or may not be dessication tolerant - Hymenophyllum-type or H-type plants; they may have evolved in damp, shady conditions. Other epiphytic fern species range between being homoiohydrous and poikilohydrous, and they are commonly desiccation tolerant and resurrection plants - Pleopeltis-type or P-type plants (Hietz et al. 2021; Aros-Mualin & Kessler 2024). These authors found that overall, some 5% of ferns are H-type, of these 61% being epiphytic and 39% terrestrial and the H-type syndrome evolving four times, while some 7% are P-type, of these only 13% being epiphytic but 87% terrestrial, this syndrome evolving some 19 times or so. Desiccation tolerance is quite common in fern sporophytes, both epiphytic and terrestrial (e.g. Lösch et al. 2007), and peltate scales may play a central role in the uptake of water, rather as in Bromeliaceae (John & Hasenstein 2016: P. polypodioides). Ferns show a variety of adaptations to the ecologically dry epiphytic habitat, including CAM photosynthesis (Keeley & Rundell 2003; Watkins & Cardelús 2012), and in some respects many epiphytic ferns are ecologically more like angiosperms than terrestrial ferns, using water quite efficiently (and having very low hydraulic conductivity) (Watkins & Cardelús 2012). Interestingly, the ratio of the surface area of the mesophyll to its volume, connected to increasing CO2 diffusion into the cell, in some xerophytic ferns is as high as that of quite a number of eudicots and monocots (Théroux-Rancourt, Roddy et al. 2020/2021). However, some ferns show poikilohydric behaviour either/both in the sporophytic or gametophytic stage (e.g. Nitta et al. 2021 for a study of Hymenophyllaceae on the island of Moorea), indeeed, fern gametophytes seem often/sometimes poikilohydrous and dessication tolerant (Aros-Mualin & Kessler 2024, but see Pinzón‐Camacho et al. 2025 and below). The sporophytes of Asplenium trichomanes are even frost tolerant, the apoplastic water content of the frond increasing with cooling, winter season poikilohydry (Lösch et al. 2007).
See also Gesneriaceae, Melastomataceae, Ericaceae, Orchidaceae, Piperaceae and Bromeliaceae, which are the other major epiphytic groups of vascular plants. It is interesting that although crassulacean acid metabolism (CAM) photosynthesis is quite often linked with the epiphytic habit in angiosperms, it seems not to be common in ferns (Holtum et al. 2007).
The tracheids of ferns tend to be long and wide, and the scalariform perforation plates may extend the length of the cell, and as a result, water transport can be relatively efficient (Pittermann et al. 2015); for surprisingly efficient water transport in conifers, another group without vessels, see Pittermann et al. (2005, 2011). For Late Embryogenesis Abundant (LEA) genes and gene families, commonly involved in desiccation tolerance throughout land plants, see Artur et al. (2018); little is known about such genes in ferns.
The stereome in ferns is a compact subepidermal layer of lignified cells commonly found in both the stem and the rachis of the frond, although it is sometimes mid-cortical or even absent (Moran 2022). Such a layer of cells would seem to prevent the movement of O2 and CO2 into the tissues below it and would hence preclude photosynthesis. Moran (2022) notes that the stereome is interrupted by aerophores at the boundary of the upper and lower surfaces of the frond in the [Cyatheales + Polypodiales] clade; since the stereome is interrupted, gas exchange can proceed. In Marattiales functionally comparable structures, porophores, are scattered over the leaf axis, and they have a central pore, not stomata (Moran 2022).
Ferns often grow in shaded and rather humid conditions, and like other plants that grow with them, they show a variety of adaptations to such conditions. Thus genera like Diplazium, Lindsaea (Malesian), Danaea and Trichomanes (New World) have iridescent leaves, and this is caused by the layering of cellulose in the cell walls (Lee 1996; Gould & Lee 1996; Graham et al. 1998). Photonic structures, helicoid structures in the cell wall, are known from a number of ferns from throughout the phylogeny: Marattiaceae, Anemiaceae, Lindsaeaceae, Pteridaceae, Aspleniaceae, Athyriaceae, Blechnaceae, Dryopteridaceae, Lomariopsidaceae, Tectariaceae and Polypodiaceae, and hey produce a distinctive blue iridescence evident on the fronds of the plant (Lundquist et al. 2024).
Pinzón‐Camacho et al. (2025) looked at the growth of fern gametophytes the sporophytes of which favoured sunnier or shadier conditions, and found that the growth of gametophytes was more sensitive to air humidity than to soil moisture, although both were important. Gametophytes of sun-tolerant species dried out more slowly that those of species living in the shade, but interestingly, gametophytes in general did not recover after even partial drying (Pinzón‐Camacho et al. 2025).
As of 2024, five families of ferns were known to have gametophytes that can live as independent populations that persist by vegetative reproduction (Yoneoka et al. 2024); the ferns involved have epiphytic or epilithic sporophytes. The families include Hymenophyllaceae (14 records) and Pteridaceae (15 records), also Aspleniaceae, Lomariopsidaceae and Polypodiaceae-Grammitidoideae, and in such cases the gametophytes are strap-like, not cordate, and with gemmae, and they may live up to 10,000 years or so (Vittaria appalachiana, Pteridaceae: Farrar & Mickel 1991; Yoneoka et al. 2024) or even up to 10,000,000 years or more (Farrar 1990).
Jin et al. (2021) looked at a number of ecologically potentially interesting features in fern sporophytes, and suggested that the relatively low LMA, large frond area and open venation of terrestrial ferns might help them to capture light on the shaded and moist forest floor of LTRF while the cost of construction per unit area was relatively low. By contrast, the high LMA, low frond area and reticulated venation more common in epiphytic ferns may help them to survive in the exposed and xeric forest canopy. Ferns other than Polypodiales, although overall showing a fair amount of variation, especially between orders, have not shown much increase in diversity over the last 100 Ma since angiosperms have risen to dominance (see Testo & Sundue 2016; Lehtonen et al. 2017; Jin et al. 2021).
A fern spike, an increase in the proportion of fern spores in the local palynoflora, is known from a number of localities globally at the time of the end-Cretaceous bolide impact. Fern spikes are also associated with the Permo-Triassic and Triassic-Jurassic boundaries, and extant ferns are commonly primary colonisers after catastropic events (B. A. Thomas & Cleal 2022).
Around 38-43% of all ferns, epecially members of the [Marattiidae + Polypodiidae] clade, accumulate aluminium (i.e. tissue concentration ≥1000 mg kg-1), a far higher percentage than in seed plants (Chenery 1949a, b; Schmitt et al. 2017).
Fertilization & Spore Dispersal. The sex of the gametophytes may be controlled by pheromones (Atallah & Banks 2015).
Spore discharge from the eusporangiate-type fern sporangium, and in some other ferns, is described below. A number of ferns have chlorophyllous spores, and these are rather short-lived relative to spores lacking chlorophyll, but germinate faster (Lloyd &am; Klekowski 1990); for further discussion see Ecology & Physiology above.
Sperm swim in water, hence dispersal is over short distances (von Aderkas et al. 2022).
Grusz (2016) and Haufler et al. (2016) discusses apomixis in ferns, where it is quite common - 2-10% of ferns may be apomictic. It is especially common in species growing in seasonally dry environments, and here sporophytes may produce unreduced spores. The resultant gametophytes produce new sporophytes by mitosis and they become evident as little buds early in the development of these gametophytes. Therefore, apomixis both accelerates the life cycle and reduces the plants' dependance on water - none is needed for the sperm to swim (Haufler et al. 2016).
Plant-Animal Interactions. Overall, there are not many records of insects eating either pteridophytes or lycophytes (Fuentes-Jacques et al. 2022; Porto et al. 2024). There is much less herbivory in ferns compared with that in angiosperms, and only some 35% of herbivores that do eat ferns are restricted to them (Hendrix 1980; see also Cooper-Driver 1978; Ehrlich & Raven 1964; c.f. Balick et al. 1978: herbivory about the same, 73% restricted; Turcotte et al. 2014: caveats). Indeed, Porto et al. (2024) found that, as wih angiosperms, plant—insect interactions in ferns were most specialized in the tropics; there was also phylogenetic signal in the interactions of endopterygote insects, but not in Thysanoptera, Hemiptera or Orthoptera, that ate ferns with these ferns. It has also been suggested that overall herbivory levels in ferns and angiosperms tend to be similar (Hendrix & Marquis 1983; Marquis 2024); in Guanacaste, Costa Rica, at least, caterpillars that eat ferns are restricted to ferns, if polyphagous there (Quicke et al. 2025). Note that despite the age of ferns, most insect groups that eat them are not notably ancient (Porto et al. 2024; c.f. in part sawflies below).
Cooper-Driver (1978, see also 1985) observed that ferns tended not to be very diverse in the way of secondary compounds that they contained. However, they quite commonly have phytoecdysones, which can defend against many herbivores (Hikino et al. 1973; Balasubramanian et al. 2006), or there may be protectants that are fairly specifically targeted, e.g. chitin-binding proteins that may have moved to ferns from bacteria via lateral gene transfer (Shukla et al. 2016; Wickell & Li 2019), although their distribution in ferns is somewhat sporadic (F.-W. Li et al. 2018). Fang et al. (2022) thought that rapid jasmonic acid-mediated accumulation of toxic secondary metabolites could lead to the high biotic resistance here. Recently J.-Z. Wei et al. (2023) found that insecticidal proteins similar to those in Bacillus thuringensis were scattered in ferns - Ophioglossaceae, Pteridaceae-Pteris and Schizaeales, however, the domain that is normally required for their activity is absent in ferns, even although the proteins were insecticidal there. In the chemically well-defended bracken, Pteridium aquilinum, herbivory is not associated with jasmonate-mediated regulation of VOC (volatile organic compound) emissions, which in flowering plants, for instance, can attract predators of herbivores, etc. (Radhika et al. 2012). Castrejó-Varela et al. (2022) discuss the defence against herbivory that is provided by a number of fern metabolites, and also by the accumulation of heavy metals, another possible defence.
Fuentes-Jacques et al. (2022) note that Dennstaedtiaceae, which include the much-studied Pteridium, also Equisetum and Pteridaceae, are well represented as hosts of herbivorous insects, groups like Polypodiaceae, Hymenophyllaceae, Dryopteridaceae and Cyatheaceae being under-represented - Dryopteridaceae and Dennstaediaceae, distantly followed by Polypodiaceae were the major hosts suggested by Porto et al. (2024). Hemiptera, Hymenoptera and Thysanoptera are the insects most commonly involved, groups like Coleoptera (Chrysomelidae, Curculionidae best represented) and Lepidoptera being under-represented (Hemiptera and Orthoptera high, Diptera low in Porto et al. 2024). Of the some 1462 records, Fuentes-Jacques et al. (2022) found that most (55%) were of leaf chewers, although Hemiptera, sap suckers, were also prominent (29%), especially on Dryopteridaceae and Pteridaceae, while of the chewers, about a quarter of the records of Lepidoptera were from Dennstaedtiaceae alone (119/470). Polypodiaceae had most mutualistic/commensal species (Porto et al. 2024).
Gelechioidea-Stathmopodidae-Cuprininae are a group of microlepidoptera in which the caterpillars eat only fern spores – some of these moths are specialists, eating spores only from a single genus/family, others are generalists (Z.-Y. Shen et al. 2024). Although the diversification rate of these moths is unaffected by the adoption of this novel habit, overall there have been more transitions from the generalist (ancestral) to specialist habit rather than vice versa, especially at the family level (Shen et al. 2024).
For herbivory by caterpillars of lithinine geometrid moths, which has involved host shifts after a single colonization event, see Weintraub et al. (1995). Z.-Y. Shen et al. (2024) noted that the larvae of the microlepidopteran Gelechioideae-Stathmopodidae-Cuprininae were distinctively specialized in that they ate fern spores, some being restricted to one or a few fern species; related subfamilies of moths are angiosperm herbivores.
Konstantinov and Knyshov (2015) noted that the heteropteran Bryocorini (Miridae-Brycorinae) fed on ferns, particularly the sporangia, but also fern foliage, although one species rather surprisingly ate mosses; it is possible that these insects can deal with the protectant phytoecdysteroids that are common in ferns. Aspleniaceae seem to be the ancestral food here, but each insect species tended to be found on more than one fern family. Two families of sawflies found on ferns - all Polypodiales, which have a crown-group age spread of around (150-)175-205(260, 303) Ma, may represent relatively ancient associations that predate the origin of angiosperms while another family seems to have moved on to ferns from angiosperms after the diversification of the latter; this third family of sawflies also includes taxa that eat mosses and monocots (Schneider 2016; Marquis 2024 - see also above).
At around 1%, the proportion of ferns with nectaries is similar to that of angiosperms with extrafloral nectaries (totals are ca 149 and 3,913 spp. respectively). Nectaries in ferns and extrafloral nectaries in angiosperms evolved at about the same time, i.e. ca 135.3 and 132.0 Ma respectively, somewhat before that of the associations of ants with plants which developed ca 115.3 Ma (Suissa et al. 2024: Fig. 4). The diversity of ferns associated with ants increased only some 100 My later after the middle Cenozoic while the diversity increase on angiosperms was more consistently high. Nectaries on ferns are commoner in epiphytes and climbers, the arboreal Cyatheaceae being the major exception, and there has been little movement of epiphytic nectar/ant ferns from trees back to the understory (Suissa et al. 2024).
M. G. Santos et al. (2018) discuss what little is known about galls and galling insects in ferns and lycophytes.. When they wrote the article, they knew of only 93 species (in 41 genera) with galls, though they estimated that around 229 species might be galled (based on what was known about galling in Costa Rica). The most heavily galled families bwere Polypodiaceae (21 host species0, Dryopteridaceae, and Athyriaceae (8 species of Diplazium alone were galled here). Some 21 kinds of galls are known from Pteridium spp., mites and four insect orders being the main gallers. Overall, of the 133 gall morphotypes that Santos et al. (2018) recorded, leaf roll and globoid galls were commonest; as is generally common in galls, host specificity was high; and of the gall morphotypes, 38 were formed by mites, Eriophyidae, 35 by the dipteran Cecidomyidae, and the remaining 60 types by members of five other insect orders.
Plant-Bacterial/Fungal Associations. For mycorrhizae in ferns, see Lehnert et al. (2010, 2016: "endophytes" s. l. include mycorrhizae and endophytes s. str.) and Pressel et al. (2010, 2016). Fine endophytes, with their distinctive fan-like arbuscules, are known from ferns; these endophytes are probably Mucoromycotina, not Glomus (Orchard et al. 2016 and references), and mucoromycotes have been reported from or are likely to occur in gametophytes of a number of ferns, glomeromycotes being commoner in sporophyte roots (Ogura-Tsujita et al. 2019). Mycorrhizal associations are commoner in ferns than in lycophytes (Pressel et al. 2010; Rimington et al. 2014: ca 2/3 vs less than 1/2 species examined; see also Lehnert et al. 2016). Mycorrhizal fungi may be found in heart-shaped fern gametophytes where they gain entry via the rhizoids and live in the central cushion, but they are not found in taxa with filamentous or strap-shaped gametophytes (e.g. Pressel et al. 2010, see above). For glomeromycote associations with fern gametophytes, see Pressel et al. (2016); note that relatively little is known about such associations in Polypodiales. Still less seems to be known about associations of fungi with the sporophytic generation, but in ferns with very thiny, wiry roots ca 1 mm across mycorrhizae are uncommon, although fungi with dark septate hyphae have been reported, especially in epiphytic taxa (Lehnert et al. 2916), however, Pressel et al. (2010, 2016) suggest that such fungi are unlikely to be members of mutualistic associations. Equisetum is not known to form any mycorrhizal associations (Read et al. 2000; Pressel et al. 2010, 2016, c.f. Lehnert et al. 2016), probably because it is frequently to be found in more or less transiently wet but nutrient-rich habitats (see also Salviniales, liverworts in similar habitats). Interestingly, Giesmann et al. (2021) noted that there was commonly movement of carbon from fungus to plant in pteridophytes (see Paris-type arbuscular mycorrhizae), perhaps connected with the shady conditions that many ferns prefer.
Vegetative Variation. For the basic patterns of stelar vascular tissue, see Suissa and Friedman (2022). Vasco et al. (2013) summarize fern frond morphology and development, noting a number of shoot-like features. Details of leaf development in Equisetum are similar to those of other ferns and seed plants rather than of lycophytes (Ambrose & Vasco 2016; see also Romanova et al. 2023b). There seem to be single adaxial (C3HDZ) and abaxial (KANADI) leaf domain regulators in both Equisetum and other ferns, other regulators (ARP - adaxial, YABBY - abaxial) having been lost (Romanova et al. 2023b). Equisetum has paracytic-type stomata of a kind unlike those of any other land plants (Cullen & Rudall 2016).
Fern apical meristems for the most part are multicellular, the apical initial(s) only rarely dividing (Ambrose & Vasco 2015; Vasco et al. 2016; c.f. Evkaikina et al. 2017: unicellular for the most part). D'Amato and Avanzi (1968; see also Gifford 1985) noted that the apical cells of Equisetum early became polyploid and did not subsequently divide (c.f. in part Gifford et al. 1979); Romanova et al. (2023b) suggest that leaf initiation in the latter genus is like that of ferns and lycophytes (i.e. Selaginellaceae) with a monoplex SAM. In fern gametophytes the apical cell is functional for a short while only, and then the apical region converts to a multicellular meristem (Takahashi et al. 2009, 2015 and references).
It is still rather difficult to understand the evolution of the apparently very unfern-like plant body of Psilotales, particularly that of Psilotum itself, which until fairly recently was considered to be perhaps the most primitive extant vascular plant; Kaplan (1997, vol. 3: chap. 10) and Siegert (1973 and references) summarize the literature. (When I was taught about Psilotum, it was compared with the Palaeozoic rhyniophytes, now thought to be entirely unrelated.) However, as, Kaplan noted, young leaves of Psilotum did have features of fronds (see also Kaplan 1977). Similarly, the leaves of Equisetum, also apparently very odd for a fern, may be secondarily simple, thus its fossil relatives such as Sphenophyllum have much larger whorled and sometimes deeply lobed leaves with dichotomous venation, and bifacial xylem has been found in this genus (Kenrick & Crane 1997; Doyle 2013 and references). For the early development of the fern sporophyte, see White and Turner (1975).
Genes & Genomes. General aspects of nuclear genome evolution in vascular plants are discussed elsewhere. For fern cytology, chromosome evolution, etc., see Manton (1950), a classic. Polyploidy is common in ferns, being involved in almost 1/3 (31%) of all speciation events (Wood et al. 2009). Almost 50 years ago it was estimated that about 1,200 species of ferns were of hybrid origin (Knobloch 1976); homoploid hybrids are often sterile, but they become fertile after alloploidy events (Pelosi et al. 2024). Members of clades that have been separated for a long time quite often hybridize, thus there has been recent hybridization between Cystopteris and Gymnocarpium, clades that diverged an estimated (76.2-)57.9(-40.2) Ma (Rothfels et al. 2015a), while hybridization in Osmunda has involved clades that may have been separated for four times as long (Bomfleur et al. 2014b; Grimm et al. 2015; H.-M. Liu et al. 2020: estimates lower, but still ca 100 Ma). See also Tseng et al. (2024: Thelypteridaceae) and Keskeniva et al. (2025: Marattiaceae-Danaea) for more on deep hybridization. The ubiquity of hybridization here makes it difficult to identify single copy loci (Pelosi et al. 2022).
Genomes in ferns tend to be consistently rather large, and genome size evolution may in fact be greater than in several angiosperm groups, although earlier work had found relative stasis (e.g. J. Clark et al. 2016); for example, it is greater than that in Brassicales, but less than in Caryophyllales (Baniaga et al. 2016). Obermayer et al. (2002), J. Clark et al. (2016: many details), Hidalgo et al. (2017c), Pellicer et al. (2018: mean 1 C genome = 14,320 Mb) and F.-G. Wang et al. (2022: not integrated with previous work, mean 2 C genome = 14.6 pg, inc. lycophytes) provide information on genome size; particularly large genomes occur in Psilotales (Hidalgo et al. 2017b), some Ophioglossales and in a few polypods, while Wang et al. (2022) noted that the aquatic heterosporic Salviniales have notably small genomes. Thus Pelosi et al. (2022) observed that overall genome size was extremely variable here, ranging from 1C = 0.26 Gb in the heterosporous water fern Salvinia cucullata to 73.19 Gb in Tmesipteris elongata. Note that genome size in heterosporous ferns is in general low, averaging only 1C = 2.43 pg, compared with a 12.05 pg average for homosporous ferns - see also angiosperms (Sessa & Der 2016; Pelosi et al. 2022; Gene and Genome Duplication, and Genome Size, especially towards the end. Chromosome numbers are also high, thus Sigel (2016) noted that in homosporous ferns n = 60.5 (n = 16 in angiosperms), corresponding 1C values being 12.05 pg and 6.1 pg respectively. Unlike the situation in other embryophytes, chromosome number and genome size are positively correlated here (Clark et al., 2016), and these authors also thought that the epiphytic habit and increased genome size in eupolypods might be connected (see also Wang et al. 2022), if so, this is the reverse of the situation in orchids. Wang et al. (2022) also noted that 2 C values in general had little phylogenetic signal, although they were connected with habitat, but that base chromosome numbers had a strong signal.
There may have been a genome duplication in the ancestor of the [Salviniales [Cyatheales + Polypodiales]] clade (F.-W. Li et al. 2018, H. Chen et al. 2022), although Pelosi et al. (2022) placed a duplication as common to all leptosporangiates other than Osmundales and another as common to all Polypodiales except for Lindsaeaceae. C.-H. Huang et al. (2019) suggests the positions of some 19 genome duplications throughout the clade, three were along the backbone of the phylogeny and were shared by two thirds or more of all ferns, and although Chen et al. (2022) advocated caution here, noting differences between the duplications suggested by O.T.P.T.I. (2019) and Huang et al. (2019), for instance, Pelosi et al. (2022, Fig. 2) noted that of the 18 larger scale events in ferns (two genera or more) that they recorded, 12 had identical placements in the phylogeny produced by Huang et al. (2019). See also Stull et al. (2023) for more on polyploidy/genome duplications. Genome duplications are also known from or within individual genera such as Equisetum (J. W. Clark et al. 2019 and references), Pelosi et al. (2022: ca 14 such events), etc..
For the evolution of the plastome, see Karol et al. (2010), Wolf et al. (2010, 2011), Grewe et al. (2013), Kuo et al. (2018b), and Lehtonen and Cárdenas (2019). X.-Y. Du et al. (2022: esp. Figs 2, 3 - not all included yet) in particular suggest that there have been some 30 or so changes in plastome structure and gene content here that involve two or more families, and this does not include what might be going on in the eupolypod I and II groups. Robison et al. (2018) emphasised the importance of mobile open reading frames (MORFs) in causing variation in the plastome of Pteridaceae is particular, and although these are not known from Equisetaceae they are otherwise quite widely distributed in ferns, being found i.a. in Ophioglossaceae, Cibotiaceae, etc., and they are also known from Lycopodium; they affect plastome size, the taxa with the largest plastomes all having MORFs (Lehtonen & Cárdenas 2019). They may also be found in mitochondria and nuclei, but their origin is unknown - they may be of viral or chloroplast-plasmid origin (Robison et al. 2018). Some inversions in the chloroplast inverted repeat (IR) area lead to its boundaries shifting, and other changes in the chloroplast genome may be high-level synapomorphies (Gao et al. 2009; Ruíz-Ruano et al. 2018; Lehtonen & Cárdenas 2019). Cárdenas (2019) also noted that these inversions were concentrated in different regions of the plastome, including the IR, and were commoner in Eupolypod I than in Eupolypod II groups. X.-Y. Du et al. (2022: 127 plastomes, all 50 families, 11 orders) found a number of "structural synapomorphies, including 9 large inversions, 7 invert repeat region ... boundary shifts, 10 protein-coding gene losses, 7 tRNA gene losses or anticodon changes, and 19 codon indels" here (ibid., p. 1) - the extension of the IR may be such that the SSC region may be minute and lack any genes. Grammitidaceae s. str. in particular (included in Polypodiaceae below) have chlorophyllous spores and accelerated plastid genome evolution, a correlation found elsewhere in ferns, although it is not 100% (H. Schneider et al. 2004b), indeed, species with spores that less obviously contain chloroplasts are quite widespread (Sundue et al. 2011).
Vangerow (1999) and especially Knie et al. (2016) focus on RNA editing (C → U) and U → C (reverse editing) in the chondrome, and the latter seems to become more prevalent as one goes up the tree. W. Guo et al. (2016b) discuss the chondromes of Ophioglossales and Psilotales in particular.
Chemistry, Morphology, etc.. The xyloglucan composition of the primary cell wall varies substantially, that of Equisetum being particularly distinctive (Hsieh & Harris 2012); for cell wall polysaccharides, see also Silva et al. (2011). Not too much is known about lignin composition, but both syringyl and guaiacyl lignins are reported; variation can be at the infrageneric level (Espiñeira et al. 2011; for more on lignins and their evolution, see also above). F.-W. Li et al. (2015: esp. Fig. 5) discuss phytochrome duplication.
Basic sporophyte morphology was outlined by Kaplan (1997, vol. 2: chap. 11, 18, vol. 3: chap. 19, 2001). The information on horizontal cell walls in early embryo/sporophyte development in ferns given by Philipson (1990) seems to be incorrect - the examples should be vertical? For the organization of the apical meristem of the stem, see Ambrose and Vasco (2015), also Tracheophytes above. The stem has a soleno-/siphonostele, the protoxylem being restricted to lobes of the central xylem strand, hence bringing to mind a beaded necklace (development of the xylem is mesarch, although it is notably variable in the Ophioglossum/Psilotum clade). The protoxylem is described as having G-type tracheids (Edwards 1993). Hernández-Hernández et al. (2012) discuss the distribution of the circumendodermal band, tannin-containing cells with thickenings on inner periclinal and also sometimes the anticlinal walls that surrounds the petiolar vascular bundles and that has a common orgin with the endodermis; they also detail the distributions of a number of other vegetative/habit features. In a comprehensive survey van Cotthem (1970b) documented extensive variation in stomatal morphology that more or less correlates with phylogeny. Note that in groups with polocytic stomata, in which a single subsidiary cell almost surrounds the guard cells (e.g. Polypod I and II groups) there are ?variants in which the cell completely surrounds the guard cells, and yet more, the surrounding cell lacks a cross wall (desmocytic and pericytic types respectively - van Cotthem 1970b). For aerophore distribution in ferns, see Davies (1991), while Wagner (1979) and in particular (Boyce ans Knoll (2002) discusses reticulate venation in fern fronds - a small amount of reticulation is common, but extensive reticulation much less so. Usually fern leaves develop by the activity of a marginal meristem, and vein endings are free and at the margin; reticulate venation with multiple vein orders and freely-ending internal veinlets suggests diffuse lamina growth elsewhere characteristic of broad-leaved angiosperms in particular, but rare in pteridophytes (Boyce & Knoll 2002; c.f. Hagemann & Gleissberg 1996 and the marginal blastozone of angiosperms).
J.-S. Xue (2023) noted a basic similarity in the composition of fern spores and seed plant pollen grains, although flavonols in the cytoplasm of the latter are glycosylated (see also Fang et al. 2022 for similarities between spores and pollen grains). Interestingly, Xue et al. (2023: Fig. 5D) suggested that eusporangiate ferns, and some angiosperms, have the flavonol isorhamnetin as well, although I do not know the distribution of this in euphyllophytes - it is certainly present in large amounts in Ginkgo biloba.
Takahashi et al. (2009, 2014 and references) describe gametophyte development in ferns. They note that the apical region converts to a multicellular meristem, which can divide - dichotomous branching - if cell division in the middle of the meristem stops; the branched, strap-shaped gametophytes of epiphytic ferns are simply an extreme variant of this morphology. Archegonia develop only after the formation of the multicellular meristem. For details of male gamete morphology and movement, etc., see e.g. Renzaglia et al. (2000b, 2002) and H. Schneider et al. (2002).
For general information on pteridophytes (note that pteridophytes in the past have often - and still may - include lycophytes), see also Raven and Edwards (2001), Kato (2005) and Ranker and Haufler (2008). For squalene cyclases, see Shinozaki et al. (2012), for comparative anatomy, see Ogura (1972), for vessels, see Sen and Mukhopadhyay (2014 and references), for phloem, see Evert (1990a), for venation, see Wagner (1979) and Boyce (2005b), for details of stelar morphology and evolution, see Beck et al. (1982), for stomata, see Mukhopadhyay (2021), for spores, see Tryon and Lugardon (1991) and Wallace et al. (2011: wall development), and for young sporophytes, etc., see Johnson and Renzaglia (2009 and references).
Phylogeny. The overall circumscription of the fern clade has only recently become clear, even if the actual position of Equisetum is still somewhat uncertain (see the three hypotheses immediately below). Below I discuss first the relationships of Equisetum and then some more general aspects of relationships within ferns.
1. Equisetum may be embedded in the monilophyte clade, perhaps being sister to Angiopteris, etc. (although with only moderate support), the combined clade in turn being sister to remaining ferns (e.g. Pryer et el. 2001a, 2004a; Wikström & Pryer 2005; Y.-L. Qiu et al. 2007; Ebihara et al. 2011; Evkaikina et al. 2017; c.f. in part Wolf et al. 1998). Lehtonen (2011: some analyses) and Z.-D. Chen et al. (2016) found that it was sister to the eusporangiate ferns, although support was not strong, indeed, the latter group recovered Marattiales as sister to all other ferns. Hennequin et al. (2008) recovered the relationships [[Ophioglossales + Psilotales] [Marattiales [Equisetales ...]]], while Lehtonen (2011) found that Equisetum was on occasion sister to [Marattiales + eusporangiate ferns]. Lehtonen & Cárdenas 2019) recovered this latter relationship
2. Equisetum may be sister to all other ferns, as in a rps4 analysis, and also 4- and 5-gene analyses, the latter two with strong support (Schuettpelz et al. 2006), analyses of several plastid genes (Rai & Graham 2010), in a matK phylogeny (Kuo et al. 2011), fossil analyses (Lehtonen et al. 2017) and in plastome parsimony analyses (Lehtonen & Cárdenas 2019). Knie et al. (2015) also find good support for the relationships [Equisetum [[Psilotum + Ophioglossum] + The Rest]], as does Rothfels et al. (2015b) in their nuclear gene analysis and Testo and Sundue (2016) in their 4,000 species analysis; see also Labiak and Karol (2017: most analyses). This sister group relationship of Equisetum was also recovered by Sessa and Der (2016), Qi et al. (2018) and references) and H. Shen et al. (2017: 69 spp., 34/48 families, 1334 or 2391 genes), the last two studies using transcriptome data, and support was strong. Indeed, this relationship is quite commonly found (e.g. O.T.P.T.I. 2019: not always; Pelosi et al. 2022). Interestingly, spore wall ultrastructure of Calamites, an extinct equisetaceous plant, is not so different from that of Ophioglossaceae and other ferns (Lugardon & Brousmiche-Delcambre 1994; Grauvogel-Stamm & Lugardon 2009). Equisetum has no mitochondrial atp1 intron, either a secondary (and parallel) loss or plesiomorphic absence, depending on the topology of the whole group (Wikström and Pryer 2005: see the character hierarchy below).
3. H. Schneider et al. (2009) noted potential morphological apomorphies such as simple leaf blade and stems with both radial and dorsiventral symmetries (= erect plus creeping stems...) suggesting a clade [Psilotales + Equisetales], which is consistent with some structural changes in the plastome (Grewe et al. 2013; see also some analyses in Karol et al. 2010; also Wolf & Karol 2012; Ruhfel et al. 2014; J.-M. Lu et al. 2015; Gitzendanner et al. 2018a; Kuo et al. 2018b; Lehtonen & Cárdenas 2019: model-based analyses; Lehtonen et al. 2017, 2020; X.-Y. Du et al. 2022; Nitta et al. 2022, all plastome analyses - more references in Qi et al. 2018).
Here Equisetum alone is placed as sister to all other monilophytes (hypothesis 2), although hypothesis 3 clearly also has support.
General Relationships.
The use of morphology alone or in combination with molecular data affects the relationships detected (Wikström & Pryer 2005 and references); see also Grand et al. (2013) for various morphological analyses. Garbary et al. (1993) looked at characters taken from male gametogenesis and found that monilophytes were paraphyetic, relationships being [Marsilea [[other monilophytes] [gymnosperms]]]. Note that in several morphological cladistic analyses (e.g. Bremer 1985; Stevenson & Loconte 1996; Rothwell 1999: fossils included or not) Psilotum has been found to be sister to all other vascular plants (see above). Although some morphological analyses (e.g. H. Schneider et al. 2009) do place Psilotum with other monilophytes, the same analyses also place flowering plants within a paraphyletic group of extant gymnosperms... You win some, lose some.
This reorganisation of monilophytes has sometimes been severely criticised (Rothwell & Nixon 2006), but it is unclear how damning such criticism is. Since the evaluation of "support" values for particular topologies is integral to the approach adopted in these pages, the decision to exclude such values by those authors makes their work difficult (for me, at least) to interpret.
There has been a fair bit of work over the last decade or so that is clarifying relationships among monilophytes, although some details of family relationships are still unclear (e.g. see H. Shen et al. 2017; Nitta et al. 2022; Pelosi et al. 2022). The strongly supported [Psilotum + Ophioglossum] clade (Tmesipteris is sister to Psilotum) is perhaps sister to all remaining ferns, as chloroplast data has broadly tended to suggest (Rothfels et al. 2015b for references). As will already be evident, Marattiales earlier moved around a bit, thus Shen et al. (2017; see also Wickett et al. 2014; Lehtonen et al. 2017) obtained a moderately well supported [Marattiales + Psilotales] clade sister to leptosporangiate ferns, but this may be a sampling issue. Qi et al. (2018) carried out a series of analyses on 136 genera (in 43 families) using a variety of data sets, the smallest having 72 nuclear genes and the largest having 935 genes. Their results are largely consistent with those described below, differences being noted only where they are important. The trees produced by H. Shen et al. (2017) in a phylogenomic analysis of 69 taxa also differed somewhat, while the tree in Kuo et al. (2018b: plastomes) showed relationships that are broadly similar to those below, except in the position of Equisetum (see above) and in that of Pteridium (sister to the Eupolypod II clade), but again sampling was skimpy. O.T.P.T.I (2019) obtained a variety of relationships towards the base of the monilophyte tree, but the topology preferred there is the same as that used here. Relationships recovered by X.-Y. Du et al. (2022: 127 plastomes, all 50 families, 11 orders) include [Equisetaceae [Ophioglossaceae + Psilotaceae]], [Marattiaceae + leptosporangiate ferns], and [[Dipteridaceae + Matoniaceae] [Schizaeales + core leptosporangiates]], and within Polypodiales broadly similar to relationships found by Du et al. (2021). On the other hand, Pelosi et al. (2022: 211 species, 43/48 families, all 11 orders) focussed on 2884 single-copy nuclear loci from 242 fern transcriptomes.
Nitta et al. (2022) began a project using analyses of plastome data (largely because such data were most commonly used by those producing fern phylogenies...) to produce a Fern Tree of Life. Recently-produced data are cumulated each year to produce a new tree, all the nodes being dated (the ages tend to be rather old, perhaps because of the number of calibration points used); the most recent version of this tree is to be found at https://fernphy.github.io, see also https://github.com/fernphy/pteridocat for a standardized list of names, and so on. This should be consulted by those working on the relationships of ferns. Cárdenas and Lehtonen (2023) looked at relationships suggested by variation in the mitogenome, finding that the markers there had much lower substitution rates than those in the plastome; overall, the results of the two analyses were similar, differences being relatively minor - e.g. [Marattiales + Ophioglossales] and [Hymenophyllaceae + Gleicheniaceae] were recovered in the mitochondrial analyses, [Ophioglossales [Marattiales ...]] and [Hymenophyllaceae [Gleicheniaceae ...]] in the plastome analyses. Y.-L. Qiu (2024) included a number of ferns in their study of relationships of embryophytes; all nodes are dated.
Classification. A. R. Smith et al. (2006, 2008) propose a phylogeny-based reclassification of the ferns, and they also include literature, ordinal and familial synonymy, and a list of accepted genera and some of their major synonyms; Prelli (2010) gives a nice account of European ferns. However, adjustments to this classification are being made as details of the phylogeny become better understood (Schuettpelz & Pryer 2007, 2008; Kuo et al. 2011; Rothfels et al. 2012b: reclassification of Eupolypods II; Rothfels et al. 2015b). Here I follow The Pteridophyte Phylogeny Group (2016) which should be consulted for details. The authors make it clear that the monophyly of some genera they accept is unclear, or the genera may even be polyphyletic, so further instalments of this classification are awaited - it is, however, a bit "splitty". A linear sequence of families and genera (Christenhusz et al. 2011a) is now dated, but more recently Christenhusz and Chase (2014) have proposed another classification as has H.-M. Liu (2016). There are substantial differences between these classifications, and this has occasioned some controversy - c.f. Schuettpelz et al. (2018) and Christenhusz and Chase (2018), splitting vs lumping, as in the classification of Cyathea and its relatives.
Previous Relationships. Psilotum and Equisetum had earlier been thought to represent lineages independent of each other and unrelated to ferns, with Psilotum and relatives considered to be the most primitive living vascular plants (e.g. Eames 1936). Their association with ferns, now very largely accepted, was unexpected (but see Kenrick & Crane 1997). Although Bierhorst (1968, see also 1977) had compared Psilotum with the extant fern Stromatopteris (Gleicheniaceae) and found some morphological similarities, most of these have turned out to be parallelisms and the two are not at all closely related.
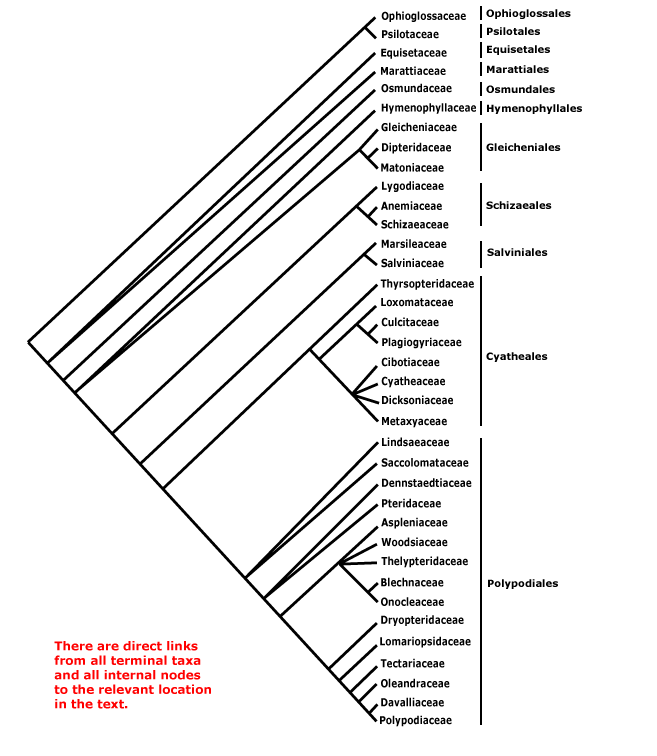
EQUISETOPSIDA / [Equisetidae + Ophioglossidae] (if this clade exists): plant with erect and creeping stems; ?leaf vernation; embryo shoot apex exoscopic [developing towards micropyle/archegonial neck, from cell], suspensor 0; chloroplast rps16 gene and rps12i346 intron lost.
Age. The clade that contains Equisetum has probably been separate from other monilophytes since the Permian, ca 250+ Ma (Stanich et al. 2009); B. Zhong et al. (2014b) thought that this clade was (370.3-)296.2(-189.9) Ma.
EQUISETIDAE Warming
EQUISETALES Berchtold & Presl
Just the one family, 1 genus, 18 species.
EQUISETACEAE L. C. Richard - Equisetum L. - Back to Polypodiopsida
Plant with erect and creeping stems, (tuberous - subg. Equisetum); roots not mycorrhizal, single from buds, triarch, with large central tracheid; cell walls with HGS lignins, also with (1→3),(1→4)-ß-D-MLGs [Mixed-Linkage Glucans], SiO2 accumulation +; stem tuber +, secondary thickening 0, intercalary meristem + [at base of leaf sheath], primary xylem endarch, (vessels +), protostelic (solenostelic), main photosynthetic organ, ridged, ridges alternating from internode to internode, carinal bundles with central canal, internodes with hollow pith; stomata paracytic, maturing basipetally, mesogenous, overlying chlorenchyma, in rows, guard cells lacking chloroplasts, subsidiary cells with radiating silicified ribs, forming stomium, epidermal cells in files; leaf vascular bundles amphicribral; leaves whorled, small, simple, 1-veined, basally connate, not photosynthetic; buds alternating with the leaves, together alternating at each node; sporangiophores peltate, attached along the internodes [= a strobilus], development basipetal; sporangial cell walls with helical secondary thickenings; spores with circular aperture [hilate], alete [not ridged], abapertural obturator + [check], chlorophyllous, wall with silica, elaters 4-6/spore, spatulate, helically-coiled; gametophyte not mycorrhizal, (bisexual), unisexual (heteromorphic); cilia with spline ≥300 microtubules across; sporophyte — gametophyte interface smooth; embryo shoot apex exoscopic [developing towards micropyle/archegonial neck], plane of first cell division variable, suspensor 0; n = x = 108, ancestral 1C genome = 19.5 pg; chondrome with C → U RNA editing (none), atp1 intron 0.
1/18: [list]. Northern Hemisphere, Southeast Asia and Malesia to New Caledonia, South America, the Canaries, East African mountains and the Mascarenes.
Age. Species in crown-group Equisetum may have separated in the Caenozoic (77.5-)64.8(-52.1) Ma (Des Marais et al. 2003), but Christenhusz et al. (2021) suggest an age of almost three times greater, (209.7-)174.9(-164.6) Ma, while the age in W. Sun et al. (2024: E. bogotense not included) is (177-)137(-95.8) Ma.
Fossils with many of the apomorphies of crown group Equisetum are known from Upper Jurassic deposits from Patagonia some 150 Ma or more (Channing et al. 2011) and the Lower Cretaceous of British Columbia ca 136 Ma (Stanich et al. 2009). The oldest fossils with tubers are of the genus Equisetites from the end Triassic—beginning Jurassic; tubers are known from other fossil sphenophytes (J. Lee 2025).
Evolution: Divergence & Distribution. For the long fossil record of Equisetum, known from deposits in the Lower to Mid Triassic of Queensland, Australia, ca 230 Ma, see Gould (1968), also Channing et al. (2011: uppermost Jurassic, ?crown group), Elgorriaga et al. (2018), Gnaedinger et al. (2020), etc..
As Stanich et al. (2009 and references) note, Equisetum, which has undergone little change in its basic morphology for a long time and which is currently widespread, has been considered to be one of the most successful genera of extant vascular plants, although there are of course various ways you can evaluate success - and generic circumscriptions vary... Speck et al. (2003: Figs 16, 20) suggest a number of apomorphies for Equisetum, its subgenera, and its immediate fossil relatives. Indeed, most of the apomorphies of the genus tend to be discussed in the context of variation in taxa like Calamitaceae rather than those of other extant ferns, which indeed is not unreasonable.
Given the suggested crown-group age of the genus (see above), it is not surprising that the biogeogeographic history of the genus suggested by Christenhusz et al. (2021) involves vicariance events connected with the break-up of Pangaea.
Not only does Equisetum in particular have a long fossil history, but equisetophytes in general (names for them vary) are well known from the Upper Devonian onwards, being derived from trimerophyte-grade plants. Early members of this clade have secondary thickening (known from stem tubers in some fossils - J. Lee 2025), their spore morphology was very different, etc. (T. N. Taylor et al. 2008; Stanich et al. 2009: esp. p. 1296). Indeed, change in spore morphology from the trilete, i.e. 3-ridged Calamites to the at first sight very different alete or unridged spores of Equisetum is convincingly demonstrated by Grauvogel-Stamm and Lugardon (2009), elaters appearing by the Middle Triassic (Schwendemann et al. 2010; see also Stanich et al. 2009). Other fossils placed in this area include Sphenophyllum, which has megaphylls, and this suggests that the tiny leaves of Equisetum may be reduced rather than being microphylls; roots of fossils also differ in morphology and can be polyarch. Neoarthropitys gondwanaensis, from Middle to Late Triassic deposits ca 240 Ma from western Argentina, is perhps intermediate between Calamitaceae, with secondary thickening and Equisetaceae, which lacks it (see also mesarch versus endarch primary xylem).
Ecology & Physiology. Equisetum tends to grow in ecologically rather stressfull habitats, including hot springs (Channing et al. 2011 and literature; Husby 2012). Its chlorophyllous spores - such spores are often associated with the epiphytic habitat in ferns - here have to do with the rather wet habitats in which Equisetum often lives (Mellado-Mansilla et al. 2022). Pressurized gas flow, so-called convective ventilation, from the stems into the rhizomes in some extant species of Equisetum and probably in their fossil calamitalean relatives from the Carboniferous, and oxygen moves in this flow via interconnected air spaces into the rhizomes - and this is accompanied by changes in position of the flow-resistant endodermis in the stem - so perhaps allowing them to penetrate deeply into the anoxic substrates commonly favoured by this group (Armstrong & Armstrong 2009). Although species of Equisetum like E. hyemale lack interconnected air spaces and have no convective ventilation, yet such species may still grow in anoxic, partly submerged conditions (Armstrong & Armstrong 2011). However, there is another way of looking at plant form and function here, and the complex system of air canals may help the plant save on materials while maintaining rigidity of the stems. To the numerous apomorphies in the characterization above could be added others like "loss of secondary thickening", the complex system of air canals helping to provide any needed plant support (Spatz et al. 1998a; Speck et al. 1998, 2003). Endodermal layers are variously positioned and thickened so if stems lose turgor in their parenchyma cells, they may collapse or not depending on these other aspects of stem anatomy (Spatz et al. 2003). The anatomy of fossils like Calamites can also be analyzed from this point of view (Spatz et al. 1998b).
The stomata are likely to be immobile, especially when older, because of the rigid radiating rib-like silica thickenings of the subsidiary cells, and the stomata may even be permanently closed then, although the chloroplasts can move from one guard cell to another (Cullen & Rudall 2016). However, stomatal opening and closure is a complex affair, depending on species, local conditions, etc., and abscisic acid response was conditional (Meigas et al. 2024). For silica deposition, see also Law and Exley (2011), however, stomata - and the plant itself (Equisetum arvense) - can develop normally in the total absence of silicic acid (Law & Exley 2011).
Equisetum arvense and at least some other species of the genus have a mixed-linkage glucan (1→3),1→4)-β-D-glucan) otherwise known from monocots... (Sørensen et al. 2008; see also Fry et al. 2008; Novo-Uzal et al. 2012; X. Xue & Fry 2012). It is found in just about all the cell walls other than those of the vascular tissues, and the walls are also rich in pectin and cellulose - and are unlike the two main types of cell wall previously described (as of 2008 - Sørensen et al. 2008).
Fertilization & Spore Dispersal. Aided by their elaters, spores of Equisetum can either jump up to 1 cm in the air as they dry, or move by short random walk-type movements along the ground (Marmottant et al. 2013). Studies on the stem of E. hyemale focussing on anatomy, node number, etc., suggest that how the stem vibrates may affect spore dispersal (Zajaczkowska et al. 2017).
Plant/Animal Interactions. Equisetum turns out to be much more nutritious than one might think, and it has been suggested that it/its relatives may have been an important component in the diet of sauropod dinosaurs (Hummel et al. 2008; Pickrell 2019). Although extant Equisetum are quite small and sauropods are quite large, at least some of the former are quite high in protein and energy.
Correia et al. (2020) review herbivory on sphenophytes in general. This includes evidence of galls on Middle Pennsylvanian Annularia and there is also a variety of other feedling guilds (ibid.: Table 2). Note, however, that when it comes to extant species of Equisetum and insect herbivory, the two main subgenera of are rather different. Poinar (2014) found a number of monophagous insects - 4 species of the dipteran agromyzid Liriomyza, all 7 species of the weevil Grypus, several species of the hymenopteran Dolerus, all three species of the chrysomelid Hippuriphila, a couple opf aphids - on species of subgenus Equisetum where they ate various parts of non-reproductive plants. On the other hand, only a single species of aphid was found on the rhizome of a species of subgenus Hippochaete (see also Correia et al. 2020: Table 3); herbivory in E. bogotense, sister to the rest of the genus, seems to be unknown.
Plant-Bacterial/Fungal Associations. For the absence of mycorrhizae in Equisetaceae, see Pressel et al. (2016, c.f. Lehnert et al. 2016). However, mucoromycote fine root endophytes and other fungal associates seem to be particularly common on six species of Equisetum growing in the Canadian Arctic, indeed, more generally from 51o N-82o N (Hodson et al. 2009; Orchard et al. 2017); see also Ogura-Tsujita et al. (2019).
Genes & Genomes. For a genome duplication in Equisetum dated to (112.5-)92.4(-75.2) - or perhaps only 70-50 Ma, the latter age roughly at the K-P boundary, see Vanneste et al. (2015) and Lohaus and Van de Peer (2016). However, J. W. Clark et al. (2019) discussed two duplications, one mid-late Carboniferous (329-307 Ma) and the other Triassic (253-233 Ma). They thought that in both any connections between the duplications and the evolution of the genus were unclear, the former being well before the K-P boundary event - perhaps they affected diversity within the whole lineage, but not disparity. Clark and Donoghue (2018) linked these early duplications to more or less distant events in the Equisetum clade/its ancestors, while a third duplication dated to somewhere around the K-P boundary was perhaps connected with the origin of Equisetaceae. For duplications, see also C.-H. Huang et al. (2022).
For genome sizes in both extinct and extant taxa, a genome size increase within Equisetum not being associated with a duplication event, see Clark et al. (2019), also Franks et al. (2012). Christenhusz et al. (2021) reconstruct the ancestral genome size for the genus, and they suggest that variation in genome size is connected with the flagellum number of the spermatozoids.
W. Sun et al. (2024) discuss plastome variation and evolution here, i.a. the GC content is low.
Chemistry, Morphology, etc.. For Si concentration, etc., see Husby (2012). For leaf morphology, see Rutishauser and Sattler (1987). Cullen and Rudall (2016) discuss the development of the remarkable stomata in detail; they are mesogenous. Details of sporophyte growth are discussed by Tomescu et al. (2017) and Tomescu & Rothwell (2022); Romanova et al. (2023b) focus on stem and leaf growth.
The tapetum seems to be involved in the formation of the elaters (Uehara & Murakami 1995).
For the gametophyte, see Hauke (1969).
Phylogeny. Equisetum bogotense is sister to the rest of the genus (Guillon 2007; Christenhusz et al. 2019, 2021; J. W. Clark et al. 2019), and a position as a distant sister to the rest of the genus was also recovered in molecular and joint analyses, but not in morphology-only analyses (see Elgorriaga et al. 2018).
Classification. The two-subgenus classification of Equisetum (e.g. Hauke 1978) needed amending given the position of Equisetum bogotense as sister to the rest of the genus, and Christenhusz et al. (2019; see also Elgorriaga et al. 2018, Satjarak et al. 2022) did the job - there is now a subgenus Paramochaete, in addition to subgenera Hippochaete and Equisetum. Previous sectional classifications have not held up.
[[Ophioglossidae] [Marattiidae + Polypodiidae]]: sporophyte (with glomeromycote associate); stomata largely cyclocytic; sporangia not aggregated into strobili, (spore aperture proximal and monolete); gametophyte (with glomeromycote associate); sporophyte — gametophyte boundary with unicellular sporophytic haustoria [?level]; chondrome rpl2 coding region with indel.
Age. Rothfels et al. (2015b) suggested an age of (390.4-)351.7(-309.7) Ma for this node and C.-H. Huang et al. (2019) an age of ca 357.1 Ma. The stem age of Ophioglossidae is estimated to be (280.4-)267(-226.1) Ma (Pelosi et al. 2022), ca 368 Ma (Testo & Sundue 2016), ca 342/324 Ma (W. Sun et al. 2024: pp. 12 and 5 respectively) or as little as ca 173 Ma (H. Shen et al. 2017).
Plant with erect and creeping stems; stem protoxylem development variable, usu. solenostelic; embryo suspensor 0; gametophyte white, subterranean, axial, mycoheterotrophic; chondrome RNA editing U → C.
Age. Magallón et al. (2013) estimated an age of around 275.6 Ma for this clade, and (316-)306(-267) Ma is the age in Y. L. Qiu et al. (2007), ca 214.5 Ma in Laenen et al. (2014), (317.1-)250.5(-141.8) Ma in Rothfels et al. (2015b) and ca 284.8 Ma in C.-H. Huang et al. (2019); see also P. Soltis et al. (2002) for estimates.
Evolution: Plant-Bacterial/Fungal Associations. For mycoheterotrophy of the gametophytes in this clade, the plants being non-photosynthetic and mycorrhizal (but not yet confirmed for the small genera in Ophioglossaceae), see Merckx et al. (2013a) and Pressel et al. (2016).
Genes & Genomes. Both Psilotum nudum and Tmesipteris obliqua have very large genomes, 1C = 142.4 and 147.3 Gb respectively, only slightly smaller than the genome of Paris japonica, while Ophioglossum reticulatum has a diploid chromosome number of ca 1440 (Bennett & Leitch 2005; J. Clark et al. 2016; F.-G. Wang et al. 2022; Heslop Harrison et al. 2022; Pelosi et al. 2022). Recently it has been found that the genome of the octoploid T. oblanceolata, at 160.45 Gbp, is the largest known (Fernández et al. 2024).
For the mitochondrial genomes of Psilotum (very large) and Ophioglossum, see W. Guo et al. (2016b); they are similar in terms of apomorphies to those of seed plants.
PSILOTALES Prantl
Just the one family, 2 genera, 17 species.
PSILOTACEAE J. W. Griffith & Henfrey - Back to Polypodiopsida
Epiphytes; silica content slight; roots 0; stem with tetrahedral apical cell; stereome mid-cortical; ?leaf vascular bundles; leaves small, simple, (laterally flattened - Tmesipteris [T.]), veins 1 or 0; sporangia 2-3, forming synangium; tapetum glandular-amoeboid; spores kidney-shaped, monolete; gametophyte with septate rhizoids; placenta with sporophyte and gametophyte cells intermingling, transfer cells [wall ingrowths] gametophyte only - T./sporophyte only - Psilotum, n = 52, 208, nuclear genome [1 C] 72.5-150.6(-?313) pg/160.45 Mbp, [2 C] 56.15 pg/147.3 Gb.
2/17: [list], Tmesipteris (15). Pantropical, inc. Pacific, warm temperate.
Age. B. Zhong et al. (2014b) estimated an age of (147.1-)72.3(-14.7) Ma, Rothfels et al. (2015b) an age of (142.5-)78.9(-28.5) Ma and C.-H. Huang et al. (2019) and age of ca 57.4 Ma for this clade.
Evolution: Plant-Bacterial/Fungal Associations. Mycorrhizal associations in Psilotum in the echlorophyllous gametophytic and subterranean sporophytic stages are with glomalean Glomus group A fungi, and they are also to be found in the autotrophic sporophyte, as in Tmesipteris (Winther & Friedman 2009; Imhof et al. 2013; Lehnert et al. 2016).
Vegetative Variation. Wardlaw (1956) noted that the highly reduced leaves of Psilotum, apparently very different to those of other vascular plants, could in fact be compared with those of Tmesipteris and other ferns. For apical growth, etc., see Renzaglia et al. (2000a).
Genes & Genomes. Tmesipteris obliqua has a huge genome of around 148.8 Gb, for which, see Hidalgo et al. (2017b, c); that of Psilotum nudum, at 142.4 Gb, is not much smaller (Obermayer et al. 2022) - Paris japonica (Melanthiaceae) has the largest genome. C.-H. Huang et al. (2019) suggest that there has been a genome duplication along the stem of this clade.
For general information, see Kramer (1990).
OPHIOGLOSSSALES Link
Just the one family, 15 genera, 190 species.
OPHIOGLOSSACEAE Martinov - Back to Polypodiopsida
Rhizome creeping, cylindrical, hairs 0; roots fleshy, ca 2 mm≤ across, 2-5-arch, not budded; root hairs 0; stem with eustele, stereome 0, cork mid-cortical, stele sympodial; tracheids with circular bordered pits; petiole vasculature U-shaped; frond vascular bundles collateral; (axillary buds +); fronds dimorphic; tropophores: compound, stalked, stalk cylindrical, 1 produced/year, vernation nodding, leaf base sheathing, sheath papery; sporophores: 1-4/rhizome, sporangia free; gametophyte early cylindrical, (with septate rhizoids); embryo (shoot apex exoscopic [developing towards micropyle/archegonial neck], first cell wall of the zygote vertical), suspensor +/0; nuclear genome [1 C] ca 28.35(?-139) pg, [2 C] 55.0 pg.
15/190: [list]. More or less world-wide.
Age. Rothfels et al. (2015b) suggested an age of (249.6-)161.7(-74) Ma for crown-group Ophioglossaceae, Gil and Kim (2018) an age of ca 256 Ma.
1. Botrychioideae C. Presl
Rhizome erect; vascular cambium +; indumentum +; tropophore [sterile part of leaf] (not stalked), (amphistomatous), leaves pinnate, ptyxis circinate [Botrychium], fine venation ± free and forked; sporophore (1-2)-4-pinnate, sporangium dehiscence various; spore exine various; n = (44) 45 (46), 6.0-30.6 Gbp/1 C.
6/100: Botrychium (42), Sceptridium (40). ± Cosmopolitan.
[Helminthostachyoideae [Mankyoideae + Ophioglossoideae]]: vascular cambium 0; sporophore simple; sporangium dehiscence transverse.
2. Helminthostachyoideae C. Presl - Helminthostachys zeylanicum Kaulfuss
leaves ternate, venation free and pinnate (anastomosing); sporophore spike-like, spore exine baculate; gametophyte early broadly ovate; n = 94, 11.7-13.1 Gbp/1 C.
1/1. Sri Lanka to the Himalayas, S. China, Taiwan, Malesia and the western Pacific.
[Mankyoideae + Ophioglossoideae]: roots budded; sporangia sunken, basally fused
3. Mankyuoideae J. R. Grant & B. Dauphin - Mankyua chejuensis B. Y. Sun et al.
Vascular cambium?; leaves pinnate, venation free; sporophore spike-like, ± divided; spore exine baculate; gametophyte ?rhizoids +; n = 130. ca 12.7 Gbp/1 C.
1/1. South Korea (Jejudo Island).
[Ophioglossoideae [Helminthostachyoideae + Botrychioideae]]: gametophyte rhizoids thread-like.
4. Ophioglossoideae C. Presl
Plant (epiphytic); (roots not budded); rhizome (erect), (tuberous/subglobose), (indumentum + (internal)), venation anastomosing; tropophores usu. amphistomatous; leaves simple (palmately lobed), venation (complex-)reticulate, sheath membranous; sporophores (-12/rhizome), simple, spore exine usu. reticulate; gametophyte (early disc-like), (rhizoids = papillae); HGT 0, (mitogenome nad1 intron 0), 2n = ?86, 120, 370-380, 16.3-136.7 Gbp/1 C.
7/ca 100: Ophioglossum (ca 50). ± Cosmopolitan.
Evolution: Divergence & Distribution. Mankyua, ca 194.8 Ma, is known only from Jejudo Island, all of ca 2 Ma and in contact with the Korean mainland during its existence (Gil & Kim 2018).
There are two bipolar geographical disjunctions within Botrychium in which polyploidy/introgressive hybridization has been involved; such distributions are uncommon in ferns. Interestingly, the gametophytes of Ophioglossaceae can self, and this may facilitate dispersal (Farrar & Stensvold 2017; see also Dauphin et al. 2017a).
L. Zhang and Zhang (2022) optimised two morphological characters on their prefered tree (see above). Morphological and cytological characters examined by L.-Y. Kuo et al. (2024) are largely congruent with the tree they present (see characterizations above); horizontal gene transfer is scattered in the family, but is not known from Ophioglossoideae.
Ecology & Physiology. Glomeromycote mycorrhizae in Ophioglossum and Botrychium are associated with the echlorophyllous gametophytic and subterranean sporophytic stages, and also the photosynthesising sporophyte; the latter may obtain some of its carbon from other conspecific or heterospecific plants with which the fungus is associated (Winther & Friedman 2007; Field et al. 2015a; Field & Pressel 2018; see also Friedman et al. 2021). Botrychium gametophytes do not develop beyond the 8-celled phase without establishing this mycorrhizal association, while sporophytes may spend the first ten years of their lives underground living in association with fungus (Winther & Friedman 2007). Even when above-ground fronds are produced, these may not appear every year (Reintal et al. 2010; Shefferson et al. 2018), and the sporangiophore of mixotrophic species like O. kawamurae may lack an associated frond (Suetsugu et al. 2020b).
HGT in both platids and especially mitochondria has involved genes from other ferns, but also those of some root-parasitic Santalales, and also a few Lamiales-Orobanchaceae (L.-Y. Kuo et al. 2024 for extensive discussion); as just mentioned, both gametophyte and sporophyte of Ophioglossaceae are associated with fungi.
Fertilization. Sperm transfer between subterranean gametophytes of Botrychium is of the order of 2 cm; selfing is likely. However, at higher altitudes water flow, perhaps from snow melt, facilitates crossing (Dauphin et al. 2024). In B. campestre, at least, gemmae are produced by the sporophytes which, like the spores, eventually produce above-ground fronds; these gemmae can help tide the species over dry years, populations apparently disappearing then (Popovich et al. 2024).
Plant-Bacterial/Fungal Associations. The Glomus involved in the mycoheterotrophic association is in the same immediate clade as species involved in similar associations in Arachnitis (Corsiaceae) and Lycopodiaceae, and at least some of the fungi are the same as those associated with the (quasi-)autotrophic sporophyte (Leake et al. 2008; Winther & Friedman 2007, 2008; Imhof et al. 2013). There are more species of fungus in the sporophyte, the fungi found in the gametophyte also being found in the sporophyte (Winther & Friedman 2007). Perez-Lamarque et al. (2019/2020) noted that the fungi in these associations were not known from any other plants (c.f. Psilotaceae). Suetsugu et al. (2024c) found that the fungus associated with the mycoheterotrophic gametophyte of Sceptridium belonged to Entrophospora-Entrophosporales- Glomeromycotina, while that of the photosynthetic sporophyte was a member of Glomeraceae-Glomerales-Glomeromycotina.
Mitochondrial genes from a presumably root-parasitic member of Loranthaceae seem to have been acquired by Botrychium virginianum, perhaps via a common mycorrhizal associate (C. C. Davis et al. 2005b). However, in general mycorrhizae are not very common on Santalales and if the gene transfer took place in Asia where the fern may have originated, as Davis et al. (2005b) suggested, the absence of extant root-parasitic Loranthaceae from that area is notable.
Genes & Genomes. Ophioglossum reticulatum, at n = 720, has the highest chromosome number of any embryophyte. There has been a genome duplication somewhere in Ophioglossaceae (C.-H. Huang et al. 2019; Pelosi et al. 2022).
Species involved in hybridization tend to be consistently donors of either paternal or maternal genomes (Dauphin et al. 2017b: esp. Fig. 4).
Both plastomes and chondromes in Ophioglossoideae are notably s,aller than in the other subfamilies (L.-Y. Kuo et al. 2024). The chondrome of Ophioglossum californicum, at least, is made up of two chromosomes (W. Guo et al. 2016b).
Chemistry, Morphology, etc.. Takahashi and Kato (1988) describe the development of lateral meristems in the family (see also M. Kato 1987), although Moran (2022) suggests that ferns do not have such meristems. Thus there is some debate as to whether there is secondary thickening in Botrychium. It certainly is not conventional secondary thickening, and the distinctive appearance of the vascular tissue - the tracheary elements are in radial series, even if there is no evidence that the stem has increased in width - may be connected with the fact that the leaves can take up to five years to develop, perhaps a record for land plants (Rothwell & Karrfalt 2008). See also Wagner and Wagner (1993) for a list of distinctive features of the family.
Bruchmann (1906 and references) described megagametophyte development, etc., in Ophioglossum and Botrychium.
Phylogeny. See Hauk et al. (2003) for a phylogeny, Mankyua not included, also Shinohara et al. (2013), Mankyua was included, but its position was unstable, and it was either sister to the rest of the family (also in the joint analysis), or to Ophioglossum s. str. alone. Similarly, Gil and Kim (2018) found that Mankyua was sister to the rest of the family in maximum parsimony analyses, but Psilotum took that position in other analyses.
L. Zhang et al. (2020b) looked at relationships throughout the family (ca 3/4 of the species included); plastid markers only were examined. Given the dismemberment of Botrychium, Botryopus was found to be paraphyletic, necessitating the description of a new genus. Zhang et al. (2020b) also found that the support for Mankyua as sister to the rest of the family was rather weak. L. Zhang and Zhang (2022) in a comprehensive study used 8 plastid markers for 184 accessions, also 22 entire plastomes and 29 morphological characters, with analyses separate and variously combined. In the 8 plastid marker analysis, support for the basal position of Mankua was still not very strong, nor that for some genera in Botrychioideae or for Ophioglossum s.l., but there was good support for the three genera into which Zhang and Zhang divided the latter. In the plastome analysis there was no maximum likelihood BS support for the position of Mankyua and that for the position of Helminthostachys was weak; in the morphology only analysis a [Mankyua + Helminthostachys] clade, part of a tritomy with the other two subfamilies, had weak support, while in the morphology + eight-marker analysis there was quite strong support for a clade [Mankyua [Helminthostachys + Botrychium etc.]] (Zhang & Zhang 2022). Overall, the relationships were [Mankyua [Ophioglossum etc. [Helminthostachys + Botrychium etc.]]]. L.-Y. Kuo et al. (2024: 83 plastid and 37 mitochondrial genes, Fig. 2A for previous studies) on the other hand, found the relationships above, although they were more poorly supported in the mitogenome analyses; some nodes in Ophioglossoideae were still poorly supported.
Classification. This is still somewhat in a state of flux. M. Kato (1987) provides an early classification of the family based on morphological phylogeny - 6 genera. Mabberley (2008) noted there were four genera and 55 species, as of iv.2017 I had 4 genera and 80 species, The Pteridophyte Phylogeny Group (2016) recognized 10 genera with 112 species while L. Zhang et al. (2020b) 11 genera placed in four subfamilies, noting also that there seemed to be problems with species limits in genera like Ophioderma. The classification followed above is that prefered by Zhang and Zhang (2022).
[Marattiidae + Polypodiidae]: plants often Al accumulators; frond vascular bundles amphicribral; scales +; frond compound, vernation circinate; sporangia abaxial; [check tapetum]; gametophyte green, surficial; nuclear genome 3.5-14.0 pg; changed gene adjacencies at borders of chloroplast IR; mitochondrial atp1i361g2 intron gain.
Age. B. Zhong et al. (2014b) suggested an age of (378.8-)336.7(-291.5) Ma and Rothfels et al. (2015b) an age of (364.1-)329(-289.2) Ma for this clade, while the age in C.-H. Huang et al. (2019) is ca 347.1 Ma and that in May et al. (2021: stem-group Marattiales) is (505.7-)414.8(-350.9) Ma.
Fossils of the marattialean Psaronius are known from the early Pennsylvanian ca 320 Ma (Rößler 2000).
Evolution: Divergence & Distribution. Suissa and Friedman (2022) suggest that the transition from a protostele to a solenostele happened here, albeit with reversals. This transition is not placed here above, largely because the solenostelic condition predominates in only one of the next four clades up the tree (ibid.: Fig. 3).
MARATTIIDAE Klinge
MARATTIALES Link —— Synonymy: Christenseniales Doweld
Just the one family, 6 genera, 110 species.
MARATTIACEAE Kaulfuss - Back to Polypodiopsida
Roots fleshy, ca 2 mm≤ across, with several protoxylem poles; root hairs few, septate [?multicellular]; mycorrhizae +; stem polycyclic stele +; mucilage canals +; rhizome with scales; foliar aeration by porophores, elongated, scattered over leaf axis, with central pore; petiole vasculature polycyclic; (stomata 125-145 x 80-110 μm - Christensenia); fronds collenchymatous-pulvinate [on rhachis/pinnae], (fine venation reticulate, with internally directed veins - Christensenia), stipules +, thin/fleshy and starchy; meiosis monoplastidic [?all]; spores bilateral or ellipsoid, monolete; gametophyte with both glomeromycotes and mucoromycotes; transfer cells 0; x = 40, genome [2 c] 6.4-10.7 pg, genome duplication +; chondrome with "normal" C → U RNA editing only.
6/140: [list], Danaea (81). Pantropical, to Hawai'i, New Zealand.
Age. Crown-group Marattiaceae are estimated to be 236-201 Ma (Lehtonen et al. 2020: c.f. Figs 2 and 4), in line with estimates by other authors given there, while J. Zhao et al. (2023) suggested an age of ca 319.5 Ma, although the age in Testo and Sundue (2016) is only 165-154 Ma while those in May et al. (2021) at (173.7-)85.5(-37.4) Ma and C.-H. Huang et al. (2019) at ca 68.4 Ma are still younger. P. Soltis et al. (2002) and Zhao et al. (2023) offer suggestions for ages of other nodes in this clade.
Evolution: Divergence & Distribution. Marattiales (as Psaroniaceae) are richly represented in the Permo-Carboniferous fossil record (Rothwell et al. 2018c; Lundgren et al. 2019; Lehtonen et al. 2020). A few fossils from the late Permian may perhaps be assigned to Marattiaceae, but the family explodes in the Triassic and Psaroniaceae almost disappear then - fossils of both Angiopteris and Marattia are recorded from the Upper Triassic, possibly around 215 Ma (Rothwell et al. 2018c). Indeed, the fossil Psaronius has tended to associate with Marattia in some studies (e.g. Grand et al. 2013 and references), but no fossils were linked to that genus by Lehtonen et al. (2020). Rothwell et al. (2018b) also include fossils in their comprehensive study of the family, but relationships between the extant genera become unclear depending on particular data/fossils included and the outgroup used. Nevertheless, Psaronius and several other fossils are in a clade (= Psaroniaceae) sister to the clade that includes all extant Marattiales (= Marattiaceae) along with a few more fossils (Rothwell et al. 2018b; see also Lehtonen et al. 2020 for fossils and phylogeny). Note, however, that May et al. (2021: total evidence/tip dating) do not think that any fossils can be associated with crown-group Marattiaceae. In any event, crown-group Marattiaceae may have a ca 100 My fuse.
Lehtonen et al. (2020) suggest that some five or so marattiaceous clades were on different parts of Pangaea before it split up, although there has also been substantial dispersal - Marattia itself is on Hawai'i, perhaps having got there from somewhere in eastern Asia. Nevertheless, the crown-group age of the speciose Angiopteris may be only some 37.6 Ma (J. Zhao et al. 2023).
Interestingly, polysymmetric sporangia, common in these fossils, are found only in Christensenia among extant taxa, all others having bisymmetric sporangia, but these are in turn uncommon in fossils (Lundgren et al. 2019); Christensenia is well embedded in the family (e.g. Lehtonen et al. 2020; J. Zhao et al. 2023) - are its sporangia a reversal, or what?
Christenhusz and Chase (2013) comment on the biogeography of Marattiaceae. There seems to have been a slow-down in the rate of evolution in this clade (Rothfels et al. 2015b and references). This (probably accompanied by extinction) was in the early Permian-Cisuralian some 298-273 Ma, and it affected the previously very diverse Psaroniaceae, of which estimates suggest that there were 1,200-2,800(!) species in the later Carboniferous (May et al. 2021).
Keskeniva et al. (2025) discussed hybridization in tropical species of Danaea, perhaps surprisingly common (c.f. H.-M. Liu et al. 2020). The ages of the parents ofD. trifoliata and D. wendlandii are estimated to be 72-14 and 62-23 Ma respectively (Keskeniva et al. 2025).
Ecology & Physiology. Aluminium accumulation is quite common in Marattiaceae (Schmitt et al. 2017).
Fertilization & Spore Dispersal. Hovenkamp et al. (2009) observed explosive short-range spore dispersal in Angiopteris. The sporangium opens relatively slowly, exposing the spherical spores. These dry out, becoming smaller; cavitation between the exospore and perispore then occurs, the spore quickly returning to its normal size and in the process jumping up to 4 mm - one hopes it gets caught up by a passing gust of wind.
Plant/Animal Interactions. Angiopteris may have been an important component of the diet of smaller sauropods (Hummel et al. 2008).
Genes & Genomes. For meiosis, see R. C. Brown and Lemmon (2001). Pelosi et al. (2022) discuss a whole genome duplication that they place along the stem of Marattiaceae.
Chemistry, Morphology, etc.. For gum in Marattia, see Lambert et al. (2016). The porophore is formed by the disintegration of stomata, etc. (Moran 2022). H. Shen et al. (2017) scored both Osmunda and Angiopteris as having capsules with a rudimentary annulus, so affecting the position of this character on the tree (see next node).
Phylogeny. For a phylogeny, see Murdock (2008a), Christenhusz et al. (2008) and Lehtonen et al. (2020); Danaea (no porophores!) is sister to the rest of the family (Murdock 2008a; Lehtonen et al. 2020). Suggested relationships in May et al. (2021) are [Danaea [[Marattia [Christensenia + Angiopteris>]] [Eupodium + Ptisana]]]. J. Zhao et al. (2023: 16 spp., plastome, chondrome and single-copy nuclear genes) examined relationships here, and had to deal with ILS and quite extensive hybridization. Keskiniva et al. (2024: 4 chloroplast markers, 301 samples) looked at relationships within Danaea; there were three well-supported subgenera, and in the past most specimens had been placed in one of three species, now each was placed in separate subgenera, however, linking morphological and molecular distinctness in this genus could be difficult.
Classification. Both Marattia and Angiopteris were early thought to be paraphyletic, but they can easily be made monophyletic (Murdock 2008b). For Danaea, see Christenhusz (2010).
POLYPODIIDAE Cronquist, Takhtajan & Zimmermann / Leptosporangiate ferns.
Blue light stomatal opening response absent; primary cell walls poor in mannans and rich in tannins; roots with 2 protoxylem poles; primary xylem with scalariform bordered pits; leaf trace single; aerophores linear, on either side of the petiole, with stomata; stomata often anomocytic; sporangium derived from periclinal division of a single epidermal cell, wall one-layered, exothecium forming an annulus, stalk 4-6 cells across [= leptosporangium]; 64-800 spores/sporangium; gametophyte cordate [level?], antheridia ± exposed; sporophyte — gametophyte placental cells interdigitating, walls labyrinthine, gametophytic cells with stacks of mitochondria, plastids pleomorphic; first cell division of the zygote longitudinal [embryo prone], with quadrant/octant formation, suspensor 0.
Age. Magallón et al. (2013) estimated an age of around (267.8-)252.7-251.4(-246.1) Ma for this clade; ca 299 Ma is the age in Schuettpelz and Pryer (2009), (327.8-)301.3(-271.5) Ma in Rothfels et al. (2015b), ca 309.5 Ma in Hennequin et al. (2008), ca 318.4 Ma in C.-H. Huang et al. (2019), (330-)323(-310) Ma in Y. L. Qiu et al. (2007), perhaps 350 Ma in H. Schneider et al. (2004a), and (357.5-)357(-356) Ma in Testo and Sundue (2016) - but only around 170 Ma in Villarreal and Renner (2014). An estimate in Pelosi et al. (2022) is (312.0-)300.9(-299.3) Ma while in Nitta et al. (2022) it is as much as ca 423.2 Ma. All told, a rather disconcerting spread, but the older ages are perhaps quite likely - see X. He et al. (2021: 298.3 Ma fossils of Gleicheniaceae...).
Evolution: Divergence & Distribution. Quite long phylogenetic fuses are common in fern families (see also Nitta et al. 2022).
Barrington (2020) discussed polyploidy and evolution in ferns, a discussion in which the evolution of polyploid ferns, especially Polystichum species, on Darwinian islands (oceanic islands, sky islands, ecological islands) figured quite largely.
Ecology & Physiology. Tolerance of extreme desiccation, sometimes facultative, is scattered through this clade, and some gametophytes, too, are similarly tolerant (Proctor & Tuba 2002; Gaff & Oliver 2013).
Foliar nectaries, often quite small and inconspicuous and on the lower surface of the frond, are scattered here, e.g. in Cyatheaceae (common in Cyathea), in some Polypodiaceae (Moran 2022); they are probably under-recorded. The composition of the nectar varies and such nectaries have evolved several times; ants have been observed taking the nectar (Mehltreter et al. 2021). These nectaries are commonly modified aerophores (Moran 2022).
Fertilization & Spore Dispersal. Noblin et al. (2012, see also Ingold 1939; Tian et al. 2021) describe the mechanics of how the annulus functions in sporangium dehiscence of leptosporangiate ferns. The spores are dispersed by a catapult-like motion: As the cells of the annulus (Polypodium aureum was the subject of the study by Noblin et al.) dry, they deform, and the annulus recurves, having separated at the stomium, and then, suddenly, the outer periclinal walls of the cells of the annulus cavitate, collapsing inwards and the annulus snaps back. The spores are ejected at a velocity of around 10 m s-1 as the rate of closure of the annulus abruptly decreases, this decrease being functionally akin to the crossbar of a catapult (Noblin et al. 2012). Gómez-Noguez et al. (2022) looked at the determinants of the terminal velocity (Vt) of fern spores, and they found that mass was more important than size, spore appendages tended to reduce Vt, and spore ornamentation might increase (e.g. smooth, reticulate) or decrease (clavate, cristate) it. Of course, work on wind-dispersed spores in all groups is relevant to what goes on here - so see spore dispersal in bryophytes, lycophytes, Gnetales, pines etc. (for which, see below), and even wind-pollinated angiosperms under Pollination & Seed Dispersal/Fertilization & Spore Dispersal. For other mechanisms of spore dispersal in ferns, see Onocleaceae, Equisetales and Marattiales.
Genes & Genomes. The GC content of the chloroplast genome is notably high around here (Y. Yu et al. 2019b).
Chemistry, Morphology, etc.. Schölch (2022 and references) discusses sorus morphology and development. For placental structures, see Duckett and Ligrone (2003, 2004).
Phylogeny. It was early found that the large clade made up of leptosporangiate ferns was monophyletic and had very strong support (see also Hasebe et al. 1994, 1995, Pryer et al. 1995; Wolf et al. 1998; Quandt et al. 2004; Schuettpelz et al. 2006; Rai & Graham 2010; etc.). Rothfels et al. (2015b) emphasised that their analyses of nuclear data broadly agreed with several plastid sequence analyses, although chondrome data may suggest different topologies (e.g. J. Zhao et al. 2023).
Within the leptosporangiate ferns, Osmundales, Osmunda and relatives, the sporangia of which have some eusporangiate features, are strongly supported as being sister to the rest of the clade. There is further substantial resolution of relationships (e.g. Pryer et al. 2004a, b and references; Schuettpelz & Pryer 2007; c.f. in part Kuo et al. 2011: positions of Gleicheniaceae, Lindsaeaceae, Nephrolepis [previously in Lomariopsidaceae] uncertain). It is unclear whether or not there is a [Hymenophyllales + Gleicheniales] clade (Knie et al. 2015 for literature; H. Shen et al. 2017; Kuo et al. 2018b; Lehtonen et al. 2017; Lehtonen 2018); relationships here are probably best represented as a tritomy. There is some evidence that Gleicheniales are paraphyletic, but support is slight (Rothfels et al. 2015b; see also Qi et al. 2018). J.-M. Lu et al. (2015: chloroplast genome, but sampling) placed Dipteridaceae and Lygodiaceae as successive branches along the leptosporangiate stem. Schizaeales and Salviniales (strong support) and Cyatheales (weak support) are successively sister to Polypodiales (e.g. Rothfels et al. 2015), and Rai and Graham (2010) suggest additional variants. For relationships in Schizaeales, see Labiak et al. (2015 and references). Relationships at the base of the eptosporangiates are structured as follows below: [Hymenophyllales, Gleicheniales [Schizaeales [Salviniales [Cyatheales + Polypodiales]]]]
Structural changes in the plastomes of Polypodiales (Wolf & Roper 2008; Wolf et al. 2010, 2011) support relationships that are largely consistent with those suggested by sequence analyses, while H. Shen et al. (2017) suggest relationships based on analyses of transcriptome data. Looking at more basal Polypodiales, Lindsaeaceae are perhaps sister to all others (see also Rothfels et al. 2015b), but the genera Cystodium, ex Dicksoniaceae, and Lonchitis and Saccoloma, both ex Dennstaediaceae and all as separate families below, are also in this area (Lehtonen 2011; Lehtonen et al. 2012; Qi et al. 2018). Relationships around here have been depicted as [Saccolomataceae [[Cystodiaceae [Lindsaeaceae + Lonchitidaceae]] [Pteridaceae [Dennstaedtiaceae [Polypod 1 + Polypod 2]]]]] - e.g. H.-M. Liu et al. (2020). Pteridaceae and Dennstaediaceae were again well supported as successive sister taxa to the Eupolypods (Lehtonen 2011; Rothfels et al. 2015b; J.-M. Lu et al. 2015; Qi et al. 2018). However, X.-Y. Du et al. (2021) in an extensive plastome analysis recovered Pteridaceae and Dennstaedtiaceae as sister taxa, while the clade immediately basal to this (it includes Saccolomataceae and Lindsaeaceae) was subtended by a short branch and the internal branches separating families were also short, data concordance being low. Faute de mieux, relationships at the base of Polypodiales below are given as [[Saccolomataceae [Cystodiaceae [Lindsaeaceae + Lonchitidaceae]]] [[Dennstaediaceae + Pteridaceae] [Eupolypods]]]. The latter are then divided into two major groups, the Eupolypod I and Eupolypod II clades.
Within the Eupolypod I clade, Lehtonen (2011) found that Polypodiaceae were not monophyletic in parsimony analyses, and other relationships were unclear. For more on relationships in the Eupolypod I clade, see L.-B. Zhang and Zhang (2015). Qi et al. (2018) included members of all Eupolypod I families in their analyses, and the following relationships were frequently recovered - [Hypodematiaceae [Didymochlaenaceae [Dryopteridaceae [Lomariopsidaceae [Nephrolepidaceae [Tectariaceae [Oleandraceae [Davalliaceae + Polypodiaceae]]]]]]]], and X.-Y. Du et al. (2011) recovered similar relationships, although the Tectariaceae area was divided - Pteridryaceae and Arthropteridaceae were recognised. Focussing on Hypodematiaceae in particular, Fan et al. (2021) found the same relatioinships between the families they included.
Lehtonen (2011) found that within the Eupolypod II clade relationships between families were mostly rather poorly supported, even if the families themselves were largely monophyletic. Cystopteris and relatives form a clade that may be sister to the rest (Rothfels et al. 2009, esp. 2012a, 2013, 2015b: Qi et al. 2018). Relationships between the members of the Eupolypod II groups sampled by Qi et al. (2018) are rather different from those below - the relationships [[Diplaziopsidaceae + Aspleniaceae] [Rhachidosoraceae [Thelypteridaceae [Woodsiaceae [Athyriaceae [Blechnaceae + Onocleaceae]]]]]] were often recovered, although the basal grouping [[Diplaziopsidaceae + Aspleniaceae] [Cystopteridaceae + the rest]] was also found.
Changes in the topology in this part of the tree are to be expected. For instance, the topology used by H.-M. Liu et al. (2020) is rather different from that below.
OSMUNDALES Link
Just the one family, around 6 genera, 18 species.
OSMUNDACEAE Martinov - Back to Polypodiopsida
Roots (≥1 mm across); SiO2 accumulation common; stem with ectophloic solenostele, with a ring of conduplicate/twice conduplicate discrete bundles, outer cortex sclerotic; cataphylls [= petiole bases] +; petiole vasculature complex U-shaped; stomata all anomocytic; fronds with fertile and sterile portions, (separate fertile and sterile fronds), stipules +; annulus a lateral group of cells; spores chlorophyllous; gametophyte bipolar, (rhizoids septate); zygote elongating; gametophyte with both glomeromycotes and mucoromycotes; n = x = 22, genome [2 C] ca 25.3 pg; chondrome with slight U → C RNA editing.
6/18 [list]: Osmunda (5(-10)). Almost worldwide, but not Middle East-Siberia or polar.
Age. The age of this clade is estimated to be around 199.6 Ma (Schuettpelz & Pryer 2009) or ca 206 Ma (Hennequin et al. 2008). However, estimates from Carvalho et al. (2013) based on fossils that can be assigned to the leptosporangioid branch of the tree suggest an age in excess of ca 265 Ma, Late Permian (see also Wilf & Escapa 2014), while the preferred age in Grimm et al. (2015: comprehensive analysis, also incorporating fossils; c.f. Carruthers & Scotland 2020) is (264-)243(-233) Ma (see also Bomfleur et al. 2017). It has also been suggested that the Osmunda clade in particular originated in the late Carboniferous, ca 323 or 305 Ma (Phipps et al. 1998; H. Schneider et al. 2004a - see also below under Gleicheniaceae) or in the late Triassic ca 210 Ma (S.-J. Wang et al. 2008: Osmunda paraphyletic).
Evolution: Divergence & Distribution. Osmundaceae are very common and diverse in the fossil record from the Permian onwards, but have become less so more recently (S.-J. Wang et al. 2003; Grimm et al. 2015). These fossils often have remarkably good preservation, thus a fossil some 180 Ma has anatomy that is remarkably like that of the extant Osmunda claytoniana (Bomfleur et al. 2014a). Detailed studies of axis anatomy have been carried out (e.g. Miller 1971; Wang et al. 2013: 28 characters; Bomfleur et al. 2014b/2015; 2017: 45 characters, states sometimes arbitrary) and used to reconstruct the phylogeny of the group (Bomfleur et al. 2017: some states arbitrary). Wang et al. (2013) suggested that the fossil clade Guaireaceae was sister to Osmundaceae, in some analyses the fossil clade [Aurealcaulis [Ashicaulis + Palaeosmunda]] was recovered as sister to extant Osmundaceae; there was substantial extinction of Osmundales at the end of the Permian of genera that lived in moister habitats. Urrea et al. (2024) reexamined the fossils, taking into account character dependance, and they also tried weighting against homoplasy; all in all relationships were rather unclear.
Todea, now known only from New Guinea, Australia, New Zealand and South Africa, has been found in the early Eocene of Patagonia in rocks ca 53 Ma (Carvalho et al. 2013; Bippus et al. 2019); see also Fagaceae.
Osmundaceous stems were stable sites for early epiphytes, or at least plants growing on more or less rotting organic remains, thus Bippus et al. (2019: Early Eocene) discuss findings on early "epiphytic" communities known largely from osmundaceous rhizomes. The plants that McLoughlin and Bomfleur (2016) found on an Early Jurassic (191-181 Ma) prostrate rhizome of Osmunda pulchella include a small herbaceous heterosporous lycopsid.
Bomfleur et al. (2014b/2015) note that Osmundastrum cinnamomea is able to hybridize with some species of Osmunda, but not with other Osmundaceae. Given an estimated date of the split of the first two of (264-)238(-233) Ma, only a little less than the age of crown-group Osmundaceae as a whole (Grimm et al. 2015), they have been separated for far longer than any other vascular plants that hybridize; they are the epitome of deep hybridization (Tseng et al. 2024); the estimate of the age of deepp hybridization in the family in H.-M. Liu et al. (2020) is less, but still possibly 100 Ma.
The chlorophyllous spores of Osmundaceae are associated with the wet habitats in which they commonly grow, an association also noted for ferns in general, but not with the epiphytic habitat, another green spore — habitat association in ferns, as in Hymenophyllaceae (Mellado-Mansilla et al. 2022).
Genes & Genomes. H. Schneider et al. (2015) reconstruct the history of genome size changes in Osmundaceae; there is some variation. Generation times in royal ferns are long, so genome change might be expected to be low, but whether or not there is "genomic stasis" (Bomfleur et al. 2014a; c.f. Schneider et al. 2015) is another issue. Indeed, the chromosome number and genome size of 180 Ma fossils similar to Osmunda claytoniana and those of extant O. claytoniana may have been similar (Bomfleur et al. 2014a).
Chemistry, Morphology, etc.. Atkinson and Stokey (1964) describe the sidtinctive morphology of the young gametophyte.
Phylogeny. It has been suggested that Osmunda is paraphyletic, with Osmunda (now = Osmundastrum) cinnamomea being sister to the rest of the family (Metzgar et al. 2008); relationships in Carvalho et al. (2013) are [Osmundastrum [Osmunda [Leptopteris, Todea, Todites]]]. However, Bomfleur et al. (2014b/2015) argue for the monophyly of [Osmunda + Osmundastrum] based on extensive data from fossils and a re-evaluation of the molecular evidence.
Classification. The classification of Osmundaceae and its fossil relatives is very complex for such a small group (Bomfleur et al. 2017: fear of "ancestors"?).
[Hymenophyllales, Gleicheniales [Schizaeales [Salviniales [Cyatheales + Polypodiales]]]]: roots originate in endodermis; protostele +; sporangia in sori, annulus ± oblique, continuous; genome duplication +; plastome with loss of trnK-UUU, trnT-UGU, trnS-CGA; chondrome with substantial U → C [reverse] RNA editing.
Age. (297-)286(-272) Ma is the age for this node in Y. L. Qiu et al. (2007), ca 280.1 Ma in Schuettpelz and Pryer (2009: Hymenophyllales sister to the rest), (306.8-)278.7(-252.3) Ma in Rothfels et al. (2015b), ca 273 Ma in Hennequin et al. (2008) and ca 287.5 Ma in C.-H. Huang et al. (2019); see also below under Gleicheniaceae.
Evolution: Divergence & Distribution. C.-H. Huang et al. (2019; see also Pelosi et al. 2022) suggested that there had been a genome duplication somewhere along the stem of this clade, which also shows increased diversification.
Evkaikina et al. (2016) noted that all these monilophytes have stems with a single apical cell, while Marattiales and Osmundales have a group of apical cells.
[Hymenophyllales + Gleicheniales] [if a clade]: stem protostelic.
Age. The age for this node in Y. L. Qiu et al. (2007) was estimated to be (283-)273(-259) Ma, in Rothfels et al. (2015b) (276.7-)237.2(-192.4) Ma and in C.-H. Huang et al. (2019) to be ca 281.4 Ma.
The fossil Hopetedia praetermissa from the Late Carnian (Jurassic, ca 230 Ma) of North Carolina is assignable to stem-group Hymenophyllaceae (Axsmith et al. 2001).
Phylogeny. Although Pelosi et al. (2022) recovered the contents of these two orders as a clade, Gleicheniales were not monophyletic (support not strong); the two are successive sister taxa in the plastome tree of X.-Y. Du et al (2022).
HYMENOPHYLLALES A. B. Frank
Just the one family, 9 genera, 435 species.
HYMENOPHYLLACEAE Martinov - Back to Polypodiopsida
Plants usu. epiphytes; mycorrhizae uncommon; stem protostelic; axillary buds +; petiole vasculature ?; fronds 1 cell thick between veins, stomata 0, cuticle 0; sorus receptacle at veinlet apex; spores globose, chlorophyllous; gametophyte filamentous or ribbon-like, irregularly branched, initially triradiate [developing from each of three cells with radiating walls dividing spore/three cells cut off from tips of spore], gemmiferous or not, mycorrhizae 0; embryo not with tetrad/octant formation; x = 36; rpl23-trnI-trnL gene sequence in the LSC.
9/540, [list, to subfamilies]. Especially tropical, temperate
Age. Crown Hymenophyllaceae are estimated to be (191.7-)173.6(-155.1) Ma (Hennequin et al. 2008), (194.7-)176.2(-156.5) Ma (Del Rio et al. 2017a), (190.4-)185.1(-174.7) Ma (Schuettpelz & Pryer 2009), ca 243 Ma (Testo & Sundue 2016), ca 250 Ma (Dubuisson et al. 2021) or ca 228.8 Ma (C.-H. Huang et al. 2019).
1. Hymenophylloideae Burnett - Hymenophyllum Smith
Frond (>2 cells thick), venation usu. anadromous; indusium ± bivalved (tubular), receptacle usu. rounded, included; n = 11 ... 36.
1/350. Tropical and N. (few) and S. temperate.
Age. Crown-group Hymenophylloideae are some (107.9-)85(-62.1) Ma (Hennequin et al. 2008).
Fossils that have been placed in or near Hymenophyllum are known from the Early Cretaceous of Mongolia 125–99.6 Ma (Herrera et al. 2017b) and also from deposits in the Atlantic U.S.A., Spain, etc. (Skog & Sender 2022: Acrostichopteris longipennis similar to Hymenophyllum fucoides).
2. Trichomanoideae C. Presl —— Synonymy: Trichomanaceae Burmeister
(Rhizome erect); (roots 0); venation usu. ana- and catadromous; indusium ± tubular, receptacle growing basally, protruding, hair-like; n = 32, 33, 36, genome duplication +.
8/190: Trichomanes (70), Didymoglossum (>30), Crepidomanes (>25). Tropics, some temperate.
Age. The age of this subfamily is estimated to be (159.6-)144(-128.4) Ma (Hennequin et al. 2008), but uncertainties in topology and different methods of analysis yield very different estimates; another estimate is around 210 Ma (Dubuisson et al. 2021).
Evolution: Divergence & Distribution. For ages of clades in the family, see also Hennequin et al. (2008). Diversification in Trichomanes is estimated to have begun in the middle of the Jurassic and that in Hymenophyllum in the middle of the Cretaceous (Schuettpelz & Pryer 2007), ca 147.3 versus ca 41.9 Ma (Schuettpelz & Pryer 2009), or ca 90.5 versus 49.8 Ma (Del Rio et al. (2017a); see also Schuettpelz and Pryer (2007) for other dates.
Dubuisson et al. (2021) discussed diversification of Trichomanes in the African-Madagascan region; T. crenatum, from West Africa, represents an independent movement from South America ca 13 Ma or so, an age in line with those of geographically isolated angiosperms from the same area of Africa (Bromeliaceae, Clusiaceae, etc.) whose immediate relatives are also in tropical America. Trichomanes subgenus Afrotrichomanes, also African, perhaps represents an older African-American vicariance event; vicariance events are rather uncommon in ferns (Dubuisson et al. 2021).
Del Rio et al. (2017a) discussed the evolution of Hymenophyllum on New Caledonia, where they found that the ten species that they sampled represented eight dispersal events, probably from New Zealand - there are 17 species of the genus in New Caledonia.
Dubuisson et al. (2021) looked at the evolution of a number of features in the family with a focus on Trichomanes s. str.. For gametophyte variation and evolution in Hymenophyllaceae, see e.g. Stokey (1940), Atkinson and Stokey (1954), Dassler and Farrar (1997), etc..
Schuettpelz and Pryer (2007) discuss the rate of molecular evolution of Hymenophyllaceae; there is an apparent slow-down in Hymenophyllum itself.
Ecology & Physiology. Around half the family are epiphytic (Zotz 2013), and there are also climbing taxa (see Dubuisson et al. 2009 for growth forms in Hymenophyllum). The epiphytic habit in Trichomanes evolved before that in Hymenophyllum, the plants probably growing on the stems of Cyatheaceae on which species of Trichomanes are still often to be found (Hennequin et al. 2008; see also Lehnert & Krug 2019). The epiphytic habit is likely to have been ancestral in Hymenophylloideae (Hennequin et al. 2008; Schuettpelz & Pryer 2009), while in Trichomanoideae the terrestrial habit is more common, but there is also a substantial clade of epiphytic taxa. When the epiphytic habit evolved is rather unclear, but some time between the early Mid Jurassic and the later Cretaceous seems likely (Hennequin et al. 2008). Lehnert and Krug (2019) emphasised that in the family as a whole the epiphytic taxa are low epiphytes, that is, they grow on the trunk and lower branches and quite close to the ground (the story in Lehnert et al. 2017 is rather complex). Despite the apparently delicate nature of the fronds of Hymenophyllaceae and their poikilohydrous metabolism, desiccation tolerance is at least sometimes well developed, rather like mosses (Proctor 2003, 2012). Indeed, the sporophytes of some epiphytic trichomanoid ferns have lost both cuticle and roots ("regressive evolution" - Dubuisson et al. 2011), taking up water via their fronds (Nitta et al. 2021), and the plants may then be functionally very similar to bryophytes; the stem stele may have just a single vascular element (Dubuisson et al. 2013; see also Dubuisson et al. 2003b). In a study of Hymenophyllaceae from the Pacific island of Moorea, Nitta et al. (2021) found that the gametophytes had similar or lower desiccation tolerance than the sporophytes, and also had lower light photosynthetic optima. Interestingly, there was no connection between desiccation tolerance and whether or not gametophytes grew outside of the range of the sporophyte here; Nitta et al. (2021) also looked at the phylogenetic signal in these attributes of the plants (there was some for desiccation tolerance). Gametophytes that can live independently of the sporophyte are quite common here (Yoneoka et al. 2024).
Fertilization & Spore Dispersal. The spores have chloroplasts and germinate while still in the sporangium (Iwatsuki & Ebihara 2023).
Plant-Bacterial/Fungal Associations. The filamentous or ribbon-like gametophytes and rootless sporophytes - and even the rhizomes of the latter - are thin and wiry, and this, associated with the epiphytic habitat, means that mycorrhizae are rather uncommon in Hymenophyllaceae (Pressel et al. 2016). However, dark septate endophytes are common in Hymenophylloideae, possibly the ancestral condition for the clade, while in Trichomanoideae arbuscular mycorrhizal associations are much more common (Lehnert et al. 2016; Lehnert & Krug 2019: optimization seems rather erratic).
Genes & Genomes. Hennequin et al. (2010) suggest as a possible base chromosome number in the family x = 36 - previous suggestions are x = 6-9, 11, 13... For a whole genome duolication in Trichomanoideae, see Pelosi et al. (2022)
Kuo et al. (2018b) outline the extensive variation in the chloroplast genome, where there are i.a. rearrangements in the long single copy region (see also Ruíz-Ruano et al. 2018; X.-Y. Du et al. 2022).
Chemistry, Morphology, etc.. For general information, see Iwatsuki and Ebihara (2023). Stokey (1940) described spore germination/the morphology of the young gametophyte, and H. Schneider (2000) rootlessness in some Trichomanoideae.
Phylogeny. For the phylogeny of Hymenophyllaceae, see Pryer et al. (2001b) and Dubuisson et al. (2003a, 2013), and for that of Trichomanes and relatives, see Ebihara et al. (2007) and Baurat et al. (2015). Hennequin et al. (2003, 2006) looked at relationships within Hymenophyllum, in the latter paper focussing on the large subgenus Mecodium; this turned out to be polyphyletic, species being in a number of basal clades. Vasques et al. (2019) found that subgenus Mecodium s. str. was sister to subgenus Hymenophyllum and was made up of two clades, both with Old World-New World distributions that are largely non-overlapping. For Pacific species of Hymenophyllum, see Ebihara et al. (2010).
Classification. This is particularly problematic in Hymenophyllaceae, anything from two to 34 genera being recognised, but I tentatively follow Ebihara et al. (2006; see also The Pteridophyte Phylogeny Group 2016), who include infrageneric groupings, a full synonymy, etc.. However, Ohlsen (2020) noted that genera in the 9-genus classification of the family were less well defined morphologically than in a 2-genus classification (Hymenophyllum + Trichomanes), so the latter does have its advantages.
[Gleicheniales [Schizaeales + Core Leptosporangiates]] (if the clade exists): (spores monolete, perine closely attached to exine).
Age. The stem group age of this node is ca 276.4 Ma (Schuettpelz & Pryer 2009), but see Gleicheniaceae.
GLEICHENIALES Schimper
Root stele with 3-5 protoxylem poles; rhizome protostelic, with scales; frond with 2 (+) axes, veins anastomosing; sporangium maturation simultaneous; gametophyte (axial - Stromatopteris), (rhizoids septate); antheridia with 6-12 narrow curved or twisted cells in walls; x = 20, ... 116, nuclear genome [1 C] 2.96 pg [?sampling]. 3 families, 10 genera, 172 species.
Includes Dipteridaceae, Gleicheniaceae, Matoniaceae - but note that relationships are [... [Gleicheniaceae [Matoniaceae + Dipteridaceae]...]] in X.-Y. Du et al. (2022) and that Hymenophyllaceae are embedded in this group in C.-H. Huang et al. (2022).
Age. Crown-group Gleicheniales are Permian and ca 263 Ma or 262.2 Ma (Pryer et al. 2004; Schuettpelz & Pryer 2009) or (252.4-)196.1(-134) Ma (Rothfels et al. 2015b).
Extensive fossil material of Chansitheca wudaensis, identified as Gleicheniaceae, is found in volcanic tuff deposits from the lowermost Permian in Mongolia that have been dated to 298.3 Ma (X. He et al. 2021), thus providing a minimum crown-group age for the order.
Evolution: Ecology & Physiology. Aluminium accumulation is quite common in Gleicheniales (Schmitt et al. 2017).
Genes & Genomes.For possible changes in the plastome around here - exactly where they might go depends on the topology - see X.-Y. Du et al. (2022).
Synonymy: Dipteridales Doweld, Matoniales Reveal, Stromatoperidales Reveal
GLEICHENIACEAE C. Presl - Back to Polypodiopsida
Rhizome with protostele (solenostele), projections of epidermis with peltate or basifxed scales [= squamophores]; petiole vasculature incurved U-shaped; frond growth indeterminate, pseudodichotomously forked (not - Stromatopteris), (plant scrambling); pseudostipules +/0; indusium 0, annulus transverse; spores (bilateral), with exospore flange, trilete or monolete; (embryo shoot apex exoscopic [developing towards micropyle/archegonial neck]), first cell wall vertical; gametophyte (white, subterranean, mycoheterotrophic, axial), with clavate hairs; x = 22, 34, etc., genome duplication +.
8/149 [list]: Sticherus (91), Dicranopteris (23). Pantropical, including New Caledonia.
Age. Lima et al. (2023) estimated that crown-group Gleicheniaceae were (125.0-)123.4(-121.8) Ma.
Fossil evidence suggests that Gleicheniaceae may be much older than the age just given, dating back to the Permian (Skog 2001), and fossils of the family are found along with those of Matoniaceae, Marattiaceae, etc., from deposits in the central Transantarctic Mountains from the early Middle Triassic ca 225 Ma (Klavins et al. 2001), while Chansitheca wudaensis (ca 298 Ma, early Permian) seems to be the first clearly gleicheniaceous fossil (He et al. 2020); see also ordinal ages above.
Evolution: Divergence & Distribution. For repeated long-distance dispersal of Gleichenia between Australia and New Zealand and New Caledonia within the last five million years, see Ohlsen et al. (2022).
Plant-Bacterial/Fungal Associations. Stromatopteris has a mycoheterotrophic gametophyte, but the identity of the fungus is not known (Merckx et al. 2013a; Imhof et al. 2013).
Genes & Genomes. Pelosi et al. (2022) discuss a genome duplication in Gleicheniaceae.
There has been a chloroplast genome inversion in the family (Wolf & Roper 2008).
Chemistry, Morphology, etc.. For general information, checklist, etc., see Lima et al. (2024).
Phylogeny. H. Liu et al. (2020b) found that Gleichenia boryi, from Madagascar and environs, in fact represented a distinct lineage (= Rouxopteris) that has remained separate since the Cretaceous. Lima et al. (2023: 70 spp., plastome and GoFlag data) found that genera were monophyletic in both analyses, but Sticherus was para- or polyphyletic; past reticulation/polyploidization is likely in the family. Lima et al. (2024) Ohlsen et al. (2022: plastid markers) found that there were three phylogenetically quite distinct but morphologically very similar lineages in the Australian Gleichenia dicarpa.
[[Dipteridaceae + Matoniaceae] [Schizaeales + Core Leptosporangiates]] (if the clade exists - see X.-Y. Du et al. 2022): plastome inversion from ndhB to psbA in the LSC region, IR expansion includes the second exon of the ndhB gene.
[Dipteridaceae + Matoniaceae]: ?
DIPTERIDACEAE Seward & E. Dale - Back to PolypodiopsidaPetiole vasculature inverted Ω-shaped bundle; stomata paracytic; frond (dissected), fine venation reticulate, with internally directed veins, density 4.4-5.6 mm/mm2, (venation dichotomous); sori (± disordered), sporangia with "short" stalks, annulus oblique; spores (bilateral, monolete); n = 33, genome duplication +.
2/11: [list], Dipteris (7). N.E. India to N.E. Australia.
Evolution: Divergence & Distribution. There are fossil Dipteridaceae from the middle Triassic in the Northern and especially Southern Hemispheres, and in the later Mesozoic/early Caenozoic they were very widespread, with at least six extinct genera (Choo & Escapa 2017; Yañez et al. 2023).
Pelosi et al. (2022) discuss a whole genome duplication that characterises the family as a whole, and there may be another in Dipteris itself.
Phylogeny. For relationships in the family, including its fossil relatives, see Choo and Escapa (2017). Vegetative features of the fossil Goepertella seem to be apormophies linking it with Dipteridaceaem reproductive features such as sporangium arrangement plesiomorphies that it has in common with Matoniaceae (Yañez et al. 2023),
MATONIACEAE C. Presl —— Synonymy: Cheiropleuriaceae Nakai - Back to Polypodiopsida
Stems solenostelic, with two vascular cylinders and a central bundle; petiole vasculature ± inverted Ω-shaped bundle; fronds or pinnae ± dichotomously branched, free-ending veinlets 0; sporangia in ring surrounding central "receptacle", sorus indusiate; x = 25, 26.
2/4: [list], Matonia (2), Phanerosorus (2). Malesia.
Age. Tomaniopteris, from the eaerly Middle Triassic of Antarctica, is the earliest fossil to be placed in Matoniaceae (Klavins et al. 2004).
Evolution: Divergence & Distribution. Fossil Matoniaceae have a world-wide distribution (Klavins et al. 2004).
[Schizaeales + Core Leptosporangiates [= Salviniales [Cyatheales + Polypodiales]]]: plant with hairs; endospore 2-layered; antheridium wall ca 3 cells across; plastome with “reversed” gene orientation [= two overlapping inversions].
Age. (281-)266(-250) Ma is the age for this node in Y. L. Qiu et al. (2007), ca 264.6 Ma in Schuettpelz and Pryer (2009), (289.4-)258.3(-235.2) Ma in Rothfels et al. (2015b) and ca 265.8 Ma in H.-C. Huang et al. (2020).
Evolution: Genes & Genomes. For the evolution of the “reversed” gene orientation in the IR region that can be placed at this node, see X.-Y. Du et al. (2022); Dipteridaceae and Matoniaceae are described as being intermediate, so apomorphies associated with this change may have to be modified.
SCHIZAEALES Schimper
Stem protostelic, solenostelic; stomata notably variable; fronds differentiated into fertile/sterile portions [hemidimorphic]; petiole vasculature?; sporangia on leaf segments lacking laminar tissue, annulus sub-apical, transverse, continuous; archegonium venter free; n = 28-504. - 3 families, 4 genera, 198 species.
Age. The crown-group age of Schizaeales is estimated to be around 218.4 (Schuettpelz & Pryer 2009), 183.6 Ma (Hennequin et al. 2008) or 208.9 Ma (C-H. Huang et al. 2020).
Includes Anemiaceae, Lygodiaceae, Schizaeaceae
LYGODIACEAE M. Roemer - Lygodium Swartz - Back to Polypodiopsida
Fronds indeterminate, climbing, pseudodichotomously branched, with a bud in angle of branch; one sporangium/sorus, subtended by antrorse indusium-like flange; n = 28-30, x = 30.
1 [list]/29 (22-49). Tropical and warm temperate.
Evolution: Divergence & Distribution. There has been extensive hybridization in Lygodium (Pelosi et al. 2024).
Plant-Animal Interactions. Species of Lygodium are noted for often having nectaries (Suissa et al. 2024).
Phylogeny. Sampling was comprehensive; matrices were based on the GoFlag 408 flagellate land plant probe set and 43 plastid loci (Pelosi et al. 2024).
Botanical Trivia. The fronds may be up to 30 m or so long.
[Anemiaceae + Schizaeaceae]: sporangia not in sori, indusium 0; chloroplast psaM gene 0.
ANEMIACEAE Link - Anemia Swartz - Back to Polypodiopsida
Hydroregulation various, plants dessication tolerant; rhizome with dictyostele or solenostele, (with pockets axillary to the fronds); spores tetrahedral, with parallel solid ridges (ridges hollow, centre spongy); x = 38.
1 [list]/130. Widespread.
Evolution: Divergence & Distribution. Anemia spores were abundant in North America after the K/P bolide impact (Berry 2020).
Phylogeny. For a largely well-resolved phylogeny of the family and optimisation of characters on to the tree, see Labiak et al. (2015: spore morphology!).
SCHIZAEACEAE Kaulfman - Back to Polypodiopsida
Inner pericyclic cells 6, 8, thickened; fronds undivided or fan-shaped, veins dichotomizing; sporangia borne on marginal projections at blade tip; spores monolete, bilateral, smooth; gametophyte filamentous [Microschizaea], (white, subterranean, mycoheterotrophic, filamentous [Actinostachys] or tuberous), rhizoids septate, (borne 2-3 together on large, vacuolated cells, = rhizoidophores); (embryo shoot apex exoscopic [developing towards micropyle/archegonial neck], first cell wall vertical); chloroplast ndh, rps 16, ycf 94 genes 0, small single copy reduced in size; x = 77, 94, 103.
3/40 [list]: Schizaea (23)Actinostachys (17). Pantropical to temperate.
Evolution: Plant-Bacterial/Fungal Associations. Actinostachys has a mycoheterotrophic gametophyte, but it is not known what the fungus is (Merckx et al. 2013a; Imhof et al. 2013).
Genes & Genomes. For the plastome of Schizaeaceae, see Labiak and Karol (2017) and Ke et al. (2022); there has been expansion and contraction of the inverted repeat, and several genes have been lost, including ndh genes (Lehtonen & Cárdenas 2019; Mower et al. 2021 for possible connections between various distinctive life styles that might affect the photosynthetic process and the loss of such genes). There has been an inversion in the plastome somewhere around here (Wolf & Roper 2008); see also the next node up.
Phylogeny. Ke et al. (2022: 27 spp., 3 plastome genes; markers from LSC region, 11 spp.) looked at relationships in the family; Microschizaea is sister to the other taxa - although not all topologies recovered placed it in this position.
Classification. For generic limits here, still somewhat unclear, see Ke et al. (2022).
[Salviniales [Cyatheales + Polypodiales]] / Core Leptosporangiates: sporangium stalk 1-3 cells across [?position]; nuclear genome duplication [CYATγ]; plastome with double overlapping inversions in the rpoB-psbZ region.
Age. The age of this node is estimated to be around 234.7 Ma (Schuettpelz & Pryer 2009), (269.1-)231.6(-190.8) Ma (Rothfels et al. 2015b), about 240.2 Ma (X.-Y. Du et al. 2021), about 224.8 Ma (C.-H. Huang et al. 2019) or about 281 Ma (X. Huang et al. (2022).
Evolution: Genes & Genomes. The rate of evolution of the 18S nuclear gene was lower than in the other vascular plants examined (Stenøien 2008: lycophytes not included). For a possible nuclear genome duplication in this area, see F.-W. Li et al. (2018), H. Chen et al. (2022), Fang et al. (2022) and Marchant et al. (2022).
For the double inversion in the plastome, see Lehtonen and Cárdenas (2019). X.-Y. Du et al. (2022) describe them as a ca 4 kb inversion from trnC-GCA to trnE-UUC together with a ca 1 kb inversion from trnD-GUC to trnE-UUC.
SALVINIALES Link
Aquatics, mycorrhizal fungi 0; roots 0; stem solenostelic; aerenchyma +; veins ± anastomosing; stomata anomocytic; sterile/fertile frond dimorphism; sporocarp +, plant heterosporous, sporangia lacking annulus; megaspore 1 per megasporangium, m with acrolamella over the exine aperture; female gametophyte development endosporic; nuclear genome [1 C] ca 2.38 pg, [2 C] 1-2.4 pg; nrDNA with 5.8S and 5S rDNA in separate clusters. - 2 families, 5 genera, 82 species.Includes Marsileaceae, Salviniaceae.
Age. Crown-group Salviniales are estimated to be around 158.9 Ma (Hennequin et al. 2008), ca 186.8 Ma (Schuettpelz & Pryer 2009), (204.5-)153.5(-150) Ma (Testo & Sundue 2016), ca 191.2 Ma (C.-H. Huang et al. 2019) or ca 163.8 Ma (X.-Y. Du et al. 2021).
De Benedetti et al. (2020: esp. Table 1, see also 2017) compare fossil sporangia and mega- and microspores (there may be more than one of the former per megasporangium in Paleoazolla) throughout the group.
Chemistry, Morphology, etc.. See Nagalingum et al. (2006, 2007: sporocarp structure) and de Benedetti et al. (2020: spores and sporangia).
Phylogeny. Nagalingum et al. (2008) discuss the phylogeny of the group.
Synonymy: Marsileales von Martius, Pilulariales Berchtold & Presl
MARSILEACEAE Mirbel —— Synonymy: Pilulariaceae de Candolle - Back to Polypodiopsida
Stereome 0/mid-cortical; stomata respond to low fluence of blue light; fronds amphistomatous, simple, linear, or to 4 divisions/frond; sori heterosporangiate, in stalked bean-shaped sporocarps [folded pinnae]; spores perine with gelatinous layer; megaspore exine compact, permeated by canals; microspore surface ± rugulate; female gametophyte with 1 archegonium, venter free; male gametophyte: gamete cilia with spline 40-50 microtubules across [Marsilea]; n = 10, 19, 20, nuclear genome [1 C] 0.83-1.34 Gb.
3 [list]/70: Marsilea (65). Aquatics, tropical to temperate.
Evolution: Divergence & Distribution. Regnellidium may have moved from North to South America and thence to Antarctica during the Cretaceous and Paleogen (K. K. Tang et al. 2022 and references).
Ecology & Physiology. Westbrook and McAdam (2020) discuss the distinctive response of stomata of Regnellidium and Marsilea to low fluence of blue light, unusual in this part of the phylgeny, noting that it may be connected with the very high (for a fern) rate of gas exchange during photosynthesis.
Phylogeny. For a phylogeny of Marsilea and character evolution there, see Nagalingum et al. (2007).
SALVINIACEAE Martinov —— Synonymy: Azollaceae Wettstein - Back to Polypodiopsida
Plant free-floating; (lignin 0 - fronds, roots, Azolla); stereome 0; stomata epi-/amphistomatous, vestigial [guard cells perpendicular to long axis of the pore]; fronds sessile, 2-ranked, <2.5 cm long, simple; (sporocarp 0 - Salvinia); megaspore exine ± lacunose/porose, microspores in 1-10 massulae per microsporangium, (massulae glochidiate); sporophyte — gametophyte boundary smooth, cells vacuolate; n = 9, 22, nuclear genome [1 C] 0.23/0.26[Salvinia]-0.75 Gb.
2 [list]/21: Salvinia (12). Aquatics, tropical to warm temperate. Map: see Hermsen et al. (2019: Fig. 2, distribution of Azolla sporophytes with vegetative structures).
Age. The crown-group age of this clade may be around 89 Ma (Metzgar et al. 2007; Hennequin et al. 2008) or 99 Ma (X. HUang et al. 2022).
Fossils of Azolla are known from the Upper Cretaceous, thus fossil floats of the North American A. montana (Hall & Swanson 1968) are perhaps 70 Ma while sporophytes of Azolla are known from rocks as old as 72-66 Ma from Patagonia, Argentina (Hermsen et al. 2019).
Evolution: Divergence & Distribution. There was substantial turnover of genera around about the K/PG transition-early Palaeogene (Collinson et al. 2013; de Benedetti et al. 2018).
Ecology & Physiology. Azolla has a cyanobacterium often called Nostoc in its tissues and this is an important nitrogen fixer in rice paddies, etc.; it can grow very fast and fix large amounts of carbon (Dijkhuizen et al. 2018). Fronds of A. filiculoides contain N. azollae (the cyanobacterium is vertically transmitted, motile filamentous hormogonia entering the sporocarp), and massive amounts of biomass are produced - ca 39 t ha-1 yr-1, around one quarter of which is protein; gram negative rhizobial bacteria are also very common in the leaf pockets where Nostoc lives, but they do not fix nitrogen (Dijkhuizen et al. 2018). The symbiosis is estimated to have begun around 100 Ma (F.-W. Li et al. 2018) or perhaps as much as 140 Ma (Ran et al. 2010).
Over a period of perhaps 800,000 years in the middle Eocene ca 50 Ma Azolla covered vast areas of the Arctic Ocean, there being episodic events when the surface water was ± fresh - this was the Azolla event (Brinkhuis et al. 2006; F.-W. Li et al. 2018). It has been suggested that Azolla sequestered so much CO2 at this tome that it contributed to global cooling, drawing down an estimated 55-470 ppm pCO2 and contributing to the beginning of the greenhouse → icehouse earth transition and the associated Grand Coupure (Speelman et al. 2009) - other estimates suggest early Eocene pCO2 dropping from perhaps 2000 ppm to ca 600 ppm (Whaley 2007). Scheffer et al. (2003) discuss the conditions in which floating aquatics come to dominate.
For the water repellancy of Salvinia fronds, see Steigleder and Roldo (2018) and references. The dense hairs are each like little egg beaters, and air is trapped inside the beater "cage", however, the very apex of the cage has no wax and attracts water; the overall result is that a layer of air close to the leaf surface is trapped, water may form droplets, but they roll off the leaf.
Plant-Bacterial/Fungal Associations. The association of Azolla with Nostoc is obligate, and there has apparently been cospeciation in this association, but with one host switch; there has been quite extensive loss or pseudogenization of some housekeeping genes in Nostoc (Ran et al. 2010; Li et al. 2018). Note that the common symbiosis pathway, involved in both arbuscular mycorrhizal and legume rhizobial associations, is not involved here (Li et al. 2018). Ran et al. (2010) and Warshan et al. (2018) discuss the relationships of N-fixing members of Nostoc, and show that N. azollae is unrelated to Anabaena or other species of Nostoc.
Vegetative Variation. Salvinia produces two floating leaves, a bud, and a submerged leaf at each node, and as the bud develops, it produces additional buds... (Lemon & Posluszny 1997). Croxdale (1978) describes the complexity of development of Salvinia leaves, including that of the stomata, which apparently remain open.
Evolution: Genes & Genomes. F.-W. Li et al. (2018) summarize genome size, chromosome numbers and many other aspects of genome evolution here in their study of the genomes of Azolla filiculoides and Salvinia cucullata (see also Sessa & Der 2016; Heslop Harrison et al. 2022). There seems not to be a genome duplication at this level of the whole order, but there is perhapa a duplication in Azolla, at least (H. Chen et al. 2022).
Chemistry, Morphology, etc.. Salviniaceae have distinctive stomata (Westbrook & McAdam 2020 and references).
Phylogeny. For relationships in Azolla, see Metzgar et al. (2007).
[Cyatheales + Polypodiales]: hydathodes +; foliar aeration by aerophores, lateral on leaf axis, stomata-bearing, stereome discontinuous; IR with several large inversions, ycf2 duplication.
Age. The suggested age of this node is ca 211 Ma in Y. L. Qiu et al. (2007), ca 223.2 Ma in Schuettpelz and Pryer (2009), (270.1-)228.8(-187.5) Ma in B. Zhong et al. (2014b), (238.1-)204.6(-179) Ma in Rothfels et al. (2015b), ca 212.0 Ma in C.-H. Huang et al. (2019) and ca 234.8 Ma in X.-Y. Du et al. (2021).
Evolution: Divergence & Distribution. C.-H. Huang et al. (2019) place a genome duplication on the stem of this node and suggest that diversification shows an increase.
Ecology & Physiology. Aluminium accumulation is quite common here (Schmitt et al. 2017). Moran (2022) discussed the evolution of aerophores, which associated with the interruption of the stereome, allows oxyggen to move into petiolar tissues.
CYATHEALES A. B. Frank
Stem (polycyclic) solenostelic; petiole vasculature complex, ± inverted Ω-shaped; stomata mostly paracytic; hairs +; sori terminal on veins, indusiate, indusium with outer and inner parts; sporangium stalk ca 5 cells across, annulus oblique; antheridium walls ³5 cells across; nuclear genome [1 C] 6.48-9.63 pg, genome duplication +. - 8 families, 13 genera, 745 species.
Includes Cibotiaceae, Culcitaceae, Cyatheaceae, Dicksoniaceae, Loxsomataceae, Metaxyaceae, Plagiogyriaceae, Thyrsopteridaceae.
Age. The crown-group age of Cyatheales is ca 186.7 Ma (Schuettpelz & Pryer 2009) or rather younger, (167.8-)109.1(-56.8) Ma (Rothfels et al. 2015b: Alsophila sister to the rest); C.-H. Huang et al. (2019) suggest an age of around 104.2 Ma.
For the fossil history of Cyatheales, see Vera and Herbst (2016: solenosteles and dictyosteles).
Evolution: Divergence & Distribution. Ramírez-Barahona (2024) integrated our understanding of the rich fossil history of Cyatheales with phylogeny when thnking about the biogeography of the order. He suggested that the phylogeny and distribution of extant members of the order suggested a Gondwanan origin, however, if one added one's understanding of the fossil record, then an origin of Cyatheales in Laurasia seemed more likely, with subsequent movement on to Gondwana, etc.; in any event, there seems to have been extensive movements of members of the group over time. Thus Ramírez-Barahona (2024) noted that Thyrsopteris, currently known only from Juan Fernandez Island and so perhaps a Gondwanan element in that flora, was once quite widely distributed in the northern hemisphere, and so agreed with the idea of a Laurasian origin of the order. Ramírez-Barahona (2024) mentioned Coniopteris as a good example of problems with the fossil record; some fossils placed in that genus had an oblique, complete annulus and were probably Cyatheales, and they were from Laurasia; on the other hand the numerous fossils from Antarctica, although rarely with sporangia, possibly belonged to Polypodiales, members of which have a vertical and incomplete annulus (see also C. Li 2019b).
There seems to have been a slow-down in the rate of evolution at the base of Cyatheales (Rothfels et al. 2015b and references). Hybridization and polyploidy are rather uncommon here, but c.f. Cyatheaceae for an example.
Nectaries are quite common in Cyatheales (Suissa et al. 2024), but it is unclear exactly what taxa include them.
Genes & Genomes. For a possible genome duplication here and elsewhere in this family group, see H. Chen et al. (2022) and Pelosi et al. (2022). Chloroplast and nuclear genes in Cyatheales have lower and less variable substitution rates that those in Salviniales and Polypodiales (Chen et al. 2022).
Phylogeny. Lantz et al. (1999) discuss the phylogeny of the group using morphological analyses of extant and of fossil + extant taxa. X.-Y. Du et al. (2022) recovered the relationships [[Metaxyaceae, Cibotiaceae, Dicksoniaceae, Cyatheaceae] [Thyrsopteridaceae [Loxsomataceae [Culcitaceae + Plagiogyriaceae]]]] - or relationships may be [[Thyrsopteridaceae [Loxsomataceae [Culcitaceae + Plagiogyriaceae]]] [Metaxyaceae [Dicksoniaceae [Cibotiaceae + Cyatheaceae]]]], while the relationships that Ramírez-Barahona (2024: 101 fossils, 442 spp. Cyatheales, 10 plastid regions) used to think about major patterns in the evolution and in particular biogeography of the families in the order are [[Thyrsopteridaceae [Loxosomataceae [Plagiogyriaceae + Culcitaceae]]] [Metaxyaceae [Cyatheaceae [Dicksoniaceae + Cibotiaceae]]]].
Classification. Note that Christenhusz and Chase (2016) recognized but a single family in Cyatheales, all the families below being subfamilies. In truth, neither the family groupings nor the families themselves are exactly bursting with apomorphies.
Synonymy: Dicksoniales Reveal, Hymenophyllopsidales Reveal, Loxsomatales Reveal, Metaxyales Doweld, Plagiogyriales Reveal
[Thyrsopteridaceae [Loxsomataceae [Culcitaceae + Plagiogyriaceae]]]: ?
Age. Estimates of the age of the clade immediately above are ca 90.7 Ma (C.-H. Huang et al. 2019) or (214.2-)179.0(-143.8) Ma (Ramírez-Barahona 2024: Table S1).
THYRSOPTERIDACEAE C. Presl - Thyrsopteris elegans Kunze - Back to Polypodiopsida
Indusium cup-shaped, receptacle columnar, clavate; n = ca 78.
1 [list]/1. Juan Fernandez I..
Evolution: Divergence & Distribution. Despite the current limited distribution of Thyrsopteris to an oceanic island, fossils assigned this family are quite widespread in the Southern Hemisphere; they have recently been found in amber from Myanmar that is ca 99 Ma (C. Li et al. 2019), indeed, in the past it was quite widely distributed in the Northern Hemisphere (Ramírez-Barahona 2024).
[Loxsomataceae [Culcitaceae + Plagiogyriaceae]]: ?
Age. This clade is maybe (168.0-)140.1(-118.4) Ma (Ramírez-Barahona 2024: Table S1).
LOXSOMATACEAE C. Presl - Back to Polypodiopsida
Petiole vasculature complex; indusium urceolate, receptacle elongate, often exserted; gametophyte with scale-like hairs; n = 46, 50.
2/2: [list]. Scattered, Costa Rica to Bolivia (Loxsomopsis), northern New Zealand.
Age. The crown age of Loxsomataceae is estimated to be (62.5-)32.9(-9.4) Ma (Ramírez-Barahona 2024: Table S1).
[Culcitaceae + Plagiogyriaceae]: genome duplication +.
Age. This clade is maybe (107.7-)74.9(-48.3) Ma (Ramírez-Barahona 2024: Table S1).
Evolution: Divergence & Distribution. Pelosi et al. (2022) noted that a genome duplication characterised this clade.
CULCITACEAE Pichi Sermolli - Culcita C. Presl - Back to Polypodiopsida
Petiole vasculature inverted Ω-shaped; outer indusium scarcely differentiated; sori with paraphyses; n = 66.
1/2: [list]. ± Tropical, scattered, to New Caledonia, also Azores to the Iberian Peninsula, not Africa.
Age. This family is some (43.0-)19.2(-3.1) Ma (Ramírez-Barahona 2024: Table S1).
PLAGIOGYRIACEAE Bower - Plagiogyrium (Kunze) Mettenius - Back to Polypodiopsida
1 root/petiole; petiole vasculature U- or V-shaped bundle, (three elliptic meristeles), pneumatophores (0); stomata anomocytic alone; fronds dimorphic, when young with dense, pluricellular, mucilage-secreting hairs; indusium 0, frond margin recurved [= false indusium]; n = 62, 64-66, 75.
1/15: [list]. Eastern India and Japan to the Solomon Islands, Mexico to Brazil, the Antilles, often montane.
Age. The age of crown-group Plagiogyriaceae is some (61.9-)39.9(-20.3) Ma (Ramírez-Barahona 2024: Table S1).
Evolution: Genes & Genomes. C. H. Huang et al. (2020) suggest that there is a genome duplication associated with Plagiogyria, at least.
Chemistry, Morphology, etc.. See X.-C. Zhang and Noteboom (1998: revision) and R-x. Wang et al. (2018: cytology).
[Metaxyaceae [Cyatheaceae [Cibotiaceae + Dicksoniaceae]]]: petiole vasculature with multiple traces coming from a ± U-shaped bundle; paraphyses +.
Age. This clade may be some 159 Ma (Hennequin et al. 2008), ca 78.4 Ma (C.-H. HUang et al. 2020) or as much as (207.9-)188.1(169.6) Ma (Ramírez-Barahona 2024: Table S1).
METAXYACEAE Pichi Sermolli - Metaxya C. Presl - Back to Polypodiopsida
Indusium 0; n = 95, 96.
1 [list]/6. Tropical South America.
Age. Crown-group Metaxyaceae is maybe (27.2-)15.9(-62.6) Ma (Ramírez-Barahona 2024: Table S1).
[Cyatheaceae [Cibotiaceae + Dicksoniaceae]]: ?
Age. The age of this clade, if it exists, is ca 150 Ma (Janssen et al. 2008) or ca 157 Ma (Noben et al. 2017) or (197.8-)178.9(-159.6) Ma (Ramírez-Barahona 2024: Table S1); Loiseau et al. (2020) suggested that stem-group Cyatheaceae were (172.4-)157.8(-147.5) Ma.
CYATHEACEAE Kaulfman —— Synonymy: Alsophilaceae Presl - Back to Polypodiopsida
Usually trees; roots diarch, not medullated; stem with polycyclic dictyostele, medullary bundles +; (pit aerophores +); scales +, large (also small), (extrafloral nectaries +), (spines + - Alsophila); fronds large; sori not marginal, indusium 0 to completely surrounding sporangia; genome duplication?; 1 C = 6.48-9.63 pg; x = 69.
3/645: [list]: Alsophila (275), Cyathea (265), Sphaeropteris (105). Pantropical (to sub-Antarctic - Alsophila).
Age. Crown-group Cyatheaceae are estimated to be ca 96 Ma (Janssen et al. 2008), (136.8-)117.8(-101.1) Ma (Loiseau et al. 2020) or(139.4-)121.8(-104.8) Ma (Ramírez-Barahona 2024: Table S1); see also H. Yi et al. (2023).
Evolution: Divergence & Distribution. For various dates within Cyatheaceae, see Loiseau et al. (2020).
Korall and Pryer (2014) outline major biogeographic patterns in the group; initially Gondwanan vicariance seems to be involved, although crown-goup diversification did not begin until ca 96 Ma with subsequent rather limited (for ferns) transoceanic long distance dispersal. The 100+ endemic species of Cyathea on Madagascar may represent a Pliocene diversification (4.24-)3.07> Ma of three separate clades each of which has a fairly lengthy sojourn on the island - thus one Malagasy clade hung around for 30 Ma before it diversified (Janssen et al. 2008, see also Korall & Pryer 2014; Testo & Sundue 2016 for very high speciation and extinction rates). For hybridization, introgression and cryptic diversity in Gymnosphaera and Alsophila, see H. Yi et al. (2023).
Bystriakova et al. (2011) discussed niche evolution. Ramírez-Barahona et al. (2016) suggested that there was parallel evolution in plant size (trunk and frond length) and that climatic niche evolution and diversification rates were correlated. Loiseau et al. (2020), on the other hand, thought that rates of evolution had been more or less constant over time. Extrafloral nectaries occur on the fronds of some species of Cyathea (Mehltreter et al. 2021).
Genes & Genomes. Although the base chromosome number for the family is high, there is little recent polyploidy, unlike other ferns (X. Huang et al. 2022). However, J. Wang et al. (2020) discussed allopolyploid hybridization that produced Gymnosphaera [= Alsophila] metteniana; there has been subsequent gene flow from the diploid parents via unreduced gametes.
X. Huang et al. (2022) suggest that there is a family-wide WGD event in addition to an older event; C.-H. Huang et al. (2019) associate a duplication with Alsophila.
The rate of plastome evolution has slowed down considerably here, perhaps because of the long generation time of tree ferns (P. Soltis et al. 2002; esp. B. Zhong et al. 2014b); this slow-down involves the nuclear genome, too (Huang et al. 2022).
Phylogeny. See Korall et al. (2006, 2007) for a phylogeny; Cyathea and Sphaeropteris are sister taxa. More recently Dong and Zuo (2018: 5 chloroplast gene regions) found four main clades, Gymnosphaera, ex Alsophila, being recognised, and it was sister to Alsophila, the combined group being sister to Cyathea.
[Cibotiaceae + Dicksoniaceae]: genome duplication.
Age. This clade is estimated to be (189.5-)166.7(-144.7) Ma (Ramírez-Barahona 2024: Table S1).
Evolution: Divergence & Distribution. For a genome duplication characterising this clade, see Pelosi et al. (2022); Metaxyaceae were not sampled.
CIBOTIACEAE Korall - Cibotium Kaulfuss - Back to Polypodiopsida
Stomata with three subsidiary cells; spores with equatorial flange, usu. parallel ridges on distal face; n = 68.
1/9: [list]. Tropical, Central America, Southeast Asia to Malesia, Hawaii.
Age. The age of crown-group Cibotiaceae is estimated to be (45.0-)23.4(-8.3) Ma (Ramírez-Barahona 2024: Table S1).
DICKSONIACEAE M. R. Schomburgk —— Synonymy: Lophosoriaceae Pichi-Sermolli
- Back to PolypodiopsidaUsually trees; not Al accumulators; sori marginal, vein-related, outer valve formed by reflexed frond segment margin and often differently coloured from the other, receptacle conoid, sporangium development basipetal; n = 56, 65.
3/35: [list], Dicksonia (20-25). Tropical America, St Helena, Malesia to the Antipodes and New Caledonia.
Age. The crown-group age of Dicksoniaceae is estimated to be ca 135 Ma (Noben et al. 2017), (152.0-)136.9(-117.3) Ma (Loiseau et al. 2020) or (155.5-)138.1(-124.3) Ma (Ramírez-Barahona 2024: Table S1).
Evolution: Divergence & Distribution. Dicksoniaceae (but not Calochlaena) have quite a rich fossil record from ex-Gondwanan continents and the family has a basically Gondwanan distribution today. Note that the three genera have diversification fuses of 80-110 Ma, and some species of Dicksonia have estimated ages older than the islands they currently inhabit, so understanding the biogeography of the family is tricky (Noben et al. 2017).
Phylogeny. For the phylogeny of Dicksoniaceae, with Calochlaena sister to the rest, see Noben et al. (2017).
POLYPODIALES Link
Roots black, wiry [?level]; rhizome dorsiventral [?level]; petiole with a single vascular bundle; sporangial maturation mixed; stalk 1-3 cells thick, annulus vertical, interrupted by stalk and stomium; neochrome/phy 3 + [?distribution]. 25 families, genera, species.
Includes Aspleniaceae, Athyriaceae, Blechnaceae, Cystodiaceae, Cystopteridaceae, Davalliaceae, Dennstaedtiaceae, Desmophlebiaceae, Didymochlaenaceae, Diplaziopsidaceae, Dryopteridaceae, Hemidictyaceae, Eupolypod I, Eupolypod II, Eupolypods, Hypodemataceae, Lindsaeaceae, Lomariopsidaceae, Lonchitidaceae, Nephrolepidaceae, Oleandraceae, Polypodiaceae, Pteridaceae, Rhacidosoraceae, Saccolomataceae, Tectariaceae, Thelypteridaceae, Woodsiaceae.
Age. This clade is estimated to be around 260 Ma (Testo & Sundue 2016), (200-)176(-163) Ma (H. Schneider et al. 2004a), ca 191 Ma (Schuettpelz & Pryer 2009), (220.1-)184.2(-149.2) Ma (Rothfels et al. 2015b), around 150 Ma (Hennequin et al. 2008), around 205.8 Ma (X.-Y. Du et al. 2021), ca 173.5 Ma (C.-H. Huang et al. (2019), ca 303.1 Ma (Nitta et al. 2022) and (222.0-)185.0(-172.2) Ma (Pelosi et al. 2022).
Evolution: Divergence & Distribution. X.-Y. Du et al. (2021: e.g. Figs 2, 3; see also Nitta et al. 2022: Fig. 5) compare ages of clades in Polypodiales using different methods, and also those suggested by other authors.
Lehtonen et al. (2017) suggest that the diversity in Polypodiales is associated with low extinction rates, not with e.g. the adoption of the epiphytic habit - epiphytes are common here (see also Sundue et al. 2015 for Polypodiacaeae themselves). Sundue and Rothfels (2014) discuss characters for this clade, particularly the Eupolypod II group. Neochromes, chimaeric photoreceptors in which red-sensing phytochrome and blue-sensing phototropin are fused into single molecules, moved from hornworts to ferns ca 179 Ma, probably by lateral gene transfer (Suetsugu et al. 2005; F.-W. Li et al. 2014, 2015; Wickell & Li 2019). Within ferns (but not hornworts) further lateral transfer of the neochrome gene has resulted in its phylogeny not matching that of its hosts. These latter include Alsophila and Plagiogyrium (Cyatheales) and Dipteris (Dipteridales) deeper in the tree (Li et al. 2014), i.e. a rather different distribution from that of the cryptochromes discussed by Cai et al. (2020) - see also Polypodiopsida above.
Genes & Genomes. Marchant et al. (2022) were unsure if a genome duplication, Pterα, could be placed at this node.
Phylogeny. For discussion of relationships within Polypodiales, see above.
Classification. Currently relationships within Polypodiales are a bit uncertain and a number of small families are being described, but the links above lead to the families as recognized by The Pteridophyte Phylogeny Group (2016).
Synonymy: Aspleniales Reveal, Athyriales Schmakov, Blechnales Reveal, Dennstaedtiales Doweld, Dryopteridales Schmakov, Lindseales Doweld, Negripteridales Reveal, Parkeriales A. B. Frank, Platyzomatales Reveal, Pteridales Doweld, Thelypteridales Doweld
[Saccolomataceae [Cystodiaceae [Lindsaeaceae + Lonchitidaceae]]] / SaCyLoLi clade: stem solenostelic (protostelic).
Age. The crown-group age of this clade is around 186.1 Ma (X.-Y. u et al. 2021) or ca 165.5 Ma (C.-H. Huang et al. 2019).
SACCOLOMATACEAE Doweld - Saccoloma Kaulfuss - Back to Polypodiopsida
Rhizome dicyclic solenostele; scales +, peltate; petiole vasculature with inverted Ω-shaped bundle; frond 1-4-pinnate; sorus submarginal, indusium ± cupuliform; spores also with distinctive ± parallel branched ridges; x = ca 63.
1/24: [list]. Tropical, but not mainland Africa.
Age. Crown-group Saccolomataceae may be ca 17.4 Ma (X.-Y. Du et al. 2021: ?sampling).
Phylogeny. Schwartsburd (2022: 3 plastome genes) found that the Old and New World species in his analysis formed sister clades.
[Cystodiaceae [Lindsaeaceae + Lonchitidaceae]]: ?
Age. The crown-group age of this clade is ca 135.9 Ma (X.-Y. Du et al. 2021).
CYSTODIACEAE J. R. Croft - Cystodium sorbifolium (Smith) J. Smith - Back to Polypodiopsida
Petiole vasculature with two complex bundles.
1/1: [list]. Malesia.
[Lindsaeaceae + Lonchitidaceae]: ?
Age. This node is ca 101.2 Ma (Schwartsburd et al. 2020: topology) or ca 117.6 Ma (X.-Y. Du et al. 2021).
LINDSAEACEAE M. R. Schomburgk - Back to Polypodiopsida
Innermost cortical layer of root usu. of 6 large cells; s protostelic, with internal phloem; petiolar vasculature V-shaped and then with two bundles; indusium opening towards margin of frond; x = 34, 38, etc., genome duplication +
7/235: [list], Lindsaea (180). Pantropical (subtropical).
Age. Crown-group Lindsaeaceae are ca 60.2 Ma (X.-Y. Du et al. 2021).
For the fossil record of Lindsaeaceae, fossils being reported from ca 99 Ma amber from Myanmar, see C. Li et al. (2018).
Evolution: Divergence & Distribution. Lindsaea and Odontosora, separated since the Cretaceous (or for ca 41.5 Ma - see Schwartsburd et al. 2020), hybridize - = xLindsaeosoria flynnii (Lehtonen 2018; H.-M. Liu et al. 2020; Stull et al. 2023).
Genes & Genomes. H. Chen et al. (2022) noted that Lindsaea had a genome duplication, but it was absent fromAdiantum (Pteridaceae) - see also Pelosi et al. (2022).
Phylogeny. See Lehtonen et al. (2010) for a phylogeny and generic classification.
LONCHITIDACEAE Doweld - Lonchitis L. - Back to Polypodiopsida
Petiole with two vascular bundles.
1/2: [list]. Tropical America and Africa, Madagascar.
Age. The two species diverged ca 14.9 Ma (X.-Y. Du et al. 2021), probable long distance dispersal.
[[Dennstaedtiaceae + Pteridaceae] Eupolypods]: genome duplication +.
Age. The age of this node is ca 150.3 Ma (C.-H. Huang et al. 2019), ca 185.1 Ma (X.-Y. Du et al. 2021) or 207.4 Ma (Shang et al. 2023).
Evolution: Divergence & Distribution. For a genome duplication here, see Pelosi et al. (2022).
[Dennstaedtiaceae + Pteridaceae]: SiO2 accumulation common; petiole vasculature with inverted Ω-shaped bundle.
Age. B. Zhong et al. (2014b) suggested an age of (217-)154.3(-93.1) Ma for this node, Schuettpelz and Pryer (2009) an age of ca 110.8 Ma, H. Schneider at al. (2016) ages somewhere around 137.4-95.6 Ma and X.-Y. Du et al. (2021) an age of ca 174 Ma. Note topology of tree in Schwartsburd et al. (2020) on which ages were estimated, but c.f. their Fig. 1, furthermore, these two families are successive branches of the tree in C.-H. Huang et al. (2019)
DENNSTAEDTIACEAE Lotsy - Back to Polypodiopsida
Cell walls with HGS lignins; medullary bundles +; hairs uniseriate [catenate]; leaf buds + [?sampling]; sori marginal, with a single vein, receptacle conoid, sporangium development basipetal.
11/265: [list]. Tropical, (temperate).
Age. Crown-group Dennstaedtiaceae are estimated to be ca 115 Ma (Schneider et al. 2004a), ca 72.2 Ma (Schuettpelz & Pryer 2009), around 98-66.2 Ma (H. Schneider et al. 2016), ca 94.2 Ma (Schwartsburd et al. 2020), ca 104.4 Ma (C.-H. Huang et al. 2019) or about 116.6 Ma (X.-Y. Du et al. 2021).
Pigg et al. (2021) summarized the fossil record of Dennstaedtiaceae which goes back to amber from Myanmar dated ca 100 Ma - Krameropteris resinatus from this amber was placed at the split between Monachosorum and other Dennstaedtiaceae (H. Schneider et al. 2016) - apparently it has an "undeniable cathetogyrate sporangium" (Becari-Viana & Schwartsburd 2017: p. 105).
1. Monachosoroideae Crabbe, Jermy & Mickel - Monachosorum Kunze
Rhizome short-creeping to ascending, dictyostelic; petiole with two vascular bundles; sorus abaxial, abaxial indusium 0; x = 28.
1/3: Central Himalayas to Japan and E. Malesia.
Age. Crown-group Monoachosoroideae are some 17.1 Ma (Schwartsburd et al. 2020).
[Dennstaedtioideae + Hypolepidoideae]: rhizome long-creeping, proto- or (polycyclic) solenostelic; petiole with one vascular bundle; epipetiolar buds + (0); sorus marginal; adaxial indusium + (0).
Age. The split of these two subfamilies is dated to ca 78.7 Ma (Schwartsburd et al. 2020).
Rhizome (branching dichotomous - Mucura); (leaf buds 0); sorus abaxial, indusium abaxial, (half) cup-shaped; x = 30-47.
4/: Microlepia (60-80), Dennstaedtia (55-70). Pantropical, W. Pacific, Hawaii.
Age. The crown-group age of this clade is ca 48.6 Ma (Schwartsburd et al. 2020).
Rhizome (ascending, dictyostelic, phyllotaxis spiral - Blotiella), with aerenchyma in lateral line; (vessels + - Pteridium); ?leaf buds; (sorus with 2 or more veins), (abaxial indusium +; spores monolete (trilete - Pteridium); x = 28.
6/: Hypolepis (69). Pantropical, islands, Pteridium ± worldwide.
Age. The crown-group age of Hypolepidoideae is ca 35.5 Ma (Schwartsburd et al. 2020).
Evolution: Divergence & Distribution. Schwartsburd et al. (2020) optimized the evolution of a number of morphological features in the family (focus on Hypolepis), as did Triana Moreno et al. (2022) for the family as a whole.
Ecology & Physiology. Dennstaedtiaceae are often plants of sunny/open habitats and can be weedy (Pigg et al. 2021), although Schwartsburd et al. (2020) noted that this was probably a feature of the [Dennstaedtioideae + Hypolepidoideae] clade.
Plant-Animal Interactions. Some 21 kinds of galls are known from Pteridium spp., mites and four insect orders being the main gallers.
Chemistry, Morphology, etc.. Beccari-Viana and Schwartsburd (2017) discuss rhizome anatomy and morphology in the family.
Phylogeny. Schwartsburd et al. (2020) looked at the phylogeny of Dennstaedtiaceae, and i.a. found that Monachosorus was sister to the rest of the family (see also C.-H. Huang et al. 2019) and Dennstaedtia was para-/polyphyletic - this latter problem was finally cleared up by Triana-Moreno et al. (2022: 93 species, 4 chloroplast markers).
Classification. Schwartsburd et al. (2020; see also Triana-Moreno et al. 2022) recognized three subfamilies, two of which seem to need names.
PTERIDACEAE E. D. M. Kirchn. —— Synonymy: Parkeriaceae Hooker - Back to Polypodiopsida
(Epiphytic), (xeric), (aquatic), (ephemeral), (plant deciduous - Adiantum); (vessels + - Ceratopteris); stem soleno-—dictyostelic; venation reticulate [?level]; indusium 0; (spores bilateral); gametophyte (ribbon-like), (gemmiferous); (mycorrhizae 0); x = 29, 30.
53/1206: [list], Pteris (250), Adiantum (225), Aleuritopsis* (100), Jamesonia (50), Myriopteris (45), Antrophyum (34), Doryopteris* (41), Haplopteris (40), Adiantopsis* (35), Pellaea (30+), Cheilanthes* (29), Gaga* (19); taxa with * were included in Hemionitis s.l.. Worldwide.
Age. Estimates of the age of this clade are around 106.3 Ma (Schuettpelz & Pryer 2009), 90 Ma (Rothfels & Schuettpelz 2014), about 99.9-87.4 Ma (H. Schneider et al. 2016), ca 97.8 Ma (C.-H. Huang et al. 2019) or ca 130.6 Ma (X.-Y. Du et al. 2021).
Evolution: Divergence & Distribution. Adiantum is quite diverse in the West Indies, the species there being the result of at least 17 colonization events and some subsequent speciation on the older islands (Regalado et al. 2018). Similarly, C.-W. Chen et al. (2023) suggested that distributions in the Old World Antrophyum could be understood in the context of around 23 dispersal- and 4 vicariance-type events. Schuettpelz et al. (2025; see also Tryon & Tryon 1982) noted the substantial geographical signal in relationships in the hemionitid ferns. Pellaea is primarily American, but it is also known from Hawaii and there is one species endemic to South Africa (J. Zhao et al. 2025).
Ecology & Physiology. Cheilanthoid ferns, some 400 or more species, can grow in very dry conditions (e.g. Grusz et al. 2014 and references), and a variety of features enable their life there, including apomixis (Schuettpelz et al. 2024 for some references). Such ferns hydroregulate in various ways, but the plants are notable dessication-tolerant (Aros-Mualin & Kessler 2024). Remarkably, free-living gametophytes of some species survive for years even when desiccated; when rehydrated, groups of living cells regenerate prothalli (Pickett 1931: ca half the examples are Pteridaceae). Kao et al. (2019) discuss the evolution of farina, whitish and powdery, made up of a variety of lipophilic flavonoid aglycones produced by glands on the margins of the gametophytes and on the lower surface of the fronds - the focus is on Notholaena, a denizen of deserts of southwest North America. The pattern of gain (?and loss) of this feature is complex, in part independent in the two generations, and there are some species that lack farina but seem to do perfectly well growing in the same conditions as their farina-producing relatives (Kao et al. 2019).
Vittarioid ferns are commonly epiphytic and are one of the major epiphytic monilophyte groups; CAM photosynthesis is known here. With their simple, strap-like fronds and long-lived ribbon-like gametophytes and polyploid (n = 60) nuclear genome, they are very different from their sister group, Adiantum (Pryer et al. 2016); for genome evolution, see below. Twig epiphytes commonly lack mycorrhizae, but mycorrhizae are more common in low epiphytes, the plants then growing quite close to the ground (Lehnert et al. 2016).
Some species of Pteris in particular and Pityrogramma hyperaccumulate arsenic (Reeves et al. 2017; Souri et al. 2017).
Pelosi et al. (2023) discuss Vittaria appalachiana, close to V. lineata but known only as gametophytes, and the likely genetic consequences of this; such gametophytes are quite common here (Yoneoka et al. 2024).
Genes & Genomes. There appear to be intergeneric hybrids in the taenitidoid group, e.g. between Syngramma and Austrogramme (C.-W. Chen et al. 2022). Marchant et al. (2022) looked at the nuclear genome of Ceratopteris richardii (Parkerioideae) and found a genome duplication (CERα) ca 62 Ma there but not in Acrostichum (also Parkerioideae); there was little intragenomic synteny evident. There is also a genome duplication in the Haplopteris-Vittaria clade (Pelosi et al. 2022). For the nuclear genome of Adiantum capillus-veneris, see Fang et al. (2022). Findings in these papers bear on general aspects of nuclear genome evolution in vascular plants and are discussed elsewhere. There are increased rates of molecular evolution in both plastomes and nuclear genomes, apparently not driven by selection (Rothfels & Schuettpelz 2014); for vittarioids, see also Pryer et al. (2016). Increased evolutionary rates in the nuclear genome are perhaps to be associated with high ploidy levels (Grusz et al. 2016).
Mobile open reading frames in the genomes of fern organelles, possibly of plastid plasmid or viral origin, are particularly common here, mainly in the plastome where they may be associated with structural changes (Robison et al. 2018). In the vittarioids in particular there are a number of of gene losses in the plastome (e.g. trnT, trnV) and a 7kb inversion (Robison et al. 2018).
Phylogeny. For phylogenies, see Crane et al. (1995), Prado et al. (2007), Schuettpelz (2007), L. Zhang et al. (2016b: Pteridoideae, 14 genera), Schuettpelz et al. (2016: vittarioids) and C.-W. Chen et al. (2022: Taenitis and relatives). Pteris has been the subject of some attention, e.g. Chao et al. (2014: position of P. longifolia, the type, unclear) and L. Zhang et al. (2015: Pteris somewhat expanded), while L. Zhang and Zhang (2017) produced a comprehensive phylogeny and infrageneric classification, most infrageneric taxa being well supported. Pryer et al. (2016) found that Adiantum is monophyletic and is sister to Vittaria and its relatives, as did Gu et al. (2020: rbcL), and the latter also looked at variation in scale morphology in the context of the phylogeny that they recovered - non-Adiantum vittarioids had transparent meshes. Pelosi et al. (2022) did not recover a monophyletic Cheilanthes in their transcriptome analysis, and monophyly of some other cheilanthoids—hemionitids has also been questioned, J. Zhao et al. (2024: variety of markers/analyses) also noting polyphyly in Paragymnopteris, Pellaea etc. (see also Schuettpelz et al. 2025). J. Zhao et al. (2025: 61 spp., 9 plastid markers) looked ta relationships in the pellaeids, and they found Pellaea itself to be in four clades. Schuettpelz et al. (2025: 223/354 species, 3 plastid markers) has clarified relationships in the hemionitids. C.-W. Chen et al. (2023: 4 chloroplast markers) looked at relationships in Antrophyum, finding for the most part quite well supported basal relationships, A. novae-caledoniae being sister to the rest of the genus; they also looked at the evolution of nine vegetative characters.
Classification. Generic limits are difficult in cheilanthoid ferns (Grusz et al. 2014; Yesilyurt et al. 2015), but Schuettpelz et al. (2025: esp. Fig. 2) discuss generic limits in the hemionitid ferns, recognizing 22 genera there, including Christenhuszia with two species (c.f. Christenhusz et al. 2018, = Hemionitis s.l. with ca 500 species) - 17 species were unplaced. J. Zhao et al. (2025) described two new genera and resuscitated one old genus to deal with the polyphyly in Pellaea.
Eupolypods / = [Eupolypod I + Eupolypod II]: stem dictyostelic; petiole vasculature V-shaped bundle, then two bundles, circum-endodermal band surrounding individual bundles + [thickened, not lignified] (0); polocytic stomata common [subsidiary cell single, almost surrounding guard cells]; fronds to 1.5 times pinnate; spores monolete, reniform, perine distinct; x = 41.
Age. This clade, which includes most ferns, has been estimated to be (124-)105(-91) Ma (H. Schneider et al. 2004a), ca 116.7 Ma (Schuettpelz & Pryer 2009), ca 100.2 Ma (C.-H. Huang et al. 2022), while Pelosi et al. (2022) suggested an age of (123.6-)114.0(-79.2) Ma and Shang et al. (2023) an age of ca 162.9 Ma.
The oldest Eupolypod fossil is from Burmese amber around 98 Ma; it was compared with Thelypteridaceae (Eupolypod II: Regalado et al. 2017), the crown-group age of which has been estimated at some 68.5 Ma (Schuettpelz & Pryer 2009).
Evolution: Divergence & Distribution. An increase of diversification seems to be associated with this node - perhaps connected with a genome duplication (C.-H. Huang et al. 2022)?
Genes & Genomes. There may have been a genome duplication (PTERa) somewhere aroud here (O.T.P.T.I. 2019, see also C.-H. Huang et al. 2022), although evidence for this seems to be mixed (Fang et al. 2022).
[Hypodematiaceae [Didymochlaenaceae [[Nephrolepidaceae + Lomariopsidaceae] [Dryopteridaceae [Tectariaceae [Oleandraceae [Davalliaceae + Polypodiaceae]]]]]]] / Eupolypod I / Polypodiineae: plants epiphytic (not); rhizome scales persistent, dense; petiole vasculature complex, two vascular bundles + 1 or more smaller bundles [circular in t.s., also with abaxial semicircular arc of smaller bundles]; perispore with thick tuberculate folds/wings.
Age. The age of this node is variously estimated to be ca 98.9 Ma (Schuettpelz & Pryer 2009) or (172.5-)170(-158.5) Ma (Testo & Sundue 2016), ca 93.5 Ma (C.-H. Huang et al. 2019), ca 140.1 Ma (X.-Y. Du et al. 2021), (155.0-)146.2(-139.6) Ma (Fan et al. 2021), ca 161.1 Ma (Nitta et al. 2022) and ca 142.4 Ma (Shang et al. 2023).
Evolution: Ecology & Physiology. Members of this clade are quite commonly epiphytic (e.g. Tsutsumi & Kato 2006; Schuettpelz & Pryer 2009) - perhaps 2,040 species of epiphytes, around 80% of the clade (Pittermann et al. 2022). For rhizome scales, perhaps protecting the plant against desiccation and aiding in the absorbtion of water and nutrients, see Tsutsumi and Kato (2008); if they are not at this position on the tree, they should be placed at the next node up (with parallel evolution within Dryopteridaceae). Fronds in which the fine venation is reticulate and with internally directed veinlets occur in some members of this clade (Boyce & Knoll 2002) - ?elsewhere?
Phylogeny. For some discussion of relationships within the Eupolypod I clade, see above.
Classification. One or two more families may still be needed in this area. Thus H.-M. Liu et al. (2014), as they placed the odd genera Pteridrys and Pleocnemia in Tectariaceae and Dryopteridaceae respectively, recognised an [Arthropteridaceae + Tectariaceae] clade (the former is synonymized under the latter here). Further work on the Tectariaceae area (see Dong et al. 2018; X.-M. Zhou et al. 2018) has suggested both more genera and new families. Thus relationships in Shang et al. (2023) are [Didymochlaenaceae [Hypodematiaceae [Dryopteridaceae [[Nephrolepidaceae + Lomariopsidaceae] [Arthropteridaceae [Pteridryaceae + Tectariaceae]] ..., i.e. with some rearrangements and some splitting when compared with the sequence immediately above.
HYPODEMATIACEAE Ching - Back to Polypodiopsida
(Rachis hairy), (fronds with glandular hairs); sori/indusia hippocrepiform; (n = 40).
2/31: [list], Hypodematium (29). Old World (sub) tropics, from Africa and Madagascar to Sakhalin and western New Guinea.
Age. Crown-group Hypodematiaceae are ca 67.3 Ma, but with huge error bars (X.-Y. Du et al. 2021) or (87.3-)77.0(-70.5) Ma (Fan et al. 2021).
Evolution: Divergence & Distribution. Hypodematiaceae are probably eastern Asian in origin; Fan et al. (2021) discuss their subsequent movements around the Old World.
Phylogeny. Fan et al. (2021) discuss relationships in Hypodematiaceae.
Previous Relationships. The two genera included here have features of several other eupolypod I families, and until recently they were assigned accordingly (Fan et al. 2021).
Age. The age of this clade is around 135.5 Ma (X.-Y. Du et al. 2021) or 148.7-)142.4(-140.2) Ma (Shang et al. 2023: sister to eupolypods I).
DIDYMOCHLAENACEAE L.-B. Zhang & L. Zhang - Didymochlaena Desvaux - Back to Polypodiopsida
Rhizome erect, plant subarborescent; sori elliptical, somewhat elongated, <10/pinnule; spores monolete; x = 41.
1/22: [list]. ± Pantropical, inc. Fiji, Madagascar and the Greater Antilles. Map: Shang and Zhang (2023: Fig. 3).
Age. The crown-group age of this clade is (87.6-)52.8(-29.7) Ma (Shang et al. 2023).
Evolution: Divergence & Distribution. Shang and Zhang (2023, see also Shang et al. 2023) found a strong geographical signal in relationships within Didymochlaena - [America [Africa + S.E. Asia/Malesia]], with further subgroupings geographically distinguishable; they optimised a number of characters on the phylogeny of the genus, noting that species groups here were well characterized morphologically. They talked about the "tripling of molecular, morphological and geographical differentiation in Didymochlaena" (ibid.: p. 283).
Phylogeny. For relationships, see Shang and Zhang (2023) and Shang et al. (2023).
Age. This clade is ca 129.0 Ma (X.-Y. Du et al. 2021).
DRYOPTERIDACEAE Herter - Back to Polypodiopsida
Terrestrial; fronds ≥3.5 times pinnate; sori vein-related, superficial, discrete; perine winged; gametophyte strap-like; x = 41.
24/2,271: [list, to subfamilies]. World-wide.
Age. Crown-group Dryopteridaceae are around 81.8 Ma (Schuettpelz & Pryer 2009), (94-)76(-58) Ma (Le Péchon et al. 2016), ca 106.5 Ma (X.-Y. Du et al. 2021) or about 104.7 Ma (Z.-Y. Zuo et al. (2024).
The recently-described Prosperifilix found in Kachin amber ca 99 Ma is considered to belong to Dryopteridaceae (X. Long et al. 2023).
[Dryopteridoideae [Ctenitoideae [Polystichopsidioideae + Polybotryoideae]]]: rhachis/costae: V-shaped groove on rhachis and adaxial pinna rhachis.
Age. The age of this clade is around 98.9 Ma (Z.-Y. Zuo et al. 2024).
1. Dryopteridoideae B. K. Nayar
(Leaf base swollen, with starch storage tissue [= trophopods] - Dryopteris).
7/1,064: Polystichum (500), Dryopteris (400), Arachnoides (78), Stigmatopteris (40), Cyrtomium (35). Tropical and Temperate.
Age. Crown-group Dryopteroideae are some 197.2 Ma (Z.-Y. Zuo et al. 2024).
[Ctenitidoideae [Polystichopsidoideae + Polybotryoideae]]: ?
Age. This clade is around 96.7 Ma (Z.-Y. Zuo et al. 2024).
2. Ctenitidoideae Z.-Y. Zuo & J.-M. Lu - Ctenitis (C. Christensen) C. Christensen
rhachis/costae: fronds with free veins, scales and multicellular ctenitoid hairs +.
1/143. Pantropical, inc. Hawaii.
Age. Ctenitoideae are about 33.2 Ma (?sampling: Z.-Y. Zuo et al. 2024).
[Polystichopsidoideae + Polybotryoideae]: ?
Age. This age of this node is ca 75.4 Ma (Z.-Y. Zuo et al. 2024).
3. PolystichopsidoideaeZ.-Y. Zuo
Hairs on stipe base and lamina.
2/8: Polystichopsis (7). Neotropics, Trichoneuron microlepioides in Yunnan, China (and included in Lastreopsis in the FoC...).
Age. Crown-group Polystichopsidioideae are about 27.8 My (Z.-Y. Zuo et al. 2024).
4. Polybotryoideae H.-M. Liu & X.-C. Zhang
Plant climbing or hemiepiphytic; fronds (slightly) dimorphic; sporangia covering surface of frond [acrostichoid] / sori discrete.
4/52: Polybotrya (39). New Woeld tropics, inc. Mexico, the Antilles, ?extinct in Florida.
Age. This clade is about 33.7 Ma (Z.-Y. Zuo et al. 2024).
[Lastreopsidoideae [Pleocnemioideae + Elaphoglossoideae]]: ?
Age. The age of this node is about 87.5 Ma (Z.-Y. Zuo et al. 2024).
5. Lastreopsidoideae Z.-Y. Zuo
Rhachis/costae: groove widened, (shallowly) U-shaped at the base; hairs on stipe base and lamina.
4/150: Megalastrum (90), Parapolystichum (30), Lastreopsis (21). Pantropical also Australia, etc.
Age. Lastreopsidoideae are about 84.2 Ma (Z.-Y. Zuo et al. 2024).
[Pleocnemioideae + Elaphoglossoideae]: ?
Age. This node is about 81.8 Ma (Z.-Y. Zuo et al. 2024).
6. Pleocnemioideae Z.-Y. Zuo - Pleocnemia C. Presl
Rhachis/costae axes adaially nearly round, prominulous or flat; fronds with anastomosing veins, scales, cylindric yellow glands +.
1/20. N.E. India and S.E. China to Malesia and the W. Pacific to Samoa.
Age. Crown-group Pleocnemioideae are ca 45.1 Ma (Z.-Y. Zuo et al. 2024).
7. Elaphoglossoideae (Pichi-Sermolli) Crabbe, Jermy & Mickel
Plant climbing or hemiepiphytic; rhachis/costae: lower stipe adaxially usu. grooved (rounded), → convex → ribbed adaxially on upper rachis and costae; fronds dimorphic, sporangia covering surface [acrostichoid],
5/834: Elaphoglossum (730), Bolbitis (66), Lomagramma (18). Tropics.
Age. This clade is about 62.3 Ma (Z.-Y. Zuo et al. 2024).
Evolution: Divergence & Distribution. Dryopteridaceae probably originated in the Americas (Z.-Y. Zuo et al. 2024).
Z.-Y. Zuo et al. (2024: Figs 3-5) optimised the distributions of a number of characters on to their plastome phylogeny.
Diversification within the speciose Polystichum began in the Eocene, (60-)46(-32) Ma (Le Péchon et al. 2016), that in Elaphoglossum began a bit more recently - and that covers about 2/3 of the family. For polyploidy and evolution in Polystichum, see Barrington (2020), a discussion in which the evolution of such ferns, especially Polystichum species, on Darwinian islands (oceanic islands, sky islands, ecological islands) figures quite largely.
There has been extensive hybridization in Dryopteridaceae, nothogenera including xCyclobotrya (Cyclodium x Polybotrya) and xSellimeris (= Selliguea x Arthromeris), both Polybotryoideae, and xDryostichum (Dryopteris x Polystichum), Dryopteroideae (H.-M. Liu et al. 2020); these are examples of deep hybridization (Tseng et al. 2024). Sessa et al. (2012b) found that the allopolyploid D. pseudofilix-mas "inherited genomes from progenitor species estimated to have diverged 39.2 million years ago" (see also Sigel 2016).
Despite the pantropical distribution of Ctenitis, long distance dispersal seems to have been uncommon (Hennequin et al. 2017). Labiak et al. (2014) found that in Lastreopsis there had been movement to and fro between Australia and South America towards the middle of the Caenozoic.
Ecology & Physiology. Elaphoglossum is the major epiphytic genus in the family - ca 400 species (ca 2/3 of the genus at 600 species, but c.f. above) are epiphytes (Zotz 2013). Some of those epiphytes collect dead leaves, etc., i.e. they are trash-basket epiphytes (Ortega-Solis et al. 2021)
Plant-Animal Interactions. Galls are relatively commonly to be found on Dryopteridaceae (M. G. Santos et al. 2018), although the family is also relatively large...
Chemistry, Morphology, etc.. For a survey of spore morphology in Elaphoglossum, more or less related to phylogeny, see Moran et al. (2007) and for that in Taiwanese Polystichum, see E. Liu et al. (2024).
For general information, see Zuo et al. (2024).
Phylogeny. For a phylogeny of the whole family, see H.-M. Liu et al. (2007, esp. 2015); three subfamilies are recognised, although two genera are unplaced. For a phylogeny of Elaphoglossum, see also Lóriga et al. (2014: 20-15 Ma fossil of ?crown-group Elaphoglossum from Dominican amber). Rouhan et al. et al. (2004) and Vasco et al. (2015) discuss relationships within Elaphoglossum. Here the Costa Rican E. amygdalifolia and the Cuban E. wrightii are successively sister to the rest of the genus. Moran et al. (2010a, b) investigated relationships within the bolbitidoid ferns with a focus on variation in perispore morphology. Li and Lu (2006a, b), L.-B. Zhang et al. (2012), Sessa et al. (2012a, 2017: sub-Saharan Dryopteris), and McHenry and Barington (2014: exindusiate Andean species monophyletic, sister to Mexican spp.) have been working on relationships within Dryopteris itself, a genus whose limits are being clarified (e.g. Zhang & Zhang 2012) and which shows considerable hybridization at all levels (Sessa et al. 2012a, b). In Ctenitis, on the other hand, polyploidy is probably rare (Hennequin et al. 2017). Le Péchon et al. (2016) examined phylogenetic and biogeographic relationships in Polystichum, while Labiak et al. (2014) looked at relationships around Lastreopsis. N. T. Lu et al. (2018) found that Arachniodes was largely monophyletic, with A. mutica, from Japan, being sister to the rest of the genus. Z.-Y. Zuo et al. (2024: 94 plastomes, all 24 genera) producded a quite well-resolved tree on which the classification above is based.
Classification. The subfamilies above can be characterised by unique combinations of characters, if not by synapomorphies (Z.-Y. Zuo et al. 2024: see subfamilial characterisations)
[[Lomariopsidaceae + Nephrolepidaceae] [Tectariaceae [Oleandraceae [Davalliaceae + Polypodiaceae]]]] / DANLOPPT clade: ?
Age. This node is ca 117.3 Ma (X.-Y. Du et al. 2021) ar ca 119.8 Ma (Shang et al. 2023).
[Lomariopsidaceae + Nephrolepidaceae]: ?
Age. The age of this clade is around 105.4 Ma (Shang et al. 2023).
LOMARIOPSIDACEAE Alston - Back to Polypodiopsida
; n = 40, 41.
5/70: [list], Lomariopsis (60). ± Pantropical.
Age. An estimate of the age of crown-group Lomariopsidaceae is ca 68.7 Ma (X.-Y. Du et al. 2021).
Evolution: Ecology & Physiology. Gametophytes that can live independently of the sporophyte are known from this family (Yoneoka et al. 2024).
Phylogeny. For relationships in Lomariopsidaceae and the vicissitudes of the family, see C.-W. Chen et al. (2017), and for the sister taxa Dracoglossum and Lomariopsis, with dissimilar sporophytes but very similar gametophytes (also, in both antheridium production is at most uncommon), see Watkins and Moran (2019). The thalloid submersed aquatic Süßwassertang, which looks rather like a liverwort, in fact belongs to Lomariopsis; the sporophytic stage is unknown (F.-W. Li et al. 2009).
[Nephrolepidaceae [Tectariaceae [Oleandraceae [Davalliaceae + Polypodiaceae]]]]]: ?
Age. This node is ca 109.0 Ma (X.-Y. Du et al. 2021).NEPHROLEPIDACEAE Pichi Sermolli - Nephrolepis Schott - Back to Polypodiopsida
Stem tubers +; leaves once-pinnate.
Age. Crown-group Nephrolepidaceae are ca 32.0 Ma (X.-Y. Du et al. 2021) or ca 59.8 Ma (Shang et al. 2023).
1/19: [list]. Tropical to subtropical.
Classification. If a sister-group relationship with Lomariopsidaceae were to hold, the two are best merged.
[Tectariaceae [Oleandraceae [Davalliaceae + Polypodiaceae]]]: frond veins free, parallel or pinnate.
Age. This node is ca 91.8 Ma (X.-Y. Du et al. 2021) or ca 98.5 Ma (Shang et al. 2023).
TECTARIACEAE Panigrahi —— Synonymy: Arthopteridaceae H. M. Liu, Hovenkamp & H. Schneider, Pteridryaceae Li Bing Zhang, X. M. Zhou, Liang Zhang & T. N. Lou - Back to Polypodiopsida
(Climbers); (rhizome slender), (stipe and pinnae articulated); (frond veins free, parallel or pinnate), with jointed usually stubby hairs; n = 40-42.
7/270: [list], Tectaria (210). Pantropical, inc. oceanic islands.
Age. Crown-group Tectariaceae (excluding Arthropteris) are around 50.4 Ma (Schuettpelz & Pryer 2009) or ca 34.5 Ma (X.-Y. Du et al. 2021) also Ar + Pt + Tec = 77.3/78.6 Ma, Pt + Te = 70.7/64.9 Ma (Du et al. 2021 and Shang et al. 2023 respectively).
Phylogeny. Although in the analysis of H.-M. Liu et al. (2013) the position of Arthropteridaceae was uncertain, F. G. Wang et al. (2014a) found that they were embedded in Polypodiaceae-Tectarioideae, as were Lomariopsidaceae (for which, see above); the latter were in a separate clade in Schuettpelz and Pryer (2009). L.-B. Zhang and Zhang (2014) placed Arthropteridaceae sister to Tectariaceae, but with less than overwhelming support, Lomariopsidaceae were again separate. The circumscription of Tectariaceae as adopted here was also found by Zhang et al. (2016a) and the monophyly of Tectaria s. str. confirmed - although three species were somewhat errant in the study of Dong et al. (2018) and were placed in a separate genus, and overall relationships were [Arthropteris [Pteridrys group + Tectariaceae sensu stricto]] - the position of Arthropteris was not that well supported. Indeed, X.-M. Zhou et al. (2018) found the relationships [Pteridrys group [Arthropteris + Tectariaceae sensu stricto]] - again, support could have been stronger - and recognised all three as families.
Classification. Given the size of the clade, the recognition of three families - Tectariaceae, Arthopteridaceae and Pteridryaceae (e.g. X.-Y. Du et al. (2022) - seemed a bit much. For an infrageneric classification of Tectaria, see L.-B. Zhang and Zhang (2018). F.-G. Wang et al. (2014a) include a broadened but monophyletic Tectariaceae as a subfamily of Polypodiaceae.
[Oleandraceae [Davalliaceae + Polypodiaceae]]: stem with perforated dictyosele; fronds abscising from rhizome.
Age. This node is ca 86.2 Ma (X.-Y. Du et al. 2021) or ca 92.9 Ma (Shang et al. 2023).
OLEANDRACEAE Pichi Sermolli - Oleandra Cavanilles - Back to Polypodiopsida
Fronds abscising just above the base [so leaving phyllopodia], often simple; x = 41.
1/15: [list]. Tropical.
[Davalliaceae + Polypodiaceae]: epiphytes predominant; rhizome with acrogenous bramching, scaly; sori not vein-related, sporangium initiation mixed; perispore thin, verrucate, granulate, etc..
Age. The crown-group age of this clade has been estimated as ca 42 Ma (Sundue et al. 2015), ca 79.6 Ma (X.-Y. Du et al. 2021) or ca 83.2 Ma (Shang et al. 2023).
DAVALLIACEAE M. R. Schomburgk - Davallia (L.) Smith - Back to Polypodiopsida
Fronds ≥3.5 times pinnate; spores warty, warts close, not constricted at their bases [= verrucate-colliculate]; x = 40.
1/65: [list]. South East Asia to Oceania, one species each in the western Mediterranean and tropical Africa.
Age. Crown-group Davalliaceae are ca 19.4 Ma (Schuettpelz & Pryer 2009), ca 25.5 Ma (X.-Y. Du et al. 2021) or ca 28.3 Ma (Shang et al. 2023).
Evolution: Divergence & Distribution. F.-G. Wang et al. (2014b) optimize spore morphology on a phylogeny of the family.
Phylogeny. Tsutsumi et al. (2016) found seven major clades in the family (one monotypic), but relationships rather depended on the marker analysed.
Classification. For a classification in which Davallia is dismembered, see Kato and Tsutsumi (2008). However, Tsutsumi et al. (2016) suggested that only a single genus should be recognised, in part because of these phylogenetic uncertainties, but they provide a sectional classification.
POLYPODIACEAE J. Presl & C. Presl - Back to Polypodiopsida
Rhizome (radial); (petiole with one or two vascular bundles - grammitids); fronds monomorphic (dimorphic); indusium 0; spores bilateral, monolete; (gametophyte strap-like); x = 35-37.
63/1,650: [list, to subfamilies/tribes].
Age. Crown-group Polypodiaceae, [Loxogramme + The Rest], are about 55.8 Ma (Schuettpelz & Pryer 2009), ca 71.2 Ma (X.-Y. Du et al. 2021) or ca 72.8 Ma (Shang et al. 2023).
1. Loxogrammoideae H. Schneider
Rhizome branching uncommon; scales clathrate [lattice-like]; frond with minute 2-celled glandular hairs; (spores tetrahedral, trilete, chlorophyllous).
2/36: Loxogramme (34). Old World, inc. Pacific Islands, L. mexicana Mexico and Central America.
Age. Crown-group Loxogrammoideae are (55.5-)54.2(-52.7) Ma (B. Xue et al. 2024).
[[Platycerioideae + Microsoroideae] [Crypsinoideae [Polypodioideae [Campyloneuroideae [Adetogrammoideae [Serpocauloideae + Grammitidoideae]]]]]]: rhizome branching common.
Age. The age of this clade is some (63.2-)58.0(-51.7) Ma (B. Xue et al. 2024), ca 60.6 Ma (Jian et al. 2024) or ca 70 Ma (J. Zhao et al. 2024).
[Platycerioideae + Microsoroideae]: ?
Age. This clade is about (59.5-)54.3(-48.8) Ma (B. Xue et al. 2024), (54.8-)53.0(-50.6 Ma (Jiang et al. 2024) or ca 57.3 Ma (J. Zhao et al. 2024).
2. Platycerioideae B. K. Nayar
(Crassulacean Acid Metabolism +); hairs stellate; rhizomes heavily sclerified; fronds not pinnately dissected, (dimorphic, plant humus-catching); (nectaries +); paraphyses ≡ stellate trichomes; n = 37.
3/86: Pyrrosia (65), PLatycerium (18). Palaeotropical, to Oceania, 1 sp. (Platycerium andinum) in the Andes.
Age. Crown-group Platycerioideae are (49.5-)45.0(-42.1) Ma (B. Xue et al. 2024), ca 44.6 Ma (Jiang et al. 2024) or ca 50.0 Ma (J. Zhao et al. 2024).
(Ant plants with swollen hollow rhizomes - Lecanopteris); rhizome scales clathrate [lattice-like].
9-16/>180: Lepisorus (80), Leptochilus (51), Microsorum (40). Palaeotropical, to the Antipodes.
Age. Crown-group Microsoroideae are ca 45.4 Ma (Jiang et al. 2024) or ca 48. Ma (J. Zhao et al. 2024).
Age. The age of this clade is around (62.7-)56.4(-48.4) Ma (B. Xue et al. 2024).
4. Crypsinoideae B. K. Nayar (inc. Drynarioideae)
(fronds with nectaries).
3/153: Selliguea (105), Drynaria (45). Old World tropics, S. South America
Age. Crown-group Crypsinoideae are ca 52.7 Ma (J. Zhao et al. 2024).
[Polypodioideae [Campyloneuroideae [Adetogrammoideae [Serpocauloideae + Grammitidoideae]]]]: ?
Age. Polypodioideae and Grammitidoideae diverged ca 40.8 Ma (Testo & Sundue 2016), and another age for this clade is (61.7-)55.4(-47.4 Ma (B. Xue et al. 2024).
Rhizome scales clathrate [lattice-like]; fronds pinnatifid, (with nectaries), sori round, in a single row, paraphyses filamentous; spores bilateral, monolete.
5/ : Pleopeltis (93), Pecluma (50), Polypodium (40). North Temperate, Africa and South America.
[Campyloneuroideae [Adetogrammoideae [Serpocauloideae + Grammitidoideae]]]: ?
Age. The age for this clade is some (59.4-)53.5(-46.0) Ma (B. Xue et al. 2024).
6. Campyloneuroideae X.-C. Zhang & R. Wei
.
3/ : Campyloneuron (50). Most South America, Microgramma mauritiana Africa to the Mascarenes.
[Adetogrammoideae [Serpocauloideae + Grammitidoideae]]: fronds monomorphic only.
Age. This clade is (57.1-)52.0(-45.0) Ma (B. Xue et al. 2024).
7. Adetogrammoideae X.-C. Zhang & R. Wei - Adetogramma chrysolepis (Hooker) T. E. Almeida
.
1/1. C. and S. Andes.
[Serpocauloideae + Grammitidoideae]: rhizome branches at most few; scales clathrate [lattice-like].
Age. The estimated age for this clade is (55.9-)50.9(-44.3) Ma (B. Xue et al. 2024).
8. Serpocauloideae X.-C. Zhang & R. Wei - Serpocaulon A. R. Smith
(fronds with nectaries); spores ± verrucate; n = 37.
1/37. Central and South America, the Antilles (S. Mexico; U.S.A. - Florida).
9. Grammitidoideae Parris & Sundue
Rhizome short-creeping to erect, usu. unbranched; scales on rhizomes, on fronds 0, (scales not clathrate); aerophores 0; petiole with single vascular strand; sori exindusiate; sporangial stalk of one row of cells; spores tetrahedral, trilete, chlorophyllous; gametophyte gemmiferous or not.
42/911: Oreogrammitis (155), Prosaptia (87), Calymmodon (65), Lellingeria (50), Grammitis (40), Tomophyllum (40). Pantropical. esp. Old World.
Evolution: Divergence & Distribution. For ages of grammitid ferns, see Schuettpelz and Pryer (2009), Sundue et al. (2015) and Bauret et al. (2017). There seem to be some rooting issues in the tree that was focused on Platycerium provided by J. Zhao et al. (2024), so some ages should be interpreted carefully.
Diversification in Polypodiaceae may be associated with the uplift of the Andes, clades with broad elevational ranges diversifying faster, speciation being related to climatic/environmental factors but less obviously associated with morphological features (Sundue et al. 2015: epiphytes in general; Kreier et al. 2008: Andean Serpocaulon, but c.f. Sundue et al. 2015). Bauret et al. (2017) noted much long distance dispersal from Madagascar to Africa within Grammitidoideae, little in the reverse direction; grammitid ferns may have originated in the New World, with several dispersals across the Atlantic. In Grammitidoideae there is a basal New World-African grade, but the majority of genera are in a Malesian-Pacific clade (Shalisko et al. 2019; X.-M. Zhou et al. 2023).
Polypodiaceae in general have high diversification rates, however, this is a complex story (Sundue et al. 2015). There seems to have been substantial diversification 10-15 Ma after the Palaeocene-Eocene Thermal Maximum (P.E.T.M.) at ca 55.8 Ma, perhaps associated with the newly-formed (sic) angiosperm-dominated rainforest. Grammitids did not diversify notably faster; although species with chlorophyllous spores have diversified rather faster, such species also include other than grammitids. The highly specialized morphologies in the family did allow the colonization of nutrient-poor epiphytic habitats, and of novel habitat types along ecological gradients (Sundue et al. 2015). Interestingly, clades whose species were epiphytes per se or had fronds that trapped humus, diversified at lower rates than the others, rather, clades with non-cordiform gametophytes diversified fastest (Sundue et al. 2015). One humus-trapping clade, Platycerium, seems to have originated in Africa (but in fact opinions differ), achieving its more or less pan-tropical distribution through long distance dispersal (B. Xue et al. 2024; J. Zhao et al. 2024).
Weber and Agrawal (2014) suggested that the evolution of nectaries in Pleopeltis was associated with an increase in diversification. Such nectaries are known from Aglaomorpha, Serpocaulon, Drynaria, Platycerium and Campyloneuron as well, i.e. taxa in a variety of subfamilies. and they may be quite distinct and vascularized or inconspicuous and unvascularized (Mehltreter et al. 2021; Sanín et al. 2021: Table 2, 2023).
Relationships within Selliguea show some major geographic structuring (He et al. 2018), while Jian et al. (2014) estimated that there had been 13 dispersal and 6 vicariance events in the Microsoroideae they studied. Janssen et al. (2005) discussed the evolution of the diverse frond morphologies in Drynaria s.l., while L. Wang et al. (2012) looked at the phylogeny, etc., of Lepisorus in the Qinghai-Tibet region. X.-M. Zhou et al. (2027) optimized the variation of a number of characters within Drynarioideae, but with suggestions linking on this variation to that in other subfamilies.
For the vegetative anatomy of Loxogramme involuta, sister to rest of Polypodiaceae, but which is sometimes like that of Davalliaceae, see Mondal and Moktan (2023).
Ecology & Physiology. Ca 87% of the species of Polypodiaceae, mainly the grammitid ferns, are epiphytic (Holtum et al. 2007; Zotz 2013), making them the major epiphytic clade in the monilophytes. A number of microsoroid ferns, now terrestrial or rheophytic, have become secondarily terrestrial (C.-C. Chen et al. 2022). CAM photosynthesis is known from some of these taxa (Silvera et al. 2010b) - C3 + CAM, strong CAM, and facultative CAM have all been recorded (Pteridaceae also have a few CAM taxa, but that seems to be it for ferns - Holtum 2023). Some polypod ferns are deciduous, while there have been a number of cases of the evolution of dessication tolerance associated with various kinds of hydroregulation here, including of course Pleopeltis (Aros-Mualin & Kessler 2024).
An apparently secondary association with mycorrhizal ascomycetes has developed in the sporophytes (unlike, say, in epiphytic Hymenophyllaceae, although in both dark septate endophytes tend to be common), and the plants seem to be dependent on this association; they are often rather small and are twig epiphytes (Lehnert et al. 2016). Interestingly, mycorrhizae are uncommon in these taxa which have been characterized as "non-mycorrhizal nutrient savers" (Lehnert et al. 2016: p. 8).
A number of epiphytic taxa catch litter - Drynaria/Aglaomorpha and Platycerium are good examples (Zona & Christenhusz 2015). They would be placed in the so-called "trash-basket epiphyte" group of Ortega-Solis et al. (2021).
Burns et al. (2021) suggest that there is primitive eusociality in Platycerium, specifically in P. bifurcatum. There is division of labour, with both nest (humus-catching) and photosynthetic fronds, generations of these fronds overlapping; water and nutrients are utilized collectively. The colonies may include plants with different genotypes, or often all the plants may be the same genotype, plants developing from buds along the profuse root system (Burns et al. 2021).
Lecanopteris (Microsoroideae) is an epiphytic myrmecophyte commonly found in ant gardens from Malesia to the Pacific (Haufler et al. 2003, Testo et al. 2019b and Jiang et al. 2024, all phylogeny; Chomicki et al. 2017a; Jiang et al. 2024). Rhizome morphology varies according to the species, in some taxa the rhizomes are monomorphic, with fronds, little branched and with a gallery and chamber system, while in others the rhizomes are dimorphic, the domatial rhizomes being frondless, much-branched and hollow (Gay 1991). The basal L. mirabilis lacks swollen rhizomes, and Suissa et al. (2024) noted that none of the species has nectaries.
For details of desiccation tolerance in Pleopeltis polypodioides, the scales functioning rather like those in Tillandsia, see John and Hasenstein (2016).
A few species are known to have persistent, vegetatively-reproducing gametophytes (Yoneoka et al. 2024).
Plant-Animal Interactions. Polypodiaceae are one of the major groups of ferns whose members are frequently associated with ants (Suissa et al. 2024), and there are more species known to produce galls than any other family of ferns (M. G. Santos et al. 2017 - although of course the family is rather large...
Plant-Bacterial/Fungal Associations. An association with the sporophyte of the ascomycete Acrospermum maxonii had been reported from a number of Polypodiaceae in Mycopteris and Ascogrammitis, the fungus reproducing on the plant (e.g. Sundue 2014; see Sundue et al. 2015). The fungus Tricharia has been reported from Serpocaulon (Sanín et al. 2023). The association does not seem to be detrimental to the fern.
Genes & Genomes. Jiang et al. (2024) found that Lecanopteris had very large plastomes; there was considerable expansion of the chloroplast IR region while variation in the SSC region was 119-21,607 bp.
Chemistry, Morphology, etc.. For root anatomy, see H. Schneider (1996, 1997), and for petiole anatomy, see Sundue et al. (2014a, esp. b). Mondal and Moktan (2023a) examined petiole and midrib anatomy - in the former the two bundles at the apices of the arms of the U or V formed by the separate petiole bundles were the largest, although a few taxa had smaller wing bundles, while the midrib of Loxogramme involuta was particularly distinctive. Some of the epiphytic taxa are soleno- or dictyostelic (Suissa & Friedman 2022); see also Mondal and Moktan (2023b) for rhizome anatomy - the presence/absence of sclereid nests, etc..
See Sanín et al. (2023: Serpocaulon. C.-C. Chen et al. (2020) describe spore morphology in Microsoroideae, and this provides some tribe-level apomorphies.
Phylogeny. For relationships in Polypodiaceae, see Janssen et al. (2007) and in particular the plastome analysis of Wei et al. (2021); in the latter, Synammia, the placement of which had previously been unclear, was sister to Drynarioideae. Polypodioideae were paraphyletic, Grammitidoideae being included, and how plastome relationships might relate to those to be obtained using nuclear data was unclear (Wei et al. 2021). Wei and Zhang (2022) carried out concatenated analyses of 88 chloroplast genes and the nuclear ribosomal complex and found some discordance in separate analyses of genes from the two compartments; the topology of the combined analysis was that of the chloroplast tree, which is hardly surprising since the plastid data contained over 25 times as many informative sites as the nuclear data - the focus here was on genera, although only 6/33 genera of Grammitidoideae were included.
Campyloneuroideae. Labiak and Moran (2017) looked at relationships in the Neotropical and largely epiphytic genus Campyloneurum.
Crypsinoideae: He et al. (2018: chloroplast genes) examined relationships around Selliguea s.l., finding it to be made up of three main clades.
Grammatidoideae. For relationships in the grammitids, see Sundue et al. (2010, 2014a, esp. b, 2015 and references: generic changes) and Bauret et al. (2017) - Grammatis, Enterosora, etc., polyphyletic. For relationships around Pyrrosia, see Zhou and Zhang (2017); there was probably ancient hybridization here, but Hovenkamp's sections have held up quite well). Shalisko et al. (2019: 208 spp, 6 chloroplast markers) clarified relationships in the basal African-American grade of the subfamily, while X.-M. Zhou et al. (2023: 412 spp., all but one genus included, 6 chloroplast markers, key to 35 Old World genera) did the same for the Malesian-Pacific clade that makes up the rest of the subfamily - the latter group finding that Ctenopterella and Grammitis in particular were polyphyletic. There appear to be a substantial number of cryptic species in the Malesian-Pacific clade (Zhou et al. 2023).
Microsoroideae. For a phylogeny of microsoroid ferns, see Kreier et al. (2008) and C.-C. Chen et al. (2019: 17 clades/genera), both groups (and others) having noted that Microsorum was not monophyletic. Testo et al. (2019b) revisit this problem and make some appropriate nomenclatural changes. L. Zhang et al. (2019: chloroplast genes) looked at relationships in the Old World Leptochilus; they found that basal relationships were unclear and that there was quite extensive infraspecific variation/polyphyly in some species, suggesting that the number of species in the genus had been seriously underestimated. Zhang et al. (2024a, also b) returned to the problem with improved sampling. For relationships in Lepisorus/Lepisoreae, see C.-F. Zhao (2019) and L. Zhang et al. (2020a) in particular. Testo et al. (2019b; see also Jiang et al. 2024: plastome and nuclear transcriptome analysis) looked at relationships around Lecanopteris, and they found that L. mirabilis, which lacks swollen rhizomes, was sister to the rest of the genus; there is extensive cytonuclear discordance here (see also Jiang et al. 2024).
Platycerioideae. B. Xue et al. (2024: plastome analysis) looked at relationships between all species of Platycerium; support was generally good. J. Zhao et al. (2024: all spp., data from plastomes and single-copy nuclear genes) found three main clades in the genus, the Afro-American (one species from America), Javan-Australian and the Malayan-Asian clades. There was gene-tree conflict, cytonuclear discordance, etc.; ancient hybridisation and incomplete lineage sorting were to be taken into account. The focus of the study by X.-M. Zhou (2017) was Pyrrosia (50 species examined, 4 plastid markers), and they found that the clade [P. costata + P. stigmosa] was sister to the rest of the genus, which was in turn made up of three large clades.
Classification. He et al. (2018) attempted to bring stability to the Selliguea area by broadening the concept of that genus - clades around there lack morphological apomorphies... C.-C. Chen et al. (2019) divided microsoroid ferns into six tribes, although not all clades that they thought were likely to be genera had generic names... X.-M. Zhou et al. (2017) revised relationships within Platycerioideae, i.a. describing Hovenkampia. L. Zhang et al. (2020a) completed the dismemberment of Lepisorus into several genera, however, C.-F. Zhao (2019; Zhao et al. 2020: corrections) provided an infrageneric classification for a broadly-delimited Lepisorus in which they recognized 17 sections, while Testo et al. (2019b) described three genera, two new, to include the sucessive sister clades to the myrmecophyte Lecanopteris. Wei and Zhang (2022) provided a new subfamilial classification of Polypodiaceae; 3 of the 9 subfamilies they recognized (see above) were new, and these new subfamilies were needed to keep Grammatidoideae and Polypodioideae separate... Note that the classification is based on the topology of a chloroplast tree, and that of the nuclear tree they show is somewhat different - things seem somewhat up in the air. Zhou et al. (2023) noted that almost three quarters of grammitidoid genera - ca 30 - had been described since 1988.
[Cystopteridaceae [[Rhachidosoraceae [Diplaziopsidaceae [Aspleniaceae [Desmophlebiaceae + Hemidictyaceae]]]] [Thelypteridaceae [Woodsiaceae [Athyriaceae [Blechnaceae + Onocleaceae]]]]]] / Eupolypod II / Aspleniineae: petiole vasculature with a V-shaped bundle changing to two, ± elongated or crescent-shaped [in t.s.]; rhachis sulcus wall confluent with the costa of pinna; sorus linear, indusium laterally attached, sporangia with 7-21 cells/annulus [?level].
Age. This node is estimated to be ca 103.1 Ma (Schuettpelz & Pryer 2009), (196-)186(-184) Ma (Testo & Sundue 2016), ca 90.2 Ma (C.-H. Huang et al. 2022), ca 150 Ma (X.-Y. Du et al. 2021) and ca 163.0 Ma (Nitta et al. 2022).
Evolution: Divergence & Distribution. For a discussion of possible apomorphies in this clade, see Sundue and Rothfels (2014).
Phylogeny. For some relationships within the Eupolypod II clade, see above.
CYSTOPTERIDACEAE Schmakov - Back to Polypodiopsida
Rhizome long-creeping; veins reaching the frond margin; indusium 0 or hood-like; n = 40, etc..
3/37: [list], Cystopteris (27). North Temperate, esp. Europe, IndoMalesia to Japan, Cystopteris fragilis to Chile.
Age. Rothfels et al. (2015a) estimated that crown-group Cystopteridaceae were (76.2-)57.9(-40.2) Ma, and the comparable age in X.-Y. Du et al. (2021) is ca 70.0 Ma.
Evolution: Divergence & Distribution. There has been hybridization between Cystopteris and Gymnocarpium, two clades separated for some 60 Ma - (76.2-)57.9(-40.2) Ma, = xCystocarpium roskamianum, there may also be hybridization between Cystopteris and Asplenium, = xAsplenicystopteris, which are members of clades that have been separated for 100(+) My or so (Rothfels et al. 2015a; H.-M. Liu et al. 2020), and there has been additional very extensive hybridization in the family, perhaps especially within Cystopteris where C. fragilis alone represents at least six distinct allopolyploid taxa (Rothfels et al. 2014).
Phylogeny. For phylogenetic relationships, see Rothfels et al. (2013). The genera are monophyletic (Rothfels et al. 2014) - but see above.
Age. This node may be ca 146.8 Ma (X.-Y. Du et al. 2021).
[Rhachidosoraceae [Diplaziopsidaceae [Aspleniaceae [Desmophlebiaceae + Hemidictyaceae]]]] / RHADD clade: pinna sulcus wall continuous with rhachis sulcus wall [?check]; frond margins translucent; sorus elongated, on one side of the vein; gametophyte: thallus margins glabrous.
Age. 141 Ma is an estimate of the crown-goup age of this clade (X.-Y. Du et al. 2021).
RHACHIDOSORACEAE X. C. Zhang - Rhachidosorus Ching - Back to Polypodiopsida
Rhizome scales clathrate; petiole bundels U-shaped; sori on one side of vein; n = 41.
1/8: [list]. East Asia to Japan, Sumatra and the Philippines.
[Diplaziopsidaceae [Aspleniaceae [Desmophlebiaceae + Hemidictyaceae]]]: rhizome scales not clathrate; scales on mature fronds 0.
Age. The crown-group age of this clade may be ca 130.6 Ma (X.-Y. Du et al. 2021).
DIPLAZIOPSIDACEAE X. C. Zhang & Christenhusz - Back to Polypodiopsida
Plant proliferous [producing plantlets by asexual reproduction]; roots pale, fleshy; fronds soft and fleshy; vein endings raised and thickened, forming a submarginal vein; sori elongated, only on one side of vein; n = 40, 41.
2/4: [list], Diplaziopsis (3). East Asia to Malesia and the Pacific.
Age. Crown-group Diplaziopsidaceae are 28.2 Ma (X.-Y. Du et al. 2021).
[Aspleniaceae [Desmophlebiaceae + Hemidictyaceae]]: ?
Age. This clade is ca 123.7 Ma (Wu et al. 2021).
ASPLENIACEAE Newman - Back to Polypodiopsida
Epiphytes common; root pericyclic sclereids with excentric lumina; rhizomes dorsiventral/radial, scales clathrate; frond trace single, circumendodermal band surrounding trace 0(?); petiole vasculature with bundles back-to-back, C-shaped / fusing and becoming X-shaped (V- or U-shaped), (circumendodermal band +), (cortex lacking intercellular spaces - Hymenasplenium); frond usu. 2-3 times pinnate, with small clavate hairs, margins decurrent and forming lateral ridge along rhachis; sori on one side of vein, (paired); indusia lateral, linear; sporangium stalk 1 cell across in the middle; spores with decidedly winged perine; n = (35) 36 (38) 39.
2/730: [list], Asplenium (700), Hymenoplesium (30). Widely distributed.
Age. Crown-group Aspleniaceae are ca 57.7 Ma (Schuettpelz & Pryer 2009) or ca 82 Ma (X.-Y. Du et al. 2021).
Evolution: Ecology & Physiology.Asplenium s.l. includes a large number of epiphytic species (Zotz 2013). A number of epiphytic Aspleniaceae also trap litter (Zona & Christenhusz; "trash-basket epiphytes" - Ortega-Salis et al. 2021). Some of the individuals of, say, Asplenium nidus can become very large, weighing ca 1 ton dry weight, and are ecologically very important, containing ca 1/2 the insect biomass of the canopy, among which ants and termites are important, making nests in the plants (e.g. Ellwood et al. 2002; Ellwood & Foster 2004).
Hymenasplenium murakami-hatanakae was recently desccribed as having persistent, gametophytes that can only reproduce vegetatively (Yoneoka et al. 2024).
Plant-Animal Interactions. Fayle et al. (2012) looked at the association of ants with Asplenium phyllitidis and A. nidus in the Danum Valley (Sabah, Borneo). The asociations of the two species were similar, and all told Fayle et al. (2012) found 71 ant species from 27 genera on the 83 individual plants they studied - there were some 248 ant colonies, and the species involved were a subset of those found on the forest floor. The ants did benfit the plant - protection against herbivores - and the ferns did benefit the ants - they provided a place to live - but there was no specific adaptation/investment by either party, and the ants formed their colonies in the fern "core", a spongy mass in the centre of the plant, part of the trash basket, that includes decomposing plant material.
Chemistry, Morphology, etc.. Helical, non-lignified wall thickenings (c.f. the velamen of monocots) occur in cortical cells of the roots of some Asplenium, mostly epiphytic species (Leroux et al. 2011). For petiole anatomy, see P.-H. Kuo et al. (2024).
Phylogeny. For the phylogeny of Asplenium, see H. Schneider et al. (2017), and they noted that some major clades are largely diploid, others are largely polyploid. See also K.-W. Wu et al. (2020: 6 plastid genes), and also Ohlsen et al. (2014) for Hymenasplenium and Asplenium from Australasia and the S.W. Pacific.
Classification. For generic limits, see Bellefroid et al. (2010 and references); Asplenium s. str. is paraphyletic.
[Desmophlebiaceae + Hemidictyaceae]: ?
Age. This node is ca 110.2 Ma (X.-Y. Du et al. 2021).
DESMOPHLEBIACEAE Mynssen, A. Vasco, Sylvestre, Moran & Rouhan - Desmophlebium Mynssen, A. Vasco, Sylvestre, Moran & Rouhan - Back to Polypodiopsida
Rhizomes erect or decumbent; petiole bundles hippocampiform, U-shaped or free; fronds unequal-pinnate, veins free, submarginal vein +, thickened; sori elongated; spores cristate; n = ?
1/2: [list]. Costa Rica to Brazil.
For information on Desmophlebiaceae, see Mynssen et al. (2016).
HEMIDICTYACEAE Christenhusz & H. Schneider - Hemidictyum marginatum (L.) C. Presl - Back to Polypodiopsida
Fronds unequal-pinnate, with submarginal collecting vein and margins with broad membranous border; vein endings raised and thickened; n = 31.
1/1: [list]. S. Mexico to S.E. Brazil.
[Thelypteridaceae [Woodsiaceae [Athyriaceae [Blechnaceae + Onocleaceae]]]] / WOBAT clade: (SiO2 accumulation common [?Athy., Onocl.]); frond once-pinnate/pinnatifid; gametophyte: thallus with papillate margins.
Age. This node is ca 140.4 Ma (X.-Y. Du et al. 2021).
THELYPTERIDACEAE Pichi-Sermolli - Back to Polypodiopsida
(Fiddlehead mucilaginous); etiole vasculature with two bundles uniting distally into a gutter shape; hairs acicular, whitish or hyaline [on fronds, also on surface and/or margins of rhizome scales]; rhachis and costa adaxially grooved, grooves not continuous from one axis to next; basal vein of frond lobe/pinnule develops on basiscopic side [catadromous]; sporangial stalk three cells across [?level]; n = 27-36.
37/1,200: [list], Amauropelta (233), Sphaerostephanos (190), Goniopteris (138), Pneumatopteris (80), Christella (70), Plesioneuron (60), Coryphopteris (47), Pronephrium (37), Reholttumia (30) - Thelypteris (2)! More or less world-wide.
Age. The age of crown-group Thelypteridaceae is estimated to be ca 68.5 Ma (Schuettpelz & Pryer 2009) or ca 90.8 Ma (X.-Y. Du et al. 2021).
The oldest fossil assigned to Thelypteridaceae with confidence is a Cyclosorus in deposits from the K-Pg boundary from Colorado (Fawcett & Smith 2021).
Evolution: Divergence and Distribution. Nothogenera here include xChrinephrium (Christella x Pronephrium), xChrismatopteris (Christella x Pneumatopteris) and xGlaphypseudosorus (Glaphyropteridopsis x Pseudocyclosorus) (H.-M. Liu et al. 2020). Indeed, Christella seems often to be involved in deep hybridizations here, that is, in intergeneric hybridization involving species that had long been separated - maybe up to 45 My or so (Tseng et al. 2024).
Chemistry, Morphology, etc.. Oliveira et al. (2017) discuss mucilage secretion by uniseriate glandular hairs - they called the hairs colleterss. Such hairs are uncommon.
Phylogeny. Fawcett et al. (2021) have recently carried out an extensive phylogenetic analysis of the family using 407 nuclear loci and 621 samples. I.a. Pneumatopteris turned out to be duodecaphyletic, and tree topology varied somewhat depending on the analysis, Christella being a particularly notable example.
Classification. For an evaluation of generic limits, which might seem to be best drawn broadly given the extensive generic polyphyly and highly homoplasious "generic" characters, especially within Cyclosorus s.l., see He and Zhang (2012) and also Almeida et al. (2016) - there have even been suggestions that just a single genus should be recognized in the family (references in Fawcett et al. 2021). Note that the classification of the Pteridophyte Phylogeny Group (2016) had been proposed at a time when major phylogenetic problems within the family were evident but for the most part had not been addressed. Fawcett and Smith (2021) have recently suggested that 37 genera should be recognized, and although identification of these genera may indeed not be easy (see also Fawcett et al. 2021), at least there is a key; Fawcett and Smith also recognize infrageneric groupings.
[Woodsiaceae [Athyriaceae [Blechnaceae + Onocleaceae]]]: ?
Age. The age of this node is around 136.1 Ma (X.-Y. Du et al. 2021).
Phylogeny. Woodsia (= Woodsiaceae) may be sister to this whole clade (Schuettpelz & Pryer 2009: Fig. S1).
WOODSIACEAE Herter - Woodsia R. Brown - Back to Polypodiopsida
Plant epipetric; rhizomes suberect; articulated hairs + (0), glandular hais +/0; petiole bases persist; circumendodermal band 0; sori/indusium saucer-shaped/more or less globose/many strap-shaped or filamentous segments/0, receptacle raised; n = 33, 37-39, 41.
1-2/65: [list]. Mostly montane, northern hemisphere, to tropical South America, a few in Africa and Madagascar.
Age. Crown-group Woodsiaceae are ca 45 Ma (A. Larsson in N. T. Lu et al. 2020) or ca 69.5 Ma (X.-Y. Du et al. 2021).
Evolution: Divergence & Distribution. N. T. Lu et al. (2020) looked at the distributions of a number of characters in the context of the phylogeny of the family.
xWoodsimatium, Woodsia x Physematium, is a nothogenus here (H.-M. Liu et al. 2020).
Phylogeny. For relationships, see N. T. Lu et al. (2020: 43 spp., plastid analyses).
Classification. Schmakov (2015) recognized seven genera in two subfamilies, N. T. Lu et al. (2020) two genera, which hybridize, and five subgenera, three in Physematium and two in Woodsia, while the Pteridophyte Working Group (2016) recognized but a single genus. Species limits are also in a mess,, estimates being 22-65.
[Athyriaceae [Blechnaceae + Onocleaceae]]: ?
Age. An estimate of the age of this node is ca 132.7 Ma (X.-Y. Du et al. 2021).
ATHYRIACEAE Alston - Back to Polypodiopsida
Mature frond with abundant anthocyanin; margins entire ["smooth"], petiole base swollen [starch-containing, ± persistent, = trophopod]; corniculae/scales at adaxial junction of pinna costa with rachis; sori on both sides of vein, indusia opening to face away from vein [two linear back to back sori or J-shaped indusium wrapped around a vein]/(on one side of vein); n = (39) 40.
3-7/650: [list], Diplazium (300-350), Athyrium (160-230), Deparia (92). Mostly terrestrial, understory.
Age. Crown-group Athyriaceae are around 78.4 Ma (Schuettpelz & Pryer 2009) or ca 121.3 Ma (Du et al. 2021).
Evolution: Divergence & Distribution. For the high diversification rate in the family, the highest in the ferns as a whole, see Testo and Sundue (2016), and for divergence dates and biogeography in Diplazium, perhaps a member of the boreotropical flora in the Eocene, see Wei et al. (2015). Nothogenera here include xCornathyrium, Cornopteris x Athyrium, and xSchnellerathyrium, Athyrium x Pseudathyrium (H.-M. Liu et al. 2020a).
Phylogeny. Wei et al. (2013) evaluated relationships within Diplazium and found i.a. that species previously assigned to Allantodia in particular were scattered through the tree; they circumscribed Diplazium broadly; Wei et al. (2024: 186 plastomes, 178 taxa with nuclear ribosomal genes, 211 taxa with three chloroplast genes) dealt with this problem in more detail. The latter found that the topology based on the nuclear ribosomal gene was rather different from those based on nuclear genes (Wei et al. 2024). Wei et al. (2017) and Moran et al. (2019) disentangled relationships in Athyrium, chipping off some small genera, while Kuo et al. (2017, 2018a) looked at relationships in Deparia.
Classification. R. Wei et al. (2013, esp. 2024) provided an infrageneric classification for Diplazium, in the latter paper four subgenera and 12 sections, most new, that are correlated with both geography and morphology being recognized. In Kuo et al. (2017, 2018a) Deparia was given an infrageneric classification, the groups being morphologically characterized.
[Blechnaceae + Onocleaceae]]: fronds dimorphic [fertile and sterile].
Age. This node is ca 97.4 Ma (X.-Y. Du et al. 2021).
Evolution: Divergence & Distribution. Suissa and Smith (2024) looked at the evolution of dimorphic frond morphology here. In some species on the one plant can be found both purely vegetative and also fertile fronds, the latter being more highly dissected, with a higher soral to laminar area ratio and with an overall somewhat reduced area. Of course, fronds in monomorphic species may sometimes be sterile, but then their laminar area tends to be nearly identical to that of the fertile pinnae. The basal condition in Onocleaceae is having at least 50% of the lamina of the fertile frond being covered by sori, and the fertile fronds also showed hygromorphic movement - that is, there is humidity-driven movement of the fronds - and the change monomorphic → dimorphic frond morpholog was irreversible. The basal condition in Blechnaceae was to lack frond dimorphism, and species that did have dimorphic fronds lacked such hygromorphic movements and the change to dimorphism was evoluionaril reversible (Suissa & Smith 2024, q.v. for details of the evolution of this feature).
BLECHNACEAE Newman - Back to Polypodiopsida
Stem (with perforated dictyostele); young fronds reddish; petiole with several vascular bundles; ?trophopods, (fronds monomorphic), basal vein of frond lobe/pinnule develops on basiscopic side [catadromous], veins forming narrow areoles near the costa [costular anastomoses]; sori linear, on subcostal commissural vein, opening towards midvein, (on arches of areolae), (acrostichoid), indusia opening towards costa, (0); perine winged; x = 34, [n = 27-29, 31-37, 40].
24/267: [list: to subfamilies], Cosmopolitan.
Age. Crown-group Blechnaceae are around 59.8 Ma (Schuettpelz & Pryer 2009), however, an age of almost 100 Ma is suggested by Vicent et al. (2017: also more dates), and in line with this Molino et al. (2019) date [Brainea + Struthiopteris] (Blechnoideae) to around 84.9 Ma. The age of the family in X.-Y. Du et al. (2021) is estimated to be ca 62.4 Ma, in Berry (2022) ca 75.7 Ma and in Testo et al. (2022) ca 74-73 Ma.
1. Woodwardioideae Gasper, V. A. O. Dittrich & Salino
3/16: Woodwardia (14). N. Hemisphere.
Age. The age of this clade is some 60 Ma (Testo et al. 2022).
[Stenochlaenoideae + Blechnoideae]: ?
Age. This clade is ca 67.8 Ma (Berry 2022) or (107.5-)89(–72.3) Ma (Testo et al. 2022).
2. Stenochlaenoideae (Ching) J. P. Roux
(Plant viny - Salpichlaena); stomata (polocytic).
3/12: Stenochlaena (8). ± Tropical, scattered.
Age. Crown-group Stenochlaenoideae are estimated to be ca 54.5 Ma (Berry 2022; see also Testo et al. 2022).
Testo et al. (2022) discuss Palaeocene fossils from North America possibly belonging to Salpichlaena.
3. Blechnoideae Gasper, V. A. O. Dittrich & Salino
19/242: Parablechnum (68), Austroblechnum (40); Blechnum (30). Mostly southern Hemisphere, esp. tropical America, New Zealand and Oceania, few Africa to Southeast Asia.
Age. Crown-group Blechnoideae are perhaps ca 59 Ma (Testo et al. 2022).
Evolution: Divergence & Distribution. Ages are particularly difficult around here, and Berry (2022) notes of Stenochlaena that it may have survived the K/Pg event - or a genome duplication in the genus and its subsequent diversification may have happened around the PETM...
Testo et al. (2022) suggested that all three subfamilies were originally Eurasian, with present-day distributions resulting from climate-mediated vicariance and a dizzying series of long distance dispersal events (ibid.: Fig. 5, 113(±63) such events). Although tending to be tropical in distribution, the family shows a fair bit of cold tolerance, and species grow at high altitudes on tropical mountains and in the far south of South America. The age of crown-group Sadleria, restricted to Hawaii and just under 15 Ma, suggests that it initially colonized now-submerged Hawaiian islands (Testo et al. 2022). For other notes on biogeography, see Moran and Smith (2001) and Vicent et al. (2017).
Moran et al. (2018) looked at spore morphology here in the context of phylogeny.
Ecology & Physiology. Salpichlaena (Stenochlaenoideae) is a genus of viny taxa.
Phylogeny. Perrie et al. (2014) discuss relationships in Blechnaceae; see also de Gasper et al. (2017) and Testo et al. (2022).
Classification. Perrie et al. (2014) circumscribed Blechnum quite broadly. For a rather splitty classification, with 24 genera and three subfamilies, see de Gasper et al. (2016, 2017: justification p. 440). For genera around Struthiopteris, see Molino et al. (2019).
ONOCLEACEAE Pichi-Sermolli - Back to Polypodiopsida
Circumendodermal band 0; fronds dimorphic, petiole basally ± swollen [trophopod], vascular bundles uniting distally into a gutter shape; sporophylls: sori enclosed by reflexed lamina margins; indusium deltate; sorus with 24-35 cells/annulus; spores chlorophyllous; n = 37, 39, 40.
4/5 [list]: Pentarhizidium (2). Northern Hemisphere.
Age. Crown-group Onocleaceae are ca 76.1 Ma (X.-Y. Du et al. 2021).
Onoclea sensibilis is known fossil from Palaeocene North America 62-58 Ma, the fossils being remarkably similar to extant individuals (Rothwell & Stockey 1991).
Evolution: Fertilization & Spore Dispersal. In Onoclea sensibilis and other members of the family, and even Blechnaceae, hygroscopic movement of the dead fern fronds in the early spring results in the leaflets uncurling, and this allows the dispersal of the spores, which have in fact matured by the autumn before (Suissa 2022).