EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]: sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; seed exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ERICALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]]: ovules lacking parietal tissue [= tenuinucellate] (present).
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID I / LAMIIDAE / [CARDIOPTERIDALES [GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]]: ?
[GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]: G [2], superposed; loss of introns 18-23 in RPB2 d copy. - check
[ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]: vessel elements with with simple perforation plates; nodes 1:1.
[[GENTIANALES + BORAGINALES] VAHLIALES, SOLANALES, LAMIALES] / CORE LAMIIDS:: herbaceous habit widespread; (8-ring deoxyflavonols +); C forming a distinct tube, initiation late [sampling!]; A epipetalous; (vascularized) nectary at base of G; style long; several ovules/carpel; fruit a septicidal capsule, K persistent. 42 families, 2,497 genera, 51,185 species.
Age. Magallón and Castillo (2009) offer estimates of ca 81 Ma for both relaxed and constrained penalized likelihood datings for this clade - but no Vahliaceae. The age for the same node in Bell et al. (2010) is (96-)87, 83(-77) Ma (no Vahlia) or (102-)92, 86(-79) Ma (Vahlia included), in K. Bremer et al. (2004a) it is ca 108 Ma, in Foster et al. (2016a: no Vahlia) it is ca 96 Ma, in Xue et al. (2012) it is only 60.4 or 57.6 Ma, and in Naumann et al. (2013: no Boraginales) it is 77.5-76.5 Ma, while in Nazaire et al. (2014) it is (102.1-)89.1(-73.3) Ma - but c.f. topologies. The age of this node is estimated to be around 113 Ma by Z. Wu et al. (2014: Boraginales sister to the rest), ca 76.3 Ma by Tank et al. (2015: Table S1) and ca 82.8 Ma - Solanales + Gentianales (Y.-L. Cao et al. 2024).
Evolution: Divergence & Distribution. Magallón and Sanderson (2001) and Magallón and Castillo (2009) found high diversification rates in the four large orders, and the herbaceous habit (see S. A. Smith & Donoghue 2008) is also common here; Magallón et al. (2018) dated an increase in diversification rate at this node to (92.7-)91.1(-89.7) Ma - Vahliales were were not included.
There are several characters of potential phylogenetic interest in this group (see also Stull et al. 2018 and discussion). The hydroxycinnamic acid depside, rosmarinic acid, is known from Boraginaceae and Hydrophyllaceae, as well as some Lamiaceae (Mølgaard & Ravn 1988). Lamiales and Boraginaceae have in common similar hydroxycinnamic acid derivatives, i.e. disaccharide esters of rosmarinic/lithospermic/caffeic acids (Mølgaard & Ravn 1988). Solanaceae are noted for the diversity of acylsugars contained in their glandular hairs. These are not known from Convolvulaceae, but Moghe et al. (2017: esp. Figs 6, 8) suggest that acylsugar acyltransferases evolved around 70 Ma, related enzymes occurring in Gentianales and Boraginales, but not in Lamiales or Convolvulaceae (Vahliales were not sampled); the evolution of these acyltransferases was perhaps associated with genome duplication. Oleaceae, Lamiaceae, and Solanaceae have (arabino)xyloglucans and some (galacto)xyloglucan hemicelluloses in the cell wall; the plesiomorphic condition for seed plants is to have (fuco(galacto))xyloglucans (O'Neill & York 2003; Harris 2005), but the sampling is very poor. Boraginaceae have callose plugs in the pollen tube, as do Solanales, but their presence varies in Hydrophyllaceae, and they are absent in Heliotropiaceae, Cordiaceae, and monosymmetric-flowered Lamiales (Cocucci 1983). Protein crystals in nuclei are common, but are apparently not known from Avicennia, etc. (Speta 1977, 1979), and information is needed for groups recently moved to Lamiales. Whether or not such crystals characterise both Lamiales and also Boraginaceae s. str. (see also Wagstaff & Olmstead 1997) needs to be confirmed. Although some Boraginaceae have protein bodies in their nuclei, they are of two very different kinds, and many Boraginaceae entirely lack them; there is also variation within Lamiales.
González and Rudall (2010) thought that a bicarpelate gynoecium might be an apomorphy for this clade, and it was perhaps derived from a pseudomonomerous gynoecium like that of Metteniusa (Metteniusaceae) - see also below for gynoecial evolution. Certainly, taxa with sympetalous flowers, epipetalous stamens, and a bicarpelate gynoecium are overwhelmingly the commonest here, and variation beyond this theme is slight.
Core lamiids include many annuals and herbaceous to shrubby perennials. Indeed, the annual/herbaceous habit is common here, there being no or only slight secondary thickening in such cases, and this seems to be the result of changes in regulatory interactions between genes; the actual genes involved seem not to have been lost, unlike the situation in monocots and some aquatics (Davin et al. 2016; Roodt et al. 2019). As a result, reacquisition of the woody habit is quite common, perhaps most notably on islands, but in many other situations, too (e.g. Carlquist 1973; Rowe & Paul-Victor 2012; Lens et al. 2012b, 2013; Davin et al. 2016). Many core lamiids have dry fruits with very small seeds of 10-2 grams or less (Moles et al. 2005a; Linkies et al. 2010), although Convolvulaceae, Lamiaceae, and Verbenaceae, for example, have a maximum of four seeds/fruit. However, even when each fruit has fewer seeds, these are still generally small. Seed coats often have a mechanical layer just a single cell thick, although Convolvulaceae are a notable exception. Haig and Westoby (1991) discuss situations in which small seeds may be at an advantage. Overall diversification rates are higher in smaller- than larger-seeded angiopserms, perhaps linked to improved colonization potential (Igea et al. 2017), and the rate of seed mass change is also higher when rates of diversification are higher. For further discussion about seed size, see core asterids and core campanulids. The flowers of core lamiids are relatively large and often monosymmetric.
Plant-Animal Interactions. Nylin et al. (2014) noted that members of all the four larger orders were hosts for nymphalid butterfly larvae, three (not Boraginales) being "important" hosts - only seven orders in this category were mentioned.
Chemistry, Morphology, etc.. Hoffman et al. (2005) found that the xyloglucans of the few Solanales and Lamiales they examined lacked fucosylated subunits; the one Gentianales they examined was intermediate. This clade (bar Boraginales) may have closed root apical meristems (Clowes 2000), but the sampling is poor.
Phylogeny. Relationships in this part of the tree have long been unclear, even if its overall composition has not changed much. Thus Bell et al. (2010; see also J. F. Walker et al. 2019: no Boraginales) suggested the grouping [Lamiales [Gentianales [Boraginaceae [= Boraginales hereafter] + Solanales]]], Lens et al. (2008a: Bayesian analyses) and Magallón and Castillo (2009) that of [Gentianales [Lamiales [Boraginales + Solanales]]], and Lens et al. (2008a: maximum parsimony) found the relationships [[Solanales + Gentianales] [Lamiales + Boraginales]]. Qiu et al. (2010: mitochondrial genes) did not find Boraginales and Gentianales to be immediately associated, although they were not strongly separated, while the two were sometimes linked, if with weak support, by Ruhfel et al. (2014: see also other positions for Boraginales). In general, relationships between Gentianales, Lamiales and Solanales remained uncertain (Albach et al. 2001b; B. Bremer et al. 2002; Janssens et al. 2009, Boraginales not included; J. Li & Zhang 2010). Even an analysis of all 79 protein-coding plastid genes and four mitochondrial genes did not clarify them (Moore et al. 2008) nor did the 17-gene 640-taxon study of Soltis et al. (2011). Finet et al. (2010) found quite good support for a [Gentianales + Solanales] clade, but no Boraginales were included.
Weigend et al. (2013b), sampling four plastid loci and 134 taxa (including 81 Boraginales), found the very weakly supported sets of relationships [Boraginales [Lamiales [Solanales + Gentianales]]] (see also Nazaire et al. 2014: Suppl. Fig. 4A, ITS + 4 plastid markers) and [Lamiales [Gentianales [Solanales + Boraginales]]] in different analyses. The [Solanales + Boraginales] clade was recovered by Luebert et al. (2016b), but the position of Gentianales was unclear, and similar relationships (with similarly low support) were also found by Luna et al. (2019). In plastome analyses, the relationships [Lamiales [Boraginales [Gentianales + Solanales]]] were recovered by Ku et al. (2013a; for [Gentianales + Solanales], see also Martínez-Alberola et al. 2013), while Boraginales were sister to the other core Lamiales in the study by Z. Wu et al. (2014), although relationships were not strongly supported. On the other hand, Refulio-Rodriguez and Olmstead (2014), sampling 1 mitochondrial and 9 plastid genes and 129 terminals (75 in Lamiales), recovered the relationships [Gentianales [Solanales [Boraginales + Lamiales]]]; the whole clade had very strong support, as did the monophyly of the orders, but bootstrap support for the two nodes along the spine was only moderate (maximum likelihood) or very weak indeed (maximum parsimony). The amount of data analyzed seems to have allowed somewhat improved support for these relationships. However, the topology [[Solanales + Gentianales] [Boraginales + Lamiales]] was recovered by Magallón et al. (2015) and Z.-D. Chen et al. (2016: support very weak), while Maia et al. (2014), in one alignment using 18S/26S nuclear ribosomal data, retrieved a polyphyletic Boraginales within Solanales, although support was not strong, while another alignment suggested a clade [Boraginales + Gentianales]. The relationships [[Boraginales + Gentianales] [Lamiales + Solanales]] were recovered by Stull et al. (2015), but again support was not strong, and also by H.-T. Li et al. (2019, 2021: chloroplast genomes, support moderate/poor for the first pairing). One factor driving these conflicting relationships may be differing signals in nuclear (e.g. = [Solanales + Gentianales]) and chloroplast (e.g. = [Lamiales + Gentianales]) (see also Ku et al. 2013b) genes (Xi et al. 2014), although both Stull et al. and Li et al. looked at chloroplasts. The three genomic compartments also yielded different topologies from each other (and differing from those in Xi et al. 2014) in Sun et al. (2014). Indeed, S. A. Smith et al. (2013) had noted that inclusion of all three orders in analyses destabilized the phylogenies obtained in their survey of seed plant phylogenies. Support in this area was generally rather weak in Gitzendanner et al. (2018a: plastome analysis). However, C. Zhang et al. (2020) in their analyses using nuclear genomes found strong support for a [Boraginales + Gentianales] clade, the monophyly of the other orders was well supported, but their relationships were not; see also Stull et al. (2020a). The [Boraginales + Gentianales] clade also had support in an Angiosperms353 analysis that focused on Gentianales (Antonelli et al. 2021; see also Leebens-Mack et al. 2019) and moderate support in the extensive plastome study by Li et al. (2021).
Furthermore, the position of Vahlia (now Vahliales: A.P.G. IV 2016) remains uncertain. Magallón and Castillo (2009), Magallón et al. (2015) and Bell et al. (2010) suggested that it was sister to all other lamiids (except Garryales, Icacinales, etc.). In analyses of combined 18S/26S nuclear ribosomal data, Maia et al. (2014) found remarkably strong - given the weak support for most relationships in their study - support for this position. Vahlia was found to be sister to Lamiales, but with only 63% bootstrap support, by Albach et al. (2001b; see also Lens et al. 2008a: Bayesian analyses; Nazaire & Hufford 2012: plastid genes; Weigend et al. 2013b; Nazaire et al. 2014: Suppl. Fig. 4A; Luna et al. 2019: little support), or it links with Boraginaceae in other analyses (Lundberg 2001e; B. Bremer et al. 2002; Hasenstab-Lehman 2017: chloroplast genes). Only in ndhF analyses was there some support for a linkage with Solanales (Olmstead et al. 1999, 2000; see also Savolainen et al. 2000a; Lundberg 2001e). Refulio-Rodriguez and Olmstead (2014) also found this position, but despite the large amounts of data in their study, there was substantial support only in the Bayesian analyses, and there was also moderate support in the plastome analyses of H.-T. Li et al. (2019: familial sampling within Solanales poor, 2021: improved sampling). Stull et al. (2015) found Vahlia to be weakly linked with a [Solanales + Lamiales] clade, while there was 91% bootstrap support for a [Vahliales [Solanales + Lamiales]] clade in Stull et al. (2018), and in Tank and Olmstead (pers. comm.) the linkage was with a [Solanales [Boraginales + Lamiales]] clade. Vahlia was consistently found to be sister to Lamiales by Luebert et al. (2016b). The relationships suggested in the Angiosperms356 tree associated with W. J. Baker et al. (2021a) were rather different because Solanales were broken up, Montiniaceae and Hydroleaceae being placed along the stem lamiids, the topology being [... [Hydroleaceae [Garryales [Icacinales [Montiniaceae [Vahliales [other Solanales, Lamiales, etc.]]]]]]]. A rather different universe? However, Vahliaceae were found to be sister to Solanales, albeit with weak support, in Version 3 (April 2023) of the Seed Plant Tree, and although Montinia was in Solanales, Hydrolea was still wandering around.
In the plastome phylogeny of H.-T. Li et al. (2021) relationships are [Lamiales [Vahliales + Solanales]] [Gentianales + Boraginales]], while in the Angiosperms353 analysis of Zuntini et al. (2024) they are [[Vahliales + Lamiales] [Solanales [Gentianales + Boraginales]]]. Hydrolea is in Solanales, sister to Sphenocleaceae. See also the Seed Plant Tree, version of ix.2024, where Vahliaceae are weakly supported as being sister to Solanales, Hydrolea is sister to Lamiales.
[GENTIANALES + BORAGINALES]: ?
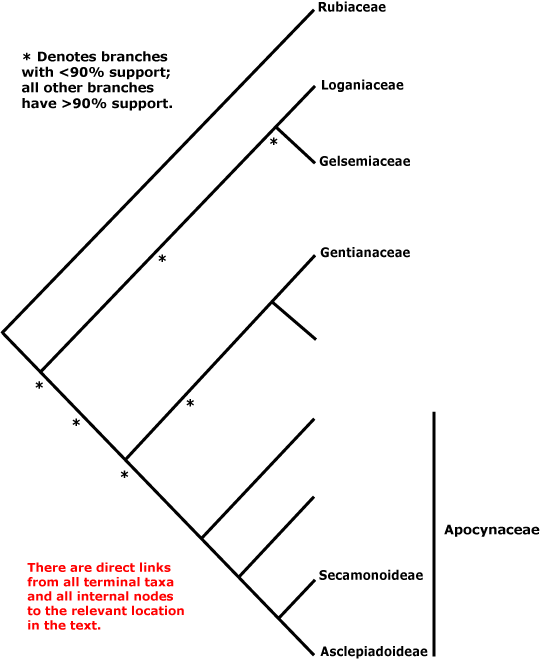
GENTIANALES Berchtold & J. Presl - Main Tree.
Route I iridoids [mostly derived from secologanin or secologanic acid], monoterpene indole alkaloids, (O-methylated) flavones and flavonols +, non-hydrolysable tannins [e.g. myricetin] usu. 0; fibre tracheids, heterogeneous rays [type IIA or IIB] +; vestured pits +; petiole bundle(s) arcuate; colleters +, glandular hairs 0; branching from current flush; leaves opposite, joined by a line across the stem; (corolla swollen at the apex in bud); pollen orbicules +; ovules many/carpel, endothelium 0; endosperm nuclear/coenocytic, copious [?level]; germination phanerocotylar, epigeal. - 5 families, 1,121 genera, 20,145 species.
Includes Apocynaceae, Gelsemiaceae, Gentianaceae, Loganiaceae, Rubiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Gentianales may be some (75-)71, 68(-65) or (68-)64, 61(-57) Ma old (Wikström et al. 2001: the first age is with Dialypetalanthus sister to the rest, the second ignores the genus). Janssens et al. (2009) date them to 79±10.2 Ma, Antonelli et al. (2009) to just under 80 My; comparable figures are 108 and 78 Ma in K. Bremer et al. (2004a) while the ages in Wikström et al. (2015), at (104-)96(-87) Ma, and Tank and Olmstead (pers. comm.), at (105.6-)91.2(-75.2) Ma, are similar. Estimates of crown group ages are (86-)73(-60) (ages in Rubiaceae table) or (75-)52(-35) Ma (ages in asterid table) in Lemaire et al. (2011b), (78-)69, 65(-54) Ma (Bell et al. 2010) and ca 67.7 Ma (Magallón et al. 2015); B. Bremer and Eriksson (2009) suggest rather greater ages of (104.7-)90.4(-76.5) Ma, end-Turonian, as do Neupane et al. (2017), at (102-)89(-78) Ma, Rydin et al. (2017), ca 90 Ma, and C. Zhang et al. 2020), ca 86 Ma, while at around 58.8 Ma the age in Tank et al. (2015: Table S2) is on the young side.
Evolution: Divergence & Distribution. Wikström et al. (2015) discuss dates suggested for the order as a whole and for Rubiaceae by earlier workers, and mention a root age for Gentianales of 98-58 Ma in B. Bremer and Eriksson (2009), but the latter give no root age. Razafimandimbison et al. (2019) suggest a number of ages for clades in Gentianales.
At about 20,000 species, this is the largest clade with the combination of fused petals and - ususually - polysymmetric flowers, often quite small in Rubiaceae and Apocynaceae-Asclepiadoideae, quite large in Gentianaceae and other Apocynaceae. Seeds are generally quite small here, but those of Henriquezia (Rubiaceae-Dialypetalanthoideae) are up to 8 cm in diameter (Cortés-B. & Motley 2015). A variety of structures are found at the nodes in all four families varying from apparently "normal" vascularized stipules common in Rubiaceae to colleters associated with the leaf base to lines across the stem at the nodes (Ye & Ronse De Craene 2024).
For iridoid synthesis, see Jensen et al. (2002) and references; there has been tandem duplication of secoiridoid biosynthetic genes in Gentianales (T. Zhou et al. 2022; Tominaga et al. 2023). Monoterpene indole alkaloids are found scattered throughout the order, but they are apparently not known from Gentianaceae themselves (they are also known from Nyssaceae-Cornales). Thus camptothecin, one of these alkaloids, is found in Ophiorrhiza (Rubiaceae), Mostuea (Gelsemiaceae) and Ervatamia (Apocynaceae), and of course Camptotheca (Nyssaceae) itself (see Lorence & Nessler 2004). These alkaloids are derived from tryptophan and the iridoid terpene secologanin, the two being condensed in Gentianales by the key enzyme strictosidine synthase, and a variety - ca 3,000+ as of 2024 - of very different terpene alkaloids are then derived from this strictosidine (O'Conner & Maresh 2008; Laforest et al. 2026 - Wink 2008 noted that strictosidine synthase is in fact quite widely distributed in flowering plants). The focus in Laforest et al. (2025) was on a group of these alkaloids, the spirooxindole alkaloids (100+ are known), mitraphyllidine in particular; these have the distinctive spiro[pyrrolidine-3,3'-oxindole] ring. Laforest et al. (2025) found such alkaloids in Mitragyne and Uncaria, both Naucleeae, and also Cinchona pubescens, and they suggested that a genome duplication in the Cinchonoideae alliance (sic) was involved; they did not find these alkaloids in Ophiorrhiza, which makes sense in this context (O. = Rubioideae), but they were absent from Coffea, the Coffeeae alliance, which makes less sense (note that Cinchona pubescens is sometimes called Coffea pubescens...).
For the evolution of pollen morphology in Gentianales, see L.-E. Yang et al. (2020), but c.f. topology, etc., e.g. the sister relationship between Oncothecaceae and Icacinaceae is supported. Endress (2011a) suggested that a key innovation in Gentianales was tenuinucellate ovules.
Plant-Bacterial/Fungal Associations. Paris-type endomycorrhizae involving Glomeromycota are common in Gentianales, although some taxa may have the Arum type or intermediates, perhaps especially in Apocynaceae (Imhoff 1999). Note, however, that Giesemann et al. (2019, 2021) have quite recently suggested that Arum and Paris AM may have play different roles in the movement of carbon through the community. For Gentianaceae and iridoid-induced A.M. hyphal branching, see that family.
Genes & Genomes. Coffea has no obvious genome duplication more recent than the eudicot/core eudicot γ whole nuclear genome duplication, the gamma triplication ( q.v.; Denoeud et al. 2014).
There is substantial variation in the presence of the mitochondrial coxII.i3 intron here (Joly et al. 2001).
Chemistry, Morphology, etc.. Jansen and Smets (2000) discuss vestured pits here. Vesturing may also be found on thickenings on the walls of septate wood fibres, as in Damnacanthus (Ohtani 1987). Colleters range from secretory palisade cells surrounding an elongated axis to much smaller, simpler, hair-like structures (some Gentianaceae, Apocynaceae-Asclepiadoideae). They are borne on leaves, the calyx or corolla (Renobales et al. 2001), or even in a ring below the leaves and encircling the stem, as well as in their normal axillary position, and they may sometimes be vascularized, as in some Rubiaceae and Apocynaceae (Fernandes et al. 2016 and references; Judkevich et al. 2017). For variation in the composition of colleter secretion, see Resmondi et al. (2015) and Ribeiro et al. (2017). There are relatively few reports of colleters in Gentianaceae (Thomas 1991). However, Dourado et al. (2022) note that they occur in three tribes there, and their morphology usually differs from that in colleters from Rubiaceae and Apocynaceae, colleters in Gentianaceae having all head cells secretory versus there being a secretory palisade epidermis in the other families. Hence although colleters are included in the ordinal characterization above, further details of their morphology and distribution may help clarify their evolution.
Tapetal variation is considerable, amoeboid tapeta being known from some species of Gentianaceae, Rubiaceae and Apocynaceae (Furness 2008a). Most families have taxa with bi- or tricellular pollen grains; for further discussion, see Williams et al. (2014).
Some information is taken from Rogers (1986: general), Erbar and Leins (1996: corolla development), Conn et al. (1997: general), Jansen and Smets (1998: wood anatomy), and Vinckier and Smets (2002a, c: orbicules).
Phylogeny. Struwe et al. (1995) suggested that Loganiaceae, even when narrowly circumscribed, i.e. excluding Buddleja, etc., were extremely paraphyletic, with clades including about 1,300 genera and 15,500 species or more (Rubiaceae, Gentianaceae, Apocynaceae + Asclepiadaceae) coming from within them; they delimited families accordingly. B. Bremer (1996a), Potgieter et al. (2000) and Backlund et al. (2000) and others have since found somewhat different relationships - Rubiaceae are consistently sister to the [Loganiaceae, Gentianaceae, Gelsemiaceae, Apocynaceae] clade.
Relationships between these last four families have been unclear for some time, and in Antonelli et al. (2021: Fig. 2) the various suggestions that have been made are spelled out. Backlund et al. (2000) early noted that C17 indole alkaloids, the number of tapetum layers, and cytology supported the relationship [Gelsemiaceae + Apocynaceae], but that the presence of quercetin and kaempferol, imbricate corolla, and horny (starchy) endosperm might support a close relationship between Gelsemiaceae and Loganiaceae. M. Endress et al. (1996) found the relationships [Gelsemiaceae [Apocynaceae [Strychnaceae + Geniostomaceae (well supported)]]], see also B. Bremer and Struwe (1992). In other analyses there is weak support for a relationship between Gelsemiaceae and Apocynaceae (Backlund et al. 2000; Jiao & Li 2007; see also B. Bremer 1999; Rova et al. 2002). However, B. Bremer et al. (2002) and Soltis et al. (2011: sampling) found some support for a sister group pair [Gentianaceae + Apocynaceae], as did Magallón et al. (2015) and Refulio-Rodriguez and Olmstead (2014: not the maximum likelihood analysis). Refulio-Rodriguez and Olmstead (2014) also found support for the pair [Gelsemiaceae + Loganiaceae] (see also Struwe et al. 2014: weak support; Wikström et al. 2015; Stull et al. 2020: chloroplast data, six taxa). On the other hand, Tank and Olmstead (pers. comm.) recovered the relationships [Apocynaceae [Gentianaceae [Loganiaceae + Gelsemiaceae]]]; L.-L. Yang et al. (2016) [Gentianaceae [Gelsemiaceae [Loganiaceae + Apocynaceae]]], but with little support; Z.-D. Chen et al. (2016) and H.-T. Li et al. (2021) the relationships [Gelsemiaceae [Loganiaceae [Gentianaceae + Apocynaceae]]] with moderate and poor support respectively; and Luna et al. (2019) [Gentianaceae [Apocynaceae [Gelsemiaceae + Loganiaceae]]], but with weak support.
Nuclear data, however, tell a slightly different story. C. Zhang et al. (2020) found strong support for the the relationships [[Gelsemiaceae + Gentianaceae] [Loganiaceae + Apocynaceae]], and they have been recovered in other studies using nuclear genomes (I.T.P.T.I. 2019; Stull et al. 2020a; Antonelli et al. 2021; the Seed Plant Tree, version of ix.2024; Zuntini et al. 2024). These latter relationships are followed below.
Previous Relationships. The circumscription and relationships of Loganiaceae are a key to understanding both the past and present circumscription and relationships of Gentianales, and genera that used to be in Loganiaceae are in several families of Lamiales. For further details, see below.
Synonymy: Apocynales Berchtold & J. Presl, Asclepiadales Berchtold & J. Presl, Chironiales Grisebach, Cinchonales Lindley, Galiales Bromhead, Loganiales Lindley, Lygodisodeales Martius, Rubiales Berchtold & J. Presl, Strychnales Link, Theligonales Nakai, Vincales Horaninow
RUBIACEAE Jussieu, nom. cons. - Back to Gentianales
Plant often woody; tanniniferous, triterpenes +, iridoids +/0; vessels mostly ≤81μm across, vascular pit borders ≤7.8μm across, aliform-confluent xylem parenchyma 0, true tracheids +, rays with multiseriate part no wider than uniseriate part; nodes ?; secretory sacs widespread; glandular hairs 0; colleters adaxial to stipules/calyx; stomata paracytic; lamina vernation usu. flat, stipules interpetiolar, (sheathing/intrapetiolar/two pairs/toothed or not), often innervated from circumferential vascular ring; inflorescences often terminal, thyrsoid, flowers often rather small and aggregated, usu. 4- or 5-merous, protandrous; K short, aestivation open/long/connate), C with early tube formation; pollen grains ± triangular in polar view [?all]; tapetal cells uninucleate; ovary inferior, nectary on top, style well developed, apically bifid, stigma wet or dry; ovules ?/carpel, integument 5-11 cells across; fruit various; seeds 1-many, exotesta alone persisting, walls variously thickened; endosperm +, horny, often hemicellulosic, at least initially with some starch; embryo straight, cotyledons relatively small [50>>% length of embryo], suspensor often uniseriate; x = 11; nuclear genome [1Cx] 0.15-0.76 pg/[1C] (0.054-)1.054(-21.818) pg.
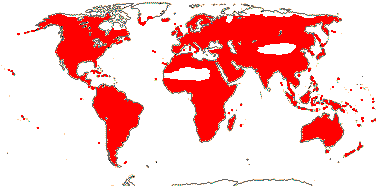
72 tribes/615/14,266 [list, to tribes] - in 72 tribes below. World-wide, but largely tropical, especially Madagascar and the Andes. Map: from Hultén (1958, 1971) and Brummitt (2007).
Age. Antonelli et al. (2009) suggested that divergence within Rubiaceae began (68.8-)66.1(-63) Ma similar to the ages in Tank and Olmstead (pers. comm.) of (83.8-)66.1(-50) Ma, although B. Bremer and Eriksson (2009) provided rather older dates of (100.8-)86.6(-72.9) Ma, and, at (98-)85(-74) Ma, also Neupane et al. (2017). Crown group ages in Lemaire et al. (2011b) are around (77-)62(-50) Ma, and (60-)56, 55(-51) Ma in Wikström et al. (2001), (69-)57(-45) Ma in Bell et al. (2010: note topology), (87.9-)84.9(-80.8) Ma in Manns et al. (2012) and (96-)87(-79) Ma in Wikström et al. (2015). At slightly over 90 Ma, the age in Rydin et al. (2017) is the oldest, but dating was not the focus of that study.
A. Graham (2009a) summarized the fossil history of Rubiaceae - there are no certain records from the Cretaceous or Palaeocene, the earliest being vegetative fossils from the Eocene of North America (Roth & Dilcher 1979) which had previously been placed in seven families and four orders. These fossils are a little odd in that Roth and Dilcher (1979), who compared them with members of Cinchonoideae and Ixoroideae (together = Dialypetalanthoideae), suggested that the stipules on the petiole bases might be single, and at least sometimes they are minutely serrulate, perhaps because of hydathodes on their margins. Martínez-Millán (2010: fossil record) dated the family to around 40.4 Ma.
Note: to be properly integrated, inc. secondary chemistry, endosperm type, embryo size, etc.
Includes Acranthereae, Airospermeae, Aitchisonieae, Alberteae, Anthospermeae, Argostemmateae, Augusteae, Bertiereae, Chiococceae, Chioneae, Cinchoneae, Cinchonodae, Clavistigmateae, Coffeeae, Coffeodae, Colletoecemateae, Coptosapelteae, Cordiereae, Coussareeae, Craterispermeae, Crossopterygeae, Cyanoneuroneae, Danaideae, Dialypetalantheae, Dialypetalanthodae, Dialypetalanthoideae, Dunnieae, Foonchewieae, Gaertnereae, Gardenieae, Glionettieae, Greeneeae, Guettardeae, Hamelieae, Henriquezieae, Hillieae, Hymenodictyeae, Isertieae, Ixoreae, Ixorodae, Jackieae, Knoxieae, Lasiantheae, Lasianthodae, Luculieae, Mitchelleae, Morindeae, Mussaendeae, Mussaendodae, Naucleeae, Octotropideae, Ophiorrhizeae, Paederieae, Palicoureeae, Pavetteae, Perameae, Posoquerieae, Prismatomerideae, Psychotrieae, Psychotriodae, Putorieae, Retiniphylleae, Rondeletieae, Rubieae, Rubiodae, Rubioideae, Sabiceeae, Schizocoleeae, Schradereae, Scyphiphoreae, Seychelleeae, Sherbournieae, Sipaneeae, Spermacoceae, Steenisieae, Strumpfieae, Temnopterygieae, Theligoneae, Trailliaedoxeae, Urophylleae, Urophyllodae, Vanguerieae.
[Luculieae [Coptosapelteae + Acranthereae]]: ?
Age. If a clade - Luculia diverged from [Coptosapelta + Acranthera] in the Late Cretaceous (Manns et al. 2012).
Luculieae Rydin & B. Bremer - Luculia Sweet: unplaced as yet.
Shrub; iridoids, oleanane and ursane type triterpenes, coumarins, flavonoids like Rubioideae; nodes 1:1; raphides 0; petiole bundle incurved U-shaped; inflorescence terminal, flowers large, 5-merous, heterostyly +; K long-lobed, deciduous, C imbricate; pollen?; secondary pollen presentation 0; ovules many/carpel; fruit baccate; seeds winged at ends; ?endosperm; embryo minute; n = ?
1/5. Himalayas, Myanmar, Thailand, S.W. China.
[Coptosapelteae + Acranthereae]: ? - unplaced as yet.
Age. This clade is around (76.0-)51.2(-26.2) Ma (Bremer & Eriksson 2007).
Coptosapelteae S. P. Darwin - Coptosapelta Korthals
Subshrubs, vines; distinctive seco-iridoids, guaiane-type sequiterpenes, and lupane-type pentacyclic triterpene lactones, anthra- and naphthoquinones, Al accumulator; raphides 0; flowers single/inflorescences +, (terminal) axillary; flowers (4-)5-merous; C right-contorted; anthers long, pollen 3-4(-10) pororate; secondary pollen presentation +; stigma fusiform; ovules many/carpel; fruit a loculicidal capsule; seeds winged; embryo straight, cotyledons short; n = ?
1/56. S.E. China, Japan, to Malesia.
Acranthereae S. P. Darwin - Acranthera Meisner
Herbs, shrubs; inflorescence terminal or axillary; flowers (4-)5-merous; C (reduplicate-)valvate; anther apices with sharply acute or spurred appendages, these connate into a tube surrounding stigma and united with it at top; stigma clavate, 10-ridged, with multicellular papillae; placentation parietal; ovules many/carpel; fruit baccate; embryo small; n = 10.
1/16. India and Sri Lanka to S.E. China and (West?) Malesia.
1. Rubioideae Verdcourt, = [Urophyllodae [Lasianthodae [Coussareeae [Rubiodae + Psychotriodae]]]]
Herbs quite common; (anthraquinones, camptothecin [pentacyclic quinoline alkaloid], cylcotide proteins, monofluoracetates +), anthraquinones from shikimic acid, indole alkaloids common, plants often Al-accumulators [esp. woody taxa], (foetid - paederoside); vessels frequently in aggregates, usually ≤50 um across; raphides + [square in transverse section]; hairs uniseriate, septate; plants heterostylous; C valvate, (tips of lobes hood-like), (tube with windows, usu. basal [= fenestrate]); pollen (grains 3-celled); ovule 1/carpel, basal, apotropous.
207/8,106: 30 tribes. Worldwide.
Age. B. Bremer and Eriksson (2009) suggested ages of (90.7-)77.9(-65.3) Ma, Wikström et al. (2015: topology) ages of (85-)76(-67) Ma, Lemaire et al. (2011b) ages of (60-)53(-48) Ma and Razafimandimbison et al. (2019) ca 73.4 Ma for crown-group Rubioideae.
Urophyllodae Ezedin / [Temnopterygieae [Colletoecemateae + Seychelleeae]] Urophylleae, Ophiorrhizeae] / Urophylleae alliance / SCOUT clade: ?
1A. Temnopterygieae Razafimandimbison & Rydin - Temnopteryx sericea Bentham & J. D. Hooker
Robust herb; ?nodes; stipules large, multifid, interpetiolar; flowers 5-merous; inflorescence ± capitate, axillary, sheathed by campanulate, multifid, basal bract; K unequal, C-like; G [3-5], stigma with linear lobes; ovules many/carpel; fruit baccate.
1/1: Cameroon, Equatorial Guinea, Gabon.
[Colletoecemateae + Seychelleeae]: flowers 5-merous; embryo long.
Age. The age of this node is ca 14.6 Ma (Razafimandimbison et al. 2019).
1B. Colletoecemateae Rydin & B. Bremer - Colletoecema E. M. A. Petit
Small trees or shrubs; ?nodes; inflorescences axillary, bracteoles 0; pollen grain cell no.?; ovules subbasal, obturator 0; fruit drupaceous, stone single, incised apically [the two carpels], 2-seeded/(?2, separate); endosperm soft, oily; n = ?
1/3. West Central tropical Africa.
Age. The crown-group age of Colletoecemateae is ca 3.2 Ma (Razafimandimbison et al. 2019).
1C. Seychelleeae Razafimandimbison, Kainulainen & C. Rydin - Seychellea sechellarum (Baker) Razafimandimbison, Kainulainen & C. Rydin
Shrubs; ?chemistry; ?nodes; raphides 0; stipules intrapetiolar, adnate to base of petiole, persistent; inflorescences terminal; A only loosely adnate to C; pollen grain cell no.?; ovule morphology?; G [5-6]; fruit drupaceous, stones separate; ?endosperm; n = ?
1/1. The Seychelles.
1D. Urophylleae Verdcourt (inc. Pauridiantheae)
Subshrubs to small trees (herbs); axial parenchyma diffuse, cells with helical thickenings, (sheath cells surrounding rays +); styloids +; (plant dioecious); inflorescences axillary, flowers often 5-merous, (bracts 0); (semaphyllous calycophylls); pollen grains 2-celled; G [2-several], with false septae [?all], stgmas ± spreading (subcapitate); placentae subpeltate, ovules many/carpel; fruit baccate, loculi with mucilage; exotestal cells massively thickened; n = x = 9, chromosomes bimodal.
6/256: Urophyllum (150), Praravinia (49). Tropics, to Japan, few America.
Age. Crown-group Urophylleae are estimated to be (56.6-)34.8(-14.2) Ma (Bremer & Eriksson 2007).
1E. Ophiorrhizeae Verdcourt
Herbs (annuals) to small trees; (indole alkaloids - camptothecin - +); nodes 1:1; stipules often fimbriate; inflorescences terminal (axillary); flowers 5-merous; (calycophylls +; A forming cone - Neurocalyx); pollen grain aperture buds +; many ovules/carpel, endothelium ?+; fruit loculicidal or septicidal capsule/indehiscent/operculate - splash cup; endosperm cellular; seeds dust-like; endosperm soft, oily, embryo long; (n = 12).
6/420: Ophiorrhiza (190), Spiradiclis (38), Xanthophytum (32). China and IndoMalesia to Fiji.
Age. Ophiorrhizeae are (70.1-)44.3(-22.4) Ma (Bremer & Eriksson 2007).
[Lasianthodae [Coussareeae [Psychotriodae + Rubiodaee]]]: chloroplast atpB promoter 0.
Age. The age of this node is around 66.6 Ma (Razafimandimbison et al. 2019).
Lasianthodae Ezedin / [Lasiantheae + Perameae] / Perameae alliance: Al accumulation common; ovule 1/carpel.
1F. Lasiantheae B. Bremer & Manen
Subshrubs to small trees; (plant foetid); axial parenchyma diffuse, cells with helical thickenings; inflorescences axillary; flowers 4-6-merous, usu. sessile; (C imbricate); pollen grains (porate); G [4-12] [Lasianthus]; ovule erect, recurved, obturator +; fruits drupaceous, often blue to black; pyrene with germination slit; endosperm soft, oily, embryo long, cotyledons short.
5/296: Lasianthus (185). Tropical, Africa and Indomalesia to Australia, 1 sp. West Indies.
Age. Lasiantheae are (53.5-)34.8(-20.4) Ma (Bremer & Eriksson 2007).
1G. Perameae S. P. Darwin - Perama Aublet
Herbs; lamina with parallel veins, stipules very small; K bilobed; ovule basal; fruit capsular; n = 9.
1/14. Antilles, Central and South America.
[Coussareeae [Rubiodae] + Psychotriodae]]: ?
Age. This node is approximately Ma (Razafimandimbison et al. 2019).
1H. Coussareeae Bentham & J. D. Hooker (inc. Cruckshanksieae, Coccocypseleae)
Axial parenchyma ± paratracheal, libriform fibres septate, sheath cells surrounding rays +; nodes 3:3 girdling/3:3; inflorescences terminal/axillary; calycophylls + [Cruckshanksia].
10/414. America, Mexico southwards, mostly tropics.
3/10: Cruckshanksia (7). Mostly Andean, Chile to S. Peru.
[Coccocypselum + Coussarea groups]: septate fibres +; flowers 4-merous.
5/72: Declieuxia (30), Coccocypselum (20). Southern Mexico to Brasil, esp. southeastern Brasil.
2/290: Faramea (170), Coussarea (120). American tropics.
Age. This clade is (62.8-)52.2(-42.6) Ma (Bremer & Eriksson 2007).
Age. This age of this node is (75.2-)63.0(-52.0) Ma (Bremer & Eriksson 2007) or (68-)62(-56) Ma (Razafimandimbison et al. 2017).
Rubiodae Robbrecht & Manen / [[Danaideae [Spermacoceae + Knoxieae]] [Anthospermeae [[Foonchewieae [Dunnieae + Cyanoneuroneae]] [Argostemmateae [Paederieae [[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]]]]]] / Spermacoceae alliance: ?
Age. The age of Rubiodae is estimated to be (65.1-)54.8(-45.4) Ma (Bremer & Eriksson 2007).
[Danaideae [Spermacoceae + Knoxieae]]: ?
Age. This node is some (65.1-)54.8(-45.4) Ma (Bremer & Eriksson 2007).
1I. Danaideae B. Bremer & Manen
Shrubs to small trees, lianes [Danais]; anthraquinones +, (plant foetid); inflorescences terminal (axillary) (subtended by large bracts - Payera); C also valvate-reduplicate, (tube fenestrate); pollen grains 2-nucleate; styles linear [D.], placentae peltate; many ovules/carpel, apex angled; capsule loculi-/septicidal [Schismatoclada], apex beaked; seeds winged; exotestal cells thickened on anticlinal walls; cotyledons≤radicle; n = ?
3/74: Schismatoclada (47), Danais (49). Madagascar (not the west and southwest), the Mascarenes, Comoros and Seychelles, 1 sp. Tanzania. Map: see Buchner and Puff (1993).
Age. Danaidae are estimated to be (41.0-)20.1(-4.9) Ma (Bremer & Eriksson 2007).
[Spermacoceae + Knoxieae]: largely herbaceous (annuals); nodes 1:1, petiole bundle arcuate; stipules fimbriate, colleters at ends of fimbriae; ovule 1/carpel.
Age. This node is estimated to be (53.7-)44.3(-35.6) Ma (Bremer & Eriksson 2007).
1J. Spermacoceae Chamisso & Schlechtendal (inc. Hedyotideae) —— Synonymy: Hedyotidaceae Dumortier, Houstoniaceae Rafinesque, Hydrophylacaceae Martynov, Lippayaceae Meisner, Spermacoceaceae Berchtold & J. Presl
Also subshrubs with xylopodia [woody underground system] to climbers or small trees; petiole vasculature arcuate; (also druses - Bouvardia); leaves (± ericoid), (axillary fascicles); inflorescences terminal/axillary; flowers often 4-merous; (calycophylls +), C (tube with windows); heterostyly 0 (+); pollen hideously variable [esp. old Spermacoce], pantoporate/3-12-colporate, etc.; stigma variously bilobed; ovule (?many/carpel), amphitropous, attached to middle (base) of septum, micropyle much elongated, integument adnate to nucellus laterally, nucellar cells anticlinally elongated, (raphal projection +), obturator +/0; archesporium multicellular or not, embryo sac elongated, antipodals not persistent to large; fruit (heterocarpous), schizocarp/septfragal capsule/circumscissile/indehiscent [baccate/drupaceous], (false carpoophore +); seeds (1), (winged), ± grooved adaxially or not, (elaiosome +); exotestal cells thickened on anticlinal and inner tangential walls/0/(outer periclinal wall punct(ulic)ate); endosperm cartilagineous or not, (ruminate), suspensor uniseriate; n = 6-17, x = 9, 14.
86/1,541: Spermacoce (275: inc. Borreria), Oldenlandia (240), Manettia (125), Hedyotis (115), Mitracarpus (58), Galianthe (55), Bouvardia (42). Pantropical, esp. Brazil, a few species outside the tropics.
Age. The age of Spermacoceae is around (36.0-)28.5(-21.7) Ma (Bremer & Eriksson 2007).
1K. Knoxieae Bentham & J. D. Hooker (inc. Triainolepideae)
Also subshrubs; septate fibres 0, axial parenchyma ± diffuse; stipules fringed; inflorescences terminal, (± capitate); flowers often 5-merous, sessile; K unequal, (foliaceous); G [1-5]; ovules pendulous; fruit a schizocarp/drupaceous, preformed germination slits +; exotestal cells with reticulate/anastomosing thickenings on inner tangential walls; n = (10), 12, (17 - Otiophora), x = 10.
16/130: Pentas (34), Pentanisia (19), Otiophora (18). Palaeotropics, esp. Africa and Madagascar.
Age. Crown-group Knoxieae are thought to be (26.5-)17.3(-9.1) Ma (Bremer & Eriksson 2007).
[Anthospermeae [[Foonchewieae [Dunnieae + Cyanoneuroneae]] [Argostemmateae [Paederieae [[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]]]]]: asperulosidic glycosides +.
1L. Anthospermeae de Candolle —— Synonymy: Operculariaceae Perleb
Herbs to shrubs (trees); (plant foetid), lignans, iridoids +; nodes 1:1; (colleters at the ends of the stipular fimbriae); lamina (amphistomatous/terete); plant monoecious/dioecious/flowers perfect [Nertera], anemophilous, (protogynous); flowers small, 4-5(-10)-merous, heterostyly 0; K minute to foliaceous; A (inserted at base of C tube); nectary 0; style short, stigmatic lobes long, short-pilose; (ovaries fused); 1 basal ovule/carpel, obturator small, finger-like; antipodal cells multinucleate [Phyllis]; fruit drupaceous/schizocarp/exocarp splitting/indehiscent, (with modified pedicel apex); suspensor multiseriate; (n = 10 - Leptostigma).
12/120: Coprosma (110), Anthospermum (40). Africa, S. China to Malesia and the Antipodes, Pacific Islands, the Antilles, Central and W. South America.
Age. Crown-group Anthospermeae are (46.6-)31.8(-16.7) Ma (Bremer & Eriksson 2007).
[[Foonchewieae [Dunnieae + Cyanoneuroneae]] [Argostemmateae [Paederieae [[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]]]]: asperulosidic glycosides +.
[Foonchewieae [Dunnieae + Cyanoneuroneae]]: inflorescence terminal; many ovules/carpel.
1M. Foonchewieae R.-J. Wang - Foonchewia guangdongensis (Dunn) Z.-Q. Song
Subshrub; ?chemistry; stipules entire; flowers 5-merous, rather small, bracteoles small, deciduous; pollen grain cell no.?, 4-colporate; fruit a porose capsule; seeds angular, exotesta with thickened anticlinal walls; ?embryo; n = ?
1/1. South China, Guangdong.
[Dunnieae + Cyanoneuroneae]: ?
1N. Dunnieae Rydin & B. Bremer - Dunnia Tutcher
Subshrub; ?chemistry; stipules bifid; bracteoles adnate to base of ovary or not, on a few marginal flowers large, petal-like [semaphyllous calycophylls - Delprete 2019]; flowers (4-)5-merous, rather small; A inserted high in tube; pollen grain cell no.?; fruit a septicidal capsule, apex beaked, (valves bifid); seeds winged; embryo minute; n = ?
1/2. India, South China (Guangdong).
1O. Cyanoneuroneae Razafimandimbison & B. Bremer - Cyanoneuron Tange
Herbs or small shrubs; ?chemistry; hairs multiseriate; upper epidermis 2-layered; stipules with long linear segments; inflorescence condensed; flowers 5-merous; pollen grain cell no.?; fruit drupaceous, stone two locular, septum +, thin, soft; seeds numerous, angled; thickening on inner periclinal exotestal walls forming a three-dimensional network; endosperm soft, thick-walled, embryo medium-sized; n = (10?) 11.
1/5. Borneo, Sulawesi.
[Argostemmateae [Paederieae [[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]]]: ?
1P. Argostemmateae Verdcourt
(Unbranched) (tuberous) herbs to small shrubs, (epiphytic); ?asperulosides; (anisophyllous), (stipules foliaceous); inflorescences often terminal (umbelliform); flowers usu. 5-merous; C (rotate); A inserted at base of C tube, anthers (connate, forming a cone), (porose, endothecium 0); pollen grains 2-nucleate [1 sp.], 3-colpate; nectary 0 [Argostemma]; placentae stoutly stalked, stigma subpunctate to capitate or disciform/2(-5)-lobed; many ovules/carpel; fruit fleshy circumscissile [edge of disc] capsule [?splash cup]/dry septicidal capsule/baccate; seeds tiny; exotestal cells thickened on radial (and outer periclinal?) walls; (n = 14).
6/251: Argostemma (110), Mycetia (45). IndoMalesia, South China southwards, 2 spp. in W. tropical Africa.
Age. Crown-group Argostemmateae are some (38.1-)21.0(-9.0) Ma (Bremer & Eriksson 2007).
Anthospermeae de Candolle - If Paederieae belong here, this node is (50.6-)41.2(-32.5) Ma (Bremer & Eriksson 2007).
[Paederieae [[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]]: ?
1OQ. Paederieae de Candolle —— Synonymy: Lygodisodeaceae Bartling
Perennial herbs to shrubs (lianes), usu. foetid; root with superficial cork cambium [Paederia]; nodes 1:1; (colleters at the ends of the stipular fimbriae); inflorescences terminal, (inflorescence bracts petal-like); flowers 4-5(-6)-merous, heterostyly ?0; C induplicate-valvate, (tube fenestrate); A inserted at different levels; pollen grains 3-(4-)colpate, no endoapertures (6-15-pantocolpate - Leptodermis); (G [2-5]), style much longer than stigma lobes; 1 basal apotropous ovule/carpel, obturator +, 2-lobed, with hairs; archesporium multicellular; fruit a (winged) schizocarp/capsule; suspensor uniseriate/biseriate; exotesta crushed [?always]; endosperm horny, radicle short, cotyledons foliaceous; n = (10, 12, 13).
6/98: Leptodermis (40), Paederia (30). Tropics and subtropics, esp. Himalayas to Japan.
Age. Crown-group Paederieae are (40.7-)30.7(-20.4) Ma (Bremer & Eriksson 2007).
[[Putorieae + Theligoneae] [Aitchisonieae + Rubieae]]: plant foetid; A inserted above the middle of the C tube; style much longer than stigma lobes; one ovule/carpel, basal.
Age. The age of this node is estimated to be (44.0-)34.4(-25.5) Ma (Bremer & Eriksson 2007).
1R. Putorieae Lange - Plocama Aiton
Herbs to shrubs; plant foetid; nodes 1:1; (short shoots +), leaves (isobifacial); anisophylly in the inflorescence; inflorescences terminal; flowers 4-7-merous, ?heterostyly; pollen grains 3-colpate; ovules apotropous, obturator 2-lobed, (with hairs); archesporium multicellular, embryo sacs elongating greatly, usu. growing up the micropyle; fruit a schizocarp, carpophore +/berry; exotestal cells unthickened [Crocyllis s. str.]; suspensor uniseriate [check], embryo long.
1/36. Canary Islands, Mediterranean, Egypt-Libya-Arabia to Pakistan, southern Namib Desert.
Age. Crown-group Plocama is ca 24.4 Ma (Rincón-Barrado et al. 2021).
1S. Theligoneae Baillon - Cynocrambe Gagnebin —— Synonymy: Cynocrambaceae Endlicher, nom. illeg., Theligonaceae Dumortier, nom. cons.
Herbs, annual to perennial; extreme anisophylly +; plant monoecious, anemophilous; flowers usu. 2-3-merous, heterostyly 0; staminate flowers: usu. paired, opposite leaves; P tubular, tube short [1.5> mm long], ridged, splitting into 2-5 segments; A 2-many, not epipetalous, in groups of 2-6, dehisced anthers spirally twisted; pollen grains (3-)4-8 zono/pantoporate; carpelate flowers: C tubular, ovary with 1 locule developing, other rudimentary, style ± gynobasic; ovules campylotropous; fruit with thin fleshy "mesocarp", basal annular elaiosome + [from apex of pedicel]; exotestal cell walls thickened, not pitted; endosperm fleshy, with starch, embryo long, U-shaped, cotyledons incumbent, suspensor uniseriate; (n = 10).
1/4. Mediterranean, Macaronesia, S.W. China and Japan.
Age. Crown-group Theligoneae are estimated to be (23.2-)13.4(-6.2) Ma (Deng et al. 2017).
1T. Aitchisonieae Bordbar, Razafimandimbison & Mirtadzadini - Aitchisonia rosea Helmsley
Shrublet; ?chemistry; hairs stalked, glandular; inflorescence ± capitate, few-flowered; flowers 5-merous, with paired fused cup-like "bracts"; K lobes evident; fruits separating into dehiscent mericarps; ?embryo.
1/1. Afghanistan, Iran, Pakistan.
1U. Rubieae Baillon - inc. Galieae, Stellatae —— Synonymy: Aparinaceae Hoffmannsegg & Link, Asperulaceae Spenner, Galiaceae Lindley
Herbs, annual to perennial, (subshrubs); (wood storied); nodes 1:1; stem angled; lamina pellucid-punctate abaxially, stipules foliaceous, axillary colleters 0/(+); flowers 4-5-merous; (K developed), C often rotate [a.k.a. "short, cup-shaped"], tube often short [<1.5 mm long]; (secondary pollen presentation [Phuopsis]); pollen grains 5-13-colpate [not Kelloggia], (micro)spinose, orbicules 0; (styles ± free); integument often adnate to nucellus laterally, nucellar cells anticlinally elongated/not; archesporium multicellular [to 200 cells], (megaspore in micropyle), (embryo sac tetrasporic, 15-16-celled, antipodal cells numerous [Crucianella - type]); fruit schizocarp/fleshy, (with hooks); testa adnate to pericarp [= cypsela!]/(not); suspensor haustoria +/0; embryo curved; n = (9-12), chloroplast atpB promoter modified.
17/1004: Galium (678), Asperula (190), Rubia (80). N. hemisphere, but ± worldwide, esp. mountains.
Age. The minimum divergence time within Rubieae has been dated ca 17 Ma (Nie et al. 2005) or (24.7-)18.1(-12.0) Ma (Bremer & Eriksson 2009), (29.8-)19.6, 14.9(-9.2) Ma (Ehrendorfer et al. 2018) - or (37-)30.1(-24) Ma (Deng et al. 2017).
A. Graham (2009) noted that fossils of Galium had been reported from rocks that were at least 55 Ma.
Psychotriodae Robbrecht & Manen / [Schizocoleeae [Craterispermeae [Gaertnereae [Palicoureeae + Psychotrieae] [Schradereae [Prismatomerideae [Mitchelleae + Morindeae]]]]]] / Psychotrieae alliance: ?
Age. The age of Psychotriodae is (60.7-)48.7(-34.9) Ma (Bremer & Eriksson 2007) or (61-)55(-49) Ma (Razafimandimbison et al. 2017).
1V. Schizocoleeae Rydin & B. Bremer - Schizocolea Bremekamp
Trees; stipular tube long, usu 8-laciniate; inflorescence axillary; flowers 5-merous, ?heterostyly; ?pollen; G septae thin; ovule ?1/carpel; fruit baccate, 1-seeded; n = ?
1/2. Tropical West Africa.
1W. Craterispermeae Verdcourt - Craterispermum Bentham
Shrubs to trees; banded axial parencyma/circumferential parenchyma bands +, septate fibres 0; stipules connate, entire; inflorescences usu. supraaxillary; flowers 5-merous; pollen grain cell no.?; placentation apical [1 pendulous ovule/carpel], funicular obturator 0; fruits baccate or drupaceous, 1-seeded; seeds adaxially concave.
1/32. Tropical Africa, Madagascar, the Seychelles.
Age. The crown-group age of Craterispermeae is estimated to be (12-)7(-5) Ma (Razafimandimbison et al. 2017).
[Schradereae [Prismatomerideae [Mitchelleae + Morindeae]]]: ?
1X. Schradereae Bremekamp - Schradera Vahl
± Epiphytic root climbers, (shrubs); nodes 3:3; stipules usu. basally connate, deciduous; inflorescences terminal, ± capitate, involucre +, showy or not; flowers (3-)5(-6)-merous; C with ring of hairs at filament insertion, (corona +, short, with erect hairs); pollen grains 2-nucleate [1 sp.], (2-)3(-4)-porate/shortly colporate; G with lysigenous mucilage cavities, placentation axile; ovules many/carpel [?all], campylotropous; fruit baccate; exotestal cells with thickened anticlinal walls; embryo quite long, cotyledons = radicle.
1/62. Malesia, tropical America, 1 sp. Sri Lanka.
Age. Crown-group Schradereae are (33-)23(-14) Ma (Razafimandimbison et al. 2017).
[Prismatomerideae [Mitchelleae + Morindeae]]: inflorescences terminal; pollen grains 2-nucleate; ovaries fused [= fruit a syncarp]/not; exotestal thickenings 0.
1Y. Prismatomerideae Y. Z. Ruan
Shrubs to trees; vessels solitary, axial parenchyma diffuse; (stipules connate); flowers (sessile), 4-5(-6)-merous; pollen grains (to 5-colporate); placentation axile; ovule 1/carpel, pendulous, apotropous, sessile, hemianatropous, massive; fruit baccate-drupaceous, endocarp membranaceous, with germination slits (forming lid); seed globose to hemispherical, adaxially ± concave; exotesta parenchyma-like, testal cells ± crushed; endosperm corneous, often dark blue, excavation filled with parenchymatous ?placental tissue, embryo (very) small, cotyledons << radicle.
2/27: Prismatomeris (17). Sri Lanka to S. China and W. Malesia.
Age. The crown-group age of Prismatomerideae is (29-)20(-12) Ma (Razafimandimbison et al. 2017).
[Mitchelleae + Morindeae]: C tube with hairs inside; fruit a drupe; endosperm soft, oily.
Age. This node is (42-)39(-36) Ma (Razafimandimbison et al. 2017).
1Z. Mitchelleae Razafimandimbison & B. Bremer
Thorny shrubs to creeping herbs, growth sympodial; axial parenchyma 0, septate fibres +; nodes 1:1; (heterophylly +); inflorescence usu. 2-flowered, G connate/not; flowers 4-merous; C basally fenestrate; pollen grains (to 6-(syn)colporate); G [4], style apically branched; ovule apical-horizontal, campylotropous, obturator massive; pyrenes pitted, stone cells ± fibres, K accrescent; exotestal cells elongated, thickenings along radial and outer periclinal walls (± 0), other cells crushed; embryo minute, cotyledons small, radicle points downwards.
2/16: Damnacanthus (12). North and Central America, India, East Asia.
Age. The crown-group age of Mitchelleae is (8-)10(-5) Ma (Razafimandimbison et al. 2017).
1AA. Morindeae Burnett
Shrubs to small trees (lianes); wood with circumferential parenchyma bands; nodes 1:1; stipules usu. connate to sheathing; inflorescence (axillary), (with petal-like bracts); flowers 5-merous, (± sessile); C (tube fenestrate); pollen grains (to 6-colporate); G [2-12], pseudoseptum developing [= massive development of placentae]; ovules 2/carpel, subbasal, obturator 0; fruit drupaceous, pyrenes with lateral germination slits; seed basally winged (not); testa ± parenchymatous; endosperm oily, soft.
5/163: Gynochthodes (95), Morinda (40). Pantropical.
Age. Crown-group Morindeae are (36.8-)25.5(-13.0) Ma (Bremer & Eriksson 2007) or (34-)26(-21) Ma (Razafimandimbison et al. 2017).
[Gaertnereae [Palicoureeae + Psychotrieae]] - if this clade exists: flowers often small; 1 ovule/carpel, erect, basal; fruit drupe/drupaceous.
1BB. Gaertnereae Endlicher —— Synonymy: Pagamaeaceae Martynov
Shrubs to trees; wood with circumferential parenchyma bands, fibre tracheids +; stipules forming long sheath; (plant dioecious); inflorescences terminal/axillary; flowers 4-5-merous; pollen grains with crescent-shaped ectexinal thickenings at ends of aperture; G secondarily superior, [2-8]; endosperm ruminate (not), starchy.
2/116: Gaertnera (70). Pantropical, but not E. Malesia. Map: Malcomber and Taylor (2009).
Age. Crown-group Gaertnereae are (29.3-)18.2(-9.1) Ma (Bremer & Eriksson 2007) or (33-)25(-17) Ma (Razafimandimbison et al. 2017).
[Palicoureeae + Psychotrieae]: fruit with pyrenes, endosperm often horny.
Age. The age of this node is estimated to be ca 63.0 Ma (Bremer & Eriksson 2009) or (51-)44(-37) Ma (Razafimandimbison et al. 2017).
1CC. Palicoureeae Robbrecht & Manen —— Synonymy Nonateliaceae Martynov
Cyclotide proteins +; foliage drying green, stipules connate, usu. persistent/marcescent; (inflorescence capitate), (bracteoles large, petal-like); (pollen grains (pantoporate), (with pollen buds); fruits often blue, pyrenes thick-walled, with adaxial furrows and preformed germination slits; testa lacking red pigment.
10/1,113(-1,220<): Palicourea (689), Notopleura (210), Chassalia (140 - but see Razafimandimbison et al. 2014; Thilén et al. 2025, inc. Geophila), Eumachia (83), Readea (80), Hymenocoleus (19). Pantropical, esp. New World. Photo Fruit.
Age. The crown-group age of Palicoureeae is (45-)38(-32) Ma (Razafimandimbison et al. 2017).
1DD. Psychotrieae Chamisso & Schlechtendal - Psychotria L. —— Synonymy: Psychotriaceae F. Rudolphi
Shrubs (myrmecophytes) to trees; (CAM photosynthesis +); (indole alkaloids +); nodes 1:1; foliage drying brown-grey, (leaves with bacterial nodules), stipules deciduous; pollen grains also inaperturate/2-4(-5) colpate/porate, (pollen buds +); fruit often red, pyrene walls thin, (a schizocarp); testa with ethanol-soluble red pigment; endosperm (ruminate), starchy/(oily), embryo short.
1/1,819. Pantropical, but esp. Old World.
Age. Crown-group Psychotrieae are (46.9-)35.6(-25.5) Ma (Bremer & Eriksson 2007) or (30-)27(-23) Ma (Razafimandimbison et al. 2017).
2. Dialypetalanthoideae Reveal / inc. the old Ixoroideae, etc.) [Cinchonodae [Dialypetalanthodae [Mussaendodae [Steenisieae [Retiniphylleae [Jackieae, Ixorodae, Airospermeae, Coffeodae]]]]]]
Plants woody; route II carboxylated iridoids +, indole and corynanthean and complex indole alkaloids + (0); crystal sand +; (1 or more K petal-like [= calycophylls]); (secondary pollen presentation + [brush type/receptaculum pollinis]); stigma unlobed/shortly bilobed.
/5,988. Pantropical.
Age. N.B. Ages for nodes/associated ideas of relationships in older literature should be checked with current ideas of relationships, Razafimandimbison and Rydin (2024) in particular, so as to clarify just what node these ages might refer. B. Bremer and Eriksson (2009: stem age of this node not the crown age for the family, c.f. Fig. 1) suggested that the split of Ixoroideae (= Dialypetalanthoideae there) and Cinchonoideae was approximately (88.7-)73.1(-58.4) Ma, Manns et al. (2012: Luculia, Cinch. Ixor.) give an age of (84.5-)78.5(-71.7) Ma, Lemaire et al. (2011b: Luculia, etc. not included) an age of (68-)60(-54) Ma, and Wikström et al. (2015) an age of (89-)78(-67) Ma - but see below for relationships around here.
B. Bremer and Eriksson (2009) estimated that divergence within Dialypetalanthoideae began (52.5-)38.7(-28.1) Ma, Manns et al. (2012: HPD estimates) gave an age of (65.6-)57.4(-50.3) Ma, and Lemaire et al. (2011b) an age of (52-)36(-24) Ma - but they also note "more recent stem node ages" of 26 Ma for Dialypetalanthoideae, all rather confusing. Antonelli et al. (2009) dated crown Dialypetalanthoideae at some (54.6-)51.3(-47.8) Ma, Wikström et al. (2015) suggested an age of (64-)51(-42) Ma and Bremer and Eriksson (2007) an age of (52.5-)38.7(-28.1) Ma.
Cinchonodae Robbrecht & Manen = [[Isertieae + Cinchoneae] [Chioneae [[Strumpfieae + Chiococceae] [Hillieae + Hamelieae]]] [[Rondeletieae + Guettardeae] [Naucleeae + Hymenodictyeae]]] / Cinchonoideae s. str. / Cinchoneae alliance: ?
[[Isertieae + Cinchoneae] [Chioneae [[Strumpfieae + Chiococceae] [Hillieae + Hamelieae]]]: ?
[Isertieae + Cinchoneae]: secondary pollen presentation 0; many ovules/carpel.
2A. Isertieae de Candolle
Shrubs to trees (lianas); indole alkaloids +; inflorescences terminal; (calycophylls + - Kerianthera); flowers to 7-merous; C valvate (imbricate); anthers locellate; pollen 3-colporoidate [colpi poorly developed, orae well developed], oncus protruding; G [2-5(-6)]; fruit drupe [ca 6-locular], stone sculpted, baccate [2-3-locular], (placental pulp +); seeds (winged), exotestal cells thickened on inner periclinal walls; n = 9, 10, 11, x = 10, 11.
2/17: Isertia (15). Tropical America.
2B. Cinchoneae de Candolle —— Synonymy: Cinchonaceae Batsch
Shrubs to small trees; (anthraquinones +), indole alkaloids +; nodes 1:1; petiole bundle prostrate D-shaped, with medullary plate; C valvate, sericeous abaxially; pollen 3-colporate, inner part of aperture [os] poorly defined; ovules with chalazal projection; fruit a capsule; seeds winged; exotestal cells thickened reticulately on inner periclinal walls/?massively pitted; n = 13, 14, 17, 18, x = 17.
8/125: Remijia (38), Ladenbergia (34), Cinchona (23). Costa Rica to tropical South America, esp. Andean. Photo Flower.
Age. Crown-group Cinchoneae are (28.6-)15.6(-5.3) Ma (Bremer & Eriksson 2009).
[Chioneae [[Strumpfieae + Chiococceae] [Hillieae + Hamelieae]]]: A inserted at base of corolla.
2C. Chioneae Razafimandimbison & Rydin
Shrubs to trees; often maroon colouration; young internodes flattened, terete when older; inflorescence terminal; flowers 4-6-merous; K connate, C imbricate; A basally connate; pollen spinulose [Chione]; secondary pollen presentation?; stigma shortly bilobed; 1 pendulous ovule/carpel; fruit drupaceous; pyrenes 2, with marginal germination slit, or 1, 2-seeded, no germination slit; pyrenes laterally compressed; seed surface granular; n = ?
2/27: Chione (?20). Mexico to Colombia, Ecuador, Peru, the Antilles.
[[Strumpfieae + Chiococceae] [Hillieae + Hamelieae]]: ?
[Strumpfieae + Chiococceae]: secondary pollen presentation 0.
2D. Strumpfieae Delprete & Motley - Strumpfia maritima Jacquin
Ericoid shrub; nodes 1:1; inflorescences axillary, racemose; flowers 5-merous, protogynous; C quincuncial; anthers connate around style, porose, connective forming apical projection; G with false septum, style with a kink [staminate phase], stigma little expanded, barely bilobed; ovules 2/carpel, erect, apotropous, obturator well developed; pyrene plurilocular; seeds 1-4; exotestal cells unthickened; endosperm oily.
1/1. West Indies (S. Florida to Venezuela).
2E. Chiococceae Bentham & J. D. Hooker —— Synonymy: Catesbaeaceae Martynov, Coutareaceae Martynov
Subshrubs to small trees (lianes); nodes 1:1/3:3 [Portlandia]; petiole vasculature arcuate + wing bundles; inflorescences often axillary; flowers 4-6(-8)-merous; K lobes ± free, C narrowly imbricate (induplicate-valvate); (secondary pollen presentation +); A adnate to base of C tube/inserted on disc, (extrorse); pollen spinulose; G ([5-20] - Erithalis); ovules 1 pendulous-many spreading/carpel; (archesporium multicellular); fruit drupaceous, (leathery) baccate, or loculicidal; pyrenes laterall compressed; seeds (winged all around); cotyledons accumbent; n = also 12-14, 20 [Catesbaea]
32/239: Phialanthus (22), Solenandra (22), Scolosanthus (20). ± amphi-Pacific: U.S.A. (Florida), Central and South America, esp. the Antilles (70% of the species), W. Pacific, Palawan (the Philippines) to New Caledonia, i.e. W. of the Andesite Line.
Age. Chiococceae are estimated to be (40.5-)27.6(-15.4) Ma (Bremer & Eriksson 2009).
[Hillieae + Hamelieae]: raphides +.
Age. This clade is estimated to be (27.4-)18.7(-11.5) Ma (Bremer & Eriksson 2009).
2F. Hillieae S. P. Darwin
Rather succulent shrubs to trees, epiphytes; plant usu. glabrous; inflorescences terminal, 1-few-flowered; C right-contorted; secondary pollen presentation 0, stigma short-subcapitate to long-bilobed; ovules many/carpel; capsule septi-/loculicidal, often with terminal beak-like appendage; seeds small, plumose [multiseriate filaments] at one end, flattened-winged; n = 17-18, x = ?18.
3/29: Hillia (25). Tropical America, Mexico to Brazil.
Age. The age of Hillieae is (18.8-)11.7(-5.1) Ma (Bremer & Eriksson 2009) or (26.6-)19.1(-11.1) Ma (Ball et al. 2025).
2G. Hamelieae de Candolle —— Synonymy: Hameliaceae Martius
Shrubs to small trees; indole alkaloids +; (exposed wood reddish); nodes 1:1; petiole vasculature (incurved-)arcuate, wing bundles +/0; (stomata parallelocytic - Plocaniphyllum); inflorescences terminal, (branches monochasial); flowers often yellow, 4-5-merous, bracteoles small-0; C imbricate/right-contorted/valvate; A at base of corolla, (anthers tailed); G [2-5], secondary pollen presentation?; many ovules/carpel, epidermal cells of ovules large; fruit baccate / dehiscent (indehiscent, 2-seeded, with pterophyll - Cosmocalyx); exotestal outer periclinal walls granular to tuberculate; n/x = 12, 14.
13/181: Hoffmannia (115), Deppea (35). New World tropics.
Age. The age of Hamelieae is (20.8-)13.5(-7.4) Ma (Bremer & Eriksson 2009).
[[Rondeletieae + Guettardeae] [Naucleeae + Hymenodictyeae]]: ?
[Rondeletieae + Guettardeae]: indole alkaloids +; C imbricate/quincuncial, lobes spathulate; secondary pollen presentation usu. 0; polyploidy prevalent.
Age. This clade may be around (37.3-)27.5(-18.3) Ma (Bremer & Eriksson 2009).
2H. Rondeletieae Burnett
Shrubs to trees; nodes 1:1; inflorescences terminal/axillary; flowers 4-6-merous, (heterostyly +); (calycophylls +), C valvate/imbricate/contorted, mouth with conspicuous fleshy ring; pollen grains (colpate); ovules (1-)many/carpel; fruit loculi(septi-)cidal capsule/indehiscent, (dry; ridged, 2-seeded, with pterophyll) or drupaceous; seeds minute, (winged; fleshy); x = 10, 11.
17/187: Rondeletia. Mostly Greater Antilles, some Central America, few South America.
17 and 187Age. Rondeletieae may be some (32.0-)22.4(-12.1) Ma (Bremer & Eriksson 2009).
2I. Guettardeae de Candolle —— Synonymy: Guettardaceae Batsch
Shrubs to trees; nodes 1:1?; hairs with Ca oxalate crystals in walls (not Machaonia); (plant dioecious - Timonius, etc.); inflorescences terminal/axillary; flowers (heterostyly +; 3-merous); C valvate (imbricate); (secondary pollen presentation + = Dichilanthe); pollen (colporate - Gonzalagunia)/porate/inaperturate; G [2-many [many T.]); ovule 1/carpel, pendulous; fruit drupaceous/schizocarp/(capsule - M.); seeds often elongated; exotestal cells thickened on anticlinal walls; embryo long, endosperm slight/0, soft, oily; (n = 9), x = 9-11.
21/790: Timonius (280), Guettarda (150), Arachnothryx (107), Chomelia (79), Stenostomum (50), Guettardella (29), Tournefortiopsis (21). Tropical.
Age. Crown-group Guettardeae are some (31.2-)23.0(-14.5) Ma (Bremer & Eriksson 2009).
[Naucleeae + Hymenodictyeae]: secondary pollen presentation +; anthers basifixed; pollen with H-shaped endapertures, protruding onci; seeds winged, wing bilobed.
Age. This clade is thought to be (25.3-)19.7(-14.9) Ma (Bremer & Eriksson 2009).
2J. Naucleeae Burnett (inc. Cephalantheae) —— Synonymy: Cephalanthaceae Rafinesque, Naucleaceae Wernham
Shrubs to trees, (lianes, with curved axillary thorns); indole alkaloids +; nodes 3:3/1:3 (1:5/1:1 - Cephalanthus, Mitragyna); cells with both crystal sand and druse(s) [= duplex idioblasts]; terminal bud flattened/not; inflorescences terminal (on plagiotropic branches)/axillary, capitate; flowers 4-5(-6)-merous; K large, apex elaborated, variously thrown off [Neonauclea], C imbricate/valvate; anthers (mesifixed); pollen grains with H-shaped endoaperture, (in tetrads); nectary inconspicuous, ± sunken in ovary; pollen presenter clavate to capitate or spindle-shaped, stigma lobed; ovules 1 pendulous-many/carpel, with chalazal projection, obturator +; fruit usu. loculi- and/or septicidal capsular, opening from the base, (schizocarp/fleshy syncarp); seeds winged/tailed/arillate/not; exotestal cells thickened on inner periclinal walls, massively pitted/(reticulate).
19/194: Neonauclea (71), Uncaria (39). Palaeotropics, few Neotropics to temperate North America.
Age. The age of Naucleeae may be (19.6-)16.0(-14.0) Ma (Bremer & Eriksson 2009).
Fossils identified as Cephalanthus are known from the Upper Eocene in Europe (Mai & Walther 1985).
2K. Hymenodictyeae Razafimandimbison & B. Bremer
Small to large trees, (epiphytic), often deciduous; indole alkaloids 0; nodes 1:1; stipule margins with large deciduous colleters; inflorescences usu. terminal, spiciform to racemiform (ultimate units subcapitate); flowers 5-merous; C valvate; stigma globose to clavate; ovules 1-14(-many)/carpel; fruit a loculicidal capsule, lenticillate, placentae accrescent and falling out with the seeds; embryo small.
2/32: Hymenodictyon (22). Tropical Africa, Madagascar (most), India and S.W. China to Thailand, E. West Malesia, inc. Sulawesi.
Age. Hymenodictyeae are estimated to be (9.0-)3.6(-0.1) Ma (Bremer & Eriksson 2009).
[Dialypetalanthodae [Mussaendodae [Steenisieae [Retiniphylleae [Jackieae, Ixorodae, Airospermeae, Coffeodae]]]]] / Ixoroideae: ?
Dialypetalanthodae Ezedin = [Dialypetalantheae, Sipaneeae [Henriquezieae + Posoquerieae]] / Dialypetalantheae alliance: ?
2L. Dialypetalantheae Reveal (inc. Condamineeae, Calycophylleae, Hippotideae, Simireae) —— Synonymy: Dialypetalanthaceae Rizzini & Occhioni, nom. cons.
Trees to shrubs; indole alkaloids +; (resin secreted by stipular colleters); lamina (pinnately lobed/pinnate); inflorescences terminal/axillary; (flowers protogynous); (semaphyllous calycophylls + [attractive K]), C also valvate/imbricate), (free - Mastixiodendron, Mussaendopsis); A (not epipetalous - Muss.), (10, developing from staminal ring - Dialy.); G (semisuperior); ovules (1-)many/carpel; fruit a septi-/loculicidal capsule, (baccate); seeds rounded/angled/winged; exotestal cells thickened on inner periclinal walls; embryo ?(long); n = ?11, 12, 17 - Dialypetalanthus: phloem stratified; cork cortical; K = C, both free, two decussate pairs, C opposite K; A (8-)16-17(-25), not epipetalous, basally connate, anthers basifixed, porose; ovules with chalazal projection.
35/310: Pentagonia (50), Simira (47), Pentagonia (37), Dolicholobium (27). S.E. U.S.A. to Neotropics, some Southeast Asia, Malesia, the Pacific.
Age. Crown-group Dialypetalantheae (as Condamineeae) are (35.4-)19.7(-7.6) Ma (Bremer & Eriksson 2007).
2M. Sipaneeae Bremekamp
Shrubs, (herbs (annuals), treelets); (raphides +); inflorescences terminal/axillary; flowers (4-Steyermarkia)5(-7)-merous, (heterostylous), C left-contorted; anthers 2-locular; pollen 3-4-colporate, reticulate to foveolate (smooth); secondary pollen presentation 0; placentae usu. stalked; many ovules/carpel; fruit septi- or loculicidal capsule, or dry, indehiscent, K persisting; exotestal cells with ± warty thickenings on anticlinal and inner periclinal walls, large pores on inner periclinal walls.
10/43: Sipanea (17), Sipaneopsis (8). Tropical Central and South America (in the latter E. of the Andes), 9 genera on the Guiana Shield.
Age. Crown-group Sipaneeae are thought to be (47.4-)30.0(-16.7) Ma (Bremer & Eriksson 2007).
[Henriquezieae + Posoquerieae]: flowers 5-merous; secondary pollen presentation 0; many ovules/carpel.
2N. Henriquezieae Bentham & J. D. Hooker —— Synonymy: Gleasonioideae, Henriqueziaceae Bremekamp
Trees (shrubs); vessels (44-)114, 172 (-341)μm across; vascular pit borders 7.8-9.8μm across, 5aliform-confluent xylem parenchyma ± developed, rays uniseriate, low, fibres with very thick walls; phloem with large resin cells; nodes 3(-7):3(-7); petiole bundle annular, with inverted plate(s) of vascular tissue and wing bundles; (axillary stipule colleters 0); (large flat glands on abaxial bases of petioles), stipules large, (intrapetiolar); flowers ± monosymmetric; K (4 - Henriquezia), (open), C induplicate-valvate/imbricate; pollen grains >50μm across, (in tetrads); (G ± superior); (2-4 collateral ovules/carpel; fruit loculicidal capsule, K deciduous, leaving a ring; seeds flattened/(not), 0.5-9.5 cm across, wings several cells thick; exotestal cells outer surface papillate/short hairs/wartlets/concave; endosperm 0, cotyledons large, cordate; n = 13-16, 20, 21.
3/21: Platycarpum (12). South America, the Amazon area.
2O. Posoquerieae Delprete
Trees (shrubs);; inflorescence terminal; flowers monosymmetric; C imbricate or left-contorted, 0.3-38 cm long[!], anthers connate, (filaments of different lengths), explosive pollination; fruit fleshy, leathery or woody baccate/loculicidal capsules; seeds large/small; exotestal cells parenchymatous/radially elongated and walls thickened; n = ?
2/23: Posoqueria (22). Neotropics, to southern Mexico.
Age. Crown-group Posoquerieae are (33.4-)17.9(-5.8) Ma (Bremer & Eriksson 2007).
[Mussaendodae [Steenisieae [Retiniphylleae [Jackieae, Ixorodae Airospermeae, Coffeodae]]]]: indole alkaloids 0[?].
Mussaendodae Ezedin = [Mussaendeae + Sabiceeae] / Mussaendeae alliance: heterostyly + (0); C (reduplicate-)valvate; many ovules/carpel; fruit baccate.
Age. This node is dated to (66.3-)47.3(-26.6) Ma (Duan et al. 2018: check sister).
2P. Mussaendeae J. D. Hooker
Shrubs to trees or lianes; (laticifers +); nodes 1:1; petiole bundle C-shaped; stipules ± bifid; (plant dioecious); inflorescences terminal; flowers 5-merous; (semaphyllous calycophylls +); C also induplicate-valvate; pollen grains (3-celled); fruit also loculicidal capsule; x = 11.
7/223: Mussaenda (132). Africa, Southeast Asia to the Pacific.
Age. Crown-group Mussaendeae are (41.8-)24.0(-9.0) Ma (Bremer & Eriksson 2007).
2Q. Sabiceeae Bremekamp (inc. Virectarieae) —— Synonymy: Sabiceaceae Martynov
Shrubs, lianas (herbs); crystal sand +/(styloids +); lamina abaxially usu. with dense indumentum; inflorescences axillary, (terminal); flowers 4-8-merous; pollen grains also 3-4-porate; G [2-5], stigma; fruit (schizocarp/loculicidal capsule); exotestal cells with (complex) verrucate thickenings on inner periclinal/(and anticlinal) walls, small/large pits; endosperm horny, embryo short; (n = 9), x = 9-11.
4/169: Sabicea (150). Neotropics, Africa, Sri Lanka.
Age. Sabiceeae are some (43.5-)26.9(-10.9) Ma (Bremer & Eriksson 2007).
[Steenisieae [Retiniphylleae [Jackieae, Ixorodae, Airospermeae, Coffeodae]]]: ?
2R. Steenisieae Kainulainen & B. Bremer - Steeniisia Bakhuisen f.
Shrub, monocaulous; hairs thick walled; inflorescences axillary, flowers 4-5-merous; pterophyllous calycophylls +, C left-contorted; anthers connate around style, with long apical appendages; secondary pollen presentation +; many ovules/carpel; seeds flattened; endosperm hard-bony; n = ?
1/5. Borneo, Malaya, Natuna Islands.
[Retiniphylleae [Jackieae, Ixorodae, Airospermeae, Coffeodae]]: ?
2S. Retiniphylleae Bentham & J. D. Hooker - Retiniphyllum Humboldt & Bonpland
Shrubs to trees; buds resiniferous; inflorescences terminal; bracteoles connat [= calyculus at apex of pedicel]; C contorted; secondary pollen presentation +; anthers with apical and basal appendages; G [(4-)5(-8)]; ovules 2/carpel [1 aborts], collateral, pendulous, with common cap-like micropylar obturator; fruit drupaceous, with 1-seeded pyrenes; testa parenchymatous; endosperm ?oily, cotyledons< 1/21. White sand, South America, esp. the Guayanan region. [Jackieae [Ixorodae, Airospermeae, Coffeodae]: ? 2T. Jackieae Korthals - Jackiopsis ornata (Wallich) Ridsdale Tree; stipules connate, sheathing, fimbriate; inflorescences usu. axillary; flowers 5-merous; K usu. 3, C ?aestivation; secondary pollen presentation?; 2-5 ovules/carpel, placentae basal; fruit a 1-seeded nutlet, 3-winged [= 3 k lobes], pink; ?embryo. 1/1. West Malesia, not the Philippines.
Ixorodae Robbrecht & Manen = [Crossopterygeae, Glionettieae, [Trailliaedoxeae + Clavistigmateae], Vanguerieae [Scyphiphoreae [Greenieae [Aleisanthieae + Ixoreae]]]] / Vanguerieae alliance: ?
2U. Crossopterygeae Bridson - Crossopteryx febrifuga (G. Don) Bentham
Smallish tree; inflorescences terminal; flowers 4-6-merous; secondary pollen presentation +; pollen 3-colpate; stigma lobes +, broad; few ovules/carpel, impressed in placentae; fruit a loculicidal capsule; seeds flattened, with fimbriate wing; n = ?
1/1. Tropical Africa, savannas.
2V. Glionettieae Razafimandimbison & Rydin - Glionnetia sericea (Baker) Tirvengadum
Shrub to small tree; secondary veins close; inflorescences terminal on lateral branches; flowers 5-merous; C long-tubular; secondary pollen presentation 0, stigma lobes +; many ovules/carpel; fruit a loculicidal capsule; exotestal cells elongated, anticlinal and inner periclinal cells massively thickened; n = ?
1/1. The Seychelles, higher altitudes.
[Trailliaedoxeae + Clavistigmateae]: ?
Age. The age of his clade is (21.7-)13.8(-7.0) Ma (Tu et al. 2024).
2W. Trailliaedoxeae Kainulainen & B. Bremer - Trailliaedoxa gracilis W. W. Smith & Forrest
Shrublet, thorny; inflorescences terminal; flowers minute [≤2.5 mm long], 5-merous; pollen tectum perforate-echinate; secondary pollen presentation ? [anthers dehiscing in bud]; 1 pendulous ovule/carpel; fruit a schizocarp; seeds elongated; endosperm 0; n = ?
1/1. S.W. China.
2X. Clavistigmateae Kainulainen & B. Bremer - Clavistigma pendula (Wallich) T. Y. Tu & P. W. Xie
Shrub; leaves 2-ranked [internodes twisted], inflorescences axillary; pollen tectum reticulate; style coiled in buds, pollen shed on stigma; stigma undivided, clavate, upper surface concave, placentae peltate; 10-15 ovules/carpel; dehiscence septicidal (also loculicidal); seeds elongated, angled, exootesta reticulate, anticlinal walls sinuous; endosperm ?; n = ?
1/1. China (Yunnan), Bhutan, N. India, Nepal, N. Thailand.
2Y. Vanguerieae Dumortier
Shrubs to trees/(climbers/geofrutices); (plant deciduous); nodes 1:1/3:3; (short sh0ots +), (thorns from supernumerary buds); branches plagiotropic, leaves secondarily biseriate; lamina (with Burkholderia in the mesophyll); (plant dioecious); inflorescences always axillary; flowers 4-5-merous; K (large), connate, C valvate, (moniliform hairs at the throat and/or deflexed unicellular hairs down tube); pollen grains por(or)ate/short colporate, (with pollen buds); G [2-5(-10)], secondary pollen presentation + [presenter hood-/knob-like, "stigmatic knob"], outer cells radially elongated, stigma recessed or not; 1 pendulous ovule/carpel, embryo sac elongated, obturator +; fruit drupaceous, stones with apical germination slit; endosperm soft, oily, embryo curved, cotyledons accumbent, suspensor multiseriate; (germination cryptocotylar).
29/640: Psydrax (100), Pyrostria (70), Rytigynia (70), Vangueria (60), Fadogia (45), Peponidium (45), Canthium (30). Africa, islands of Indian Ocean, Asia to Australia and the Pacific, tropical and subtropical.
Age. Crown-group Vanguerieae are (24.6-)15.5(-7.3) Ma (Bremer & Eriksson 2007).
[Scyphiphoreae [Greenieae [Aleisanthieae + Ixoreae]]]: ?
2Z. Scyphiphoreae Kainulainen & B. Bremer - Scyphiphora hydrophyllacea C. F. Gaertner
Shrub to small tree; nodes 1:1; veinlet endings with distinctive tracheids; petioles articulated, stipules basally connate; inflorescences axillary; flowers 4(-5)-merous; K tubular, connate, secondary pollen presentation + [on style/outside of stigma]; placentae horizontally T-shaped; ovules 2/carpel, 1 ascending, epitropous, 1 descending, apotropous, chalazal projection +, funicular obturator +; embryo sac bisporic, 8-nucleate [Allium type]; fruit dry, indehiscent, longitudinally ridged, 4-seeded; K persistent, mesocarp well developed; exotestal cells with pitted U-shaped thickenings; endosperm oily; embryo long, cotyledons ± = radicle.
1/1. Mangroves, India to China (Hainan), Malesia, Australia and New Caledonia.
[Greenieae [Aleisanthieae + Ixoreae]]: inflorescences usu. terminal; flowers 5-merous; K connate, secondary pollen presentation + [on style/outside of stigma].
2AA. Greeneeae Mouly, J. Florence & B. Bremer - Greenea Wight & Arnott
inflorescence branches scorpioid; flowers 5-merous, protogynous; K not connate; secondary pollen presentation 0; many ovules/carpel; fruit capsular; n = ?
1/9. Indochina to Sumatra.
2BB. Aleisanthieae Mouly, J. Florence & B. Bremer
Shrubs to trees; lamina often with woolly indumentum abaxially; inflorescences (axillary), branches scorpioid; pollen grains (pororate, etc.); many ovules/carpel; fruit capsular, endocarp hard; exotestal walls with thickenings; n = ?
3/10. Malay Peninsula, Borneo, the Philippines.
2CC. Ixoreae A. Gray - Ixora L.
Trees and shrubs; xylem with banded parenchyma; nodes 1:1/3:3; (styloids +); petiole articulated; (plant cauliflorous); flowers 4-merous; G ([-7]), stigma bilobed; one ovule/carpel; fruit drupaceous; seed adaxially concave.
1/564. Tropical, inc. the Pacific.
Age. Ixora is (23.4-)14.4(-6.0) Ma (Bremer & Eriksson 2007).
2DD. Airospermeae Kainulainen & B. Bremer
± Shrubby (monocaulous); inflorescences terminal; flowers 5-merous; C left-contorted; secondary pollen presentation?; 1 pendulous ovule/carpel; fruits drupaceous, 2 pyrenes; ?embryo; n = ?
2/7. Flores, Philippines, Papua, Fiji.
Coffeodae Ezedin = [Augusteae [Alberteae [[Bertiereae + Coffeeae] [Gardenieae, Cordiereae, Pavetteae, Octotropideae, Sherbournieae]]]] / Coffeeae alliance: C left-contorted.
2EE. Augusteae Kainulainen & B. Bremer
Shrubs to trees, (rosette plants); nodes 1:1; inflorescence terminal, flowers (4-)5-merous; C (imbricate - FoC); secondary pollen presentation ?, stigma ± bilobed; many ovules/carpel, placentae peltate; fruit septi-/loculicidal capsule; seeds minute, flattened (narrowly winged); endosperm fleshy; (n = ?12).
2/104: Wendlandia (90). Scattered, Tropical America, Southeast Asia to Australia and Fiji, northeast Africa.
2FF. Alberteae Sonder
Shrubs (semisucculent) to trees; xylem with banded parenchyma; nodes 1:1/3:3; plant monopodial (often), inflorescences terminal on lateral branches; flowers 5-merous, mono-/polysymmetric; semaphyllous/pterophyllous calycophylls +, C contorted, indfundibular/tubular; anthers with basal appendages (not); secondary pollen presentation 0, stigma lobes short; 1 pendulous apotropous ovule/carpel, obturator +; fruit indehiscent/2 mericarps separating from base, stone (with apical germination slit); exotestal cells thickened on inner periclinal/(and anticlinal) walls; endosperm soft, oily, suspensor massive, multiseriate; (n = 10).
3/9: Razafimandimbisonia (5). Madagascar, South Africa (Alberta).
[[Bertiereae + Coffeeae] [Gardenieae, Cordiereae, Pavetteae, Octotropideae, Sherbournieae]] : pollen 3 colporate?
[Bertiereae + Coffeeae] : C left-contorted.
Age. This clade is (33.3-)27.3(-21.3) Ma (Bremer & Eriksson 2009).
2GG. Bertiereae Bridson - Bertiera Aublet
Shrubs to small trees (climbers, stoloniferous herbs); elongated cells with crystal sand in leaf; growth monopodial; inflorescences usu. terminal; flower usu. 5-merous; secondary pollen presentation +; anthers with apical connective appendages; placentae peltate, style longitudinally ridged, shortly bifid; many ovules/carpel; fruit baccate/(sudrupaceous)/wall ± dry; exotestal cells with much thickened (pitted) inner periclinal and ± anticlinal walls; endosperm oily, walls quite thick, embryo ?short, radicle much longer than cotyledons.
1/57. Tropical America and Africa to the Mascarenes.
2HH. Coffeeae de Candolle —— Synonymy: Coffeaceae Batsch
Shrubs to trees (lianes), (deciduous); growth monopodial (sympodial); nodes 1:1/3:3; (leaves with bacterial nodules); inflorescences axillary, sessile, paired (flowers single); calyculus + [= fused bracts and bracteoles]; flowers 4-8(-12)-merous; secondary pollen presentation +/0; (pollen 4-5-colporate); stigma bilobed; 1-2(-10(-20)) ovules/carpel; fruit drupaceous/?baccate/winged); (placentae fleshy, surrounding seeds); seed (ventrally grooved); exotesta 0/± crushed/endotesta crushed + ±isolated fibres/walls thickened; endosperm (ruminate), radicle superior.
12/311: Coffea (125: inc. Psilanthus), Tricalysia (90). Tropical Africa, Madagascar etc., Sri Lanka and India to West Malesia, E. New Guinea and N.E. Australia.
[Gardenieae, Cordiereae, Octotropideae, Pavetteae, Sherbournieae]] : ?
2II. Gardenieae de Candolle —— Synonymy: Gardeniaceae Dumortier, Randiaceae Martynov
Shrubs to trees, lianes, (epiphytes); large resin cells in phloem; nodes 1:1/3:3/5:5; (buds extraxillary, alternate); petiole bundle U-shaped/annular (with inverted adaxial bundles), foliar sclereids +; inflorescences terminal [inc. pseudoaxillary]; C (right-contorted); C (3-)5-6(-13); anthers with placentoids; pollen grains (in tetrads/massulae/also porate/pantoporate - esp. Randia, Alibertia), orbicules 0; secondary pollen presentation +, G [2-9(-16)], placentae ± stalked, peltate/(parietal), (stigma bilobed); ovules 1-many/carpel; fruit baccate, placentae fleshy/(drupe - Duperrea); seed (adaxially excavated); exotesta fibrous[?]; endosperm (ruminate), horny, embryo long, cotyledons short, broad [add]; (n = ?10, ?17).
63/643: Gardenia (200/60), Randia (100), Atractocarpus (53). Pantropical.
63 and 6432JJ. Cordiereae de Candolle
Shrubs to trees; plant dioecious; inflorescences terminal [female - 1-few flowered]; flowers usu 5-merous; secondary pollen presentation 0; male: pollen grains (-7)-aperturate, also por(or)ate; female: G [2-7], stigma ± fusiform, lobed; (1-)3-many ovules/carpel; fruit baccate, placentae fleshy; n = ?
12/123: Alibertia (35), Duroia (25). Central and tropical South America.
Age. The age of Cordiereae is (22.7-)14.4(-5.7) Ma (Bremer & Eriksson 2009).
[Octotropideae, Pavetteae, Sherbournieae] - if this clade exists: C left-contorted; secondary pollen presentation +.
2KK. Octotropideae Beddome (inc. Hypobathrideae, Cremasporeae)
Trees to shrubs; nodes 1:1; petiole base articulated; inflorescences supraaxillary; flowers 4(-5)-merous; [secondary pollen presentation +; anthers long-apiculate; pollen 3-colpate - all?]; placentae very variable/(parietal), stigma shortly 2-lobed; 1 pendulous-many epi(?apo)tropous ovules/carpel; fruit ± baccate/(leathery); (seeds winged); exotesta appearing fibrous or striate, cells thickened on anticlinal/(also outer periclinal) walls/not; endosperm (ruminate), horny; embryo small [?all], cotyledons << radicle [Oct]; (n = 12).
33/139: Hypobathrum (35). Largely tropical Africa, Madagascar and associated islands, Cape Verde Islands, also India to West Malesia.
2LL. Pavetteae Dumortier
Trees to shrubs; nodes 1:1/3:3; plant usu monopodial; (leaves with bacterial nodules); inflorescence usu. terminal; flowers 4-5(-6)-merous; (anthers with transverse septae), (pollen grains circular to quadrangular in polar view, often 4-colporate); ovules 1-many/carpel, apotropous/?, obturator massive; fruit a drupe, pyrenes bilocular, 1-several seeds/loculus, (slight placental pulp); seeds with adaxial hilar excavation, testa often forming a thickened ring around the hilum, exotestal cells thickened on outer periclinal walls/parenchymatous; endosperm cellular, ruminate/not, embryo short to mid-sized [1/2 length of seed]; (n = 10).
22/658: Pavetta (340), Tarenna (200), Rutidea (22). Old World tropics inc. NE Australia, esp. Africa and Madagascar.
Age. The age of Pavetteae is some (17.3-)11.8(-6.5) Ma (Bremer & Eriksson 2009).
2MM. Sherbournieae Mouly & B. Bremer
Plant ± shrubby, lianescent; nodes 1:1; inflorescence pseudoterminal; pollen grains (in tetrads), por(or)ate; many ovules/carpel, placentation parietal; fruit baccate, placentae pulpy; exotesta folded, walls with thickenings; n = ?
4/57: Oxyanthus (34). Tropical and southern Africa.
Pubistylus andamanensis Thothathri - does anything need to be done with this?
Small tree to shrub; ?nodes; inflorescences axillary; flowers 5-merous; C imbricate; secondary pollen presentation?; stigma 2-lobed; ovules 1-2/carpel, pendulous, epitropous; fruit drupaceous; exotestal cells "thick-walled"; endosperm ruminate, embryo short, cotyledons very short; n + ?
1/1. Andaman Islands.
Evolution: Divergence & Distribution. For dates in Rubiaceae, see e.g. Bremer and Eriksson (2009), Antonelli et al. (2009: divergences within South American Cinchonoideae, especially [Isertieae + Cinchoneae]), Wikström et al. (2015: many dates), Rydin et al. (2017), Neupane et al. (2017: esp. Spermacoceae) and Razafimandimbison et al. (2019: esp. Rubioideae).
Wikström et al. (2020: Fig. 1) provide a useful summary of ideas of relationships in Rubiaceae, suggesting species numbers for all tribes. A number of papers have discussed the evolution of Rubiaceae, but since some of both the broad patterns and details of relationships (and ages) in the family as a whole in Rydin et al. (2017) differ from those in earlier papers, talking about evolution in the family is rather difficult. Furthermore, and compounding the problem, clades like Luculieae, Coptosapelteae, and [Colletoecemateae + Seychelleeae] (for the last, see Razafimandimbison et al. 2019) are species-poor, morphologically interesting, and at/near the base of the tree, if details of their positions are not always certain.
Antonelli et al. (2009) thought that Rubiaceae had a boreo-tropical origin and then moved into South America from the Old World via a North Atlantic land bridge. Manns et al. (2012), however, suggested that Ixoroideae and Cinchonoideae (together = Coptosoapeltoideae) originated in South America ca 78.5 Ma, with substantial subsequent long distance dispersal - not so much by land bridges - of taxa with both wind- and animal-dipersed seeds. In particular, the palaeotropical [Hymenodictyeae + Naucleeae] probably moved there from the New World in the Eocene (Manns et al. 2012). The speciose [Theligonieae + Rubieae] may have evolved along the northern coast of the Tethys (Deng et al. 2017). Long distance dispersal is quite often invoked to explain broad patterns of distribution, the plants thought to be involved often having drupaceous fruits (B. Bremer & Eriksson 1992; see also Willis et al. 2014a), although it can be difficult to decide exactly what kind of fruit a plant has (Rutishauser et al. 1998)...
Lower-level studies also emphasize frequent long distance dispersal events. The dioecious Coprosma, with around 110 species, may have originated in New Zealand (or perhaps New Guinea), and in the former it is the second most diverse woody lineage after Hebe (= Veronica). Stem [Coprosma + Nertera] has been dated to (47-)39(-30) Ma, before the Oligocene marine transgression, but well after the separation of Zealandia from Gondwana (Wallis & Jorge 2018), but diversification began in New Zealand less than 14 Ma (Coprosma and Nertera diverged just over 25 Ma); from there it was dispersed, apparently by birds, widely across the Pacific perhaps some 16 times, although surprisingly, perhaps, it is absent from New Caledonia (e.g. Cantley et al. 2014, 2016). It has been suggested that there were some 30 or more long distance dispersal events, including two to the Hawaiian archipelago where there were subsequently eight or more dispersal events to the one island within the archipelago (Cantley & Keeley 2012; Cantley et al. 2014, 2016; Hembry 2018: Marquesas, also other Rubiaceae). All this makes for dizzying reading. Suggestions that Jurassic to Cretaceous Gondwanan vicariance events have shaped distributions in Anthospermeae, vicariance effects also being responsible for the distribution of Coprosma with which Cantley et al. grappled (Heads 1996, 2017, 2018: metapopulation vicariance) would seem to be decidedly unlikely. Coprosma is also known for the extent of the variation it shows in wood anatomy (Jansen et al. 2002c).
Looking at relationships in the pantropical Lasiantheae, Smedmark et al. (2014) emphasized how difficult it was to reconstruct biogeographical events if more than one resolution was allowed for nodes whose support was weak - indeed, little could be said. For the speciose Rubieae, the Old World is a possible place of origin (Soza & Olmstead 2010a). In Putorieae, close to Rubieae (Plocama is the only genus), Mediterranean taxa were sister to the rest of the tribe, within which diversitification began some 14 m. years later; the southern African P. crocylli and the yemenensis group, members of the Rand Flora inhabiting the periphery of Africa, separated ca 5.2 Ma (see Rincón-Barrado et al. 2021 for a detailed discussion of the biogeography of the genus). In both Coffeodae and Psychotriodae, common in the western Indian Ocean area, there have been numerous dispersal events to Madagascar in particular, mostly from the west. Many of these are thought to have occurred within the last 15 Ma at a time when ocean currents, etc.,have rather perversely been flowing from the east, furthermore, there are not many frugivorous birds on Madagascar/migratory frugivorous birds in the area, so how dispersal has occured is unclear (Wikström et al. 2010; Razafimandimbison et al. 2017; Kainulainen et al. 2017). Interestingly, a clade of Malagasy dry-fruited Spermacoceae including Astiella (it is sister to a clade that includes Houstonia) may have moved there from the New World during the Oligocene, although the whole tribe is largely distylous, normally not conducive to long distance dispersal (Janssens et al. 2015). Despite some biogeographic connections between Madagascar and the east, there is as yet no evidence that either the Mascarenes nor the Seychelles were involved, and Rubiaceae on the Comoros and the Mascarenes seem to have had an origin in Madagscar (Wikström et al. 2010). Tosh (2009) summarized biogeographical studies on Madagascan Rubiaceae.
There is considerable variation in fruit and seed morphology in Rubiaceae, and I have not done it justice. Overall, there may be a correlation between fruit type, plant habit, and diversification. Thus clades that are shrubs or trees and in which winged seeds are apomorphic, shrubs with animal-dispersed fruits, and herbs with abiotic dispersal (but not winged seeds), are all notably speciose (Eriksson & Bremer 1991; B. Bremer & Eriksson 1992). Fleshy fruits may have evolved about twelve times in the family, especially during the Eocene-Oligocene period (B. Bremer & Eriksson 1992), however, this figure is likely to be an underestimate, fleshy fruits having evolved at least four times in a New World clade of Galium alone (Soza & Olmstead 2010b, q.v. for other fruit morphologies there). Ehrendorfer et al. (2018) also deal with some aspects of the evolution of fruits in Rubieae in the course of their extensive discussion on the evolution of the tribe - initial diversification in western Eurasia? For fruit evolution in Cinchonoideae in the old sense, see Torres-Montúfar et al. (2018b), but note that terms used to describe fruits with superior ovaries, as in that paper, cannot be readily used for fruits with inferior ovaries, as in Rubiaceae. For the evolution of schizocarpous fruits in Psychotria and of many-seeded carpels from one-seeded carpels, see Razafimandimbison et al. (2014). In Mussaenda there seems to be an association between diversification and dioecy (Duan et al. 2018; see also Käfer et al. 2014), which has evolved perhaps 4 times here, with heterostyly the plesiomorphic condition, but not with plant habit, etc.. Note that diversification within Mussaenda, which contains over 80% of the species in the tribe, did not begin until ca (12.6-)9(-5.6) Ma, ca 40 Ma after the origin of the tribe (Duan et al. 2018).
- Vicentini (2016) discussed the evolution of Pagamea (Rubioideae-Gaertnereae), one of the more prominent clades that has diversified largely on white sand in South America; species limits there can be very tricky (Prata et al. 2018). Fine and Baraloto (2016) noted that in such habitats there had been investment in dense, long-lived tissues that were well-defended both structurally and chemically.
- See Nowak et al. (2012) for the biogeography of Coffea (Coffeeae-Coptosoapeltoideae), which has gametophytic self incompatibility, even on Mauritius. There the initial colonization is likely to have involved at least four, probably 14 or more seeds - and thus 2-7 or more fruits - arriving more or less simultaneously (Nowak et al. 2014). Coffea probably moved from Africa to Madagascar (Hamon et al. 2017), and the Madagascar+Mascarene species form a clade in the combined analysis of A. P. Davis et al. (2011) - there are around 65 or more species of Coffea on the island.
- Rondeletieae (Coptosapelt.) are largely (120/180 spp.) Caribbean endemics (Torres-Montufár et al. 2017a); this tribe is difficult to distinguish from Guettardeae, its sister clade (e.g. Torres-Montúfar et al. 2020a).
- Hillieae. Ball et al. (2025) suggested that hawkmoth pollination was plesiomorphic in the genus, but there is also polination by hummingbirds and bats in Central America, while in the Andes of northern South America hawkmoth pollination is the norm, speciation perhaps being driven by topographic diversity.
Jansen et al. (2002c) provide an extensive analysis of wood anatomy and phylogeny in Rubiaceae; although there seemed to be a general correlation of "types" of wood anatomy with tribes, relationships in the family need to be clarified before the variation in wood anatomy can be placed in the conext of a tree and, one hopes, understood. Koek-Noorman (1997) had found that taxa might have fibre tracheids or septate libriform fibres, and that this correlated with variation in other features of wood anatomy - group I- and II-type woods.
For chemistry and phylogeny, see Young et al. (1996); Dessein et al. (2005a) discussed the phylogenetic significance of palynological variation in the family. Endress (2011a) thought that the inferior ovary of Rubiaceae might be a key innovation.
Finally, Psychotria. The old Psychotria was a very large genus of small to smallish shrubby plants that formed species swarms in the lowland tropics. It is now divided into largely Old and New World clades; the former are Psychotria s. str. and the latter are included in Palicourea (Taylor 2017 for references). In the Old World there are 250+ species of Psychotria s.l. throughout the New Guinea/Pacific area (Andersson 2002; Nepokroeff et al. 2003: Hawaiian radiation - note that these two papers have different taxonomies; Barrabé et al. 2013; esp. Razafimandimbison et al. 2014 and references; Lim & Marshall 2017: diversity still increasing, even on old islands). The genus is abundant throughout Malesia (Nepokroeff et al. 1999; Andersson 2002), and is to include taxa like Calycosia and Amaracarpus as well as the highly derived ant plants like Myrmecodia, Hydnophytum and relatives. Indeed, in the South-East Asian—West Pacific region there has been considerable diversification of myrmecophytic Rubiaceae (see below), and genera like Squamellaria and Myrmecodia (both = Psychotria s.l.) are commonly found in ant gardens - assemblages of plants on trees that are the result of the activities of the ants - that evolved perhaps (7.8-)5.8(-3.8) Ma (Chomicki et al. 2017a). Squamellaria has diversified on islands in the southwestern Pacific within the last 12 Ma or so (Chomicki & Renner 2016a; Chomicki et al. 2019b). Psychotria in New Caledonia, with some 85 species, represents yet another major radiation (Barrabé et al. 2013), interestingly, its relationships are not with the Pacific Psychotria, 78/87 of the New Caledonian species belonging to a clade that has diversified there within the last ca 7 Ma and that is sister to an Australian clade, while the remaining nine species belong to three separate clades (Barrabé et al. 2013, q.v. for many dates). In a clade of New World Psychotria (= subgenus Psychotria) older species tend to occupy larger areas than younger species (Paul et al. 2009); c.f. J. C. Willis's "age and area" hypothesis (e.g. Willis 1922), but the conceptual baggage is presumably very different in the former. Diversification in Palicourea seems to have begun a little over 10 Ma (Bedoya et al. 2023), and Sedio et al. (2013) examined the movement of Palicourea and Psychotria in the New World at about the time of the biotic interchange between North and South America (there dated to ca 3 Ma) and noted that the ecological preferences of taxa that moved did not change, and that in Central America in particular the ecological diversity of these genera was increased by immigrants from South America with ecological preferences other than those of the natives. Bedoya et al. (2023) also noted that Palicourea showed extensive climatic niche conservatism despite its movements around the Neotropics, although lability in the biotic niche area was substantially greater and there was parallel variation here as the genus moved into new areas, for instance, there was parallel variation in inflorescence types.
There are considerable differences in foliar secondary chemistry between closely related species in the 20 species of Psychotria found on Barro Colorado island (B.C.I.), Panama. Here, like several other taxa that grow in swarms of ecologically similar species in LTRF, these differences are implicated in defence against a variety of herbivores and help to contribute to the latitudinal gradient in diversity (Sedio et al. 2017, 2021; Sedio 2017, see also Endara et al. 2017). Secondary metabolites found in the fruits are different yet again (G. F. Schneider et al. 2023). These sympatric species of Psychotria also have very different endophytic fungi - this depends on the plant's chemistry - and in turn the endophytes affect their host's chemistry (Christian et al. 2020). Furthermore, species growing together in B.C.I. were more closely related than expected by chance, and the hydraulic traits that were being studied were conserved phylogenetically (Sedio et al. 2012) - a common pattern in such plants, where variation in chemical defence strategies tends not to be correlated phylogenetically and is orthogonal to factors involved in plant growth and nutrient allocation (Sedio et al. 2021).
For similar systems, see also Eugenia, Inga, Ocotea, Passiflora, Piper, Protium, sundry Solanaceae, etc.. For myrmecophytism in Rubiaceae, especially Psychotria s.l., see below
Ecology & Physiology. Rubiaceae are an important component of the understory vegetation of tropical forests in both Malesia and the New World. Thus they include the second highest number of tree species (i.e., plant with a single stem >2 m tall, of if two or more stems, one erect stem >5 cm d.b.h.) of any family(!), over 4,800 species (Beech et al. 2017) - are no. 1, and they also have the second highest numbers of species overall in Amazonian forests (Cardoso et al. 2017) and also in Madagascar (Kainulainen et al. 2017). Of course, they include very few of the larger trees, being represented by only -- of the 277 species that make up half the stems 10 cm or more d.b.h. in those forests (ter Steege et al. 2013). The family is not uncommonly epiphytic, and it represents an appreciable component of the woody epiphytic flora in the tropics. CAM photosynthesis has recently been detected in some species of the epiphytic and myrmecophytic Squamellaria (= Psychotria s.l.) from Oceania (Chomicki & Renner 2016a). For rubiaceous epiphytes, see Zotz, Weigelt et al. (2021: list), while Hietz et al. (2021) and Zotz et al. (2021) discuss the ecophysiological characteristics of epiphytes in general. Climbing Rubiaceae are notable components of tropical vegetation in the New World, at least (Sperotto et al. 2023).
Razafimandimbison et al. (2012) looked at the evolution of growth habit in Morindeae; the liane habit is plesiomorphic there (ca 100 species in the Old World), with independent evolution of the self-supporting habit, perhaps connected with the diversification of the clade in the Neotropics. Secondary woodiness may have evolved three times in Spermacoceae growing in tropical uplands (Neupane et al. 2017), and it has also evolved in Rubieae and Anthospermeae (Jansen et al. 2002c). Rowe and Speck (2015) discuss the biomechanics of climbing in the herbaceous Galium aparine, a plant that is covered in small prickles that act as grapnels.
Quite a number of Chiococceae, e.g. Schmidtottia, are to be found growing on serpentine or limestone in Cuba, and also over serpentines in New Caledonia (e.g. Motley et al. 2005). McCartha et al. (2019) discuss hyperaccumulation of nickel in some species of Psychotria found from Mexico to Ecuador, Venezuela and the Greater Antilles - and not simply when grown on soils over ultramafic rocks. A few nickel hyperaccumulators are known in Psychotria from the Malesia-New Caledonia area (McCartha et al. 2019).
Rubiaceae, in particular Rubioideae, are notable among non-monocot angiosperms in having species in which raphides may be found in just about any part of the plant; there are five monocot families like them in this respect (Lawrie et al. 2023).
In South East Asia Rubiaceae are important food resouces for frugivores because they produce crops of sugar-rich fruits more or less aseasonally (Leighton & Leighton 1983), indeed, they are the most important gentianid food resource for smaller unspecialized avian frugivores in general (Snow 1981, see also below).
For the association of species of Psychotria s.l. with ants, see Plant/Animal Interactions below.
Pollination Biology & Seed Dispersal. Many Rubiaceae have rather small flowers, but they are often more or less closely aggregated; taxa like Cephalanthus and many Morindeae and Naucleeae have flowers aggregated into spherical heads. Cephaelis (= Palicourea: Rubioideae) has a condensed inflorescence immediately subtended by paired and coloured inflorescence bracts, while large, coloured calyx lobes (= semaphyllous calycophylls) are scattered in Dialypetalanthoideae in particular and help to attract the pollinator (these calycophylls often fall off, and the fruit is dehiscent - Delprete 2019). The Old World Mussaenda has single calycophylls on a few flowers in the quite lax inflorescence (the genus may be polyphyletic - see Alejandro et al. 2005; also Duan et al. 2018), while the New World Warszewiczia, often cultivated, has similarly conspicuous individual sepals. Claßen-Bockhoff (1996a) surveyed the more flower-like inflorescences that are quite common here; Cruckshanksia (Rubioideae-Coussareeae), from southern South America, is a particularly notable example, as is Stipularia africana (see also Baczynski & Claßen-Bockhoff 2023). Genera like Wittmackanthus, Calycophyllum, Nematostylis, etc., also have pseudanthium-type inflorescences; Delprete (2019) provides a review of calycophylls.
In some species of Spermacoce (Rubioideae-Spermacoceae) the corolla is extensively modified, for example, lobes/pockets developing on either side of the apices of the corolla, the inside of the tube having various modifications, etc., and pollination can be explosive (pollen: Dessein et al. 2000: pollen in related Knoxieae; Vaes et al. 2006: corolla in Australian Spermacoceae; Harwood & Dessein 2005: extreme palynological variation in Spermacoceae; Dessein et al. 2002, 2005b; Dessein 2003). Explosive pollination is also known from Posoquerieae (Dialypetalanthoideae), pollen being catapulted onto the pollinating insect; the flowers are monosymmetric and may be inverted (Puff et al. 1995; Delprete 2009; Cortés-B. & Motley 2015). Here corolla length ranges from 38 cm in Posoqueria, pollinated by sphingids, to 3 mm in the monotypic Molopanthera (M. paniculata), probably bee pollinated, the other genus in the tribe (Delprete 2009). Bird pollination is scattered in the family, as in some Palicourea and Psychotria (Silva et al. 2010; Betancourt et al. 2023), although insects are common pollinators. Buzz pollination is thought to occur in a number of genera, including Argostemmma, that have united stamens (Puff et al. 1995), although few observations have been made. Wind pollination characterises Theligonieae and is common in Anthospermeae.
Secondary pollen presentation is notably common in Dialypetalanthoideae (Nilsson et al. 1990; Igersheim 1993c; Puff et al. 1996: much information; de Block & Igersheim 2001; see also Tilney et al. 2011, 2014; Kainulainen et al. 2013; Romero et al. 2022; El Ottra et al. 2023: 38 genera). Here pollen is presented on the style, a deposition-type pollen presentation mechanism. Tilney et al. (2014) suggested that in some taxa at least, hydrophilic pectic thickenings of anticlinally elongated cells of the surface of the pollen presenter, the thickenings of Igersheim, might interact with protruding onci/pollen buds of the pollen grains (they looked at Vangueria infausta - Vanguerieae) and be involved in the transfer of water, etc., from the plant to the pollen on the pollen presenter.
Many species of Rubioideae, overall, perhaps half of the whole family, are heterostylous (Bir Bahadur 1968; Robbrecht 1988; Betancourt et al. 2023), more genera being heterostylous than in any other family (Simón-Porcar et al. 2024: 130/247 known cases, somewhat under a quarter of the family). Hardly surprisingly, perhaps, taxa with secondary pollen presentation are never heterostylous and there have been several reversals to homostyly (Ferrero et al. 2012). Anderson (1973) suggested that heterostyly had evolved several times from protandrous (Rubiaceae are overwhelmingly protandrous) self-compatible ancestors, and overall there have been 20 or so origins of the condition here (Ferrero et al. 2012; Cohen 2019: general); dioecy may evolve from heterostyly (Betancourt et al. 2023). Heterostyly is suposed to increase the precision of deposition of pollen on the stigma, and in angiosperms as a whole there have been around 152 gains of this condition - and 137 losses (Simón-Porcar et al. 2024). Furtado et al. (2021) noted that the efficiency of legitimate pollen transfer was improved if the stigma-style length was rather precisely controlled and the stigmatic lobes were large. Corolla lobe size can vary considerably between these morphs in Rubiaceae (Betancourt et al. 2023), who also noted that pollen flow between flowers wih different style lenghs was commonly asymmetrical. Trevizan et al. (2024) noted substantial differences in absolute stigma length beween long- and short-styled flowers in Palicourea (especially) and Psychotria - stigmas were longer in the short-styled flowers - although there was not a comparable difference in pollen deposition between the different flower types in the Palicourea studied.
There is dioecy in a number of Neotropical Gardenieae like Randia that have large fleshy fruits (C. Taylor, pers. comm.) and in Mussaenda (Duan et al. 2018), and monoecy is scattered, in particular occuring in a few wind-pollinated Rubioideae. In Vanguerieae and Mussaenda there are apparent reversals from dioecy to hermaphroditism (Razafimandimbison et al. 2009; Duan et al. 2018), and in New World Galium there may have been reversals to polygamy (Soza & Olmstead 2010b). For the evolution of breeding systems in Galium, see Goldberg et al. (2017).
Taxa in which the fruits have one or more expanded calyx lobes are scattered in the family. These are usually not coloured and attractive in flower (c.f. above), but are involved in seed dispersal alone, the fruits being indehiscent; Delprete (2019, q.v. for a list) calls them pterophyllous calycophylls. Seeds may be wind-dispersed, thus winged seeds have evolved some eleven times in the Galianthe s.l. clade (Spermacoceae) alone (Florentin et al. 2025). In Morindeae and some other taxa many-seeded fleshy fruits are in fact multiple fruits, the inferior ovaries of two or more flowers fusing (e.g. Razafimandimbison et al. 2012). The seeds of of Henriquezia (Dialypetalanthoideae-Henruqueziae) are up to 8 cm in diameter (Cortés-B. & Motley 2015). Ophiorrhiza, usually a plant of the forest floor, has splash-cup dispersal, the cups opening only when wet; vivipary may also occur here, with the plantlets being dispersed in the water drops (Dintu et al. 2014).
Plant-Animal Interactions - Ants. Myrmecophytes are quite common in Rubiaceae, some 140 species of Rubiaceae in 22 genera having the myrmecophytic habit (Razafimandimbison et al. 2005), and around 105 species of these are Hydnophytinae (sic) from Australasia (Chomicki & Renner 2017a). (Genera like Myrmecodia, Hydnophytum and three other related genera are well-known ant plants; they are to be included in Psychotria s.l., and they make up the bulk of the myrmecophytes (see also) Chomicki & Renner 2015, 2017; Chomicki 2020 for a useful summary.) Overall relationships are [Squamellaria [Anthorrhiza [Hydnophytum (paraphyletic), Myrmephytum, Myrmecodium]]] (Chomicki & Renner 2016b), interestingly, CAM photosynthesis has been reported from this group (Gilman et al. 2023). About 90 species in crown-group Hydnophytinae have been dated to around 13.7 Ma (Chomicki & Renner 2015: Fig. S7, 17 species of Hydnophytinae in tree, 2016), or perhaps (9.3-)6.3(-3.3) Ma; for other rubiaceous myrmecophytes, ages are younger (Chomicki & Renner 2017a; Chomicki et al. 2017a). Myrmecophytic Naucleeae (Dialypetalanthoideae) are estimated to be ca 16 Ma - or much younger (Chomicki & Renner 2015: Fig. S11), but the sampling, although focused on myrmecophytes, is slight.
Most is known about myrmecophytism in Psychotria s.l.. Here the ants, whether specialists or generalists, live in chambers in the grossly swollen stem (hypocotyl) base. About 45 species of ants are generalists, nesting inside the swollen base (= domatium). The gross morphology of some of these swollen stems is remarkable. Thus Hydnophytum caminiferum has curious, almost J-shaped, funnel-like projections of uncertain function developing from an irregularly swollen stem base (Wistuba et al. 2014, see also Jebb & Huxley 2019), while H. [= Squamellaria] kajewskii has an almost boat-shaped stem base, the vegetative stems at the top being rather like a mast, while along each side there is a row of projecting holes, almost like rowlocks - no ant association was noted there (Chomicki & Renner 2016a). Other species like M. arfakianum has blue, 6-merous flowers, and on it goes. The plants receive nutrients from the ants (for ant domatia in general, see Chomicki et al. 2024). However, only a few species of ants are involved in specialized symbioses; the ants also receive rewards from the plants, they may protect the plants against herbivores, facilitate their gemination and establishment, etc. (Chomicki 2020; Chomicki et al. 2025). In some symbioses, material brought in by the ants is stored in chambers with warty walls and nutrients from this material and from ant excreta are taken up by the plant; on the other hand, the chambers with smooth walls are used by the ants to rear their offspring. The ants involved include the dolichoderines Pseudomyrmex and Anonychomyrma (Chomicki & Renner 2019). Some of these myrmecophytes have more or less branched thorns or spines arising from the root, stem, inflorescence, and even the torn and eaten surfaces of the leaves (Huxley 1978; Jebb 1991; Huxley & Jebb 1991 for the taxonomy of the group). The mutualisms may break down, mainly at higher altitudes, and other organisms such as frogs may be found in the domatia (Chomicki & Renner 2017a). At these higher altitudes there are fewer ants and the walls of the domatia are not differentiated, all being smooth (Chomicki & Renner 2019).
A set of ant-plant relationships that has received a lot of recent attention is that between some species of Fijian Squamellaria (also = Psychotria s.l.; 6 species in Chomicki et al. 2025) and the dolichoderine ant Philidris nagasau (Chomicki et al. 2019b; Wcislo 2020), which cannot make carton, so needing the plant to construct its domatia (Chomicki & Renner 2016b, 2019). The floral disc keeps on producing nectar, rich in sugars and amino acids, for ten or more days after anthesis, the ants, but not other insects, being able to take this nectar from the plant; fruit development does not begin until after nectar secretion stops (Chomicki et al. 2016a). Furthermore, P. nagasau actively disperses the seeds of these Squamellaria, putting them in cracks in the trunk of the host trees and protecting them before they germinate, while related but non-farmed species of Squamellaria are dispersed by birds (Chomicki & Renner 2019). The ant even fertilizes the seedlings by excreting into the hypocotylar domatium with its warty walls (there are no smooth walls at this stage) before they can be occupied by the ant (Chomicki & Renner 2019). Squamellaria seedlings can develop a long hypocotyl which enables them to grow more easily out of the cracks in which the seeds have been placed (Chomicki & Renner 2016b). Interestingly, P. nagasau puts the seeds on trunks in full sun, and plants that grow in such conditions flower profusely and provide the ant with copious nectar, and the ant protects the plants effectively from the seedling stage onwards (Chomicki et al. 2020). However, at the same time fertilization of the plant with nitrogen by the ant is reduced because of the high-nectar, low-insect diet of the ants in such sunny conditions; faeces of ants growing in shadier conditions are richer in N because of their higher-insect diet (Chomicki et al. 2020). However, neither insect nor plant seems to be negatively affected by these high-light conditions. There is just a single large colony of ant on each plant, all the galleries in the domatium being interconnected (see also Chomicki et al. 2025). However, in the three species of Squamellaria immediately basal to the P. nagasau-associated plants, there may be up to five colonies of ants on a single plant, overall 18 species, 13 genera and 3 subfamilies of ants being involved, and most of these ants benefit the plant (Chomicki et al. 2025). These Squamellaria species grow in a different way, the galleries being compartmentalized, each compartment having a separate entrance. This arrangement allows independent colonizations of the one plant, indeed, if the compartmentalization of the domatium is broken down in experiments, the ants that were in separate compartments start fighting, mayhem ensues and the nutritional symbioses break down (Chomicki et al. 2025).
Three other clades of hydnophytes grow in similar high-light environments, the largest being Myrmecodia (Chomicki et al. 2020) which has around 26 species; here the association of the plant and ant (e.g. Iridiomyrmex cordata) is taken advantage of by larvae of the blue butterfly, Hypochrysopsis apollo (Dunn & Dunn 1991; Forster 2000). See above for more information on rubiaceous myrmecophytes.
Close associations with ants have arisen several other times in Rubiaceae. Thus there are several myrmecophytic Naucleeae, the ants - a variety of species are involved (Razafimandimbison et al. 2005) - living in less grotesquely swollen stem domatia. For example, some eight species of Neonauclea from Western Malesia have a three-way relationships - plant-ant-pseudococcid (for honeydew -Cladomyrma, see Moog 2009). Interestingly, some of these myrmecophytic clades have diversified notably slowly and/or have very limited distributions; about 18/68 species in this group of Nauleeae are myrmecophytes and there have been about three origins of the habit (Razafimandibison et al. 2005). Focussing on Neonauclea s.l., Ordas et al. (2021) suggested two origins of myrmecophytism there, one in Myrmeconauclea s.str., but also several losses of myrmedomes/swollen internodes in Neonauclea s. str.. In the western Amazon, Duroia hirsuta (Cordiereae-Dialypetalanthoideae) is often associated with the ant Myrmelachista schumanni which forms monospecific "devil's gardens" by poisoning the surrounding vegetation with formic acid; there is a single polygynous ant colony in each garden, which can persist for hundreds of years (Davidson & McKey 1993; Frederickson et al. 2005; Frederickson & Gordon 2007; Salas-Lopez et al. 2016 and references). Ascomycete fungi, Chaetothyriales, often grow inside the domatia (Vasse et al. 2017). Perhaps rather surprisingly, herbivory of ant-infested Duroia may be quite high, and it increases as the garden getrs larger - basically, a version of the Janzen-Connell effect (Frederickson & Gordon 2007). Plant growth in these gardens may also be inhibited by allelopathic compounds like the tetracyclic iridoid lactone (plumericin) that is produced by Duroia (Page et al. 1994).
Myrmecophytes are often in species-poor clades, but exceptions in Rubiaceae include Hydnophytinae and Neonauclea, as well as Macaranga (Euphorbiaceae) and Cecropia (Urticaceae: Chomicki & Renner 2015).
Other Insects. Rubiaceae are not often eaten by caterpillars (Ehrlich & Raven 1964), although some sphingids (Semanophorae) do prefer members of the family (Forbes 1956; Quicke et al. 2024, 2025: Guanacaste area, Costa Rica). Recent work suggests that ome 817 species of Rubiaceae are associated with mites and develop acarodomatia (Chomicki et al. 2024).
Psychotria species are noted as growing in species swarms in various parts of its range, including Panama. For similar tropical systems, see also Eugenia, Inga, Ocotea, Passiflora, Piper, Protium and Bursera and sundry Solanaceae - the discussion about Inga is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
Plant-Bacterial/Fungal Associations. There are bacterial leaf nodules in some 80 species of Psychotria (Rubioideae), mostly from east Africa-Madagascar, and their presence is correlated with development of distinctive dendroid colleters both here and in unrelated genera like Pavetta (Lersten 1974a, b and references). Leaf nodulation seems to have originated in mainland taxa which then moved to Madagascar and the Comoros independently, but not to the Mascarenes; nodulation has sometimes been lost, and also independently acquired in the Malagasy P. bullata (Razafimandimbison et al. 2014). Bacteria are initially to be found in mucilage secreted by the colleters, and they then move into substomatal cavities in the leaf, where they grow between intrusive thick-walled mesophyll cells, but not in the cells themselves (Lersten & Horner 1967; Pinto-Carbó et al. 2018). Bacteria of the ß-proteobacterium Burkholderia have been isolated from the nodules (van Oevelen et al. 2004), and there is vertical transmission of the bacterium in the seed near the plumule (Sinnesael et al. 2018). Although some species of Burkholderia are nitrogen-fixing symbionts in the root nodules of Fabaceae-Faboideae, nitrogen fixation has not been detected in Psychotria (Miller 1990). Carlier and Eberel (2012) found that the genome of the nodule bacterium Candidatus B. kirkii, at 4 Mb, was small for a Burkholderia and had large proportions of pseudogenes and transposable elements. Genes affecting a variety of functions seem to have been negatively impacted. There are indeed no genes involved in nitrogen fixation, but on a 140 kb plasmid there were genes involved in secondary metabolite synthesis perhaps protecting against herbivores or pathogens (Carlier & Eberel 2012); protection against herbivory by the production of toxic chemicals by the bacteria seems quite likely (Verstraete et al. 2017). In P. umbellata, at least, Burkholderia-free plants grew less well than plants with the bacteria (Sinnesael et al. 2019) - herbivory would not have been involved here.
There are also about 440 species of Pavetta (Pavetteae) and Sericanthe (Coffeeae) (both Dialypetalanthoideae) that have leaf nodules (all told, ca 650 species of Rubiaceae in at least four tribes have foliar endophytes - Verstraete et al. 2023). Although specificity of Burkholderia, a bacterium commonly in the nodules, is high and its transmission is largely vertical, again there is also horizontal movement, and the association here is not of very long standing (Lemaire et al. 2011b; Pinto-Carbó et al. 2018). Members of two groups of Burkholderia also grow free in the leaf between mesophyll cells in some African - from South Africa, also elsewhere - Vanguerieae (Dialypetalanthoideae), an association that also seems largely specific from the plant's point of view but not from that of the bacteria (Verstraete et al. 2011b, 2013a, b). This association seems to have evolved three times, and although again any benefits to the partners are unclear - but Burkholderia can cause fatal acute heart failure in cattle that eat infected Vanguerieae - the diversity of the infected clades seems to be higher than that of their non-infected sister taxa (Verstraete et al. 2013b, 2017); overall, some 150 species of Vanguerieae may be involved. For more information, see I. M. Miller (1990), C.-J. Yang and Hu (2018), Verstraete et al. (2023: Caballeronia endophytic in leaves of Empogona and Tricalysia - Coffeeae) and Cheek and Onana (2024: Keetia nodulosa - Vanguerieae, Africa).
Vegetative Variation. Although most Rubiaceae can be recognised by a distinctive combination of vegetative characters - opposite entire leaves + interpetiolar stipules will do it - vegetative variation in the family is quite extensive, even apart from the remarkable morphologies of the myrmecophytic taxa just mentioned.
A number of taxa are lianes, and as might be expected, their anatomy can be distinctive. Leal et al. (2020: q.v. for more literature on cambial variants here) described additional vascular cylinders that might develop in the cortex or in dilated ray regions in both the stem and the root of Chiococca alba. Herbaceousness is prevalent in Rubioideae, but some Spermacoceae may be secondarily woody, with xylopodia, or lianes/climbers, and the related Knoxieae are thought to be both primarily and secondarily woody (Lens et al. 2009a, b; Neupane et al. 2017; Carmo et al. 2021 - see also Divergence & Distribution above). "Latex" is not uncommon.
Some taxa have 1:1 nodal anatomy, and Sinnott (1914) and Sinnott and Bailey (1914) had suggested that the basic condition for Rubiaceae was to have such nodes, as did Neubauer (1981), although this might seem rather odd for a family with well-developed stipules. Howard (1970) added a number of examples of Rubiaceae that had more complex nodes. Vascular bundles may encircle the node and they may come from one or several gaps, hence complicating the interpretation of nodal anatomy (c.f. Patil & Patil 2012: few examples taken from this source). Robbrecht and Puff (1986) thought that that 3:3 nodes were basic in Rubiaceae, and they distinguished between 1:1 and 1:3 nodal morphologies, variation associated with how stipules were innervated - both are included in the 1:1 "type" here (see also Mitra 1948 for innervation of the stipule). Genera with trilacunar or more complex nodes (Neubauer 1981; Robbrecht & Puff 1986) do not seem to correlate with phylogeny, indeed, Saha et al. (2014) proposed a correlation with habit: Woody taxa tended to have 3:3 nodes, herbaceous taxa 1:1 nodes. Rogers (2005) suggested that nodal anatomy of the family might repay closer investigation; the nodes of his Henriquezieae are definitely not the 1:1 type... However, overall we still know remarkably little about nodal anatomy in the family, although Robbrecht and Puff (1986) found a variety of nodal morphologies in Gardenieae and Sana and Maiti (2012) and especially Saha et al. (2014) have looked at the nodes of a number of Indian taxa. For the nodal anatomy of seedlings, see Bailey (1956) and Sisode and Patil (2004).
As to Gardenieae, the axillary buds of Gardenia itself are often extra-axillary, although still opposite. However, in the recently-described Thaigardenia the leaves are opposite, but the extra-axillary buds from a leaf pair end up at quite different heights on either side of the stem, and so branching appears to be alternate - there may also be supernumerary axillary buds, but not necessarily the same number in opposing leaf pairs (Sunkaew et al. 2024). Altogether remarkable; nodal anatomy might repay investigation.
Petiole anatomy is variable, and although the vascular bundles may be more or less arcuate in taxa with 1:1 nodes, in taxa with both 1:1 and 3:3 nodes they may also be annular, with or without wing bundles and with or without inverted vascular plates (Martínez-Cabrera et al. 2009; esp. Sana & Maiti 2012; Saha et al. 2014). Little is known about details of features like veinlet presence and venation density, although there may be some phylogenetic signal here (Pacheco-Trejo et al. 2009). Saha et al. (2014) looked at node and petiole anatomy and also foliar venation patterns in some 76 species in 17 tribes of Indian Rubiaceae.
The nature of the whorled leaves of many species of Galium and relatives has been a matter of some dispute; Ehrendorfer et al. (2018) optimised some relevant vegtative variation on a tree of the tribe (see also L.-E Yang et al. 2018 for a phylogeny). They often have whorled leaves but lack obvious stipules, however, there are only two opposite branches and two 1:1 nodes per whorl, suggesting that the basic construction of the plant is having paired, opposite leaves each subtending a bud. Indeed, the nodes of most species (e.g. Majumdar & Pal 1958; Neubauer 1981), including those of genera like Phuopsis (pers. obs.), are of the 1:1 type, but branches separate from these single traces and form a vascular collar around the stem from which the stipular bundles diverge. However, taxa like G. rubioides have four leaves at a node which are all independently directly vascularized from the stele (Neubauer 1981; Rutishauser 1999); this is likely to be a derived condition (Soza & Olmstead 2010a). Soza and Olmstead (2010a) suggested that the basic condition in Rubieae was to have six leaves per whorl (c.f. L.-E Yang et al. 2015), although there was frequent evolution of four-membered whorls (and even reversals to six again). Leaves and stipules may come from separate primordia developing from a ring primordium encircling the stem (e.g. Pötter & Klopfer 1987), and it has even been suggested that "leaf-like stipules are independent structures, not part of the leaf" (Soza & Olmstead 2010a: p. 768). At the same time species like G. paradoxa sometimes have opposite leaves with paired, interpetiolar stipules (I do not know what their anatomy is) - and there is similar variation in the unrelated Limnosipanea (C. Taylor, pers. comm.; = Galium s.l.) and also in Rubia (L.-E Yang et al. 2015). Kelloggia, sister to the rest of the tribe (Nie et al. 2005), has opposite leaves, as do Didymaea and Theligonum.
Anisophylly is well known in Rubiaceae, occurring in herbaceous taxa like Theligonum and Argostemma. It is especially marked in taxa like A. cordifolia, where the adult plant grows on shady, mossy rocks and seems to to have but a single leaf (Nuraliev et al. 2017b), all in all looking rather like Monophyllaea (Gesneriaceae), a plant of similar habitats. A number of genera, especially those growing in more shaded conditions, have plagiotropic branches where all the leaves are in one plane because of twisting of the internodes; species include some Coffea, Psychotria, Amaracarpus, and Vanguerieae (e.g. Wong et al. 2018). Lobed leaves - leaves in the family never have teeth - have long been known from genera like Genipa and Pentagonia, species of the latter even having a fenestrate lamina (P. lanciloba). However, the discovery of taxa like P. osapinnata, which has fully compound imparipinnate leaves, the leaflets being subopposite to alternate and with undulate margins, came as something of a surprise (e.g. Hammel 2015). Thus it is interesting that the simple leaves of Coffea show a cryptic compound-leaf developmental program (KNOX1 is reactivated) early in their development (Bharathan et al. 2002; Nakayama et al. 2022).
Stipule morphology and position show considerable variation (see above - Galium, etc.. There are sometimes two pairs of stipules, one more or less intrapetiolar, the other interpetiolar.
Colleters in the axils of the stipules are common in the family (e.g. Lersten 1974; Miguel et al. 2010 and references; Tresmondi et al. 2015; Lopes-Mattos et al. 2015; Judkevich et al. 2017: Spermacoceae). The exudate from the colleters covers the bud (e.g. Tremondi et al. 2015), and that of Kindia (Pavetteae) is bright orange and contains a variety of triterpenes (Cheek et al. 2018a). Colleters of the standard type may nevertheless differ at the subcellular level, and colleters of some forest Rubiaceae in Brazil, at least, produced hydrophilic secretions while savanna species produced lipophilic/mixed secretions (Tresmondi et al. 2015, esp. 2017). Although Henriquezia and Platycarpum (Henriquezieae) lack colleters, they have glands on the leaves that may secrete nectar (Cortés-B. & Motley 2015). Rubieae also lack colleters in the axils of their foliaceous stipules, although the leaf blade has more or less pellucid glands, while in a number of taxa with fimbriate stipules the colleters are not axillary, but are borne on the ends of the fimbriae (Krause 1909). Colleters are also common in the flowers, thus in Cephalanthus they are found in the sinuses of both calyx and corolla (Romero et al. 2017), but I know little about details of their distribution there.
Genes & Genomes. For a survey of chromosome numbers in Rubiaceae, see Kiehn (1995), for chromosomes of some Thai Rubiaceae, see Puangsomlee and Puff (2001), of Neotropical Rubioideae, see Kiehn (2010) and Kiehn and Berger (2020), and from Africa-W. Indian Ocean, see Kiehn and Berger (2023). Polyploidy is widespread (e.g. Kiehn & Berger 2023), thus chromosome numbers in Coprosma range from n = 16 to n = ca 110 or so, with higher numbers from Hawai'i, Tasmania and Macquarie Island (Dawson 1995). x = 11 for the family, perhaps (e.g. Raghavan & Rangaswamy 1941).
Laforest et al. (2025) suggest that there has been a chromosomal duplication in a clade including Uncaria, Mitragyne and Cinchona, all members of Dialypetalanthoideae-Cinchonodae.
As might be expected, molecular evolution in the herbaceous Rubioideae seems to be faster than that in the woody members (Rydin et al. 2009b); note the laggard woody Dunnia within the herbaceous clade!
The chloroplast atpB promoter region, etc., vary in Rubioideae (Manen & Natali 1996; Natali et al. 1996).
Economic Importance. For the origin of Coffea arabica (Coffeeae-Dialypetalanthoideae), arabica coffee, an allotetraploid hybrid, see Bawn et al. (2021) - C. canephora and C. eugenioides are the likely parents, the hybridization event being dated to between 1.08 Ma and 543,000 years ago. There is also robusta coffee (C. canephora), while a third species, C. liberica var. dewevrei, which yields excelsa coffee, is also used (A. P. Davis et al. 2025).
The purine alkaloid, caffeine, is toxic to invertebrates, including bees, and is in high concentration in the fruits and seeds of Coffea. The weevil Hypothenemus hampei is nevertheless an important pest of coffee beans, destroying up to 80% of the crop. This is because it has the gammaproteobacterium Pseudomonas in its gut, and Pseudomonas detoxifies caffeine, caffeine in fact being its main source of C and N (Ceja-Navarro et al. 2015). Adding further complexity to caffeine-plant-animal interactions, Stevenson (2020: p. 606) observed, "bees fed caffeine at ecologically relevant concentrations during a learning experiment were three times more likely to recall a trait associated with a food reward than bees fed a control diet" - but graduate students already knew this.
Chemistry, Morphology, etc.. For cyclotide proteins, found in Oldenlandia and Psychotria and relatives in Rubioideae, see Gruber et al. (2008) and Koehlbach et al. (2013).
Judkevich et al. (2020a) described the development of dioecy in some Brazilian Gardenieae. In some taxa the calyx begins to develop after the corolla, but this is only when the calyx lobes are much reduced; the calyx tube always develops later (de Block & Vrijdaghs 2013). Floral - especially corolla - development has been carefully analysed in Spermacoceae, where the corolla may be fenestrate; corolla tubes are variously produced from an annular intercalary meristem (a stamen-corolla tube), an intercalary meristem in a different position produces a corolla tube sensu stricto, or corolla lobes may fuse postgenitally, and it can be difficult to distinguish between early and late corolla tube development (Vrijdaghs et al. 2015). In a subsequent study that focused on Rubieae very much the same set of variables was found to produce the corolla tubes there (Vrijdaghs et al. 2020). Ronse Decraene and Smets (2000) discussed floral development in the family, emphasizing variation in the relative development of a stamen-corolla tube with a common meristem and a corolla tube s.s.; in some taxa, filaments fuse postgenitally with the corolla tube. See Gonçalves and Mariath (2022) for floral morphology in Psychotria. A few Rubiaceae like Theligonum and Dialypetalanthus have more than twice the number of stamens as perianth/sepals/petals (e.g. Endress 2003a; see also euasterids); Wunderlich (1971) noted that the stamens of Theligonum were in groups of 2-6, and suggested that each group represented a separate male flower. Taxa with connate anthers are discussed by Puff et al. (1995), and those with locellate anthers by Judkevich et al. (2020b). Harwood and Dessein (2005) noted that palynological variation in Spermacoceae was extreme, almost as much as that in the rest of the family combined (see also Dessein 2003; Dessein et al. 2002, 2005b). Prominent onci are scattered in the family, including in Spermacoceae (see also Romero et al. 2017). While nearly all Rubieae have 5-13 zonocolpate pollen grains (e.g. Huysmans et al. 2003, q.v. for other distinctive features of the pollen), this is not so in Kelloggia, sister to the rest of the tribe. While nearly all Rubieae have 5-13 zonocolpate pollen grains (e.g. Huysmans et al. 2003, q.v. for other distinctive features of the pollen), this is not so in Kelloggia, sister to the rest of the tribe.
Taxa like the sister genera Gaertnera (Malcomber 2002) and Pagamea (Vicentini 2016) have secondarily superior ovaries (Igersheim et al. 1994). Details of the origin of the ovary septum vary, whether from the carpel walls and/or the placental column (Figueiredo et al. 2017). Variation in ovule morphology and development is considerable (e.g. Maheshwari 1950; de Toni & Mariath 2008, 2010; Figueiredo et al. 2013a, b, 2017 and references), and in Galieae and Spermacoceae, for example, the integument is adnate to the nucellus, while in taxa like Houstonia the ovule consists of an embryo sac that develops inside an ellipsoid structure with practically no other structures visible - no integuments, etc. (Andronova 1977). However, much of the work is heavily typological and the ovule "types" (for which see e.g. Fagerlind 1937; Andronova 1977; Galati 1991), which can include details of placenta, origin of septae, etc., need to be decomposed into the individual variables. The integument is ca 15 cells across in Petitiocodon (Octotropideae), and Didymosalpinx, also in that tribe, is apparently the only member of the family to have a multilayered testa (Tosh et al. 2008); the chalaza in the Psychotia examined by Gonçalves and Mariath (2022) is massive, and those authors also discussed variation in ovule morphology, position, etc., in Psychotria and relatives, noting that Mitchella had a four-locular ovary, there being fusion of ovaries in pairs. There is confusing discussion on the presence of arils/strophioles/second integuments in Rubiaceae, see e.g. Wunderlich (1971), von Teichman et al. (1982) and de Toni and Mariath (2004) - it has even been suggested that the primitive condition for Rubiaceae is to have two integuments (Gonçalves & Mariath 2022). These structures have been described as coming from placental tissue, and/or being well developed very early on, and/or being more or less absent in the mature seed - at least sometimes they would seem to be rather massive obturators, and are described as such above. Fagerlind (1936a) found that Putoria had a remarkable multicellular archesporium in which several embryo sacs developed, elongating greatly and growing up the long micropyle. In Rubieae in particular understanding is confused by the anticlinally-elongated nucellar cells (not embryo sacs!), although here the megaspore may end up in the micropyle (Mariath & Cocucci 1997; see also Andronova 1977, 1988 and references); some Spermacoceae also have an odd nucellus (Mariath & Cocucci 1997). The ovules are sometimes more or less beaked at the chalazal end, and this is associated with the subsequent development of a winged seed, the beak representing the start of the development of the wing (Buchner & Puff 1993). There is considerable variation in the pattern of thickening of the exotestal cells which is generally best developed on the inner periclinal walls; as might be expected, an exotesta is more poorly developed in taxa with drupaceous fruits (Robbrecht & Puff 1986). Salazar-Duque et al. (2021) looked at "fruit" development, i.e., that of pericarp + epicarp, the latter probably hypanthial in Rubiaceae, of species from both major clades (or all three subfamilies of some phylogenies) and noted that comparison of the activities of the genes involved here and the canonical genes involved in fruit s. str. development in Arabidopsis showed a number of differences, indeed, some of the Arabidopsis genes were missing, others in different copy numbers, and so on.
For additional general information on Rubiaceae, see Verdcourt (1958), Bremekamp (1966), S. P. Darwin (1976), Robbrecht (1988, 1993), Robbrecht et al. (1996), Delprete (2004), T. Chen et al. (2011: Chinese taxa) and Razafimandimbison and Rydin (2024), also Bremekamp (1957: Henriquezieae), Puff (1982: Anthospermeae et al.), Puff and Mantell (1982: Putorieae), Ridsdale (1978a, b: Naucleeae; 1979: Jackieae), Puff et al. (1984: Alberteae), Rogers (1984, 2005: Henriquezieae), Tirvengadum (1984: Glionettia), B. Bremer (1987: Hamelieae - Argostemmateae sister!), Puff and Robbrecht (1989: Knoxieae), Buchner and Puff (1993: Danaideae), Deb and Rout (1993: Pubistylus), Igersheim (1993b: Strumpfieae), Igersheim and Robbrecht (1993: Prismatomerideae), Igersheim and Rohrhofer (1993), Delprete (1998: Hamelieae), Neupane et al. (2009, 2015), Carmo et al. (2021) and Nuñez Florentin et al. (2022), all Spermacoceae, Puff and Rohrhofer 1993: Scyphiphoreae), Robbrecht et al. (1991: Mitchelleae and relatives), 1993a: Octotropideae, 1993b: Bertiereae), Igersheim and Rohrhofer (1993: Otiophora), Darwin (1994) and J. Chen and Wong (2023), both Timonius, Delprete (1996: Chiococceae, Condamineeae/Dialypetalantheae), Tange (1998b: Cyanoneuron), Dessein (2003: Spermacoceae s.l.), Delprete and Cortés-B. (2004: Sipaneeae), Razafimandimbison and Bremer (2006: Hymenodictyeae), Sridith (2007: Argostemma), H.-Z. Wen and Wang (2012: Foonchewieae), Kästner & Ehrendorfer (2016: European taxa), Persson and Delprete (2017: Gardenieae), Wong et al. (2018: Vanguerieae), Löfstrand et al. (2019: Coussareeae), Mendoza-Cifuentes et al. (2020: Dialypetalantheae), Delprete (2022: Sipaneeae) and Matheka et al. (2024: Vanguerieae).
For chemistry, see Martins and Nunez (2015: comprehensive), Berger (2012: Psychotria and relatives), Schinnerl et al. (2012) and Kornpointer et al. (2010: alkaloids and iridoids, esp. in Palicourea) and Rasoarivelo et al. (2018: Anthospermum), for alkaloids, see Aniszewski (2007) and Berger et al. (2012) and for spirooxindole alkaloids, see Laforest et al. (2025), for Al and Si accumulation, see Jansen et al. (2002a, 2003a), and for toxic monofluoracetates, see Lee et al. (2012). Accorsi (1949: epidermis and stomata), Koek-Noorman and Hogeweg (1974), Jansen et al. (1996: Gaertnereae, 2001b: Rubioideae, 2002c: the whole family and a complete bibliography), Martínez-Cabrera et al. (2010), León H. (2013) and Martínez-Cabrera et al. (2015) all deal with wood anatomy, Gamalei et al. (2008: phloem), Lersten and Horner (2011: calcium oxalate crystals in Naucleeae), Krause (1909: colleters), Romero et al. (2019: leaf, esp. Cephalanthus), Judkevich et al. (2020c:Randia leaf anatomy) and Martínez-Cabrera (2009: leaf anatomy, esp. Hamelieae) all discuss other aspects of anatomy. See also Rutishauser (1984: stipules), Weberling (1977: inflorescences), Martínez-Cabrera et al. (2013: Hamelieae, etc., floral morphology), Galati (1991: Spermacoceae, floral anatomy, sporogenesis), Vrijdaghs et al. (2022 and references: floral development, Puff et al. (1993a: pollen, fruits in Mussaenda et al., 1993b: Schradereae) and Salazar‐Duque et al. (2021: fruit anatomy and development), Puff and Buchner (1998: Schradereae), Huysmans et al. (1997: the old Cinchonoideae), Vinckier et al. (2000: the old Ixoroideae) and Verstraete et al. (2011a), all orbicules, Johansson (1992: Psychotria), Pire (1996: Borreria), Huysmans et al. (1998b: Isertieae, 1999: Chiococceae, 2003: N.W. European taxa), Dessein et al. (2000: Knoxieae), D'Hondt et al. (2004), Verellen et al. (2007), Kuang et al. (2008: Naucleeae), X. Guo et al. (2020: parallel evolution of pantocolpate grains), Torres-Montúfar et al. 2020b: Rondeletieae) and Delprete et al. (2023: Sipaneae), all pollen, Puff (1993), number of pollen grain nuclei, Rakotonasolo and Davis (2006), some odd placentation types, Lloyd (1899, 1902, 1906 and references), Fagerlind (1936b), Nagl (1962), Periasamy and Parameswaran (1965), Tan and Rao (1988), all embryology, Fagerlind (1937: embryology and much else), and Takhtajan (2013: esp. seeds).
Phylogeny. Note that in the following discussion, Cinchonoideae refers to a group now included in Dialypetalanthoideae, but here I refer to the names originally used... B. Bremer (2009) summarized phylogenetic work on the family (see also Bremer 1996b). The basic phylogenetic structure was something like [Rubioideae [[Luculia [Acranthera + Coptasapelta]] [Cinchonoideae + Dialypetalanthoideae]] (see B. Bremer 1995, 1999; Rova et al. 2002; Robbrecht & Manen 2006; Rydin et al. 2009a; etc.). The clade of Luculia and Coptosapelta - and now Acranthera - seems moderately well supported. However, Bremer and Eriksson (2009) suggested that the three might not form a single clade, and while they do form a clade in Manns et al. (2012), it is sister to Rubioideae, while Wikström et al. (2015: no Acranthera) found Luculia and Coptasapelta successively sister to that subfamily. In L.-L. Yang et al. (2016) the basal phylogenetic structure in the family was [[Acranthera + Coptasapelta] [Rubioideae [Luculia [Cinchonoideae + Dialypetalanthoideae]]]], or some other variation on the theme, although support was very weak, while in Z.-D. Chen et al. (2016) relationships were [[[Rubioideae [Acranthera + Coptasapelta]] Luculia] [Cinchonoideae + Dialypetalanthoideae]], but with weak support at the base of the Rubioideae side of the tree. Refulio-Rodriguez and Olmstead (2014) found moderate support for the placement of Luculia as sister to the other four Rubiaceae they examined (these included representatives of all three subfamilies). Wikström et al. (2020: plastomes) found that Luculia, etc., were perhaps closest to Rubioideae while Antonelli et al. (2021: Angiosperms353) suggested that they were closest to Cinchonoideae (= Dialypetalanthoideae). R.-R. Gao et al. (2021) looked at the chemistry of C. diffusa and thought that the genus was closest to Rubioideae. However, the six seco-iridoids they found there were most similar to such iridoids in other Gentianales, not Rubiaceae, the sesquiterpenes, particularly the guaiane-type sesquiterpenes they found, were hardly common in other Rubiaceae, and although lupane-type pentacyclic triterpene lactones were common in other Rubiaceae, again the particular lupane-type lactones they found in Coptasapelta were not. On balance, the distinctive chemistry of Coptasapelta described by Gao et al. (2021) would not seem to suggest any particular relationships within Rubiaceae.
Luculia and Coptasapelta are not close morphologically. As Robbrecht and Manen (2006) emphasized, the two differ in having (Coptosapelta) or not (Luculia), raphides, accumulating (or not) aluminium, hairs T-shaped (or not), pollen pororate, acolumellate (tricolporate), and distyly (secondary pollen presentation) (Verellen et al. 2004; for pollen presentation, see Puff et al. 1996). Although Acranthera may be sister to Coptasapelta (see also Bremer & Eriksson 2009), these two also have little morphologically in common (Rydin et al. 2009a), Acranthera in particular having a remarkable androecium/stigma-style complex (Puff et al. 1995). Thus assigning polarity to many features in the family is rather tricky. Thureborn et al. (2023: Angiosperms353 analysis) found the three genera just mentioned to be at the base of a clade made up of those Rubiaceae other than Rubioideae in their study - Rubioideae was the focus there.
But there is more. Rydin et al. (2017), using mitochondrial genes and single representatives of the great majority of hitherto recognized tribes, found relationships differing in some important aspects from those previously often suggested, in particular, part of Cinchonoideae, including Cinchoneae, migrate into Ixoroideae (= Dialypetalanthoideae), and with strong support. In addition, there is support for the previously unplaced Coptosapelta as sister to the combined [Cinchonoideae, Dialypetalanthoideae] clade (the relationships of Luculia are still unclear - ?sister to Rubioideae), the next clade is represented by a grade in e.g. Antonelli et al. (2009), there is an unexpected association of [Airospermeae + Jackieae] which is placed in the old Ixoroideae part of the tree, and within Rubioideae [Colletoecemateae + Urophylleae] are sister to all other Rubioideae, and there are other rearrangements (Rydin et al. 2017). Since tribes were represented by single species, what happens in more detailed analyses - and when using nuclear genes - are very important open questions. Wikström et al. (2020) looked at whole-plastome data, highlighting taxa whose relationships varied depending on the nature of the analysis of the plastid data and those whose position was different when nuclear rDNA data were analyzed. Antonelli et al. (2021), using Angiosperms353 data, has taken the next step, if the sampling is still rather slight. Thus Antonelli et al. (2021) found that Steenisieae were migratory, their position depending on the analysis, the Vanguierieae Alliance was probably paraphyletic, Colletoecemateae, Urophylleae and Ophiorrhizeae might form a clade, the latter also being a possibility from the results discussed by Wikström et al. (2020), who found little resolution of relationships in the nuclear data they used in Cinchonoideae. These last two papers include many further hints of relationships that more or less modify some of those suggested in the account above.
More recently, Thureborn et al. (2022) used an Angiosperms353 probe set to look at relationships within Rubioideae (101 spp., all but two tribes included, all but one non-monogeneric tribe has 2 or more genera included). Overall, relationships here have not had to change much, and most nodes are well supported in both coalescence and concatenation analyses, although the position of Ophiorrhizeae moves. Thureborn et al. (2022) found a novel SCOUT clade sister to the rest of the subfamily. This clade includes the monotypic African Temnopteryx as sister to the other members - previously it was of uncertain position, but was placed somewhere around here. The rest of the subfamily is made up largely of Ixorodae and Psychotriodae, Coussareae are somewhat migratory, perhaps sister to Ixorodae, but with little support, and there are some changes suggested within these supertribes - for instance, the position of Knoxieae depends on the analysis. Ball et al. (2023) are developing a Rubiaceae2270x probe set, specific to Rubiaceae; preliminary findings using this probe set show no strongly supported conflicts with previous relationships, although are paraphyletic.
Rubioideae. Ophiorrhiza has the atpB promoter region that is lacking in other Rubioideae examined by Manen and Natali (1996; see also Natali et al. 1996) and so it was thought to be sister to the rest of the subfamily. However, Rydin et al. (2009a) found that the monotypic African Colletoecema (Colletoecemateae) was sister to the whole of Rubioideae, and with strong support (see also Piesschart et al. 2000: near basal, actual position uncertain; Sonké et al. 2008; L.-L. Yang et al. 2016); does it have an atpB promoter region? Colletoecema linked with Seychellea, a fugitive from the Psychotria area, in the recent 5 plastid gene analysis of Razafimandimbison et al. (2019). Sonké et al. (2008) and Rydin et al. (2009a) also found that Ophiorrhiza was close to Urophylleae, the latter mainly because of support from ITS, not chloroplast genes, while the former placed Neurocalyx (now in Ophiorrhizeae itself) in this area, and Lasiantheae and Coussareeae were also close. Relationships are similar in Razafimandimbison et al. (2019), and although Temnopteryx was sister to Ophiorrhizeae in the study, in other analyes it was sister to remaining Rubioideae, if with little support (see also Piesschaert et al. 2000; Sonké et al. 2008). Rydin et al. (2008) discussed the placement of some other small and little-known genera of Rubioideae (see also Razafimandimbison & Rydin 2019); they considerably affect our understanding of the evolution and diversification of the clade. See also Rydin et al. (2017) for relationships within Rubioideae, including a reevaluation of the position of Colletoecema, and for further general information, see Andersson and Rova (1999), B. Bremer and Manen (2000: phylogeny and classification), Backlund et al. (2007), Wikström et al. (2015: note basal clades). Rubiodae (e.g. L.-L. Yang et al. 2016; Ezedin 2025) includes about thirteen tribes, including the following four. Rincón-Barrado et al. (2021) found the relationships [Rubieae [Theligoneae + Putorieae]]; the [Theligoneae + Putorieae] clade had stong maximum likelihood but weak Bayesian inference support (see also Antonelli et al. 2021 for this topology). Plocama rosea, included in earlier analyses, turns out to have been misidentified. Bordbar et al. (2021) found that the real P. rosea was Aitchisonia rosea and was sister to Rubieae, although not in all analyses; they described it as a new tribe, Aitchisonieae.
Elsewhere in Rubioideae, Cantley et al. (2016) worked out relationships within Coprosma (Anthospermeae); earlier infrageneric groupings (see Heads 1996 and references) were not supported and C. moorei and C. talbrockei, which had caused problems for earlier workers, were placed well outside the genus. Within Anthospermeae, the Cape genus Carpacoce is sister to the rest of the tribe (see also Thureborn et al. 2019). Nuclear and plastid data tend to be in conflict, but not at deeper nodes (Thureborn et al. 2019).
Argostemmateae (Rubiodae): Ginter et al. (2015) discussed relationships around Argostemma.
Coussareeae have recently been examined by Löfstrand et al. (2019), and although the three main clades that they found could be characterised (see above), the tribe as a whole could not; many relationships were quite well supported, but the classical infrageneric taxa of Faramea and Declieuxia were not supported. Löfstrand et al. (2021) found two main clades in a more detailed analysis of Faramea, but again classical groupings were not recovered. Interestingly, only 11/21 of the species they included that had more than a single specimen in the analysis and whose mono/polyphyly could be established were in fact monophyletic (Löfstrand et al. 2021) - two specimens is a pretty low bar for suggesting monophyly...
Danaideae (Rubioidae). Razafimandimbison et al. (2022: 3 plastid and nrITS markers) found that the three genera in this Madagascan tribe were monophyletic and included well supported subgroups, however, relationships between the genera were unclear.For relationships in Knoxieae (Rubiodae), see Kårehed and Bremer (2007).
Xiao and Zhu (2007) and Smedmark et al. (2015) examined relationships within the pantropical Lasiantheae.
Morinda (Morindeae), with its distinctive capitate inflorescences and compound fruits, is in fact polyphyletic (Razafimandimbison et al. 2009b, 2012).
Ophiorhizeae: Razafimandimbison and Rydin (2019) discuss the circumscription of and relationships within this tribe, basal relationships being [Neurocalyx [Xanthophytum [Ophiorrhiza ...]]].
The relationships and limits of Psychotria and its relatives (Psychotriodae) pose problems. The Psychotria and Palicourea complexes are sister taxa. Psychotrieae are largely divided into Old and New World clades. A Malesian-Pacific clade of Psychotria includes perhaps five "genera" of morphologically distinctive myrmecophytes whose recognition makes Psychotria paraphyletic, as does the recognition of genera like Amaracarpus (Nepokroeff et al. 1999; Andersson 2002: the optimisation of marginal preformed germination slits on the pyrenes is questionable; Razafimandimbison et al. 2008, 2014). Within the myrmecophytes, relationships are [Squamellaria [Anthorrhiza [Hydnophytum + Myrmecodia]]], the penultimate group very paraphyletic and including Myrmephytum (e.g. Chomicki & Renner 2020).
Palicoureeae (Psychotriodae) include some erstwhile species of Psychotria (Taylor 2017 and references; Taylor et al. 2017); Cephaëlis groups with Palicourea. Barrabé et al. (2012) focussed on relationships of a clade of Palicoureae, the Malesian-Pacific-American Margaritopsis, while Bedoya et al. (2023: 94/650 species, 810 exons) obtained a fair amount of resolution within Palicourea itself despite rather extensive gene tree discordance. Thilén et al. (2025: Angiosperms353 dataset) confirmed the monophyly of the African Hymenocoleus, but Chassalia was paraphyletic and included Geophila.Prismatomerideae: Razafimandimbison et al. (2020) looked at relationships here and redrew generic boundaries.
Putorieae. Rincón-Barrado et al. (2021) examined relationships within Plocama, the only genus; Mediterranean taxa (Putoria s. str.) were sister to the rest of the genus - see Bordbar et al. (2021) for the consequences of the misidentification of Ploc. rosea (= Aitchisonieae).
Both morphology and molecular data strongly support the monophyly of Rubieae (Rogers 2005 for literature). There is now considerable phylogenetic resolution within the tribe, and the basal relationships are likely to be [Kelloggia [[Rubia + Didymaea] [the rest]]] (e.g. Nie et al. 2005; Ehrendorfer et al. 2018). Relationships between a number of strongly-supported (both bootstrap and posterior probabilities) major clades are themselves well supported (Soza & Olmstead 2010a, 2010b: New World Galium; Ehrendorfer et al. 2018: the whole tribe; see also Manen & Natali 1995; Manen et al. 1994; Natali et al. 1996). De Toni and Mariath (2011) thought that flowers and fruits suggested that Galium and Relbunium were sister taxa and both worthy of generic status. However, Soza and Olmstead (2010b) noted that the fleshy fruits of Relbunium were distinctive (but not unique) in Galium, and the Relbunium group was embedded in a larger South American clade that is firmly part of Galium (see also Ehrendorfer et al. 2018). Asperula, within which Sherardia is nested, is also part of this problem (Gargiulo et al. 2015), while L.-E Yang et al. (2015, see also 2018) looked at relationships within Rubia — Didymaea, they thought, was its sister clade, and the South African R. horrida was sister to a large eastern Asian clade (see also Deng et al. 2017). Much the same general relationships were recovered by L.-E. Yang et al. (2018), who in a comprehensive analysis of Galium noted that most classical sections were not monophyletic.
Relationships within the 1000+ species of Spermacoceae are difficult to disentangle. Kårehed et al. (2008) investigated the phylogeny here, and they suggested that Hedyotis should be restricted to Asian taxa. Wikström et al. (2013) is another important step forwards, and for further studies in Spermacoceae, see e.g. Groeninckx et al. (2009a, esp. 2009b: [Pentodon + Dentella] sister to the rest of the tribe), Rydin et al. (2009b), Guo et al. (2013: Asian taxa), Salas et al. (2015: Brazil), Neupane et al. (2015: whole Asia/Pacific area), Florentín et al. (2017: Galianthe) and Nuñez-Florentin et al. (2021a: esp. Caribbean taxa, 2021b: New World genera). Carmo et al. (2021: three markers, two chloroplast, one nuclear) found gene tree discordance, but fortunately Diadorimia, the new genus they described, was consistently sister to the rest of the tribe; polyphyly was common, and [Dentella + Pentodon] were embedded in the fourth branch of the tree. Gibbons (2020) examined Australian taxa placed here, and found that all the native Oldenlandia were to be excluded from the genus, and a clade [Dentella + Pentodon], with 5-merous flowers, might be sister to one of the two main clades that make up the tribe as a whole. For relationships in Brazilian Spermacoceae including the description of new genera, see Nuñez-Florentin et al. (2023a, b). Nuñez-Florentin et al. (2023a) found that Galianthe, sister to the rest of the tribe in this local analysis, was monophyletic, even if infrageneric groups in that genus were not holding up - confirmed by Florentín et al. (2025: 41 spp., 3 markers). Nuñez-Florentin et al. (2023b: 2 nuclear, 4 plastid markers) obtained little resolution along the spine, and two genera were still polyphyletic. Nuñez-Florentin et al. (2024) found that Denscantia was polyphyletic, and polyphyly in Borreria, Hexasepalum and Spermacoce was evident in Florentín et al. (2025).
Relationships between major clades in Urophylleae are clarified by Smedmark (2008) and Smedmark and Bremer (2011: species-level relationships uncertain).
Dialypetalanthoideae. For phylogenetic relationhips within the old Cinchonoideae, see Andreasen and Bremer (1996), Rydin et al. (2009), and especially Manns et al. (2012) and Manns and Bremer (2010); the latter recognise nine tribes within the subfamily, all of which are strongly supported as being monophyletic, however, some of the relationships between groups of tribes are as yet poorly resolved (Manns & Bremer 2010; see also Paudyal et al. 2104; Rydin et al. 2017). [Naucleeae + Hymenodictyeae] are a well supported clade sister to the only moderately (jacknife) supported remainder of Cinchonoideae (Andersson & Antonelli 2005: Luculia and Coptasapelta excepted; L.-L. Yang et al. 2016). See also Razafimandimbison & Bremer (2001, 2002), Razafimandimbison et al. 2004, Wikström et al. 2010, and especially Löfstrand et al. (2014) for Naucleeae and Hymenodictyeae. Generic and tribal limits are diffficult around Rondeletieae and Guettardeae (Rova et al. 2009; Manns & Bremer 2010; Torres-Montúfar et al. 2020a: immediate outgroups seem to be all over the phylogenetic map). L.-L. Yang et al. (2016) discuss relationships in Chinese members of Cinchonoideae s. str.. Ixoroideae (= Dialypetalanthoideae). The six-plastid gene study of Kainulainen et al. (2013: Dialypetalanthus not included) provided substantial support for relationships through the old Ixoroideae, although those in the "basal" Dialypetalantheae-Mussaendeae area remained somewhat unclear. However, few deep relationships in the subfamily had strong support - nor did the subfamily itself - in the study by L.-L. Yang et al. (2016), which focussed on its Chinese representatives. Relationships within the old Gardenieae are becoming clarified, and Pavetteae, Gardenieae, and two small tribes may form a clade, although without bootstrap support (Mouly et al. 2014: chloroplast data). Sipanea and Posoqueria form a basal clade (Razafimandimbison et al. 2011; Mouly et al. 2014); Cortés-B. and Motley (2015) discussed relationships around Henriquezia, Sipanea and Posoqueria, perhaps [Sipaneeae [Henriquezieae + Posoquerieae]], although Gleasonia (Henriquezieae) tended to wander, and how the extensive variation in flower and fruit in particular might relate to living on the nutrient-poor soils of the Guayana region is unclear.
As already mentioned the findings by Rydin et al. (2017) add further uncertainties to what we think we know about tribal relationships. However, Ly et al. (2020) looked at plastomes of 28 species (23 genera) from members of Ixoroideae s. str. and recovered most of the tribes for which they had more than one species as monophyletic, the only exception being Sherbournieae, the two representatives of which (Sherbournia and Mitriostigma) were associated with a [Pavetteae + Gardenieae] clade, but were not close to being sister taxa. Overall the relationships were [Mussaeneae, Condaminieae [Coffeeae [Gardenieae + Pavetteae]]]. Interestingly, these relationships were only partly recovered (although Sherbounieae were still polyphyletic) in a semi-parallel analysis of the nuclear genomes (Ly et al. 2020).
Tribes in the Cinchonoideae s.l., i.e. Cinchonoideae s. str and Dialypetalanthoideae combined, are treated alphabetically here.
Kainulainen et al. (2009) discuss relationships in Alberteae.
Chiococceae have included Strumpfia in the past, perhaps sister to the rest, but it lacks their spiny pollen (Manns & Bremer 2010) - see Strumpfieae below. For phylogenies, see Motley et al. (2005) and Paudyal et al. (2018: 2 nuclear, 2 plastid markers); the latter group found that support along much of the spine was rather weak.
Chilquillo et al. (2023) found that genera in Cinchoneae were monophyletic, with the exception of Ladenbergia.
For the circumscription of Coffeeae, see A. Davis et al. (2007), Maurin et al. (2007: 84 spp. Coffea, 7 spp. Psilanthus) and Arriola et al. (2018: Asian taxa). Coffea is to include Psilanthus (Maurin et al. 2007: 84 spp. Coffea, 7 spp. Psilanthus; see also Davis et al. 2009: 5 markers, 45 spp. Coffea, 11 spp. Psilanthus; Nowak et al. 2012; Hamon et al. 2017). However, the relationships between these genera are complex (see also Davis et al. 2009), and Charr et al. (2020) found that chloroplast data supported the synonymization of the two genera, while extensive nuclear data suggested that the two could be kept separate. Tosh et al. (2009) adjusted the limits of the African Tricalysia (see also Tosh 2009).
Dialypetalantheae (was Condamineeae) as circumscribed by Kainulainen et al. (2010) are very heterogeneous and include Mastixiodendron, the morphologically even more distinctive Dialypetalanthus (see also Feng et al. 2000), several genera with calycophylls, etc.. Relationships within the tribe are poorly resoved, consisting of a tetratomy made up of Ferdinandusa, a small Malesian-Pacific clade and larger septicidal- and loculicidal-dehiscence clades (Kainulainen et al. 2010; see also Vridaghs et al. 2022).
In Gardenieae the Madagascan Melanoxerus links with African taxa in plastid and ribosomal analyses, but with Neotropical taxa in nuclear gene analyses (Kainulainen & Bremer 2014); Mouly et al. (2021) expand the limits of Atractocarpus on New Caledonia. In a study of New World taxa of the tribe, Borges et al. (2021) found that both Randia and Casasia were para-/polyphyletic.
Guettardeae. Torres-Montúfar et al. (2020a) examined relationships here. Guettarda itself is polyphyletic (Achille et al. 2006; Manns & Bremer 2010), as is Antirhea; relationships in the latter have been disentangled by Chavez et al. (2021). Manns and Bremer (2010) found that Rogeira was sister to other Guettardeae and with strong support.
Stranczinger et al. (2014) offer some preliminary suggestions about relationships in Hamelieae; generic limits can be tackled when sampling is improved.
Hillieae. A recent study by Bell et al. (2025: 25 spp., Rubiaceae-specific probe set (Rubiaceae2270a), 1,375 loci) found that the three genera here were monophyletic, although several species were questionably monophyletic.
Ixora s. str. (Ixoreae) is paraphyletic (Mouly et al. 2009a, b, see also Andreasen & Bremer 2000; Tosh et al. 2013: Afro-Madagascan species).
Mussaendeae have received quite a bit of attention (e.g. Alejandro et al. 2005; Duan et al. 2018).
In Naucleeae, Cephalanthus is sister to the rest (Löfstrand et al. 2014 and references). For relationships in Neonauclea s.l., N. brassii sister to the rest, see Ordes et al. (2021).
Tosh et al. (2008: Didymosalpinx sister to the rest) and Alejandro et al. (2011) looked at relationships within Octotropideae.
There are four main clades within Pavetteae, and Tarenna in particular is polyphyletic, Lepactina paraphyletic (de Block et al 2015, 2018: Afro-Madagascan clade).
Cortés-B. et al. (2009) looked at Retiniphylleae.
Within Rondeletieae there is a fair bit of well-supported structure, although Rondeletia is polyphyletic (Manns & Bremer 2010). Torres-Montúfar et al. (2017a, 2020a) also looked at relationships here, although extensive sampling in the large genus Rondeletia is still needed before clade limits can be understood.
For relationships in Sabiceeae, see Khan et al. (2008) and in particular Zemagho et al. (2016).
Because of pollen morphology - and many other reasons - Strumpfia, sister to Chiococceae, has been placed in the monogeneric Strumpfieae (Paudyal et al. 2014).
For relationships within Vanguerieae, see Lantz and Bremer (2005) and references, Razafimandimbison et al. (2009) and Wikström et al. (2010). Razafimandimbison et al. (2011) found that at the base of the clade encompassing the ca 1100 species of Vanguerieae there were successively four monogeneric clades (three in Kainulainen et al. 2013, Glionettia unplaced). See Razafimandimbison et al. (2009a) for the phylogeny of dioecious Vanguerieae.
Apart from the myrmecophytic Psychotrieae, two genera are particularly odd morphologically:
Classification. Robbrecht and Manen (2006) and B. Bremer (2009) should be consulted for early discussions on taxon limits, formal classification, and the like. B. Bremer and Manen (2000) outline a tribal classification for Rubioideae and Manns and Bremer (2010) one for Cinchonoideae, both assigning nearly all genera to those subfamilies, at least provisionally (see also Paudyal et al. 2014). There is a tribal classification of Dialypetalanthoideae (as Ixoroideae) in Kainulainen et al. (2013) where genera in 21 of the 24 tribes are enumerated; one genus (Glionettia) is unplaced. See also Govaerts et al. (2013) for a World Checklist of Rubiaceae and Verstraete et al. (2025) for a generic list (with orthographic variants) of the family (at Zenodo - see https://zenodo.org/).
I had just (12.x.2017) begun putting in a tribal classification of Rubiaceae, but reading Rydin et al. (2017) a week later suggested that this might be premature, certainly as to subfamilial limits and relationships between some of the tribes. It now seems prudent to recognize only two subfamilies. Antonelli et al. (2021) also suggested recognizing just the two subfamilies and not assigning Luculia, etc., to either; judging by the Angiosperms353 phylogeny presented by Antonelli et al. (2021) for the two subfamilies, further changes to relationships and circumscriptions of tribes may be necessary, but in that paper only one genus per tribe was included, with a few exceptions. For the tribal classification of Galioideae, see Razafimandimbison et al. (2022). After a general evaluation of the previous literature, Razafimandimbison and Rydin (2024, see above) produced a tribal classification for the whole of the family that is followed here; relationships there are largely in agreement with the comments immediately above.
Bremer (2009) noted that of the ca 611 genera in the family, 1/3 were monotypic; others were very large and they are turning out to be para- or polyphyletic. Not surprisingly, generic limits are problematic in a number of places, indeed, if preserving the names of some of these small genera seems desirable, then wholesale dismemberment of larger genera and ever more small genera will be the logical if perhaps unfortunate result. Within Rubieae, the circumscriptions of both Asperula and Galium are currently difficult (Soza & Olmstead 2010a). In Galium in particular, fruit morphology is a poor indicator of sectional relationships (Soza & Olmstead 2010b; see also Abdel Khalik et al. 2009; Deng et al. 2017). Ehrendorfer et al. (2014) sketched out a possible taxonomic solution for Galium that allowed paraphyly of genera (see also Kästner & Ehrendorfer 2016: pp. 56-58), however, Ehrendorfer et al. (2018) later realized that paraphyly was not really an option, and suggested that if monophyletic genera were to be recognized around here, in addition to various segregate genera that had been recognized at times in the past, some 10 new genera would be needed - "moderate splitting" (ibid.: p. 17). The recent recognition of a monotypic Pseudogalium sister to the rest of Galium s.l. seems quite unnecessary (c.f. L.-E Yang et al. 2018) but would be in line with this approach. Also in Galium s.l., del Guacchio and Caputo (2020) have recently described three genera based on sections of Asperula since their phylogenetic position was "deemed stable" - what the future might hold in terms of the classificatory consequences of this is unclear (and one genus seems to be a later homonym...). However, a broad circumscription for Galium is adopted above, largely following the phylogeny in Sosa and Olmstead (2010a) and Ehrendorfer et al. (2018).
Thureborn et al. (2019) rework subtribal limits in Anthospermeae, some generic adjustments will have to be made (e.g. Nenax = Anthospermum?). A Pacific-Malesian clade of Psychotria also includes myrmecophytes like Myrmecodia and Hydnophytum as well as genera like Amaracarpus (Andersson 2002); Andersson (2002) seemed inclined to split the clade, while e.g. Nepokroeff et al. (1999) and particularly Razafimandimbison et al. (2014) would include them in Psychotria s.l., and the latter group formally synonymized the genera (see synonymy), although many species combinations still have to be made. The nomenclatural changes needed as relationships within and between Palicoureeae and Psychotrieae are clarified are under way (e.g. Taylor 2017 and references; Taylor et al. 2017). Generic limits in Spermacoceae are very difficult with considerable both lumping and splitting having occurred in the past. Oldenlandia appears to be wildly polyphyletic, for example, it appears in eleven or so places in Carmo et al. (2021: see Fig. 2), and Spermacoce itself is also very paraphyletic (Kårehed et al. 2008; Wikström et al. 2013); new genera are being described in the context of local phylogenetic analyses (Groeninckx et al. 2010a, b). Thus X. Guo et al. (2013) described three new genera from the Asian part of the Spermacoce complex, but another 10-17 names (several do already exist) will then be needed to describe just this part of the tree, while Gibbons (2020) described new genera based on Australian species of Oldenlandia, that genus in fact not being native on the continent despite what was previously thought. Neupane et al. (2015) summarized generic relationships in the general Hedyotis-Oldenlandia area, while Nuñez Florentin et al. (2021b) keyed out all the New World genera. Nuñez Florentin et al. (2024) discussed generic limits here - they prefer narrow limits, genera with broad limits being difficult to recognize, etc.. For generic limits in Urophylleae, see Smedmark and Bremer (2011: nine of Bremekamp's small genera still unsampled).
Within Dialypetalanthoideae, there are conflicts over generic limits in Chiococceae, in the Exostema/Coutarea area in particular (Delprete & Paudyal 2023). Chiococceae have been somewhat dissected (Paudyal et al. 2018), although there is considerable diversity in corolla, fruit, etc., in the tribe (see also Delprete 1996; Motley et al. 2005). Within Coffeeae, Coffea is to include Psilanthus (Maurin et al. 2007). Generic limits pf Gardenieae from New Caledonia are being reevaluated (Mouly et al. 2021). Ixoreae: The limits of Ixora have been clarified, and are broadly drawn (Mouly et al. 2009a, b). Pavetteae in Madagascar are diverse, de Block et al. (2018) describing four small new genera from there, but more changes are to come, especially in Tarenna (see also de Block 2020). Generic and tribal limits are diffficult around Rondeletieae and Guettardeae (Rova et al. 2009; Manns & Bremer 2010), and both Rondeletia and Guettarda are polyphyletic (Achille et al. 2006; Manns & Bremer 2010). Zemagho et al. (2016) provide an infrageneric classification of Sabicea (Sabiceeae).
Previous Relationships. Rizzini and Occhioni (1949) were sure Dialypetalanthaceae were in Myrtales, while Cronquist (1981) placed them adjacent to Pittosporaceae in his Rosales, but Theligonaceae were at least placed in Rubiales. Both families were kept separate by Takhtajan (1997) and were included in his Rubiales.
Thanks. I am grateful to Elmar Robbrecht for help with the synonymy and to Charlotte Taylor for many useful comments.
[[Gelsemiaceae + Gentianaceae] [Loganiaceae + Apocynaceae]]: route I secoiridoids +; (included phloem +), internal phloem + [intraxylary phloem/bicollateral vascular bundles]; C tube formation late; syncarpy postgenital; testa with anticlinal walls thickened.
Age. Bell et al. (2010) give an age of (69-)61, 57(-47) Ma and Magallón et al. (2015) around 60 Ma for this clade, but see relationships within it; (93-)73(-49) Ma are the ages in Wikström et al. (2015).
Chemistry, Morphology, etc.. Backlund et al. (2000) early noted that C17 indole alkaloids, the number of tapetum layers, and cytology supported the relationship [Gelsemiaceae + Apocynaceae], but that the presence of quercetin and kaempferol, imbricate corolla, and horny (starchy) endosperm might support a close relationship between Gelsemiaceae and Loganiaceae. Gentianaceae and Apocynaceae are the only families in which postgenitally fusing carpels are flat, not plicate (Kissling et al. 2009b). Bouman and Schrier (1979) noted that exotestal cells with thickenings on their anticlinal walls are common around here; Gelsemiaceae and Loganiaceae were not mentioned.
[Gelsemiaceae + Gentianaceae]: ?
GELSEMIACEAE L. Struwe & V. Albert - Back to Gentianales
Pollen surface ± reticulate; ?testa; x = 10 (?9).
3 [list]/11. ± Pantropical (map: from Leeuwenberg 1961; van Steenis & van Balgooy 1966; Sobral & Rossi 2003).
Age. Crown-group Gelsemiaceae are (61.7-)36.8(-13.9) Ma (Tank & Olmstead pers. comm.).
1. Gelsemieae G. Don
Shrubs or vines/lianes; camptothecin-type alkaloids +; true tracheids +; stomata?; leaf (margins serrate), (stipules 2, interpetiolar/short sheathing); (flowers single); flowers heterostylous; A latrorse (extrorse - Gelsemium); pollen pores with distinct lateral extensions [?level]; nectary +, ?position; placentae fleshy [Gelsemium], style twice branched, stigma punctate; ovules 2-8/carpel; fruit a loculi- and/or septicidal capsule, (muricate), K usu. persistent; seeds winged or flattened, surface hairy/rugose/smooth; n = 4, 10.
2/10. ± Pantropical. Map: from Leeuwenberg (1961), van Steenis and van Balgooy (1966) and Sobral and Rossi (2003). Photo: Gelsemium Collection © M. Dirr, Flower (Pin), Flower (Thrum).
2. Pteleocarpa lamponga (Miquel) Heyne —— Synonymy: Pteleocarpaceae Brummitt
Tree; ?nodes; stomata anomocytic; leaves spiral; flowers not heterostylous; C ?quincuncial; pollen grains small, 20.3-22.5 uµ long; ?nectary; G stipitate, styles largely separate, stigma subcapitate; ovules 2/carpel; fruit a 1-seeded samara; n = ?
1/1. Southern Peninsula Thailand and West Malesia, but not the Philippines.
Evolution: Divergence & Distribution. For the phylogeny and biogeography of the family (Pteleocarpa not included), see Jiao and Li (2007).
Pollination Biology & Seed Dispersal. Considerable work has been caried out on the nectar of Gelsemium, containing as it does alkaloids, to work out its effects on (potentially) pollinating bees, both adults and larvae, and what effect it might have on selfing, etc. (Irwin & Adler 2008 and references). Almost the whole plant of Pteleocarpa may turn yellow when it is in flower. How nectar is secreted there is unclear (Struwe et al. 2014).
Chemistry, Morphology, etc.. Pteleocarpa has mainly apotracheal parenchyma in uniseriate bands that do not often span the rays, solitary vessels, vestured pits, fibre tracheids with bordered pits, etc. (Gottwald 1982).
For general information, see Struwe (2018), and on Pteleocarpa, see Struwe et al. (2014) and Rueangsawang and Chantahanothai (2014).
Phylogeny. The association of Pteleocarpa with this clade is strongly supported. Refulio-Rodriguez and Olmstead (2014) suggested that it was sister to the two other genera, Struwe et al. (2014) did not place it.
Classification. Although Pteleocarpa is a morphologically distinct genus - Brummitt (2007, 2011) recognized it as a separate family - it is best included in Gelsemiaceae (see Struwe et al. 2014).
Previous Relationships. Pteleocarpa has been included in Boraginaceae-Ehretioideae, as by Takhtajan (1997), among other places; the other genera are ex-Loganiaceae.
GENTIANACEAE Jussieu, nom. cons. - Back to Gentianales
Monoterpene indole alkaloids 0; starch 0, oligosaccharides +, tannins 0; root hairs 0 [perennial species], exodermis with l0ng and short cells; (vessel elements with scalariform perforation plates), rays often 0; cork position?; medullary bundles/isolated phloem strands +; parenchyma septate; mucilage cells + (0); plant glabrous, glands 0; (stomata anisocytic); leaves sessile, usu. connate basally, lamina vernation variable, secondary veins ± palmate/plinerved (pinnate); flowers 4-5-merous, K 1-trace [?all], K and C traces dividing into three, "disc-like" structure between K and C, C right-contorted [dextrorse], marcescent, (tube formation intermediate), petal epidermal cells elongated and flat; A basally connate, (extrorse), (placentoids +); tapetum (amoeboid), cells uninucleate; G ?orientation, placentation parietal, stigma broadly 2-lobed (capitate), wet; funicle with at best poorly developed vascular tissue, (outer epidermal cells of integument early on massive), hypostase +; (antipodal cells diploid to polyploid/multiplying, persistent); K often prominent in fruit; seeds small; exotestal cells (± elongated), inner walls variously thickened (not), other layers ± disappear; x = 10 (9), nuclear genome [1C] (0.115-)1.56(-21.092); platsome 100 bp deletion in trnL gene.
102 [list, to tribes]/1,750 - 7 tribes below. World-wide, but especially temperate, about half the genera in South America. Map: from Gillett (1963), Hultén (1958, 1971), van Steenis and van Balgooy (1966), Klackenberg (1985), Ho & Liu (2001), Struwe and Albert (2004) and Kissling (2012). Photo: Flower, Flower.
Age. The crown age of the family may be some 50 Ma (Y.-M. Yuan et al. 2003), (78.2-)59.5(-40) Ma (Tank & Olmstead pers. comm.), or (78.6-)66.2, 57.8(-47.3) Ma (Merckx et al. 2013c); there is also a suggestion of an age as much as (125-)100(-75) Ma (Kissling in Struwe 2014).
Although fossils with pollen like that of Macrocarpaea are reported from the Eocene ca 45 Ma, their identity is questionable (Stockey & Manchester 1985; Struwe et al. 2002).
1. Saccifolieae Struwe, Thiv, V. A. Albert & J. Kadereit —— Synonymy: Saccifoliaceae Maguire & Pires
Herbs (holomycoheterotrophic, associated with glomeromycotes) (annual) to subshrubs; ?chemistry; (stomata anisocytic); (colleters +); cauline and foliar extrafloral nectaries +; (leaves spiral); flowers (heterostylous), (4-)5(-6)-merous; base of style persistent in fruit; (dust seeds +); (endosperm cellular - Voyriella), (cotyledons 0); n = 10-14.
5/20: Tapeinostemon (10). Mexico, Central and tropical South America.
Age. Crown-group Saccifolieae are estimated to be (46.1-)30.8, 27.5(-14.6) Ma (Merckx et al. 2013c).
[Exaceae [Voyrieae [Chironieae [Potalieae [Helieae + Gentianeae]]]]]: ?
Age. This node was dated to some (57.4-)49.1, 48.6(-40.1)Ma by Merckx et al. (2013c) and (71.7-)49.6(-34.4) or ca 64.4 Ma by C. Chen et al. (2023).
2. Exaceae Colla
Herbs (holomycoheterotrophic, associated with glomeromycotes), (annual), to shrubs; xanthones 0; (flowers monosymmetric/enantiostylous - Exacum [E.], Orphium, oblique monosymmetry - E.); (median petal adaxial); K connate or not, usu. prominently keeled, C (imbricate - E.), epidermal cells rounded and convex; anther (porose), with glands and/or prolonged connective (0), (endothecium 0 - E.); ovary ± bilocular, (?oblique - E.), (placentae 4, pendulous), (style with commissural secondary stigmas towards base [= diplostigmaty] - Sebaea); (ovule straight), (endothelium + - E.); (dust seeds +), exotestal cells with sinuous anticlinal walls or not; x = 7, n = 9, 11, 15, etc.
8/169: Exacum (69), Sebaea (60). Mainly Africa, esp. Madagascar, also Indo-Malesia to Australia and New Zealand (some Sebaea).
Age. The crown-group age of Exaceae may be some 40 Ma (Y.-M. Yuan et al. 2003) or (41.6-)32.1, 31.7(-21.7) Ma (Merckx et al. 2013c).
[Voyrieae [Chironieae [Potalieae [Helieae + Gentianeae]]]]: placentation parietal, (placentae bilobed).
Age. The age of this node was estimated to be (65.2-)54.0, 46.8(-40.1) Ma (Merckx et al. 2013c) or (70.4-)60.3(-50.9) Ma (Gomes et al. 2022b).
3. Voyrieae Gilg - Voyria L.
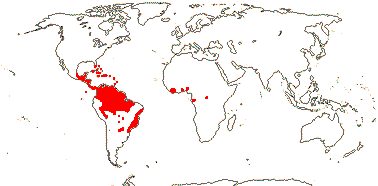
Herbs, fully mycoheterotrophic, associated with glomeromycotes, (epiphytic); ?chemistry; axis may lack nodes but bear roots and shoots; roots and shoots both exogenous/both endogenous; roots up to 15 mm across, runner-like to star-shaped at base of shoot, 5-7-arch, unmedullated; shoots root-borne, (stelar vascular bundles separate [subgenus Leiphaimos - L.]/vascular cylinder +; stomata anomocytic [subgenus Voyria - V.]/0; (leaves not connate basally), colleters +/0; flowers (single), (4-)5(-7)-merous; K connate, C (with simple hairs); A (extrorse), thecae (initially) connate or not, (with ± long (hairy) basal tails), (filaments ± 0); pollen variously clumped, grains (asymmetric), 1-2(-6)-porate, exine smooth to scabrate, orbicules 0; G with paired (stipitate) capitate glands - ?L./0, placentae strongly bilobed, (style shorter than G), stigma expanded, usu. undivided, rotate/capitate/infundibular; ovules straight, no integument - L./anatropous, one integument, endothelium +, nucellar cap +; embryo sac with inverted polarity - L.; C marcescent or not; seeds rather tubby, dust-like, to filiform, 200-600(-2000) μm long, embedded in the swollen placenta or not, (aborted, = paraphyses - L.); exotesta +; endosperm cellular or initially nuclear, present to almost absent [L.], embryo undifferentiated [L.]; n = 16-20.
1/20. S. Florida, the Antilles, tropical America, Voyria primuloides in West (also Central) Africa. Map: from Maas and Ruyters (1986) and Raynal-Roques (1967).
Age. The age of crown-group Voyria is (59.0-)47.1, 40.7(-31.9) Ma (Merckx et al. 2013c) or (65.6-)56.1(-45.2) Ma (Gomes et al. 2022b).
[Chironieae [Potalieae [Helieae + Gentianeae]]]: O-glycosylxanthones, L-(+)-bornesitol +; (interxylary phloem +).
Age. The age of this node is perhaps (49.4-)42.6, 41.8(-36.7) Ma (Merckx et al. 2013c).
4. Chironieae Endlicher —— Synonymy: Chironiaceae Berchtold & J. Presl, Coutoubeaceae Martynov
Herbs (fully mycoheterotrophic, associated with glomeromycotes) (annual) to shrubs; distinctive 6-substituted xanthones; (interxylary phloem +); (glandular hairs +); extrafloral nectaries complex, aggregated; flowers (2-)4-5(-12)-merous, monosymmetric by androecium or not; K connate; anthers and style branches coiling; (pollen in tetrads); nectary usu. 0; n = 10, 13-15, 17, etc.
26/160: Centaurium (20), Sabatia (20). Tropics and N. temperate.
Age. Crown-group Chironieae may be (42.2-)34.4, 33.1(-24.4) Ma (Merckx et al. 2013c).
[Potalieae [Helieae + Gentianeae]]: ?
Age. This node is some (46.0-)40.2, 39.8(-35.6) Ma (Merckx et al. 2013c) or about 64-37.7 Ma (Favre et al. 2016, q.v. for other estimates).
5. Potalieae Reichenbach —— Synonymy: Potaliaceae Martius
Herbs, annual to perennial, (vines/lianas +), (large trees), (prickly); C-glucoflavones +; rays uni(bi - multi)-seriate [Potaliinae]; nodes 5 or more:5 or more; epidermal and cortical sclereids + [?all]; leaves (with ± serrulate margins), (massive sheathing stipule-like structure/paired flaps at leaf base); flowers 3-16(-24)-merous; K basally connate; (anthers versatile), (filaments basally connate); pollen often porate; nectary 0/(base of ovary); syncarpy congenital [?all], stigma often capitate; (fruit a berry); n = ?
20/163: Fagraea (50), Lisianthus (30). Pantropical.
Age. Crown-group Potalieae are (38.7-)36.8, 36.6(-34.7) Ma (Merckx et al. 2013c) or 40.3-33.7 Ma (Favre et al. 2016).
[Helieae + Gentianeae]: (colleters +).
Age. This node is perhaps (38.7-)32.1, 31.7(-25.0) Ma (Merckx et al. 2013c), 60.7-32.2 Ma (Favre et al. 2016) or (71.7-)49.6(-34.4) Ma (C. Chen et al. 2023: stem Gentianeae), older than other estimates .
6. Helieae Gilg
Herbs (shrubs); vessels often in multiples; extrafloral nectaries +, simple; (inter/intrapetiolar sheaths, stipules +); flowers (4-)5(-6)-merous, (monosymmetric by androecial arrangement); K lobes with abaxial glandular areas; C in bud pointed (rounded); (separate corolline flaps or fully tubular corona at adaxial base of filaments - Symbolanthus); anthers (coiling), connective projecting apically; pollen in tetrads/polyads/(monads), (triporate), elaborate exine projections/spines/globules; G with basal glandular areas, style often long, stigmas bilamellate, twisted and flattened when dry; (C persistent in fruit); exotestal cells dome-like+/concave, thickenings ± reticulate/band-like; n = ?
23/226: Macrocarpaea (118), Symbolanthus (30). Tropical Central and South America, Caribbean.
Age. Crown-group Helieae are (26.6-)20.0, 18.9(-13.1) Ma (Merckx et al. 2013c) or 30-13.3 Ma, quite similar to other estimates (Favre et al. 2016).
7. Gentianeae Colla —— Synonymy: Obolariaceae Martynov
Annual to perennial herbs; distinctive xanthones, C-glucoflavones +, (fructans/inulin +); nodes (1:3/3 or more:3 or more); lamina (± serrulate); anther tapetal cells uni-(bi)nucleate; pollen striate (echinate); nectary at base of G/on stipe; G stipitate, style often short or 0, (hollow); (ovules straight, hemitropous, etc.), integument 2-20 cells across; seeds larger; embryo small; n = 5≤, very variable.
18/975. North temperate, esp. eastern Asia, to New Guinea and the Antipodes, South America, few Africa.
Age. Crown-group Gentianeae are (32.8-)26.9, 26.5(-20.6) Ma (Merckx et al. 2013c) or 52.8-29.7 Ma, somewhat older than other estimates (Favre et al. 2016); C. Chen et al. (2023) suggest ages of around 43.8 and 37.8 Ma.
7A. Gentianinae G. Don
C with folds between lobes, tube development early-late intermediate [Gentiana].
Gentiana (360). North Temperate, New Guinea (Tripterospermum).
7B. Swertiinae Grisebach
Annual to perennial herbs (climbers), , (plant hemi- or holomycoheterotrophic, coralloid roots +, root hairs 0); flowers (4-merous); C (margins dentate to fringed), (usu. with one or two nectaries, naked or variously enclosed, with vascularized or non-vascularized fimbriate appendages, or scales), (in C spurs - Halenia [= Sw.]), 0; pollen (striate(-perforate) / echinate); antipodal cells multinucleate [H.] or polyploid [S. s. str.]; seed shape various, (winged), surface often warty; n = 6-13, ...
5/531: Swertia (507), Pterygocalyx (19). North Temperate, esp. Eurasia, to South America, the Antipodes, few Malesia, Africa.
Evolution: Divergence & Distribution. Merckx et al. (2013c) and Matuszak et al. (2015) give other dates, etc., for the family.
Klackenberg (Exaceae, in Struwe et. al. 2002) suggested that some of the distribution patterns in Exaceae in particular, but also Saccifolieae and Chironieae, reflected vicariance/continental drift events. Such suggestions were taken up by Pirie et al. (2015), who noted that in this context Gentianales would be over 250 Ma with the divergence between Saccifolieae and the rest of the family being at the Jurassic—Triassic boundary ca 200 Ma (ibid., Fig. 1, ?dates there), and even so there would also have been a number of dispersal events.
- The origin of Voyrieae, and perhaps Gentianaceae as a whole, was perhaps in the Guianas area (Gomes et al. 2022b; see also Albert & Struwe 1997). Since Voyria primuloides, the only African species of the holomycoheterotrophic and otherwise Neotropical genus (≡ Voyrieae), diverged from the rest of the genus somewhere between 28-12 Ma, LDD across the Atlantic is probably responsible for its disjunction (Merckx et al. 2013c).
- Von Hagen and Kadereit (2001) suggested that Gentianella (Gentianeae) moved into South America from the north and diversified considerably in the Andean region; there are ca 170 species in South America, of which ca 48 are restricted to the páramo (Sklenár et al. 2011), versus 42 in the whole of the northern hemisphere. From the Andes Gentianella seems to have moved on to New Zealand by long distance dispersal. Von Hagen and Kadereit (2003) found that there was an increase in diversification of Halenia (Gentianeae) only after it moved into Central and South America within the last 1 Ma, not when it first acquired corolla spurs; there were three separate colonizations of South America. The genus may originally have been from Central or East Asia, and its stem group age is ca 11.8 Ma (von Hagen & Kadereit 2003). For diversification and "key innovations" in the small (south)east Asian and largely alpine clade of subtropical taxa sister to the speciose Gentiana, see Matuszak et al. (2016), while Favre et al. (2016) suggested that Gentiana itself has been on the Qinghai-Tibet plateau region for the last ca 34 Ma, whence it dispersed to montane regions in much of the rest of the world, indeed, the Qinghai-Tibet plateau region has been very important for the evolution of Gentianeae as a whole. Favre et al. (2021) identified a number of correlates of diversification of Gentiana in the European Alps, an enterprise somewhat confused by hybridization.
- Vieu et al. (2021) noted that diversification in the speciose Macrocarpaea (Helieae) began a mere (9.7-)7.2(-5.2) Ma and is centred on mid-elevation Andean rainforests; a clade restricted to the Brazilian Atlantic Forest is sister to the rest of the genus, but given the distributions of the immediate relatives of Macrocarpaea, its place of origin is unclear. For species limits in the genus in populations from southern Ecuador / northern Peru - not an easy problem - see Vieu et al. 2023).
- The wide distribution of Exacum (Exaceae) was probably attained by dispersal (Y.-M. Yuan et al. 2005).
C. Chen et al. (2023) noted that many floral traits seemed to be almost randomly distributed on a phylogeny of Gentianeae-Swertiinae, but still rather little is known about the evolution of that subtribe (but see Kadereit & von Hangen 2025).
For polyploidy, (recurrent) hybridization and evolution in Centaurium, see Valdés-Florido et al. (2024).
Ecology & Physiology. Holomycoheterotrophic taxa have evolved in the three basal clades in the family (Bidartondo et al. 2002; Merckx et al. 2013c). The holomycoheterotroph Voyria in Panamanian forests is sensitive to the amount of P in the soil, disappearing when P increased above 2 mg/kg-1 (Sheldrake et al. 2017) - possibly common in holomycoheterotrophic plants associated with glomeromycotes. Roots vary greatly in length, varying from being long and like runners to small and almost star-like (Imhof 1997, 1999a); root hairs are generally absent, but they are present in V. primuloides. They are also found in V. aphylla where its roots abut those of other plants and also litter. Fungal penetration occurs in the former situation, while the roots of these other plants are described as being decomposed at the point of fungal attachment, although apparently only locally, and carbon exchange may occur via the fungi (Imhoff 1999a), so perhaps there is indirect parasitism? For more on all shades of mycoheterotrophy, see Orchidaceae in particular, Hynson et al. (2013: holomycoheterotrophy), etc.. The ?eutrophic Eustoma grandiflora (Lisianthus-Chironieae) forms a Paris-type arbuscular mycorrhizal association (like the holomycoheterotrophs), and the development, etc., of this association has been studied in some detail. Here branching of the hyphae of AM fungi likeRhizophagus. but not Glomus, is caused by monoterpene glucosides/secoiridoids like gentiopicrosides; gibberellic acid, but not strigolactones, induce these secoiridoids (see Tominaga et al. 2021, 2023, also below).
Gentians commonly produce numerous tiny seeds, so similarities in the relationships between both orchids and gentians and their associated fungi are perhaps not unexpected. Suetsugu et al. (2020c) found evidence that adult plants of Pterygocalyx volubilis (Gentianeae), a vine, were mixotrophic, they were partial mycoheterotrophs, a state well known to occur in Orchidaceae. Seedlings of Gentiana zollingeri grew underground for quite some time where they were associated with and apparently supported by Glomeraceae fungi, and the same fungi were also linked with the adult plant, although this was photosynthetic - and similar fungi were associated with the holomycoheterotrophic Voyria. Not all Gentiana, at least, show such an association (Yamoto et al. 2021). Genera like Bartonia and Obolaria (not immediately related) do have chlorophyll although their leaves are reduced and again the adults are mixotrophic (Cameron & Bolin 2010). Both autotrophic and mycoheterotrophic species are found in genera like Exochaenium and Exacum; some species of Exochaenium may even be parasites (Kissling 2012).
As might be expected, Paris-type endomycorrhizae involving Glomeromycota are common in Gentianaceae, including the holomycoheterotrophic members (Imhoff 1999a, 2009; Imhoff & Weber 2000; Franke et al. 2006; Imhoff et al. 2013). The Glomus involved in holomycoheterotrophic relationships in Voyria and Voyriella are quite closely related and also to the Glomus in other mycoheterotrophic vascular plants like Corsiaceae and Psilotaceae, but not to fungi on their autotrophic immediate relatives (Winther & Friedman 2008; Perez-Lamarque et al. 2020), and fungal specificity has been reported to be high (Bidartondo et al. 2002; Sýkorová 2014 and references). However, these fungi were unlike those on their nearest non-mycoheterotrophic relatives (Perez-Lamarque et al. 2020: other Gentianaceae not in the comparison). At least in less vegetatively reduced gentians the presence of fungi is not needed for germination (Sýkorová 2014), but the situation in the genera just mentioned is unclear. For other details of these associations, see Imhof et al. (2013).
For more information on the morphology, etc., of holomycoheterotrophic Gentianaceae, see Oehler (1927) and Merckx et al. (2013a); for information on Voyria in particular, see Merckx et al. (2013a), general, Imhof et al. (2013: roots, mycorrhizae), Waterman et al. (2013: pollination), Johow (1885, 1889: anatomy, seed, etc.), Maas and Ruyters (1986), Bouman and Devente (1986), and Bouman and Louis (1990), seeds, and Franke 2002 (V. flavescens).
Pollination Biology & Seed Dispersal. Swertia often has fimbriate appendages and other structures associated with nectaries on the petals, which may be one of two per peral. Halenia (= Swertia s.l.) has flowers with a nectar spur on each petal, uncommon in angiosperms (von Hagen & Kadereit 2003). Most species of Sebaea s. str. (Exaceae) have a pair of commissural secondary stigmas at the base of the style (Kissling et al. 2009b; Kissling & Barrett 2013), apparently unique in the angiosperms; these remain functional after the terminal stigmas become non-functional and they apparently allow self-fertilization to occur; Hill (1913) suggested that they are fused and displaced lateral lobes on the stigmas. There is buzz pollination in Exacum (Russell et al. 2015; Amorim et al. 2019).
Pollination of the mycoheterotrophic Voyria is mostly by bees. The pollen is often clumped, the clumps being held together by the tubes of pollen grains that have already germinated and/or by secretions from the anthers; the anthers are borne close together around the style or stigma (Hentrich 2008; Hentrich et al. 2010a).
Species of Voyria have exceptionally small dust seeds, and wind dispersal may help explain the quite large ranges in this genus (Gomes et al. 2022b; see also Eriksson & Kainulainen 2011).
Plant-Animal Interactions. Tachia guianensis (Helieae) may be associated with a variety (ca 37 spp!) of ants which live in the stems and protect the plant against termite attack and the depradations of leaf-cutting ants. These 37 species of ants were almost entirely different fropm those on three species of specialized myrmecophytes in the same area, and these three species had ants that were largely different from each other (Dejean et al. 2017, 2018).
Plant-Bacterial/Fungal Associations. See Ecology & Physiology above.
Genes & Genomes. A genome duplication, the EXAFα event, some 29.6 Ma, may be associated with the node [Exaceae [Voyrieae + the rest] (Landis et al. 2018), however, the ages of the duplication and those along the spine/tribes of Gentianaceae do not match.
Z. Li et al. (2020) found that the plastome of the holomycoheterotroph Cotylanthera (= Exacum) paucisquama was small and highly degraded, only 21 of the normal ca 113 genes being present, and the inverted repeat had considerably expanded.
Chemistry, Morphology, etc.. Gentianaceae often taste bitter because of the iridoids they contain, while Exacum may be foetid. Flavone-O-glycosides are known from two species of Exacum, alone in the family.
The wood of Helieae shows paedomorphic characteristics (Carlquist & Grant 2005). 1:3 nodes are scattered through the family, e.g. Exacum, Gentiana and Sabatia, while in Frasera and Swertia at least there is extensive variation in nodal anatomy within an individual, leaves produced at different times in the season having different nodal morphologies (Post 1958). Although Gentianaceae are not supposed to have stipules, Potalieae in particular, and especially Fagraea, have a variety of inter/intrapetiolar sheaths and auriculate structures at the nodes. For stipule-like structures in Macrocarpaea (Helieae), see e.g. Grant and Weaver (2003), here M. zophoflora is described as having deciduous lunate interpetiolar stipules 7-10 x 10-12 mm in size, while taxa like M. maguirei have interpetiolar ridges 4-10 mm high. Taxa in Helieae and Potalieae often have winged, vaginate petioles. There are different kinds of extrafloral nectaries in the family, both on the leaf and stem, and the survey begun by Dalvi et al. (2013) could usefully be extended.
Corolla tube formation for some taxa has been described as being late-early (Erbar & Leins 1996b). Exacum has an imbricate corolla (and calyx), and the flower is monosymmetric because of the orientation of the androecium and style and curvature of the pedicel (Ronse de Craene 2010). There are sometimes two almost stamen-like appendages on either side of the ovary of Voyria, perhaps modified nectaries, indeed, I have not done justice to the variation in nectaries in the family; as Pringle (2014: p. 6) noted, "the flowers of most species produce nectar". In one major clade of Sebaea secondary stigmas develop towards the base of the style; this is associated with the postgenital fusion of flat carpels (Kissling et al. 2009b). Gentiana has ovules over almost the entire inner surface of the loculus.
Cotylanthera (= Exacum) has straight, ategmic ovules, as have some Voyria. The polarity of the embryo sac in such cases is reported to have been inverted 180o, the egg cell being near the chalaza (Bouman et al. 2002; see also Suessenguth 1927). However, it is perhaps as likely that the ovule is anatropous, but the ovules are so highly reduced that few landmarks are left to be able to tell the orientation. The funicles may also lack vascular tissue (Bouman et al. 2002). From illustrations in Johow (1895) there appear to be two parietal cells in Voyria, and although he emphasized that the embryo sac developed from the uppermost (micropylar) megaspore, he illustrated the lowermost cell in that role. An endothelium has been reported from Gentianaceae (Kapil & Tiwari 1978), but as Shamrov (1996) describes this, it consists of one or two layers of cells of the integument that are elongated periclinally, not more or less anticlinally enlarged, as is common. The multiplicative integument of Orphium frutescens is about 6 cells across at anthesis (Hakki 1999), indeed, the considerable variation in integument thickness in the family needs to be put into a phylogenetic context. The embryos of some of the mycoheterotrophic taxa have very reduced cotyledons.
For additional information about Gentianaceae, see Wood and Weaver (1982), Ho and Liu (2001: Gentiana, 2015: Swertia, etc.), Kadereit and von Hagen (2025: Swertiinae), papers in Struwe and Albert (2002), Struwe et al. (2002), Pringle (2014), Struwe (2014), Struwe and Pringle (2018), the Gentian Research Network and Gomes et al. (2022b: Voyria), all general, for chemistry, see Jensen and Schripsema (2002) and Kshirsagar et al. (2019: Swertia), for anatomy, see Dalvi (2014: Saccifolieae), for colleters, see Dourado et al. (2022: Helieae), for the corona in Symbolanthus, variable in morphology and position, see Struwe et al. (2003), for orbicules, see Vinckier and Smets (2000a), for pollen, see Nilsson (2002), Nilsson et al. (2002), Chassot and von Hagen (2008: Swertia) and de Sousa et al. (2018: Helieae), for the gynoecium, see Shamrov and Gevorkyan (2010b), for general embryology, see Maheswari Devi (1963) and Hakki (1997), for ovule development, etc., see Stolt (1921: integument thickness varies within Gentiana, postament +), Shamrov (1996), Bouman and Schrier (1979), Vijayaraghavan and Padmanaban (1968), Akhalkatsi and Wagner (1997), Xue et al. (2007) and Li et al. (2015) and for seed morphology, see Whitlock et al. (2010).
We need more basic anatomical, chemical and developmental information about Saccifolieae, Exaceae and Voyrieae in particular if the evolution of Gentianaceae is to be understood.
Phylogeny. Relationships between some of the tribes remain rather poorly supported (Molina & Struwe 2009; Merckx et al. 2013c; Struwe 2014), in particular, the position of Voyria is still somewhat uncertain - it may have diverged before Exaceae, not after it, as suggested above. Indeed, although there is evidence from pollen, etc., that Voyria is not particularly similar to the mycoheterotrophic Voyriella, etc., its immediate relationships are unclear, although it does have bicollateral vascular bundles like many other Gentianales in this part of the tree. [Saccifolieae [Exaceae [Chironieae [Gentianeae + Helieae + Potalieae]]]] is probably the best way to summarize other relationships in the family (e.g. Struwe 2014; L.-L. Yang et al. 2016; see also Lam et al. 2018). Z.-D. Chen et al. (2016) do not re+cover quite the same basal relationships in Chinese Gentianaceae, but the vast majority of species they examined are Gentianeae.
Chironieae-Chironiinae: Mansion and Struwe (2004) and Mansion (2014) have clarified relationshipshere, and many of the relationships are moderately to well supported.
For the phylogeny of Exaceae, see Y.-M. Yuan et al. (2003) and Kissling et al. (2009a: Sebaea s.l. is paraphyletic), however, within Sebaea s. str., there are two major clades; in the one that has secondary stigmas, S. pusilla, sister to the rest of the clade, lacks such stigmas (Kissling et al. 2009b).
Within Gentianeae-Swertiinae, relationships in the general Swertia/Halenia area are poorly understood (H.-C. Xi et al. 2014). Thus Gentianella seems to be polyphyletic (von Hagen & Kadereit 2001), as does Swertia, too (Chassot et al. 2001; Kadereit & von Hagen 2003) along with Lomatogonium - indeed, all three are polyphyletic in both nuclear and plastome analyses, and there is also extensive genome conflict (G. Chen et al. 2023). Kadereit and von Hagen (2025) developed this last study to clarify relationships here producin a kind of composite hylogent, and they obtained the topology [[Obolaria + Latouchea] [Megacodon [Pterygocalyx + Swertia s.l.]]] (see also Classification below). Ho et al. (1996) carried out a morphological phylogenetic analysis of Gentiana. Relationships within Gentianinae are being clarified, however, Metagentiana, only recently described, is probably polyphyletic (Chen et al. 2005b; Favre et al. 2010, 2014; Matuszak et al. 2016). Favre et al. (2020) obtained very largely well supported relationships in Gentiana using 294 unlinked anchored loci, plastomes, and the nuclear ribosomal DNA cistron, although relationships suggested by the latter differed somewhat from those suggested by the first two (see also Favre et al. 2021 for incongruence between nuclear and plastome relationships in European Gentiana).
For relationships in Helieae, morphologically variable and all members Neotropical, see Struwe et al. (2009) and especially Calió et al. (2016). Vieu et al. (2021: 2 ribosomal 4 plastid markers, good sampling) looked at relationships within Macrocarpaea.
Potalieae: Relationships within Fagraea have recently been clarified, and the variation in tree architecture there makes more sense (Wong & Sugumaran 2012).
Voyrieae: Albert and Struwe (1997) provide a morphological phylogenetic analysis of Voyria - much useful information, while Gomes et al. (2022b: ITS and 4-marker analyses) looked at molecular relationships.
Classification. For an infra-familial classification of Gentianaceae, see Struwe et al. (2002) and especially Struwe (2014), also the Gentian Research Network.
For discussion about generic limits in Helieae, very problematic for a long time, see Calió et al. (2016). Kissling et al. (2009a) and Kissling (2010) have divided the polyphyletic Sebaea into three genera. Ho and Liu (2015: p. 21) suggested that "all infrageneric categories [of Swertia] ... might be created as independent genera if they were shown to be monophyletic units", but it is unclear why this might be be necessary or useful; demonstration of monophyly, whether or not associated with morphological distinctions, does not entail any particular nomenclatural actions. Indeed, Kadereit and von Hagen (2025) examined taxon limits in Swertiinae in considerable detail, in particular examing the relative merits of a Swertia s.l. or s.str.. Like C. Chen et al. (2023), they found that floral traits were not particularly correlated with clades here and opted for the recognition of a Swertia s.l. (see also above). There are suggestions that the monophyletic Fagraea s.l. should be dismembered (Wong 2012 and references) and members of a small clade of subtropical gentians sister to Gentiana s. str. have been placed in five genera (Favre et al. 2014), however, this does seem a little excessive. Favre et al. (2020) presented a classification of Gentiana, sections and series, which is quite similar to that of Ho and Liu (2001).
Previous Relationships. Anthocleista, Fagraea and Potalia used to be in Loganiaceae (Struwe & Albert 2000), but details of their iridoid chemistry are very gentianaceous and they are well embedded in the family (e.g. Backlund et al. 2000). Emblingia has been placed here (Savolainen et al. 2000a), but a position in Brassicales in its own family (q.v.) is justified on both molecular and morphological grounds.
[Loganiaceae + Apocynaceae]: ?
LOGANIACEAE Martius, nom. cons. - Back to Gentianales
Tryptophane-derived alkaloids +; nodes also 3(+):3(+) (and split laterals); stomata?; lamina vernation ± flat, (sheathing stipule +); flowers 4- or 5-merous; K basally connate or not, C valvate, often hairy at the mouth; tapetum (amoeboid); pollen grains tricellular [?all], with lateral extensions at the endocolpus; nectary 0/poorly developed/ gynoecial; G collateral, (-5), often partly inferior and partly apocarpous (congenitally syncarpous), placentae peltate, (stylar fusion postgenital), style branches +/0, stigma capitate/long-clavate/2-lobed/punctate; ovules (1-)many/carpel, epitropous [?always], integument 4-6 cells across; fruit a follicle, loculi- and/or septicidal capsule, drupe or berry;; exotestal cells papillate or hairy, ± thick-walled and lignified except outer wall; x = 11 (?10), nuclear genome [1C] (0.05-)0.976(-19.221) pg; seedings epigeal and phanerocotylar.
13 [list]/420. Pantropical, esp. Australia and New Caledonia. Map: from Leenhouts (1962), van Steenis and van Balgooy (1966) and Leeuwenberg (1969). [Photo - Flower, Fruit]
1. Antonieae Pohl —— Synonymy: Antoniaceae Hutchinson, Gardneriaceae Perleb
Iridoids 0; rays uniseriate, homocellular; flowers (monosymmetric; C aestivation various; A (1, abaxial - Usteria); fruit a capsule; n = 11.
4/14: Bonyunia (10). Tropical: South America, West Africa, West Malesia.
Age. The clade [Spig. + Strych.] is some (70.8-)44.7(-18.3) Ma (Tank & Olmstead pers. comm.).
2. Spigelieae Dumortier - Spigelia L. —— Synonymy: Spigeliaceae Berchtold & J. Presl
(Annual) herbs (shrubs); secoiridoids 0; lamina venation palmate; endosperm ruminate; fruit circumscissile, etc; n = 8, 10, 13.
1/ca 75. Southern U.S.A. to ca 35o S in South America.
3. Strychneae Dumortier —— Synonymy: Strychnaceae Perleb
Lianes with branch tendrils; iridoids +; (included phloem +); Leaves often pseudoverticillate, lamina venation palmate; tapetal cells polyploid [Strychnos]; C valvate; pollen lacking lateral extensions at the endocolpus; fruit a berry or drupe (seed winged - Neubergia); n = 11-12.
3/220: Strychnos (200). Circumtropical.
4. Loganieae Endlicher —— Synonymy: Geniostomataceae L. Struwe & V. Albert
(Annual) herbs (aquatics) to shrubs; iridoids +; flowers (4-merous); (median K abaxial - Logania), C contorted/quincuncial; styles separate; (seeds embedded in fleshy placenta - Geniostoma); n = 19, 20.
8/150: Mitrasacme (55), Geniostoma (40). The Mascarenes, South East Asia to Malesia, especially the Antipodes, Pacific islands, inc. Hawaii.
Evolution: Pollination & Seed Dispersal.There is secondary pollen presentation (stylar) in two genera (El Ottra et al. 2023).
Chemistry, Morphology, etc.. The wood of Strychnos has included phloem (van Veenendaal & den Outer 1993). The plant has branch tendrils, although they may be described as being modified leaves ().
Colporate pollen without lateral extensions at the endocolpus is reported to be a character restricted (in this group) to Strychnos and its immediate relatives. The ovules of Mitrasacme have an endothelium and the endosperm is "intermediate" in development, while Mitreola oldenlandioides has straight ovules and cellular endosperm (Reddy et al. 1999).
For information, see Leeuwenberg (1980) and Struwe et al. (2018), both general, Hakki (1998: Usteria), Aniszewski (2007: alkaloids), Keller (1996: "stipules"), Hasselberg (1937: nodes and stipules), and Dahlgren (1922), Bendre (1975) and Maheswari Devi and Lakshminarayana (1960: ?Strychnos with an endothelium), all embryology.
Phylogeny. L.-L. Yang et al. (2016) found the relationships [Antonieae [Spigelieae embedded in Strychneae + Loganieae]], with quite good support. For relationships in Loganieae, see also Gibbons et al. (2012: generic limits will have to be adjusted), Antonelli et al. (2021: still not much in the way of clear signal), while Setubal et al. (2021) recovered the relationships [Gardneria, [Bonyunia paraphyletic, Antonia, Norrisia], [[Logania + Strychnos] [Spigelia [Mitrasacme (on a long branch) [Geniostoma [Mitreola + Logania]]]]].
Setubal et al. (2021) looked at relationships within Strychnos; the old sections have not held up.
Classification. Four tribes are recognized above, but this is clearly unsatisfactory.
Previous Relationships. In the past Loganiaceae have seemed to show relationships all over the sympetalous map, and Bentham (1856) compared Loganiaceae, then notably more broadly delimited than now, to a less-wooded area from which obvious forests representing more distinctive families such as Rubiaceae, Solanaceae, etc., had been removed. Loganiaceae in this sense (e.g. Leeuwenberg 1980) had ca 22 genera and 310 species. Of these genera, Buddleja s.l., Androya, Peltanthera, Sanango, Plocospermum, Nuxia and Retzia, and Polypremum, are now in seven or so separate clades in Lamiales, i.e. Scrophulariaceae (Buddleja s.l. and Androya, not immediately related), Peltantheraceae (near Gesneriaceae), Gesneriaceae themselves, Plocospermataceae, Stilbaceae (Nuxia and Retzia) and Tetrachondraceae, while Desfontainia (Columelliaceae) is in Desfontainiales, a campanulid (for references, see those families), so it is not surprising that Loganiaceae seemed to be such a "central" family. Chemical variation within Loganiaceae s.l. strongly supports its break-up (e.g. Jensen 1999). However, Loganiaceae are morphologically quite heterogeneous even in their much more restricted circumscription.
APOCYNACEAE Jussieu, nom. cons. - hierarchy below below very much under construction - - Back to Gentianales
Tryptophane-derived, steroidal indole alkaloids [pseudalkaloids, pregnane skeleton], route II decarboxylated iridoids +, (tanniniferous); pericyclic fibres 0 [always?]; vessel (elements with scalariform perforation plates), single or in radial groups, rays with multiseriate part no wider than uniseriate part; tracheids in ground tissue; laticifers +, articulated, latex white; (petioles also with adaxial bundles); stomata usu. paracytic (anomocytic, actinocytic); lamina vernation usu. flat or conduplicate; K quincuncial, colleters alternisepalous and/or basal-adaxial/0, C sinistrorse-contorted [left-handed], postgenital connation forming the upper tube [above the insertion of the A], corolline corona + [opposite A]; anthers ± connivent, entirely fertile, filaments shorter than anthers; pollen transported in foam; pollen surface usu. smooth, ± perforate, intine in interapertural areas 3-layered, (in apertural areas thin), (tetrads +, acalymmate); nectary +, from base of G [some rauwolfioids], 2-5 separate/± fused/disc-like; G apocarpous, (collateral), styluli short, apices alone postgenitally syncarpous, style head swollen, not differentiated [receptive, also epidermis secretory, pollen often stuck to style head by secretions, also stuck to pollinator], wet or dry; ovules (hemitropous), integument 6-9 cells across, obturator + [?all]; exotestal cells with all walls thickened, (flattened mesotestal crystalliferous cells); (chalazal endosperm haustorium +); x = 11, nucleus with protein crystalloids, genome [1C] (0.074-)0.788(-8.439) pg; also sporophytic incompatibility system present.
400 [list - tribal classification]/4,555 (5,100) - 5 subfamilies and ca 25 tribes below; of the subfamilies, the first two are wildly paraphyletic. Largely tropical to warm temperate (map: from Hultén 1968; see also maps below). [Photo - Flower, Fruit.]
N.B. There is considerable variation in the morphology and distribution of colleters on the plant, but little of this is mentioned below. Similarly, I refer to a corona that varies considerably in morphology and is associated with the corolla, and there is also a corona associated with the stamens in particular. Similarly, mention is made of nectaries, and this refers to nectaries that are 2-5 separate lobes, some kind of annular structure, whether free from or adnate to the gynoecium, or structures pretty much indistinguishable on the gynoecium, but nectar is secreted; mention is made when the nectary is associated with filaments or is somewhere on the gynostegium.
Age. Rapini et al. (2007) thought that crown-group Apocynaceae were ca 54 Ma, but Bell et al. (2010) suggested an age of only ca 21 Ma - ca 85.6 Ma is the estimate in Fishbein et al. (2018) and Bitencourt et al. (2021).
1. "Rauvolfioideae" Kosteletzky / rauvolfioid grade ("Carissoideae" Endlicher) - see the next 11 tribes, 1A-1K.
1A. Aspidospermateae Miers
Trees or shrubs, (root buds +); indole alkaloids +; leaves spiral (opposite); calycine colleters 0; C (right-contorted, dextrorse), postgenital conntion behind A incomplete; anthers lacking appendages; pollen 4-6 colporate, pseudocolporate, ridged, ridges formed by unequal deposition of infratectum layer in the exine that delimit apertures; (nectary 0); style head bifid, (with basal collar - Haplophyton); fruit a drupe, berry or follicle; one (two) carpel developing; seeds winged/not/(with micropylar and chalazal coma - Haplophyton); n (= 10).
6/70: Aspidosperma (50). Extreme southwest and southeast U.S.A. to Argentina, the Antilles.
Age. The crown-group age is estimated to be ca 49.6 Ma (Fishbein et al. 2018).
[Alstonieae [[Pycnobotrya [Vinceae [Willughbeieae + Tabernaemontaneae]]] [Melodineae, Hunterieae, Amsonieae, Alyxieae, Diplorhynchus [Plumerieae [Carisseae + APSA clade]]]]]: apical appendages of stigmatic head not secretory.
Age. The age for this node is (82.8-)63.2(-45) Ma (Tank & Olmstead pers. comm.) or ca 79 Ma (Fishbein et al. 2018).
1B. Alstonieae G. Don
Trees or shrubs; alsostrine alkaloids +; leaves usu. (-9-)whorled; (calycine colleters + - Dyera); C (right-contorted); anthers (with apical and basal appendages - Dyera); style (head with basal collar - Alstonia); fruit a follicle; seeds with hairs around the margins/winged; much polyploidy (n = 10).
2/47: Alstonia (45). Africa, Southeast Asia, Malesia to Australia, the Pacific.
Age. Crown-group Alstonieae are estimated to be ca 61.5 Ma (Fishbein et al. 2018).
[[Pycnobotrya [Vinceae [Willughbeieae + Tabernaemontaneae]]] [Melodineae, Hunterieae, Amsonieae, Alyxieae, Diplorhynchus [Plumerieae [Carisseae + APSA clade]]]]: uniseriate rays common.
[[Pycnobotrya [Vinceae [Willughbeieae + Tabernaemontaneae]]]: ?
Liana; ?K colleters; anthers with basal and elongate apical appendages; ?stylar head; ovules 4/carpel; seeds winged; n = ?
1/1. Tropical Central and West Africa.
[Vinceae [Willughbeieae + Tabernaemontaneae]]: style +.
1C. Vinceae D. Don —— Synonymy: Ophioxylaceae Martius, Vincaceae Vest
Trees, shrubs (lianes, herbs); K (colleters on margins); C (right contorted), corona inconspicuous; anthers (with apical appendage); pollen (asymmetrical), pore position variable; style filiform, stylar head differentiated, unlobed, integument 7-10 cells across [Rauvolfia]; seed with apical wreath of hairs, basal collar +; fruit a drupe/moniliform with several drupelets/follicle, often only 1G developing; seeds usu 1-4(-6)/carpel, (warty, hairy or winged); (endosperm 0 - Kopsia); n 8-10 (23).
9/160: Rauvolfia (60), Ochrosia (40). Pantropical, esp. Old Word (temperate - Vinca).
Age. The crown-group age of Vinceae is estimated to be ca 69.7 Ma (Fishbein et al. 2018).
[Willughbeieae + Tabernaemontaneae]: G [2] [could be placed here]; fruit (a berry).
Age. The age of this node is ca 70.5 Ma (Fishbein et al. 2018).
1D. Willughbeieae A. de Candolle —— Synonymy: Pacouriaceae Martynov, Willughbieaceae J. Agardh
Large lianes with tendrillate axillary/terminal branches/inflorescences, (trees, or shrubs (rhizomatous)); (calycine colleters +); anther appendages ?0; (nectary 0); G [2] [et seq., = fusion congenital], placentation axile to parietal, style usu. filiform, head usu. undifferentiated; fruit a berry, placentae usu. fleshy; (seed 1); cotyledons usu. cordate.
18/140: Landolphia (60), Lacmellea (23). Pantropical.
Age. The crown-group age of this clade is estimated to be ca 25.2 Ma (Fishbein et al. 2018).
1E. Tabernaemontaneae G. Don
Shrubs or trees (lianes); fibres septate, axial parenchyma slight; leaves (anisophyllous), petioles usu. basally connate, (forming an ochrea); calycine colleters in several rows; A connective apex triangular to deltoid, anthers sagittate, with lignified lateral-basal appendages [= guide rails], basal part enlarged, sterile; G ([2]), placentation axile to parietal, or G 2, style filiform to thick, stylar head upper crest five-lobed, basal flange thickened (uniformly receptive, tip bilobed); fruit a follicle - Tabernaemontaninae/berry/berrylet, (K persistent); seed arillate, ± ruminate, with deep hilar groove/embedded in pulp.
15/170: Tabernaemontana (122). Central to South America, Amazonia (Ambelaniinae) and pantropical, to Australia (Tabernaemontaninae).
Age. Crown-group Tabernaemontaneae are estimated to be ca 42.3 Ma (Fishbein et al. 2018), but c.f. the estimate of ca 20.8 Ma in Bitencourt et al. (2021) - Tabernaemontana–Voacanga.
Age. The age of this node is around 72.3 Ma (Fishbein et al. 2018).
Diplorhynchus condylocarpon (Müller Argoviensis) Pichon
Shrub or small tree; ?colleters; anthers with apical and basal appendages; G ±[2], placentation parietal, ?style, ?head; ovules 4/carpel; seed winged at one end.
1/1. Tropical and southern Africa.
[Melodineae [[Hunterieae [Amsonieae + Alyxieae]] [Plumerieae [Carisseae + APSA clade]]]]: ?
1F. Melodineae G. Don
Lianes or trees (shrubs); (calycine colleters +); corolline corona +/0, (postgenital connation behind A incomplete); pollen (in tetrads); G [2], placentation axile, or apocarpous, style slender (short), style head undifferentiated; fruit a berry, placentae pulpy/follicle; seeds winged or not; n also 10.
3/28: Melodinus (25). Palaeotropics.
Age. The crown-group age is estimated to be ca 55.4 Ma (Fishbein et al. 2018).
[Hunterieae [[Amsonieae + Alyxieae] [Plumerieae [Carisseae + APSA clade]]]]: ?
1G. Hunterieae Miers
Shrub or small trees (lianas); calycine colleters +/-; G 2-5, style long, head with long, slender, non-receptive apices/(0); fruit berrylets/(follicles); seeds embedded in pulp/(winged); n also 10.
4/20: Hunteria (12). Africa, 1 sp. Sri Lanka to southeast Asia
Age. Crown-group Hunterieae are around 42 Ma (Fishbein et al. 2018).
[[Amsonieae + Alyxieae] [Plumerieae [Carisseae + APSA clade]]]]: ?
[Amsonieae + Alyxieae]: calycine colleters 0 (see Fishbein et al. 2018).
Age. This node is ca 70.5 Ma (Fishbein et al. 2018).
1H. Amsonieae M. E. Endress - Amsonia Walter
Small shrubs to perennial herbs; leaves spiral; K colleters 0; ?style, head with basal collar, apical wreath of hairs; fruit a follicle; seeds not flattened or winged; n also 8, 16.
1/18. Southern U.S.A., 1 sp. Japan, 1 sp. E. Mediterranean.
Age. The crown-group age of Amsonieae is estimated to be ca 9.7 Ma (Fishbein et al. 2018).
1I. Alyxieae G. Don
Shrubs, trees or lianes; indole alkaloids 0, coumarins +; (leaves spiral); corona 0-rudimentary; pollen grains (2-)3(-5)-porate, triangular (barrel-/irregularly-shaped), ectoapertures with thickened margins, (inaperturate, in tetrads - Condylocarpon); G also [3-5], style slender, head undifferentiated; fruit berry/drupe/moniliform with several drupelets/follicle; seed (with corky aril)/deep hilar groove/(winged at both ends); (± ruminate); n = 9.
7/140: Alyxia (106). Madagascar, India, southeast Asia to the Pacific basin, Condylocarpon Central America, northern Brazil and Guyana.
Age. Crown-group age Alyxieae are ca 56.2 Ma (Fishbein et al. 2018).
Distinctive pollen of the Alyxia type is known from Palaeocene sediments ca Ma from Borneo (Muller 1981).
[Plumerieae [Carisseae + APSA clade]]: indole alkaloids 0; style long; late-acting self-incompatibility [?all].
Age. This node is ca 69.5 Ma (Fishbein et al. 2018).
1J. Plumerieae E. Meyer —— Synonymy: Cerberaceae Martynov, Plumeriaceae Horaninow
Shrubs, trees (lianes); iridoids, cardenolides [cardiac glycosides] +; leaves spiral (opposite), lamina revolute [Thevetia]; calycine colleters 0/+; corona (0); anther apex sterile, long-filiform (plumed), connective broad; nectary (0);(G ([2])/(postgenitally connate)/(semi-inferior), (placentation parietal), style (stout), head with enlarged free apices, (apical wreath + - Allamanda), basal collar/lobes/(0); 2-many ovules/carpel; fruit a drupe/samara/follicle; seeds winged/not; n = 9, 10, 16.
10/60: Allamanda (14). Central and tropical South America, the Caribbean, Madagascar to Japan, Australia (Queensland) and Pitcairn I., New Caledonia (Cerberiopsis).
Age. Crown group Plumerieae are ca 59.6 Ma (Fishbein et al. 2018).
Age. This node is ca 65 Ma (Fishbein et al. 2018).
1K. Carisseae Dumortier —— Synonymy: Carissaceae Bertolini
Shrubs to trees; (cardenolides [cardiac glycosides] +); (branched thorns +); calycine colleters usu. 0; (C right-contorted), corona 0; G [2], placentation usu. axile, style head undifferentiated; ovules usu 1-5/carpel; fruit a berry, placentae ± indurated.
2/20: Carissa (7-20). Old World tropics.
Age. Crown-group Carisseae are ca 31 Ma (Fishbein et al. 2018).
APSA clade [Apocynoideae, Periplocoideae, Secamonoideae, Asclepiadoideae] / [Wrightieae [Nerieae [Malouetieae [[Echiteae [Odontadenieae + Mesechiteae]] [Apocyneae [Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Aristolochioideae]]]]]]]: iridoids 0, deoxyhypusine synthase/homospermidine synthase duplication, (pyrrolizidine alkaloids +), (cardenolides [cardiac glycosides] +); anthers sagittate, with lignified lateral-basal appendages [= guide rails], basal part enlarged, sterile, firmly adnate [postgenitally] to style head by trichomes on connective [= gynostegium], adhesive used in pollen transport secreted between anthers [foam, etc.]; pollen porate; stylar head differentiated both radially and vertically, two small apical appendages, below secretory and non-secretory zones alternating [see adnation of anthers], receptive basally; fruit a follicle; endosperm thin.
Age. The crown-group APSA clade is ca 57.4 Ma (Fishbein et al. 2018).
"Apocynoideae" Burnett, = the Apocynoid grade, includes the next nine tribes, and Periplocoideae, Secamonoideae and Asclepiadoideae, the latter with 5 tribes, are embedded in this grade.
Wrightieae G. Don
Shrubs, trees or lianes; cardenolides +; latex often translucent; C left- or right-contorted; corona +, (opposite)/alternate C/(0); A attached to style head by sterile basal part; nectray 0/adnate to G, indistinct; style head with apical wreath of hairs, basal collar; (G with postgenital fusion); chalazal coma +; n also 10.
3/30: Wrightia (25). Old World tropics.
Age. Crown-group Wrightieae are ca 6.4 Ma (Fishbein et al. 2018).
[Nerieae [Malouetieae [[Echiteae [Odontadenieae + Mesechiteae]] [Apocyneae [Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]]]]]]]: C right-contorted [dextrorse]; exine infratectum granulate [?all]; micropylar coma +.
Age. This node is dated to ca 54.6 Ma (Fishbein et al. 2018).
Nerieae Baillon
(Succulent) shrubs or trees, (lianas); pyrrolizidine alkaloids [Alafia], iridoids, cardenolides +; (cork cambium deep-seated - Rhazya); (leaves spiral); (corona +); anthers with long apical appendage, (weakly) attached to style head by connective; (nectary 0); G (with postgenital fusion), style head with (poorly developed) apical wreath, basal collar; coma also chalazal, deciduous (0); n also 10.
6/70: Strophanthus (38), Alafia (23). Africa (most) and Europe to Japan and Malesia.
Age. The age of crown-group Nerieae is ca 43 Ma (Fishbein et al. 2018).
[Malouetieae [[Echiteae [Odontadenieae + Mesechiteae]] [Apocyneae [Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]]]]]]: nectaries 5, (basally connate), surrounding base of ovary; seeds laterally compressed.
Age. This node is ca 54.2 Ma (Fishbein et al. 2018).
Malouetieae Müller Argovensis
Shrubs to trees (stem succulent, with trifid spines), (lianes/perennial herbs); (CAM photosynthesis - Pachy.); steroidal and pyrrolizidine alkaloids +; corona usu. 0; pollen (1-polypantoporate); (anther bases weakly attached to style head), (guide rails poorly developed); (nectary 0); style usu. apical wreath 0, basal collar 0, stigmatic zone below adnation of anthers; (coma also chalazal, deciduous/0); n also 9.
13/100: Malouetia (27), Pachypodium (21). Palaeotropics, to the western Pacific, also Cuba, Bahamas, northwest South America.
Age. The crown-group age of this clade is ca 49.2 Ma (Fishbein et al. 2018).
[[Echiteae [Odontadenieae + Mesechiteae]] [Apocyneae [Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]]]: plant vine/liane; few wide vessels and several narrow vessels in clusters; vasicentric tracheids +; fibres [not tracheids] in ground tissue.
[Echiteae [Odontadenieae + Mesechiteae]] / New World clade: ?
Echiteae Bartling
Woody lianes (small trees; perennial herbs); pyrrolizidine alkaloids + [Ech]/0 [Od], (cardenolides - O); latex often translucent; colleter 1, at base of K; C (valvate), corona +/0; (filaments spirally twisted - some Parsonsia); (theca base adnate to style head); (post-genital syncarpy), style head (apical wreath)/(equatorial flange)/(basal collar); n also = 6-10, 12.
23/200: Parsonsia (120), Prestonia (58). New World, tropical, southeast and southwest USA, also New Caledonia (two genera) to Australasia and South East Asia (Parsonsia).
[Odontadenieae + Mesechiteae]: cardenolides +; corona +
Odontadenieae Miers
Herbs with persistent woody base, subshrubs, (twiners); lamina lacking adaxial colleters, stipules +; K colleters opposite C or annular; anthers adnate by pads of hair to the style head, with slender, acuminate basal appendages; nectary various; style-head usu. fusiform or spool-shaped, diaphanous basal collar ± developed; n = ?
8/39, 3 subribes: Odontadenia (22).
Mesechiteae Miers
Perennial herbs, vines/lianes to subshrubs, with xylopodium, perennial herbs; (corona +); stigmatic head with five ± basal arms to which anthers attach by cellular fusion, (with long ribs), stigma in lower region of head; n also 10.
5/235: Mandevilla (170), Forsteronia (42). New World.
Age. The crown-group Mesechiteae are ca 33.4 Ma (Fishbein et al. 2018).
[Apocyneae [Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]: microsporogenesis successive, grains in tetrads.
Apocyneae Reichenbach / Old World clade
Shrubs, lianes, or herbs; cardenolides, (pyrrolizidine alkaloids + - Anodendron, Amphineurion); leaves (spiral), domatia +; K with basal colleters in a single ring; C (left-contorted/valvate), corona 0 (+, small); A-C tube short; (pollen polypantoporate); theca base adnate to style head; (post-genital syncarpy +), style head with equatorial flange, (basal collar +), stigma below adnation of anthers; n also 8, 10, 12.
24/117: Urceola (20). Largely Indo-Malesian to China, also North Temperate (Apocynum).
Age. Crown-group Apocyneae are ca 44.7 Ma (Fishbein et al. 2018).
[Rhabdadenieae [Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]]]: microsporogenesis successive, grains in tetrads.
Rhabdadenieae M. E. Endress - Rhabdadenia Müller Argoviensis
Slender lianas to perennial herbs; ?chemistry; wood fibres very thin-walled, parenchymatous; calycine colleters 0; guide rails truncate, fused to filament; style head apically hairy, basal collar +; n = ?
1/3. Tropical America, Florida, the Caribbean.
Age. The crown-group age of Rhabdadenieae is ca 27 Ma (Fishbein et al. 2018).
[Baisseeae [Periplocoideae [Secamonoideae + Asclepiadoideae]]]: colleters on adaxial surface of petiole; A-C tube very short; G initially half inferior, style short and thick, head without basal flange/collar.
Baisseeae M. Endress
± Large lianes, (rhizomatous); spermidine alkaloids +; hairs branched; lamina with domatia; K colleters +/0, corona +; (filaments long, spirally twisted first in one direction and then the other - Dewevrella); style (filiform - Dewvrella), head apex elongate-tapering; n = ?
4/29: Baissea (18). Tropical Africa, Madagascar.
Age. Crown-group Baisseeae are ca 30 Ma (Fishbein et al. 2018).
[Periplocoideae [Secamonoideae + Asclepiadoideae]]: ?
3. Periplocoideae Endlicher —— Synonymy: Periplocaceae Schlechter, nom. cons.
Herbs, shrubs (small trees), lianes or slender climbers, (roots tuberous); cardenolides; cork endodermal (not Cryptolepis)); flowers to 10 mm long (to ca 9 cm -Cryptostegia); colleters in sinuses of K; C (valvate/imbricate), corona usu +; A-C tube very short; anthers triangular, ± connate laterally, lignified guide rails 0, , adnate to style head by cellular fusion, staminal feet running down C tube and forming ring above the ovary; nectar secreted on margins of/between staminal feet [alternistaminal]; tapetal cells uni(bi)nucleate; pollen in T-shaped/rhomboidal tetrads, ectexine forming a common covering [calymmate], inner and outer walls differentiated [check], grains (4-)6(-16) porate; pollinaris ± horizontal; (pollinia +, 2/loculus, wall 0); pollen collected on spoon-like structure [= translator, from secretions in groove between anthers], basal sticky disc [= viscidium]; stylar head with narrow often bifid apex, stigmatic zones 5; seeds flat on both sides [?all], exotestal cells unthickened [Periploca]; seeds (winged), (coma around entire margin of seed); embryo chlorophyll?
33/180: Raphionacme (35), Pentopetia (23). Old World: Canary Islands, southern Europe to India, China and Australia, but esp. Africa-Madagascar (Map: Good 1952).
Age. The crown-group age of Periplocoideae is estimated to be (40-)31.5(-23.4) Ma (Joubert et al. 2016) or ca 14.9 Ma (Fishbein et al. 2018).
[Secamonoideae + Asclepiadoideae] / asclepiads: cardenolides, (fructans/inulin) +; leaves succulent; C tube formation intermediate; A inserted well below bases of C lobes, staminal corona + [common vascular supply with A], filaments 0, anthers [inserted on top of fused tube/specialized ring corona/staminal feet], adnate to style head by cellular fusion; nectary staminal, [produced in alternistaminal sections of A tube behind guide rails formed by wings of adjacent anthers, = "stigmatic chambers"], usually accessible at or near base of guide rails; endothecium not fibrous; pollinia + [pollinia of the one pollinarium from half anthers of adjacent stamens], pollinaria erect, joining hardened apical clip-like corpusculum, sticky disc [= viscidium] +; pollen tetrads +, tectum surrounds the whole tetrad or each pollen grain [calymmate], grains inaperturate, exine 3-layered, orbicules 0; style short, canals +, head with five stigmatic zones; seed with a distinct margin, often winged; endosperm nuclear (cellular), embryo chlorophyllous.
Age. This node was dated to ca 42 Ma (Rapini et al. 2007) or ca 44.5 Ma (Fishbein et al. 2018).
4. Secamonoideae Endlicher
Lianes/vines, climbing by twining, (shrubs); (colleters on adaxial surface of leaf); K (with single trace), C valvate, (left-contorted); corona +/0; pollinia 4, no waxy outer wall; microsporgenesis simultaneous, outer and inner walls of tetrad differentiated [inner walls have intine bridges], granular layer thick; style head various, secretions pale [→ sot translatopr, lacking definite arms (long, recurved - Seamonopsis)].
8/160: Secamone (100). Old World, esp. Madagascar, tropics to temperate.
Age. The crown-group Secamonoideae are some 21.6 Ma (Fishbein et al. 2018).
5. Asclepiadoideae Burnett
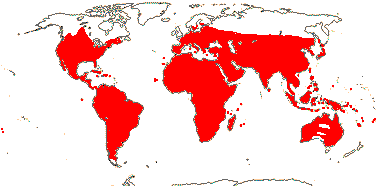
(Interxylary phloem +); inflorescences extra-axillary; colleters in sinuses of K; C corona 0 (inconspiucuous), staminql and interstaminal corona +, connate; anthers bisporangiate, dithecal, pollinia 2, with a waxy covering, connective apex ± membranous; tapetal cells uni-/multiseriate, uni-/multinucleate; microsporogenesis successive, pollen first as linear tetrads, exine granular, thin; (nectary also on corona); stigmatic nectary +; pollen receptive areas on the anther wings [not stigmatic], compitum usu 0; integument 5-6-8 or more cells across, ?multiplicative, (endothelium 0), obturator as hairs; antipodal cells multiplicative, ephemeral; stigmatic head broad, secretions dark [→ hard translator, with arms]; follicles often single; seed convex [rapahal side]/concave, raphe running from apex to centre; suspensor uniseriate, long; mitochondrial PEP subunit β rpoC2 pseudogene [from the chloroplast]; n = (9-14).
181(-214)/(2365-)3445. Tropics to temperate, drier areas esp. in Africa. Map: see Good (1952). Photo: Flower, Flower, Flower, Fruit.
Age. Crown group Asclepiadoideae were estimated to be ca 37 Ma (Rapini et al. 2007) or ca 42 Ma (Fishbein et al. 2018: fig. 1). Note that Del Rio et al. (2020: p. 135) cite the age that Fishbein et al. (2018) give for "the origin of the Asclepiadoideae" as being ca 55 Ma, but even the stem-group age of Asclepiadoideae in Fishbein et al. 2018 would be less that 45 Ma.
Fossil seeds placed in two species of Asclepiadospermum have been found in early Eocene deposits ca 48 Ma from China (Del Rio et al. 2020).
5A. Fockeeae H. Kunze, Meve & Liede
Plants twining, with massive tuberous roots; connective appendages inflated; pollinaria with caudicles ± 0; pollinium wall + [of sporopollenin]/0, pollen grains appear to be single when mature - Cibirhiza); stylar head umbonate, caudicles 0; seeds winged.
2/9. Drier parts of southern and eastern Africa, Arabia (Oman).
Age. The crown-group age of this clade is estimated to be around 8 Ma (Fishbein et al. 2018).
[[Eustegieae + Asclepiadeae] [Marsdenieae + Ceropegieae]]: (often epiphytic); (CAM photosynthesis + - ?Eustegieae); (phenathroindolizidine alkaloids); (leaves spiral); pollinium with sporopollenin wall, pollinaria with translator arms [caudicles]; outer and inner walls of tetrads not differentiated, pollen in monads when mature.
Age. This node is ca 41.5 Ma (Fishbein et al. 2018).
[Eustegieae + Asclepiadeae]: pollinaria horizontal or pendent; mitochondrial rpll pseudogene [from the chloroplast].
5B. Eustegieae Liede & Meve
Herbaceous; lamina linear/hastse/palmately lobed; C ?aestivation; corona 3-seriate; style head rostrate or not; fruit (indehiscent - Emicocarpus, seed 1/carpel, wingless); seeds winged; n = ?
2/4. Mozambique, South Africa (the Cape).
5C. Asclepiadeae Duby —— Synonymy: Asclepiadaceae Borkhausen, nom. cons., Cynanchaceae G. Meyer
Herbs (stem succulents) to small trees, vines, (tuberous/rootstocks thickened/rhizomatous); (latex clear); C valvate/imbricate, (corona +), (tube development early-late intermediate - Vincetoxicum); endothecium + (0 - Calotropis); follicles usu. 1 (2 - Orthosia); seeds (also with wing-like margin); n also 9, 10; germination (hypogeal).
87/1,840. Cynanchum (330), Matelea (180), Vincetoxicum (150), Ditassa (140), Gonolobus (130), Oxypetalum (125), Asclepias (100), Oxypetalum (125), Matelea (75), Metastelma (75), Orthosia (55), Scyphostelma (50+), Xysmalobium (46). Tropical to subtropical (temperate).
Age. The crown-group age of Asclepiadeae is estimated to be ca 38.6 Ma (Fishbein et al. 2018).
[Marsdenieae + Ceropegieae]: pollinaria erect.
Age. This node is around 39.5 Ma (Fishbein et al. 2018).
5D. Marsdenieae Bentham
Usu. vines, plants herbaceous or not, (erect shrubs), (lithophytes, epiphytes); cork endodermal; C valvate/imbricate, (corona +); pollinia lacking insertion crests (+, on outer side), (pollinia with pellucid margin along proximal side - 0 Hoya clemensiella group); style head convex to conical; follicles often 1; seeds with coma (elaiosome), (exotestal cells with outer walls unthickened - Hoya).
36/790. Hoya (350-450), Ruehssia (130), Leichhardtia (85), Dischidia (80), Gymnema (40). Largely Palaeotropical to Oceania, Cioneura southern Europe, Ruehssia Central and South America.
Age. Crown-group Marsdenieae are some 22.8 Ma (Fishbein et al. 2018).
5E. Ceropegieae Orban —— Synonymy: Stapeliaceae Horaninow
Plants (annual), ± erect, stems reed-like and photosynthetic, to large shrubs, vines, stem succulents [esp. stapeliads], (root tubers +); latex clear; leaves often much reduced or spines, glands or membranous structures in stipular position; C valvate, (free), (tubular); corona +, in two series, (0); connective appendages 0 (+ - Caudanthera); pollinia with pellucid distal germination zone ["germination mouth"], insertion crests +, on inner side; pollen (orbicules + - Riocreuxia); seeds with coma and wing-like margin; ventral testa with elongated pitted cells; (hypocotyl massive, cotyledons very small).
11/800: Ceropegia (695), Heterostemma (45). Mostly Africa, some Canaries, also southern Arabian Peninula and southern Europe to China, Malesia and 1 sp. in Australia.
Age. The crown-group age is estimated to be about 35.4 Ma (Fishbein et al. 2018).
Evolution: Divergence & Distribution. For dates of divergences within the family, etc., see Rapini et al. (2007) and Liede-Schumann et al. (2012), and for dates around Ceropegieae see Rapini et al. (2007) and Meve et al. (2016). A number of dates from Fishbein et al. (2018: App. S9, see also p. 499) are added above, but see cautions there; ages in Bitencourt et al. (2021) are the same as in Fishbein et al. (2018) and for the most part have not been repeated).
Bitencourt et al. (2021) provided a comprehensive account of the biogeography and diversification of Apocynaceae, suggesting that they had a pantropical origin, with current distribution patterns reflecting numerous subsequent dispersal events, especially from Africa (there were 64.2 of them...); note that their work used the topology suggested by Fishbein et al. (2018). The whole of the APSA clade (i.e. Apocynoideae, Periplocoideae, Secamonoideae and Asclepiadoideae) may have an African origin (Livshultz et al. 2007), and 11/12 increases in diversification in the family detected by Bitencourt et al. (2021) were in this clade. Features like comose seeds (but there may have been a long period between origin and diversification here), the twining habit and pollinarium development may have promoted dispersal and establishment in habitats that had become drier, cooler, more open, etc., as a result of the Eocene-Oligocene climate transition (Bitencourt et al. 2021). Asclepiadoideae, often herbs, are derived from "Apocynoideae", which are often more or less woody (Livshultz et al. 2011); the origin of the subfamily was perhaps in Africa (Rapini et al. 2007), about 700 species having been recorded from there (Johnson 2010). (Note that Asclepiadeae, with around 1820 species, includes perhaps 2/5 of the species in the whole family.) Diversification of Ceropegieae, with some 780 species, may have occurred a mere 3 Ma as Africa became more arid (Bruyns et al. 2015: estimate younger than in Rapini et al. 2007), and they are notably diverse in southern Africa in particular (Ollerton et al. 2003). There are relatively few Ceropegieae basal to Ceropegia itself, but they are nevertheless quite diverse both vegetatively and florally; overall, although species with a tubular corolla are common in the tribe, this condition is probably derived from a rotate corolla (Bruyns et al. 2022, q.v. for characters of the tribe). Interestingly, these southern African Asclepiadoideae have more specialized pollination systems (fewer species of pollinator per species of plant) than their European and North American counterparts, and this may be connected with species diversity (Ollerton et al. 2006). There is substantial geographical structuring of clades within groups like e.g. Asclepiadoideae (Goyder 2006 for a summary), thus basal clades of the large genus Cynanchum are African (Khanum et al. 2016; see also M. Endress et al. 2018). Meve and Liede (2002b) discuss a number of apparently quite recent Africa → Madagascar movements in Asclepiadoideae, there being the odd movement in the opposite direction.
For diversification in Periplocoideae, see Joubert et al. (2016); Phyllanthera grayii, from northeast Australia (other species up to South East Asia), and Petopentia, from South Africa, are successively sister to the rest of the subfamily. Genera in major clades within "Apocynoideae" are usually from either the Old or the New World, with little overlap between the two (Livshultz et al. 2007).
Farrell and Mitter (1994, 1998) and Farrell (2001) thought that there was some kind of co-evolution, perhaps escape-and-radiate, between the longicorn cerambycid beetle Tetraopes and Asclepias, the latter producing cardenolides, normally a deterrent to herbivores; this association may date to ca 40 Ma. The beetle larvae eat roots, the adults eat leaves and flowers, and the genus Phaea is also involved; its larvae bore in stems (Farrell & Mitter 1998). For more on latex and plant defence, see below under Plant-Animal Interactions.
P. K. Endress (2011a) thought that a key innovation within Gentianales was the evolution of pollinia/pollinaria, presumably to be optimized to the [Secamonoideae + Asclepiadoideae] node; Kuang et al. (2024). These pollinia may provide increased pollination efficiency by generalist pollinators in the rather small populations growing in the dry areas thaT many members of that part of the family prefer - a reduction of the Allee effect. (Pollen morphology and development is very variable in Apocynaceae, and the optimization of palynological characters above is only tentative.) Endress (2016: comparison with Orchidaceae) emphasized the development of synorganization/complexity in the flowers here, synorganization laying the foundation for the development of novel structures, however, the corona, one of the most conspicuous novel structures in Apocynaceae, seems not to be directly the product of synorganization and its origin in different parts of the family varies - corolline?, androecial? (Heiduk et al. 2020).
For characters and phylogeny, see Sennblad (1997). Fishbein et al. (2018) in particular examined the evolution of a number of vegetative and floral characters in the family, at the same time noting that competing topologies, e.g. in the APSA area, yielded very different understandings of evolution of these characters. Fruit and seed morphology in "Rauvolfioideae" in particular is quite variable, but follicular fruits with comose seeds characterise the APSA clade (Fishbein et al. 2018). Pyrolizidine alkaloids are also found only in the APSA clade, but their presence there is sporadic, and although they may be consistently present or absent in some tribes, overall there seem to be too many uncertainties/outright gaps in our knowledge to worry too much about details of their distribution on the tree - see Livshulz et al. (2018a) and especially Barny et al. (2021) for information. Indeed, C. R. Smith et al. (2025) suggested that it is if anything more likely that homospermidine synthase, an enzyme involved in pyrrolizidine alkaloid biosynthesis, may well have evolved here several times, with subsequent losses. Kuang et al. (2024) looked at the variation of a number of characters of the pollinaria of Hoya in the context of the topology of a phylogenetic tree taken from Wanntorp et al. (2014); the variation of some characters was correlated with the groups recognized there, although pollinarium type was only poorly correlated.
All tribes in "Rauvolfioideae" can be more or less readily distinguished by a combination of features in their wood anatomy (Lens et al. 2008b), but such characters have not been integrated into the phylogeny. However, Beckers et al. (2022) looked at wood anatomy in Secamonoideae and Asclepiadoideae, estimating that there had been some 28 or more transitions to woodiness within Asclepiadoideae alone, mostly within Asclepiadeae, perhaps in response to drought. There were differences between tribes and subtribes, for instance, Asclepiadeae had rather shorter uniseriate rays, fibres and vessel elements than Marsdenieae, although details of their phylgenetic significance still remained to be worked out (Beckers et al. 2022). Beckers et al. (2024) took up the challenge, integrating variation in 17 characters of wood anatomy taken from wood anatomical descriptions of 275 species (46 new descriptions) with a tree having the relationships above (for which, see Fishbein et al. 2018).
Ecology & Physiology. Members of the old Asclepiadaceae are the most speciose scandent group in New World tropical forests, those of the old Apocynaceae somewhat less so. There are all told ca 1,350 species of climbers, some quite small plants, in Apocynaceae (see also Sperotto et al. 2023). To put these numbers in context, there are around 10,000 species of climbers in the New World alone - see also elsewhere. In Africa Apocynaceae are perhaps the most prominent group of scandent species (Gentry 1991), and they are aso notable components of the viney vegetation of the deserts in S.W. North America (Krings 2000). Livshultz et al. (2011) proposed that [Asclepiadoideae + Secamonoideae] had moved into drier habitats, the large rainforest lianes of Baisseeae representing the ancestral habit/habitat of the whole clade, while Meve et al. (2016) suggested that the Indo-Australian rainforest liane Heterostemma was sister to (and the habit/habitat ancestral to) the arid-zone succulent Ceropegieae from Africa (although Bitencout et al. 2021 suggested the movement was in the opposite direction). Fishbein et al. (2018) discuss the lability of growth form evolution in Apocynaceae. Beckers et al. (2022) suggested that twining → erect was the general direction of evolution here, and they noted that a number of anatomical features commonly associated with the climbing habit were not to be found in the Apocynaceae that they examined.
The Hoya-Dischidia clade, found from Indo-Malesia to the Pacific, also includes a large number - I estimate ca 310 species - of epiphytic climbers. However, details of the evolution of this distinctive habitat preference/habit are not known, phylogenetic relationships here currently being unclear (Wanntorp et al. 2014; Rodda et al. 2020), although Wanntorp et al. (2014) thought that the radiation of Hoya was connected with its adoption of the epiphytic life style. Dischidia in particular is often to be found growing in the nests of arboreal ants, and Hoya species may also be associated with ants. A number of species of Dischidia in particular are shingle-leaved plants, having more or less orbicular leaves closely adpressed to the stem of the host, the plant being attached by roots that grow out along the stem or only at the nodes (Zona 2020); some of these plants are also associated with ants (see Plant-Animal Interactions below for more on ant associations). For epiphytes here, see Zotz, Weigelt et al. (2021: list), while Hietz et al. (2021) and Zotz et al. (2021) outline the ecophysiological characteristics of epiphytes in general. Sousa-Baena et al. (2018b) discuss tendrils in the family.
More or less erect growth forms have evolved from lianes in some Secamonoideae (Lahaye et al. 2005). Speck et al. (2003) discuss the biomechanics of the changes in life form involved; dwarf shrubs here are biomechanically rather different from "normal" shrubs, and there are several differences in developmental anatomy between shrub and liane. Heterochrony is involved, these self-supporting shrubs having many of the features of young lianes, which themselves have more or less rigid searching shoots with dense wood, and this dense wood phase is prolonged in the shrubs (Speck et al. 2003; LaHaye et al. 2005).
Succulence is quite widespread in Apocynaceae, particularly in Periplocoideae (root succulents) and Asclepiadoideae-Ceropegieae (notable stem succulents, but other growth forms - Bruyns et al. 2022), and there is also leaf succulence, for instance, in Hoya and Dischidia (Marsdenieae); leaf and stem succulence have been lost far faster than they have been gained (Mauseth 2004b; Fishbein et al. 2018). Nyffeler and Eggli (2010b) estimated that there are 74 genera containing 1151 species of succulents; 65 of these genera, mostly small, include only succulent taxa (Meve & Liede-Schumann 2010: many of these genera are now in Ceropegia - Bruyns et al. 2017). There are about 400 species of stem-succulent Ceropegieae in the Old World, with centres of distribution in Arabia, East Africa and southern Africa (Meve et al. 2004). Stapeliads, which make up ca 340 of these species, seem to have evolved in the northern hemisphere, then moved into southern Africa, and then fanned out through the drier parts of the continent, Madagascar and Arabia (Bruyns et al. 2014a) - and this all in the last eight million years or so (Rapini et al. 2007). The embryo in a number of these succulent taxa has a massive hypocotyl and very small cotyledons, and the leaves in the adult plant may be reduced to minute projections (Bruyns 2005). CAM photosynthesis is known from a number of Asclepiadoideae and has been associated with succulence there (Keeley & Rundel 2003); see Holtum (2023) and Gilman et al. (2023) for CAM photosynthesis, and Zotz et al. (2023) for possible connections between CAM and the epiphytic habit.
Pollination Biology & Seed Dispersal. For a general survey of pollination in Apocynaceae, see Ollerton & Liede (1997) and especially Ollerton et al. (2018), for details of the complex association between the stamens and the stigma head, see the account in M. E. Endress et al. (2018), for glandular structures in the flower of asclepiads, see Demarco (2017b), and for the evolution of corolla form, see Fishbein et al. (2018). In the family as a whole, the anthers are closely associated with a swollen stigma head, but in various ways, and the morphology of the head is variable, with crests, lobes, etc. (e.g. Nilsson et al. 1993; Fishbein et al. 2018). In genera like Alyxia all or most (not the very apical) cells of the stigmatic head secrete a sticky polysaccharide-terpenoid material to which the pollen adheres, a variant of secondary pollen presentation (El Ottra et al. 2023: 15 genera); there are no localized pollen-receptive and secretory areas on the stigma. Taxa with such stigmas, perhaps the basic condition for the family, predominate in the basal "Rauvolfioideae", however, it has also been suggested that they are derived, and more than once, the differentiated stigma described below being plesiomorphic for the whole family (Simõs et al. 2007a; see especially Schick 1980, 1982, also Shamrov & Gevorkyan 2010a and Morokawa et al. 2015 for the morphology of the stigm head). Taxa with an otherwise undifferentiated stigma head may have a pair of apical lobes, although these do not function as stigmas (Albers & van der Maesen 1994).
In "Rauvolfioideae" and "Apocynoideae" with spatial differentiation within the stigma head, pollen is deposited onto the apex or in bands down the side of the swollen head, sticky material is secreted immediately below, the sticky material enabling the pollinator to pick up the pollen, and pollen is deposited/germinates only at the base of the head (e.g. Albers & van der Maesen 1994). Hairs on the anthers or stigmatic head and lignified guide rails on the side of adjacent anthers are also involved in pollen presentation and guiding the pollinator so that pollination is effective (e.g. Fallen 1986). An annulus or flange around the middle of the head aids in the removal of the pollen from the proboscis of the pollinator, scraping it off, the receptive stigma itself being a ring around the base of the head below the annulus. The stigmatic flange and the lignified guide rails on the anthers together form a trap-and-guide pollination mechanism. In Tabernaemontaneae several features - an androecium with thick, lignified guide rails, a stylar head with a five-lobed upper crest and a thickened basal flange, and paired nectaries - are all associated, all being lost together some five times on the tree (Simões et al. 2010).
In "Apocynoideae" with anthers adnate to the stigma head pollen from two thecae of adjacent anthers commonly mixes; pollen from the two thecae of the one anther do not mix because the intervening connective is adnate to the stigma head. However, the stigma head between the anthers secretes foam, etc., that is involved in the transport of the pollen from the anther to the stigma. In Secamone, Baissea, etc., the whole stigmatic head is glandular (Safwat 1962: check). In asclepiads, the translator of the pollinaria is made up of a corpusculum, a sticky mass produced in the interstaminal area, and two caudicles (stalks linking the pollinia with the corpusculum), all formed from stigmatic secretions produced between the anthers that vary in composition (Demarco 2014, 2017b). Thus the basic spatial arrangement of the androecium in "Apocynoideae" where the pollen on the stigmatic head is in bands coming from adjacent anthers is the same as that in Asclepiadoideae where the two unisporangiate thecae that make up the pollinaria come from adjacent anthers (see also Schill & Jäkel 1978: survey of pollinaria; Schick 1982).
Pollen is commonly aggregated in a variety of ways in Apocynaceae (see Harder & Johnson 2008; Fishbein et al. 2018). In plants with pollinia, the efficiency of transfer of pollen from the stamens to the stigma is increased, but even in Apocynum cannabinum, which lacks pollinia, it is quite high (Harder & Johnson 2008; Livshultz et al. 2018b: the plant does have tetrads). Indeed, the pollen:ovule ratio in A. cannabinum is low when compared with most other Apocynaceae other than asclepiads (Livshultz et al. 2018b). Within Periplocoideae, pollinia seem to have evolved at least three times (Ionta & Judd 2007), and independently of the evolution of pollinia in asclepiads (Straub et al. 2014; Fishbein et al. 2018), and the old idea of the evolution of the pollinia and complex flowers of Asclepiadaceae via Periplocaceae/Periplocoideae as some sort of intermediate needs to be revised (see also Potgieter & Albert 2001; Sennblad & Bremer 2002; Ionta & Judd 2007; esp. Livshultz et al. 2007). The intimate association of the androecium and gynoecium to form the gynostegium and pollinaria that characterize [Asclepiadoideae + Secamonoideae] (asclepiads below) is postgenital, although variation in the pollinarium of Fockeeae, sister to all other Asclepiadoideae, somewhat confuses the issue (see Verhoeven et al. 2003). Kuang et al. (2024) discuss details of the variation in pollinaria in a number of species of Hoya.
There is a great variety of coronal appendages of one sort or another in Apocynaceae flowers. These may develop from the corolla tube (Nerium, Allamanda, Matelea), and then in the same radius as the stamens, or from the apices of the anthers (Adenium, Nerium). Strophanthus can have appendages at the apices of the anthers or more or less bilobed coronal appendages in the angles of the corolla lobes, furthermore, the apices of the corolla lobes narrow into thin, dangling processes that in some species are almost 30 cm long; there is no nectary here. Sometimes, however, what is called a corona is no more than a transverse ridge on the side of the corolla tube or a longitudinal ridge down the tube. The diversity of form produced by tissues from the corolla, stamens, and staminal feet in asclepiads, the gynostegial corona, is remarkable (e.g. Kunze 1982, 1990, 2005a; Liede & Kunze 1993 for terms used; Fishbein et al. 2018). Within Asclepiadoideae, the corona develops very late and is clearly staminal (Monteiro & Demarco 2017), Heiduk et al. (2020) also noting that its was not vascularized and secreted little nectar in Ceropegia sandersonii, the species they examined in detail, and this is consistent with the pattern of gene expression in the flower (Livshultz & Kramer 2009), although Kunze (2005b) suggests that the corona was an organ sui generis - which it is, if from another point of view. As with the relationship between anthers and pollinaria, the staminal corona lobe in Asclepiadoideae is in the same basic position as the corolline corona in more basal Apocynaceae. The gynostegium covering the gynoecium is formed by the post-genital fusion of anthers and stigma. It represents a modified part of antherine connective tissue (the staminal retinacle) that becomes adnate to the stigmatic head (e.g. Simões et al. 2007b). This develops into a very complex system, and there is much variation in the details of the gynostegium in Asclepiadoideae (e.g., see the photographs in Pilbeam 2014). Kunze and Wanntorp (2008a) discuss corona and anther skirt evolution and they puzzle over the morphologically distinctive gynostegium of the molecularly unremarkable Hoya spartioides (Kunze & Wanntorp 2008b). For coronal morphology, see e.g. Ollerton and Liede (2003: Cynanchum), etc.. In the hierarchy above I distinguish between a corona, qualified as calycine or corolline, and I also mention a gynostegial corona - variation here is incompletely digested.
Nectar in basal Apocynaceae is commonly secreted by nectaries at the base of the gynoecium; these vary in number and may be more or less connate or free; some taxa have nectaries in the ovary wall (Morokawa et al. 2015), but little distinction is made between different kinds of nectaries in the character hierarchy above. Sucrose-dominated nectar seems to predominate in the family (Galetto 1997). Some taxa produce no nectar, and here there is likely to be deceit pollination, as in the rauvolfioid Plumeria (Endress et al. 2018 and references), but particularly in Ceropegieae (S. D. Johnson & Schiestl 2016 - remember, generic limits are problematic here). However, in asclepiads in particular nectar may be secreted in "staminal outgrowths" from the petal-like staminal feet (i.e. the corolla + filament tissue below the anther) that completely surround the gynoecium. Kunze (e.g. 1991, 1997; Kunze & Wanntorp 2008b; see also Monteiro & Demarco 2017; Demarco 2017b) show that this nectar is secreted beneath the guide rails, although it may be accessible to the pollinator only elsewhere in the flower, the nectar moving from where it is secreted via an intricate capillary system (e.g. Kunze 1997; Demarco 2017b; see also below for other functions of this nectar). Thus nectar secreted in stigmatic chambers in Asclepias syriaca moves to the cuculli of the gynostemium (Kevan et al. 1989). Fahn (1979) suggested that the stigma itself might secrete nectar in Asclepias. Nectar may also be secreted on the corona, as in some species of Marsdenia (Marsdenieae), and here it is picked up by the pollinators (moths: Domingos-Melo et al. 2018). The presence of defensive compounds in the nectar is discussed in Plan-Animal Interactions below.
In asclepiads, the proboscis or leg of the insect is guided to the viscidium, and this attaches the pollinia to the pollinator (e.g. Kunze 1991); insects may even become trapped in the process and die on the flower (Liede 1996). Thus the spider-hunting wasp Hemipepsis dedjas, pollinator of the South African asclepiad Pachycarpus appendiculatus, commonly loses its maxillary and/or labial palps to which the pollinaria become attached as the insect forages for nectar; the palps are broken off as the insect tries to remove them from the stigmatic groove (Shuttleworth & Johnson 2009b). In some species, mucilage/lipid exudate is produced by the margins of the guide rails before anthesis and may lubricate the movement of the insect's appendage (Demarco 2017a), or the insect itself may fall down the as in the deceptive pitfall traps of Ceropegia sandersonii (Heiduk et al. 2020).
In Catharanthus roseus direct cell-to-cell communication via plasmodesmata developing in the epidermal cells was established early as the apices of the carpels fused, and here and elsewhere in Apocynaceae pollen is potentially able to fertilize ovules in either carpel wherever it lands on the stigma (van der Schoot et al. 1995). However, most asclepiads lack a compitum (it is present in Secamone and Tylophora) and the stylar canals are usually separate (but not in those two genera - Kunze 1991), and it is common for ovules in just a single carpel per flower to be fertilized. However, the situation becomes complex, because in many Asclepiadoideae, at least, the receptive area for the pollinia is on the anther wings at the end of the guide rail, and pollen of a pollinium germinates in one of the chambers into which it has been deposited (Vieira & Shepherd 2002). In Asclepias syriaca, at least, but also in other asclepiads, nectar secreted by the nectaries in the stigmatic chambers is needed for the pollen to germinate (Kevan et al. 1989; see also Monteiro & Demarco 2017; Demarco 2017b; Domingos-Melo et al. 2018), although little has been said about how the pollen tubes are guided from the pollinia to the stigma/style. Nevertheless, the end result is that there are effectively five separate stigmatic regions in a flower with two carpels (there are also five stigmatic zones in some Periplocoideae).
Pollination might seem to be quite a precise process in many Apocynaceae, and particularly in asclepiads with their complex floral morphologies. Pollination by bees, butterflies and (hawk)moths is scattered throughout the family, however, many species of Secamonoideae, Asclepiadoideae and Periplocoideae in particular are pollinated by wasps, beetles or flies, and are cases of what we think of as generalist pollinators pollinating specialized flowers (Ollerton et al. 2018; ). A variety of floral volatiles from the family has also been characterized, including those that mimic the presence of carrion (Jürgens et al. 2006, 2009a, 2013). Several species of insects may be effective pollinators of the one species of asclepiad, so if the flowers seem morphologically specialized they are functionally generalists (e.g. Ollerton et al. 2003, 2007: Table 1, but c.f. Ollerton et al. 2017 in part). Thus there were 41 (low end) to 185 (high end) species of visitors to flowers of five species of Asclepias in North America, 17 to 116 respectively being effective pollinators (Fishbein & Venable 1996). Ollerton et al. (2003) looked at pollination of some asclepiads in South Africa, 8/9 of which were Asclepiadeae, and found that flowers pollinated by specialist insects had abundant and easily accessible nectar and were also visited by many other insects; furthermore, such asclepiads quite often set fruit - and apparently not by selfing - in cultivation well away from their native habitats and pollinators (Bruyns 2005). Asclepiads with specialised flowers may indeed specialise, but on common, ubiquitous visitors (Ollerton et al. 2003).
Meve and Liede (1994; see also Bruyns 2005) surveyed pollination in stapeliads in general (= Ceropegia s.l., 700+ species, see Bruyns et al. 2017 and below for a phylogeny); these taxa are much photographed (e.g. Pilbeam 2014; Bruyns 2005; de Kock 2017; Frandsen 2017). As Bruyns et al. (2014a) note, the flowers may be more or less hidden under bushes or in litter (the smell of these flowers may help in their detection, see Jürgens et al. 2006), and how the very complex morphologies of the small flowers - the coronal-gynostegial area is quite often less than 4 mm across - relate to pollinator activities needs investigation. Fungus gnats are implicated in the pollination of some small concealed flowers (Bruyns 2000). Bruyns et al. (2015) suggested that flies of one type or another were pollinators in this group, with large flies pollinating stapeliads, and since 10 or more asclepiad species may grow together (Bruyns 2000), details of what pollinators do in terms of con- and heterospecific pollinaria transmission is of considerable interest. Fly pollination has been studied in detail in Ceropegia (e.g. Vogel 1961); it is widespread in Ceropegieae (and also some Asclepiadeae). Flies can be attracted in various ways - foul smell, dark colour, dangling appendages, whether from the the tips of the corolla lobes, the corona, etc. (Vogel 2001), nectar, and the like - which are all found in stapeliads and other groups (Oelschlägel et al. 2014; Plachno et al. 2010: osmophores). For chemical mimicry of oviposition sites, see e.g. Jürgens et al. (2013: carrion) and Heiduk et al. (2016: kleptoparasites). Heiduk et al. (2023) discussed kleptomyophily in Ceropegia, the focus being on C. gerrardii which has green and rather undistinguished flowers with "bleeding petals"; here the scent of the petal exudate was that of distressed honey bees and attracted predatory Milichiidae flies, and the exudate also contained sugar and proteins. Ceropegia sandersonii has complex deceptive pitfall trap-type flowers pollinated by flies that slide down slippery zones on the petals (Heiduk et al. 2020). Overall, there are around 400 species of mostly dipteran-pollinated carrion flowers in the Old World Ceropegieae alone, and the flowers smell like decaying animal carcasses, decaying fruits and/or rotten organic material, rotting fish, excrement (p-cresol - herbivore faeces; polysulphides or heptanal and octanal dominant - carnivore/omnivore faeces or carcasses) or urine (much hexanoic acid), rarely do they smell of honey or lack any detectable scent; colour is also important (Jürgens et al. 2006, 2009a, 2013). Interestingly, nearly all the species studied by Jürgens et al. (2006) have at least some nectar as a reward, Stapelia s. str. being an exception. In one example, about 60% of the some 60 species of Ceropegia examined were pollinated by a single genus of flies (Ollerton et al. 2009b: female biting midges were also quite common), the flowers being of the pitfall type (Heiduk et al. 2017); such plants can be thought of as being ecologically quite specialized (Ollerton et al. 2007). Ollerton et al. (2017) summarized information on pollination in some 69 taxa of Ceropegia and found that although sixteen families of flies had been recorded as pollinators, generally one species of Ceropegia was pollinated by members of only one family of fly (although 10/16 of the fly families visited 2 or more species of plant). Interestingly, muscid and calliphorid flies, visitors of only a single species of Ceropegia each, were common visitors to stapeliads (Ollerton et al. 2017).
Despite the apparently functionally generalized flowers with easily-accessible nectar of Pachycarpus grandiflorus (Asclepiadeae), just a single species of Hemipepsis, a spider-hunting wasp, was found to be its effective pollinator (Shuttleworth & Johnson 2009a, see also b), and the wasp was not put off by the bitter nectar which detered other pollinators (S. D. Johnson 2010). Yamashiro et al. (2008) studied details of pollination of Japanese species of Vincetoxicum, also Asclepiadeae; tipulids, other dipterans, moths, etc., were all involved. A variety of insects also visited V. sangyyojarniae, but effective pollination was at night and was carried our by cecidomyiid flies; attracted by the fungus-like scent, they also partook of the viscous, sucrose-rich nectar, and that the flies also "fitted" the flowers was also important (Kidyoo et al. 2024; see also Liede-Schumann et al. 2027). Overall, there is a fair bit of geographic and phylogenetic structuring to plant:pollinator relationships in Apocynaceae (Ollerton et al. 2018), although it should not be forgotten that some Asclepiadoideae, despite their complex flowers, are generalists (e.g. Ollerton et al. 2007, 2017; Yamashiro et al. 2008). Indeed, much remains to be found out about pollination, for example, what pollinates the spherical, fig-like flowers of Heterostema ficoides (Ceropegieae), from Thailand, that are about 1.7 cm across with just a small opening at the apex and inside are filled with a mass of fleshy hairs coming from the petals, other hairs, coronal appendages, etc. (Kidyoo 2019)?
Wyatt and Lipow (2007, see also 2021) suggest that the evolution of pollinia and secondary apocarpy in Asclepiadoideae in particular, and the latter in Apocynaceae s.l., is connected with the post-zygotic incompatibility systems that characterises Apocynaceae (?all) and at least some other Gentianales - a system that wastes both pollen and ovules. In such situations a syndrome develops that may involve a combination of low fruit set (I had wondered why only one carpel/flower developed in Asclepias syriaca and other asclepiadoids, even only one carpel/inflorescence), apocarpy, restricted stigma surfaces, reduced ovule number, and aggregation of pollinia into polyads/pollinia - having thousands of pollen grains in a single pollinium acslepiads is perhaps connected with a single carpel having multiple ovules. Note, however, that <1>Plumeria and a variety of other Apocynaceae for which about the only characters in common are separate carpels are recorded as having late-acting self incompatibility (Gibbs 2014). In Ceropegieae, at least, the situation is rather like that in many Orchidaceae - natural hybrids are uncommon, but it is quite easy to make successful artificial crosses between species in different "genera" (Meve et al. 2004 for references). This may be connected with a pollination mechanism that is normally so precise that interspecific incompatibility barriers evolve only belatedly...
Plant-Animal Interactions.
Introduction.For latex, which occurs throughout the family, and plant defence in general, see e.g. Agrawal (2017) and Ramos et al. (2019) and references. Latex of a variety of asclepiads, at least, may contain proteases, effective against insect herbivores (Arribére et al. 1998), thus cysteine protease breaks down the chitin in the peritrophic membrane surrounding the food boluses in the gut, with deleterious effects on the insect; also involved in defence are the cardenolides and of course the latex itself - being sticky, it can totally gum up small insects (Agrawal et al. 2008; see Mason et al. 2018 for the importance of the peritrophic membrane, also below).
Although various toxic metabolites are very common in the latex of Apocynaceae, there are about 50 separate groups of herbivorous insects that eat the plants (Farrell 2001 and references). Insects, larvae or adults, may cut leaf veins (they "trench" the leaves), so locally interrupting the translocation of cardenolides, pyrrolizidine alkaloids, etc., to the leaf tissue and so apparently allowing the insect to eat it (Dussourd & Eisner 1987); interestingly, the photosynthetic cost to the plant of this leaf-cutting behaviour may be greater than if the insect had simply eaten the leaf (Delaney & Higley 2006). There is evidence of both trenching and vein-cutting behaviour in Eocene-age leaves (= Apocynophyllum neriifolium, but family-level identity somewhat unclear) in Geiseltal deposits 47.5-42.5 Ma from Germany (McCoy et al. 2022). For more on trenching and foliovory in the family, see Dussourd (2016 and references) and Agrawal (2017: esp. pp. 100-106).
Many insects that eat Apocynaceae sequester cardenolides, metabolites that are usually highly toxic, from the latex; both larvae and adults have bright aposematic warning colouration and are involved in Batesian- and Mullerian-type mimicry systems (Bowers 1993 for aposematic caterpillars). Thus brightly-colored orange and black danaine caterpillars and bright orange aphids are found on Asclepiadoideae in both North America and southern Africa; the New World longicorn Tetraopes whose larvae eat roots, usually of Asclepias (Farrell & Mitter 1998) is often orange and black, and so on. Insects that eat cardenolide-containing Apocynaceae show convergence at the amino acid level (asparagine changes to histidine in several entirely unrelated insects at position 122 of the K-ATPase β subunit) that appears to promote cardenolide resistance; normally cardenolides inhibit the sodium-potassium (Na/K) pump by binding with this subunit (Zhen et al. 2012; Dobler et al. 2012, 2015; Whiteman & Mooney 2012; Agrawal 2017 for a summary). Cardenolides may not be toxic to a few species of herbivores on Apocynaceae whose caterpillars do not sequester them, but species that do sequester cardenolides have modifications to their Na/K pump that non-sequestering species lack (Petschenka & Agrawal 2015). The ability of some insects to eat cardenolide-containing plants is then a separate issue (see also Dobler et al. 2015).
The interactions of the monarch butterfly, Danaus plexippus, with its food plant, Asclepias syriaca, have become one of the best known insect-plant relationships (for details, see Agrawal 2017). The monarch butterfly is involved in a cardenolide syndrome; the cardenolides are noxious and protect both the caterpillar and the adult butterfly, being retained after metamorphosis, although they are not used in pheromones (e.g. Malcolm 1991; see also Hartmann & Witte 1995; Dobler et al. 2011; Agrawal 2017). Monarch caterpillars can eat leaves of A. curassavica although the latter contain large amounts of the cardenolide voruscharin that is poisonous to the caterpillar, but the latter is neither resistant to it nor does it sequester it - although it can convert it to cardenolides that it does sequester, but at a metabolic cost (Agrawal et al. 2021); note that local adaptation to such compounds in the butterfly is unlikely given its migratory behaviour and so it encounters a variety of potential host plants. These cardiac glycosides target the sodium pump, Na+/K+-ATPase, that is central to the transport of ions across the plasma membrane of animal cells, hence their toxicity to herbivores, indeed, to most animals. However, monarchs, like several other cardenolide-resistant insects (cardenoloide resistance has evolved independently over 20 times in six orders), have accumulated mutations in the α subunit of this pump that make them insensitive to the cardenolide glycosides - and using CRIPR-Cas9 editing, Drosophila melanogaster could also be made resistant to cardenolides, the adults even retaining some cardenolides, like monarchs (Karageorgi et al. 2019). In those lepidoptera tolerant to/sequestering cardenolides, Faldwyn et al. (2018) discuss the interactions between species of Asclepias, cardenolide uptake by the monarch, and changing temperatures. Monarch caterpillars currently do better on introduced A. curassavica than on native A. incarnata, but if growing on A. curassavica they will ingest a detrimentally large amount of cardenolides at higher temperatures - the current preferences of the caterpillars could be some kind of ecological trap (Faldwyn et al. 2018). A complicating factor is that monarchs also have to deal with a protozoan parasite, and infected butterflies preferentially oviposit on A. curassavica, its high cardenolide concentrations reducing the effects of the parasite on the next generation (Lefevre et al. 2010). Milkweed butterfly tolerance of and resistance to particular species of parasite depends on the species of Asclepias they are eating; tolerance and resistance are conferred separately by the plant (Sternberg et al. 2012).
Agrawal & Fishbein (2006) looked at the different defence syndromes of Asclepias, and overall the story is very complex. Although the resprouting ability of Asclepias - or simply its tolerance of herbivory - may be an effective defence against specialist herbivores (Agrawal & Fishbein 2008), production of a variety of phenolics changed with herbivory, showing an overall increase even as cardenolide production decreased (Agrawal et al. 2009a). Cardenolides vary in how effective they are in detering potential herbivores, and Züst et al. (2019) noted that structural variation in cardenolides showed around 100 times as much difference in reduced inhibition of the NA+/K+ ATPase in adapted herbivores when compared to non-adapted herbivores; for the reaction of monarch caterpillars to cardenolides in Pachypodium (Malouetieae), see Agrawal et al. (2018). In Asclepias, latex exudation and the activity of the cysteine protease mentioned above are positively correlated, but are negatively correlated with cardenolide concentrations - some kind of trade-off (Agrawal et al. 2008); indeed, overall cardenolide concentration in latex is not related to the amount of latex produced, there may be little cardenolide to be found in latex, but much in leaf tissue, etc. (Züst et al. 2019 and references). One interpretation of the initial diversification of North American Asclepias is that this was accelerated by reduced investment in defensive traits like latex and cardenolide production (Agrawal et al. 2009b). Interestingly, although Agrawal et al. (2008) found that most speciation events in Asclepias were associated with an increase in latex production, overall there was a reduction, the result of a few large changes. Furthermore, more derived milkweeds are likely to have lower amounts of the more toxic cardenolides, and monarch caterpillars grow faster on such plants, even if they sequestered less in the way of cardenolides, and so are presumably more palatable to birds... (Agrawal et al. 2015); suggestions that "the goal to make monarchs more vulnerable to predators" is a driver of such declines make evolution sound positively Machiavellian (Züst et al. 2019: p. 58; see also Agrawal 2017). An additional wrinkle is that monarchs preferentially sequester the more toxic cardenolides, and they can even convert less toxic to the most toxic cardenolides (e.g. Seiber et al. 1980; Züst et al. 2019 and references). Both induced and constitutive cardenolide production - amount and diversity - increase at lower latitudes (Rasmann & Agrawal 2011; Agrawal et al. 2012 for a summary, 2022), although Moles et al. (2011b) suggested that generally such protective compounds decrease at lower latitudes. N-containing cardenolides are potentially very toxic to otherwise resistant insects, having negative effects on adapted sodium pumps, interestingly, less so on non-adapted pumps (Agrawal et al. 2022). However, monarchs can detoxify voruscharin, one of these cardenolides, although at some cost, and lygeid bugs (Oncopeltus) can deal with labriformin, even sequestering the less toxic cardenolides into which that bug converts labriformin (Agrawal et al. 2022: costs to the insect are less clear here). Some lepidoptera that sequester cardenolides have larvae with cardenolide-sensitive Na+/K+-ATPase, but here it was suggested that protecion by the perineurium allowed the organism to remain functional - for two sphingids, the perineurium was either a diffusion barrier for polar cardenolides (e.g. ouabain), probably the normal condition, or it was an active efflux barrier for non-polar cardenolides (e.g. digoxin) (Petschenka et al. 2013).
Farrell and Mitter (1994, 1998) and Farrell (2001) studied the possible co-evolution of the longicorn cerambycid beetle Tetraopes with Asclepias; Phaea is paraphyletic with respect to Tetraopes. This beetle-plant association - cardenolides are involved - is dated to around 20-40 Ma (Farrell & Mitter 1994, 1998). Species in another part of this beetle complex eat Ipomoea, in Convolvulaceae but also laticiferous. More recently-derived beetle species are found on younger species of Asclepias which have more toxic cardenolides... (Pellmyr 2002 for a summary). For more on detoxification of cardenolides, see Groen and Whiteman (2022).
Finally, there is the question of how the presence of defensive compounds like cardenolides in nectar might affect pollination, although little seedms to be known about this. However, P. L. Jones and Agrawal (2016) examined the interrelationship of the two in an experimental setting. Bumblebees, Bombus impatiens, effective pollinators, seemed initially unaffected by nectar that had cardenolide concentrations that were as high as any they would normally encounter, but over time and at the colony level there was some negative effect. Milkweed butterflies, poor pollinators, were unaffected by cardenolides in the nectar, although in subsequent oviposition they showed a preference for plants of Asclepias syriaca with intermediate levels of cardenolides. To say that this whole system is very complex is an understatement.
Burzynski et al. (2015) discuss the rather scattered distribution of pyrrolizidine alkaloids in the family, and note the insects, which include some sphingid caterpillars, that seem to be able to tolerate them. Caterpillars of the two main clades of Nymphalidae-Danainae (Danaini, Ithomiini, milkweed and clearwing butterflies) can be found on Apocynaceae, probably their ancestral host family (Edgar 1984; Janz et al. 2006; c.f. Wahlberg et al. 2009); the danaine clade diverged from other butterfly clades ca 89 Ma (Wahlberg et al. 2009). It has been suggested that only basal Danainae and Ithomiinae sequester pyrrolizidine alkaloids, although caterpillars of monarchs, for example, can also sequester them (Opitz & Müller 2009). Almost all Danaini, some 160 species, are to be found on Apocynaceae (Agrawal et al. 2012; Petschenka & Agrawal 2015), 1,2-dehydropyrrolizidine alkaloids, found in some Apocynaceae, attracting the butterflies, mostly males, which use them as the basis of their pheromones and for defence (Boppré 2005; Brehm et al. 2007s; Hartmann & Ober 2008). Caterpillars of Danainae-Danaini relish Apocynaceae (Ehrlich & Raven 1964; see Ackery & Vane-Wright 1984 for a comprehensive treatment; Brower et al. 2010 for a phylogeny), but they also eat other plants with latex, including several Moraceae and Carica; Danainae-Tellervini also eat Apocynaceae (Wahlberg et al. 2009; Nylin et al. 2013). Most (702/716) records of herbivory by Danainae are from members of the APSA clade, although they also eat taxa in Melodineae, etc. (Livshultz et al. 2018a). There has been a duplication of the deoxyhypusine synthase gene, and one of the copies, the homospermidine synthase (hss) gene, is involved in the synthesis of the pyrrolizidine alkaloids that are found scattered in members of the APSA clade (see also Barny et al. 2021). As with defence genes in the insects, there are widespread molecular-level parallelisms with other plants that have evolved the hss gene (Livshultz et al. 2018a). However, the ability to synthesize these alkaloids had been repeatedly lost, and some Apocynaceae have hss pseudogenes, interestingly, about half of the apocynaceous genera eaten by Danainae caterpillars, specialist herbivores, have these pyrrolizidine alkaloids which otherwise may be a defence against generalized herbivores (Livshultz et al. 2018a). Other Danainae obtain pyrrolizidine alkaloids only as adults and by pharmacophagy of unrelated plants (Beran & Petschenka 2022).
Adult ithomiine butterflies are also attracted to Apocynaceae from which they take up alkaloids, and Edgar (1984) and Brehm et al. (2007) suggested that the pyrrolizidine alkaloids of Echiteae may have been originally involved in these butterflies' pharmacophagous behaviour. (Note that these alkaloids are also found in Crotalaria (Fabaceae), Heliotropaceae and some Asteraceae-Asteroideae.) Larvae of only a few species of ithomiines are found on Apocynaceae, mostly on Echiteae (Edgar 1984: as Parsonsieae). In a comprehensive morphological analysis (Wilmott & Freitas 2006) these apocynaceous-eating ithomiines, which include Tellervo, the only Old World member of the group, came out at the base of the tree (unrooted); ithomiines otherwise eat mostly New World Solanaceae, q.v..
Arctiid moths (e.g. tiger moths, wooly bears; Arctiinae, previously Arctiidae) are another group that forms associations with Apocynaceae (Opitz & Müller 2009), and as with some danaiids the pyrrolizidine alkaloids are the basis for their pheromones - and in Creatonotos there are brush-like scent-producing coremata which can be the length of the moth (Morales et al. 2017a for references). Furthermore, caterpillars of arctiids like Grammia incorrupta self-medicate on pyrrolizidine alkaloid-containing plants, prefering food with more of these alkaloids when they themselves are heavily infected by endoparasites, their survival thereby being enhanced (Singer et al. 2009; see other articles in Conner et al. 2009); Zaspel et al. (2014) discuss self-medication (pharmacophagy) and its evolution by caterpillars of Arctiinae on food containing high concentrations of pyrrolizidine alkaloids. For more on tiger moths, wooly bears and pyrrolizidine alkaloids, see Hartmann (2009). Pyrrolizidine alkaloids and pentacyclic triterpene saponins variously sequestered and modified are also found in the secretions of the defensive glands of some Chrysolina and Platyophora beetles (Chrysomelidae), both very speciose genera (Pasteels et al. 2001; Termonia et al. 2002; Hartmann et al. 2003). Pyrrolizidine alkaloids are sometimes expressed in the root only, where they protect the plant against generalist root feeders, while the absence of the alkaloids in the leaves means they do not attract specialized herbivores (Livshultz et al. 2016).
Hosts of the some 600 species of seed-eating Hemiptera-Lygaeidae-Lygaeinae bugs - a successful group - are predominantly members of the old Apocynaceae (Slater 1976); an association with Apocynaceae is an apomorphy for the family (Bramer et al. 2015; Petschenka et al. 2022). Lygaeids more or less tolerate cardenolides in their diet since they have cardenolide-resistant Na+/K+-ATPase (as evident in its insensitivity to the cardenolide ouabain), and they may also sequester cardenolides as a defence against predators; the two features are probably apomorphies for Lygaeinae, a speciose clade. Lygaeids have moved on to other families that also produce cardenolides, or sometimes on to plants producing other noxious metabolites, perhaps being able to use the same sequestration mechanism in both; some lygaeids are generalists, and may occur on plants that produce no cardenolides (Bramer et al. 2015; Petschenka et al. 2022) - certainly this close association with Apocynaceae is no evolutionary dead end for the lygaeids (Termonia et al. 2001; Bramer et al. 2015).
Aphids (e.g. oleander aphid, Aphis nerii) may also be conspicuous on milkweeds, and here resistance to cardenolides may have evolved rather differently (Zhen et al. 2012). These aphids seem to feed preferentially on internal phloem/adaxial phloem of leaf bundles, which is apparently the cardenolide transport system, so they acquire both food and protection at the same time (Botha et al. 1977). However, which bundles are targeted may also depend on the age of the leaf (Botha et al. 1975). Aphids feeding on Asclepiadoideae may on occasion even induce cardenolide production by the plant (Martel & Malcolm 2004).
Some Malesian species of Dischidia in particular, and also some Hoya, are myrmecophilous. The plants, along with a variety of other angiosperms and some ferns, live in ant gardens that are formed by the activity of ants - the clades of the latter involved have been aged at a mere (7.9-)4.9(-1.9) Ma (Orivel & Leroy 2011; Chomicki et al. 2017a, see also Chomicki & Renner 2015 for dates). In taxa like D. rafflesiana (= D. major) the ants (genera like Iridiomyrmex and the dolichoderine Philidris) inhabit modified leaves that are shaped like the finger of a glove and they bring in dead insects, etc., into the leaf cavity; the roots of the plant grow into the decaying material, the ants feeding the plant as much as protecting it (e.g. Janzen 1974c). Thus ca 39% of the carbon in the leaves in D. major comes from ant respiration and ca 29% of the nitrogen from the debris that the ant brought in (Treseder et al. 1995), and also from ant excreta. Philidris ants may build soil-covered runways between pitchers, extending the effective size of the colony, and they also build plant- and dead arthropod-based partitions within the pichers, using the roots as scaffolding - and the roots have access to nutrients from the partitions; food for the ants includes dead and dying arthropods that they scavenge (Peeters & Wiwatwitaya 2014). Interestingly, bacterium-eating nematodes are not associated with the rubbish dumps inside the domatia, as they are in some other myrmecophytes (Maschwitz et al. 2016). Blatrix et al. (2021) noted that the inside of the pouch/pitcher leaves of D. major had a green (algae - Trebouxia, Trentepohliaceae) and black ("black fungi" - commonly members of ant-plant associations, in this case members of Chaetothyriales and Capnodiales). However, some species of Dischidia are effectively free loaders on this and other plant-ant associations, since although lacking domatia themselves, their roots grow into the domatia of these other plants. Ants may also live under the convex shingle leaves of other species of Dischidia, roots of the plant taking up nutrients from ant excreta, etc. (Zona 2020), while yet other species are quite independent of ants. Although the seeds of Hoya are plumed and so are apparently wind dispersed, they also attract ants that take them to their nests (Janzen 1974c; Weir & Kew 1986).
The African crested rat, Lophiomys imhausii, eats the cardenolide-rich roots and bark of Aconkanthera schimperi (Carisseaea, rauvolfioid), and spreads its poison-containing saliva on its back, where it is taken up by modified hairs. When attacked, the rat displays these hairs, black and white, but if such deterrence is ineffective and it is bitten, the attacking e.g. dog can be killed by the poison in these hairs (Kingdon et al. 2011).
There is an inverse correlation between the presence of leaf surface waxes and that of indumentum, the presence of either making it more difficult for potentially noxious insects to alight on the plant, although other plant traits are also involved (Agrawal et al. 2009c).
Plant-Bacterial/Fungal Associations. Although Paris-type mycorrhizae are found in Gentianaceae, Loganiaceae, and Rubiaceae, Arum-type endomycorrhizae, or intermediates, are common in Apocynaceae, especially in Asclepiadoideae (Imhoff 1999).
Vegetative Variation. Root buds in Aspidosperma are described by Kataoka et al. (2019). The vascular cambium is occasionally storied.
There is discussion as to whether the laticifers are articulated or not (for the latter, see e.g. Endress et al. 1918), but the concensus of recent work is that they are articulated and anastomosing (Gama et al. 2017; see also Ramos et al. 2019; Pirollo-Souza et al. 2019); Agrawal (2017) thought that the whole complex laticiferous system in the plant was made up of only 16 cells. The cork cambium in both Periplocoideae and Asclepiadoideae can be superficial or deep seated, depending on the species (Treiber 1891); that in the root of Aspidosperma is immediately subexodermal (Kataoka et al. 2019). The exudates produced by the colleters may protect meristematic areas against dessication, as well as being fungicides and being able to gum up the feet and/or mouthparts of herbivorous insects (Ribeiro et al. 2017).
Seedlings of genera with opposite leaves, like Hoya, may have spiral leaves. On the other hand, taxa like Absolmsia (= Hoya) spartioides and Vallesia have spiral leaves even at the flowering stage; in the former the leaves are much reduced and the main photosynthetic organ of the plant is the stem/peduncle. Flat foliar vernation is common in the family (Cullen 1978). 10 major stomatal types and 36 subtypes were found on the leaves of Vincetoxicum arnottianum (Nisa et al. 2019).
There is some discussion as to whether Apocynaceae really have/have "real" stipules. In taxa such as Vallesia there are cauline stipules, apparently colleters in a stipular position. Indeed, colleters - the epidermal cells are palisade and produce mucilage - are common in the family (Rio et al. 2022); in Apocynaceae like Alstonia there is an adaxial excavation at the base of the petiole in which the axillary bud is enclosed and this latter is often encased by secretions from the colleters. Rio et al. (2022) found that some colleters in Prestonia coalita were vascularized "which correspond with modified stipules" (ibid. p. 339). Nitra (1950) noted that in Ervatamia (= Tabernaemontana) divaricata each leaf had intrapetiolar stipular flanges innervated by four vascular bundles from the leaf traces. Mandevilla has a distinctive ring of large, radiating, almost fleshy projections encircling the stem immediately below the leaves, and in other taxa there may be adaxial, scale-like structures on the petiole (Thomas & Dave 1991). A variety of stipule-like structures is also found in Stapelia and relatives; in Edithcolea grandis (= Ceropegia sordida) the "stipule" is thought to be represented by a single hair (Bruyns 2000, see also 2004), while there is a variety of hairs and other structures in the stipular position in Caralluma (Bruyns et al. 2010 - all to be included in Ceropegia, see Bruyns et al. 2017). do Valle Capelli et al. (2017) describe a variety of stipule-type structures and associated colleters from species throughout the family (see also Ye and Ronse De Craene et al. 2024).
Vascular variants are common in climbing Apocynaceae (e.g. Cunha Neto 2023 and references), as might be expected, however, there is no synthesis of the literature. Speck et al. (2003) and Lahaye et al. (2005) discuss the evolution of the shrub habit from a viny/lianescent ancestral habit; see Ecology & Physiology above.
Branching in a number of taxa is complex. The apical bud may abort, or be converted into an inflorescence. However, there may be a pair/whorl of very reduced leaves separated by a very short internode from a whorl of normally-developed leaves at the end of each innovation, and these reduced leaves may subtend vegetative branches (as in Alstonia - Mueller 1985) or inflorescences which then appear at first sight to be in an extra-axillary position (Troll & Weberling 1990). However, the "lateral" inflorescences of at least some stapeliads may be displaced-terminal (Bruyns 2004), and such inflorescences are also found in Apocynum, Asclepias, etc., although Leptadenia (Asclepiadoideae-Ceropegieae) does have axillary inflorescences. The terminal inflorescence is evicted, and the continuing growth of the stem is by an axillary meristem. Nolan (1969) described the division of the apical meristem of Asclepias syriaca that resulted in the meristem that produced an inflorescence (not associated with any kind of leaf) and stem as possibly being dichotomous - this structure might have evolved from a monopodial, sympodial, or "primitively" dichotomising ancestor... Although the literature on such inflorescences is quite extensive, e.g. Woodson (1935), Holm (1950), Liede and Weberling (1985) and Steck and Weberling (1982), there is no recent synthesis. Cymose inflorescences of some sort are the norm in the family, and some Marsdenieae like Hoya in particular have very long-lived but contracted umbel-like inflorescences in which single flowers or whorls of flowers open at intervals over a year or more (Meve et al. 2009).
Genes & Genomes. Albers and Meve (2001) discuss the karyology of the family, particularly that of the asclepiads and Periplocoideae. Variation in chromosome length between the subfamilies (1.5-1.0> μm average for the subfamilies) that represents an overall decrease in length within the family as a whole.
In their study of the plastomes of the Hoya group, Rodda and Niissalo (2021; figures below supplemented by those in Odago et al. 2022) noted a number of differences when comparing them with those of other Apocynaceae: The IR was much increased in size, (39,168-)41,241-42,233 bp vs ca 26,000 b.p., and at 2,265-2,306 bp and with only a single gene, the ndhF gene, the small single copy region is almost the smallest known in flowering plants - but see also Lamprocapnos (Papaveraceae-Fumarieae) and Geraniaceae). For more on plastome genes included/lost, GC content, 6 types and 12 subtypes of IR/SC boundaries, etc., see Y. Wang et al. (2022). The much-expanded IR, etc., of the Hoya group may be synapomorphies there; they are apparently not to be found in Marsdenia (= Leichhardtia) flavescens, also sampled by Rodda and Niissalo (2021); Leichhardtia is sister to the Hoya group (Liede Schumann et al. 2022: support could be stronger). Odago et al. (2022: Table 1, but c.f. text) found that the LSC was (80,795-)90,038-92,363 bp long, interestingly, H. thomsonii had the smallest IR and LSC.
Extensive intragenomic polymorphisms in high copy loci were detected in the chondrome of Asclepias, and the total number of polymorphisms was correlated with phylogeny (Weitmier et al. 2015; see also J. Song et al. 2012). A rpll pseudogene (this gene is mitochondrial) is found in the plastid in Asclepiadeae, etc. (Straub et al. 2013), while the chloroplast PEP subunit β rpoC2 gene is present as a mitochondrial pseudogene throughout Asclepiadoideae, and something funny is also going on in Secamone. At 718,734 bp, the chondrome of Hoya lithophytica is the largest in the family (Rodda & Niissalo 2021).
Chemistry, Morphology, etc.. For distinctive fatty acids in the seed, see Badami and Patil (1981). Agrawal et al. (2011) summarize information on cardiac glycosides in the family; they note that the steroidal alkaloids found in Apocynaceae are strictly speaking pseudoalkaloids, since the nitrogen does not come from amino acids.
There is variation in the direction of contortion of the corolla lobes (e.g. Eichler 1874). How the corolla tube is initiated in Periplocoideae is unknown. Much has been written about the homology of the various petaloid floral structures; deciding which structures on different flowers are directly comparable (see Remane's criteria) can indeed be very difficult. If the staminal feet in Periplocoideae are considered similar to the fused basal tube of [Secamonoideae + Asclepiadoideae] in that both are outgrowths of the stamen-corolla tube, then there is a potential synapomorphy for the larger group, or a parallelism if they do not form a single clade (Livshultz et al. 2007). The single staminal bundles of Ceropegia sandersonii stamens are formed by the fusion of petaline bundles from either side of the stamen, and the staminal bundles themselves produce bundles that innervate the carpel walls (Heiduk et al. 2020).
There is quite a lot of variation in anther development and pollen morphology and development in Apocynaceae (e.g. Alves et al. 2024), independent of pollinarium formation, although it is not well integrated into the characterizations above. Alyxieae in particular have grains with large pores, and some species have remarkable barrel-like pollen grains, the pores forming the ends of the barrel (M. Endress et al. 2007a). In Vinceae there is considerable variation in pore number and position, Chonemorpha in particular having remarkable irregular-lumpy pollen grains looking like misshapen potatoes (Livshultz et al. 2018c). Although the surface of the grains is often smooth, it can also be rugose or have projections (Nilsson 1990). Periplocoideae and Secamonoideae are reported to have T-shaped and tetragonal tetrads and simultaneous microsporogenesis; when microsporogenesis is successive, the tetrads are linear, but rhomboidal tetrads are also reported (Safwat 1962; Maheshwari Devi 1964; Omlor 1998; Nilsson et al. 1993; see also Matomoro-Vidal et al. 2014). There has been considerable discussion about the nature of the paired nectaries of Vinca in particular, and because of their vascularization, etc., there are even suggestions that they are modified carpels (e.g. Woodson & Moore 1938; Rao & Ganguli 1963; Fahn 1979); this is unlikely (see also Erbar 2014). When the carpels are connate, placentation may be axile or parietal (for the latter in Allamanda, see Fallen 1985). Syncarpy seems to have evolved more than once in the family, and it is, for example, congenital in Acokanthera and postgenital in Allamanda (Sennblad & Bremer 1996). The carpels may be collateral (Spichiger et al. 2002). An endothelium has been reported in the ovules of Apocynaceae s. str. (Kapil & Tiwari 1978; Cronquist 1981), but it is absent according to Rohwer (1996). There are a few reports of obturators of various morphologies in the family (Morokawa et al. 2015; Demarco 2017b).
Apocynaceae are a much-studied group, e.g. M. Endress and Bruyns (2000), Leeuwenberg (1983: Plumerioideae, 1994), Albers and Meve (2002: enumeration of succulent taxa), Livshultz et al. (2007) and especially M. E. Endress et al. (2018), for much information on Periplocoideae, Secamonoideae, and Asclepiadoideae, see a site run by S. Liede-Schumann and U. Meve while F. d'Alessi and L. Viljoen provide information on stapeliads (= Ceropegia pro parte) in particular; for Fockeeae, see Jankalski (2014), for Marsdenieae, see Liede-Schumann et al. (2022), and for a magnificent revision of southern African stapeliads, see Bruyns (2004). See also Aniszewski (2007) and Colegate et al. (2016), both alkaloids, Demarco and Castro (2008 and references: laticifers), Lens et al. (2008b, 2009c: wood anatomy, to be integrated), Cremers (1973: growth of some lianes), Rajput et al. (2024a: stem anatomy of the climber Gymnema), Glück (1919) and Ribeiro et al. (2017 and references), both colleters. There is much on floral morphology - Fallen (1983: floral morphology and evolution, not asclepiadoids), Allorge (1996: 25 genera), Erbar and Leins (1996b), P. K. Endress (1994b) and Swarupanandan et al. (1996), both Asclepiadoideae, Meve et al. (2009: Marsdenieae), Bruyns (2000) and Bruyns et al. (2012), both stapeliads, Kunze (1990, 1995: Gonolobeae [= Asclepiadeae], 2005a: corona) and Galeto (1997: some Argentinian taxa) - and on pollen - Nilsson (1986), Sampson and Anusarnsunthorn (1990: Parsonsia), Verhoeven and Venter (1994, 2001: useful comparisons), Van der Ham et al. (2001: Alyxieae), Vinckier and Smets (2002b: orbicules), van de Ven and van der Ham (2006: Melodinus, etc.), Van der Weide and Van der Ham (2012: Tabernaemontaneae) and Volkova and Severova (2015: Vinca), and Kunze (e.g. 1993, 1994, 1996) and Demarco (2014), both stamen development, Civeyrel et al. (1998: pollinaria variation), Omlor (1996: translator structure in Periplocoideae and Secamonoideae, 1998: floral morphology and testa anatomy), P. K. Endress et al. (1983) and Shamrov and Gevorkyan (2010b), both gynoecium, Andersson (1931), Anantaswamy Rau (1940b), Venkata Rao and Rama Rao (1954) and Maheshwari Devi and Lakshminarayana (1977), all embryology, Sarikaya and Güven (2024: seed and fruit) and Hillebrand (1872: seed hairs - how different are the two coma types?).
Phylogeny. Both Rauvolfioideae and Apocynoideae are paraphyletic (Sennblad & Bremer 1996, 2002); see Livshultz et al. (2007) for the phylogeny of "Apocynoideae" and Simões et al. (2007a) for that of the basal "Rauvolfioideae", particularly paraphyletic. The relationships of seven tribes in the latter are more or less clear, but those of the remaining five tribes remain to be established; there is good support for Aspidospermateae and Alstonieae as successively sister to all other Apocynaceae (Simões et al. 2007a). Z.-D. Chen et al. (2016) took a comprehensive look at Chinese Apocynaceae and recovered many of the relationships discussed here, although with a few differences, e.g. Nerieae were paraphyletic. Overall relationships in the extensive analysis by L.-L. Yang et al. (2016) are also quite similar to those discussed below, and many support values were quite high. Absences like that of Rhabdadenieae are unsurprising given the geographical focus (China) of the analysis, relationships between Odontadenieae and Echiteae were unclear, Periplocoideae were of uncertain position, and there was some support for a [Marsdenieae + Ceropegieae] clade in Asclepiadoideae. The analysis by Fishbein et al. (2018) produced largely similar results, as did that by Nazar et al. (2019: PHYA + trnL-F), only differences being noted; in the latter study in particular, there was little support for many relationships along the spine. In Nazar et al. (2019) a [Hunterieae + Amsonieae] clade switched places with a [Tabernaemontaneae + Vinceae] clade, depending on the analysis, and Rhabdadenieae, whether or not associated with Maiouetieae, branched off before Periplpocoideae; an [Odontadenieae [Mesechiteae/Echiteae + Apocyneae]] clade branched off separately immediately afterwards. Within Asclepiadoideae there was a [Eustegieae [Asclepiadeae [Marsdenieae + Ceropegieae]] clade. In the analysis by Y. Wang et al. (2022: 101 plastomes, 26/27 tribes, 77 genes), there were substantial differences from these nuclear relationships. In terms of the sequence above, it is relationships in the middle of the tree that differed: ... [Pycnobotyra (weak support) [[Willughbeae + Tabernaemontaneae] [[Vinceae (paraphyletic) + Willughbeae]] [Melodineae (paraphyletic) + Alyxieae]] [[Hunterieae + Amsonieae] ..., furthermore, a subtribe and even some genera were not monophyletic, although Wang et al. (2022) noted that these were known problems. However, in a nuclear analysis using the Angiosperms353 probe set, Antonelli et al. (2021) had recovered relationships like [Apocyneae [Echiteae + Mesechiteae]] (c.f. above - [Echiteae/Odontadenieae, Mesechiteae] [[Rhabdadenieae + Apocyneae]), although they included only 31 Apocynaceae in 23 tribes. Another case of "wait and see".
Apocynoideae, Periplocoideae, Secamonoideae, and Asclepiadoideae, i.e. basically the old Apocynoideae, Periplocoideae/Periplocaceae, and Asclepiadaceae combined, form the APSA clade. A question has been whether or not Periplocoideae were immediately related to [Baisseeae [Secamonoideae + Asclepiadoideae]], nearly all having pollinaria. However, one suggestion was that relationships were [Periplocoideae [[Asian clade + New World clade] [Baisseeae [Secamonoideae + Asclepiadoideae]]], with the position of Rhabdadenia unclear (e.g. Livshultz 2010); she thought that ca 8 times the current amount of parsimony-informative data might solve the problem... Straub et al. (2014) examined plastome sequences of 13 taxa in the APSA clade, and they suggested that that Periplocoideae were not immediately related to the old asclepiads; Fishbein et al. (2018: e.g. Odontadenieae polyphyletic...) came to a similar conclusion, but plastid data suggested a closer relationship of Periplocoideae and the asclepiads. However, the former set of relationships are followed above. Baisseeae are strongly supported as being sister to [Secamonoideae + Asclepiadoideae] (Livshultz 2009, esp. 2010). Below Baisseeae is a polytomy including a largely Asian clade (but including Apocynum) of ex-Apocynoideae, a largely American clade of that group, Rhabdadenia, and Periplocoideae (Livshultz et al. 2007; Livshultz 2010; see also Lahaye et al. 2007).
For a phylogeny of Alyxieae, see M. Endress et al. (2007).
For relationships in Apocyneae, see Livshultz et al. (2018c).
There is small mostly African clade, Baisseeae, in which Dewevrella, with coiled filaments, is sister to the rest - and it is morphologically rather different from them (Livshultz 2009, esp. 2010).
Parsonsia and Echites are included in Echiteae (Sennblad & Bremer 2002); relationships there are discussed by Morales et al. (2017a). Prestonia was the focus of the study by Morales et al. (2017b), who found that the previously-recognized sections were all unsatisfactory.
Burge et al. (2013) clarified relationships within the much-cultivated Pachypodium (Malouetieae); see also Agrawal et al. (2018)
For phylogenetic relationships in Mesechiteae, see Simões et al. (2004, 2006b, 2007b); in an earlier circumscription, the tribe was polyphyletic.
Odontadenieae. Morales and Endress (9 genera, 26 spp, 2 nuclear (ITS) and 7 plastid markers) clarified relationships here, adjusting generic limits accordingly.
Simões et al. (2010, see also 2006a) have clarified relationships within Tabernaemontaneae, i.a. circumscribing Tabernaemontana broadly to include Stemmadenia; major clades within that genus are correlated with geography.
For relationships in Vinceae, see Simões et al. (2016); Kopsia is sister to the rest, although taxa outside basal Apocynaceae were not included.
Within Periplocoideae Phyllanthera is sister to the rest, although only the Australian species has been examined (see also Ionta & Judd 2007; Joubert et al. 2016 and Nazar et al. 2019, in both relationships slightly different; see also Venter & Verhoeven 2001).
Secamonoideae: Secamone may be paraphyletic (Lahaye et al. 2005, 2007: Secamonopsis part of a polytomy, Toxocarpus et al. not included; Nazar et al. 2019: Toxocarpus included, but sampling?).
Surveswaran et al. (2014) found relationships within Asclepiadoideae to be [Fockeeae [Eustegieae + Asclepiadeae] [Ceropegieae + Marsdenieae]], although some support values were not very high, relationships also found by Y. Wang et al. (2022); relationships within New World Asclepiadoideae have been clarified by e.g. Liede-Schumann (2005) and Rapini et al. (2007).
Asclepiadeae. 1. Goyder et al. 2007) suggested that Asclepias was polyphyletic; Asclepias in the New World is sister to an Old World clade of Asclepiadeae within which the Old World Asclepias are embedded (there are some 16 genera in the African Asclepias complex), however, support for the two clades is not strong, and basal relationships within the Old World clade also have little support - and more recently a 3-gene chloroplast tree of the African taxa was most notable in that that most species were part of a 22-tomy... (Chuba et al. 2017). A study focussing on New World Asclepias found that Trachycalymma was sister to both the Old and New World clades, although none of these relationships had much non-parametric bootstrap support (Fishbein et al. 2011); in Goyder et al. (2007) Trachycalymma was embedded within the Old World clade. 2. Relationships around Astephaninae are unclear (Liede 2001). 3. In Cynanchinae, for the delimitation and relationships of Cynanchum itself, see Liede and Kunze (2002), Liede and Täuber (2002) and especially Khanum et al. (2016). 4. For the Gonolobus area, see Krings et al. (2008); Gonolobinae are proving difficult, conflicting topologies perhaps being due to incomplete lineage sorting (McDonnell et al. 2018). 5. Silva et al. (2012) found that most genera of Metastelmatinae are not monophyletic. 6. Liede-Schumann and Meve (2024) looked at relationships around Orthosia (Orthosiinae) (37 spp., 6 plastid markers) and found that with slight adjustments it, Scyphostelma and Jobina were monophyletic (see also Fishbein et al. 2018). 7. Vincetoxicum is not monophyletic (Yamashiro et al. 2004), however, Liede-Schumann et al. (2012) clarified relationships in the whole Tylephorinae, to which it belongs; see also M. Liao et al. (2022: 2 mitochondrial and 5 chloroplast genes).
Within Ceropegieae relationships are complex, Ceropegia itself occurring all over the tree and Brachystelma being embedded in it (Meve & Leide 2001, 2002a; Meve & Liede-Schumann 2007; Ribeiro et al. 2014; Bruyns et al. 2015, 2017: important taxonomic paper; Meve et al. 2016); the genera had been delimited using gross floral morphology. The stapeliads are monophyletic, but are embedded in Ceropegieae (Bruyns et al. 2014a, 2015, 2017), and the general relationships are [Heterostemma/Heterostemminae [Leptadeniinae [Anisotominae + Stapeliinae]]] (Meve et al. 2016; Bruyns et al. 2022). Livshultz et al. (2013) outlined relationships within Marsdenieae; the Old World Hoya and Dischidia are fairly close but could be separated by morphological features, possibly apomorphies, such as pollinarium morphology, inner guide rail presence/absence, and nectary position (Wanntorp & Kunze 2009). However, whether Dischidia is sister to or embedded within Hoya remained unclear (Wanntorp et al. 2014; Rodda et al. 2020: the position of Oreosparte is also unclear). Estimates of species numbers in these two genera in particular vary widely, although Hoya is indeed very speciose; see also Wanntorp et al. (2006a, b, 2009) for phylogenies, Wanntorp and Forster (2007: floral morphology in general), Kunze and Wanntorp (2008a: corona and anther skirt) and Kunze and Wanntorp (2008b: focus on Hoya spartioides). Relationships along the backbone of Hoya remained poorly resolved (Wanntorp et al. 2011a), although Wanntorp et al. (2014: 154 spp., 2 plastid and 2 nuclear markers) recovered a number of (quite) well supported clades that included most of the species they examined. Do Espírito Santo et al. (2019) focussed on relationships in New World Marsdenieae and confirmed that Marsdenia was polyphyletic; although the positions of Rhyssolobium and two species of Marsdenia depended on the particular analysis, they placed the New World species of Marsdenia in Ruehssia. Liede-Schumann et al. (2022: 5 nuclear and four chloroplast markers) looked at some 171 species of Marsdenieae from throughout the range of the tribe, and they recovered three main clades, the Asian-Pacific, African-Madagascan and Cosmopolitan clades; the position of the second clade was unclear. In general, earlier results were confirmed, thus Marsdenia was polyphyletic, species occurring in all three main clades, and Hoya was paraphyletic, Dischidia being embedded in it, while [Papuahoya + Oreosparte] were sister to the combined group, although support values around there were generally rather low (Liede-Schumann et al. 2022). Interestingly, Rodda and Niissalo (2021) had found the relationships [Papuahoya [Dischidia [Hoya (= Clemensiella s. str.) [Oreosparte + the rest of Hoya]]]] in an analysis of plastome exons 90≤ bp long including 40 species of the Hoya group. These relationships were also recovered by Odago et al. (2021: 72 protein-coding chloroplast genes, 55 taxa), although support was not always strong, and they also recovered similar species groups within Hoya, although H. ignorata and H. exilis did not link with any group.
Classification. The suprageneric classification here is largely based on that of M. Endress et al. (2007a, esp. 2014, 2018; see also Endress & Bruyns 2000; summary in Nazar et al. 2013); for subtribes, see also Fishbein et al. (2018: Asclepiadoideae). No attempt here has been made to develop a rational classification throughout the family since this must await clarification of relationships, e.g. at the base of the tree, within the old Rauwolfioideae, but given that groups like Asclepiadoideae and Periplocoideae are well embedded in the tree, this is going to be quite a challenge.
Generic limits need much attention in Apocynaceae (see e.g. Liede & Täuber 2000; Liede et al. 2002; Rapini et al. 2003; Goyder et al. 2007; Meve & Liede-Schumann 2007). For genera - and subtribes - in Apocyneae, see Livshultz et al. (2018c), for generic limits in Tabernaemontaneae, see Simões et al. (2010), and for an infrageneric classification of Prestonia, see Morales et al. (2017b). Generic limits in Asclepiadoideae are particularly difficult, and current genera can seem to be distinguished by floral minutiae of dubious significance. Asclepias itself is definitely polphyletic, the New World clade including the type of the genus (Goyder et al. 2007), but morphology alone is not clarifying relationships too much; Goyder (2009) provisionally included even Trachycalymma (see above) in an admittedly unsatisfactorily circumscribed African Asclepias, while Chuba et al. (2017: p. 148) note that "the species comprising the African Asclepias complex cannot be readily partitioned into monophyletic genera", and thought that extending the limits of Asclepias might be the way to go. Liede-Schumann et al. (2012) extended the limits of the Old World Vincetoxicum to include Tylophora (see Liede-Schumann & Meve 2018 for the formal reclassification). Ceropegieae are another very difficult area, indeed, various genera are embedded within Caralluma, in turn embedded in Ceropegia (Meve & Leide 2001, 2002; Meve & Liede-Schumann 2007; Bruyns et al. 2010: see de Kock & Meve 2007 for a checklist). Bruyns et al. (2015: p. 50) noted that Surveswaran et al. (2009) "found that Brachystelma is not monophyletic and also that Ceropegia is not monophyletic unless Brachystelma and the stapeliads are included in it"; they found the same relationships, and seemed to be inclining to an enlarged Stapelia with around 670 species. For the same classificatory conclusion formally codified, but under Ceropegia, see Bruyns et al. (2017), q.v. for a discussion of the problem and the 63 sections that they recognized in Ceropegia. For subtribes here, see Bruyns et al. (2022) and references. Liede and Täuber (2000) included Sarcostemma in Cynanchum (Asclepiadeae), and the limits of the latter have reasonably been drawn quite widely (Khanum et al. 2016). For genera in Asclepiadoideae-Marsdenieae, see Omlor (1998), Meve et al. (2009) and in particular Liede-Schumann et al. (2022); it was early realized that to make Marsdenia monophyletic, the whole tribe, which includes Hoya, etc., would have to be just a single genus (Livshultz et al. 2013), or... However, Do Espirito Santo et al. (2019) moved 110 species of New World Marsdenia into Ruehssia, combinations being made for the Brazilian taxa. Liede-Schumann et al. (2022) largely confirmed this classification, Marsdenia being reduced to three, perhaps eight, species, and they noted (as have others) that Hoya and Dischidia were not separating cleanly, Dischidia being well embedded in Hoya. For a very useful generic synonymy of Periplocoideae, Asclepiadoideae and Secamonoideae, see http://www.bio.uni-bayreuth.de/planta2/research/databases/delta_as/triblist.html
Thanks. I thank M. Endress for comments.