EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID I / FABIDAE / [ZYGOPHYLLALES [the COM clade + the nitrogen-fixing clade]]: endosperm scanty.
[the COM clade + the nitrogen-fixing clade]: ?
[FABALES [ROSALES [CUCURBITALES + FAGALES]]] / the nitrogen-fixing clade / fabids: (N-fixing by associated root-dwelling bacteria); tension wood +; seed exotestal.
[ROSALES [CUCURBITALES + FAGALES]]: (N-fixing bacteria actinomycetes, nodules branched, vasculature central [pericyclic in origin])/0; styles separate; ovules 1-2/carpel, apical.
Age. The age of this node is thought to be ca 115 Ma (Gu et al. 2024).
[ROSALES + CUCURBITALES]: (N-fixing bacteria actinomycetes, nodules branched, vasculature central [pericyclic in origin])/0; styles separate; ovules 1-2/carpel, apical.
Age. This node is estimated to be (110-)105(-100) or (94-)89(-84) Ma (H. Wang et al. 2009); a very different age of around 429-199 Ma is suggested by Jeong et al. (1999), while ages of around 92.2-78.9 Ma are offered by Naumann et al. (2013), of (110-)97(-92) Ma by Bell et al. (2010: c.f. topology), about 106.4 Ma by Tank et al. (2015: Table S1), around 107.4 Ma by Hohmann et al. (2015), about 113 Ma by Foster et al. (2016a: q.v. for details), ca 126 Ma by Z. Wu et al. (2014) and about 102 Ma by J.-Y. Xue et al. (2020).
Fossils of Prunus [sic] aspenensis from Wyoming and thought to be 113.0-100.5 Ma are considered to represent stem Rosaceae (Gu et al. 2024) - a position around about here follows...
Evolution: Divergence & Distribution. It is difficult to optimize ovule number in this part of the tree; see also D. W. Taylor et al. (2012) for possible apomorphies. The character "fruit indehiscent" could be placed at this node rather than the next one up, but since the gynoecial morphology at the two nodes is likely to be rather different, I did not place the dehiscence feature here.
Ecology & Physiology. Nitrogen fixing in this clade usually involves symbioses with the actinomycete Frankia (= β-rhizobia of some) rather than with rhizobia, and this is discussed further on the Fabales page.
Plant-Bacterial/Fungal Associations. The actinorhizal Frankia, filamentous and more or less aerobic, forms nodules on a number of taxa in the [Rosales [Fagales + Cucurbitales]] clade. The bacterium enters through the middle lamella of intact adjacent epidermal cells, a rare mode of entry in Fabaceae (Venado et al. 2020, q.v. for more details of the whole process). The nodules are indeterminate branched/coralloid structures, develop in the pericycle and appear to be modified lateral roots, although they lack root caps and have superficial cork cambium (Pawlowski & Demchenko 2012; H.-L. Li et al. 2015; S. Liu et al. 2020). The nodules have central vascular tissue surrounded by cortical cells containing the bacteria; nodule development definitely has similarities with root development, and a root may even develop from the tip of the nodule (Shen et al. 2020). The cortical tissue may be made up of infected cells alone (Cucurbitales) or of a mixture of infected and uninfected cells as in Rosales and Fagales (S. Liu et al. 2020). For more on N-fixation in this clade, see papers in Adv. Bot. Research 94. 2020.
Normand et al. (2006) discuss the considerable gene/genome divergence within Frankia. Strains of Frankia form four main clusters, of which three are associated with N-fixing plants in this clade (Pawlowski & Demchenko 2012; van Nguyen et al. 2019). Strains of cluster I nodulated only members of Fagales, those forming cluster II, sister to all other strains in the genus, nodulated Cucurbitales and some Rosales, and strains forming cluster III nodulated members of Rosales and also two members of Fagales (Clawson et al. 2004; van Nguyen et al. 2019); no members of cluster IV are nodulators. Interestingly, in some cluster II infections, two or more strains could be isolated from the one plant. Although the common symbiotic signaling pathway is involved in plant infection, some strains in all three clusters lacked the ability to produce lipochitooligosaccharides, i.e. no NOD signaling factors, but they are common in cluster II (Pawlowski & Demchenko 2012; van Nguyen et al. 2019; Rutten et al. 2020). Details of infection vary, whether occurring via a root hair or by penetration via the anticlinal walls of epidermal cells, and the morphology/anatomy of the nodules also vary considerably (Pawlowski & Demchenko 2012). For more information about Frankia, see papers in Pawlowski and Newton (2008) and de Bruijn (2015).
Rose (1980) noted that vesicular-arbuscular mycorrhizae co-occurred on all plants with actinorrhizal Frankia nitrogen fixation that she studied - seven families, with 7+ origins of the association, and in four there were also/only associations with ectomycorrhizal fungi.
Phylogeny. For relationships, see above.
(Isoflavonoids, dihydroflavonols +); mucilage cells +; roots diarch [lateral roots 4-ranked]; prismatic crystals in ray cells; (sieve tubes with non-dispersive protein bodies, lacking starch or protein inclusions); inflorescence cymose; hypanthium +, nectariferous, K valvate, C clawed; stigma dry; ovule 1/carpel, epitropous, micropyle endostomal; fruit indehiscent, K and/or hypanthium persistent; (polyembryony +); 4bp duplication near 3' end of chloroplast rbcL gene. - 9 families, 263 genera, 8,010 species.
Includes Barbeyaceae, Cannabaceae, Dirachmaceae, Elaeagnaceae, Moraceae, Rhamnaceae, Rosaceae, Ulmaceae, Urticaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) dated crown Rosales at (79-)76(-73) Ma, while other estimates are rather older: (96-)93, 88(-85) Ma (two penalized likelihood dates, Bayesian relaxed clock estimates to 103 My: H. Wang et al. 2009), ca 94 Ma (Magallón & Castillo 2009), (104-)85, 82(-73) Ma (Bell et al. 2010) and ca 97 Ma (Z. Wu et al. 2014). However, the estimate in Xue et al. (2012) is only 40.8 or 31.9 Ma, that in Hohmann et al. (2015) is ca 87.7 Ma, in Tank et al. (2015: Table S2) is ca 89.4 Ma, in Q. Zhang et al. (2018) is (107-)105.5(-102.8) Ma, and in H.-L. Li et al. (2015) it is (112.6-)106.5, 106.1(-100.2) Ma, in X. Chen et al. (2020) it is (97.0-)93.5(-89.8) Ma, while in J. Zheng et al. its is (124.5-)113.8(-103.7) Ma and in Gu et al. (2024) it is ca 106.9 Ma. An early estimate in Jeong et al. (1999) is 367-170 Ma, so take your pick.
Evolution: Divergence & Distribution. Rosales contain ca 1.9% of eudicot diversity (Magallón et al. 1999). A. G. Simpson et al. (2022) looked at diversification in the order as a whole, although the focus on the genus "level" and the fact that the tree used (ibid. Fig. 2, but c.f. Fig. 1) had very extensive (near) polytomies made life rather difficult.
Ronse De Craene (2003, see also 2010) suggested that the absence of petals might characterise Rosales, the "petals" that were apparently there occupying the position of stamens and being modified stamens, their evolution allowing e.g. Rosaceae to diversify. However, if Rosales are sister to Fabales, as Ronse de Craene (2003) thought, the latter are not a notably diverse group in terms of species numbers, the more so since almost 4,000 species of Rosales - about half - are in the Ulmaceae-Urticaceae group, which lack petals of any sort. This latter group forms a well-supported clade, and the wind pollination that is so common there cannot be considered basic to Rosales as a whole (c.f. Ronse de Craene 2010). Even with the relationships [Fabales [Rosales [Cucurbitales + Fagales]]] (see above) it seems that no argument connecting petals in Rosales with its diversity can be made (see also Ronse de Craene & Brockington 2013).
A granular exine infratectum may be a synapomorphy for the clade (see also Doyle 2009); here it is placed as a synapomorphy for the [Ulmaceae [Cannabaceae [Moraceae + Urticaceae]]] clade, but it is found elsewhere in the order, too. Jiang et al. (2019) discuss the evolution of aspects of pollen morphology throughout the order; I have not yet attempted to integrate their work here.
Ecology & Physiology. Van Velzen et al. (2018) suggest that there may have been a change in symbiont from Frankia to rhizobium in a recent ancestor of Parasponia andersonii (but see below under Cannabaceae-Parasponia) in addition to repeated losses of the ability to fix N in other Rosales (and Fagales and Cucurbitales), rather than independent acquisitions of N-fixation via Rhizobium here and in Fabaceae; it is almost as if some underlying tendency were involved (e.g. Soltis et al. 1995b; Werner et al. 2014). For a summary of what is known about N fixation by actinorhizal plants in in this clade, see Santi et al. (2013), also references above, for rhizobial N fixation in Parasponia, see Cannabaceae, and for N-fixation in general, see elsewhere.
Plant-Animal Interactions. Quite a number of butterfly larvae - especially caterpillars of "basal" groups and Lycaeninae - feed here (Fiedler 1995; Janz & Nylin 1998) - see also below, especially Rosaceae.
Plant-Bacterial/Fungal Associations. Some taxa in at least Rosaceae, Rhamnaceae, Elaeagnaceae and Ulmaceae are ectomycorrhizal (see Malloch et al. 1980; Rose 1980; S. E. Smith and Read 1997).
Genes & Genomes. The position of the duplication that resulted in two copies of the granule bound starch synthase I (gbss I) nuclear gene is unclear; two copies are found in all Rosaceae and also in Frangula (Rhamnaceae) (Evans & Campbell 2002).
Soltis et al. (1995b) noted that there was a duplication in the chloroplast rbcL gene; it was absent from the other rosids they examined.
Chemistry, Morphology, etc.. Roots are commonly diarch in Rosaceae, but are also tetrarch, etc.; sampling elsewhere is poor, although less so in Ulmaceae and relatives, and diarch roots are found throughout the order. Tracheary members in Rosaceae commonly have pseudotori (thickenings in pit membranes associated with plasmodesmata), while true tori occur in Rosaceae (Cercocarpus) and also Cannabaceae and Ulmaceae (Jansen et al. 2007; Coleman et al. 2004; Dute 2015), although these tori are formed in two different ways (Dute et al. 2010a) and the torus is only weakly lignified. Sieve tube plastids lacking both starch and protein inclusions are quite common in Rosales, although they are rare outside (Behnke 1991a) and the sieve tubes quite often have non-dispersive protein bodies, although not in Rhamnaceae (Behnke 1973).
Mucilage cells of various kinds are quite commonly reported from the flowers, although not in families like Rosaceae and Moraceae (de Barros et al. 2023). For polyembryony, sporadic here, see G. Dahlgren (1991).
Kubitzki (2004) provides a summary of the order; there is much useful information in Thulin et al. (1998).
Phylogeny. In early molecular studies Rosaceae appeared as sister to the rest of the order (strong support: Savolainen et al. 2000a; H. Wang et al. 2009), while Ulmaceae and relatives (the old Urticales) and Rhamnaceae and relatives formed two clades (also Thulin et al. 1998; Savolainen et al. 2000b; Richardson et al. 2000b; Sytsma et al. 2002: position of Rosaceae, etc., uncertain; Wang et al. 2009; Soltis et al. 2011), as in the tree below. The position of Elaeagnaceae was sometimes rather labile (Richardson et al. 2000b: successive approximation weighting), even being embedded in Rhamnaceae in a rbcL analysis, and there was quite strong support for a clade [Barbeyaceae + Dirachmaceae]. S.-D. Zhang et al. (2011) looked at variation in twelve genes (10 from the plastids) in 25 taxa of Rosales, and the major relationships within the order are very well supported, although somewhat less so in the subclade that includes Rhamnaceae; the relationships that Zhang et al. (2011) found are followed here. This grouping had also been suggested by C. S. Campbell (pers. comm. 2003), Sytsma et al. (2002: support weak).
Relationships in part of Rosales in fact remain unclear. Richardson et al. (2000a) thought that Rhamnaceae, Barbeyaceae and Dirachmaceae might form a clade, and although this was not confirmed by Zhang et al. (2011), the maximum parsimony bootstrap values for the relationships there are not strong, nor is the maximum likelihood support for [Elaeagnaceae [Barbeyaceae + Dirachmaceae]], although posterior probabilities are all 1.0. H.-L. Li et al. (2015) and M. Sun et al. (2016) found weak support for the relationships [Dirachmaceae [Rhamnaceae [Barbeyaceae + Elaeagnaceae]]], while relationships in the rather exiguous sample of Z. Wu et al. (2022) - although this was not the focus of their work - were [Rosaceae [Elaeagnaceae [Rhamnaceae + Cannabaceae, etc.]]]. H.-T. Li et al. (2021: plastome sequences) found the relationships [Rosaceae [[Rhamnaceae [Elaeagnaceae [Barbeyaceae + Dirachmaceae]]] [Ulmaceae, etc.]]]. In the Seed Plant Tree of Life (i.2022 version) relationships were [Rosaceae [Barbeyaceae [[Rhamnaceae [Elaeagnaceae + Dirachmaceae]] [Ulmaceae, etc.]]]], although support for the [Rhamnaceae etc.] clade was not strong; another suggestion is [Rosaceae [Dirachmaceae *[[Rhamnaceae [Barbeyaceae + Elaeagnaceae]] [Ulmaceae, etc.]]]] (Zuntini et al. 2024; Seed Plant Tree of Life ix.2024 version - see Sun et al. 2016 above).
For relationships in the Ulmaceae-Moraceae area, see the phylogenies suggested by Sytsma et al. (2000) and Song et al. (2001), and they find that Cannabis is in the old Celtidaceae (see also e.g. Ueda et al. 1997b); Cannabaceae is the earliest name for the combined group. They also place Cecropia within Urticaceae, and this set of relationships has been strongly supported by a more comprehensive analysis (Sytsma 2002) and other studies since. See H.-L. Li (2015), M. Sun et al. (2016) and Z.-D. Chen et al. (2016) for quite comprehensive molecular phylogenies of the whole group, Judd et al. (1994) for a morphological phylogeny, and Zavada and Kim (1996) for a molecular phylogeny with a focus on the old paraphyletic Ulmaceae s.l.. However, the rather surprising relationships found by Z.-Y. Wu et al. (2018) were [[Cannabaceae [Ulmaceae + Moraceae]] [Gironniera + Urticaceae]].
There is also the issue of the placement of the holoparasitic Cynomoriaceae. Z.-H. Zhang et al. (2009; see also Moore et al. 2011) placed Cynomoriaceae in Rosales and sister to Rosaceae based on analyses of chloroplast inverted repeat sequences; support was strong, but Moraceae were the only other family in the order examined. However, there are problems with this analysis (see Bellot et al. 2016) and Cynomoriaceae are here included in Saxifragales, whether sister to the rest of the order or embedded in it, q.v. for further details of relationships.
Previous Relationships. In the past, Urticales (Urticaceae, Moraceae, etc.) were kept well separate from Rosaceae, largely because their very reduced and usually wind-pollinated flowers suggested relationships to the old Amentiferae.
Synonymy: Rhamnineae Shipunov - Amygdalales Link, Artocarpales Martius, Barbeyales Takhtajan & Reveal, Cannabales Döll, Dryadales Link, Elaeagnales Berchtold & J. Presl, Ficales Dumortier, Frangulales Wirtgen, Morales Martius, Rhamnales Link, Sanguisorbales Link, Spiraeales Link, Ulmales Link, Urticales Berchtold & J. Presl - Barbeyanae Reveal & Doweld, Rhamnanae Reveal, Rosanae Takhtajan, Urticanae Reveal - Frangulopsida Endlicher, Rhamnopsida Brongniart, Rosopsida Batsch, Urticopsida Bartling - Rosidae Takhtajan
ROSACEAE Jussieu, nom. cons. - Back to Rosales
Triterpenes +, alkaloids 0; cork deep seated; (vessel elements with scalariform perforation plates); (true) and fibre tracheids +; petiole vasculature of arcuate or annular bundles, or annular; leaf cuticle waxes as clustered tubules; leaves spiral (opposite), lamina vernation usu. conduplicate, teeth hydathodal/water pores, (secondary veins palmate), stipules often petiolar; inflorescences racemose (determinate); flowers protogynous or condition ± undecided; (C 0); A ca 20 common, = 10 in parapetalous pairs + 5 + 5; (pollen porate); G free, stigmas punctate to expanded, or decurrent down stylulus, compitum 0; ovules with parietal tissue 2-4 cells across, nucellar cap ca 4 cells across; megaspore mother cells several; fruit aggregate of achenes; exotestal cells periclinally elongated, radial walls thickened, or palisade or tabular, walls with spiral or reticulate thickenings, outer wall often becoming mucilaginous, endotegmic cells slightly thickened, or seed coat undistinguished; chalazal endosperm haustorium +; x = 7, nuclear genome [1 C] (0.065-)0.712(-7.808) pg/[2 C] (0.2-)0.42-3.0 pg, duplication of GBSSI [granule bound starch synthase I] gene.
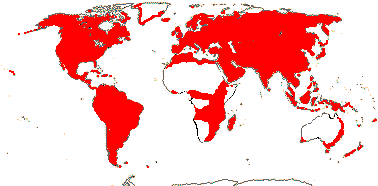
92 [list]/2,805 [species numbers in particular ± notional]. World-wide, but esp. N. hemisphere, often not deserts or tropical rainforest. Map: from Vester (1940: overly optimistic - or inc. Chrysobalanaceae?), Hultén (1971), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 2 (2006) and FloraBase and Australia's Virtual Herbarium (both consulted i.2013). Photos: Collection, Collection.
Age. Crown-group Rosaceae are dated to (92.8-)88.3(-84.2) Ma (Chin et al. 2014), around 61.2 Ma (Hohmann et al. 2015), or as little as ca 46 Ma (Murat et al. 2015b); also 76.9 Ma (Dobes & Paul 2010), or as much as 108-93 Ma (Töpel et al. 2012), (103-)92.2(-85.8) Ma (Gehrke et al. 2015) or ca 101.6 Ma (Y. Xiang et al. 2016) - see also S.-D. Zhang et al. (2017) for more dates and who offer an estimate of ca 95.1 Ma themselves, X. Chen et al. (2020) who suggest an age of (96.0-)89.6(-79.6) Ma, J. Zhang et al. (2021) an age of (108.7-)92.2(-81.7) Ma and W. Su et al. (2021) an age of only 67.7-41.3 Ma.
Turonian fossils some 90 Ma old are assignable to Rosaceae (Crepet et al. 2004 for references).
Age. Divergence at this node is dated to (96.1-)87.6, 86.1(-63.3) Ma (H.-L. Li et al. 2015), (94.3-)92.9(-92.1) Ma (S.-D. Zhang et al. 2017), (78.3-)69.8(-61.3) Ma (X. Chen et al. 2020) or ca 94.5 Ma (Gu et al. 2024).
1. Dryadoideae Juel —— Synonymy: Cercocarpaceae J. Agardh, Dryadaceae Gray
Roots with N-fixing Frankia, (ectomycorrhizal); sugar alcohol sorbitol [carbohydrate transport], cyanogenic glycosides [dhurrin] +; (torus-margo pits + - Cercocarpus [= C.]); leaves usu. simple; C (0 - C., = A); A often many; G 1-many, on ± flat receptacle; ovules straight (anatropous, apotropous - Dryas), (with chalazal projection - C.); styles persistent, hairy (0); n = 9.
4/19: Cercocarpus (8). W. North America, Dryas circumboreal.
Age. Crown-group Dryadoideae are dated to ca 73 Ma (Zanne et al. 2014; see Tedersoo & Brundrett 2017), (83.2-)63.1(-44.7) Ma (Chin et al. 2014), (48.7-)40.7(-39.7) Ma (S.-D. Zhang et al. 2017), ca 38 Ma (Y. Xiang et al. 2016) or (48.9-)39.3(-34.4) Ma (X. Chen et al. 2020).
2. Rosoideae Arnott
Herbs to shrubs; 2-pyrone-4,6dicarboxylic acid, ellagic acid +; rays often narrow; cuticle waxes as narrow ribbons and triangular rodlets; leaves usu. compound; (epicalyx +), G many, on ± raised receptacle; fruits achenes; ovule (straight), unitegmic; x = 7; plant with phragmidiaceous rusts.
21/2,187. Especially temperate (to Arctic) areas.
Age. Crown-group Rosoideae are dated to (83.2-)71.1(-64.3) Ma (Chin et al. 2014) or ca 82 Ma (Y. Xiang et al. 2016); other dates are 66.5-50 Ma (Dobes & Paul 2010), (78.1-)75.8(-74.5) Ma (S.-D. Zhang et al. 2017), 95-70 Ma (Topel et al. 2010), (75.8-)65.5(-55.2) or 43.8-36.5 Ma (Gehrke et al. 2015) and (78.3-)69.8(-61.3) Ma (X. Chen et al. 2020).
2A. Ulmarieae Lamarck & de Candolle - Filipendula Miller —— Synonymy: Ulmariaceae Gray
Plant herbaceous; receptacle enlarged, nectary 0; ovules 2/carpel, superposed.
1/10. Eurasia.
Age. Crown-group Ulmarieae are (26.3-)13.5(-5.1) Ma (X. Chen et al. 2020).
Rosodeae T. Eriksson, Smedmark, & M. S. Kerr / [Rubeae [Colurieae [Roseae [Potentilleae + Agrimonieae]]]]: ?
Age. This node can be dated to ca 75 Ma (Y. Xiang et al. 2016), ca 61.6 Ma (X. Chen et al. 2020: Colurieae sister to rest) or ca 65≤ Ma (Carter et al. 2019: Coluria sister to Rubus).
2B. Rubeae Dumortier - Rubus L. —— Synonymy: Chamaemoraceae Lilja
Scrambling shrub/herbs, prickly/not; plant deciduous (not), leaves (simple), stipules free from petioles/adnate; (plant dioecious); A (20-)many; receptacle enlarged, stigma capitate (slightly 2-lobed); ovules 2/carpel, collateral, 1 aborts, integument ca 6 cells across; fruit an aggregate of drupelets.
1/1,732, inc. apomicts. ± Worldwide, esp. N. Temperate.
Age. Crown-group Rubeae are estimated to be (32.3-)18.4(-9.0) Ma (X. Chen et al. 2020) or around 20 Ma (Carter et al. 2019).
Fossils of Rubus are anything from Eocene in age, ca 55 Ma, or younger (Graham 2018).
[Colurieae [Roseae [Potentilleae + Agrimonieae]]]: ?
2C. Colurieae Rydberg
A (many); ovule apotropous [Geum]; hypanthium (with hooks); seed coat vascularized [Waldsteinia].
3/42: Geum (40). Temperate, inc. montane tropics, Chile.
Age. Crown-group Colurieae are estimated to be (59.1-)44.9(-28.5) Ma (X. Chen et al. 2020).
[Roseae [Potentilleae + Agrimonieae]]: ?
Age. The age of this clade is (86.2-)73.8(-61.2) Ma (Töpel et al. 2012) or ca 50.5 Ma (Debray et al. 2021).
2D. Roseae Lamarck & de Candolle - Rosa L.
Shrub, often ± climbing, prickly; A (30-)many; fruit with hypanthium fleshy, urn-shaped, nectary poorly developed; carpellary vascular supply recurrent; extragynoecial compitum +; integument ca 8 cells across.
1/ca 150(?-200). N. temperate, also N.W. Africa, most Asian.
Age. The crown-group age of this clade is (19.7-)10.4(-4.7) Ma (X. Chen et al. 2020) or ca 21.5 Ma (Debray et al. 2021).
[Potentilleae + Agrimonieae]: ?
Age. The age of this node is ca 62 Ma (Y. Xiang et al. 2016).
2E. Potentilleae Sweet
Herbs, usually perennial, (shrubs); (stipules with adaxial flange - ligular stipules); (epicalyx +); receptacle enlarged; (anthers [?pseudo]unithecal); integument ca 4 cells across.
18-19/1,740. N. temperate to Arctic (montane tropics to S. temperate)
Age. Crown-group Potentilleae are aged at 50-25 Ma (Gehrke et al. 2015), (52.4-)44.9(-36.1) Ma (Feng et al. 2017) and (50.1-)45.0(-38.4) Ma (X. Chen et al. 2020).
2E1. Potentillinae J. Presl —— Potentillaceae Berchtold & J. Presl, Tormentillaceae Martynov
(A 1), (extrorse); style often lateral/gynobasic.
5-6/540: Potentilla + Argentina et al. - Potentilla s.l. (505). N. temperate to Arctic (montane tropics to S. temperate).
2E2. Fragariinae Torrey & A. Gray —— Synonymy: Alchemillaceae Martinov, Fragariaceae Nestler
(Leaves simple); (flowers 4-merous); C (0); A (1, 4, 5, etc.), thecae more or less confluent; parietal tissue ca 2 cells across; (G 1); (receptacle massive, fleshy); nucellar cap ca 7 cells across; phragmidiaceous rusts 0 (+, F.); x = 7, n = 7(-8 ... 78).
13/1,200: Alchemilla (734(-1000+?)), Fragaria (25). N. temperate, esp. Europe, tropical mountains, some S. temperate.
2F. Sanguisorbeae de Candolle / Agrimonieae Lamarck & de Candolle - earlier?
A 1-many; pollen (6-colpate), surface striate or microverrucate, colpi operculate; G 1-5; (2 ovules/carpel); integument 6-8 cells across; phragmidiaceous rusts 0.
12/274. ±Worldwide, esp. temperate conditions.
Age. The crown-group age of this clade is (49.2-)40.6(-30.4) Ma (X. Chen et al. 2020), ca 40 Ma (Jauregui-Lazo & Potter 2021) or ca 37 Ma (G.-J. Zhang et al. 2022).
2F1. Agrimoniinae J. Presl —— Synonymy: Agrimoniaceae Gray
A folded in bud; nectary 0; stigma expanded, margin digitate; integument 1.
5/20: Agrimonia (15). N. Temperate, Africa.
Age. Crown-group Agrimoniinae are (30.6-)22.3(-15.0) Ma (G.-J. Zhang et al. 2022).
2F2. Sanguisorbinae Torrey & A. Gray —— Synonymy: Poteriaceae Rafinesque, Sanguisorbaceae Berchtold & J. Presl
Herbs to small trees, (ericoid shrubs), (short shoots +); periderm anatomy/stem protection various, periderm initiated deep in the cortex, phellem cells with dark contents; (leaves simple/bifolioliate), (stipules sheathing/0); plant monoecious/dioecious; inflorescence capitate/densely spicate (racemose); flowers (2-)4-merous; C 0; A 1-many, filaments twisted in bud; pollen hexacolpate; G 1(-3), (inferior - Polylepis), hypanthium enclosing G, stigma conspicuous, capitate-peltate-elongated, margin penicillate; (integument 1 [inner integument 0]); hypanthium in fruit hardening, (with barbed spines), enclosing achene; n = 6.
7/254: Cliffortia (124), Acaena (60), Polylepis (26), Poterium (18), ?inc. Sanguisorba (15). ± Worldwide (few Indo-Malesia and lowland tropics in general), also Hawai'i.
Age. The age of crown-group Sangisorbinae is (28.6-)20.5(-13.3) Ma (G.-J. Zhang et al. 2022).
3. Amygdaloideae Arnott
Plant woody, (ectomycorrhizal); sugar alcohol sorbitol [carbohydrate transport], cyanogenic glycosides, flavones +, ellagic acid 0; cuticle waxes as tubules or platelets; leaves simple (compound); G <5, opposite K/C, stigma usu. wet; ovules 2/carpel, epitropous, funicular obturator +, papillate; fruit a follicle; x = 9.
57/1,540. Mostly North Temperate.
Age. Crown-group Amygdaloideae are (91-)90.2(-89.4) Ma (S.-D. Zhang et al. 2017) or (91.8-)87.0(-82.8) Ma (Chin et al. 2014), a similar age is suggested by Töpel et al. (2012), ca 125 Ma by N. Su et al. (2023) and, somewhere around about this node, ages of (51-)47-46(-42) Ma (Wikström et al. 2001) or (59-)44, 40(-27) Ma (Bell et al. 2010) have also been suggested.
3A. Neillieae Maximowicz —— Synonymy: Neilliaceae Miquel
Cyanogenic glycosides?; vessel elements with scalariform perforation plates; leaf teeth colleter-like; pollen surface smooth, perforate, orbicules +; G (1 - Niellia); ovules 2-4/carpel, apical, apotropous (-5, pleurotropous), micropyle exostomal, obturator +, funicular; seeds hard, shiny; endosperm copious.
2/27: Neillia (17). E. and W. North America, northeast Asia.
[Spiraeeae [[Lyonothamneae + Amygdaleae] [Sorbarieae [Kerriodae + Pyrodae]]]]: ?
3B. Spiraeeae Candolle —— Synonymy: Spiraeaceae Bertuch
Vestured pits +; nodes 1:1 [?all]; stomata various, (amphistomatous); stipules 0; (flowers single); pollen surface striate, perforate, orbicules +/0; ovules 6-8/carpel, unitegmic, integument 3-4 cells across, obturator +, funicular; fruit (achene - Holodiscus / pod - Kelseya); testa with thin walls [Pentactina]; n = 18.
8/106: Spiraea (80-100). N. temperate, to Columbia, (S. and) E. Africa, West Malesia.
Age. The age of this clade is estimated to be around (62.9-)59.2(-56.0) Ma (Chin et al. 2014).
[[Lyonothamneae + Amygdaleae] [Sorbarieae [Kerriodae + Pyrodae]]]: ovules 2/carpel, collateral.
Age. Crown-group Prunus + The Rest are dated to 36.1-34.3 Ma (Naumann et al. (2013: too young), (72.4-)67.8(-63.7) Ma (Chin et al. 2014), about 49.6 Ma (Hohmann et al. 2015) and 52.8-32.0 Ma (W. Su et al. 2021).
[Lyonothamneae + Amygdaleae]: ?
3C. Lyonothamneae A. Gray - Lyonothamnus floribundus A. Gray
Cyanogenic glycosides 0; leaves opposite, compound, leaflets long, margins regularly lobed, stipules deciduous; G seminferior, placentation apical; ovules 4-6/carpel; n = 27.
1/1. California Islands (off S. California).
3D. Amygdaleae Jussieu - Prunus L. —— Synonymy: Amygdalaceae Marquand, Prunaceae Martinov
Tree to shrub, (deciduous); (ectomycorrhizal); cork superficial; true tracheids 0; pith solid; lamina vernation laterally or vertically conduplicate, marginal extrafloral nectaries + [on petiole/lamina margin towards base], (abaxial surface0, cork warts +/0 [black spots], leaf teeth colleter-like; (plant dioecious); inflorescence raceme (corymbose/flowers single); bracteoles 0; (K, C, similar, ± K-like); G 1, stigma bilobed; micropyle bistomal, outer integument 6-8 cells across, inner integument 3-6 cells across, nucellar cap 2-3 cells across, (integument 1, ca 12 cells across), parietal tissue ca 8 cells across, obturator +, from loculus wall; fruit a drupelet, (stones 2), (outer pericarp [= hull] dry, dehiscent), endocarp and inner mesocarp lignified; seed coat mostly pachychalazal; n = 8 (-8x), nuclear genome [1 C] 0.28-3.65 pg; germination hypogeal/epigeal.
1/ca 275 (250-400). Temperate, also tropical montane.
Age. The beginning of diversification within Prunus has been dated to (67.4-)62.4, 60.7(-55) Ma (Chin et al. 2014), the species sampled by Y. Xiang et al. (2016) started diverging ca 30 Ma, L. Zhao et al. (2016c: p. 17) dated "the first formation of the racemose group" (basal, paraphyletic) at (66.3-)55.4(-45.1) Ma, J. Zhang et al. (2021) estimated the age of Prunus to be (57.8-)49.8(-42.4) Ma while N. Su et al. (2023) offered an estimate of (79.8-)67.3(-55.7) Ma.
There are well-preserved ca 49.5 Ma fossil flowers of Prunus from Washington State (Benedict et al. 2011).
[Sorbarieae [Kerriodae + Pyrodae]] [if this exists]: ?
Age. The age of this split is ca 92 Ma (Y. Xiang et al. 2016).
3E. Sorbarieae Rydberg
(Flavone C-glycosides - Adenostoma); petiole bundle deeply U-shaped (with wing bundles)/± annular/bundles 3, arcuate; leaves compound (simple: Adenostoma); pollen surface striate, otherwise perforate, microechinate, etc., orbicules +; G (1, Adenostoma), 5, opposite K; placentation apical; ovules 6-8/carpel, micropyle exostomal, (unitegmic - Spiraeanthus); (fruit an achene: Adenostoma).
4/8: Sorbaria (4). Central to East Asia, W. North America.
Kerriodae Ezedin / [Exochordeae + Kerrieae]: phragmidiaceous rusts 0; non-cyanogenic nitrile glucosides +.
3F. Exochordeae Reveal
Cork superficial; pith chambered; stipules deciduous/obsolete; inflorescence with terminal flower; styles lateral; ovules (pleurotropous), micropyle endostomal, obturator +, from ovary wall; fruit a drupe, or septicidal capsule, the carpels also opening adaxially [Exochorda]; seed coat vascularized [Exochorda, Oemleria]; n = 8.
3/8: Exochorda ((1-)4(-7)) - also Prinsepia. Central to East Asia, Taiwan, Oemleria W. North America.
Age. Well-preserved fossil flowers of Osmaronia from ca 49.5 Ma are known from N.W. Washington State, U.S.A. (Benedict et al. 2011).
3G. Kerrieae Focke —— Synonymy: Coleogynaceae J. Agardh, Rhodotypaceae J. Agardh
Wart-like projections on lamina; nectary 0 [?all]; C (0 - Neviusia [= N.]); A >40; G 1-5; G on ± flat receptacle; ovule 1 (2) carpel; ?obturator; fruit a drupaceous achene; n = 9 (8).
Note: [Coleogyne + Rhodotypos] Leaves opposite; G (1 -C.), surrounded by rim or sheath-like structure; ovules 1-2/carpel; (n = 8 - C.).
4/4. East Asia, W. North America, Alabama.
Pyrodeae Ezedin / [Gillenieae + Maleae]
Flavone C-glycosides +; cork superficial [?Gillenia]; rays often narrow; colleters + [probably elsewhere]; G ± connate, adnate to base of hypanthium, opposite K or odd member abaxial, gynoecial ring primordium +; ovules 2/carpel, ± apotropous, (micropyle bistomal), funicular obturator +; exotesta ± thickened, often mucilaginous, mesotesta thick, sclerotic; hybridization between n = 9 ancestors; Gymnosporangium rust common.
Age. The split of the two tribes below can be dated to (67.1-)60.6(-55) Ma (Chin et al. 2014), ca 54 Ma (Y. Xiang et al. 2016; see also S.-D.Zhang et al. 2017) or a mere 27.1-13.5 Ma (W. Su et al. 2021).
3H. Gillenieae Maximowicz - Gillenia Moench
Leaves compound; n = 9.
1/2. E. North America.
3I. Maleae Small
Isochlorogenic acid +; x = 17; genome duplication, four copies of GBSSI gene.
33/1,010. Northern Hemisphere, few S. South America; ± temperate.
Age. The crown-group age of Maleae is estimated to be (54.4-)50.1(-49.4) Ma (S.-D. Zhang et al. 2017) or (55.8-)53.9(-51.5) Ma (L. Zhang et al. 2023).
3I1. Lindleyinae Reveal —— Synonymy: Lindleyaceae J. Agardh
(Plant dioecious); 4-many pleurotropous ovules/carpel; (ovules with with chalazal projection, obturator 0 - Kageneckia); fruits dehiscing adaxially; n = 17; Gymnosporangium rust 0.
2/4: Kageneckia (3). Mexico, Peru, Chile. Photo: Kageneckia Flower, Fruit.
Age. The age of this node is (50.5-)48.5(-46.5) Ma (L. Zhang et al. 2023), and stem-group Malinae are (64-)58, 53(-43) Ma (Lo & Donoghue 2012).
3I2. Vauqueliniinae B. B. Liu - Vauquelinia Bonpland
Tannin-containing cells pervasive; micropyle exostomal; capsule septicidal, opening adaxially (and partly abaxially as well); ovules with chalazal projection; x = n = 15.
1/3. S.W. North America.
3I3. Malinae Reveal (= Pyrinae) —— Synonymy: Cydoniaceae Schnizlein, Malaceae Small, nom. cons., Mespilaceae Schultz-Schultzenstein, Pyraceae Vest, Sorbaceae Brenner
(Plant ectomycorrhizal); flavone C-glycosides + [?level]; (phloem stratified [± sclereidal] - Malus); crystals in axial parenchyma; (nodes 5:5 - Sorbus); leaves often deciduous (imparipinnately compound), (teeth colleter-like), stipules persisting over the winter (not), (intrapetiolar, connate); G at least half inferior, (carpels laterally free), styles (connate basally); micropyle endostomal [Photinia], outer integument 5-14 cells across, inner integument 3-6 cells across, nucellar cap ca 4 cells across; hypanthium fleshy in fruit [= pome, endocarp cartilaginous, seeds several], (endocarp woody [pyrenes]); n = 17, nuclear genome [1 C] 1.35-2.25 pg.
27-30/912-1,000 (45 genera - Rushforth 2018): Cotoneaster ((50-)261-294(-370)), Crataegus (222-260, inc. Mespilus), Aria (97), Sorbus (92-250: generic limits?), Pyrus (73-83), Photinia (65), Malus (47), Rhaphiolepis (42) - see Robertson et al. (1991) for genera. Largely North Temperate. Photo: Flower.
Age. Crown-group Malineae are (35.9-)34.2(-32.5) Ma (L. Zhang et al. 2023); the age of the [[Aronia + Pyrus] Malus] clade is around 56.3 Ma (B.-B. Liu et al. 2021b/2022) and that of the [[Eriobotrya + Pyrus] Malus] clade is (45.8-)44.9(-44.1) Ma (J. Zhang et al. 2021).
Floral formula: * ⚥ K 5; C 5; A (10-)15<; N; G (1-)10< / [3-5].
Evolution: Divergence & Distribution. See S.-D. Zhang et al. (2017) for additional ages for tribes and groups of tribes, and Dobeš and Paule (2010), Gehrke et al. (2015), Feng et al. (2017), X. Chen (2020) and G.-J. Zhang et al. (2022) for ages within Rosoideae and B.-B. Liu et al. (2021b/2022) for ages of a number of Malinae, especially Malus itself. Prunus endocarps have been found in the early Eocene of China ca 55 Ma, and there was considerable diversification of the family in the Eocene - thus Hesperomeles, found in Central America to Peru, and Photinia, now known from Southeast Asia, along with 11 other genera (two extinct) of Rosaceae are reported in deposits ca 50 Ma from the Okanogan Highlands in W. North America (Wehr & Hopkins 1994; see also DeVore & Pigg 2007; Benedict et al. 2011; Q.-Y. Li et al. 2011; Greenwood et al. 2016: Early Eocene fossils ca 50 Ma from British Columbia).
Rosaceae are described as being "extraordinarliy species-rich" (Magallón & Sanderson 2001: p. 1773), and this depends on clade sizes and ages - here ca 3,250 species, 44-25 Ma - when compared with those of other angiosperms (Magallón & Sanderson 2001).
Given the extensive hybridisation - sometimes rather deep - in Rosaceae, and so conflicting chloroplast and nuclear topologies of subfamilial and tribal relationships (the latter especially in Amygdaloideae - see Genes & Genomes and Phylogeny below) it is rather difficult to talk much about some aspects of evolution here. The strawberry, Fragaria × ananassa, is an octoploid, and is a cross between F. virginiana and F. chiloensis, themselves both octoploids. A parent of three of the genomes involved is F. iinumae, from eastern Asia (Rousseau-Gueutin et al. 2009) and so with a distribution that does not not overlap with either of these two polyploid species, while the parent of the remaining genome is F. vesca, which overlaps with both of them in North America (Jin et al. 2023).
X. Chen et al. (2020) suggested that 3-9 dispersal, 8 vicariance and 2 extinction events had been involved in shaping the distribution of Rosoideae, the subfamily perhaps being of Asian origin. Within Rosoideae, Töpel et al. (2012) looked at diversification in a clade of North American Potentilla s.l. in the context of changing climate since the late Oligocene ca 23 Ma. Nearly all the >120 species of the shrubby Cliffortia (Agrimonieae) are to be found in the Cape Floristic Region of South Africa (Linder 2003). Interestingly, the South African Acaena latebrosa is sister to the rest of the genus, Acaena being sister to Cliffortia (Jauregui-Lazo & Potter 2021). Acaena is a young genus, the stem being (15.7-)13.6(-11.7) Ma, and it probably originated in South Africa, with several extensive long distance dispersal events explaining its widespread distribution mostly in the Southern Hemisphere. These dispersal events include back and forth dispersals between New Zealand and South America, dispersals to a number of isolated oceanic islands, and also to Hawaii, California, etc. (Jauregui-Lazo & Potter 2021). Rubus, quite a young genus (ca 20 Ma), probably originated in North America, and subsequently there has been extensive migration using land bridges (to South America, around the northern hemisphere) and quite possibly long distance dispersal to the Antipodes (Carter et al. 2019). Within Rubus, the small subgenera Chamaemorus and Dalibarda are successively sister to the rest of the genus in both nuclear and plastid trees, and both include species that are unarmed, mostly herbs, and that have stipules free from the petiole (see T.-R. Huang et al. 2023 for morphology) - perhaps the basal condition for the genus?
All but two of the tribes of Amygdaloideae diverged between 96-88 Ma, and then nothing much seems to have happened for the next over 20 Ma - and in Amygdaleae, nothing for almost 60 Ma (Y. Xiang et al. 2016). The inferior-ovaried Malinae may represent a rapid but ancient radiation (Campbell et al. 2007; c.f. in part Xiang et al. 2016) perhaps associated with a whole genome duplication in the stem lineage of Malineae, and there was another in the stem lineage of Maleae) and also with the climatic changes that occurred at the end of the Palaeocene, e.g. the Paleocene-Eocene Thermal Maximum and the Early Eocene Climatic Optimum, to the beginning of the Oligocene, the cooling event that happened then (Xiang et al. 2016); see also L. Zhang et al. (2023) for events around here, including an increase in the rate of diversification both at the base of Malinae and of Malus itself. Lo and Donoghue (2012) dated stem group Malinae to late Palaeocene, with subsequent divergence in the Eocene and Oligocene, a substantial amount of movement around the northern hemisphere, probably via the Beringian land bridge, and much heterogeneity in clade size that is independent of age. Aldasoro et al. (2005) also suggest biogeographic relationships in this group. Rosa minutiflora, from Baja California, and the Asian R. berberifolia diverged ca 24.3 Ma (Fougère-Danezan et al. 2015).
Hybridization, polyploidy and apomixis, all more or less connected, are common in Rosaceae. For the relationship between polyploidy and diversification - perhaps direct - see Vamosi and Dickinson (2006). Here, as elsewhere in angiosperms, this relationship is probably not because speciation is faster in polyploids (the reverse may be more likely), rather, it is more likely to be the result of a ratchet mechanism since polyploidy is irreversible (Scarpino et al. 2014). Within Crataegus clades are linked with geography, but parents of genomes may have very disparate geographic origins (Lo et al. 2009). Full-sized trees are restricted to Amygdaleae and Maleae where they are associated with the acquisition of fleshy disseminules (Xiang et al. 2016). There may a connection between genome duplication, habit, disseminule type and diversity (Xiang et al. 2016), but as these authors noted, although Rosoideae have fleshy fruits and are even more speciose than Maleae, they are mostly herbs to shrubs and there is not the same association with genome duplications.
There is intergeneric hybridization in Rosoideae (e.g. Smedmark et al. 2003; Töpel et al. 2011) and very widespread in Amygdaloideae-Maleae (Robertson et al. 1991), and there is interclade hybridization within Potentilla s.l. (Eriksson et al. 2022), Rubus (Carter et al. 2019), etc.. Debray et al. (2021, see also references) have begun to work out hybridization in Rosa where polyploidy is common - for instance, Täckholm (1920) had early described how permanent pentaploids in section Caninae were the result of the fusion of haploid male gametes and tetraploid female gametes. Allopolyploidy in Fragariinae and its taxonomic implications were studied by Lundberg et al. (2009). Deep hybridization within Prunus has been invoked to explain contrasting phylogenies obtained when using nuclear and plastid data, and it also fits with chromosome numbers (Chin et al. 2014), and from the analysis of L. Zhao et al. (2016c) there appear to be four hybrid clades/subgenera at the base of the tree that are allopolyploids (and have racemose inflorescences). However, N. Su et al. (2023) suggested the scenario here of racemose-flowered x solitary-flowered → corymbose-flowered (these were the three groups that he detected). The Maddenia group of Prunus and the ancestor of the [Lyonothamneae + Amygdaleae] clade may have hybridized, hence the very different positions of the former tribe in nuclear and chloroplast trees (Hodel et al. 2022: see also Y. Xiang et al. 2016; Hodel et al. 2021, also Phylogeny below). B.-B. Liu et al. (2021b/2022) documented extensive hybridization/introgression in Maleae in general and in Malus in particular, with substantial disagreement in the topologies of nuclear and chloroplast trees. For more on hybridization, see Stull et al. (2023).
Further complicating the issue of hybridization is the frequency of apomixis, the two often being associated. Indeed, the combination of apomixis, unequal partitioning of the chromosomes at meiosis and hybridization is common in Rosaceae, as in taxa like Rubus (e.g. Sochor et al. 2015), Potentilla and Alchemilla (e.g. Dobeš et al. 2015), all Rosoideae, and Amelanchier, Sorbus, Cotoneaster and Crataegus, all Amygdaloideae-Pyrodeae (Dickinson et al. 2007; see also Asker & Jerling 1992; Hörandl et al. 2007; Talent & Dickinson 2007; Coughlan et al. 2017; Majeský et al. 2017; Dickison 2018 and other papers in Taxon 67(6). 2018; Lepší et al. 2019).
In Crataegus the hybrids have various ploidy levels (there are also autotriploids). There were ca 17 North American species of Crataegus in 1896, while 30 years later there were over 1,000, and at least some are triploid apomictic hybrids; C. S. Sargent described over 700 of these species ("I know Crataegus is in a mess - I put it there" - ?apocryphal). In general, the evolution of apomixis seems to have preceded hybridisation (Dickinson et al. 2007). For more on apomixis, hybridization, etc., in Crataegua see e.g. Lo et al. (2009a, 2010, 2013). In pentaploid Rosa unequal division occurs at meiosis, and as a result megaspores have 4/5th of the genome and microspores 1/5th (Wissemann & Ritz 2007 and references). There are ca 755 species of European brambles (Rubus) - 863 total - of which six are diploid (and two of these may be extinct), although there are four more diploids in surrounding areas, as well as others in North America, etc. (Sochor et al. 2015); Carter et al. (2019) suggested that the ancestor of the genus was North American, and they discussed its subsequent migration. In Europe crosses between sexual (series Glandulosi: ovulate parent) and apomictic (Discolores: pollen) species of Rubus have generated novel apomicts (series Radula: Šarhanová et al. 2017). For diversity among West Asian Rubus subgenus Rubus, an edifice built on ca 4 sexual diploid and 2 sexual tetraploid species, see Kasalkheh et al. (2024). As to the question, what is a taxonomic species among the apomicts?, the answer may be biotypes that have a distribution range of at least 50 km or so (Kasalkheh et al. 2024). In Amelanchier the diploid taxa behave as species according to several definitions of the term, however, polyploid apomicts break down the relative simplicity of the variation patterns at the diploid level (Burgess et al. 2015). In Sorbus diploids are sexual while polyploids show largely pseudogamous apomixis; both gametes may fuse with the central cell, hence the endosperm is tetraploid.
There are about 34 species of Lachemilla (= Alchemilla, Potentilleae), herbs of various kinds to small shrubs, that grow in the páramo of South America, the group being a very important component of the vegetation there (Gehrke et al. 2008; Sklenár et al. 2011; Morales-Briones et al. 2018a; see also Schley et al. for hybridization, etc.). The shrubby habit has also evolved (and been lost) in Alchemilla growing in alpine regions on African mountains (Gehrke et al. 2015).
Koski and Ashman (2016a, b) carried out extensive studies on floral UV absorbtion patterning in Potentilla s.l. and also flower size, linking variation in the former to environmental factors (high UV, petals "black" ≡ UV absorbtion), and variation in the latter to reproductive character displacement.
Chin et al. (2013) looked at the various glandular/apparently glandular structures on the leaf surface and margin and also petiole in Prunus and optimized the variation that they found - quite extensive - on a phylogeny of the genus. Interestingly, leaf teeth in several species of the genus, as well as in Neillia and Physocarpus, are colleter-like both in terms of their structure and what they secrete (mucilage). M. de S. Silva et al. (2022) summarized the variation in leaf teeth in Rosaceae and found records of hydathodes/water glands throughout the family, but colleteriform leaf teeth were indeed restricted to some Amygdaloideae (they do not mention such teeth in Physcocarpus); teeth in Prunus can be colleters, extrafloral nectaries or hydathodes.
Much attention has been paid to the androecium of Rosaceae and its development and evolution. Early studies include those by Murbeck (1941), Kania (1973) and Lindenhofer and Weber (1999a, b, 2000: the series was apparently never finished). Initially it was thought that taxa like Neurada (now in Malvales) were "basal" (e.g. Murbeck 1941), while later Quillaja (now in Fabales) was another candidate for this position; attempts were then made to relate the considerable staminal diversity in Rosaceae, including the multistaminate androecia of a number of species, to the diplostemony of these plants. Alternatively, given that the androecia of some Rosaceae seemed to have paired stamens, it seemed that there had been dedoublement (Lindenhofer & Weber 1999a). However, these latter authors noted (as had others) that the stamens in front of the petals in many taxa were almost between the petals and sepals rather than being opposite the petals, a position that Lindenhofer and Weber called parapetalous, overall, the androecium (and perianth) seemed to be basically spirally arranged. Indeed, taxa commonly had around 20 stamens, although infraspecific variation was common - neither diplostemony nor polystemony, but what Lindenhofer and Weber (1999a, b, 2000) called pluristemony.
Optimisation of characters on the tree presents problems. Potter et al. (2007) used DELTRAN, and so being host to Gymnosporangium rusts is not an apomorphy of Pyrodeae; using ACCTRAN (as here) it is. They divided up the presence of sorbitol (see e.g. Waalart 1980; Rennie & Turgeon 2009) into two states; it might be present in only small amounts (Dryadoideae), or it was more abundant (Amygdaloideae); presence of at least some sorbitol could be used to characterize the larger clade. However, given the relationships suggested by Y. Xiang et al. (2016) and followed here, I have placed the acquisition of sorbitol as features of both subfamilies, but both it, and the presence of cyanogenic glucosides, could characterize the family as a whole and be lost in Rosoideae. L. Zhang et al. (2023: Figs 7, 8) optimise the evolution of a number of characters in Maleae.
Ecology & Physiology. Some, but not all, Dryadoideae, are N-fixers, although the widespread Dryas integrifolia does not fix N (Markham 2009). Nodules and association with Frankia have also been reported from other Rosaceae, e.g. from Rubus ellipticus (Markham 2009). Species of the N-fixing Cowania (Dryadoideae) and the non N-fixing Fallugia (Rosoideae-Colurieae) can be successfully intergrafted; when Fallugia is the stock the combination will not fix N (Kyle et al. 1986).
Dryas is ectomycorrhizal and is one of the eight major biomass accumulators in tundra vegetation. Four other species of these eight are also ectomycorrhizal, and the apparently endomycorrhizal Rubus is also on this list (Chapin & Körner 1995; Gardes & Dahlberg 1996). For literature on mycorrhizae, see Brundrett (2017a) and for their ages, etc., see Tedersoo and Brundrett (2017) and Tedersoo (2017b).
The synthesis of triterpenes in Eriobotrya japonica (Amygdaloideae-Maleae) has been linked with the genome duplication in that tribe (W. Su et al. 2021), although ursane and oleanane triterpenoids, members of the two major pathways there, are also to be found in the immediately unrelated Rosa odorata, in Rosoideae (Lv et al. 2021).
Potentilla arguta has glandular hairs and may be protocarnivorous, and the plant is able to digest proteins (?source of enzymes) and take up at least some of the products (Spomer 1999); the ability of plants with such hairs to digest at least some proteins is quite widespread in flowering plants.
There are prominent basal nectary glands on the lamina of fossils identified as Prunus from the Okanogan Highlands formation of western North America ca 49.5 Ma (DeVore & Pigg 2007; Benedict et al. 2011), apparently the earliest example of foliar extrafloral nectaries.
Rubus in particular readily invades natural areas (Daehler 1997).
Pollination Biology & Seed Dispersal. Beetle pollination - the Meligethes group (Nitidulinae) is involved - occurs in genera like Rosa, Rubus (Rosoideae), Malus, Pyracantha and Prunus (Amygdaloideae), especially in the eastern Palaearctic. The move on to Rosaceae occured maybe 13-11 Ma; the beetle larvae eat the pollen (M. Liu et al. 2021). J.-R. Wang et al. (2020) discuss possible wind pollination in Agrimonieae-Sanguisorbiinae.
There is a period of about 19 days between pollination and fertilization in Prunus persica (Herrera & Arbeloa 1989).
Gametophytic self incompatability occurs in Rosaceae (Prunus: Zhang & Xue 2008; McClure 2008). For apomixis, common in the family, see below.
Y. Xiang et al. (2016) discuss the evolution of fruit type in the family in some detail (see above). For masting in Sorbus aucuparia and factors that might affect it, see Bogdziewicz et al. (2020).
Plant-Animal Interactions. Galls caused by cecidomyid midges are quite common in North American Rosaceae (Gagné 1989). Some 60 species or more of cynipid gall wasps of the tribe Diplolepidini are restricted to Rosa, Diplolepis rosae forming the well-known robin's pincushion or bedeguar gall (Csoka et al. 2005; Redfern 2011; Y. M. Zhang et al. 2020). These gallers have only one generation per year, and leaves may be the initial structure galled, host shifts being relatively uncommon (Zhang et al. 2020). Diplolepidini are perhaps 39 Ma and are not immediately related to the oak-galling Cynipini, rather, they are sister to Pediaspini, found on Acer, those two clades separating perhaps 142-133 Ma and together forming a small clade outside Cynipidae (Blaimer et al. 2020). There are some rose gallers in Diastrophini, which are indeed members of Cynipidae.
Caterpillars of a variety of groups of ditrysian Yponomeutoidea moths are recorded as eating Rosaceae (Sohn et al. 2013). Prunus, Malus, Crataegus and Rubus are important host plants for lepidopteran larvae in the contiguous United States of America, being included in the top 20 such plants (Narango et al. 2020.
Aphids are notably diverse on Rosaceae, with some 300 species being known to occur on the family, and it has been suggested that they were the original hosts for Aphididae as a whole (Peccoud et al. 2010).
For literature on the evolution of the dipteran tephritid Rhagoletis as it moved from Crataegus to introduced Malus in North America, see Feder and Forbes (2008), Forbes et al. (2017), Ragland et al. (2017) and Doellman et al. (2018).
Plant-Bacterial/Fungal Associations. At least some Dryadoideae and Amygdaloideae are ectomycorrhizal (ECM) plants. 154 ECM OTUs have been recorded from Dryas integrifolia alone in a Low to High Arctic transect, many of these also being widely distributed outside the Arctic (Gardes & Dahlberg 1996; Timling & Taylor 2012), however, Bjorbækmo et al. (2010) found considerable geographical partitioning in the ECM fungi of D. octopetala. In southern Oregon Geopora sp. (Pezizales, a hypogeous ascomycete) forms ECM associations with Cercocarpus ledifolia (Amygdaloideae) in particular, and other generalist ECM species from the same habitat associated with C. ledifolia also form associations with Quercus garryana (Fagaceae) and Arctostaphylos spp. (Ericaceae) (McDonald et al. 2010), the result being a complex mycorrhizal network. Ascomycetes are also associated with other ECM Rosaceae, including Purshia (McDonald et al. 2010). Rose (1980) recorded vesicular-arbuscular mycorrhizae on Purshia and Cercocarpus.
Savile (1979b; see also Jackson 2004b: possible codivergence) discusses the distribution of phragmidiaceous rusts within Rosaceae-Rosoideae; they are found on no other Rosaceae, and only rarely on plants from other families. Most of these rusts are autoecious, that is, their entire life cycle occurs on the one species. Gymnosporangium rusts, found scattered on Amygdaloideae-Pyrodeae, are heteroecious. Here the telial stage (the teliospore is a thick-walled resting spore that germinates to produce basidiospores) is common on some Cupressaceae, the aecial stage, which produces thinner-walled binucleate aeciospores, is found on Pyrodeae (Lo & Donoghue 2012 for "gains" and "losses" of infestation). Fruits of Amygdaloideae can be seriously damaged by Monilinia (polyphyletic - ascomycete-Sclerotiniaceae), also found on Ericaceae (Holst-Jensen et al. 1997). Interestingly, Rosaceae rarely produce phytoalexins, protective compounds induced by e.g. fungal infection (Harborne 1999).
Genes & Genomes. The basic chromosome number of the family may be x = 7 (Shulaev et al. 2010) or x = 9 (Jung et al. 2012; Murat et al. 2015b), with subsequent rearrangements. The nuclear genome has evolved in different ways in different parts of the family (small rearrangements - Fragaria; more translocations - Malus); of the taxa examined, Prunus (n = 8) has the most conserved genome in the family (Jung et al. 2012). See Kajewski (1957) for classic cytological work on Geum, Goldblatt (1976a) for some important counts.
It has long been suspected that Pyreae (= Maleae) were of wide hybrid origin, as their chromosome number might imply (n = 9 [Rosoideae] x n = 7 [Spiraeaoideae] → n = 16 [some Maloideae]) (Evans et al. 1998; Hodel et al. 2022 for references). However, Evans and Campbell (2002) suggested that polyploidisation with subsequent aneuploidy (9 x 2 → 18, 18 - 1 → 17) within the diploid Gillenia clade (herbaceous, with compound leaves) or something similar was more likely to explain the chromosome numbers. Gillenia is sister to Maleae (Potter et al. 2002; Evans & Dickinson 2002) - together they make Pyrodeae - and is host to the same rusts that are found on other Pyrodeae, but it has only two copies of the GBSSI gene, as in the rest of the family; other Pyrodeae have four copies of this gene. Hodel et al. (2022) suggested that there had been hybridization between a member of Spiraeeae (x = 9), probably the pollen donor, and an ancestor of the clade [Sorbarieae (x = 9) + Exochordeae (x = 8) + Kerrieae (x = 9)], probably the carpellate plant (see the different positions of these groups in the nuclear and plastome trees), and they noted that over 45% of the nuclear genes (203/448) of Spiraeeae sampled supported the chloroplast topology, i.e. the topology [Spiraeeae [Gillenieae + Maleae]], not Spiraeeae subbasal in the whole Amygdaloideae. (Depending on the analysis, [Exochordeae + Kerrieae + Sorbarieae] or an ancestor of Sorbarieae alone were sister to Spiraeeae - Hodel et al. 2022.) The result of this hybridization event was the clade [Gillenieae (x = 9) + Maleae (x = 17)], the latter tribe being polyploid-aneuploid, although Hodel et al. (2022) somewhat favour the idea that there was initially a genome doubling in the Gillenia clade as well. L. Zhang et al. (2023: Fig. 2A) also suggest that there was a duplication/hybridization at the base of Maleae, even though some genome duplications map to the first three nodes of the tree.
There may have been a genome duplication around Malus and Pyrus some (21.3-)19.9, 18.3(-16.4) Ma, yet the oldest fossils assigned to these genera are about 2½ times as old (Vanneste et al. 2014a), indeed, on balance they think that their ages are an underestimate of at least 28 Ma. Landis et al. (2018) date the MADOα event involving all three clades in J. Sun et al. (2018: see below) to ca 9.4 Ma, while W. Su et al. (2021) date the duplication to less than 27.1-13.5 Ma. Lachemilla and immediately related genera (= Alchemilla s.l.) may share a genome duplication (Morales-Briones et al. 2018b), the result of an ancient autopolypoidy event, and three more genome duplications (allopolyploidies) are thought to have occured within this clade (Morales-Briones et al. 2020/2021). Aphanes, although deeply nested within this Alchemilla clade, nevertheless has few paralogs and mostly diploid chromosome numbers; a suggestion is that a diploidization event occured after the initial autopolyploidy, then there were the allopolyploidy events just mentioned, Aphanes, however, descending directly from this diploidized ancestor (Morales-Briones et al. 2020/2021). A duplication in Rosoideae-Colurieae on up (see above, the ROPAα event, has been dated at 60.2 Ma (Landis et al. 2018). Genome sizes in Rosaceae as a whole are rather low, minimum holoploid genome sizes being the lowest in those angiosperms examined by Elliott et al. (2022b: Fig. S11), although in Pyreae genome sizes are rather higher (Dickson et al. 1992).
Hybridization, polyploidy and apomixis, all connected, are discussed under Divergence & Distribution above.
For sex chromosomes in North American octoploid Fragaria, see Tennessen et al. (2018). Here a sex-determining region, a gene cassette, is found in female individuals alone - but on different chromosomes in different taxa. This the result of sequential translocations of the one casette, which picks up some of sequences from the adjacent part of the host chromosome each time it moves (Tennessen et al. 2018).
J. Zhang et al. (2021) discuss plastome variation in the family, especially Amygdaleae. This is mostly a matter of the extensive variation in the sizes of the various parts of the plastome in Prunus s.l., however, Maleae show little variation and while Rosoideae tend to have small plastomes, G.-J. Zhang et al. (2022) found little variation in Sanguisorba.
Economic Importance. Rosaceae are noted for producing a variety of fruits, very different morphologically - e.g. strawberries (swollen receptacles), blackberries and raspberries (drupelets), plums, etc. (drupes), and apples and pears (pomes). Fan and Whitaker (2023) discuss aspects of the genomic history of the cultivated strawberry. A number of taxa, particularly roses, are of importance for their flowers. The codling moth, the tortricid Cydia pomonella and relatives, is a major pest of apples and their relatives.
Members of Rosaceae are over-represented among clades that have become naturalized and/or are invading natural areas, and they include 5 of the top 50 genera with the most naturalized species (Pysek et al. 2017).
Chemistry, Morphology, etc.. For general chemistry, see Hegnauer (1973, 1990), and for 2-pyrone-4,6dicarboxylic acid distribution, see Wilkes and Glasl (2001). Okuda et al. (1992) discuss tannin distribution in the family; chlorogenic acid was found in nearly all taxa examined. For cyanogenesis, see also Thodberg et al. (2018); non-cyanogenic glucosides are apparently restricted to Kerriodae (Lechtenberg et al. 1996). Challice (1974) looked at chemotaxonomy in the context of the origin of Pomoideae. Triterpenes are common (W. Su et al. 2021; Lv et al. 2021).
S.-Y. Zhang (1992) carriued out a comprehensive survey (62 genera) of wood anatomy in the family; this needs to be integrated with the clades recognized above. A polyderm is common, although perhaps not in Pyreae (Mylius 1913), while Oskolski et al. (2023) described a variety of phelloderms in Polylepis and relatives in Sanguisorbinae. In this latter group there are also different sclerification patterns that may involve bands of sclereids in the secondary phloem, although Oskolski et al. (2023: p. 21) also noted several characters of stem anatomy in common between members of Sanguisorbeae (sic) - schizo-rhexigenous intercellular spaces in the cortex, periderm initiation deep in the cortex, phellem cells with dark contents, etc.. Evert (1963) noted a distinctive stratified phloem in Malus 1:1 nodes occur in Spiraea, which also lacks stipules, and there are five traces in the base of the petiole of Sorbus, for example (Rushforth 2020), although normally Rosaceae have stipulate leaves and 3:3 nodes (see e.g. Sinnott & Bailey 1914). In deciduous taxa like Micromeles and other taxa growing in warm temperate conditions the stipules fall off with the rest of the leaf, but in taxa growing in cooler conditions the leaf abscises just above the stipules, the remainder falling off in the subsequent spring (Rushforth 2020). Development of the compound leaf of Potentilla is basipetal (Hagemann & Gleissberg 1996); Guédèes (1968) described the stiples here with their adaxial flange as being ligular stipules. There are extensive data on cuticle waxes (Fehrenbach & Barthlott 1988), but they are recorded only as summaries in the context of conventional subfamilies; Barthlott (pers. comm.) kindly provided a more detailed breakdown. Leaf cuticle waxes as clustered tubules are common in the family. For a foliar endodermis in several Rosoideae and Spiraea, at least, see references in Trapp (1933).
In Sanguisorba flowers of the heads/botryoids initiate acropetally (the terminal flower is initiated first) but open basipetally (J.-R. Wang et al. 2020). Descriptions of inflorescences can be confusing. Thus Evans and Dickinson (1999) and X. Wang et al. (2019) both describe the inflorescences of Osmaronieae and Amygdaleae as being racemes; the Osmaronieae illustrated have well-developed terminal flowers, while species of Prunus may have functional, aborting or no terminal flowers - and in Prunus bracteoles may or may not be present. The epicalyx in some Rosaceae seems to represent stipules associated with the calyx members, and in Agrimonia, at least, it originates later than the calyx (Ronse DeCraene & Smets 1995d; Remizowa 2019). For hypanthium development, see Rauh and Reznik (1951). Ronse de Craene (2007) wondered whether the petals were modified staminodes, while Lindenhofer and Weber (1999, 2020a, b) suggest that on occasion the outer whorl of stamens are modified petals. In some species of Prunus sepals and petals are similar and look more or less like sepals - this is a derived feature, and more than once (X. Wang et al. 2021). Erbar and Söte (2022) discuss the morphology of the nectary in some detail. There are often five traces to each carpel. Rosa setigera is reported to have a synstigma like that of Ficus (Teixeira et al. 2018), but a synstigma in Ficus involves stigmas of different flowers - here the styles are free and no fusion of the stigmas of the two whorls of separate carpels is mentioned, although they are close to each other (Kemp et al. 1993), so even the existence of an extragynoecial compitum is unclear. Even in taxa with inferior ovaries, there is great variation in whether or not the carpels are connate, or what parts are connate, and in whether or not the carpels are adnate to the axial tissue enveloping the carpels/hypanthium. Thus Cotoneaster has an inferior ovary, and there the carpels are more or less separate from each other although adnate to the hypanthium, c.f. also Pyracantha. The odd genus Dichotomanthes, also included in Pyreae, has a single carpel that is superior in position (Rohrer et al. 1994); this must represent a reversal. In general, the styles of the separate carpels are somewhat off-centre, and a gynobasic style is but an extreme form of this asymmetry. The single, basal ovule of Chamaebatia (Dryadoideae) lacks an obturator (Evans & Dickinson 2002), while ovules of Rhodotypos have a protruding nucellus (c.f. Rhamnaceae). Prunus may have one or two integuments, in the latter case, these may be free only above the micropyle (Lora et al. 2015), so the single integument may represent two fused integuments. However, J.-R. Wang et al. (2020) suggested that the single integument of Sanguisorba tenuifolia may have developed by the reduction of the inner integument. The lignified exotesta common in the family can be found even in the seeds of the drupaceous Prunus.
Some general information is taken from Decaisne (1874), Robertson (1974), Hess and Henrickson (1987: Vauquelinia), Judd et al. (2002), Kalkman (2004) and especially Potter et al. (2007). There is information on cork initiation and bark anatomy in Lotova and Timonin (e.g. 1998, 1999, 2002) and Weiss (1890), on stipule morphology in Guédèes (1968), on petiole vasculature in Morvillez (1917) and J.-H. Song and Hong (2018: the "nodes" mentioned are not nodes), on stomata etc. in Spiraeeae (Song et al. 2024), on inflorescence morphology and development in Weberling (1989) and Bull-Hereñu and Claßen-Bockhoff (2011b), and on floral morphology in Kania (1973), Evans and Dickinson (1999a, 1999b, 2005), Ronse de Craene (2018: Geum), X. Wang et al. (2018: Prunus), J.-H. Song et al. (2020) and J.-R. Wang et al. (2020), both Sanguisorba); pollen morphology in Chung et al. (2010: Sanguisorbeae [= Agrimonieae]), Song et al. (2016: Sorbarieae, 2017a: Neillieae, 2017 b: Spiraeeae), Xiong et al. (2019: Rubus) and Pathak et al. (2019: esp. Photinia), carpel orientation and general morphology in Focke (1888), Schaeppi (1951: peltate and plicate carpels), Schaeppi and Steindl (1950: Rosoideae gynoecium), Sterling (1966, 1969 and references), van Heel (1981, 1983: development), and Smedmark and Eriksson (2006: stylar hook), ovule morphology in Péchoutre (1902: quite comprehensive, also seeds), Juel (1918: see comments on Péchoutre; orientation of carpels) and Ahn et al. (2023), and exotesta in Frohne and Jensen (1992) and J.-H. Song et al. (2020: Spiraeeae).
Phylogeny. Initially Rosoideae and a number of clades within it, Amygdaloideae, Amygdaloideae minus Lyonothamnus, [Pyrodeae + Sorbarieae] and Pyrodeae were all well-supported, but little could be said of other larger patterns of relationship (e.g. Morgan et al. 1994; Potter et al. 2002; Potter 2003; Potter et al. 2007a). The position of Dryadoideae was uncertain, other than being a rather basal branch in the tree (Potter et al. 2002: Evans et al. 2002). Potter (2003: several genes) found that Dryadoideae were fairly well supported as sister to other Rosaceae, but their position was not secure in Potter et al. (2007a), although a sister group relationship with Spiraeaoideae (= Amgydaloideae) was perhaps most likely (see also Töpel et al. 2012; Z.-D. Chen et al. 2016). However, Chin et al. (2014), H.-L. Li et al. (2015, 2016), M. Sun et al. (2016), S.-D. Zhang et al. (2017: analysis of whole plastomes, position not stable) and X. Chen et al. (2020: chloroplast genes) all suggested that Dryadoideae and Rosoideae formed a clade, although support was not always that strong. Y. Xiang et al. (2016: nuclear genes) found that Dryadoideae were sister to the rest of the family, and they thought that other positions were unlikely. W. R. Baker et al. (2021a: see Seed Plant Tree, ca 350 nuclear genes) recovered a [Dryadoideae + Amygdaloideae] clade in their initial analysis, although their sampling of Rosoideae was skimpy; Dryadoideae were rather weakly associated with Rosoideae in a later version (ii.2022). Hodel et al. (2022) found Dryadoideae and Rosoideae were associated in plastome analyses, but in all nuclear analyses Dryadoideae were sister to the rest of the family.
Dryadoideae. Here Dryas is sister to the rest of the subfamily (e.g. H.-L. Li et al. 2015; M. Sun et al. 2016; S.-D. Zhang et al. 2017; Seed Plant Tree ii.2022).
Rosoideae. Eriksson et al. (2003) provide a phylogeny of Rosoideae from which the tribal relationships above were taken; see also Y. Xiang et al. (2016: nuclear data) and S.-D. Zhang et al. (2017) and X. Chen et al. (2020), both the latter plastome data, for phylogenies of the subfamily. The relationships of the tribes recognized above may vary, however, Filipendula (Ulmarieae) is generally found to be sister to other Rosoideae, although Rubeae and Roseae in particular tend to be somewhat migratory, nuclear and plastome data telling different stories. Zhang et al. (2017) suggest the grouping [Ulmarieae [Colurieae [Rubeae [Agrimonieae [Roseae + Potentilleae]]]]]. However, other positions are not so stable, thus Y. Xiang et al. (2016) found the relationships [Ulmarieae [Rubeae [Colurieae ...]]] in their nuclear analyses, while the relationships of Agrimonieae, Roseae and Potentilleae are also somewhat fluid.
Agrimonieae: For relationships, see Y. Xiang et al. (2016) and S.-D. Zhang et al. (2017). Previous infrageneric groupings did not hold up in Acaeana, furthermore, the paraphyly of Acaena was confirmed, Sanguisorba was para-/polyphyletic, and hybridization may be confusing things in Acaena (Jauregui-Lazo & Potter 2021), as in its sister group Cliffortia (see Whitehouse 2021). For some plastome-based relationships, see G.-J. Zhang et al. (2022), and for relationships in Polylepis, see Boza Espinoza and Kessler (2022).
Potentilleae: Alchemilla, Fragaria, and other genera form a well-supported clade outside Potentilla and relatives (see also Dobeš & Paule 2010). Potentilla (including a few segregate genera) is sister to a small clade including Argentina (= Potentilla sect. Anserina), the two forming a clade sister to the other Potentilleae (all relationships with strong support: Dobeš & Paule 2010; see also Eriksson et al. 2003). T.-T. Xue et al. (2023: 131 spp., coding/non-coding plastome regions, 30 mitochondrial genes) recovered 8 well-supported clades here, including the Anserina clade; the Argentina clade was by far the largest, and with proportionally many more uncertain nodes and also more differences between the topologies resulting from the chloroplast and mitochondrial analyses. Eriksson et al. (2015) and Feng et al. (2017) noted the polyphyly (five or more separate clades!) of the small genus Sibbaldia while Töpel et al. (2011) and Koski and Ashman (2016a) discussed relationships within Potentilla s.l. - Fragariinae are sister to Alchemillinae. For relationships within Alchemilla s.l., which have a strong geographic signal, see Gehrke et al. (2008). Andean Lachemilla, often placed in Alchemilla, includes some 61 species all told, and there has been extensive hybridization, one clade, which includes species with orbiculate leaves, probably being of hybrid origin (Morales-Briones et al. 2018a, b).
Roseae: For Rosa, see Bruneau et al. (2007), Wissemann and Cox (2007) and Koopman et al. (2008); relationships here are not easy to disentangle. There is still rather little support in the quite comprehensive analysis of Fougère-Danezan et al. (2015), again, hybridization complicates the issue; see also M. Sun et al. (2016). Debray et al. (2021: ca 126 species, 4 plastid single copy orthologous tags, 92 nuclear tags) carried out separate analyses of chloroplast and nuclear tags, the latter initially including only non-hybrid diploids, and then on to the latter they grafted (as it were) hybrids and polyploids.
Rubeae: It had been known for some time that the infrageneric classification of Rubus, based on that of Focke of over 100 years ago, did not reflect relationships (Alice & Campbell 1999) and the genus was not even monophyletic in Z.-D. Chen et al. (2016). Recent studies on the genus include those of Carter et al. (2019: 87 wild spp., ca 1000 low copy nuclear genes, plastome analyses) and T.-R. Huang et al. (2023: chloroplast genes, ca 1/6 the genus, including species from Carter et al. 2019). Carter et al. (2019) found gene tree/species tree and cytonuclear discordance, the latter due to hybridization and incomplete lineage sorting. Indeed, Huang et al. (2023) noted that although the plastome data that they used and nuclear data allowed the recovery of the same major clades, relationships both between and within these clades differed. For diversity among West Asian Rubus subgenus Rubus, an edifice built on ca 4 sexual diploid and 2 sexual tetraploid species, see Kasalkheh et al. (2024), also Genes & Genomes above.
Amygdaloideae. Here Exochorda forms a small clade along with Oemleria and Prinsepia (Evans & Dickinson 1999a for information); they have been placed near Prunus (Potter et al. 2002, see also Lee & Wen 2001) to which they do show some morphological similarity; Lyonothamnus, Neillieae, and Amygdaleae were basal in the subfamily. The coverage of genera in Amygdaloideae was quite extensive in Töpel et al. (2012), and the sequence of divergence of the clades differed, Neillieae and Amygdaleae in particular moving higher into the tree, while S.-D. Zhang et al. (2017) preferred the relationships [Lyonothamneae [Neillieae [[Exochordeae + Kerrieae] [Amygdaleae [Sorbarieae [Spiraeeae [Gillenieae + Maleae]]]]]]; see also Chin et al. (2014), H.-L. Li (2015), M. Sun et al. (2016) and Z.-D. Chen et al. (2016: relationships again rather different from those above) for phylogenies. Y. Xiang et al. (2016) found the relationships above which for the most part were well supported. Spiraeeae in particular had moved basally in the tree, which is rather less pectinate than earlier suggestions, and the position of the tribe is also not stable in the analysis of S.-D. Zhang et al. (2017). Hodel et al. (2022) looked at relationships here in some detail, and they found extensive differences when comparing nuclear and chloroplast trees. Relationships based on nuclear data were [Neillieae [Spiraeeae [[Lyonothamneae + Amygdaleae] [[Sorbarieae [Kerrieae + Exochordeae]] [Gillenieae + Maleae]]]]], and although the nodes along the backbone tended to have low ASTRAL quartet support and low to very low gene tree support, monophyly of those tribes with two or more species included was strong. Relationships obtained using chloroplast data were [Lyonothmneae [Neillieae [[Kerrieae + Exochordeae] [[Amygdaleae + Sorbarieae] [Spiraeeae [Gillenieae + Maleae]]]]]], and bootstrap support was generally good (Hodel et al. 2022) - this is discussed further under Genes & Genomes above. Gene-tree support for the position of Spiraeeae in plastome analyses was higher than that for its nuclear position (Hodel et al. 2022).
Amygdaleae. For phylogenies of Prunus s. l., see Lee and Wen (2001), Bortiri et al. 2001 (ITS and trnL-trnF together produced some structure), Wen et al. (2008: some conflict between ITS and ndhF), Chin et al. (2013), Yazbek and Oh (2013: subgenus Amygdalus), L. Zhao et al. (2018: Malesian section Mesopygeum) and J. Zhang et al. (2021: plastomes, focus on true cherries). Shi et al. (2013) had fairly good sampling, some 84 species, concatenated data, and recovered three major clades (subgenera), while Chin et al. (2014, see also 2013) recovered two main clades, a tropical clade with racemose inflorescences and a temperate clade, of which a subclade also had racemose inflorescences. The analyses of L. Zhao et al. (2016c) suggested that there were four clades of hybridogenous origin at the base of the tree that had racemose inflorescences, and species like P. africana were particularly peripatetic. In an analysis of the plastomes of 32 species, L. Wang et al. (2021) recovered the largely well-supported relationships [[Laurocerasus and Padus groups] [[Cerasus group] + [Amygdalus group, Prunus group, P. humilis]]]. The distribution and nature of calcium oxalate crystals correlated quite well with relationships here (see Lersten & Horner 2000); Maddenia (dioecious, apetalous) is also to be included in Prunus (Potter et al. 2007a). Although a recent phylogenomic analysis recovered the racemose group as being monophyletic, sampling was poor (only two racemose species were included, but over half the species in Prunus have such inflorescences), gene trees were inclined to suggest other relationships (elsewhere in the genus this was even more pronounced), and in general hybridization/allopolyploidy seemed to have been common (Hodel et al. 2021). N. Su et al. (2023: 32 spp., plastome and RADSeq data) recovered three main clades, the racemose (often polyploid), solitary-flowered (usually diploid) and corymbose clades; there perhaps was hybridization between the first two that resulted in the third, and there were different topologies/extensive hybridization within clades when analyses using different data types were compared. Finally, there is evidence for hybridization between the Maddenia group of Prunus and the ancestor ]of the [Lyonothamneae + Amygdaleae] clade (Hodel et al. 2022). There is still a way to go before we understand what is going on around here.
Maleae. It is almost the norm here for analyses of chloroplast and nuclear data to give (very) different topologies (e.g. H. Wang et al. 2024: Figs 1, 2). Malineae (Pyrinae) include the bulk of the tribe and many taxa are ornamentals or have edible fruit. Aldasoro et al. (2005) suggested relationships based on an analysis of morphological and anatomical data. Generic limits have remained difficult, since there is little molecular divergence between many of the genera but considerable divergence within them (Dickinson et al. 2007; Q.-Y. Li et al. 2012: little support for most relationships discussed). Lo and Donoghue (2012) looked at over 330 species and large amounts of data, and they found substantial resolution of relationships, although support along the backbone could still be improved. S.-D. Zhang et al. (2017) found largely similar results with four main groups, but they, too, noted incongruence between their results and those using nuclear markers - hybridization... Relationships immediately outside the inferior-ovaried Malinae in J. Sun et al. (2018: 15 chloroplast genes) are the same as those above, inside that group they found three main clades, Crataegus-Amelanchier, Sorbus-Cotoneaster and Malus-Aria, although support generally was weak except for relationships in the first group. Wang et al. (2024: plastomes of 370 spp., 26 genera) found that Amelanchier, Malus, Sorbus s.l. and Stranvaesia were not monophyletic; they recovered the same three major groupings in the tribe as have commonly been found in analyses of both nuclear and plastome data. B.-B. Liu et al. (2020) found that Raphiolepis was embedded in Eriobotrya using both chloroplast and nuclear data, although other relationships in this area were not so stable; they expanded the limits of the former genus. L. Zhang et al. (2023: 62 transcriptomes/genomes, 23 genera, 2114 orthologous genes, 10564 gene families) recovered 11 clades, mostly well supported although half the nodes along the backbone of the Malinae part of the tree were ± poorly supported. Malus, Sorbus and Pourthiaea were not monophyletic even although sampling within genera was scanty, and topologies based on nuclear and chloroplast data differed considerably (Zhang et al. 2023).
- F. Li et al. (2014) looked at relationships within Cotoneaster, where nuclear and chloroplast genes tended to suggest different relationships, again perhaps because of past hybridization - interestingly, Cotoneaster forms grafts with Crataegus... Meng et al. (2021) in an extensive plastome/nucleome study of Cotoneaster also found evidence of hybridization, and the previously recognized sections were not supported.
- Lo et al. (2007, 2009b), Zarrei et al. (2015), Liston et al. (2021) and others have focused on Crataegus s.l. where hybridization and apomixis confuses the issue (see also Zarrei et al. 2014, also Divergence & Distribution above). Interestingly, overall relationships in Crataegus based on analyses of both nuclear - some 244 genes - and plastome data for 24 species that Liston et al. (2021) obtained were similar, although there was hybridization between species in different subgenera, etc.. X.-H. Lin et al. (2025: 58 spp.) examined plastome-based relationships, and the subgeneric relationships that they obtained were [[Mespilus# [Sanguineae + Americanae]] [Crataegeae + Brevispineae#]] (# = 1 species only), support quite strong.
- B.-B. Liu et al. (2021b/2022) and G.-N. Liu et al. (2023) discuss relationships in the Malus area; hybridization again.
- For relationships around Photinia, see W. Guo et al. (2020) and Wang et al. (2024: plastome data, paraphyly of Stranvaesia not discussed).
- Z.-T. Jin et al. (2024) looked at relationships within Pyrus (32 spp., 32 plastid coding and 801 single-copy nuclear genes), and found that although there were two main groupings (≡ subgenera) there was hybridization between members of the two, also, different specimens of the one species often did not group together.
- M. Li et al. (2017) examined relationships within Sorbus s. str.; Ulaszewski et al. (2021) suggested that plastome data supported generic segregates in the Sorbus area, however, in a plastome analysis by J.-H. Ma et al. (2023) five of the genera segregated by Rushforth (2018) from Sorbus were found to be paraphyletic.
Spiraeeae. S.-X. Yu (2018) discused relationships within Spiraea, and they found previously-recognized infrageneric taxa to be unsupported.
Classification. Fruit types are certainly not as good indicators of relationships within Rosaceae as was for a long time thought. Thus the old Spiraeoideae (here = Amygdaloideae; changed iii.2014) used to be considered a very natural group (e.g. Kalkman 1988) characterised by follicular fruits, but they have turned out to be strongly paraphyletic, here including taxa from both Prunoideae/Amygdaloideae and Maloideae; Prunoideae were characterized by their drupaceous fruits and Maloideae by their pomes (a pretty useless term). However, chemistry, chromosomes and fungal associations all support the realignments suggested by molecular data (especially Morgan et al. 1994), as does developmental work by Evans and Dickinson (1999a, b, 2002). Note that tribes in the old Spiraeoideae may represent clades (see especially Evans et al. 2002; Potter et al. 2007a). The classification here largely follows that of Potter et al. (2007a).
Clade limits in Potentilleae (Rosoideae) are becoming clearer (Dobes & Paule 2010; c.f. Soják 2008 for an alternative). Eriksson et al. (2022) analysed the consequences (number of genera to be recognized, typification issues, number of transfers needed, recognizability of genera, etc.) of various possible limits for Potentilla. These ranged from including all Potentilleae in Potentilla - probably 800 or so combinations would be needed, even if the resultant genus might be quite easy to recognize - to recognizing a small clade that includes the type of Potentilla, P. reptans, as Potentilla s. str. - what happens to the rest of Potentillinae is then left very much to the imagination, but see Kechaykin and Schmakov (2016) for one solution. Alchemilla is to include Aphanes and Lachemilla (Gehrke et al. 2008). The solution that Eriksson et al. (2022) prefered seems reasonable, i.a. it would include all the lower-level hybridization events known from this part of the tree in the one genus - see the synonymy of Potentilla in the family list. Boza Espinoza et al. (2022) recognized five sections in Polylepis, one with four and another with three subsections. T.-R. Huang et al. (2023) divided Rubus into ten subgenera, representing the ten groups that were recovered in both plastome and nuclear analyses, however, relationships both within and between these groups differed in the two sets of analyses, so they stopped at subgenera.
Generic limits in Malinae (Amygdaloideae) may reflect the European origin of taxonomy and the fact that the group is common in Europe (e.g. Walters 1961). However, there is now support for many of the main clades within Malinae and one can aspire (perhaps) to a stable taxonomy; Sorbus and some other genera have turned out to be polyphyletic (e.g. Lo & Donoghue 2012; H. Wang et al. 2024), although it does not make deciding on generic limits any easier (Gehrke in Kadereit et al. 2016). Robertson et al. (1991) recognised 28 genera in Maloideae (= Malinae), Rushforth (2018, see also 2020) 45 genera (and counting); Christenhusz et al. (2018) made 843 species combinations in Pyrus to accomodate their broad view of that genus (≡ Malinae), it included around 1,000 species while J.-H. Ma (2023) synonymized five of Rushforth's new genera under Micromeles, sister to Sorbus... H. Wang et al. (2024) recognized three subribes, one new, within Maleae; they provided a checklist for Photinia while noting the potential hybrid origin of this genus (and Cotoneaster). Altogether something of a disaster area.
Liston et al. (2021) noted that subgenera in Crataegus (for which, see Ufimov & Dickinson 2020: 5 subgenera, subgenus Sanguineae with three sections; there are also series) had both morphological and molecular support, but not the sections. For a synopsis of Rhaphiolepis, see B.-B. Liu et al. (2020). Prunus is circumscribed broadly below, and although Shi et al. (2013) produced an infrageneric classification, it does not agree that well with the relationships suggested by L. Zhao et al. (2016c). J. Zhang et al. (2021) thought that at least Cerasus should be kept separate, but the nomenclatural consequences of doing this were not explored. Malus is also circumscribed rather broadly - see G.-N. Liu et al. (2023) for a justification; two subgenera are recognized in Pyrus (Z.-T. Jin et al. 2024).
Previous Relationships. Rosaceae as circumscribed above are holding together very well despite their morphological heterogeneity, but there have been departures. Chrysobalanaceae, often associated with Rosaceae in the past, are well embedded within Malpighiales, Quillaja rather unexpectedly is an isolated clade within Fabales, while the poorly-known Guamatela is correspondingly isolated in Crossosomatales (Oh & Potter 2006).
And what about Brachycaulos (B. simplicifolius)? This little plant from the Himalayas was originally placed in Potentilleae by Dixit and Panigrahi (1981), but they later moved it to its own family: It is a tufted, almost glabrous plant with simple leaves that have stipules adnate to the petiole, and the flowers are terminal and with 5 stamens opposite the sepals and 2 carpels with terminal styles. Kalkman (2004: p. 384) thought that it "almost certainly" was not Potentilleae, the name was not mentioned by Eriksson et al. (2022), while Mabberley (2017) suggested that it might even be Saxifragaceae...
Botanical Trivia. Trees of Polylepis tarapacana grow at some 5,100 m in Bolivia, the highest altitude for any tree (Hoch & Körner 2005) - see also Tibetan Juniperus (Cupressaceae).
[[Rhamnaceae [Elaeagnaceae [Barbeyaceae + Dirachmaceae]]] [Ulmaceae [Cannabaceae [Moraceae + Urticaceae]]]]: styles branched; ovules apotropous, trans-spliced intron in nad1 gene [cis-spicing elsewhere].
Age. The age for this node is some (70-)67, 65(-62) Ma (Wikström et al. 2001), (86-)76, 73(-65) Ma (Bell et al. 2010), ca 68.4 Ma (Naumann et al. 2013), ca 78.1 Ma (Tank et al. 2015: Table S2) or ca 102.4 Ma (Gu et et al. 2024).
Evolution: Genes & Genomes. For nad1 intron splicing, see Qiu et al. (1998).
[Rhamnaceae [Elaeagnaceae [Barbeyaceae + Dirachmaceae]]]: petiole bundle arcuate; A = and opposite C/alternate with P; capsule septicidal; ovule basal, ascending, parietal tissue 5-6 cells across; coat multiplicative, exotesta palisade, thick-walled; cotyledons large.
Age. Estimates of the age of this node are (67-)64-62(-59) Ma (Wikström et al. 2001), (81-)71, 69(-60) Ma (Bell et al. 2010), (315-)287(-239) Ma (T. He & Lamont 2022: Rham + Elaeagn), (178.1-)144.4(-116.7) Ma (Y.-S. Chen et al. 2023) and ca 97.9 Ma (Gu et al. 2024).
Evolution: Divergence & Distribution. Note that most of the apomorphies mentioned above are those of the [Dirachmaceae + Rhamnaceae + Elaeagnaceae] clade for the pre vi.2011 version of the site; they must now reverse - or be uncertain - in the poorly-known Barbeyaceae if it is embedded in that group... Dense, curly hairs on the abaxial surface of the leaf blade could be an apomorphy here (Sytsma et al. 2002), but Qiu et al. (1998) put this feature at a higher node. For changes in infratectum morphology on the tree, see also Doyle (2009).
Chemistry, Morphology, etc.. The wood anatomy of Dirachmaceae is particularly similar to that of Rhamnaceae (Baas et al. 2001).
A granular layer below the tectum is more or less developed in both Rhamnaceae and Dirachmaceae. The flattened seed with an antiraphal vascular bundle of Dirachmaceae is similar to seeds in Rhamnaceae (Boesewinkel & Bouman 1997); the distribution of testal and tegmic epidermal cells with sinuous anticlinal walls could be interesting.
Phylogeny. Relationships in this group are discussed above.
RHAMNACEAE Jussieu, nom. cons. - Back to Rosales
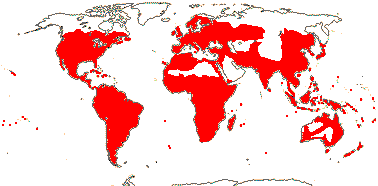
Shrubs to trees; chelidonic acid +; saponins, biflavonyls, benzylisoquinoline alkaloids +, myricetin, ellagic acid 0; (vessel elements with scalariform perforation plates); libriform fibres +; sieve tubes lacking non-dispersive protein bodies, plastids with starch grains only; lysigenous mucilage cavities +; leaves opposite or spiral, lamina vernation conduplicate(-plicate) or involute, (margins entire), venation pli-nerved or strong veins at base, (stipules 0), colleters +; (plant dioecious); flowers small, 4-5(-6)-merous; K (connate), with a longitudinal median ridge adaxially, C cucullate, enfolding A; A adnate to base of C; tapetal cells uni/binucleate; exine infratectum granulate-intermediate, nectary as disc on ovary or revolute and annular or undistinguished on hypanthium; G [2-3(-5)], opposite K or odd member adaxial, style + or styles separate, with canals as many as carpels (one; 0), stigma papillate; ovules (2/carpel), (median), (apotropous), exostomal (bistomal), outer integument 4-10 cells across, inner integument 3-4 cells across, nucellus conical, parietal tissue 4-7 cells across, nucellar cap to 6 cells across, nucellus ± protruding through micropyle, hypostase +, funicular obturator +; often several megaspore mother cells, (embryo sac bisporic, 8-nucleate - Allium type), antipodals degenerate; fruit with raised annular rim at base [= deciduous hypanthium]; seeds often laterally flattened, (arillate); testa with median integumentary antiraphe bundle +/0, (mesotesta with a few sclerotic cells), endotegmen of cuboid cells, with scalariform thickenings (slightly lignified); endosperm +, (starchy), (perisperm +?), polyembryony common, embryo chlorophyllous; x = 12 (?6), nuclear genome [1 C] (0.025-)0.737(-21.66) pg.
63 [list: to tribes]/1055 - three main groups below. World-wide, especially tropics and warm temperate regions. Map: see van Steenis and van Balgooy (1966), Meusel et al. (1978), Frankenberg and Klaus (1980), Richardson et al. (2003), Australia's Virtual Herbarium (consulted xii.2012), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5. (2010: C. Asia?). [Photo - Flower, Dry fruit, Fleshy fruit.]
Age. Bell et al. (2010) thought that crown Rhamnaceae were (73-)62, 59(-46) Ma, Richardson et al. (2004), 49.5-43.6 Ma, while at (100.6-)92.6, 91.4(-84.1) Ma the age in Onstein et al. (2015) was (up to 2022) more compatible with the fossil record; (293-)259(-218) Ma (T. He & Lamont 2022), (164.5-)135.3(-110.2) Ma (Y.-S. Chen et al. 2023), ca 113.5 Ma (Tian et al. 2024) and ca 72.1 Ma (Gu et al. 2024) are recent estimates, so things are up in the air.
Fossils from the Cretaceous-Cenomanian, some 94 Ma, have been identified as Rhamnaceae (Crepet et al. 2004 for references). Calvillo-Canadell and Cevallos-Ferriz (2007) placed Mexican fossils, Coahuilanthus (c.f. Rhamneae), from the late Campanian (ca 73 My) onwards in Rhamnaceae, while the age spread in Gu et al. (2024) is 83.6-72.1 Ma; fossils from the late Campanian itself have spathulate petals ca 0.7 mm long. Apparent rhamnaceous fossils with puzzling fruits and leaves from the Cretaceous-Maastrichtian of Colombia that are dated to some 68 Ma are also in serious conflict with dates of diversification within the family if assigned to tribes, but not if they are placed incertae sedis in the family (Correa et al. 2010). The fossils in amber from Myanmar identified as Phylica by Shi et al. (2022, see also below) lead to a suggestion that the crown-group age for Rhamnaceae, at (293-)259(-218) Ma, was over double previous estimates (Lamont & He 2022). See also Friis et al. (2011) and Jud et al. (2017) for early fossils.
[Rhamnoideae + Ampeloziziphoids]: ?
Age. The age of this clade is ca 96.3 Ma (Y.-S. Chen et al. 2023).
1. Rhamnoideae Eaton
Ovary inferior; fruit indehiscent; nuclear genome [1C] ca 1.33 pg.
Tropical, north temperate.
[Maesopsideae [Fenghwaia + Rhamneae]]: ? leaf venation pinnate.
Age. The age of this node is estimated to be (113-)91(-70) Ma (T. He & Lamont 2022) or ca 76.5 Ma (Y.-S. Chen et al. 2023).
1A. Maesopsideae Engler & Weberbauer - Maesopsis eminii Engler
Leaves (opposite), lamina margin strongly glandular-toothed, venation pinnate; G single-celled, style lateral; fruit a drupe, 1-seeded; endosperm copious n = 9.
1/1. Tropical Africa.
Age. This node is ca 73.8 Ma (Y.-S. Chen et al. 2023).
Fenghwaia gardeniicarpa G.-T. Wang & R.-J. Wang
Inflorescence subfasciculate; pollen smooth to perforate; G inferior, 3-locular, stigmas ± separate, capitate; fruit capsular, dehiscence down septal radii, with five longitudinal ridges, K persistent; seeds verrucose, flattened, with pronounced basal appendage [?funicular]; endosperm slight, embryo chlorophyllous.
1/1. Guangdong Province, S. China.
1C. Rhamneae Horaninow —— Synonymy: Frangulaceae de Candolle
(Trees deciduous), (climber); (thorns +); (hypanthial nectary - Frangula); C (0); (reproductive cycle >1 yr - Berchemia); fruit fleshy; (endosperm 0); n = 6, 10, 12, 13.
15/306: Rhamnus (103), Berchemia (47). World-wide, especially tropics and warm temperate; not southern South America. (Map: see Hauenschild et al. 2018b).
Age. Crown group Rhamneae are some (31.2-)28.5, 27.6(-24.9) Ma (Richardson et al. 2004), ca 48.5/46.4 Ma (Onstein et al. 2015), (69.7-)55.6(-40.5) Ma (Y. Yang et al. 2019) or ca 55.5 Ma (Y.-S. Chen et al. 2023)..
Age. The age of this clade is estimated to be ca 66 Ma (Jud et al. 2017).
Flowers of Notiantha grandensis, from Patagonia and dated to just after the K/P boundary (there are also rhamnaceous leaves in the sediments), have been associated with this node (Jud et al. 2017) - check.
2A. Ventilagineae Bentham & J. D. Hooker
Lianes, ± twining; leaves 2-ranked, lamina with secondary venation palmate; inflorescence fasciculate to cymose; G [2], semi-inferior; fruits (?dehiscent), ± winged, wing apical; endosperm 0; n = 12.
2/51: Ventilago (40). Esp. Indo Malesia to the Pacific and Australia, 2 spp. Africa and Madagascar. Map: see Hauenschild et al. (2018b).
Age. This clade - Ventilago only - is some 33.4 Ma, and if sister to Maesopsideae, etc., that clade is ca 96.3 Ma (Y.-S. Chen et al. 2023).
[Bathiorhamneae [Doerpfeldieae + Ampelozizipheae]]: ?
Age. This clade is ca 72.2 Ma (Y.-S. Chen et al. 2023).
2B. Bathiorhamneae J. E. Richardon - Bathiorhamnus Capuron
lamina with secondary venation palmate; inflorescence dichasial or fasciculate; (G inferior); fruit with 3 mericarps; n = ?
1/7. Madagascar.
Age. This age of this clade, which includes some erstwhile species of Ziziphus, is ca 48.9 Ma (Y.-S. Chen et al. 2023).
[Doerpfeldieae + Ampelozizipheae]: ?
Age. This clade is ca 33.6 Ma (Y.-S. Chen et al. 2023).
2C. Doerpfeldieae J. E. Richardson - Doerpfeldia cubensis Urban
Stem climber; lamina with secondary venation palmate; inflorescence dichasial or fasciculate; (G inferior); fruit a drupe; n = ?
1/1. Cuba.
2D. Ampelozizipheae J. E. Richardson - Ampelozizyphus Ducke
lamina with secondary venation palmate; inflorescence dichasial or fasciculate; (G inferior); fruit explosive septicidal capsule; n = ?
1/2. Northeast South America.
3. Ziziphoideae Luersson
Lamina (with secondary venation palmate)); nectary position variable; G semi-inferior to inferior; (embryo sac bisporic, eight celled [Allium type]).
Pantropical, warm temperate.
Age. Ziziphoideae have been dated to ca 121 Ma (Y.-S. Chen et al. 2017: [Sarcomphalus + Ziziphus]), (93.2-)89.8(-74.6) Ma (Hauenschild et al. 2018b) and ca 230 Ma (T. He & Lamont 2022) or ca 101.3 Ma (Chen et al. 2023).
Roots with N-fixing Frankia; (leaves spiral, venation palmate).
1/53. North America. Map: see Hauenschild et al. (2018b).
Age. Crown group ages range from (34.7-)24.4, 23(-0.3) Ma (Tedersoo & Brundrett 2017; Onstein et al. 2015) to (15.7-)12.5(-12.9) Ma (Hauenschild et al. 2018a) and ca 26.2 Ma (Y.-S. Chen et al. 2023).
3. Alphitonia Endlicher
4/23: Alphitonia (15). Malesia, Australia (not the S. and S.E.), Pacific Islands to New Caledonia and Hawaii.
Age. The age of Alphitonia s.l. is some (40.8-)29.9(-18.1) Ma (Hauenschild et al. 2018b) or ca 50.4 Ma (Y.-S. Chen et al. 2023: inc. Emmeno.).
[Phyliceae [Gouanieae + Paliureae]]: ?
3C. Phyliceae Endlicher —— Synonymy: Phylicaceae J. Agardh
Ericoid shrubs; lamina linear, revolute/flat, underside tomentose, (stipules 0 - nearly all Phylica); inflorescences very variable, inc. capitate/flowers axillary/terminal; flowers (tiny, 3> mm long); C (0), linear to ovate, (clawed); G inferior, stigma terete/capitate/± 3-lobed; fruit a ?loculicidal capsule; seeds arillate; n = ?
4/135: Phylica (132). Southern and Eastern Africa, especially the Cape of South Africa, Madagascar, scattered on islands from St Helena to New Amsterdam and Réunion. Map: see Hauenschild et al. (2018b).
Age. Crown group ages are (41.7-)31.1, 27.5(-22.7) (Onstein et al. 2015), 36.5-10.3 (Onstein et al. 2014), ca 15 Ma (Richardson et al. 2001a), 26.7-19.8 Ma (Richardson et al. 2004: check), (32-)20.3(-14.7) Ma (Hauenschild et al. 2018b) or ca 38.9 Ma (Y.-S. Chen et al. 2023).
Fossil remains in 99 Ma amber from Myanmar have been named Phylica piloburmensis and Eophylica priscastellata, the former placed in crown-group Phylica, the latter sister to Phylica (C. Shi et al. 2022) - conifers or green algae (Electrophycus astroplethus - see Poinar & Brown 2021) were their previous incarnations. Note that 99 Ma is three to nine times older than previously suggested ages for Phylica... This fossil is discussed further below.
[Gouanieae + Paliureae]: tapetal cells with large polysaccharide-containing vesicles.
Age. The age for this node may be around (89.5-)77.8(-70.6) Ma (Hauenschild et al. 2018b).
3D. Gouanieae Reichenbach —— Synonymy: Gouaniaceae Rafinesque
Lianas, stem tendrils (shrubs), ((annual) herbs - Crumenaria); (leaves ± 0 - C.); G inferior; fruit usu. longitudinally winged, K persistent, schizocarpic, (; n = 11.
7/75: Gouania (60). Pantropical, some subtropical. Map: see Hauenschild et al. (2018b).
Age. Crown-group Gouanieae are (56.3-)46.9(-21.5) Ma (Hauenschild et al. 2018b) or ca 76 Ma (Y.-S. Chen et al. 2023).
Age. Nge et al. (2024: Fig S1a) suggested that the age of a [Granititus (?near Alphitonia) + Pomaderreae] clade is ca 47.8 Ma and that of a [Hovenia (Paliureae) + Pomaderreae] clade is ca 53.2 Ma.
3E. Paliureae Endlicher —— Synonymy: Ziziphaceae Adanson
Plant (climbing); (thorns, ?stipular spines +), ectomycorrhizal; inflorescence cymose; G (superior); fruit dry/fleshy; (endosperm 0); n = 6, 12, 13, 18, 20...
3/108: Ziziphus (100). ± Pantropical to warm temperate (Europe to East Asia). (Map: see Hauenschild et al. 2018b).
Age. Crown-group Paliureae date to (34.7-)31.6, 30.6(-27.5) Ma (Richardson et al. 2004), (82.6-)75.8(-68.4) Ma (Hauenschild et al. 2018b) or even ca 89.8 Ma (Y.-S. Chen et al. 2023).
Fossils assigned to Paliurus are reported from ca 66 Ma deposits in the Deccan Traps (Manchester & Kapgate 2014).
3F. Pomaderreae Endlicher
Plant ecto- (dual) mycorrhizal; hairs stellate; (plant thorny - Crypt.); leaves (opposite); flowers (solitary); fruit a ?loculicidal capsule; seeds arillate; n = 12, 18.
12/242: Pomaderris (70), Cryptandra (65), Spyridium (40), Stenanthemum (26). Australia: see map in Hauenschild et al. (2018b).
Age. Crown group ages are ca 41 Ma (34.4, 32.3 Ma?: Onstein et al. 2015), ca 30 Ma (Zanne et al. 2015: see Tedersoo & Brundrett 2017), (28.4-)21.8(-16.8) Ma (Hauenschild et al. 2018b), ca 41.7 Ma (Y.-S. Chen et al. 2023) or ca 35.3 Ma (Nge et al. 2024: Fig. S1a).
3G. Colletieae Endlicher
Plant thorny [thorns from the upper serial buds]; roots with N-fixing Frankia; stems green, (thorns = cladodes); serial buds +; leaves opposite, often much reduced/0, stipules also petiolar [Colletia]; hypanthium (long and tubular), nectary +; G (superior); fruit (fleshy), (capsular, explosive); seeds arillate, aril often separating from seed; n = 11.
7/25: Discaria (6). Most South America, also North America and the Antipodes. Map in Hauenschild et al. (2018b).
Age. This clade is some (24.6-)10.3(-8.6) Ma (Hauenschild et al. 2018b) or ca 50.9 Ma (Y.-S. Chen et al. 2023: inc. Schistocarpaea, ca 29.0 Ma not).
Floral formula: * ⚥ K 5; C 5; A 5 opp. C; N; G [3] / [3].
The capsules of Rhamnaceae are rather similar to those of Euphorbiaceae, Phyllanthaceae and Picrodendraceae; in all these groups there are taxa that have 1-2 seeds per loculus and a capsule that falls to bits as it dehisces. The fruit wall often separates into two layers; the inner layer, woody, twists as dehiscence occurs so helping to eject the seed. However, since the seeds are basal in Rhamnaceae there is no columella as is so typical of the other families, and the latter also lack a hypanthium. Phyllanthaceae and Picrodendraceae have two seeds per carpel, Euphorbiaceae have one seed per carpel.
Evolution: Divergence & Distribution. Millan and Crepet (2014) discuss a Solanaceous fossil from the Eocene that they confidently assign to Rhamnaceae. On the other hand, the 99 Ma Rose Creek flower, previously thought to be close to Rhamnaceae, has been described as Dakotanthus cordiformis and with ten stamens is certainly not Rhamnaceae, rather, it is perhaps close to Fabales-Quillajaceae (Manchester et al. 2018a); see Friis et al. (2011) for other Caenozoic fossils. For more dates, see Hauenschild et al. (2018b) and Y.-S. Chen et al. (2023: Fig S1) - the topology in this latter differs somewhat from that above.
Richardson et al. (2004, see also Richardson et al. 2001a) discuss the evolution and historical biogeography of the family. They noted i.a. the rapid diversification of the speciose Phylica (Ziziphoideae) within the last ca 8 My; nearly all the species of Phyliceae as a whole are to be found in the Cape Floristic Region of South Africa (Linder 2003). However, how Phylica gets around is unclear since the genus also inhabits a number of isolated oceanic islands; some 8,000 km separates P. arborea on Gough island - midway between South America and the southern tip of Africa - and Amsterdam island - midway between Australia and the southern tip of Africa (Richardson et al. 2003).
This introduces the small matter of Eophylica priscastellata and Phylica piloburmensis, flowers placed in Rhamnaceae that were found in 99 Ma amber from Burma (C. Shi et al. 2022), P. piloburmensis being unusual in that, despite its age, it was described in an extant genus. For Eophylica, see also Poinar and Chambers (2022), who discussed its "so-called 'pseudanthial head'". Given these findings, Lamont and He (2022: Fig 1; T. He & Lamont 2022) suggested an origin of the family on Gondwanan Antarctica and its migration north during the Cretaceous. He and Lamont (2022) looked at some features characteristic of fire-prone vegetation and noted that they characterized Ziziphoideae, which includes Phylica, indeed, they thought that Rhamnaceae as a whole might have evolved in such vegetation. These authors noted that Belcher et al. (2010) had found that fire was particularly prone to develop in the period 340-250 Ma, and they suggested that this might be associated with the evolution of Rhamnaceae then - that is, the family, not to mention angiosperms as a whole, would be very old, even older than many molecular ages. However, two recent papers help clarify the situation. Oskolski et al. (2024), with a focus on Rhamnaceae and to a certain extent other Rosales, suggested that E. priscastellata, with its epicalyx, octomery and pendent ovules, hardly fitted in Rhamnaceae - Dirachmaceae were perhaps possible, but... As to P. piloburmensis, its imbricate petals and stamens with longer filaments and no obvious petal-stamen complexes also made its identity suspect. Beurel et al. (2024) re-examined the original images of the flowers of P. piloburmensis, noting that they had "a spiral phyllotaxis, undifferentiated perianth, a floral cup, stamens with a pair of basal filament appendages (glands), bilocular valvate anthers with apical flaps, inner staminodes and a likely unilocular (semi-inferior or superior) ovary", that is, it seems a rather different plant from that described by Shi et al. (2022). Given this list of features, it is not surprising that Beurel et al. (2024) thought that the affinities of the fossil might be lauralean, and they redescribed it as Nothophylica piloburmensis. Indeed, a position in Laurales near Hernandiaceae-Lauraceae was retrieved by Beurel et al. (2025) in an analysis of morphological characters, there were a number of positions up to three steps longer, but all were in Laurales, exclusing Calycanthaceae. (Interestingly, fossil buds of this plant had been identified as a gymnosperm seed, and Eophylica itself (sterile) had earlier been described as Electrophycus astroplethus (Poinar & Brown 2021) and considered to be a new genus of green alga and apparently the earliest name for this plant (but c.f. Poinar & Chambers 2022).)
Hauenschild et al. (2018a, esp. b) looked at the biogeography of other Ziziphoideae and suggested that vicariance might be involved in the distribution of some taxa that are now widespread, including Paliureae, Pomaderreae, and Alphitonia s.l.. Y.-S. Chen et al. (2017) thought that Paliurus itself was on India as it rafted north during the Cretaceous, and indeed it has been found fossil from all three northern continents in the Caenozoic (Eocene and younger); extant taxa are known from the Mediterranean (including North Africa) to Japan. However, other genera like Gouanieae and Phyliceae seemed to have nice Gondwanan distributions, although they were young (but see above); long distance dispersal has apparently been involved in the achievment of their ranges. Alphitonia and relatives probably originated in Australia (Hauenschild et al. 2018a).
Diversification in Rhamnaceae has been looked at in the context of its response to climate change at the end of the Oligocene (cooler, drier conditions then), particularly as the family moved into seven (warm) temperate areas with Mediterranean climates, and there was high diversification in such areas, less so in the tropics (Tian et al. 2024). These movements were mostly between 35.0 and 24.5 Ma (upper limits of 4/6 ages ca 83.5 Ma), and the Cape and South West Australia had notably high species:area ratios; diversification was somewhat later and there may have been an element of preaption involved (Tian et al. 2024). Diversification in the Southern China area was somewhat older, ca 49 Ma, and the evolutionary dynamics there differed, while in tropical areas, also older, there had been lower diversification and higher extinction rates (see also southwestern Australia and the Pomaderreae - Nge et al 2024).
Rhamnaceae are quite conspicuous in extant Mediterranean ecosystems (for such ecosystems in general, see Rundel et al. 2016). Onstein et al. (2015) estimate that about 112 species in five groups are found only there, with another 187 species found in those areas and also elsewhere - the five groups involved are 1, Ceanothus in California, 2, Phyliceae in the Cape, 3, Pomaderreae especially in western Australia, 4, Colletieae in Chile and 5, Rhamneae in the Mediterranean.
1. Over three quarters of the species of Ceanothus in the Californian chaparral are reseeders (Wells 1969), although some species in subgenus Ceanothus there have massive lignotubers (Keeley et al. 2024). Colonization of and diversification in these Mediterranean ecosystems was, Onstein et al. (2015) thought, not simultaneous, furthermore, Rhamnaceae were to be found in all these areas well before the onset of the winter rainfall regime, indeed, their diversification rates seem unaffected by the timing of this onset. Thus most diversification within Ceanothus has been within the last ca 6 Ma, before the origin of the Mediterranean-type vegetation the genus now favours, although proto-Mediterranean climates developed in the Californian region ca 15 Ma (Burge et al. 2011; Onstein et al. 2015).
2, 3. In the Cape (for its climate in the context of changing ocean curents, see Dupont et al. 2011) and Western Australia there seem to have been very low extinction rates, and Phyliceae and Pomaderreae there are xeromorphic, a connection being the nutrient-poor soils in both areas on which the plants grow (Onstein & Linder 2016).
3. Pomaderris (Pomaderreae) is a largely Australian genus. Here vicariance via the uplift of the Nullarbor Plain may have set the stage for its diversification, and there appear to have been at least six independant dispersal events to New Zealand in the last ca 5 Ma (Nge et al. 2021b). Indeed, species distributions/relationships in Pomaderris and in Cryptandra (also Pomaderreae) within Australia seem to fit the peripheral vicariance pattern, the genera becoming restricted to the periphery of the continent after the drying out of the centre, a process that began in the Eocene (Nge et al. 2023: Fig. 1 for other examples). The speciation rate in Cryptandra has declined as aridity in Australia has expanded (Nge et al. 2023). In Pomaderreae as a whole there has been much polyploidy (44% of the species, 7/10 genera), but this is associated neither with notable effects on diversification rates nor with an increased niche occupancy, although in Southeast Australia polyploid Pomaderris grows in wetter niches and the diversification rate there is quite high (Nge et al. 2024). Interestingly, these authors found that although there are a notable number of Pomaderreae in southwest Western Australia they are not polyploids, rather, the stable climate and geology has allowed species to accumulate there, and extinction has been low.
4. Ochetophila trinervis (Colletieae) is found both in the southern Andes and on the subantarctic Marion Island, some 7,500 km or more distant, and in this case fairly recent long distance dispersal by the swallow Hirundo rustica (it apparently eats seeds on occasion) is suspected (Kalwig et al. 2019).
Y.-S. Chen et al. (2023) looked at correlations between diversification rates (= speciation minus extinction) and propagule dispersal, and found that these rates were highest in myrmecophilous and diplochorous clades; even although speciation rates were low here (lower than in taxa with wind or vertebrate distribution, for example), extinction rates were still lower.
Ecology & Physiology. There seem to have been three origins of the climbing habit in the family (Richardson et al. 2000a), and in two of these the plants have winged fruits; for twining stem climbers here, see Sousa-Baena et al. (2008b).
Recently C. Shi et al. (2022: p. 135) have noted both the evidence for fire in amber from Myanmar ca 99 Ma and the presence of what they thought was fossil Phylica in that amber: "Our results demonstrate that a key element of the xeromorphic fynbos vegetation [Phylica] existed as long as 99 Ma ago, as well as the great antiquity of open fire-prone vegetation in Gondwana" (see also He & Lamont 2022; Lamont & He 2022). Phylica is now largely southern African, but Shi et al. (2022: Fig. 5b) suggest that it may have been on both southern Africa and then-adjacent India ca 100 Ma, moving north on the latter; however, this whole scenario is rather problematic, and for further discussion, see above.
Origins of N-fixing in Rhamnaceae are quite recent compared to those in other families - late Eocene to Oligocene are the dates suggested (H.-L. Li et al. 2015: support for sister-group relationships low).
Pollination Biology & Seed Dispersal. Pseudanthia are known from Rhamnaceae, including in Spyridium, Phylica plumosa, etc. (Baczynski & Claßen-Bockhoff 2023). Nectary position is particlarly variable in Colletieae, members of which have a long hypanthial tube, sometimes with a ligule; the nectary can be on the inside of the tube or on the underside of the ligule (Medan & Aagesen 1995; see also Gotelli et al. 2016b for nectaries).
Perhaps a third of the family, all in the Ziziphoideae, have myrmecochorous seeds, thus Pomaderreae, with their arillate seeds, are myrmecochorous (Lengyel et al. 2010). Dispersal of the disseminules is by wind, vertebrates, ants, explosive discharge from the fruits, or diplochory (a combination of the last two); Y.-S. Chen et al. (2023, esp. Table S2) gives dispersal information for all species in the family for which this is known. See Diversity & Distribution above for possible connections between dispersal types and diversification.
Berchemia sinica - indeed, most of the genus - have prolonged reproductive cycles, maybe 14 months long, the zygote being quiescent for nine months or so (F. Ma et al. 2024).
Plant-Bacterial/Fungal Associations. The North American Ceanothus is associated with N-fixing actinomycetes, as are many Colletieae; N-fixing genera may form a monophyletic group, although there is no strong evidence for this yet (Richardson et al. 2000b).
Ectomycorrhizae have been reported from Rhamnus and Pomaderris (Malloch et al. 1980; Tedersoo et al. 2008), while Rose (1980) recorded vesicular-arbuscular mycorrhizae in the N-fixing Ceanothus. Dual mycorrhizal plants are quite common in Ziziphoideae-Pomaderreae (Teste et al. 2019: Table S3); they are not recorded from Ceanothus (the AM—N-fixing correlation again).
For the complex ansamycin maytansinoids found in Colubrina and - the precursors, at least - probably synthesized by a bacterium, see references in Cassady et al. (2004); these have a 19-member ring that i.a. incorporates a chlorinated benzene ring.
Genes & Genomes. cox1 introns are common in Rhamnaceae (Sanchez-Puerta et al. 2008).
Chemistry, Morphology, etc.. Growth patterns and "spine" morphology in Rhamnaceae is variable. Thus looking at the few Colletieae discussed by Tourn et al. (1992, see also earlier papers) and Nesom (2023a) one find variation in spine (= stipule) and thorn presence, in thorn morphology, in the number, disposition and fate of axillary buds, various aspects of shoot growth including whether or not the apex aborts and presence or absence of short shoots, and so on, and even in the apparently more conventional Rhamnus cathartica there are substantial changes during development (Charles-Dominique et al. 2012). Ziziphus jujuba has thorns, but they are in the stipular position. Phyllotaxis in Berchemiella (Rhamneae) is of the complex orixoid type (see elsewhere). Leaves in Colletieae are often at most poorly developed; photosynthesis is carried out by the much-branched, green, thorny stems; in species like Colletia paradoxa, the thorns are cladodes, being laterally-flattened and triangular, the thorn proper being the very tip. The lamina of New World species of Gouania has glands at the base while that of Karwinskia has pellucid dots.
Stamens and petals develop from a common primordium, and the petals are initially much smaller than the stamens (Bennek 1958). Tapetal cells vary in the number of nuclei that they contain and may have large polysaccharide-containing vesicles (Gotelli et al. 2020: Table 1, also references in Gotelli et al. 2023). For pollen tube growth in the hollow styles of Colletieae, which is sometimes through the cells, see Gotelli et al. (2012); hollow styles have been reported elsewhere (Medan 1985: list; Medan & Hilger 1992: Colubrina) and may be an apomorphy for all or part of the family, but they may also be single, or even absent. Is there some kind of chalazal haustorium (see Srinivasachar 1940)? The outer epidermis of the outer integument of the ovule can have rather large cells (Juel 1929). The parietal tissue is ca 13 cell layers across in Colletia, although this may include a nucellar cap (Laguna & Cocucci 1971). The fruit of Fenghwaia has five longitudinal ridges, but three carpels, and is variously described as being dehiscent or indehiscent - the former seems to be correct (G.-T. Wang et al. 2021).
General information has been taken from Brizicky (1964), Richardson et al. (2000a) and Medan and Schirarend (2004), also Kellermann and Udovic (2020: Colletieae); Hegnauer (1973, 1990) summarized chemistry, Cremers (1973, 1974) growth of lianescent taxa, Bennek (1958) and Nair and Sarma (1961), floral morphology and anatomy, Medan and Aagesen (1995), Vikhireva (1952: not read) and Medan and Hilger (1992) comparative floral and fruit morphology, Schirarend and Köhler (1993a, b) and Gotelli et al. (2016a), pollen morphology, Medan (1985, 1988) discussed gynoecial development, Juel (1929) and Arora (1953) ovules and Gama-Arachchige et al. (2013) seed coat anatomy, especially the water gap.
Phylogeny. There are three main clades in the family. Richardson et al. (2000b) found weak support for the basic relationships [rhamnoids [ziziphoids + ampeloziziphoids]], but relationships between the major clades in the ziziphoid group in particular were poorly understood. Basic relationships were better supported in Hauenschild et al. (2016a), and although there was some resolution of relationships in the rhamnoid and ziziphoid groups, the former had one major polytomy and the latter two. H.-L. Li et al. (2015) found the relationships [ziziphoids [rhamnoids + ampeloziziphoids]], and Schistocarpaea was sister to the zizyphoids examined; see also M. Sun et al. (2016). Ziziphus is wildly para/polyphyletic, species occurring in all the three main groups above (Islam & Simmons 2006; Hauenschild et al. 2016a; Z.-D. Chen et al. 2016: Hovenia similar). The tree in Y.-S. Chen et al. (2023: 4 markers) is pretty detailed and some taxa are polyphyletic; many of the tribes above agree with the topology there, but some relationships differ.
For the phylogeny and morphology of Colletieae, see Aagesen (1999: 63 characters, morphological analyses) and Aagesen et al. (2005: morphology plus 1 plastid gene), also Hauenschild et al. (2016a, 2018b); relationships there are [Kentrothamnus, [Retanilla + Trevoa], The Rest].
Phylicieae are monophyletic, but Richardson et al. (2001a) found that Phylica itself might be paraphyletic, Nesiota sometimes being included - but then generic limits can be adjusted; [P. plumigera + P. buxifolia] were sister to the rest of the genus.
Pomaderreae. Kellermann et al. (2005) and Kellermann and Udovicic (2008) looked at relationships here. Nge et al. (2023: 60/65 spp., 30 orthologous nuclear loci) examined relationships in Cryptandra, while Nge et al. (2024) extended this analysis to 215/240 species of the whole Pomaderreae, including all 10 genera.
Rhamneae. For relationships in Rhamnus and relatives, see Hauenschild et al. (2016b) and Y. Yang et al. (2019a), although support for many relationships in the latter is weak.
Classification. For a phylogeny-based classification of the family, see Richardson et al. (2000a). The rhamnoids include three tribes, [Ventilagineae [Maesopsideae + Rhamneae]]. The ziziphoids are made up of five tribes, Phyliceae, Pomaderrieae, Gouanieae, Colletieae, and Paliureae, as well as unplaced genera like Ceanothus and Alphitonia, and include most of the rest of the family. The ampeloziziphoids include three tribes, three genera, and four species (Richardson et al. 2000b), which seems a trifle redundant, hence the tribes are not recognised above. Hauenschild et al. (2016a) adopt the same classification.
Nesom (2023a) discusses generic limits around Sarcomphalus; he splits the genus, monophyletic, into three and suggests that more changes might be in the offing.
[Elaeagnaceae [Barbeyaceae + Dirachmaceae]]: ?
Age. Bell et al. (2010) estimated the age of this node to be (77-)65, 62(-51) Ma and Gu et al. (2024) ca 94.9 Ma - topology in the latter [Dirach [Barb + Elae]], age of the latter pair (90.5-)89.4(-88.4) Ma.
ELAEAGNACEAE Jussieu, nom. cons. - Back to Rosales —— Synonymy: Hippophaeaceae G. Meyer
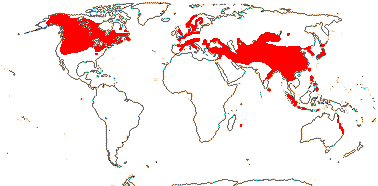
Trees or shrubs, often deciduous; N-fixing Frankia +; dihydroflavonols?, 0-methyl flavonoids, ellagic acid +, myricetin 0; (cluster roots +); cambium storied; phloem stratified; true and fibre tracheids +, vestured pits + [not all], fibre pits bordered; wood with broad rays, prismatic crystals in ray cells; sieve tube plastids lacking both starch and protein inclusions; nodes 1:1; petiole bundles arcuate or annular; no mucilage cells in leaves; hairs lepidote or stellate; leaves spiral or opposite, lamina vernation conduplicate-flat, margins entire, stipules 0; plant dioecious [Hipp. - H.] of monoecious; inflorescence a raceme, or flowers axillary; flowers (2-)4(-6)-merous; hypanthium long to short, nectary + [Elaea.]; P +, uniseriate, 2, 4, 5, ± petal-like; A also 2 x P, borne in throat of tube; pollen 3-nucleate; G 1, stylulus long, stigma decurrent or capitate; compitum necessarily 0; ovule epitropous, micropyle?, outer integument 5-16 cells across, inner integument 3-4 cells across, funicular obturator +; megaspore mother cells several; hypanthium accrescent, fleshy, closely investing fruit; pericarp thin [H.]; testa very thick, exotesta with sinuous anticlinal walls at least in part, (not palisade), mesotesta ± thick-walled; endosperm with chalazal haustorium, (starchy), cotyledons usu. unequal; n = 6, 10, 11, 13, 14, x = 7, nuclear genome [1 C] (0.061-)1.083(-19.358) pg.
3 [list]/102: Elaeagnus (92 - Gu et al. 2024). North Temperate, warm tropical; Malesia and Australia - also quite widely cultivated and/or escaped. Map: from Meusel et al. (1978), Hultén and Fries (1986) and Australia's Virtual Herbarium (consulted ix.2014). [Photos - Collection, Shepherdia Fruit © R. Kowal.]
Age. Bell et al. (2010) estimated that the age of crown-group Elaeagnaceae was (30-)20(-10) Ma while the age suggested in Gu et al. (2024) is (41.3-)40.8(-40.3) Ma.
Floral formula: * ⚥ P (2, 4, 5); A 5 alt. P, 10; N; G 1.
Evolution: Divergence & Distribution. The ancestral area of Elaeagnaceae seems to have been on the Qinghai-Tibet Plateau, and there has been substantial subsequent dispersal, for instance, five times to Japan alone - ?birds (Gu et al. 2024, q.v. also for many dates within the family). Gu et al. (2024) also noted that there was a phylogenetic fuse of around 50 Ma in the family (extinctions), and also a substantial fossil record, which makes the place of origin of the stem group difficult to work out; there was also a phylogenetic fuse of ca 25 Ma within Elaeagnus, which started diversifying a mere ca 14.7 Ma. The family may have originated in mid to high latitudes in the Northern Hemisphere, although divergence within it may have begun in the Qinghai-Tibet Plateau area (Gu et al. 2024). Subsequent movement of the family was probably facilitated by land bridges, although long distance dispersal (birds) is also possible (Gu et al. 2024).
Ecology & Physiology. All genera are associated with N-fixing Frankia, and cluster roots have been reported from Hippophae (Shane & Lambers 2005); carboxylate exudation may help in phosphorus acquisition (Lambers et al. 2012b). For the genome of H. rhamnoides with an emphasis on N fixation, see Z. Wu et al. 2022). The maximum age for the acquisition of N fixation has been put at (101.8-)91.1, 82.8(-64.5) Ma (H.-L. Li et al. 2015: support for sister-group relationship with Barbeya low), compatible with ages suggested by Gu et al. (2024).
Pollination Biology & Seed Dispersal. Hippophae is wind pollinated and not surprisingly lacks nectaries (Erbar & Söte 2022). Dispersal of the fruite by birds has been reported (Gu et al. 2024).
Plant-Animal Interactions. Some lycaenid larvae eat Elaeagnaceae (Fiedler 1995).
Plant-Bacterial/Fungal Associations. Both ecto- and arbuscular mycorrhizal associations have been reported from Elaeagnaceae (e.g. Rose 1980). For the host preferences of rusts, see Savile (1979).
Genes & Genomes. There is a genome duplication in Hippophae rhamnoides, although its exact position in uncertain; there are also a number of gene duplications (Z. Wu et al. 2022).
There are very extensive differences between the nuclear coalescent and plastome trees in the main clade of Elaeagnus (>70% of the genus), i.e. there is substantial cytonuclcear discordance - five reticulations have been detected here (Gu et al. 2024: e.g. Fig. 3).
Chemistry, Morphology, etc.. The androecium is obdiplostemonous according to Huber (1963). When stamens are equal in number to the perianth, they alternate with the lobes; this is consistent with an interpretation of P = K, with antepetalous stamens... Seed anatomy is rather like that of Rhamnaceae (Corner 1976).
For general information, see Bartish and Swenson (2004), for wood anatomy, see Jansen et al. (2000b) and for fruit and seed anatomy of Hippophae, see Harrison & Beveridge (2002).
Phylogeny. Elaeagnus is sister to the rest of the family (M. Sun et al. 2016; Gu et al. 2024; etc). Within Elaeagnus, analysis of nuclear data (75 spp., 83 nuclear genes) suggests that there are three main clades; the proportion of gene trees in concordance with the main tree is ususally low here (Gu et al. 2024: also 71 plastome samples, 78 coding sequences). There is extensive conflict between the nuclear and the plastome trees in both Elaeagnus and Hippophae, hybridization having been likely (Gu et al. 2024).
Previous Relationships. Elaeagnaceae have been difficult to place. They were included in Proteales by Cronquist (1981) because of superficial floral similarities, and in Elaeagnales-Rhamnanae, next to Proteanae, in Rosidae, by Takhtajan (1997).
[Barbeyaceae + Dirachmaceae]: anther connective produced; ovules apotropous, outer integument 3-5 cells across, parietal tissue 5-6 cells across.
Evolution: Divergence & Distribution. Diversification here seems to have slowed down (Magallón et al. 2018).
Chemistry, Morphology, etc.. In both families the embryo sac is quite deep seated, and in Dirachma socotrana in particular the suprachalazal nucellar tissue is very well developed.
BARBEYACEAE Rendle, nom. cons. - Barbeya oleoides Schweinfurth - Back to Rosales
Trees; ellagic acid +; libriform fibres +; sieve tubes with compound perforations, sieve tube plastids with starch grains only; prismatic crystals in ray cells 0; nodes 1:1; no mucilage cells in leaves; stomata laterocytic; hairs unicellular, spirally twisted; leaves opposite, lamina vernation supervolute-curved, margins entire, stipules 0; plant dioecious; inflorescence fasciculate, bracts and bracteoles 0; hypanthium 0; P +, uniseriate, sepal-like, 3-4; A (6-)9-12; pollen exine infratectum granulate-intermediate; nectary 0; G 1-2(-3), ± separate, styluli long, stigma long-clavate, decurrent, ?type; ovule subapical, pendant, micropyle long-endostomal, inner integument 5-6 cells across, nucellar cap ca 5 cells across, hypostase +; fruit a nutlet, P accrescent, wing-like; seed coat undistinguished, exotesta perforated, not palisade, anticlinal walls sinuous, endotegmen tanniniferous, anticlinal walls sinuous; n/x = ?
1 [list]/1. N.E. Africa, Arabia. Map: from Aubréville (1974) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Floral formula: * [♂] [♀] P 3-4; A 9-12. /// P 3-4; G 1-2(-3).
Chemistry, Morphology, etc.. The sieve tubes have compound perforations, unlike Ulmaceae and its immediate relatives and other Rosales.
The perforated seed coat is rather like that common in the Ulmaceae group (Bouman & Boesewinkel 1997).
Additional information is taken from Friis (1993: general), Dickison and Sweitzer (1970: morphology), Tobe and Takahashi (1990: hairs and pollen), and Bouman and Boesewinkel (1997: ovule and seed); Hegnauer (1990) has a little information on chemistry.
Previous Relationships. The monotypic Barbeyaceae were placed in Hamamelididae-Urticales by Cronquist (1981) and in Hamamelididae-Barbeyanae by Takhtajan (1997).
DIRACHMACEAE Hutchinson - Dirachma Balfour f. - Back to Rosales
Shrub; stalked glands +/0; chemistry?; phloem stratified; sieve tube ?plastids, ?non-dispersive protein bodies; nodes ?lacunar; mucilage cells in leaves; leaves spiral, stipules subulate, persistent; flowers single, terminal; epicalyx of 4-8 lobes (in middle of "pedicel"); flowers 5-8-merous; hypanthium short, (K-C tube +), K valvate, basally connate, C contorted, initial development slow, vasculature fan-shaped; nectaries on/near base of C, lacking stomata; A obdiplostemonous, anthers extrorse, long, opening from apex; G [8], deeply longitudinally ridged, opposite the K, style +, stigma clavate or elongated; ?ovule orientation, micropyle zig-zag, inner integument ca 2 cells across, nucellar cap 0, hypostase ?+; fruit beaked, segments opening from the base, wooly inside, columella +, K deciduous above the hypanthium; seeds laterally flattened, integumentary antiraphe bundle +, exotesta with anticlinal walls thickened, tegmen ?multiplicative, endotegmen tanniniferous; endosperm slight, embryo colour?; n/x = ?
1 [list]/2. Socotra, Somalia (map: from Link 1991b).
Floral formula: * ⚥ K (5-8); C 5-8; N; A 5-8 opp. C; G [8].
Chemistry, Morphology, etc.. The single flowers of Dirachma may represent a reduced, cymose inflorescence, the epicalyx consisting of bracts and bracteoles (Ronse De Craene & Miller 2004: Dirachma socotrana). A hypanthium is described as involving just the sepals and petals but perhaps also the stamens (Ronse De Craene & Miller 2004: pp. 117, 123); it is unclear that there is much of a conventional hypanthium. Petal initiation is later than that of the stamens, as is common is rosids, and until quite late in development they are very much shorter than the stamens. There is a lot of starch in the pollen grains (Boesewinkel & Bouman 1997). The presence of an antiraphal vascular bundle means that the outer integument is very thick (6-9 cells or more) when viewed in the abaxial median plane; integument measurements are taken from the sides of the ovule (see Boesewinkel & Bouman 1997). There is a structure on the seed described as a small, funicular aril, perhaps similar to that found in some Rhamnaceae (Ronse De Craene & Miller 2004).
For additional information, see Link (1991b [c.f. 1990], 1994) and Bayer (2004), all general, Baas et al. (2001: wood anatomy), Ronse De Craene and Miller (2004: floral morphology), and Boesewinkel and Bouman (1997: ovule and seed).
Previous Relationships. The exotestal seeds with straight embryos suggest that Dirachma is not close to Geraniaceae (Geraniales), with which Dirachma had been linked, as by Cronquist (1981) - see e.g. Boesewinkel (1985) and Boesewinkel and Bouman (1997). Takhtajan (1997) included Dirachmaceae in his Malvales because of its similarity in gross morphology.
[Ulmaceae [Cannabaceae [Moraceae + Urticaceae]]]: flavonols and their glycosides, myricetin [some Ulmaceae, Cannabaceae] +, ellagic acid 0; latex system + [± throughout the plant]; hairs unicellular and multicellular-glandular; cambium ± storied; libriform fibres +; phloem stratified; cystoliths common, made up of calcium carbonate, epidermal and hair cell wall silicification and calcification common; at least one prominent prophyllar bud; lamina with secondary veins proceeding straight to non-glandular teeth and higher-order veins convergent on those teeth [urticoid], stipules cauline; flowers small [7> mm across], usu. protogynous, often imperfect; hypanthium?, P +, uniseriate, K-like, imbricate; A equal and opposite P; nectary 0; staminate flowers:pollen porate, exine infratectum granular, intine thickening at the apertures [= oncus]; pistillode +[?]; carpelate flowers: staminodes usu. 0; G [2], superposed, abaxial only fertile, stigma/styles spreading, receptive area extending down adaxial surface and ± confluent basally; ovule 1, apical, pendulous; fruit a drupe; testa thin, vascularized, perforated [rarely in Ulmaceae]; x = 14; germination phanerocotylar.
Age. This node may be (61-)57-55(-51) Ma (Wikström et al. 2001), (76-)66, 64(-55) Ma (Bell et al. 2010), ca 70.9 Ma (Tank et al. 2015: Table S2), ca 90 Ma (Manchester 1989b), ca 97.2 Ma (Gu et al. 2024), or still older, (124.7-)118.3(-109.9) Ma (Q. Zhang et al. 2021).
Evolution: Divergence & Distribution. The copious information (but there is less about embryology) on the four families awaits synthesis. Sytsma et al. (2002) should be consulted for details of character evolution; they note that inflexed stamens and their dehiscence, fruit type, and laticifers need detailed study; hypanthium presence and other characters can be added to this list. For laticifers, see recent work by Leme (2018), also Marinho and Teixeira (2018) and Ramos et al. (2019). The walls of the laticifers in Ampelocera glabra and Zelkova serrata (Ulmaceae) for instance, are thick, if unlignified, and look superficially like fibres, and starch and terpenes are in the exudate that they produce (Leme 2018). It is not clear which taxa have a hypanthium; at least some species of Ulmus and Pilea do, but other species of Ulmus and also Zelkova, Ampelocera and Trema show no obvious signs of one (see also Bechtel 1921; Leme 2018). The single perianth whorl, described as sepals here, may indeed be equivalent to the sepals of other Rosales (e.g. Leme et al. 2020; Teixeira et al. 2020c; Pedersoli et al. 2022). Two-ranked leaves may be an additional synapomorphy (or pegged at a still higher level), as well as urticoid teeth. For the evolution of fruits, see Tiffney (1986a).
Dottori (1994) described the features separating the two parts of the old Ulmaceae, Ulmaceae s. str and Cannabaceae (Celtidaceae).
Ecology & Physiology. 12/42 of the commoner species in west Amazonian rainforests are members of this clade (as Urticales: Pitman et al. 2001).
Pollination Biology & Seed Dispersal. For discussion of flowers and inflorescences, the insects that may breed in them, and pollination, see Berg (1990).
Plant-Animal Interactions. Some Nymphalinae-Nymphalini butterflies and other nymphalids like Apaturinae, Libytheinae, Pseudoergolinae, etc., have larvae that eat members of these families (see also under Urticaceae) - but also on the immediately unrelated Euphorbiaceae (Malpighiales: see Ehrlich & Raven 1964). Similarly, caterpillars of Acraea (Acraeinae, also Nymphalidae) are quite common on Urticaceae (including Cecropia), and also on Moraceae, etc.; this particular genus is also commonly found on Passifloraceae and their relatives. The ancestor of Nymphalinae may have fed on Urticaceae and relatives (Nylin & Wahlberg 2008; Wahlberg et al. 2009; Nylin et al. 2014), and some members of the clade shifted to Lamiales around the K/P boundary (Nylin & Wahlberg 2008).
Chemistry, Morphology, etc.. Raffinose and stachyose are common oligosaccharides in phloem exudate in Ulmaceae, Moraceae and Cannabaceae sampled (Zimmermann & Ziegler 1975).The whole group has rather homogeneous wood anatomy: Rays are relatively broad, pits are simple, intervessel pitting is alternate, fibres are septate, and parenchyma is paratracheal (Baas et al. 2000). For torus-margo pits, see above. For the distinctive composition of the cystoliths, calcium carbonate (CaCO3), rather unusual in flowering plants, see Karabourniotis et al. (2020). Ampelocera seems to be the only member of Ulmaceae recorded as having cystoliths (Fernández Honaine et al. 2023; Fernández Honaine et al. 2023). Indeed, minerals of various kinds are deposited in the leaves, and cystoliths are common (Piperno 2006; see Satake 1931 for spodograms). The cystoliths are globose to elongated, and they are amorphous CaCO3 concretions with a stalk and/or centre part made up of silica (Pierantoni et al. 2018 for cystoliths and other kinds of foliar mineralizations in Ficus).
Because of the well-developed prophyllar bud(s), the inflorescences are often paired, with a bud between them (Berg 1989), and/or the branches may have a bud on one or both sides at the base. Ulmus does not appear to show this arrangement, and it is tentatively placed as an apomorphy at the [Cannabaceae [Urticaceae + Moraceae]] node below. The "stipular buds" of Cannabis (Miller 1970 and references) are really prophyllar buds.
Teixeira et al. (2020c) survey floral construction in the group as a whole. Staedler (1923) discussed the absence of an anther epidermis, although it is present in Ficus (other Rosales?); there is no obvious link with dehiscence mechanisms. The pollen here is often described as bein porate, although Basak et al. (2023) suggest that that at least of Cannabis and some Urticaceae is pororate. Starchy pollen is common, but apparently not in Urticaceae. Bechtel (1921) and Eckardt (1937) described gynoecial morphology in considerable detail. Taxa with a perforated testa are quite common, although this feature may have arisen more than once (Kravtsova & Oskolski 2007; M.-Q. Yang et al. 2013).
Note that older literature on Urticales refers to this whole clade while that on Ulmaceae may also include information about Celtis and relatives in Cannabaceae. For further information, see Berg (1989: general), Tippo (1938) and Sweitzer (1971), both anatomy, Giannasi (1978, 1986: chemistry), Terabayashi (1991: vernation), Hennig et al. (1994: cuticle waxes), Tobe and Takaso (1996) and Behnke and Barthlott (1983), both hairs, Berg (1990: inflorescences and pollination),Punt and Malotaux (1984: pollen), Omori and Terabayashi (1993: gynoecial vascular anatomy), Mohan Ram and Nath (1964) and Dottori (1994), both embryology, Kravtsova and Wilmot-Dear (2013: fruit anatomy) and Takaso and Tobe (1990: testa).
Phylogeny. Relationships in this group are discussed above.
ULMACEAE Mirbel, nom. cons. - Back to Rosales
Trees, deciduous; (growth sympodial, apex of innovation aborts); (ectomycorrhizae +); lignans +; (wood fluoresces); unicellular hairs smooth; torus-margo pits +; cystoliths usu. pegless; terminal bud of innovation aborts; leaves two-ranked, lamina vernation laterally (vertically) conduplicate-plicate, secondary veins going into teeth, (margin entire), stipules extrapetiolar; flowers perfect and mixed; 4-5(-8)-merous; P spiral, (connate); A extrorse; tapetal cells 2-3-nucleate; endothecial thickenings U-shaped; pollen 4-7-porate, exine rugulose; at least one stigma/style with 3(-5) vascular bundles; ovule (with bistomal micropyle), outer integument ca 4 cells across, inner integument ca 4 cells across, parietal tissue ca 5 cells across, nucellar cap ca 2 cells across; fruit a samara; seeds flattened, coat undistinguished, exotestal cells elongated, unthickened; chalazal endosperm haustorium +, (polyembryony +); x = 14, often terminal/subterminal diffuse-complex centromeres, nuclear genome [1 C] (0.014-)1.162(-39.572) pg; 69bp ndhF deletion.
7 [list]/56. ± Word-wide, but not Arctic, South temperate, Madagascar, or the Antopdes and the Pacific. Map: from Soepadmo (1977), Hultén and Fries (1986), Fl. N. Am. 3 (1997), Todzia (1989, 1992) and Trop. Afr. Fl. Pl. Ecol. Distr. 5 (2010). Photos: Collection.
Age. Crown-group Ulmaceae may be (95.2-)85.4(-76.0) Ma (Q. Zhang et al. 2021) or ca 76 Ma (Gu et al. 2024).
Fossil pollen from the Maastrichtian has been associated with Ulmaceae, although it is not associated with any particular genus (Muller 1981a).
1. Ulmeae Dumortier / The Temperate Clade
(Bark flaky - Phanera0; wood ring porous; vessel elements with simple and scalariform perforations, spiral thickenings +; giant/D-type stomata [Hemiptelea, Zelkova]; (embryo sac tetrasporic, Drusa/Adoxa types - Ulmus, Zel.); fruit (achenial - Zel.), (wing small, one-sided - Hemi.; drupe. with fleshy prickles - Plan.); (embryo curved - Zel.).
4/43: Ulmus (20-40). North temperate, scattered in Central Asia, also Central America, scattered Western Malesia. Map: see Fragnière et al. (2021: Fig 1: d-g).
Age. The age of crown-group Ulmeae may be (83.1-)72.59(-63.3) Ma (Q. Zhang et al. 2021).
The extant genus with the oldest known fossils is Ulmus itself, which has leaves and fruits in Early Eocene deposits in northeastern China some 50 Ma old (Q. Wang et al. 2010; see also Friis et al. 2011 for Cenozoic fossils), while the age of the genus based on fossils from western North America (Manchester 1989a, b) was a little older, around 57 Ma. This suggests an appreciably greater age for crown-group Ulmaceae as a whole.
2. Holoptelea, etc. / The Tropical Clade
Wood diffuse porous; vessel elements with simple perforations, spiral thickenings 0; first leaf of branch adaxial; A 12-16; outer integument ca 6 cells across [Holoptelea]/integument 1, 7-10 cells across [Phyllostylon]; (fruit a drupe - Ampelocera); exotesta thick-walled [H.]; (germination cryptocotylar - P.).
3/13: Ampelocera (9). Tropical, scattered; Afica, West and Central, continental Southeast Asia. Map: see Fragnière et al. (2021: Fig 1: a-c).
Age. The crown-group tropical clade may be some (68.4-)43.9(-22.1) Ma (Q. Zhang et al. 2021).
Evolution: Divergence & Distribution. For a review of the fossil history of Ulmaceae, see Manchester (1989a, b), and for that of Cedrelospermum in particular, crown-group Ulmaceae, around 48 Ma and widely distributed in the Northern Hemisphere, see Manchester (1989b) and Jia et al. (2018).
Ulmus is known fossil from western North America, and Hemiptelea, now restricted to eastern Asia, from eastern Europe (Fragnière et al. 2021). Indeed, both Ulmus and Zelkova, at least, may be of Boreo-Tropical rather than South East Asian in origin (Xing et al. 2016; Q. Zhang et al. 2021).
Ecology & Physiology. The majority of Ulmaceae grow in tropical humid forests or in alluvial and riparian forests, quite often in rather wet conditions. Fragnière et al. (2021, see also Neubig et al. 2012b) discuss the ecology and distribution of the species in the family in some detail.
Pollination Biology & Seed Dispersal. Breeding systems in the family are variable, but at least some flowers are perfect. Fruit dispersal is by wind (most genera), water (Planera) or animals (Ampelocera), or perhaps even as a tumbleweed (Zelkova) (Fragnière et al. 2021). Seeds seem to remain viable for up to about 1 year only (Dottori 1994).
Plant-Bacterial/Fungal Associations. Species of the ascomycete Ophiostoma cause Dutch Elm disease which has been decimating elm populations worldwide; the fungus is spread by phloem-eating bark beetles, e.g. Scolytus. For more on such beetles, see Pinaceae, also Vega and Hofstetter (2015).
Genes & Genomes. There is little variation in the size, structure, etc., of the plastome in Ulmaceae (Y. Gao et al. 2022).
Chemistry, Morphology, etc.. There are distinctive fatty acids in the seeds of some Ulmaceae, but not in those of Cannabaceae or Moraceae (Badami & Patil 1981: sampling).
The terminal buds of the innovations abort (Berg 1989) and there is cladoptosis in Ulmus (Manchester 1989b). Ulmus lacks well-developed prophyllar buds and has one of the two stipules intrapetiolar, they are both intrapetiolar in seedlings of some species, and the leaves may also be opposite, as in seedlings. Hemiptelea has pegged cystoliths.
For the literature on embryo sac morphology and development in Ulmus and Zelkova, see Dottori (1991) and Haig (2020) and literature; an additional embryo sac and even egg may develop at the chalazal pole. Nawaschin (1895) suggested that chalazogamy occurred in Ulmus.
For general information, see Todzia (1993), for floral morphology, see Leme (2018) and Leme et al. (2018: Ampelocera), for embryology, see Dottori (1991, 1994), for gynoecial morphology, see Okamoto et al. (1992), and for a summary of fruit morphology, see Herrera et al. (2014).
Phylogeny. The poorly-known Ampelocera is to be included here (see Ueda et al. 1997b; also Wiegrefe et al. 1998); although its hairs are smooth, its leaves have ascending veins. A clade including [Ampelocera [Phyllostylon + Holoptelea] is sister to [Hemiptelea [Zelkova + Ulmus]] (Sytsma et al. 2002; Neubig et al. 2012b; see also M. Sun et al. 2016: resin ducts/laticifers). A topology in which [Phyllostylon, Ampelocera, Holoptelea] were sister to the rest of the family was also recovered in the plastome study of Y. Gao et al. (2022), while Holoptelea ended up in Cannabaceae is some analyses of Y. Xiang et al. (2024). Indeed, the position of Planera varied depending on whether plastome or nuclear data were examined - an ancient hybrid? - and there was also some other infrageneric variation depending on the kind of data and mode of analysis (Q. Zhang et al. 2021). Focussing on Ulmus, Whittemore et al. (2021) found that chloroplast and RAD-seq phylogenies differed appreciably.
Classification. For an infrageneric classification of Ulmus - three subgenera, two of these divided into six sections - see Whittemore et al. (2021).
[Cannabaceae [Moraceae + Urticaceae]]: C-glycoflavones also +; (sieve tube plastids with starch grains); unicellular hairs usu. micropapillate; secondary veins palmate (pinnate), stipules cauline-intrapetiolar; plant monoecious; (inflorescences paired at nodes) [?level]; staminate flowers: P = A, filaments incurved in bud [explosively straightening]; carpelate flowers: stigma/styles with single vascular bundle; fruits not or barely flattened; embryo curved.
Age. The age for this clade has been estimated at (52-)49, 42(-39) Ma (Wikström et al. 2001: c.f. topology), (65-)56, 54(-45) Ma (Bell et al. 2010), around 59.2 Ma (Tank et al. 2015: Table S2), (96.4-)92.6(-90.5) Ma (J.-J. Jin et al. 2019) or ca 89.8 Ma (Gu et al. 2024).
The Malaysian pollen Triorites micropori, aged at some 89.8 Ma, has been placed in stem Cannabaceae (see Gu et al. 2024).
Evolution: Pollination and Seed Dispersal. Pedersoli et al. (2019: see also references) give details of explosive pollen release in taxa of all three families. Among other aspects of this process, they emphasize the occurrence of mucilage-producing cells (these may be in different parts of the flower), the mucilage perhaps causing the pollen grains to aggregate and so to get thrown further when the filaments abruptly straighten, keeping the flower parts attached before dehiscence, and so on. The adaxial cells of the incurved filaments are turgid and deeply folded (e.g. ibid., Figs 6D-F), considerable mechanical force accumulating, the folds disappearing on the straightening of the filaments. Furthermore, the pistillode is often an integral part of the dehiscence mechanism, being hollow, and/or with an apical cavity, and with an apical opening; the pistillode often deflates during the explosion process, of which this deflation is an integral part (Teixeira et al. 2020c). The perianth members are rigid (and basally connate in Moraceae and Urticaceae) and this stops the movement of the filament as it straightens, causing the anthers to invert and throw out the pollen. Interestingly, the stamens explode sequentially (Pedersoli et al. 2019).
Members of all three families are quite often dispersed by phyllostomid bats in the New World (Lobova et al. 2009). Trema, and Ficus and Cecropia in particular, are all important food sources for frugivorous birds (Snow 1981; Fleming 1986; Messeder et al. 2020a).
Chemistry, Morphology, etc.. For resin ducts and laticifers, see Prado and Demarco (2018).
CANNABACEAE Martynov, nom. cons. - Back to Rosales —— Synonymy: Celtidaceae Link
Trees (shrubs), (thorny); (roots with rhizobia), (plant ectomycorrhizal); (flavonols - Aphananthe), sesquiterpene lactones +, (flavonols 0); (torus-margo pits + - Celtis), true tracheids +; (sieve tubes lacking non-dispersive protein bodies); cystoliths usu. with pegs [level?]; leaf vernation (laterally) conduplicate-plicate (conduplicate; supervolute); stipules connate or not; P with a single trace; staminate flowers: tapetal cells 2-4-nucleate; endothecial thickenings U-shaped/annular/spiral; pollen 3(-2) porate, exine scabrate to verrucate; carpelate flowers: P (valvate); stigma 2-/4-branched, bifacial [?level]; ovule development precocious, campylotropous (anatropous/hemitropous), micropyle bistomal/zigzag, outer integument 2-4(-8) cells across, (elaborate - Celtis), inner integument 2-3 cells across, parietal tissue 4-6 cells across, nucellar cap 2-6 cells across; embryo sac haustorium +; fruit (achene, samara); exotestal cells tangentially elongated, with arms, unthickened; (polyembryony +); n = (8-11, 13-15), x = 7, centromeres medial/submedial (not Gironniera), simple, nuclear genome [1 C] (0.043-)1.008(-23.682) pg.
10/117: [list], Celtis (74). Worldwide, but not Arctic, distribution of Humulus lupulus in Asia unclear. Map: from Wickens (1976), Soepadmo (1977), Hultén and Fries (1986), Fl. Austral. vol. 3 (1989), Fl. N. Am. vol. 3 (1997) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010). [Photos - Collection, Celtis Flower.]
Age. Crown-group Cannabaceae may be around 75 Ma (Q. Zhang et al. 2018), (81.3-)71.5(-66.6) Ma (M.-Q. Yang et al. 2017), (94-)87.4(-78.8) Ma by J.-J. Jin et al. (2019) or ca 78.6 Ma (Gu et al. 2024).
Pollen from the Turonian some 90 Ma is placed in this family (see Muller 1981a). Fossil fruits like those of Gironniera have been found in European Maastrichtian deposits (Knobloch & Mai 1986: see Manchester 1989a), and J.-J. Jin et al. (2019) suggest that the stem age of this genus is ca 68.7 Ma, consistent with that identification.
Cannabis L. + Humulus L. —— Synonymy: Humulaceae Berchtold & J. Presl, Lupulaceae Link
Lianes [H.]/herbs [.]; leaves opposite [C.]/spiral [H.], lamina strongly palmately-lobed or -compound, veins proceeding to the apex of lobes; P 2, connate; staminate flower: A straight; G 0; carpellate flower: A 0; G collateral, stigma unifacial; embryo coiled [H.]; n = 8, 10, sex via X-autosome balance system.
Evolution: Divergence & Distribution. J.-J. Jin et al. (2019) give ages for all the major branching points in Cannabaceae (see also Bell et al. 2010; M.-Q. Yang et al. 2017). Note that Boutain (2016) suggested a late Cretaceous age for Cannabaceae s. str., i.e. the [Cannabis + Humulus] clade, while ca 53.4 and 25.4 Ma were the stem and crown group ages for this group in Jin et al. (2019).
Manchester (1989a: as Ulmaceae) reviewed the fossil history of Cannabaceae. The Caenozoic fossil history of Cannabaceae that are now East Asian endemics is discussed by Manchester et al. (2009); see also the general summary in Friis et al. (2011). Leaves of Celtis ameghinoi are perhaps the most common leaf type in the Laguna del Hunco deposits ca 52 Ma in Argentinian Patagonia, however, recent work by Wilf et al. (2024) suggests that they are better placed in the Dobinea (Anacardiaceae) area...
J.-J. Jin et al. (2019) discuss the evolution and diversification of Cannabaceae in some detail. Richly represented in the Early Tertiary fossil record, movement of genera across the North Atlantic and Beringian land bridges helps explain some of the more northerly and temperate distributions/disjunctions in the family, while long-distance dispersal was probably involved in the tropical intercontinental disjunctions and the occurremce of genera like Trema on islands throughout the Pacific (see also M.-Q. Yang et al. 2017). Although there seem to be no particular adaptations facilitating long distance dispersal in the family, small, fleshy fruits are common here (Jin et al. 2019). Interestingly, diversification in Celtis began ca 23.6 Ma, but only after a phylogenetic fuse of ca 35 Ma (Jin et al. 2019), although earlier fossils are known, e.g. wood from ca 36 Ma is known from Oregon (Wheeler & Manchester 2021). The fuse in Aphanathe is even longer, e.g. ca 52.4 Ma (e.g. Yang et al. 2017).
For the optimization of a number of characters in Cannabaceae, see M.-Q. Yang et al. (2013).
Ecology & Physiology. The liane, Celtis iguanea, is a reliable keystone food resource in Cocha Cashu, Peru, along with two species of Ficus, not so reliable, and a few other taxa (Diaz-Martin et al. 2014).
Pollination and Seed Dispersal. For details of explosive pollination, see above, also Cuéllar (1967). Note that Todzia (1993) noted that the stamens were straight, as did Kubitzki (1993), although the latter was reporting on Cannabaceae s. str..
Fruits of Trema, a plant that is common in secondary vegetation, are favoured by frugivorous birds of all sorts, both specialists and generalists (Snow 1981), for Celtis, see above.
Plant-Animal Interactions. Caterpillars of most Apaturinae (Nymphalidae) are found on Cannabaceae, although those of Hestinalis eat Urticaceae, Sephisa, Fagaceae, while Apatura, the spectacular purple emperor, has moved on to salicin-containing Salicaceae (Salix and Populus: Ohshima et al. 2010). Ulmus is an important host plant for lepidopteran larvae in the contiguous United States of America, being included in the top 15 such plants (Narango et al. 2020).
Plant-Bacterial/Fungal Associations. Parasponia andersonii, and some other species of the genus (= Trema) is the only non-legume nitrogen fixer that is associated with other than actinomycetes (see also Rutten et al. 2020), and its rhizobia - a variety of taxa are involved - remain in infection threads like those of some Fabaceae, especially basal Caesalpinioideae (de Faria 1987). Van Velzen et al. (2018) suggest that there may have been a change in symbiont from Frankia to rhizobium in a recent ancestor of P. andersonii. On the other hand, Tutten et al. (2020) suggested that there had been two ancestral duplications of Lys-motif lipochitooligosaccharide (= Nod factor) receptors in rhizobia-associated plants (both Fabaceae and Parasponia) that predated and coincided with the evolution of N-fixing nodules. Unlike Fabaceae, Nod factor recognition leading to nodule formation as well as arbuscular mycorrhizal symbioses are controlled by the same receptor kinase enzyme; infection of the plant is by entry of bacteria through cracks in the epidermis. The nodules are modified lateral roots and have central vascular tissue, the bacteria being in the cortex; this is unlike the nodules of Fabaceae but like the Frankia-induced nodules found elsewhere in this general part of the N-fixing clade (Streng et al. 2011; Behm et al. 2014 for literature). For further discussion, see Rutten et al. (2020 and references), also above and Fabales.
Gironniera is reported to be ectomycorrhizal (Smits 1994).
Genes & Genomes. Cannabis and Humulus have an X-autosome balance system determining the 'sex' of the plant (Westergaard 1958; Parker & Clark 1991; Divashuk et al. 2014: sex chromosomes ± homomorphic in Cannabis; Prentout et al. 2019). Although Prentout et al. (2019) suggest that dioecy is ancestral for Cannabaceae, evidence for this is at best ambiguous (see Q. Zhang et al. 2018: sampling exiguous, dioecy uncommon in Cannabaceae s.l.). However, the same system is shared by both Cannabis and Humulus, and sex chromosomes had stopped recombining in their common ancestor; other aspects of dioecy are similar in both, and dioecy may have arisen here 25-21 Ma (Prentout et al. 2021). Aspects of the control odf sex expression in the Salix-Populus (Salicaceae, q.v.) system are older, but details of its evolution of dioecy there are complex, more than one kind of dioecy being involved.
n = 10 is common in Cannabaceae.
Economic Importance. For the biology of Cannabis sativa, see Small (2015), it provides oil, grain, high-quality fibres (hemp) and hallucinogens (marijuana). Cannabidiolic acid (CBDA) is favoured over delta-9-tetrahydrocannabinolic acid (THCA) in medications, but CBDA is produced in only "modest" quantities by hemp, very little THCA being produced, but the latter, and cannabinoids in general, are produced in large quantities by marijuana plants. Recent interest in CBDA has led to the development of cultivars in which some of the hemp genome containing genes involved in the synthesis of CBDA have been introgressed into the marijuana genome (Grassa et al. 2018/2021).
Chemistry, Morphology, etc.. Only the "basal" Aphananthe and Gironniera have flavonols.
It may be that the laticifers of Cannabis etc., are not so similar to those of Urticaceae and Moraceae; there is no milky exudate and they occur throughout the plant, and given the derived position of Cannabis, they perhaps have evolved independently - but our ideas about the morphology and distribution of laticifers in the [Ulmaceae etc.] clade is changing (e.g. Leme 2018). Humulus, with its opposite leaves, has split laterals/a commissural vascular bundle (Colomb 1887). The "distinctive" camptodromous (to semicraspedodromus) venation of Celtidaceae s. str. is disturbed by the inclusion of Cannabis, etc., in the same immediate clade, however, strictly craspedodromous venation is reported from Palaeocene Celtis itself (Manchester et al. 2002).
The primordia of the two perianth members of the staminate flowers of Humulus and Cannabis soon become connate, forming a ring around the flower (Leme 2018), and monoecy, not andromonoecy, in Celtis was confirmed by Leme et al. (2021a). The anticlinal walls of the testa of Humulus are sinuous and its embryo is green. Little seems to be known about the embryology of Cannabaceae, but there are reports of e.g. chalazogamy in Celtis occidentalis, an antipodal haustorium in the embryo sac of of Cannabis, etc. (see e.g. Modilewski 1908). The stones of the fruits of Celtis show a considerable amount of variation in colour, shape and surface (Zamengo et al. (2020).
For general information, see Grudzinskaya (1967), Todzia (1993: as Ulmaceae), and Kubitzki (1993b: as Cannabaceae), for chemistry, see Hegnauer (1973, 1990, as Ulmaceae; also 1964, 1989), also Teixeira et al. (2020b: laticifers), Leins and Orth (1979: Cannabis flowers), Leme et al. 2020 (floral development: Cannabis, Celtis, Trema), Mohan Ram and Nath (1964: Cannabis ovules and seeds), Modilewski (1908) and Dottori (1991, 1994), all embryology, and Kravtsova and Wilmot-Dear (2013: fruit anatomy).
Phylogeny. Pteroceltis, Humulus, and Cannabis are close, and they and some other members of this clade have sieve tube plastids with starch grains (Behnke 1989). Studies suggested that Lozanella was sister to Aphananthe or sister to the rest of the family (Wiegrefe et al. 1998; Soltis et al. 2002), but M.-Q. Yang et al. (2013, 2017; see also van Velzen et al. 2006; H.-L. Li et al. 2015; M. Sun et al. 2016) found that Aphananthe was indeed well supported as sister to the rest of the family, with either Lozanella and/or Gironniera sister to the remainder, while in a plastome analysis H. Zhang et al. (2018) found the well-supported relationships [Aphananthe [[Lozanella + Gironniera] [the remainder]]] (see also J.-J. Jin et al. 2019). [Gironniera [Chaetachme + Pteroceltis] ...]] were the basal branches in those Cannabaceae examined by Q. Zhang et al. (2021), who discussed the controversy over the placement of Chaetachme. Trema is paraphyletic, the N-fixing Parasponia being embedded in it (Sytsma et al. 2002; van Velzen et al. 2006; Yang et al. 2013; van Welzen et al. 2018; Jin et al. 2019: q.v. for relationships in general here), while the position of Celtis was only weakly supported in the study by H. Zhang et al. (2018). For relationships in Celtis, in which there is a temperate grade that includes a tropical American clade, see Jin et al. (2019) and Zamengo et al. (2020).
[Moraceae + Urticaceae]: (calcium carbonate crystals); P valvate (imbricate); staminate flowers: pollen 2- or 5-porate; pistillode +; carpelate flowers: P ± connate; seed coat undistinguished.
Age. The age for this node was estimated to be (122.9-)100(-78.4) Ma by X. Huang et al. (2019), Zerega et al. (2005) suggested an age of at least 89 Ma, (93.3-)87.5(-81.7) Ma was estimated by Q. Zhang et al. (2018), around 80.4 Ma by J.-J. Jin et al. (2019), ca 82.5 Ma by Gu et al. (2024), (73.4-)70.9(-68.5) Ma by Magallón et al. (2018), while ca 58.8 or 55.3 Ma are ages in Tank et al. (2015: Table S1, S2).
Evolution: Divergence & Distribution. There may be an uptick in the diversification rate at this node (Magallón et al. 2018).
There is a group of genera of Moraceae with explosive pollen dispersal and they were included in a paraphyletic Moreae that was part of a basal polytomy within Moraceae in early phylogenetic reconstructions of the family. For dioecy, see Datwyler and Weiblen (2004).
Genes & Genomes. Rates of molecular evolution are likely to have increased at least twice in this clade, e.g. in most Urticaceae and also in Dorstenia, the increase being associated with the adoption of the herbaceous habit (Smith & Donoghue 2008).
Chemistry, Morphology, etc.. Marinho and Teixeira (2018) discuss the anatomy of the latex system and its distribution in various parts of the flower, finding that in some Urticaceae laticifers occur both in the perianth and inflorescence axis, not just in the bark, as was previously thought, moreover, they note that lipids are common in the latex of Moraceae, phenolics in that of Urticaceae. Laticifers are less branched in Urticaceae. Details of the distribution of laticifers may also be interesting: they are absent from the flowers of Urticaceae-Cecropieae studied, they are generally present at least in the carpelate flowers in Moraceae, but they were absent from the flowers of the one species of Ficus studied (Marinho & Teixeira 2018).
Scattered in the group are taxa in which the tepals are persistent (e.g. Pilea) or accrescent (e.g. Morus), and in the latter the fruits are anthocarps in the strict sense. For inflorescence development, which can be very similar in genera in the two families, see Bernbeck (1932).
Phylogeny. For a morphological phylogeny with a focus on Urticaceae s.l., i.e. Moraceae and Urticaceae here, see Kravtsova and Oskolski (2007).
Classification. The two families have much in common, and Corner (1952) even included Moraceae in Urticaceae. One might go further - Lozanella, with its opposite leaves and boxy venation, looks rather like Urticaceae, but it is a member of Cannabaceae...
MORACEAE Gaudichaud, nom. cons. - Back to Rosales
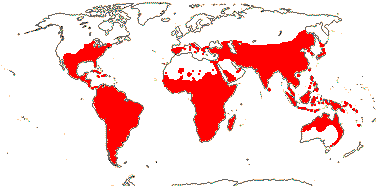
Largely woody; (isoflavonoids +); (cork in outer cortex); latex milky; small stalked glandular hairs +; (stomata aniso- and cyclocytic); leaves spiral, lamina vernation variable; plant dioecious (monoecious); inflorescences congested, often ± spicate [staminate] or ± globose [carpelate]; flowers 4-merous, (P 0-10); staminate flowers: bracts peltate, (filaments straight); G (0); carpelate flowers: A 0; (G inferior), stigmss 1 or 2, if 2, often unequal, papillate; ovule (subapical; campylotropous), outer integument 3-4 cells across, inner integument ca 3 cells across, nucellar cap ca 5 cells across [nucellar beak]; infructescence/fruit various; exotesta ± tanniniferous; n = 12 upwards, esp. 13, 14), x = 14, chromosomes 0.5-2.7 µm long, centromeres both terminal and median, nuclear genome [1 C] (0.036-)0.833(-19.536) pg.
39 [list: to tribes]/1,137 - seven groups below. Mostly tropical to warm temperate. Map: from Jalas and Suominen (1976), Wickens (1976), Frankenberg and Klaus (1980), Fl. Austral. vol. 3 (1989), Fl. N. Am. vol. 3 (1997), and Wilmott-Dear and Brummit (2007: Asia and South America only approximate).
Age. Zerega et al. (2005) dated crown-group Moraceae to (110-)89.1(-72.6) Ma, while (100.6-)93.1(-85.9) Ma is the age in Gardner et al. (2017), (84.7-)79(-73.2) Ma that in Q. Zhang et al. (2018), ca 84.7 Ma in Gardner et al. (2021) and ca 73.2 Ma in Gu et al. (2024). However, given that Misiewicz and Zerega (2012) suggested that the age of crown-group Dorstenia was (132.0-)112.3(-84.8) Ma, the sky - or somewhere near it - might be the limit.
[Chlorophoreae [Artocarpeae + Moreae]]: inflorescence axis initially narrowly convex, becoming ± spicate to globose; style sublateral, stigmas with unicellular hairs [?Artocarpeae]; "fruit" = syncarp, fleshiness from accrescent P.
1. Chlorophoreae A. de Jussieu (= Maclureae) - Maclura Nuttall
Trees to shrubs (lianes), axillary thorns +; (terminal bud aborts); leaves (2-ranked), stipules often small; inflorescence globose, flowers closely packed, with golden dye; staminate flowers: bracts not peltate; carpelate flowers: (bracts peltate); stigmas unequal/1 ca 3 mm long; P adnate to G or not, fruit (a drupe).
1/11: Tropical, 1 species to temperate North America. Photo - Inflorescence.
Age. The age of crown group Chlorophoreae is (73.4-)61(-49.1) Ma (Gardner et al. 2017), ca 50 Ma (Zerega et al. 2005), ca 54.8 Ma (Gardner et al. 2021) or as little as (41.1-)24.7(-8.9) Ma (Q. Zhang et al. 2018).
Age. This clade has been estimated to be (92.7-)83.8(-74.8) Ma (Williams et al. 2017) or ca 84 Ma (Gardner et al. 2017).
2. Artocarpeae Lamarck & de Candolle —— Synonymy: Artocarpaceae Berchtold & J. Presl
(Lianes); lamina (with glandular spots with a waxy surface), (margin spiny), (stipules ensheathing stem [amplexicaul]); plant monoecious; staminate inflorescence: flowers condensed; carpellate inflorescence: flowers usu. condensed; (P connate, that of adjacent flowers adnate in the middle portion [completely]); staminate flowers: bracts with yellow dye glands or not; flowers 2-merous; A 1(-3), filaments straight [at least Neotropical taxa]; ?pistillode; (exotesta several thickened layers - Prainea s. str.); syncarp with infructescence axis also expanded; germination hypogeal.
4/76: Artocarpus (ca 70). Indo-Malesia (Artocarpus) and Central and South America. Photo: Fruit].
Age. Crown-group Artocarpeae are (65-)59.7(-55.2) Ma (Williams et al. 2017), (78.5-)69.6(-61.4) Ma (Gard+ner et al. 2017), (80.6-)65.1(-52.2) Ma (Zerega et al. 2005), (68.6-)65.6(-64) Ma (Q. Zhang et al. 2018) or ca 64.0 Ma (Gardner et al. 2021).
3. Moreae Dumortier
Plant (with thorns), (herbaceous - Fatoua); (terminal bud aborts); lamina venation (palmate), vernation conduplicate-plicate; inflorescence (1-flowered), spicate or racemose, (globose); staminate flowers: (bracts not peltate); (G 0); carpelate flowers: style (1); fruit dehiscent drupe/drupe/berry, (P basally fleshy); testa below hilum usu. thick, vascularized; (endosperm +), cotyledons straight/folded, (unequal - Trophis).
10/66: Sorocea (19), Morus (16), Paratrophis (12). Tropical, especially Asia to Australia, some temperate.
Age. This clade has been estimated to be (77.3-)67.1(-57) Ma (Gardner et al. 2017), (75.2-)58.6(-44.2) Ma (Zerega et al. 2005), ca 64.8 Ma (Gardner et al. 2021) or only (46.8-)40.3(-34.9) Ma (Q. Zhang et al. 2018).
[Parartocarpeae [Dorstenieae [Ficeae + Olmedieae]]]: ?
4. Parartocarpeae Zerega & E. M. Gardner
Lamina (margin entire); plant monecious; inflorescences globose, unisexual, involucre +, uniseriate, bracts connate; bracts ?0; flowers sunk in fleshy receptacle, P 0; staminate flowers: (5-merous - Pseudostreblus), A (1-)2-3, basally connate, filaments straight, anthers extrorse, G 0; carpellate flowers: stigmatic branch 1; syncarp fleshiness receptacular; germination epigeal.
3/5. South China and Malesia to the Solomon Islands.
Age. The age of this clade is (45.4-)30.2(-17.1) Ma (Gardner et al. 2017) or ca 75.3 Ma (Gardner et al. 2021).
[Dorstenieae [Ficeae + Olmedieae]]: radial latex tubes +; plant monoecious, inflorescence bisexual; inflorescence axis initially broadly convex, becoming broadly convex to ± urceolate; pistillode conical.
Age. The age of this clade is estimated to be (82.1-)77.3(68.7) ma (Q. Zhang et al. 2019).
5. Dorstenieae Dumortier —— Synonymy: Dorsteniaceae Chevallier
(Herbs, rhizomatous/tuberous), (annuals), (stem ± swollen); (terminal bud aborts); unicellular hairs uncinate; lamina (palmately compound), (peltate), (stipules 0 - Fatoua); plant (dioecious/andromonoecious); inflorescences bisexual [?apo], axis various; staminate flowers: (3-merous), P (connate), (0 - Trilepisium); A 2-5, filaments (straight)/(curved, not explosive - Dorstenia); pistillode 0; (pollen pantoporate); carpelate flowers: (2-merous), (embedded in receptacle); P (0 - Treculia), connate; ovule apotropous, campylotropous, (with massive swelling on one side [see below]), parietal tissue 4-10 cells across; antipodals 8-25; syncarps various: axis barely developed, fruit dehiscent drupe [Fatoua]/axis enclosing inflorescence, carpelate flower 1, P adnate to fruit, fruit achene-nut [Trilepisium]/axis horizontally expanded, P adnate to fruit, fruit dehiscent drupe [Dorstenia, Fatoua, etc.]/axis surrounded by flowers, developed somewhat, interfloral bracts persistent, hardened, achenes numerous [Treculia]; (endosperm + - Dorstenia), embryo (curved), cotyledons flat or thick, unequal; n = 12-16, etc..
12/156: Dorstenia (117), Brosimum (15). Pantropical. Photo: Fruit.
Age. Crown Dorstenieae are ca 71 Ma (Zerega et al. 2005), (72.5-)61.5(-50.6) Ma (Gardner et al. 2017), (70.2-)60.5(-51.4) and (79.8-)75.4(-65.2) Ma (Q. Zhang et al. 2018, 2019 respectively) or ca 67.6 Ma (Gardner et al. 2021).
[Ficeae + Olmedieae] / involucrata clade: insect pollination +; involucre + [involucral bracts pluriseriate, imbricate]; staminate flowers: filaments straight, G often 0 or rudimentary; carpelate flowers: bracts not peltate; P free.
Age. The age of this clade is estimated to be (88.2-)72(-59.6) Ma (Zerega et al. 2005), (68.6-)62.2(-56) Ma (Q. Zhang et al. 2018), (65.8-)57.8(-50.1) Ma (Gardner et al. 2017), (66.2-)59.3(-52.7) Ma (Pederneiras et al. 2018) or ca 63.8 Ma (Gardner et al. 2023).
6. Ficeae Dumortier - Ficus L. —— Synonymy: Ficaceae Berchtold & J. Presl
Also hemiepiphytes, lianes, stranglers, stoloniferous, etc.; (phenanthroindolizidine alkaloids +), (cysteine proteases +); wood with conspicuous apotracheal parenchyma bands; (latex 0); leaves (opposite), with petiolar or laminar gland [?= nectary], lamina (venation ± palmate), (vernation conduplicate-plicate), glandular spots with a waxy surface, stipules ensheathing stem [amplexicaul], open in leaf axil; plant monoecious, functionally dioecious; inflorescence axis hollow-spherical, flowers enclosed; staminate flowers: aggregated around ostiole (not); bracts not peltate; P 2-4, connate or not; A 1-2, (unithecate), epidermis collapses [?all]; laticifers 0; pistillode 0/+; carpelate flowers: P 3-5, connate or not; A 0; G laticifers 0, style sublateral, stigma (tubular/infundibular), dry; nucellar cap to 10 cells across; syncarp axis fleshy [fruit = syconium], fruits proper achenial, numerous; (perisperm +).
1/876. Pantropical, especially Borneo, New Guinea. Photo: Syconium.
Age. Estimates of the age of crown-group Ficus range from around 86.7 to (92.6-)69.9(-60) to (80.8-)63.4(-47.8) Ma (L. Xu et al. 2011, Chantarasuwan et al. 2016 and Machado et al. 2018 respectively), (51-)43.3(-40.1) Ma (Zerega et al. 2005) and (42.8-)28.7(-15.8) Ma (Gardner et al. 2017); Cruaud et al. (2012b) suggest an age of (101.9-)74.9(-60.0) Ma, while (50.4-)42.8(-34.5) Ma is the estimate in Pederneiras et al. (2018), (55.9-)48.5(-40.6) Ma in Q. Zhang et al. (2018), ca 30.7 Ma that in Gardner et al. (2021, but sampling) and ca 44 Ma in Garner et al. (2023).
For the fossil record of Ficus, see Chandra et al. (2023). These authors described three species of Ficus from leaves in ca 60 Ma deposits from Rajasthan, N.W. India, placing them in subgenus Pharmacosyces sections Sycamorus and Oreosycea.
7. Olmedieae Trécul (Castilleae)
(Branches spreading), cladoptosis + (- Poulsenia, Antiaropsis); (cardiac glycosides +); wood fibres septate (not); cystoliths 0; leaves 2-ranked; stipules ensheathing stem [amplexicaul]/not; inflorescence unisexual, axis disc-like to hollow-spherical, bracts imbricate, (not involucrate), flowers enclosed; P connate (free - Antiaropsis); staminate flowers: bracts 0/trilobed; pistillode usu. 0; carpelate flowers: (bracts 0); inflorescence axis or P fleshy, (latter adnate to fruit), fruit a (dehiscent) drupe; seeds "large", testa vascularized; cotyledons (unequal - Mesogyne).
13/63: Naucleopsis (22), Perebea (9), Pseudolmeda (9). Pantropical, esp. America.
Age. Estimates of the age of crown-group Olmedieae are (68.5-)53.3(-39.8) Ma (Zerega et al. 2005), (47.1-)34(-21.3) Ma (Gardner et al. 2017), (47-)31.2(-18.7) Ma (Q. Zhang et al. 2018: phylogenetic fuse is ca 30 My) or ca 39.1 Ma (Gardner et al. 2021).
Evolution: Divergence & Distribution. For all aspects of the evolution, ecology, pollination, etc. of Ficus, see below.
For additional dates, see e.g. Q. Zhang et al. (2019: Dorstenieae). Note that the three dating methods compared by Gardner et al. (2021) gave rather different results.
Opposing continental drift and dispersal scenarios dominate the literature here, and clearly dating is crucial. Berg (2005) suggested that diversification in Moraceae occurred on a still physically coherent tropical supercontinent, but Zerega et al. (2005) advanced a more complex set of hypotheses to explain the distribution and diversification of the family. Maclura (Chlorophoreae, see below for inflorescences) is a small but old genus (Gardner et al. 2017), nevertheless, crown-group ages make drift an unlikely explanation for current distributions here. Clement et al. (2020) suggested that Olmedieae had moved once from the Old to the New World. Although Borneo is the centre of diversification for Artocarpus, it has been suggested that the genus may have moved there from the New World (Williams et al. 2017).
Berg and Hijman (1999) suggested that the distribution of Dorstenia, found in both Africa and South America, could be explained by continental drift; however, Zerega et al. (2005) estimated that the crown-group age of the genus was a mere 18.4-3.5 Ma, and Berg and Hijman's suggestion is also incompatible with ages in Gardner et al. (2017). However, in a more complete analysis, Misiewicz and Zerega (2012) dated the crown age of Dorstenia at (132.0-)112.3(-84.8) Ma, with the age of the New World crown group, (44.8-)30.3(-16.5) Ma - and in fact the latter was the age that Zerega et al. (2005) had earlier thought might be the crown-group age for the whole genus, given their sampling. On the other hand, Q. Zhang et al. (2019) suggested that (67.3-)61.3(-49.8) Ma might be the crown group age of the genus and Barreto et al. (2023) agreed... Dorstenia may have originated in Africa, moved to South America and thence twice to Central/North America by long distance dispersal, and back to Africa (Misiewicz & Zerega 2012; see also Zhang et al. 2019). Woody taxa with rather few large seeds per infructescence are basal in the genus, which otherwise is largely herbaceous, unusual for Moraceae, and it includes stem succulents, rhizomatous and tuberous plants, and even an annual species (Berg & Hijman 1999; Zhang et al. 2019).
Dioecy may be the plesiomorphic condition for the family, although that depends in part on how gains and losses of dioecy are weighted and of course on the phylogeny (Datwyler & Weiblen 2004; Q. Zhang et al. 2018: likely; Gardner et al. 2021 for phylogeny). Zhang et al. (2018) suggest that there have been five or so transitions to monoecy with various reversals and further transitions - certainly, dioecy is no dead end here (see also Goldberg et al. 2017; Sabath et al. 2015), and they also note "dioecy in Moraceae is ancestral to a larger clade including at least Cannabaceae, Urticaceae and Moraceae" (ibid.: p. 201).
Ecology & Physiology. Moraceae are the second to eighth most speciose family in lowland tropical rainforest in both America and Africa, and in Amazonian forests they include quite a large number of the species (11/227) that make up half the stems 10 cm d.b.h. or more (Gentry 1988; ter Steege et al. 2013).
Very high photosynthetic rates have been recorded from some species (references in Pierantoni et al. 2018). Most species have cystoliths on one or both sides of the leaf blade, and these may be involved in redistributing light in the blade - although some species, including F. religiosa, lack them, so absolute statements about any functions of these and other mineralizations in the lamina are difficult to make (Pierantoni et al. 2018).
Pollination Biology & Seed Dispersal. Aside from Ficus (see below), there are other remarkable pollination systems in Moraceae. Ficus is sister to Olmedieae. Female inflorescences of Olmedieae such as Antiaropsis are urceolate. The pollinators (thrips) breed among the flowers of the male inflorescences, eating pollen, etc., pollination occurs when the pollen-coated thrips visit female inflorescences (Datwyler et al. 2003; Datwyler & Weiblen 2004; esp. Zerega et al. 2004; Clement & Weiblen 2009); insect pollination here is a reversal from wind pollination. Pollination of Artocarpus integer is by dipteran cecidomyiid gall midges: The larvae eat fungi growing on the male inflorescences, and pollination occurs when the adult midges visit female inflorescences, perhaps attracted by scent (Sakai et al. 2000; see also Gardner et al. 2018). Pollination in Dorstenia is poorly known, but apomixis and myophily have both been suggested - flies may lay eggs in the inflorescences, pollinating as they do so (de Araújo et al. 2017). P. E. Taylor et al. (2006) describe explosive pollen release in Morus alba; for further details of similar pollen release in other taxa, see above. Pseudanthia are known from Antiaropsis and Dorstenia (Baczynski & Claßen-Bockhoff 2023). Q. Zhang et al. (2018) discuss the evolution of breeding systems in the family as whole, but with a focus on Ficus.
Seed dispersal in Moraceae is often by animals, but just what part of the plant contributes to the fleshiness that attracts the disperser varies. In Morus the fruits, achenes, are surrounded by the fleshy perianth and are closely packed on an erect inflorescence axis. The more or less elongated inflorescences of Artocarpus - it includes the bread and jack fruits - can weigh up to 50 kg, and the fleshy part is made up of both the perianth members and the inflorescence axis itself (see Zerega et al. 2010: phylogeny and morphology). Broussonetia papyrifera has heads of bright red, dangling drupes, while Dorstenia, etc., have "dehiscent drupes" in which the stone is shot out of the turgid fruit, the separation following a line of weakness in the mesocarp tissue (Schleuss 1958). In both these cases the developing fruits are initially extruded by the turgid, closely-packed perianth members. For additional information about seed dispersal here, see de Araújo et al. (2017: q.v. for variants) and Thorogood et al. (2018: Dorstenieae not sister to Ficeae). In Antiaropsis (Olmedieae) the inflorescence axis is concave at first, but spreading ("dehiscing") when ripe, and then the exposed drupes contrast in colour with the bracts, etc. (e.g. Zerega et al. 2004). Leite et al. (2020b) discuss general infloresence morphology, e.g. the shape of the receptacle, in Moraceae; they suggest that changes in the morphology of the receptacle in the adult inflorescence are driven by pollinators. Maclura pomifera (Chlorophoreae) is known for its large (15 cm or more across), multiseeded fruits in which the perianth becomes large and fleshy; these may have been dispersed by now-extinct megafauna, and they may also have dispersed other species in the genus, although ripe fruits there are usually less than 7 cm across (Gardner et al. 2017: see also Fabaceae and Arecaceae fruits).
Plant-Animal Interactions. Lim et al. (2021) found that members of Moraceae were the most abundant food plant of primates, justahead of Fabaceae. Interestingly, it was the fruits of Fabaceae that were primarily eaten, as with three other of the ten commonest families eaten (Lim et al. 2021).
Bombyx mori (the silkworm) caterpillars can eat quite a number of species of Moraceae, but not those of most other members of the Ulmaceae group - although they will eat Ulmus itself (Fraenkel 1959). Caterpillars of danaine butterflies quite commonly eat Moraceae; both they and their usually preferred Apocynaceae are rich in latex, although Moraceae do not often have the cardenolides that are quite common in Apocynaceae (Ackery & Vane-Wright 1984).
Genes & Genomes. Chromosome numbers in Dorstenia are very variable (Berg & Hijman 1999). The base chromosome number for the African species may be x = 12, and x = 13 for the American species; genome sizes (2C) = 2.50-5.47 pg (Barreto et al. 2023). For more on chromosomes in the family, see Oginuma and Tobe (1995).
Chemistry, Morphology, etc.. Laticiferous cells elongate and branch and intrude between other cells, the nuclei divide, but cell walls are not formed. Some species of Dorstenia have small, cauline stipules that do not overlap the petiole.
Zerega and Gardner (2019: p. 262) describe the inflorescences of Olmedieae as having "an involucre of multiple layers of involucral bracts", perhaps comparable to the ostiolar bracts of Ficus. The flowers of Parartocarpeae are apparently sunk into the receptacle and lack a perianth, and it is receptacular tissue that makes the fleshiness of the syncarp (Zerega & Gardner 2019); this should be confirmed. Indeed, floral/infructescence morphology and anatomy need attention. Leite et al. (2018) described the flowers of five taxa, of which two seem to be distinctly odd. Thus the staminate flower of Brosimum gaudichaudii is described as having an adaxial bracteole while both staminate and carpelate flowers of Clarisia ilicifolia are described as having two lateral sepals; Castilla elastica has staminate flowers that lack a perianth but are each associated with a trilobed bract. Leite et al. (2020a) look at the development of the gynoecium; two carpels are always involved, but there is just a single ovule with axile placentation (not parietal, as Leite et al. suggest), and the stigma may be formed by one or two carpels, but if just one, which carpel is involved depends on the species, if two, the stigma lobes may be equal or unequal. Ovule morphology and embryology in Dorstenia need examination (Berg & Hijman 1999). In some taxa integuments are not evident on the side of the ovule adjacent to the funicle, rather, there is a massive pad-like structure called a "bourrelet" or "wulst" (de Granville 1971).
For general information, see Rohwer (1993a), Berg (2001: American taxa), Berg et al. (2005, 2006: Malesian taxa), Zerega and Gardner (2019) and Gardner et al. (2021); for chemistry, see Hegnauer (1969, 1990), for wood anatomy, see ter Welle et al. (1986: was this series ever finished?) and for pollen, see Burn and Mayle (2008).
Phylogeny. Datwyler et al. (2003), Datwyler and Weiblen (2004), Gardner et al. (2017) and especially Clement and Weiblen (2009) and Gardner et al. (2023) discuss the phylogeny of Moraceae. Some of the old and paraphyletic Moreae with their incurved stamens are now placed in Chlorophoreae, Olmedieae (Castilleae) are sister to Ficeae, and relationships between the tribes (see above) are on the whole strongly supported (Clement & Weiblen 2009). However, analyses of morphological characters alone provided little resolution, only Ficeae (the one genus!) being well supported, and although most Olmedieae formed a clade, support was rather poor (Clement & Weiblen 2009); Clement et al. (2020) sampled extensively within Olmedieae and formalized the relationship between this tribe and Ficeae. Zerega et al. (2010) recovered a [Parartocarpus + Hullettia] clade, which was also found by Gardner et al. (2017), although support for its position could be stronger - in other studies this clade is part of Dorstenieae. The position of Maclureae (= Chlorophoreae) has been a little unclear, and they were sister to [Dorstenieae [Olmedieae + Ficeae]] in a two gene analysis by Chung et al. (2017), [Artocarpeae + Moreae] forming a separate clade. However, an analysis of 25 species and 333 nuclear genes [Parartocarpus + Hullettia] were found to be sister to [Dorstenieae [Olmedieae + Ficeae]] and with strong support, and although support for the clade [Maclureae [Artocarpeae + Moreae]] was rather less, other relationships were strongly supported (Zerega & Gardner 2019). Gardner et al. (2021, also 2023) carried out a yet more extensive analysis, with sampling complete to good (except in Ficus!) and again many nuclear genes included and with a variety of analyses; by and large support was strong. Morus, Trophis and in particular Streblus were para/polyphyletic, species of the latter appearing just about everywhere in the tree; hybridization did not cause major problems (Gardner et al. 2021).
Zerega et al. (2010) provided a detailed phylogeny of Artocarpeae, which they recircumscribed, and they followed up with an analysis using nuclear genomes - Jarrett's subgenera and sections largely held up (Gardner et al. 2020). Williams et al. (2017) looked at the phylogeny of Artocarpus, and A. altissima may be sister to the rest of the genus. Indeed, the position of this species is unclear, while in the New World, the genus Batocarpus seems to be embedded in Clarisia (Gardner et al. 2020, see also Gardner & Zerega 2021 and references). In Dorstenieae, most infrageneric taxa of Dorstenia itself turned out not to be monophyletic in the analysis of Misiewicz and Zerega (2012), interestingly, D. psilurus var. scabra was recovered as sister to the rest of the genus, separated by a ca 20 Ma internode from all other taxa examined - that included D. psilurus var. psilurus... Chung et al. (2017) examined relationships around Broussonetia and found that the genus was paraphyletic; see also W.-H. Kuo et al. (2022) for relationships in the Broussonetia area. Clement et al. (2020) noted that individual gene trees provided little support for relationships in Dorstenieae; the clade [Sparattosyce + Antiaropsis], from New Caledonia and New Guinea respectively, was sister to the rest of the tribe (but not in all analyses), and the American representatives formed a clade.
Classification. Clement and Weiblen (2009) suggest tribes and their delimitation (note the likely position of Treculia); they also discuss one or two problems with generic limits. Gardner et al. (2021) return to the issue of tribal and generic delimitation, the limits Streblus in particular needing adjustment and Moreae being reworked; their classification is followed above. W.-H. Kuo et al. (2022) discuss the genera to be recognized around Broussonetia.
Botanical Trivia. The straightening stamens and reflexing tepals of Morus alba are reported to show the fastest movement of any plant parts known, over half the speed of sound (P. E. Taylor et al. 2006).
Fig-Insect Associations other than Pollination and Herbivory.
Divergence & Distribution. There are over 800 species of figs, and they are closely associated with perhaps 10,000 or so species of fig wasps (Cook & West 2005), and these latter include pollinators, gallers, inquilines, and parasitoids. Fig seeds are commonly dispersed by bats and birds, and other animals such as monkeys are also involved. All in all, figs are major elements in the diets of a number of very different kinds of organisms on which they in turn often depend. The habit of Ficus varies - its species are usually trees, sometimes with masses of stilt roots, a few are epiphytes, while there are ca 16 species of so-called earth figs, smallish trees and mostly from Borneo, that bear their figs on branched stolons, sometimes subterranean, that are up to 10 m long; the figs themselves are often leafy (Gardner et al. 2025).
There are various ideas about the biogeography of Ficus (Chantarasuwan et al. 2015 and references). The origin of crown group Ficus may be in Late Cretaceous Eurasia, while most diversification has been in the Caenozoic, dispersal rather than vicariance best explaining details of the current distribution of the genus (Cruaud et al. 2012b; Chandra et al. 2023). Ficus subgenus Pharmacosycea is largely restricted to the New World, as is Urostigma section Americana, the latter being sister to the African section Urostigma, while Urostigma-Malvanthera is Australasian (e.g. Cruaud et al. 2012b; Bruun-Lund et al. 2017b; Machado et al. 2018). Machado et al. (2001) suggested that the age of the fig-fig wasp association was some (100.3-)87.5(-74.7) Ma, and they thought that the mutualism originated in the southern hemisphere, the distribution and diversification of both wasps and figs being at least partly shaped by the fragmentation of Gondwana. Ficus may have moved directly to America from Africa (Machado et al. 2018) and also via the North Atlantic (Pederneiras et al. 2018, q.v. for further details). Clement et al. (2020) thought that Ficus had moved once from the Old to the New World.
Lopez-Vaamonde et al. (2009) discuss dating and biogeography of fig wasps, so closely associated with Ficus, suggesting that their ancestors lived in Asia, dispersal rather than Gondwanan break-up being important for their subsequent distribution (see also Cruaud et al. 2012b); Xu et al. (2012) suggested a Gondwanan ancestry, but with much subsequent dispersal. 24 species of figs of section Oreosyce (subgenus Pharmacosycea) and their pollinating Dolichoris agaonid wasps are found on New Caledonia and elsewhere in the Old World, including Australasia, perhaps an old vicariant distribution. However, Cruaud et al. (2012c) date the age of the wasps in New Caledonia to around 45.9-32 Ma, suggesting that they arrived in New Caledonia from Sundaland by island-hopping - and the ages of the other New Caledonian taxa in their survey also very largely ruled out vicariance.
Diversification seems to have been largely constant over the >70 Ma life of Ficus (Bruun-Lund et al. 2017b), although there may have been small positive rate shifts in America; Gardner et al. (2023) suggests that there have been five rate shifts in Ficus and there may be an association between monoecy and rather higher diversification rates in that genus (Bruun-Lund et al. 2017b). Moraceae, and in particular Ficus, include a number of (hemi)epiphytes, stranglers and lianes (for how Ficus pumila climbs, see Groot et al. 2003). Machado et al. (2018) and Bruun-Lund et al. (2017b) discuss ecological interactions, particularly the role of hemiepiphytism, and diversification; overall, hemiepiphytes tend to have larger ranges if lower population densities than other taxa (see also Harrison & Rasplus 2006).
Ecology & Physiology. Ficus is noted for the variation in habit that it shows, whether free-standing trees, hemiepiphytes or epiphytes, and they are prominent components of many tropical forests. Z. Zhang et al. (2020) looked at climbing species in subgenus Synoecia, a group of climbers, and found that the climbing habit had evolved four or so times, species with the climbing habit being phylogenetically separated by non-climbing species of subgenus Ficus; climbers evolved from shrubs or trees. In an analysis in southern China figs with different habits differed in various foliar characteristics such as toughness, C:N ratio, indumentum, and alkaloid content in the latex and in the rest of the leaf, and also in the insect herbivores that they supported; herbivores were fewest on epiphytes and they tended to be more specialized there (J. Zhao et al. 2020 - see also Plant-Animal Interactions, etc., below).
Teixeira et al. (2025) discuss the role that maculae - small dark- or light-coloured areas on the surface of the fig - might have in fig ecology, with a focus on Ficus citrifolia. They suggest that they may function as extrafloral nectaries attracting ants to the nactar that they secrete, and/or they help keep the internal temperatures of the fig at a reasonable level by transpiring water - stomata in the macular areas are denser that elsewhere on the fig. Interestingly, oviposition by non-pollinating fig wasps was more frequent in the areas away from the maculae.
Figs are a very important and dependable food resource for frugivores, both birds and mammals, in many places in the tropics (e.g. Shanahan et al. 2001), and the diversity of their growth forms means they are encountered throughout the forest. Fig "fruits" are perhaps surprisingly nutritious (Herre et al. 2008 for references), although there is some argument about this (see Fleming 1986; Shanahan et al. 2001) and I know of no comparative study of the nutritional content of Old and New World figs (Snow 1981). In at least some communities figs may be especially important and reliable food resources when other plants are not fruiting; whether or not fruits on individual fig trees ripen synchronously, individual species often do not fruit synchronously (e.g. Marshall 1985). "All accounts agree that figs are among the most important foods of specialized frugivores in Africa, southeast Asia, and Australia..." (Snow 1981: p. 9); in the New World, however, figs tend to be eaten by unspecialized frugivores. Figs are indeed ecologically very important in both the New (e.g. Cocha Cashu, Peru: Diaz-Martin et al. 2014; see also Messeder et al. 2020a: they did not look at fruit availability in times of resource scarcity, but see below) and Old (e.g. sub-Saharan Africa, Kissling et al. 2007; West Malesia, Lambert & Marshall 1991) Worlds, although perhaps less so in India, and they may have considerable local diversity - for example, there are 77 species in Lambir Hills National Park in Sarawak alone (Shanahan et al. 2001; Harrison & Shanahan 2005). The species richness of Ficus is correlated with that of their avian frugivores, particularly that of their specialised frugivores, and the frugivores also select on particular aspects of the morphology of the fig species (Shanahan et al. 2001; Kissling et al. 2007; Herre et al. 2008). Leighton and Leighton (1983) called the relationship between Ficus and its frugivores a keystone mutualism, since the loss of the figs would deplete the populations of animals that dispersed both Ficus and the fruits of other plants. Monoecious figs in Indo-Malesia, even if at low densities, can be very large plants; within a population, the species is in fruit continuously, although the period of fruiting of any particular individual may be quite short (e.g. Lambert & Marshall 1991); individuals may produce very large numbers of figs when other canopy trees are not fruiting, so they "provide famine food for the herbivorous vertebrates" (Ashton 2014: p. 359). Similarly, Terborgh (1986: p. 339) observed, "[s]ubtract figs from the ecosystem and one could expect to see it collapse", and he noted that in some New World ecosystems figs, making up less than 1% of the species (12 out of ca 2,000), sustained nearly the entire frugivore community for three months of the year. Janzen (1979) had calculated that seven species of figs on Barro Colorado Island produced 200,000 ± 75,000 figs ha-1/yr - maybe 195 kg dry weight. Kattan and Valenzuela (2013, see also Snow 1981) discuss the arguments pro and con as to whether figs really are keystone species for frugivores. Be that as it may, their importance is obvious, and the role that they play in ecosystems can be compared with that of Miconia (Melastomataceae) - and don't forget Cecropia (Urticaceae).
Pollination Biology & Seed Dispersal. As is well known, pollination of figs is by fig wasps, the relationship between plant and pollinating wasp being very close (if not quite as close as we used to think), and as mentioned the relationship between the fig and the animals that disperse its fruits can also be quite close and of considerable general ecological importance. But in addition to the pollinating fig wasps, a number of other insects, up to 30 or more species of non-pollinating fig wasps - gallers, parasites, parasitoids, etc. - are part of the community that depend on the one species of fig - and they all depend on the fig wasp to chew an exit hole in the fig so the adults can move on to other figs (Jansen-González et al. 2014; see also below).
Pollination Biology. Borges (2021) provides a good recent account of fig pollination, a classic example of brood-site pollination mutualism, and the numerous outstanding problems with which she ends emphasize the complexity of the interactions involving figs, fig wasps, and associated insects. (Other major brood-site mutualisms include the Glochidion/Phylllanthus -Epicephalus and the yucca-yucca moth systems, q.v..) The basic details of the association between figs and their agaonid chalcid fig-wasp pollinators (Agaonidae) may be summarized as follows. Fig wasps have a haplo-diploid breeding system, males are haploid and develop from unfertilized eggs and the diploid females from fertilized eggs. Female wasps are fertilized by the flightless males inside the fig; adult wasps of neither sex feeds. In passive pollination, as in section Pharmacosycea, pollen from the staminate flowers becomes passively attached to the recently-hatched female wasps as they wander around the inside of the fig, there is not much if anything in the way of obvious adaptations of the fig wasp for pollen transport. However, in actively-pollinated species female wasps actively pick up pollen from the staminate flowers - these are at least sometimes aggregated around the ostiole - using their coxal combs and store it in thoracic pockets specialized for this purpose (e.g. Ramírez B. 1969; Machado et al. 2001). In both cases the figs show extreme protogyny, the staminate flowers maturing only after the adult wasps appear, and this happens long after - maybe three months after - the females that laid the eggs that produced these wasps and belonging to the previous generation have died; development of the plant and pollinator have to be coordinated (Jansen-González et al. 2012). The female wasps leave via exit holes in the wall of the fig that are prepared by the males (e.g. Kjellberg et al. 2001; Cook & Rasplus 2003) and are attracted to receptive figs usually by a mixture of terpene volatiles (e.g. C. Chen & Song 2008). They enter via the ostiole, having specialised jaws and a head that is shaped so as to allow them to squeeze between the inflorescence bracts that surround the ostiole, although they often lose their wings and antennae in the process (van Noort & Compton 1996). Castro-Cárdenas et al. (2022) describe the ostioles in some detail; they note that the ostiolar bracts are associated with colleters, the exudate of which perhaps helps lubricate the entry of the wasps, and there are also osmophores in the ostioles. Carpellate flowers in monoecious figs are layered, that is, the flowers tend to have styles of different lengths. Pollination, too, is passive or active. When pollination is passive it occurs during the general course of the egg-laying activities of the pollen-coated wasp in the new fig. In active pollination the wasps remove pollen from their coxal pockets and use their fore legs to deposit pollen on the stigmas of the flowers. In both cases, eggs are laid between the inner integument and nucellus, the wasp at the same time depositing a few drops of liquid from her "poison" sacs. This latter is necessary for the development of the gall on which the larvae feed (see below for galling wasps), so allowing them to develop into the next generation of wasps (e.g. Martinson et al. 2013; see also Jansen-González et al. 2014). Both long- and short-styled flowers in monoecious figs get pollinated, and both flower types can produce seeds, but the wasps lay eggs in those flowers with short styles. Long-styled flowers may also have thin styles and long stigmas, a combination not particularly conducive for egg-laying but good for pollination (Kerdelhué et al. 2000; Jousselin & Kjellberg 2001; Jousselin et al. 2002, 2004). In figs with active pollination there are fewer male flowers in the syconium than in those with passive pollination (for pollen:ovule ratios in these different pollination types, see Kjellberg et al. 2001), so active pollination may allow the fig to economize on resources, however, there are reversals from active to passive pollination (Machado et al. 2001). The larva of the developing wasp depend on endosperm, whether in fertilized or parthenogenetic flowers (Jansen-González et al. 2014). G. Wang et al. (2014) suggested that there are possible differences in pollen morphology between passively and actively pollinated flowers. Whether active or passive pollination is ancestral for Ficus is unknown (Cruaud et al. 2012b; see also G. Wang et al. 2021), although one might think the latter was more likely.
The life cycle in gynodioecious/dioecious figs follows the same basic principles. "Male" figs produce at most few fruits but many wasps, the female wasps picking up pollen before they leave, while "female" figs produce lots of fruits but few if any wasps - from the point of view of the wasp they are sterile (see Janzen 1979 for a good early account; Kerdelhué et al. 2000; Cook & Segar 2010). Wasps pollinating dioecious figs have shorter ovipositors than those pollinating monoecious figs; the sterile female flowers in "male" figs all have short styles and produce wasps, while the female flowers in "female" figs have longer styles and so do not have eggs laid in them (Cook & Segar 2010). Pollination of female figs is by deceit, male and female figs usually producing identical scents if flowers in figs of the two kinds open together, however, the scents may differ if the two kinds of figs open at different times (Hossaert-McKey et al. 2016). Indeed, such differences are seen even at the infraspecific level, as in Ficus carica itself. Here only one of the flowering events that occur in male trees happens at the same time as the single flowering event of female trees, and the scent of male figs that open by themselves differs from that of male figs when they open along with female figs, and in the latter case male and female figs have similar scents (Soler et al. 2012). R. Wang et al. (2021) described pollination Of F. pumila var. pumila by the wasp Wiebesia pumilae. Here the aldehyde decanal (C10H20=O) is the attractant, binding to a particular odorant-binding protein in the wasp; interestingly, changes in the secondary metabolites involved in defence are similar in both galled and seed-producing ovules (Wang et al. 2021; see also Jansen-González et al. 2014). On occasion dioecy reverts to monoecy (Machado et al. 2001). For more on monoecy and dioecy, see Q. Zhang et al. (2018: dioecy = gynodioecy)
Machado et al. (2001) looked at monoecy and dioecy from the point of view of the interests of the fig and its pollinating wasp, and these can be at odds. For instance, from the point of view of the wasp, being able to gall all the flowers in a monoecious fig would be advantageous, but that would not benefit the fig. Hence the question: What happens to the fig if there is no pollination, so the fig produces no fruits, but the wasp lays eggs, so potentially producing more wasps? If pollination is active and there is no or only little pollination, the whole fig may be aborted, or the plant allocates fewer resources to the fig, some of the developing insects dying and/or the size of the adult wasps being reduced8 - but adult wasps may develop in these pathernogenetic flowers. If, however, pollination is passive, sanctions by the plant are generally not so dramatic, although adult wasps may be somewhat smaller (e.g. Jandér & Herre 2010, 2016; Martinson et al. 2013; also Jansen-González et al. 2024 for general issues of galling in such situations).
Stigmas of passively-pollinated Ficus are bilobed and papillate, in actively-pollinated dioecious species the stigmas are tubular, while in actively-pollinated monoecious species they are more or less bilobed and papillate adaxially (Teixeira et al. 2018). In the latter two groups, at least, the stigmas of separate flowers may become entangled by stigmatic papillae, stylar hairs, or the stylar branches themselves, and they form a synstigma, functionally analogous to the extragynoecial compitum of some apocarpous taxa (Basso-Alves et al. 2014; Teixeira et al. 2018, 2020a; Delgado-Pérez et al. 2022; see also Procris - Urticaceae). Teixeira et al. (2020a, see also 2020c) found that in section Americanae, at least, stigmas of 2-20 flowers might be aggregated (elsewhere in the genus the synstigma may include all the flowers inside the fig, and most species, perhaps two thirds of the genus, form some sort of synstigma). Although the synstigmas do not produce abundant secretion, exudates from stigmatic cells and pollen tube transmitting tissue may help in pollen tube growth (Jousselin & Kjellberg 2001: the pollen may be only 10μm in diameter). Of course, functionally the synstigma may result in the production of seeds without associated larval predation. Indeed, there is a good example of this in the functionally dioecious F. fistulosa from southern China. Here male figs had flowers with short, separate styles, the wasp laid an egg and deposited a single pollen grain deep in the style; the wasp larva developed on the endosperm produced by the fertilized egg. Flowers in female figs had flowers with long styles so the wasp could not lay an egg in the ovule, but it deposited a number of pollen grains - and because the female flowers had a synstigma, pollination and hence seed production of several flowers resulted from the attempt at laying a aingle egg (Miao et al. 2023). A possible complication is introduced in cases like F. tuerckheimii where the pollen can germinate in the anther (Delgado-Pérez et al. 2022).
Since fig development is often more or less synchronized within trees but asynchronous between trees, and within a single fig staminate flowers mature later than carpelate flowers, out-crossing of the fig is favoured (Ahmed et al. 2009). Interestingly, although oviposition by fig wasps may be staggered, adults tend to hatch at the same time (e.g. Kerdelhué et al. 2000; Lopez-Vaamonde et al. 2001; Jousselin et al. 2003; Jackson 2004a; Kjellberg et al. 2005; Rønsted et al. 2005b, 2008a for references). Agaonid wasps are tiny, and they can be carried by air currents for up to 14 km even in the rainforest, and the breeding units of monoecious figs in particular may be an order of magnitude larger - covering some 100 square kilometres or more - than those of other forest plants (Herre 1996; Nason et al. 1998; Ashton 2014; Souto-Viláros et al. 2019). Even more remarkably, pollen is transported by wasps for distances over 160 km in riparian populations of the monoecious Ficus sycomorus in the Namib desert (Ahmed et al. 2009); that the wasps are small and interactions between fig and wasp often precise and seemingly delicate does not convert to notably narrow ranges for species of fig, and overall fig wasp distributions are surprisingly dynamic (H. Yu et al. 2019). In general pollinators of monoecious figs fly further than those of dioecious figs (Harrison & Rasplus 2006; Bruun-Lund et al. 2017b). Monoecious figs tend to be large trees with (very) low population densities and wide distributions while dioecious figs are more local, and are smaller trees with higher population densities, often pioneers in large gaps (Ashton 2014; L.-Y. Yang et al. 2015). The wasps are attracted to the figs by fragrances secreted by glands on the ostiolar bracts or the outside of the fig, depending on the species (Souza et al. 2015; G. Wang et al. 2016 and references); there are a variety of other glands in figs, some of which may produce phenolics that may protect the fig against overheating.
Feinsinger (1983), Hembry et al. (2014) and many others (see also papers in J. Biogeog. 23(4). 1996, also Plant-Animal Relationships below for further details) have discussed the fig-fig wasp association from the point of view of the extent to which it represents coevolution. Until recently, the link between fig and fig-wasp seemed to be an excellent example of an obligate one-on-one association between the plant and pollinator (e.g. Herre 1996 and references), and this is often how it is depicted in general biological textbooks. There is strict co-evolution, both figs and their pollinating agaonid wasps are monophyletic, and the two speciate/change morphologically almost in synchrony, i.e. co-evolution = cospeciation. Indeed, there is a strong general association between species of figs and wasp species, and in groups like section Ficus sect. Galoglychia cospeciation seems likely, even in the non-pollinating wasps that are also members of the system (Jousselin et al. 2008; Cruaud et al. 2012a, b; c.f. Cook & Segar 2010 in part). However, elsewhere things are not so simple. Recent work suggests that there may be rather less specificity in the association between fig and wasp than was previously thought (Cook & Rasplus 2003; Machado et al. 2005; Jackson et al. 2008; P. Yang et al. 2012; L.-Y. Yang et al. 2016; Hembry & Althoff 2016; Haren et al. 2023). Thus there may be up to nine (or even 20 or more) species of wasps pollinating the one species of fig, and a single species of wasp may visit several species of figs growing in the one area (Darwell et al. 2014; G. Wang et al. 2016; Wachi et al. 2016; H. Yu et al. 2019; Souto-Viláros et al. 2019). In the case of the nine species of Valisia found on the dioecious Ficus hirta, the wasps were parapatric while the fig showed clinal variation (Yu et al. 2019). Pollinator-sharing between fig species seems to be more common in monoecious Neotropical figs than in dioecious palaeotropical figs (Moe et al. 2011, but c.f. G. Wang et al. 2016); it also occurs in some African taxa, where one wasp may reproduce in a number of closely related figs. There is also lineage duplication, wasp speciation occuring within the one species of fig (McLeish & van Noort 2012). Yu et al. (2019 and references) note inbreeding in the wasps is common, the male and female wasps in a single fig, which mate before the latter leave, probably being descendents of a single pollinating female wasp. Moe and Weiblen (2012) also suggest that in some situations the fig wasp may evolve without there being a corresponding change in the host; one interpretation is that the wasp is adapting to its pollinator, rather than the opposite or both changing together. Souto-Viláros et al. (2019) looked at six species pairs (from four different sections) growing on Mt Wilhelm, Papua New Guinea, and suggested that it was an inherent part of the speciation process for the fig wasps to accumulate differences faster than the figs, so disturbing any simple 1:1 fig:fig wasp relationship, but subsequent divergence could restore the relationship. Overall, about 30% of Ficus species are pollinated by more than one species of wasp (L.-Y. Yang et al. 2015: this figure is likely to be an underestimate), and in dioecious Ficus, where the figure may be over 40%, the different wasps pollinating the one species of Ficus may be sister species, and there co-/sympatric speciation is possible, however, in monoecious Ficus around two thirds of the co-pollinators were not sister species. In parallel with this difference, phylogenies of plant and insect in dioecious figs may be largely congruent, less so in monoecious figs (Yang et al. 2015). Recent work on Neotropical strangler figs using a probabilistic approach suggests that when it came to speciation, host switching predominated, cospeciation being no more than expected by chance, the authors noting that there was "[s]trong evidence for host specificity in ecological time combined with strong evidence for host switching in evolutionary time", which may reconcile phylogeny with observations on pollination biology (Satler et al. 2019: p. 2305; see also Z. H. Su et al. 2022). Overall the wasps have speciated more than the figs (e.g. Yu et al. 2019, there being perhaps over 1,000 species of agaonids, although the great majority are undescribed (Cook & Segar 2010; Darwell et al. 2014).
One possible implication of the absence of host specificity just mentioned is hybridization. There has indeed been evidence for hybridisation in Ficus for some time. Thus Ramírez B. (1994) noticed hybridization of introduced F. religiosa with F. septica and F. aurea in Florida and the Philippines respectively - and depending on the phylogeny, the clades to which the protagonists belong may have been separated for over 70 Ma (see G. Wang et al. 2021: Figs 2-5). Hybridization and gene flow has been reported in dioecious figs (Wang et al. 2016: species limits may also be an issue here). Recently, Wang et al. (2021) found that phylogenetic analyses of the nuclear genomes, plastomes and chondromes of the fifteen species of Ficus in their study showed extensive incongruence (and the topology of the wasp phylogeny differed yet again); host shifts by the pollinators seem to have been frequent. As they concluded (ibid.: p. 10, see also Fig. 2), "little in the evolution of figs and their wasps appears to make sense except in the light of hybridization and introgression (mediated by pollinator host-switches)".
Indeed, understanding hybridization and its consequences helps in the interpretation of both the phylogeny and evolution of Ficus. As Gardner et al. (2023) noted, hybridization provides the opportunity for genetic introgression, the incorporation of genetic material from one species by another. Cytoplasmic introgression may also occur, the evidence for this being nuclear-chloroplast phylogenetic incongruence. Both seem to have occurred in Ficus. There have been pulses of nuclear introgression punctuating evolution within otherwise phylogenetically quite stable lineages; these seem to have happened quite a long time ago (Gardner et al. 2023: Fig. 3). There is also extensive cyto-nuclear discordance with nuclear and plastome trees having rather different topologies (Gardner et al. 2023: Fig. 2), for example, species of Oreosycea are sister to most/all species of three (out of the four) major clades of the chloroplast tree. The transfer of Oreosycea plastomes may have been mediated by Blastophaginae fig wasps, which pollinate both Oreosycea and some of the species they are associated with in at least two of these chloroplast lineages. Interestingly, Blastophagineae pollinate both Oreosycea and Pharmacosycea which previously made up the polyphyletic subgenus Pharmacosycea; Pharmacosycea subg./sect. Pharmacosycea is sister to the rest of the genus in both trees.
To conclude: Although the association of individual species of figs and their pollinators may be close, diversification in Ficus seems not to be the result of simple 1:1 co-speciation, but host-switching, hybridization and introgression are an integral part of the whole process. Finally, note that Asparagaceae-Agavoideae, Ranunculaceae, Phyllanthaceae, Saxifragaceae and Caryophyllaceae have similar unorthodox interactions between plant and pollinator (see Hembry & Althoff 2016 and Kawakita & Kato 2017f for reviews of diversification and coevolution in these systems).
Obviously knowing the timing of diversification of the two partners is important in thinking of the fig-fig wasp association as an example of general co-evolution/cospeciation, and a variety of crown group ages for Ficus are suggested above. Note that one recent estimate of the crown group age of a clade made up of Agaonidae and associated galling wasps was only (89-)49(-27) Ma (Peters et al. 2017b: ages for parasitoids, etc., still younger - see below). However, other estimates of the age of crown group Ficus, (101.9-)74.9(-60.0) Ma, and that of the fig wasps, (94.9-)75.1(-56.2) Ma, are quite similar to each other (Cruaud et al. 2012b), if different from the dates just mentioned, although Cruaud et al. (2012b), comparing the ages of figs and pollinators at 36 nodes, found that in eight of these the estimates of the ages of the figs were older, while in the others the wasps were older, the ages often being quite different, which of itself makes thinking of strict cospeciation difficult. Machado et al. (2001) suggested that the age of the association was (100.3-)87.5(-74.7) Ma, in this case the wasps being older than the figs. However, given current problems with dating, ultimately it is perhaps less the timing of the diversification of the parties involved that matters right now, more the patterns of relationships they show, and as mentioned above, there are changes underway.
Fig-Insect Associations other than Pollination and Herbivory. Ficus is susceptible to a variety of herbivores, as is discussed elsewhere. There are also a variety of insects specifically associated with figs. Along with fig wasps, there are many other species of chalcids that are part of the fig-fig wasp ecosystem. These non-pollinating fig wasps can be gallers, parasitoids of both pollinating and galling wasps, inquilines (species living commensally in figs) or even seed predators. Interestingly, the adults of the different species in the one fig emerge synchronously (Farache et al. 2018 and references) - all members of this community have to leave the fig, and they can only exit through the hole that the pollinating wasps chew, being unable to chew holes themselves. There can be up to 40 species of chalcid wasps associated with a single fig species (Cook & Segar 2010; Cruaud et al. 2011), although the diversity of the fig wasp community is lower in dioecious figs (Kerdelué & Rasplus 1996; see also Cook & Rasplus 2003), and the phylogenetic diversity of non-pollinating fig wasps is greater in the Old than in the New World (Cook & Segar 2010). Many of these wasps seem to be specialists, in one study 55% of the wasp species emerging from figs of a host species were found only on that fig (Farache et al. 2018). Jansen-González et al. (2014) looked at galling of the Brazilian Ficus citrifolia by a member of the Idarnes Flavicollis group. Here there is proliferation of the nucellus in particular, also integument and endothelium, by the galler, and if the ovules have been fertilized, the embryo and endosperm de-differentiate; in other situations involving passive pollination, the galler may induce parthenogenetic development of the endosperm (Jansen-González et al. 2014), but there seem to be few examples. Gall wasps penetrate the fig wall when ovipositing, and ovipositor length depends on when they lay their eggs and where galls develop - if early in fig development when the wall of the fig is thin, the ovipositor is short, if late, then it is longer (Silvieus et al. 2008); in the Idarnes case just mentioned, the egg ends up in the same place as the egg of a pollinating wasp would, between the inner integument and nucellus (Jansen-González et al. 2014). Externally-ovipositing non-pollinating wasps like Idarnes and Critogaster tend to have the highest "poison"/gall stimulant:egg ratio, and they lay their eggs in short-styled flowers, i.e., the same flowers that pollinating wasps lay eggs in; this "poison" is needed if the galls that the larvae eat is to develop (Martinson et al. 2013). R. Wang et al. (2021), Y. Yang et al. (2023) and others discuss the delicate balance involved here: the fig needs to attract the pollinating wasp, yet at the same time not attract anatogonists/gallers - indeed, the wasp has few odorant binding proteins, and only one volatile organic compound produced by the plant attracts the wasp. For instance, larvae of sycophaginae gall wasps in particular produce galls on individual flowers of figs (none is a parasitoid), and they may have have moved on to figs in Australia some 50-40 Ma, i.e. they may be half the age of Ficus, with subsequent dispersal to other areas (Cruaud et al. 2011). However, Peters et al. (2017b) dated a clade of pollinating and galling wasps to (89-)49(-27) Ma, and of parasitoids and inquilines to a mere (39-)26(-15) Ma; they did not include any Sycophaginae in their study. Parasitoid wasps may have very large geographic ranges, even larger than those of pollinating wasps, and they are least host specific (Silvieus et al. 2008; Sutton et al. 2015; see also above). The parasitoid wasps that parasitize fig wasps may be of considerable importance in preserving the relationship between the pollinating wasp and fig (Dunn et al. 2008). For more information on figs and wasps, as well as gallers and parasitioids, see also Kjellberg et al. (2005), the papers in Symbiosis 45, 1-3 (2008), and especially Herre et al. (2008).
Another element in this microcosm are the drosophilid flies that in Africa, at least, have a very close association with figs and oviposit either on the stomium or the exit holes made by the male fig wasps (Harry et al. 1996, 1998). Finally, it has been noted that fig wasp communities in both Australia and Panama have very high incidences - around 60% or so - of infection by Wolbachia bacteria that variously affect reproduction in their hosts and may also influence their speciation (Haine & Cook 2005). Both figs and bacteria in these two systems studied were immediately unrelated to one another, and horizontal transfer of the bacterium inside the fig is likely (Haine & Cook 2005). C.-Y. Yang et al. (2012) found seventeen different species of fig wasps, one a pollinator, the others inquilines, parasitoids, gallers, etc., from four families and eight genera living in Ficus benjamina figs, and thirteen of these were infected by Wolbachia strains, several of them by more than one strain, and there were frequent recombinations between strains and considerable horizontal transfer.
Seed Dispersal. Figs are commonly dispersed by bats (Muscarella & Fleming 2008) and birds (Snow 1981; see also Fleming 1986), and overall, the morphology of the figs they eat is quite diverse. The small achenial fruit proper is borne on the inside of the fleshy, invaginated inflorescence axis that is eaten by the disperser - that is, the fig is functionally a berry from the animal's point of view.
Pesquet's parrot, Psittrichas fulgidus, resricted to montane habitats in New Guinea, has an obligate association with species of figs like Ficus sterrocarpa and other species of section Melananthera. The figs are hard, and the parrot cracks them open with its massive bill (it is also called the vulturine parrot) and eats the pulp inside, although other birds feeding on the left-overs may be the real seed dispersers (Mack & Wright 1998). Figs that are eaten by birds are often small and red, orange or purple, and they do not smell (Ashton 2014 for the Indo-Malesian species), and in America, at least, propagule dispersal by birds may be more effective than that by bats (Machado et al. 2018). Ficus microcarpa is eaten by the Indian Hill Mynah, and the fruits have a lipid-containing exocarp that seems to remain intact as the seeds pass through the bird - and when the seeds are on the ground, the lipids attract ants which are involved in their local dispersal (Kaufmann et al. 1991).
New and Old World bats search for food in different ways and have selected figs with different qualities; overall fig morphology is quite diverse. Bat-dispersed fruits in the New World often tend to be relatively large (>2.5 cm), rather dull, and greenish or yellowish in colour, and they smell (Compton 1996 [a whole series of papers]; Korine et al. 2000; Shanahan et al. 2001; Harrison 2005; Machado et al. 2018). Some 21 species of Artibeus bats (phyllostomids) are the predominant ficivores there. The bats are slow feeders and spit out larger seeds, fibre, etc., but they commonly disperse the tiny fig achenes. Like other bat-dispersed taxa in the New World, including Cecropia (Urticaceae) and Trema (Cannabaceae), particularly in Mexico (Lobova et al. 2009: other genera dispersed by phyllostomids include Piper, Solanum and Vismia - Fleming 1986)), species of Ficus can be found in early successional communities (Muscarella & Fleming 2008). The altitudinal ranges of the bats and figs are similar (Fleming 1986). In the Old World bat-dispersed fruits are often yellow, etc., and are sometimes quite large (Kalko et al. 1996; Harrison & Shanahan 2005; Lomáscolo et al. 2008, 2010); bat-dispersed figs in Malesia, also dull-coloured, may not smell, and pteropodids (fruit bats) are the dispersers there (Hodgkinson et al. 2003).
Herbivory. Herbivores of Ficus in New Guinea include a couple of specialist moth groups, the noctuoid erebid (Erebidae-Aganinae) Asota and the metalmark choreutoids, and they can tolerate protease activity in the latex (Volf et al. 2018). Caterpillars of Asota chew into the main vein to interrupt the latex flow before eating the rest of the leaf (Compton 1989 and references) and are found on plants the latex of which has polyphenol oxidative ability and also alkaloids, the larvae being brightly coloured and perhaps sequestering these alkaloids, while the choreutoid caterpillars can handle species of figs with dense hairs (Volf et al. 2018); Erebidae, and Asota in particular, are also by far the main caterpillars on Ficus in the Xishuangbanna Tropical Botanical Garden in southern China (J. Zhao et al. 2020). The cysteine protease ficin is found in the latex of F. virgata and affords protection against herbivory there (Konno et al. 2004; see Agrawal et al. 2008 and Mason et al. 2018 for more on such defences). Volf et al. (2018) suggested that radiation of the choreutids occurred ca 70 Ma, shortly after the beginning of diversification in Ficus, and there might have been sequential coevolution of these moths (Rota et al. 2016 estimate a crown group age - Brenthia versus the rest of the family - of (96-)76(-58) Ma, with much subsequent long distance dispersal). Although larvae of these moths (some adults mimic jumping spiders) are common on Ficus, they also eat plants in a variety of other families - check out Hosts. For a review of plant defences in Ficus, see Villard et al. (2019).
Economic Importance. Natural rubber is the polymer cis-1,4-polyisoprene produced by Hevea brasiliensis in particular, and it, or its variants, are produced by a variety of plants; it was first synthesized artificially at the beginning of the twentieth century.
Chemistry, Morphology, etc.. Bauer et al. (2014) discuss details of the latex and its coagulation in Ficus benjamina. Ficus spp. differ considerably in the kinds and arrangement of mineral deposits - silica, calcium oxalate, and amorphous calcium carbonate - in their leaf blades, and these seem to be little affected by the light regime of the plant, its age, etc. (Pierantoni et al. 2019).
Berg and Corner (2004) provide general information in the course of their revision of Malesian species of Ficus. For fig/syconium development, see Rauh and Reznik (1951); Basso-Alves et al. (2014) describe the development of flowers in Ficus. The comparative anatomy of the wall of the fig syconium is little known, but there is considerable variation (Fan et al. 2019). Anther wall development varies, being either the basic or monocot type (Delgado-Pérez et al. 2022). For embryology, see Johri and Konar (1956); there is a persistent perisperm in the seeds of Ficus tuerckheimii (Delgado-Pérez et al. 2022).
Phylogeny. Cruaud et al. (2012b) noted that relationships along the backbone of Ficus remained rather poorly supported, although the American section Pharmacosycea was likely to be sister to the rest of the genus (see also Pederneiras et al. 2015: the hemiepiphytic F. crassivenosa sister to the section, 2018; Bruun-Lund et al. 2017a; Machado et al. 2018: F. nevesiae sister; Q. Zhang et al. 2018: F. tonduzii sister, sect. Pharmacosycea paraphyletic). Clement et al. (2020) included 307 species in their 6-gene analysis and found that subgenera Ficus, Pharmacosycea and Urostigma were polyphyletic, section Pharmacosycea was perhaps sister to the rest of the genus, but overall deeper relationships were not well supported. Note that Bruun-Lund et al. (2017a) had found that there was conflict between chloroplast and nuclear studies, and G. Wang et al. (2021) in their comparative study of the nuclear genomes, plastomes and chondromes of fifteen species from throughout the genus found extensive incongruence in the relationships they obtained from analyses of the three compartments separately (and for good measure from the wasp phylogeny, too). Z. Zhang et al. (2020), focusing on the climbing species (subgenus Synoecia) and using three nuclear markers again found little support along much of the backbone of the tree, although quite strong support for their expansion of subgenus Synoecia itself (see below). Subgenus Pharmacosycea remained polyphyletic, the American species being poorly supported as sister to the rest of the genus - and F. tonduzii was sister to that group (Zhang et al. 2020). For relationships in section Americanae, see Machado et al. (2018: F. bonijesulapensis sister to the rest). In subsection Urostigma Chantarasuwan et al. (2015) reccovered a strict concensus tree in theie analysis of 60+ morphological and anatomical characters that was one gigantic polytomy; this was another of the sections that were para/polyphyletic in the study of Q. Zhang et al. (2018). Rasplus et al. (2020) assembled data for 102 morphological characters and 402 kb DNA data from restriction site-associated DNA sequences, and depending on the analysis of the latter Pharmacosycea might again be sister to the rest of the genus - but long branch attraction? Overall, Rasplus et al. (2020) suggested that the relationships [gynodioecious clade [Pharmacosycea [Oreosycea [2 clades of Urostigma]]]] were in bettter agreement with morphological data and pollinator relationships, even if ancestrally there was active, not passive, pollination - and their favoured topology was different yet again.
The recent study by Gardner et al. (2023) was based on 235 species of Ficus, about a quarter of the genus, and some 1751 nuclear loci; there were also plastid analyses. Relationships recovered there can be summarized (ibid.: Table 1 and Fig 1) as follows [subg. Pharmacosycea (sect. Pharmacosycea) [[subg. Urostigma/subg. Spherosuke/Mixtiflores, inc. subsect. Urostigma [[Conosycea subsect. Cordifoliae + sect. Malvanthera] [Madagascar clade [sect. Platyphyllae/Galoglychia + sect. Americanae]]]] [Oreosycea/subg. Pharmacosycea sect. Oreosycea [[subg. Ficus (= subsect. Ficus)/Caricae + subg. Sycidium (subg. Terega)] [subg. Sycomorus [subg. (sect.) Frutescentiae [subg. (sect.) Eriosycea + subg. Synoecia]]]]]]].
Classification. Pederneiras et al. (2015, see also Berg & Corner (2005) and Clement et al. (2020) discussed the infrageneric classification of Ficus, still very much a work in progress. Z. Zhang et al. (2020) attempted a partial reclassification, subgenus Synoecia being expanded to include most of the species formerly placed in subgenus Ficus, itself much reduced; subgenus Pharmacosycea remained polyphyletic, with three separate clades. However, the tree in Gardner et al. (2023: see immediately above for topology) agrees quite well with the Berg-Corner-Pederneiras classification, only minor taxonomic adjustments being needed.
URTICACEAE Jussieu, nom. cons. - Back to Rosales
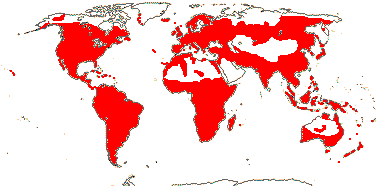
(Furanocoumarins) +; (wood fluoresces); (wood parenchyma not lignified); laticifers (throughout the plant), latex not milky (milky); petiole bundle(s) annular or arcuate; bundle sheath extensions 0; stomata often anisocytic (paracytic, etc.); cystoliths [in lamina, stem] punctiform; lamina base often asymmetric, vernation laterally or vertically conduplicate, venation palmate, trinerved, stipules interpetiolar; K (0-)4, 5(-6), with single trace [?level]; staminate flowers: anther endothecium 0; carpelode +, inflated, with aerenchyma; carpelate flowers: staminodes 0; G 1, rudiment of second carpel +; ovule basal, ± straight, both integuments often 2 cells across, (protruding into the stylar canal), (inner integument obturator, of several 1-celled thick projections in t.s.), parietal tissue 4-6 cells across, nucellar cap 2-4 cells across; fruit often a nut or achene, straight; seed coat perforated, ± crushed, but various testal/tegmic layers persisting; endosperm ± copious, (starchy), chalazal haustorium +, embryo straight; x = 7, chromosomes 0.9-1.6 µm long, protein bodies in nuclei, nuclear genome [1 C] (0.039-)0.568(-8.35) pg.
54 [list: to tribes]/2,625 - five groups below. World-wide, but mainly tropical. Map: from Frankenberg and Klaus (1980), Hultén and Fries (1986), Fl. Austral. vol. 3 (1989) and Fl. N. Am. vol. 3 (1997): ??Arabia, Central Asia). Photo: Shoot, Flower, Fruit.
Age. The age of crown-group Urticaceae is estimated to be (81.7-)68.7(-56.2) Ma (Z.-Y. Wu et al. 2018), (104.8-)84.9(-66.3) Ma (X. Huang et al. 2019) or ca 60.4 Ma (Gu et al. 2024).
[Cecropieae s.l. + Boehmerieae]: ?
Age. The age of this node is some (68.5-)54.9(-42.5) Ma, see Z.-Y. Wu et al. (2018).
[Cecropieae s. str. + [Leucosyke + Maoutia]]: ?
Age. The age of this clade is (60.1-)44.1(-27.3) Ma (Z.-Y. Wu et al. 2018).
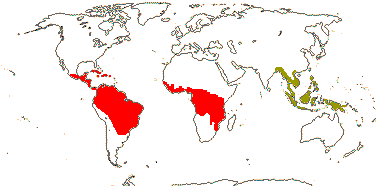
1a. Cecropieae Gaudichaud s. str. —— Synonymy: Cecropiaceae C. C. Berg
Trees to shrubs (lianas); (stilt roots +); mono- or diC-glycostlated flavones +; laticifers in flowers 0; bundle sheath extensions +; stomata anomocytic; hairs arachnoid, unicellular; cystoliths 0; leaves spiral, lamina palmately compound or -lobed, (venation pinnate - some Coussapoa), stipules intrapetiolar, connate, ± sheathing the stem [open on side of stem opposite leaf]; plant dioecious; inflorescences densely spicate/capitate, subumbellate/digitate; staminate flowers: (P connate); A ± connate [basally only to ± complete], filaments straight in bud (not), pistillode usu. 0; carpelate flowers: stigma lingulate/capitate-penicillate/peltate; (endosperm 0), (cotyledons thick, radicle short - Pouruma, Myrianthus); n = 14
4/145: Cecropia (61), Coussapoa (50). African and esp. American tropics. Map: red, see Bonsen and ter Welle (1963) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010).
Age. The age of Cecropieae s. str. is around (30-)19.5(-11) Ma (Z.-Y. Wu et al. 2018).
1b. [Leucosyke + Maoutia] = Cecropieae s.l.
Shrublets to small trees; cystoliths associated with hair bases only; stipules intrapetiolar, connate, inflorescences capitate; staminate flowers: pistillode woolly (glabrous); carpelate flowers: stigma ± sessile, penicillate-long papillate; n = ?
1-2/60. China (Taiwan), tropical Southeast Asia and Malesia to Polynesia. Map: see above, green.
Age. For the age of this clade, (43-)28.3(-14.7) Ma, see Z.-Y. Wu et al. (2018).
2. Boehmerieae Gaudichaud - inc. Parietarieae, Forsskaoleeae
Plant shrub to small tree, herbs (annual); leaves often opposite; (stipules 0 - Soleirolia, etc.); (inflorescence capitate, involucral bracts free/connate); staminate flowers: (P 1, A 1 - Forsskaolea, etc.); pistillode +, often lanate; carpelate flowers: (P 0 - Phenax); (rudiment of second carpel 0), stigma persistent or not, stigma various [sessile-penicillate/filiform/villous]; ovule bistomal [Parietaria]; n = 13-14, 21.
Boehmeria (52), Pipturus (30).
Age. The age of Ma, see Z.-Y. Wu et al. (2018).
Flowers of Boehmerieae (= Ekrixanthera) are reported from Mexican and Dominican amber 26-22.5 Ma; the flowers are 5-merous and the perianth members are heteromorphic (Poinar et al. 2016b, see also 2022).
[Elatostemateae + Urticeae]: leaves 2-ranked, (venation pinnate); staminate flowers: pistillode +; achenes asymmetrical (not).
Age. The age of this node is some (72.3-)59.2(-47) Ma (Z.-Y. Wu et al. 2018) or around 70 Ma (X. Huang et al. 2019); see also Tseng et al. (2025).
3. Elatostemateae Gaudichaud
Herbs (annual), (shrubs), often ± fleshy; cystoliths linear; leaves opposite, one sometimes much reduced [= nanophyll] - Pilea [P.], borne in one plane (not), (2-ranked), (spiral), (margin entire), 3-veined (pinnate), stipules intrapetiolar, connate [P., etc.]; inflorescence with receptacle or not, if yes, usually involucrate; P 2-3(-5), usu. not isomerous [P.]; staminate flowers: P with apical appendage; pistillode 0 (small); carpelate flowers: (monosymmetrical - P.); P (0), (shorter than ovary); staminodes +, inflexed, (minute); (rudiment of second carpel 0), stigma penicillate (capitate - Sarcopilea); fruit explosively ejected by inflexed staminodes (not); n = 12-14 (16).
6/1,300: Elatostema (652, or many more), Pilea (613 [Moonlight et al. 2014], or -?715), Elatostematoides (20-40). Pantropical (warm temperate), most Old World to west Pacific
Age. The age of crown-group Elatostemateae is (66-)53.3(-41.5) Ma (Z.-Y. Wu et al. 2018) or around 59 Ma (Tseng et al. 2025).
4. Urticeae Lamarck & de Candolle
Herbs (annual)/shrubs (climbers/hemiepiphytic scramblers/trees); plant with stinging hairs (not); (cork cortical - Urtica); cystoliths (± linear); leaves (spiral/opposite - Urtica), stipules intrapetiolar, connate, (stipules 2, interpetiolar); P (1); staminate flowers: (filaments straight - Poikilospermum); carpelate flowers: (disymmetrical - Urtica); (P free); (rudiment of second carpel ?0), stigma sessile/capitate-penicillate/linear; ovule with inner integument growing into style base [Laportea], nucellus 4-5 cells across, nucellar cap 2-4 cells across; antipodal cells several [Urtica]; fruit (explosively ejected, mucilage from exocarp and P - Nanocnide, P.); exotestal cells elongated, tegmen ± obliterated; endosperm rather slight; n = 11-13.
Urtica (63), Dendrocnide (37), Urera (35), Poikilospermum (20).
Age. The age of crown-group Urticeae has beeen estimated as (60.3-)47.1(-34.6) Ma (Z.-Y. Wu et al. 2018) and (70.1-)57.4(-45) Ma (X. Huang et al. 2019).
DeVore et al. (2020) found leaves of c.f. Giardinia ca 48.7 Ma with distinctive stinging hairs from British Columbia.
Evolution: Divergence & Distribution. For additional ages in the family, see Z.-Y. Wu et al. (2018) and Schüßler et al. (2019: Parietaria, etc.).
Wu et al. (2018) estimated that there had been around 92 long distance dispersal events in Urticaceae - the family may have originated in Eurasia - and in some 76 of these at least one ocean must have been crossed. Although neither wind nor animal dispersal seemed particularly likely here, Wu et al. (2018) noted that the fruits could float, sometimes for long periods and remaining viable after 10 months in seawater, long enough for most detected LDD events, according to modelling of ocean currents. Ecological traits analyses indicated that preferences for disturbed habitats in the family might facilitate LDD, however, nearly half of all LDD events involved dioecious taxa, and dioecy might well complicate establishment (Wu et al. 2018).
For the biogeography of Urticeae, which may have originated in tropical Asia, see X. Huang et al. (2019). Migration via land bridges followed by climate-induced vicariance has been involved, and latterly long distance dispersal. Urtica, although a genus of only moderate size, is widely distributed and has many island endemics. Of the two clades of Urticeae on Hawai'i, one is around 19.1 Ma, and although the two species that make it up diverged ca 10 Ma, even this is much older than the ages of the main islands, however, the other clade involved, represented by Hesperocnide (= Urtica) sandwicensis, is much younger (X. Huang et al. 2019). Surprisingly, the small annual U. lobulata, from South Africa, is sister to the shrubby New Zealand U. ferox, the clade they form being associated with clades that are basically Eurasian in distribution (Grosse-Veldmann et al. 2016b, see also X. Huang et al. 2019).
Phylogeny and geography seemed to be quite congruent in Pilea, contributory factors perhaps being localized pollen and seed dispersal (Monro 2006). Similar patterns were found in taxa growing in seasonally-dry Neotropical forests but in not those epiphytic on larger trees in rainforests (Dexter et al. 2017). L.-F. Fu et al. (2022) noted that the species-rich Pilea had small flowers with explosive pollen dispersal; the achenes were little ornamented, and seed dispersal was also explosive, animal dispersal here being unlikely.
Tseng et al. (2025) thought that the origin of Elatostema was in tropical Asia, with two shifts to Africa in the Eocene. They suggested that Elatostema was wind pollinated, but noted that the genus prefers shady, moist and sheltered conditions... Diversification has been high, especially in Karst country (as also in Begonia), and phylogeny and geography, but less so morphology, seemed to be associated (Tseng et al. 2025, q.v. for ages). For Elatostema and its relatives, see also Tseng et al. (2018).
For the evolution of characters in Urticaceae, see the detailed discussion in Z.-Y. Wu et al. (2015); 16/19 characters they examined showed parallelisms and/or reversals, with some, like the external morphology of the achene, having as many as 40 of these events. For the evolution of Urticeae and Elatostemateae, see C. Kim et al. (2015a).
146 species of Elatostema in China were recognized in FoC (2003), 278 species about a decade later by W.-T. Wang (2014)...
Ecology & Physiology. The ant-plant Cecropia (see also below) is a fast-growing pioneer tree of the Neotropics that dominates the early succession after forest clearing in South America, but the forest soon changes composition - c.f. situations where Vismia (Hypericaceae), also with fleshy fruits, is common (Mesquita et al. 2001). Ecologically, it is a New World analogue of Macaranga (Euphorbiaceae), except the latter does not have fleshy fruits.
Pollination Biology & Seed Dispersal. Explosive dispersal of the pollen as the filaments abruptly straighten is common in Urticaceae. Indeed, the name "artillery plant", used for cultivated species of Pilea, refers to the little puffs of pollen produced by this explosive dispersal when the inflorescence is jogged. For further details of this pollination mechanism, see above. A synstigma is reported in the inflorescences of Procris (Teixeira et al. 2018) - see also Ficus. Grosse-Veldmann and Weigend (2018) discuss the variety of breeding systems in Urtica, in particular the variety of different ways that staminate and carpelate flowers are arranged in the inflorescences of monoecious species, while Z.-Y. Wu et al. (2018) estimated that monoecy had arisen at least 84 times during the course of the evolution of Urticaceae - the breeding system is pretty labile. Derelomine weevil pollination - brood-site pollination mutualisms - is known from Cecropia (Haran et al. 2023a).
In Pilea the achenes are explosively dispersed by the abrupt straightening of the fleshy, inflexed filaments of the staminodes, the mechanism being similar to that involved in pollen dispersal (see also above) (Tseng et al. 2025). Some species of Poikilospermum have explosive "seeds", pressure from mucilage from the enxocarp and perianth building up, the endocarp and enclosed seed finally being ejected (Kravtsova et al. 2020).
The phyllostomid bat Artibeus eats Cecropia in Mexico in particular (Lobova et al. 2009 for records), but Neotropical phyllostomid bats in general rely mainly on this genus, Ficus, Piper, Solanum and Vismia (Fleming 1986). Smaller frugivorous birds of many kinds also favour the genus, 24 species of birds having been recorded on Cecropia at Barro Colorado Island in Panama, where they ate the tips of the infructescences (Eisenmann 1961; see Snow 1981; also Charles-Dominique 1986 and other papers in Estrada & Fleming 1986; Messeder et al. 2020a). The genus is a prominent component of secondary vegetation in the New World (see also Musanga in Africa). Myxospermy occurs here and elsewhere in the family (Western 2012).
Plant-Animal Interactions. As mentioned, Cecropia is a keystone species, along with Ficus and Miconia in particular, also Byrsonima, in the context of the maintainance of general plant—frugivore (bird) interactions throughout the Neotropics (Messeder et al. 2020a).
It has been suggested that caterpillars of Nymphalini butterflies have a plesiomorphic association with Urticaceae as food plants (Janz et al. 2001); caterpillars in a clade of Nymphalidae-Heliconiinae-Acraeini utilise members of this family, probably switching from host plants in the Passifloraceae area (Silva-Brandão et al. 2008; see also Nylin & Wahlberg 2008; Wahlberg et al. 2009; Nylin et al. 2014).
About three quarters of the 61 species of Neotropical Cecropia are associated with Azteca and some other ants that live in the stems and eat glycogen-rich food bodies (Müllerian bodies) produced by the plant at the abaxial base of the petiole (Davidson & McKey 1993). For a phylogeny of Cecropia, see Treiber et al. 2016; Gutiérez-Valencia et al. 2017). The age of this association is unclear (c.f. the topology in Chomicki & Renner 2015: fig. S3), although Gutiérez-Valencia et al. (2017: a variety of estimates) date it at around (16.7-)11.1, 8.4, 6(-4.3) Ma. The distributions of Azteca and Cecropia in the New World are largely conguent (Y. Luo et al. 2023). Glycogen is uncommon in plants, and Bischof et al. (2013) describe how it is synthesized. Some beetles also eat these food bodies, along with ant eggs, etc. (Jolivet 1991; Longino 1991; Yu & Davidson 1997). One ant may replace another within this association (Davidson & McKey 1993), but the association between plant and ant can also break down, especially on islands and at high altitudes (Janzen 1973; Gutiérez-Valencia et al. 2017); Musanga, e.g. M. cecropioides, from Africa, also lacks ants but is otherwise very similar to Cecropia. Some studies suggested that Musanga might be derived from within Cecropia, and its African sister species, perhaps C. sciadophylla, was also not myrmecophilous (Treiber et al. 2016). Although Gutiérez-Valencia et al. (2017) found that the two genera were sister taxa (the position of the related Myrianthus, also African, was unclear), the presence of prostomata, preformed ant entry holes, in the non-myrmecophilous C. sciadophylla, perhaps sister to the rest of Cecropia, made understanding the evolution of myrmecophily difficult (Gutiérez-Valencia et al. 2017); extensive co-speciation is unlikely (Davidson & McKey 1993). Roubik (2021) found that both bees - the meliponine Plebeia - and ants might be associated with C. sciadophylla and C. ficifolia, the bees depending on the honeydew and wax provided by scale insects (Coccidae: mainly Cryptostigma) associated with the ants. For chaetothyrialean ascomycete fungi, also involved in the association between ant and plant, although details are unclear, see Vasse et al. (2017). As with species of Macaranga (Euphorbiaceae) in Malesian forests, bacteria living off colony debris are eaten by rhabditid nematodes that may in turn be eaten by ants (Maschwitz et al. 2016). Myrmecophytes often are in species-poor clades, exceptions including Psychotria s.l., Neonauclea (both Rubiaceae), Cecropia and Macaranga (Chomicki & Renner 2015)
The parasitoid chalcoid wasps Aximopsis parasitize only Azteca queens, laying their eggs through the entrance to the colony soon after the queen has closed it (Gates & Pérez-Lachaud 2012 and references).
The common name of the stinging nettle, Urtica dioica, emphasizes the painful results of brushing against the plant and being injected with the histamine, formic acid, etc., in the bulbous-based stinging hairs. Not that much is known about the mechanism of stinging in Urticeae, although H.-Y. Fu et al. (2006) found histamine and formic acid in the stinging hairs of U. thunbergiana (see also Fu et al. 2003). Dendrocnide excelsa, also Urticeae, grows to 30 m, and its stings can last for weeks; the venom in its hairs is rather like that of spiders and cone shells, having small, disulphide-rich peptides that modulate the activity of sodium channels and enhance neuronal excitability (Gilding et al. 2020). For the morphology and mineral composition of the stinging hairs of four genera of Urticeae, see Mustafa et al. (2018b), the hairs were quite variable in their mineralization.
Genes & Genomes. Ogoma et al. (2022) looked in some detail at plastome variation in Urticeae. There was some variation in the size and boundaries of the inverted repeat, some intron loss, gene inversion, etc., but nothing major.
Chemistry, Morphology, etc.. Groups of cells in the vascular tissue may be unlignified and the pericyclic sheath may also be late in lignifying; the presence of unlignified apotracheal parenchyma was used to divide the family (minus Cecropiaceae) into two main groups (Bonson & ter Welle 1984); Cecropia et al. have lignified parenchya (Bonson & ter Welle 1983). Leaves of isophyllous Pilea studied by Dengler and Donnelly (1987) hav e 3:3 nodes, while in the anisophyllous Pellionia the large leaves had 4:3 nodes and the small leaves 1:1 nodes (for anisophylly, see also Samra et al. 2025). Variation in stipule position and morphology around Urtica is considerable (Deng et al. 2013); no mention of prophylls...
Pedersoli and Teixeira (2020) discuss floral morphologies in Parietaria debilis; the ovules are more or less orthotropous (c.f. ibid., p. 713). Carpelate inflorescences of Zhengyia are borne towards the apex of the flowering stem, staminate inflorescences are basal (Deng et al. 2013). Boehmeria has a fleshy perianth. The connation of the perianth and androecium in Cecropieae is illustrated by Teixeira et al. (2020c). For the absence of an endothecium, see Staedtler (1923); this character needs more extensive sampling. Although the gynoecium is basically bicarpelate, one carpel is highly reduced (e.g. Eckardt 1937) and there is a single style/stigma that shows a great deal of variation especially in Boehmerieae and Urticeae, although those of Elatostemateae are almost entirely penicillate (C.-j. Chen (1985). Pedersoli et al. (2022) found that the gynoecium of only two of the seven species they examined had two vascular bundles entering its base, while in three, Boehmeria cylindrica, Phenax sonneratii and Pilea cadierei, there was only a single vascular bundle and no lobing of the gynoecium - hardly even pseudomonomerous. Shamrov (2004) shows the inner integument of Leucosyke becoming much elaborated and functioning as an obturator, while Kravtsova et al. (2020b) record an integumental obturator and hypostase from Poikilospermum. The seeds of Dendrocnide may lack holes in the exotesta, and the whole seed coat is relatively well developed, while Kravtsova (2006) found ingrowths in cell walls of both the pericarp and seed coat, observations that should be extended. Kravtsova et al. (2020b) discuss the fruits and seeds of Poikilospermum in some detail.
See also Miller (1971), Berg (1977: African genera, 1978: Cecropiaceae), Kubitzki (1993b: Cecropiaceae) and Friis (1993), all general, Hegnauer (1973, 1990: chemistry), Bigalke (1933: cystoliths and hairs), Pedersoli et al. (2020: Cecropia floral development), Modilewski (1908) and Fagerlind (1944a: Elatostema, apomixis, ovules quite variable), both embryology, and Kravtsova (1995, 2001 [check], 2003: seed coat anatomy, 2009: also fruit wall anatomy) for additional information.
Phylogeny. Urticaceae minus Cecropiaceae are paraphyletic (e.g. Sytsma et al. 2000, 2002 - three genes; Monro 2006 - two genes). Datwyler & Weiblen (2004 - one gene) and Zerega et al. (2005) found strong support for Poikilospermum (map above - green) as sister to the rest of the family - [Poikilospermum [Cecropiaceae s. str. [rest of Urticaceae]]], while Chomicki and Renner (2015: fig. S3) grouped Poikilospermum with Cecropia et al., but this was probably a sampling issue. In an extensive seven-gene/three compartment study four main clades were evident: Cecropieae, only moderately supported, were sister to [Leucosyke + Maoutia], while Poikilospermum was part of a well supported clade that includes Urticeae, etc., and this was sister to the speciose Elatostemateae, and finally Boehmerieae make up most of another clade where Forsskaoleeae and Parietarieae are sister taxa and in turn are sister to the other Boehmerieae (Z.-Y. Wu et al. 2013, see also 2015, 2018). Hadiah et al. (2008) had found similar relationships, Poikilospermum being associated with Urtica, etc. (see also C. Kim et al. 2015a; Z.-Y. Wu et al. 2018). Indeed, Poikilospermum is most often included in in Urticeae in molecular studies, even being placed within Urera (Wu et al. 2015; see also Kravtsova et al (2020a: closest to Dendrocnide). There is now some basis for thinking about diversification in the family.
Boehmerieae. Schüßler et al. (2019) looked at relationships around Parietaria, the annual species of which form a clade sister to [the other species + ± woody Gesnouinia and Soleirolia]. Boehmeria itself is polyphyletic (e.g. Z.-Y. Wu et al. 2018; Monro et al. 2020; c.f. Liang et al. 2020). For relationships in Cecropieae, see Gutiérez-Valencia et al. 2017); the position of Myrianthus was unstable.
Elatostemateae. For a preliminary phylogenetic study of Elatostema, see Hadiah et al. (2003); Procris might have to be included (see also Hadiah et al. 2008). However, in a more comprehensive analysis Tseng et al. (2012, esp. 2018) found Pellionia to be sister to a clade of Arican species of Elatostema, with Elatostema subgenus Weddellia and the genera Elatostematoides and Procris successively sisters to that clade. See Monro (2006) for suggestions as to how to proceed with the phylogenetic analysis of the large genus Pilea; infrageneric taxa previously recognized were not holding. L.-F. Fu et al. (2022: two chloroplast, one nuclear markers, 22 morphological charaters displayed) looked at some 100+ species in the genus, and obtained quite good phylogenetic resolution; they found that the African P. tetraphylla, with decussate leaves, was sister to the rest of the genus. Tseng et al. (2025: 99 spp., nuclear ITS, 2 plastid markers) found relationships in the tribe to be [Pilea [Lecanthus [[Pellionia + Procris] [Elatostematoides [Weddellia [Pellionia [core Elatostema + African species]]]]]]].
Urticeae. Deng et al. (2013) and in particular C. Kim et al. (2015a) discuss relationships around Urtica; Laportea and Urera are not monophyletic, and Hesperocnide (California, Hawaii!) is embedded in Urtica. Similar relationships were recovered by X. Huang et al. (2019). For a comprehensive phylogeny of Urtica, which has important implications for species limits there, see Grosse-Veldmann et al. (2016b) and Grosse-Veldman and Weigend (2018); X. Huang et al. (2019) found that relationships at the base of the genus were [Zhengyia [[U. pilifera + U. neubaueri] [[clade with Urtica and Hesperocnide mixed] etc.]]]] - see also Z.-Y. Wu et al. (2018). Although few species from any one genus were sampled, W. R. Baker et al. (2021a: see Seed Plant Tree) found notable polyphyly in Urera and Laportea. Ogoma et al. (2022: analyses here plastome + nrITS, ITS + trnL-F intergenic spacer) again found that Laportea, Urera and Urtica were para- or polyphyletic, and perhaps Hesperocnide should be added to the list. Note, however, that there is a fair amount of movement of clades around the tree when the various analyses of Ogoma et al. (2022) are compared.
Classification. The four main clades recovered in the phylogeny of Z.-Y. Wu et al. (2013) are recognised as tribes above, except that [Leucosyke + Maoutia], sister to Cecropieae, are separated from that tribe. Wu et al. (2015) suggest that four subfamilies should be recognised, and these then divided into tribes. This may - perhaps - be the way to go, but clarification of the phylogeny would first be useful.
The limits of several genera including Boehmeria, Pouzolzia and Urera are particularly problematic. The polyphyly of Boehmeria means that breeding that focuses on the fibre plant, ramie (B. nivea), that does not take this into account is compromised (Monro et al. 2020). For Elatostema and genera around it, see Tseng et al. (2018), the classification in W.-T. Wang (2014) will need to be reworked; L.-F. Fu (2022) clarify generic limits around Pilea. Parietaria is biphyletic (Schüßler et al. 2019). Generic limits in Urticeae - see the phylogenies in Ogama et al. (2022) - are overdue for some serious attention.
L.-F. Fu et al. (2022) divided Pilea s. str. into eight sections, two with rather poor support - section Pilea has ca 570 species...