EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ± globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megasporangium indehiscent, megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [≤2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID I / FABIDAE / [ZYGOPHYLLALES [the COM clade + the N-fixing clade]]: endosperm scanty. - Back to Main Tree
[the COM clade + the N-fixing clade]: ?
[[FABALES + FAGALES] [CUCURBITALES + ROSALES]] / the N-fixing clade / fabids: N-fixing by associated root-dwelling bacteria, fixation threads + (not); tension wood +; seed exotestal.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) dated this node to (96-)94, 89(-87) Ma, but other estimates are a little older - Moore et al. (2010: 95% highest posterior density) suggested ages of (107-)104(-100) Ma and Bell et al. (2010) ages of (107-)99(-91) Ma. Stem-group ages for Fabaceae (the ages just mentioned) offered by H. Wang et al. (2009), Magallón and Castillo (2009) and Foster et al. (2016a), are not much different, nor, at (119-)115, 111(-109) Ma, are those of of H.-L. Li et al. (2015), while Xue et al. (2012) suggested ages of a mere 68.6 or 62.2 Ma for this clade and Hohmann et al. (2015) an age of 109.1 Ma. (100.2-)94.6, 70.6(-61.6) Ma are ages suggested by Pfeil and Crisp (2008), around 96.8 or 86.3 Ma is suggested by Naumann et al. (2013) and ca 103.7 Ma by X. Guo et al. (2021); the oldest estimates are ca 132 Ma (Z. Wu et al. 2014) and (170.3-)150.5(-146.2) Ma (Y. Xiang et al. (2024: Table S4).
Evolution: Divergence & Distribution. Practically alone among land plants, a number of species in the N-fixing clade have associations with N-fixing bacteria and play a key role in the terrestrial N cycle (see below). The bacteria involved are of two kinds, rhizobial bacteria, which include the alphaproteobacterial Rhizobia and the betaproteobacterial Burkholderia, both gram negative bacteria, and the filamentous gram-positive actinomycete/actinorhizal Frankia. The ability to fix N is uncommon elsewhere in seed plants, although Nostoc, a N-fixing blue-green algae, is associated with Gunneraceae and Cycadales, and also with hypnalean feather mosses and particularly with hornworts, see Anthocerophyta. In vitro N fixation by Azotobacteria vinelandii endophytic in Mammillaria has been recorded (Lopez et al. 2011), and Burkholderia, a genus which can fix N in Fabaceae, forms associations in leaf nodules with some Primulaceae-Myrsinoideae and Rubiaceae, while diazotrophic bacteria can fix N in sugar cane, teosinte, a race of maize, and a few other grasses (Van Deynze et al. 2018). However, the literature below shows how difficult it is to understand the evolution of N-fixation in the N-fixing clade.
N fixation in this group of four orders would seem to be a classic example of a "tendency" or "predisposition" (e.g. Soltis et al. 1995b; Kistner & Parniske 2002; Vessey et al. 2004; Marazzi et al. 2012; Nagy 2018; Parniske 2018). Werner et al. (2014) introduced a different set of terms, but the principle is the same; they thought that a state precursory to N fixation was necessary, and that this was subsequently lost >16 times. Be this as it may, it has been suggested there have been some nine independent establishments of symbioses with Frankia alone (H.-L. Li et al. 2015: three chloroplast genes, very good sampling), and at least eight more with proteobacteria, particularly rhizobia, all bar one in Fabaceae, and there have been losses in the ability to fix N within Faboideae, at least (e.g. Swensen 1996; Clawson et al. 2004; J. J. Doyle 2011; Santi et al. 2013; Werner et al. 2014: c.f. topology; J. J. Doyle 2016). Frare et al. (2018) thought that the acquisition of the aquaporin ammonium channel NOD26 allowed toxic ammonium to flow from the bacteria to the plant under anaerobic conditions. N-fixing angiosperms have more aquaporins compared to plants that cannot fix N, the number having been increased by tandem duplications; the channel was "a good candidate to be a key molecular innovation for the emergence of N-fixing symbiosis" (Frare et al. 2018: p. 555). The concept of "deep homology" has also been invoked to explain such underlying similarities (Doyle 2011; Ibáñez et al. 2016); nodules in diferent families might share various homologous elements that had been coopted in separate, therefore non-homologous, origins of nodulation. As Battenburg et al. (2018) noted, even if there were some sort of predisposition for N fixation around here, the predisposition itself must have had some function, otherwise it would not have persisted for 30 million years or more (Doyle 2011, see also below). Battenburg et al. (2018) looked at root and nodule transcriptomes in Medicago truncatula (with rhizobia) and also Ceanothus thyrsiflorus (Rosales) and Datisca glomerata (Cucurbitales), both actinorhizal, and found quite extensive overall similarities in nodule gene expression patterns, some perhaps connected with AM symbiosis, and additional changes that were associated with nodulation in each of the hosts - a two-step origin of N fixation.
Elements of the discussion in the preceding paragraph can be incorporated into recent suggestions (e.g. van Velzen et al. 2018; Griesmann et al. 2018; Shen & Bisselin 2020; Mbengue et al. 2020, etc.) that the common ancestor of the whole N-fixing clade had nodules and fixed N, but there has been subsequent widespread loss of the ability to fix N. The bacteria were initially in fixation threads, and the number of losses of the ability to fix N could be in three figures(!) (see also Shen & Bisseling 2020; Y. Zhao et al. 2021; also de Faria et al. 2022 for especially common losses of N fixation in taxa that had fixation threads in Fabaceae). An association with the actinorhizal Frankia is likely to be ancestral (Zhao et al. 2021). Thus Van Velzen et al. (2018) thought that Parasponia (= Trema, Cannabaceae) had changed its symbionts from Frankia to rhizobia only recently, and this in addition to repeated losses of the ability to fix N in other Rosales, not independent acquisitions of N-fixation via Rhizobium here and in Fabaceae as if some underlying tendency were involved (e.g. Soltis et al. 1995b; Werner et al. 2014). Interestingly, a common set of some 290 symbiosis genes are involved in N-fixation in both P. andersonii and legumes (Medicago), while NFP2, NIN and RPG genes, all essential for the establishment of N-fixing nodules, are found as pseudogenes in at least half of the six non N-fixing members of Rosales outside Cannabaceae that were examined (van Velzen et al. 2018). Interestingly, nodules developed in M. truncatula that has a homeotic mutation in the regulator gene NODULE ROOT 1 share a number of similarities with actinorhizal nodules, perhaps suggesting that the latter are ancestral - loss of function discloses the original function (Shea et al. 2020). Similarly, Z. Wu et al. (2022) noted that there were a number of nodulation genes shared by Hippophae rhamnoides (Rosales) and M. truncatula, although at the same time there were also different gene families involved in actinorhizal and rhizobial N fixation. Thus the former had some 67 gene families not in legumes that were involved mostly in stress response and cell wall modifications, while there were some 433 legume-specific gene families that were involved in cell wall biogenesis and aspects of N metabolism (Wu et al. 2022). In any event, if there has been a single origin of root nodule symbiosis, whether a predisposition or not, there have been numerous subsequent losses/changes in the ability to fix N throughout the N-fixing clade, and its current distribution in this clade seems on the surface to be rather unparsimonious.
Kates et al. (2022/2024), however, return to the idea of tendencies/predispositions in their comprehensive study of the N-fixing clade. They do not deal with the assembly of the detailed wherewithall to fix N, rather, they provide a possible evolutionary scenario. They suggest that an ancestral precursor state for N fixation evolved from a non-precursor state some time in the rosid lineage. This precursor state then evolved into an intermediary hidden state which in turn led to a gain of root nodule symbiosis; each gain of symbiosis was associated with the separate origin of an intermediary hidden state, so N fixation in the various groups of plants that have N-fixation would be non-homologous in the strict sense (special properties differ). Overall, Kates et al. (2022/2024) thought that there had been 16 independent gains and 10 losses of the ability to fix N; of the latter, eight had been in Fabaceae-Caesalpinioideae, two in -Faboideae. Furthermore, "Loss of [root nodule symbiosis] through a transition to a non-precursor state, an irreversible change, occurs at a rate 14 times slower than the rate at which the precursor transitions to the intermediary hidden state and 150 times slower than the rate at which RNS is then gained from the intermediary hidden state" (ibid. 2024: p. 5). For hidden states, see also the evolution of holoparasitism in Orobanchaceae.
Finally, Mavima et al. (2025) have also recently looked at the long-term history of the evolution of N-fixation, with a focus on the betaproteobacteria as a whole which originated around 2175-1000 Ma; the N-fixing β-rhizobia, Cupriavidus (the oldest), Trinickia and Paraburkholderia (their focus was on the last) originated 2135-514 Ma. The most recent common ancestor of Paraburkholderia is some 555-285 Ma (other estimates are a mere 20 Ma), and the genus now includes 90 or more species, of which 24 are rhizobial; rhizobial Central and South American species - which are found on Caeslpinioideae-Mimoseae - form a clade separate from their South African relatives - which are found mostly on Faboideae. Mavima et al. (2025) suggested that a predisposition to harbour nodulation genes evolved in the alphaproteobacterium Bradyrhizobium 1098-348 Ma and then moved into Paraburkholderia et al.; there was perhaps a precursor state to nodulation of over 400 Ma. Nodulation in Papilionoid legumes was dated to ca 103 Ma and that in Mimosoid legumes (sic) to perhaps 48 Ma. Endemic rhizobia in biomes in both South America and Africa grew in areas with poor soil and had acquired a predisposition to harbour nodulation genes before or soon after the split of those two continents. However, another scenario suggested by Mavima et al. (2025) - and they seemed to prefer it - was that nodulation loci in African and American taxa of Burkholderia had independent origins... They also suggested that Frankia might have had a/the precursory state to nodulation in the N-fixing clade.
It has quite recently been found that a set of some 290 symbiosis genes are involved in N-fixation in both Parasponia (= Trema) andersonii and the legume Medicago, both associated with rhizobia, a number that is unlikely to be the result of chance; at the same time, NFP2, NIN and RPG genes, all essential for the establishment of N-fixing nodules, were found as pseudogenes in three or more of the six members of Rosales outside Cannabaceae that were examined, however, the NIN gene was not pseudogenized in the non-N-fixing Zizyphus (van Velzen et al. 2018). Griesmann et al. (2018) found that the NIN gene, which plays a central role in nodule formation in both rhizobial and actinorhizal symbioses, is absent in several non-nodulating members of the N-fixing clade, even though adjacent genes were present.
The haemoglobin to be found in N-fixing nodules is an example of the complexity of the changes involved in nodulation. The pinkish colour of the nodules is because of the presence of haemoglobin (leghaemoglobin in Fabaceae). It is involved in oxygen transport and both helps to preserve the largely oxygen-free micro-environment that the rhizobial bacteria in particular need for N fixation (nitrogenase is inactivated by oxygen), but it also maintains enough oxygen for respiration (Ott et al. 2005). mRNAs of these leghaemoglobins are found just about throughout the plant from pod to root (Larrainzar et al. 2020). The haemoglobins have evolved independently in different N-fixing clades, all having a distinctive pentacoordinate haeme iron rather than the hexacoordinate haeme iron of the other plant haemoglobins from which they evolved (Sturms et al. 2010). Not only have different haemoglobins been co-opted, but not all Frankia nodulators - Ceanothus (Rhamnaceae) is an example - have haemoglobin in their nodules (van Velzen et al. 2018 and references); indeed, in Ceanothus any haemoglobin involved may be synthesized by Frankia itself (Vessey et al. 2004), not the plant. For more on haemoglobins, see Quilbé et al. (2021). Legumes also contain phytoglobins, of which Glb-1 controls nitric oxide concentrations during symbiosis (Larrainzar et al. 2020).
Thus the molecular bases for the restriction of these N-fixing bacterial associations to the N-fixing clade - or, more accurately, parts of it - are being dissected, although we are still some way from really understanding the history of N fixation (van Velzen et al. 2018; Griesmann et al. 2018; Nagy 2018; see also Werner et al. 2014; H.-L. Li et al. 2015); there are definitely a number of loose ends that must be cleared up.
Given the great ecological importance of N-fixing plants, the timing of their evolution is of considerable interest. Under the multiple origins of N-fixation hypothesis, Jeong et al. (1999) and Clawson et al. (2004) compared phylogenetic relationships within Frankia with those of its hosts. Clawson et al. (2004) suggested that all three clades of Frankia that they recognised might have diverged before the evolution of the angiosperms, while Jeong et al. (1999) thought that the Frankia clades had diverged after the plant clades, being about one third the ages of the latter. However, the plant ages were extraordinarily old, thus the [Rosales [Fagales + Cucurbitales]] clade was estimated to be 429-199 Ma (Jeong et al. 1999). H.-L. Li et al. (2015: n.b. stem-group ages for N-fixing clades, and they are sometimes very stemmy) suggested that associations with Frankia were established during two periods in the Late Cretaceous and Eocene when both global temperatures and atmospheric CO2 concentrations were high; by their estimates, no new Frankia relationships had been established since the beginning of the Oligocene. Under the single origin hypothesis, the minimum time at which N fixation appeared would be the crown-group age of the N-fixing clade, and this is commonly estimated to be (119-)115-89(-87) Ma (see above), in line with the estimate of 100 Ma for the acquisition of N-fixation offered by van Velzen et al. (2018). The first fossil nodules, from deposits in the eastern U.S.A. - both the plant and its bacterial associates are unknown - are ca 84 Ma (Herendeen et al. 1999); to Parniske (2018) this suggested that the common ancestor of the N-fixing clade did not have nodules. However, the stem group age of the N-fixing clade, i.e. the age of [the COM clade + the N-fixing clade] node, after which N-fixation could have evolved, is likely to be appreciably older, although relationships around here are a little uncertain. Using age estimates of Bell et al. (2010), J. J. Doyle (2011) noted that the common ancestor of the N-fixing clade was about 100 Ma, but the first symbiosis in extant clades was likely to be at most ca 70 Ma. i.e. there was a 30 Ma lag. Datiscaceae and Elaeagnaceae may be particularly old N-fixing clades, but since both have very long stems exactly when N-fixing actually evolved there - if it evolved independently - is anyone's guess. Place of origin? There are suggestions that the N-fixing clade originated on Gondwana (van Nguyen et al. 2019).
See D. W. Taylor et al. (2012) for possible apomorphies of the whole clade, and Jiang et al. (2019) for pollen evolution.
Ecology & Physiology. Nitrogen-fixing members of the four orders of the N-fixing clade - but Fabaceae are most important - grow throughout the world and their activities are central in understanding the global N cycle, being involved in the movement of N from the atmosphere to the terrestrial (and ultimately aquatic) parts of the cycle. Thus over 100 kg N ha-1 y-1 can be added to the system (Carlsson & Huss-Danell 2003 and references), similarly, estimates of biological N fixation in terrestrial environments, much of which is by bacteria associated with Fabaceae, are 90% of the 100-140 Tg [1 Tg = 1012g) of the total N fixed per annum (Gage 2004 and references). Once N has entered biological cycles, the activities of Metarhizium and related ascomycete fungi in moving N from insects eating plants back to plants should also be taken into account; they may be another important element in the global N cycle (Behie & Bidochka 2014), along with N fixation in thunderstorms and loss of N in denitrification (NO3→N) that is mediated by a variety of bacteria in the soil.
There are major geographic patterns in the kinds of N fixation. Overall, rhizobial N-fixing plants, i.e. Fabaceae, are commonest in tropical savanna and dry tropical broadleaf forest at around 30o N and S (Steidinger et al. 2019). Rhizobial N fixation in Fabaceae is generally facultative and N-fixing fabaceous trees are uncommon north of 35oN despite N limitation there; the costs of constructing, maintaining and regulating the N-fixing pathway interact with temperature and the obligate/facultative difference (Menge et al. 2014, 2017a, b). Many herbaceous Faboideae fix N, (Interestingly, strains of Bradyrhizobium, close to Rhizobium, are the dominating bacteria in pine forests across North America, and although they are unable to fix N (other strains can) and N-fixing plants are vanishingly uncommon in such forests, they do seem to be able to metabolize aromatic carbon sources - VanInsberghe et al. 2015.) Frankia-associated plants are woody, and they favour high light and soils with low available N and are early successional for the most part (H.-L. Li et al. 2015). Frankia associations are generally obligate and are commoner in cooler areas of the globe. Such ecological constraints may be the limiting factors in the distribution of the different kinds of N fixation (Menge & Crews 2016). De Faria et al. (2022) suggested that loss of ability to nodulate may have occured as temperatures and atmospheric CO2 concentrations decreased during the Cenozoic, early Cenozoic conditions being especially suitable for N fixation. Fixation-thread mediated symbiosis is likely to be plesiomorphic (De Faria et al. 2022) and thus plants with such threads were presumably likely to be present in the early Cenozoic and to live in these warmer conditions. However, the distributions of fixation types in extant Fabaceae just mentioned does not really fit this scenario.
N-fixing plants in general - although Fabaceae make up the majority of these - have very high concentrations of N in their leaves, and this has been linked with high levels of both carbon fixation and photosynthesis (see M. Adams et al. 2016). However, Adams et al. (2016) did not confirm these correlations, rather, water-use efficiency was linked with high leaf N in woody N-fixing plants. There is no evidence that Fabaceae in general have a high demand for N, moreover, inoculation of plants with crushed rhizobia affected plant N concentrations independently of any fixation, which suggests a rather complex interaction between the plant, bacterium and N metabolism (Wolf et al. 2016). But N is just one important element, and there is also a link between plant growth, enhanced P acquisition, lower soil pH and increased rock weathering in both N-fixing Betulaceae and Fabaceae, q.v..
Ectomycorrhizal (ECM) associations are also common in the N-fixing clade, for instance, in Fabaceae-Detarioideae and most Fagales, and ECM associations have been reported from a number of taxa like Casuarinaceae which harbour Frankia (e.g. Rose 1980). This association with ECM fungi has evolved here at least seven times (as well as in other seed plants), but apparently not in Cucurbitales. Plants with ECM fungi rarely fix N, although Casuarinaceae are an exception. Interestingly, ECM associations also involve a perturbation of the N cycle in that N may move directly from humus to the fungus, and then to the plant, rather than moving in inorganic form from the soil into the root hairs (see also below). There is a final wrinkle in the association of N-fixing trees with ECM. In Alnus carboxylate exudation by the roots may help in phosphorus acquisition (Lambers et al. 2012), indeed, A. rubra caused a substantial increase in bedrock weathering, and hence in the access of the plant to the nutrients this would provide (Perakis & Pett-Ridge 2019).
Plant-Animal Interactions. There are associations of particular groups of butterflies and plants (as food sources for caterpillars) within Fabales and Rosales in particular (Ackery 1988, 1991). Indeed, it has been suggested, as by Scott (1985) and Janz and Nylin (1998; see also Braby & Trueman 2006) that the ancestral food plant for larvae of butterflies as a whole may perhaps have been somewhere in the N-fixing clade or the malvids or their (immediate) ancestors (Nylin et al. 2014), however, caterpillars are common in the former only on Fabaceae, a fairly young, mostly Caenozoic group (see below); stem Fabales are rather older (see above). Malvales are another possibility (Ackery 1991), as are Rosids as a whole (e.g. Powell 1980; Berenbaum & Passoa 1999). See below for the ages of some of the butterfly families involved.
Megachile (Megachilidae) has some 1,478 species in 52 subgenera, and includes the leaf-cutter bees, as well as resin and mortar bees. Female leaf-cutter bees surround the cells in which their larvae develop with the parts of leaves that they cut - but these leaves are not any old leaf, rather, ca 72% of the bees' leaf sources were members of the rosid clade, indeed, Sinu and Bronstein (2018) found in a study involving sites in three quite different parts of the world that 42 % of the species visited by the bees were members of Fagales, Rosales and Fabaceae, all members of the nitrogen-fixing clade. Thus leaf-cutter bees cut the leaves of 41/42 species of Fabaceae growing at the University of Arizona arboretum (Sinu & Bronstein 2018). In a recent paper by Luizzi et al. (2025), the leaf-cutter bee M. lippiae was found to prefer the leaves of Floribunda roses, on which the fungus Aspergillus (Ascomycota-Eurotiomycetes) was to be found, compared with leaves of other roses - leaves with Bacillus and Alternaria were not favoured. Aspergillus in the larval bee cells reduced the activity of Ascosphaera (also Eurotiomycetes), a fungus that causes chalkbrood, a serious disease of these larvae (Luizzi et al. 2025). Interestingly, latex-containing leaves were untouched by the bees - and as an aside, leaf-cutting bees are not very often pollinators of Asteraceae.
Plant-Bacterial/Fungal Associations. Pawlowski and Sprent (2008) compare actinorhizal and rhizobial symbioses; see also papers in de Bruin (2015, e.g. chapters 3, 12, 24, 42, 55) and in Adv. Bot. Research 94. 2020. (Note that Rhizobium and relatives, α-proteobacteria, and Burkholderia and relatives, ß-proteobacteria, are sometimes (but usually not here) called α-rhizobia and β-rhizobia respectively.) Rhizobial nodules are anatomically rather like stems in having peripheral vascular bundles, the bacteria being in the pith (Franche et al. 1998), although there is often an association of nodule origination with lateral roots, at least in Faboideae (op den Camp et al. 2011). On the other hand, actinorhizal nodules develop in the pericycle and appear to be modified lateral roots, although they lack root caps and have superficial cork cambium (Pawlowski & Demchenko 2012; H.-L. li et al. 2015; L. Liu et al. 2020).
Rhizobial small RNAs derived from rhizobial tRNA (tRFs) seem to be involved in the very first stages of nodulation, and species of Faboideae examined by B. Ren et al. (2019) differed in their target sites for these tRFs. A number of the genes involved in the establishment of the symbioses with the various bacteria involved, both rhizobial and actinorhizal, are the same as those involved in arbuscular mycorrhizal (AM) and ECM associations, the "SYM" (symbiosis) or CSSP (common symbiotic signalling pathway) being involved in all (Markmann & Parniske 2008; Gherbi et al. 2008; Bonfante & Genre 2010; Hocher et al. 2011; J. J. Doyle 2011; Svistoonoff et al. 2013, 2014, 2015; F. M. Martin et al. 2017; Gough & Bécard 2017; Cope et al. 2019; Mbengue et al. 2020). There are connections between the signalling genes involved in AM symbioses and rhizobial Nod factors (these are lipochitooligosaccharides, LCOs) that allow the bacterium to be recognised by the plant (Maillet et al. 2010; Op den Camp et al. 2010; Streng et al. 2011; Young et al. 2011; Roberts et al. 2012; Rutten et al. 2020), and the rhizobia may acquire the nod genes by horizontal transfer within Fabaceae (Suominen et al. 2001). However, horizontal transfer may range more widely, occuring both between and within the main groups of N-fixing bacteria, involving species of both α- and β-ptoteobacteria (Dludlu et al. 2018: the Cape; Mbengue et al. 2020). Both the AM establishment and nodulation pathways involve the recognition of LCOs (N-acetylglucosamine units - see chitin - with a fatty acid at one end and 2-O-methyfucose at the other), part of the common symbiosis signalling pathway (CSSP), and nuclear Ca2+ oscillations ("spiking") occur in both (Garcia et al. 2015; Kawaharada et al. 2017). The CSSP may be involved in the establishment of actinorhizal associations, with nuclear Ca2+ oscillations in both (Barker et al. 2017). Interestingly, Nod factors are not found in many actinorhizal associations (Normand et al. 2007; Nguyen et al. 2019; Rutten et al. 2020), although they are found in the cluster II strains (basal in the genus), but even some species of the faboid legume Aeschynomene (Faboideae-Dalbergieae) do not have them (Giraud et al. 2007; Chaintreuil et al. 2013, 2016; see also A. Taylor & Qiu 2016).
There are further links between nodulation and pathogen resistance which involve chitooligosaccharides (Liang et al. 2014; Mbengue et al. 2020); for an association between oomycete resistance and AM absence, see Delaux et al. (2014). Other flowering plants respond to the rhizobial Nod factor by suppressing MAMP (microbe-associated molecular pattern) immunity - and this is the initial response even in rhizobial infection in legumes (Liang et al. 2013).
The symbiosis receptor-like kinase gene exists in a particularly distinctive form in the N-fixing clade - and also in Tropaeolum, but not rice, tomato or poppy. This gene in the three latter genera rescued mycorrhizal formation in defective forms of the gene in Fabaceae, but it did not rescue nodule formation, whereas the Tropaeolum gene restored the ability to form nodules (Markmann et al. 2008; see also Chen et al. 2007, 2009; Gherbi et al. 2008; Yano et al. 2008; Markmann & Parniske 2009). There is notable similarity at the transcriptional level in the genes involved in the establishment of N-fixing endosymbioses in Casuarina, Alnus, Rhamnaceae (all with Frankia), legumes, etc. (Hocher et al. 2011), and many of these genes have been coopted from other pathways that are widely functional in land plants, including hornworts and liverworts (Svistoonoff et al. 2013).
Although there is considerable variation in nodule morphology, this does not correlate with bacterium type, and the formation of nodules and N-fixation can be disassociated (van Velzen et al. 2018). In general in Fabaceae the nodules arise from the cortex and develop peripheral vascular tissue, ultimately of cortical origin, with bacterial infected cells, also of cortical in origin, in the centre; they are perhaps shoot-like structures (Hirsch & Larue 1997; Franche et al. 1998; Couzigou et al. 2012). However, in Faboideae there seems to have been co-option of genes originally involved in lateral root origination in nodule formation (op den Camp et al. 2011; J. J. Doyle 2011; Magne et al. 2018; Soyano et al. 2019). Nodules in all other taxa, whether associated with Frankia or rhizobia, are modified lateral roots, although they lack a root cap. The vascular tissue is central and of pericyclic origin, there is an endodermis, although it differentiates relatively late in the development of the nodule; initiation of the nodule is pericyclic, cortical cells that contain bacteria surround the vascular tissue, and a root may develop from the end of the nodule (Soltis et al. 1995b; Gualtieri & Bisseling 2000; Raven & Edwards 2001; Vessey et al. 2004; Shen et al. 2020). When it comes to actual N fixation, in all actinorhizal symbioses and in a number of rhizobial associations (including Parasponia, Cannabaceae) the bacterioids remain in apoplastic fixation threads (the wall of the thread may be made up of endoplasmic reticulum membranes) between the cell wall and plasmalemma; symbiosomes are restricted to Fabaceae (de Faria et al. 2022); see below.
Intercellular penetration of the root epidermis by Frankia occurs in both Rosales and perhaps in Cucurbitales; in Fagales infection is through root hairs (Clawson et al. 2004; c.f. Santi et al. 2013); another kind of entry is via cracks in the epidermis, while entry by root hairs with the formation of infection threads is restricted to some Fabaceae (Svistoonoff et al. 2014; Ibáñez et al. 2016; Coba de la Peña et al. 2018: Fig. 4). Ibawe et al. (2016) discuss the different variants of intercellular invasion by both rhizobia et al. in Fabaceae (ibid. Table 1) and by actinorhizae in other families; in the latter, and even some of the former, nodulation (Nod) factors are not involved. Details of how infection patterns map on to phylogeny are unclear (see also Soltis et al. 2005a; J. J. Doyle 2011), but as the legume phylgeny is clarified, this will change (e.g.) de Faria et al. 2022). Parniske (2018) saw the general problem faced by members of the N-fixing clade as dealing with positive cell turgor pressure as the cell wall was penetrated by bacteria, and the need to somehow plug the hole.
Chemistry, Morphology, etc.. Whether or not the presence of stipules is plesiomorphic in the clade depends in part on its phylogeny, but there is clearly a variety of structures borne at the node - and nodal anatomy is also variable. Thus in Rosaceae variation in nodal anatomy and stipule presence seem to be linked, and variation in nodal anatomy within Surianaceae is also correlated with presence/absence of stipules.
Phylogeny. For the composition of the N-fixing clade, a rather unexpected group, see Chase et al. (1993, 1999), Savolainen et al. (1997), Soltis et al. (1995b, 1997, 1998), and Swensen (1996). This clade was not recovered in some analyses of the complete chloroplast genome, e.g. Bausher et al. (2006), although poor sampling - no other fabids/rosid I taxa were included - may well be reponsible. The N-fixing clade was also found not to be monophyletic in Duarte et al. (2010) or in the genome-level analysis of Burleigh et al. (2011). The clade had little support in the mitochondrial matR analysis of Zhu et al. (2007), but support was much strengthened when two chloroplast genes were added; it was also monophyletic in the mitochondrial analysis of Qiu et al. (2010; see also Z.-D. Chen et al. 2016).
An additional wrinkle is the possibility of an ancient hybridization involving the N-fixing clade and the malvids, the COM clade being the result; see the Dilleniales and particularly the Zygophyllales pages for more discusssion. However, much of the nuclear genome of the COM clade, the possible product of this hybridization, seems to be malvid in origin (Sun et al. 2015) and the possibility of such a hybridization is now considered to be low.
Relationships within the N-fixing clade have been somewhat unclear (e.g. Xue et al. 2012). Although Zhu et al. (2007) could not even recover a monophyletic Rosales using the mitochondrial matR gene, the other three orders were, however, all strongly supported, and in most other analyses all orders are well supported.
1. Sytsma et al. (2002) recovered a topology [Cucurbitales [Fabales [Fagales + Rosales]]] (= [C [Fab [Fag + R]]]).
2. Ravi et al. (2007) examined data sets including 61 protein-coding genes from the plastome (no Fagales) and four other genes (Fagales included), and they found good support for [Fab + R] and some support for the broader grouping [C [Fag [Fab + R]]], however, apart from Fabales (three Fabaceae-Faboideae included), the other orders were represented by single exemplars. A [Fab + R] clade was also obtained by Jansen et al. (2007) and Moore et al. (2007), but no Fagales were included.
3. Zhu et al. (2007: four genes) and Lee et al. (2011: focus on nuclear genome) relationships were [Fab [C [Fag + R]]], albeit there was little support for the topologies found (see also Bell et al. 2010). In other analyses there is some support for a [C + Fag] clade (see Chase et al. 1993; Setoguchi et al. 1999; Schwarzbach & Ricklefs 2000; Soltis et al. 2000, 2003a; L.-B. Zhang et al. 2006).
4. For studies of relationships the results of which largely supported the topology adopted here until xii.2021, i.e. [Fab [R [C + Fag]]], see Jeong et al. (1999: no Fabaceae included), Moore et al. (2008, 2011), H. Wang et al. (2009), Soltis et al. (2011), Z. Wu et al. (2014), Foster et al. 2016a (support weak), H.-L. Li et al. (2015: very good generic sampling), Valencia-D et al. (2020: plastid genomes, poor sampling), and so on. Recently, L. Liu et al. (2023: 122 single copy nuclear genes, 36 spp., all 18 superrosid orders, concatenation RAxML analysis) recovered this grouping, and with quite good support, although obviously sampling was minimal.
5. L. Zhao (2016) found strong support for the topology [[Fab + Fag] [R + C]] in a large-scale nuclear gene analysis, and although their sampling was rather exiguous, this topology has been quite frequently recovered recently, e.g. Koenen et al. (2019b/2020a: support could be stronger), W. R. Baker et al. (2021a: see Seed Plant Tree), X. Guo et al. (2021), Z. Wu et al. (2022) in their nuclear analyses and Zuntini et al. (2024) in their extended Angiosperms353 analysis. This topology is followed here.
6. L. Liu et al. (2023: c.f. 4 above) recovered the relationships [Fag [Fab [C + R]]] in ASTRAL coalescence analyses, but support was not very strong.
Botanical Trivia. It has been suggested that N-fixing plants, including Gunnera with its blue-green algae, are over-represented among species that are invading natural areas (Daehler 1997).
Age. The age of this node is about 99.7 Ma (X. Guo et al. 2021).
Ellagic acid 0; vessel elements with simple perforation plates; wood often fluorescing; nodes?; styloids +; K initiation helical; C clawed; tegmen ± crushed/disintegrating, embryo chlorophyllous. - 4 families, 762 genera, 20,410 species.
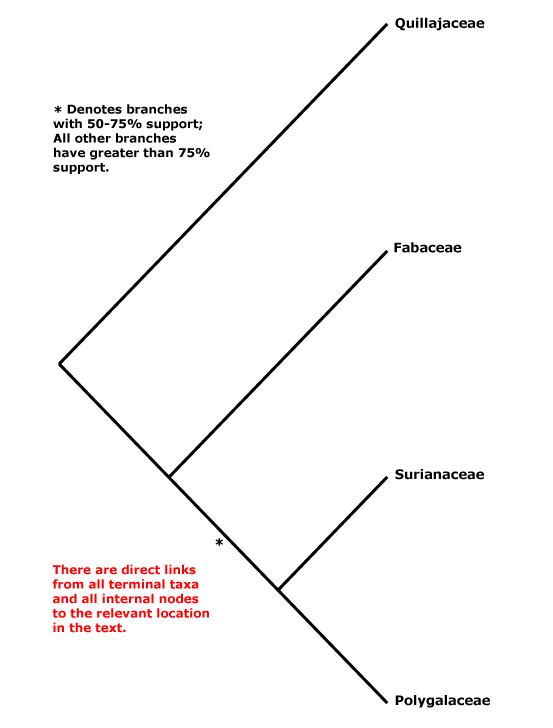
Includes Fabaceae, Polygalaceae, Quillajaceae, Surianaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) date crown-group Fabales to (83-)79, 74(-71) Ma; other estimates are (90-)87(-84) or (75-)72(-69) Ma (penalized likelihood dates), Bayesian relaxed clock estimates being slightly older, (107.1-)104, 101.7(-91.6) Ma (H.-L. Li et al. 2015), ca 100 Ma (Hengcheng Wang et al. 2009), ca 90.3 Ma (Koenen et al. 2013), ca 71.1 Ma (Tank et al. 2015: Table S2), (76.7-)75.0(-69.3) Ma (Uluer et al. 2022) or (88.3-)78.0(-71.9) Ma (L. Cai et al. 2025).
At around 100 Ma, the age of Dakotanthus, perhaps to be associated with Quillajaceae (Manchester et al. 2018), would question some of the estimates above. A recent formal phylogenetic analysis placed Dakotanthus in number of positions (equal maximum parsimony) in the rosids, both Rosales and Sapindales, but also in Fabales including Quillajaceae, and that despite not being able to include some characters that are particularly characteristic of extant Quillajaceae (Schönenberger et al. 2020).
Evolution: Divergence & Distribution. Fabales contain ca 9.6% of eudicot diversity (Magallón et al. 1999), of which the bulk is made up of Fabaceae. If [Quillajaceae + Surianaceae] are sister to Fabaceae, then a strong slow-down in the rate of evolution occurred in the former clade, although if Duparquetia is sister to the rest of Fabaceae (although this seems unlikely), things get more complicated. Indeed, given the uncertainty over the relationships between all four families in Fabales and the subfamilies in Fabaceae, thinking of their evolution and optimisation of characters is a particularly fraught enterprise. -However, it does seem that diversification rate increases in the order are nested - 7/8 such events (Zuntini et al. 2023).
Bello et al. (2012) suggested a number of apomorphies for Fabales, however, Krameria (Zygophyllales, immmediately unrelated) was used as the outgroup because similarities between it and Polygalaceae had been suggested in the past, and most of the apomorphies listed may be plesiomorphies. Despite the floral differences between Polygalaceae and Fabaceae, there are some developmental similarities between them (Prenner 2004d), however, the keel/papilionoid flowers in the two have arisen probably independently since the parts making them up and their arrangement are quite different (Bello et al. 2012). The evolution of papilionoid flowers in Fabales is discussed in more detail under both Polygalaceae.
Lamont et al. (2018b: fig. 10, [Poly [Fab [Quill + Sur]]]) discuss the evolution of Fabales, with fires and hard seeds playing important roles, while Jiang et al. (2019) discuss the evolution of pollen morphology. .
Ecology & Physiology. About a quarter of all records of extra-floral nectaries come from members of this clade (Weber & Keeler 2013).
Genes & Genomes. The rpl22 gene is in the nucleus in Polygalaceae and Fabaceae (i.e. it is absent from the chloroplast), but other members of the order have not been studied and its presence in other angiosperms is sporadic (J. J. Doyle et al. 1995; Su et al. 2014; Dugas et al. 2015). Many Fabaceae-Faboideae have lost the rps16 gene, and it is also absent from Polygala (Downie & Palmer 1992: again, sampling).
Chemistry, Morphology, etc.. The distribution of a number of features may be of systematic significance in Fabales, but sampling is poor - thus the chemistry of Quillajaceae and Surianaceae is poorly known. Although styloids, along with druses, are reported from Surianaceae, Quillajaceae and Fabaceae, details of their distribution within Fabaceae are unclear; they are certainly quite common in Faboideae (Lersten & Horner 2005), apparently less so in the rest of the family.
Pollen grains of Quillajaceae and some Surianaceae have exine protruding at the apertures, and these and some Fabaceae-Cercidoideae have striate pollen, although this is perhaps derived within the latter group (Banks et al. 2003; Claxton et al. 2005); these features are unlikely to be high-level apomorphies. It would be nice to know if Surianaceae or Quillajaceae had starchy endosperm.
Phylogeny. Fabales as circumscribed here were rather unexpected, but they are quite strongly supported (Morgan et al. 1994; Källersjö et al. 1998; etc.). Although Hilu et al. (2003) found Larrea (Zygophyllaceae) to be weakly associated with Fabaceae, the latter were the only Fabales included in their rbcL analysis.
Within Fabales, Persson (2001) suggested the relationships [Polygalaceae [Surianaceae [Quillajaceae + Fabaceae]]], but with little support (this tree was used in versions 1-6 of this site), although elements of it have also been obtained by Cannon et al. (2014: Surianaceae not sampled) and Z.-D. Chen et al. (2016: Quillajaceae not sampled). Forest et al. (2002, see also Qiu et al. 2010) found weak support for the topology [Q [F [S + P]]], and Banks et al. (2008) also found strong support for the relationship [Q [the rest]]. However, Wojciechowski et al. (2004: ?sampling) suggested the possibility of a [S + Q] grouping. The unrooted topology in Bruneau et al. (2008a) is [P [Q + S] F]. Bello et al. (2009) in a careful analysis of matK and rbcL data, preferred the relationships [P [F [S + Q]]] obtained in a maximum parsimony analysis, however, support was poor, and if anything was still poorer for any relationships obtained in Bayesian analyses of the same data (see also M. Sun et al. 2016). Wang et al. (2009) did not obtain well supported relationships in Fabales in their twelve-gene analysis of the rosids, while in a megaphylogeny of angiosperms S. A. Smith et al. (2011) found some support for a clade [Q + F]. Relationships remained unclear in the study by Soltis et al. (2011). Bello et al. (2012) returned to the problem and included morphological data with a two-gene data set. In various analyses [S + Q] were usually at least moderately supported, but support for Fabaceae and Polygalaceae as being successively sister to that pair was weak (Bello et al. 2012); the topology recovered by Bello et al. was also preferred by Cardoso et al. (2013b) and Koenen et al. (2013), although their focus was on Fabaceae. Cardoso et al. (2013b) also placed Vauquelina (Rosaceae!) in this area as part of a tritomy. That genus has indeed been associated with Quillaja in the past, but some time ago in an ITS phylogeny it grouped with Rhaphiolepis and Eriobotrya within Rosaceae-Amgydaloideae (Campbell et al. 1995), and it is to stay in Rosaceae (see also Goldblatt 1976a: cytology). H.-L. Li et al. (2015, 2016) obtained the groupings [[S + Q] [P + F]], although support for the latter family pair was not good, and H.-T. Li et al. (2019, 2021) in their plastome analyses recovered a [[F + S] [P + Q]] grouping, but in both cases with poor support. L. Zhao (2016: Surianaceae not included) found the relationships [Q [P + F]]. Aygören Uluer et al (2020) ran a variety of analyses, including a supermatrix with 678 taxa and three plastid genes, another with 69 taxa (no outgroups) and one nuclear and one plastid gene. The relationships [[F + P] [S + Q]] (see also Uluer et al. 2022: 3 plastid markers) were obtained in many analyses, but the topology [F [P [Q + S]]] had strong support in a molecular clock analysis. Aygören Uluer et al. (2020) also looked more specifically at variation in some nuclear and chloroplast genes and were unable to reject any of the possible topologies for relationships between the four families - perhaps, they thought, their evolution had been nearly simultaneous. In the plastome analysis of H.-T. Li et al. (2021) relationships are [[F + S] [Q + P]]. The Angiosperms353 topology recovered by W. J. Baker et al. (2021) showed [P [Q + F]] (no Surianaceae), in Version 2 (2022) they were [Stylobasium [P [S [Q + F]]] - support for the position of Quillajaceae was weak - and in the version of ix.2024 (see the Seed Plant Tree) they are [P [[S + Q] F]] (no Stylobasium). Zuntini et al. (2024: Stylobasium included, embedded in S) also recovered the relationships [P [S [Q + F]]], but support was weak.
Synonymy: Caesalpiniales Martius, Cassiales Link, Mimosales Link, Polygalales Berchtold & J. Presl, Quillajales Doweld, Surianales Doweld - Fabanae Reveal, Polygalanae Doweld - Polygalopsida Endlicher
[Quillajaceae + Surianaceae] (if this clade exists): ?
Age. The crown-group age of this clade is ca 56.8 Ma (Koenen et al. 2013) or (73.9-)68.6(-50.2) Ma (Uluer et al. 2022).
[Fabaceae [Surianaceae + Polygalaceae]] (if this clade exists): suspensor persistent, also connected to wall of embryo sac [?Surianaceae].
Age. Crown group ages of this clade are (82-)79, 74(-71) Ma (Wikström et al. 2001) or ca 70.6 Ma (Naumann et al. 2013).
[Fabaceae + Surianaceae] (if this clade exists): ?
Age. The crown-group age is ca 70 Ma (Tank et al. 2015: Table S1, S2) or ca 92.1 Ma (Magallón et al. 2015).
QUILLAJACEAE D. Don - Back to Fabales
Small evergreen tree; saponins, leucodelphinidin, flavone C-glycosides +; vessel elements with scalariform perforation plates; cork cambium ?deep-seated; storying?; styloids in phloem +; mucilage cells +; nodes 1:3; petiole bundles arcuate, pericyclic fibres 0; hairs warty; leaves spiral, blade vernation conduplicate, margins toothed [hydathodal?], (entire), stipules petiolar; inflorescence terminal, cymose; hypanthium +; K valvate, nectary on hypanthium and lower half of K, C contorted, spathulate; A unidirectional in initiation, 5A opposite K above nectary + 5A opposite C below nectary; pollen striate; G [5], deeply longitudinally ridged, opposite K, styles ± impressed, stigmatic zone elongated down style; ovules several/carpel, apotropous to pleurotropous, in two marginal rows, micropyle?, outer integument ?3 cells across, inner integument?; fruit strongly asymmetrically lobed, follicular/loculicidal [i.e. opening down the entire lobe], K moderately accrescent; seeds winged; testa with 3 outer layers thickened, sclerotic; endosperm type?, cotyledons investing radicle, conduplicate; n = 14, x = ?, nuclear genome [1 C] ca 0.42 pg.
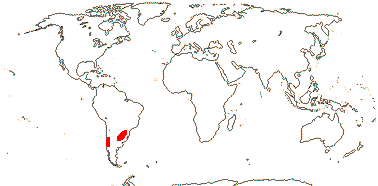
1 [list]/2. Temperate South America, not Peru (map: from Donoso Z. 1994; Luebert 2013). [Photo - Flower, Fruit.]
Evolution: Divergence & Distribution. Dakotanthus cordiformis, from ca 100 Ma Late Albian to Cenomanian deposits in the Dakota Formation, shows a "particularly close association" with Quillajaceae, at least when compared with extant angiosperms (Manchester et al. 2018: p. 27), but the pollen surface and also details of the floral diagram differ. López-Martínez et al. (2023a) suggest that its relationships are uncertain.
Genes & Genomes. There is a genome duplication here (Cannon et al. 2014). Robertson (1974, see reference, but no voucher - Goldblatt 1976a) noted that n = 17 but Goldblatt (1976a) and Stau et al. (2019) found that n = 14.
Chemistry, Morphology, etc.. For flavonoids, see Bate-Smith (1965), for saponins, abundant here, see van Setten and van de Werken (1996). The leaves are amphistomatous.
The flowers of Quillajaceae, with the distinctive arrangement of their nectary and androecium, may be interpreted as having a hypanthium. Androecial development is unidirectional and is rather like that of Fabaceae (Bello et al. 2007/8); the carpels are definitely connate axially, but are largely free laterally, c.f. earlier versions of this site. There appear to be only three traces to each carpel, although Sterling (1969) observed that there were also "intermediate" bundles. Embryologically the family is poorly known.
See also Lindenhofer and Weber (1999a), Culham (2007) and Kubitzki (2006b) for general information, Bate-Smith (1965) and Hegnauer (1973, 1990), all as Rosaceae, for chemistry, Marchiori et al. (2009) for wood anatomy (intercellular canals, included phloem), Lersten and Horner (2005) for vegetative anatomy, Kania (1973) for gynoecial morphology, and Péchoutre (1902, as Rosaceae) for seed morphology. Additional data from: Aronson 7897 (anatomy, embryo).
Phylogeny. Cardoso et al. (2013b: matK phylogeny) placed Vauquelina in this area.
Previous Relationships. Quillaja was included in Rosaceae-Quillajoideae (Takhtajan 1997) or, more usually, in Spiraeaoideae, e.g. as Quillajeae (Robertson 1974). It is indeed superficially quite similar to the South American Kageneckia (Spiraeaoideae), but wood anatomy, molecular data, etc., suggest that it should be removed from Rosaceae (Lotova & Timonin 1999; c.f. S.-Y. Zhang 1992).
FABACEAE Lindley, nom. cons. // LEGUMINOSAE Jussieu, nom. cons. et nom. alt. - Back to Fabales
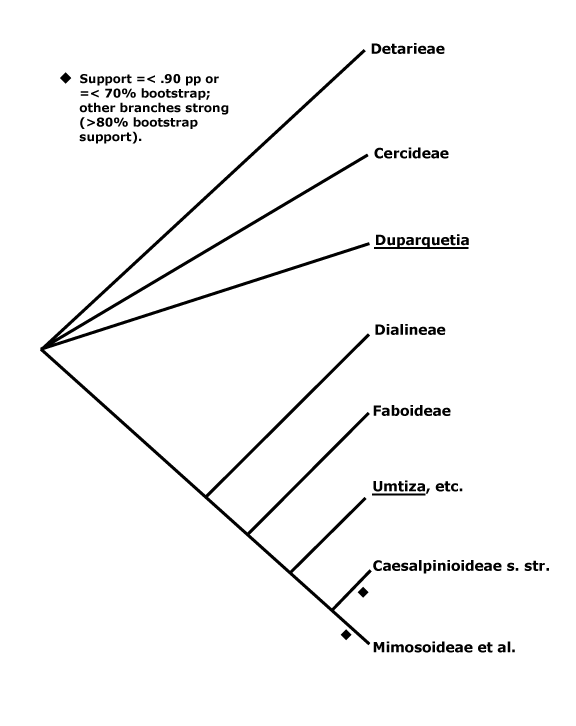
Trees or lianes to annual herbs; coumarins/furanocoumarins, 5-deoxyflavonoids, C-glycosylflavonoids, pinitol [cyclitol] +/0, lectins [haemagglutinins] and gums, esp. in seeds; cork also in outer cortex; (wood storied); secretory cells common, sieve tube plastids with protein crystals and several small starch grains; parenchyma (+; diffuse/terminal), (rays heterocellular); nodes 3:3; cuticle wax platelets as rosettes; stomata various; branching from previous flush; (colleters +), hairs often uniseriate (mesifixed); (extrafloral nectaries +); leaves odd pinnate, petiolules and base and apex of petiole pulvinate, leaflets ± opposite, blade with conduplicate vernation, stipules +, cauline; inflorescence racemose, bracteoles often v. small; flowers monosymmetric, resupinate [median sepal abaxial], (3-)5(-6)-merous, floral developmental sequence K-G-C-outer whorl A-inner whorl A [G initiation/development much advanced]; K ± connate to free, adaxial-median C with patterning/different in colour from the other C (not); A unidirectional in initiation, 10, heteranthy common, filaments connate to free, anthers basifixed to dorsifixed; endothecial ribs 6>/cell, tapetal cells bi(multi)nucleate; (spines/baculae supratectal), exine columellate; G 1, stipitate, with adaxial furrow, stylulus long, (hollow), young stylulus abaxially curved (straight), stigma wet, compitum necessarily 0; ovules 1-several/carpel, one-ranked, micropyle zig-zag, outer integument 2-5 cells across, inner integument 2-3 cells across, endothelium +, parietal tissue to 5 cells across, nucellar cap 2-3 cells across, hypostase +; (megaspore mother cells several), antipodal cells persistent; chalazal embryo haustorium +; fruit dehiscing both ad- and abaxially, explosive or not; seed symmetric, with radicular projection, lens + [= small, ± raised structure usu. near hilum across from micropyle]; raphe and antiraphe ± same length, vascular bundle in antiraphe [v.b. surrounds whole seed]; testa multiplicative, fracture lines +, exotesta palisade, linea lucida + [light line, separating much thickened outer anticlinal walls from the thinner inner walls], at least at hilum, near median, subhilar cells thick-walled, outermost mesotestal layer of stellate/hourglass cells, endothelium ± persistent; endosperm cells thick-walled, with galactomannans [= Schleimendosperm], (0); embryo ± straight [hilum opposite chalaza], cotyledons investing radicle, accumbent, venation palmate, (cell walls thick), (with amyloid), (starch +); whole nuclear genome duplication, x = 7, nuclear genome [1 C] (298-)2129(-26797) Mb/(0.061-)0.943(-14.54) pg; plastome rpl22 gene transferred to nucleus, infA gene lost, chondrome rpo7, 11, 13 genes 0.
766 [list, to subfamilies, some tribes, see also Faboideae]/19,580 - discussed under six subfamilies below. World-wide.
[N.B.: in general, distinguish clearly between ovary with stipe or gynophore/0, adnate ad/abaxially to hypanthium/not.]
Age. Wikström et al. (2001, 2004) date crown-group Fabaceae to (71-)68(-65) or (59-)56(-53) Ma; Bruneau et al. (2008a, b; slightly younger estimates in Bello et al. 2009) thought that Fabaceae began diversifying in the Palaeocene ca 64 Ma. Crown Fabaceae are dated to ca 59 Ma by Lavin et al. (2005), (77-)63, 61(-47) Ma by Bell et al. (2010), (87.1-)80.6, 56.8(-48) Ma by Pfeil and Crisp (2008), and ca 92.9 Ma by Hohmann et al. (2015). These ages are based on Cercidoideae being sister to all other Fabaceae; Duparquetia was not included. Y. Zhao et al. (2021) estimated a crown-group age for the family of some 67.3 Ma, Koenen et al. (2019b/2020a) include an estimate of 86.5-65.5 Ma while L. Cai et al. (2025) suggested (66.8-)66.0(-64.7) Ma - but c.f. Diversity and Distribution below.
Legume pods and leaflets not assignable to a particular subfamily have been found among post-bolide impact early Eocene fossils from Corral Bluffs, Colorado, and they are dated to 65.3 ma (Lyson et al. 2019), while somewhat older deposits ca 64.6 Ma from southern South America include remains of Leguminosae (Iglesias et al. 2007); see also Herrera et al. (2019b) and Koenen et al. (2019b/2020a) for other early records of the family.
[Cercidoideae + Detarioideae]: gynophore/stipe adnate adaxially to hypanthium.
Age. One estimate for the age of this node in Koenen et al. (2019b/2020a: T. S5) is 80.8-57.5 Ma; Y. Zhao et al. (2021) estimated an age of around 66.5 Ma.
1. Cercidoideae Legume Phylogeny Working Group —— Synonymy: Bauhiniaceae Martynov
Trees, shrubs, lianes climbing by branch tendrils, (cladodes + - Breniera); nodules 0; (distinctive secondary thickening), (intraxylary phloem/bicollateral vascular bundles +); (axillary extrafloral nectary [prickle or spine] - Bauhinia - B.), colleters +; leaves 2-ranked, apparently simple [bilobed or not]/bifoliolate, with single pulvinus; (?inflorescence part-cymose - B.); (keel flowers + - Cercis [C.])/polysymmetric, heterostyly [Tylosema]; hypanthium +, ± elongated (0), (nectary inside); K (connate, spathaceous), C (0), with adaxial-median member innermost [ascending cochleate]; A (1-9), (filaments partly connate, mono-/diadelphous), anthers dorsifixed (porose), staminodes 0-7, (branched; staminodial disc +, fleshy); pollen (in tetrads), (with non-sporopollenin viscin threads), inaperturate/3-porate/3-6-colp(oror)ate/3 (syn)colporate, surface variable, supratectal gemmate processes common, (operculum spiny); G (gynophore +, adnate to hypanthium abaxially), (style 0), stigma ±punctate, (capitate), wet, papillate [?some]; (ovules initiated when carpels are open), parietal tissue to 20 cells across, (nucellar beak +), nucellar cap to 10 cells across; fruit (samara/indehiscent, multiseeded), seeds 1-many, (asymmetric), funicle (around most of the seed), (funicular aril 2-lobed), lens +, inconspicuous, micropyle, hilum crescentic (circular - C.), apical [note the order: lens-micropyle-hilum]; (post-chalazal vascular bundle 0 - B.), exotesta mucilaginous, mesotesta lacking stellate/hourglass cells; endosperm + (with galactoglucomannans - C.)/0, (embryo curved); whole nuclear genome duplication, n = 7[Cercis], (12-)14(-15, etc.).
?14/335: Phanera (ca 80), Schnella (47). Pantropical (temperate). Map: from Meusel et al. (1965), Sales and Hedge (1996) and Trop. Afr. Fl. Pl. Ecol. Distr. 3 (2008). [Photo - Bauhinia, Cercis, © D. Kimbler.]
Age. Possible ages for crown-group Cercidoideae are estimated to be ca 34 Ma (Lavin et al. 2005), ca 47.3 Ma (Bruneau et al. 2008a), ca 62.7 Ma (Meng et al. 2014), 54.0-36.0 Ma (Koenen et al. 2019b/2020a), ca 34.0 Ma (Y. Zhao et al. 2021) or (55.1-)54.8(-48.1) Ma (Uluer et al. (2022).
See Meng et al. (2014) for a list of fossils of Bauhinia.
2. Detarioideae Burmeister
Trees (shrubs), evergreen; plant ectomycorrhizal/dual mycorrhizal (not); vestured pits +; leaf phloem transfer cells + (0); leaflets (with flat extra-floral nectaries on the surface/marginal EFNs/etc.); bracteoles generally caducous; hypanthium +, ± elongated; (K "4" [2 adaxial K connate]), C (0-7), adaxial-median member outermost [= descending cochleate]; A (2-many), initiation time of the two whorls overlapping, (ring meristem +), anthers dorsifixed or basifixed; pollen surface variable, (pectic substances below aperture - Zwischenkörper/oncus); fruit with adaxial median K last to be lost, hilum minute, slit-like, etc.; testa cracked; endosperm 0, cotyledon cell walls commonly thick, amyloid +, with xyloglucans [?level]; whole nuclear genome duplication, x = (8, 10, 11) 12, etc..
70/990. Tropical.
Age. The crown age of this clade is estimated to be ca 29.2 Ma (Lavin et al. 2005), ca 53.6 Ma or only ca 17.3 Ma when there were no constraints (Bruneau et al. 2008a), but 68-64 Ma in de la Estrella et al. (2017), (68-)65.5(-63) Ma in Schley et al. (2018), 43.0-25.5 Ma (Koenen et al. 2019b/2020a), ca 51.9 Ma (Y. Zhao et al. 2021) or (55.8-)57.7(-53.1) Ma (Uluer et al. 2022: ??).
60-58 Ma fruits with resin glands have been found in Colombia (Herrera et al. 2019b).
[Detarieae [Schotieae + Barnebydendreae]]: ?
2A. Detarieae de Candolle —— Synonymy: Detariaceae J. Hess
(Ecto-/arbuscular mycorrhizae +), (nodules +); cut bark resiniferous [with bicyclic diterpenes]; leaves (bifoliolate - Hym., Colophospermum, etc.), leaflets punctate (not, ?level); bracteoles often imbricate; K 2 [Col., "4", 5, (petal-like); C (3 + 2 minute/0); A (many); (gynoecial stipe 0), stigma peltate/capitate; ovule with obturator [?all], outer integument ca 6 cells across; fruit indehiscent, often a samara, follicle, (legume); (seeds arillate); (chromosomes acrocentric - Peltogyne).
21/185: Copaifera (35), Peltogyne (25), Hymenaea (16). Pantropical, but 1/2 the genera from Africa-Madagascar.
[Schotieae + Barnebydendreae]: ectomycorrhizae?; plant deciduous.
2B. Schotieae Estrella, L. P. de Queiroz & Bruneau - Schotia Jacquin
Nodules 0; flowers polysymmetric; K ± petal-like, "4", two adaxial K connate; C 5, ≥1 reduced, filamentous; A basally connate or not; ovules several to many/carpel; fruit with replum ["persistent sutural frame"]; seeds arillate.
1/4. Southern Africa.
2C. Barnebydendreae Estrella, L. P. de Queiroz & Bruneau
Flowers ± polysymmetric; K (± petal-like), (4), C 5, (1-3 much reduced); A 10/9 connate + 1 - Barnebydendron; gynoecial stipe free; ovules (3-)5-10(-12)/carpel; fruit samara, 1-2(-3) seeded.
2/2. Guatemala to Bolivia and the S.E. Atlantic coast of Brazil.
[Saraceae [Afzelieae + Amherstieae]]: ?
2D. Saraceae Estrella, L. P. de Queiroz & Bruneau
Arbuscular mycorrhizae + [Saraca], (nodules +); bracts/bracteoles (petal-like); flowers monosymmetric (polysymmetric); (K petal-like), C 0-3, 2 or more much reduced; A <10 [2 + staminodes/(3-)4-8(-10) - Saraca], basally connate; (gynoecial stipe free); ovules 2-8(-12)/carpel; fruit valves elastixc.
4/16: Saraca (11). S.W. China to Malesia and the West Pacific.
[Afzelieae + Amherstieae]: ectomycorrhizae common; nectaries on abaxial surace of the leaflets.
2E. Afzelieae Estrella, L. P. de Queiroz & Bruneau
Ecto- and arbuscular mycorrhizae, (nodules) +; flowers monosymmetric; C 5/1 + 4 much reduced; A 3/7-9/10, usu. basally connate; fruit valves not elastic; seeds arillate.
3/15: Afzelia (11). Pantropical.
2F. Amherstieae Bentham —— Synonymy: Tamarindaceae Martinov
(Vines, with leaf tendrils); (arbuscular mycorrhizae +), nodules 0; (stipules intrapetiolar); leaves (unifoliolate); bracteoles quite often C-like, ± persistent (connate, enveloping K/0); flowers mono-(poly)symmetric, (all develop synchronously - Brownea); K (1 [Aphanocalyx], 2), 4 [= 3 + 2 adaxial connate], C 2-5; (ring primordium + [C, 10 A, order of initiation of latter various]; A 3-10(-many), usu. basally connate/diadelphous, (staminodes +); (pollen 4-porate); (gynoecial stipe free); fruit valves elastic, (indehiscent - Tamarindus); (pleurogram +, closed - Tamarindus); (first seedling leaves opposite).
49/770: Macrolobium (80), Crudia (55), Cynometra (36), Gilbertiodendron (30), Brachystegia (29), Brownea (27). Pantropical, >70% genera continental Africa.
Age. The age of this clade is ca 30 Ma (Schley et al. 2018).
Reports of fossils of extant genera in Africa from the Eocene 46-34 Ma, perhaps as much as 52.1 Ma, include Brachystegia and Cynometra (Epihov et al. 2017 and references).
[Duparquetioideae [Dialioideae [Caesalpinioideae + Faboideae]]]: ?
Age. The age of this clade is ca 67.1 Ma (Y. Zhao et al. 2021).
3. Duparquetioideae Legume Phylogeny Working Group - Duparquetia orchidacea Baillon
Liane; nodules 0; ?chemistry; wood not storied; vestured pits 0; leaves odd pinnate; inflorescence terminal; flowers papilionoid-asymmetric, developmental sequence K-C-A-G; hypanthium 0; K 4 [adaxial-lateral member missing], free, ± petal-like, abaxial-lateral K bilobed, C development simultaneous, enclosing the rest of the flower in bud, fringed with glands, adaxial-median member outermost [descending cochleate], two abaxial C small, strap-like, fringed with glands; A 4, opposite K, free, anthers basifixed, postgenitally connate, porose, thecae apically aristate; pollen grains asymmetric, ectoaperture encircling the equator, with two endoapertures; nectary 0; G developing immediately after A; ovules 2-5/carpel, outer integument broadly encircling rest of ovule; endocarp hairy; seeds 2-5/fruit; ?galactomannans, radicle ± obscured by cotyledons; n = ?
1/1. Tropical W. Africa.
Age. The stem-group age of Duparquetia is ca 62.1 Ma (Koenen et al. 2013: c.f. topology) or (68.5-)69.1(-63.3) Ma (Uluer et al. 2022: ??, D. + Cercidoideae?).
[Dialioideae [Caesalpinioideae + Faboideae]]: ?
Age. An estimate of the age of this node from Koenen et al. (2019b/2020a: t. S5) is 84.7-63.5 Ma and from Y. Zhao et al. (2021) it is ca 66.8 Ma.
4. Dialioideae Legume Phylogeny Working Group
Trees (shrubs); ?chemistry; (stomata paracytic); leaves odd pinnate, (extrafloral nectaries +), (leaflets alternate); (stipules 0); inflorescences thyrsoid, branches, etc., 2-ranked, ultimate axillary flowers lacking bracteoles; K initiation bidirectional, C initiation simultaneous; A initiation simultaneous; stigma ± punctate (peltate); ovules (1-)2(-many)/carpel; fruit indehiscent, (dehiscent), usu. drupe or (narrowly winged) samara; seeds 1-2/fruit; (seed coat undifferentiated); whole nuclear genome duplication, n = (12) 14.
17/85. Pantropical.
Age. The crown-group age of this clade is 30±3 Ma (Lavin et al. 2005), ca 34 Ma (Bruneau et al. 2008a) or (44.7-)45.2(-38.8) Ma (Uluer et al. 2022: ??).
Fruits identified as Dialioideae that are 60-58 Ma have been found in Colombia (Herrera et al. 2019b), while Cynometroxylon tanganyensis was found in Tanzania in deposits 52±2 Ma (Cantrill et al. 2013).
4A. Poeppigieae Britton & Rose - Poeppigia procera (Sprengel) C. Presl
Nodules 0; leaves 2-ranked, equal-pinnate, minor vein phloem transfer cells +; K initiation unidirectional, connate; anthers dorsifixed, opening by slits; G stipitate, style short, stigma small, punctate, papillate; ovules 6-8/carpel; fruit flattened, dry.
1/1. Southern Mexico to Brazil, Cuba.
[Baudouinieae + Dialieae]: leaves unequal-pinnate; A (dimorphic), anthers usu. basifixed, porose.
?Floral development; A (7-9), (5 connate); fruits fleshy, (-7 seeded - Eligmocarpus); smooth/deeply horizontally furrowed.
2/?8: Baudouinia (6). Madagascar.
Nodules 0; vestured pits 0 (+ - Mendoravia); bracteoles usu. 0 [ultimate triads], (racemose); flowers (single, axillary), (papilionoid), (radially symmetrical), (3-merous), (bracteoles 0); relative timing of organ formation variable; hypanthium usu. 0; K (3-7), free, initiation various, not unidirectional, C (0-4), (usu. not clawed), adaxial-median member innermost [ascending cochleate]; A (2-)5 [= whorl opposite K](-15), (staminodes 0-4), anthers (polysporangiate), often porose, (filaments short); pollen (± syncolporate, surface rugulate/striate - Martiodendron); (G 2), (stipe 0/adnate adaxially to hypanthium), style glabrous, (complex, petaloid), stigma subcapitate to punctate, papillate; ovules (initiated when carpels are open), 2/carpel; (pleurogram + - Apuleia).
14/76: Dialium (28). Pantropical.
[Caesalpinioideae + Faboideae]: (roots with branched nodules, vasculature peripheral, cortical in origin, bacteria α- or β-proteobacteria), lacking hypodermis [?level]; vestured pits +; (stipules 0), stipels +/0; (hypanthium 0); anthers basifixed or dorsifixed; chalazal endosperm haustoria + [?level]; (seed arillate).
Age. This node has been dated to (62-)59, 53(-50) or (36-)34(-31) Ma (Wikström et al. 2001), (67-)50, 49(-30) Ma (Bell et al. 2010), ca 61.3 Ma (Bruneau et al. 2008a), ca 55 Ma (Lavin et al. 2005; quite similar ages in Bouchenak-Khelladi et al. 2010b), 81.7-60.6 Ma in Koenen et al. (2019b/2020a) and 66.5 Ma in Y. Zhao et al. (2021).
5. Caesalpinioideae de Candolle
Shrubs, trees, lianes (herbs); (plants susceptible to Ravenelia rusts); sieve tube plastids also with fibres; extrafloral nectaries common, on petiole/rhachis; (leaves bipinnate); hypanthium cupular, (G stipe adnate adaxially to hypanthium); C with adaxial-median member innermost [ascending cochleate]; stigma ± punctate; ovules usu. campylotropous [up a level?], outer integument with vascular strand; seed (aril 0), funicle long and thin to stout and thick; testa cracked; x = 14; whole nuclear genome duplication, sucrose synthase gene [SUSY] duplicated.
163/ca 4,680 - 11 tribes below. Predominantly tropical, esp. Africa and America. [Photos - Collection.]
Age. The [Umtiza group (= Gledistieae)+ The Rest] clade has been dated to ca 58.6 Ma (Bruneau et al. 2008a), Koenen et al. (2019b/2020a: T. S5) thought the age was around 74.5-54.1 Ma, Y. Zhao et al. (2021) suggested that it was 63.4 Ma and Uluer et al. (2022: ??) gave an age of (64.1-)64.5(-58.2) Ma. Check: node?
Includes I. Albizzia clade, I. Archidendron clade, Caesalpinieae, I Calliandra clade, Campsiandreae, Cassieae, I Cedrelinga, Ceratonieae, M Chidlowia, I Cojoba clade, Core Mimoseae (CM), CM Cylicodiscus clade, CM Dichrostachys clade, Erythropheeae, Gleditsieae, Inga clade (I), CM Ingoid clade, I Jupunba clade, CM Mimosa clade, Mimoseae, CM Neltuma clade, M Newtonia grade, CM Parkia clade, I Pithecellobium clade, CM Prosopis clade, Pterogyneae, I Pseudosamanea, Samanea clade, Schizolobieae, Sclerolobieae, I Senegalia etc. grade, CM Strypnodendron clade, I Zapoteca clade.
The hierarchy below, especially that in the Mimoseae area, was initially based on that in Koenen et al. (2020b), but modifications and elaborations suggested by Ringelberg et al. (2022) were then included as were correct generic names; see also PhytoKeys 205 (2022) for some of the many polyphyletic genera in this area. A summary - and more, some new tribal names, etc. - of all this work and much else is to be found in Bruneau et al. (2024). Note that in this latter work there are dot-map distributions for all the genera.
5A. Ceratonieae Reichenbach —— Synonymy: Ceratoniaceae Link
Trees; leaves bipinnate, with terminal pinna (not -C.), leaflets sessile/petiolulate; plant dioecious (not)); flowers polysymmetric; (median K adaxial - C.), (K minute, C 0 - C.), C (green - Acrocarpus): A = C, 2xC, many; (ovules initiated when carpels are open); fruit (indehiscent, ± fleshy), (unequally 4-winged - T.); seed coat undifferentiated, pseudopleurogram + [no fracture of exotesta]; n = 12, genome size [1 C] ca 0.57 pg, large deletion in plastid trnL intron (not).
4/6: Ceratonia (2), Tetrapterocarpon (2). Scattered: Madagascar, Mediterranean, southern Arabia, the Horn of Africa, India and China to Indonesia, Hispaniola.
Age. The age of this clade is estimated to be around 45 Ma (Bruneau et al. 2008a).
Fossils identified as Arcoa, with the same distinctive terminal pinna, have been found in early Eocene deposits perhaps ca 50 Ma from Wyoming (A. lindgreni) and from younger late Eocene deposits in Colorado (Herendeen & Herrera 2019).
Age. Marazzi et al. (2012) suggested that the age of this clade was 63 or more Ma, Y. Zhao et al. (2021) thought that it was around 62.4 Ma.
5B. Gleditsieae Nakai
Trees, (deciduous); nodules 0 [Umtiza?]; branched thorns on trunk (0); supernumerary axillary buds conspicuous; leaves bipinnate, (not U.); plants ± dioecious (flowers perfect); flowers (3-4-merous), (bracts 0); helical initiation of floral parts [G.]; flowers polysymmetric; K spreading in young bud, (median K adaxial - G.); (ovules initiated when carpels are open), orientation of G. various [Gl.], placental suture to top of style; n = 14.
3/20: Gleditsia (13), Gymnocladus (6). E. North America, temperate South America, mainland S.E. Asia (scattered in WWestern Malesia, the Near East), Umtiza South Africa, the eastern Cape.
Age. The crown Umtiza group is 55-52 Ma (Bruneau et al. 2008a).
Age. Pterogyne may have diverged from the rest of this clade some 60-57 Ma (Y. Zhao et al. 2021).
5C. Pterogyneae Legume Phylogeny Working Group - Pterogyne nitens Tulasne
Tree; guanidine alkaloids +; leaves imparipinnate, stipels +; flowers small [to 5 mm across], polysymmetric; fruit a samara; n = 10.
1/1. South America - southern Brazil and Bolivia to northern Argentina.
[Cassieae [Caesalpinieae [[Schizolobeae, Sclerolobieae, Dimorphandreae] [Campsiandreae [Erythrophleeae + Mimoseae]]]]]: (nodules +, growth indeterminate, bacterioids in fixation threads); post-zygotic incompatibility system [?all]; endosperm slight. - Check all this...
Age. This clade is ca 59.9 Ma (Y. Zhao et al. 2021).
5D. Cassieae Bronn —— Synonymy: Cassiaceae Vest
Herbs (annuals) to trees; (nodules +, rhizobia also in membrane-bounded symbiosomes - Ch.; colleters +; (leaflets amphistomatous); (extrafloral nectaries petiolar - Ch.); (inflorescence cymose - Ch.); (bracteoles 0); (flowers enantiostylous, asymmetric), A heteromorphic (not), (anther sutures hairy - Ch.), filaments curved/straight; (hypanthium and nectary 0); (anthers porose); stigma often chambered/crateriform, (dry - Cassia); micropyle zig-zag, outer integument 2-9 cells across, inner integument 2-3 cells across, parietal tissue ca 6 cells across, hypostase +; fruit (explosively dehiscent); lens ± elliptic; suspensor poorly developed; n = 8, 10, 14.
7/695: Chamaecrista (361), Senna (287), Cassia (39). Tropics, esp. South America, also temperate, the four small genera all South American.
Age. Cassieae are around 53 Ma (Bruneau et al. 2008a).
Leaves of Cassia fistula and leaves, fruits and seeds of C. angustifolia are reported from Eocene (thus ≤34 Ma) lignite deposits at Gurha, Rajasthan, India (Harsh & Shekhawat 2022).
Age. The age of a Caesalpineae etc., clade is ca 59.5 Ma (Y. Zhao et al. 2021).
5E. Caesalpinieae Reichenbach —— Synonymy: Caesalpiniaceae R. Brown
Nodules +, terete [caesalpinioid], (flattened - crotalarioid)/0; (prickles, spines, or glandular hairs); (foliar glandular idioblasts +); leaves bipinnate (pinnate), (with terminal pinna); flowers monosymmetric (polysymmetric - Pterolobium); abaxial K often distinctive [enlarged, fringed - Tara; cucullate], adaxial C often patterned; A ± surrounding G; pollen (with non-sporopollenin viscin threads); ovules initiated when carpels are open; (fruit a samara); (pleurogram + - Caes.); n = (11) 12, chromosomes ca 2 μm long, nuclear genome [2C] (0.92-)2.46-2.76(-7.11) pg.
27/225: Erythrostemon (31), Hoffmannseggia (24), Mezoneuron (24), Centostigma (15). Pantropical, inc. S.W. U.S.A., Florida, the Arabian Peninula, E. Australia to Fiji.
Age. Caesalpinieae are about 56 Ma (Bruneau et al. 2008a) or ca 54.8 Ma (Gagnon et al. 2018).
Bipinnate leaves identified as Caesalpinioideae have been found fossil in deposits 60-58 Ma from Colombia (Herrera et al. 2019).
[[Schizolobeae, Sclerolobieae, Dimorphandreae] [Campsiandreae [Erythrophleeae + Mimoseae]]]: bracteoles 0; K imbricate; C imbricate.
Age. This clade is about 56.3 Ma (Bruneau et al. 2008a) or ca 57.1 Ma (Y. Zhao et al. 2021).
Age. This clade is about 56.9 Ma (Y. Zhao et al. 2021).
5F. Schizolobieae Nakai
Nodules 0; azetidine-2-carboxylic acid +; (abaxial K cucullate - Parkinsonia); anthers latrorse, connective apiculate; pollen (with non-sporopollenin viscin threads); (ovules initiated when carpels are open); n = (11-)13, 14.
8/42: Delonix (12), Parkinsonia (12), Bussea (7), Peltophorum (7). S.W. U.S.A. to South America, the Antilles, Africa, Madagascar, S. Arabian Peninsula, Sri Lanka, S. China to E. Malesia, N.W. Australia.
Age. This clade is about 56.7 Ma (Y. Zhao et al. 2021).
5G. Sclerolobieae Bentham & Hooker (Tachigalieae Nakai)
(Nodules + (0), fixation threads + [mucunoid]; leaves pinnate, (growing for a long time - Tachigali), stipules foliaceous or unusually divided; flowers (like those of Malpighiaceae, fertile A 1, staminodes 9 - Moldenhawera); pollen (in tetrads - Dipty.), with sporopollenin viscin threads [Jaqueshuberia]; cotyledons with amyloid; n = 12.
5/108: Tachigali (76-90: inc. Sclerolobium), Moldenhawera (12). Central and South America.
5H. Dimorphandreae Bentham / Dimorphandra Group A
(Nodules + - Dimorphandra)/0; (leaves once pinnate); median sepal adaxial; C protective in bud, C + A initiation simultaneous; (A 5, staminodes +, opposite K); pollen tricolpate; (pleurogram +); n = 14.
4/35: Dimorphandra (paraphyletic: 26). Costa Rica to South America, the Antilles, mainland Africa.
Age. This clade is about 56.0 Ma (Y. Zhao et al. 2021).
[Campsiandreae [Erythrophleeae + Mimoseae]]: ?
5I. Campsiandreae Legume Phylogeny Working Group
(Nodules + - Camp.)/0; hypanthium cupular; C imbricate; A long-exserted; pollen (in tetrads - Din.); (ovules initiated when carpels are open); n = 13 (14).
2/17: Campsiandra (?15). Tropical South America, not the West nor South Brazil, etc..
[Erythrophleeae + Mimoseae]: extrafloral nectaries on petiole and/or rhachis (0), elevated; leaves usu. bipinnate, leaflets 2-ranked; inflorescences dense, spicate/racemose; flowers small, pedicellate, hypanthium +/0; K/C imbricate; n = 12, 14.
5J. Erythrophleeae Legume Phylogeny Working Group
Alkaloids, saponins + [very toxic]; sap reddish; nodules +, with fixation threads [E.]; (median K adaxial - E.).
2/13: Erythrophloeum (12). Tropical Africa, Madagascar, S.E. Asia S. China to Thailand, N. Australia.
5K. Mimoseae Bronn (Mimosoideae de Candolle)
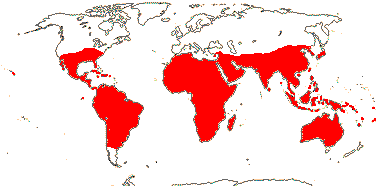
Shrubs or trees (herbs); (cluster roots +), nodules +, terete [caesalpinioid], with membrane-bounded symbiosomes (0); albizziine and other non-protein amino acids +, exudates mostly gums; sieve tube plastids also with circular fibres, one/large starch grains; wood not storied; (septate fibres +; aliform axial parenchyma +); rays usu. >20 cells high; (leaflets fold forwards at night); inflorescences also capitate, floral development ± synchronous, organ initiation simultaneous; flowers rather small, polysymmetric, K C and A with three separate series of vascular gaps up the receptacle, K connate, initiation simultaneous, valvate, (much reduced), median K adaxial, C protective in bud (not), initiation simultaneous, valvate, initially free, ± coherent, not clawed, not patterned, (reduced); A 3-10-many, long exserted, conspicuous, (heteranthy +), basifixed, introrse, polysporangiate [with subloculi], terminal often stipitate gland +/0, surfaces of gland/connective cells sculpted; endothecial cells with base plate, ribs >6/cell, tapetal cells uninucleate; pollen in tetrads/polyads, grains colporate/porate/porate + pseudocolpi; (nectary 0); G (stipitate), stylar groove 0, stigma cup-shaped, (peltate); nucellus 2-3 cells across, nucellar cap +, (apex exposed); seed (arillate), funicle long, thin; pleurogram +, U-shaped [= open] (O-shaped - closed)/(0), lens tiny, raised above surface [shorter palisade cells, thinner cuticle]; (exotesta mucilaginous); endosperm 0, (tubular chalazal haustoria +), suspensor vestigial at cotyledon stage, detached from wall, cotyledons ± cover radicle; n = (8, 12) 13 (14, 16 or more); plastome IR with ca 13 kb expansion [= IREC].
100/3,510. Tropical and warm temperate, esp. Africa and America. Map: from Vester (1940), Maslin et al. (2003) and Trop. Afr. Fl. Pl. Ecol. Distr. 3. (2008a). [Photos - Collection.]
Age. Mimoseae have been dated to (49.5-)42.4, 40.5(-31.3) Ma (Lavin et al. 2005: inc. Pentaclethra; ages in Bouchenak-Khelladi et al. (2010b) are (61-)59.5(-58) Ma, ca 46 Ma in Bruneau et al. (2008a), while (62.7-)51.4(-15.0) Ma is the estimate in Miller et al. (2013).
Brea et al. (2008) report wood of Paracacioxylon frenguellii - there are also pulvinate leaves - from early Palaeocene rocks in Argentina 57-54 Ma that they identified as belonging to Mimosoideae, and in particular being similar to Acacia s.l., although there are no pollen polyads known from this period in Argentina. Acacia s.l. is known from woodland habitats in Tanzania ca 46 Ma (Jacob 2004).
5K1. Adenanthera clade (Xylia clade) / Adenantherinae Bentham
(leaflets 2-ankled); (inflorescence capitate - Xylia); K (imbricate); (C free - Pentaclethra); endosperm +, slight; seeds brightly (bi)coloured [Adenanthera], (pleurogram 0).
6/45: Tropical, to the Pacific, few America. Xylia (20, inc. Calpocalyx), Adenanthera (12), Pseudoprosopis (7).
[Sympetalandra, Chidlowia [Entada clade [Newtonia grade + Core Mimosoids]]]: ?
C imbricate; A basally adnate to C; fruit dehiscent.
1/5. West Malesia, inc. Lesser Sunda Islands.
5K3. Chidlowia Hoyle - Chidlowia sanguinea Hoyle
Nodules +; leaves once paripinnate; flowers rather distant along inflorescence axis; C imbricate; A dorsifixed; fruit explosively dehiscent; pleurogram 0.
1/1. Tropical West Africa, Sierra Leone to Ghana.
[Entada clade [Newtonia grade + Core Mimosoids]]: ?
5K4. Entada clade.
Twining liane - Entada, (geoxylic, swollen stem base - Elephantorhiza, = Entada), to tree, (deciduous - Piptadeniastrum); pollen grains monads; stigma tubular to cupuliform; fruit lomentum with persistent replum [= craspedium]; (seeds winged, elongated, pleurogram 0; embryonic axis perpendicular to length, funicle enters the middle - P.).
3/43: Entada (40). Pantropical, esp. Africa. Map: O'Donnell et al. (2022: Fig. 3).
[Newtonia grade i/ii/iii/iv + Core Mimosoids]: ?
Anonychium (Bentham) Schweinfurth [= Prosopis sect. Anonychium [= Newtonia grade i] - A. africanum (Guillemin & Perrottet) C. E. Hughes & G. P. Lewis
C linear, free, glabrous; anther gland sessile, ventral, between thecae, as triangular hood-shaped protrusion, cells papillate; pollen costate; mesocarp thick, spongy.
1/1. Africa, the Sahel (Senegal to Ethiopia).
[Newtonia grade ii/iii/iv + Core Mimosoids]: ?
Platyhymenia Bentham - Plathymenia reticulata Bentham [= Newtonia grade ii]
Pollen grains single; seeds winged.
1/1. Central-eastern South America.
[Newtonia grade iii/iv + Core Mimosoids]: ?
Fillaeopsis Harms - Fillaeopsis discophora Harms [= Newtonia grade iii] [These last two may be sister taxa - see Pan et al. 2012: Fig. 3.]
Pollen in tetrads; seeds winged, elongated, pleurogram 0; embryonic axis perpendicular to length, funicle enters the middle.
1/1: Tropical Central West Africa, few records from the Congo.
Age. Pollen tetrads (Fillaeopsidites) from Cameroon dated to the Oligocene or earlier belong to this genus (Guinet & Salard-Cheboldaeff 1975; Pan et al. 2012).
[Newtonia grade iv + Core Mimosoids]: ?
iv. Newtonia Baillon [= Newtonia grade iv]
Seeds winged, elongated, pleurogram 0; embryonic axis parallel to length, curved and narrowed near base, funicle enters seed asymmetrically near base.
1/11. Tropical Africa, not the southwest. Map: See Pan et al. (2012).
Age. Seeds 22-21 Ma of Newtonia have been found in Ethiopia northeast of their current range (Pan et al. 2012).
[Cylicodiscus clade [Prosopis clade [Neltuma clade [Dichrostachys clade [Parkia clade [Stryphnodendron clade [Mimosa clade + Ingoid Clade]]]]]]] / Core Mimosoids: ?
5K6. Cylicodiscus clade - Cylicodiscus gabunensis Harms
Leaves bi-imparipinnate, leaflets few, large; seeds winged, elongated, pleurogram 0; embryonic axis parallel to length, not curved and narrowed near base.
1/1: West/Central Africa, Guinea-Congo forests.
Prickles (massive) +; mesocarp spongy; n = 14.
2/4: Prosopis (3). North Africa to N.W. India, Nepal and Azerbaijan.
Prostrate shrubs to small trees; thorns (from prophyllar buds)/stipular spines; (stem photosynthetic); (leaves amphistomatous); (inflorescence capitate); mesocarp thick, mealy/pulpy/spongy (thin); n = 14.
3/41: Neltuma (20, = Prosopis sects. Algarobia and Monilicarpa), Strombocarpa (10, = Prosopis sect. Strombocarpa]). Tropical and Subtropical U.S.A. to Peru-Argentina, mostly the western part (few E. Brazil), also southwest Africa (Xerocladia).
[Dichrostachys clade [Parkia clade [Stryphnodendron clade [Mimosa clade + Ingoid Clade]]]]: ?
5K9. Dichrostachys clade / Desmanthinae Bentham
Shrubs to trees, (herbs) (aquatics); leaves (single pair of pinnae, each with three leaflets - Kanaloa); inflorescence (elongated) capitate, flowers often heteromorphic [perfect at apex, then staminate, then sterile at base with C-like staminodes]; (K imbricate - Mimozyganthe); anthers (long-hairy - Leucaena), glands terminal, caducous, stipitate/connective acute, produced/0; pollen grains monads (tetrads/acalymmate polyads, etc.), col(porate); (seeds winged, elongated, embryonic axis perpendicular to length, funicle enters the middle - Lemurodendron), (pleurogram 0).
12/116: Leucaena (24), Desmanthus (23), Neptunia (22), Dichrostachys (13). Tropical and subtropical America and Africa, India and Sri Lanka (few), Java and the Philippines E., inc. Australia, Pacific Islands (Hawaii - Kanaloa, ?extinct).
[Parkia clade [Stryphnodendron clade [Mimosa clade + Ingoid Clade]]]: pollen acalymmate [exine not forming a common covering over polyad].
Age. This clade is ca 46.6 Ma (Conceição Oliveira et al. 2021a, b).
5K10. Parkia clade / Parkiinae Wight & Arnott
(Nodules + - Anadenanthera); (stem spines + [stipular position, (also swollen)]) - Vachellia); inflorescences capitate, (with trimorphic flowers, outer flowers with petal-like staminodes - Parkia); K (imbricate); (A 10≤ - Vachellia); polyads with internal space, pores facing internally.
3/202: Vachellia (164), Parkia (35). Pantropical, Asia - only some Parkia.
Age. The age of a clade [Par. + Anadenanthera] is ca 33.6 Ma (Conceição Oliveira et al. 2021a, b).
[Stryphnodendron clade [Mimosa clade + Ingoid Clade]]: ?
Age. The age of this clade is ca 45 Ma (Conceição Oliveira et al. 2021a, b).
Polyads (8-)16 grained, oval, grain ornamentation various; (seeds winged, elongated; embryonic axis perpendicular to length, funicle enters the middle - Pseudopiptadenia, etc.).
7/55: Stryphnodendron (28), Pityrocarpa (7), Gwilymia (7). Tropical America, Mexico southwards.
[Mimosa clade + Ingoid Clade]: ?
5K12. Mimosa clade / Mimosinae J. Presl —— Synonymy: Mimosaceae R. Brown
Herbs (annuals) to shrubs (trees), (plant sensitive); (prickles +), hairs stellate and variants; petiolar nectaries often 0; inflorescence capitate (spicate); merism various; K connate, C long, basally connate, apical closure by papillae, hairs; A (= and opposite K), anthers not polylocellate [lacking sublocules], filaments folded in bud; polyads 4(-16) grains, acalymmate, grain surface areolate(and/or verrucate), grain arrabgement various; stigma porate; fruit (lomentum with persistent replum [= craspedium]); endosperm slight; n = 13.
3/730: Mimosa (689), Piptadenia (30). Pantropical (warm temperate), esp. America.
5K13. Ingoid Clade / [Senegalia i/ii/iii/iv grade [Calliandra clade [Zapoteca clade [Cojoba clade [Pithecellobium clade [Archidendron clade, Cedrelingia, Pseudosamanea [Jupunba clade [Samanea clade [Inga clade + Albizzia clade]]]]]]]]]: A >10, from ring primordium, basally connate; polyads 16-grained.
5K13a. Senegalia, etc., grade i. Senegalia s. str., = S. sects Senegalia, Monacanthea (= M) s. str. [= Senegalia grade i]
Shrubs to trees (± lianes - M.), prickles 1-3, nodal, (cauline, M.); inflorescences single, axillary, ± spicate; postgenital fusion of C; staminal disc at the base of the filaments.
1/55 (M. 4). Old World: Africa to Laos, esp. Afican mainland.
5K13b. Parasenegalia clade [= Senegalia grade ii]
Shrubs to trees or lianes; inflorescences spicate (capitate), aggregated; (anther glands 0); stigma filiform [?M].
3/27: Mariosousa (14), Parasenegalia (11). New World, inc. southwest U.S.A. and the Caribbean.
5K13c. Albizia leonardi [= Senegalia grade iii]
1/1. Haiti. Parasenegalia vogeliana, also from Haiti, goes somewhere around here.
5K13d. "Senegalia pro parte = S. sect. Monacanthea pro maxime parte, ?= Manganaroa Spegazzini [= Senegalia grade iv]
Lianes [stem tendrils], shrubs to trees; prickles ± cauline (nodal, forked); inflorescence variously aggregated, terminal, capitate (spicate); C valvate, development simultaneous..
1/164. Pantropical, esp. America, inc. S.W. U.S.A. and N.E. Australia
[Calliandra clade [Zapoteca clade [Cojoba clade [Pithecellobium clade [Archidendron clade, Cedrelingia, Pseudosamanea [Jupunba clade [Samanea clade [Inga clade + Albizzia clade]]]]]]]]: A >10, from ring primordium, basally connate; polyads 16-grained. - check position.
Age. The age of Ingeae [?here] is (21.3-)18.8(-16.3) Ma (E. R. de Souza et al. 2013).
Subshrubs to trees (rhizomatous, xylopodia); (thorns, stipular spines + - Afrocalliandra - Af.); ; leaves 2-ranked [?all], petiolar nectary +/0; inflorescence capitate-umbellate; (hypanthium +); polyads usu. 8-grained, calymmate, (7-grained, acalymmate - Af.), flattened, ± ovoid, apex appendiculate, with sticky gland (not); cytokinesis successive; stigma ± expanded/capitate [wide area receptive to polyads], surface various; (cotyledons sagittate); n = 8, 11.
3/162: Calliandra (150), Acaciella (10). Warm temperate to tropical America, C./S. U.S.A. southwards, 2 spp. in Africa.
Age. The age of the [Calliandra + Afrocalliandra] clade is (17.5-)14.7(-11.7) Ma (E. R. de Souza et al. 2013).
Stipular spines +/0; A = 30-60; polyads 16-(20-)grained, lens-shaped structure in central cells; fruit an explosive legume (not); n = 13.
5/45: Viguieranthus (22), Zapoteca (18). Africa inc. E. Mediterranean, Arabia, esp. Madagascar (Viguieranthus), tropical America (Zapoteca), scattered Sri Lanka to Vietnam.
3/25: Cojoba (18), Lysiloma (8). Southernmost U.S.A. to N.W. South America, the Caribbean.
[Pithecellobium clade [Archidendron clade, Cedrelingia, Pseudosamanea [Jupunba clade [Samanea clade [Inga clade + Albizzia clade]]]]]: (C ± connate).
Shrub or tree; wood with septate fibres, parenchyma not confluent; stipules spinescent; inflorescences capitate (to spicate)); A -3((-7)-9) cm long, anthers elliptic [not round]; pollen porate; nectary as callosities (0, or strongly disciform [Sphinga]); funicles straight/sinuous/sigmoid; (aril + - Pithecellobium).
7/33: Pithecellobium (19). Southern U.S.A. and the Caribbean to Peru and N.E. Brazil, all seven genera in Mexico.
Age. The age of this clade is some (24.5-)21.0(-17.2) Ma (E. R. de Souza et al. 2013).
5K13f. Archidendron clade / Acaciinae Wight & Arnott —— Synonymy: Acaciaceae E. Meyer
(Plants dual-mycorhizal - Acacia); (phyllodes +), (stipules 0); A (10-)30<, ± connate, (ring meristem + - Ac. celastrifolia; G (5, opposite K - Archidendron lucyi); (aril +), pleurogram +/0; (seedlings with bipinnate leaves).
9/1,240: Acacia s. str. (1,082), Archidendron (120), Serianthes(18). Indo-Malesia, Ryukyu Islands southwards, Australia (esp. Acacia), Hawaii to New Caledonia, Réunion, etc..
Two unplaced genera go around here, and the position of the Samanea clade is also unclear:
Cedrelinga cateniformis (Ducke) Ducke
1/1. Central South America.
1/3. Mexico to N.W. South America, S. Cuba.
p>[Jupunba clade [Samanea clade [Inga clade + Albizzia clade]]]: ?Inflorescences congested to lax, spiciform to racemose, with sylleptic branches; flowers dimorphic or not; polyads with 16 grains; fruit follicle, lomentum or legume; pleurogram (0); embryo blue [delphinidin] or not.
4/70: Jupunba (37), Pseudalbizia (18). American tropics (Mexico southwards), the Caribbean, two species of Hydrochorea in Africa.
[Samanea clade [Inga clade + Albizzia clade]]: ?
inflorescences with dimorphic flowers.
2/13: Chloroleucon (10). Tropical America (Mexico southwards), the Caribbean
p>[Inga clade + Albizzia clade]: ?Indumentum dense, ferrugineous; (leaves once pinnate - Inga); inflorescences capitate, (from old wood - Zygia); median K abaxial, C &plusm; connate; A many, from ring meristem; polyads with 16/18/24/32 grains; G (2); epicarp ± farinose, seeds covered by sweet white powder [= dry pulp] [Inga]; pleurogram +/0.
8/385: Inga (300), Abarema (110), Zygia (60). Neotropics, Mexico southwards, the Caribbean, Osodendron Central and tropical West Africa.
Wood with septate fibres, parenchyma not confluent; midrib of leaflets asymmetrically displaced; inflorescences with dimorphic flowers [outer flowers with larger C]/not; A connate; polyads with 16(32) grains.
3/100: Albizia (90), Enterolobium (8). Largely tropics, to New Caledonia, S.W. Russia.
6. Faboideae Rudd / Papilionoideae de Candolle, nom. alt.
Nodules with infected and uninfected cells mixed, indeterminate; isoflavonoids [pterocarpans and isoflavans], prenylated flavonoids, exudates mostly gums; wood often ring porous, vessels with helical thickenings, vascular tissue storied (not); sieve tubes with spindle-shaped non-dispersive protein bodies [forisomes]; (cork cambium deep seated); A free to connate; ?tapetal cells; pollen (endexine ± 0), (exine granular); stigma semi-dry; ovules campylotropous; seed asymmetrical, raphe shorter than the antiraphe, funicle short, hilum long, hilar groove + [break in palisade, = faboid split]/(0 - usu. overgrown seed), micropyle conspicuous [discoloured] (not), rim aril +; testa fracture lines 0, counter palisade +, tracheid bar in subhilar tissue, exotestal cells with near apical linea lucida; embryo curved (straight), radicle long, cotyledons do not cover radicle; whole nuclear genome duplication.
503/ca 14,000 [list: in progress, to tribes, for the rest of the family, see above]. World-wide, esp. (warm) temperate. Map: from Vester 1940; Meusel et al. 1965; Hultén 1971; Trop. Afr. Fl. Pl. Ecol. Distr. vol. 3 (2008a), vol. 4 (2008b). Photo: Flower, Fruit, Collection.]
Age. Crown group Faboideae may be about 59±8.6 Ma (Lavin et al. 2005: clade = Ateleia + Albizia), ca 45 Ma (Bruneau et al. 2008a), ca 55 Ma (Cannon et al. 2014), 73.6-55.2 Ma (Koenen et al. 2019b/2020a: t. S5), about 63.8 Ma (Y. Zhao et al. 2021), (64.9-)67.2(-62.5) Ma (Uluer et al. (2022: ??) or (62.1-)60.9(-59.7) Ma (L. Cai et al. 2025).
Includes - but this is all a bit tentative - Abreae, ADA clade, Aldina, Amburaneae, Amorpheae, Andina, Angylocalyceae, Astragaleae, Baphieae, Bossiaeeae, Brongniartieae, Cadieae, Camoensieae, Carmichaelieae, Cicereae, Cladrastideae, Coluteeae, Crotalarieae, Dalbergieae, Diocleeae, Dipterygeae, Exostyleae, Fabeae, 50 kb Inversion Clade, Galegeae, Genisteae, Hedysareae, Hypocalypteae, Indigofereae, Inverted Repeat Loss Clade/IRLC, Leptolobieae, Loteae, Medicageae, Millettieae, Mirbelieae, Myroxylodae, Non-Protein Amino Acid Accumulating Clade, Ormosieae, Phaseoleae, Psoraleeae, Robinieae, Sesbanieae, Sophoreae, Swartzieae, Trifolieae, Vataireoids, Wisterieae.
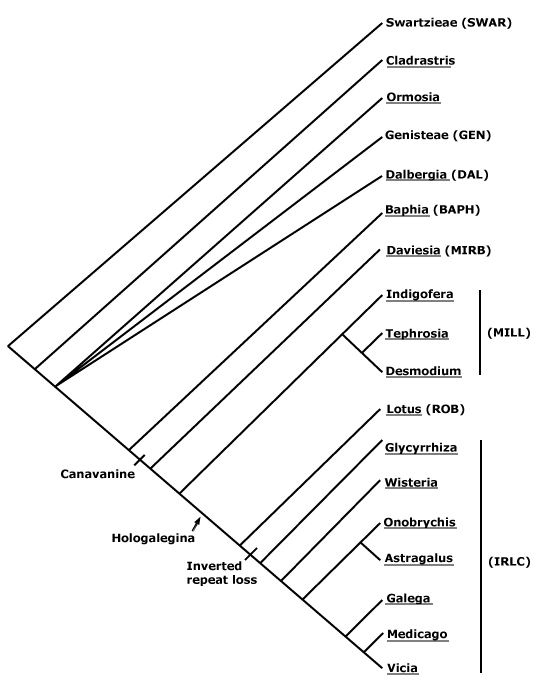
6A. Myroxylodae Ezedin / [Angylocalyceae [Dipterygeae + Amburaneae]] / ADA Clade: ?testa anatomy.
Age. Estimates for the age of this clade are 50.8±3.8 Ma (Lavin et al. 2005) or about 61.1 Ma (Y. Zhao et al. 2021).
6A1. Angylocalyceae (Yakovlev) Cardoso et al.
K, hypanthium enlarged; C thickened, red (white); A exserted; n = 13.
5/21: Alexa (9), Angylocalyx (7). Africa, Madeira, northern South America, Castanospermum eastern Australia, western Pacific.
Age. Mid to Late Eocene fossil seeds, Jantungspermum gunnellii, from S.W. Borneo have been compared with seeds of Castanospermum (Spagnuolo et al. 2024).
[Dipterygeae + Amburaneae]: secretory cavities + [other than in the flower].
Age. This clade is about 54.9 Ma (Y. Zhao et al. 2021).
6A2. Dipterygeae Polhill
Tanniniferous cells +; (secretory cavities 0 - Monopteryx); (leaflets amphistomatous); leaflet margin slightly recurved; keel flowers + (no - M.); 2 adaxial K much enlarged, petal-like, (connate - M.), 3 abaxial K as small teeth; A connate (free - M.), (glands +, with secretory cavity); endosperm 0; n = 8.
4/25: Dipteryx (12). Neotropical.
Age. Crown-group Dipterygeae are about (47.3-)40.0(-32.6) Ma (Carvalho et al. 2023b).
6A3. Amburaneae Nakai
Plants with balsam/resin/coumarin; idioblasts in the midrib; (leaflets glandular-punctate); keel flowers +/flowers polysymmtric/etc.; C (1 large, 4 very small); endosperm +, cotyledonary areole 0; n = 11, 13, 14.
8/30: Dussia (9), Cordyla (7). Neotropical.
[Swartzieae [Cladrastideae + 50 kb inversion clade]]: ?Age. This clade is around 63.6 Ma (Y. Zhao et al. 2021).
6B. Swartzieae de Candolle —— Synonymy: Swartziaceae Bartling
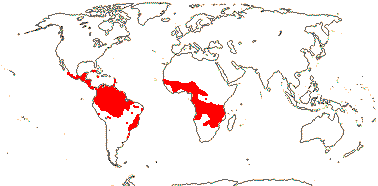
Trees, shrubs; nodules +/0, terete [caesalpinioid], ?infected cell mixture; (leaflets alternate), stipels +/0; (plant dioecious - Ateleia); (bracteoles 0); three series of vascular gaps up the receptacle [K, C, A: ?always], hypanthium 0; K (completely connate, opening irregularly), C 1 (0, 2, 5), three bundles developing [Swartzia]; A (8-)many, from (incomplete) ring meristem, free, development centripetal or centrifugal, heteranthy usu. conspicuous, anthers dorsi(basi)fixed; pollen grains often syncolpate; post-zygotic incompatibility system [?all]; nectary 0; G (1-4), long-stipitate, (jointed with G), style (very short - Ate.); ovules anatropous, micropyle zig-zag, outer integument 6-8 cells across, inner integument 5-6 cells across, ?parietal tissue; seed arillate or not; (testa thin, cracking); (endosperm +, cotyledonary areole + - Bobgunnia), (embryo straight); n = 8, 13[Swar.], 14, 20.
8/253: Swartzia (210), Ateleia (27). Mostly Central and South America, the Caribbean; Bobgunnia Africa and Madagascar. Map: from Cowan (1967) and Kirkbride and Wiersema (1997).
Age. The age for a crown group around here is 48.9±2.8 Ma (Lavin et al. 2005: inc. Ateleia) or ca 45 Ma (Bruneau et al. 2008a: inc. Lecointea).
[Cladrastideae + 50 kb inversion clade]: keel flowers + (0); K connate, adaxial-median C outermost [= standard] [aestivation descending cochleate], (lateral C [= wings] variously sculpted), 2 abaxial C connate [= keel], epidermal micromorphological differentation associated with C differentiation; A variously connate, (not)
Age. The crown-group age of this clade is about 63.0 Ma (Y. Zhao et al. 2021) or (60.2-)59.0(-57.9) Ma (L. Cai et al. 2025).
6C. Cladrastideae L. Duan & J. Wen
Trees to shrubs, deciduous (evergreen); flavonol O-glycosides/highly glycosylated flavonols +; axillary bud enclosed by swollen petiole base; leaves chartaceous (sclerophyllous), (stipules 0), stipels +; A ± free; fruit flattened, winged, (tadily dehiscent)/moniliform, fleshy; endosperm 0, cotyledonary areole ?0; n = 14.
4/20: Styphnolobium (7). East Asia, inc. Japan, S. central and S.E. U.S.A., Mexico to Colombia.
Age. This clade started diversifying 47.4±2.6 Ma (Lavin et al. (2005) or (54.8-)49.9(-45.1) Ma (Duan et al. 2019).
Age. The 50 kb inversion clade is ca 61.9 Ma (Y. Zhao et al. 2021).
271/22,650.
[Exostyleae + Vataireoids]: leaflet (margins serr(ul)ate).
6D1. Exostyleae Nakai (= lecointeoids)
Stomata with heavily cutinized cuticular flanges flanking guard cells; leaves (unifolioliate), leaflets (spinescent), margins serrate; flowers poly(mono)symmetric; colleters +; (hypanthium +); C aestivation various; A (opposite C develop first), anthers basifixed; G and C initiated together; fruit a drupe; n = 11.
6/21: Zollernia (10). Neotropical.
Plant deciduous; flavonol O-glycosides/ highly glycosylated flavonols +; branching subwhorled; leaves in groups at ends of twigs, (leaflets serrate); inflorescence terminal; flower standard with colour splotch; A ± connate (flowers small, ± polysymmetric; K valvate; A free), anthers dorsifixed; fruit an apically-winged samara, (also low lateral wings form over the seed); plastid 400bp deletion in trnL-F intergenic spacer.
4/27: Luetzelburgia (13). Neotropics.
Amphimas Harms (Not sampled by L. Cai et al. 2025.)
Tree; resin +, red, flavonol O-glycosides/ highly glycosylated flavonols +; stipels well developed; flowers ± polysymmetric; C 3, deeply bilobed; A connate only basally; fruit thin walled, 2-winged, dehiscent.
1/3. West and Central Africa.
[Genistodae [Dalbergioids [Andira Clade [Baphieae + NPAAA clade]]]]: plastome rpl22 to nucleus [check].
Genistodae Ezedin / [Ormosieae [Brongniartieae [Cabari [Diplotropideae [Camoensieae [Haplormosia [Sophoreae [Podalyrieae [Crotalarieae + Genisteae]]]]]]]] / genistoids s.l.: quinolizidine alkaloids + [all major pathways, α pyridones, etc.] (0); bacterial infection through the epidermis, nodule morphology very various; flowers (polysymmetric); x = 9.
Shrubs, trees (lianas); nodules indeterminate, bacterioids in infection threads, infected and uninfected cells mixed; pentacycline quinolizidine Ormosia-type alkaloids; leaves (unifoliolate), (stipules 0); (bracts 0), (bracteole 1); C (keel joined by trichomes, spirally twisted - Spirotropis); A free (basally connate), (some staminodial); (stigma ± bilobed); pod swollen [Ormosia], dehiscent (not), valves woody; seeds red/black-red bicoloured/black-brown; testa hard/fleshy.
3/160: Ormosia (150). Mexico and the Caribbean, South America, esp north and west, India and China to Australia.
Age. The age of crown-group Ormosieae is estimated to be (53.1-)44.5, 40.8(-31.2) Ma (Torke et al. 2021).
6E2. Brongniartieae Hutchinson
Shrubs, small trees; nodules with fixation threads; pentacycline quinolizidine Ormosia-type alkaloids; colleter-like glands in axils of stipules or on leaflet pulvinuli; leaves (unifoliolate, with stipels); inflorescence paniculate, flowers (solitary), (resupinate); K bilabiate, (C keel spirally twisted); pollination brush-type (explosive); A (mono-)diadelphous; A heteranthous [dimorphic: dorsi-/basifixed - short A, long A basifixed]; pollen operculate, endoaperture indistinct; (nucellar cap ca 2 cells across, parietal tissue 3-7 cells across - Hovea); pods with (septae)/spongy tissue between seeds (seed 1); seeds arillate; embryo straight.
15/171: Brongniartia (65), Hovea (38), Harpalyce (35). S.W. North America (Dermatophyllum) to tropical America (inc. Cuba), Australia, Haplormosia (= H. monophylla) W. and W. C. Africa.
Age. Crown-group Brongniartieae are ca 58 Ma (Cardoso et al. 2016) or ca 53 Ma (Sa˜o Mateus et al. 2024) - inc. Dermatophyllum?.
[Diplotropideae [Camoensieae [Haplormosia [Sophoreae [Podalyrieae [Crotalarieae + Genisteae]]]]]]: ?
6E3. Diplotropideae Yakovlev (inc. Leptolobieae (Bentham) Cardoso et al.)
Colleter-like glands in axils of stipules or on leaflet pulvinuli; flowers weakly or not keel type [± symmetrical, abaxial 4 C free]; A free; pollen (3-4-brevicolpate), (tectum psilate); fruit (samara),(with narrow marginal wing); seeds 1-several.
5/29: Leptolobium (13), Diplotropis (10). South America, 1 sp. Central America.
Age. Late Palaeocene fossils ca 56 Ma from Wyoming have been placed sister to Bowdichia (Paleobowdichia lamarensis) or associated with Guianodendron and Stemonanthus (Tobya claibornensis), i.e. in both cases embedded in the tribe (Herendeen et al. 2022).
[Camoensieae [Haplormosia [Sophoreae [Podalyrieae [Crotalarieae + Genisteae]]]]]: ?
6E4. Camoensieae (Yakolev) Cardoso - Camoensia Bentham & J. D. Hooker
Lianes; leaf tendrils; leaves trifoliolate, stipellate; flowers huge [for a pea; to ca 20 cm across], hypanthium long; petals crimped, ± spreading, standard +; filaments connate at the base; ovary very long-stipitate; fruit dehiscent, valves ± elastic; testa undifferentiated [seed overgrown]; n = 9; chloroplast inverted repeat lost.
1/2. Africa, the Gulf of Guinea.
[Haplormosia [Sophoreae [Podalyrieae [Crotalarieae + Genisteae]]]]: quinolizidine alkaloids + leaves unfolioliate; A dimorphic [basifixed, shorter, dorsifixed].
6E5. Haplormosia monophylla Harms
Tree; A ± free, dimorphic [basi-/dorsifixed]; pods 1-seeded, woody, ± dehiscent.
1/1. ± Coastal West Africa - Sierra Leone to south Gabon, not Ghana to Benin.
[Sophoreae [Podalyrieae [Crotalarieae + Genisteae]]] / core genistoids: piperidine alkaloids +, canavanine 0; crystals in wood usu. 0; A dimorphic [long, basifixed, shorter, dorsifixed]; micropyle inside hilum or in rim, punctate [ypsiloid]; plastome with 36 kb inversion inside the 50 kb inversion.
Age. This clade is some 45.2±2.3 Ma or (55.3-)51.2(-43.9) Ma (Boatwright et al. 2008).
6E6. Sophoreae de Candolle (inc. Thermopsideae, Euchresteae) —— Synonymy: Inocarpaceae Berchtold & J. Presl, Sophoraceae Berchtold & J. Presl
Herbs, shrubs or small trees, (rhizomatous), (deciduous), (spines/?thorns, inc. on rhizomes); nodules irregular/thin-walled bacterial infection threads and bacterioid infection packets; (pentacycline quinolizidine Ormosia-type alkaloids - Thermopsideae), matrine-type quinolizidines, ?canavanine; bracteoles 0; K with trifid lower lip [abaxial 3 K much fused]; A free, (diadelphous - ex-Euchresteae); pollen grains (syncolporate), exine of tectum only; fruits (moniliform, indehiscent); aril with longitudinal extension; antiraphe bundle 0; endosperm 0, polysaccharide reserves various; cotyledonary areole 0; (germination hypogeal).
12/137: Sophora (65), Thermopsis (23), Baptisia (17). Tropical and subtropical (temperate; few eastern South America), Sophora also Pacific Islands.
Age. Sophoreae are (43.2-)40.8(-38.4) Ma (Lavin et al. 2005) or around 46.7/41.4 Ma (M. Liao et al. 2023: Baptisia sister to rest).
[Podalyrieae [Crotalarieae + Genisteae]]: nodules indeterminate.
6E7. Podalyrieae Bentham (inc. Cadieae Baillon, Liparieae)
Shrubs (small trees), (deciduous); a-pyridone alkaloids 0, quinolizoidine alkaloids, vicenin-2 [seed flavone], isoflavone 3'-hydroxydaidzein [seed], esters of cyanidin with coumaric or acetic acid [purple-flowered taxa] +; nodulation via root hairs, nodule central tissue with infected and uninfected cells [Burkholderia involved]; wood with prismatic navicular crystals/acicular crystals/crystal sand; leaves simple (linear), (trifoliolate, pinnate e.g. Cadia); inflorescence racemose, (flowers single); bracteoles reduced/0; (flowers polysymmetric - C.); A weakly (strongly - Podalyria) dimorphic, mono-/diadelphous, (A free, filament bases swollen - C.); (seed with single recurrent bundle, doubled at raphe; pods twist - C.); seed arillate, collar aril around hilum; n = 9 [P.].
10/137: Amphithalea (42), Cyclopia (23), Liparia (20), Podalyria (17). South Africa, mostly the Cape, but Cadia Madagascar, also N.E. Africa, S.W. Arabia.
Age. Edwards and Hawkins (2007) date diversification in Podalyrieae to 44.6±2.4 or ca 45.2 Ma and Boatwright et al. (2008) 30.5±2.6 Ma and (44.1-)34.7(-25.1) Ma.
[Crotalarieae + Genisteae]: nodules indeterminate, bacteria enter via cracks, nodule with infected cells only; (pyrrolizidine alkaloids +); leaves uni-/trifoliolate, minor veins with phloem transfer cells.
Age. Boatwright et al. (2008) suggest that these two tribes diverged 36.9±2.5 Ma.
6E8. Crotalarieae Hutchinson —— Synonymy: Aspalathaceae Martynov
Herbs to shrubs; nodules (0), branched, infection threads 0 (nodules indeterminate, girdling the root [lupin type] - Listia); a-pyridone alkaloids 0, monocrotalines [pyrrolizidine alkaloids, produced in the nodules], vicenin-2 + [seed flavone], (cyanogenesis + - Lot.); leaves simple (some Crot., Asp.), (spiny / fleshy, cylindrical - Asp.); K bilabiate or not, C (standard blade with appendages), beak spirally twisted/not; A mon-(pseudo mono-/diadelphous); (stigma wet - Crot.).
16/1,285: Crotalaria (760), Aspalathus (290), Lotononis (90), Lebordea (51), Rafnia (19). Tropical and subtropical, largely African-Madagascan, Southern/Eastern Africa with almost half the species of the tribe.
Age. Edwards and Hawkins (2007) date diversification in the Cape Crotalarieae to 46.3±2.4 or ca 45.2 Ma, the age of Crotalarieae as a whole being around 50 Ma; estimates in Boatwright et al. (2008) are 31.2±3.4 Ma and (45.6-)35.2(-23.3) Ma.
6E9. Genisteae Bronn (inc. Cytiseae, Crotalarieae-Argyrolobium group) —— Synonymy: Cytisaceae Berchtold & J. Presl
Crack entry, nodules flattened [crotalarioid], (girdling the root - lupin type); 5-0-methylgenistein [isoflavone]/(0), (cytisine) +; (nodes 1:1); (thorns + - Ulex); leaves (palmately compound); K bilabiate [bifid upper lip, trifid lower lip, abaxial 3 K much fused]; nectary 0/from phloem, on outside of A tube; A connate, dimorphic [basi-/dorsifixed]; aril with extension on the short side of the seed (0).
25/983: Lupinus (650), Genista (90), Argyrolobium (80), Cytisus (65), Ulex (20). Mostly North Temperate, Lupinus also South America, esp. the Andes, Argyrolobium esp. southern Africa, east African mountains, Madagascar.
Age. Estimates of the age of crown-group Genisteae in Boatwright et al. (2008) are 32.3±2.9 Ma or (46.8-)37.5(-27.6) Ma.
[Dalbergiodae [Andira clade [Baphieae + NPAAA clade]]]: ?
Dalbergiodae Ezedin / [Amorpheae + Dalbergieae] / Dalbergioids: fruit indehiscent; x = 10.
Age. The age of this clade is 55.3±0.5 Ma (Lavin et al. 2005).
6F1. Amorpheae Borissova —— Synonymy: Daleaceae Berchtold & J. Presl
(Plant deciduous); glands +, schizogenous; inflorescences terminal; C adnate to A [stemonozone], or ± polysymmetric, [C 1, A 10 [open on one side]/C 0, A [10]/etc.]; ovules 1-2/carpel; fruits 1-seeded; n = 7, 8, 10.
8/247: Dalea (165), Marina (38). New World, Canada to Argentina.
Age. The age of this clade is 36.9±3 Ma (Lavin et al. 2005).
6F2. Dalbergieae de Candolle (inc. Adesmieae, Aeschynomeneae) —— Synonymy: Dalbergiaceae Martinov, Geoffroeaceae Martius
Plants (lianas - Dalbergia, Machaerium), ± aquatic; nodules (0) cauline but associated with a lateral root, crack entry, (Nod genes 0), small, oblate, determinate, lenticillate or not, with infected cells only, short-lived [= desmodioid/aeschynomenoid nodules], bacterioids pleiomorphic, differentiation irreversible [they cannot divide], nuclear endoreduplication / nodules indeterminate, terete [caesalpinioid]; (ectopic cambia localized, in phloem - Machaerium), (sieve tube plastids with starch grains only - Pterocarpus, etc.); epidermal mucilaginous idioblasts +/0, (mesophyllar secretory cavities/phenolic idioblasts +); (leaves opposite - Platymiscium), (leaflets fold forwards at night); pedicel and K continuous []; flowers (polysymmetric); A free/connate half way, mono-/pseudomono-/dia-/tetradelphous [4 + 1 + 4 + 1], (colleters +); hypanthium ± 0/long [Arachis], nectary on top/stipe of G adnate to hypanthium/0, (G with 10-12 vascular bundles - Ara.); (ovules initiated when carpels are open - Aes.); fruit lomentum/1-seeded samara/nut-like; (endosperm 0, cotyledonary areole 0); x = 10, n = (8-)10(14, 16-20).
49/1,335: Dalbergia (250), Adesmia (206), Machaerium (130), Aeschynomene s. str. (?100), Zornia (75), Arachis (70), Ctenodon (66 -?120), Stylosanthes (48), Pterocarpus (40), Humularia (33),Chaetocalyx (29). Tropical, but few Asian (c.f. Dalbergia itself).
Age. Dalbergieae may have started diversifying 50.7±0.8 Ma (Lavin et al. 2005); Aeschynomene and Arachis separated ca 49 Ma (Quilbé et al. 2021).
Annual to perennial herbs, shrubs; aeschynomenoid nodules [Pterocarpus]; epidermis with secretory, mucilaginous idioblasts; leaves (digitate - Z.), (pellucid glands, etc. +); (inflorescence spicate - Z.), (bracts paired - Amicia, ?= stipules), bracteoles (foliaceous, peltate - Z.)/0; (flowers polysymmetric); (K initiation adaxial→abaxial); A free/mon-/diadelphous; fruit a lomentum, (apical unit winged); n = 19. 5/360.
Adesmia (206), ?Zornia (75), Chaetocalyx (29) - Amicia, Nissolia, Poiretia. South America, some Central and North America, Z. pantropical.
Age. Lavin et al. (2005) suggest that the age of this node was 35.3±2.3 Ma. Rooting of tree with dates in Fortuna-Perez et al. (2013: fig. 4) is incorrect.
[Genistodae [Baphieae + NPAAA clade]: plastome rpl22 to nucleus [?where].
[Andira clade [Baphieae + NPAAA clade]]: ?
[Aldina + Andira clade]: flavone C-glycosides common; leaves ± clustered at the ends of branches; inflorescence terminal; 1-4 ovules per carpel; fruit indehiscent, usu. 1-seeded; testa undifferentiated [seed overgrown]; endosperm 0.
Aldina Endlicher (Not included in L. Cai et al. 2025)
Tree; plant ectomycorrhizal, with nodules; sap red; flowers polysymmetric; K completely connate, opening irregularly, C imbricate; A many, free, dorsifixed; pollen with lamellate endexine adjacent to apertures; G 1(-3), gynophore +, long, jointed with G, (± 0); fruit "nucoid legume", ± globose, (tardily dehiscent); seeds 1-4.
1/18. Northern Amazonia - Colombia, Brazil, Guyana, esp. Venezuela.
Tree; nodules + [astragaloid type - And.], bacterioids in infection threads; fruit indehiscent, drupe (samara - Hym.); n = 11.
2/46: Andira (29), Hymenolobium (17). Neotropical.
Age. This clade is 17.9±3.8 Ma (Lavin et al. 2005).
Age. This clade is around 55.3±0.5 Ma (Lavin et al. 2005).
Shrubs, trees, lianas; nodules +; vessel elements >250μm long; leaves unifoliolate; K split to base on one or both sides; A free, anthers ± basifixed; n = 11.
7/57: Baphia (47). Largely tropical Africa, esp. west-central, Madagascar, N.E. India, Bangladesh, South China to South Vietnam, Borneo, southern Philippines. Map: see Goncharov et al. (2013).
[[Hypocalypteae [Mirbelieae + Bossiaeeae]] Galegodae] / Non-Protein Amino Acid Accumulating Clade/NPAAA clade (Canavanine-accumulating clade): alkaloids 0, non-protein amino acids + [e.g. canavanine]; anther glands common; x ?= 12, ? whole genome duplication.
ca 10,200 spp.
Age. The age of this clade is around 61 Ma (Snak et al. 2016) or ca 59.1 Ma (Koenen et al. 2013).
[Hypocalypteae [Mirbelieae + Bossiaeeae]: leaves not pinnate.
Age. The age of this clade is 54.1±1.2 Ma (Lavin et al. 2005).
6I. Hypocalypteae (Yakolev) Schutte and Van Wyk - Hypocalyptus Thunberg
Shrubby; nodule central tissue with infected and uninfected cells [Burkholderia involved]; NPAA canavanine + [in seed], wood with tanniniferous tubes, crystals 0; leaves trifoliolate; inflorescence racemose; A connate, anthers alternately dorsi- and basifixed; antipodal cells ephemeral; hilar aril continuous, micropyle outside; endosperm 0, cotyledonary areole ± 0; n = 10.
1/3. The Cape, South Africa.
6J. [Mirbelieae + Bossiaeeae]: plants with ectomycorrhizae (0); stem photosynthetic; (thorns +); leaves phyllodinous (lobed-hastate)/± ericoid/much reduced; inflorescence pseudoracemose; C often yellow, red guide marks; anther connective broad, dark coloured; antipodal cells giant; micropyle punctate [ypsiloid].
Age. The age of this clade is 48.4±1.3 Ma (Lavin et al. 2005: note topology).
6J1. Mirbelieae (Bentham) Polhill & Crisp
(Herbs) shrubs (small trees) (thorns +); (fluoroacetate + - Gastrolobium); (cluster roots + [P acquisition]); vessel elements 250> μm long; leaves phyllodinous (scales, spines, trifoliolate, pinnate [Ptychoselma]), (margin serrulate), (stipules 0); (raceme terminated by a vegetative bud, aborting floral bud immediately below, several peduncular bracts +, bracteoles 0); free, heteranthous [?extent]; stigma punctate-papillate; ovules 2 (several)/carpel; embryo sacs several [Gastrolobium], bisporic, (5 nucleate), antipodal cells 0 (giant); (fruit obtriangular; seeds arillate); n = 8, 9.
23/688: Pultenaea (>150), Daviesia (131), Gastrolobium (110), Jacksonia (75), Gompholobiumm (46), Chorizema (27), Sphaerolobium (21). Australia, esp. the S.W. and (S.)E..
Age. Mirbelieae are around 48.4±1.3 Ma (Lavin et al. 2005).
6J2. Bossiaeeae Hutchinson
Shrubs; cladodes +, leaves unifoliolate, reduced); A [9] + 1/(monoadelphous); embryo sac with giant antipodal cells.
6/77: Bossiaea (60). Australia, esp. the southwest.
[Indigofereae [Clitorieae [Phaseoleae, etc. [Abreae [Diocleeae + Millettieae]]]]] / Old World Clade: homoglutathione + [= γ-glutamyl-cysteinyl-β-alanine], albumin-1 gene.
[Phaseolodae Ezedin = Abreae Baill.; Diocleeae Hutch.; Indigofereae Benth.; Millettieae Miq.; and Phaseoleae DC.]
Age. The age of this clade - but note topology - has been estimated at around 79 Ma (Hohmann et al. 2014) or some 52.8±1.0 Ma (Lavin et al. 2005).
[Indig. + Mill.-Phas. + Rob. + IRL Clade]: tyrosine-insensitive alternative biosynthetic pathway +; x = 9. [Glycine, Medicago]
6K. Indigofereae (Bentham) Hutchinson
(Nodules +); ?albumin-1 gene; indospicinine + [?level - NPAA]; hairs unicellular, ± T-shaped; early expression of monosymmetry; colleters +; A mono-/diadelphous; antipodal cells very large; x = 7, 8.
6/857: Indigofera (787), Microcharis (40). Indigofera tropical-warm temperate, 1/4 species southern Africa, other genera Africa and environs.
Age. Crown-group Indigofereae are some 30.0±3.3 Ma (Lavin et al. 2005) or ca 33 Ma (Schrire et al. 2009).
Age. This clade (inc. Xeroderris) is some 45.2±1.7 Ma (Lavin et al. 2005).
Other ages around here: [[Phaseolus + Vigna] Desmodieae] ca 39.2 Ma, [the whole lot [Mullera + Fordia]] ca 48.7 Ma (Jabbour et al. 2017).
[Xeroderris, etc. [Abreae [Diocleeae + Millettieae]]]: ?
Age. The age of this clade is around 36.9±2.3 Ma (Lavin et al. 2005).
Xeroderris, etc.
[Abreae [Diocleeae + Millettieae]: inflorescence pseudoraceme [two or more flowers per node].
6L1. Abreae Hutchinson - Abrus Adanson
Lianes, vines; nodules indeterminate, terete [caesalpinioid]/determinate, often with lenticels [desmodioid]; leaves pinnate; A [9]; pod elastic; seed hard, red and black, black, white, etc..
1/17. Old World, mostly Africa-Madagascar.
Age. The age of stem Diocleeae is estimated to be (35.6-)33(-30) Ma (Snak et al. 2016).
6L2. Diocleeae Bentham (inc. Galactieae)
Shrub, vine, robust liana; leaves (uni-)trifoliolate, leaflets stipellate, bases asymmetrical; ?inflorescence; hypanthium +; flowers (resupinate - Canavalia, Cleobulia); K (4), abaxial lobe longest/all same length; A diadelphous/pseudomonoadelphous [A connate, vexillary A free at base, two fenestrations], (4/5 A staminodial); style (swollen); pod elastic (not/indehiscent); antiraphe bundle 0 [Canavalia].
13/202: Canavalia (60), Galactia (58), Macropsychanthus (46). Mostly tropical, S.E. U.S.A., Pacific islands.
6L3. Millettieae Miquel (inc. Tephrosieae, see also Andira for wood).
(Nodules determinate, lenticillate [desmodioid]), (bacterioids in persistent fixation threads - Dahlstedia); canavanine 0; (leaflets with pellucid glands); (flowers heterostylous); early expression of monosymmetry; A mono-/pseudomono-/diadelphous; nectary annular; n = 11, 12; plastome rps12 intron moved to the nucleus.
29: ca 1,000: Tephrosia (350), Lonchocarpus (100), Pongamia (56), Derris (55), Millettia s. str. (7). Pantropical.
Age. This age of this clade (excl. Galactia) is about 26.1±2.0 Ma (Lavin et al. 2005).
6M. [Apios, etc., Desmodieae [Psoraleeae + Phaseoleae]: (sieve tube plastids with starch grains only); inflorescence pseudoraceme [two or more flowers per node].
Age. This clade is about 27.8±1.6 Ma (Lavin et al. 2005), but including Platycyamus (colleters) it is 39.7 ±2.0 Ma.Vines (tuberous), (annual), shrubs; leaves (trifoliolate).
2/119: Mucuna (112). Pantropical/warm temperate, inc. E. Asia, E. North America.
[Desmodieae [Psoraleeae + Phaseoleae]: leaves tri-(uni-)foliolate.
6M2. Desmodieae HutchinsonShrub or tree (herbs; annuals); nodules (0), small, oblate, determinate, often with lenticels [desmodioid (usu.)/aeschynomenoid nodules], always associated with a lateral root [desmodioid/aeschynomenoid nodules]; nodes multilacunar; leaves (pinnate), with stipels; pollen (syncolporate); nectary annular; (1 ovule/carpel); fruit a lomentum (indehiscent/dehiscent pod), often ≤6-seeded; (endosperm 0, cotyledonary areole 0); chloroplast rps12 intron 0.
32-48/530: Desmodium (260), Grona (40), Lespedeza (40), Campylotropis (37).
Age. This clade is estimated to be (32.1-)28.3(-24.5) Ma (Jabbour et al. 2017).
[Psoraleeae + Phaseoleae]: nodules determinate, with lenticels [desmodioid]; A mon-/pseudomon-/diadelphous.
Age. This node is 19.2±1.4 Ma (Lavin et al. 2005).
6M3. Psoraleeae Bentham
Herbs to shrubs, (trees); epidermal foliar oil glands + [anticlinal divisions]; leaves (tritfoliolate/palmate/pinnate, 0), (linear, with linear segments), minor veins with phloem transfer cells, (hypo-/hypoamphistomatic); inflorescence branches 3-flowered cymes; (cupule + [= 2-5 ± fused bracts], bracteoles small - terminal flowers of Psoralea), (standard with paired callosities); A [9] + 1, dimorphic; stigma penicillate; 1 ovule/carpel; fruit indehiscent; seed (adherent to pericarp).
9/250: Psoralea (129), Cullen (24),Pedomelum (22). Africa, esp. the Cape, South Africa, Arabia, Australia, North America to Mexico (the Andes, Eurasia).
Age. The age of crown group Psoraleeae is some (11.0-)8.2(-)5.9) Ma (Bello et al. 2022).
6M4. Phaseoleae de Candolle —— Synonymy: Galedupaceae Martynov, Phaseolaceae Martius
Vines/lianas (shrubs), (xylopodia +); nodules (0), (indeterminate, terete); (canavanine 0 - Erythrina), nitrate reductase constitutive; ectopic cambia +; sieve tube plastids with starch grains only; nodes multilacunar; stipules (peltate); extrafloral nectaries common in inflorescence; abscission zone = swollen scar/trichomatic; (bracteoles 0); flower (held upside down), (asymmetric, keel twisted laterally); K tubular (teeth v. small - Ery.); pollen (triporate), surface reticulate; post-zygotic incompatibility system [Strongylodon]; nectary annular, with 10 traces, ±bilabiate, (adnate to hypanthium - Ery.); (stigma capitate); fruit (2-seeded), dehiscence explosive [?level]; seed (with several layers of hourglass cells in circumhilar region), (hilar tongue +), (counter palisade 0 - Ery.); suspensor very large [>100 cells], club-shaped [Phaseolus]; plastome (with 78 kb inversion - Phaseolineae), rps16 gene lost [?level].
89/1,600: Rhynchosia (230: polyphyletic), Eriosema (150), Erythrina (110), Mucuna (105), Vigna (90), Phaseolus (75), Psoralea (70), Canavalia (60), Clitoria (60), Dolichos (60), Galactia (60).
Galegodae Ezedin / [Loteae + Sesbanieae + Robinieae] + IRL Clade] / Hologalegina clade / temperate herbaceous group: plant herbaceous; cyanogenic glucosides/0, (non-cyanogenic β- and γ-hydroxynitrile glucosides +); x = 9.
5,300 spp.
Age. Galegodae have been dated to 56±0.9 Ma (Lavin et al. 2005), ca 51.0 Ma by Azani et al. (2019) and ca 55.2 Ma by Y. Zhao et al. (2021).
[Loteae + Robinieae + Sesbanieae] / Robinioids: albumin-1 gene 0; nodules often determinate, often lenticillate [desmodioid].
Age. This node is around 48.3±1.0 Ma (Lavin et al. 2005) or ca 48.2 Ma (Azani et al. 2019).
6N1. Loteae de Candolle —— Synonymy: Coronillaceae Martynov, Lotaceae Oken
Nodules (indeterminate, terete or flattened [caesalpinioid, crotalarioid]); leaves 2-ranked; (basal pair of leaflets stipule-like), (stipules reduced/0); peduncle with foliage leaf, partial inflorescence capitate/umbellate; A 9 + 1, (monadelphous), (antesepalous) filaments swollen, pump-type secondary pollination presentation; (fruits lomenta); n = 6-8; cotyledons foliaceous, (plumule aborts).
22/285: Lotus (125, inc. Coronilla), Anthyllis. Temperate, especially Macaronesia and the Mediterranean Basin.
Age. The age of the clade [Sesbania + Anthyllis] is ca 42.7 Ma (Azani et al. 2019).
6N2. Robinieae Hutchinson —— Synonymy: Robiniaceae Vest
Often shrubs or trees; nodules usu. indeterminate; colleters +; A pseudomono-(di-)adelphous; (legume resupinate); n = 7, 8 (9-11.
11/69: Coursetia (35). North and Central America, the Antilles, few in South America (map: see Lavin & Sousa 1995).
Age. Crown Robinieae are some 45 Ma (Lavin & Sousa 1995), 38.5±1.5 Ma (Lavin et al. 2005) or ca 39.4 Ma (Azani et al. 2019: Poitea + the rest, but something is wrong here).
6N3. Sesbanieae (Rydberg) Hutchinson - Sesbania Scopoli
Annual/perennial herbs, soft shrub; exudate +, dark; stem nodules +; leaves paripinnate, leaflets stipellate; fruit transversely septate (not, seeds 2), dehiscent, valves not elastic; hilum ± circular, rim aril +; n = 6; reversion of 50 kb inversion.
1/85. ± Tropical, esp. Africa-Madagascar.
Inverted Repeat Loss Clade / IRL Clade: isoflavones + (0); nodule growth indeterminate [?all], bacterioids pleiomorphic, differentiation irreversible [they cannot divide], nuclear endoreduplication, surrounding membranes with leaky cysteine-rich peptides; nodes commonly 3:3; petiole lacking wing bundles; stomata anomocytic; leaves often 2-ranked, pulvini 0, odd pinnate; stipels uncommon, stipules adnate to the petiole; FLO/LFY genes expressed also in the leaf; CA primordia +; A initiation bidirectional, overlap in the timing of C, A, and G initiation; (stigma wet); tapetal cells uninucleate; outer integument 3-5 cells across, endothelium +; x = (7), 8; plastid transmission biparental; plastome inverted repeat 0, rps16 gene 0, clpP intron 1 0; seedling with first two leaves alternate.
Ca 45/4,500. Especially northern and temperate.
Age. The IRLC is estimated to be 39.0±2.4 Ma (Lavin et al. 2005), ca 38.5 Ma (Azani et al. 2019) or ca 48.9 Ma by Y. Zhao et al. (2021).
Woody lianes, stem twining/sprawling shrubs; plate-like cortical ectopic cambia [Wis.]; vessels dimorphic [in terms of dimameter]; persistent subepidermal periderm [no rhytidome]; phellem stratified; inflorescences true panicles (racemes); bracts often enclosing buds; callosities on standard above the claw boss-like (morphology otherwise); A pseudomono-(diadelphous); nectary annular [Wis.]; fruit with endocarpial septae ± developed; hilum ± elliptic; n = 8; germination hypogeal.
13/36: Callerya (?13). Temperate China and Japan to Malesia, islands to New Caledonia and Cook Islands, eastern Australia, eastern U.S.A. (Wisteria frutescens).
Age. [Wisteria + Callerya] were estimated to be ca 22.4 Ma (Azani et al. 2019) or (39.3-)30.3(-22.9) Ma (Duan et al. 2021a: the tribe)
Adinobotrys Dunn (Not included in L. Cai et al. 2025.)
Tree; inflorescence paniculate; standard boss callosities +; pods (inflated), endocarp subseptate, hilum circular to elliptic.
1/2. "India", Myanmar and Vietnam to West Malesia (not the Philippines).
[All Other IRLC]: rps12 intron lost.
Glycyrrhizeae Rydberg - Glycyrrhiza L.
Herbs; leaflets (margins serrulate); inflorescence racemose; A "mono-"/diadelphous; fruits indehiscent.
1/20. Scattered: Eurasia, North Africa, North America, temperate South America, S.E. and S.W. Australia.
Age. A clade [Glycyrrhiza + Wisteria, etc.] is thought to be ca 30.3 Ma (Azani et al. 2019).
Age. The age of the rest of the IRLC is ca 44.3 Ma (Y. Zhao et al. 2021).
[Hedysareae...]: minor veins with phloem transfer cells + [?level].
Age. 1: Hedy + Ast. This node is ca 32.8 Ma (Azani et al. 2019). 2. Hedy + Fab/Cara + Ast - This clade is ca 30.3 Ma (Azani et al. 2019). 3. Hedy + Fab - This node is ca 29.8 Ma (Azani et al. 2019:Cara + Ast).
[Hedysaroids + Vicioids] were dated to ca 30.3 Ma by Azani et al. (2019).
Hedysareae de Candolle —— Synonymy: Hedysaraceae Oken
(Annual) herb to shrubs (trees); leaves (even-pinnate - Caragana); inflorescence racemose; A pseudomono-/diadelphous; o.i. 4-5 cells across; fruit a lomentum/1(-2)-seeded [Onobrychis]/dehiscent, valves elastic [Caragana].
12/427: Hedysarum (160), Onobrychis (130), Caragana (75). Eurasia, North Africa, the Horn of Africa and Socotra, some North America.
Age. The age for Hedysareae in Juramurodov et al. (2024) is ca 21.1 Ma (+ Caragana ca 23.8 Ma, + Astragalus ca 28.9 Ma) (Juramurodov et al. 2024).
Plant shrubby; leaves (im)paripinnate, (palmate), (stipules spine-like); inflorescence racemose, peduncles asymmetrical, axillary, or pedicels attached to the slightly gibbous calyx; pods dehiscent; "glandular trichomes in pods"; n = 8.
3/106: Caragana (100). Eastern Europe to China and Mongolia.
Chesneyeae (Ranjbar, Hajmoradi & Waycott) Ezedin
Plant ± herbaceous, perennial; leaves imparipinnate; flowers usu. single, axillary; n = 7, 8
2/33: Chesneya (21), Gueldenstaedtia (12). S>W. and Central Asia to Mongolia and the Sino-Himalayan region to Siberia.
Herbs to shrubs (lianes); (swainsonine +); (cladodes +); leaves (unifoliolate/petiole and lamina 0, stipules connate); A diadelphous, (anthers unilocular).
5/113: Swainsona (83), Carmichaelia (24). The Antipodes, including Lord Howe and Norfolk Islands.
Age. This node is 14.8±2 Ma (Lavin et al. 2005), ca 24.5 Ma (Moghaddam et al. 2017), ca 17.2 Ma (Azani et al. 2019) or (20.5-)16.1(12.5) Ma (C. Su et al. 2020: inc. Carmich, Oxytr).
Coluteeae Hutchinson
Herbs, shrubs; (swainsonine +); leaves (tri-/unifoliolate//lamina 0, stipules fused, stem photosynthetic); A diadelphous [?all]; stylar pollen brush +.
9/198: Swainsona (84), Lessertia (55), Colutea (28). Carmichaelia (24). The Antipodes, Mediterranean and Central Europe to N. China, The Himalayas, Africa.
Age. Crown-group Coluteeae are ca 20.4 Ma (Moghaddam et al. 2017) or ca 13.4 Ma (Azani et al. 2019).
Astragaleae Dumortier —— Synonymy: Astragalaceae Berchtold & J. Presl
(Annual) perennial herbs to shrubs; (swainsonine +); hairs (mesifixed to ± bifurcate); (leaves as spines); (bracteoles 0); A diadelphous, (5 fertile - Biserrula); o.i. 2-3; pod ± longitudinally septate; suspensor a plate of ca 20 cuboid cells, then row of 10 elongated cells; n = 8 (11-13 - New World Astragalus).
3/4,010: Astragalus (3,239), Oxytropis (654).
Age. This node is ca 20.8 Ma (Moghaddam et al. 2017) or ca 16.1 Ma (Azani et al. 2019) - both Ast. + Oxy., ca 12.4 Na (Wojciechowski 2005), (17.0-)14.2(-11.5) Ma (Azani et al. 2019) or (16.0-)12.5(-9.4) Ma (C. Su et al. 2020), all Ast. A. annul./A. epigl sister).
Vicioid Clade.
Cicereae Alefeld - Cicer L. —— Synonymy: Ciceraceae W. Steele
Herbs, shrubs (vines, with leaf tendrils); leaflet margins serrulate; A diadelphous.
1/43. Mediterranean to Central Asia, N.E. Africa, Canary Islands.
Galegeae Bronn - Galega L.
Herbs (vines, tendrils terminal), stipules deeply 2-5-lobed; inflorescence racemose; A mon-/diadelphous; nectary 0.
1/6. Europe to Pakistan, North Africa, mountains to Kenya.
, Hedysaroids to ca 21.6 Ma, Vicioids + Parochetus to ca 26.7 Ma, and the vicioids (Fabeae, ?etc.) to ca 20.5 Ma - Azani et al. (2019).
[Trifolieae [Medicageae + Fabeae]]:
Age. This node is estimated to be ca 36 Ma (Robledillo et al. 2020). [Tif + Med. ca 31.6 Ma
Next two: mitochondrial rps1 gene 0.
Trifolieae Endlicher - Trifolium L. —— Synonymy: Trifoliaceae Berchtold & J. Presl
Nodules flattened [crotalarioid]; stem smooth; leaves trifoliolate, leaflet margins closely serrulate, stipules adnate to petiole; inflorescence ± capitate; A 9 + 1/pseudomonadelphous; ovules epitropous.
1/240. North Temperate, esp. Mediterranean to Turkey, the Andes, African mountains.
Medicageae Alefeld
Nodules flattened, indeterminate [crotalarioid], (terete - Ononis [caesalpinioid]); stem ± angled; leaves trifoliolate (pinnate - some Ononis), leaflet margins serrulate, stipules adaxially connate; A pseudomon-/diadelphous (monadelphous, nectary 0 - Ononis); ovules apotropous; x = 8.
Medicago (87), Ononis (75).
C standard with 3 clusters of veins [M.]/3 well-definied basal veins), tripping mechanism [M.], wings united by tooth-like appendages [M.]; staminal tube thickened [M.]; stigma fungiform [M.]; cotyledons with swollen bases {T.].
Trigonella (137), Medicago (83).
Fabeae Reichenbach (inc. Vicieae) —— Synonymy: Lathyraceae Burnett, Papilionaceae Giseke, Viciaceae Oken
Herbs, annuals (perennial), often vines, tendrils terminal; nodules terete (flattened) [crotalarioid, caesalpinioid]; phytoalexins pisatin and wyerone widespread; leaves pinnate, (stipules large); A [9] + 1, (mon-/pseudomonadelphous); style dorsiventrally (laterally) compressed, evenly hairy (inwardly hairy - Lathyrus); x = 7 / n = 5-7, centromeric satellites very diverse, nuclear genome [1 C] 2.02±0.07-12.93±0.66 pg.
5/330: Vicia (160), Lathyrus (150). North Temperate to subArctic, temperate South America, North and East Africa, Macaronesia, Hawaii.
Age. The age of crown-group Fabeae is estimated to be 23-16 Ma (Schaefer et al. 2012) or ca 22.1 Ma (Robledillo et al. 2020).
Glycine: 5-7/4; testa multiplicative, tegmen multiplicative, crushed.
Floral formula: ↑ K 5; C 5; A 10; N; G 1.
Floral formula: * K [5]; C [5]; A (3-)10-many/[10-many]; N; G 1.
Floral formula: ↑ K 5; C 5; A 10/[9] + 1/[10]; N; G 1.
Evolution: Divergence & Distribution. For ages of nodes pretty much throughout the tree, see e.g. Lavin et al. (2005), Bruneau et al. (2008), Koenen et al. (2013) and Y. Zhao et al. (2021). For ages of clades around Acacia see Gómez Acevedo et al. (2015) and Comben et al. (2020), and in Phaseoleae, H. Li et al. (2013). Koenen et al. (2019b/2020a: see t. S5, figs S10-S17) give additional dates for some deeper nodes.
For the fossil record of Fabaceae, see Herendeen and Dilcher (1992) and especially Herrera et al. (2019). The latter found that deposits in Colombia 60-58 Ma were very rich in remains of legumes, with eight fruit and six leaf morphotypes. For fossils of Cercis, see Jia and Manchester (2014); the oldest, from Oregon, date from about 26 Ma. Mimosoid pollen is known fossil from Africa (Late Eocene), South America (Oligocene) and New Zealand (early Miocene); its first appearance in Australia was at the end of the Oligocene (Martin 1994). Fossils that have been compared with the South American Dinizia are known from Eocene deposits in southeast U.S.A. (Herendeen & Dilcher 1999).
Kawahara et al. (2023) and others have suggested that the ancestral host plants of butterflies (Papilionoidea) as a whole were Fabaceae, and since they estimate that the age of butterflies is ca 101.4 Ma, Fabaceae would have to be that age (or a bit older). However, although most age estimates for the family (see above) are younger, sometimes substantially so, others are in this area, e.g. ca 92.1 Ma for the stem age of Fabaceae (Magallón et al. 2015; see also H.-T. Li et al. 2019).
Bruneau et al. (2008a, b) thought that the major clades in Fabaceae had separated by 58-55 Ma; the crown ages of the major clades are 56-34 Ma, but note that the constrained (given above) and unconstrained ages that they found differed considerably, the latter sometimes being one seventh of the former (Bruneau et al. 2008a). The family has a fairly long fuse, but the subfamilies originated very close togther, so close that Koenen et al. (2019a: p. 1355) suggested that "the prevailing view of some subfamilies as 'basal' or 'early-diverging' with respect to others should be abandoned" - which of course makes it impossible to think of initial character evolution in the family. Koenen et al. (2020b) linked genome duplications in Fabaceae and its diversification with events at/just after the K-P boundary, along with the evolution of mammals and birds. There was perhaps a duplication at the base of [Caesalpinioideae + Faboideae] in particular; subfamilies separated early in the Palaeocene, although Detarioideae may have begun to diversify only in the Eocene, and the family itself may be late Cretaceous in origin. Similarly, the evolution of Fabaceae has been placed in the later part of the Cretaceous some 100 Ma, the high oxygen levels in the atmosphere then favouring fires, and also the characteristic hard legume seeds (Lamont et al. 2018b). Y. Zhao et al. (2021) thought that if Fabaceae started diverging ca 67.3 Ma, then all six subfamilies had diverged within the subsequent 0.82 Ma; separate genome duplications occured in the stems of all subfamilies (except of course Duparquetioideae).
Koenen et al. (2013) note that by practically any measure of success, Fabaceae are successful - pretty much a fair statement, as we shall see. They are notably speciose, particularly Caesalpinioideae (especially Mimoseae) and Faboideae, and within the latter, perhaps especially the 50 kb inversion and IRL (= Inverted Repeat Loss) clades (Magallón & Sanderson 2001), and overall include ca 9.4% of eudicots. Magallón et al. (2018) thought that there had been increases in the diversification rate in this part of the tree (92.1-)88.1(-84.8) Ma and also ca 5 Ma before - but in terms of pinning these increases to nodes, branches are short and there is uncertainty about relationships. Y. Zhao et al. (2021) noted that, thinking about the family as a whole, it was largely two or so N-fixing clades within Caesalpinioideae and Faboideae just mentioned (or with their limits adjusted) that contain the bulk of the diversity within the family, basal branches in both those subfamilies and the other subfamilies in their entireties including relatively few species. Details of the evolution of nitrogen fixation in the family are problematic, gain of the ability to associate with rhizobia - but exactly where in the tree is unclear - was followed by multiple losses, as with some recent scenarios of the evolution of nitrogen fixation as a whole (Koenen et al. 2019a; Zhao et al. 2021: see also above). In part, the considerable diversity of the family is best explained by number of separate radiations - see Koenen et al. (2013) for a number of diversification rate shifts - rather than any particular One Thing. Thus even the very speciose Astragalus is perhaps not particularly notably so when considered in the context of its immediate relatives (Sanderson & Wojciechoswki 1996). Interestingly, monosymmetry is associated with higher diversification rates in Faboideae, but not in the "caesalpinioids", i.e. Mimoseae (Prenner & Cardoso 2016).
A pre-Gondwanan breakup age for Detarioideae-Amherstieae of perhaps 130 Ma, and so a proportionally older age for the family as a whole, was suggested because of their amphiatlantic distribution and common possession of ectomycorrhizae (ECM) (Henkel et al. 2002; Moyersoen 2006). However, this seems unlikely, indeed, recent estimates of the crown-group age of Detarioideae are anything from 68-64 Ma (de la Estrella 2017) to ca 17.3. Ma (Bruneau et al. 2008a: see also above). There are reports of fossils of several extant genera in Africa from the Eocene 46-34 Ma, and they include Brachystegia (ECM), and Cynometra, both Detarioideae-Cynometreae, the latter with arbuscular mycorrhizae (AM), and they seem to have been dominants even then; Fabaceae seem to have had pretty much a worldwide distribution by the Eocene (Epihov et al. 2017 and references).
One way to think about the diversification of Fabaceae and their biogeography is in terms of vicariance of biomes rather than of the classical geographical areas (Lavin et al. 2004; Schrire et al. 2005). Changes in diversification rates of clades in the family can quite frequently be linked to biome shifts (Koenen et al. 2013). Fabaceae, many of which are deciduous, are very important components of the vegetation of seasonally dry tropical forests (= the Succulent Biome) and of savannas (Oliveira-Filho et al. 2013; see also Schrire et al. 2015). Stem ages of species tend to be old in such habitats (Pennington & Lavin 2016), indeed, members of the Caesalpinia group ca 55 Ma may have been part of vegetation that only later (Oligocene to late Miocene) became what we call the Succulent Biome (Gagnon et al. 2018; Donoghue 2019). These authors suggest that within the Caesalpinia group, a quite small if pantropical clade of around 225 species, there have been no fewer than 49 transcontinental disjunctions, of which all bar two are within biomes, and 27 are within the Succulent Biome in particular. Phylogenetic (ecological) conservatism is pretty extreme here, intercontinental dispersals have been easier than than biome shifts (see also Donoghue 2008), and any shifts between biomes that have occurred are associated with changes in the growth form of the plant (Gagnon et al. 2018: Fig. 3 in particular). Savanna expansion in Africa has been dated to 15 Ma and subsequently (T. J. Davies et al. 2020). It is noteworthy that the phylogenetically-based Dry Forest grouping of Slik et al. (2018) also includes forests in Africa, Madagascar and India.
In the Neotropics species diversity of Fabaceae is associated with temperature (Punyasena et al. 2008). Here Dexter et al. (2017) found little correlation between geography and phylogeny in two rainforest genera, the mimosoid Inga (except in Central America) and the basal faboid Swartzia (except in Central America and the Brazilian Atlantic Forest). There is little evidence of a connection between vicariance events and diversification, and the potential sources of species found in any particular area (= the local community assembly) within Amazonia seemed to be the whole of the rest of Amazonia. However, as mentioned, other Fabaceae are major components of the Neotropical seasonally dry forest biome. Unlike Amazonia, this biome is rather widely scattered and relationships of the plants growing there often show geographical structuring, with clades confined to single dry forest regions (E. R. de Souza et al. 2013; Dexter et al. 2017). Interestingly, Dexter et al. (2017) suggest that the Amazonian rainforest is surprisingly unstable - ages of co-occuring species are quite variable - ca 1.5 Ma for Inga and ca 18 Ma for Burseraceae-Protieae.
Long distance dispersal has often been invoked to explain various disjunct distributions in Fabaceae, and Schaefer et al. (2012) estimated that there had been as many as 7 long distance dispersal events per 10 million years in Fabeae alone. There are a number of transoceanic disjunctions within the family, and 51/59 of those listed by Schrire et al. (2005) are only 1-22 Ma old (see also Bouchenak-Khelladi et al. 2010b; Vatanparast et al. 2013: Dalbergia; Tosso et al. 2017: Guibortia, transoceanic dispersal and habitat shifts). Indeed, some 16 (7% of the total) American-amphitropical disjuncts occur in Fabaceae (Simpson et al. 2017a). Brongniartieae and Haplolobieae are largely tropical American taxa, and this is their probable place of origin; in the former both the widely disjunct West African Haplormosia monophylla and the Australian taxa are probably the result of L.D.D. events (Sa˜o Mateus et al. 2024 - however, c.f. L. Cai et al. 2025 for a rather different poitiion for Haplormosia). Sa˜o Mateus et al. (2024: Figs 4-6, focus on Harpalyce) optimized the evolution of a number of characters in Brongniartieae on their phylogeny, including biome evolution (ibid. Fig. 8). The North Atlantic land bridge may have been important in the Caenozoic dispersal of the family (Lavin et al. 2000). One particularly interesting connection is in Hymenaea, whose resin forms valuable amber deposits. The majority of the species are New World, but there is a single Old World species, H. verrucosa. Both the Mexican and Dominican members seem to have evolved from extinct species whose immediate relationships are likely to be with the Old World H. verrucosa (Poinar & Brown 2002); see also Seyfullah et al. (2018).
Cercidoideae. Diversification in Cercis (Cercidoideae) began ca 35 Ma and its spread was from east to west - and across the Atlantic (Fritsch & Cruz 2012). Cercidoideae as a whole may be Tethyan in origin, initially favouring seasonally dry habitats (Sinou et al. 2009, c.f. Meng et al. 2014: sampling poor, note dates). Other than Cercis itself, basal members of both the Phanera s. str. and Bauhinia s. str. clades are African (Sinou et al. 2020). The pantropical Bauhinia s.l. includes a number of climbers (= Phanera s. str.), and Bauhinia s. str. shows geographically-restricted clades - [Asia [Asia [Africa + America]]], the split between Cercis and Bauhinia being dated to ca 62.7 Ma and B. yunnanensis diverging from the rest of the genus soon afterwards (Meng et al. 2014: ?sampling, c.f. Sinou et al. 2009, 2020: B. yunnanensis = Phanera, well embedded in the Phanera clade).
Detarioideae.Increase in diversity of the Brownea clade (Amherstieae) has been gradual for some 30 Ma - the museum hypothesis, although interestingly Ecuadendron and Brachycylix, which diverged only ca 0.6 Ma, are on a branch over 27 Ma (Schley et al. 2018). Hoorn et al. (2023) also discuss diversification in this clade, and in the Andira clade (Faboideae), both clades showing similar patterns of increase, but the latter is notably less speciose - but also younger.
Caesalpinioideae. Diversification in the Cassia, etc., complex may have begun ca 54-53 Ma (de Souza et al. 2019). Marazzi and Sanderson (2010) suggest an age of 53-47.5 Ma for stem group Senna, (47-)45(-41.7) Ma for the speciose crown group. Within the speciose Chamaecrista rainforest trees seem to have given rise to shrubs, extrafloral nectaries probably evolving once here (De Souza Concição et al. 2009; Silva et al. 2017 and Marazzi et al. 2019 for extrafloral nectary morphology, etc.).
Early divergence within Mimoseae seems to have occurred in Africa (Bouchenak-Khelladi et al. 2010b). The age of the crown-group clade [Ingeae + Acacieae] is estimated to be (24.5-)21(-17.2) Ma by A. O. de Souza et al. (2013), q.v. for other dates in the Calliandra area; diversification and plant-insect interactions in Inga are discussed below. For diversification in Mimosa, a two-step affair, see Koenen et al. (2013). See also Iganci et al. (2015) for diversification and biogeography in the Abarema area - note that Abarema is polyphyletic. The Jupunba clade is very largely New World in distribution, but two species of Albizia/Cathormion from Africa were found to be embedded in Hydrochorea (Soares et al. 2022).
Pedley (1986) rather improbably (with the advantage of hindsight) suggested that Acacia and Senegalia were widespread by the mid-Cretaceous. Recent estimates of the ages of crown-group Acacia s. str., at some 1,000+ species the largest genus in Australia, range from 26.6-3.3 Ma, older ages being driven by recent fossil discoveries (Miller et al. 2013, q.v. for dates of other genera that used to be in Acacia, etc., see also M. A. M. Renner et al. 2019; note that fossils have been described by by Jacobs and Herendeen 2004 as A. mahengensis from deposits ca 46 Ma in East Africa). Renner et al. (2019) noted increases in climatic disparity in the genus ca 7.5 Ma and again ca 2 Ma, although the overall diversification rate in the genus seems to have been largely constant - i.e., little change over the course of ca 20 Ma. Acacia seems initially to have been adapted to ± dry conditions, perhaps equivalent to modern semi-arid environments, rainforest members of the genus being derived; Paraserianthes and Paraarchidendron, closely related to Acacia and both monotypic, are also rainforest taxa (Renner et al. 2019). González-Orozco et al. (2013) plotted the distribution of Australian Acacia (practically the whole genus) in the context of various environmental factors, and they thought that climatic variables were correlated with the largest-scale patterns. Interestingly, fossils of Acacia are known from New Zealand until the early Pleistocene (Renner et al. 2019). Mishler et al. (2014) looked at patterns of endemism there taking into account clade ages, etc., and found areas of palaeoendemism scattered in the continent, with neoendemism particularly apparent in southwestern Australia. The very close similarity between A. koa, from Hawai'i, and A. heterophylla, from Réunion Island and some 18,000 km distant, is surprising, but perhaps Austronesians moved a species like A. melanoxylon from eastern Australia (G. K. Brown et al. 2012). However, given the structure of relationships - A. heterophylla is embedded in A. koa, the combined clade being sister to A. melanoxylon - long distance dispersal from Hawaii to Réunion, implausible although it may seem, is perhaps more likely (Le Roux et al. 2014).
Faboideae. Shoemaker et al. (2006) and Soltis et al. (2009) think, although with some hesitation, that diversification in Faboideae may be connected to a genome duplication immediately basal to the split between mirbelioids and the rest, the NPAAA clade, although exactly where that duplication is to be placed is unclear. This duplication has also been implicated in nodule formation in Faboideae, perhaps helping to explain nodule diversity there (Q.-G. Li et al. 2013). See also rate shifts suggested by S. A. Smith et al. (2011) and discussion below under Ecology & Physiology. The evolution of the distinctive keeled pea flowers with their enclosed androecia in Faboideae (see Pollination Biology below: such flowers are also found in some Cercidoideae - and Polygalaceae) seems not to be directly connected wih diversification here, rather, a variety of intrinsic and extrinsic factors including nodulation, chemical defenses and dispersal into areas with new biomes appear also to be involved, that is, evolution is a matter of synnovation rather than key innovations, interestingly, there are a notable number of rate shifts in Faboideae in the Miocene (L. Cai et al. 2025).
- Within Faboideae, (Tribes are treated alphabetically below; those of the IRLC are at the end.)
- For evolution in the quite old but not very speciose Cladrastideae which may have originated in North America, see Duan et al. (2020); the stem of Platyosprion, sister to the rest, is ca 40 Ma. Cladrastideae, with around 20 species, is sister to the 50 kb inversion clade, with around 22,650 species. Hoorn et al. (2023) discuss diversification in the Andira clade, in the 50 kb inversion clade, not very speciose, but young.
- For biogeographical relationships in Desmodieae, see Jabbour et al. (2017); the tribe may have originated in Asia.
- Lupinus is a major genus in Genisteae, in the 50 kb inversion clade, and it includes several major clades that are correlated with geography (Aïnouche & Bayer 1999, support not very strong; Aïnouche et al. 2004). Within the North American perennial clade there has been a recent (ca 2.7 Ma) Central American/Andean radiation that is now represented by over eighty species, some 56 of which grow in the páramo, and the rate of diversification increases as the genus moved into Andean South America from Central America (Moore & Donoghue 2009; see also Silvestro et al. 2011; Sklenár et al. 2011). Lupinus, both in the Andes and in alpine North America, where there had been an earlier burst of diversification, are largely perennials (Drummond 2008; Drummond et al. 2012), the annual habit being plesiomorphic. Alpine taxa in South America in particular show much variation in habit, etc., ranging from tussocks to stem rosettes to shrubs 6 m tall, and there have also been reversals to the annual habit; diversification is estimated to be (5.6-)4.6(-1.7) Ma (Nürk et al. 2019). Contreras-Ortiz et al. (2018) discuss the evolution of habit here, noting that plants with a fistulose inflorescence and leaf rosettes had evolved several times, diversity in high-altitude lupins perhaps being generated by recurrent evolution. Evolution in these Andean species has been adaptive, although with a geographic component (Nevado et al. 2016; Contreras-Ortiz et al. 2018). Both overall disparification, the equivalent of Simpsonian adaptive radiation (and here plant height was emphasized), and diversification, that is, species number, have increased rapidly, the latter despite an increase in generation time which might be expected to slow things down (Hughes & Atchison 2015; Nürk et al. 2019). However, as Givnish (2015b) notes, understanding the reasons for diversification s.l. in Andean Lupinus is not easy. For diversification in Lupinus compared with that of some other explosive radiations, see Knope et al. (2012); Hypericum, Echium, Hawaiian Lobelioideae and Asteraceae-silverswords show similar diversification on (sky) islands, and Dianthus and Espeletia have very rapid radiations. Perennial Lupinus also diversified in eastern South America ca 6.5 Ma; these Lupinus are separately related to east North American annuals (Drummond et al. 2012). These South American radiations may also be connected with the movement of bumble bees, pollinators of Lupinus, from North to South America some 6 Ma (Hughes & Eastwood 2006). All told, there are ca 171 species of Lupinus in South America versus ca 90 species in the whole northern hemisphere (von Hagen & Kadereit 2003). Ree et al. (2003) studied aspects of LEGCYC gene evolution in the context of variation of floral morphology in the genus.
- Indigofereae have diversified considerably in succulent biomes, and clades growing there tend to be geographically more restricted than those common in grassland biomes. Crown group ages of the four major clades into which species of Indigofera fall, the Cape, Tethyan, Pantropical and Palaeotropical Clades, are ca 15.5 Ma or less (Schrire et al. 2009) or (22.6-)21.8-19.7(-12.3) Ma (Du Preez et al. 2024, see also 2025a). All told, Indigofera has around 787 species, of which ca 550 are from the Africa-Madagascar region; there are some 140 species in the Greter Cape Floristic Regin (GCFR). Du Preez et al. (2024) suggested that Indigofera as well as its four main clades originated in Africa ca 38 (stem) or 31 Ma (crown age), and that its current wide distribution has been achieved via a combination of stepping stone and long distance dispersal events, the latter, for instance, between Africa and Madagascar and perhaps between Africa and the New World and Australia. Note that of these four main clades, only the Cape Clade has a distribution that is more or less resricted, being found largely in (GCFR). Overall, species in the Cape Clade prefer drier conditions, and most diversification there has happened in the last 12.3 My; interestingly, this diversification began in the Succulent Biome, the genus moving into the Grassland and Temperate biomes - rather unusually, there was no movement back from the latter (Du Preez et al. 2025b). Note that the position of I. nudicaulis has occasioned some concern, in some analyses being found outside the four main clades, however, it seems to have diverged early from the rest of the Cape clade ca 22.6 Ma (du Preez et al. 2024, for further discussion, see du Preez et al. 2025a). The Indigofera Cape Clade can be characterized by being shrubs to small trees with (stalked) biramous hairs, its leaves are pinnate (digitate), its flowers are pink-red-purplish and the plant is a resprouter (seeder) (see du Preez et al. 2025a for the optimization of a number of morphological characters on to a phylogeny of the group).
The other two most speciose genera in the GCFR are Aspalathus, with ca 290 species the most speciose member of Fabaceae there and found mostly in the Cape Core Region (Du Preez et al. 2025b), and Psoralea, with ca 140 species. In Aspalathus and Indigofera, at least, species are ro be found in both the Fynbos and Renosterveld (Du Preez et al 2025b). Almost half the species of Crotalarieae are to be found in southern Africa, esp. Aspalathus, and Crotalaria is also very diverse here (its origin is in Africa) with over 500 of its ca 700 species in the Africa—Madagascar region, and it is especially rich in the Afromontane area in East Africa, but it is also to be found elsewhere (Rockinger et al. 2017). Of the almost 300 species of a clade of Crotalaria, nearly all are restricted to the Cape Floristic Region (Linder 2003), and Edwards and Hawkins (2007) date diversification in the Cape part of Crotalarieae to about the same time, 46.3±2.4 or ca 45.2 Ma (estimates of the age of crown-group Crotalaria itself are of the order of (42.4-)29.1, 17.7(-14.3 Ma - Rockinger et al. 2017). There have been at least nine movements of the genus to Madagascar and at least five to Australia. Interestingly, truly simple leaves are found in perhaps 29% of Crotalaria, being most common in species growing in the humid tropics, and also in the monotypic Euchlora, while species with unifolioliate leaves predominate in dry climates, furthermore, polyploid taxa (n = 16) are to be found in a New World subgroup of a clade characterised by a bilabiate calyx (Rockinger et al. 2017), Psoraleeae - Bello et al. 2022
- Lotus (Loteae), speciose in the Mediterranean and especially Macaronesia, seems to have diversified within the last ca 7.6 Ma, and there have been several colonizations of Macaronesia and one back from there probably to North Africa (Jaén-Molina et al. 2020).
- In the mirbelioids it has been suggestedd that apparent independent increases in the rate of diversification in Mirbelieae, Podalyrieae and relatives may rather be the results of extinctions caused by cooling climates and increased seasonality ca 32-30 Ma in the early Oligocene (Crisp & Cook 2009), and Crisp and Cook (2007) date the development of SW/SE Australian disjunctions to vicariance events caused by the development of aridity in the Nullarbor plane some 14-13 Ma (other climatic events could also be implicated). Consistent with such ideas, Nge et al. (2021c: Fig. 1) note that species distributions/relationships in Bossiaea (Bossiaeae) and Jacksonia (Mirbelieae) within Australia seem to fit the peripheral vicariance pattern, species now being found predominantly on the periphery of the continent after the drying out of the centre, a process that began in the Eocene. Indeed, the Australian Mirbelieae s.l., with over 800 species, include two thirds of all Australian Faboideae, and 470 of those species are included in Pultenaea s.l., which seems to have undergone a rapid radiation 25-20 Ma (Crisp et al. 2004; Orthia et al. 2005a, b; for the limits of the genus, now rather reduced, see R. L. Barrett et al. 2024). Plants in this clade have variously reduced/modified (phyllodinous) leaves, sometimes unifoliolate, and photosynthetic stems (cladodes).
- In Podalyrieae, Schnitzler et al. (2011) found that diversification of the ca 128 species from the Cape region began ca 33 Ma at the end of the Eocene and was connected with shifts in how the plants survived fires, either by resprouting or germination of seeds (note that Edwards & Hawkins 2007 date diversification here to 46.3±2.4 or ca 45.2 Ma).
- Basic geographic relationships within Ormosieae are [New World [Old World [Old World + New World]]], problems these cause aside, Torke et al. (2021) discuss the biogeography of the well-sampled New World Ormosieae in particular in some detail; the story is centred on Greater Amazonia whence Ormosia achieved the rest of its Neotropical distribution. Torke et al. (2021) think that this clade may initially have arrived in the New World by long distance dispersal (O. panamensis, perhaps related to Old World taxa, was not sampled).
- Divergence of woody clades in the Old World Phaseoleae (crown group age ca 28.6 Ma) has been associated with Late Oligocene warmness and aridity, and of herbaceous members with tropical arid climates in the Early Miocene (H. Li et al. 2013: Apios sister to the rest).
- Robinieae may have been diversifying for some 30 Ma in the Neotropical seasonally dry tropical forest (Pennington et al. 2009; Pennington & Lavin 2016).
- Finally, Psoraleeae (Phaseoleae), the age of which is only some 8.2 Ma, seem to have originated in the Mediterranean biome of southern Africa, their current wide distribution being the result of long distance dispersal events coupled witn biome shifts, etc. (Bello et al. 2022) - certainly no biome conservatism here.
- The Inverted Repeat Loss Clade (IRLC) is predominantly Old World (Dormer 1946b, before the discovery of the IRL...). Duan et al. (2021a) examine the historical biogeography of Wisterieae in some detail. Things are complicated here, because although relationships immediately around Wisteria are the same in both chloroplast and nuclear trees, there are otherwise quite substantial differences within the genus; the discussion in Duan et al. (2021a) is based on the topology of the chloroplast tree, although the nuclear tree seems to have been important in the justification of the nomenclatural changes they made.
- In the IRLC the speciose Astragalus, the largest plant genus by some 850 species and with some 3,900 (as of viii.2025) species, characterises drier areas of both hemispheres, growing in wooded and steppe habitats (Azani et al. 2017); there are about 2,300 species in Southwest and Central Asia and ca 1,500 species in the Irano-Turanian region alone. A number of taxa have leaf rhachis spines (see subgenus Astracantha), and are spiny cushion plants. The Neo-Astragalus clade, endemic to North America, grows in low‐nutrient soils and under rather harsh climatic conditions in the lowlands, and there has been extensive aneuploid evolution here (n = 11-15); polyploidy based on n = 8 is common in the Eastern Hemisphere and the circumboreal region (Folk et al. 2024). Overall diversification rates in Astragalus are not linked to particular ecological strategies (a non‐adaptive radiation – see Givnish 2015), although areas in which neo-Astragalus grows have strong diurnal temperature ranges and particularly isothermality, and its species prefer a different spectrum of soil types than do Eurasian species. Astragalus has a West Asian origin with a crown group age of ca 11.5 (14.2) Ma (Folk et al. 2024) - or (17.0-)14.2(-11.5 Ma (Azani et al. 2019), (16.0-)12.5(-9.5) Ma (Su et al. 2021; see also Shahi Shavvon et al. 2017; Amini et al. 2018). Astragalus separated from Oxytropis (20.8-)16.1(-12.3)/16-12 Ma; the latter has a beak on its keel and some 600 species, and diversification occurred a mere 3.8 Ma. Azani et al. (2019) suggested that for Astragalus to reach its current distribution from an ancestral area in western Asia (including the eastern Mediterranean) where most of its early-diverging clades originated, there must have been ca 196 dipersal but only 14 vicariance events. Annuals have evolved several times in both the Old and New Worlds, although the diversification rates of these annual lineages have been low and there have also been reversals to perenniality, as in some other Faboideae (Azani et al. 2017, 2019; C. Su et al. 2020: see below). Radiation in the speciose aneuploid New World Neoastragalus clade (ca 500 species), which may have originated from a Mediterranean annual clade (Azani et al. 2019), started ca 4.4 Ma (Wojciechowski 2004); there were two invasions of west South America - there are over 100 species there - that happened a mere 2.07-1.62 Ma (the larger invasion) and 1.23-0.79 Ma (the smaller invasion), both invasions being followed by very high diversification rates (Scherson et al. 2008; see also Koenen et al. 2013). Azani et al. (2019) suggest three upticks in diversification, one ca 11.2 Ma involving most of the genus, another ca 5.2 Ma in the Hypoglottis clade, and a third ca 2.7 Ma in the distinctive Astracantha clade species of which have a spiny cushion habit. This last group, along with Old World annuals, may represent responses/adaptations to aridity and strong winds, but in groups living at different altitudes, the Astracantha group prefering higher altitudes (Azani et al. 2019). Interestingly, species of Astragalus in the South American páramo vegetation may have moved up from the south (Sklenár et al. 2011). See more on the annual habit, selenium accumulation, etc., in Ecology & Physiology below.
To integrate. 1. Schaefer et al. (2012) suggested that Fabeae, believed to be of eastern Mediterranean origin and originally with an annual habit, have moved at least 39 times into Eurasia, and also to the New World, the Atlantic islands, and elsewhere (ca 12 events). 2. There are four monotypic genera in Mexico, two in Caesalpinioideae-Schizolobieae and two in -Mimoseae (Hughes et al. 2022b). 3. There are America-Africa disjunctions in Caesalpinioideae which are made up of just one or a few African taxa but more American taxa, and distributions within America may also be distinctive - examples include Haematoxylum and Pomaria (Caesalpinieae), Parkinsonia (Schizolobieae) and Xerocladia within the Mimoseae-Neltuma clade (Hughes et al. 2022a). 4. M. Liao et al. (2023) discussed the evolution and biogeography of Faboideae-Sophora, i.a. noting that geography and relationships of species in section Edwardsia, common denizens of oceanic islands, could be represented [Chilean lineage [Juan Fernandez Islands lineage, La Réunion lineage [Pacific lineage [Hawaiian lineage + New Zealand lineage]]]] - pretty remarkable.
An alternative pathway leading to the synthesis of L-tyrosine has been recorded in some Faboideae - it probably occurs in most of the clade, but nowhere else in flowering plants, and another enzyme in this pathway is found here in a non-canonical form, but it also occurs rather more widely in pentapetalous plants (Schenck et al. 2017, 2019/2020). The normal pathway is the arogenate dehydrogenase pathway, ADH/TyrAa, but in some legumes the prephenate dehydrogenase pathway - PDH/TyrAp - is found. Wink and Mohamed (2003) had earlier plotted the distributions of a number of secondary metabolites on a rbcL phylogeny of the family - mostly Faboideae.
The non-protein amino acid accumulating (NPAAA) clade (or clade with non-proteogenic amino acids) includes most Faboideae and is characterised by the presence of canavanine, (NH2)2C=N-O-CH2-CH2-C(NH2)COOH (the O along the spine is the only difference from arginine). A number of these NPAAs are involved in plant defence against insects (e.g. T. Huang et al. 2011). Gibson et al. (2025: the topology of the tree and generic limits bear scrutiny) recently looked at the distributions of eight NPAAs across all flowering plants, although Fabaceae were central (NPAAs are especially common throughout most of Faboideae and also in Caesalpinioideae-Mimoseae). Gibson et al. (2025) suggested that djenkolic acid, a sulphur compound, was found in some Mimoseae, all records being in Acacia, with but two exceptions. However, it would seem to have a broader distribution in Minoseae; Pilak et al. (2001) had looked at the synthesis of this compound which they found more widely in Mimoseae, and they noted that its presence was associated with the production of carbon disulphide, which is responsible for the -S-C-S- motif in the centre of djenkoic acid. Azetidine-2-carboxylic acid seems to be commonest in the Asparagaceae-Convallarioideae (Nolinoideae in Gibson et al.) area, but is also found in Caeasalpinioideae-Schizolobieae, basal to Minoseae (and perhaps also in Baphia, Dendrobium and even Beta...). Thives Santos et al. (2024) discussed how azetidine, a proline mimic, variously reduced root growth in other angiosperms as it became mistakenly incorporated into nuclear proteins. Other NPAAs noted by Gibson et al. (2025) include indospicine (in the Indigofera area) and mimosine (Leucaena, Senegalia berlandieri), other NPAAs being uncommon.
Extrafloral nectaries are common in Fabaceae and are very variable in both position and morphology, however, as will be evident in the characterisations, there is some phylogenetic signal in their morphology/position (McKey 1989; Weber & Keeler 2013; Gonzalez & Marazzi 2018; esp. Marazzi et al. 2019). These extrafloral nectaries may have evolved some 35 times, and they have also subsequently been lost and even regained (e.g. Marazzi et al. 2011, 2015; see also McKey 1989). They are notably common in Mimoseae, perhaps only a single origin, and they are to be found towards the base of the petiole, on the rachis, and sometimes on the petiolules (e.g. Delgado et al. 2017; Marazzi et al. 2019), although interestingly, they are absent from the majority of Mimosa itself (Simon et al. 2011). They are less common in "caesalpinioids", although they may be represented by tufts of hairs or other kinds of nectaries; they are found on the lower surface of the leaflets in Detarioideae-[Afzelieae + Amherstieae] or as odd structures at the node in Bauhinia (Pascal et al. 2000; Marazzi et al. 2019). They are still less common in Faboideae, although they have originated several times even there (Marazzi et al. 2014, 2019). Marazzi et al. (2012) suggested that in a clade making up most of Caesalpinioideae (Gleditsia/Chamaecrista/Mimoseae) the origin of extrafloral nectaries could be thought of as a facilitating "deep homology" that was manifest in the numerous subsequent independent acquisitions of the nectaries there. Extrafloral nectaries characterise a major clade within South American Senna, where they may be a "key innovation" that is involved in the diversification of that clade; it is more speciose than its sister clade (282 species with nectaries versus 68 species lacking nectaries) and it has also speciated significantly faster. The extrafloral nectary-bearing clade may have moved to new habitats that became available after the Andean uplift and speciated there (Marazzi et al. 2006, 2013b; Marazzi & Sanderson 2008, esp. 2010; Weber & Agrawal 2014). Marazzi and Sanderson (2010) suggest a crown-group age for this clade of some 40.8-30.6 Ma, that is, somewhat before the Andean uplift (ca 30 Ma). However, Marazzi and Sanderson (2010) also noted that Simon (2008) had found that the loss of extra-floral nectaries characterized a large clade of Mimosa that was far more speciose than its sister clade - indeed, the numbers of species in the sister taxa with and without extrafloral nectaries are 15 and 515 respectively (Simon et al. 2011). Furthermore, a less conspicuous and organized kind of extrafloral nectary characterizes a separate clade of Senna (Marazzi et al. 2013b). For more on extrafloral nectaries and their associated ants see below.
Papers by Shirley Tucker are an essential starting point for any understanding of the extensive floral diversity in Fabaceae (e.g. Tucker 1987a, 1989, 1996a, 2000a, 2003a; Tucker & Douglas 1994: phylogeny, for more general accounts). Over half the species in the family are in the Faboideae-NPAAA clade (most have keel flowers) and Caesalpinioideae-Mimoseae (which have polysymmetric brush flowers), and both clades are well embedded in the phylogeny. For the great diversity of floral morphology in other parts of the family, the images in the paper by the Legume Phylogeny Working Group (2017) are a good introduction; see also Ojeda et al. (2019: Detarioideae, also Falcão et al. (2020) and Falcão and Mansano (2021) - many have notably odd flowers, but not so Poeppigia). Interestingly, in Faboideae clades with polysymmetric flowers below the NPAAA clade tend to be small (Klitgård et al. 2013) - c.f. Mimoseae. Clarifying basal relationships in the family, relationships in the IRL clade, and those between Fabaceae and its immediate relatives is particularly important here.
Although the pea-type keel flower may seem to preponderate in Fabaceae, at least for members that live in more temperate parts of the world, there is quite extensive variation even within this flower "type" (although rather little in the gynoecium at anthesis). Variation in floral morphology and development is considerable in basal Faboideae, e.g. Cardoso et al. (2013a, b), although within Dipterygeae there ia a clade ca 30 Ma with papilionoid flowers that are quite keel-like (Carvalho et al. 2023a, b: some characters optimized on tree). For glands in various parts of the plant, both leaves and flowers, in Dipterygeae and Amburaneae, especially in anthers of the former, see Leite et al. (2019, also Palermo et al. 2017: Dipterygeae, glands/cavities and canals). Pennington et al. (2000) discussed floral evolution in basal Faboideae, some of which, like Swartzia (Swartzieae), have flowers with very derived morphologies - again, floral variation around here is considerable. Androecial initiation in Swartzia and relatives can be both centripetal and centrifugal (Tucker 1990, 2003b); the androecium here is heteromorphic, stamens have different morphologies both within flowers and between species, differing in features that may attract insects (Basso-Alves et al. 2022a). Swartzia usually has only a single petal and lacks even rudiments of the others, but Amburana (Myroxylodae, closely related), also with just one petal, does have rudiments of others (Leite et al. 2015). Although the flower of Petaladenium (Amburaneae) is keel/papilionoid, the keel petals are not fused and they lack locking devices, perhaps suggesting that these flowers were the result of an early "experimental" phase in floral evolution (e.g. Tucker 1993; Leite et al. 2014; Prenner et al. 2015). There is extensive floral variation in Amorpheae - petals may be lost, or all petals may look rather similar, a stemonozone may be developed, etc. (see McMahon & Hufford 2002, 2004, 2005; McMahon 2005). Floral variation is also very considerable in the old "Caesalpinioideae" (e.g. Prenner & Klitgaard 2008b; see also Zimmerman et al. 2013b, 2017). Klitgård et al. (2013) found that polysymmetric flowers, sometimes with long, linear petals, had evolved about four times in the Pterocarpus clade (Dalbergieae) alone, which otherwise has monosymmetric keel flowers (see Bento et al. 2021 for an example of the development of the two flower types in the tribe, Table 2 for some general comparative data). It has also been suggested that mimicry may have been involved in the evolution of the keel flowers of Cercis (Tucker 2002a). Overall, L. Cai et al. (2025) suggest that keel-type flowers have evolved some six time in Fabales, including once in Polygalaceae, and lost some 32 times, including 30 times in Fabaceae-Faboideae alone, but the exact number will depend on details of the phylogeny. Interestingly, only three of the losses are in the NPAAA clade and all three are in the largely bird-pollinated Erythrina (Cai et al. 2025). Indeed, keel-type flowers are pollinated very largely by bees (see below), but include a variety of morphologies, including valvular, brush and explosive mechanisms, and pollination in the various losses of keels just mentioned are by bats and various kinds of birds in particular (Cai et al. 2025).
There is discussion above about diversification in Faboideae and its possible connection with the evolution of keeled, pea-type flowers (L. Cai et al. 2025). Of course, Cercidoideae-Cercis and Polygalaceae-Polygaleae in particular also have keel flowers, and Uluer et al. (2022) think about their evolution in the latter - mimicry? (see below). Cai et al. (2025) noted that there had been 18 rate shifts in Fabales, of which 11 were in Faboideae and 9 of these occurred in the Miocene - interestingly, however, the diversity shifts did not appear to be state-dependent, they were not associated with the evolution of keel flowers. Shifts are associated with groups like the mimosoids, Genisteae, Lupinus, the NPAAA, IRL and Vicioid clades, Astragalus, Diocleeae, and smaller clades such as Psoraleeae and Phaseolinae (Cai et al. 2025: Fig. 2).
Normal floral orientation is to be found Caesalpinioideae like Ceratonia, while inverted orientation occurs in Cercidoideae (see Tucker 1989; Herendeen et al. 2003; Luckow et al. 2005; Alvarado-Reys 2024), Duparquetia, many other Caesalpinioideae, and in Faboideae. The normal order of development of the parts of the flower (simply outside inside) is not found in most Fabaceae, and although it is in Duparquetia, rather basal in the family, this genus has a highly derived rather keel-type papilionoid-looking (but quite differently constructed) flowers - if the order of development may be "normal" here, not much else is (Prenner & Kligaard 2008b). Detarioideae show extensive loss of sepals and/or petals and/or stamens (Bruneau 2000; Mackinder 2005: genera), and linked with the first two changes in particular there can be increase in size of the bracteoles and in stamen number (Tucker 1992b, 2000a, etc.). Thus in Monopetalanthus durandii the flower is surrounded by bracteoles and the floral formula is K 1 (minute), C 1; A 10; G 1, and Brachystegia glaucescens also has large bracteoles and a floral formula of K 5 (all small), C 0; A 10; G 1 (Tucker 2000a). Ojeda et al. (2019) looked at the evolution of floral morphology in the quite small Anthonotha clade (Amherstieae) and found 35 transitions in the seven floral characters examined, some variation even being infraspecific. In Faboideae there are a number of near-basal clades that include both taxa with polysymmetric flowers, sometimes with numerous stamens, and taxa with pea flowers (e.g. Cardoso et al. 2012a, b). Thus near-basal in the 50 kb inversion clade we find Aldina, with polysymmetric flowers, which is sister to [Andira + Hymenolobium], with papilionoid flowers (Ramos et al. 2015, 2022). Dialioideae - but not the two basal clades - vary considerably in the numbers of their parts and in floral symmetry (Zimmerman et al. 2017 and references; Falcão et al. 2020), with the carpel sometimes being initiated in the inner staminal whorl, perhaps homeosis (Falcão et al. 2020). All this perhaps suggests an early "experimental phase" in the evolution of floral morphologies in the family with a lack of canalization in floral development (Prenner & Klitgaard 2008; see also e.g. Polhill et al. 1981; Tucker 2001, 2003a, c; Ramos et al. 2015; Zimmerman et al. 2017), and papilionoid flowers have evolved at least five times in the family (Bukhari et al. 2017). Endress (2012) suggested that floral asymmetry was a key innovation in Phaseoleae - here the keel is rather like an elephant's trunk and there is a pump-type secondary pollination mechanism. CYCLOIDEA-like genes vary extensively in the family and its immediate relatives (Z. Zhao et al. 2018), although it is as yet unclear how this might relate to floral evolution.
The flowers of Mimoseae - often rather small, radially symmetrical, sympetalous, with normal floral orientation, and aggregated into dense heads or spikes - seem at first sight to be rather different from those of other Fabaceae, hence in the past the group was put in its own family or at least subfamily. However, molecular data placed them firmly within Caesalpinioideae s. str., many of which have very different-looking flowers (see below). Pedersoli et al. (2023) examined corolla development in 16 species scattered through Mimoseae, and found that the corolla primordia were initially free, but became fused either by postgenital formation of a single tissue (connation) and/or the interlocking of epidermal papillae (coherence); connation may be derived (they found it in members of the ingoid clade alone), but even there taxa may also have more or less coherent corollas.
Indeed, polysymmetric flowers are scattered throughout much of Faboideae, although less so in the IRLC, and prior to the advent of molecular phylogenies such flowers were often thought of as being "primitive" and so taxa with them tended to be placed together in classifications. It has been suggested that floral development is more canalized in the IRLC clade (e.g. Pennington et al 2000, 2001), althought it is certainly not in other Faboideae like the vataireoids (Cardoso et al. 2013a; Calvacante de Oliveira et al. 2022: pollen). The old Caesalpinoideae have been described as having a "distinct experimental phase" in their floral development (Prenner & Klitgaard 2008: p. 1363), and Duparquetia, the subject of their paper, is just one instance. Variation in number and position of parts is very considerable in what are now Dialioideae, development seeming not to be canalized (e.g. Tucker 1998; Zimmerman et al. 2013) - although this lack of canalization may perhaps be derived (Zimmerman et al 2017). For floral development in Caesalpinia itself, see Tucker et al. (1985). In any event, from this floral heterogeneity, the mimosoid and papilionoid flowers emerged. Of the ca 116 genera of Faboideae in clades below the NPAAA clade most (ca 70%) are small, with 10 or fewer species. Floral morphology there is very diverse, many genera having other than papilionoid flowers (Cardoso et al. 2013a, b, 2015).
Bello et al. (2012) suggest a couple of apomorphies for the family and for clades within it. For the direction of curvature of the young style in Fabaceae, see Prenner and Cardoso (2016). Zimmerman et al. (2017) provide an apomorphy scheme for Dialioideae; see also Falcão et al. 2020), and for possible subfamilial apomorphies. Poeppigia, sister to the rest of the subfamily, has a number of plesiomorphic features including fairly ordinary flowers, as do both genera in Baudouinieae (see Falcão & Mansano 2021), hence many features that are common or distinctive in the subfamily are in Dialieae and are derived (Zimmerman et al. 2017, see also above); little is known about the [Eligmocarpus + Baudouinia] clade, the next branch up from Poeppigia (Falcão et al. 2020: Table 1).
Much work details apomorphies for individual clades in Faboideae, but we await a general synthesis, and this probably depends on nuclear phylogenies - and they have begun. Schutte and van Wyk (1998b) provide an apomorphy scheme for clades around Faboideae-Hypocalypteae, while root nodule morphology may also help to delimit groups of genera in Faboideae (e.g. Lavin et al. 2001; Wojciechowski 2003; Doyle 2011; Sprent et al. 1989, 2013); nodule morphology is independent of the particular bacterium involved and is controlled by the plant (Angus et al. 2013; Agapakis et al. 2014). Wojciechowski et al. (2003, 2004, see also Bell 1971; Wojciechowski 2003) note that the distribution of some non-protein amino acids are systematically interesting in Faboideae. Dormer (1946b) offers a number of suggestions for vegetative features characterizing clades here. Silvério Pena Bento et al. (2020) optimised a number of vegetative/anatomical features on the phylogeny of Amburaneae, idioblasts in the midrib perhaps being an apomorphy for the tribe, while Carvalho et al. (2023b) did the same for some floral features. Epidermal micromorphological differentation associated with corolla differentiation (standard, wings, keel) and sculpting of the wings are perhaps features to be associated with the 50 kb inversion node (see also Chemistry, Morphology, etc. below).
Finally, I finish this section by thinking about Inga (Mimoseae) and herbivory, a rather complex story. Herbivory by foliovorous insects is often quite marked in Fabaceae, despite the diversity of their defensive secondary metabolites; herbivores in general prefer to feed on N-rich plants, which Fabaceae certainly are (Simonsen & Stinchcombe 2014 and references). Cassia fistula is sometimes almost defoliated by caterpillars of pierid butterflies (pers. obs.), while up to one third or more of the developing foliage of some species of Inga may be eaten by herbivores (Kursar et al. 2009; Coley et al. 2019). Plant-insect interactions in Inga, with some 350 species, have been much studied over the last few years. Inga has diversified in the New World l.t.r.f. only within the last 10-2 Ma (Richardson et al. 2001b; Pennington et al. 2009; see aso Nicholls et al. 2015). Up to 43 species of Inga may coexist at a single site, perhaps in part because species, even sister species, may differ considerably in antiherbivore defences (see also Kursar et al. 2009; Nicholls et al. 2015; especially Endara et al. 2021, 2022, 2023 and Forrister et al. 2022). These defences can represent a major investment by the plant, perhaps 37±11% (of total dry weight) of expanding leaves, and they consist mainly of soluble phenolics and saponins in particular; overall, some 9-10,000 different secondary metabolites have been found in about 100 species of Inga, any one species having 194±103 such metabolites (Endara et al. 2022; Forrister et al. 2022). These metabolites are almost twice as abundant in young than in mature leaves - herbivores consume most leaf area in the young leaves (Wiggins et al. 2016; Endara et al. 2022). Characters like delayed greening of the leaves during growth, etc., can also be important in this context (Lokvam & Kursar 2005; Kursar et al. 2009; Endara et al. 2015; see also Richardson et al. 2001b; Pennington et al. 2009; Sedio et al. 2017), and here secondary metabolites as defences are less prominent. Indeed, there are two main modes of defence here. Inga umbellifera has the "escape" strategy - there is rapid leaf expansion, simultaneous flushing of the leaves (which are low in nutrients; it does have some rather odd secondary metabolites), very rapid leaf expansion and delayed greening - and the plant is less effectively defended against herbivores in terms of the variety of herbivores found on it, and these herbivores were generalists. On the other hand, taxa like I. goldmanii have a "defence" strategy - extrafloral nectaries attract ants, there are foliar flavanoids, etc.; this latter type of plant is more effectively defended, and this despite the more protracted juvenile stage of its leaves. Thus lepidopteran larvae grew less well when fed a diet containing crude extracts of the plant and there was lower herbivore diversity, the herbivores being more specialists. Overall, there is rather more extensive herbivory in the taxa wih the escape strategy during leaf expansion, brief though the vulnerable period may be. Leaves in general once expanded are tougher, contain distasteful metabolites and are better defended (Kursar & Coley 1991, 1993; Coley et al. 2005; etc.). Forrister et al. (2022) note that closely related species have divergent chemical profiles, and this is even more pronounced in sympatric sister species than when the species are parapatric; there is little phylogenetic signal in secondary chemistry, the herbivores eating plants that have similar defences, and these are unlikely to be closely related (Endara et al. 2022). Indeed, Endara et al. (2021) emphasized that it was chemistry, not phylogeny per se of the plant that drove diversity of herbivores and associated plants and structured the local community. Specialist herbivores stopped individual species of Inga from becoming abundant, hence rainforest diversity; closely related species of Inga in the one locality differed in their chemistry, and this was true across Amazonia (and patterns in Panama were similar). Inga formed a giant metacommunity, dispersing across the Amazon; things like flower, fruit and seed morphology were relatively invariant (Endara et al. 2021), overall, Inga was an example of diversification despite floral uniformity (see Vasconcelos et al. 2018). Each species of Inga maximized its phytochemical diversity by producing structurally unrelated compounds, probably by regulating gene expression; evolution here is not stepwise, although that had previously been the assumption (Forrister et al. 2022). Thus comparing the phylogeny, chemical profile, etc., of Inga with the phylogenies of three of its major lepidopteran herbivores, caterpillars of gelechioid leaf miners, erebid noctuid moths, and riodinid butterflies, it seems that simple coevolution has not been involved, rather, the defences of Inga are evolutionarily labile and responded quickly to the attentions of the particular herbivores, the latter tracking/living on those Inga that they could - another way of putting it is that they were preapted to some species (Endara et al. 2017, 2023). Indeed, this lack of a strong correlation between herbivore phylogeny and the nature of secondary compounds is quite common (Kariñho-Betancourt et al. 2015; Endara et al. 2023, but c.f. swallowtail butterflies, etc..) Despite very different defences, the overall level of herbivory might be similar (Lokvam & Kursar 2005). As mentioned, with older leaves, their toughness may be their major defence - for instance, the large amounts of tyrosine in some young leaves discussed below had effectively vanished in older leaves (Coley et al. 2019). However, older leaves may also contain a greater variety of defensive metabolites, and the amount of these metabolites may show quite considerable infraspecific variation; since the leaves are on the plant for a relatively long time, the overall variety of herbivore-plant interactions could be quite large (Wiggins et al. 2016). The general result is that a diversity of niches are evident in just a few species of plants; such niche packing is an important stimulus in herbivore diversification, speciation too, but perhaps less so (see especially Albrecht et al. 2023, also Forrister et al. 2015; Forrister et al. 2022). Coley et al. (2019) looked at a clade of Inga in which overexpression of a primary metabolite, the amino acid tyrosine (there were sometimes also tyrosine-derived secondary metabolites), which here could be up to 20% of the dry weight of young leaves, was a feeding deterrent to generalist herbivores. Of the specialists, riodinid caterpillars preferred hosts which overexpressed tyrosine itself; these amino acids are important for the caterpillar in its association with ants (see e.g. Pierce 1985; Pierce et al. 2002; Pellissier et al. 2012), noctuids (macromoths) preferred hosts with the tyrosine-derved secondary metabolites, while gelechioids (micromoths) tended to avoid both (Coley et al. 2019, see also Endara et al. 2022). Ants are attracted to extrafloral nectaries on the trees on which riodinid caterpillars feed, and the dorsal glands of some of these riodinid caterpillars secrete nitrogen- and sugar-rich exudates that they ultimately obtain from the plant, and ants both consume these exudates and protect the larvae - both ants and caterpillars were to be found on young, rapidly-expanding leaves, high in nitrogen - and ants might also deter other herbivores (e.g. Fiedler 2006; Pellissier et al. 2012; Coley et al. 2019; Endara et al. 2022). Endara et al. (2018) did invoke a form of co-evolution to explain the relationships between sawflies (Argidae, genus/genera unclear) and the species of Inga on which their larvae fed. Sawfly larvae found saponins distasteful, preferring plants with amine-type (tyrosine-derived) metabolites, and their preferences largely determined the Inga species that they ate; most host shifts were between species with similar chemistry, whether or not they were related to the original host. Beyond that, speciation in these sawflies was largely allopatric. The sawflies were aged at (7.9-)6.3(-4.8) Ma, and if younger than their hosts then resource tracking could be an explanation for their radiation; interestingly, they did not eat basal Inga species (Endara et al. 2018). Any effect of nodulation, which may be absent in Inga in some species/situations, is unclear (Coley et al. 2019). Dexter et al. (2010) and Nicholls et al. (2015) discuss species limits/infraspecific variation in Inga.
Endara et al. (2021, 2023) and others have linked Inga-type systems to the generation of diversity in the tropics in general, and to the latitudinal gradient of diversity that is so obvious now. They noted that herbivores in tropical areas tended to be specialised and lived on plants with similar chemical profiles - which might not be closely related. Species of plants living together often had different defences, herbivores preventing individual species from becoming abundant; chemical rarity was advantageous to the plant, which overall helped drive plant diversity, and abiotic niche variation was less important. Insect herbivores showed resource tracking. Elsewhere species of plants in boreal forests, for example, tended to be chemically similar, and mammalian herbivores there were generalists, and they also ate chemically rare species, so there was no advantage in being distinctive chemically since it offered less protection (Endara et al. 2023). For other similar tropical systems, see Eugenia, Ocotea, Passiflora, Piper, Protium and Bursera, Psychotria, and sundry Solanaceae - the discussion about Inga here is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
Ecology & Physiology.
Introductory.
Nitrogen Fixation, Nitrogen Metabolism and the Nitrogen Cycle.
Ectomycorrhizae (ECM).
Lianes and Vines.
Other.
Fabaceae grow in closed lowland tropical rainforest, but they are also often members of more open vegetation, including early successional communities, in both tropical and temperate regions of the world, and they include more tree species (= single stem >2 m tall, of if >2 stems, one erect stem >5 cm d.b.h.), ca 5,400, than any other family (Beech et al. 2017), for what that is worth. They often dominate in tropical or subtropical (maximum annual temperature >35o C) deciduous arid and semi-arid woody vegetation types, and these often have alkaline soils (e.g. Rundel 1989; Lewis et al. 2005; Schrire et al. 2005; M. Adams et al. 2016; Steidinger et al. 2019). Thus the Caesalpinia group in particular, robinioid legumes (Pennington et al. 2009), etc., often "spiny" plants of one sort or another, make up an important component of the seasonally dry Succulent Biome in America and Africa-Arabia - other major groups here include Bursera and co., Cactaceae, Didiereaceae, some clades of Euphorbia, etc. (Gagnon et al. 2018 and references). The Fabaceae that grow there are deciduous, but relatively small amounts of precipitation trigger leaf break (Oliveira-Filho et al. 2013; Gagnon et al. 2018). There has also been much diversification within a number of geographically-restricted clades of Indigofereae that grow in succulent biomes (Schrire et al. 2009). Around 203 species of Mimosa are found in the Brazilian cerrado (de Mendonça et al. 2008), eleven separate clades having moved into this vegetation (Simon et al. 2009; see also Borges et al. 2022b). Similarly, about 1/3 the species of Calliandra (46/142) are to be found in campos rupestres vegetation, especially in the Chapada Diamantina mountain range (E. R. de Souza et al. 2013). In the South African Cape Fynbos and associated vegetation there are some 250 species of Aspalathus alone and in the West Australian kwongan over 135 species of Acacia (Cowling et al. 2000). Australia is unusual in that woody Fabaceae, especially Acacia, are to be found in nearly all vegetation types, at least for part of the succession (Orians & Milewski 2007); vestured pits are common in Acacia and other plants growing in drier conditions (Carlquist 2017b).
In Africa "acacia", i.e. species of Senegalia (for the widespread S. senegal, see Bakhoum et al. 2018), also Vachellia, form a major component of the ca 2.5 x 106 km2 of microphyllous dry forests on relatively richer soils (Kalahari, E. Africa, the latter forests also with Commiphora), and also in the drier Sudanian woodlands to the north (Timberlake et al. 2010). African savannas are physiognomically distinctive because Senegalia and Vachellia trees, although not very tall, have very broad crowns, almost the shape of an open umbrella (Troll's model), and so this savanna looks different from those on other continents (Moncrieff et al. 2014). In eastern Africa there is a strong correlation between the diversity of "acacia" and the mammals that browse it, and mammalian diversity is thought to drive the diversity patterns of the plants (Greve et al. 2012). Jacobs (2004) noted that Fabaceae (= caesalpinioids) were a dominant family in both forest and savanna vegetation. Various Detarioideae in particular dominate the ca 2.6 x 106 km2 of Miombo woodland (Timberlake et al. 2010; see also below), indeed, ca 43% of all Detarioideae occur in Africa and Madagascar (some Faboideae clades are also quite diverse there), Detarioideae perhaps originating in Africa or in South America (de la Estrella et al. 2017).
Perhaps 16% of all woody species in Neotropical l.t.r.f. are members of Fabaceae, especially Caesalpinioideae and Detarioideae (Burnham & Johnson 2004), which together made up the old caesalpinioid legumes, and they are prominent in low diversity forests on poor soils in the tropics (e.g. Maisels & Gautier-Hion 1994 and references). Fabaceae are notably relatively more common in Guiana than in western Amazonia - to 49 vs 10% of all trees (ter Steege et al. 2006); they are #1 in individuals, Sapotaceae are next, and then Lecythidaceae. They are overwhelmingly the most common family in Amazonian forests in terms of numbers of species and individuals with stems 10 cm or more across, although they do not have a proportionally high number of locally abundant species (Gentry 1988; Hedin et al. 2009; Table 1; ter Steege et al. 2013; see also Cardoso et al. 2017; c.f. in part Levis et al. 2017; Maezumi et al. 2018); those species which do have high abundance values tend to be ECM plants (see below). They make up 1/4 of the 20 species with most above-ground woody biomass (5 species, 2 known to be ECM, = 4.96% of the total biomass), and 6/top 20 species ranked by productivity (Fauset et al. 2015); most of these are probably AM plants (Béreau & Garbaye 1994). Interestingly, Dinizia excelsa (Caesalpinioideae) is only #931 in abundance, but #25 in above-ground biomass (Fauset et al. 2015) - it is a huge emergent.
When did all this happen? Epihov et al. (2017) link the rise of tropical forests rich in N-fixing legumes in the Palaeocene-Eocene 58-42 Ma to a genome duplication that occurred 58-42 Ma, perhaps at the NPAAA node (q.v.). This duplication facilitated the evolution of nodulation in Faboideae, hence N enrichment of the soil, stimulation of community-level primary productivity (particularly because atmospheric CO2 concentrations were quite high, especially around the Palaeocene-Eocene Thermal Maximum), increase in respiration of microbes in the soil, decrease in soil pH, and ultimately to increased silicate weathering - and sequestration of C. It has also been suggested that this nodulation may involve the coöption of genes from this duplication event (see the NPAAA clade: op den Camp et al. 2011; J. J. Doyle 2011; Q.-G. Li et al. 2013). However, N-fixing clades such as Chamaecrista (n = 8) lack this duplication, although they may have another (Cannon et al. 2010, 2014), and Caesalpinioideae-Mimoseae are a major N-fixing clade that has to be taken into account in the evolution of nodulation. Overall any simple causal connection between genome duplications and nodulation is unclear (Cannon et al. 2014). Although nothing is mentioned about mycorrhizae, the Detarioideae-Amherstieae found in the African Eocene 46-34 Ma include Brachystegia (ECM) and Cynometra (AM), and they seem to be dominants even then - Epihov et al. (2017) should be consulted for further details. Herendeen and Jacobs (2000) found both Detarioideae-Amherstieae (Cynometra, Aphanocalyx) and Caesalpinioideae-Mimoseae (Acacia s.l.) in deposits ca 45.8 Ma from East Africa.
When and where the associations between Fabaceae and their N-fixing bacteria developed is unclear (but see J. J. Doyle 2011: N-fixation arose 6-7 times; Sprent et al. 2013). The first association presumably occurred some time after the Late Cretaceous crown-group age of the family, so ca 65 Ma is the very earliest. However, given current ideas that N fixation involving Frankia and rhizobia evolved just once, the ancestral legume may have fixed N, probably in nodules with fixation threads, but N fixation was subsequently frequently lost (de Faria et al. 2022). N fixation is known from Caesalpinioideae (esp. Mimoseae), Detarioideae, Dialoideae and Faboideae. Werner et al. (2015) discuss "symbiotic persistence" in legumes, in which taxa remain able to form symbioses over evolutionary time, even if the association has remained facultative; in the regular fixing state there is a low possibility of loss, but in the stable fixing state loss is very infrequent. In temperate-polar conditions, areas with lower quality soils, symbioses tend to be stable; the concentration of N and P in the leaf is positively correlated with stable associations. Interestingly, Mimoseae, despite their symbiosomes (see below) show regular fixation (Werner et al. 2015: 5/7 examples in Fig. 1, see also Fig. 2).
In Caesalpinioideae, at least, nodules with fixation threads were the original condition; symbiosomes are found pretty much throughout Mimoseae, but are uncommon in more basal Caesalpinioideae. The evolution of symbiosomes - one or a few bacterioids are enclosed by a host-derived membrane probably coming from the plasmalemma - in Mimoseae is dated to 46.5-40.7 Ma (de Faria et al. 2022); Coba de la Peña et al. (2018) discuss the morphological changes involved in the evolution of the symbiosome, and these authors suggest that is it becoming some kind of organelle (c.f. mitochondria, chloroplasts)... Details of the evolution of nodulation types in Faboideae, the other major nodulating group, are less clear, but symbiosomes are very common there, too (some taxa do have fixation threads), and in the Inverted Repeat Loss Clade the bacterioids may be enlarged (endoreduplication), but cannot divide (Casaes et al. 2024). Indeed, the evolution of symbiosomes may be associated with the great diversification of Fabaceae most noticeable in Mimoseae and a subset of Faboideae (de Faria et al. 2022). In Chamaecrista symbiosomes are more likely to be found in species that grow in open habitats, while section Apoucouita, species of which are found in woody habitats, has infection threads (Casaes et al. 2024). Interestingly, although Chamaecrista with infection threads may be associated with the bacterium Paraburkholderia and those with symbiosomes with a diversity of Bradyrhizobium, in general, nodule type and bacterial species are not associated (Casaes et al. 2024). The evolution of the symbiosome seems to have improved the evolutionary stability of nitrogen fixation in legumes, to paraphrase the title of the paper by de Faria et al. (2022).
A number of Faboideae and Caesalpinioideae-Mimoseae have some kind of underground perennating structures and can tolerate fires (Lamont et al. 2018b). Fire had little effect on physical dormancy of seeds of Cerrado species (Daibes et al. 2019); Detarioideae, with the largest seeds, were least affected by heat. In general Fabaceae are fire-prone but have heat-stimulated germination of their hard seeds (Lamont et al. 2018b).
Nitrogen Fixation, Nitrogen Metabolism and the Nitrogen Cycle.
The dominance of Fabaceae in certain habitats takes on more importance when one factors in the ability of many members of the family to fix N in their nodules; all members of around 76% of genera whose N-fixing proclivities are known fix N, somewhere around 100-290 x 106 tons of N being fixed annually (Afkhami et al. 2017). Legumes have a distinctive N metabolism (see M. Adams et al. 2016 and references), the family as a whole being noted for its high foliar N concentrations (e.g. H. Xu et al. 2018) and a high rate of photosynthesis (the enzymes involved in N fixation themselves need N), and although herbaceous groups in general tend to have higher N, Fabaceae are the only woody group with this high N strategy (McKey 1994). A N-rich economy is perhaps particularly noticeable in Faboideae (Waterman 1994). There is a substantial increase in leaf N at the crown-group Fabaceae node, along with a preference for less seasonality in precipitation (Cornwell et al. 2014). Epihov et al. (2017) found that N-fixing legumes had higher foliar N concentrations than non-fixing legumes, which in turn had higher foliar N than other angiosperms that did not fix N. High foliar N has also been linked to increased water use efficiency in woody N-fixing plants, and also to plant defence (it is involved in the synthesis of alkaloids, non-protein amino acids, etc. - Waterman 1994), rather than increasing levels of photosynthesis (see also Wink 2013; M. Adams et al. 2016). However, in the seasonally dry Succulent Biome and similar habitats favoured by the family the rapid growth of N-rich leaves is soon repaid because of the high rate of photosynthesis of those leaves, indeed, legume leaves tend to be short lived (McKey 1998). N moves around the plant during growth, and extrapolating from agricultural legumes, it is likely to be remobilized from the leaves, ending up in large quantities in the seed. High N in the seed may i.a. allow for rapid initial growth and the early development of photosynthesizing leaves (McKey 1994), and N is also a component of the defensive compounds in those seeds (Waterman 1994).
Non-protein amino acids (NPAAs) are common in many Fabaceae (see e.g. Bell 1971; Fowden et al. 1979), and N in the xylem sap is transported as a mixture of amino acids, amides, and sometimes also ureides; very little is transported as nitrate. L-canavanine, which can be taken up in place of the normal amino acid L-arginine, may have potent effects on herbivores, although some can detoxify it (e.g. Rosenthal 1990, 2001 and references; Kergoat et al. 2005b; Huang et al. 2011: NPAAs in general). The NPAA L-canaline is rather like the amino acid ornithine; both L-canaline and L-canavanine serve as N reserves for the plant. A number of Fabaceae have cyanogenic glucosides. Here plant-insect interactions have been studied in detail, for instance, those between the cyanogenic host, Lotus corniculatus, and caterpillars of the burnett moth, Zygaena filipendulae; the latter can also synthesize the cyanogenic compounds itself (Zagrobelny et al. 2008; Møller 2010; Zagrobelny & Møller 2011: other similar systems). The genes involved in cyanogenic glucoside synthesis are clustered, and although cyanogenic glucosides are perhaps an apomorphy of Galegodae/the Hologalegina clade, where they may offer protection against small, generalist herbivores at mid latitudes and elevations, they are rather uncommon, the cluster having frequently been lost or replaced by other forms of defence (Takos et al. 2011; Olsen & Small 2018; see van Velzen et al. 2018 for a similar pattern in N-fixing plants). Jensen et al. (2011) found that the pathways by which the glucosides linamarin and lotaustralin are synthesized in plant and insect use the same biochemical intermediates - a nice example of convergent evolution. Alkaloid glucosides are known, e.g. from Vicia, and Pentzold et al. (2014) discuss ways insects have of getting around such defences. Interestingly, production of the NPAA canavanine and alkaloids is mutually exclusive, while within Genistodae quinolizidine and pyrrolizidine alkaloids are similarly mutually exclusive (Wink 2008). For the toxic indolizidine alkaloid, swainsonine, found in a few IRLC members, see below under plant-fungal relationships.
Associations with a diversity of N-fixing bacteria are very common in Fabaceae, the bacteria living in the nodules (see also Bacterial/Fungal Associations below). Estimates of biological N fixation in terrestrial environments, much of which is by bacteria associated with Fabaceae, are 90% of the total of 100-140 Tg [= 100-140 x 1012 g) N fixed per annum (Gage 2004 and references: humans have doubled this). Substantial amounts of N can be fixed as NH3 and moved to the plant, and then to the community when the legume dies or is eaten (e.g. Batterman et al. 2013: Panama). However, the role of legumes in the N cycle of tropical forests is not simple. Non-nodulating species are proportionally less common in the humid tropics (Simonsen et al. 2017: fig. 3A). N fixation is more or less facultative in that the amount of N that a plant fixes can change (both the fixation rate and nodulation) depending on N and P availability in the soil, light, temperature and water conditions (e.g. Taylor & Komatsu 2024). The identity of the plant is also important, thus Wurzburger and Hedin (2015) found that 44% of Fabaceae in tropical forests did not fix N, two species were superfixers. Even grazing may affect N fixation (Batterman et al. 2013; Menge et al. 2014; Dovrat et al. 2018).
Overall, N-fixing trees are commonest in the tropics, and within the tropics, notably more common in the American rather than the Asian tropics (Menge et al. 2019: Africa not included; Menge 2017a). This difference held however it was measured, and especially north of 35o in North America there are very few N-fixing trees (see also Menge et al. 2017b). There are other legumes - particularly frequent in Faboideae and often herbaceous plants - that are stable N fixers in which bacterial associations have persisted over evolutionary time, and these are commonest in areas with cooler temperatures, whether cool because of altitude or latitude, and are the commonest legumes in these conditions (Werner et al. 2015; see also Menge & Crews 2016 for latitudinal patterns in N fixation). Note, however, it is not always clear what species habitually nodulate, and this is discussed further under Bacterial/Fungal Associations below. Interestingly, free-living bacteria closely related to N-fixing rhizobia are very common in northern conifer-dominated forests in North America (VanInsberghe et al. 2015; for further details, see below).
The relationship between N fixation and forest successional stage and growth and diversity is complex. Thus in some old, relatively nutrient-rich forests, N fixation by legumes (in this case, species of Inga) was lower when compared with species growing in seasonally flooded forests and in light gaps (Barron et al. 2011; Hedren et al. 2009 for the "leaky nitrostat" model). Other species of Fabaceae also have low fixation rates in mature forests (Högberg 1986; Barron et al. 2011 and literature; Sprent et al. 2013 for a summary; B. N. Taylor et al. 2019). However, species like Tachigali versicolor continue to fix N even in 300 y.o. forests, being something of a gap specialist (Batterman et al. 2013). Winbourne et al. (2025) found that Inga was a major fixer of N (fixing around 40-83% of the N) in the secondary Atlantic forests of Brazil, while in mature forests the liane genera Macropsycanthus and Dioclea (both Faboideae-Diocleeae) fixed maybe 76% of the N, although fixation by Inga was still appreciable, around 15%. In Costa Rican secondary forests legumes grew more quickly, died more slowly, and recruited more poorly (?seed size) than plants that did not fix N (Menge & Chazdon 2015). In Costa Rican forests the abundance of N-fixing legumes was associated with reduced overall forest growth, the legumes outcompeting other trees and not promoting biomass increase because of their presence, although this effect was not found in all studies (B. N. Taylor et al. 2017), their basal area might be relatively higher in older forests, but in this latter case it was rather asymbiotic N fixation that increased (Taylor et al. 2019). Along similar lines, H. Xu et al. (2018) found that legumes in forests on Hainan growing on richer soils fixed more N and were associated with a more diverse local community, while legumes that fixed less N grew on poorer soils and local diversity there was less, perhaps suppressed by competition. Interestingly, nodulation in Amazonian Fabaceae is inversely correlated with their dominance there, and so is proportionally less in the poorer soils of the Guianan Amazon where Fabaceae are abundant (ter Steege et al. 2006); McKey (1994) had earlier noted that non-nodulators may grow in N-poor communities. The effectiveness of nodulation is often reduced in more acid soils, although Lupinus and Mimosa in Brazil, at least, may be exceptions (Lin et al. 2012). N-fixing tropical forest legumes may have a competitive advantage over non-nodulators as atmospheric CO2 concentration increases (Cernusak et al. 2011), which might affect how one thinks of the history of the family. See also Terpolilli et al. (2011) for the efficiency of N fixation.
Interactions between legumes and their N-fixing bacteria go far beyond any simple association of the two and resultant fixation of N. Nodules in Medicago can be formed both by N-fizing or non-N-fixing ("exploitative") rhizobia, and under conditions of no herbivory plants with mixture of exploitative and non-exploitative rhizobia grew more poorly than plants with only N-fixing rhizobia, but the two performed equally poorly if there was herbivory (Simonsen & Stinchcombe 2014). Taylor & Komatsu (2024) looked at N fixation in Ulex and Spartina, both Genisteae, and found i.a. that the identity of the plant and the identity and diversity of the N-fixing bacteria affected N-fixation, as did N concentration, but here higher N might lead to higher N fixation (c.f. comments elsewhere on this page). Furthermore, phosphorous acquisition is also important, and Marcellus et al. (2024) found that root phosphatase activity increased four times per one unit increase of N fixation.
There are also interactions with endomycorrhizal (AM) fungi, with complex interactions at the level of gene expression (Afkhami & Stinchcombe 2016). In Acacia mangium nodulation and leaf N are increased if the plants are ECM (Diagne et al. 2013), while in AM Fabaceae phosphorus (P) uptake by the fungus may affect N fixation (Walker et al. 2013 and references), for instance enabling growth of nodulated seedlings in the nutritionally harsh conditions of sand dunes (van der Heijden et al. 2015b).
Recent work on successional legumes in tropical forests in Panama suggests additional dimensions to the relationships between N-fixing legumes and the environment. There Epihov et al. (2021) found that primary silicate weathering doubled under the legumes, the soil pH became acid because of the reduction of Fe--- by Acidobacteria, a common bacterium being Candidatus Acidoferrum, placed in an order by itself. Ultimately this weathering led to the release of inorganic P from very insoluble ferric-P compounds and ultimately the enhancement of plant growth and C sequestration - not only of the legumes, but also that of other nearby trees. Epihov et al. (2021: p. 9) emphasize the "central role of fast-growing N2-fixing legume trees and their soil microbiomes in tropical forest nutrient cycling".
There may also be a trade-off between extrafloral nectaries and N fixation. Godschalx et al. (2015) found that N-fixing rhizobia in Phaseolus lunatus reduced the amount of nectar produced in these nectaries and hence the attractiveness of the plant to ants. Indeed, N fixation utilises much of the host's photosynthesate which otherwise could be used to produce nectar, but the N fixed was used in the production of another form of defence, cyanogenic compounds (Godschalx et al. 2015; Olsen & Small 2018). However, Marazzi et al. (2019) note that the trade-off is unclear. Finally, synthesis of pyrrolizidine alkaloids in Crotalaria occurred as a result of the reprogramming of the plant genome that occurs during nodulation (Benedito et al. 2008: ??correct reference); if N levels were high, there was no nodulation and no alkaloid production (Irmer et al. 2015). However, details of such interactions between plant and bacterium are poorly known.
Mostly Ectomycorrhizae (ECM), but also Arbuscular Mycorrhizae (AM).
The ECM habit has evolved at least four times in the family - [Mirbelieae + Bossiaeeae], Aldina, both in Faboideae (what about Gleditsia?), some Acacia (Caesalpinioideae-Mimoseae) and in Detarioideae, especially Amherstieae (M. E. Smith et al. 2011). Some Detarioideae - most records are from the Old World, particularly Africa - that are ECM plants do not fix N (e.g. Onguene & Kuyper 2001; for further details see clade asymmetries). Estimates of the number of Detarioideae involved range from 250 (Brundrett 2009) to 450 (B. Mackinder pers. comm. viii.2012) species. Indeed, Fabaceae are the main group of tropical African ECM plants, and overall the family has most genera (more even than Dipterocarpaceae!) among tropical ECM plants as a whole (Corrales et al. 2018). In Africa, some 36 of the ca 82 genera included in Detarioideae are reported to be at least locally dominant (e.g. Letouzey 1968; Mackinder 2005), and 11 of these genera are in the Berlinia clade of only 16 genera (Amherstieae: see also Wieringa & Gervais 2003); a number of others are characteristically gregarious. Van der Burgt et al. (2020) found 42 species of Detarioideae growing in clusters in the Korup National Park, Cameroon; at least 29 of those species were group-forming, and of these, at least 21 were known to be ectomycorrhizal. Dominance is quite often associated with the ECM habit (e.g. Torti et al. 2001; Corrales et al. 2018), however, some Amherstieae are AM plants (see e.g. Cynometra below), and New World Browneopsis in particular (Amherstieae: mycorrhizal status?) are reported to dominate, especially along streams (Klitgaard 1991). For literature, including that for Acacia and Mirbelieae, see Brundrett (2008, 2017a) and for ages, etc., see Tedersoo and Brundrett (2017) and Tedersoo (2017b).
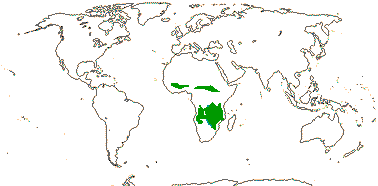
All told, Detarioideae occupy ca 2.8-3.3 M km2 in the Zambezian region alone (estimated from White 1983). In Miombo forests they represent 20-90% of the trees, 30-96% of the basal area, and with biomass estimates in the range of 35-97 Mg ha-1 (Högberg & Piearce 1986; Frost 1996; see also Malimbwi et al. 1994). Genera like Isoberlinia, Julbernardia and Brachystegia (all ECM plants, Amherstieae) are very important components of the widespread deciduous Miombo forests which grow on often rather poor soils over some 2.7 M km2 or more of central Africa; the trees have a very distinctive umbrella shape, and their leaflets are on the small side, so the forests appear quite uniform. They form the largest block - albeit sometimes interrupted - of such forests in the world (B. Campbell et al. 1996), and are the centre of diversity of Brachystegia (e.g. White 1983; Högberg 1990; S. E. Smith & Read 2008; Timberlake et al. 2010; Boom et al. 2024). Brachystegia plastomes, although not reflecting species identity, suggest an overall westwards expansion of miombo, or at least of Brachystegia itself (Boom et al. 2021). Interestingly, Boom et al. (2024) suggested that the age of Zambezian Brachystegia was only some (3.49-)2.95(-2.44) Ma (and the age of the whole genus 3.82-2.69 Ma), the Zambezian group in particular perhaps forming a syngameon. The ages just mentioned are younger than those of many C4 grasses and raise the question of what grew in the Miombo area before Brachystegia, and also how to interpret papers such as those by Epihov et al. (2017), Bianconi et al. (2020), Pan et al. (2023), etc., which suggest much older ages for Brachystegia and its relatives. Thus de la Estrella et al. (2020) noted that there was a marked reduction in area of the Congo rainforest starting ca 10 Ma, Brachystegia splitting from its relatives ca 8 Ma (but c.f. Figs 2, 4, & p. 10). Many other species, both trees and herbs, also grow in these communities, and the trees include other ECM taxa like Monotes (Dipterocarpaceae) and Uapaca (Phyllanthaceae), but also AM taxa like Colophospermum mopane (Detarieae), which often forms pure stands, and Pterocarpus (Dalbergieae) (White 1983; Högberg & Piearce 1986).
Gomes et al. (2021) looked at Miombo woodlands in Angola emphasizing the importance of P uptake in this system (for P uptake, see also Lupinus, N-fixing early successional Fabaceae in Panama, etc., below). The association of detarioid legumes in the woodland with ECM allows them to take up P from organic matter and so maintain high photosynthetic rates. There are also grassier areas where geoxylic plants are to be found, and although geoxylic ECM Fabaceae have lower leaf P than the woodland Fabaceae, leaf P is higher than in nodulating legumes, in turn higher than in non-legumes (Gomes et al. 2021). Of course, not only is atmospheric [CO2] increasing now, but it was (much) higher in the past, and how Miombo woodlands may have responded to higher [CO2] is unclear, given that the woody plants are ECM and the grasses, also common in such woodlands, are AM. Terrer et al. (2021) looked at C sequestration, N uptake, etc., in temperate ECM (woody) and AM systems (grasses, some woody). In AM systems when atmospheric CO2 increased, the above-ground biomass increased only slightly, but soil organic matter increased because root production increased, yet nutrients in the soil organic matter were less accessible to microbial (AM) decomposition and the C:N ratio increased. In ECM plants, on the other hand, above-ground biomass strongly increased, fungi mobilizing nutrients from the soil, the soil C:N ratio decreasing (Terrer et al. 2021). Adding a historical dimension to biomass change and nutrient uptake in Miombo woodlands and similar systems makes life rather complicated. For more on Miombo woodlands, see the papers in Campbell (1994), especially Frost, (1996), also Malimbwi et al. (1994) and Abdallah and Monela (2007).
The AM Colophospermum mopane (Det-Detarieae), an important food for elephants, dominates ca 380,000 km2 of mopane woodland (e.g. Högberg & Piearce 1986; Torti et al. 2001; Mackinder 2005; Newbery et al. 2006), and the AM Pterocarpus (Fab-Dalbergieae) is also to be found there. Such woodlands, along with Miombo forests sensu stricto, form a major part of the Zambezian Region. The ECM Isoberlinia (Detarieae) is a major component of Sudanian Woodland (White 1983) which forms an interrupted band south of the Sahara from Mali to Uganda (see map above: White 1983; map: very approximate, from White 1983). This forest is biogeographically closest to Miombo woodlands among other African vegetation types (Linder et al. 2012). Finally, Baikiaea plurijuga, also Detarieae, along with Acacia s.l., are common on Kalahari sands from Angola-Namibia to Zimbabwe.
In Africa, LTRF can include a substantial element of ECM Fabaceae. Indeed, a "caesalpinioid" Biafran forest subtype has been recognised, and here, of 34 genera recorded, 28 are members of Detarioideae, and 11 of these are described as being characteristically gregarious (Letouzey 1968; see also Bâ et al. 2011a). Microberlinia (Amherstieae) dominates Guineo-Congolian forests in Cameroon, and other Detarioideae dominate parts of the coastal forest from Sierra Leone to western Gabon, and again in the periphery of the Zaire basin (White 1983). Newbery (1997) noted that Detarioideae in Cameroon LTRF grew on poor soils and seemed to be able to control the flow of P through the ecosystem to their own benefit, however, fertilization with P had little effect on their growth (Newbery et al. 2002, see also Nérée & Kuyper 2001: myccorrhizal associations in southern Cameroon). Similarly, Van der Burgt and Eyakwe (2008) give information about a ca 35 km2 "caesalpinioid"-dominated area in the Korup Forest while Chuyong et al. (2002) described the slow leaf decomposition of the ECM Detarioideae there and various aspects of nutrient cycling. Bastin et al. (2015) noted that aside from Gilbertiodendron (see next paragraph), five other genera of Fabaceae were included in the 18 species dominating in the eight Central African rainforest sites examined, Dialium (2.3 % of above-ground biomass, #5), Scorodophloeus (1.7%, #12), Julbernardia (1.4%, #13), Pentaclethra (1.2%, #15) and Erythrophleum (1.0%, #16), scattered in Dialioideae, Caesalpinioideae (inc. Mimoseae) and Detarioideae.
Gilbertiodendron dewevrei (Detarioideae-Amherstieae), whose foliage, seeds, and perhaps even mycorrhizae are major food sources for elephants, etc. (Blake & Fay 1997), alone dominates ca 10,000 ha in the eastern Congo. There it has above-ground biomass of 394-411.1 Mg ha-1, about 74% of the total (Makana et al. 2011); it may occupy 88% of the total basal area in the forests it dominates (Hart et al. 1989). Bastin et al. (2015) found that it represnted 20% of the aboveground biomass in the 8 Central African sites they examined; found in three forests, it was overwhelmingly #1, #2 a mere 3.6%. Torti et al. (2001) discussed the factors that seemed to facilitate dominance of Gilbertiodendron, including the dense shade it cast, deep leaf litter, handling the potentially low availability of N, etc.. Peh et al. (2011a) and Lokonda et al. (2018) found little difference in the soil of Gilbertiodendron forests in Cameroon when compared with that of adjacent forests, the former noting that there might be differences in such forests elsewhere and that ECM fungi might be involved in the dominance relationships of these forests and the latter the much thicker litter layer under Gilbertiodendron than under AM trees. The value of the ECM habit to these tropical legumes is unclear. One study found that although nutrient uptake was increased, there was not a parallel increase in plant growth, however, there are suggestions that ECM facilitate accumulation of nutrients that are later expended in masting events (Corrales et al. 2018). See also below under Miombo woodlands.
Relatively little is known about details of N cycling in Australia. Interestingly, a number of species of Australian Acacia are both EM and AM plants, as are members of Mirbelieae. In addition, some species also have cluster roots, these, too, being involved in nutrient uptake, perhaps especially that of phosphorus, and these are found in plants growing on poor soils (Sprent 1994; Skene 1998).
In the New World, the ECM Aldina (Faboideae: 50 kb inversion clade) and the coppicing Dicymbe (Detarioideae-Amherstieae) dominate forests in the Pakaraima Mountains in the central Guiana Shield region (ter Steege et al. 2006; McGuire 2007b; M. E. Smith et al. 2011). The latter in particular supports a rich community of fungi (M. E. Smith et al. 2011; Henkel et al. 2012) and has a remarkably high basal area of 38.4-52.5 m2 per hectare, around 25(-40) m2 being more normal figures (Henkel 2003). Aldina is also common in Amazonian rainforest, some species favouring white sand habitats (ter Steege et al. 2013; see also Ramos et al. 2015, 2022). The ECM Peltogyne (Detarioideae-Detarieae) is one of the rare monodominants of the northern Amazon, where it occupies ca 53% of the basal area of trees 10 cm or more in d.b.h. on Maraca Island, Roraima, the proportion increasing when the trees are larger (Nascimento & Proctor 1997; Nascimento et al. 1997). The Guianan upland ECM/AM Eperua falcata, along with E. leucantha (mycorrhizae?), also Detarieae, are 50% more abundant (usually far more) than any other non-palm of the 20 most abundant hyperdominant trees (10 cm d.b.h. or more) of the Amazonian rainforest (Peh et al. 2011b; ter Steege et al. 2013; Fauset et al. 2015, see also above; E. falcata perhaps AM - e.g. Béreau & Garbaye 1994), although the interpretation of such hyperdominance is complicated by the effect that pre-Columbian humans had on the vegetation (e.g. Levis et al. 2017; Maezumi et al. 2018).
ECM (or mixed AM/ECM) plants are common in Australian Faboideae-Mirbelieae and -Bossiaeeae, and they are dated to 45-50 Ma (Toon et al. 2015; Zanne et al. 2014: see also Tedersoo & Brundrett 2017; Tedersoo 2017b).
Not all/only monodominant legumes are ECM (Torti & Coley 1999; Torti et al. 1997, 2001; Henkel et al. 2002; Peh et al. 2011b; Corrales et al. 2018: 10 species listed). Legumes like the caesalpinioid AM Mora excelsa (it also harbours endophytes) dominates ca 37,000 hectares in Trinidad (Beard 1946; Hart et al. 1989). Interestingly, as is common in ECM plants, both foliar and litter N contents are low, there is foliar resorbtion of N, soil nitrate concentrations are low, litter decomposes slowly and accumulates - perhaps the roots get the N the plant needs from the litter layer (Brookshire & Thomas 2013). Two other species of AM Mora may also be monodominants, as is the AM (and clonal) Pentaclethra (Mimoseae-Xylia) and Prioria copaifera (Detarioideae-Detarieae), Pentaclethra macroloba representing 16-18% of the above ground biomass in some Costa Rican forests (Torti et al. 1997; Henkel 2003; Peh et al. 2011b; Menge & Chazdon 2015). The Old World Cynometra (Det-Amherstieae) also appears to be AM (e.g. Connell & Lowman 1989; Peh et al. 2011b), and C. alexandri dominates ca 11,000 ha in Uganda (Eggeling 1947) and in some forests in the eastern Congo where it comes close to dominating (ca 31.5% of above-ground biomass) the ECM Julbernardia secretii (Det-Amherstieae: also AM) adds another ca 12% (Makana et al. 2011: the next species, not a legume, adds only 4.8%). Cynometra forests do not accumulate large amounts of litter, but that is also true of the ECM Julbernardia (Torti et al. 2001). Talbotiella gentii (?= Hymenostegia: Det-Amherstieae) forms monodominant stands in dry forests in Ghana, but the soil composition in unremarkable; its seedlings tolerate shade and there is some vegetative reproduction (Swaine & Hall 1981), and although its mycorrhizal status is unknown, that of its immediate relatives is usually AM (Bechem et al. 2014).
Although the relationship between mono- or oligodominance of legumes in tropical forests in particular and the nature of their fungal associations is not simple, phylogeny, monodominance and mycorrhizal type are often combined. Of the nine dominant tropical species listed by Hart et al. (1989), five are caesalpinioids, of which three are ECM Detarioideae (two more are ECM dipterocarps), while all the dominants listed by Connell and Lowman (1989) were legumes. 12/22 of the tropical classical monodominants listed by Peh et al. (2011b: Table 1) are legumes, of which 4/9 whose mycorrhizal status is known are ECM plants (the others are Cynometra, Pentaclethra and three species of Mora). Of the other ecological attributes of these legumes, all had poor dispersal, masting occurred in all but two (two of the three species of Mora), and the seeds were medium-sized to large, being 5-117 g in weight (Peh et al. 2011b). However, the sample size is small. Overall, dominant tropical legumes seem to be characterized by a syndrome of ecological features that includes growing on poor soils, having large seeds with poor dispersal, accumulating litter and being able to access organic N, tolerating shade, etc. (e.g. Alexander 1989b; Hart 1990; Torti et al. 2001; Bâ et al. 2011b). However, Newbery (2005, see Hogberg 1986) noted that mast fruiting had not been recorded from East African forest dominated by caesalpinioids, while Nascimento and Proctor (1997) observed that there was little difference between the soils on which the ECM Peltogyne dominated and those in adjacent more diverse forests. Note that Gentry (1993) thought that the ecological roles of ECM dipterocarps in the Old World and "caesalpinioid" legumes in the New World, particularly on poor soils and under seasonal climates, were similar.
There are a few aspects of AM fungi-legume associations worth mentioning. Legumes tend to accrue less benefit from their AM associations than do other seed plants, and this may be connected with their ability to fix N, since benefits to seed plants decrease overall when N is added; details of the plant-fungus interactions depend on the identity of the AM fungus (Hoeksema et al. 2019: meta-analysis). However, NO3- may increase in AM symbioses in some Fabeae (S. Wang et al. 2020).
Fabaceae are perhaps the most important family of lianes, both ecologically and in terms of number of species, in the New World, with about 720 climbing species recorded from there alone (Gentry 1991: Bignoniaceae and Sapindaceae are the two next most important families); Rhynchosia is one of the ten most species-rich genera of seed-plant climbers (Sperotto et al. 2023). Fabaceae are prominent in drier forest types in both America and Africa (Lewis et al. 2005; Schrire et al. 2005), and lianes also are prominent in such forests (Gentry 1988; Schnitzer 2005); Fabaceae are prominent vines in the deserts of S.W. North America (Krings 2000). Fabaceae quite commonly have positive root pressures, which may reduce the susceptibility of liane stems to irreversible cavitation (Fisher et al. 1997, but c.f. Knipfer et al. 2016). It is noteworthy that lianes in general maintain high hydraulic conductance yet are not compromised by the development of embolisms as would happen in trees growing under comparable conditions (van der Sande et al. 2019), overall, they use water very efficiently (Schnitzer et al. 2019; see also Dias et al. 2019 and references). As a result, lianes grow remarkably well in the dry season/dry conditions and they grow proportionally much more than trees, even in the face of El Niño events (Schnitzer et al. 2019). Lianes are also abundant in forest edges, treefall gaps, and similar habitats where their growth habits will also be advantageous, and they may have negative effects on the growth of co-occurring trees by smothering them (Schnitzer 2018). Leme et al. (2021) compared liane with tree Fabaceae-Faboideae in three tribes, Dalbergieae, Phaseoleae and Diocleeae, and found that species with the former habit have wider (but varying considerably in size) and more numerous vessels that occupied much more of the wood (25% versus 6% in t.s.), more axial and radial parenchyma compared to fibres, taller rays, longer fibres (perhaps increased flexibility), and so on - the lianescent vascular syndrome, found in other plants, and evolving in parallel.
Entada (Caesalpinioideae-Mimoseae) and Bauhinia (= Phanera: Cercidoideae) are lianes that are stem climbers, the former having a twining stem and the latter tendrillate short shoots (Sousa-Baena et al. 2018b), although tendrils in the latter are variously described - “in pairs at leaf axils”, “leaf opposing” “opposite”, “axillary” (Gu et al. 2024). Phanera in particular is noted for having older woody stems that are flattened and undulating, while vessels in the stem of Entada are up to 700 μm across (Ewers et al. 2015). For additional information about leguminous lianes, see Angyalossy et al. (2015), for their anatomy, see Rajput et al. (2012b), Schnitzer et al. (2015), also Carlquist (2013) and Fisher and Blanco (2014), both Bauhinia. For the evolution of rainforest and savanna lianes in the Caesalpinia group, predominantly inhabitants of the Succulent Biome, see Gagnon et al. (2018).
A number of Faboideae, e.g. Vicia and Lathyrus (inc. Pisum), are tendrillar vines, the tendrils being modified leaflets, and in Lathyrus aphaca the whole leaf (bar the enlarged stipules, the major photosynthetic organs) is an unbranched tendril (Sousa-Baena et al. 2018b for a summary). Hofer et al. (2009) discussed the evolution of tendrils in Faboideae.
Fabaceae are the number three family when it comes to species numbers, and they are #2 in terms of the number of angiosperm holoparasites that they host - some 14 genera in four main clades, 63/373 (16.9%) of the total records (Hatt et al. 2024c) - basically, quite close to what one might expect for a clade this size.
Fabaceae make up the largest element in the Neotropical seasonally-dry tropical forest (Pennington et al. 2006b and papers therein; Banda-R et al. 2016).
N-fixing taxa like some species of Lupinus, e.g. L. albus, as well as Stylosanthes, Aspalathus and the mirbellioid Viminaria, may also form cluster/proteoid roots, and these can vary in morphology. In at least some cases they facilitate P uptake in P-poor or P-unavailable soils (e.g. Dinkelaker et al. 1995; Shane et al. 2004b; Lambers et al. 2006, 2012b, 2015c; Epihov et al. 2021), developing when foliar P concentrations are low (X. Wang et al. 2015). Both L. albus and some other species of the genus that cannot form cluster roots cannot form mycorrhizal associations (Delaux et al. 2014), hence the need for alternative means of P uptake. The overall appearance of cluster roots here is rather regular and dauciform, but this carrot-like shape is made by the dense, widely-spreading lateral roots themselves (they do not branch), not by root hairs as in dauciform roots proper (Watt & Evans 1999; Shane & Lambers 2005; Shane et al. 2006). Species of Lupinus with such roots (especially common in the Old World), and some with ordinary roots (from the New World) release massive amounts of carboxylates (organic anions) such as citrate into the soil, the cumulative amounts being up to 23% of the dry mass of the adult plant, and these can replace organic or inorganic P on soil particles and so mobilize it (Skene 1998; Lambers et al. 2013 and references), indeed, carboxylate production and P uptake are associated in both Faboideae and Caesalpinioideae-Mimoseae (Teste & Laliberté 2018 and references). In L. albus both cluster root and nodule formation are negatively controlled by the constitutive cluster root 1 gene (Marquès et al. 2025). Cluster root formation in L. albus in particular has been much studied. The soil-borne proteobacterium Klebsiella pneumoniae has been found to promote the formation of cluster roots by reducing ethylene production (Q. Zhang et al. 2022). Carboxylates like malate (especially in young nodules) and citrate (in older nodules) are produced and they make the soil acid, the phosphate then becoming solubilized; the plant produces phenolic isoflavonoids, protecting these carboxylates, and also antifungal glucanases and chitinases active in the immature cluster root stage (Weisskopf et al. 2006). Weisskopf et al. (2011) found that pseudomonad Burkholderia spp. were very common in the cluster roots, although overall the abundance and diversity of bacteria had decreased. Interestingly, these Burkholderia could metabolize oxalates, unlike pathogenic members of the group. The bottom line is that since Lupinus can also fix N, it can be an aggressive pioneer on volcanic and other skeletal and nutrient-poor soils (Lambers et al. 2013).
There are about 120 species of annuals in Astragalus, a habit that has evolved up to ca 8 times in the Old World taxa alone, the plants tending to grow in drier or even xeric areas, also in disturbed vegetation; the annual habit has also evolved independently in both North and South America (Azani et al. 2017, 2019; C. Su et al. 2020). (In Trifolium, too, the annual habit has evolved several times - Ellison et al. 2006.) About 27 species of Astragalus in North America are hyperaccumulators of selenium (Se), the sulphur-antagonist (El Mehdawi et al. 2012; Schiavon & Pilon-Smits 2016), which is stored as γ-glutamyl-methyselenocysteine. This may be associated with nodulation and also help protect the plant from herbivores (Alford et al. 2014), but Se does not seem to affect the drought tolerance of the plant (Statwick et al. 2017); Reynolds et al. (2020) discuss the effects of selenium (Se)-accumulating plants on vegetation. Endophytic bacteria are involved in Se uptake, and Se can be up to ca 1.5% dry weight, 30% elemental Se concentration (Lindblom et al. 2013; Sura-deJong et al. 2015); see also Steven and Culver (2019) for different levels of Se accumulation reflecting different kinds of relationships between herbivores and plants. Se accumulation seems to have evolved more than once here, and Astragalus includes the largest complex of Se accumulators in seed plants (White 2016); some other Fabaceae, including Neptunia amplexicaulis (Caesalpinioideae-Mimoseae), also accumulate large amounts of Se. For Se tolerance, see also Stanleya, etc. (Brassicaceae: Ecology and Physiology).
Knoblauch et al. (2001) discuss the function of the distinctive spindle-shaped non-dispersive protein bodies, forisomes, that are common in the sieve tubes of Faboideae (e.g. Behnke 1981b; Behnke & Pop 1981; Peters et al. 2010; Müller et al. 2014). The forisomes can block the pores of the sieve plates when turgor pressure changes, and they can change their shape and volume very quickly depending on the concentration of Ca2+ ions; ATP is not needed for this shape change (Peters et al. 2007, 2008, 2010). Within Faboideae, forisomes are absent from a number of Galegeae, many members of which - including those species of Astragalus studied - also lack calcium oxalate crystals (Peters et al. 2010).
Pollination Biology & Seed Dispersal.
Pollination Biology.
For a general account of pollinatiom, see Kalin Arroyo (1981) and for the evolution of the evolutionary history of the leguminous flower, see Sinjushin (2021a). Although one often thinks of the monosymmetric pea-type or papilionoid keel flower and its variants as characterising the whole family except Mimoseae, this very much underestimates the great variation in floral morphology in many of the clades that make up the old Caesalpinioideae (now included in basal Caesalpinioideae s. str., Cercidoideae, Detarioideae and Dialoideae in particular) and in the basal clades in particular of Faboideae. As Bruneau et al. (2005: p. 201) noted of caesalpinioid legumes, "zygomorphy is expressed as a multitude of homoplasious morphs". Detarioideae in particular show much floral variation, with the complete loss of organs or whorls of organs often being associated with major dislocations of developmental patterns, or there may be fusion of parts, as in several species that apparently have only four sepals (Tucker 2000a, b; Bruneau et al. 2014; Ojeda et al. 2019), while corolla development in the speciose Inga (Caesalpinioideae-Mimoseae) is notably labile, the median petal being adaxial or abaxial depending on the species (Paulino et al. 2017). Within Faboideae (= Papilionoideae), keel flowers are found mainly in the 50kb inversion clade, and are probably derived within the subfamily (see L. Cai et al. 2025 for a discussion, also Divergence & Distribution above). Thus the flowers of Swartzia, basal in Faboideae, are very different from those of all other Faboideae, indeed, from those in Fabaceae as a whole, in having a single banner petal, numerous, free, mostly infertile and very dimorphic stamens, and no nectar (Tucker 2003b), while the related Ateleia herbert-smithii is wind pollinated (Tucker 1990). There is considerable floral variation, although rarely including the evolution of keel-type flowers, in other basal Faboideae (e.g. Mansano et al. 2002, 2004).
Hardly surprisingly, Fabaceae attract a diversity of pollinators that visit the flowers for various rewards, however, the speciose 50kb inversion clade in Faboideae (it includes are overwhelmingly pollinated by bees, although reversals form this morph are pollinated by a variety of other pollinators (L. Cai et al. 2025). Lewis et al. (2000) summarize what was then known about pollination in Fabaceae, especially in "Caesalpinioideae" and Marinho et al. (2018b) discuss floral odours, often terpenoid in nature.
As mentioned, Mimoseae have flowers that are very different from those of most other Fabaceae, the reason why they were once put in a separate family by themselves. Numerous, often small, polysymmetric flowers are grouped together in a dense raceme, spike or head, and all the flowers open at about the same time; this inflorescence unit is the unit of attraction. There may be differentiation of flowers within the head, thus in some species of the largely bat-pollinated Parkia the basal flowers have long, conspicuous staminodes, the flowers immediately above secrete nectar, while the rest of the inflorescence has perfect flowers with a small perianth (Conceição Oliveira et al. 2021a, b), and in taxa like Calliandra and Neptunia there are pseudanthia (Baczynski & Claßen-Bockhoff 2023). In Mimoseae the pollen grains are frequently aggregated into polyads which are transferred by the pollinator to a cup-shaped stigma that is of the appropriate size for the polyad of that species, furthermore, there are also about as many ovules in the ovary as there are pollen grains in the polyad (Kenrick & Knox 1982; Kenrick 2003: implications of this pollination mechanism for the breeding system; Banks et al. 2010; Guglielmi & Texeira 2024); Banks et al. (2011) note that all the pollen grains of a polyad form a single harmomegathic unit. Variation in both pollen and anther morphology in the Dichrostachys clade is considerable (Hughes 1997). Many of these features seem to be connected with the post-zygotic incompatability system common in Mimoseae, a system that is rather wasteful of both pollen and ovules. In Mimoseae the syndrome includes some combination of low fruit set, apocarpy, restricted stigmatic surfaces, reduced ovule number, and aggregation of pollinia into polyads/pollinia (Wyatt & Lipow 2021, see also 2007). However, the same incompatability mechanism is also known from taxa like Caesalpinia and Senna (Gibbs 2014), with rather different floral morphologies, so details of the evolution of what is going on in Mimoseae remain to be established.
Anthers in many Mimoseae have terminal glands that vary in morphology/anatomy, although little is known about any functions they might have (Luckow & Grimes 1997; de Barros & Teixera 2015; de Barros et al. 2016) - perhaps they produce a smell (Tybirk 1997)? In Calliandra the polyads often seem to be sticky, the adhesive being rather like tryphine and produced by parenchymatous and tapetal cells (Capucho & Texeira 2013). The polyads attach to the pollinator, but in this case the stigma is much larger and capitate and the polyads adhere to its surface (Prenner & Teppner 2005; Greissl 2006, c.f. in part Teppner 2007b). For nectaries, which are on the inside of the staminal tube/hypanthium, see Ancibor (1969); they are, for example, absent from Acacia, although found in taxa now placed in Senegalia (e.g. Pedley 1986; Tybirk 1997). Many Mimoseae have polyads, which vary in arrangement, pollen grain number (4->20), etc. (e.g. Guinet 1969, 1981b, 1990; Sorsa 1969; Feuer 1987; Banks et al. 2011; Ribeiro et al. 2018; Soares et al. 2021 and references); this variation will need to be integrated with the developing phylogeny of the tribe (see Ringelberg et al. 2022). De Souza et al. (2013) described Afrocalliandra that rather surprisingly has a 7-grained polyad (it has recently included in Calliandra - Thulin 2023); I have no idea how this develops. For more information on locellate anthers (scattered in the clade), polyads and pollen morphology, anther dehiscence, etc., see Guinet (1981a, 1986, 1990), Prenner and Teppner (2005), Teppner (2007a, b), Teppner and Stabentheiner (2007, 2010), and Capucho and Texeira (2020: esp. polyad development) and references. There is sequential flowering of different species of "Acacia" at the one locality in Africa (Stone et al. 1998); see Hosaka et al. (2016) for such sequential flowering in general.
Bee Pollination. Interestingly, within the tropics bees seem to be commonest in the New World (Michener 1979), and there woody Fabaceae are especially diverse. Bee pollination is particularly common in Faboideae, and is associated with the monosymmetric papilionoid flowers common there. Such flowers have a more or less erect banner petal outside the others, two wing petals, and paired interlocking keel petals enclosing the stamens (see Uluer et al. 2022 for papilionoid-type flowers in both Fabaceae and elsewhere). The micromorphology of the petal epidermis varies considerably (and not only in papilionoid flowers), even differing between the petals of a single flower. In the IRLC studied, although all petals have cells with tabular-rugose-striate sculpturing, this varies between petals along the dorsi-ventral axis of the flower (Ojeda et al. 2009, 2019). Furthermore, the outer surfaces of the wing petals in particular are variously sculpted (Tewari & Nair 1979), with pockets and folds that afford footholds for the pollinator (Stirton 1981; Le Roux & van Wyk 2012; Alemán et al. 2018 and references). Colour patterning of the corolla is conspicuous, as in Lupinus, and is always on the adaxial standard/banner petal; these petals may also absorb ultraviolet light; the colour patterning on the standard of Hardenbergia violacea even appears to mimic an anther (Lunau 2006). Scaccabarozzi et al. (2021) looked at the diversity of bees (and some scarabeid beetles) that pollinated legumes with papilinoid flowers in southwest Australia, and within this general floral type the insects showed specificity. Flowers of the southern/austral plant families Myrtaceae, Fabaceae, and Proteaceae, are often pollinated by members of groups of colletid bees, also long-term denizens of Australia, such as Euryglossinae (esp. Myrtaceae), Hylaeinae, and Neopasiphaeinae (Slattery et al. 2023). For pollination of keel flowers in general, see Westerkamp (1996, 1997), and for false colour floral reconstructions (by combining u.v. and colour photographs) so as to see flowers of Faboideae more as a bee might see them, see Lunau et al. (2021).
Papilionoid flowers of sorts are scattered outside Faboideae (see in particular below). Although the flowers of Cercis (Cercidoideae) are only superficially similar to the papilionoid flowers of Faboideae (Tucker 2002a), they both have keels and seem similar functionally, although flowers of the former lack the sculpturing on the wing petals that is common in Faboideae and their stamens are basically free. Colour patterning can be conspicuous as in Caesalpinia and Bauhinia, again, it is on the adaxial banner petal. In some Caesalpinia s.l. the abaxial sepal is coloured and more or less functions as a keel. Although papilionoid flowers may not be as widespread in Fabaceae as one might have thought, flowers with some kind of banner petal are indeed quite common in the family outside of Mimoseae.
Within Fabaceae, Xylocopa (carpenter bees) alone visit some 52 genera (Leppik 1966; Hurd 1978; Kalin Arroyo 1981; Lewis et al. 2000). Pollination in "Caesalpinioideae" is predominantly by polylectic bees, while oligolectic bees are commoner pollinators of Mimoseae and some Faboideae. Oligolectic bees are more speciose in (warm) temperate regions, especially Mediterranean climates (Michener 1979; Kuhlmann & Eardley 2012), and that is where the latter two groups are particularly common. Faboideae are pollinated mostly by polylectic bees and in a variety of ways, and the plants are a major sources of both nectar and pollen (Goulson 2010: U.K. species) - indeed, it was estimated that in 2007 Trifolium vulgare alone was responsible for some 30% of the nectar produced by 260 species of flowers in the U.K. and ingested by bees (Baude et al. 2015). Bee pollination is the norm in the megadiverse Astragalus (Bombus, Osmia, Anthophora: Soltani et al. 2021). Dorchin et al. (2021) note that long-horned bees (Apidae, the Eucera complex) have diversified on Faboideae-IRLC. Keel flowers are pollinated by medium-sized to large bees, since they can "work" the flowers more easily, and large bees also pollinate Faboideae with inverted keel flowers (Amaral-Neto et al. 2015, q.v. for floral modifications involved); inverted keel flowers are found in genera like Canavalia. Fabaceae, along with Aizoaceae, Asteraceae and Zygophyllaceae, in the drier areas of southwestern Africa, including Namibia, are much visited by non-Apis bees (Kuhlmann & Eardley 2011).
The nature of the reward for the bee varies, as is how the pollen is presented and where any nectar is to be found. When the androecium is monadelphous, i.e. the filaments of all the stamens are connate, there is no nectar immediately available and the reward for the pollinator is then often pollen. If the androecium is diadelphous - nine stamens are connate and a single adaxial stamen is free - the reward is generally nectar, the polinator accessing the nectar via the slit of the androecial tube (see e.g. Prenner 2004c, 2013b; note that details of androecial initiation may vary (Prenner 2004c)). The flowers of course are often monosymmetric and the nectary lies between the filament tube and gynoecium and is quite variable in morphology - it is often better developed on the abaxial side of the flower, and the nectary vasculature - it comes from the staminal bundles - may appear before the nectary is visible (see also Leite et al. 2015 for access to nectar; Sinjushin 2024a [Phaseoleae], esp. 2024b for nectary morphology). However, androecium development shows considerable variation in detail, and the free stamen may become more or less fused to the other nine, although leaving slits on either side of the filament (e.g. Sousa & Sousa 1981; Tucker 1981; Rodríguez-Rieño et al. 1999). The anther glands in Mimoseae may produce nectar as a reward for the pollinator, although other functions for these glands have also been suggested (Luckow & Grimes 1997 - see above), while in Faboideae-Dipterygeae there are a variety of glandular structures on the flower apart from anther nectary glands (Leite et al. 2019). Indigofera even has short, nectar-secreting spurs (see also A. R. Davis et al. 1988; Vogel 1997).
Secondary pollen presentation occurs in Fabeae and a number of other Faboideae in particular - some 46 genera all told, and with a variety of pollination mechanisms (El Ottra et al. 2023). Here the pollinator may pick up pollen from a pollen brush at the end of the style; this mechanism has evolved perhaps eight times, the brush-type pollination device (e.g. Lavin & Delgado-S. 1990). In the asymmetrical flowers characteristic of Phaseolinae the labellum is twisted, forming a tube that looks rather like an elephant trunk; this is why Vigna caracalla is called the corkscrew vine (c.f. Pedicularis: Orobanchaceae). When the bee lands on the keel, it depresses it and the style then forces pollen out of the tip of the keel - the end of the tube - in a tooth paste-like strand (Delgado-Salinas et al. 2011), this is a pump-type secondary pollen presentation device. Genera like Lupinus also have a pump-type mechanism, although here the apex of the keel is not twisted. Taxa like Cytisus, Medicago, Desmodieae, Indigofera and some Mucuna have explosive pollination devices. Here the style, held under tension, is released when the pollinator lands on the keel, its weight as it were breaking the flower, pollen being flung out and scattered over the insect (e.g. Polhill 1976); the pre-pollination morphology of the flower is often not restored and such flowers can be visited only once (c.f. Polygala with explosive pollination - see below). For more on explosive pollination, see e.g. Suzuki (2003: Cytisus), Solomon Raju & Purnachandra Rao (2006: Pongamia pinnata), Alemán et al. (2014: Desmodium), etc..
Buzz pollination is quite common in Fabaceae. It occurs throughout Cassia, Chamaecrista and Senna (Caesalpinioideae), all told maybe some 700 species (Teppner 2018; Lewis et al. 2000; Nogueira et al. 2018; see also Venkatesh 1958: anther morphology; Amorim et al. 2019: floral morphology in general). Westerkamp (2004) and Amorim et al. (2017, 2019) suggest that in some species of both Senna and Chamaecrista the orientation of the anthers of the abaxial stamens is such that the pollen ejected when the symmetric flower is vibrated initially misses the bee entirely, but it bounces off specially modified deflector petals and then lands on the bee's back - ricochet pollination; monosymmetric flowers lack these modified petals. The bee may indeed have difficulty in removing the pollen from its back, but it is removed by the stigma. Anthers are basifixed and porose and have at least four different modes of development (Tucker 1996b), and there are three (or more) stamen morphs: three abaxial staminodes, four middle medium-sized stamens from which pollen is taken by the bees, and three longer abaxial stamens that produce the pollen that is actually involved in pollination, or three abaxial stamens and seven staminodes (see Tucker 1996b; Marazzi & Endress 2008; Marazzi et al. 2007; Amorim et al. 2017, 2019; Dellinger et al. 2019c). Cassia s. str., with dorsifixed anthers, also has three stamen morphs. Saab et al. (2021) looked at pollination in Ca. fistula, disentangling a rather complex sytem. The medium anther morph reflected u.v. and attracted the pollinators, and the pollen was in fact sterile although it was collected by the bees; the pollen in the three anther morphs differed in size, etc., and the reserves were sometimes predominantly starch, but not in the fertile grains. The anthers were basi- or dorsifixed, variation being within an anther morph, and so on (Saab et al. 2021). The filaments are curved and the anthers have slits or basal pores. Enantiostyly is sometimes an integral part of this remarkable pollination mechanism, especially in species of Senna; it is likely to have been acquired once, although also subsequently lost. The largely herbaceous Chamaecrista is also enantiostylous; the stamens may have two morphs in different whorls, the filaments are short, and the basifixed anthers dehisce by pores and have a velcro-like line of hairs (Tucker 1996b). For monomorphic (just the one enantiomorph on the one plant) and dimorphic enantiostyly here, see Almeida et al. (2018) and de Almeida and de Castro (2019), and Almeida et al. (2015a, also b) provide a classification of enantiostylous flowers in Cassieae - they list seven types. Morphologies can be quite bizarre. Thus in Ch. planifolia an abaxial petal that would otherwise deflect pollen is strongly asymmetrical and folded-tubiform at the apex; the ten anthers open inside the base of the tube and the pollen from all ten comes out of the apex of the tube (Amorim et al. 2019). There is considerable variation in stigma morphology in the group, but the stigmas are usually porose or crateriform; an exudate initially covered by cuticle may or may not be present, ditto a crown of short hairs surrounding the stigma (Owens & Lewis 1989; Dulberger et al. 1994; Marazzi et al. 2007). How the pollen actually gets to the receptive stigmatic surface which may be at the bottom of a chamber in the style is poorly known. Arceo-Gómez et al. (2011) suggested that in Ch. chamaecristoides buzzing by the pollinator might loosen the hairs around the stigmatic cavity, so allowing pollen grains inside, or the hairs might act as scrapers, or...; in their experiments, vibrations from an electric razor were much more efficient than those from a tuning fork in moving the pollen. Pollen orbicule micromorphology may also be important, and it has been suggested that taxa with microechinate orbicules are likely to be buzz pollinated, electrostatic charges at the tips of the spinules helping to keep the pollen grains separate (Galati et al. 2019; see also Buchmann & Hurley 1978). Interestingly, very similar flowers are found in Petalostylis cassioidea where pollen from the three fertile anthers hits the flattened, almost bowl-like style with its incurved margins whence it may be deflected onto the bee (Tucker 1998; Amorim et al. 2019). Although P. cassioidea used to be placed in Cassieae, recent work (e.g. Zimmerman et al. 2017) suggests that it should be included in Dialioideae where it is sister to Labichea, its Cassieae-like flowers being an excellent example of convergence. For more on buzz pollination, see elsewhere.
North temperate megachilid osmiine bees like Hoplitis species of the Annonosmia-Hoplitis group collect pollen from concealed-pollen flowers of Fabaceae and Boraginaceae; polylecty is derived in these bees (Sedivy et al. 2013; for megachilid pollination and Crotalaria, see Rockinger et al. 2017). Flowers of these two groups of course have very different morphologies, but both have pyrrolizidine alkaloids and/or particular nutrients that in this case are essential for the growth of the bee larvae - hence the visits of the bees (Sedivy et al. 2013). On the other hand, the quinolizidine alkaloid lupanine in the pollen of Lupinus may have a negative effect on bumblebee colony development - males were fewer and smaller than normal when lupin pollen was fed to the colony (Arnold et al. 2014; see also Lucchetti et al. 2018).
A final variant of bee pollination is found in Moldenhawera nutans (Caesalpinioideae-Sclerolobieae) and maybe other species of the genus, and perhaps in genera like Schizolobium, Peltophorum, etc., of the related Schizolobieae. The flowers of M. nutans are very similar to those of Malpighiaceae, which they mimic; the two grow in the same habitats and are both visited by oil-collecting Centris bees. However, the flowers of M. nutans have no oils, but the bees vibrate the flowers (as they do with Malpighiaceae) and collect pollen from the staminodes (de Queiroz et al. 2023) - mimicry in M. nutans is intermediate between Batesian and Müllerian. For similar mimicry systems see Orchidaceae-Epidendroideae-Oncidiinae.
Bat Pollination. Bat pollination is quite common in tropical members of the family (Fleming et al. 2009), bats visiting flowers with a variety of morphologies. In the Central American Mucuna holtonii (Faboideae-Phaseoleae) the concave erect standard acts a sonic reflector, guiding the bat to the flower, and when it lands on the flower the latter "explodes", covering the bat with pollen (von Helversen & von Helversen 1999). Bats may pollinate Mimoseae, as in some Parkia (Bumrungsri et al. 2008 and refs). Parkia is one of the few taxa that is both pollinated by bats and pantropical (so different bats are involved in the Old and New Worlds), other genera being Mucuna (de Moura et al. 2015, de Moura et al. 2016), Cayaponia (Cucurbitaceae) and Ceiba (Malvaceae: Bombacoideae). Interestingly, there is a small clade of Brazilian Parkia that is no longer bat pollinated, rather, it is pollinated by bees and Thysanoptera (Conceição Oliveira et al. 2021b). The pollen of bat-pollinated Fabaceae often has verrucate ornamentation (Stroo 2000 for references) although that author did not find such a correlation across angiosperms in general.
Bird Pollination. Pollination by birds is scattered in Fabaceae. The some 105 species of the pantropical/warm temperate Erythrina (Faboideae-Phaseoleae) are pollinated by both perching (sun) and hovering (humming) birds. Floral morphology and how the flowers and inflorescences are held varies according the requirements of these different visitors, and there may have been four or more shifts from passerine to hummingbird pollination here. Interestingly, a number of New World taxa are pollinated by perching birds (orioles, honeycreepers), and their morphology and nectar composition differs from those of the hummingbird-pollinated species (Steiner 1979; Bruneau 1997; Mabberley 2017: summary; Bilbao et al. 2021; de Souza et al. 2024). The nectary in Erythrina is 10-lobed and adnate to the hypanthium (Sinjushin 2024a). Bird pollination has originated ca 20 times in the Australian bacon-and-eggs peas (Faboideae: [Mirbelieae + Bossiaeeae]) alone, although the bird-pollinated clades are rather smaller than their bee-pollinated sister clades. Here it has been suggested that evolution of the meliphagid pollinator and of the plants involved may have been contemporaneous (Toon et al. 2014: Dollo model of evolution preferred, no strong evidence from phylogeny or morphology that bee-pollinated flowers are derived).
Butterfly Pollination. Just to round things off, Bauhinia galpinii is wing-pollinated by swallowtail butterflies (A. V. S. Silva et al. 2024).
Heteranthy. Variation in stamen size in the one flower, heteranthy (Swartzia is a good example), is notably common in Fabaceae, although less so in Mimoseae (Vallejo-Marín et al. 2010), and Paulino et al. (2016) describe how this works in some Faboideae. Variation in pollen morphology in the one flower is also considerable in basal "Caesalpinioideae" (e.g. Graham & Barker 1981; Banks et al. 2003; Banks & Lewis 2018), and Banks and Rudall (2016) speculate on the functional significance of this variation. In Lupinus the different stamen morphs, members of different whorls, produce different kinds of pollen, the inner whorl, opening later, producing sterile pollen that contains starch but no lipids, while in Cytisus the pollen from the two morphs - both found in anthers of the same whorl - is deposited on different places on the pollinator, and although pollen of both types of anthers germinated in vitro, this was not so in vivo (Paulino et al. 2016). However, heteranthy is not necessarily associated with differences in pollen morphology/behaviour (Nogueira et al. 2018).
There is considerable variation in stigma morphology; the stigmas are porose to crateriform and may produce an exudate (e.g. M. F. B. Costa et al. 2014).
For self-incompatability, etc., in Astragalus, see Soltani et al. (2021).
Seed Dispersal.
Van Staden et al. (1989) provide a useful general account of seed morphology and anatomy in relation to dispersal and germination. The legume s. str. is a single-carpelate fruit that dehisces explosively, the two valves of the carpel twisting in opposite directions as they separate down both sides (described as "elastic" in the characterizations above). Such fruits have two layers of lignified fibres at least one of which is oblique to the long axis of the fruit (Fahn & Zohary 1955); Armon et al. (2011) describe the geometry and mechanics of the opening of such fruits. A legume so defined is common in European-North American Faboideae, but also in Bauhinia, Duparquetia, Detarioideae, where seeds of the emergent tree Tetraberlinia moreliana (Amherstieae) may be thrown some 60 m (e.g. van der Burgt 1997), and so on. The fruits of Cercis are not explosively dehiscent, but are otherwise similar - and they are also typologically rather similar to the fruits of Myristica, the nutmeg! Overall there is a great diversity of fruit morphology in the family, including variously winged fruits, fleshy fruits, fruits breaking up into single-seeded units in different ways (i.e. lomentum and variants), and fruits modified for external animal transport with spines and hooks, for example, the velcro-like hooks on the lomenta of Desmodium s.l. (hence its common name, beggar's ticks). For lomenta in Desmodieae, see also Jabbour et al. (2017) and Nemoto and Ohashi (2003) and for the indehiscent, spiny, spirally-coiled fruits of many species of Medicago, see Fourquin et al. (2013: also their evolution). Entada has lomentum-type fruits, and the huge fruits (to 2 m long or so) of species like E. gigas break up, the units floating in the sea for months or more (references in O'Donnell et al. 2022). In Trifolium the calyx and corolla are both involved in fruit dispersal. Lonchocarpus has wind-dispersed fruits (Augspurger & Hogan 1983), although not obviously highly specialized.
Arillate seeds are common and are eaten by birds in particular, and legumes with such seeds are an important food source for specialized frugivorous birds in Africa and Southeast Asia-Malesia in particular (Snow 1981). However, seeds of taxa like Abrus precatorius and Pithecellobium have red and black colour patterns on their testa and mimic the colour contrasts of arillate seeds; seed dispersal is by deceit (M.-F. Jin et al. 2024). Such mimicry, whether of an aril (seed bicoloured) or of a fleshy fruit, also occurs in Erythrina, Rhynchosia and Ormosia, all sizeable genera (McKey 1975; Foster & Delay 1998; c.f. transference of function, Corner 1958), however, chewing the seeds of Abrus precatorius is not recommended. There is considerable colour variation within Ormosia, where the hard seed can be entirely red/orange, or the red-black colour contrast can be reversed, the red or the black surrounding the funicular scar, depending on the species, or the colour of the seed contrasts with that of the inside of the fruit; however, some species do have a fleshy seed coat (Torke et al. 2021). Close to a thousand Faboideae are myrmecochorous, myrmecochory occuring in many species of Acacia s. str. and other Australian Fabaceae, for example (Mckey 1989; Orians & Milewski 2007; Lengyel et al. 2009, 2010); independent evolution of myrmecochory has occurred several times within the family. Dispersal of seeds by water has evolved more than once in Canavalia (Snak et al. 2016).
In many taxa, especially those with explosively dehiscent fruits, the seed coat is very hard and may need scarification for germination to occur; a water gap has been reported from several taxa (Gama-Arachchige et al. 2013). For other aspects of the ecology of seed coats, see Souza and Marcos-Filho (2001).
Lim et al. (2021) found that members of Fabaceae were the second most abundant food plant of primates, just behind Moraceae. Interestingly, it was foliage of Fabaceae that was primarily eaten, but also other parts of the plant, as with three others of the ten commonest families eaten (Lim et al. 2021).
Fabaceae are noted for the number and variety of their associations with ants, and also with the larvae of a variety of other insects, some, like the larvae of lycaenid butterflies, taking advantage of legume/ant associations (see also immediately below).
Rather generalized legume/ant associations are very common in Fabaceae and are mediated by the extrafloral nectaries that are widespread in the family (McKey 1989; Koptur 2005; Weber & Keeler 2013; especially Gonzalez & Marazzi 2018 and Marazzi et al. 2019). The nectar secreted, which usually contains sucrose (see e.g. Wäckers 2005), attracts ants that can protect the plant against herbivorous insects; a variety of ants may be attracted to the nectaries of a single legume species (e.g. Schemske 1983; Seufert & Fiedler 1996). For the evolution of such nectaries, see Divergence & Distribution above. Larvae of lycaenid butterflies are part of the equation, being involved in phoretic associations with ants and being common on members of Fabaceae. Thus 40% of the host-plant records of lycaenid/riodinid caterpillars are from Fabaceae (for Inga in particular, see Endara et al. 2022; for further details, see Pierce & Dankowicz et al. 2022a, b), and over 90% of the caterpillars are myrmecophilous; larvae of 10 species of blues were recorded from Saraca thaipingensis alone growing in Ulu Gombak in Peninsula Malaysia (Seufert & Fiedler 1996) while riodinids are one of the three main lepidopteran groups associated with the Neotropical Inga (Endara et al. 2022).
There are a number of intimate ant/legume associations where generally but a single species of ant is involved (Schemske 1983). The close association of Pseudomyrmex ants with some members of the old Acacia subgenus Acacia (= Vachellia), including American swollen-thorn acacias such as V. sphaerocephala and V. cornigera and the African V. drepanolobium, are well known. The plant provides protein-rich Beltian bodies at the ends of each leaflet (for their development, see Rickson 1969: the leaves have notably many leaflets, even for Acacia s.l.) as food for the ants, and the ants also take nectar from the petiolar extrafloral nectaries; the swollen stipular thorns serve as their homes (e.g. Gijsman et al. 2021). Leichty and Poethig (2019) looked at the development of these structures on seedlings, and perhaps not surprisingly, extrafloral nectaries were the first to appear, however, details of colonization of the seedling by the ant are still unclear (Janzen 1967b). The ants patrol the plants removing any insects they find, and they may even clear the ground around the host (c.f. devil's gardens), the width of clearance depending on the species (Janzen 1966, 1967b, 1974b; Webber & McKey 2009; Amador-Vargas 2019). In some associations ants prune their hosts, farm nectar-feeding scale insects, or eat the extrafloral nectaries (C. Baker et al. 2017). Interestingly, Vachellia produces new leaves (and thus Beltian bodies) even during the dry season so providing a stable food resource for its ant associates, while the biomass locked up in the swollen thorns, which persist on the plant and do not break down quickly, is considerable (e.g. Janzen 1966). Sucrose in normal extrafloral nectar is broken down by invertase in the ant gut into glucose and fructose, however, nectar produced by Vachellia is sucrose-free (Kautz et al. 2009) and the ant's invertase is inhibited by chitinase, a major protein in the extrafloral nectar of the host (Heil et al. 2014; Heil 2015). Complex interactions between protease inhibitors in the plant and proteases in insects may help deter other than the mutualist ants from eating the Beltian bodies (Orono-Tamayo et al. 2013), and Rubin and Moreau (2016) discuss the interaction between molecular evolution and mutualism in Pseudomyrmex. The ages of Pseudomyrmex and the main clade of Vachellia that it inhabits is about the same, ca 5 Ma, and there seems to have been but a single gain of domatia there (Chomicki et al. 2015; Chomicki & Renner 2015), although there may have been two origins of this association in Pseudomyrmex (Ward & Branstetter 2017). In V. erioloba, at least, two or even more species of ants are commonly found inhabiting different domatia on the one plant (Campbell et al. 2015). The plant can adjust the rewards it produces depending on whether it is associated with an ant, or not; interestingly, Crematoaster crinosa may be found on Central American Vachellia, although it is not much good at protecting the plant (it is a "parasitic" ant), however, extrafloral nectaries at the apex of the leaf may be produced by the V. collinsii that it inhabits (unusual for that species), perhaps to encourage the ant to patrol the length of the leaf (Gijsman et al. 2021). As The Economist (July 10th, 2021, p. 74) noted "lazy insects get bigger rewards", however, this is probably a more complex situation than it seems at first sight, i.a., the ant moves on to trees that had previously been inhabited by Pseudomyrmex (Janzen 1967; Gijsman et al. 2021).
There are several other examples of close ant/plant relationships in Fabaceae (McKey 1989 for a list; Davidson & McKey 1993; McKey & Davidson 1993). It is estimated that there have been at least 15 gains of ant domatia in the New World legumes Platymiscium (Faboideae-Dalbergieae - ?4 gains: see Chomicki & Renner 2015, origins ca 13.4 Ma, 6.1 Ma, and more recently) and Tachigali (Caesalpinioideae-Sclerolobieae: 9 origins; see Fonseca 1994, also Davidson & McKey 1993 for relationships of the Pseudomyrmex involved and Huamantupa-Chuquimaco et al. 2024 for relationships in Tachigali). Evolution of ant and plant seems to have been more or less contemporaneous, but with later bouts of colonization by both the same and by different ant clades (Chomicki et al. 2015). Note that herbivore activity may result in the induction of extrafloral nectar, with movement of ants on to the plant (Heil 2015; see also Heil et al. 2001, etc.). In the whistling thorn tree, Vachellia drepanolobium, dominant in East African savannas Crematogaster ants defend the tree against herbivores, including elephants and young giraffes (the ants have nasty stings); if herbivores are excluded, the plant invests less in ant rewards such as extrafloral nectaries and swollen thorns (Palmer et al. 2008), while if the invasive ant Pheidole megacephala displaces the Crematogaster, the Vachellia population is much reduced and the sebra population increases - lions kill fewer zebras, which can see the lions stalking them better (Kamaru et al. 2024)... In the common African Leonardoxa africana (Det-Amherstieae) a third party, an ascomycete (Chaetothyriales - see Vasse et al. 2017), is central to the relationship. Nitrogen from the ant, Petalomyrmex phylax, initially moved more into the fungus than the plant (Defossez et al. 2010), but ant larvae ate the fungi (Blatrix et al. 2012) and over time the N became distributed about equally among all three partners via the larval excreta (Defossez et al. 2010). Brouat et al. (2001) thought that the size and shape of the prostoma, the more or less unlignified area of the stem of Leonardoxa through which the ant entered the plant, could in some cases be linked with comparable attributes of the head of the ant involved in the association (see also Davidson & McKey 1993). Here there are connections between the size of the associated ants, the age of the plant when the association begins, the size of the domatium that the plant produces, and the habitat in which the plant grows, all varying within L. africana (Blatrix et al. 2002b). For plant-ant signalling in this association, see Vittecoq et al. (2011). There are similar plant-ant-fungus associations in Tachigali (Blatrix et al. 2012: the ant is Pseudomyrmex penetrator). There are distinctively different fungal communities in the domatia inhabited by the three species of ants commonly found on the African Vachellia drepanolobium (but Chaetothyriales, commonly associated with ants, are not prominent), and these are found in the infrabuccal cavities of the ant alates, although what these fungi might do is unclear (Baker et al. 2017). Finally, in the southeast Asian Saraca thaipingensis (Detarioideae) bacteria living off colony debris are eaten by rhabditid nematodes that are possibly in turn eaten by the ants living in the plant (Maschwitz et al. 2016).
Ants do not always provide benefits to the plant. Thus in Leonardoxa africana (Detarioideae) the ant Cataulacus effectively parasitises the association by taking nectar from the extrafloral nectaries yet proving little in the way of protection (Gaume & McKey 1999). Perversely, species of Vachellia with low nectar rewards are derived from those with higher rewards, and species that offer only low rewards are often colonized by exploiter ants that also do not defend the plant (Heil et al. 2009), while Seufert and Fiedler (1996) recorded a diversity of ants on Saraca thaipingnsis only some of which provided protection against herbivory. Indeed, caterpillars of lycaenid butterflies (see below) in particular may take advantage of the ant-Fabaceae relationships. Specialized ant/domatia systems can be exploited by parasitic ants recently derived from generalist rather than specialist relatives (Chomicki et al. 2015). Amador-Vargas et al. (2020) found that trees of Vachellia collinsii that had parasitic ants produced spines with narrower diameter/lower volume. The salticid spider, Bagheera kiplingi, lives almost exclusively off the Beltian bodies of Vachellia in S.E. Mexico and Costa Rica (Meehan et al. 2009). For more on ants and plants, see elsewhere.
Other Insects.
Associations of other insects and Fabaceae are often quite close. Caterpillars of Lycaenidae-Curetinae, -Riodininae-Riodinini and especially -Lycaeninae-Lycaenini (the blues) butterflies are often found on Fabaceae (Ehrlich & Raven 1964; Fiedler 1991, 1995, 2001, 2006). This is in part because of the close association between many lycaenid caterpillars and ants, often conspicuously active on Fabaceae (see above): Ca 40% of lycaenid host plant records are from Fabaceae, and over 90% of the caterpillars involved are myrmecophilous (see also Pierce et al. 2002). For lycaenid (Riodinidae) caterpillars, major herbivores of taxa like Inga, see above. Caterpillars of ten species of blues were recorded on the one legume, Saraca thaipingensis (Detarioideae), from Ulu Gombak in Peninsula Malaysia; details of the relationships depended on the particular species of blue and ant, and although the plant might be protected against most larvae, on occasion it suffered more or less extensive defoliation (Seufert & Fiedler 1996). It has also been suggested that Fabaceae are the ancestral host of butterflies as a whole, Rhopalocera (?ref.).
Similarly, larvae of the some 260 species in 15+ genera of Coliadinae and Dismorphiinae (Pieridae) butterflies are found on Fabaceae, although diversification rates in Coliadinae are lower than in the brassicalean-associated Pieridinae (Braby & Trueman 2006: a quarter of the records; Wheat et al. 2007) - see also Brassicales for pierids and Santalales for pierids and lycaenids (the African Pseudopontiinae are recorded from Acanthaceae and Opiliaceae - G. S. Robinson et al. consulted vii.2015). Fabaceae may even be the original food plants of Pieridae (Braby & Trueman 2006; Wheat et al. 2007; Fordyce 2010); Pieridae started diversifying (108-)87(-67) Ma (Espeland et al. 2018). Caterpillars of the skipper group Eudaminae are also quite common on Fabaceae (Warren et al. 2009); Quicke et al. (2025) note that in Guanacaste, Costa Rica, non-hesperine skippers are notable herbivores on Fabaceae. The diversity of caterpillars on Fabaceae, especially that of basal butterfly groups, including the monotypic Baronia, perhaps sister to all Papilionidae and the only swallowtail found on Fabaceae, its caterpillars eating Acacia (Heikkilä et al. 2011; see also Ehrlich & Raven 1964), is such that Janz and Nylin (1998) and Braby and Trueman (2006) suggested that Fabaceae might be a springboard for host-plant diversification of Pieridae, e.g. Dismorphinae (see also Quicke et al. 2025: on Mimoseae). This would then suggest that diversification of these butterflies would be very largely Caenozoic, given suggestions for the age of Fabaceae (see above). Ages of various major clades of butterflies are given in Chazot et al. (2019), the age of butterflies as a whole being (129.5-)107.6(-89.5) Ma and that of most families is Cretaceous, while ages of groups particularly active on Fabaceae are Pieridae, (92.4-)76.9(-63.1) Ma, Riodinidae, (85.2-)70.9(-57.2) Ma and Lycaenidae, (88.1-)73.4(-60.3) Ma (see Chazot et al. 2019 for other ages suggested for these groups).
Turning now to moths, Trifurcula, perhaps 60 species, a genus of small leaf miner moths belonging to the monotrysian Nepticulidae, are known only from Faboideae (Doorenweerd et al. 2016). X. Li et al. (2021) suggested that the ancestral host plant of the speciose Gracillariidae (close to 2,000 species, 105 genera), predominantly leaf miners, were Fabaceae, and diversification here may have begun (103.7-)103.1(-97.7) Ma, while with over 1,200 species, the leaf rolling tortricid Grapholitini may have evolved only ca 44.3 Ma - they were probably initially monophages or oligophages and were also on Fabaceae (G. L. Hu et al. 2023). Caterpillars of Notodontidae-Hemiceratinae eat Inga in the Guanacaste area of Costa Rico (Quicke et al. 2025).
Which leads on to thinking about Inga and herbivory. Herbivory by foliovorous insects is often quite marked in Fabaceae, despite the diversity of their defensive secondary metabolites; herbivores in general prefer to feed on N-rich plants, which Fabaceae certainly are (Simonsen & Stinchcombe 2014 and references). Cassia fistula is sometimes almost defoliated by caterpillars of pierid butterflies (pers. obs.), while up to one third or more of the developing foliage of species of Inga may be eaten by herbivores (Kursar et al. 2009; Coley et al. 2019). Plant-insect interactions in Inga in particular have been much studied over the last few years. Inga, with some 350 species, has diversified in the New World l.t.r.f. only within the last 10-2 Ma (Richardson et al. 2001b; Pennington et al. 2009; see aso Nicholls et al. 2015). Up to 43 species of Inga may coexist at a single site, perhaps in part because species, even sister species, may differ considerably in antiherbivore defences (see also Kursar et al. 2009; Nicholls et al. 2015; also below). These defences represent a major investment by the plant, perhaps 37±11% of the total dry weight of expanding leaves, and they consist mainly of soluble phenolics and saponins in particular. This is almost twice as much in as mature leaves, most leaf area being lost in the young leaves (Wiggins et al. 2016; Endara et al. 2022). Characters like delayed greening of the leaves during growth, etc., can also be important in this context (Lokvam & Kursar 2005; Kursar et al. 2009; Endara et al. 2015; see also Richardson et al. 2001b; Pennington et al. 2009; Sedio et al. 2017). Any effect of nodulation, which may be absent in Inga in some species/situations, is unclear (Coley et al. 2019). Forrister et al. (2022) note that closely related species of Inga have divergent chemical profiles, and this is even more pronounced in sympatric sister species than when the species are parapatric; there is little phylogenetic signal in secondary chemistry, the herbivores growing on plants that have similar defences, and these are unlikely to be closely related (Endara et al. 2022). Each species of Inga maximizes its phytochemical diversity by producing structurally unrelated compounds, and this is probably the result of the regulation of gene expression; evolution here is not stepwise, although that previously been the assumption (Forrister et al. 2022). Thus comparing the phylogeny, chemical profile, etc., of Inga with the phylogenies of three of its major lepidopteran herbivores, caterpillars of gelechioid leaf miners, erebid noctuid moths, and riodinid butterflies, it seems that simple coevolution has not been involved, rather, the defences of Inga are evolutionarily labile and have responded quickly to the attentions of the herbivores, the latter tracking/living on those Inga that they could - another way of putting it is that they were preapted to them (Endara et al. 2017). (Indeed, this lack of a strong correlation between herbivore phylogeny and the nature of secondary compounds is quite common - Kariñho-Betancourt et al. 2015, but c.f. swallowtail butterflies, etc..) Despite very different defences in different species, the overall level of herbivory might be similar (Lokvam & Kursar 2005). With older leaves, their toughness may be their major defence - for instance, the large amounts of tyrosine in some young leaves discussed below had effectively vanished in older leaves (Coley et al. 2019). However, older leaves may also contain a greater variety of defensive metabolites, and the amount of these metabolites might show quite considerable infraspecific variation; since the leaves are on the plant for a relatively long time, the overall variety of herbivore-plant interactions could be quite large (Wiggins et al. 2016). Different forms of herbivory/plant defence are discussed by Coley et al. (2005). Inga umbellifera, a more basal species [check] with an "escape" strategy, had rapid leaf expansion, simultaneous flushing and delayed greening (and some rather odd secondary metabolites); the plant was less effectively defended in terms of the variety of herbivores found on it, and these herbivores were generalists. Inga goldmanii has a "defence strategy": Its extrafloral nectaries attracted ants that presumably protected the plant and the leaf flavanoids were more effective in defence; lepidopteran larvae grew less well when fed a diet containing crude extracts of the plant and there was less herbivore diversity, the herbivores being more specialists. Overall, however, Coley et al. (2005) found that similar amounts of hebivory occured in the two during leaf expansion. Coley et al. (2019) looked at a clade of Inga in which overexpression of a primary metabolite, the amino acid tyrosine (there were sometimes also tyrosine-derived secondary metabolites), which here could be up to 20% of the dry weight of young leaves, was a feeding deterrent to generalist herbivores. Of the specialists, riodinid caterpillars preferred hosts which overexpressed tyrosine itself; these amino acids are important for the caterpillar in its association with ants (see e.g. Pierce 1985; Pierce et al. 2002: Pellissier et al. 2012), noctuids (macromoths) preferred hosts with the tyrosine-derved secondary metabolites, while gelechioids (micromoths) tended to avoid both (Coley et al. 2019, see also Endara et al. 2022). Ants are attracted to extrafloral nectaries on the trees on which riodinid caterpillars feed, and the dorsal glands of some of these riodinid caterpillars secrete nitrogen- and sugar-rich exudates that they ultimately obtain from the plant, and ants both consume these exudates and protect the larvae - both ants and caterpillars were to be found on young, rapidly-expanding leaves, high in nitrogen - and ants might also deter other herbivores (e.g. Fiedler 2006; Pellissier et al. 2012; Coley et al. 2019; Endara et al. 2022). Endara et al. (2018) did invoke a form of co-evolution to explain the relationships between sawflies (Argidae, genus/genera unclear) and the species of Inga on which their larvae fed. Sawfly larvae found saponins distasteful, preferring plants with amine-type (tyrosine-derived) metabolites, and their preferences largely determined the Inga species that they ate; most host shifts were between species with similar chemistry, whether or not they were related to the original host. Beyond that, speciation in these sawflies was largely allopatric. The sawflies were aged at (7.9-)6.3(-4.8) Ma, and if younger than their hosts then resource tracking could be an explanation for their radiation; interestingly, they did not eat basal Inga species (Endara et al. 2018). There is not much variation in floral morphology here, so Inga is an example of diversification despite floral uniformity (see Vasconcelos et al. 2018). For other similar systems, see Piper, Eugenia, Protium, etc., Passiflora, sundry Solanaceae and Psychotria.
The lectin proteins that are often so prominent in the seed are probably involved in defence against granivorous insects (Peumans & van Damme 1995; Vandenborre et al. 2011), however, it is clear from the description of groups of insects that specialize of seeds of Fabaceae that many insects seem unaffected by them. Indeed, some 70% of the 1,700 seed beetles, bruchids, Chrysomelidae-Bruchinae (they used to be Bruchidae), are associated with Fabaceae. Two clades, made up largely of New World Acanthoscelides and predominantly Old World Bruchidius (neither genus is monophyletic), dominate, and their hosts are mostly Mimoseae and a diversity of Faboideae (and also in Acanthoscelides some Malvaceae, and in Spermophagus Malvaceae and Convolvulaceae in particular). In Faboideae bruchids detoxify the non-protein amino acid L-canavanine (Kergoat et al. 2005b, 2006), characteristic of the Faboideae-Non-protein amino acid accumulating clade. The larvae are specialized seed-eaters, and particular groups of bruchids may be associated with particular groups of Fabaceae (e.g. Kergoat et al. 2006, 2007, 2011 and references: Bruchus and Vicieae (= Fabeae), Sennius on Cassia). Individual bruchid genera tend to be found on seeds of legumes that are in adajcent pectinations of the mimosoid phylogenetic tree (Kergoat et al. 2007 - but check...), however, in in the bruchid Spermophagus, which does not eat Fabaceae, there is less specificity (Kergoat et al. 2015). For estimates of when particular groups of bruchids diversified on particular clades of Faboideae, see Kergoat et al. (2011). Divergence of the beetles is estimated at around 60 Ma, near the time of origin of Fabaceae, and of Acanthoscelides and Bruchidius in particular ca 49.5 Ma (Kergoat et al. 2005b, 2011). Stem Bruchinae are rather older, and may initially have eaten members of Arecaceae (q.v.); from Faboideae, probably their original host within Fabaceae, they moved on to other groups following the chemistry of the plants (esp. Kergoat et al. 2005a, b). See also Janzen (1969) for the complexity of the association, Southgate (1979: general), Johnson (1989, 1990: Acanthoscelides) Birch et al. (1989) for the chemistry of the interaction), Kato et al. (2010) for the importance of female oviposition preferences and Soltani et al. (2021: bruchids and Astragalus).
Psyllidae (jumping plant lice; Hemiptera-Sternorrhyncha) have diversified on Macaronesian Faboideae-Genisteae (Percy et al. 2004). Psyllids are quite common there, but their diversification has been dated to around 3 Ma, well after that of their hosts which is dated at ca 8 Ma (Percy et al. 2004). Aphids can sequester quinolizidine alkaloids from Genisteae like Lupinus, Genista, etc. (Opitz & Müller 2009).
Six subtribes including about 60% of the almost 1,000 species in the straight-snouted weevil Brentidae-Apioninae-Apionini are found on Fabaceae-Faboideae, perhaps moving there from the [[Frankeniaceae + Tamaricaceae] [Plumbaginaceae + Polygonaceae]] clade no later than the Upper Cretaceous (Winter et al. 2016: numerous dates for weevil diversification). Pinzón-Navarro et al. (2010) discuss the weevils found on Inga; for diversification of this genus, see above.
In another variant of insect-plant relationships, the flowers of Crotalaria are visted by Danainae (butterflies) and Ctenuchidae (arctiid moths). The pheromones of the latter are based on the pyrrolizidine alkaloids that the plants contain (for which, see Irmer et al. 2015 - they are also to be found in some Asteraceae and in wilting plants of some Boraginaceae and Heliotropaceae: Edgar et al. 1974; Pliske 1975; Boppreé 1986; Opitz & Müller 2009; Livschulz et al. 2018a: molecular-level parallelism). Crotalaria is associated with arctiids such as Utetheisa, its secondary metabolites providing defence for the caterpillars, etc. (Eisner & Meinwald 1995; Hartmann 2009). Singer et al. (2009; see other articles in Conner et al. 2009; Zaspel et al. 2014) discuss self-medication (pharmacophagy) and its evolution in caterpillars of Arctiinae on food containing high concentrations of pyrrolizine alkaloids.
In Australia ca 250 species of a clade of thrips have an obligate association with phyllodinous species of Acacia (Crespi et al. 2004). Hooked hairs may capture and kill leaf hoppers, sciarid flies, etc., as in Phaseolus vulgaris (Rebora et al. 2020 and references).
For insect vein cutting (trenching) and its effect of the photosynthesis of the leaf, see Delaney and Higley (2006). Interactions between rhizobial infections, nectaries, and the effects of herbivory are discussed above.
Plant-Bacterial/Fungal Associations.
Bacteria and N-Fixation.
For much information, see papers in de Bruijn (2015) and Adv. Bot. Research 94. 2020/Frendo et al. (2020).
There are lists of nodulating bacteria in e.g. Checcucci et al. (2019) and M. Tasng and Capela (2020). Note that there is currently a tendency (e.g. M. Tang & Capela 2020) to call the two unrelated groups of nodulating bacteria, the α-proteobacteria Rhizobium and relatives and the ß-proteobacteria Burkholderia and relatives, α-rhizobia and β-rhizobia respectively. Sprent et al. (2013, 2017) summarise the relationships of the 14 bacterial genera known to be able to nodulate legumes; see below for suggestions as to the timing of the evolution of these associations. Many nodulating bacteria in legumes are members of the proteobacteria α-2 subclass (Sprent et al. 2017), but they do not form a monophyletic group there, four clades and several "species" being involved, furthermore, bacteria like Agrobacterium (crown gall tumour, but also involved in horizontal gene transfer within seed plants) and others are also members of this group (J. J. Doyle 1998; Sprent 2001). Bradyrhizobium, associated with Fabaceae both in Australia and Africa, is very diverse with 15 or so main clades (the number is increasing), although many clades lack names (Stepkowski et al. 2012; Beukes et al. 2016 and references), and there is substantial temperate/tropical differentiation in the bacteria (Stepkowski et al. 2012). Other important N-fixing bacteria associated with Fabaceae include Burkholderia and relatives, which are ß-proteobacteria and not at all close to Rhizobium. These are quite common in the tropics and can tolerate alkaline conditions (Sprent 2007; Angus et al. 2013; Ardley et al. 2015); not all fix N, for instance, some are human pathogens. Bacteria like Burkholderia phymatum and Cupriavidus form nodules that fix N in Mimosa from South America and some Fabaceae-Faboideae from South Africa, at least, although most fixers in Faboideae are rhizobia (Walker et al. 2015: Fig. 89.2). The effective N-fixing symbionts in Burkholderia form two groups, one, involved in symbioses with New World Mimosa and Mimoseae, has the nod and nif genes on plasmids, the other, B. tuberum, nodulating African Faboideae-Crotalarieae and -Phaseoleae, has the nod and nif genes on its chromosomes (Bontemps et al. 2010; Agapakis et al. 2014). Interestingly, transfer of nod genes from α proteobacteria to South American Burkholderia has been dated to 60-50 Ma (Walker et al. 2015). Other ß-proteobacteria form nodules, albeit ineffective, with Mimoseae (Moulin et al. 2001; Sprent 2002; Elliott et al. 2007 and references). Yet others are pathogenic, but these latter bacteria rarely nodulate. Furthermore, Kost et al. (2014) found that species of Burkholderia that were plant pathogens could not metabolise oxalate secreted by the root, while beneficial species could; they used it as a source of C and they could also colonize the roots (plants involved: lupin, maize). Overall, a number of bacteria have "independently" become involved in N fixation in Fabaceae, but see below for movements of genes via gene casettes, symbiosis islands and plasmids; rhizobia that are nodulation-factor-dependent are likely to have a single origin of nodulation, but with subsequent extensive horizontal transfer (e.g. M. Tang & Capela 2020). Surprisingly, a Burkholderia growing on Solanum was able to form nodules and fix N when on Phaseolus vulgaris (Martínez-Aguilar et al. 2013).
A single plant may form associations with more than one species of bacterium, and these may be members of both main groups just mentioned (Sprent et al. 2013 and references), and closely related species of legume may form associations with a variety of bacteria (Ardley et al. 2013: the Lotononis group-Crotalarieae). At least some pioneer legumes form nodules with a variety of bacteria, thus perhaps enhancing their success as colonizing species (Behm et al. 2014 for references). Indeed, species of legumes that have obligate associations with rhizobia spread into non-native habitats less readily than do legumes lacking such associations, suggesting that appropriate bacteria, whether α-2 or ß-proteobacteria, can be lacking (Simonsen et al. 2017). Symbiont specificity tends to be greatest in the IRLC clade (Faboideae), although genera like Astragalus are exceptions (Howard & Wojciechowski 2006; Sprent et al. 2017). Dormer (1946b) noted that the same strain of bacterium did not infect both pulvinate and epulvinate species of Faboideae; the latter are the members of the IRL clade, of course.
It is not entirely clear which legumes nodulate. S. Liu et al. (2020) suggested that only Faboideae and Caesalpinioideae form nodules, but lists in e.g. Sprent et al. (1989) suggest that the ability to nodulate is more widespread, if sporadic. Note that from table 1 in Diabate et al. (2005) listing nodulation in West African rainforest legumes it seems that either there is infraspecific variation in nodulation, and/or that it can be difficult to determine whether a plant has nodules or not. There is also intragneric variation in nodulation. In Mimoseae exactly which bacteria form associations depends on soil conditions (Sprent et al. 2017), while in Medicago truncatula nodule-specific cystein-rich peptides control the bacteria that can form nodules with the plant by causing early nodule senescence in some bacterial strains (S. Yang et al. 2017). Indeed, the balance between the plant and its nodulating bacteria is complex. For instance, at the time of infection the host plant cannot determine if the bacteria infecting it are likely to be effective in fixing N, and it may resort to a variety of post-infection strategems(!) to control the bacteria (Sachs et al. 2018; see also Tsikou et al. 2018; Wendlandt et al. 2018: host sanctioning against ineffective fixers, differential investment in effective fixers). And of course we should not forget that legumes are associated with a variety of other endophytic bacteria (Peix et al. 2015).
These irregular, pinkish-coloured (because of haemoglobin, see above) nodules nearly always grow on the roots (Sprent 2009; Sprent et al. 2017: summaries), and although their basic construction is somewhat stem-like (H.-L. Li et al. 2015), Shen et al. (2025) noted that in Lotus japonicus, for instance, nodules developed in that part of the root where the Casparian strip was developing, and Casparian strips were also associated with root nodules, the apoplastic barrier that they formed helping to control nutrient uptake and nodule functioning. Corby (1988) provided an invaluable summary of nodule morphology (see also papers in South African J. Bot. 89. 2013). The α-proteobacterium Rhizobium is the best-known nodulator, but Bradyrhizobium (both = α rhizobia) and Burkholderia (= β rhizobium) are other genera involved. Some aquatic legumes in both Mimoseae (e.g. the aquatic Neptunia natans - it lacks root hairs) and Faboideae like Aeschynomene s. str. (Bradyrhizobium is the bacterium involved) and Sesbania form stem nodules, albeit associated with adventitious roots, and in the polyphyletic Aeschynomene in particular the ability to form such nodules has evolved more than once (Subba-Rao et al. 1995; Arrighi et al. 2013; Chaintreuil et al. 2013, 2016; Brottier et al. 2018). Rhizobia fix N, but only when in association with their host, indeed, one of the components of the cofactor of the nitrogenase which actually fixes the N comes from the host (Hakoyama et al. 2009). Nodulation is especially widespread in Faboideae where the nodulating Swartzia and immediate relatives form a clade near the base of the subfamily (see below: Ireland et al. 2000; Pennington et al. 2000; Lavin et al. 2005), however, members of other basal clades seem not to be nodulated. Mimoseae are also much nodulated (Sprent 2000, 2001, 2007), but nodulation is sporadic in more basal Caesalpinioideae, although it occurs in Chamaecrista and several other genera (Manzanilla & Bruneau 2014). In general, nodulation is very common indeed in Fabaceae from outside the tropics, less so in those from the humid tropics (Sprent et al. 2013; Simonsen 2016: fig. 3A; see also de Faria et al. 1987b and references).
Details of the evolution of nodulation in Fabaceae are still not well understood (J. J. Doyle 2013), and the implications of recent suggestions about how N-fixation evolved in the N-fixing clade as a whole (van Velzen et al. 2018; Griesmann et al. 2018; see above) will take a little time to sink in; the following account may well have to be modified. R. Walker et al. (2015) suggested that Burkholderia might originally have been an endosymbiont of an AM fungus. Thus candidatus Glomeribacteria gigasporarum is found in the glomerimycote Gigaspora margarita; genome analysis placed Glomeribacteria within Burkholderia, while analysis of metabolic pathways put it close fo Wolbachia (Walker et al. 2015). In Faboideae, nodulation involves the cooption of genes originally involved in lateral root origination, and it develops after a genome duplication event ca 54 Ma, 56.6 Ma, 58 Ma, or (67-)63.7, 57(-56) Ma (Schmutz et al. 2010; op den Camp et al. 2011; Q.-G. Li et al. 2013; Vanneste et al. 2014a, 2014b; J. J. Doyle 2011; Werner et al. 2014; Quilbé et al. 2021). This duplication occured in Faboideae after their divergence from Caesalpinioideae; N-fixing taxa such as Chamaecrista and many Mimoseae are found in the latter clade, but it lacks this duplication, although it may have another (Cannon et al. 2010, 2014), the nodules there probably being plesiomorphic in morphology (Vanneste et al. 2014b). In a phylogeny of NOOT/NBCL proteins (NOOT = NODULE ROOT, NCBL = NOOT-BOP-COCH-like) there seem to have been independent duplications in Caesalpinioideae and Faboideae; Cercidoideae have but a single copy, the two copies cluster separately, those of Caesalpinioideae and Faboideae again forming separate clusters (Shen et al. 2020). Causal connections between genome duplications and nodulation seem to be unclear (Cannon et al. 2014, see below for genome duplications in Fabaceae, about which there is some discussion...).
Nodulation involves the assembly of a co-expression network, a nodule-related module, in which a variety of genes that initially may have other functions become co-regulated (Z. Wu et al. 2019). Nodule formation often involves the production of Nod factors (NFs) by the bacterium. NFs are lipochito-oligosaccharides (LCOs), made up of a chain of 3-5 N-acetyl-D-glucosamine units, variously substituted and with a 16-20 C fatty acid attached. However, Gourion et al. (2015), Ibáñez et al. (2016) and others have emphasized that NFs are not always involved in nodulation - in Aeschynomene, for example, there is infraspecific variation in Nod dependency (see also below), that during infection there may be cell death as in the hypersensitive response to pathogens, and that in general, modulation of the normal immunity defence reactions of the legume are important in the initial stablishment of the plant/bacterium association. Parniske (2018) also questioned the centrality of LCOs, certainly when thinking about associations in the N-fixing clade as a whole, There is a common symbiotic signalling pathway (CSSP) in both baterial and mycorrhizal pathways, with a number of genes being shared by the two (Kawaharada et al. 2017 for details). Initial stages of nodulation are accompanied by the reprogramming of the host's genome, and the expression levels of large numbers of genes changes, including the repression of genes which might be involved in defence (Benedito et al. 2008). Venkatheshwaran et al. (2012) discuss a mutation that improves nuclear calcium signalling at an early stage in the development of symbioses (for which, see Barker et al. 2017) - IRLC clade only (Lotus, Medicago)? For more details, see Venado et al. (2020) and other papers in Adv. Bot. Research 94. 2020.
The initiation of nodules starts with the exudation of flavonoids, isoflavonoids and other bacterial attractants by the host and the subsequent infection of a root hair by a bacterium (e.g. Oldroyd 2013). The plant may also become infected through cracks ("natural" cracks, or wounds) in the epidermis (= crack entry), perhaps the plesiomorphic condition, as in Faboideae-Dalbergieae and -Genisteae (Cannon et al. 2010; Vessey et al. 2004; Okubo et al. 2012; Terpolilli et al. 2012; Chaintreuil et al. 2013; Ibáñez et al. 2016). There is also nodulation near lateral roots (Sprent et al. 2013 for a summary). Infection in Sesbania rostrata is both through cracks and via root hairs, the particular mode depending on the interaction of ethylene and water conditions in the soil (Oldroyd & Downie 2008), and infraspecific variation in entry is not so uncommon (Venado et al. 2020). Photosynthetic Bradyrhizobium can form nodules in stem tissue, as in American species of Aeschynomene (the genus is polyphyletic, but see Cardoso et al. 2020). Interestingly, here the plants have lost canonical nodABC genes; the bacteria may enter via cracks in the stem near root primordia, but in some species of Aeschynomene - less prolific nodulators - the epidermis is continuous (Giraud et al. 2007; Arrighi et al. 2014; Chaintreuil et al. 2013, 2016). The species of Aeschynomene are more or less aquatic (= Aeschynomene s. str.), an environment favoured by Bradyrhizobium, and the association that the two have formed, despite the lack of Nod genes in the bacterium, may have evolved three times, in the A. evenia and A. afroi clades and in A. fluminensis (Brottier et al. 2018). Aside from the lack of Nod genes, Quilbé (2021) discuss other differences in nodulation between A. evenia and the model species Medicago truncatula and Lotus japonicus. Like Arachis, there is an intercellular symbiotic infection process (as in about a quarter of N-fixing legume species) and stem nodulation occurs in other semi-aquatic genera. The Nod-independant clade, the A. evenia clade, is largely made up of diploid species.
Nodule morphology is usually controlled by the plant (Angus et al. 2013; Agapakis et al. 2014; S. Liu et al. 2020). Infection threads are invaginations of the wall of the root hair through which bacteria reach the apoplastic area beneath the epidermis, and how they develop is not that dissimilar from how root hairs themselves develop. The bacteria synthesize exopolysaccharide, Epr3 being the receptor gene in the plant, and they move down the thread in part by successive divisions. The overall development of the thread results from quasi-independent events in successive cells, so the thread reaches more deeply into the root, eventually branching in the nodule primordium (for details, see de Faria et al. 1987a; Gage 2004; Oldroyd and Downie 2008; Kawaharada et al. 2017). The bacteria eventually move by endocytosis into the nodule cell in which they will reside. The plesiomorphic infection morphology is that of persistent fixation threads. Here the N-fixing bacterioids, two or more per cell, are retained within structures bounded by a membrane analagous to that found in a symbiospme (see below) and are thin cell wall-bounded structures (de Faria et al. 2022). The nodules may be long-lived or indeterminate and have a persistent meristem, and they are initiated in the pericyclic region or inner cortical cells and acropetal auxin transport is initially inhibited (for this and other aspects of auxin involvement in nodule development, see T. T. Xiao et al. 2025); the bacterioids are able to divide (see also Parasponia - Cannabaceae, nodulation is also by Frankia here). Persistent fixation threads and nodules of indeterminate growth are found in some Caesalpinioideae (not Mimoseae) and also in some Faboideae like Andireae and Brongniartieae (Naisbitt & Sprent 1992; Rae et al. 1992; S. Liu et al. 2020); assimilated nitrogen is exported as amides (Larrainzar et al. 2020). Alternatively, one or a few bacterioids are enclosed by a host-derived membrane probably coming from the plasmalemma, the whole forming an organelle-like symbiosome that is in the cytoplasm (Rae et al. 1992; Gage 2003; Hirsch 1992; Streng et al. 2011, summary; Sprent & James 2007; Sprent et al. 2013, 2017; Coba de la Peña et al. 2018; S. Liu et al. 2020; Mergaert 2020; de Faria et al. 2022). The bacteria-infected cells may undergo mitosis, there is a single bacterioid per cell, the nodule has a short life span, i.e. they are of determinate growth, they are initiated in cells of the outer or middle part of the cortex and there is initial acropetal auxin transport; nitrogen is exported as ureides (or asparagine) (de Faria & Sprent 1995; Corby 1988: survey of nodule morphology; Sprent 2005: nodule distribution, see also J. J. Doyle 1994, 1998; Sutherland et al. 1994: Sophoreae, etc.; Doyle et al. 1997; Lavin et al. 2001; Oono et al. 2010; Terpolilli et al. 2012; Larrainzar et al. 2020: esp. haemoglobins). De Faria et al. (2022) noted numerous examples of loss of fixation thread-type nodules in Caesalpinioidese and two examples of the evolution of symbiosomes there. However, symbiosomes, very common in Mimoseae, were less fequently lost. Chomicki et al. (2020b) discuss the stability of such symbiotic associations, a stability that is promoted by their compartmentalization in the organism that they inhabit.
Although symbiosomes are formed in cells that are themselves no longer meristematic, the nodule meristem continues to produce a supply of cells that become serially infected by the persistent infection threads (for infection threads, see de Faria et al. 1987a). In determinate nodules such as occur in Phaseolus, but which are usually found in tropical legumes (Gage 2003), symbiosomes form in cells of the nodule meristem, and transmission is by cell division (Rae et al. 1992). In Medicago, at least, with indeterminate nodules, repeated endoreduplication of the genome of the host cell is needed for invasion of the bacterium to occur and the whole nodulation process to be effective (Maluszynska et al. 2013 and references). The nodules are anatomically rather like stems in having peripheral vascular bundles, the bacteria being in the pith (Franche et al. 1998), however, nodule origination occurs where lateral roots develop, at least in some Faboideae (op den Camp et al. 2011). Bacterioid differentiation is either reversible, little morphological change occurring, or irreversible, in the latter case the bacterioids becoming swollen and there is only one per cell; N-fixation by such bacterioids is more efficient than that by the non-swollen bacterioids (Oono & Denison 2010; Sprent et al. 2013). The plant can produce nodule-specific cysteine-rich [NCR] antimicrobial peptides, and these cause endoreduplication of the bacterial genome; the bacteria stop growing and become dependent on the host for many basic activities; they have been called ammoniaplasts and are effectively plant organelles (Gage 2004; Terpolilli et al. 2012; Czernic et al. 2015). This is an irreversible change and it has occured several times, as in the IRLC (Oono et al. 2010). Interestingly, the shape of such bacterioids in species of Aeschynomene depends on whether the plant has Nod factors or not (Czernic et al. 2015). Many of the later aspects of nodule development, including symbiosome development, N fixation, leghaemoglobin production, and so on are ultimately controlled by a master regulator, NODULE INCEPTION (NIN) (Feng et al. 2021; Jiang et al. 2021).
Checcucci et al. (2019) discuss the fluidity of rhizobial-α and -β genome structures (see also Geddes et al. 2020; M. Tang & Capela 2020). A relatively small chromosome-born "symbiosis island" in which genes involved in nodulation and N fixation are aggregated can move from one bacterial genome to another, and this may faciltate nodulation, although not all nodulation genes may occur on the island (Sullivan & Ronson 1998; J. J. Doyle 1998; see also Batstone 2021); such islands can be serially incorporated into the one genome (Checcucci et al. 2019). Similarly, mobile plasmids in which there are aggregations of nodulation genes are exchanged as units between bacteria via horizontal gene transfer (e.g. Agapakis et al. 2014; Gedes et al. 2020; Tang & Capela 2020; see also Ormeño-Orillo et al. 2013 for horizontal transfer). Particular legumes may select particular bacterial variants for nodulation, yet all the bacteria may have similar symbiosis islands (Parker 2012). It is often suggested that the genes involved in nodulation moved from α- to ß-proteobacteria, but it appears that nodIJ genes, at least, may have evolved following a gene duplication in ß-proteobacteria and then became part of the nodABCIJ operon that is common in α-rhizobia (Aoki et al. 2013). Genes integral to nodulation may have a single origin, but this is accompanied by substantial parallel evolution as they move between unrelated rhizobial strains (Tand & Capela 2020). Indeed, aggregation of genes in rhizobia may enable them to become endosymbionts in a single step (Maclean et al. 2007), although there is often a period after movement of the plasmid (or symbiosis island) during which these genome blocks become integrated into the receptor rhizobial genome (Tang & Capela 2020).
In the field it has been found that there is both vertical transmission of these gene complexes as well as horizontal transmission of symbiosis islands from introduced strains of nodulating bacteria to native (in this example, Ethiopian) strains (Aserse et al. 2012; see also Beukes et al. 2016). Along the same lines legume hosts in eastern North America select for particular Bradyrhizobium strains, and independent of this particular variants of the symbiosis island are exchanged between the different bacteria (Parker 2012). Strains of Bradyrhizobium may be metabolically quite different, conversely, quite different legume bacterial symbionts may share more genes than Rhizobium leguminosarum, for example, shares with the closely related but non-symbiotic Agrobacterium (Maclean et al. 2007). Hirsch and LaRue (1997), Couzigou et al. (2012), Chaintreuil et al. (2013) and others also discuss the complex origin of the nodule developmental pathway. Finally, it has ben noted that the exchange of these symbiosis islands and plasmids can also cause symbionts to become pathogens and vice versa (Sprent et al. 2017), indeed, which rhizobial species can colonize a particular plant species and how effective those rhizobia are in N fixation are variables that are not easy to predict (Boivin & Lepetit 2020).
Further complicating the issue: Associations between bacteria and plant may break down, e.g. the bacteria no longer form nodules, or the nodules they do form do not fix N (Gano-Cohen et al. 2020). Also, rhizobia seem not to form resting spores, and how they persist in the soil is unknown - perhaps in biofilms (Hirsch 2010)? This raises the important point that to understand the overall fitness of rhizobia one has to take into account their life in the soil apart from the host, and that the host root grows into a mixed inoculum of different rhizobial genera, species and strains varying in their fitness with respect to the host, and a single host may become associated with more than one rhizobial strain; interestingly, agricultural practices based on crop rotation make it difficult for host and bacterium to become co-adapted (Burghardt 2020).
For selenium accumulation, in which bacteria are also involved, see above.
Fungi.
There are many ecologically important fungus-legume interactions some of which have systematic and biogeographical implications (see also above under Ecology & Physiology). ECM plants are found in Caesalpinioideae-Mimoseae (phyllodinous Acacia, Senegalia), Faboideae such as Aldina, Gleditsia, and a group of five genera around Mirbelia, and in particular Detarioideae, and sometimes they are also AM, being dual-mycorrhizal plants (e.g. Sprent & James 2007; S. E. Smith & Read 2008; Bâ et al. 2011a, b; M. E. Smith et al. 2011; Bennett et al. 2017; Teste et al. 2019: Table S2, 21 AM/ECM genera) - Sprent et al. (1987: Table 7) had noted that a number of Detarioideae were AM plants. Over one hundred species of mostly basidiomycete fungi were identified in the ECM associations of three species, two of Dicymbe and one of Aldina (Detarioideae and Faboideae respectively), dominating in New World forests (M. E. Smith et al. 2011). ECM networks, as in Dicymbe forests in Guyana and in Gilbertiodendron dewevrei (another detarioid) forests in Cameroon, can be complex, and adult-seedling networks may be established as the seed germinates, even if there is no evidence of the movement of nutrients between adult and seedling (McGuire 2007b; Michaëlla Ebenye et al. 2016). Interestingly, ECM fungi from African Fabaceae and those on Uapaca (Phyllanthaceae) group together in phylogenetic analyses (Tedersoo et al. 2014a), perhaps because their hosts grow together. For further details of ECM fungi and Fabaceae, see Ecology & Physiology above and clade asymmetries, however, little seems to be known about mycorrhizal associations in Mimoseae, although at least Acacia s.l. can be AM or ECM (Hayman 1986; Teste et al. 2019). AM fungi are known from several N-fixing Fabaceae-Faboideae (Hayman 1986), and have been found in root nodules in several species although their importance there is unclear (Scheublin & van der Heijden 2006). O'Dell and Trappe (1992) list Faboideae that do not form AM associations; this often varies within a species. Some species of Lupinus that have no AM associations still have the genes that are part of the AM symbiosis toolkit, although these now have non-symbiotic roles (Delaux et al. 2014, see also above).
Lupinus can produce phomopsins, toxic macrocyclic hexapeptides that cause serious poisoning (lupinosis) when the plants are eaten by sheep and other animals - or rather, these hexapeptides are produced by the ascomycete Diaporthe toxica/Phomopsis leptostromiformis (the anamorph) which is variously an endophyte/pathogen/saprophyte in/of the plant (e.g. Allen 1998). Similarly, in Oxytropis kansuensis (and some other species of the genus) the toxic indolizidine alkaloid, swainsonine, is synthesised by the endophyte Undifilum, an imperfect stage of Pleosporaceae, Dothidiomycetes (= Alternaria), another ascomycete (Pryor et al. 2009; see also Reyna et al. 2012). Swainsonine is also found in some species of Astragalus and of course in Swainsona itself, also members of the IRLC, and in the former, at least, general endophyte richness was inversely related to plant size and endophyte presence (Ralphs et al. 2008; Harrison et al. 2018). In North America the legumes producing swainsonine are often called locoweeds, and they cause a serious, sometimes fatal, neurological disease in cattle that eat them. Note that fungal generic names are rather in flux, thus Rhizoctonia leguminicola (grows on clover, also produces swainsonine) = Slafractonia leguminicola (see also Poaceae-Pooideae and Convolvulaceae-Ipomoea for similar "plant" metabolites).
Rusts show patterns of distribution on Fabaceae that mirror systematic patterns in the family. Uromyces is found predominantly on herbaceous Faboideae (but also on Bauhinia and one or two other non-Faboideae), while Ravenelia is found on woody members of the family, Caesalpinioideae s.str. (but there is one record from Bauhinia) and especially on Mimoseae (Savile 1976, 1979a, b; El-Gazzar 1979). In some species of Ravenelia the teliospores, thick-walled spores in which nuclear fusion and then meiosis occur, are aggregated into groups, and these telial heads may mimic the groups of pollen grains (polyads) that are common in Mimoseae. Stingless Trigona bees pick up both telial heads and polyads as they forage for pollen, so helping disperse the fungus. Interestingly, the distributions of rusts, acacias and trigonid bees all break at about Wallace's Line; thus Ravenelia is not native to Australia while Acacia s. str. is centred there (El-Gazzar 1979; Savile 1979b).
Fabaceae include most ECM plants that also fix N, a rather uncommon combination (for Alnus, Betulaceae, see Walker et al. 2013), although relatively little is known about their eco-physiology. AM Fabaceae are also commonly associated with N-fixing bacteria (Bâ et al. 2011b). Annual legumes obtained less benefit from joint associations with AM and rhizobia than did perennials; in the latter the benefits from the joint association were enhanced, not additive or less (Primieri et al. 2021) - but annuals in general seem less likely to have AM associations. In taxa like Acacia rostellifera AM, EM and rhizobia may be found together (Teste & Laliberté 2018); in this case foliar Mn increased with age, suggesting that carboxylates were being used for P acquisition.
The establishment of AM associations and a variety of aspects of the nodulation process starting with root hair curling are connected and can be linked to an autophagy-related kinase, precursors for all these activities being produced by autophagy (Estrada-Navarrete et al. 2016: Phaseolus vulgaris). There are also connections with plant defence, the recognition of pathogen-associated molecular patterns (PAMPs), receptors related to the lysine motif - and these receptors are also involved in nodulation - effectors are involved in cutin metabolism, suppressing plant defences, whether in facilitating establishment of AM symbioses or the entry of pathogens (Oldroyd 2013; Desaki et al. 2017; see also AM fungi below).
Vegetative Variation. Although most Fabaceae have spirally-arranged, once- or twice-compound leaves, leaflets that are opposite and with entire margins, and pulvini associated with leaves and leaflets, there is extensive variation on this theme. Thus palmate leaves occur in Lupinus, leaflets with serrate margins in Cicer, Medicago, etc., and unifolioliate leaves are scattered throughout the family, perhaps most notably in Bauhinia, named after the botanical brothers Caspar and Jean Bauhin because the lamina is bilobed, and Cercis and relatives, all in Cercidoideae. Here bifoliolate leaves are reduced to a single leaf, that is often, but not always, lobed (Owens 2000) - Brenierea insignis, close to sister to Bauhinia s. str. has flattened stems (cladodes) and a leaf that is quite fleshy and unlobed. The single leaf in Cercis, at least, is the result of the fusion of two leaflets (Champagne et al. 2007) and it is not surprising that the leaf has a cryptic compound developmental program early in its development (Nakayama et al. 2022). The leaves of Tachigali (Caesalpinioideae) grow more or less continuously - rather like those of Chisocheton (Meliaceae) - and may live for seven years or so (Fonseca 1994).
Angiosperm leaf development is usually associated with the activity of the KNOX1 gene, and this is also true of plants with compound leaves incuding many Fabaceae. However, in the IRLC (Faboideae) the KNOX1 gene is not - or rather differently - expressed in the developing leaves, while it is the FLORICAULA/LEAFY (FLO/LFY) genes, normally floral meristem identity genes, that largely control leaf development, as well as being expressed in the flowers (Hofer et al. 1997; Gourley et al. 2000; Gleissberg 2002; Champagne et al. 2007; Wang et al. 2008; Peng et al. 2011; Townsley & Sinha 2012). Interestingly, pulvini are lacking in the leaves of the IRLC, but are present in nearly all other Fabaceae (see Dormer 1946b; Champagne et al. 2007; Rosin & Kraemer 2009). J. Chen et al. (2010) and Hu et al. (2020) and others have looked at leaf development in Medicago, the latter group looking at the development of mutants in M. truncatula that have palmate or pinnate leaves.
For lianes and vines in Fabaceae, see also "Ecology & Physiology" above; the taxa discussed here are all Faboideae. As might be expected, there is extensive both morphological and anatomical variation in climbing Fabaceae. Although successive ectopic cambia and interxylary phloem are quite common in lianes/vines (Moya et al. 2018), the correlation is not perfect. Nejapa et al. (2021) and Nejapa and Pace (2023) discuss the distinctive vascular anatomy of the liane Wisteria, the former also noting other climbing members of the family with similar anatomy - evolved independently. A recent study of Phaseolus lunatus showed ectopic cambia developing in just about every tissue in the stem (Rajput et al. 2023). Sousa-Baena et al. (2018b) discuss tendrillate plants in general. In some mutants of Pisum (= Lathyrus oleraceus) the leaf consists of nothing but a tendril with two orders of branching and the foliaceous stipules, the tendrils being modified abaxialized leaflets (Hofer et al. 2009), while in L. aphaca the photosynthetic function of the leaf is taken over by the large stipules, the rest of the leaf being an unbranched tendril; on the other hand, in L. nissolia the leaf is phyllodinous, entirely lacking tendrils (and leaflets) (see Kenicer et al. 2005 for a phylogeny). Sousa-Baena et al. (2014b, 2018a) also discussed the evolution of tendrils and the molecular control of their development, noting that in Faboideae FLO/LFY genes were largely involved, while in tendrillate leaves of Bignoniaceae-Bignonieae KNOX1 genes were also expressed - as would follow from the preceding paragraph.
In Acacia s. str. (the old subgenus Phyllodineae), the leaves of the mature plant are undivided structures that are flattened at right angles to the plane of flattening of a normal lamina; they are phyllodes. A long-standing question has been, what "is" this structure morphologically? - Gardner et al. (2008) summarized of the history of this controversy. In the early development of normal leaves of Fabaceae two rows of adaxial meristems produce the leaflets/pinnae, and these become lateral in position by secondary reorientation. In the phyllodes of Acacia there is a single, broader adaxial meristem that develops into the entire leaf (Kaplan 1980); there is no reorientation, hence the plane of flattening of the phyllode. In species like A. verticillata these phyllodes are densely set along the stem, some are associated with stipules and buds and have an extrafloral nectary, but others are simply flattened, needle-like processes (Kaplan 1980); the relationship between the two kinds of phyllodes is unclear. Rutishauser (1999) discusses the morphology of whorled leaves in Acacia, and Rutishauser and Sattler (1986) and Sattler et al. (1988) the development of the leaves of A. longipedunculata where there are only seven traces and 1-3(-4) axillary buds per whorl of up to 27 phyllodes, and colleters and other structures are also involved. Rutishauser (2016b) discusses the variety of vegetative phyllotactic arrangements to be found in Acacia, especially evident when the plant is producing phyllodes. The phyllodes often have stipules, and their position depends on the insertion of the phyllodes; the phyllodes may lack associated stiples, and then their vasculature differs from those that have them (one- versus three-trace). Seedlings and regeneration shoots of Acacia can have normal-looking and normally-arranged once- or twice-compound leaves as well as intermediates between such leaves and phyllodes. Pasquet-Kok et al. (2010) looked at the complex functional aspects of this change from regular leaves to phyllodes during development in the Hawaiian A. koa, and they found i.a. that phyllodes were more drought tolerant but regular leaves might grow faster and be more shade tolerant.
Daviesia (Mirbelieae area), a scleromorphic Australian member of Faboideae, also shows extensive foliar variation. Here Crisp et al. (2017) suggest that the leaves are neither simple nor compound, even in seedlings, all foliar structures being phyllodinous. Venation can be linear or strongly reticulate, the latter in D. latifolia. Members of the D. cardiophylla group are described as having three node-like thickenings at the bases of the midrib and of the marginal veins, while the leaves of species like D. stricta and D. crenata are drawn with apparent articulations where the petiole joins the stem (Crisp et al. 2017). Furthermore, a number of species have anomalous secondary thickening in their roots made up of concentric layers of interconnected vascular strands that result from the activity of successive cambia (Pate et al. 1989), as also in some Acacia. Cluster roots, probably involved in the uptake of phosphorus, are common here and in related genera (Nge et al. 2020). Jacksonia floribunda, also Mirbelieae, has well-developed phylloclades which look like simple leaves with reticulate venation and serrate margins (Dörken et al. 2024).
Some species of Mimosa and other genera have leaves that are sensitive to touch (= thigmonasty), stimulus transmission occurring as membrane depolarisation propagates down the petiole and along the stem (Volkov & Markin 2014 for a summary; Tian et al. 2021 for the application of Mimosa-type bending). The folding of the leaf is caused by turgor changes in the cells of the pulvini at the bases of the leaf and leaflets; for the anatomy of the pulvinus, which has an endodermis, see e.g. Weintraub (1952) and Rodrigues and Machado (2007). Simon et al. (2011) suggest that such sensitive leaves have evolved about six times in Mimosa alone. In Codariocalyx (= Desmodium) gyrans the single pair of lateral leaflets move intermittently without being touched, the speed of movement increasing with the temperature. A full understanding of the evolution of such features depends on more extensive studies on this and related phenomena in legume leaves. Thus the leaves of the mimosoid Albizia (Samanea) saman show nyctinastic movements, the leaflets folding together towards the evening when the light is failing, or just when there is heavy cloud cover, this behaviour being responsible (in some tellings of the tale) for the common name of this plant, the rain tree. Nyctinastic movements of various kinds are quite widespread in the family and may correlate with phylogeny (e.g. Lavin 1988; Farruggia et al. 2018 and references). Takahara et al. (2022/2023) note that there are distinctive circumferential slits in the cortical motor cells of the pulvini in the Fabaceae that they examined, members of Faboideae and Caesalpinioideae, the former including Medicago, Glycine and Trifolium that belong tp the IRLC - a distinctive feature of which is that it lacks a well-developed pulvinus... The walls of these pulvinar slits were low on cellulose, for example, but had much de-methyl-esterified homogalacturonan without calcium crosslinking; these slits are involved in anisotropic cell proximodistal extensability, their width increasing or decreasing under hypotonic and hypertonic conditions respectively (Takahara et al. 2022/2023).
Phyllodes aside, there is a fair amount of phylogenetic signal in whether or not the leaves are unifoliolate, bifoliolate (and/or connate), once-pinnate, or twice pinnate. In some Caesalpinioideae-Ceratonieae and -Caesalpinieae with twice-pinnate leaves there is also a terminal pinna, the leaf ending in a triad of pinnae (Herendeen & Herrera 2019). Not enough attention has been paid to whether or not the leaflets are sessile or more or less petiolulate.
Nodal anatomy is more variable than might be thought. Multilacunar nodes are scattered in the family, while on the other hand Ulex (= Genista s.l.) has 1:1 nodes (see especially Watari 1934 for nodal and petiolar anatomy - 133 species examined). The pattern of change in nodal anatomy during ontogeny in Vicia is complex, and in adult plants the lateral bundles sometimes arise a full internode below the node they innervate (Kupicha 1975). Slade (1952) looked at nodal anatomy, etc., in the cladodes of Carmichaelia (Fab-Galegeae, also complex.
Genes & Genomes. There may have been a whole genome duplication in Fabaceae prior to the divergence of Dalbergieae (Bertoli et al. 2009; Schmutz et al. 2010; Cannon et al. 2014: Copaifera; see also Young et al. 2011; Wang et al. 2017), and subsequent chromosomal rearrangements have been extensive (Murat et al. 2015b: Glycine, Lotus). However, exactly when this duplication - if it is a single event - occured and where it is to be placed are unclear. What may be this duplication, the COPOα duplication, ca 69.1 Ma and involving much of the family, is mentioned by Landis et al. (2018). There may be additional duplications somewhere in Cercidoideae and in the ancestor of [Chamaecrista + Mimoseae] (e.g. Young et al. 2011; Landis et al. 2018: ca 52.9 Ma, GYDIα, Gleditsia), although it is again not clear exactly where they occurred and what evolutionary consequences, immediate or otherwise, there might be for the clades involved (Cannon et al. 2014: Swartzieae s.l. not sampled). A genome duplication (67-)63.7-54 Ma (Schmutz et al. 2010; op den Camp et al. 2011; Q.-G. Li et al. 2013; Vanneste et al. 2014a, 2014b; Werner et al. 2014; Landis et al. 2018: GLSOβ, Cladrastis etc.) seems connected with Faboideae in particular rather than Fabaceae in general. A genome duplication near the NPAAA crown group has been dated to ca 54 Ma (op den Camp et al. 2011; Q.-G. Li et al. 2013), (67-)63.7, 57(-56) Ma (Vanneste et al 2014a), or ca 53 Ma, but again, exactly where it is to be placed is unclear (Murat et al. 2015b). This is all rather confusing, and the extreme hypotheses seem to be a family within which there have been separate deep duplication events (e.g. Cannon et al. 2014) versus a single duplication event that may also have involved Quillajaceae and Polygalaceae as well (Wong et al. 2017), although these hypotheses could be combined... Indeed, Y. Zhao et al. (2021) suggest that there have been some 28 whole genome duplications here, one involving the family as a whole, one for each of the subfamilies (excluding the monotypic Duparquetioideae), and the rest (actually, including a couple of triplications) within Caesalpinioideae and Faboideae, most of these latter being fairly shallow.
Jiang et al. (2013) examined the fate of duplicated genes in Glycine max, interestingly, there is no evidence of fractionation bias or genome dominance associated with this duplication, so it may have been an autoploid event, while there was evidence of fractionation bias, at least, for a duplication in Medicago, suggesting alloploidy (Garsmeur et al. 2013). For genome duplication, N fixation and the rise of tropical forests rich in N-fixing legumes (e.g. Epihov et al. 2017), see above.
It has been suggested that the ancestral chromosome number for the family is x = 7 (e.g. Goldblatt 1971; Carta et al. 2020). Cercis is diploid, everything else is polyploid (chromosome numbers of Duparquetia are unknown). There has been subsequent polyploidization within Cercidoideae - perhaps Cercis x extinct clade → allopoyploid Bauhinia, etc. (Stai et al. 2019), while the rest of the family was also polyploid because of independent polyploid events that had occurred at the base of each subfamily (see also Cannon et al. 2014; L. Ren et al. 2019 ), however, c.f. in part the preceding paragraph. The CYCLOIDEA gene is duplicated in all Cercidioideae except Cercis (Sinou et al. 2020). Thus Cercis at n = 7, may represent the ancestral genome of the family, and it also has a very small genome (at 1C = 375 pg/367 Mbp, the equal smallest in the whole family) that has evolved slowly (Stai et al. 2019). However, if a clade [Cercidoideae + Detarioideae] is sister to the rest of the family, it complicates thinking about genome evolution (Koenen et al. 2019b/2020a).
L. Ren et al. (2019) focussed on genome evolution in Faboideae, where there is a fair bit of variation, perhaps especially in the basal members (whose relationships are rather uncertain: Note also that in Stai et al. (2019) and Ren et al. (2019) variation in chromosome numbers is dealt with by emphasizing modal numbers and Polygalaceae are not mentioned as a possible outgroup for Fabaceae.) Overall, there is little polyploidy in Faboideae, and there has been extensive reduction in chromosome numbers. However, within Trifolium there has been extensive polyploidy and aueuploidy (e.g. Ellison et al. 2006). For chromosome numbers, see also Goldblatt (1981), and for karyological features in Swartzia, see Pinto et al. (2016), for those of Hymenaea and relatives (Detarioideae-Detarieae) see Serbin et al. (2019), while Moraes et al. (2020) looked at chromosome number evolution in the speciose Dalbergieae, noting both ascending and descending diploidy there.
Souza et al. (2019) found that genome size increased with latitude in the Caesalpinia Group, and this and related correlations are quite common in flowering plants. Lathyrus (inc. Pisum, Faboideae-IRLC-Fabeae) have diffuse centromeres/holocentric chromosomes, and this follows a duplication of the CenH3 gene (Neumann et al. 2015). In Fabeae there is extreme centromeric satellite diversity (Robledillo et al. 2020). Finally, there are a number of interesting corrlations in Fabeae: small genomes with annual habit, fewer flowers, fewer ovules/flower, and self compatability versus larger genomes with perennial habit, more flowers, more ovules/flower and self incompatability (Sinjushin 2021b). Taxa in Trifolieae, Genisteae and Phaseoleae - and hence, perhaps, much of Faboideae - have lost microRNA827 (Lin et al. 2017). Bertioli et al. (2009) link genome regions that are variable with the presence of retrotransposons. For heterochromatin variation in Caesalpinieae, see Van-Lume et al. (2017). Lyu et al. (2017) found that the genome size of the mangrove associate Pongamia pinnata was unexceptionable.
There is little variation in the plastomes of Caesalpinieae (Aecyo et al. 2021). The plastomes of many Mimoseae, Faidherbia, Inga, etc., but not Parkia, Prosopis and the Inverted Repeat Expansion Clade, are 10-15 kb larger than those of other legumes, partly because of the extension of the inverted repeat into the small single copy region and partly because of tandem repeat expansions (Dugas et al. 2015; Y.-H. Wang et al. 2017, 2018).
There have been major changes in plastome organisation in Faboideae in particular that, apart from their intrinsic interest, provide a considerable amount of phylogenetic structure (e.g. C. Lee et al. 2021). The plastome here is notably labile, both in terms of sequence (Kua et al. 2012) and structure (G. E. Martin et al. 2014 for a summary). Thus most Faboideae have a 50 kb inversion in the large single-copy region of their plastomes, however, taxa like Swartzieae, Cladrastis, and a few others, lack this inversion (J. J. Doyle et al. 1996, 1997; Pennington et al. 2001; Wojciechowski et al. 2004). In Sesbania there has been a reversion of this inversion (lee et al. 2021), while in Genisteae and their immediate relatives there has been a 36 kb inversion inside this inversion (Martin et al. 2014)... In the 50 kb inversion clade the plastid genomes have become highly rearranged, there is plastid genome incompatability and there are also increased nucleotide substitution rates in both plastid and mitochondrial genomes. Note that there is coevolution of nuclear and organellar genes - plastid proteostasis - because of the close interactions of the two, for instance, several chloroplast proteins are synthesized by nuclear genes, etc., nonsynonymous substitution rates are higher in both plastid-encoded and nuclear-encoded plastid-targeted ribosomal protein genes and these genes have significantly different nonsynonymous substitution rates in the 50 kb inversion clade compared with those in other legumes (see Forsythe et al. 2021; Tressel et al. 2025).
Of course, the best known plastome change in Fabaceae is the loss of the inverted repeat which characterises a major clade within the Faboideae 50 kb inversion clade (Kolodner & Tewari 1979; Lavin et al. 1990), the IRLC, the Inverted Repeat Loss Clade above; the inverted repeat has been lost independently in Camoensia scandens (C. Lee et al. 2021). The IRLC is a largely temperate, herbaceous and very speciose group (Wojciechowski 2003 and references). Temperate members of the IRLC lack the clpP intron, while the rps12 intron has been lost from all members of the clade examined except Wisteria, Callerya and Afgekia, but not Glycyrrhiza (Jansen et al. 2008; Wojciechowski et al. 2008; see also Saski et al. 2007; Cai et al. 2008; c.f. Sabir et al. 2014 in part). In Medicago minima, a member of the IRLC, a new IR, albeit only ca 7 kb long, has developed (I.-S. Choi et al. 2019a). Charboneau et al. (2021) looked at the plastomes of some NeoAstragalus (= New World Astragalus) and found a number of gene/intron losses and inversions there and elsewhere in the IRL clade; inversions, they thought, might be the result of microhomology-mediated break-induced replications, and repeat clusters, etc., showed a fair amount of phylogenetic signal.
Overall, nucleotide substitution rates in the plastomes of Faboideae were higher than those of other Fabaceae, and in part this may be because many of the former are herbaceous. Substitution rates in IRLC plastomes tend to be higher than those in other taxa, and overall plastome evolution there has been considerable (Magee et al. 2010; Sabir et al. 2014; Schwarz et al. 2015, 2017). Desmodium and possibly related genera have lost the rps12 intron, and it has moved to the nucleus (Doyle et al. 1995; Bailey et al. 1997; Jansen et al. 2008). ORF 184 has been lost many times, especially in the MILL clade, and accD (= ORF 512, zpfA) has also been lost (Doyle et al. 1995). Both the rps16 and ycf4 genes are lost in many Faboideae (Doyle et al. 1995; Jansen et al. 2007), the former being absent in all members of the IRLC, but it is also absent in four other clades in Faboideae (Schwarz et al. 2015). Y.-H. Wang et al. (2018) summarize much of the work on the evolution of the plastome in Fabaceae.
Transmission of plastids may be biparental (Phaseolus unclear: Corriveau & Coleman 1988; Q. Zhang et al. 2003), perhaps predominantly paternal in Medicago (Matsushima et al. 2008; for Lathyrus (inc. Pisum), see Bogdanova et al. (2021). Indeed, in cases of hybridization in Medicago and Pisum s. str. in particular incompatability between chloroplasts from one parent and the hybrid genome (plastome-genome incompatability - PGI) may result in the death of those chloroplasts and thus to variegation (Ruhlman & Jansen 2018 and references). Heteroplasmidy, two plastid types differing in this case in the orientation of a ca 45 kb segment of the plastome in the accD coding region, is known from within M. truncatula ssp. tricycla (Gurdon & Maliga 2014).
I.-S. Choi et al. (2019b) found that legume mitochondrial genome varied extensively in size, variation perhaps correlated with relationships, although sampling was somewhat exiguous from this point of view; even taxa with similarly-sized genomes might share little DNA. G. Petersen et al. (2020) noted that here has been extensive movement of the legume mitochondrial genome, particularly that of Mimoseae, into the parasitic Lophophytum (Balanophoraceae).
Cytisus purpureus forms a well-known graft hybrid with Laburnum anagyroides (+ Laburnocytisus adamii; see Herrmann 1951 for another example); the epidermis alone is Cytisus tissue, and any seeds, being derived from cells from deeper layers, will give Laburnum plants. However, the graft hybrid often breaks down, resulting in branches that are pure L. anagyroides or pure C. purpureus.
Economic Importance. Seeds of Fabaceae-Faboideae in particular are a major source of proteins for humans; Weeden (2007) discusses the diversity of genetic changes involved in domestication of legumes. Soybeans provide around 2.1% of the caloric intake of the human population; they are #2 of the non-grain crops. For information on the domestication of soybean (Glycine max: Phaseoleae), common bean (Phaseolus vulgaris: Phaseoleae), pea (Pisum sativum, = Lathyrus oleraceus: Fabeae), the azuki bean (Vigna angularis: Phaseoleae, see also V. radiata and V. unguiculata) and relatives, see papers in Ann. Bot. 100(5). 2007, for these taxa and lentils (Lens [= Vicia] culinaris: Fabeae), see Fuller (2007) and for the soybean, see da Silva and de Majo (2020). For the peanut, Arachis hypogea (Dalbergieae), an allopolyploid having A. duranensis and A. ipaënsis as its probable parents, see Krapovikas and Gregory (1994), for its domestication, see Dillehay et al. (2007) and for its phylogeny, see Friend et al. (2010) and Moretzsohn et al. (2013), for the domestication of the lima bean, Phaseolus lunatus, see Serrano-Serrano et al. (2010), for much information on the forage crop, alfafa (Medicago sativa-Medicageae), see Small (2011), also M. truncatula, for the relatives of soybean, see Sherman-Broyles et al. (2014). The black-eyed pea or cowpea, Vigna unguiculata, is an important crop in drier regions, especially in Africa and Asia; see also pigeon pea (Cajanus cajan), chickpea or garbanzo bean (Cicer arietinum). For oils from soybean and peanuts, see papers in Vollmann and Rajcan (2009), and for gum arabic, the exudate of Senegalia senegal (Mimoseae), see Bakhoum et al. (2018).
In Africa the obligate hemiparasite Striga gesnerioides, morphologically distinct from the rest of the genus and the only species not to be found on grasses, is an important parasite - along with the holoparasite Alectra vogeli - of the cowpea, Vigna unguiculata (Spallek et al. 2013).
Fabaceae are over-represented among clades that have become naturalized and/or are invading natural areas. Thus Leucaena leucocephala is a particularly notable invasive species, and 6 of the top 50 genera with the most naturalized species belong here (Daehler 1997; Pysek et al. 2017).
Chemistry, Morphology, etc.. The diversity of secondary metabolites in Fabaceae, perhaps especially in Faboideae, is remarkable (see also above) - for instance, about 28% of all known flavonoids and about 95% of the isoflavonoid aglycone structures, over 1,000 of these alone, have been found here (see also Barbero & Maffei 2017, Arimra & Maffei 2017 for references). Isoflavonoids are restricted to Faboideae and are involved in plant defence (phytoalexins); they may also play a role in nodulation (Hegnauer & Grayer-Barkmeijer 1993; Reynaud et al. 2004). Flavonoids lacking the 5-hydroxy group are characteristic of Fabaceae (Seigler 2003), but I do not know at what level they might be apomorphic. Pea albumin, a small sulphur-rich peptide involved in food storage - it also has insecticidal properties - is known only from Faboideae, and the albumin-1 gene may be a synapomorphy for the [Galegodae + millettioid] clade (Louis et al. 2007); it is not to be found in some/all robinioids, and it has been transferred at least twice to parasites, Cuscuta, and Orobanche and relatives (Y. Zhang et al. 2013). Colville et al. (2015) linked the presence of homoglutathione to the genome duplication in Faboideae and to the Old World clade; the function of homoglutathione, unlike that of glutathione, was unclear.The Australian Gastrolobium (Mirbelieae) produces the toxic sodium monofluoroacetate (Chandler et al. 2001). Resins found in some Detarieae contain distinctive bicylic diterpenes (Fougère-Danezan et al. 2007). Overall, however, despite the diversity of secondary metabolites in the family, their correlation with clades is for the most part poor (Wink 2013).
Characters of "caesalpinioid" woods include rays that are usually more than 20 cells tall, presence of silica bodies, and axial canals (Evans et al. 2006). Some Mimoseae and Faboideae have leaves that are rich in silica (Westbrook et al. 2009). There is sometimes an endodermis in the stem - Lathyrus, Neptunia etc. - [check Seago]; Colleters have been reported from Caesalpinioideae, especially Chamaecrista (Coutinho et al. 2015; Silva et al. 2018 and de Barros et al. 2017b and refs). Stomatal morphology varies a great deal within Dipterygeae (Silva 2018).
Both Inga and Tachigali, Caesalpinioideae and quite diverse, have notably short generation times, some species of the latter even being monocarpic (T. R. Baker et al. 2014); for relationships in the former, see Nicholls et al. (2015), and for those in the latter, see Huamantupa-Chuquimaco et al. (2024).
Inflorescences in a few tribes in Faboideae are pseudoracemes, that is, the main inflorescence axis is indeterminate, but the flowers are in axillary groups, often made up of three, but up to twelve or so flowers and in a more or less fasciculate-cymose arrangement (Tucker 1987b, 2006; Tucker & Stirton 1991: several "bracteolar" structures associated with a single flower; Teixeira et al. 2009). Dialioideae also have distinctive inflorescences, the ultimate axillary flowers on the inflorescence branches being subtended by bracts, but themselves having no bracteoles (Falcão et al. 2020) - a modified cymule? Krüger et al. (1999) interpreted the flowers of Colophospermum as having two lateral prophylls and two vertical sepals - not four sepals.
Although there have been fairly extensive studies on the floral vasculature of Fabaceae, (see e.g. J. A. Moore 1936a: 42 genera, 60 species - Faboideae: Randhawa 1969: 89 genera, 149 species) they are relatively poorly known. Moore distinguished between unihiate taxa with common KCA traces, the Phaseolus type (Phaseoleae, inc. Wisteria, probably Millettia), and dihiate taxa, with KC and A traces in separate whorls, the two whorls either being close together or even overlapping, the Baptisia type (Sophoreae, Podalyrieae, Genisteae), or quite well separated, the Lathyrus type and the common condition. The Phaseolus vascular type had distinctive annular nectaries (Moore 1936b). Trihiate development in which the K, C and A whorls are separate, is known from Mimoseae. (Note that in the characterisations and discussion above I refer directly to the number/nature of the whorls evident during development - it seemed simpler.) Zalko et al. (2022a, especially 2022b) noted additional features like the relation between the perianth and stamen whorls on origination, etc., however, given our current state of knowledge there seems to be rather little systematic signal in the data. Zalko et al. (2022b) also discuss ideas as to the nature of the nectary disc. Independently from the issue of their vascularization, the parts of the flowers of most Fabaceae often develop in unusual sequences, i.e. not simply from the outside in, but often sepals-carpel-petals-outer stamens-inner stamens, and there are other distinctive features of their development, including unidirectional organ initiation and primary common primordia which give rise to antesepalous stamens and secondary common primordia, the latter then giving rise to petals and antepetalous stamens (e.g. Tucker 1984; Feng et al. 2006; Wang et al. 2008; Movafeghi et al. 2011; Falcão et al. 2020: c.f. Table 1 and p. 38). The pattern of initiation of the sepals and stamens is variable, by no means always being unidirectional (e.g. Prenner 2004a; de Chiara Moço & de Araujo Mariath 2009; Leite et al. 2015). Prenner (2013b) found that petal initiation in Abrus, as in some other Faboideae, was simultaneous. There are CA primordia in the flower, A initiation is bidirectional, and there is overlap in the timing of C, A, and G initiation is members of the IRLC (Naghiloo & Dadpour 2010). Flowers in which all parts initiate in a helix, as in Gleditsia, are very uncommon (Tucker 1991). For more on variation in general patterns of floral development, see Prenner (2004a: development largely centripetal and whorled, except antesepalous stamens; Prenner 2004e: Lespedeza, but c.f. Tucker 2006). Prenner and Klitgaard (2008b) emphasized the diversity of developmental patterns even within the corolla whorl, thus although both Duparquetia and Faboideae have the adaxial petal in the outermost position, the two get there by developmentally different pathways; Bento et al. (2021: Table 2) give an idea of the variation in corolla initiation in early-diverging Faboideae. For the diversity of secretory structures - beyond just nectaries - in developing flowers of a variety of Fabaceae, see de Barros et al. (2017b).
Mirror image flowers are scattered in Fabaceae, and are quite common in some Cassieae (Tucker 1996b). In a number of Mimoseae the flowers are heteromorphic, the outer flowers of the capitate inflorescences being more conspicuous, either having a larger corolla, as in some Albizia (Rico Arce et al. 2008), or petal-like staminodes, as in Neptunia pubescens, a species that can have three floral morphs in the one inflorescence (Tucker 1988b). Details of hypanthial evolution within Fabaceae are unclear; the hypanthium seems to have become much reduced and lost several times. The "normal" (for flowering plants) floral orientation with the median sepal adaxial and the median petal abaxial is found in Minoseae (see also Gleditsia, Ceratonia, Ertythrophloeum), however, in some 4-merous Mimoseae things get complicated and the median petal is adaxial (Prenner 2011; see also Alvarado-Reyes et al. 2024). For development in the rather odd flowers of Bauhinia galpinii, see A. V. S. Silva et al. (2024). There is self incompatability in Tylosema esculentum (Bauhinioideae) (Hartley et al. 2002).
In Duparquetia the sequence of development of the floral parts is "normal" (although not normal for Fabaceae), but not much else is (Prenner & Klitgaard 2008b). When there is the complete loss of individual floral structures in evolution, floral development can be greatly changed (e.g. Tucker 1988a, 1990, 2000a), and in Gleditsia the inflorescences may start developing under the bark - development is rather odd there, too (Tucker 1991). However, as Bruneau et al. (2014) noted, in Detarioideae organs in one whorl can be lost without much affecting the development of the rest of the flower - as with the complete loss of a calyx member in Duparquetia and Goniorrhachis (Prenner & Klitgaard 2008b; Prenner & Cardoso 2016). For additional information about floral development in "caesalpinioids", see also Mair (1977: monosymmetry), Tucker (1996a, b, 2000b: Detarioideae), 2001, 2002b, c, 2003c [all Detarioideae]), Tucker (1998) and Zimmerman et al. (2013a, b), all Dialioideae, where complete organ loss is common, Pedersoli et al. (2010: Copaifera, Detarioideae), and Bruneau et al. (2014: Detarioideae). Prenner and Cardoso (2016) note that in Detarioideae a 4-merous calyx may be the result of fusion of two members or, in Goniorrhachis, the absolute suppression of one member (long plastochrons, no space left), much as in Dialioideae (Zimmerman et al. 2017). Falcão et al. (2020: p. 42) suggested that in Apuleia leiocarpa the carpel is in the same whorl as the stamens, the original central carpel having aborted and the carpel that we see is in fact "one of the three stamens [that] is modified into [the] carpel". Bauhinia has "staminodial" structures at the base of the ovary (Endress 2008c) that may have something to do with colleters.
Faboideae. For basic floral anatomy in Faboideae, see and above). Prenner (2013a, b: esp. androecium) surveyed floral development in Faboideae and suggested that in some species there was a slight asymmetry in the early development of the androecium (the adaxial median stamen is initiated slightly off the median axis) in more basal Faboideae, and also in some "Caesalpinioideae", rather than exactly on the median plane (Prenner 2004c). The complex floral vasculature in Swartzia is described e.g. by Zalko (2025), and see also Tucker (1990, 2003b) and Paulino et al. (2013). The flowers of some Amorpheae have a stemonozone, not a hypanthium, i.e. the staminal tube is adnate to the petals (McMahon & Hufford 2002). For floral and inflorescence morphology, especially in Faboideae-Loteae, see Sokoloff et al. (2007a). See also Mansano et al. (2002) and Mansano and Teixeira (2008: Lecointea clade, Exostyleae), Song et al. (2011: Clianthus), Paulino et al. (2011: Indigofera), de Chiara Moço and de Araujo Mariath (2009: Adesmia) and Bento et al. (2021), both Dalbergieae, in the latter see also non-papilionoid flowers in Faboideae, and Table 2, initiation of monosymmetry, shape of floral primordium, etc..
Caesalpinioideae. Casanova et al. (2020) looked at floral development in Tachigali-Sclerolobieae. Within Mimoseae, the calyx of Parkia multijuga (Mimoseae) is quite monosymmetric, especially in bud (Pedersoli & Texeira 2015). Ramírez-Domenéch and Tucker (1988) found that Mimoseae have centripetal organ initiation, the three whorls initiating in sequence, although there was variation in the details of the initiation of the outer (= sepaline) whorl. For the adaxial sepal of Mimoseae, see Ramírez-Domenéch and Tucker (1990); they describe a variety of developmental pathways that result in the connate calyx of that clade. For more on floral development in Mimoseae, see Gemmeke (1982); stamens may develop centripetally on five main primordia. Gonçalves et al. (2024) emphasized the variation in meristicity in Mimosa, noting how often the organs involved were entirely absent, lacking even a trace of rudiments, however, in staminate flowers the gynoecium was usually present, if rudimentary. De Barros et al. (2017a: useful tables) describe floral development of taxa in and close to Mimoseae. The cochlear-descending calyx aestivation, helically-initiated androecium, etc., of Calliandra s. str. are distinctive (Prenner 2004b). Luckow and Grimes (1997) and de Barros and Texeira (2015) and de Barros et al. (2016) describe the diversity of the remarkable apical glands, sometimes vascularized, on mimosoid anthers, noting also the sculpting of the connective cells.
Guinet (1981a) outlined some major patterns of pollen variation in the family. Hesse (1986) noted that both Bauhinia and Cercis - and Caesalpinia and Delonix - had pollen-connecting threads made up of something other than sporopollenin. For pollen variation in "Caesalpinioideae", see Graham and Barker (1981), Banks et al. (2003), Banks and Rudall (2016) and Banks and Lewis (2009, esp. 2018 and references), for that in Stryphnodenron and relatives, see Barduzzi et al. (2024) and for that in Mimoseae, see Liau-Kiang et al. (2024). For polyads, anther dehiscence, etc., in Mimoseae see also under Pollination Biology & Seed Dispersal above, for pollen, see also Banks and Klitgaard (2000: Detarioideae) and Banks et al. (2013, 2014: Cercidoideae). Pollen of Cercidoideae and in particular Detarioideae (Banks & Lewis 2018) is notably variable, while that of Duparquetia is unique among angiosperms (Banks et al. 2006). Aperture position in this clade does not follow Fischer's rule (Banks et al. 2010). Ferguson and Skvarla (1981) discuss pollen of Faboideae (see also Diez & Ferguson 1996; Kuriakose 2007). Calvacante de Oliveira et al. (2022) found considerable variation in shape and exine ornamentation in vataireoid pollen, and, like other floral characaters in that area, they contained little phylogenetic signal.
Compared with the variation in other parts of the flower, that of the unfertilized gynoecium is slight: There is nearly always just a single carpel with the same orientation, although it is rarely resupinate. Paulino et al. (2014) noted that polycarpellary gynoecia were commonest in Mimoseae, but rare in Faboideae with keel flowers. The style is at least sometimes hollow, although the cavity arises in various ways, including by lysigeny (Lersten 2004). Heterostyly is known from Tylosema (Bauhinioideae) (Hartley et al. 2022). The carpels may have five traces and are quite often open during development, the ovules being exposed, in taxa now placed in non-mimosoid Caesalpinioideae and in Detarioideae-Dialieae, but not, apparently, in Cercidoideae or Mimoseae; there is one record from Faboideae (Tucker & Kantz 2001). The embryo sac of some Faboideae (?elsewhere) more or less protrudes into the micropyle, as in Archevaletaia (Maheshwari 1950). Both a true (integumentary) endothelium and a nucellar endothelium may be present in Faboideae (Rodriguez-Pontes 2008 for discussion and references).
After fertilization a considerable amount of variation in fruit, seed and embryo morphology and anatomy develops, as is clear from the illustrations of fruit and seed from the endpapers of Lewis et al. (2005). In Astragalus (quite commonly) and Oxytropis (rarely) the fruit is longitudinally more or less septate, the septum being either a funicular flange developing on the adaxial side of the fruit and/or a septum developing from the abaxial commissure (e.g. Barneby 1964). Nemoto and Ohashi (2003) discussed variation in lomentum anatomy in Desmodieae, recognizing seven "types", and also finding that pericarp anatomy suggested the removal of two genera from the tribe. Van Staden et al. (1989) discuss seed morphology and anatomy in the context of dispersal, germination, etc., while Kashyap et al. (2021) and Darzi and Zarre (2024) focus on the seeds of Astragalus - there seems to be relatively little variation, at least in the surface of the exotesta. Seeds of Fabaceae are commonly physically dormant, and for the role that the testa plays in dormancy, see Smýkal et al. (2014). Burrows et al. (2018) described the behaviour of the lens - a tiny structure on the other side of the hilum to the micropyle - in seeds of Australian species of Acacia, which after a mild stimulus might pop open or otherwise change, affecting subsequent imbibition by the seed and its germination; water may also enter via the hilar fissure in Faboideae, or via the hilum, or cracks in the testa may develop, and so on (Smýkal et al. 2014). The pleurogram, found mostly in Caesalpinioideae, is also at least sometimes involved in germination, being another pathway for the entry of water (Rodrigues-Junior et al. 2019: esp. Senna, 2020; also De-Paula and Oliveira 2008, 2012: esp. Chamaecrista). The absence of a pleurogram in Mimoseae in particular is often associated with hydrochory and anemochory, and also the seeds have a thin coat (Bruneau et al. 2024). Testa anatomy is quite complex in Fabaceae - see especially Corner (e.g. 1951, 1976) and Manning and van Staden (1987). However, a few taxa scattered throughout the family have so-called overgrown seeds; here the seed coat is largely undifferentiated and the growth of the seed is almost unconstrained except by the walls of the carpel - as Corner (1951: p. 141) noted, such seeds "have the nature of tumours" (see also Jordaan et al. 2001: Colophospermum). The two recurrent vascular bundles lateral to the hilum are absent in basal Faboideae (Lackey 2009). The cotyledonary areole, found in a number of Faboideae that also have some endosperm as seed reserve, consists of cotyledonary cells that differ in size, shape, stainability, etc., from the others; the size of the areole is partly linked to the amount of endosperm (Endo & Ohashi 1998; Lackey 2011). Rodriguez-Pontes (2007) mentions the pattern of cell wall formation in the endosperm in Senna. There is a great deal of variation in the embryo suspensor, even within Faboideae, especially in Vicia and Phaseolus coccineus where endopolyploidy in the suspensor can reach 8,192 C (e.g. Nagl 1962, 1974; Brady 1973; Lersten 1983 and references; also Tucker 1987; Yeung & Meinke 1993; Rodriguez-Pontes 2008; Endo 2012b; Shi et al. (2015: coleorhiza in some taxa?). This variation needs to be integrated with the phylogeny. There is amyloid in the cotyledons in Detarieae (Hegnauer & Grayer-Barkmeijer 1993), also in Sclerolobieae, the old Tachigalieae (Kooiman 1960; Meier & Reid 1982). D. L. Smith (1981) discussed cotyledon vasculature and anatomy; quite variable. For the aborting plumule in seedlings of Lotus and Coronilla and their relatives, see Dormer (1945a).
For additional general information see Polhill and Raven (1981), Ferguson and Tucker (1994), Crisp and Doyle (1995), Doyle and Luckow (2003) and Lewis et al. (2005: geographic distributions, illustrations, etc. of all genera); for ingoid Caeesalpinioideae, see Barneby and Grimes (1996, 1997) and Barneby (1998), for much information about South African Faboideae, see Moteetee and van Wyk (2015), for Cercidioideae, Wunderlin et al. (1987), for Genisteae, Polhill (1976), for Erythrina, Allertonia 3(1). 1982 (Erythrina Symposium IV), for Millettieae, Geesink (1984), for Sesbanieae, Lewis (1988), for the Caraganeae area, see Ranjbar (2014) and the Mirbelieae area, see R. L. Barrett et al. (2024), for Acacia s.l., Pedley (1986), for Robinieae, Lavin and Sousa (1995), for Inga, Pennington (1997), for Podalyrieae and Hypocalypteae and relatives, Schutte and van Wyk (1998a: inc. chemistry, 1998b respectively), for Dialioideae, see Zimmerman et al. (2017) and Falcão and Mansano (2021) and for Lathyrus, see Kenicer and Parsons (2021). For secondary metabolites in general, about which much is known, see e.g. Hegnauer (1994, 1996), Southon (1994), and Hegnauer and Hegnauer (2001), also Frohne and Jensen (1992), Waterman (1994), Wink and Waterman (1999: evolution), Dixon and Sumner (2003), and Wink (2003, 2013), for alkaloids, see Greinwald et al. (1996: Brongniartieae), Aniszewski (2007) and van Wyk (2003: Genistodae, esp. Table 1, useful), for quinolizidine alkaloids in particular, see Greiwald et al. (1966, 1992), Kinghorn and Balandrin (1984), Ricker et al. (1994), Bunsupa et al. (2012: synthesized from cadaverine) and Kite (2017: in genistoids), for gums and resins, see Lambert et al. (2009, 2013), for galactomannans, see Nadelmann (1890), Reid (1985), Meier and Reid (1982: Lupinus), and Buckeridge et al. (1995, 2000a, b; Lackey 2011; ratio of galactose to mannose varies, of phylogenetic interest?), for glucosylceramides, see Minamioka and Imai (2009), for glycosylated flavonoids, see Kite et al. (2013), for polysaccharides and flavonoids in particular, see Hegnauer and Grayer-Barkmeijer (1993) and Harborne and Baxter (1999), for terpenoids, see Langenheim (1981, 2003), for non-cyanogenic hydroxynitrile glucosides, see Bjarnholt et al. (2008 and references), and for xyloglucans, see Kooiman (1960). For starch, see Czaja (1978) and for epidermal wax crystals, see Ditsch et al. (1995). Feitoza and Lima (2020) look at the chemosystematics of the core genistoid clade.
For wood anatomy, see Baretta-Kuipers (1981), Wheeler and Baas (1992: esp. fossil woods), Gasson et al. (2000 [Faboideae], 2003 ["Caesalpinioideae"], 2009 [Caesalpinieae], and references), Evans et al. (2006) and Lewandrowski et al. (2022), both Mimoseae, latter also phenols and lignification, Oskolski et al. (2014: Crotalarieae), and Stepanova et al. (2013a: Hypocalyptus, 2013b: Podalyrieae, 2017: Baphieae + Mirbelieae), for roots, including nodule morphology, see Malpassi et al. (2015), for foliar variation in basal "Caesalpinioideae", see Lersten and Curtis (1994), for that in the Hymenaea clade, see Pinto et al. (2018), flor leaflet anatomy in the Pterocarpus clade, see Varilla González et al. (2023), for stem anatomy, see Dormer (1945b), for the diversity of crystals in the bark of African genistoids and their possible evolution, see Kotina et al. (2015), and for foliar glands/idioblasts, see Turner (1986) and Fortuna-Perez et al. (2021: Dalbergieae, Adesmia group). Luckow et al. (2005) discuss variation in flower and seed in Mimoseae; for general floral and inflorescence morphology, see Endress (1994b), Naghiloo et al. (2012: variability), and Prenner (2013a: Faboideae, 2013b: Faboideae, esp. androecial variation), for floral anatomy, see Rao et al. (1958), for floral morphology, see Schleiden and Vogel (1839), Tucker (1993: taxa that were in Sophoreae, 2000a: some Amherstieae - see also above), Crozier and Thomas (1993: Glycine), Kantz and Tucker (1994: Caesalpinia s.l.), Teixeira et al. (2009: some Millettieae), Sinjushin (2018a: Cordyla pinnata, 2023: 5-merous "flag blossoms"), Kochanovski et al. (2018: Hymenaea) and Pedersoli and Texeira (2015, also references: two mimosoids), for endothecial thickenings, see Manning and Stirton (1994), for tapetum, see Wunderlich (1954: c.f. Caesalpinia) and Buss and Lersten (1975), for pollen, see Ferguson and Pearce (1986 and references: Cercidoideae), Ferguson and Skvarla (1991: Swartzieae), Ferguson and Stirton (1993: Leptolobieae), dos Santos et al. (2011: Cercidoideae), Oliveira et al. (2019: Phaseoleae), Medina-Acosta et al. (2019: Mimosa), Lattar et al. (2019: Aeschynomeneae [= Dalbergieae]), Antonio-Domingues et al. (2022: Aeschynomene s.l.) and de Almeida et al. (2024: Brazilian Dialioideae), for carpel development, see van Heel (1981, 1983), van Heel (1993: Archidendron), and Sinjushin (2014: polymerous gynoecia in peas), for embryology, etc., see Guignard (1881), Newman (1934), James (1950: Astragalus), Dnyansagar (1957, 1958, 1970), Rugenstein (1983: Cercidieae), Cameron and Prakash (1990: giant antipodals, 1994: Faboideae megagametophyte v. variable), Miller et al. (1999: Glycine), Riahi et al. (2003), Rodriguez-Pontes (2007) and Tanaomi et al. (2016) and references, all Astragalus and relatives, De-Paula and Oliveira (2012: Chamaecrista ovules), and Endo (2012a: funicle morphology). For fruit anatomy in Crotalaria and relatives, see Le Roux et al. (2011), also Pfeiffer (1891), Kapil et al. (1980), etc., for seed coat morphology and anatomy, see e.g. Pammel (1899), Corner (1951, 1976), van der Pijl (1956), Kopooshian and Isely (1966), Gunn (1981a, b, 1984, 1991: "Caesalpinioideae"), Kirkbride and Wiersema (1997), Kirkbride et al. (2003), Jordaan et al. (2001: esp. Colophospermum), Moïse et al. (2005), and Lackey (2009), for endosperm, see e.g. Anantaswamy Rau (1953), Johri and Garg (1959) and Rodriguez-Pontes (2008), both haustoria, for embryo anatomy, see D. M. T. Oliveira (1999), and for seedlings, see Compton (1912: also anatomy), Léonard (1957: African Detarioideae, a classic) and Duke and Polhill, 1986).
Phylogeny. For a rbcL phylogeny of the family, especially Papilionoideae, see Wink & Mohamed (2003). There, and up through the latter part of the twentieth century, Fabaceae were divided into three groups variously considered to be subfamilies or families - Faboideae (≡ Papilionoideae), Caesalpinioideae and Mimosoideae. The Legume Phylogeny Working Group (L.P.W.G. 2013a) provide a good summary of relationships in the family as understood then: Fabaceae are monophyletic in both molecular and morphological analyses, although support might not be strong, "Caesalpinioideae" were wildly paraphyletic at the base of Fabaceae, while Mimosoideae and Faboideae were clearly monophyletic and separately embedded in "Caesalpinioideae". Cercidoideae, Duparquetia and Detarioideae were all candidates for being sister to the rest of the family (Bruneau et al. 2008a, b; Cardoso et al. 2012a: Duparquetia not included; L.P.W.G. 2013a). Duparquetia was found to be sister to Dialioideae by Herendeen et al. (2003b), while Cardoso et al. (2013b) found some support for the topology [Duparquetia [[Cercidoideae + Detarioideae] [Dialioideae [Caesalpinioideae inc. Mimoseae + Faboideae]]]]; the [Dialioideae [Caesalpinioideae + Faboideae]] clade was well supported. Wojciechowski et al. (2004) placed Cercideae sister to the rest of Fabaceae, and within the latter Dialieae were sister to the remainder. Two main clades made up the rest of the family. One includes the old Mimosoideae, to which Ceratonia, Gleditsia, etc., Caesalpinieae and Cassieae (all Caesalpinioideae s.str.) were more or less successively sister taxa, and the other is made up of Faboideae. Bruneau et al. (2008a, b) found a rather similar set of relationships, [Detarieae [Duparquetia, Cercideae, [Dialiieae [Faboideae [Caesalpinioideae + Mimosoideae]]]]]. Cercis and Bauhinia may be sister to all other Fabaceae (e.g. J. J. Doyle et al. 2000 and references; Bruneau et al. 2001), although they are also placed sister to Detarioideae, if sometimes with only with moderate support (Wojciechowski et al. 2004; Lavin et al. 2005; Forest et al. 2007b; Cardoso et al. 2013b; Cannon et al. 2014: only one Detarioideae sampled). Aygören Uluer et al. (2020) included 678 taxa and three genes in a maximimum likelihood analysis in which the clade [Dialioideae [Caesalpinioideae + Faboideae]] was consistently recovered, but Duparquetioideae tended to wander about, being sister to the whole family or variously placed relative to Cercidoideae and Detarioideae. See also M. Sun et al. (2016) for relationships - "Caesalpinioideae" were in eleven separate clades - and Z.-D. Chen et al. (2016).
Recent work using chloroplast (R. Zhang et al. 2020: 187 plastomes) or both chloroplast and nuclear genomes (Koenen et al. 2019a, 2019b/2020a) is clarifying the situation insofar as it is becoming likely that there is a hard polytomy or something close to it at the base of the family, incomplete lineage sorting being a likely culprit (Koenen et al. 2019a). As Koenen et al. (2019a) noted, there was no phylogenetic signal in any chloroplast and most nuclear genes, and the signal in those nuclear genes that did have some signal was conflicting. Zhang et al. (2020) often recovered a [Duparquetioideae [Dialioideae [Caesalpinioideae + Faboideae]]] clade, and perhaps a [Cercidoideae + Detarioideae] clade was sister to them (see also Koenen et al. 2019a: Duparquetia not included in nuclear analyses), however, relationships between the subfamilies were not that clear; as Zhang et al. (2020) noted, plastome data alone were not solving all the problems... Y. Zhao et al. (2021) - 333 genera included (ca 43% of the family) and somewhat over 1,500 nuclear genes examined - also found similar, if very close, relationships between the subfamilies. Uluer et al. (2022) recovered the relationships [[Detarioideae [Duparquetioideae + Cercidoideae]] [Dialioideae [Caesalpinioideae + Faboideae]]]; they analyzed three plastid gene regions, and although monophyly of all the subfamilies was well supported, relationships between the first three subfamilies were not.
Duparquetioideae. For Duparquetia, see Forest et al. (2002) and Tucker et al. (2002). The genus is highly derived. Interestingly, its carpel begins to develop after the stamens are initiated, unlike other Fabaceae but like the usual situation in angiosperms (Prenner & Klitgaard 2008a, esp. b).
Within Cercidoideae, Cercis is sister to all other members of the clade, Adenolobus is sister to the remainder, and then perhaps Griffonia (Sinou et al. 2009, 2020; Y.-H. Wang et al. 2018; Y. Zhao et al. 2021). Gu et al. 2024) suggest relationships [Cercis, Adenolobus, [[Griffonia [Piliostigma + Bauhinia]] [etc.]]. Within Cercis, C. chungii is sister to the rest, the North American and European species being embedded in the tree (Jia & Manchester 2014). Generic limits around Bauhinia are discussed by Sinou et al. (2008, esp. 2009, 2020), Y.-H. Wang et al. (2018), etc.; the two small basal clades here are Piliostigma (African) and Breniera (Madagascan). Bauhinia s. str. and immediate relatives, but not other Cercidoideae, lack the plastid rpl2 intron (e.g. Lai et al. 1997; Sinou et al. 2009; Meng et al. 2014: ?sampling). Many other species that were included in the old Bauhinia are now in the Phanera clade (Sinou et al. 2020); sister to Phanera s. str., or to be included in it, is the [Cheniella + Lasiobema] clade (Clark et al. 2017; Sinou et al. 2020).
Detarioideae. Although M. Sun et al. (2016) suggested that Gigasiphon might be sister to other Detarioideae, that genus is in Cercidoideae here... De la Estrella et al. (2017, 2018) found [Schotia [Barnebydendron + Goniorrhachis]] (two tribes) to be sister to Detarieae/resin-producing members of the subfamily, and the Saraca and Afzelia clades (two tribes) successively sister to the large tribe Amherstieae, basal relationships within which were unclear. Relationships in Bruneau et al. (2008a) were similar, except there was a basal tetratomy made up of Schotia, Barnebydendron, the resin-producing taxa, and the rest. Relationships in Y. Zhao et al. (2021) were a little unclear; Detarieae might be sister to the rest of the subfamily. Amherstieae. Redden et al. (2010; see also Schley et al. 2018) examined relationships in the Brownea clade, possible synapomorphies for it being an unchanneled leaf rachis, thread-like stipules, connate bracteoles, four sepals, and introrse anthers. For relationships in the important Amazonian Brownea itself, where there has been hybridization, see Schley et al. (2020) - B. coccinea and B. grandiceps are both scattered more or less throughout the whole generic tree. Redden and Herendeen (2006) and Redden et al. (2018) focus on Paloue. Boom et al. (2021, 2024: 28/29 spp., various analyses) looked at relationships in Brachystegia; there seems to have been hybridization here, and plastome and nuclear data suggest different relationships. Anthonotha was monophyletic in the analysis of Ojeda et al. (2019) but Englerodendron s. str. was para/polyphyletic (see Isomacrolobium and Pseudomacrolobium, both reduced to Englerodendron - de la Estrella 2019a). For relationships around Gilbertiodendron, see de la Estrella et al. (2014), while Radosavljevic et al. (2017) examined relationships around Cynometra, which turns out to be diphyletic, Cynometra s. str. including Maniltoa (see also Temu 1990: morphological analysis; Radosavljevic 2019). Murphy et al. (2017) found two main clades in Macrolobium, one from Central and N.W. South America, and the other, overlapping geographically somewhat, from tropical South America; resolution of relationships in the latter was not very good and some sections were not monophyletic. W. J. Baker et al. (2021: see Seed Plant Tree) looked at relationships around here, and with good generic sampling - [Tamarindus [Intsia [Detarieae [Schotia group + The Rest]]]], quite well supported, are their somewhat unexpected findings.
Dialioideae. The monotypic Neotropical Poeppigia is sister to all other Dialioideae (Bruneau et al. 2008a; M. Sun et al. 2016; Zimmerman et al. 2017); [[Eligmocarpus + Baudouinia] [Zenia + rest of clade] - with a fair number of polytomies and Petalostylis (ex-Cassieae) sister to Labichea and well embedded - complete the relationships as known (Zimmerman et al. 2017; see also Y. Zhao et al. 2021, but sampling poorer). However the [Petalostylis + Labichea] clade had rather weak support in the complete plastome analysis of Bai et al. (2020), being best represented as a tritomy with Strockiella; sister to that clade was the clade [Zenia [Distemonanthus [Dialium + Dicorynia]]].
There are then two large clades.
1. Caesalpinioideae, = the old Mimosoideae + part of the old Caesalpinioideae. Mimosoideae, now Mimoseae, have distinctive small, closely-aggregated, polysymmetric flowers, but basal to them are several clades made up of some of the old Caesalpinioideae. These include genera like Caesalpinia itself, Cassia, and Dimorphandra with large more or less monosymmetic/asymmetric flowers. The poorly-supported Umtiza clade may be sister to the rest of this whole clade, and it includes taxa like Gleditsia, Gymnocladus and Ceratonia, several of which are dioecious and have smallish, greenish flowers sometimes with a poorly differentiated calyx and corolla - not plesiomorphic features (Herendeen et al. 2003a; Forest et al. 2007b; see also Redden & Herendeen 2006: morphological analysis; Fougère-Danezan et al. 2003, 2007, 2010: molecular and morphological studies; Manzanilla & Bruneau 2011; L.P.W.G. 2013a, 2017: matK only; Cardoso et al. 2013b). Caesalpinia, Cassia and relatives, Sclerolobieae, etc., are also near-basal in Caesalpinioideae, and relationships in this area in Y. Zhao et al. (2021) were strongly pectinate. Ringelberg et al. (2022a) looked at relationships in Caesalpinioideae using 997 nuclear genes (821 single-copy genes) sequenced from 147 of the 152 genera currently recognised in the subfamily; all told, they examined 420 species). ASTRAL trees based on analysis of the 821 single-copy genes are shown; ASTRAL can deal with multi-species coalescence better than can concatenation analyses, and it also handles the extensive, if local, conflict between gene trees found in the analyses by Ringelberg et al. (2022a) better. Basal clades here are, as in other analyses, include ex-Caesalpiniaceae, and Ceratonieae rather than Gleditieae (the Umtiza clade), are sister to the rest of Caesalpinioideae. Pterogyne is associated with Caesalpinieae in plastid analyses but with Cassieae in nuclear analyses (Manzanilla & Bruneau 2012); in an Angiosperms353 analysis Y. Zhao et al. (2021) found strong support for a position of that genus by itself immediately basal to those two tribes, but it was not included in the analysis of Ringelberg et al. (2022a).
Caesalpinieae. For relationships here, see Simpson et al. (2003) and Gagnon et al. (2018). Gagnon et al. (2013, esp. 2016) discussed relationships around the old Caesalpinia; the genus is either poly- or paraphyletic (Manzanilla & Bruneau 2012). R. P. Clark et al. (2022) summarize relationships in the tribe. In the plastome analyses of Aecyo et al. (2021), relationships were [[Haematoxylum [[Caesalpinia, etc.] [Guilandila etc.]]] [Cenostigma [[Arquita etc.][Libidibia etc.]]]], although relationships in the Haematoxylum clade were somewhat problematical.
Cassieae. For phylogenetic relationships within Senna, see Marazzi et al. (2006), and for relationships within Chamaecrista, see de Souza Conceição et al. (2009), Rando et al. (2016) and A. O. de Souza et al. (2019, 2021), the classical sections, etc., do not map on to the phylogeny at all closely. De Souza et al. (2021) suggest some apomorphies for Chamaecrista.
Sclerolobieae. Huamantupa-Chuquimaco et al. (2024: 64 taxa, nuclear ITS, plastid matK and trnL intron) found relationships within Tachigali for the most part to be poorly resolved.
There are some caesalpinioids that have racemose inflorescences with small, more or less simultaneously-opening and more or less polysymmetric flowers with free sepals and petals (and ten stamens) borne close together, e.g. Dimorphandra, Dinizia, Pachyelasma, Peltophorum and Erythrophleum, that are immediately above this area of the tree and immediately basal to Mimoseae. Although there are a number of groups of genera here, details of relationships between them were for some time unclear (Luckow et al. 2003; Wojciechowski 2003; Lavin et al. 2005; Bruneau et al. 2008a, b; Bouchenak-Khelladi et al. 2010b; Cardoso et al. 2012c, 2013; Manzanilla & Bruneau 2012; L.P.W.G. 2013a, b; Kyalangalilwa et al. 2013; M. Sun et al. 2016). They may also show considerable similarity to Mimoseae in wood anatomy (Evans et al. 2006) and also in pollen, which is rather homogeneous although nearly always in monads (Banks & Lewis 2009). For Peltophorum and its relatives, see Haston et al. (2005).
Within Mimoseae, = the old Mimosoideae/Mimosaceae, there were two groups named for convenience. The first was the large clade Mimoseae I, in which Ingeae, derived, with a valvate calyx and many connate stamens forming a tube, at least basally, are embedded in Acacieae (e.g. Clarke et al. 2000; Robinson & Harris 2000; Miller & Bayer 2000, 2001; Luckow et al. 2003; Jobson & Luckow 2007; G. K. Brown 2008; Brown et al. 2008; Bouchenak-Khelladi et al. 2010b; Miller & Seigler 2012; Kyalangalilwa et al. 2013). In the skeletal tree shown by R. Zhang et al. (2020), relationships are [paraphyletic Mimoseae [paraphyletic Ingeae + Acacieae]]. The diversification of Inga has been much studied (e.g. Richardson et al. 2001b; Kursar et al. 2009; Dexter et al. 2012), although with rather little resolution, especially between closely-related species and also along the spine. However, targeted enrichment of nuclear genes bids fair to deal with both these problems (Nicholls et al. 2015); in that study, I. huberi was sister to the rest of the genus, while there is fascinating variation within I. umbellata. Acacia subgenus Acacia (now = Vachellia), which includes the bull's horn acacias, seems to be monophyletic, but Acacia s.l. is highly poly/paraphyletic (e.g. Pedley 1986; see Murphy 2008). Kyalangalilwa et al. (2013) and Gómez Acevedo et al. (2015) looked at the whole complex, emphasizing the extent of para- and polyphyly, and not only in Acacia. Siegler (2003) summarized the phytochemistry of the complex, Evans et al. (2006) detailed wood anatomy, and Kergoat et al. (2007) noted what bruchids had to say about systematics of Acacia s.l.; see also Muelleria 26(1). 2008, a special issue on Acacia, and also the World Wide Wattle website. Murphy et al. (2000, 2003, 2010: relationships still only moderately resolved) and Miller et al. (2003) discuss the phylogeny of Acacia s. str., the old subgenus Phyllodineae. Mishler et al. (2014) found little support for basal relationships here, although there was more in a later study using complete chloroplast genome sequences (Williams et al. 2016). G. K. Brown et al. (2012) focussed on relationships of Acacia s. str. outside Australia, while Brown et al. (2006b) found complex relationships between bipinnate-leaved and phyllodinous acacias, the situation not being helped by somewhat different topologies, albeit with little support, when morphological data were added. Miller and Bayer (2003) looked at relationships in Vachellia (the old subgenus Acacia), and Senegalia (the old subgenus Aculeiferum), although support for the monophyly of this is weak (Miller & Seigler 2012). Terra et al. (2017) focused at relationships within Senegalia, although no Asian species were included; American and African species were in different clades, although the African S. adenocalix and S. schweinfurthii were embedded in the American clade; S. atraxantha and its immediate relative were sister to other African species, although they are in different sections and the American species are in the same section as S. atraxantha (see also below), Miller et al. (2017) focussed on relationships of three small ex-Acacia American genera; paraphyletic in Miller and Seigler (2012), they may form a monophyletic group? Albizia is also in Mimoseae I, and it seems potentially quite polyphyletic (Kyalangalilwa et al. 2013). Mimosa may be monophyletic and sister to Piptadenia (Besseger et al. 2008); this relationship was also found by Simon et al. (2015), whose focus was on Stryphnodendron. Simon et al. (2011: 259 spp, trnD-trnT marker, see also 2009) provide an extensive phylogeny of Mimosa, optimizing various characters on the tree; Grohar et al. (2021) emphasized distinctive characters (hairs branched, A = C) in section Calothamnos and Borges et al. (2022b: 73 taxa, 75 morphological characters, 3 plastid loci) looked at species from the Brazilian Cerrado. De Souza et al. (2013) provided a comprehensive phlyogeny of Calliandra - largely, if not entirely, New World - and its immediate relatives (see also Thulin 2023). Ferm et al. (2019) found that our current understanding of relationships in this area was unsatisfactory, and although Zygia, their focus, was largely monophyletic, none of its sections for which they included two or more species was monophyletic.
The second group was Mimoseae II, a paraphyletic grade basal to Mimoseae I. They include Parkia, a polyphyletic Neptunia, Leucaena, etc., as well as genera like Pentaclethra (nodules +/0; Kyalangalilwa et al. 2013: see also above). Conceição Oliveira et al. (2021a) provide a phylogeny of Parkia; the relationships of the sections and the limits of section Parkia are unclear. Catalano et al. (2008) provide a phylogeny of the ecologically important New World genus Prosopis, also in this area (and see Burghardt & Espert 2007 for morphology). Iganci et al. (2015) looked at Abarema, highly polyphyletic, Soares et al. (2021: Jupunba and Punjuba), and the latter also emphasized the polyphyletic nature of Barneby and Grimes's Ingeae. [Genera like Pentaclethra are also to be included (c.f. Bouchenak-Khelladi et al. 2010b, but some confusion there?)].
Koenen et al. (2020b) used 964 low-copy nuclear genes to look at relationships in Mimoseae. Mimoseae I above are largely the same as the core mimosoids of Koenen et al. (2020b), Mimoseae II are the rest; the major groups that Koenen et al. (2020b) mention are summarized above. The ingoid clade largely consists of what currently appears to be a hard hexa- or heptatomy that includes some 1,750 species. Koenen et al. (2020b) evaluate their results in some detail, so for further details consult their paper; they note that members of the Samanea clade in particular are perhaps rogue taxa. Ringelberg et al. (2022a) looked at relationships in Caesalpinioideae in particular. Relationships in Dimorphandreae were unclear, Dimorphandra being polyphyletic in nuclear, but not chloroplast analyses; lumping or splitting will be needed, although the clade is quite small... (Ringelberg et al. 2022a: Appendix 1). Some 25% of the genera in Mimoseae, many more than in other members of the subfamily, were found to be non-monophyletic - and this figure is perhaps an underestimate given the low density of the species-level sampling (Ringelberg et al. 2022a). Further details of relationships here and how they are being clarified (see especially papers in PhytoKeys 205. 2022) are given below. Note that the names for the clades are those in Koenen et al. (2020b), a few names being added because of changes to our ideas of relationships since, the contents of the clades come from Ringelberg et al. (2022a), again as modified (esp. name changes) by the papers in PhytoKeys.
Before discussing individual clades in Mimoseae (the sequence below is alphabetical), a paper by Terra et al. (2022) should be mentioned. This looks at relationships around Senegalia as in the nuclear analyses of Ringelberg et al. (2022a) in considerable detail. There is conflict between plastome and nuclear trees, Senegalia being monophyletic in the former, but Terra et al. (2022; see also Miller et al. 2017, Wei et al. 2021; etc.) suggest that the topology in the nuclear analyses be followed. All told there are three, perhaps four, clades here, and although Terra et al. (2022) did not suggest any nomenclatural changes given the then current state of sampling, the clade characterizations in this area above are based on their discussion. Note also that Alvarado-Reyes et al. (2024) suggested that Senegalia sect. Monacanthea pro parte could be characterized (apomorphies) by the postgenital fusion of the corolla and the presence of a staminal disc at the base of the filaments - there were stomata in the apical part of this tube, perhaps involved in nectar secretion. And now for the individual clades.
The Albizia Clade. É. R. de Souza (2022) looked at some relationships here as they divided Leucochloron; most of the genus remains in the Inga clade.
The Archidendron Clade. G. K. Brown et al. (2022) focussed on Archidendron, and they found that it formed two main clades, one largely Western Malesian + Asian, the other Eastern Malesian + East Australian, and Archidendropsis also formed two immediately unrelated clades. However, details of relationships in Archidendron remained unclear, so they left its names unchanged. Demeulenaere et al. (2022: 77 loci) looked more at the Serianthes area where relationshps were [Wallaceodendron [Falcataria + Serianthes]]; relationships within this clade were identical in concatenated ML and and ASTRAL partition trees, although elsewhere in the Archidendron clade there were quite substantial differences. Discordance developed in the analyses as one went deeper in the trees, and in analyses carried out by others there are yet other topological differences (Demeulenaere et al. 2022: Fig. 8; see also Ringelberg et al. 2022a for discussion).
The Calliandra clade. This was the subject of an extensive study by E. R. de Souza et al. (2013: nuclear ITS + the trnL-trnF area), who included 98/142 species in the genus in their study, as well as representatives of immediately related genera and other genera in the Inga alliance; they also looked at a number of morphological characters, including pkynoilogical variation. Calliandra (including Guinetia) was monophyletic, and de Souza et al. (2013) described Afrocalliandra, which they placed sister to Calliandra, the two differing in a number opf features (see above).
The Dichrostachys clade. Relationships in part of this clade were [Leucaena [Schleinitzia [Mezcala [Kanaloa + Desmanthus]]]], and a suggestion was made that Schleinitzia is an hybrid between the ancestor of Mezcala and something else in the [M. + K. + D.] clade. Neither Dichrostachys nor Alantsilodendron are monophyletic and generic rearrangements are in the offing (Ringelberg et al. 2022a: Appendix 1).
The Entada Clade. O'Donnell et al. (2022) discuss the circumscription of Entada.
The Jupunba Clade. Here Aviles Peraza et al. (2022) placed the New World Albizia, sister to the rest of this clade, in Pseudalbizia (the bulk of the rest of Albizia is in the Inga group). Soares et al. (2021) found that Punjuba was sister to the remainder of the clade, and within Jupunba P. langsdorfii was sister to the rest of the genus. Branch lengths in the Jupunba-Balizia-Hydrochorea area are short, quartet support is low, and there apppears to have been ILS; Balizia was sometimes placed in Hydrochorea (Soares et al. 2022: extensive exon analyses).
The Inga clade. The limits of this clade have been variously drawn iun the past... Ringelberg et al. (2022a: Appendix 1) discuss the work still to be done on Zygia, a genus some of whose species have already been moved elsewhere in the larger ingoid clade.
The Neltuma Clade. Hughes et al. (2022a) formalized the dissolution of the triphyletic Prosopis of which the bulk is included in this clade; the two other parts are nearby, but in separate clades along the extensive pectination that makes up Mimoseae. Within this clade relationships are [Neltuma [Strombocarpa + Xerocladia]].
The Pithecellobium Clade. Tamayo-Cen et al. (2022: nuclear ribosomal ITS and ETS) recovered the relationships [Havardia [Sphinga + Pithecellobium]] plus the rest of the group; two new genera ere described, but relationships here differ somewhat from those in Ringelberg (2022a: see Appendix 1).
The Stryphnodendron clade. Studies by de Lima et al. (2022) and Borges et al. (2022a) have lead to the description of three new genera and the synonymization of one old genus. De Lima et al. (2022: 1 nuclear and 3 chloroplast markers) recovered the relationships (generic names and circumscriptions are those of both groups above) [Marlimorimia [Naiadendron [Parapiptadenia + Pityrocarpus]]] [Stryphnodendron [Microlobius + Gwilymia]]; in a 977 gene analysis of 11 taxa they noted a substantial number of conflicting gene trees. Borges et al. (2022a: 1 nuclear, 2 chloroplast markers) recovered the relationships [Pityrocarpa [Stryphnodendron (inc. Microlobius, Naiadendron and Gwilymia) [Parapiptadenia + Marlimorimia]]], although they noted that backbone support was poor, e.g. in Stryphnodendron itself. See also Barduzzi et al. (2024) for relationships here, and also optimization of a number of pollen characters on the tree (ibid., Figs 3, 40.
The Zapoteca clade. Various species of Calliandra have been moved here, and yet one more may have to be moved, and it may need a new genus (Ringelberg et al. 2022: Appendix 1). Ferm (2019: nuclear ITS and ETS, trnL-trnF region) had carried out a preliminary phylogenetic analysis of Zapoteca, and found that Z. nervosa, with distinctive pollen morphology was sister to the rest pf the genus
2. Faboideae/Papilionoideae are monophyletic. The following topology (simplified) aids in the discussion of relationships here: [Myroxylodae, Swartzieae, etc. [Cladrastis, etc., [(= 50 KB inversion clade) Andira et al., Vatairea et al., Exostyleae/Lecointea et al., Genistodae: GEN, [Amorpheae + dalbergioids = dalbergioids s.l.: DAL], [baphioids: BAPH [(NPAAA clade, = Non-Protein Amino Acid Accumulating clade) mirbelioids: MIRB [[Indigofereae + millettioids: MILL] [robinioids: ROB + Inverted Repeat Loss Clade, = IRLC]]]]]]], seems moderately well supported (see also Liston 1995; Wink & Mohamed 2003: rbcL; Wojciechowski 2003; McMahon & Sanderson 2006; L.P.W.G. 2013a). The [robinioids + IRLC] clade make up Galegodae (see Farruggia & Howard 2011 for possible nuclear markers; Ezedin 2025). The tree here is based largely on Wojciechowski et al. (2004), Peters et al. (2010) and Cardoso et al. (2012c, 2013b); see also the L.P.W.G. (2013a), McMahon and Sanderson (2006: a supermatrix analysis of 2228 species) and M. Sun et al. (2016) for more information. Details of relationships differ somewhat in the nuclear analysis of Y. Zhao et al. (2021). I.-S. Choi et al. (2022) carried out a comprehensive series of analyses of 237 Faboideae (174 genera) with plastomes and matK analyses of 534 species; in the plastome analyses many of the nodes along the spine were well supported, except some in the middle, e.g. at the base of the 50kb inversion clade. L. Cai et al. (2025: 279 spp. Faboideae, 1456 low-copy nuclear genes) focused on the backbone relationships in Faboideae and recovered some quite well supported relationships - again, some are different from those based on analyses of plastome data - and they used these to constrain relationships in a matK dataset of Fabales - 3,326 species. As of iv.2025 these relationships have very largely been incorporated in to the phylogeny above.
Swartzieae may be sister to other Faboideae, but support in some studies is weak (Ireland et al. 2000; Pennington et al. 2000, 2001; Lavin et al. 2005; R. Zhang et al. 2020); Duan et al. (2019) also recovered the relationships [Swartzieae s.l. [Myroxylodae [Cladrastis clade, etc.]]]. Cardoso et al. (2012a, 2013b) had found the well-supported [Angylocalyceae [Dipterygeae + Amburaneae]] clade, Myroxylodae/the ADA clade, to be sister to all other Faboideae, and that topology is followed below (see also M. Sun et al. 2016; Y. Zhao et al. 2021; L. Cai et al. 2025). Carvalho et al. (2023a, 2023b: 4 markers, sampling good) provide a phylogeny of Dipterygeae; the four genera are monophyletic. Cardoso et al. (2013b; also M. Sun et al. 2016; Zuntini et al. 2024) found some support for a Swartzieae s.l., i.e. [Swartzieae s. str. + Ateleia, etc.], that was sister to remaining Faboideae (see Torke & Schaal 2008 for a phylogeny). Mansano et al. (2004) looked at relationships in the lecointeoids (= Exostyleae), but these were on the whole poorly supported; Holocalyx might be sister to the rest of the tribe. For the vataireoids, with Vataireopsis sister to the rest, see Cardoso et al. (2013a: not in ITS analyses). Cardoso et al. (2012a, esp. 2013b) discussed other relationships among Faboideae basal to the NPAAA clade. Cladrastis and relatives (= Cladrastideae) are sister to the 50 KB inversion clade (e.g. Cardoso et al. 2013b).
Duan et al. (2020) found that Platyosprion was sister to the rest of Cladrastideae.
The 50 KB inversion clade. Relationships at the base of this clade were found to form an extensive polytomy (9-furcate in Cardoso et al. 2013b; see also Ramos et al. 2015). However, a number of recent studies are suggesting that [Vataireoids + Exostyleae] are sister to the rest of this clade (Zuntini et al. 2024; Kates et al. 2024; Cai et al. 2025). Gai et al. (2025) were uncertain as to what should be the next branches: Concatenation analyses suggested that there was a clade [Dalbergioids s.l. + Genistodae], although support was not strong, but it was rather stronger in coalescent analyses for the set of relationships [Genistodae [Dalbergioids [Andira [Baphieae + NPAAA clade]]]]. The well-characterized clade [Crotalarieae + Genisteae + Podalyrieae + Sophoreae] included Haplormosia (Cai et al. 2025).
Vataireoids. In the vataireoid clade, Cardoso et al. (2013a) found the relationships [Lutzelbergia [Sweetia [Vatairea + Vataireopsis]]].
Genistodae (Genistoids). In the early study by Käss and Wink (1997a) Sophoreae appeared to be very much para-/polyphyletic. Crotalarieae and Genisteae have long been thought to be sister taxa (e.g. Käss & Wink 1997a; Hunter et al. 2024). Crisp et al. (2000) outline relationships in Genisteae s.l./core genistoids, including Cytiseae; see also Castellanos et al. (2017). Ormosieae, Brongniartieae and Leptolobieae are successive sister groups to other Genistodae (L. Cai et al. 2025). Gregório et al. (2023) found that the small genus Clathropsis was polyphyletic; the new genus that they described, Cabari, was unplaced, along with Dermatophyllum. For the relationships of Orphanodendron and Camoensia, both ex-Caesalpinioideae, but to be placed somewhere around the genistoids, the two genera perhaps being sister taxa, see Castellanos et al. (2016). There are some very distinctive non-papilionoid floral morphologies around here. For instance, Camoensia scandens has petals with crinkly margins where scent glands are to be found, there is a very large standard, the four other petals are long and narrow, and there are eleven stamens, two stamens being opposite the standard (see Leite et al. 2021 for more details) - the related Orphandendron has closer to radially symmetrical flowers, Pericopsis and Uleanthus are also near. Other morphological features may also be odd - thus Sellocharis (Genisteae) has 5-7 "leaflets" in whorls (Hunter et al. 2024).
For relationships within Crotalarieae, see Boatwright et al. (2008b, esp. c, 2009), for those in Cape Crotalarieae, see Edwards and Hawkins (2007: resolution along spine not too good) and of Lotononis and relatives in particular, Boatwright et al. (2011: also character evolution). Le Roux et al. (2013) found little support for relationships along the backbone of Crotalaria, although several well-supported clades were recovered. Rockinga et al. (2017) looked at 3 plastid and 2 nuclear genes in 338 species of the genus (ca 48%, plus 33 spp. in 15 other genera of the tribe), recovering i.a. the relationships [[Eochlora [Bolusia + Crotalaria]] [rest of Crotalarieae]], while within Crotalaria itself there was a fair bit of phylogenetic structure (see Diversity & Distribution above).
Genisteae. Genista is perhaps to include Ulex and other genera, but relationships between the major groups in Genista were initially poorly supported. It was thought that Cytisus was paraphyletic (Pardo et al. 2004), and might include Ulex (Cubas et al. 2002; Cristofilini & Troia 2006). For relationships in Canary Island Genisteae, see Percy and Cronk (2002). I.-S. Choi et al. (2022) also suggest chloroplast-based relationships. Hunter et al. (2024: 48 spp., 24/25 genera, plastomes + 1,547 low-copy nuclear genes) recovered the groupings [Argyrolobium etc. [Anarthrophyllum etc. [Lupinus + the Cytisus-Genista clade]]]; relationships in the Anarthrophyllum clade are [Anarthrophyllum [Sellocharis + Polhillia]], while another major clade is [Argyrolobium [Melolobium +Dichilus]]. The topologies of the nuclear and plastome trees differed somewhat wihin these four major groupings, although the groupings were the same, perhaps especially in the Cytisus-Genista clade where Genista was all over the place in the nuclear tree but monophlyetic in the chloroplast tree. Relationships in Lupinus have been much studied (e.g. Aïnouche et al. 2004; Moore & Donoghue 2009; Silvestro et al. 2011; Sklenár et al. 2011; Contreras-Ortiz et al. 2018); see also above; relationships found by authors like Drummond et al. (2012) and Hunter et al. (2024) are [Lupinus N.W. [3 spp Lupinus W. North America + Lupinus Old World]].
Ormosieae. Torke et al. (2021) outlined relationships here; the main clades in Ormosia have strong geographical signals, but Torke et al. found that practically none of the previous infrageneric groupings/segregate genera held up. De Souza Gregório et al. (2025) clarified the content of the tribe and plotted the variation of a number of characters on the tree; Ormosia is rather different from the other two genera.
Podalyrieae. Edwards and Hawkins (2007) discussed relationships in Podalyrieae, although these for the most part had little support; see also Boatwright et al. (2008: support still not strong). Cadia may be sister to Podalyrieae (Pennington et al. 2001; Wink & Mohamed 2003; Boatwright et al. 2008).
Sophoreae. Early work suggested that this tribe was very much para/polyphletic (Käss & Wink 1997a). M. Liao et al. (2023) extended the limits of Sophora somewhat; here nuclear and chloroplast genes tell rather different stories, e.g. in the arrangement of the main clades/sections. There are similar problems in the two main clades of Thermopsis in North America, perhaps here there is infraspecific hybridization; that genus is not monophyletic, and there are other generic problems in Thermopsidae (Farmer & Jansen 2024).
Pennington (2003) looked at relationships in the New World Andira.
Relationships within the dalbergioid legumes, Dalbergieae, are discussed by Lavin et al. (2000, 2001) and Klitgård (2013); Moraes et al. (2020) recovered the groupings [Adesmia et al. [Pterocarpus et al. + Dalbergia et al.]]; Aeschynomene was polyphyletic. Indeed, Machaerium is more related to Aeschynomene section Ochopodium than to Dalbergia, so the apparent similarities in habit, fruit, etc., between Machaerium and Dalbergia need re-evaluating, furthermore, the (semi)aquatic habit in Aeschynomene has evolved twice (Ribeiro et al. 2007; Arrighi et al. 2013; Chaintreuil et al. 2013, 2016; Vatanparast et al. 2013). Chaintreuil et al. (2013) and Brottier et al. (2018) confirmed the polyphyly of Aeschynomene and the general complexity of relationships in the area. Polyploid species of Aeschynomene (one of the Aeschynomene clades here includes the type, A. aspera), along with genera like Smithia and Kotschya, form a polytomy; the parents of this whole group may have been the diploids A. semperflorens and A. patula (Brottier et al. 2018). Cardoso et al. (2020: 54, 67 spp., ITS/5.8S and 2 chloroplast markers) also recovered the clade [Machaerium + Aeschynomene section Ochopodium], while Aeschynomene section Aeschynomene might include seven small genera, mostly African, although relationships were uncertain around there. Monteiro et al. (2024) looked at relationships in Adesmia series Adesmia. Cardoso et al. (2012a) and Klitgård et al. (2013) noted that taxa with polysymmetric flowers had evolved several times in Dalbergieae; see Bento et al. (2021) for the floral ontogeny of one of these taxa, Riedeliella. The large genus Dalbergia itself is likely to be monophyletic (Vatanparast et al. 2013). For relationships in Arachis, see Krapovickas and Gregory (2007), and for those within Zornia, see Fortuna-Perez et al. (2013) who found that the topology of the nuclear gene tree was rather different from that of the nuclear+chloroplast tree. For relationships within Amorpheae and its floral evolution, see McMahon and Hufford (2002, 2004, 2005), McMahon (2005) and Becklund and Ayers (2022).
Baphieae. Goncharov et al. (2013) looked at relationships here, which were [Dalhouseia [[Airyanthe + Baphia subg. Macrosiphon] [The Rest]]]; Baphia was polyphyletic.
The NPAAA clade. Hypocalyptus (= Hypocalypteae) is sister to all other members of the NPAAA clades, and then come the Mirbelioids, Hologalegina (= Galegodae), and the [Indigofereae + Millettioid-Phaseoloid] clades (L. Cai et al. 2025).
Mirbelieae. For relationships in or revisions of Mirbelia s.l., see Crisp and Cook (2003a, b), of Gastrolobium, Chandler et al. (2001), Pultenaea, Orthia et al. (2005b), and of Jacksonia, Chappill et al. (2007). In an ITS + trnL analysis of the Bossiaea area there are three main groups, the Bossiaea,Daviesia and Mirbelieae s. str. groups, the first and last were monophyletric, the second a polytomy; in Mirbelieae s. str. Isotropis was sister to the rest, Pultenaea and Podolobium were polyphyletic and Mirbelia paraphyletic (Crisp & Cook 2003b). For relationships in Daviesia, see Crisp et al. (2017: 118 spp., nrITS, ndhF and trnL–trnF); the basal topology here is [D. anceps [D. microcarpa + The Rest]], and there is a fair amount of structure in the relationships obtained. R. L. Barrett et al. (2024: Angiosperms353 data set) focussed on relationships in the Pultenaea area.
For characters of the millettioid clade, see Tucker (1987a). Da Sila (2012) provides a phylogeny of part of this clade, Lonchocarpus is split. Kajita et al. (2001: rbcL) also focused on the phylogeny of Phaseoleae, Millettieae and what were thought to be their relatives (including Wisteria, see the IRLC below) as part of a broader study. In an extensive analysis de Queiroz et al. (2015) found the relationships [Indigofereae [Clitorieae [Phaseoleae, etc. [Abreae [Diocleeae + Millettieae]]]]], although details of the relationships obtained depended on the markers used (see also Egan et al. 2016).
Stefanovic et al. (2009: eight chloroplast genes) concentrated on determining relationships among the some 2,000 species of phaseolids, finding substantial resolution, i.a. Mucuna was sister to Desmodium and its relatives (c.f. Jabbour et al. 2017), the combined clade being sister to the rest of the group, which also includes Cajanus, Vigna, Erythrina, and so on. De Queiroz et al. (2003) produced a morphology-based phylogeny of Diocleinae, then in Phaseoleae, as did Maxwell and Taylor (2003). Although Canavalia was monophyletic, genera likeGalactia, Camptosema and Dioclea itself were not, while other genera grouped with taxa that are now included in Phaseoleae and Millettieae.
Brongniartieae. Thompson et al. (2001) looked at relationships within this largely Australian-South American clade; see also Cardoso et al. (2016) and de Queiroz et al. (2017) for characters, relationships and circumscription. The African Haplormosia is sister to other members of the tribe (Cardoso et al. 2016); however, c.f. L. Cai et al. (2025) where Domatophyllum appears to be in that position, while Haplormosia is sister to the whole [Sophoreae ... Genisteae] clade. Sa˜o Mateus et al. (2024: nuclear and plastid markers) looked at the evolution of Brongniartieae, especially Harpalyce, which is monophyletic; these authors included several accessions of a number of taxa, and in Harpalyce in particular the species were often not monophyletic - ILS?
Desmodieae. Jabbour et al. (2017: 2 plastid, 1 nuclear marker, 25/32 genera, 56 spp.) examined relationships here; several genera, including Desmodium itself, came out in more than one place in the tree, the New Calesonian taxa seemed to be in a muddle, and there was some conflict between topologies obtained from nuclear and chloroplast markers. The focus of Ohashi et al. (2019, 2020, 2022, 2025 and references) has been on Desmodium s.l. - again there may be substantial conflict between trees, and clearly a good nuclear tree is much to be desired here.Diocleeae. De Queiroz et al. (2015) examined relationships here in some detail and found that genera like Camptosema and Galactia were very much polyphyletic; for relationships in Canavalia, see Snak et al. (2016: stem, ca 15.8 Ma, ?= tribe, crown, (11.1-)8.7(-6.7) Ma) and for those around Dioclea itself, with generic adjustments, see de Queiroz and Snak (2020). De Queiroz et al. (2003) and Maxwell and Taylor (2003) discuss morphology and morphology-based relationships.
Indigofereae. Barker et al. (2000) and Schrire et al. (2009, see also 2003) disentangle relationships within the tribe, finding considerable phylogenetic structure (i.a. there are four major clades within Indigofera itself) that can be linked with both morphology and ecology. Du Preez et al. (2024: 393 taxa, ITS, see also 2025a, b) further developed ideas of relationships here, i.a. they found that I. nudicaulis was sister to the remainder of the Cape clade, but separated by a long branch.
Millettieae. For the delimitation of Millettieae, see Lavin et al. (1998); Hu (2000) and Hu et al. (2000) studied their phylogeny, and Abrus (with nine stamens) is (near-)basal (Prenner 2013b and references). Clades around the old Millettia are unclear (Cooper et al. 2019), the genus being para/polyphyletic in analyses such as those of Lavin et al. (2005) and Duan et al. (2021a). For the phylogeny of Derris and its immediate relatives, see Sirichamorn et al. (2012, 2014a, b).
Leptolobieae. For relationships around Bowdichia, see Cardoso et al. (2012a). Leptolobium is polyphyletic (Gregório et al. 2023).
Phaseoleae. For a phylogeny of Phaseolus itself, see Delgado-Salinas et al. (1999, 2006). Of other Phaseoleae, Vigna has to be dismembered (Delgado-Salinas et al. 2011), as does Pueraria (of kudzu vine fame), members of which are in five widely separate clades (Egan et al. 2016). Horton et al. (2024: 67 spp., chloroplast and nuclear markers) looked at relationships in Vigna s. str.; there was a fair amount of resolution, although some conflict between plastid and nuclear trees; Physostigma is the sister genus (there is quite considerable floral variation around here, i.e. including Phaseolus). Recognition of Paracalyx makes Eriosema paraphyletic and Rhynchosia is polyphyletic, the type, R. volubilis, being in a clade by itself separate from the two other clades containing the other 31 species examined - and one of these clades was paraphyletic (Cândido et al. 2020). H. Li et al. (2013) carried out a quite detailed analysis of Phaseolineae in which ¾ genera were included. They found that Apios was sister to the rest, and Desmodieae made up the next pectination, however, the once and future(?)) Psoraleeae were deeply embedded in the phylogeny, Egan et al. (2024: p. 7) noting that they were "nested within tribe Phaseoleae subtribe Glycininae" - clearly, tribal limits around here should be taken with a grain or so of salt. For relationships in the pantropical Mucuna, nearly all of which are lianes, see de Moura et al. (2015) and for the optimisation of some fruit and seed characters which correlate nicely with the subgenera, see de Moura et al. (2016). The pantropical Erythrina is not monophyletic (de Moura et al. 2011).
Psoraleeae. Dludu et al. (2013) examined relationships around Psoralea and Otholobium and with a focus on taxa from the Cape area, and they found that Otholobium might be para-/polyphyletic, including Psoralea; see also Egan and Crandall (2008). Bello et al. (2022: ITS, 7 plasid markers) found that South American Otholobium were clearly separate from the rest of the group; nuclear and plastid trees told pretty much the same story. Egan et al. (2024: ITS and three plastid genes) again looked at the phylogeny here in some detail, changing some generic limits/describing a new genus; Otholobium is a subgenus of Psoralea.
Galegodae. For the phylogeny of Robinia and its relatives, which include Lotus and Sesbania, two genera that are quite close, see Wojciechowski et al. (2000). Within Robinieae s. str. [Hebestigma + Lennea] are sister to the rest (Lavin et al. 2003). Sesbanieae. Farruggia and Wojciechowski (2009) and Farruggia et al. (2018) examined relationships within Sesbania itself. Loteae. For members of this tribe from the Canary Islands, see Allan et al. (2004) and for Lotus in Macaronesia in general, see Jaén-Molina et al. (2020). Degtareva et al. (2012) looked at relationships of Anthyllis and Allan et al. (2003) at those around Lotus, with Hammatolobium being sister to Old World Lotus, and the unrelated New World Lotus perhaps including Ornithopus and other genera in a basal polytomy (see also Degtjareva et al. 2006b, 2008; Kramina et al. 2016).
Included in Galegodae is the Inverted Repeat Loss clade (IRLC). Wojciechowski et al. (2000) outlined relationships in this speciose clade, as did Duan et al. (2021a). In the latter, where the focus was on Wisterieae, trees based on analyses of 75 protein coding sequences from chloroplast DNA were compared with those based on analyses of much less than 1/10th the sequence data from nuclear ribosomal RNA genes; there was an appreciable amount of conflict in the topologies of the two trees, support being rather weaker in the tree based on nuclear data. Duan et al. (2021b) looked at the immediate relatives of Wisterieae. However, it seems that worrying about relationships, producing classifications, etc., depends of having well-sampled full nuclear and plastome trees to compare, so little further can be said at present (vi.2022). Wojciechowski et al. (2000) found that at the base of the IRLC relationships were [Glycyrrhiza [Wisteria s.l. + ...]], Wisteria being embedded in Callerya, although C. atropurpurea tended to wander between Wisteria s.l. and Glycyrrhiza (J. Li et al. 2014), etc.. However, Duan et al. (2021a, b: plastome data) found that Wisterieae were sister to Glycyrrhizeae (= Glycyrrhiza and Glycyrrhizopsis), but Adinobotrys might be sister to Glycyrrhizeae or to [Glycyrrhizeae + Wisterieae]. Indeed, Adinobotrysagrees with Wisterieae in having the cp rps12 intron although it differs from them in habit (Compton et al. 2019); note that Glycyrrhiza and the other members of the IRLC lack this intron (Compton et al. 2019). C. Su et al. (2020) also recovered a clade [Glycyrrhiza + Wisteria]. Sister to Astragaleae are Coluteeae (or maybe the two should be a single tribe) (Moghaddam et al. 2017; for relationships around here, see also M. Zhang et al. 2009a).
Relationships in Wisterieae are being sorted out: they form a clade (Wojciechowski et al. 2000) which was studied in some detail by Duan et al. (2021: esp. Fig. ), although there were substantial differences in topology in the nuclear and chloroplast trees.
Astragaleae. Extensive phylogenetic studies (e.g. Wojciechowski 1993, 2004; Liston & Wheeler 1994; Wojciechowski et al. 1999; Kazempour Osaloo et al. 2003, 2005) showed that Astragalus was largely monophyletic, although bits, like the old subgenus Pogonophace (= Phyllolobium), have had to be removed (M.-L. Zhang & Podlech 2006). Most New World taxa are aneuploid (n = 11-15) and are also monophyletic (there are non-aneuploid members there too - C. Su et al. 2020), other species are base 8; the Old World [A. pelecinus + A. epiglottis] clade - the two are annual species - may be sister to the rest of the genus (Liston & Wheeler 1994; Azani et al. 2017; see also C. Su et al. 2020). For general relationships in Old World Astragalus, see Kazempour Osaloo et al. (2003, 2005), Kazemi et al. (2009), Riahi et al. (2011), Dizkirici et al. (2014: ITS, fair resolution), Maassoumi et al. (2016: ITS sequences) and especially Azani et al. (2017, 2019) and C. Su et al. (2020: plastomes, eastern Asian taxa). Glottis, Ophiocarpa/Pseudosesbanella and Phaca are successively sisters to the rest of the genus. However, Khalili et al. (2020) found considerable conflict between relationships based on plastome and those based on nuclear genes in their study of the spiny sect. Acanthophace, so one awaits genus-level analyses that incorporate variation in the nuclear genome. Amini et al. (2018: nuclear + plastid marker) examined relationships in the large Old World section Incani. For relationships in New World Astragalus, see Scherson et al. (2005, 2008: 1 nuclear, 2 plastid markers). Note that there is high discord along the backbone of the Neo-Astragalus part of the tree, quartet scores being close to zero there (Folk et al. 2024). Bueno et al. (2025) looked at 107 carefully-chosen species using about 781 nuclear loci and plastome data, and once again, considerable nuclear-plastome conflict was evident, hybridization being likely (ibid. Fig. 2); however, there are around 229 supraspecific groupings in the genus, and these authors estimated that analyses of Astragalus from the eastrn hemisphere alone would have to include about 900 species if sense was to be made of its phylogeny. The immediate relatives of Astragalus are unclear, as is obvious from the summary in Sun et al. (2020).
Oxytropis may be sister to Astragalus; for some relationships in the former, see Archambault and Strömvik (2012), Dizkirici Tekpinar et al. (2016) and Shahi Shavvon et al. (2017); the latter two groups used ITS and one plastid gene and produced superb examples of phylogenetic combs, although Shahi Shavvon et al. (2017) suggested that anchored hybrid enrichment might be the way to go in the future... Variation in Oxytropis was less than in Astragalus.
Hedysareae, Galegeae, etc., may be mixed, relationships being complicated in this area. For instance, Duan et al. (2015: 138 spp., 1 nuclear and 3 chloroplast markers) found the relationships [[Vicioid clade inc. Galega] [[Galegeae I inc. Astragalus and Oxytropis] [[Caragana + Galegeae II] [Alhagi [Sulla [Hedysarum p. pte [[Hedysarum p. pte + Sartoria] [[Taverniera + Ebenus] [Onobrychis + Eversmannia, etc.]]]]]]]].
For relationships in Hedysareae, see P.-L. Liu et al. (2017: 56 spp., five nuclear and 5 plastome markers); nuclear data suggested that Hedysarum was polyphyletic, part to be placed in Sartoria, the combined group being in turn sister to Tavernieria, while in plastid analyses, Hedysarum was monophyletic. Juramurodov et al. (2024: 3 plastid, 1 nuclear markers) emphasized relationships within Hedysarum (110 species included), with a focus on Central Asian taxa; they found that the three sections largely held up and many Central Asian taxa were to be placed in section Multicaulia subsection Crinifera, although relations there were largely unresolved. Again, there was nuclear/chloroplast conflict, Onobrychis, Eversmannia and Cadia being embedded within Hedysarum in nuclear ITS analyses, although support was not strong, while using plastid data these genera were outside Hedysarum, even if Eversmannia itself was sometimes embedded in Onobrychis (Juramurodov et al. 2024).
Caraganeae. Duan et al. (2016: 75 species, nuclear ITS and 3 plastid markers) discussed the phylogeny and diversification of Caragana in the context of the Qinghai-Tibetan Plateau uplift. they suggested that Chesneya (= Chesneyeae) was the result of a chloroplast capture event that involved the ancestors of Chesniella (staminate parent) and the GUT clade – [Gueldenstaedia + Tibetica].
Within Coluteeae the monotypic Podlechiella is sister to the rest (Moghaddam et al. 2017)
For Galega, see also Duan et al. (2021a). Within the combined clade, Safaei Chaei Kar et al. (2014) looked at relationships within Onobrychis and found that current infrageneric groupings had little support, Amirahmadi et al. (2016) providing an elaborated ]phylogeny. M. Zhang et al. (2009b, 2015), M. Zhang and Fritsch (2010) and Duan et al. (2016) discussed the phylogeny and diversification of Caragana in the context of the Qinghai-Tibetan Plateau uplift. Within Hedysareae Alhagi may be sister to the rest (Amirahmadi et al. 2014). Steele and Wojciechowski (2003), Lavin et al. (2005), Dangi et al. (2015), Koenen et al. (2019a), and R. Zhang et al. (2020) discuss the limits of the tribe. Vicieae (= Fabeae) may be embedded in Trifolieae s.l., so it is unclear whether Trifolieae should be monogeneric, restricted to Trifolium, or include the genera placed in Medicageae above; the tribes, etc., are circumscribed narrowly here. This group is sister to everything except Wisteria et al. in the plastome analysis of Sun et al. (2020), where there is the phylogenetic structure [Cicer [Hedysarum [[Melilotus + Medicago]...]]].
Trifolieae. Within Trifolium itself, the American species form a monophyletic group (Steele & Wojciechowski 2003; Ellison et al. 2006; Liston et al. 2006). Medicageae/Trigonellinae. Phylogenetic relationships within Medicago have turned out to be highly reticulating (de Sousa et al. 2016 and references) and using different data sets/methods of analysis results in different topologies (de Sousa et al. 2014). The genus perhaps includes Trigonella; for its limits, see Bena (2001), Steele et al. (2010) and Dangi et al. (2015). Note that Mittal and Dangi (2025: ITS and 3 plastid markers) found that Trigonella was to include Melilotus.
Fabeae (inc. Vicieae). A preliminary phylogeny of Lathyrus suggested that the ca 20 South American species might represent a single clade derived from Northern Hemisphere ancestors (Asmussen & Liston 1998; see also Kenicer et al. 2005). Relationships in the Vicia/Lathyrus area are complex, and both Vicia and Lathyrus are para/polyphyletic, i.a. Lens and Pisum respectively being embedded in them, and classical infrageneric groupings were not holding up (e.g. Steele & Wojciechowski 2003; Lavin et al. 2005; Dangi et al. 2015; Koenen et al. 2019a; R. Zhang et al. 2020). In Pisum s.str. analyses of chondromes and plastomes suggested very different relationships; there was also hybridization and plastid-nuclear incompatability (Bogdanova et al. 2021). Fabeae are also the subject of an extensive study by Schaefer et al. (2012), which focused on taxa from the Atlantic Islands off N.W. Africa.
Classification. In the past, Fabaceae have usually been divided into three groups, i.e. Fabaceae/oideae (= Papilionaceae/-oideae), Mimosaceae/-oideae and Caesalpiniaceae/-oideae, but the latter have turned out to be paraphyletic; the L.P.W.G. (2013b) give a fascinating introduction to the dynamics of the reclassification of the family. Relationships at the deeper nodes are poorly known, but the old Mimosoideae are deeply embedded in part of Caesalpinioideae (= Caesalpinioideae s. str), so changes in the names for the major elements that make up the scaffolding of the family were to be expected. The L.P.W.G. (2017) suggest a revised classification, largely followed above, and i.a. the old Mimosoideae were to be refered to as "the mimosoid clade" pending clarification of their relationships. Given what is known now, this clade can be called Mimoseae, and the question is how to name the clades it contains as their relationships become better understood; the informal names used (ix, 2022) here are those in Koenen et al. (2020b) and Ringelberg et al. (2022a), with some minor additions. Lewis et al. (2013) provide a linear sequence of legume genera recognised as of March, 2013.
A fair bit of adjustment to generic limits has been going on, perhaps especially in Caesalpinioideae-the mimosoid clade/Mimoseae. Ringelberg et al. (2022a: see also Appendix 1) found that a quarter of the genera there were not monophyletic, and some of these problems have been cleared up by papers in PhytoKeys 205. 2022. In some cases, in the past in particular, although it was obvious that there would be changes, sampling was not yet good enough to know what to do (e.g. see Percy & Cronk 2002; Allan et al. 2004; Ribeiro et al. 2007; Cardoso et al. 2012a; Dludlu et al. 2013; Terra et al. 2022). Variation in fruit morphology used to be considered to be an important generic character in Mimoseae, however, genera so delimited are not holding up (e.g. Ringelberg et al. 2022; Borges et al. 2022a; Soares et al. 2022, and other papers in PhytoKeys 205. 2022), although distinctive fruit types are being used to help justify the separation of genera for whose recognition there are other reasons (e.g. Hughes et al. 2022b; Koenen 2022). Interestingly, as Hughes et al. (2022a) retooled generic limits in the old Prosopis (most species are now in the Neltuma clade), they found that the relationships of the mimosoid hosts of bruchid beetles (see Kingsolver et al. 1977) agreed quite well with the revised generic limits. Ultimately, some version of monophyly, not morphology, is the arbiter, and morphology may simply not be able to deliver the goods - as É. R. de Souza et al. (2022: p. 445) noted, "Boliviadendron [a new genus that they were describing] is similar in almost all respects to the genus Leucochloron and is segregated first and foremost because these two lineages are phylogenetically not closely related". The complex infrageneric classification of Mimosa (5 sections, 41 series, 39 subseries) by Rupert Barneby is not holding up, but Borges et al. (2022b) have begun to rework the series. Calliandra and surrounding genera were studied by de Souza et al. (2013; see also Thulin 2023). The old Acacia subgenus Acacia, which includes the bull's horn acacias, seems to be monophyletic, but Acacia s.l. is polyphyletic. As Maslin (2001) noted sadly of the 955 or so species placed in Acacia for the Flora of Australia, "we are obliged to present the flora treatment in the absence of a more meaningful classification". However, things had already begun to change, although Pedley's (1986) solution following strict nomenclatural lines was proving only partly acceptable, and the argument became what names to use for the bits into which Acacia s.l. had to be divided (Maslin et al. 2003). The speciose Australian subgenus Phyllodineae is now Acacia s. str., see Miller and Bayer (2003; also Boatwright et al. 2015) for Vachellia, the old subgenus Acacia, and Senegalia, the old subgenus Aculeiferum, also Siegler et al. (2006, 2017) for other segregates. This nomenclatural solution, although less than ideal for some, is taking hold. Iganci et al. (2015) found that Abarema was very much polyphyletic, and Soares et al. (2021) are renaming the bits into which it has been divided. Elsewhere within Caesalpinioideae, generic limits in the Caesalpinia group were initially unclear (Gagnon et al. 2013), but see Gagnon et al. (2017) in particular, also R. P. Clark et al. (2022), for a solution.
Bruneau et al. (2024) should be consulted for details of a classification of the whole of Caesalpinioideae that is followed above. Given the highly pectinate nature of the tree in this part of the phylogeny the Linnaean ranking system broke down, although perhaps subtribes could have been used. Within Mimoseae numerous monophyletic groups are referred to simply as "Genus name clade" (phylogenetic definitions are also given), indeed, there are only a relatively few small areas where they thought that relationships were unclear. But note that just about any classification system here will seem to have serious failings... Within Caesalpinioideae, A. O. de Souza et al. (2021) provide an infrageneric classification of Chamaecrista - four sections, one of which is divided into three subsections, in place of the previous 6 sections + 3 subsections + 39 series. Aviles Peraza et al. (2022) provide a sectional classification of Pseudalbizia. E. R. de Souza et al. (2013) found that Barneby's infrageneric classification of Calliandra did not hold, and they recognized six sections, while Ferm (2019) found that the five subgenera into which Zapoteca had been divided largely did not hold.
For generic limits in Cercidoideae, where there is some disagreement over genera to recognize but perhaps a developing concensus, see e.g. Wunderlin et al. (1987), Lewis and Forest (2005), Wunderlin (2010) and Sinou et al. (2009 and especially 2020: also sections in Bauhinia).
De la Estrella et al. (2018) provide a tribal classification of Detarioideae, which is followed above. For the limits of Cynometra (Amherstieae), see Radosavljevic (2019).
In Faboideae, Cardoso et al. (2013b) listed the early-branching clades, i.e., those below the NPAAA clade, as well as the genera and numbers of species that they contain. For a sectional classification of Neotropical Swartzia, see Torke and Mansano (2009), for that of an expanded Cytisus, see Cristofilini and Troia (2006). Du Preez et al. (2025a) recognized 8 sections in the Indigofera Cape clade of which three were monotypic and two had four subsections each. The sections previously recognized in pantropical Crotalaria - le Roux et al. (2013, 2014) - are not holding up in molecular analyses (Rockinga et al. 2017). Unique combinations of floral characters can be used to recognize genera around Crotalaria (Le Roux & van Wyk 2012). For a sectional classiification of Onobrychis, see Amirahmadi et al. (2016). De Moura et al. (2016) provide an infrageneric classification for Mucuna. The limits of Desmodium are being adjusted (Ohashi et al. 2019), principles there following Ohashi et al. (1981: p. 293) in "maintaining most familiar generic names and adding relatively few additional segregates that are, for the most part, fairly readily recognizable". One can but hope: At least seventeen genera (as of iv.2021) had been added since 2005, including two genera recently segregated from Desmodium where together they make up a small clade sister to the rest of the genus (Ohashi et al. 2018) - and less than 20% of the tribe was included in that phylogenetic analysis. The trees produced by Jabbour et al. (2017) also suggested that attention needed to be paid to generic limits here. There is a fair bit of interest in Aeschynomene because of the way it forms associations with N-fixing bacteria, however, that genus is polyphyletic and generic limits in the Aeschynomene area are in some disorder (but see Cardoso et al. 2020); there has been fairly deep hybridization here (see e.g. Chaintreuil et al. 2013; Brottier et al. 2018). Thompson (2001) provides a careful study of E. Australian Hovea (Brongniartieae). For generic limits around Gastrolobium, see Chandler et al. (2001), for those around Vigna, see Delgado-Salinas et al. (2011), for those around Lotononis, see Boatwright et al. (2011); the limits of Derris have been redrawn (Sirichamorn et al. 2014b). Horton et al. (2024) provide a subgeneric/sectional classification of Vigna s. str.. M. Liao et al. (2023) extended the limits of Sophora somewhat and recognized 9 well-supported sections. See Orthia et al. (2005a, b) for the expanded generic limits of Pultenaea. Adesmia (Dalbergieae) has been divided into 2 subgenera and 43 series; Monteiro et al. (2024) hope to simplify this classification... Generic limits in Amorpheae seem rather unsatisfactory, but relationships also have to be clarified before there can be any progress (see Becklund & Ayers 2022). R. L. Barrett et al. (2024) clarified generic limits in some Mirbelieae.
Within the IRLC, Compton et al. (2019) provide a classification of Wisterieae with rather narrowly drawn generic limits; Duan et al. (2021b) adjust tribal limits around here, recognizing a Glycyrrhizeae (= Glycyrrhiza and Glycyrrhizopsis) and Adinobotryeae (Adinobotrys). However, changes in tribal relationships in the IRLC awaits analyses of the nuclear genome. Schaefer et al. (2012) suggest that one solution to the unexpected phylogenetic relationships they found in Fabeae is to include Lens in Vicia (as V. culinaris) and Pisum in Lathyrus (as L. oleraceus: see Kenicer & Parsons 2021)... For sectional, etc., classifications of Astragalus, see e.g. Barneby (1964: New World - all told, some 93 sections and lower-level groups recognized here) and Podlech and Zarre (2013: Old World - ca 136 sections etc.), and there may be some 350 sections in total, but there is much to be done on relationships here. Degtjareva et al. (2006b) suggested a sectional classification for Lotus. Duan et al. (2016) divided Chesneya (Caraganeae) into three monophyletic sections and Caragana itself into seven sections. Mittal and Dangi (2025) suggested that 17 sections were to be recognized in their expanded concept of Trigonella.
Previous Relationships. Fabaceae s.l. have often been placed in their own order, as in both Cronquist (1981) and Takhtajan (1997), and then they are usually divided into three families. Fabaceae have also been linked with Connaraceae, here in Oxalidales, and with Sapindaceae (e.g. Dickison 1981b), here in the Sapindales. However, there is little morphological support for such associations other than the common posession of compound leaves which, indeed, are pulvinate in Connaraceae, and that family also has a somewhat pod-like fruit. Sapindaceae, like Fabaceae, also have non-protein amino acids.
Botanical Trivia. There has been as much diversification of the ycf4 protein, involved in photosystem 1 assembly, within Lathyrus as there has been between cyanobacteria and other angiosperms (Magee et al. 2010).
The seeds of Mora megistosperma (Caesalpinieae) are, at ca 18 x 12 cm, perhaps the largest of any broad-leaved angiosperm (Lewis et al. 2005), and the embryo is the largest of all angiosperms.
And when the kudzu vine was thought to be a useful ground cover, soil stabilizer, etc., there were homecoming Kudzu Kings and Queens...
[Surianaceae + Polygalaceae]: embryo chlorophyllous.
Age. This node is dated to (71-)68, 66(-63) Ma (Wikström et al. 2001).
SURIANACEAE Arnott, nom. cons. - Back to Fabales —— Synonymy: Stylobasiaceae J. Agardh
Woody; ellagic acid?; storying +/0, wood fluorescing?, vessels in radial multiples; (sieve tube plastids with starch grains and protein filaments forming a peripheral shell - Stylobasium); cork also in inner cortex; nodes 3:3 (1:1 - Suriana); (medullary vascular bundles - Recchia); sclereids +; colleters + [Suriana]; petiole bundle arcuate to annular; leaves spiral or two-ranked, (unifacial), (pinnate, leaflets alternate, articulated), stipules + (0 - Suriana); inflorescence cymose, usu. terminal; pedicels articulated; K connate basally or not, quincuncial, C (0), contorted, shortly clawed or not; ?receptacular tissue ± forming a ring around the C base; A = and opposite K [= obdiplostemonous]; pollen suboblate, exine striate [Suriana], vermiform [Cadellia]; nectary 0; (gynophore +, nectariferous - Recchia); G 1-5, when 5 opposite C, styluli separate, ± gynobasic, stigma clavate to capitate, compitum 0; ovules surrounded by mucilage, 1-5/carpel, apotropous [Suriana], campylotropous to amphitropous, unitegmic, integument 3-7 cells across, parietal tissue 4-5 cells across, (nucellar cap +), hypostase +; megaspore mother cells several, antipodal cells ± degenerate; fruit indehiscent, berry, drupe or nut, endocarp with outer layer of palisade sclereids, other cells apart from the thin-walled inner epidermis isodiametric, K persistent, accrescent or not; exotestal cells enlarged, cuboidal, tanniniferous, rest crushed [ca 7 cells thick], or seed tegmic; chalazal endosperm haustorium +, endosperm 0, embryo curved or folded, cotyledons incumbent; x = 7, nuclear genome [1 C] (0.045-)0.887(-17.65) pg; germination epigeal, phanerocotylar.
5 [list]/8 Mostly Australian, also Mexico and the Osa Peninsula, Costa Rica (Recchia); Suriana maritima littoral, pantropical. Map: from van Steenis and van Balgooy (1966: blue - Suriana maritima) and FloraBase (consulted xi.2010). [Photo - Flower.]
Age. The age of crown-group Surianaceae is (50.4-)38.7(-27.0) Ma (Bello et al. 2009) or (53.1-)47.6(-33.2) Ma (Uluer et al. 2022).
Chemistry, Morphology, etc.. The family is vegetatively heterogeneous, although its wood anatomy is quite homogeneous (Webber 1936). The bark parenchyma of Cadellia and Recchia has sclereids (Crayn et al. 1995).
There is no compitum (Armbruster et al. 2002). The exotesta of Suriana is described as being green (Rao 1970). Both Cadellia and Recchia have thickened cell walls in the exocarp (Crayn et al. 1995)
For more information, see Gutzwiller (1961), Weberling et al. (1980), and Schneider (2006), all general, Hegnauer (1973, as Simaroubaceae), chemistry, Behnke et al. (1996), sieve tube plastids, Jadin (1901) and Boas (1913), both vegetative anatomy, Mauritzon (1939), Wiger (1935), Anantaswamy Rau (1940a), Rao (1970) and Heo and Tobe (1994), all embryology, etc., Gadek and Quinn (1992: pericarp); for floral development, see Bello et al. (2007/8) and for fruit anatomy Fernando and Quinn (1992), both Suriana only), and for seed coat anatomy, see Gama-Arachchige et al. (2013: esp. water gap). Additional data from: Cadellia - Benson s.n. = NSW 408528 (anatomy); Stylobasium - Latz 12864 (fruit) and Strid 20708 (anatomy).
The vegetatively "atypical" Suriana is the only genus whose embryology has been studied and Surianaceae as a whole are little known chemically.
Phylogeny. [[Recchia + Cadellia] [Suriana [Guilfoylia + Stylobasium]] are suggested relationships in the family (Forest et al. 2007b); c.f. also Crayn et al. (1995) and Bello et al. (2009). Notice, however, that Stylobasium is placed sister to the rest of Fabales in Seed Plant Tree, Version 2 (2022) - support strong.
Classification. Although the sieve tube plastids of Stylobasium are distinctive (Behnke et al. 1996), there seems little reason to recognise Stylobasiaceae as a family, i.a. four families for five genera would then be needed if the commonly recovered relationships above hold.
Previous Relationships. Surianaceae were included in Rosales-Simaroubaceae (here in Sapindales) by Cronquist (1981) and in Rutales (here Sapindales), but as a separate family, by Takhtajan (1997).
POLYGALACEAE Hoffmannsegg & Link, nom. cons. - Back to Fabales
Saponins +; nodes 1:1; styloids 0; (tracheidal/fibrous/sclereidal cells); (stomata other than anomocytic); plant glabrous or with unicellular hairs; branching from previous flush; often paired glands [crateriform extrafloral nectaries] or thorns at nodes; axillary buds 2 or more/node; lamina entire, glands +; inflorescence racemose, (flowers in cymose clusters along axis); flowers monosymmetric; K quincuncial, C 5, (not clawed), keel ± apparent [= abaxial C]; A 8, ± connate, basally adnate to C, median adaxial A often absent; pollen polycolporate, surface smooth or foveolate; (disc excentric); G connate, style long, stigma dry; ovules (initiated when carpels are open), epitropous, micropyle zigzag (endo-, exostomal), exostome often long, outer integument 2-6 cells across, inner integument (1-)2(-3) cells across, parietal tissue 1-3 cells across, nucellar cap 2-3 cells across, suprachalazal region ± massive; testa multiplicative, exotesta subsclerotic or otherwise distinct, endotestal cells ± palisade, U-thickened, with crystals or not; endosperm 0-copious; x = 12 (?11), nuclear genome [1C] (0.039-)0.763(-14.856) pg; rpl22 gene transferred from chloroplast to nucleus [?sampling].
29 [list]/1,236 - four tribes below. World-wide, except the Arctic and New Zealand. [Photo - Flower.]
Age. Diversification of Polygalaceae began in the early Caenozoic (65.5-)57.4(-49.3) Ma (Bello et al. 2009), (62.7-)63.6(-58.2) Ma (Uluer et al. 2022: ??) or (64.9-)62.8(-50.6) Ma (L. Cai et al. 2025: p. 9).
1. Xanthophylloideae Chodat - Xanthophyllum Roxburgh —— Synonymy: Xanthophyllaceae Reveal & Hoogland
Shrubs or trees; growth sympodial, terminal bud aborts; plants Al-accumulators; wood parenchyma apotracheal, diffuse; petiole with cortical sclereids (0), bundle annular, with inverted central plate/arcuate with wing bundles/etc.; glands at nodes; tracheoid foliar idioblasts ± at ends of veins, stomata various; (conspicuous domatia on leaf blades); inflorescence indeterminate; K unequal, C contorted, (no keel), (adaxial petals with colour patterning); A (7-10); G [2], placentation parietal, stigma small, bilobed (capitate); ovules 2-8(-20)/carpel, in two rows, outer integument 4-12 cells across, hypostase massive [?level]; fruit a berry, (irregularly loculicidally dehiscent), K deciduous; testa vascularized, strongly multiplicative (not), (± crushed); (endosperm starchy); n = 8 [1 species].
1/110. Indo-Malesia. Map: from van der Meijden (1982).
2. Polygaloideae Eaton
inflorescence cymose; A often monadelphous, anthers opening apically [pores/slits]; ovule 1/carpel; seed hairy (glabrous), exostomal/funicular aril + (0).
More or less world-wide. Map: from Wickens 1976; Frankenberg & Klaus 1980; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; GBIF 2009; Flora of China Vol. , Australia's Virtual Herbarium (consulted xii.2012) - orange from Paiva (1998).
Age. The age of this clade is (95.5-)84(-74.5) Ma (Pastore et al. 2019) or (56.6-)56.1(-55.5) Ma (L. Cai et al. 2025: as Polygalaceae).
2A. Diclidanthereae Reveal (= Moutabeeae in older literature) —— Synonymy: Diclidantheraceae J. Agardh, Moutabeaceae Pfeiffer
Shrubs to trees, lianes; plants Al-accumulators; successive cambia +; included phloem +/0, banded apotracheal parenchyma +; petiole bundle annular, with wing bundles; glands on leaves (and at nodes); inflorescence often racemose; K adnate to C, abaxial C not keeled; A (6-10), connate/adnate to C/free, anthers with short confluent apical slits; G [2-8], stigma capitate; fruit?; funicular aril +; nucellar epidermis persistent, embryo large, curved, cotyledons foliaceous, chlorophyllous, accumbent; n = 14.
4/17: Moutabea (11). Panama, South America, New Guinea to New Caledonia. Photos: Flowers, Flower - Close-up, Petioles, Branch, Petioles with ants, Flower with moth.
[Carpolobieae + Polygaleae]: ?
2B. Carpolobieae Eriksen
Shrub to small tree, liane; (glands at nodes); (C contorted); A (4) 5, anthers with short confluent apical slits; G [3], stigma capitate; fruitBerry-drupe, 1-3 locular; exotesta fleshy [?both]; endosperm copious; n = 9-11.
2/7: Carpolobium (4). Tropical Africa, Madagascar.
2C. Polygaleae Chodat
Herbs (annuals), (echlorophyllous mycoheterotrophs - Salomonia), lianes, shrubs; (ergoline alkaloids +), (methyl salicylate + [wintergreen]), tannins 0 [Polygala]; (successive cambia +); vestured pits +, banded paratracheal parenchyma +; petiole bundle arcuate to annular; foliar glands 0; flowers (asymmetrical),; (2 abaxial lateral K, minute), two adaxial lateral K = wings, two adaxial C connate [= the standard], abaxial C carinate, (± deeply lobed/apically fringed), 2 abaxial-lateral C minute/0; (A [2-7]), anthers with apical pores; G [2] (adaxial member suppressed), stylar canal + [Polygala], stigma bilobed, ± asymmetric, wet, one lobe sterile [?always]; ovule with (postament - Epirixanthes?), (antiraphe +); fruit capsule, often flattened, berry, drupe or samara, (K persistent, green - Polygala, etc.); seeds 2, hilar/chalazal/exostomal elaiosome + (0); testa (hairy), (mesotesta +); cotyledons chlorophyllous; x = 12 (?11), nuclear genome [1 C] (0.039-)0.763(-14.856) pg.
29/1,100: Polygala (349/673 - Moonlight et al. 2024), Senega (229), Monnina (158), Muraltia (121), Securidaca (56). More or less World-wide. Map: from Wickens (1976), Frankenberg and Klaus (1980), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), GBIF (consulted 2009), Flora of China, and Australia's Virtual Herbarium (consulted xii.2012); orange from Paiva (1998).
Age. Some (84.0-)76.0(-68.5) Ma (Pastore et al. 2019) or (44.7-)45.2(-38.8) Ma (Uluer et al. 2022: ??) are estimates; the [Polygala + Securidaca] node is estimated to be (42-)40, 28(-26) Ma (Wikström et al. 2001).
The distinctive Paleosecuridaca curtisii, from the Late Palaeocene of North Dakota ca 60 Ma, has fruits remarkably like those of Securidaca and seeds with a testa that has a well developed palisade layer, however, there are two seeds per carpel (Pigg et al. 2008b).
Evolution: Divergence & Distribution. N.B. The names of the genera mentioned below are those in the papers cited. I have not attempted to clean up the nomenclature, which is anyhow in a state of transition.
The "papilionoid"/keel flower in Polygalaceae is quite differently constructed from that of Fabaceae (Westerkamp & Weber 1997, 1999; Bello et al. 2010, but see Prenner 2004d), although quite often both looking and being functionally similar. Bello et al. (2012) suggest that the evolution of this flower type may have been responsible for rapid diversification within Polygaleae, the pollinators of keel flowers in Fabaceae moving on to those in Polygaleae. Note that the flowers of Polygala, which in overall appearance most approach those of some Fabaceae, are derived within Polygalaceae, and general floral variation in the family is considerable.
It has beeen suggested that papilionoid flowers in Polygaleae in particular are the result of mimicry by Polygaleae of such flowers in Fabaceae, probably some kind of Mullerian mimicry, although parallel evolution could not be ruled out (Uluer et al. 2022; for parallel evolution, see e.g. Ruxton & Schaefer 2011). Uluer et al. (2022) made comparisons with eight clades of South American Fabaceae - they thought that Polygaleae (and Faboideae and Fabales) originated there (and adding Africa, also Fabaceae) - and these included four clades in Dalbergieae, two that involved some or all Millettieae, and finally one that involved Leptolobieae and Camoensieae, and they were dated to (53.8-)48.4-45.5(-30.1) Ma. Uluer et al. (2022) thought that papilionoid flowers in Polygaleae, which have a crown-group age of 45.2 (44.7-38.8) Ma (Uluer et al. 2022: ??) were younger by some 10-22 My than those in Fabaceae, so geography and timing seemed to be consistent with the mimicry hypothesis. Of course they are also consistent with the idea of parallel evolution, moreover, the dates given are confusing, thus although crown Faboideae are estimated to be 67.2 (64.9-62.5) Ma (Uluer et al. 2022), papilionoid flowers are not the ancestral condition for the subfamily as a whole.
The evolution of elaiosomes in Polygalaceae is dated to (69.9-)54-50.5(-35.2) Ma, well after that of the ant clades concerned (by some estimates, at least), and it may have spurred diversification in the family (Forest et al. 2007b; Lengyel et al. 2009). Much of Muraltia, also myrmecochorous and with some 120 species found mostly in the Cape Floristic Region of South Africa (Linder 2003), may have diversified quite recently, mostly within the last ca 10 Ma, although diversification began around 14.8±3.6 Ma (Forest et al. 2007a); Verboom et al. (2009) thought diversification started in the Fynbos (21.4-)18.5(-14.1) Ma and in the Succulent Karoo (4-)2.5(-1.3) Ma.
Xanthophyllum is one of the five most speciose genera in West Malesian l.t.r.f. (Davies et al. 2005).
Bello et al. (2012) list a number of apomorphies for the family and of several clades within it.
Ecology & Physiology. For fires and hard seeds in Polygalaceae, see Lamont et al. (2018b).
Pollination Biology & Seed Dispersal. The flowers of Polygala are complex, and details of pollination are correspondingly so. In many species of Polygala pollen is presented on the sterile lobe of the often rather complex, asymmetric stigma, i.e. secondary pollen presentation on a stylar brush (Weekley & Brothers 1996; see Brantjes 1982; Castro et al. 2008a for further details; Bello et al. 2010 for stigma morphology). Other secondary pollination mechanisms known are explosive and pump pollination, and these two may both occur in a single flower, explosive pollination first followed by pump pollination - and there may even be tertiary pollen presentation (Westerkamp & Weber 1997; El Ottra et al. 2023 for secondary pollination in general).
Ant dispersal is quite common in Polygaleae in particular, and hilar/chalazal elaiosomes (the former are called caruncles) may be an apomorphy for the tribe. All told, there may have been at least six origins of myrmechochory in the family (Forest et al. 2007b; Lengyel et al. 2009, 2010).
Plant-Bacterial/Fungal Associations. For details of the association between glomeromycotes and echlorophyllous mycoheterotrophic species of Epirixanthes (= Salomonia), see Imhof (2007) and Imhof et al. (2013), and for general information on mycoheterotrophy, see elsewhere.
Vegetative Variation. Although genera like Xanthophyllum, some Diclidanthereae, etc., may have paired glands at the nodes, other genera seem to lack anything even faintly like stipules. De Aguiar-Dias et al. (2011) suggested that the paired nectary glands at the base of the leaf in Polygala laureola were true stipules because they received a vascular bundle from the single foliar vascular trace; the glands of Xanthophyllum may be similarly vascularized (Dickison 1973b). Xanthophyllum also has distinctive tracheoidal foliar idioblasts (Dickison 1973b), and although something seems to be going on at the ends of the veinlets in Diclidanthereae, whether the cells involved can be compared with the tracheoidal cells is unclear since Styer (1977), who discusses these latter cells, uses a different set of terms and does not cite Dickison (1973b).
Genes & Genomes. There is a genome duplication somewhere around here (Cannon et al. 2014), and it may be at the base of the family (the POLUβ event, ca 60.1 Ma), as suggested by Landis et al. (2018).
Chemistry, Morphology, etc.. AlthoughPolygala myrtifolia has eight stamens; the two stamens in the median plane, so on opposite sides of the flower, appear to have been lost (Prenner 2004d); see Bello et al. (2010) for other floral diagrams. The degree of connation of the filaments varies, as does that of their sometimes rather slight adnation to the petals. For floral morphology and development of Polygaleae, see Krüger and Robbertse (1988) and Krüger et al. (1988), and for that of the family as a whole, see Bello et al. (2010, 2012). The tricolpate pollen of Balgooya is probably derived; some Polygalaceae such as Heterosamara have asymmetric, almost boat-shaped pollen grains (Banks et al. 2008). Some species of Polygala, at least, have a stylar canal (Castro et al. 2008b). Monnina seems to have a nucellar cap ca 6 cells across, while the inner integument of Securidaca is up to 9 cells across in the endostomal region (Verkeke 1985). In indehiscent fruits the testa is more or less crushed (Rodrigue 1893; but c.f. Verkeke 1984, 1985). Verkeke (1985) distinguished between epitropous-dorsal ovules (Xanthophyllum) and epitropous-ventral ovules (the rest).
Additional information is taken from van der Meijden (1982: Xanthophyllum), Paiva (1998: Polygala, especially in Africa and Madagascar), Eriksen (1993a) and especially Eriksen and Persson (2006), and Chodat (1891, 1893) is still worth consulting - all general. For chemistry, see Hegnauer (1969, 1990), for nodal and leaf anatomy of Xanthophyllum, see Dickison (1973b), for wood and leaf anatomy of Moutabeae/Diclidanthereae, see Styer (1977) and for anomalous secondary thickening in Securidaca, see Rajput et al. (2012a), also Banks et al. (2008: pollen morphology and evolution), Manning and Stirton (1994: endothecial thickenings), and Verkeke and Bouman (1980), Verkeke (1991) and Takhtajan (2000), all ovule and seed.
Phylogeny. Of the four groups mentioned above, Diclidanthereae appeared to be paraphyletic in early analyses (Persson 2001: trnL-F), although adding rbcL data suggests they are monophyletic (Forest, in Eriksen & Persson 2006), and morphology also points in this direction (Eriksen 1993b); the other three groups appear to be monophyletic (although Carpolobieae are only weakly supported). However, all four tribes are strongly supported in a three-gene analysis (Forest et al. 2007b; see also Mota et al. 2019: 4 genes), and Xanthophylleae are sister to the other three tribes; relationships between these three were unclear and have remained so, as in Bello et al. (2012) and Mota et al. (2019). Polygala and Bredemeyera are grossly para/polyphyletic (Persson 2001; Abbott 2011; Pastore et al. 2017, 2019). See Eriksen (1993b) for a morphological phylogeny.
Classification. Because of the polyphyly of Polygala and Bredemeyera in particular, generic adjustments are under way (see Pastore 2012; Abbott et al. 2011, 2013; Pastore et al. 2017, 2023; Mota et al. 2019). Three subgenera, one with 16 sections, are recognized in Senega, ex Polygala (Pastore et al. 2023). Moonlight et al.(2024) include Polygala in their list of Big genera - 500 spp. or more.
Previous Relationships. The Polygalales of Cronquist (1981) included seven families, the mutual affinities of five of which were described as being "widely accepted". Along with Polygalaceae, these are Xanthophyllaceae (here = Polygalaceae), Vochysiaceae (Myrtales), Malpighiaceae, Trigoniaceae (both Malpighiales), Krameriaceae (Zygophyllales) and Tremandraceae (Oxalidales-Elaeocarpaceae)... For Emblingiaceae, another group that was often included in (e.g. Cronquist 1981; Mabberley 1997) or near (e.g. Takhtajan 1997) Polygalaceae, see Brassicales.