EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); root apical meristem closed, (root hairs also from radially alternating files of cells with and those without hairs [type III root hairs]); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. For further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ? - Back to Main Tree
Age. The age of this node has been estimated at (125-)121, 111(-107) Ma (Wikstr&m et al. 2004; similar in Jian et al. 2008); the age in Anderson et al. (2005: see positions of Crossosomatales included) is ca 108 Ma, it is ca 160 Ma in Z. Wu et al. (2014: [Vit. + Sax.] sister to rest, ca 151 Ma divergence between the two), while the comparable figures in Foster et al. (2016a: q.v. for details) are 128 and 125 Ma respectively. Magallón and Castillo (2009) provide estimates of around 114.5 Ma, Moore et al. (2010: 95% highest posterior density) an age of (111-)108(-103) Ma; ages of (135-)128, 117(-111) Ma are suggested by Bell et al. (2010), (112-)107(-101)Ma by N. Zhang et al. (2012), 110.5 Ma by Magallón et al. (2013), 114-111 Ma by M. Sun et al. (2014) and ca 117.7 Ma by Tank et al. (2015: Table S1) - or ca 58 Ma (Palazzesi et al. 2012, but c.f. Sytsma et al. 2014 - ca 104 Ma; Fan et al. 2019 - ca 126.3 Ma).
Fossils are somewhat younger, and those assignable to rosids as a whole are ca 94 Ma, and those to Saxifragales ca 90 Ma old (Crepet et al. 2004, see also Friis et al. 2011).
Evolution: Divergence & Distribution. Major relationships at the base of the Pentapetalae clade remain uncertain, and there is discussion of this problem and of the variation of a number of characters whose position on the tree depends on a resolution of these relationships at the Pentapetalae node.
Much of the initial crown group diversification within this clade may have occurred within a narrow time interval of 5-15 Ma around 117-93 Ma in the late Aptian early Turonian (Wang et al. 2009; see also Jian et al. 2008). M. Sun et al. (2020) noted that diversification rates in temperate rosids had increased greatly since the Miocene ca 15 Ma and was about twice that of tropical clades; species in the latter were older. Thus there are substantial temperate clades even within Malpighiales, otherwise an iconic clade of LTRF. Indeed, Malpighiales have slightly over 50% tropical species, while Cucurbitales and Myrtales have somewhat under 50% (Sun et al. 2020). Igea and Tanentzap (2019/2020) found similar patterns when looking at angiosperms as a whole.
Ecology & Physiology. There is a particularly noteworthy increase in seed size at this node that is associated with large leaf size; plants with such leaves and seeds commonly grow in equatorial areas that are both warm and well-watered (Cornwell et al. 2014: but c.f. Saxifragales). A relatively large embryo over half the length of the seed may be a feature of this clade (Forbis et al. 2002), but there is considerable variation in embryo size in Saxifragales while Vitales have small embryos. The character has been pegged to a higher level in the tree, to the rosid s. str. node.
Maherali et al. (2016) note that ectomycorrhizal associations in angiosperms are commonest in this clade.
Plant-Animal Interactions. Butterfly caterpillars are common on members of the group, occurring about twice as frequently as might be expected going by species number alone, but the tree habit is also common here, and trees perhaps can support a correspondingly disproportionately large number of larvae (Janz & Nylin 1998). However, Menken et al. (2009; see also Ward et al. 2003) reported on an extensive survey of larval host plants of British lepidoptera which they thought could be extended - with care - more globally, noting that caterpillars of basal Lepidoptera-Glossata (butterflies and moths with a coilable proboscis) tended to be found on woody rosid I (Fabidae) plants, normally as leaf miners or other non-exposed life styles (see also Ward et al. 2003). Since mines attributed to basal glossatan Neptulicidae have been reported from fossil magnoliid and protealean leaves, among others (see Menken et al. 2009), and these plants have a rather different chemistry from that of the rosids, the deep history of such lepidoptera-plant associations is unclear.
Genes & Genomes. For a possible whole genome duplication, see Tuskan et al. (2006) and Jaillon, Eury et al. (2007); it is probably to be placed immediately basal to the core eudicot node. There are quite a number of gene duplications in the general Dilleniales-Vitales area, perhaps this genome duplication was involved (e.g. Litt & Irish 2003; Kramer et al. 2004; Kim et al. 2004; Zahn et al. 2005b; Howarth & Donoghue 2006; especially Kramer & Zimmer 2006; Shan et al. 2007. Duplications include: euAP1 + euFUL + AGL79 genes [duplication of AP1/FUL or FUL-like gene], PLE + euAG [duplication of AG-like gene: C class], SEP1 + FBP6 genes [duplication of AGL2/3/4 gene]. The euAP1clade includes key regulators that have been implicated in the specification of perianth identity (Litt & Irish 2003). However, not all major core eudicot groups have been sampled for this gene, the situation in Santalales, for example, being unknown; there has been another duplication of this gene (and also of the AGL1/2/3 gene) perhaps immediately below the Pentapetalae node, but above the Ranunculales node. The roles such genes may play in many eudicot groups is unknown. Duplications of the CYC2 gene clade are widespread, and they are often associated with the evolution of monosymmetric flowers (Howarth et al. 2011 and references).
Where the loss of the chloroplast infA gene is to be placed is unclear (see Millen et al. 2001).
Chemistry, Morphology, etc.. Taxa that have cuticle wax platelets as rosettes are scattered through this group, but they are especially common in Fabaceae and they are also to be found in several Malpighiales (see Ditsch & Barthlott 1997 for details). Type III root hairs in which there are radially alternating cell files of cells with and those without hairs are scattered here, being known from e.g. Saxifragales, Caryophyllales, Ericales, Boraginales - but not from campanulids (K. Lee et al. 2022). Type I root hais are also scattered here; these are known from some campanulids.
Distinctive mucilage cells with a much thickened mucilaginous inner periclinal wall and distinct cytoplasm are found in flowers in particular in this broader group, but they are not yet reported from Geraniales (Matthews & Endress 2006b). Petal development is often retarded relative to that of other parts of the flower (also in Cabomba and Saruma).
Phylogeny. Basal relationships within what are now called the superrosids remain somewhat unclear, particularly the positions of Vitales and Saxifragales (N. Zhang et al. 2016: Fig. 1 for four common hypotheses, fabids = the N-fixing + the COM clade). Nickrent et al. (2005) found the position of Saxifragales to be particularly uncertain, although Vitales tended to go with other rosids. Soltis et al. (2007a) recovered a grouping [Saxifragales [Vitales + other rosids]], both groupings with 1.0 p.p. (see also Zhu et al 2007, but little support). Earlier work had suggested similar relationships, thus Saxifragales were sister to the rest of the group (e.g. P. Soltis et al. 1999: Vitales not included), albeit with little support. Moore et al. (2010, 2011) found that although [Saxifragales, Vitales, other rosids] formed a strongly-supported clade, it was unclear whether Vitales were sister to Saxifragales, to other rosids, or to [Saxifragales + other rosids]; support for the latter position increased with reduced taxon sampling. Moore et al. (2010) also recovered a [Saxifragales + Vitales] clade sister to other rosids in maximum likelihood but not in maximum parsimony analyses, and this also appeared in most analyses in Ruhfel et al. (2014), in both nuclear and chloroplast, but not in mitochondrial, analyses in M. Sun et al. (2014: divergence 114-110 Ma, 2015), and in the chloroplast genome analyses of Z. Wu et al. (2014) and N. Zhang et al. (2016: good support). Zhang et al. (2016; see also Soltis et al. 2013) suggested that a bicarpelate gynoecium might be a synapomorphy for the clade, but support for this must at best be weak.
H. Wang et al. (2009: Dilleniales not included) in a 43,000 bp analysis, largely of chloroplast sequences, found substantial resolution within the superrosids, and the relationships that they suggest, [Saxifragales [Vitales [rosid I/fabids + rosid II/malvids]]], were in part followed in immediately subsequent versions of this site, although the position of Vitales was only moderately supported (72% bootstrap in a ML analysis); they analysed a twelve-gene and inverted repeat data sets separately and in combination, preferring ML over MP analyses. The topology in Davies et al. (2004), Bell et al. (2010), Soltis et al. (2011) and X. Yang et al. (2017) is similar. Saxifragales are also well supported as sister to [Vitales + other rosids] in the 12-gene plus plastid inverted repeat analyses of Wang et al. (2009) and that position is quite well supported (91% bootstrap) in the chloroplast genome analysis of H.-T. Li et al. (2019), but relationships tend to be [Vitales [Saxifragales...]] in O.T.F.T.I. (2019).
Vitaceae were placed sister to other rosids, but with only moderate support by D. Soltis et al. (2000), and even this moderate support weakened in a subsequent four-gene analysis (D. Soltis et al. 2003a); however, Jansen et al. (2006a, b) using complete plastome sequences found quite strong support for this position, although members of Berberidopsidales, Dilleniales, Santalales and Saxifragales were not included (see also Ruhlman et al. 2007; Jansen et al. 2007; Moore et al. 2007; M. Sun et al. 2016). Hilu et al. (2003: matK analysis [incomplete sequence] alone) suggest relationships between Vitales and Dilleniales (only moderate support in parsimony analysis, but 100% posterior probability in Bayesian analyses), the combined clade being just above Malpighiales and below Saxifragales in a pectinate tree of major clades within the core eudicots. Although this relationship was not recovered in the analysis of the matK gene by Worberg et al. (2007), Vitales and Dilleniales do have a similar and rather distinctive testa anatomy (see also Kubitzki 2006a). Indeed, on balance Vitales are likely to be sister to the other rosids (e.g. Jansen et al. 2007; H.-T. Li et al. 2019), although support was only weak in Wang et al. (2009). For a palaeohexaploidy event that seemed to link Vitales with rosids in particular, see Gunnerales.
The positions of Geraniales and Myrtales have also tended to be uncertain. Geranium, the only representative of Geraniales included, was sister to all other rosids except Vitaceae in a study by Zhu et al. (2007, support weak); their position was also unstable in a rbcL analysis of all angiosperms (Hilu et al. 2003). In some earlier trees, Crossosoma (Crossosomatales) was also included or was nearby, see e.g. Morgan and Soltis (1993), Chase et al. (1993), while in Price and Palmer (1993: rbcL analysis) Biebersteinia (see Sapindales here) was still tentatively included in Geraniales. Savolainen et al. (2000a) found Geraniales to be monophyletic, but with only 52% support (see also Savolainen et al. 2000b); Crossosomatales were still its sister group, but with still less support. However, Soltis et al. (2011, see also Moore et al. 2011) find strong support for relationships in the clade as shown in the Summary Tree, those for [Malvales + Brassicales] at 85% ML bootstrap and for [Geraniales + Myrtales] at 79% being the weakest; all other relationships along the spine have ³99% ML bootstrap (see also Ruhfel et al. 2014; M. Sun et al. et al. 2016: support for the two basal internodes weak; Logacheva & Shipunov 2017: strong support, Huerteales not included). Relationships [Geraniales [Myrtales [Sapindales etc.]]] were recovered by Hohmann et al. (2015) and Foster et al. (2016a: support weak). Using mitochondrial and chloroplast genes, Zhu et al. (2007) found that Myrtales and Geraniales were successively sister to all other rosids - but with little support (Zhu et al. 2007). S.-B. Lee et al. (2006) found some support for the clade [Geraniales + Myrtales] sister to the fabid/rosid I clade, although sampling was poor. Jansen et al. (2007; see also Z. Wu et al. 2014) recovered this [Myrtales + Geraniales] clade as sister to the malvids, albeit with weak support. Xi et al. (2014) found that Eucalyptus was weakly supported as sister to all rosids in analyses using nuclear data, while in those using chloroplast data, the genus was sister to the malvids, and with strong support, however, representatives of Geraniales were not included and sampling in general was a bit sketchy (this was not the main focus of their work). There is a fair amount of variation in relationships in this area in the trees provided by M. Sun et al. (2014).
Fernando et al. (1995) placed Picramniaceae, ex Simaroubaceae, between the [Fabid + COM] clade, which includes Surianaceae (fabid) and Irvingiaceae (COM clade), both ex-Simaroubaceae, and malvids, which include Simaroubaceae themselves, but a position along the spine of the malvid clade is best. Picramnia was placed in the position followed by the A.P.G. group (2009, 2016) by e.g. Moore et al. (2011); see also Logacheva and Shipunov (2017: plastomes).
D. Soltis et al. (2003a) found 79% support for rosids s.s., i.e., excluding Vitales and Saxifragales. Within rosids s. str., relationships have been somewhat unclear (e.g. Soltis et al. 2005b; Jansen et al. 2006a; Bausher at al. 2006; Zhu et al. 2007; versions of this site up to March 2009), but the topology seemed to be becoming clearer (e.g. H. Wang et al. 2009). The relationships of the rosid I clade (= [fabid/N-fixing clade + the COM clade, = [Oxalidales [Celastrales + Malpighiales]]]), have been particularly problematical. In an analysis including the mitochondrial matR and two chloroplast genes, the COM clade were sister to the fabids/N-fixing clade, with weak to moderate support; Crossosomatales were weakly supported as sister to the rosid II clade (= malvids) (Zhu et al. 2007). Jansen et al. (2007) recovered a malvid clade with strong support (weaker using maximum parsimony), in turn strongly supported as sister to the [COM + fabid] clade, albeit with sketchy sampling. Ruhfel et al. (2014) recovered a variety of relationships around here, including a [COM + fabid] clade, and Z. Wu et al. (2014: chloroplast genomes) also recovered a [COM + fabid] clade, and with Zygophyllaceae sister to the former.
However, in some analyses of four mitochondrial genes, Qiu et al. (2010) found that the [COM + fabid] clade was not monophyletic, there being quite strong support for a [COM + malvid] clade (see also Duarte et al. 2010; Burleigh et al. 2011). Consistent with such ideas, in an analysis of 154 protein-coding genes Shulaev et al. (2011) found that Populus was sister to [Carica + Arabidopsis], rather than to the four taxa from the nitrogen-fixing clade included in the study, and the same basic relationships were found by E. K. Lee et al. (2011: better sampling, but no Celastrales or Oxalidales). Burleigh et al. (2011) in a genome-level analysis found that Malpighiales were embedded in the malvids, although again no representatives of Celastrales or Oxalidales were examined (see also Duarte et al. 2010). Similar relationships were rejected by all tests in the combined analysis of Zhu et al. (2007), although they were found in the analysis of matR data alone.
Soltis et al. (2011) discussed the influence of mitochondrial genes on relationships in this part of the tree; mitochondrial genes alone placed a weakly supported COM clade as sister to core malvids with quite strong support. In analyses of large amounts of chloroplast data Malpighiales grouped with the N-fixing clade, while in analyses of nuclear data they grouped with the malvids (Xi et al. 2014). A [COM + malvid] clade was also obtained (just) in an analysis of 31 (30 eudicot) complete chloroplast genomes (Fajardo et al. 2013). Finally, Gitzendanner et al. (2018a) and Valencia-D et al. (2020) obtained the relationships [[Zygophyllales + COM clade][Fabales + Rosales]] in plastome analyses.
Thus the COM clade in general, or Malpighiales in particular, do not have stable relationships. In an important study by M. Sun et al. (2014, see also 2016) the sampling of taxa with genome data from different compartments was matched as carefully as possible. Sun et al. (2014) found that a [COM + fabid] clade was obtained in analyses of chloroplast data, while a [COM + malvid] clade was recovered in analyses of mitochondrial and nuclear data. (The mitochondrial tree showed a number of idiosyncracies, not that surprising for such trees; e.g. Lonicera was sister to other campanulid taxa included, Crossosomatales and Zygophyllales formed a clade outside the [fabid [COM + malvid]] clade, Garryaceae and Aquifoliaceae switched positions, Platanus was sister to Ranunculales, etc., although overall support values for those positions were very low.) Sun et al. (2014) suggested that the COM clade might be the result of a very ancient hybridization between a fabid and malvid, with the chloroplast genes coming from the former and much of the rest of the genome from the latter, an idea supported by the much larger number of nuclear genes that grouped with the malvids rather than the fabids. The inclusion of Caryophyllales within the superrosids was slightly disconcerting, and the non-inclusion of taxa in clades like Picramniales and in particular Dilleniaceae in the study might have affected some placements. Zeng et al. (2014: 59 genes, 61 taxa) also found that the COM clade (Malpighiales alone included) was sister to the malvids, however, in other analyses they retrieved a [COM + fabid] clade (ibid.: suppl. Fig 14: plastid genes), and L. Zhao et al. (2016), too, recovered relationships similar to those of Sun et al. (2014), i.e. [[[Celastrales + Malpighiales] [Oxalidales + malvids]] [fabids]], in a large-scale analysis of nuclear genes. Buddenhagen et al. (2016), using an anchored phylogenomics approach, joins the list of those finding support for a [Malpighiales + malvid] clade, and in their case the support was robust. Recent relationships recovered by W. J. Baker et al. (2021a: see Seed Plant Tree), X. Li et al. (2021) and others also suggest complications. Thus Oxalidales minus Huaceae linked with Sapindales, Malvales and Brassicales.
The relationships of Zygophyllales have been unclear. Hilu et al. (2003: rbcL) found that Larrea (Zygophyllaceae) was weakly associated with Fabaceae, the only member of Fabales they included; they noted that the possession of anthraquinones was a possible synapomorphy between Zygophyllaceae and the N-fixing clade (see also Sheahan & Chase 2000). The position of Zygophyllales was rather labile in the comprehensive analysis of H. Wang et al. (2009). They sometimes appeared to be linked with the rosid II clade, the malvids (maximum parsimony), or sometimes sister to the rosid I clade, and with reasonable support (maximum likelihood), but the former position could be rejected (Wang et al. 2009). Bell et al. (2010) placed Zygophyllales in a polytomy with the COM and N-fixing clades (see also Magallón & Castillo 2009), and several analyses, including the 17-gene analysis of Soltis et al. (2011), place it sister to the rosid I clade (as here: see also Sun et al. 2013; Zeng et al. 2014: suppl. Fig. 14; Z.-D. Chen et al. 2016). Qiu et al. (2010: mitochondrial genes) suggested that Zygophyllales were embedded in Crossosomatales, but with only moderate support, the combined clade being sister to all rosids, while it is sister to the fabids in the plastid analyses of Sun et al. (2014). L. Zhao et al. (2016) found that Larrea tridentata, the only member of Zygophyllales that they included, was sister to a [[Celastrales + Malpighiales] [Oxalidales + malvids]] clade in coalescence analyses, but embedded in a [Geraniales + Myrtales] clade when concatenation was applied; although they preferred the first position, in neither case was support overwhelming. Analyses by Ruhfel et al. (2014) also found a Zygophyllales that tended to wander around, while in Baker et al. (2021: see Seed Plant Tree) Zygophyllales were sister to Myrtales, but with very poor support.
In a study in which the focus was on relationships along the spine of the Pentapetalae, Zeng et al. (2017) found that Oxalidales were sister to the malvids included in the analysis or sister to the malvids minus Crossosomatales, but since no Geraniales, Myrtales, Picramniales, Huerteales or Zygophyllales were included, it is difficult to make much sense of this result. Rather later, L. Liu et al. (2023: 122 single copy nuclear genes, 36 spp., all 18 superrosid orders, concatenation RAxML analyses) recovered Vitales as being sister to all other superrosids, where relationships were [Saxifragales [[Crossosomatales + Geraniales] [[Myrtales + Zygophyllales] [[N-fixing clade/Fabids], [Picramniales [[Celastrales + Malpighiales] [Huerteales [Oxalidales [Sapindales [Malvales + Brassicales] (= malvids s.l.); overall support was quite good. However, Liu et al. (2023) found ASTRAL coalescence analyses differed in detail, albeit with poor support, furthermore, relationships suggested by analyses of plastomes and nuclear genomes differed, which they suggested was because of a mixture of ILS and hybridization. The COM clade was not recovered, rather, COM members grouped with the malvids, not fabids, as noted above, indeed, an [Oxalidales + Sapindales] clade was found by these authors in their ASTRAL analyses. Interestingly, Endress and Matthews (2006a; also Endress et al. 2013) had early suggested that some morphological characters were consistent with the relationships [[COM + malvids] fabids]. These include the frequency of features such as a contorted corolla, a polystaminate androecium and polycarpy, and the inner integument tends to be thicker than the outer in the [COM + malvid] clade. Q. Ma et al. (2024: 230 single copy gene families, 15/17 rosid orders) found that that there was a clade [Zygophyllales + Myrtales] which they dated at 101-83 Ma. The overall relationships there are [Crossosomatales [[Zyg. + Mal] [[N-fixing clade (Fab + Cuc sampled)] [[Celastrales + Malpighiales] [Huerteales [Oxalidales] [Sapindales [Brassicales + Malvales]]]]]]].
For further discussion of major patterns in relationships within the rosids, see the Pentapetalae node.
Classification. The circumscription of the rosids could usefully include both Saxifragales and Vitales if they form a single clade; they are morphologically quite similar. However, given the general uncertainty over relationships at the base of the rosids and asterids (see above) any such grouping could at best be considered tentative.
SAXIFRAGALES Berchtold & J. Presl - Main Tree.
Ellagic acid, myricetin, flavonols +, (silicon concentration high [?level]); (tension wood +); branching from the previous flush [woody members]; cuticle waxes as clustered tubules; petiole bundle annular; lamina margins serrate, teeth with gland broadening distally and with apical foramen, higher order lateral veins joining it; A ?, anthers basifixed, with basal pit, sagittate; carpels free, at least apically, styluli short, stigmas decurrent, at most slightly wet; ovules ³2/carpel, with bistomal micropyle, (outer integument largely dermal in origin); fruit dry; seeds ± exotestal; embryo size?; unique 1 BP [adenosine] insertion in 18S rDNA. - 15 families, 112 genera, 2,600 species.
Includes Altingiaceae, Aphanopetalaceae, Cercidiphyllaceae, Crassulaceae, Cynomoriaceae, Daphniphyllaceae, Grossulariaceae, Haloragaceae, Hamamelidaceae, Iteaceae, Paeoniaceae, Penthoraceae, Peridiscaceae, Saxifragaceae, Tetracarpaeaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Jian et al. (2006, esp. 2008) estimate the crown-group age for Saxifragales at 103-83 My; ca 108 Ma is the age in Tank et al. (2015: Table S1, S2) .
Evolution: Divergence & Distribution. For additional dates in the order, see Jian et al. (2008).
Saxifragales contain ca 1.3% of eudicot diversity. They have a very poor representation in the tropics, which makes the inclusion of the small lowland tropical Peridiscaceae as sister to the rest of the order (see below) the more notable.
Evolution: Divergence & Distribution. Saxifragales are characterised by having notably small seeds (Moles et al. 2005a; Linkies et al. 2010; Sims 2012: as Saxifragales); The tropical Peridiscaceae, which have rather larger seeds, were not included in these studies.
Bertilanthus scanicus, from Late Cretaceous rocks in Sweden and previously associated with Paracryphiaceae, seems better associated with Saxifragales, where its closest match was with Heuchera, but other positions in the order were also suggested (López-Martínez et al. 2023a).
D. Soltis et al. (2013) looked at diversification in Saxifragales in the context of a 900+-species supermatrix, focussing particularly on woody←→herbaceous and annual←→perennial transitions, while Rubio de Casas et al. (2016) looked at the relationship between habitat and diversification, finding the ancestral habitat to be forest, while shifts to cliffs and shrublands and to the tundra habitat in particular were accompanied by high net diversification rates. More recently Folk et al. (2019) rather unexpectedly found that diversification increases ca 15 Ma predated by around 5 Ma increases in ecological and phenotypic evolution and that diversification rates had not slowed down - hardly the Standard Model. Gu et al. (2022) found that diversification in saxifragalean families was not correlated with their ages, rather, they thought, global cooling might have promoted diversification of herbaceous and deciduous woody taxa.
Because many relationships within Saxifragales have been difficult to resolve, it has been suggested that they represent an ancient and rapid radiation (Fishbein et al. 2001; Fishbein & Soltis 2004). More recently Jian et al. (2006, esp. 2008) estimated that early diversification in the clade perhaps occurred over a period as short as 3-6 Ma, while Hermsen et al. (2006b: topology resolved but different in detail from that below, support slight) thought that much diversification occurred around 90-84 Ma in the Late Cretaceous. A genome duplication seems not to have occurred here (L. Liu et al. 2023).
Saxifragales have notably small seeds compared with those of other angiosperms, however, Peridiscaceae, not included in these seed size studies, have larger seeds, so this character is pegged to the next node up below.
D. Soltis et al. (2007b), Endress (2010c) and Carlsward et al. (2011) all suggested possible apomorphies for the clade - or at least features common in the clade - other than those given above, while Hermsen et al. (2006b, see list of characters) thought that Saxifragales lacked any "diagnostic" morphological features. Carlsward et al. (2011) provide apomorphies for all internal branches of Saxifragales, and many of these are flagged as such below (pollen characters are not yet included). Doyle (2012) suggested that tricolpate pollen was "retained" in many Saxifragales.
Chemistry, Morphology, etc.. Roots are diarch (Van Tieghem & Douliot 1888). Saxifragales commonly have scalariform perforation plates, lateral pitting that is mostly scalariform or opposite, bordered pits, etc., but whether these are synapomorphies is unclear; Jian et al. (2006) characterised the largely unresolved woody members and the [Saxifraceae + Crassulaceae] clade in terms of their wood anatomy. Leaf teeth are basically rosid, although those of Cercidiphyllum are described as being more or less chloranthoid (not very different), while Hamamelidaceae can have teeth with a clear, glandular apex (fothergilloid) and those of Altingiaceae are platanoid, basically, the higher order lateral veins do not quite make it to the tooth (Hickey & Wolfe 1975; Tetracarpaea is similar - Hils et al. 1988). Despite appearances, the floral apex in nearly all taxa studied is reportesd to be flat or concave (Fishbein et al. 2000; Soltis & Hufford 2002; D. Soltis et al. 2003b; Soltis et al. 2005b), although Wurdack and Davis (2009) suggested that this was not the case for Peridiscaceae.
For information on the hamamelids as it was beginning to be realised that they might have to be split, see Crane and Blackmore (1989). For chemistry, see Giannasi (1986) and Jay (1971), for anatomy, see Watari (1939), Ramamonjiarisoa (1980) and Cutler and Gregory (1998), for general morphology, see Hermsen et al. (2006b), for floral anatomy and morphology, see Gaümann (1919), Bensel and Palser (1975a-d), Hufford and Endress (1989), Drinnan et al. (1995) and Fishbein et al. (2000), for pollen, see Hideux and Ferguson (1976) and Zavada and Dilcher (1986), and for seed coat, see Krach (1976).
Phylogeny.The group, Saxifragales, as circumscribed here has long been apparent in molecular phylogenies (e.g. D. Soltis et al. 1997; D. Soltis & P. Soltis 1997), although support for the clade has not always been very strong (D. Soltis et al. 2013). Within Saxifragales relationships other than the Saxifragaceae/Crassulaceae clade ("S.-C. clade" below) have been unclear for some time, and even now many of the deeper nodes remain poorly supported (D. Soltis et al. 2013; Dong et al. 2018) - better, many of the nodes in general here could do with improved support (H.-T. li et al. 2021). However, support for the S.-C. clade and relationships within it is generally strong. The relationships [[Crassulaceae [Tetracarpaeaceae [Penthoraceae + Haloragaceae]] [[Saxifragaceae [Iteaceae + Pterostemonaceae]]] Grossulariaceae]] were found by Morgan & Soltis (1993); support for a [Pterostemon + Itea] clade (= Iteaceae) is strong (e.g. Soltis et al. 2007a). Aphanopetalum (ex Cunoniaceae) is to be included in the Crassulaceae et al. clade. Somewhat different topologies for this part of the tree are sometimes recovered (e.g. Stubbs et al. 2020a: c.f. S7 and S8; Gu et al. 2022).
Although Hilu et al. (2003: matK) did not recover the S.-C. clade, there was no strong support for alternative placements; Cercidiphyllaceae and Daphniphyllaceae were sister taxa, with moderate jacknife support. Hermsen et al. (2006b), who included both molecular and morphological (the latter also from selected fossils) data, also recovered a S.-C. clade, while all other families were in a clade sister to this. [Paeonia + Daphniphyllum] and [Cercidiphyllaceae + Altingiaceae] were clades, but with very weak support (<50% bootstrap). Paeonia was linked with moderate support to the Crassulaceae clade, or, more weakly, with the S.-C. clade in some analyses in Fishbein et al. (2001); the latter relationship also appeared in a study by Fishbein and Soltis (2004), while Z.-D. Chen et al. (2016) found some support for a position of Paeonia as sister to a clade [Cercidiphyllaceae [Dapniphyllaceae [Altingiaceae + Hamamelidaceae]]], relationships with even less support. H.-T. Li et al. (2019, see also 2021) recovered this general group, but found that Altingiaceae had some support as being sister to Daphniphyllaceae.
In most of these analyses Peridiscaceae were not included, indeed, they had been placed in Malpighiales by Savolainen et al. (2000a: see A.P.G. II 2003). However, Davis and Chase (2004; see also Soltis et al. 2007a) found that the family belonged here, adding Soyauxia, previously placed in Medusandraceae, while Wurdack and Davis (2009) added Medusandra itself. Paeonia was linked with low support to Peridiscus by Davis and Chase (2004). D. Soltis et al. (2007b) were unable to recover stable relationships among the woody Saxifragales, long branch attraction (to Paeoniaceae and Peridiscaceae) possibly occurring; depending on the analysis, a [Peridiscaceae + Paeoniaceae] clade made Hamamelidaceae paraphyletic, or Peridiscaceae were sister to all other Saxifragales. Despite the addition of more data, Jian et al. (2006) still found it difficult to resolve relationships between members of the woody members (i.e. Altingiaceae, Hamaelidaceae, Cercidiphyllaceae, Daphniphyllaceae), although it appeared that Peridiscaceae might be sister to the rest of the order, and Paeoniaceae sister to the S.-C. clade. Similar relationships were recovered by Soltis et al. (2011, see also Moore et al. 2011), but support for the position of Paeoniaceae was weak; other relationships with Saxifragales that they found agree with the topology described below. Li (2008) also found little support for many relationships apparent in the order, including an association of Paeoniaceae with the C. clade that appeared in some analyses. Dong et al. (2013: 18 chloroplast regions) found the relationships below, although [Dapniphyllaceae [Altingiaceae...]] (poorly supported) was the structure at the base of the woody clade. Paeoniaceae were again found to be associated with the S.-C. clade, but with little support, by M. Sun et al. (2016).
However, Jian et al. (2008: see also Qiu et al. 2010: slight differences, placement of Hamamelidaceae), using a variety of large (for 2008) data sets (some with over 50,000 bp) and analyses, found strong maximum likelihood and Bayesian support for the topology used here, although Paeonia in particular moved around the tree in some parsimony analyses. There is little morphological support for the basal branches, pretty much par for the course, although characters can be optimised to positions on a number of the shallower branches (c.f. Hermsen et al. 2006b). A study by Qi et al. (2012) that focussed on Cercidiphyllum found a rather different set of relationships, including the paraphyly of Hamamelidaceae, but posterior probabilities were low, while in a rbcL analysis by Breteler et al. (2015), with a focus on Medusandraceae (= Peridiscaceae), but not including Paeonia, not only were Medusandraceae not basal (but there was no particular support for an alternative position), but Leea was placed within the order... Dong et al. (2018) carried out a variety of analyses using whole chloroplast genomes and found that the position of Paeoniaceae (sister to woody clade or to the S.-C. clade?) depended on the assumptions of the analyses, and overall they preferred the tritomy [Paeoniaceae + S.-C. clade + woody clade], while Ding et al. (2019), also using plastome analyses, found quite good support for a [Paeoniaceae + woody] clade, although in the latter there was a [Daphniphyllaceae + Altingiaceae] clade, albeit with little support. Gu et al. (2022) found the relationships [[Peridiscaceae [Paeoniaceae + woody taxa]] [S.-C. clade]], but again, details of relationships did not fit any general pattern. Analysis of Angiosperms353 data suggest that a clade [Peridiscaceae [Paeoniaceae [Daphniphyllaceae [Hamamelidaceaeae [Altingiaceae + Cercidiphylaceae]]]]] was sister to the [S.-C. clade].
Some early molecular analyses placed Cynomoriaceae in Saxifragales, perhaps in the Crassulaceae area, although with little support (Nickrent 2002; Nickrent et al. 2005), however, Barkman et al. (2007) found no support for a position in this order - but none for any particular position at all. A position in Saxifragales was rejected by Jian et al. (2008), who preferred to place them in Santalales; Balanophoraceae, with which Cynomoriaceae have been associated in the past, are certainly to be included there (see also Nickrent et al. 2005). Recently Cynomoriaceae have been placed in Rosales as sister to Rosaceae based on analysis of chloroplast inverted repeat sequences (Moraceae were the only other family in Rosales examined), and with strong support; Cynomoriaceae were certainly to be excluded from Saxifragales (good sampling) and several other rosid orders (Z.-H. Zhang et al. 2009; see also Moore et al. 2011). Depending on the particular mitochondrial gene analyzed by Qiu et al. (2010), Cynomoriaceae were placed with Saxifragales (matR, nad5) or Sapindales (atp1, rps3). Naumann et al. (2013) placed Cynomoriaceae in Saxifragales as sister to [Paeoniaceae + Altingiaceae], the only other Saxifragales in the study, but representatives of the other clades to which Cynomoriaceae might be related were also included, while they are sister to the S.C. clade in Z.-D. Chen et al. (2016) and sister to the C. clade in H.-T. Li et al. (2019), but in both cases with little support. The inclusion of Cynomoriaceae in Saxifragales has been confirmed by Bellot et al. (2016) using genes from all three compartments, although again exactly where they were to be placed was unclear. W. J. Baker et al. (2021: see Seed Plant Tree) found that Cynomoriaceae were sister to all other Saxifragales, although support was low (but not too bad in other analyses), and H.-T. li et al. (2021: plastome analyses) placed them as sister to the [Crassulaceae ... Haloragaceae] clade, but with very low support. The family is placed at the end of the account of the order below.
Comparing two important recent studies, we find substantial differences. Thus in the plastome study of H.-T. Li et al. (2021) relationships at the base of the rosid clade were [Dilleniales [Saxifragales [Vitales [[Geraniales + Myrtales]...]]]]. However, in the Angiosperms353 analyses of Zuntini et al. (2024) they were [Vitales [Saxifragales [[Geraniales + Crossosomatales]...]]]. Arrangements within Saxifragales also differ in the two. Thus Li et al. (2021) show relationships as being [Peridiscaceae [[[Daphniphyllaceae + Altingiaceae] [Cercidiphyllaceae + Hamamelidaceae]] [Paeoniaceae [[Iteaceae [Grossulariaceae + Saxifragaceae]] [Cynomoriaceae [Crassulaceae [Aphanopetalaceae [Tetracarpaeaceae [Penthoraceae + Haloragaceae]]]]]]]]] On the other hand, Zuntini et al. (2024: Suppl. Fig. 4) suggest the relationships [Cynomoriaceae [[Peridiscaceae [Paeoniaceae [Daphniphyllaceae [Hamamelidaceae [Cercidiphyllaceae + Altingiaceae]]]]] [[Iteaceae [Grossulariaceae + Saxifragaceae]] [Crassulaceae [Tetracarpaeaceae [Aphanopetalaceae [Penthoraceae + Haloragaceae]]]]]]] - rather different.
Classification. Dong et al. (2013) suggested that a much expanded Hamamelidaceae, Saxifragaceae and Haloragaceae should be recognised, along with Paeoniaceae, Peridiscaceae and Crassulaceae...
Previous Relationships. Saxifragales include Hamamelidaceae, classically thought to be a key group linking the Englerian Amentiferae (usually dioecious or monoecious woody plants with an ament or catkin and small flowers, and sometimes believed to be primitive) to "dicots" with more conventional flowers (e.g. Endress 1967; Frohne & Jensen 1992). However, the old Amentiferae, included in Cronquist's (1981) Hamamelidae, are now in several bits, mostly in the rosids, of which one is here - see also Fagales, the major part of Amentiferae, Malpighiales (Salicaceae), Rosales ("Urticales"), etc. (Qiu et al. 1998a). For the woody Saxifragaceae, now similarly widely distributed, see below; many iridoid-positive and/or tenuinucellate members are now in the asterids, but most iridoid-negative, herbaceous and/or crassinucellate members remain here. Ironically, three families of Saxifragales s. str. are reliably reported to have iridoids (how many origins?) and are the only families outside asterids with them. Daphniphyllanae, Saxifraganae and Hamamelidanae, in which Takhtajan (1997) placed most of the families that are in Saxifragales here, are all in his Hamamelididae.
Synonymy: Hamamelidineae Thorne & Reveal - Altingiales Doweld, Cercidiphyllales Reveal, Crassulales Link, Cynomoriales Burnett, Daphniphyllales Hurusawa, Fothergillales Link, Grossulariales Berchtold & J. Presl, Haloragales Link, Hamamelidales Link, Iteales Doweld, Medusandrales Brenan, Paeoniales Heinze, Peridiscales Doweld, Sedales Reichenbach f., Sempervivales Berchtold & J. Presl - Daphniphyllanae Takhtajan, Hamamelidanae Takhtajan, Paeonianae Doweld, Saxifraganae Reveal - Hamamelididae Takhtajan, Paeoniidae C. Y. Wu - Crassulopsida Brongniart, Hamamelidopsida Brongniart, Saxifragopsida Brongniart
PERIDISCACEAE Kuhlmann, nom. cons. —— Synonymy: Medusandraceae Brenan, nom. cons., Soyauxiaceae Barkley - Back to Saxifragales
Trees; plants Al-accumulators, ?chemistry; vessel elements with scalariform perforation plates; apotracheal (paratracheal, diffuse) parenchya +; secretory canals + [Medusandra - M.]; petiole bundles with wing bundles [Soyauxia], also an adaxial plate [Peridiscus - P.] or an adaxial [Whittonia] or medullary [M.] annular bundle; crystals +; hairs unicellular, lignified [M.]; epidermal wax crystals in rosettes; leaves two-ranked, (spiral), lamina margin serrate [?tooth morphology], (entire), (secondary veins palmate, petiole pulvinate at both ends - P., M.), stipule single and adaxial, or paired and lateral; inflorescences axillary, racemose(-spicate) or fasciculate, flowers small; P 4-7, or K 5(-6), C 5(-6); A many, at most slightly connate basally, (anthers monothecal); nectary ?large, annular, hairy/0, (or: A 5, opposite C, staminodes 5, opposite K, filaments long, shortly hairy; nectary 0 - M.); G [3-4], 1-locular, (with central column), stigmas punctate; ovules 1-2(-3)/carpel [6-8 in total], apical, pendulous, epitropous; fruit a drupe or capsule, 3-4-valved, wall expanding early [M.], P deciduous or K much enlarged, accrescent, recurved (M.); seed 1, large, coat tanniniferous, walls thin, ± collapsed; endosperm ?development, copious, cell walls thick, pitted, embryo very small; n = x = ?
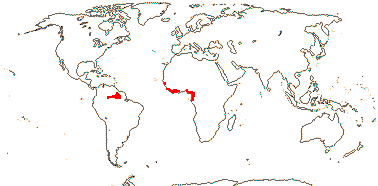
4 [list]/11: Soyauxia (7). South America, tropical W. Africa (map: from Aymard C. & Arellano P. 2018; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010).
Age. Crown-group Peridiscaceae are thought to be ca 110.8 Ma (Gu 2022: stem 110.8 Ma).
Evolution: Plant-Bacterial/Fungal Associations. For mycorrhizae in Soyauxia, see Bechem et al. (2014).
Chemistry, Morphology, etc.. Peridiscaceae are a very poorly known and superficially heterogeneous group. I have not seen Whittonia, but Metcalfe (1962) gives some details of its anatomy. The vascular pitting of Medusandra is scalariform and the pits are bordered (Metcalfe 1952). Note that the leaves are almost certainly simple, not unifolioliate (c.f. Brenan 1952; Hutchinson 1973); the rather swollen apex of the petiole is like that of many Euphorbiaceae, Hydnocarpus, Octoknema, etc., which are not usually described as being possibly unifoliolate. Petiole anatomy in the region of the pulvinus is complex. For epidermal wax crystals, see Ditsch and Barthlott (1997); Prado and Demarco (2018) suggest that the family has laticifers.
The bracteoles are often inconspicuous (c.f. Cronquist 1981). Peridiscus and Whittonia have monothecal anthers, probably derived within the family. The flowers of Medusandra have long and conspicuous staminodes borne opposite the sepals, hence the generic name. Breteler et al. (2015) discuss stamninodes and nectaries in Peridiscaceae; they suggest that in Soyauxia, at least, what had been called a disc is staminodial. The basic seed morphology/anatomy of Soyauxia and Peridiscus, from either side of the Atlantic, are almost identical, although the two are vegetatively very different - Peridiscus is sometimes identified as Menispermaceae!
See Metcalfe (1952b, 1962) and Miller (1975) for anatomy and Soltis et al. (2007b), Bayer (2006), and Bayer and Dressler (2014), all for general information.
Phylogeny. Relationships are [Medusandra [Peridiscus + Soyauxia]] (Wurdack & Davies 2009; Breteler et al. 2015).
Previous Relationships. Peridiscaceae were included in Violales by Cronquist (1981) and Takhtajan (1997), and a similar position was suggested by A.P.G. III (2003); Soyauxia in particular has been associated with Flacourtiaceae. Medusandra was tentatively included in Malpighiales by Soltis et al. (2005b), certainly, the serrate leaf blades and the cauline stipules suggest relationships other than to Santalales or Santalanae, where it had been placed (Cronquist 1981; Takhtajan 1997; Thorne 2007).
[[Paeoniaceae [Altingiaceae [Hamamelidaceae [Cercidiphyllaceae + Daphniphyllaceae]]]] [[Crassulaceae [Aphanopetalaceae [Tetracarpaeaceae [Haloragaceae + Penthoraceae]]]] [Iteaceae [Grossulariaceae + Saxifragaceae]]]]: floral apex flat-concave early in development; hypanthium +/G often (semi-)inferior.
Age. Magallón and Castillo (2009) estimated an age of ca 106.7 Ma for this node, Bell et al. (2010) an age of (111-)103, 95(-92) Ma. Other estimates are from Wikström et al. (2001, 2004) at (116-)111, 92(-87) Ma and Anderson et al. (2005) at ca 102 Ma, while 109-107 Ma is the age suggested by M. Sun et al. (2014) and ca 108.3 by Gu et al. (2022: note topology); at 122.4-110.8 Ma, Shenk and Hufford (2010) is the oldest estimate.
Evolution: Genes & Genomes. For chloroplast genomes in this clade, se Ding et al. (2019).
[Paeoniaceae [Altingiaceae [Hamamelidaceae [Cercidiphyllaceae + Daphniphyllaceae]]]]: buds perulate; leaves with basically palmate venation; mitochondrial coxII.i3 intron 0.
Age. The age of this clade is estimated to be around 72.6 Ma by Naumann et al. (2013) and ca 103.3-103.6 Ma by Tank et al. (2015: Table S1 and S2).
PAEONIACEAE Rafinesque, nom. cons. - Paeonia L. - Back to Saxifragales
Perennial herbs, shortly rhizomatous to shrublets; ethereal oils, flavones +, hydrolysable and non-hydrolysable tannins 0; cork "subcortical" [Tiagi 1970]; stem with cortical vascular bundles; vessel elements with simple or scalariform perforation plates; nodes also 5:5; petiole bundles forming a ring; calcium oxalate as crystals; wax tubules with palmitone predominating; palisade mesophyll with arm cells; indumentum 0 (hairs +, unicellular); leaves spiral, compound, ultimately ternate, lamina vernation variable, leaf base broad, stipules 0; inflorescence terminal, flowers 1-few; flowers large [³5 cm across], with cortical vascular system; P many, spiral, 3:3 vascularization, [= "K" (3-)5(-7), tough, "C" 5-8(-13)], not sharply distinguished; A many, from 5 trunk bundles continuing spiral of P, centrifugal, anthers with basal pits?; nectary cone-like structures/sheath-like/0; G free, (2) 3-5(-15), stylulus 0, stigma expanded, rather oblique, wet; ovules usu. many/carpel, micropyle exo-/bistomal, obturator +, outer integument 10-30 cells across, inner integument 3-6 cells across, endothelium +, parietal tissue ca 5 (10) cells across, nucellar cap ca 12 cells across/?0, nucellus mostly absorbed before anthesis, hypostase +; archesporium multicellular, embryo sac often more than 1, elongate; fruit a follicle, K persistent; funicle fleshy, with apical rim-aril (0); testa fleshy, vascularized, exotestal cells palisade, variously thickened, the hypodermis palisade, ± lignified, (some mesotesta thickened); endosperm cell walls with xyloglucans [thick, pitted - amyloid], chalazal endosperm haustorium +, zygote initially coenocytic, several embryos initially developing, one matures, minute; n = 5, x = 5, chromosomes 10-15 µm long, nuclear genome [1 C] (2.595-)16.814(-108.944) pg; plastome infA and rpl32 genes lost; chondrome coxII.i3 intron 0; germination hypogeal.
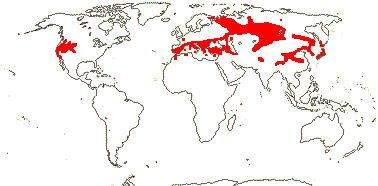
1 [list]/33. N. Temperate, especially East Asia. Map: from Stern (1946) and Hultén and Fries (1986). [Photo - Fruit]
Age. Crown-group Paeoniaceae are estimated to be ca 12.1 Ma (Gu et al. 2022).
Evolution: Divergence & Distribution. For the evolution and biogeography of the genus, see Sang et al. (1997).
Seed Dispersal. The testa is thick, fleshy and coloured, and in at least some species (Paeonia anomala, P. mlokosewitschii) its blackish colour contrasts with the red of the testa of partly developed and unfertilized seeds when the follicle opens. The funicle is also fleshy.
Chemistry, Morphology, etc.. Information about Paeonia in the older literature may be found under Ranunculaceae, as with petiole anatomy (Ezelarab & Dormer 1963).
According to Hiepko (1965, see also Endress 2010c) Paeonia lacks petals - presumably because of the similar spiral arrangement and vasculature of the perianth members. For details of the distinctive androecium, which may appear fasciculate in development and opposite the petals, see e.g. Leins and Erbar (1991), Rudall (2010) and Remizowa (2019). There is a prominent lobed disc, but in P. officinalis it does not secrete nectar (Hiepko 1966; Erbar 2014 and references), however, in some species the disc does secrete nectar (Bernhardt et al. 2013 and references). Johri et al. (1992) called the micropyle exostomal, however, the inner integument, too, partly forms the micropyle.
Both the embryo sac and embryo development are very distinctive. The embryo sacs (there are often more than one per ovule) develop from megaspore mother cells that are deeply embedded in a massive nucellus plus nucellar cap, and they elongate considerably towards the micropyle as they develop; the secondary endosperm nucleus is huge (see Vinogradova & Zhinkina 2020 and Vinogradova 2022 for the archesporium). The initial cell divisions of the embryo are free-nuclear (see e.g. Yakovlev & Yoffe 1957; Cave et al. 1961; Walters 1962), and although Murgai (1962) thought that they were cellular, this has not been confirmed (see e.g. Mu & Wang 1985). Murgai (1962) also illustrated a very thick layer of nucellar tissue but no nucellar cap; to be confirmed. The funicle is fleshy, and there may be a small rim-aril at the apex - or this could be an obturator, it is visible very early - and there is no tegmen.
For general information, see Tamura (2006) and Hong (2012); for general floral morphology, see Hiepko (1964, 1966), for the perianth, see Brouland (1935)c, for possible polyembryony, etc., see Sapunova and Vinogradova (2025) and for ovules and seeds in general, see Tiagi (1970) and Takhtajan (1988).
Phylogeny. Sang et al. (1997: plastid genes) suggest phylogenetic relationships in the genus.
Previous Relationships. Paeoniales were included in Ranunculidae (Takhtajan 1997), and a relationship between Paeoniaceae and Ranunculaceae in particular has often been suggested (Takhtajan 1997; Mabberley 1997 included Glaucidium [see Ranunculaceae here] in Paeoniaceae) because of gross floral similarities between the two. However, they differ in the nature of the petals and nectaries, the development of the androecium, numerous embryological features, etc. (e.g. Tiagi 1970); there are no dipteran agromyzid leaf miners on Paeoniaceae, although they are common on Ranunculaceae. Dilleniales, in which Paeoniaceae were placed by Cronquist (1981; see Corner 1946), have multistaminate and centrifugal androecia, but differ in gynoecial development, nectary morphology, etc..
[Altingiaceae [Hamamelidaceae [Cercidiphyllaceae + Daphniphyllaceae]]]: (route I iridoids +); cuticle waxes as tubules, nonacosan-10-ol the main wax; buds perulate; inflorescence racemose, flowers sessile; anthers ± valvate, connectives apically protruding; fertlization delayed; seeds winged; embryo long; germination epigeal and phanerocotylar.
Age. Moore et al. (2010: 95% HPD) suggested crown group ages of (103-)98(-94) Ma for this node; aroud 98.7 Ma is the age in Tank et al. (2015: Table S2) and (113.7-)106(-99.3) or 90 Ma (Jian et al. 2008).
Evolution: Divergence & Distribution. Maslova (2010; see also Maslova et al. 2012) saw fossil Platanaceae and Hamamelidaceae, in particular Hamamelidaceae-Altingioideae, as being related and springing from a polymorphic ancestral group. Maslova's Hamamelidales included Hamamelidaceae, with a number of fossil genera, Platanaceae and several more fossil genera, and Bogutchantaceae (for further details, see Platanaceae) while Sarbaicarpaceae N. Maslova and Kasicarpaceae N. Maslova were both placed in the extinct order Sarbaicarpales N. Maslova, part of the immediately larger group. Variation in this whole group is complex, and whether some characters reflected common ancestry or parallel variation was unclear (Maslova 2010: p. 1456 for a summary). Vavilov's Law of parallel variation was invoked (Maslova 2010: table 2), and it helped explain the "isomorphic polymorphisms" observed in platanoids and altingioids. Maslova (2010) was inclined to reject molecule-based hypotheses of relationships, and Proteaceae barely entered into the discussion; it is unclear what to make of these fossils and ideas.
Diversification of the major clades here may have occurred within a mere 3-6 Ma (Jian et al. 2008).
Pollination Biology. There are a number of reports of delayed fertilization from members of all four families, although not in Paeonia (Endress 2110c; esp. Sogo & Tobe 2006d and references), hence the placement of this feature here.
Chemistry, Morphology, etc.. Raffinose and stachyose are common oligosaccharides in phloem exudate in this clade (Daphniphyllaceae not studied: Zimmermann & Ziegler 1975). Hufford and Endress (1989; see also Hersen et al. 2006b) discuss anather morphology and anatomy in detail; members of this clade can have obviously valvate anthers, as in Hamamelidoideae, or the stomium may simply divide at the two ends of the theca, or at least at the base of the theca. Wheeler et al. (2011) summarize what is known of wood anatomy in the clade.
ALTINGIACEAE Horaninow, nom. cons. - Liquidambar L. - Back to Saxifragales
Trees, evergreen or deciduous; resins, route I iridoids +; secretory canals + [containing terpenoid resins]; petiole with 3-5 annular bundles, (with medullary bundles); stomata paracytic; leaves spiral, lamina lobed, vernation flat, lobes conduplicate, stipules on leaf base; plant monoecious, inflorescence ± capitate; P 0; staminate flowers: A 4-10, (anthers with longitudinal slits), ?filament length; pollen grains pantoporate, spherical, surface fine-reticulate; pistillode +; carpelate flowers: intercarpellary protrusions [= phyllomes] in a single series; G [2], unsealed, (semi)inferior, (transverse), styluli short to quite long, stigmatic their entire length, with multicellular protrusions, but no papillae; ovules 20</carpel [only the lower ones fertile], straight, (micropyle endostomal), outer integument ca 2 cells across, inner integument ca 5 cells across; fruit a septicidal (and loculicidal or ventricidal) capsule; exotesta lignified or not, mesotesta ± sclerotic, endotestal cells oblong, lignified; endosperm slight; n = 15, 16, x = 16.
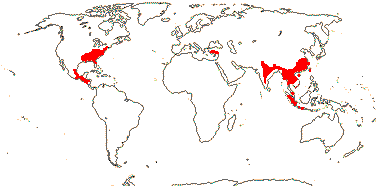
1 [list]/13. E. Mediterranean, East Asia to Malesia, Central America. Map: see Vink (1957), Wood (1972), Rzedowski (1978) and esp. Ickert-Bond et al. (2005). [Photos - Collection.]
Age. Ickert-Bond and Wen (2006) suggested that the crown-group age for the family can be dated to somewhere between 54 and 19.5 Ma, while Gu et al. (2022) offer an estimate of ca 22.4 Ma.
Altingiaceae have a rich fossil history. Microaltingia (ca 90 Ma) has 2-3 whorls of phyllomes, tiny, prolate, tricolpate pollen grains with a coarsely reticulate exine, a more or less superior ovary, ovaries with 8 or more ovules per carpel, and perhaps unwinged seeds; it may have been pollinated by insects (Z.-K. Zhou et al. 2001). If correctly assigned here - sister to the extant representatives of the family (Ickert-Bond et al. 2005, 2007) - it is yet another fossil with interestingly plesiomorphous features (see also Calycanthaceae, Platanaceae, Fagaceae, etc.). Friis et al. (2011) i.a. note that there are 2-3 series of sterile organs on the ovary. The ca 89 Ma Protoaltingia comoxense was recently described by Scharfstein et al. (2020) from Vancouver Island and linked withAltingia in morphological analyses, although such analyses may be problematic (see below). Paleoaltingia, with globose infructescences a mere 3 mm across, has recently been described from 94-90 Ma deposits in New Jersey; it and Microaltingia were placed immediately basal to the [Altingiaceae [Cerciciphyllaceae + Daphniphyllaceae]] clade in a combined analysis by Lai et al. (2021). See also Maslova et al. (2019) for fossils.
Evolution: Divergence & Distribution. Ickert-Bond and Wen (2006) give dates for divergence of clades within Altingiaceae; the basal split in the family is between the European + American and East Asian clades.
Chemistry, Morphology, etc.. There can be confusion between the exudates of Styracaceae, and the genus Styrax in particular (from which benzoin oil, gum benjamin, etc., come), and Altingiaceae (from which storax comes) - not to mention those of Lindera benzoin (Lauraceae). Secretory canals are also reported from Mytilaria (Hamamelidaceae s. str.).
The strongly vascularized structures ("phyllomes") interior to the staminal whorl of Liquidambarf have occasioned considerable discussion; these may be staminodia, organs sui generis, or reduced female flowers, and they may function as nectaries (e.g. Z.-K. Zhou et al. 2001; Ickert-Bond et al. 2005; Lai et al. 2021). The orientation of the carpels varies (Bogle 1986). This and Hamamelidaceae - "micropyle faces upwards"?
Endocarpial cells are thickened and elongated transverse to the long axis of the fruit, and they look almost palisade in transverse section. The testa is notably thinner than that of most Hamamelidaceae. Ickert-Bond et al. (2005) suggest that in Liquidambar the exotegmen "constitutes most of the seed coat", although this is not immediately evident in the sections presented (e.g. Ickert-Bond et al. 2005: Fig. 9, G-J) nor in Melikian (1973) and Z.-Y. Zhang and Wen (1996). However, if confirmed (see e.g. Ickert-Bond et al. 2007), it will be another sharp difference from the more or less massively mesotestal seeds of most Hamamelidaceae; there is no exotegmen in most Hamamelidaceae, a point also made by others (e.g. Mohana Rao 1975a).
For information about Hamamelidaceae s.l., see Bogle (1986: floral morphology, etc.), Ferguson (1989: general, esp. fossils), Skvortsova (1960: petiole), Melikian (1973: seed coat anatomy), Zavada and Dilcher (1986: pollen), and Endress (1993: general).
Phylogeny. Shi et al. (2001) present a molecular phylogeny of Altingiaceae (see also Ickert-Bond & Wen 2006), and this suggests that there is only one genus, in contrast to morphological phylogenies (Ickert-Bond et al. 2005, 2007; Scharfstein et al. 2020).
Classification. It is best that a single genus be recognised, both because of phylogeny (see above) and because species of the two genera that have been recognized, Liquidambar and Altingia, can hybridize in the wild, resulting in the genus Semiliquidambar, S. cathayensis in particular having much reduced fertility and perhaps even representing a F1 hybrid (W. Wu et al. 2010); see also Maslova et al. (2019). Ickert-Bond and Wen (2013) provide a taxonomic synopsis of the family.
Previous Relationships. Altingiaceae have often been included in Hamamelidaceae (e.g. Cronquist 1981).
[Hamamelidaceae [Cercidiphyllaceae + Daphniphyllaceae]]: ?
Age. The age of this node is ca 98.2 Ma (Tank et al. 2015: Table S2).
HAMAMELIDACEAE R. Brown, nom. cons. - Back to Saxifragales
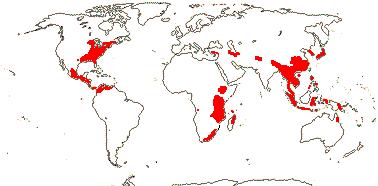
Trees or shrubs, evergreen; (C-glycosylflavones +); (vestured pits +; true tracheids +); sclereids common; petiole bundle (±) annular (with adaxial bundle; arcuate); stomata often paracytic, but variable, inc. laterocytic; hairs stellate (other); leaves= two-ranked (opposite, spiral), lamina vernation ± conduplicate-flat or -plicate, (margins entire), stipules cauline; flowers (2-)4-5(-7)-merous; K free to connate; A = and opposite K (3-many); staminodia opposite C, anthers with valves, two (one) pairs, (connective not prolonged); pollen tricolpate, (6-rugate); nectary ± annular, staminodial, or on base of C; G [2], stigmas with multicellular protrusions, but no papillae; ovules ca 6/carpel, often epitropous, outer integument 6-12 cells across, inner integument 2-3 cells across, (micropyle zig-zag), hypostase +; fruit a loculicidal and septicidal capsule, K often persistent; tegmen tanniniferous; endosperm slight, perisperm +, (polyembryony +); x = 6 (?7, ?8), nuclear genome [1 C] (0.029-)1.252(-53.933) pg.
27 [list: to subfamilies]/82 - four groups below. Tropical to temperate, esp. East Asia to Australia, not South America. Map: from Vester (1940), Vink (1957), Ying et al. (1993), Fl. N. Am. vol. 3 (1997), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1. (2003) and Coates Palgrave (2002). [Photos - Collection] [Photo - Flower.]
Age. An estimate of the crown-group age of Hamamelidaceae is a mere (42-)27, 25(-13) Ma (Bell et al. 2010) or a very different (110-)104, 87(-81) Ma (Wikström et al. 2001), while ca 38.7 Ma is the estimate in Gu et al. (2022); all these ages are in the context of relationships in the order that differ from those followed here.
Archamamelis, from the Upper Cretaceous, has 6-7-merous flowers, rather small, almost triangular petals, anthers with flaps, and a tricarpelate gynoecium, and may be stem-group Hamamelidaceae (Endress & Friis 1991; Friis et al. 2011).
1. Exbucklandioideae Harms —— Synonymy: Exbucklandiaceae Reveal & Doweld, Rhodoleiaceae Nakai
(Venation pinnate), (stipules 0); inflorescence capitate; (flowers monosymmetric); (C 0); anthers (bisporangiate); ; G half inferior, (styluli short); (ovules to 20/carpel); outer integument ca 2 cells across, much elongated [Exbucklandia]; seed winged, wing terminal [prolongation of testa - Exbucklandia], or lateral [Rhodoleia]; exotestal cells alone thickened, endotegmen +, tanniniferous; n = 12, 16.
2/ca 7. East Himalayas and South China to Sumatra.
[Mytilarioideae [Disanthoideae + Hamamelidoideae]]: seeds not winged, hilum large, coat often with often discoloration near the hilum; testa multiplicative, thick, hard, exotestal cells thickened (not), mesotesta massive, usually of ± fibrous sclerotic cells.
2. Mytilarioideae H. D. Chang
(Secretory canals +); nodes 5:5; (stipule 1, tubular - Chunia); (K 0, C 0 - Chunia); (C and A basally fused, forming a tube); G ± inferior; ovules 2/carpel; n = ?
2/2. China (Kwangsi, Hainan), Laos.
[Disanthoideae + Hamamelidoideae]: C ribbon-like, adaxially circinate.
Age. The age of this node is estimated to be (92-)84, 61(-53) Ma (Wikström et al. 2001).
3. Disanthoideae Harms - Disanthus cercidifolius Maximowicz —— Synonymy: Disanthaceae Nakai
Plant deciduous; flowers paired; nectary at base of C; anthers with slits; styluli short, stout; n = 8.
1/1. E. China, Japan.
4. Hamamelidoideae Burnett —— Synonymy: Fothergillaceae Nuttall, Parrotiaceae Horaninow
Plant evergreen or deciduous; (stomata anomocytic); (buds naked), (perula 1), prophyll 1 (2), basal, then an internode; lamina with pinnate/craspedodromous venation, vernation conduplicate-plicate; inflorescence racemose, spicate, (with 3-flowered lateral cymes); (K [5-9]); (C not circinate), (0); A 4-23, centripetal or centrifugal, anther (thecae unisporangiate), with 1 or 2 pairs of valves, or slits; tapetal cells multinucleate; G (to inferior); (styluli long); ovules 1/carpel (-3, 2 sterile), (apotropous), parietal tissue 8-10 cells across, (nucellar cap ca 2 cells across); seed dispersal ballistic; (endosperm cellular - Parrotiopsis); n = 12, nuclear genome [1C] 1.06-2.31 pg.
23/78: Corylopsis (30). Tropical to temperate, esp. East Asia to Australia, not South America.
Age. Allonia decandra, a fossil probably to be placed in crown-group Loropetalineae (Hamamelidoideae), was collected from ca 83 Ma rocks of the Cretaceous-Campanian in the eastern U.S.A., it has twice as many stamens as petals and a lobed disc adaxial to them (Magallón-Puebla et al. 1996: Friis et al. 2011); the seeds are rather angled, so there may have been more than one per loculus. Other Cretaceous fossils can perhaps be associated with the family (Radtke et al. 2005).
Corylopsis is known fossil from outside S.E. Asia. Leaf fossils, C. reedae, have been described from deposits ca 49.4 Ma from Republic, Washington (Radtke et al. 2005). Reported also from Greenland, Alaska and Europe, seeds as young as have recently been found in Tennessee (Quirk & Hermsen 2020). Radtke et al. (2005: Table 1, Fig. 5) discuss the fossil history of Hamamelidaceae; fossils are widespread and identifiable to genus in Tertiary deposits.
Evolution: Divergence & Distribution. For the early Caenozoic fossil history of what are now East Asian endemic members of the family, see Manchester et al. (2009); thus fossils of Corylopsis, now in Southeast Asia, are reported in deposits ca 50 Ma from the Okanogan Highlands in W. North America (Princeton, Republic: see Wehr & Hopkins 1994). For other literature on fossils, see Magállon et al. (2001: Androdecidua endressii), Benedict et al. (2008: Hamawilsonia), L.-C. Zhao and Li (2008: Corylopsis) and Friis et al. (2011: family).
L. Xie et al. (2010) note that there is not really much variation within Hamamelis, and the genus, which has diversified only within the last ca 9.7 Ma, has a stem/fuse of ca 41.5 Ma. However, W. Zhou et al. (2022: H. mollis or perhaps H. japonica sister to rest, in turn Foth. Parr.) estimate a crown-group age of 34.0-27.5 Ma with a fuse of around 16-23 Ma.
For the evolution of the flower in Hamamelidoideae, see Magallón (2007; fossils included, optimisation on to more than one topology).
Pollination Biology. There is considerable variation in floral morphology, and Parrotiopsis and Rhodoleia have pseudanthia (Baczynski & Claßen-Bockhoff 2023); Parrotiopsis has showy inflorescence bracts, and these are bright red in Rhodoleia, and there the whole inflorescence is very like the flower of, say, Calycanthaceae. Corylopsis has rather ordinary-looking flowers with obovate petals, although this morphology is probably derived. Petals may be lost, as in Fothergilla where the inflorescence is made conspicuous by the plump and showy white filaments (see Endress 1978 for a discussion of the floral morphology of those Hamamelidaceae without a perianth). Nectar is a common reward, and the nectaries are very variable in morphology (Endress 1993 and references). Eustigma has quite long white styluli and massive, purplish stigmas that are the most conspicuous parts of the flower. Disanthus in Japan is pollinated by fungus gnats (Mochizuki & Kawakita 2017).
Fertilization in Hamamelidaceae is often much delayed.
Plant-Animal Interactions. For the phylogeny of hormoraphidine aphids, notable gallers on Hamamelidoideae with ca 13 genera of aphids known from Distylium alone, see J. Chen et al. (2014).
Chemistry, Morphology, etc.. What is going on with the growth of Exbucklandia?
There is variation in the direction of initiation of the stamens in multistaminate androecia (Endress 1976); it can be centripetal (Matudaea) or centrifugal (Fothergilla). Exbucklandia has a remarkably long outer integument, although it is developed on only on one side of the micropyle, and it develops into the seed wing (Kaul & Kapil 1974).
Much additional information can be found in Endress's early work (1967a [general, comparison with Betulaceae, Corylopsis is the link], 1970b [inflorescence], 1971 [inflorescence, flower], 1976, [floral development], 1993 [general]). For petiole anatomy, see Skvortsova (1960), for ovary/fruit anatomy in Fothergilla, see Bobrov et al. (2025) and for seed anatomy, see Mohana Rao (1975a), Z.-Y. Zhang and Wen (1996) and Benedict et al. (2008: also fruits, etc.).
Phylogeny. A clade including Exbucklandioideae and Mytilarioideae was apparent only in the analysis of ITS data and good sampling (75% bootstrap, better if gaps scored as a fifth character state: J. Li et al. 1999b; c.f. Shi et al. 1998); the genera included have in the past been placed in three subfamilies. With rbcL data, Mytilaria alone was rather weakly supported as sister to [Disanthoideae + Hamamelidoideae] (Li et al. 1999a). A later two-gene analysis resulted in strong support for the clades represented by the four subfamilies above and their relationships (Li 2008) as did the study by Z.-D. Chen et al. (2016). However, H.-T. Li et al. (2021: plastome analyses) found only poor support for the monophyly of the family as a whole, the only case in angiosperms apart from Aristolochiaceae where there were problems with monophyly.
Within Hamamelidoideae [Corylopsideae (monotypic) + Loropetaleae (weak support)] were sister to the rest, but tribal interrelationships had for the most part only weak support (J. Li & Bogle 2001; Li 2008); see also Ding et al. (2019: beginning of a plastome phylogeny). See Xie et al. (2010; also W. Zhou et al. 2022) for the phylogeny and biogeography of Hamamelis, which, despite having similar petals to those of Loropetalum, is not immediately related.
Classification. For a classification of Hamamelidoideae, see J. Li and Bogle (2001).
[Cercidiphyllaceae + Daphniphyllaceae]: plant dioecious; flowers small, C 0; staminate flowers: , filaments at most barely longer than anthers; carpelate flowers: endosperm cellular.
Age. The age of this node is ca 91.8 Ma (Tank et al. 2015: Table S2).
Chemistry, Morphology, etc.. The filaments are at most only slightly longer than the anthers, quite common in wind-pollinated flowers.
CERCIDIPHYLLACEAE Engler, nom. cons. - Cercidiphyllum Siebold & Zuccarini - Back to Saxifragales
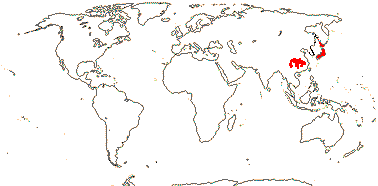
Deciduous trees, with short shoots; chalcones, dihydrochalcones +; cork in outer cortex; primary stem with continuous cylinder; prophyll adaxial; leaves usu. opposite, lamina vernation involute, (margins entire), stipule adaxial-petiolar; inflorescence capitate/fasciculate; P 0, floral apex?; staminate "flower": A 16-34 [= several flowers], anthers long; pollen tricolpate; carpelate flower: G free, single, suture abaxial, ["G 1-8", = 1-8 flowers, each subtended by a bract (perianth member?)], stylulus long, stigma decurrent its entire length, esp. laterally, papillate; ovules many/carpel, outer integument 4-5 cells across, inner integument 2-3 cells across; fruit a follicle; chalazal appendage +, with hair-pin loop vascular bundle; testa undistinguished, exotestal cells enlarged, slightly thickened, tegmen tanniniferous; endosperm slight, suspensor single-celled, cell notably enlarged; n = 19, x = 9 (?10, ?8), nuclear genome [1 C] (0.041-)1.265(-39.306) pg.
1 [list]/2. China and Japan. Map: from Heywood (1978) and Fu and Hong (2000). [Photos - Collection.]
Age. Gu et al. (2022) estimate that the two species separated ca 3.0 Ma.
Evolution: Divergence & Distribution. For the early Caenozoic fossil history of Cercidiphyllum, see Manchester et al. (2009) and Friis et al. (2011). Cercidiphyllum was quite common plant in streamside habitats in the Late Cretaceous to Eocene; frequent associates included Platanus, Metasequoia and Ginkgo (Royer et al. 2003).
Palaeocene fossils (Joffrea) have 2-carpelate flowers borne on an elongated axis with the adaxial sutures of the carpels facing each other (Crane & Stockey 1985, 1986; see also Friis et al. 2011). Staminate flowers of fossils may have five stamens and a pentamerous perianth (Kvacek 2008).
Qi et al. (2012) found substantial genome structure in populations of Cercidiphyllum, which is by no means a relict.
Chemistry, Morphology, etc.. Takhtajan (1997) described the venation of leaves on the long shoots as being pinnate, but the main secondary veins all arise within 5(-10) mm of the base.
Krassilov and Lowen (2007) thought that the flower of Cercidiphyllum was unlike that of other Saxifragales (see also Maskova 2010). The "flowers" of today's species can be interpreted as pseudanthia. Both individual carpels and groups of stamens are subtended by bracts and are more or less decussately arranged. Each carpel represents a carpelate flower, indeed, the carpels are sometimes slightly separated from one another on the stout green "pedicel" = inflorescence axis. However, for Yan et al. (2007; see also Jin et al. 2018) this bract structure was a tepal because it was developmentally so different from the vegetative leaves, although it might have teeth or be almost bilobed. In any event, the suture of the carpel is adaxial with respect to the floral axis that originally bore it, abaxial to the inflorescence axis. Soltis et al. (2005: fig. 6:11) suggested perhaps inadvertently that the gynoecium is partly inferior.
For variation in nodal anatomy between leaves on long and short shoots, see Howard (1979), and for general information, see Endress (1993) and Crane and Du Val (2013).
DAPHNIPHYLLACEAE Müller Argoviensis, nom. cons. - Daphniphyllum Blume - Back to Saxifragales
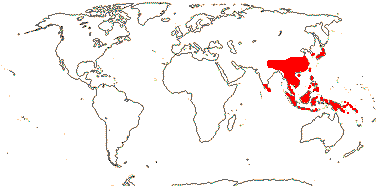
Evergreen trees or shrubs; plants Al-accumulators, route I iridoids, triterpene [squalene] alkaloids +, myricetin, hydrolysable tannins 0; pits bordered; true tracheids +; pith at least sometimes septate; pericyclic fibres 0; stomata paracytic (laterocytic, anomocytic); plant glabrous; leaves ± pseudoverticillate, spiral, lamina vernation ± flat, margins entire, secondary veins pinnate, stipules 0; flowers pedicellate; P +, uniseriate, ± sepal-like, 2-6; staminate flowers: A 5-12(-24), anthers with slits, basal pit indictinct, filaments with 3 traces, (staminodes +); pistillode 0; carpelate flowers: P free/connate/0; staminodes 0/+; G [2(-4)], placentation apical-axile to parietal, styluli short, recurved, stigmas rather massive, with multicellular protrusions but no papillae; ovules (1)2/carpel, ± apical, pendulous, micropyle exostomal/zig-zag?, outer integument 3-6 cells across, inner integument 4-5 cells across, hypostase +; fruit a 1-seeded drupe; seeds not winged, seed coat persistent but thin-walled and crushed, or endotegmen tanniniferous, walls thickened; perisperm slight, embryo short, cotyledons same width as radicle, (polyembryony +); n = 16, x = 9 (?10, ?8).
1 [list]/30. East Asia to Malesia. Map: from Huang (1997). [Photo - Fruit]
Age. Crown-group Daphniphyllaceae may be ca 19.9 Ma (Gu et al. 2022).
Evolution: Divergence & Distribution. Daphniphyllum pollen is reported from middle Miocene deposits in Austria ca 14 Ma (Grísson et al. 2015b).
Plant-Animal Interactions. Some epiplemine Uraniidae (moths) have caterpillars that eat Daphniphyllaceae - as well as assorted asterids - probably because of the iridoids they have in common (Lees & Smith 1991).
Chemistry, Morphology, etc.. The flowers may be secondarily superior (D. Soltis et al. 2003b). Bhatnagar and Kapil (1982) described the endotegmic cells as being thickened and variously cutinised or sclerotic.
For general information, see Z.-Y. Zhang and Lu (1989) and especially Kubitzki (2006b), for information on stomata, etc, see Tang et al. (2009), for embryology, see Bhatnagar and Kapil (1994); for a monograph, see Huang (1965).
Phylogeny. M.-S. Tang et al. (2022: 2 chloroplast markers, nuclear ITS gene) found that chloroplast and nuclear trees had different topologies, but support values were weak; the sections previously recognized morphologically based, were not recovered.
Previous Relationships. Daphniphyllaceae have been difficult to place, sometimes being associated with Euphorbiaceae, etc., or placed in a separate order in the Hamamelidae (Cronquist 1981). Balanops (here Balanopaceae-Malpighiales) and Daphniphyllum were included in a bigeneric Daphniphyllanae by Takhtajan (1997).
[[Crassulaceae [Aphanopetalaceae [Tetracarpaeaceae [Penthoraceae + Haloragaceae]]]] [Iteaceae [Grossulariaceae + Saxifragaceae]]]: vessel elements with simple perforation plates; petiole bundle(s) arcuate; cuticle waxes not tubular; lamina venation ± pinnate; stigma not decurrent; ovules apotropous [all?]; K persistent, withered.
Age. The crown-group age of this clade is some (96-)91, 78(-73) Ma (Wikström et al. 2001, 2004), (88-)80, 77(-69) Ma (Bell et al. 2010), or (114-)96.8(-90.1) Ma (L.-Y. Chen et al. 2014a).
Chemistry, Morphology, etc.. Mauritzon (1933) provides information on the ovules and endosperm development for many taxa in this clade.
[Crassulaceae [Aphanopetalaceae [Tetracarpaeaceae [Penthoraceae + Haloragaceae]]]]: stem with endodermis, stipules 0; inflorescence cymose; flowers 4-merous; A 2X K/C, obdiplostemonous; G opposite C; endosperm cellular.
Age. The crown-group age of this clade is around (88-)80, 77(-69) Ma (Bell et al. 2010), (82-)77, 69(-64) Ma (Wikstöm et al. 2001), (109.7-)94(-85.6) Ma (L.-Y. Chen et al. 2014a), ca 89.6 Ma (Tank et al. 2015: Table S2), (121.4-)10.5(-93.9) Ma (Messerschmid et al. 2020) or ca 106.4 Ma (Gu et al. 2022).
Chemistry, Morphology, etc.. For obdiplostemony and associated features, see Ronse De Craene and Bull-Hereñu (2016) and references.
CRASSULACEAE Jaume Saint-Hilaire, nom. cons. - Back to Saxifragales
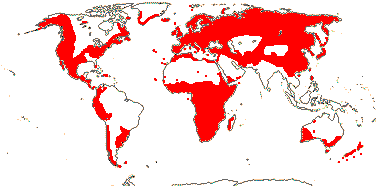
Succulent herbs to soft-stemmed shrubs; mycorrhizae 0; crassulacean acid metabolism common; flavones, acylated flavonol glycosides, sugar reserve as sedoheptulose, non-hydrolysable tannins +, hydrolyzable tannins 0; red pigment common, even in roots; (cork cortical); young stem with separate bundles; (medullary bundles +); sieve tube plastids lacking starch and protein inclusions; xylem rayless; nodes also 1:1-3, 3:3, etc.; cuticle waxes very variable; stomata usu. anisocytic; leaves succulent, lamina vernation flat to curved, margins entire, hydathodes +; inflorescence cymose; anthers median sagittate, latrorse; nectaries ± finger-like, at bases of carpels [outgrowths of carpels]; G ± free, opposite C, with 5 vascular bundles, (G ± connate, placentation parietal), stigmas punctate to moderately capitate, (wet); ovules 1-many/carpel, micropyle bi-(exo-/endo-)stomal, outer integument ca 2 cells across, inner integument 2-3 cells across, nucellar cap 2-7 cells across, nucellar epidermal cells enlarged, apical cells ?tanniniferous, appearing subpalisade [for all, or Crassuloideae?], hypostase +; (megaspore mother cells several); fruit a follicle; exotestal cells with outer wall ± thickened, inner pigmented layer +; endosperm cellular and variants, (nuclear), chalazal haustorium +, embryo long, suspensor biseriate, short [?dist.], basal cell much enlarged, with mycelium-like haustorial branches; x = 8 (?7, ?9), nuclear genome [1 C] (0.053-)0.848(-13.468) pg; germination epigeal and phanerocotylar.
34 [list]/1,480 - three subfamilies below. Cosmopolitan, esp. the Cape region and Mexico, but few in S. South America and the Antipodes, not in Polynesia, frequently in drier regions. Map: see Hultén (1958), Bywater and Wickens (1983), Jürgens (1995), Thiede (1994, 1995), Fl. China Vol. 8 (2001) and Trop. Afr. Fl. Pl. Ecol. Distr. Vol. 1 (2003). [Photo - Flower.]
Age. Diversification in this clade began (44-)41, 39(-36) Ma (Wikstöm et al. 2001), while Bell et al. (2010) estimate an age of (63-)50, 47(-36) My; around 45 Ma is the estimate in J.-Q. Zhang et al. (2014b), (95.3-)72.9(-60.7) Ma in Magallón et al. (2018), (99.2-)81.7(-65.4) Ma in Messerschmid et al. (2020) and ca 79.1 Ma in Gu et al. (2022).
1. Crassuloideae Burnett - Crassula L. —— Synonymy: Tillaeaceae Martynov
(Aquatics), (geophytes), (annuals); leaves opposite, (internodes very short); lamina (small), with several marginal hydathodes only, (densely hairy/papillate); flowers 3-9-merous; A = and opposite K, slightly introrse at anthesis; pollen surface striate; G (3 - odd G abaxial - Tillaea s. str.); parietal tissue 1(-2) cells across, soon disappearing; follicles releasing seeds through apical pore, seeds dust-like; testa cells with sinuous anticlinal walls, unipapillate; first division of micropylar endosperm cell in horizontal plane; n = 7, 8 (11).
1/200. Esp. Southern Africa to S.W. Arabia, "Tillaea" more or less world-wide, the only representative of Crassulaceae in the Antipodes.
Age. Around 33 Ma is the estimate of the crown-group age of this clade in J.-Q. Zhang et al. (2014b: sampling), (56-)46.1, 31.6(-19.5) Ma in Bruyns et al. (2018), (51.3-)39.5(-28.9) Ma in Messerschmid et al. (2020) and (49.0-)35.3(-23.4) Ma in M. Lu et al. (2021).
[Cotyledonoideae + Sempervivoideae]: leaves spiral, lamina with single (sub)apical hydathode; A obdiplostemonous, introrse only in early bud; (placentae lobed); parietal tissue 1-4 cells across; seeds costate; first division of micropylar endosperm cell in vertical plane.
Age. Messerschmid et al. (2020) estimated that the age of this clade was (84.5-)70.5-(56.9) Ma.
2. Cotyledonoideae A. Berger
Plant ± woody, (deciduous), (heraceous; annuals); bufadienolides + [cardiac glycosides]; (stem roots with multicellular hairs - "Bryophyllum"); crystal sand +; leaves (opposite), (margins with teeth); inflorescence (spike-like); flowers 4-5(-6)-merous; C ± connate; A ± adnate to C, with spherical connective prolongation; (styluli long); (nucellus with central strand); seeds 4-6-costate, with a micropylar corona; x = 9 [n = 9, 17 (18)].
4/240: Kalanchoë (145), Tylecodon (46). Old World, especially the Karoo in southern Africa, but extending to South East Asia and Malesia, not Australasia.
Age. The crown-group age of this clade is estimated to be (42.3-)23.2, 22.9(-10.1) Ma (Bruyns et al. 2018) or (44.3-)32.9(-22.8) Ma (Messerschmid et al. 2020).
3. Sempervivoideae Arnott —— Synonymy: Cotyledonaceae Martynov, Rhodiolaceae Martynov, Sedaceae Roussel, Sempervivaceae Jussieu
(Plants annual); (pyrrolidine and piperidine alkaloids +), (non-cyanogenic β- and γ-hydroxynitrile glucosides +), (nonhydrolyzable tannins 0 - esp. Sedum [S.] acre group); (leaves opposite); flowers 4-32-merous; (C connate); (stamens = and opposite K); (infra-stylar extra-gynoecial compitum/pollen tube growth - S.); nucellus elongated, with central strand; (megaspore haustoria + - S., Rosularia); (follicle with abaxial dehiscence - Diamorpha); seeds ³6-costate; suspensor (uniseriate, 7-10 cells long, terminal cell relatively small - S. sect. Rupestria/Phedimus); n = >5 (up to n = 320 - S. suaveolens).
30/1,005: Sedum (755: polyphyletic), Sempervivum (65), Aeonium (34). Largely northern hemisphere.
Age. Crown-group Sempervivoideae are (48.2-)31.7, 28.3(-17.9) Ma (Bruyns et al. 2018) or (79.5-)65.9(-53.4) Ma (Messerschmid et al. 2020), very different estimates.
Age. An estimate of the age of Telephieae is (46.9-)35.4(-24.6) Ma Messerschmid et al. 2020).
Age. The age of Umbiliceae is (71.1-)56.5(-41.5) Ma (Messerschmid et al. 2020).
Age. Crown-group Sedeae are some (57.9-)48.0(-38.8) Ma (Messerschmid et al. 2020).
Evolution: Divergence & Distribution. For dates in and around Sedum s.l., see Messerschmid et al. 2020).
Diversification may have increased at the crown-group Crassulaceae node (95.3-)72.9(-60.7) Ma (Magallón et al. 2018). Diversification within Crassula has predominantly been within the last 10 Ma or so (see also Ecology & Physiology) and within the old subgenus Crassula (M. Lu et al. 2021).
Although Crassulaceae are found on relatively few oceanic islands, they have evolved quite a few single island endemics (Lenzner et al. 2017). In particular, Aeonium, largely Macaronesian, i.e. from the Canary Islands, the Azores and Madeira, has the most endemic species of any genus there, and the species have striking growth forms, some arborescent, each of which Mes and t'Hart (1996) and Jorgensen and Olesen (2001), at least, thought had evolved just once. Indeed, with Aichryson, Monanthes and Greenovia, the Aeonium alliance makes a group of about 60 species that have diversified within the last ca 15.3 Ma (S.-C. Kim et al. 2008), or ca 40 species that have diversified within the last (8.1-)4.6(-1.7) Ma (Messerschmid et al. 2023). Relatives are North African species of Sedum, e.g. section Monanthoidea, and a few species of the Aeonium alliance have themselves moved back to Africa, where they are found in Morocco, the East African mountains, and the Yemen (Mes et al. 1996; Mort et al. 2007; Cristini 2022; Messerschmid et al. 2023 - see also Dracaena-Convallarioideae-Asparagaceae). Diversification in the largely European Jovibarba/Sempervivum clade is a result of a combination of vicariance, long-distance dispersal, and edaphic specialization (Klein & Kadereit 2015). Rhodiola, embedded in the Telephium clade (see Messerschmid et al. 2020), is likely to have evolved on the Quinghai-Tibet Plateau (J.-Q. Zhang et al. 2014b).
The distinctive wood of Crassulaceae, which lacks rays and has very short vessel elements with annular and helical thickening, is probably paedomorphic (t'Hart & Koek-Noorman 1989); plant chemistry, in particular the presence of hydrolyzable tannins and the absence of non-hydrolyzable tannins, as in other woody Saxifragales, is consistent with this idea (Thiede & Eggli 2006).
There have been several origins of sympetaly in Sempervivoideae ('t Hart et al. 1999; Carrillo-Reyes et al. 2009), however, both it and epipetaly tend to be weak. The increase in numbers of flower parts in some Sempervivoideae - some have a multistaminate androecium - is in the context of an increase in merosity of the whole flower; the relation between the number of parts of each whorl is like that of other basic core eudicot flowers (see also the euasterid clade), i.e. K = C = G; A = 2x C. Messerschmid et al. (2020: Fig. 1) plot the distributions of several of these and other characters previously used to separate genera in Sedum s.l. and other Sempervivoideae.
All ca 200 species of the Echeveria group (well embedded in Sedum s.l. - Messerschmid et al. 2020) in Sempervivoideae appear to be interfertile, a remarkable situation apparently without parallel in flowering plants (Uhl 1992). For hybridization in Sempervivum, see Klein and Kadereit (2015).
A number of species of Kalanchoë, notably some from Madagascar, produce plantlets in notches at the margin of the leaf blade, whether because of damage to the leaf or not; these have rather aptly been called foliar embryos (Yarborough 1932). Both embryogenic and organogenic pathways have been co-opted here, and the young plantlets have cotyledon-like first leaves; however, unlike seeds, these plantlets show no dormancy, thus a gene that induces dormancy in Arabidopsis is not functional here (Garcês et al. 2014; see also J. Guo et al. 2015). Species in which development of plantlets is constitutive, i.e. plantlets are produced without the plant being damaged, do not produce viable seed (Garcês et al. 2007). For more on the control of the development of these plantlets, see G. F. Smith et al. (2021). There is considerable variation in habit in the genus, from annual to perennial and herbs to trees (almost) (Smith et al. 2023).
Ecology & Physiology. Along with Aizoaceae-Ruschieae, Crassula (and a few other Crassulaceae, esp. Kalanchöoideae) make up an important component of the vegetation of the winter rainfall Succulent Karoo of south west Africa (Ogburn & Edwards 2010), and some 66 of the 120+ Crassula species that grow there are endemics - although compared with Ruschieae there are few very narrow endemics, perhaps because of the very small and dust-like seeds of Crassula (Bruyns et al. 2018). Crassula includes over 25 annuals, and these are members of several basal clades that overall show little diversification (see also Aizoaceae-Ruschioideae!); the annual habit may even be ancestral for the genus (M. Lu et al. (2021). Much diversification has been within the last 10 M years or so, although crown-group Crassula/Crassulaceae themselves are considerably older, and compact perennials, etc., have evolved in the very speciose clade C of the genus (Bruyns et al. 2018; Lu et al. 2021). About the time of this diversification, which is similar in other succulent clades like Aizoaceae, Cactaceae, Aloe, succulent Euphorbia, etc. (M. Lu et al. 2021), there was notable global cooling, and in western southern Africa (about half the species of Crassula are to be found there) this was also accompanied by aridification (Lu et al. 2021).
Crassulacean acid metabolism (CAM: see Kluge & Ting 1978; Chomthong & Griffiths 2023: recent developments; other papers in Ann. Bot. 132(4). 2023) is common throughout the family (Nelson et al. 2005 and Earles et al. 2018 for morphology; Winter & Smith 1996a and references; Pilon-Smits et al. 1996; Holtum 2023), and it has been reported in everything from aquatic species of Crassula to the so-called tree houseleeks, Aeonium (e.g. Keeley 1998; Keeley & Rundel 2003; Mort et al. 2007)t, rather similar to its general pattern of occurrence in vascular plants as a whole (Holtum 2023). Bräutigam et al. (2017) outline the general principles of the evolution of CAM photosynthesis, which seems be a response to drying conditions, cooling and declining atmospheric CO2 concentrations in the Miocene ca 20 Ma - temperatures were indeed still warm, and so conditions for photorespiration become favourable. Overall, CAM photosyntheis has been detected in 38 families and 370 genera of vascular plants (33 families of angiosperms) with some 114 or so origins of the habit, although these numbers are very much subject to change (Gilman et al. 2023: Table 1 for a list of the taxa involved) CAM plants prefer conditions where rainfall is regular; overall nearly 7% of vascular plants are CAM plants, and they are widely distributed both taxonomically, ecologically and geographically. CAM in epiphytic plants is usually obligate CAM while CAM annuals often have inducible or facultative CAM - there are a number of CAM variants, and it can be very difficult to detect some of them - there is a CAM continuum in most CAM lineages (Messerschmid et al. 2021; Holtum 2023; Gilman et al. 2023). Indeed, establishing the presence of a particular CAM mechanism in any one species presents problems because its expression there may depend on local environmental conditions (= facultative CAM) - and add phenotypic lability, a variety of CAM types, and so on (e.g. Hancock et al. 2019; Winter et al. 2019). There are some aquatic CAM plants like Isoetes - CAM here may be quite old, its evolution being at a time when CO2 concentrations were much higher than in the Pliocene, etc. - and Gilman et al. (2023) also note that aquatic plants in particular can move between different photosynthetic pathways and use multiple carbon concentrating mechanisms simultaneously. The evolutionary sequence C3 → C3 + CAM → strong CAM has been established in a number of taxa including Orchidaceae, Cactaceae, Clusiaceae and Asparagaceae-Agavoideae (references - and much else - in Gilman et al. 2023). Overall, origins of CAM are commonest after 25 MA or so when CO2 concentrations had dropped to around 500 ppm and peaking around 10-5 Ma, the origin in Crassulaceae being decidedly older (Sage et al. 2023). Crassulaceae are of course a major CAM family (for the evolution of CAM in Crassulaceae, see Holtum 2023; Gilman et al. 2023), but overall almost half the CAM species are epiphytic Orchidaceae (q.v.). CAM is commonest in herbaceous plants, although less so in annuals; CAM trees are infrequent, akthough they are found in Clusia while columnar cacti can be quite massive plants... Decreasing CO2 concentrations leads to reduced CO2 availability as a substrate for photosynthesis, greater photorespiration, and decreasing water use efficiency because of stomatal opening, and although dry conditions would seem to favour the evolution of CAM, deserts have been around longer than most CAM plants (Sage et al. 2023).
There are four phases in the strong form of CAM: In phase I, most of the night, there is CO2 uptake, carboxylation occurs and malic acid forms; in phase II, the early morning, stomata open and carboxylation via RuBisCO occurs; in phase III, most of the day, the stomata are closed, malate moves out of the vacuole and is decarboxylated, high concentrations of CO2 developing around the RuBisCO; and in phase IV the stomata open and RuBisCO carboxylation is driven by a draw-down of malate. Overall, evapotranspiration is low and water use is very efficient (Heyduk 2022). E. J. Edwards (2019) looked at CAM evolution while emphasizing possible rate-limiting steps in the process, which may have evolved late in the whole process of CAM development. Note that depending on how "intermediates" are scored in Aeonium, the ancestral state for photosynthesis may differ, furthermore, there is no obvious connection between ancestral states and the habit of the plant (rosettes, rosette and candelabra trees, shrubs), etc. (Mort et al. 2007); A. spathulatum seems to have reverted to C3 photosynthesis. Gehrig et al. (2001), Kluge and Brulfert (1996) and Mioto et al. (2014) discuss CAM variation within Kalanchoë; see Boxall et al. (2017) for its circadian control, and Males and Griffiths (2017) discuss the stomatal biology of CAM plants. For CAM in Kalanchoë compared with that in some other CAM plants, with both sequence convergence and in particular a number of changes in the temporal expression of genes, see X. Yang et al. (2017). For the evolution of CAM, see e.g. Silvera et al. (2010b), Edwards (2019, 2023) and I. Y. Y. Tang et al. (2021), Bräutigam et al. (2017) and Hermida-Carrero et al. (2020) for the molecular evolution of RuBisCO, and Males and Griffiths (2017) and Cheng and Raissig (2023) for the stomatal biology of CAM plants, which is rather poorly known.
Pollination Biology & Seed Dispersal. In Sedum lineare, at least, pollen tubes can leave a carpel in which the ovules have been fertilized via an opening at the base of the carpel and thence into an adjacent carpel - an extragynoecial compitum (X.-F. Wang et al. 2011). Monocarpy has evolved perhaps six times, no losses, or four times with two losses in Aeonium (Mort et al. 2007) - another way of dealing with the same problem? G. F. Smith et al. (2021) review reproduction in Kalanchoe, particularly the vegetative reproduction that results in plantlets developing along the margins of the leaves in some species of Kalanchoë; see also Diversity & Distribution above.
Plant-Animal Interactions. Species of the papilionid butterfly Parnassius subgenus Parnassius have caterpillars that eat Crassulaceae (and Saxifragaceae), and they may have moved onto these plants from Papaveraceae (Michel et al. 2008; Condamine et al. 2012, 2018). For swallowtails in general, see Aristolochiaceae.
Vegetative Variation. There are multicellular hairs (bi-, uniseriate, cells not elongated) on the aerial stem-borne (= "adventitious") roots of some species of Kalanchoë (Popham & Henry 1955).
Genes & Genomes. There is a genome duplication (the CRAPα event) ca 70.3 Ma that has been associated with Crassulaceae (Landis et al. 2018).
For suggestions as to the base chromosome number of the family and of its major clades, see Mort et al. (2001).
Chemistry, Morphology, etc.. Anthocyanin is also found in the roots of Saxifragaceae, as well as Melastomataceae, Balsaminaceae, Asteraceae, Droseraceae, and Francoaceae (Krach 1976; Molisch 1928). Sedoheptulose is the most abundant sugar in Crassulaceae; isocitrate is common, unlike other succulents (Thiede & Eggli 2006).
The extent of rayless wood in the family should be confirmed (Carlquist 2015b). The sieve tube plastids are a distinctive variant, lacking starch, the S0 type (Behnke 1988a). For (mistaken) reports of cortical vascular bundles, see Thiede and Eggli (2006). The leaf blade usually lacks palisade tissue, and there are often stomata on both sides. The stomata may also be heliocytic, with an additional ring of distinct cells outside a basically anisocytic configuration.
There is sometimes only a single vascular trace to the sepals (t'Hart & Koek-Noorman 1989). Anthers early in development are introrse, but often at maturity the sporangia are equidistant from one another (Wassmer 1955); for anther anatomy and development in Crassula, etc., where there is quite considerable variation in position and extent of development of cells with fibrous thickenings, see Anisimova and Shamrov (2023 and references). According to D. Soltis et al. (2003b), the ovary is secondarily superior. Although this seems unlikely, the very base of the ovary is sometimes inferior, and the carpels, apparently free, are slightly connate at the base; a small amount of axial tissue is also apparent in the gynoecial region of some taxa (Wassmer 1955, q.v. for gynoecial details). The nectary is ± receptacular in origin (Erbar 2014 and references). The nucellar epidermal cells tend to be large and more or less radially elongated in Crassuloideae (in particular) and Cotyledonoideae; in some Sempervivoideae megaspores elongate, a.k.a. "megaspore haustoria", sometimes even those from the same megaspore mother cell - and grow towards the micropyle, and there and in Kalanchoë two embryo sacs may develop (Mauritzon 1933: much information; Subramanyan 1968). Haustoria from places other than the massive suspensor are reported for Crassulaceae (Mickesell 1990). Distinctive compound plasmodesmata in the suspensor cell walls have been found in some Sempervivoideae, although their distribution within the family is unclear - the variables here seem to be plasmodesmatal distribution and branching and the presence or absence of associated electron-dense material (Kozieradzka-Kiszkurno et al. 2011, 2020). Kozieradzka-Kiszkurno et al. (2020) discusses the distribution of different suspensor morphologies in the family; the relatively long uniseriate suspensors of Sedum section Rupestria (= Phedimus) seem to be unique, at least in Sempervivoideae. The testal cells of Crassula often have a single papilla and sinuous anticlinal walls (e.g. Bywater & Wickens 1983). There is no tegmen. Spongberg (1977) noted that the endosperm is usually scanty, while Mabberley (1997) described it as being copious; the former is correct.
For general accounts of Crassulaceae, see 't Hart & Eggli (1995), Eggli (2003: enumeration of all species), Descoings (2006: Kalanchoe), Frandsen (2017: photographs of Crassula), Cristini (2022: Aeonium) and in particular Thiede and Eggli (2006); for chemistry, see Stevens (1995) and Bjarnholt et al. (2008: non-cyanogenic glucosides), for anatomy, see Gregory (1998), Jensen (1968: nodal anatomy, variable) and Melo-de-Pinna (2016: leaf development), for floral development, see Nelson (1990), and for embryology, etc., see Rombach (1911), Vignon-Fétré (1968), Subramanyam (1970) and Anisimova and Shamrov (2021).
Phylogeny. The basic phylogenetic structure of Crassulaceae seems fairly well established (e.g. van Ham 1995; van Ham & 't Hart 1998; Mort et al. 2001, 2010 for a summary; Mayuzumi & Ohba 2004). The rather highly derived Crassula/Crassuloideae are sister to the rest of the family, and there Kalanchoë and relatives are sister to Sempervivoideae (e.g. Mort et al. 2001; Mort 2002; Thiede & Eggli 2006).
Within Crassuloideae, Tillaea is polyphyletic and embedded within Crassula (Mort et al. 2009; Mort et al. 2010). Bruyns et al. (2018) looked at relationships within Crassula focussing on the southern African species; of two "classical" subgenera, one was paraphyletic, and only three of the sixteen sections of which they included more than one species was monophyletic. M. Lu et al. (2021: ITS plus 2 chloroplast markers) sampled widely in the genus (119 species) and recovered three major clades. The old subgenus Disporocarpa is paraphyletic, making up two of those clades and including the clade [C. bergioides + C. glomerata] that is sister to the rest of the very speciose clade 3, otherwisee made up of species of subgenus Crassula (Lu et al. 2021).
Kalanchoideae. For the phylogeny of Kalanchoë, see Gehrig et al. (2001) and Kluge and Brulfert (1996). For relationhips in the subfamily, see Mort et al. (2005 and references) - Adromischus is sister to the rest of the subfamily, and this was confirmed by Han et al. (2024: plastomic and 1054 nuclear gene datasets), where relationships were [Adromischus [Kalanchoe [Cotyledon + Tylecodon]]], although within Kalanchoe Eukalanchoe was not monophyletic and there was some conflict between nuclear and plastome relationships.
Sempervivoideae. Sedum appears in five of the seven main clades apparent in phylogenetic analyses of Sedoideae (= Sempervivoideae: van Ham 1995; van Ham & 't Hart 1998; Mayuzumi & Ohba 2004; Nikulin et al. 2016), and to make it monophyletic, genera like Aeonium, Echeveria, Villadia, Sempervivum and Dudleya would all have to be included. Within the Acre clade of Sedum, most New World Sedoideae as well as all the old Echeverioideae in the study formed a single clade, although it was only poorly supported (Carrillo-Reyes et al. 2009); for relationships of the Acre clade and other east Asian Sedoideae, see also Mayuzumi and Ohba (2004). Acevedo-Rosas et al. (2004) found that the limits of Graptopetalum were unclear and that there was little strong support along the backbone of the tree. For relationships within Dudleya, probably monophyletic, see Yost et al. (2014), and for those within Rhodiola (= Sedum, ca 60 spp.), see J.-Q. Zhang et al. (2014a, b) and D.-N. Zhao et al. (2023: plastome analyses, 23 spp.), the latter recovering two main clades, one with all dioecious taxa, within the genus. Klein and Kadereit (2015) resolved relationships within the Jovibarba/Sempervivum clade, but chloroplast and nuclear data gave trees with different topologies. De la Cruz-López et al. (2019) examined relationships within Echeveria and related genera from Mexico, finding that Pachyphytum was sister to three clades including both Echeveria and genera like Graptopetalum and Reidmorania - and there was also the odd species of Sedum around. A recent study by Messerschmid et al. (2020) used ITS and three chloroplast markers taken from 298 species of Crassulaceae and included 148/ca 470 species of Sedum, these representing 144/186 of the infrageneric taxa into which species of Sedum have been placed; they also recovered different topologies when nuclear and chloroplast data were analyzed.
Classification. The main groupings in the family seem stable, although what they have been called varies (see Eggli et al. 1995). Within Sempervivoideae, generic limits are unclear (an understatement, e.g. Eggli et al. 1995), Van Ham and t'Hart (1998: p. 123) noting that "Sedum is generally considered as a hold-all taxon that encompasses the least derived Crassulaceae as well as homoplastic or transitional phenotypes to nearly every other genus of the family". Furthermore, many genera, some previously placed in what were considered to be different subfamilies, e.g. Sedoideae and Echeverioideae, hybridise (e.g. Uhl 1976; 't Hart et al. 1999). t'Hart (1995) and Thiede and Eggli (2006) provide a guide through the chaos, and although the latter prefer to retain a paraphyletic Sedum pro tempore, the genus will have to be dismembered or its circumscription much enlarged. The extremes are a Sedum that is monotypic or that encompasses almost two thirds of the whole family; Klein (in Kadereit et al. 2016) is inclining towards the former solution and Messerschmid et al. (2020) towards the latter. In the generic synonymy here a rather broadly-circumscribed Sedum is recognized, and depending on the generic circumscription here, the recognition of nothogenera may be an option (G. F. Smith et al. 2018). See also Holtum (2023) for generic groupings within Sempervivoideae. Smith and Figueiredo (2018) provide an infrageneric classification for Kalanchoe.
Previous Relationships. Crassulaceae have been linked with Rosaceae and Podostemaceae (Rombach 1911) and also Ranales and Saxifragales old style (Subramanyam 1962) because of embryological similarities.
[Aphanopetalaceae [Tetracarpaeaceae [Penthoraceae + Haloragaceae]]]: ?nodes 1:1; fruit indehiscent.
Age. The age for this node is estimated to be (92-)82.5(-75.7) Ma (L.-Y. Chen et al. 2014a) or some 71.3 Ma (Tank et al. 2015: Table S2).
APHANOPETALACEAE Doweld - Aphanopetalum Endlicher - Back to Saxifragales
Scrambling shrub; chemistry?; vessel elements with scalariform perforation plates; pericyclic fibres 0; petiole with 3 (1) bundles; leaves opposite, lamina margin serrate, teeth with a single vein and a distinct dark apex, or entire, colleters in stipular position ; inflorescence axillary, or flowers solitary; hypanthium short; K large, petal-like, C 0/reduced, linear; pollen with rugulate-stellate surface; ?nectary; G seminferior, opposite C, style single, with four canals, branches short; ovule 1/carpel, apical, pendulous, apotropous, micropyle bistomal, outer integument ca 8 cells across, inner integument ca 2 cells across, parietal tissue ca 4 cells across; archesporium multicellular; fruit a nut, K enlarging; seed 1; seed coat?; endosperm development?, embryo curved, size?; n = x = ?
1 [list]/2. W. and E. Australia (map: from Australia's Virtual Herbarium [outliers omitted] i.2014). [Photo - Flower.]
Age. The two species may have diverged (19.6-)11(-4.7) Ma (L.-Y. Chen et al. 2014a) or ca 10 Ma (Gu et al. 2022).
Chemistry, Morphology, etc.. The ray parenchyma stores starch. Two small bundles soon diverge from the main leaf trace. It is unclear if the stipules "are" stipules or colleters (Dickison 1980b; Kubitzki 2006b).
As in Saxifragaceae and Iteaceae, the vascular trace in the petal plane gives a branch to the lateral sepal position, also carpel wall and lateral carpel traces and a single stamen trace; the trace in the sepal plane supplies the carpel wall and median carpel bundle and provides a stamen bundle.
Some information is taken from Kubitzki (2006b: general), Jensen (1968: vascular system), Dickison (1980b: nodal anatomy), Dickison et al. (1994: anatomy), Bensel and Palser (1975b: floral anatomy), and Mauritzon (1939a: embryology).
Previous Relationships. Aphanopetalum used to be included in Cunoniaceae (Cronquist 1981), latterly as a separate subfamily (Takhtajan 1997).
[Tetracarpaeaceae [Penthoraceae + Haloragaceae]]: nectary 0 [to confirm].
Age. The age of this node is some (72-)61, 58(-46) Ma (Bell et al. 2010), (62-)58, 53(-49) Ma (Wikström et al. 2001), or (85.3-)78.1(-72.9) Ma (L.-Y. Chen et al. 2014a).
Classification. Although combination of these three rather small families was an option in A.P.G. II (2003), there seems to be little or nothing holding them together morphologically (see also Moody & Les 2007) and they are kept separate in A.P.G. III (2009).
TETRACARPAEACEAE Nakai - Tetracarpaea tasmannica Hooker - Back to Saxifragales
Evergreen shrub; chemistry?; plant glabrous; leaves spiral, petiole short; inflorescence terminal, racemose; (flowers 5-merous), floral apex convex; C spatulate; A 4-8, if 4, opposite K, anthers with basal pits?, dehiscing by somewhat elongated apical pores, fibrous endothecium 0; G free (basally connate), 4 (5), opposite C, styluli short, stigma not expanded; ovules many/carpel, micropyle exostomal, parietal tissue ca 4 cells across; fruit a follicle; "testa" ca 4 cells across, cells ± elongated longitudinally, cuticle well developed, no mechanical layer; embryo small; n = x = ?
1 [list]/1. Australia, Tasmania only. [Photo - Habit.]
Age. Gu et al. (2022) mention that the crown age of Tetracarpaeaceae is ca 41.7 Ma and the stem age is ca 83.5 Ma.
Chemistry, Morphology, etc.. The lamina teeth are perhaps hydathodal, but there are no water pores. The ovary is apparently secondarily superior (D. Soltis et al. 2003b). It is unclear whether the seed coat is testal or testal + tegmic; Takhtajan (2009) described the ovules as being unitegmic.
See Kubitzki (2006b: general), Hils et al. (1988) and Gornall et al. (1998: as Escalloniaceae), both anatomy, and Mauritzon (1933: ovules) for information.
Previous Relationships. Tetracarpaea used to be included in Grossulariaceae (Cronquist 1981), i.e., it was thought to be a woody saxifrage.
[Penthoraceae + Haloragaceae]: ± herbaceous; embryo long.
Age. The age of this node is estimated at (63-)51, 48(-35) Ma (Bell et al. 2010), (51-)47, 43(-39) Ma (Wikström et al. 2001), (74.4-)71.3(-70.1) Ma (L.-Y. Chen et al. 2014a), or around 54.5 Ma (Tank et al. 2015: Table S2).
PENTHORACEAE Britton, nom. cons. - Penthorum L. - Back to Saxifragales
Rhizomatous herbs; flavonoids +, flavones, myricetin, non-hydrolysable tannins 0; cork ?; young stem with pseudosiphonostele; endodermis?; pericyclic fibers 0; leaves spiral, lamina amphistomatic, vernation supervolute, teeth hydathodal, colleters +; inflorescence terminal, branched, units monochasial cymes; flowers 5-7-merous, hypanthium +; C 0(-7); A lacking a basal pit, latrorse; pollen oblate; ?nectary; G [5-8], half inferior, opposite sepals, apical parts free, becoming superior, placentae intrusive, styluli submarginal, stigmas capitate; ovules many/carpel, micropyle?, funicles long; fruit with free part of each carpel basally circumscissile; exotestal cells with outer wall ± thickened, papillate, micropylar operculum endostomal, tegmen otherwise crushed; seeds minute, first endosperm division very asymmetrical, cell at end of suspensor large; n = 8 (9), x = 8.
1 [list]/2. East and South East Asia, E. North America. Map: from Hong (1993) and FoC vol. 8 (2001). [Photo - Penthorum Inflorescence.]
Age. Crown-group Penthoraceae are only 6.5-2.4 Ma (Thiede 2006 for references), (5.8-)2.9(-1) Ma (L.-Y. Chen et al. 2014a), 12.6-4.2 Ma (N. S. Lee et al. 1996) or ca 8.5 Ma (Gu et al. 2022).
Evolution: Divergence & Distribution. The E. North American/East Asian disjunction is dated to a mere 6.5-2.4 Ma (Thiede 2006 for references).
Chemistry, Morphology, etc.. Cork in the root is initiated in a superficial position (van Tieghem 1899).
The sepals are unequal in size and the bracts are lateral to the pedicels. There is a much-enlarged but non-dividing micropylar cell in the embryo suspensor - c.f. Haloragaceae and their haustorial suspensor. Danilova (1996) shows the carpels as opposite to the calyx. The first two pairs of seedling leaves are opposite.
See Spongberg (1972: as Saxifragaceae) and Thiede (2006), both general, Haskins and Hayden (1987) and Gornall (1998: as Saxifragaceae), both anatomy, Mauritzon (1933: endosperm), and Nemirovich-Danchenko (1994b: seeds) for information.
Previous Relationships. Penthorum used to be included in Saxifragaceae (Cronquist 1981).
HALORAGACEAE R. Brown, nom. cons. - Back to Saxifragales
Flavones +, flavonols 0; cork ?; often calcium oxalate crystals in hair-like cortical cells; cuticle waxes 0 (parallel platelets); leaves opposite (spiral), lamina (deeply pinnately lobed), vernation conduplicate-flat, colleters +?; inflorescence ± determinate, (branches dichasial cymes)/fasciculate/flowers solitary; flowers small [petals 4> mm long], 4-merous; K valvate, C deciduous; A (= and opposite K), anthers much longer than filaments, (apiculate), basal pits?; pollen grains tricellular, 4-6 colpate, tectum distantly verruculate, otherwise smooth and with minute pores; ovary inferior, (sub 1-locular), opposite petals or odd member adaxial, styluli (0), with swollen bases, stigmas capitate or not, penicillate, dry; ovule 1/carpel [fertile], apical, pendulous, apo(epi-)tropous; fruit often indehiscent; exotesta (and hypodermal layer) persistent, thin-walled, rest obliterated; endosperm starchy, (nuclear), haustorial suspensor +, embryo (short), cotyledons rather short; x = 7, nuclear genome [1C] (0.047-)0.411(-3.6) pg.
9 [list]/145 - two groups below. World-wide, but especially Australia.
Age. Crown-group Haloragaceae are estimated to be (56.3-)47.1(-37.3) Ma (L.-Y. Chen et al. 2014a).
Hernández-Castillo and Cevallos-Ferriz (1999) described Tarahumara sophiae, from Mexico in deposits ca 70 Ma, as having four carpels free from one another but adnate to a hypanthial wall, while its fruit is described as being drupe-like, with one seed per carpel. Although perhaps assignable to this part of the tree, its morphology is unlike that of any extant Haloragaceae (see also Friis et al. 2011 for fossils). It is older than most estimates for stem-group Haloragaceae.
1. Glischrocaryon Endlicher
Herbs with woody rootstock to small trees; flowers perfect; whole flower (inflorescence) brightly coloured; (petals to 4 cm long); pollen apertures crassimarginate; ?embryology; fruit winged, 1-seeded; n = 7.
1-2/9. Southern and Western Australia, Northern Territory.
Age. The crown-group age of this clade may be some (14.8-)10.3(-6.4) Ma (Chen et al. 2014).
2. Halorageae Bartling —— Synonymy: Cercodiaceae Jussieu, Myriophyllaceae Schultz-Schultzenstein
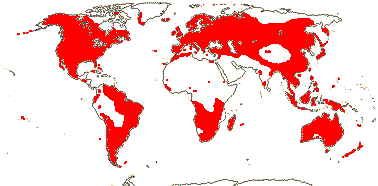
Aquatic (ephemeral) herbs to small shrubs; stem with endodermis [Myriophyllum - M.]; plant monoecious (dioecious), or flowers perfect; K (0), C (0), (hooded); pollen 4-6(-20)-aperturate, (porate), apertures tenuimarginate; (ovules 2/carpel); (integuments free only towards the apex - M.), outer integument 2-3 cells across, inner integument ca 2 cells across, parietal tissue 2-3 cells across, nucellar cap +/0, postament +, funicular obturator +, poorly developed; embryo sac with antipodal cells persistent; fruit nut-like, 1-4-seeded, (schizocarp), exocarp often ornamented; haustorial suspensor + [terminal cells divides longitudinally, the two cells becoming massive, nuclei huge]; n = 6, 7 (8).
7/136: Myriophyllum (60), Gonocarpus (41), Haloragis (28). Largely Australian, but Proserpinaca and some Myriophyllum making the distribution more or less cosmopolitan (map: from van Steenis 1962; Hultén 1958, 1971; van der Meijden 1971; Wood 1972; Orchard 1981; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Chen et al. 2014). [Photo - Collection.]
Age. The crown-group age of this clade is estimated to be (50.9-)42(-33.5) Ma (L.-Y. Chen et al. 2014a).
Evolution: Divergence & Distribution. Proserpinaca fossils are common in Europe and Asia, but the genus is now restricted to the New World, where it is scattered (L.-Y. Chen et al. 2014a: Fig. 1).
The family may have an Australian origin (e.g. Moody & Les 2007), and there has since been substantial dispersal, especially of the aquatic taxa (L.-Y. Chen et al. 2014a, q.v. for more details and dates). For the suggested ages of some intercontinental disjunctions within Haloragaceae, see Les et al. (2003). Moody and Garcia (2021) examine diversification in Gonocarpus which may have begun some 22 Ma, but that in S.W. Australia, for example, is Late Miocene, probably in response to aridification then.
Ecology & Physiology. The aquatic habit has evolved at least twice in the family (L.-Y. Chen et al. 2014a). There is absorbotrophic mixotrophy, in which the plants obtain some of their carbohydrates (for example) from the water, in Myriophyllum, as in some other aquatics (see Firmin et al. 2022 for references).
Pollination Biology. In some species of Glischrocaryon the whole inflorescence is coloured. However, details of its pollination are unknown; wind pollination is likely in other Haloragaceae.
Birds are the likely dispersal agents, especially of the aquatic taxa (L.-Y. Chen et al. 2014a).
Chemistry, Morphology, etc.. Nodal anatomy was observed in Haloragis erecta and Laurembergia, vernation in the first. Pelargonidin occurs in leaves, as in Saxifragaceae (Doyle & Sogin 1988). Adventitious roots arise between the leaves in Haloragis.
Trihaloragis has flowers in which all whorls are trimerous, very unusual in eudicots; Moody and Les (2007) point out the extensive variation in floral merosity in the family. Myriophyllum appears to have endostomal ovules, at least initially, but they become bistomal as embryo sac development proceeds (Batygina et al. 1985). Bawa (1969, 1970) noted that the archesporial cell was hypodermal, i.e. the ovule is tenuinucellate/there is no parietal tissue, although there come to be two cell layers above the embryo sac. Nijalingappa (1975) described the embryo sac of Haloragis micrantha as having a hypostase; there is definitely a postament. Corner (1976) described the endosperm as being starchy.
For general information, see Orchard (1975: Antipodean taxa, inc. much detail about floral anatomy, etc.), Orchard (1990; Australian taxa), and Kubitzki (2006b); for other information, see Orchard and Keighery (1993: Meziella), Praglowski (1970: pollen) and Kapil and Bala Bawa (1968), Bawa (1969), Nagaraj and Nijalingappa (1974) and Nijalingappa (1975), etc. (all embryology).
Phylogeny. Moody and Les (2001, 2004 and especially 2007) discuss relationships within the family; in the latter study nuclear ITS and chloroplast genes were found to be in some conflict - thus in the ITS tree [Haloragis + Proserpinacea clade were sister to the rest of the family, while in the plastome tree the [Glischrocaryon + Haloragodendron] clade - the plants are woody - were sister to other Haloragaceae (Moody & Les 2007; see also L.-Y. Chen et al. 2014a). The reciprocal monophyly of the two latter genera was not certain, and the latter genus has now been synonymized under the former. The rest of the family forms a single clade, but relationships within it are uncertain, Meionectes and Proserpinaca in particular not having well-supported positions (Moody & Les 2007); the latter two genera have been found to be sister taxa, the combined clade being sister to the remainder (L.-Y. Chen et al. 2014a). The trimerous Trihaloragis is sister to all other members of this clade (Moody & Les 2007). For the phylogeny of Haloraghis (and the disappearance of Meziella) see Moody and Les (2009), and for that of Gonocarpus, where G. eremophilus, from northern West Australia, is sister to the rest of the genus, see Moody and Garcia (2021).
Previous Relationships. The monotypic Haloragales were placed near Saxifragales by Takhtajan (1997) or linked with Gunneraceae and placed next to Myrtales, as by Cronquist (1981). Historically Haloragaceae and Gunneraceae have often been associated, although their pollen is quite different (e.g. Praglowski 1970), the perianth of Gunneraceae is not differentiated into sepals and petals, etc..
[Iteaceae [Grossulariaceae + Saxifragaceae]]: (vessel elements with scalariform perforation plates); leaves spiral; inflorescence terminal; hypanthium +; stamens = and opposite K; nectary ± gynoecial; ovules many/carpel; fruit a septicidal capsule.
Age. The age of this node may be (86-)81, 73(-68)) Ma (Wikström et al. 2001: c.f. topology), somewhere between (103.3-)96, 87.6(-81.3) Ma (Jian et al. 2008: other estimates), ca 84.8 Ma (Tank et al. 2015: Table S2) or (98.3-)91.6(-89.1) Ma (Ebersbach et al. 2017a). Silvestro et al. (2020) estimate the time-of-origin of Iteaceae to be ca 130.6 Ma.
The fossil Divisestylus brevistamineus, from Late Cretaceous rocks some 90 Ma in New Jersey, is perhaps to be placed somewhere around here. It has only five stamens and they are opposite the sepals, an inferior ovary and in particular it has fused stigmas yet separate styles - just like Itea. However, the pollen is tricolpate and striate, suggesting that if Divisestylus is to be placed around here, it is stem-group Itea - indeed, cladistic analyses of morphological data placed it with Iteaceae, but usually outside [Itea + Pterostemon] (Hermsen et al. 2003; see also Friis et al. 2011; López-Martínez et al. 2023a).
Evolution: Divergence & Distribution. The peltate glandular hairs of Pterostemon are similar to those in Grossulariaceae and so could perhaps be optimized to this node.
Chemistry, Morphology, etc.. Nectaries are common here, and are often associated with the gynoecium, although in Itea and Ribes, for example, they extend to the hypanthium, and in some Saxifragaceae to the receptacle (Erbar 2014 and references).
ITEACEAE J. Agardh, nom. cons. - Back to Saxifragales
C-glycosylflavones +; K valvate; anther connective with apical protrusion; placentation axile, style well developed; micropyle?; endosperm slight, ?type, x = 11.
2 [list]/21. Rather scattered, warm temperate to tropical.
Age. The age of crown Iteaceae is (51-)46, 39(-34) Ma (Wikström et al. 2001) or ca 63.9 Ma (Gu et al. 2022).
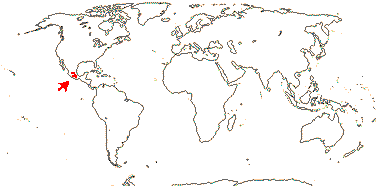
1. Pterostemon Schauer —— Synonymy: Pterostemonaceae Small, nom. cons.
Shrubs; ?chemistry; ?nodes; unicellular hairs with rough walls, glandular hairs +, conical to peltate; lamina vernation subpalmate, stipules cauline, minute; inflorescence a corymbose cyme; bracteoles +, hypanthium short; C shortly clawed or not; A 5, opposite K, anthers ?dorsifixed, connective apiculate, filaments flattened, apex of each wing ± toothed, staminodes 5, opposite C; pollen oblate (to subspherical); nectary 0; G [5(-6)], largely inferior, orientation?, stigmas (± radiating), capitate, ?type; ovules 4-8/carpel, ascending, apotropous, parietal tissue ca 8 cells across; fruit indehiscent, 1(-2) seeded, C also persistent; seed coat "cartilaginous", exotegmen "tristratified"; n = ?
1/3. Mexico. Map: from N. Smith et al. (2004).
2. Itea L.
Trees to shrubs; allitol +, flavonols, ellagic acid 0; hairs unicellular only; young stem with separate bundles; pith chambered; stomata paracytic; lamina vernation conduplicate, margins spiny- or gland-toothed, stipules small, on petiole base or adjacent stem; inflorescence (branched) racemose; flowers rather small; C valvate; anthers dorsifixed, connective globular; pollen grains oblate, bilateally symmetric, heteropolar, 2-porate, ektexine homogeneous; G [2] to subinferior, styles postgenitally fused at least at the stigma, stigma punctate-lobed, wet; parietal tissue 3-4(?-7) cells across; exotestal cells with outer walls thickened; (endosperm moderate - Itea rhamnoides), cotyledons incumbent; n = 11.
1/18. South East Asia to W. Malesia, E. North America, E. and S. Africa. Map: from Mai (1985), Aubréville (1974a), Coates Palgrave (2002), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) . [Photo - Itea Flower.]
Age. The distinctive pollen of Itea is reported from Eocene West North America in deposits 59-42 Ma (no illustrations), and somewhat later, 35.7 Ma, from Western Europe (Hermsen et al. 2013), while leaves are known fossil from Yunnan 32-30 Ma (Tian et al. 2021).
Chemistry, Morphology, etc.. Itea: For the chemistry of Itea, see Bohm et al. (1999). Pterostemon also has flavones, in this being like other Saxifragales. Choristylis (= Itea) lacks axial parenchyma.
The inflorescences of Itea may be terminal on rather short shoots. Its ovules are sometimes described as being unitegmic, with the integument 6-7 cells across, but this appears to be incorrect (Kubitzki 2006b). Although the carpels are free initially, they become connate, even along the style (Ge et al. 2002), so the styles are perhaps more accurately described as being postgenitally connate styuli.
For additional information, see Spongberg (1972), Gornall et al. (1998), and Kubitzki (2006b), all general, Ramamonjiarisoa (1980) and Gornall et al. (1998: as Escalloniaceae), both anatomy, Bensel and Palser (1975b) and Ge et al. (2002), both floral anatomy and development, and Mauritzon (1933: ovules).
Pterostemon: The pericyclic fibres of Pterostemon seem to be weakly developed and the androecium is obdiplostemonous.
For more information, see Goldberg (1986), Wilkinson (1994, 1998) and especially Kubitzki (2006b) and Guzmán et al. (2013), but Pterostemon is not well known.
Classification. Combination of Iteaceae and Pterostemonaceae was optional (as Iteaceae s.l.) in A.P.G. II (2003), and a broadened circumscription was formally adopted by A.P.G. III (2009).
[Grossulariaceae + Saxifragaceae]: glandular hairs +; petiole base broad, ensheathing ca 1/2 stem or more, lamina with secondary veins ± palmate; G [2]; (postament +); endosperm ± cellular, first division asymmetrical [chalazal chamber small], (micropylar chamber nuclear); germination epigeal, cotyledons expanded.
Age. Estimates of the age of this node are ca 75.8 Ma (Tank et al. 2015: Table S2), (93-)84(-74) Ma (Ebersbach et al. 2017a), 91.3-71.0 Ma (Stubbs et al. 2020a) and ca 85.7 Ma (Gu et al. 2022).
Tylerianthus crossmanensis, ca 90 Ma from the Upper Cretaceous of New Jersey, has been compared with Grossulariaceae, although it does not have a hypanthium (Friis et al. 2011 for references), and with Saxifragaceae s. str. (and also Parnassia, Gandolfo et al. 1998b). The analyses of López-Martínez et al. (2023a: Table 3) always placed it in Saxifragales, but always with other groups that pretty much spanned the "dicots" - see also Hydrangeaceae. In the angiosperm-wide analysis recently carried out by Schönenberger et al. (2020) the single maximum-parsimony position obtained was in crown-group Saxifragaceae.
Chemistry, Morphology, etc.. For endosperm development, see Gaümann (1919) and Dahlgren (1930).
GROSSULARIACEAE de Candolle, nom. cons. - Ribes L. —— Synonymy: Ribesiaceae Marquis
- Back to Saxifragales
Shrubs; non cyanogenic β- and γ-hydroxynitrile glucosides; cork cambium outer cortical/pericyclic; underground stems with endodermis; pericyclic fibres 0; axial parenchyma 0; petiole bundles ± connate; lamina vernation conduplicate-plicate, margins also lobed, some teeth hydathodal, leaf base with thin margins, (paired prickles at the nodes); (plant dioecious); inflorescence axillary, leafy below, racemose; pedicels articulated, bracteole single; (flowers 4-merous), hypanthium forming an obvious tube, nectary at base, or hypanthium very short, flowers rotate; C small, aestivation open; (A 4, 10), tapetal cells binucleate, (staminodes +); pollen pantoporate/4-7 colp(di)orate, with distinctively rugose ectoapertures, tectum complete; ovary inferior, carpels usu. superposed, nectary on top of G, placentation parietal, style single, long, stigma capitate, wet; ovules with exostomal micropyle, outer integument 3-5 cells across, inner integument ca 2 cells across, parietal tissue ca 3 cells across, nucellar cap 0, postament +, hypostase +; fruit baccate; seeds hard, arillate, exotestal cells palisade, mucilaginous, then a layer 3-6 cells across, endotestal cells crystalliferous, radial and inner walls lignified, ?tegmen cells elongated, tanniniferous; endosperm hemicellulosic, embryo short to long, cotyledons accumbent; n = 8, x = 8, chromosomes 1.5-2.5 µm long, nuclear genome [1 C] (0.141-)0.969(-6.682) pg.
1 [list]/150. Temperate N. hemisphere, also along the Andes (map: from Hultén 1968; Hultén & Fries 1986; Jalas et al. 1999; Fl. China 8. 2001; Malyschev & Peschkova 2004). [Photos - Collection.]
Age. Gu et al. (2022) estimate the crown-group age of Grossulariaceae to be ca 20.7 Ma.
For Tylerianthus crossmanensis, ca 90 Ma, see above and also Hydrangeaceae.
Evolution: Divergence & Distribution. The genus probably originated in North America (references in Ebersbach et al. 2017a); Schultheis and Donoghue (2004) suggested that within subgenus Grossularia there had been movement from west North America to eastern Asia.
Pollination Biology & Seed Dispersal. For the pollination of Ribes speciosum by the migratory hummingbird Calypte anna, see Abrahamczyk et al. (2017). Goldberg et al. (2017) discuss the evolution of breeding systems in the genus.
The fruits of Ribes are an important food for Andean frugivorous birds (Weigend 2006).
Plant-Animal Interactions. Several species of insects have been recorded as being herbivorous on Ribes (Weigend 2006).
Plant-Bacterial/Fungal Associations. A number of fungi, including the telial stage of the economically very important white pine blister rust (the basidiomycete Cronartium ribicola), spend part of their life cycle on the plants of this genus. In some places in North America attempts - largely unsuccessful - have been made to eradicate Ribes so as to disrupt the life cycle of this damaging fungus.
Chemistry, Morphology, etc.. For non-cyanogenic β- and γ-hydroxynitrile glucosides, which are found along with cyanogenic α-hydroxynitrile glucosides, see Bjarnholt et al. (2008). Stem collenchyma is well developed.
Nectar glands on the filaments occur in some species (Weigend 2006). There is considerable variation in pollen morphology, and Ribes divaricatum has pentacolpo-diorate grains (Weigend 2006; see also Gavrilova & Tikhonova 2019). The stylar bundles are ventral carpellar (Saxena 1969). Wronska-Pilarek (2001) described a cellulose-pectinous layer surrounding the seed; it was ca 6 cells across and made up of thin-walled, elongated cells, perhaps tegmic/nucellar in origin. Wronska-Pilarek (2001) also thought that the aril sometimes developed from placental tissue.
Additional information is taken from Weigend (2006: general), Stern et al. (1970) and Gregory (1998), both anatomy, Klopfer (1969a, 1973) and Gelius (1967), floral morphology, and Mauritzon (1933) and Shamrov (1998), ovule/embryology.
Phylogeny. Spiny gooseberries (the old Grossularia) are well embedded in Ribes (Schultheis & Donoghue 2004). Weigend et al. (2002) found that R. aureum and R. odoratum (subgenus Symphyocalyx) were sister to the rest of the genus; a number of clades were well supported, but there was little support for the positions of branches along the spine of the tree. However, R. aureum and R. odoratum were embedded in the genus in the phylogeny provided by Messinger et al. (1999: chloroplast restriction sites) and the latter species was similarly positioned in that of Senters and Soltis (2003: ITS), but in both cases support was very weak.
Previous Relationships. Grossulariaceae as circumscribed by Cronquist (1981) are very heterogeneous, and include genera now placed in Phyllonomaceae and Escalloniaceae (both campanulids), Montiniaceae, Tribelaceae (both lamiids), and Tetracarpaeaceae, Iteaceae (including Pterostemonaceae), and Celastraceae (all rosids).
SAXIFRAGACEAE Jussieu, nom. cons. - Back to Saxifragales
Herbs; mycorrhizae 0 [?how common]; cork also pericyclic; young stem with separate vascular bundles; petiole bundles also annular (with medullary or adaxial bundles); hairs (uni-)multiseriate with multicellular glandular head; lamina vernation variable, (secondary veins pinnate), hydathodes +, (margins entire), colleters +; (hypanthium ± +); (C clawed); A 10; tapetal cells (1-)2-4-nucleate; nectary +, ± annular (0); G [2], superior to inferior, (free), carpels with 5 bundles, apical parts quite often free, orientation variable, placentation axile, styluli ± long, adaxially channeled, stigma spatulate to capitate, wet or dry, compitum 0 [?all]; outer and inner integuments ca 2 cells across, parietal tissue 2-6 cells across, nucellar cap +/0, (hypostase +); fruit also a follicle; exotestal cells with outer wall (radial walls) ± thickened, variously ornamented, inner pigmented layer +, (endotegmen crystalliferous); endosperm (helobial), moderate, embryo medium (large), cotyledons incumbent; radicle with anthocyanin; n = (5-)7(+), x = 8 (?9, ?7), nuclear genome [1 C] (0.041-)1.233(-36.865) pg; plastome rpl2 intron 0.
41[list, to tribes]/855 - ten tribes below. Mostly N. temperate and Arctic (S. temperate, some tropical mountains). Map: from Hultén (1958, 1971), Meusel et al. (1965), Fl. China vol. 8 (2001) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photos - Collection]
Age. The age of crown-group Saxifragaceae may be (50-)38(-26) Ma (Bell et al. 2010), (59-)54, 49(-44) Ma (Wikström et al. 2001), (46.1-)38.4(-31) Ma (Deng et al. 2014) or as much as (71.8-)61.8(-52) Ma (Ebersbach et al. 2017a, q.v. for other published dates, all much younger), 87.4-51.8 Ma (Stubbs et al. 2020a: c.f. S7 and S8) and ca 64.6 Ma (Gu et al. 2022).
For Tylerianthus crossmanensis, in ca 90 Ma deposits from the Upper Cretaceous, see above, but also Hydrangeaceae.
1. Cascadieae R. A. Folk & D. E. Soltis
(Plant annual); leaves cauline; (flowers monosymmetric), flowers often single, axillary; C not clawed; (A 6, 5 opposite K, 1 opposite C - Saxifragodes); testa tuberculate/echinate.
2/2. W. U.S.A., S. Chile (Tierra del Fuego, Patagonia).
Age. This clade is around 32.9 Ma (Folk et al. 2019).
2. Saxifrageae Dumortier - Saxifraga L.
Plant (annual), (rhixomes thin), (tusssock-forming, (stems woody); (root periderm producing ca 20 layers of phellem); (foliar druses - Irregulares); leaves (opposite), hydathodes on upper surface, (producing chalk); (axillary bulbils +); infloresences thyrse/cyme; flowers ((obliquely) monosymmetric), hypanthium ± 0; C (0), clawed; (A 5, 8); (pollen grains tricellular- Ciliatae), surface granular/striate, nexine ca 1/2 thickness of sexine; testa smooth to papillate, exotestal cells with bumps ["Zellwandhöcker"], anticlinal walls often sinuous; endosperm often helobial, suspensor (with large terminal cell); n = 5, etc..
1/561. Mostly Arctic and northern montane/alpine, Saxifraga magellanica and a few other species Andean, S. hederifolia in Ethiopia and S. gemmipara in Thailand.
Age. The age of crown-group Saxifrageae is (39.3-)30.8(-23.5 Ma (Deng et al. 2014), or about twice that age, (71.8-)61.8(-52) Ma (Ebersbach et al. 2017a).
Age. The crown-group age of this clade is (37.2-)30(-23.9) Ma (Deng et al. 2014) or 87.2-51.8 Ma (Stubbs et al. 2020a: S7 Ma - even crown-group Micranthes is about twice the age of the preceding estimate for the whole group...).
[Chrysosplenieae + Micrantheae]: ?
3. Chrysosplenieae Dumortier —— Synonymy: Chrysospleniaceae Berchtold & J. Presl
Rhizomes thin/thick; (nodes 1:1 [Chrysosplenium/Chr.]; C 0 [Chr.]; (splash cup dispersal); n = 4, 7-9 ["x = 11, 12"], 11
2/68: Chrysosplenium (66). Circumboreal-North Temperate, the Andes.
4. Micrantheae R. A. Folk & D. E. Soltis - Micranthes Haworth
(Rhizomes +, thin); nodes 1:2; hydathodes in leaf margin, not producing chalk; leaves with druses; inflorescence scapose, thyrse/cyme; flowers (monosymmetric), hypanthium ± 0; C clawed; placentation axile; ovules unitegmic; seeds longitudinally ribbed (ribs 0); exotesta with straight anticlinal walls; endosperm helobial; suspensor thin, (terminal cell large); n = 5, etc..
1/80. Circumboreal.
; flowers (3-10-merous); (hypanthium 0); K 0-10; pollen colpate, colporate, or 6-9-porate, exine often reticulate, nexine ca = thickness of sexine; nectary disc-like (0); G [(3-5)].
[Darmereae [Heuchereae [Leptarrheneae [Boykinieae, Saniculiphylleae, Astilbeae]]]]: ?
5. Darmereae R. A. Folk & D. E. Soltis
Rhizomes large, (scaly); (druses in leaves); leaves (pinnately/palmately compound - Rodgersia), stipules +; inflorescence (scapose), compound cymes, (bracts/bracteoles 0 - Bergenia); flowers 4-7-merous; C (0), clawed or not; A (= and opposite K), (6-8) (12-14); integument single, 4-6 cells across [Darmera]; seeds smooth (tuberculate/winged), (endotegmen thickened - Rodgersia); endosperm ± helobial [Bergenia]; n = (15) 17 (18),
6/19: Darmera (10). (Central) East Asia, (W. U.S.A.).
[Heuchereae [Leptarrheneae [Boykinieae, Saniculiphylleae, Astilbeae]]]: ?
6. Heuchereae R. A. Folk & D. E. Soltis —— Synonymy: Pectiantiaceae Rafinesque
Leaves ± palmately lobed, venation palmate, (stipules +); inflorescence raceme (indeterminate, thyrsoid); hypanthium +; C (linear (and with linear lobes)); A also 5, opposite K, (opposite C), (flower monosymmetric, C 4, A 3, opposite K, adaxial - Tolmeia); G [2 (-3, Lithophragma)], (styluli 0); placentation parietal; fruit (circumscissile), often a splash-cup: seeds black to brown, smooth to echinate or pitted; endotegmen thick-walled [Heuchera, Tolmeia]; x = 7.
16/83: Heuchera (43), Asimitellaria (11), Lithophragma (10). North America to Mexico, esp. western coastal, also east Asia.
[Leptarrheneae [Boykinieae, Saniculiphylleae, Astilbeae]]: ?
7. Leptarrheneae Schulze-Menz
Rhizomes thin; stipules 0; anthers bisporangiate, dehiscence apical; seeds smooth/ribbed; x = 7.
2/2. Pacific Northwest to the Aleutians, Japan, S.E. China.
[Boykinieae, Saniculiphylleae, Astilbeae]: rhizome scaly, secondarily thickened.
8. Boykinieae R. A. Folk & D. E. Soltis
Inflorescences cymes; C often clawd (linear); A often 5, opposite K; seeds tuberculate (reticulate, smooth).
8/19: Boykina (7). Western U.S.A. (especially) and Canada, some Japan and the Andes - Bolivia and Argentina). <)
9. Saniculiphylleae C. Y. Wu & T. C. Ku - Saniculiphyllum guangxiense C. Y. Wu & Ku
Stipules 0; inflorescence cymose; C clawed; A 5, opposite K; G [3-5]; seeds smooth.
1/1. China (E. Yunnan, W. Guangxi).
10. Astilbeae Schulze-Menz
Nodes multilacunar [Astilbe]; leaves ternately compound (simple, articulated - Saxifragopsis), stipules +; K with a single trace [Astilbe], C (0-)5, ovate to linear; A 10; seeds smoth/ribbed/wrinkled/striate.
2/19: Astilbe (18). Himalayas and southern Kurile Islands to New Guinea, eastern and western U.S.A..
Evolution: Divergence & Distribution. Deng et al. (2014) give dates for spilts in Saxifragaceae, while Ebersbach et al. (2017a) provide numerous times of divergences within Saxifraga in particular. See also the ages in Stubbs et al. (2020a: S7, S8); ages need to be sorted out.
Folk et al. (2019, 2021) discussed the distribution and ecology of the family in some detail; the family probably originated in North America under cold, alpine conditions and then moved into warmer, more mesic environments. Deng et al. (2014) suggested that Saxifragodes albowiana, from southern South America, was sister to Cascadia nuttallii, from the Pacific Northwest, the two diverging slightly over 20 Ma (Deng et al. 2014); c.f. the topology of Fig. 3, the dated DIVA tree, and Fig. 1; in the former [Saxifragodes + Cascadia] were sister to all other Heucheroideae, in the latter, they were part of the Astilbe et al. clade. Be that as it may, these two genera form the geographically somewhat unlikely Cascadieae.
Much speciation in Saxifraga section Ciliatae in particular is quite recent and is associated with the uplift of the Qinghai-Tibet plateau, and the ca 110 species of subsection Hirculoideae arose within the last two million years (Gao et al. 2015). In the comprehensive study by Ebersbach et al. (2017a), it was suggested that Saxifraga (and Saxifragaceae) originated in North America, although it is now particularly diverse in the Qinghai-Tibet area, with 200+ species, most in Saxifraga sections Ciliatae and Porphyrion. Most diversification in these clades has been dated to to (10-)7, 6(-4) Ma, and orogenesis of the Hengduan Mountains in particular is implicated (Ebersbach et al. 2017a). For diversification in section Ciliatae with its some 175 species, especially subsection Hirculoideae, see Ebersbach et al. (2017b, 2018); broad niches and a higher rate of niche evolution may have driven diversification in the largest clade of this section. Overall, a mixture of geographic factors and adaptations (see below) seem to have driven diversification here (Ebersbach et al. 2017b). Ebersbach et al. (2020, also below) noted that in section Porphyrion subsection Kabschia there has been the evolution of the cushion-habit and lime-secreting hydathodes; hybrid swarms occur in this section, and they may have been part of the process. Deng et al. (2014) suggested that Saxifraga bicuspidata, from the Tierra del Fuego area, diverged from the rest of the genus ca 22 Ma, while S. magellanica, from southern South America, is embedded within the otherwise European section Saxifraga (Ebersbach et al. 2017a). There is no simple story about diversification to tell here (Ebersbach et al. 2020).
It has been suggested that the lability of ovary position in Saxifragaceae (e.g. Klopfer 1972b) is connected with selection by insects that both pollinate the flower and lay eggs in the ovary at the same time (Soltis & Hufford 2002), since it seems that protected, i.e. inferior, ovaries would be favoured. Indeed, variation in ovary position within the family is extreme, occurring within genera and even between the different morphs of heterostylous flowers (e.g. Kuzoff et al. 1999, 2001; Soltis & Hufford 2002).
Ecology & Physiology. Despite being quite a small family, Saxifragaceae have about 100 species (largely Saxifraga) that are cushion plants adapted to cold and dry environments, often growing at high altitudes (Boucher et al. 2016b; Ebersbach et al. 2017a). Ebersbach et al. (2017b, see also 2020) noted particularly high diversification rates in section Porphyrion subsection Kabschia, and these seemed to be associated with the cushion habit and lime-secreting hydathodes that are common there - although the latter in particular are also found in species of section Ligulatae and have a less than perfect association with base-rich habitats. The only other family with a comparable number of cushion species, Caryophyllaceae, has three times as many species overall and cushion plants are more taxonomically scattered there.
Pollination Biology & Seed Dispersal. Mitella, along with a few other Saxifragaceae, is very largely pollinated by fungus gnats, and this association has evolved in parallel in the family, as in the Japanese Micranthes fusca (Mochizuki & Kawakita 2017). Flowers of these species are often more or less broadly saucer-shaped, purple in colour, and the petals have very narrow lobes (Okuyama et al. 2008) that are grasped by the pollinators as they land (Katsuhara et al. 2017). Cryptic species are being discovered in the fungus gnat-pollinated Mitella sect. Asimitellaria (= Asimitellaria: Okuyama & Kato 2009). Larvae of the fungus gnats Gnoriste and Boletina eat mosses and liverworts, not fungi, that grow with the plants the adults pollinate (Okuyama et al. 2018). Greya, a moth, has a close asociation with Lithophragma, a genus from western North America, the flowers of which the adult moth pollinates and the developing seeds of which its caterpillars eat - for further details, see Plant-Animal Interactions below.
The seeds of a number of forest-dwelling Saxifragaceae are dispersed by rain, whether by a splash-cup mechanism, as in Mitella and Chrysosplenium, or ballistically, the seeds being thrown from the fruit as it moves violently after being hit by a drop of water, as in Heuchera (see also Savile 1975 for dispersal mechanisms).
Plant-Animal Interactions. The moth Greya (Prodoxidae, a rather basal glossatan lepidopteran, and related to Tegeticula of yucca moth fame), is both a seed predator and pollinator of some Saxifragaceae (Segraves & Thompson 1999), i.e. it is an obligatory brood-site pollination mutualist. Its association with Lithophragma (Heuchereae) has been studied in considerable detail, clarifying the diversification of both the plant and the pollinator/seed parasite moth (Thompson 2005; Rich et al. 2008; Thompson et al. 2013, 2017). Although other insects may also pollinate the flowers, mostly in the northern parts of the range of the two partners (Pellmyr & Thompson 1996; Thompson et al. 2017), throughout most of their ranges plant (two clades of Lithophragma, ca 8 species: Thompson et al. 2013; Deng et al. 2014) and moth (two species of Greya) are mutually dependent, the moths living on (or in) Lithophragma flowers practically their entire lives (Thompson et al. 2017). The female moth pollinates the flower as it lays eggs in the ovary, and the caterpillar eats the developing seeds; Greya also obtains nectar from Lithophragma, but pollination does not occur then. This association is at least 5-10 Ma (Rich et al. 2008; Deng et al. 2015; Thompson et al. 2017), while Pellmyr and Leebens-Mack (1999) suggested that stem Greya are (102.9-)84.6(-66.3) Ma. There is great - and unusual, at least in taxa with comparable floral biologies - variation in floral scent in Lithophragma, and this varies sharply at levels from population on up (Friberg et al. 2013, 2019). J. M. Brown et al. (1997), Hembry and Althoff (2016) and Kawakita and Kato (2017f) and others review host-switching, diversification and coevolution here. See also Asparagaceae-Agavoideae, Ranunculaceae, Phyllanthaceae-Glochidion, etc., Moraceae-Ficus and Caryophyllaceae for similar pollinator-seed eater interactions.
In addition to Greya, caterpillars of Yponomeutoidea-Yponomeutini moths eat Saxifragaceae (Sohn et al. 2013). Parnassius (Papilionidae-Parnassiinae) caterpillars, particularly diverse in eastern Asia, eat Saxifragaceae (and Crassulaceae) (Simonsen et al. 2011; Condamine et al. 2011, 2018).
Plant-Bacterial/Fungal Associations. Short-cycle Puccinia rusts (Uredinales, basidiomycetes) are frequently found on Saxifragaceae (Savile 1975, 1979a, b).
Genes & Genomes. A genome duplication event ca 53.4 Ma characterises Heucheroideae (ORRPα), while the SASTβ event ca 57.9 Ma seems to be a family-level event (Landis et al. 2018).
Introgressive hybridisation in the Heuchera clade is extensive and there are various combinations of chloroplast and nuclear genomes. For example, the plastome of Mitella is found in some species of Heuchera, and although parents of the two genomes involved are now some 1,300 km apart this was not so in the Palaeocene (Folk et al. 2016, 2018b; Stull et al. 2023), and the plastome of Tellima is found in Mitella (see also D. Soltis et al. 1993; Soltis & Kuzoff 1995; Okuyama et al. 2013). For similar conflict within the Heuchera-Tiarella group, see Ding et al. (2019 and references). There has been hybridization in e.g.Saxifraga section Saxifraga, and over 2,000 hybrids are known horticulturally (Tkach et al. 2015b).
In Saxifraga s. str. the diploid chromosome number varies from 12-ca 200, in Micranthes from 10-120.
Chemistry, Morphology, etc.. The root periderm of alpine species of Saxifraga (sensu?) growing at high altitudes way produce 20 layers or more of phellem, perhaps protecting the roots from extreme temperature fluctuations (Serra et al. 2022, see Luhan 1952. The distribution of druses and acicular crystals is of systematic interest (Gornall 1987), however, only Saxifraga s.l. has been studied in detail. Over 50 vascular bundles may enter the petiole base in some taxa. Hydathodes are common.
Saxena (1973) suggested that the androecium of Saxifragaceae was not obdiplostemonous; from Gelius's (1967) description of androecial development, the relationship between the whorls can change during development. The distinction between striate and reticulate pollen in Saxifraga does not seem to be that great (Kaplan 1981). The stylar vascular supply is from dorsal and ventral carpellar bundles; the vascularization of the nectary is variable (Saxena 1973). In at least some species of Saxifraga, and in Astilbe and Rodgersia, the two carpels are oblique, and in the latter two genera this is associated with inverted floral orientation, the odd K being abaxial; many other taxa have superposed carpels (Eichler 1878; Engler 1930a; Eckert 1966). In Chrysosplenium one carpel is open, the other closed. There is much variation in endosperm development, some taxa having helobial endosperm, and there are intermediates (Mauritzon 1933; Kaplan 1981).
For general information, see Morf (1950) and Spongberg (1972); McGregor (2008) and Webb and Gornall (1989) provide well-illustrated summaries of the ornamentally important Micranthes and Saxifraga. Some details of vegetative anatomy are taken from Thouvenin (1890) and Gornall (1998), of venation from Z. Zhang et al. (2015b: focus on Saxifraga), of floral anatomy from Bensel and Palser (1975b), of floral morphology from Klopfer (e.g. 1968, 1970a, b, 1973), Klopfer and Ziesing (1971) and Ronse Decraene et al. (1998c: Chrysosplenium), of pollen from Z. Zhang et al. (2015a: mostly Saxifraga), of embryology from Pace (1912), Mauritzon (1933: much detail), Vignon-Fétré (1968) and Gornall (1989), and of seed morphology from Kaplan (1981: also pollen, etc., Saxifraga s.l.) and Knapp (1998). Given the history of the circumscription of Saxifragaceae, early references to it may contain information about several other families, too.
Phylogeny. There are two major clades in Saxifragaceae (Folk et al. 2019, 2021). One clade is almost entirely made up of Saxifraga s. str. which alone includes some 63% of the species in the family; for relationships, see Winkler et al. (2013: subsection Oppositifoliae, nuclear differentiation despite hybridization and plastid sharing), Gao et al. (2015), Tkach et al. (2015b) and Ebersbach et al. (2017a). In molecular phylogenies (and in horticulture!) there is evidence for hybridization in section Saxifraga (Tkach et al. 2015b). Sections [[Irregulares - it may have scapose inflorescences + monotypic Heterisia] [Saxifragella [Pseudocymbalaria ...]]] are sister to the rest of Saxifraga (Tkach et al. 2015b: detailed discussion). Two monotypic genera making up Cascadieae may form a clade sister to Saxifraga (Folk et al. 2021).
Most of the morphological variation in the family is in the other clade wich includes most of the genera (37/40), many very small, but only ca 37% of the species (Soltis et al. 2001; Xiang et al. 2012; Prieto et al. 2013; but see topologies in Stubbs et al. 2020a: c.f. S7, S8). Relationships here are becoming somewhat better resolved, with a topology of [Astilbe et al. [Heuchera et al. [Darmera et al. [Chrysosplenium et al. + Micranthes]]]] (Deng et al. 2014: Bayesian p.ps all 1.0 or close). Relationships in Tkach et al. (2015b) were somewhat different in detail, although these were not the focus of that paper, in particular, Cascadia and Saxifragodes, separately or together (= Casacadieae), were on long branches somewhere near the base of this part of the tree. Phylogenies obtained by Folk et al. (2019: Fig. S1, 301 protein-coding genes, ExaML tree, Fig. S2, PHLAWD, 24 loci) are the basis of the classification provided by Folk et al. al. (2021). The topologies of those trees is consistent with that used above, however, note that support values along the spine in the ExaML tree of the second clade tended to be rather low, while the tritomy [Boykinieae, Saniculiphylleae, Astilbeae] above was indeed shown as such in the PHLAWD tree (Folk et al. 2019). Mitella was polyphyletic in both trees, Peltoboykinia was separated by a long branch from the rest of Chrysosplenieae, [Micranthes holmiei + M. merkii] may be sister to the rest of the genus, etc. (Folk et al. 2019, which should be consulted for these and other details).
Micranthes used to be part of Saxifraga, but it is well embedded in this second clade and is not at all close to Saxifraga. The inflorescences of Saxifraga s. str. often have inflorescence bracts, however, those of Micranthes and Darmera, which also used to be in Saxifraga, are scapose, and there are also differences in pollen and testa surface between the latter two in particular. For relationships within Micranthes, see Tkach et al. (2015a: quite well resolved), Stubbs et al. (2020a, 2020b), the latter finding extensive hybridization within the genus (Stubbs et al. 2020a: comparison of nuclear and plastome phylogenies), and Folk et al. 2019: Figs S1, S2). Folk and Freudenstein (2014) looked at relationships in the Heuchera area; Mitella (see also Okuyama 2016) was polyphyletic.
Classification. Generic limits in the Heuchera clade were for some time unclear (Soltis et al. 1996 and refs; Okuyama et al. 2008). However, there has been estensive work on the family as a whole over the last 20 years or so, and for genera and tribes, see especially the discussion in Folk et al. (2021, based on Folk et al. 2019). For an infrageneric classification of Saxifraga, see Tkach et al. (2015b), who recognized 13 sections, of which two were divided into 9 subsections.
Brachycaulos simplicifolius may belong somewhere in Saxifragaceae (Mabberley 2017); it is provisionally included in Rosaceae below.
Previous Relationships. In the past, genera "intermediate" between what was thought to be a very variable Saxifragaceae and other families tended to be included in Saxifragaceae. It has long been recognized that this was because the inclusion of more genera in Saxifragaceae would have little effect on the family description since there was already so much variation included in it (e.g. Pace 1912). However, if placed in the more homogeneous Crassulaceae, for example, such genera would greatly affect the description of that family and hence make it less discrete (e.g. Penthorum). However, many tenuinucellate and unitegmic genera that used to be included in Saxifragaceae or Grossulariaceae have turned out to be entirely unrelated to each other. There was clearly a division between Saxifragaceae s. str. + Grossulariaceae s. str., with petals that remain very small for quite some time during development, and Hydrangeaceae (also classically placed in this area), with their relatively faster-developing petals, as is common in the asterids (Gelius 1967), and that flags a major separation. Of Saxifragaceae in the old sense, Parnassia is in Celastraceae-Celastrales (a conclusion in agreement with data from floral anatomy - e.g. Bensel & Palser 1975a, d), Francoaceae-Francoeae are in Geraniales, Vahlia is in Vahliales, a monogeneric order in the lamiids, and so on. However, the unitegmic Darmera is properly to be retained in Saxifragaceae (e.g. Gornall 1989).
Age. An age of (117-)100.2(-76) Ma was suggested for the clade [Cynomoriaceae [Paeoniaceae + Altingiaceae]] in Naumann et al. (2013), and there are stem ages of ca 67.2-65.8 Ma (Cusimano & Renner 2019: note spreads, some strict clock ages far older).
CYNOMORIACEAE Lindley, nom. cons. - Back to Saxifragales
Echlorophyllous, root parasite; tracheary elements with annular bands and simple perforations; sieve element plastids 0 [haustorium]; vascular bundles in the stem scattered; cork?; stomata +, ?morphology; plant glabrous; cauline scale leaves +; plant (polygamo)monoecious; inflorescence clavate-capitate, indeterminate, branches cymose; inflorescence bracts peltate, floral bracts recaulescent; flowers minute; P +, uniseriate, (0-)4-5(-8), basally connate or not; staminate flowers: A 1, introrse; pollen colporate; concave-cristate stylodium +; carpelate flowers: staminodia 0; G 1, inferior, style long, channeled down one side, with 2 vascular bundles, stigma not expanded, papillate; ovule single, apical, pendulous, straight/semianatropous, unitegmic, integument 5-8 cells across, parietal tissue 1-3 cells across; fruit an achene; seed minute [≤ 1.5 mm long]; testa ca 7 cells across, persistent, exotesta slightly developed, walls little thickened; endosperm cellular, copious, thick-walled, embryo undifferentiated; n = 14, size strongly bimodal; germination with a germ-tube like structure.
1 [list]/2. Mediterranean to C. Asia. Map: from Jalas & Suominen (1976), Jäger et al. (1985), Flora of China [approx.] 13 (2007) and Trop. Afr. Fl. Pl. Ecol. Distr. 5 (2010). Photo: Habit, © D. L. Nickrent.
Evolution: Ecology & Physiology. In the Mediterranean area the hosts of Cynomoriaceae are often members of Cistaceae or Amaranthaceae, also Plumbaginaceae, elsewhere Cynomorium parasitizes Amaranthaceae, Tamaricaceae, Nitrariaceae, etc. (Jäger at al. 1985). Fahmy and Hassan (2020) document contact between the tracheary elements of host and parasite, although this is uncommon; the sieve elements of the two show no continuity.
Pollination Biology & Seed Dispersal. Flowers of Cynomorium songaricum produce a variety of compounds that have carrion-type smells, and they are visited by by a variety of dipteran pollinators; there may also be wind pollination (D. Wang et al. 2020).
Genes & Genomes. Cusimano and Renner (2019) document successive horizontal gene transfers from host plants as Cynomorium moved east to west from Asia to the Canary Islands; Sapindales and Caryophyllales were the sources of the transferred genes.
Chemistry, Morphology, etc.. The root has root hairs. The stem seems to develop inside a cavity in the tuber-like haustorial structure attached to the host (Solms-Laubach 1867). Weddell (1860) specifically noted that he did not find stomata on foliar organs, and these structures will bear closer examination.
Perfect flowers are also known. There are a number of questions about the floral morphology of Cynomorium, but very few new data have appeared in the last 150 years. The perianth is less well developed in pistillate than in staminate flowers, and there is debate as to its morphological nature - is it perianth, or (partly) bracteate, and if the latter, what is the structure of the inflorescence? The distinctive concave-cristate stylodium lacks vascularization; it may be nectariferous. The pistillode in staminate flowers may be appear to be superior or inferior and the gynoecium in carpelate flowers has been drawn as being semi-superior (Hooker 1856; A. Li 2008). The style in pistillate flowers is channelled its entire length and has two vascular bundles, a rather odd combination; Hooker (1856) suggested that there are two carpels. It is unclear whether the ovule has parietal tissue, or whether the cells above the embryo sac represent a nucellar cap; the former is more likely.
For general information, see the Parasitic Plants website (Nickrent 1998 onwards), Heide-Jørgensen (2008), Nickrent (2020) and Weddell (1860), for some chemistry, see references in Z.-H. Zhang et al. (2009), for pollen nucleus number, see Hansen (1986), for ovule, etc., see Juel (1902: ?nucellar cap), for ovule and seed, see Teryokhin et al. (1975), Baskin and Baskin (2021) for seeds, etc, and for details of seed anatomy, see Takhtajan (2000).
Previous relationships. Cynomoriaceae have usually been included in Balanophoraceae or Balanophoranae (e.g. Cronquist 1968; Takhtajan 1997), or their position has seemed to be completely uncertain (see above).