EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] // CORE ASTERIDS // EUASTERIDS // GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID I / LAMIIDAE / [CARDIOPTERIDALES [GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]]: ?
[GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]: G [2], superposed; loss of introns 18-23 in RPB2 d copy. - check
[ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]: vessel elements with with simple perforation plates; nodes 1:1.
[[GENTIANALES + BORAGINALES] VAHLIALES, SOLANALES, LAMIALES] / CORE LAMIIDS: herbaceous habit widespread; (8-ring deoxyflavonols +); C forming a distinct tube, initiation late [sampling!]; A epipetalous; (vascularized) nectary at base of G; style long; several ovules/carpel; fruit a septicidal capsule, K persistent.
Evolution: Divergence & Distribution. For the complex patterns of variation in a number of characters in this part of the tree, see the Gentianales page.
SOLANALES Berchtold & J. Presl - Main Tree
O-methyl flavonols (flavones) +, myricetin 0; inflorescence terminal; K connate; A adnate to C; anther sacs with placentoids; pollen tube usu. with callose; endosperm development?, chalazal endosperm haustorium +. - 5 families, 165 genera, 3,945 species.
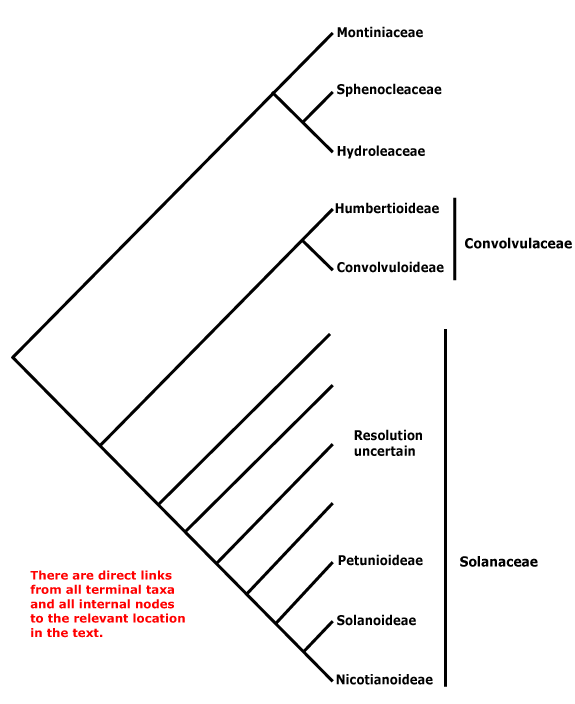
Phylogeny. For the circumscription and relationships of Solanales, see discussion under Gentianales. The contents of the order may change, with [Convolucaceae + Solanaceae] being all that remains.
Includes Convolvulaceae, Hydroleaceae, Montiniaceae, Solanaceae, Sphenocleaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Solanales may date from (82-)78, 76(-72) Ma (Wikström et al. 2001) or (102-)90(-75) Ma (Wikström et al. 2015); Bremer et al. (2004) date them to ca 100 Ma, Tank and Olmstead (2017) to (110.1-)93.6(-78.9) Ma, and Lemaire et al. (2011b) to (93-)71(-50) Ma, Nylinder et al. (2012: suppl.) suggest ages of around 87.7 and 85.4 Ma, Magallón et al. (2015) ages of around 79.2 Ma, Magallón and Castillo (2009) ca 73 Ma, C. Zhang et al. (2020) ca 106.6 Ma and Bell et al. (2010) suggest ages of (85-)76, 71(-62) Ma.
Evolution: Divergence & Distribution. For suggestions as to the evolution of features of the pollen, see L.-E Yang et al. (2020), but c.f. topology, etc..
Phylogeny. In part, this is unclear. Within Solanales, Montiniaceae were found to be sister to [Solanaceae + Convolvulaceae] (B. Bremer 1996, see also Soltis & Soltis 1997). D. Soltis et al. (2000) found strong support for the association of Montinia and Hydrolea; Sphenoclea was not included. With the inclusion of the latter and broader sampling (all three genera) in Montiniaceae, B. Bremer et al. (2002) found strong support for the association of Sphenoclea and Hydrolea, but only just above 50% for the association of Montiniaceae with that pair; support was stronger in Soltis et al. (2011); see also Refulio-Rodriguez and Olmstead (2014) and C. Zhang et al. (2020). the topology of the tree here follows that of these latter papers. However, it should not be forgotten that relationships in W. J. Baker et al. (2021) were very different, Montiniaceae and Hydroleaceae being separate clades in an extensive pectinate section below the core lamiids (Sphenocleaceae were not examined), while in Version 3, April 2023, Hydrolea was sister to Lamiales and relationships in Solanales are [Sphenocleaceae [Montiniaceae [Convolvulaceae + Solanaceae]]]. In the plastome phylogeny of H.-T. Li et al. (2021) relationships are [Lamiales [Vahliales + Solanales}, while in the Angiosperms353 analysis of Zuntini et al. (2024) they are [[Vahliales + Lamiales] [Solanales [Gentianales + Boraginales]]] - however, the content and arrangement of Solanales in the two is the same. See also the Seed Plant Tree version of ix.2024 where Vahliaceae are weakly supported as being sister to Solanales, Hydrolea is sister to Lamiales.
Synonymy: Cestrales Martius, Convolvulales Berchtold & J. Presl, Cuscutales Martius, Hydroleales Martius, Nolanales Lindley, Sphenocleales Doweld
[Montiniaceae [Sphenocleaceae + Hydroleaceae]]: route I secoiridoids +; petiole bundle(s) arcuate; stigma ± capitate.
Age. The age of this node is estimated to be (79-)65, 61(-47) Ma (Bell et al. 2010), ca 63.2 Ma (Tank et al. 2015), (71-)66, 65(-60) Ma (Wikström et al. 2001), ca 72 Ma (Magallón et al. 2015), (94-)78(-58) Ma (Wikström et al. 2015), (102.2-)83.2(-60.8) Ma (Tank & Olmstead pers. comm.) and ca 92 Ma (Bremer et al. (2004).
Evolution: Divergence & Distribution. Not a very diverse clade (Tank et al. 2015; Magallón et al. 2018).
The character "pits vestured" may be best placed at this node.
MONTINIACEAE Nakai - Back to Solanales —— Synonymy: Kaliphoraceae Takhtajan
Shrubs, trees (lianes); plants with a peppery smell; tannin slight; cambium storied or not; young stem with a vascular cylinder (separate bundles); (medullary bundles +); pericyclic fibres 0; crystal sand, acicular crystals and styloids usu. all +; nodes 1:1-11; petiole arc of (rounded) bundles (+ additional strands); axillary tuft of usu. uniseriate hairs at nodes; (stomata anisocytic - some Grevea); leaves also opposite; inflorescence cymose, (carpelate flowers single, terminal), bracteoles 0; flowers imperfect, small; K ± connate; C free [absolutely so - Montinia], imbricate/valvate; nectary +, vascularized; staminate flowers: 3-4(-5)-merous; A free, anthers basifixed/dorsifixed, becoming extrorse, filaments short; pollen grains (large), columellate-reticulate, reticulum smooth/with supratectal spinules, granular elements belween; pistillode minute; carpelate flowers: 4-5-merous; staminodes + (0); ovary (semi)inferior, placentation basal/intrusive parietal-subaxile, style short, stout, hollow, stigma 2 lobed/divided, styles separate, recurved. stigma decurrent [Kaliphora]; ovules 1 ascending/4-many per carpel, (campylotropous, apotropous - Kaliphora), integument 7-9 cells across, parietal tissue ca 1 cell across, chalazal end with projection [Montinia]; fruit a capsule; seeds winged, exotesta lignified, periclinal walls thickened, (adjacent wall of mesotesta also thickened [Montinia)]; or ?drupe, placentae at least initially fleshy; testa thin-walled, ± pulpy when wetted, exotesta not persistent [Grevea]; or drupe, 2-seeded [Kaliphora]; endosperm +/0, ?development, hemicellulosic, walls thick, layered, cotyledons accumbent, foliaceous; cotyledonary petioles connate [Montinia]; n = 16 [Kaliphora], 34 [Montinia], x = ?
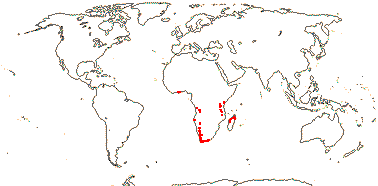
3 [list]/5. Africa and Madagascar (map: from Milne-Redhead & Metcalfe 1955; Verdcourt 1975; Bosser 1990; Brummit 2007: C. and W. Africa). [Photos - Kaliphora, Montinia Fruits © Serban Procheŝ.]
Age. The age of crown-group Montiniaceae is around 42 Ma (Bremer et al. (2004), (60-)40(-23) Ma (Wikström et al. 2015) or (63.6-)38(-14.3) Ma (Tank & Olmstead pers. comm.).
Chemistry, Morphology, etc.. Pericyclic fibres are poorly developed in Kaliphora, ?others; Grevea has vascular bundles in the pith. The axillary tufts of hairs are least well developed in Kaliphora. Kaliphora is anisophyllous, and the leaves are subopposite; successive leaves may be borne on the same side of the stem.
See Milne-Redhead and Metcalfe (1955) and Ronse de Craene (2016), both general, Hegnauer (1973, 1990, as Saxifragaceae) for chemistry, Dahlgren et al. (1977) for germination and iridoids, Ramamonjiarisoa (1980), Carlquist (1989), and Wangerin (1906), Gregory (1998) for vegetative anatomy, Ronse Decraene (1992) and Ronse Decraene et al. (2000a) for details of floral morphology, Hideux (1972) and Ferguson (1977: Kaliphora) for pollen, Mauritzon (1933: Montinia) for embryology, and Krach (1976, 1977) and Takhtajan and Trifonova (1999) for testa anatomy.
Phylogeny. Relationships are [Kaliphora [Grevea + Montinia]]; support is strong (B. Bremer et al. 2002), although the group is very heterogeneous.
Previous Relationships. Montiniaceae have been hard to place, and have generally ended up somewhere around Saxifragaceae s.l.. Thus Cronquist (1981) included them in his heterogeneous Grossulariaceae, while Takhtajan (1997) placed Montiniaceae and Kaliphoraceae next to each other in his Hydrangeales. The pollen seemed rather like that of Escalloniaceae to Hideux and Ferguson (1976).
[Sphenocleaceae + Hydroleaceae]: placentae swollen; ovules many/carpel; antipodal cells degenerate; endosperm at most scanty, with multicellular micropylar haustoria.
Age. The age for this node is estimated to be around (91-)64.5(-37.9) Ma (Tank & Olmstead pers. comm.), 56.5 Ma (Magallón et al. 2015) or 54.6 Ma (Tank et al. 2015: Table S1).
SPHENOCLEACEAE Baskerville - Sphenoclea Gaertner - Back to Solanales
Herbs, rather fleshy, annual; fructose with isokestose linkages, cyclic thiosulphinates [zeylanoxides] +, alkaloids 0; vestured pits 0; cork ?mid-cortical; cortical air spaces +; stomata tetracytic; inflorescences spicate; flowers small, ca 3 mm across; K imbricate, C quincuncial, tube formation early, lateral veins of adjacent lobes connate and commissural; tapetal cells binucleate; pollen grains tricellular; nectary 0; ovary ± inferior, placentae massive, style short, stigma subcapitate, wet; integument "massive", hypostase 0; synergids elongated; fruit capsular, capsule circumscissile; seeds tiny [ca 0.5 mm long]; exotestal cells polygonal, inner walls thickened and with radial spine-like processes; endosperm cell walls thick; n = 12, 16, 20, etc., x = 6 (?7, ?12).
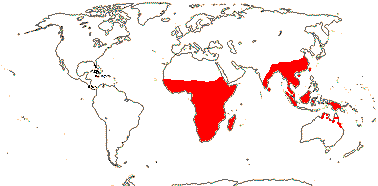
1 [list]/2. Old World tropics. Map: from Brummit (2007); but c.f. Australia's Virtual Herbarium consulted v.2013; esp. Carter et al. (2014). [Photo - Habit © B. Hammel]
Evolution: Ecology & Physiology. The cyclic thiosulphinates, and perhaps also things like secologanic acid, seem to be responsible for the potent allelopathic efect of Sphenoclea zeylanica, both on rice and other plants (Hirai et al. 2000 and references.
Chemistry, Morphology, etc.. Corolla tube formation is of the early type, and the corolla lobes are characteristically incurved; the lateral veins of adjacent lobes are fused producing commissural veins (Erbar 1995). The anticlinal walls of the testa are shown as being massively thickened in Takhtajan (2010).
Some information is taken from Subramanyam (1950b), Monod (1980), Carter et al. (2014) and Lammers (2016), all general, and Kausik and Subramanyam (1946) and Tobe and Morin (1996), both embryology.
Previous Relationships. Sphenocleaceae, along with Hydrolea, another genus of uncertain position, were placed near Boraginaceae by Cosner et al. (1994). However, in morphological studies (e.g. Gustafsson & Bremer 1995) Sphenocleaceae are placed well within Asterales; they have often been associated with Campanulaceae (e.g. they are placed in Campanulales by Takhtajan 1997: p. 408 - "it definitely belongs", see also Cronquist 1981), although they lack latex.
HYDROLEACEAE Edwards - Hydrolea L. - Back to Solanales —— Synonymy: Sagoneaceae Martynov
(Annual) herbs to shrubby; mycorrhizae 0; chemistry?; cork?; (vessel elements with scalariform perforation plates); cortex aerenchymatous; thorns +/0, axillary-sublateral; lamina margin toothed to entire; flowers 4-5-merous, medium sized [ca 1.5 cm across]; K basally connate, C connate, tube formation late; A versatile, filament base abruptly broadened/lobed; nectary 0/+; G diagonal, [2(-4)], placentae bilobed or not, styles separate, ± spreading, stigma slightly funneliform or capitate; ovules mostly pleurotropous, funicular bundle absent, integument 6-8 cells across; fruit a septi-(+ loculi-)cidal capsule, (irregularly dehiscent); seeds longitudinally ridged and ruminate, exotestal cells thin-walled, endotestal cells tanniniferous, with a cuticle; n = (9) 10 (12), x = 6 (?7, ?12).
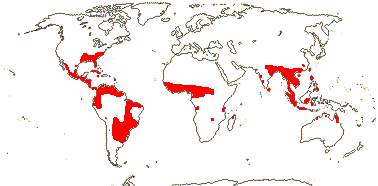
1 [list]/12. Tropical, warm temperate (map: from Davenport 1988; FloraBase 2007). [Photo - Hydrolea Flower © B. Kenney]
Evolution: Plant-Bacterial/Fungal Associations. Hydrolea appears to lack mycorrhizae.
Chemistry, Morphology, etc.. The axillary inflorescences may be cymose. Davenport (1988) suggested that there is no nectary disc. The two carpels are shown as being oblique by Schnizlein (1843-1870: fam. 147), and this was confirmed by Erbar et al. (2005), even for Hydrolea palustris, which has flowers with the median sepal abaxial. Indeed, Erbar et al. (2005) noted that there the first sepal arose in the adaxial-lateral position, the second was abaxial, an unusual sequence - other species in the genus need study. Di Fulvio (1997) notes that the four ventral bundles of the two carpels are all connate in the center of the ovary - c.f. Hydrophyllaceae/Nameae where there are often two or four such bundles, rarely a single bundle (di Fulvio 1997). There are no nuclear inclusions (di Fulvio 1991).
For general information, see Bittrich and Amaral (2016) and Davenport (1988: monograph), for wood anatomy, see Carlquist and Eckhart (1984), for pollen, see Constance and Chuang (1982), and for embryology Svensson (1925) and di Fulvio (1989b, 1990); also, check Boraginales/Hydrophyllaceae, since relevant literature - e.g., on vestured pits - may be there.
Previous Relationships. Hydrolea has usually been included in Hydrophyllaceae (e.g. Cronquist 1981; Takhtajan 1997). Not only molecular differences but also axile versus parietal placentation and embryological differences (see di Fulvio de Basso 1990) separate the two.
[Convolvulaceae + Solanaceae]: coumarins, caffeic acid esters, polyhydroxylated nortropane alkaloids [calystegines], acyl pyrrolidine, etc., ornithine-derived alkaloids [inc. hygrines], sesquiterpenoid phytalexins, flavonol and flavone glycosides, acylated anthocyanins +, condensed tannins, iridoids 0; internal phloem + [intraxylary phloem; bicollateral vascular bundles]; leaves with conduplicate vernation; flowers large [>1.5 cm across/long]; C-tube formation late, C contorted-plicate or induplicate-valvate; tapetal cells multinucleate; placentae massive, style undivided; ovules many/carpel, integument (5-)9-20(-40) cells across; K persistent in fruit; testa often multiplicative; young seeds starchy, endosperm haustoria 0, ?amount, cotyledons incumbent; plastome infA gene 0.
Age. The two families may have diverged (91-)86(-81) Ma (K. Bremer et al. 2004a), (96.9-)80.4(-60.9) Ma (Tank & Olmstead pers. comm.), ca 75.2 Ma (Hoshino et al. 2016), (89-)70(-49) Ma (Wikström et al. 2015), (71-)66, 65(-61) Ma (Wikström et al. 2001), ca 66.6 Ma (Magallón et al. 2015), around 64.3 Ma (Naumann et al. 2013), (83-)64(-49) Ma (da Silva et al. 2017), (69.7-)62.1(-54.4) Ma (Paape et al. 2008), (74-)62, 59(-49) Ma (Bell et al. 2010), (57.2-)52(-46.8) Ma (Magallón et al. 1999), ca 55 or (53.5-)49.2(-46) Ma (Särkinen et al. 2013, see also Dupin et al. 2017), ca 57.3 Ma (Nylinder et al. 2012: suppl.), or ca 56.2 Ma (Tank et al. 2015: Table S2) - or a mere ca 39.9 or 37.5 Ma (Xue et al. 2012). An age of over 150 Ma is suggested by Eserman et al. (2013: Convolvulaceae-Ipomoeeae the focus), and although the range bars are huge, they do not overlap with any of the other dates just mentioned except those in Bremer et al. (2004). See, however, Carruthers and Scotland (2020).
Evolution: Divergence & Distribution. Given the recent findings of Palaeocene Ipomoea from India (Srivastava et al. 2018) and of early Eocene Physalis from Argentina (Wilf et al. 2017a, see also Deanna et al. 2020), there are suggestions of an east Gondwanan origin of this clade (Srivastava et al. 2018); these fossils have been placed in clades that are well embedded in their respective families. Ages within Solanaceae in particular tend to be in conflict with the ages above.
Flowers with an oblique plane of symmetry may be an apomorphy at this level, or even higher, although J. Zhang and Zhang (2016) and Zhang et al. (2017) suggest that monosymmetry in Humbertia, sister to other Convolvulaceae and with a monosymmetric flower, develops differently from that in Solanaceae.
Eich (2008) provided an extensive summary of the distribution of secondary metabolites in these two families placed in the context of phylogeny.
Plant-Animal Interactions. Chrysomelidae-Cassidinae+Hispinae and -Criocerinae beetle larvae like members of this clade, especially Convolvulaceae (Schmitt 1988; Jolivet 1988; Buzzi 1994; Vencl & Morton 1999).
Chemistry, Morphology, etc.. Pyrrolizidine, tropane and pyrrolidine alkaloids, common in this clade, are all synthesised from an ornithine precursor (Hegnauer 1973; Dahlgren 1988). For calystegines, see Schimming et al. (2005). Gemeinholzer and Wink (2001) discuss the sporadic distribution of tropane alkaloids in Solanaceae; they are known from Schizanthus and other clades; Schimming et al. (1998) discussed the distribution of polyhydroxynortropanes, found in most Convolvulaceae, but not in Cuscutoideae, unknown in Humbertia, and scattered in Solanaceae. Putrescine N-methyltransferase, derived from the common spermidine synthases, is the first specific enzyme in the nicotine, nortropane and tropane alkaloid pathways; for more information on tropane alkaloid synthesis, see Y.-J. Wang et al. (2023) - that the smae metabolites are found in two plants does not necessarily mean that the same enzymes are involved. These alkaloids i.a. deter feeding by insects; widely distributed in Solanaceae, they have also been found in Calystegia sepium; nicotine alkaloids, also synthesized via PUTRESCINE N-METHYL TRANSFERASE, are of course to be found in Solanaceae (Junker et al. 2013).
For inter-/intraxylary pohloem, see Carlquist (2013). The corolla lobes have a thicker central area that is distinct from the margins because of the contorted-plicate or induplicate-valvate aestivation of the corolla; c.f. the "winged" corolla scattered in Asterales. For nectaries, see Erbar (2014). Corner (1976) did not mention an endothelium for Convolvulaceae, but c.f. Kaur (1969) and Kaur and Singh (1970).
CONVOLVULACEAE Jussieu, nom. cons. - Back to Solanales
Plant laticiferous, suberin in laticifer wall, resin glycosides [?level]; stomata usu. paracytic; lamina margins entire; K quincuncial, large, free; anther placentoid 0; pollen tectum imperforate; nectary vascularized, receptacular [?level]; stigma dry; ovules erect, apotropous; K ± dry, scarious in fruit; seeds 4/fruit, hilum 10%> of the seed; exotesta with papillae or hairs, usu. little thickened, outer hypodermis of small cells, little thickened, inner hypodermis of 1+ palisade layers, thickened; x = 7, nuclear genome [1 C] (0.119-)2.211(-41.163) pg, plastid rpl2 intron 0.
59 [list: tribal assignments]/1,880 (1,660) - six main clades below. World wide. Map: from Meusel et al. (1978), Lebrun (1977) and Staples and Brummitt 2007).
Age. Crown-group diversification may have begun around 68 Ma (Dillon et al. 2009), 70-47.8 Ma (Eserman et al. 2013) or (73.8-)56.5(-39.9) Mya (Olmstead & Tank 2017). Note that the clade age in Dillon et al. (2009) that is compared with Merremieae is the crown-group age of the whole family, not just a subclade of it, and the age in Wikström et al. (2001) is that of stem-group Convolvulaceae.
1. Humbertioideae Roberty - Humbertia madagascariensis Lamarck —— Synonymy: Humbertiaceae Pichon, nom. cons.
Large tree; chemistry?; wood dense, hard; vascular bundles collateral; petiole bundle annular; latex cells in the flowers alone; flowers single, axillary, strongly obliquely monosymmetric [rotated 108o to axis]; A adnate to base of C, connective tissue lignified, filaments bent in bud; ?pollen; style ?hollow, stigma clavate; ovule many/carpel, ?morphology; fruit a drupe, 1-2 seeds carpel; hilum crescentic; endosperm copious, cotyledons flat; n = ?; ?plastome; seedling?
1/1. Madagascar.
[Cardiochlamyeae [Eryciboideae, Cuscutoideae [Convolvuloideae + Dichondroideae]]]: vine or liane, climbing by twining [sinstrorse]; fibriform vessels +; ssecondary thickening anomalous [e.g. successive cambia; included phloem; xylem and phloem inverted]; latex canals +, usu. articulated; lamina with ± palmate venation, base ± cordate/hastate; pollen grains 3-colpate; ovules (1-)2(-4)/carpel, exotestal cells bulging; cotyledons complexly folded or coiled; plastid rpl 2 intron 0.
2. Cardiochlamyeae Stefanovic & Austin
Usu. lianes; hairs T-shaped; inflorescence racemose; bracts foliaceous, sessile; pollen (pantoporate - Cardiochlamys); stigma capitate (slightly two-lobed); fruit indehiscent, 1-seeded [= utricle], K accrescent, forming a wing; n = ?; seedling with small ovate cotyledons.
5/24. Madagascar, Southeast Asia, West Malesia. Map: from Staples (2006).
[Eryciboideae, Cuscutoideae [Convolvuloideae + Dichondroideae]]: ?endosperm.
3. Eryciboideae (Endlicher) Roberty - Erycibe Roxburgh —— Synonymy: Erycibaceae Meisner
Lianes; lamina not cordate, venation ± pinnate; inflorescence ?racemose, branched (± fasciculate); corolla lobes with thin margins, two-lobed; ovary 1-locular, style 0, stigma conical-radiate; fruit a berry, 1-seeded; exotestal cells fleshy, mesotestal cells little elongated and thickened; n = ?; germination cryptocotylar.
1/70. Southeast Asia, Indo-Malesia to Australia. Map: from Hoogland (1953a), Flora China vol. 16 20) and Australia's Virtual Herbarium (consulted xii.2012).
4. Cuscutoideae Link - Cuscuta L. —— Synonymy: Cuscutaceae Dumortier, nom. cons.
Plant parasitic, host-parasite connection via phloem/xylem, plant annual, vine, twining counter-clockwise; endomycorrhizae 0; stems white, yellow to red (green); internal phloem 0; stomata on stem transversely oriented [?all]; leaves reduced to scales; inflorescence monochasial cymes, variously grouped; K connate, C imbricate, C-A tube +, [C fusion very late in development]; infrastaminal scales + (0), at base of tube, unvascularized, margin ± fimbriate or crenulate, membranous [= corona], alternating with C; anther wall 3 cells across; tapetum (amoeboid), cells binucleate; pollen grains (bi-)tricellular, (-12-colpate), (surface reticulate); styles separate (unequal) / single, connate / ± 0, (gynobasic), stigmas capitate/elongated; ovules with integument (?8-)15-17 cells across, usu. unvascularized, parietal tissue none, endothelium 0; megaspore mother cells several, competition between the developing embryo sacs, embryo sac bisporic, spores chalazal, eight-celled [Allium type] (normal); fruit basally circumscissile/(indehiscent); testa multiplicative, water gap in hilar tissue; embryo spirally coiled, acotyledonous (almost), radicle absent, (chlorophyll 0); n = (4-)7(<), chromosomes 0.4-23 µm long, (holocentric), genome 1.16-65.54 pg/2C [342-34,734 Mbp/1C - monocentric chromosomes]; cotyledons 0.
1/195. More or less world-wide, ca 3/4 species New World.
Age. The age of crown-group Cuscuta is estimated to be 22.5-19.9 Ma (Neumann et al. 2023: Fig. S10).
[Convolvuloideae + Dichondroideae]: (cork pericyclic); (fibers or sclereids +); unicellular T-shaped hairs common; lamina vernation conduplicate; inflorescence cymose; integument vascularized, with unbranched bundle, 5-10 cells across, parietal tissue 1-3 cells across, placental obturator common; fruit capsular, 4-valved; water gap in testal bulges near micropyle [?distribution]; endosperm nuclear/coenocytic, storing galactomannans [?always], embryo curved/folded, cotyledons bifid/bilobed, suspensor haustorium +; n = 7-15+; chloroplast atpB gene with 6-15 bp deletion, ycf15 absent, trnF with 150 bp deletion; incompatibility system sporophytic.
49/1375. World-wide (see family map). [Photo - Flower, Fruit.]
5. Convolvuloideae Burnett
Homospergmidine synthase gene +, (pyrrolizidine alkaloids + [esp. ipangulines]); (margin of leaf blade serrate); inflorescence a dichasial cyme; C with interplical veins; style +; hilum >10% of the seed, peripheral, hilar pad heart-shaped; (exotestal cells not bulging).
5A. Aniseieae Stefanovic & D. Austin
Vines; lamina (not cordate); inner K smaller than the outer; filaments swollen, pubescent; pollen grains prolate, (to polycolpate); stigmas elongated (globose); seeds (hairy).
3/6. New World Tropics, including the Antilles.
[Convolvuleae ["Merremieae" + Ipomoeeae]]: ?
5B. Convolvuleae Dumortier
Vines; ergoline alkaloids +; filaments swollen, pubescent (not); pollen grains (pantoporate - Calystegia, Xenostegia), (exine plurigemmate-echinate - X.; stigma subulate, 2-lobed (dissected - Polymeria); cotyledons large [often >2 cm long], strongly bilobed.
2/225. Convolvulus (215). ± World-wide, esp. temperate.
"Merremieae" D. F. Austin - note the problems in tribal circumscription in this area (see Simões et al. 2015).
(anthers spirally twisted at anthesis); pollen (pantocolpate/12-colpate); (styluli +), style (gynobasic), stigmas globose, smooth to papillate; fruit (schizocarp - Remirena)/(operculate - Operculina); nuclear genome ca 1.51 pg/2C; (seedling cryptocotylar - Didstimake tuberosus, eophyll 5-lobed, 5-foliolate).
Distimake (35). Pantropical (warm temperate).
Age. This clade is some (70-)55.3(-47.8) Ma (Eserman et al. 2013).
5C. Ipomoeeae Hallier f. - Ipomoea L.
Vines to lianas (annuals), (small trees), (roots tuberous/xylopodium); (ergot (and indole) diterpene alkaloids), (swainsonine) +; indumentum sericeous/various/0; lamina (compound), (margins serrate/± deeply pinnately-/palmately-lobed), (extrafloral nectaries on petiole/leaf base/K); (flowers monosymmetric); filament base swollen, pubescent; pollen pantoporate, echinate supratectal elements with "basal cushions"/0, metareticulate; G ([3-4]), stigma capitate-lobed; fruit (indehiscent, outer layer ± fleshy) (with false septum), K ± accrescent; seed (variously hairy); embryo sac much elongated [?level].
1/621. Pantropical (warm temperate), most segregate genera Old World.
Age. Crown-group Ipomoeeae are estimated to be ca 35 Ma (Eserman et al. 2013), ca 19.4 Ma (Wilf et al. 2017a) or ca 8.4 Ma (Magallón et al. 2015 - see Muñoz-Rodríguez et al. 2019).
The 58.7-55.8 Ma Ipomoea meghalayensis, from western Meghalaya, India, has recently been described (Srivastava et al. 2018).
6. Dicranostyleae Stefanovic & D. F. Austin (= Dichondroideae Endlicher)
Cyme monochasial (dichasial); (hilum >10% of the seed); reversion to a non-edited start codon for the psbL gene; cotyldons small [usu. <2cm long], unlobed.
6A. Jacquemontieae Stefanovic & D. Austin - Jacquemontia Choisy
Vines; hairs stellate; filaments swollen, pubescent; pollen grains tri- to pantocolpate, spines 1-3(-5)-tipped; style sortly divided, stigma ellipsoid and flattened; capsule 8-valvate.
1/110. Mostly the Americas, tropical to warm temperate.
[Dichondreae, Maripeae, Cresseae]: style ± bifid.
Dichondreae G. Don —— Synonymy: Dichondraceae Dumortier, nom. cons., Poranaceae J. Agardh
Prostrate herbs to lianes; lamina (base not cordate), (venation pinnate); (inflorescence racemose - Calycobolus), (flowers adnate to bracts - Neuropeltis, etc.; K enlarged in fruit); filaments swollen, pubescent; style (gynobasic), stigmas globose; fruit an utricle; cotyledons linear [Dichondra].
6(?9)/: Calycobolus (25), Neuropeltis (14). Tropical-warm temperate, Africa-Madagascar, the Americas, also Indo-Malesia (Porana).
6. Maripeae Webb & Berthelot
Lianes; lamina elliptic, venation pinnate; filaments swollen, pubescent; style ± bifid; fruit ligneous-baccate; water gap 0 [Maripa]; exotestal cells fleshy [black liquid!], mesotestal [palisade] cells little elongated and thickened.
3/35: Maripa (19). Central and South America.
6. Cresseae C. B. Clarke —— Synonymy: Cressaceae Rafinesque, Evolvulaceae Berchtold & J. Presl
Herbs or small shrubs (lianes); lamina base not cordate, ?venation; (plant dioecious); filaments straight, glabrous; pollen grains (pantocolpate); style ± bifid, stigma globose/lobed; fruit (utricular, unilocular); cotyledons very deeplyand narrowly lobed [Stylisma].
8(?12): Evolvulus (100), Bonamia (45). ± Pantropical.
Evolution: Divergence & Distribution. The 58.7-55.8 Ma age of the recently described Ipomoea meghalayensis (Srivastava et al. 2018) suggests a very considerably older crown-group age for the family than several of the estimates above. For some more dates, see Eserman et al. (2013) and Muñoz-Rodríguez et al. (2019: Supplementary Information), both Ipomoea.
Srivastava et al. (2018) thought that Convolvulaceae had an east Gondwanan origin; the basal clades in Convolvulaceae are all Old World, including Madagascar but not the African mainland (M. A. García et al. 2014). Muñoz-Rodríguez et al. (2019) discuss diversification in the Ipomoeeae-Argyreieae area. Long distance dispersal is quite common in this part of the family (Muñoz-Rodríguez et al. 2018, 2019).
As Stefanovic et al. (2003) noted, characters for their /Dicranostyloideae (= Dichondroideae) will depend very much on the position of Jacquemontia, currently unresolved in that clade. Eserman et al. (2013) discuss the evolution of a number of characters in Ipomoeeae. Cheek and Simão-Bianchini (2013) recently described the distinctive Keraunea brasiliensis that has fruits rather like those of Neuropeltis, although overall it was clearly an odd plant. Preliminary molecular data suggested that the type was to be placed in Malpighiaceae and a paratype in Ehretiaceae (see Muñoz-Rodríguez et al. 2022). The former placement may have been because an errant leaf, probably of Mascagnia cordata, that had inadvertently become associated with the type specimen was sampled (de Almeida et al. 2023a); the latter placement is where the genus belongs (Moonlight & Cardoso 2023; Cheek et al. 2023b).
Cuscuta. Cuscuta (dodder) is such a a morphologically and ecologically distinctive plant that it is treated separately below.
Many Convolvulaceae contain alkaloids and are toxic to herbivores. Ergoline alkaloids and indole diterpene alkaloids of Convolvulaceae like Ipomoea and Turbina appear to be synthesized by associated ascomycete clavicipitalean fungi (Eserman et al. 2013). About 20% of Ipomoeeae s. str. (= Ipomoea s.l.) form associations with the clavicipitacous asomycete endophyte Periglandula and are ergot alkaloid-positive (Schardl et al. 2013: P. ipomoeae; Cook et al. 2019), and about half these species also produce indole diterpene alkaloids (Quach et al. 2023). Concentrations of the ergot alkaloids, at 1,600 to 5,100 µg/g, are up to 1000 times higher than in poöid grasses (Beaulieu et al. 2013); these grasses are associated with the same fungus. Furthermore, the indolizidine alkaloid swainsonine is synthesized by chaetothryialean ascomycete fungal associates of some Ipomoeeae (= Ipomoea s.l.), and although it is not very common there, it is very largely found in species other than those that produce ergot alkaloids (Cook et al. 2014, 2019; Quach et al. 2023). Interestingly, species producing these alkaloids tend to have larger seeds than those that do not, and the alkaloids may help protect against seed predators or herbivores (Quach et al. 2023). See also Fabaceae-Faboideae-IRLC clade and Poaceae-Pooideae for other examples of "plant" secondary metabolites synthesized by fungal associates.
Pyrrolizidine alkaloids (PA), on the other hand, are synthesized by the plant, and homospermidine synthase (HSS) is the first gene in the PA biosynthetic pathway. PA alkaloids may have evolved more than once in the family, but HSS evolved only once (an apomorphy!) in Convolvuloideae (see Stefanovic et al. 2003) following a gene duplication, as in other plant groups like Fabaceae, Asteraceae, and Apocynaceae that have PAs (Reimann et al. 2004; Langel et al. 2010; Kaltenegger et al. 2013; Livschulz et al. 2018a: widespread molecular-level parallelism). Nortropane alkaloids like calystegines are also synthesized by the plant (Schimming et al. 1998). Kaltenegger et al. (2013) also discuss the evolution of these alkaloids in Convolvuloideae; their distribution is sporadic.
Pollination Biology & Seed Dispersal. Convolvulaceous flowers often last for only a single day. Thomson and Wilson (2008) discuss bird pollination in Ipomoea. S. D. Smith et al. (2010) noted that white corollas were relatively uncommon in Ipomoea subg. Quamoclit because clades in which they evolved speciated relatively less than the others, while McDonald et al. (2011) discuss the numerous origins of self- from cross pollination (and reversals) in Ipomoea. Some Convolvuloideae have flowers with slight oblique disymmetry (Lefort 1951), while the monosymmetry of the flowers of Humbertia is largely positional, indeed, they are drawn as being polysymmetric by Pichon (1947); for the plane of monosymmetry here, different from that of Solanaceae, see J. Zhang et al. (2017).
For sporophytic self incompatibility, especially in Ipomoea, see Kowyama et al. (2008).
The bracts may be adnate to the pedicel and accrescent, the fruit being indehiscent-utriculate (wind dispersal: Neuropeltis and relatives), or the bracteoles may be much enlarged (e.g. Calystegia s. str., = Convolvulus).
Convolvulaceae are the only gentianid family in which the seeds have physical dormancy (Baskin et al. 2000), and this is caused by the thick, hard seed coat found in nearly all members of the family. The convolvulaceous seed coat is perhaps the most complex of that of any other gentianid; it is up to about 30 cells thick and consists of several types of cells, some much thickened and lignified; see Govil (1971), Kaur and Singh (1970, 1987) and Jayasuriya et al. (2008a, esp. 2009, the bulges and bulging cells and cell layers all a bit confusing) for details. In Humbertia there is a thick layer of collapsed cells underneath the palisade layer; the testa of the seed examined showed no ligification (Deroin 1993). indeed, thick and complex seed coats are an apomorphy here. The seeds normally need scarification before they take up water, and, if unscarified, water initially penetrates the seed only at particular places in the coat (Jayasuriya et al. 2009). However, in Erycibe and Maripa there is no physical dormancy and the seeds are recalcitrant, being unable to stand either drying or freezing (Jayasuriya et al. 2008b, 2009); what about Humbertia?
Plant-Animal Interactions. Larvae of the bruchid Spermophagus (Chrysomeloidea-Bruchinae) eat seeds; the genus has diversified on and is roughly contemporaneous with Old World Convolvulaceae, although its primary hosts are Fabaceae (Kergoat et al. 2015: c.f. Malvoideae hosts). Other bruchids such as Megacerus are also seed predators on Convolvulaceae (Reyes et al. 2009).
Some chysomelid beetle larvae sequester isoprenoids from Convolvulaceae they are eating that are then converted to triterpene saponins in the defensive glands of the adults (Opitz & Müller 2009).
Plant-Bacterial/Fungal Associations. The ergoline alkaloids of Convolvulaceae like Ipomoea and Turbina (= Ipomoea s.l.) are synthesized by the associated ascomycete clavicipitalean fungi Periglandula (P. ipomoeae: Schardl et al. 2013) and are found in tissues where those fungi also occur (e.g. Ahimsa-Müller et al. 2007; Markert et al. 2008; see also above, and Leistner & Steiner 2009; Eserman et al. 2013). Ergoline alkaloids occur in 1/4 or probably more of the species of Ipomoea, and they are particularly common in four clades there (one of these clades has been dated to ca 15 Ma), the alkaloids differing somewhat between the clades (Beaulieu et al. 2021). These alkaloids are particularly to be found in seeds, and they are more concentrated in larger seeds, perhaps being involved in protection against herbivores (Beaulieu et al. 2021). These fungi may grow on the surface of the leaf (Beaulieu et al. 2012, 2015), possibly facilitating horizontal transmission of the fungus; vertical transmission also occurs (Beaulieu et al. 2021). The indolizidine alkaloid swainsonine is synthesised by other ascomycete fungal associates (in Chaetothyriales) of some Convolvuloideae, interestingly, not in clades of Ipomoea that synthesize ergoline alkaloids (Cook et al. 2014; Beaulieu et al. 2021).
One or more genes from the gram-negative Agrobacterium are found in the genome of cultivars of the sweet potato, Ipomoea batatas, having moved there by horizontal transport (Kyndt et al. 2015).
Genes & Genomes. Cuscuta and Ipomoea have a genome triplication in common (G. Sun et al. 2018), this is the IPQUα duplication that is estimated to have happened ca 32.8 Ma (Landis et al. 2018). Speta (1977) and Thaler (1966) mention a few mostly old records of protein crystalloids in the nucleus.
Substitution rates of nucleotides in the plastome of Convolvulaceae are extremely variable and probably cause the topological uncertainty in plastome-based relationships here (C.-S. Wu et al. 2022). There are numerous rearrangements of the plastome, including inversions, duplications, contraction and expansion of the IR and gene and intron losses. Rather unusually, there is DNA of mitochondrial origin in the plastome of Jacquemontia (Wu et al. 2022). Liqiong et al. (2023) found that the plastome ranged from 113273-164112 bp and contained (66-)78-79 protein-coding genes. However, J. Sun et al. (2019 and references, see below) found little variation in the plastome in Convolvulaceae in other than in Cuscuta... Interestingly, although Liqiong et al. (2023) found a reduced number of genes in the Cuscuta plastomes they examined (figures in parentheses above), the plastome sizes of the two species of Erycibe that they included was 113273 and 123094 bp, i.e., similar to those of Cuscuta and unlike those of other Convolvulaceae.
Cuscuta L.
Evolution: Divergence & Distribution. There are some remarkable geographical disjunctions within Cuscuta, including C. kilimanjari, an African member of an otherwise South American clade (M. A. García et al. 2014). Ho and Costea (2018) discuss fruit type in Cuscuta in the context of geography and diversification.
Species limits in Cuscuta are difficult (Costea & Stefanovic 2009; Costea et al. 2011 and references); for hybridization, etc., see M. A. García et al. (2014 and references).
Ecology & Physiology. D. M. Watson et al. (2022) suggested that being a twining annual that had direct phloem contact with its host were key innovations for Cuscuta that have driven its diversification. The plant body consists largely of a white (although most species do have some chlorophyll), counter-clockwise twining stem that is attached to the host by haustoria, and a single plant may produce about a kilometer of stem a year (Watson et al. 2022); roots and leaves are rudimentary. Cuscuta exaltata and a number of other species have retained most of the genes associated with photosynthesis despite the loss of about half the plastome (McNeal et al. 2007a; Barrett et al. 2014; Sun et al. 2018; Banerjee & Stefanovic 2020), but photosynthesis in the parasite is much reduced (see Funk et al. 2007 for the gradual loss of functionality in the chloroplast genome). Much of the photosynthesis that does occur is carried out in the seed where the chlorophyll is concentrated, and photosynthesis there is largely involved in the synthesis of lipids that are i.a. seed reserves that are used up during seedling establishment (McNeal et al. 2007a, b; Tesitel 2016); overall CO2 fixation is at most low (e.g. van der Kooij et al. 2000). In other cases any photosynthesis may facilitate fixation of CO2 produced by the plant (e.g. Hibberd et al. 1998; Vogel et al. 2018). Taxa that appear to have no chlorophyll at all have a very different reproductive biology - they have large, scented, flowers, no selfing, low seed set, etc. (McNeal et al. 2007b); Banerjee and Stefanovic (2023) discuss two groups in Cuscuta subgenus Grammica, including section Subulatae in its entirety, which appear to be unable to photosynthesize.
Jhu and Sinha (2022b, see also Balios et al. 2024) review haustorium development, noting the importance of tactile stimuli and light signals for stem haustorium induction. Dodder haustoria have often been described as being modified roots, although elements of the development of the two seem rather different (Alakonya et al. 2012). Dodder produces de-esterified pectins which form a kind of cement that helps Cuscuta attach to its host, and these pectins are substrates for pectate lyases involved in remodelling the host cell wall as the parasite penetrates the host (Jhu et al. 2022a). Recently it has been found that changes in the expression of genes involved in root initiation and development, including the important gene LATERAL ORGANS BOUNDARIES DOMAIN 25 (LBD25), are among those involved in the development of the stem haustoria in Cuscuta (G. Sun et al. 2018; Jhu et al. 2021). Furthermore, the growth of the searching hyphae, anticlinally-elongated cells that establish connection with the vascular tissue of the host, can be linked to genes involved in pollen tube growth (see also Masumoto et al. 2021). Indeed, there are parallels here with haustorial development in root parasites in Orobanchaceae and Santalaceae, both having similar elongated cells and in the former at least there is LBD25. In both tomato variants that are resistant to infection by Orobanchaceae, and also in Vicia (Fabaceae) that is similarly resistant, it is the development of lignification that seems to prevent haustorial penetration, and it is the outer cortex that becomes lignified in Cuscuta (Jhu et al. 2022b). Interestingly, most genes that have been involved in horizontal gene transfer are strongly expressed in the haustoria (Z. Yang et al. 2019). There are direct host—parasite phloem connections that are marked by a labyrinthine rather transfer cell-like morphology in the Cuscuta phloem (Dörr 1990; see also Joel 2013a).
Hibberd and Jaeschke (2001) provide a model of nutrient flow between host and parasite; stomata on the flower and/or special protuberances on the stem may increase parasite transpiration and hence nutient/water flow (Clayson et al. 2014: stomata are also found on extra-floral nectaries). There is also more or less extensive (depending on the host) bidirectional exchange of mRNA, etc., between Cuscuta and its hosts, although how this affects the functioning of the partners, whether or not the mRNA is expressed, etc., was initially unclear (G. Kim et al. 2014). However, it has recently been found that microRNAs only 22 nucleotides long are induced at the haustorial connection, and these miRNAs change host gene expression in various ways to the advantage of the parasite (Shahid et al. 2018; see also Z. Yang et al. 2019). However, it is unclear how this bidirectional movement occurs (Balios et al. 2024). Conversely, flowering in dodder is controlled by the host; although dodder has no functional flowering time genes, signals from those of its host cross to the parasite and interact with a dodder transcription factor, causing the dodder to flower (Shen et al. 2020). N. Liu et al. (2019) found that mobile proteins and mRNAs moved between host and parasite, even between different hosts that were bridged by the parasite individual, and even ending up in seeds. Landi et al. (2022) described changes occurring in the host as C. campestris parasitized Artemisia campestris: The volatile organic compounds produced by the latter changed, its stomata remained open, and its internal defences were based more on primary metabolites rather than on the more effective secondary metabolites, all features of the Cuscuta parasite.
As just mentioned, dodders can bridge two or more hosts, form haustoria on themselves or on other parasitic plants, including other species of Cuscuta (see Krasylenko et al. 2021 for such behaviour; Chitralekha 2024 for anatomy, literature, etc.), furthermore, viruses can be transmitted from one plant to another via the dodder stem (Hosford 1967), indeed, all plants infected by the one dodder individual form a sort of community. Thus a host plant infested with dodder and also experiencing herbivory can generate signals that cause extensive transcriptomic changes elsewhere in that plant, so activating its defence responses to the herbivory. Fichman et al. (2024) looked at the transfer of stimuli between one Arabidopsis thaliana plant and another via the parasite, again detecting such movements - this happened over half an hour or less - and suggested that in such circumstances calling Cuscuta a parasite might not be correct. This defence activation also occurs in other plants to over 1 m away that are connected by the dodder individual, whether or not those other plants are related to the initial host (Hettenhausen et al. 2017) - similar phenomena have been noticed in mycorrhizal associations (Jung et al. 2012 and references).
Cuscuta species tend to have broad host ranges and, unlike Orobanchaceae and other root parasites, germination needs no specific stimulants - their seeds can remain viable for ten years or so and it is tactile stimuli and light signals that are important in haustorium induction (Jhu & Sinha 2022a, 2022b). Their loss of flowering-time genes means that they flower when their host flowers (Shen et al. 2020), and this is no disadvantage to the parasite. They can increase plant diversity by parasitizing and suppressing the dominant species in the community, thus the dominant Salicornia virginica was suppressed by C. salina allowing other species to grow, although in lower areas of the saltmarsh the dodder seemed to prefer less dominant species (Pennings & Callaway 1996), similar effects being seen in other parasitic groups like Orobanchaceae (D. M. Watson et al. 2022); grazing can have the same effect. For details of the resistance of some plants to dodders - at least sometimes hypersensitivity is involved - see Hegenauer et al. (2016) and Mutuku et al. (2020) and references. The tomato, Solanum lycopersicum, can recognize a variety of species of Cuscuta as being pathogenic, and it resists colonization by them (Hegenauer et al. 2020).
Pollination Biology & Seed Dispersal. In some temperate species of Cuscuta the seeds have both physiological and morphological dormancy (see Gama-Arachchige et al. 2013 for a water gap in such seeds). The indehiscent fruit of some species of Cuscuta can float and water transport may have been involved in the dispersal here (McNeal et al. 2007b).
Hardly surprisingly Cuscuta has lost the ability to form endomycorrhizal associations (Delaux et al. 2014; see also Vogel et al. 2018).
Genes & Genomes. There is remarkable genome variation in Cuscuta. Overall genome size ranges from 342-34,734 Mbp/1 C, i.e. 102-fold, in species with monocentric chromosomes, but only 533-1,545 Mbp/1 C in those with holocentric chromosomes (subgenus Cuscuta) while x, the base chromosome number, decreased from 15 or 16 to 7 (Neumann et al. 2020) - yet only 12 species were included in this study! In species with large genomes, up to about 80% of the genome is made up of various kinds of repeats. Neumann et al. (2020) compare genome variation here with that in other groups of parasitic plants, noting that here (and in Santalaceae, for example), some species have quite small genomes, so clearly there is no simple correlation between large genomes and the parasitic habit. Cuscuta campestris has 44,303 genes, while C. australis a mere 19,671 genes, about the fewest protein-coding genes known, and there has been little expansion of gene families in the latter (G. Sun et al. 2018; Vogel et al. 2018). L. Cai et al. (2021) noted that gene loss here was in functional categories similar to the genes lost in other hemi/holoparasitic plants. There is also a rather low number of Benchmarking Universal Single-Copy Orthologs, near-universal single-copy orthologues, aand in this Cuscuta is rather similar to the situation in groups like Utricularia (Lentibulariaceae) and Wolffia (Araceae), also lacking much in the way of conventional leaves and roots (Michael et al. 2020). Many protein-encoding genes have been lost in some holoparasitic clades in Cuscuta, also, genes involved in soil-root interactions, etc. (Braukmann et al. 2013; see also Krause 2011; A. Vogel et al. 2018: nuclear genome sequence). Sun et al. (2018) found that genes controlling flowering time, nutrient uptake, photosynthesis, Casparian strip development, leaf patterning, root origination and development, etc., have also been lost in C. australis. Gene number in parasitic/carnivorous plants in general is discussed further by Palfalvi et al. (2020: Fig. S3).
In Cuscuta, the nuclear-encoded SSL (strictosidine synthase-like) gene came via HGT from Brassicales (D. Zhang et al. 2014), while the albumen-1 gene had moved from Fabaceae-Faboideae (Y. Zhang et al. 2013). A. Vogel et al. (2018) suggest that there may have been some 36 transfers of genes from different hosts to the nuclear genome of C. campestris, with Fabales being involved in a third (12) of these events and Caryophyllales in another seven, while Z. Yang et al. (2019) estimated that there had been around 108 HGTs, 16-20 of which could be placed along the stem of the genus (c.f. Phelipanche - Orobanchaceae), and genes transferred were probably functional. Yang et al. (2019) noted that over 90% of these genes came from Malvales, Caryophyllales, Malpighiales and Fabales, and a number of these transferred genes are involved in haustorial development, as in Phelipanche (= Orobanche-Orobanchaceae, but root haustoria there). Given the extent of mRNA movement between host and parasite and the way one Cuscuta individual can bridge different host plants, possibly belonging to different species, the possibilities are almost endless (G. Kim et al. 2014). See also C. C. Davis and Xi (2015), Orobanchaceae and Rafflesiaceae.
Neumann et al. (2023) found that the kinetochore, where chromosomes normally attach to microtubules, has degenerated in Cuscuta subgenus Cuscuta, species of which are holocentric/have diffuse centromeres, and the spindle assembly checkpoint there is no longer functional. Interestingly, details of kinetochore degeneration differed in the two species of Cuscuta studied, and the chromosomes of both differed substantially from those of the holocentric Rhynchospora pubera (Cyperaceae) (Neumann et al. 2023).
For the plastome and its extensive evolution in Cuscuta, gene loss, etc., see McNeal et al. (2007a, b) and Braukmann et al. (2013: subgenus Grammica), for the rpl2 intron, see Downie et al. (1991) and Stefanovic et al. (2002), and for more on plastome evolution in Cuscuta see e.g. Hibberd et al. (1998), Stefanovic and Olmstead (2005), Park et al. (2019: variation at the subgeneric level?), Banerjee and Stefanovic (2020) and Q. Liu et al. (2022a). Mower et al. (2021) discussed possible connections between various distinctive life styles that might affect the photosynthetic process and the loss of ndh genes. Banerjee and Stefanovic (2020) found that the two species of subgenus Cuscuta whose plastomes they examined had lost the chloroplast inverted repeat and in the genus as a whole the ndh genes were lost (or in a couple of cases pseudogenized), although the photosynthetic genes were mostly retained. However, in subgenus Grammica, the ability to photosynthesize has been completely lost in all species in section Subulatae and some species in section Ceratophorae (Banerjee & Stefanovic 2020).
Variation in the chondrome is notably less than in the plastome. However, mitochondrial genes, at least, may move from Cuscuta to its host, an example being in Plantago (Mower et al. 2010). There is also some movement in the reverse direction, and the atp1 gene, involved in the construction of the protein pump Na+/K+ ATPase, has moved from an host in core Lamiales to the chondrome of subgenus Pachystigma and the gene seems to be functional there (Q. Lin et al. 2022a; see also Sanchez-Puerta et al. 2023).
Chemistry, Morphology, etc.. Kuijt (1969), Heide-Jørgensen (2008), the Parasitic Plants website (Nickrent 1998 onwards) and the Digital Atlas of Cuscuta (Costea 2007 onwards), provide general information about dodders; for some anatomy, see Solms-Laubach (1867), for stomata, see Poisson (1874), for pollen, see Welsh et al. (2010), for embryology, see Sastri (1956: embryo sac haustoria), see also Johri and Nand (1935: parietal cells), Tiagi (1951), and Vázquez-Santana et al. (1992), for seeds, etc., see Johri and Tiagi (1952), and Sherman et al. (2008) for germination. For other stem hemi/holoparasites, see e.g. Cassthya and mistletoes and other Santalales.
Phylogeny. Although Cuscuta is clearly monophyletic, exactly what its relationships are within Convolvulaceae is unclear, as will be evident below. For early ideas of relationships within Cuscuta, see e.g. Stefanovic et al. (2007), M. A. García and Martín (2007), McNeal et al. (2007b), Stefanovic and Costea (2008) and Braukmann et al. (2013); the trees in Revill et al. (2005) may be compromised by some misidentifications of the material examined (see McNeal et al. 2007b); Costea et al. (2015) provides a summary tree. M. A. García et al. (2014) found that the predominantly Asian subgenus Monogynella was sister to the rest of the genus, [Cuscuta + Grammica] (see also Neumann et al. 2020). Q. Lin et al. (2022a) noted the overall subgeneric relationships of [Monogynella [Cuscuta [Pachystigma + Grammica]]] in plastid + nuclear analyses and [Cuscuta [Pachystigma + Grammica]] in analyses of 30 chondrome genes. However, in analyses of atp1, not only did HGT affect the position of species of subgenus Pachystigma examined, but species of subgenus Cuscuta were well embedded in the genus, rather than being sister to the rest (Lin et al. 2022a). Species of section Subulatae, entirely lacking photosynthetic genes, were sister to the rest of subgenus Grammica (Lin et al. 2022a). Looking at plastome variation, Pan et al. (2023) found that neither subgenera Cuscuta nor Grammica were monophyletic.
And back to the rest of the family...
Economic Importance. Ipomoea batatas, the sweet potato, is a major root crop (root tubers are also quite common elsewhere in Ipomoea). Its closest relative/progenitor is I. trifida; I. batatas itself is an autopolyploid, and subsequent chloroplast capture from I. trifida resulted in the two chloroplast races in the species. Polynesian representatives of I. batatas probably arrived there by long distance dispersal, and they do not have the I. trifida plastome (Roullier et al. 2013; Muñoz-Rodríguez et al. 2018, 2019).
Convolvulaceae include a disproportionally large number of notably serious and widespread weeds (Daehler 1997). The effect of Cuscuta on crops - mostly eudicots - is in addition to this imbalance (for C. campestris, see Parker 2021 and other entries in the Invasive Species Compendium. CAB International);.
Chemistry, Morphology, etc.. Glycine betaines are rather commonly accumulated in Convolvulaceae (Rhodes & Hanson 1993), perhaps surprising since it is not a family of halophytes; glycosides are known only from Cuscuta and Convolvuloideae. Wood fluorescence occurs, but not often. Humbertia has hard wood with the odour of sandalwood. Successive cambia have been found in some species of Ipomoea (Terrazas et al. 2011) and Carlquist (2013) noted the occurrence of interxylary phloem in that genus and Turbina; note that interxylary cambia have recently been reported from Ipomoea (Pace et al. 2018) and inter- and intraxylary phloem in Argyreia (Lawand et al. 2023). For successive cambia, etc., Merremia being especially variable, see Rajput et al. (2016, 2024b) and Lekhak et al. (2018 and references). Rajput et al. (2024c: see Table 1) described the very extensive vascular variation in the family; the various reports of cambial activity will have to be reconciled and placed in the ccontext of the family phylogeny. Adventitious roots on the stem may develop in two lines sublateral to and below the petiole; adventitious shoots develop from the roots of the sweet potato.
The sepals of Humbertia have five traces, but in Convolvuloideae there are fewer; secretory cells are apparently restricted to the flower in the former (Deroin 1993). The corolla tube of some Cuscuta and some other members of the family is strictly speaking a corolla-stamen tube, both being integral parts of the tubular structure; fusion of the corolla happens only late in development (Prenner et al. 2002). The distinctive infrastaminal scales found here are initiated only after the stamens have developed, the scales being parts of the corolla-stamen tube; they do not secrete nectar, but may have laticiferous cells, and they may protect the nectar or the ovary (Riviere et al. 2013). Other Convolvulaceae may have thickened filament bases, or even scale-like structures, although the few taxa with the latter are unrelated to Cuscuta (Riviere et al. 2013). The diversity of style and stigma morphology in Cuscuta alone is as great as that in the rest of the family (e.g. Wright et al. 2011). Nectaries in the family, including those of Humbertia, are discussed by Deroin (1993). Weberling (1989) described the ovary as being fundamentally gynobasic, although with an apical septum.
There is general information on the family in Staples and Brummitt (2007), Hoogland (1953b), articles in Rheedea 34(5). 2024 and especially Convolvulaceae Unlimited, see also J. R. I. Wood et al. (2020: New World Ipomoea) and Nickrent (2020: Cuscuta). Some information about Humbertia is taken from Pichon (1947) and K. Kubitzki and H. Manitz (pers. comm.), but the genus is poorly known, especially embryologically. For chemistry, see Hegnauer (1964, 1989) and Eich (2008), for galactomannans and their like, see Kooiman (1971) and Reid (1985), for floral anatomy, see Deroin (2004 and references), for pollen, see Sengupta (1972), Tellería and Daners (2003: S. South American taxa), Buril et al. (2014: Jacquemontia), Simões et al. (2019: Operculina) and de Man and Simõs (2022: Xenostegia), for ovary morphology, see Deroin (1999b), for embryology, see Raghava Rao (1940), Kaur (1969), Kaur and Singh (1970) and Yana and Rao (1993), for seed reserves, see G. Dahlgren (1991), and for seedling morphology, see Austin (1973) and Staples et al. (2025).
Phylogeny. For a morphological phylogeny of the family, see Austin (1998). The distinctive Humbertia is consistently recovered as sister to the rest of the family (e.g. Refulio-Rodriguez & Olmstead 2014; M. A. García et al. 2014). Relationships within the rest of the family are still rather uncertain. Despite the sequencing of over 6800 bp, the position of Cuscutoideae was unclear (Stefanovic & Olmstead 2001, 2004; Stefanovic et al. 2002 and esp. 2003), however, they may be close to a clade containing species with bifid styles, Dichondroideae or perhaps [Convolvuloideae + Dichondroideae] (Stefanovic & Olmstead 2004; Wight et al. 2011). It has been suggested that part of Poraneae (Cardiochlamyeae: Porana itself is polyphyletic) and Erycibeae s. str. are successive sister taxa (Stefanovic et al. 2002); Erycibe in particular can look very unlike other Convolvulaceae and herbarium specimens are often misidentified. Ipomoea, Convolvulus, and their relatives form a clade that is sister to a rather unexpected clade made up of Poraneae, Cresseae, Dichondreae (with gynobasic styles), some Erycibeae (Maripeae), etc., as well as Jacquemontia. Several members of this latter clade have styles divided to the base or only an at most shortly connate style with long branches (but Jacquemontia has a long style), and leaf blades with more or less pinnate venation; Jacquemontia could be sister to the other taxa (see also Stefanovic & Olmstead 2004). However, support along the spines of these two clades is mostly rather weak; Merremia (in particular) and Convolvuleae, Cresseae and Poraneae are polyphyletic (Stefanovic et al. 2002). Relationships in Z.-D. Chen et al. (2016: Chinese taxa) seem somewhat scrambled, but support is poor, while in Park et al. (2019: 50 protein-coding chloroplast genes) the relationships [Cressa [Ipomoea + Cuscuta]] were recovered, but taxon sampling was skimpy (Cuscuta was the focus).
More recently, C.-S. Wu et al. (2022) looked at 78 plastomes in the family, 19 new, but only 9/12 tribes and 17 genera. Basal relationships (Humbertia was not included) were [[Cardiochlamyeae + Erycibeae] [Cuscuteae...], but suppoprt was not very strong. A Dicranostyloideae was made up of [Cresseae [Dichondreae + Jacquemontieae]] while Merremieae included both Convoluleae and Ipomoeeae. A number of relationships in Simões et al. (2022: Angiosperms353 nuclear data, 34 genera, 11 tribes) had quite good support, although the positions of Erycibe and Cuscuta were unclear, and other relationships they found point out how much remains to be done. Thus relationships in Dicranostyloideae were [[Maripeae + Poraneae = Neuropeltis] [Jacquemontia [Poraneae s. str., also inc. Dichondreae + Cresseae]] while Convolvuloideae were made up of a paraphyletic Merremieae that included Ipomoeeae (Simões et al. 2022). Neither paper makes entirely satisfactory reading, i.a. sampling being slight. Liqiong et al. (2023) examined the variation in the plastome of some 40 species, noting that trees based on different parts of the plastome, e.g. SSC, LSC, IR, had different topologies. Liqiong et al. (2023) preferred the tree resulting from the analysis of the whole genome - there relationships were [[Cardiochlamys + Erycibe] [Cuscuta [[Dichondra + Cressa] [[Merremia [M. hainanensis + Convolvulus]] [M. quinquefolia = Ipomoea]]]]] - clearly, Merremia needed attention... Note that W. Yu et al. (2023) preferred relationships based on the analysis of single copy sequences rather than of whole plastomes.
Relationships within Convolvuloideae have also been unclear. Merremieae - and Merremia itself - are not monophyletic, Ipomoeeae being embedded in the former, while Merremia is widely dispersed in that part of the tree - and there is little support along the backbone (Stefanovic et al. 2002; Sosef et al. 2019; esp. Simões et al. 2015). Ipomoea itself in the old sense was very much paraphyletic; note that there was practically no resolution in the strict concensus tree in the morphological analysis by Wilkin (1999). For relationships in Ipomoeeae/Ipomoea based on variation in plastid sequences, see R. E. Miller et al. (1999: Ipomoea s. str., resolution poor, classic subgenera in particular not monophyletic), Eserman et al. (2013) and J. Sun et al. (2019). Within Ipomoea s.l., the clade sister to the rest is largely Old World (and contains most of the generic segregates), there is a clade and a couple more species in the New World, and the next three clades are largely African-Madagascan; there are two small clades in Australia, and these seem to be related to the very largely New World taxa which form the last major clade in the genus (Muñoz-Rodríguez et al. 2019; see also Manos et al. 2001a; J. R. I. Wood et al. 2020). Rattanakrajang et al. (2022: 3 chloroplast markers + ITS) recovered two main clades here, an Argyrieae that also included Stictocardia, Lepistemon and a few errant species of Ipomoea, including the type(!), and a clade that included Lepistemon and the bulk of Ipomoea. Although a large problem, it will be interesting to see what a good nuclear analysis with sufficient sampling may suggest. There seem to be two main clades in a largely monophyletic Convolvulus, although C. nodiflorus did not link with the rest of the genus (Carine et al. 2004: sampling needs to be extended), however, perhaps mercifully, the latter is in fact a species of Jacquemontia. In an extensive study Williams et al. (2014) recovered a Calystegia that was well embedded in the Convolvulus clade, while Simões et al. (2015) found that general relationships in this area were [Convolvulus [Parameria ["Merremieae" + Ipomoeeae]]]. Pollen variation and conventional sections in Jacquemontia are at odds (Buril et al. 2014). Clearly, much remains to be done, and although Cuscuta and Ipomoea are large genera and have attracted much interest, an understanding of the phylogeny of the whole family remains elusive. Indeed, Kagume et al. (2025) atttacked the phylogeny of Ipomoea by using relationships evident in an analysis of 57 species of Ipomoea s.l. using an Angiopserms353 data set - there was an emphasis on African taxa - as a constraint tree for relationships evident in a subsequent analysis of 830 accessions of members of the group using an ITS dataset.
Classification. How many subfamiliers to recognize - two or more - seems immaterial, especially given the current uncertainty in relationships, and the classification here is very much provisional. For the tribes above, some of which may be paraphyletic, see the classification in Stefanovic et al. (2003 - there is a version of this in Staples & Brummitt 2007). Given the extensive paraphyly of Merremieae immediately below Ipomoeeae and the low support along the spine of that part of the tree (Simões et al. 2015), Simões and Staples (2017) suggest that Merremieae should no longer be recognised, and they provide a generic reclassification of its members (barring a few small clades/individual species that it was premature to place) that is followed here. Unfortunately, tribal limits in this area remain up in the air, Simões et al. (2015: p. 382) reasonably noting that "attempts at tribal delimitation in the Convolvuloideae clade to which [Merremieae] belongs are likely to be problematic". The problem of Ipomoeeae/Ipomoea is also discussed below, but if Ipomoeeae are recognized and the topology in Simões et al. (2015) is confirmed, it seems that at least seven more tribes (including Merremieae s. str., which would now be a rather small tribe) will be needed.
What to do with Ipomoea - to split, or to lump (it includes about half the species in the family) - is difficult; neither of the options is without its drawbacks (Stefanovic et al. 2003). Although Muñoz-Rodríguez et al. (2019) elected to delimit the genus broadly (see also Wilkin 1999), Rattanakrajang et al. (2022) prefer narrow limits, but that entails new genera, conserved types, etc., since Ipomoea in the old sense is polyphyletic - the argument can be followed in the pages of Taxon. As can be seen above, Ipomoeeae have no characters apart from distinctive pollen (that can indeed be recognized using a hand lens!), but broadening the circumscription of the genus may the way to go... (see J. R. I. Wood et al. 2020). However, Simões et al. (2024) reinstate some palaeotropical genera - this can be followed in the synonymy - overall, some 20 genera or more will be needed. Kagame et al. (2025) in a fairly lengthy discussion also opt for narrower generic limits, suggesting that the two clades evident in the old Ipomoea should be subtribes, Astripomoeinae and Argyreiinae (the first had no obvious diagnostic characters), and proposing to recognize a number of genera which "should be diagnosable and of manageable size" (only part of the argument, of course, and what "manageable size" might mean is unclear). We shall see.
Costea et al. (2015) provide an infrageneric classification (4 subgenera, 18 sections) for the speciose Cuscuta.
Thanks. To George Staples, for interesting conversations, information, corrections, etc..
SOLANACEAE Jussieu, nom. cons. - Back to Solanales
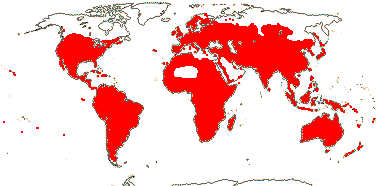
Herbs to shrubs, branching sympodial; hygroline alkaloids, oligosaccharides, (myricetin) +; roots diarch [lateral roots 4-ranked]; wood commonly fluoresces; pits vestured; cystoliths +; stomata various; leaves simple to compound; inflorescence terminal, monochasial-like, lax; flowers rotated 36o to axis; K often valvate; A (sub)basifixed; endothecial thickenings reticulate; G often pseudo-4-locular, 36o to the median, placentae swollen, stigma wet; ovules many/carpel, campylotropous; testa to 30 cells across, ± multiplicative, embryo sac with chalazal haustorium; K often ± accrescent; exotestal walls thickened on inner periclinal and anticlinal walls in particular, anticlinal walls sinuous, endotesta [= endothelium] ± persistent, walls ± lignified; embryo long, often ± curved, cotyledons ½-¼ its length, radicle same width; x = 12 (?6, ?11), chromosomes usu. 1.5-5 µm long, protein bodies in nuclei, nuclear genome [1 C] (0.128-)1.972(-30.478) pg, genome triplication [ca 81 mya].
102 [list, tribal assignments]/2,280 - treatment below needs some work. World-wide, but overwhelmingly tropical America. Map: from van Steenis and van Balgooy (1966), Meusel et al. (1978), van Balgooy (1984) and Heywood (2007).
Includes Anthocercideae, Benthamielleae, Browallieae, Brunfelsieae, Capsiceae, Cestreae, Cestroideae, Duckeodendroideae, Goetzioideae, Hyoscyameae, Jaboroseae, Lycieae, Mandragoreae, Nicandreae, Nicotianoideae, Nolaneae, Petunieae, Petunioideae, Physalideae, Salpichroa, Salpiglossideae, Schizantheae, Schizanthoideae, Schwenckioideae, Sclerophylax, Solandreae, Solaneae, Solanoideae.
Age. Crown Solanaceae have been dated to (45-)41, 36(-32) Ma (Wikström et al. 2001); J. Huang et al. (2023a) and Zamora-Tavares et al. (2016) think that they are quite old, dating them to (74.3-)73.3(-72.0) and (82.4-)61.9(-47.8) Ma respectively, Janssens et al. (2009) date them to 58±9.1 Ma, Paape et al. (2008) to ca 51 Ma, Olmstead and Tank (2017) to (65.3-)42.6(-21) Ma, De-Silva et al. (2017) to (55.2-)42.2(-26.8) Ma, and Bell et al. (2010) to (49-)38, 37(-29) Ma; still younger estimates are around 35 Ma (Dillon et al. 2009), and (34.0-)30.4(-26.3) Ma (Särkinen et al. 2013, see also Dupin et al. 2017). From Fig. 1 in Clarkson et al. (2017) an estimate of around 26.2 Ma can be suggested - but there are huge error bars and the focus was on Nicotiana...
Note that most of these ages - and those below - are questioned by the discovery of ca 52.5 Ma early Eocene fossils of Physalis infinemundi and P. hunickenii from Argentina (Wilf et al. 2017a and Deanna et al. 2020 respectively). At the same time, Cantisolanum daturoides, from the London Clay and thought to be the oldest fossil identifiable as Solanaceae, may in fact be a commelinid monocot (Särkinen et al. 2013), similarly, for the identities of other fossils from the Eocene that are putatively solanaceous, see Millan and Crepet (2014), and although none of them belongs, try Rhamnaceae...
1. Schizanthoideae Miers - Schizanthus Ruíz & Pavón
Annual to biennial herbs; pyrrolidine alkaloids [hygrine, etc.], distinctive tropane alkaloids +; cork cambium pericyclic; pericyclic fibres 0; flowers strongly monosymmetric, functional A developing before the C; K ± free [see Conv.], C cochleate, margins laciniate, abaxial pair connate, forming a keel; A 2 [abaxial-lateral], anthers explosive, staminodes 3; pollen tricellular, ektexine foot layer well developed; stigma very small; fruit a septicidal capsule; exotestal cells individually inpushing; endosperm nuclear/coenocytic, copious, embryo curved, cotyledons incumbent; n = 10.
1/14. Chile, adjacent Argentina (Mendoza, Nequén). Photo: Flower.
Age. Crown-group Schizanthus has been dated to ca 5.5 Ma, certainly under 10 Ma (Särkinen et al. 2013) or (8.5-)8.2(-7.9) Ma (J. Huang et al. 2023a).
[[Goetzeoideae + Duckeodendroideae] [[Schwenckioideae + Cestroideae] [Petunioideae [Nicotianoideae + Solanoideae]]]]: (crystal sand +, esp. in stem); K connate [?here]; ektexine foot layer not well developed.
Age. This clade is (69.5-)68.0(-66.7) Ma (J. Huang et al. 2023a).
[Goetzeoideae + Duckeodenroideae] : ?
Age. A clade including Goetzioideae and Duckeodendron has been dated to (39-)35, 33(-29) Ma (Wikström et al. 2001) or around 22.7 Ma (Särkinen et al. 2013).
2. Goetzeoideae Thorne & Reveal —— Synonymy: Goetzeaceae Miers
Trees to shrubs; (internal phloem 0 - Tsoala - T.); leaves xeromorphic; flowers single, lateral-axillary/terminal; C (tube narrow tube, >15 cm long - T.); pollen tricolpate, exine echinate, tectum perforate; stigma capitate to bi(tri)lobed; (G with 1 ovule); fruit a berry/4-valved capsule; seeds to 5/loculus; endosperm at most slight, (quite copious - T.), embryo straight, cotyledons large, fleshy, relatively very long; n = 13, 24; deletion in the chloroplast trnL-F spacer.
6/8. Most Greater Antilles (not Jamaica), also E. Brazil, Madagascar (Tsoala). [Photo - Flower.]
Age. Crown-group Goetzioideae are ca 15.6 Ma (Särkinen et al. 2013).
3. Duckeodendroideae Reveal - Duckeodendron cestroides Kuhlmann —— Synonymy: Duckeodendraceae Kuhlmann
Large tree; wood with large intercellular radial canals [c.f. Apocynaceae s. str.]; pith sclerified; bracteoles ?0; K imbricate, C quincuncial; pollen striate; stigma slightly bilobed; 1 ovule/carpel; fruit a drupe, pericarp complex, seed 1, K not accrescent; endosperm slight, embryo U-shaped, cotyledons very small; n = ?; deletion in the chloroplast trnL-F spacer.
1/1. Brazil, Manaus.
[[Schwenckioideae + Cestroideae] [Petunioideae [Nicotianoideae + Solanoideae]]]: acylsugars +/0: whole genome duplication; exotestal cells tabular [?level].
Age. This clade is (64.6-)64.2(-61.1) Ma (J. Huang et al. 2023a).
[Schwenckioideae + Cestroideae]: ?
Age. These two clades diverged around (62.4-)62.0(-59.0) Ma (J. Huang et al. 2023a).
Herbs (annuals) to shrubs; pericyclic fibres +; inflorescence a raceme, or with ± cymose branches; flowers monosymmetric; C aestivation valvate-con/induplicate, lobes 3-lobed; A 4, didynamous, or 2 + 3 staminodes; (G with 1 loculus, 1 ovule - Melananthus); fruit a capsule (achene); endosperm copious to scanty, embryo ± straight; n = 12.
3/31: Schwenckia (25). Mexico to Argentina, the Antilles, ?S. americana also W. Africa
Age. Crown-group Schwenckioideae are ca 16.6 Ma (Särkinen et al. 2013).
5. Cestroideae Burnett
(Low concentrations pyrrolidine-type nicotinoids); bordered pits +; pericyclic fibres +; C cochlear; A 4 or 5, often didynamous, staminode +/0, anthers dorsifixed; endosperm copious.
10/210. South, Central (and North) America.
Age. Crown-group Cestroideae are around 27.0 Ma (Särkinen et al. 2013).
5A. Benthamielleae Hunziger
Herbs, inc. cushion plants, to shrubs; cork cambium pericyclic; (stem, petiole, massively lignified - Pantacantha); leaf base sheathing (not); bracteoles +; C valvate-induplicate or contorted-conduplicate; pollen tricolp(oroid)ate, surface rugulate, rugulae striate; ovules 4-10/carpel; (fruit partly loculicidal); <7 seeds/capsule; endosperm copious, embryo annular-curved; n = 11.
3/15. Argentina, Chile.
Age. Benthamielleae are about 8.7 Ma (Särkinen et al. 2013).
[Salpiglossideae [Browallieae + Cestreae]: ?
Age. This clade is ca 24 Ma (Särkinen et al. 2013) or (54.8-)54.2(-51.2) Ma (J. Huang et al. 2023a).
5B. Salpiglossideae Burnett —— Synonymy: Salpiglossidaceae Hutchinson
Herbs (annuals) to shrubs; cork cambium pericyclic; flowers ± monosymmetric; A (4 or 2 + 2 adaxial staminodes, abaxial stamens with unequal divergent thecae - Reyesia); pollen (in tetrads - R.); style (hollow), spathulate apically, style hollow (solid), stigma with expanded 3-crested apex; capsule 2-4-valved, septi-loculicidal; embryo coiled, cotyledons incumbent, ca 1/5 enbryo; n = 11.
2/7: Reyesia (4). Mexico, Andean Chile and Argentina.
Age. Crown-group Salpiglossideae are estimated to be (21.5-)21.0(-19.0) Ma (J. Huang et al. 2023a).
Age. The [Browallieae + Cestreae] clade is around 21.7 Ma (Särkinen et al. 2013) or (47.3-)46.3(-44.1) Ma (J. Huang et al. 2023a).
5C. Browallieae Burnett - Browallia L. —— Synonymy: Browalliaceae Berchtold & J. Presl
Herbs to shrubs; (withanolides +); flowers ± strongly monosymmetric; A 4, 2 with anthers 2 thecate, filaments long, slender, 2 with anthers 1-thecate, filaments dhort, broad; pollen 3-7 colp/oroid/ate, surface striate to reticulate; style swollen horizontally corrugated and recurved apically, stigma expanded, ± 4-lobed; seeds many; embryo slightly curved, cotyledons ca 1/3 its length; n = 10-12.
3/10: Browallia (7). U.S.A. (Arizona) to Bolivia.
Age. Crown-group Browallieae are estimated to be ca 19.5 25.2 Ma (Särkinen et al. 2013) or (11.0-)10.6(-10.3) Ma (J. Huang et al. 2023a).
5D. Cestreae Dumortier —— Synonymy: Cestraceae Schlechtendal
Shrubs to trees (vines), plant odoriferous; (steroidal alkaloids - Cestrum); leaves often unequal; C (subcontorted-)valvate-induplicate; G ± stipitate; fruit a berry/(septi- + loculicidal capsule), raised annular rim at base [= remains of C]; seeds (1-)2-many, (narrowly winged); exotestal cells somewhat thickened on all walls, anticlinal walls straight - Cestrum; embryo ± straight, cotyledons quite broad, (>50% embryo length - Cestrum); n = 8, chromosomes 6-14 µm long, Arabidopsis-type telomeres absent.
3/190: Cestrum (175). Tropical and subtropical America.
Age. Cestreae are around 10.7 Ma (Särkinen et al. 2013) or (11.2-)10.4(-9.8) Ma (J. Huang et al. 2023a).
[Petunioideae [Nicotianoideae + Solanoideae]] (if this clade exists): low concentrations pyrrolidine-type nicotinoids; branching particularly distinctive [see below].
Age. This node has been dated to (28-)25, 23(-20) Ma (Wikström et al. 2001), ca 28.0 Ma (Särkinen et al. 2013), ca 21 Ma (Clarkson et al. 2017) and (63.9-)63.5(-60.2) Ma (J. Huang et al. 2023a).
A genome triplication sometimes placed here has been estimated to be anything from 95-39 Ma or so, and it has also been placed at the family level - see Genes & Genomes below.
6. Petunioideae Thorne & Reveal
(Annual) herbs to shrubs, (tussock plants [shrubby]); (root cork cambium superficial); (tracheids +); (stem cork cambium pericyclic); bordered pits +; pericyclic fibres +(0); druses 0(+); (plant ± aphyllous, resinous - Fabiana); (flowers monosymmetric); (K with hypodermal crystals); C cochlear, contorted, reciprocative [anterior C induplicate, covers other 4, conduplicate]; A 4(-5), usu. didynamous; (pollen in tetrads); (oil flowers, nectary 0 - Nierembergia); fruit a capsule, 4(2)-valved; exotesta with straight (sinuous - F.; closely and shortly sinuous - Petunia) anticlinal walls; endosperm copious, embryo straight to slightly curved, 55-25%; n = 7-11; plastid transmission biparental.
13/160: Brunfelsia (45), Petunia (35), Nierembergia (21). Central and South America, the Andes.
Age. Crown-group Petunioideae are some 25.2 Ma (Särkinen et al. 2013) or (50.7-)50.0(-45.4) Ma (J. Huang et al. 2023a).
[Nicotianoideae + Solanoideae]: (cotyledons accumbent); x = 12.
Age. The age of this clade has been estimated at ca 23.7 Ma (Wu & Tanksley 2010; Y. Wang et al. 2008), (17-)14, 12(-9) Ma (Wikström et al. 2001), (25.5-)24.0(-23.0) or ca 29 Ma (Särkinen et al. 2013), ca 30.2 or 26.2 Ma (Naumann et al. 2013), (72.8-)44.7(-23.0) Ma (Eserman et al. 2013), ca 17.5 Ma (Clarkson et al. 2017), (26.0-)25.0(-24.0) Ma (S. Wang et al. 2022) or (61.8-)61.3(-58.0) Ma (J. Huang et al. 2023a).
7. Nicotianoideae Miers —— Synonymy; Nicotianaceae Martynov
Embryo straight [?all].
9/128. Mostly Australia, few New World, Africa.
Age. Estimates of the age of crown-group Nicotianoideae are ca 14.6 Ma (Särkinen et al. 2013), ca 15 Ma (Clarkson et al. 2017) and (51.5-)49.6(-42.3) Ma (J. Huang et al. 2023a).
Unplaced - Symonanthus - 15.1 Ma/2 spp. 1.3 Ma (Särk. et al. 2013).
7A. Nicotianeae Dumortier
- Nicotiana L.Herbs (annual) to small trees; pyrrolidine-type nicotinoids; (cork cambium pericyclic); C contorted-conduplicate; A 4 (staminode +), 5, (didynamous); fruit a 4-lobed septi-loculicidal capsule, K at most weakly accrescent, seeds many; endosperm copious, embryo straight to slightly (strongly) curved; n also = 9, 10, 16.
1/95: Nicotiana. S.W. North America to South America E. of the Andes, Australia, 1 sp. Africa.
Age. Crown-group Nicotiana is around 9.0 Ma (Särkinen et al. 2013), ca 13 Ma (Clarkson et al. 2017), (13.8-)10(-6.4) Ma (S. Wang et al. 2022) or (20.6-)19.3(-13.2) Ma (J. Huang et al. 2023a).
7B. Anthocercideae G. Don
Shrubs to trees; pericyclic fibres +/0; (cork cambium pericyclic); (K with hypodermal crystals); C valvate-supervolute; anthers (pseudomonothecal, reniform); pollen tricolpate, colpus membrane granular, surface striate; fruit a septi-loculicidal capsule (berry), seeds 2≤; endosperm copious, with oil sector (oil 0, starchy), embryo curved, cotyledons <1/7th its length; n = 16, 18, 28, 30....
7/31: Anthocercis (10). Australia, 1 sp. New Caledonia.
Age. Crown-group Anthocercideae are estimated to be 9.6 Ma (Särkinen et al. 2013) or 11.2-11.3 Ma (Clarkson et al. 2017 - inc. Symonanthus, 15.1 Ma/2 spp. 1.3 Ma (Sä)).
8. Solanoideae Kosteletzky
Pyrrolidine alkaloids [hygrine, etc.], withanolides + [substituted 28C steroidal lactones]; pits not vestured; (interxylary phloem +); C valvate, cochlear, contorted; A 5 (4); integument 7-13 cells across; fruit a berry, (K very accrescent), seeds flattened, hilum ± lateral; exotestal cells with sinuous anticlinal walls, (radially elongated); endosperm cellular, ± copious, embryo curved, often coiled; chromosomes 1-14 µm long.
62/1,946. World-wide, but esp. South America.
Age. The age of crown-group Solanoideae has been estimated to be (23.3-)21.0(-19.0) Ma (Särkinen et al. 2013), ca 54 Ma (Dupin & Smith 2018) or (57.3-)56.3(-54.4) Ma (J. HUang et al. 2023a).
Fossils perhaps to be assigned to Solanoideae are dated at ca 33.9 Ma (Martínez-Millán 2010); see also below under Physalideae.
[Jaboroseae [Hyoscyameae [Sclerophylax [Nolaneae + Lycieae]]]] / Atropina clade: seeds many/fruit.
Age.The crown-group age of this clade is around 18.0 Ma (Särkinen et al. 2013) or (53.3-)50.5(-30.4) Ma (J. Huang et al. 2023a).
8A. Jaboroseae Miers (inc. Latueae)
Herbs to shrubs (thorns); C valvate in-/conduplicate; anthers mesifixed, filaments uncinate; pollen tricellular; stigma capitate-bilobed; fruit a bery; endosperm slight to abundant, embryo curved.
2/23: Jaborosa (22). Temperate South America, esp. Chile.
Age.The crown-group age of Jaboroseae s.l. is ca 15.3 Ma, and of Jaborosa itself ca 7.0 Ma (Särkinen et al. 2013); J. Huang et al. (2023a) estimate the age of Jaboroseae s.l. to be (48.9-)45.4(-27.9) Ma.
[Hyoscyameae [Sclerophylax [Nolaneae + Lycieae]]]: ?
Age. This clade is around 16.9 Ma (Särkinen et al. 2013) or (44.4-)41.9(-27.7) Ma (J. Huang et al. 2023a).
8B. Hyoscyameae Endlicher —— Synonymy: Atropaceae Martynov, Hyoscyamaceae Vest
Perennial herbs, with thick rhizomes/fleshy roots; tropane alkaloids +, hyoscyamine-type ; flowers usu. solitary; C aestivation cochleate; stigma discoid-capitate (depressed); K accrescent, capsule circumscissile; (endosperm helobial); cotyledons ca 50% or somewhat less; n also = 14, 17, 18..., whole genome duplication.
7/43. Eurasia, North Africa, Madeira, Canary Islands. Photo: Przewalkskia Fruiting Calyx.
Age. Crown-group Hyoscyameae are around 11.8 Ma (Särkinen et al. 2013) or (23.9-)22.3(-13.9) Ma (J. Huang et al. 2023a).
[Sclerophylax [Nolaneae + Lycieae]]: flowers rather small.
Age. The crown-group age of this clade is ca 12.2 Ma (Särkinen et al. 2013, see also Chiarini et al. 2022).
8C. Sclerophylax Miers —— Synonymy: Sclerophylacaceae Miers
Annual (perennial) herbs; (raphides and crystal sand +); stomata ± anisocytic [Cru]; plant procumbent/decumbent (erect); leaves unequally geminate, succulent, (linear-rhomboidal); ovules 1(2)/carpel, pendulous, apotropous, integument ca 10 cells across; fruit indehiscent, usu. sessile (pedicellate - sect. Caducifructus), ± embedded in the stem, K much accrescent and lignified, spiny; pericarp ca 2 cell layers across; seeds 1-3/fruit; testa 1 cell across, anticlinal walls sinuous; embryo straight (curved).
1/12. Argentina, adjacent Paraguay and Uruguay; halophytes.
Age. The age of crown-group Sclerophylax may be ca 4.5 Ma (Särkinen et al. 2013), although Chiarini et al. (2022) suggested that it was somewhat older, (12.4-)8.3(-4.4) Ma.
Age. This clade is ca 10.9 Ma (Särkinen et al. 2013) or (30.2-)28.4(-18.6) Ma (J. Huang et al. 2023a).
8D. Nolaneae Reichenbach - Nolana L. f. —— Synonymy: Nolanaceae Berchtold & J. Presl, nom. cons.
Herbs (annuals) to shrubs; crystal sand +; (leaves succulent); A 2 long 2 short [heteranthy]; tapetal cells binucleate; G (3-)5, (tangentially divided), (style gynobasic); ovules 1-several/carpel; fruit nutlets, 1-several seeded; exotesta with anticlinal walls sinuous; endosperm moderate, cots ca 60%.
1/ca 80. W. South America, N. Peru southwards, esp. near the coast (Loma), also the Galapagos.
Age. Crown-group Nolaneae are thought to be ca 6.3 Ma (Särkinen et al. 2013: N. sessiliflora sister to the rest) or (13.2-)12.4(-8.0) Ma (J. Huang et al. 2023a).
8E. Lycieae Lowe - Lycium L. —— Synonymy: Lyciaceae Rafinesque
Shrubs (thorns +); imidazolic alkaloids only, glycine betaines +; C aestivation cochleate(-plicate); ovules 1-many carpel; fruit a berry (drupe, stones 1-4-seeded), basal portion of C persistent; cotyledons incumbent; n = 12, also = 18, etc..
1/101. ± Worldwide, esp. southern South America, southern Africa and S.W. North America.
Age. Crown-group Lycium is 29.4 ± 9.7 Ma in Fukuda et al. (2001), about 4.9 Ma in Särkinen et al. (2013), (16.2-)15.1(-9.9) Ma in J. Huang et al. (2023a) or ca 25 Ma (H. Chen et al. 2024).
[Solandreae [Mandragoreae [[Nicandreae + Datureae] [Solaneae [Capsiceae + Physalideae]]]]] or - J. Huang et al. (2023a) - [Mandragoreae [Solandreae [Datureae [Nicandreae [Solaneae [Salpichroa [Jaltomata [Capsiceae + Physalideae]]]]]: ?
Age. This clade is some 20.2 Ma (Särkinen et al. 2013) or (56.8-)56.2(-54.0) Ma (J. Huang et al. 2023a).
J. Huang et al. (2023a):
Solandreae + = (56.0-)55.4(-53.5) Ma,
Datureae + = (55.3-)54.8(-53.1) Ma,
Nicandreae + = (54.9-)54.5(-52.9) Ma,
Salpichroa + Jaltomata + Phys. Cap. = (53.5-)53.2(-52.5) ma,
Jaltomata + Phys. Cap. = (53.0-)52.8(-52.2) Ma, Jaltomata (14.3-)13.1(-6.5) Ma.
8F. Solandreae Miers (inc. Juanulloeae)
Hemipiphytes, shrubs/lianes, climbing by roots; (myrmecophytes); leaves (on short shoots), lamina membranous/chartaceous/coriaceous, (margin entire), (venation indistinct); bracts (foliaceous), ?bracteoles (0); flowers often weakly monosymmetric, often large [C (2-)7->40 cm long/across], pendulous; C cochleate/valvate, (margins fimbriate); pollen grains oblate to spherical, surface various, inc. spinose; G (semi-inferior), [2-5] (4-10-locular); fruit a berry; seeds many; endosperm (sparse), embryo (slightly curved - Markea), cotyledons (broad), ac-/incumbent/oblique.
11/80: Solandra (10). Mexico to S. Brazil, Antilles, mostly Andean Ecuador and Colombia.
Age. Crown-group Solandreae are some 15.8 Ma (Särkinen et al. 2013) or (36.8-)32.1(-12.8) Ma (J. Huang et al. 2023a).
[Mandragoreae [[Nicandreae + Datureae] [Solaneae [Capsiceae + Physalideae]]]]]: ?
Age. The age of a clade [Mandragora [[Nicandreae + Datureae] ...]]] is ca 19.7 Ma (Särkinen et al. 2013).
8G. Mandragoreae Reichenbach - Mandragora L.
Perennial herbs, roots tuberous; C cochleate; pollen cryptoaperturate; stigma capitate; fruit a berry; endosperm slight, cotyledons unequal, >50% length
1/3-4. Mediterranean Europe to the Sino-Himalayan region; distribution not continuous.
Age. Crown-group Mandragoreae are ca 7.0 Ma (Särkinen et al. 2013) or (19.9-)18.2(-11.2) Ma (J. Huang et al. 2023a).
[[Nicandreae + Datureae] [Salpichroa [Solaneae [Capsiceae + Physalideae]]]]: ?
Age. The crown-group age of this clade is ca 17.9 Ma (Särkinen et al. 2013).
[Nicandreae + Datureae]: flowers solitary; anthers with hairs; embryo curved.
Age. This clade is some 15.9 Ma (Särkinen et al. 2013).
8H. Nicandreae Lowe - Nicandra Adanson
Annual herbs; K segments auriculate, C cochlear-plicate, internode between K and C; G [3-5], stigma capitate-lobed; fruit a berry, K strongly accrescent; cots <1/2 length; n [rather odd] = 10, 19, etc..
1/3. Peru to N. Argentina.
8I. Datureae Dumortier —— Synonymy: Daturaceae Berchtold & J. Presl
Annual herbs to small trees; (thorns +); (short shoots + - Trompettia); flowers large [>3cm long]; C contorted-conduplicate; A latrorse/extrorse; pollen surface ± striate, striae with longitudinal or oblique ornamentation; stigma ± bilobed; fruit a septi-loculidal capsule/berry, (K deciduous); seeds many, flattened, with elaiosome / ± tetrahedral, ± corky; (cotyledon apex recurved).
3/20: Datura (9). Southwest U.S.A. and Mexico, Venezuela to Bolivia, Brazil (Atlantic Forest).
Age. The age of Datureae is (46.9-)34.7(-23.8) Ma (Dupin & Smith 2018), the [Dat. + Brug.] split is dated to (32.2-)29.1(-15.1) Ma (J. Huang et al. 2023a).
[Salpichroa [Jaltomata [Solaneae [Capsiceae + Physalideae]]]]: fruit a berry.
Age. This clade is estimated to be 17 Ma (Särkinen et al. 2013) or (54.0-)53.7(-52.5) Ma (J. Huang et al. 2023a).
Herbs (annual) to shrubs (climbing); flowers single (paired) at a node; K divided ± to base; anthers connate around style, (filaments expanded at apex); pollen tricellular; anticlinal walls of exotesta tall, hairlike/not; endosperm rather slight, embryo subcoiled.
1/20. Mexico, The Andes.
Age. This clade may be (53.0-)52.8(52.2) Ma (J. Huang et al. 2023a).
[[Jaltomata [Capsiceae + Physalideae]] Solanum] - if this clade exists: ?
Age. The Solanum/Capsicum split, described as corresponding to the common ancestor of Solanum and Physalis, has been dated to (21-)19(-17) Ma (Särkinen et al. 2013), who suggest that Solaneae are sister to Capsiceae, etc., and in turn to the [Nicandreae + Datureae] clade. The age of the Solanum/Capsicum clade is also estimated to be ca 27.6 Ma (Hoshino et al. 2016) or ca 19.6 My (Wu & Tanksley 2010; Y. Wang et al. 2008), while the age of the [[Jaltomata [Physalis [Lycianthes + Capsicum]]] Solanum] clade is estimated to be ca 53.2 Ma (Messeder et al. 2024).
[[Jaltomata [Capsiceae + Physalideae]] - if this clade exists: ?
8K. Jaltomata Schlechtendal
Herbs (annual) to shrubs; nectary ducts/"corona" + [between filament bases]; genome duplication + [?: unlikely].
1/47. S. U.S.A., the Neotropics.
Age. The crown age of Jaltomata may be (14.3-)13.1(-6.5) Ma (J. Huang et al. 2023a).
Age. The age of this clade is ca 16.7 Ma (Särkinen et al. 2013) or ca 52.2 Ma (J. Huang et al. 2023a).
8L. Capsiceae Dumortier
Herbs (annuals) to trees (vines); leaves (amphistomatous); K margin often truncate, ± entire, appendages (0-)5-10, (in 2 series), linear/laterally flattened/etc., C rotate to stellate, valvate; A (4 + 1 larger), spreading/erect, anthers dehiscing by pores/slits; nectary ducts + [between filament bases]; stigma discoid to bilobed; K not accrescent, fruit (a drupe, with 1-2-seeded pyrenes); cots ca 50%; (n = 13).
2/243: Lycianthes (150/200), Capsicum (43). Southern U.S.A. to Argentina, East Asia. Photo: Flower.
Age. Diversification in Capsiceae began around 13.2 Ma (Särkinen et al. 2013) or (45.4-)43.5(-31.0) Ma (J. Huang et al. 2023a).
Deanna et al. (2023) describedLycianthoides calycina from fossils ca 51 MA collected in the Green River Area, Colorado.
8M. Physalideae Miers
Perennial herbs to small trees; inflorescence axillary fascicles; K (entire - Witheringia), C valvate (contorted); (stapet auriculate/filaments winged); (nectary ducts +) [between filament bases]; stigma capitate to bilobed; K not/some/very [= inflated] accrescent in fruit; endosperm (scanty), cotyledons = or much shorter; chromosomes asymmetrical (± symmetrical - Iochrominae).
29/ca 300: Physalis (90), Deprea (50), Iochroma (34). The Americas, Withania scattered in the Old World, also St Helena, Hawai'i and the Canaries. Photo: Iochroma Flower.
Age. Crown-group Physalideae are about 14.7 Ma (Särkinen et al. 2013: Cuatr. + Wither. sister to rest) or (42.7-)40.4(-27.8) Ma (J. Huang et al. 2023a: different topology).
Wilf et al. (2015, esp. 2017a; see also Deanna et al. 2020) described ca 52.2 Ma fossil fruits from Chubut, Argentina, which they placed in crown Physalis and Deanna et al. (2023) the fossil Eophysaloides inflata from deposits 51.5-49.5 Ma in Colombia.
8N. Solaneae Dumortier - Solanum L.
Herbs (annuals) to shrubs; (primary root to hexarch); steroidal glycoalkaloids +; (stellate hairs +), (prickles +); pedicels articulated/not; C rotate, (tubular, campanulate), aestivation valvate or valvate-induplicate; anthers dehiscing by pores, ± connate, (pollen exiting communal apical hole), (A free, with slits), filaments enlarged at base; endothecium 0; nectary 0; stigma capitate or bilobed; seed (hairy [hairs = strips of secondary thickening - "baguettes"]); (n = 11, 15), nuclear genome [1C] 1.26-2.08 pg.
1/1,238. ± Worldwide, esp. South America, Australia, but not cool N. temperate, North Africa or central Asia.
Age. Crown-group Solaneae are estimated to be (19-)17(-15) Ma (Särkinen et al. 2013), (41.7-)39.0(-21.7) Ma (J. Huang et al. 2023a) or (45.2-)45.0(-44.7) Ma (Messeder et al. 2024).
Evolution: Divergence & Distribution. The ca 52.2 Ma fossil fruits from Chubut, Argentina, placed in crown Physalis (Wilf et al. 2017a; Deanna et al. 2020) rather confuse dating and, if confirmed, must change our ideas about the evolution of Solanaceae: Not only are these findings over twice the molecular ages of crown Solanoideae above, but the fossils are in a quite derived clade within Solanoideae. Even if Särkinen et al. (2018) are correct in their suggestion that these fossils should be assigned to stem Solanoideae, there are still major problems with dating, since stem Solanoideae have estimated to be a mere (25.5-)24.0(-23.0) or ca 29 Ma (Särkinen et al. 2013), however, a more recent estimate of the age of crown-group Solanoideae taking recent fossil findings into account is ca 54 Ma (Dupin & Smith 2018), while Särkinen et al. (2018) suggest that the fossil Solanispermum and Solanum arnense, 48-44 Ma, also might represent stem Solanoideae, however, the ca 50 Ma Cantisolanum daturoides is probably the seed of a commelinid monocot... (for many more ages throughout Solanaceae, see Särkinen et al. 2013: Additional file 2). Ages in J. Huang et al. (2023a) are considerably older than those in Särkinen et al. (2013), as might be expected. But even in Huang et al. (2023a) there was considerable variation in ages for the same branching point. Thus age estimates for crown-group Solanaceae were 83.3, 73.3 or 65.1 Ma depending on the calibration method - Huang et al. (2023a) prefered a calibration that gave the intermediate ages. Recent findings of fossils of Physalideae from Colombia ca 50 MA and of Lyciantheae from Colorado, U.S.A., also ca 50 Ma (see Deanna et al. 2023), confirm the older ages with which this paragraph started. Indeed, a recent estimate of the crown-group age of Solanum is around (45.2-)45.0(-44.7) Ma (Messeder et al. 2024), so clearly ages are being nudged upwards, and the origin of the family is now thought to be in the Palaeocene or Late Cretaceous (Deanna et al. 2023).
The early-diverging clades in the family are temperate and/or Andean-South American in distribution, perhaps reflecting the original climatic preferences of Solanaceae (Olmstead 2013: much on possible niche conservatism there). The family is most diverse in the New World, particularly South America, where it grows in a variety of habitats, including along the foggy west coast, where it is particularly common - with ca 75 endemic species - in Lomas vegetation (Barboza et al. 2016). Solanaceae are less common elsewhere, particularly in Africa. Solanaceae may have had a New World origin, with perhaps 8-9 dispersal events to the Old World (Tu et al. 2010). Olmstead (2013) suggested that dispersal was involved in shaping the distributions of ten clades, but he could not find any connection between the likelihood of dispersal and disseminule type (dry versus fleshy). The Malagasy endemic Tsoala (Goetzeoideae) was found to be sister to Metternichia, from Minas Geraes, Brazil (Särkinen et al. 2013: Add. File 2), and Janssens et al. (2015) suggested that long distance dispersal (America to Madagascar) was involved in setting up this disjuction. Interestingly, self-incompatible (SI) Lycium dispersed to Africa, presumably with a dispersal-caused bottleneck in the diversity of SI alleles, but there was subsequent restoration of this diversity (J. S. Miller et al. 2008). Over one third of the some 256 estimated dispersal events in the family were from South to Central or North America, and vicariance was relatively uncommon (Dupin et al. 2017: biogeographical stochastic mapping). Thus Datureae spread from Andean South America to Brasil and Central and North America (Dupin & Smith 2019). There were a number of trans-oceanic dispersal events, ages in Solanaceae (see also Särkinen et al. 2013) being substantially post continental drift (Dupin et al. 2017). However, accepting the early Eocene age of the Patagonian fossils of Physalis, old continental configurations, for example, those allowing movement between South America and Australia via Antarctica, might well have facilitated the early distribution of the family (Wilf et al. 2017a; Deanna et al. 2020, 2023); dispersal might initially not have been so important.
The [Nicotianoideae + Solanoideae] clade includes ca 85% of the species in the family (Olmstead & Sweere 1994), and Schranz et al. (2012) suggested that there was a lag time between a genome duplication event that characterizes this clade and its subsequent diversification, this latter largely represented by the speciose Solanoideae with their fleshy fruits (see also Vanneste et al. 2014b). Similarly, Bombarely et al. (2016) suggested that genes involved in floral pigmentation patterns might have evolved considerably after a genome triplication event. Soltis et al. (2009) place a genome duplication as an apomorphy of the species-rich Solanoideae, although with hesitation - however, ages for the event vary (see also Genes and Genomes below), and it may even have nothing particularly to do with Solanaceae at all. Vanneste et al. (2015) link the evolution of fruit fleshiness - especially evident in Solanoideae - in the family to the genome triplication event. Deanna et al. (2018b/2019) looked at the repeated evolution of inflated calyces in the fruits of Physalideae; this has occurred some 24 times, but it has been lost only twice (the step from non-accrescent to accrescent-appressed calyces is never reversed). L. Wang et al. (2015) also discuss the development and evolution of fruit morphology in the family.
Given the age estimates of Särkinen et al. (2013), there seems to have been a ca 20 Ma hiatus or "fuse" between the origination of the family and its crown-group diversification, although this is not evident in J. Huang et al. (2023a). Indeed, times they are a-changing - Huang et al. (2023a) note that nine or so clades in Solanoideae had originated between 56.7 and 52.2 Ma. The crown-group age for the ca 1,200 species of Solanum (inc. tomato, potato and eggplant) was initially estimated to be a mere 15.5 Ma or so (add Capsicum - ca 19.6 My: Wu & Tanksley 2010; Y. Wang et al. 2008); Paape et al. (2008: see also estimates for other nodes) gave ages of (20.6-)16.1(-12.2) Ma, while (17.5-)15.5(-13.3) Ma ((18.7-)17.0(-14.5 My) - Jaltomata sister, (21.0-)19.1(-17.0) Ma - add Capsicum) are estimates in Särkinen et al. (2013, q.v. for much else), while Echeverrí-Londoño et al. (2020) suggest an age of (18-)ca 15(-13) Ma, although other estimates using recent fossil findings as calibrations are much older (J. Huang et al. 2023a), and in general older ages seem likely here - see also the beginning of this section. Solanum is New World in origin, and that is where most species and overall diversity are to be found; divergence within the genus may have been after a lag (Huang et al. 2023a). There have been at least seven shifts to the Nearctic (Solanum does not like conditions much cooler than temperate, but c.f. the Petota group in part) and perhaps four to the Old World. In the genus as a whole diversification is currently highest in the Australian spiny tomato clade - movement was perhaps initially from America to Africa (7-)6(-5) Ma - although there are no distinctive features obviously/immediately associated with this radiation (Echeverrí-Londoño et al. 2020). However, relationships in the genus immediately above subgenus Thelopodium are unclear, and may even best be represented by a polytomy (hard), which makes thinking about biogeography and character evolution rather difficult (Gagnon et al. 2022). Indeed, it had been thought that the ancestors of potatoes and tomatoes were plants of the hyperarid lomas vegetation, but the revised phylogeny suggests that they were plants of tropical montane to subtropical biomes (Gagnon et al. 2022). Adaptation in Solanum sect. Lycopersicon has been linked to introgression, recruitment from ancestral variation, and de novo mutations (Pease et al. 2016). Recently the evolution of the some 107 species of the petota clade, to which the potato belongs, has been studied in detail (Z. Zhang et a. 2025). There was an homoploid hybridization event around 9.0-8.4 Ma between species of the rhizomatous etuberosum (3 species) and the tomato (17 species) clades that resulted in the evolution of stem tubers on stolons, a distinctive feature ("key innovation") of the petota clade; subsequent diversification of this clade has been quite rapid as it moved ito higher elevation areas with colder climates as the Andean uplift occured, now being widely distributed in Central America and west South America in particular (Z. Zhang et al. 2025).
Medium-sized (1.3-3 cm) green fruit seem to be ancestral in Solanum, and they are perhaps dispersed by fruit-eating phyllostomid bats, diversification both of Solanum with such fruits and of the bats at ca 14 Ma being more or less contemporaneous; in addition to the medium-sized green fruits there are small, variously coloured (black, purple red) fruits, often dispersed by birds, and larger, yellowish to greenish fruits, often dispersed by non-flying mammals, and in these latter smell as a cue may be important. There is phylogenetic conservatism in fruit type in Solanum, although other factors are clearly also involved in fruit evolution here (Messeder et al. 2024; c.f. Valenta & Nevo 2020); overall, there have been around 83 changes in fruit colour during the evolution of Solanum, the most frequent shift being from green to yellow.
It has proved difficult to understand inflorescence morphology, indeed, its construction appears to be unique. The basal condition for the family, an apomorphy, is to have monochasial-like cymes with extended inflorescence internodes, while dichasium-like cymes and clustered inflorescences (here these internodes are reduced, and in taxa like Solanum bracts, etc, are lost) are derived (J. Zhang et al. 2022). Furthermore, the basic plane of symmetry in flowers like Salpiglossis and Schizanthus is oblique/inverted, and the abaxial (and also two adaxial) stamens are sterile (see also Zhang et al. 2022 for oblique orientation). The complex flower of Schizanthus is described as having oblique rather than inverted symmetry (Cocucci 1989b), and the two are functionally equivalent, but c.f. Ampornpan (1992: p. 87), "Schizanthus bipinnatus showed no indication of any oblique orientation of either type", i.e., when the abaxial sepal was slightly oblique to the subtending bract and/or flowers were displaced by growth of axillary buds, while for Walters (1969: p. 17), the gynoecium was "oriented obliquely to the symmetry of the corolla". For CYC gene expression here, see Preston et al. (2011b). For floral evolution in Schizanthus, not very speciose, see Pérez et al. (2006: midpoint rooting, 2007: functional floral integration and pollination mode; Chinga et al. 2021: complex heterochronic changes, sometimes differences within the one corolla primordium). Pérez (2011) found that morphological and genetic divergence here were not always in synchrony; for more on floral development, see Chinga and Pérez (2016).
J. Zhang and Zhang (2016) and Zhang et al. (2017) noted that in Solanaceae monosymmetry is more frequently expressed in the androecium than in the corolla (as heteranthy) - it is almost twice as frequent in the former, although monosymmetry in the two is correlated. Overall, monosymmetry has been gained 17-20 times in the corolla and lost 24-26 times in the androecium (Zhang et al. 2017: findings depend on breaking down the character "monosymmetry"). The basic plane of symmetry of the flower is at 36o to the vertical, and it is the abaxial stamen along that plane that is first to be modified (Robyns 1931; Zhang et al. 2017). More or less well developed 3:2 monosymmetry is quite common (see also Eichler 1875; Robyns 1931; Cocucci 1989a, b; Hunziker 2001; Knapp 2002a; Ampornpan & Armstrong 2002; Bukhari et al. 2017). In the complex monosymmetric flowers of Schizanthus (see above) dehiscence of the two functional anthers is usually explosive (Cocucci 1989a).
Clarkson et al. (2017) discuss diversification in Nicotiana, interestingly, there was a 4-6 Ma lag in the polyploidy event associated with the origin of section Suaveolentes, with ca 26 species, and its subsequent diversification, although the cause of this is unclear; N. africanum, from Namibia, is sister to the rest of the group. Younger ploidy events here are not associated with much diversification at all.
For the evolution of Sclerophylax, see Chiarini et al. (2022: quite some detail); the genus is likely to have originated in the Prepuna area of N.W. Argentina.
Lycium, with some 80 species, is described as having "one significant mass extinction around 23 mya" soon after the origin of the genus, and its diversification rates were "among the slowest diversification rates in the plant kingdom" (H. Chen et al. 2024: pp. 5, 9). The mass extinction is not specified further, and there are several older clades even within Solanaceae that have (far) fewer species, so the import of what is going on with diversification rates here is unclear - "most gradual" is probably meant (c.f. title). Earlier suggestions of a Gondwanan distribution (Symon 1991) have no support; South America is the likely place of origin of the genus and long distance dispersal is favoured over vicariance as the way in which its broad distribution was achieved (Chen et al. 2024) - but again, understanding geography and climate at the time when the genus evolved is critical.
Larter et al. (2018) looked at changes in colour of the corolla in the Iochroma group (Solanoideae-Physalideae) and found developmental parallelisms at the level of regulatory control.
For withanolides - steroidal lactones, many based on a C28 ergostane with a δ-lactone/lactol side chain - and phylogeny, see Misico et al. (2011; also Burton and Oberti 2000; L. Chen et al. 2011; Pigatto et al. 2014). However, it has quite recently been found that an enzyme involved in an early step of withanolide synthesis is also involved in the synthesis of C28 petuniasterones, i.e. in taxa with capsular fruits, although it has not been found in Nicotiana (Knoch et al. 2018), so the story is becoming more complex.
Ecology & Physiology. Solanaceae are an important component of understory vegetation in the l.t.r.f. of the New World. Solanum in particular, with its relatively nutritious fruits, is an important food source for Sturnira, a phyllostomid bat (Fleming 1986; Lobova et al. 2009 for records). The bats are slow feeders and spit out seeds, fibre, etc.; Solanum, like other bat-dispersed taxa in the New World, tend to be early successional plants (Muscarella & Fleming 2008), and the altitudinal ranges of the bats and plants are similar (Fleming 1986).
Dillon et al. (2009) looked at the evolution of Nolana, a clade of the coastal deserts (lomas) in the Atacama Desert of W. South America; the genus was perhaps originally from Peru. Nolana is the most speciose genus in this remarkable Peruvian-Chilean vegetation. The hyperarid deserts in which it grows have ≤5 mm rain/year and have been dated to ca 8 Ma; a clade of Nolana invaded the Atacama desert ca 3.8 Ma, and another five clades rather later, ca 2 Ma. Water uptake in N. mollis has been studied in some detail, and is predominantly via roots deep in the soil; previous suggestions were that atmospheric water condensed on salt exuded by the leaves, and then was taken up by the plant via the leaves, or water dripping from the leaves was taken up by shallow roots (Gersony et al. 2025). Interestingly, this region has been semiarid, with ≤250 mm rain/year, since the Late Jurassic ca 150 Ma (Guerrero et al. 2013).
In Datura, there was little correlation between animal defences and phylogeny (Cacho et al. 2015), and different defences had different ontogenetic trajectories (Kariñho-Betancourt et al. 2015). Boccia et al. (2024) observed that a cellulose synthase-like protein, GLYCOALKALOID METABOLISM 15 (GAME15), a member of the cellulose synthase-like M subfamily in Solanum nigrum, was located on the endoplasmic reticulum where it is an essential scaffold protein ensuring the synthesis of steroidal saponins and glycoalkaloids (= SGAs), cholesterol being a precursor; scaffold proteins like GAME15 bind to other proteins, organizing them into a functional unit; they improve signaling efficiency, ensure that the steps in a synthetic sequence occur in the right order (GAME 15 et al. form a metabolon), etc.. Jozwiak et al. (2024) found that GAME15 was also a cholesterol glucuronosyltransferase. Both steroidal saponins and glycoalkaloids are involved in plant defence, e.g. against herbivores, and such metabolites are found in a variety of species of Solanum (Boccia et al. 2024), and in plants glycoalkaloids/steroidal saponins are commonest in Solanaceae, especially Solanum, and they are also scattered in many other families, including members of Liliales, Asparagales, etc. (Roddick 1996).
Paungfoo-Lonhienne et al. (2010) found that nitrogen from Escherichia coli and Saccharomyces cerevisiae that had entered intact tomato (Solanum esculentum) roots was used by the plants as the protists were broken down, so tomatoes are perhaps technically carnivorous (see also Selosse et al. 2017c).
Pollination Biology & Seed Dispersal. Cocucci (1999) and Knapp (2002a, 2010) summarise information on pollinators and basic floral morphology of Solanaceae. Nierembergia (Petunioideae), with ca 21 species, has oil flowers, the oil being secreted by multicellular hairs, and ther flowers attract a variety of oil bees (Coccucci 1991; Renner & Schaefer 2010; Possobom & Machado 2017a; Tölke et al. 2019 and references; Tate et al. 2009 for a phylogeny). It has recently been shown that the Carolina sphinx/tobacco hornworm, Manduca sexta, is preferentially attracted to Nicotiana flowers that have a corolla tube the "right" length for its proboscis by the particular volatiles produced by those flowers, but not those by flowers with tubes of different lengths: Is this co-evolution (see Haverkamp et al. 2016)?; what is going on in the other flowers that the Carolina sphinx pollinates?
Within Solanoideae, the Andean Iochrominae are notably diverse florally and flowers here have a variety of pollinators; there is significant variation in flower colour in both bee- and bird-pollinated species when in sympatry (S. D. Smith & Baum 2006; Muchhala et al. 2014; Smith et al. 2018). Ibañez et al. (2018) examined the evolution of corolla tube length in Andean Salpichroa, finding both increases (pollination by Ensifera ensifera) and decreases. Ng and Smith (2015) look at the evolution of red flowers in the family - ca 34 species have such flowers, and they have originated some 30 times, and all within the last 11 Ma. Although the red colour is produced in three main ways, closely related red-flowered species are likely to have the same biosynthetic pathway (Ng & Smith 2015). A number of species of Cyphomandra (= Solanum subgenus Leptostemon) are pollinated by orchid bees that collect fragrances from the connectives, but also simultaneously pushing against the thin walls of the anthers which behave as little bellows, directing jets of pollen on to the bees (Sazima et al. 1993). For the evolution of floral scents, see Martins et al. (2007). In Jaltomata, sister to Solanum, there is considerable variation in corolla morphology as well as in the dynamics of nectar secretion that can be interpreted in terms of heterochronic shifts (Kostyun et al. 2017); nectar here may be red (R. J. Miller et al. 2013). Indeed, thte nectar in Jaltomato calliantha may be held in well-like structures in which the nectar pools develop between the stamens; called a corona, they appear to be a novel recruitment of the stamen identity program, although some C-class genes are also involved, and they originate from the bases of adjacent stamens (Kostyun et al. 2019). A number of Solanoideae have capillary nectary grooves on the bases of the petals along which nectary moves, sometimes to dark spots on the corolla whence it is removed (Dong et al. 2013); for nectar secretion, see I. W. Lin et al. (2014) and Solhaug et al. (2019) and references. Oil may be collected by bees, as in Nierembergia (Cocucci 1991).
Buzz pollination is common in Solanum in particular, indeed, Solanum and relatives probably make up the largest group of buzz-pollinated angiosperms (see Cocucci 1999; Teppner 2005: pollination of the tomato; Harter et al. 2002; Carrizo García et al. 2008; Vallejo-Marín et al. 2021/2022; Falcão & Stehmann 2018; Vallejo-Marín 2019; Cardinal et al. 2018 - the last three buzz pollination in general), although in Jaltomata buzz pollination is uncommon. The corolla is often rotate, the flowers lack nectar, there is a central cone of anthers dehiscing by terminal pores, etc., all features of buzz-pollinated flowers (= the solanoid flower - Faegri 1986). There is considerable variation in androecial anatomy, whether the anthers are connate, forming a tight cone, and if so, in how the anthers become connate, and so on. Thus in S. lycopersicum the anther cone is held together by interlocking anther hairs while in S. dulcamara the closely appressed anther surfaces are in part held together by extracellular secretions, not hairs, indeed, if hairs are induced on the anthers, the cone falls apart; both species are considered to have a well developed cone of the "pepper pot" type (Glover et al. 2004). Although Glover et al. (2004) thought that species with the pepper-pot androecium formed a clade, the plesiomorphous condition being a less coherent androecium, the "salt shaker" type, from the phylogeny in Weese and Bohs (2007) the two cone variants seem to have evolved independently. Zygomorphy and heteranthy, which in this context (there is only a single whorl of stamens) is really a kind of zygomorphy (see also Zhang et al. 2017), have evolved several times in Solanum, also in other Solanoideae like Sclerophylax, etc. (Bohs et al. 2007). In heteranthous flowers some of the anthers may be feeding anthers, while others deposit pollen on the pollinator in such a way that pollination can take place (Stern & Bohs 2012); the pollen grains from the two anther morphs are primarily involved either in pollination or in feeding the pollinator (Vallejo-Marín et al. 2014). The morphology of the androecium varies, with consequences for details of the pollination process - thus when anthers form cones, transmission of the bee-induced vibrations is improved, and there is increased pollen release (e.g. Nevard et al. 2021; Vallejo-Marín et al. 2021/2022). However, Nunes et al. (2020/2021) found that the fundamental frequencies of the buzzes of different species of bees and the natural frequencies of the vibrations of the anthers of different species of Solanum did not always match. Over a million pollen grains can be produced by a single flower (G. J. Anderson & Symon 1988). C. D. Moore et al. (2024) looked at changes in scent production in Solanum flowers after the removal of pollen - they were uncommon (1/7 species). Lycianthes is another genus that has buzz pollination - it is interesting that Capsicum, in which nectar is the main reward, may be derived from within it (Särkinen et al. 2013; Carrizo García et al. 2016; Spalink et al. 2018). For more on buzz pollination, see elsewhere.
The common ancestor of Solanaceae is likely to have had RNase-based gametophytic self incompatibility (SI) (Paape et al. 2008; see also Zhang & Xue 2008; McClure 2008), and self-compatability (SC) has since evolved many times, but never SI from SC; for a detailed study of the frequent loss of gametophytic incompatibility in Solanaceae, see Igic et al. (2006). Overall diversification of SI clades is greater than that of SC clades, yet SC species are frequent perhaps because of the frequency of the SI → SC transition and high speciation within those clades - and they also have high extinction rates (Goldberg & Igic 2012: scoring of dioecious taxa?; Goldberg et al. 2010), although other models fit the data (Bromham et al. 2015b). For the loss of SI in Jaltomata, which happened a relatively long time ago (>3 Ma), see M. Wu et al. (2019). The evolution of polyploidy and self-compatibility in the family are correlated (J. S. Miller at al. 2008; Robertson et al. 2010). For the evolution of breeding systems in Lycium and Solanum, see Goldberg et al. (2017), and for gametophytic SI in the former, see Y.-L. Cao et al. (2021).
Androgenesis, an uncommon condition in which the male gamete produces an embryo in maternal cytoplasm, basically asexual reproduction of the male nuclear genome, has been recorded in at least Petunia, Nicotiana and Capsicum, in Petunioideae, Nicotianoideae and Solanoideae respectively (Chat et al. 2003 for references; Hedtke & Hillis 2011; c.f. paternal apomixis in Cupressus).
How seeds are dispersed is very much that which fruit morphology might suggest (see Knapp 2002b for a summary; also Vanneste et al. 2015: evolution of fruit fleshiness; L. Wang et al. 2015: evolution of fruit morphology). In Physalideae in particular, taxa with an inflated calyx ("Japanese lanterns") are common, and although the function of such a calyx is unclear, there have been ca 24 transitions to a fully inflated calyx - and very few reversals (Deanna et al. 2018b/2019). Neotropical phyllostomid bats rely mainly on fruits of SolanumCecropia, Ficus, Piper and Vismia (Fleming 1986), plants often of secondary vegetation. The berries of Solanum sect. Gonatotrichum are explosive... (Stern & Bohs 2012).
Plant-Animal Interactions. Most Solanaceae synthesize a variety of metabolites including nicotinoids, capsaicinoids, and steroidal alkaloids and withanolides (a steroid backbone to which a lactone is attached) of varying degrees of toxicity that defend the plant against herbivores - these are not produced by all taxa, by any means (Wink 2003; Misico et al. 2011: withanolides; Y.-J. Wang et al. 2023). The multiple lines of defence in the plants cause most insect herbivores to avoid them (Harborne 1986; Hsiao 1986), nevertheless, New World Solanaceae are eaten by larvae of some 390 species of Nymphalidae-Danaeinae-Ithomiini (or Ithomiinae) butterflies, and the butterflies seem to have switched host plants from Apocynaceae q.v., perhaps from Parsonsieae in particular (they also eat a few Gesneriaceae: Ehrlich & Raven 1964; Edgar 1984; Brown 1987; Drummond & Brown 1987; Willmott & Freitas 2006). Diversification of the ithomiines may have picked up some time after the switch to Solanaceae, since the basal ithomiine subtribes Melinaeina and Mechanitina are not particularly speciose (Peña & Espeland 2015). There seem to be no records of caterpillars eating Schizanthoideae, Goetzeoideae or Schwenckioideae, but Solanum is particularly favoured comprising ca 70% records of Neotropical Solanaceae food sources and ca 89% those of all Ithomiini (Willmott & Freitas 2006; see also Brower et al. 2006; Garzón-Orduña et al. 2015; Peña & Espeland 2015). Interestingly, apart from the possible exceptions mentioned, most species of Solanaceae are eaten by ithomiine larvae, perhaps suggesting that the host plant niche is almost saturated by the butterfly (Willmott & Elias, in Elias et al. 2009). Strict co-evolution seems not to be involved, i.e. there is no co-speciation (see also De-Silva et al. 2017), but the diversification rate of the butterflies seems to have temporarily increased with this shift (Fordyce 2010) which occurred within a larger clade of butterflies that utilizes Solanaceae (Hamm & Fordyce 2015). The mimicry rings in which Ithomiini are involved may be associated with particular solanaceous host plants (Willmott & Mallet 2004).
However, the timing of this ithomiine radiation is problematic. Their move on to Solanaceae has been estimated as happening 46-37 Ma (Nylin et al. 2013 and references), with diversification beginning at middle elevations on the Andes in the middle Miocene some 15 Ma, while The origin and diversification of ithomiine clades in the northern Andes has occurred within the last 15-10 Ma (see above: De-Silva et al. 2017). The ithomiine Pteronymia and relatives seem to be quite young, younger than their hosts - thus the age of crown Solanum is some (29.5-)20.9(-14.5) Ma, and Solanaceae are common all along the Andes today (Elias et al. 2009). Wahlberg et al. (2009) had suggested that Ithomiini were (40.3-)37.1(-34) Ma old, but using calibrations derived from the Solanaceae phylogeny of Särkinen et al. (2013), Garzón-Orduña et al. (2015: Nylin et al. not mentioned) estimated that the ages for nodes within Ithomiini in particular were about half the ages of those suggested by Wahlberg et al.. In another estimate, ithomiines as a whole started diversifying ca 33 Ma (De-Silva et al. 2017; see Wahlberg et al. 2009; c.f. Garzó-Orduña et al. 2015). Mechanitina—Godyridina are a major ithomiine clade (see Garzón-Orduña et al. 2015) for which feeding on Solanum may be basal, and its age was estimated to be around 30 Ma by Wahlberg et al. (2009), far older than the (20.6-)16.1, 15.5(-12.2) Ma ages of Solanum mentioned above. Younger ithomiine ages are not only more in line with host-plant ages, but they fit scenarios of Andean uplift better. However, if there are 52 Ma fossils of crown Physalis from Argentina (Wilf et al. 2015, esp. 2017a), what then?
Some ithomiine larvae may be distasteful because of tropane alkaloids, etc., they obtain in the leaves they eat, although this may be at best uncommon, except in basal ithomiines (Drummond 1986; Brown 1987; Opitz & Müller 2009). However, the noxious solanaceous chemicals may guide both oviposition by adults and the feeding preferences of the larvae: "Though the butterflies may be able to recognise their food plants, biologists have greater difficulty in Solanaceae identification" (Brown 1987: p. 373). Adult ithomiine butterflies are also distasteful because of the 1,2-dehydropyrrolizidine alkaloids monoesters that they obtain mostly from Apocynaceae, Heliotropaceae and Asteraceae-Asteroideae (especially Eupatorieae), and these can also be the precursors of the pheromones that they produce. The adult butterflies are quite palatable immediately after hatching, but that soon changes, and massive amounts (to ca 20% dry weight) of these chemicals may be sequestered (Brown 1987; see also Bowers 1993). Interestingly, Ithomiini preferentially visit bait with withered flowers, while Arctiinae moths, who also go after these alkaloids, prefer crushed roots. Arctiine caterpillars may self-medicate on material high in alkaloids (Singer et al. 2008). For a survey of the insecticidal properties of alkaloids, especially steroidal glycoalkaloids and tropane alkaloids, found in Solanaceae, see Chowanski et al. (2016), and for the synthesis of such alkaloids, see Y.-J. Wang et al. (2023: note that Aacutangulus acutangulus = A. acutangulus var. acutangulus = Anisodus acutangulus var. acutangulus), with more details under Erythroxylaceae.
Tobacco hornworm caterpillars were found to prefer members of the [Solanoideae + Nicotianoideae] clade as food sources, although they didn't like Nicandra much; they died on Petunia, and did not grow on Browallia and Brunfelsia. Other plant feeders show similar distinctive patterns (e.g. Fraenkel 1959), thus some sphingids are found here and on Oleaceae (Forbes 1958). Rauscher and Huang (2015) note that a gene duplication of threonine deaminase in Nicotianoideae and Solanoideae is involved in herbivore defence, sometimes by depleting threonine in the gut of the caterpillar; selection on this gene may have been very prolonged, for around 25 Ma or far more. Phytophagous Chrysomelidae beetles (perhaps especially Criocerinae) are notably more common on New World than Old World Solanaceae, perhaps because the beetles first used the family as a food source in the former area (Jolivet & Hawkeswood 1995; see also Hsiao 1986); Criocerinae may have moved onto Solanaceae from monocots; some chrysomelids found on New World Solanaceae are covered by faecal shields (Vencl & Morton 1999; see Jolivet 1988; Gómez-Zurita et al. 2007). Finally, in Solanum dulcamara nectar-like substances exude from wounds and attract ants that protect the plant against herbivores; this exudate is largely sucrose, so differing from phloem sap which contains other sugars, amino acids, etc., and, like extrafloral nectar itself, wound secretions are induced by jasmonate (Lortzing et al. 2016; also Heil et al. 2015, see also Fagaceae).
Solanaceae, perhaps especially Solanoideae, have many hair types (see Seithe 1962, 1979; Seithe & Sullivan 1990; Watts & Kariyat 2021). Touch-sensitive trichomes are common in Solanoideae (or perhaps they have simply been much studied here), as are glandular hairs of various types. The secretions that are produced when the sensitive hairs are brushed by the insect may contain poisonous/deterrent metabolites such as sesquiterpenes, or they may rapidly oxidise and become sticky, so trapping and killing small insects, or they hydrolyse, and attract insects that then target caterpillars eating the plant (van Dam & Hare 1998; Kellogg et al. 2002; Weinhold & Baldwin 2011; Bleeker et al. 2011; Kim et al. 2011; LoPresti et al. 2015 and references). The secretions of glandular hairs in the tomato are involved in plant communication and defense, and bacterial diversity in the phyllosphere is also greater when there are such hairs (Kusstatscher et al. 2020). Karban et al. (2019) found that in Nicotiana attenuata with its sticky hairs that the number of insects trapped ∝ (positively) the number of predators of those insects ∝ the number of seed capsules produced. Stellate, uncinate, etc. hairs in Solanaceae may also be involved in biomechanical defence (Bar & Shtein 2019 and references), while the morphology and composition of cuticle waxes also affect the feeding behaviour of caterpillars and their like (Watts & Kariyat 2022); for hairs and caterpillar herbivory, also see Kaur et al. (2022).
Recent work shows the complexity of some of these herbivore-plant interactions. Polydnaviruses in the larvae of braconid wasps that parasitize noctuid corn earworm caterpillars eating tomato plants are able to manipulate various aspects of what may be a tri- or tetratrophic interaction to their own benefit. Here the effect that the caterpillar saliva normally has in inducing plant defences is reduced, ultimately to the benefit of the parasitoid and its associated viruses (Tan et al. 2018). Oher viruses of this group suppress the reaction of the host caterpillar to the wasp eggs, again to the benefit of the parasitoid - and these viruses (Tan et al. 2018). Similar interactions are likely to be widespread. Complexity of another type is evident in the interaction of different hair types during defence. Thus long trichomes in Solanum lycopersicum sense mechanical stimuli like those produced by caterpillars crawling over the plant, and such stimuli are transduced via inter-trichome communication: The basal cells of these long hairs produce a calcium wave that induces jasmonic acid synthesis in shorter, glandular hairs or in leaf tissue, and this in turn activates terpene and alkaloid synthesis, such metabolites helping to repel insects (C. Sun et al. 2024).
In Solanum in particular acylsugars, with acyl groups variously attached e.g. to pyranose or furanose rings of a disaccharide, protect against both herbivores and fungal pathogens (Fan et al. 2019). (Acyl groups - R-C(=O)- - attach as follows: R-C(=O)-O-R'.) Quite simple changes in the enzymes involved in transferring the acyl groups to the sugars result in changes in the positions to which these acyl groups are transferred, hence increasing the diversity of the acylsugar protectants (Fan et al. 2019). Some plants have 300 or more different kinds of these acylsugars, within Solanaceae being produced by the clade [Salpiglossis + Solanum] (Moghe & Last 2015; Moghe et al. 2017; Vendemiatti et al. 2024: Fig. 1). Indeed, there seems to be an increase in the length of the acyl chains from 2-5 C long in Salpiglossis (Cestroideae-Salpiglossideae) to up to 18 C long in some species of Solanum, although sampling is rather poor (Vendemiatti et al. 2024). Acylsugars are not found in basal Solanaceae or in Convolvulaceae, but see also elsewhere for the evolution of acylsugars which are known from a number of other angiosperm groups.
Sonawane et al. (2023) discuss the synthesis of the various steroidal glycoalkaloids involved in plant defence in Solanum. Interestingly, odorless-2 mutants of tomatoes produce only very small amounts of metabolites like monoterpenes, sesquiterpenes and flavonoids, although they do produce protectants like acyl sugars and glycoalkaloids, and they were found to be susceptible to herbivory by the Colorado potato beetle and Manduca sexta larvae in the field, interestingly, these mutants produce only a few of a particular type of glandular hairs presumably involved in the compromised protectant pathway (Kang et al. 2010).
There is another wrinkle concerning the posession of dense, glandular trichomes (Glas et al. 2012). Mirid bugs of subtribe Dicyphini in particular are able to walk easily on the plant despite these hairs and they may eat the insects trapped there (Wheeler & Krimmel 2015); nitrogen from the excreta of the bugs may be taken up by the leaf (Spomer 1999), so this may be a form of indirect carnivory (B. Anderson 2005). There has been much discussion concerning latitude, secondary metabolites and plant diversity (e.g. Schemske 2009; Sedio et al. 2018, 2021; Sedio 2017; etc., and discussion elsewhere). For other similar tropical systems, see Eugenia, Inga, Ocotea, Passiflora, Piper, Protium and Bursera, and Psychotria; the discussion about Inga is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
Plant-Bacterial/Fungal Associations. The distinctively pungent capsaicanoids of chilis (Capsicum spp.) are involved in the protection of the fruit against the fruit-destroying Fusarium fungus (Tewksbury et al. 2008). Capsaicins can be synthesized by the ascomycetous endophytic fungus Alternaria (Devari et al. 2014).
Vegetative Variation. Recent work describes extensive parallelism at the level of gene control - a member of the cytokinin biosynthetic gene family is involved - in the evolution of prickles in Solanum. They have evolved at least 26 times in angiosperms alone, and have been lost many times - 31 times in Solanum, in almost half of these instances this gene being implicated; in Solanum the very speciose (over half the genus) monophyletic spiny [sic] Solanum clade, the Leptostemon clade, is an example (Satterlee et al. 2024). Unusual stomata with degenerate guard cells have often been reported in the family (Cammerloher 1920; D'Arcy & Keating 1973).
Leaves in the fertile part of the stem of Solanaceae, perhaps especially in Solanoideae, are often unequally geminate and/or branching is not simply axillary. Petunia can have ordinary-looking cymose inflorescences, but Schwenckia, Schizanthus and many other taxa are described as having more or less concaulescent or recaulescent bracts, only one branch of the cymose-type inflorescence is developed at each node, or the two branches develop in different ways, etc. (e.g., for Schizanthus, see Grau & Gronbach 1984; for concaulescence/recaulescence, see Barboza et al. 2016). This makes interpretation of the construction of the plant difficult, but see especially the exhaustive study of Danert (1958; also Child 1979; Child & Lester 1991; A. D. Bell & Dines 1995). Castel et al. (2010) suggest that there are similarities in the inflorescences of at least members of Petunioideae and Solanoideae, and they note that the absence of bracts may be only apparent. Detailed studies on Solanum lycopersicum show that after germination and a brief monopodial growth phase only a few phytomers (= leaf, axillary bud, internode, terminal meristem) in duration, the terminal meristem becomes an inflorescence in which the phytomers consist of a terminal flower plus an "axillary" bud but no subtending leaf, and the axillary bud continues the growth of the inflorescence, the process repeating itself and the result being a monochasial cymose structure. An axillary bud from the last cauline leaf also grows out, produces a few phytomers with leaves, and then the whole process repeats iself (see esp. Sawhney & Greyson 1972 and Périlleux et al. 2014; also Danert 1967; Lippman et al. 2008). Heterochrony during meristem maturation may help generate the diversity of inflorescence morphologies around Solanum (Lemmon et al. 2016); Capsicum and Nicotiana (N. benthamiana) were the two species with "single-flowered inflorescences" examined, and they have flowers that are not axillary. Lemmon et al. (2016) found that genes involved in floral meristem identity were precociously expressed in the single-flowered species. On the other hand, delayed maturation of the inflorescence in Solanum lycopersicum led to much more branching in the inflorescence (Park et al. 2012, see also 2014). It has recently been suggested that taxa like Petunia and Solanum esculentum have floral unit meristems rather than inflorescence meristems, and the former, indeterminate like flower meristems, may produce flower-analogues like the capitulum of Asteraceae and Nyssaceae-Davidia (T. Zhang & Elomaa 2020). Note that the growth pattern of the old Nolanaceae (= Solanoideae-Nolaneae) is very like that of other Solanoideae (see also Eichler 1874; Danert 1958). Finally, Goetzea has an odd growth pattern, the leaves being more or less two-ranked and the single flowers being lateral to the leaf, although how this relates to the other growth patterns just discussed in Solanaceae is unclear; it was not studied by Danert (1958). Zhang et al. (2022) summarize inflorescence morphology; see also above.
There are similarities at the genetic level between shoot branching and leaf dissection, and the abscission zone of the fruit, made up of arrested meristematic cells, is also involved in the same regulatory network (Busch et al. 2011; Périlleux et al. 2014). Although Barboza et al. (2016) describe the nodes as being one trace, one gap, in the flowering part of the stem, things may get a lot more complicated (see e.g. di Fulvio 1961).
Genes & Genomes. A genome duplication event specific to Solanaceae has been dated to ca 52-50 Ma (Schlueter et al. 2004) or (64.8-)63.7, 59.6(-57.5) Ma (Vanneste et al. 2014b) - see also Gao et al. (2018); Y.-L. Cao et al. (2021) talk about a whole genome duplication for the family, but it is placed at the [Petunioideae [Nicotianoideae + Solanoideae]] node, the limit of their sampling. A genome duplication (?the same) has been dated to ca 67 Ma (Potato Genome Sequencing Consortium 2011) and a triplication to (90.4-)71(-51.6) Ma (Tomato Genome Consortium 2012) or at least 30 Ma (Bombarely et al. 2016; see also Gao et al. 2018 for estimates); there is perhaps another duplication event at 23-18 Ma (Blanc & Wolfe 2004). Where the possible family-level duplication actually goes is unclear, indeed,, F. Wu et al. (2006) dismissed the possibility that there had been a genome duplication either on the branch leading to or on those within Solanaceae (see also Robertson et al. 2010), although they did allow the possibility of a duplication well before the divergence of Solanales and Gentianales - perhaps the γ nuclear genome/gamma triplication of the core eudicots (see also Bombarely et al. 2016 for this duplication in Solanaceae). Things are complicated: Landis et al. (2018) mention the LYBAα duplication estimated to have occurred ca 14.2 Ma in the ancestor of the [Nicotianoideae + Solanoideae] clade and another duplication within/at the base of Solanoideae - but the latter includes Pyrenacantha (Icacinaceae)... Y.-L. Cao et al. (2021) discuss a genome triplication within Solanaceae but basal to all (as of 2021) sequenced genomes. Within Nicotiana increases in diversification - see especially the Suaveolentes group - have been associated with genome duplications, but they occurred only after a lag, the duplications being more basal in the tree (Clarkson et al. 2017). J. Huang et al. (2023a) also evaluated a number of potential genome duplications in the family and suggested that there had been duplications around 81, 37, and 20 Ma - see the characterizations above, although I am a little unclear as to exactly where the first duplication should be placed. P. Wang et al. (2018) examined gene family size in Solanaceae, and found that gene families involved in things like fruit ripening and secondary metabolism were very variable and tended to differ between species; such genes had evolved by tandem duplications, while housekeeping genes were less variable and tended to evolve by genome duplication.
F. Wu and Tanksley (2010) reconstructed the ancestral genome of the [Nicotianoideae + Solanoideae] clade and looked at the various changes that have occurred in the genomes of Nicotiana, tomato, pepper, etc.. Within Nicotiana, there has been reticulate evolution both at the diploid and polyploid level, as was evident in an attempt to understand the origin of allopolyploid species of the section Suaveolentes (Kelly et al. 2012); for hybridization in the genus, see Soltis et al. (2016b and references). Hybridization is also common in Solanum, thus there has been much hybridization and introgression in species of Solanum with tubers and in S. tuberosum itself, genomes in the latter showing very extensive diversity (Hardigan et al. 2017; see also Ovchinnikova et al. 2011). There is much reticulation evident in the ancestry of the tomato, too, and no analyses of any of the 100 kb segments of the transcriptome - 2,743 of them - gave the species tree (Pease et al. 2016)... M. Wu (2019) looked at the genome of Jaltomata and found that includes various amounts of the genome of related clades; it is perhaps unlikely to have a whole genome duplication (J. Huang et al. 2023a). For chromosome numbers in Solanoideae, see Robertson et al. (2010) and Chiarini et al. (2010); Chiarini et al. (2018) think about chromosome evolution in Solanum. Deanna et al. (2018a) looked at chromsome evolution in Iochrominae, and Rodríguez et al. (2021) at that in its sister taxon, Physalidinae, x = 12 and with a more asymmetric karyotype. For chromosome numbers in Cestrum et al., see Las Penas et al. (2006); see also Knapp et al. (2004b).
Arabidopsis-type telomeres are absent from some Browallioideae (Sýkorová et al. 2003a). Cestreae in particular, which lack these telomeres, have chromosomes that at 7.21-11.51 µm long are considerably larger than those of the rest of the family, which are much smaller, e.g. 1.5-3.52 µm long in Nicotianoideae (Acosta et al. 2006; Tate et al. 2009). The genome of Solanum-Cyphomandra, at 2 C = 49.6 pg DNA, is the most massive of any woody angiosperm (Schneider et al. 2015). For genome size variation in Jaltomata and neighbouring genera - Copia and Gypsy transposable elements are involved - see M. Wu et al. (2019).
Chung et al. (2023) noted that maternal inheritance of the plastome when male gametogenesis was at low temperatures.
One or more functional genes from Agrobacterium rhizogenes are found in many, but not all, species of Nicotiana and may have coevolved with the plant genome (Intrieri & Buiatti 2002); horizontal gene transfer is relatively quite common in Solanaceae (Talianova & Janousek 2011). It has also occurred quite extensively and recently in the mitochondrial Cox-1 intron both within the family and from outside (Sanchez-Puerta et al. 2011).
Interestingly, Petunia (Petunioideae) and Hyoscyamus (Solanoideae) can be intergrafted (Taiz & Zeiger 2006). Cybrids, cytoplasmic hybrids containing genomes of two species, have been formed between Nicotiana tabacum (Nicotianoideae) and Hyoscyamus niger (Solanoideae) (Sanchez-Puerta et al. 2014), i.e. they are members of clades that diverged (90.4-)71-12(-9) Ma (see above)...
Economic Importance. Potatoes, Solanum tuberosum, provide perhaps 1.7% of the world's caloric intake (#3 of the non-grain crops), and the origin and evolution of the petota clade, to which it belongs, was recently discussed by Z. Zhang et al. (2025). Note that there has been little recent improvement in potato cultivars, the main cultivars being a century or so old (but see Stokstad 2019); for information on potatoes, rich in proteins and other nutrients, see also Spooner et al. (2014). For the domestication of the tomato, S. esculentum, see Bai and Lindhout (2007) and for its phylogeny, see Pease et al. (2016), the latter emphasizing the importance of both mutation and introgression in generating adaptive genetic variation here. Cooley and Vallejo-Marín (2021) discuss the role of sonication by bees in ensuring a good crop of tomatoes. Grafting is extensively involved in the production of tomatoes, eggplants and peppers (Kyriacos et al. Chili peppers (Capsicum annuum) were domesticated in Mexico, quite possibly in a number of places (Aguilar-Meléndez et al. 2009); other species of the genus are also economically important (Perry et al. 2007 and references; see also Carrizo García et al. 2016: relationships in the genus). Not all species are pungent - see Haak et al. (2012) for hot peppers.
Chemistry, Morphology, etc.. Lycium is recorded as accumulating glycine betaines, and at least some members of the genus are halophytic (Levin & Miller 2005). For alkaloids in Datureae, see Doncheva et al. (2006); Schizanthoideae and some other groups have distinctive tropane alkaloids (Hunziker 2001; Y.-J. Wang et al. 2023).
There is substantial variation in tissue patterns in the stem, for which see especially Cosa [de Gastiozoro] et al. (1991, 1993, 1994), Liscovsky and Cosa (2005) and Liscovsky et al. (2002) and references; I have incorporated a few details of variation in tissue distributions into the characterizations above. Cosa de Gastiazoro (1994) noted that cork cambium in the roots of three genera of Petunioideae she examined was "subexodermal" - superficial (see also Barboza et al. 2016: Fig. 56); the broader distribution of this feature is unclear. Liscovsky et al. (2001) noted that the parenchyma cells around the protoxylem in Datura ferox divided forming files of cells and became considerably enlarged. Chromosomal endoreduplication may be involved in the development of tubers in Solanum, and tuber enlargement there is the result of cell expansion, not cell division (Hardigan et al. 2017 and references). Leaf development in Nicotiana is basipetal (Hagemann & Gleissberg 1996).
For floral development, see Ampornpan (1992, but c.f. Cocucci 1989b), for some floral anatomy, see Armstrong (1986). Knapp (2010) surveyed the considerable floral diversity in Solanaceae. The calyx and corolla are often open in development (Baehni 1946). In floral development, petal and stamen primordia together are lifted by zonal growth and the carpel primordia develop on a flat apex; in this respect there are some similarities between Solanaceae, Scrophulariaceae and Gesneriaceae, few with Montiniaceae (Huber 1980: 66-69; Ronse Decraene et al. 2000a). For floral development in Datureae, see Yang et al. (2002). There is considerable variation in corolla aestivation in the family (Ampornpan 1992), the patterns of endothecial thickenings are very diverse (Carrizo García 2002), and quite large and complex stigmas are common (Cocucci 1991, 1995).
The two carpels so common in Solanaceae are often in the plane determined by the first sepal initiated; this is one of the abaxial pair and so is somewhat off the median (= bract-determined) plane. Indeed, the basic plane of symmetry in flowers like Salpiglossis and Schizanthus (for the latter, see above) is oblique/inverted, and the abaxial (and also two adaxial) stamens are sterile (see also Ampornpan & Armstrong 2002 for flowers of Salpiglossis, G median, odd K and staminode abaxial). Nolaneae, often separated from Solanaceae because of their distinctive gynoecium, have five carpels borne opposite the petals, but their number is secondarily increased by tangential division; stamen and petal number are unaffected. Souèges (1907: 206 illustrations) described seed coat morphology and development, the chalazal end of the embryo sac "herniating" (sic). He described all the tissues surrounding the embryo sac/developing embryo as being testal; the innermost layer was the endotesta (= endothecium, sometimes not persistent), and the 4-6 cell layers immediately outside this, the inner zone, was digested by the endotesta, becoming the endosperm. In many cases the testa seems to be multiplicative, and he described the complex patterns of thickening of the exotestal cells in detail, i.a. noting that the bands of thickening on the anticlinal walls of the exotesta of Solanum might become hair-like structures as the exotesta broke down, although he preferred to call them "baguettes" (for seed anatomy, see also Barboza et al. 2022 - Capsicum; for the seed coat mostly of Physalideae, see Axelius 1992).
For generic descriptions and much else, see Hunziker (2001), Barboza et al. (2016) and many volumes of Kurtziana; Goodspeed (1954) remains the classic account of Nicotiana, also see Barboza et al. (2022: Capsicum) and di Fulvio (1961: Sclerophylax). Solanaceae Source includes information currently mostly about Solanum, but its coverage will expand. For general chemistry, see Hegnauer (1973, 1990, also 1966, 1990 as Nolanaceae) and Eich (2008), for the evolution of secondary metabolites, see Wink (2003 and references), and for polyhydroxylated nortropane alkaloids like calystegines, see e.g. Dräger (2004), for wood anatomy, see Carlquist (1987a, 1988a, 1992d) and Jansen and Smets (2001: vestured pits - do Petunioideae and Nicotianoideae have them?). For floral vascularization, see Liscovsky et al. (2009 and references), for floral development, see Payer (1857), Sattler (1977), Mair (1977: Schizanthus), Bondeson (1986: Nolana) and Kostyun et al. (2017: Jaltomata), for floral development and inflorescence morphology, see Huber (1980), for nectaries, see Vogel (1998b), for pollen morphology, see Barboza (1989: tricellular pollen), Persson et al. (1994: Juanulloeae; 1999: Datureae), Knapp et al. (2000: Anthoboleae), Stafford and Knapp (2006: basal monosymmetric taxa), Z.-Y. Zhang et al. (2009: Hyoscyameae) and Gavrilova (2014) and Finot et al. (2018), both Nolana and relatives, for embryology, see di Fulvio (1969, 1971: Nolana), for fruit anatomy, see Pabon Mora and Litt (2007), and for seed coat and embryo, see Wojciechowska (1972: European/cultivated taxa)
Phylogeny. Relationships along the spine of Solanaceae are still poorly known; for early studies, see Olmstead & Palmer (1992) and Fay et al. (1998b). However, the clade [Petunioideae [Solanoideae + Nicotianoideae]] is well supported in Olmstead et al. (1999), although less so in Olmstead and Santiago-Valentin (2003); see also Särkinen et al. (2013). In the summary tree of Olmstead and Bohs (2007), immediately below the clade [Solanoideae + Nicotianoideae] was a polytomy including Petunioideae, Cestroideae and Schwenckioideae (see Dillon et al. 2009 for another topology). Relationships between these latter clades had only weak support; Schwenckia might be sister to the rest of the family (Olmstead et al. 1999). Using the nuclear gene SAMT (salicylic acid methyl transferase), Martins and Barkman (2005) found Schizanthus in this position, and with rather strong support (see also Olmstead & Sweere 1994), with Schwenckia weakly linked with Cestroideae (see also Olmstead & Bohs 2007), yet Schizanthus has also been strongly associated with Nicandra (Zamora-Tavares et al. 2016: ?sampling). A Goetzeoideae clade has included Duckeodendron as sister to the rest, but with only moderate support (Santiago-Valentin & Olmstead 2001, 2003); here Duckeodendron is left unaffiliated. Wu et al. (2006) found a strongly supported grouping of [Solanoideae [Petunioideae + Nicotianoideae]], the sequences analyzed coming from ten orthologous loci each on a different chromosome - but no other clades of the family were included. The two-gene tree in a study by Olmstead et al. (2008) is rather like that of Martins and Barkman (2005): [Schizanthoideae [Goetzeoideae, Duckeodendron [[Cestroideae/Browallioideae, including Benthamiella et al.; their relationships have previously been unclear], Petunioideae, Schwenckioideae [Nicotianoideae + Solanoideae]]]], but support is strong for relationships between the last pair of taxa alone (see also Ng & Smith 2015: Reyesia with Salpiglossis and Bouchetia - support moderate).
Särkinen et al. (2013: ca 1000 taxa, 2 nuclear and 5 chloroplast genes) added Reyesia to the taxa at the base of the tree whose relationships were uncertain, although it may link with Salpiglossis (Salpiglossideae). In a tree used for dating, clades including Cestrum, Browallia, Benthamiella, etc., and Schizanthus, Duckeodendron, etc., were successively sister to the rest of the family, made up of [Petunioideae [Nicotianoideae + Solanoideae]], although many details of relationships at base of Solanoideae were poorly supported (Särkinen et al. 2013: for details of the phylogeny and most ages used above, see additional file 2): See also the maximum likelihood tree in Dupin et al. (2017). The distinctive Sclerophylax is to be included in Solanoideae (see also Olmstead et al. 2008). J. Huang et al. (2023a) looked at almost 1700 nuclear genes, 180 species, and 14/15 tribes; Benthamielleae were not included, nor was Duckeodendron cestroides, a monotypic genus that is rather basal in the family in those analyses in which it has been included. Names used for clades here and in Särkinen et al. (2013) sometimes differ.
Petunioideae. For the phylogeny of Brunfelsia, which moved to the Antilles, where it radiated, from South America, see Filipowicz and Renner (2012). [Särkinen et al. (2013) recognized two tribes. Petunieae and Brunfelsieae here, and Mäder and Freitas (2019) recovered the relationships [Nierembergia [Calibrachoa [Fabiana + Petunia]]]. In the nuclear analyses of J. Huang et al. (2023a) relationships are [Nierembergia [Brunfelsia [Petunia [Fabiana + Calibrachoa]]]]. There was weak support for a [Nierembergia + Nicotiana] clade using plastid data, strong support for a [Nierembergia + Bouchetia] clade using nuclear waxy data, and for [Nierembergia [Petunia + the rest]] using combined data (Alaria et al. 2022: these relationships not the focus of their study). Alaria et al. (2022: 3 chloroplast, 1 nuclear markers, 24 spp.) also consistently recovered the relationships [Petunia [Fabiana + Calibrachoa]]. Pezzi et al. (2024: 37 spp., transcriptomic data) found that there had been hybridization and incomplete lineage sorting within Petunia, Calibrachoa and Fabiana (all three are southern South American) and also deeper hybridization between the first two genera, despite their different base chromosome numbers (x = 7 and 9 respectively); Fabiana is morphologically rather different from the other two genera.
Nicotianoideae. For a phylogeny of Nicotiana, sister to the largely Australian Anthocercideae, see Clarkson et al. (2004, 2017). There has been quite a bit of hybridization - thus sections Sylvestres (n = 12) x Tomentosae (n = 12) → Nicotiana (n = 24), e.g. Clarkson et al. (2004) and Knapp et al. (2004a). The eastern Andean Nicotiana section Tomentosae was found to be sister to the rest of the genus (e.g. Clarkson et al. 2017). Largely similar relationhips were recovered in the plastome analysis of 24 species of Nicotiana by S. Wang et al. (2022).
Solanoideae. Carrizo García et al. (2018 and references) emphasized that Salpichroa and Jaborosa were not related, the former perhaps being sister to [Solaneae + Physalideae], the latter close to the Mandragora area; they suggest a phylogeny for Salpichroa. Messeder et al. (2024) recovered a [[Jaltomata [Physalis [Lycianthes + Capsicum]]] Solanum] clade. Jaltomata has often been found to be sister to Solanum (but see above, introduction to Solanoideae), and the two show extensive genomic similarity, and although the Jaltomata genome is somewhat less similar to that of Capsicum, there has probably been introgression between these last two (M. Wu et al. 2019), even although they are now in separate tribes.
Capsiceae generally includes just two genera, but of the two Lycianthes is paraphyletic (see also J. Huang et al. 2023a), Capsicum being embedded in it (Särkinen et al. 2013; Carrizo García et al. 2016; Spalink et al. 2018).
Datureae: for relationships between all species included in this tribe, see Dupin and Smith (2018, 2019).
Jaltomata. The three major clades in Jaltomata are each characterised by fruit colour, and the red-fruited [J. antillana + J. auriculata] clade is sister to the rest of the genus (R. J. Miller et al. 2011).
Lycieae. Lycium is paraphyletic (Levin & Miller 2005; Levin et al. 2007, 2009, 2011); L. bridgesii may be sister to the rest of the genus, but this depends on the analysis. Y.-L. Cao et al. (2021) show the relationships between 13 species based on variation in the nuclear genome; the trees in H. Chen et al. (2024) are based on analyses of 4 plastid and 2 nuclear markers for 50 or so species.
Nicandra (Nicandreae) has been associated with Datureae (Sarkinen et al. 2013; Dupin & Smith 2018); see above.
Nolaneae. For relationships within the distinctive Nolana, see Tago-Nakawaza and Dillon (1999), Dillon et al. (2007, 2009), Tu et al. (2008) and Guerrero et al. (2013); N. sessiliflora may be sister to the rest of the genus.
Physalideae. Relationships around Physalis are still poorly understood (Whitson & Manos 2005; Zamora-Tavares et al. 2016; Rodríguez et al. 2021); for relationships within the florally diverse Deprea, see Deanna et al. (2017). Iochroma is polyphyletic (S. D. Smith & Baum 2008; Olmstead et al. 2008; Gates et al. 2018). Improved sampling is beginning to clarify things, thus Deanna et al. (2018b/2019) found that 8/27 sampled genera, including Physalis itself, and Withaninae (out of the three subtribes) were not monophyletic. Sandoval-Padilla et al. (2025: 55 plastomes, 20 genera) found the relationships [Tubocapsicum [Cuatresia [Witrhaninae [Physalidinae + Iochromineae]]]] here, and of the 8 genera with two or more species included four were para- or polyphyletic, all from Iochromineae...; overall there was little sequence variation.
The Sclerophylax Clade. In an analysis of 13/14 species of Sclerophylax and using four markers Chiarini et al. (2022) found quite strong support for the position of S. caduciformis as sister to the rest of the genus, although resolution of relationships between the other taxa tended not to be too good.
Solandreae. Orejuela et al. (2017) discuss relationships in Solandreae; those along the spine are not well supported making generic delimitation a perilous occupation, but Orejuela et al. (2022) are clearing things up.
Solaneae. The limits of Solanum have been expanded to include Cyphomandra and Lycopersicon (see Bohs 2005, 2007; Levin et al. 2006; Weese & Bohs 2007: three genes, S. thelopodium sister to the rest, or unresolved in Bayesian analyses; Poczai et al. 2008; Särkinen et al. 2013, 2015). The latter recovered two main clades, the three species of subgenus Thelopodium beiong sister to those clades. Gagnon et al. (2022) carried out a series of analyses - ITS, waxy and seven plastid markers; plastomes; 303 genes of the Angiosperms353 group. Although in some analyses the same basic groups were recovered, overall there were major differences in the structure of clade I such that it generally appeared as a grade whose topology differed according to the analysis and was best represented by a polytomy, perhaps hard, and there were also problems with the stem of the Leptostemon clade and of the Eastern Hemisphere spiny group; other relationships, including the basal position of subgenus Thelopodium, were largely unchanged (Gagnon et al. 2022). Relationships within the speciose Solanum subgenus Leptostemonum, characterised by stellate hairs and prickles, are outlined by Stern et al. (2011) and Vorontsova et al. (2013: African taxa); there have been three invasions of the Old World (Aubriot et al. 2016a). Särkinen et al. (2015) tackled relationships within the large black nightshade clade (morelloid Solanum species). Tepe et al. (2016) discussed relationships in the potato clade; section Petota, which includes the potato, S. tuberosum, is now at around 112 species and less than half the size it was twenty five years ago. Z. Zhang et al. (2025) looked at relationships here. including 3/3 species of the Etuberosum group, 12/17 species of the Lycopersicum lineage and 44/107 species of sect. Petota using haplotype genomes and clarified the homoploid hybrid origin of the Petota clade. Messeder et al. (2024: 233 spp., various analyses of sets of 889-1786 low copy nuclear genes) were able to clarify relationships in Solanum - like some other studies the small Thelopodium group (S. thelopodium, S. dimorphandrum) was recovered as sister to the rest of the genus, interestingly, there was good support for realignments that Messeder et al. suggested like the placement of the Regmandra clade along with six minor clades (c.f. Särkinen et al. 2013; Gagnon et al. 2022).
Classification. For the main outlines of the classification above, see Olmstead et al. (2008), also Särkinen et al. (2013: names used there may differ from those above) and Barboza et al. (2016). R. O. Olmstead in Satfford and Knapp (2006) included Benthamielleae in Petunioideae, but see Särkinen et al. (2013); for the circumscription of Lycium (= Lycieae), see Levin et al. (2011), and for that of Solandreae, see Orejuela et al. (2017). Generic limits in Physalideae need adjusting (see also Sandoval-Padilla et al. 2025 for problems in Iochromineae in particular) - monotypic genera that make others paraphyletic are causing problems, and perhaps all Iochromineae should be placed in Iochroma (Deanna et al. 2018b/2019).
Knapp et al. (2004a) provide an infrageneric classification of Nicotiana that deals with the hybrid origin of some clades; these are put in sections separate from those to which their parents belong. This classification seems to be holding. Within Solanum Tepe et al. (2016) noted that the potato clade was made up of ca 11 sections and 194 species, and most of these latter were in section Petota (this includes the potato, S. tuberosum). For comments on Solanum and its classification, see Muñoz-Rodríguez et al. (2023) who note that there is an informal infrageneric classification here (see Bohs 2005) as has happened in some other large genera; see also Messeder et al. (2024) for some rather different relationships which may affect the classification.
Previous Relationships. Hutchinson (1973) placed Duckeodendraceae in Boraginaceae, but doubtfully; Cronquist (1981) kept it as a poorly-known family; Takhtajan (1997) placed it as a separate family in Solanales. Its carpels are oblique to the main axis of the flower (Kuhlmann 1934), as is appropriate for Solanaceae.
Botanical Trivia. Tomatoes can be grafted on to potatoes; the result is a pomato, Tom Tato, tomtoe or "ketchup and fries", similarly, eggplants can be grafted on to potatoes - the result, "egg & chips".