EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
[DILLENIALES [SAXIFRAGALES + ROSIDS]]: stipules + [usually apparently inserted on the stem].
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID II / MALVIDAE / [[GERANIALES + MYRTALES] [CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]]: ?
[GERANIALES + MYRTALES]: ellagic acid +; K persistent in fruit.
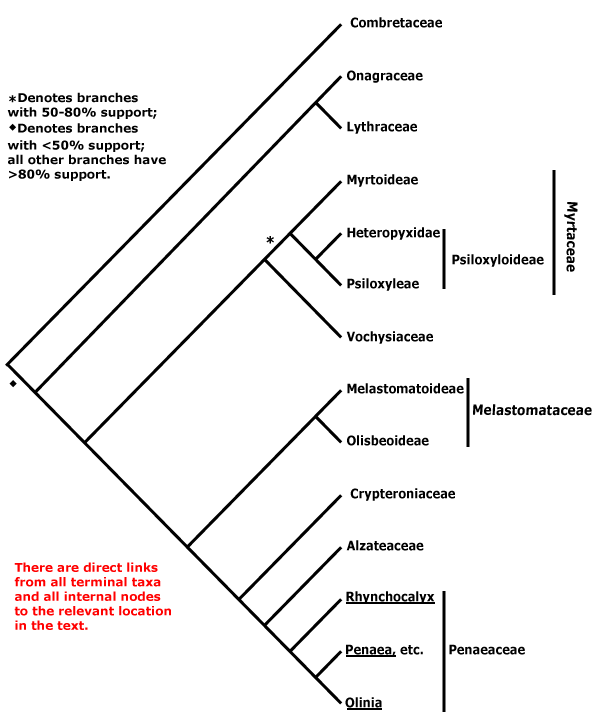
Phylogeny. See the Dilleniales page for major patterns of relationships within Pentapetalae and the superrosid node for major patterns of relationships within the rosids, particularly the relationships of Geraniales and Myrtales.
MYRTALES Reichenbach - Main Tree.
Bark flaky; flavonols only, myricetin, methylated ellagic acid +; vessel elements?, vestured pits +, (rays with multiseriate part no wider than uniseriate part); tension wood +; secondary phloem stratified; (interxylary phloem +), internal phloem + [= intraxylary phloem, vascular bundles bicollateral]; cork cambium deep seated, (polyderm +); nodes 1:1; (vein endings with spirally-thickened tracheoids); cuticle waxes often 0; branching from current flush [all?]; leaves opposite, lamina with secondary veins joining an intramarginal vein [brochidodromous venation], margin entire, colleters + (?inc. small stipuliform structures); inflorescence racemose; (flowers 4-merous); hypanthium +, nectariferous; K valvate, C clawed; filaments incurved in bud; pollen grains small [(40.94-)19.5-2(-9.93) μm long], ± spherical [see Kriebel et al. 2017], with pseudocolpi; ovary inferior, (transseptal bundles +), style long, minor stylar bundles +, stigma wet; ovules many/carpel, micropyle bistomal and zig-zag, inner integument ca 2 cells across; antipodal cells ephemeral; (mesotesta sclerotic), endotesta crystalliferous, exotegmen cells tracheidal; endosperm at most slight; x = 12. - 9 families, ca 380 genera, 13,005 species.
Includes Alzateaceae, Combretaceae, Crypteroniaceae, Lythraceae, Melastomataceae, Myrtaceae, Onagraceae, Penaeaceae, Vochysiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001: note topology) dated crown Myrtales to (83-)79, 75(-71) Ma, Hengchang Wang et al. (2009) to (89-)85, 78(-74) Ma, although a Bayesian relaxed clock estimate was as much as 99 Ma, Tank et al. (2015: Table S2) ca 86 Ma, and Bell et al. (2010: note topology) offered dates of (99-)89 Ma. Thornhill et al. (2012a, 2015) suggest several dates, but they are all clustered around (98-)93.3-91.6(-85.7) Ma; an age of ca 111 Ma was suggested by Sytsma et al. (2004), (118.8-)116.4(-113.7) Ma by Berger et al. (2015: topology), ca 90 Ma by Sytsma and Berger (2011), (130.9-)125.5(-120.3) Ma by Gonçalves et al. (2020a) and (114.2-)104.9(-88.0) Ma by X.-F. Zhang et al. (2021).
The oldest fossils assignable to Myrtales are some 65 Ma old (Crepet et al. 2004). However, fossil flowers of Platydiscus peltatus found in Late Cretaceous rocks from Sweden ca 83 Ma have been linked with Myrtales (López-Martínez et al. 2023a); earlier placements included Cunoniaceae.
Evolution: Divergence & Distribution. Myrtales contain ca 6% core eudicot diversity (Magallón et al. 1999), and Magallón et al. (2018) suggested that there was an increase in the diversification rate at this node that they dated to (116.4-)105.5(-96.6) Ma.
Berger et al. (2015) discuss the biogeography and diversification of Myrtales in some detail, noting that most families were restricted to or most diverse in the southern hemisphere, ancestrally being in South America (Lythraceae, Melastomataceae, Onagraceae, Vochysiaceae) or Africa (the [Crypteroniaceae [Alzateaceae + Penaeaceae]] clade, Myrtaceae). Energetically costly elements of floral displays (e.g. large flowers) have evolved in parallel in Myrtales, and perhaps paradoxically low cost floral elements, e.g. small flowers, shallow flowers, show phylogenetic inertia (i.e. are plesiomorphic?) (Vasconcelos & Proença 2015).
Myrtales, but not including Combretaceae, have distinctively small seeds (Cornwell et al. 2014).
Several characters common in Myrtales may be apomorphies here. Raffinose and stachyose are common oligosaccharides in phloem exudate in Myrtaceae, Onagraceae, Lythraceae and Combretaceae, at least (Zimmermann & Ziegler 1975). Both superficial and deep-seated cork cambium is reported in Melastomataceae-Melastomatoideae; species with deep-seated cork cambium do not necessarily have flaky bark (Milanez et al. 2021). Polyderm (alternating endodermal and parenchymatous layers laid down by a pericyclic meristem) is known from families like Onagraceae, Lythraceae, Myrtaceae, and probably Penaeaceae, at least (Mylius 1913). Wood fibres are usually non-septate (e.g. Combretaceae), but those of Lythraceae, at least, are septate. In roots of some aquatic Lythraceae, Melastomataceae, Myrtaceae and Onagraceae (and Euphorbiaceae and Fabaceae) there is a distinctive lacunate cork produced from a pericyclic cork cambium (Little & Stockey 2006), and there may also be lacunae in the stem polyderm produced in response to flooding (Lempe et al. 2001). The cork cambium is sometimes initiated in the superficial or mid-cortical position (e.g. Myrtaceae, Melastomataceae). Tracheoidal sclereids with spiral wall thickenings that are associated with the vein endings are known from Vochysiaceae, Lythraceae, Combretaceae, Melastomataceae, Alzateaceae and Penaeaceae (Sajo & Rudall 2002); their more general distribution needs to be checked. Since there is internal phloem, petiole and midrib bundles are often bicollateral; Carlquist (2013) noted the occurrence of interxylary phloem in some Combretaceae, Melastomataceae, Onagraceae and Lythraceae. Weberling (2000) notes that "true rudimentary stipules" occur in Myrtaceae and most myrtalean families; stipules, when they occur, are indeed generally small, often not vascularized, and are likely to be colleters s. str. (see also Carr & Carr 1966; LaFrankie 2010; da Silva et al. 2012).
In Myrtaceae the calyx and corolla originate at about the same time, while in Lythraceae and Onagraceae the calyx is visible considerably before the corolla (Mayr 1969); it will be interesting to know the general distribution of this feature. Many Myrtales, including some Myrtaceae, have notably narrow petal bases, i.e., they are close to being clawed; the definition and distribution of clawed petals in Myrtales may have to be amended, but I have provisionally put "clawed petals" as a feature of the whole clade. In those taxa that have filaments that are straight in bud, the filaments are usually short; the length of the style is correlated in part with the length of the hypanthial tube. Although "inferior ovary" is put as a feature for the whole order, there is a fair amount of variation in ovary position in families like Vochysiaceae, Myrtaceae and Melastomataceae, indeed, in some Vochysiaceae the ovary becomes superior during development, although starting off as inferior (Litt 1999; Litt & Stevenson 2003a). Distinctive winged fruits are found in Combretaceae and rarely in Oenothera, and even more distinctive fruits that open by the placentae breaking through the ovary wall occur throughout Cuphea (Lythraceae) and in some Sonerila (Melastomataceae). The basic chromosome number for the order may be x = 12 (S. A. Graham et al. 1993).
L. A. S. Johnson and Briggs (1985) provide a morphological phylogeny for the group. For the evolution of pollen shape and size, see Kriebel et al. (2017); they use characters that are continuous variables and analyze the measurements in a phylogenetic framework - one of the few examples of this approach.
Ecology & Physiology. Salt tolerance has evolved quite often in Myrtales, most notably in Combretaceae (12 spp.) Lythraceae (21 spp.) and Myrtaceae (47 spp.: Moray et al. 2015).
Plant-Animal Interactions. The host plants of the moth group Mimallonoidea-Mimallonidae, which has some 290 species and is restricted to the American tropics, are predominantly Myrtales (St Laurent et al. 2021), and there the caterpillars live inside shelters/cases ("sack bearers": St Laurent et al. 2021). Woody Myrtales, especially Combretaceae, are ancestrally hosts of Mimallonidae, and there may be connections between diversification in these two groups - but note the some 60 Ma stem of Mimallonidae, which have a crown-group age of a mere (48.5-)44.0(-39.7) Ma, which makes it difficult to know what their caterpillars originally ate (St Laurent et al. 2021; for the Myrtales phylogeny, etc., used here see Berger et al. 2016). Interestingly, sister to Mimallonoidea are Macroheterocera, which St Laurent et al. (2021) estimate as having at least 74,000 species, and they are external feeders - the two groups diverged perhaps (108.4-)105.2(-100.3) Ma.
Lycaenid caterpillars are quite commonly to be found on Myrtales, especially on Lythraceae, Myrtaceae, Melastomataceae and Combretaceae, and most of them are myrmecophilous (Fielder 1991, 1995).
Genes & Genomes. Almeda and Penneys (2022) suggest base chromosome numbers for all families and some other clades in the order; unless these numbers differ from other such suggestions, they are generally not mentioned. Although a number of genome duplications have been reported in the order, Low et al. (2022) suggest that they represent separate sightings of the Pan-Myrtalean WGD.
Plastome variation in the order is not very great, being mainly in size - thus the inverted repeat is 23,902-36,747 bp long (c.f. in part X.-F. Zhang et al. 2021). In most taxa the IR is around 26,000 bp long, but in Lythraceae it is only 23,000-25,000 bp long. Other variables include features such as exactly what genes are at the junctions of the various architectural features of the plastome, etc. (X.-F. Zhang et al. 2021).
Chemistry, Morphology, etc.. For further information, see Dahlgren and Thorne (1985: general; also other papers in Ann. Missouri Bot. Gard. 71(3). 1985, also Lowry (1976), anthocyanins, Weiss (1890) and van Tieghem (1891b), both cork cambium position, Jansen et al. (2008), Carlquist (2017a) and Carlquist and Raven (2018: inc. vesturing spreading on to vessel walls - scattered), all vestured pits, Meylan and Butterfield (1978: wood anatomy), Lourteig (1965), Venkateswarlu and Prakash Rao (1971: wood anatomy), Beusekom-Osinga and Beusekom (1975: morphology etc. around Crypteroniaceae), van Vliet and Baas (1985: vegetative anatomy), Weberling (1988: inflorescence morphology), Ronse Decraene and Smets (1991b: polyandry), Mauritzon (1939a: embryology), Tobe (1989) and Tobe and Raven (1983a, 1985a, 1985b, 1987a, 1990), all embryology and ovule morphology (ordinal characters are mostly plesiomorphous), Boesewinkel and Venturelli (1987: ovule and seed), and Solt and Wurdack (1980) and Almeda (1997), both chromosome numbers.
Phylogeny. The position of Myrtales within the rosids was unstable in a rbcL analysis of all angiosperms (Hilu et al. 2003). However, there was some support for a position sister to all other rosids except Geraniales, Vitales and Saxifragales (Zhu et al. 2007), while Wang et al. (2009) suggested that is was sister to Geraniales, the combined group being sister to all other malvids. See also the Pentapetalae and Saxifragales pages for further discussion on the relationships of Myrtales.
Relationships within the order have been extensively studied by Conti et al. (1996, 1998, 1999, 2002), Sytsma et al. (1998, esp. 2004), Clausing and Renner (2001: Melastomataceae), Schönenberger and Conti (2001, 2003: esp. Penaeaceae area, etc.), P. G. Wilson et al. (2005: Myrtaceae s.l.), and X.-F. Zhang et al. (2021: no Crypteroniaceae, etc.), and the tree is based on these publications. The position of Combretaceae has been somewhat unclear (see also Maurin et al. 2010; M. Sun et al. 2016; Kriebel et al. 2017). However, at least some support for a position sister to [Onagraceae + Lythraceae] was found by Berger and Sytsma (2010), Bell et al. (2010), Soltis et al. (2011), H.-T. Li et al. (2019: 98% bootstrap), Gonçalves et al. (2020a), X.-F. Zhang et al. (2021), X. Wang et al. (2021) and H.-T. Li et al. (2021) - last three, plastome analyses; support in the last four was strong, even if the branch subtending the clade was short in some. On the other hand, Maurin et al. (2021: Angiosperms353 nuclear analyses) found thaat the position of Combretaceae was unclear, hence the basal tritomy below.
Anatomy (vestured pits), some morphological features (general leaf type and insertion) and molecular data all strongly suggest that Vochysiaceae are to be included in Myrtales. However, at first sight the distinctive monosymmetric and spurred flowers of that family are quite unlike those of the rest of the order.
Synonymy: Melastomatineae J. Presl - Circaeales Martius, Combretales Berchtold & J. Presl, Epilobiales Martius, Henslowiales Martius, Lythrales Link, Melastomatales Berchtold & J. Presl, Memecylales Martius, Myrobalanales link, Oenotherales Bromhead, Onagrales Berchtold & J. Presl, Penaeales Lindley, Trapales J. Presl, Vochysiales Link - Myrtanae Takhtajan - Myrtopsida Bartling, Oenotheropsida Brongniart
[Combretaceae [Onagraceae + Lythraceae]]: raffinose and stachyose common oligosaccharides in phloem exudate; interxylary phloem +; polyderm + [alternating endodermal and parenchymatous layers laid down by pericyclic meristem]; fibres with at most minutely bordered pits; petiole bundle arcuate; exotegmen fibrous.
Age. The age of this clade is estimated to be (128.7-)123.5(118.5) Ma by Gonçalves et al. (2020a), while X.-F. Zhang et al. (2021) suggested an age of (109.3-)96.2(-81.0) Ma.
COMBRETACEAE R. Brown, nom. cons. - Back to Myrtales
tannins often not abundant, soluble oxalate accumulating; vessels grouped; fibres with at most minutely bordered pits; (flowers vertically monosymmetric); tapetal cells binucleate; (pollen at anthesis with starch); K persistent; starch grains in nucellus. - ?where
Evergreen, woody; 5-desoxyflavonoids, flavonoid sulphates +; (cork epidermal); vesturing spreading over inside of vessel [?level], fibres non-septate; sclereids +/0; mucilage ducts + [?level]; petiole bundle also annular (wing bundles +); hairs appressed, unicellular, thick walled, apex pointed, with basal compartment, 2-armed; stipules at most small; (plant monoecious); flowers 4-5(-8)-merous; A obdiplostemonous, inserted below or at the hypanthial apex; nectary annular; G [2-5(-8)], alternate with K or odd member abaxial, unilocular, placentation apical, stigma punctate; ovules 2/carpel, outer integument 2-5 cells across, inner integument 2-4 cells across, parietal tissue 5-10 cells across, nucellar cap to 8 cells across, hypostase +, funicles long, usu. with obturator; megaspore mother cells several; fruit indehiscent, dry; seed single, large; (testa multiplicative), endotesta tracheidal or sclerotic, crystals ?0; embryo often green; x = 12 (?13), nuclear genome [1 C] (0.35-)3.817(-41.585) pg.
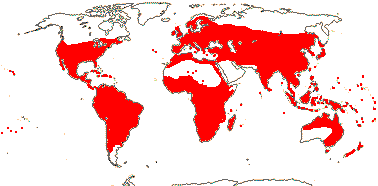
10 [list: to tribes]/500: 3 groups below. Largely tropical. [Photo - Flower, Flower, Fruit.]
Age. Crown Combretaceae are ca 46 Ma (Sytsma et al. 2004) 0r (106.5-)102.6(-98.9) Ma (Berger et al. 2015). Sytsma and Berger (2011) suggested that Strephonema diverged soon after the origin of stem Combretaceae at ca 90 Ma.
Fossils of Esgueiria, assigned to Combretaceae, are widespread in the Northern Hemisphere in Late Cretaceous deposits ca 90-70 Ma old. They seem to have inferior unilocular ovaries with apical placentation and glandular-peltate hairs (Friis et al. 1992, 2011), there is no hypanthium, of the eight stamens, five are in one whorl and three in another, the styles are more or less separate, and the surface of the unicellular hairs of the Japanese, but not the Portugese, material is distinctly rough (Takahashi et al. 1999). The nature of the nectary is unclear; in some specimens there are structures outside the androecium that have been interpreted as possible nectaries (Takahashi et al. 1999) - they would be unlike the nectaries in extant Combretaceae. The identity of these fossils should be confirmed - to be compared with Hydrangeaceae? Dilcherocarpus, from the Albian-Cenomanian of the Dakota Group, Kansas, and ca 100 Ma old, has been assigned to Combretaceae (Manchester & O'Leary 2010).
1. Strephonematoideae Engler & Diels - Strephonema J. D. Hooker
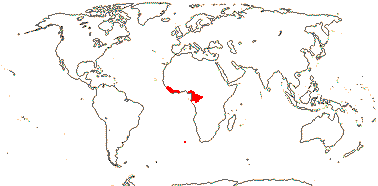
Trees; vesture elements globular; imperfect tracheary elements with bordered pits; internal phloem 0; stomata paracytic, accessory cells subdivided; lamina with closely parallel tertiary venation ± at right angles to the midrib, domatia marginal, revolute; pollen lacking pseudocolpi [tricolporate], only semitectate; G half inferior; ovules 2; fruit largely superior; cotyledons hemispherical, large, conduplicate; n/x = ?; germination hypogeal.
1/3. West Africa. Map: from Jongkind (1995) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
2. Combretoideae Beilschmied
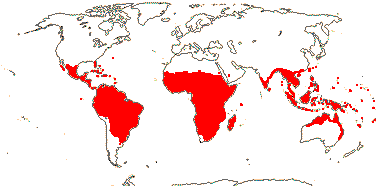
(hairs also lepidote), (stalked glands +); (petiole with glands); (plant dioecious); inflorescence often spicate; pollen (lacking pseudocolpi), (surface microechinate); cotyledons flattened; nuclear genome [1 C] (6259-)2919(-1223) Mb.
13/500. Largely tropical. Map: from van Steenis and van Balgooy (1966), Wickens (1976), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), FloraBase (consulted 2006) and Stace (2010).
Age. The age of this clade was estimated to be some (103.6-)101.6(-100.5) Ma by Gonçalves et al. (2020a), however, X.-F. Zhang et al. (2021) estimated an age around 25 Ma, although with huge error bars - and an odd topology.
2a. Laguncularieae Engler & Diels
Trees or shrubs; leaves often amphistomatous, stomata cyclocytic/anomocytic; lamina (with glands); bracteoles ± adnate to G, large to small; flower (monosymmetric - Danseia); K with three traces; nectary also bilobed, inside hypanthium/± 0; (pollen tricolporate); ovules 2-20, hypostase +; fruits flattened, (winged by the bracteoles); cotyledons spirally folded/convolute; n = 13; germination hypogeal/epigeal.
4/8. Tropical, mangroves, also N. and N.E. Australia.
Age. Ricklefs et al. (2006: ?sampling) dated crown Laguncularieae to ca 23 Ma.
2b. Combreteae Engler —— Synonymy: Bucidaceae Sprengel, Myrobalanaceae Martinov, Sheadendraceae G. Bertolini, nom. invalid., Terminaliaceae Jaume Saint-Hilaire
Trees or shrubs (lianes), short shoots and "spiny" structures common; included phloem + [= interxylary phloem]; (mucilage ducts - Terminalia); stomata anomocytic; lamina vernation conduplicate or supervolute, domatia pocket- or bowl-shaped; K with one trace, C (small/0); A (= and alternate with/opposite to K), (-15); stigma (capitate); (micropyle endostomal - Guiera), (parietal tissue ca 10 cells across, pachychalazal - Combretum coccineum), funicular obturator papillate; (embryo sac tetrasporic, 16-nucleate); fruit ± winged, (drupaceous); (integuments multiplicative); (megaspore mother cells several); n = (7, 11) 12, 13.
5/490. Largely tropical. Combretum (255), Terminalia (190).
Age. This node is about 33 Ma (Berger et al. 2015).
Leaves of four species of Terminalia including T. chebula and T. bellirica have been described from Eocene (or Palaeocene?) Gurha lignite deposits in Rajasthan, India (Harsh & Shekhawat 2023, 2024), which seems rather unlikely.
Evolution: Divergence & Distribution. For the fossil record of winged fruits that can be assigned to Combretaceae, see Manchester and O'Leary (2010).
Diversification of most of the family is a Caenozoic phenomenon (Sytsma & Berger 2011), indeed, indeed, Berger et al. (2015) found that there had been a significant shift in speciation rates in Combreteae, but when that occurred was unclear. Anest et al. (2024) found that there was a correlation between the development of short shoots (resulting in a cage-like plant architecture), the subsequent evolution of spiny structures of various kinds, and herbivore pressure in Combretaceae, specifically Terminalia and Combretum (Combreteae). However, in Africa, but not globally, there seems to have been no interaction between the evolution of spines, amount of herbivory and diversification.
Ecology & Physiology. Lumnitzera and Laguncularia are mangrove taxa (see articles in Ann. Bot. 115(3). 2015; Suvarna Raju 2021: pollination); the radicle of Laguncularia appears during dispersal (Rabinowicz 1978), although the seeds of Lumnitzera at least, soon sink (Clarke et al. 2001). For more on the mangrove habitat, see Rhizophoraceae.
Combretum is one of ten largest genera of climbing plants (Sperotto et al. 2023).
Seed Dispersal. The flattened and/or winged fruits of Combretaceae are often wind- or water dispersed, and Systma and Berger (2011) note substantial dispersal of the family in the Pacific.
Chemistry, Morphology, etc.. The islands of included phloem in Combretum are connected in a reticulating fashion along the stem (Robert et al. 2011); den Outer and van Veenendaal (1995) noted that this system was more important in the transport of assimilates that the phloem outside the xylem - and that it was interesting that these plants were shrubs or trees, not lianas where odd vascular construction is almost the norm. Keating (1985) describes the stomata as being paracytic while Dahgren and Thorne (1985) call them anomocytic; in any event, variation in stomatal morphology is extensive (Stace 1965; Tilney 2002).
There are hairs lining the ovary loculus walls in Combretum. Guiera (Combreteae) is distinctive embryologically - not only does its anther have a single middle layer, but its micropyle endostomic, but the nucellus and nucellar cap are thin, the synergids and antipodals are persistent, the latter sometimes being multiplicative, there is no obturator, the cotyledons are convolute, etc. (Venkateswarlu & Prakasa Rao 1972: separate tribe?).
Some general information is taken from S. A. Graham (1964), Venkateswarlu and Prakash Rao (1971) and Jongkind (1995), both Strephonema, Tomlinson (1986), and Stace (2006, 2010: New World taxa); see also Lowry (1976: chemistry), Verhoeven and van der Schijff (1974: anatomy, inc. root cork cambium), Venkateswarlu and Prakasa Rao (1970: floral anatomy), Eckert (1966: obdiplostemony), El Ghazali et al. (1998) and El Ghazali (2022), pollen, and Mauritzon (1939a), Fagerlind (1941a) and Venkateswarlu (1952b), all embryology.
Phylogeny. Strephonema is likely to be sister to the rest of the family (e.g. Maurin et al. 2017, 2021). Lumnitzera and Laguncularia, both mangrove plants, are sister taxa, but Conocarpus, found in similar habitats, is not immediately related (Tan et al. 2002; Maurin et al. 2010, 2921); Maurin et al. (2017) had found that the three genera of Laguncularieae were paraphyletic at the base of Combretoideae, although support for that position was not strong and it has not been confirmed. Furthermore, M. Sun et al. (2016) suggested that Conocarpus was sister to the rest of the family, but this, too has not been confirmed; the genus is sister to Terminalia s.l. in Maurin et al. 2010, 2021), although otherwise relationships are as noted above. Maurin et al. (2010) in particular discuss details of relationships within the family, especially in the large genera Terminalia and Combretum while Maurin et al. (2017) clarify the limits of Terminalia; see also Maurin et al. (2021).
Classification. For generic limits, especially around Combretum, see Stace (2007), while Maurin et al. (2010) suggest that the limits of Terminalia, currently paraphyletic, be expanded; support values underpinning change in neither case are very high. For a classification down to subtribes, see Maurin et al. (2010).
[Onagraceae + Lythraceae]: tannins often not abundant, soluble oxalate accumulating; vessels grouped; petiole bundle arcuate; tapetal cells binucleate; (pollen at anthesis with starch); nucellus with starch grains, hypostase +; megaspore mother cells several; K persistent; x = 8.
Age. The two families are estimated to have separated at the end-Cenomanian ca 93 Ma (Sytsma et al. 2004) or earlier, around (115.8-)101(-84.5) ma (Gonçalves et al. 2020a), (109.1-)104.6(-100.2) Ma (Berger et al. 2015) and ca 107.3 Ma (Inglis & Calvacanti 2018), or somewhat later, about 72.2/68.2 Ma (Tank et al. 2015: Table S2), (80-)67, 63(-49) Ma (Bell et al. 2010), (71-)67, 57(-53) Ma (Wikström et al. (2001), (108.9-)89.6(-81.0) Ma (X.-F. Zhang et al. 2021) or ca 104.6 Ma (Inglis et al. 2023). The youngest ages conflict with ages of fossils of Lythraceae, q.v..
Evolution: Divergence & Distribution. For attempts to relate the development of the female gametophyte of Onagraceae to that of Lythraceae, see e.g. Mauritzon (1934e).
Chemistry, Morphology, etc.. Both Trapa and some species of Ludwigia are aquatics with distinctive floating rosettes of expanded leaves. Decodon is the only typically pentamerous genus in Lythraceae (S. A. Graham 2006), as is Ludwigia (Onagraceae). Since both may be sister to the rest of their respective families, working out where floral merosity changes on the tree becomes difficult. A number of Lythraceae, including Trapa, have capitate stigmas, and this could be another feature uniting the two families.
ONAGRACEAE Jussieu, nom. cons. - Back to Myrtales
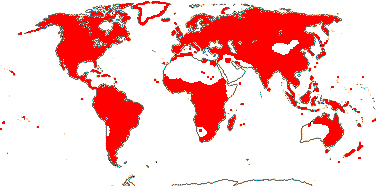
Plants herbaceous; flavonoid sulphates +; phloem loading via intermediary cells [specialized companion cells with numerous plasmodesmata, raffinose etc. involved]; raphides +; leaves (spiral), lamina vernation ± flat to involute, margins toothed, stipuliform structures +; bracteoles often 0; C deciduous, ± clawed; anthers polysporangiate, versatile, filaments straight in bud; pollen grains relatively large [55.25 (25.58 S.D.) μm long], oblate, colpi short, wide, protruding, pseudocolpi 0, starchy, surface psilate [smooth], ektexine paracrystalline, beaded, endexine massive, electron-dense, tectum, columella, foot layer all 0, viscin threads + [= sporopollenin, attached proximally]; nectary on G/base of hypanthium; G = to & alternating with K, stigma capitate (± lobed); ovules with outer integument 2-5 cells across, inner integument 2(-3) cells across, (epistase +), parietal tissue ca 2(?-13 - Vesque) cells across, nucellar cap ca 2 cells across, hypostase +/0; micropylar megaspore functional, embryo sac 4-nucleate [Oenothera type]; fruit loculicidal, opening down the sides; exotestal inner walls thickened and lignified, mesotesta 2 or more cells across, (cells thickened, ± sclerotic), endotesta crystalliferous; endotegmic cells longitudinally elongated, "tanniniferous", inner walls thickened); endosperm nuclear/coenocytic, diploid; x = 10 (?8, ?7, ?11), nuclear genome [1 C] (0.031-)0.847(-23.474) pg/(3081-)1309(-147) Mb.
22 [list, to tribes]/656 - two subfamilies below. World-wide. Map: based on Raven (1963a, 1967), Meusel et al. (1978), Trop. Afr. Fl. Pl. Ecol. Distr. 1. (2003), also P. Hoch and W. Wagner (pers. comm.).
Age. Crown-group Onagraceae are estimated to be ca 82 Ma (Sytsma et al. 2004), (96.2-)85.4(-73.3) Ma (Berger et al. 2015), (88.4-)71(-54.3) Ma (Gonçalves et al. 2020a) or (74.1-)46.9(-2.7) Ma (X.-F. Zhang et al. 2021).
1. Jussiaeoideae Beilschmied - Ludwigia L. —— Synonymy: Isnardiaceae Martinov, Jussiaeaceae Martinov
Herbs (annuals), (shrublets); stem with endodermis; flowers 4-5-merous; hypanthium 0; (A 10); pollen in tetrads (monads; large irregular clumps), colpi vertically elongated, columellae 0, viscin threads ± simple, smooth; G (3-)4-5(-7), with central vascular bundles, (style 0), minor stylar bundles +; nectary on top of ovary; ovules with parietal tissue 3-6 cells across, hypostase +; megasporocyte 1; capsule (apically porose); seed (chalaza expanded/= wing); much polyploidy.
1/82: World-wide, esp. America, commonly moist habitats. [Photo - Flower.]
2. Onagroideae Beilschmied
Included phloem +; flowers 4-merous, hypanthium long; K reflexed; pollen oblate, viscin threads usu. compound, variously beaded, twisted, etc.; transseptal vascular bundles + (0), minor stylar bundles 0, (stylar bundles 0), (stigma 4-lobed; dry); ovules (parietal tissue 10-25 cells across); hypanthium separating at base in fruit.
21/574. World-wide, but esp. western North America. [Photo - Flower, Flower.]
Age. This age of the node (Hauyeae + Circaeeae) is at least ca 52 Ma (Berry et al. 2004).
2A. Hauyeae Raimann - Hauya de Candolle
Shrubs to trees; leaves spiral; flowers axillary; pedicels short; pollen por(or)ate; seeds asymmetrically winged; mesotesta 0; n/x = 10.
1/2. Mexico (Hidalgo, Guerrero) to Costa Rica.
[Circaeeae [Lopezieae [Gongylocarpeae [Epilobieae + Onagreae]]]]: x = 11.
2B. Circaeeae Dumortier
Pollen columellae (±) 0; fruit indehiscent [very different types].
2/113. Northern Hemisphere, Mexico to the Andes, New Zealand, Tahiti.
Age. This node is at least ca 41 Ma (Berry et al. 2004).
2B1. Circaea L. —— Synonymy: Circaeaceae Berchtold & J. Presl
Rhizomatous herb; hypanthium constricted/very short; flowers 2-merous; K 2, lateral, C 2, ± lobed; A 2, opposite K; pollen with smooth viscin threads; ovules 1/carpel; fruit indehiscent, dry, with uncinate hairs.
1/8. Northern Hemisphere.
2B2. Fuchsia L —— Synonymy: Fuchsiaceae Lilja
Plant woody (lianes/epiphytes/trees), (deciduous); flowers (axillary/sessile), protogynous; C (0); pollen (blue), 2 aperturate, colpi horizontally elongated; fruit baccate; seeds embedded in pulp (not).
1/105. Mexico to Tierra del Fuego, esp. the northern Andes, New Zealand, Tahiti.
Age. Crown-group Fuchsia is at least ca 31 Ma (Berry et al. 2004).
[Lopezieae [Gongylocarpeae [Epilobieae + Onagreae]]]: ?
2C. Lopezieae Spach
Leaves usu. spiral; flowers monosymmetric [very different types]; pollen grains [Lopezia] blue (cream), triangular in polar view, apertures rimmed-porate.
2/23. Mexico to Central America.
2C1. Megacorax gracielanus S. González & W. Wagner
Shrubs; leaves fasciculate, blades ± linear; C as presented monosymmetric; hypanthium very short; n/x = 15.
1/1. Mexico (Durango).
2C2. Lopezia Cavanilles —— Synonymy: Lopeziaceae Lilja
(Annual) herbs/shrubs; (adaxial C pair auriculate/with glands); A 1 [abaxial] (held under tension), (2), staminode 1 (0), petaloid [vertical pair]; pollen (blue), columellae 0 [?tribe]; ; (stylar vasculature anatomosing); seeds tuberculate, (winged), wax granules on surface (0); mesotesta 1 cell layer; n = 7-11.
1/22. Mostly Mexico, to Panama.
[Gongylocarpeae [Epilobieae + Onagreae]]: stipuliform structures 0.
2D. Gongylocarpeae Donnell Smith & Rose - Gongylocarpus Schlechtendal & Chamisso
Herbs, annual/shrubs; leaves spiral; flowers sessile; pollen porate, protrusions 0, viscin threads beaded; ovules 1/carpel, parietal tissue "thick"; fruit in pith of stem, indehiscent.
1/2. Mexico and Guatemala.
[Epilobieae + Onagreae]: leaves ± spiral; pollen (columellae 0); mesotesta 0; OEGRα genome duplication.
Age. This clade may be ca 26 Ma (Landis et al. 2018).
2E. Epilobieae Endlicher —— Synonymy: Epilobiaceae Ventenat
Annual/perennial herbs to subshrubs; (floral tube 0), (with scales towards base); K erect (spreading); pollen (blue), in tetrads, viscin threads often smooth; stigma dry, lobes commissural, papillae multicellular; parietal tissue 0; seeds with chalazal hairs (not); n = 9-10, 12-13, 15-16 [x = ?9].
2/173: Epilobium (165). Widely distributed, few in lowland tropics.
2F. Onagreae Dumortier —— Synonymy: Oenotheraceae C. C. Robin
Anuual to perennial herbs (shrubs); lamina (deeply pinnately lobed); flowers (sessile), (hypanthium short); pollen (porate0, (protrusions notably prominent), (0), surface (beaded-linear), (viscin threads smooth - Clarkia); stigma (with basal indusium - Oenothera), (strongly lobed); (ovules to 1/carpel); (seeds winged); n (5-)7(-9) [x = 7], translocation rings/0, (plastid transmission biparental - Oenothera).
13/260: Oenothera (145), Clarkia (42). North and South America, especially western North America.
Evolution: Divergence & Distribution. For the fossil history of the family, see Grímsson et al. (2011a) and Lee et al. (2013) and references; the fossil pollen is very distinctive and is known from the Maastrichtian, at least 66 Ma. Pollen of Corsinipollenitis from Late Cretaceous sediments in India perhaps ca 66 Ma has been linked with a couple of Old World species of Ludwigia like L. perennis (Farooqui et al. 2019) that are quite closely related. Two other Indian species, L. adscendens and L. octovalvis, have been associated with Ludwigia pollen from Pleistocene deposits in India; these species are quite unrelated (see S.-H. Liu et al. 2017: phylogeny of Ludwigia). Corsinipollenitis has also been described from Palaeocene deposits in Venezuela (Pocknall & Jarzen 2009).
The apomorphy scheme above is based in part on that in Wagner et al. (2007), q.v. for more details about individual genera in particular.
Pollination Biology & Seed Dispersal. For details of floral morphology in Onagraceae and its relation to pollination, see Wagner et al. (2007). Just about all the New World species of Fuchsia are likely to be pollinated by humming birds, and even species from New Zealand are pollinated by the local birds (e.g. Berry 1989; Wagner et al. 2007 and references). Pollination in North American members of the family has been studied in great detail (e.g. references in Linsley et al. 1973; Clinebell et al. 2004). The strongly monosymmetric flowers of Lopezia have only a single abaxial stamen that may become extrorse during explosive dehiscence and a petal-like adaxial staminode; the two adaxial petals may be recurved and have pseudonectaries on their claws (Eyde & Morgan 1973). Some 12 species of oligolectic Andrena bees are major visitors to Camissonia campestris alone (Linsley et al. 1973), while Clinebell et al. (2004) documented a wide variety of potential pollinators visiting the four species of Onagraceae and three other species that they studied. It has been suggested that plants of Oenothera drummondii can sense pollinators (by vibration), and that this caused a rapid increase in the sugar concentration of the nectar produced (Veits et al. 2018/2019); this must be confirmed, and in its native habitat. How petal spots develop in Clarkia gracilis has been worked out (Martins et al. 2012), and heteranthy in C. unguiculata, at least, is not as extreme as had been thought (Peach & Mazer 2019).
Protogyny is quite common in a few clades; the non-protogynous taxa are either protandrous or undecided in equal numbers (Newman 1993). For a general survey of reproductive biology in Onagraceae, see Raven (1979).
The fruits of a number of species of Oenothera open only when moist - they are ombrohydrocharous. When wetted, the fruit opens from the apex along the locular radii, the four segments so formed recurving, and when dry the fruit closes (Poppendieck 1995). The fruits are winged (down the middle of the valves), and the seeds of such taxa seem to be notably uniform (Poppendieck 1995).
Plant-Animal Interactions. Some caterpillars are found on both Vitaceae and Onagraceae (Forbes 1956), both groups that contain raphides that have been suggested to deter feeding (Hyles lineata was defoliating Onagraceae - California 2019). Similarly, the gelechioid moth Momphis has diversified on Onagraceae, where various species eat flowers and fruits or are gallers or leaf miners, etc., and the moth is also found on raphide-bearing Rubiaceae, and a few species on other families including Melastomataceae - which may not have raphides but of course is also Myrtales (Bruzzese et al. 2019).
M. T. J. Johnson (2014) discussed proanthocyanidins, ellagitannins and caffeic acid derivatives, deterrents against herbivory, in Oenothera. Interestingly, the control of flavonoid biosynthesis is affected in permanent translocation heterozygotes (see below), which are functionally asexual, both flavonoids and attacks by generalist mites increasing (Johnson et al. 2009, 2014), although any connection between the principal components of variation in flavonoid defences and plant phylogeny was at most moderate (Johnson et al. 2014), as is common in such interactions (e.g. Cacho et al. 2015).
Plant-Bacterial/Fungal Associations. Rusts on Onagroideae and Jussiaeoideae differ (Savile 1979b).
Genes & Genomes. A genome duplication for the [Epilobeae + Onagreae] clade, the OEGRα event, has been dated to ca 26 Ma (Landis et al. 2018).
Almeda and Penneys (2022) suggest that x = 11 in Onagraceae. For general cytological studies, see Tanaka et al. (1988) and for chromosome numbers, see Wagner et al. (2007).
All the chromosomes in some species of Oenothera form a ring at meiosis being joined by a series of permanent translocations; the whole genome then forms a single linkage unit (Cleland 1972), and reproduction is effectively asexual. Golczyk et al. (2014) found that breaks occurred subterminally between between two distinct chromatin regions and that there were no normal telomeres; translocations did not involve whole arms. Although the permanent translocation heterozygotes/hybrids (PTH) self, they show increased diversification rates over sexual species, interestingly, there are frequent reversals from PTH to sexuality, and the PTH condition has arisen ca 20 times (M. T. J. Johnson et al. 2011). The whole system sometimes breaks down (see Harte 1993: the contributions of Oenothera to biology; Stubbe & Steiner 1999 and references: translocation, etc; Wagner et al. 2007).
Particular combinations of genome and plastome may be incompatible, and the resultant inviability of some of these combinations may provide genetic barriers between taxa (Stubbe & Steiner 1999). Incompatability between chloroplasts from one parent and the hybrid genome (plastome-genome incompatability - PGI) may result in the death of those chloroplasts and thus to variegation (Snijder et al. 2007; Ruhlman & Jansen 2018 and references). AB-I plants cannot acclimate to high-light intensities (Zupok et al. 2021). Chiu and Sears (1993 and references) discuss biparental transmission of plastids in Oenothera. Sobanski et al. (2019) looked at the control of plastid competition and inheritance (the accC and ycf2 genes are involved), and they suggested that aggressive plastomes were derived, having evolved twice in the genus (once with a reversal would also be possible?). There is basically no hybrid incompatability involving nuclear loci, and hybridization is widespread in the genus (references in Zupok et al. 2021). The American school recognises ca 13 species in the genus that are based on particular combinations of nuclear and chloroplast genomes, while in the largely morphological species definition of the European school ca 80 species are recognized... (Zupok et al. 2021).
Erixon and Oxelman (2008b) found elevated positive substitution rates in the clpP1 gene in Oenothera, and there was also a great increase in the size of some exons, loss of introns, etc..
Chemistry, Morphology, etc.. The morphology, anatomy, etc., of Onagraceae are rather better known than that of most comparable families thanks to extensive comparative studies carried out from the 1960s onwards by P. H. Raven and his associates.
The development of the leaf in Oenothera is basipetal (Hagemann & Gleissberg 1996). The "stipules" of Ludwigia can be quite prominent.
The ovary of Gongylocarpus becomes completely enveloped by stem tissue after pollination (Carlquist & Raven 1966). Tobe and Raven (1986a) noted that the septae in the polysporangiate anthers of Onagraceae might be of tapetal or (largely) parenchymatous origin; the former was likely to be an apomorphzy for the family, and the latter morphology has been derived several times. The pollen grains of some species of Oenothera are very large indeed, triangular in polar view, and with strongly protruding pores (Kriebel et al. 2017). The viscin threads that characterize the family vary considerably in morphology, often being annular-vermiform, but they are also smooth or irregularly beaded (Skvarla et al. 1976); rather unusually, but like the threads of Ericaceae, they are made up of spooropollenin (Halbritter et al. 1997). In Oenothera, more than one spore from the same megaspore mother cell may germinate and - presumably - compete (Noher de Halac & Harte 1977). Carlquist and Raven (1966) described seeds of Gongylocarpus fruticulosus that had elongated ?exo/mesotegmic cells with gelatinous wall thickenings running at right angles to elongated endotegmic cells with their ?tanniniferous contents.
For general information, see the Onagraceae website and Wagner et al. (2007: superb summary), also Balfour and Philipson (1962: Godetia nodal anatomy), Tobe and Raven (2018: vestured pits), Eyde (1982: floral anatomy), Maheshwari (1947), Tobe and Raven (1986b, 1987d, 1996) and Hoch et al. (1993b: variation in anther septum development, embryology), Praglowski et al. (1983, 1987, 1989, 1994: pollen), Skvarla et al. (1977: tetrads, 1978: viscin threads), Johansen (1928: hypostase and environmental correlations), and Tobe et al. (1987d: seed coat anatomy).
Phylogeny. For morphological phylogenies, see Hoch et al. (1993a: Lopezia, 1993b: family. Ludwigia is sister to the rest of the family. Within Ludwigia, taxa with five and those with ten stamens form separate clades (Barber et al. 2008), although support for the 10-stamen clade was strong only in Bayesian analyses (S.-H. Liu et al. 2017). Support for relationships in the 5-stamen clade was quite strong when using chloroplast data, but that along the backbone in particular of the rest of the tree was rather weak whether using chloroplast, nuclear or combined data, and especially in parsimony analyses (Liu et al. 2017). There was some conflict in the topologies of trees obtained using nuclear markers and those using chloroplast markers, but allopolyploidy is common in the genus (Liu et al. 2018).
Knowledge of relationships along the backbone of the tree seems to be stabilising, i.e. on the relationships [Hauya [[Fuchsia + Circaea] [Lopezia [Gongylocarpus [Epilobeae + Onagreae]]]]] (Levin et al. 2003, 2004), and this is the topology followed above. However, Ford and Gottlieb (2007) found a clade [Hauya [Fuchsia + Circaea]] that was sister to other Onagroideae (see also M. Sun et al. 2016). Relationships are similar to those in Lenin et al. (2003, 2004) in the Angiosperms353 analysis of Maurin et al. (2021: Hauya not included), although the clade [Gongylocarpus [Epilobeae + Onagreae]] is not well supported within Onagreae relationships are somewhat different from those previously recovered.
Berry et al. (2004: ca 1/3 species included) examined relationships in Fuchsia and found that all the sections with two or more species sampled except section Fuchsia (paraphyletic) were monophyletic, and the species from Tahiti and New Zealand (with blue pollen) formed a clade sister to the rest of the genus. However, relationships along the spine of the tree had rather little support. For relations in and the circumscription of Oenothera, see e.g. Hoggard et al. (2004) and Levin et al. (2004) and references.
Classification. Wagner et al. (2007) describe all supraspecific taxa in the family and so they include sectional classifications of Fuchsia, Oenothera, etc.. Reflecting the new, but for the most part well supported phylogeny of the family, generic limits have been adjusted, so Oenothera has been expanded and Camissonia very much cut up (e.g. Levin et al. 2004); for all the nomenclatural changes involved, see Hoch and Wagner (2007). The Onagraceae website contains a largely up-to-date summary of the classification, etc..
Botanical Trivia. In 1827 Robert Brown recorded the phenomenon that is now called Brownian motion when observing the pollen grains of Clarkia pulchella.
Hugo de Vries thought that the abrupt appearance of Oenothera lamarckiana was an example of normal evolution, which for him was a process in which mutation = major change = speciation, natural selection not being involved. However, O. lamarckiana is a morphological variant caused by the breakdown of the permanent translocation system mentioned above (c.f. Linnaeus and Peloria [= Linaria, Plantaginaceae]).
LYTHRACEAE Jaume Saint-Hilaire, nom. cons. - Back to Myrtales
Herbs to trees; quinolizidine alkaloids +; fibres +, septate; mucilage cells common; hairs uni- or bi(multi)cellular; leaves (spiral), lamina vernation flat to conduplicate, stipules +/0; (inflorescence determinate); pedicel articulated [?always]; flowers (3) 4 (5) 6(-16)-merous, heterostyly common; hypanthium + (spurred), (0, but with K + C tube), often strongly ribbed and/or appendages [= epicalyx] alternating with K, C crumpled in bud, (0), initial development slow [?level]; A developing before C, basically obdiplostemonous, (1- = and opposite K/C -many, centrifugal or centripetal), inserted just below C to near ovary, heteranthy +; (tapetal cells multinucleate); pollen grains (porate), (pseudocolpi 0, 6), (surface striate); G superior, [2-6(-many)], (inferior), orientation variable, (placentation parietal), stigma capitate to punctate, also dry; ovules few/carpel), outer integument 2-7(-9) cells across, inner integument 2(-3) cells across, parietal tissue 2-9 cells across, "chalazal strand" +, (postament +); (embryo sac much elongated); fruit a capsule, dehiscence irregular/circumscissile/loculicidal (indehiscent; berry), K often ± enclosing fruit; seeds usu. flattened; testa multiplicative, many-layered, exotesta various, invaginated hairs + (0), endotestal cells often elongated and tracheidal/sclerotic, (crystalliferous), (endotegmen of crossing fibres); (cotyledons folded); n = (5-)8(-11, + polyploids), chromosomes 0.5-4 µm long; x = 8, n = 15, nuclear genome [1 C] (0.044-)0.78(-13.878 pg/(333-)673(-963) Mb; plastome rpl2 intron 0.
28/687 [list]. Tropical, but some temperate, esp. America. Map: from van Balgooy (1975), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), S. A. Graham et al. (2005) and Inglis et al. (2023: Figs 2, 3). Photo: Flower.
Age. Estimates of the age of the family are (65-)50, 46(-29) Ma (Bell et al. 2010: minus Deocodon), ca 60 Ma (Sytsma et al. 2004) or rather older, ca 80 Ma (Inglis & Calvacanti 2018) and even (99.7-)95.5(-91.7) Ma (Berger et al. 2015); Gonçalves et al. (2020a) estimated an age of (77.6-)68.6(-63.8) Ma, X.-F. Zhang et al. (2021) an age of (86.8-)72.2(-63.8) ma, but these ages refer to a number of different topologies, and Inglis et al. (2023) an age of ca 95.7 Ma.
Note that fossil evidence suggests that a crown group age for the family is likely to be at least 85 Ma, most fossils coming from Clade 2 below. Pollen from the Montana group, Wyoming, dated to the Lower Campanian 82-81 Ma has reliably been identified as Lythrum or its segregate, Peplis; there is somewhat younger (72-78 My) pollen from Siberia (Grímsson et al. 2011b: exquisite micrographs). Enigmocarpon paijai, known from both flowers and fruits in rocks 67-65 Ma from the Deccan Traps, India, belongs to but is unplaced within Lythraceae (S. Y. Smith et al. 2015 and references). The fossil Trapago is found in deposits 73-65 Ma, and other fossils assignable to Myrtales in general are perhaps slightly older (Crepet et al. 2004 and references). S. A. Graham (2013) summarized the earlier literature on fossils here (see also Inglis et al. 2023).
Clade 1/South American —— Synonymy: Punicaceae Berchtold & J. Presl, nom. cons.
(flowers monosymmetric - Cuphea), (nectary at base of G/enclosed by spur - C.); parietal tissue (-15 - C.) cells across; (invaginated hairs often with spiral structure), (sarcoexotesta - Punica)
15/383: Cuphea (255), Diplusodon (100).
Age. The age of this clade is ca 70.6 Ma (Inglis et al. 2023).
Clade 2/North American —— Synonymy: Ammanniaceae Horaninow, Blattiaceae Engler, Duabangaceae Takhtajan, Lagerstroemiaceae J. Agardh, Lawsoniaceae J. Agardh, Sonneratiaceae Engler, nom. cons., Trapaceae Dumortier
vesturing spread over inside of vessel [Sonneratia]; (lamina margins dentate - Trapa); A centrifugal - Lagerstroemia; ovules (1/carpel - T.); (fruit a drupe - T.); (testa not many layered - Duabanga); (embryo anisocotylous, radicle 0 - T.).
13/304: Ammannia (108), Rotala (72), Lagerstroemia (48), Lythrum (39).
Age. Inglis et al. (2023) estimated the crown-group age of this clade to be ca 92.4 Ma.
Evolution: Divergence & Distribution. Sonneratia, a mangrove genus, has a long fossil record, its pollen, Florschuetzia, being distinctive (J. Müller 1978, 1984), and it may be Late Eocene-Oligocene in age. Duabangaxylon, associated with the Indo-S. Chinese-Malesian Duabanga (it grows in Eastern India eastwards), is reported from deposits ca 65 Ma in Kutch, far western India (Shukla & Mehrotra 2017). Decodon, now restricted to eastern North America, was widely distributed in the Northern Hemisphere from the Eocene onwards and was more diverse than it is now (Ferguson et al. 1997 and references; Little et al. 2004); it is also known from the late Cretaceous of Mexico (Grímsson et al. 2012). See S. A. Graham (2013) for a summary and evaluation of the fossil record of the family.
The 100+ species of Diplusodon, most of which grow in campos rupestres and cerrado vegetation in Brazil and a number of which have xylopodia, are thought to have evolved some (4.6-)3.5(-2.5) Ma, while stem Diplusodon is ca 58 Ma (Inglis & Cavalcanti 2018)... S. A. Graham et al. (2006) suggested that Cuphea originated and initially diversified in eastern Brazil.
Ginoria, from southern Mexico and the Caribbean, and the monotypic Tetrataxis, from Mauritius, separated ca 24.5 Ma; Inglis et al. (2023) discuss this and other distributions in the family, emphasizing apparent adaptations of the seeds for water or wind dispersal. Inglis et al. (2023) also emphasized the heterogeneity in clade size in Lythraceae - there are six monotypic genera that are anything from ca 40-85 Ma (although fossil and extant distributions may not match here), while Cuphea, which includes over 250 species, is only some 20 Ma.
The testal hairs of a number of Lythraceae - they appear only when the seed is wetted - are apparently unique in the angiosperms. They are tentatively placed as an apomorphy for the family as a whole above, but matters are complicated. Not all genera of Lythraceae have such hairs, and there are two different types (S. A. Graham & Graham 2014: Table 2). In one of them the hairs are in spiral coils, as is shown by Stubbs and Slabas (1982: esp. Figs 3, 5, 6) in some remarkable images; taxa with these spiral hairs are apparently restricted to Clade 1 above. However, spirals are also mentioned by Panigrahi (1986) in taxa that apparently lack these coils, and one wonders what internal anatomy might disclose in some taxa in which the hair surface shows no obvious structure. For more on morphology and phylogeny, see Tobe et al. (1998) and Graham and Graham (2014).
Ecology & Physiology. Sonneratia, with ca 7 species, is a notable element of mangrove vegetation from Southeast Asia to the Pacific. The nuclear genomes (1 C) of the mangrove species are (329.6-)326, 259(-255.4) Mb, about half the size of those of non-mangrove species (). Pemphis lives in back-mangrove habitats, on rocky shores, etc., from East Africa to the Pacific; it has been dated to somewhat over 45 Ma, Sonneratia to about 40 Ma (Z. He et al. 2022); Mao and Fuong (2013) discussed the pollen record of the latter (as Florschuetzia). For the evolution of the mangrove habitat, see Rhizophoraceae, also articles in Ann. Bot. 115(3). 2015, Suvarna Raju (2021: pollination), etc..
Pollination Biology & Seed Dispersal. Heterostyly is known from some Lythraceae (Barrett & Shore 2008).
Taxa whose seeds have hairs may be more or less myxospermous; the hairs evert when the seed is wetted (Western 2011; S. A. Graham & Graham 2014). Mucilage is also produced by the testal cells in other taxa like Sonneratia. The fruits of Cuphea open by the placenta expanding and moving laterally, breaking through both the thin ovary wall and the hypanthium; the seeds - there are only a few - are exposed on the placentae (Graham & Graham 2014). In a number of cases, seeds seem adapted to wind or water dispersal - for the latter, there may be aerenchyma or tissue in the testa that aids in flotation (Graham & Graham 2014; Inglis et al. 2023).
Genes & Genomes. A genome duplication, the LAINα event that has been dated to ca 68 Ma, involves both Lagerstroemia and Punica (Landis et al. 2018). S. A. Graham and Cavalcanti (2001) suggest that x = 8 is the basic chromosome number for the family, while x = 11 for the speciose Cuphea. In this latter genus there has been extensive polyploidy, evident in the terminal branches, also dysploidy (Feitoza et al. 2024).
Nuclear genomes [1 C] of the non-mangrove species are (963-)673(-333) Mb compared with (329.6-)326, 259(-255.4) Mb for the mangrove species ().
Chemistry, Morphology, etc.. The phyllotaxis of Lagerstroemia indica is the complex orixate type (see elsewhere).
The epicayx of Lythrum salicaria is initiated after the calyx, the inner stamens after the carpels, etc., and there are stamen fascicles opposite the petals (Cheung & Sattler 1967; Ronse Decraene & Snets 1991b; Rudall 2010); the petals here and in Trapa and Lagerstroemia initiate on time, but their early development is notably slow (Remizowa 2019). The stamens may be held on one side of the flower (also in some Onagraceae) and so causing rather weak monosymmetry; this is much more marked in Melastomataceae. However, some species of Cuphea, e.g. C. glutinosa, have quite strong monosymmetry that involves the corolla. Androecial development is both centrifugal (e.g. Lagerstroemia) and centripetal (Weberling 1989 and references; Ronse Decraene & Smets 1991). Pollen is notably variable (Graham 2006), and the oblate pollen of Cuphea may be triangular in polar view, being rather like pollen of Onagraceae in these respects (Kriebel et al. 2017). Species of the large genus Cuphea consistently have eleven stamens. When G = K, the carpels may alternate with or be opposite to them, when G = 2, the carpels may be transverse or median, when G = 3, the odd carpel is adaxial (Eichler 1878; Baillon 1877; Spichiger et al. 2002). Ovule morphology varies considerably. In taxa like Trapa, Sonneratia and Cuphea the embryo sac is much elongated, the nucellus sometimes being massive. There is usually a postament, and the base of the endosperm may be abruptly narrowed and protrude into the hypostase (see e.g. Täckholm 1915; Tischler 1917; Mauritzon 1934e; Joshi and Venkateswarlu 1936; Venkateswarlu 1937; Ram 1956: Trapa, and references).
The inferior ovary of Punica granatum is unique in flowering plants, appearing to have two (or three) superposed layers of carpels, the basal series (3) having axile placentation and upper series having intrusive parietal placentation and at least sometimes being 5 in number (Roth & Lindorf 1972). The placentation is fundamentally axile, the appearance of parietal placentation being the result of growth at the base of the ovary (Sinha & Joshi 1959 for vasculature), although this is difficult to understand if the series have different numbers of units. See Leins (1988) for the centripetal development of the androecium. In the other species of the genus, P. proto-punica, there is a more ordinary semi-inferior ovary. The seeds of Punica have a sarcotesta (e.g. Roth & Lindorf 1972), although this is often described as being an aril, not least by Wikipedia (1.2019)!
For general information, see S. A. Graham (1964, 2006) and Sinjushin (2018b: Trapa); for some vegetative anatomy, see Little et al. (2004), for pollen, see J. Muller (1981b) and A. Graham et al. (1990), for development, see Cheung and Sattler (1967: Lythrum), for ovules, see Mauritzon (1939a), for seed development, see Nagl (1962) and Titova et al. (1997), both Trapa and for seed anatomy and morphology, see Grütter (1893) and S. Graham and Graham (2014).
Phylogeny. S. A. Graham et al. (2005) found maximum parsimony support for the topology [Decodon [[Lythrum + Peplis (= Lythrum)] [remainder of the family]]]; the remainder of the family formed two large clades. However, support along the back-bone was weak, and in maximum likelihood analyses there was some support for the three genera just mentioned being sister to one of these two clades. Z.-D. Chen et al. (2016) found Rotala to be sister to the rest of the family, but support for this position was weak. The relationships found by Gonçalves et al. (2020a) were [Heimia [Amannia [Trapa + Lagerstroemia]]], but this family was not the focus of their work. The old Sonneratiaceae (Sonneratia and Duabanga), plants of mangroves, are not monophyletic (Shi et al. 2000; Huang & Shi 2002; Graham et al. 2005; Narzary et al. 2016); Sonneratia itself may be sister to Trapa (e.g. Z.-D. Chen et al. 2016; X.-F. Zhang et al. 2021). The distinctive Punica was found to be sister to Woodfordia (Narzary et al. 2016), while [Woodfordia [Punica + Pemphis] were sister to the rest of the family in the plastome study of X.-F. Zhang et al. (2021).
Relationships found in the Angiosperms353 study by Maurin et al. (2021: good generic-level sampling) should be consulted. There were two main clades in the family, the basal branches of both having moderate quartet support. In one, Decodon and Lythrum (see above) were in a clade, there was also a clade [[Sonneratia + Trapa] [Lagerstroemia + Duabanga]], and Rotala, Lawsonia and Ammannia were also in this first main clade, but all separated and the last paraphyletic. The other clade was made up of a clade [Pemphis [Punica [Capuronia + Galpinia]]] that was sister to a clade [Diplusodon [... Woodfordia ... [Pleurophora + Cuphea]]] (Maurin et al. 2021). The same two main clades were recovered in the plastome analysis of Inglis et al. (2023: 1 species/genus), and details of the relationships in those clades were largely similar, differences being at three poorly supported nodes in the latter study.
S. A. Graham et al. (2011) found that Rotala and Ammannia, previously thought to be close, were well separated, and they clarified relationships within the former genus. For a phylogeny of Cuphea, see Graham et al. (2006: 53 spp., morphology and ITS), although there was little support for relationships between the major clades in the ITS analyses and little support for any relationships in analyses of the morphological data. Feitoza et al. (2024: 44 spp.) found a fair bit of resolution in their analyses using nuclear ITS plus two plastid genes. Inglis and Cavalcanti (2018) found that Diplusodon showed a fair bit of resolution at deeper nodes and could be divided into four main clades, each with a geographical signal; D. virgatus, which has a white corolla, was sister to the rest of the genus, which has a red corolla.
Two taxa with particularly distinctive morphologies:
Classification. The current classification of the family will have to be retooled if the tree in Maurin et al. (2021) holds up - two subfamilies and a few tribes would make sense. For likely changes in generic limits around Ammannia, see S. A. Graham et al. (2010, esp. 2011).
Previous Relationships. Some morphologically distinctive taxa that were until quite recently separated as their own families - Trapaceae (water chestnut), Sonneratiaceae, Punicaceae (pomegranate) - nestle firmly within Lythraceae.
[[Vochysiaceae + Myrtaceae] [Melastomataceae [Crypteroniaceae [Alzataeaceae + Penaeaceae]]]]: inflorescences with at least the branches cymose.
Age. This node has been dated to around 88.2 Ma (Tank et al. 2015: Table S1), (84-)79, 74(-69) Ma (Wikström et al. 2001: internal relationships ± scrambled), (90-)75, 73(-59) Ma (Bell et al. 2010), (107-)95.1(-95.1) Ma (Berger et al,. 2015), (111.6-)100.8(-90.5) Ma (Gonçalves et al. 2020a) and (106.9-)94.2(-83.5) Ma (X.-F. Zhang et al. 2021).
Evolution: Divergence & Distribution. Vochysiaceae, Myrtaceae and Melastomataceae are notable components of tropical forests, particularly in the New World.
Chemistry, Morphology, etc.. Oil glands are found in the anthers of many Myrtaceae, and a number of other taxa in the Melastomataceae-Crypteroniaceae also have a very much expanded connective. Whether some staminal features - perhaps linked with pollination - are a higher-level apomorphy in this clade awaits further study.
[Vochysiaceae + Myrtaceae]: crystals in wood rays 0; hairs simple, 1-2-celled; K and C imbricate; post-zygotic incompatibility system [?all]; style depressed in apex of gynoecium; fruit a capsule.
Age. The two families diverged 100-93 Ma (Sytsma et al. 2004), (107-)101(-95) Ma (Berger et al. 2015), (96.5-)91.7(-89.9) Ma (Thornhill et al. 2012a: combined constraints), 72.3/61.9 Ma (Tank et al. 2015: Table S2) or (106.9-)86.4(-83.5) Ma (X.-F. Zhang et al. 2021).
Evolution: Divergence & Distribution. Sytsma et al. (2004) discussed the age and biogeographic history of the whole group in some detail.
VOCHYSIACEAE A. Saint-Hilaire, nom. cons. - Back to Myrtales
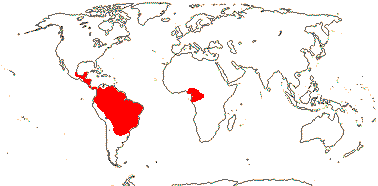
Trees (lianes); 5-deoxyflavonoids +; plants Al-accumulators; pericyclic fibres at most few; small phloem bundles inside xylem, sclerified bundles in pith; sclereids, mucilage cells/ducts +/0; leaf traces run along stem before entering petiole; cuticle waxes ± grouped parallel platelets; stomata also paracytic; indumentum often brown, hairs unicellular; leaves leathery, lamina vernation conduplicate, stipules cauline; inflorescence terminal, lateral cincinni +; flowers strongly mono- or asymmetric, plane of symmetry oblique; hypanthium 0, K basally connate, quincuncial, one adaxial-lateral K larger, variously nectariferous; C quincuncial [when 5]; fertile A 1, opposite abaxial-lateral C [on plane of symmetry], anther about as long as filament, filaments straight in bud; (pollen striate); G [3 (4)], odd member adaxial, ovary ± tapering into style, stigma punctate to subcapitate; ovules epitropous, outer integument 2-3 cells across, inner integument ca 2 cells across; n = 11, 12, x = ?11, ?12, chromosomes ca 1 μm long.
8-10 [list]/250. Tropical America, W. Africa. Map: from Stafleu (1954) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photo - Flower.]
Age. The age of crown-group Vochysiaceae is estimated to be some 36-33 Ma (Sytsma et al. 2004), (52-)39(-28) Ma (Berger et al. 2015), around 20 Ma (X.-F. Zhang et al. 2021) or (66.7-)50(-34.9) Ma, rather considerably older (Gonçalves et al. 2020a).
Fossils from northern Peru in rocks ca 39 Ma has been named Qualeoxylon lafila and carefully compared with that of other Vochysiaceae and of Myrtaceae (Woodcock 2024).
1. Erismadelphus Mildbraed + Korupodendron Litt & Cheek
Wood rays uniseriate, included phloem [Eris.]; non-wood anatomy?; (3 K C-like - Korup.), C 5, nectariferous cavity running down one side of the inferior ovary, (spurs 2, short, stout - E.); anther connective massive [E.], staminodes 2; G inferior, unilocular, (stigma unilateral); ovule single; ?embryology, etc.; fruit samaroid, with 5 wings [= accrescent sepals]; seed coat compressed, vascularized; cotyledons ± plano-convex; testa undifferentiated, with vascular bundles; germination ?hypogeal [K.].
2/3. West Africa (see Map above).
Age. The crown-group age of this clade is (27.2-)15.6(-5.8) Ma (Gonçalves et al. 2020a).
[Vochysieae + Qualea, etc.]: some rays multiseriate; nectariferous spur ± well developed; C 1; stigma wet; micropyle long, hypostase +, placental obturator +; embryo sac much elongated; cotyledons ± convolute.
Age. This clade is (53.2-)40.3(-28.8) Ma (Gonçalves et al. 2020a).
2. Vochysieae Dumortier
(Rhizomatous herbs); cork outer cortical; (included phloem +); midrib bundle semicircular/interrupted annular, secretory ducts in midrib/0; (hairs stellate - E.); (bract/eoles recaulescent - V.); C (5, 3, 0, also -4 reduced); A (initially median, moving out of plane of symmetry - E.), inserted on K [S., V.], (staminodes 2); (exothecium unthickened, secondary pollen presentation + - some V.), (staminodes 2); pollen grains (tricellular - S.), (almost smooth - V.); G (inferior, 1 loculus developing - E.), stigma (unilateral), with multicellular uniseriate hairs; ovules 2/carpel, outer integument 7-12 cells across, inner integument 3-4(-8 - S.) cells across, parietal tissue -40 [V.] cells across (integuments vascularized - S.); (spurred K deciduous - E.); fruit (samaroid, with 4 wings [= sepals] - E.), (nut), loculicidal capsule; seed 1; (seed winged, wing of hairs); seed coat largely exotestal, undifferentiated, crystal layer 0, tegmen 0, (with vascular bundles - E.); (cotyledons massive, plano-convex E.). E. = Erisma, S. = Salvertia, V. = Vochysia.
4/143: Vochysia (144), Erisma (17). South America, mostly Amazonian, also Panama and the Atlantic Forest.
Age. Crown-group Erismeae are some (24.2-)14.3(-5.5) Ma (Gonçalves et al. 2020a: ?sampling).
3. Qualea Aublet, etc. / QRC clade.
(Plant deciduous - M.); (included phloem +); cataphylls +/0; midrib bundle shallowly arcuate, stomata paracytic; (leaves held in one plane - C.), (venation eucamptodromous - C.), stipules as glands/with extrafloral nectaries/minute; (inflorescence compact, axillary, flowers small, <3.2 mm long - M.), (bracteoles 0 - some Q., 0-3 - M.); enantiostyly [C.]; A not in plane of symmetry; (nectary spur 0 - some Q.); C 5/3/1 (+ reduced C), (not clawed - M.); A (thecae attached their length to connective), (with hairs); staminodia 0-4; pollen striate-reticulate; style solid, stigma papillate; ovules (2-)several/carpel, 2-ranked, outer integument 2-4 cells across, inner integument 2-4 cells across, parietal tissue ca 7-20 cells across, (nucellar cap +), (postament + - Q.), placental obturator +; megaspore mother cells several, delayed fertilization; fruit a loculicidal capsule, (outer layer of pericarp separating irregularly); seeds (winged, wing circumferential - C.; one-sided - M.); mesotesta ?not sclerotic, endotestal cells ± U-thickened, pectic, crystalliferous or not, exo/mesotegmic cells fibrous, thick-walled or not, endotegmen tanniniferous [Q.], or multiplicative - Q. to ca 20 cells across, ± undifferentiated, tegmen ± obliterated, or exotestal hairs +, thick-walled, a few other layers persisting, but rest and tegmen disorganised. C. = Callisthene, M. = Mahechadendron, Q. = Qualea, R. = Ruizterania.
2-4/54: Qualea (50). Tropical Central and South America
Evolution: Divergence & Distribution. For additional ages within Vochysieae, see Gonçalves et al. 2020a).
Gonçalves et al. (2020a) could not be sure of the identity of the leaf fragments described as Qualea siwalica, from Miocene deposits from the Siwaliks in western Nepal. Given that its relatives would be New World taxa, this must come as something of a relief.
The phylogenetic "fuse" of Vochysiaceae is around 50 (Gonçalves et al. 2020a) to 60 (Berger et al. 2105) million years. The present distribution of Vochysiaceae on either side of the Atlantic was thought likely to be the result of dispersal to Africa from America (Sytsma et al. 2004; Berger et al. 2015: differences only in detail). However, Gonçalves et al. (2020a) found that the African Vochysiaceae were sister to the rest of the family, and suggested [Africa + South America] was the ancestral area for both [Vochysiaceae + Myrtaceae] and Vochysiaceae, and for the New World taxa the Cerrado seems to be central.
Although Vochysiaceae are a rather small family, they encompass a substantial amount of variation - vegetative, floral, seed coat - and their phylogenetic relationships are poorly understood.
Pollination Biology & Seed Dispersal. Visitors to the flowers are various and include bees, hummingbirds and hawkmoths (Carmo-Oliveira et al. 2017 for literature). In at least some Qualea and relatives, fertilization is delayed; the ovules develop slowly, and the pollen tubes spend some time in the obturatal area (Carmo-Oliveira et al. 2020). Carmo-Oliveira and de Morretes (2009) discussed variation in incompatibility mechanisms in Brazilian Vochysiaceae, Vochysia showing gametophytic self incompatibility while Qualea and Callisthene had some kind of late-acting self-incompatibility mechanism (see also Oliveira 1998).
Plant-Animal Interactions. Swollen stem domatia in Vochysia vismiaefolia developed only after the ant Pseudomyrmex sp. starts excavating the internodes, and these domatia can also be induced by simply drilling little holes into the internodes (Blüthgen & Wesenberg 2001).
Genes & Genomes. For cytological information, see Yamagishi-Costa et al. (2018).
Chemistry, Morphology, etc.. At least in Vochysia guatemalensis there are conspicuous, symmetrically-arranged mucilage canals in the pith. Qualea has trilacunar nodes (pers. obs.).
There appear to be colleters at the adaxial bases of the petioles and sepals (Boesewinkel & Venturelli 1987). Leaves of small saplings may have short petioles and swollen leaf bases. The leaves on the branches of Callisthene, although opposite, form monolayers by twisting of the stem/petioles (c.f. Coffea, etc.).
The flowers vary in the number and arrangement of their parts and perhaps also in their basic symmetry, however, the literature is somewhat confusing, as is that about floral development; for early work, see e.g. Baillon (1874), Eichler (1878) and in particular Warming (1875). More recently, floral diagrams are to be found in Stafleu's monograph (1952: Fig. 1; 1953: Fig. 1; 1954: Fig. 4; Keay & Stafleu 1952: Fig. 1), Kopka and Weberling (1984), etc.. The single stamen may be opposite the abaxial-lateral C or closer to the adjacent K; in the latter case, it is off the plane of floral symmetry, while the staminodes are generally (?always) opposite members of the corolline whorl (Stafleu; Kawasaki 1998; also Litt & Cheek 2002; Litt & Stevenson 2003b). Note that Litt and Stevenson (2003b: Fig. 2) and Gonçalves et al. (2020a: Fig. 6) show the single stamen as being opposite or somewhat oblique to what one would interpret as being the abaxial C (but see below), although Litt and Stevenson (2003b) had found that the anther of Erisma was initially opposite the petal and on the plane of symmetry of the flower, becoming displaced only late in development; Stafleu (1953) suggests that the two positions of the stamens being discussed reflect the fact that they belong to different staminal whorls... Litt and Stevenson (2003b) and Gonçalves et al. (2020a) illustrated the spur in all taxa as being associated with the adaxial sepal (a simplistic interpretation of their diagrams), while in Kawasaki (1998: Fig. 6), Kopka and Weberling (1984), Stafleu, etc., it comes from or is opposite to an adaxial-lateral sepal; the first authors show the symmetry of the flower in Vochysieae as being strongly oblique in relation to the inflorescence axis, although the floral axis was not indicated, however, bracteoles are generally indicated by Stafleu. Neither Litt and Stevenson (2003b) nor Gonçalves et al. (2020a) indicated the position of the inflorescence axis in other taxa, Kawasaki (1998) did not indicate the positions of either axis, while Stafleu indicated perianth aestivation, the positions of bracteoles and the floral axis, showing (nearly) all flowers as being oblique to the axis in the same way. Litt and Stevenson (2003b) and Gonçalves et al. (2020a) indicated that the fertile stamen in Salvertia and Vochysia was slightly oblique to the petal that was drawn as being abaxial, while Kopka and Weberling (1984) and Kawasaki (1998) showed that stamen as being opposite the petal, furthermore, Kawasaki (1998) showed variation in the positional relationships of the carpels to the rest of the flower while in Litt and Stevenson (2003b) and Gonçalves et al. (2020a) this relationship was shown as being invariant. Litt and Stevenson (2003b) and Gonçalves et al. (2020a) did not indicate overlap of any perianth members while Kawasaki (1998) and Stafleu did. In their description of Mahechadendron Ariza-Cortés et al. (2023) show quincuncial aestivation, an apparently adaxial spurred sepal (but the position of the axis is not shown), an abaxial petal, and the stamen opposite one of the sepals adjacent to the petal - enantiostyly. The odd carpel is abaxial. Finally, the spur in Vochysieae is described as being receptacular in origin by Kopka and Weberling (1984) and calycine in origin in Carmo-Oliveira et al. (2018); the two African taxa hardly have spurs, rather, there is a nectariferous cavity on one side of the inferior ovary. It will be a relief to have a well-supported phylogeny to try and make sense of all this.
The ovary is initiated in an inferior position, the superior ovaries in mature flowers of Vochysieae being secondary (Litt 1999; Litt & Stevenson 2003a). Carmo-Oliveira and de Morretes (2009) found that the stigmas of the Brazilian Vochysiaceae they examined were wet, those of Vochysia and Salvertia having multicellular uniseriate hairs while those of Callisthene and Qualea were papillate.
Carmo-Oliveira et al. (2020) noted that the members of the [Qualea-Ruitzerania-Callisthene] clade that they examined had a multicellular archesporium, often more than one embryo sac, and delayed maturation of the embryo sac and of fertilization - the pollen tubes might hang out for several days on the obturator. Corner (1976) described the ovules of Qualea sp. as being long-exostomal (see also Carmo-Oliveira et al. 2020: e.g. Callisthene). The integuments in Vochysieae vary considerably in thickness, but the outer integument is always thicker than the inner (Carmo-Oliveira et al. 2020); Castro et al. (2024) describea "tegument" (not defined) in Qualea grandiflora that was 8-10 cells across; ca 20 cells seems more likely from the images provided.
For additional information, see Warming (1875: general), Kawasaki (1998: Erisma, 2006: general), for wood anatomy, see Quirk (1980) and Sajo and Rudall (2002, also leaf anatomy), for bark, see Roth (1972), and for ovules and seeds, see Boesewinkel and Venturelli (1987).
Phylogeny. Relationships have been unclear here for some time. Erismeae, containing the tropical American Erisma and the West African Erismadelphus and Korupodendron, were thought to be monophyletic. They have G inferior; 1-2 lateral to apical ovules/ovary; fruit samaroid, with persistent enlarged K; seed single, testa undifferentiated, with vascular bundles. However, Vochysieae are probably not monophyletic (Litt 1999), and a recent plastome analysis by Gonçalves et al. (2020a) showed rather different relationships, with the African Korupodendron and Erismadelphus being sister to the rest of the family, within which Erisma was sister to the remainder - i.e. the old Erismeae are paraphyletic in this telling of the story (note, however, that Gonçalves et al. were cautious in inferring much from their tree, noting that they had looked at chloroplast data alone). Indeed, Baker et al. (2021: see the Seed Plant Tree, also x.2024 - all genera of Vochyiaceae included) found that Erisma was embedded in Vochysieae, and sister to Vochysieae was a clade including Callisthene, Qualea, etc., that lacks a name. However, Maurin et al. (2021) found the relationships [[African taxa] [Erisma [[Vochysia + Salvertia] [Callisthene, etc.]]]], although support for the relationships between the American clades was rather weak. The recognition of Ruizterania (Marcano-Berti 1969) may make Qualea paraphyletic (Gonçalves et al. 2020a). Ariza-Cortés et al. (2022) tried to place their Mahechadendron using variation in the rbcL gene, and they obtained a pentatomy; adding 12 morphological characters cleared things up only a little - i.a. relationships were [[QRC clade] [[Erisma + African taxa] [[Salvertia + Vochysia]]]. They thought that Mahechadendron might be close to Callisthene.
Previous Relationships. Because of their distinctive monosymmetric, spurred flowers Vochysiaceae were often associated with families that have more or less similar flowers but that are now no longer thought to be at all closely related, Euphronia (see Malpighiales-Euphroniaceae) being one frequent associate (e.g. Mabberley 1997; Takhtajan 1997). Takhtajan's (1997) Vochysiales also included families like Malpighiaceae (Malpighiales), Tremandraceae (= Oxalidales-Elaeocarpaceae) and Krameriaceae (Zygophyllales); Cronquist's (1981) Polygalales, in which he included Vochysiaceae, were even more heterogeneous.
MYRTACEAE Jussieu, nom. cons. - Back to Myrtales
Ethereal oils + [usu. terpenes]; wood fibres with distinctly bordered pits; leaves with glands, glands schizogenous; apex of anther connective glandular [terpene-producing]; pollen grains small [11.6 (13.94 S.D.) μm long, 10-18 µm across], oblate, triangular in polar view, parasyncolpate [colpi margins forming triangular apocolpial polar area], polar island ± developed, pseudocolpi 0; x = 12 (?11, ?6), nuclear genome [1 C] (0.063-)0.614(5.989) pg/(234-)488.4(-1785) Mb [?level].
131 [list: to tribes]/5,900) - three main groups below. Worldwide, mostly tropical-warm temperate.
Age. Crown Myrtaceae may date to 87-85 Ma (Biffin et al. 2010a), 95-84 Ma (Sytsma et al. 2004), or (88-)85(-84) Ma (Berger et al. 2015); ages in P. G. Wilson (2011) are largely similar. In a comprehensive analysis also using fossil pollen, Thornhill et al. (2012a: dates for root-only calibrated tree much younger, 2015) suggest ages for crown group Myrtaceae of (97-)90.6, 84.6(-73.7) Ma, Gonçalves et al. (2020a) ages of (91.5-)86.3(-83.5) Ma and X.-F. Zhang et al. (2021) ages of (83.6-)67.5(-52.4) Ma.
For a summary of fossils attributed to Myrtaceae see Biffin et al. (2010a; also Crepet 2008); Thornhill and Macphail (2012) evaluate the fossil pollen record. For interesting Eocene fossils from Australia, see Basinger et al. (2007) and from Colorado, see Manchester et al. (1998). Pigg et al. (1992) described a fossil from the Palaeocene that they thought was close to Myrtaceae-Myrteae-Psidium, and Cretaceous pollen (P. G. Wilson 2011; see also Thornhill & Macphail 2012) and wood (Myrteae) (Vasconcelos et al. 2017) can also be assigned to the family.
Includes Backhousieae, Chamaelaucieae, Cloezieae, Eucalypteae, Heteropyxideae, Heteropyxidoideae, Kanieae, Leptospermeae, Lindsayomyrteae, Lophostemoneae, Melaleuceae, Myrteae, Myrtoideae, Metrosidereae, Osbornieae, Psiloxyleae, Syncarpieae, Syzygieae, Tristanieae, Tristaniopsideae, Xanthmyrteae, Xanthostemoneae.
1. Heteropyxidoideae Reveal (Psiloxyloideae in older literature)
Plant "tanniniferous"; leaves spiral; plant dioecious; A erect in bud, each A with separate trace; staminate flowers: anther sacs each opening separately; pollen with close-fitting polar island in apocolpial area; pistillode +; carpelate flowers: staminodia +; G superior, base narrow; embryo sac bisporic, 8-nucleate [Allium type]; endotesta crystalliferous, cells periclinally elongated; x/n = 12.
2/4. S.E. Africa, Mascarenes.
Age. Around (45-)40(-38) Ma is the suggested crown-group age of this clade in Sytsma et al. (2004) and (64.5-)42.6(-22.7) or (60.6-)39.7(-20.2) Ma in Thornhill et al. (2012 and 2015 respectively).
1A. Heteropyxideae Harvey - Heteropyxis Harvey —— Synonymy: Heteropyxidaceae Engler & Gilg, nom. cons.
Trees; terpenes 0; axial xylem parenchyma 0; epidermal wax crystalloids as small platelets; leaves with domatia, stipules minute; (plant monoecious); staminate flowers: stamens = and opposite C (+ 1-3 opposite K); carpelate flowers: G [(2-)3], stigma capitate; fruit a capsule, style green, persistent; ovules hemitropous; seeds with narrow wings at either end, exotesta with tangentially elongated cells, walls scalariform-reticulately thickened, exotegmic cells elongated.
1/3. South eastern Africa. Map: from MO herbarium records, green.
1B. Psiloxyleae (Croizat) A. J. Scott - Psiloxylon mauritianum (J. D. Hooker) Baillon —— Synonymy: Psiloxylaceae Croizat
Trees; secretory canals in the young stem; vestured pits?, axial xylem parenchyma?, fibres septate, crystalliferous; nodes ?; glands not producing ethereal oils; plant glabrous; stipules colleter-like; (plant polygamodioecious), pedicels articulated; flowers 4-5(-6)-merous; C coriaceous, caducous, punctate, staminate flowers: A 2x C, anthers versatile; carpelate flowers: G [3(4)], style 0, stigma large, lobed; ovules hemicampylotropous; fruit a berry, punctate; exotesta cells large, exotegmen crushed.
1/1. Mascarenes. Map: red, see above.
2. Myrtoideae Sweet
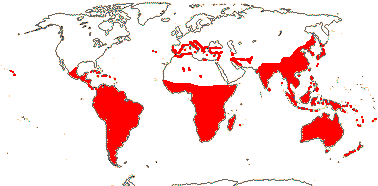
Trees and shrubs; terpenes diverse and abundant, exudates gums [kinos]; (plants Al accumulators); (cork cambium superficial); axial parenchyma +; sieve tubes with non-dispersive protein bodies; (stomata paracytic); hairs unicellular, rather thick-walled; lamina vernation variable, stipules 2/several, colleter-like, or 0; flowers (3-)4-5(-8)-merous; K (with a single trace), C (0-)4-5(-12), often deciduous; A many, conspicuous, locule pairs open by common slit; pollen grains syncolpate, with apocolpial area, polar island 0, exine rugulate, also other surfaces; G partly superior, opposite K or C, if 3, odd member abaxial, base broad, placentae well-developed, minor stylar bundles 0, stigma punctate to capitate (peltate), also dry; micropyle zig-zag [?always], outer integument 2-6(-12) cells across, inner integument 2(-4) cells across, (unitegmic, integument 6-8 cells across, vascularized), parietal tissue 2-12 cells across, nucellar cap 0 (-3 cells across - v. rare), hypostase 0/+, (postament +), (obturator +); (seed pachychalazal), exotesta variously thickened, endotesta thickened or not, crystalliferous, (sclerotic palisade cells at the micropyle), (exotegmen 0); embryo green or white, straight or curved, cotyledons very variable; x = 11, chromosomes 2> μm long; seedlings with ring of hairs/coleorhiza at base of hypocotyl.
129/5,894 [Nic Lughadha et al. 2016]. Tropical (temperate), esp. Australia. Map: from Meusel et al. (1978), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Lucas (2007).
Age. Thornhill et al. (2012a) gave ages for crown-group Myrtoideae of (90.3-)83, 71.3(-64.9) Ma, the lower age, some (78.3-)71.5(-65.4) Ma, was preferred by Thornhill et al. (2015); (86-)80(-70) Ma are the ages suggested by Sytsma et al. (2004) and around 62Ma by Berger et al. (2015).
For wood of Myrceugenelloxylon antarcticus, known fossil from Antarctica in Late Cretaceous deposits, see Poole et al. (2003). Woods of Callistemoxylon, Eucalyptus (E. dharmendrae) and Tristania from Early Palaeogene rocks of the Deccan Traps can all be referred to Myrtoideae (Wheeler et al. 2017).
[Lophostemoneae + Xanthostemoneae]: leaves spiral.
Age. The age of this clade is around (69.7-)56.3(-37) Ma (Thornhill et al. 2012a: combined constraints).
2A. Lophostemoneae P. G. Wilson
A fasciculate; fruit (baccate - Kjellbergiodendron); seeds (1/fruit), flattened or linear; cotyledons folded, vernation various.
4/7. Borneo, E. Malesia, eastern Australia.
Age. Kjell/Lopho diverged (57.4-)39.4(-21.2) Ma (Thornhill et al. 2012a: combined constraints).
2B. Xanthostemoneae P. G. Wilson
(Leaves opposite); A free, anther "point of attachment enclosed by connective"; pollen with close-fitting polar island in apocolpial area; seeds flattened (and winged); cotyledons accumbent.
3/48: Xanthostemon (45). Eastern Malesia, the Philippines, to Australia and New Caledonia.
[Osbornieae + Melaleuceae]: flowers 1 or 3/axil; pollen 14-18 µm across; embryo straight.
Age. The age of this clade has been dated to (68.9-)55.3(-42.9) Ma (Thornhill et al. 2012a: combined constraints).
2C. Osbornieae P. G. Wilson - Osbornia octodonta F. Mueller
Shrub; axillary bud enclosed by swollen petiole base; lamina isobilateral; K and C similar, bases broad, subpetal-like adaxially; G 2, collateral, placentation basal-axile; to ca 8 ovules/carpel; fruit subbaccate, leathery, indehiscent, P persistent; seed 1/fruit; embryo straight, radicle short, cotyledons large, ± orbicular, flat.
1/1. Borneo to Palau, N. Australia.
2D. Melaleuceae Burnett —— Synonymy: Melaleucaceae Vest
Leaves usu. spiral, lamina (venation parallel); inflorescence spike-like or capitate; flowers (calyptrate - fused K); A often fasciculate, (anthers with pores or transverse/oblique slits); pollen (close-fitting polar island in apocolpial area); ovules 1-many/carpel; capsule (dehiscence tardy); seeds linear, (winged); x = (8-10).
2/335: Melaleuca (334). Mostly Australia, esp. southwest, few Malesia.
[Backhousieae [Kanieae + Metrosidereae]: ?
2E. Backhousieae P. G. Wilson
A free; G semi-inferior; ovules 1-2/carpel; fruit thin-walled, indehiscent, seeds 1-2/fruit; embryo curved, cotyledons incumbent.
2/10: Backhousia (8). Eastern Australia.
[Kanieae + Metrosidereae ]: hypanthium with 5 main veins to K; A in single whorl; pollen parasyncolpate; ovules scattered on placentae; seeds linear, cotyledons face-to-face.
2F. Kanieae Engler - Kania Schlechter —— Synonymy: Kaniaceae Nakai
Stomata clumped, clumping irregular; A with connective apically expanded; placentae basal, distant from style base.
1/6. New Guinea, the Philippines
2G. Metrosidereae P. G. Wilson - Metrosideros Gaertner
Vesturing spread over inside of vessel; C usu. red; A free, in single whorl; pollen (with polar apocolpial islands of sorts); G semi-inferior; placentae adjacent to style base, even if basal; n = 11.
1/60. The Philippines, New Guinea to Bonin Is, New Zealand, Hawai'i and Pitcairn Island, also South America (Tepualia s. str.: C. and S. Chile, adjacent Argentina).
Age. Fossils identified as Metrosideros, some perhaps belonging to the monophyletic subgenus Metrosideros and some that would have been called Mearnsia in the past, have been discovered in Oligocene-Miocene deposits ca 30 Ma, perhaps as young as 16 Ma, in Tasmania (Tarran et al. 2016, 2017), while there are also fossils of the latter type in New Zealand (see Pole et al. 2008).
[Tristanieae, Cloezieae, Xanthomyrteae]: cotyledons lying face to face
2H. Tristanieae P. G. Wilson
Flowers yellow to red; A free/fascicles opposite C; pollen 5-10.2µm across, triporate, smooth; G half-inferior, placentae adjacent to style base.
2/4: Thaleropia (3). New Guinea, eastern Australia.
Age. Wood identified as that of Tristania is known from Early Palaeogene rocks of the Deccan Traps (Wheeler et al. 2017) - for ex-Tristania, see Kanieae, Lophostemoneae...
2I. Cloezieae P. G. Wilson - Cloezia Brongniart & Gris
Flowers yellow or white; stamens in one whorl, anthers versatile, (connective expanded apically); pollen parasyncolpate; placentae distant from style base; ovules/carpel few, in circular series.
1/5. New Caledonia.
2J. Xanthomyrteae P. G. Wilson - Xanthomyrtus Diels
Stomata clumped, in bands, water stomata 0; flowers yellow, usu. 4-merous, sessile; stamens 1(–2)-seriate, free; pollen parasyncolporate; placentae adjacent to style base; ovules around placental margins; fruit a berry; seeds many, small; testa crustaceous; cotyledons accumbent.
1/23. Borneo and the Philippines to New Caledonia, not Australia.
2K. Tristaniopsideae P. G. Wilson
Oil glands in pith (0); flowers whitish to yellow; A in several whorls (not), in groups (fascicles) opposite C; pollen (with small irregular polar islands, etc.); placentae distant from style base; ovules often in (semi)circular series.
7/59: Tristaniopsis (50). Myanmar and Thailand to Malesia, East Australia and New Caledonia.
[Syncarpieae [Syzygieae [Lindsayomyrteae + Eucalypteae]]]: ?
2L. Syncarpieae P. G. Wilson - Syncarpia P. G. Wilson
Buds with scales; plant usu. glabrous, (hairs when young); flowers sessile, in groups, connate basally; G inferior, placentae basal; ovules several; seeds linear; embryo straight, cotyledons obvolute, radicle ca 1/2 their length.
1/3. Eastern Australia.
[Syzygieae [Lindsayomyrteae + Eucalypteae]]: ?
2M. Syzygieae P. G. Wilson - Syzygium Gaertner
Plant usu. glabrous; stomata (cyclostaurocytic); (K calyptrate), (C calyptrate); pollen (with close-fitting polar island in apocolpial area)y, (surface smooth); G usu. [2]; fruit baccate, green, purple-black (± dry), (fibre bundles in hypanthium wall), usu 1-seeded; testa apparently 0/leathery; embryo straight, cotyledons thick, ruminate/foliaceous, obvolute; n = 11.
1/1,193. Tropical and subtropical Old World. Photo - Flower.
Age. The age of crown-group Syzygium is estimated to be ca 51.2 Ma (Low et al. 2022), while ages for crown groups of subgenera Sequestratum, Perikion, Acmena, the S. rugosum clade (= subgenus Oborapi), and Syzygium date to 34.2, 24.1, 15.8, 7.0 and 9.4 Ma respectively (Low et al. 2022).
21.7-21.5 Ma leaves from S.E. Australia have been identified as Syzygium (Tarran et al. 2018).
[Lindsayomyrteae + Eucalypteae]]]: ?
2N. Lindsayomyrteae P. G. Wilson - Lindsayomyrtus racemoides (Greves) Craven
Leaves spiral, remarkable purple(-blue) when young; androecium fasciculate; pollen 10-14 µm across, syncolpate [no apocolpial zone], surface granulate-scabrate; G [3]; pericarp thin, fruit dehiscing irregularly; seeds 1-3, 1/carpel; embryo straight, cotyledons massive, unequal, largely flat.
1/1. Moluccas, New Guinea, Australia (N. Queensland).
2O. Eucalypteae P. G. Wilson
Plant ectomycorrhizal and/or arbuscular mycorrhizal [dual mycorrhizal]; cyanogenic glucosides + [Eucalyptus]; hairs on (young plants), (multicellular, branched), plant usu. glabrous, epicuticular waxes as tubules [β-diketones] and/or platelets; leaves (spiral), (isobifacial); calyptra common [= K + C (+ hypanthium)] / [K] [C] / [C] / free, (C keel + corolline portion [= "compound"!]); A fasciculate, (arising from stemonophore)/not, (staminodes numerous, abaxial); G [2-7], style (barely impressed); 3-many ovules/carpel; fruit (1-seeded); cotyledons deflexed, obvolute / large, reniform, peltate, much contorted, juvenile leaves opposite.
Age. The age of this clade (Stock, Euopsis sister) is estimated to be (58.7-)55.3(-52.5) Ma (Thornhill et al. 2012a: combined constraints) or ca 59.2 Ma (Thornhill et al. 2019).
There are fossils of Eucalyptus (E. dharmendrae) from Early Palaeogene rocks of the Deccan Traps (Wheeler et al. 2017).
4/1,000: Eucalyptus (843). Australia, few Malesia, 1 sp. New Caledonia.
[Leptospermeae + Chamaelaucieae]: pollen syncolpate.
Age. This clade is ca 50 Ma (Thornhill et al. 2012a) or (88.8-)63.5(-47.8) Ma (Nge et al. 2025a).
2P. Leptospermeae de Candolle —— Synonymy: Leptospermaceae Berchtold & J. Presl
Leaves usu. spiral; flowers (in heads), sessile or not; A (10-), shorter than C or not, (basally connate), (fasciculate); G [(-12)]; ovules 1-many/carpel; fruit indehiscent or not; seeds winged, linear [Leptospermum]; testa thin; cotyledons broad, flat, longer than hypocotyl.
13/178: Kunzea (62), Leptospermum (44), Gaudium (22). Australia, esp. S.W. (genera), a few species in Malesia, New Zealand.
Age. This clade (Kunzea sister) is around (42.5-)33.3(-25.2) Ma (Thornhill et al. 2012a).
2Q. Chamaelaucieae de Candolle —— Synonymy: Baeckeaaceae Berchtold & J. Presl, Chamelauciaceae Rudophi
Shrubs (small trees); usu. glabrous; leaves (spiral); flowers solitary; K (long-aristate/margins variously lobed or fimbriate), (= C), C (margins fimbriate); A (4)/5 [opposite K or C]-15/(-many), (fasciculate, opposite K), (not inflexed in bud), anthers dorsifixed (basifixed), versatile, usu. with connective gland, (staminodes 10); (secondary pollen presentation + [stylar hairs], oil from anther gland mixes with pollen helping adhesion to stylar hairs); pollen (porate, brevicolpate, dicolpate, etc.), exine other than rugulate, often smooth; ovules 1-many/carpel; fruit (indehiscent, nut-like), seeds reniform/angular, with elaiosome/aril, testa (thin); embryo curved, hypocotyl massive, cotyledons small, deflexed; n = (5-)11.
37/700 (+ ca 100 to be described), 12 subtribes: Verticordia (100), Calytrix (75), Thryptomene (56), Darwinia (45), Scholtzia (40). Australia, a few species Malesia to China, New Caledonia.
Age. Crown-group Chamaelaucieae (Ochrosperma sister) are (51.9-)46.8(-42.2) Ma (Thornhill et al. 2012a); Nge et al. (2025a) estimated the crown-group age to be (61.1-)42.4(-31.2) Ma.
2R. Myrteae de Candolle —— Synonymy: Eugeniaceae Berchtold & J. Presl, Myrrhiniaceae Arnott
(Small) shrubs (with lignotubers) to trees; leaves (amphistomatous); lamina (venation triplinerved); (hypanthium +); K (connate [= calyptra], circumscissile), C (fall as unit); A (4), ± straight (incurved), (polysporangiate); pollen brevicolpate, 10-14 µm (etc., to 50 μm - Octamyrtus) across, exine granulate (-verrucate); G inferior, [(2-18)], transseptal vascular bundles +; fruit baccate, (placenta also fleshy - Psidium); ovules 1-many/carpel; seeds 1-many/fruit, shiny (dull), operculate; testa multiplicative, ± sclerotic [Psidium [outer cells pulpy], Myrtus] or thin, crustaceous [Eugenia]; embryo various, curved or not, (radicle very short), cotyledons fleshy, (fused), (variously folded)/foliaceous/small; (seedlings lacking ring of hairs/coleorhiza at base of hypocotyl - Psidium).
47/2,500-2,930: Eugenia (1,284), Myrcia (744), Calyptranthes (130), Psidium (100), Campomanesia (80), Myrcianthes (50), Myrceugenia (40), Plinia (40). Tropical (temperate), esp. South America. Photo - Bark, Flower, Fruit.
Age. Crown-group Myrteae have been dated to (53.6-)50.9(-50) Ma (Thornhill et al. 2012a: combined constraints, Myr comm sister) and ca 90 Ma (Murillo-A et al. 2016), but as young as the Miocene ca 18 Ma (Berger et al. 2016; see also Vasconcelos et al. 2017).
Evolution: Divergence & Distribution. For ages of various clades in the family, see Biffin et al. (2010a) and Thornhill et al. (2012a, 2015, 2019: Eucalypteae), also Vasconcelos et al. (2017: Myrteae). The pollen record suggests the existence of at least some tribes in the late Cretaceous (P. G. Wilson 2011), but this is inconsistent with many molecular divergence dates (see also Vasconcelos 2017 for an evaluation of much fossil literature). Some dates for the same node diverge widely. Thus crown-group Myrteae are dated to the Cretaceous ca 90 Ma by Murillo-A et al. (2016) and the Miocene ca 18 Ma by Berger et al. (2016; Vasconcelos et al. 2017 for literature and dates); Thornhill et al. (2012a) also emphasized similar differences.
Thornhill et al. (2015) thought that although Myrtaceae might have been Gondwanan in origin, none of the 22 disjunctions they examined could unambiguously be linked to geological vicariance events; Myrtoideaea may have originated in Australia. Myrteae are the largest tribe in the family and include species with fleshy fruits; the tribe may have originated in the New Zealand-New Caledonia-Australia area, either in the early Palaeocene ca 65.5 Ma (macrofossil evidence) or substantially later in the mid-Eocene ca 40.8 Ma (palynological evidence), and then achieved their broader largely austral distribution via Gondwana (Vasconcelos et al. 2017). De la Estrella et al. (2019b) also suggested that Myrtoideae, and Myrteae in particular (see Vasconcelos et al. 2017 for a phylogeny), originated in the Australia-New Zealand area; Myrtaceae make up the third commonest angiosperm family among the fossils found in Antarctica, which may have been important in enabling the distribution of the tribe. However, not only are dates uncertain, but the status of New Zealand and New Caledonia during this period is unclear: Were they always emergent, or submerged for part of the time, and/or were there metapopulations of Myrteae on ephemeral islands (Vasconcelos et al. 2017; see also Condamine et al. 2016, etc.)? Mazine et al. (2018) suggest that Myrtus was originally associated with dry non-tropical climates in South America, as were South American Myrteae in general; the Brazilian Atlantic Forest was important in their susbsequent diversification. The Neotropical Myrteae Working Group (2023/2024) suggested that LDD had been involved in the pantropical distribution of Myrtus section Jossinia, while Antarctica might have been used by other groups. Mediterranean Myrtus itself is part of a clade that is otherwise from Central and South America. For diversification rate shifts within Myrteae, see Vasconcelos et al. (2017). Eugenia is the most species-rich tree genus in the Altantic Forest of Brazil (Mazine et al. 2014). The ca 200 Old World species of Eugenia in sect. Jossinia appear to represent a single movement of the genus (c.f. van der Merwe et al. 2005; Bernardini et al. 2014), perhaps originally from Atlantic and dry forests in South America, to the Old World, possibly via New Caledonia, where E. brongniartiana, sister to the rest of the section, is to be found; the genus is ca 40.9 or ca 30.3 Ma (Mazine et al. 2018). However, only 14 species of Jossinia were examined, and relationships in the trees presented varied substantially (Mazine et al. 2018), so more news from this area can be expected.
The speciose Myrcia s.l. with 700-800 species that are often montane plants (also New World Myrteae), may have begun diversifying in the Brazilian Atlantic forest ca 28 (36-21) Ma; it is notably diverse in eastern Brazil, the Caribbean, and the Guyana Highlands (Santos et al. 2017). Vasconcelos et al. (2018) found that there was remarkably little floral variation in Myrcia s.l. and the little variation that the group did show was not particularly correlated with phylogeny. There is some variation in fruit anatomy (Galan et al. 2016), but there is, however, considerable variation in chemistry (Stefanello et al. 2011), vegetative feaures and plant habit, and variation in such features in particular help separate the species of the genus (Vasconcelos et al. 2018). Interestingly, in Myrcia s.l., ontogeny and phylogeny are inversely correlated, that is, characters that appear later in ontogeny are more indicative of deeper relationships (Vasconcelos et al. 2016). Amorim et al. (2019) discuss the evolution of some characters here, and suggest that the Atlantic Forest was the ancestral area of the genus.
Fossils identified as Metrosideros, some perhaps belonging to the monophyletic subgenus Metrosideros and some that would have been called Mearnsia in the past, are known from Oligocene-Miocene deposits ca 30 Ma, perhaps as young as 16 Ma, in Tasmania (Tarran et al. 2016, 2017). However, Metrosideros now grows in eastern Malesia, the Bonin Islands, throughout Melanesia and Polynesia, New Caledonia (>20 species), Hawai'i, and New Zealand, but it is unknown from Australia; most of this wide distribution is attributable to the spread of Metrosideros subgenus Metrosideros. This may have radiated from New Zealand, its small seeds being dispersed by wind (Wright et al. 2000b, 2001; see also Pillon et al. 2015), and its arrival in Hawai'i is estimated to be a mere (6-)3.9 Ma, probably on Kaua'i (Percy et al. 2008) or ca 3.1 Ma (Dupuis et al. 2020). However, understanding the biogeography of the genus is difficult, partly because relationships within it are unclear, and partly because it also includes the South African M. angustifolia and the Chilean M. stipularis, perhaps sister species (Pillon et al. 2015). However, Dupuis et al. (2019) found that M. laurifolia, from New Caledonia, was sister to the rest of the genus, although there was litle resolution of relationships between the Hawai'ian accessions. Metrosideros collina, the only species known from the Marquesas Islands, may have given rise to the Hawai'ian clade with its some 25 "taxa" that show a considerable amount of variation in habit in particular - or perhaps 4 species, M. polymorpha being most notable. Indeed, Choi et al. (2021) emphasized the rapid evolution of these Hawai'ian taxa, both hybridization as well as diverse recombinations of a rich pool of ancestral variation being involved. This seems not to fit with the involvement of M. collina in the story; there substantial long distance dispersal had been integral to its arrival in the Marquesas, but perhaps the adaptive radiation on Hawai'i occurred as Metrosideros took advantage of the greater ecological opportunity there (Choi et al. 2021).
Overall, a shift in speciation rates in Myrtaceae has been provisionally placed around the K/P boundary in stem Myrtoideae (Berger et al. 2015), although its exact position and cause(s) remained somewhat unclear. The shift to fleshy fruits in Syzygieae and Myrteae seems to have been accompanied by increased diversification rates (Biffin et al. 2010a). The Malesia-centred Syzygium is notably diverse on New Caledonia, with ca 70 species there (see Biffin et al. 2006 for a phylogeny). The genus may have originated in the Australia—New Guinea area, with subsequent migrations to the rest of the Old World (perhaps twelve from the Sahel to the Sunda shelf alone and beginning ca 17.1 Ma, some leading to rapid and substantial diversification) and the Pacific (Low et al. 2022).
There are fossils named Eucalyptus dharmendrae from Early Palaeogene rocks of the Deccan Traps (Wheeler et al. 2017). However, note that Hermsen et al. (2012, see also Macphail and Thornhill 2016 and other papers in Australian J. Bot. 64(7-8). 2016) in their evaluation of the fossil evidence of Eucalyptus, thought that crown-group Eucalypteae were early Palaeocene or Eocene in age, while Thornhill et al. (2015) dated crown-group Eucalyptus to the Late Eocene (35-)34.4(-33.9) Ma. Importantly, there are fossils of Eucalyptus, possibly of the relatively widespread subgenus Symphyomyrtus, in early Eocene deposits of Argentinian Patagonia ca 51.9 Ma old (Wilf et al. 2010; esp. Gandolfo et al. 2011; Hermsen et al. 2012), and there are also fossils of Eucalyptus from Early Miocene rocks in New Zealand (Rozefelds 1996; Pole 2003). Eucalypteae are no longer found in either South America or New Zealand, but see elsewhere for discussion about such south South American/Australian-Malesian distributions.
Chamaelaucieae (excluding subtribe Ochrospermatinae, sister to the rest of the tribe) probably originated in south-west Western Australia, most biogeographic events being dispersal (ranges expansions rather than founder events) or within-area speciation, and only around 10% vicariance events (Nge et al. 2025a). Overall, temperature-dependent diversification models fitted the data best, and the extensive diversification of the tribe in Southwest Australia is probably connected to the climatic stability there - as with a number of other groups (Nge et al. 2025a, but see caveats). The increasing aridity of the Oligocene-Miocene may have led to the rapid divergence of major clades within the family (P. G. Wilson 2011); decrease in diversification rates had been especially rapid before the Eocene-Oligocene boundary (Nge et al. 2025a, c.f. in part 2020b). Indeed, species distributions/relationships in Calytrix (Chamaelaucieae) within Australia seem to fit the peripheral vicariance pattern, the genus becoming restricted to the periphery of the continent after the drying out of the centre, a process that began in the Eocene. Vicariant speciation subsequently occured in the areas in which the genus still grew (Nge et al. 2021a: Fig. 1 for other examples, 2024).
Heteropyxidoideae join other examples in the family of species-poor geographically restricted taxa that are sister to much more diverse clades (Lucas et al. 2007; Thornhill et al. 2015; Vasconcelos et al. 2017). Sytsma et al. (2004) suggested that Psiloxylon may have been hopping around on islands in the Indian Ocean for almost 40 My (see also Berger et al. 2015: ca 10 My; Thornhill et al. 2015). Heteropyxideae are perhaps most similar to Myrtoideae-Leptospermeae; both wood anatomy (e.g. bordered pits) and pollen are like those of Myrtoideae (Stern & Brizicky 1958); Heteropyxis apparently lacks terpenes (Mohammed et al. 2009). The stamens have separate traces and the androecium shows no signs of being fasciated.
Perianth parts (calyx and/or corolla) show striking variation. Thus in a number of Eucalyptus and some other taxa they may be undifferentiated and/or variously fused into a calyptra or operculum (e.g. Pryor & Knox 1971; Drinnan & Ladiges 1989a, b; Bohte & Drinnan 2005; Proença et al. 2022); circumscissile abscission of the hypanthium/calyptra occurs in various ways. The development of the corolla in Angophora in particular is complex. There is an abaxial more or less apiculate keel supplied by a largely unbranched vascular bundle and an adaxial corolline portion which can be more or less connate (e.g. Drinnan & Ladiges 1988); the corolla is here described as being "compound" (e.g. Crisp et al. 2024), which seems to be close to a misnomer... Crisp et al. (2024) think about the phylogeny of Eucalyptus s.l. and the variation of such characters. There may also be a calyptrate calyx in Eugenia, and the corolla has also been described as being calyptrate, although here free petals surround the rest of the flower and fall off as a unit (). Vasconcelos et al. (2019a) summarized the extensive floral variation in Myrteae (some species are dimerous, there may be a hypanthium, the calyx may be calyptrate, the filaments straight or incurved, and so on) in the context of the phylogeny of the tribe. Syzygieae also have a calyptra, either made up of a connate calyx or a morphologically quite distinct structure, the petals may all fall off together although they are not connate at all... (Vasconcelos et al. 2019b). Thornhill and Crisp (2012) describe pollen evolution in the family; some features they discuss can be optimized on the tree as it becomes better resolved.
Ecology & Physiology. Most of the family, ca 4,600 species, are trees (= single stem >2 m tall, or if ≥2 stems, one erect stem ≥5 cm d.b.h.), the third highest number after Fabaceae and Rubiaceae, but proportionally a higher number than in those families (Beech et al. 2017). Although Myrtaceae have notably many species with stems at least 10 cm across in the Amazonian tree flora, individual species seem never to be locally common (ter Steege et al. 2013), overall, the family is the sixth most abundant in terms of species numbers in Amazonia (Cardoso et al. 2017).
Closely related species of Eugenia from Barro Colorado island, along with Inga, Piper, Burseraceae, Psychotria, etc., growing as swarms of ecologically similar species in LTRF, also drier forests, show considerable differences in their foliar secondary chemistry, the differences being implicated in defence against herbivores (Sedio et al. 2017). Along the same lines, Vasconcelos et al. (2018) found little floral variation in Myrcia s.l., but again, there was variation in chemistry , etc..
There are around 1,600 Australian species of Myrtaceae, including ca 800 species of Eucalyptus and friends (Crisp et al. 2011a), Eucalyptus s.l. in particular dominating over 90% of the fire-dependent savannas, woodlands and forests there (Lawler & Foley 2002). Although Thornhill et al. (2019) thought that Eucalyptus began its rise to dominance only ca 20 Ma, Crisp et al. (2011) suggested that resprouting and the development of the distinctive fire-controlled eucalypt-dominated vegetation could be dated to 62-60 Ma. Ladiges et al. (2011, see also 2012) suggested that within two clades of Eucalyptus s.l., Late Cretaceous-Palaeocene in age, there had been independent adaptation to arid/semi-arid conditions. Species of Eucalyptus, with their epicormic buds, volatile oils, open canopies, bark shedding in large amounts, etc., can be thought of as fire-promoting plants (Keith 2012; Grootemaat et al. 2017: comparison of leaf and bark decomposability, flammability, etc.), but they themselves survive these fires. Indeed, although initial bark growth was rapid, overall it was slower than isometry, and eucalypts have relatively thin bark compared to non-eucalypts (Lawes & Neumann 2022). This is because about 90% of Eucalyptus s.l. species have epicormic strands in their bark, meristematic strands up to several centimetres long, and although they do not often have organised buds, resprouting occurs from the strands even after quite severe fires; resprouting is also associated with the development of additional axillary buds in the young stems (Burrows 2013, and references; Eucalyptus may also have lignotubers. Resprouting after fire happens in many species of Eucalyptus s.l. (but not in e.g. E. regnans) as well as in some other Australian Myrtaceae - importantly, it occurs in the canopy (Burrows 2002; Burrows et al. 2010). In fact different species of Eucalyptus develop bark of varying thickness depending on the particulars of the fire regime; thin bark may mean that the species devotes resources to growing tall more quickly (Lawes & Neumann 2022). Crisp et al. (2011a: 95% HPD) link the evolution of these epicormic strands/buds with biome evolution in Australia some (62-)62, 60(-58) Ma, vegetation dominated by the highly flammable Eucalyptus originated then (dates for crown-group Eucalypteae in Thornhill et al. 2012 are a little younger - (66.2-)60, 55.3(-50.9) Ma). For fire in Australia, see Bradstock et al. (2012), Bowman et al. (2012), Carpenter et al. (2015, 2016, also Hill & Jordan 2016; c.f. in part Bond & Scott 2010); for epicormic buds in Myrtaceae, see Clarke et al. (2012, esp. Fig. 5). Biomass accumulation in eucalypt woodlands can be great. Average figures of 1,867 tonnes C ha-1 living and dead biomass have been recorded for Eucalyptus regnans-dominated forests (Keith et al. 2009), but the highest values are ca 1,000 tons more - that is why they make such a good bonfire. Eucalyptus-dominated savanna responds differently to fire than do other savannas, in part because in the latter woody species with epicormic buds are vanishingly uncommon (Moncrieff et al. 2016), and Australian Eucalyptus-dominated savanna is also distinctive becase eucalypts tend to be tall and with narrow crowns compared to trees in savannas elsewhere (Moncrieff et al. 2014); height may affect the prevalence of crown fires. Eucalyptus is now to be found in just about every biome on the continent (see also M. A. Renner 2019; Hühn et al. 2024). Note that crown-group diversification of the Australian Banksia (Proteaceae), many of which are also fire-associated (see also below), is also quite early (e.g. He et al. 2011; Lamont et al. 2018b); Proteaceae are late Cretaceous in age. For more on fire in Eucalpus and Myrtaceae, see Keeley et al. (2012) and Lamont et al. (2018b), also Clarke et al. (2012: esp. Fig. 5) for epicormic branching in the family.
As mentioned above, eucalypt woodland was once geographically more widespread, occurring along with palms in New Zealand at the end of the Early Miocene (Pole 2003) and in Patagonian South America in the early Eocene ca 52 Ma (Hermsen et al. 2012), and its subsequent disappearance in those places may be because climates became wetter (Crisp et al. 2011a). Interestingly, González-Orozco et al. (2016) found that areas of Australia currently harbouring palaeo-endemic species of the Eucalyptus group were particularly likely to shift or even disappear as the climate warms. Eucalypts growing in arid environments have thick leaf blades with apparently overly-closely spaced veins ca 24 mm mm-2, i.e., the distance between veins is less that the distance between veins and stomata (de Boer et al. 2016; c.f. Zwieniecki & Boyce 2014). This may enable such leaves to achieve maximum photosynthetic rates very soon after rain falls; these leaves are also amphistomatous (de Boer et al. 2016). For the possible ecological significance of juvenile/adult differences in leaf morphology (= heteroblasty) in eucalypts, see Vlasveld et al. (2018). There are physiological similarities in the comparison between AM Juniperus/ECM Pinus edulis, which live together in North America, and between Eucalyptus, often ECM, and AM Callitris species which may live together in Australia (Larter et al. 2017).
Dry-fruited Myrtoideae, including Melaleuca and Eucalyptus, also species of the fleshy-fruited Syzygium, are often ectomycorrhizal (e.g. Chilvers & Pryor 1965; Smith & Read 1997; Adams et al. 2006; Tedersoo et al. 2008; Bâ et al. 2011a; Laliberté et al. 2015; see also Moyersoen et al. 2001; Brundrett 2017a and Plant-Bacterial/Fungal Associations below). These plants are especially abundant in Australia, but although Brundrett (2009) estimated that 1,800 species may be involved, little is known about the eco-physiological dimensions of these associations and the role that they might play in the ecological dominance of Myrtaceae in parts of Australia.
Nine species of Eucalyptus, not immediately related to one another, along with Pinales and a few Dipterocarpaceae, include the majority of giant trees (trees ≥70 m tall) known (Tng et al. 2012). The giant eucalypts may be an odd sort of rain forest pioneer. They usually depend on fire for their regeneration, and several of them are seeders rather than resprouters; rain-forest trees later move in under the eucalypt canopy, which is not very dense (Tng et al. 2012). Eucalyptus regnans is one of these giant trees and it has a very narrow canopy, much light penetrates, and there is a well-developed understory.
Osbornia octodonta is a mangrove plant from Borneo, the Philippines and northern Australia. For more on managroves and their evolution, see elsewhere.
Pollination Biology & Seed Dispersal. In many Myrtaceae the numerous stamens with their white or brightly coloured filaments are the main visual attractant for the pollinator, and in taxa such as Callistemon (= Melaleuca), the bottle-brush, the flowers of the one inflorescences all open simultaneously. Many Myrtaceae have such brush-type inflorescences, not that dissimilar functionally from the large polystaminate flowers of Eucalyptus s.l.. Calothamnus (also = Melaleuca) has similarly aggregated flowers in which the stamen fascicles form flattened structures, the individual flowers being more or less monosymmetric (Westerkamp & Claßen-Bockhoff 2007), Verticordia has elaborately-fringed sepals - and sometimes also petals and staminodes, and some species of Darwinia have coloured inflorescence bracts. Most Myrtaceae have distinctive oil glands in their anthers, and the composition of the oil often differs from one species to another – and also from that of glands in the vegetative parts of the plant (Ladd 2024). These glands (see also Beardsell et al. 1993; Landrum & Bonilla 1996) produce oils which may attract pollinators (?aroma, ?food). The oils may also help in the attachment of pollen to stylar hairs, as in the secondary pollen presentation devices of Verticordia and a number of other Australian taxa of the Chamaelaucium alliance (deposition devices - see El Ottra et al. 2023 for secondary pollen presentation - they note that five genera are involved), or in the protection of the flowers (Slater & Beardsall 1991; Howell et al. 1993; Ladd et al. 1999; esp. Ladd 2024). Interestingly, in species in which anthers/stamens are the main floral advertisement, no oil is released from the glands (Ladd 2024).
Some 320 or more species of Myrtaceae in Australia may be pollinated by birds, especially by lorikeets (ca 1/2 of Eucalyptus s.l., perhaps 40 species of Melaleuca, some Darwinia, etc.: Ford et al. 1979; Beardsell et al. 1993), and both pollen and nectar may be rewards (Stiles 1981 and references). There seem to be simultaneous radiations of Myrtaceae (inc. Eucalyptus), parrots and euryglossine bees, although many details of the interacations involved are unclear (Slattery et al. 2023). Comparison of New World (= many of the old Myrtoideae) and Australian taxa (= many of the old Leptospermoideae) is interesting, since in the former the flowers often last for only a single day, nectar is not often produced, the stigmas are dry, bird pollination is uncommon, etc., while in the latter the flowers can be quite long-lived, nectar is often produced, the stigmas are wet, and bird pollination is quite common (c.f. Nic Lughadha & Proença 1996 and Beardsell et al. 1993). Metrosideros polymorpha from Hawai'i is a very important nectar source for birds there, however, it is estimated that the genus has been on the islands for a rather short time, perhaps (6-)3.9 Ma (Percy et al. 2008) or ca 3.1 Ma (Dupuis et al. 2020), and the birds have been around for longer than that. In the South American Myrrhinium, close to (or perhaps even in) Psidium, pollination is by frugivorous birds that visit the flowers to eat their fleshy petals (Roitman et al. 1997; Nadra et al. 2018). For summaries of pollination in Western Australian Myrtaceae, see Keighery (1982); honeyeaters, etc., may obtain carbohydrates from sources along the stems of plants like Eucalyptus. Indeed, an often overlooked set of plant-animal interactions occurs between nectarivorous birds like honeyeaters, etc., and eucalypts (and other taxa). The birds may acquire substantial amounts of carbohydrates from the plant, either as manna, dried exudates from wounds (rate of removal by New Holland honeyeaters, e.g. 9.1 blobs/minute), as honeydew, produced by young aphids, etc., and/or as lerp, a protective covering produced by some psyllids (Paton 1980). Such carbohydrate complements that acquired from other sources, including flowers; importantly, birds apparently looking for insects to eat as they probe Eucalyptus bark may well be looking for these nectar sources (Paton 1980). For bird pollination in Chamaelaucieae, e.g., bell inflorescences in Darwinia, see Nge et al. (2025a)
Bee pollination is common, especially in South American Myrteae, where meliponines in particular collect pollen from Myrcia, and they and other bees are the major pollinators of the unspecialized but mass flowering Myrteae that are common there (Gressler et al. 2006; Vasconcelos et al. 2018), although bees of a variety of sizes also pollinate Psidium in which flowering is mostly steady state (Proença et al. 2022). Interestingly, night-flying bees are reported to be the pollinators of Campomanesia and some species of Eugenia in South America (Cordeiro et al. 2019). Bee pollination also occurs in a number of Australian Myrtaceae, including Syzygium, and there Colletidae-Euryglossinae are notable pollinators (Beardsell et al. 1993; Nic Lughadha & Proença 1996; Biffin et al. 2010a). Indeed, flowers of the southern/austral plant families Myrtaceae, Fabaceae and Proteaceae are often pollinated by members of groups of colletid bees, also long-term denizens of Australia, such as Euryglossinae (esp. Myrtaceae), Australasian endemics, Hylaeinae, and Neopasiphaeinae (Slattery et al. 2023).
The berry-like fruits common in Myrtaceae, especially in Myrteae and Syzygieae (unrelated), are commonly dispersed by bats (Muscarella & Fleming 2008), monkeys and especially birds (Gressler et al. 2006; Proença et al. 2022). The fruits are an important food resource for small animals in general in the New World in particular, and Myrtaceae in the Brazilian Atlantic Forest vary considerably both in basic fruit morphology and in fruiting times (Galan et al. 2016; Staggemeir et al. 2017, 2015b and references: phenological patterns in Myrteae). Wind dispersal is also common, if only over short distances, thus Booth (2017) reviewed the extensive literature bearing on seed dispersal in the capsular-fruited Eucalyptus and found that dispersal probably averaged only 1-2 m/year. See also Beardsell et al. (1993) and Nic Lughadha and Proença (1996) for seed dispersal.
Plant-Animal Interactions. Myrtoideae are noted for the amount and diversity of the terpenes that they produce (Keszei et al. 2010), and these compounds were thought to be involved in defence against herbivores (Lawler & Foley 2002). Thus the monoterpenoid 1-8-cineole, antimicrobial and an insecticide, is apparently a major component of the essential oil of Eucalyptus (Hendry et al. 2009; Pichersky & Raguso 2018). However, at least some of the terpenes may be involved in signalling, a variety of formylated phloroglucinol compounds, for the most part biosynthetically unrelated to terpenes, being involved in herbivore deterrence (B. D. Moore et al. 2004).
About half the galls on Australian plants have been recorded from Myrtaceae (Mani 1964). Thus gall-inducing Eriococcidae (scale insects, Hemiptera) are widely distributed on Eucalyptus and other members of the family (Gullan et al. 2005), and Crisp et al. (2024) even suggest that infestation by Cystococcus is an apomorphy for [Eucalyptus subgenus] Corymbia. Another set of galls are found on species of Eucalyptus s.l. as well as other unrelated largely Australian Myrtaceae such as Syzygium, Melaleuca and the like - a few Myrtaceae from India to New Zealand are also involved. These genera are part of a three-way mutualistic association that involves the plant, the actual gall-former, the nematode Fergusobia (see Ye et al. 2007; Davies et al. 2010 for phylogenies, etc.), and the dipteran Fergusonina. The nematode parasitises Fergusonina for part of its life cycle and is responsible for gall formation while the fly larvae determine the internal structure of the gall; nematodes without fly larvae and vice versa do not survive, indeed, the female fly carries the nematode to new sites (Taylor et al. 2005; Nelson et al. 2014; Scheffer et al. 2017). Although the relationship is often 1:1:1, deep cospeciation appears not to be involved, the stem age of Fergusoninidae being around 42 Ma, much younger than the ages of its hosts, however, lower-level cospeciation is possible (Nelson et al. 2014); around 3/4 of the flies are restricted to a single host plant species, and all to a single host genus (Scheffer et al. 2017). Little more than the outline of this fascinating tritrophic association is currently understood, and there may be several hundred different fly-nematode associations (Nelson et al. 2014) - around 170 species of flies is a number derived from figures in Scheffer et al. (2017: p. 151), with one plant species hosting up to eight Fergusonina species. Hansen et al. (2024) noted that some 36 species of the endemic hemipteran psyllid Pariaconus were to be found on the Hawai'ian Metrosideros polymorpha but on no other species, some of the Pariaconus feeding on the leaf surface, others in open galls and yet others in closed galls, and showing interesting changes in their associated microbiomes and the various essential amino acids that the various symbiotic bacteria there produced. (For other insects that eat Metrosideros on Hawai'i, which has quite recently moved there, perhaps only ca 3.1 Ma (Dupuis et al. 2020), see Gruner 2004.)
Larvae of 90 or more species of a family of sawflies, Pergidae, eat Eucalyptus, far more species than are found on non-myrtaceous/rainforest plants (Schmidt & Walter 2014). Rainforests, at least, are where species-poor clades of the family are to be found, and radiation of two clades on eucalypts is dated to to the Palaeocene, roughly the time that eucalypts themselves were diversifying (Schmidt & Walter 2014; see also above). Tortricine moths (Epitymbiini) larvae feed on Myrtaceae leaf litter in Australia, and some other moth groups are also foliovores on this family (Powell et al. 1999). Some 150+ species of the moth Pectinivalva, leaf miners belonging to the monotrysian Nepticulidae, and with a few other genera making up an Australian clade, are known only from Myrtaceae (Doorenweerd et al. 2016).
As discussed elsewhere, oscine birds, a major group of Passeriformes, initially radiated in Australasia, and they can taste sweetness and are involved in the pollination of Myrtaceae; there is perhaps a connection between early oscine diversification and the evolution of Myrtaceae and Eucalyptus (see also Pollination and Seed Dispersal above).
In Eugenia closely related species may vary in details of their secondary metabolites, which in turn determines what insects, etc., eat the plants, speciation in the genus, etc.. For similar tropical systems, see also Inga, Ocotea, Passiflora, Piper, Protium and Bursera, Psychotria, and sundry Solanaceae - the discussion about Inga is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
For the gut biota of koala bears, which eat nothing but Eucalyptus leaves, and its possible role in dealing with the secondary metabolites of that plant, see Shiffman et al. (2017: zoo animals, bacterial family S24-7 abundant in all koalas, c.f. p. 20). Johnson et al. (2018) discuss the possible genetic basis for the koala's ability to tolerate levels of toxins in eucalypt leaves that would be lethal to most other mammals.
Plant-Bacterial/Fungal Associations. Dry-fruited Myrtoideae from Australia, including Melaleuca and Eucalyptus, are often ectomycorrhizal (ECM) or dual-mycorrhizal - ECM-arbuscular mycorrhizal (AM) plants (e.g. Chilvers & Pryor 1965; Smith & Read 1997; Adjoud-Sadadou & Halli-Hargas 2000; Tedersoo et al. 2008; Bâ et al. 2011a; Kariman et al. 2012; Teste & Laliberté 2018; Teste et al. 2019: Table S2); see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, ages, etc.); Myrtaceae are the main ECM group in Oceania (Corrales et al. 2018). The beneficial effects of ECM fungi are strong in Myrtaceae, and the boletes Pisolithus and Scleroderma are particularly evident (Hoeksema et al. 2019: meta-analysis). Adams et al. (2006) noted that younger plants of Eucalyptus were more likely to be AM, the mature plant becoming ECM - in some cases, at least, the C costs to a seedling of a P-acquiring AM symbiosis may be lower than in an early development of an ECM asoociation, although not if conditions are cold or soil nutrients low (Teste et al. 2019). Similar ECM fungi are also to be found on Nothofagaceae, another southern group, perhaps because ECM fungi moved from Nothofagaceae on to Myrtaceae as the climate became drier in the Tertiary (Tedersoo et al. 2008, 2014a). Basal clades of the ECM false truffle group Hysterangiales are found predominantly on Australian Myrtaceae (Mesopelliaceae grow on Eucalyptus) (Hosaka et al. 2008); for more on ECM and other fungal associations, see elsewhere. Vellinga et al. (2009) discuss introductions of ECM fungi in general, over a quarter of which have been on to Myrtaceae/Eucalyptus. Bacterial mycorrhization helpers are known for both ecto- and endomycorrhizal Eucalyptus (Duponnois & Plenchette 2003).
Genes & Genomes. A genome duplication, the SYMIα event ca 44.7 Ma, has been reported (Landis et al. 2018: Syzy. Euc.), but this may be the Pan-Myrtalean WGD (see Low et al. 2022). Almeda and Penneys (2022) suggest that x = 11 is the base chromosome number of this group. For cytology, see Rye (1979) and Costa and Forni-Martins (2007 and references).
Economic Importance. For Eucalyptus s.l., now very widely grown for its timber and essential oils, see Coppen (2002). The fruits of Psidium guajava, the guava, are widely eaten (Proença et al. 2022 and references). Domestication here occured in the Brazilian Amazonian region at least 6505 years b.p., and from there it moved to elsewhere in South and Central America, movement to the Andes perhaps being especially early (Arévalo-Marín e al. 2024).
Chemistry, Morphology, etc.. For polyhydroxyalkaloid distribution (pyrrolizidine, pyrrolidine, and piperidine alkaloids), see Porter et al. (2000); they occur both in Psiloxylon and in some Myrtoideae. A number of Myrtoideae produce gums s.l. (these have been called kinos - Lambert et al. 2007b, 2013) and especially terpenes (Keszei et al. 2010), while some have high silica content in their leaves (Westbrook et al. 2009). The Eucalyptus group is rich in tannins, especially ellagic acid, and as elsewhere there is an inverse correlation between the production of hydroyzable tannins/ellagitannins and condensed tannins/proanthocyanidins; tannin chemistry and phylogeny are correlated (Marsh et al. 2017: comprehensive survey); cyanogenesis occurs in Eucalyptus (Hansen et al. 2018).
Vestured pits are common in dry-fruited Myrtoideae (?Syzygium as well) (Carlquist 2017b). Lid cells are distinctive epidermal cells covering oil glands that i.a. can be used to recognise fossil Myrtaceae (Lange 1980). The secretory glands examined by Richit et al. (2023) were schizogenous, not lysigenous, apparent lysigeny resulting from how the material had been prepared for study, and their contents were various: terpenes, flavonoids, triketones, resins (sometimes), etc.. There is considerable variation in stomatal morphology in Myrtoideae (Tarran et al. 2018 and references: esp. App. S1B); cyclostaurocytic stomata have four or more cells more or less radially arranged around the stomata. P. G. Wilson et al. (2022) note that the stomata are sometimes in strands or irregular clumps independent of the underlying vasculature; normally the stomata are all over the leaf surface or are restricted to the areoles. Tarran et al. (2018) also discuss the distribution of eglandular hairs in the family. Da Silva et al. (2012) noted that there was a diversity of colleter morphologies, although they did not record any colleters from Eucalypteae; see Pimentel et al. (2014) for colleters in the flowers.
For inflorescence morphology in Myrteae, see McVaugh (1956). Floral development has been much studied. The androecium shows much variation and its morphology can be difficult to characterise. As Orlovich et al. (1996: p. 716) noted, "In both Lophostemon and Xanthostemon, polyandry is beyond the concepts of haplostemony and diplostemony in both their ontogenetic and phylogenetic senses." Taxa with a fasciculate androecium often have a prestaminal bulge, and the distribution of stamens around the circumference of the flower is also affected by differential expansion of the hypanthium, stamens tending to group opposite the calyx or corolla (e.g. Orlovich et al. 1996; Belham & Orlovich 2003). Flowers with apparently oppositisepalous stamens are developmentally derived from an oppositipetalous androecium (see e.g. Carrucan & Drinnan 2000; Drinnan & Carrucan 2005), and fasciculate and non-fasciculate androecia intergrade (Orlovich et al. 1999). In some species of Eucalyptus the androecium is displaced, arising from a stemonophore, the base of the corolla limb, or development may be more conventional (see e.g. Drinnan & Ladiges 1989a, b, 1991). Some species in Eugenia sect. Umbellatae have polylocellate anthers (Giaretta et al. 2024). Anther glands occur throughout the family, and Ladd (2024) discusses their morphologyy, the composition of the oils they secrete, and their possible functions. Thornhill and collaborators have carried out a comprehensive pollen survay of Myrtaceae: Thornhill et al. (2012b) - Eucalypteae, Lophostemoneae, Syncarpieae, Xanthostemoneae, Psiloxyloideae, ibid. (2012c) - Backousieae, Melaleucieae, Metrosidereae, Osbornieae, Syzygieae, ibid. (2012d) - Chamaelaucieae, Leptospermeae, Linsayomyrteae, and Thornhill et al. (2012e) - Kanieae, Myrteae, Tristanieae. Microsporogenesis in some Eucalyptus may take six months (Beardsell et al. 1993). Both anther and pollen morphology in Verticordia are very variable, and the pollen is dispersed in pollenkitt, sometimes also with oil (Ladd et al. 1999, 2000).
L. A. S. Johnson and Briggs (1984) emphasized the fully superior nature of the ovary in Heteropyxidoideae (= their Psiloxylaceae), with its relatively narrow base, comparing it with the more or less inferior ovary of Myrtoideae (Myrtaceae), which always had a broad base. Pimentel et al. (2014; see Martos et al. 2017 for more on floral development of Myrteae) suggest that the inferior ovary in Myrteae may be either appendicular or receptacular (also the placentae may be cauline or carpellary), while Harthman et al. (2018) suggest that the distinction between axile and parietal placentation is not that sharp. There is considerable variation in ovule morphology and orientation, the latter even on the one placenta (Bohte & Drinnan 2005), and the parietal tissue varies greatly in thickness, being very thick in Eugenia (van Wyk & Botha 1984) and Eucalyptus s.l. (Bohte & Drinnan 2005). Corner (1976) described the micropyle as being exostomal; it is variable, but perhaps most commonly bistomal.
Seed coat anatomy (see also ovules) and embryo vary greatly, and testa anatomy correlates with fruit type: Capsular fruits tend to have exotestal seeds; baccate fruits have seeds with a generally sclerotic testa (c.f. Nic Lughadha & Proença 1996 and Beardsell et al. 1993; see also Gauba & Pryor 1961 and references: Eucalyptus s.l.; van Wyk & Botha 1984; Biffin et al. 2006). Myrtaceae, especially Myrteae, have a variety of embryo/cotyledon types, including some taxa in which the cotyledons are barely developed although the rest of the embryo seems ordinary - thus genera like Myrciaria are described as having homogeneous embryos (c.f. Lecythidiaceae, see Kausel 1955; Amshoff 1958). McVaugh (1956) described Myrciinae as having contortuplicate, foliaceous cotyledons and an elongate radicle, Pimentinae as having a spiral/curved embryo with an elongate radicle and very short cotyledons, and Eugeniinae as having fleshy, distinct conferruminate cotyledons, or an undivided embryo with a very short radicle - and noted that Eugenia and Syzygium could be distinguished by their embryos (in the latter, more or less normal). There is commonly a more or less elaborated/swollen basal portion of the hypocotyl that bears root hairs, however, in Angophora a broad, disc-like structure (= coleorhiza) in the same position lacks such hairs (Baranov 1957).
For general information, see Schmid (1980) and especially P. G. Wilson (2011), also Blake (1977: Allosyncarpia et al.), Van Wyk (in Dahlgren & Van Wyk 1988), Heteropyxis, Snow et al. (2003), Austromyrtus, etc., McKinnon et al. (2008), Eucalyptus and immediate relatives, Parnell et al. (2007), Syzygium s.l., Slee et al. (2020), Eucalyptus, Proença et al. (2022), Psidium, and De Souza Neto et al. (2022), Neotropical Myrteae; also Keszei et al. (2008: terpenes, Australian Myrtaceae), Hallam and Chambers (1970: epicuticular waxes in Eucalyptus), van Wyk et al. (1980: cork cambium initiation), Soh and Parnell (2011: Syzygium) and Cardoso et al. (2009: Brazilian Myrtoideae) both lamina anatomy, Brooker and Nicolle (2013: venation and oil glands), for inflorescence structure of Myrtoideae, see Briggs and Johnson (1979), for floral development, see Belsham and Olovich (2003), Drinnan et al. (2001) and Orlovich et al. (1996), for filament curvature in Myrteae, see Vasconcelos et al. (2015: correlation with major clades), and for embryology, in which there is considerable variation, see Mauritzon (1939a: integuments in bitegmic ovules consistently 2 cells across), Narayanaswami and Roy (1960 and references), Prakash (1969: Darwinia) and references in Bohte and Drinnan (2005), also see van Wyk and Botha (1984: seed coat, etc., Eugenia) and other papers by van Wyk and collaborators.
Phylogeny. Conti et al. (1996) found a well supported [Heteropyxidaceae + Psiloxylaceae] sister to [Myrtaceae + Vochysiaceae]; note, however, that the pollen grains of the first two are similar to those of Myrtaceae (Dahlgren & Thorne 1985). Monophyly of Myrtaceae s. str. (= Myrtoideae) was not strong (Conti et al. 1996, 1998). However, P. G. Wilson et al. (2005: matK only) found Myrtaceae s. str. to have 80% jacknife support, while [[Heteropyxidaceae + Psiloxylaceae] + Myrtaceae s. str.] (all together = Myrtaceae here) had ³95% support; a similar set of relationships were found by Sytsma et al. (2004: matK and ndhF) and Maurin et al. (2021: nuclear genomes).
The old Myrtoideae, capsular-fruited, are paraphyletic (Sytsma et al. 1998; P. G. Wilson et al. 2001; Salywon et al. 2002), while fleshy-fruited taxa (Myrteae) are largely derived and monophyletic, although the large genus Syzygium s.l., Syzygieae, represents an independent acquisition of fleshy fruits (see also L. A. S. Johnson & Briggs 1984); for relationships in these plants, see also Biffin et al. (2010a). The limits of major clades (= tribes) in Myrtoideae are similar in Wilson et al. (2005: matK) and Biffin et al. (2007: ITS), but the relationships between the clades are less so, although the differences are poorly supported. Vasconcelos et al. (2017) found relationships around Myrteae to be [[Leptospermeae + Eucalypteae - outgroup] [Syzygium [Metrosideros [Xanthomyrtus [Myrtastrum and other Myrteae]]]]].
Within Myrtoideae as currently delimited, Maurin et al. (2021) found relationships that differed depending on the particular analysis of Angiosperms353 data. Although [Lophostemoneae + Xanthostemoneae] were consistently sister to the rest of the subfamily, the topology then seems to revolve around Melaleuceae (probably plus Osbornieae). In exon coalescent ASTRAL analyses Myrteae, Lysicarpus, etc., formed a clade above Melaleuceae, and Chamaelaucieae, Leptospermeae, etc., formed a clade below; these two clades switched positions while largely retaining the same internal relationships in exon RAxML supermatrix and coalescent supercontig ASTRAL analyses - although support values were in general poor (Maurin et al. 2021). Tribal limits and internal relationships suggested by Maurin et al. (2021: q.v. for details) do sometimes differ (and they may vary between analyses); only some of their findings are mentioned here. Note that they found Kanieae were polyphyletic, with Kania separate from the rest of the tribe. Relationships around Eucalypteae may be [Syncarpieae [Syzygieae [Lindsayomyrteae + Eucalypteae]]] (Maurin et al. 2021) - clade size varies greatly. P. G. Wilson et al. (2022) concentrated on the relationships of Kania and the old Kanieae; topologies tended to change depending on the analysis/type of data (nuclear/chloroplast).
Chamaelaucieae. X. Ma et al. (2011) carried out a preliminary study on Verticordia, recovering two main groups (prior work suggested that there were three subgenera). Rye et al. (2020: 1 nuclear, 2 choroplast markers) provide an extensive phylogenetic analysis of the tribe. Nge et al. (2025a: Angiosperms353 probe set) included 36/37 genera and 131 species of the tribe; data sets included 292 loci (exons) and 341 loci (supercontigs), the latter over twice as long. Most subtribes were monophyletic, but not Rinziinae s. l.; the strength of the relationships between the subtribes depended on the analysis, but support for the monophyly of the tribes tended to be quite good, although there was variation in the topology of the tree; the species sampling was designed to recover polyphyly, and genera like Verticordia and Darwinia were indeed polyphyletic (Nge et al. 2025a, q.v. for extensive discussion). Within Chamaelaucieae, Nge et al. (2021a) paid attention to relationships in Calytrix; Homalocalyx was its sister, while within Calytrix itself, C. superba and C. erosipetala tended to be basal, depending on the analysis.
Eucalypteae. Phylogenetic relationships around Eucalyptus s.l. have been much discussed, e.g. by Byrne (2008), Parra-O. et al. (2009), Steane et al. (2011), Bayly et al. (2013a: chloroplast genomes), Rutherford et al. (2016), Jones et al. (2016: much low-level hybridization, species polyphyly, etc.), González-Orozco et al. (2016: esp Extended Data Figs 6, 7), Healey et al. (2018: chloroplast capture between quite separate taxa), and Thornhill et al. (2019). Ochieng et al. (2007) recovered the monophyly of Corymbia using nrITS pseudogene sequences, corroborating relationships using morphology, microsatellites, etc., however, González-Orozco et al. (2016) found that Angophora was nested within Corymbia, although support was not strong, while within Eucalyptus, Symphyomyrtus was paraphyletic. Indeed, the position of Corymbia with respect to Angophora seems particularly uncertain (Schuster et al. 2018; Thornhill et al. 2019; Maurin et al. 2021), and within Corymbia morphology and nrDNA tell one story, chloroplasts another - here, as in Eucalyptus itself, hybridization and chloroplast introgression rather than lineage sorting seem to be involved (Schuster et al. 2018). Series and sections in neither Corymbia nor Eucalyptus are monophyletic (Thornhill et al. 2019). McLay et al. (2023b) looked at relationships within subgenus Eudesmia - "extreme gene tree discordance ... hybridization, introgression, and incomplete lineage sorting blur deep evolutionary relationships despite clear species groupings" (from the title) says it all. See also Crisp et al. (2024: 266 spp., 100 low copy nuclear genes) for a phylogeny of Eucalyptus s.l..
Leptospermeae: Binks et al. (2021) found that both chloroplast and nuclear ribosomal trees confirmed the polyphyly of Leptospermum - the species it includes are probably to be placed in five separate genera, or perhaps four of those clades will go into Kunzea. P. G. Wilson and Heslewood (2023: 6 loci), looking at 98 taxa from all nine genera currently recognised in Leptospermeae, confirmed these results.
Melaleuceae, another predominantly Australian group, are strongly supported as being monophyletic. The three main clades making it up have high posterior probabilities but only moderate to low bootstrap support; most of the small genera previously recognised in this tribe fall into one of these clades, along with a group of species of Melaleuca s. str. (Edwards et al. 2010; see also Brown et al. 2001).
Metrosidereae: Wright et al. (2000a), Pillon et al. (2015) and especially Dupuis et al. (2020) evaluate relationships in the largely East Malesian-Pacific Metrosideros, still poorly understood but which has been expanded to include taxa from South America (Tepualia) and New Caledonia. Dupuis et al. (2020) found that M. laurifolia, from New Caledonia, was sister to the rest of the genus.
Myrteae. Vasconcelos et al. (2017) found that Myrteae included three major clades, [Australasian [[Myrtus group + Main Neotropical Lineage]], but the relationships between the three were somewhat unclear as was the position of the New Caledonian Myrtastrum rufopunctatum; the latter could be sister to all the rest of the tribe (Vasconcelos et al. 2017). Amorim et al. (2019) suggested the relationships [paraphyletic Blepharocalyx [Myrtus group [PAM clade = Myrceugenia group [Plinia group + Myrcia s.l.]]]]. The Neotropical Working Group (2023/2024) looked at 2 nuclear and 7 plastid markers, 712 species, and all genera. Proença et al. (2022) looked at relationships within Psidium, and phylogenetic structure is developing there; it is unclear if Myrrhinium will remain separate. For relationships in Myrceugenia and its immediate relatives like Blepharocalyx, etc., see Murillo-A et al. (2012, 2013). The fleshy-fruited Myrcia s. str. is strongly paraphyletic and forms a large clade with i.a. Calyptranthes (Lucas et al. 2011, also P. G. Wilson et al. 2016; Santos et al. 2016; Vasconcelos et al. 2016), while Marlierea, which it also includes, is notably polyphyletic (Staggemeier et al. 2015a). There have been 9 usually well-supported major clades (= sections) within Myrcia in its broad sense (Lucas et al. 2011; Santos et al. 2017), and another clade was added by Amorim et al. (2019); see also Lima et al. (2021: section Aguava). Mazine et al. (2014, 2018, see also Mazine Capelo et al. 2011; Bernardini et al. 2014) have begun the task of disentangling relationships in the largely New World Eugenia; it initially appeared that there were two major clades in the genus, but more detailed analyses suggest a rather more pectinate structure, albeit support for some nodes tends to be rather weak, while within the large persistently bracteolate clade the Old World section Jossinia is sister to the rest. Van der Merwe (2005) discuss relationships in Eugenia, mostly African, de Lange et al. (2010) provide a phylogeny of the Antipodean Kunzea. Flickinger et al. (2020) take a broad look at Myrteae in the Greater Antilles. Maurin et al. (2021) sampled Myrteae quite extensively, and they found that the odd New Caledonian Myrtastrum rufopunctatum is sister to the clade that contains all Neotropical species. Proença et al. (2022: 30 species, 2 chloroplast and two nuclear markers)
Syzygieae. Eugenia (connate cotyledons, especially New World) and Syzygium (free cotyledons, Old World) were confused in the past, both having fleshy fruits; the two are not immediately related (see Schmid 1972 for a pre-molecular resolution of the problem; for Eugenia, see above). After disentangling the two, the relationships of Syzygium to genera like Acmena had to be worked out (Harrington & Gadek 2004: ?rooting). As Biffin et al. (2006, see also Biffin & Craven 2011) found, Acmena and Waterhousea are in a well supported clade along with Syzygium s. str., while the poorly supported sister clades to this group are also largely made up of species of Syzygium. Low et al. (2022: ca 240 spp., 1/5 the genus) carried out a variety of analyses focussing on single nucleotide polymorphisms and single-copy nuclear genes and found that the five subgenera (one not yet described, but see Hatt et al. 2023: 7 morphological/anatomical characters examined, subgenus described) were all monophyletic, furthermore, there was a substantial amount of well supported phylogenetic structure in the genus over which the analyses largely agreed. Topologies from plastid analyses, however, showed some incongruence (Low et al. 2022).
Classification. Myrtaceae s. str. (excluding Heteropyxidoideae) were traditionally divided into Leptospermoideae - leaves spiral to opposite, and fruit dry, dehiscent, and Myrtoideae - polyhydroxyalkaloids common, leaves opposite, terpenoid-containing glands in the apex of the connective, stigma dry, and fruit fleshy, indehiscent. This distinction is untenable (see above). For a classification of Myrtoideae in which fifteen tribes are recognized, see P. G. Wilson et al. (2005, 2022: dismemberment of the polyphyletic Kanieae) and Wilson (2011). Rye et al. (2020) divided Chamelaucieae into 11 subtribes, mostly new.
Generic limits in Myrteae have been problematic (Lucas et al. 2005, 2007; P. G. Wilson 2011), but see Vasconcelos et al. (2017) and in particular Lucas et al. (2019) for genera and a subtribal classification, the former pointing out a number of para- and polyphyletic genera, the latter recognizing 9 subtribes. Thus the limits of and infrageneric groupings within Myrcia/Calyptranthes have needed attention (Lucas et al. 2011; Wilson et al. 2016). Indeed, Myrcia, paraphyletic, is to be expanded to include Marlierea and Calyptranthes, genera that were based on characters of the calyx that have turned out to be homoplastic (Staggemeier et al. 2015a; Vasconcelos et al. 2016). Biffin et al. (2006; see also Craven 2001; Biffin et al. 2007; Biffin & Craven 2011) suggest that Syzygium should be delimited broadly (just the one genus in the tribe), at least pending a better understanding of the morphological variation of this clade; an infrageneric classification needed to be put in place, although some of the groups that were becoming apparent were difficult to recognise - but that of course would be true if Syzygium were split and these groups were recognised as genera. Eucalyptus may be in the process of being dismembered (Parra-O. et al. 2009 and references; Crisp et al. 2024); although for a classification of Eucalyptus s.l., see Nicolle (2024) and Nicolle et al. (2024) - broad limits, as in Brooker (2000), but here the immediate stimulus was Crisp et al. (2024) and their segregation of Blakella. (The disposition of Corymbia had not been immediately settled; should it be included in Angophora, or separated, with yet more genera having to be recognized - see Schuster et al. 2018 for earlier nomenclatural arguments in the Australian eucalypt community; Thornhill et al. 2019; Crisp et al. 2024.) The limits of Melalauca are being expanded; if genera were segregated they would both be small and undiagnosable, distinctive characters being highly homoplastic in the group (Edwards et al. 2010). Generic limits in Chamelaucieae are in a state of flux (Rye 2015 and references). Following on the molecular studies of Binks et al. (2021), P. G. Wilson and Heslewood (2023) added three new and one resuscitated genera to Leptospermeae.
For a subtribal classification - twelve of them - of Chamaelaucieae, see Nge et al. (2025a and references). Lucas et al. (2018) provide a sectional classification of Myrteae-Myrcia (see also Santos et al. 2024, 9 sections, also Myrciinae as a whole), and Mazine et al. (2016, 2018) an infrageneric classification of Eugenia, the majority of species being placed in section Umbellatae; note that the limits of Neotropical Eugenia are best expanded (Mazine Capelo et al. 2011 for a summary). There is an infrageneric classification of Syzygium in Craven and Biffin (2010), although since 80-90% of the species belong to subg. Syzygium within which relationships are poorly understood, there is still plenty to do (see Hatt et al. 2023 for one more subgenus). Proença et al. (2022) suggest that Psidium can be divided into four sections. Within Eucalyptus s.l., Nicolle (2024) recognises 11 subgenera, of which 5 are monotypic; in addition, there are some 26 sections, 110 series and 44 subseries.
Botanical Trivia. Trees of Eucalyptus regnans, the snow gum, are the tallest known angiosperms, growing up to 101 m (ca 330 feet) tall, however, before the logging of the last century and a half there may have been individuals substantially over 400 feet (122 m) tall (Carder 1995). However, in mass such trees are much smaller than individuals of Sequoia sempervirens or Sequoiadendron giganteum (Cupressaceae) or of clones of bamboo or Populus.
Thanks. I am grateful to Z. Rogers for discussion about Heteropyxis.
[Melastomataceae [Crypteroniaceae [Alzataeaceae + Penaeaceae]]]: plants Al accumulators; fibre tracheids +; (nodes swollen); branched or unbranched sclereids +/0 within same family; C clawed?; connective abaxially much expanded; endothecial thickening absent/atypical; nectary 0; exotestal cells ± longitudinally elongated.
Age. Estimates of the age of this node are (99-)91(-82) Ma (Berger et al. 2015), ca 84 Ma (Morley & Dick 2003), ca 82 Ma (Sytsma et al. 2004), about 80 Ma (Renner et al. 2001), about 68.1/65.4 Ma (Tank et al. 2015: Table S1, S2) or (107.6-)94(-80.4 Ma (Gonçalves et al. 2020a).
Evolution: Divergence & Distribution. Endothecium evolution may be more complex than simply scoring its absence as an apomorphy for the clade might imply (e.g. Cortez et al. 2014; see esp. Melastomataceae).
Chemistry, Morphology, etc.. A number of taxa, but apparently not Melastomataceae, have more than a single branch from the leaf axil. Nodes other than simple unilacunar are quite widespread, however, a survey of nodal anatomy, particularly that of Melastomataceae, is much needed.
The anther connective is least expanded in Penaeaceae-Rhynchocalyx.
Phylogeny. For relationships in this area, see Conti et al. (2002).
MELASTOMATACEAE Jussieu, nom. cons. - Back to Myrtales
Trees or shrubs; (plants Al-accumulators); veinlets with terminal sclereids[?]; stomata hideously variable; leaves with 2 or 4 strong secondary veins, from (near) the base [acrodromous]; inflorescence axillary; K valvate, C right-contorted [dextrorse, right-handed], spreading; A = 2 x K, usu. longer than C, dimorphic, bent to one side of the flower (not), anthers with branched vascular trace [?all]; tapetal cells uninucleate; nectary 0; G opposite K, stigma punctate; ovules many/carpel; micropyle zig-zag, outer and inner integuments ca 2 cells across; seeds "small"; radicle bent; x = 11 (?10), nuclear genome [1 C] (0.038-)0.829(-18.202) pg.
177/5,750: [list, to tribes]. Very largely tropical, also subtropical, 70% New World (Veranso-Libalah et al. 2018). Three subfamilies, 22 tribes below.
Age. Renner et al. (2001, also Renner & Meyer 2001) suggested that crown group diversification began about 53 Ma and Wikström et al. (2001) offered the somewhat younger age of (51-)47, 41(-37) Ma, Morley and Dick (2003) suggested the substantially older date of ca 82 Ma; dates suggested by Bell et al. (2010) are (65-)48, 41(-28) Ma, while Systma et al. (2004) suggested an age of ca 56 Ma, Berger et al. (2015) an age (75-)64.5(-56) Ma, Gonçalves et al. (2020a) an age of (80.4-)66.7(-53.6) Ma, Reginato et al. (2020) an age of (77.3-)63.4(-56.1) Ma, X.-F. Zhang et al. (2021) an age of (71.5-)45.8(-13.7) Ma and Amarasinghe et al. (2021) an age of (85.9-)77.1(-67.5) Ma.
Includes Astronieae, Bertolonieae, Cyphostyleae, Dinophoreae, Dissochaeteae, Eriocnemeae, Feliciadamieae, Henrietteeae, Kibessioideae, Lavoisiereae, Lithobieae, Marcetieae, Melastomateae, Melastomatoideae, Merianieae, Miconieae, Olisbeoideae, Pedoconnective Clade, Pyramieae, Pyxidantheae, Rhexieae, Rupestreeae, Sonerileae, Stanmarkieae, Trioleneae.
[Kibessioideae + Olisbeoideae]: included phloem +; vascular bundles surrounded by sclerenchymatous ring; anthers dehiscing by slits; strong development of differential growth in the flower [see Chemistry, Morphology, etc.]; G opposite C; fruit (±) a berry.
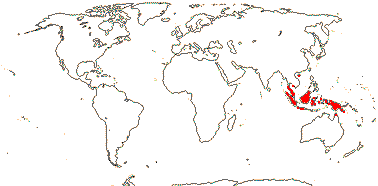
1. Kibessioideae Naudin (inc. Pternandreae Gilg) - Pternandra Jack
Small trees; cork cambium superficial; hairs uniseriate; petiole bundle arcuate; flowers 4-merous; K connate, cupuliform/truncate, tube ± echinate/scaly/tuberculate (smooth); A not dimorphic, shorter than C, endothecium restricted to inner wall of inner sporangium only, connective thickened, spur near the filament (0); placentae on walls of G, G divided by septae [= dorsal medial placentae]; fruit surface setose/umbonate-tesselate-echinate; n/x = ?
1/15. India, S. China to N.E. Australia, most Malesian. Map: based on Maxwell (1981: incomplete).
Age. Crown-group Pternandra is some (16.7-)9.9(-4.4) Ma (Reginato et al. 2020).
2. Olisbeoideae Burnett —— Synonymy: Memecylaceae Candolle, Mouririaceae Gardner
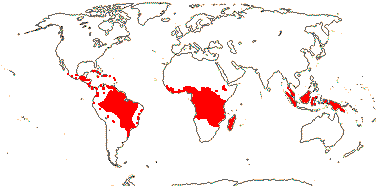
Vessel ray pits half bordered; crystal styloids +, large; (nodes 1:3); sclereids at node and base of petiole (etc.); petiole bundles (deeply) arcuate to annular plus wing bundles; indumentum ± 0 [Mem.]; sclereids +, at ends of veinlets filiform/branched/spheroidal (0), small crystal styloids + (0); petiole bundle arcuate, annular; (leaf veins lacking fibrous sheath); stomata (in crypts - many - Mou.); plant usu. glabrous, (hairs uniseriate); stem apex frequently aborting, branching (complex) from previous flush; lamina vernation flat [Mem.] or revolute [Mou.], secondary veins pinnate (pli-nerved); inflorescences (terminal), often fasciculate, pedicels articulated; flowers small, 4-5-merous; K (imbricate), (truncate), (calyptrate), C (protective in bud - most Mem.), often asymmetrical [scalloped/fringed on one side]; A (straight in bud - Votomita), anther endothecium + [cells thickened all around], connective enlarged, with oil gland (0); placentation various [basal/parietal/axile/free-central, 2- or 4-locular], stigma wet; ovules 1-14(-many)/carpel, (campylotropous), apotropous, funicle 0, (outer integument 4-6 cells across - Mou.), parietal tissue ca 3 cells across; seeds large, 1-3(-12)/fruit; testa (multiplicative), (some sclerotic hypodermal exotestal cells: Me.), exotegmen fibrous, massively sclerotic subhilum [?W.]; embryo large (small), green, cotyledons thin, crumpled, hypocotyl long [Mem.], or cotyledons thin, variously curved, hypocotyl medium, seed coat in subhilar region massively thickened [Spathandra], or anisocotyly extreme, hypocotyl short, local thickening of seed coat [W.]; n = 7, 12, x = 7, 12, seedling hypocotyl elongated or not [W.], cotyledons lobed or not, stipules +.
6/556: Memecylon (391), Mouriri (89), Warneckia (49). Tropical. Map: from Morley (1976: Fig. 1), Schatz (2001) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photo - Flower, Fruit.]
Age. Crown-group Olisbeoideae are (44.9-)31.3(-20.1) Ma (Reginato et al. 2020), while Amarasinghe et al. (2021) estimated that the Memecylon + Mouriri clade was ca 44.4 Ma.
3. Melastomatoideae Seringe
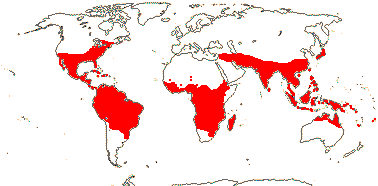
Acylated anthocyanins + [?level]; anthocyanins in the root tip; libriform fibres +, vessel-ray pits simple; medullary vascular bundles +; (nodes 1:3; split laterals); leaf veins lacking fibrous sheath; lamina vernation conduplicate or supervolute, tertiary veins at right angles to the midrib [?level]; flowers ± monosymmetric [largely by androecial and stylar orientation]; abaxial K projections + [callose to acicular] (0); G developing before A; anthers opening by pores, epidermis persisting, endothecium 0, 3 middle layers of wall with thickened cells, (descending abaxial branch of thecal vascular bundle), connective with a basal appendage or not; pollen 3-colporoidate; G opposite K, often spaces between ovary wall and hypanthial tube, style impressed; ovule micropyle zig-zag, outer integument (2-)3(-4) cells across, inner integument 2(-3) cells across, parietal tissue 2-7 cells across, ?nucellar cap +, ?endothelium +, hypostase +: embryo sac long and thin, curved or not; fruit loculicidal capsule/dehiscing down its inferior part/baccate; seeds small, many, with hilar operculum, radicle in testal pocket, exotesta palisade to cuboid, lignified, (sclerotic mesotesta +), tegmen crushed; cotyledons often unequal, incumbent; n = (8-)9(-)12(-)17 (23, 31), x = 12, chromosomes 0.5-3 μm long.
181/4,455. Largely tropical and subtropical, esp. South America - Colombia and Brazil diverse, ca 400 spp. endemic to the Caribbean alone. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Quian and Ricklefs (2004), FloraBase (consulted 2007) and Woodgyer (2007). Photo:Flower, Fruit, Fruit.
Age. Crown-group Melastomatoideae are (72.0-)58.9(-47.3) Ma (Reginato et al. 2020).
Fossil leaves, Xystona simonei and identified as Melastomatoideae, have been found in Palaeocene deposits 60-58 Ma from Central Colombia (Carvalho et al. 2021b).
[Lithobieae [Henrietteeae + Astronieae]]: A not dimorephic.
Age. This clade is (53-)39.6(-28.4) Ma (Reginato et al. 2022).
3A. Lithobieae Penneys & Almeda - Lithobium cordatum Bongard
Herb, rosulate, rhizome +, tuberous; flowers single, long-pedicellate, ?axillary; flowers polysymmetric, 3(-4)-merous; abaxial K teeth 0; anthers uniporose; G semi-inferior; fruit loculicidal capsule; seeds smooth; testal anticlinal walls sinuous; n/x = ?
1/1. Brazil, Minas Gerais. Map: Reginato et al. (2022: Fig. 2:3).
[Henrietteeae + Astronieae]: stomata mostly anomocytic; large styloids in lamina; anthers dolabriform [= like an axe head].
Age. The age of this clade is (36.1-)23.6(-12.9) Ma (Reginato et al. 2020) or (37.6-)27.8(-19.7) Ma (Reginato et al. 2022).
3B. Henrietteeae Penneys, Michelangeli, Judd & Almeda
Shrubs to trees; medullary vascular bundles 0; inflorescence along stem or branches, cymose/fasciculate/flowers axillary; flowers 4-8-merous; K calyptrate to valvate; G (3-)4-8(-15)-locular, ± inferior; fruit a berry; n = 15, 20, 28.
3/95: Henriettea (71). South Mexico to Bolivia and Brazil, the Caribbean. Map: Reginato et al. (2022: Fig. 2:5).
Age. Henrietteeae are (17.4-)12.9(-8.4) Ma (Reginato et al. 2020) or (21.7-)16.9(-12.2) Ma (Reginato et al. 2022).
3C. Astronieae Triana
Shrubs to trees; petiole with bundles in arc, + adaxial bundles; hairs non-glandular peltate scales; inflorescence often terminal; flowers polysymmetric (monosymmetric by androecium - Tessmanianthus), K open, abaxial K teeth 0; A (many - Astrocalyx), (anthers with terminal slits, thecae prolonged into a short tube); G opposite C, placentation basal to basal-axile; n = 20.
5/143: Astronidium (67), Astronia (59). Indomalesia and Pacific, Tessmannianthus Panama, Colombia to N. Peru. Map: Reginato et al. (2022: Fig. 2:4), Mancera et al. (2022: Fig. 2).
Age. Crown-group Astronieae are (12.1-)8.0(-4.7) Ma (Reginato et al. 2020) or (28.1-)21.1(-15.5) Ma (Reginato et al. 2022).
Fossil Astronia has been reported from North America: A. cumingiana from the Turonian of late Cretaceous (94-90 Ma) age was found at Crossman site, New Jersey, northeastern U.S.A., albeit erroneously referred to in the discussion as Astronium Jacq. (Anacardiaceae) (Crepet 2008).
[Merianieae [Eriocnemeae + Miconieae]]: ?
Age. Note that Reginato et al. (2020) dated the clade (including Octhephilus, then thought to be in Eriocnemeae, and Physeterostemon, also Eriocnemeae) at (12.5-)8.2(-3.8) Ma; the former genus is now placed in Merianieae by Maurin et al. (2021), hence the date can go here. Reginato et al. (2022) suggest that the age of this clade is (51.3-)40.2(-30.1) Ma.
3D. Merianieae Triana
Herbs to trees (lianes; roots from internodal areas/epiphytes); glandular scales; (hairs with subulate cells, ± mesifixed); (stem with nodal flaps/4-winged internodes); (lamina/petiole junction area with processes); (K calyptrate); A appendages conspicuous; (anthers polysporangiate); (outer integument ca 3 cells across); capsule triangular; testa smooth to tuberculate; n = 12, 13, 15, 17, 19, x = ?12.
8/301. Meriania (120), Graffenrieda (68), Axinaea (41). Central and South America, the Caribbean. Map: Reginato et al. (2022: Fig. 2:(6).
Age. Crown-group Merianieae are some (28.5-)21.4(-15.3) Ma or (37.9-)30.0(-23.5) Ma (Reginato et al. 2020, 2022 respectively).
[Eriocnemeae + Miconieae]: fruit indehiscent.
Age. The age of this clade is around (18.8-)13.3(-8.9) Ma (Reginato et al. 2020) - note that these authors dated the clade that included Octhephilus, then thought to be in Eriocnemeae and Physeterostemon, also Eriocnemeae, at (12.5-)8.2(-3.8) Ma; the former genus is now placed in Merianieae by Maurin et al. (2021). Reginato et al. (2022) suggested that the age was (28.6-)20.9(-15.4) Ma.
3E. Eriocnemeae Penneys & Almeida
Herbs to shrubs; druses +; ?stomata; inflorescences terminal; flowers poly/monosymmetric; anthers uniporose, (pedoconnectives +), appendages 0/various; G to semi-inferior; fruit dry; testa smooth; n = 17.
3/7: Physeterostemon (5). S.E. and N.E. Brazil, Guyana. Map: Reginato et al. (2022: Fig. 2:7).
Age. The age of crown-group Eriocnemeae is estimated to be (10.8-)7.8(-4.1) Ma (Reginato et al. 2022) or (7.7-)5.5(-3.6) Ma (Bacci et al. 2022b).
3F. Miconieae de Candolle - Miconia Ruíz & Pavón —— Synonymy: Miconiaceae Martius
(Herbs-)shrubs to trees, (root lianas/epiphytes); nodes 1:1; petiole bundles arcuate; foliar raphides 0; (foliar ant domatia +); inflorescences (axillary); K (calyptrate), C white; A (many), anthers pink/yellow, (polysporangiate), connective elaboration slight/0, (pedoconnective +), (glands on connective at base of thecae); G (superior), (-21), (placentae linear/branched), (stigma massive, crateriform); fruit baccate, (1 seed/carpel); outer integument (2)/3(-7) cells across, (multiplicative); n = (8-10), (15-)17(18), x = 17.
1/1,940: Miconia (inc. Clidemia, Conostegia, Leandra, etc.). Tropical America, esp. the Atlantic Forest, also the Antilles. Map: Reginato et al. (2022: Fig. 2:8).
Age. Crown-group Miconieae are estimated to be (15.8-)11.3(-7.4) Ma (Reginato et al. 2020) or (20.6-)16.7(-13.3) Ma. And if there is a clade [Bertolonieae + Pyxidantheae, etc], it is estmated to be (62.3-)51.3(-43.1) Ma (Reginato et al. 2022).
3G. Bertolonieae Triana - Bertolonia Raddi
Rhizomatous herbs, subshrubs, (epiphytes); stomata anisocytic (diacytic); hairs glandular, (etc.); inflorescence scorpioid cymes/helicoid racemes; A monomorphic, pore 1, pedoconnective +, with short dorsal/no appendage; G [3(-4)]/subinferior; capsule triquetrous, enveloped by K; testa tuberculate; n = (12) 14.
1/36. S.E. Brazil, Atlantic Forest. Map: Reginato et al. (2022: Fig. 2:9) and Bacci et al. (2022: Fig. 1). Not Venezuela, see Bacci et al. 2022)
Age. Crown-group Bertolineae are some (20.3-)11.6(-4.6) Ma (Reginato et al. 2020), (38.1-)29.8(-21.9) Ma (Bacci et al. 2021b), (21.6-)16.1(-10.6) Ma (Reginato et al. 2022) or (11.6-)8.4(-5.9) MA (Bacci et al. 2022b).
Age.Ages for the clade [Medinilla (Sonerileae) [Rhexia (Rhexieae) + Tibouchina (Melastomateae)]] in Rutschmann et al. (2004) were around 111.7-25.3 Ma, depending on fossil calibrations, etc..
[Pyxidantheae, Stanmarkieae [Sonerileae + Cyphostyleae]]: ?
Age. The age of this clade is some (49.0-)40.1(-34.6) Ma (Reginato et al. 2022). Note that the topology there is different from that in Penneys et al. (2022).
3H. Pyxidantheae Grisebach (inc. Blakeeae) —— Synonymy: Blakeaceae Barnhart
Shrubs or trees, habit various (lianas, hemiepiphytes, epiphytes); inflorescence axillary, cymose/flowers single or fascicles, bracteoles 4(6); flowers usu. 6-merous, monosymmetric or not; K with external projections/calyptrate, no projections, valvate (imbricate); A isomorphic; S superior to inferior; fruit baccate; n = 28, 31...
2/212: Blakea (200). Mexico (Chiapas) to Bolivia and Brazil, Lesser Antilles, Jamaica. Map: Reginato et al. (2022: Fig. 2:12).
Age. Crown-group Pyxidantheae are estimated to be (9.5-)6.5(-3.7) Ma (Reginato et al. 2020) or (26.8-)16.3(-8.3) Ma (Reginato et al. 2022).
3I. Stanmarkieae Penneys & Almeda
Suffrutescent herbs to small trees, (epiphytic); ?anatomy; flowers 5-merous; K open; (pedoconnectives + - Centradeniastrum); G [3]; testa densely tuberculate, raphe elongated, arilloid; n = 20.
2/4: Centradeniastrum (2). Mexico, Guatemala, N.W. South America (Colombia to Peru). Map: Reginato et al. (2022: Fig. 2:10).
Age. Crown-group Stanmarkieae are (20.4-)11.6(-3.0) Ma (Reginato et al. 2020).
[Sonerileae + Cyphostyleae]: ?
3J. Sonerileae Triana (inc. Medinillleae, Oxysporeae)
Herbs to small trees, (epiphytes), (climbers; stems succulent; root/stem bulbils; etc.), (swollen-root myrmecophytes); xylem lobed in transverse section (not - Medinilla), (inter-vessel pits +, scalariform); (medullary vascular bundles 0); (raphides +); glandular hairs sessile or stalked; stomata anisocytic; (anisophylly + [extreme]), (leaves alternate), lamina (margin serrulate), (stipules +); inflorescence scorpioid, (branching alternate); (flowers 3-, 4-merous); A (isomorphic), (= and opposite K/many); pollen tricolpate, tricellular; G almost superior to inferior, (with apical annulus [= "crown"]); ovule micropyle bistomal; fruit a capsule, triangular/not, dehiscence septicidal/lateral, (old placentae fimbriate ["thready"]), (berry - M., etc.); endocarp with U-shaped thickenings; antipodal cells ± persistent; n = 9-21.
44/1,084: Medinilla (384), Sonerila (184), Gravesia (116), "Phyllagathis" (74), Anerincleistus (39), Oxyspora (39), Perilimnastes (20). Tropical, Old World, esp. Madagascar and Malesia-India-S.E. Asia, few New World (6/12). Map: Reginato et al. (2022: Fig. 2:13).
Age. The crown-group age of Sonerileae is estimated to be (29.3-)21.7(-14.6) Ma (Veranso-Libalah et al. 2018), (33.1-)25.4(-18.8) Ma (Reginato et al. 2020) or (44.1-)36.0(-29.2) Ma (Reginato et al. 2022).
3K. Cyphostyleae Gleason
((Rosette) herbs), shrubs (trees); ?anatomy; (lamina venation pinnate); inflorescence terminal or axillary; K calyptrate (not - Quipuanthus); flowers 4-10-merous; C (small - ≤5 mm long), A = and opposite K, filaments (S-shaped), straight, with apical "elbow", thecae at right angles to the filament, connective appendages 0; G (2-)3(-5), inferior, style (curved); fruit dry, breaking ± irregularly, (capsular, lateral-loculicidal), centraal columella persistent; n/x = ?
4/25: Allomaieta (10). Low altitude to montane rainforest and inter-Andean valleys from Colombia to S. Peru. Map: Reginato et al. (2022: Fig. 2:11).
Age. The crown-group age of this clade is (21.5-)>14.2(-7.1) Ma (Reginato et al. 2020) or (25.2-)17.4(-11.1) Ma (Reginato et al. 2022; Michelangeli et al. 2022c: Fig. 2).
[Trioleneae [[Pyramieae + Dissochaeteae] [Pedoconnective Clade]]]: staminal appendages conspicuous.
Age. This clade is some (58.3-)47.8(-40.5) Ma (Reginato et al. 2022).
3L. Trioleneae Bacci, Michelangeli & R. Goldenberg
Rhizomatous herbs (subshrubs), (epiphytes), (rhizomes scaly, fleshy - M.); leaves anisophyllous/not; inflorescence scorpioid/helicoid cymes; A dimorphic, pedoconnectives +, 1/3 [T.] ventral appendages; G [3]/semiinferior; capsules triquetrous, apical collar +, truncate, splash cup +; testa papillate/tuberculate; n = 8 (9, 17).
2/45: Triolena (29), Monolaena (16). Mexico south to Peru and adjacent Bolivia, western Amazonia. Map: Reginato et al. (2022: Fig. 2:14) and Bacci et al. (2022a: Fig. 1A).
Age. Crown-group Trioleneae are (32.4-)22.1(-12.4) Ma (Reginato et al. 2020), (49.7-)37.7(-26.1) Ma (Bacci et al. 2021b) or (35.7-)28.0(-20.9) Ma (Reginato et al. 2022).
[[Pyramieae + Dissochaeteae] [Pedoconnective Clade]]: ?
[Pyramieae + Dissochaeteae]: ?
Age. The estimated age of this clade is ca 39 Ma (Kartanegoro et al. 2021) or (34.8-)26.6(-18.8) Ma (Reginato et al. 2022).
3M. Pyramieae Naudin (inc. Cambessedesieae)
Shrubs, trees, (lianas), (stems succulent); (cortical bundles +, medullary bundles 0 - Cambessedesia - C.); flowers 4-7 merous; hypanthium (apically constricted); (C bicoloured - C.); A (not heteranthous), appendages 0/1 dorso-basal, linear/2, bilobed; G superior-inferior, [2-5(-6)]; fruit a capsule; seeds pyramidal/oblong (winged); testa (striate); n = 13, 15 21-24.
4/70: Huberia (37), Cambessedesia (25). Brazil, esp. S.E., few in Andean Ecuador and adjacent Peru. Maps: Reginato et al. (2022: Fig. 2:15) and Bochorny et al. (2022: Fig. 2)
Age. Crown-group Pyramieae are (34.8-)26.6(-18.8) Ma or (37.0-)29.9(-21.8) Ma (Reginato et al. 2020, 2022).
3N. Dissochaeteae Triana
Plants root climbers, scramblers (epiphytes) to small trees; (anomalous secondary thickening + [wood deeply 4-several lobed]); inter-vessel pits alternate [?level]; (rays multiseriate); indumentum stellate/bristles; leaves anisophyllous (extremely so)/not, with massive interpetiolar stipuliform structures, (margins serrulate), venation basal, acrodromous; (plant cauliflorous - Myrianthemum); flowers 4-merous; K free to connate and truncate; A 8/4 + 4 staminodes/4 opposite K/4 opposite C, (connectivce appendages 0); G semi-inferior; fruit baccate/dry indehiscent, sclereids +.
7/89: Dissochaeta (30), Macrolenes (27). Bhutan and S. China, inc. Hainan, to New Britain; also tropical W. Africa (mostly - Myrianthemum mirabile. Maps: Reginato et al. (2022: Fig. 2:16) and L. Chen et al. (2023: Fig. 2).
Age. Crown-group Dissochaeteae (n.b. Myrianthemum not included) are thought to be (26.5-)17.5(-10.3) Ma (Veranso-Libalah et al. 2018), (37.3-)28.5(-19.0) Ma (Reginato et al. 2020) or (30.7-)22.6(-14.9) Ma (Reginato et al. 2022).
[[Marcetieae + Lavoisiereae], Melastomateae, [Dinophoreae [Rupestreeae + Rhexieae]]] / Pedoconnective Clade: cortical vascular bundles +; abaxial K teeth 0; pedoconnective + [= basal-ventral extension of connective] (0).
Age. C.f. the topology of tribes in the Pedoconnective Clade in Reginato et al. (2022) with that in Penneys et al. (2022).
[Marcetieae + Lavoisiereae]: ?
Age. The age of this clade is ca. 30.0 Ma (Reginato et 2020).
3O. Marcetieae da Rocha, Guimarães & Michelangeli
Annual or perennial herbs to subshrubs (small trees); flowers 4(-5)-merous; A (= and opposite K (+ staminodes), anther pedoconnective bipartite, (not), (long); G [(2-3)4], (semi-inferior), glabrous; outer integument ca 3 cells across; fruit a capsule, (berry); seeds cochleate to reniform, (winged, flattened - Acanthella); n = (9-)12, (18).
20/169: Marcetia (36), Macairea (23), Comolia (16), Aciotis (15), Siphanthera (15). Mexico and the Antilles to Argentina, esp. Brazil (31 spp. Marcetia from Bahia). Map: Reginato et al. (2022: Fig. 2:21).
Age. Crown-group Marcetieae are estimated to be (32.1-)26.3(-20.1) Ma (Reginato et al. 2020) or (35.5-)29.5(-24.0) (Reginato et al. 2022).
3P. Lavoisiereae de Candolle (inc. Microlicieae)
Shrubs to small trees, (xylopodium +), (herbs - Poteranthera); cortical vascular bundles +, medullary vascular bundles 0; petiole bundles 3-7, arcuate, in a line, (7, amphicribral, incurved C-shaped arrangement); lamina often amphistomatous, palisade tissue isobifacial; flowers often solitary, 4-10-merous, (bracts 0); K not conspicuous; A (inner whorl staminodial/0), anthers rostrate, (polysporangiate, septae parenchymatous); G (2-)3(-10), (inferior - Lavoisiera); fruit capsule, dehiscence basipetal [?level], (capsular, dehiscence lateral, acropetal); seeds ellipsoid-reniform (cochleate), hilum linear; testa reticulate-foveolate [any testal processes 1-celled]; n = 9-13, 17, 18, x = 12.
3/273: Microlicia (253). South America, mainly E. Brazil (over 200 species from the Cadeia do Espinhaç), also Bolivia; drier and more open environments. Map: Reginato et al. (2022: Fig. 2:17) and Pacifico and Almeda (2022: Fig. 2A).
Age. The crown-group age of this clade is 19-16 Ma (Fritsch et al. 2004), (34-)25.2(-17.2) Ma (Veranso-Libalah et al. 2018) and (22.7-)16.0(-9.3) Ma or (29.2-)22.4(-16.4) Ma (Reginato et al. 2020 and 2022, respectively), see also Pacifico and Almeda (2022).
3Q. Melastomateae Bartling
(Interxylary phloem + - Dissotis); (cortical vascular bundles 0); large styloids in lamina [Centradenia]; (stipules + - Tibouchina); flowers (terminal, solitary - Cailliella), (pendulous - Brachyotum [B.]; intersepalar appendages +; K lobes caducous; C (± tubular -B.); A isomorphic, (A opposite C largest - some Monochaetum), (included - B.), ventral appendage linear, (nectar + - B.); (fruit a berry), (placentae fleshy); seeds cochleate, surface tuberculate, hilar operculum round; embryo strongly curved; n = 8-12, 15, 17, x = 12.
44/823: Pleroma (157), Chaetogastra (117), Melastoma (80), Brachyotum (55), Monochaetum (54), Osbeckia (51), Tibouchina s. str. (30). Pantropical, drier and more open environments. Map: Reginato et al. (2022: fig. 2:22).
Age. Crown-group Melastomateae are (36-)29.2(-23) Ma (Veranso-Libalah et al. 2018, q.v. for other dates in this area), (30.4-)24.9(-19.2) Ma (Reginato et al. 2020) or (38.5-)33.0(-29.4) Ma (Reginato et al. 2022).
[Dinophoreae [Rupestreeae + Rhexieae]]: ?
Age. This clade is some (49.5-)41.4(-35.3) Ma (Reginato et al. 2022).
3R. Dinophoreae Penneys & Veranso-Libalah
Shrubs; cortical vascular bundles 0 [confirm]; leaves sub-/unequal; A with various appendages, (pedoconnective 0); G (±) inferior; (fruit a berry - Dinophora); seed (cochleate - D.); n = 12.
3/8: Ochthocharis (5). West and Central tropical Aftica, Indochina to New Guinea. Map: Reginato et al. (2022: Fig. 2:20).
Age. Crown-group Dinophoreae are some (13.7-)7.4(-2.4) Ma (Reginato et al. 2022).
Age. This clade is estimated to be (46.3-)37.0(-27.6) Ma (Reginato et al. 2022).
3S. Rupestreeae Penneys & Goldenberg - Rupestrea Goldenberg, Almeda &aamp; Michelangeli
Shrubs; ?anatomy; abaxial K teeth +; anther pore minute, "encrypted", pedoconnective +, inconspicuous, connective appendages +; G 3(-4), semi-inferior; ovules 1/carpel; fruit dry, indehiscent, turgid cells in inner pericarp, sclereids 0; seeds 1/carpel, large [2.5-3.5 mm long], raphal scar long, antiraphe long, depressed, with incomplete septum internally; testa smooth; embryo curved; n/x = ?
1/2. Atlantic Forest, Bahia, Brazil. Map: Goldenberg et al. (2015: Fig. 5), Reginato et al. (2022: Fig. 2:18).
3T. Rhexieae de Candolle —— Synonymy: Rhexiaceae Dumortier
(Annual) herbs to shrubs; cortical vascular bundles +; A not dimorphic, (pedoconnectives 0); (G opposite C - Rhexia); seed reniform; testa surface complexly costate-tuberculate [tubercles multicellular]; n = 11, 12, 15, 18, x = 12.
3/21: Rhexia (13). E. North America from Nova Scotia southwards to tropical America, the Antilles. Map: Reginato et al. (2022: fig. 2:19).
Age. Divergence within Rhexieae started (40.8-)32.3(-23.3) Ma (Veranso-Libalah et al. 2018), (25.0-)22.2(-20.1) Ma (Reginato et al. 2020) or (37.5-)30.0(-21.1) Ma (Reginato et al. 2022).
Probably Melastomatoideae, ?relationships:
3. Feliciadamieae Penneys - Feliciadamia stenocarpa (Jacques-Félix) Bullock
Plant ±herbaceous; ?anatomy; petiole longer than lamina; flowers 5-merous; K lobes keeled, abaxial K teeth 0; A dimorphic, larger A with pedoconnective shortly produced, connective basi-dorsally elongated, subulate, basi-ventrally divided, spurred; G [3], inferior, much elongated, ridged, placentae angular-sinuous; ovules few, uniseriate, pendulous, "droits"; ?fruits; ?seeds; n/x = ? Note that the flowers are described as being purple, while in the image on POWO they are basically white...
1/1. West Africa (Guinea).
Evolution: Divergence & Distribution. The North American Rhexia - well, North American now - has been found fossil (as the distinctive seeds) throughout Eurasia in the Caenozoic (Michelangeli et al. 2012 and references), which has important implications for both dating and biogeographical scenarios. The identification as Melastomatoideae of leaves from deposits dated 60-58 Ma from Colombia, the first fossils of the family from South America, is also potentially important.
The topology of the tree in Reginato et al. (2020), and the taxa examined in the tribes recognized, should be compared with subsequent topologies for the family. Papers in Goldenberg et al. (2022) make it clear that relationships between the subfamilies, and between several of the tribes in Melastomatoideae, and genera within those tribes, are not yet fully understood, even if all genera have finally been placed in tribes; again, talking about evolution is difficult.
Renner et al. (2001, also Renner & Meyer 2001) thought that there was little substantial diversification within Melastomataceae until ca 30 Ma, and movement into Africa occurred still more recently some ca (18-)14(-10) Ma. Morley and Dick (2003), on the other hand, were inclined to think that the broad outlines of diversification in the family could be linked with major tectonic (drift) events, and there was much diversification, including the separation of the African/Malagasy clades, before ca 68 Ma, roughly when Madagascar and India separated; the family may have been of African origin. Clearly, these are incompatible scenarios. Renner (2004a, b) again suggested that dispersal, not drift, was more likely, with separate Miocene dispersal events resulting in the species found on Madagascar, for example, and dispersal, whether by long distance dispersal or across N-S and E-W land bridges, is favoured by Reginato et al. (2022), the family perhaps being of South American origin with subsequent dispersal events Neotropics → Afrotropics, and some further on to Indo-Malesia, etc., being dated to around 29.5-24.4 Ma (ibid. Table 5). Berger et al. (2015) date a shift in speciation rates in the family to after the divergence of Kibessieae, which they date to around 64 Ma, and place this in South America. The placement of the African Myrianthemum mirabile as sister to the otherwise Indo-Malesian Dissochaeteae is interesting; the New World Pyramieae are sister to the combined clade (L. Chen et al. 2023).
For a summary of diversity and distribution in Melastomataceae, see Ulloa Ulloa et al. (2022). Melastomataceae s.l. are centred in the New World tropics where some 70% of the family are to be found; with approaching 700 species in Amazonia alone, they are the fourth most diverse family there (Cardoso et al. 2017). There is a fair bit of geographical structuring of the major clades in the family, thus Goldenberg et al. (2008) found substantial geographical signal correlating with major clades in Miconia and its relatives (see also Penneys & Judd 2005; Michelangeli et al. 2008b, 2012, 2013; and Reginato & Michelangeli 2015, for example). For the diversification of highly speciose Miconia s.l., some 1,900 species, see Michelangeli et al. (2022d). The genus probably originated in Amazonia, the Brazilian M. mirabilis group being sister to the rest of the genus, and other basal clades are Brazilian. Then Miconia moved to the Andes and to east Brazil, and then to Mesoamerica and the Greater Antilles. Other major groups of Andean melastomes include Cyphostyleae, Melastomeae, Merianeae and Pyxidantheae; the Andes had reached its current height some time before the diversification of Miconia, etc. (Michelangeli et al. 2022d). Michelangeli et al. (2012) had noticed a rather similar distributional pattern in Melastomateae, which may have originated in white sand savannahs in northwest South America. Miconia is a major element in the Antillean flora, and Bécquer-Granados et al. (2008) and Michelangeli et al. (2008b) discuss the complex biogeography of the Antillean melastomes. It is estimated that 495 species of the family grow there, some 383 of which are Miconia (ca 18% of the genus), 342 being endemic, and largely made up of 4-5 major clades (Majure et al. 2022a, q.v. for much detail; 452 species from the Greater Antilles alone is the estimate in Ulloa Ulloa et al. 2022). The Caribbean Clade of Miconia includes ca 160 species, and Cuba plays an important role in its diversification, and also in that of other groups - Hispaniola is also important, while montane forests also factor in (Majure et al. 2022a). In Brazil, some 580 species of Melastomataceae (14% of the whole family), largely in nine major clades, grow in the Atlantic Forest (Michelangeli et al. 2015; similar estimates in Bacci et al. 2022b) with around 560 species in Miconia alone, over a quarter of the genus (Caddah et al. 2022). Some 138/215 species of Leandra (= Miconia s.l.) are from eastern Brazil (Reginato & Michelangeli 2015; Bacci et al. 2022b). Bacci et al. (2021b) note how the phylogeny/distribution of Bertolonia, with some 36 species restricted to the Atlantic Forest, reflects a major N/S break in the Atlantic Forest. Note that the crown-group age in Bacci et al. (2020) is around 30 Ma (the stem-group age is based on a mis-rooting), but Bacci et al. (2022b) later estimated the ages of 8/9 major clades of Melastomataceae there, including Bertolonia (the oldest), as being only (11.6-)8.4-1.0(-0.6) Ma, only a clade in Merianieae at (26.8-)18.6(-11.5) Ma being somewhat older. Merianeae seem to have diversified in the mid Miocene ca 12 Ma; along with the Andean uplift there was abiotic climatic niche evolution, facilitating evolution of the the clade, and there was a substantial uptick in diversification (Dellinger et al. 2024); diversification elsewhere in extra-Andean regions was more or less continuous (see also Meseguer et al. 2022). The evolution of the woody habit and of relatively leage flowers - pollinated by medium-sized bees - may be preaptions for the Andean diversification (Dellinger et al. 2024). Around 200 species of Lavoisiereae have radiated into the fire-prone Cerrado (see also Ecology & Physiology below), and other melastomes also grow there.
Basal in the largely Old World Sonerileae are New World genera (Wurdack & Michelangeli 2019; Y. Liu et al. 2022). However, the recently-described Benna, from Guinea, West Africa, is embedded in a clade of these New World taxa and may represent an independent migration from the New to the Old Worlds (van der Burgt et al. 2022) - as these authors note, such a distributional patten is not that uncommon.
Within Olisbeoideae, a clade of Memecylon from western and central Africa is sister to the rest of the genus (Stone 2014). (Renner & Meyer 2001; Renner et al. 2001; Renner 2004b) similarly, Amarasinghe et al. (2021) found two small African clades sister to the rest of the genus; they suggest that the origin of the genus is African, with subsequent long distance dispersal. Memecylon is particularly diverse in Madagascar, where there are about 140 species, many of which are very localized (Stone 2012, 2023: 136 species; Amarasinghe et al. 2021); other Melastomataceae are to be found in l.t.r.f. on Madagascar, and they have an overall high level of endemism (Muasya et al. 2011). In the Old World, endemicity is also high in Sri Lanka and India, for example (Stone 2022).
It has been suggested that buzz-pollinated Melastomataceae and their pollinators are trapped on an adaptive peak or plateau (e.g. Berger et al. 2015; Dellinger et al. 2018, but c.f. Gavrutenko et al. 2020: see also Solanaceae, Malpighiaceae), but the overall diversification of the family is quite extensive - so what is the trap? Furthermore, Dellinger et al. (2019a) found that in Merianieae, despite the presence of long-tubular porose anthers characteristic of buzz-pollinated flowers throughout the tribe, there have been two shifts to pollination by passerine birds in particular (see Dellinger et al. 2014 for the complex mechanisms evolved) and two to pollination by vertebrates (birds, bats, rodents) more generally. In Merianieae these shifts have occurred at high elevation locations in the Andes and probably within the last 5 Ma, and there exothermic bees are less efficient pollinators that endothermic vertebrates (Dellinger et al. 2021). However, the situation is complicated, because even at these higher elevations the floral morphology of some 2/3 Merianieae suggest pollination by bees, and there have been reversals to bee- from vertebrate-pollinated flowers (Dellinger et al. 2021). Somewhat similar correlations in Miconieae were found by Gavrutenko et al. (2020) in their extensive reconstructions of the evolution of 12 core plus 11 other floral characters scored for 358 species. Generalized floral characters, of which a polysymmetric androecium was perhaps central, were associated with taxa growing at higher altitudes, generalized pollination (again, problems with bees and buzz pollination), and rapid diversification rates (Gavrutenko et al. 2020). Compared with other floral organs, the androecium shows the most variation (Dellinger et al. 2019a; Melo et al. 2022 and references). For floral evolution in Leandra, see Reginato and Michelangeli (2016).
Abiotic dispersal is plesiomorphic in the family, while biotic dispersal has arisen some eighteen times and in both the Old and New Worlds, perhaps in association with passerine radiations, but there have also been reversals to abiotic dispersal (Reginato et al. 2020). Within Melastomatoideae, capsular fruits are associated with superior ovaries and fleshy fruits with inferior ovaries, although this is certainly not an absolute correlation (e.g. Clausing et al. 2000; Basso-Alves et al. 2017a). There is no particular connection between dispersal mode and range sizes or shifts in diversification rates, these latter appearing to be rather idiosyncratic (Reginato et al. 2020).
Basso-Alves et al. (2017b) looked at the development of the remarkable biseriate calyx of Leandra melastomoides, noting that there was an abaxial/upper/dorsal portion (a Vorläuferspitze?, = teeth in the characterizations above) and a broad basal/adaxial/ventral portion (= lobes above). There is only a single vascular bundle supplying each calyx member, and this divides unequally and periclinally; the larger, abaxial branch proceeds undivided into the tooth, while the smaller adaxial branch divides and innervates the lobe (Basso-Alves et al. 2017b). The young calyx primordium is largely made up of tissue that becomes the tooth, the lobe initially being a small projection on its adaxial side. Many Melastomatoideae have such teeth, although exceptions at the tribal level are common, and they have been placed as an apomorphy there. Those tribes that are recorded as entirely lacking teeth are considered to have lost them, the loss being an apomorphy for the pedoconnective clade - although all this is somewhat notional. There is more on floral Vorläuferspitze in Baum (1950, 1951a).
For wood anatomy and phylogeny, see van Vliet et al. (1981). Vessel:ray pits are simple; in Olisbeoideae, vessel:ray pits are half bordered and the rays are 2-5 cells wide and heterocellular, compared to 1(-4) cells wide and homocellular elsewhere in this clade. Considerable work has been carried out on S.E.M. studies of seed morphology in the family. Thus Ocampo and Almeda (2013) looked at the seeds of 234 species of Miconieae, attempting to standardize descriptions; they rejected the use of seed "types" (c.f. Groenendijk et al. 1996). Ocampo et al. (2014) compared phylogenetic analyses of molecular and morphological data, examining 364 species of Miconieae and 17 seed characters with up to 8 states, and found little phylogenetic signal in the seed characters (see their Fig. 1 for a spectacular polytomy), although there were perhaps some correlations with ecology. See also Ocampo et al. (2022) for seed characters and confirmation of the limited phylogenetic signal they have here.
Ecology & Physiology. Melastomatoideae in particular are an important component of the understory vegetation of tropical forests, especially in the New World. Thus genera like Blastus, Sonerila and "Phyllagathis", all Sonerileae, have a combination of modifications like variegation and other colouration patterns, iridescence and distinctive surface textures of their leaves, along with plastid modifications (Lee 1997, 2001; Y-S. Chen et al. 2017). For more on variegation, etc., in the leaves of taxa growing on the forest floor, see J.-H. Zhang et al. (2020), Begoniaceae-Begonia, Marantaceae, Orchidaceae, etc.. Melastomataceae in Amazonian forests are represented by many species with stems at least 10 cm across, but no species is common (ter Steege et al. 2013). Melastomataceae are also quite often early successional, and apomixis was found to be more common in pioneers or invasives (dos Santos et al. 2012). Epiphytes are quite common in Melastomatoideae, also in Ericaceae, another woody group, Piperaceae and Gesneriaceae in broad-leaved angiosperms, and also Orchidaceae, Bromeliaceae and ferns,. These epiphytes include ca 300 species or more in the palaeotropical Medinilla (Sonerileae). A 1986 estimate was that ca 230 species in the New World were obligate or facultative epiphytes, and these were particularly common in montane habitats; interestingly, obligate epiphytes, ca 85% of the total, have fleshy fruits (Renner 1986; Clausing et al. 2000). New World epiphytic melastomes may have distinctive anatomical traits that can be connected with water stress, but they do not have CAM photosynthesis (Ocampo & Almeida 2013b). For more on epiphytes in Melastomataceae, see Zotz, Weigelt et al. (2021: list), while Hietz et al. (2021) and Zotz et al. (2021) discuss the ecophysiological characteristics of epiphytes in general. Some Melastomataceae are scramblers, whether with hook-shaped roots, or climbing by roots that attach directly to the support; some of the latter taxa show extreme heterophylly, with pseudo-2-ranked leaves, one leaf of each pair being much reduced (see Zeigler 1925; Clausing & Renner 2001a; Gamba-Moreno & Almeida 2014 for anisophylly). Samra et al. (2025) found that anisophyly in Triolena, at least, was associated with conditions of high precipitation and high isothermality, but not with low light and understory habitats. Bacci et al. (2019) noted that herbaceous taxa tended to have angled fruits.
The family is overwhelmingly mesophytic. However, many Microlicia-Microlicieae (= Lavoisiereae) are adapted to the seasonally dry and fire-prone campos rupestres Cerrado vegatation of Brazil where they are notably diverse (over 200 species are known from the Cadeia de Espinhaço alone); the habitats that the genus favours are often rocky and with poor soils. Pacifico and Almeda (2022) list a number of xeromorphic features found in Microlicia and suggest that diversification here is associated with variation in niche preferences - up to ten species can grow together. Fritsch et al. (2004) thought that Rhynchanthera, which prefers rather more mesic conditions, was sister to other members of the tribe that they examined, other studies suggest that it is embedded in Microlicia itself (see also Simon et al. 2009; de Carmo et al. 2020: anatomy; Versiane et al. 2021: phylogeny, but c.f. Versiane et al. 2021; Pacifico & Almeda 2022). Some other melastomes grow in the Cerrado, and diversification of Microlicia, etc., has occurred within the last (ca 14-)9.8(-ca 6) Ma (Simon et al. 2009) or ca 3.7 Ma (Fritsch et al. 2004, 2007). Indeed, much diversification in this species-rich habitat has been within the last 5 Ma or so (Simon et al. 2009). Interestingly, ca 12 shifts to open habitats have occurred in African Melastomateae alone since the middle Miocene (Veranso-Libalah et al. 2018).
Pollination Biology & Seed Dispersal. For a summary of pollination in Melastomataceae, see Dellinger et al. (2022). Monosymmetry of the flower is common in Melastomatoideae and is most evident in the androecium, and the stamens, often distinctively different in colour from the petals, may form a serried rank on one side of the flower; the petals are usually more or less widely spreading. Buzz pollination is prevalent, flowers being visited by females of many species of bees in search of pollen and for which the flowers are an important pollen resource (Renner 1989; Harter et al. 2002; Melo et al. 2021). In Merianieae, bees may buzz individual anthers when the staminal appendages are large, or the whole androecium when they are smaller (Dellinger et al. 2021). Heteranthy (differences in shape between stamens of the one flower) and stamen dimetrism (size differences) are also common; these conditions represent extremes of a continuum (c.f. Melo et al. 2021: Fig. 1) and are often associated with differences in pollen size, ornamentation, quality, etc. (Trevizan et al. 2023). The colours of the anthers of the two whorls may also be strikingly different, e.g. yellow anthers in the inner whorl, dark-coloured anthers in the outer whorl (Microlicia s.l. - Pacifico et al. 2022). Valadão-Mendes et al. (2021) found that stamen dimorphism was linked with larger flowers and a greater diversity of pollinating bees. Heteranthy and associated features of the flower have been interpreted in the context of the "pollen dilemma", the pollen being needed both by the plant for pollination and by the pollinator for food (e.g. Westerkamp 1996; Velloso et al. 2018). The flowers are usually nectarless, and stamen dimetrism is less pronounced when rewards are other than pollen (Melo et al. 2021). In Dissochaeta the anthers of oppositisepalous stamens provide feeding pollen for the bees, while it is pollen from the oppositipetalous stamens that is involved in actual pollination (check: Kartonegoro et al. 2018). Pollen from stamens differing in morphology may differ in fertility, and in at least some cases it is pollen that is less fertile but borne in more conspicuous anthers that is more likely to be collected by the bee (Luo et al. 2008), or there may be more pollen in the pollinating oppositipetalous stamens (Velloso et al. 2018; see also Melo et al. 2021, 2022). Different species of bees tend to visit different melastomes, although the bees involved are not oligolectic; thus mass-flowering Miconia cinerascens in Brazil is visited by the stingless Melipona (Harter et al. 2002) which also visits other plants. Goldenberg et al. (2008) suggest quite complex relationships between anther morphology and pollinator in Miconia and relatives, and in Andean Miconia, not only has there been independent evolution of broader anther pores, but also white anthers (from variously coloured), dioecy, and polysymmetric flowers (Burke et al. 2012). Selfing in Bertolonia (Bertolonieae) involves germination of the pollen while still in the anthers, the pollen tubes reaching the stigma via the anther pores - a "pollen tube shower" (Passos et al. 2021). For more on buzz pollination, see Dellinger et al. (2019c) and elsewhere, and for anther dehiscence see Cortez et al. (2014).
Within Merianieae there are three quite distinct pollination syndromes, and these varied as much in characters specific to Merianieae as those in classical pollination syndromes - thus all three syndromes, the bee, "mixed vertebrate" (bird, bat, rodent) and "passerine" syndromes, had porose anthers (Dellinger et al. 2018). Transition from buzz pollination involves changes in how the pollen is released and in the rewards offered to the pollinator (e.g. Dellinger et al. 2018, 2021), and has been thought of as an escape from specialization (de Brito et al. 2016). Parts of the flower other than the tubular anthers (a "developmental constraint") evolved more quickly; the flower as a whole was not evolving, rather, functional modules within it were (Dellinger et al. 2019b). Although lacking conventional nectaries, some Melastomataceae have nectariferous anther connectives, the nectar exuding through cracks in the tissue, or more commonly through stomata (e.g. Blakea, Penneys & Judd 2013b); staminal nectar is also produced by bird-pollinated species of Brachyotum (Meyer et al. 2021). Nectar can also be produced on the corolla (Medinilla), the surface of connectives, epidermis of the inner wall of the hypanthium, or even on the stigma or on top of the ovary (some Miconia) - again, nectarostomata are generally involved (Varassin et al. 2008). In Axinaea nectar may be contained within expanded anther connective appendages. In such cases the contorted petals, although free, do not reflex, rather, they form a tube, and the anthers often open by longitudinal slits (although lacking an endothecium?); pollination is likely to be by birds and perhaps rodents (Renner 1989; Stein & Tobe 1989; Vogel 1997; Varassin et al. 2007, esp. 2008; Penneys & Judd 2013b). Pollination by tanagers (passerines) has recently been demonstrated in Axinaea, and there the birds eat a sucrose-rich appendage on the anthers which also acts as bellows, pollen being puffed out of the anther pores and dusting the bird's head at the same time (Dellinger et al. 2014). Axinaea grows at 1000-3600 m in the Andes, and some other Melastomataceae, mostly from higher altitudes, have also adopted vertebrate pollination and they, too, produce nectar as a reward (Varassin et al. 2008; Dellinger et al. 2014 and references; Brito et al. 2016; de Brito et al. 2017). There is bird pollination in Blakea, and here pollination is explosive, the pollen being discharged when the anthers are touched (Wester et al. 2016). Flowers of Miconia sect. Cremanium, whose stomatiferous anthers have broad pores, are visited by a variety of pollinators, and generalist pollination is derived from more specialized buzz pollination in this genus (Kriebel & Zumbado 2014). In Miconieae as a whole generalist pollination, involving flies, has evolved several times from specialist pollination, involving wasps and/or bees alone. Generalist vertebrate pollination is linked with short anthers, large anther apertures, nectar as a reward, altitude, and also with seed size, flowers pollinated by generalists tending to have fruits with fewer, larger seeds (Brito et al. 2016, 2017). However, in some species of Meriania at least, pollination seems to be bimodal rather than generalized, and the one flower has adaptations to pollination by separate functional groups of pollinators, here hummingbirds/bats, hummingbirds/rodents and flower piercers/rodents (Dellinger et al. 2019a, b), i.e. groups of rather different - one would have thought - vertebrates. For generalist pollination, see also Manrique Valderrama et al. (2022). In Miconia on Trinidad there is sequential flowering of different species of Melastomataceae at the one locality (Snow 1965); see Hosaka et al. (2016) for sequential flowering in general. Pseudanthia have been reported (Baczynski & Claßen-Bockhoff 2023: Table 1).
In many Olisbeoideae oil is the reward for the pollinator, and genera of Centridini, Exomalopsini, Euglossini and Tetrapedini collect exudate from the oil-producing anther glands, also collecting pollen by buzz pollination. Flowers with these glands (the great majority of species) are blue, rarely yellow, flowers without them are white (Buchmann & Buchmann 1981; Buchmann 1987), however, there is much infraspecific variability in flower colour (Morley 1976). Amarasinghe et al. (2021) discuss cauliflory, pollination and fruit dispersal in Memecylon.
Apomixis/polyembryony is common in Melastomatoideae growing in Cerrado and Campo Rupestre vegetation types in Brazil and it has been found in about one third of the species there; apomixis was more common in the widely-distributed species (dos Santos et al. 2012; Rodrigues & Oliveira 2012). In Miconieae, a tribe in which apomixis is common, apomictic species were little visited by pollinators (Brito et al. 2016); they total ca 70% of the whole tribe (Caetano et al. 2018; Melo et al. 2021 and references). Apomixis is autonomous, i.e. fertilization is unecessary, endosperm being at most slight in the whole order, so pollen fertility is quite often very low, and polyembryony is not uncommon (Caetano & Oliveira 2022). For apomixis and also hybridization in Leandra, see Reginato and Michelangeli (2015).
Within Melastomatoideae, capsular fruits are often associated with superior ovaries and fleshy fruits with inferior ovaries (e.g. Clausing et al. 2000; Basso-Alves et al. 2017a); for more on frugivory in Melastomataceae and its general ecological importance, especially for birds, see the next section. Thus 400 species of Sonerileae have fleshy fruits, and such fruits have probably evolved three times there (Quakenbush et al. 2024). In the New World, taxa with dry fruits tend to be commoner at higher altitudes and in savannas and more seasonal forests (Stiles & Rosselli 1993). A few taxa with dry, dehiscent fruits do have inferior ovaries, and in such cases the outer fruit wall may fall away, the fruit proper then functioning as an ordinary capsule (Michelangeli et al. 2008a); a group of genera with such fruits form a clade (Michelangeli et al. 2011: they also have five stamens opposite the sepals). Indeed, the ripe fruit, that is, a capsule, the product of the superior ovary, may be completely surrounded by the persistent hypanthium, and this has occasioned the description of several fruit "types" here (Bacci et al. 2019 and references). Splash-cup dispersal is known from a few Melastomataceae that are forest herbs, and in some vivipary is also recorded; seeds that are not dispersed may germinate in the capsule (Bacci et al. 2021a). In Bertolonia there is a variant of this mode of dispersal, the so-called "squirt corner" mechanism; here the falling raindrop forces the tiny seeds to the corners of the strongly trigonous capsules whence they get squirted out (Pizo & Morellato 2002). This mechanism is also known from Trioleneae (Bacci et al. 2022a).
Plant-Animal Interactions. Throughout the Neotropics fleshy fruits of Melastomatoideae are a major resource for frugivorous birds, smaller species in particular - i.e. unspecialized frugivores (Stone 1981; Messeder et al. 2020b). Thus fleshy fruits of Miconieae in the Colombian Andes comprise about 11.5% by weight of fleshy fruits but ca 32% of all fruit eaten by birds (Kessler-Rios & Kattan 2012). Taxa with fleshy fruits and small seeds are much eaten by tanagers, which, being "mashers", tend to remove seeds more than 2 mm long before swallowing the fruit, while manakins, "gulpers", eat fruits with larger seeds and eat the fruit whole; both groups of birds are restricted to the New World (Stiles & Rosselli 1993; see also Renner 1986). There seems to be no particular correlation between the thickness of the outer integument and the fleshiness of the fruit (Caetano et al. 2017). Miconia is a major keystone species (along with Cecropia and Byrsonima) in the context of the maintainance of general plant—frugivore (bird) interactions throughout the Neotropics (Messeder et al. 2020a). Messeder et al. (2020b) built up a database of animals that ate New World Miconia, and they suggested that the genus was a significant food source for 13% of all Neotropical birds, in particular some 70% of the primary frugivores - not to mention mammals, especially primates, reptiles, fish and ants, the latter attracted to the fruit pulp (seeds of Melastomatoideae have no arils) as well as being granivores. Thus Land (1983) recorded 23 species of birds eating fruits of a tree of Miconia trinervis in Guatemala. As mentioned, such birds tend to be rather small, while species of Miconia, notably common in secondary forest, produce many small fruits with numerous small seeds (Snow 1971; Messeder et al. 2022: Table 1, fruit and seed size for the whole family). Sympatric species of Miconia have evolved to ripen fruit at different times of the year (Messeder et al. 2020a, b), and Snow (1965) emphasised that this happened in the rather equable climate of Trinidad, where there were some 20 species of that genus. Messeder et al. (2020b: p. 589) highlighted "the role of Miconia in providing consistent and reliable food resources to frugivores over the years, as well as being one of the plant taxa most likely to sustain many animal species during periods of food scarcity in seasonal ecosystems [but see above] throughout the Neotropics". A comparison they make is with Ficus (Moraceae) which, along with Cecropia (Urticaceae), is another particularly important resource for frugivorous birds and other animals (see also Marshall 1985). In Old World Malesian forests sugar-rich fruits of the largely aseasonally-fruiting Melastomatoideae in the understorey are an important food source for birds (Leighton & Leighton 1983), for example, Melastoma has fruits which dehisce to expose mounds of small seeds with fleshy testas that are eaten by birds. Olisbeoideae also have fleshy fruits, but with fewer and larger seeds.
Clidemia heterophylla and species of Tococa like T. guianensis in western Amazonia live in close association with the ant Myrmelachista which creates mono- or oligospecific "devil's gardens" by injecting formic acid into the leaves of the surrounding vegetation, which is thus suppressed; there may be 1-2 million ants in these colonies (Morawetz et al. 1992; Davidson & McKey 1993; McKey & Davidson 1993; Frederickson et al. 2005; Salas-Lopez et al. 2014). Bacteria living off colony debris in Tococa may be eaten by rhabditid nematodes that are possibly in turn eaten by the ants (Maschwitz et al. 2016). Petiolar or laminar ant domatia are quite common in Pyxidantheae and Miconieae; in Maieta guianensis (Miconieae) ca 80% of the host plant's nitrogen is derived from the waste deposited by the ant Pheidole minutula that lives in the domatia (Solano & Dejean 2004). Other ants, small in size, may live between the long, dense hairs covering the plant - in some eight Neotropical genera, for instance (Davidson & McKey 1993). Pearl bodies, eaten by ants, are found on a number of Asian Sonerileae, and species of Pachycentria are common inhabitants of ant gardens, their seeds being attractive to ants (Clausing 1998). A few taxa, including most species of Medinilla from Magagascar, have swollen roots, but ants =do not often make their nests there (Clausing 1998). Chomicki and Renner (2015: Fig. S10) estimate that there may have been eight gains of myrmecophily in Miconieae alone, and almost as many losses.
Plant-Animal Interactions. Insects may get trapped on Melastomataceae that have glandular hairs - see LoPresti et al. (2015), also elsewhere.
Widespread and sometimes very extensive damage to anthers of Brazilian Melastomataceae can be caused by pollen-robbing Trigona bees (Renner 1983).
Plant-Bacterial/Fungal Associations. Graffenrieda emarginata, frequent on fungus-rich ridges in southern Ecuador, forms associations with both ecto- and endomycorrhizal fungi (Haug et al. 2004).
Vegetative Variation. A survey of nodal anatomy is needed; split laterals are probably quite common (pers. obs., see also R. A. Howard in van Vliet & Baas 1975), and in some taxa cortical bundles run along the raised angles of the stem. Hairs are very diverse here: "Melastomataceae ... has the greatest diversity of vestiture in the angiosperms" (Wurdack 1986: p. 1). Mentink and Baas (1992) carried out an extensive survey of foliar anatomy which needs to be integrated with tribal limits as they stand now (see their Table 1 - close to 6,000 entries). Van der Burgt et al. (2022) suggested that the leaves of Benna, a genus that they described, were alternate; more or less extreme anisophylly occurs in Melastomataceae, including Sonerileae, but alternate-leaved taxa are very uncommon here. In genera like Dissochaeta and Pternandra there can be prominent interpetiolar flanges even below the leaves, while in Meriania nobilis the flange forms a large-cup-shaped structure at the node (?anatomy). Vernation sometimes seems to be plicate because the secondary veins are so prominent; the leaves of Mouriri remain folded as they elongate. In some Sonerila in particular, stem and root bulbils and other means of vegetative propagation develop (Sekarathil et al. 2025).
Genes & Genomes. The basic cytology of Melastomataceae is poorly known, but Almeda and Penneys (2022) summarize what is known about chromosome numbers and they suggest a base number of 12 for several tribes; tetraploidy is common here. Almeda and Penneys (2022) also discuss the possible effects of apomixis on chromosome number.
Chemistry, Morphology, etc.. The roots have anthocyanins. Poay et al. (2011) described galloylated cyanogenic glucosides from Phyllogathis. The bark anatomy of species of Miconia from the Brazilian Cerrado varies (Milanez et al. 2021); a broad survey of this feature would be useful. For foliar sclereids, common in Olisbeoideae, see Rao (1957, 1983); they are associated with the endings of the veinlets, and some span the width of the lamina.
For general floral morphology and development, with a bibliography, see Basso-Alves and Teizeira (2022). The floral vasculature of Mouriri is distinctive (Morley 1953, 1976), with strong differential growth in the inferior ovary resulting in the branching points of the main carpel bundles being as it were drawn upwards and outwards - variation in placentation in Mouriri is extreme. It is as if the central part of the flower (the stylar base region) had become depressed, the lower central parts moved laterally, and the outer parts upwards; there may be two vascular bundles suppying each placenta that enter separately from each end (Morley 1953). Kibessioideae also have this kind of floral development, and they do have rather odd placentation, although overall with little variation, and there may be approaches to this kind of growth in some Melastomatoideae (Morley 1953, see also literature cited). Venkatesh (1955) found that the anthers of Memecylon had a normal endothecium, while in those of Mouriri the walls of the cells of the hypodermal layer were thickened all around. Turning to Melastomatoideae, Eggli and Almeda (2023) suggest that the androecium is usually obdiplostemonous. Basso-Alves et al. (2022b) described details of how the connective was modified in representative taxa; they noted terminal prolongations of the connective in Chaetogastria and Microlicia, the presence of a descending abaxial branch of the thecal vascular bundle, that pedoconnectives were not restricted to the so-called Pedoconnective Clade, and so on. The pedoconnective, an extension of the connective between the base of the thecae and the insertion of the anther on the filament, can be quite elaborate, sometimes having an appendage at the base. In Miconia, at least, the anther epidermis has a thick cuticle, except that in the pore area, which lacks a cuticle (Cortez et al. 2014). Anthers and anther wall development in Miconia are very diverse, some species having bisporangiate anthers, monocot wall development, crystals in the tapetal cells, etc. (Cortez et al. 2015). Polysporangiate anthers are found in some Melastomataceae, especially in Microlicia (Caetano et al. 2020). Ovary position varies extensively in the family, and Basso-Alves et al. (2017a) studied the formation of the hypanthium (= "perigynous hypanthium", when the floral apex changed from convex to concave) and the development of the inferior ovary (= "gynoecial hypanthium"), largely due to intercalary meristematic activity. Little is known about floral development, although Wanntorp et al. (2011b) studied that of Conostegia, in which there has been increase in floral meristicity, and some other genera; stamen primordia opposite the petals may split. For the development and anatomy of the fleshy fruit of Miconia, see Cortez and Carmello-Guerreiro (2008). The seed coat in Miconia varies considerably, but there seems to be little phylogenetic signal here (Ocampo & Almeda 2013a), and any similarities with that of Lithobieae (Penney et al. 2020) are likely to be parallelisms. The reports of stipules in seedlings of Olisbeoideae need confirmation - are they colleters?
For general information, see Penneys (2004 onwards)
Phylogeny. The clade [Mouriri + Memecylon] is sister to Pternandra, and these are in turn sister to the rest of the family in ndhF trees (Renner 1993); van Tieghem (1891b), Morley (1953, 1976), van Vliet et al. (1981) and others had early suggested this relationship, some characters of wood anatomy agreeing with it, and it was also recovered by Veranso-Libalah (2018: no comment, general relationships not the focus) and by Maurin et al. (2021) in a comprehensive analysis of relationships in Myrtales using nuclear genomes. X.-F. Zhang et al. (2021: plastomes, only one species of Olisbeoideae included) and W. J. Baker et al. (2021a: nuclear genomes, see Seed Plant Tree of Life) also found Pternandra linking with Olisbeoideae - in the latter Oxyspora (Sonerileae!) was sister to the whole family, but with very little support... In the Seed Plant Tree version i.2022 the Pternandra-Olisbeoideae connection remained - support was strong - andOxyspora is very much polyphyletic (five species included), but at least all the species are within Melastomatoideae. Note that Pternandra has also been found to be sister to all other Melastomatoideae, Astronieae perhaps next (Clausing & Renner 2001b: moderately good support in a 3-gene analysis; Renner 2004b; Wurdack & Michelangeli 2019). Recently Penneys et al. (2022) have provided a 9-gene (7 chloroplast, 2 nuclear) analysis of 2,435 species and 158/177 genera of melastomes, and the topology they found has largely been followed above (other than the arrangement of the first two subfamilies); branches with less than 50% bootstrap support have been reduced to polytomies.
Within Olisbeoideae, Spathandra may be sister to the large, palaeotropical Memecylon, although support is weak and it sometimes links with a [Votomita + Mouriri] clade, Lijndenia and Warneckia are successively sister to the whole group, although sometimes the two form a single clade (Stone 2006, 2002; Stone & Andreasen 2010). In both the 9-gene and other analyses of Penneys et al. (2022: Suppl. figs 1, 2) relationships are [Votomita [Mouriri [Memecylon [Warneckea [Spathandra + Lijndenia]]]]. Stone (2006) found that all six morphologically-based genera of Olisbeoideae had molecular support, although later Stone (2022) found that details of relationships based on the nuclear GapC gene differed substantially from those just mentioned - [[[Votomita + Mouriri] [[Spathandra + Memecylon]] [Lijndenia + Warneckea]], although support was quite often only moderate. Within Memecylon, the small African subgenus Mouriroidea was found to be sister to the rest (Stone 2014), although branch lengths along the spine of the phylogeny tended to be short, while Amarasinghe et al. (2021) found two African species to be successively sister to the rest of the genus, although support was weak, and Penneys et al. (2022) recovered M. occultum as sister to the rest of the genus.
Broad studies in Neotropical Melastomatoideae in particular over the last decade or so have used largely chloroplast genes to provide ideas of relationships (Guimaraes et al. 2010; Penneys & Judd 2010, esp. 2011: 111 morphological characters; Judd et al. 2010; Wurdack & Michelangeli 2019, etc.). A clade that included Merianieae and Miconieae was stable, and was sister to clade in which Bertolonieae were sister to a group that included Melastomateae, but further relationships there were not entirely stable (Reginato et al. 2016). These relationships are quite similar to those in Michelangeli et al. (2014) and Goldenberg et al. (2015). However, in the latter in particular Henriettieae associated with Astronieae, while the odd genus Rupestrea, there newly described (now = Rupestreeae), was strongly supported as being sister to a clade that included Lavoisiereae, Rhexieae, and Melastomateae, although more detailed relationships within that clade, the pedoconnective clade, were not well supported (Oldenberg et al. 2015); this clade was also recovered by Maurin et al. (2021), who also emphasized the instability of relationships in it. In a quite recent plastome analysis Henriettea (Henriettieae) was found to be sister to the 15 other Melastomatoideae in the study, which represented representatives of most major clades (Reginato et al. 2016). Veranso-Libalah et al. (2018) recovered relationships quite similar to those found by other authors, i.e. [[[Henrietteeae + Astronieae] [Merianieae + Miconieae]] [Pyxidantheae [[Cambessedesia [Dissochaeteae + Sonerileae]] [Rhexieae ... Melastomateae]]]], and also by Penneys et al. (2020), who in some analyses found the relationships [[Henrietteeae + Astronieae] [Lithobium [Merianieae [Eriocnemeae + Miconieae]...]]]. Lithobium (= Lithobieae) tends to move around the tree (Maurin et al. 2021). For Schwackaea, see Kriebel (2016a). See also van der Burgt et al. (2022; 2 nuclear ribosomal, three plastid markers, 245 spp.) for relationships; Sonerileae were the focus here. Maurin et al. (2021) found that in the Angiosperms353 nuclear analyses the content of the tribes were largely stable, even if some ideas of their relationships and of relationships within them might have to be revised. They also emphasized that Dissochaeteae and Sonerileae were unlikely to be sister taxa - c.f. Veranso-Libalah et al. (2018). Details of relationships in Kartonegoro et al. (2021) are somewhat different from the above, but such relationships were not the focus of their work.
Of tribal studies in Melastomatoideae, first Astronieae. Penneys (2013) suggested that the genera included were likely to be monophyletic, based on his preliminary analysis. Maurin et al. (2021) found that Tessmannianthus, a small Neotropical genus of quite large trees, was sister to other Astronieae, all Old World taxa; this position was confirmed by Mancera et al. (2022: Fig. 1), although one species of that genus may be outside the tribe.
Bertolonieae. Bacci et al. (2020, 2021b) looked at the phylogeny of Bertolonia in the context of its distribution, floral evolution, etc..
Dissochaeteae. Relationships in this clade have been clarified by Kartonegoro et al. (2021, 2022: Fig. 2, summary tree), Creochiton and Pseudodissochaeta are successively sister to the rest of the tribe,Medinilla, Dinophora and Ochthocharis being excluded (see Sonerileae for the first, Dinophoreae for the other two). L. Chen et al. (2023) found that the erstwhile Medinilla mirabilis was sister to the rest of the tribe.
Fritsch et al. (2004) looked at relationships within Lavoisiereae and i.a. found that Rhynchanthera was sister to the rest of the tribe. More recently, Versiane et al. (2021) looked at the phylogeny of the tribe in some detail, and extended the limits of Microlicia because of its paraphyly; Pacifico et al. (2022: 2 nuclear markers) also found a broad circumscription of Microlicia compatible with a tree in which generic segregates were scattered within a Microlicia s.l. clade, Tremblya being reduced to 11 species.
Lithobieae. The relationships of Lithobium cordatum, a distinctive species known for some 180 years, have long been unclear, but in a seven-gene analysis that included 24/27 genera that had previously been associated with it, Lithobium turned out not to link with any of them (Penneys et al. 2020).
Marcetieae are based on some Neotropical genera that used to be in Melastomateae; here nodes along the spine tend to have somewhat weak support, but the monophyly of the genera had stronger support (da Rocha et al. 2017). On the other hand, Pacifico et al. (2023: 8 markers, 35 spp. Marcetia) found four genera to be para- or polyphyletic; within Marcetia (monophyletic) M. acerosa was sister to the rest.
Melastomateae. Michelangeli et al. (2012; see also Renner & Meyer 2001) and Guimarães et al. (2020) looked at relationships here (the limits of the tribe have been restricted). Veranso-Libalah et al. (2017, esp. 2018, 2020: Dissotis area) examined he phylogeny of African Melastomateae, while Meyer et al. (2021) examined relationships in the New World Brachyotum-Chaetogastra area. For the dismantling of the polyphyletic Tibouchina - at least the members of the old sections usually held together - see Guimarães et al. (2020), and for Tibouchina s. str., see Jantzen et al. (2021). Veranso-Libalah et al. (2022: Fig. 1) provide a summary phylgeny of the tribe.
Generic limits in Merianieae have long been known to be difficult, and the composition of the tribe has fluctuated considerably over the yeare (Schulman & Hyvönen 2003; Michelangeli et al. 2022b). Bacci et al. (2019) found that genera like Macrocentrum and Meriania were polyphyletic in their study of the phylogeny of the general Sonerila area, and this was confirmed by Michelangeli et al. (2022b: Fig. 1 - 88 gene analysis) who also emphasized the considerable variation in the tribe.
Miconieae. For a phylogeny of the fleshy-fruited, speciose, and paraphyletic Miconia, see Michelangeli et al. (2004), Martin et al. (2008), Majure et al. (2015), Skean et al. (2018); previously delimited genera and sections are not monophyletic, in part because of over-reliance on anther morphology when delimiting taxa (Goldenberg et al. 2008; Burke et al. 2012). For the polyphyletic Leandra, see Martin et al. (2008); Pleiochiton and Leandra were recovered as successively sister to other members of the tribe (Maurin et al. 2021). Gamba and Almeida (2014) looked at relationships in the Octopleura clade of Miconia, the old Octopleura/Ossea area, where there were three main groups and much variation in indumentum, ovary position and seed morphology in particular. Goldenberg et al. (2018) examined relationships in section Chaenanthera from the Mata Atlantica; there seems to have been hybridization here. The M. discolor clade, almost one quarter of Miconia in the Atlantic forest, was studied by Caddah et al. (2022: 3 chloroplast and 2 nuclear genes). Judd (1989) and Judd and Skean (1991) provide morphology-based phylogenetic analyses of axillary- and terminal-flowered Miconieae respectively. For relationships in a somewhat expanded Conostegia, see Kriebel et al. (2014: variation in position of major clades, nice morphology) and especially Kriebel et al. (2016b), and for those in the speciose Leandra, see Reginato and Michelangeli (2015) - and of course these will end up in Miconia. Gavrutenko et al. (2020: 3 nuclear, 6 plastid markers) looked at relationships in 1,083 species of Miconieae, although character optimisation was the focus of their study - relationships were [[Mecranium [Cremanium[Conostegia + Caribbean species]]] [Tococa etc. [Clidemia + Miconia]]]. Michelangeli et al. (2022b: Fig. 3) summarize relationships in the tribe, emphasizing the rampant para-/polyphyly.
Pyramieae (Cambessedesieae). Cambessedesia rupestris, Merianthera burlemarxii and Huberia comosa are all sisters to the other species in these genera, but relationships between the genera are not well supported (Bochorny et al. 2019). The presence of cortical bundles and the absence of medullary bundles in Cambessedesia (Penneys et al. 2022) is an odd combination for this part of the tree - see also the Pedoconnective Clade
Penneys and Judd (2013b) analyzed both molecular and morphological characters in Pyxidantheae (Blakeeae).
Understanding relationships within Sonerileae has been complicated by the frequent inclusion of Dissochaeteae (even in 2022) and other tribes (Y. Liu et al. 2022: Table 1). Futhermore, genera like Phyllagathis are still very much polyphyletic - its species were found in eight of the fourteen clades recovered by Q.-J. Zhou et al. (2019a, see also b; Y. Liu et al. 2024) even though seven taxa of the genus still remained unplaced, and other genera in the tribe were also polyphyletic. Y. Liu et al. (2022: Fig. 2) provided a summary phylogany of the tribe, discussing the Old World members in 37 groups. Van der Burgt et al. (2022: 2 nuclear, 3 plastid markers, 145 spp.) also found extensive para-/polyphyly here: Amphiblemma, Bindia, Blastus, Medinilla, Oxyspora, Phyllagathis, and so on. More detail is provided by Q.-J. Zhou et al. (2022: 332 single-copy orthologs, SNP analyses; plastomes) who carried out a series of analyses on about 1/5 of the tribe, 205 spp.; they found 34 major clades. The focus in Quakenbush et al. (2024: 385 nuclear genes, 272 samples, and 81 plastid protein-coding genes, 224 samples) was on fleshy-fruited taxa, most of which belong to Medinilla. They found quite extensive conflict between plastome and nuclear trees, however, in an extensive discussion on relationships they characterized 15 groups in Medinilla which hopefully will serve as a basis for an infrageneric classification of that genus.
Classification. The inclusion of Memecylaceae in Melastomataceae was an option in A.P.G. II (2003), and this was formalized in A.P.G. III (2009). However, given the position of Kibessieae either sister to or immediately basal to Memecylaceae/oideae, Maurin et al. (2021) question whether Melastomataceae should be divided into subfamilies - "each of the main clades could be recognized as tribes" (ibid., p. ). If by this is meant that Melastomatoideae could be considered a single tribe, the nomenclatural implications of this would be unfortunate indeed - some formal grouping of clades in Melastomatoideae seems desirable. Indeed, over the last decade or so sampling of New World Melastomatoideae in particular has rapidly been extended and so needed generic and tribal changes are being made - e.g. Penneys et al. (2010: Henrietteeae), Michelangeli et al. (2011: Cyphostyleae), Penneys and Judd (2013a: Blakeeae (= Pyxidantheae)), Schulman and Hyvönen (2003: Merianieae), Veranso-Libalah et al. (2017, 2020: African Melastomateae, Dissotis pulverized in the latter paper), Bochorny et al. (2019: Cambessedesieae/Pyramieae, three monogeneric tribes vs an extended but undiagnosable tribe - the latter option seems reasonable) and Guimarães et al. (2020: Tibouchina divided). The limits of Microlicia have been extended (Versiane et al. 2021).
For generic limits in Miconieae, see Goldenberg et al. (2008 and references) and in particular Michelangeli et al. (2022a); up to 25 genera have been recognized here in the past. Majure et al. (2013) discuss the homoplasy of previously-used "generic" characters and argue for a broadly delimited Miconia (see also Majure et al. 2015, 2018; Skean et al. 2018, etc.); 583 species from generic segregates - which were mostly para- or polyphyletic - have recently been transferred to the genus (Michelangeli et al. 2019) and a broad circumscription of the genus seems reasonable - and is being accepted (Michelangeli et al. 2022a). An infrageneric classification is gradually being developed - thus Caddah et al. (2022) placed the 77 species of the M. discolor clade from the Brazilian Atlantic Forest in Miconia supersection Discolores, which they divided into three sections, however, some species were not placed in sections. Within Sonerileae, Y. Liu et al. (2024) help in the dismemberment of Phyllagathis, recognizing Perilimnastes while Quakenbush et al. (2024) extended the limits of Medinilla.
For a summary of the classification of the family, see Ulloa Ulloa (2022), and for individual tribes, see papers in Goldenbrg et al. (2022); for names in the family, see also MEL names (Renner et al. 2007c).
Thanks. I am grateful to S. Renner for comments.
[Crypteroniaceae [Alzateaceae + Penaeaceae]]: vessel-ray and vessel-parenchyma pits half bordered; foliar sclereids +; stipules +, minute; A = and opposite C; endothecium at most ephemeral; fruit a loculicidal capsule; endotegmen not fibrous.
Age. Renner et al. (2001) thought that this node was only ca 21 Ma, Tank et al. (2015: Table S2) about 39.8 Ma, while Conti et al. (2002: calibration in part based on drift events) estimated it to be 141-106 Ma. The crown group estimate in Moyle (2004) was (78.6-)68(-57.4) Ma, and that in Morley and Dick (2003) was ca 68 My; ca 52 Ma was the estimate in Sytsma et al. (2004) and (66-)53(-40) Ma that in Berger et al. (2015). Rutschmann et al. (2004) used a variety of analytic methods, and the ages they obtained spanned almost all the others, around (152.6-)109.0-58.9(-35.9) Ma.
Evolution: Divergence & Distribution. Renner et al. (2001) thought that the dispersal was responsible for the distributions of taxa in this clade, while Conti et al. (2002) thought that many of the distribution patterns reflected vicariance events, specifically continental drift (see also Morley & Dick 2003).
Schönenbergerand Conti (2003) discuss the floral evolution of the whole group.
Chemistry, Morphology, etc.. For gross morphology, see van Beusekom-Osinga and van Beusekom (1975). Anatomy is very variable, and van Vliet and Baas (1975) provide detailed comparisons within Myrtales. Laterocytic stomata are known i.a. from Alzatea, Dactylocladus, and Rhynchocalyx (Baranova 1983). For perianth morphology, see Schönenberger and von Balthazar (2006). Details of internal exine structure of Alzatea are similar to those of some Crypteroniaceae; for pollen of much of this group, see J. Muller (1975).
Phylogeny. Some of the relationships within this clade are only weakly supported (see Schönenberger & Conti 2003).
Classification. Van Beusekom-Osinga and van Beusekom (1975) included Alzateaceae and Rhynchocalycaceae in their expanded Crypteroniaceae. However, such expansion was deemed too radical, but Penaeaceae has been moderately broadened to include Oliniaceae and Rhynchocalycaceae (A.P.G. III 2009).
CRYPTERONIACEAE A.-L. de Candolle, nom. cons. - Synonymy: Henslowiaceae Lindley - Back to Myrtales
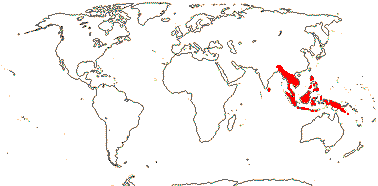
Trees; cork also subepidermal; septate fibres 0, tracheids +, pits bordered; nodes 3:3 [by split laterals], 1:3 with girdling bundles, cortical bundles ± developed or 0; sclereids +/0; plant glabrous (hairs unicellular); petiole bundles annular (with medullary bundle) or arcuate, wing bundles +; stomata paracytic (anomocytic - Dactylandra); lamina with secondary veins pinnate to palmate; plant polygamodioecious, or flowers bisexual; inflorescence racemose (spicate), with long branches; flowers 4-5-merous; C 0, 4-5; (A 2x K), connective thickened apically or not; (pollen bisyncolporate); G [2-6], ± inferior, placentation parietal or basal, (transseptal bundles +), style usu. slender, stigma capitate; ovules 1-3 or many [Crypteronia]/carpel, parietal tissue 2 cells across, nucellar tissue disintegrates early, (endothelium + - Axinandra); fruit a capsule, flattened or not, K deciduous; seeds winged, exotestal cells elongated, endotesta crystalliferous, endotegmen tanniniferous, other layers ± degenerate; n = 8, x = 8.
3/10: [list] - Crypteronia (4). South East Asia, Malesia, Sri Lanka. Map: from van Beusekom-Osinga (1977).
Age. Conti et al. (2002, see also Conti et al. 2004; Rutschmann et al. 2004, esp. 2007) suggested an origin any time from the Early to Late Cretaceous, a later date being favoured (60-45 Ma in Conti et al. 2002). Again, crown-group estimates in Moyle (2004) are much younger (48.6-)39(-29.4) Ma, while those in Rutschmann et al. (2004, see also 2007) were 82.6-17.9 Ma.
Evolution: Divergence & Distribution. Conti et al. (2002, see also Conti et al. 2004; Rutschmann et al. 2004, esp. 2007; Datta-Roy & Karath 2008) suggest that Crypteronia and relatives rafted from Gondwana (Africa) to Asia via India. For Moyle (2004), given the younger ages he proposed for the group, drift could not be involved.
Plant-Animal Interactions. In West Malesia, at least, there are interactions between Crypteronia, the ant Cladomyrma, and pseudococcid scale insects (Moog 2005). Bacteria-eating nematodes are also conspicuous inhabitants of rubbish dumps inside the domatia in C. griffithii (Maschwitz et al. 2016).
Chemistry, Morphology, etc.. Some information on Crypteronia and relatives is taken from Tobe and Raven (1983b, 1987b, c) and Renner (2006b: general), also Mentink and Baas (1992: foliar anatomy).
Phylogeny. Dactylocladus is sister to the other two genera, but with only weak support (Conti et al. 2002).
Previous Relatiosnhips. Crypteroniaceae have been included in Melastomataceae in the past (e.g. van Vliet et al. 1981; Mentink & Baas 1992).
[Alzateaceae + Penaeaceae]: vesturing of pits globular [?level]; septate fibres +, fibre tracheids ?; style stout.
Age. Ages for this node in Rutschmann et al. (2004) were around (135.6-)92.4-53(-26) Ma and in Gonçalves et al. (2020a) (62.7-)36.3(-14.3) Ma.
Chemistry, Morphology, etc.. For vestured pits, see Carlquist (2017a).
ALZATEACEAE S. A. Graham - Back to Myrtales - Alzatea Ruíz & Pavón
Trees or shrubs; plants not Al accumulators, myricetin 0; vesturing spread over inside of vessels, vessel-ray and vessel-parenchyma pits simple; branches tending to be several together; nodes 3:3, cortical bundles +; sclereids +; petiole bundle annular and with wing bundles; stipules 0; flowers small, 5(-6)-merous; K pointed in bud, C rudimentary even in bud; anther thecae along apical margin of connective, connective wide, with backwards-directed appendage, filaments short; pollen pseudocolpi 0 (+, faint0; G [2], placentation intrusive parietal and with an incomplete septum, transseptal bundles +, stigma capitate; ovules many/carpel, micropyle endostomal; megaspore mother cells several, embryo sac bisporic [chalazal dyad], eight-celled [Allium-type]; capsule flattened, K persistent; seeds several, winged all around, with hair-pin bundle; exotestal cells low, with irregularly sinuous anticlinal walls, everything else collapsed; suspensor small; n = 14, x = 14.
1 [list]/?2. Costa Rica to Peru. Map: see Silverstone-Sopkin and; Graham (1986). [Photo - Flowers.]
Evolution: Plant-Bacterial/Fungal Associations. Roots of Alzatea verticillata have a considerable variety of mycorrhizal morphologies and have a comparable variety of mycorrhizal associates; they are reported to have ectendomycorrhizae, with both a Hartig net and arbuscules formed by a glomeromycote (Peterson 2012 and references), and mucuromycotes have also been found here (Beck et al. 2007).
Chemistry, Morphology, etc.. See S. A. Graham (1985, 2006) for general information on Alzatea.
PENAEACEAE Guillemin - Back to Myrtales
Trees or shrubs; lamina with "glandular" tip; anthers polusporangiate [walls tapetal]; parietal tissue 3-4 cells across; x = 10.
9 [list]/29. E. and S. Africa, overwhelmingly South African, also St Helena. 3 groups below.
1. Rhynchocalyceae Beusekom —— Synonymy: Rhynchocalycaceae L. A. S. Johnson & B. G. Briggs - Rhynchocalyx lawsonioides Oliver
Myricetin 0; branches tending to be several together; cork cortical; petiole bundle arcuate, sclereids + [not in stem?]; stipules colleter-like; flowers 6-merous; K pointed, C lobed; A loculi all opening separately, epidermis only persisting; G [2 (3)], placentation parietal, transseptal bundles +, stigma ± punctate; ovules many/carpel, micropyle endostomal, nucellar cap ca 3 cells across; megaspore mother cells several; capsule flattened; seeds several, winged, embryo basal; exotesta tanniniferous, outer wall lignified, exotegmen not fibrous.
1/1. South Africa, coastal Natal and Transkei.
[Penaeeae + Olinieae]: plants not Al accumulators; non-hydrolysable tannins +; hypanthium well developed; pollen grains smooth, foot layer and tectum thick; exotegmen fibrous.
2. Penaeeae A. de Candolle —— Synonymy: Plectroniaceae Hiern
± Ericoid shrublets; tracheids +, with vestured pits; mesophyll with tracheoidal cells and/or ± brached fibres/sclereids; leaves often sessile, lamina (isobifacial), (lacking "glandular" tip), stipules ± colleter-like; flowers axillary, 4-merous; K petal-like, C 0; (connective with branched vascular bundle), (filaments straight in bud); G 4, opposite petals, style also filiform, stigma capitate or lobed; ovules 2-4/carpel, parietal tissue ca 3 calls across, chalazal nucellar region massive; embryo sac tetrasporic, 16-celled [Penaea type]; seeds with funicular elaiosome; exotestal cells much developed or endotestal cells much elongated, other layer crushed, endotegmen fibrous[?]; endosperm pentaploid, chalazal ["basal"] endosperm haustorium +, embryo suspensor 0, hypocotyl massive, cotyledons tiny, root cap 0
7/23: Stylapterus (8). South Africa, S. and S.W. parts of the Cape. [Photo - Habit.]
3. Olinieae Horaninow - Olinia Thunberg —— Synonymy: Oliniaceae Harvey & Sonder
Cork subepidermal; cyanogenic compounds +; veinlets with terminal sclereids; stomata variable; stipules cauline-on leaf base; flowers (4-)5-merous; ?epicalyx +, small, K ± spatulate, C concave, thick ["scale-like"], hairy; anthers polysporangiate, filaments very short; pollen heteropolar; G [(2-)4-5], opposite sepals, transseptal bundles +, stigma ± clavate, (commissural); ovules 2-10/carpel, apotropous, straight/campylotropous, outer integument 3-5 cells across, chalaza strongly vascularized, hypostase +; fruit drupaceous, 1-seeded, K not persisting; exotegmic cells fibrous; "conspicuous suspensor" 0, cotyledons spirally twisted or irregularly folded; n = 12, ?15, ?20.
1/5. Africa, St. Helena. Map: from Coates Palgrave (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. 1. (2003). [Photo - Fruit, Flowers.]
Evolution: Divergence & Distribution. Penaeeae are restricted to the Cape Floristic Region (Linder 2003), and the rest of the family has African connections - and also with St Helena...
Pollination Biology & Seed Dispersal. Penaeeae often have ant-dsipersed seeds (Lengyel et al. 2010).
Genes & Genomes. The haploid chromosome number of Olinia is 12, according to Takhtajan (1997), but c.f. Goldblatt (1976).
Chemistry, Morphology, etc.. Crushed or broken plant parts of Olinieae smell of almonds; they contain the cyanogenic glucoside, prunasin.
The interpretation of the perianth of Olinia is a matter of some dispute; here I follow Schönenberger and Conti (2003) although the exact nature of the outer whorl of very small appendages is still unclear (described as "?epicalyx" above). Nectar is secreted from the base of the hypanthium. From the various accounts in Kubitzki (2006, see below; Basso-Alves & Teixeira 2022) the anthers often seem to be incurved, at least in bud. The ovules have been reported as being apotropous and ascending (Baillon 1877).
See Van Wyk (in Dahlgren & Van Wyk 1988), Schönenberger (2006: Rhynchocalyx), Schönenberger et al. (2006: Penaea), etc., and von Balthazar and Schönenberger (2006: Olinia) for general information; for anatomy, see Dickie and Gasson (1999: Penaea etc.), for embryology, see Tobe and Raven (1984b, c, 1985b) and Stephens (1909b).
Phylogeny. M. Sun et al. (2016) found that a clade [Olinia + Rhynchocalyx] was weakly supported as sister to the rest of the family. Maurin et al. (2021) noted that the position of Glischrocolla was not stable.