EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CHLORANTHALES [[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]]]: sesquiterpenes +; (microsporogenesis also simultaneous); seed endotestal.
[[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]] / MAGNOLIIDS / MAGNOLIANAE Takhtajan: (neolignans +); root cap meristem open; vessels solitary and in radial multiples, (with simple perforation plates in primary xylem); (sieve tube plastids with polygonal protein crystals); lamina margins entire; A many, spiral [possible position here], extrorse; ovules with hypostase, nucellar cap +, raphal bundle branches at the chalaza; antipodal cells soon die.
[CANELLALES + PIPERALES]: flavonols, aporphine alkaloids +; nodes 3:3; G whorled.
PIPERALES Dumortier - Main Tree.
Plant ± herbaceous, growth sympodial; sesquiterpenes [e.g. γ-elemene] +; stele initially with distinct bundles; wood storied, with broad rays, interfascicular cambium producing secondary rays [lacking fusiform initials], vessel elements in radial files, with simple perforation plates; starch grains compound; nodes often swollen, ?anatomy; ?stomata; leaves two-ranked, lamina heart-shaped, secondary veins palmate; A in 3's; G occlusion?; seed ± tegmic, endotegmen tanniniferous; PHY E gene absent. - 4 families, 17 genera, 4,170 species.
Includes Aristolochiaceae, Hydnoraceae, Piperaceae, Saururaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Magallón and Castillo (2009: note topology) offer estimates of 175 and 119 Ma for relaxed and constrained penalized likelihood datings of crown group Piperales, Wikström et al. (2001) an age of (139-)133, 122(-116) Ma, Bell et al. (2010) ages of (138-)119, 104(-87) Ma, and Magallón et al. (2013, 2015) ages of around 110 Ma and 105.4 Ma respectively; ages of (124-)101(-76.5) Ma were suggested by Naumann et al. (2013), (158.1-)151.6, 105.6(-88.1) Ma by Massoni et al. (2015a), (174-)148(-124) Ma by Salomo et al. (2017: a variety of other ages, too), and as little as 103.5-51.5 Ma by Morris et al. (2018).
For an Early Cretaceous fossil showing some similarities with this group, see Friis et al. (1995). Silvestro et al. (2021) estimated that the time-of-origin of Aristolochiaceae was about 104.5 Ma.
Evolution: Divergence & Distribution. Friis et al. (2005b) suggested that Appomattoxia, from the Early Cretaceous, might belong somewhere around Piperales (c.f. Friis et al. 1995, 2011), but relationships with Chloranthaceae or Amborella have also been suggested (Doyle & Endress 2010).
For the evolution of woodiness in the order, see Trueba et al. (2015). Although I have tentatively called Piperales as a whole herbaceous, this depends on the definition of "woody" (see also Wagner et al. 2014; Trueba et al. 2015). For some thoughts on floral evolution in Piperales, see Remizowa et al. (2005b). Piperales are characterised by having notably small seeds, other magnoliids and ANITA grade angiosperms having substantially larger seeds (Moles et al. 2005a; Sims 2012). Although the Piperales are commonly divided into a group without a perianth, i.e. Piperaceae and Saururaceae, and a group with a perianth, the rest of the order (e.g. Joste & Wanke 2024), the latter feature is of course a plesiomorphy.
Ecology & Physiology. G. Liu et al. (2014) noted that the litter of Piperales tended to break down fast compared with that of other magnoliids, and they noted that many members of this order were smallish plants and hence are unlikely to have a substantial effect on nutrient cycling.
Pollination Biology. There are a number of reports of delayed fertilization in Piperales, including in some Piperaceae (Sogo & Tobe 2006d for references).
Genes & Genomes. Jost and Wanke (2024) looked critically as the variation of the plastome in autotropic Piperales and found problems in many earlier publications. They did fid variation in the boundaries of the IR and large SC region, the SSC-IR junction being relatively stable. There is a fair amount of variation in the ycf15 gene and the ccmA gene seems to have pseudogenized in a number of Piperales (Jost & Wanke 2024). For the PHY gene, see Matthews et al. (1995).
Chemistry, Morphology, etc.. Carlquist et al. (1995) suggest a number of wood anatomical characters that may be common to this clade, for instance, wood in some Aristolochiaceae and Piperaceae is storied (Carlquist 1992a, 1993); Trueba et al. (2015: esp. Table 1) compared wood anatomy across the order. Peperomia is described as having 3:3 nodal anatomy, while "five or seven separate strands" enter the petiole of Piper (Sinnott 1914). Isnard et al. (2012) disucuss growth form and anatomy in Piperales in some detail; Aristolochia and Piper are particularly variable in growth habit. Most taxa in the clade are sympodial in that the stem is put together by succesive axillary innovations. Inflorescences are in general terminal, rarely axillary, although Isnard et al. (2012: Fig. 17B) suggest that growth in Piperaceae is monopodial; the climbing stage of Asian Piper is monopodial, but the inflorescences are terminal in sympodial plagiotropic branching systems.
The much reduced flowers of the [Piperaceae + Saururaceae] clades can be described as monosymmetric (Tucker 1984), and so it is conceivable that monosymmetry is an apomorphy for the whole order. Variation in embryo sac morphology in the whole clade is very considerable, but there are now attempts to put this in a phylogenetic context (e.g. Madrid & Friedman 2008a, 2008b, 2009; see also Haig 2020).
For some information on lianes, etc., see Rowe and Speck (2005), on crystals, etc., Horner et al. (2013, esp. 2015 - considerable variation), and for floral development, see Tucker and Douglas (1996).
Phylogeny. The pairing [Piperaceae + Saururaceae] has usually been strongly supported as sister to Aristolochiaceae (e.g. Neinhuis et al. 2001, Nickrent et al. 2001). Discussion over other relationships in the order hinge on the circumscription of Aristolochiaceae. Neinhuis et al. (2000) suggested that Lactoridaceae were not to be included in Aristolochiaceae, although subsequent analyses have tended in the opposite direction (e.g. Neinhuis et al. 2005: Lactoridaceae sister to Aristolochioideae, support weak). Similar relationships were found by Davis et al. (2004: support rather weak - ±70%, Hydnoraceae not included [= −Hyd.]). In the two-gene analysis of Wanke et al. (2007: −Hyd.) support for Lactoridaceae as sister to Aristolochioideae was quite strong (82% bootstrap: see also Borsch et al. 2005; Qiu et al. 2005; Soltis et al. 2007a), however, the position of Asaroideae was uncertain; it might be sister to [Lactoridaceae + Aristolochioideae] (most common) or to [Piperaceae + Saururaceae]; Hilu et al. (2003: matK analysis alone, −Hyd.) also thought that Aristolochiaceae were paraphyletic and included the rest of the order. Kelly and González (2003) claimed that a morphological phylogenetic analysis strongly refuted the idea that Aristolochiaceae s. str. were not monophyletic; molecular data and the coding of morphological data were to "blame" (ibid.: p. 240) for the possibility that Lactoris might end up in the family. Nickrent and Blarer (2005) found moderate support for the clade [Hydnoraceae + Aristolochioideae]. See also the various analyses in Naumann et al. (2013: supplementary material). Massoni et al. (2014) recovered the relationships [Asaroideae [Hydnora [Lactoris + Aristolochioideae]]], although support was mostly rather weak. H.-T. Li et al. (2021: plastome analyses) also found relationships within Piperales somewhat uncertain, but this may in part be a sampling issue, and along the same lines, B. Zhu et al. (2020) recovered the plastome relationships [Asarum [[[Sarcandra + Chloranthus] [Aristolochia + Houttuynia]]]. For relationships in the order, see also an Angiosperms353 probe set analysis of the magnoliids as a whole by Helmstetter et al. (2024: 199 genera, ca 3/4 the total).
As is evident from the paragraph above, relationships of the parasitic Hydnoraceae (often placed in Hydnoroideae) have been uncertain, although they have quite consistently been placed in this general area (e.g. Barkman et al. 2007: see also Nickrent & Duff 1996; Blarer et al. 2000; Nickrent et al. 2001, 2002). However, W. J. Baker et al. (2021a: see Seed Plant Tree, −Hyd.) found that Lactoris by itself was on the stem of the whole magnoliid clade - relationships were [Lactoris [Ceratophyllum [Chloranthaceae [[Canellales + remaining Piperales] [Laurales + Magnoliales]]]]]. The two (Sauruma, Aristolochia) other Aristolochiaceae they sampled were sister to [Piperaceae + Saururaceae]. In a complex series of analyses involving seven Aristolochiaceae s.l. (and nine taxa from Piperaceae and Saururaceae), 83 (or 21 - Hydnoroideae) plastid genes, 44 mitochondrial genes and 10 nuclear genes, Jost et al. (2021) recovered the relationships [Asaroideae [Hydnoroideae [Lactoris + Aristolochioideae]]]. Support was generally strong, although less so for the [Lactoris + Aristolochioideae] clade, while Hydnoroideae were on a very long branch (Jost et al. 2021). Things get more complicated in the i.2022 version of the Seed Plant Tree where Hydnora was associated with Ceratophyllum, Lactoris was the next branch up and sister to monocots, while Prosopanche was embedded in Orchidaceae; other Aristolochiaceae s.l. were in their expected positions. Indeed, where Hydnoroideae were to go was often unclear. However, Helmstetter et al. (2024) found that Hydnoroideae=Hydnoraceae were sister to all other Piperales, although not in all analyses, while in the Seed Plant Tree viii.2024 version both Lactoris and Hydnoraceae (both genera were included) are very much isolated, the former even being placed in the eudicots near Proteales... See also Zuntini et al. (2024), where relationships in Piperales are [Hydnoraceae [[[Saruma + Asarum] [Lactoris + Aristolochia]] ...]. The nuclear analysis in Hatt et al. (2024a) was focused on Piperales, and there Hydnora and Prosopanche were in a clade that included Aristolochia and that was sister to the rest of the order.
Classification. Classification is another issue in the area surrounding Aristolochia. Having recovered the relationships [Asaroideae [Hydnoroideae [Lactoris + Aristolochioideae]]] (names are those in APWeb as of xii.2021), some, like Jost et al. (2021) and Helmstetter et al. (2024), prefer to recognise all four as families. However, it seemed then best to change nothing, and given the relationships this still seems the best course of (in)action; having Hydnoraceae as sister to the rest of the order (e.g. Helmstetter et al. 2024, also consistent with the topology in Zuntini et al. 2024) would be incorrect and splitting Aristolochiaceae unnecessary.
Previous Relationships. Takhtajan (1997) placed Aristolochiales in Magnolianae, his Lactoridanae were monotypic, although placed immediately after Laurales and before Aristolochiales. In some floral details, Saururaceae are very like Acoraceae (Buzgo & Endress 2000), e.g. they both have monosymmetric flowers, but these probably represent convergences. Similarly, the three-merous perianth and adaxial prophylls that seem to suggest a relationship between Piperales and monocots (and Nymphaeales), the now unlikely palaeoherb hypothesis (for which see e.g. Donoghue & Doyle 1987), also represent parallelisms. Interestingly, the foliar vasculature of Piper, with its mixture of acropetally and basipetally developing traces (Balfour 1958b), is also monocot-like, while Rousseau (1927) thought that Piperaceae were a "famille par enchainement", in this case linking Chloranthaceae and Saururaceae (Peperomia was rather more different).
Synonymy: Aristolochiales Berchtold & Presl, Asarales Horaninow, Hydnorales Reveal, Lactoridales Reveal, Saururales Martius - Piperineae Shipunov - Aristolochianae Doweld, Lactoridanae Reveal & Doweld, Piperanae Reveal - Piperidae Reveal - Aristolochiopsida Bartling, Asaropsida Horaninov, Piperopsida Bartling
ARISTOLOCHIACEAE Jussieu - Back to Piperales
Flavonols +; ring of fibres (+ sclereids) in cortex; nodes 3:3-5; stomata anomocytic; hairs uniseriate; prophyll single, adaxial; lamina vernation conduplicate; inflorescence cymose, monochasial, flowers leaf-oppposed, quite large, polysymmetric; P with odd member adaxial, uniseriate, 3, valvate, connate; A 6, in P-opposed pairs, anthers extrorse, filaments ± 0, connective extended apically; carpels basically free; ovules many/carpel, micropyle endostomal, outer integument ca 2 cells across, inner integument 2-3 cells across, nucellus somewhat beaked, parietal tissue 4-6 cells across, nucellar cap +; fruit a follicle; exotestal cells enlarged and thickened or not, endotesta thickening various, containing crystals or not, exotegmen and mesotegmen crossing fibres, (exotegmen radially elongated), endotegmen often with reticulate thickenings, (tanniniferous); endosperm oily, embryo undifferentiated; x = 7 (?6), nuclear genome [1 C] (0.02-)1.329(-86.525) pg.
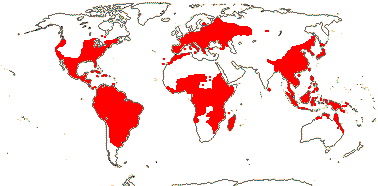
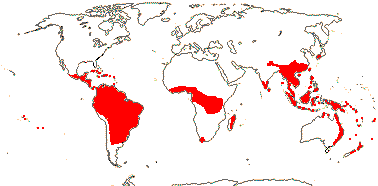
7-9 [list: as subfamilies]/682 - three groups below. World-wide, not Arctic. Map (Asaroideae + Aristolochioideae): from Poncy (1978), Fl. N. Am. vol. III (1997), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), de Groot et al. 2006 - S. America?, and Australia's Virtual Herbarium (consulted xii.2012).
Age. Wikström et al. (2001) suggested an age for crown Aristolochiaceae of (128-)122, 108(-102) Ma, Bell et al. (2010) ages of (126-)104, 91(-72) Ma, Naumann et al. (2013) an age of about 103.4 Ma, Massoni et al. (2015a) ages of (147.3-)140, 81.7(-52.4) Ma and Salomo et al. (2017) ages of (157-)129(-100) Ma; an age of ca 102.6 Ma for a clade [Lactoridaceae + Aristolochiaceae] was suggested by Tank et al. (2015: Table S2).
Assign to appropriate hierarchical level: cuticle waxes as annular rodlets, palmitone the main wax; (filaments slender), style hollow
1. Asaroideae O. C. Schmidt
Plants rhizomatous; sieve tube plastids lacking starch, with cuneate protein crystalloids and a large polygonal protein crystal; (pericyclic fibres 0); crystals and crystal sand +; (terminal cells of hairs glandular); flowers solitary, terminal; G inferior, stigma with multicellular papillae; (ventral carpellary bundles antiseptal); K/P persistent; elaiosome extending along the raphe.
2/129. North Temperate.
Age. Wikström et al. (2001) suggested an age of (52-)44, 36(-28) Ma for crown-group Asaroideae and Naumann et al. (2013) an age of about 14.8 or 13.7 Ma; Takahashi and Setoguchi (2017) dated the "initial divergence of Asarum" to (21-)13(-6.8) Ma.
1A. Saruma henryi Oliver
Wood rays, axial parenchyma 0, interfascicular area with upright cells; pericyclic fibres in groups; petiole with 3 arcuate bundles, fibers 0; P = K + C; A incurved [so dehiscence introrse]; pollen grains monosulcate, semitectate; G 6, ± free but adnate to hypanthium, opposite K and C; fruit a follicle; n = 26.
1/1. Central China, ± the length of the country. [Photo: Flower.]
1B. Asarum L. —— Synonymy: Asaraceae Ventenat
(Plant deciduous); (inner whorl of P +, at most minute); pollen 2-polyporate, 3-4-colpate or inaperturate; G superior-inferior, styles free to ± connate, hollow, compitum +; fruit an irregularly dehiscent capsule; n = 6, 12, 13, 18, 20, 26; nuclear genome [2 C] 6.12-10.4 pg.
1/128. N. Temperate, esp. East Asia, 1 sp. in Europe. [Photo: Flower.]
[Lactoris [Hydnoroideae + Aristolochioideae]: growth monopodial; plant ± woody; inflorescence axillary; bracts distinct.
Age. Wikström et al. (2001) suggested an age of (112-)107, 85(-80) Ma for the [Lactoris + Aristolochia] clade, Bell et al. (2010) an age of (114-)91, 78(-54) Ma, and Naumann et al. (2013) an age of about 99.4 or 98 Ma.
2. Lactoridoideae [not published] - Lactoris fernandeziana Philippi —— Synonymy: Lactoridaceae Engler, nom. cons.
Plant a shrublet; ?chemistry; ?cork; wood rayless [internodal regions], interfascicular area with upright cells; sclereids associated with pericyclic fibres; nodes 1:2; crystal sand +; petiole?; plant glabrous; cuticle waxes as parallel platelets; lamina elliptic, secondary veins subpinnate, stipule +, sheathing, intrapetiolar, adnate to the petiole; plants polygamo-dioecious; inflorescence axillary; thyrsoid, bracteoles 0; flowers small; P separate, members with but a single trace; A in two whorls, 6, (inner or both whorls staminodial); pollen in tetrads, saccate, ektexine granular, (subcolumellate); G 3, basically free, alternating with P; ovules 4-8/carpel, pendulous, epitropous, parietal tissue ?0, endothelium +, funicle long; seed coat cells collapsed, two cuticular layers persisting, endothelium also ± persistent; endosperm nuclear/coenocytic, with chalazal haustorium; n = 20.
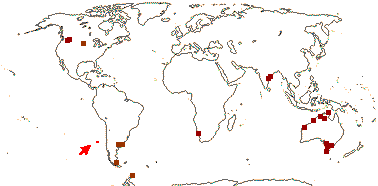
1/1. Chile, the Juan Fernandez Islands. Map: see opposite, for the distribution of fossils (brown squares), see Gamerro and Barreda (2008). Photo: Specimen.
Age. Lactoripollenites (= Rosannia) is widespread in the fossil record, first occurring in Late Cretaceous deposits from S.W. Africa (Turonian-Campanian), i.e. from around 92 Ma and later (Zavada & Benson 1987; Macphail et al. 1999; Gamerro & Barreda 2008; Srivastava & Braman 2010).
>[Hydnoroideae + Aristolochioideae]: G inferior.Age. The age of this node is variously estimated to be (105-)91.4(-78) Ma (Naumann et al. 2013), their preferred estimate, (124.4-)101.4(-76.5) Ma ([Piperaceae + Saururaceae] sister - Table 2), or (122-)111(-99) Ma old.
3. Hydnoroideae Walpers
Root parasites, echlorophyllous, ± herbaceous, rhizomatous; starch grains?; sieve tube plastids without starch or protein inclusions, perivascular fibres 0; mucilage cells +; stomata 0, cuticle wax crystalloids 0; vegetative hairs 0?; leaves 0; flowers arising endogenously from roots, 3-4(-5)-merous, large, polysymmetric; P very thick and fleshy, 3-4(-5) merous; A = P, adnate to and opposite P, anthers polythecate [>5 thecae], (staminodes +, ± deeply lobed, alternating with P, retrorse and ligule-like, below A - ? - Pro.); pollen (extruded in threads), monosulcate, 2-3-porate, or trichotomocolpate, ektexine homogeneous; G inferior, alternating with P, placentation lamellate, parietal or apical, ?compitum, style 0, stigma broad, cushion-shaped; ovules straight, unitegmic, integument 2-4 cells across, parietal cells 0, nucellar epidermis persistent, nucellar cap?; embryo sac bi-/tetrasporic and 8-nucleate [Adoxa type], antipodals not persistent; fruit baccate, ± circumscissile or not; seeds minute [≤1 mm long]; exotestal cells with U-thickened inner walls (not), anticlinal walls ± sinuous; endosperm cells with thick walls, arabinose and starch +, perisperm +, ca 1 cell layer across, embryo undifferentiated, ca 30 cells,; n = 12; seed germination via germ tube.
2/17. Africa, the New World. [Photo - Prosopanche Staminate Flower © L. Musselman, Flower © R. Polhill & Paolo, Fruit © G. Williams.]
Age. Crown-group Hydnoroideae are estimated to be (74-)55(-36) Ma (Naumann et al. 2013: (86.9-)58.2(-29.5) Ma - Table 2) or around 54 Ma.
3A. Hydnora Thunberg
Rhizome angled, tubercles in lines/rounded, tubercles scattered, flowers or haustoria developing from tubercles; [anatomy - if stem angled] rhizome with corky cap; cork well developed, outer cortical; cells of cortex and pith, tanniniferous, vascular bundles ca 6, collateral, vascular bundles to tubercles forming ray-like structures alternating with central va=scular bundles; xylem elements with scalariform walls, perforation plates simple; osmophores on midpoint/apex T; , antheral ring formed by connate inverted V-shaped anther lobes; seeds ruminate; proembryo a chain of ca 15 cells, embryo develops from the middle.
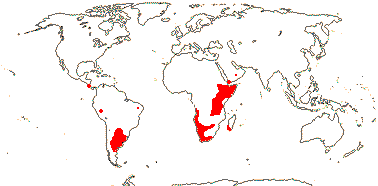
1/10. Arabian Peninsula, southern and eastern Africa, Nigeria, Madagascar and Réunion, Map: from the Parasitic Plants Website (2004), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010) and Hatt et al. (2022, 2024a: Map 1).
3B. Prosopanche de Bary
anthers adaxially connate, forming solid body.
1/7. Costa Rica and S. South America. Map: from the Parasitic Plants Website (2004) and Machado and de Queiroz (2012).
4. Aristolochioideae Link
Plants lianes or vines (shrubs; herbs); benzylisoquinoline alkaloids +; (sieve tube plastids also with polygonal protein crystalloids and peripheral protein fibres); (secondary thickening odd), interfascicular area parenchymatous; ring of pericyclic fibres; groups of silicified cells +; (nodes 3:3, 5:3); druses + (0 - Thottea); hairs with hooked, silicified terminal cell; petiole with a ring of (three) bundles or incurved U-shaped; axillary buds several, superposed, prophyll well developed, stipule-like; (lamina lobed), base of petiole U- or V-shaped; inflorescence usu. axillary; (flower with median tepal abaxial), floral primordia monosymmetric, (flowers monosymmetric - Aristolochia [A.]); P (margins fimbriate), (nectaries on adaxial surface, as hairs/glandular patches); A 3-12(-40<, centripetal - Thottea), (in a single whorl); (microsporogenesis successive - A.); pollen inaperturate; G [(2-)4-6], inferior, (alternate with A - A. s.l.), apically constricted, compitum +, stigma dry or wet, (commissural - A.); (funicle massive - A. bracteata); fruit opening laterally, acropetally/basipetally septicidal, segments also opening adaxially, (schizocarp, berry), K not persistent; seeds winged, (arillate); n = (4-)6-7(8...13); nuclear genome [2 C] 0.67-1.83(-4.41) pg.
2-5/535: Aristolochia (524, inc. Isotrema (98), Thottea (45)). Tropics (temperate), relatively less diverse in Africa (inc. Madagascar), few in N. Australia. Map: see family above. Photos - Flowers, Fruits.
Age. The age of crown-group Aristolochioideae is estimated to be around 39.5 or 35.1 Ma (Naumann et al. 2013), while Allio et al. (2020/2021) date divergence in Aristolochia s.l. to (72.8-)55.5(-39.2) Ma.
Friis et al. (2022a) found seeds with the distinctive anatomy of those of Aristolochiaceae (crystalliferous endotesta; crossing tegmic fibres) from the early Cretaceous of Portugal (Aristospermum huberi, Aptian to early Albian) and from rather younger Cretaceous deposits in Maryland (Siratospermum mauldinense).
Evolution: Divergence & Distribution. The recently-described fossil Hexagyne philippiana whose 3-merous flowers have 6 carpels and 3 (?or 6) perianth parts ends up in Aristolochiaceae in morphological analyses, although positions in basal eudicots and even in monocots are found in trees only one step longer (Coiffard et al. 2014: note topology). The discovery of this plant, found in deposits in eastern Brazil 115-112 Ma, perhaps supports a Gondwanan origin of Aristolochiaceae (Coiffard et al. 2014). Molecular ages of stem-group Lactoris are Cretaceous, and fossil pollen attributed to the genus (= Rosannia) is of comparable age and is widely distributed both around the southern hemisphere and also in the Northern Hemisphere, e.g. in Alberta, Canada (e.g. Srivastava & Braman 2010; Quattrocchio 2017). However, the oceanic Juan Fernandez islands, on which Lactoris currently resides, are young, up to ca 4 Ma, although seamounts nearby that are up to ca 10 Ma may have been emergent at some stage (Sontag & Stuessy 2017). The ancestral region of Aristolochia s.l. may have been in the Central American and the Western Nearctic and Eastern Palaeoarctic area (Allio et al. 2020/2021).
Polyploidy may have been important in the evolution of Aristolochia (Wanke et al. 2017). Within Aristolochia subgenus Isotrema there are two North American-East Asian disjuctions; these and other events are dated by González et al. (2014) to around 30 Ma, the initial divergence of the two herbaceous species from the rest of the subgenus, which is woody, is earlier. Diversification of Asarum subgenus Heterotropa seems to be linked to features like fungal brood site mimicry and stems with shorter internodes and the production of a single expanded leaf per innovation, the other species producing two subopposite expanded leaves at the end of the innovation; self pollination by movement of the stamens as the flower ages, as in other species of Asarum, no longer occurs here (Sinn et al. 2015a, b).
Friis et al. (2019b) suggest that an endothelium or something similar may be plesiomorphic here.
Physiology & Ecology. All told, there are perhaps 395 species of twining climbing/lianoid Aristolochioideae (see also Gentry 1991). Speck et al. (2003) summarized biomechanical studies in Aristolochia, relating them to anatomy. Perennial herbs evolved from shrub and liane/vine growth forms several times; the shrubs themselves differ somewhat biomechanically from shrubs in other families. Wagner et al. (2012) also discuss the evolution of (weakly) shrubby members of Aristolochia which nevertheless retain many elements of the distinctive stem anatomy, including the broad rays, of their primitively climbing relatives. The climbing habit is likely to be an apomorphy for the genus, and woodiness perhaps derived (Wagner et al. 2014, q.v. for much other information, see also Speck et al. 1997). Busch et al. (2010) describe how the pericyclic cylinder is repaired when it breaks; cells adjacent to the break may divide and their walls become lignified.
Naumann et al. (2016; see also Hatt et al. 2022, 2024a) summarize the hosts recorded for Hydnoroideae. Prosopanche bonacinae parasitizes quite a variety of taxa, P. americana parasitizes primarily the mimosoid Prosopis (= Neltuma) in Argentina (Mares et al. 1977), and two other rarer species are also found on Fabaceae. Hydnora is found mostly on mimosoid legumes and Euphorbia, in the latter case different species of Hydnora from the one locality parasitizing different species of Euphorbia (Bolin et al. 2009b), and H. sinandevu is known from Commiphora-Burseraceae (Bolin et al. 2018; Hatt et al. 2024a: Table 1).
Pollination Biology & Seed Dispersal. There are reports of thermogenesis in the flowers of some Aristolochiaceae (Cocucci & Cocucci 1996; Seymour 2001; Bolin et al. 2009a; Seymour et al. 2009). Various forms of fly pollination are common in the family (Bliss et al. 2013; Oelschlägel et al. 2014; Gottsberger 2016a and references). Many taxa of Aristolochia s.l. trap the flies in chambers or kettles, specialized multicellular hairs allowing insects to enter, but not leave, the floral chamber, although they attempt to escape through the "windows" on the side of the chamber. Flies remain there until the hairs wither, and the corolla also often changes colour then (Sakai 2002; Oelschlägel et al. 2009; Rintz 2009). Nectar in Aristolochia may be produced on the inside of the perianth tube to feed the temporarily-trapped pollinators and/or help guide them to the exit (Erbar 2014 and references; Erbar et al. 2017), and it has even been suggested that flies may sup from the copious stigmatic exudate (Baker et al. 1973). Flies, including phorid flies that use the flowers as brood sites, may oviposit on the flowers, and the relationship between plant and pollinator may be specific (Sakai 2002; Bliss et al. 2013 and references; Jürgens et al. 2013 for the various odour syndromes involved). In a wrinkle on fly pollination, chemicals in the rather weak (to us) scent of the European Aristolochia rotunda specifically attract dipteran chloropid flies - the same chemicals are produced by recently-dead mirid bugs. The chloropid flies are kleptoparasitic, stealing food from other insects, and also eating the secretions produced by mirid bugs when they are eaten by other arthropods (Oelschlägel et al. 2014). Aristolochia includes species with pitfall trap flowers. The inside of the perianth tube of Aristolochia arborea looks as if it has a small mushroom growing in its mouth, and this and a number of species of Asarum subgenus Heterotropa in particular with similar fungus-like structures are pollinated by fungus gnats, etc., and the volatiles they produce may include alpihatic compunds, benzenoids and terpenoids (Vogel 1978a; Sinn et al. 2015a, b; Okuyama et al 2025). Both in Asarum and Aristolochia there is brood site mimicry (see also Okuyama et al. 2020), but there are also brood-site mutualisms here (S. D. Johnson & Schiestl 2016). Kakishima and Okuyama (2020) found that the odours produced by Asarum tamaense, visited by female Cordyla fungus gnats, were primarily those of carrion and fermenting fruits... The focus of the study by Okuyama et al. (2025) was on pollination in Asarum by flowers that were carrion or dung mimics, and as is common in such cases the volatiles were dominated by oligosulphides, here dimethyl disulphide and largely secreted by the perianth members; Okuyama et al. (2025) estimated that there had been at least 18 gains and losses of the ability to produce such volatiles in their system alone, not to mention their production in quite unrelated plants, for example, Eurya (Theaceae).
Pollination of the foetid flowers of Hydnora is by flies and dermestid and scarab beetles; there is deceit pollination here, and oviposition on the flowers may also occur (Bolin et al. 2006b, 2009a; Gottsberger 2016a; etal. 2024; see also Hatt et al. 2024a). In Hydnora triceps both flower and fruit are underground. Weevils (perhaps) and nitidulid beetles are pollinators of Prosopanche americana, and here, too, taxa like the weevil Hydnorobius hydnorae [sic] oviposit in the flowers (Mares et al. 1977; Cocucci & Cocucci 1996; Sequeira et al. 2018). Recent work suggests that in Prosopanche pollination relationships are mutualistic, nitidulid sap beetles both pollinating the flowers (they are attracted by volatiles the flower produces) and laying eggs on them, the larvae eating flowers, the mesocarp of the fruit (but not the seeds) and so on (Rocamundi et al. 2023). Thermogenesis, although sometimes at rather low levels, has been recorded in both genera, and it may be involved in scent production/volatilization (Seymour et al. 2009). Some species of Hydnora have pitfall trap flowers.
Seeds of Aristolochiaceae with arils or elaiosomes are probably dispersed by ants.
Each flower of Hydnora has up to 35,000 ovules; the fruits are eaten byr by jackals and other smaller mammals (Hatt et al. 2024a), which presumably disperse the seeds. Solms-Laubach (1874) described the thickened testa cell walls of Prosopanche as being "schaumige" (frothy); dispersal here is also by a variety of mammals (Bolin et al. 2009b: Hydnora).
Plant-Animal Interactions. Aristolochia is eaten by caterpillars of the magnificent birdwing butterflies (e.g. Ornithoptera), the largest butterflies known, of the Papilionidae-Papilioninae-Troidini (birdwings s.l., ca 135 spp.; birdwings s. str, ca 36 spp.), and in Guanacaste, Costa Rica, troidines are again Aristolochia specialists (Quicke et al. 2025). Many other papilionids, e.g. the pipe-vine swallowtail (Battus philenor), also eat Aristolochia (Berenbaum & Feeney 2008). The association between caterpillars of these butterflies and Aristolochiaceae - they are apparently not found on Saruma - has been studied in some detail (e.g. Weintraub 1995). Based on relationships in a morphological phylogeny [Cressida [[Pharmacophagus + Ornithoptera] [Battus etc.]]] and correlation with geography, Parsons (1996) invoked continental drift to explain the distributions of birdwings and their relatives, but the butterfly relationships he found are very different from those suggested elsewhere - even if there is still hardly any general agreement over this. The larvae of these butterflies may sequester the poisonous aristolochic acid, although the story is not a simple one (diMarco & Fordyce 2017). Larvae of Luehdorfia and other genera of the Parnassiinae (Luehdorfiini and Zerynthiini, but not Parnassiini, also swallowtails) are also found on Aristolochiaceae, perhaps the original host for this clade, although they are not very speciose (Nazari et al. 2007; Michel et al. 2008; Condamine et al. 2018). Aristolochiaceae may be the original food for the larvae of Papilionidae as a whole (but perhaps not Baronia: Condamine et al. 2011; Allio et al. 2020/2021; c.f. K. S. Brown et al. 1995; Michel et al. 2008; Simonsen et al. 2011: two shifts on to the family; see also Ehrlich & Raven 1964), and the larvae of many taxa that do not normally eat Aristolochiaceae can nevertheless be reared on the family. Indeed, Papilionidae are just about the only butterflies whose larvae eat Aristolochiaceae (Condamine et al. 2011), but there seems to be no particular connection between details of the phylogeny or chemistry of that family and the phylogeny of the butterflies (Silva-Brandão & Solferini 2007; Simonsen et al. 2011). Although furanocoumarins, important secondary compounds in Rutaceae, for example, and on which Papilionidae are also notably diverse, are apparently not known from Aristolochiaceae, they do have other distinctive compounds like benzofuran neolignans (Z.-B. Zhou et al. 2013). In a number of Aristolochia-eating papilionids aristolochic acids (nitro phenanthrolene [= phenanthrene, a tricyclic aromatic hydrocarbon, C14H10], with nitrogen at the 4 and 5 positions) carboxylic acid, also toxic to humans, being nephrotoxins and carcinogens) are found in the osmeteria, an eversible organ found on the prothoracic segment of the caterpillar that is used in defence (Opitz & Müller 2009). Papilionid larvae may sequester toxic compounds from their host, and these compounds may be passed on to the adult, too; Rothschild (1979: p. 91) suggested that a specimen of the giant Papilio antimachus could store "sufficient cardiac glycosides to kill five cats", although nothing seems to be known about what the larvae eat; cats aside, swallowtails may be mimicked (Batesian mimicry) or be Mullerian mimics themselves.
Swallowtails are also to be found on Annonaceae, Apiaceae, Papaveraceae, Rutaceae and Crassulaceae in particular, and there are one or two species (literally) on Fabaceae, Magnoliaceae and Zygophyllaceae (q.v.), and in part the host plant variation follows host plant chemistry; see also papers in Scriber et al. (1995), Fordyce (2010), Simonsen et al. (2011) and Condamine et al. 2011). Movement on to some of these families may be accompanied by increases and then subsequent decreases in diversification, and the evolution of new cytochrome P450 monooxygenases that are involved in the detoxification of plant secondary compounds may play an important role in facilitating this colonization of new hosts (Berenbaum et al. 1996). However, Nishida (1995) noted the complexity of the chemical bouquets that stimulated oviposition in the female, furthermore, Allio et al. (2020/2021) observed that there were genome-wide changes associated with these host shifts. Larvae of Baronia brevicaulis, belonging to a monotypic clade perhaps sister to all other Papilionidae, eat Vachellia campechiana (previously Acacia cochliacantha); ?chemistry. For more on plant chemistry and the papilionids, see Berenbaum and Feeny (2008).
Allio et al. (2020/2021) suggest that radiations of Papilionidae and Aristolochia were synchronous, dating them at (71.0-)55.4(-47.8) Ma and (72.8-)55.5(-39.2) Ma respectively, and this occurred in the Central American-west North American/Nearctic-east Palaeoarctic area - perhaps suggesting some kind of coevolution of the two. However, estimates of when Papilionidae diversified vary by a factor of over two (Simonsen et al. 2011 for literature), for instance, Condamine et al. (2011) suggested that diversification began in the early Caenozoic (62.5-)52(-46) Ma, Chazot et al. (2019: also other estimates) suggested an age of (84.3-)68.4(-53.5) Ma, and Espeland et al. (2018) an age of (109-)84(-63) Ma. Furthermore, basal relationships are somewhat unclear (e.g. Espeleand et al. 2018) - and this is not to mention the if anything even greater variation in estimates of the age of Aristolochia (see above).
Quicke et al. (2024) noted that very few caterpillars were to be found on Aristolochia in the Guanacaste (Costa Rica) area, and those that were there were oligophages.
Vegetative Variation. The anatomy of Hydnoroideae needs attention; careful comparative studies in Aristolochiaceae and and Piperaceae, at least, ares very much needed. One problem is knowing exactly the part of the plant at which one is looking. In Prosopanche burmeisteri and Hydnora africana the vascular bundles of the angled "rhizoid" (= rhizomes) are in a strongly medullated and star-like arrangement with a varying number of rays (Schimper 1880). The vascular bundles on the rays of the star face away from each other, and in P. burmeisteri the ring/rays are interrupted so there seem to be two systems of vascular bundles. The outer cells of the rhizoid have suberin, and cork develops somewhat later. "Appendages" of P. burmeisteri, which develop in rows along the angles of the rhizoids, had a small amount of vascular tissue in the centre; these may become haustoria or flowers, or remain undeveloped (Hatt et al. 2022). S. T. Wagner et al. (2014) confirmed the cauline nature of the rhizoids. Interestingly, the rhizoids of H. abyssinica are shown as being round in t.s. and with numerous scattered vascular bundles (Schimper 1880), an anatomy which would seem to have nothing to do with that of either Prosopanche or H. africana. However, Tennakoon et al. (2007: H. triceps with angled rhizoids, see also 2005) noted that anatomical features of H. abyssinica are seen in some Piperaceae, for example, and that the absence of an interfascicular cambium, the collateral vascular bundles, etc., of H. triceps are consistent with a position here; the cork- and cap-like structure at the apex of the rhizoids is presumably protective, and it is interesting that the cork cambium has a quite superficial position.
Genes & Genomes. A genome duplication, the SAHEα event that occurred some 122.2 Ma involves the whole family (Landis et al. 2018), although Qin et al. (2021) suggested that there has been no whole genome duplication in Aristolochia fimbriata other than the ε/epsilon event that occurred in the ancestor of all angiosperms. For the cytology of Asarum s.l., see Sugawara (1982, 1987 and references), for nuclear genome size, see Bliss et al. (2013), and for chromosome number evolution in Aristolochia, see Freyman and Höhna (2017).
Much of the large single copy of the plastome of Asarum is inverted, and there have been three independent and more or less complete incorporations of the small single copy region into the inverted repeat; all in all, the plastome is labile here, perhaps because of the long regions made up of AT-repeats (Sinn et al. 2018). Some species of Asarum seem to lack quadripartite plastomes (see also Jost & Wanke 2024). Plastomes in Aristolochiaceae have an average GC content of 38.4% (Jost et al. 2022).
Plastomes in Hydnoroideae range from 24,479-28,658 bp (Jost et al. 2022); at some 24 kb, the plastome in Hydnora visseri is among the smallest known. Plastomes in Hydnoroideae have an average GC content of 20.4—24.1% (Jost et al. 2022). Some plastid genes, now non-functional, are scattered in the mitochondrial genome of (Naumann et al. 2014). However, like several other taxa with much reduced plastomes, genes that code for functions such as producing plastid ribosomes remain functional, and the basic gene order of the plastome is unchanged, so despite its very small size, it remains partly functional (Naumann et al. 2016). There is a small inverted repeat, but not in Prosopanche, although perhaps a small inverted repeat such as in Hydnora itself has become inverted there (there are two direct repeats), and there are additional inversions and repeats; scenarios for explaining plastome structure are complex (Jost et al. 2020, 2022). Hydnoroideae have lost all but the rps12 intron (Jost et al. 2022). For plastome evolution in parasitic flowering plants in general, see Wicke and Naumann (2018).
Chemistry, Morphology, etc.. Aristolochic acid is closely related biosynthetically to benzylisoquinoline alkaloids (Gershenzon & Mabry 1981). Aristolochia has cuticular wax rodlets, but other genera lack crystalloids.
The vegetative anatomy of Hyddnoroideaee is not well understood. Ding Hou (1984) notes that the leaves wither on the plant and do not abscise. The shrubby habit is derived within Aristolochia, and the cork is (eventually) deep seated (Wagner et al. 2014), although in the illustrations in Carlquist (1993, q.v. for anatomical features common in the family) it appears to be outer cortical. Dickison (1992) noted that the floral vascular cylinder of Saruma had internal phloem, and vascular bundles there were amphicribral, i.e. they had phloem all around; for more on the vascular tissue of the family, see Cunha Neto (2023). The central leaf trace of the woody Aristolochia arborea sometimes divides into three parts, or is this a single trace broken up by broad rays? Nair and Narayanan (1962) discuss nodal anatomy in some detail, although things are still not entirely clear; the central bundle of the basically trilacunar node is inclined to divide while Dormer (1955) looked at nodal anatomy in Asarum europaeum - his findings need to be confirmed/extended. Aristolochia clematitis appears to have lateral prophylls. González and Rudall (2001) suggested that the stipule of Lactoris is initially paired, and there has been discussion over the nature of the stipuliform structures in Aristolochia (Ye & Ronse De Craene 2024 and references. Lobed leaves are known from Aristolochia.
There has been much discussion about the nature of the perianth in Aristolochiaceae. The uniseriate perianth may well be derived from the outer whorl of a biseriate perianth (González & Stevenson 2000). In any inner whorl, whether in Asarum, Saruma or Thottea, "petal" bases are narrow, while the bases of members of the outer whorl are very broad and encircle the floral axis. It has been suggested that "petals" are derived from stamens (see Leins et al. 1988: Thottea; Leins & Erbar 1985b, 1995; Kelly 2001; Ronse De Craene et al. 2003); they were drawn as staminodes and described as petal appendages by Ronse de Craene (2010). Their position in some species of Asarum, in the angles of the outer whorl, makes any staminodial origin unlikely (the position would suggest oppositipetaly...) and would also suggest that the perianth tube is a K + C tube. Jaramillo and Kramer (2004) describe the basic perianth condition for the family as being unipartite (= uniseriate), with its ancestors having "multiple" whorls. In the development of monosymmetry in Aristolochia CYC genes are not much in evidence (Horn et al. 2014). Madrigal et al. (2019) suggested that the involvement of RADIALIS and DIVARICATA genes in monosymmetry in Aristolochia was rather different from that in monosymmetric flowers of monocots and core eudicots. Qin et al. (2021) thought that CINCINNATA genes were "responsible for the heterogeneous growth and morphological deformation of the perianth" of Aristolochia (ibid. p. 1244). See also Bliss et al. (2013 and references) for the perianth in the family.
The median outer perianth member is adaxial (González & Stevenson 2000a) in some taxa, although it is abaxial in Aristolochia s. str. (although not in A. grandiflora) and also in Pararistolochia (Neinhuis et al. 2005: ?other taxa). Spichiger et al. (2004) show a floral diagram for Aristolochia where the six stamens and carpels are not opposite to the perianth members - nor would be opposite sepals or petals, if such were present. In Asarum, there are stamens more or less adnate to the style. Mkala et al. (2021) described some species of Hydnora as having "bait bodies" between the perianth lobes or even "well developed petals that are concave in shape" (ibid. p. 4). Carpel orientation in Hydnoroideae is suggested by stigma position (see Baillon 1888).
González and Stevenson (2000b) note that the stigmas of Aristolochia are commissural (see also Eichler 1878; Leins & Erbar 1985b), and that when there is only a single whorl of stamens in the flower, it is the inner whorl. Endress (1994c) suggested that the androecium in Lactoris was adnate to the gynoecium, as in other Aristolochiaceae, but at most it is adnate to the stipe of the gynoecium; ovary position is variable around here. Thottea has four placentae and presumably four carpels, but there are about twice as many - or even more - styles; these surround an open gynoecium (Leins et al. 1988; c.f. Endress 2014). Leins and Erbar (1995) described the flowers of Saruma, which are rather different from those of the rest of the family (see also Dickison 1992; Sinn et al. 2015a): sepals and petals are quite distinct and the nine carpels are adnate to the hypanthium, but are largely free from one another. All in all, rather confusing. An illustration in Engler (1888) shows a bistomal micropyle.
For general information, see Engler (1887), Carlquist (1964), Ding Hou (1984: Malesian taxa), Kubitzki (1993), Huber (1993), and Bliss et al. (2013), Hegnauer (1964, 1966, 1989), Chen and Zhu (1987) and Crawford et al. (1986) for chemistry, Metcalfe (1987), Carlquist (1990b: Lactoris; 1993: other genera) and Dickison (1996: Saruma) for anatomy, Behnke (2001, esp. 2003) for sieve tube plastids, González (1999) for inflorescence morphology, Tucker and Douglas (1996), Leins et al. (1988) and Mair (1973: Aristolovhia) for floral development, Sugawara (1987) for floral anatomy of Asarum, Mulder (2003) for pollen, González et al. (2001) for microsporogenesis, Wyatt (1955), Johri and Bhatnagar (1955) and Nair and Narayanan (1961, 1963) all for embryology, González and Rudall (2001) for ovule and seed development and Huber (1985) for seed characters. For more about Lactoris, see Bouman (1971: ovule), Tobe et al. (1993: embryology and karyomorphology), and González and Rudall (2001: morphology).
Hydnoroideae: For general information, see Engler (1887), Meijer (1993), Nickrent (2020), and Cocucci and Cocucci (1996) and Matt et al. (2022), both Prosopanche and Matt et al. (2024: Hydnora), Hegnauer (1966, 1989) for what little is known about chemistry, Schimper (1880) for vegetative anatomy, Solms-Laubach (1874), Dastur (1921: Hydnora) and Cocucci (1976), all embryology, for germination, see Bolin et al. (2006a) and for seeds, see Baskin and Baskin (2021: Hydnora). Other information may be found at the Parasitic Plants website (Nickrent 1998 onwards) and Heide-Jørgensen (2008).
Phylogeny. For the circumscription of Aristolochiaceae and major relationships within it, see above.
Morphology and molecules (ITS) suggested similar relationships in Asarum (Kelly 1998, c.f. 1997), and A. epigynium, from Taiwan, may be sister to the rest of the genus (Sinn et al. 2015a). However, different relationsips may come from analyses of plastid and nuclear sequences (Sinn et al. 2018; see also Okuyama et al. 2020), thus although the Hexastylis group was polyphyletic in both the ITS and matK analysis, the large Heterotropa clade was polyphyletic only in the latter (Takahashi & Setoguchi 2017). Okuyama et al. (2020: ddRAD-seq, c.f. other analyses, e.g. plastome genes) obtained quite well resolved relationships in section Heterotropa, where the deciduous A.forbesii, from China, was sister to the rest of the section (Japan, Taiwan, etc.). Although the monophyly of Aristolochia s.l. is not in question, it encompasses quite a lot of variation. There are four main clades that are all well supported, one of which includes just two species (González & Stevenson 2002; Neinhuis et al. 2005; Wanke et al. 2006b, 2017; Ohi-Toma et al. 2006). González et al. (2014) looked at relationships within subgenus Isotrema and found that the herbaceous North American A. reticulata and A. serpentaria are sister to the rest of the group, but in an otherwise quite extensive study by X.-X. Zhu et al. (2019) these species were not included. Within Thottea, T. piperiformis is sister to the rest of the genus (Oelschlägel et al. 2011).
Classification. See Huber (1985) for an infrafamilial classification. However, there are suggestions that the family should be more or less split up (et al. 2024 for a recent example), and relationships are somewhat uncertain: See elsewhere.
Asarum can be circumscribed broadly, as here (Sinn et al. 2015a provides an infrageneric classification), or divided into a number of genera. Huber (1993) suggested that Aristolochia could be divided into eight genera, some of which would be well characterised morphologically. Some splitting, perhaps into four genera, all with synapomorphies, seems to be favoured (González & Stevenson 2002; Neinhuis et al. 2005; Wanke et al. 2006b; X.-X. Zhu et al (2019: Isotrema), but recognition of some genera will have extreme nomenclatural consequences (González 2012). Aristolochia s.l. is immediately recognizable (Buchwalder 2014).
Matt et al. (2024) recognized four subgenera in Hydnora.
Previous relationships. Hydnora was initially described as a fungus. Hydnoraceae were placed in Rafflesiales by Cronquist (1981) and in Hydnorales in Rafflesianae by Takhtajan (1997); Cocucci and Cocucci (1996) saw connections between Hydnoraceae and Annonaceae. Lactoris had until recently been placed in its own family, Lactoridaceae, and included in Magnoliales (Cronquist 1981), or put in the monotypic Lactoridanae - but on the page after Aristolochiaceae (Takhtajan 1997).
Thanks. I thank Mauricio Diazgranados for comments.
[Piperaceae + Saururaceae]: root epidermis from inner layer of cap; ?nodes; stomata tetracytic; cuticle wax crystalloids usu. 0; lamina vernation supervolute, leaf base ± sheathing stem, (stipules +, intrapetiolar, ± on petiole); inflorescence indeterminate, spicate, flowers dense, sessile, small [8> mm across], monosymmetric, A initiated in pairs; P 0; filaments rather slender; microsporogenesis simultaneous; pollen grains <20 µm; G with odd member adaxial [when 3], primordium initially annular, stigma dry, papillate; ovules straight; seed coat exo- and endotegmic; perisperm +, starchy, endosperm ?type, scanty, persists around embryo, embryo short, broad; germination phanerocotylar.
Age. Wikström et al. (2011) suggested an age of (106-)100, 90(-84) Ma for this node, Magallón et al. (2013, 2015) ages of around 64.1 Ma and 65.5. Ma (wide confidence intervales) respectively, and Bell et al. (2010) an age of (96-)78, 67(-45) My; 76.5 and 62.2 Ma are the ages in Naumann et al. (2013), ca 74.8 Ma in Tank et al. (2015).
Evolution: Divergence & Distribution. There was a major decrease in the rate of diversification at this node (Massoni et al. 2015a).
Rudall (2023a) suggested that this clade had anisocytic/cyclocytic stomata. Jaramillo et al. (2004) discussed the complexities of floral evolution in this group; it is possible that a four-carpelate gynoecium is the basic condition. Madrid and Friedman (2009) thought that embryo sac development was bisporic, although it might be more accurate to say that it is unclear. There may be a connection between the evolution of perisperm and the great variability in embryo sac morphology in this clade (Madrid & Friedman 2010), however, other clades with perisperm such as core Caryophyllales and Zingiberales are much less adventurous in terms of such variation.
Pollination Biology & Seed Dispersal. Generalist pollination predominates here (Gotttsberger 2016).
Genes & Genomes. A genome duplication, the PEPRα event, dated to some 114.2 Ma, is to be placed at this node (Landis et al. 2018).
Chemistry, Morphology, etc.. There is an endodermis in the stem of Saururus, Houttuynia and at least some Piperaceae (Seago 2020). Nodal anatomy in some members of this clade cannot be described in terms of the conventional trace/gap formula used elsewhere for seed plants. In Piperaceae, for example, 3-11 traces supply the leaf, and in Piper, at least, the lateral and median traces develop in completely different ways, foliar innervation being more similar to that in monocots (e.g. Balfour 1958b). In Houttuynia the innervation of the prophylls and the regular leaves is very different (Murty 1961).
Tucker (1982) noted that details of the order of initiation of the androecium in Piperaceae and Saururaceae differed, although both could be described as having monosymmetric flowers with stamens initiated in pairs. Some taxa in both families have pollen with punctate ectexine, the punctae being surrounded by papillae (S. Y. Smith & Stockey 2007a).
Some information is taken from Murty (1961: morphology), Blanc and Andraos (1983: growth), Tucker et al. (1993: morphology and development), Tucker (1984: floral development), Rudall et al. (2009b: seed development) and Baskin and Baskin (2018: germination).
PIPERACEAE Giseke - Back to Piperales
Growth monopodial, habit various; piperamides + [R-(C=O)-NH2, one or two H atoms variously replaced], flavonols, tannins 0; root cap meristem closed [epidermis from inner layer of cap complex]; trichoblasts in vertical files, proximal cell smaller; (cork in outer cortex); cambium storied; nodes with (5-)9(-20) traces; mucilage canals +; petiole bundles arcuate; prophyll single, basal, adaxial to lateral, often ± reduced, with a fairly prominent axillary bud; petiole (margins ± sheathing), (ligule +); bracts peltate or clavate; A (1-)2(-10, or 3 + 3, latrorse to extrorse, thecae not dehiscing their entire length, endothecium from outer secondary parietal cell layer, inner secondary parietal cell layer dividing; pollen ektexine tectate, endexine 0; G [2-5], styluli/style branches +; ovule single, basal, parietal tissue 2-5 cells across; embryo sac tetrasporic, sixteen-celled, eleven cells at the chalazal end; fruit fleshy, drupe; seed with exo- and endotegmic layers well developed, former in particular thick-walled; x = 12 (?13), nuclear genome [1 C] (0.061-)1.317(-28.571) pg.
5 [list]/3,630 - three subfamilies below. Pantropical. Map: from Jaramillo and Manos (2001), Trop. Afr. Fl. Pl. Ecol. Distr. Vol. 1 (2003), Wilson (2007) and M. A. Jaramillo (pers. comm.).
Age. Ca 54.4 Ma is the age for crown-group Piperaceae in Naumann et al. (2013) and (96.8-)77.7, 58(-37.8) Ma in Massoni et al. (2015a).
1. Verhuellioideae Samain & Wanke - Verhuellia Miquel
Herbs; stem with single central amphicribral bundle, protoxylem elements only, secondary thickening 0; pericyclic lignification 0; druses +; inflorescence axillary; bracts peltate; A 2-3, apex of connective expanded, thecae short, widely separated, dehiscence latrorse; pollen 8-10 μm across, inaperturate, tectum discontnuous; with ± spherical microechinate processes [like mace heads], orbicules +; G stipitate; ovule unitegmic, integument ca 3 cells across; fruit longitudinally ca 4 ridged, surface with rounded protuberances; n = ?
1/3. Cuba and Hispaniola.
[Zippelioideae + Piperoideae]: cauline vascular bundles in 2(+) rings; (mucilage canals in stem +); raphides in leaves +.
Age. The age for this node is given as around 45.9 Ma in Naumann et al. (2013).
2. Zippelioideae Samain & Wanke
Shrubs, woody root climbers; (cambium ± 0); undulating sclerenchymatous band internal to outer ring of bundles; inflorescence ?; A 4, 6; outer integument poorly developed, inner integument 4-5 cells across, parietal tissue 4-8 cells across; fruit glochidiate [Zippelia]; endosperm nuclear; endotegmen palisade in t.s., tanniniferous, becoming lignified, near micropyle interface with perisperm convoluted; zygote uninucleate; n = 19.
2/6. China to Malesia, Central and South America.
Age. This node has been dated to around 28.5 Ma (Naumann et al. 2013).
3. Piperoideae Arnott
(Cambium 0), (undulating sclerenchymatous band internal to outer ring of bundles +); stem endodermis + [?level]; also druses or crystal sand +; lamina (venation ± pinnate); inflorescence terminal, (racemose); micropyle endostomal, outer integument 2-5 cells across, inner integument 3-5 cells across; (chalazal cells fewer, triploid); plane of first cleavage of zygote obliquely vertical/(zygote uninucleate when seed is dispersed).
Age. Wikström et al. (2011) suggested an age of (51-)47, 41(-37) Ma for this node; the age is about 91.2 Ma or perhaps older in J. F. Smith et al. (2008), ca 90.8 Ma in Chomicki and Renner (2015), but only 34.3 Ma in Naumann et al. (2013). Symmank et al. (2011) dated stem Peperomia to ca 57 Ma. See other ages in Piperaceae - the family is another clade with severely conflicting estimates of ages (see also below).
3A. Piper L.
Herbs, shrubs, smallish trees, woody root climbers; petiole bundles forming a ring; plant dioecious [Old World]; A (1-)2-6(-10); micropyle (bistomal - Heckeria), (inner integument to 7 cells across - Macropiper); (chalazal cells fewer, triploid); (endosperm nuclear); plane of first cleavage of zygote obliquely vertical/(zygote undivided); n = x = 13, polyploids esp. Old World, chromosomes 0.7-2.27(-2.45 - Pothomorphe) μm long, heterochromatin blocks conspicuous.
1/2,170. Pantropical, but few in Africa. Photo: Piper - Flower, Fruit.
Age. J. F. Smith et al. (2008) suggested that crown-group Piper was ca 71.25 Ma, while Martínez et al. (2014) dated crown Piper to (114-)111(-110) Ma.
3B. Peperomia Ruíz & Pavón —— Synonymy: Peperomiaceae A. C. Smith
Herbs (annual), (± stoloniferous), cormose geophytes, (subshrubs), (vines), often epiphytes; (CAM photosynthesis +); cauline vascular bundles scattered, (forming a ring); petiole bundles 3-5, forming an arc; leaves (opposite), lamina ± fleshy, peltate, elliptic, etc., (venation ± pinnate); inflorescence terminal, (racemose), A 2, bisporangiate, unithecal; pollen inaperturate, with irregular bluntly microechinate processes, orbicules 0; G 1 ([2]), stigma often lobed, 1 lobe sterile, fertile part penicillate; ovule unitegmic, integument ca 2 cells across, (chalazal cells fewer, triploid); fruits with sticky unicellular processes/spiny/smooth to irregular, (with a beak or pseudopedicel); plane of first cleavage of zygote obliquely vertical/(zygote undivided); n = x = 11, chromosomes (0.71-)1.05-3.50 μm long, heterochromatin blocks inconspicuous; germination (one cotyledon not exposed).
1/1,384. Pantropical (warm temperate), esp America. Photo: Peperomia - Flower.
Age. The crown-group age of Peperomia is estimated to be ca 88.9 Ma (J. F. Smith et al. 2008).
Evolution: Divergence & Distribution. For ages for various clades within both Piper and Peperomia, see J. F. Smith et al. (2008). Fossil evidence led Martínez et al. (2012, esp. 2014) to think that Piper originated in the early Cretaceous, a crown-group age of (117-)111(-109) Ma being driven by the attribution of a Late Cretaceous Colombian fossil to the stem group of the extant Schilleria clade of Neotropical Piper.
The discovery that Verhuellia is sister to the rest of the family (Wanke et al. 2007b) changes hypotheses about the apomorphies here. Moreover, of the three genera in the two clades that are successively basal to Piper and Peperomia, we know rather little about two.
Ulloa Ulloa et al. (2018) estimate that there are some 1,890 species of Piper in the New World alone (including around 62 species at La Selva Botanical Station in Costa Rica), Peperomia has 1,133 species; there were only three other New World genera with over 1,000 species. Certainly speciose, but how this might work out in comparisons with clades of the same age is unclear, futhermore, species limits in groups like the Old World Piper sect. Muldera are problematic, to say the least (Asmarayani 2022; see also J. F. Smith et al. 2008). Nevertheless, there seems to have been a major uptick in diversification rates at the Piperoideae node (Massoni et al. 2015a; see also Tank et al. 2015); an increase of diversification in Piperoideae was dated to (65.5-)48.8(-39.4) Ma by Magallón et al. (2018). Although crown group diversification in Piper and Peperomia began ca 71.75 and 88.9 Ma respectively, much species diversification was mid-Caenozoic and later, in Peperomia being dated to ca 57 Ma (J. F. Smith et al. 2008) Martínez et al. (2012, esp. 2014: note topology, comparison with Lactoris, etc., not relevant) estimated that the stem age of New World Piper was (106-)93, 69(-67) Ma, and with the older estimate diversification in South America was well under way by the end of the Eocene, although much diversification has been considerably more recent - thus they suggest that that the Macrostachys and Radula clades, which between then include around half the Neotropical species of the genus, began diversifying around ca 45 Ma, the average age of the species examined being 7 and 11 Ma respectively. Although Symmank et al. (2011) dated stem Peperomia to ca 57 Ma, estimates of diversification were again much later. However, Sen et al. (2019) put the origin of Piper at (116-)110(-105) Ma.
J. F. Smith et al. (2008) noted the extent of geographical signal in the phylogenies of the clades; there has been extensive migration in both genera. Thus Piper berteroana is found both on the Juan Fernandez Islands and on Tristan da Cuhna, over 4,000 km distant (Valdebenito et al. 1992), which may be something of a record, if confirmed. Diversification in the largely South American Peperomia subgenus Tildenia - distinctive, the plants are cormose and have peltate leaves - started ca 15 Ma, and it twice dispersed to Central America (Symmank et al. 2011; see also Naumann et al. 2011). For ranges of Neotropical species of Piper and their distribution patterns, see Quijano-Abril et al. (2006: track-compatability analyses) and Paul and Tonsor (2008); see also Marquis (2004) for its biogeography there. There is geographical signal in Old World Piper, too, even if distributions like that of Macropiper - New Guinea to the Pacific, also Africa - are perplexing (Asmarayani 2018).
Within Peperomia diversification rates vary, increasing notably in subgenus Micropiper (sic) which has 5-25 times more species than other clades in the genus (Franzke et al. 2016b). Although a major epiphytic genus, neither the adoption of the epiphytic habit (it predates diversification) nor the evolution of fruits with obvious means of dispersal (some have sticky papillae and pseudopedicels) seem to have driven diversification here, indeed, epiphytes are scattered in all six clades immediately below subgenus Micropiper, being unknown only in the two basalmost clades in the genus. Although Micropiper has the most epiphytic species of any subgenus, they make up only somewhat over 60% of its 800 or so species (Frenzke et al. 2016b). Perhaps the general diversity of life forms in the genus facilitated its diversification; the epiphytic habit is derived, as is the geophytic habit - several times (Symmank et al. 2008; Frenzke et al. 2016b). Little is known about fruit dispersal, although there are suggestions that there may have been some very long distance dispersal in Peperomia, for example, from South America to Masafuera (Juan Fernandez Islands) to Tristan da Cunha, more than 5,000 km (Valdebenito et al. 1990, 1992; J. F. Smith et al. 2008), however, what disperses the small fruits of terrestrial taxa - although some are sticky, a few are spiny, and the mesocarp is very thin - is unclear.
Jaramillo and Manos (2001) discussed the phylogeny and morphology of Neotropical Piper, classical taxa holding up quite well, although this is not the case in Palaeotropical taxa (Asmarayani 2018). J. F. Smith et al. (2008) noticed that species of Peperomia in their study might not be monophyletic, and accessions of other species might have separated over 50 Ma.
Ecology & Physiology. Peperomia, with some 1,385 species, is a notable element of the epiphytic flora in the Neotropics, and Piperaceae, with around 500 species of epiphytes, are the 5th most speciose epiphytic family globally (Holtum et al. 2007; Zotz et al. 2021b; Svahnström et al. 2025, q.v. for how common epiphytic taxa are relative to related non-epiphytic taxa). Carmona-Higuita et al. (2025) found that most "endemic" Piperaceae were to be found in the Paramo (14.2%), Rondonia (13.3%) and Yungas (9.7%) biogeographic provinces in the Neotropics; Peperomia tetraphylla grew almost throughout the area. Hietz et al. (2021) and Zotz et al. (2021a) discuss the ecophysiological characteristics of epiphytes in general. Crassulacean acid metabolism (CAM) is common in epiphytic Peperomia, as in a number of other epiphytes (Holthe et al. 1992; Ting et al. 1996; Symmank et al. 2008; Holtum 2023). See also Melastomataceae, Ericaceae and Gesneriaceae are other big epiphytic families in the eudicots, Araceae, Bromeliaceae and Orchidaceae in the monocots, and also ferns.
Piperaceae are also notable climbers. Thus Gentry (1991) estimated that there were some 125 species of scandent Piperaceae in the New World, while practically all Old World Piper are climbers of one sort or another (for additional references, see Schnitzer et al. 2015). All told, there are around 675 climbing species of Piper ranging from vines to quite large lianes (Gentry 1991; Rani Asmarayani pers. comm. xii.2015). In Peru the direction of climbing of piperaceous climbers may be left-handed, the unusual condition for lianes (Burnham et al. 2019).
Finally, Piper is one of the major components of the understory in Neotropical lowland rainforests, with sixty or more species sometimes occurring within quite limited areas (Marquis 2004), local diversity increasing around bat roosts (Salazar et al. 2013). As noted below, it is a member of several associations involving numerous species of diverse groups of herbivorous insects and other organisms (references in Dyer & Palmer 2004) and it is a major source of food for frugivorous Carollia bats. However, by some measures, it has low overall ecological diversity (Salazar et al. 2016). Thies and Kalko (2004) noted that in Panama, at least, species tended to prefer growing either in gaps or in the forest, and depending on where they grew, the pattern of fruit ripening differed.
All the evidence suggests that diversification of Piper in the New World began a long time before that of the bats, ca 72 Ma (see above) vs (26-)20(-18) Ma, the latter being the stem-group age for the bats (Fleming 2004; Datzmann et al. 2010), so how the current apparently close relationship between the two developed is unclear. Old World species of Piper are bird-dispersed (Fleming 2004). See also Clade Asymmetries.
Neotropical Piper in particular has numerous associations with insects, and they are in turn associated with other insects; all in all Piper is an important contributor to Neotropical insect diversity. Both the number of species of Piper and the diversity of their secondary metabolites are contributing factors to these insect associations (Richards et al. 2015; G. F. Schneider et al. 2021: secondary metabolites; Quicke et al. 2025: Costa Rico). Maybe as many as 500 or so species of the geometrid moth Eois (species numbers are very uncertain, only ca 254 species have been described, there may be over 1,000 species in the New World alone - Jahner et al. 2017) feed on Piper in the Neotropics, its ancestral host there, and the crown age of the Neotropical members of the genus (they form a clade) has been dated to around (36-)32(-16) or (28.4-)24.2(-20.3) Ma (Strutzenberger & Fiedler 2011, Strutzenberger et al. 2017 respectively). This age is generally in line with that of extensive diversification of Piper itself, estimated at being somewhat after 21.5 Ma (J. F. Smith et al. 2008, but see above), and also with the uplift of the Andes. Most diverse at mid elevations, Eois may comprise 10% of the geometrids there. Diversification in the moth may have occurred within the last 23 Ma or so, and sometimes small clades of Eois have radiated on single species of Piper (lineage duplication). Such mini-radiations are Pleistocene in age, i.e. they have happened in the last 2.6 Ma, however, there is no signal of strong co-evolution of moth and plant (Strutzenberger & Fiedler 2011), or, as Strutzenberger et al. (2017) put it, "Eois is more likely an assemblage of co-evolutionary scenarios limited to smaller clades which may be confined to small geographic scales". This diversification may have begun in Central America at low elevations, with subsequent movement to South America, but shifts in elevation, group of Piper eaten, etc., seem to be uncommon and overall rates of diversification show little variation (Jahner et al. 2017), although there may have been a slowdown in speciation in South America in the Eocene (Strutzenberger et al. 2017). These moths moved an estimated six times on to Peperomia (Strutzenberger et al. 2017), and with a few shifts elsehere, e.g. on to Chloranthaceae, but there is only a single record from a plant not containing essential oils (!Gesneriaceae, see Strutzenberger et al. 2010). Seifert et al. (2015) found 10 species, 7 undescribed, of Eois on the Peperomiathey were examining in southern Ecuador. Perhaps 17% of Eois are found in the Old World (Jahner et al. 2017), where the genus probably originated; Euphorbiaceae are its host plants there (Strutzenberger et al. 2017). For similar systems, see e.g. Sedio et al. (2021), For similar tropical systems, see Eugenia, Inga, Ocotea, Passiflora, Protium and Bursera, Psychotria, and sundry Solanaceae - discussion about Inga is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
These relationships are complex. Many of the plant-animal interactions both of Piper and Peperomia have been linked to the possession by the plant of piperamides, a class of nitrogenous compounds with the general formula R-(C=O)-NH2, where one or two of the H atoms are variously replaced (Dyer et al. 2004). Piperamides deter generalist herbivores, but like similar siutuation (e.g. glucosinolastes and Brassicacaeae) individual species of Piper may have distinctive piperamides to which particular species of Eois, for example, may be adapted (Dyer et al. 2004; Richards et al. 2010). The coexistence of members of species groups of Piper, even if the most abundant species in an area may be immediately unrelated, interacts with herbivory and interspecific diversity in secondary metabolites. In particular, there is a mismatch between plant phylogeny and the nature of the secondary metabolites the plants contain, the two not being immediately connected, and this may help enhance the local diversity of Piper (Salazar et al. 2016; Sedio et al. 2017; see also Endara et al. 2017). As M. J. Kato and Furlan (2007: p. 529) note, "groups of species specialize in the production of amides, phenylpropanoids, lignans and neolignans, benzoic acids and chromenes, alkaloids, polyketides, and a plethora of compounds of mixed biosynthetic origin" - and there may be differences between seedlings and adult plants. With increased chemical diversity comes an increase in the diversity of specialized herbivores - although not necessarily an increase in specialist folivory - and a decrease in overall leaf damage (Salazar et al. 2013; Richards et al. 2015). Herbivores that may eat plants other than Piper also attack a number of species of Piper, while specialist herbivores are found where Piper species are diverse chemically; predators avoided these Piper herbivores since they contained toxic chemicals from Piper that they had sequestered, but their parasitoids attacked them since there were no predators around (Richards et al. 2015). Indeed, a potentially very large number of braconid wasps (Parapanteles) are parasitoids on Eois caterpillars (of course, there are also other parasitoids), and although less is known about the radiation of these wasps, this, too seems be Pleistocene in age (J. S. Wilson et al. 2012a). Bats eat Eois (also primarily at lower elevations) and also the fruits of Piper (see above), beetles eat the ants, while mirid bugs, herbivory by leaf cutter ants and other generalist herbivores, etc., are all part of the complex set of associations centred on Piper (Gastreich & Gentry 2004; Rico-Gray & Oliveira 2007; Fincher et al. 2008; Richards et al. 2010; J. S. Wilson et al. 2012a).
The four to five (to nine?) species of Carollia bats (Phyllostomidae) are abundant, wide-ranging New World bats that preferentially eat and disperse the relatively high-quality (nutritionally) fruits of Piper, and perhaps some Peperomia. There are a lot of species of Neotropical Piper - maybe up to ca 1,500, with up to 64 species at a single l.t.r.f. location (Marquis 2004). However, these few species of Carollia preferentially disperse the seeds of a number of species of Piper, thus 45-47% of the diet of C. perspicillata is made up of Piper, an individual eating maybe 34 spikes a night, while over a third of the annual diet of C. sowelli is made up of P. sancti-felicis alone at La Selva, Costa Rica (Fleming 1988, 2004; Santana et al. 2021); the bats are fast feeders, ingesting the whole fruit, and the seeds, thousands per night, are dispersed in the faeces. Piper lives in the understory and in early successional habitats, and the altitudinal ranges of the bats and plants are similar (Fleming 1986). Fruits of Neotropical Piper emit volatile compounds that the bats found attractive, for instance, 2-heptanol, CH3-(CH2)-4CHOH-CH3 identified in the fruits of two species of Piper being particularly attractive (Santana et al. 2021); the bats may be attracted by essential oil extracts or other volatiles (see also Mikich et al. 2003; Muscarella & Fleming 2008; Lobova et al. 2009). Baldwin et al. (2019) shows how for these bats the gut retention time was decreased and fruit handling time increased by the presence of amides, in both cases leading to a reduction of dispersal distances. G. F. Schneider et al. (2021) noted the diversity of distinctive metabolites in the fruits of Piper in Costa Rica. Although Piper specialists eat Piper when it is available, local stocks of fruits may soon be exhausted and the bats then turn to a variety of other genera for food (Fleming 1986; Muscarella & Fleming 2008), Cecropia, Ficus, Solanum and Vismia are also favoured, and many species of these genera are plants of secondary vegetation (Fleming 1986). Indeed, Piper growing in gaps is visited by a variety of frugivores, while it was the forest-growing species that were visited almost entirely by Carollia (Thies & Kalko 2004). Carollia is on occasion insectivorous (Datzmann et al. 2010), and it may also disperse larger-seeded plants (Melo et al. 2009), while the phyllostomid Sturnira, usually a Solanum specialist, sometimes also eats Piper (Fleming 1986). For further details of bats and Piper, see Salazar et al. (2013).
There is considerable variation in the nature (druses, raphides) and pattern of oxalate deposition in the leaves of Piper and Peperomia (Horner et al. 2009, 2012, 2015 - spectacular under polarizing light). Although there is not that much correlation with phylogeny (other than the presence of raphides), Kuo-Huang et al. (2007) suggested how the druses might be involved in photosynthesis. In the single-layered palisade tissue in Peperomia a druse in the centre of each cell may help deflect light to the surrounding chloroplasts; the thylakoids in the chloroplasts are at right angles to the druse (Horner 2012). For window-leaved Peperomia in the Andes, see Horner et al. (2016).
Pollination Biology & Seed Dispersal. For a summary of pollination, see Gottsberger (2016a; see also Valentin-Silva et al. 2018); pollinators are unspecialized. Valentin-Silva et al. (2020) looked at polllination of 17 species of Piper growing together in Brazil, and found that although 16 species were pollinated by generalized visitors, in half of these there was in fact self pollination; pollinators did not seeem to be driving what diversification there was in floral traits of Piper. Sex expression, both in the Old and New Worlds, is rather labile; for the evolution of breeding systems, see e.g. Goldberg et al. (2017) and Asmarayani (2018).
For fruit dispersal, see Ecology & Physiology above. In the Neotropics, at least, a variety of ants may also disperse the seeds of Piper. Thus species of Ectatomma ants in particular, but altogether 11 species of ants from 7 genera, take seeds and surrounding pulp from the spikes of P. sancti-felis (a bat-dispersed species - see above!) in Costa Rica, dispersing the former; ants are less or not attracted to either bat faeces or cleaned seeds (Clemente ∧ Whitehead 2020).
Peperomia, the other big clade within Piperaceae, has completely different dispersal mechanisms. Species are very largely epizoochorous, attaching to dispersers either by hooks on the fruit, or more commonly by sticky secretions, as in P. polystachya; this is very unusual emong epiphytic taxa, where dispersal is either by wind or internal animal transport (Frenzke et al. 2016b), although some species in genera like Vaccinium, do have sticky seeds. The sticky substances produced by the glands are pressure-sensitive (Frenzke et al. 2016a).
Plant-Animal Interactions. See also Ecology & Physiology above. Ants are associated with some species of Piper at lower elevations and protect them (e.g. Letourneau 2004; Chomicki & Renner 2015, q.v. for dates - quite recent); obligate myrmecophytism has evolved more than once in the genus (Tepe et al. 2004, 2007: anatomy of Piper). Nitrogen may move from the ant to Piper, although amounts seem to be small and their significance unclear (Fischer et al. 2003). Production of food bodies for the ants by Piper seems to be facultative, that is, they are produced only when the appropriate Pheidole ants are around (Risch & Rickson 1981; Davidson & McKey 1993). Interestingly, first instar larvae of the clerid beetle Phyllobaenus can also induce the development of plant food bodies, but older instars are predators of Pheidole bicornis, and they can decimate the colony (Letourneau 1991). Some species of Peperomia are to be found growing in New World ant gardens (Orivel & Leroy 2011). For ants and domatia, see Chomicki et al. (2024).
Genes & Genomes. Samuel and Morawetz (1989) discuss chromosome numbers, size, variation in heterochromatin distribution, etc., and their evolution in Piperaceae; overall, Piper s.l. and Peperomia show a number of differences (see above). Qin et al. (2021: Fig 4d) found three genome duplications in Piper nigrum, Pn-α, Pn-β and Pn-γ, none of which was to be found in Aristolochia fimbriata.
Economic Importance. For the black pepper, Piper nigrum, see Ravindran (2000); it has been of major economic importance for thousands of years. The synthesis of the alkaloid piperine, the major component of the spice, has recently been clarified (L. Hu et al. 2019), and several gene clusters involved were found to have expanded. The leaves of P. betle are chewed along with betel nut (Areca catechu) or tobacco, while kava drink is made out of the roots of P. methysticum.
Chemistry, Morphology, etc.. Aerial roots of Piper have superficial cork cambium and a vascular cylinder with a very broad pith (Raman et al. 2012). The anatomy of the stem is complex, for example, in Piper s.l., which has distinctive procambial variants. Thus Cunha Neto (2023: Fig. 3. ?Manekia) shows a stem with an outer ring of sclerenchyma, and internal to that three rings of vascular bundles. Bundles of the middle ring become joined by a vascular cambium from which outer phloem and inner xylem develops - "normal", but note that the interfascicular areas remain parenchymatous. All bundles of the outer and inner rings develop secondary thickening individually, i.e they are ectopic cambia. Suwanphakdee et al. (2024) looked at stem and leaf anatomy in a number of Asian Peperomia and found that most species had scattered bundles in the stem, although a few were more like Piper - rings of vascular bundles, an undulating ring of sclerenchyma and a central mucilage canal... There was also variation in indumentum and leaf anatomy - in the latter,the adaxial hypodermis was massiva and often multiseriate, and the palisade layer was distinctive, if the cells that made it up were small; stomatal variation was considerable. See also Datta and Dasgupta (1977a - twigs, b - leaves) and Souza et al. (2004) and Nugroho et al. (2019: Indonesian Piper) for leaf and stem anatomy.
In Piper, at least, the midrib bundle is acropetal and the lateral bundles are basipetal, joining with the large medullary bundles in the stem which otherwise have no connection with the leaves; there are two rings of vascular bundles in the stem, secondary thickening occuring mostly in the outer ring (Balfour 1958a, b; c.f. de Bary 1884). Some confusion surrounds the terms used to describe the leaf. The petiole is more or less broadly sheathing and has lateral flanges for all or some of its length. Prophylls, at least on fertile plagiotropic branches I have seen, are comparable with this basal part of the petiole, although they may also be very much reduced; structures called stipules (the lateral flanges) or ligules are not homologous with such structures elsewhere. The prophylls of Piper are drawn as being lateral and with curved-involute vernation (Rousseau 1927; Blanc & Andraos 1983; see also Gardner 1997, 2003). The leaves of Piperaceae may be rich in silica (Westbrook et al. 2009).
The inflorescence of Zippelia is described as being racemose, but with the flowers being arranged sympodially (Lei et al. 2002). For the development of the peltate bracts, see Endress (1975). Syncarpy is weak; Piper has separate carpel primordia. Each carpel has a single ventral bundle. The margin of the young integument is beautifully crenate in some species of Piper (Tucker 1982). The embryo at least sometimes lacks a suspensor, but I am not sure of the distribution of this feature, while in Zippelia the zygote remains as such up to the maturity of the seed and in Peperomia it may not be much bigger (Madrid & Friedman 2010). In Zippelia and some Piper the endotegmen alone is persistent.
There is extensive variation in the differentiation of the embryo in Piperaceae, and the polarity of evolution of this feature is unclear, as is that of micropylar morphology, etc.. There is also considerable variation - some infraspecific - in the particular kind of tetrasporic embryo sac development that occurs (Arias & Williams 2008: Verhuellia not yet studied). The embryo sac of Peperomia is very variable, ranging from three-celled (but with 14 polar nuclei) to a common condition of ten cells with seven polar nuclei (e.g. Fagerlind 1939a, b and references; Madrid & Friedman 2010), that of Zippelia is 16-celled, while that of Piper is 8-celled, the antipodals being polyploid. Madrid and Friedman (2008a, 2009) suggest that the basic embryo sac for the family - at least all the family minus the currently unstudied Verhuellia - may be the Drusa type, which is tetrasporic and with sixteen cells, 11 of which congregate at the chalazal end (three of the megaspores migrate there first). The endosperm ranges from 15n (in Peperomia) to triploid. Kanta (1963) noted that there was extensive division of the antipodal cells during early seed development. The nucellar cells of Peperomia, at least, are in radiating files (Fagerlind 1939a).
Some information is taken from Bornstein (1991), Tebbs (1993) and Dyer and Palmer (2004), all general; see also Hegnauer (1969, 1990), M. J. Kato and Furlan (2007) and de Araujo et al. (2018: essential oils) for chemistry, searching Natural Products Alert (NAPRALERT) may also help, Piperno (2006: phytoliths), Weberling (1970: stipules), Burger (1972: Central American Piper), Blanc and Andraos (1983, 1984: growth patterns), Schmitz (1872: floral development), Johnson (1914), Murty (1959a, b), Kanta (1963), and Johri et al. (1992), all floral morphology and embryology, and Lei et al. (2002: embryology of Zippelia); for floral development, see Lei and Liang (1998: Piper; 1999: Peperomia), Tucker et al. (1993: Zippelia), and Samain et al. (2010a: Verhuellia).
Phylogeny. Relationships are likely to be [Verhuellia [[Zippelia + Manekia] [Piper + Peperomia]]] (Jaramillo & Callejas 2004; Wanke et al. 2006a, 2007a, b; Naumann et al. 2013: these relationships not always obtained; Z.-D. Chen et al. 2016; Jost et al. 2021); this entails redrawing the old subfamilial boundaries. Massoni et al. (2015a) also recovered a clade [Zippelia + Manekia].
Jaramillo and Callejas (2004) and J. F. Smith et al. (2005, 2008) found that Piper s. str. was divided into New and Old World clades, the latter, Piper s. str., being divided into a mainland Asian clade, containing both the two endemic African species and a species from Australia, and also a Pacific islands Macropiper clade including the economically very important Piper methysticum (Jaramillo & Callejas 2004 found that one African species they examined grouped with their Pacific clade - see also Jaramillo et al. 2008; Smith et al. 2008). This Pacific clade, the Macropiper clade, was either sister to the rest of the genus or sister to the Asian clade (Jaramillo et al. 2008). However, Asmarayani (2018) found no support for the monophyly of New World Piper (although its monophyly could not be excluded), and in a study with a focus on Old World Piper she recovered the relationships ["New World species" [Macropiper (including African species) ["West of Wallace's Line Piper"/"East of Wallace's Line Piper"]]]. Rather similarly, Simmonds et al. (2021: chloroplast + nuclear ribosomal DNA, 28 taxa) found plastome relationships to be [the Neo-tropical lineage [P. commutatum from Mexico [the Macropiper clade, inc. P. capense + the Asian Tropic clade, Piper s. str.]]]. Paul and Tonsor (2008) discuss aspects of the diversification of Piper in the New World, interestingly, classical infrageneric groups are holding up better in New than Old World Piper (Asmarayani 2018). Notice, however, that Simmonds et al. (2021) found some hard incongruence beween the nuclear and chloroplast phylogenies they obtained, indeed, P. commutatum showed signs of wanting to move. In a trnK/matK analysis, Wanke et al. (2007a) found much less resolution within Piper than Peperomia. For the phylogeny of Peperomia, see Wanke et al. (2006a, 2007a), Samain et al. (2009: but c.f. outgroup, also characters used), Naumann et al. (2011), Frenzke et al. (2015, 2016b) and Simmonds et al. (2021). Kobayashi et al. (2019) looked at relationships within Peperomia subgenus Micropiper; in a subsequent study they found extensive incongruence between the same plastid data set and nuclear ITS sequences, and they suggested that hybridization was the cause (Kobayashi et al. 2022). Many of the characters previously considered to be systematically important in Peperomia have evolved in parallel (Samain et al. 2009).
Classification. For the classification of Piperaceae followed here, see Samain et al. (2008, 2010a); unfortunately, the subfamilies are not easily characterisable. Although Peperomia is indeed distinctive, its recognition as a separate family would make Piperaceae paraphyletic, and two more very small families (total - 9 species, 3 genera) would need to be described.
Frenzke et al. (2015) assigned 80% of the species of Peperomia to 14 well-supported monophyletic subgenera that they characterized in detail. Bornstein (2024) recognized nine subgenera in Neotropical Piper based on his work and that of Jaramillo et al., etc..
Botanical Trivia. Peperomia has the dubious distinction of having the most herbarium names of any genus, about 1,530. Most of these names were coined by William Trelease - and are mostly synonyms (Mathieu 2007).
Thanks. I am grateful to Diego Salazar for information on what eats New World Piper, Rani Asmarayani for information on Old World Piper, and to S. Wanke for estimates of species numbers.
SAURURACEAE Richard - Back to Piperales
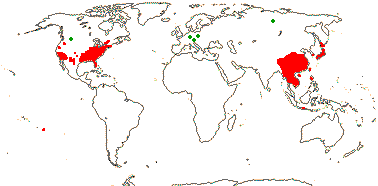
Plant rhizomatous or herbaceous; leucanthocyanins +, alkaloids 0; cambium in fascicular areas only, wood ?not storied, rayless [?always], vessel elements with scalariform perforation plates; druses or crystal sand +; nodes 5:7 or 7-9:7-9; petiole bundles arcuate (annular); cuticle waxes as parallel platelets; stipules +, intrapetiolar; inflorescence terminal/leaf opposed; common bract/flower primordium +/0; A often 3, or 6 or 8 in two whorls, ± connate in pairs, introrse; pollen (trichotomosulcate), often boat-shaped, 20> µm long, ektexine tectate-columellate, punctate, punctae surrounded by papillae; G [3-4], placentation parietal, filaments ± adnate to G, styluli +, recurved, margins papillate; ovules 4-13/carpel, micropyle zig-zag (exostomal), outer integument 2-3 cells across, inner integument 3-4 cells across, parietal tissue 1-2 cells across; fruit dry, dehiscing apically; exotestal and tegmic cell walls thickened, former lignified or not; endosperm (helobial), unicellular chalazal haustorium +, embryo minute, only slightly differentiated; x = 12 (?11), nuclear genome [1C] (0.036-)0.952(-24.965) pg.
4 [list]/6. Temperate North America, East Asia to West Malesia. Map: from Z.-Y. Wu (1983), Ying et al. (1993) and Fl. N. America vol. 3 (1997), fossil distribution from S. Y. Smith (2007, green crosses). Photo: Collection.
Age. Possible crown-group ages for the family are (84-)78, 75(-69) Ma (Wikström et al. 2001), (77-)54, 47(-26) Ma (Bell et al. 2010), or (80.8-)63.9, 51.8(-46.7) Ma (Massoni et al. 2015a).
Grímsson et al. (2017c) described tiny (3-5 x 6-11 μm!) 82-81 Ma pollen grains from the Campanian of Wyoming that they thought was practically identical to that of Saururus; they named it Saururus aquilae but were agnostic where on the Saururaceae tree it should be placed - perhaps stem Saururaceae.
1. Saururus L. + Anemopsis Siebold & Zuccarini
Rhizomatous or stoloniferous perennial; (vascular bundles in two rings - S.); (vessel elements with simple perforation plates - A.); lamina vernation involute; inflorescence lax, nodding when young, (uppermost foliage leaves ± white - S. chinensis); (A basal - S.); (G free - S.); (ovules 1-2/carpel - S.); (fruit a schizocarp, surface warty, units 1-seeded - S.); n = 9, 11.
2/3: Saururus (2). Temperate North America, eastern India to China, Japan, Korea, Luzon. Photos: Saururus Habit © E. Pontieri, Saururus Inflorescence © E. Pontieri.
Age: S. Y. Smith and Stockey (2007b) described a fossil assigned to Saururaceae, Saururus tuckerae, from the Middle Eocene of British Columbia ca 44.3 Ma, and although its stamen number (5, basally adnate to the carpels) differs from those normally associated with the family, there is clearly much variability here (see also Massoni et al. 2015b; López-Martínez et al. 2023). The fossil has also been associated with monocots, etc. (López-Martínez et al. 2023a). For other records of fossils, see Friis et al. (2011).
2. Houttuynia Thunberg + Gymnotheca Decaisne
Rhizomatous perennials; inflorescence dense, erect, inflorescence bracts petal-like; (floral bracts petal-like - ); (pollen lacking papillae around punctae - G.); (parietal tissue 0 - H.); n = 11, 12-64 - H..
2/3: Gymnotheca (2). Eastern India to China, South Korea and Japan, south to Thailand, introduced into Java?
Evolution: Pollination Biology. The small individual floral bracts of Anemopsis are petal-like; it and Houttuynia are described as having pseudanthia (Baczynski & Claßen-Bockhoff 2023). Saururus cernuus has a stigmatic self-incompatibility mechanism (Pontieri & Sage 1999). Armbruster et al. (2002) described Houttuynia as being partly syncarpous and having a compitum.
Chemistry, Morphology, etc.. Anemopsis, alone in the family, has a relatively well developed vascular cambium and also vessel elements with simple perforations (Carlquist et al. 1995). The nodal anatomy of Houttuynia is 5:7, but only a single trace supplies the prophyll, and its bud is supplied by vascular tissue leaving from the sides of the gap that the trace produced (Murty 1961); nodes of Saururus are 7-9:7-9 and the central bundle may temporarily split (Murty 1959c). The stomata of Houttuynia are surrounded by cells that are arranged spirally (Peterson et al. 2010: rosette of 4-6 cells - Murty 1961). According to Murty (1961) the single intrapetiolar stipule represents two connate stipules (see also Lactoris above).
Ovules of Houttuynia lack parietal tissue (Murty 1961). Each carpel has two ventral bundles, whether or not they are fused.
Some information is taken from Wood (1971) and Wu and Kubitzki (1993), both general, Hegnauer (1963, 1990: chemistry), Datta and Dasgupta (1977a, b: vegetative anatomy), Carlquist et al. (1995: wood anatomy), Quibell (1941), Tucker (1981 and references), Liang and Tucker (1990) and Liang et al. (1996), various aspects of floral development and anatomy, S. Y. Smith and Stockey (2007a: pollen ultrastructure) and Raju (1961: embryology).
Phylogeny. [Saururus + Gymnotheca] was recovered in all analyses, but [Houttuynia + Anemopsis] only in analyses of chloroplast data (Meng et al. 2002, 2003). [Houttuynia + Anemopsis] was also found in a three-gene analysis, but the support is poor; [Saururus + Gymnotheca] is a better-supported clade (Jaramillo et al. 2002). The relationships [[Saururus + Gymnotheca] [Houttuynia + Anemopsis]] are also recovered in other molecular analyses (e.g. Neinhuis et al. 2005; Massoni et al. 2014; Jost et al. 2021), although they are not found in morphological studies. The latter genus pair is also apparent in the Angiosperms353 tree, Jan. 2023 version (see Seed Plant Tree of Life; Gymnotheca was not included.
)