EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
Age. The approximate age for this node is 191 Ma (Wu et al. 2014) or only 130.3 Ma (Magallón et al. 2015); Tank et al. (2015: Table S1) dated this clade to around 131.4 Mya, while around 144-140 Ma was the estimate in Zeng et al. (2017) and (137-)131(-123) Ma in P.-L. Liu et al. (2020). In many phylogenies Sabiaceae are adjacent to other members of the order along the eudicot spine, but whatever the topology, ages are rather younger. Estimates for a topology [Proteales [Sabiales [Buxales...]]] range from (143-)129, 126(-116) Ma (Bell et al. 2010 for details), while Xue et al. (2012) estimate (126.4-)121.4(-110.2) Ma, Naumann et al. (2013) around 124.8 Ma, and Magallón et al. (2013) about 121.5 Ma. Wikström et al. (2001) estimated (150-)144-130(-124) Ma for the stem Nelumbo, etc., clade and (145-)140, 128(-123) Ma for stem-group Sabiaceae; Anderson et al. (2005) dated stem group Sabiaceae to 122-118 Ma, and it would be slightly older than the stem Nelumbo, etc., clade
Evolution: Divergence & Distribution. Endress (2011a) suggested that syncarpy might be a key innovation somewhere around here; optimization on the tree is not easy. Positioning of other apomorphies is also difficult. Although the androecial feature "stamens numerous, but then usually fasciculate and/or centrifugal" is placed at the [Rosids et al. + Asterids et al.] / Pentapetalae node, there is no particular reason why it should not be placed here. If CRABSCLAW expression is found in the nectaries of Sabiaceae and Proteaceae, this, to could be placed at this node, and it would be interesting to look at what is going on in Buxaceae, too (for nectaries, see also Tölke et al. 2019); along the same lines, sucrose synthesis and secretion is similar in the floral nectaries of the Brassicaceae and Solanaceae examined, which are extrastaminal and gynoecial nectaries respectively (Lin et al. 2014). See also the Pentapetalae page.
Chemistry, Morphology, etc.. For the distinction between gynoecial (supposedly asterids only) and receptacular nectaries, see Smets (1988), Smets et al. (2003) and Erbar (2014: the two not always easy to distinguish); for a general survey of nectaries, see also Bernadello (2007). Nectary vascularization can vary between quite closely related taxa (e.g. Saxena 1973; de Paula et al. 2011).
PROTEALES Berchtold & J. Presl - Main Tree.
Lamina margin serrate, ?tooth morphology; stigma dry; ovules 1-2/carpel, ± apical, pendulous, apotropous; seed coat?; endosperm development?, slight or 0, embryo long. - 4 families, 85 genera, 1,750 species.
Includes Nelumbonaceae, Platanaceae, Proteaceae, Sabiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Sauquet et al. (2009b) suggested ages for this node of around (125.1-)123.4(-119.6) Ma, Magallón and Castillo (2009) suggest ca 122.8 and 123.6 Ma, the age in Magallón et al. (2015) is about 127.5 Ma, that in Tank et al. (2015: Table S2) about 130.3 Ma, those in Zeng et al. (2017) 137-127 Ma, and that in T. Yang et al. (2018) (129.4-)122.8(-111.6) Ma, but the estimates in Foster et al. (2016a: q.v. for details), at ca 141 Ma and especially in Z. Wu et al. (2014), at ca 189 Ma, are considerably older.
Evolution: Divergence & Distribution. Although the order is small, it is morphologically heterogeneous. Endress (2011) discusses ovule curvature and integument thickness; see M.-Y. Zhang et al. (2017) for the evolution of pollen features, while the study of Nelumbo fruit morphology by Romanov et al. (2021) bears on variation in the order as a whole.
Chemistry, Morphology, etc.. Fot discussion on stomatal morphology, etc., here, see Gobo et al. (2023).
Phylogeny. For discussion of the monophyly and relationships of this very unexpected clade, see the eudicot node.
Previous Relationships. Thorne (2007) includes the order, variously broken up, along with Buxales, in his heterogeneous Ranunculidae, however, most authors (e.g. Cronquist 1981; Takhtajan 1997) have not seen any connections at all between the four families included below.
Classification. The inclusion of Sabiaceae in Proteales seems the sensible thing to do, assuming that its relationships continue to hold up (Y. Sun et al. 2015; A.P.G. IV 2016), however, because support for its position was not very strong in the plastome analysis of H.-T. Li et al. (2021), they inclined towards placing it in its own order. Ovule number and embryo are similar in the combined group (see also Romanov et al. 2021 for possible apomorphies).
Synonymy: Proteinae Reveal - Meliosmales C. Y. Wu et al., Nelumbonales Martius, Platanales Martius, Sabiales Takhtajan - Nelumbonineae Shipunov - Proteanae Takhtajan, Nelumbonanae Reveal, Sabianae Doweld - Nelumbonidae Takhtajan - Nelumbonopsida Endlicher, Proteopsida Bartling
SABIACEAE Blume, nom. cons. - Back to Proteales
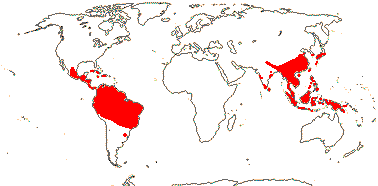
Woody; pentacyclic triterpenoids +, tanniniferous, benzylisoquinoline alkaloids?; vessel elements with simple/scalariform perforation plates, bars few (-30); (true tracheids +); secondary phloem with broad or flaring rays; (sieve tube plastids also with protein crystalloids); cuticle wax crystalloids 0; stomata also paracytic; lamina venation often brochidodromous; flowers 5-merous, bracteoles ?0; P = K + C, (K) C A opposite each other, K quincuncial, with single/no trace, C quincuncial; pollen tricolporate, orbicules +; nectary +, receptacular, thin, ± lobed, annular; G connate, [2(-3], completely closed (also secretory canal), when 2, oblique or median, stigma punctate, wet, no papillae, compitum +; ovules (1-)2/carpel, campylotropous or ± straight, unitegmic, integument 3-6 cells across, nucellus apex exposed, intraovular hairs +; ?antipodal cells; fruit a drupelet to ± dry, style subbasal; endocarp usu. thick, surface often ± ornamented; seed coat undistinguished; endosperm helobial[?], chalazal endosperm haustorium +, embryo curved, cotyledons folded, suspensor ± 0; x = 8 (?7, ?6).
4 [list]/119 (66). Sri Lanka and South East Asia to Malesia, tropical America. Map: from van Beusekom (1973), see also T. Yang et al. (2018), Sinimbu, and pers. comm. Rafael Sühs.
Age. Anderson et al. (2005) date crown group Sabiaceae at 119-91 Ma; (135-)129, 114(-108) Ma is the figure in Wikström et al. (2001) and (92.7-)86.2(-83.5) Ma in T. Yang et al. (2018).
Fossils identified as Sabiaceae are known from the Cretaceous-Cenomanian ca 98 Ma (Insitiocarpus, c.f. Meliosma) and -Turonian (Sabia) of Europe (Knobloch & Mai 1986; Friis et al. 2011).
1. Sabioideae Y. W. Law & Y. F. Wu - Sabia Colebrook
Lianes; wood rays 0; buds perulate; leaves simple, 2-ranked [?often], lamina margins entire (minutely toothed); inflorescence various, (1-flowered), axillary; filaments flattened, anther dehiscence vertical, valvate; G fusion postgenital, style usu. +; drupelets 1-2, flattened, schizocarpic when 2; seeds with numerous dark reddish glands; n = 12.
1/30. Sri Lanka and South East Asia to Malesia.
Age. Crown-group Sabioideae are estimated to be a mere (19.5-)11.6(-4.8) Ma (T. Yang et al. 2018).
Fossils from the Czech Republic and named Sabia menispermoides are dated to around 93.9-83.4 Ma
2. Meliosmoideae Masters —— Synonymy: Meliosmaceae Meisner, Wellingtoniaceae Meisner
Shrubs to trees; wood rays broad; (pits vestured - Meliosma); nodes complex unilacunar [Meliosma]; buds naked; leaves spiral, imparipinnate (simple), leaflet vernation conduplicate [Meliosma], margins toothed/entire; inflorescences terminal (axillary); flowers obliquely monosymmetric, (4-merous); (K not vascularized); C ± unequal, (inner C deeply lobed); A (adnate to base of C - Meliosma), 2 fertile, anther dehiscence (transverse), valvate, filaments swollen apically, opposing C ± orbicular, 3 staminodial, opposing C small, bifid or not; nectary appendages alternating with A, G fusion congenital, style +, (styluli +, ?not vascularized, marginal, ovary roof + - Ophiocaryon); placentation axile, integuments (2); drupelet usu. 1, asymmetrically subspherical, ridged, endocarp (splitting[?]), (with long canal including vascular bundle); (cotyledons coiled - Ophiocaryon), (radicle folded); n = 16.
3/89: Meliosma (80). Sri Lanka and South East Asia to Malesia, tropical America. [Photo - Flower, Fruit.]
Age. The age of crown-group Meliosmoideae is around (69.6-)67.3(-66.1) Ma (T. Yang et al. 2018).
Evolution: Divergence & Distribution. >Given the wide distribution of fossil Sabiaceae in the northern hemisphere in the Paleogene, migration by northern landbridges seems a reasonable way to explain the Indo-Malesian - Neotropical distribution of the family, although the amphipacific disjunction of Kingsboroughia alba, dated to only (6.7-)2.4(0.05) Ma, is more likely to be the result of long distance dispersal (T. Yang et al. 2018). Crown-group Sabia has a 70 My+ stem...
Pollination Biology & Seed Dispersal. Most species of Meliosma have explosively dehiscent anthers that are held under tension by the complex staminodes, but there is also a kind of secondary pollination presentation in which pollen collects on the broad connective between the anther sacs (Ronse de Craene & Wanntorp 2008; Zúñiga 2015). Wong Sato and Kato (2018) noted that the flower of M. tenuis appears polysymmetric to the pollinator; pollination cannot take place until after the two anthers explode and after the staminodes fall off, which is a day or so later. Ronse de Craene et al. (2015b) and Thaowetsuwan et al. (2017) suggested that anther dehiscence in Sabia and Ophiocaryon respectively was similar.
Genes & Genomes. A genome duplication ca 9.7 Ma is associated with Sabiaceae (Landis et al. 2018).
Chemistry, Morphology, etc.. Sabiaceae are distinctive among members of the eudicot grade in that the perianth is differentiated into a calyx and corolla (Drinnan et al. 1994; Hoot et al. 1999) and there is a nectary that appears to be axial/receptacular (see Erbar 2014). However, the interpretation of the flower of Meliosma, especially of the nature of the perianth members, is difficult. Two sepals are smaller than the others and have been called bracteoles, as by Endress (2010c), who would then interpret the flower as being basically monosymmetric and trimerous, and the calyx whorl and the two whorls of both corolla and androecium as all alternating (one member of each is reduced). Others, e.g. Zúñiga (2015), incline to thinking of the flower as being 5-merous. According to Baillon (1874), the two carpels of Sabia are median; Warburg (1896) drew the two carpels of Meliosma as being oblique to the vertical axis of the flowers, but median to the plane between the two bracteoles; van Beusekom and van der Water (1989) show the carpels as being oblique both to the vertical axis and to the plane between the bracteoles, and the flower could be called obliquely monosymmetric. Wanntorp and Ronse de Craene (2007) illustrate the carpels as being more or less collateral, and Ronse de Craene (2010) as slightly oblique, bracteoles are not shown, but their position is described as being variable - but there is discussion as to whether or not the flowers have bracteoles. Thus Ronse De Craene et al. (2015a) noted substantial differences in the floral development of the two species of Sabia they examined that they suggest is connected to the incorporation of a bracteole into the flower in S. japonica as a "sepal", while Thaowetsuwan et al. (2017), although noting variation in size between members of the two perianth whorls in Ophiocaryon, thought that bracteoles might be absent, there being a 5-merous calyx and corolla. See also Zúñiga (2015) and in particular Sokolov et al. (2018) for more discussion on floral phyllotaxis here.
There may be variation in the number of anther loculi/thecae. Ophiocaryon paradoxum has a coiled embryo; it is known as the snake nut.
For a general account, see Kubitzki (2006b) and Nadiah (2023), for chemistry, see Hegnauer (1973, 1990), and for wood anatomy, which is very variable, see Carlquist et al. (1993). For a revision of Sabia, see van de Water (1980), for Meliosma, see van Beusekom (1971).
Phylogeny. Relationships are [Sabia [[Meliosma alba + Ophiocaryon] other Meliosma]] (Zúñiga 2015: analyses of chloroplast/combined data, but not nrITS). These relationships were confirmed - and quite well supported - in the more extensive analysis of T. Yang et al. (2018: chloroplast data only), but earlier sectional groupings do not hold up.
Classification. The classification here follows that in T. Yang et al. (2018).
[Nelumbonaceae [Platanaceae + Proteaceae]]: epidermal waxes with tubules [2/3], nonacosan-10-ol the main wax; nodes?; stipules sheathing [2/3]; connective extended beyond anther loculi.
Age. Wikström et al. (2001) estimate this node to be (143-)137, 125(-119) Ma, Bell et al. (2010) suggested ages (131-)116, 110(-101) Ma, Xue et al. (2012) ages of (122.8-)109.3(-75.2[- 16.3 My!]) Ma, almost the age of fossils reliably assigned to Nelumbonaceae, Anderson et al. (2005) date this node to around 121-115 Ma and Tank et al. (2015: Table S2) to about 119.8 Ma. Magallón and Castillo (2009) and Magallón et al. (2015) suggest an age of around 117 Ma, Magallón et al. (2013) an age of around 105 Ma and Xue et al. (2012) ages of (123.8-)ca 109(-16.3) Ma; (125-)121.7, 119.9(-114.3) Ma are some ages in Sauquet et al. (2009b), around 120-115 Ma in Zeng et al. (2017), a low ca 82.6 Ma in Naumann et al. (2013), close, ca 93.1 Ma, in Y. Li et al. (2020) and a high ca 177 Ma in Z. Wu et al. (2014).
The oldest fossils of this clade (Nelumbonaceae, Nelumbites) are around 107-99.6 Ma (Upchurch & Wolfe 2005; see also Doyle & Endress 2010; Friis et al. 2011). Silvestro et al. (2021) estimated that the time-of-origin of Nelumbonaceae was about 122.7 Ma.
Evolution: Divergence & Distribution. Barthlott et al. (1996) noted that the cuticle waxes of Platanaceae and Nelumbonaceae were very different. However, Hayes et al. (2000) emphasised that there were only two sepals in Nelumbo, so the [Nelumbonaceae [Platanaceae + Proteaceae]] clade could be characterised as having dimerous flowers (see Doyle & Endress 2000 for Proteaceae). However, fossils assignable to Platanaceae are very variable in their floral morphologies, and some seem to have much more conventional, almost core eudicot-like flowers (von Balthazar & Schönenberger 2009). Fossils like Exnelumbites, from Late Cretaceous Mexico, have chloranthoid leaf teeth, that is, teeth with a glandular apex, a vein proceeding to the apex and joined by branches from above and below (Estrada-Ruiz et al. 2011). In the platanoid teeth of Platanus higher order veins approach but do not enter the apex, and so they are perhaps not so different from chloranthoid teeth (Doyle 2007; Estrada-Ruiz et al. 2011).
Chemistry, Morphology, etc.. For variation in microsporogenesis and pollen morphology, see Furness and Rudall (2004) and Denk and Tekleva (2006); successive microsporogenesis has been reported from both Nelumbonaceae and Platanaceae.
NELUMBONACEAE A. Richard, nom. cons. - Nelumbo Adanson - Back to Proteales
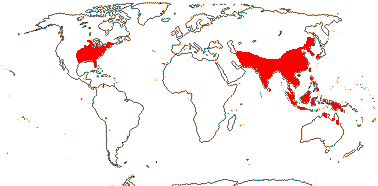
Aquatic herbs, rhizomatous; mycorrhizae 0; benzylisoquioline, aporphine alkaloids +; laticifers +, articulated; root stele polyarch; cork?; plant with air canals; vascular cambium 0; vascular bundles lacking fibrous sheath; stem endodermis +; tubular P-protein and rod-shaped bodies +; nodes ?; cuticle waxes as clustered tubules, nonacosan-10-ol and nonacosanediols, stomata mostly adaxial, adaxial epoidermal cells papillate; prophyll adaxial; leaves vertically two-ranked, in groups of three along the stem, sheathing cataphyll on one side then cataphyll and expanded leaf on the other side; leaf peltate, lamina with a central disc, vernation involute, margin entire, venation actinodromous, midrib unbranched, other main veins dichotomising, festooned brochidodromous, freely ending veinlets 0, stipule sheathing, open; pedicel with central ring of bundles derived from central stele in stem, other cylinders from cortical bundles; flowers single, ?axillary, protogynous, large [>4 cm across], complex cortical vascular system +; P = K + C, K 2, 4, C 10-30, spiral; A many, from ring meristem, development chaotic, at least outer extrorse, connective with a terminal appendage, filament often with more than one bundle; tapetal cells multinucleate; microsporogenesis also successive, pollen with membranous granular layer + [innermost endexine]; receptacle massive, obconical, with emergent druses; G (2-)10-30, carpels ascidiate, immersed in receptacle, occluded by secretion, cells surrounding stylar canal long-papillate, stylulus 0, stigma expanded, papillate, wet; ovule 1/carpel, submarginal-apical, anatropous, ?pachychalazal, outer integument ca 30 cells across, inner integument (?5-)8-10 cells across, integuments slightly lobed, parietal tissue 3-5 cells across, nucellar cap ca 4 cells across, chalaza massive, postament +, hypostase +, funicle massive, funicular obturator +, of hairs; antipodal cells multiplying, multinucleate, persistent; fruit a nutlet, with an apical pore, pericarp with outer and inner epidermis 2-layered, subdermal exocarp layer with light line, outer mesocarp lignified, inner mesocarp spongy, with air spaces; testa ± obliterated; endosperm slight, embryo large, chlorophyllous, differentiated, cotyledons massive, tubular but basically two, several leaf primordia; n = 8, x = 8, nuclear genome [1 C] (0.024-)0.539(-11.918) pg; radicle aborting, adventitious roots +.
1 [list]/2. Temperate, E. North America and E. Asia. Map: from Fl. N. Am. vol. 3 (1997), Fu and Hong (2000), Sculthorpe (1987) and Wu (1983) - the last two include all Malesia and N. Australia in the range, but the plant is not in N.W. Australia, at least, in FloraBase 2006, the range in Sculthorpe (1987) also includes the Antilles and NW South America...]). [Photo - Nelumbo Flower © J. Manhart, Collection.]
Age. Crown-group Nelumbonaceae may be (6.5-)1.6(-0.1) Ma (Xue et al. 2012).
A fossil recently described as Notocyamus hydrophobus has been collected in ?Lower Aptian rocks of the Crato Formation in N.E. Brazil estimated to be ca 121 Ma (Gobo et al. 2023). The fossil is a whole plant in fruit, and it has the distinctive fruit type of Nelumbo, although with marginal leaf attachment, secondary thickening, etc.. Morphological phylogenetic analyses place it with Nelumbo, although allowing a single step more it might end up sister to [Magnoliales + Laurales]; the fosssil does have the palinactodromous leaf venation and marginal leaf attachment of Platanus (Gobo et al. 2023).
Floral formula: * K 2, 4; C 10<; A many; G 10-30.
Evolution: Divergence & Distribution. Like Nymphaeaceae, Nelumbonaceae were much more diverse in the past. Cretaceous fossils - probably to be assigned to stem-group Nelumbonaceae, since the leaves lack the central disc and may even have chloranthoid teeth, and the floral receptacle is shaped differently - have been placed in several species and four genera (Estrada-Ruiz et al. 2011 and references; Friis et al. 2011). Fossils of Nelumbonaceae - known from leaves and fruits, although these are not connected - are also known from southern Argentina in late Upper Cretaceous rocks of Campanian-Maastrichtian age (Gandolfo & Cuneo 2005). Fossil Nelumbonaceae, as Nelumbites, the leaves with rather different venation but the flowers with the distinctive expanded floral receptacle of extant Nelumbo (but different in shape), are reported from the mid to Late Albian (late Lower Cretaceous) ca 107-99.6 Ma (Upchurch & Wolfe 2005; see also Doyle & Endress 2010; Friis et al. 2011; Doyle & Upchurch 2014). Thus although Nelumbo is currently restricted to the northern hemisphere, these early fossils from the southern hemisphere suggest that the evolutionary history of the family will need to be interpreted with care.
Given the great age of the clade, 100 Ma or substantially more, Nelumbo has been called a living fossil, at least from the molecular point of view, and it also shows considerable morphological stasis (Sanderson & Doyle 2001; Xue et al. 2012).
Ecology & Physiology. Vogel (2004a) provide a fascinating account of air circulation in Nelumbo, i.a. suggesting the air flows in different directions in different halves of the leaf, similarly in the petiole, and that air may move down one petiole and then up another two nodes behind on the stem (see also Matthews & Seymour 2006). The central disc/central plate/navel has many giant stomata and is the site of air exit for the petiolar canals (see also Estrada-Ruiz et al. 2011); if covered by water, air from the petiolar canals bubbles up through it. This circulation probably supplies the submersed roots and rhizomes with oxygen (c.f. Armstrong & Armstrong 2009). Furthermore, Nelumbo is noted for its extremely hydrophobic upper surface of the lamina (that is where most of the stomata are, they are smaller/normal in size), the result of the micromorphology of the epidermal surface, which has papillae, wax tubules, etc. (Ensikat et al. 2011).
Pollination Biology & Seed Dispersal. The flowers are thermogenic, starch breaking down in the expanded receptacle, and halictid bees and especially chrysomelid bettles are the likely pollinators (Vogel & Hadacek 2004; Watling et al. 2006; Li & Huang 2009; Dieringer et al. 2014). The progamic phase, the time between pollination and fertilization, is notably short, as in at least some other aquatic angiosperms (including Nymphaea: see Williams et al. 2010). The sharply pointed and often six-rayed epidermal druses on the surface of the receptacle may protect it against herbivores (Vogel 2004b).
Lotus fruits are noted for their longevity, and fruits 1350±220 yrs old have been germinated (Shen-Miller et al. 1995). This may be connected with the chemical composition of the fruit wall which is distinctive in its high polysaccharide (galactose, mannose) and tannin content, compared to the lignin + cellulose composition of (e.g.) the seed coat of Nymphaeaceae (ven Bergen et al. 1997). For a water gap in the fruit, see Gama-Arachchige et al. (2013).
Vegetative Variation. Understanding how Nelumbo grows is difficult. The cataphylls more or less surround the stem and presumably represent the stipular portion of the leaf. The sheathing stipule associated with the foliage leaf is open on the side of the stem opposite to the leaf insertion. Axillary branches show the same arrangement as the leaves as described above, but with the addition of the prophyll which is on the same side of the branch as the first cataphyll. Flower buds develop in the axis of the second cataphyll, axillary branches in the axil of the expanded leaf, however, other interpretations are also possible. Despite its loss of cambial activity, Nelumbo shows little evidence of this at the genomic level (Povilus et al. 2018, 2019/2020 - c.f. Nymphaeaceae, monocots). For some literature on the growth pattern of Nelumbo, see Eichler (1878), Wigand and Dennert (1888), Miki (1926), and Esau and Kosakai (1975).
Vessels arise first in the roots, but are also found in the rhizome, and the trend of their specialisation is similar; this is the monocot pattern. Details of the root cortex of Nelumbonaceae differ considerably from those in Nymphaeales (Seago 2002). Although the vascular bundles are scattered in the stem, they are inside an endodermis (Seago 2020), although the genus is also scored "N" - no stem endodermis. For foliar venation, see Estrada-Ruiz et al. (2011).
Genes & Genomes. There has been a very slow rate of substitution in the nuclear genome of Nelumbo, perhaps because of its long generation time (vegetative reproduction is common, also long-lived fruits), and there was a genome duplication, the λ duplication, some (76-)65(-54) Ma (Ming et al. 2013). P.-L. Liu et al. (2020) also suggest that there was a Nelumbo-specific duplication that is unconnected with the duplication evident in the core eudicots.
Z. Wu et al. (2014) described the chloroplast genome of Nelumbo.
Chemistry, Morphology, etc.. For benzylisoquinoline alkaloids, see Y. Li et al. (2020).
For a summary of pedicel/floral vasculature, which differs considerably from that found in Nymphaeales, see Moseley et al. (1993). Although the floral vasculature is complex because of the presence of rings of cortical bundles, the vascularization of individual parts of the flower is undistinguished; the sepals may, however, have but a single trace that quickly divides (Moseley & Uhl 1985). Hayes et al. (2000) noted that the two sepals are inserted in the vertical plane; Moseley and Uhl (1985) found that there may be four, decussating sepals. The stamens develop from an androecial ring, and they and the carpels may be irregularly whorled (Hayes et al. 2000). Gupta and Ahluwalia (1978) noted that there was sometimes a very small "third integument" between the two normal integuments, there was also an hump on one side of the carpel beneath which air spaces developed. Cronquist (1981) described the stamens as being introrse-latrose; Endress (1995) as extrorse, Takhtajan (1997) as extrorse (the outer members) and introrse (the others). There is also disagreement over endosperm development which has been variously described as nuclear, cellular, or helobial, and over pollen morphology (Kreunen & Osborne 1999).
Titova and Batygina (1996) described the morphology of the embryo in some detail - in the context of the relationships between Nelumbo and other(!) Nymphaeaceae, Ceratophyllum and monocots. I.a. they mention that the embryo is dorsi-ventrally symmetrical, the two cotyledons originating on a common cotyledonary ridge, etc..
Some general information is taken from Wigand and Dennert (1888), Williamson and Schneider (1993) and Hayes et al. (2000), for chemistry, see Hegnauer (1969, 1990: as Nymphaeaceae), for floral anatomy, see Ito (1986b), for stamens in particular, see Moseley (1958), for embryology, etc., see Cook (1909), Khanna (1965) and Batygina et al. (1982), for fruit morphology, development, etc., see Romanov et al. (2021) and for seed coat anatomy, see Gama-Arachchige et al. (2013: esp. water gap).
Previous Relationships. In the past, Nelumbonaceae were usually associated with Nymphaeaceae (e.g. Cronquist 1981), the two having superficially similar flowers and vegetative body (both are "water lilies") - and it turns out that floral gene expression patterns in Nymphaea and Nelumbo are remarkably similar (Yoo et al. 2010). Takhtajan (1997) removed Nelumbonaceae from Nymphaeales, but placed them alone in his subclass Nelumbonidae. Both the morphology of the cuticle waxes and plant chemistry suggest a relationship with Ranunculales, but there the waxes are nonacosan-10-ol rather than 4-10- or 5-10-diol (Barthlott et al. 1996, 2003: the difference not emphasised).
[Platanaceae + Proteaceae]: woody; non-hydrolysable tannins, myricetin +, benzylisoquinoline alkaloids 0; (pits vestured); wood with broad rays [8+-seriate]; stomata laterocytic; flowers 4-merous [but see fossil Platanaceae]; P +, not differentiated; stamens = perianth, opposite them; carpels with 5 vascular bundles, hairy, postgenital fusion complete, stylulus long; ovules straight, inner integument 3-5 cells across; endosperm nuclear.
Age. Wikström et al. (2001) estimate this node to be (124-)117, 108(-101) Ma, ages in Sauquet et al. (2009b), at (121-)114.6, 113.3(-107.6) Ma are similar while those in Bell et al. (2010), at (102-)99, 88(-97) Ma and Tank et al. (2015: Table S2), at around 93.4 Ma, are a little younger. Anderson et al. (2005) date the node to 119-110 Ma, Barker et al. (2007b) to (126.7-)118.5(-110.3) Ma, while ca 106.2 Ma is the age in Magallón et al. (2015).
Age. Platanocarpus (most = Friisicarpus) is known fossil from the Lower Cretaceous 113-98 Ma (Crane et al. 1993), and other fossils are associated with Platanaceae in the constrained morphological analysis of Doyle and Endress (2010).
Evolution: Divergence & Distribution. That Platanaceae and Proteaceae are sister taxa may explain why there are so many leaf fossils from the southern hemisphere that are "platanoid" in their general aspect (K. Johnason, in Drinnan et al. 1994; Hoot et al. 1999).
Chemistry, Morphology, etc.. The wood anatomy of Proteaceae and Platanaceae is very different, perhaps because of the different climatic conditions under which the two grow (Baas et al. 2003); however, they both have broad rays, and the former have concave vessel-parenchyma festoons and the latter concave growth ring boundaries. For stomatal morphology, see Carpenter et al. (2005).
Although flowers of Platanaceae and Proteaceae look very different, von Balthazar and Schönenberger (2009; see also Ronse De Craene 2015b) suggest similarities; both consist of perianth, stamens, and fleshy structures. In Platanaceae the latter may represent an outer staminal whorl, in Proteaceae an inner whorl. This hypothesis needs further study, but if the difference in their positions are confirmed they will not be an apomorphy at this level. Endress (2011) suggests that the outer integument is 2(-3) cell layers thick here.
PLATANACEAE T. Lestibudois, nom. cons. - Platanus L. - Back to Proteales
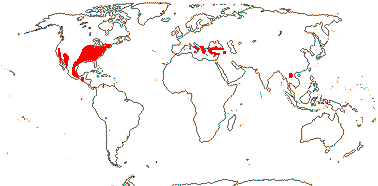
Growth sympodial; plant deciduous (evergreen); cork in outer cortex; nodes multilacunar; petiole bundle annular, wing bundles +; ?resin ducts +; hairs candelabriform, basal cell conoid, over the junction of epidermal cells; leaves two-ranked (spiral), lamina vernation plicate-revolute, teeth glandular, with a terminal cavity, higher order veins approach but do not enter it, (margin entire), 2 strong secondary veins near base (venation pinnate; also in seedlings), petiole enclosing the axillary bud (not), stipule tubular, closed (adaxial-sheathing, open); plant monoecious; inflorescences capitate; flowers sessile, small [≤5 mm across], 3-4(-7) merous; P uniseriate, connate or not, often lacking vasculature; staminate flowers: outer whorl staminodial, ca 3, tiny; anthers valvate, connective with subpeltate apex; pollen semitectate-reticulate, 16-22 µm long; (pistillodes +); carpelate flowers: staminodes +; G (3-)5-8(-9), in two whorls, stigma decurrent in two crests, unicellular-papillate, ± dry; ovules 1(-2)/carpel, lateral-subapical, outer integument 3-4 cells across, integuments slightly lobed; fruit an achene, with basal tuft of hairs; mesotesta thick-walled, sclereidal; seed reserves hemicellulosic, endosperm moderate, embryo suspensor uniseriate, ca 4-celled; n = 16-21, x = 7, chromosomes 1.4 µm long [mean], nuclear genome [1 C] (0.033-)1.601(-78.113) pg.
1 [list]/10. North Temperate. Map: from Fl. N. Am. vol. III (1997), Jalas et al. (1999), Europe, and Feng et al. (2005). [Photo - Leaves & Stipules, Collection.]
Age. Quadriplatanus georgianus, in Coniacian to Santonian deposits ca 86 Ma in Georgia, U.S.A., has been linked with Platanaceae (Magallón-Puebla et al. 1997), and also with Malpighiales, etc. (López-Martínez et al. 2023a). There are numerous fossil remains identified as Platanaceae that go back to the Valanginian-Hauterivian, i.e. to some 137.1-125.8 Ma (Magallón-Puebla et al. 1997 and references).
Fossils with the distinctive hollow, sheathing petiole bases of subgenus Platanus are abundant in the Palaeocene some 60 Ma (Feng et al. 2005); this puts a lower limit on the crown-group age of the family. However, leaves with such petioles may have small, fugaceous, triangular stipules very unlike those of extant taxa (Wang et al. 2011). Furthermore, it is unclear that such petiole bases are restricted to subgenus Platanus; they could have been lost in P. kerrii - but this is reported to have a hollow petiole base... (Leroy 1982; Nares et al. 2024).
Maslova (2010) included Platanaceae in Hamamelidales, along with a number of fossil genera, some placed in the extinct Platanaceae-Gymnoplatananthoideae and also in Bogutchantaceae (sic) N. Maslova (= Bogutchanthaceae) - see also Hamamelidaceae).
Evolution: Divergence & Distribution. The morphology of fossil Platanaceae sometimes differs substantially from that of their extant relatives (see also Kvacek 2008: whole plant reconstruction; Friis et al. 2011; Midell et al. 2014). Thus the leaves may be tri- or pentafoliolate (Kvacek et al. 2001a, = Platanites, 5 species known from west North America to western Scotland in rocks from the Cretaceous to the Eocene, Nares et al. 2024), imparipinnnate/ly lobed (= Sapindopsis: for this genus, see Doyle 2014b; Sender et al. 2016 and references) or unlobed, if with the distinctive swollen petiole base common here (Langeria: Huegele & Manchester 2022). It may be that Platanus is ancestrally deciduous (Nares et al. 2024).
Indeed, in Upper Cretaceous plants there is extensive floral variation, and plants can have inflorescences with sessile or pedunculate heads: Staminate flowers = P 4, basally connate, stamens equal and opposite perianth members and arising from a short ring of tissue, alternating with ?staminodes, perhaps of an outer whorl, or two 4- or 5-membered whorls of perianth present, A 5, pistillode consistently present, or P in 2 whorls, connate, outer more or less completely so, or free; G 8, 2 opposite each member of inner perianth, ovules perhaps anatropous, stylulus 0. Mindell et al. (2014) described the Late Cretaceous Ambiplatanus with very small heads and some perfect flowers; the 5-merous perianth was in two whorls with the stamens opposite members of the inner whorl (no comment was made about this position) and also the carpels - the latter had no style, and it is possible that the seeds were winged and the fruit dehiscent. The Middle Cenomanian Verneda hermaphroditica has, as its name implies, perfect flowers, also a perianth with at least three whorls, filaments that are fused basally, and a eusyncarpous and pentalocular gynoecium, yet it is placed in this area in morphological analyses (Moreau et al. 2016: esp. pp. 833-834). Some fossils have rather smaller pollen than that of extant taxa - are they wind-pollinated? - insect pollination is the suggestion for some of them. Tricolporate pollen has even been found in situ in fossils assigned to Platanaceae (e.g. Manchester 1986; Crane et al. 1993; Pedersen et al. 1994; Friis et al. 1988, 2011; Magallón-Puebla et al. 1997: Mindell et al. 2006: Crepet et al. 2004; von Balthazar & Schönenberger 2009; Taylor et al. 2009; Doyle & Upchurch 2014 for other references). Cretaceous Platanaceae do not have hairy fruits (von Balthazar & Schönenberger 2009; Friis et al. 2011), and the fruits may even be follicles (Crane et al. 1993).
From the molecular point of view, at least, Platanus can be considered a living fossil (Sanderson & Doyle 2001).
Subgenus Castaneophyllum (Platanus kerrii, from Thailand) differs in lamina morphology, venation, petiole base and stipule morphology from subgenus Platanus.
Ecology & Physiology. In the Late Cretaceous-Miocene Platanus was a common plant of disturbed streamside habitats; frequent associates included Ginkgo, Metasequoia and Cercidiphyllum (Royer et al. 2003); for further details see above. Some species of Platanus grow in such habitats today.
Pollination Biology. There is about five weeks between pollination and fertilization in Platanus racemosa (Floyd et al. 1999), while in P. × acerifolia, at least, flower buds form the year before anthesis (X. Yan et al. 2024).
Genes & Genomes. There is evidence from the stomatal size of fossils that polyploidization occurred within this clade (Masterson 2004), indeed, X. Yan et al. (2024) sequenced the genome of Platanus × acerifolia and found that it was an ancient hexaploid, and they dated it to ca 49 Ma - in fact very young compared to the ages suggested immediately above for the family. None of the three subgenomes showed dominance. The reconstructed ancestral eudicot karyotype was very similar to the karyotype of P. × acerifolia in terms of gene content, presence of colinear blocks, etc., and it was less similar to the karyotypes of Buxus sinica, etc.; there has been karyotypic stasis here (Yan et al. 2024) - c.f. Asteraceae.
Chemistry, Morphology, etc.. Hennig et al. (1994) described the cuticle wax as lacking crystalloids, Fehrenbach and Barthlott (1988) as having rodlets and platelets. There is some variation in stomatal morphology (Carpenter et al. 2005). Platanoid leaf teeth are supposed to be a variant of chloranthaceous teeth (for which, see e.g. Nelumbonaceae) in which the accessory veins approach but do not fuse with the central vein which ends in the tooth (Doyle 2007; Estrada-Ruiz et al. 2011).
Von Balthazar and Schönenberger (2009) described the parts of the flower as alternating regularly; the androecium was biseriate, the outer, very much reduced whorl appearing late in development. Developmental studies suggest that flowers of Platanus are basically 4-merous (A. Douglas in Hoot et al. 1999).
Some information is taken from Kubitzki (1993b); see Floyd et al. (1999) and Floyd and Friedman (2000) for embryology and endosperm development and Denk and Tekleva (2006) for pollen of extant and fossil taxa. See Smets (1986) for nectaries, Melikian (1973) and Takhtajan (1991) for testa anatomy, and Floyd et al. (1999) for embryology; for chemistry, see Hegnauer (1969, 1990), and for vegetative characters, see Doyle and Upchurch (2014).
Phylogeny. For a phylogeny of Platanus, see Grimm and Denk (2008).
Previous Relationships. Platanaceae were included in Hamamelidales by both Cronquist (1981) and Takhtajan (1997).
PROTEACEAE Jussieu, nom. cons. - Back to Proteales
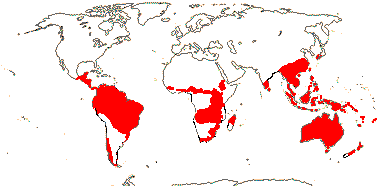
Trees or (acaulescent) shrubs; leaf [P] low, ca 0.4 mg g-1 [?here]; lateral roots of limited growth, forming clusters [= proteoid roots], mycorrhizae 0; vessel elements with simple perforation plates (scalariform, bars few), true tracheids and libriform fibres +, banded and unilateral paratracheal parenchyma, rays of two distinct sizes; phloem stratified or not; nodes (1:1), 3:3, 5:5; petiole bundles numerous, pattern complex; sclereids common; hairs with 2 short cells, one in epidermis, with doughnut-shaped base, apical cell elongated, bifid or not; stomata parallel to long axis of leaf; leaves spiral (opposite), (odd-pinnately, rarely palmately, compound or lobed), lamina often coriaceous, vernation usu. conduplicate, margins spiny toothed to entire, base of petiole often swollen, stipules 0; inflorescence various; flowers 4-merous; P = C-like, valvate, decussate-diagonal; A (connective appendage 0); tapetal cells binucleate; microsporogenesis also successive, cleavage centrifugal; pollen triangular in polar view, oblate, triporate, pores broadly operculate, apertures in threes at four points of the young tetrad [Garside's Rule], (colpate), tectum forms pore membrane, foot layer forming irregular blocks, exine with ektexine only, endexine 0; G 1, orientation adaxial, stigma terminal or lateral, often slit-like, secretory; ovules submarginal to submarginal-basal, long, vascular bundles forming a ring in the chalazal region, outer integument 2(-9) cells across, inner integument 3-4(-6) cells across, integuments slightly lobed [level], parietal tissue (?0-)2-16 cells across, nucellar cap 2-7 cells across, ± endothelial, hypostase +; (antipodals not persistent); (exotesta +), endotesta palisade, with crystals , (exotegmen fibrous); cotyledons large, embryo suspensor 0; x = 7, nuclear genome [1 C] (0.245-)2.478(-25.102) pg.
80 [list]/1,615 - five subfamilies below. Largely southern hemisphere, esp. Australia and S. Africa. Map: from Johnson and Briggs (1975), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Weston (2006) and Prance et al. (2007).
Age. Anderson et al. (2005) date crown-group Proteaceae at 96-85 Ma, Barker et al. (2007b) at (126-)118(-110) Ma, (97-)91.4, 88.2(-82.6) Ma is the estimate in Sauquet et al. (2009b: Bellendena sister to Persoonioideae), (115.5-)107.2(-96.1) Ma in Onstein et al. (2016) and around 125.6-110.9 Ma (Skeels et al. 2025b).
Hill et al. (1995) and Weston (2006) summarize the fossil record of the family, Carpenter (2012) that of leaf fossils in particular. Leng et al. (2005) discuss small but mature capsular fruits from late Cretaceous (late Santonian/early Campanian) Sweden which have several attributes of Proteaceae, e.g. the flowers are paired, the stigma is somewhat abaxial on the fruit. However, there are also differences, e.g. there are only three vascular bundles per carpel and there seems to be little in the way of a perianth; one species has the most remarkable papillate seeds. Proteaceae fossils are known from sediments ca 94 Ma old in Australia, i.e., shortly after the separation of Australia from Antarctica some 97 Ma (Hill & Brodribb 2006), while pollen grains, Triorites africaensis and identified as Proteaceae (but some species are not - see Wanntorp et al. 2011c), are known from deposits ca 93 Ma in northern Africa and Peru (Walker & Doyle 1994).
1. Bellendenoideae P. H. Weston - Bellendena montana R. Brown
Plants Al-accumulators; inflorescence terminal, bracts 0; secondary pollen presentation; ovules 2/carpel; fruit dry, indehiscent, 2-winged; n = 5, chromosomes ca 6.7 µm long, nuclear genome size ca 1 pg DNA (means).
1/1. Australia (Tasmania).
[Persoonioideae [Grevilleoideae + Symphionematoideae + Proteoideae]]: stomata brachyparacytic; P connate; A adnate to P, more or less sessile; nectary +, receptacular, ± vascularized, [(2-)4-lobed, annular, unilateral]; stylulus long; endosperm +; (cotyledonary blade cordate).
Age. Wikström et al. (2001) estimated that this node was (67-)60, 47(-40) Ma old.
2. Persoonioideae L. A. S. Johnson & B. Briggs
Proteoid roots 0; tepals with Vorlaüferspitze; pollen discharge passive; 1-2(+) ovules/carpel; fruit a drupe (follicle - Placospermum); cotyledons obreniform; n = 7, chromosomes 9.1-14.4 µm long, nuclear genome size 2.5-4.3 pg DNA (means).
4/110. Australia, New Caledonia and New Zealand.
Age. This clade may be (84.1-)72.3(-60.5) Ma (Barker et al. 2007b) while (66.3-)49, 47.2(-31) Ma is the age in Sauquet et al. (2009b).
2a. Placospermeae C. T. White & W. D. Francis - Placospermum coriaceum C. T. White & W. D. Francis
Plants Al-accumulators.
1/1. N.E. Queensland, Australia.
Cluster roots 0; amphistomaty common; polycotyly [Persoonia].
3/110: Persoonia (101). Mostly Australia, also New Caledonia and New Zealand.
Age. The age of this clade is (38-)22.2, 20.8(-10) Ma (Sauquet et al. 2009b) or (79.1-)45.7(-21.6) Ma (Onstein et al. 2016).
Pollen of Persoonieae is known from the later Cretaceous (Maastrichtan) onwards (Dettmann & Jarzen 1998).
[Grevilleoideae [Symphionematoideae + Proteoideae]]: (tyrosine-derived cyanogenic glycosides +); T orthogonal [?level]; flowers vertically or obliquely monosymmetric [P split to base on one side (4:0), or 3 P connate, 1 free (3:1)]; (ovules anatropous); x = 14, chromosomes 0.5-5 µm long, nuclear genome size 0.05-0.27 pg DNA (means).
Age. This clade is some (93.7-)88.3, 86.2(-80.8) Ma (Sauquet et al. 2009b).
3. Grevilleoideae Engler
Plants Al accumulators; sieve tubes with rosette-like non-dispersive protein bodies; (hairs biramous); stomata randomly oriented [parallel - Sphalmium]; (leaves deeply pinnately-lobed (with small teeth)); paired flowers subtended by a common bract (not); (A not adnate to P); pollen diporate, (boomerang-shaped), also with abundant endexine, also in the apertural region; (carpel orientation diagonal), stigmatic head swollen ellipsoid to disciform [Lomatia]; ovules (1-)2+/carpel; fruit a drupe or follicle, the latter with winged seeds [wing from outer integument]; seeds (pachychalazal), (endotestal cells short); endosperm with nuclear chalazal haustorium; cotyledons basally auriculate; n = (10-)14(-15), chromosomes 1-2.6 µm long [mean].
46/950. Australia and the S.E. Pacific to Southeast Asia, S. India and Sri Lanka, South America, few South Africa and Madagascar. Photos - Grevillea Flower, Embothrium Flower, Fruit, Habit. unplaced: Sphalmium — Carnarvonia
Age. Crown-group Grevilleoideae are some (86.1-)80.4, 78.1(-73.8) Ma (Sauquet et al. 2009b), (109.6-)90.1(-87.2) Ma (Onstein et al. 2016) or 113.5-97.4 Ma (Skeels et al. 2025b).
Pollen from 4/7 tribes of Grevilleoideae is known from the later Cretaceous (Dettmann & Jarzen 1998). For example, Late Cretaceous pollen identified as Macadamia (Macadamieae) is known from southeast Australia and the Antarctic peninsula (Dettmann & Jarzen 1990); Macadamia in the old sense includes the New Caledonian Virotia. Pollen of the Gevuina/Hicksbeachia type has a similar distribution; Gevuina from Australia and eastern New Guinea is now placed in Bleasdalea. Knightia pollen is also known from Australia (Dettmann & Jarzen 1990); Knightia from New Caledonia is now placed in Eucarpha. The oldest leaf fossil of Proteaceae from the Americas, Proteaceaefolia araucoensis, perhaps a member of Grevilleoideae, was described from Palaeocene-Thanetian deposits ca 55 Ma from the Lota–Coronel flora from central Chile (Carpenter & McLoughlin 2025, q.v. for caveats about a number of vegetative fossils from this flora supposedly of Neotropical affinities); it lacks a cuticle, but is similar otherwise to the E. Australian Orites excelsus.
3A. Embothrieae Meisner
Leaves (isobifacial, linear, divided or not); (secondary pollen presentation, pollen on the style apex - Hakea); (serotiny: fruits massive, woody).
10/: Hakea s.l. (520: inc. Grevillea)
[Macadamieae [Roupaleae + Banksieae]]: ?
3B. Macadamieae Venkata Rao
(tapetal cells uninucleate - Macadamia).
16/:
3C. Roupaleae Meisner
(cluster roots 0); leaves (opposite); ovule 1/carpel; seeds (winged).
13/150: Helicia (110), Roupala (34). India and Sri Lanka, China to Australia, Mexico to Argentina.
3D. Banksieae Dumortier —— Synonymy: Banksiaceae Berchtold & J. Presl
3/229: Banksia (225: see Stimpson et al. 2016). Australia, 1 sp. New Guinea.
[Symphionematoideae + Proteoideae]: fruit indehiscent.
Age. This clade is some (90.4-)85.1, 83.6(-78.3) Ma (Sauquet et al. 2009b) or ca 122 Ma ("median time" - Lamont et al. 2023).
4. Symphionematoideae P. H. Weston & N. P. Barker
Proteoid roots 0; T orientation?; pollen discharge passive/by vibration from anthers; nectaries 0; ovules 1-2/carpel; fruit dry; n = 10.
2/3. S.E. Australia, inc. Tasmania.
Age. The crown-group age of this clade is slightly less than 80 Ma (Barker et al. 2007b), (70.3-)56, 44.9(-20.5) Ma (Sauquet et al. 2009b), or (78.4-)25.0(-7.3) Ma (Onstein et al. 2016).
5. Proteoideae Eaton —— Synonymy: Lepidocarpaceae Schultz Schultestein
(Herbaceous), (plants Al-accumulators); sieve tubes with non-dispersive protein bodies; flowers sessile; (hypanthium +); T free; (neectary 0); ovules 1(2)/carpel), (bistomal); fruit often single-seeded, drupe or nut, (follicle, serotinous or not), (surrounded by bracts, branches); n = (10-)11-13(-14), chromosomes 1.2-3.4 µm long [mean].
25/655. Africa S. of the Sahara, esp. the Cape region, Australia, esp. the West.
Age. Proteoideae are estimated to be some (86.4-)80.6, 80.6(-75.3) Ma (Sauquet et al. 2009b), (92.2-)85.5(-74.8) Ma (Onstein et al. 2016) or ca 101.1 Ma (Lamont et al. 2023).
Pollen identified as that of Beauprea, now from New Caledonia, is known from Australian deposits some 80 Ma or more (Dettmann & Jarzen 1990: Fig. 7).
Proteoideae II: leaves various, inc. linear, bifurcating-spiny; secondary pollen presentation +, style apex bearing the pollen, pollen discharge explosive.
fibres relatively narrow [Leuc.]Unplaced: Eidothea — Beauprea — Beaupreopsis — Dilobeia — Cenarrhenes — Franklandia - most Madagascar, New Caledonia, Tasmania, etc..
Eidithoea Beauprea, Stirlingia etc. - pollen tricolpoidate, endexine 0.
5A. Proteeae Dumortier
(rays distant [6> rays/mm - Prot.]); nectar with up to ca 1/3 xylose [pentose].
2/130: Protea (115). Africa, Guinea and the Sudan southwards, Madagascar, but mostly the Cape.
5B. Conospermeae Endlicher - ex Proteoideae I
(cluster roots 0); (plant herbaceous - Stirlingia); flowers (monosymmetric: Conospermum); pollen discharge explosive [anthers and stigma held under tension, separate when touched, pollen ejected], A 3, staminode + 2 monothecate A / passive.
3/113: Synaphea (56), Conosperma (50). Australia, esp. the west.
5C. Petrophileae P. H. Weston & N. P. Barker
(plant dioecious - Aul.); seeds hairy [Pet.].
2/69: Petrophile (66). Petrophile Australia, mostly western, Aulax South Africa.
5D. Leucadendreae P. H. Weston & N. P. Barker
(fibres relatively narrow - Leuc.); (flowers single, axillary - Aden.); (pollen discharge passive - esp. Leuc., wind pollination).
11/280: Leucadendron (96), Serruria (57), Leucospermum (48), Isopogon (40), Adenanthos (33), Spatella (20). South Africa, mostly the Cape, to East Africa, Isopogon and Adenanthos mostly S.W. Australia.
Floral formula: */⚥ T [2 + 2]; A 4; G 1.
Evolution: Divergence & Distribution. For additional ages of clades within Proteaceae, see Sauquet et al. (2009b: Table S2) and Onstein et al. (2016).
There is a great diversity of pollen assigned to Proteaceae from the late Cretaceous (Campanian-Maastrichtian) in central and southeast Australia (Dettmann & Jarzen 1991, 1996: 31 species in 5 morphogenera from the Otway Basin, 1998: a number of taxa restricted to the Eocene; see also Friis et al. 2011; Ladd & Bowen 2020), and Proteaceae seem to have been very diverse and ecologically important in at least parts of that continent (see also Itzstein-Davey 2004; Lamont & He 2012, but c.f. Hill & Jordan 2016 in part). Fossil Proteaceae are also prominent in Patagonia (Barreda et al. 2012), southern Africa (Scholz 1985: Namaqualand), the Antarctic Peninsula (Bowman et al. 2014; Barreda et al. 2019: pollen ca 83 Ma), and elsewhere in Gondwana by the later Cretaceous (see also Askin 1989; Dettmann 1989); they are second (after Nothofagaceae) as fossils from Antarctica (de la Estrella et al. 2019b). Carpenter et al. (2015) suggest that late Cretaceous Proteaceae may have dominated the vegetation in which they grew, and fire was very important then. But particularly with the older fossils, it can be difficult to understand the ecology of the plants. Thus Ladd and Bowen (2020) note that fossil pollen identified as Embothrium is dated to around 78 Ma; the genus is now pollinated by birds, members of clades that are quite young, and so what pollinated such plants in the Cretaceous is unclear. Similarly, Ladd and Bowen (2020) are inclined to think that explosive pollination mechanisms may have been quite important in the early history of the family, but who exactly was getting dusted with pollen becomes a question. Kooyman et al. (2014) discuss earlier possibly wider ranges of southern proteaceous genera. Thus He et al. (2016a) suggested that Beauprea (Proteoideae), known fossil - it has distinctive pollen - from throughout eastern Gondwana and including New Zealand up to a mere 1 Ma, but now known only from New Caledonia, was on Zealandia 82 Ma as it split from Gondwana, and so it may have been an original inhabitant both of New Caledonia and New Zealand (but c.f. Giribet & Baker 2019: cautions as to whether these islands have always been emergent) - see also Beaupreopsis. Indeed, there are extensive pollen records of Proteaceae on New Zealand from the later Cretaceous and through the Neogene (Wanntorp et al. 2011c), but there has been much subsequent extinction and only two species are to be found on the islands now (Lee et al. 2001; for the history of the New Zealand/New Caledonian flora (and fauna), about which there has been much discussion, see e.g. Nothofagaceae and Amborellaceae). Even in end-Cretaceous deposits on Campbell Island, now an isolated Antarctic island south of New Zealand, no fewer than twelve species of proteaceous pollen were recovered (Wanntorp et al. 2011c).
Although some transoceanic disjunctions in the family, for example that of the sister taxa Cardwellia in Australia and Gevuina in South America, could reflect vicariance/continental drift events, others, like Brabejum in Africa which is sister to Panopsis in South America involve genera whose estimated time of divergence is later than the geological events that might seem to have caused their distribution patterns (Barker et al. 2007b). The crown-group age of Grevilleoideae-Embothrieae was estimated to be around 70.8 Ma (Milner et al. 2015; 68.1-66.6 Ma in Sauquet et al. 2009b), but although current distributions of Lomatia and Embothrium/Oreocallis (= Embothriidae) are Gondwanan, at least some details of diversification are unconnected with the breakup of Gondwana (Sanmartín & Ronquist 2004; Milner et al. 2015; see also Weston & Crisp 1994). Indeed, Proteaceae are a noted southern family, and pre-freeze Antarctica may well have been importantly involved in their dispersal, although ecologically many hardly fit the description of "palaeo-Antarctic rainforest lineages" (see elsewhere for more discussion).
Reyes et al. (2014) suggested that Proteaceae often diversified in hotspots of Mediterranean vegetation (for the age of such vegetation, see He et al. 2016b and references). Floral monosymmetry seems to have led to both enhanced speciation and extinction, so overall no change (Reyes et al. 2014). Sauquet et al. (2009b, q.v. for much more detail, note topology) compare diversification in the Cape and S.W. Australian hotspots, and find that although more clades are to be found in the latter area, not all of them had diversified much. Fires may have spurred diversification, as in Banksia, where flower retention on inflorescences and leaf retention on plants may increase the intensity of fires, even if exactly how this might benefit a species is not entirely clear (He et al. 2011; see also Bond & Midgley 1995). Midgley and Bond (2011: p. 6) suggested that "ancient fire is uniquely Australian", and fire-dominated eucalypt vegetation may have begun to develop there in the earliest Caenozoic, a little before Banksia diversified (Crisp et al. 2011), but both heath vegetation with numerous Proteaceae, including Banksia (but no Myrtaceae) and also evidence of fires from Central Australia has been dated to around 89-65.5 Ma (Lamont & He 2012; Carpenter et al. 2015; Cowan et al. 2023 for post-fire regeneration of Banksia; see also below). Indeed, Lamont et al. (2018b) suggested that fire-stimulated germination originated in the Proteoideae ca 81 Ma, the family becoming (sic) fire-prone 15 Ma before that (Lamont et al. 2018b).
Several lines of molecular evidence indicate that there may have been rapid diversification within Grevilleoideae (Hoot & Douglas 1998; Skeels et al. 2025a). The stem age of the very largely Australian Banksieae is estimated at (94.9-)87.9(-80.9) Ma (Barker et al. 2007b, q.v. for other ages). Banksia itself may be ca 60.8 Ma old, the crown group being (56.9-)44.5(-36.6) Ma; serotiny here is coeval with the origin of the genus (He et al. 2011). More recent diversification within Banksia may have been instigated by vicariance events such as the aridification of the Nullarbor Plain some 14-13 Ma which led to the separation of what became eastern and western clades (Crisp & Cook 2007; see also Ladiges et al. 2012). However, although Banksia is diverse in areas with Mediterranean climates, it does not seem to have undergone particularly rapid speciation, rather, unexceptionable rates over a long period of time in a stable habitat may have resulted in the numbers it has (Cardillo & Pratt 2013; see also Skeels et al. 2025b). Crown group Hakea s. str. (ca 150 species) may be as young as (14.0-)9.6(-6.4) Ma (Mast et al. 2012, see also 2009), although Lamont et al. (2016a) suggested an age of around 14 Ma while Hanley et al. (2008) and especially Mast et al. (2015) suggested ages in excess of 20 Ma, maybe to 35 Ma. The origin of this clade may be in southwest Australia (there are around 315 species of Hakes s.l. there), and there may have been around twelve west to east movements in the clade, indeed, it has been estimated that there have been around 47 shifts from one biome to another, a remarkably high number when compared with other Mediterranean taxa, including Banksia and Protea (Cardillo et al. 2017; Skeels & Cardillo 2017); phylogenetic niche conservatism would seem to be an alien concept here. Diversification has been+ most rapid in a clade of Hakea s. str. rather than in Grevillea s. str. (Mast et al. 2015). Extensive studies have been carried out on the interaction between traits such as serotiny, spiny leaves, pollination by meliphagids, fruit size, conspicuousness and aggregation, and seed predation by cockatoos in S.W. Australian species of Hakea s. str.. It transpires that large fruits, even if conspicuous and not protected by spiny leaves, etc., are largely impervious to the attention of cockatoos despite these fruits being serotinuous and so remaining on the plant for a long time (even decades); the flowers of such plants were often pollinated by meliphagids, features such as non-spiny leaves, etc., allowing them easy access to the flowers (Lamont et al. 2016b).
Skeels et al. (2025b) take up the issue of the biogeography of Grevilleoideae in the context of their densely-sampled nuclear phylogeny of the subfamily interpreted in the context of the changing climates, etc., of the Sahul area over the last 90 My or so, and they note that all four tribes and most of the subtribes had diverged by the K/P boundary event of ca 66 Ma. The ancestor of Grevillioideae is likely to have been a plant growing around 113.5-97.4 Ma in the tropical biome as the Sahul area was developing. The highly speciose Hakea s.l., which has getting on to 1/3 of the species in the whole family, started diverging a little over 40 Ma at the time of a major mass extinction; this clade has a stem of over 20 Ma (Dettmann & Jarzen 1998 noted that a number of pollen taxa were restricted to the Eocene). Skeels et al. (2025b) note that in long-occupied biomes like S.W. Australia diversification may be low but overall diversity is high. In general, diversification tends to be high in new/emerging or expanding biomes. The climate in which taxa in S.E. Australia grow is more or less Cretaceous in age, and although overall diversity may be lowet than in S.W. Australia, generic diversity is higher (Skeels et al. 2025b). Overall, diversity of the subtropical and temperate biome has remained roughly constant, that in Mediterranean biome remained constant from ca 80 Ma to the later Oligocene, when it increased, that in semi-aris biome remained low and constant from 75-40 Ma, with an increase 40-20 Ma, and that in arid region developed in the mid-Oligocene ca 30 Ma, overall diversity in the various biomes being constant for the last 15 Ma or so (Skeels et al. 2025b: Fig. 2B).
Protea, speciose in southern Africa with some 70 out of its 115 species being restricted to the Cape, has been studied by Barraclough and Reeves (2005), although they found it difficult to pin down dates for its diversification. Sauquet et al. (2009a, b) suggest that diversification in Protea may have occurred within the last 18 Ma although it may have been there since the late Cretaceous, on the other hand, Leucadendrinae had started diversifying in southern Africa as much as 39 Ma, even though they had been in the Cape region itself for a shorter time than Protea; there had been considerable diversification within Proteoideae even back the Cretaceous (Sauquet et al. 2009b, see also Linder 2003: ca 270 spp. in Cape Floristic Region). On the other hand, Schnitzler et al. (2011; Mitchell et al. 2017 for a phylogeny) thought that diversification in Protea began ca 28 Ma in the middle of the Oligocene and was connected with edaphic (soil mediated) speciation; rates of diversification throughout its range were similar (Valente et al. 2010b; see also Silvestro et al. 2011; Lamont et al. 2017). Mitchell et al. (2018) found a number of associations between various aspects of vegetative construction and temperature, elevation, rainfall, and the like in the diversifiction of Protea, thus plant and leaf size are positively correlated with temperature and leaf construction costs are negatively correlated with rainfall. Proteaceae are more plants of the Fynbos rather than the Renostervels (du Preez et al. 2025b). See below for fire and the evolution of Cape Proteoideae.
A recent paper by Lamont et al. (2023) suggests a rather different interpretation of at least part of the biogeographical history of Proteaceae. They note that the pollen record of Proteaceae in Africa is very rich, starting off with Triorites africaensis grains known from 107-94 Ma (Lamont et al. 2023), the whole African pollen record suggesting gradual southwards migration of plants that could be assigned to Proteoideae. This prompted these authors to disregard the molecular relationships in Proteoideae which linked particular African and Australian groups, rather, they thought that “probable parallel evolution ... in matched but disparate habitats (e.g. sclerophyll shrublands in Australia and South Africa) accompanied by extensive extinction over such long expanses of geological time may give the wrong impression that [molecularly] linked clades are siblings. Thus, molecular phylogenies may sometimes create relationships that are more apparent than real.” (Lamont et al. 2023: p. 656). These authors thought that tere was parallel molecular evolution between members of Proteoideae on the two continents; clades currently in similar habitats on the Cape and in S.W. Australia probably arose from a common ancestor in N.W. Africa.
Interestingly, leaf fossils of Eocene-Miocene Persoonioideae-Persoonieae are hypostomatic, while most extant species are amphistomatous, the plants then probably growing in more open habitats - and perhaps in communities unlike those in which Persoonieae are now generally found (Carpenter et al. 2017). Stomatal size could easily be added to the list of characters; it is in part correlated with chromosome numbers, etc. (Carpenter et al. 2010a).
Ecology & Physiology. Proteaceae are most diverse in rather dry climates on nutrient-poor old soils in Australia and southern Africa, especially soils poor in phosphorus (P) (see Orians & Milewski 2007; Lawes & Neumann 2022) - old, climatically-buffered, infertile landscapes (OCBILs: Hayes et al. 2021). Salt tolerance is uncommon (Flowers et al. 2010). It is estimated that fire-proneness in Proteoideae arose around 90 Ma, with a variety of fire-associated traits evolving over the next 20 My or so (Lamont et al. 2018a, b). Fire-adapted and Proteaceae-dominated heathlands in Australia have been dated at ca 89-84 or 75-65.5 Ma (Lamont & He 2012; Carpenter et al. 2015 respectively; see also Carpenter et al 2016b), temperature and atmospheric oxygen concentrations being high then. There were numerous species of Proteaceae in these early heathlands, judging by pollen and vegetative remains, Proteoideae and Grevilleoideae being prominent components of the vegetation, and Banksia may have evolved by then, however, neither Eucalyptus nor other Myrtaceae were recovered in the deposits examined. This suggests that the sclerophyllous biome is much older than previously thought (Carpenter et al. 2015; c.f. in part Lamont & He 2012); perhaps surprisingly, Sphagnum was a important component of this vegetation, although it is not to be found in such places today and crown-group Sphagnum has been estimated to be only 104-30 Ma (Shaw et al. 2010a) or ca 25 or even a mere 14 Ma (Shaw & Devos 2014). For fire in Australia, see Bradstock et al. (2012), Bowman et al. (2012), Hill and Jordan (2016), Lamont et al. (2018b), etc., c.f. in part Bond and Scott (2010). Many species of Australian Proteaceae grow in fire-prone habitats, and they have thick leaves, serotiny, wind-dispersed seeds and germination stimulated by heat and smoke, and resprouting is also common (the oldest resprouter may be Franklandia, as much as 81.5 Ma (He et al 2016a; Lamont et al. 2018b). Pausas and Lamont (2018) and Lamont et al. (2018b) integrate these and other variables (soil, biotic) in their analysis of the ecological proclivities of the family in Australia. See also Pausas et al. (2017), conifers in general and Pinaceae in particular for adaptations to fires.
In the Cape area, the speciose Protea diversified 34-22 Ma, initially in winter-wet shrublands where sclerophylly and serotiny were favoured. The genus moved in to summer-wet grasslands where larger leaves (and stature), and especially non-retention of seeds and resprouting, were favoured, speciation beginning (10.7-)7(-3.3) Ma (Lamont et al. 2013: surely P. sulphurea plesiomorphically a shrubland species?). Lignotubers, common in plants growing in habitats where fire is common, have evolved several times in Proteaceae, and Lamont et al. (2017) discuss the role that fires, not frost, are likely to have had in the evolution of subshrub geoxyles, i.e. subshrubs with lignotubers, in African Protea in the last 11 Ma; however, non-sprouters, as Lamont et al. (2018b) suggested, might produce more seeds and/or store them better. There is a similar complex of characters in the Cape genus Leucadendron, also Proteoideae, and their evolution was correlated and trade-offs were evident. Thus species that had a persistent seed bank in the soil tended to show reduced resprouting after fires, if species had seeds that were dispersed over long distances, their pollen was less so, and so on (Tonnabel et al. 2014a, especially b; see also Barker et al. 2004). Stepanova et al. (2021) looked at the distribution of a number of ecologically-important wood characteristics in Proteoideae, and noted a correlation between narrow rays and higher vessel frequency and reproduction by seeding after fires, and broad rays, lower vessel frequency and resprouting. These broad rays appear to be where endogenous epicormic buds, important in resprouting, are initiated (Stepanova et al. 2021).
Proteaceae are notable in having a relatively large seed mass and both very low leaf nitrogen (N) and high leaf mass per area (i.e. they have a low SLA), the latter features tending to be linked (Cornwell et al. 2014). From examination of fossil leaves, scleromorphy may have evolved in the late Palaeocene, xeromorphy in the late Eocene/Oligocene (Hill 1998; Hill & Brodribb 2001). Jordan et al. (2005, 2008) outline the evolution of scleromorphic leaf anatomy, in particular, aspects of stomatal morphology like sunken stomata, associated papillae, etc., and amphistomaty is common. Sclerophylly itself is implicated in photoprotection and occurs in association with some combination of open, oligotrophic, cold and dry conditions, while sunken stomata help reduce water loss (Weston 2014); genera like Hakea s. str. are extreme sclerophylls (Lamont et al. 2016a). The family as a whole shows great variation in aspects of leaf anatomy and morphology like cell size, stomatal and fine vein density, leaf size and thickness, that affect productivity, transpiration, etc. (Brodribb et al. 2013), interestingly, there is no simple correlation between productivity and success. Bellendena, perhaps sister to the rest of the family (see below), has medium-sized stomata, while Persoonioideae (perhaps sister to Bellendena) and Protea in particular have large stomata and other anatomical features that are common in taxa of more open habitats (Brodribb et al. 2013). Looking at stomatal size in fossil Proteaceae, there is a correlation of larger, less dense stomata with cooler temperatures, and, surprisingly, more numerous, smaller stomata with higher CO2 concentrations (Jordan et al. 2020). Aspects of leaf anatomy that facilitate the movement of water through the leaves are correlated with annual precipitation (Jordan et al. 2013).
The P metabolism of Proteaceae is very distinctive, as already suggested, even if much still remains to be understood (Lambers et al. 2015a, b, c; Hayes et al. 2021). Both Southwest Australia and the Greater Cape region are noted centres of plant diversity outside the tropics, and both have Mediterranean climates and OCBILs in which the soils are notably poor in P - and Proteaceae have diversified in both areas (Hayes et al. 2021). Proteoid roots/cluster roots (absent in Persoonia, for example) are short-lived (at most around a month) bottle-brush like clusters of roots of determinate growth, and these clusters may represent a single order of branching of the subtending root, or they may be more complex, being made up of hundreds or thousands of roots, and these have very long root hairs (e.g. Dinkelaker et al. 1995; Laliberté et al. 2015). They account for up to 50% to perhaps 80% of the mass of all the roots of a plant at any one time, a single cluster having a surface area some 25 times that of an equivalent mass of axial roots, root hairs excluded (Shane & Lambers 2008; see also Purnell 1960; Dinkelaker et al. 1995). These roots are initiated opposite the xylem poles of the main root, and in Hakea prostrata they form up to seven longitudinal rows down the root (the rows may be double). Although cluster roots eventually lose their root caps and so are determinate in growth (Watt & Evans 1999; Shane et al 2004b), the caps - perhaps of young roots - are also described as secreting considerable amounts of mucilage (Dinkelaker et al. 1995). They exude organic acids like citric acid, especially as tricarboxylates, into the soil in large amounts over a short period of time, and this enables the plant to mobilize phosphate and perhaps other nutrients like manganese that are at a premium in the P-poor soils on which Proteaceae often grow (Skene 1998; Watt & Evans 1999; Shane et al. 2004b; Weston 2006; Lambers et al. 2015c); they are P miners (Lambers et al. 2008). Interestingly, cluster roots may form in response to the presence of organic matter near the root (Watt & Evans 1999 and references). (Such cluster roots also develop in Lupinus, Arabidopsis, a number of Fagales, etc., under conditions of low P and as soils age, i.e. conditions common in soils where Proteaceae grow - see López-Bucio et al. 2003; Lambers et al. 2008; Turner et al. 2013; Kang et al. 2014: development of cluster roots in Arabidopsis. In other taxa, including Cyperaceae, long root hairs are the response of the plant to low inorganic P, etc. - V. A. S. Jones & Dolan 2012; Lambers et al. 2015c; Salazar-Henao et al. 2017.) Furthermore, when P becomes scarce Proteaceae may remove the P component of their cell membranes (as phospholipids) and divert it to maintaining their photosynthetic rates (Lambers et al. 2012a, b), while species of Banksia and Helicia in particular show very high levels of P resorbtion prior to leaf senescence (Denton et al. 2007; see also Hayes et al. 2014; Lambers et al. 2015b). Interestingly, many members of the family develop P toxicity when growing on relatively P-rich soils; they cannot down-regulate their uptake of P, and it accumulates in palisade mesophyll cells in particular, eventually causing the death of the plant (Shane et al. 2004a; Shane & Lambers 2008; Lambers et al. 2011 for a general summary). Genera of Proteaceae growing in P-rich soils tend to have few species (Hayes et al. 2021). Particularly in Australia, and there perhaps particularly in resprouting/serotinous species, P is preferentially moved into the seeds (Denton et al. 2007; Groom & Lamont 2009). Late leaf greening and development of higher levels of rRNA, changes in levels of foliar lipids over time, very high efficiency of use of P (and N) in photosynthesis, etc. (so photosynthetic rates are relatively high), are additional factors that contribute to this distinctive P metabolism (Lambers et al. 2015b: list on p. 1; Hayes et al. 2021). In South America soils may be richer in P, but it is not readily available (Lambers et al. 2012b). However, cluster root formation in the Patagonian Embothrium coccineum was induced by low nitrogen rather than low P, even if P uptake was also subsequently enhanced (Piper et al. 2013; see also Delgado et al. 2014).
Shen et al. (2024) suggest that there are three main P-acquisition strategies in S.W. Australian Proteaceae - cluster roots (discussed above), facilitation and fire. In facilitation, taxa like Adenanthos cygnorum as it were piggy-back on the activities of other Proteaceae; although they lack cluster roots themselves, they acquire P mobilized by neighbouring plants that do have cluster roots. On the other hand, taxa like A. barbiger use P mobilized by fires, and this species, too, lacks cluster roots. Normally a genus has but a single P acquisition strategy, Adenanthos is unusual in having three (Shen et al. 2024).
Lomatia tasmanica appears to be represented by a single clone about 1.2 km across, however, only recently the same clone may have been far bigger, ca 6 km across, and fossil records suggest that it may be up to 43,600 years old (Lynch et al. 1998). Centenaro et al. (2023: Appendix S1) thought that the oldest clonal plants - indeed, the oldest seed plant known, period - known was Gaylussacia (= "clonal herb") at ca 13,000 years, however, clones of Posidonia oceanica in the Mediterranean may be up to 15 km across and thousands to 200,000 years old (Arnaud-Haon et al. 2012).
Ritmejerytè et al. (2023) looked at the distribution of cyanogenic glycosides in flowers of eleven species of Grevilleoideae, and found extensive variation both between species, between tissues and there was little correlation with taxonomy and between floral and foliar glycoside concentrations. Dhurrin and proteacin were glycosides involved, and the highest concentrations that Ritmejerytè et al. (2023) found were 2,359-5,578 μg CN g-1 dry weight, the latter figure in the flowers of Grevillea robusta.
Pollination Biology & Seed Dispersal. Brush-type secondary pollen presentation involving various regions at the apex of the style is common (Sedgley et al. 1993; Ladd 1994; Ladd & Bowen 2020; El Ottra et al. 2023: ca 60 genera with this mechanism, but it is unclear where apomorphies might be). The pollen may even initially cover the stigma, sometimes mixed with cells that have become detached from the stigmatic surface and whose walls have spiral thickenings (Ladd et al. 1996, 1998), the stigma proper often being slit- or groove-like, however, selfing is uncommon. Indeed, in only a few taxa does pollen move directly from the anthers to the pollinator, while in Proteoideae I in particular there are a number of species with explosive anther dehiscence (Ladd & Bowen 2020). Tilney et al. (2016) suggested that there might be interactions between onci/pollen buds that are found on the pollen grains of some Proteaceae with epidermal cells of the pollen presenter, perhaps involving the hydration of the pollen grains. The aggregation of flowers into dense inflorescences is common, and monosymmetric flowers are also common in the family, this feature having evolved 10-18 times or more (and with 4-12 reversals: Reyes et al. 2016; Citerne et al. 2016: CYC-like genes involved). The Cape species Mimetes cucullatus and M. fimbriifolius have monosymmetric pseudanthium-type inflorescences made up of groups of polysymmetric flowers with asymmetric inflorescence bracts forming a bilabiate involucrate structure at the base, the whole borne under a more or less brightly coloured concave standard-type "petal", actually the cauline leaf subtending the inflorescence above it (Rourke 1984; see also Baczynski & Claßen-Bockhoff 2023 for pseudanthia). Furthermore, flowers of the southern/austral plant families Myrtaceae, Fabaceae and Proteaceae are often pollinated by members of groups of colletid bees, also long-term residents of Australia, Euryglossinae (esp. also on Myrtaceae), with many Australasian endemics, also Hylaeinae and Neopasiphaeinae (Slattery et al. 2023). A few genera like Leucadendron are anemophilous (Ladd & Bowen 2020).
There are about 1,100 Australian species of Proteaceae, and many, including members of the large genera Grevillea (?to be included in Hakea) and Banksia, also Telopea and relatives, are likely to be pollinated by birds (Maynard 1995; Ladd & Bowen 2020). Although it has been suggested that ca 300 species of Proteaceae in Australia may be bird-pollinated (Ford et al. 1979), a moderately conservative estimate is that there are ca 350 species of bird-pollinated Proteaceae there (E. T. Miller pers. comm. 12.ii.2017). Two recent studies have examined bird pollination in Hakea s. str., its evolution and possible relationship with cyanogenesis in the flowers. From the phylogeny provided by Mast et al. (2012), insect pollination would seem to be derived from bird pollination, while Hanley et al. (2008) suggested the opposite, however, the phylogeny of the latter was based on an earlier morphological study that could not be confirmed by Mast et al. (2012). Dates, as well as a possible association between bird pollinators and the presence of cyanogenic glycosides and insect pollination and spiny leaves suggested by Hanley et al. (2008) also need confirmation, but see Mast et al. (2015). In southern Africa perhaps 87 species of Proteaceae in the Cape Fynbos are pollinated by a few species of nectariniids, and particularly by the sugarbird, Promerops cafer (Promeropidae) (Rebelo et al. 1984: Rebelo 1987); there are two species of Promerops, one of which (P. gurneyi) is more broadly distributed, in Africa, as are Proteaceae themselves (the Promerops clade is quite old, around 39-33 Ma - Beresford et al 2005; Fleming & Kress 2013). In both Australia and the Cape, the prominence of ornithophily in the family may be connected with the nutrient-poor soils on which Proteaceae there grow; nectar - basically C, H, and O - costs the plant little (Rebelo 1987; Orians & Milewski 2007).
In both Australia and southern Africa non-flying mammals (rodents, marsupials) are also among the pollinators (Collins & Rebelo 1987). Pollination in the speciose Persoonia, an Australian genus that lacks secondary pollen presentation, was found to be by bees (Bernhardt & Weston 1996); for bee pollination, see also Wallace et al. (2002). The inflorescences of some species of the Australian Conospermum (Proteoideae) look rather like those of Anigozanthus (Haemodoraceae), and both have monosymmetric flowers. In at at least some species of Conospermum there is exposive pollination; here colletid bees, Leioproctus, touch the staminodes when visiting the flowers, and this triggers the style which rapidly moves in an arc across the mouth of the flower, and the anthers dehisce; small insects trapped by the movement may die (Houston 1989). The association between Leioproctus and Conospermum is very close (Houston 1989). The large Cape genus Leucadendron, dioecious, is commonly pollinated by nitidulid beetles, although a few species have become wind-pollinated (Welsford et al. 2016 and references).
Some species of both Baksia and Hakea s.l. (Grevilleoideae) have nectar that is coloured (Hansen et al. 2009). The pentose monosacchariude xylose is found in nectar of Proteaceae-Proteeae (ca 1/3 the sugars or more), although birds and mammals cannot assimilate it well (Jackson & Nicolson 2002; Lotz & Schondube 2006).
White and Midgley (2022) suggested that Proteaceae in the Cape had polymorphic seeds when it came to colour, different colours reflecting different substrates, and granivorous birds drove this colour variation. Serotiny is scattered in Proteaceae (see also above), the follicles opening only after a fire. Banksia is a major serotinous clade, although with some reversals (He et al. 2011: Dryandra not included, other features associated with serotiny examined). A number of the species involved have massive fruits, but there are only two seeds inside, while in other taxa the fruit is enclosed by bracts, branches, etc.. For myrmecochory in Hakea s.l., Leucadendron, etc. (Grevilleoideae and Proteoideae), see Lengyel et al. (2009, 2010), it may have first originated in Proteaceae (58-)44.5(-32) Ma (Lamont & He 2012).
Plant-Animal Interactions. Given the size of the family, caterpillars of Lycaenidae butterflies seem to eat its members quite frequently (see Fiedler 1991). Caterpillars of the dryandra moth, Carthaea saturnioides, the only member of a family close to the saturnines, are found only on Proteaceae.
Plant-Bacterial/Fungal Associations. In Australia, the fungal pathogen Phytophthora cinnamomi is proving especially destructive to members of this family in the Mediterranean climates of the southwest. The African Faurea saligna is reported to be ectomycorrhizal (Högberg & Piearce 1986), but absence of mycorrhizae seems to be the common condition here, interestingly, other plants lacking mycorrhizae are most often herbaceous, unlike Proteaceae (Maherali et al. 2016).
Vegetative Variation. Leaf morphology and anatomy are very variable both within and between species in Proteaceae, particularly in Australian taxa, although it is difficult to relate this to ecological factors (Nicotra et al. 2011).
Genes & Genomes. That palaeo-polyploidy has been involved in the evolution of chromosome number in the family is questionable (Stace et al. 1998). There is very considerable variation in chromosome and genome size that correlates with phylogeny/habitat, but much less with chromosome number; there have been changes in stomatal size (in part) both subsequent to and independent of changes in genome size and chromosome number (Stace et al. 1998; Carpenter et al. 2010a; Jordan et al. 2014).
Chemistry, Morphology, etc.. For distinctive fatty acids in the seeds, see Badami and Patil (1981); for cyanogenic glycosides, see Swenson et al. (1989).
The roots have 4-7 protoxylem poles (e.g. Watt & Evans 1999). Sieve tubes have sieve areas for most of their length. Nodes in Panopsis appear to be pentalacunar, while those of Finschia are trilacunar, although with some variation in the number of traces departing from each gap (Catling 2010). Cotyledonary nodes commonly have split laterals (Naubauer 1991), however, the distribution of this character is unclear. Stem lenticels are often horizontally elongated (Keller 1996). Antipin and Choob (2019) outline variation in hair morphology in the family, and they note that the basal cells of the 3-celled hairs so prevalent here may undergo anticlinal divisions. For stomatal orientation on the lamina, see Carpenter et al. (2010a).
The flower-pairs common in the family represent reduced inflorescence branches (Douglas & Tucker 1996a). For variation in floral orientation, see Douglas and Tucker (1996a, b); the flowers may be mirror images, as in Orites. In Grevilleoideae, the basic floral orientation is orthogonal to the bract immediately subtending the flower, but the position of this bract relative to the axis that bears it varies and the carpel is often (?always) oblique (Douglas & Tucker 1996a; Mast et al. 2015). Placospermum has vertically monosymmetric flowers in which there are three staminodes and only the single adaxial stamen is fertile - see image. Proteaceous flowers can be interpreted as being dimerous (Ronse De Craene 2016b), although there is little evidence that the perianth is derived from a biseriate structure, and the individual perianth members - of Macadamia, at least - have three traces (Puri 1951 and references); for the family, 1-5 bundles supplying each tepal are reported (Weston 2006). A small, almost spine-like process that is described as a Vorlaüferspitze (Douglas & Tucker 1996c) occurs just abaxial to the apex of the perianth members (see Kaplan 1973) in Persoonioideae and also elsewhere in the family. Anthers of Stirlingia, Franklandia, Conosperma and Synaphea have an exothecium, but no endothecium (Venkata Rao 1971). The nectary has very variable vasculature and it is also very variable in morphology; it is best considered to be an enation rather than (a) modified stamen(s) (Venkata Rao 1967; Douglas & Tucker 1996c). The stigma may be papillate, or it is more or less enclosed, with exudate (Matthews et al. 1999); due to the early growth of the carpel, it may be in a more or less abaxial position on the carpel/style.
Although the ovules are straight, the funicles can be quite long and strongly curved (Venkata Rao 1962). The position of the embryo sac in the ovule varies considerably (Venkata Rao 1971). Although a nucellar cap seems to be common, it can be difficult to distinguish from files of cells produced by the division of hypodermal nucellar cells (e.g. Jordaan 1945a, see also Venkata Rao 1962). Manning and Brits (1993) suggested that there had been much misinterpretation of fruit/seed anatomy; their interpretation is followed here. Testa and tegmen variation is extensive, partly because the fruits are sometimes indehiscent (Venkata Rao 1971; Corner 1976). The ovary wall may also be adnate to the integuments (so the fruit is a sort of caryopsis?!). The testa and/or the tegmen may be multiplicative. Endosperm and endosperm haustorium development is variable; the endosperm of Lomatia (Grevilleoideae) has 1-celled outgrowths over its surface (Venkata Rao 1962). Cotyledons of Bellendena are not cordate are they often are elsewhere in the family.
For additional general information, see Johnson & Briggs (1963, 1975: including chromosome lengths!), Venkata Rao (1971) and especially Weston (2006), for general chemistry, see Hegnauer (1969, 1990), for polyol distribution, see Bieleski and Briggs (2005), for nodes, see Catling and Gates (1998), for Al accumulation, see Webb (1954), for the life history of Grevillea s. str., see Brough (1933), for flowers, see Douglas and Tucker (1997: Proteoideae-Conospermeae, considerable developmental variation) and Mair (1977: monosymmetry), for pollen, see Dettmann (1998), Sauquet et al. (2006), Sauquet and Cantrill (2007), and Blackmore and Barnes (1995: development, Garside's Rule), for some embryology, see Venkata Rao (1959) and Jordaan (1945b), and for a survey of seed coat anatomy, see Takhtajan (2000).
Phylogeny. For a summary of phylogenetic studies in the family, see Weston (2014). Although the subfamilies recognised here seem to be fairly solidly supported, relationships between them are less clear. Thus the summary tree in Weston and Barker (2006) and Weston (2006) suggest that Persoonioideae and the monotypic Bellendenoideae are sister taxa (see also Sauquet et al. 2009b); the distinctive Carnarvonia (fully compound leaves) and Sphalmium (stylulus short), sometimes segregated as subfamilies (as in The Flora of Australia), may be immediately related, and although definitely to be included in Grevilleoideae, further relationships within that clade are unclear. Although understanding relationships between the major clades was not the focus of the study by Mast et al. (2012), they recovered the relationships [[Persoonoideae + Bellendenoideae] [Grevillioideae [Proteoideae + Symphionematoideae]]].
Persoonioideae is largely Persoonia itself; for relationships in the subfamily, see Holmes et al. (2018).
Relationships in Proteoideae are explored by Barker et al. (2002); the Australian Isopogon and Adenanthos are sister to a clade that represents most of the subfamily, but not including Protea and Faurea (support strong for this set of relationships); unfortunately Eidothea, a distinctive genus segregated as Eidotheoideae in The Flora of Australia, was not included. Sauquet et al. (2009b) found some support for [Agastachys + Symphionema] as sister to Proteoideae, within which Eidothea was involved in a basal tritomy. For the phylogeny of Leucadendron, see Tonnabel et al. (2014a, b), also Barker et al. 2004; the backbone has little support, but L. argenteum was treated as sister to the rest of the genus in character reconstructions (Tonnabel et al. 2014b: comments on groupings recognised by earlier authors). For relationships in Protea, see Mitchell et al. (2017 & references).
Within Grevilleoideae, Sauquet et al. (2008b) found some support from the topology [Macadamieae [Banksieae + Roupaleae]]; Carnavonia was weakly supported as sister to Macadamieae. Skeels et al. (2025b: ca 700 spp., 458 nuclear loci) recovered similar relationships, finding that Embothrieae were sister to the three tribes just mentioned.
Embothrieae. For relationships in Hakea, which may well include Grevillea and a couple of other small genera, see Mast et al. (2012, esp. 2015); G. endlicheriana, from S.W. Australia, is sister to the whole of the rest of the clade and Hakea s. str. is monophyletic. In a careful analysis Skeels et al. (2025a: 537 spp. Hakeinae, 216 nuclear loci) found that the two genera were reciprocally monophyletic in concatenation analyses, but Hakea was embedded in Grevillea in coalescent analyses. There was extensive gene tree discordance, low phylogenetic signal, probably rapid radiation and ILS.
Classification. For a subfamilial/tribal classification, the outlines of which are followed here, and a generic checklist, see Weston and Barker (2006) and Weston (2006).
Banksia is to include Dryandra (Mast et al. 2005; see also Thiele et al. 2015). Hakea may well be expanded to include Grevillea and Finschia (Mast et al. 2015 for possible options; Skeels et al. 2025a); for combinations of species of Grevillea in Hakea, see Christenhusz et al. (2018).
Previous Relationships. The testa anatomy of Proteaceae is similar to that in Papaveraceae, Chloranthaceae and Aristolochiaceae, for what that is worth.