EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tectum reticulate-perforate, nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID II / MALVIDAE / [[GERANIALES + MYRTALES] [CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]]: ?
[CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]: ?
[PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]: ovules 2/carpel, apical.
[SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]: flavonols +; vessel elements with simple perforation plates; (cambium storied); petiole bundle(s) annular; style +; inner integument thicker than outer; endosperm at most scanty.
Age. Suggestions for the age of this node are (88-)71(-63) Ma (N. Zhang et al. 2012; see also Xue et al. 2012), (102-)96(-90) and (80-)76(-72) Ma (H. Wang et al. 2009), ca 98.25 Ma (Magallón & Castillo 2009), around 93.6-89.9 Ma (Naumann et al. 2013), about 103.5 Ma (Hohmann et al. 2015), ca 111 Ma (Foster et al. 2016a: q.v. for details), (116.9-)111.5(-106.2) Ma (Muellner-Riehl et al. 2016) and (199-)186/-174)/(137-)131(-124) Ma (Joyce et al. 2023: S. M. B.).
Evolution: Divergence & Distribution. For integument thickness, a possible apomorphy, which, however, reverses, see Endress and Matthew (2006a), moreover, its condition is unclear in Huerteales, etc..
Genes & Genomes. Based on a study of the genome of Arabidopsis, De Bodt et al. (2005, see also Maere et al. 2005) suggest there was a duplication of the whole genome some 109-66Ma before present, although given the uncertainty over the dating of this duplication and relationships within rosids, exactly where the duplication should go on the tree is unclear. A position at this node is one possibility.
There are suggestions that the chloroplast infA gene was lost or became a pseudogene at this node (Logacheva & Shipunov 2017; see also Millen et al. 2001; Su et al. 2014).
Phylogeny. Relationships between the malvid clades have been somewhat uncertain. The clade [Malvales + Sapindales] was sister group to Brassicales (Soltis et al. 2000; Peng et al 2003: both weak support; Bell et al. 2010), and Endress and Matthews (2006) noted that there are some features perhaps more common in these first two families than elsewhere in this affinity. Other studies suggest that [Malvales + Sapindales] may be sister to [Brassicales + Huerteales] (Soltis et al. 2007a: support weak for the latter pair; Bell et al. 2010). Although Bausher et al. (2006) in an analysis of whole chloroplast genomes found strong support for the clade [Brassicales + Malvales], only one species from the three larger orders and no Huerteales were included (but see also S.-B. Lee et al. 2006: sampling even more exiguous; Jansen et al. 2007; Moore et al. 2007; Muellner-Riehl et al. 2016). There was also some support for this topology in analyses by Savolainen et al. (2000) and Hilu et al. (2003). Alford (2006), when describing his Gerrardinaceae, found that Huerteales (Perrottetia not included), Brassicales and Malvales formed a tritomy, the combined group being rather poorly supported as sister to Sapindales, while Worberg et al. (2007b, 2009) recovered the relationships [Sapindales [Huerteales [Brassicales + Malvales]]], with strong support, and they found that each of the four orders was monophyletic. In studies including the mitochondrial matR gene, although the malvid clade was recovered, relationships within it were unclear (Zhu et al. 2007). I follow Worberg et al. (2009).
SAPINDALES Berchtold & J. Presl - Main Tree.
Interesting secondary compounds, ethereal oils, myricetin +; tyloses 0, vessel:ray pits bordered, marginal parenchyma delimiting growth rings (0); secretory cells/tissue +); mucilage cells + [with swollen and layered inner periclinal walls - position in plant varies]; branching from previous innovation, petioles leaving a prominent scar; leaves spiral, odd-pinnately compound, leaflets opposite, vernation conduplicate; inflorescence branches, at least, cymose; A 2 x K, (± obdiplostemonous); tapetal cells polyploid; (pollen exine distinctly striate); nectary well developed; G = and opposite petals, or 3, odd member adaxial, stigmatic head from postgenitally united free carpel tips; ovules few/carpel, epitropous, nucellar cap + [?all]; exotegmen not fibrous; (embryo chlorophyllous); x = 5-7. - 9 families, 479 genera, 6,570 species.
Includes Anacardiaceae, Biebersteiniaceae, Burseraceae, Kirkiaceae, Meliaceae, Nitrariaceae, Rutaceae, Sapindaceae, Simaroubaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age of crown-group Sapindales has been variously estimated as 117.4, 104.9 or 90.5 Ma (Muellner et al. 2007: c.f. topology), (110.5-)105(-99) Ma (Muellner-Riehl et al. 2016) and (187-)172(-151)/(130-)124(-117) Ma (Joyce et al. 2023).
Evolution: Divergence & Distribution. Sapindales contain ca 3% eudicot diversity (Magallón et al. 1999) and show quite high diversification rates (Magallón & Castillo 2009); Magallón and Sanderson (2001) described this as a very species-rich clade.
Muellner-Riehl et al. (2016: Table S1) discusss dating here - they provide dates for hundreds of nodes - in some detail. They prefered ages from analyses using three maximum constraints over those using one or no such constraints; ages in the latter case were ca 1.7-3 times those when three constraints were used - e.g. ca 441 versus ca 148 Ma for the eudicot stem age... Their ages for families were older than those in earlier general studies that included Sapindales, but younger for the most part than ages in studies that focussed on a single family (Muellner-Riehl et al. 2016: esp. Table 5). Joyce et al. (2023) provide two sets of ages in Sapindales, one fairly conventional, while the other is 30-40 Ma older and is based on a calibration that used reported angiosperm pollen from 247 Ma (see elsewhere for more on such pollen) - both estimates are given here.
There is extensive variation in gynoecial morphology in Sapindales summarized by El Ottra et al. (2022). Although it is likely that the common ancestor of Sapindales had only a few ovules per carpel, exactly how many is unclear. Although having many ovules per carpel is likely to be derived here, there is otherwise quite extensive variation in ovule number, often at the familial to tribal level, but given all the variation, it is difficult to suggest apomorphies. For some embryological s.l. features possibly characterizing Sapindales, see Yamamoto et al. (2016), q.v. for cautions on numbers of nuclei in/polyploidy of tapetal cells. For possible high-level apomorphies in features of wood anatomy within Sapindales, see the comprehensive study by Pace et al. (2022: Table 4); many of these characters have been included below. 23 characters were examined (a few are divisions of continuous variation). The species examined are as far as possible those used by Muellner-Riehl et al. (2016), and the characters were optimized on their tree (Pace et al. 2022: Figs 3-18) and possible functional aspects discussed.
Pollination Biology. There is notable variation in dichogamy here, see e.g. Bertin and Newman (1993), Routley et al. (2004). Tölke et al. (2018b, see also 2022) discuss the little that is known about osmophores in sapindalean flowers. Nectar varies considerably in composition (Tölke et al. 2022).
Plant-Animal Interactions. Associated with the frequent accumulation of noxious secondary metabolites in Sapindales, specialised herbivores are found on many of this group. Thus the hemipteran psyllid Calophya eats largely Anacardiaceae, Burseraceae, Simaroubaceae and Rutaceae (Burckhardt & Basset 2000) - plus a couple of records from entirely unrelated families. A notable diversity of monoterpene synthase genes have been found in Sapindales studied, and the products of these genes may be involved either directly in plant defence, or indirectly by signalling to parasitoids of herbivores, but studies of these genes are currently only preliminary (Zapata & Fine 2013 and references). Galls are quite common, perhaps especially on Sapindaceae and Anacardiaceae (Mani 1964; see also Price et al. 1998).
Genes & Genomes. Guimarães & Forni-Martins suggested that the base chromosome number for the order was x = 5, 6, or 7; this was based on the relationships [Biebersteiniaceae [Nitrariaceae [etc.]]].
Chemistry, Morphology, etc.. Gums and resins occur in both the Rutaceae-Meliaceae-Simaroubaceae and Burseraceae-Anacardiaceae groups (Nair 1995; see also Töke et al. 2022).
Stratified phloem may be quite widespread (in some Meliaceae, Burseraceae and Simaroubaceae, at least: M. Ogburn, pers. comm.), also Sapindaceae. Leaf teeth, when present, have a clear glandular apex broadening distally and with a foramen and two accessory veins (or one, the other going above the tooth (Hickey & Wolfe 1975). Stipular structures that vary in morphology are scattered through the order (Cruz et al. 2015 for examples and references), and these may be modified basal leaflet pairs; some have been described as pseudostipules or metastipules, the latter being defined as structures having the morphology of true stipules, yet there was good reason to believe that they were derived from pseudostipules... (Weberling & Leenhouts 1965). Tölke et al. (2022) discuss secretory structures, "ducts, cavities, laticifers, floral and extrafloral nectaries, osmophores, colleters, idioblasts, and trichomes" in Sapindales (ibid., p. 251, also Fig. 1, summary; Fig. 13, various substances secreted); see also Maximo et al. (2023: extrafloral nectaries, esp. Sapindaceae).
Bachelier and Endress (2009) note some floral developmental features found widely in this clade, while Yamamoto et al. (2014: esp. Table S1) compare embryological features; for some details of embryology, see also Mauritzon (1936). Inconspicuous oblique monosymmetry may be common in the order; many Sapindaceae, for example, are more strongly monosymmetric, but it is oblique in e.g. Aesculus (Cao et al. 2017). The flowers are often imperfect, but since staminate and carpelate flowers have well-developed pistillodes and staminodes respectively, they can be difficult to distinguish. Floral tubes formed by connate or closely adpressed and flattened filaments occur throughout Meliaceae, in a number of Rutaceae, and in Boswellia dioscorides (Burseraceae); they are uncommon elsewhere. Pollen with striate exine is scattered through the order. Septal cavities have been noticed in Cneorum (Rutaceae) and Koelreuteria (Sapindaceae), but they do not secrete nectar (Caris et al. 2006b, c.f. septal nectaries in monocots).
Brazilian J. Bot. 45(1). 2022 is devoted to articles on Sapindales, especially South American taxa. Pace et al. (2022) cover wood anatomy, Gonçalves-Esteves et al. (2021) pollen morphology.
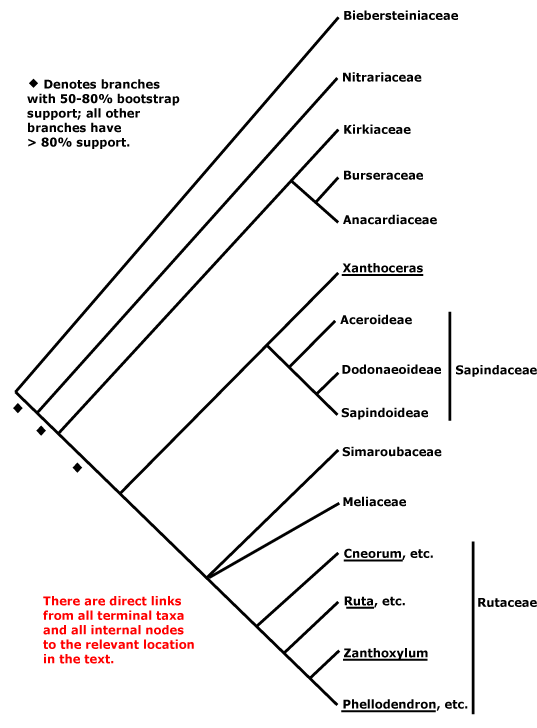
Phylogeny. For general relationships, see Gadek et al. (1996), while Pell (2004) notes some deletions and insertions that may characterise groupings within the clade. Molecular data had early placed Biebersteinia, ex Geraniaceae, in Sapindales, albeit with a long branch (Bakker et al. 1998). Its herbaceous habit is rather unusual for Sapindales, but its ethereal oils (no oxygenated sesquiterpenes, high proportion of aliphatic hydrocarbons - Bate Smith 1973; Greenham et al. 2001), single ovule/carpel, etc., are all consistent with a position here. Muellner et al. (2007) present a two-gene tree with quite good sampling; their results, albeit poorly supported, suggest the basal relationships in the tree here (also poorly supported in Soltis et al. 2011), and the position of Sapindaceae is unresolved (see esp. Muellner-Riehl et al. 2016). There was also a fair bit of resolution elsewhere, excepting an only moderately-supported sister group relationship between Meliaceae and Simaroubaceae (see also Koenen et al. 2015; Muellner-Riehl et al. 2016; but c.f. Gadek et al. 1996; Soltis et al. 2011: ?sampling). H.-T. Li et al. (2019) found strong support for a [Meliaceae [Simaroubaceae + Rutaceae]] clade, relationships which have been recovered before (e.g. Magallón et al 2015; Zeng et al. 2017); Sapindaceae were sister to this group, and with quite good support (Li et al. 2021). Relationships are somewhat different in Wang et al. (2009), but support was weak and sampling poor, and support was again mostly poor in M. Sun et al. (2016), although there was some support for a [Nitrariaceae + Biebersteiniaceae] clade sister to the rest of the order (see also Muellner-Riehl et al. 2016; H.-T. Li et al. 2019: support very poor), while the relationships [Nitrariaceae [Biebersteiniaceae ...]] in Z.-D. Chen et al. (2016). In Li et al. (2021) we find [Nitrariaceae [Biebersteiniaceae [Kirkiaceae [Burseraceae + Anacardiaceae]] [Sapindaceae [Meliaceae [Simaroubaceae + Rutaceae]]]]]
W. J. Baker et al. (2021a: Angiosperms353 Seed Plant Tree) found the relationships [Biebersteiniaceae [Nitrariaceae ...]] in a preliminary analysis of Angiosperms353 nuclear data. In a subsequent analysis involving 448 samples and some 85% of the genera, Joyce et al. (2023) suggested that relationships in Sapindales might need a substantial overhaul. For instance, Sapindaceae, which had been sister to [Meliaceae [Simaroubaceae + Rutaceae]] in pre iv. 2023 versions of this site, were thought to be part of a basal tetratomy in the order, i.e., [Nitrariaceae, Biebersteiniaceae, Sapindaceae, the rest]; Simaroubaceae were also misplaced, relationships being [Simaroubaceae [Rutaceae + Meliaceae]] (Joyce et al. 2023). Similar relationships were evident in another Angiosperms353 analysis; there was weak support for a [Biebersteiniaceae + Nitrariaceae] clade sister to the rest of the order. Joyce et al. (2023) found that Rutaceae-Cneoroideae of earlier versions of this site were paraphyletic (see Cneoroideae, Spathelioideae), which has morphological and taxonomic implications. These - or very similar - relationships were also recovered in the v.2023 version 3 and ix.2024 versions of the Seed Plant Tree and in Zuntini et al. (2024) and are followed below (the positions of Biebersteiniaceae and Nitrariaceae may flip, but support tends to be poor) - overall relationships are [[Biebersteiniaceae + Nitrariaceae] Sapindaceae [[Kirkiaceae [Anacardiaceae + Burseraceae]] [Simaroubaceae [Meliaceae + Rutaceae]]]]. Interestingly, in the Seed Plant Tree Picrolemma (not included in Zuntini et al. 2024) was sister to [other Simaroubaceae [Rutaceae + Meliaceae]]]. Joyce et al. (2023) found large numbers of paralogous loci in both Meliaceae and Rutaceae, so casting doubt on some of the relationships they had recovered, and they suggested that trees were not the best way to represent relationships here. Some additional details of proposed relationships will be found under individual families, but clearly, more work is needed.
Relationships. Bretschneideraceae and Akania (= Akaniaceae here, see Brassicales) have been associated with Sapindales, Bretschneidera in particular looking very like a member of Sapindaceae and its myrosin cells were not considered to be all that important (Cronquist 1981; Takhtajan 1997).
Synonymy: Rutinae Reveal - Acerales Berchtold & J. Presl, Aesculales Bromhead, Amyridales J. Presl, Aurantiales Link, Biebersteiniales Takhtajan, Burserales Martius, Cedrelales Martius, Citrales Dumortier, Cneorales Link, Diosmales J. Presl, Hippocastanales Link, Julianales Engler, Leitneriales Engler, Meliales Berchtold & J. Presl, Nitrariales Martius, Pteleales Link, Rutales Berchtold & J. Presl, Simaroubales Berchtold & J. Presl, Spondiadales Martius, Terebinthales Dumortier, Zanthoxylales J. Presl - Burseranae Doweld, Rutanae Takhtajan, Sapindanae Doweld - Aceropsida Endlicher, Aesculopsida Brongniart, Rutopsida Meisner - Rutidae Doweld
[Nitrariaceae + Biebersteiniaceae]: ?.
Age. The age of this node (if it exists) is (107.6-)94.5(-79.4) Ma (Muellner-Riehl et al. 2016).
NITRARIACEAE Lindley - Back to Sapindales
β-carboline indole alkaloids + [Peganum], ethereal oils?, saponins 0; cork in inner cortex; wood storied; nodes?; petiole bundle arcuate, with wing bundles; mucilage cells +, throughout plant or not; cuticle waxes 0 (platelets, rodlets); leaves thick/fleshy, ?vernation, simple, ± deeply lobed, stipules +; inflorescence terminal; K (connate); C protects the flower in bud; A 15, filament bases broad, flattened; tapetal cells binucleate; nectary as sunken glands; G [3 (4)], stigma as commissural compital lines ± decurrent down style, dry; ovule micropyle zig-zag or bistomal, outer integument 2-4 cells across, inner integument 2-3 or 4-7 cells [which?] across, parietal tissue 2-4 cells across; exotesta cells inflated or not; endotesta short palisade, or not; x = 12 (?11, ?6).
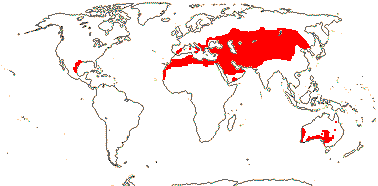
3 [list]/13. Usu. ± arid regions from North Africa to East Asia, also S.W. Australia and E. Mexico (map: from Brummitt 2007; modified by Frankenberg & Klaus 1980; Pan et al. 1999; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Fl. Austral. vol. 26. 2013). [Photo - Flowers.]
Age. The age of crown-group Nitrariaceae is around 96.5, 86.1, or 57.7 Ma (Muellner et al. 2007), (83.8-)63.1(-41.8) Ma (Muellner-Riehl et al. 2016) or (145-)103(-63)/(102-)72(-48) Ma (Joyce et al. 2023).
1. Nitraria L.
Plant shrubby, (with thorns); xylem parenchyma aliform-confluent; short shoots +; lamina (toothed or lobed at apex), stipules scarious [?not associated with all leaves], ?intrapetiolar; nectary +; A in triplets, opposite K; G [(6)], style broad at base, tapering; ovules 1/carpel, apotropous; fruit a 1-seeded drupe, mesocarp woody, pock-marked; endosperm slight, embryo chlorophyllous; n = 12 and polyploidy.
1/6. Europe to Asia, the Sahara, Australia.
Age. Crown-group Nitraria is some (51.5-)33.4(-17.6) Ma (Muellner-Riehl et al. 2016).
[Peganum + Tetradiclis]: plant herbaceous; raphides +; stomata in longitudinal bands of small cells; leaves deeply lobed; style impressed; funicle long; testa mucilaginous.
Age. This node is (76.9-)53.4(-33.1) Ma (Muellner-Riehl et al. 2016).
2. Peganum L. —— Synonymy: Peganaceae Takhtajan
Plant also subshrub; mycorrhizae 0; mucilaginous cells few; two adjacent leaves/node, or one leaf/node, (barely lobed), stipules tiny; flowers single, leaf opposed; K valvate; outer A in pairs opposite C; ovules many/carpel; megaspore mother cells several; fruit a loculicidal capsule or berry; testa weakly multiplicative, exo- and endotesta palisade, endotegmen ± fibrous; endosperm +; n = 12
1/6. Europe to Asia, east Mexico.
3. Tetradiclis M. Bieberstein —— Synonymy: Tetradiclidaceae Takhtajan
Plant annual; leaves opposite or spiral, very fleshy, (entire), stipules small; inflorescence spike-like, cymose; flowers (3-)4-merous; A = and opposite K, filaments subulate; nectary 0; G [4], each divided into three parts, placentation basal, placentae long, style ± gynobasic, ± hollow; ovules to ca 6/carpel [4 ovules in central locellus, 1 each in lateral locelli]; fruit a loculicidal capsule [seeds in central locellus only released], endotegmen not fibrous; endosperm slight; x = n = 7.
1/2. Eastern Mediterranean to Central Asia (Pakistan), North Africa.
Evolution: Divergence & Distribution. Given the phylogeny of the family, the distinctive flowers and fruits of Tetradiclis may be derived.
Ecology & Physiology. Members of this family are often to be found in salt deserts, salt (NaCl) concentration in Nitraria in particular reaching 14% (Sheahan 2011 for references).
Chemistry, Morphology, etc.. Takhtajan (1997) says that stipules are absent in Tetradiclis; they are present, if small. There may be colleters here and elsewhere in the family. In general, leaf morphology and nodal anatomy need attention.
Bachelier et al. (2011) discuss floral morphology in detail, e.g. features like androecial morphology that have been interpreted in various ways in earlier literature. The androecium of Peganum is described as being obdiplostemonous by Eckert (1966); the 15 stamens may be in groups of three opposite the sepals, or there may be paired stamens opposite the petals (Ronse Decraene & Smets 1991a, 1992, 1996a; Ronse Decraene 1992; Ronse Decraene et al. 1996).
For general information, see Weberling and Leenhouts (1965), Hussein et al. (2009) and Sheahan (2011: as Nitrariaceae and Tetradiclidaceae); for chemistry, see Hegnauer (1973, 1990), Sheahan and Cutler (1993) provide details of anatomy, Bachelier et al. (2011: all three genera) of floral morphology; for the embryology of Peganum, see Kapil and Ahluwalia (1963), and of Tetradiclis, see Kamelina (1994), for endosperm development, etc., see Batygina et al. (1985), and for seed anatomy, see Danilova (1996).
Phylogeny. Molecular data suggest the relationships [Nitraria [Tetradiclis + Peganum]] (Sheahan & Chase 1996; Savoilainen et al. 2000; Muellner et al. 2007; Muellner-Riehl et al. 2016; c.f. M. Sun et al. 2016 in part).
Relationships. Nitrariaceae and Zygophyllaceae agree in general appearance, wood anatomy, and perhaps also chemistry (Nag et al. 1995). Indeed, the two families eere often placed in an expanded Zygophyllaceae (e.g. Cronquist 1981), while Takhtajan (1997) included the genera in Nitrariaceae as three separate families in his Zygophyllales. Zygophyllales-Zygophyllaceae as circumscribed here are not remotely close to Nitrariaceae, and their similarities may be because both grow in dry and warm habitats; note that no endothelium has been recorded in members of Nitrariaceae (Kapil & Tiwari 1978: c.f. Zygophyllaceae s. str.).
Botanical Trivia. For some reason (?smell) even camels will not eat Peganum (Sheahan 2011).
If there is a clade [Biebersteiniaceae, Sapindaceae, Kirkiaceae etc., Simaroubaceae etc.], its age is estimated to be (182-)169(-153)/(128-)122(-114) Ma (Joyce et al. 2023).
BIEBERSTEINIACEAE Schnitzlein - Biebersteinia Stephan - Back to Sapindales
Rhizomatous perennial herbs; wood anatomy?; nodes?; hairs glandular; leaves (2-3-compound), leaflets lobed, margins toothed, stipules +, petiolar, lobed or not; inflorescence terminal, axis indeterminate; C clawed, (denticulate); nectary glands separate, opposite K; anther thecae opening by single slit; tapetal cells up to 12-nucleate, nuclei fuse; pollen 3-celled, exine striate; gynophore +, short, G [5], styles separate, impressed, apically connate, stigma capitate; ovule 1/carpel, initially apotropous, unitegmic, integument 4-5 cells across, (nucellar cap ca 2 cells across), nucellus apex exposed, parietal tissue 3-4 cells across, funicle massive, bent; embryo sac tetrasporic, 16-nucleate, 13-celled [Penaea type]; fruit a schizocarp, columella persisting, K ± accrescent; exotesta ± collapsed, endotesta lignified, cells polygonal; endosperm +/-, pentaploid, embryo somewhat curved, cotyledons foliaceous, incumbent; x = n = 5.
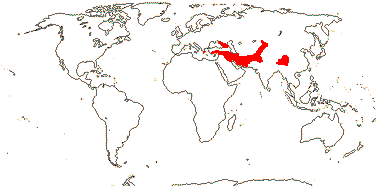
1[list]/5. Greece to Central Asia. Map: from Heywood (2007) and Muellner et al. (2007). [Photos - Collection.]
Age. The age of crown-group Biebersteiniaceae is estimated as 63.3-54.8 Ma (Muellner et al. 2007) or (55-)34.7(-16.1) Ma (Muellner-Riehl et al. 2016).
Chemistry, Morphology, etc.. At least some species of Biebersteinia are foul-smelling.
The antipetalous stamens are longest. Takhtajan (1997) and Yamamoto et al. (2014) both described the ovules as being unitegmic; however, Boesewinkel (1988, 1997) thought that the ovules were bitegmic and the micropyle was bistomal. There are distinctive changes in the ovule as it develops, and at the time of pollination the nucellus apex is exposed (Yamamoto et al. 2014).
See also Baillon (1874), Kunth (1912) and Muellner (2011), all general, Hegnauer (1989, as Geraniaceae) and Tzakou et al. (2001: fatty acids), both chemistry, and Kamelina and Konnova (1989: embryology).
Biebersteinia is little known.
Relationships. Biebersteinia has often been more or less closely associated with Geraniaceae (Geraniales) in the past (e.g. Cronquist 1981; Takhtajan 1997). Boesewinkel (1988, 1997), who thought that the ovules were bitegmic (but see above), saw a similarity of the seed coat of Biebersteinia and that of Vivianaceae (= Geraniales-Francoaceae-Vivianeae), especially when young, with both exotesta and endotegmen being tanniniferous.
SAPINDACEAE Jussieu, nom. cons. - Back to Sapindales
Woody; quebrachitol [cyclitol], steroidal saponins, cyclopropane amino acids + [non-protein amino acids], ellagic acid 0 (+); cork also outer cortical; (petiole bundle with cortical or adaxial bundles); (epidermal cells mucilaginous), cuticle waxes 0 (platelets, rodlets); leaves spiral, odd pinnate, leaflets articulated [check basal pectinations], vernation also conduplicate-plicate, margins often serrate, colleters common; inflorescence paniculate, the flowers often in clusters, imperfect; pedicels articulated; flowers 5-merous; C clawed; nectary extrastaminal; A often around 8, filaments hairy; (tapetal cells 1-3-nucleate); pollen grains spherical, tricolporate; G [(2) 3(-6)], stigma various, dry or wet; ovules variously curved, sessile, campylotropous, micropyle bistomal, outer integument thicker than the inner integument, parietal tissue 4-15 cells across (?0); fruit a loculicidal capsule/other; seed often pachychalazal; testa vascularized, exotesta palisade (not), unlignified, (mesotestal cell walls thickened and lignified; endotesta crystaliferous), tegmen (multiplicative), limited to radicular pocket, (exotegmen fibrous, lignified or not); endosperm starchy, embryo curved, radicle in pocket formed by seed coat, cotyledons unequal; x = 10 (?8, ?9), 16, nuclear genome [1 C] (0.038-)0.802(-16.721) pg.
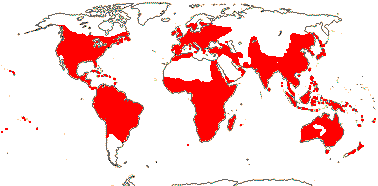
144 [list, to tribes]/1,925 - four subfamilies below, beginning of tribal classification. ± World-wide. (map: from Herzog 1936; Meusel et al. 1978; Fl. Austral. Vol. 25. 1985). [Photo - Flower, Fruit, Fruit.]
Age. Wikström et al. (2001) date crown-group Sapindaceae to (43-)39, 36(-32) Ma, Bell et al. (2010) suggested an age (53-)42, 41(-30) Ma, and Muellner-Riehl et al. (2016) an age of (96.9-)87.2(-77.4) Ma. Crown and stem ages of 36 and 55 Ma respectively were suggested by Quirk et al. (2012). Alternatively, it is mid Cretaceous and (very approximately) 116-98 Ma (Buerki et al. 2010c), while ages of (151-)135(-117)/(113-)103(-94) Ma, so possibly yet older, were suggested by Joyce et al. (2023); Arias (2023/2024) suggested an age of ca 104.3 Ma for the "origin" of Sapindaceae.
Fossils that can be placed in Sapindaceae are known from the later Cretaceous (Coetzee & Muller 1984).
Includes Acereae, Athayaneae, Blomieae, Bridgesieae, Cupanieae, Dodonaeeae, Dodonaeoideae, Doratoxyleae, Guindilieae, Haplocoeleae, Hippocastaneae, Hippocastanoideae, Koelreuterieae, Melicocceae, Nephelieae, Paullinieae, Sapindeae, Sapindoideae, Schleicherieae, Stadmanieae, Thouinieae, Tristiropsideae, Ungnadieae.
1. Xanthoceratoideae Thorne & Reveal - Xanthoceras sorbifolium Bunge —— Synonymy: Xanthocerataceae Buerki, Callmander & Lowry
Plant deciduous; wood ring porous; phloem stratified; pericyclic sheath?; stomata anomocytic; buds perulate; leaves imparipinnate; flowers large [ca 3 cm across], polysymmetric; C 5, ca 2 cm long; nectary of golden, horn-like glands alternating with C; A 8, anthers with apical gland; pollen spiny; post-zygotic incompatibility system; stigma capitate, 3-sulcate; ovules 6-8/carpel, arranged in parallel, outer integument 6-8 cells across, inner integument 3-4 cells across, hypostase +, obturator 0; fruit a capsule; hilum large, broadly arcuate; mesotestal cell walls thickened, tegmen multiplicative, with inner layers thick-walled; n = 15; germination epigeal.
1/1. N. China.
[Hippocastanoideae [Dodonaeaoideae + Sapindoideae]]: pericyclic sheath of phloem fibres and stone cells; laticifers + [?], (tanniniferous idioblasts +); flowers ± strongly monosymmetric (not), (4-merous); C with basal appendages or not; (style hollow), (branched); ovules (1-)2/carpel, apotropous, (obturator -); (megaspore mother cells several); (fruit septicidal), seed usually 1/carpel; nuclear genome [1 C] (367-)1197(-11149) Mb.
Age. Wikström et al. (2001) dated this node to (36-)33, 29(-26) Ma, Bell et al. (2010) suggested that it was (46-)37, 35(-26) Ma - alternatively, its age is mid Cretaceous between (very approximately) 116 and 98 Ma (Buerki et al. 2010c), or somewhere in between, variously around 75.5, 65.8, or 58.8 Ma (Muellner et al. 2007) or (91.7-)81.8(-71.8) Ma (Muellner-Riehl et al. 2016).
2. Hippocastanoideae Dumortier
Plant deciduous; ("latex" +), cyanogenic glucosides 0; stomata actinocytic (anomocytic); buds perulate (perulae 0); leaves opposite, basically palmate organization, vernation conduplicate-plicate, deciduous; protogynous; A (5-)6-8(-12); stigma ± capitate-lobed, dry, papillate; nucellar cap 8-10 cells across; aril 0; (embryo chlorophyllous); x = 20, nuclear genome [1 C] 0.6-1.18 pg.
5/144. North temperate, some tropical and then usually montane.
Age. Wikström et al. (2001) dated crown-group Hippocastanoideae to (29-)26, 20(-17) Ma, Bell et al. (2010) to (37-)25(-14) Ma - or they may be (89.8-)88.2(-83.8) Ma (Du et al. 2019), 83±20.5 Ma old (Buerki et al. 2013b), (75.8-)66.4(-60) Ma (Muellner-Riehl et al. 2016) or ca 78.1 Ma (Arias 2023/2024).
2A. Acereae (Durande) Dumortier —— Synonymy: Aceraceae Jussieu
(Pericyclic sheath 0); cuticle wax crystalloids quite common [Acer]; leaves simple, (palmate), (odd pinnate); flowers polysymmetric; K with a single trace, C (0, 4)5(6), not clawed, (0), basal appendages 0; nectary annular, also inside A/0; A (4, 5)8(10, 12); ; G [2(-5)]; outer integument 3-5 cells across, ?obturator +; style ± 0, branches long; fruit a schizocarp, samaroid; cotyledons (plicate), ?unequal in size; germination epigeal.
2/122: Acer (120[-150 - J. Li et al. 2019]). North Temperate, esp. China, Korea and Japan.
Age. The split between Acer and Dipteronia has been dated to (98-)78(-63.5) Ma (Renner et al. 2007b) or ca 67.0 Ma (Arias 2023/2024).
The distinctive fruits of Dipteronia (now found only in China, = Acer s.l.), are known fossil from North America as long ago as late Palaeocene, 63-60 Ma, and have been found quite commonly there since, but they are not known from Europe (McCain & Manchester 2001).
2B. Hippocastaneae (de Candolle) Dumortier —— Synonymy: Aesculaceae Burnett, Hippocastanaceae A. Richard, Paviaceae Horaninow
(Plant evergreen - Billia); (fructan sugars accumulated as isokestose oligosaccharides [inulins] - Aesculus); (vessel elements with scalariform perforation plates); leaves palmately compound; flowers monosymmetric, "large"; K connate (free), C 4, 5, with basal appendages; nectary on one side of flower; outer integument 8-10 cells across, inner integument 3-6 cells across, hypostase 0 [Handeliodendron]; fruit a capsule; hilum large, (double arillode + - Handeliodendron); cotyledons incumbent [?level]; germination hypogeal [?all].
3/16: Aesculus (13). Discontinuously north temperate, S. to Ecuador.
Age. Crown-group Hippocastaneae are estimated to be (89.6-)85.9(-80.4) Ma (Du et al. 2020) or ca 28.1 Ma (Arias 2023/2024).
Leaves, fruits, and perhaps pollen of Aesculus hickeyi, some specimens of which were originally identified as Carya (Juglandaceae), are known from Palaeocene deposits 60-55 Ma from North Dakota and Wyoming (Manchester 2001).
[Dodonaeoideae + Sapindoideae]: leaves usu. evergreen, even-pinnate, (bicompound; simple), leaflets opposite or not, (margins entire), (rachis winged); 1 common A-C primordium; seeds often with sarcotesta or chalazal/integumentary arils, (dormancy physical, water gap near hilum).
Age. The age of this node may be mid-Cretaceous very approximately 116-98 Ma (Buerki et al. 2010c) or (88.2-)77.4(-66.5) Ma (Muellner-Riehl et al. 2016).
3. Dodonaeoideae Burnett
Flowers with oblique plane of symmetry [?distribution]; K initiation spiral, with gland-tipped marginal trichomes, C 4, appendages uncommon; A 8; tapetal cells uni-/binucleate; nectary (semi)annular; ovule outer integument 8-10 cells across, inner integument 3-4 cells across, parietal tissue 7≤ cells across.
24/140. Pantropical-warm temperate.
Age. Crown-group Dodonaeoideae are 80.5±12.75 Ma (Buerki et al. 2013b), (71-)53.2(-36) Ma (Muellner-Riehl et al. 2016) or ca 79.3 Ma (Arias 2023/2024).
3A. Dodonaeeae (Kunth) de Candolle —— Synonymy: Dodonaeaceae Small
Cork pericyclic [Dodonaea]; stomata cyclocytic [Dod.]; flowers obliquely symmetrical, polysymmetric (monosymmetric); C (0 - esp. Dod.); A (many - esp. Distichostemon), glabrous; (pollen in calymmate tetrads - Magonia); (nectary - Dod.); (inflated gynophore - Dod.); ovules superposed (?other subfams), (8/carpel, in parallel - Magonia), outer integument (3-4 - Mag.)/8-10 cells across, chalaza pointed [Mag.]; fruit a capsule/(schizocarp, dehiscemce septicidal); seed (arillate; sarcotestal; winged); n = 10, 12, 14-16.
16/126: Dodonaea (65), Harpullia (26). Pantropical-warm temperate, esp. Australia, to W. Pacific.
Age. The age of this clade is ca 68.1 Ma (Arias 2023/2024).
3B. Doratoxyleae Radlkofer
Flowers polysymmetric, K (monosymmetric); A 5-8; ovules ± anatropous, epitropous, bistomal, outer integument ca 5 cells across, nucellar beak + [?= nucellar cap], funicular obturator +, of hairs; fruit indehiscent, drupe or berry; aril/sarcotesta 0; outer and inner integuments multiplicative, exotesta lignified; endosperm ruminate, 0 at maturity, chalazal haustorium +, cotyledons large, foliaceous, suspensor 2-3-seriate, ca 5 cells long; n = 16.
8/19: Doratoxylon (6). Pantropical, mostly New World, but barely South America, and Africa-Madagascar (Ganophyllum to Australia).
Age. This clade is around 38.7 Ma (Arias 2023/2024).
4. Sapindoideae Burnett
4. Sapindoideae Burnett
(Vessel elements with scalariform perforation plates); stomata various; C (0, 5+), ± complex appendages +/0; ovules (epitropous), outer integument 4-12 cells across, inner integument 2-7 cells across; chromosomes 0.62-4.36 µm long.
111/1,365: Guioa (65), Cupaniopsis (60), Talisia (42), Cupania (50), Matayba (50). Pantropical.
Age. The crown-group age of this clade is estimated to be (75.6-)63.7(-51) Ma (Muellner-Riehl et al. 2016: Koelreuteria sister) or ca 99.1 Ma (Arias 2023/2024: Suppl. Fig. 12).
For Late Cretaceous/Early Palaeocene fossil woods (India, Deccan Traps) and seeds (Europe) that may well be Sapindoideae, see Wheeler et al. (2017).
4A. Ungnadieae Buerki & Callmander
Shrub to small trees; leaves trifoliolate/parpinnate; flowers functionally unisexual; monosymmetric; K 5, imbricate, C 4-5, clawed, basal appendages tufted, filiform, above the claw; A glabrous.
2/2. S. North America, S.W. China and North Vietnam.
Age. Ungnadieae are ca 24.3 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Koelreuterieae [Schleichereae [Nephelieae [Cupaniodae + Paulliniodae]]]]: ?
4B. Koelreuterieae Radlkofer —— Synonymy: Koelreuteriaceae J. Agardh
(Wood ring porous - Koelreuteria); leaflets with rosoid teeth; flowers with oblique monosymmetry; K spirally initiated; C 4 (+ 1 reduced; C:A primordia +); 1 A outside nectary; androgynophore +; micropyle zig-zag, nucellus >10 cells across, nucellar cap +, funicular obturator +; capsules inflated; aril/sarcotesta 0; cotyledons convolute.
3/13: Erythrophysa (9). Africa, Madagascar, E. Iran, Afghanistan, China and Korea to Japan, Fiji.
Age. Crown-group Koelreuterieae are ca 59.3 Ma (Arias 2023/2024: Suppl. Fig. 12).
Fossil fruits of Koelreuteria are known from the early Eocene Green River deposits in Colorado, etc., that are ca 52 Ma (Q. Wang et al. 2013, q.v. for a critical evaluation of the whole fossil record); there are also Eocene records for East Asia and widely in the Northern Hemisphere by the Oligocene (P.-R. Chen et al. 2022: fig. 7).
[Schleichereae [Nephelieae [Cupaniodae + Paulliniodae]]]: marginal parenchyma 0; ovule 1/carpel.
4C. Schleichereae Radlkofer
Flowers polysymmetric (monosymmetric)
6/11. Tropical Asia, ?Malesia.
Age. Crown-group Schleichereae are estimated to be ca 59.3 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Nephelieae [Cupaniodae + Paulliniodae]]: ?
4D. Nephelieae Blume
K whorled [Litchi]; seed with sarcotesta [?Nephelium]/aril.
16/116: Chytranthus (30), Nephelium (22). Africa, Madagascar, Tropical Asia, Malesia.
Age. Crown-group Nephelieae are around 43.7 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Cupaniodae + Paulliniodae]: ?
Cupaniodae Ezedin / [Stadmanieae + Cupanieae]: ?
4E. Stadmanieae Buerki & Callmander
Flowers unisexual, polysymmetric; K (0), C (0), basal appendages +: A 5(-10); fruit ± indehiscent.
10/>50. Tropical Africa, Madagascar to the Seychelles.
Age. Crown-group Stadmanieae are some 27.5 Ma (Arias 2023/2024: Suppl. Fig. 12).
4F. Cupanieae Blume
?
36/470: Guioa (64), Cupaniopsis (60), Cupania (58), Matayba (50). Indo-Malesia and Australia to the western Pacific, Madagascar and the Mascarenes, New World tropics, few spp. Africa.
Age. The age of crown-group Cupanieae is ca 34.3 Ma (Arias 2023/2024: Suppl. Fig. 12).
Pollen of Cupania is known from Eocene deposits in Argentina dated to ca 40 Ma, P.E.T.M. age (Fernández et al. 2021).
Paulliniodae Avecedo-Rodríguez et al. / [Sapindeae [Tristiropsideae [Haplocoeleae [Melicocceae [Blomieae [Guindilieae [Athyaneae [Paullinieae [Bridgesieae + Thouinieae]]]]]]]]]: ?
4G. Sapindeae (Kunth) de Candolle
(Wood ring porous - Sapindus); fruit indehiscent, (berry - Sapindus); aril 0 (fruit dehiscent; dry arils +).
13/130: Deinbollia (40), Lepisanthes (24), Toulicia (14). Pantropical.
Age. Crown-group Sapindeae are some 46.2 Ma (Arias 2023/2024: Suppl. Fig. 12).
4H. Tristiropsideae Buerki & Callmander Tristiropsis Radlkofer
Flowers monosymmetric, (functionally unisexual); K 5, C 5/0, basal appendages +; A 8(-13); G (4, 5-carpellate); fruit a drupe, 2-3-locular; aril 0.
1/3. Malesia, E. Australia, Pacific Islands.
4I. Haplocoeleae Buerki & Callmander
Flowers polysymmetric, unisexual; K 4-7, C 0: A 4-7; disc hemispherical; style short; fruit 1-seeded, at most slowly dehiscing; seed arillate.
2/7. Tropical Africa.
Age. Haplocoeleae are estimated to be around 30.6 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Melicocceae [Blomieae [Guindilieae [Athyaneae [Paullinieae [Bridgesieae + Thouinieae]]]]]]: ?
4J. Melicocceae Blume
Flowers polysymmetric; C (appendages 0); fruit indehiscent, ± dry; sarcotesta +; n = 16.
4/60: Talisia (50). New World tropics.
Age. Melicocceae are ca 36.2 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Blomieae [Guindilieae [Athyaneae [Paullinieae [Bridgesieae + Thouinieae]]]]]: ?
4K. Blomieae Buerki & Callmancder - Blomia prisca (Standley) Lundell
Flowers polysymmetric, (unisexual); K valvate; C at most vestigal, appendages vestigial; disc annular, lobed; G 1, style short, stigma capitate; fruit at most slowly dehiscing; aril thin.
1/1. S.E. Mexico, Guatemala, Belize.
[Guindilieae [Athyaneae [Paullinieae [Bridgesieae + Thouinieae]]]]: C with hood-shaped, basal appendage creacentic; disc unilateral; A 8; pollen tricolporate; fruit a schizocarp, septicidal; aril 0.
4L. Guindilieae Buerki, Callmander & Acevedo-Rodríguez - Guindilia Hooker & Arnott
Leaves opposite, simple, (tridentate at apex); flowers (unisexual); K imbricate; disc pyramidal, 2-lobed; pollen (syncolporate), surface striate; fruit mericarps subglobose, crustose; n = 10, genome 1C 1.16-1.20 pg.
1/3. Andean Chile and N.W. Argentina.
[Athyaneae [Paullinieae [Bridgesieae + Thouinieae]]] / Paulliniodae: leaves odd pinnate; inflorescence a thyrse with lateral cincinni; pedicels articulated, flowers monosymmetric; C 4; A 8; x = 15.
Age. The age of this clade is (58-)51(-45) Ma (Urdampilleta et al. 2025).
4M. Athyaneae Acevedo-Rodríguez - Athyana (Grisebach) Radlkofer
Plant monoecious; K 4, 5, valvate, imbricate; C basal appendage hood-shaped; disc semi-annular; fruit mericarps winged; n = 15.
1/3. Brazil, Bolivia, Paraguay, Argentina.
Age. Athyaneae are around 21.2 Ma (Arias 2023/2024: Suppl. Fig. 12).
[Paullinieae [Bridgesieae + Thouinieae]]: ?
4N. Paullinieae (Kunth) de Candolle
Shrubs, vines, lianes climbing by branch tendrils; secondary thickening anomalous [vascular strands separate by the initiation of new cambia or dissection of original cambium/phloem wedges, stem lobed/etc.], not; (stem endodermis +); (latex +, laticifers articulated, non-anastomosing [distribution various]), (callose in laticifer wall); trifoliolate (imparipinnate/ternate), stipules +, minute to large; flowers obliquely monosymmetric (polysymmetric - Thinouia); K 4 (5), C 4, (5), basal appendage (little expanded), petal-like; disc annular or unilateral, arcuate/not, 2- (corniculiform), 4-, 6-lobed; androgynophore +/0; pollen spherical, tricolporate [Thinouia]/asymmetrical, trihemi- or syncolporate proximally, strongly oblate or perprolate, with 2 colpi diorate [Lophostigma]); stigma lobes conspicuous, papillate; o.i. 5-9, i.i. 4-7, obturator large; (antipodal cells persistent, multinucleate - Cardiospermum); fruit samaroid, or ± capsular, (inflated), long-stipitate; (sarcotesta ± developed, margin free); (amyloid [xyloglucans] in seed - Cardiospermum); n = 7, 9, 10, 11, 18 [all Cardiospermum], 12, 14; germination hypogeal (epigeal).
7/480: Serjania (230), Paullinia (220), Urvillea (20). Mostly New World tropics, 2 spp. Africa and Madagascar.
Age. Crown-group Paullinieae are some 46.2 Ma (Arias 2023/2024: Suppl. Fig. 12) or (43-)37(-31) Ma (Urdampilleta et al. 2025).
4O. Bridgesieae Acevedo-Rodríguez - Bridgesia incisifolia Cambessèdes
Shrub; leaves simple, venation pinnate; C basal appendage hood-shaped; disc semi-annular; pollen striate-perforate (and rugulate); fruit stipitate, shortly winged, inflated; seed with large hilum; n = 14, genome 1 C ca 1.28 pg.
1/1. Andean Chile.
Age. The fossil Bridgesia bovayensis was described from Eocene deposits in Holly Springs, Mississippi (!: Manchester & O'Leary 2010).
4P. Thouinieae Blume —— Synonymy: Allophylaceae Martynov
Leaves tri-(uni)foliolate; venation pinnate; infloreascence (branches 1-flowered/racemose); pedicels (not articulated - Allophylastrum); C (0); disc semiannular/cupular; A (6); pollen 3-6 porate (brevicolporate), oblate to spherical; fruit samaroid/individual carpels berry-like; n = 14.
3/279: Allophylus (1-255), Thouinia (28). Mexico to South Amarica, Allophylus pantropical.
Age. Crown-group Thouinieae are about 56.2 Ma (Arias 2023/2024: Suppl. Fig. 12).
Evolution: Divergence & Distribution. Cupaniopsis-type pollen is widespread in the fossil record, including from several sites in Africa, although Sapindaceae with such pollen are no longer to be found there (Coetzee & Muller 1984). Wehrwolfea, with striate pollen, a floral formula of K 4 C 4 A 10(?+) G [3-4], and placed in Sapindaceae, is known from the middle Eocene of western Canada (Erwin & Stockey 1990), however, Acer and Dipteronia are older (see above). For the early Caenozoic fossil history of what are now East Asian endemics, see Manchester et al. (2009). Muellner-Riehl et al. (2016) suggest dates for more clades and Du et al. (2019) dates for clades in Hippocastaneae.
Buerki et al. (2010c, 2013b) outline the biogeography of the family, in which much dispersal is involved. The subfamilies of Sapindaceae spread in the mid Cretaceous 116-98 Ma, initially from Laurasia, with South East Asia remaining an important area in the evolution of the family (Buerki et al. 2010c, 2013b). Sapindaceae may not be a simple example of a temperate radiation embedded in an otherwise tropical group (c.f. Judd et al. 1994). Xanthoceratoideae and Hippocastanoideae are both predominantly temperate (Du et al. 2019 suggest that Hippocasataneae originated in eastern Asia), although the rest of the family is largely tropical, and the samaras of Acereae (see e.g. Harris et al. 2017b) evolved independantly from those in the rest of the family. Arias (2023/2024e) also examined the biogeography of the family, dispersal remaining a major factor in explaining its distribution; Antarctica has been particularly important by enabling plants to move around the southern end of the world.
For diversification in Acereae, see Renner et al. (2007b) and Y. Feng et al. (2018), although Dipteronia has only two species, these may have separated around 58.2-46.5 Ma, before diversification in the far larger Acer began. Sapindaceae seem to have moved into New Caledonia ca 10 times or more, or there is yet a more complex pattern of movement to and from the island; the relatives of the Mauritian Cossinia pinnata (Dodonaeoideae) grow in the New Caledonian area (Buerki et al. 2012a).
Harris et al. (2009) found that Aesculus parryi, from Baja California, diverged ca 49 Ma and A. californica, rather more widespread, diverged ca 61.2 Ma, in both cases the sister clades being east North American. The very widespread Dodonaea viscosa has achieved its range within the last two Ma (Harrington & Gadek 2009). Koelreuteria is known only from east Asia and Fiji, but it has a fossil record throughout much of the Northern Hemisphere - perhaps its origin was in the North Pacific rim area, with subsequent long distance dispersal to Fiji (Q. Wang et al. 2013)?
Medina et al. (2020) discuss the distribution of what they call laticifers in Sapindaceae, and these are tentatively placed above as an apomorphy for the family minus Xanthoceratoideae. However, laticifers are not found in all genera, and Medina et al. (2017, 2020) suggest that articulated non-anastomosing laticifers had five independent origins, and with some subsequent losses (see also Montes 2017). The main constituents in these laticifers are terpenes, found as essential oils and resins, although apparently not rubber (so from this point of view they are not laticifers in the strict sense), and a variety of less abundant substances; there may be callose or suberin in the walls. The secretory tubes can be hard to distinguish from idioblasts, which may also be in longitudinal series in the stem so look like the cells that make up the laticifers - and the two may be found in the same plant; taxa that lack tubes have these idioblasts, and they contain phenolics, uncommon in other secretory structures here (Medina et al. 2020). For laticifers, see also Prado and DeMarco (2018).
Acevedo-Rodríguez et al. (2017) suggest a number of apomorphies for clades in Paulliniodae; many of these have been incorporated into the hierarchy above. However, given the broader phylogenetic context here, the positions of some are moving down the tree, furthermore, it is difficult to know what to do with the extensive - and remarkable - variation in pollen morphology in Paullinieae, for example.
Although most Sapindoideae have but a single ovule per carpel, this is not an apomorphy for the subfamily since basal taxa like Ugnandia and Koelreuteria have two ovules per carpel. There are usually 1-3 seeds per fruit, and the size of animal-dispersed fruits depends on the size of the frugivores in the area, as was shown by Brodie (2017) looking at seed and frugivore size across Malesia - Sapindaceae from Sulawesi and Moluccas, with smaller frugivores, had significantly smaller fruits than Sapindaceae from New Guinea or the region of the Sunda shelf, although obviously this is not likely to be unique to Sapindaceae (see also Arecaceae). Paullinia is a valuable resource for frugivorous birds on Barro Colorado Island, Panama, the fruits tending to ripen in the dry season when other trees were not fruiting (Chery et al. 2019a).
Ecology & Physiology. Sapindaceae, along with Bignoniaceae and Fabaceae, are the major components of the liane/vine vegetation of the Neotropics (e.g. Gentry 1991; Sperotto et al. 2023). The largely Neotropical Paullinieae (Sapindoideae) include 8 genera of climbers, both vines and lianes, notably Serjania and Paullinia; Paullinieae contain one third of the species in the family, including around 470 species that are climbers - as a clade very largely of climbers, it is one of the biggest in the angiosperms. As might be expected, many of these climbers have stems with anomalous secondary thickening (see Vegetative Variation below). Lianes in general use water very efficiently and grow remarkably well in the dry season/dry conditions and proportionally much more than trees (van der Sande et al. 2019; Schnitzer et al. 2019; Dias et al. 2019). Lianes are also abundant in forest edges, treefall gaps, and similar habitats where their growth habits will also be advantageous, and they may have negative effects on the growth of co-occurring trees (Schnitzer 2018).
Pollination Biology & Seed Dispersal. Species of Acer like A. rubrum are known for having very labile breeding systems (Blake-Mahmud & Struwe 2019: A. pennsylvanicum); Renner et al. (2007b) suggested that dioecy had evolved several times in the genus and that the sex of the flower might be determined by environmental cues. Interestingly, fully carpellate trees tended to be unhealthy, and although these trees produced fruits, they might die... (Blake-Mahmud & Struwe 2019: see also Castanea, although monoecy there), furthermore, if the tree was badly damaged (defoliated, severely pruned) trees might well become female, but never male (Blake-Mahmud & Struwe 2020). Goldberg et al. (2017) looked at the evolution of breeding systems in Dodonaea and suggested that dioecy might be plesiomorphic there (is not Dodonaea polygamodioecious?), and this and other floral features may be associated with wind pollination (L. Cao et al. 2025). The latter group suggested that a disc-like structure inside the androecium was unlikely to represent the nectary of the insect-pollinated ancestors of Dodonaea; the nectary in those species is outside the androecium. For pollination by hemipterans, see Plant-Animal Interactions below.
A number of Sapindaceae have winged fruits rather like those of maples (Acer), although constructed in a variety of ways - for example, the position of the wing varies. Lentink et al. (2009) studied the aerodynamics of the fruits of three species of maple, and showed that they developed a vortex at the leading edge of the rotating wing that generated lift, so allowing the descending fruit to travel greater distances (Lentink et al. 2009). The wings on the fruits of Paullinia are brightly coloured and may help attract frugivorous birds; the fruits are capsules and contain "arillate" seeds (Chery et al. 2019a).
Plant-Animal Interactions. Hemipteran rhopalid seed predators have recently switched from native to introduced Sapindaceae in both Australia and Florida (Thompson 2005; Forbes et al. 2017 and literature). Work by Cenzer (2017) paints a complex picture in Florida, at least, where maladaptive plasticity in hybrid red-shouldered soapberry bugs (Jadera haematoloma) masks differences developed between populations of those bugs feeding on the different sapindaceous species (native Cardiospermum, introduced Koelreuteria). Interestingly, Western boxelder bugs, Boisea trivittata, which fancy the seeds of Acer negundo (and other species of the genus, also Fraxinus) respond to infrared cues in the laboratory (Takács et al. 2017); these bugs are all soapberry bugs, Serinethinae, a group of about 63 rhopalids that specialize on Sapindaceae, especially Sapindoideae, but they are not found on Dodonaeoideae and Xanthoceratoideae (Carroll & Loye 2012 for insect-host associations). Recent work (focus on J. haematoloma) suggests that these bugs also take nectar from the plants whose seeds they eat, and that they also pollinate them, albeit not terribly efficiently, so rather than being simple seed predators, they are pollinating seed predators (Comerford et al. 2025) - c.f. yucca moths, fig wasps, Epicephala-Phyllanthus/Glochidion association, etc..
The cynipoid Pediaspini, found on Acer, are sister to those Diplolepidini found on Rosa, the two clades separating perhaps 142-133 Ma and together forming a small clade outside Cynipidae (Blaimer et al. 2020). Acer is an important host plant for lepidopteran larvae in the contiguous United States of America, being included in the top 10 such plants (Narango et al. 2020).
Ants visit the extrafloral nectaries of Urvillea and Nephelium lappaceum (Maximo et al. 2023)
Vegetative Variation. For the vegetative anatomy of two species of Acer compared with that of 12 other species of Sapindaceae, see Salim et al. (2025); Acer fitted in quite nicely with the rest of the family in UPGMA analyses. Stem tendrils of Paullineae are found in leaf axils, and there is a basal bud on the side opposite to the direction in which the tendril is growing. Tendrils often develop on inflorescences, and they appear to be modified branches that grow from the axils of prophylls.
Cauline secondary thickening in Paullinieae is often strikingly anomalous - the liana syndrome. There are some ten unusual patterns of cambial origin, some species developing several independent vascular cylinders whether surrounding each other (ectopic, including successive, cambia) or not while in others there is just a single cambium; cambial activity is also variable, that of some species resulting in xylem outside and cambium inside; furthermore, there may be heteromorphic vessels of two size classes, or parenchyma-like fibre bands alternating in amount of thickening; etc. (Tamaio & Angyalossy 2009; Angyalossy et al. 2015 and other references in Schnitzer et al. 2015; Lopes et al. 2017; da Cunha Neto et al. 2018; Pellissari et al. 2018; Chery et al. 2019b, 2020; Rizzieri et al. 2021; Pace et al. 2022: Figs 2, 19). The cambial variants, and even some taxa with apparently ordinary vascular tissue (ordinary vascular tissue is found in some taxa of all genera), have a 5-lobed star-shaped primary vascular body (Chery et al. 2019b). Interestingly, clade I of Paullinia, sister to the rest of the genus, appears to have ordinary secondary thickening (Chery et al. 2019a), while ordinary secondary thickening may be derived in Urvillea, for example (Cunha Neto et al. 2023). For vascular variants, see Cunha Neto (2023) in particular; many of the variants here are some kind of ectopic cambium, including successive cambia. Vessel dimorphism - very wide and very narrow vessels - is common, perhaps ensuring rapid movement of water yet at the same time affording some protection against embolisms (Bastos et al. 2016 and references; Bouda et al. 2019). Anatomical variation in yet other characters and also other lianescent members of Paullinieae like Serjania increases the complexity of variation patterns of stem anatomy in the tribe (see e.g. da Cunha Neto et al. 2017; Chery et al. 2020; Pace et al. 2022; Cunha Neto et al. 2023). The root anatomy of these vines/lianas shows similar dimorphism in vessel diameter but usually not the cambial variation of the above-ground parts of the same plant (Bastos et al. 2016). For more on such anomalous secondary thickening, see a couple of places (one, two) elsewhere.
Genes & Genomes. It has also been suggested that the base chromosome number for Sapindaceae is x = 7 (Ferrucci 1989) or 16 (Guimarães & Forni-Martins 2021; Urdampilleta et al. 2025). For chromosome numbers, see also Lombello and Forni-Martens (1998), for chromosome size, see Ferrucci (1989), and for genome size, see Coulleri et al. (2014: not much correlation with anything). Urdampilleta et al. (2025) looked at chromosome numbers and their evolution in Paulliniodae in particular.
W. Wang et al. (2020b) note that size and genome order of plastomes in Acer are similar, but the boundary of the inverted repeat may vary quite considerably.
Economic Importance. The aril of the lychee (variously spelled: Litchi chinensis) is a popular fruit, especially in the Indo-Malesian area.
Chemistry, Morphology, etc.. Gibbs (1958) noted that sections Cissifolia and Negundo of Acer had very low amounts of syringyl lignin, confirming their distinctiveness. A cauline endodermis is reported from some climbing Paullinieae, being recognised as such by its position and the large size of its cells and the starch grains they contain rather than by any distinctive pattern of wall thickening (da Cunha Neto et al. 2018). For laticifers in Paullinieae, which can be clustered, see da Cunha Neto et al. (2017); latex is reported to have evolved several times here (Prado & Demarco 2018).
Aesculus has large bud scales, Billia has naked buds, but both branch from the previous flush. "Ordinary-looking" stipules are known only from climbing species like Serjania, but leaflets looking like stipules (pseudostipules) occur elsewhere in the family. Maximo et al. (2023) described distinctive extrafloral nectaries on the foliar serrations of Urvillea. They consist of a multi-layered epidermis with a layer of transfer cells immediately below, and the nectar was found to contain ca 14% amino acids and almost 20% of the sugar alcohol, xylitol HOCH(CH(OH)CH2OH)2, an alditol - rather unusual.
Radlkofer (1892-1900) shows Serjania as having strongly obliquely symmetric flowers, with the odd gynoecial member abaxial on the plane of symmetry. The abaxial corolla member is absent, but the stamens are abaxial, the two adaxial(?)-lateral members being missing. Flowers of Eurycorymbus change from being obliquely symmetric to polysymmetric during the course of development (Cao et al. 2017). In Acer, the samaras are shown as being oblique by Schnizlein (1843-1870), while Ronse de Craene (2010) depicts gynoecial orientation as varying within an inflorescence. The petals of Sapindaceae are often rather complex and have a similarly complex set of terms used to describe them; see "appendages" above.
There is extensive variation in pollen morphology within Sapindaceae - the pollen is often triangular, heteropolar, prolate, and syntricolporate, and Paullinieae are particularly variable (see J. Muller & Leenhouts 1976; van den Berg 1978; Ferrucci ∓ Anzótegui 1993; Acevedo-Rodríguez et al. 2011, 2017; Gonçalves-Esteves et al. 2021), and this variation is being integrated into the phylogeny. I have not attempted to integrate variation in stigma/style morphology with the phylogeny in other than a cursory fashion. Brizicky (1963) reported that the ovules may be epitropous (see also Gulati & Mathur 1977; Tanaka et al. 2016); those of Koelreuteria and other taxa are both epitropous (the lower ovule) and apotropous (the upper ovule) in the same loculus (Mauritzon 1936; Danilova 1996). Several aspects of the embryology of Filicium are distinctive (Gulati & Mathur 1977), but sampling is too poor to understand the significance of this. Corner (1976) noted that the outer integument of Nephelium lappaceum was slightly thinner than the inner integument, and that there was a definite funicle in Aesculus, at least after fertilization. Tanaka et al. (2016) described how the ovule became campylotropous as an invagination developed in the raphal region; this also produced the radicular pocket characteristic of sapindaceous seeds.
Comparing the ovules of Thinouia mucronata and Serjania meridionalis, both Paullinieae, Solís and Ferrucci (2021) found numerous differences. These included basic ovule type, obturator position, presence of hypostase and nucellar cap, and so on - and whether the flowers were poly- or monosymmetric.
Weckerle and Rutishauser (2005) describe all stages of the fruits and seeds of Paullinieae from ovule to germination - a rather remarkable piece of work. There is considerable variation in the morphology of the pollen tube transmitting tissue and in the degree of development of the srcotesta (sometimes called an aril by Weckerle & Rutishauser 2005). The fruit can look like a follicle when only one carpel develops; dehiscence is, however, down the abaxial side and rudiments of other carpels are quite often visible. In many Sapindaceae (and also Anacardiaceae) the pericarp grows much faster than the seed, so what seem to be almost mature fruits can contain seeds that are still very small. I do not understand details of the morphology and anatomy of the seed very well, a subject that has been bedeviled by terminological issues (e.g. van der Pijl 1957; Weckerle & Rutishauser 2005; see also Guérin 1901). It seems that fleshiness, etc., may reflect varying developments of the testa/sarcotesta at the micropylar end of the seed. Turner et al. (2009) document a water gap near the hilum in the hard seeds of Dodonaea (see also Gama-Arachchige et al. 2013).
For general accounts/information, see Radlkofer (1890, 1933 to 1934, etc.), Acevedo-Rodríguez et al. (2011), Urdampilleta (2016: Bridgesia, Guindilia) and Buerki et al. (2021), for chemistry, see Hegnauer (1964, 1966, 1973, 1989, 1990, also under Aceraceae and Hippocastanaceae), for wood anatomy, see Klaassen (1999) and Agarwal et al. (2005), for epidermal features, see L.-M. Cao and Xia (2008) and Pole (2010), for floral anatomy of Acer, see Hall (1951), for floral morphology of Litchi and Dimocarpus, see S. X. Xu (1990, 1991), of Koelreuteria, see Ronse Decraene et al. (2000b) and Cao et al. (2018), of Delavaya, Cao & Xia (2009), of Handeliodendron, Cao et al. (2008), of Acer, etc., Gostin and Minea (2007) and Leins and Erbar (2010), of Xanthoceras, Zhou and Liu (2012), and for that of Dodonaea, see Cao et al. (2025 - also references to other genera), for nectaries, which are variously vascularized, see Hall (1951), Solís and Ferrucci (2009), Zini et al. (2014a) and Solis et al. (2017), for endothecial thickenings, see Manning and Stirton (1994), for pollen, see Ferrucci and Anzótegui (1993 and references), for style morphology, see Lersten (2004), for embryology, see Nair and Joseph (1960), Tobe and Peng (1990), González et al. (2017) and Avalos et al. (2019: Koelreuteria) and for seeds, see Turner et al. (2009: germination).
Phylogeny. Buerki et al. (2009, 2010b: 81 and 104 genera respectively) carried out extensive phylogenetic studies on Sapindaceae. Preliminary studies suggested that Xanthoceras might be sister to all other Sapindaceae, general relationships being [Xanthoceras [[erstwhile Aceraceae + Hippocastanaceae] [the remainder of the family]]] (see Klaassen 1999; Savolainen et al. 2000a; Soltis et al. 2007a). Subsequent two-gene studies (Harrington et al. 2005, 2009: information about secondary structure of ribosomal DNA, extensive sampling in Dodonaeoideae but no Sapindoideae) largely confirmed these results. Harrington et al. (2005) found that Xanthoceras was not sister to the rest of the family in single gene analyses, being somewhat embedded, but without strong support; it was only in the joint analysis that it was sister and with 70% bootstrap and ³95% posterior probability support (see also Buerki et al. 2010a, 2010b, support still very low; M. Sun et al. 2016; Muellner-Riehl et al. 2016). Early morphological analyses (Judd et al. 1994) suggested a rather different set of relationships, while in an analysis of ca 350 nuclear genes Baker et al. (2021: see Seed Plant Tree) found relationships that were rather different, thus Dodonaeoideae are sister to the rest of the family, while Aesculus hippocastanum was quite far separate from the Hippocastaneae (which include A. pavia). However, in the Angiosperms353 target genes study by Buerki et al. (2021) with very extensive generic-level, but not infrageneric, sampling these problems were not evident, and they recognized the four subfamilies and divided them into tribes; support was strong.
Hippocastanoideae. For the phylogeny of Acer, see J. Li et al. (2006), Renner et al. (2007b), Li (2011) and Harris et al. (2017b). Evidence that Dipteronia, with pinnate leaves and cyclic samaras, is derived from within Acer, with more regulation-type samaras and usually palmate/ly lobed leaves, is ambiguous. A recent transcriptome analysis (Li et al. 2019: 500 nuclear loci) suggested that the two were sister taxa, and although Harris et al. (2017b) found that A. japonica was sister to [other Acer + Dipteronia], that species was well embedded within Acer both in Renner et al. (2007b: one analysis, ?incomplete sequence) and in Li (2011). For relationships within Acer, where groupings generally agree with sections currently recognized, see Li et al. (2019: ca 2/5 species included). In a plastome analysis representatives of section Negundo was found to be sister to those of the other seven sections included (W. Wang et al. 2020b). Harris et al. (2009) examined the phylogeny of Aesculus as did Aygören Uluer and Alshamrani (2019), the latter discussing hybridization there.
Dodonaeoideae. Dodonaeeae. Relationships within Dodonaea are discussed by Harrington and Gadek (2010).
Sapindoideae. Delevaya and Koelreuteria are successively sister to the rest of Sapindoideae (Buerki et al. 2013b), M. Sun et al. (2016) found Ungnadia to be sister to the rest of the subfamily, and Koelreuteria was in this position in the study by Muellner-Riehl et al. (2016: the two other genera not included).
Cupanieae. For relationships around Cupania, see Buerki et al. (2012a).
Paulliniae. Acevedo-Rodríguez et al. (2017) looked at relationships here. Chery et al. (2019a) clarified relationships within Paullinia itself, which came out as sister to Cardiospermum, in turn sister to [Urvillea + Serjania], although that clade had little support; seven main clades, mostly well supported, were recovered within Paullinia, Radlkofer's sections holding up reasonably well. Cardiospermum itself may be polyphyletic, and this is supported by laticifer distribution - laticifers are not found in all the species currently included in the genus (Medina et al. 2021) and a new genus was described to deal with this in Urdampilleta et al. (2025). Urdampilleta et al. (2025) were looking at relationships in Paullinieae (ca 87 spp., 3 markers...); the two species of the new genus, Acevedoa, were on a long branch and some species ofUrvillea were associated with Serjania. Steinmann et al. (2022: 48 spp. - ca 1/5 the total, ITS and trnL-F) in a study of Serjania had found that none of the sections included that were represented by two or more specimens was monophyletic, and capsules had evolved more than once from schizocarps and also reversed. Cunha Neto et al. (2023: 12 markers, 10 nuclear) examined relationships in Urvillea.
Classification. The phenetically distinctive Aceraceae and Hippocastanaceae are here included in Sapindaceae, with which they have much in common; Buerki et al. (2010b) prefered to recognize them (and Xanthoceras, as Xanthoceraceae [sic]) as separate families; for subfamilies, see Buerki et al. (2009). There is extensive polyphyly of the classically-recognized tribes (Buerki et al. 2010b), while generic limits in the Cupania group (Sapindoideae) are unclear (Buerki et al. 2012a). However, Buerki et al. (2021) have provided A complete classification of the family down to the tribal level; very few genera are unplaced.
For sections in Acer, see de Jong (2004); they are slightly modified in J. Li et al. (2019).
Previous Relationships. Sapindaceae are chemically similar in some respects to Fabaceae, e.g. both have non-protein amino acids (for a summary, see Fowden et al. 1979), and both have compound leaves, their seeds may be arillate, etc., but they are not closely related.
[[Kirkiaceae [Anacardiaceae + Burseraceae]] [Simaroubaceae [Meliaceae + Rutaceae]]]: wood silicified or with SiO2 grains; tension wood +; resin canals +; persistent floral apex in the center of the gynoecium [?this level]; ovules often 2/carpel, superposed, micropyle endostomal, inner integument elongated, S- or Z-shaped; x = 14.
Age. Wikström et al. (2001) dated this node (Sapindaceae included) to (66-)62-57(-53) Ma and Bell et al. (2010) suggested an age of around (75-)71(-70) My; about 61.75 Ma is the age in Naumann et al. (2013), ca 73.4 Ma in Tank et al. (2015: Table S2), (107.8-)102(-96.1) Ma in Muellner-Riehl et al. (2016), and (83.4-)81.5(-79.9) Ma in Magallón et al. (2018). Suggested ages in Joyce et al. (2033: Sapindaceae excluded) are (176-)162(-142)/(125-)118(-110) Ma.
Evolution: Divergence & Distribution. Diversification rates may have increased at this node in a nested fashion, (83.4-)81.5(-79.9) Mya and ca 4 Ma before (Magallón et al. 2018).
Ecology & Physiology. All families in this clade (bar Kirkiaceae) include common trees at least 10 cm across in Amazonian forests and at least one of the 227 species that make up half the stems in Amazonian forests (for a total of 24 species; ter Steege et al. 2013).
Genes & Genomes. Guimarães and Forni-Martins (2021) suggest that the base chromosome number at this node is x = 14; given numbers for more basal nodes, there may have been a genome duplication here.
Chemistry, Morphology, etc.. For resin canals in this clade, perhaps only dubiously an apomorphy, see Prado and Demarco (2018).
[Kirkiaceae [Anacardiaceae + Burseraceae]]: tyloses +, vessel:ray pits simple/borders much reduced, marginal parenchyma 0, axial parenchyms scanty; cuticle waxes often 0; inflorescence thyrsoid [panicle of cymes]; flowers small [≤1 cm across]; K ± connate, C protective in bud; pollen (exine striate); G adnate to central receptacular apex, synascidiate, stigma with uniseriate multicellular papillae, wet; fruit with 1 seed/carpel, endocarp well developed.
Age. The age of this node is somewhere around 93.6, 83.6 or 74.1 Ma (Muellner et al. 2007), (127-)116(-105) Ma (Weeks et al. 2014), (102.9-)94.6(-85.6) Ma (Muellner-Riehl et al. 2016) or (185-)148(-131)/(119-)109(-100) (Joyce et al. 2033).
Chemistry, Morphology, etc.. Syllepsis is uncommon in this clade (Keller 1994). For some general information, see Bachelier and Endress (2008b).
KIRKIACEAE Takhtajan - Kirkia Oliver - Back to Sapindales
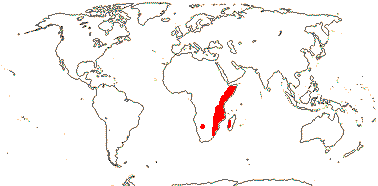
Tree or shrub, roots often tuberous; ellagic acid +, Si in wood?; resin canals?; nodes?; petiole with medullary bundles; glandular hairs with multiseriate stalks; stomata ?anomocytic; leaves ± opposite to spiral, leaflet margins serrate; plants monoecious; inflorescence subdichasial, ultimate branches monochasial; flowers 4-merous; K basally connate, decussate, valvate → open, C with adaxial-basal multicellular glandular hairs; staminate flowers: A = and opposite K; pollen syncolpate; nectary broad, well developed; pistillode +; carpelate flowers: staminodes +; G [4 (8)], ?orientation, extra "loculus" ± developed, receptacle apex convex, swollen, glandular, styluli closely adpressed, erect, finally spreading, apices postgenitally connate, stigmas ± punctiform; ovule usu. 1/carpel, micropyle bistomal, long [to 2 x length of nucellus], outer integument 2-3 cells across, cells much swollen in micropylar region, inner integument 3-4 cells across, parietal tissue ca 14 cells across; fruit a schizocarp, inner part of pericarp with horizontally and vertically oriented elongated sclereids, endocarp lignified, mericarps pendulous from columella; testa "very thin"; endosperm ?type, embryo curved; x = 15.
1 [list]/8. Tropical and S. Africa, Madagascar. Map: from Brummitt and Stannard (2007).
Age. Crown-group Kirkiaceae may be as little as (5-)2(-0.2) Ma (Joyce et al. 2023).
Chemistry, Morphology, etc.. The family is chemically unexceptionable, lacking distinctive secondary metabolites found elsewhere in the order (Mulholland et al. 2003). The wood of Pleiokirkia is reported to smell like honey (Schatz 2001).
The lower order inflorescence branches have carpelate flowers, while flowers on higher order branches are staminate (Bachelier & Endress 2008b). The endocarp of the fruit has elongated and variously oriented sclereids (Fernando & Quinn 1992).
For general information, see Muellner (2011), and for some information on anatomy, see Jadin (1901), on chemistry, see Nooteboom (1967); for the floral morphology of Kirkia, see Bachelier & Endress (2008a, esp. b) and for fruit anatomy, see Fernando and Quinn (1992).
Previous Relationships. Kirkiaceae were previously placed in (Cronquist 1983, but with some doubt) or near (Takhtajan 1997) Simaroubaceae, but i.a. they lack quassinoids and limonoids.
[Anacardiaceae + Burseraceae]: biflavonoids; phloem with vertical intercellular schizogenous secretory canals, surrounded by a light-coloured, sinuous, sclerenchymatous band [not easy to see], radial canals +; (vessel elements with scalariform or reticulate perforations); ray cells with prismatic crystals; glandular hairs with uniseriate stalks; (plants dioecious); C little longer than K; (nectary extrastaminal); tapetal cells bi- (uni-, poly-)nucleate; central receptacular apex ± exposed in the center of the flower; ovule pachychalazal, obturator placental, short, broad, fruit a drupe, (developing well before seed), stone operculate, 2≤ layers across, cells not oriented, walls lignified.
Age. Bell et al. (2010) suggested that the two families diverged (73-)64(-56) or (51-)50(-49) Ma, Tank et al. (2015: Table S1, S2) around 59.6 Ma, while Wikström et al. (2001) gave ages of (56-)51, 47(-42) Ma, Muellner-Riehl et al. (2016) ages of (97-)87.5(-78.5) Ma, and Weeks et al. (2014) ages of (121-)108(-95) My; a mere 37.7 Ma is the age in Naumann et al. (2013) while ages in Joyce et al. (2033), at (151-)134(-116)/(111-)100(-89) Ma, are about three times that.
Fossils assignable to Burseraceae/Anacardiaceae are known from the early Eocene in England ca 50 Ma (Collinson & Cleal 2001) and from the Deccan Traps of India ce 66 Ma (Wheeler et al. 2017).
Evolution: Divergence & Distribution. Weeks et al. (2014) compared the path of evolution in Burseraceae and Anacardiaceae using clades of the same age and about the same size, and they noted the comparatively greater diversity of fruit morphologies and expanded climatic tolerances in the latter (see also Donoghue & Edwards 2014 for biome shifts).
Chemistry, Morphology, etc.. Anacardiaceae like Pachycormus have thin, brown, flaking bark that looks quite like that of Burseraceae; the wood anatomy of the two is very similar (Daly et al. 2011).
Burseraceae and Anacardiaceae are palynologically indistinguishable. Bachelier and Endress (2009) discuss the floral morphology and anatomy of this clade in detail, i.a. they describe the ponticulus of the anacardiaceous ovule, but not found in Burseraceae (c.f. Michell et al. 2022). The basic endocarp condition for [Anacardiaceae + Burseraceae] seems to be that of unoriented sclerified and often crystalliferous cells (Wannan & Quinn 1990), as found in Anacardiaceae-Spondiadoideae, and also in Buchanania, Campnosperma and Pentaspadon, included in Anacardioideae (e.g. Pell 2004: Campnosperma not sequenced), as well as in Burseraceae. An operculum may be derived twice in Anacardiaceae (Pell & Urbatsch 2001), but it is also found in fruits of some Burseraceae and perhaps it, too, is plesiomorphic within the whole clade. Hill (1933, 1937) and others describe germination of such fruits, noting how the operculum gets pushed off by the germinating seed, and also that opercula might have different morphologies, even within the one family.
For chemistry, see Hegnauer (1964, 1989), for general developmental information, see Bachelier and Endress (2007a, especially 2008a, b).
ANACARDIACEAE R. Brown, nom. cons. - Back to Sapindales
Trees or shrubs; exudate resinous, variously coloured, black or becoming blackish when dermatatitis-inducing, deoxyflavonoids +; crystals in xylem; wood often fluorescing; pith loose, shining; nodes usu. 3:3; colleters +; leaflets not articulated, margins usu. entire, base of petiole often swollen; breeding system various; flowers (3-)5(-7)-merous, often imperfect, protogynous; K ±connate basally, C protective in later bud; staminate flowers: A 10; pistillode +; (andro/gynophore +); carpellate flowers: staminodes +; styluli ± separate, terminal to gynobasic, (apex postgenitally connate), stigma capitate (lobed), dry; ovule 1/carpel, apical, apotropous, ± anatropous, micropyle zig-zag (endostomal), funicle long, massive, ponticulus + [= dorsal funicular bend/projection toward style base]; fruit a drupe, inner part complex, layered, endocarp crystalliferous; seed often ± pachychalazal, vascular bundle in raphe amphicribral; endosperm oily (and starchy), usu. little-0 at maturity, cotyledons large; x = 15 (?16), nuclear genome [1 C] (0.031-)0.605(-13.019) pg/(440-)1819(-9144) Mb.
80 [list]/873 - five groups below. Tropical, esp. Palaeotropics, also temperate. Map: from Heywood (1976), modified by Barkley (1937), Fl. Austral. vol. 25. (1985), Wickens (1976), Meusel et al. *1978), Arbonnier (2002), Nie et al. (2009) and Trop. Afr. Fl. Pl. Ecol. Distr. 6. (2011). Photo Flower, Fleshy fruit and Dry fruits.
Age. Estimates of ages of crown-group Anacardiaceae are 72.7, 65.2, and 54.8 Ma (Muellner et al. 2007), (128-)97(-83) Ma (Weeks et al. 2014), (87-)75(-63.5) Ma (Muellner-Riehl et al. 2016) and (131-)114(-96)/(100-)88(-72) Ma (Joyce et al. 2023).
Spondiadoideae de Candolle —— Synonymy: Spondiadaceae Martynov
staminate flowers: (styluli basally connate - Tsiramiramias; carpelate flowers: G [(3-)5(-12)]; fruit a bony pyrene, 1-5(-12) locular, opercula external.
28/
(Plant deciduous); (dermatitis-inducing metabolites + - Spondias). biflavonoids 0; pedicels often articulated; G [(3-)4-5] (1 G fertile - Tapirira), (± free), (style 1), (styluli well separated), stigma little expanded; hypostase +; fruit >2-(1-)seeded, pericarp with lacuna(e)/not, inner mesocarp of encircling fibres (also/or brachysclereids), operculum +/0; exotestal cells (and hypodermis) sclereidal (not), tegmen ± 0, hypostase persistent, saddle-shaped; embryo straight; n = 15, 16, 18.
6/31: Spondias (16). Mexico to southeast Brazil and Bolivia (some Spondias), Old World tropics to the Pacific.
Age. Fruits of Dracontomelon (as Pseudosclerocarya) are known in early Eocene deposits of the London Clay (references in Herrera et al. 2019a).
Buchanania, Campnosperma: biflavonoids 0.
Campnosperma Thwaites
Dermatitis-inducing metabolites +; hairs scales/stellate; leaves simple, lamina (auriculate at base); pedicels articulated; G 1, style 0, stigma disciform; fruit 1-seeded, germination pores, etc. 0, central lacuna +, fibres 0, inner mesocarp isodiametric sclereids, locular envelope 0; embryo strongly curved [U-shaped]; n = ?
1/16. Tropical, not mainland Africa, Thailand to the Solomon Islands.
Buchanania Sprengel
(Plant deciduous); leaves simple; flowers perfect; anthers sagittate; G 4-6, free, 1 fertile, styluli +, stigma obliquely truncate; fruit 1-seeded, germination pores, etc. 0, lacunae 0, inner mesocarp 2-4 layers palisade brachysclereids over irregularly-oriented sclereids, locular envelope +; n = ?
1/22. Sri Lanka, India, south China through Malesia to northeast Australia, west Pacific.
The Sclerocarya Hochst. complex
(Plant deciduous); leaves (unifoliolate), (leaflets serrate); (pedicels articulated); A (-many - Sclerocarya); G (1 [Solenocarpus, Haplospondias]-)4-5(-12)]; hypostase +; fruit usually 2≤ seeded, pericarp with lacuna(e)/not, inner mesocarp of encircling fibres [locular envelope], operculum +/0; exotestal cells (and hypodermis) sclereidal (not), tegmen ± 0, hypostase persistent, saddle-shaped; (embryo C-shaped - e.g. Lannea), (cotyledons reniform); n = 12(-15, 20).
13/72: Lannea (40). Tropical, inc. Baja California, northeast Australia, west Pacific.
Embryo curved - Dracontomelon
(Vines, secondary thickening normal; perennial herbs), (plant deciduous); exudate gums and resins, 5-deoxyflavonoids, also alkylcathechols and alkylresorcinols [phenols with unsaturated side chains - dermatitis-inducing metabolites] +; (cork cortical); giant/D-type stomata on veins = Mangifera); leaves (opposite), frequently simple, (lobed); (flowers monosymmetric), pedicels usu. articulated; (hypanthium +); K and/or C (0), (P []/K and C [Toxicodendron] single trace); (nectary + - Mang./glandular hairs on C/staminal tube - Anacardium); A (1 [+ staminodes]), 5 [opposite K], 10 (many), (basally connate); nectary + (0 - Pistacia); G pseudomonomerous, [3(-6)], (inferior), (highly) asymmetric, one G fertile, symplicate zone?, styluli terminal to gynobasic, stigma ± capitate/± lobed/punctate, with multicellular papillae; ovule apical to basal, (unitegmic, usu. apically bifid, 4-5 cells across), nucellus 5-20 cells across, (apex exposed - Pistacia), ovule (almost circinotropous), (funicle with "knees" and other outgrowths), (ponticulus +); (chalazogamy +); fruit 1-seeded, often asymmetric, ± flattened, (K much accrescent, forming wings), (hypocarp developed); exocarp thin, epidermis lignified, endocarp with up to three layers of palisade lignified sclereids, internal to these a crystalliferous layer [= stratified], (not), (operculum 0); testa collapsed, tegmen undifferentiated/endotegmen lignified; (embryo chlorophyllous), (cotyledons folded - Mang.); n = (7-12)15(-16), etc..
45/735: Searsia (120), Semecarpus (72), Mangifera (68), Schinus (50), Ozoroa (40[+]). Largely tropical, also temperate.
Pentaspadon J. D. Hooker
Plant deciduous; dermatitis-inducing metabolites +; flowers perfect; A 5 opposite K/5 + 5 staminodes/10; G 1, style short, stigma subcapitate; endocarp with irregularly-oriented sclereids; seeds somewhat flattened; n = ?
1/6. Vietnam to Malesia and the Solomon Islands.
Dobineeae Engler —— Synonymy: Podoaceae Franchet
Perennial herbs [Campylopetalum], shrubs; leaves simple, lamina margin (doubly) serrate; plant dioecious; staminate flowers: 4-5-merous; pistillode +; carpellate flower: bract foliaceous, pedicel adnate to bract; K, C, A, 0; style 1, terminal, stigma linear.
2/3: Dobinea (2). Bhutan, NE India, Nepal, S.W. China, N. Thailand.
Age. Leaves initially identified as Celtis ameghinoi are perhaps the most common leaf type in the Laguna del Hunco deposits ca 52 Ma in Argentinian Patagonia, however, recent work by Wilf et al. (2024) suggests that they are better placed in Dobinea (Anacardiaceae, not Cannabaceae...). However, since the distinctive fruits of Dobinea have not been found in these Argentinian deposits, Wilf et al. (2024) named the fossils Dobineaites ameghinoi; the plant may well have been a tree, although extant Dobinea is a shrub.
Age. Woods of Anacardioxylon and Dracontomeloxylon from Cretaceous-Maastrichtian/Palaeocene-Danian deposits in the Deccan Traps ca 66 Ma may belong to Anacardioideae (Wheeler et al. 2017).
Synonymy: Blepharocaryaceae Airy Shaw, Comocladiaceae Martynov, Julianaceae Hemsley, Lentiscaceae Horaninow, Pistaciaceae Martinov, Rhoaceae Sadler, Schinaceae Rafinesque, Vernicaceae Schultz-Schultestein
Evolution: Divergence & Distribution. For many ages in the family, see Muellner-Riehl et al. (2016); note the narrow circumscription of the Spondias clade there. For the early Caenozoic fossil history of what are now East Asian endemic Anacardiaceae, see Manchester et al. (2009) - Choerospondias, now growing from N.E. India eastwards, has been found in Lower Eocene deposits of the London Clay. Middle Eocene deposits from Germany include fossils of the distinctive fruits of the New World Anacardium, with their much-swollen pedicels; although the African Fegimanra, sister to Anacardium, also has swollen pedicels, they are clearly different (Manchester et al. 2007b; Pell et al. 2011; Collinson et al. 2012 for this and other fossil records). Distinctive fruits that have been identified as the Old World Dracontomelon are known from the Late Eocene of Panama in deposits some 40-37 Ma old and also younger, ca 20 Ma (Herrera et al. 2012, 2019a), while wood identified as that of the Old World Mangifera is reported from Late Middle Eocene deposits ca 39 Ma on the Pacific side of Peru (Woodcock et al. 2017; for Palaeocene leaf fossils from India, see Mehrotra et al. 1998; Q. Li et al. 2019 - ?drift).
The Argentinian fossil Dobineaites, mentioned above, may be related to Dobinea, from the Eastern Himalayas and southwest China; the latter genus has small, dry fruits attached to midrib of orbicular, reticulately-veined accrescent floral bracts. Indeed, it probably belongs to a group of taxa including Eucalyptus that have moved from southern South America to the general Australian-S.E. Asian region (see elsewhere).
Weeks et al. (2014) emphasized the diversity of fruit dispersal types in the family and the extent of long distance dispersal, and they also noted that the ability to live in cooler (i.e. with some freezing) conditions has evolved here.
Pell (2004) looked at the morphology of the whole family from a phylogenetic point of view. Herrera et al. (2018) catalogued the extensive variation in fruit morphology of Spondiadoideae, in which several different structures are involved in germination, there are often various lacunae, and the woody/stony layer (endocarp s.l.) is nearly always well developed and sometimes remarkably elaborated. Overall, ovule and pericarp variation in the family is considerable and needs to be put in a phylogenetic context.
Pollination Biology & Seed Dispersal. Pistacia and Amphipterygium (see Julianaceae below) are both wind pollinated, dioecious, and have reduced flowers (Bachelier & Endress 2007b). Tölke et al. (2018a) summarized the diversity of nectar and nectaries in the family, noting the variation in nectar composition, both infraspecific and between closely related taxa, while Tölke et al. (2018b) described the osmophores that produce the very different floral scents of Anacardium and Mangifera. Goldberg et al. (2017) looked at the evolution of breeding systems in Rhus. Chalazogamy s.l. (technically, perhaps, funiculogamy - Gonzalez 2016) is known from Schinopsis, Pistacia, Toxicodendron and Anacardium, the pollen tube moving into the ovule from the funicle via the ponticulus, an outgrowth of the funicle that bridges the gap between it and the chalaza (e.g. Martínez-Pallé & Herrero 1995; Bachelier & Endress 2009; Mitchell et al. 2022).
Disseminules of Anacardioideae are often modified in various ways for wind dispersal. The wings of the fruit may be formed from broad bracts that are adnate to it (Dobinea), the flattened peduncle of the inflorescence (Amphipterygium), much enlarged sepals (Parishia) or petals (Swintonia), or the fruits may be samaras (Loxopterygium), while in Cotinus hairs on the pedicels help in the wind dispersal of the small, nut-like fruits. The evolution of these fruit types seems to be correlated with the adoption of drier habitats (Pell & Mitchell 2007). More or less fleshy drupaceous fruits are also common (for fruits in general, see Mitchel et al. 2022). In Anacardium the fleshy swollen pedicel is part of the attractive unit. Germination of the stones of the drupaceous fruits of Spondiadoideae is often faciltated by various opercula, etc., that have developed (see Herrera et al. 2018 for references).
Plant/Animal Interactions. Aphids (Fordinae) that form distinctive galls are closely associated with species of Pistacia and Rhus (H. C. Zhang & Qiao 2007, 2008; Inbar 2009; Z. Ren et al. 2017, 2019), aphid-plant associations often being specific (Ben-Shlomo et al. 2022). The sometimes massive, cauliflower-shaped (but not coloured) galls on Pistacia induced by Slavum wertheimae (Pemphigidae) produce terpenes that dissuade goats, at least, from eating them (Rostás et al. 2013). Melaphidina aphids have diversified in part by occupying different sites on the one plant; the primary host of Fordinae-Melaphidina is Rhus, the secondary hosts are mosses, and those of Fordina are Pistacia and the roots of Poaceae respectively (Zhang & Qiao 2007). Batovska et al. (2025) compare the metabolites of different galls on Pistacia palaestina. There galls formed by Paracletus cimiciformis look rather like ungalled leaflets and the metabolites in the galls are also rather similar to those of the leaflets; the galls support only a few aphids. On the other hand, galls formed by Baizongia pistaciae look rather like bananas (and they are as big as a banana), they have very distinctive metabolites and thousands of aphids live inside. There are 6 genera and 11 species, largely East Asian, in Melaphidina and the crown-group has been dated to (79-)73.3(-68.3) Ma (Ren et al. 2017).
The development of galls by caterpillars of the lepidopteran Eucecidoses minutanus on Schinus engleri has been followed in detail, and different larval stages are associated with distinct gall morphologies (Ferreira et al. 2021; Guedes et al. 2023 for other lepidopteran galls). A gall-forming jumping psyllid plant louse, the hemipteran Calophya, is notably common on Schinus, and related psyllids occur both on that genus and other Anacardiaceae (Burckhardt & Basset 2000; Burckhardt 2005). For more on galls, see Michell et al. (2022).
Genes & Genomes. Guimarães and Forni-Martins (2021) suggested that the base chromosome number for Anacardiaceae was x = 14.
Economic Importance. Galls on Rhus produce industrial tannins and are also used for medicines (Wool 2004).
Chemistry, Morphology, etc.. Anacardiaceae are well known for the sometimes extremely violent allergenic reactions their exudates cause; catechols, resorcinols and other types of phenolic compounds - often in a mixture, as in urushiol - are involved. About a third or so of the genera may have such compounds, although Mitchell (1990) found that only half of these had been studied in any detail, so it is unclear if Spondias should be included in this list; see also Ding Hou (1978), Aguilar-Ortigosa et al. (2003) and Aguilar-Ortigosa and Sosa (2004).
Schweingruber et al. (2011) emphasize the abundance of tension wood here. Branching in Anacardium may occur on the current flush. Tölke et al. (2021a) discuss secretory canals in the family.
Flowers in Anacardiaceae are small but show a considerable amount of variation. Hardly surprisingly, wind-pollinated taxa often lack a nectariferous disc, also petals. Mangifera has one or two stamens borne inside the nectariferous disc; normally the stamens are outside the nectary. In Anacardium a single large stamen is on an oblique plane of symmetry; the other smaller stamens are also fertile (Bachelier & Endress 2009; Sokoloff et al. 2017 for discussion). More generally, the position of the carpel, when single, suggests that the flower is obliquely symmetric (Ronse de Craene 2010). Tölke and Demarco (2020) describe the various kinds of monomerous androecia in the family. For infraspecific variation in style number - 1, 3 - see Gonzàlez and Vesprini (2010). In Anacardioideae the floral/receptacle apex is sometimes quite short (Bachelier & Endress 2009). In Lithraea, Schinopsis, Pistacia and Amphipterygium the ovules are unitegmic, etc. (Bachelier & Endress 2007b; Gonzalez 2016). Sometimes the second integument is represented by a small protrusion towards the apex of the otherwise single integument (e.g. Grundwag 1976; Robbertse et al. 1986). A ponticulus, a protrusion from the funicle, is common, and it appears to be a strongly developed portion of the outer integument that is otherwise adnate to the funicle; it also occurs in taxa with single integuments like Lithraea (Carmello-Guerreiro & Sartori Poli (2005). The fruits are commonly described as drupes, and the wall, and the stone in particular, are layered, some of the layers being produced from division of the endocarpial layer, however, the fruits may not be drupes in the strict sense (Wannan & Quinn 1990; Gonzàlez & Vesprini 2010). The fruit often develops well before the seed, and the testa well before the embryo, so for some time the fruit, although quite large, is almost empty (Copeland 1955, 1962).
For general information, see Ding Hou (1978), Mitchell et al. (2006, 2022) and Pell et al. (2011). For general chemistry, see Young (1976), for chemistry of Julianaceae, see Hegnauer (1966, 1989), for exudates, see Lambert et al. (2013), for wood anatomy, see Gupta and Agarwal (2008), for multiseriate ± capitate colleters, see Lacchia et al. (2016), for inflorescences, see Barfod (1988), for floral morphology, Wannan and Quinn (1991) and Tölke et al. (2021b: Spondias, Tapirira), for some embryology, see Grimm (1912), Copeland and Doyel (1940) and Copeland (1955), for fruit anatomy, see Pienaar and von Teichman (1998), for ovules, fruit and seed, see von Teichman and van Wyk (1988) and Carmello-Guerreiro and Sartori Poli (1999), for seed anatomy, see von Teichman (1991, 1994, and references), and for germination of Spondiadoideae, see Hill (1937).
Phylogeny. Spondiadoideae-Spondiadeae and some Rhoeeae, including Pegia, Tapirira and Cyrtocarpa (see Aguilar-Ortigosa & Sosa 2004; Pell 2004) have been recovered as sister to the rest of the family. However, the situation is now rather complicated. Buchanania in some analyses is quite well supported as sister to other Anacardioideae (Aguilar-Ortigosa & Sosa 2004; Wannan 2006), consistent both with its chemistry, endocarp anatomy (it lacks a stratified endocarp), carpel number of 4-6, and different position of the fertile carpel, but its phylogenetic position is not fixed in other analyses (Pell & Mitchell 2007, c.f. abstract). Campnosperma, included in a study by Chayamarit (1997: sampling limited, relationships different from other studies, no support values), has an endocarp similar to that of Buchanania and the fruit is sometimes two-locular; it was not sequenced by Pell (2004). Pell et al. (2011) suggested that Spondiadoideae may be polyphyletic, and Weeks et al. (2014) found that Spondiadoideae were paraphyletic, Campnosperma being between the two parts, Buchanania ending up sister to one of those parts, and Pentaspadon was sister to the whole family - however, support was not strong. M. Sun et al. (2016) also did not recover a monophyletic Spondiadoideae, and although relationships within a portion of the subfamily were quite well resolved, that portion did not include genera like Pegia and Spondias itself... On the other hand, Z.-D. Chen et al. (2016) found Spondias, Dracontomelon, and Buchanania to be in the same clade and sister to the rest of the family (moderate support) while relationships in Muellner-Riehl et al. (2016) are [[Spondias + Dracontomelon] [[Buchanania + Lannea, etc.] [other anacards]]]. Fruit anatomy (Herera et al. 2018) suggested that although Buchanania might be in this region, Campnosperma was unlikely to be.
In the remainder of the family, there are four main clades, with [Dobinaea + Campylopetalum] sister to the whole lot and support for the scaffolding quite good (Weeks et al. 2014). In the old Anacardioideae (Pell & Urbatsch 2000, 2001) wind-dispersed taxa do not form a single group (Pell & Mitchell 2007, also Muellner-Riehl et al. 2016; c.f. Pell & Urbatsch 2001).
In the Angiosperms353 analysis (Seed Plant Tree of Life, Jan. 2022 version), relationships were [[Burseraceae] [Attilaea [Santiria [[Pentaspadon [Campnosperma [[Spondias, etc.] [Buchanania, Pouquartia, etc.]]] [[Campylopetalon + Dobinaea] [[Anacardium, Mangifera, etc.] [all other Anacardioideae]]]]]]]]. There is obviously work to do: Attilaea (see also Gómez 2009) is the only two-carpellate anacard; although it is unclear what Santiria is doing, otherwise relationships in Burseraceae are unexceptionable.
For relationships within Rhus, from which the allergenic Toxicodendron has been excluded, see Yi et al. (2007) and Andrés-Hernández et al. (2014). Silva-Luz et al. (2018: much morphology, previous sections, etc., do not hold) examined relationships in Schinus, closely related to Lithraea, Mauria and Euroschinus; [S. terebinthifolia + S. weinmanniifolia] form a clade sister to the rest of the genus.
Classification. See Mitchell et al. (2006) for a list of genera and Pell et al. (2011) for a classification, etc., the latter included 21 genera in their polyphyletic Spondiadoideae. Buchanania and Campnosperma are included in Anacardioideae above, and this robs the subfamily of much in the way of apomorphies, but obviously the current classification is decidedly temporary. Overall, ca 25% of the genera recognized (out of some 80+ total) are monotypic, relationships being very pectinate (Mitchell et al. 2023).
For the limits of Rhus, which seem best narrowly drawn (i.e., restricted to ca 35 species), see Moffett (2007), Yi et al. (2007) and references, and for a sectional classification of Schinus, see Silva-Luz et al. (2018).
Previous Relationships. A number of anacardiaceous genera have highly reduced flowers and inflorescences, and in the past they have been segregated in separate families. These include Blepharocaryaceae, with their compact, involucrate inflorescences, Julianaceae, dioecious, the staminate flowers with extrorse anthers and carpelate flowers that lack a perianth but are surrounded by an involucre, and finally Podoaceae, with opposite leaves and carpelate flowers that also lack a perianth.
BURSERACEAE Kunth, nom. cons. - Back to Sapindales
Trees or shrubs; bark flaky, light grey; exudate colorless to white, resinous, ellagic acid +; pith cells heterogeneous; nodes usu. 5:5; sclereids in stem; indumentum very various; epidermis with mucilage cells; leaflets (with pellucid dots), ?vernation, base often ± symmetrical, veinlets ending free in areoles, petiolules and/or petioles often pulvinate; dioecy common; K induplicate-valvate, ± connate, C valvate; A obdiplostemonous; ventral carpel bundles fused bundles of adjacent placentae, style usu. short; ovules 2/carpel; fruit septifragal [outer pericarp], pseudaril + [mesocarpial], with columella, stone with valves, K deciduous; (exotesta with shortly radially elongate but unthickened cells), endotesta lignified, ± tracheidal; embryo reserves hemicellulosic; x = 13 (?14), nuclear genome [1 C] (0.033-)0.701(-16.429) pg.
19[list]/775 (860) - four groups below. Tropical. [Photos - Leaf, Flower, Fruit.]
Age. De-Nova et al. (2012) dated crown-group Burseraceae to the early Palaeocene (69.7-)64.9(-60.3) Ma; the estimate in Weeks et al. (2005: in text as the divergence between Anacardiaceae and Burseraceae) is (61.9-)60(-58.1) Ma, in Muellner-Riehl et al. (2016) it is (85.5-)75.2(-64.5) Ma, and in Weeks et al. (2014) the age is (106-)91(-78) Ma; an age of 120 Ma plus can be estimated from the discussion in Becerra (2005) while (130-)108(-84)/(99-)85(-71) Ma are the ages suggested by Joyce et al. (2023).
See Daly et al. (2011, 2022) for the fossil record of the family.
1. Beiselieae Thulin, Beier & Razafimandimbison - Beiselia mexicana Forman
Plant deciduous; (vessel elements with scalariform perforation plates); leaf base much swollen, persistent, spinose [spine = petiole apex/rhachis base], axillary bud on base of spine, venation cladodromous [freely branching towards margin], margins serrate; carpelate inflorescence racemose; G [9-12], symplicate zone short, ovary strongly 9-12-furrowed, style ± 0; ovules superposed; fruit a pseudocapsule, pericarp septifragal, columella massive, strongly ribbed, pyrenes free, apically radially winged [between the ribs]; germinal epigeal, cotyledons entire.
1/1. Michoacan, Mexico. Map: below in blue, see Daly et al. (2022: Fig. 1).
[Garugeae [Bursereae + Protieae]]: (plant deciduous); oleoresins with mono- and bicyclic monoterpenes, triterpenes with ursane and oleanane components; silica bodies +/0; (pith cells homogeneous); snail glands + [curled ± uniseriate glandular hairs]; lamina margins serrate to entire, venation often brochidodroumous, ((pseudo-)stipules +); (K with single trace); A (apparently in a single whorl); (pollen psilate); G [(2-)3-5], opposite C, (one carpel developing), symplicate zone well developed, receptacle enclosed by the gynoecium; ovules collateral, campylotropous, outer integument 3-5 cells across, inner integument 3-4 cells across, (integument 1, ca 5 cells across, apically bifid), parietal tissue 5-20≤ cells across, nucellar cap 1-30 cells across (?0); fruit pyrenes often angled, usu. with pericarpial pseudo-aril,; vascular bundle in outer integument; cotyledons straight to variously folded, entire to palmately lobed.
18/754. Tropical, but esp. America and N.E. Africa. Map: from Rzedowski (1978), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 6 (2011), D. C. Daly (pers. comm.) and Daly et al. (2022: Fig. 1).
Age. The crown-group age of this clade is some (60.2-)58(-55.8) Ma (Weeks et al. 2005), (71.7-)62.9(-54.7) Ma (Muellner-Riehl et al. 2016) or (66.5-)63.4, 54.2(-48.8) Ma (Fine et al. 2014), but some estimates, at (116-)98, 92.7(-74.8) Ma, are older (Becerra et al. 2012), and yet older in Becerra (2003, 2005).
[Canarieae [Bursereae + Protieae]]: ?
2. Canarieae Engler
(Plant decidous); (cork cambium deeper - Santiria); petiole bundle with medullary strands; (leaflets not pulvinate), ("stipules" +, petiolar or cauline, laciniate to entire); dioecious, or flowers perfect; flowers often 3-merous, (hypanthium +); (C connate); A (connate - Canarium), basifixed [?level], (connective massive), (anthers horizontal); (pollen striate); fruit fleshy, often indehiscent, pyrenes operculate, (winged), developed/aborting seeds 1/2, 2/3, etc.; n = (22-24); if germination hypogeal, often phanerocotylar.
11/295: Canarium (120), Dacryodes (90). Tropical, esp. Old World.
Age. Crown-group Canarieae are estimated to be (54.5-)45.6(-36.9) Ma (Federman et al. 2015) or (60.3-)52.5(-47) Ma (Muellner-Riehl et al. 2016).
[Bursereae + Protieae]: petiole bundle with/without medullary strands; lamina (venation semicraspedodromous); fruit with pyrenes usu. separating/separable.
Age. The age of this node can be dated to (66.8-)63.2, 48.6(-46) Ma (Fine et al. 2014) or (67.2-)59.3(-51.8) Ma (Muellner-Riehl et al. 2016).
3. Bursereae de Candolle —— Synonymy: Balsameaceae Dumortier
Plant usu. deciduous, (thorny); snail glands 0; (petiole bundle arcuate - Commiphora); leaves (bipinnate), free-ending veinlets usu. much branched; plant (polygamo-)dioecious; pollen colpi short; fruit usu. dry, endocarp fused, exocarp dehiscing by valves, (pyrenes tangentially winged), (pseudoaril +); n = (11, 12).
3/286: Commiphora (185), Bursera (110). Tropical America, Africa, 150 spp. Commiphora in Africa, 100 spp. Bursera in Mesoamerica.
Age. Crown-group Bursereae may be around (58.3-)52.5(-47.4) Ma Muellner-Riehl et al. 2016).
4. Protieae Marchand - Protium Burmann f.
Leaves (unifoliolate); C induplicate-valvate, (connate); (stamens = and opposite K); fruit usu. fleshy, endocarp not fused; (columella ribbed); n = (11).
1/145 (+ 60 to describe). Mostly Neotropical, a few Madagascar and Malesia
Age. Crown-group Protieae can be dated to (43.2-)32.6, 25.7(-18) Ma (Fine et al. 2014) or (40.2-)24.4(-11.7) Ma (Muellner-Riehl et al. 2016).
Evolution: Divergence & Distribution. Dates for the split between Bursera and Commiphora (both Bursereae) vary from ca 120 to ca 60 Ma - c.f. Becerra (2005) and Becerra et al. (2012); with the earlier age, distributions could be affected by continental drift; De-Nova et al. (2012) dated this split to (59.0-)54.7(-50.6) Ma, while it is estimated to be (58.3-)52.5(-47.4) Ma by Muellner-Riehl et al. (2016: q.v. for ages of other clades). Fossils 66-65 Ma of both flowers and fruits from the Deccan Traps of India have recently been identified as Burseraceae (Debursera and Bursericarpum respectively, see Kumar et al. 2023; Sahniocarpon - Manchester et al. 2024a); wood from the same area has also been associated with Burseraceae. Kumar et al. (2023) thought that the family moved from India to Europe and North America (the Out of India hypothesis) via land bridges or perhaps long distance dispersal; continental drift was probably not involved.
Weeks and Simpson (2007) suggested that divergence of Commiphora from the clade now represented by the E. Asian B. tonkinensis occurred some 53-42 Ma in the Eocene; Commiphora itself did not diversify until 32.3-23.2 Ma, Neogene aridification of Africa occurring more or less at that time. Weeks et al. (2005), Weeks and Simpson (2007: much detail) and Weeks et al. (2014) discuss the complex biogeographic relationships within Burseraceae, the latter emphasizing the paucity of biome shifts in the family and the importance of Miocene radiations in both Protieae and Bursereae. Crown-group Commiphora may be (45.8-)36.6(-47.4) or (32.3-)27.8(-22.3) Ma (Gostel et al. 2016 and Weeks & Simpson 2007 respectively), but Gostel et al. (2016) suggest that diversification began around 9.5 Ma later, there being a very long branch above C. lasiodiscus. There are four quite separate clades of Commiphora on Madagascar, indeed, the Malagasy C. lasiodiscus is sister to the rest of the entire genus (Gostel et al. 2016).
For general remarks about the biogeographical relationships in New World Burseraceae, see Daly et al. (2022). De-Nova et al. (2012) thought that crown group Bursera was ca 49.4 Ma, although diversification within the genus did not really get going until (23-)20 Ma, and Becerra et al. (2009) suggested that Bursera, speciose in the seasonally-dry, tropical forests of Mexico, had diversified most within about the last 25 Ma. De-Nova et al. (2012) estimated the age of most species of Bursera in these Mexican forests to be ca 7.5 Ma - more or less as predicted for species in such forests (Pennington et al. 2009; Dick & Pennington 2011). (Old Worls Bursera = Protium.) As is quite common with trees in tropical rainforests (e.g. see also Annonaceae, Fabaceae), there is little geographic structuring of relationships in Protium (= Protieae) in tropical South America, although it is evident in Central America (Dexter et al. 2017, c.f. in part Fine et al. 2014 - the geographic units used are larger there). (Geographic structuring is more evident in at least some forest herbs and in plants of the seasonally dry Neotropical forest biome.) Diversification within Protium seems to be temperature-dependant, in part, at least; increase in diversity here began around the Oligocene, but it has been largely flat in the Pleistocene. Interestingly, co-occuring species of Protium diverged around 18 Ma, while in co-occuring species of Inga (Fabaceae-Caesalpinioideae), similarly unstructured geographically, the figure is ca 1.5 Ma (see also below; Dick & Pennington 2019).
Federman et al. (2015) suggest that Canarium (Canarieae) arrived in Madagascar drifting in ocean currents from the Southeast Asian region only about 8.4 Ma; diversification in Madagascar, where the genus is now a prominent and speciose (over 30 species) component of the rain forest, diversification being dated to within the last 6 Ma or so. (The Malagasy Tseboneae, which includeCapurodendron (Sapotaceae), have 34(described)-48 species and are probably the most diverse endemic Madagascan plant clade (Boluda et al. 2022.)
Protium heptaphyllum is thought to be the 12th most common tree species in the Amazon basin (ter Steege et al. 2013). However, Damasco et al. (2021; see also Daly et al. 2022) suggest that P. heptaphyllum was probably best divided into eight separate species, some quite common, others perhaps endangered, that differed in soil and climate preferences, etc..
Ecology & Physiology. Burseraceae are a notable component of the Amazonian forests, and include a disproportionally large number of the common tree species with stems at least 10 cm across - Protium is the #2 tree genus (ter Steege et al. 2013); 11 species were hyperdominants, although the number will depend on species limits (Daly et al. 2022, see also above). Fine et al. (2014 and references) have studied the diversification of the Protieae, an important element of Neotropical forests, in some detail; this began (43.2-)32.6, 25.7(-18) Ma, well before the uplift of the Andes. 35 species of Burseraceae, mostly Protium, in the western Amazon largely separated out ecologically, preferring either fertile clay, white sand, or terrace soils (Fine et al. 2005), whle within species like P. subserratum there is extensive local-scale parapatric morphological and edaphic variation (Misiewicz & Fine 2014). As in groups like Eugenia, Inga, Piper and Psychotria differentiation of secondary metabolites may also be involved (see also below). Indeed, there is substantial variation in monoterpene composition in species of Protium; this is largely stable within a plant over time, although within a species there may be some variation, and some monoterpene combinations are found in more than one species (Piva et al. 2019).
Bursereae, very largely made up of Bursera and Commiphora (see above for ages), are predominantly denizens of drier forests in the New World and Africa-Madagascar respectively, the grass-poor Succulent Biome (Pennington et al. 2006b and papers in that volume; Gagnon et al. 2018 and references). Thus Becerra et al. (2009) noted that some 85 of the ca 100 species of Bursera, often quite narrowly distributed, were to be found in seasonally-dry tropical forests in Mexico alone. De-Nova et al. (2012) suggested that there had been nine shifts to xerophytic scrublands there, seven to oak forests, and one to tropical forests; overall they discussed the habitat preferences of the genus in terms of niche conservatism. Other major groups in the Succulent Biome include Fabaceae, Cactaceae, including Pereskia, some clades of Euphorbia, etc. (Gagnon et al. 2018 and references).
Pollination Biology & Seed Dispersal. Daly et al. (2022) summarized visitors to the flowers of New World Burseraceae; small insects, especially bees, are commonly involved.
Burseraceae are one of the three most important food sources (the other two are Lauraceae and Arecaceae) for specialized avian frugivores, especially in the New World (Snow 1981). Interestingly, in a study carried out in Colombia the seeds of Dacryodes olivifera at 33 × 21 mm were among the largest dispersed by the oilbird Steatornis caripensis and were dispersed up to 32.3 km (Stevenson et al. 2021). Daly et al. (2022) noted that the pseudarils of Protiumyielded a low-energy food, those of Bursera a high-energy food. The fruits of Malagasy Canarium are quite large for the genus and are dispersed by large lemurs, most of which have recently been made extinct (Federman et al. 2016).
For the evolution of the breeding system in Bursera, dioecy probably being plesiomorphic, see Goldberg et al. (2017).
Plant-Animal Interactions. For possible coadaptive relationships between Burseraceae, especially Bursera itself, and the herbivorous chrysomelid beetles Blepharida, and how the latter deal with the toxic terpene-containing resins the plants contain, see Becerra (1997, 2003 and references) and Becerra et al. (2001, 2009: particularly interesting). There are about 45 species of Blepharida, a number of then feeding on but a single species of Bursera, and the insect phylogeny matches host plant chemistry rather than its phylogeny (Becerra 1997; Pellmyr 2002). Becerra (2003) suggested that insects and plants had been co-evolving for about 100 Ma, although other estimates for the age of the family (see above) suggest that this figure is very much an over-estimate. In plants that have a squirt defence toxic material in their tissues is under pressure and is ejected up to 2 m when the tissue is perforated by the insect; such species have a rather simple terpenoid-based exudate (Becerra et al. 2009). Locally, species of Bursera tend to be chemically more dissimilar than would be expected at random (Becerra 2007), perhaps promoting niche differentiation and local diversity (see also Endara et al. 2017). Overall chemical diversity in Bursera has increased with time/speciation, if dropping off when considered from a per-speciation-event point of view, and terpene variation seems to have become a matter of permuting combinations of chemicals in the local ecological context (Becerra et al. 2009). Overall, correlation between terpenes and phylogeny is not strong (Becerra et al. 2009), bu his is not uncommon when thinking of defensive compounds and plants in general (Cacho et al. 2015; Forrister et al. 2022).
Protieae (= Protium s.l.) invest heavily in secondary metabolites, and they represent some 22-58% dry weight of the leaves, average 40%, although most of the compounds were not herbivore-active metabolites... (Salazar et al. 2018). Protium species with more diverse metabolites that affect herbivores, either positively or negatively, may commit less to defence, and herbivore species richness is negatively correlated with metabolite richness (Salazar et al. 2018). Those metabolites that reduced herbivory were more conserved across the plant phylogeny, but there was no particular correlation between the metabolite composition of the plant and herbivore phylogeny, as might be expected for a system such as this where the herbivores are largely generalists, although individual herbivores by no means feed on all species (Salazar et al. 2018). Zapata and Fine (2013) found there were 3-5 copies of monoterpene synthase genes in Protium, one copy being very old, the other copies representing duplication events that occurred 50-70 Ma, i.e. before the diversification events discussed here (Fine et al. 2014). The products of these genes might have functions other than direct defence against herbivores, rather, they might attract predators and parasitoids of these herbivores (Zapata & Fine 2013). Daly et al. (2022) noted that in both Protium and Bursera closely related species, also species growing together, were likely to differ in their chemical defences. For other similar tropical systems, see Eugenia, Inga, Ocotea, Passiflora, Piper, Psychotria, and sundry Solanaceae (discussion about Inga is perhaps particularly detailed). For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
Species of Protium and other members of the family are rich in aromatic oleoresins, terpenoids, etc., and exudate from Protium is collected by euglossine orchid bees (Henske et al. 2014).
Economic Importance. Members of Burseraceae are noted for the diversity of aromatic compounds that can be obtained from them - e.g. myrrh comes from Commiphora, elemi incense from Canarium, frankincense from Boswellia and linaloe and copal from Bursera itself (Rüdiger et al. 2007).
Chemistry, Morphology, etc.. Phytoliths are commonly found in Burseraceae (Piperno 2006). Some Burseroideae have foliaceous stipule-like structures; these are usually interpreted as being the reduced basal pair of leaflets of a compound leaf.
A few genera (e.g. Garuga) have a well-developed hypanthium; the disc is rarely extrastaminal (Triomma). The odd carpel is drawn as being abaxial in 4-merous Amyris (Schnizlein 1843-1870, fam. 244). Srivastava (1968) thought that the ovules of Bursera delpechiana were straight, but they do not appear to be so from his illustration. The embryo sac is often very deeply seated in the ovule, with up to 85 cell layers between it and the nucellar epidermis; the shape of the embryo sac at maturity is very variable (Wiger 1935; see also Mauritzon 1935; Wiger 1936).
For additional general information, see Lam (1931, 1932), Leenhouts (1956), Forman et al. (1989: Beiselia) and in particular, Daly et al. (2011). For some chemistry, see Khalid (1983) and Lambert et al. (2013: exudates), for pollen morphology, see Harley and Daly (1995: Protieae) and Harley et al. (2005: considerable variation), for embryology, Narayana (1960 and references), for pseudaril anatomy, see Ramos-Ordoñez et al. (2013) and for mature fruit anatomy in Canarieae, see Martínez-Habibe (2022).
Phylogeny. The quite recently-described Beiselia is sister to the rest of the family (Clarkson 2002; Weeks et al. 2005; Thulin et al. 2008; etc.). This has considerable implications for character evolution; Beiselia also has several probably autapomorphic features.
Thulin et al. (2008) found that Protieae, Bursereae, and Garugeae (the latter including Canarium, etc., i.e. = Canarieae) all had strong support individually, but relationships between them were unclear; again, although Becerra et al. (2012) suggested the relationships [Canarieae [Protieae + Bursereae]], support for the position of Canarieae was not very strong (see also Federman et al. 2015; Muellner-Riehl et al. 2016: support quite strong).
Bursereae. In some studies Commiphora has been found embedded in Bursera, but with weak support (Weeks et al. 2005; see also Muellner-Riehl et al. 2016). Becerra et al. (2012) and De-Nova et al. (2012, but c.f. some analyses in the latter) found that a monophyletic Bursera was sister to Commiphora; what about B. tonkinensis? For relationships within Commiphora, with several well-supported clades that do not correspond to previous infrageneric groupings, see Gostel et al. (2016). Garugeae. Canarium was polyphyletic in the analyses of Federman et al. (2015), although the great bulk of the genus formed a single clade, while Muellner-Riehl et al. (2016) questioned the monophyly of Canarium and Dacryodes. Protieae. In a comprehensive analysis of Protium and relatives, Fine et al. (2014) found that Tetragastris and Crepidospermum were well embedded in Protium.
Classification. For a classification of the expanded Protium (= Protieae) recognizing nine sections, see Daly and Fine (2018).
[Sapindaceae [Simaroubaceae [Meliaceae + Rutaceae]]]: gums +; anthers with a pseudo-pit; nucellar cap +, tapetal cells multinucleate, nuclei fusing to form polyploid mass; outer integument over five cells across, obturator + [?level]; testa multiplicative.
Age. Wikström et al. (2001) dated this node to (61-)57, 55(-51) Ma, Magallón and Castillo (2009) suggested an age of around 70.7 Ma, Tank et al. (2015: Table S1, S2) an age of about 69 Ma, and Bell et al. (2010) an age of (70-)64(-57) or (54-)51(-49) Ma, while (106.5-)100.5(-94.4) Ma is the age in Muellner-Riehl et al. (2016).
Chemistry, Morphology, etc.. For an extensive tabulation of variation in anther, ovule and seed characters of Sapindaceae along with those of Simaroubaceae, Rutaceae and Maliaceae, see Tobe (2011a).
[Simaroubaceae [Meliaceae + Rutaceae]]: alkaloids, triterenoids + ; (axial ducts/canals of traumatic origin); cuticle waxes 0; leaves (trifoliolate), (simple); inflorescence branches cymose; C imbricate; x = 9.
Age. Wikström et al. (2001) dated this node to (51-)47, 45(-41) Ma, while an age of ca 53.6 Ma was suggested by Tank et al. (2015: Table S2) - note the topology in these two - and an age of (100.3-)93.5(-86.6) Ma was suggested by Muellner-Riehl et al. 2016).
Evolution: Divergence & Distribution. The triterpenoid limonoids (see Rutaceae), meliacins (Meliaceae), cneorids (Rutaceae), and quassinoids (Simaroubaceae) are biosynthetically related; for this group of compounds, see e.g. Connolly et al. (1970), Evans and Taylor (1983), papers in Waterman and Grundon (1983) and Waterman (1983, 1993), all general, da Silva and Gottlieb (1987), Vieira and Braz-Felho (2006: quassinoids), Roy and Saraf (2006: limonoids), da Silva et al. (2021) and de la Peñ et al. (2023: limonoid synthesis), and they often have a bitter taste.
For trans-octadecanoic acids in seed oils, see Stuhlfauth et al. (1985). For trauma-induced ducts/canals, see Pace et al. (2022); they are scattered in this clade alone in Sapindales, but they are uncommon, except in Meliaceae-Swietenioideae (= Cedreloideae), so as an apomorphy, they are hardly satisfactory. For the base chromosome number here, see Paetzold et al. (2018) - x = 14 in Carta et al. (2020). Note also the topologies of the tree in the various reconstructions of such chromosome numbers.
Chemistry, Morphology, etc.. For some information on carpel development, see van Heel (1983).
SIMAROUBACEAE Candolle, nom. cons. - Back to Sapindales
Trees; bark very bitter, quassinoids, ellagic acid +, gums 0; (cambium storied), wood often fluorescing, fibers with distinctly bordered pits; (nodes multilacunar); pith conspicuous, medullary secretory canals +; sclereids common, oil cells uncommon; leaflets not articulated, vernation also supervolute-curved; breeding system various; plant polygamous/dioecious; inflorescence thyrsoid, (pedicels articulated), flowers rather small, 1> cm across; K usu. basally connate, C yellow-green; A obdiplostemonous; G (1-)5(-8), G ± free, style +, often short, stigmas ± recurved, ± pointed, receptive zone elongated, dry; ovules 1(-2)/carpel, (hemitropous), (micropyle zig-zag), (inner integument very long, folded), outer integument 3-10 cells across, inner integument 2-8 cells across, parietal tissue 6-22 cells across, nucellar cap 2-7 cells across; fruit drupelets, laterally flattened-lenticular, inner pericarp sclereidal, endocarp s.s. lignified; seed (pachychalazal), with undistinguished testa or scattered lignified cells, endotesta often slightly lignified, tegmen crushed, (mesotegmen with reticulate thickenings); (endosperm with hemicellulose reserve), (perisperm +, thin); x = 8 (?9)/12, 14, nuclear genome [1C] (0.032-)0.924(-26.595) pg.
19-22 [list]/110 - seven groups below. Largely tropical and esp. the New World; a few (e.g. Ailanthus) temperate. Map: from Nooteboom (1962), Heywood (1978), Thomas (1990) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5 (2010); fossils of Ailanthus as black crosses, from Corbett & Manchester 2004 and Clayton et al. 2009, also Japan; fossils of Leitneria as blue crosses, from Clayton et al. 2009). [Photo - Flower, Fruit, Fruit.]
Age. Crown-group Simaroubaceae are dated to around the Cretaceous-Maastrichtian, a little more than 65 Ma (Clayton et al. 2009), or somewhat older, (84.5-)74(-63) Ma (Muellner-Riehl et al. 2016).
Woods (Ailanthoxylon, Simarouboxylon) from the Deccan Traps of Late Cretaceous and Early Palaeocene age may be simaroubaceous, and if confirmed this would have interesting biogeographic implications (Wheeler et al. 2017).
1. Casteleae Bartling —— Synonymy: Castelaceae J. Agardh, Holacanthaceae Jadin, nom. inval.
Thorny shrub to tree; vessels with spiral thickenings; wood with libriform fibers; leaves simple, (scale-like), margins ± toothed to entire; plant mon- or dioecious; inflorescence fasciculate; C usu. red; A (connate basally); style short; drupelets (± carinate); n = 13.
2/14: Castela (12). Southern U.S.A. to Argentina, the Caribbean and Galápogas Islands.
Age. Crown-group Casteleae are some (40.9-)23.6(-9.3) Ma (Muellner-Riehl et al. 2016).
[Picrasmateae [Ailantheae [Leitnerieae [Nothospondias [Picrolemma + Simaroubeae]]]]]: leaf rhachis collapses at the petiolular nodes.
Age. The age of this clade is around (80.9-)70.3(-60.1) Ma (Muellner-Riehl et al. 2016).
2. Picrasmateae Engler - Picrasma Blume
β-carboline indole alkaloids +; wood with exclusively uniseriate rays; pseudostipules +, cauline; leaf margins ± toothed; plant mon- or dioecious; C valvate; A 5; n = ?
1/8. ± Tropical, America and the Caribbean, Asia to Malesia.
Age. Crown-group Picrasma may be (33.6-)16.2(-3.5) Ma (Muellner-Riehl et al. 2016: quass. jav.).
[Ailantheae [Leitnerieae [Nothospondias [Picrolemma + Simaroubeae]]]]: sclereids in leaflet mesophyll; leaves with flat surface glands/(0).
Age. The age of this clade is variously suggested to be around 61.1. 52, or 47.5 Ma (Muellner et al. 2007: inc. Soulamea, check), (66.8-)58.2(-52.2) Ma (Muellner-Riehl et al. 2016) or about 23-21 Ma (Pfeil & Crisp 2008: stem age of Ailanthus).
3. Ailantheae Meisner - Ailanthus Desfontaines —— Synonymy: Ailanthaceae J. Agardh
Leaves (paripinnate), stalked nectaries at base of petiole; plant dioecious; C induplicate-valvate; (androgynophore +); tapetal cells binucleate; fruit a samara; n = 32 [= 2 x 16?].
1/5. Turkestan to Indo-Malesia, N. Australia.
Age. Crown-group Ailanthus is (53.6-)35.3(-15.8) Ma (Muellner-Riehl et al. 2016).
[Leitnerieae [Nothospondias [Picrolemma + Simaroubeae]]]]: disc usu. 0; (seeds with starch).
Age. This clade is around (58.9-)49(-38.9) Ma (Muellner-Riehl et al. 2016).
4. Leitnerieae Baillon —— Synonymy: Leitneriaceae Bentham & J. D. Hooker, Soulameaceae Pfeiffer
(Ellagic acid 0 - Leitneria); oil cells +; (vessels with spiral thickenings - L.); stomata paracytic; leaves (simple), (stipules +, cauline - some Soulamea); plant usu. dioecious; (inflorescence ± catkinate - L.; (flowers 3-merous - S./4-merous - Brucea); nectary (0 - L.); staminate flowers: (P 0; A (1-)4 - Leitneria); G 1 [L.], [2 - S.], ca 5, style ±0-short; fruit drupelets, ± flattened and carinate, (2-seeded samara - S.); (endosperm +); n = 16.
5/?30: Soulamea (14). S.E. U.S.A. (Leitneria), Africa, tropical and subtropical Asia and Malesia to N. Australia and Polynesia, inc. New Caledonia.
Age. Crown-group Leitnerieae are (55.8-)45.3(-33.3) Ma (Muellner-Riehl et al. 2016: Leitneria sister).
[Nothospondias [Picrolemma + Simaroubeae]]: gynophore +, nectariferous, disc 0.
Age. The age of this clade is (54.2-)44(-33.2) Ma (Muellner-Riehl et al. 2016).
5. Nothospondias Engler - Nothospondias staudtii Engler
Foliar glands 0; plant dioecious; flowers 4-merous; K ± connate; ?styles; fruit ± globose; inner mesocarp ?fibrous.
1/1. Tropical West Africa.
[Picrolemma + Simaroubeae]: stigma at most shortly lobed.
Age. This clade is some (45.5-)34.7(-24.8) Ma (Muellner-Riehl et al. 2016).
6. Picrolemma J. D. Hooker
Mesophyll sclereids 0; plant dioecious; K ± connate; staminate flowers: A 5, opposite K, alternating with staminodes; G completely free.
1/2. Peru, Brazil.
7. Simaroubeae Dumortier —— Synonymy: Quassiaceae Bertolini, Simabaceae Horaninow
(Geophyte, shrub, unbranched tree); (rays uniseriate - Quassia); leaves (paripinnate), (simple), (lacking glands), (leaflets articulated - Quassia); (flowers perfect); K with a single trace, C (8 - Iridosma), (contorted - e.g. Quassia, Simaba), (valvate), (long, coherent into tube - Q.); A (5, alternating with staminodes - Eurycoma, Simaba), (many), filaments with lateral or basal-adaxial appendages, (0 - Perriera, Gymnostemon); (disc + Perriera); G (2) 4-5(-6); (style long, with separate canals or not); (endothelium + - Simaba trichilioides), tissue below embryo sac massive (with central elongated cells - Samadera); fruits (nutlets), drupelets, ± bicarinate, (single, 7≤ cm long), stone from inner mesocarp fibres, or massive, also numerous fibres associated with vascular bundles, etc. - Homalolepis); n = 15 (16).
11/54: Homalolepis (30). Pantropical.
Age. Crown-group Simaroubeae are (40.3-)30.1(-21.3) Ma (Muellner-Riehl et al. 2016).
Evolution: Divergence & Distribution. For more ages in Simaroubaceae, see Muellner-Riehl et al. (2016; also Clayton et al. 2009). In the map above it is obvious that distributions of some genera in the past and the present are very different. Thus Ailanthus, now known only from Asia to Australia, is a widespread fossil in the Eocene ca 52 Ma (Corbett & Manchester 2004). Clayton et al. (2009) discussed the fossil history of Leitneria and Chaneya, the latter not certainly Simaroubaceae; fruits identified as Leitneria, a genus now endemic to the southeast U.S.A., are reported from eastern Siberia (Ozerov 2012).
The bulk of the diversification within Simaroubeae has occured within the last (27.4-)19.5(-12.1) Ma (Muellner-Riehl et al. 2016). Despite (or because of?) the fairly good fossil history of the family in the northern hemisphere, the biogeographic history of Simaroubaceae is of considerable complexity with much dispersal (and some extinction) needed to explain the current distribution of taxa (Clayton et al. 2009, see also 2007).
I have put in some phylogenetic structure above because of its effect on our understanding of character evolution; Simaroubeae are quite well known and have some distinctive features, but they are well embedded in the family, so these features are not family-level apomorphies. See also Devecchi et al. (2017) for the optimization of some 17 characters - the focus is on Simaroubeae, but there are broader implications, while Alves et al. (2022) examined the evolution of 20 features of inflorescence, flower and fruit. J.-X. Ren et al. (2020), looking at the chemistry of Picrasma quassioides, found similarities with Rutaceae - and Asteraceae.
Pollination Biology & Seed Dispersal. There are reports of other than porogamous fertilization in the family (also in Anacardioideae: Wiger 1935; Rao 1970). Flower type is variable (Devecci et al. 2017; Pirani et al. 2021). For pollination - usually by small insects - and seed dispersal - generally by animals, see Pirani et al. 2021).
Plant-Animal Interactions. Leaf webbing caterpillars of the yponomeutoid moths Attevidae are notably common here (Sohn et al. 2013).
Genes & Genomes. Romero-da-Cruz et al. (2021) suggested that the base chromosome number of Simaroubaceae was 12, Guimarãraes and Forni-Martins (2021) that it was 14.
Chemistry, Morphology, etc.. Han et al. (2023) note that a number of Simaroubaceae have β-carboline indole alkaloids; their status as an apomorphy above is provisional. The adult plant of Holacantha is basically a huge, intricately-branched thorn; the leaves are reduced to scales. Development of the leaflets in Ailanthus is acropetal, and although the leaflets appear to be transversely inserted, that simply reflects how they develop (Hagemann & Gleissberg 1996). Devecchi et al. (2018) described a variety of extrafloral nectaries, perhaps all modified foliar apices, from vegetative perulae, leaflets and bracts of Homalolepis.
Whether the androecium of Picrolemma is haplostemonous or obhaplostemonous is unclear (c.f. Clayton 2012 and Pirani et al. 2021). Although the carpels are often more or less free except basally, there is often only a single style. The gynoecium of Leitneria is described as having a single carpel with two ovules, of which only one is fertile (Tobe 2011a).
For additional information, see Clayton (2011: general), Devecchi et al. (2018: Homalolepis) and Pirani et al. (2021: American taxa), for chemistry, see Hegnauer (1973, 1990, also 1966, 1989, as Leitneriaceae), da Silva and Gottlieb (1987), Waterman (1993) and Vieira and Braz-Felho (2006), all quassinoids, and Leite and Castilho (2020); see also Jadin (1901) and Boas (1913), both vegetative anatomy, Webster (1936: wood anatomy), Alves et al. (2016: floral morphology of Simaba), Endress et al. (1983: carpel morphology), Wiger (1935; see also Mauritzon 1935; Wiger 1936), all embryology, and Fernando and Quinn (1992: pericarp anatomy), also Abbe (1974) and Tobe (2011a, 2013), inflorescence, floral morphology/anatomy and embryology of Leitneria.
Phylogeny. Relationships in the family are rather pectinate. The topology [Picrasma etc. [Ailanthus [[Soulamea, etc.] [Nothospondias* [Picrolemma [Quassia* [Samadera* + Simarouba etc.]]]]]]] is mostly quite well supported, although support for the first clade is not that strong (it may be two clades, as in the phylogeny above: Muellner-Riehl et al. 2016; Majure et al. 2021) and that for some nodes along the backbone (the genera that might move have an asterisk above) could be improved (Clayton et al. 2007; see also M. Sun et al. 2016; Muellner-Riehl et al. 2016). Relationships recovered by Joyce et al. (2023) in their Angiosperms353 target capture analysis are also very pectinate, with Castela again being sister to the rest of the family. However, the next clade up includes both Ailanthus and Picrasma, the former including the latter, and so on - however, detailed comparisons must wait until genera like Leitneria and Brucea are sampled.
Majure et al. (2021) discuss relationships in Castela. Leitneria is well embedded in the family (Clayton et al. 2007) and is embryologically similar to Brucea, in the same clade (= Leitnerieae). Picrolemma is strongly supported as being sister to Quassia (Devecchi et al. 2017). Within Simaroubeae, Quassia and Samadera are successively sister to the remainder. For some relationships in Simaroubeae, see Devecchi et al. (2017: 2 nuclear ribosomal and 3 chloroplast markers) - Simaba turns out to be polyphyletic; they resuscitated Homalolepis, and basal relationships in that genus tend to be quite well supported.
Gumillea (ex Cunoniaceae) may belong to Simaroubaceae, although the stamens do not appear to have scales and there are many ovules per carpel - the latter feature in particular is rather odd for any putative sapindalean plant. It has stamens alternate with the petals, so making membership in Picramniales unlikely (and ovule number also militates against this, too).
Classification. For the dismemberment of Quassia, see Clayton et al. (2007). Devecchi et al. (2017) suggested that part of the biphyletic Simaba was to be called Homalolepis; given the support for higher-level relationships in Surianeae, extending the limits of Simaba would probably have entailed the whole tribe being placed in a single genus.
Previous Relationships. Molecular data have suggested the excision of Suriana and its relatives (see Fabales-Surianaceae), Harrisonia (Rutaceae), and Picramnia and Alvaradoa (Picramniales-Picramniaceae) (e.g. Fernando et al. 1995) from the old Simaroubaceae; its limits always had been rather problematic.
Botanical Trivia. Note the demise - alack! - of Leitneriaceae, the only family previously thought to be restricted to the continental U.S.A..
[Meliaceae + Rutaceae]: limonoids/protolimonoids [pentanortriterpenes, degraded triterpenes] +, flavones +; style-head discoid or capitate, lobed.
Age. The age of this node is estimated to be (157-)139(-123)/(114-)104(-96) Ma (Joyce et al. 2023).
Chemistry, Morphology, etc.. For triterpenoids in general, also limonoid synthesis, etc., in particular see above.
Botanical Trivia. Roy and Saraf (2006: p. 191) noted of limonoids that they were "confined to only plant families of order Rutales and that too more abundantly in Meliaceae and Rutaceae, and less frequently in Cneoraceae and Harrisonia sp. of Simaroubaceae" - and now both are included in Rutaceae-Cneoroideae...
MELIACEAE Jussieu, nom. cons. - Back to Sapindales
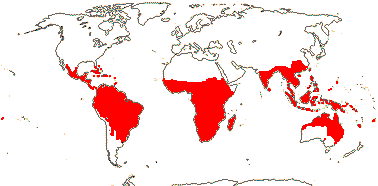
Trees; bark often rather bitter; (cambium storied), (spiral thickenings on vessels); secretory cells with resin, etc. + [= oil idioblasts]; nodes 5:5; leaves (even-pinnate), leaflets not articulated (articulated); flowers (3-)5(-8)-merous; K not enclosing C [?level], often connate, (vascular trace single); A 2 x C, connate, forming a tube, anthers on margin; G (1) [(2-)4-5(-many)], postgenitally united, opposite C, hairy, (placentation parietal), stigma capitate, wet; ovules anatropous/straight/campylotropous, (micropyle exo-/bistomal), outer integument 2-5 cells across, inner integument 2-4(-5) cells across, parietal tissue 3-9(-18) cells across, nucellar cap 3-5(-9) cells across, placental obturator common; seeds often pachychalazal, coat vascularized, testa usu. undistinguished but thick, endotesta crystalliferous, (tegmen multiplicative); embryo white, cotyledons collateral; x = 6, 7?/13/14, nuclear genome [1 C] (0.027-)0.602(-13.394) pg/(269-)541(-856) Mb.
58 [list: subfamilies]/740 - 2 main groups below. Pantropical, but largely Old World; plants of the lowlands. Map: see Wickens (1976), Pennington (1981), FloraBase (2006), Flora of China vol. 11. (2008), GBIF (consulted x.2009), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 6 (2011) and Fl. Austral. vol. 26. (2013).
Age. Bell et al. (2010) suggested that the two subfamilies diverged (48-)39, 38(-27) Ma; Muellner et al. (2007, see also 2006) thought that diversification within the family had begun considerably earlier, (98.5-)96, 73.5(-61.5) Ma, Koenen et al. (2015) gave ages of (91.5-)80.5, 59.5(-54) Ma, Muellner-Riehl et al. (2016) ages of (89.8-)79.6(-69.7) Ma, while Wikström et al. (2001) suggested another later date of (40-)36, 30(-26) Ma.
For fossil Meliaceae, see Mabberley (2011) and Muellner-Riehl and Rojas-Andrés (2021).
1. Melioideae Arnott
(Suckering shrublets); (hairs stellate - Aglaia); (nodes 3:3); buds naked; (leaves with terminal bud [= pseudogemmula]); plants dioecious; K (connate, opens irregularly), C (-14), (connate); (nectary disc 0), (style hollow); ovules (1-)2(-many)/carpel, collateral or superposed, (straight); archesporium multicellular; (fruit dry, winged [Quivisianthe], inflated); seeds with sarcotesta/aril/0; (exotesta +), endotegmen crushed/multilayered; n = 8, 11, 12, 14, 15, 18 ... 140.
40/605. Guarea (75), Ruagea (17), Chisocheton (55), Didymocheton (43). Pantropical, but largely Old World.
Age. Diversification within Melioideae began (91-)78, 70(-58.5) Ma (Muellner et al. 2006, 2007 - age in latter a little older), (81.8-)69.9(-57.5) Ma (Muellner-Riehl et al. 2016) or (83-)72.5, 54(-49) Ma (Koenen et al. 2015).
Fossil fruits named as Manchestercarpa vancouverensis found in deposits 79-72 Ma from Vancouver Island, British Columbia, were placed in Melieae, being thought to being very close to Melia itself (Atkinson 2020), although Atkinson does note that the endocarp of the fossil consists of interlocking sclereids, not interwoven bands of fibers, the central hollow is of the same length throughout, rather than being constricted in the middle, etc., features of Melia.
1A. Melieae de Candolle
Plant (deciduous); leaves (to 3-pinnate); polygamous; fruit a drupe; endosperm (slight).
3/10. Tropical to temperate Asia, Iran eastwards, Australia.
1B. Aglaieae Blume, etc.
A (tube cyathiform), anthers on inner side; (gynophore +); (testa multiplicative, all cell walls thick, tegmen cells collapse - Lansium); (cotyledons superposed).
Aglaia (125)
1C. Trichilieae de Candolle
(inc. Turraeeae, Munronia onwards) —— Synonymy: Aitoniaceae R. A. Dyer, nom. illeg.leaves (two-ranked, simple - Turraea); plants hermaphroditic/polygamous; A (± free); (exotegmen fibrous - Trichilia); (embryo chlorophyllous - Trichilia, Nymania).
8: Trichilia (107), Turraea (60).
2. Cedreloideae Arnott (Swietenioideae) —— Swieteniaceae E. D. M. Kirchner
Buds perulate (naked - Capuronianthus); ducts of traumatic origin; leaves (opposite), (leaflets ± serrate); plants monoecious; (C connate); (nectary 0); ovules (2 - Capuronianthus) 3-many/carpel, collateral; fruit a septifragal capsule, valves falling off, columella persisting; seeds winged, (fruit ± fleshy, seed single - Walsura); (exotegmen fibrous - Swietenia); x = ?14, n = 13, 18, 23, 25, 26, 28.
14/56: Entandrophragma (11), Khaya (9). Pantropical, but largely Old World. [Photo - Flower, Fruit.]
Age. Diversification within Cedreloideae began (86-)75, 67.5(-58) Ma (Muellner et al. 2006, 2007), (75.2-)64.8(-55.6) Ma (Muellner-Riehl et al. 2016), or (59.3-)48.5, 38.6(-33) Ma (Koenen et al. 2015).
2A. Chukrasia A. de Jussieu + Schmardaea H. Karsten
Plant deciduous; C contorted [?S]; (connective forming long apical appendage - Schmardaea); (gynophore + - Chukrasia); columella 0 [Schmardaea]; endosperm +.
2/2. India, Sri Lanka, and China to Malaysia, northwest South America.
[Cedreleae [...Xylocarpeae]]: ?
2B. Cedreleae de Candolle —— Synonymy: Cedrelaceae R. Brown
Plant (deciduous); midrib of C adnate to androgynophore; A =C, free; androgynophore +.
2/14: Cedrela (8). Indo-Malesia, tropical America.
Plant (subdeciduous); leaves ± even pinnate; C contorted; staminal tube globose, anthers on inside [these three in sister taxon Swiet]; ovules 3-4/loculus, superposed; pericarp fleshy, columella 0; sarcotesta +, spongy, testa (massive, woody or corky - Xy.); cotyledons fused; x = ; nuclear genome [1 C] ca 388 Mb {Xy.].
2/8: Tropical, inc. the western Pacific, the greater Antilles.
Evolution: Divergence & Distribution. For additional divergence dates, see Muellner-Riehl et al. (2016). Atkinson (2020) questioned the identity of a number of fossils previously included in Meliaceae, some near Melia. However, fossils that can be placed in or very close to Melia, now known mainly from the Indo-Malesia-Australian area, have been found in localities scattered throughout the northern hemisphere (Atkinson 2020). Muellner-Riehl and Rojas-Andrés (2021) evaluated much of the fossil record of the family.
Muellner-Riehl and Rojas-Andrés (2021) noted that Meliaceae had a similar number of species throughout the tropics, although the number of endemic genera in Africa and Madagascar, at 20, was far higher than in the New World (6) or Indo-Malesia (7) while in terms of overall generic diversity Africa and Indo-Malesia were similar. Muellner et al. (2006) discussed the biogeography of Meliaceae, proposing an origin in Africa and subsequent dispersal. In a later study that focused on New World Meliaceae, Muellner-Riehl and Rojas-Andrés (2021) suggested that Carapa and Trichilia might have arrived in South America via dispersal across the Atlantic, other genera may have moved across land bridges in the northern hemisphere, subsequently utilizing the various links that developed between North and South Americas. Koenen et al. (2015) also suggest an Old World origin for the family, and although one thinks of it as being characteristic of l.t.r.f., they proposed that its common ancestor was a deciduous tree of seasonal or montane habitats. Atkinson (2020) thought that the Melia-like fossil that he described from Campanian-aged deposits in North America, although to be referred to an important tropical clade, nevertheless did not imply that l.t.r.f. existed then. Indeed, crown-group ages of rainforest clades in the family are a mere 23 Ma (Late Oligocene/Early Miocene), their stem-group ages are Eocene and they may have been quite species-rich in pre-Late Oligocene times, but with extinction then and subsequent diversification (Keunen et al. 2015). Heads (2019a) provided a comprehensive account of the biogeography of the family based on range expansion-vicariance dynamics, and he also emphasized "atypical" habitats in which some Meliaceae are to be found. Monthe et al. (2019) looked at shifts from rain to dry forests in African Cedreloideae, but noted that differing dates were obtained from plastid and ribosomal analyses.
For the biogeography of Aglaia, see Muellner et al. (2008b) and Grudinski et al. (2014a), the latter suggesting Oligocene-Miocene rather than Eocene diversification; movement was from West Malesia eastwards.
Gama et al. (2020) discussed the evolution of various floral traits in the family, optimising them on the tree in Muellner-Riehl et al. (2016). Oil-containing idioblasts are said to be an apomorphy for the family by Tölke et al. (2022).
Ecology & Physiology. Although quite a small family, Meliaceae make up 17% of all trees >10 cm d.b.h. in Sumatra (Mabberley 2011). Carapa procera is one of the four common species mentioned growing in the ca 145,500 km2 of peat in the Cuvette Centrale in the Congo (Dargie et al. 2017). Xylocarpus is a well-known mangrove genus, and for its seeds and germination, see Clarke et al. (2001); there is some information in Ann. Bot. 115(3). 2015 and also the discussion under the mangrove habitat below.
Pollination Biology & Seed Dispersal. Most Meliaceae have a well-developed floral tube which is formed by the connation of the filaments - a rather uncommon way of forming a tube. The pistillode in staminate flowers is well developed, the result being that staminate and carpelate flowers are very similar functionally, although the staminal tube in the former is often somewhat narrower; the staminal tube and the large stigmatic head seem to be integral parts of the pollination mechanism. The whole apex of the style is commonly more or less massively swollen (see also Gama et al. 2020) and is sometimes involved in secondary pollen presentation, as in Vavaea (Ladd 1994). Gama et al. (2020) summarize what little is known about pollinators in the family.
Animal dispersal is common in Melioideae; for detailed studies of the dispersal of arillate-type seeds of Malesian Aglaia, see Pannell and Koziol (1987). Wind dispersal is common in Cedreloideae.
Plant/Animal Interactions. For the diversity of ant-attended extrafloral nectaries in Cedreloideae, especially Carapa, see Kenfack et al. (2014). Some species of Chisocheton are myrmecophytes.
Caterpillars of the pyralid moth Hypsipyla are stem borers and can cause serious damage; all the host records I have seen are members of Cedreloideae.
Vegetative Variation. Munronia is ± herbaceous. Most species of Guarea (tropical America) and Chisocheton (Malesia), both Melioideae, have indefinitely growing leaves, additional pairs of leflets being produced from the apex of the leaf, although despite this distinctive similarity they are not closely related (Koenen et al. 2015). In Guarea the apical part of the leaf is shoot-like in its gene expression (Tsukaya 2005). The leaves of Chisocheton can be rooted (Fisher & Rutishauser 1990) and can continue to grow for a long time, although I do not know that a tree has ever been produced from a leaf. Species of Chisocheton such as C. pohlianus have epiphyllous inflorescences, flowers appearing between the leaflets, and specimens of this species have been misidentified as Rubiaceae... Capuronianthus (Cedreloideae) has opposite, compound leaves, while the simple-leaved Vavaea and Turraea (both Melioideae) look rather unmeliaceous except when in flower; the leaves of some species in the latter genus can even be two-ranked, borne on short shoots, and lack articulations.
Economic Importance. Azadirachta indica (Melia azadirachta) is the neem tree, the limonoids, notably azadirachtin and including 1β, 2β, 21,23-depoxy-7α-hydroxy-24,25,26,27-tetranor-apotriculla-14,20,22-trien-3-one, in the leaves and seeds of which have insecticidal properties (for accounts, see Roy & Saraf 2006: Table 2; Singh et al. 2009). A number of species yield important woods, and these include three species of mahogany, especially Swietenia mahogani and S. macrophylla, American/Spanish cedars, Cedrela fissilis and C. odorata, Australian red cedar, Toona ciliata, also Entandrophragma and Carapa spp. (Pace et al. 2022).
Genes & Genomes. For a base chromosome number for the family of x = 14, see Carta et al. (2020) and Guimarãraes and Forni-Martins (2021). For genome sizes, see Lyu et al. (2017).
Chemistry, Morphology, etc.. Over one third of the limonoids in Meliaceae (= meliacins) are to be found in Azadirachta indica and Melia azedarach alone; limonoids in the family are notably diverse, with a number of limonoid groups unique to them, e. g. C-seco meliacins (Roy & Saraf 2006). Although it was thought that the two subfamilies above could be separated by their limonoid types, work on Quivisianthe (Melioideae) suggests that any distinction may not be that simple (Mulholland et al. 2000).
Resin canals are not reported from Meliaceae by Prado and Demarco (2018). Sieve tube plastids with protein crystalloids and starch occur in Melia and Azederach. Walsura often has leaflets with ± pulvinate petiolules and prominent reticulate venation.
Although the flowers are often apparently perfect, dioecy is widespread; carpelate flowers are the first to be produced in the cymose inflorescences. Gouvêa et al. (2008b) drew the flowers of Swietenia as being inverted. The filaments of Vavaea are largely free, as are those of Cedrela, Toona and Walsura (Cedreloideae-Cedreleae). Indeed, Cedreleae are rather different florally from other Meliaceae, but features found there such as the more or less free stamens may be derived, not plesiomorphous as one might think (c.f. Gouvêa et al. 2008a). A multicellular archesporium is quite common in Melioideae (Prakash et al. 1997). There is considerable variation in seed morphology and development (e.g. Wiger 1935; Corner 1976), even within the subfamilies, and this will have to be integrated with the phylogeny as it develops.
For general information, see van Wyk (in Dahlgren & van Wyk 1988: Nymania), T. D. Pennington and Styles (1975: generic monograph), Pennington (1981: Neotropical Meliaceae), Pannell (1992: Aglaia), Mabberley et al. (1995: esp. Malesia), and Mabberley (2011), for chemistry, see Hegnauer (1969, 1990) and Mulholland et al. (2000), for floral anatomy, see Murty and Gupta (1978: both subfamilies), and for embryology, etc., see Wiger (1935; see also Mauritzon 1935; Wiger 1936), Paetow (1931), Nair and Kanta (1961) and N. C. Nair (1962, 1970 and references).
Phylogeny. Cedreloideae and Melioideae are both monophyletic (Oon et al. 2000: one gene, Cedreloideae not well supported; Muellner et al. 2003: three genes; Muellner et al. 2006: rbcL alone, sampling better; Koenen et al. 2015; Muellner-Riehl et al. 2016). The clade [Schmardaea + Chukrasia] is sister to the rest of Cedreloideae (Atkinson 2020). Within Melioideae, Melieae (including Owenia) are sister to the rest, but with only moderate support (stronger in Muellner-Riehl et al. 2016; see also Atkinson 2020); relationships along the backbone of the rest of the rather pectinate ITS tree are poorly supported, but rather better resolved by rbcL data (Muellner at al. 2008a). Owenia, paraphyletic and including Melia, is sister to other Melioideae in Koenen et al. (2015), who suggested that there may be four more small clades successively sister to other Melioideae. A similar structure was recovered by Atkinson (2020), who also found a larger clade that included Turraea and Trichilia and sometimes (total evidence trees) another that included Guarea and Aphanamixis. Ruagea in close to [Guarea + Turraeanthus] (Rojas-Andrés et al. 2023: 2 nuclear ribosomal and 2 chloroplast markers). For relationships around Dysoxylum, which has turned out to be polyphyletic, see Holzmeyer et al. (2021). Two Malagasy genera previously segregated as separate subfamilies, Quivisianthe and Capuronianthus, have consistently been found to be well embedded in Melioideae and Cedreloideae respectively (e.g. Muellner et al. 2003, 2006; Koenen et al. 2015; M. Sun et al. 2016, q.v. for more details). Oyedeji Amusa et al. (2025: focus on South African taxa) recovered this basic structure; Turraea was paraphyletic.
For relationships in Aglaia, see Muellner et al. (2005) and Grudinski et al. (2014a, b), in Chisocheton, see Fukuda et al. (2003), and in Trichilia, where relationships are [T. havanensis [African species + American species]], see Clarkson et al. (2016: ITS only).
Within Cedreloideae the clade [Chukrasia + Schmardaea] is sister to other members of the subfamily (Koenen et al. 2015; Muellner-Riehl et al. 2016). For relationships in Neotropical Cedreleae, see Muellner et al. (2009); although immediate generic associations are similar to those in Muellner-Riehl et al. (2016), broader relationships within the subfamily differ. In the African Khaya in particular, but also in Entandrophragma, there is conflict between phylogenies obtained from analyses of plastid and ribosomal sequences (Monthe et al. 2019).
Classification. The classification suggested by Penningron and Styles (1975) is being substantially amended, as is evident from the mismatch between their tribes and the phylgeny in Muellner-Riehl et al. (2016; see also Gama et al. 2020). Generic limits in Melioideae in particular are uncertain, genera like Owenia, Lepidotrichilia, Dysoxylum, and Aglaia all being more or less para/polyphyletic (Koenen et al. 2015), and the limits of Trichilia will also bear rexamination (Clarkson et al. 2016). Holzmeyer et al. (2021) have reworked generic limits in the Dysoxylum area, while Muellner-Riehl and Rojas-Andrés (2021) listed the genera that they recognized in the family.
The connection between species limits in Aglaia and phylogenetic relationships as they are currently understood there is somewhat unclear (Grudinski et al. 2014b).
Thanks. I am grateful to David Kenfack for useful information.
RUTACEAE Jussieu, nom. cons. - Back to Sapindales
Furanocoumarins, distinctive limonoids; vessel elements (with spiral thickenings), (scalariform perforation plates); libriform fibres +; wood often fluorescing; (nodes 1:1); (cuticle waxes platelets, rodlets, etc.); stomata various; petiole bundle annular; oils +; leaves (simple), pulvinate/articulated, vernation also flat, margins entire to crenate (serrate); flowers often perfect; (3-)5-merous; K not enclosing C in bud [?level], (2-4), connate or free; A filaments ± flattened; (gynophore +); G variously (postgenitally) connate to almost free, styluli connate or not, style impressed to ± gynobasic, stylar canals as many as carpels, stigma discoid or capitate, dry or wet; ovules 1-2/carpel[?], (apical), micropyle also bistomal, zig-zag, nucellar cap 2-6(-18) cells across; chalazal embryo sac haustorium +; seed with chalazal aperture in the sclerotesta at base or raphe [?Aurantioideae]; exotegmen tracheidal + (0); x ?= 9, , nuclear genome [1 C] (0.115-)1.061(-9.818) pg.
161 [list]/2,085 - six or so subfamilies below. Largely tropical.
Age. Bell et al. (2010) suggested that the age of this node was (51-)40(-29) Ma; however, other dates are (87-)82(-74) Ma (Appelhans et al. 2012a), around 93.3, 82.1, or 72.9 Ma (Muellner et al. 2007, see also 2006), (93.4-)84.6(-75.9) Ma (Muellner-Riehl et al. 2016), (72.7-)62.7(-53.3) Ma (Pfeil & Crisp 2008), or (43-)39, 37(-33) Ma (Wikström et al. 2001), so there is quite a range with which to work. The spread has been increased by Joyce et al. (2023), who estimate ages of (148-)128(-111)/(107-)97(-87) Ma.
1. Cneoroideae Webb —— Synonymy: Cneoraceae Vest, Ptaeroxylaceae J.-F. Leroy
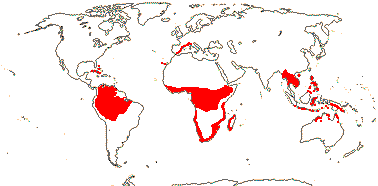
(Scandent) shrubs to trees; pyranochromones, diterpenoid cneorubin, quassinoids, limonoids, prenylated coumarins +; solitary oil cells +; petiole bundle more or less cylindrical, of two opposed plates, (arcuate - Bottegoa); stomata anomocytic to cyclocytic; (stipules, stipular spines +); leaves simple - twice-pinnate(plant dioecious); C valvate; (andro)gynophore ± +, A = and opposite K, (8-10 - Harr.), filaments (with adaxial appendage - Harr.); pollen reticulate, (tricellular - Cn.); G [2-5], style short to long, (with canals - Harr.), stigma various; ovules 1-2(3)/carpel, also apotropous, (becoming) campylotropous, micropyle endo-/bistomal, outer integument ca 2 cells across, inner integument 3-4 cells across, parietal tissue 4-5 cells across, hypostase +/0, obturator +; fruit a loculicidal capsule, with ± separate/separating druplelets seeds (winged), [endo]testa multiplicative, exotestal cells large, outer walls thickened, endotestal cells small, thick-walled, with crystals, (oil cells +), exotegmen fibrous (not); endosperm +/0; embryo curved; n = 18 [Cn.].
5/16: Old World tropics, inc. Madagascar, also W. Mediterranean, the Canaries. Map: from Appelhans et al. (2012a: Old World), Australia's Virtual Herbarium (consulted xii.2012) and Fl. Austral. vol. 26 (2013).
Age. Crown Cneoroideae have been dated to (78-)74(-58) Ma (Appelhans et al. 2012a) or (84.6-)67.2(-46.9) Ma (Muellner-Riehl et al. 2016).
[Spathelioideae [Zanthoxyloideae [Rutoideae [Amyridoideae [Haplophylloideae + Aurantioideae]]]]]: multicellular oil glands on leaflet margins.
2. Spathelioideae Engler - Spathelia L. —— Synonymy: Spatheliaceae J. Agardh
Unbranched/small trees; pyranochromones, diterpenoid cneorubin, quassinoids, limonoids, alkaloids + +; petiole bundle more or less cylindrical, of two opposed plates; solitary oil cells + (0 - Dictyoloma), stomata anomocytic to cyclocytic; leaves pinnate (bicompound), (stipules and stipular thorns +); (bracteoles 0 - D.); C valvate/quincuncial; A = and opposite K, filaments winged at base; pollen reticulate; G [2-3(4-5)], style short, stigma lobed; ovules 1(-2, 4-5)/carpel, apotropous, (becoming) campylotropous, micropyle endo-/bistomal, outer integument ca 2 cells across, inner integument 3-4 cells across, parietal tissue 4-5 cells across, hypostase +/0, obturator +; fruit 2-3 winged, indehiscent, (follicle); [endo]testa multiplicative, exotestal cells large, outer walls thickened, endotestal cells small, thick-walled, with crystals, (oil cells +), exotegmen fibrous (not); endosperm +/0; n = ?.
1/19. Central America, Bahamas, the Greater Antilles, some N. South America. Map: from Appelhans et al. (2012a: New World).
[Zanthoxyloideae [Rutoideae [Amyridoideae [Haplophylloideae + Aurantioideae]]]]: dihydrocinnamic acid derivates; oil glands +, schizolysigenous, leaflets usu. prominently punctate; pollen (exine striate); (ovules hemitropous, campylotropous), (apotropous), outer integument 3-10 cells across, inner integument 2-4(-6) cells across, parietal tissue (3-)5-12(-16) cells across; archesporium often multicellular; (endocarp area persisting at adaxial base of seed); (exotesta mucilaginous); nuclear genome [1 C] (196-)1278(-8509) Mb.
Map: from Meusel et al. (1978), Brummitt (2007) and Groppo et al. (2012).
Age. The age of this node may be (56.8-)47.6(-36.4) Ma (Pfeil & Crisp 2008), (73.5-)69.5(-62.3) Ma (Appelhans et al. 2012a), (90-)74(-58) Ma (Salvo et al. 2010: q.v. for more dates) or (83.2-)74.5(-66.1) Ma (Muellner-Riehl et al. 2016).
3. Zanthoxyloideae Arnott —— Synonymy: Boroniaceae J. Agardh, Diosmaceae Bartling, Diplolaenaceae J. Agardh, Flindersiaceae Airy Shaw, Fraxinellaceae Nees & Martius, Jamboliferaceae Martynov, Pilocarpaceae J. Agardh, Pteleaceae Kunth
Shrubs to trees; quinolone and acridone [derived from anthranilic acid], (furo-)pyranoquinoline and 1-benzyltetrahydroisoquinoline alkaloids, (limonoids 0); (distinctive tracheal veinlet endings); (petiole with medullary vascular bundles - Conchocarpus), (foliar sclereids +), oil cells also commonly solitary; (colleters +); leaves (opposite), (stipules +, intrapetiolar/hooded sheath - Metrodorea); flowers (vertically/obliquely monosymmetric), (4 merous); C (connate), apex inflexed (not); A (connate), (4), (2, with basal anther appendages, + 3 staminodes - Angostura alliance); ([andro]gynophore +); G often connate by the styluli (styluli terminal), (connate only at apex); usu. 2-10 ovules/carpel, (obturator +); fruit follicle/capsule; seeds (winged), detached, (forcibly expelled with endocarp); exotesta often mucilaginous/sarcoexotesta [spongy], irregularly palisade, (variously lignified), (mesotesta sclerotic), exotegmen with crossed lignification bars (meso- and endotegmen tracheidal), (nucellar polyembryony +); x = 18 (n = 7-11...72); 2n genome 0.65-0.87 pg.
111/1,580: Agathosma (150 +), Boronia (160), Vepris (80), Zieria (60), Acronychia (48), Conchocarpus (48), Philotheca (34). Pantropical, some (warm) Temperate. [Photo - Flower, Flower, Fruit.]
Age. Crown-group Zanthoxyloideae are (79-)70.7(-62.8) Ma (Muellner-Riehl et al. 2016).
3A. Dictamneae Bartling —— Synonymy: Dictamnaceae Vest
Subshrubs to trees; leaves (in pairs on opposite sides of the stem - Orixa); (flowers monosymmetric - Dictamnus); A = K (A = 2 x K); 1-5 ovules/carpel; exotegmen lacking crossed lignification bars [Skimmia].
4/16: Casimiroa (10). Central America to Texas (U.S.A), warm temperate Europe to China, Japan and the Philippines.
Age. Estimates for the age of this clade are (60-)38(-18) Ma (Muellner-Riehl et al. 2016) and (33-)22(-12) Ma (Appelhans et al. 2012).
3B. Zanthoxyleae Dumortier / Protorutaceae —— Synonymy: Zanthoxylaceae Martinov
Shrubs to trees, (lianes), (deciduous); benzylisoquinoline alkaloids +; prickles + [phloic, on stem (stipular position) or leaf rhachis]; leaves 1-3-foliolate or (im-)paripinnate; plant dioecious/flowers perfect; P = T, 4-10-merous) / K + C, 3-5-merous, K ± connate; G 1-5, opposite K, styluli gynobasic to terminal, stigmas often connate; 2 ovules/carpel, 1 aborts; fruit follicular / capsular (drupes); (mesotesta fibrous - Phellodendron), meso-/endotesta thickened [Zanthoxylum]; endosperm copious, embryo short to long, slender; n = 16-18.
4/245: Zanthoxylum (233), Tetradium (9). Tropics (temperate), esp. Southeast Asia to Australia.
Age. Zanthoxyleae are estimated to be (70.4-)66.3(-62.6) Ma (Appelhans et al. 2018a).
3C. Melicope J. R. & G. Forster - ?Tribe
leaves (whorled), tri/unifoliate [latter inc. all 55 species from Hawaii];); plant dioecious (flowers perfect); flowers 4-merous; K valvate; A 4, 8 (4 + 4 staminodes); G apocarpous-syncarpous; ovules (1)2(-8)/carpel; fruit follicle/capsule/(drupaceous), (C persistent); sclerotesta thick, sarcotesta spongy; endosperm +, cotyledons flattened.
1/235. Madagascar, India to Japan, Malesia and the Pacific, 1 sp. Australia.
[Rutoideae [Amyridoideae [Haplophylloideae + Aurantioideae]]]: (leaves simple, trifoliolate); C imbricate, clawed; endosperm 0.
Age. The age for this node is estimated to be some (75.3-)62.9(-50) Ma (Muellner-Riehl et al. 2016).
4. Rutoideae Arnott
Perennial herbs to shrubs (trees); acridone alkaloids reduced at C-1 and C-3, napthalene coumarins +; limonoids 0; C (valvate - Chloroxylum), (fringed - Ruta); (gynophore +); (G [2-5]), [postgenitally united/(styluli connate)]; ovules 4-8(-12)/carpel; fruit loculicidal often ventricidal capsule, (septicidal, with mericarps); seeds detached, reniform), (winged); seeds angled and slightly curved/reniform, surface ornamentation conspicuous, various; endosperm usu. ± copious, embryo straight to curved; n = (9), 10.
5/20: Ruta (10). North (warm) temperate, some southern Africa, not the Antipodes or South America, Chloroxylum tropical (Madagascar, Sri Lanka).
Age. Crown-group Rutoideae are estimated to be some (51.9-)40.4(-29.5) Ma (Muellner-Riehl et al. 2016: Claus. Gly.).
[Amyridoideae [Haplophylloideae + Aurantioideae]]:
5. Amyridoideae Link —— Synonymy: Amyridaceae Kunth
Shrub to tree; leaves simple to unequal pinnate; flowers (3-)4-5 merous; (A = and alternating with C); G 1; ovules 1-4/carpel; fruit drupe/berry [Cneoridium]; endosperm (+); n = 18.
3/42: Amyris (40). Southern U.S.A. to South America, the Antilles.
[Haplophylloideae + Aurantioideae]:
6. Haplophylloideae Appelhans, Bayly, Heslewood, Groppo, Verboom, P. I. Forster, Kallunki & Duretto - Haplophyllum A. de Jussieu
Perennial herbs (subshrubs); anom. (amphistomatous), leaves usu. simple; A connate to free, filaments hairy adaxially/not; pollen strongly triangular in polar view, pores/endapertures protruding, surface usu. striate; gynophore short, thick; G (3-)5, styluli basal, connate, stylar canals separate; ovules (1-)2-4(-8)/carpel; fruit loculicidal capsule, (with apical appendages/prominent glands); seeds detached, reniform, surface rugose; dorsal exotestal cells with sinuous anticlinal walls; endosperm copious, embryo long, curved; n = 9.
1/66. The Mediterranean, North and N.E. Africa, Arabia to China and Siberia. Map: Townsend (1986: Map 1).
7. Aurantioideae Eaton —— Synonymy: Aurantiaceae Jussieu, Citraceae Roussel
Shrubs to trees; methylcarbazole alkaloids, distinctive flavonoids by polymethoxylation; (thorns +); leaves unifoliolate to imparipinnate; (rhachis winged), (leaflets alternate); C (valvate - Micromelum), (A many); G [2-5(-20)], (placentation parietal); ovules 1-many/carpel, (unitegmic, nucellus apex exposed, integument ca 5 cells across - Glycosmis), hypostase, obturator +, with hairs; embryo sac (4-nucleate - onagrad type, Glycosmis); fruit a ± dry berry with mucilaginous pulp directly from endocarp/(loculi filled with fleshy multicellular exotestal hairs [= hesperidium]); seed (pachychalazal - Glycosmis), exotesta in part mucilaginous (not), of laterally compressed fibres, inner walls lignified, (testa sclereidal-fibrous - Atalanta), (ecto-, meso- and) endotesta with crystal-containing cells, exotegmen fibrous; (multicellular chalazal haustorium), (nucellar polyembryony +), embryo (curved), (chlorophyllous), cotyledons thick, not folded; n = 9.
27/206: Glycosmis (50), Citrus (30). The Mediterranean region to Indo-Malesia and the Pacific, also Africa.
Age. The age of crown-group Aurantioideae is estimated to be (28.2-)19.8(-12.1) Ma (Pfeil & Crisp 2008), ca 30 Ma (Muellner et al. 2007) or (52-)40.5(-29.5) Ma (Muellner-Riehl et al. 2016).
Evolution: Divergence & Distribution. For the early Caenozoic fossil history of what are now East Asian endemic Rutaceae, see Manchester et al. (2009); Gregor (1989) discussed Caenozoic fossil seeds. For other dates of diversification within Rutaceae, especially Aurantieae, see Pfeil and Crisp (2008; c.f. in part Muellner et al. 2007) and Appelhans et al. (2018a).
Rutaceae are relatively young, and distributions are unlikely to be much affected by continental drift (but c.f. Kubitzki et al. 2011; Hartley 2001a, 2001b; Ladiges & Cantrill 2007). Appelhans et al. (2018a) suggest that the family may be Eurasian in origin, and Ang et al. (2025) think that the large genus Zanthoxylum is of Old World origin.
Appelhans et al. (2014b) thought that the shiny black seeds common in the Acronychia-Melicope clade (probably bird-dispersed - see Appelhans et al. 2018a) might be a key innovation, and this clade has 5 (Appelhans et al. 2018b) to 17 times () as many species as Tetracomia and the Euodia clade, successively its sisters. The exotesta of seeds of members of the Acronychia-Melicope clade is edible (birds) and the sclerotesta is thick, while members of the Euodia clade have explosively-dehiscent follicles and seeds with a thin testa (Hartley 2001a; Appelhans et al. 2018b). Melicope s.l. itself has radiated extensively across the Indian and Pacific Oceans, where species are to be found from Madagascar to the Austral islands (Appelhans et al. 2018b). There has been a major radiation of Melicope on Hawai'i of some 55 species, the largest radiation of a woody clade there. The beginning of this radiation predates the ages of the main islands (Paetzold et al. 2018, 2019), and from Hawai'i there seem to have been two dispersals to the Marquesas Islands over 3,500 km away (Paetzold et al. 2018, 2019). The source area for the Hawaiian plants (and for the whole group) is likely to be in the general Australia-New Guinean region, although Vanuatu and New Caledonia also figure prominently as secondary dispersal hubs (Harbaugh et al. 2009b; Appelhans et al. 2014a, 2018b; Hembry 2018). Paetzold et al. (2018) discuss how to reconcile the apparent ease with which Melicope undergoes long distance dispersal with features such as its dioecy on Hawai'i and elsewhere, woodiness and polyploidy (albeit apparently with subsequent diploidization).
Ca 275 species of Diosmeae are restricted to South Africa, very largely to the Cape Floristic Region (Linder 2003; Trinder-Smith et al. 2007). About 1/4 (>400 spp.) of the species in the family are to be found in Australia (see Bayly et al. 2013b for a phylogeny), where most have narrow distributions; movement seems to have been from rainforest habitats to more sclerophyllous/xerophytic vegetation, but there were only four or five of these shifts (Bayly et al. 2013b). However, species distributions/relationships of Boronia within Australia seem to fit the peripheral vicariance pattern, species now being found on the periphery of the continent after the drying out of the centre, a process that began in the Eocene (Nge et al. 2021c). A number of genera show the common southeast-southwest Australia vicariance patterns, while relationships in Lasionema are [Queensland [New Zealand + Southeast Australia]] - just a single species in the first two areas (Orel et al. 2023; Duretto et al. 2023). The crown-group age of Zanthoxylum on Hawai'i (hybridization may have been involved in its origin - see also Reichelt et al. 2021) is around (17.5-)11.8(-6.9) Ma (Appelhans et al. 2018a). How Zieria arrived in New Caledonia is unclear, especially if the estimates of the age of Zieria of less than 20 Ma are correct: Z. chevalieri is the only species there, and is sister to the rest of the genus, all Australian (Barrett et al. 2015 and references). Citrus may have moved from west to east Malesia and Australia some time in the Miocene/Pliocene (Schwartz et al. 2015). Haplophyllum is an Irano-Turanian genus and has colonized the Mediterranean area more than once (Salvo et al. 2011; Manafzadeh et al. 2011).
Poon et al. (2007) looked at variation in characters of secondary chemistry and morphology in the light of phylogeny. Appelhans et al. (2011: many original observations) plotted a number of morphological characters on the tree, with a focus on Cneoroideae; the clade is morphologically quite heterogeneous - like the rest of the family. See also Bayly et al. (2013b) for morphology in Australasian members of the family.
Ecology & Physiology. Rutaceae have very diverse secondary metabolites, some of which (essential oils, coumarins, etc.) are similar to those in Apiaceae, Asteraceae, Papaveraceae, etc. (Hegnauer 1971; Kubitzki et al. 2011), while their alkaloids are like those found in some magnoliids - and are produced via nine or more different biosynthetic pathways. Thus 1-benzyltetrahydroisoquinoline alkaloids are found in a small group of related Rutoideae, and also in Papaveraceae (and a couple of other families), a distribution that has exercised phytochemists' imaginations in the past (Kubitzki et al. 2011). Robertson et al. (2018) discuss the much greater diversity of alkaloids found in Australian Flindersia growing in drier woodlands/vine thickets over those in more mesic habitats.
Groups of genera of Australian Zanthoxyloideae tend to live in similar habitats in the same biome, although there is of course some switching. Interestingly, genera in sclerophyllous communities tend to have an order of magnitude more species than those that live in rainforest (Duretto et al. 2020).
Pollination & Seed Dispersal. El Ottra et al. (2016) discussed pollination in Conchocarpus rubrus (Galipeinae), with its tubular flowers; hummingbirds and to a lesser extent butterflies were its main pollinators. Overall, butterflies seem more important pollinators of these tubular-flowered New World Galipeinae, while in the Old Word Boronieae (polyphyletic), meliphagids visit the (red) tubular flowers of Correa, for example (Armstrong 1979). Diplolaena in particular has pseudanthia (Baczynski & Claßen-Bockhoff 2023).
Adventitious polyembryony is quite common in Rutaceae, including Zanthoxylum (not pseudogamous) and Citrus (pseudogamous, the common condition) (Desai 1962b).
Bayley et al. (2013) and Appelhans et al. (2014b) discuss the dispersal of the black, shiny, fleshy seeds in the Acronychia-Melicope clade. Diosmeae (South African), Boronia and relatives (Australian), and some other Rutaceae have seeds with elaiosomes at the base that are endocarpial in origin and are dispersed by ants (Kubitzki et al. 2011; Bayley et al. 2013).
Plant-Animal Interactions. Caterpillars of Papilionidae-Papilioninae-Papilionini butterflies are notably common on Rutaceae, about ca 1/3 of the records being from here, and 80% of the ca 200 species of Papilio will eat Rutaceae (Aubert et al. 1999; Zakharov et al. 2004). The first time I saw the giant swallowtail, P. cresphontes, I also found its host plant, Ptelea trifoliata, about twenty yards down the path (Shaw Nature Reserve, Missouri). Rutaceae may have been the original food plants for Papilio, since as mentioned even those species whose caterpillars now eat Magnoliales will eat Rutaceae if they have to, and they can detoxify Rutaceae (furanocoumarins are the compounds involved) using cytochrome P450 monooxygenase (Zakharov et al. 2004, but c.f. Fordyce 2010; see also Berenbaum & Feeney 2008; Simonsen et al. 2011; Condamine et al. 2012). As with the magnoliids, e.g. Aristolochiaceae, perhaps the original food plants of Papilionidae-Papilioninae, and Magnoliaceae, it is the alkaloids in part that attract the butterflies, although overall other receptor cues picked up by the ovipositing females are very important in this association (Nishida 1995; Berenbaum et al. 1996). Ehrlich and Raven (1964) noted that P. demodocus was rather anomalous, since it fed on Ptaeroxylon, which then was included in Meliaceae, but since it is now placed in Rutaceae, all is right with the world. Apiaceae (q.v.) are another group with furanocoumarins, and caterpillars of some species of Papilio eat them, too. For more on swallowtails and Rutaceae, see papers in Scriber et al. (1995), and for more on swallowtails in general, see Aristolochiaceae.
Isobutyl glucosinolate is reported from Luvunga scandens, from Thailand (Sirinut et al. 2017); this should be confirmed.
Genes & Genomes. For the evolution of chromosome numbers in the family, an endeavour that should be reworked, see Stace et al. (1993).
There has been extensive hybridization in Citrinae. Apomixis (nucellar polyembryony) has evolved more than once, and there are also reversals to full sexual reproduction. Apomixis and the associated lack of recombination can lead to the accumulation of deleterious mutations, Muller's ratchet, although a mere 5% of sexual reproduction counteracts these deleterious genetic effects - and indeed apomixis is often not obligate. Furthermore, if apomixis is obligate, the number of generations of this obligate apomixis affects whether or not there is recovery of population fitness when sexual reproduction resumes (e.g. Hodac et al. 2019: Ranunculus; N. Wang et al. 2022a).
There is mitochondrial heteroplasmy associated with unexpected paternal leakage, the chondrome can show extensive structural variation, and there is also cytoplasmic male sterility in mandarins in particular (Wang et al. 2022b).
Economic Importance. For the origin of limes, lemons, etc., probably in the foothills of the Himalayas in the late Miocene, see G. A. Wu et al. (2014, 2018) and Curk et al. (2016), and for papers on various aspects of the genus Citrus, see Talon et al. (2020). The citrus greening disease is a major threat to growers of Citrus fruits, and in Florida an introduced psyllid, Diaphorina citri, something of a pest in its own right, transmits the gram negative gracilicute Candidatus Liberibacter americanus that lives in the phloem. This bacterium is devastating the Citrus industry there, and it has also spread from Florida; other "species" of Liberibacter attack Citrus elsewhere in the world (Gutierrez & Ponti 2013; Wikipedia 19.xii.2020). See also papers in Tropical Plant Pathology 45(3). 2020.
Chemistry, Morphology, etc.. For secondary metabolites, see Hegnauer (1971) and Kubitzki et al. (2011). Da Silva et al. (1988) surveyed the distribution of some secondary metabolites, suggesting that an overhaul of the infrafamilial classification was in order. Adsersen et al. (2007) noted the value of prenylated acetophenones as a marker for Xanthoxyleae (inc. Melicope, etc.), and Braga et al. (2012) the distinctive dihydrocinnamic acid derivates common in Rutoideae.
Cruz et al. (2015, see also 2017) describe the development of a hood-shaped leaf base in Metrodorea from initially paired primordia and characterise it as stipular in nature; it is vascularized, and leaflets may arise directly from it (Kaastra 1977). Prickles of Zanthoxylum can be in the stipular position, and they are initiated in the phloic region of the vascular tissue (Reynel 2017), so they are spines s. str.. Orixia japonica has very odd leaf insertion, in which two leaves separated by internodes of ordinary length appear to arise from the same side of the stem, followed by a pair of leaves that arise from the other, and so on - another way of describing this pattern is in terms of the divergence angles of successive leaves, i.e. 180o, 90 o, -180o, -90o, etc. - orixate phyllotaxis (Yonekura et al. 2019). Unfortunately, the stem anatomy of this plant appears not to have been studied. Pulvini s.l. are to be found in a number of Rutaceae, there septate fibres lacking lignin and gelatinous fibres are to be found surrounding the phloem, while elsewhere in the petiole the perivascular fibres are completely lignified (Ferreira et al. 2022). Whether or not these pulvini are involved in leaf movement is largely unknown. In the middle of the petiole the vascular tissue forms an annular bundle, interruptedly so in Ruta graveolens, sometimes with medullary (Conchocarpus macrophyllus) or wing (Citrus x Limonia) bundles (Ferreira et al. 2022).
The cavities in the secretory glands found in the fruit of Citrus grandis are described as forming by schizolysigeny, as by Bai et al. (2020), and programmed cell death was involved in their formation, chromatin degradation and nuclear rupture of the cells occurring. Machado et al. (2016) looked at glands in Metrodorea nigra (Zanthoxyloideae), and here the inner epithelial cells peeled away releasing oil bodies into the gland lumen, and these cells were replaced by cells from the parenchyma layer that surrounds the oil gland - schizolysigeny was involved here as well. H. Wang et al. (2024) found that there were neither marginal leaf glands nor were the leaf margins crenate, linked features (the crenations are associated with glands), in a cultivar (Hua Pi) of kumquat, Fortunella (= Citrus) crassifolia - interestingly, leaflets of this glandless mutant, but not those of the wild type, were happily eaten by caterpillars of the noctuid tobacco cutworm, Spodoptera litura. Wang et al. (2024: esp. Figs 4B, G, S16A) followed details of the development of these glands; initially epidermal, they became more deep seated and sheath and epithelial cells were evident (schizogeny?), and although there seems to have been lysigeny in older glands this was not specifically mentioned. CsMYC5 promoted both cavity formation and the expression of genes involved in essential oil biosynthesis here (Wang et al. 2024). For more on oil glands, see elsewhere.
Rutaceae are particularly variable in flower and fruit (Boesewinkel 1980b). Thus The flowers of Galipeinae (the Angostura alliance of Kubitzki et al. 2011), to which Erythrochiton (but not the tube-forming Correa) belongs, may have radially symmetrical to monosymmetric flowers, the latter obliquely symmetrical or not, with a lip or not; the corolla postgenitally connate by hairs or papillae, filaments connate and forming a tube, or a tube formed by the serial adnation of filaments and petals; five, three or only two stamens plus three to five staminodes, A/staminodes opposite C described as corolline appendages ("apêndices petalares" - Pirani et al. 201: p. 314); disc vascularized; gynoecium fluted, postgenitally connate apically, style ± impressed, stigma capitate to lobed; ovule variously oriented, outer integument 4-6 cells and inner integument 2-4 cells across (or only one integument), a nucellar beak or not, an obturator or not, the chalazal zone massive, etc. (Pirani & Menezes 2007; Pirani et al. 2010; El Ottra et al. 2011, 2013, esp. 2019; Bruniera et al. 2015)... See also below.
Erythrochiton hypophyllanthus has epiphyllous inflorescences on the abaxial side of the leaf (c.f. sometimes in Ruscus). Peltostigma has a floral formula K3 C3 A9 G [?5], and looks almost lauraceous; Pilocarpus has an erect raceme and the calyx is reduced to a rim. Monosymmetry is scattered in the family, occurring in Dictamnus and Erythrochiton, for example. Kallunki (1992) illustrates the flowers of Erythrochiton fallax as having the median sepal adaxial, but their exact orientation and how they are held in nature is unclear since the inflorescence can be pendulous and up to 1.5 m long. Wei et al. (2011) thought that the plesiomorphic condition for Rutaceae was to have five stamens.
Triphasia has three carpels, the odd member being adaxial, and the same is true of Cneorum tricoccon, which has 3-merous flowers (see Caris et al. 2006b for floral development). Carpel (stylar) fusion may be postgenital (Gut 1966). Ovule development is notably variable (Mauritzon 1935b: Cneoroideae not included; Desai 1962a). Although anatropous ovules are common, various degrees of hemitropy and campylotropy occur, and of two ovules in a loculus, one may be apotropous and the other epitropous (Mauritzon 1935b), as in some other Sapindales. The micropyle can be exo-, endo-, or bistomal-zigzag or the ovule apex may even be naked, and in bitegmic taxa, either integument may be slightly thicker than the other (e.g. Corner 1976). However, Mauritzon (1935b) suggested that the outer integument is often thicker - 3-10 cells across (outer) versus 2-4 cells across (inner), and the outer integument is sometimes (Aegle) multiplicative. Nucellar cells above the embryo sac may be in series, and nucellar polyembryony is quite widespread (e.g. Mauritzon 1935b; Mahabalé & Chennaveeraiah 1958). The embryo sac can be relatively quite small relative to a massive nucellus, and in Aegle there is a layer of crystalliferous cells below the exotegmen and 2-3 layers of such cells below the endotegmen (Mahabalé & Chennaveeraiah 1958). Desai (1962a) drew the ovule of Coelonema as having three integuments, the two outer each having but a single layer of anticlinally elongated cells but the middle integument with a massive excentric-apical cap-like structure that he compared with an obturator- remarkable, but confirmation is needed. The endocarp divides periclinally during development (Hartl 1957) resulting in a pronounced layering of the mature capsule, especially in Rutoideae.
For general information, see van Wyk (in Dahlgren & van Wyk 1988: Ptaeroxylaceae), van der Ham et al. (1995), White and Styles (1966), Reynel (2017: Zanthoxylum, and especially Kubitzki et al. (2011), Appelhans et al. (2021: esp. subfamilies, data matrix) and Groppo et al. (2022: (South) American taxa), also Straka (1976), Dahlgren and van Wyk (1988), van der Ham et al. (1995) and White and Styles (1966) for Cneoroideae. For chemistry, see also Hegnauer (1973, 1990, also 1964, 1989 as Cneoraceae), Straka et al. (1976), Waterman and Grundon (1983), da Silva and Gottlieb (1987: limonoids), Mulholland et al. (2000, esp. Ptaeroxylaceae), Yan et al. (2011: Harrisonia in particular) and da Silva et al. (2021), and for alkaloids, Fish and Waterman (1973: esp. Zanthoxylum) and Waterman (1975, 1999). For wood anatomy of Cneoroideae, see Appelhans et al. (2012b: phylogenetic signal within the subfamily), for colleters, see Macêdo et al. (2016), for floral development, Zhou et al. (2002) and Wei et al. (2011), for floral orientation, see Eichler (1878), for pollen, see Morton and Kallunki (1993: Cuspariinae), for gynoecium/perianth of Zanthoxylum, see Beurton (1994), for gynoecial morphology, see Gut (1966), Endress et al. (1983), and Lersten (2004), for ovules of Harrisonia, see Wiger (1935; see also Mauritzon 1935; Wiger 1936), for ovules and testa, see Honsell (1954), Banerjee and Pal (1958), Johri and Ahuja (1957), Boesewinkel (1977, 1978), and Boesewinkel and Bouman (1978: see raphal vascular tissue), and for fruit anatomy, see Brückner (1991). For the chalazal opening (?vascular bundle) in the seed, see Wilson (1998) and Hartley (2003); for seed anatomy in Rutoideae, see Gallet (1913).
Phylogeny. In a two-gene analysis, the [[Spathelia + Dictyoloma] [[Cneorum + Ptaeroxylum] Harrisonia]] clade (= Cneoroideae) was sister to all other Rutaceae (Chase et al. 1999), although the position of Harrisonia - sequences from only one gene - was somewhat unclear (see also Groppo et al. 2008, 2012). Morton (2015) found Cneoroideae to be paraphyletic and basal to the rest of the family, although it was not the focus of her study. Recent work suggests that the basic relationships in the rest of the family are [Zanthoxyloideae [Aurantioideae + Rutoideae]] (Groppo et al. 2012; Morton & Telmer 2014; Muellner-Riehl et al. 2016). For general relationships in the family, see also M. Sun et al. (2016) and Z.-D. Chen et al. (2016: Chinese taxa, quite good support). [Aurantioideae [Chloroxylon, Boenninghausenia, Ruta, etc. (Ruteae plus!, = Rutoideae)]] form a poorly to well-supported clade (e.g. Morton et al. 2003; Groppo et al. 2008, 2012; Salvo et al. 2010; Appelhans et al. 2012a; Bayly et al. 2013b; Morton & Telmer 2014; Appelhans et al. 2016). Joyce et al. (2023) found strong support for the paraphyly of Cneoroideae which broke up into two clades successively sister to the rest of the family: [Harrisonia [Cneorum [Bottegoa [Cedrelopsis + Ptaeroxylon]]], Cneoroideae s. str., and [Dictyoloma [Sohnreyia + Spathelia]], Spathelioideae.
Within the old Cneoroideae Spathelia (chromones) and Dictyoloma (C valvate) are a strong genus pair (in Appelhans et al. 2011 Dictyoloma is embedded in Spathelia); secretory cavities are reported from them (Groppo et al. 2008). Jadin (1901) had noted that anatomically Harrisonia was rather different from other Simaroubaceae (in which it was then placed) in its heterogeneous pith and its lack of medullary secretory canals. Although it does not seem to have pellucid foliar gland dots, Fernando and Quinn (1992) found secretory cavities in the fruits while Waterman (1993) noted that the genus contained no quassinoids, which are unique to Simaroubaceae. Fernando et al. (1995) suggested that its removal to Rutaceae was justified on both molecular and morphological grounds. Razafimandimbison et al. (2010) found a weakly/moderately supported clade that included the old Ptaeroxylaceae and in which [Spathelia + Dictyoloma] were sister to the rest. Appelhans et al. (2011: denser sampling, five chloroplast genes, 2012a) again found this basic topology; support for the groups was strong, and within the two major clades in Cneoroideae, both strongly geographically circumscribed, in one Sohnreyia was sister to Neotropical taxa and in the other Harrisonia was sister to palaeotropical taxa (Appelhans et al. 2012a).
Rutoideae. For the circumscription and contents of Rutoideae, now a rather small subfamily, see Appelhans et al. (2016, 2021); Chloroxylon, ex Flindersioideae and a tree, is sister to the other four genera.
Haplophylloideae. Salvo et al. (2011) and Manafzadeh et al. (2011) discussed relationships in Haplophyllum; Townsend (1986) monographed the genus.
Aurantioideae. For general relationships, see Pfeil and Crisp (2008) and Bayer et al. (2009). Appelhans et al. (2016) suggested that Haplophyllum and Cneoridium, ex Ruteae, are at the base of Aurantioideae, which rather changes the apomorphies for that group, however, those two groups are now in separate subfamilies, Haplophylloideae and Amyridoideae (Appelhans et al. 2021, see above). Clauseneae may not be monophyletic (Morton 2009, 2015); Glycosmis and/or Micromelum may be sister to other Aurantioideae (Groppo et al. 2012; Schwartz et al. 2015; Shivakumar et al. 2017: ?sampling, rooting; Appelhans et al. 2021), and both have (1-)2 ovules/carpel, similar chromosome numbers (Mou & Zhang 2012) and see characterization above - perhaps best put in a separate tribe when phylogeny is firmed up. Murraya is very polyphyletic (Z.-D. Chen et al. 2016: Chinese taxa). For other relationships in Aurantieae/-oideae, see Morton (2009) and especially Appelhans et al. (2021), and for relationships around Citrus, see Scott et al. (2000), Samuel et al. (2001), Araújo et al. (2003), Bayer et al. (2009), Carbonell-Caballero et al. (2015: chloroplast genomes) and Schwartz et al. (2015: Feroniella not in Citrus). Citrons, some of which have quite large or "fingered" fruits, link up with the Australian Microcitrus and Eremocitrus, Fortunella links with Citrus madurensis, and Poncirus is then sister to the remainder of Citrus, or at least its maternal parent is (Carbonell-Caballero et al. 2015; see also G. A. Wu et al. 2018: esp. nuclear data); relationships in this area are complex, with much hybridization, introgression, apomixis, etc. - see also Genes & Genomes above. Different relationships within Citrus are suggested by analyses of chloroplast, mitochondrial and nuclear data (N. Wang et al. 2022b: c.f. in part p. 3, Fig. 2A-C).
Other genera in the family form a single clade, Zanthoxyloideae, within which the classical subfamilies and genera, largely based on variation in fruit morphology (Engler 1931; c.f. Hartley 1981; But et al. 2009) have not been confirmed, although Aurantioideae (above) are a partial exception (Appelhans et al. 2021). Note that as of x.2025 there is no sectional classification here. Hartley (e.g. 1981, 1997, 2001a, b) had early suggested some generic realignments in Malesian-Pacific Rutaceae that largely ignored the then-conventional subfamilies; this work has held up fairly well in molecular studies. Thus neither the large genus Melicope nor Acronychia are monophyletic (Appelhans et al. 2014b, 2021). Within Melicope Paetzold et al. (2019: 41/54 spp. examined, RAD-seq) looked at relationships in the Hawaiian clade; they found five main clades, although mostly not corresponding to the previous sectional classification, and there was evidence of hybridization. Appelhans et al. (2025) further clarified relationships here, noting that both Acronychia and Macluroderndron might be close to Melicope. For relationships within Acronychia, see also Appelhans et al. (2018b). Salvo et al. (2008, also 2010; Groppo et al. 2008, 2012) found that Dictamnus was widely separate from the other members that had been included in Ruteae, rather, it linked with Casimoroa and Skimmia (see also Morton & Telmer 2014; Morton 2015). Other Rutaceae not included in the old subfamilies formed a clade [Dictamnus et al. [[Pilocarpus + Ravenia] The Rest]]], i.e. most of Zanthoxyloideae (see Poon et al. 2007; Groppo et al. 2008, 2012; Salvo et al. 2010; Morton & Telmer 2014) or were part of a polytomy including them (Bayly et al. 2013b; Morton 2015). One large clade is mostly Old World-Oceanian in distribution, although it includes the Chilean Pitavia (Groppo et al. 2012; in clade C4d: Appelhans et al. 2021). Flindersia (clade C4c) and relatives have secretory cells in the stem only and septifragal capsules that are perhaps reminiscent of Meliaceae, but their furoquinoline alkaloids, schizogenous cavities, and subterete filaments are consistent with a position in Rutaceae. Euodia and relatives form another moderately to well supported clade (Salvo et al. 2010; Groppo et al. 2012) perhaps sister to a clade including most of the Australian taxa that were included in the old Boronieae (Bayly et al. 2013b; Morton 2015; clade C4f, Appelhans et al. 2021), and a [Zanthoxylum + Toddalia] clade (Groppo et al. 2012: support poor), in turn sister to the Flindersia group (Bayly et al. 2013b); see also Appelhans et al. (2021) for relationships around here, Zanthoxylum is paraphyletic, and includes Toddalia and Fagara. Indeed, for relationships in Protorutaceae, largely composed of Zanthoxylum, see Appelhans et al. (2018a, 2021). Reichelt et al. (2021: 354 nuclear genes) looked at relationships in 44 species of Zanthoxylum and found that although some sections of the genus recognised by Reynal (2017) were not monophyletic, there were four main clades made up of species from Africa, Asia-the Pacific-Australia, America-east Asia, and Z. asiaticum. Relationships between these clades were not that well supported, and hybridization (the Hawaiian species) and incomplete lineage sorting seemed to be confusing things (Reichelt et al. 2021). Ang et al. (2025) used nuclear data to examine relationships here, 122/233 species being included; there was a certain amount of hybridization and incomplete lineage sorting in parts of the tree, and support in concatenation was stronger than in coalescence analyses. Duretto et al. (2020) looked at relationships around Boronia and found that the genus was polyphyletic (see also Bayly et al. 2015; Appelhans et al. 2021), so some species have been placed in Cyanothamnus. Orel et al. (2023: plastomes of 60 spp., esp. Philotheca) and Duretto et al. (2023: esp. Leionema and Phlebalium, 3 chloroplast 2 nuclear markers, 128 spp., 18 genera) looked at relationships in the Eriostemon clade with its 16 genera and 209 species often growing in sclerophyllous vegetation; this group used to be in Boronieae. Orel et al. (2023) found Philotheca to be extensively para- and polyphyletic, there was a major polytomy that precluded further progress and there was cytonuclear discordance between phylogenies based on plastid and nuclear ribosomal DNAs; the age of Myrtopsis, only in New Caledonia, was uncertain. Duretto et al. (2023) did not find polyphyly in the genera on which they focused, and overall Halfordia and Neoschmidea were successively sister to the rest of the clade. Duretto et al. (2025: Angiosperms353 universal bait kit, 25/54 spp. Philotheca, 14/16 genera from this area) returned to the Philotheca problem, and despite the extensive cytonuclear discordance (see e.g. their Fig. 5) that they found they were able to clarify generic limits and relationships, and they provide a key to the 19 genera that they recognize in the Eriostemon group. Another clade (see e.g. Bayley et al. 2013b) includes the North American Ptelea, a largely African Diosmeae (for relationships, see Trinder-Smith et al. 2007, Agothosma is polyphyletic, see also Appelhans et al. 2021), and a largely Central and South American Galipeinae (for relationships, see Kallunki & Groppo 2007: Bruniera et al. 2015). However, support for some of these groups, and of relationships between and within them, was still rather weak (Groppo et al. 2012; Bayly et al. 2013b; Morton & Telmer 2014). For relationships in the largely Australian Zieria, see Morton (2015) and Barrett et al. (2015); the latter found that the single New Caledonian species, Z. chevalieri, was sister to the rest of the genus, that about one quarter of the morphological species appeared to be other than monophyletic, and that relationships suggested by cpDNA were incongruent with those based on morphology. Barrett et al. (2018) found major incongruences between relationships based on cpDNA and those based on nrDNA. For relationships in Clauseneae, see Shivakumar et al. (2016).
Classification. Although Cneoroideae (also called Spathelioideae in some recent literature) form a fairly distinct group, inclusion within Rutaceae s.l. is reasonable (Groppo et al. 2008, 2012; Appelhans et al. 2011, A.P.G. 201); for a tribal classification of Cneoroideae, see Appelhans et al. (2011). It was evident that tribal and subfamilial limits for the most part needed overhauling (e.g. Salvo et al. 2008; Poon et al. 2008). Groppo et al. (2012) recognised only two subfamilies, Rutoideae being split into tribes, but the clades are largely the same as those above (see also Morton & Telmer 2014; Muellner-Riehl et al. 2016). However, Appelhans et al. (2021) have recently provided a subfamilial classification of the family that is followed here. They noted that although some clades in the large subfamily Zanthoxyloideae could perhaps be recognized as tribes, relationships along part of the backbone of that subfamily were unclear, so it was premature to do this (Dictamneae are recognized above since they are strongly supported as being sister to the rest of the subfamily; the next "clade" up, Clade C2 + C3, could be broken up into eight or so tribes...).
Kubitzki et al. (2011) noted that a quarter of the genera in the family are monotypic - for instance, there are ten monotypic genera within the small subtribe Galipeinae (Bruniera et al. 2015). Beyond this, generic limits are difficult, especially around Citrus (Carbonell-Caballero et al. 2015 and references), also in Galipeinae (Kallunki & Groppo 2007), Diosmeae (Trinder-Smith et al. 2007) and around Melicope (Appelhans et al. 2014b); the necessary nomenclatural changes are gradually being made. Thus Duretto et al. (2023) reduced two small genera to synonymy and provided infrageneric classifications for some genera in the Leionema-Phlebalium area while Orel et al. (2025) modified the limits of genera in the Phebalium-Philotheca group, describing two as new - Orel et al. (2025) noted problems with generic delimitation in the family especially if plastid data alone were to be used. Appelhans et al. (2025) broaden the limits of Melicope and place the species in the genus in 7 sections. Ang et al. (2025) review the infrageneric classifications of Zanthoylum and propose the beginning of a new one.
Previous Relationships. Cronquist (1981) placed Cneoraceae in his Sapindales which included Rutales more or less as above and then some; Airy Shaw (1966) associated Kirkia with Ptaeroxylaceae, but with hesitation. Hegnauer (1990) included Ptaeroxylum in Meliaceae, although he noted it was chemically more similar to Rutaceae. Thorne (1992) included Harrisonia (ex Simaroubaceae), in Rutaceae, although he gave no reasons for this. Savolainen et al. (2000b) suggested that Lissocarpaceae should be included here, but a position in Ericales-Ebenaceae is now strongly supported (e.g. Berry et al. 2001).
Botanical Trivia. Ehrlich and Raven (1964) predicted, based on the caterpillars that ate it, that Ptaeroxylon would be found to have alkaloids - it has (e.g. Muscarella et al. 2008).