EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
[DILLENIALES [SAXIFRAGALES + ROSIDS]]: stipules + [usually apparently inserted on the stem].
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
Age. Moore et al. (2010: 95% HPD) suggest ages of (104-)102(-97) Ma for crown-group malvids, Xue et al. (2012) an age of around 84 Ma, while about 103.2 Ma is the age in Naumann et al. (2013), ca 118.3. Ma in Hohmann et al. (2015: note topology) and ca 121 Ma in Foster et al. (2016a: q.v. for details).
Evolution: Divergence & Distribution. Hengchang Wang et al. (2009: penalized likelihood dates) suggested that rapid radiation within Malvidae occurred (113-)107-83(-76) Ma.
Genes & Genomes. The chloroplast infA was found to be lost lost in members of this clade (Bauscher et al. 2006: no members of Geraniales or COM clade included).
Phylogeny. See the Saxifragales pages for further discussion on the relationships of Geraniales and Myrtales. See the Dilleniales page for major patterns of relationships within Pentapetalae. An ancestor of the malvids may have been involved in an ancient hybridization with an ancestor of the fabids, for which, see the Zygophyllales page.
The immediate relationships of the core malvids are unclear. Using mitochondrial and chloroplast genes, Zhu et al. (2007) found that Myrtales and Geraniales were successively sister to all other rosids - but with little support. S.-B. Lee et al. (2006) found some support for the clade [Geraniales + Myrtales] sister to the rosid I clade, although sampling was poor. Jansen et al. (2007; see also Z. Wu et al. 2014; Zeng et al. 2014: suppl. Fig. 14; Z.-D. Chen et al. 2016; Logacheva & Shipunov 2017: 100% support) recovered [Myrtales + Geraniales] as sister to the rosid II/malvids, albeit with weak support. Xi et al. (2014) found that Eucalyptus was weakly supported as sister to all rosids in analyses using nuclear data (see also Zeng et al. 2014: transcriptome data), while in analyses using chloroplast data, the genus was sister to the malvids, and with strong support, however, representatives of Geraniales were not included and sampling in general was a bit sketchy (this was not the main focus of their work). There is a fair amount of variation in relationships in this area in the trees provided by Sun et al. (2014), while L. Zhao et al. (2016) consistently recovered a [Geraniales + Myrtales] clade, whether with or without Zygophyllales, as sister to all the rosids, although support was not strong. All relationships within the malvid clade below are also very strongly (100% bootstrap) supported in the chloroplast analyses of H.-T. Li et al. (2019).
[GERANIALES + MYRTALES]: ellagic acid +; K persistent in fruit.
Age. The age of this node is variously 89-83 Ma (Anderson et al. 2005), (103-)100(-97) Ma (Wikström et al. 2001); (114-)107(-100) or (90-)83(-76) Ma (Hengchang Wang et al. 2009); ca 108 Ma (Magallón & Castillo 2009: topology uncertain); (121-)115, 107(-103) or (122-)116, 108(-104) Ma (Bell et al. 2010: topology uncertain); (99-)91(-77) Ma (N. Zhang et al. 2012), or about 79 Ma (Xue et al. 2012). Ca 123 and 88.2 Ma are ages suggested by Sytsma et al. (2004 and 2014 respectively), (124.8-)123.6(-122)Ma by Berger et al. (2015), around 92.5 Ma by Naumann et al. (2013) and (118.6-)111.6(-95.5) Ma (X.-F. Zhang et al. 2021).
GERANIALES Berchtold & J. Presl - Main Tree.
Vessel elements with simple perforation plates; nodes also 3<:3<; lamina margins gland-toothed; inflorescence cymose; C contorted; nectary outside A, opposite K; A obdiplostemonous; G opposite C, ?style, stigma dry; outer integument [largely dermal in origin], 2-3 cells across, inner integument 2-3 cells across; fruit capsular, ?dehiscence; seed testal. - 2 families, 17 genera, 742 species.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Geraniales can be dated to 86-80 Ma (Anderson et al. 2005); other estimates are provided by Wikström et al. (2001), (98-)94, 88(-84) Ma, Hengchang Wang et al. (2009), ca 106.5 Ma, and Hohmann et al. (2015), (109-)103(-97), (74-)68(-62) My (a muddle here -sorry), Bell et al. (2010), (106-)93, 87(-74) Ma, and Park et al. (2015a) ca 95.4 Ma, while ca 104 Ma is the estimate in Sytsma et al. (2004). Palazzesi et al. (2012) suggested just under 50 Ma, but c.f. Sytsma et al. (2014) who reanalyzed the same data and came up with an age of 89-76 Ma, while around 109.5 Ma is the estimate in Tank et al. (2015: Table S2), which is around the common age for the order - see also ca 101.9 Ma in van de Kerke et al. (2019).
Evolution: Divergence & Distribution. Geraniales are poorly understood, and although a smallish group, they are morphologically quite heterogeneous, which, coupled with lingering uncertainty over their exact phylogenetic position, makes thinking of apomorphies difficult (Kubitzki 2006a). Palazzesi et al. (2012) discuss the evolution of the whole group in some detail, but Sytsma et al. (2014) should be consulted fot a reanalysis of their data, which yielded a number of different and mostly significantly older dates; adding rosid stem priors was a major cause of the differences. Jeiter et al. (2017a) discuss the evolution of nectaries in Geraniales - they vary considerably in morphology.
Genes & Genomes. Details of the distribution of the chloroplast rpl16 intron in the order are unclear (see Geranioideae: c.f. Downie & Palmer 1992b).
Chemistry, Morphology, etc.. Bortenschlager (1967) provides a comprehensive pollen survey of Geraniaceae in the old sense, i.e. including things like Dirachmaceae (Rosales), also Vivianiaceae which, although no longer in Geraniaceae, at least are still in Geraniales. For references to obdiplostemony, see Ronse De Craene and Bull-Hereñu (2016).
Phylogeny. Savolainen et al. (2000a) found that the order was monophyletic, but with only 52% support (see also Savolainen et al. 2000b). The tree prior to Oct. 2012, but names as used here - [Geraniaceae [[Bersameae + Francoeae] [Vivianieae + Ledocarpeae]]] - was based on these and other studies, although sampling was inconsistent, etc.; Bersameae included Greyia. However, the clade [Greyieae + Francoeae] has been strongly supported (Morgan & Soltis 1993; Price & Palmer 1993; Soltis & Soltis 1997), but Soltis et al. (2007a) found the set of relationships [Geraniaceae [Viviania [Bersama [Francoa + Greyia]]]] (see also Bell et al. 2010). Palazzesi et al. (2012: trnL-F, ITS) on the other hand, recovered a tree [Geraniaceae [Bersameae [Vivianieae [Francoeae + Greyieae]]]], with support for all clades strong except that for the [Vivianieae [Francoeae + Greyieae]] clade, although the chronogram there is based on a tree with the topology [Geraniaceae [Vivianieae [Bersameae [Francoeae + Greyieae]]]].
However, the genome tree associated with W. J. Baker et al. (2021: see Seed Plant Tree) suggests that Francoaceae may be falling to bits. [[Geraniaceae + Francoa] [Viviania [Crossosomatales ...]] are successive sisters to a large clade containing Zygophyllales, Myrtales, Malpighiales, the N-fixing clade, and so on. They looked at only two members of the old Francoaceae, but this is clearly another place to keep an eye on. That there may be problems here can be seen in the two trees recovered by X. Wang et al. (2021), one, a protein-coding tree, shows fairly conventional relationships, the other, based on whole chloroplast genomes, shows the relationships [Monsonia [[Hypseocharis + Erodium carvifolium] [[more Erodium] [[Monsonia + Pelargonium transvaalense] [more Pelargonium]]]]].
Classification. Geraniaceae and Francoaceae-Vivianieae have a layer of small hypodermal druse-containing cells in the calyx (Kenda 1956), and Weigend (2006: 217) suggested that there might be "a close and possibly exclusive relationship between Geraniaceae and Ledocarpaceae [= Vivianieae]." He lists numerous characters suggesting such a relationship, such as that in both the basal ovules in the ovary tend to develop into seeds (although Boesewinkel 1997 mentioned that it was the upper ovules of Caesarea that developed into seeds), and their fruits are septicidal or septifragal (Boesewinkel 1997 describes them [apart from Rhyncotheca] as being loculicidal). However, the nature of the campylotropy of the ovules differs and the fruits of Rhyncotheca differ rather strongly from the superficially similar fruits of Geraniaceae (although both do have a column), etc.. Nevertheless, there are a number of similarities between the two.
Previous Relationships. None of the nine geranialean families studied by Oltmann (1971: table on p. 145) is still included in Geraniales; Geraniales were previously a very heterogeneous group! The circumscription of the clade adopted here is somewhat unexpected, largely because Geraniaceae and Oxalidaceae have previously been considered very close (e.g. Cronquist 1981). Indeed, although Cronquist included only five families in his Geraniales, they are here placed in four orders in both asterids and rosids. Geraniaceae in particular included genera now widely dispersed in the rosids, but it has not previously been associated with a number of taxa included in Francoaceae below.
Includes Francoaceae, Geraniaceae.
Synonymy: Francoales Martius, Greyiales Takhtajan, Ledocarpales Doweld, Melianthales Doweld - Geranianae Reveal - Geraniopsida Meisner
GERANIACEAE Jussieu, nom. cons. - Back to Geraniales
Herbs; hydrolysable tannins +; leaves spiral, pinnate, leaflets not articulated, secondary veins [inc. on leaflets] often palmate; inflorescence cymose, often pseudoumbellate; nectaries vascularized; pollen ?often starchy; ovules often campylotropous, micropyle zig-zag; K persistent in fruit; endotesta palisade, crystalliferous, much thickened, with light line, unlignified, exotegmen palisade, lignified, anticlinal walls sinuous; x = 11 (?10, ?12); nuclear genome [1 C] (0.035-)0.989(-27.655) pg; 4 kb inversion in plastome, reduced single copy region.
6/743: [list], 2 groups below. Temperate and warm temperate. Photos: Collection.
Age. Palazzesi et al. (2012) opined that Hypseocharis diverged from the rest of the family (42.8-)36.9(-5.7) Ma, although a reworked estimate is (72-)62(-52) Ma (Sytsma et al. 2014), while Fiz-Palacios et al. (2008) suggested an age of 55 Ma, Park et al. (2015a) an age of ca 51 Ma, van de Kerke et al. (2019) an age of ca 35.8 Ma and Sytsma et al. (2004) an age of around 85 Ma for this split.
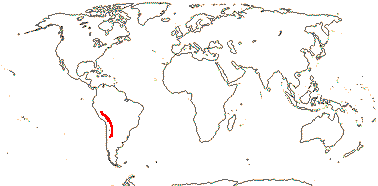
1. Hypseocharis Remy —— Synonymy: Hypseocharitaceae Sweet
Perennial ± acaulescent herbs, tuberous, or thick taproots; anatomy?; stipules 0; A 5, 15 [10 in pairs opp. C], ca 12 ovules/carpel, style filiform, stigma capitate, ?surface; fruit a loculicidal capsule, (septicidal, almost with mericarps - H. tridentata), filaments also persistent; endosperm scanty, embryo cochlear, cotyledons spirally twisted; n = ?
1/1-3. S.W. Andean South America. Map: from Slanis and Grau (2001).
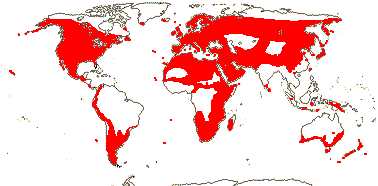
2. Geranieae Arnott —— Synonymy: Erodiaceae Horaninow
(Shrubs; stem succulents, geophytes); (CAM photosynthesis + - Pel., Mon.); plant often aromatic, (petroselenic acid + - Ger.); root cortical phi [φ] cell wall thickenings hypodermal; young stem with separate vascular bundles; (vessel elements with scalariform perforation plates); wood often rayless; ?nodes; stem often jointed; petiole bundles annular (medullary bundles +); hairs glandular; cuticle waxes 0 (rodlets); (stomata anisocytic); leaves (opposite), also simple and lobed, lamina vernation conduplicate-plicate, (petiolar spines + - Mon.), stipules 2, often well developed, interpetiolar or cauline, colleters +; (inflorescence subumbellate); (flowers monosymmetric - Pel.); K quincuncial [Ger.], aristate; (anthophore [prolongation between K and the rest of the flower] +); C (0-4), often salverform, (long-fringed); nectary opposite K, (?opposite C - Mon.), (adaxial nectary only, receptacular, forming tube - Pel.); initially common A-C primordia [Ger.]; A 5/2-7 [Pel.]/10/15 [10 in pairs opp. C], (antepetalous whorl staminodial), filaments ± connate basally; (tapetum amoeboid); pollen grains (tricellular), (tricolpate - Mon.), surface baculate/clavate/gemmate or rugulose-(interwoven-)striate, 40-140 µm long; G [(2-)5], true style short, stout, hollow [?always], stigma lobed; ovules 1-2/carpel, apical, apotropous, campylotropous by development of cells of the inner integument, (micropyle exostomal, parietal tissue ca 4 cells across, (nucellar cap +); embryo sac invades and obliterates apical nucellar tissue; fruit septicidal, upper part of ovary elongating [= "stylar" beak, awn], mericarps curl upwards and separate from columella, seed/"achene"/mericarp dispersal unit; exotestal cells undistinguished, stellate, stomata +, (endotegmen slightly lignified); endosperm 0 (some), suspensor pluriseriate, embryo chlorophyllous [Ger.], curved, cotyledons accumbent, longitudinally folded (incumbent, flat - Pel.), radicle ± as long as rest of embryo; n = 4, 7-14, etc; plastid transmission biparental, inversions in plastome, (ndh genes 0), trnT-GGU gene loss, group II intron between nad1 b/c exons, rpl16 and rps16 intron loss, etc.; sporophytic incompatibility system present.
5/742: Geranium (307), Pelargonium (280), Erodium (80), Monsonia (40: inc. Sarcocaulon). Temperate and warm temperate, esp. southern Africa (map: from van Steenis & van Balgooy 1966; Hult én 1971; Wickens 1976; Meusel et al. 1978; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Aedo et al. 2005).
Age. The crown group age of Geranioideae is estimated at (71-)54, 48(-33) Ma (Bell et al. 2010) or some (50-)48, 37(-34) Ma (Wikström et al. 2001); Palazzesi et al. (2012) suggest an age of slightly less than 30 Ma and Park et al. (2015a) an age of ca 35 Ma, but (57-)48(-39) Ma is the figure in Sytsma et al. (2014), see also 47-38 Ma in Fiz et al. (2008), ca 39.5 Ma in Marcussen and Meseguer (2017) and (45.1-)35.8(-29.5 Ma in van de Kerke (2019), but as much as ca 67.8 Ma in Hohmann et al. (2015), similar to Sytsma et al. (2004).
Evolution: Divergence & Distribution. Diversification of Pelargonium (and Monsonia), some 155 species of which are succulents (Nyffeler & Eggli 2010b) in South Africa seems to have occurred ca 30-10 Ma as aridification set in, while diversification of the Geranium-Erodium clade occurred at roughly the same time in Eurasia and the Mediterranean, perhaps in response to climate change and mountain uplift there (Fiz et al. 2008); however, Fiz-Palacios et al. (2010) suggest that the ancestral area of Erodium was Asia, where it arose ca 18 Ma, while Marcussen and Meseguer (2017) think that diversification of Geranium itself began in the Mediterranean area. Palazzesi et al. (2012) in general offer younger estimates for splits within Geranioideae, but c.f. Sytsma et al. (2014) for a reanalysis of their data and van de Kerke et al. (2019: Fig. 3) for very different dates possible in the Pelargonium region in particular.
The phylogeny and diversification of Pelargonium in South Africa, which has about 150 species in the Cape Floristic Region alone (Linder 2003), has been much discussed (e.g. Struck 1997; Bakker et al. 1999, 2000, 2004, 2005; Röschenbleck et al. 2014; van de Kerke et al. 2019); all told, some 222 of the 280-300 species of Pelargonium are restricted to southern Africa (ca 290 species for the family as a whole there - Johnson 2010; see also Albers & Becker 2010 for a summary). There is striking vegetative variation, almost 100 species being geophytes (Procheŝ et al. 2006), and leaf shape and scent is notably variable, C. S. Jones et al. (2009) and Nicotra et al. (2007) discussing foliar evolution i.a. in the context of temperature and photosynthetic rates (see below for photosynthesis). Floral variation in the genus is also striking, P. apetalum lacking petals, other species having almost polysymmetric flowers, there is great variation in the colour and margins of the petals, the nectar tube is 1.5> mm - 10 cm, and so on (e.g. van de Kerke et al. 2020, see also Pollination Biology & Seed Dispersal below). Divergence of the Cape fynbos clades of Pelargonium may have begun some (13.6-)10.6(-3.7) Ma, diversification in the Succulent Karoo starting a little earlier (Verboom et al. 2009); the first split in the genus as a whole is estimated to be a mere (10.5-)9.7(-9.0) Ma by van de Kerke et al. (2019), with further diversification not beginning for another million years. There are a number of substantial disjunctions between the Cape and the East African mountains, and Pelargonium also grows in Asia Minor (Bakker et al. 2005; van de Kerke et al. 2019); and the disjunction P. karooicum (Cape) - P. caylae (Madagascar) - P. endlicherianum (Asia Minor) has been dated to ca 5 Ma (van de Kerke et al. 2019).
Ecology & Physiology. For photosynthetic rates in the numerous South African species of Pelargonium, of which most grow in the winter rainfall zone, see Nicotra et al. 2008); the species with more dissected leaf blades tend to have higher rates of carbon gain and of water loss that those with less dissected blades. If water stress is extreme, CAM cycling can occur in some species (C. S. Jones et al. 2003); for CAM in Geranium and Monsonia, see Gilman et al. (2023).
Geranium viscosissimum has glandular hairs and may be protocarnivorous, being able to digest proteins (?source of enzymes) and take up some of the products (Spomer 1999); the ability of plants with such hairs to digest at least some proteins is quite widespread.
Pollination Biology & Seed Dispersal. Some southwest African Geraniaceae are pollinated by three-five species of (extremely) long-tongued dipteran Nemestrinidae flies, Prosoeca, perhaps 25% of the species being so pollinated (Manning & Golblatt 1996, 1997; Goldblatt & Manning 2000; Pauw et al. 2020: pollinator probiscis and nectary tube lengths do not always match). Overall, Pelargonium in southern Africa is pollinated by a diversity of visitors (see esp. Struck 1997, c.f. groupings there with Röschenbleck et al. 2014). Thinking about pollination, etc., Van de Kerke et al. (2020) carried out geometric morphometric analyses of floral shape in virtual 3D for a number of species; despite the great variation that they captured, they were unable to include some of the more "extreme" species in their analyses. The two adaxial petals of Pelargonium, not the abaxial petal(s), may bear markings and be the flag petals, and although the latter is the common condition in flowering plants, the single adaxial petal of flowers that are presented inverted may be the flag petal (Bukhari et al. 2017). The single adaxial receptacular nectary forms a tube sometimes running the entire length of the (quite long) pedicel (Tsai 2016). Jeiter et al. (2017b) suggest that it forms because of intercalary receptacular development immediately below the calyx, but since this does not occur in the area of the nectary the latter comes to sit in the tube. The length of the tube in two species compared depended on the rate and especially duration of this intercalary growth (Tsai et al. 2018). (For nectaries, see also Vogel 1998c and Jeiter et al. 2017a.) McDonald and Van der Walt (1992) looked at species in the P. tricolor group, and found that the dark warty areas on the adaxial petals were rich in anthocyanidins and guided the behaviour of the long-tongued fly pollinators - they were effectively pseudonectaries. Darwin (1859) noted that the central flower of a Pelargonium inflorescence might lose its adaxial markings and also its nectary. This would be expected of a peloric flower which has become "ventralised", i.e. the morphology of the abaxial sector of the flower extends to the adaxial sector, so the adaxial petals with markings and the adaxial nectary disappear.
A number of species of Geranium, at least, are revolver flowers, and in species like G. robertianum with a salverform corolla the flower develops in such a way that each "tube" is made up of six adjacent parts from four floral whorls - a remarkable example of synorganisation that does not involve any fusion of parts (Endress 2010d; see also Jeiter et al. 2017b). Pollination in G. thunbergii, at least, is promiscuous, bees, flies and butterflies all being involved and the particular species involved differ from year to year (Kandori 2002). There may be deceit pollination in some species of Erodium, where glistening globose hairs may mimick nectaries, or flattened hairs glisten as if they are soaked with nectar (Aldasoro et al. 2000).
Whether individual seeds of Geranioideae are catapulted or achenes or whole mericarps are the unit of dispersal, the fruit wall has a stiff outer layer and an inner layer that contracts as it dries, the awn (the upper part of the ovary) coiling and bending as a result (Abraham & Elbaum 2013). In still conditions in Erodium and some species of Geranium the whole mericarp, the seed + awn, are thrown about half a metre (initial launch velocity 4±2 m s-1), and then hygroscopic movements of the awn, twisting and untwisting as humidity decreases and increases, result in the seeds being "planted" in the soil (Stamp 1989; Evangelista et al. 2011) - c.f. some Poaceae. In other species fine hairs on the awn may aid in wind dispersal of the fruit (Abraham & Elbaum 2013). Yeo (1984) discussed fruit dehiscence in Geranium in particular, linking it to the taxonomy of the genus; see also Marcussen and Meseguer (2017). Physical dormancy of seeds is common in the family (Gama-Arachchige et al. 2010).
Plant-Animal Interactions. For trenching by insects on the leaves of Pelargonium, see Hurley and Dussourd (2015). Exudate from the glandular hairs of Pelargonium x hortorum is toxic to the caterpillars of the soybean looper, but final instar larvae exhibit vein-cutting behaviour.
Vegetative Variation. Leaf morphology is notably variable within Pelargonium (Nicotra et al. 2011), while Sarcocaulon (= Monsonia) is almost cactus-like, with fleshy, spiny stems, and the photosynthetic leaves and flowers are borne on the short shoots that the spines subtend. Pelargonim zonale is a popular and variously variegated ornamental (Baur 1909), the layers of the apical meristems differing in their ability to produce functional plastids.
Genes & Genomes. A genome duplication, the GEMAα event, ca 58.4 Ma for crown-group Geraniaceae was noted by Landis et al. (2018). Within Pelargonium A 2-7, either x = 11, chromosomes 1.5³ µm long or x = 9, chromosomes 1.5-3.0 µm long, etc. - there is a large and small chromosome clade (Röschenbleck et al. 2014; van de Kerke et al. 2019 and references).
The plastomes of Geraniaceae have been much studied, and Weng et al. (2013) and Ruhlman and Jansen (2018) provide useful summaries. Palmer et al. (1987) had early found a large expansion of the inverted repeat (IR) in the chloroplast genome of Pelargonium, and subsequent work shows remarkably extensive changes in the chloroplast genome of Geranieae (least in California), but not in Hypseocharis. The genome may greatly expand, largely because of the IR expansion, and although the IR of Pelargonium is the largest of any flowering plant (P. hortorum also has the largest plastome known), it is sometimes entirely absent (in Erodium). Indeed, the IR may have been lost twice in Erodium, and an IR has also been regained, as in E. absinthoides (Blazier et al. 2016b: see also Thismia-Thismiaceae). There are also very extensive rearrangements, duplications (whole genome or smaller-scale), and increases in GC content (Erodium has the highest GC content in angiosperms - Blazier et al. 2016b) and substitution rates, as well as other changes, and genes like the ndh genes may be lost (Chumley et al. 2006; Guisinger et al. 2008, 2011: sampling not bad; Jansen 2009: summary; Blazier et al. 2011: recent loss in some Erodium; Sloan et al. 2012b; Weng et al. 2013; J. Wang et al. 2015; Ruhlman et al. 2015; Zhu et al. 2015). It is noteworthy that this variation occurs within genera and species, taxa like Erodium carvifolium and California showing little change, although the former has lost its inverted repeat (Weng et al. 2013). Indeed, as Jin et al. (2020b) noted. the loss of the IR here may not be associated with other changes in the plastome as it often is elsewhere, e.g. in Fabaceae-Faboideae and the Putranjivaceae-Lophopyxidaceae family pair. Guisinger et al. (2011) suggest that this variation is the result of relaxed selection after improper DNA repair caused by mutation(s) in nuclear DNA-repair genes. For the PEP subunit α rpoA gene, see Blazier et al. (2016a).
Biparental transmission of plastids has been recorded from Pelargonium and Geranium (Tilney-Bassett &anp; Birky 1981; Corriveaux & Coleman 1988; Q. Zhang et al. 2003). In hybrids of the former, incompatability between chloroplasts from one parent and the hybrid genome (plastome-genome incompatability - PGI) may result in the death of those chloroplasts and thus to variegation (Weihe et al. 2009; see also Wicke et al. 2011a; Apitz et al. 2013; J. Zhang et al. 2015; Ruhlman & Jansen 2018), and such variegation has long been known in Pelargonium (Bauer 1909). There are parallels with the plastomes and plastome inheritanbce of Passiflora, etc. (Shrestha et al. 2019, 2021)
Parkinson et al. (2005) and Bakker et al. (2006a) found great increases in the rate of evolution of the mitochondrial gene nad1 throughout Geranieae, especially Pelargonium, but not in Hypseocharis (see also Palmer et al. 2000). Such rate increases have been observed in other mitochondrial genes, as well, and there has also been extensive gene and intron less throughout Pelargonium (Mower et al. 2007; Guisinger et al. 2008; Choi et al. 2020; Zwonitzer et al. 2024: see also Characters); rates of evolution are also notably high in genera like Plantago, Ajuga and Silene. Indeed, the chondrome in Geranium is in a considerable state of flux. Not only have mitochontrial genes like ribosomal protein and succinate dehydrogenase genes moved from the mitochondrion to the nucleus in Erodium (Adams & Palmer 2003), but the chondrome has acquired material from both parasitic (Orobanchaceae, Cuscuta) and non-parasitic (Euphorbiaceae-Acalyphoideae, Rubiaceae-Rubioideae) flowering plants alike within the last 6 Ma or so (Park et al. 2015a). There are extensive rearrangements within the Monsonia chondrome, the rate of these rearrangements being very variable, although substitution rates here, on the other hand, are both low and clock-like (Cole et al. 2018).
Weng et al. (2012) observed that both plastid and mitochondrial genes had accelerated substitution rates, but noted that the rate might not be the same throughout the phylogeny or throughout the one genome and it might be the result of either increased mutation rate or selection. J. Zhang (et al. 2014) found coordinated evolution between nuclear and plastid genes that are both involved in the synthesis of plastid-encoded RNA polymerase (PEP).
Chemistry, Morphology, etc.. The presence of cortical phi [φ] cell wall thickenings in the root hypodermis in Geranieae has been known for some time (Collings et al. 2020) - its extent, etc., is unclear. For nodal anatomy in Geranieae, see Kumar (1977). Although the nodes are described as being trilacunar and variants, from the illustrations at least some species seem to have split laterals. Growth can be sympodial, axillary buds successively evicting the terminal buds (Kumar 1977).
Boesewinkel (1988) described the corolla of Hypseocharis as being often imbricate, while Weddell (1861, Vol. 2: 289) illustrated it as being contorted (see also Takhtajan 1997). Monsonia may have nectaries axillary to the sepals or on the abaxial bases of the stamens (Aldasoro et al. 2001). When there are fifteen stamens, there are antepetalous stamen pairs, as is common in obdiplostemony (Ronse Decraene et al. 1996; see also Rama Devi 1991), or there may be five groups of three connate stamens (Aldasoro et al. 2001). There is a single, adaxial nectary of Pelargonium that initially forms a concavity in the receptacle that elongates - sometimes greatly elongates - by intercalary growth (Tsai 2016). Campylotropy is by inpushing of the inner integument (Albers & Van der Walt 2006 and references).
Some general information is taken from Yeo (1990) and Albers and Van der Walt (2006), and see also Monsonia, perhaps paraphyletic (Aldasoro et al. 2001), Hypseocharis (Slanis & Grau 2001), Geranium (Aedo 2023) and succulent Geraniaceae (Meve & Albers 2023); for chemistry, see Lis-Balchin (2002), for petroselenic acid in particular, see Tsevegsuren et al. (2004), for floral morphology, see Erbar (1998), for nectaries, see Link (1990), for androecial development, see Ronse Decraene and Smets (1995c), for pollen, see Verhoeven and Marais (1990), for ovule morphology, see Mauritzon (1934), for embryology, see Nagl (1962) and L. L. Narayana (1970), for ovule and testa development, see Boesewinkel and Been (1979), and for seed coat, see Tokarski (1972: Geranium, also fruits), Meisert et al. (2001) and Gama-Arachchige et al. (2010: also dormancy).
Phylogeny. Hypseocharis is sister to the other members of the family (e.g. Price & Palmer 1993; Bakker et al. 1998). The genus is in need of further study; if H. tridentata, with septicidal (and ventricidal) capsule dehiscence, is sister to the rest of the genus, simple optimisation of fruit characters on the tree becomes interesting. Other relationships are [Pelargonium [Monsonia [Geranium + Erodium]]] (see also Palazzesi et al. 2012; J. Zhang et al. 2015); the position of the monotypic California is unclear (Fiz et al. 2008).
For a phylogeny of Erodium, see Fiz et al. (2006); there has been substantial dispersal. For a well-sampled phylogeny, of Pelargonium, see Röschenbleck et al. (2014) and especially van de Kerke et al. (2019: largely plastome based). Relationships in the Robertianum part of Geranium were quite well supported, but those in the rest of the genus tended to be less well so (Marcussen & Meseguer 2017).
Classification. Recognising Hypseocharis as a family was an option in A.P.G. II (2003) and Palazzesi et al. (2012) keep Hypseocharitaceae separate, but the family is small and reasonably included in Geraniaceae (see A.P.G. 2009). Hypseocharis has also been placed in a monotypic order, Hypseocharitales (Takhtajan 1997), although mercifully placed near Geraniales.
Röschenbleck et al. (2014, see also some comments in van de Kerke et al. 2019) provide an infrageneric classification of Pelargonium, some taxa are not monophyletic, and not all species are placed yet. For an infrageneric classification of Geranium, see Aedo et al. (1998) and especially Aedo (2023); four subgenera and 17 sections are recognised, and in subgenus Geranium there are many informal species groups. However, phylogenetic relationships in the genus are very poorly known.
Previous Relationships. Hypseocharis used to be included in Oxalidaceae (Hutchinson 1973; Cronquist 1981), but nectaries, testa anatomy, etc., place it unambiguously here (e.g. Boesewinkel 1988; Rama Devi 1991). Also, its leaflets are not strongly articulated with the petiole, as they are in Oxalidaceae. On the other hand, Geraniaceae used to include taxa like Biebersteiniaceae (Sapindales) and Dirachmaceae (Rosales). For suggested relationships between Geraniaceae and Francoaceae-Vivianieae, see below.
Botanical Trivia. The geranium of the window sill is really a Pelargonium.
FRANCOACEAE A. Jussieu, nom. cons. - Back to Geraniales
Trees to perennial herbs; at least traces of inulin +; leaf insertion rather broad; inflorescence terminal, racemose; micropyle endostomal; x = ?
9 [list]/31 - four groups below. Africa, South America.
Age. Wikström et al. (2001) estimated the age of this node at (74-)67, 59(-52) Ma, and while comparable estimates in Palazzesi et al. (2012) are only (42.8-)31.4(-20.2) Ma, those in Bell et al. (2010) are (94-)77, 70(-51) Ma, in Sytsma et al. (2004) around 85 Ma, and that in Tank et al. (2015: Table S2) is around 94.4 Ma, while another age for this node is estimated to be a mere ca 21.3 Ma (van de Kerke et al. 2019), but c.f. the topology, [Viv. [Franc. + Ber.], as also for the ages in Sytsma et al. (2004) and Park et al. (2015a).
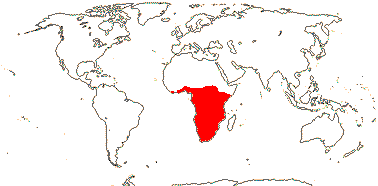
1. Bersameae Planchon —— Synonymy: Bersamaceae Doweld, Melianthaceae Horaninow, nom. cons.
Shrubs to trees; odoriferous [nasty, mustard], ellagic acid, glucuronide triterpenoid saponins, bufadienolides + [cardiac glycosides]; cork cambium subepidermal to pericyclic; medullary vascular bundles + (0); nodes 5-10:5-10; petiole with ring of bundles (cortical or medullary bundles +); styloids +; cuticle waxes usu. 0; leaves odd-pinnate, leaflets articulated, vernation conduplicate, secondary venation pinnate, margins strongly serrate to entire, (base not very broad - Bersama) stipules lateral, or single, intrapetiolar; inflorescence terminal or axillary, (with conspicuous sterile flowers at apex); flowers ± monosymmetric, resupinate [median sepal abaxial]; K 5, or [2] + 3, adaxially weakly spurred or not, C 4-5, ?imbricate, clawed, unequal, recurved or not; nectary large, adaxial, vascularized, (nectar black, rich in iron, ellagic acid - Melianthus); A 4-5, (connate basally), dorsifixed, anther endothecium not fibrous [M.], tapetal cells 3-nucleate; G [(3-)4-5(-7)], glandular hairs inside the loculus, style impressed, long, stigma punctate or capitate; ovules 1-5/carpel, axile or basal, apotropous to pleurotropous, ?campylotropous, parietal tissue ca 11 cells across, outer integument 16-20 cells across; fruit a loculicidal capsule; seed (arillate - B.), testal, exotesta palisade, crystalliferous, outer wall thick, tegmen crushed; endosperm thick- or thin walled, starchy or with xyloglucans [thick, pitted - amyloid - M.], embryo small to medium, suspensor 0; n = (18) 19, x = ?
2/14. Africa: Bersama (8). Map: modified from Culham et al. (2007), see Trop. Afr. Fl. Pl. Ecol. Distr. vol. 6 (2011). [Photo - Inflorescence, Inflorescence.]
Age. Crown-group Bersameae are (33.8-)26.9(-20.0) Ma (Linder et al. 2006), (14.9-)10(-5.7) Ma (Palazzesi et al. 2012) or (20-)14.4(-10) Ma (Sytsma et al. 2014).
[Vivianieae [Greyieae + Francoeae]]: stipules 0; anthers basifixed; fruit a septicidal capsule.
2. Vivianieae Klotzsch —— Synonymy: Ledocarpaceae Meyen, Rhyncothecaceae Jussieu, Vivianiaceae Klotzsch
(Thorns +); ?inulin; ?nodes; wood rayless; leaves opposite, usu. pinnate, a line across the stem at the node; K aristate, C (margin crenulate); pollen grains spherical, 23-40 µm long, pantoporate, with microspines, (metareticulate - Viviania); G [2-3], style 0/short, style branches long, stigma dry, margins ± revolute; ovules 1-2/carpel, parietal tissue ca 6 cells across, (nucellar cap 2 cells across), obturator as hairs on funicle; exotesta and endotegmen more or less tanniniferous; endosperm ± copious, walls thick, pitted, embryo curved; x = 6 (?10, ?9).
4/18. W. South America, S. Brasil.
Age. Crown-group Vivianieae are about 27.5 Ma (Palazzesi et al. 2012) or (44-)35(-28) Ma (Sytsma et al. 2014).
2A. Viviania Cavanilles
Woody herb (annual); chemistry?; cork?, cambium storied; nodes 1:1; glandular hairs +; cuticle waxes ± band-like or 0; lamina white-hairy below, secondary veins subpalmate; (flowers 4-merous); K valvate, basally connate, strongly 8- or 10-ribbed; (C 2-lobed); nectary glands alternating with C; (A 4, 5, 15); G [(2) 3], odd member abaxial; ovules 2/carpel, superposed; seed (hairy), raphe tanniniferous; (exotegmen thick-walled, elongated); endosperm copious, initially with some starch, embryo chlorophyllous, root long; n = 7.
1/6. Chile, S. Brazil. Map: from Lefor (1975).
Shrubs; anatomy?; cuticle waxes as platelets; (leaves simple); epicalyx + or 0; (K acute), (C 0); (filaments long - Rhynchotheca); (pollen inaperturate - Balbisia, Wendtia); nectary 0; G [3, 5], opposite sepals, stigmas drying dark; ovules 2 collateral or superposed-many/carpel, nucellar cap +; (fruit septicidal or more or less septifragal); (exotesta of slime cells - Balbisia); endosperm scanty (thin-walled), cotyledons spiral (straight); embryo colour?; n = 9.
3/12: Balbisia (10). W. South America, especially the Andes. Map: modified from Culham (2007).
[Greyieae + Francoeae]: inflorescence with sterile bracts at apex; bracteoles 0; A weakly secondarily obdiplostemonous, endothecium surrounds thecae; ovary sulcate; ovules many/carpel, pleurotropous; endotesta fibrous, anticlinal walls thickened and lignified.
Age. This node is dated at (45-)38(-31) Ma (Wikström et al. 2001), (17.7-)11.2(-5.9) Ma (Palazzesi et al. 2012; Sytsma et al. 2014 estimate the age to be (35-)27(-15) Ma and Bell et al. (2010) (32-)19(-8) Ma.
3. Greyieae Gürke - Greyia —— Synonymy: Greyiaceae Hutchinson, nom. cons.
Shrubs to small trees; ellagic acid, B-ring deoxyflavonoids +; cork cambium ± pericyclic; (largely phloic medullary bundles +); cambium storied; pericyclic fibres +; nodes ca 9:9, some bundles beginning to leave at the bottom of the internode below; petiole bundle arcuate, also peripheral cortical bundles; raphides +; cuticle waxes usu. 0; leaves simple, outer cortex detaching from stem along with petiole and lamina, lamina vernation conduplicate, secondary veins palmate, margin bluntly serrate, teeth hydathodal, petiole terete; flowers resupinate [median sepal abaxial]; K 5, imbricate, C 5, imbricate; nectaries 10, stalked, peltate- or anvil- shaped; A long-exserted; G [(4-6)], ovary furrowed, opposite C or K, style continuous with ovary, long, stigma slightly expanded and ridged, wet; micropyle exo- or endostomal, outer integument 3-7 cells across, inner integument 2-4 cells across, hypostase massive; fruit valves also opening internally; exotesta crystalliferous, outer wall thick; endosperm starchy, thick-walled; n = 16-17, x = 6 (?10, ?9), nuclear genome [1 C] (0.03-)0.929(-28.486) pg.
1/3. South Africa, Swaziland. [Photo - Inflorescence.]
Age. Divergence between the extant species of Greyia is put at a mere (0.8-)0.4(-0.07) Ma (Palazzesi et al. 2012).
4. Francoeae Spach
Perennial herbs, (root tuberous - Francoa); flavonols, tannins of any sort 0, anthocyanin in roots, inulin?; cork ?; young stem with pseudosiphonostele, endodermoid layer +; nodes 3:5; petiole bundle arcuate, with lateral annular bundles; hairs uniseriate, secretory; leaves pinnate or simple, (petiole persistent, swollen - T.), lamina vernation conduplicate-plicate; inflorescence (scapose - F.), (branched); (flowers monosymmetric - T.), 4(-5)-merous; K induplicate-valvate, C clawed or not; A (= and opposite K - some F.); tapetum multinucleate [F.]; pollen with complex endapertures; nectaries interstaminal, lobed; G [4(-5)], placentation parietal [F.], style short, ± impressed, stigmas capitate, commissural [not T?]; ovules with bistomal micropyle, outer integument ca 2 cells across, inner integument ca 3 cells across, parietal tissue 3-6 cells across, nucellar cap ca 2 cells across, postament +, funicular obturator +; embryo sac elongated; exotestal cells elongated, tegmen of pigmented cells, exotegmic cells ± elongated, (with all walls thickened); endosperm nuclear/coenocytic, embryo short, radicle with anthocyanin [F.]; n = 20, 26, x = ?
2/2. Chile. [Photo - Francoa Habit © Gardenweek.org]
Age. The split between Francoa and Tetilla is dated at ca 4 Ma (Palazzesi et al. 2012).
Evolution: Divergence & Distribution. Diversification within Vivianieae began in southern South America prior to the Andean orogeny (Palazzesi et al. 2012; Sytsma et al. 2014). Diversification within Melianthus happened some (26.2-)19.7(-12.2) Mya (Linder et al. 2006, q.v. for more dates, etc.), but c.f. the later dates suggested by Palazzesi et al. (2012) and Systma et al. (2014), who link diversification to the beginning of aridifiaction in South Africa.
Pollination Biology & Seed Dispersal. There is bird pollination in Melianthus, the black nectar there probably attracting passerine birds, but not bees; it seems to have evolved three times (Pauw & Stanway 2014; Magner et al. 2023). This nectar is quite alkaline, and is a combination of ferric iron and ellagic acid (Magner et al. 2023).
Genes & Genomes. A genome duplication ca 89.9 Ma, the FRAPα event, is associated with Francoaceae (Landis et al. 2018).
The chloroplast NADH dehydrogenase-like complex has been more or less lost in Melianthus, at least (Ruhlman et al. 2015).
Chemistry, Morphology, etc.. For nectaries, see Jeiter et al. (2017a).
Bersameae: Hutchinson (1973) described a disc lining the inside of the K; for the black nectar of at least some species of Melianthus see Magner et al. (2023). The number of nuclei in the pollen grain is unclear (Dahlgren & van Wyk 1988). For the ovules of Bersama, which face away from each other, see Danilova (1996). Khushalani (1963) described a nucellar cap 8-11 cells acros in Melianthus major, but it apparently developed from hypodermal cells and so is considered to be nucellar tissue as defined here.
For general information, see Van Wyk (in Dahlgren & Van Wyk 1988) and Linder (2006), for anatomy, see Hilger (1978a, b), Gornall and Al-Shammary (1998) and Steyn for floral development, see Ronse Decraene et al. (2001b), for anthers, see Endress & Stumpf (1991), for embryogenesis, see Steyn et al. (1986), for flower and fruit, see Doweld (2001a: c.f. micropyle type), for the embryogeny of Melianthus, see Steyn (1974), and for seeds of Melianthus, see Guérin (1901) and Corner (1976).
Vivianieae: The vessel elements of Viviania sometimes have a single bar across the perforation. The inflorescence of Viviania can be replaced all or in part by a branched thorn, and the stamens are much longer than the stigma/styles. Weigend (2005) detailed some aspects of floral morphology and pollination.
See Narayana and Rama Devi (1995) and Weigend (2006) for general information, Carlquist (1985b) for wood anatomy, M. S. Dunthorn (pers. comm.) for nodal anatomy of Viviania, Palazzesi et al. (2012) for pollen, Mauritzon (1933) for embryology and Boesewinkel (1997) for ovules and seeds; for carpel orientation, see Baillon (1874) and Knuth (1931). Endosperm type/development of Vivianieae is unknown.
Greyieae: The distinctive abscission of leaf plus attached cortex of the internode below as a single unit in Greyia, facilitated by the activity of the cork cambium, is described by Steyn (1974). However, Van Wyk (in Dahlgren & Van Wyk 1988) disagrees with her interpretation, and show rudimentary stipules on a single plant(!) of Greyia sutherlandii.
For general information, see Linder (2006), further information is taken from Bohm and Chan (1992: B-ring deoxyflavonoids), Hilger (1978a), Ramamonjiarisoa (1980) and Gregory (1998), all anatomy, Steyn and van Wyk (1987) and Ronse Decraene and Smets (1999), both floral development, Endress and Stumpf (1991: stamens), Hideux and Ferguson (1976: pollen), Steyn et al. (1986: embryogenesis), and Nemirovich-Danchenko (1995: seed coat anatomy).
Francoeae: There is a large chalazal endosperm sac in Francoa that remains free-nuclear longer than the rest of the endosperm (Gaümann 1919). For general information, see Linder (2006).
For vegetative anatomy, see Gornalll and Al-Shammary (1998), for floral morphology and development of Francoa, see Klopfer (1972a, 1973) and Ronse Decraene and Smets (1999), for embryology, see Mauritzon (1933), and for seed anatomy, see Krach (1976), Nemirovich-Danchenko (1994a) and Danilova (1996).
Phylogeny Within Vivianieae, Palazzesi et al. (2012; see also Price & Palmer 1993) found the relationships [Viviania [Rhynchotheca + Balbisia]] (Fig. 4), but support for the sister-group relationship of the latter pair of genera was not strong, and in the chronogram (Fig. 5) relationships are [Balbisia [Viviania + Rhynchotheca]] (for the latter topology, see also Systma et al. 2014). Within Bersameae, Melianthus major is probably sister to the rest of the genus (Linder et al. 2006).
Classification. Melianthaceae and Francoaceae were placed in a single family by Savolainen et al. (2000b); many of the characters given there that linked the two may be plesiomorphic. A.P.G. II (2003) suggested as an option separating Melianthaceae and Francoaceae, an option which was followed in early versions of this site; later the two were combined (see A.P.G. III 2009, also A.P.G. IV 2016).
Previous Relationships. Melianthaceae were included in Sapindales by Cronquist (1981), Greyiaceae were included in Rosales, while Francoa was placed in Saxifragaceae, also Rosales. Krach (1976) compared the testa anatomy of Francoa with that of Cunoniaceae.