EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; female gametophyte initially syncytial, walls then surrounding individual nuclei; seeds "large" [ca 8 mm3], but not much bigger than ovule, with morphological dormancy; embryo cellular ab initio, suspensor short-minute, embryo with roots arising at the end of the main axis [plant allorhizic, shoot and root at opposite ends], white, cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[LILIALES [ASPARAGALES + COMMELINIDS]]: (inflorescence branches cymose); protandry common.
[ASPARAGALES + COMMELINIDS]: style long; whole nuclear genome duplication [τ/tau event].
COMMELINIDS
unlignified cell walls with >3.5 mg g-1 ferulate [ester-linked to non-cellulosic glucuronoarabinoxylans; unlignified cell walls fluorescing blue under UV, green with NH3],pcoumarate acylates lignin [mostly on syringyl units], also glucuronoarabinoxylans; exodermal cells monomorphic; (vessels in stem and leaves); SiO2 bodies +, in leaf bundle sheaths; stomata para- or tetracytic, (cuticular waxes as laterally aggregated rodlets [looking like a scallop of butter]); inflorescence branches determinate, peduncle bracteate; P = K + C [stamens adnate to/inside corolla/inner whorl only]; pollen starchy; ovary superior; embryo short, broad.
[POALES [COMMELINALES + ZINGIBERALES]]: primary and secondary cell walls mostly with (glucurono)arabinoxylans; stomata subsidiary cells with parallel cell divisions; endosperm reserves starchy.
[COMMELINALES + ZINGIBERALES]: inflorescences with cincinnal branches [helicoid cymes]; P = T 3 + 3; A opposite individual T members; tapetum invasive/amoeboid; pollen orbicules 0 [?sampling].
ZINGIBERALES Grisebach - Main Tree.
Giant herbs; no aerial stem except when flowering; vascular bundles collateral [not amphivasal]; sieve tube plastids also with starch grains; cystoliths globular and tabular, in bundle sheath, the cell lumen; petiole bundles in arcs; cuticular waxes as aggregated rodlets; leaf ?arrangement, air channels +, with sheath, pseudopetiole and blade, lateral veins S-shaped, more than a single order, fine transverse venation; inflorescence branches spiral; inflorescence bracts large; flowers large [>2 cm long], monosymmetric; T whorls not/slightly differentiated; anthers long [>5 mm long]; pollen inaperturate, not resistant to acetolysis [exine at most thin, spinulose, to 0], outer intine thick, channeled; G inferior, (septal nectaries labyrinthine), style long, stigma large, ± elongate-clavate, wet; ovule with outer integument >5 cells across, epidermal cells of nucellus apex radially elongated [= nucellar pad], suprachalazal tissue well developed; fruit dehiscing laterally, loculicidal; seeds with germination valve [= operculate], micropylar collar + [outer integument forming annular inpushing in perisperm surrounding operculum], endotesta sclerotised [thickenings often U-shaped in t.s.], silicified; endosperm nuclear, perisperm s.l. +, reserves starchy, ?embryo; chromosomes holocentric, whole nuclear genome duplication [γ/gamma event], genome size 0.3-6 pg (1 C); chloroplast with 12-b.p. insertion at 3' end of matK gene, six nucleotide deletion in atpA gene; cotyledon not photosynthetic, ligulate, collar roots +. - 8 families, 92 genera, 2,185 species.
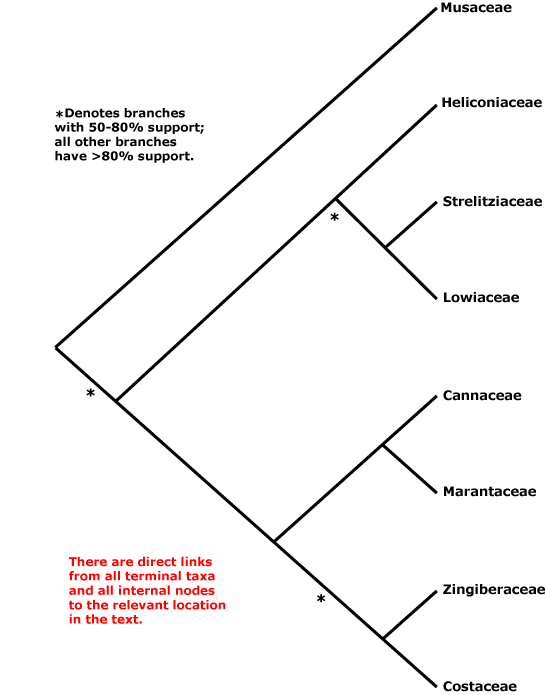
Includes Cannaceae, Costaceae, Heliconiaceae, Lowiaceae, Marantaceae, Musaceae, Strelitziaceae, Zingiberaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Zingiberales are dated to ca 88 Ma (Janssen & Bremer 2004). Comparable figures are 62 Ma in Bremer (2000b) and (66-)62, 57(-54) and (42-)38(-34) Ma in Wikström et al. (2001). Magallón and Castillo (2009) estimated ages of 87 and 79.5 Ma and Bell et al. (2010) (89-)84 My; other figures are around 83.5 Ma (Tank et al. 2015: Table S2, Strel. 86.2 My), ca 83 Ma (Givnish et al. 2018b), (103.5-)80.5(-57.5) Ma (Givnish et al. 2016), (96-)67(-52) Ma (Merckx et al. 2008a) and 75-30 and 66-26 Ma (Mennes et al. 2013, 2015 respectively). However, Kress and Specht (2005, 2006) gave rather older crown group ages of ca 96.6 Ma and 110-106 Ma respectively and late Jurassic/early Cretaceous around 150 Ma is the age in Fleming and Kress (2013, but c.f. their Fig 7.3), however, (85-)76(-65) and (60-)56(-54) Ma are ages in Hertweck et al. (2015), Ma in Givnish et al. (2018b). Of course, in nearly all cases the topology differs from that suggested by Carlsen et al. (2018) and Givnish et al. (2018b).
The distinctive seeds of Spirematospermum are known from the Late Cretaceous and Palaeogene. They have in the past often been placed with Musaceae or Zingiberaceae or left unplaced other than to order (Collinson & van Bergen 2004; Fischer et al. 2009; Benedict 2011; Friis et al. 2011: also other fossils; S. Y. Smith et al. 2013b). However, it has recently been suggested that a position in stem Zingiberaceae (Iles et al. 2015) or, for some species, even crown-group Zingiberoideae (Smith et al. 2018) is best; crystal sand has been found in the fossils, and this is common only in Zingiberaceae (S.-T. Chen & Smith 2013). S. Y. Smith et al. (2021) discuss other fossils, some also erstwhile Musaceae, which are best placed in Zingiberaceae; Callistemonites is best left unplaced.
Evolution: Divergence & Distribution. S. Y. Smith et al. (2018) integrate seed characters as visible in fossils in a total evidence phylogeny of the order (Musaceae sister to the rest: ?rooting) and particularly note at least eight characters that unite Musaceae, Costaceae and Zingiberaceae that are also often to be seen in the fossils; these characters, they thought, might be plesiomorphic for the order, or perhaps parallelisms. Thinking about character evolution (maximum parsimony reconstruction, deltran option) is clearly tricky, features like "seed coat tapering at the micropyle" having six origins across the order, most in the banana clade, but seven reversals, three in fossils, in Zingiberaceae alone (Smith et al. 2018). Overall, support is strong only for Zingiberaceae and perhaps Musaceae in the total-evidence all-fossil analyses (Smith et al. 2018). There is considerable diversity in seed morphology/anatomy across the order, and Benedict et al. (2016) examined the distribution of 51 seed characters, and suggested that Zingiberaceae occupied the largest seed morphospace of all the families - but of course over half the species in the order belong to Zingiberaceae...
There is quite likely to have been a rapid radiation of Zingiberales in the late Cretaceous/early Caenozoic (Carlsen et al. 2018), perhaps some 65 Ma (see also Christelova et al. 2011); families other than Cannaceae and Marantaceae had all diverged by ca 60 Ma (Kress & Specht 2005), or all families had diverged by 86-74 Ma (Kress & Specht 2006). However, Janssen and Bremer (2004) found divergence dates within Zingiberales to show a wide spread; those estimated under the DELTRAN optimisation were notably younger than under the two other regimes used. Given the uncertainty in relationships and ages in Zingiberales, it is difficult to think about ancestral areas, etc. (but see e.g. Kress & Specht 2006: Southeast Asia + Gondwanan vicariance; Deng et al. 2016: Australia).
A nice Zingiberales "tree" at the website of the Smithsonian Institution ("Zingiberales Research") had for well over a decade (as of 2018) depicted relationships in Zingiberales as [Musaceae [[Strelitziaceae + Lowiaceae] [Heliconiaceae [[Cannaceae + Marantaceae] [Costaceae + Zingiberaceae]]]]], the first four families being the banana group of families, the last four the ginger clade. Such a tree allowed Rudall and Bateman (2004) to suggest that there had been a change in symmetry in the order. The basic condition for the order was the suppression of the adaxial median stamen of the inner whorl, Pattern 1 monosymmetry, which tended to be linked with labellum formation (see Lowiaceae). The abaxial median stamen of the outer whorl was similarly suppressed in the [Heliconiaceae + the Ginger Families] clade, Pattern 2 monosymmetry (see also Kirchoff et al. 2009), and there was a transition between the two (1 → 2). Of course, the adaxial median stamen would need to regain its fertility, not mentioned by Rudall and Bateman (2004), and the flowers of Heliconiaceae are inverted compared with those of the Ginger Families. Using the same tree, Yockteng et al. (2013) emphasized the role of the duplication of SEP-like genes, two copies of SEP3 showing balancing selection, two LOFSEP copies showing balancing selection, while AGL6 genes - only a single copy, and not expressed in staminodes of the Ginger families - were involved in stamen development. Almeida et al. (2018) examine the floral transcriptomes of six species of Zingiberales (=6 families) in the context of the relationships above, noting some differences between the banana group and ginger clade.
When the two whorls of tepals are differentiated, both are still more or less petal-like, although the inner are usually larger and sometimes differently coloured, although in Phenakospermum (Strelitziaceae), and both Heliconiaceae and Musaceae, the tepals are largely undifferentiated (see also Payer 1857). Stamens are individually opposite members of both whorls in these families in particular. However, in this context the recently-described Musa nanensis, with its more or less polysymmetric flowers, six tepals in two distinct whorls (the inner tepals are much smaller) and all connate, and six basally connate stamens (Swangpol et al. 2015) is decidedly odd. In the ginger families clade the two perianth whorls individually encircle the floral apex as is common in the commelinids. Variation in stamen number and morphology is very great (Fleming & Kress 2013 for a summary). More or less flattened and petal-like filaments are common, and this is associated with balanced ad/abaxial expression of polarity genes, as in many leaves (Almeida et al. 2014). Interestingly, the short, broad, concave staminode of Heliconia and inner adaxial tepal of Strelitzia and most species of Musa look quite similar; at one level the flowers are similar, yet at another, rather different. For further discussion on monosymmetry see especially the ginger families clade below.
Given the variation in features like symmetry and perianth type within the sister Commelinales (see also Rudall & Bateman 2004) - oblique monosymmetry, a tepaloid perianth with stamens individually opposite perianth members and flowers in which both perianth whorls individually surround the apex are found there, too - thinking about floral evolution in Zingiberales gets tricky. Indeed, given the extensive floral variation in Zingiberales, they are certainly an order in which a well-supported phylogeny is needed before we can think much about morphological evolution - but, as discussed below, such a phylogeny has been elusive, and the topology of the best supported phylogeny (see Carlsen et al. 2018; Givnish et al. 2018b) is unlike any previously suggested.
Endress (2011a) thought that an inferior ovary might be a key innovation for the clade. For a still interesting general discussion on the evolutionary morphology of the order, focussing primarily on vegetative morphology, see Tomlinson (1962a). S.-T. Chen and Smith (2013) noted that the whole order had globular and tabular cystoliths, but other cystolith types had more limited distributions.
Ecology & Physiology. Leaves of plants of Musaceae, Heliconiaceae and Strelitziaceae growing in more or less open conditions tear along the veins in the wind, but with rather little damage to the rest of the leaf, so producing a kind of compound leaf (c.f. Arecaceae). They can be conspicuous members of this vegetation, Phenakospermum, for example, being notably common in Amazonian rainforest (Fauet et al. 2015). The air spaces so common in the petioles, etc. of Zingiberales may be involved in the movement of oxygen (or carbon dioxide) through the plant, although perhaps as much (or more) in strengthening mechanisms of the tissues in which they occur (W. T. Williams & Barber 1961; Hejnowicz & Barthlott 2005 and references).
Givnish et al. (2005, 2006b) noted that net venation, animal-dispersed propagules and tolerance of shady habitats are linked in this group, as elsewhere in monocots. Tanks of various sorts (phytotelmata), whether in the axils of leaves, as in Musa, or of inflorescence bracts, are scattered throughout the order (Fish 1983).
Pollination Biology. Animal pollination, and bird pollination in particular, pervades the order (Cronk & Ojeda 2008). Indeed, in general vertebrate pollination predominate in the banana clade (see below) and insect pollination in the ginger clade (Carlsen et al. 2018). In hummingbirds, much involved in the pollination of Heliconiaceae, crown-group diversification in lowland South America is (24.7-)22.4(-20.3) Ma (Bleiweiss 1998a; McGuire et al. 2007, 2014) or (29.9-)28.8(-28.4) Ma (Tripp & McDade 2014), more or less compatible with the age of Heliconiaceae (Iles et al. 2016; see below). Euglossine bees are important pollinators of Neotropical Zingiberales (Zucchi et al. 1969; Williams 1982), and the bees began diversifying some 42-27 Ma (Ramírez et al. 2010). However, Fleming and Kress (2013) suggested that understanding the evolution of pollination was difficult, given a common ancestor of the order that they thought lived in the late Jurassic/early Cretaceous around 150 Ma. For them vertebrate pollination, possibly by early mammalian multituberculates or even small dinosaurs, was ancestral in the order - "this conclusion is not unreasonable" (Fleming & Kress 2013: p. 271, c.f. their Fig. 7.3, crown-group Zingiberales are less than 100 My). Given the likely topology of relationships in the order (Carlsen et al. 2018; Givnish et al. 2018b) suggestions as to the nature of the ancestral pollinator will depend on what has been going on in Commelinales. The distinctive pollen of Zingiberales, with its reduced exine and expanded intine, does not seem to be associated with any aspect of pollination (Kress 1986b).
Plant-Animal Interactions. McKenna and Farrell (2005, 2006) discuss the diversification of the chrysomelid hispine beetle Cephaloleia, a rolled-leaf beetle, on Neotropical Zingiberales; it also eats some other commelinids (Arecaceae, Poales) as well as Cyclanthaceae and Orchidaceae (see also Staines 2004; García-Robledo et al. 2013a; Staines & García-Robledo 2014). Both feeding on Zingiberales and specialisation of the larvae and particularly adults on the young, rolled leaves may each have evolved once. McKenna and Farrell (2005) suggested that some Cephaloleia specialized on Heliconiaceae and Marantaceae - not immediately related, of course - and others were more generalists on Zingiberales. In a more local study at La Selva, Costa Rica, where there are 5 families of Zingiberales, perhaps 5 species of Cephaloleia were generalists (but none ate Costaceae that grow there), while all 13 others, and the two species of Chelobasis in the study, were found only on a single family; all told, there were 58 species of Cephaloleiini (see also Staines 2011). In Costa Rica, at least, there are also numerous cryptic species of these beetles, all with rather limited altitudinal ranges and thermal tolerances (García-Robledo et al. 2016; Staines & García-Robledo 2014: 214 species in the genus). Interestingly, the beetles also ate introduced Zingiberales, but nearly always these were members of the same family that the beetles ate in Costa Rica (Garcia-Robledo et al. 2017: three Cephaloleia the exceptions). Blankenship et al. (2018) looked at the microbiome of the beetles in the context of the specialist/generalist and native/introduced dichotomies, and, rather surprisingly, they found that the specialist beetles had more diverse microbiomes - also found in the eggs - than the generalists.
The beetles involved in the New World belong to Arescini and Cephaloleiini, and the association between these chrysomelid beetles and Zingiberales may date from the very late Cretaceous, fossil feeding damage being described from North American material (Wilf et al. 2000; McKenna & Farrell 2006). However, it was suggested that ascribing feeding patterns in fossil material to the particular activities of hispine beetles might not be possible, since other insects could produce similar feeding patterns on these plants (García-Robledo & Staines 2008), so dating this association was not straightforward. Nevertheless, Labandeira and Currano (2013) stood by the initial identification, noting that it was i.a. driven by the combination of adult and larval feeding marks. It has also been suggested that the beetles diversified ca 20 Ma after diversification of their hosts in the Neotropics (Gómez-Zurita et al. 2007; c.f. McKenna & Farrell 2005; Suchan & Alvarez 2015), and that the beetles were preapted to their hosts, perhaps something of a tangent (Garcia-Robledo et al. 2017: "ecological fitting"; Agosta 2006).
Caterpillars of Hesperiinae-Calopodini skippers quite often eat Zingiberales - caterpillars of most other skippers eat Poaceae (Warren et al. 2009).
Genes & Genomes. For the γ genome duplication, see D'Hont et al. (2012: see also below under Musaceae). The MALEα duplication, dated at 106.9 Ma, characterizes the whole clade (Landis et al. 2019). There has been a triplication of the CYC-like gene here (Bartlett & Specht 2009, 2011) as well as duplications of GLOBOSA-like genes (Bartlett & Specht 2010) that are perhaps involved in floral diversification and the evolution of monosymmetry.For genome size, see Leitch and Leitch (2013); there are about 30 measurements. For the atpA deletion, see Davis et al. (2004).
Mahanty (1970) and Song et al. (2004) suggest that the base chromosome number for the order is 11, while Satô (1960) established the primitive karyotype by comparison with the moss Takakia... The families from which holocentric chromosomes have not yet been reported are Marantaceae, Costaceae and Lowiaceae (Bures et al. 2013; Escudero et al. 2016b).
Chemistry, Morphology, etc.. The phenol zingerone (C11H14O3) has apparently been isolated from Eocene fossils of Spirematospermum (see above); is its presence a synapomorphy for the order (van Bergen & Collinson 1999)? The roots tend to have V-shaped aggregations of xylem, with an especially large metaxylem element at the angle (von Guttenberg 1968), and although the central stele of Strelitziaceae and Musaceae, with intermingled xylem and phloem occupying more of less the entire pith, seems very distinctive, careful reading of Tomlinson (1969) shows that other taxa commonly have a similar anatomy. Arber (1925) suggested that the cauline vascular bundles are not amphivasal, but I have not checked this against recent anatomical literature. Korn (2006) noted that Musa and Calathea ornata were unusual in that in all the individuals that he examined the foliar genetic spiral proceeded in the same direction, although in plants of other species clockwise and counter-clockwise spirals occurred in equal frequencies; asymmetry of the base of the leaf blade is also notable in this order (see also Martinez et al. 2016). For a summary of leaf venation, see Salvi et al. (2014).
Zingiberales have various kinds of determinate inflorescences (Kunze 1985), at least, the branches are determinate (see also Eichler 1975). Bracteoles are more or less lateral in Canna, Costus, Heliconia, etc., and the flowers may seem to have inverted orientation (see also Heliconia below), while the flowers of Marantaceae may have an oblique plane of symmetry - at one level, not very different (see also Eichler 1875). There is variation in the stage at which monosymmetry is evident in the flower (see also Kirchoff 2003; Kunze et al. 2005c). Endress (1994b) noted that there may be massive development of endothecial and/or lignified tissue on the connective side of the anther. Although pollen grains of the order are apparently inaperturate, they range from functionally monoaperturate or omniaperturate (Kress 1986b; Furness & Rudall 2000b). How widely distributed stamens supplied by several vascular traces are in the order is unclear (see Rao et al. 1954 for some records).
For leaf flavonoids, see Williams and Harborne (1977), for phenylphenalenones, see Otálvaro et al. (2002), for sieve tube inclusions, etc., see Behnke (1994), for vegetative anatomy, see Tomlinson (1969), for phytoliths, see Piperno (2006) and Benvenuto et al. (2015), and for vessel and tracheid micromorphology, see Carlquist and Schneider (2010); for information on floral anatomy, see Rao et al. (1954), on nectary morphology and position, see Kirchoff (1992) and Stauffer et al. (2009), on the tapetum, see Furness and Rudall (2001), on ovules, see Mauritzon (1936d), and on seed morphology and anatomy, Humphrey (1896), Mauritzon (1936d), Grootjen and Bouman (1981b), Manchester and Kress (1993) and Liao et al. (2004).
Phylogeny. For a discussion of the relationships of Zingiberales, very clearly monophyletic, see the commelinid page.
Phylogenetic relationships within the order have been much studied, but they remained uncertain for some time - see especially Kress (1990b, 1995) and Andersson and Chase (2001: Costaceae and Zingiberaceae not obviously sister taxa) for early work. Musaceae were weakly (barely over 50%) supported as sister to the rest of the order in Kress et al. (2001: 2 genes + morphology, successive approximation weighting, see also Janssen & Bremer 2004), and slightly better, but still not that well (78%) supported as member of a clade [Heliconiaceae, Musaceae, [Lowiaceae + Strelitziaceae]] in Givnish et al. (2006b: one gene), while Wikström et al. (2001: three genes) found the relationships [Musaceae [Heliconiacaeae [[Lowiaceae + Strelitziaceae] [the ginger families]]]] and in Magallón et al. (2015) Strelitziaceae are sister to the rest of the order. Even the Ginger Families were not retrieved as monophyletic in some analyses (e.g. Davis et al. 2004: support values very low; Soltis et al. 2007a; Bell et al. 2010; Iles et al. 2016: focus on Heliconiaceae), or relationships within this group were scrambled (Wikström et al. 2001). Johansen (2005), looking at six DNA regions (plastid, nuclear), suggested that the relationships [Lowiaceae [Strelitziaceae [[Musaceae + Heliconiaceae] [the ginger families]]]], which would make understanding character evolution of the flowers in particular difficult; however, support was not strong and sampling other than in Orchidantha, the focus of the paper, was poor. Yockteng et al. (2013) prefered the relationships [Musaceae [[Strelitziaceae + Lowiaceae] [Heliconiaceae + the ginger families]]], although they obtained other relationships, as well as between Zingiberales and remaining commelinids, in separate analyses of the SEPALLATA, AGL6 and LOFSEP genes.
Barrett et al. (2012b) found the relationships [Heliconiaceae [Musaceae + Zingiberaceae]] among the four taxa whose complete chloroplast genomes they analyzed; support was not strong. After adding plastid genomes from nine other members of the order (Barrett et al. 2013: codon-based likelihood analyses), the relationships *[Heliconiaceae [*[Musaceae [Strelitziaceae + Lowiaceae]] [the Zingiberaceae group]]] were obtained, although support for the clades with asterisks was slight. Sass et al. (2016) analyzed 308 nuclear gene exons and 68 plastid genes for 53 Zingiberales, and in most analyses they retrieved the relationships [Musaceae [[Heliconiacaeae [Lowiaceae + Strelitziaceae]] [the Ginger Families]] (see also Deng et al. 2016), however, in perhaps suspect coalescent analyses, support for the basal position of Musaceae dropped below 50%, the most dramatic drop in the whole tree. Relationships among Chineae taxa of Zingiberales in Z.-D. Chen et al. (2016) are [Lowiaceae [Musaceae [the Ginger Families]]], while Tang et al. (2016) i.a. suggested a [Musaceae + Heliconiaceae] clade, the two diverging less than 30 Mya ago. However, in a very recent study a clade [Musaceae [Heliconiaceae [Strelitziaceae + Lowiaceae]]], the banana families, was quite often sister to the ginger families (Carlsen et al. 2018: 378 nuclear genes, 518,442 bp). Although a topology [Musaceae + the rest of the order] was sometimes recovered, the more data used, the more likely became the recovery of the banana clade, although many trees from analyses of individual genes were unresolved. Carlsen et al. (2018) suggested that an entirely unambiguous resolution of relationships in this clade might be very difficult to obtain, but their topology, [banana families + ginger families], is in fact quite well supported and is followed here. Givnish et al. (2018b: plastid phylogenomics, see Fig. 2) also recovered this set of relationships in their targeted chloroplast genome analysis of Zingiberales and with around 84% bootstrap support, although in sampling that was less complete both in terms of taxa and genes the topology [[Musaceae + the ginger families] [other Zingiberales]] was recovered. Timilsena et al. (2022) also recovered the relationships [banana families + ginger families], but relationships between the families in the former group tended to be poorly supported; sampling was minimal, and genera like Siphonochilus (?Costaceae) were not included.
Synonymy: Amomales Lindley, Cannales Berchtold & J. Presl, Lowiales Reveal & Doweld, Marantales Martius, Musales Berchtold & J. Presl
[Musaceae [Heliconiaceae [Strelitziaceae + Lowiaceae]]] / the banana clade: guard cells symmetric [inner and outer ledges of stomatal chamber equal]; T whorls C-like; A 5 [but not always same stamens], median [adaxial] A of inner whorl not fertile/0; embryo plug-like.
Age. Th age of this node is ca 57 Ma (Givnish et al. 2018b) or perhaps ca 85 Ma (Burgos-Hernández et al. 2019).
Evolution: Divergence & Distribution. The development of the adaxial member of the outer perianth whorl is delayed and there is a sterile prolongation of the apex of the ovary in all families except Heliconiaceae (see also Kirchoff et al. 2020), althouth the sterile prolongation is barely evident in Musaceae like Ensete and Musella. In any event, such distributions makes these characters difficult to polarize.
MUSACEAE Jussieu, nom. cons. - Back to Zingiberales
Plant cormose; steroidal saponins, (phenylphenalenones) +; SiO2 bodies also ± trough-shaped; roots not medullated, centre portion occupied by scattered wide vessels and strands of phloem; rhizome with endodermis; sieve tube plastids also with peripheral protein fibres; laticifers +, articulated; mucilage cells +; petiole with 1 series of ± abaxial air canals; stomata oriented transverse to long axis of leaf; plant glabrous; prophylls lateral; leaves spiral, buds leaf-opposed; plant monoecious; inflorescence bracts deciduous, cincinni at right angles to the main axis, floral bracts and bracteoles 0; 5 T connate, connation congenital, development of adaxial member of outer whorl delayed, median [adaxial] inner T ± reduced, hooded [cucullate], free, (all 6 T connate, whorls strongly differentiated - Musa nanensis); septal nectary labyrinthine; staminate flowers: median [adaxial] A of inner whorl absent, (A 6; basally connate), with several vascular bundles; anther wall formation of the basic type [Musella s. str.], tapetum glandular, exothecium +, endothecium poorly developed; pistillode +; carpelate flowers: staminodes +, G loculi mucilaginous, with intra-ovarian trichomes, stigma capitate; ovules (micropyle exostomal), outer integument "massive", inner integument 2-3 cells acoss, cells anticlinally elongated, hypostase +; fruit baccate/(laterally ?loculicidally dehiscent); seed with chalazal chamber, aril?; outer periclinal walls of exotesta pulling away from the rest of the seed, exposing silica crystals in inner periclinal wall, mesotesta 20-25 cells across, sclerotised; embryo short, straight, ± plug-like; n = 7, 9-11, x = 6 (?8, ?9), chromosomes 1.2-2.9 µm long, nuclear genome [1 C] (0.059-)0.927(-14.594) pg; plastid transmission biparental; collar at right angles to cotyledon.
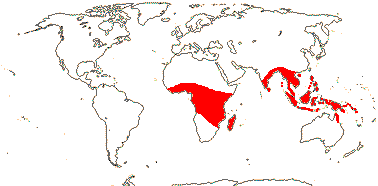
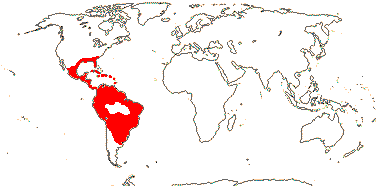
3[list]/93: Musa (85). Africa, Himalayas to South East Asia, Philippines and N. Australia. Map: J. Kress (pers. comm.). [Photos - Collection.]
Age. Crown-group Musaceae are dated to ca 61 Ma (Janssen & Bremer 2004), and ages suggested by Christelova et al. (2011) are similar, being (80.5-)69.1(-57.8) Ma; those in Bell et al. (2010) are younger, (51-)36, 34(-20) Ma, as are those in in Wikström et al. (2001) - (55-)50, 48(-43) or (35-)39(-25) Ma. Kress and Specht (2005, 2006) offered crown group ages of around 47.5 and ca 87 or 51 Ma respectively, while estimates in Burgos-Hernández et al. (2019) are (80.1-)61(-45.9) Ma and in Janssens et al. (2016) are (61.2-)51.9(-45.6) Ma.
Evolution: Divergence & Distribution. For divergence dates within Musaceae, see Janssens et al. (2016) and Burgos-Hernández et al. (2019 and references); diversification probably began in the earlier Eocene.
Ensete oregonense, although where in the family it is actually to be placed is unclear (S. Y. Smith et al. 2018) - is known fossil in Eocene deposits some 43 Ma from west North America (Manchester & Kress 1993; Iles et al. 2016), and this is quite a range extension when compared with the distributions of extant taxa. Note that most Spirematospermum, known from the Cretaceous and widespread and sometimes abundant in the northern hemisphere in the Palaeogene, has been associated with Musaceae and even placed in its own subfamily, Parietimusoideae. However, such fossils are now placed in stem or crown-group Zingiberaceae (c.f. T. C. Fischer et al. 2009; S. Y. Smith et al. 2018), and Musa cardiosperma, from the Deccan Intertrappean deposits, is also to be placed in Zingiberaceae (S. Y. Smith et al. 2021). On the other hand, Burgos-Hernández et al. (2019) in their summary of the rich record of fossils associated with Musaceae accept the Late Cretaceous (Santonian) S. chandlerae from North America as belonging to the family. The fossil record still needs to be sorted out.
A boreotropical origin of Musaceae is perhaps likely (Burgos-Hernández et al. 2019) followed by more recent diversification on the Southeast Asian mainland (extant taxa). Janssens et al. (2016) more specifically suggest an origin in the northern Indo-Burman region ; this article should be consulted for further details of the evolution and dispersal of the family.
Ecology & Physiology. Ennos et al. (2000) looked at the functional morphology of the petiole of Musa textilis noting i.a. that the petiole, although rigid, twisted easily; the inner tisuues, only 9% of the dry mass, were made up of stellate parenchyma and isolated reinforcing strands that together allowed the leaves to twist away from the wind, the blade folding. The blades also tear easily along/between the veins, reducing their resistance to the wind without greatly affecting their photosynthetic capacity.
Pollination Biology. As with Heliconiaceae, the inflorescence may be erect or pendent, depending on the species, and insects, birds, bats and tree shrews are all known pollinators (Nur 1976; Marshall 1985; Liu et al. 2002; Xue et al. 2005 and references). Comparing sympatric bat- and bird-pollinated species, not only how the inflorescence was held, but flower colour, tepal position, and nectar consistency and composition, all differed (Ito et al. 1991).
Apomixis is reported from Musa (Lim 2016a).
Genes & Genomes. There may have been one (or more) genome duplications in this clade some 60 Ma (Lescot et al. 2008; see also McKain et al. 2016; Qiao et al. 2019: three duplications; Zwaenepoel & Van de Peer 2020). Vanneste et al. (2014a) dated a genome duplication in Musa to (68.9-)66.1(-62.8) Ma, while D'Hont et al. (2012) date two duplications, the alpha and beta events, to around 65 Ma. Interestingly, there is no evidence of fractionation bias or genome dominance associated with the alpha duplication, suggesting it was an autoploid event, while there is for the beta duplication, suggesting alloploidy there (Garsmeur et al. 2013).
The mitochondria, but not the chloroplasts, are paternally inherited in Musa (Fauré et al. 1994).
Economic Importance. For general information on the domestication of the banana (Musa spp. and hybrids), see Heslop-Harrison and Schwarzacher (2007), for breeding, etc., see Pillay and Tenkouano (2011) and for hybridization and hybrid genomes, see Cenci et al. (2019); see Piperno (2006) for phytoliths and domestication.
Chemistry, Morphology, etc.. The changes in the anatomy of the leaves (in particular) during development of the Gros Michel banana was described by Skutch (1927); the Vorlaüferspitseis is up to ca 15 cm long.
Swangpol et al. (2015) describe the remarkable polysymmetric flowers of Musa nanensis. Does the endosperm have a small chalazal chamber?
Some information is taken from Andersson (1998: general), Tomlinson (1959, 1969: anatomy), Fahn (1983: inflorescence), Xue et al. (2005: microsporogenesis, etc., 2007: embryology of Musella), Fahn et al. (1961: nectary), Kirchoff (1992: ovary), and Graven et al. (1996: seed anatomy and macromolecular composition).
Phylogeny. Liu et al. (2010) and Li et al. (2010) discuss the phylogeny of the family, in which there are two main clades; the suckering Musella is derived from the non-suckering Ensete in the former. However, in some analyses in the latter the two were sister taxa (see also Givnish et al. 2018b and especially Burgos-Hernández et al. 2019). Janssens et al. (2016: sampling very good) found Musa, with two main clades, to be sister to the [Ensete + Musella] clade.
[[Heliconiaceae [Strelitziaceae + Lowiaceae]] [[Cannaceae + Marantaceae] [Costaceae + Zingiberaceae]]]: ?Age. Suggested ages for this node are (61-)52, 50(-43) Ma (Bell et al. 2010), (61-)57, 53(-49), (37-)33(-29) Ma (Wikström et al. 2001), around 96.6 and 109.3 Ma (Kress & Specht 2006, 2006 respectively), and 75-30 Ma (Mennes et al. 2013).
[Heliconiaceae [Strelitziaceae + Lowiaceae]]: leaves 2-ranked; inflorescence branches 2-ranked, the bracts persistent; micropylar collar 0; embryo medium to long, ± curved.
Age. This node is dated to (101-)86(-73) Ma (Iles et al. 2016) or ca 48.2 Ma (Givnish et al. 2018b).
HELICONIACEAE Vines - Heliconia L. - Back to Zingiberales
Rhizome with endodermoid layer; SiO2 bodies also decorated and trough-shaped; anticlinal walls of epidermal cells sinuous, stomata polycytic, neighbouring cells with oblique divisions; petiole long; inflorescence (pendulous), inflorescence bracts 2-ranked or spiral, flowers resupinate or not, obliquely monosymmetric; 5 T connate, connation postgenital, median member of outer whorl ± free, recurved; A 5, basally adnate to T, staminode +, = median member of outer whorl, ± hooded; tapetum at least initially non-syncytial; pollen asymmetric, heteropolar [± hat-shaped], functionally monoaperturate; ovule 1/carpel, basal, apotropous, micropyle bistomal; fruit fleshy, usu. blue, schizocarp or drupe, endocarp well developed, operculate, operculum off-centre, derived from funicle; aril 0; seed surface ± ruminate; testa and tegmen thin, undifferentiated; n = (11) 12, x =12, chromosomes 1.4-4.5 µm long, nuclear genome [1 C] (0.084-)0.486(-2.802) pg; coleoptile 0, but sheath lobed, collar at right angles to cotyledon.
1/187: [list] - 5 subgenera, 17 sections. Mostly tropical America, a few Celebes to the Pacific. Map: Old World from Kress (1990a) and New World, W. J. Kress (pers. comm.). [Photo - Flower, Flower.]
Age. Estimates of the age of divergence of crown group Heliconia are ca 87 or 32-28 Ma, depending on the method used (Kress & Specht 2006), ca 32 Ma (McKenna & Farrell 2006), or (47-)39(-32) Ma (Iles et al. 2016).
Kapgate (2013) reported Heliconiaites mohgaoensis from Deccan Intertrappean deposits 70.6-65.5 Ma; both the age and locality of this report are somewhat surprising.
Evolution: Divergence & Distribution. The predominant pollinators of New World Heliconia are hummingbirds and most Heliconia are from the New World; Heliconiaceae make up one of the single most important hummingbird-pollinated clades. Here ages become important. Most estimates of the age of crown-group Heliconiaceae are 40-30 Ma (see above), and diversification in New World Heliconia is estimated to have begun a little over 30 Ma (Iles et al. 2016). Diversification of crown-group hummingbirds, currently one of their major pollinators, may have started in South or Central America, perhaps in lowland South America, as late as the early Miocene (24.7-)22.4(-20.3) Ma, much speciation occurring about 13-12 Ma along with the uplift of the Andes, hummingbirds being found at high elevations there (Bleiweiss 1998a; McGuire et al. 2007, 2014; Abrahamczyk & Renner 2015; Prum 2015); Tripp and McDade (2014a) estimated crown-group diversification of hummingbirds to have begun (29.9-)28.8(-28.4) Ma. However, the first hummingbird fossils in the New World may date to ca 32 Ma (Barreto et al. 2024). Iles et al. (2016) suggested that there was diffuse coevolution between birds and plants (sensu Tripp & McDade 2014a; see also Ehrlich & Raven 1964), ecological interactions between hummingbirds and Heliconia driving adaptations in both, the two becoming associated early in the history of hummingbirds in the New World (see also Temeles et al. 2009; Abrahamczyk et al. 2017a).
However, things become a little complicated. Much of the early fossil history of stem-group hummingbirds is from the Oligocene in Europe where they are found in deposits ca 34.3 Ma (Mayr 2004, 2009; Louchart et al. 2008) and from the Late Eocene of the Caucasus (Louchart et al. 2008). Furthermore, Heliconia itself may have a very long stem history - ca 47 Ma (Iles et al. 2016, but much less in Givnish et al. 2018b), about which precisely nothing is known. Be all this as it may, perhaps hummingbirds that early evolved in association with Heliconia became the templates, as it were, for a variety of younger plant clades as they adopted bird pollination, the evolution of these hummingbird-pollinated plants being "facilitated by this pre-existing relationship" (Iles et al. 2016: p. 161). Gesneriaceae-Gesnerioideae are also commonly pollinated by hummingbirds, and the association there, beginning ca 22.4 (Roalson & Roberts 2016) or (25.5-)18.5(-5) (Serrano-Serrano et al. 2017) Ma, is also quite old, as is that in Bromeliaceae, ca 14 Ma (Givnish et al. 2014a) and Nepetoideae-Salviinae, ca 20 Ma (Kriebel et al. 2019), while the little-known Ericaceae-Vaccinieae may be another example. For more dates see Iles et al. (2016) and Barreto et al. (2024) for more on hummingbird pollination in general, see below.
Ecology and Physiology. Phytotelmata in the axils of bracts of species of Heliconia from French Guiana were found to have a substantial component of anoxygenic phototrophic bacteria, perhaps nitrogen-fixing, rather like the bromeliad phytotelmata from the same area that were studied (Vergne et al. 2021). For the insects living in the water that accumulates in the inflorescence bracts of H. caribaea, see Machado-Allison et al. (1983).
Pollination Biology & Seed Dispersal. Variation in floral and inflorescence morphology in Heliconiaceae is considerable (Berry and Kress 1991), and as in Musaceae, the inflorescences may be erect or pendant; in the latter case, the flowers are not resupinate (Iles et al. 2016). Pollen-connecting threads derived from the break-down of cell walls allows the pollen to be held together in clumps (Rose & Barthlott 1995; Simão et al. 2007); in Heliconia psittacorum, at least, Nascimento et al. (2018) suggest that there is secondary pollen presentation, the pollen being held in the stylar hairs. Hummingbird pollination is prevalent, and Heliconia is a major nectar resource for sickle-bill hummingbirds (Eutoxeres) and other trap-lining hermits at lower altitudes in the New World (they may also nest underneath the leaves), and there may be sequential opening of flowers of different species at the one locality. Indeed, in the West Indies males and females of the one species of hummingbird Eulampis jugularis may take nectar from different species of Heliconia - H. caribaea (short bill, the male) and H. bihai (long bill, the female) are involved, although details depend on the locality (Temeles & Kress 2002; Temeles et al. 2019). At higher altitudes, as in the Andes, where Heliconia does not grow sickle-bill hummingbirds take nectar from Centropogon (Campanulaceae-Lobelioideae) (Stiles 1975; Stein 1992; Kress & Beach 1994; Pedersen & Kress 1999; Fleming et al. 2005; Abrahamczyk et al. 2017a; Hosaka et al. 2016 for sequential opening in general; Mansaray et al. 2025). For the diversity of bird-pollinated taxa of Gondwanan origin in tropical and premontane parts of the northern Andes, see Gentry (1982).
Betts et al. (2015) suggested that the plant can recognize when it is being visited by trap-lining rather than territorial hummingbirds, pollen tube growth being enhanced and hence pollination is more successful; the plant apparently senses the greater amount of nectar removed by trap-liners. The distinctive pollen of Heliconia does not seem to be particularly associated with any aspect of pollination (Kress 1986b).
Water collects in the inflorescence bracts of species with erect inflorescences. The mouth of the corolla is held above the surface of the water and so the flower is accessible to the pollinator, although the ovary may be under water; the thick and fleshy pedicel later elongates so raising the fruits above the water and making them accessible for the seed disperser. The liquid may be a protection against herbivores (Wootton & Sun 1990), and the system is functionally analogous to a water calyx (see also some Bromeliaceae).
Plant-Animal Interactions. The herbivorous Cephaloleia beetles (Cassidinae+Hispinae, Chrysomelidae) seem to have diversified in the Oligocene, perhaps coincident with crown Heliconia diversification (McKenna & Farrell 2006; see also Iles et al. 2016), but c.f. Garcia-Robledo et al. (2017 and references) who suggest that beetles diversified later. Chelobasis eats only plants of Heliconiaceae (Staines & García-Robledo 2014).
Economic Importance. Although Heliconia is a popular horticultural plant, almost half the species are endanged; most species in cultivation are those that have broader distributions (Kress et al. 2025b). Colombia has the most - 96 - species of Heliconia (37 are endemic) of which 11 species are critically endangered and 17 are endangered (Kress et al. 2025b).
Chemistry, Morphology, etc.. The laterally-aggregated wax rodlets of the "Strelitzia-type" found here may form little chimneys above the stomatal apertures (Barthlott et al. 1998).
Kirchoff et al. (2009) suggest that the flower of Heliconia is obliquely monosymmetric (the characterization above follows this interpretation) while the floral diagram in Eichler (1875) shows an inverted orientation; clarification is in order (see Dworaczek & Claßen Bockhoff 2016 for reports of resupinate flowers here). In Kress et al. (2025a) the flowers are described either as being resupinate or not - this does not depend on wheher the inflorescence is erect or pendant. The parietal tissue soon disintegrates. For operculum morphology, see S. Y. Smith et al. (2018).
Additional information is taken from Andersson (1998; general), Tomlinson (1959, 1969: anatomy), and Kirchoff (1992: ovary); see Stone et al. (1979), Prakash et al. (2000), Kress (1986b) and Simão et al. (2007) for pollen and anther and Simão et al. (2006) for ovule and seed.
Phylogeny. Iles et al. (2016: 7 nuclear and plastid markers) provided a comprehensive analysis of the family; about 3/4 of the species then known were included. A clade made up of the Old World subgenus Heliconiopsis and a small group of Ecuadorian species is sister to the rest of the family; in general, classical subgenera were not monophyletic, atthough sections fared somewhat better. However, the backbone of the tree is very poorly supported - vanishingly little ML support, some Bayesian (Iles et al. 2016). Kress et al. (2025a: 452 nuclear loci, 136 spp.), however, obtained much better support, although there were three tetra- and pentatomies and the morphology of five species did not agree with their molecular placements; subgenus Heliconiopsis, which was made up of an Old World and a New World clade, was sister to the rest.
Classification. Kress et al. (2025a) divided the genus into 5 subgenera and 17 sections.
[Strelitziaceae + Lowiaceae]: leaf pseudopetiolate, petiole with adaxial and abaxial series of air canals; inflorescence a truncated polytelic synflorescence; T whorls differentiated, both petal-like, development of adaxial member of outer whorl delayed, 2 abaxial-lateral members of inner whorl enclosing stamens, median member of inner whorl ± reduced, hooded [cucullate], free; tapetum glandular; apex of ovary prolonged, sterile [= "floral column"], stigma 3-lobed; fruit longitudinally loculicidally dehiscent; outer integument 14-20 cells across; tegmen at most poorly developed.
Age. Suggested ages for this node are (52-)48, 45(-41) or (30-)26(-22) Ma (Wikström et al. 2001; for the low end of the latter ages, see also Tang et al. 2016), (53-)42, 40(-30) Ma (Bell et al. 2010), and ca 78 Ma (Janssen & Bremer 2004); ages in Kress and Specht (2005, 2006) are around 49.1 and 96-80 Ma respectively.
Evolution: Divergence & Distribution. Kirchoff et al. (2020) suggested that the complex inflorescences of Lowiaceae and Strelitziaceae (but not Phenakospermum in the latter) in which there was no main inflorescence axis, but rather a coflorescence made up of a series of many flower-bearing axillary branches, a truncated polytelic synflorescence, might be a syapomorphy at this level; it its so treated above. However, the "normal monocot-type" inflorescence of Phenakospermum then becomes a reversal.
Chemistry, Morphology, etc.. The exostomal aril is lobed or fimbriate. For details of anatomy, see Tomlinson (1959), and of the so-called floral column, the result of intercalary growth at the top of the ovary, see Kirchoff and Kunze (1995).
STRELITZIACEAE Hutchinson, nom. cons. - Back to Zingiberales
Plant (arborescent, growth monopodial), (stem dichotomizing); steroidal saponins, phenylphenalenones +; roots medulla with scattered wide vessels and strands of phloem; stem also with vessels (not Ravenala), often lacking endodermis; SiO2 bodies also ± druse-like; petiole with several arcs of air canals; anticlinal walls of epidermal cells sinuous, hypodermis 3-6-seriate; 2 lateral members of inner T whorl basally connate, large, median [adaxial] member small; (A 6 - Ravenala), staminodes 0; tapetal cells to 32-ploid; stigma long-turbinate; ovules with bistomal micropyle, suprachalazal area massive; capsule woody; aril ± of hairs; operculum rudimentary/0, endotesta with U-shaped thickenings, tegmen only a cuticle; (perisperm 0), embryo ?long; n = (7, 9) 11, x = 7 (?6, ?8), chromosome length?, nuclear genome [1 C] (0.049-)0.738(-11.004) pg; plastid transmission biparental [Strelitzia]; primary root well developed.
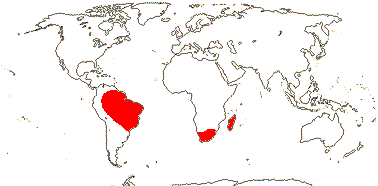
3/7:[list] - Strelitzia (ca 5). Tropical South America, E. southern Africa, Madagascar. Map: J. Kress, pers. comm.. Photo: Flower.
Age. Crown-group Strelitziaceae are dated to (33-)29(-25) or (22-)18(-14) Ma (Wikström et al. 2001), ca 59 Ma (Janssen & Bremer 2004), or (36-)25, 23(-14) Ma (Bell et al. 2010); ages in Kress and Specht (2005, 2006) are around 25.3 and ca 74 or 58-55 Ma respectively.
Evolution: Divergence & Distribution. Vicariance has been invoked to explain the distribution of Strelitziaceae (Sanmartín & Ronquist 2004; ca 83 Ma), but most of the ages for the family given above suggest that dispersal must have been involved.
Pollination Biology. Pollination in the group has been much studied (e.g. referemces in Andersson 1998), although it is unclear what the plesiomorphic condition might be (c.f. Kress et al. 1994).
Chemistry, Morphology, etc.. Stomatal morphology may vary depending on where on the plant the stomata are; the basic morphology seems to be brachyparacytic, with cells at the two ends of the stomata often being shorter than other epidermal cells, but there may be other associated and more or less thick-walled cells (Tomlinson 1960).
Growth in Phenakospermum is described as being monocarpic (Kirchoff et al. 2020), but in fact it grows like most other monocots, including Zingiberales; see above for inflorescence structure in [Strelitziaceae + Lowiaceae]. Thread-like structures are found in the anthers of Strelitzia; these are formed from rows of epidermal cells (Kronestedt & Bystedt 1981).
Some information is taken from Andersson (1998: general); for anatomy, see Tomlinson (1969), branching and buds in Strelitzia reginae, see Fisher (1976), floral morphology of Strelitzia, see Kronestedt & Walles (1986), pollen, see Kronestedt-Robards and Rowley (1989), the remarkable arils of Strelitzia, see Pfeiffer (1891), Serrato-Valenti et al. (1991) and Pirone et al. (2010) and references, and for embryology, see Mauritzon (1936d).
Phylogeny. Relationships are [Ravenala [Strelitzia + Phenakospermum]] (e.g. Kress & Specht 2006); Cron et al. (2012) found that whether Strelizia or Ravenala was sister to the rest of the family depended on the analysis.
LOWIACEAE Ridley, nom. cons. - Orchidantha N. E. Brown - Back to Zingiberales
Kaempferol 0; SiO2 bodies also ± conical [hat-shaped]; starch grains angular; endodermoid layer in rhizome; stomata paracytic, guard cells asymmetric [inner and outer ledges of the chamber unequal]; fiber cells or bundles of fibers in leaf, palisade tissue 0, large and small cells mixed; foliar lateral [cross] veins +, abaxial to longitudinal veins, (pseudopetiole 0); inflorescence modular, modules 1-flowered 6-leaved units, 4 leaves expanded, other 2 reduced, flower from the 4th leaf, branching from leaf/leaves below the flower, flower single, axillary, prophyll 0; flowers resupinate or not; outer T basally connate or not, inner T unequal, abaxial-lateral members small, inner [adaxial] median T large [= labellum]; A 5, basally adnate to inner T, grouped around style, median [adaxial] A of inner whorl ?absent; ?tapetum; septal nectary 0/non-functional; ovary prolongation several times the length of the ovary, stigma flared-tubular, unequally three-lobed, lobes ± fimbriate, (lobed), U-shaped secretory tissue on ventral/adaxial side at base [= viscidium]; inner integument ca 4 cells across; seeds ampulliform [flask-shaped], aril micropylar, lobes long; testa shortly hairy to smooth, vascularized, lignified, exotesta cells isodiametric, endotesta of radially elongated sclereids, tegmen 0; perisperm slight; n = 9, x = 8 (?6, ?7), chromosomes 4.3-6.6 µm long, ?holocentric; seedling?
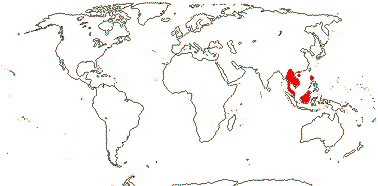
1/34: [list]. S. China to Borneo. Map: J. Kress (pers. comm.) and Sakai and Inoue (1999).
Age. Crown-group Lowiaceae may be around 3 or 19-13 Ma (Kress & Specht 2005, 2006 respectively: ?sampling) or ca ca 14.3 Ma - ca 46.1 Ma another estimate (Niissalo et al. 2022).
Evolution: Pollination Biology. The flowers last one day. They are (?always) held in an inverted position, and the positionally abaxial median petal is large and forms a labellum. The flowers often smell foul, and the dark-coloured and apparently nectarless Orchidantha inouei is pollinated by scarabeid dung beetles (Sakai & Inoue 1999), pollination in some species occuring at night (Vislobokov et al. 2017). Other species of Orchidantha have lighter-coloured flowers that smell less foul, and these are pollinated by Nitidulidae, fruit or sap beetles (Vislobokov et al. 2017); deceit pollination seems to be involved. Nopun et al. (2025) describe the anatomy and histochemistry of the labella of O. foetida and O. siamensis, dark- and white-coloured respectively, witha focus on the anatomy and secretions (foul smelling) of their osmophores.
Chemistry, Morphology, etc.. The longitudinal and horizontal vascular bundle systems of the leaf blades appear independent of one another in cross section.
The complex inflorescence morphology of Orchidantha has been described by Kunze (1986), Nagamasu and Sakai (1999), Kirchoff et al. (2020) and others. The inflorescence is polytelic, the basic unit consisting of a prophyll, two leaves, one or both of which may subtend axillary repeating basal units, a leaf/bract subtending a flower, then sometimes additional reduced leaves, and finally an aborted axis. The stamens are individually opposite each tepal member (Kirchoff & Kunze 1995). The apical prolongation of the ovary is traversed by the stylar canal (Kirchoff & Kunze 1995, c.f. Larsen 1998). It is not clear if the endotesta is silicified.
Much information is taken from Larsen (1998), also Leong-Skornicková et al. (2021); see Tomlinson (1969: anatomy), Kunze (1986: Orchidantha maxillarioides), Pedersen (2001), Pedersen and Johansen (2004) and Kirchoff et al. (2020), all flowers, and Wen et al. (1997: seed).
Lowiaceae are very poorly known.
Phylogeny. Johansen (2005) provided a phylogeny of the family, and she found that the ca seven Bornean species she sampled formed a well supported clade sister to the other five species included, all from mainland South East Asia, and these formed a less well supported clade.
[[Cannaceae + Marantaceae] [Costaceae + Zingiberaceae]] / the ginger clade: raphides 0; petiole with one series of air canals; stomata paracytic, guard cells asymmetric in transverse section [inner and outer ledges of stomatal chamber unequal]; petiole short; P fully bicyclic, inner T connate, A 1 [= median (adaxial) member of inner whorl], 2 A of both whorls staminodial, staminodes ± C-like; (tapetum multilayered), non-syncytial; micropyle endostomal; seeds arillate, aril micropylar; testa with stomata, endotestal cells large, sclerified; chalazosperm + [= perisperm of some authors], endosperm slight, embryo long.
Age. Ages suggested for this node are (51-)47(-43) and (32-)28(-24) Ma (Wikström et al. 2001: note topology), ca 47.7 Ma (Magallón et al. 2015) or ca 84 Ma (Janssen & Bremer 2004). Similar, if rather older, ages are suggested by Kress and Specht (2005, 2006), the crown group being estimated at ca 88.5 and 106-100.5 Ma old respectively.
Chemistry, Morphology, etc.. Costus, Canna and Kaempferia and at least some other genera have more or less lateral floral prophylls (e.g. Rüter 1918). Zingiberaceae and Cannaceae have anther placentoids (Weberling 1989).
Some information on seed anatomy is taken from Tang et al. (2005); there is no mention of starch in the endosperm. For some floral morphology, see Endress (1995b). Judd et al. (2007) provide useful information.
[Cannaceae + Marantaceae]: kaempferol 0; vessels in stem; oblique cells at apex of petiole [in longitudinal view]; flowers asymmetric, semi-inverted; A bisporangiate, monothecal, staminodes free; stigma not notably expanded; endosperm absent or almost so, embryo ± curved x = 9.
Age. The Cannaceae and Marantaceae clades diverged 101-91 Ma (Kress & Specht 2006), ca 68 Ma (Janssen & Bremer 2004), (54-)45, 43(-36) Ma (Bell et al. 2010: note position of Cannaceae) or as little as 38.2 Ma (Magallón et al. 2015); ages in Kress and Specht (2005, 2006) are around 80.1 and 96-91 Ma respectively.
Evolution: Pollination Biology. Although both Cannaceae and Marantaceae have asymmetric flowers and secondary pollen presentation, details are quite different in the two families.
Chemistry, Morphology, etc.. CiGLO, a B-class MADS-box gene, is, rather surprisingly, expressed in the petals, stamens, and also gynoecium (Yu et al. 2014).
For flowers, see Eichler (1874: both families together in Marantaceae), Kirchoff (1983: table of equivalencies of different parts of flowers of Cannaceae and Marantaceae) and Kunze (1984), for ovules, etc., see Johri et al. (1992), for the micropylar collar, see Boesewinkel and Bouman (1984).
CANNACEAE Jussieu, nom. cons. - Canna L. - Back to Zingiberales
Chelidonic acid, aromatic resin +; mucilage canals in stem; SiO2 bodies ± druse-shaped; (leaves spiral); inflorescence branched; flowers short-lived, paired, not enantiostylous; fertile ½ stamen with petaloid appendage, staminodes 1-4(-5); tapetal cells 2-6-nucleate; microsporogenesis also successive; G muricate, style flattened, secondary pollen presentation [pollen deposited on abaxial surface of style], stigma on one edge; outer integument ca 10 cells across; fruit glandular-muricate; seed pachychalazal, funicle hairy, aril 0, imbibition lid on raphe, micropylar collar 0, operculum 0; malpighian layer formed by exotesta and also epidermis of chalaza, mesotesta sclereidal, endotesta 0; n = 9, x = 9, chromosomes 2.1-3.4 µm long, nuclear genome [1 C] (0.051-)0.871(-14.945) pg; primary root well developed, collar roots +.
1/10: [list]. New World (sub)tropics. Map: Maas-van de Kamer and Maas (2008). [Photo - Flower]
Age. Crown group Cannaceae may be around 32-24 Ma (Kress & Specht 2005, 2006).
Evolution: Divergence & Distribution. Diversification in Cannaceae has slowed down (Hertweck et al. 2015).
Pollination Biology & Seed Dispersal. Pollen is deposited on the abaxial surface of the flattened style whence it is picked up by the pollinator.
The seeds may retain their ability to germinate for some 600 years (references in Grootjen & Bouman 1988).
Chemistry, Morphology, etc.. Floral diagrams in Eichler (1875) suggest that the bracteole is lateral and the plane of symmetry of the flower is inverted. The flowers are paired, but are usually not enantiostylous and do not show pendulum symmetry - they are modified cincinni (X. Tian et al. 2021). The nature of the androecium is unclear. Miao et al. (2014a, b) suggested that in Canna indica the fertile ½ stamen represents two primordia, one member of the outer whorl (the fertile bit) and one member of the inner whorl (the petaloid bit); the labellum then consists of another member of the outer whorl and another member of the inner whorl. On the other hand, Almeida et al. (2013) thought that the fertile and petaloid parts were together produced by a single half anther (see also Tian et al. 2017 - what the parts "are" is unclear, two thecae of one anther?). ABC-type floral genes have very broad expression patterns across the various floral organs (Almeida et al. 2013).
The micropyle becomes zig-zag after fertilization of the flower. Grootjen and Bouman (1988) described a pachychalaza in Cannaceae, with mitosis occurring during ovule development in the chalaza and basal part of the nucellus; this is unlike the pachychalaza in other zingiberalean families. An aril appears to be absent (e.g. Grootjen & Bouman 1981b), and the hairy funicle (see above) is unique in Zingiberales.
Additional information is taken from Tomlinson (1961b, 1969: anatomy), Kubitzki (1998d: general), Tanaka (2001: revision), Maas-van de Kamer and Maas (2008: monograph), Rowley and Skvarla (1986: pollen development) and Tanaka et al. (2009: cytology).
Phylogeny. For phylogenetic relationships in the genus, see Prince (2010); the North American Canna flaccida is sister to the rest of the clade, whose origin is perhaps to be sought in South America.
MARANTACEAE R. Brown, nom. cons. - Back to Zingiberales
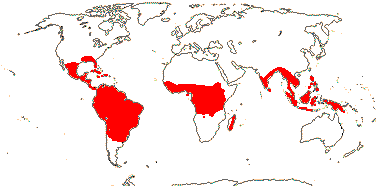
(Aerial stem +), (climbers); myricetin, flavone C-glycosides, flavonoid sulphates +; SiO2 bodies also hat-like; (stomata anomocytic); leaf sheath closed; petiole often long, pulvinate at the apex [oblique cells]; (inflorescence bracts deciduous); flowers in mirror image pairs, from a common primordium splitting, enantiostylous, of moderate size; outer whorl P sepal-like, median member subadaxial, inner whorl petal-like, it and A develop before outer whorl T and A; C, A and style all basally fused; length:width ratio of C tube usu. <5; outer whorl A staminodes 2, 1, petal-like (0), inner whorl A one staminode hooded, with trigger appendage and basal plate [= staminodium cucullatum], another ± fleshy and with callosities [= staminodium callosum], one stamen with half anther fertile, often a petal-like lateral appendage; (only 1 G fertile), style under tension, becoming curved [pollination explosive], secondary pollen presentation via the style [pollen deposited on secretory area on adaxial surface of style, the "stamp"]; ovule 1/carpel, basal, becoming amphitropous, outer integument 6-8(-12) cells across, (nucellar cap ca 2 cells across), lateral epidermal cells dividing periclinally; seed operculum endotestal; mesotesta tanniniferous, (tegmen with thin elongated sclereids), cells intruding into perisperm degenerate forming perisperm canal; endosperm not starchy, embryo strongly curved; n = 10-14, x = 7 (?9) 13, chromosomes (0.8[Maranta]-)1-1.9 µm long [mean length], ?holocentric, nuclear genome [1 C] (0.103-)0.574(-3.211) pg; (mesocotyl +), collar at right angles to cotyledon.
28/665: [list, to groups]. Tropics, esp. American, not in Australia. Map: from Heywood (1978), Andrew Ford, pers. comm., Fl. N. Am. vol. 4 (2003) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). [Photo - Leaf, Flower.]
Age. Divergence within the crown group is dated to ca 56.7 or 71.5-61 Ma (Kress & Specht 2005, 2006: Marantochloa + Mar.) or ca 57 Ma (Janssen & Bremer 2004); ages of (26-)23(-20) and (17-)14(-11) Ma (Wikström et al. 2001: Cal. + Mar.) have also been suggested, but note sampling in all these studies.
1. Sarcophrynium clade (= Phrynium clade)
Bracteole +, gland-like; 2 (0) petaloid staminodes, cucullate staminode appendages 2, divergent, unequal [1 long, rolled lengthwise; sword-type trigger appendage - not Haumania]; fruit indehiscent, caryopsis-like
6/24: Hypselodelphys (8), Sarcophrynium (6). Tropical Africa.
[Calathea [Donax [Maranta + Stachyphrynium]]] clades: cucullate staminode appendage simple; fruit a capsule.
Bracteole + (0), length:width ratio of C tube usu >5; 1(0) petaloid staminode.
5/328: Goeppertia (248), Calathea (37), Monotagma (37). Ischnosiphon (35). New World.
[Donax [Maranta + Stachyphrynium]] clades: petaloid staminodes usu. 2.
(mucilage canals - Thalia); bracteole +, gland-like; petaloid staminode (1 [Thalia, some Phrynium]), cucullate staminode with large, flat trigger appendage [ear-type], (appendages 2, long [tentacle-type trigger appendage - Thalia]; (micropyle bistomal - Phrynium); (fruit indehiscent, fleshy - Thalia).
4/48: Phrynium (37). New World - Illinois to south Argentina - few Old World (to Melanesia).
[Maranta + Stachyphrynium] clades: bracteole usu. 0; C tube length variable.
4. Maranta clade (= Myrosma clade)
(bracteole +); (fruit indehiscent - Halopegia).
7/137: Maranta (52), Strumanthe (20), Ctenanthe (15). Central and South America, West Indies (Madagascar, Java, Indochina).
Cucullate staminode with 2 appendages [some Marantochloa, Ataenidia]; seeds 3/fruit, arillate.
3/27: Marantochloa (18), Stachyphrynium (8). Africa (+ 1 sp. Madagascar and Comoros), China and Sri Lanka to West Malesia.
Unplaced: Monophrynium, Monophyllanthes, Sanblasia (latter with C tube very long and narrow).
Evolution: Divergence & Distribution. Marantaceae may have originated in Africa, with subsequent dispersal to South East Asia and the New World (Andersson & Chase 2001; Ley & Claßen-Bockhoff 2011b). Although there are about 450 species known from the New World, the bulk of the family, generic diversity is not correpondingly high. Marantaceae are considerably more speciose than Cannaceae, perhaps because of their distinctive explosive pollen transfer mechanism (Ley & Claßen-Bockhoff 2009), although it is likely that a variety of factors have shaped diversification here (Ley & Claßen-Bockhoff 2011b). Figueiredo et al. (2022) looked at diversification in seven clades of Amazonian Marantaceae in the Calathea clade - although not Calathea itself, mostly Goeppertia. They noted that species in quickly-growing clades were inhabitants of fertile soils associated with the Andean orogeny, but that diversification in these clades increased only when the Pebas system dried out some 10 Ma.
Pischtschan et al. (2010) describe the extensive variation in the cucullate inner staminode in the family, recognizing ten main variants/types. The correlation between this variation and relationships is quite good, if somewhat below the level that is the focus here.
There is considerable asymmetry of clade size within the family. Thus the oligospecific Thalia and Haumania are both sister to far more speciose clades, and both these genera have very different cucullate staminodes - they are both unique in the family - from the rest of the clade to which they belong...
Ecology & Physiology. A notable number of Marantaceae growing on the forest floor have beautifully patterned and coloured leaves (see J.-H. Zhang et al. 2020 for a classification of variegation types). In this they are similar to Begonia where blue iridescence of the leaves of plants growing in such conditions has been associated with increased photosynthetic efficiency (Jacobs et al. 2016 and references), and also to other groups growing in this habitat.
Pollination Biology & Seed Dispersal. Marantaceae have complex, highly integrated, enantiostylous, asymmetric flowers (paired flowers of Thalia form a pseudanthium - Baczynski & Claßen-Bockhoff 2023). Pollination is explosive. The style is held under tension by the hooded inner staminode (the staminodium cucullatum) that has various lobes and appendages (Pischtschan et al. 2010), while the other inner staminode (the staminodium callosum) is firm and fleshy, with knobs, etc., on its adaxial surface. Sticky pollen is deposited on the flattened stamp on the adaxial surface of the style by the early-maturing anther while the flower is still in bud, and there is an adjacent secretory area. The progress of the pollinator in the flower is guided by the knobs, etc., of the staminodium callosum, and the flower is tripped by the pollinator when it comes into contact with an appendage on the staminodium cucullatum. The style then abruptly curves and pollen from the stamp of that flower, aided by the secretions of the adjacent secretory area, is deposited on the pollinator, and pollen from another flower deposited on the stigma itself, which is depressed (Ley & Claßen-Bockhoff 2011b, 2012; see also El Ottra et al. (2023).
During pollination, the sensitive style of Thalia geniculata moves across the flower in some 0.33 seconds, most of the movement occurring within about 0.0033 seconds (Claßen-Bockhoff 1991b). The anatomy of the style is distinctive, with a combination of collenchymatous cells, large intercellular spaces, extensive elliptical openings on the walls, and separation of the cells by breakdown of the primary wall starting before the flower opens. As the style moves, there is extensive redistribution of water between the cells (Pischtschan & Claßen-Bockhoff 2010).
Long-tongued, trap-lining euglossine bees are the main pollinating agents in the New World, and the floral tube lengths of New World Marantaceae are appreciably longer than their Old World representatives, ca 17.6 mm long versus ca 4.6 mm long. Interestingly, there are no intrinsic barriers to selfing (see Claßen-Bockhoff 1991b for floral morphology and function and Kennedy 2000 for general information, also Andersson 1998; Classen-Bockhoff & Heller 2008 for a developmental study on the diversity of form of some New World Marantaceae). African Marantaceae are pollinated by large and small bees and sunbirds, and there has been parallel evolution of the various morphologies involved when compared with New World taxa with bee and bird pollination (Ley & Claßen-Bockhoff 2010, 2011a, esp. 2009 for details). Variation in tube length alone may allow effective pollination by very different kinds of pollinator within the one floral morphology (Ley & Claßen-Bockhoff 2010, 2011b). Hooded staminodes with a rather simplified morphology may be derived (Pischtschan et al. 2010; Ley & Claßen-Bockhoff 2011b).
For seed dispersal in some New World Marantaceae, by birds or by ants, see Horvitz et al. (2002 and references).
Genes & Genomes. Andersson (1998) questioned the chromosome numbers reported for Marantaceae because of the small size of the chromosomes - (0.8[Maranta]-)1-1.9 µm mean lengths - Winterfeld et al. 2021) and problems with the identity of the material counted. Winterfeld et al. (2020) surveyed the family, reporting basic numbers from 9 to 14; there has been quite extensive polyploidy and aneuploidy, and it is a count of 2n = 72 that gives a haploid number of 9...
There has been a rapid acceleration in mitochondrial DNA substitution rates in Sarcophyrnium, and also some genera in other families (Zwonitzer et al. 2024: see also Characters).
Chemistry, Morphology, etc.. The plant body of Marantaceae is made up of modules consisting of a prophyll, a reduced leaf (both with short internodes), and then expanded leaves. These latter vary in number and internode length (although the first is often longest) and also orientation, since the plane of distichy of a unit may be parallel to or at right angles to that of its parent axis. The leaves are usually counterclockwise-convolute, and the blades are asymmetric, having a wider right half - reversed in clockwise-convolute leaves - and there can also be anatomical asymmetry along with the morphological asymmetry (de Albuquerque et al. 2019; see also Tomlinson 1961a - c.f. in part).
The inflorescence is almost mind-bogglingly complex (Tomlinson 1961a; Andersson 1976; Kunze 1985); the latter suggests that the apparently indeterminate units that bear the paired flowers are modified from determinate structures. There has been much recent work on floral morphology and development. The basic orientation of the flower is more or less inverted, the median sepal being subadaxial (Pischtschan & Claßen-Bockhoff 2008; Ley & Claßen-Bockhoff 2011b, 2012). Depending on factors like whether the inflorescence is held erect or pendulous, there are various kinds of changes in the orientation of the flowers, as in Thalia, but the flowers are not resupinate in a strict sense, not all rotating 180o (Dworaczek & Claßen Bockhoff 2016). According to those authors, the two flowers of the flower pair are the result of a common primordium splitting into two equaal halves. For variation in the branching of the perisperm canal, see references in Benedict et al. (2018).
Some general information is taken from Eichler (1884), Andersson (1981, 1998) and Prince and Kress (2006a). For morphology and anatomy, see Tomlinson (1969) and for seed morphology, see Grootjen (1983a).
Phylogeny. Prince and Kress (2006a) found that relationships between the Sarcophrynium, Stachyphrynium, Maranta, Donax and Calathea groups into which the family could be divided was for the most part unclear (very low bootstrap values, mostly high posterior probabilities alone), and support for these five groups other than the Stachyphrynium and Maranta clades (also well supported as sister taxa) was little better (Prince & Kress 2006a, b: two plastid genes, 80 taxa, eight genes, all three compartments). For relationships among Asian members of the Stachyphrynium and Donax clades, see Suksathan et al. (2009). Borchsenius et al. (2012) looked at relationships in Calathea (3 plastid markers, ITS, 42 spp., all main groups), confirming its polyphyly; in Goeppertia, which they resuscitated, they recovered six main clades, Calathea/Goeppertia straminea, a distinctive species that i.a. has inflorescences borne on leafless shoots, being sister to the rest of the genus. The major clades in the family that Prince and Kress (2006b) had recovered also appeared here, although Haumania moved from the Calathea group to the Sarcophrynium group (Borchsenius et al. 2012), which makes sense. The focus in Fernandes et al. (2023: 1 nuclear, 3 plastid markers, 32 spp.) was on Maranta, which, as in earlier studies, was found to be non-monophyletic, and the current infrageneric classification did not have much support. Luna et al. (2025: 41spp., 3 plastid and 1 nuclear markers) looked at relationships in the Maranta group; Halopegia was sister to the rest, and on a very long branch.
Classification. Andersson and Chase (2001) provided a phylogenetic classification of the family, but this now hardly reflects what is known about phylogeny; Prince and Kress (2006a) suggested that five informal groups be recognized (see above). Studies on Asian members of the Stachyphrynium and Donax clades has led to generic realignments - Phrynium was paraphyletic (Suksathan et al. 2009). Calathea is polyphyletic, and Borchsenius et al. (2012) placed most of its species in Goeppertia. Fernandes et al. (2023) extended the limits of Maranta.
[Costaceae + Zingiberaceae]: fibrous sheath in stem; leaf ligulate; bracteole lateral; outer T = K connate; stamen with three or more vascular bundles [?sampling]; filament flattened, connective prolonged, abaxial member of outer A whorl staminodial, all 5 staminodes connate ["lateral staminodes fused to labellum"], forming labellum, with narrow tube and distinct open limb; exine + [so pollen resistant to acetolysis]; epigynial nectaries 2, vascularized; style slender, running between two half anthers, stigma cup- or funnel-shaped, ± bilobed; ovule with hypostase; embryo sac with postament; endotesta well developed; endosperm helobial, persistent, not that copious; seedling with well developed hypocotyl.
Age. Estimates for the time of divergence of these two families are ca 83 or 105-99 Ma (Kress & Specht 2005, 2006 respectively), ca 79 Ma (Janssen & Bremer 2004), (84.9-)74.2(-65.2) Ma (Carlsen et al. 2018), ca 84.9 Ma (Böhmová et al. 2022) and (90.3-)80.4(-72.8) Ma (Xavier et al. 2024).
Evolution: Divergence & Distribution. For possible additional floral synapomorphies, see Specht et al. (2001).
Chemistry, Morphology, etc.. Pancharoen et al. (2000) summarize the phyochemistry of Zingiberaceae s.l.. The fibrous sheath in the stem may be outside the vascular bundles, or somewhat more interior (Tomlinson 1969). Wijesinghe and Perera (2025) describe some remarkable phytoliths in some plants belonging to these two families from Sri Lanka.
For floral development, see Rao et al. (1954) and Kirchoff (1988a). The massive stamens of Costus and of some Zingiberaceae have several vascular bundles (Rao et al. 1954). Van Heel (1988) described the gynoecium of Costus as having septal nectaries, that of Zingiberaceae as lacking them. However, Rao (1963; see also Burtt 1972b) showed that in Costus there were three complex nectar-secreting septae in the upper part of the ovary, but only two epigynial glands, that is, free-standing structures in the uppermost part of the inferior ovary, while in Zingiberaceae there were either two (long-)linear free-standing epigynial nectaries at the base of the corolls tube, or these were variously connate and shaped (see also Rao et al. 1954; Newman & Kirchoff 1992). The embryo sac sometimes has supernumerary (?nucellar) nuclei (Panchaksharappa 1962 and references).
COSTACEAE Nakai - Back to Zingiberales
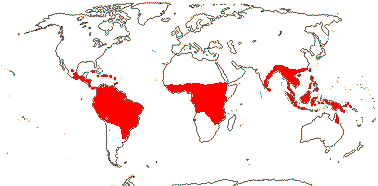
(Plant epiphytic); aerial stem +, (branched); benzoquinones, flavone C-glycosides, steroidal saponins +; SiO2 bodies also ± druse-shaped; sheath with 1 series of adaxial air canals, no canals in petiole and blade, vascular bundles adaxial; hypodermis ³1 layered; (hairs multicellular); leaves spiromonostichous, sheath closed; inflorescence spicate-capitate, (axillary), unbranched, flowers (single), bracts often with abaxial nectaries; anthers with several vascular bundles, filaments medium; pollen resistant to acetolysis, pantoporate/spiraperturate [spirocolpate]/etc.; (G [2]), nectary above ovary loculi, stigma with adaxial projection, fimbriate; outer integument 5-6 cells across; seed with sunken chalaza, chalazal chamber + [surrounded by testa]; endosperm oily, starch 0, embryo ± straight; n = 9 (14), x = 9, chromosomes 2.3-3.7 µm long, ?holocentric, nuclear genome [1 C] (0.104-)1.24(-14.79) pg; cotyledonary hypophyll blade-like, photosynthetic, with apical backwardly-directed process, first leaves foliaceous, sheath closed.
7/143: [list] - Costus (108). Pantropical, esp. America and Papuasia-Australia. Map: Maas (1972) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). Photos Costus © L. Brothers, Dimerocostus © L. Brothers.
Age. Divergence within crown group Costaceae can be dated to (30-)27, 26(-23) or (18-)15(-12) Ma (Wikström et al. 2001), ca 47 Ma (Janssen & Bremer 2004), 23.2 Ma and 74 or 52-47 Ma (Specht 2005, 2006b respectively), (38.9-)33.6(-29.3) Ma (Carlsen et al. 2018) and 29.3-24.3 Ma (Böhmová et al. 2022: see outgroup).
Evolution: Divergence & Distribution. Specht (2006b) discussed the diversification and biogeography of Costaceae in detail. There are two major clades in the Neotropics, members of one, the less speciose and consisting of generic segregates, have largely allopatric distributions, while members of the other, Costus s. str., show a much higher degree of sympatry (André et al. 2016). In this latter group, Vargas et al. (2020) found that drivers of speciation in lowland (Amazon) and mountain-dwelling (up to 2,000 m) taxa were rather similar, allopatry and ecogeographic speciation being involved in both. American Costus appears to have arrived there from Africa (Maas-van de Kamer et al. 2016; see also Böhmová et al. 2022).
For floral evolution and pollination, see Specht (2005; also Kay et al. 2005; Kay & Schemske 2003). Hummingbird pollination seems to have been particularly important in facilitating diversification of Neotropical Costus, but euglossine bees are also effective pollinators (Salzman et al. 2015); euglossine pollination is probably the ancestral condition, but ca half the genus (26 species or more) are pollinated by hummingbirds, with around a dozen origins and at least one reversal (Vargas et al. 2020).
Ecology & Physiology. For the spiromonostichous leaf phyllotaxis, unique in flowering plants, its effect on leaf shading, and its association with features of foliar anatomy, see Salvi and Smith (2016; also von Veh 1931). Yonekura and Sugiyama (2024) developed a model to explain the development of thisd distinctive phyllotaxis, the leaf primordia having both an inductive and an inhibitory effect on future primordia; note that their model also generated one-sided distichy patterns, 'charaterized by its primordium initiation position on two angled orthostichies' (ibid. p. 152) - leaves 2-ranked, but on the same side of the stem - a very unusual pattern found in the inflorescences of Thalia and Ctenanthe, in the related Marantaceae.
Pollination Biology & Seed Dispersal. Around half the species (26) of New World Costus are pollinated by hummingbirds, the rest by euglossine bees (Kay et al. 2005; Vargas et al. 2020; see also Thomson & Wilson 2008). There are often nectaries on the inflorescence bracts that are visited by ants.
Species with seeds that are dispersed by ants are common here (Lengyel et al. 2010).
Plant-Animal Interactions. Quicke et al. (2025) found caterpillars of tiget moths and skippers eating Costus in Guanacaste, Costa Rica.
Chemistry, Morphology, etc.. The bracteole is described as being lateral and consistently anodic by Kirchoff (1988b) and is drawn in an adaxial-oblique position by Ronse de Craene (2010). The pollen is particularly variable in morphology and the grains are resistant to acetolysis (Stone et al. 1981 and references). There are two to four rows of ovules (Newman & Kirchoff 1992).
Some information is taken from Larsen (1998: general), Tomlinson (1969: anatomy), Kirchoff (1988b: floral morphology) and Panchaksharappa (1963) and Grootjen and Bouman (1981b), ovule and seed development, etc..
Phylogeny. Specht (2006a) provided a detailed phylogeny of Costaceae (see also Specht et al. 2001); the clade [Chamaecostus [Dimerocostus + Monocostus]] is sister to the rest, within which the distinctive Tapeinochilos is embedded. For relationships in Costus, see Maas-van de Kamer et al. (2016), also Kay et al. (2005) and Vargas et al. (2020), both focus on the American taxa. J. Chen et al. (2022: full plastomes around Hellenia, 2 chloroplast markers in broader analysis) looked at relationships among Asian taxa.
W. J. Baker et al. (2021: see also Seed Plant Tree) found that Siphonochilus (here placed as sister to all other Zingiberaceae) was embedded in Costaceae; as of iii.2023 relationships there were [Costus woodsonii [S. aethiopicus [Paracostus aenigmaticus [four other genera]]]] - but see below under Zingiberaceae. Böhmová et al. (2022: HybSeq and plastome data) carried out a comprehensive analysis of the family - Siphonochilus was the outgroup - and recovered the monophyly of all the genera except that Tapeinochilos was embedded in Cheilocostus; Paracostus was sister to the rest of the family. Some relationships along the spine - and between species - were not very well supported. However, Costus woodsonii was back to its position (Costus was paraphyletic) in the Seed Plant Tree version ix.2024.
Classification. A generic revision (Specht & Stevenson 2006) is based on a phylogeny of the family in which Costus turned out to be polyphyletic (Specht 2006); the genera that they recognize can be characterized morphologically. Böhmová et al. (2022) thought that Tapeinochilos should be recognized, and something of a nomenclatural mess has developed around Tapeinocheilos (J. Chen et al. 2022) and Cheilocostus (Nguyen et al. 2023).
ZINGIBERACEAE Martinov, nom. cons. - Back to Zingiberales
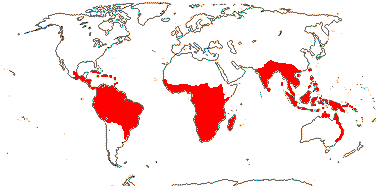
SiO2 bodies also epidermal; (hairs with sunken bases); (leaf sheath closed); hypodermis 0-1-layered; (inflorescence bracts deciduous); anther crest petaloid, filament short, median A of outer whorl 0, lateral staminodes of outer whorl C-like; nectaries free-standing in base of floral tube [variously connate and shaped]; ovules (1-)many/carpel, outer integument (5-)7-13 cells across; (nucellar cap +), lateral epidermal cells dividing periclinally, epistase +; fruit fleshy [but usu. dehiscent]; (hairs on seed); exotesta of fibriform cells, uniseriate, micropylar hilar rim [external tube or flange], endotestal cells parenchymatous, chalazal pigment cells disciform; chalazosperm 0; embryo ± length of seed, (curved) [plumular end L- or J-shaped]; x = 12 (?8, ?13), nuclear genome [1 C] (0.169-)1.696(-17.026) pg; cotyledon haustorial, seedling collar not prominent, first leaves haustorial.
58/1,957 (POWO - also 1,100-1,430, 1,600, 1,800): [list: to tribes] - four groups below. (Sub)tropical, esp. South East Asia-Malesia. Map: from Maas (1977), Heywood (2007) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). [Photo - Fruit.]
Age. The age of crown-group Zingiberaceae is somewhat more than 70 Ma (Auvray et al. 2010, age of Zingiberopsis), although since Spirematospermum chandlerae has been included in crown-group Zingiberoideae (S. Y. Smith et al. 2018) and is perhaps 80 Ma (Friis 1988), dates are unclear.
1. Siphonochiloideae W. J. Kress
Plants show dormancy; rhizome fleshy, often ± vertical, (roots tuberous); ?plane of distichy of leaves; inflorescence on leafless shoot, a raceme, bracteoles 0; filament of fertile stamen adnate to base of labellum, forming a tube above point of insertion of petals; ?lateral staminodes of outer whorl; seed raphe externally visible, aril solid, micropylar; (exotesta multiseriate), micropylar collar 0; n = 13, 14, 21.
2/20. SubSaharan Africa and Madagascar.
[Tamijioideae [Alpinioideae + Zingiberoideae]]: 12-b.p. insertion at 3' end of matK absent.
2. Tamijioideae W. J. Kress - Tamijia S. Sakai & Nagamasu
Rhizome fibrous; plane of distichy of leaves transverse to rhizome; inflorescence axes separate from vegetative axes, long, prostrate; lateral staminodes of outer whorl adnate to labellum; placentation parietal/axile; seed unknown; n = ?
1/2. Borneo, Sarawak and Brunei.
[Alpinioideae + Zingiberoideae]: phenylpropanoids and related curcumins, ethereal oils +; SiO2 usu. also as sand; (root ± not medullated, centre with phloem strands); (vessels also in stem); sieve tubes with nuclear non-dispersive crystalline protein bodies; oil cells +; lateral staminodes ± free from labellum, (anther crest 0); aril usu. surrounding more than half the seed, adpressed, (chalazal chamber +); endosperm without starch.
Age. Divergence of these two subfamilies has been dated to ca 26 Ma (Janssen & Bremer 2004) and a mere (11-)10(-9) and (6-)5(-4) Ma (Wikström et al. 2001); ages of (54.7-)35.6(-17.9) Ma (Eguchi & Tamura 2016), ca 65 Ma (Kress & Specht 2005, 2006: calibration point) and (63.9-)57.0(-49.5) Ma (Xavier et al. 2024) have also been suggested.
3. Alpinioideae Link
Plants evergreen, no dormancy; rhizome fibrous; plane of distichy of leaves transverse to rhizome; (inflorescence axes separate from vegetative axes); filament medium; median A of outer whorl 0, lateral staminodes of outer whorl 0/very small; micropylar hilar rim usu. 0, (chalazal chamber +); endotesta lignified, endotestal chalazal pigment cells non-disciform [trumpet-shaped, etc.]; perisperm with simple starch grains, endosperm lacking starch, embryo medium to long; x = 12, chromosomes 0.7-4.5 µm long.
20/1,013. Mainly Indo-Malesia, also tropical Australia and American and African tropics.
Age. Fossils of Spirematospermum chandlerae and S. wetzleri are assigned to crown-group Alpinioideae (S. Y. Smith et al. 2018) and those of the former are early Campanian, perhaps 80 Ma (Friis 1988); other species of Spirematospermum may be stem [Alpinioideae + Zingiberoideae] (Smith et al. 2018) - on the other hand, S chandlerae fossils 83.6-72.1 Ma have been considered to be stem Zingiberaceae (Iles et al. 2015.
3A. Alpinieae A. Richard —— Synonymy: Alpiniaceae Link, Amomaceae Jaume Saint-Hilaire
Vessel elements in roots also with simple perforation plates; (styloids + - Aframomum); (placentation free-central), flexistyly widespread; (fruit indehiscent); seed (raphe externally visible); n = (11 - Renealmia, 24 - Amomum).
16/830: Alpinia (200), Etlingera (110), Renealmia (75), Amomum (65), Aframomum (60), Hornstedia (50), Meisteria (42). Esp. Indo-Malesia (West of Wallace's Line), some temperate, also American and African tropics (Renealmia), African tropics (Aframomum).
3B. Riedelieae W. J. Kress
Blade with extrafloral nectaries on adaxial midrib; (placentation parietal); fruits elongated, opening to the base; aril micropylar, solid.
4/105. Riedelia (75). Eastern Malesia, also Australia (Queensland) and Thailand, both one species.
4. Zingiberoideae Hasskarl
Plants show dormancy; rhizomes fleshy, (starchy roots or tubers); plane of distichy of leaves parallel to rhizome; (inflorescence branches spiral); filament short to long; lateral staminodes of outer whorl free from (adnate to) labellum; (tapetum amoeboid); style with 2 vascular bundles; seed hairy, (micropylar collar 0); endosperm with starch [aleurone]; chromosomes 2.1-5.8 µm long; plastid inheritance biparental.
33/922. Indo-Malesia; tropical Australia.
4A. Zingibereae Petersen —— Synonymy: Curcumaceae Dumortier
Pseudostem common; (steroidal saponins, myricetin + - Hedychium); (pollen sulcate - Zingiber); (anther crest wrapped around style); (placentation basal or free-central); fruits globose or ovoid, fleshy, (indehiscent); seed (hairy), (not operculate - Hedychium), aril (carunculate); U-shaped cells in endotesta 0; starch grains of perisperm compound; n = 11, 12, 17, 18, etc..
30/655. Curcuma (100), Zingiber (100), Boesenbergia (95), Hedychium (90). India-S. China-Malesia, some some temperate/high altitude, tropical Australia.
4B. Globbeae Petersen
Roots tuberous (not); blade (decuurent o n pseudopetiole); infloresecnce terminal on leafy shoot/basally on separate shoot, with cincinni/spicate; anther (crest spurred), (hinged at junction with filament; lateral staminodes adnate to labellum and filament adnate (not); anther long-exserted on arched filament (filament ±= anther); placentation parietal; seeds (arillate); exotesta usu. multiseriate; starch grains of perisperm simple; n = (8, 10, 11, 12, 14) 16, 24, etc..
3/125: Globba (ca 100). Sri Lanka, India and S. China to Malesia, Queensland and the Bismarck Archipelago.
Evolution: Divergence & Distribution. The distinctive seeds of species of Spirematospermum, known from the Late Cretaceous and Palaeogene seem best placed in stem Zingiberaceae (Iles et al. 2015) or, for some, crown-group Zingiberoideae (Smith et al. 2018) is best; crystal sand has been found in the fossils, and this is common only in Zingiberaceae (S.-T. Chen & Smith 2013). S. Y. Smith et al. (2021) discuss other fossils from around here. Momordiocarpon is to include Musa cardiosperma, from the Deccan Intertrappean beds, and it and Orthogonosperma, also from these beds, are to be included in Zingiberaceae, some analyses even placing them close to Aframomum.
The origin of the family was Mid-Cretaceous and probably in northern Africa, and that of Alpinioideae and Zingiberoideae in particular in the Indo-Burma area, diversification in these latter being linked to Tertiary climatic changes (J.-L. Zhao et al. 2022).
Xavier et al. (2024) looked at correlations between various aspects of the cytology of Zingiberaceae - chromosome number, genome size (these two varied by factors of 6 and 49 respectively and were not correlated), frequency of polyploidy/hemiploidy, dysploidy (ascending dysploidy was commonest), and so on, with geography, genus size and age, etc. - altogether a complex story.
Renealmia is the only genus of Zingiberaceae in South America, and it seems to have migrated there from Africa within the last 16 Ma (Särkinen et al. 2007). Aframomum is its sister taxon, and diversification in that genus may have begun ca 34.3-25.2 Ma, although some estimates are much more recent (Auvray et al. 2010).
Ashokan et al. (2022a, b) looked at diversification within the speciose Hedychium. The basal clades there tend to have whitish, often scented flowers; brightly coloured and less often scented flowers are commonest in the speciose H. coronarium clade, although this seems not to convert to obvious pollinator adaptations; there is no simple division between diurnal/bird and nocturnal/moth pollination.
There are 0, 2, 4 or 6 flaring appendages on the anthers of Globba (hence the apt name of a segregate, Mantisia), and their development was studied by Cao et al. (2018), who also optimised them on a phylogenetic tree of the genus.
Benedict et al. (2018) noted that the considerable variation in seed characters in Zingiberaceae was not linked to climate; both Zingiberoideae and Alpinioideae have moved into temperate/high altitude habitats several times. Benedict et al. (2015b) optimised a number of seed characters on the tree.
Species of Curcuma are quite often of hybrid origin. In the case of C. vamana, members of one putative parent group are currently ca 2,000 km distant, yet most of the features of C. vamana are of this group (Záveská et al. 2012, see also 2015).
Much "familial" information like sieve tube morphology is properly to be placed at the [Alpinioideae + Zingiberoideae] node, the other two subfamilies being poorly known.
Ecology & Physiology. Although one often thinks of Zingiberaceae as being a tropical family, species in genera like Hedychium and in particular Roscoea can tolerate extreme conditions in the Himalayas. Furthermore, the distributions of fossil Zingiberaceae suggest that the group was initially subtropical, but that there were quite extensive changes as climate shifted during the Tertiary (Quirk et al. 2024).
Pollination Biology. For a detailed study of floral morphology and pollination (pollinators: two kinds of bees and a nectariniid spiderhunter) in some Bornean gingers, see Sakai et al. (1999b, 2013). Ley and Harris (2014) looked at floral morphology in African Aframomum where most species had purple, trumpet-shaped flowers that were probably pollinated by bees. Flexistyly (the style changing its orientation during anthesis) is scattered through Alpinieae (Kress et al. 2005a; Raju 2019 and references), while the style becomes spirally-twisted in the flowers of Roscoea schneideriana. What goes on here is unclear - the base of the floral tube is occluded by the style and there is no nectar (B. Wang et al. 2020); Paudel et al. (2019 and references) studied pollination in other members of the genus. There are staminal (thecal) appendages in Roscoea (Paudel & Li 2020) which are integral to the pollination process in cross-pollinating species of the genus. Etlingera elatior has pseudanthia (Baczynski & Claßen-Bockhoff 2023).
Genes & Genomes. Beltran and Kiew (1984) discussed the cytology of Zingiberaceae. Hlavatá et al. (2022) looked at genome size and its evolution in Amomum; genome size tended to increase in the genus. Xavier et al. (2024) examined a number of aspects of cytology of the family (nothing is known about Tamijioideae) - see above under Divergence & Distribution.
Records of plastid inheritance are to be found in Q. Zhang et al. (2003) and references.
Economic Importance. For Zingiber, ginger, Curcuma, turmeric, and Elettaria, cardamom, see Ravindran et al. (2007 and references).
Chemistry, Morphology, etc.. The plane of distichy of leaves of the axillary bud is commonly parallel to the rhizome, only sometimes is it at right angles (Weisse 1932, 1933; R. M. Smith 1985), while Bai and Xia (2024) note that both variants occur in Zingiber - this feature, used to characterize subfamilies above, needs re-evaluation. For variegation in the leaves of juvenile Kaempferia, see Y.-S. Chen et al. (2017).
Although Larsen et al. (1998) suggested that Hedychieae lack an operculum in the seed, Grootjen and Bouman (1981b) had reported one from Hedychium itself. Pommereschea (Zingibereae) has a parenchymatous endotesta (Liao & Wu 2000).
Some information is taken from Larsen et al. (1998), Kress et al. (2002) and Tan et al. (2020: Globbeae), general, Tomlinson (1956, 1969: anatomy), Uma and Muthukumar (2014) and Gevú et al. (2017), both root anatomy, Harling (1949) and Panchaksharappa (1962), both embryology, Liao and Wu (1996) and Benedict et al. (2015a: Alpinioideae, 2015b, whole family, X-ray tomographic microscopy), all seed anatomy, Wood et al. (2000: Hedychium and relatives), Poulsen (2012: Etlingera) and Sakai and Nagamasu (2000: Tamija). For floral morphology and development, see Rao (1963: nectaries), Rao et al. (1954), Kirchoff (1997: Hedychium, i.a. distinctive floral orientation), Box and Rudall (2006) and Kong et al. (2007), both Globba, and Iwamoto et al. (2020: Globbeae).
Phylogeny. Basic relationships in the family are [Siphonochiloideae [Tamijioideae [Alpinioideae + Zingiberoideae]]]; all clades have strong support (Kress et al. 2002: two genes; D. J. Harris et al. 2006). Zuntini et al. (2024) found that Aulotandra trigonocarpa (Alpinieae) alone was sister to the rest of the family, including Tamija, but sampling was exiguous. Although as mentioned above Siphonochilus was embedded in Costaceae in early version of the Kew Tree of Life, in the Seed Plant Tree consulted 2/ii/2024 a clade [Siphonochilus + Aulotandra] was sister to all other Zingiberaceae. Z.-D. Chen et al. (2016) examined relationships in Chinese Zingiberaceae - there was some phylogenetic structure in Alpinioideae, little in Zingiberoideae.
Alpinioideae. For a phylogeny of part of Alpinioideae, see Pedersen (2004) and especially Kress et al. (2005a). Alpinieae. Pedersen (2004) and Kress et al. (2005a) found that Alpinia, Etlingera, and Amomum, all part of this tribe, are all more or less strongly para/polyphyletic. Xia et al. (2004), de Boer et al. (2018) Poulsen et al. (2018: Elettaria triphyletic) examined relationships around Amomum and Hlavatá et al. (2022) compared relationhips obtained from analyses of 449 nuclear genes, complete plastomes, the rDNA cistron and ITS; although the same four major groupings, and three subgroups of one of those groups, were obtained, relationships between those groups differed, differences in the nuclear and plastome analyses being least (a pair of major groups switched positions). Auvray et al. (2010) looked at relationships around the African Aframomum. Särkinen et al. (2007) provided a phylogeny of the African-American Renealmia, but in a more comprehensive study by Valderrama et al. (2017) the deeper relationships within the genus were not well supported, although those within some groups were; it was unclear whether the African taxa were paraphyletic with respect to the American taxa, but the latter probably formed a single clade.
Zingiberoideae. Ngamriabsakul et al. (2004) discuss relationships within Zingibereae; for a phylogeny of Globbeae, see Williams et al. (2004), Záveská et al. (2012) clarify the limits of Curcuma and Sam et al. (2016) looked at relationships in the Scaphochlamys area. The monophyly of Globbeae is as yet poorly supported (Tan et al. 2020). There are five main clades in Hedychium, with some 90 species, and clade V, the H. coronarium clade, is notably speciose; H. pauciflorum by itself is sister to these five clades (Ashokan et al. 2022a). As Ashokan et al. (2022b: p. 1424) note, Hedychium "is a young, messy genus with poorly defined species boundaries".
Classification. The classification above and the genera are largely taken from Larsen et al. (1998) and Kress et al. (2002). However, it has been evident for some time that generic limits in Alpinioideae needed attention (e.g. Xia et al. 2004; Kress et al. 2007). De Boer et al. (2018) placed the species in the Amomum area in several genera to keep Alpinia, etc., apart, although it is unclear exactly what the options for classification around here really are. New genera are being described from Malesia/South East Asia (e.g. San 2016; Lim 2016b). Záveská et al. (2012) include four small genera in Curcuma and provide an infrageneric classification.