 |
QUICK SEARCH
MO PROJECTS:
Africa
Asia/Pacific
Mesoamerica
North America
South America
General Taxonomy
Photo Essays
Training in Latin
America
MO RESEARCH:
Wm. L. Brown Center
Bryology
GIS
Graduate Studies
Research Experiences
for Undergraduates
Imaging Lab
Library
MBG Press
Publications
Climate Change
Catalog Fossil Plants
MO DATABASES:
W³MOST
Image Index
Rare Books
Angiosperm
Phylogeny
Res Botanica
All Databases
INFORMATION:
What's New?
People at MO
Visitor's Guide
Herbarium
Jobs & Fellowships
Symposium
Research Links
Site Map
Search
Canastra, a New Genus of Paniceae (Poaceae, Panicoideae) Segregated from ArthropogonNovon 11(4): 429-436, f. 2. 2001. Osvaldo Morrone and Fernando O. Zuloaga
Gerrit Davidse
Tarciso S. Filgueiras
ABSTRACT. Based on morphological, anatomical, and molecular evidence, the Brazilian endemic grass Arthropogon lanceolatus is reclassified in the newly described, monotypic genus Canastra. Canastra lanceolata is illustrated, and the genus is compared with the putatively related taxa in the Paniceae: Altoparadisium, Achlaena, Homolepis, Streptostachys, Panicum sects. Laxa, Lorea, and Prionitia, and Cliffordiochloa Key words: Brazil, Canastra, Paniceae, Panicoideae, Poaceae. During a recent revision of Arthropogon Nees and the establishment of the new genus Altoparadisium Filgueiras, Davidse, Zuloaga & Morrone (Filgueiras et al., 2001), it became evident that Arthropogon lanceolatus Filgueiras was erroneously placed within that genus. However, at that time it was not possible to ascertain a clearly defined position within the Paniceae for this taxon. New phylogenetic studies in the tribe, using molecular data of the gene ndhF (Giussani et al., in press), together with previous morphological analyses (Zuloaga et al., 2000; Filgueiras et al., in prep.) allowed us to establish its relationships within Paniceae more clearly, and we now describe a new genus to place this species. MATERIALS AND METHODS. Herbarium specimens were examined from IBGE, MO, R, and US. Transverse sections of dried leaf blades were prepared after desilicification in 10% hydrofluoric acid (Breakwell, 1914); the sections were then stained in safranin and fast green. Abaxial epidermal peels of leaf blades were prepared by removing the mesophyll and vascular tissue with a scalpel following the method of Metcalfe (1960). The epidermis was then stained in safranin. The standardized terminology of Ellis (1976, 1979) was used to describe the anatomical structure of the leaves. Canastra Morrone, Zuloaga, Davidse & Filgueiras, gen. nov. TYPE: Canastra lanceolata (Filgueiras) Morrone, Zuloaga, Davidse & Filgueiras. Spiculae dorsaliter compressae, callus glaber haud pilosus, gluma infera et supera aristatae, gluma infera redacta, 1-nervia, anthoecium superum cartilagineum, quam gluma supera et lemma inferum crassius, lemma superum paleae superae apicem includens, lamina anatomia non-Kranz ("C3" dicta), mesophyllum cellulis fusiformibus manifestis praeditum. Homolepis, Streptostachys, Cliffordiochloa et Panicum sect. Laxa spiculis dorsaliter complanatis, callo glabro, cellulis fusiformibus mesophyllo, cellulis distinctis nullis ("Kranz" dictae) similis sed glumis aristatis, anthoecio supero cartilagineo absimilis. Canastra lanceolata (Filgueiras) Morrone, Zuloaga, Davidse & Filgueiras, comb. nov. Basionym: Arthropogon lanceolatus Filgueiras, Bradea 3(36): 307. 1982. TYPE: Brazil. Minas Gerais: Furnas, Ribeirão das Pacas-Cascata, na margem do rio sobre quartzito, 28 July 1966, Legerunt Luiz Emygdio, Aydil Andrade, Nobre de Melo, E. Lessa & F. Medeiros 2308 (holotype, R-116424; isotypes, IBGE-19804, MO-5102622, US-5102622). Fig. 1 (line drawing). [ enlarge (39K) / enlarge (16K) ] Plants perennial, caespitose. Culms 50--60 cm tall, simple or branched at the basal nodes, erect; internodes compressed, striate, glabrous; nodes glabrous. Sheaths overlapping, longer than the internodes, laterally compressed, keeled on the back, ciliate, with papillose-pilose hairs on the lower portion of the membranous margins, otherwise glabrous; lower sheaths usually laciniate. Ligules 1.4--1.6 mm long, membranous-ciliate, the membranous portion ca. 0.2 mm long, the hairs 0.9--1.3 mm long, whitish; collar glabrous. Leaves linear, 15--30 × 0.4--0.7 cm, involute, rigid, narrowed at the base, the apex subulate, densely papillose-pilose on the basal portion near the ligule, glabrous on the rest of the surface, the margin involute, papillose-pilose toward the base. Inflorescences terminal or axillary, borne from the uppermost nodes, many-flowered; peduncle up to 25 cm long, glabrous. Terminal panicle congested, exserted, 10--22 × 2--5 cm; main axis angular, scaberulous to smooth, glabrous; pulvini long-pilose; primary branches alternate to subopposite, the lower ones 4--5 cm long, divergent from the axis; secondary and tertiary branches adpressed, axis of the branches triquetrous, scabrous; pedicels triquetrous, scabrous; axillary panicles partially exserted, 8--10 × 1--2 cm. Spikelets long ellipsoid, 3--3.2 mm long, exclusive of awns, 0.8 mm wide, dorsiventrally compressed, 2-flowered, scabrous, greenish and tinged with purple. Lower glume awned, 0.4--1.2 mm long without the awn, 1-nerved, scabrous, the awn up to 1.7 cm long, scabrous with antrorse hairs, flexuous, purplish. Upper glume as long as the spikelet, awned, herbaceous, 5-nerved, the nerves prominent and scabrous, the awn up to 1.5 cm long, scabrous with antrorse hairs, purplish. Lower lemma 2.7--3 mm long, 3-nerved, acute, glabrous. Lower palea lanceolate, 2--2.2 × 0.5 mm, hyaline, 2-nerved, glabrous, the margins smooth to scaberulous. Lower flower staminate, stamens 3, purplish; anthers 1--1.8 mm long. Upper anthecium long-ellipsoid, acute, cartilaginous, 2.4--2.7 × 0.4 mm, glabrous. Upper lemma not enclosing the apex of the palea, 3-nerved, the nerves not prominent. Upper palea 2-nerved, glabrous. Upper flower bisexual; lodicules 2, ca. 0.2 mm long, hyaline, conduplicate; stamens 3; anthers 1.4--1.6 mm long; styles 2, free from the base; stigmas plumose, purple. Caryopsis long ellipsoid, 1.2 × 0.3 mm, plano-convex; hilum punctate, basal; embryo 1/3--1/2 as long as the caryopsis. Additional specimens examined. BRAZIL. Minas Gerais: Furnas, cerrado próximo às eclusas, pelas estada Belo-Horizonte-Furnas, 6 July 1995, Lombardi 877 (IBGE, MO); São Roque de Minas, Parque Nacional da Serra da Canastra, 14 July 1997, Lombardi 1895 (IBGE); nascente do Rio São Francisco, 11 Sep. 1999, Filgueiras & Rodrigues-da Silva 3588 (IBGE, MO, SI, SP); Casca d’Anta, 11 Sep. 1999, Filgueiras & Rodrigues-da Silva 3589 (IBGE, MO, SI, SP); Rio das Pedras, 11 Sep. 1999, Filgueiras & Rodrigues-da Silva 3590 (IBGE, K, ICN); Rio do Peixe, 11 Sep. 1999, Filgueiras & Rodrigues-da Silva 3591 (B, IBGE, NY, R), 11 Sep. 1999, Filgueiras & Rodrigues-da Silva 3592 (IBGE, MO, SI, US). LEAF BLADE ANATOMY Histofoliar analyses of this species allowed us to conclude that Canastra lanceolata is a C3, non-Kranz species, characterized by two sheaths around the vascular bundles, the outer parenchymatous sheath without specialized chloroplasts, and an inner mestome sheath, also without specialized chloroplasts. The mesophyll is irregularly radiate, with 8 to 12 cells between consecutive vascular bundles. This genus is also distinguished by the presence of fusoid cells in the transverse section of the blade. Fusoid cells were previously reported in the tribe Paniceae in Homolepis Chase, Streptostachys Desvaux, and Panicum L. (for a detailed discussion see Zuloaga et al., 1992).   Figure 2. (above) Leaf blade anatomy of Canastra lanceolata, transverse sections. ---A. Outline of the leaf blade. ---B. Detailed view of A showing first-, and second-order vascular bundles, bulliform cells, and fusoid cells. (Filgueiras & Rodrigues-da Silva 3588, SI.) [Click on image to enlarge.] LEAF BLADE IN TRANSVERSE SECTION (FIG. 2) Outline: V-shaped; leaf thickness at mid-lamina 90--152 µm, arms of the lamina symmetrical. Ribs and furrows: adaxial ribs and furrows slightly developed, abaxial ribs and furrows indistinguishable; ribs associated with the vascular bundles, the apex flat; furrows 1/4--1/3 deep in relation to the width of the lamina. Median vascular bundle: midrib with a first-order vascular bundle, structurally distinguishable from the lateral first-order vascular bundles, solitary, associated with adaxial colorless parenchyma. Vascular bundle arrangement: 14 to 16 vascular bundles in the entire blade; 2 or 3 second-order vascular bundles between contiguous vascular bundles. First-order vascular bundles circular to elliptical in outline; metaxylem vessel elements narrow, with angular walls; phloem tissue adjoining the inner bundle sheath. Second-order vascular bundles elliptical in outline, with xylem and phloem tissue distinguishable; all vascular bundles situated in center of blade; 8 to 12 mesophyll cells between contiguous vascular bundles. Vascular bundle sheaths: outer first-order bundle sheaths consisting of 8 to 12 parenchyma cells, with abaxial interruption of sclerenchyma girders; outer second-order bundle sheaths completely surrounding the vascular bundle, with 8 to 10 parenchyma cells; parenchyma sheath cells inflated with thin walls and lacking chloroplasts; mestome bundle sheaths complete, surrounding the xylem and phloem tissue or present with a few cells abaxially in the second-order bundles. Sclerenchyma: minute, adaxial and abaxial girders associated with first- and second-order vascular bundles; fiber thick-walled, lignified. Small sclerenchyma cap present in the margin of the lamina. Mesophyll: chlorenchyma irregularly radiate around the vascular bundles; chlorenchyma cells tabular, raquimorphous. Fusoid cells present. Adaxial epidermal cells: bulliform cells present in adaxial furrows between all vascular bundles, fan-shaped, in restricted group of 4 to 6 cells, occupying up to 1/2 of the blade thickness. Epidermal cells small, regular in size, the outer walls flattened and with continuous and thickened cuticle. Macrohairs, papillae, and prickles absent. Abaxial epidermal cells: bulliform cells absent; cuticle thickened; macrohairs, papillae, and prickles absent. ABAXIAL EPIDERMIS IN SURFACE VIEW (FIG. 3C, D) Zonation. Costal and intercostal zones distinguishable; costal zone 5 to 9 cells wide, the intercostal zone 12 to 18 cells wide. Intercostal long cells: rectangular in shape, more than three times as long as wide, the anticlinal walls parallel and undulate, thickened, the end walls vertical; short cells solitary, disposed in a row between each successive long cell. Stomata: 27--29.5 × 19--24.6 µm, in 3 or 4 rows adjoining the costal zones; subsidiary cells triangular-shaped. Microhairs: fusiform, panicoid type, bicellular, 54--66.5 µm long, distal cell with very thin walls and tapered at the apex; basal cells equal or slightly longer than the distal cells. Macrohairs: absent. Papillae: absent. Hooks and prickles: absent. Costal silica bodies dumbbell-shaped or cruciform; intercostal silica bodies tall and narrow, transversely elongated. ADAXIAL EPIDERMIS IN SURFACE VIEW (FIG. 3A, B) Epidermal cells similar to the abaxial surface, except for the presence of a central band of cells in the intercostal cells, 4 to 8 cells wide; isodiametric or rectangular cells up to 3 times as long as wide. 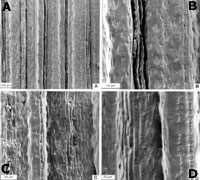 Figure 3. (above) Scanning electron micrographs of the leaf blades of Canastra lanceolata. ---A. Adaxial epidermis showing costal and intercostal zones. ---B. Detailed view of A. ---C. Abaxial epidermis showing costal and intercostal zones. ---D. Detailed view of C. (Filgueiras & Rodrigues-da Silva 3588, SI.) [Click on image to enlarge.] DISTRIBUTION AND ECOLOGY Canastra lanceolata is known from eight populations, all in the Brazilian state of Minas Gerais (Fig. 4). Two collections were made in Furnas, including the type collection (Emygdio et al. 2308 and Lombardi 877). The remaining six collections come from Parque Nacional da Serra da Canastra, in Minas Gerais. Plants grow typically among rocks, or very close to the margins of puddles, streams, or small rivers. They are found in shallow and extremely sandy soils. Plants are robust, tussock-like, with conspicuous upright purple inflorescences. One of the populations sampled inside the Parque grows at the headwater of the great São Francisco River, one of the major rivers in Brazil. Other populations of C. lanceolata may be found along the river where it eventually leaves the limits of the Parque. A live plant of Filgueiras & Rodrigues da Silva 3500 was brought to Brasília and planted on the grounds of Reserva Ecológica do IBGE, where it is growing well. The genus is named after the Parque Nacional da Serra da Canastra, where six populations of its single species were found. These populations are the only ones legally protected and geographically referenced. The only other population known to us outside the park is situated at Furnas (Minas Gerais). The Portuguese word "canastra" means "canister" in English and, in this context, alludes to a rocky outcrop that gives its name to the Parque. This outcrop is a curious landmark and bears a striking similarity to a canister when viewed from certain angles. Canastra is also the common name in Brazil for the giant armadillo (Priodontes maximus), often seen in the Parque.   Figure 4. (above) ---A. View of the Rio Preto site, Parque Nacional da Serra da Canastra, Brazil. Plants of Canastra lanceolata growing on rocks in the river (lower left; photo by TSF). ---B. Plants of Canastra lanceolata at the headwater site of the São Francisco river, Parque Nacional da Serra da Canastra (photo by TSF). [Click on image to enlarge.] DISCUSSION AND CONCLUSIONS Filgueiras (1982) described Arthropogon lanceolatus and stated that this species differed from other taxa of Arthropogon by the absence of a hairy callus in the base of the spikelet. This author also studied briefly the anatomy of the species but did not mention the fact that A. lanceolatus was the only C3 species of the genus. Filgueiras (1982) related Arthropogon lanceolatus to Arthropogon piptostachyus (= Achlaena piptostachya) by the presence of well-developed bulliform cells. Nevertheless, the latter is a C4, MS species with distinctive Kranz cells and laterally compressed spikelets. In a recent cladistic analysis of the tribe Paniceae, using morphological characters, Zuloaga et al. (2000) found that Canastra lanceolata was located in a different clade from the one including other species of Arthropogon. In this analysis, C. lanceolata was related to Homolepis, Streptostachys asperifolia Desvaux, Panicum sect. Laxa Hitchcock & Chase ex Pilger, and the Australian genus Cliffordiochloa B. K. Simon (the latter likely to be conspecific with Panicum laxum of sect. Laxa), all taxa with fusoid cells present in the transverse section of the leaves. Zuloaga et al. (2000) stressed that Arthropogon is not a monophyletic genus and should be segregated into three monophyletic units, which correspond to Altoparadisium, Arthropogon, and Canastra. Subsequently, Filgueiras et al. (2001), after the collection of a previously undescribed grass in central Brazil, conducted a detailed study of Arthropogon and related genera, including a phylogenetic analysis. They segregated the new genus Altoparadisium (with two species), related to Arthropogon, and indicated that the cladistic analyses of the group clearly supported the idea of separating A. lanceolatus from the genus Arthropogon. They also noted that further studies were necessary to ascertain the systematic position of this taxon within the tribe. In their cladistic analyses, C. lanceolata was placed together with Homolepis aturensis (Kunth) Chase, and Streptostachys asperifolia, again in a clade supported by the presence of fusoid cells in the three taxa already cited. Finally, in a recent phylogenetic study of the tribe, using molecular data of the gene ndhF, Giussani et al. (in press) determined that Canastra lanceolata appears in a separate clade from Arthropogon. Canastra lanceolata is related to Panicum euprepes Renvoize (Panicum sect. Lorea Zuloaga) and P. prionitis Nees (Panicum sect. Prionitia Zuloaga), and this clade is supported by 85% bootstrap value. It should be mentioned that within this clade the length of each terminal branch is 15, 20, and 11 steps long, for C. lanceolata, P. euprepes, and P. prionitis, respectively. Therefore, these values probably reflect a low similarity among these taxa (Giussani et al., in press). Canastra is characterized by its dorsiventrally compressed spikelets without a hairy callus, with the lower and upper glume awned, the lower glume reduced and 1-nerved, the upper anthecium cartilaginous, thicker than the upper glume and lower lemma, and the upper lemma enclosing the tip of the upper palea. Anatomically, the genus is non-Kranz, with distinctive fusoid cells in the mesophyll. The new genus and related taxa within the Paniceae are compared in Table 1 [ pdf file / html file ]. 1. Lower and upper glume awned ..... 2
2(1). Spikelets dorsiventrally compressed ..... 3
3(2). Hairy callus absent; lower flower present; upper anthecium cartilaginous; plants non-Kranz with fusoid cells ..... Canastra
4(2). Spikelets stipitate; distinctive Kranz cells present ..... Achlaena
5(1). Hilum punctiform; lower glume 1/5 to 3/4 the length of the spikelet ..... Panicum
6(5). Spikelets stipitate; lower lemma without glandular microhairs ..... Streptostachys
Acknowledgments. We gratefully thank IBAMA for granting TSF free access to Parque Nacional da Serra da Canastra to collect specimens of C. lanceolata and to study local populations. The Director of the Parque and his staff were especially interested and helpful. The Departamento de Botânica da Universidade de Brasília (through Augusto Franco) provided a field vehicle and a driver for the 1999 trip to the Parque. We acknowledge the help given by Robson Rodrigues-da Silva (TSF’s graduate student) and by Sr. Mendes (driver) during the long, hasty trip to the Parque in 1999. TSF also thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a productivity research grant (Process nr. 301190/86-0). We also thank Vladimiro Dudás for the illustrations. Literature Cited
|
© 1995-2025 Missouri Botanical Garden, All Rights Reserved
4344 Shaw Blvd.
St. Louis, MO 63110
(314) 577-5100
Technical Support