EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [[LILIALES +ASPARAGALES] COMMELINIDS]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[LILIALES + ASPARAGALES] COMMELINIDS] - if this node exists: (inflorescence branches cymose); protandry common; style long; whole nuclear genome duplication [τ/tau event].
Age. This node has been dated to 118-116 Ma (Bremer 2000b; Leebens-Mack et al. 2005). Other estimates are are around 159 Ma (Paterson et al. 2004), ca 135.6 Ma (Tank et al. 2015: Table S1), ca 128 Ma (G.-Q. Zhang et al. 2017: sampling), (133.9-)123.6(-113.1) Ma (Eguchi & Tamura 2016), ca 122 Ma (Janssen & Bremer 2004), (126-)122(-98) Ma (Merckx et al. 2008a), about 121 Ma in Foster et al. (2016a: q.v. for details), (112-)107, 98(-93) Ma (Wikström et al. 2001) and ca 133.1 and 118.6 Ma (Magallón & Castillo 2009); ages a mere 76.6 or 75.4 Ma in Xue et al. (2012) and 88.9-78.2 Ma in Good-Avila (2006), (130-)121(-116) or (121-)115(-110) Ma in Hertweck et al. (2015), a group of similar estimates, 116-94 Ma in Mennes et al. (2013, see also 2015), about 120-90 Ma in S. Chen et al. (2013: conflicting estimates), about 114.6 Ma in Magallón et al. (2015) and 93.0-79.6 Ma in Bentz et al. (2024a).
Evolution: Divergence & Distribution. For possible ecophysiological and morphological changes here associated with the τ duplication (see below), and in more or less contemporaneous duplications in Magnoliales + Laurales, Nymphaeales and the core eudicots, see elsewhere.
Genes & Genomes. A genome duplication, the τ/tau genome duplication event, may be pegged to the ancestor of the monocots as a whole (Jiao et al. 2014), or at least to the clade [Asparagales + commelinids] (Deng et al. 2015); the latter position seems more likely (McKain et al. 2016; see also Olsen et al. 2016; H. T. Lee et al. 2016) and the base number may have been x = 5 (see also Murat et al. 2017). For this duplication, see also L. Zhang et al. (2020) and X. Guo et al. (2021), the latter suggesting an age of 138.8-101.8 Ma. T. Shi et al. (2022) placed this duplication two nodes down the tree (the common ancestor of Dioscoreales and everything else), and also suggested that the base chromososome number at that node was x = 5. Indeed, there may have been 5 (Murat et al. 2017) or 7 (Ming et al. 2015) preduplication protochromosomes, so what happened may be x = 7 → x = 14 (tetraploidy: τ/tau genome duplication event) (Ming et al. 2015: pineapple) or x = 5 → x = 10 (Murat et al. 2017). P. Soltis and Soltis (2016) note that uncertainty over exactly where this event should be placed makes it difficult to think about any evolutionary implications it might have. Note that the ORSAγ duplication event (Landis et al. 2018) is placed two nodes down the tree at the [[Dioscoreales + Pandanales] [Liliales...]] node.
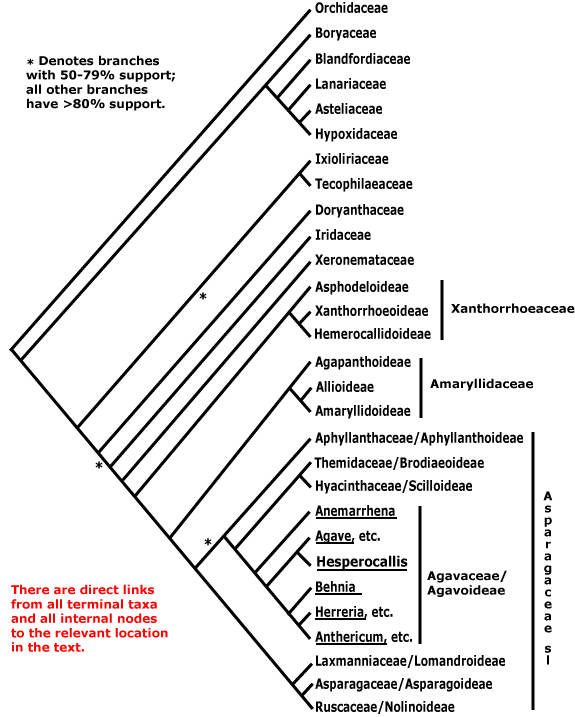
ASPARAGALES Link - Main Tree.
Chelidonic acid +, steroidal saponins 0 [exact position where?]; root hairs from unmodified rhizodermal cells, (velamen +), hypodermal cells dimorphic; anthers longer than wide; microsporogenesis simultaneous; ovules many/carpel; seeds with phytomelan (0), exotestal, tegmen not persistent; endosperm helobial; mitochondrial sdh3 gene lost. - 14 families, 1,122 genera, 36,265 species.
Includes (= Asparagaceae-Agavoideae, Amaryllidaceae, Anthericaceae (= Asparagaceae-Agavoideae-Anthericeae), Aphyllanthaceae (= Asparagaceae-Aphyllanthoideae), Asparagaceae (see also -Agavoideae, -Aphyllanthoideae, -Asparagoideae, -Brodiaeoideae, -Convallarioideae, -Lomandroideae and -Scilloideae), Asphodelaceae, Asteliaceae, Blandfordiaceae, Boryaceae, Convallariaceae (= Asparagaceae-Convallarioideae), Doryanthaceae, Eriospermaceae (= Asparagaceae-Convallarioideae-Eriospermeae) Hypoxidaceae, Hyacinthaceae (= Asparagaceae-Scilloideae-Hyacintheae), Iridaceae, Ixioliriaceae, Lanariaceae, Laxmanniaceae (= Asparagaceae-Lomandroideae-Sowerbaea group), Lomandraceae (= Asparagaceae-Lomandroideae), Orchidaceae, Ruscaceae (= Asparagaceae-Convallarioideae-Rusceae), Tecophilaeaceae, Themidaceae (= Asparagaceae-Brodiaeoideae), Xeronemataceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown group Asparagales may be ca 125.3 Ma (Tank et al. 2015: Table S2; see also C. Q. Tang et al. 2016), ca 119 Ma (Janssen & Bremer 2004) or (118-)112, 110(-106) Ma (Hertweck et al. (2015; see also Givnish et al. 2015, 2016a), although Wikström et al. (2001: note topology) had suggested dates of 101-92 Ma; Magallón and Castillo (2009) suggested ages of ca 112.6 Ma and Bell et al. (2010) ages of (114-)103, 92(-83) Ma. Other estimates are (127-)119(-101) Ma in Merckx et al. (2008a), 96-93 Ma or 113-63 Ma in Mennes et al. (2013, 2015 respectively), 101-93 or 85.1 Ma in S. Chen et al. (2013), about 109 Ma in Magallón et al. (2015) and (125.8-)112.9(-98.4) Ma in Eguchi and Tamura (2016). The rather young age in Smith et al. (2008) is (95.6-)90.3(-85) Ma and that in D.-F. Xie et al. (2020) is (78.3-)74.6(-70.8) Ma, but the lowest suggested age seems to be 69-60 Ma (Good-Avila et al. 2006). Ages in Serna-Sánchez et al. (2021: Orchidaceae sister to the rest?) are ca 123.6 (strict clock) or ca 113.7 Ma (relaxed clock) and in C. I. Smith et al. (2021) they are (124.0-)104.0(-83.7) Ma. Silvestro et al. (2020) estimate the time-of-origin of Orchidaceae to be ca 137.7 Ma, Ji et al. (2022) estimate it to be (127.5-)108.9(-92.2) Ma, G. Zhang et al. (2023) (138.1-)131.9(-131.2) Ma and Pérez-Escobar et al. (2023) (126-)120(-114) Ma. Although Janssen and Bremer (2004) did not recover Orchidaceae as sister to the rest of Asparagales, its stem-group origin was near the beginning of divergence within the order and was ca 119 Ma.
Evolution: Divergence & Distribution. S. Chen et al. (2013) give age estimates for many nodes in Asparagales in particular, but differences between the two methods that they used were often substantial, the older ages (from BEAST) being about half as much again as the younger age (PATHd8) in a third or so of the cases.
Asparagales may have the highest diversification rate in the monocots, about the same as Poales, but in both the rate is little over half that of Lamiales, the angiosperm clade with the highest rate (Magallón & Castillo 2009); Magallón and Sanderson (2001) did not give estimates for the group. Within the clade, the diversity of Orchidaceae is remarkable, but to understand that diversity it makes as much sense to focus on clades within Orchidaceae as on the family as a whole (e.g. Givnish et al. 2015; see below). Endress (2011a) thought that an inferior ovary might be a key innovation in Asparagales, and Rudall (2001a, see also 2002, 2003a) included an inferior ovary as a synapomorphy of the order, noting that in "higher" Asparagales there might be a reversal to superior ovaries that is associated with the presence of infralocular septal nectaries (as in Xanthorrhoea and Johnsonia (Asphodelaceae-Xanthorrhoeoideae and -Hemerocallidoideae). Tobe et al. (2018) thought that inferior ovaries had evolved two nodes down the tree (see the [[Dioscoreales + Pandanales] [Liliales...]] node), but in either case inferior ovaries are scattered through the Asparagales and fitting ovary evolution to the tree is difficult; ovary position is a very labile character. Remizowa (2022) described interlocular septal nectaries in Ledebouria and suggested that such nectaries were common in Asparagales that had superior ovaries. I do not pretend to understand gynoecial/septal nectary evolution here.
The some 2,550 species in five major clades of Asparagales in southern Africa, e.g. in Iridaceae and Asphodelaceae, but not Orchidaceae-Epidendroideae, make the group notably diverse there (Johnson 2010).
Plant-Animal Interactions. Food plants of caterpillars of the [Heteropterinae-Trapezetinae-Hesperiinae] clade of skippers are common here, but most are found on Poaceae (Warren et al. 2009).
Plant-Bacterial/Fungal Associations. Asparagales commonly have Arum-type arbuscular mycorrhizae where the hyphae are intercellular, and also form coils, pelotons and particularly branched arbuscules within cells, while in Liliales these mycorrhizae are commonly Paris-type with intercellular hyphae that form coiled structures between the cells (F. A. Smith & Smith 1997; Rasmussen & Rasmussen 2014).
Genes & Genomes. Asparagales have a very wide spread in genome sizes - 0.3-82.2 pg (1C: Leitch & Leitch 2013). For cytology here, see Tamura (1995).
There has been much duplication of genes in the TEOSINTE BRANCHED and PROLIFERATION CELL FACTOR gene families in this clade, and they are commonly expressed in the ovary/fruit and sometimes in the leaf, etc., in Orchidaceae and Hypoxidaceae (see below for what also goes on in Orchidaceae). Interestingly, CYC and TB1 genes (the former are not duplicated) seem not to be involved in the development of monosymmetry in the flowers of Asparagales, unlike their roles in Commelinales, Zingiberales and Liliales (Madrigal et al. 2017). The YABBY transcription factor family are not involved in foliar bifaciality/abaxial development in Asparagales, etc., unlike in other seed plants, but what goes on more basally in monocots seems not to be known (e.g. Romanova et al. 2021).
Chemistry, Morphology, etc.. For fructan sugar accumulation, quite common outside Orchidaceae, see Pollard (1982). Storage mannans in the vegetative tissues are reported from Asphodelaceae-Asphodeloideae, Amaryllidaceae and Orchidaceae; they are uncommon elsewhere (Meier & Reid 1982). A source for many anatomical features of the root is Kauff et al. (2000); characters like velamen are mentioned below only when they are present. There is no starch in the chloroplasts of guard cells of members of Amaryllidaceae, Iridaceae, and "Liliaceae" (= Allium) and no chloroplasts at all in those of Paphiopedilum (Orchidaceae) (D'Amelio & Zieger 1988; Willmer & Fricker 1996).
There are three-trace tepals in Orchidaceae, Amaryllidaceae-Amaryllidoideae and -Agapanthoideae, Iridaceae, Asphodelaceae-Asphodeloideae (but not Kniphofia, Ashpodelus) and -Hemerocallidoideae, Asparagaceae-Agavoideae; one-trace tepals in Amaryllidaceae-Allioideae and Asparagaceae-Convallarioideae (but not Maianthemum stellatum), -Aphyllanthoideae, and -Asparagoideae. Asparagaceae-Scilloideae have tepals with both one and three traces, Urginea (= Drimia) even having five traces in the outer whorl and three in the inner (Chatin 1920; Carpenter 1938). Where changes in microsporogenesis are to be placed on the tree is not clear.
For flavonoids, see C. A. Williams et al. (1988), for general morphology, see Rudall (2003a), for root morphology, see Kauff et al. (2000), for cladodes, see Schlittler (1953b), for inflorescences, see Schlittler (1953a), for pollen of Japanese representatives, see Handa et al. (2001), for ovule and seed, see Shamrov (1999a) and Oganezova (2000a, b), and for the distribution of taxa with phytomelan and/or with baccate fruits, see Rasmussen et al. (2006).
Phylogeny. For a discussion about the relationships of Asparagales, see Petrosaviales. Note that H.-T. Li et al. (2019) place Petrosaviaceae (q.v.) as sister to Orchidaceae, and both contain holomycoheterotrophic taxa, but this relationship may well not hold up (e.g. W. R. Baker et al. 2021a: see Seed Plant Tree).
Relationships within Asparagales are based largely on those already evident in the analyses in Chase et al. (2000a) and Fay et al. (2000: successive weighting). These studies differ little in detail, although the analysis of Fay et al. (2000) hardly surprisingly had more nodes in the core Asparagales with strong support; they are consistent with relationships in Chinese Asparagales as detailed in Z.-D. Chen et al. (2016) and the relationships in McLay and Bayly (2016), although the focus of the latter was Asphodelaceae. For the [Amaryllidaceae + Agapanthaceae] clade, see Meerow et al. (2000b), and relationships between Aphyllanthaceae, Themidaceae and Hyacinthaceae might be better represented as tritomy (see also S. Chen et al. 2013); these families are now subsumed in a broadly-drawn Amaryllidaceae and Asparagaceae respectively. The relationships in McPherson and Graham (2001) and D.-K. Kim et al. (2012) are largely congruent with those below, although their sampling is poorer and more geographically constrained. In some phylogenetic reconstructions of Hilu et al. (2003) Asparagales were paraphyletic, Orchidaceae being separate from the rest.
Understanding the relationships between Boryaceae and Orchidaceae is important since it considerably affects our ideas of diversification. Boryaceae have been placed as sister to Orchidaceae (e.g. Chase et al. 1995a; McPherson & Graham 2001), although with rather weak support. Janssen and Bremer (2004) found the relationships [[Boryaceae, Blandfordiaceae, etc.] [Orchidaceae [Ixoliriaceae [Tecophilaeaceae [Doryanthaceae + The Rest]]]]], while Orchidaceae were embedded in a Boryaceae-Hypoxidaceae clade (X.-X. Li & Zhou 2007), but again, with little support. In Wikström et al. (2001) Orchidaceae were sister to Hypoxidaceae, while Rudall (2003a: morphological data) also suggested that there was a close relationship between Hypoxidaceae and Orchidaceae in particular, and also between Boryaceae and Blandfordiaceae and Iridaceae and Doryanthaceae. Seberg et al. (2012) largely recovered the topology found by earlier workers; mitochondrial data provided little support for the backbone of the tree and mitochondrial and chloroplast data agreed only after removing edited mitochondrial sites.
Most more recent work suggests that Orchidaceae are sister to all other Asparagales (e.g. 76% bootstrap support in Graham et al. 2006; about the same in Givnish et al. 2006b; stronger [96-99%] in Pires et al. 2006: good sampling, seven genes from two compartments; Chase et al. 2006; S. Chen et al. 2013). There was more or less strong support for the [Boryaceae, Blandfordiaceae et al.] clade in these analyses. All in all, the topology [Orchidaceae [[astelioids] [all other Asparagales]]], seems the best hypothesis. This affects the characterisation of Asparagales, since some characters previously considered to refer to Asparagales as a whole move to the next node up (c.f. versions 6 and younger of this site; there Orchidaceae were not sister to the rest of the order). However, if recent suggestions that Petrosaviaceae are sister to Orchidaceae (H.-T. Li et al. 2019, but c.f. 2021) are confirmed, the positions of these characters will have to be rethought.
Within the astelioids, a clade [Asteliaceae [Lanariaceae + Hypoxidaceae]] was recovered by S. Chen et al. (2013), while in Chase et al. (2006) Boryaceae were placed immediately above the Blandfordiaceae et al. clade, albeit with very little support. For some reason I had earlier had a grouping [Lanariaceae [Asteliaceae + Hypoxidaceae]], but this has been corrected (see also H.-T. Li et al. 2019). The clade [[Ixoliriaceae + Tecophilaeaceae] [Doryanthaceae [Iridaceae [Xeronemataceae + The Rest]]]] is strongly supported in analyses using data from four plastid genes (Fay et al. 2000; see also Chase et al. 2000a; Soltis et al. 2007a), but no morphological characters have yet been found for it. The positions of [Ixoliriaceae + Tecophilaeaceae] and Doryanthaceae are reversed in Kim et al. (2011) and in Fig. 2 in S. Chen et al. (2013), althouggh in their Fig. 3 the three families are shown forming a clade [Doryanthaceae [Ixoliriaceae + Tecophilaeaceae]], and they do have a few features in common. Givnish et al. (2018b) found that the clade [Doryanthaceae [Iridaceeae ... Asparagaceae]] had rather poor support. Nearly all nodes in the topology below were well supported in the plastome analysis of H.-T. Li et al. (2021), and also in Zuntini et al. (2024), however, in the Seed Plant Tree of Life ix.2024 version Orchidaceae are separate, and are to be found between Araceae and Dioscoreales.
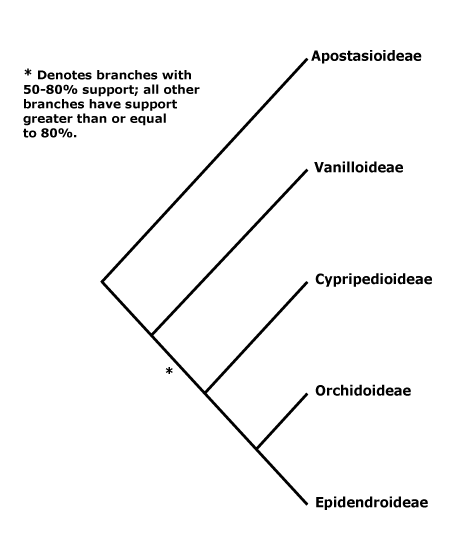
Classification. Quite extensive changes in names and group circumscriptions in the order have made for a less than an ideal situation, but one still hopes for stability. However, there are clearly problems in the acceptance of the APG classification in the Asphodelaceae-Amaryllidaceae-Asparagaceae area (Nyffeler & Eggli 2010b, 2020; Nordal & Sletten-Bjorå 2016), and with the Asparagaceae s.l. in particular one can see what one of the issues is - nothing characterises that clade. There have been suggestions that Orchidaceae should be placed in their own order, either because of their age and/or diversity and morphology (e.g. Eguchi & Tamura 2016), and they have sometimes been separated into three families, Apostasiaceae, Cypripediaceae and Orchidaceae s. str. (Vermeulen 1966).
Previous Relationships. Dahlgren et al. (1985) took important steps in reorganizing the relationships of the "lily-like monocots". They recognized two groups, Asparagales and Liliales, which were separable by features including patterning of the tepals and absence of phytomelan, both features of their Liliales. However, they still included Iridaceae and Orchidaceae in Liliales.
Synonymy: Asparagineae J. Presl, Asphodelineae Thorne & Reveal, Hyacinthineae Link, Iridineae Engler, Scillineae J. Presl - Agavales Hutchinson, Alliales Berchtold & J. Presl, Amaryllidales Link, Apostasiales Martius, Asphodelales Doweld, Asteliales Dumortier, Gilliesiales Martius, Hypoxidales Martius, Iridales Rafinesque, Ixiales Lindley, Narcissales Dumortier, Orchidales Rafinesque, Tecophilaeales Reveal, Xanthorrhoeales Reveal & Doweld
ORCHIDACEAE Jussieu, nom. cons. - Back to Asparagales
Hemimycoheterotrophic herbs; flavone C-glycosides, flavonols +, chelidonic acid?; roots with velamen and exodermis, little branced [?all]; stomata frequently tetracytic; leaf vascular bundle sheaths with fibres, (also fibre bundles in leaves); flowers rather weakly monosymmetric, median outer T adaxial [flowers described here as if upside-down]; T free, lateral outer T first to develop, median member the last, median [abaxial] inner T differentiated [= labellum]; A [3], [median of outer whorl and laterals of inner whorl], basally adnate to style; endothecial thickenings annular; tapetal cells uninucleate; septal nectaries 0, placentation intrusive parietal, placentae bilobed, style solid [?all], stigmas commissural, wet; ovules ca 1500+/carpel, outer integument 1(-2) cells across, inner integument 2-3 cells across, parietal tissue none, funicle not vascularized; time from pollination to first zygote division long [7≤ days or far more: ?here]; fruit erect, dehiscing laterally, loculicidal, lipid layer +, interplacental areas separating, T deciduous; seeds minute, dry, testal, (testa more complex, even fleshy); endosperm barely developing, none at maturity of seed, embryo minute, undifferentiated [usu. ≤700 cells], suspensor various/0, not persisting; x = 10 (?11), nuclear genome [1 C] (0.182-)3.529(-68.513) pg; whole genome duplication, ca 270 bp transfer from fungal to orchid mitogenome; young seedling with a protocorm [radicle and plumule 0, cotyledon at most inconspicuous; from the middle (chalaza)], holomycoheterotrophic.
Ca 880/26,000 (30,543 - POWO 2021): [list, to tribes] - five subfamilies below. World-wide. [Photo - Flower]
Age. Crown group Orchidaceae have been dated to ca 111 Ma (Janssen & Bremer 2004) or (121-)93.7(-75) Ma (Chomicki et al. 2014c; see also X.-G. Xiang et al. 2017). The estimates of Ramírez et al. (2007, see esp. Supplementary Table) are somewhat younger at (90-)84-76(-72)Ma but (105-)80(-56) Ma when recalculated by Gustafsson et al. (2010). Other crown group estimates include (105-)80-77(-56) Ma (Gustafsson et al. 2010; Leopardi-Verde et al. 2016: similar, less spread; G.-Q. Zhang et al. 2017: ca 81 Ma, wider spread), while ages in Givnish et al. (2015, 2016a), at (99.5-)90(-79.7) Ma (and with a ca 20 Ma stem) and Bouetard et al. (2010) were slightly older, and the youngest, (82-)68(-54) or ca 51.6 Ma, are those in S. Chen et al. (2013) and (85.5-)68.9-)55.6) Ma in Y.-K. Kim et al. (2019). Ages in Serna-Sánchez et al. (2021) are ca 88.2 (strict clock) or ca 89.8 Ma (relaxed clock; phylogenetic fuses ca 35.4 and 23.9 Ma respectively), in Mayer et al. (2021) the age is 102-79 Ma, G. Zhang et al. (2023) estimate (102.6-)101.5(-97.1) Ma and Pérez-Escobar et al. (2023) (93-)83(-73) Ma.
Note: in the characterisations below, "P [3]", "P [5]", "P [5 + lab.]", etc., refer to the fusion of the perianth members. Although they are all more or less petal-like, "inner P distinct" refers to the condition where the two inner adaxial T members are strongly differentiated from the three outer members, whether or not they are fused.
1. Apostasioideae Horaninov —— Synonymy: Apostasiaceae Lindley, Neuwiediaceae Reveal & Hoogland
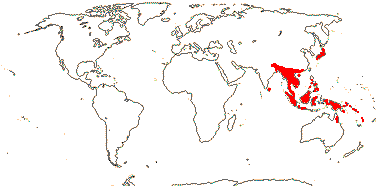
Plant (rhizomatous, rhizomes with scales), roots (with scattered tubers, tubers with irregular papillae), (rootlets wire-like); plant ?chemistry; root velamen uniseriate, pith with scattered vascular bundles, vessel elements often with simple perforation plates; (vessels in stem +); conical SiO2 bodies in bundle sheath cells; leaves spiral, vernation plicate; flowers (not resupinate, ± polysymmetric - Apostasia); T apiculate, carinate [prominent midrib], plain coloured, (labellum 0 - Apostasia), develop from a ring primordium, lateral members of inner whorl develop first; A (staminode +/0 - Apostasia); pollen surface reticulate, colpus operculate; placentation axile (with central cavity), stigma papillate, lobes spreading; micropyle bistomal; (embryo sac bisporic, the spores chalazal, 8-celled [Allium type] - Neuwiedia); fruits baccate / dehiscing (irregularly); seeds ovoid/elongated; exotesta with cuticular layer, cells isodiametric, thin, transparent, collapsing, endotesta cells ± isodiametric, thickening U-shaped, sclerified, dark-coloured/testa cells elongated, thin-walled; endosperm ?+; n = 24; genome (1 C) 341 Mb-5.83 Gb.
2/16: Apostasia (8), Neuwiedia (8). Sri Lanka, N.E. India to N.E. Australia, Japan. Map: from Pridgeon et al. (1999).
Age. Estimates of the age of crown-group Apostasioideae are (54-)49-45(-41) Ma (Ramírez et al. 2007), or (66-)43(-23), (61-)41(-23) Ma (Gustafsson et al. 2010) and ca 29.1 Ma (Y.-K. Kim et al. 2019). Ages in Serna-Sánchez et al. (2021) are ca 39.8 (strict clock) or ca 36.4 Ma (relaxed clock), phylogenetic fuses ca 48.4 and 53.4 Ma respectively.
[Vanilloideae [Cypripedioideae [Orchidoideae + Epidendroideae]]]: roots ± fleshy; C-glycosyl flavones, (saponins), 6-hydroxy flavonols +; vessel elements with scalariform perforation plates; leaves two-ranked; flowers strongly monosymmetric; ?abaxial outer tepal develops after inner whorl, labellum strongly differentiated, develops before other members of the inner whorl; the style and A almost completely congenitally fused [column, = gynostemium]; anthers to 2x as broad as long; pollen sticky; median G initiated first, initially much larger, placentation parietal, hairs/papillae on inside of carpel wall [?level], stigma asymmetric [lateral lobes, + part median lobe]; ovules not fully developed at time of pollination (mature), fertilization takes 6≤ days [to some months]; (embryo sac 6-nucleate); dust seeds [small; exotesta usu. alone present, cells ± elongated].
Age. The age of this node is around 71 Ma (Gustafsson et al. 2010), (92.9-)84(-74.4) Ma (Givnish et al. 2015, 2016a) and (73.7-)59.3(-44.7) Ma (Y.-K. Kim et al. 2019). Ages in Serna-Sánchez et al. (2021) are ca 85 (strict clock) or ca 89.8 Ma (relaxed clock) and in G. Zhang et al. (2023) ca 93.5 Ma.
2. Vanilloideae Szlachetko
Velamen uniseriate; plant glabrous; sclerenchyma in leaves; stomata notably variable; (leaf blade venation reticulate); (calyculus +); T often carinate; A 1 [= median [abaxial] member of outer whorl], staminodes 2 [= lateral members of inner whorl], anther incumbent [bent forward] by massive expansion of the apical column/connective; "pollinia" +, soft [not highly organized], viscidium 0; pollen inaperturate[?], (polyporate), (in tetrads), smooth or not; (placentation axile), rostellum [ridge, part of median stigmatic lobe] +; (micropyle bistomal), outer integument 4-5- cells across, inner integument 2-4 cells across; ovules do not develop before anthesis; (T persistent in fruit); seeds also often relatively large, circumferentially winged/ellipsoid/spherical, crustose, [exotestal - outer wall well developed, cells often polygonal, tegmen persisting or not]/filamentous protrusions at either end, tegmen persisting, (exotesta with cuticular layer); endosperm to 16-nucleate (0); n = 9, 10, 12, 14-16, 18, etc..
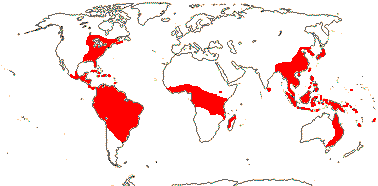
16/235. Pantropical (temperate). Map: from Pridgeon et al. (2003).
Age. Crown group Vanilloideae are (76-)71-65(-61) Ma (Ramírez et al. 2007), or (79-)58, 57(-43) Ma as recalculated by Gustafsson et al. (2010), while ca 71 Ma is the estimate in Bouetard et al. (2010) and around abour 77 Ma in Givnish et al. (2016a). Ages in Serna-Sánchez et al. (2021) are ca 81.0 (strict clock) or ca 67.7 Ma (relaxed clock), phylogenetic fuses ca 4.0 and 12.7 Ma respectively, in Mayer et al. (2021) crown-group Vanilloideae are 84-45 Ma and in G. Zhang et al. (2023) (76.5-)74.3(-68.4) Ma.
2A. Vanilleae Blume —— Synonymy: Vanillaceae Lindley
(Plant holomycoheterotrophic, associated with ECM fungi - Aphyllorchis); (CAM photosynthesis - some V.); viny [root climbers], (hemi)epiphytes/epilithic, monopodial - Vanilla); (lignin with catechyl units - V.); stem photosynthetic, (leaves much reduced/±0 - V.); (margins of labellum fused with column); outer integument 3-6 cells across - V.; (fruit ± fleshy); (plastome Mat K + - Aph.).
9/175: Vanilla (110), Lecanorchis (22), Epistephium (21). Pantropical (to subtropical).
2B. Pogonieae Garay & Dunsterville
Lip margin laciniate.
5/60: Cleistes (45), Pogonia (6). Eastern North America, South America, China, Taiwan, Japan, Korea, the Moluccas.
[Cypripedioideae [Orchidoideae + Epidendroideae]]: (velamen multiseriate), tilosomes +/0; exotesta lacking cuticular layer.
Age. The age of this clade is estimated at only 37-26 Ma by Wikström et al. (2001), but (69-)48, 42(-23) Ma by Bell et al. (2010), around 69-68 Ma by Gustafsson et al. (2010), (87.4-)76(-64.6) Ma by Givnish et al. (2015, 2016a) and (66.1-)52.9(-42.9) Ma by Y.-K. Kim et al. (2019). Ages in Serna-Sánchez et al. (2021) are ca 70.1 (strict clock) or ca 76.9 (relaxed clock) Ma, in G. Zhang et al. (2023) ca 89.1 Ma and in Lagou et al. (2024) the estimate is (88.2-)66.9(–50.3) Ma.
3. Cypripedioideae Kosteletzky —— Synonymy: Cypripediaceae Lindley
Plant (epiphytic, epilithic); root with persistent hairs, (pith 0 - some Cypripedium); stem bundles amphivasal; stomata anomocytic - Cypripedium; leaf blade vernation (plicateconduplicate); (flowers not resupinate); outer T valvate/open, 2 abaxial T of outer whorl connate, labellum saccate; A 2 [= lateral members of inner whorl], (pollinia +), staminode single, conspicuous [= median member of outer whorl]; (tapetal cells binucleate); microsporogenesis successive?; pollen sulcate, ± smooth [psilate], (foveolate), (in tetrads), sticky or not; (placentae axile, with central cavity [or not?]), stigma lobes spreading, (papillate), median lobe largest; (micropyle bistomal); ovules early- to mid-development at anthesis [mature - Cypripedium], embryo sac bisporic [chalazal dyad], eight-celled [Allium-type]; (T persistent in fruit); (outer periclinal wall of testa sclerified - Selenipedium); n = 9 or more; (chloroplast ndh genes not functional).
5/170: Paphiopedilum (86(-110?)), Cypripedium (48). Mostly (warm) temperate N. Hemisphere, East Malesia and tropical South America (and S. India). Map: from Hultén (1958) and Pridgeon et al. (1999). Photo: Flower.
Age. Crown group Cypripedioideae are (54-)49-44(-39)/(66-)43-41(-23) Ma (Ramírez et al. 2007), but recalculated as being rather younger, (50-)33, 31(-17) Ma by Gustafsson et al. (2010), while from Fig. 1 in Givnish et al. (2016a) their age is around 63 Ma and it is ca 68 Ma in Guo et al. (2012: Fig. 4, several younger ages also suggested) while in Y.-K. Kim et al. (2019) it is only ca 27.5 Ma... Ages in Serna-Sánchez et al. (2021) are ca 31.5 (strict clock) or ca 38.5 Ma (relaxed clock), phylogenetic fuses ca 38.5 and 31.5 Ma respectively, in Szlachetko et al. (2021) it is ca 51 Ma, while in Mayer et al. (2021) estimates are 38-15 Ma, in Lagou et al. (2024) they are (33.2-)31.3(–29.4) Ma and in M. Liao et al. (2024).
[Orchidoideae + Epidendroideae]: leaves withering on the plant; flowers resupinate [ovary twisted]; floral primordium transversely elliptic-oval; labellum initiated first; A 1 [= median [abaxial] member of outer whorl], sporangia 2 [?level], (staminodes 2 [from outer whorl]); pollinia +, attached to sticky viscidium, pollinium/pollinarium stalk variously formed; co-occurrence of successive and simultaneous cytokinesis, pollen in tetrahedral tetrads (monads), inaperturate (porate or ulcerate), polysaccharide layer +, with sporopollenin [= second intine layer - ?level]; rostellum + [ridge, part of median stigmatic lobe, viscidium is also part of it]; (embryo sac bisporic [chalazal dyad], eight-celled [Allium-type]/tetrasporic [Adoxa type]); T persistent in fruit; phytomelan 0, organized tegmen not persisting; n = 9 or more [19 common].
Age. The age of this node is estimated to be around 59-51 Ma (Gustafsson et al. 2010), (73.7-)64.0(-54.8) Ma (Givnish et al. 2015, 2016a), (72.5-)61.5(-51.7) Ma (Leopardi-Verde et al. 2016), (55.8-)44.7(-36.2) Ma (Y.-K. Kim et al. 2019) or (69.3-)60.4(-54.3) Ma (M.-H. Li et al. 2022). Ages in Serna-Sánchez et al. (2021) are ca 63.2 (strict clock) or ca 68.4 Ma (relaxed clock), in Pérez-Escobar et al. (2023) around 65-64 Ma and in G. Zhang et al. (2023) somewhat older, ca (79.7-)77.7(-74.7) Ma.
4. Orchidoideae Eaton
Root tubers +/0, (fleshy rhizomes +); (glucomannans +); (amyloplasts with numerous minute starch grains [= spiranthosomes]); (tilosomes 0); sclerenchyma in leaf and stem rare; stomata anomocytic; leaves (spiral), soft, herbaceous; anther erect (incumbent), apex acute, staminodes of inner whorl reduced; pollinia soft/sectile, (hamulus +, caudicle +); pollen usu. intectate; x = 7?
204/3,755 (ca 5,000 - J. B. Thompson et al. 2023). World-wide, esp. temperate. Map: from Pridgeon et al. (2001, 2003); distribution in N. Asia and N. North America unclear.
Age. Crown-group Orchidoideae are (63-)58, 52(-48) Ma (Ramírez et al. 2007), or, as recalculated by Gustafsson et al. (2010), (67-)50(-34) Ma and (64-)53(-42) Ma. Ages in Serna-Sánchez et al. (2021) are ca 57.8 (strict clock) or ca 60.0 Ma (relaxed clock), phylogenetic fuses ca 5.4 and 8.4 Ma respectively, in Mayer et al. (2021: ?sampling) the estimate is 68-38 Ma and in G. Zhang et al. (2023) it is ca 61.3 Ma.
[Cranichideae + Diurideae]: ovules early- to mid-development at anthesis.
Age. The ages for this clade in Serna-Sánchez et al. (2021) are ca 55.8 (strict clock) or ca 53.2 Ma (relaxed clock), Smidt et al. (2021) suggest an age of ca 47 Ma and G. Zhang et al. (2023) ca 58.2 Ma.
4A. Cranichideae Pfeiffer
(Plant holomycoheterotrophic); pollinia soft, caudicle 0, 2nd intine layer 0 [Pteroglossa roseoalba]; stylar canal + [Spiranthinae].
33/746[?]: Microchilus (235), Pterostylis (215), Cyclopogon (83), Pelexia (77), Zeuxine (74), Goodyera (70), Cheirostylis (53), Sarcoglottis (48), Anoectochilus (47), Vrydagzynea (43).
Age. Ages in Serna-Sánchez et al. (2021) are ca 46.0 (strict clock) or ca 41.6 Ma (relaxed clock), phylogenetic fuses ca 9.8 and 11.6 Ma respectively; Smidt et al. (2021: divergence of Goodyerinae) estimate an age of ca 43 Ma and G. Zhang et al. (2023) ca 49.1 Ma.
4b. Diurideae (Endlicher) Meisner
.
Caladenia (270), Corybas (140), Prasophyllum (131), Thelymitra (110), Diuris (70).
Age. Ages in Serna-Sánchez et al. (2021) are ca 54.4 (strict clock) or ca 40.9 Ma (relaxed clock), phylogenetic fuses ca 1.4 and 12.4 Ma respectively and in G. Zhang et al. (2023) ca 49.0 Ma.
[Codonorchideae + Orchideae]: ?
Age. This clade is ca 53.8 (strict clock) or ca 54.3 Ma (relaxed clock) in Serna-Sánchez et al. (2021)
4C. Codonorchideae P. J. Cribb - Codonorchis Lindley
?
1/2. S.E. Brazil, E. Argentina and Chile, Falkland Islands.
4D. Orchideae Small —— Synonymy: Neottiaceae Horaninow, Limodoraceae Horaninow, Liparidaceae Vines, Ophrydaceae Vines
(Plant holomycoheterotrophic); pollinia sectile, well developed caudicular stalks; ovules early-development to mature at anthesis.
Habenaria (908), Platanthera (136), Disa (182), Ophrys (10-354),Cynorkis (156), Peristylus (105), Satyrium (86), Disperis (78), Ponerorchis (55), Herminium (49).
Age. Suggested ages for Orchideae in Serna-Sánchez et al. (2021) are ca 38.5 (strict clock) or ca 41.1 Ma (relaxed clock), phylogenetic fuses ca 15.3 and 13.2 Ma respectively, and in G. Zhang et al. (2023) ca 45.2 Ma.
5. Epidendroideae Kosteletzky
Plant fleshy [stems, leaves - ?level]; roots (with pneumathodes), (velamen 0); conical/(spherical) SiO2 bodies in bundle sheath cells/0; bicellular mucilage-secreting floral hairs +; sclerenchyma in leaf [as fibre bundles or associated with vascular bundles], stomata often paracytic; leaves (unifacial, terete/isobifacial), (articulated and deciduous above sheathing base), vernation conduplicate (plicate); anther incumbent [bent forwards], (strongly convex), with beak, operculate; pollen (semitectate); endothecial thickenings often other than annular; pollinia hard/(soft/sectile), 2-12, clavate, with a waxy surface, frenicular caudicle and stipes +; (embryo with cotyledon visible); n = 5+; (chloroplast ndh genes not functional); ca 8 kbp transfer from fungal to orchid chondrome.
650/21,800 (16 tribes below). More or less world-wide, but most diverse in the tropics, rather poorly developed in Australia. Map: from Pridgeon et al. (2005).
Age. Crown group Epidendroideae are estimated to be (67-)59, 51(-44) Ma (Ramírez et al. 2007), or recalculated as (62-)49, 44(-29) Ma (Gustafsson et al. 2010), ca 48 Ma (Givnish et al. 2016a) and (58.9-)48.6(-39.1) Ma (Leopardi-Verde et al. 2016); other estimates include (115-)97.7(-83) Ma (Sosa et al. 2016, q.v. for discussion), (49.5-)39.8(-32.0) Ma (Y.-K. Kim et al. 2019) and 47-26 Ma (Mayer et al. 2021). Ages in Serna-Sánchez et al. (2021) are ca 44.5 (strict clock) or ca 60.2 Ma (relaxed clock), phylogenetic fuses ca 18.7 and 8.2 Ma respectively, in G. Zhang et al. (2023) ages are (74.2-)72.0(-68.1) Ma and in L. Simpson et al. (2022/2024) the age is ca 54.8 Ma.
Succinanthera baltica, consisting of hard pollinaria attached to a fungus gnat found in Baltic amber ca 55-45 Ma, has been placed in Epidendroideae, perhaps belonging to a basal clade (Poinar & Rasmussen 2017).
5A. Neottieae Lindley
Terrestrial, (plant holomycoheterotrophic); silica bodies 0 (+), stomata anomocytic, sclerenchyma in leaf 0; labellum = hypochile + epichile (not), spur +/-; pollinia 2, 4, soft and mealy; ovules early-development to mature at anthesis; (plastome MatK + - Aphyllorchis).
5/200: Epipactis (70), Neottia (65: inc. Listera), Palmorchis (21), Aphyllorchis (20). Largely temperate/subtropical Northern Hemisphere, few tropical, Palmorchis Central and South America.
Age. Ages in Serna-Sánchez et al. (2021) are ca 35.6 (strict clock) or ca 56.3 Ma (relaxed clock), phylogenetic fuses ca 8.9 and 4.0 Ma respectively.
[Sobralieae [Wullschlaegelieae, Thaieae, [Triphoreae [Xerorchideae [[Gastrodieae + Nervilieae] [Tropidieae [Arethuseae [Malaxideae [[Collabieae + Podochileae] [Vandeae [Cymbidieae + Epidendreae]]]]]]]]]]: ?hard pollinia + (Pérez-Escobar et al. 2023)>
Age. This clade is ca 34.8 Ma (Y.-K. Kim et al. 2019) or ca 67.2 Ma (G. Zhang et al. 2023); fossil hard pollinia are dated to 55–40 Ma (Poinar & Rasmussen 2017).
5B. Sobralieae Pfitzer
(Plant epiphytic [apo.?]), (CAM photosynthesis +).
3/265: Sobralia (150), Elleanthus (110). New World tropics.
Age. Ages of Sobralieae in Serna-Sánchez et al. (2021) are ca 7.7 (strict clock) or ca 11.5 Ma (relaxed clock), with phylogenetic fuses ca 33.1 and 41.8 Ma respectively, while a crown-group age of a mere ca 8.7 Ma associated with a fuse estimated to be ca 58.5 Ma was suggested by G. Zhang et al. (2023); the crown-group age in Collobert et al. (2023) is estimated to be (17.1-)7.1(–1.7) Ma .
5C. Wullschlaegelieae Dressler - Wullschlaegelia Reichenbach f.
Plant holomycoheterotrophic; P [5] / 5; pollinia 2, sectile, viscidium +, stipes 0; plastome IR 0, Mat K +.
1/2. Southermost Mexico, Central and South America, West Indies.
5D. Thaieae K.-H. Jin & X.-G. Xiang - Thaia saprophytica Seidenfaden
Plant holomycoheterotrophic; labellum with strong median ridge.
1/1. Laos, Thailand.
Age. This clade is ca 66.8 Ma (G. Zhang et al. 2023).
5E. Triphoreae Dressler
(Plant holomycoheterotrophic - Pogoniopsis); stomata perigenous; leaves plicate or conduplicate; anther ±erect; (ovule ategmic - P.); seed ovoid, (nucellar epidermis → hard seed coat, funicle enlarged - P).
5/31: Triphora (19). E. North America to tropical South America and the Antilles, also West and Central Africa (Diceratostele gabonensis).
Age. This clade is ca 66.7 Ma (G. Zhang et al. 2023).
5F. Xerorchideae P. J. Cribb - Xerorchis Schlechter
pollinia 8
1/2. Non-Andean tropical South America.
Age. The age of this clade is ca 65.9 Ma (G. Zhang et al. 2023) and that of a clade [Gastrodieae, Vandeae, Malaxidae] is estimated to be ca 67 Ma (Yuan et al. 2018); ca 48.0 Ma is the estimate in L. Simpson et al. (2022/2024).
[Gastrodieae + Nervilieae]: rhizomes swollen; pollen sectile.
Age. This clade is estimated to be ca 35.9 Ma (Y. Wen et al. 2022) or (64.6-)63.6(-58.9) Ma (G. Zhang et al. 2023).
5G. Gastrodieae Lindley
Plant holomycoheterotrophic; P [3], ([5]), (inner P distinct); pollinia 2/4, sectile/granular, viscidium +; (ovule unitegmic - Gastrodia); plastome (IR 0 - Ga.), (MatK + - some Ga), rrn block + [rRNA genes].
5/122: Gastrodia (100≤), Didymoplexis (20). Tropics, inc. the Antipodes, scattered, to warm temperate, mainland South America only 2 spp. (Uleiorchis).
Age. The age of a [Gastrodia + Didymoplexis] clade is estimated to be ca 29.9 Ma (Y. Wen et al. 2022).
5H. Nervilieae Dressler
Plants holomycoheterotrophic / unifoliate, leaf not persisting; leaf petiolate, blade cordate, venation palmate; (flowers not resupinate - Epipogium); pollinia 2, sectile, viscidia 0; (ovule unitegmic - E.),(plastome IR 0 - E.).
3/75: Nervilia (70). Europe and Africa to the Pacific islands; Southeast Asia, Japan, E. Russia.
Age. Crown-group Nervilieae are ca 57.3 Ma Ma (G. Zhang et al. 2023).
Age. This clade is ca 65.0 Ma (G. Zhang et al. 2023).
5I. Tropidieae (Pfitzer) Dressler
(Plant holomycoheterotrophic), rather woody; leaves plicate; anther erect; pollinia sectile.
2/39: Tropidia (30), Corymborkis. Tropical.
Evolution: Divergence & Distribution. The holomycoheterotrophic habit seems to have evolved twice in this small clade (Sabino Kikuchi et al. 2020).
Phylogeny. For relationships in Tropidieae, see Sabino Kikuchi et al. (2020).
[Arethuseae [Malaxideae [[Collabieae + Podochileae] [Vandeae [Cymbidieae + Epidendreae]]]]] / "upper" epidendroids: (CAM photosynthesis +): pseudobulbs +; water-storing hypodermis; pollinia hard, stalks well developed, cellular [?here]; ovules often only at earliest stage of development at anthesis [?here].
Age. This clade is 37.9-30.8 Ma (Givnish et al. 2015), ca 30.8 Ma (Y.-K. Kim et al. 2019), ca 57.2 Ma (G. Zhang et al. 2023) or ca 41.2 Ma (L. Simpson et al. 2022/2024).
5J. Arethuseae Lindley
Plants terrestrial - esp. Arethusinae, (annually deciduous pseudobulbs - Pleione), also corms +; inflorescence terminal, inc. on reduced leafless shoots; peduncle often 1 internode; (flowers not resupinate); (inner P distinct); labellum lamellae plane/papillate/denticulate/trichome-like, spur usu. 0; pollinia (4) 8, accessory structures +, viscidium 0/indistinct; column foot +/-, apex often marked by expanded margins [= petaloid]; endocarp elaters 0.
16/745: Coelogyne (595), Glomera (130). Tropical Asia and Malesia to the Pacific, some E. North America to the Caribbean.
Age. Serna-Sánchez et al. (2021) suggest that the crown-group ages for this clade are ca 19.8 (strict clock) or ca 22.5 Ma (relaxed clock), phylogenetic fuses here are ca 16.9 and 23.6 Ma respectively; G. Zhang et al. (2023) estimated Arethuseae to be rather older, (51.0-)37.2(-33.5) Ma.
[Malaxideae [[Collabieae + Podochileae] [Vandeae [Cymbidieae + Epidendreae]]]] / the MCVECP clade: plant epiphytic; tegulum + [= pollinarium stipes formed from epidermis (and underlying cell layers) of rostellum; ?level]; ustilaginalean mitochondrial DNA.
Age. This clade is ca 28.6 Ma (Y.-K. Kim et al. 2019), (51.7-)39.0(-48.1) Ma (Collobert et al. (2023) or (55.8-)54.9(-48.1) Ma (G. Zhang et al. 2023).
5K. Malaxideae Lindley —— Synonymy: Pycnanthaceae Ravenna
(Plants terrestrial - Malaxidinae), (corm-like structures +); leaves plicate/conduplicate; (ovules unitegmic); (suspensor lobed).
Bulbophyllum (2,190), Dendrobium (1,647), Liparis (320-430), Oberonia (325), Crepidium (260), Malaxis (185). Pantropical, but esp. Southeast Asian-Malesian, few temperate.
Age. Ages in Serna-Sánchez et al. (2021) are ca 33.7 (strict clock) or ca 37.0 Ma (relaxed clock), phylogenetic fuses ca 2.2 and 7.9 Ma respectively, and in L. Simpson et al. (2022/2024: Oberonia sister) the age is ca 36.5 Ma. Note estimates of the age of the speciose Dendrobium-Bulbophyllum clade of (37.6-)31.6(–26.2) Ma (A.-Q. Hu et al. 2022), (34.0-)30.3(–26.9) Ma (X.-G. Xiang et al. 2016) and (39.0-)33.2(-27.7) Ma (Simpson et al. 2022/2024).
[[Collabieae + Podochileae] [Vandeae [Cymbidieae + Epidendreae]]: endocarpial elater-hairs +.
Age. The age of this clade is around ca 26.8 Ma (Y.-K. Kim et al. 2019) or 52.8 Ma (G. Zhang et al. 2023).
Age. This clade is estimated to be ca 40.8 Ma (G. Zhang et al. 2023) or (37.8-)33.4(-26.a) Ma (P. Zhou et al. 2023).
5L. Collabieae Pfitzer
Plant (holomycoheterotrophic - Risleya), terrestrial; rhizomatous, (pseudobulbs); root hairs +; inflorescenece usu. lateral; (labellum saccate/spurred); rostellum elongate, pollinia 8 (4), hard to soft, viscidium + (0).
24/487: Calanthe (260), Plocoglottis (40-45), Phaius (40), Spathoglottis (40). Tropical Old World (to temperate Asia), Calanthe calanthoides alone in Central America, The Antilles and N. South America.
Age. Ages in Serna-Sánchez et al. (2021) are ca 17.3 (strict clock) or ca 22.5 Ma (relaxed clock), phylogenetic fuses ca 14.6 and 13.5 Ma respectively, and in P. Zhou et al. (2023) Collabieae are (34.5-)28.9(-22.3) Ma.
5M. Podochileae Pfitzer
(Plants terrestrial); pseudobulbs 1-3 leaves; leaves (deciduous), (2-ranked); pollinia 8 (4); ?endocarp elater hairs.
28/1,297: Phreatia (220), Pinalia (210), Appendicula (150), Ceratostylis (150), Trichotosia (80), Podochilus (62), Cylindrolobus (60), Octarrhena (52), Porpax (42), Mycaranthus (40). Indo-Asia to the Pacific, Stolzia (= Porpax) Africa.
Age.
[Vandeae [Cymbidieae + Epidendreae]]: ?
Age. This clade is (31.1-)25.3(-21.2) Ma (Y.-K. Kim et al. 2019) or ca 50.7 Ma (G. Zhang et al . 2023).
5N. Vandeae Lindley
(Pyrrolizidine alkaloids +); growth monopodial; (roots the main photosynthetic organs).
>150/2,715: Polystachya (240), Taeniophyllum (190), Thrixspermum (180), Angraecum (176), Vanda (87), Campylocentrum (70), Phalaenopsis (70), Trichoglottis (70), Gastrochilus (69), Aerangis (58).
Age. Ages in Serna-Sánchez et al. (2021) are ca 25.6 (strict clock) or ca 30.8 Ma (relaxed clock), phylogenetic fuses ca 8.9 and 9.3 Ma respectively, while the age in Y.-K. Kim et al. (2020: Thrix + Gast) is ca 13.8 Ma, in Y. Li et al. (2022: An + Cy) it is somewhat over 21 Ma and in G. Zhang et al. (2023) it is ca 45.6 Ma.
Age. G. Zhang et al. (2023) suggest an age for this clade of ca 50.1 Ma.
5O. Cymbidieae Pfitzer
Leaves (plicate), (amphistomatous), (mesophyll homogeneous); seeds (with hooks/capitate protrusions - esp. Oncidiinae).
Maxillaria (658), Oncidium (374), Eulophia (267), Telipogon (205), Catasetum (180), Cyrtochilum (140), Dichaea (120), Gomesia (120), Camaridium(80), Gongora (75), Cymbidium (70), ?Kefersteinia (70), Scaphyglottis (70), Trichocentrum (70), Brassia (64), Coryanthes (60), Ornithidium (60), Stanhopea (60), Notylia (56), Ornithocephalus (55), Fernandezia (51), Cyrtopodium (50), Maxillariella (50).
Age. Ages in Serna-Sánchez et al. (2021) are ca 31.6 (strict clock) or ca 35.4 Ma (relaxed clock), phylogenetic fuses ca 2.9 and 4.7 Ma respectively and the age in G. Zhang et al. (2023) is ca 40.3 Ma.
5P. Epidendreae Lindley
(Plant mycoheterotrophic - Danxiaorchis; Yoania), (pseudobulbs laterally flattened); stem (with single leaf and tubular sheaths); inflorescence (axillary), (abaxial to leaf); (pedicel articulated below G); (flowers not resupinate); pollinia (2-)8, (soft); (testa hard - Y.); n = x = 20.
Epidendrum (1,872), Encyclia (170), Anathallis (155), Dracula (135), Prosthechea (120), Cattleya (115), Platystele (110), Trichosalpinx (110), Agrostophyllum (100), Sophronitis (60), Dryadella (55), Muscarella (55), S.E. U.S.A. and Mexico to N. Argentina, the Antilles.
Age. Ages in Serna-Sánchez et al. (2021) are ca 32.2 (strict clock) or ca 35.5 Ma (relaxed clock), and phylogenetic fuses are ca 2.6 and 5.2 Ma respectively, and the age in G. Zhang et al. (2023) is ca 48.3 Ma.
5P1. Pleurothallidinae G. Don
Single- (multi-)flowered coflorescences + [all growth units can flower annually], peduncle very short; colleters on G [Pleur.].
44/>5,114: Stelis (1,338), Lepanthes (1,189), Masdevallia (656), Pleurothallis (556), Acianthera (200), Octomeria (160), Pabstiella (135), Anathalis (120), Specklinia (100), Brachionidium (80), Andinia (70), Porroglossum (53), Restrepia (53), Myoxanthus (50).
Age. An estimate of the stem-group age - Epidendrum + Cattleya sister, is (23.6-)18.7(-15.5) Ma, crown group variously (20.9-)14.9(-13.6) or (11.7-)8.1(-6.6) Ma
GLOSSARY (for definitions, see e.g. Rasmussen (1986), Freudenstein (1994).
carapace (of a seed) = protective, ± persistent collapsed inner integument, maybe with phenolics, etc..
caudicle = pollinium stalk, made up of elastoviscin and pollen grains from basal extension of pollinia.
column = a structure extending above the ovary and incorporating stigmas, styles and stamens, i.e. a gynostemium.
epichile = the apical or distal part of the labellum.
granular pollinia = pollinia with air spaces, tetrads usually with exine, opaque and often light yellow.
hamulus = pollinarium stipes, derived from the entire apical part of rostellum, ± recurved or hook-like, c.f. tegula.
hard pollinia = compact pollinia with few or no air spaces, exine only on the surface tetrads, deep yellow or orange, translucent.
hypochile = the basal or proximal part of the labellum.
incumbent (of anthers) = bent adaxially/inwards at ca 90o.
labellum = the usually very highly modified adaxial inner tepal, usually abaxial in position because the flower is resupinate.
massulae = a group of pollen tetrads of irregular number and so size, derived from a single archesporial cell.
mentum = structure produced by the lateral sepals, labellum base and column tissue.
pollinaria = a complex structure where two or more pollinia are attached by translators to a central corpusculum.
rostellum = apical portion of the median stigmatic lobe, or only the non-receptive part.
sectile pollinia = pollinia that have a soft and friable ("mealy") texture and are made up of massulae.
soft pollinia = pollinia made up of relatively few pollen grains with a low degree of cohesion
stipes (pl. stipites) = pollinarium stalk, cellular in nature and derived from the rostellum, see hamulus and tegula.
tegula = pollinarium stipes, derived from the dorsal epidermis of the rostellum, c.f. hamulus.
translator, of a pollinarium = caudicle or stipes.
viscidium = sticky basal portion of pollinium stalk, derived from rostellar cells.
Evolution: Divergence & Distribution. Neither Mycophoris, a putative orchid seed in Dominican amber at least 20-15 Ma, and its associated fungus (Poinar 2016a) are likely to have been correctly identified (Selosse et al. 2017b). However, Iles et al. (2015) give dates for three fossils reliably placed within Orchidaceae and Poinar (2016b) described pollinaria attached to beetles and stingless bees in Dominican and Mexican amber some Ma respectively.
See Pérez-Escobar et al. (2017a: Fig. S7-S9) for ages, the focus on Epidendreae and Pleurothallidinae, also Fischer et al. (2007) and L. Simpson et al. (2022/2024) both Bulbophyllum, Sosa et al. (2016: Epidendreae), X.-G. Xiang et al. (2017) and H.-T. Li et al. (2019), both Dendrobium, Andriananjamanantsoa et al. (2016), Pessoa et al. (2018) and Farminhão et al. (2021, 2023), all Angraecum and relatives, etc.. Serna-Sánchez et al. (2021) suggest a number of ages for major clades in the family, but these differ, and sometimes quite substantially, depending on whether strict or relaxed clock methods are being used, and this also applies to the stem branch lengths (= phylogenetic fuses) of these clades. Thus 13/17 crown group ages are older if relaxed clock methods are used, while 11/17 stem-group ages are older (the clades with younger ages differ in the two methods); as an example, strict clock estimates of the age of Diurideae are ca 54.4 Ma for the crown group and ca 1.4 Ma for the stem group, while with a relaxed clock the comparable ages are ca 40.9 and 12.4 Ma respectively (Serna-Sánchez et al. 2021). There is also the issue of the data used to construct the tree in the first place. Surprise: evolutionary stories that depend on these ages will be different.
Diversification may have increased at this node around (108.8-)73.1(-59.7) Ma (Magallón et al. 2018) - but see below.
We still know rather little about the origin and biogeography of the family (see also Chase 2003). Givnish et al. (2016a) suggested that Orchidaceae originated in Australia ca 112 Ma and then spread via Antarctica to South America where Vanilloideae and Cypripedioideae had originated by 64 Ma. Ramírez et al. (2007) also thought that the subfamilies had diverged by the end of the Cretaceous, ca 65 Ma (see also Givnish et al. 2015, 2016a; G. Zhang et al. 2023: mycoheterotrophic taxa at the base of Epidendroideae evolving in response to K/P event?), or perhaps slightly later in the early Palaeocene, and that orchid radiation has been a largely Caenozoic phenomenon. Thus Zhang et al. (2023) saw dramatic expansion of tropical forests ca 56 Ma near the Palaeocene-Eocene Thermal Maximum, shortly after the development of closed-canopy LTRF ca 60 Ma; the epiphytic MCVECP clade began to evolve ca 55 Ma. Dates suggested by Gustafsson et al. (2010) are somewhat younger, major diversification perhaps occurring during the cooler period at the end of the Eocene and into the Oligocene rather than during the thermal maximum earlier in the Eocene (c.f. Ramírez et al. 2007). J. B. Thompson et al. (2023) using the estimate of ca 64 Ma by Givnish et al. (2015) for the divergence of Orchidoideae and Epidendroideae suggested that global cooling from the Miocene onwards rather than any other factor had driven diversification of Orchidoideae, with several rate shifts (increases) beginning around 26.2 Ma. (Note that other estimates for the same initial divergence event published since 2015 range from (79.7-)77.7-44.7(-36.2) Ma - see above; these were not mentioned). Another suggestion is that Orchidaceae originated in Laurasia around about 120 Ma (there was subsequently an evolutionary fuse of ca 30 My). Southern Central America in particular has been a hotspot for orchid speciation since the Pliocene, indeed, most species in the family have evolved within the last 5 My (Pérez-Escobar et al. 2024).
Despite the minute size of orchid seeds, long distance dispersal (l.d.d.) seems not to be notably common in the family (c.f. Vollering et al. 2015), and orchid species may be narrowly distributed despite the wide distribution of some of their fungal associates (Davis et al. 2015; see also Weiß et al. 2016: Sebacinales-Serendipitaceae the fungi). Indeed, there can be surprisingly little genetic differentiation between orchid populations, despite the possibility for l.d.d. of the seeds and resultant founder effects (Phillips et al. 2012), perhaps because l.d.d. swamps potential geographic variation... However, epiphytic taxa in tropical mountains may show quite a bit of local genetic divergence (Givnish et al. 2015 for literature). Pérez-Escobar et al. (2017a, see also b) noted that the Andes seems not to have been much of a barrier to the dispersal of Cymbidieae and Pleurothallidineae whose diversification they were studying. There are a few cases where l.d.d. does seem to have been a factor. Givnish et al. (2016a) estimated that there had been around 73 l.d.d. events in his study of the family, even if the distributions of over 97% of orchid species are restricted to single continents. Bouetard et al. (2010) estimated that Vanilla started to diversify ca 34 Ma, at least three instances of l.d.d. being needed to explain its present distribution, while l.d.d. seems also to be quite common in Spiranthes with S. romanzoffiana occuring on both sides of the Atlantic, movement having been from west to east (Dueck et al. 2014 and refs). Kirby (2016) looked at possible l.d.d. in tropical American orchids. L.d.d. is a common way in which plants get to oceanic islands, and here the relatively low diversity of orchids compared to groups like Asteraceae (Lenzner et al. 2017) is perhaps connected with the obligate association of germinating orchid seeds with fungi, but it at first sight does seem odd. That orchids arrived on Krakatau, Indonesia (Partomihardjo 2003), quite soon after its devastating eruption is of doubtful relevance since Krakatau is preeminently a continental island. Traxmandlová et al. (2017 and references) suggested that species richness of orchids on islands depended in considerable part on area and altitude, i.e. habitat diversity.
Recent work (J. B. Thompson et al. 2023) suggests that diversification in Orchidoideae has increased during the recent period of global cooling that began in the Miocene ca 26 Ma, a number of rate increases being associated with this. These shifts are most obvious in Orchidoideae of temperate regions, perhaps especially in the Antipodes and in Habenaria, Pterostylis and Diuridae; there is a possible connection between these cooler temperatures and deceit pollination (for which, see below). Smidt et al. (2021) had proposed that there had been two dispersal events between Southeast Asia and America in the Cranichideae-Goodyerinae, and in one, Nearctic Goodyera s.l., there had been movement back to Southeast Asia.
Diversification in the six or seven tribes of the speciose core/advanced/"upper" Epidendroideae, largely epiphytic, began 37.9-30.8 Ma (Givnish et al. 2015: inc. Arethuseae; see also Freudenstein & Chase 2015); for the MCVECP clade (= [Malaxideae [[Collabieae + Podochileae] [Vandeae [Cymbidieae + Epidendreae]]]]), (55.8-)54.9(-48.1) Ma, see G. Zhang et al. (2023). Speciation may have increased in these clades, e.g. in Epidendroideae-Malaxideae-Dendrobiinae, partly because of the adoption of the epiphytic habit (Gravendeel et al. 2004) and also movement into montane areas (Givnish et al. 2015), and because of changes in the rate of uplift of the Andes (Pérez-Escobar et al. 2017a). The evolution of the epiphytic habit is thought to be an apomorphy of the MCVECP clade by Zhou et al. (2023); although Givnish et al. (2015) put this one tribe lower in the tree, they thought that the time of evolution of this habit was substantially younger - and although gained but once it was lost multiple times (Givnish et al. 2015), rather unlike some other epiphytic groups (G. Khan et al. 2024). Some of these epiphytic clades do show increased rates of diversification, but not the clade as a whole. There are ca 8,450 (and counting) Neotropical species of Cymbidieae and Pleurothallidinae, mostly Andean. Clades of Cymbidieae, most diverse below 800 m, are largely derived from Amazonian lowland taxa (41-)34(-27) Ma (stem age), most diversification being after ca 25 Ma, while Pleurothallidinae are most diverse between 400 and 2200 m, clades largely being derived from lineages preadapted to cool conditions, comparable ages being (27-)20(-13) and ca 17 Ma (Pérez-Escobar et al. 2017a). On the other hand, in the largely terrestrial (a reversal) Epidendreae-Calypsoinae there is a decrease in the rate of speciation (Givnish et al. 2015, see also 2016a); see also Sosa et al. (2016) for another example of the derived terrestrial habit in Epidendreae. P. Zhou et al. (2023) looked at diversification in the tropical terrestrial Collabieae, and they thought that it had to do with higher diversification rates under more stable humid climates in Asia, less with colonization times; they suggested that there had been 5 dispersal and 4 vicariance events in this tribe (which has one species in the New World). For more on epiphytism, see below
In Epidendroideae-Malaxideae, Bulbophyllum, largely Old World and very diverse with some 2,200 species, the few (ca 60) New World species form a clade sister to the African taxa, the overall geographical pattern being [S. E. Asia [Madagascar [Africa [Africa + New World]]]]; the genus may have initially evolved in Southeast Asia (Gravendeel et al. 2004; Smidt et al. 2011; esp. B. Gravendeel, E. de C. Smidt, G. Fischer & J. J. Vermuelen in Pridgeon et al. 2014); there are some 500 species in New Guinea alone. However, relationships in Gamisch and Comes (2019, see also Fischer et al. 2007; Pridgeon et al. 2014) are somewhat simpler - [S. E. Asia [Madagascar [Africa + Neotropics]]] (a few species of the Madagascan clade are to be found on mainland Africa), with long-distance dispersal - possibly even separately and sequentially from S. E. Asia - resulting in the current distribution of the genus - overall, however, as in other studies there is a strong biogeographical element in the major groupings. The Madagascan, African and Neotropical clades seem to have had high but constant rates of diversification and high rates of extinction (intrinsic extinction factors?), and there have been major range expansions and contractions in all four groups during the Quaternary which may have affected speciation and extinction (Gamisch & Comes 2019). The stem age of Bulbophyllum is perhaps (37.0-)29.3(-23.3) Ma or ca 33.2 Ma and the crown age (25.6-)20.6(-16.2) Ma or (30.7-)23.6(-20.1) Ma (Gamisch & Comes 2019 and L. Simpson et al. 2022/2024 respectively). Diversification in the largely Indo-Malesian Dendrobium with its 1,600+ species is estimated to have begun (31.6-)28.2(-25.1) Ma (X.-G. Xiang et al. 2016) or (47.7-)37.4(-29.5) Ma (M.-H. Li et al. 2019: split between Asian and Australasian clades). Simpson et al. (2022/2024) found the largely Australian sections [Adelopetalum + Minutissimum s. str.] to be sister the rest of the genus. Fischer et al. (2007: Madagascan species), A.-Q. Hu et al. (2019: the Cirrhopetalum group) and Thawara et al. (2024: the Asian clade) have looked at aspects of character evolution in Bulbophyllum - in most cases there is extensive homoplasy, while Y. Li et al. (2025) discuss genome evolution.
- In Vandeae a major epiphytic group from Africa-Madagascar is the angraecoid orchids (all told, some 360 species). Diversification here began in the Miocene (24.0-)17.6(-11.1) Ma; Farminhão et al. (2021) noted that there was some similarity in the patterns of diversification of the Angraecum group and Bulbophyllum. The Angraecum group is overall far less speciose, if with sometimes spectacular flowers, and it is noted for its extensive dysploidy (but Bulbophyllum is not), and although this seems not to be correlated with its diversification, there may be links between chromosome number change and features like leaflessness and rostellum structure (Farminhão et al. 2021).
- Interestingly, Pedersen et al. (2020) found small monophyletic groups of Dendrochilum (280 species, = Coelogyne-Arethuseae), mainly West Malesian, on the same mountain.
- Epidendrum and Lepanthes (both Epidendreae) are New World, and with 1,549 and 1,035 species respectively they are two of the five largest genera there (Ulloa Ulloa et al. 2017).
- About half the 180 species of the tuberous Disa (Orchidoideae-Orchideae) are restricted to the Cape Floristic Region, and they moved into subalpine/grassy habitats where they often flower immediately after fires, a habit that has been dated to around 12.9 Ma; they have also diversified on the Drakensbergs (Bytebeier et al. 2011, see also Linder 2003; Galley et al. 2007). Satyrium (also Orchideae) has diversified in the Fynbos region, also in the Cape (van der Niet & Linder 2008; Verboom et al. 2009; Goldblatt & Manning 2002b also discuss orchids in the Cape flora).
- The ancestors of Cypripedioideae/Cypripedium may have been plants of both the Old and New Worlds or just the latter, depending on the model used; the clade may have originated in the late Cretaceous, but then there was a phylogenetic fuse of some 35 My... (Lagou et al. 2024). There seem to have been around ten hybridization events along the phylogenetic backbone of Cypripedium (Lagou et al. 2024: Fig. 3D). Similarly, M. Liao et al. (2024) noted "Cypripedioideae appeared to have arisen in South America and/or the adjacent Qinghai-Tibet Plateau and Hengduan Mountains ∼35 Mya".
There are some 295 species of more or less echlorophyllous holomycoheterotrophs in Orchidaceae, somewhat under half of all such species (ca 664 spp.: Jacquemyn & Merckx 2019, modified by P.F.S. vi.2024, also, see esp. Thismiaceae s.l.), and they have evolved some 25-30 (or many more) times (Molvray et al. 2000; Merckx & Freudenstein 2010; Freudenstein & Barrett 2010; Merckx et al. 2013c; Feng et al. 2016; Lam et al. 2018). It has been suggested that Aphyllorchis (Neottieae), all ca 20 species of which are holomycoheterotrophs and one of the larger such genera, diverged from Cephalanthera ca 28 Ma, however, A. montana, the only species studied by Feng et al. (2016), showed signs of only recently having become a holomycoheterotroph, several of its photosynthesis genes apparently being functional (see also Barrett et al. 2018). For more on mycoheterotrophy, obligate or not, see below.
Endress (2016: comparison with Apocynaceae) emphasized the development of synorganization/complexity in the flowers of Orchidaceae; synorganization lays the foundation for the development of novel structures, although it could be argued (see also below") that within the highly speciose [Orchidoideae + Epidendroideae] there is little real floral novelty, just infinite variation on a theme (see also Freudenstein 2025). Monosymmetry of the flower in many, but not all orchids - and in Hypoxidaceae and Doryanthaceae - is evident even in the earliest primordia (Kurzweil & Kocyan 2002 and references); although the flowers are usually (but not always) presented inverted in all subfamilies, resupination in the sense of twisted ovaries seems not to occcur in the three basal subfamilies (Koopowitz 2017). Recent work suggests that PROLIFERATION CELL FACTOR and CINCINNATI gene families are much duplicated and are involved in the development of monosymmetry of flowers here (Madrigal et al. 2017: comparison between Cattleya trianae and Hypoxis decumbens), unlike the situation in other monosymmetric flowers. However, in their analysis of the complex evolutionary hostory of the RADIALIS and DIVARICATA gene lineages, Madrigal et al. (2019) found a common gene regulatory network involved in the control of floral symmetry in monocots and core eudicots. In Orchidaceae there were complex patterns of gene duplication that differed between the two groups of genes. A few Orchidaceae have more or less polysymmetric flowers, and in Telipogon (Epidendroideae-Oncidiinae) a polysymmetric perianth becomes evident only late in development (Pabón-Mora & González 2008). Duttke et al. (2012) discuss the remarkable terata to be found in Neofinetia falcata (Vandeae: Aeridinae) that have been accumulated in Japan over the last 350 years. For floral development and the expression of B-, C- and D-class genes in particular in Dendrobium, see Y. Xu et al. (2006); genes of all three classes are expressed in the column. Hsu et al. (2015) suggest how labellum development might be controlled; there was gene duplication after divergence of the lipless Apostasioideae, while Madrigal et al. (2017) note that there was a large-scale duplication event prior to the divergence of Epidendroideae and Orchidoideae. The sequence of organ initiation varies considerably within the family (Pabón-Mora & González 2008). Thus Apostasioideae and Cypripedioideae have simultaneous initiation of members of the inner tepal whorl, the plesiomorphic condition for Asparagales (Kocyan & Endress 2001a); have Vanilloideae been studied? Rudall et al. (2013b) attempt to work out what some of the parts of orchid flowers "are". They suggest connections between the auricles, which often have raphides, with inner whorl anthers of Apostasioideae, and they show that the bursicle, which had also been thought to be of staminodial origin, develops from lateral lobes of the median carpel. The labellum, which in its lobing they describe as being analogous to a compound leaf, has three traces - two come from the lateral sepals or perhaps originally from stamens of the outer whorl (Rudall et al. 2013b).
Endress (2011a) thought that the inferior ovary in Asparagales might be a key innovation, although where this feature should be placed on the tree is unclear - perhaps here is indeed one place. The presence of pollinia is another feature that he mentioned; this is probably best placed as a synapomorphy of the [Orchidoideae + Epidendroideae] clade. Indeed, orchid diversity is most often attributed to the nature of the association of the plant with its pollinator, as is discussed below. For details on the distinctive pollinaria of many epiphytic Epidendroideae, see Freudenstein and Chase (2015), who noted that "vandoid" anthers may increase pollinator specificity and so promote diversification. Mosquera-Mosquera et al. (2019) suggested that the ancestor of Epidendroideae had a pollinarium with four granular pollinia, caudicle, tegulum and viscidium.
But as to which of all these features might indeed be key innovations, that is another matter. Overall, species richness in tropical orchids is highest in montane habitats, usually in the 1,000-2,000 m zone although in the 2,000-3,000 m zone in New Guinea (Vollering et al. 2015), and the orchids involved are largely epiphytic members of the core Epidendroideae (Givnish et al. 2015, 2016a; see also Kirby 2016). (Orchidaceae are only the third most diverse family in lowland Amazonia, behind Fabaceae and Rubiaceae - Cardoso et al. 2017.) For the link between habitat diversity and orchid species richness on the islands of the tropical southwest Pacific, see Keppel et al. (2016). Normally neither orchids nor pollinating insects are diverse on oceanic islands, but angraecoid orchids are surprisingly diverse on the Mascarene islands, of which Réunion in particular also has a diverse insect fauna (Micheneau et al. 2008). I return to the issue of the apparent species richness of Orchidaceae and the factors that may cause it below.
Further discussion on diversity in the family is structured as follows:
Ecology and Physiology.
Fungi and Germinating Orchids in Particular, and Mycoheterotrophy in General.
The Epiphytic Habit.
Pollination & Seed Dispersal.
Orchid Pollination Biology.
Orchid Flowers.
Rewards - or Lack Thereof.
Pollinators.
Pollination - General.
Seed Dispersal.
Plant-Animal Interactions.
Bacterial/Fungal Associations.
Vegetative Variation.
Possible Syntheses.
For mycorrhizae in orchids, see e.g. Burgeff (1959) and Favre-Godal et al. (2020: biochemistry, molecular interactions, little on seedlings). Here I begin by discussing the seedling stage of Orchidaceae, echlorophyllous and mycoheterotropic, a condition uncommon elsewhere. There will then be an apparent digression on Paris-type arbuscular mycorrhizal (AM) fungi and mixotrophy in orchids, after which much of the focus will be on holomycoheterotrophy in adult plants, a condition that is scattered elsewhere in angiosperms but is commonest in Orchidaceae, and as as will become clear, this is not just because they are a large family. So see
1. Fungi and Germinating Orchids,
2. Mixotrophy, and
3. Holomycoheterotrophy in Adult Plants in General.
1. Fungi and Germinating Orchids. Just about all orchids have obligate associations with mycorrhizal saprotrophic or ECM fungi, mostly basidiomycetes (see also Bacterial/Fungal Associations below) during germination and as young seedlings, and this is central to understanding the ecophysiology of the orchid plant (for reviews, see Rasmussen 2002; Imhoff 2009; Girlanda et al. 2011; Dearnalay et al. 2012, 2017; Oberwinkler et al. 2013; Hynson et al. 2013; Rasmussen et al. 2015; S. Zhang et al. 2018; Jacquemyn & Merckx 2019). This is the "initial mycoheterotrophy" of Ogura-Tsujita et al. (2021).
Yeung (2022) summarized the development of the embryo in Orchidaceae. There is usually no endosperm in the mature orchid seed, although there may be one or two endosperm cells - and the embryo is nearly always apparently undifferentiated. Although Arditti (1967; see also Yeung 2017) did suggest that a cotyledon was recognizable in the embryos of a few species, the species mentioned are not basal in the tree (see also below). Importantly, the sometimes rather protracted obligate and at least initially echlorophyllous subterranean holomycoheterotrophic phase of the young plant compensates for the absence of reserves in the minute seeds. Miura et al. (2023) looked at Bletilla striata (Epidendroideae-Arethuseae) and found that germination and the establishment of the mycorrhizal association were simultaneous, and unlike most other seed plants germination was not promoted by giberellic acid, rather, this became inactivated while mycorrhizal symbiosis was autoactivated at the same time. In apparently all species, the very young plant depends entirely on its fungal associates for nutrients. Thus the enzyme trehalase is expressed by the orchid seedling, and this ensures its carbohydrate supply by converting the fungal trehalose (two glucose molecules) into glucose, which then may be converted into sucrose, in which form carbohydrates commonly move around the plant (M.-H. Li et al. 2022; D.-K. Zhao et al. 2024: what about amino acids). This plant-fungus association results in the formation of a protocorm (Peterson et al. 1998), a tuberous mass that basically consists of hypocotyl plus plumule, and although it lacks roots, it may have tufts of root hairs (Weber 1981; Whigham et al. 2008; Bustam et al. 2014), and these root hairs ("rhizoids") are sometimes described as being branched (Rasmussen ?1999); the protocorm develops from the middle part of the seed, occasionally from the chalazal end (Balashova et al. 2022). The fungi enter the very young seedling via the scar of the suspensor or via the rhizoids, depending on the orchid species; fungi that enter the "wrong" way may end up killing the seedling (Yeung 2017). The fungal hyphae form pelotons, complex hyphal coils, inside the host plant cells, and these are digested by the host; there may also be movement of nutrients from regular hyphae to the orchid. This association with fungi is essential for the establishment of the orchid seedling, and it does not appear to be antagonistic (Rasmussen 1995; Johnson & Edwards 2000 in part; Eriksson & Kainulainen 2011; Leake & Cameron 2012; Perotto et al. 2014; Rasmussen & Rasmussen 2014; Yeung 2017). Depending on the orchid species, various fungi may be associates, and the species involved may change even during the seeding stages or later on as the orchid matures. Rammitsu et al. (2023a) found that Ceratobasidiomycetaceae isolates were essential for in vitro germination and early development of the epiphyte Vanda falcata, while in 12/13 sites where the adult orchid grew the same Tulasnellaceae O.T.U. was to be found, in five of those sites being the only fungus there, and although a variety of ceratobasidiomycete OTUs were to be found, none was widespread, and four sites had none at all. Even in epiphytic species with chlorophyllous protocorms (also found in some terrestrial species), an association with a fungus is still needed if the young orchid is to survive (Hynson et al. 2013). Stöckel et al. (2014) noticed that 13C and 15 N were higher in young orchid seedlings with ECM associates (rather like the holomycoheteotrophic adults) than in species with rhizoctonia associates (the adults here were photosynthetic, possibly mixotrophic), although overall patterns of nutrient uptake here were somewhat confusing. Ghirardo et al. (2020) have documented the extensive metabolomic changes in both fungus and plant at the protocorm stage in the association between the agaric Tulasnella calospora and Serapias vomeracea (Orchidoideae-Orchideae) - interestingly, a variety of lipids were to be found in the fungal mycelium growing outside the protocorms while there were chito-oligosaccharides in the protocorms themselves. Z.-X. Xu et al. (2023) described the association between the basidiomycete Serendipita indica, also an endophyte in other circumstances, early in the germination of six species (2 genera) of epiphytic Epidendroideae, noting the importance of ADH, alcohol dehydrogenease, genes catalyzing alcoholic fermentation, and hypoxia-responsive genes like DcADH, etc. - indeed, the induction of hypoxia seemed to be important early in the germination of these plants. Some 356 genes were differentially expressed in symbiotic compared to aseptic protocorms, overall the number of up-regulated and down-regulated genes being similar (Xu et al. 2023). A few orchids can germinate in the absence of a fungus, and in vitro germination of a number of terrestrial Australian orchids using variously doctored "asymbiotic" media could be as effective as when using the standard "symbiotic" medium (Bustam et al. 2014). Jolman et al. (2022) summarized the quite extensive literature on asymbiotic germination which has been recorded for some 270 species.
Light commonly inhibits germination, even in epiphytic species (Rasmussen et al. 2015; Balashova et al. 2022). In vitro experiments suggest that seed germination in at least some orchids, especially those from oligotrophic habitats, is suppressed by nitrate (and the growth of the adult plant can also be negatively affected), perhaps associated with the preference of orchids for organic nitrogen (Figura et al. 2019 and references), and this in turn is likely to be linked to their association with fungi.
Which came first, the dust seeds or the holomycoheterotrophic seedling association? This is a chicken-or-egg question, although Rasmussen and Rasmussen (2014) suggest that a developing association with ECM fungi was the spur. Sugars and nitrogen, the latter predominantly as nitrogen-rich amino acids, move from the fungus to the orchid (Zimmer et al. 2007; Kuga et al. 2014; Focha et al. 2016). Orchid fungi that are saprotrophic can break down cellulose, but not lignin, and nutrients, including sugars (c.f. ericoid mycorrhizae), that ultimately move into the orchid come from these saprotrophic activities; the plant cell wall degrading enzymes of the fungi may also be involved in penetrating the cell walls of the orchid (Dearnaley et al. 2012; Kohler et al. 2015; Teixeira da Silva et al. 2015 for references). The number of copies of carbohydrate-active enzymes and proteins with a cellulose-binding domain in these fungi was as high as in white-rot fungi, and higher than in brown-rot fungi (Kohler et al. 2015). In orchid—fungal associations nutrients move to the orchid by tolypophagy or ptyophagy. In the former nutrients move from the fungal pelotons inside the cell to the orchid; groups of collapsed hyphae remain, or the hyphae lyse. In ptyophagy breakdown affects both hyphae and orchid tissue (see Rasmussen 2002; Rasmussen & Rasmussen 2014). Overall, Tulasnella (Cantharellales, a basidiomycete) is prominent in both terrestrial and epiphytic orchids (Oberwinkler et al. 2017), and Sebacina, Ceratobasidium and Atractiellomycetes (all basidiomycetes) somewhat less so (Kottke & Kovács 2013; see also Sieber & Grünig 2013 for fungal root endophytes). However, as more is found out about this association, it seems that the plant may synthesize some metabolites needed by the fungus (Z.-X. Xu et al. 2023), so to think of this association as being a form of parasitism by the orchid may oversimplify the issue. However, the young orchid does depend on the fungus for its survival.
2. Mixotrophy/Hemimycoheterotrophy. The fungi associated with the plant as it germinates may be quite different from those associated with the adult plant (e.g. Hashimoto et al. 2012; Rasmussen et al. 2015; Y.-I Lee et al. 2015; Bayman et al. 2016). A variety of fungi, among which "Rhizoctonia" is important, usually form the initial fungus-orchid association, later on, specificity may be higher (Roy et al. 2009; Hynson et al. 2013; Rasmussen & Rasmussen 2014); Z.-X. Xu et al. (2023: p. 2534) notes that "orchid mycorrhizal fungi are often highly specific". (The "rhizoctonia" that is often mentioned in the orchid literature, especially the older literature, is a common anamorph or form genus that encompasses a multitude of sins (Dearnalay et al. 2012), and it includes a number of basidiomycetes, e.g. Ceratobasidiaceae, Sebacinales-Serendipitaceae and Tulasnellaceae (Weiß et al. 2016), which all have a rhizoctonia stage - note that Rhizoctonia s. str. = Cantharellales-Ceratobasidiaceae (see below). However, for reasons that are not understood, Serendipitaceae (near-basal Agaricomycetes-Sebacinales), not ECM but saprophytic and endophytic, are important in seedling (see above) but not adult mycoheterotrophy, while Sebacinaceae, often ECM, may be found on adult but not seedling mycoheterotrophic orchids and their relatives (Weiß et al. 2016). Y.-I Lee et al. (2015), Rasmussen et al. (2015 and references), Bayman et al. (2016) and others discuss changes in partners (this also occurs in Ericaceae-Pyroloideae) and Whigham et al. (2008) for change, change = addition of partners, or no change at all. Interestingly, one effect of burning an orchid habitat may be a change in the fungi associated with the orchids; thus in Victoria, Australia, before the burning Tulasnella calospora was found in quadrats along with the orchid Pterostylis revoluta, but afterwards Ceratobasidium was a major fungus - and the orchids were not flowering (Jasinge et al. 2018). See Martos et al. (2012) for the literature on the phylogenetic signal of orchid and fungus. In the process of disentangling relationships of Rhizoctonia within Cantharellales, D. Gónzalez et al. (2016, also Selosse et al. 2021) recovered the relationships [[Botryobasidiaceae + Ceratobasidiaceae] [Tulasnellaceae + Other Cantherallales]]. For ascomycetes, e.g. Tuber (Pezizales) and orchids, e.g. Epipactis, see Selosse et al. (2004) and Dearnalay et al. (2012).
The relationship between fungus and orchid is certainly not one-to-one (e.g. Roy et al. 2009; Martos et al. 2012: identification method important; Leake & Cameron 2012; Jacquemyn et al. 2013); it can even seem pretty random, at least in the ECM pezizalean associates (Tuber: ascomycetes) of Epipactis studied by Tesitelova et al. (2012) or be quite specific, as in the association of individuals of Anoectochilus sandvicensis with a single OTO of Ceratobasidium (Zahn et al. 2023). Shefferson et al. (2005, 2010) found rather narrow host breadth in fungi associated with Cypripedium and Goodyera respectively, which they thought was common in Orchidaceae and might be considered to be phylogenetic conservatism (Shefferson et al. 2010), however, a close/narrow association does not convert to a unique association from the fungus's point of view - orchids can be specialists, and their fungal associates, generalists (and vice versa). Some orchids do have a wider suite of associates, indeed, there is variation in this within Cypripedium, which also has nice examples of orchid—fungal associations switching (Shefferson et al. 2007). Individual North American clades in the mycoheterotroph Corallorhiza striata complex (Epidendroideae-Epidendreae-Maxillariinae) are associated with different sets of the fungus Tomentella (Thelephoraceae: Barrett et al. 2010), and Bidartondo et al. (2004) discussed potential host specifity in several European orchids. Roche et al. (2010) studied the specificity of the Tulasnella-Chiloglottis association (see also Otero et al. 2011: Pterostylinae).
Overall, orchid fungal networks are not nested, that is, specialist fungi, forming associations with only a few species of orchids, are only rarely also found on generalist orchids growing in the same area - fungi in these latter also grow on other generalist orchids (in this orchid mycorrhizae are like ECM and ericoid mycorrhizal associations - Toju et al. 2016 and references). However, recent work on orchids growing on Réunion suggests that the nature of the mycorrhizal network in epiphytic and terrestrial orchids differed, with only 10 of the 95 fungal species (= taxonomic units) recorded in this study being found in members of both groups of orchids, fungal associations were nested in epiphytic orchids (most species of Angraecum) and specificity was higher there. Furthermore, most of the fungal species, whether restricted to one of the groups of orchids or occuring in both, were found on only one or two orchid species (Martos et al. 2012). Differences between the species of fungi found in the two habitats, or in features of the orchids that affect colonization by the fungi, may both explain this striking pattern (Leake & Cameron 2012). A final issue more or less alluded to in the preceding paragraphs is whether or not the association between orchid and fungus is constant, and what circumstances might cause it to change (Lespiaucq et al. 2021).
Orchids that have "normal" green leaves may nevertheless also obtain some of their nutrients from the fungal associates being discussed, and ultimately this may come from other plants - "fungal cheating" (e.g. Suetsugu & Matsubayashi 2021: p. 2032). (Note, however, that plant → fungus → plant nutrient transfer in other mycorrhizal associations has often yet to be conclusively demonstrated - Henriksson et al. 2023; Karst et al. 2023 - but on the other hand, this may occur in Paris type arbuscular mycorrhizae - Giesemann et al. 2019, 2021.) Indeed, even aside from the seedling stage, some kind of fungal association is also pervasive in adult orchids, hence orchid-type mycorrhizae are to be found in ca 9% of all flowering plants (Brundrett 2009; Brundrett & Tedersoo 2018); the part of the plant that hosts the fungus can vary (Ramsay et al. 1986). Tulasnella, mentioned above, is associated with a number of adult orchids, these are (largely) autotrophic (Summer et al. 2012; Ogura-Tsujita et al. 2012) and can fix their own C. But adult orchids may be decidedly mixotrophic/partly mycoheterotrophic (see Ogura-Tsujita et al. 2021), obtaining some C from their own photosynthetic activities and some from their fungal associate(s), and other nutrients like N may move, too, whether the fungus is parasitic on other plants, like Armillaria, saprotrophic or mycorrhizal (e.g. Rasmussen 2002: Table 1; Hynson et al. 2013). For example, Yagame et al. (2021) found that in Crematogaster variabilis (Epidendreae, = C. appendiculata var. variabilis) such mixotrophy was established only between Psathyrellaceae, the various rhizoctonias with which the plant was also associated not being involved. Suetsugu et al. (2020a) established that C from decaying plant material had moved to the orchid via saprotrophic fungi by finding 14C spikes from atomic bomb tests of the mid twentieth century in the orchid. Mixotrophy may be an adaptation to the low-light conditions of the forest floor where many orchids grow (Roy et al. 2009 and references), as with Paris type AM associations, another case where the C in the plant at least sometimes has two sources (Giesemann et al. 2019). Plants of two species of Cephalanthera acquired about half as much fungus-derived C under low-light conditions compared with holomycoheterotrophic species, but much less than that as light increased, indeed, they became almost completely autotrophic under high light conditions (Preiss et al. 2010) - species like C. subaphylla have much-reduced leaves and take up substantial amounts of C, while C. falcatay, with normal-sized leaves, has an albino form (Sakamoto et al. 2016). Indirect associations with trees via ECM orchid associates are well known (e.g. Bidartondo & Read 2008; see also Bidartondo et al. 2004; Selosse et al. 2021), and there can be bi- or unidirectional movement of C and nitrogen between chlorophyllous orchids and their fungi (e.g. Bidartondo et al. 2004; Cameron et al. 2008a, esp. 2008b; Hynson et al. 2009a, 2013). Recent work suggests that mixotrophy is very widespread (Selosse et al. 2017a for a review; Lallemand et al. 2018; Suestsugu & Okada 2025); it occurs in orchids associated with rhizoctonias (Gebauer et al. 2016), with up to 20% of the C needs of the plant coming from the fungus (Schweiger et al. 2018), even in orchids growing in high-light conditions in meadows (Schiebold et al. 2017a). Interestingly, photosynthesates produced by the mixotrophic orchids themselves may not move into the perennating underground parts (D. L. Taylor et al. 2002), rather, C, etc., in these latter comes from the associated fungus, which in turn for the most part does not supply the above-ground parts of the plant. However, Cameron et al. (2008a, b) found that C moved from Goodyera repens to its associated fungus Ceratobasidium cornigerum, which is not an ECM fungus, while in some orchids C in the young above-ground shoots in the spring may also come from the fungus (Gonneau et al. 2014; Lallemand et al. 2018); the relationship between plant and ECM fungus in the protocorm and in the underground parts of the adult plant are quite similar. All told, these compartmentalizations are unlike the situation in other mycorrhizae (but see some AM associations), and details of the movement of C and N between plant and fungus are complicated (see also Gebauer & Meyer 2003; Liebel et al. 2010). Shoots of a number of mycohetero-/mixotrophic orchids do not appear above ground every year, the phenomenon of vegetative dormancy (Shefferson 2003, 2018; Reintal et al. 2010), the record apparently being 18 year's dormancy in an individual of Epipactis helleborine. Small plants may continue to grow during this period, perhaps because of their fungal associations, although in larger plants there is generally a cost to the plant (Hurskainen et al. 2018).
The evolution of OrM associations may be via mixotrophy as the nature of the association between the fungus and the plant changes, and the fungi involved in such mixotrophic associations may initially have been saprobic - this is the Waiting Room hypothesis (e.g. Selosse et al. 2009, 2021). Details of fungus-orchid associations in Apostasioideae have been unclear, however, it has recently been found that C, and perhaps aLso N, moves from the fungal associate Ceratobasidium to Apostasia nipponica as an adult, i.e. the latter is a mixotroph (Suetsugu & Matsubayashi 2020), as in a number of other orchids. Apostasia wallichii is similar, and there Botryobasidium, close to Ceratobasidiaceae, has been found (Suetsugu et al. 2025a). Tulasnellaceae and Ceratobasidiaceae, ex-rhizoctonias, were found to be associated with Nieuwedia, Ceratobasidiaceae in particular belonging to hemi- or holomycoheterotrophic associations throughout Orchidaceae, and also belonging to ECM associations, and the suggestion that mixotrophy might be the ancestral condition for Orchidaceae as a whole (Suetsugu & Matsubayashi 2020; see also M.-H. Li et al. 2022) seems quite reasonable. Although Selosse et al. (2021) allowed that rhizoctonia mycorrhizae might have been ancestral in Orchidaceae, Serendipitaceae seemed not to occur in Apostasia and they thought that it was difficult to assess how many shifts to mixotrophy there might have been. Zahn et al. (2024) found that protocorms of N. malipoensis were associated with saprotrophic Ceratobasidiaceae, seedlings and adults of the orchid being associated with saprotrophic Tulasnellaceae, however, at these later stages at most little C or N seemed to be moving from the fungus to the orchid. On balance, however, it seems not unreasonable to think of Orchidaceae as being ancestrally mixotrophic (see also Suetsugu et al. 2025a).
Any connection between the specificity of mycorrhizal associations and the diversification of Orchidaceae in general (Otero & Flanagan 2006) or speciation in holomycoheterotrophic taxa in particular (Kinoshita et al. 2016) is unclear. Some specificity of the fungal associations has been noted (see also above), thus Shefferson et al. (2005) found quite high specificity between orchids like Cypripedium and their fungal associates (Tulasnella), although this was less in C. californicum (see also Rasmussen 2002). It has been suggested that differentiation of fungal communities on different species of orchids may contribute to niche partitioning (Waterman et al. 2011; McCormick & Jacquemyn 2013; Jacquemyn et al. 2013 and references). Thus Nurfadilah et al. (2013) found that fungi varied in their ability to utilize nutrients in phosphorus-poor West Australian soils, suggesting that this might help explain diiferences in how locally common species of orchids might be. However, Davis et al. (2015) thought that any specificity of fungus:orchid relationships was unlikely to explain the endemism of southern Australian orchids, rather, pollination by sexual deception and specific edaphic requirements might be a better explanation. But if the estimate by van der Heijden et al. (2015a) that around 25,000 species of fungi are associated with orchids is confirmed, this may open up all sorts of evolutionary possibilities if at the same time futher questioning ideas of specificity (for more on the specificity of mycorrhizal associations, etc, see also Selosse et al. 2010 and below).
Finally, to return to an issue mentioned briefly above, there are two kinds of presumed autotrophic AM associations, the Arum and Paris types, the former representing ca 60% of AM associations, the latter the remainder (Giesemann et al. 2019; c.f. Dickson 2004). Until recently it seemed that any distinction between the two was not that important. Glomeromycote hyphae are aseptate, and the Arum type has densely-branched intracellular arbuscules and sparsely-branched hyphae running between the cells, while the Paris type has intracellular hyphal coils and at most few intercellular hyphae (e.g. Gallaud 1905; F. A. Smith & Smith 1997; Torti et al. 1997; Peterson & Massicotte 2004; Imhof 2007; Imhof et al. 2013; Giesemann et al. 2019), and there are vesicles, etc.. The morphological distinction between these two kinds of mycelia depends in part, at least, on whether or not there are intracellular spaces in the tissues of the host plant; there are intermediates between these types (Brundrett 2004). However, Giesemann et al. (2019, 2021) have recently suggested that Arum and Paris mycorrhizae/fungal associates may play quite different roles in facilitating the movement of C through the community. It seems that Paris-type mycorrhizal associations alone are involved in both holomycoheterotrophic and mixotrophic associations, and there is some level of mixotrophy between apparently "normal" heterotrophic adult plant hosts and fungus, and at least sometimes the seedling - that is, nutrients move from one plant to another. In both Orchidaceae, and in in Gentianaceae in particular, there can be a close association between glomeromycote and seedling, and also various degrees of mixotrophy/mycoheterotrophy in the adult - see Gentianaceae Ecology & Physiology and elsewhere for such associations - see also Tominaga et al. (2021, 2023), who suggest that there is different fungus-plant signalling in these two AM types.
Giesemann et al. (2019) found that almost 50% of the C in the leaves of Paris quadrifolia (Paris-type AM) came from its associated fungus, while in Arum maculatum (Arum type) all its C came from its own photosynthetic activities. The fungal associates of these two plants represent the two main AM morphotypes, so a question is, do these morphotypes consistently behave differently in their C metabolism? Is P. quadrifolia a part-mycoheterotroph that habitually obtains some of its C from other plants by way of the fungus asssociated with it? Do photosynthesates often move from one plant to another by way of links provided by Paris-type AM fungi, particularly in low light conditions (Giesemann et al. 2019)? Giesemann et al. (2019) found that there was some movement of C into Anemone nemorosa, also with Paris-type AM, but not into other taxa with Arum-type AM; in a broader study, Giesemann et al. (2020) found some movement of C in about half the species with Paris-type AM that they examined, but not in plants with Arum-type mycorrhizae. Note that the association between plant and glomeromycote AM fungus is very often obligate whether the plant is a holomycoheterotroph or a (part-)autotroph (Kohler & Martin 2017).
To conclude: returning to orchid-fungal associations in particular, there is certainly no sharp distinction between holomycoheterotrophy, mixotrophy and autotrophy, as least based on current evidence. As Zahn et al. (2023: p. 1458) noted, in species like Epipactis palustris that are associated with rhizoctonia-type fungi there might be "a cryptic manifestation of partial mycoheterotrophy", but in species like E. atrorubens, associated with an ectomycorrhizal fungus, partial mycoheterotrophy/mixotrophy was more obvious.
3. Holomycoheterotrophy in Adult Plants in General. And now on to holomycoheterotrophy in adult orchids, and in this context thinking about the fungal associates of holomycoheterotrophic land plants in general, especially those of angiosperms (for which, see e.g. Imhof et al. 2013) is interesting. Holomycoheterotrophy is not easy to study, not least because holomycoheterotrophic species are quite often very uncommon. Thus Thismia americana (Thismiaceae) was found near Chicago over 100 years ago, but never since, while Epipogium aphyllum (Orchidaceae-Epidendroideae), was declared extinct in Great Britain in 2009 - but was found there again in 2010. Holomycoheterotrophic plants, whatever the details of the association involved - they are an ecological grouping, like water plants, marine angiosperms, parasites, mistletoes, carnivorous plants, and their like - are in general often small, inconspicuous and highly modified morphologically, and working out details of their anatomy, pollination and seed dispersal, let alone physiology, is not at all easy. Below I discuss first, the association between holomycoheterotrophs and glomeromycote fungi, then the association between orchidaceacous holomycoheterotrophs and basidiomycete ectomycorrhizal (ECM) fungi (there are parallels between these two groups that were not evident a few years ago), holomycoheterotrophic Ericaceae also being associated with basidiomycetes, and finally the association between some orchids and saprotrophic fungi.
In holomycoheterotrophs other than Orchidaceae (and Ericaceae) the associated fungi are commonly all Glomus group A (for fungi, see e.g. Merckx et al. 2012; Renny et al. 2017; Gomes et al. 2022). Although there is quite a diversity of fungal form, very often one can see fungal coils inside the host plant cells, as with Paris type AM, and in their survey Imhof et al. (2013) noted specifically that there was no obligate mycoheterotroph with Arum-type AM.
Perez-Lamarque et al. (2020) looked at general interactions between holomycoheterotrophic plants ("mycoheterotrophic cheater plants") and fungi and suggested that some of these plants interacted with globally specialized fungi; Glomus in other mycoheterotrophic vascular plants like Gentianaceae, Corsiaceae and Psilotaceae, were similar, but unlike the fungi on their immediate relatives, autotrophs (Winther & Friedman 2008; Perez-Lamarque et oal. 2020). On the other hand, fungi associated with Burmanniaceae, Polygalaceae and Triuridaceae, and also Lycopodiaceae and Ophioglossaceae, showed the reverse set of relationships. However, Gomes et al. (2022) examined a more local situation in some detail, the relationships between some holomycoheterotrophs belonging to Triuridaceae, Gentianaceae and Burmanniaceae, their associated fungi, and also the AM fungal associates of the trees, in a plot in the forests of French Guiana. They found that the mycoheterotrophs were mostly associated with Glomeraceae, especially members of the Rhizophagus irregularis group, also Acaulosporaceae and Gigasporaceae. The holomycoheterotrophic taxa targeted fungi that were widely associated with the local autotrophic plants, different mycoheterotrophs targeting different fungi; overall, the C acquired nefariously by the mycoheterotrophs came from many different autotroph species, i.e., their carbon source was assured. For additional information, see Johow (1889), Hynson et al. (2013), papers in Merckx et al. (2013a), and Henriksson et al. (2023), and for more on particular glomeralean-associated mycoheterotrophs, see Burmanniaceae, Corsiaceae, Gentianaceae, Petrosaviaceae, Polygalaceae, Thismiaceae and Triuridaceae.
About 880 species of Orchidaceae are full/obligate mycoheterotrophs, i.e. holomycoheterotrophs, of which over 120 are in Epidendroideae-Gastrodieae alone. There have been around 25, perhaps over 30, even over 40, separate transitions to mycoheterotrophy in the family, about 3/4 of all such transitions, and probably half or more of these are in Epidendroideae (Merckx & Freudenstein 2010; Merckx et al. 2013b; Ogura-Tsujita et al. 2021; M.-H. Li et al. 2022; etc.) - overall there must be more than one thousand species of holomycoheterotrophs. Note that Ogura-Tsujita et al. (2021) include taxa which have chlorophyll in their reproductive shoots in their class of full mycoheterotrophs - but however one cuts it, there is a continuum of variation. Simard et al. (2012) also offer a reminder that there is a continuum between autotrophs and echlorophyllous holomycoheterotrophs (see also Schiebold et al. 2017a), and so between autotrophs, hemiparasites, and holoparasites. In Orchidaceae, holomycoheterotrophs are commonest in ground-dwelling Epidendroideae, which include about 1 in 10 such species (Freudenstein & Barrett 2010; Merckx et al. 2013b). Holomycoheterotrophic orchids lack chlorophyll (they usually grow in shady conditions) and they are totally dependent on the fungus for all carbon (and nitrogen) (e.g. Dearnalay et al. 2012; Hynson et al. 2013). As might be expected, the adoption of holomycoheterotrophy seems to be irreversible (Hynson et al. 2013), however, holomycoheterotrophic and leafy taxa of Cymbidium can hybridize (C. macrorhizon x C. ensifolium: Ogura-Tsujita et al. 2014), and mixotrophy may reverse to autotrophy (Lallemand et al. 2019b). Timilsena et al. (2022b) looked at the holomycoheterotroph Corallorhiza striata and found that as with other holomycoheterotrophs (they examined taxa in Pandanales and Dioscoreales, and also included discussion on the orchid holomycoheterotroph Gastrodia elata - see also Yuan et al. 2018) there had been extensive loss of genes (see also Y. Wen et al. 2022, etc.). In none of the holomycoheterorophs were they able to detect 174/1375 Benchmark Universally Conserved Orthologous (BUSCO) nuclear genes (the total numbers of undetected BUSCO genes in individual species ranged from 322-393); these are the Missing in Mycoheterotrophs (MIM) genes that are involved in photosynthetic or plastid membrane protein production among other things (Timilsena 2022b; Yuan et al. 2018: Fig. 1d). The plastomes themselves were also much reduced, for instance, Yuan et al. (2018) could find only 19 protein-coding genes in G. elata, and one inverted repeat was missing. The evolution of the plastome in mycoheterotrophic Orchidaceae is discussed further below. On the other hand, the chondrome in G. elata is very large (Yuan et al. 2018), but I do not know how common this is in holomycoheterotrophs. Eriksson and Kainulainen (2011) and Merckx et al. (2013c) discuss the ecological drivers of the evolution of holomycoheterotrophy. See also Ericaceae for holomycoheterotrophic plants that live in association with ECM fungi.
The evolution of various photosynthetic life styles has been much studied in Epidendroideae, as in Neottieae (e.g. T. Zhou & Jin 2018; Feng et al. 2016; in particular Lallemand et al. 2019b), a tribe where there are all variants between apparent autotrophy and holomycoheterotrophy (the latter has evolved at least three times here; see also Ogura-Tsujita et al. 2021: Table 2). There seems to have been rapid evolution at the base of this clade which makes understanding the evolutionary story difficult, but there may not be a simple progression, autotrophy → mixotrophy → holomycoheterotrophy (Zhou & Jin 2018; Lallemand et al. 2019b). Indeed, Lallemand et al. (2019b) found that photosynthesis genes might be under positive selection in mixotrophic species, and they suggested that such genes might still be important in fruit development there; Kobayashi et al. (2021) noted that photosynthesis occured in the capsule of the apparently holomycoheterotrophic Cymbidium macrorhizon (Cymbidieae), although its importance for the plant was unclear (see also Suetsugu et al. 2018). There are also associations with ECM fungi in mixotrophic/holomycoheterotrophic Neottieae, autotrophic taxa being associated with saprotrophic and endophytic fungi (Roy et al. 2009; Selosse et al. 2010; Dearnaley et al. 2012; Lallemand et al. 2019b). Members of the Hexalectris spicata complex (Arethuseae) are each associated with different members of the ECM Sebacinales-Sebacinaceae (Kennedy et al. 2011; Weiß et al. 2016), and Barrett et al. (2019b) thought that there had been four or five independent losses of photosynthesis in this genus alone, interestingly, Hexalectris has an epiphytic ancestry (Sosa et al. 2016). Several species of Russula form both ECM associations with adjacent trees and endomycorrhizal associations with Corallorhiza (Epidendreae-Calypsoineae) (Taylor & Bruns 1999; Freudenstein et al. 2017; see also Z.-H. Li et al. 2020), and in such mycorrhizal networks it is the tree that is the ultimate source of the orchid's carbon (see also Dearnaley 2007). Selosse et al. (2021: Table 1) emphasized the association of ectomycorrhizal fungi with orchids, ranging from major associates in holomycoheterotrophs to uncommon associates along with rhizoctonia-type fungi in mixotrophs. Corallorhiza includes both leafless species that photosynthesize and those that cannot. Interestingly, in roots of albino individuals of Epipactis helleborine (Neottieae) there was some bidirectional flow of carbon between fungus (the ectendomycorrhizal ascomycete Wilcoxina) and plant; roots of green individuals were associated with similar fungi and overall differed little (Suetsugu et al. 2017; see also Kinoshita et al. 2016: Gastrodia-Gastrodieae). Lallemand et al. (2019a) looked at a comparable situation in the leaves of three species (including E. helleborine) and found that in albino leaves, too, there were no major changes in the transcriptome, but most amino acids greatly accumulated, although branched-chain amino acids and lysine in particular less so, being used as energy sources, there was a permanent starvation response and related autophagy. In Cymbidium the evolution of mixotrophy and then mycoheterotrophy depends on the establishment of associations between orchid and ECM fungus (Ogura-Tsujita et al. 2012). Julou et al. (2005) compared albino and green individuals of Cephalanthera damasonium (Neottieae) and found little difference in fertility between the two, and noted that a number of changes in the albinos were still needed for there to be full mycoheterotrophy. However, in Goodyera velutina (Orchidoideae-Cranichideae), associated with both ECM fungi and cantharellalean rhizoctonia, green individuals were autotrophic and white individuals obtained their C from their cantharellalean, not ECM, associates (Suetsugu et al. 2019). M.-H. Li et al. (2022) looked at the evolution of holomycoheterotrophy in Platanthera (Orchideae) and found that the holomycoheterotroph P. guangdongensis expressed trehalase, which converted the fungal trehalose to glucose (as noted, trehalase is also expressed at the seedling stage), so ensuring the carbohydrate supply for the plant. Li et al. (2022) noted that plants of P. guangdongensis (and Gastrodia elata at least) lacked roots. The identity of the fungal associate may change with the establishment of holomycoheterotrophy (Hynson & Bruns 2010).
Ogura-Tsujita et al. (2009, 2021: Fig. 2, Table 1) drew attention to a small group of orchid holomycoheterotrophs that are associated with saprotrophic fungi, mostly Agaricales, some involved in litter decay and some in wood decay and whence the nutrients for the orchids ultimately come (see also Yukawa et al. 2009; Y.-I Lee et al. 2015; Bayman et al. 2016; Lebreton et al. 2021). Such saprotrophic associations with holomycoheterotrophs are known only in Orchidaceae, and this life style may have evolved nine times or so; some holomycoheterotrophs are associated with a variety of fungi that have different life styles. The specificity of the saprotrophic fungi is variable (Ogura-Tsujita et al. 2021). There seems to be a connection between the adoption of the holomycoheterotrophic habit and the movement of epiphytic orchids (Epidendroideae) to the ground (Martos et al. 2012).
Yagame et al. (2016) suggested that in Neottia there has been an evolutionary shift from associations with saprophytic/endophytic Sebacinales-Serendipitaceae (in the autotrophic taxa) to associations with Sebacinaceae (in the echlorophyllous holomycoheterotrophic taxa); this shift would be consistent with the Waiting Room hypothesis (e.g. Selosse et al. 2009, 2021). In tropical holomycoheterotrophs associations with saprotrophic wood-decay fungi are common (Roy et al. 2009; Garbaye 2013; Hynson 2013; Bayman et al. 2016). Thus non-mycorrhizal but lignin-decaying fungi like the basidiomycete Mycena (Mycenaceae) are members of such associations, Mycena, for example, supports the holomycoheterotrophic Gastrodia confusa (Epidendroideae) in the manner to which it has become accustomed, at least initially, and in G. elata IAA from Mycena is essential for sugar nutrition of the germinating seed (Ogura-Tsujita et al. 2009 and references; Q.-S. Yuan et al. 2025). Note that saprotrophic fungi other than rhizoctonia are commonly associated with mycoheterotrophic orchids on Taiwan (Selosse et al. 2010; Y.-I Lee et al. 2015: ECM fungi only sometimes involved), and the associates of Galeola [= Erythrorchis] altissima, at up to 10 m or so tall the largest mycoheterotroph known, are largely wood-decaying basidiomycetes (Ogura-Tsujita et al. 2018). Umata et al. (2022 and references) noted that in Gastrodia there was a switch from Mycena, an associate up to the protocorm stage, to the parasite/facultative saprophyte Armillaria, while they found that in Cyrtosia septentrionalis (Vanilloideae) the polypore Phyllosporinus was more likely to be an associate at early stages, Armillaria later on. However, in Cyrtosia details of how the orchid obtained its nutrients were unclear given that Armillaria seemed to be associated with zones of active growth on the main lateral roots, up to 5 m long, or on some of their branches, but they were rather sparse (Umata et al. 2022). D. Wang et al. (2021 and references) noted that there had been some 17 shifts to holomycoheterotrophy in Orchidaceae and these within the last 35 Ma or so, shifts to saprotrophic or ECM fungi being prerequisites - and evidence suggests that there have been only around 40 such shifts in land plants as a whole. See also Selosse et al. (2010), Simard et al. (2012 and references) and Ogura-Tsujita et al. (2021) for fungal specificity.
The three species of the Australian Rhizanthella (Orchidoideae-Diuridae) are subterranean holomycoheterotrophs, practically the only plants known to have this life style (there are one or two other plants with subterranean flowers, or the ovary/fruit, but not the whole plant), and their flowers even open underground (Delannoy et al. 2011); the plants are described as having pseudanthia (Baczynski & Claßen-Bockhoff 2023). The orchids form an association with ECM fungi (Ceratobasidiaceae) also associated with Melaleuca uncinata and M. scalena, and they receive their nutrients indirectly, either from Melaleuca via the fungus or from the soil, also via the fungus (Bougoure et al. 2010; Rasmussen et al. 2015 and references). The fungus has high concentrations of N-rich proteins and chitin, and Rhizanthella acquires N after the lysis of the fungal pelotons; overall, N concentrations in the orchid are high, despite its having no chlorophyll (Bougoure et al. 2010). However, the N metabolism of holomycoheterotrophic and mixotrophic orchids is poorly understood, although both groups of orchids are noted for having very high N concentrations in their tissues, and C and N uptake may be linked if amino acids move from the fungus to the orchid, with some, like lysine, providing a source of C (Hynson et al. 2013, 2016 for literature; Zimmer et al. 2007; Kuga et al. 2014; Focha et al. 2016; Lallemand et al. 2019a). Schiebold et al. (2017b) looked at 15N enrichment in species of Epipactis and found that it depended on the fungus, the orchid and the environment, varying from 3.2±0.8%-24.6±1.6% in the species studied, the higher values being when the associated fungus was Glomus, of the independent fungal group Glomeromycota.
As mentioned, holomycoheterotropic orchids - and other such plants - are terrestrial, even in Epidendroideae in which the epiphytic habit is so common, and they tend to live in low-light conditions. Gastrodia (E.-Gastrodieae), with around 100 species, is one of the most speciose mycoheterotrophic genera, and seed is set here via self-pollination, pollination by drosophilids through brood-site mimicry or mutualism, or pollination by small bees possibly attracted by pseudopollen; some kind of selfing is quite common in such conditions (Suetsugu & Yamamoto 2024). Thus in G. elata there is both agamospermy and pollination during the course of collection of starch-rich pseudopollen from the base of the callus on the labellum by Lasioglossum bees (Suetsugu & Yamamoto 2024). The fruits of Rhizanthella are fleshy, as is quite frequent in other holomycoheterotrophs, and the seeds are crustose (Cameron & van den Berg 2017), in one case at least being dispersed by camel crickets (Rhaphidophoridae-Tachycines elegantissima) as in the unrelated holomycoheterotroph Yoania (Epidendroideae-Epidendreae: Suetsugu 2017, see also 2020b). Germination may take almost a year or so (Bougoure et al. 2010), and of course there may be a lengthy period between pollination and fertilization (see below).
There are around 20,700 species of epiphytic Orchidaceae, around 3/4 of all angiosperm epiphytes (Zotz et al. 2021b: checklist, hemiepiphytes included; see also Benzing 1983; Zotz 2013); overall almost 70% of orchids are epiphytic (Silvera et al. 2009, 2010a, b), genera like the epiphyte-dominated Bulbophyllum having over 2,100 species. Epiphytic orchids make up the largest group of epiphytes (e.g. Kress 1989; Holtum et al. 2007). Svahnström et al. (2025) discuss the commoness of epiphytic relative to non-epiphytic taxa here, indeed, epiphytes had fewer specimens and smaller extent of occupancy (range, number of specimens/species) than did terrestrial species. In the Neotropics, Carmona-Higuita et al. (2025) found that some 83% of all "endemic" epiphytic species there belonged to Orchidaceae, and most of them were to be found in the Paramo (22.5%), Cauca (12.9%), Guatuso-Talamanca (8.4%) and Yungas (7.4%) biogeographic provinces; Stelis, Lepanthes, Epidendrum, Masdevallia, Pleurothallis, Telipogon and Acianthera together included 827 such endemic species, and only Isochilus linearis was found almost throughout the Neotropics.
Core Epidendroideae are commonly epiphytic plants (they include ca 67.6% of all vascular epiphytes), CAM photosynthesis is also quite common there (Holtum 2023: Fig. 3; Collobert et al. 2023) and overall the group is very speciose, including around 19,560 species, both epiphytic and terrestrial (figures from Pridgeon et al. 2005, 2009, 2014), i.e., about two thirds of the whole family. Although Neottieae and some other basal Epidendroideae are not epiphytes (e.g. Freudenstein & Chase 2015), in the hyperdiverse northern Andes Epidendroideae may comprise 30-50% of the epiphytic species (references in Pérez-Escobar et al. 2017a). Epiphytic species of Cymbidium have a higher leaf mass per unit area, thicker leaves that take longer to dry and a thicker epidermis than do terrestrial species, and venation and stomatal density were also linked in the epiphytic species, however, the floral traits of the two groups were similar when it came to thinking about water loss (F.-P. Zhang et al. 2025; see also T.-Y. Gao et al. 2025). Mycorrhizal associations, very small seeds facilitating dispersal, etc., may be other elements in the success of epiphytes in Epidendroideae (Hietz et al. 2021; hemiepiphytes excluded), however, note that root traits, seed mass, dormancy, etc., were largely absent in these studies, although there were some such traits included in the study of Panamanian epiphytes by K. Wagner et al. (2021). (The other main groups of vascular epiphytes are bromeliads and ferns; compared with them orchids have smaller and more numerous stomata, and although genome size in epiphytic orchids is smaller than in other Orchidaceae, it is larger than that in epiphytic bromeliads, while ferns further differ in having thinner fronds, etc.. See also Benzing (1990) and Zotz et al. (2021a, 2023) for more on the ecophysiological features of epiphytes in general - they are on the slow end of the Leaf Economic Spectrum.)
Ke, Y. [et al. 2024], Zhang, Y. B., Zhang, F. P., Yang, D., Wang, Q., Peng, X. R., Huang, X. Y., Sher, J., & Zhang, J. L. 2024. Monocots and eudicots have more conservative flower water use strategies than basal angiosperms. Plant Biol. (Stuttgart) 26: 621-632. https://doi.org/10.1111/plb.13637
Chomicki et al. (2014c) estimated that the epiphytic habit had been acquired four to seven times in Orchidaceae and subsequently been lost rather more often, although details of the evolution of this trait were unclear. Zhou et al. (2023) discuss the evolution of epiphytism in detail, and they estimate at least 14 origins in the family as a whole, with reversions to the terrestrial habit, reaquisitions of the epiphytic habit, also adoption (and loss) of the epilithic habit (some 46 times, most in epiphytic Epidendroideae, but some in terrestrial Cypripedioideae, etc.). Thus there have been some reversals from epiphytism to the terrestrial habit including in Bletia and its relatives (Epidendreae), one of which, Hexalectris, is mycoheterotrophic and grows in quite dry conditions in North America (Sosa et al. 2016), and there have also been reversals to the terrestrial habit in Malaxideae (Cameron 2005), Catasetinae (Martins et al. 2017/2018), etc.; see also Collobert et al. (2023) for reversals. However, other apomorphies, maybe deeper in Orchidaceae, while having no obvious immediate effect on diversification, may have combined to affect diversification at these upper levels (Givnish et al. 2015: see also below, pseudobulbs). A CAM-associated radiation in Epidendroideae may have happened some 65 Ma and be linked with the decline of atmospheric CO2 then (Silvera et al. 2009). Some evidence suggests that epiphytic orchids tend to have narrower ranges than their terrestrial relatives (Martins et al. 2017/2018), although the reverse is true in some other groups (Reimuth & Zotz 2020). Collobert et al. (2023) noted that both the evolution of the CAM habit and of succulence - initially stem succulence of various sorts, latterly leaf succulence - is evident in drought-adapted terrestrial lineages, i.e. before the adoption of the epiphytic habit (note that differences in the topology of the tree there and that above do not affect their conclusions). For possible connections between the epiphytic habit and the success of the family, see below, and for other major epiphytic groups, see Araceae, Bromeliaceae, Gesneriaceae, Melastomataceae, Ericaceae, ferns and Piperaceae.
Relatively rather little seems to be known about the ecology of epiphytic orchids, for instance, problems that they may face as their host tree grows larger, so changing the conditions of their existence, or more generally, the nature of the interactions, if any, between host/phorophyte and orchid (Rasmussen & Rasmussen 2018: literature on apparently quite specific associations between orchid and host). Nyffeler and Eggli (2010b) estimated that some 50+ genera and 2,200 species of orchids, or perhaps double that number, especially epiphytic species, were succulent (see also S. Zhang et al. 2018 and Eggli 2020a for general accounts). They have often fleshy leaves that may be terete (Balachandar et al. 2019 - Luisia) or bifacial, and quite often a fleshy stem, whether corm or pseudobulb, although this feature is quite often absent (Freudenstein & Chase 2015). Pseudobulbs are common, and may be heteroblastic, consisting of a single swollen internode and 1-2 terminal leaves, e.g., Oncidium, Bulbophyllum (around >2,500 species in these two genera alone), or homoblastic, with two or more swollen internodes and leaves along the length of the pseudobulb, e.g. Dendrobium, Grammatophyllum, etc., although stems may be rather little swollen, as in the latter (e.g. Ng & Hew 2000). Thawara et al. (2024) look at pseudobulb and leaf number evolution (i.a.) in Asian species of Bulbophyllum; the plediomorphic condition in that group was for there to be distinct pseudobulbs with but a single leaf (note, they used their maximum likelihood tree which has a rather different topology from their Bayesian inference tree). Epiphytic orchids, like other epiphytic plants, have to deal with periodic drought and lack of nutrients (Gravendeel et al. 2004, see also Motomura et al. 2008: epilithic orchids similar), as in the dry terrestrial habitats where succulence is also common (see also Figueroa et al. 2008). Terrestrial orchids have thinner leaves, and drought tolerance and leaf water storage capacity increases (S.-B. Zhang et al. 2014); see also Collobert et al. (2023) for succulence and the epiphytic/terrestrial habits.
As mentioned, associated with the drier conditions of the epiphytic habitat and the succulent habit of many orchid epiphytes is the evolution of crassulacean acid metabolism, CAM photosynthesis, which is uncommon in terrestrial orchids. A very approximate estimate is that ca 9,700 species of Epidendroideae have CAM photosynthesis (see Winter & Smith 1996b; also Eggli 1991a), and variants of CAM photosynthesis such as CAM-cycling are also common (see Silvera et al. 2008 for Oncidiinae; Winter et al. 2015). However, the actual number of taxa involved is unclear (Zotz et al. 2023), there seems to be some confusion over CAM and the epiphytic habit in Cymbidium (c.f. Motomura et al. 2008; Zhang et al. 2014), and the importance of weak CAM is hard to judge (Zotz et al. 2023). In a survey of 1,002 Costa Rican orchids, only some 10% of Vanilloideae and Epidendroideae were found to show signs of strong CAM, perhaps 30% more had weak CAM; CAM was not found in Orchidoideae and Cypripedioideae (Silvera et al. 2010a). Similarly, in a survey of 1079 orchids - mostly Epidendroideae, mostly Colombian, and three quarters epiphytic - only 8.9% (9.4% of the epiphytic species) had the carbon isotope signature of CAM plants (Torres-Morales et al. 2020). Such figures might suggest that overall there are somewhat fewer than 2,500 CAM orchids, but Silvera et al. (2010b) estimate that there are some 7,800 species of orchids involved, one quarter of which have strong CAM. Givnish et al. (2015) thought that CAM might have evolved four times or so in the family, C. E. Martin et al. (2010) suggested that CAM has evolved perhaps ten times, but also with reversals to C3 photosynthesis, and there were also intermediates (see also Motomura et al. 2008; Silvera et al. 2010b), however, A.-Q. Hu et al. (2022) thought that reversals of CAM to C3 photosynthesis were very uncommon. M.-H. Li et al. (2019: support along the spine tends to be weak) proposed some eight origins of CAM in Dendrobium alone. In this last study, the first origin of CAM was dated to (28.9-)21.9(-16.0) Ma in the Asian clade, and origins there were in general older than those in the Australasian clade (Li et al. 2019). For a general survey of the evolution and distribution of CAM photosynthesis in orchids, see Holtum (2023).
Although Silvera et al. (2009) found CAM radiations in Cymbidieae-Oncidiinae and Epidendreae-Laeliinae in particular, there is a question to what extent CAM is associated with increased diversification and/or can be considered a key innovation. Thus A.-Q. Hu et al. (2022) noted that the extinction rate of species in the Cirrhopetalum group of Bulbophyllum with strong CAM was ten times that of C3 species (see also Bromeliaceae), and they thought that CAM could not be considered a key innovation. Gamisch et al. (2021) looked at Malagasy species of Bulbophyllum, and there CAM developed as the genus populated the wetter lowlands, although it also moved in to the seasonally dry Sambirano forest. The evolution of CAM did indeed result in a widened spatio-ecological amplitude for the genus on the island, but there was no increase in diversification (Gamisch et al. 2021).
Cauline pseudobulbs, common in epiphytic orchids, may carry out CAM even although they lack stomata. CAM occurs in Bulbophyllum minutissimum, however, since the pseudobulb of Bulbophyllum is a modified leaf base, even if lacks a blade, one presumes it has stomata (Kerbauy et al. 2012 and references). The photosynthetic pathway in root and pseudobulb and in the leaf may be different, the former being C3 while the latter is CAM (C. E. Martin et al. 2010), or vice versa (Rodrigues et al. 2013). Adoption of CAM is predominantly by epiphytic Epidendroideae growing at low altitudes and drier conditions (Silvera et al. 2009; Kerbauy et al. 2012; M.-H. Li et al. 2019: cooling perhaps also involved) and has been linked to the Caenozoic radiation of that subfamily (Silvera et al. 2009). However, CAM photosynthesis has evolved perhaps four times (and reversed once) in terrestrial Eulophiinae (Epidendroideae-Cymbidieae) alone (Bone et al. 2015b). In the Madagascan Eulophiinae adoption of CAM may have allowed the orchids to move into drier habitats (Bone et al. 2015b). Species of Madagascan Angraecum (Vandeae) growing in humid conditions/more substrate have δ13C values suggesting that C3 photosynthesis is going on in the plants, while in those growing in drier conditions/less substrate the values suggest C4 photosynthesis (Kluge et al. 1998). Gene duplication has been implicated in the functional diversification of genes like phosphoenolpyruvate carboxylase (PeP C) that are involved in CAM photosynthesis (Silvera et al. 2014), PeP C in particular is involved in the dark fixation of CO2, and Silvera et al. (2010b: Fig. 5) suggests how these genes might evolve in Orchidaceae, although Deng et al. (2015) thought that the number of duplicate genes may be irrelevant as regards photosynthesis type. See also Bräutigam et al. (2017) and Hermida-Carrero et al. (2020: molecular evolution of RuBisCO) for the evolution of CAM photosynthesis, Males and Griffiths (2017) for the stomatal biology of CAM plants, and Crassulaceae for a major terrestrial CAM family and the evolution of CAM.
Epiphytic orchids often have very thick roots with a very well developed outermost velamen and exodermis (= a sort of modified hypodermis, a hypodermis with a casparian band, apparently widespread in angiosperm roots - see Peterson & Perumalla 1990) with tilosomes on top of the living passage cells; there are sometimes pneumathodes, and any root hairs present are primarily for anchoring the plant to the twig (e.g. von Guttenberg 1968; Pridgeon 1987: comprehensive; Siegel 2015: a readable account; Collobert et al. 2023 for velamen thickness and plant habit - connection not strong); there may be chloroplasts in the cortical cells (e.g. Benzing 1996). Zotz and Winkler (2013) found that in a number of epiphytic epidendroid orchids both water and nutrient uptake into the velamen was very quick; the velamen dried out slowly, and nutrients moved into the root cortex via the mitochondrion-rich passage cells of the exodermis. However, there was a fair bit of variation in nutrient uptake between the species examined, and overall the function/behaviour of the velamen is not all that well understood. Along with the velamen and exodermis of epiphytic orchids, many also have distinctive often branched phi (φ) thickenings on their anticlinal walls; lignified, they are best developed on the cortical cells immediately adjacent to the endodermis, but they may be found more or less throughout the cortex. They also also found in epilithic orchids, but are at best uncommon in terrestrial species; their function is not well understood, but they may be involved in tissue support and/or response to abiotic stresses (Idris & Collings 2019; Collings et al. 2020). There is normal CAM in the photosynthetic aerial roots (no stomata) of the leafless Campylocentrum tyrridion (Vandeae) (Winter et al. 1985).
Another general problem faced by epiphytic plants is damage to tissues caused by UV-B radiation. Twig epiphytes in particular, here concentrated in a clade of the New World Epidendroideae-Cymbidieae-Oncidiinae, face such problems. They grow on twigs less than 2.5 cm in diameter and in very exposed and high light conditions. Recent work suggests that chalcone synthase genes can be induced by UV-B light in the root tips of epiphytic orchids, and as a result UV-B-absorbing flavanoids are synthesised (Chomicki et al. 2014c: for sunlight and epiphytism, see also Bromeliaceae). A leafless orchid was included in this study and it showed behaviour similar to that of the two leafy epiphytic taxa examined.
Other than succulence and associated CAM photosynthesis and the ability of a few epiphytic orchids to capture humus, whether in "trash basket roots" formed by negatively geotropic roots (e.g. Cyrtopodium saintlegerianum (dos Santos et al. 2023), Grammatophyllum) or by leaves (Zona & Christenhusz 2015), orchids would seem to have few obvious adaptations to growing in an environment where water is often at a premium. A recent study examining phorophyte (host tree) preferences of orchids in southeast Mexico found that orchids did not prefer trees with fissured bark, where water would seem to be able to remain in the fissures. Rather, the amount of water persisting after the excess drained away was linked to the density of porose structures in the bark, and these trees were preferred (Zarate-García et al. 2020).
Mycorrhizal associations are usually less common in epiphytes than in other vascular plants (e.g. Janos 1993; Desirò et al. 2013; Mellado‐Mansilla et al. 2022). However, the basidiomycetes Sebacinales-Serendipitaceae, Tulasnellales (= Cantharellales), Ceratobasidiomycetaceae and Atractiellomycetes are ECM associates of epiphytic orchids (e.g. Favre-Godel et al. 2020). Species of Tulasnellaceae and Ceratobasidiaceae, at least, colonize more than one species of orchid (Kottke et al. 2008; see also Martos et al. 2012; Gowland et al. 2013; Balachandar et al. 2019; Rammitsu et al. 2020, 2023). Although Rammitsu et al. (2020) found 24 fungal OTUs to be associated with the epiphytic Thrixspermum japonicum in a broad survey across 20 sites in Japan spanning well over 1,000 km, the one species of Ceratobasidium predominated at 12 of those sites, being about half of all samples collected - indeed, it is probably globally distributed; all told, Ceratobasidium spp. were almost 60% of the samples, Tulasnellaceae were another 17%. In montane South America mycorrhizal Serendipitaceae are in clades close to but separate from clades that form distinctive modified ericoid mycorrhizae in Ericaceae growing in the same habitats (Setaro et al. 2013). It has been suggested that a reason for the success of orchids as epiphytes is their ability to form associations with free‐living saprotrophic fungi (Yukawa et al. 2009). On Réunion relationships between epiphytic fungi (including Ceratobasidiaceae) and their host epiphytic orchids (34 spp., angraecoids, most Angraecum itself) were found to be nested, but not those in terrestrial orchids (25 genera); there were different fungi in different habitats, and low specificity in the terrestrial orchid-fungal associations (Martos et al. 2012). Basidiomycete fungi are to be found in the photosynthetic roots of the leafless epiphyte Dendrophylax lindenii (Chomicki et al. 2014b). In addition to the implications such mycorrhizal associations have for the nutrition of the epiphytic orchid, there may also be associations between the aerial roots of the orchids and nitrogen-fixing cyanobacteria and interactions with other bacteria from the rhizosphere (Teixeira da Silva et al. 2015: focus on Dendrobium). See also Rammitsu et al. (2023) for clades of fungi, some very widespread, associated with epiphytic orchids.
The twig epiphytes common in Oncidiinae are distinctive. Their seed coats have little grapnel-like structures, perhaps aiding in their attachment to the twigs of their host (Chase & Pippen 1988). The plants are very small and may mature within a year (Chase 1987; Chase and Palmer 1997; Neubig et al. 2012a), indeed, many of these epiphytes are exposed to light immediately on germination, rather than having an initial slow-growing subterranean mycotrophic phase (Leake & Cameron 2012). Furthermore, Jolman et al. (2022) noted that seed dormancy in epiphytes was less marked than in terrestrial species; in the latter, dormancy could be quite protracted. Dormancy of Cattleya seeds, rich in linoleic acid, has also been demonstrated (Francisqueti et al. 2024), The leaves of epiphytic Oncidiinae are isobifacial or otherwise strongly laterally flattened and are arranged like a small fan or in two ranks (the psygmoid habit) and the plants have no pseudobulbs. All in all, they look like very young plants of other Oncidiinae and are more or less paedomorphic (Chase 1987; Neubig et al. 2012a for references: see Bromeliaceae-Tillandsioideae for a comparable situation). Chase et al. (2005) found that genome sizes in annual Oncidiinae were not particularly small when compared with those of their immediate relatives.
The leaves of some epiphytic Epidendroideae-Vandeae, including species in all three subtribes, but especially in Aeridinae, are very small and not photosynthetic and/or are soon deciduous, and the vegetative plant consists largely of photosynthetic roots; any stem is minimal, except the stem that forms the inflorescence axis (c.f. leafless species of Vanilla where the stem is the photosynthetic organ). These roots may be quite thick (ca 5 mm across) and are often terete, as in Dendrophylax and Microcoelia, while those of the aptly-named Taeniophyllum are distinctively flattened (e.g. Carlsward et al. 2006b) and those of Vanilla may reach 15 m in length (Botomanga et al. 2024). There are about 340 species of leafless Epidendroideae, all epiphytes; overall, leaf loss is estimated to have occured 20 or more times in Orchidaceae (Carlsward et al. 2006a; Freudenstein 2012) although adjustments to the number come along with improved phylogenies (Farminhão et al. 2023). Cortical cells of the roots of these epiphytes have chloroplasts, but how carbon dioxide and water flux are controlled here is not entirely clear especially since the roots lack stomata. However, aeration units along these roots may be stomata analogues (Benzing et al. 1983; Cockburn et al. 1985; Benzing 1996). Roth-Nebelsick et al. (2021) looked at the aeration units in the roots of three leafless and one leafy species of Angraecinae, noting in the former group the single, dead, thin-walled exodermal aeration cells (they are overlain by non-wettable velamen - pneumathodes) and the particular arrangement of the specialized cortical cells underneath; there can be gaps between these latter cells. Both the exodermal aeration cells and/or the specialized cortical cells may close as the root dries out and shrinks (see also Carlsward et al. 2021). CAM occurs in the stomata-less roots of leafless orchids like Campylocentrum tyrridion (Winter et al. 1985, 2015; Kerbauy et al. 2012; Carlsward et al. 2021). Suetsugu et al. (2023a) found that the roots of Taeniophyllum aphyllum fix CO2 during the might, malate concentrations becoming reduced during the course of the day, quite like the leaves of the related Thrixspermum japonicum, also a CAM plant. The roots of the latter did fix carbon, although photosynthesis was of the C3 type and chlorophyll amounts were lower than in Taeniophyllum. However, photosynthesis in orchid roots, including those of the many leafy epiphytic taxa which also have very thick, green, photosynthetic roots, remains poorly understood, although as just mentioned CAM in root and stem are not necessarily correlated - see also C. E. Martin et al. (2010). The roots of some leafless epiphytic orchids develop a thick covering in which nitrogen-fixing cyanobacteria, especially members of Nostocales, Oscillatoriales and Synechococcales, as well as fungi, etc., grow (Tsavkelova et al. 2022: greenhouse conditions). Botomanga et al. (2024) discuss the anatomy of the various kinds of aerial roots of Vanilla (see also Stern & Judd 1999: vegetative anatomy in general).
Epiphytic orchids in general have smaller genomes that those of their terrestrial relatives (Chase et al. 2005; Moraes et al. 2022, Maxillariinae - see p. 11: "the association between small genome size and epiphytism [is] a key innovation to Neotropical orchid diversification"), although the significance of this is obscure (epiphytic ferns tend to have quite large genomes...). However, if nitrogen is at a premium for epiphytes (see also carnivorous plants), then reduction in genome size - and hence in the amount of nitrogen used in its synthesis - to a functional minimum might be an advantage (Vesely et al. 2013).
Interestingly, there are spiral-crack root hairs, root hairs whose walls break down and form a ribbon-like spiral, in some epiphytic Orchidaceae (e.g. Stern 2014; Bernal et al. 2015). In Araceae such hairs are thought to help dissipate energy from strains on the roots that are likely in climbers (X. Yang & Deng 2016) - and such strains are also likely in epiphytes, too, although in Orchidoideae-Spiranthinae, at least, not all the species with such hairs are epiphytes (Bernal et al. 2015); see also Posidoniaceae.
Not many orchids are climbers, one exception being Vanilla and the related Pseudovanilla. Climbing here is by means of leaf-associated roots that attach the plant to its support.
Plant-ant associations are known from Myrmecophila (sic: Epidendroideae-Epidendreae), which grows in dry and open environments, even on sand dunes (Rico-Gray et al. 1989; Pridgeon et al. 2005). Gegenbauer et al. (2023) found that N from material collected by ants and placed inside the pseudobulbs (they function as ant domatia here) of the related orchid Caularthron bilamellatum (also New World, Epidendreae) moved to the plant via an endophytic hypocrealean fungus with thin hyphae, and other endophytic fungi in the same plant with thick black hyphae (black = melanin; ascomycetes, but unrelated) may also be involved. Rico-Gray et al. (1989) had found that C moved from dead fire ants (they had been fed 14C-labelled honey) placed in the domatia of what would now be called M. grandiflora to the plant (note that discussion there is about minerals).
A number of orchids have photonic structures, in these cases reflecting helicoid arrangements of components of the cell wall; perhaps basically involved in providing strength and support for the plant, the blue iridescence of the leaves that results would be a secondary "product" of such structures (Lundquist et al. 2024). The orchids with this iridescence are in genera from Epidendroideae-Epidendreae and -Malaxideae.
Flowering may be stimulated by fire in a number of ground-dwelling Orchidoideae, the trait perhaps originating as long ago as 60 Ma (Lamont & He 2017; Lamont et al. 2018a).
Pollination Biology & Seed Dispersal.
Orchid flowers may be notably long-lived, even lasting for months, although some last for only a single day; flowers that are pollinated by deceit (see below) tend to live longer than flowers that offer a reward (Nunes et al. 2017 and literature; F.-P. Zhang et al. 2021). Within Cypripedioideae, the flowers of Paphiopedilum last notably longer than those of Cypripedium, and the former i.a. having a thicker stem endodermis, which may even be absent in the latter; conserving (not storing) water seems to be important in Paphiopedilum (Zhang et al. 2021).
In the brief discussion about orchid pollination below, I first talk about basic orchid floral morphology, then emphasize rewards of various kinds (see Rewards - or Lack Thereof), then the pollinator itself (see Pollinators), finally, some other general issues are mentioned at the end (see Pollination - General).
Flowers of Orchidaceae are commonly resupinate, the ovary being twisted about 180°, the labellum ending up in the abaxial position (Ernst & Arditti 1994; Yam et al. 2009 for reviews). However, the degree of resupination often varies within a plant when the inflorescence is arching, all flowers of the one inflorescence ending up being oriented so that their labellum is in the same position with respect to gravity, with the ovary sometimes being twisted 360° (as in Angraecum, etc.) or not at all. Aside from such variation, the labellum may have switched from abaxial to adaxial in position ca 5 times in Angraecum alone, speciation rates being higher in clades with the latter position (Andriananjamanantsoa et al. 2016). Fischer et al. (2007) discuss the some ten different ways - of which twisting of the pedicel is but one of the mechanisms involved - that flowers in the speciose Bulbophyllum present themselves in the Madagascar region. Catasetum has resupinate staminate flowers, but the carpelate flowers are not resupinate (see also below). Flowers of Stelis, which commonly have a very short inner perianth that may appear to be little differentiated, may be presented with the odd member of the outer perianth whorl abaxial (Karremans 2019). In genera like Calopogon the flowers are never resupinate, and all flowers on the erect inflorescence show "normal" monocot orientation, the labellum being adaxial. Koopowitz (2017) discussed the absence of resupination s. str. (= twisted ovary) in basal clades like Cypripedioideae, etc., but the flowers are still presented to the pollinator inverted, i.e. with the median sepal adaxial and the median petal, the laballum, abaxial.
The outer and inner whorls of the tepals are more or less distinguishable, if both are petal-like, but the most conspicuous element of the flower is often the labellum. This is a member of the inner whorl of tepals and shows a truly remarkable diversity of form and colour (Rudall & Bateman 2002; see also 2004); duplication of B-class genes may be involved (Mondragón-Palomino & Theißen 2008; Mondragón-Palomino 2013 and references). The column is formed from the androecium and stigma/style. For a general discussion on floral evolution in the family, with an emphasis on terata and homeosis s.l., see Rudall and Bateman (2002); Rudall and Bateman (2004: outgroup a Hypoxis-type flower, but this is of no consequence) emphasize the various processes involved.
There is generally only a single stamen with the pollen grains variously aggregated into pollinia. The spatial relationships of the labellum and column in particular force the pollinator to approach the flower in a particular way, and the result is that in general, pollinaria are usually very precisely placed on the pollinator, closely related orchid species differing in exactly where their pollinaria are placed (e.g. Maad & Nilsson 2004). After the pollinarium is attached to the pollinator, the pollinia may move, so bringing them into the proper position for pollination; for pollinia, pollinaria, and pollen deposition - not always in one go even when there are pollinia, e.g. Listera (= Neottia, Epidendroideae), see Rasmussen (1982), Freudenstein and Rasmussen (1996, 1997), Nazarov and Gerlach (1997), Johnson and Edwards (2000), Pacini and Hesse (2002), Freudenstein et al. (2002), Harder and Johnson (2008), Selbyana 29: 1-86. 2008, and so on. The plesiomorphic condition for Orchidaceae is to have elastoviscin (quite different from the viscin threads of, say, Onagraceae) associated with the pollen grains. It is very similar to pollenkitt, and the two may be the same, so it has not been placed as an apomorphy for the family, however, tapetal plastids seem not to be involved in its synthesis as they are in that of pollenkitt and its composition is poorly understood (Purgina et al. 2024b). Elastoviscin makes up the caudicula of the pollinium in some orchids, or it can be entirely lost (Still & Wolter 1986). The surfaces of the pollinia develop a callose wall and sporopollenin is deposited; however, in the individual tetrads, in which meiosis is simultaneous (but c.f. Purgina et al. 2024b), there is neither the development of a callose wall nor sporopollenin (Cui et al. 2023). For details of pollen grain and pollinium morphology and development, see Purgina et al. (2024a: 5 spp. Epidendroideae, b: 6 spp. Orchidoideae).
A distinctive feature of many orchid species is that the ovules are not fully developed at anthesis and may not even be recognizable as such then, and fertilization is then necessarily delayed relative to pollination (Nishimura & Tamura 1993 and references). The time between pollination and fertilization ranges from four days to ten months (in Vanda), the normal time being one week to six months (Swamy 1949b; Wirth & Withner 1959; Veyret 1965; Yeung & Law 1997; also Sogo & Tobe 2005, 2006d for references; Duarte et al. 2019: ?Apostasioideae), and even after fertilization there is 2-45 days before the first cell division in the zygote (Yeung 2022). A recent survey by Mayer et al. (2021) found that in species that were tropical/epiphytic/had deceptive pollination the ovules were usually least developed at anthesis, so in many Epidendroideae (not Neottieae) the ovules had barely started developing when the flowers were already at anthesis, while in temperate orchids ovule development is normally faster, perhaps to take the change in season into account (see also Ortúñez et al. 2025). Perhaps surprisingly, the life cycle of a number of full mycoheterotrophs is speeded up. Some of them have embryo sacs that are fully developed at anthesis and/or the time between fertilization and the first division of the zygote is relatively short, the range being 2-15 days (Y.-Y. Li et al. 2016; Yeung 2022). For orchid seed morphology, etc., see below.
Finally, it may be mentioned that a few Orchidaceae are completely cleistogamous, i.e. they always self. These include some five species of Gastrodia, a holomycoheterotroph, and the two species studied by Suetsugu et al. (2022c) could be considered partly neotenous from the point of view of their transcriptome profiles, but with full neoteny or progenesis from the point of view of their morphological features. Suetsugu et al. (2022c) also noted that the complete absence of the rostellum, a feature that enabled the selfing, was more obviously the result of such processes.
The plesiomorphic condition for the family may be to lack nectar (Jersáková et al. 2006). Apostasioideae provide pollen as a reward for pollinators (Kocyan & Endress 2001); there is buzz pollination in Apostasia. However, deceit-type pollination may have been a feature of the common ancestor of the rest of the family (Weston et al. 2014; Givnish et al. 2015). For floral mimicry in general, which includes deceit pollination, see S. D. Johnson and Schiestl (2016) for a valuable review; they include many examples from Orchidaceae. It has been suggested that about one third or somewhat more orchid species - estimates range from 6,500 to 10,000 species or even more, perhaps 9/10 of the species of angiosperms that practice deceit pollination belonging to Orchidaceae (Karremans 2023) - have some kind of deceit or mimicry-type pollination systems where either food or sex is the ostensible reward (Safni 1984; Ackerman 1986; Schiestl 2005, 2010 for reviews, the latter brief; Renner 2006a: rewardless flowers in general; Schlüter & Schiestl 2008: molecular mechanisms; Peakall 2009: deceit and speciation; Chase 2009: deceit in Oncidiinae; Schaefer & Ruxton 2010: plant exploitation of the pollinator's perceptual biases; Gaskett 2011: the pollinator's point of view in sexual deception; Xu et al. 2012; Pinheiro & Cozzolino 2013: deceit in Epidendrum; Peakall 2023: sexual deception). Although Shrestha et al. (2020) emphasised that we really have very little idea as to the proportion of orchids that practice deceptive pollination, Ackerman et al. (2023) estimated that there was deceit pollination in some 46.1% of non-autogamous orchid species. Polymorphism in flower colour is notably common in species that practice deceptive pollination (Arida et al. 2025). Note that species with rewards may be more common than has been thought (Karremans et al. 2015; Jia & Huang 2021: Dendrobium), although at around 54.2% of chasmogamous species it is lower than some estimates (Ackerman et al. 2023). Pansarin (2021b) found that 6/10 of the Neotropical species of Vanilla that he examined produced nectar as a reward, another had food hairs on the labellum; osmophores were also common.
Deceit comes in various guises, from sexual deceit, where the orchid mimics a female insect and pollen exchange occurs during pseudocopulation (e.g. Ophrys below), to domicile deceit, as in species of Serapias (Orchideae: Bellusci et al. 2008 for a phylogeny) and Cypripedium (Cypripedioideae: Pemberton 2013) that attract pollinators by mimicking a nest hole, to oviposition/brood site mimicry (the large genera Bulbophyllum and Pleurothallis, ca 2,000 and 550 species respectively, are brood-site mimics), to mimicry of other taxa that have rewards, and so on. More generally, flowers pollinated by deceit do appear to have rewards of some sort, whether a fungal body, aphids, carrion, etc., that the pollinators' larvae can eat, or flowers that look like female insects to the visiting male, etc. (Kagawa & Takimoto 2015 and references). Sonkoly et al. (2016) found that orchids with deceit pollination may set fewer fruits than orchids with other sorts of pollination mechanisms, but they set more seeds per fruit, so similar numbers of seeds per plant are produced. See also the section on pollinators below, especially dipterans.
Pollination by sexual deceit, the orchid flower attracting male insects that pollinate in the course of attempting copulation, occurs in hundreds of species ot Orchidaceae, although elsewhere it is known only from Iris paradoxa and Gorteria diffusa (Asteraceae) (Peakall 2023). Deceit pollination is quite common in orchids, mostly ground-dwelling, from the Europe-Mediterranean area, Australia, and to a less extent in Central and South America (although perhaps under-recorded - e.g. situation in Lepanthes?), including epiphytic taxa, and South Africa (Peakall 2023).
Flowers of the largely European Ophrys (Orchidoideae-Orchideae) are well known for deceit pollination, their labella mimicking female bees, wasps, even beetles (e.g. Kullenberg 1961; Paulus 2006; S. D. Johnson & Schiestl 2016). They also produce semiochemicals that are very similar to insect pheromones; for example, alkenes (hydrocarbons with at least one double bond) are part of the chemical component of this mimicry, attracting the insect (e.g. Stökl et al. 2009; Dötterl & Vereecken 2010; Ayasse et al. 2011; S. Xu et al. 2012; Bohman et al. 2016; Joffard et al. 2020; Baguette et al. 2020; Peakall 2023); Joffard et al. (2016) discuss floral scent bouquets in these orchids and species limits. Morphology and scent together enable the Ophrys flower to mimic female insects (Cortis et al. 2009 and references; Francisco & Ascensão 2024), pollination occurring as the male insects attempt to copulate with the flowers (Kullenberg 1961; Kullenberg et al. 1984 and references; S. D. Johnson & Schiestl 2016). Barriers to crossing - floral form and scent again - are pre-zygotic, acting before pollination, indeed, few deleterious effects of hybridization, which occurs in the wild, have been noted (S. Xu et al. 2011). Scent chemicals are common in related orchid genera as well (Schiestl & Cozzolino 2008), and Ophrys is part of a larger clade in which food deception seems to be the basic condition (Inda et al. 2012). However, there is currently much discussion about species limits in Ophrys, with estimates of species numbers ranging from 9 - data from DNA sequence homology - to over 400 - prezygotic isolation by attraction of species-specific pollinators (Bateman et al. 2006a, esp. 2011a, 2018a; Devey et al. 2008; Vereecken et al. 2011; Bradshaw et al. 2010, Vignolini et al. 2012a: labellum colour due both to cell structure and pigment; Delforge 2016; Alibertis 2015: photographs of the species/"species" half the book; Baguette et al. 2020; Cuypers et al. 2022; Peakall 2023). Paulus (2006: p. 315) thought that "species formation in Ophrys always proceeds with the aquisition of a new species of pollinating males", and species that had different pollinators might show "genic rather than genome-wide differences", speciation had barely begun (Sedeek et al. 2014: p. 6202). If such differences did result in the differentiation of new species, then emphasis on monophyly - there are only a few more or less strictly monophyletic species (Bateman et al. 2018a) - would be side-stepped, and it could be argued that invoking strict monophyly is inappropriate. The genus began diversifying ca 4.9 Ma, rapid diversification in the Pleistocene accompanying shifts to different pollinators, i.a. wasps, apid and andrenid bees all being involved (Breitkopf et al. 2015), and the whole story is almost mind-numbingly complex.
Around 100 species of Australian Caladenia (Orchidoideae-Diuridae) are similarly pollinated by male thynnine wasps (Phillips et al. 2009, other articles in Australian J. Bot. 57(4). 2009; see also Brown & Brockman 2015). Drakaea and Chiloglottis, also Australian and Diuridae, are other thynnine mimics, pollination happening as the wasp attempts to fly away with the flightless female wasp = lower part of the labellum (S. D. Johnson & Schiestl 2016 for references); again, scent plays a role in maintaining reproductive isolation and attracting the male wasp (Peakall & Whitehead 2013). These orchids often show a high degree of pollinator specificity and overall little hybridization (Peakall 2023). In Chiloglottis there is a fair degree of congruence between the phylogenies of the orchids and the deceived wasps (Mant et al. 2002, 2005; see also Weston et al. 2011; Miller & Clements 2014). Understanding species limits is critical; there may be cryptic species in the pollinating wasps, hybridization occurs, etc. (see also D. L. Jones et al. 2001: Caladenia; Griffiths et al. 2011: Chiloglottis). In a number of such sexually-deceptive orchids both morphological and genetic differences between species or even genera are slight (Schiestl 2005 and references; Mant et al. 2005; see also below) and post-pollination reproductive barriers may be nonexistent (Whitehead & Peakall 2014).
Chemical signalling between plants and insects involved in pollination occurs in many situations, not only enhancing floral mimicry in pseudocopulation. Thus wasps may be attracted to orchid flowers that produce chemicals similar to those produced by damaged plant tissue - the wasps visit the flower expecting to find caterpillars, but pollination occurs instead. Similarly, Dendrobium sinense, pollinated by a hornet, has a floral bouquet that includes the same chemicals as in the alarm pheromones of Apis, which the hornet commonly catches (Brodmann et al. 2009). The volatile emissions that some orchids produce suggest carrion, so attracting insects looking to lay eggs in the non-existent carrion but that pollinate the flowers in their search (see Jürgens et al. 2013 for the syndrome).
Other kinds of food deceit are quite common, as with pollen mimicry in Vanilloideae-Pogonieae where it characterises a clade that includes temperate species; there appears to be pollen available for removal (Pansarin et al. 2012). Drosophila seems to be a common pollinator of Paphiopedilum. In some species females are attracted to the staminode, whether because of its resemblance to a colony of aphids or because of odour cues, and they lay eggs there. In other cases the insects may be attracted by structures on the staminode looking like pollen or nectar (references in S. D. Johnson & Schiestl 2016). Nectar deceit is also common in Caladenia (Phillips et al. 2009).
A number of species of Epidendroideae-Oncidiinae have flowers that resemble those of oil-producing Malpighiaceae, q.v.; they have radiating, clawed, yellow or purple "petals" that are similar in both shape and in colour (bee-UV-green) to petals in flowers of Malpighiaceae. Overall, there may be a Batesian mimicry system here, both groups of plants being visited by bees like Centris, although the orchids often have no reward for the bee (Neubig et al. 2012a; esp. Papadopulos et al. 2013; c.f. in part Pansarin et al. 2021b; see also below). The mimicry unit of the orchid is formed largely by the labellum, while the column is equivalent to the banner petal of a malpighiaceous flower. M. P. Powell (in Neubig et al. 2012a) has estimated that such mimicry may have evolved at least 14 times within Oncidiinae, indeed, it may be both lost and regained (Papadopulos et al. 2013) - see also Fabaceae-Caesalpinioideae-Tachigalieae for comparable mimicry. Other Oncidiinae mimic Calceolaria, another oil flower (Neubig et al. 2012a). Floral mimicry is also known from the largely Australian Thelymitra which has sub-polysymmetric flowers (Edens-Meier & Bernhardt 2014a).
Orchid flowers that are attractive, but that lack any rewards, may be polymorphic, if the pollinator is not that good at discriminating colours (and is a rather slow learner), or more continuous variation, if the pollinator is better at distinguishing colours (Kagawa & Takimoto 2015).
Flowers of many orchids do have rewards for the pollinator, and such flowers are derived from flowers lacking rewards (Cozzolino et al. 2001; Cozzolino & Widmer 2005; Smithson 2009; Pansarin et al. 2012; S. D. Johnson et al. 2013). Thus the production of rewards is a derived feature in Vanilloideae-Pogonieae (c.f. Pansarin et al. 2012), mimics of Malpighiaceae in Epidendroideae-Oncidiinae (see above), and elsewhere. In a number of species of Maxillaria hairs on the labellum contain protein and perhaps also starch and function as pseudopollen, so rewarding the pollinator (Davies et al. 2000; Davies 2009; also Arévalo et al. 2017b for labellar micromorphology in Mormolyca-Maxillariinae). Pansarin et al. (2022) suggested that edible trichomes have evolved some five times in Maxillariinae. Maxillariella produces small quantities of resins along with sugars and/or proteins, etc., that may serve as a food reward, interestingly, the main components of resins in other Maxillariinae were not found here.
Nectar flowers are quite common (Bernadello et al. 2007 and references), and although Orchidaceae do not have septal nectaries, nectar spurs are scattered in the family. Thus in the speciose African Disa (Orchidoideae), there have been several transitions from deceit to nectar rewards (nectar stomata in spurs, secretory epidermis on perianth) and subsequent loss of nectar (there are independent gains and losses of spurs), but all without having much of an effect on diversification rates (S. D. Johnson et al. 2003) or on mating (selfing vs wide outcrossing), etc. (Hobbhahn et al. 2017; see also Smithson 2006). For spurs, nectariferous and otherwise, in Orchidoideae-Orchidinae, including Habenaria, see Bell et al. (2009); the flowers of African Satyrium are not resupinate and have twin nectar spurs (S. D. Johnson et al. 2011a). For other nectar spurs, which develop from the adaxial sepal, in the largely Australian Diurideae, see the summary in Weston et al. (2011, 2014), and here various kinds of mimicry, and nectar production, sometimes from the labellum, have evolved, and then perhaps been lost many times (see also the non-resupinate Disa - Hobbhahn et al. 2013). Spurs are also common in Vandeae, as in Angraecum (Angraecinae, see above), and Aeridinae (Topik et al. 2005), for example. Tissue on the tepals may also produce nectar (Davies et al. 2005). Hobbhahn et al. (2013) discuss the evolution of nectaries of various types (a secretory nectariferous epidermis is here descibed as being "recapitulatory") in Disa, which has happened some eight times there alone.
Indeed, resins and oils are quite common rewards; for a summary of oil flowers in Orchidaceae, which have evolved probably at least a dozen times, eight times in Cymbidieae- Oncidiinae alone, and are pollinated bvy a variety of bees, see references in Renner and Schaefer (2010, also Chiron 2010; Arévalo et al. 2017b); flowers of some species mimic the presence of resin rewards (Whitten et al. 2007; Davies & Stpiczynska 2012, 2017). The flowers of orchids that have oil as a reward may show convergence with those of other oil-pollinated plants. Thus some 70 or more species of Oncidiinae have elaiophores, often on the labellum; these may be epithelial or tufts of unicellular secretory hairs (Blanco et al. 2013; Davies et al. 2014; Tölke et al. 2019 and references) and be visually similar to elaiophores on the calyx of Malpighiaceae, or the flower as a whole may be like those of Malpighiaceae. The distinctive oil secreted by the orchid is also very similar to that produced in the flowers of Malpighiaceae (see above; Reis et al. 2007 and references), and there may be some kind of Müllerian mimicry system operating (e.g. Papadopulos et al. 2013), but such systems are difficult to categorise (see also Policha et al. 2014); Centris and Tetrapedia visit oncidiine flowers with different floral syndromes (Gomiz et al. 2017). Note, however, that Castro et al. (2021) tested this mimicry hypothesis comparing Gomesa flexuosa (Epidendroideae-Cymbidieae-Oncidiinae) and Janusia guarantica (Malpighiaceae) and suggested that such orchid flowers, which also occur in other oncidiine orchids that do not grow with Malpighiaceae (as is sometimes the case even with this species pair) were not so much involved in some kind of mimicry system, rather, they were exploiting the sensory biases of their pollinating bees. Note that some Fabaceae-Caesalpinioideae (see there) have flowers that appear to mimic those of Malpighiaceae. All told, oil flowers have been reported from 8 epidendroid subtribes, of which Oncidiinae include by far the most examples (Possobom & Machado 2017 and references). Pollination of some of the mostly South African Orchidoideae-Diseae-Coryciinae, e.g. Disperis, is by oil-collecting Rediviva bees, as with other African oil-pollinated plants (Renner & Schaefer 2010: Table 1); the flowers have paired, pouch- or spur-like structures like those of another local oil plant, Diascia (Scrophulariaceae: Pauw 2006). The South African Huttonaea, perhaps immediately unrelated, also has oil flowers (Steiner 2010); Steiner et al. (2011) analyzed scent composition of many southern African oil-secreting Diseae; see also Chase et al. (2009) and Steiner (2010) for oil flowers.
There are a number of cases where orchid flowers with more or less conventional rewards have evolved from deceit-type flowers (S. D. Thomson & Schiestl 2016). There are also complex situations where flies (Drosophila in this case) are attracted to the flowers of Specklinia by aggregation pheromones and the flies may copulate when on the flower, and there is also a nectar reward (Karremans et al. 2015). The flower of Gastrodia pubilabiata looks like the fruiting body of the ECM fungus (Mycena) that is associated with it, and which may even be growing nearby; if so, the decaying mushrooms attract pollinators (deceit), so the plant benefits from the fungus both nutritionally and in its pollination (Suetsugu 2018a); here there is also self-pollination, bee (small) pollination, and nurse pollination, the decaying flowers of G. foetida being a food source for larvae of Drosophila, especially D. bizonata (Suetsugu 2023). Apparently rewardless flowers may well turn out to have rewards on closer examination, for instance, some Maxillariinae like Camaridium cucullatum did in fact produce lipids or resins on their labella (Davies & Stpiczynska 2019; Shrestha et al. 2020), while 75% of the small-flowered Oncidiinae examined by Pansarin et al. (2021) produced oil, nectar or perfume, the latter collected by euglossine bees. Indeed, male euglossine bees are pollinators of many species of Neotropical orchids (see below) which they visit to collect fragrances that they subsequently use in their courtship displays.
Pollination is largely by insects, and overall, perhaps 56% of orchids are pollinated by single species of insects (Karremans 2023).
It has been estimated that perhaps 60% of Orchidaceae are pollinated by bees (Schoonhoven et al. 2005), whether deceived or not (see Lunau et al. 2021 for false colour floral reconstructions by combining u.v. and colour photographs so as to see what a bee sees). N. H. Williams (1982) discussed the general importance of male euglossine bees in particular in the pollination of Neotropical Epidendroideae (see also Roubik 1988, 2014). There are perhaps 246 species of orchid bees (Cardinal 2018) and they pollinate perhaps up to 25% of tropical American Orchidaceae, hence their common name. Pollination by these euglossine bees is especially common in orchids growing at lower altitudes, and anywhere from 900-2,000 species may be involved (Cameron 2004 and references; Zimmermann et al. 2009; Ramírez et al. 2011 - Photo: bee pollinators). Ramírez (2009) thought that some 700 species of orchids had fragrances that attracted male bees, about 85% of all plants with such fragrances (see also Pemberton 2010); another estimate is that perhaps 2,000 species of Epidendroideae (i.e. almost all Stanhopeinae, Zygopetalinae and Catasetinae) are visited by male euglossines for fragrances (see e.g. N. H. Williams 1982; numbers from Pridgeon et al. 2009); J. W. Liu et al. (2023) estimated that ca 800 species of orchids are pollinated by these bees (they also visit flowers in several other families). Euglossine bees can be attracted by scent over considerable distances (e.g. Janzen 1971; Dötterl & Vereecken 2010). However, very little is known about details of the pollination of most of these orchids, thus Nunes et al. (2017) found that the flowers of the Zygopetalum species they examined were pollinated by bumblebees and those of Dichaea by weevils, although both genera had been thought (tentatively) to be pollinated by orchid bees (Pridgeon et al. 2009). Henske et al. (2024) observed that euglossines in fact obtain only a relatively small amount of these scents from orchids, rather, in addition to the flowers of a few other angiosperms, they obtain resins from a variety of other sources including door hinge grease, even if the ultimate source of some of the fragrances may be unclear - bacteria? Furthermore, female bees have been observed to collect these fragrances (Henske et al. 2024). Note that scent in general is important in bee pollination (Dötterl & Vereecken 2010). For further discussion on euglossine pollination, see Clade Asymmetries.
Orchid bees are vigorous fliers and may range up to 23 km from their nests (Janzen 1971). Male bees pollinate the flower as they collect fragrances that they store in their hind tibial pockets, these fragrances perhaps being involved in pre-mating isolation mechanisms in the bees; they act as both attractants and rewards for the bees (J. W. Liu et al. 2023: their focus was on orchids in Catasetinae and Stanhopeinae, both Epidendroideae-Cymbidieae). Closely related and sympatric species of Euglossa did show greater disparity in the fragrances they preferred than might be expected, but overall, the most dominant compounds in the fragrances were highly homoplasious (Zimmermann et al. 2009). Most of the fragrances that the bees pick up from the orchids can also be found elsewhere. Liu et al. (2023) noted that the fragrances were often dominated by phenylpropanoids and terpenoids, and there was evidence of both rather rapid and parallel evolution. Pollination in Gongora (Cymbidieae) occurs as the bee slides down the column having lost its hold on the epichile, slipping on the wax that covers the epidermis (Adachi et al. 2015; c.f. Nepenthes). Hetherington-Rauth and Ramírez (2015, 2016) found that species in different sections or subgenera might have similar scent bouquets, but this was less likely in more closely-related species, indeed, in some species there were sympatric chemotypes that attracted different species of bees. Coryanthes has remarkable flowers in which the pollinating bees cannot grasp the smooth surfaces, become wetted by secretions from the base of the column, fall in to liquid in the apical part of the labellum, and crawl slowly out between the apex of the labellum and that of the column, pollination occurring then (Gerlach 2017). Finally, focussing on Cirrhaea and Stanhopeinae, Pansarin et al. (2018) found that one species of orchid could be pollinated by two or more species of bees, and one species of bee visited two or more species of orchids. For orchid bees and pollinated orchids, see also Feinsinger (1983), who thought that reciprocal evolution was unlikely.
That Cymbidieae-Catasetinae, Catasetum in particular, are pollinated by male euglossine bees is well known (Darwin 1862; Chase & Hills 1992; Pérez-Escobar et al. 2015, 2017b, 2017c for phylogenies; Gerlach 2013), and its flowers are quite remarkable, even for an orchid. Here staminate flowers are resupinate and carpelate flowers are not, and there are many other striking differences between the two, especially in labellum morphology; indeed, plants with staminate and carpelate flowers were once placed in separate genera, Myanthus and Monachanthus respectively. Bees, mostly Euglossa and Eulaema, visit the flowers of Catasetum for fragrances (see e.g. Dressler 1982), and since different species of orchids tend to have different combinations of frangrances this reduces the diversity of bees visiting them (Milet-Pinheiro & Gerlach 2017). Franken et al. (2016) describe the osmophores, however, details of floral scent chemistry are both complex and poorly understood (see also Milet-Pinheiro et al. 2015). The attachment of the pollinia on the bees is by a trigger-activated explosive mechanism, the bees touching the filiform antennae on the sides of the rostellum as they collect the fragrances, and this triggers the explosive discharge of the pollinia (Nicholson et al. 2008). The bee is startled, and Romero and Nelson (1986) suggested that as a result it subsequently avoided staminate flowers, hence the very different morphologies of the carpelate flowers, which, however, are more similar between the species: "The battered pollinator will remember the negative experience with the staminate flower" (Gerlach 2012: p. 39). A final wrinkle is that whether staminate or carpellate flowers are produced is determined, at least in part, simply by light, bright light tending to favour the production of carpelate flowers (Gregg 1975; Nickerson 2023); Pérez-Escobar et al. (2015, see also 2017c) found that environmental sex determination had evolved three times here, 164 species being involved (Catasetum, Cynoches, some Mormodes).
Although euglossine bees are effective pollinators and the morphologies of the flowers that they pollinate are often nothing short of unbelievable, the relationships between orchids and bees are non-specific, particularly on the side of the bee (Ackerman 1983; Cameron 2004; Ackerman & Roubik 2012), although Stebbins (1970) thought that there had been extensive speciation in both bee and orchid because of their association. Importantly, crown-group euglossines can be dated to 42-27 Ma, with especially rapid diversification 20-15 Ma (Ramírez et al. 2010), (35-)28(-21) Ma (Cardinal & Danforth 2011) or (30-)26(-23) Ma (Cardinal 2018), although estimates of the age of stem-group euglossines vary considerably - Cretaceous (e.g. Grimaldi & Engler 2005) or later Eocene (40-)37(-35) Ma (Danforth 2018). The orchids these bees pollinate speciated about 12 Ma, (31-)27-18(-14) Mya (Ramírez et al. 2011) or 22-16 Ma (Givnish et al. 2015: two origins), later, the estimates being from Catasetinae and Zygopetalinae plus Stanhopeinae, immediately unrelated clades - although from the range of ages just given, the connection between orchid and bee diversification is unclear. Pollination by euglossine orchid bees may be largely restricted to two clades in core Epidendroideae. Lubinsky et al. (2006), noting that a number of genera in the three basal clades of orchids had aromatic fruits, and that orchid bees pollinated Vanilla, even thought that orchid-orchid bee relationships could have involved both pollination and seed dispersal, the latter being the ancestral condition for the family (see also Rodolphe et al. 2011; Pansarin 2021a, b).
Fly pollination is also common (Christensen 1994; Siegel 2016 for a readable summary; ca 20% - and likely to increase - Ackerman et al. 2023), especially in Epidendroideae. Thus the very speciose and largely Old World Bulbophyllum (Epidendreae-Bulbophyllinae) often has dark, purplish-coloured flowers, the labellum is mobile, and there may be dangling structures (hairs of various kinds, labellum tips), and it attracts a variety of flies (Vogel 2001: also Pleurothallidinae; see also A.-Q. Hu et al. 2019). A number of taxa have sweet, fruity scents and lighter-coloured flowers and are pollinated by fruit flies - which may also be commercially important pests (Tan 2008 and references, see also Texeira et al. 2004; Fischer et al. 2007 for resupination). Stpiczynska et al. (2015, 2018 and references) found some African species to have scented flowers and nectar (secreted by the labellum), or lipids as a reward, and the labellar tissue might be aerenchymatous, presumably increasing its mobility. Some species have flowers with a carrion scent suggesting pollination by necrophagous insects, as Siegel (2016: p. 87) noted of the flowers of B. fletcherianum, they smell "like a herd of dead elephants" - although elephants, dead or alive, are not known from New Guinea, where this orchid is from. However, as with other megagenera, little is really known about pollinators and floral rewards, yet the floral diversity of the genus beggars description (see e.g. the illustrations in Vermuelen et al. 2015). A rather similar set of features, including a mobile labellum, etc., characterize the new World Pleurothallidinae, Arévalo-Rodrigues et al. (2022) describing colleters and a variety of hairs producing oil, nectar, or scents, etc., on/in the flowers here, and again fly pollination, especially by drosophilids, is prevalent, perhaps contributing to diversification (Karremans & Díaz-Morales 2019: see also Ackerman et al. 2023; Gil-Amaya et al. 2025, etc.). In the large New World genus Lepanthes, pollination during pseudocopulation with fungus gnats (dipterans, often Sciaridae; overall 8 families including thousands of species - Bernhardt & Kuiter 2022) has been reported (Blanco & Barboza 2005). How this system might function is unclear since there is no obvious connection between the morphology of the orchid flower and that of the fungus gnat (Singer 2011). (Pridgeon 1982 noted that in myophilous and sapromyophilous Pleurothallidinae pollinium number was reduced from 8 to 4 or 2.) Bernhardt and Kuiter (2022; see also Kuiter 2016: Victoria, Australia Pterostylis) summarize the literature on fungus gnat pollination, noting that it is at most uncommon for the gnats to lay eggs in orchid flowers, even if it is the female gnat that is carrying out the pollination. Biting midges have been implicated in the pollination of Trichosalpinx (related to Lepanthes), again, the flowers have a syndrome very like that mentioned above for Bulbophyllum (Bogarín et al. 2018). Fungus-visiting drosophilids pollinate Dracula, which can have a distinctively-patterned outer perianth as well as a labellum that looks (and smells) very much like a fungus with gills, and other flies are also involved (Policha 2014; Policha et al. 2014, esp. 2016). Stimulation of the labellum by ?flies visiting the flowers of Porroglossum causes it to snap shut, imprisoning the insect against the column whence they remove the pollinaria, and the trap reopens after some minutes (Pridgeon et al. 2005; McDaniel & Cameron 2016). Indeed, a variety of dipteran groups pollinate orchids, sometimes nectar is a reward, or the orchid simulates decaying material or a fungus (sapro- or mycomyophily), or insects, etc., captured by another predator, but that serve as food for kleptoparasitic flies (kleptomyophily). Such modes of pollination are likely to predominate in the some 4,000+ species of Epidendroideae-Pleurothallidinae, to which Lepanthes and Dracula belong (Pridgeon et al. 2005 and references; Karremans et al. 2016; Davies & Stpiczynska 2017: Mormolyca s. str., = Maxillaria s.l.; Bogarín et al. 2018). Here self-incompatibility tends to be developed, as in some other Epidendroideae (Borba et al. 2011; Duque-Buitrago et al. 2014; Karremans et al. 2015). Pollination by Drosophila and Scatophaga is also known in Cypripedium (P. Li et al. 2012; see Edens-Meier et al. 2014 for Cypripedioideae in general), while carrion flies pollinate Satyrium pumilum (Orchidoideae-Diseae: van der Niet et al. 2011). See also above (Rewards - or lack thereof).
Moth, butterfly and even bird pollination are also well known in the family. Angraecum sesquipedale (Epidendroideae-Vandeae), from Madagascar, is a classic example of moth pollination. There the spur is 30-45 cm long, and the pollinator of the plant remained unknown for some time, although Darwin (1862) had early suggested that some moth with a proboscis that long would be found. Indeed, Xanthopus morgani praedicta, with a proboscis about 25 cm long, was subsequently discovered (Nilsson et al. 1987; Nilsson 1988; Wasserthal 1997; Arditti et al. 2012). Wasserthal (1997) thought that mutual co-evolution in the strict sense was not involved here, rather, the moth may have moved on to the plant as its spur became longer (long-tongued insects perhaps can survive on short-tubed plants; the reverse is unlikely); Armbruster (2017) discussed this and similar situations. Micheneau et al. (2010) summarized pollination and evolution in the over 200 species of angraecoid orchids, while Farminhão et al. looked at sphingid pollination throughout the Afrotropics, emphasizing the complexity of pollinator shifts in the angraecoids in particular (Farminhão et al.). For the great floral and pollinator diversification in some genera of Orchidoideae-Orchidinae, including Disa, see S. D. Johnson et al. (1998, 2013) and Bytebier et al. (2007); Hapeman and Inoue provide an extensive discussion of pollination in Platanthera, a genus that has turned out to be polyphyletic (Jin et al. 2014). E. Hágsater and Soto Arenas (in Pridgeon et al. 2005: T. 301.2) summarize pollination of Epidendrum; there lepidoptera are particularly common, but also hummingbirds, flies, etc., are involved.
The literature on orchid flowers and their pollinators is very extensive - an understatement - and only some has been mentioned here; see also above under Diversity & Distribution. For summaries of pollination in Orchidaceae, see van der Pijl and Dodson (1966), van der Cingel (1995, 2001), Endress (1994b), Biol. J. Linnean Soc. 173: 713-773. 2013, Pridgeon et al. (1999, 2001b, 2003, 2005, 2009, 2014), Pansarin (2016: Vanilloideae), Claessens and Kleynen (2016: European orchids), Valencia-Nieto et al. (2018: Epidendreae), Kuiter (2016: Australian orchids), Karremans (2023: general survey), etc. - and of course the classic study by Darwin (1862a) is still worth reading (see also Yam et al. 2009; Edens-Meier & Bernhardt 2014b).
The connection between orchid — pollinator relationship and orchid diversification remains a matter of active discussion. Tremblay et al. (2005) reviewed the evolutionary consequences of the diversity of the pollination mechanisms of Orchidaceae and the remarkable variation shown by their flowers. Orchid diversification is often explained in terms of the close association between pollinators and individual species of orchids, whether or not cospecation or coevolution is involved, and pollination relationships are usually thought of as being very precise. Of course, whether mutual change/adaptation, coevolution in the strict sense, has been involved in individual orchid-pollinator relationships is another matter - probably only rarely (see also N. H. Williams 1982). Reproduction in orchids may often be pollinator-limited (Tremblay et al. 2005), with few flowers on an inflorescence producing seeds, however, the production of huge numbers of seeds by each fruit may compensate for this - as Pérez-Hérnandez et al. (2011) noted, orchids "specialize in chance". Interestingly, nectarless orchids have a lower reproductive success than orchids that have nectar as a reward (Kindlemann et al. 2007: sample size small, latitude unimportant), and reproductive success of tropical orchids may be lower than that of their temperate relatives, perhaps because it is more difficult for the pollinator to find the orchid in diverse tropical vegetation (Kindlemann et al. 2006); these patterns will interact with factors like pollinator specificity, etc..
The presence of well-developed and effective premating/prezygotic barriers in many Orchidaceae may have obviated any pressure for the selection of postmating barriers (e.g. Whitehead & Peakall 2014), furthermore, given that endosperm does not develop in (?any) orchids, there can be no problems caused by endosperm developmental anomalies in hybrids (Yeung 2017). In any event, artificial crosses are often easy to make (some 110,000 have been made), and hybrids may have three or more genera in their parentage, although how these will look when generic boundaries are redrawn is unclear. Hybridization in the wild - and sometimes also polyploidy - has been noted in a number of cases, e.g. Epidendrum (Marques et al. 2014), Ophrys (Xu et al. 2011) and Chiloglottis (Miller & Clements 2014). Many genera in Epidendreae-Laeliinae can be crossed artificially (van den Berg et al. 2000, 2009). Many orchids are exquisitely adapted to individual pollinators whose sensory biases they may exploit (Schiestl 2010; Ramírez et al. 2011), but even here individual species of orchid bees, for example, may pollinate several species of orchids (N. H. Williams 1982), plant-pollinator relationships being highly nested; furthermore, few of the fragrances sought by male bees are unique to the orchids (Ramírez et al. 2011). Indeed, plant-pollinator specificity in euglossine orchid bees is lowest (from the bee's point of view) when a bee species is common and lowest (from the plant's point of view) when its flowering season is long, and seems to have little to do with in some way reducing extinction risk as by reducing dependence on unreliable pollinators - specificity is more apparent than real and is in part a matter of sampling (Ackerman & Roubik 2012).
However, pollinator specificity in orchids can be overemphasized. A single species of Epipactis may be visited by over 100 species of pollinators (Tremblay 1992), while in another study, pollinator specificity in orchids, although greater than that in Ranunculaceae (but that hardly says much), was less than that in Polemoniaceae (Waser et al. 1996). There may also be connections with geography here. Thus southern African orchids have more specialized pollination systems than their European-North American counterparts, and in the latter there are fewer orchids pollinated by only a single species of pollinator (Ollerton et al. 2006). Cozzolino et al. (2004) found that in sympatric species pairs of Mediterranean orchids, if pollinators were shared, then post-zygotic cytological barriers tended to develop, but these barriers were not evident when the species had different pollinators. In European orchids with generalized food-deceptive mating mechanisms, barriers to crossing may be postzygotic, whereas those that practice sexual deception have prezygotic reproductive barriers, and introgression is more likely in this latter situation (Cozzolino & Scopece 2008).
Given the timing of evolution of orchids and bees, and the often asymmetric dependency relationships of the two, recent work strongly suggests that strict insect-orchid co-speciation is unlikely to be an important explanation for orchid diversification (e.g. N. H. Williams 1982; Szentesi 2002; Jersáková et al. 2006; Ramírez et al. 2011; Karremans & Díaz-Morales 2019). That the generation of diversity by floral specialization is relatively uncommon in flowering plants in general, although perhaps occurring here in orchids (Armbruster & Muchhala 2009), is not inconsistent with this argument. Furthermore, factors other than floral variation may have contributed to orchid diversification - see also below and "Ecology & Physiology" above.
Recent studies suggest that when pollinators visit orchid flowers in the course of deceptive pollination or to pick up scent rewards - specialized pollination mechanisms - pollinator specificity is greater and orchid species richness is greater than when pollinators visit for nectar (Schiestl & Schlüter 2009; Xu et al. 2011; Schiestl 2012: sister-group comparisons; see also Dressler 1968; Scopece et al. 2010a for pollination efficiency). Thus deceit pollination may under certain situations increase outcrossing and speciation, the latter perhaps because of the specificity of the pheromones produced by the plants (Jersáková et al. 2006; see also Ledford 2007). However, this does not necessarily mean that there has been coevolution/cospeciation (Karremans & Díaz-Morales 2019), and although Baguette et al. (2020) mention "asymmetrical coevolutionary dynamics" in this context, the pollinators of orchids like Ophrys seem little affected, even if there are hundreds of species of the latter.
But all this talk about pollination may be for nought. Autonomous self pollination in orchids is quite common, being recorded in some 31% of the species for which pollination systems are known (as of 2009 - see Peter & Johnson 2009; also Gamisch et al. 2014, 2015). The bee-mimic Ophrys apifera may self-pollinate if not visited by bees; there the pollinia curve downwards and meet the stigma. There are other selfing mechanisms, too, as in Paphiopedium parishii where the contents of the anther liquefy and slop on to the stigma (L.-J. Chen et al. 2013). In Madagascan Bulbophyllum selfing varies infraspecifically and is associated with the loss of the rostellum, as it often is elsewhere in the family (Gamisch et al. 2014; see also e.g. Peter & Johnson 2009). For selfing in Angraecum, normally pollinated by sphingids, from the Mascarenes, an area where there are no sphingid pollinators, see Michenau et al. (2014: many other orchids from these islands also self-pollinate, see references).
The seeds are generally minute and the exotesta alone commonly persists (wall thickenings are notably variable), the endotesta degenerating, although in some taxa it does persist as a layer (= the carapace) around much of the embryo and the endotesta shows metabolic activity (Yeung 2017); note that taxa with well-developed suspensors lack carapaces (Y.-I Lee & Yeung 2023). Seeds with such a protective carapace are quite common in temperate orchids (Lee & Yeung 2023), although this was not mentioned for the species studied by Ortúñez et al. (2025). Interestingly, the ovule is initially surrounded by the inner integument, the outer integument developing notably later than the inner (Lee & Yeung 2023). Notably distinctive seeds are to be found in Selenipedium (Cypripedioideae) where there is a hard, dark testa, although apparently it lacks phytomelan, while seeds of Vanilloideae are notably variable in testa morphology (Cameron & Chase 1998). Seeds with quite well developed, fleshy coats are found in a few taxa scattered throughout the family, although more common in basal clades (Lee & Yeung 2023). Taxa with baccate fruits have rather larger seeds and the walls of the seed coat are isodiametric and quite heavily thickened.
Although nearly all orchids do have very small seeds, particularly when compared with those of their immediate relatives (Moles et al. 2005a), these seeds show a considerable amount of variation as a glance at general seed surveys makes abundantly clear (see e.g. Beer 1863; Barthlott & Ziegler 1981; Barthlott et al. 2014; Collier et al. 2023), and there may be variation even in quite closely-related taxa (e.g. Chase & Pippen 1988, 1990). Orchid seeds are often produced in huge numbers, up to 4,000,000 seeds per fruit or 74,000,000 seeds per plant, and they may be as small as 150 µm or less long (Arditti & Ghani 2000; Yam et al. 2009). The seed coat of orchids is usually largely exotestal (e.g. Moles et al. 2005a). After the delay between pollination and fertilization (see above), there may nevertheless still be a month or more before embryo development begins, as in Sarcanthinae (= Vandeae-Aeridinae), delays of a fortnight or more being recorded in other Epidendroideae such as Malaxideae and Cymbidieae (Swamy 1949b; Wirth & Withner 1959 for references); in Orchidoideae any delay was only a couple of days or so. Seeds of Orchidaceae usually lack endosperm; Swamy (1949b; see also Yeung 2017) noted that Vanilla might have a 10-celled endosperm, that of Cypripedium had 2-4 cells and of Chamorchis (Orchideae) 2 cells; this endosperm, insofar as it exists, is nuclear. The embryo is small, generally less than 700 cells in size, and at most with a poorly-developed cotyledon (Yeung 2017). Seeds of Eulophia cristata are less than 250 μm long, and there does not seem to be room for much of anything inside (Chase & Pippen 1990).
Much of the seed, small as it is, is in fact empty ("air space"), estimates of this space being (16 - Pogonia-)43-93(-98 - Gymnadenia)%, and so most orchid seeds are well suited for wind dispersal, trvaelling maybe up to 2,000 km (Arditti & Ghani 2000 - but see below). Fan et al. (2019, see also Ortúñez et al. 2025) found that air spaces tended to be larger in terrestrial than in epiphytic orchids (terrestrial orchids including ex-epiphytic orchids that had become secondarily terrestrial), seeds with large air spaces tending to fall more slowly, so enhancing their dispersal, perhaps particularly important in terrestrial orchids where seeds are dispersed relatively close to the ground. Despite their small size, the seed coats of these dust seeds show quite a lot of variation in shape, pattern of wall thickening, etc., thickening on the inner periclinal walls being better developed in terrestrial than epiphytic orchids (Ortúñez et al. 2025).
It has been known for about 200 years that some Orchidaceae have hairs on the endocarp (intra-ovarian trichomes), but this has hardly been common knowledge (they are not mentioned by Rudall et al. 1998c, and I did not realize their importance until 2021...). These trichomes are quite commonly found in Epidendroideae, and they may function as elaters and aid in seed dispersal (Kodahl et al. 2015). Swamy (1949b) mentions such hygroscopic hairs as being common in Sarcanthinae (= Vandeae-Aeridinae); they might be 2 cm long. These elater-trichomes, usually attached on the ovary wall between the placentae but on occasion on the placental stalks, are unicellular, with a swollen foot that has perforations (sometimes also found elsewhere on the hair), and they twist when humidity changes (Gamarra & Ortúñez 2021); in taxa like Maxillaria nardoides the hairs along with entangled seeds are extruded from the opening capsule as a capillitium, and this can form a ribbon some 6.5 cm long (Blanco et al. 2006). Overall, elater-trichomes are particularly common in epiphytic Epidendroideae, being found in numerous epiphytic genera placed in the last four of the tribes in the subfamily mentioned above, for instance, they were found in all Vandeae studied (Gamarra & Ortúñez 2021). Horowitz (1902) and Rasmussen and Johansen (2006) and others also mention such hairs, the latter noting that the terrestrial Prasophyllum (Orchidoideae-Diurideae - one species is called "the little laughing leek orchid") also had such hairs; this should be confirmed. Some genera previously thought to belong to Epidendroideae-Podochileae (Hallé 1986) that have these hairs are now included in Epidendreae. There are hook-like structures or capitate protrusions on the seeds of Cymbidieae, especially Oncidiiinae, that perhaps help in the attachment of the seeds to trees, or slow the fall of the seeds (Gamarra et al. 2018).
Other orchids with rather different kinds of seeds not surprisingly include the subterrananean holomycoheterotroph Rhizanthella (Orchidoideae-Diurideae) has baccate fruits with relatively few, large, crustose seeds (Weston et al. 2011), and in another holomycoheterotroph, Yoania amagiensis (Epidendoideaeae-Epidendreae), rather similar seeds are dispersed by camel crickets (Rhaphidophoridae: Tachycines elegantissima) (Suetsugu et al. 2017); in general, Orchidaceae with fleshy fruits have crustose seeds (Pansarin 2021a; Y. Zhang et al. 2021). Members of the two basal orchidaceous clades also often have distinctive seeds. Those of Apostasioideae are sometimes rather larger than seeds of other orchids: they have a lignified exotesta, the fruits are fleshy and dispersal is via endozoochory. The seeds of Apostasia nipponica are dispersed by crickets and camel crickets (Suetsugu 2020b, q.v. for other records) while those of Neuwiedia singapureana are eaten by birds, and after passage through their guts the germination percentage increased (Y. Zhang et al. 2021). (Interestingly, N. veratrifolia has dry capsular fruits with linear seeds that are about nine times longer than the ovoid seeds of N. singapureana, but the latter have over 25 times the volume - Zhang et al. 2021). In Vanilleae in general flattened seeds with circumferential wings are common, although Lecanorchis has linear seeds (Barthlott et al. 2014). In Vanilla imperialis a white foamy substance exudes from the fruit, carrying the seeds along with it (Kodahl et al. 2015), while Rodolphe et al. (2011) suggested that the unwinged seeds of Vanilla that have quite thick-walled cells might be dispersed by euglossine bees; the genus has resiniferous papillae on the inside of the ovary, perhaps a reward for the bee (Rasmussen & Johansen 2006). Pansarin (2021a: experimental conditions) found that birds ate the seeds of six species of Vanilla, and that after scarification of the seeds germination began in three rather than nine months. Cyrtosia, also Vanilloideae, has similar seeds (seeds of C. javanica are ca 6 mm long, the testa is 4 cell layers across, and the exotesta is lignified, with inverted U-shaped thickenings), and is dispersed by birds and perhaps also bats (C.-K. Yang & Lee 2014; Suetsugu et al. 2015). Pansarin and Suetsugu (2022) noticed that during the night the fruits of V. bahiana were visited by rodents and marsupials - hence, perhaps, the aromatic odour - and during the day by birds; they ate the sugar-rich inner part of the pericarp, placentae, etc.. Lubinsky et al. (2006) had noted that a number of genera in the three basal clades of orchids had aromatic fruits and suggested that they might be dispersed by orchid bees, and that this might even be the ancestrral condition for the family.
Nakanishi (2022) surveyed the seeds of orchids from the warm temperate region of Japan and noted correlations between seed morphology and the habits/habitats of the orchids. Saprophytes (= holomycoheterotrphs) had very long seeds up to 4.39 mm long (Lecanorchis japonicus), the tails being very narrow and the embryos tending to be very small, to a mere 0.07 mm long (Epipogium roseum). Evergreen orchids had similar seeds but with somewhat shorter tails, while summer-green terrestrial orchids had still shorter tails, although here they were often rather broad. Finally, epiphytic orchids had very short or no tails and relatively large embryos, up to 0.42 mm long (Dendrobium catenatum). Nakanishi (2022) noted that members of this last group grew high up in trees, obviously an advantage for wind dispersal, and in many species the capsules had hygroscopic trichomes (see above), but not, he thought, Dendrobium or Bulbophyllum. Note than embryo length is unambiguous when the embryo is more or less ellipsoid, but in some taxa illustrated by Nakanishi (2022) the embryos had relatively long and more or less linear protrusions; depending on one's goals, these can perhaps be ignored. Papers like this need to be integrated with those of e.g. Arditti and Ghani (2000) and Barthlott et al. (2014). Indeed, Barthlott et al. (2014) optimized some of the variation they observed on phylogenies of the family, noting that a number of the characters they described were constant at about the tribal level. Rewicz et al. (2022) looked at seed morphology in some 111 species of Habenaria and relatives; that genus was hopelessly paraphyletic in morphological analyses, and UPGMA did not support the division of Orchideae into two subtribes. However, further integration of seed characters with phylogeny will be of interest, and more is to be said about seed morphology and the ecology of seed dispersal; see also Vij et al. (1992) for seed morphology, orcid relationshiups, ecology, etc..
Plant-Animal Interactions. Orchidaceae are not often eaten by caterpillars (Janz & Nylin 1998) or by insect herbivores in general, although Riodininae-Riodininae larvae may be found on them (Hall 2003 and references).
Coryanthes - the individuals are rather short-lived - is always to be found growing in ant gardens, and the plant has numerous extrafloral necatries, even on the flower buds. However, details of how the association develops are unclear (Gerlach 2017).
For pyrrolizidine alkaloids, scattered here, and homospermidine synthase (HSS), an early gene in the pathway that produces them that has evolved in the same way in other pyrrolizidine alkaloid-producing plants, see Nurhayati et al. (2009), Langel et al. (2010) and Livschulz et al. (2018a). The HSS gene has diverged considerably from the deoxyhypusine gene, from which it is derived, and Nurhayati et al. (2009) suggested that this was evidence of an ancient separation, although they were not sure where/when this happened. Pyrrolizidine alkaloids occur in genera like Phalaenopsis and Pleurothallis (see also Nurhayati et al. 2009) and are known to be involved in the defence of plants against herbivory, but the alkaloids are also sequestered by some herbivores for their own defence.
Plant-Bacterial/Fungal Associations. Orchids characteristically have very close associations with basidiomycete and some ascomycete fungi, but not with glomeromycotes (Imhof et al. 2013). Yukawa et al. (2009) suggested that Cantharellales (basidiomycetes) may have been the fungi first associated with Orchidaceae. The families of fungi involved with orchids pretty much throughout the family are Tulasnellaceae, perhaps the most important (Martos et al. 2012), Ceratobasidiaceae and Serendipitaceae (see Currah et al. 1997 and Yukawa et al. 2009 for lists of fungi; Otero et al. 2002; Roy & Selosse 2009; Weiß et al. 2009, 2013). Some Neotropical Epidendroideae have the basidiomycete Atractiellomycetes (in the same clade as Puccinia) as mycobionts (Kottke et al. 2010), and Sebacinaceae and ECM basidiomycetes are other partners (Weiß et al. 2016).
Rinaldi et al. (2008) thought that only 10 species of fungi might be involved in fungus-orchid associations. However, the number is far greater, even on a single species of orchid and/or orchids in a relatively small area (e.g. Martos et al. 2012; Jacquemyn et al. 2013), while Duffy et al. (2019) found 75 species of orchid mycorrhizal fungi associated with Spiranthes spiralis sampled from a number of locations along a transect over 3,000 km long, diversity decreasing with increasing latitude. Overall, van der Heijden et al. (2015a) estimated that about 25,000 species of basidiomycete fungi alone were involved in associations with orchids, as many as in the mycorrhizal associations of all other embryophytes combined.
Chomicki et al. (2014b) discussed the association of the root-photosynthetic and epiphytic orchid Dendrophylax with endomycorrhizal fungi. For more on plant-fungal relationships, see above, also Jacquemyn and Merckx (2019), etc..
The ECM basidiomycete Tulasnella, also found in Cypripedium, etc., is involved in associations with Apostasioideae; other fungi are in Ceratobasidiaceae (Kristiansen et al. 2001, 2004: Neuwiedia; see also Roche et al. 2010). Ceratobasidiaceae associated with Apostasia (Yukawa et al. 2009) are found in the stomatiferous root tubercules that occur there (but not in Neuwiedia). The stomata are permanently open, the tubercles lack a velamen and exodermis and may make the plant better able to deal with the wet conditions in which it often grows (Stern & Warcup 1994, q.v. for other distinctive anatomical features of these tubercles, including a stele that is 3-6-arch rather than 18-34-arch).
For endophytic fungi, see Bayman and Otero (2006). Very little is known about them, and the one fungus may even be both pathogen, endophyte, and mycorrhizal symbiont.
Vegetative Variation. Orchidaceae show considerable diversity in habit and other vegetative features despite their generally modest size. However, some Sobralieae are slender, cane-like plants up to about 10 m tall, while viny Vanilloideae, including the mycoheterotrophic Galeola [= Erthyrochis] altissima, may be several metres long. The geophytic habit is quite common in Orchidoideae in particular. Stems, leaves and even roots may be succulent (Nyffeler and Eggli 2010b; also Figueroa et al. 2008: see above). Leaves are variously arranged (but not opposite), and may be terete or isobifacial, while individual leaf blades of taxa like Bulbophyllum fletcherianum may be up to 2 m long, some 15 cm across and about 5 mm thick (B. minutissimum, on the other hand, is 3-4 mm tall). Tatarenko (2007) summarized the extensive vegetative variation of temperate orchids, i.e. especially Orchidoideae.
Orchidaceae are one of the few non-commelinid clades with SiO2 bodies, and here they are borne in cells immediately adjacent to sclerenchymatous tissue, here in bundle sheath cells (e.g. Prychid et al. 2003b); the silica bodies show some variation in form, e.g. conical or spherical, and are not infrequently absent (Møller & Rasmussen 1984; Freudenstein & Rasmussen 1999). Indeed, silica deposition has been observed only in members of Apostasioideae and Epidendroideae, although not much is known about the latter group, see Rudall et al. (2025); ferulic acid associated with unlignified cell walls seems to be unknown from the family (c.f. commelinids).
The roots of mature plants of "leafless" Epidendroideae-Vandeae and those of many other Epidendroideae appear at first sight to lack root hairs, although they may develop on the side of the root facing the substrate (von Guttenberg 1968; Pridgeon 1987). Bernal et al. ((2015) report distinctive spiral thickenings on root hairs of some Orchidoideae-Spiranthinae, the hairs being described as a continuation of the velamen, and they are interspersed among other root hairs - which also look rather distinctive. The vegetative body of a number of epiphytic Epidendroideae-Vandeae consists largely of photosynthetic roots. These roots may be stout (ca 5 mm across) and terete, as in Dendrophylax, while those of the aptly named Taeniophyllum are distinctively flattened (e.g. Carlsward et al. 2006b: see also above). Leafless Vandeae have aeration units, distinctive exodermal cells, a space beneath, and a pair of thin-walled cortical cells in their roots; such aeration units are also found in related leafy Vandeae (Benzing et al. 1983; Carlsward et al. 2006a, b). Inflorescences and flowers of these plants are more normal, although the bracts would seem to carry out little in the way of photosynthesis. Moreira and Isaias (2008) found that the terrestrial orchids they examined had thicker roots than did the epiphytic orchids, but the latter do often have very thick roots as can readily be seen when walking around the orchid houses at MoBot. In epiphytic orchids in particular, but also in some terrestrial orchids growing in seasonal climates (see Umata et al. 2022), a velamen, made up of dead cells with spiral thickenings on the cell walls, is well developed around the outside of the root (von Guttenberg 1968; see below). Porembski and Barthlott (1988) describe the root velamen and its systematic significance; there is some doubt as to whether or not Apostasioideae have a velamen, and here I follow Pridgeon (1987). Furthermore, Porembski and Barthlott (1988) noted that all Apostasioideae, also a number of Orchidoideae, Cypripedioideae, etc., had a rhizodermis, but Pridgeon (1987) scored only the single Apostasioideae he examined and no other orchid as having a simple epidermis. Rodrigues et al. (2021) looked at a number of features of root anatomy in Pleurothallidinae in the context of a phylogeny of the Brazilian species, similarly, Bonfante et al. (2024) examined various aspects of the vegetative anatomy of 57 species of Pabstiella, common in the Atlantic Forest, amd thought of the variation of 35 characters in the context of the relationships suggested by Morales et al. (2020). There does indeed seem to be some phylogenetic signal in a number of these characters: velamen 2-/3 or more-layered (also details of the thickening patterns on velamen/epi-/endovelamen cell walls), tilosome [lignification of the inner periclinal wall of the endovelamen next to a passage cell in the exodermis] presence/absence; there are different kinds of tilosomes, and they may have complex often lignified excrescences developing from the wall; variation in foliar characters may also be useful (Pridgeon 1982: 200 spp., 22 genera, 4-20 protoxylem poles, endodermis thickening various, etc., 1987; Pridgeon et al. 1983; Porembski & Barthlott 1988). Thickenings of the walls of the exodermis may be O- or U-shaped or none, and the parenchyma may have amyloplasts and/or there may be sclerenchyma in the pith (Rodrigues et al. 2021). See also Pridgeon (2014) for more on the root anatomy of Orchidaceae; I have not done justice to the variation here. Pneumathodes are common in Epidendroideae. Roots of New World epiphytic Epidendroideae in particular have distinctive tilosomes, cells of the innermost layer of the velamen that are adjacent to the passage cells of the exodermis. However, such cells are also to be found in ground-dwelling Orchidoideae-Spiranthinae (Figueroa et al. 2008) and their exact function is unclear. Betekhtina et al. (2023) noted that the roots of orchids from the Middle Urals that they examined (Cypripedioideae, Orchidoideae?) were thick, unbranched and had few, long root hairs - c.f. Cyperaceae, Iridaceae, Poaceae.
The vernation of orchid leaves varies, being flat to plicate. The blade may be quite thin to very thick, bifacial, isobifacial or unifacial (terete), and the leaf base is sometimes massively swollen (Bulbophyllum). Leaves may be spirally arranged to 2-ranked, and are sometimes deciduous, being articulated with the sheathing base (common in Epidendroideae). Extrafloral nectaries are scattered, for instance being found on the stems opposite the leaves in Vanilla and at the bases of the pedicels in Cymbidium. Some ground- and shade-dwelling species have distinctively-coloured and -patterned leaves - variegated, purple-mottled, two surfaces of different colours (e.g. Bone et al. 2015b: variegation = absence of chlorophyll?) - that makes them particularly attractive to horticulturists. Thus Anoectochilus (Epidendroideae-Cranchideae) is a plant growing in deep shade, and its veins make a conspicuous whitish reticulum (sometimes there is a central white stripe) that contrasts with rest of the dark greenish blade - similar features are common in other plants from this habitat.
Possible Syntheses. We can now return to the question, Why are there so many species of orchids? Gravendeel et al. (2004 and references; see also Peakall 2007) list the numerous hypotheses that have been advanced to explain the diversity of Orchidaceae, not all mutually exclusive. These include pollinator specialization, niche partitioning, habitat fragmentation, and wide dispersal of the seeds. A genome duplication shared by all orchids has been implicated in the evolution of many of the adaptive traits found in the family (Unruh et al. 2016), thus G.-Q. Zhang et al. (2017) thought that the unbranched aerial roots of epiphytic orchids with their velamen, the absence of endosperm, and labellum and pollinium evolution could all perhaps be linked to particular gains and losses in the orchid genome when compared with that of other monocots. Note that some of the distinctive features of the family seem to be biologically connected, for example, pollinia ensure the fertilization of numerous ovules, the minute seeds that result are usually devoid of endosperm or a differentiated embryo, and the holomycoheterotrophy of the young plant may compensate for the absence of seed reserves and undifferentiated embryo (Johnson & Edwards 2000 in part; Eriksson & Kainulainen 2011).
As noted above, diversification may have increased in Orchidaceae (108.8-)73.1(-59.7) Ma (Magallón et al. 2018), and the family is distinctive in several ways, of which their flowers and fruits - inverted/resupinate, monosymmetric flowers with a column and labellum; minute, apparently undifferentiated endospermless seeds; and the formation of an echlorophyllous protocorm associated with fungi on germination - being just some. However, these are pretty much common throughout the family and are unlikely to be the immediate causes of major diversification given the sizes of the basal clades (see next paragraph), although they form the basis of the subsequent radiation of the family (Givnish et al. 2015). Vegetative and physiological variation, more or less associated with habit and habitat, is almost equally striking, and features of the core Epidendroideae such as shifts to the epiphytic habit and perhaps the adoption of CAM photosynthesis (see above, are as likely to have been as important in orchid diversification as anything else (Gravendeel et al. 2004; Givnish et al. 2015) - however, A.-Q. Hu et al. (2022) specifically dismiss the notion that CAM might be a key innovation here. Indeed, one could argue that it is the highly speciose and commonly epiphytic (some CAM) core Epidendroideae with their eight or so tribes and around 18,850 species (figures from Pridgeon et al. 2005, 2009, 2014), about two thirds of the entire family, that are distinctive (e.g. Freudenstein & Chase 2015). The main clades of core Epidendroideae may have diverged from each other only 37.9-30.8 Ma (rather older in G.-Q. Zhang et al. 2017), and diversification rates for the clade as a whole and even more so for some clades within it are notably high (Givnish et al. 2015, 2016a: c.f. stem age of subfamily, 2018).
A number of other features, most not immediately associated with core Epidendroideae, may also have contributed to this increased diversification.
- Waterman et al. (2011) distinguish between speciation and coexistence in orchids, and note that shifts in details of pollination (placement of pollinaria, pollinating insect) occur with speciation of the orchid, although associations with different fungi may promote the coexistence/co-occurrence of immediately unrelated orchid species.
- A genome duplication is thought to have shortly preceded the origin of crown-group Orchidaceae ca 81 Ma (G.-Q. Zhang et al. 2017: Asparagus the only out-group, huge error bars), in any event, Zwaenepoel and Van de Peer (2020) question the existence of this event.
- Epidendroideae in particular are diverse in montane habitats in New Guinea, South America, etc., and the geography/topography of such areas may have facilitated speciation (Givnish et al. 2015; see also Rhododendron), and in both the Old (Bulbophyllinae) and New (Pleurothallidinae) Worlds there are very large clades in which fly/deceit pollination is prevalent (see above). Note, however, that differences in the topology when nuclear and chloroplast phylogenies are compared may affect where on the tree acquisition of deceit pollination is to be placed (Pérez-Escobar et al. 2020/2021). Givnish et al. (2016a) discuss the biogeography and diversification rates of the family in some detail, emphasizing the role that long distance dispersal has played, and also the importance of the evolution of pollinia, the epiphytic habit and invasion of the northern Andes by the pleurothallid orchids. Indeed, the Andean uplift and current great Andean diversity stand in a similar relationship to Neotropical diversity as a whole - no uplift, no exceptional Neotropical diversity (Gentry 1982).
- Cozzolino and Widmer (2005; see also Schiestl 2005; S. D. Johnson & Schiestl 2016) suggested that orchid diversification is associated with the mimicry/deceptive pollination mechanisms that are so common in the family.
But to return to a point made earlier, looking at the numbers of species of orchids and of particular clades within the family is just one way to approach the problem. One cannot think about orchid diversification without also thinking about the evolution of the whole [Asparagales + commelinid] clade. Putting the numbers above in this broader context allows an argument to be made that orchids are rather less diverse than advertised. Orchidaceae, with around 26,085 species, are indeed a very speciose family, but of course the family rank is meaningless in such comparisons. However, sister-group relationships within the [Asparagales + commelinid] clade immediately allow one to see the problem in a somewhat different light. Orchidaceae are sister to the rest of Asparagales, which include ca 7,100 species, far fewer than Orchidaceae but nevertheless an appreciable number (c.f. Sargent 2004: Orchidaceae compared with Hypoxidaceae - only 100-220 species!). Continuing the numbers game, Asparagales as a whole, with around 33,185 species, may be sister to commelinids, which have some 24,500 species - not that different, and the commelinids encompass a considerable amount of variation. And as we have seen, within Orchidaceae, clades of 14, 245, and 170 species are successively sister to the rest, so also suggesting a rather more nuanced story about diversification (Givnish et al. 2015; see also in part S. A. Smith et al. 2011; P. Soltis et al. 2019), and this would be further emphasized if Petrosaviales were to be sister to Orchidaceae (see H. T. Li et al. 2019) although this would seem to be unlikely, but if Liliales were sister to Asparagales (e.g. Timilsena et al. 2022a, b), which indeed sounds rather more likely... Aspects of the phylogenetic/diversity patterns of Orchidaceae with their distinctive flowers are rather similar to those of angiosperms as a whole with their distinctive flowers, and numbers alone may suggest no immediate connection between flowers and success.
Ackerman et al. (2023) return to the issue of orchid diversity focussing on floral variation and using a data base of orchid reproduction that includes over 2,900 species - ca 1/10th of the family, all but one of the tribes, somewhat over half the genera (much of the world poorly covered, especially Asia, Mexico southwards). Pollinator specificity is usually high - just one pollinator per species, although in temperate terrestial orchids with nectar as a reward, the figures may be higher - and they mention Neottia ovata with its some 136 species of visitors. Hymenoptera and diptera are probably equally prominent visitors, the latter, which include fungus gnats, being under-recorded, but they are important pollinators of species-rich clades like Bulbophyllum. The amount of self pollination is unclear - perhaps 3-19% (Ackerman et al. 2023).
Of course, Orchidaceae have long been recognised as a family because they are florally, at least, all rather similar in some ways, indeed, floral variation in the family at one level can be thought of as being a series of remarkably intricate variations on a rather limited theme (see also Mondragón-Palomino 2013 and below). Most species have but a single anther, a labellum, a very similar gynoecium, etc., with variation centred on the pollinaria and labellum, so another way of looking at the problem is to think of causes both for their conservatism and for the incredible elaboration of floral form (and also seed morphology) that they show. Other Asparagales, although they have in total only about one third the number of species as orchids, could perhaps be considered vegetatively and even florally more diverse, and that is why they have been placed in a number of families, and the same is even more true of the commelinids. Of course, it is very hard to make such comparisons, and Burleigh et al. (2006) suggest that by some measures Orchidaceae do show a notable increase in complexity. The bottom line is that answers to a question like "Are orchids particularly diverse, and, if so, why?", are not straightforward, but for some variations of this question, the answer is simply "no".
Genes & Genomes. For a possible genome duplication in the common ancestor of Orchidaceae, see G.-Q. Zhang et al. (2017, q.v. for the Apostasia shenzhenica genome) and references. The VAPLα event, some 119.7 Ma, can be linked with the [Vanilloideae [Cypripedioideae [Orchidoideae + Epidendroideae]]] clade (Landis et al. 2018: ?Cyp). See also Yuan et al. (2018) for duplications.
Chromosome number and size vary considerably. Thus Apostasioideae, Epidendroideae and Orchidoideae have small chromosomes, while larger chromosomes occur in Cypripedioideae and Vanilloideae. Felix and Guerra (2010) survey chromosome number variation in Epidendroideae and I. de Oliveira et al. (2015) that in Pleurothallidinae; Epidendreae as a whole may be x = 20. Farminhão et al. (2021) discuss the extensive variation in chromosome numbers in the Epidendroideae-Vandeae-Angraecum group where i.a. there seems to have been a shift n = 17, 18 → 25, that number being unique in the subfamily; there has been substantial ascending and descending dysploidy, etc., here although chromosome number changes are not associated with shifts in diversification rates.
For genome sizes, which vary 168-fold, see Chase et al. (2005: epiphytic Oncidiinae), Leitch et al. (2009), Jersáková et al. (2013) and Yin et al. (2016); Moraes et al. (2017)found that the largest genomes in Maxillariinae arise in different ways and tend to be associated with environments that are drier in one way or another.
The rate of molecular evolution in the plastome is notably high in Orchidaceae when compared with that of other Asparagales (Barrett et al. 2015b). MatK in Apostasioideae may be in transition from a possibly functional gene to a pseudogene; in other Orchidaceae examined (but the sampling is poor) it is a pseudogene (Kocyan et al. 2004). There have been three or four losses of nuclear ndh genes (present in Apostasioideae), and details of the loss of nuclear transcripts encoding NDH proteins vary (Ruhlman et al. 2015). ndh genes may also hve been lost in the nucleus in genera like Phalaenopsis and Dendrobium, and of course they are absent in heterotrophic taxa (see e.g. Barrett et al. 2014b; Lin et al. 2015, 2017). Y.-K. Kim et al. (2019) lookrd at plastomes across the family and found that all 11 ndh genes were lost/pseudogenized in the 10 species of Aeridinae they examined (and in a few other Epidendroideae) - see also McLay et al. (2023). Mower et al. (2021) suggested possible connections between various distinctive life styles that might affect the photosynthetic process and the loss of such genes.
Wicke et al. (2016), Graham et al. (2017), Lallemand et al. (2019b) and others have looked at the overall picture of changes in the plastid genome that are associated with the adoption of mixotrophy and mycoheterotrophy. The plastome of the subterranean mycoheterotroph Rhizanthella (Orchidoideae-Diuridae) is very small, about 59 kbp, but there is still a core group of functioning genes (Delannoy et al. 2011), and over two dozen functional genes were found in the still smaller genome of Epipogium, to 19 kbp (E. roseum), the particular genes that remained functional depended on which essential genes had moved to the nucleus, etc. (Schelkunov et al. 2015). Barrett and Davis (2012), Barrett et al. (2014a, b, 2018) and Z.-H. Li et al. (2020) discuss the gradual degeneration of the chloroplast genome in mycoheterotrophs in Calypsoinae with a focus on plastome degradation in Corallorhiza; there the amount of the plastome retained is about proportional to how much photosynthesis is going on in the plant and pseudogenization and gene loss in the chloroplast can be extensive. Thus Li et al. (2020) found that the plastome of Danxiaorchis zingchiana was reduced to 26/27 housekeeping genes and the three photosynthesis-related genes, while that of Risleya atropurpurea (Collabieae) had only 25 housekeeping genes; Barrett and Kennedy (2018) also discuss genome size in the parasite-autotroph continuum. In Hexalectris (Calypsoinae) there are four to five transitions to mycoheterotrophy and with (infraspecific) variation in genes lost, etc., and in one accession of H. arizonica the IR has been lost (Barrett et al. 2019b), as it has independently in Gastrodia longistyla-Gastrodieae (Q. Liu et al. 2021). McLay et al. (2023) oberved that the plastome was unchanged in the holomycoheterotroph Dipodium roseum and was similar to that of the autotroph D. ensifolium, although in both all ndh genes were lost (most) or changed. McLay et al. (2023) note similar cases where the stem may in fact be green - the beginning of holomycoheterotrophy? Several small inversions occur in the plastomes of Vandeae-Aeridinae (Y.-K. Kim et al. 2019). For more on plastome evolution, see also Dong et al. (2018) and Wicke and Naumann (2018).
Sinn and Barrett (2019) discuss mitochondrial gene transfer from a smut fungus, Ustilago, to orchids. There was a small-scale primary transfer probably in the ancestor of all orchids and also a secondary and far larger transfer that replaced the first; the latter is so far known only from Epidendroideae, although what is going on in Orchidoideae and Cypripedioideae is unknown (Sinn & Barrett 2019). Valencia-D. et al. (2023) examined these transfers in more detail, finding that the larger transfer, which included some 16 genes, was restricted to a speciose group of tribes in Epidendroideae where it had since become variously modified and on occasion lost; the place(s) of origin and fate of the smaller transfer was unclear. In Corallorhiza maculata, at least, fungal-derived tRNA mitochondrial genes may be transcribed, post-transcriptionally modified, and aminoacylated (Warren et al. 2025: there are also several genes of plastid and a couple of genes of bacterial origins in these genomes). H. Wang et al. (2025) looked at the mitogenome and its evolution in mycoheterotrophic species of Gastrodia, comparing it with mitogenomes of other mycoheterotrphic orchids and of parasitic plants.
Chemistry, Morphology, etc.. For lignin with catechyl units, see F. Chen et al. (2012).
It is noteworthy that raphides may be found in just about any tissue in Orchidaceae (Lawrie et al. 2023). Paphiopedilum, but not other Cypripedioideae, have no chloroplasts in their stomatal guard cells (D'Amelio & Zeiger 1988: other anatomical oddities in the family).
Cardoso-Gustavson et al. (2014; see also Bogarín et al. 2018) discuss the occurrence of bicellular hairs ("colleters") that often secrete mucilage in the flowers of Epidendroideae. They note that these may be on the outside of the flower, and suggest they may be connected with the extrafloral nectaries on the inflorescences of some Epidendroideae.
Incumbent anthers are recorded from many orchids, especially Epidendroideae, where such anthers are of two types. Anthers can be bent forwards by column elongation quite late in development, or by very early anther bending, the latter in vandoids; a third way in which anthers can become incumbent occurs in Vanilloideae, and there cells of the anther connective elongate considerably (e.g. Kurzweil 1987; Freudenstein et al. 2002; Valencia-Nieto et al. 2018). In Epidendreae, anthers became incumbent early in Calypsoinae, late in other subtribes. Anthers of some species appear to be bisporangiate in early development (Freudenstein & Rasmussen 1996). Far too little attention here has been paid to the diversity and evolution of pollinaria (made up of pollinia, a caudicle/stipes, and viscidium); although a distinction is often made between sectile or soft pollinaria and hard pollinaria, a variety of morphologies is included in "sectile pollinaria" in particular (Freudenstein & Rasmussen 1996, esp. 1997, see also Vermuelen 1965; Rasmussen 1982, 1985; Johnson & Edwards 2000; Freudenstein et al. 2002; Freudendtein & Chase 2015; Mosquera-Mosquera et al. 2019). At least some Orchidaceae have placentoids in the anther locului (Weberling 1989). For details of the pollen dispersal units in Orchidoideae and Epidendroideae, see Purgina et al. (2024a, b).
Prutch and Schill (2000) discuss variation in the morphology and ultrastructure of the stigma; variation seems to be at about the subfamilial level.
There has been some uncertainty over gynoecial construction. The capsule often has 6 valves, and authors like Vermuelen (1966) suggested that the gynoecium had six parts, the placentae being on the parts opposite the petals (see also the sterile valves of Mosquera-Mosquera et al. 2019). However, there are only three (or fewer) stigmatic lobes, and Kurzweil (1999), for example, thought that there were three carpels (indeed this is the likely condition), the outer three of the six valves being made up of the midveins of the sepals and surrounding tissue, the other three each consisting of two half carpels, the adjacent halves of neighbouring carpels, so with two "half placentae" in the middle. Rasmussen and Johansen (2006; see also Pramanik et al. 2023) suggested that these outer three valves represented tissue of the outer tepals/calyx, and this separated from the fruits/inner tepals when the capsule was mature, the outer valves alternating with the adjacent halves of neigbouring carpels just mentioned - the split-carpel theory. Dirks-Mulder et al. (2019: Fig. 1) examined the development of the fruits of a member of Orchidoideae and two of Epidendroideae in some detail, one species an epiphyte (Epidendroideae) and two terrestrial, and largely agreed with Robert Brown and Rasmussen and Johansen (2006). During development the part of the "fruit" that included the placentae developed more than the part that represented the outer tepals; abscission zones developed on both sides of the members of the latter. When the fruit was ripe, the three outer parts separated from the rest along the abscission lines, while the remainder formed three valves each with seeds down the middle; this is effectively a kind of loculicidal dehiscence (Dirks-Mulder et al. 2019 suggested that it was septifragal). Although most Orchidaceae have similar capsular fruits dehiscing laterally, epiphytic taxa tending to have thicker walls than terrestrial taxa, the fruits of Apostasioideae are variable, being either baccate or, basically, just falling to bits, but the fruits of Nieuwiedia veratrifolia are lateral-septicidal, the valves falling off, but rib-like structures in the position of the outer three valves just mentioned persist (see de Vogel 1969). Pramanik et al. (2023) noted that there was often a lipid layer in the fruit valves here (see also Dirks-Mulder et al. 2019), although a lignified inner layer (as in Arabidopsis thaliana) seems to have evolved more than once. I have placed this character as a family-level apomorphy, but like other complex characters, how things will be interpreted in a few years is unclear; Dirks-Mulder et al. (2019) outlined what was known about the genetic control of fruit development here, comparing it with that of some core eudicots - these have fairly conventional superior ovaries.
We turn now to aspects of embryo sac, embryo, endosperm and seed morphology and development. Vij and Sharma (1986) and Kodahl et al. (2015) discuss embryo sac formation (6-nucleate embryo sacs are common in the family), double fertilization (extent unclear) and endosperm development (or lack thereof - seeds normally lack endosperm). Swamy (1949a) noted that Polygonum-type embryo sacs might be modified in that the chalazal nucleus remained undivided, chalazal cells fuse, or two diploid nuclei result from spindle fusion, the end result commonly being a six-nucleate embryo sac. See also Wirth and Withner (1959), Abe (1972a, b) and Duarte et al. (2019 and references) for embryo sac development; infraspecific variation in endosperm "type" is quite common, and, aside from this, there is considerable variation in the number of nuclei in the embryo sac (Vij & Sharma 1986). Orchidaceae usually lack endosperm, although Clements (1999) was unclear exactly how common double fertilisation was. Alves et al. (2019) discuss aspects of ovule and seed morphology in holomycoheterotrophic taxa, esp. in the ategmic Pogoniopsis schenckii. Swamy (1949b) discussed embryogenesis, and in particular the formation of the suspensors. These may be absent (e.g. Spiranthes, Cyrtosia javanica), single-celled (Gastrodia) or uniseriate (see also C.-K. Yang & Lee 2014; Yeung 2022; Y.-I Lee & Yeung 2023, etc.), sometimes protruding into the micropyle.
For general information, the series of volumes edited by A. M. Pridgeon, P. J. Cribb, M. W. Chase and F. N. Rasmussen deserve special notice. For subfamilies and their characters, see Vermeulen (1966) and Dressler (1979), for Apostasioideae and Cypripedioideae, see Pridgeon et al. (1999), for Vanilloideae, Pridgeon et al. (2003), for Orchidoideae, Pridgeon et al. (2001b, 2003), and for Epidendroideae, see Pridgeon et al. (2005, 2009, 2014), and also Schlechter (1992, 1996, 2003), Dressler (1993), Szlachetko (1995) and Pridgeon (2014), and for Apostasioideae, see de Vogel (1969), Pridgeon et al. (1999), Stern et al. (1993: anatomy) and Y. Li et al. (2023), for Cypripedioideae, see Atwood (1984) and Koopowitz (2017: easy-to-read account of their evolution), for Epidendroideae-Malaxidinae, see Margonska et al. (2012), for terrestrial orchids, see Rasmussen (1999), for mycoheterotrophic taxa, see Merckx et al. (2013a), general, Imhof et al. (2013: roots, mycorrhizae), and Waterman et al. (2013: pollination), for an illustrated generic account, see Alrich and Higgins (2008), for Cattleya see articles in Renziana vol. 4 (2014) and for Dendrobium, see J. J. Wood (2014) and Schuiteman (2013). Also: Anatomy, general, see Stern (2014), for roots, see Moreira and Isaias (2008) and Siegel (2015), for Epidendroideae, see Stern et al. (2004), Stern and Carlsward (2006, 2009), and Morris et al. (1996: Dendrobium), Orchidoideae, see Stern (1997a, b), Stern et al. (1993b), and Andreota et al. (2015: Cranichideae), Smidt et al. (2013: New World Bulbophyllum) and Avi and Rodrigues (2018: Pleurothallidinae), Vanilloideae, see Cameron and Dickison (1998: reticulate leaf venation), and for Apostasioideae, see Stern et al. (1993a). See also Rasmussen (1987: stomata), Mayer et al. (2011: colleters, uncommon), Aybeke (2012: rhizome and roots of Orchidoideae and Epidendroideae, Limodorum rhizome with a ring of phloem surrounding a ring of xylem?), dos Santos et al. (2023), Cyrtopodium vgetative anatomy.
In addition, see Rojas-Alvarado and Karremans (2024: inflorescence morphology), Hirmer (1920), Kurzweil (esp. 1987a, b, 1988, 1993, 1998: useful summary), Endress (1994b), Kristiansen et al. (2001), Cameron (2002), Kocyan and Endress (2001a), Johansen and Frederiksen (2002), Kurzweil and Kocyan (2002), Box et al. (2008: paedomorphosis and its effect on development in Gymnadenia), Bateman et al. (2013), Royer et al. (2020: homologies in some complex Oncidiinae), Figueroa et al. (2021: Spiranthinae, gynostemium) and Y. Zhu et al. (2024: Paphiopedilum), all floral morphology and development, Bell et al. (2009: nectar spurs in Orchidoideae), Swamy (1948a: floral vasculature), Rao (1973: floral anatomy), Franken et al. (2016: osmophores), Valencia-Nieto et al. (2016: anther development, Epidendreae). For pollen morphology, see Newton and Williams (1978: Cypripedioideae, Apostasioideae), Schill and Pfeiffer (1977: general), Ackerman and Williams (1980: Neottieae), Zavada (1990 and references: general), P. Li et al. (2012: pollinia in Cypripedioideae), also Freudenstein (1991: endothecium), Clements (1995: embryology, etc.), Carlson (1945) and Yeung and Law (1997), embryology, Kurzweil (2000), Molvray et al. (2000), Cameron and Chase (2000), Szlachetko and Rutkowski (2000) and Szlachetko and Mytnik-Ejsmont (2009 and references), all gynostemium, the papers by Szlachetko alone involving ca 1,250 pages, Yeung (2005: embryogeny), Swamy (1949a: gametophytes, 1949b: embryogeny), Rasmussen and Johansen (2006: fruits), Gamarra et al. (2012: seed morphology and classification) and Nishimura and Tamura (1993: Apostasia seed coat).
Phylogeny. Cameron (2007) provided a summary of phylogenetic work on the family. With the advent of molecular studies, it was quite soon clear that the then current subfamilial limits were going to need adjustment (e.g. Kores et al. 1997). Most recently Sánchez et al. (2021) looked at variation in 78 plastid coding genes in 117 genera distributed between all subfamilies, 18 tribes and 28 subtribes; although around 67 genera are included in the nuclear analyses of W. R. Baker et al. (2021a: see Seed Plant Tree) less can be said about relationships, although there seem to be few if any major conflicts with the tree based on the plastome data just mentioned.
There was initially some uncertainty over the position of Cypripedioideae (e.g. Cameron 2004), and in some analyses they grouped (albeit weakly) with Vanilloideae (Freudenstein & Chase 2001) or were sister to Orchidaceae minus Apostasioideae, which might make sense if thinking about androecial evolution alone (Cameron et al. 1999: one gene, successive weighting). However, they are usually placed sister to Orchidaceae minus Apostasioideae and Vanilloideae (e.g. Kocyan et al. 2004; Cameron & Chase 2000; Cameron 2002, 2005b, 2006: two genes, in a basal tritomy with atp alone; Górniak et al. 2010: nuclear gene Xdh; Givnish et al. 2015; Y.-K. Kim et al. 2019: plastomes of 45 spp.; Serna-Sánchez et al. 2021: 78 plastome genes for 117 genera, all subfamilies, 18 tribes; etc.). This latter hypothesis, followed here, suggests that the monandrous condition may have evolved twice (see also Freudenstein et al. 2002, 2004). There are also suggestions that Codonorchis is sister to [Epidendroideae + Orchidoideae] (e.g. Clements et al. 2002) or basal in Orchidoideae (Cameron 2006: it has whorled leaves; c.f. Jones et al. 2002); the genus was sister to one of the two major clades that made up Orchidoideae in Givnish et al. (2015; see also Serna-Sánchez et al. 2021: sister to Orchideae). In an analysis of chloroplast genomes, Dong et al. (2018) suggested that Orchidoideae were paraphyletic, but genera like Listera and Epipactis which they included in their Orchidoideae are basal in Epidendroideae here. Perez-Escobar et al. (2020/2021: Angiosperms353 nuclear analyses) obtained the same relationships that are recognized above in their nuclear analyses (294 genes, 89 taxa); all subfamilies were monophyletic. The position of Vanilloideae seemed uncertain in the nuclear analysis of W. R. Baker et al. (2021a: see Seed Plant Tree); sister to Cypripedioideae perhaps remains a possibility, although this position was not recovered by Wong and Peakall (2022) in their phylotranscriptomic study. G. Zhang et al. (2023) looked at relationships throughout the family using 610 species - including representatives 0f 19/22 tribes and 44/51 subtribes - using 1450 low-copy nuclear genes that were variously analyzed; their focus was on Epidendroideae. Pérez-Escobar et al. (2023: 285 genera, 448-1921 spp., depending on analysis, Angiosperms353 probe set, 339 low-copy nuclear genes) carried out an extensive series of analyses on the family, although they warned against making any classificatory changes until sampling could be improved.
For phylogenies of Apostasioideae, see Kocyan et al. (2004) and Yin et al. (2016).
Relationships within Vanilloideae are now fairly well resolved (Cameron 2004, 2009; Cameron & Molina 2006; Pansarin et al. 2008; Cameron & van den Berg 2017). Included are Pogoniinae, Vanillinae, Galeolinae and Lecanorchidinae, the last two both mycoheterotrophs). Pansarin et al. (2012) evaluated the phylogeny of Pogonieae; Pogoniopsis is to be placed in Epidendroideae. Bouetard et al. (2010) provide a phylogeny for Vanilla; see also Pansarin and Menezes (2023), with a focus on Brazilian species.
For relationships in Cypripedioideae, including also a morphological survey, see Albert (1994). Commonly-retrieved relationships are [Cypripedium [Selenipedium [Paphiopedilum [Mexipedium + Phragmipedium]]]] (Guo et al. 2012; Koopowitz 2017). J.-h. Li et al. (2011) found that morphology sometimes had misled about relationships in Cypripedium, and there was some conflict over relationships suggested by plastid and ITS data (Szlachetko et al. 2021). This conflict was also very much evident in Lagou et al. (2024: 913 nuclear loci from orchid bait set Orchidaceae963 and transcriptomic data, 80 plastid loci - see Fig. 3A), who found rather little support for relationships along the backbone of the tree, although the sections themselves were monophyletic. Cypripedium irapeanum, of sect. Irapeana, the only Neotropical section in the genus, was sister to the rest, and there had been hybridization (maybe ten hybridization events, ibid. Fig. 3) and rapid radiation and consquentially incomplete lineage sorting (Lagou et al. 2024; see also M. Liao et al. 2024 for relationships). For a phylogeny of Paphiopedilum, see Chochai et al. (2012).
Orchidoideae include the erstwhile Spiranthoideae, the latter having incumbent anthers (as in Epidendroideae) with apical rostellar tissue; Spiranthes et al. are now placed in Cranichideae. Relationships within Orchidoideae are becoming fairly well resolved (e.g. Cameron 2004; see also Inda et al. 2010: cox1 intron; Givnish et al. 2015). Four tribes can perhaps be recognizrd [[Cranichideae + Diurideae] [Codonorchideae + Orchideae]]; Codonorchis is monotypic (Serna-Sánchez et al. 2021).
For the phylogeny of Cranichideae, see Salazar et al. (2003: monophyly and characters of subtribes), while Górniak et al. (2006). Smidt et al. (2021: 1 nuclear and 1 plastid marker) looked at relationships within Goodyerinae with a focus on New World taxa (Microchilus s.l.) - c.f. S.-P. Chen et al. (2019: 2 nuclear, 5 plastid markers) in part. Salazar et al. (2011a, 2016) discuss relationships in Spiranthinae, most frequent in the Neotropics; Salazar et al. (2018, see also 2019) found that Cotylolabium lutzii was sister to the rest of the subtribe. Cisternas et al. (2012) discuss relationships in the American Chloraeinae. Salazar et al. (2011a) examined relationships around Dichromanthus et al. where adaptation to bird pollination has occurred in parallel, confusing generic limits, while Dueck et al. (2014) focussed on Spiranthes and its distribution. For relationships in Pterostylis and relatives (Pterostylidinae), see Clements et al. (2011).
Clemens et al. (2002; see also Miller & Clements 2014; Weston et al. 2014) clarify relationships of Diurideae, a few of which are to be placed in Epidendroideae; for information about relationships in the speciose Caladenia, see Australian J. Bot. 57(4). 2009, also Brown and Brockman (2015) for literature.
For relationships in Orchideae, see Bateman et al. (1997), Pridgeon et al. 1997), Inda et al. (2012) and Ngugi et al. (2020: the big picture). Raskoti et al. (2016) exmined relationships in Herminium and surrounds and Le Péchon et al. (2020) those in Holothrix. For relationships within Orchidinae and Habenariinae, see Bateman et al. (2003), Habenaria was polyphyletic and Orchis triphyletic (the other bits go in Anacampseros and Neottia). In Asia Habenaria is diphyletic and Platanthera triphyletic (Jin et al. 2014), the former problem being confirmed by Jin et al. (2017) in their study of Orchidinae, however, New World species of Habenaria are monophyletic, although sectional limits need revision (Batista et al. 2013; Pedron et al. 2014). Y. Tang et al. (2015) found a certain amount of conflict between nuclear and chloroplast data, and current genera of East Asian Orchidinae did not map well onto the clades that they were finding. Bateman et al. (2018b) emphasized the conflict between the relationships suggested by molecules and morphology in the [Dactylorhiza + Gymnadenium] area; these genera are close and also hybridize to a certain extent. Satyrium is sister to other Orchidinae (Jin et al. 2017). For relationships in the African Disa (Disinae), see S. D. Johnson et al. (1998) and Bytebier et al. (2007), and for a phylogeny of Satyrium, see van der Niet and Linder (2008; Verboom et al. 2009). Prescottiinae s.l. have diversified at very high altitudes - up to 4,900 m - in the Andes (Álvarez-Molina & Cameron 2009).
Epidendroideae. Van den Berg (2005), Górniak et al. (2010), more especially Freudenstein and Chase (2015), Givnish et al. (2015) and Serna-Sánchez et al. (2021), Wong and Peakall (2022) and in particular G. Zhang et al. (2023) discuss general phylogenetic relationships in Epidendroideae. Support for branches along the spine of Epidendroideae initially were rather weak (e.g. Cameron et al. 1997; Pridgeon et al. 2001b; Cameron 2004), although less so in Givnish et al. (2016a). Relationships in Zhang et al. (2023) could be stronger, but resolution was good and their suggestions are followed below; tribes for the most part were well supported as being monophyletic. Neottieae, a clade of terrestrial orchids, were found to be sister to all other Epidendroideae (Rothacker & Freudenstein 2006; Freudenstein & Chase 2015; Y.-K. Kim et al. 2019). X.-G. Xiang et al. (2012; see also Freudenstein 2012) found at least three more clades, including Sobralia and Elleanthus, Nervilia, and Tropidia respectively, to be successively sister to the rest at the base of the "higher", "upper" or "core" epidendroids. These taxa were all in a single clade in some analyses of Freudenstein and Chase (2015), while Triphora formed another clade; Nervilieae and Tropidieae formed a single clade in Givnish et al. (2015), as did Triphoreae and Sobralieae. Perez-Escobar et al. (2020/2021: nuclear loci) found the relationships [Neottieae [Xerorchideae [Gastrodieae [Nervilieae (nodes up to here usually with rather poor support) [Tropidieae (the last two sister taxa?) ...]]]]], while relationships in Serna-Sánchez et al. (2021) are along the lines of [Sobralieae, Triphoreae, Tropideae, Nervilieae (the last three perhaps a clade) [Arethuseae ...]]. Basically, the polytomy in Freudenstein and Chase (2015) was persisting. Barrett et al. (2024: Fig. 8) compared relationhips in this area eith a focus on holomycoheterotropic taxa, and plastome data suggested to them that Triphoreae were triphyletic, etc., although support was low and in plastome analyses in particular, branches were very long, but as in other cases, plastome data seems not to be very helpful in establishing relationships, and heterotachy - lineage-specific evolutionary rate heterogeneity over time - might be a problem.
The basal taxa/clades in Epidendroideae often lack articulated leaves, their pollinia are sectile/mealy (e.g. Freudenstein & Rasmussen 1997, see also above; Pridgeon et al. 2005), many are ground-dwelling plants and so their roots have no velamen, and holomycoheterotrophy is quite common (Barrett et al. 2024); all told, they are morphologically and ecologically rather diffferent from the highly speciose core Epidendroideae. These Core Epidendroideae contain eight or so tribes (Freudenstein & Chase 2015; Givnish et al. 2015). In the plastome analysis of Y.-K. Kim et al. (2019: 30 species included), relationships recovered above the basal clades just mentioned were [Arethuseae [Malaxideae [Collabieae [Vandeae [Cymbidieae + Epidendreae]]]]], with strong support. Perez-Escobar et al. (2020/2021) obtained similar relationships in some of their nuclear analyses, although the relationships between the last three tribes were unclear and some genera there moved around - thus the positions of Coelia and Earina at the base of Epidendreae were uncertain, and in chloroplast analyses relationships between the last three subfamilies were [Epidendreae [Cymbidieae + Vandeae]]. In the chloroplast analyses of Serna-Sánchez et al. (2021) core epidendroid relationships are similar - [Arethuseae [Malaxideae [[Podochileae + Collabieae] [Epidendreae [Cymbidieae + Vandeae]]]]].
Arethuseae.W.-C. Huang et al. (2021: 6 chloroplast regions) looked more at relationships within Arethusinae, also characters (several) disinguishing major groups in Arethuseae. For diversification in Coelogyninae, see Gravendeel et al. (2005), Freudenstein et al. (2017), Z.-H. Li et al. (2020) and others. Coelogyne is not monophyletic (e.g. Gravendeel et al. 2001; Chase et al. 2021b and references). Indeed, Coelogyne and genera segregated from it form an in places poorly-supported paraphyletic group within which a monophyletic Dendrochilum is embedded.
Collabieae. For relationships in the Calanthe group, with plicate leaves and also largely terrestrial, see Zhai et al. (2014). X.-G. Xiang (2014b: 4 plastome genes, support not always that strong) found that the epiphytic Eriodes was sister to the rest of the tribe, which was terrestrial; they preferred to delimit Calanthe broadly. P. Zhou et al. (2023: 24 plastid, 2 nuclear markers) looked at 21 (out of 24) genera and 127 species.
Cymbidieae. For studies here, see M.-H. Li et al. (2016: general, relationships [Cymbidiinae [Cyrtopodiinae + The Rest]], support in the middle part of the tree rather weak), and Serna-Sánchez et al. (2021). Cieslicka (2006), Martos et al. (2014) and Bone et al. (2015a, b), all looked at Eulophia and its relatives (Eulophiinae) - see Chase et al. (2021a for a resolution. Yukawa et al. (2002) examined relationships in Cymbidium; the infrageneric classification here did not hold up in the analyses of G.-Q. Zhang et al. (2021: 7 plastid genes and nrITS), furthermore, the topologies yielded by analyses of the nuclear and plastid genes differed, possibly reflecting past hybridization. Several papers, e.g. Chase (1987), Chase and Palmer (1997), N. H. Williams et al. (2001a, b, 2005), Chase et al. (2009) and especially Neubig et al. (2012a), have had a focus on Oncidiinae and the polyphyletic Oncidium, while Szlachetko et al. (2019) suggest that Wulfenia should be dismembered. For studies around Maxillaria, see Whitten et al. (2000), N. H. Williams and Whitten (2003), Sitko et al. (2006), Arévalo and Cameron (2013), and especially Whitten et al. (2007: Maxillaria to be restricted, generic realignments needed - for which, see Blanco et al. 2007). For relationships in Catasetinae, see Pérez-Escobar et al. (2015, 2017b). Mauad et al. (2022: 2 nuclear, 4 plastid markers) looked at Catasetum and found that infrageneric groupings there, based in large part on variation in the antennae (the triggers for pollen release), did not hold. Whitten et al. (2005) and Neubig et al. (2008: esp. Dichaea) looked at relationships in Zygopetalinae.
For a major study of Epidendreae-Pleurothallidinae, see Pridgeon et al. (2001b); Pleurothallis is not monophyletic (also Chiron et al. 2012: focus on Brazilian species) nor is Trichosalpinx (Bogarín et al. 2018). In an earlier morphological study, Freudenstein (1994) founf that 8/10 genera of Calypsoinae included formed a monophyletic group, the other two, although sister taxa, were not iummediately related. Serna-Sánchez et al. (2021: plastome data) also discuss relationships in Pleurothallidinae in some detail. Abele et al. (2005) and Matuszkiewicz and Tukallo (2006) discussed the phylogeny of Masdevallia, Karremans et al. (2012) examined relationships in Stelis, which has been extended to include "a few hundred" species of Pleurothallis (see Karremans 2019), while Karremans et al. (2016a) looked at Specklinia and its immediate relatives. Morales et al. (2020) looked at 58 species of Pabstiella (nrITS and 2 plastid markers) with a focus on its diversification in the Atlantic Forest; there were no strong incongruencies in the trees based on plastid and nuclear data. For phylogenetic relationships in Laeliinae, see van den Berg et al. (2000); Vieira et al. (2024: 80/120 spp., 3 chloroplast, 1 nuclear markers) looked at relationships within Prosthechea. Encyclia was studied by Leopardi-Verde et al. (2016), who found conflict between phylogenetic signals from ITS and chloroplast genes. Nuclear and plastid DNA also give conflicting signals in Cattleya (van den Berg 2015; see also Renziana 4. 2014); Gustafsson et al. (2010: 40/41 spp., nrITS) obtained good resolution in their study of Hoffmannseggella (= Cattleya). Epidendrinae: Pinheiro and Cozzolino (2013) summarize what is known about the phylogeny, etc., of Epidendrum itself; see also E. Hágsater and M. A. Soto Arenas in Pridgeon et al. (2005: Fig. 301:2). Sosa et al. (2016; see also Sosa 2007) discuss relationships within the terrestrial (and some mycoheterorophic) Bletiinae. Calypsoinae are another group that includes largely temperate and terrestrial taxa (see Freudenstein et al. 2017 for a phylogeny), and Zhang et al. (2013) discuss relationships there; Corallorhiza and Oreorchis are interdigitated (Li et al. 2020). For relationships around Hexalectris, see Sosa et al. (2016) and Barrett et al. (2019b). Pedersen et al. (2020) discuss relationships within Dendrochilum, where they found that D. pallidiflavens was sister to the rest of the genus. For Epidendreae see also Kulak et al. (2006).
Malaxis and Liparis (Malaxideae-Malaxidinae), both with terrestrial species in temperate as well as tropical regions, may not be monophyletic, but are closely intertwined (e.g. Cameron 2005a; Margonska et al. 2012; L. Li et al. 2020; Kumar et al. 2022: esp. evolution of corm-like structures). Malaxis is to include the recently-described Pycnantha (Pycnanthaceae) (Nicola 2012). Within Dendrobiinae, the speciose Dendrobium and many of its sections are turning out to be polyphyletic (Yukawa & Uehara 1996: vegetatively variable, florally perhaps less so; Yukawa et al. 1993, 1996, 2000; Clements 2003: ITS study, see also earlier work; Adams 2011: esp. Australian taxa; X.-G. Xiang et al. 2013); Xiang et al. (2016) recovered a split between an Australasian clade and an Asian-Malesian clade. For studies on Bulbophyllum, see Gravendeel et al. (2004), Smidt et al. (2011) and B. Gravendeel, E. de C. Smidt, G. Fischer & J. J. Vermuelen in Pridgeon et al. (2014); Fischer et al. (2007) and Gamisch et al. (2015) studied the Madagascan species, Hosseini et al. (2016) those from Peninsula Malaya and A.-Q. Hu et al. (2019, 2022) taxa of the Cirrhopetalum alliance with their subumbellate inflorescences and twisted and more or less connate lateral outer tepals. Gamisch and Comes (2019: ITS) provided an outline phylogeny of the whole genus, although the very speciose Asian-Pacific clade remains rather poorly sampled. However, this omission was partly rectified by L. Simpson et al. (2022/2024: 89 samples, 70 plastid coding regions, nuclear rDNA cistron) who found the largely Australian [sects Adelopetalum + Minutissimum s. str.] to be sister to the rest of the genus; ten of the sections they included were not monophyletic. The focus in Thawara et al. (2024: 196 spp., nuclear ITS and 2 plastid loci) was on the Asian species; there was a subbasal decatomy in the Bayesian inference tree, and the topology there was notably different from that in the maximum likelihood tree, the latter being used in character state reconstructions, probably because it had fewer polytomies.
Relationships in Neottieae have been much studied. Palmorchis is sister to other members of the tribe (T. Zhou & Jin 2018; Lallemand et al. 2019b). However, relationships between these other genera are rather unclear, branch lengths being very short (Feng et al. 2016; c.f. Lallemand et al. 2019b and references), although the genera do appear to be monophyletic.
Within Podochileae Y. P. Ng et al. (2018: nuclear ITS and 4 plastome regions) concentrated on Eriinae. Eria itself was very much polyphyletic, and they found six main clades, of which the monotypic Ridleyella might be sister to the rest of the tribe - or belong elsewhere.
For Sobralieae, see Neubig et al. (2011).
Vandeae. Russell et al. (2010) discuss phylogenetic relationships in the widely-distributed Polystachya (Polystachyineae: monopodial), where there is some correlation of polyploidy with broad species distributions. Topik et al. (2005) investigated relationships in Aeridinae; characters conventionally used to establish relationships showed little congruence with the tree they obtained (see also Hidayat et al. 2005; Zou et al. 2015). Y. Li et al. (2022) looked at the phylogeny of Gastrochilus in the context of rainfall changes, etc.. For relationships in and around Vanda itself, see Gardiner et al. (2013). For angraecoid orchids (Angraecinae) in general, see Stewart et al. (2006) and in particular Farminhão et al. (2020). Szlachetko et al. (2013: ITS), also Micheneau et al. (2008) and Andriananjamanantsoa et al. (2016), both with a focus on Madagascan taxa, as well as Simo-Droissart et al. (2018), Farminhão et al. (2020, 2023: Microceolia) and others have looked at relationships around Angraecum; the genus itself and just about all its sections were polyphyletic (see also Martos et al. 2018). Within Angraecinae, there has been one shift to the New World, the ancestor of [Campylocentrum + Dendrophylax moving there (via the Antilles) within the last 10 Ma or so (Pessoa et al. 2018).
Classification. Chase et al. (2015; c.f. Chase et al. 2003) provide a higher-level phylogenetic classification for the family with practically all genera placed into subfamilies and tribes (see also World Checklist of Monocots). For genera and synonymy in Apostasioideae, see Pridgeon et al. (1999), Cypripedioideae, Pridgeon et al. (1999), Vanilloideae, Pridgeon et al. (2003), Orchidoideae, Pridgeon et al. (2001b, 2003), and in Epidendroideae, Pridgeon et al. (2005, 2009, 2014). Ngugi et al. (2020) suggest the division of Orchideae into nine subtribes. Y. Wang et al. (2024) provide a useful review of recent work on classification in the family - subtribes and above - which clarifies what we know - and don't know.
Generic limits in the family are in the middle of a major overhaul to make them consistent with molecular findings, many of which have implications for clade/generic circumscriptions. Disagreements over generic circumscriptions in part reflect fundamental differences in classificatory philosophies and differing beliefs in the ability of morphology when used alone alone to disclose relationships. In the past the importance of floral differences in separating genera was emphasized, but here, as elsewhere, there has been widespread homoplasy (e.g. Chase et al. 2009 and references), for example, the distinctive lip-like appendage that was a defining feature of the old Corycinae (Waterman et al. 2009) seems to have evolved in parallel. Szlachetko et al. (2005 and references) give a statement of the "floral" position, maintaining that variation in column form, etc., yields taxonomically important characters (see also Szlachetko 1995; Rutkowski et al. 2008). However, clade limits suggested by molecular studies and generic limits suggested by floral features alone by no means always agree (Kocyan et al. 2008 and references). The features characterising the erstwhile broadly-delimited and polyphyletic Oncidium - mimicry of oil flowers of Malpighiaceae, whether or not the orchid itself also offers oil as a reward - is a good example of this (N. H. Williams et al. 2001; Neubig et al. 2008, esp. 2012a; Stpiczynaska & Davies 2008; Chase et al. 2009), similarly, in Aeridinae there is also probably widespread parallelism in the floral characters used to delimit genera (Hidayat et al. 2005; Salazar et al. 2011a, b for other examples). In Goodyerinae-Cranichideae, Smidt et al. (2021) expanded the limits of Microchilus to include six genera described by Leslie Garay diferences betwen which reflected the adoption of diferent pollination mechanisms. It is not that floral morphology is inherently taxonomically useless, however, undue reliance on it may well lead us seriously astray if our interest is in understanding relationships. Indeed, in some Epidendroideae vegetative variation may correlate better with clades evident in molecular phylogenies (e.g. Cameron 2005a), although anatomical variation by itself may suggest little major phylogenetic structure (Stern et al. 2004; Stern & Carlsward 2006).
However, even having a phylogeny and agreeing over basic taxonomic philosophy does not automatically lead to agreement about generic boundaries. Thus Clements (2003, 2006) suggested a wholesale pulverization and reorganization of generic limits in Dendrobium and its relatives, placing them in some 50 genera in three subtribes. The species numbers given above do not reflect this, since Burke et al. (2008), Janes and Duretto (2010), Schuiteman and Adams (2011), Schuiteman (2012, see also 2013: New Guinea), X.-G. Xiang et al. (2013) and L. Simpson et al. (2022/2024) all suggested that a broader circumscription of the genus would be preferable. Sectional limits (ca 97 sections), ten of which were not monophyletic in a study with a focus on Malesian-Australian taxa (Simpson et al. 2022/2024) are also at issue here (see also Adams 2011: focus on Australia), although Thawara et al. 2024) thought they were holding up quite well. Jones and Clements (2002a, esp. 2002b) divided Pterostylis, Clements et al. (2011) noting that there are nine or so identifiable groups around there, although that may not be quite the point - all identifiable groups do not have to be called genera. Since the monophyly of Pterostylis s.l. has been confirmed, its division is questionable (if one likes broadly-drawn generic limits), and indeed Janes and Duretto (2010) and Jones et al. (2010) suggest returning to the old circumscription of the genus (see also Clements in Chase et al. 2015). Jones et al. (2001) also dismembered the monophyletic Caladenia and Clements et al. (2002) divided a monophyletic Corybas, as did Jones et al. (2002 - also much else). Such cases simply reflect conflicting preferences for narrow or broad genus limits, so they are something of a pain. In any event, the result of nomenclatural changes made for these and other reasons in Australia was that about 45% of the species and subspecies in the entire orchid flora acquired new generic names between 2000 and mid-2009 (Hopper 2009). Vieira et al. (2024) noted that four genera had been resurrected and two new genera described to deal with variation in Prosthechea (Laelinae), but to no avail. Finally, Reveal (2012) came up with an earlier name for Epidendroideae (10 versus 58,500 hits - Google search iv.2012), although it seems to have returned to blessed obscurity.
There have been extensive discussions about generic limits in European Orchidinae in which many of the issues concerning classification in the context of phylogenies have been raised (e.g. Bateman et al. 1997; Bateman 2001, 2009, 2012; Tyteca & Klein 2008, 2009; Scopece et al. 2010b; M. Kropf in Kadereit et al. 2016). In an attempt to make generic limits there more objective, Scopece et al. (2010b) found that clade membership correlated well with post-zygotic reproductive isolation (embryo death). A phylogeny-based classification in which this and other evidence was incorporated could, they thought, be defended on more explicit grounds, and this would allow morphologically distinctive taxa previously segregated as separate genera be incorporated into their proper clades (Scopece et al. 2010b). This approach is somewhat reminiscent of that of Danser (1929), and although perhaps useful in Orchidaceae - but it is going to be interesting to see how widely it can be applied even here, and what the taxonomic consequences are - it may be inapplicable to orchids with different breeding behaviours. For an account of Anacamptis, Orchis, etc., see Kretzschmar et al. (2007). Pace (2020) looked at generic limits around Goodyera (Cranichideae).
For generic limits in Maxillariinae, c.f. Whitten et al. (2007) and Szlachetko et al. (2012). Blanco et al. (2007) made many new combinations in genera of Maxillariinae, reducing the large genus Maxillaria in size by about an half, while Schuiteman and Chase (2015) adopted a broad circumscription for Maxillaria, interestingly, 19 of the 42 generic names they placed in synonymy had been described in the last ten years. Chase et al. (2021a) took a similarly broad view as to the limits of Eulophia and Chase et al. (2021b) ditto of Coelogyne, in part because narrower concepts would yield unrecognizable taxa, entail new genera, etc.; around 330 combinations in Coelogyne were needed. For a reclassification of Pleurothallidinae, see Pridgeon and Chase (2001), although Pleurothallis may not be monophyletic, c.f. Karremans et al. (2012). Karremans et al. (2016a) discuss the synonymy of Specklinia. Karremans (2016) enumerated genera in Pleurothallidinae as a whole, Karremans et al. (2016b) provided an infrageneric classification of Acianthera, while Karremans (2019) provided an infrageneric classification for a somewhat broadly circumscribed Stelis, with some 1423 species, 1030 of which are in Stelis s. str., the largest genus of the subtribe. This is a huge group, and sampling is still relatively very poor. There is some controversy over generic limits in the Masdevallia area, c.f. Luer (2006) and Pridgeon (2007). Y. P. Ng et al. (2018) provide a classification for Podochileae that involved quite substantial changes - species from the old genus Eria ended up in 16 genera, and was reduced in size from around maybe 350-500 species to 48 (see Ng et al. 2018 for a discussion about generic limits; Moonlight et al. 2024). For the circumscription of genera around Angraecum, see e.g. Szlachetko et al. (2013) and Simo-Droissart et al. (2018); for those around Cattleya, see van den Berg (2014) and articles in Renziana vol. 4 (2014), including an infrageneric classification; for those around Bulbophyllum, see Pridgeon et al. (2014) and Vermuelen et al. (2014); for an infrageneric classification of Vanilla, see Soto Arenas and Cribb (2010), although modification seems to be in order (Pansarin & Menezes 2023). Generic limits in Habenariinae are discussed by Batista et al. (2013 and references) while Y. Tang et al. (2015: pp. 22-24; see also Jin et al. 2017) include an interesting discussion about generic limits around the polyphyletic Amitostigma (Orchidinae) - they end up with a broadly circumscribed Hemipilia. Further changes are in the offing, as is clear from the discussion in Chase et al. (2015), especially in genera like Angraecum (a broad circumscription is adopted in the generic list), Habenaria, etc.. Indeed, in the Angraecum group, as sampling of both taxa and genes improves, relationships change, and the limits of genera proposed five years before are modified (Simo-Droissart et al. 2018), while Farminhão et al. (2021) used phylocode-type names to talk about particular clades within the group (see also Farminhão et al. 2020).
Frosch and Cribb (2012) provide a sectional classification of Cypripedium (13 of them) which seems to be holding up. Morales et al. (2020) placed the 58 species of Pabstiella that they examined, about half the genus, in 10 sections.
I do not pretend to have dealt with hybridization in Orchidaceae satisfactorily, including hybridization by horticulturalists. Those interested should consult Sander's list of registered hybrids (Royal Horticultural Society 2008, also see Shaw (2021) and the quarterly updates by the Society ("Quarterly Supplement to the International Register and Checklist of Orchid Hybrids (Sander's List)". Generic name changes can have considerable consequences here. Thus the acceptance of generic name changes in some Epidendroideae in Pridgeon et al. (2005) necessitated over 10,000 changes to the names of hybrids which had to be transferred to a hybrid genus other than the one in which they were originally registered (Royal Horticultural Society 2008). It is a relief that names of hybrid genera can have no more that eight syllables, so Sophrolaeliocattleya gets in just under the wire.
The final issue has to do with species numbers, numbers that depend both on the general state of our knowledge of orchid diversity, and more particularly on issues of how to draw species limits - not totally unconnected, of course. As to our general knowledge of orchid diversity, Karremans and Davin (2017) summarize the rate of publication in Pleurothallidinae since the publication of Luer's Icones pleurothallidinarum beginning in 1975, a rate that has held constant over the ensuing 40+ years at around 85-90 new and accepted species/year. There are over 8,000 published names of Pleurothallidinae of which over 3,550 are currently accepted, and Luer was the sole or joint author of 2,634 names during this period (Karremans & Davin 2017). The rate of publication of novelties shows no signs of abating, and Karremans and Davin (2017) estimate that by 2026 there will be close to 6,000 accepted species in the subtribe, up from around 1,650 in 1975. As to species limits, here is just one example already mentioned, but by no means unique: Are there 10 or so or 350 or more species of Ophrys (Bateman et al. 2006a; Cuypers et al. 2022: see also above)? Indeed, some species numbers suggested for Orchidoideae seem somewhat high (see J. B. Thompson et al. 2023).
Botanical Trivia. Some orchids have ovules with developing embryo sacs in capsules that also have more or less mature seeds (Duarte et al. 2019).
[[Boryaceae et al.] [[Ixoliriaceae + Tecophilaeaceae] [Doryanthaceae [Iridaceae [Xeronemaceae [Asphodelaceae [Amaryllidaceae + Asparagaceae]]]]]]]: (monocot secondary thickening +); stem fructans +; (cuticular waxes as platelets transversely arranged in parallel series); (T ± connate), (trichomes at apex); (A inserted on T tube); tapetal cells bi- to tetranucleate; seeds exotestal, phytomelan +.
Age. This node is dated to around (97-)89(-79) or 85.1 Ma (S. Chen et al. 2013), about 102.9 Ma (Magallón et al. 2015), about 119 Ma (Tank et al. 2015: Table S2) and (117.4-)98.9(-82.1) Ma (Ji et al. 2022).
Evolution: Divergence & Distribution. I have put the character "outer tepals with apical trichomes" - they may be papillae or hairs, they vary in their exact position, etc. - here, although it is not flagged as an apomorphy. This feature has recently been studied in some detail, and presence or absence of these trichomes seems to vary at quite a high level, although if an apomorphy, it has been lost several times; the trichomes are involved in holding the tepals together in bud. Macfarlane and Conran (2017) give details of this character and its distribution.
For the distribution of fructose oligosaccharides, see Pollard (1982) and Meier and Reid (1982). Although recorded there only for some Hypoxidaceae in the [Boryaceae [Blandfordiaceae [Lanariaceae [Asteliaceae + Hypoxidaceae]]]] clade, and not for some of the smaller families elsewhere in Asparagales, fructans seem to be widespread in this part of the tree; they have not been recorded from Orchidaceae. Van den Ende (2022) recently mentioned the occurence of fructans with an internal glucose residue, including the neo-inulin type, as being a distinctive feature of the [Amaryllidaceae + Asparagaceae] clade (see also Shen et al. 2023). Fructans are mentioned quite often below, and they are provisionally considered to be an apomorphy for this part of the order, however, it would be nice to see a review of fructan type and synthesis for the whole order.
Vegetative Variation. Monocot secondary thickening, a distinctive pattern of secondary growth, is scattered in this clade (e.g. see list in Carlquist 2012a), where it has evolved perhaps ten times, and elsewhere it has also been reported from Eriocaulaceae and Rapateaceae (Poales: Scatena et al. 2005). A meristem cuts off tissue primarily to the inside, and vascular bundles embedded in ground tissue differentiate in it, some tissue may be cut off to the outside (Tomlinson 1970; Tomlinson & Zimmermann 1969; Zimmermann & Tomlinson 1972; Rudall 1991, 1995b; Jura-Morawiec et al. 2021). It can perhaps be thought of as the continued activity of the primary thickening meristem (see also Cheadle 1937; Carlquist 2012a), although there is some discussion as to what exactly that is (see above). There is sometimes a transition from collateral to amphivasal vascular bundles as the secondary thickening phase takes over, but this is by no means always so (e.g. Diggle & DeMason 1983; Rudall 1984). There are complex patterns of branching and fusion of the vascular bundles of the stems and branches (Haushahn et al. 2014), and Mangin (1882) had suggested there might be a connection between the origin of this secondary thickening and the reticulum of vascular bundles in association with which lateral roots arise in monocot stems. In monocots genes involved in secondary thickening via a normal bifacial cambium have been lost and reacquisiition of the woody habit has involved the evolution of novel mechanisms of secondary thickening (Davin et al. 2016; Roodt et al. 2019), although Zinkgraf et al. (2017) suggested that some of the genetic mechanisms involved in the regulation of ordinary secondary thickening had become reactivated here. Jura-Morawiec et al. (2021: table 2, Asphodelaceae, Asparagaceae studied) discuss the differences between monocot vascular cambia and those in other angiosperms - in the former, the cambial cells are of a single type, they are much shorter, there is no intrusive growth, etc.. Of course, palms and bamboos in particular have become woody without developing secondary thickening.
[Boryaceae [Blandfordiaceae [Asteliaceae [Lanariaceae + Hypoxidaceae]]]] / Boryaceae et al.: septal nectaries external; ovules with hypostase; embryo sac with chalazal constriction, antipodal cells persistent.
Age. This node can be dated around (87-)67(-47) or 42/65.2 Ma (S. Chen et al. 2013: last two numbers should be the same - c.f. Table 3), Ma (Janssen & Bremer 2004: but c.f. topology), ca 93.3 Ma (Magallón et al. 2015) or (90.0-)75.2(-61.0) Ma (Birch & Kocyan 2021).
There are many dates for clades in this area in both Janssen and Bremer (2004) and Wikström et al. (2001) - the topologies there differ from that above - and Birch and Kocyan (2021).
Evolution: Divergence & Distribution. This clade may have originated in Australia; long distance dispersal probably accounts for the major geographic disjunctions between clades, common and very evident here (Birch & Kocyan 2021).
There is extensive homoplasy in this little clade, so exactly where features like "septal nectaries external" are to be placed is unclear.
Chemistry, Morphology, etc.. For some information, see Kocyan and Birch (2011).
Phylogeny. The [Boryaceae [Blandfordiaceae [Lanariaceae [Asteliaceae + Hypoxidaceae]]]] clade is an at most moderately well-supported - if consistently appearing - group (Rudall et al. 1998a; Chase et al. 2000a; Fay et al.2000; Davis et al. 2004 - Lanariaceae not included; Graham et al. 2006; Chase et al. 2006; Birch & Kocyan 2021; etc.). Relationships between Milligania (Asteliaceae), Lanaria and Blandfordia were suggested by Bayer et al. (1998a). Kocyan and Birch (2011: all genera studied) found that Lanariaceae, Asteliaceae and Hypoxidaceae formed a tritomy, while Seberg et al. (2012) found that jackknife support for the clade was poor, and that Asteliaceae and Lanariaceae reversed their positions, i.e. to [Asteliaceae [Lanariaceae + Hypoxidaceae]], and relationships were also scrambled in Janssen and Bremer (2004) and Wikström et al. (2001). Birch and Kocyan (2021) found that relationships between the families in this clade as given below were strongly or very strongly supported.
BORYACEAE M. W. Chase, Rudall & Conran - Back to Asparagales
Plant xeromorphic, (growth monopodial - Alania), (aerial pseudostems +), rhizome often short, (with stilt roots); roots mycorrhizal, velamen + [?all], raphides 0; (monocot secondary thickening - Borya); endodermis much thickened; vascular bundles with lateral phloem; leaves spiral, base sheathing; inflorescence scapose, involucrate, capitate; T tube short; A adnate to tube [not Alania]; anthers (centrifixed), little longer than wide; micropyle bistomal, parietal tissue 1 cell across; T persistent in fruit; (seed hairy - Borya); endosperm without starch, embryo short, ovoid; n = 11, 14, x 7 (?6, ?8); seedling?
2 [list]/12: Borya (11). Australia, scattered. Map: see Brittan et al. (1987). [Photo - Borya Habit © M. Fagg]
Age. Crown group Boryaceae have been dated to 54 Ma (Janssen & Bremer 2004) and (53.7-)37.8(-22.3) Ma (Birch & Kocyan 2021).
Evolution: Ecology & Physiology. The arborescent Borya can tolerate extreme dessication, but with some interesting wrinkles - the response is facultative in some species, or only a small part of the leaf may be dessication tolerant (e.g. Barthlott 2006; Gaff & Oliver 2013 and references). The plant is poikilochlorophyllous (the chloroplasts ± break down on drying), and the leaves can remain living but completely dessicated for four years or so (e.g. references in Gaff 1981).
Plant-Bacterial/Fungal Associations. Borya has tuberculate roots that may have the coil-forming rhizoctonia fungus in them (c.f. Orchidaceae).
Chemistry, Morphology, etc.. The stems of Borya have monocot-type secondary thickening (Porembski & Barthlott 2000). There are vessels with almost simple perforation plates in the stem (Carlquist 2012a). The pedicels of Alania have several bracteoles.
Additional information is taken from Dahlgren et al. (1985) and Conran (1998), both general, Gaff (1981 and references), anatomy/physiology, Porembski and Barthlott (2000), velamen, and Conran and Temby (2000), floral morphology.
Previous Relationships. Genera of Boryaceae have often been included in Anthericaceae (= Asparagaceae-Agavoideae), as by Takhtajan (1997).
[Blandfordiaceae [Asteliaceae [Lanariaceae + Hypoxidaceae]]]: leaf blade with distinct midrib; nucellar cap ca 2 cells across.
Age. The age of this clade is estimated at (98-)81, 74(-56)Ma by Bell et al. (2010: note topology), at (79-)58(-35) or ca 38.3Ma by S. Chen et al. (2013), about 84.5 Ma by Magallón et al. (2015) and (82.6-)69.0(-55.6) Ma by Birch and Kocyan (2021).
Chemistry, Morphology, etc.. See Conran and Temby (2000) for general information, especially about ovules.
BLANDFORDIACEAE R. Dahlgren & Clifford - Back to Asparagales
Rhizome short; chemistry?; velamen ?+; raphides ?0; plant glabrous; leaves two-ranked, vernation flat-curved, sheath?; inflorescence a raceme; pedicels articulated; T large, tubular; (A adnate below middle of tube), anthers latrorse, centrifixed; pollen trichotomosulcate; septal nectaries external; G stipitate, style short, stigma ± punctate, dry; outer integument 3-4 cells across, parietal tissue ?1 cell across, hypostase +; T persistent, capsule septicidal; exotesta densely short-hairy; embryo short; n = 17, 27, x = ?; cotyledon photosynthetic.
1 [list]/4. E. Australia (map: see Brittan et al. 1987). [Photo - Blandfordia Flower © B. Walters.]
Age. The age of this clade was estimated to be (7-)4(-1.5) Ma by S. Chen et al. (2013) or (5.2-)3.2(-1.4) Ma by Birch and Kocyan (2021).
Evolution: Divergence & Distribution. Hardly a terribly diverse clade (Tank et al. 2015: Table S1).
Pollination Biology & Seed Dispersal. Bird pollination (honeyeaters) occurs here, but there is also self-pollination mediated by ants (Ramsey 1995).
Chemistry, Morphology, etc.. Clifford and Conran (1998) describe the young root, at leastas havingan uniseriate, unthickened velamen, although Kauff et al. (2000) think that it is absen.
Information is taken from Clifford and Conran (1998: general), Di Fulvio and Cave (1965) and Prakash and Ramsey (2000: both embryology) and Kocyan and Endress (2001b: some floral morphology.
Previous Relationships. Rudall (2003a) suggested that there was a close morphological relationship between Boryaceae and Blandfordiaceae.
[Asteliaceae [Lanariaceae + Hypoxidaceae]]: plants ± rosette-forming or caespitose; hairs multicellular, often branched; stomata paracytic; G ± inferior, septal nectaries internal; ovule with bistomal micropyle, micropyle zig-zag.
Age. The age of this clade 57.4 (46.4–69.0) (Birch & Kocyan 2021).
ASTELIACEAE Dumortier - Back to Asparagales
Plant ± rhizomatous; saponins +; indumentum branched-lepidote-stellate; leaves 3-ranked, base sheathing, closed or open; plant dioecious (polygamodioecious/flowers perfect), inflorescence branched raceme or spike, inflorescence bracts large; flowers (rather small), (10-14-merous); T connate basally (free); staminate flowers: A adnate to base of T/free; (nectaries on outside of ovary), pistillode [staminate floweres] +; staminodes + [pistillate flowers], G superior (subinferior), (5-7), (placentation parietal), intra-ovarian trichomes +, style branched or not (short), stigmas lobed-capitate to decurrent, dry; ?nucellar cap; fruit a berry/capsule; funicle distinct [= "long"], (with ± well developed mucilaginous hairs); endosperm oily, thin-walled, no hemicellulose, embryo "well developed"; n = 30, 35, ...105, x = ?, chromosomes 4-6 µm long; cotyledon not photosynthetic, ligule long, primary root well developed.
3 [list]/37: Astelia (31). Australia and New Zealand to New Guinea, Pacific Islands E. to Hawai'i,Chile, the Mascarenes. Map: see van Steenis and van Balgooy (1966) and Fl. Austral. vol. 45 (1987). [Photos - Milligania & Astelia Flowers © C. Howells - Australian Plants Society, Tasmania.]
Age. Crown-group Asteliaceae are dated to ca 92 Ma (Janssen & Bremer 2004); Birch et al. (2012) date it to (76-)55.4(-36.0) Ma and S. Chen et al. (2013) 32.6 or (51-)29.5(-12.5) Ma, while (76-)53.8(-36) Ma is the estimate in Birch and Keeley (2013) and (61.7-)49.3(-37.1) Ma in Birch and Kocyan (2021).
Fossils from New Zealand identified as stem Astelia are ca 23.2 Ma (Iles et al. 2015).
Evolution: Divergence & Distribution. Birch and Keeley (2013) provide numerous dates for clades within the family.
For the biogeography of Asteliaceae, see Birch et al. (2008, 2011, esp. 2012) and Birch and Keeley (2013). The initial divergence of Astelia may have been in New Zealand in the Oligocene at a time when the island may have been all(!) or mostly under water... One species of Astelia is known from Réunion - it is older than the island (see also Rousseaceae, Arecaceae, Monimiaceae, etc.) - one from southern South America, etc.. Asteliaceae may have initially been Australian, but with subsequent very extensive long distance dispersal (Birch & Keeley 2013; Birch & Kocyan 2021). For the Mascarenes/Africa-Hawaii/Antipodes connection, see also Malvaceae (Kokia), Asteraceae (Hesperomannia), Fabaceae (Acacia); Keeley and Funk (2011) give a list of Hawaiian endemics.
Given current ideas of relationships in the family (see below), character evolution in it will repay investigation.
Chemistry, Morphology, etc.. Carlquist (2012a) suggested that vessels were practically absent, except perhaps in the roots - although this might depend on the technique used to prepare the material.
For additional information, see Brittan et al. (1987) and Bayer et al. (1998a), both general, and Prakash and Ramsey (2000: embryology).
Phylogeny. The phylogeny of the family was studied by Birch et al. (2009); Milligania, with loculical capsular fruits, a semi-inferior ovary and no intra-ovarian trichomes, perfect flowers, etc., and often considered rather different from other Asteliaceae, seems to be embedded in Astelia, as do the other small genera previously recognized in the family (Birch et al. 2008, esp. 2009). However, Birch et al. (2012) and Birch and Keeley (2013) found the relationships [Neoastelia [Milligania + Astelia]] while in Birch and Kocyan (2021) relationships are [[Neoastelia +Milligania] Astelia], and with strong support.
Classification. Astelia is to include Collospermum (Birch et al. 2012); for an infrageneric classification of the former, see Birch (2015).
[Lanariaceae + Hypoxidaceae]: ?
Age. The age of this clade is perhaps (61.4-)50.5(-40.3) Ma (Birch & Kocyan 2021).
Evolution: Divergence & Distribution. This clade may have originated in southern Africa, having moved there from Australia (Birch & Kocyan 2021: but see also immediately more basal node).
LANARIACEAE R. Dahlgren - Back to Asparagales
Plant with vertical rhizome; biflavones +; raphides 0, (styloids +); indumentum dendritic; leaves two-ranked to spiral, sheath ?closed; inflorescence branched; T connate half-way; A adnate in mouth of tube; style long, stigma shortly three-lobed; ovules 2/carpel, apotropous, outer integument 5-7 cells across, parietal tissue ca 3 cells across, obturator +; fruit ± indehiscent; seed 1, black; exotesta palisade, other cells rounded, tegmen persists, develops at micropyle; endosperm initially with starch, embryo medium; n = 18; seedling?
1 [list]/1: Lanaria plumosa. Cape Province, South Africa. Photo: Habit, flower.
Evolution: Divergence & Distribution. There may have been a slowing of diversification in this clade (Hertweck et al. 2015, but c.f. topology).
Chemistry, Morphology, etc.. Information is taken from De Vos (1963) and Steinecke and Hamann (1989), both embryology, Dora and Edwards (1991 - chemistry) and Dahlgren (in Dahlgren & Van Wyk 1988) and Rudall (1998), both general.
HYPOXIDACEAE R. Brown, nom. cons. - Back to Asparagales
Plant monopodial; leaf bases persisting; contractile roots common; fructan sugars accumulated, saponins 0; velamen 0, dimorphic root hypodermis 0; scape with a single ring of vascular bundles; epicuticular waxes 0; stomata (tetracytic), with oblique or parallel cell divisions; leaves 3-ranked, sheaths also closed; inflorescence scapose, axis flattened; (flowers 2-merous); T free to long-tubular; (A many), (dehiscence latrorse), (basi- or centrifixed, sagittate); tapetum amoeboid, microsporogenesis successive [tetrads tetragonal]; nectary 0; G (apical beak +), stigmas commissural, ± 3-radiate, dry or wet; ovules apotropous, (outer integument to 4 cells across), (nucellar cap 0), (parietal tissue 0); antipodal cells soon die; fruit various; seeds globose, smooth to spiny, micropyle protrudes, apex of funicle persists; exotesta palisade or not, (endotegmen persistent), raphe prominent; endosperm thin-walled, (perisperm +, slight), embryo short, ± undifferentiated; x = 9, chromosomes 2-5 µm long, nuclear genome [1 C] (?0.125-)1.588(-?) pg; cotyledon not photosynthetic, ligule long.
5(-7)[list]/163 (100-220). Seasonal tropics, esp. southern Africa (more temperate). Map: see Fl. N. Am. vol. 26 (2002) and Australia's Virtual Herbarium (consulted xi.2012). [Photo - Inflorescence, Flower.]
Age. Crown group Hypoxidaceae have been dated to as much as ca 78 Ma (Janssen & Bremer 2004), as little as (33-)23(-16)/ca 15.6 Ma by S. Chen et al. (2013), or (40-)32.5(-25.8) Ma (Birch & Kocyan 2021).
1. Hypoxideae Bernhardt
Rhizomes tuberous, vertical, (corm); (bulliform cells); hairs tufted or two-branched, on back of T; T (basally connate); A outer whorl longer than inner, anthers (near) basifixed, connective swollen; pollen (inaperturate, 2-sulcate); fruit irregularly dehiscent/(and) loculicidal/circumscissile; seed regularly conoid-papillate/echinulate; n = 6-9, 11...
1/95: Hypoxis. Warm temperate to tropical, esp. Southern Hemisphere.
2. Curculigeae Dumortier
Rhizomes +, inc. vertical; hairs tufted or two-branched; leaves (spiny), petiolate, blade plicate, (divided); anthers porose, filaments sigmoid; pollen (disulcate); stigma asymmetrical [?all], papillae uniseriate; fruit baccate; seeds black, shiny, smooth or irregular, (strophiolate); n = 9(-11).
2(4)/26: Curculigo (19(16)). Tropical, inc. the Seychelles.
3. Empodium Salisbury, Pauridia Harvey
Corms, replaced each year; stomata (isobifacial); mucilage canals usu. adaxial to vascular bundles [Pauridia]/in foliar mesophyll [Empodium]; leaf (blade ± terete - Em.), (plicate); plant glabrous or not; flower (single - Em.); T (basally connate); A (inner whorl longer than outer), (3, opposite inner T, 3 staminodes adnate to style [= gynostemium] - Pa.), (apical appendage +, mucilage filled); pollen (2-3-sulcate), microechinate; (stigma massive); (placentation parietal - Em.); (embryo sac bisporic, 8-celled [Allium type] - Em.); fruit indehiscent/irregularly dehiscent/circumscissile; seed black to brown, ± densely conoid-papillate, (strophiolate - Em.); (endosperm nuclear - Pa.); n = 6-8.
2/42: Pauridia (35). Southern Africa, esp. South Africa (the Fynbos), the Antipodes.
Evolution: Divergence & Distribution. Pauridia occidentalis and P. salina, West Australian, are embedded in the rest of the genus (Birch & Kocyan 2021), largely to be found in the South African Cape flora - long distance dispersal again? For additional suggestions concerning the biogeography of the family, see Birch and Kocyan (2021).
Pollination Biology & Seed Dispersal. Pollen is the main reward, and flies of various kinds, beetles and bees seem to be the pollinators (Kocyan et al. 2011 for a summary; Ren et al. 2018); Snijman (2014) noted that the drak spots in the middle of the flower of Pauridia suggested hopliine (scarabeid) beetle pollination. At least some species of Hypoxis are apomictic (Nordal 1998).
Chemistry, Morphology, etc.. In Pauridia immediately adaxial to the vascular bundles there are structures variously described as being formed by the breakdown of large central mesophyll cells (Snijman 2014) or as mucilage canals (M. F. Thompson 1976) - not necessarily different things. The lamina may be very narrow, but with vascular bundles all oriented normally, or the bundles may be arranged in a ring; the leaf blade in Pauridia aquatica is flat at the base and with normally-oriented vascular bundles but terete at the apex and with a ring of bundles (Thompson 1976).
Kocyan (2007) found that some flowers of Curculigo racemosa were polyandrous, however, the stamens were not fasciculate. The staminodes of Pauridia that are adnate to the style rather surprisingly appear to represent the outer androecial whorl - and they are responsible for reports of a 6-lobed stigma in the family. The rostrum, a narrowed, beak-like apical part of the ovary, appears to have evolved more than once, but its function is uncertain; it may be up to 7 cm long in P. alticola or 8 cm long in Curculigo (Friedmannia) seychellarum. A beak may also be formed by the connate tepals.
There is some controversy over the tapetum type in the family and in the numbers of nuclei in the cells, and whether or not there is a velamen in the root. The ovules have a parietal cell, so are not tenuinucellate [?incorrect - not in the literature I have read]. The endosperm is reported as being nuclear or helobial; if the former, then the antipodal cells tend to persist (de Vos 1948, 1949).
Additional information is taken from Hilliard and Burtt (1978), Nordal (1998), Judd (2000), Wiland-Szymanska (2009: east Africa) and Snijman (2014: African Pauridia), all general, Rudall et al. (1998a: anatomy), M. F. Thompson (1978: floral morphology/anatomy), Kocyan and Endress (2001b: floral morphology), Stenar (1925: embryology) and Arekal (1967: ovule and seed).
Phylogeny. Kocyan et al. (2011) recovered three main clades - Curculigo et al., Pauridia et al., and Hypoxis (most) - in the family, a slight modification of the results obtained by Rudall et al. (1998a). Relationships between these clades were unclear, as they are in Birch and Kocyan (2021); Hypoxis is para-/polyphyletic and Curculigo and Spiloxene are even more so, the latter (as Pauridia) even including the Antipodean Hypoxis sect. Ianthe, and within the Curculigo group the three species from the Seychelles are sister to the rest (see also Kocyan et al. 2011).
Classification. For genera, see Kocyan et al. (2011) and Snijman and Kocyan (2013). Note that the three species of Curculigo from the Seychelles have been placed in two separate genera (Kocyan & Wiland-Szymanska.
Previous Relationships. Rudall (2003a) suggested that there might be a close morphological relationship between Hypoxidaceae and Orchidaceae. In older classifications, Hypoxidaceae were often included in Amaryllidaceae.
Age. The age of this node is estimated at ca 84 Ma by Eguiarte (1995), (98-)87, 78(-68) Ma by Bell et al. (2010), ca 80 Ma by S. Chen et al. (2013: Fig. 3), and around 89 and (88.9-)84.3(-80.7) Ma by Magallón et al. (2015 and 2018 respectively).
Evolution: Divergence & Distribution. Diversification may have increased at this node (Magallón et al. 2018).
[Ixioliriaceae + Tecophilaeaceae]: cormose; root pith 0; leaves spiral, shortly cylindrical at apex [Vorläuferspitze]; flowers quite large; outer T mucronate to aristate, T tube short; A inserted at mouth of tube; fruit a loculicidal capsule; embryo long; x = 12.
Age. For the age of this node, some (93-)79, 70(-59) Ma, see Bell et al. (2010), while (79.3-)64.1(-46.5) or ca 34.1 Ma is the estimate in S. Chen et al. (2013) and ca 79.7 Ma in Magallón et al. (2015). The divergence of Ixoliriaceae is dated to ca 112 Ma and that of Tecophilaeaceae to 108 Ma (Janssen & Bremer 2004: note topology).
Evolution: Divergence & Distribution. The rate of diversification may have slowed in this clade (Hertweck et al. 2015: all three).
Chemistry, Morphology, etc.. The outer tepals in at least some Iridaceae (and Orchidaceae!) are also mucronate to aristate.
Phylogeny. There is weak to moderate support for this taxon pair in Chase et al. (2000a), Pires et al. (2006), Givnish et al. (2006) and Seberg et al. (2012), and stronger support in Graham et al. (2006: sampling poor); they have a very long branch in the three-gene analysis of Fay et al. (2000). Davis et al. (2004) found some support for the clade [Ixoliriaceae + Iridaceae], although sampling was poor; Chase et al. (2006) found strong support for this relationship. Janssen and Bremer (2004) found the pectinate relationships [Ixoliriaceae [Tecophilaeaceae [Doryanthaceae + the rest]]], while the relationships [Tecophilaeaceae [Doryanthaceae [Ixoliriaceae + Iridaceae]] the rest] in Chase et al. (2006) had very little support.
Previous Relationships. Both Dahlgren et al. (1985) and Takhtajan (1997) recognised relationships between Ixoliriaceae and Tecophilaeaceae, as well as with a selection of other asparagalean families.
IXIOLIRIACEAE Nakai - Ixiolirionm Herbert - Back to Asparagales
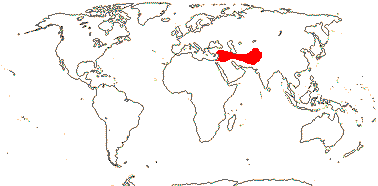
Plant a tunicated corm [surrounded by scales]; saponins 0?; root dimorphic exodermis 0, peduncle with a sclerenchymatous ring; mucilage cells +; leaf base ?type; inflorescence subumbellate, leafy; A centrifixed; tapetal cells uninucleate; stigma 3-lobed, dry; outer integument 3-4 cells across, parietal tissue ca 2 cells across, nucellar cap ca 2 cells across; seeds angled, phytomelan +; endosperm walls pitted, starch in cells surrounding embryo; x = 6 (?8, ?7), nuclear genome [1 C] (0.096-)3.291(-112.341) pg; cotyledon remains white even when exposed to light!
1[list]/3. Egypt and Turkey to Central Asia and Pakistan. Map: from Traub (1942), rather approximate). [Photo - Flower © A. Shoob]
Chemistry, Morphology, etc.. The vascular bundles in the leaf are unequal in size, some in the inflorescence axis are arranged in a circle, enclosing additional scattered bundles.
Information is taken from Kubitzki (1998b: general), Arroyo (1982) and Arroyo and Cutler (1984: both anatomy), Dönmez and Isik (2008: pollen), Stenar (1925: embryology), and Tillich (2003: seedling morphology).
Previous Relationships. The inflorescence axis is leafy, the flowers are blue and the plant lacks alkaloids, all unusual features for Amaryllidaceae, where Ixiolirion has often been included (e.g. Takhtajan 1997).
TECOPHILAEACEAE Leybold, nom. cons. - Back to Asparagales —— Synonymy: Androsynaceae Salisbury, Conantheraceae Pfeiffer, Cyanastraceae Engler, Cyanellaceae Salisbury, Walleriaceae Takhtajan
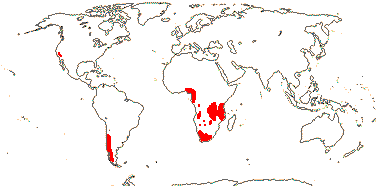
Corm, tunicated [surrounded by scales], (tuber); saponins +?, fructan sugars accumulated [Cyanastrum]; roots raphides 0; leaf vessels 0; stomata anomocytic; leaf usu. linear-elliptic, more than one order of parallel veins, (transverse veins branching, reticulated), sheaths closed; inflorescence a raceme, branched or not/flowers axillary; (bracteoles 0); flowers variously monosymmetric (polysymmetric); (T tube moderately long); A (strongly heteranthous), anthers dehiscing by apical pores/short slits; tapetal cells binucleate; pollen operculate, heteropolar, foveolate; (septal nectaries +); G semi-inferior, stigma punctate; ovules many/carpel, ana-campylotropous, micropyle bistomal, parietal tissue 3-5 cells across, chalazal end of nucellus ± swollen, funicular obturator +; testa multilayered, (exotesta palisade), thick-walled, (chalazal tissue proliferates, = massive chalazosperm); endosperm nuclear, cells thick-walled, pitted or not, (± absent), ?starch, embryo also short; x = 12, chromosomes 2-4 µm long, nuclear genome [1 C] (0.074-)2.498(-84.385) pg; cotyledon not photosynthetic, (coleoptile +), primary root long .
7 [list, two groups below]/27. Africa, Chile, and W. U.S.A.. Map: from Carter (1962), Scott (1991), Brummitt et al. (1998) and Fl. N. Am. vol. 26 (2002). [Photo - Flower, Flower.]
Age. Crown-group Tecophilaeaceae have been dated at ca 87 Ma (Janssen & Bremer 2004), ca 77 Ma (Buerki et al. 2013a: Fig. 1), and (41-)30(-20) or ca 20.4 Ma (S. Chen et al. 2013).
1. Conanthereae D. Don —— Synonymy: Conantheraceae Pfeiffer
(Chelidonic acid + - Conanthera); (fertile A 3, 4, abaxial, with bsaal caudate appendage); pollen (rugulose - Zephyra); ovules with outer integument ca 5 cells across, suprachalazal zone massive, postament + [Tec.]; n = 12.
2/9: Conanthera (5). Chile, Peru (Lima Province).
Age. The crown-group age of this clade (Tecophilaea inc. in Zephyranthes) is around 53 Ma (Buerki et al. 2013a: Fig. 1, see error bars).
2. Odontostomeae Baker —— Synonymy: Androsynaceae Salisbury, Cyanastraceae Engler, Cyanellaceae Salisbury, Walleriaceae Takhtajan, nom. cons.
(Corm non-tunicate), (plant climbing by leaf tendrils - Walleria), (± prickly); leaf (petiolate, blade ovate, base cordate - Cyanastrum [Cy.]); (large stamens 1, 3/a single isolated abaxial stamen); pollen (not operculate - Kabayea, Cy.); (ovules 2/carpel), outer integument "thick", vascularized [Cy.], 4 cells across [Cyanella], inner integument ca 3 cells across; fertilized embryo sac with postament, chalazal haustorium [Cyanella]; seeds black to brown to yellow, (pitted), (finger-like structures, each with apical tuft of minute trichomes - Walleria); (endosperm helobial - Odontostomum), (0 - Cy.); (hypocotyl and primary root 0 - Cy.); n = (8, 10) 11, 12, 14.
5/18: Cyanella (9). Africa, the U.S.A. (California - Odontostomum).
Age. The crown-group age of this clade is Late Cretaceous (Buerki et al. 2013a: Fig. 1).
Evolution: Divergence & Distribution. Buerki et al. (2013a) discussed the complex eco-biogeographical history of this small clade, i.a. they noted that its colonization of what are now Mediterranean ecosystems occurred before the origin of the Mediterranean climate, as seems to be common (see also Vargas et al. 2014). Note overall distribution: [Chile [California + Africa]].
Pollination Biology & Seed Dispersal. The floral morphology of Tecophilaeaceae suggests that buzz pollination is common (see also Russel et al. 2015).
Chemistry, Morphology, etc.. This is a heterogeneous group. Tomlinson (1974) described cells adjacent to stomata in Cyanastrum as having parallel cell divisions.
The monosymmetry of the flower is largely caused by the androecium; enantiostyly also occurs in a few species of Cyanella. Odontostomum has been reported to have six staminodia alternating with the six stamens, but these "staminodia" are some kind of corona or enation from the tepals (M. G. Simpson & Rudall 1998). The nature and function of the so-called chalazosperm in the mature seed is unclear (Simpson & Rudall 1998).
Some information is taken from Dahlgren (in Dahlgren & van Wyk 1988), Rudall (1997), M. G. Simpson and Rudall (1998), Brummitt et al. (1998) and Manning and Goldblatt (2012: African taxa); for leaf anatomy, see Arroyo (1986), for some embryology, etc., see Nietsch (1941), Cave (1952) and A.-m. Lu et al. (1985: Tab. 1 - summary), and for seedlings, variable in morphology, see Tillich (1996a, 2003).
Phylogeny. Brummitt et al. (1998) found that Tecophilaea was sister to the rest of the family, although with only moderate support; other relationships along the backbone were poorly resolved. More recently, Buerki et al. (2013a) recovered a clade [Conanthera + Zephyra] (both genera are from Chilean South America, and Tecophilaea belongs around here) that was sister to a clade including the rest of the family and where Odontostomum was sister to the rest.
Classification. Given the poor support for many relationships here, a classification is premature (c.f. Brummitt et al. 1998); despite a more resolved phylogeny, Buerki et al. (2013a) quite reasonably elected not to develop any formal suprageneric hierarchy. I have put in a couple of tribes that correspond to the two main clades evident in Buerki et al. (2013a).
[Doryanthaceae [Iridaceae [Xeronemataceae [Asphodelaceae [Amaryllidaceae + Asparagaceae]]]]]: ?
Age. this node has been dated to ca 107 Ma (Janssen & Bremer 2004); the separation of Doryanthaceae from Iridaceae (sic) has been estimated to be ca 82 Ma (Goldblatt et al. 2008).
Pollen fossils assigned to Iridaceae-Isophysis or to Doryanthes have been found in Late Cretaceous rocks ca 75-70 Ma old from Eastern Siberia (Hoffmann & Zetter 2010).
Phylogeny. There is only moderate support for this node in Fay et al. (2000) and practically no support in Seberg et al. (2012), but 92% bootstrap support in Graham et al. (2006: note sampling); see also Janssen and Bremer (2004).
DORYANTHACEAE R. Dahlgren & Clifford - Back to Asparagales
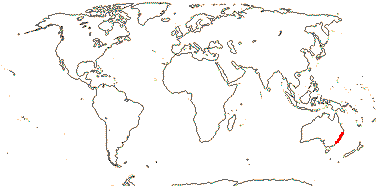
Huge sub-bulbous tufted perennial; steroidal saponins +; root cortex with fibres; stem vascular bundles encased in fibres; styloids +, raphides 0; cuticular wax rodlets parallel, stomata paracytic, subsidiary cells with oblique divisions; leaves spiral, apex cylindrical [= Vorläuferspitze], when older fray, as dry threads; inflorescence with inflorescence bracts, (subumbellate); T large, with two rows of vascular bundles, apex [esp. of outer T] cucullate, tube long; (A also adnate to the base of the tepal lobes), anthers latrorse, centrifixed, endothecium thick; pollen surface reticulate; G inferior, apex 6-ridged, stigma 3-angled, punctate, dry; ovules in two ranks, outer integument ca 5 cells across, inner integument ca 2 cells across, parietal tissue ca 5 cells across, nucellar cap ca 2 cells across, suprachalazal tissue extensive, postament +; antipodal cells to 5, ± persistent; fruit dehiscing laterally, loculicidal, T and A deciduous; seeds flattened, winged; testa multiplicative, many-layered, with phlobaphene; endosperm thin-walled, embryo flattened; n = 17, 18, 22, 24, x = 6 (?8, ?7), karyotype bimodal, nuclear genome [1 C] (0.337-)3.134(-29.176) pg; seedling with laterally compressed haustorium, coleoptile +.
1 [list]/2. E. Australia. Map: from O. Seberg (pers. comm). [Photo - Habit.]
Age. The divergence of the two species in the family is estimated to have occurred (8-)4(-1) Ma (S. Chen et al. 2013).
Chemistry, Morphology, etc.. The genus is described as being monocarpic by Forster and Eggli (2020), although usually it is pleonanthic - i.e., all the rosettes making up the one genotype do not flower at once. Kocyan and Endress (2001b) note that the connective is massive, each stamen being supplied by 2-4 "vascular complexes", although these were not observed by Newman (1928, 1929). There may be a hypostase immediately beneath the embryo sac (Newman 1928).
General information is taken from Wunderlich (1950), Clifford (1998) and Forster and Eggli (2020), Blunden et al. (1973) described leaf anatomy and Tillich (2003) seedling morphology.
[Iridaceae [Xeronemataceae [Asphodelaceae [Amaryllidaceae + Asparagaceae]]]]: (vegetative fructans +); (seeds with glucomannans as reserves); Arabidopsis-type telomeres lost, (TTAGGG)n [human-type telomeres] common.
Age. This node is estimated at (92-)81, 72(-62) Ma by Bell et al. (2010), (83-)75(-65) or ca 51.2 Ma by S. Chen et al. (2013), about 80.7 Ma by Magallón et al. (2015), (93-)83.1(-73.4) Ma by Joyce et al. (2018), about 98.1 Ma by Tank et al. (2015: Table S2), (68.0-)62.7(-58.0) Ma by D.-F. Xie et al. (2020) and (101.4-)83.4(-67.4) Ma by Ji et al. (2022).
Evolution: Divergence & Distribution. Givnish et al. (2018b) suggest that there has been an acceleration of speciation in this clade.
Genes & Genomes. The loss of Arabidopsis-type (A-type) telomeres is not simple; human-type telomeres ((TTAGGG)n) may predominate, but there are other types, too. Asparagaceae-Scilloideae agree with other members of this clade, although the A-type telomere is somewhat more common than in the other members sampled (Adams et al. 2001; especially Sýkorová et al. 2003b, 2006a, b). Acanthocarpus, alone among the taxa discussed there as being an out-group, also lacks A-type telomeres, but it belongs to Asparagaceae-Lomandroideae (ex Laxmanniaceae), so it is an ingroup, not Arecales-Dasypogonaceae, a commelinid.
Chemistry, Morphology, etc.. The plants may have distinctive carbohydrates (Meier & Reid 1982; Buckeridge et al. 2000a). Apparently there are only tracheids in the xylem (Fahn 1990).
Glucomannan seed reserves are scattered in this clade, being associated with thick-walled endospermal cells, but I do not know details of their distribution. They are reported from Iridaceae, Amaryllidaceae-Allioideae, Asparagaceae-Asparagoideae and -Scilloideae-Ornithogaleae - and they are also known from some Liliales (see e.g. Elfert 1894; Jakimow-Barras 1973; Reid 1985).
Phylogeny. This group has quite strong support in Fay et al. (2000) and Soltis et al. (2007a), etc., but lacking much jackknife support in Seberg et al. (2012: bootstrap support better).
IRIDACEAE Jussieu, nom. cons. - Back to Asparagales
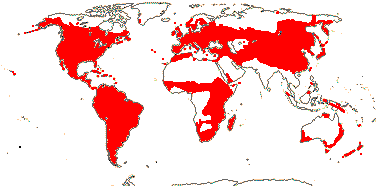
Plant rhizomatous; roots mycorrhizal; flavone C-glycosides, flavonols +, chelidonic acid 0?; dimorphic root hypodermis +; (stem endodermis +); raphides 0, styloids +; cuticular wax rodlets parallel; leaves two-ranked, ventralized isobifacial [oriented edge on to the stem]; flowers usu. large [1≤ cm across]; T ± free, apex often aristate; A 3, opposite outer T, extrorse, endothecial cells with U-shaped thickenings; G opposite inner T, nectary ?septal, style branched, stigma dry; micropyle endo- or exostomal, outer integument 4-6 cells across, parietal tissue 1(-2) cells across (absent); seed ± angled, coat testal and tegmic, phytomelan 0, phlobaphene +, endotesta pigmented, with lipids; endosperm thick-walled, hemicellulosic, embryo quite large; x = 10 (?9, ?8), nuclear genome [1 C] (0.145-)3.014(-62.805); cotyledon not photosynthetic, (hypocotyl short).
66 [list, as subfamilies]/2,145 (2,244) - seven subfamilies below. World-wide. Map: see Heywood (1978: S. America), Hultén and Fries (1986), Mathew (1989), Fl. N. Am. vol. 26 (2002), Bahali et al. (2004), FloraBase (2005), Davies et al. (2005: Fig. 2b suggests that Iridaceae grow throughout Africa, much of the Arabian Peninsula, etc.), Rodrigues and Sytsma (2006) and Alexeyeva (2008). [Photos - Collection.]
Age. Crown group divergence is estimated to have begun ca 96 Ma (Janssen & Bremer 2004), (82.9-)71.6(-60.5) Ma (Joyce et al. (2018), 70 or 66 Ma (Goldblatt et al. 2008), or (68-)58.5(-49) or 51.2 Ma (S. Chen et al. 2013).
1. Isophysidoideae Thorne & Reveal - Isophysis tasmanica (J. D. Hooker) T. Moore —— Synonymy: Hewardiaceae Nakai, nom. illeg., Isophysidaceae F. A. Barkley
Amentoflavone + [= biflavonoid]; vessel elements in roots with scalariform perforation plates; leaf cells, esp. epidermal, thick-walled, lignified; flower solitary, with spathes; T spreading; endothecium with radially elongated walls; microsporogenesis?; nectary 0; ovary ± superior [P, A, adnate basally], style branches ± spiralling, commissural; endosperm ?helobial; n = ?; seedling?
1/1. Tasmania.
[Iridoideae [Patersonioideae [Geosiridoideae [Aristeoideae [Nivenioideae + Crocoideae]]]]]: xanthone + [mangiferin], fructan sugars accumulate; vessel elements in roots with scalariform and simple perforation plates; leaf (vernation plicate); inflorescence with cymose units [flowers from the axils of successive prophylls, so alternating, = a rhipidium]; flowers short- [open ca 1 day]/long-lived; (style branches bifid), stigma on the edges of the complex/expanded branches; fruit laterally apically (completely) loculicidal, endosperm nuclear.
Age. The age of this node is around 62 Ma (Goldblatt et al. 2008).
2. Iridoideae Eaton
γ-glutamyl peptides, (steroidal saponins +), (bufadienolides +), meta carboxy aromatic amino acids +; vessel elements in root with simple perforation plates; (leaf vernation plicate); rhiphidia simple; flowers (long-lived), T whorls differentiated; endothecial cells with spiral thickenings; (pollen grains with encircling aperture); style branches long, tubular.
30/920. Worldwide, but esp. the spine of Central and South America.
Age. The age of the clade is suggested to be ca 57 Ma (Goldblatt et al. 2008).
2A. Diplarrheneae Goldblatt - Diplarrhena Labillardière
Stomata perigenous; leaf margin with vascular bundle; flowers monosymmetric; A 2; pollen inaperturate, spherical, intectate; septal nectaries +.
1/2. S.E. Australia, Tasmania.
[Irideae [Sisyrinchieae [Trimezieae + Tigridieae]]]: flowers polysymmetric; T (whorls similar); nectaries/oil glands on T, esp. outer T [base of outer T, surface of inner T]/0.
Plant (corms +, axillary/terminal, central stele 0), (bulbs +), (acaulescent); mangiferin + [= xanthone]; (stem ± winged); (stomata perigenous - Dietes); leaf blade (rounded), (dorsiventral); (inner whorl T bearded - some Iris), (T-ovary tube +, to 20 cm long - I. unguicularis); (filaments connate); (pollen zonasulcate); ovules with nucellar cap, obturator from funicle/inner integument; n = (5-9)10...
5/550: Iris s.l. (280), Moraea (230). Iris esp. Eurasia and North America, the rest Africa, esp. the Cape, but one sp. (Dietes robinsoniana) Lord Howe Island.
[Sisyrinchieae [Trimezieae + Tigridieae]]: (floral elaiophores + [unicellular hairs]/nectaries +).
Vessels in stems and leaves; (styloids 0); stem (± compressed); leaf (with (sub)marginal vascular bundle - Libertia), (fibres at the xylem pole only); T whorls similar (differentiated); anthers (porose), filaments ± connate; endothecial cells?; (oil flowers + [elaiophores on staminal column, Sisyrinchium]); style branches commissural (style undivided, stigma capitate - Solenomelus); seed (rounded, umbiculate [depression in raphal area]), (black), (± winged); embryo photosynthetic; n = 8-11, 16, 18-20...
6/243: Sisyrinchium (140-180-216 [205 Thode et al. 2021]). America, few in Australasia.
[Trimezieae + Tigridieae]: oil flowers + [inner T in particular with elaiophores on adaxial surface].
Age. This clade may be as much as 35 Ma (Renner & Schaefer 2010).
(Stem ± winged); leaves (terete), pseudomidrib +; n = 8, 9, 13, 14...
2/64: Trimezia (62: inc. Neomarica). New World, ±tropical, inc. Florida.
Plant with bulbs; mangiferin + [= xanthone]; leaf (blades pleated); (elaiophores on outer T); A (connate), anthers (poricidaL), (nectaries on connective - Cypella); pollen (zonasulcate/disulcate/trichotomosulcate); stigma (bifid); seedling (with ligule or coleoptile - e.g. Tigridia); n = (6) 7.
14/167: Tigridia (35), Cypella (30), Mastigostyla (27). (Sub)tropical America, inc. S. U.S.A., esp. Mexico.
[Patersonioideae [Geosiridoideae [Aristeoideae [Nivenioideae + Crocoideae]]]]: rhipidia 2, fused [binate], each unit with 2-many flowers; extra codon in rps4 gene.
Age. The age of this node is about 70 or 55 Ma (Goldblatt et al. 2008).
3. Patersonioideae Goldblatt - Patersonia R. Brown
Plant ± woody and rhizomatous; amentoflavone + [= biflavonoid]; (monocot secondary thickening +); vessel elements in roots often with scalariform perforation plates; leaf margins with subepidermal fibres/irregular multicellular projections; inner tepals reduced to scales or 0; endothecial cells with base-plate thickenings, filaments ± connate; pollen spherical, inaperturate, intectate; nectary 0; stigma lobes broad; ovules apparently uniseriate; embryo small; n = 11, 21; two extra codons in rps4 gene.
1/24. More or less open conditions, scattered in Malesia, New Caledonia, and the periphery of Australia. Map: partly from Fl. Austral. vol. 46 (1986).
[Geosiridoideae [Aristeoideae [Nivenioideae + Crocoideae]]]: ?
Age. This node is about 48 Ma (Goldblatt et al. 2008) or (64.5-)53.2(-42.2) Ma (Joyce et al. 2018).
4. Geosiridoideae Goldblatt & Manning - Geosiris Baillon ——
Synonymy: Geosiridaceae Jonker
Plant echlorophyllous, mycoheterotrophic; ?chemistry; styloids 0 [leaves]; leaves heterobifacial; flowers sessile; T connate basally only; microsporogenesis successive; nectary 0; placentae branched, (style swollen towards the apex, stigma truncate); ovules with outer integument 2-3 cells across, parietal tissue 1-2 cells across; seeds minute, dust-like, mesotesta 0; endosperm starchy, walls thick, hemicellulosic, embryo small; n = ?
1/3. Madagascar, the Comores (Mayotte I.), and Australia (Queensland), also the Philippines? (Joyce et al. 2018).
Age. Crown-group Geosiris is at least (43.9-)29.9(-16.1) Ma (Joyce et al. 2018).
[Aristeoideae [Nivenioideae + Crocoideae]]: ?
Age. The age of this node is estimated to be around 40 Ma by Goldblatt et al. (2008) and (49-)34, 31(-17) Ma by Bell et al. (2010).
5. Aristeoideae Vines - Aristaea Aiton
Plant rhizomatous; plumbagin + [= acetogenic naphthoquinone]; (monocot secondary thickening +); vessel elements in roots often with scalariform perforation plates; (stem ± winged); leaf bundles embedded, midrib 0, marginal sclerenchyma 0; leaves isobifacial; inflorescence units binate riphidia; flowers fugaceous; T connate basally only; pollen (in tetrads), also di(zono)sulcate/trisulcate-spiraperturate; nectary 0 (perigonal - A. spiralis), stigmatic lobes fringed; seeds v. variable, inc. arillate; outer integument 2-3 cells across [?all]; embryo small; x = 16.
1/58. Mostly southern Africa, ore or less open conditions, sub-Saharan Africa and Madagascar.
[Nivenioideae + Crocoideae]: flowers sessile, long-lived; T connate; septal nectaries +.
Age. The age of this node is ca 36 Ma (Goldblatt et al. 2008) or (46.5-)36.7(-27.6) Ma (Joyce et al. 2018).
6. Nivenioideae Goldblatt
Plant with woody stem; monocot secondary thickening +; vessel elements in roots with scalariform perforation plates only; leaves with non-vascular fibrous strands, isobifacial; inflorescence (capitate), units binate riphidia, unit of rhipidium with 1-2 flowers; (heterostyly - Nivenia); flowers long-lived, T long-tubular, (short-tubular, T long-linear - Klattia); endothecial cells with basal anastomosis of U-shaped thickenings; pollen (supratectal gemmules + - Witsenia); stigmas simple, not or somewhat expanded; ovules 2/carpel (-6), endostomal, outer integument 2-3 cells across; 1 shield-shaped seed per loculus [seed tangentially flattened]; exotesta transparent, mesotesta 0-1 cell across, tegmen surface undulate; x = 16; seedling with compact cotyledon, cataphylls 0.
3/15: Nivenia (11). Only in the S.W. Cape region, South Africa.
Age. Crown-group Nivenioideae are estimated to be (33.5-)23.3(-13.6) Ma (Joyce et al. 2018).
7. Crocoideae G. T. Burnett
Plant with corms [central vascular tissue; producing roots], covering fibrous-reticulate; vessel elements in root with simple perforation plates; mesophyll cells elongated at right angles to leaf axis, leaf margin with a subepidermal sclerenchyma strand, epidermal cells with row of papillae; first leaves bladeless cataphylls; leaf sheath closed; inflorescence spicate, rhipidium with a single flower, pair of "bracts" at base of G; flowers long-lived, variously monosymmetric; endothecial cells with spiral thickenings; pollen sulcus operculate [when monosulcate, with one or a pair of longitudinal exine bands], exine tectate-perforate, supratectal spinules +; septal nectaries +; nucellar columella +, hypostase prominent, postament +; exotestal cells outlines prominent; chalazal endosperm haustorium +; seedling cataphyll +, tubular.
32/1,170. Overwhelmingly southern Africa, to Europe, Arabia, and Central Asia.
Age. Crown-group Crocoideae are only ca 24 Ma (Goldblatt et al. 2008) or (34.6-)26.7(-19.7) Ma (Joyce et al. 2018).
7A. Tritoniopsideae Golblatt & Manning - Tritoniopsis L. Bolus
Corm axillary; leaves with several major veins; inner bract larger, with single main vein; (oil flowers +); ovules hemitropous; n = 15, ?16; seedling with tubular cotyledon sheath.
1/24. South Africa, the Cape.
[Ixieae + Watsonieae]: corm terminal [developing from base of flowering stem], new stems/corms from axillary buds; leaf with pseudomidrib; inner bracts with two main veins, often bicarinate, apex ± bifid; flowers (polysymmatric); (style branches divided); ovules campylotropous.
7B. Ixieae Dumortier (inc. Croceae, Freesieae) —— Synonymy: Crocaceae Vest, Galaxiaceae Rafinesque, Gladiolaceae Rafinesque, Ixiaceae Horaninow
(Corm covering ± woody); (roots produced from ridge on corm); leaf (dorsiventral, keel with schizogenous cavity - Crocus), margins with columnar epidermis or subepidermal sclerenchyma, (epidermal cells papillae 0 - Dierama); (leaves plicate - Babiana); inflorescence (axis subterranean), (single-flowered); flowers poly-/monosymmetric; (pollen in-/spiraperturate); style branches undivided or not; (chalazal bundle ± separated from ovule, epidermal cells there thin-walled); seeds (smooth), (black), (circumferentially winged), (chalazal ridge +); n = 6, 7, 9-13, etc., x = 15, (karyotype bimodal); (seedling with tubular cotyledon sheath, cotyledonary chlorophyll 0 - Crocus, Romulea).
20/940: Gladiolus (270), Crocus (80-100-235), Geissorhiza (104), Ixia (100), Romulea (100), Babiana (93), Hesperantha (87), Dierama (45), Tritonia (30), Freesia (16), Sparaxis (16). Africa, inc. Madagascar, especially the south, some Canary Islands, Europe to W. China (N.W. Xinjiang).
Corm axillary, rooting from below [?all]; (stem ± winged); basal leaves cataphylls [?all], lowermost foliage leaves borne on corm (stem), leaves (plicate); pseudomidrib + (0 - Pillansia), marginal epidermal cells unspecialized, marginal vein with sclerenchyma cap (0 - P.); inflorescence spike (panicle - P.); pollen (zonasulcate - Micranthus/trisulcate), (surface reticulate); styles usu. branched; n = (3-)10, x = 10; seedling with compact cotyledon.
8/124: Watsonia (53), Lapeirousia (27). Africa, tropical, inc. Socotra, esp. southern Africa.
Evolution: Divergence & Distribution. It has been suggested that Iridaceae were originally Antarctic-Australia, the family achieving its current distribution by a mixture of long-distance dispersal across a proto-Indian Ocean and migration via west Antarctica to Africa and the New World where the family is currently very diverse (e.g. Sanmartín & Ronquist 2004; Goldblatt et al. 2008).
Davies et al. (2005) noted that in Iridaceae diversification was greater in areas like southern Africa than in the northern hemisphere, and that there were clades with a disproportionately large number of species in e.g. southern Africa. The Cape area is notably diverse from a global point of view (Kreft & Jetz 2007), and Iridaceae are one of the major geophytic groups of the Cape flora (Procheŝ et al. 2006, also Linder 2003) with more than 650 species there in at least two major clades, or 1,050 species in southern Africa as a whole (S. D. Johnson 2010). The great majority of these species have specialized pollination mechanisms and are typically pollinated by only a single pollinator species, pollinator shifts apparently helping to drive diversification (e.g. Johnson & Steiner 2003; Goldblatt & Manning 2006, 2007, 2015; Forest et al. 2013; see also Aizoaceae-Conophytum). Davies et al. (2004c) see this diversification as the result of the interaction of local features such as the ecological and climatic heterogeneity of the area, and this affects reproductive isolation. Valente at al. (2012) suggested that pollinator shifts had helped drive speciation in southern African Gladiolus, although other factors were also involved. For the radiation of the Cape genus Moraea, both cytologically and florally diverse, see Goldblatt et al. (2002, 2005: pollination; Galley et al. 2007: also diversification on the Drakensbergs); radiation in this and other iridaceous Cape genera may have begun in the fynbos in the Miocene some 25 Ma, divergence in the succulent Karoo being more recent (Verboom et al. 2009). Diversification of two Cape genera, the geophytic Babiana (Croceae), with ca 92 species nearly all from the Greater Cape floristic region, and Moraea (Irideae), with over 150 species in the Cape region, may in part be connected with soil type preferences changing during speciation; here diversification began a mere 17-15 Ma in the mid-Pliocene (Schnitzler et al. 2011); diversification rates in the Cape region and outside are largely similar (Silvestro et al. 2011).
In the New World, the large genus Sisyrinchium seems to have originated at medium to high elevations in the Central Andean to Central American area; climate and changes in altitudinal range seem to have influenced its subsequent diversification (Thode et al. 2021). Long distance dispersal resulted in the single species of Sisyrinchium (S. acre) that is to be found on Hawaii and simple range extension resulted in the colonization of North America (both events in section Hydastylus), and there also seems to have been long distance dispersal (three sections perhaps involved) to North America from the Pampa + Chaco area of South America (Thode et al. 2021).
Peter Goldblatt (in Renner & Schaefer 2010 and references) suggested that oil flowers were a synapomorphy for [Tigridieae + Trimezieae]. Oil flowers are also well known from South American species of Sisyrinchium and the evolution of oil flowers in the family may date back to 35 Ma (Renner & Schaefer 2010). However, placing the posession of elaiophores as a synapomorphy for [Trimezieae + Tigridieae] definitely needs to be confirmed (see also Pastori et al. 2021).
Manning and Goldblatt (1991) suggest apomorphies for Nivenioideae.
Pollination Biology & Seed Dispersal. Iridaceae show considerable floral variation, ranging from the widely cup-shaped flowers common in Sisyrinchium to the meranthia of Iris et al. and the tubular flowers of Gladiolus et al. (e.g. Bernhardt & Goldblatt 2006; Goldblatt & Manning 2006 and references; Rodrigues & Sytsma 2006; C. A. Wilson 2006). In species of Iris and its relatives the flower may appear to the pollinator as if were really three separate monosymmetric flowers (e.g. Westerkamp & Claßen-Bockhoff 2007; Guo 2015b). The tepaloid style overarches the stamen opposite it and the landing platform for the pollinator is the member of the outer perianth whorl that lies directly underneath the style/stamen complex. However, in Cypella (Tigridieae) the three landing platforms for the pollinating bees are members of the inner perianth whorl. Here the pollen that is deposited on the backs of the bees comes from half anthers of adjacent stamens and is deposited on the receptive surfaces of two adjacent half-stigmas (Vogel 1974).
The flowers of Gladiolus (Crocoideae) are obliquely monosymmetric, although this is hardly apparent in the open flower due to changes in orientation as the flower and inflorescence grow. Tepal patterning, where it occurs, is usually on an adaxial lateral tepal of the outer whorl and adjacent members of the inner whorl and is clearly on the adaxial side of the flower, but it may be on an adaxial lateral and the abaxial member of the outer whorl and a tepal of the inner whorl between them (Eichler 1875; Choob 2001). Although other Crocoideae may have the same oblique monosymmetry, Lapeirousia can have monosymmetric flowers with normal monocot orientation. Flowers in Diplarrena (Iridoideae) are also normal from this point of view, but they have very strongly differentiated inner tepals, only two stamens and one staminode. Interesting infraspecific variation occurs. In some flowers of Crocosmia x crocosmiiflora the odd member of the outer whorl was adaxial while in others it was abaxial; the patterning of the tepals, etc., varied accordingly (pers. obs.). All told, well over half the family has monosymmetric flowers of one sort or another, and the evolution of monosymmetry in the family will repay further study (see also Davies et al. 2004b).
Much work on pollination in Iridaceae has been carried out by Peter Goldblatt, John Manning, Peter Bernhardt and their collaborators, species from the sub-Saharan region of Africa, especially from South Africa, being their focus. Most species in the family as a whole have morphologically specialized flowers and are pollinated by non-specialist (if sometimes highly specialized) pollinators (Goldblatt & Manning 2006, 2008 for general accounts; S. D. Johnson 2010). Floral homoplasy is very extensive, similar floral morphologies pollinated by similar pollinators having repeatedly evolved independently in Iridoideae and Crocoideae (e.g. Bernhardt & Goldblatt 2006; Goldblatt & Manning 2006), as in Iridoideae-Tigridieae (Rodrigues & Sytsma 2006), -Trimezieae (Lovo et al. 2012), and -Irideae (for Iris itself, see C. A. Wilson 2006). Many different kinds of pollinators are involved, and even in quite small southern African genera there may be a variety of different pollinators, e.g., dung flies, long-tongued bees, wasps and beetles all pollinate Ferraria, which has only 17 species (Goldblatt et al. 2009, see also 2000a: Ixia, 2000b: Sparaxis). Babiana (Crocoideae) is pollinated by birds, scarab beetles, bees, moths, etc. (Bernhardt & Goldblatt 2006; esp. Goldblatt & Manning 2007), while hopline scarabeid monkey beetles pollinate the flowers of three Cape genera of Iridaceae including Aristea and Moraea that have distinctive dark/iridescent markings at the bases of the tepals (Goldblatt et al. 1998b) - some species of Drosera and other genera have similar flowers (e.g. Steiner 1998; van Kleunen et al. 2007). Valente at al. (2012; also Golblatt & Manning 1998a, b, 2002a) examined pollination in southern African Gladiolus Crocoideae-Gladioleae), recording numerous gains and losses of five of the seven pollination syndromes (the other two were decidedly uncommon), with pollination by long-tounged bees being acquired at least four or twelve times (depending on how this feature was optimized), but lost twice as frequently or more (see also Alexandersson & Johnson 2002). There were 17 pollinator shifts in the 23 species of Lapeirousia (-Watsonieae) studied (Forest et al. 2014), and these were more important in speciation than were changes in substrate specificity. Lovo et al. (2021) looked at the development of some Brazilian Trimezieae in the context of their phylogeny, pollination, etc., and found that simple flowers that were little elaborated, largely concolourous (yellow) and rewardless (no nectar or oil) were in fact derived from more elaborate ornamented flowers that often have rewards; the former are probably paedomorphic. Elaborated flowers attracted a greater variety of pollinators, so from that point of view they are generalists, while the simple flowers are specialists, being visited by pollen-collecting bees (see Armbruster 2017 for ecological, evolutionary and phenotypic specialization); simple flowers are also restricted to campos rupestres vegetation, rather inhospitable, and pollen flowers are a prominent component of this flora (Lovo et al. 2021). Interestingly, rather similar largely concolourous flowers, but blue in colour, are scattered in the family (Goldblatt et al. 2008), e.g. Sisyrinchium, the blue-eyed grass - and there is even a yellow blue-eyed grass. For a discussion on the evolution of the complex appendages, etc., on the sepals of bearded irises and their ilk, see Guo (2015a, b). Iris paradoxa is one of two species other than Orchidaceae with deceit pollination (Peakall 2023).
Oil flowers are quite common in Iridaceae, especially in a number of species from the New World - Mexico and South America in particular. Thus Centris, Paratetrapedia and Tetrapedia are the pollinators of Trimezieae, etc. (Renner & Schaefer 2010). Pastori et al. (2021) also discuss such oil flowers. Oil flowers are also well known from some 35 South American species of Sisyrinchium, Cypella, and genera like Tigridia, all members of Iridoideae-Sisyrincheae, -Trimezieae and -Tigridieae (Cocucci & Vogel 2001; Renner & Schaefer 2010; Chauveau et al. 2012; Possobom & Machado 2017a, Oleques et al. 2020 and references). In nearly all cases, the elaiophores are made up of mats of unicellular capitate trichomes (Tölke et al. 2019), and these appear to have evolved twice in Sisyrinchium alone (Chauveau et al. 2011). They vary both in details of morphology and position; in Sisyrinchium they are usually found at the base of the staminal column, while in Trimezieae and Tigridieae there are tufts of these hairs (= elaiophores) on the adaxial surfaces of the tepals, the inner tepals in particular. Cocucci and Vogel (2001) recorded female bees as being the main vistors in the species they studied; the flowers may simultaneously be buzzed by the bee, and this may also occur in taxa that are visited for their pollen alone (Cocucci & Vogel 2001). Nectar may also be produced in flowers with elaiophores, and perhaps nectar and/or pollen are also rewards in other taxa in all three tribes (Silvério et al. 2012; Báez-Lizarazo et al. 2021). Thus Cypella has elaiophores on its tepals and it may also have nectaries on its anther connectives, although these appear to be barely recognizable as such ("unstructured") and it is unclear if the nectar they produce is used as a conventional floral reward (Pastori et al. 2021). Evolution of floral rewards in these taxa shows a complex pattern of gains, changes and losses (Chauveau et al. 2012). Solitary Tapinotaspidini (Apidae, Apinae) bees are particularly important pollinators, and the bees have special scrapers on their front legs to collect the oil (Cocucci & Vogel 2001; Aguiar et al. 2020).
At least 34 species of southwest African Iridaceae are known to be pollinated by three species of (extremely) long-tongued dipteran nemestrinid flies, and all told slightly over 10% (117 species) of the 1,050 species of Iridaceae in southern Africa have such pollinators. The long-tubed monosymmetric flowers pollinated by these flies have evolved several times, both here and in unrelated groups (Manning & Goldblatt 1996, 1997; Goldblatt & Manning 2000, 2006), and Karolyi et al. (2013) discuss how the flies take in the nectar. Only 64 species of Iridaceae in the same region are bird pollinated, but over 550 species are pollinated by long-tongued Apidae (Goldblatt & Manning 2006).
Some species of Nivenia are heterostylous (Sánchez et al. 2010; Cohen 2019), a rather uncommon condition in the monocots.
Plant-Animal Interactions. Bufadienolides (cardiac glycosides), noted inhibitors of the animal enzyme Na+/K+-ATPase, is known from Moraea (Iridoideae) (Harborne & Williams 2001). Spilostethus saxatalis (Hemiptera-Lygaeidae) has moved on to Colchicum autumnale where it sequesters alkaloids which are used in plant defence much like the cardenolides sequestered by other lygaeids (Petscehnka et al. 2022).
Plant-Bacterial/Fungal Associations. Details of the nature of the presumably holomycoheterotrophic association of Geosiris are unclear (Imhof et al. 2013).
Vegetative Variation. Some Iridaceae are more or less woody and have monocot-type secondary thickening (see above); the vessel elements in the roots of such plants often have predominantly scalariform perforation plates (Cheadle 1964: inc. Klattia). Betekhtina et al. (2023) noted that the roots of Iridaceae they examined from the Middle Urals (Iris) were thick, branched and had short root hairs - c.f. Cyperaceae, Orchidaceae, Poaceae.
Galil (1968: focus on Colchicum) noted that during germination in some species of Iris the first-year corm ended up quite deeply planted in the ground (see also Rodionenko 1953: n.v.). This was possible because the cortex of the root disintegrated and the base of the first leaf (and associated plumule) grew down the space so formed (they remained in vascular connection with the root), the plumule then forming a cormlet quite deep underground (some species of Oxalis and Colchicum establish themselves in a similar way).
Foliar variation in Iridaceae is considerable (see Arber 1921 for a still useful early study). Within Crocoideae alone, leaves may be terete, unifacial and hollow (e.g. some Micranthus - Goldblatt et al. 2013b, also other leaf morphologies), or apparently ordinary and heterobifacial (e.g. also some Iris), and very commonly they are ensiform and isobifacial, as in Gladiolus and most Iris. Leaves genera like Crocus can be strongly ribbed or T-shaped (e.g. Rudall & Mathew 1990), but in transverse section they all seem to be variations of a basic isobifacial leaf theme (e.g. Ross 1892, 1893; Arber 1921, 1925; Rudall 1991). Some of this variation is ontogenetic. In Iris, for example, seedlings may have terete leaf blades, while those of adults are ensiform and isobifacial (Rudall & Buzgo 2002). Geissorhiza alone has ligulate leaves. There is further variation in the presence or not of cataphylls in the seedling and, indpendently, in the adult; in the latter, the first leaf of the new corm in Crocoideae is usually a cataphyll. In adult plants of taxa from Namaqualand, South Africa, there are water-catching leaves with very distinctive morphologies (Vogel & Müller-Doblies 2011). For leaf morphogenesis, including the development of plications, see Rudall (1990b) and for variation in foliar anatomy, see Rudall and Goldblatt (1991, as Ixioideae). Both terete and laterally flattened isobifacial leaves are also to be found in Juncaceae.
Genes & Genomes. A genome duplication event, the SIANα event, date at ca 81.3 Ma, characterizes the Iridaceae (Landis et al. 2018). Moraes et al. (2015) looked at the evolution of chromosome numbers (very variable) in Iridoideae, and also gave some 2C values. Although polyploid species had the highest 2C values, both polyploids and diploids had medium-sized to small genomes. For cytological evolution in Crocus, n = 3≤, see Harpke et al. (2013); multiple hybridizations, dysploidy events, and evolution of B chromosomes are all involved.
For the biparental transmission of plastids in crosses of Louisiana irises, see Cruzan et al. (1993). Joyce et al. (2018) discuss the evolution of the chloroplast genome in the mycoheterotrophic Geosiris.
Economic Importance. Mykhailenko et al. (2019) provide an entry to the literature on saffron, the stigmas of Crocus sativus; at around $600-$1000/kg (retail is higher), it is a very expensive spice...
Chemistry, Morphology, etc.. Several distinctive metabolites occur here, e.g. plumbagin in Aristea and Homeria (Harborne & Williams 2001). Iris contains a greater diversity of isoflavonoids than any other group outside Fabaceae (Reynaud et al. 2005); for xanthones, especially in Iris, see C. A. Williams et al. (1997b).
Goldblatt (1990) interpreted the paired "bracts" below the single flowers of Isophysis as representing a reduced rhipidium, a monochasial cymose inflorescence - a rhipidium may then be another synapomorphy for the whole family. Aristea is palynologically very variable, some members even having disulcate pollen (see Goldblatt & Le Thomas 1997; le Thomas et al. 2001). Crocus has spiraperturate pollen or variants of this, and again there is quite substantial infraspecific variation in pollen morphology (Candan & Özhatay 2013 and references: Crocus chrysanthus, also variation in chromosome number). The occurrence of septal nectaries needs to be checked. In Sisyrinchium and its relatives the style branches are commissural and alternate with the stamens; elsewhere the two are on the same radius. For a discussion of the caruncles/arils of Iris, see C. A. Wilson (2006). Galil (1968 and references) noted that in at least some species of Iris the plumule descended down the hollowed primary root, the corm thus developing below the surface of the ground. Tillich (2003a) and Goldblatt and Manning (2008) note the diversity of seedlings here; this needs better integration into the characterizations.
Additional general information is taken from Goldblatt et al. (1990: Sisyrinchieae, 1998a: family), Goldblatt (2001: family, esp. Iris), Goldblatt and Manning (2008: family, 2020: Iridaceae of Southern Africa) and Merckx et al. (2013a); see also Rodionenko (1987), Mathew (1989), Goldblatt and Manning (1998: Gladiolus) and Crespo et al. (2015), Iris s.l., Rübsamen-Westenfeld et al. (1994), Imhof et al. (2013: roots, mycorrhizae) and Gray and Low (2017), all Geosiris, Kerndorff et al. (2016a), Crocus, and Munguía-Lino et al. (2017), Tigridieae. For meta-carboxy aromatic amino acids, see Larsen et al. 1981) and for the phytochemistry of Crocus, see Mykhailenko et al. (2019), Rudall et al. (1986) and Rudall (1984: secondary thickening, 1995a) discuss anatomy, Goldblatt et al. (1984) crystals; see also Cocucci and Vogel (2001) and Rudall et al. (2003a), both nectary evolution, Manning and Goldblatt (1990) endothecial thickenings, Dönmez and Isik (2008: pollen), Venkateswarlu et al. (1980), C. A. Wilson (2001), Steyn (1973a, b) and Dorofeeva and Zhurbenko (2020: Iris), all embryology, Kerndorff et al. (2016b), seeds of Crocus), Goldblatt et al. (1989), seeds of Sisyrinchieae, and Hasenstein (2016) seed coat stomata.
Phylogeny. Iridaceae are monophyletic in nearly all studies (but c.f. Chase et al. 1995a). Initial results suggested that the monotypic Isophysidoideae were sister to the rest of the family, Crocoideae and Iridoideae appeared to be monophyletic, but the status of Aristeoideae was unclear. Reeves et al. (2001a, b: four genes) found that Patersonia, Geosiris, and Aristea were successively sister to a large clade making up [Aristeoideae + Crocoideae]; support was mostly moderate (see also Teixeira de Souza-Chies et al. 1997). If these relationships were confirmed, either the circumscription of Crocoideae would have to be considerably extended, or three more subfamilies would be needed. Goldblatt et al. (2008: five plastid genes) took this latter option; they found strong support for the pectinations basal to Crocoideae s. str., albeit using successive weighting, which tends to leave one a little uneasy; the topology above was also recovered by Joyce et al. (2018) in a generic-level reanalysis of Goldblatt et al.'s data. See Rudall (1994c) for a morphological phylogeny.
Within Iridoideae the morphologically distinctive Australian Diplarrhena may be sister to the rest (Reeves et al. 2001a, b; Rudall et al. 2003a). The five tribes in Iridioideae are quite well supported and have well-resolved relationships: [Diplarreneae [Irideae [Sisyrinchieae [Trimezieae + Tigridieae]]]] (Goldblatt & Manning 2008; also Golblatt et al. 2004, 2006). Irideae. For a phylogeny of Iris s.l., see Tillie et al. (2001) and C. A. Wilson (2004); there is phylogenetic resolution of the major groups in the genus (Wilson 2011), although some of the characters used to distinguish groups in the past, such as sepal crests (ridges or more elaborate structures down the midrib of the outer perianth whorl) have turned out to be homoplasious (Guo & Wilson 2014). Wilson et al. (2016) looked at relationships within the royal irises (sect. Oncocyclus) and Wilson (2017) at relationships within the bearded irises (subgenus Iris). Schnitzler et al. (2011) and Goldblatt et al. (2013a) looked at relationships in Moraea - those along the spine of the genus are rather poorly supported, although somewhat less so in Bayesian analyses. Sisyrincheae. Karst and Wilson (2012) obtained a fair degree of resolution in relationships within Sisyrinchium, although species limits there are in a considerable state of disarray (see also Chauveau et al. 2011). Inácio et al. (2017) provided a detailed phylogeny of the genus, and found that S. chilense and S. elegantulum were the result of wide crosses, while Thode et al. (2021: 102 spp., 8 markers) obtained a topology with a fair bit of geographic structure the deeper nodes of which were quite well supported. For diversification in the American Tigridieae, see Rodrigues and Sytsma (2006). Relationships within Trimezieae are being clarified (Lovo et al. 2012, 2018).
Five tribes of Crocoideae were mostly only moderately supported and their relationships poorly resolved in a study by Goldblatt and Manning (2008; also Goldblatt et al. 2004). Tritoniopsis may be sister to the rest of the subfamily, but support is at best moderate (Goldblatt et al. 2006; Golblatt & Manning 2008). For a phylogeny of Crocus see Petersen et al. (2008, c.f. in part Frello et al. 2004), and especially Harpke et al. (2013) and Kerndorff et al. (2016a, b). For relationships within Gladiolus, see Valente et al. (2011, 2012); although there was some resolution towards the base of the tree, many species, especially those from southern Africa, could not be distinguished.
Classification. In part I follow the classification suggested by Goldblatt et al. (2008; see also Goldblatt et al. 1998a; Goldblatt & Manning 2008: family, 2015: Lapeirousia and relatives, 2020: southern African taxa); the subfamilies are for the most part well characterised. For tribes in Crocoideae, see Goldblatt et al. (2006); as they noted, "We wonder whether it serves any purpose to adjust the tribal classification of Crocoideae in the absence of any real morphological discontinuities among them" (ibid. p. 407), and although Goldblatt and Manning (2008) recognised five tribes here, only three (see above) were recognized by Goldblatt and Manning (2020: notice their subtribal classification). Lovo et al. (2018) circumscribe genera in Trimezieae, while Chauveau et al. (2012) discuss generic limits in Tigridieae.
There is an infrageneric classification of Sisyrinchium in Inácio et al. (2017). C. A. Wilson (2011, also e.g. 2017) provided an infrageneric classification of Iris, a genus that Crespo et al. (2015, see also 2024) split into twenty five genera (and there is another classification in Rodionenko 2009). See Goldblatt et al. (2013a) for an infrageneric classification of Moraea and Goldblatt and Manning (2020) for infrageneric classifications of genera like Aristea, Freesia, Ixia, Romulea, Tritonia and Watsonia.
[Xeronemataceae [Asphodelaceae [Amaryllidaceae + Asparagaceae]]]: ovary superior; mitochondrial rpl2 gene lost.
Age. Estimates of the age of this node are around (84-)74, 67(-57) Ma (Bell et al. 2010), ca 100 Ma (Janssen & Bremer 2004), and (78-)69(-60) or ca 55.8 Ma (S. Chen et al. 2013).
Evolution: Divergence & Distribution. The loss of the mitochondrial rpl2 gene occurs either at this node or the next up the tree (see Adams et al. 2002b).
Phylogeny. This is a strongly supported group in Fay et al. (2000) and Soltis et al. (2007a); see also Janssen and Bremer (2004).
XERONEMATACEAE M. W. Chase, Rudall & Fay - Xeronema W. R. B. Oliver - - Back to Asparagales
Plant rhizomatous; leaves two-ranked, ventralized isobifacial [oriented edge on to the stem]; inflorescence branched, with inflorescence bracts, densely spicate; flowers large; stamens long-exserted, anthers centrifixed; pollen boat-shaped; style solid; ca 8 ovules/carpel, ?embryology; seeds bluntly papillate; n = 17, 18, x = 9 (?8), nuclear genome [1 C] (0.277-)3.803(-52.157) pg.
1 [list]/2. New Zealand (Poor Knights Island) and New Caledonia.
Chemistry, Morphology, etc.. The family is little known, although there is some information in Chase et al. (2000c); the style is scored as if it is hollow in Rudall (2003a).
Previous Relationships. Xeronemataceae were provisionally included in Asphodelaceae by Takhtajan (1997) and in Hemerocallidaceae by Clifford et al. (1998).
[Asphodelaceae [Amaryllidaceae + Asparagaceae]]: (pedicels articulated); septal nectaries infralocular; ovules with parietal tissue 2-3 cells across.
Age. The age of this node is some (72-)64, 58(-49) Ma (Bell et al. 2010), ca 93 Ma (Janssen & Bremer 2004), 75.9 or 81.4 Ma (Tank et al. 2015: Table S2), 61-54 Ma (Wikström et al. 2001), (72-)63(-55) or ca 43.6 Ma (S. Chen et al. 2013), 67.6 Ma (Magallón et al. 2015), (60.4-)54.2(-50.4) Ma by D.-F. Xie et al. (2020) or(89.6-)72.4(-57.6) Ma (Ji et al. 2022).
Evolution: Divergence & Distribution. For the optimisation of characters like "septal nectaries infralocular" and "ovary superior", see the beginning of this page. The optimisation of successive microsporogenesis on the tree is also uncertain (Chase et al. 2000a); for instance, microsporogenesis varies within Asphodelaceae.
Chemistry, Morphology. Gatin (1920: broad sampling across Liliaceae s.l.) found that taxa that have tepals with single vascular traces are common in this clade, although some have three or more traces; she also studied many other details of pedicel vasculature. Schnarf and Wunderlich (1939) provide embryological details and El-Hamidi (1952) some for the gynoecium from scattered taxa in "Asphodeloideae"; the latter found substantial similarity between all the taxa he examined except Aphyllanthes (Asparagaceae-Aphyllanthoideae). For chromosome sizes of a number of taxa in the group, see Vijayavalli and Mathew (1990 - as Liliaceae).
Phylogeny. This clade was early found to have strong support (Fay et al. 2000; Chase et al. 2000b).
ASPHODELACEAE Jussieu, nom. cons. - Back to Asparagales
Anthraquinones +, fructan sugars accumulated; roots (vessel elements with simple perforation plates), styloids +; leaf sheath closed; inflorescence scapose; pedicels articulated; (A not adnate to T); late-acting self incompatibility [all?]; outer integument ³3 cells across, hypostase +; x = 8 (?7), nuclear genome [1 C] (0.263-)3.209(-39.203) pg; cotyledon not photosynthetic.
41/900-1,060. Esp. Old World, not Arctic, western South America.
Age. This crown group is dated to ca 90 Ma (Janssen & Bremer 2004). Bell et al. (2010), on the other hand, estimate an age of (66-)52, 47(-36) Ma, S. Chen et al. (2013) a variety of ages - (66-)56(-48), ca 47, or ca 39 Ma, while (85-)74, 68(-60) Ma is the age in Crisp et al. (2014), 52.3 Ma in Magallón et al. (2015) and (73/3-)71.3(-69.4) Ma in McLay and Bayly (2016).
1. Asphodeloideae Burnett —— Synonymy: Aloaceae Batsch
Rosette-forming leaf succulents, geophytic (rhizomatous) herbs to pachycaul trees, (climbers); (CAM photosynthesis +); tetrahydroanthracenones + [e.g. chrysophanol], anthrone-C-glycosides [in leaves], 11-methyl-8-hydroxyanthraquinone [in root]; (velamen +); (monocot secondary thickening +); sieve tube plastids with central protein crystal + peripheral fibres - Aloë group); foliar vascular bundles often inverted, parenchymatous cells in the inner bundle sheath adjacent to the phloem [aloin cells], (cells sclerenchymatous); leaves spiral, two- (or four-)ranked, margins often with spines, deltoid to linear, (bases not sheathing); inflorescence racemose, branched or not, (spicate), (not scapose); (pedicels not articulated), monosymmetry +, ± weak [Haworthia et al.]; flowers tubular, T ± free (connate - Kniphofia, etc.), (with a single trace); (anthers centrifixed); microsporogenesis simultaneous; (pollen mixed with raphides); stigma dry (wet); ovules 1-many/carpel, hemitropous, (± straight - Asphodelus clade), outer integument 3-4 cells across, parietal tissue 1 (2) cells across, hypostase +; (embryo sac tetrasporic, three chalazal megaspores fuse, divide twice [Fritillaria-type] - Eremurus); (fruit a berry); seed ± angled/(winged), aril +, funicular (thin/0); endosperm cells thick-walled, hemicellulosic[?], (perisperm +, slight), embryo long; n = (6 - Kniphofia) 7, chromosomes 1.5-20 µm long, karyotype usu. bimodal; 3'-rps12 intron lost; (coleoptile +).
19 [list]/1,005: Aloë (625), Kniphofia (70), Bulbine (60), Trachyandra (50), Eremurus (45), Haworthia (42). Africa, esp. South Africa; also the Mediterranean to Central Asia, Australia, New Zealand. Map: see Reynolds (1966), Frankenberg and Klaus (1980) and Seberg (2007). Photo: Collection, Inflorescence, Flowers.]
Age. Crown-group Asphodeloideae are estimated to be (46-)34(-25) or ca 22.5 Ma (S. Chen et al. 2013) or (75-)69-58(-51) Ma (Crisp et al. 2014: see discussion after Xanthorrhoeoideae, the two models not differing here).
[Xanthorrhoeoideae + Hemerocallidoideae]: raphides 0.
Age. The age of this node is (63-)52.5(-45) or ca 46.4 Ma in S. Chen et al. (2013) or rather older, (71.2-)67.1(-62.5) Ma, in McLay and Bayly (2016). Note that the age of Xanthorrhoea stem in Crisp et al. (2014) is based on the topology [Hemerocallidoideae [Xanthorrhoeoideae + Asphodeloideae]].
The 47.8-38 Ma fossil Dianellophyllum eocenicum from Central Australia has been placed on the stem node of Hemerocallidoideae (Iles et al. 2015).
2. Xanthorrhoeoideae M. W. Chase, Reveal & M. F. Fay - Xanthorrhoea Smith —— Synonymy: Xanthorrhoeaceae Dumortier, nom. cons.
Stem thick, woody, erect (not); plant resiniferous; monocot secondary thickening +; layer of sclerenchyma below epidermis in leaves, stomata paracytic; leaves spiral, unifacial/± quadrangular, persistent, leaf base not sheathing, internodes short; inflorescence long-scapose, densely spike-like, branches cymose, congested; flowers sessile, not articulated; flowers ± tubular, T = 3 dry + 3 subpetal-like, free; stamens long exserted, spreading; microsporogenesis successive [tetrads tetragonal]; pollen extended sulcate; stigma ± punctate, ?wet; ovules several/carpel, outer integument ca 3 cells across, apex of nucellus pointed, hypostase?; inner cuticle of tegmen +; seeds flattened; endosperm quite thick-walled, development?, little hemicellulose, embryo transverse to long axis of seed; n = 11, (karyotype bimodal); hypocotyl short.
1[list]/30. Australia. Map: from Australia's Virtual Herbarium (consulted i.2015). [Photo - Habitat, - Habit, Inflorescence.]
Age. Crown-group Xanthorrhoeoideae are estimated to be a mere (3.8-)1.7(-0.3) Ma (S. Chen et al. 2013) or (59-)35-24(-13) Ma (Crisp et al. 2014), although the latter also obtained some very young ages, most in the range of (13-)6.4, 3.3(-1.8) Ma, one somewhat older. Crisp et al. (2014) preferred the older estimates, which came from using a random local clocks model, over the younger ages, which came from an uncorrelated lognormal relaxed clock model; the latter, they thought, could not handle the substitution rate changes.
3. Hemerocallidoideae Lindley —— Synonymy: Dianellaceae Salisbury, Eccremidaceae Doweld, Geitonoplesiaceae Conran, Hemerocallidaceae R. Brown, Johnsoniaceae J. T. Lotsy, Phormiaceae J. Agardh
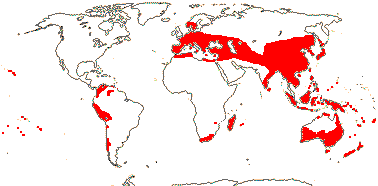
Habit various; flavonols, naphthoquinones, saponins +; roots often swollen; (vessels in the stem); mucilage cells 0; stomata anomocytic, cuticular wax rodlets parallel; leaves (spirally) two-ranked (equitant), vernation conduplicate to flat-conduplicate or plicate, (semi-ensiform, isobifacial), (margin serrulate); (inflorescence not scapose); (bracteoles lateral), flowers (monosymmetric); (median tepal of outer whorl adaxial - Hemerocallis), T tube short (1/2 connate - Hemerocallis/0); filaments often ornamented/swollen, anthers (centrifixed), dehiscing by pores/coiled after anthesis - Dianella and relatives); microsporogenesis simultaneous (successive), pollen trichotomosulcate (tetrachotomosulcate, monosulcate), usu. <30µm across, pollenkitt +; stigma dry (wet), (3-parted - Pasithea); ovules 1-many/carpel, outer integument 4-7 cells across, inner integument 2-4 cells across, parietal tissue none, nucellar cap ca 2 cells across (0), podium well developed, hypostase 0; antipodal cells large, persistent; fruit also a berry (nut); seeds ovoid, (flattened - Phormium), (with strophiole/aril - Johnsonia et al.); endosperm hemicellulosic, usu. helobial, embryo not central [Geitonoplesium], also short; n = 4 [Agrostocrinum], 8, 9, 11, 12, chromosomes 0.8-17.33 µm long; (cotyledon not photosynthetic - Dianella), epicotyl long or not, (hypocotyl 0; collar +), primary root well developed, branched or not.
20[list]/89: Dianella (40 or more). Papuasia to New Zealand and the Pacific, esp. Australia (e.g. all 8 genera of Johnsonieae s. str.), also Europe to Asia, Malesia, India, Madagascar, Africa; two genera and two species in South America (map: from Fl. Austral., Wurdack & Dorr 2009; Muscat et al. 2019: figs 2, 3 - Dianella). [Photo - Habit, Flower, Flower].
Age. Crown-group Hemerocallidoideae are (53-)45(-36) or ca 39 Ma (S. Chen et al. 2013) or (63.6-)58(-52.4) Ma (McLay & Bayly 2016).
A fossil, Dianellophyllum eocenicum, from the Middle Eocene has been collected from Lake Eyre, in Central Australia, and is quite similar to Dianella (Conran et al. 2003).
Evolution: Divergence & Distribution. For additional divergence dates within Hemerocallidoideae, see McLay and Bayly (2016). The subfamily is Australian with the exception of the Eurasian Simethis and Hemerocallis, and one species of Dianella in Zimbabwe (see Muscat et al. 2019: Figs 2, 3 for the distribution of the genus) which makes things biogeographically interesting (McLay & Bayly 2016).
Asphodelaceae-Asphodeloideae are very diverse (ca 340 species) in southern Africa (Johnson 2010) where Aloe started diversifying ca 16 Ma (Grace et al. 2015), moving north in Africa and with a major accumulation of species (>130, representing several dispersal events) on Madagascar (Dee et al. 2018). Eccremis and Pasithea represent independent migrations of the phormioid clade (Hemerocallidoideae) to South America (Wurdack & Door 2009), while Bulbinella (Asphodeloideae) is to be found in South Africa and New Zealand.
Some diversification in Xanthorrhoea may be associated with the aridification of the Nullarbor Plain some 14-13 Ma that separated eastern and western clades (Crisp & Cook 2007).
Chomicki et al. (2017b) look at the evolution of plant architecture in the Aloe area - most species have Tomlinson's and Corner's mpdels, some Leeuwenberg's. Both Hemerocallidoideae and Xanthorrhoeoideae have ovaries that can be interpreted as being secondarily superior and that have infra-locular septal nectaries (Rudall 2002, 2003a).
Ecology & Physiology. For an ecological account of Xanthorrhoea, see Lamont et al. (2004); the genus has quite deep roots. The plant grows in flammable savanna/shrubby vegetation and itself burns easily, but the apical meristem is unharmed and there is often post-fire flowering (fast-flammable: Pausas et al. 2017).
Radiations in succulent groups in general - Asphodeloideae in particular here - seem to have occurred quite recently, around 10 Ma or so; other clades include Aizoaceae, Cactaceae, succulent Euphorbia, Crassula, etc. (M. Lu et al. 2021 - see Ecology & Physiology in all these taxa).
The stomata in Phormium tenax are blocked by wax (Wulff 1898).
Pollination Biology & Seed Dispersal. Many species of the large genus Aloë (Asphodeloideae), perhaps some 85 species in southern Africa alone, but also to Arabia, islands of the Indian Ocean, are pollinated by birds (Rebelo 1987; McCoy 2019), short-billed birds other than typical nectar-eaters visiting some species with dark-coloured and bitter-tasting nectar that are not deterred by its taste (Johnson et al. 2006). There is also insect pollination, perhaps especially among the short-tubed species (Symes et al. 2009; Hargreaves et al. 2008, 2012; McCoy 2019), as in other groups of Asphodelaceae, and also bat pollination (Dee et al. 2018). Kniphofia, with its red, tubular flowers, is predominantly bird-pollinated, and like Aloe it has hexose-rich nectar (M. Brown et al. 2010). However, a variety of insects, birds, and mammals have been seen visiting flowers of Kniphofia, including the Ethiopian wolf (Canis simensis) observed licking the flowers of K. foliosa and getting pollen on its snout (Lai et al. 2024). Buzz pollination probably predominates in Hemerocallidoideae, and the small pollen grains (but c.f. Arnocrinum and Hemerocallis), although not the presence of pollenkitt, is consistent with this (Furness et al. 2014).
A number of Hemerocallidoideae have myrmecochorous seeds (Lengyel et al. 2010). Interestingly, the baccate fruits of the Lomatophyllum group of Aloë have winged seeds (Dee et al. 2018).
Vegetative Variation. Most members of the Hemerocallidoideae have leaves that are more or less isobifacial immediately above the sheath, but higher up they become dorsiventrally flattened and more "normal" in appearance; Pasithea, sister to the rest of the clade, lacks this isobifacial zone (Wurdack & Dorr 2009), so it is unlikely to be an apomorphy for the subfamily. The leaves of Geitonoplesium are resupinate.
Members of Asphodeloideae have more or less succulent leaves, and species of Aloë and Haworthia in particular are commonly rosette plants with massively fleshy leaves (e.g. Melo-de-Pinna 2016); these can be borne in spirals or be distinctively two-ranked, and seedlings/young plants may have two-ranked and adults spiral leaves (e.g. McCoy 2019; Molteno 2021). Species of Kniphofia have a variety of phyllotactic arrangements, including orixate (Yonekura et al. 2019). The vascular bundles in the leaf of Aloe may form a circle and there are globules in the outer bundle sheath (also in Kniphofia); the central cells of the leaf are gelatinous. As with Aizoaceae from southern Africa, there is great variation in the micromorphology of their epidermis (Cutler 1982); the two grow in similar extreme habitats. For the remarkable water-catching leaves in taxa growing in foggy deserts in Namaqualand, South Africa, see Vogel and Müller-Doblies (2011).
Clifford (1998) noted that seedlings of Xanthorrhoea had two-ranked leaves, those of the adult are spirally arranged. Phyllotaxis in Kniphofia can be tetrastichous, and the leaves develop in a sequence that represents the complex orixate phyllotaxis (see elsewhere).
Aloin cells are reported from Dianella (Hemerocallidoideae: see Rudall 2003a); on the other hand, Kniphofia lacks them, having a well developed sclerenchymatous cap in their place (as have some other Asphodelaceae, even some Alooideae). It is unclear if aloin cells are secretory (Beaumont et al. 1985: survey and chemistry).
Genes & Genomes. A genome duplication of crown-group Hemerocallidoideae, the PHTEα event, has been dated to 37.8 Ma (Landis et al. 2018).
Some species of Bulbine have a bimodal karyotype of n = 7, 4 long and 3 short (Spies & Hardy 1983), rather like the karyotype of Aloeae (4L + 3S: Chase et al. 2000a; Devey et al. 2006; Pires et al. 2006); they probably evolved independently, and the plants also have similar medicinal properties...
Chemistry, Morphology, etc.. The old Alooideae (= Asphodeloideae, part) are chemically very distinctive (Klopper et al. 2010 for a summary). Aloin, an anthraquinone glycoside, is a laxative commonly found in Aloë. 1-methyl-8-hydroxyanthraquinones, e.g. chrysophanol, are commonly found in the roots and anthrone-C-glycosides in the leaves (e.g. Manning et al. 2014). In other Asphodeloideae, Bulbine, Trachyandra, and Kniphofia all have knipholone, an anthraquinone derivative (van Wyck et al. 2005), but it appears not to have been reported from the Asphodelus clade. There is chelidonic acid in Johnsonia (Hemerocallidoideae) (Ramstad 1953).
The apical meristem of the stem in Xanthorrhoea media is massive - 580-1283 µm across (Staff 1968, q.v. for details of stem growth). The old Alooideae are reported to have tetracytic stomata (e.g. Cutler 1972), although this is questioned by G. Smith and van Wyk (1992).
The inflorescences of Xanthorrhoea are described as being terminal (Clifford 1998); they are sometimes axillary in Asphodeloideae. Within Hemerocallidoideae the flowers of Hemerocallis seems to have a lateral bracteole, as do those of Dianella; both may have "inverted" flowers (e.g. Eichler 1875; Ehrhardt 1992), although in Hemerocallis, at least, this seems to be variable. Hemerocallis flowers with the median outer tepal adaxial are common, but the seal of the Daylily Society shows a flower with the normal monocot orientation. The number of vascular bundles supplying the tepals in members of this subfamily varies from (1-)3-9(-25) (Clifford et al. 1998a) and the stamens are often rather elaborate. In at least some species of Aloë the larger stamens are opposite the inner whorl of tepals.
Microsporogenesis in Hemerocallis was described as being successive and the endosperm as being nuclear by Di Fulvio and Cave (1965, but c.f. Cave 1948, 1955) and Yan et al. (2017: intermediate microsporogenesis). Hemerocallis also has isoflavones, monosulcate pollen and a wet stigma, but it lacks a nucellar cap and septal nectaries. There are conflicting reports about embryo sac development (Yan et al. 2017 and references). In pollen morphology Hemerocallis was considered to be derived by Chase et al. (1996); with Simethis, which has trichotomosulcate pollen, it is sister to the rest of Hemerocallidoideae (see also McPherson et al. 2004; Wurdack & Dorr 2009; Furness et al. 2014: microsporogenesis in Simethis?), so monosulcate pollen in Hemerocallidoideae may be a reversal. Since Chamaescilla also has monosulcate pollen (McLay & Bayly 2016), the story becomes more complicated.
Ovule orientation at the basal node in the family is unclear (c.f. Steyn & Smith 1998). Kniphofia has a bistomal micropyle and a nucellar endothelium (Takhtajan 1985). Daru et al. (2013) noted that seedlings of Aloë and Gasteria have two-ranked leaves, whatever the leaf arrangement in the adults.
For general information, see G. Smith and Van Wyk (1998), Clifford and Conran (1998: Johnsoniaceae), Clifford et al. (1998a: Hemerocallidaceae), Grace and Rønsted (2017: Asphodeloideae), also Reynolds (1966, 2004), Carter et al. (2011), Frandsen (2017), esp. Aloë, well illustrated, and Smith and Figueirwedo (2020: Asphodeloideae), also Gilman et al. (2023: CAM photosynthesis), Riley and Majumdar (1979: biosystematics), Van Wyk et al. (1996, 2005), Viljoen et al. (1998: Aloe flavonoids), Grace et al. (2010) and Kite et al. (2000: anthroquinones), all chemotaxonomy, G. Smith et al. (1992: anatomy), Ely and Luque Arias (2006: anatomy of Eccremis), Kosenko (1994: pollen of Phormium), Shamrov (2014a: gynoecium of Hemerocallis), Eunus (1952), Raju (1957), Berg (1962), di Fulvio and Cave (1965), Cave (1975) and Zuo and Ma (2014), all embryology, Steyn and Smith (1998: ovule morphology, 2001) and Thadeo et al. (2015: fruit anatomy).
For Xanthorrhoea, some information is taken from Chanda and Ghosh (1976: pollen), Rudall (1994b: embryology), Rudall and Chase (1996: phylogeny) and Bedford et al. (1986) and Clifford (1998), both general.
Phylogeny. There is strong support for Asphodelaceae s.l. (in much recent literature the name used has been Xanthorrhoeaceae s.l.) in Fay et al. (2000), Wurdack and Dorr (2009), etc.. However, relationships within the clade were initially unclear. There is slight support for a [Xanthorrhoeoideae + Asphodeloideae] clade in the three-gene tree of Chase et al. (2000a; see also Fay et al. 2000; Crisp et al. 2014: chloroplast genes); some analyses in Chase et al. (2000a) also suggested an [Asphodeloideae + Hemerocallidoideae] clade. Hemerocallidoideae, and perhaps also Asphodeloideae, were paraphyletic in a rpb2 analysis of Crisp et al. (2014: ?rooting). However, Devey et al. (2006) found support for a [Xanthorrhoeoideae + Hemerocallidoideae] clade (see also Pires et al. 2006; Graham et al. 2006; Wurdack & Dorr 2009: good-moderate support; Seberg et al. 2012; Steele et al. 2012: strong support; Hertweck et al. 2015; S. Chen et al. 2013). Rudall (2003a) suggested that there were close morphological relationships between Hemerocallidaceae and Asphodelaceae - and between Xanthorrhoeaceae and Iridaceae...
Within Asphodeloideae, Aloë and its immediate relatives (= Alooideae s. str.: Klopper et al. 2010 for a summary) seem distinct and form a monophyletic group. However, more ordinary-looking Bulbine (for a phylogeny, see Devey et al. 2006) is sister to this clade, and then come other Asphodeloideae, including Kniphofia et al. and Eremurus et al., which together form a clade (Naderi Safar et al. 2014); an [Asphodelus + Asphodeline] clade (n = 14) is sister to the rest of the subfamily, and with good support (see Devey et al. 2006, also references below; McLay and Bayly 2016). Ramdhani et al. (2009) discussed the phylogeny of Kniphofia. For relationships around Aloë, which remained poorly understood and little resolved for some time and still present problems, see Treutlein et al. (2003a, b), Grace et al. (2015) and Dee et al. (2018). Ramdhani et al. (2011) looked at relationships around Haworthia, and Daru et al. (2013) and in particular Manning et al. (2014) have clarified relationships there, although support for some of the basal branches could be improved, and species of both Aloë and Haworthia are still scattered through the tree.
Rudall and Chase (1996) and Crisp and Cook (2007) have looked at the phylogeny of Xanthorrhoea.
There are two well supported clades within Hemerocallidoideae, the phormioid and johnsonioid (= hemerocalloid + johnsonioid) clades (e.g. Wurdack & Dorr 2009; also Seberg et al. 2012; Crisp et al. 2014). The loss of the 3'-rps12 intron characterises the johnsonioid clade (= [Johnsonieae [Hemerocallis + Simethis]]), see McPherson et al. (2004) and Chase et al. (2000b). Chamaescilla (ex Asparagaceae-Lomandroideae) has quite recently been found to be sister to the [Hemerocallis + Simethis] clade (McLay & Bayly 2016). Pasithea, from South America, is sister to all other phormioids (Wurdack & Dorr 2009; Seberg et al. 2012; Crisp et al. 2014). The other New World genus, Eccremis, previously of uncertain relationships (e.g. Clifford et al. 1998a) because of interpretations of morphology, is sister to Dianella (Wurdack & Dorr 2009), of the phormioid clade; similar relationships were also recovered by McLay and Bayly (2016) and Muscat et al. (2019), the latter group carrying out an extensive study of relationships in Dianella.
Classification. A.P.G. II (2003) suggested as an option the inclusion of Asphodelaceae, Xanthorrhoeaceae and Hemerocallidaceae in Xanthorrhoeaceae s.l., and this circumscription was adopted by A.P.G. III (2009), but because of nomenclatural issues the name of this clade has been changed to Asphodelaceae (A.P.G. IV 2016), otherwise, see Chase et al. (2009b). The recognition of an Alooideae (= Asphodeloideae-Aloeae) would make Asphodeloideae paraphyletic and necessitate the recognition of several other weakly characterized subfamilies.
G. Smith and Steyn (2004) discuss the taxonomy of Alooideae; generic limits around Aloë are decidedly unsatisfactory. Grace et al. (2013) and in particular Manning et al. (2014) have revised the classification of the whole group, recognising 11 genera (see also Rowley 2015), but it is clear that there is still work to do, the species of genera like Lomatophyllum being scattered around Aloë and sectional limits in the latter are problematic (Dee et al. 2018). Several intergeneric hybrids have been described from Asphodeloideae (Smith & Figueiredo 2020 and references), but their existence depends on generic limits...
Species limits are particularly difficult in Aloë, Kniphofia (Ramdhani et al. 2009) and in Haworthia (Bayer 2009; Ramdhani et al. 2011: ?hybridization), all Asphodeloideae. Species estimates in Dianella (Hemerocallidoideae) range from 25-350+ (Carr 2007); Muscat et al. (2019) suggest that the number will be well above 40.
Previous Relationships. Three genera that used to be placed in Asphodelaceae s. str., i.e. in Asphodelaceae-Asphodeloideae, are now in Hemerocallidoideae (Simethis), Asparagaceae-Asparagoideae (Hemiphylacus), and Asparagaceae-Agavoideae (Paradisea, Anthericaceae s. str.) respectively, while Chamaescilla, which used to be in Asparagaceae-Lomandroideae, has moved to Asphodelaceae-Hemerocallidoideae - the evidence is largely molecular (Chase et al. 2000b; McLay & Bayly 2016).
Thanks. I thank Syd Ramdhani and Matt Ogburn for useful discussions.
[Amaryllidaceae + Asparagaceae]: microsporogenesis successive [possible place]; endosperm development?
Age. This node is ca 91 Ma (Janssen & Bremer 2004), ca 71.5 Ma (Tank et al. 2015: Table S2), 58-51 Ma (Wikström et al. 2001), (69-)60, 54(-45) Ma (Bell et al. 2010), (67-)41(-50) or ca 41.6/40.6 Ma (S. Chen et al. 2013), about 62.5 Ma (Magallón et al. 2015), (50.6-)49.4(-48.2) Ma (D.-F. Xie et al. 2020), (86.9-)65.5(-52.2) Ma (Ji et al. 2022) or (52.6-)51.1(-49.5) Ma (Dennehy-Carr et al. 2025).
Evolution: Ecology & Physiology. Grime and Mowforth (1982) early noted links between large genomes in the taxa they examined (focus on the British flora), the geophytic habit, and fast growth by cell expansion of large cells (link to large genomes) under cooler conditions in the spring - geophytes are quite common in this clade.
Genes & Genomes. For chromosome size in Liliaceae s.l. and relatives, i.e. including some taxa in this area, see Vijayavalli and Mathew (1990).
Chemistry, Morphology, etc.. Steroidal saponins are particularly common in taxa in this part of the tree; records in older literature can be found under Liliaceae. Van den Ende (2022) mentions the occurence of fructans with an internal glucose residue, including the neo-inulin type, in the [Amaryllidaceae + Asparagaceae] clade.
Microsporogenesis is uniform here. In other Asparagales with successive microsporogenesis, details of wall formation (centrifugal cell plates) is similar to those members of this clade that have been studied, however, plate formation may also be centripetal when microsporogensis is simultaneous (Nadot et al. 2006). Johri (1966) provides some infomation about stylar morphology; the style can become solid in more than one way.
Septal nectaries are common here, as elsewhere in the monocots. However, Vogel (1998b) noted a distinctive path that nectar might take in such flowers - he mentioned taxa in Amaryllidaceae-Alliodieae-Leucocoryneae (but Allium itself seemed normal) and in Asparagaceae-Brodiaeoideae (q.v.) and -Convallarioideae-Polygonateae. Quite commonly there are nectar tubes or more or less enclosed channels in the flower, running down the ovary (nectar appears at the top) and sometimes also down most of the length of the gynophore, in both cases ending up at the bottom of the corolla tube.
Phylogeny. This is a strongly supported clade (e.g. Chase et al. 1995a; Fay et al. 2000; Chase et al. 2000b; Graham et al. 2005), however, inclusion of Aphyllanthes in analyses has tended to decrease support for the clades within it (Graham et al. 2006; Givnish et al. 2018a). Kim et al. (2011: seven genes, three compartments) found that Amaryllidaceae grouped with Asparagoideae, Lomandroideae and Convallarioideae; other members of this clade formed a separate group.
AMARYLLIDACEAE J. Saint-Hilaire, nom. cons. - Back to Asparagales
Fructan sugars accumulated; root vessel elements with scalariform perforation plates; leaves two-ranked; inflorescence scapose, umbellate [indeterminate primary axis, cymose lateral branches], inflorescence bracts 2 or more, scarious, floral bracts small; pedicels not articulated; flowers large [>1.5 cm long and across]; (T free); (A connate basally); style long, style solid, stigma dry; parietal tissue none; endosperm nuclear or helobial; x = 8 (?9), nuclear genome [1 C] (0.674-)8.187(-99.495) pg; hypocotyl 0.
73/1,605. Worldwide - three subfamilies below.
Age. Estimates of the age of crown-group Amaryllidaceae are ca 87 Ma (Janssen & Bremer 2004), (62-)51(-42) or ca 33.7 Ma (S. Chen et al. 2013), (51-)44.7(-42) Ma (Han et al. 2019), (77.2-)67.9(-58.5) Ma (Costa et al. 2020) or (50.3-)48.6(-46.6) Ma (Dennehy-Carr et al. 2025).
Evolution: Ecology & Physiology. Ma et al. (2018) noted that Amaryllidaceae, despite their herbaceous habit, had rather thick first-order roots.
Plant-Bacterial/Fungal Associations. Fungi on Allium and other Allioideae are rather different from those on Amaryllidoideae (e.g. Savile 1962).
Genes & Genomes. For cytological evolution, see Costa et al. (2020). Very large genomes with a C value of some 350 picograms or more are found in some Amaryllidaceae-Allioideae and -Amaryllidoideae - and also in Asparagaceae-Scilloideae (Leitch et al. 2005).
Chemistry, Morphology, etc.. Distinctive, mannose-binding lectins (the specificity is absolute) are found in Allioideae and Amaryllidoideae (van Damme et al. 1991: known from Agapanthus?; see also Peumans & van Damme 1995; Vandenborre et al. 2011).
Martínez-Gómez (2022) give references to the umbellate inflorescences found in practically all Amaryllidaceae (with rare exceptions - see Allium spicatum - subgenus Cyathophora); this inflorescence may be derived from a bostryx or cincinnus. For tapetal cells, see Wunderlich (1954), for inflorescence structure, see Weberling (1989).
1. Agapanthoideae Endlicher - Agapanthus L'Héritier —— Synonymy: Agapanthaceae F. Voigt
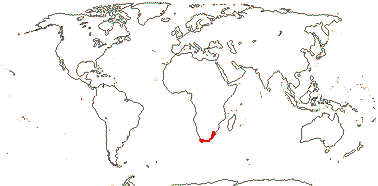
Plant rather shortly rhizomatous, rhizome horizontal to vertical; steroidal saponins and sapogenins +; multiple velamen +; laticifers +?; leaves usu. 2-ranked, vernation flat; inflorescence bracts 2, connate, "bracteoles" filiform; flowers weakly monosymmetric; T ± connate basally, blue; anther middle layer of wall from outer secondary parietal cells; ovules biseriate, apotropous; capsule pendulous; seeds with phytomelan [?level], flat, winged; endosperm with starch/hemicellulose, embryo short; n = (14) 15 (16), chromosomes 4-9 µm long; seedling as in Allioideae?
1 [list]/8. South Africa. Map: from Leighton (1965). [Photo - Habit, Flower.]
Chemistry, Morphology, etc.. Information is taken from Kubitzki (1998b) and Duncan (2021), both general and D. Zhang et al. (2010: reports of occasional embryos with two cotyledons, 2011: embryogeny).
[Allioideae + Amaryllidoideae]: plants geophytes, bulbous, bulbs tunicated [surrounded by scales], with contractile roots; lectins binding mannose; (corona +); (embryo sac bisporic, eight nucleate - Allium type).
Age. This node is estimated to be (62-)50, 46(-35) Ma by Bell et al. (2010) and (56.5-)47(-38), ca 30.3 Ma by S. Chen et al. (2013), (47.6-)41.9(-34.5) ma (D.-X. Xie et al. 2020) or (48.0-)45.5(-42.0) Ma (Dennehy-Carr et al. 2025). Janssen and Bremer (2004) estimate that the stem-group age of this clade is ca 91 Ma.
2. Allioideae Herbert
Flavonoids, cysteine-derived sulphur compounds +; root vessel elements often with simple perforation plates; raphides often 0, styloids +; laticifers +; leaves spiral, sheath closed, long; inflorescence bracts 2, ± connate; flowers medium sized [<1 cm long, <1.5 cm across]; T ± connate; A connate or adnate to free; style hollow, (stigma wet); ovules 2-many/carpel, collateral/2-seriate, campylotropous (anatropous), (micropyle bistomal), parietal tissue 0, nucellar cap +/0, obturator +; embryo sac bisporic, eight nucleate [Allium type]; seeds angular, exotestal, other layers of testa collapsed or not; (endosperm pitted), suspensor 2-tiered; chromosomes 2-20 µm long; (cotyledon not photosynthetic).
14/795: [list, to tribes]. Mainly South America, some Africa, Allium esp. N. Temperate-Eurasia - four groups below. [Photo - Collection] [Photo - Inflorescence, Flower, Flower.]
Age. Crown-group Allioideae are estimated to be ca 87 Ma (Janssen & Bremer (2004), (44.5-)37(-28) or ca 30.3 Ma (S. Chen et al. 2013), (44.5-)41.4(-35.8) Ma (Han et al. 2019), (67.5-)63.2(-53.7) ma (Costa et al. 2020), (55.3-)40.1(-28>5) Ma (Namgung et al. 2021) or (40.4-)34.5(-23.8) Ma (Dennehy-Carr et al. 2025).
2A. Allieae Dumortier - Allium L. —— Alliaceae Borkhausen, Cepaceae Salisbury, Milulacaeae Traub
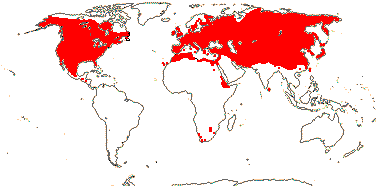
Bulbs with membranous scales, (rhizomes +); starch 0, spiranostanol steroidal saponins +, (cysteine-derived sulphur compounds 0); root exodermis multilayered, cortex not differentiated (yes), (walls with reticulating bands of thickening), stele narrow, usu. unmedullated; (longitudinally elongated arm cells with short protuberances [= peg cells]); leaves (± terete, with bifacial vascular bundles), (shortly ligulate); inflorescence enclosed by 2-5 bracts, (spike-like); T basally connate/free, with one trace; A basally connate, adnate to C, filaments often winged, at least basally, wing terminating in projection, tapetal cells uninucleate; (G semi-inferior), style ± gynobasic, solid, (paired projections from the ovary); ovules 2-many/carpel, in two rows, epi-/apotropous, outer integument 4-6 cells across, inner integument ca 3 cells across, (suprachalazal zone massive - A. fistulosum); (seed with caruncle); endosperm cellular, embryo long, curved; n = (7) 8 (9-11), chromosomes 9.0-19.3 µm long, TTAGGGn [human-type] telomeric repeats lost; (germination cryptocotylar).
1/1,020. North temperate, often seasonally dry, esp. the Mediterranean to Central Asian region and in west North America, scattered in Africa; not native in Iceland. Map: from Hultén (1962), de Wilde-Duyfjes (1976), Hanelt (1990), Hanelt et al. (1992) and Fl. N. Am. vol. 26 (2002).
Age. Crown-group Allieae are (45.8-)34.3(-24.3) Ma (Q.-Q. Li et al. 2016), (39.9-)33.5(-27.3) Ma (Han et al. 2019), (58.1-)52.2(44.4) Ma (Costa et al. 2020), (34.8-)22.2(-15.2) Ma (D.-F. Xie et al. 2020) and (28.8-)21.3(-14.4) Ma (Namgung et al. 2021), while the estimate in Hauenschild et al. (2017) is a mere (14.4-)12.8(-11.2) Ma. Friesen et al. (2024) noted that the first divergence within the Amerallium clade, basically resulting in the North American and The Rest clades, was ca 39.5 Ma, the two small subgenera associated with Amerallium having already split off ca 45.8 Ma.
Paleoallium genseli was described by Pigg et al. (2018) from deposits ca 49.4 Ma in Washington, U.S.A.; although it does look superficially like an Allium, its identity was disputed by Friesen (2022) and the fossil was not used for time calibration in the analysis of Dennehy-Carr et al. (2025).
[Tulbaghieae [Leucoryneae + Gilliesieae]]: bulbs with starch; corona +; endosperm helobial; embryo short; x = 6.
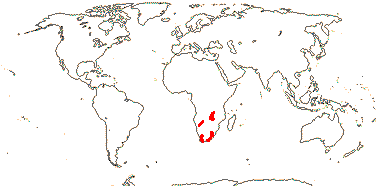
Age. This node is ca 37-32 Ma (Sassone & Giussani 2018), (65.1-)54.1(-37.1) Ma (Costa et al. 2020) or (39.1-)25.3(-11.5) Ma (Namgung et al. 2021).
The age of this node is estimated to be ca 26.8 Ma (Dennehy-Carr et al. 2025).2B. Tulbaghieae Meisner - Tulbaghia L. —— Synonymy: Tulbaghiaceae Salisbury
Plant rhizomatous; leaf sheath "short"; flowers bracteate; T rather strongly connate, corona massive, 3-lobed, lobes connate or not [opposite inner T]; A sessile, adnate to T tube and/or corona; style solid by enlargement of epidermal cells of cavity; ovules many/carpel [?all]; seeds ± flattened; n = 6, chromosomes 11.5-14.7 µm long; pseudogenization of cemA gene.
1/20. Southern Africa. Map: from Vosa (1975) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012).
[Leucocoryneae + Gilliesieae]: cysteine-derived sulphur compounds usu. 0; T ± connate; ovules 2/carpel, collateral; style gynobasic; stigma ± 3-lobed; x = 6/?5, karyotype bimodal, nuclear genome [1 Cx] 23.1-23.9 pg.
Age. The age of this node is ca 31.8 Ma (Sassone & Giussani 2018), (61.2-)ca 45(-32.2) Ma (Costa et al. 2020), ca 50.0 Ma (Escobar et al. 2020), (28.5-)16.5(-5.0) Ma (Namgung et al. 2021) or ca 22.0 Ma (Dennehy-Carr et al. 2025).
2C. Leucocoryneae (Ravenna) Sassone, S. C. Arroyo & Giussani
Steroidal saponins +; inflorescence (1-)many-flowered; A 6 (3), in 1 or 2 series (variously basally connate/adnate to T), (staminodes 3, 6); G (shortly stipitate), stigma (capitate), trilobed, papillate; ca 4-5 ovules/carpel; (embryo sac monosporic, eight nucleate [Polygonum type] - Nothoscordum); seeds black; x = 5, n = (4-)5(-7, etc.), nuclear genome [1 Cx] 9.07-30.46[-60.6(-79.3)?] pg.
7/65-100: Nothoscordum (25-80). South U.S.A., Mexico to Chile, most South America, but not in the NE. Map (South America): see Garcia et al. (2022a: Fig. 5).
Age. Crown-group Leucocoryneae are (35-)27.5(-25) Ma (Sassone & Giussani 2018) or ca 37.1 Ma (Escobar et al. 2020).
2D. Gilliesieae Baker —— Synonymy: Gilliesiaceae Lindley
Flowers ± monosymmetric/not; T (3 - Schickendantziella)/(3 + 3 reduced); (corona/petaliferous appendages +); A (2/3), ± free, (connate), (staminodes 3); septal nectaries 0; ?embryo sac; n = 6, 7, 10, acro- and metacentric, chromosomes 5.5-14.9 μm long, nuclear genome [1C] 18.4-31.1 pg.
8/26: Miersia (9). Peru to Chile.
Age. Crown-group Gilliesiae are (29-)18(-7) Ma (Sassone & Giussani 2018) or ca 25.6 Ma (Escobar et al. 2020).
3. Amaryllidoideae Burnett - Back to Asparagales
Norbelladine alkaloids, non-protein amino acids, chelidonic acid +, saponins 0; roots contractile, (velamen +, 2-4-layered), medulla often 0; sclerenchymatous ring in scape, bundles in rings; foliar (vascular bundles inverted), basal bundles in arc; (lacunae formed by breakdown of parenchyma); leaves (spiral), vernation flat or revolute to involute, (base sheathing); bracts equitant[?]; flowers large; T with median member of outer whorl adaxial, ± free; anther middle layer of wall from outer secondary parietal cells; (tapetal cells uninucleate); G inferior, stigma capitate to deeply trifid, (wet); (ovule with outer integument ³3 cells across), (inner integument to 4 cells across), (nucellar cap to 3 cells across); endosperm starchy or with hemicellulose, (thin-walled), embryo poorly differentiated, small; x = 11, chromosomes (1.5-)3-28 µm long; cotyledon bifacial, (not photosynthetic), primary root well developed, contractile.
Ca 75 [list: to tribes]/900 - fourteen tribes below. Tropical (temperate), esp. South America and Africa, also Mediterranean. Map: from Alan Meerow and O. Seberg (pers. comm.), Snijman (1984). Fl. N. Am. Vol. 26 (2002) and de Castro et al. (2012). Photo: flower, fruit.
Age. Crown-group Amaryllidoideae are (39-)28.5(-19), ca 15.9 Ma (S. Chen et al. 2013) or (42.3-)37.5(-30.5) Ma (Dennehy-Carr et al. 2025).
3A. Amaryllideae Dumortier —— Synonymy: Crinaceae Vest, Strumariaceae Salisbury
(Growth monopodial - Crinum); stomata paracytic, subsidiary cells with oblique divisions; extensible [helically-thickened] fibres in leaf; leaves follow the flowers, (perennial - many Crininae), spiral or 2-ranked; filament cup + [of webbing joining filaments basally, or small appendages developed from filaments]/ 0 [Crinineae]; tectal cells binucleat; pollen bisulcate, exine gemmate, with scattered spinules, intectate-columellate; style solid apically [Crinum], (laterally displaced); ovules unitegmic (ategmic; parietal tissue 0 - Crinum); embryo sac bisporic, 8-celled [Allium type], (antipodal cells persistent); seeds water-rich, non-dormant, phytomelan 0, testa multiplicative, to 25 cells thick, chlorophyllous, with stomata/± collapsed/0, cork layer in endosperm [Crinum]; endosperm chlorophyllous, usu. with a corky layer, starchy, embryo chlorophyllous; (n = 10, 12, 15), chromosomes 5.3-20.5 µm long.
11/146: Crinum (65), Strumaria (23). SubSaharan, especially South Africa, Crinum Pantropical.
Age. The age of crown-group Amaryllideae is estimated to be some (26.4-)17.4(-9.5) Ma (Dennehy-Carr et al. 2025).
[[Calostemmateae [Cyrtantheae + Haemantheae]] [Eurasian Clade + American Clade]]: bundle sheath cells parenchymatous.
[Calostemmmateae [Cyrtantheae + Haemantheae]]: ?
Age. The age of this clade is ca 29.6 Ma (Dennehy-Carr et al. 2025).
3B. Calostemmateae D. & U. Müller-Doblies
Ovules 2-3/carpel; embryo germinates precociously producing a bulbil; fruit dry, indehiscent; phytomelan 0; n = 10, chromosomes 3.3-8 µm long.
2/4. Australia and the S.W. Pacific, Malesia.
Age. Calostemmateae are estimated to be 23.3-)16.3(-9.6) Ma (Dennehy-Carr et al. 2025).
[Cyrtantheae + Haemantheae]: 1-layered rhizodermis +, velamen 0; scape lacking sclerenchymatous ring, subepidermal collenchyma +; fruit indehiscent.
3C. Cyrtantheae Traub —— Synonymy: Cyrtanthaceae Salisbury
Leaves two-ranked; scape hollow; flowers poly- to strongly monosymmetric; (filment cup + [basal appendages of filaments connate, forming 12-lobed structure]; late-acting self incompatibility; seeds flat, winged, horizontally stacked; n = (7) 8 (11).
1/56: Cyrtanthus. Africa, mainly the South.
Age. The age of Cyrtantheae is some (18.2-)9.6(-3.4) Ma (Dennehy-Carr et al. 2025).
3D. Haemantheae Hutchinson —— Synonymy: Gethyllidaceae Rafinesque, Haemanthaceae Salisbury
(Plant rhizomatous - Clivia, Scadoxus); (alkaloids 0 - Gethyllis [= G.]); leaves spiral, blade spirally twisted/narrowly linear/with dense long white hairs/etc.; scape 2-angled; inflorescence bracts several, equitant, (T-like); (flowers single - G., etc.), (densely aggregated - Haemanthus); (A 6-fasciculate, 6-8 A/fascicle - G.); (filament cup +); late-acting self incompatibility [all?]; fruit baccate; seeds angled/embedded in fleshy pulp/etc.; phytomelan 0 (+ - Cryptostephanus); n = 6, 8, 9, 11, 12; chromosomes 3.0-24 µm long.
6/80: Gethyllis (39, inc. Apodolirion), Haemanthus (22). Tropical Africa, mostly in the South.
Age. Crown-group Haemantheae are thought to be (29.9-)22.2(-15.2) Ma (Dennehey-Carr et al. 2025).
Age. This clade has an age of around (28.8-)22.0(-15,5) Ma (Dennehy-Carr et al. 2025).
[Lycorideae [Galantheae [Pancratieae + Narcisseae]]] / Eurasian Clade: seeds subglobose, turgid.
Age. The age of the Eurasian Clade is estimated to be (24.8-)18.3(-12.2) Ma (Dennehy-Carr et al. 2025)
3E. Lycorideae D. Müller-Doblies & U. Müller-Doblies
Spathe 2-valved; stigma ± capitate/3-lobed; 2-many ovules/carpel; (seeds irregular discs - Ungernia); n = (8, 10) 11.
3/27: Lycoris (20). Temperate to subtropical East Asia, to Iran.
[Galantheae [Pancratieae + Narcisseae]]: ?
3F. Galantheae Parlatore —— Synonymy: Galanthaceae G. Meyer, Leucojaceae Borkhausen
(Roots not medullated); (inflorescence bracts connate along one side); (anthers dehiscing by pores); parietal tissue +; embryo sac bisporic, 8-celled [Allium type; elaiosome + (0); n = 7-9, 11, 12, nuclear genome size [1Cx] -82.2 pg [Galanthus lagodechianus].
8/31: Galanthus (17). Europe to N. Africa, Crimea and the Caucasus.
3G. Pancratieae Dumortier —µ Synonymy: Pancratiaceae Horaninow
Leaves spiral; filament cup +; style solid apically; n = 11, chromosomes 8.7-22 µm long.
1/20: Pancratium. Mediterranean, southern Asia, to sub-Saharan Africa.
3H. Narcisseae Lamarck & de Candolle —— Synonymy: Narcissaceae Jussieu
(Roots not medullated); inflorescence bracts basally connate; spathe 1/2-valved; (staminal corona + [tubular - N.]); (heterostyly + [N.]); stigma 3-lobed to ± capitate; late-acting self incompatibility [N.]; parietal tissue to 2 cells across, ?nucellar cap +; embryo sac bisporic, 8-celled [Endymion-type]; seeds (flattened), elaiosome + (0); n = (7) 10, 11 (13), etc.; nuclear genome (14.3-)14.5-67.7(69.7) ... (93.6-)96.3(-99) pg.
2/58: Narcissus (?50). Europe to W. Asia and N. Africa.
Age. Crown-group Narcisseae are ca 23.6 Ma (Santos-Gally et al. 2012) or ca 13.8 Ma (Marques et al. 2017).
[[Griffinieae + Hippeastreae] [Eustephieae [Eucharideae [Hymenocallideae + Clinantheae]]]] / Andean + Extra-Andean/American Clade: 1-layered rhizodermis +; scape lacking sclerenchymatous ring, subepidermal collenchyma +; bracts obvolute; (seeds flat, horizontally stacked), phytomelan common.
Age. The American clade is thought to have an age of (25.2-)18.6(-12.6) Ma (Dennehy-Carr et al. 2025).
[Griffinieae + Hippeastreae]: arm-palisade cells +.
3I. Griffinieae Ravenna
Velamen + [Worsleya]; flowers blue; seeds whitish, globose, turgid [Griffinia]; n = 10, 21.
2/22: Brazil.
3J. Hippeastreae Sweet —— Synonymy: Brunsvigiaceae Horaninow, Oporanthaceae Salisbury, Zephyranthaceae Salisbury
Stomata anomocytic; palisade tissue isobifacial/adaxial/0; (leaves pseudopetiolate); inflorescence bracts 2, ± connate or not; flowers (very strongly) monosymmetric/polysymmetric, odd member of the outer whorl adaxial/abaxial; T tube short to long, (corona +, short, morphology various); A declinate (not), of varying lengths; style solid [Zephyranthes], stigma capitate or 3-lobed; seeds flattened, winged or D-shaped, (elaiosome +); n = 6-9, 11, chromosomes 3-16.7 µm long.
6/210: Zephyranthes (175), Hippeastrum (116). S.E./S.W. U.S.A., the Caribbean, and Central and South America.
[Eustephieae [Eucharideae [Hymenocallideae + Clinantheae]]] / Andean Tetraploid Clade: palisade leaf mesophyll absent; flowers polysymmetric (slightly monosymmetric); seeds flattened, winged; x = 23 [tetraploid], nrITS1 with two indels.
Age. Meerow et al. (2020) suggest that this clade may be ca 30.9 Ma, although at the same time they wonder if this is somewhat too great an age.
3K. Eustephieae Hutchinson
Scape ± flattened/angled; A of two lengths; (corona +); (n = 21, etc.).
3/15: C. Andes (Peru, Bolivia, Argentina).
Age.The age of crown-group Eustephieae is estimated to be around 24.1 Ma (Meerow et al. 2020).
[Eucharideae [Hymenocallideae + Clinantheae]]]: nrITS2 with indel.
Age. This node is ca 28.7 Ma (Meerow et al. 2020).
3L. Eucharideae Hutchinson (inc. Stenomesseae)
Leaves petiolate, elliptic; (flowers monosymmetric); filament cup + (0); pollen (in tetrads); (globose nectary glands at base of A - Eucrosia); seeds (globose, turgid, coat lustrous; endosperm oily); chromosomes 2.3-10.7 µm long; plastome ndhF 0, other ndh genes pseudogenized.
6/90: Stenomesson (35), Eucharis (17). Central America, the Andes S. to Bolivia.
Age. This clade is around 12 Ma (Meerow et al. 2020).
[Hymenocallideae + Clinantheae]]]: ?
Age. The age of this clade is approximately 28.1 Ma (Meerow et al. 2020).
3M. Hymenocallideae Small
Leaves (pseudopetiolate); scape ± flattened/angled; filament cup +; pollen (>100 μm long), ends of grains narrowed, with different sculpture [± auriculate]; ovules (many/carpel); testa thick, spongy, chlorophyllous, vascularized, phytomelan 0 (+ - Leptochiton); embryo starchy, (polyembryony +); (n = 12, 17, 19, 20, 22, 23), chromosomes 4-11.8 µm long.
3/65: Hymenocallis (50). S.E. U.S.A., the Antilles, Southern Mexico to Bolivia.
Age. Crown-group Hymenocallideae are around 26.1 Ma (Meerow et al. 2020).
3N. Clinantheae Meerow
(Epiphytic/epilithic); (velamen + - Pamianthe); seeds flattened, obliquely winged.
3/. Columbia to Bolivia and Peru.
Age. The age of this clade is ca 26.9 Ma (Meerow et al. 2020).
Evolution: Divergence & Distribution. There is quite extensive discussion about ages in Amaryllidaceae in Dennehy-Carr et al. (2025). For additional dates in Leucocoryninae, see Sassone and Giussani (2018) and in Allium, see Han et al. (2019) and D.-F. Xie et al. (2020).
Costa et al. (2020) obtained rather older dates for/within Allioideae than other workers, and with a focus on the Southern Hemisphere, they interpreted the biogeography of the group partly in terms of vicariant events. Thus they thought that Allium, for example, might have rafted north on India, so leading to an an "out of India" distribution. However, Namgung et al. (2021) suggested that Allioideae originated in Africa, then moving to Europe in the mid-Miocene; it may have arrived in South America by long distance dispersal.
Nguyen et al. (2008) found that most Old and New Word species of Allium are to be placed in two geographically separate clades. Basal to the clade containing all North American members of Allium (in subgenus Amerallium) are European taxa, and there are two other main clades that are made up of Eurasian and Central-West Asian taxa respectively (see also e.g. Friesen et al. 2006; Han et al. 2019). Diversity within North America Allium is centred in the west, especially in California, and a number of species there are serpentine endemics (Nguyen et al. 2008). Q.-Q. Li et al. (2016) looked at biogeographical relationships within subgenus Anguinum, which is made up of a clade restricted to eastern Asia and another that is found throughout the northern hemisphere. Yusupov et al. (2022) suggested that the area from the Caucasus to Central Asia and Iran was where Allium originated; they place the distributions of five seed characters on their tree, which includes representatives of about two thirds of the sections. Han et al. (2019) suggested that polyploidy was involved in the diversification of the genus (clades with more polyploids had higher diversification rates), along with shifts to habitats that had different soils; see also Babin and Bell (2022) for chromosome number change here. Jang et al. (2023) looked at the floral morphology of 74 species of Allium (30 sections, 9 subgenera, comparison with ITS tree), providing illustrations of the floral variation for all the species they examined. All told, they looked at 11 quantitative and 17 qualitative characters (many of the latter are reified quantitative characters); there is clearly some important variation here to use as the phylogeny of the genus gets straightened out.
Meerow (2010) discussed diversification in American Amaryllidaceae in terms of the interplay of canalization and genome doubling, emphasizing the floral and vegetative diversity encompassed by the Andean tetraploid-derived clade. Santos-Gally et al. (2012) examined the biogeography of the Mediterranean-centred Narcissus, the two main clades in the genus diversifying within the last 10 Ma as dry grassy vegetation became established in the western Mediterranean (Marques et al. 2017). Meerow et al. (2020) discussed the biogeography of the Andean Tetraploid Clade, which has reached the southeastern United States (Hymenocallis) in some detail. About a third of the subfamily, 240-280 species, grow in southern Africa, and around 85% of these are found nowhere else (Johnson 2010; Duncan et al. 2016, 2020).
García et al. (2014, 2017) thought that there had probably been extensive and ancient hybridization in Hippeastreae-Hippeastrinae, ca six events being likely; they noted cytonuclear discordance and non-monophyletic genera, and also incomplete lineage sorting in the stem clades, however, relationshiups in -Traubiinae were tree-like (see also Stull et al. 2023). Marques et al. (2017) emphasized that there had been much hybridization, including between members of different subgenera, in Narcissus. For more on intergeneric hybridization, see Z.-H. Feng et al. (2024).
Petiolate leaves have evolved at least six times in the family (?ref).
Ecology & Physiology. Although there has been a fair amount of work carried out on the possible selective advantage of bulbs, corms and tubers versus rhizomatous and other plants, it is unclear to what extent having bulbs, etc., leads to increased diversification: as Howard et al. (2020: p. 1023, see also 2019) noted "Geophytism shows higher rates of diversification relative to nongeophytes but we found little support for the hypothesis that the evolution of the bulb, corm or tuber appears to provide a diversification increase relative to rhizomatous and nongeophytic taxa." All very confusing.
Pollination Biology & Seed Dispersal. Gilliesia has very strongly monosymmetric flowers with only two stamens; the flowers may mimic insects (Rudall et al. 2002). For septal nectaries and where nectar ends up in the flower, see Vogel (1998b). Amaryllidoideae like Haemanthus have pseudanthia (Baczynski & Claßen-Bockhoff 2023).
Vegetative Variation. The apparently bifacial leaves of at least some species of Allium have inverted vascular bundles along the adaxial surface and vascular bundles with normal orientation along the abaxial surface (Mathew 1996). In a comprehensive study, Mashayekhi and Columbus (2014) looked at the leaf anatomy of 67 species of Allium, finding i.a. that species with terete, unifacial leaves might have a ring of bundles or a single series of normally oriented bundles, and species with flattened leaves sometimes had two series of normally-oriented bundles.
Genes & Genomes. Messeri (1931) and Vosa (1975, 2000: Tulbaghia) discuss aspects of the cytology of Amaryllidaceae. Within Leucocoryninae there has been hybridization, polyploidy, and also Robertsonian translocations (i.e., karyotype change by chromosome fusion or breakage at the centromere), and the extremes of genome size are represented by Ipheion (low) and Leucocoryne (high) - altogether genome evolution has been very active in this clade (Sassone et al. 2017). Indeed, at over 6,000 Mbp Amaryllidaceae have by far the largest minimum holoploid genome size of any angiosperm family (Liliaceae, at ca 3,500 bp, are next), although only ca half the size of the genome of Zamiaceae (Elliott et al. 2022b: Fig. S11, observations from at least 5 genera and 20 species/family).
Han et al. (2019) noted that polyploidy was scattered throughout Allium, while Babin and Bell (2022) examined the mechanisms involved in the change in chromosome numbers, and from a base number of x = 8, they found that there had been quite extensive ascending dysploidy (somewhat unusual), and also descending dysploidy and demipolyploidy, the latter being the result of the fusion of gametes with different ploidy levels. Allium has lost its TTAGGGn telomere minisatellite terminal sequence repeats, and although some species do have this repeat, it is not telomeric (Sýkorová et al. 2006a). Centromere variation is quite extensive, and at ca 10.6 Mb the centromere of garlic (A. sativum) is the biggest of any monocentric organism (as of 2025) - it has very large chromosomes - and there are gypsy-type LTR retrotransposons, CRs, although whether there are true CRs is unclear (Nagaki et al. 2025). Furthermore, in onion, A cepa,centromeres vary in position, although on subsequent hybridization with A. fistulosum its position stabilized (Nagaki et al. 2025). Friesen et al. (2000) thought that the rate of ITS evolution here was high.
The plastome in Allium varies from 145,819 to 157,735bp, greater than the variation in the rest of the family (Namgung et al. 2021). Munavvarov et al. (2022) found that there has been quite extensive gene loss and especially pseudogenization in the plastome of Allium (see also Namgung et al. 2021).
There has been a major movement of several ribosomal protein genes and of the succinate dehydrogenase gene from the mitochondrion in Allium (Adams et al. 2002b; Adams & Palmer 2003). The chondrome is in three circles, and the gene involved in cytoplasmic male sterility, at least in the S-type cytoplasm of "Momiji-3" (A. cepa), is orf 725 (Tsujimura et al. 2018).
Ecology & Physiology. Amaryllidoideae are an important component of the distinctive Cape geophytic flora (Procheŝ et al. 2006) having about 100 species endemic there. For water-catching leaves with very distinctive morphologies that are found especially in taxa from Namaqualand, South Africa, see Vogel and Müller-Doblies (2011).
In Allium, rhizomatous taxa are to be found in forested, mesic conditions, and bulbs in sunnier, more xeric habitats with a shorter growing season.
J. Zhou et al. (2024) found that the amaryllidaceae alkaloids of Lycoris radiata affected the microbiome of the plant. Some endophytic bacteria and pathogenic fungi were susceptible to the alkaloids, others not, and these latter might increase the alkaloid content of the plant - to the benefit of both plant and bacterium.
Pollination Biology & Seed Dispersal. Monosymmetry is thought to be ancestral in Amaryllidoideae (Meerow & Snijman 1998; Meerow 2010). It is certainly very labile, reversals and parallelisms being common, and it is perhaps under simple genetic control (Meerow et al. 1999). Some kind of corona is quite common, but its morphological nature varies (see below). The flowers are protandrous. A number of species of Amaryllis are di- or tristylous (Graham & Barrett 2004; Santos-Gally et al. 2013 and references).
Bird pollination is quite important in Amaryllidoideae. A. Meerow (pers. comm. ii.2014) estimated that around 100-150 species in South America (genera like Brunswigia, Hippeastrum, Stenomesson) may be pollinated by hummingbirds, while in southern Africa ca 13 species of Cyrtanthus alone are pollinated by sunbirds (Snijman & Meerow 2010). Several other kinds of pollinators service Cyrtanthus, including hawk moths, which may pollinate ca 22 species of Amaryllidoideae from southern Africa (Manning & Snijman 2002). Pollination of some red- and orange-flowered taxa there is by largish butterflies, which get pollen on the lower surfaces of their wings from the brush-type flowers/inflorescences and transmit it to other flowers; this type of pollination may be derived from bird pollination (Butler & Johnson 2020 and references).
Almost three hundred species in Amaryllidoideae have myrmecochorous seeds (Lengyel et al. 2010). Wind dispersal of the seed is common in Amaryllideae, and the rigid, radiating pedicels of taxa like Boophone allow the infructescences to bowl along in the wind. The testa is commonly massive, green and photosynthetic, and with anomocytic stomata in Amaryllidinae, while in Crinum the endosperm is also green and photosynthetic. Seeds of some species lack a testa but have a corky outer endosperm, and they can float and remain viable in sea water for up to two years; seeds of other species lack the corky layer and sink fast - in fact they can germinate without very much in the way of water at all (Snijman & Linder 1996; Bjorå et al. 2006). In Boophone and Cybistetes the seeds germinate while still in the fruit, while in Calostemmateae the bulbil, actually a precociously-germinated embryo, is the dispersal unit. Gethyllis (Haemantheae) has a single-flowered inflorscence and the flowers have a subterranean ovary. Here the fruit is baccate and grows just above the surface of the ground and is sometimes sweetly scented when ripe; the seeds are reported to be embedded in fleshy pulp (Duncan et al. 2016, 2020), perhaps dispersal by small mammals?
Vegetative Variation. Agapanthus has two primordia on its cotyledonary tube, Cyrtanthus has four (see also Buchholz 1919). Robertson (1906) described the inflorescences of Galanthus and Leucojum as being lateral, the stem being monopodial; stalked bulblets were produced. Gethyllis (Haemantheae) in particular shows remarkable foliar variation, and some species have collars (?= a sheathing leaf base) surrounding the leaves, which are narrow to broad, spirally twisted or not, and with variable indumentum (Duncan et al. 2016, 2020). Vogel and Müller-Doblies (2011) describe water-catching leaves with very distinctive morphologies that are found especially in Namaqualand, South Africa.
Genes & Genomes. A genome duplication for crown-group Amaryllidaceae, the AGAFα event, occurred an estimated 50.1 Ma, and a duplication for Amaryllidoideae, the NAVIβ event, ca 41.2 Ma (Landis et al. 2018). x = 11 may be the basal chromosome number for the family (Meerow et al. 2006). There has been a reduction in the GC content of the genome, perhaps associated with the large genome sizes found here (Smarda et al. 2014). For genome size in Narcissus and its correlation with taxonomy, see Zonneveld (2008; see also Marques et al. 2017). Poggio et al. (2014) found that the bimodal karyotype of Hippeastrum remained largely constant in morphology despite changes in chromosome numbers and genome size/genome, and overall genome size still less. For the extensive karyotypic evolution in Gilliesieae, where there has been much Robertsonian change (i.e. chromosome fusion or breakage at the centromere) and polyploidy, see Pellicer et al. (2017).
Economic Importance. Garlic, leeks and onions...
Caterpillars of the leek moth, Acrolepiopsis assectella (Yponomeutoidea-Glyphipterigidae), are major pests of leeks and onions (Sohn et al. 2013).
Chemistry, Morphology, etc.. Norbelladine alkaloids, unique to Amaryllidoideae, are tyrosine derivatives, and several show pharmaceutical promise. There are over 500 different structures, of which 79 or more are found in Narcissus alone, that have been placed in 118 different classes (Martin 1987; Bastida & Viladomat 2002: other references in the same volume; Rønsted et al. 2008b, 2012; Kilgore et al. 2016; papers in South African J. Bot. 136. 2021; Nair & van Staden 2021). These alkaloids cause i.a. acetylcholinesterase inhibition, etc., in Haemantheae (Bay-Smidt et al. 2011) and Calostemmateae (Jensen et al. 2011); see also Nair et al. (2016). For the synthesis of norbelladine by the condensation of tyramine and 3,4-dihydroxybenzaldehyde, see Tousignant et al. (2022). Allium is well known for producing a diversity of steroidal saponins, perhaps some 290 or so kinds (Sobolewska et al. 2016).
Because of the leaf fibres in Amaryllideae, the coverings of the bulbs produce highly-extensible cotton-like fibres when torn. There are often crystals of calcium oxalate in the foliar epidermis. Weiglin (2001) documented quite a variety of epicuticular wax morphologies in Gethyllis. For the leaf and root anatomy of Allium, see Fritsch (1988, 1992: p. 129), the cortical cell walls of roots are sometimes thickened "in a knotty manner". Garlic, A. sativum, has quite complex bulbs (Tribble et al. 2021).
The flowers of Allium are shown with the median member of the outer whorl in the adaxial position (Spichiger et al. 2004). Schickendantziella (Gilliesiae) has only three tepals; they are caudate. Coronal structures in Gilliesiae vary in number and are often more or less linear; it has been suggested that they may be staminodial in some species (ref.?), but this is perhaps unlikely since the number of stamens + linear processes usually is more than six, and taxa with more than six stamens are uncommon in this part of the tree. Do all Allioideae have apotropous ovules?
In Allioideae, some information is taken from Rahn (1998) and Sassone et al. (2014: Leucocoryneae), both general, and Sundar Rao (1940), Berg (1996) and Berg and Maze (1966), all embryology. For Allium, see Rabinowitch and Currah (2002: more horti-/agricultural), R. M. Fritsch and Friesen (2002 - and many other papers in same book), all general, R. M. Fritsch and Keusgen (2006: cysteine sulphoxide distribution), Choi et al. (2011: floral development, esp. epidermis), Messeri (1931: embryology), Specht et al. (2001: polyembryony), Vinogradova (2018: endosperm development), and Druselmann (1992: much info. on germination).
The umbellate inflorescence of Amaryllidaceae has been much studied, as references in Martínez-Gómez et al. (2022) attest. In Amaryllidoideae the flowers of Galanthus are shown with the median member of the outer whorl in the adaxial position (Spichiger et al. 2004), see also the similar position in Hippeastrum and several other monosymmetric members of the subfamily. Some species of Phaedranassa have slit-monosymmetric flowers, with all the stamens, etc., leaving the flower via an abaxial slit in the perianth tube; I do not know details of the symmetry there. Flowers of some species of Crinum are monosymmetric.
The morphologies of stuctures that have been called coronas in Amaryllidoideae, e.g. Hymenocallis, paired evascularized outgrowths of the filaments, and Narcissus, vascularized and tubular (see also Scotland 2013) and not immediately associated with the stamens, are quite different (e.g. Arber 1937). The corona may also be a tube, sometimes toothed (Pancratium), on which the stamens are born. In this account, structures borne on the perianth are called a corona, while cup-like structures on which the anthers are borne are called staminal cups; Garcí et al. (2019) call the corona a paraperigone.... Waters et al. (2013) found that the corona tube in Narcissus bulbocodium was initiated on the hypanthial/perianth/tepal tube in six spots alternating with the stamens/tepals; these six outgrowths later fused and formed a tube quite distinct from the perianth tube. The hypanthium, stamens and corona all have similar ABC gene expressions and very much stamen-like tissue identity, however, the corona is a late-developing structure (Waters et al. 2013). In taxa like Hymenocallis festalis the filaments arise from indentations in the margin of a flaring tube borne at the apex of the narrowly tubular perianth tube, the perianth lobes becoming evident at the base of the flaring tube; in other Hymenocallideae the perianth lobes may arise from the back of this distinctive flaring tube. In taxa like Hymenocallis festalis the filaments arise from indentations in the margin of a flaring tube borne at the apex of the narrowly tubular perianth tube, the perianth lobes becoming evident at the base of the flaring tube; in other Hymenocallideae the perianth lobes may arise from the back of this distinctive flaring tube. Although genes involved in staminal development are involved in the development of the corona, as with the fimbrieae and related structures in Passifloraceae (q.v.), strict homology cannot be invoked.
In Galanthus in particular the inner whorl of tepals is very different from the outer whorl, although both are petal-like. Haemanthus has tepals with a single trace. In Strumaria and Carpolyza the bases of the filaments are adnate to the style, while in Strumaria and Tedingia the base of the style may be much inflated, even bulbous. Flowers of Gethyllis may have numerous stamens.
Vosa (2000) implied that Tulbaghia has an Allium-type embryo sac. Haig (2020) discussed embryo sac morphology and development in Allium. Although the antipodal cells might not persist, in some cases a chalazal embryo sac formed - and perhaps even produced an embryo, although apomictic polyembryony also occurs in the genus. Some bisporic species of Allium have diploid endosperm, the chromosomes from the lower polar nucleus being extruded (Haig 2020). Johri et al. (1992) suggest that the embryo sac in Amaryllidoideae is the common monosporic 8-celled Polygonum-type, but this is questionable. Although the embryo sac of Crinum flaccidum, with ategmic ovules and nuclear endosperm, is said to be Polygonum-type, that is not confirmed by the description given; it is also conceivable that the species has parietal tissue 2-3 cells across (c.f. Howell & Prakash 1990; see also Dutt 1962). Sternbergia (Narcisseae) is reported to have a bisporic embryo sac, but this develops from the micropylar member of the dyad (Dane 1999), so is the Endymion type, while Leucojum aestivum (Galantheae) has an Allium-type embryo sac (Ekici & Dane 2005). Hymenocallis caribaea has parietal tissue, the micropyle is zig-zag and mostly made up of the very long inner intgument which is almost as long as the body of the ovule, and there is a massive, chlorophyllous, stomata-bearing, vascularized outer integument (Raymúndez et al. 2008). Ovule and embryo sac morphology and development here needs a review.
Yusupov et al. (2022 and references) carried out extensive work on seed morphology in Allium, although despite the numerous SEM images provided there and their Fig. 15, where seed characters are placed on the tree, it is difficult to work out the relationship between seed characters and phylogeny, partly because some of the characters represent divisions of continua and others are difficult to understand, and partly because more work is needed on the phylogeny. For more on seed morphology, see Kruse (1988), Celep et al. (2012), Ariunzaya et al. (2022: machine learning groupings), and Nour et al. (2025), all s.e.m. studies.
A very long-tubular dropper cotyledonary sheath may develop during germination; this ensures that the bulb develops well below the soil surface.
For general information, see Markötter (1936: some South African taxa), Meerow and Snijman (1998) and Duncan et al. (2016, 2020: southern African taxa - spectacular images), for anatomy, see Arroyo and Cutler (1984) and Campos-Rocha et al. (2022: Amaryllideae), for pollen, see Dönmez and Isik (2008), and for embryology, see Stenar (1925).
Phylogeny. Amaryllidaceae are a very strongly supported clade (e.g. Fay et al. 2000, but c.f. McPherson et al. 2004; Thomas et al. 2005[?]), and they do have characters in common! Meerow et al. (1999), Fay et al. (2000: strong support), Givnish et al. (2006), Pires et al. (2006) and Seberg et al. (2012: support weak) suggest a set of relationships [Agapanthaceae [Alliaceae + Amaryllidaceae]]. However, Meerow et al. (2000a) found Agapanthaceae to be sister to Amaryllidaceae, albeit with weak support, and these relationships were also recovered by Costa et al. (2020) and dated to (68.4-)62.7(-55.8) Ma. Dennehy-Carr et al. (2025: 52 genera, 150 spp., 78 protein-coding plastid genes) recently looked at relationships within the whole family, recognizing that adding a nuclear component to the analysis would be useful.
Allioideae. Fay and Chase (1996) discuss relationships within the subfamily; the topology is [Allieae [Tulbaghieae + Gilliesieae]], although the support for the clades is rather weak, while relationships in Escobar et al. (2020) are [[Allieae + Tulbaghieae] Gilliesieae].
Allieae. Nguyen et al. (2008) provide a phylogeny for Allium and find that there are three main clades (see above, also Friesen et al. 2000: inclusion of Milula, 2006: ITS; Hirschegger et al. 2010: section Allium; Huang et al. 2014). The relationships of members of the small subgenera Nectaroscordum and Microscordum have been unclear (Nguyen et al. 2008; Mashayekhi & Columbus 2014). For relationships in subgenus Amerallium, which includes nearly all North American species and some from the Old World, see Choi et al. (2012), Q.-Q. Li et al. (2012) and Mashayekhi and Columbus (2014: nuclear and plastome genes, most sections not monophyletic); Friesen et al. (2024) suggested that subgenera Nectaroscordum and Microscordum, along with subgenus Amerallium, together formed a clade that was sister to the rest of Allium. For relationships within the small subgenus Anguinum, see Li et al. (2016). In the large subgenus Melanocrommyum, many species of which are to be found in the Qinghai-Tibet region (111 species of the genus grow there), there seems to be extended incomplete lineage sorting, and morphological sections are not supported by molecular data (Gurushidze et al. 2008, esp. 2010); see also Hauenschild et al. (2017), where the subgenera were not monophyletic and Gurushidze et al. were not mentioned, Han et al. (2019), a comprehensive analysis, and Babin and Bell (2022: 2 nuclear ribosome and 5 plastid markers), again, many subgenera were not monophyletic and the nuclear and plastid data sets were incongruent. Along the same lines, Munavvarov et al. (2022) found quite extensive conflict between the relationships in plastome analyses and those implied by the current infrageneric groupings; they noted that ITS analyses did not show such conflicts, and suggested that hybridization might be involved. In the ITS analysis of Yusupov et al. (2022: all but one subgenus, ca 2/3 sections included), of the 8 sections with two or more species that they looked at, three were polyphyletic; a clade [subgenera Nectaroscordum + Amerallium] was sister to the rest of the genus, which was made up of two clades, one containing subgenus Melanocrommyum (for which, see Fritsch et al. 2010 for an ITS phylogeny and classification) plus four subgenera, and the other was made up of subgenera Allium and Cepa plus six more subgenera. Z. Z. Zhang et al. (2024: transcriptomes of 48 spp., 10 subgenera, one with only one species) looked in to the phylogeny of Allium. In the nuclear analyses, they found that representatives of subgenus Cepa were always polyphyletic, those of subgenus Allium were mono- to polyphyletic, and so on. There was extensive conflict in the various analyses, including between plastome and nuclear analyses, and Zhang et al. (2024) pointed to incomplete lineage sorting as one important cause of this, but suggested that hybridization had also been much involved, as in the formation of the second evolutionary line (in their analyses A. hirtifolium and A. victorialis were always successively sister to the rest of that clade) and of subg. Butomissa, which is sister to the rest of the speciose evolutionary line 3 in particular (Zhang et al. 2024).
Gilliesieae. Gilliesia and Miersia are paraphyletic (Escobar et al. 2020), and this has been confirmed by N. García et al. (2022b); note, however, that analyses of nuclear and plastid data may give conflicting results. Miersia s.l. is sister to the rest of the tribe. For Gilliesieae, see also N. García et al. (2022b).
Within Leucocoryneae, Fay et al. (2006b) found that part of Ipheion was embedded in Nothoscordum, sister to the rest of the group. For relationships around Leucocoryne, see Souza et al. (2015, 2016), Sassone et al. (2017), Sassone and Giussani (2018) and Escobar et al. (2020); [Leucocoryne + Latace] are sister to the remainder of the group. These two genera were not sister taxa in the analyses of N. García et al. (2022a), indeed, there were differences in the topologies of the nuclear ITS and two-gene chloroplast trees. For the contents of Leucocoryneae, see Sassone et al. (2014).
Amaryllidoideae. Phylogenetic relationships within Amaryllidoideae have been suggested as being [Amaryllideae [Cyrtantheae [Calostemmateae, Haemantheae, Gethyllideae [Eurasian Clade + Andean Clade, Extra-Andean Clade]]]] (Meerow et al. 1999, 2000a, 2000b; see also Ito et al. 1999). Relationships between major clades of American and some southern African members are not well understood, furthermore, Meerow et al. (2006) found that the inclusion of Hannonia, Lapiedra and Vagaria destabilised relationships in the European clade; Lledó et al. (2004) included the last two in Galantheae. Meerow and Clayton (2004) discussed relationships among African taxa. A study using genes from all three compartments and sampling 108 species recovered the relationships [Amaryllideae [[Calostemmateae [Cyrtantheae + Haemantheae]] [Eurasian Clade [Andean Clade, Extra-Andean Clade]]]], although support for some nodes was poor (Rønsted et al. 2012). Meerow et al. (2006) provided a phylogeny for the Eurasian Clade, which includes daffodills, snowdrops, etc. There the main dichotomy separates the Central and East Asian Lycorideae from the rest, which centre on the Mediterranean region. ITS and ndhF phylogenies were found not to be congruent (Meerow & Snijman 2006). Meerow et al. (2020: nuclear and chloroplast data) discuss relationships in the Andean Clade; there is some hybridization, but it seems not to have affected the detection of major (= tribal) relationships, but note that Pamianthe tends to wander around the tree. Dennehy-Carr et al. (2025: 150 spp, 52 genera; 78 plastid protein-coding genes) did not attempt to recognise tribes in the Eurasian, Andean and Extra-Andean Clades; some 10 genera were polyphyletic, Nerine alone appearing in four places (see also earlier literature on polyphyly in Dennehy-Carr et al. 2025).
Amaryllideae. Meerow and Snijman (2001, see also 2006) discuss relationships within Amaryllideae; Amaryllis and Boophone are successively sister to the rest of the tribe; Amaryllis differs from other Amaryllidineae in not having a green testa, etc.. Meerow et al. (2003) outline the phylogeny of Crinum, the only pantropical member of Amaryllidaceae; see also Kwembeya et al. (2007).
Cyrtantheae. For a phylogeny of Cyrtanthus and discussion on its evolution, see Snijman and Meerow (2010); molecules and cytology, but less so morphology, tend to agree, and the old species groupings, based on floral (pollinator) morphology, have broken down.
Galantheae. For alkaloids and phylogeny, see Lledó et al. (2004) and Larsen et al. (2010).
Haemantheae. For relationships here, see Conrad et al. (2006) and Bay-Smidt et al. (2011); Gethyllis is embedded in Haemantheae (Rønsted et al. 2012).
Hippeastreae. Relationships are reticulating in many Hippeastreae-Hippeastrinae in particular, and species numbers are very uncertain (García et al. 2014, 2017, 2019).
Narcisseae. For Narcissus, see Rønsted et al. (2008b: acetylcholinesterase-inhibiting alkaloids), Santos-Gally et al. (2012) and Marques et al. (2017).
Worsleya and Griffinia are morphologically and phylogenetically isolated (Meerow et al. 2000a).
Classification. Combining the three families Agapanthaceae, Alliaceae and Amaryllidaceae into Alliaceae s.l. was an option in A.P.G. II (2003), an option that was exercised in A.P.G. III (2009), although under the name of Amaryllidaceae. The infrafamilial classification above largely follows that in Chase et al. (2009b).
For a classification of the Andean Tetraploid Clade, see Meerow et al. (2020), for that of Amaryllideae, see Meerow and Snijman (2001), for that of Hippeastreae, see N. García et al. (2019: comments on making a hierarchical classification for a group in which relationships are best represented as networks), for generic limits in Galantheae, see Lledó et al. (2004), and for an infrageneric classification of Narcissus, see Marques et al. (2017). Christenhusz et al. (2018) took a broad view of Hippeastrum, providing 180 or so new combinations; this treatment has not been followed.
Botanical Trivia. The "amaryllis" of many a windowsill is really Hippeastrum.
ASPARAGACEAE Jussieu, nom. cons. - Back to Asparagales
?Steroidal saponins; x = 9 (?8), nuclear genome [1C] (0.403-)4.7(-36.649) pg.
153/2,595 (2,900). World-wide, but not Arctic - seven subfamilies below: Agavoideae, Aphyllanthoideae, Asparagoideae, Brodiaeoideae, Convallarioideae, Lomandroideae and Scilloideae (see also Anthericaceae (Agavoideae-Anthericeae), Eriospermaceae (Convallarioideae-Eriospermeae), Hyacinthaceae (Scilloideae-Hyacintheae), Laxmanniaceae (Lomandroideae-Sowerbaea group, and Ruscaceae (Convallarioideae-Rusceae), family names in the literature that are rather different from the subfamilial names that are used below).
Age. Divergence within crown-group Asparagaceae began ca 89 Ma (Janssen & Bremer 2004). Eguiarte (1995: Agavaceae and Nolinaceae), however, suggested an age of only some ca 47 Ma, Bell et al. (2010) suggested a crown-group age of (66-)56, 51(-42) Ma, while estimates in S. Chen et al. (2013) are (65-)56(-48) or ca 36.4 Ma, in C. I. Smith et al. (2021) they are (90.6-)63.8(-44.7) Ma, and in Ji et al. (2022) they are (77.2-)61.4(-49.1) Ma. An estimate of the age of the Dasylirion—Yucca clade is ca 63.1 Ma (Ayala-Hernández et al. 2025).
Evolution: Divergence & Distribution. Note that some of the ages given for nodes in this clade by S. Chen et al. (2013) are for nodes not recognized here.
There are no obvious apomorphies for Asparagaceae s.l., however, "endosperm thick-walled, pitted, hemicellulosic" might be placed at this level. The homoisoflavanones found in Scilloideae are rather uncommon in flowering plants, but they are also found in Camassia (Agavoideae, ex Chlorogaloideae) and Ophiopogon (Convallarioideae).
Phylogeny. These seven subfamilies form a rather well supported clade in Fay et al. (2000). Aphyllanthes has a very long branch in the three-gene analysis of Fay et al. (2000), and its phylogenetic position is unclear; its removal from analyses can rather dramatically changes support values (Chase et al. 2006; Givnish et al. 2018b). A position close to Hyacinthaceae (= Scilloideae) was found by McPherson and Graham (2001), but Pires et al. (2006) place it sister to Laxmanniaceae (= Lomandroideae), albeit with weak support. Seberg et al. (2012) found the relationships [[Brodiaeoideae + Scilloideae] [Aphyllanthoideae + Agavoideae]], but the position of Aphyllanthes had no support. Steele et al. (2012) also found Aphyllanthes associating with this group, but again with little support. In the nuclear analyses of Timilsena et al. (2022a), there were also problems with this genus: It was either sister to all other Asparagaceae, with strong support, or sister to [Agavoideae [Brodiaeoideae + Scilloideae]], but with very weak support.
Fay et al. (2000), Pires et al. (2001), and Pires and Sytsma (2002) discuss uncertainties as to the immediate sister taxon to Themidaceae (= Brodiaeoideae). The [Themidaceae + Hyacinthaceae] clade is moderately well supported (Fay & Chase 1996; Meerow et al. 2000), but support in the two-gene analysis of Jang and Pfosser (2002: Aphyllanthes not included) is only weak (see also Chase et al. 2006; Pires et al. 2006; for more suggestions about relationships, see also Bogler et al. 2006). Steele et al. (2012) recovered a clade [Lomandroideae [Asparagoideae + Nolinoideae (= Convallarioideae)]] that had good support (for this clade, see also Fay et al. 2000: moderate support; Seberg et al. 2012, strong support). A placement of Eriospermum (Convallarioideae here) as sister to Asparagoideae has quite strong support (Seberg et al. 2012); this position must be confirmed. Eguchi and Tamura (2016) retrieved a clade [Convallarioideae [Asparagus + Cordyline], although relationships at this level were not their focus, and relationships in Timilsena et al. (2022a) were either [Asparagoideae + Ruscoideae] or [Asparagoideae + Lomandroideae (just one species included)], and it was notable that 5/8 nodes within Ruscoideae were poorly supported and that Peliosanthes moved within the subfamily depending on the analysis. In the plastome analyses of Ji et al. (2022) there was essentially a polytomy involving Ophiopogoneae, Nolaneae, Convallarieae and Polygonateae, perhaps reflecting ILS or ancient hybridization. Bentz and Leebens-Mack (2024) discuss their new Asparagaceae1726 Hyb-Seq reads, and note that they recovered the relationships [Nolinoideae [Lomandroideae + Asparagoideae]] in ASTRAL-III coalescent analyses but [Lomandroideae [Asparagoideae + Nolinoideae]] in ML concatenation analyses; they found the relationships [Agavoideae [Brodiaeoideae + Scilloideae]] in both analyses, but they could not get material of Aphyllanthoideae. When these last are sequenced, relationships below may have to be adjusted.>
Classification. This is a rather unsatisfactory family. Thus nothing characterises it, and while some of the subfamilies do indeed have several distinctive apomorphies and are also easy to recognise, others are difficult to recognise. However, any way one might want to divide - or not - the group would seem to be unsatisfactory. As it is, Convallarioideae and Agavoideae are particularly heterogeneous, several families having been segregated from them in the past. The flowers of the whole group are for the most part a rather undistinguished "lily"-type, and quite often are rather small. The subfamilial classification below follows that in Chase et al. (2009b), but I also mention familial names for some subfamiliea (and there are links above) because the roots of the two sets of names differ in over half the cases and both will be encountered in the current literature (e.g. Eggli & Nyffeler 2020; Vázquez-García et al. 2024, and references).
[Aphyllanthoideae [Agavoideae [Brodiaeoideae + Scilloideae]]: ?
Age. The age of this node is (59-)50(-41) or ca 40.5 Ma (S. Chen et al. 2013: c.f. dates for Agavoideae) or (71.1-)55.7(-43.7) Ma (Ji et al. 2022). If there is a clade [Aphyllanthoideae + Agavoideae], its age is estimated to be ca 47.6 Ma (Givnish et al. 2018b) or (69.5-)54.1(-41.7) Ma (Ji et al. 2022).
1. Aphyllanthoideae Lindley - Aphyllanthes monspeliensis L. - Back to Asparagales —— Synonymy: Aphyllanthaceae Burnett
Flavonols +, ?saponins; root cortex sloughs off, endodermis becomes superficial, vessel elements often with simple perforation plates; monocot secondary thickening +; stems alone photosynthetic, with parallel wax scales; leaves two-ranked, scaly, non-photosynthetic, ligulate, vernation supervolute-subinvolute, base?; inflorescence scapose, flowers multibracteolate, sessile; T marcescent, basically free, with a single trace; A adnate to base of T; pollen spiraperturate; ovary sulcate down middle of loculus; infra-locular septal nectaries +, stigma trifid, dry; ovule 1/carpel, micropyle?; seeds slightly flattened, exotestal cells large, isodiametric; endosperm ?, 0; n = 16; cotyledon photosynthetic, terete, first leaf terete.
1[list]/1. W. Mediterranean. Photo: Flower © E. Bourneuf.
Chemistry, Morphology, etc.. The stomata are in bands down the scape. The tepals have but a single bundle. Is there chelidonic acid?
General information is taken from Conran (1998); he mentions helobial endosperm development here, but c.f. Schnarf and Wunderlich (1939), apparently the only source of embryological information.
Phylogeny. Remember that the immediate relationships of Aphyllanthoideae are unclear, although it is to be included in Asparagales.
[Agavoideae [Brodiaeoideae + Scilloideae]]: ?
Age. The age of this node is estimated at (62-)51, 46(-37)Ma by Bell et al. (2010) and at ca 49.8 or 33.5 Ma by S. Chen et al. (2013).
2. Agavoideae Herbert - Back to Asparagales
Plant rhizomatous; root exodermis often multilayered; outer integument 4-6 cells across; hypostase +; embryo sac with chalazal constriction; endosperm thick-walled, pitted, hemicellulosic.
23/637 [list: to tribes] - five groups below. More or less world-wide, esp. S.W. North America, few in Malesia, N. Australia, not cold temperate, New Zealand, etc.. Map: see Ying et al. (1993), García-Mendoza and Galván V. (1995), Fl. N. Am. vol. 26 (2002) and Seberg (2007).
Age. Crown-group Agavoideae can be dated at (76-)61.5(-47.5) Ma (McKain et al. 2016c) or ca 22.0 Ma (Givnish et al. 2015), ca 19.9 Ma (S. Chen et al. 2013: perhaps), (66.5-)48.0(-31.9) Ma (C. I. Smith et al. 2021) or (61.7-)45.8(-31.9) Ma (Ji et al. 2022: inc. Anem.). Once again, one is pretty much left hanging.
2A. Anemarrheneae Reveal - Anemarrhena asphodeloides Bunge —— Synonymy: Anemarrhenaceae Conran, M. W. Chase & Rudall
Steroidal saponins +; leaves ?spiral, base?; inflorescence subspicate, branched; T ± free; A 3, opposite and adnate to middle of inner T; ovules 2/carpel, apotropous; seeds angled; endosperm haustoria +; embryo curved; n = 11; hypocotyl 0.
1/1. W. China, Mongolia, Korea.
[Agaveae [Behnieae [Herrerieae + Anthericeae]]]: (vessels in stem); nucellar cap, hypostase +.
Age. The age of this node is some (56-)41.5(-26.5) Ma (McKain et al. 2016c), (48-)40, 33(-23) Ma (Bell et al. 2010), 36-35 Ma (Wikström et al. 2001) or 34.2-29.1 Ma (Good-Avila et al. 2006).
2B. Agaveae Dumortier / the ABK clade [Agavoideae Bimodal Karyotype clade] —— Synonymy: Agavaceae Dumortier, nom. cons., Chlorogalaceae Doweld & Reveal, Funkiaceae Horaninov, Hesperocallidaceae Traub, Hostaceae B. Mathew, Yuccaceae J. Agardh
Plants rhizomatous, to trees, (bulbs, tunicated [surrounded by scales] or not); (CAM photosynthesis +); steroidal saponins, non-protein amino acids, (homoisoflavanones - Chlorogalum), flavonols +, (CAM photosynthesis +); root exodermis multilayered/0, medulla with xylem or not; (monocot secondary thickening +); (silica bodies in bulb - Polianthes); adaxial bundles in leaf inverted [?level]; also styloids +; (stomata para- or tetracytic), cuticular wax rodlets parallel; (rhexigenetic lacunae + - Chlorogalum, etc.), leaves spiral, (pseudopetiolate), (often fleshy), (margins serrate), apex (pungent-)pointed, base ?, (petiolate); inflorescence usually branched, and/or flowers in pairs or fascicles, (pedicel articulated - Chlorogalum, etc.); flowers large, (monosymmetric); T ± connate; A adnate to T; tapetal cells several-nucleate; pollen semitectate, (operculate); ovary superior to inferior, (style 3-branched, with 3 canals - Camassia), stigma wet to dry; ovules many/carpel, outer integument (4-)9-14 cells across, (parietal tissue 1-2 cells across), (nucellar cap 2 cells across), ± postament, obturator +; fruit (septicidal capsule - some Yucca), (berry), T marcescent; seeds flattened/globose, with phytomelan; (endosperm thin-walled - Hosta), (perisperm +, oily - Yucca, Agave); n = 30, karyotype bimodal [25 short + 5 long] (7, 12, 18 S + 5, 3, 6 L respectively), 0.4-10 µm long; genome duplication; (cotyledon non-photosynthetic - Funkia), hypocotyl to 4 mm long, collar rhizoids +, primary root often branched.
10/425 (?600): Agave (287), Yucca (50), Hosta (?23). Central U.S.A. to N. South America, mostly S.W. North America, also East Asia (Hosta). [Photo - Flower.]
Age. Crown-group Agaveae are (37.5-)27.9(-20.7) (McKain et al. 2016c), (33.4-)25.8, 20.5(-18.4) (Good-Avila et al. 2006), (33.4-)23.7(-14)/(15.1-)14.5(-13.3) Ma (C. I. Smith et al. 2008) or (29.1-)21.9, 21.6(-16.3) Ma (Smith et al. 2021); the comparable estimate in M.-J. Yoo et al. (2021) is (46.2-)35.6(25.9) Ma.
[Behnieae [Herrerieae + Anthericeae]]: ?
Age. The age of this node is some (34-)24, 22(-13) Ma (Bell et al. (2010).
2C. Behnieae Reveal - Behnia reticulata (Thunberg) Didrichsen —— Synonymy: Behniaceae Conran, M. W. Chase & Rudall
± Sprawling, (dextrorsely twining stems), rhizomatous; tannin cells 0; velamen 1-layered; vessel elements also in the stem; leaves two-ranked, "supervolute", petiolate, blade broad, with midrib and transverse tertiaries, leaf base not sheathing; plant dioecious; staminate flowers: A adnate to base of T, tapetal cells binucleate, pistillode +; pistillate flowers: staminodes +; stigma 3-lobed, wet; ovules 2-3/carpel, micropyle endostomal, outer integument 3-4 cells across, parietal tissue ca 1 cell across; fruit a berry, T marcescent, not twisting; seeds angular, phytomelan 0, testa and tegmen thin-walled, (exotesta exfoliates); endosperm walls thick, pitted, aleurone +, embryo "large, capitate"; n = ?; cotyledon green.
1/1. Zimbabwe to eastern South Africa.
[Herrerieae + Anthericeae]: ovules 1-many/carpel.
2D. Herrerieae Baillon —— Synonymy: Herreriaceae Kunth
Usu. climbers, prickly; saponins +, chelidonic acid?; root (exodermis multilayered); (vessel elements +); mucilage cells 0; cuticular wax rodlets parallel; leaves spiral, fasciculate, sheath?; pedicels not articulated; T and A free; parietal tissue?; fruit a septicidal capsule; seeds flattened; "embryo short"; n = 27, dimorphic [one large], chromosomes 0.7-3.7 µm long [Herreria]; "germination epigeal".
2/9. South America (Brazil southwards), Madagascar. [Photo - Fruit.]
2E. Anthericeae Bartling —— Synonymy: Anthericaceae J. Agardh
Rhizome short; chelidonic acid +; (velamen +); (vessel elements in the stems); mucilage cells +, tannin cells 0, (styloids + - Chlorophytum); cuticular wax rodlets parallel; leaves spiral to two-ranked, base sheathing; inflorescence thyrsoid, flowers in groups along the axis, (raceme); (pedicels not articulated); (flower monosymmetric), (T tube 0); (pollen mixed with raphides); stigma dry; outer integument ca 4 cells across, parietal tissue 1-2 cells across, nucellar cap + [Leucocrinum]; embryo sac haustoria common; T persistent in fruit; seeds angular or flattened, black [?level]; tegmen?; embryo curved or angled, suspensor cells flattened; n = 7, 8, 10, 11, 13-15, etc., chromosomes 2-10(-13.8) µm long, genome duplication [Chlorophytum]; cotyledon not photosynthetic, coleoptile + [Chlorophytum]; plastid transmission biparental [Chlorophytum].
8/285: Chlorophytum (150), Anthericum (65), Echeandia (60). More or less worldwide, but not cold temperate, few in Malesia, N. Australia, not New Zealand, etc. [Photo - Inflorescence, Flower.]
Evolution: Divergence & Distribution. Good-Avila et al. (2006) suggest that Agave et al. are only some 26-20 Ma, and Yucca is younger, 18-13 Ma old. Rocha et al. (2006) suggested ca 12.75 Ma as the age of Agave etc. and ca 10.2 Ma for Agave s.l. (Hesperaloe and everything above in the tree - Bogler et al. 2006; c.f. also Smith et al. 2008); there are yet other possibilities for dates.
Good-Avila et al. (2006) discussed diversification in both Agave, which they thought was connected with the adoption of bat pollination, and Yucca (see also Rocha et al. 2006); C. Smith et al. (2008) suggested that diversification was not significantly different in Yucca, with 34(-50) species, and its sister taxon, Agave s.l., with some 250 or more species. Pulses of diversification in agaves may have happened a mere 9-6 Ma, a time when other succulent clades were diversifying (e.g. Good-Avila et al. 2006; Arakaki et al. 2011). Ayala-Hernández et al. (2025) found that geography (in part) and fruit type agreed with phylogeny in Yucca, but other morphological characters did not.
Ecology & Physiology. Nobel (1988) looked at the eco-physiology of agaves and their relatives. Over 300 species are succulents, mostly leaf succulents (Nyffeler & Eggli 2010b); drought tolerance is common, and some species in the Chlorogalum area grow on serpentine soils, themselves often subject to drought (Halpin & Fishbein 2014). Agave is notably common in arid regions of Mexico and the S.W. U.S.A., where it “play[s] a critical role in maintaining soil stability in arid and semi-arid ecosystems” (Gómez-Ruiz & Lacher 2019; Jiménez-Barron et al. 2020). Arakaki et al. (2011) suggested that succulents in general radiated/diversified in the mid to late Miocene to Pliocene, even if the clades involved had originated substantially earlier, and they mentioned several radiations in Euphorbia, also core Ruschioideae and Cactaceae-Opuntioideae and Cactoideae in this context (in all, see Ecology & Physiology).
The CAM photosynthetic pathway has evolved ca three times in Agaveae, a major CAM clade (or it evolved once, and was then lost three times or so), and is found in nearly all species of Agave, Yucca subgenus Sarcocarpa and Hesperaloe - there seems to have been a reversal in Polianthes [= Yucca] tuberosa, at least (Heyduk et al. 2016, 2023; Gilman et al. 2023); there is considerable variation even here in how different species became CAM plants (Heyduk et al. 2022). Interestingly, succulent leaves with a three-dimensional venation system that are characteristic of the CAM species are also found in related C3 species, and CAM morphology may have evolved before CAM photosynthesis itself (Heyduk et al. 2016), although some genes involved in CAM were also expressed there before CAM had developed (Heyduk et al. 2019). Leaf anatomy and photosynthetic type are not correlated in hybrid Yucca (Heyduk et al. 2020), however, infraspecific variation in photosynthetic types, perhaps the result of hybridization, and the general diversity of CAM phenotypes, makes this a very inetresting group to study (Heyduk et al. 2023). Yin et al. (2018) also discussed the evolution of CAM in Yucca noting that regulatory proteins involved in carbon fixation and metabolite transportation had to be reprogrammed to fit the different light/dark schedule needed in CAM photosynthesis. There seems to have been the evolutionary sequence C3 → C3 + CAM → strong CAM in Agavoideae (Heyduk et al. 2022; Gilman et al. 2023).
Pollination Biology & Seed Dispersal. The Yucca-yucca moth association, an obligatory brood-site pollination mutualism, is well known. Yucca moths are in the genera Tegeticula and Parategeticula (Prodoxidae), a rather basal glossatan (= with proboscis) moth clade. Attracted to the yucca flower by scents in which homoterpenes, which are often also involved in plant defence, are a major component (Svensson et al. 2005), the moths mate in the flower. The female moth collects a ball of pollen using structures called tentacles to scrape the pollen from the anther; she lays eggs in the ovary and then puts pollen from the pollen ball on to the stigma. The ovules are fertilized, and although some of the developing seeds are eaten by the caterpillar, the others escape that fate and mature. The ancestral condition for yucca moths may have been to eat ovaries (Yoder et al. 2010a). Species of the sister group of yucca moths, Prodoxus, eat fruits, flower pedicels, or are leaf miners, mainly on Yucca spp., but also on Agave, etc., and more than one species of moth is sometimes found on the one species of plant (Pellmyr et al. 2005). Other close relatives of yucca moths eat various parts of Dasylirion and Nolina (both Convallarioideae-Nolineae); the age of the Dasylirion—Yucca clade is ca 63.1 Ma (Ayala-Hernández et al. 2025).
The yucca-yucca moth association has been used as a textbook example of mutualism or co-evolution, where the two partners, Yucca and Tegeticula, show reciprocal evolutionary changes (see Althoff et al. 2012 for details). The two parties are indeed closely linked, but details of the association suggest that the links are not simple. Thus Prodoxus, not a pollinator but a specialist herbivore on Yucca, and Parategeticula, another pollinator, are also involved; all told, there are over 22 species of pollinating moths, 2-3 species of cheater moths, and over 35 species of Yucca (Althoff 2016 and references). Initial diversification in Yucca may have been in association with Parategeticula, a poor flier and now rather uncommon (Althoff et al. 2012). Pellmyr and Leebens-Mack (1999) estimated that the beginning of the association was around (51.3-)41.5(-31.7) Ma, active pollination by Tegeticula and Parategeticula beginning (44.5-)35.5(-26.5) Ma (see also Pellmyr et al. 1996, 2007; Pellmyr 2003; Gaunt & Miles 2002: association arose ca 32 Ma; Althoff et al. 2006), but there may have been another and more recent radiation of yucca moths only 3-2 Ma. Crown Yucca has also been aged at (14.5-)12.5(-11.5) Ma, the stem age at ca 20 Ma, the stem age of Hesperoyucca being (24-)16.5(-9) Ma (McKain et al. 2016c, but see below). Other estimates include diversification of Yucca 10-6 Ma, crown Yucca, i.e. excluding Y. queretaroensis which had no fixed position, being only (6.8-)6.4(-6.1) Ma (C. I. Smith et al. 2008), while Smith et al. (2021, q.v. for much on the ages of the protagonists) dated a Yucca that included Y. queretaroensis at (6.3-)4.1, 3.0(-1.2) Ma; Ayala-Hernández et al. (2025) - Y. queretaroensis well embedded in group 3, but support values mostly low - thought that the age of crown-group Yucca was (11.3-)7.5(-3.5) Ma (stem - ca 7 My). All this makes any co-evolutionary scenario complicated, to say the least. Indeed, by several accounts much of the divergence in Yucca seems to have occurred before that of its main pollinator, yet only a mere 6-4 Ma, and in addition to the conflicting ages, it is difficult to imagine how strict (reciprocal speciation) co-evolution might work here given the vagility of the moth (see also Feinsinger 1983; Godsoe et al. 2010; Starr et al. 2014; Hembry et al. 2014; C. I. Smith et al. 2021). Tegeticula also pollinates Hesperoyucca, not at all close to Yucca (e.g. Smith et al. 2021: Fig. 3, Protoyucca shadishii sister to Yucca; Ayala-Hernández et al. 2025: Hesperoyucca sister to Hesperaloe), the particular species of Tegeticula that is the pollinator being sister to the rest of the genus. Although a close pollinator/plant association may be quite old, it seems to have evolved independently in Hesperoyucca and Yucca (Pellmyr & Leebens-Mack 1999; McKain et al. 2016c). For a recent review of the yucca—yucca moth association, see C. I. Smith and Leebens-Mack (2023). Ages are indeed rather difficult to understand. Thus Smith and Leebens-Mack (2024: Fig. 1 – c.f. branching sequence and ages associated with the sequence) suggest that moths started feeding on Agavoideae ca 44.1 Ma, pollination starting ca 35.6 Ma, crown-group Tegeticula itself being ca 41.7 Ma; pollination thus is anything from 2-8 times the age of Yucca, furthermore, to talk about the yucca-yucca moth association simply as a mutualism is an oversimplification...
Interestingly, yet other Prodoxidae are found on Lithophragma (Saxifragaceae), where they are involved in associations similar to those they have with Yucca. See also Ranunculaceae, Phyllanthaceae-Glochidion, etc., Moraceae-Ficus and Caryophyllaceae for similar pollinator-seed eater interactions; Hembry and Althoff (2016) and Kawakita and Kato (2017f) review diversification and coevolution in them, also other papers in American J. Bot. 103(10). 2016.
For the pollination biology of Agaveae, see Rocha et al. (2006); bat pollination is common in the large genus Agave and its relatives (Fleming et al. 2009). The phyllostomid bats Leptonycteris yerbabuenae and in particular L. nivalis are pollinators of Agave spp., although they also visit other plants, including columnar cacti. Pregnant females of the latter bat in particular migrate north along the “nectar corridor” to the S.W. U.S.A., and they and their pups later go back south to Mexico, and of the migratory bats L. nivalis visits the highest elevations and so pollinates other species of Agave (Fleming et al. 1993; Gómez-Ruiz & Lacher 2019; Lear et al. 2024).
Both the ovary and fruit of Leucocrinum (Anthericeae) are below the surface of the ground (Bogler et al. 2006).
Plant-Animal Interactions. Caterpillars of the giant skippers Agathymus and Megathymus, found in Mexico and the adjacent southwest U.S.A., bore tunnels in the roots and leaves of Agave and Yucca (Warren et al. 2009).
Genes & Genomes. A genome duplication here, the CHPOα event of ca 48.7 Ma, in Agaveae involves Hesperaloe and Chlorogalum (Landis et al. 2018). For a connection between the evolution of the bimodal karyotype of Agave, Hesperocallis and their relatives with polyploidy, see McKain et al. (2011, 2016a, esp. 2012) and Halpin and Fishbein (2014). Hesperocallis has 4 long, 2 medium and 18 short chromosomes, and there are several other combinations (Halpin & Fishbein 2014; McKain et al. 2016c), but details of how bimodality interacts with polyploidy are unclear. The switch from the 25 short + 5 long chromosomes in North American Agaveae (this is a very common combination) tends to be linked with the adoption of more mesic habitats and corresponding morphological changes (McKain et al. 2016c).
There is an ITS2 deletion in Yucca (Bogler & Simpson 1996).
At least some mitochondrial genes show an accelerated rate of change (G. Petersen et al. 2006).
Chemistry, Morphology, etc.. Agave is rich in saponins and sapogenins (Sidana et al. 2016). The raphides of Agave are hexagonal in transverse section, each with a lamellated (?unit membrane) sheath (Wattendorff 1976).
For variegation in Hosta, see Zonneveld (2007).
The flowers of Agave are shown with the median member of the outer whorl in the adaxial position (Spichiger et al. 2004). Camassia at least has single-trace tepals, Agave, etc. have three, while Hosta may have as many as 13 traces (Lin et al. 2011). The outer tepals of Herreriopsis have sac-like bases - possibly tepalline nectaries. In Hosta the stamens are sometimes inserted on the ovary. The tapetal cells of Polianthes (= Agave) are multinucleate. Germination of the pollen grain via the proximal pole has been reported in Beschorneria (Hesse et al. 2009a). Furcraea has nuclear endosperm.
Some information on Anthericeae is taken from Conran (1998); ovule morphology is apparently known from Leucocrinum alone in this group. Ubisch bodies are present in Anemarrhena, so there is probably a glandular tapetum; information for this genus is taken from Conran and Rudall (1998: confusion over stamen position) and Rudall et al. (1998b). For information about Behnia (Behnieae) and Herreria and Herreriopsis (Herrerieae), see Conran (1998); details of ovules/embryology are unknown. The leaves of Herreria and Herreriopsis are described as being cladode-like (Conran 1998) or cladodes (D. W. Stevenson in Takhtajan 1997).
See also Verhoek (1998), Judd et al. (2013) and Thiede and Eggli (2020) for general information especially on what has been considered Agavaceae s. str. in the past, i.e. Agave, Yucca and their immediate relatives, see also Lynch et al. (2001: c.f. Scilloideae!) and Solano et al. (2013: Polianthes anatomy), Alvarez & Köhler (1987: pollen), Fagerlind (1941b), Cave (1948, 1955, 1974: variation in endosperm development), Wunderlich (1950: also floral morphology) and di Fulvio and Cave (1965), all embryology; see also Kubitzki (1998b: Hostaceae), Speta (1998: Hyacinthaceae-Chlorogaloideae).
Phylogeny. Pires et al. (2004) and especially Bogler et al. (2006: 2- and 3-gene analyses, the latter with more missing data, but overall the same topology) discussed relationships within Agavoideae. I have followed the latter - which see for details - above. Support for the subfamily as a whole is only 75%, that for the [Behnia + Herreria, etc. + Anthericum, etc.] clade 87%, and that for [Herreria, etc. + Anthericum, etc.] only 51% (and still less in the two-gene tree); however, other nodes have close to 100% support. Largely similar relationships were found by G. Petersen et al. (2006c) in their analysis of variation of four mitochondrial genes that are evolving particularly quickly here. C. Smith et al. (2008) included Hosta, etc., in their Agavaceae and excluded Anthericaceae, although support for Agavaceae so delimited was weak; that for the still broader circumscription adopted here was stronger. The [Agaveae [Behnieae [Herrerieae + Anthericeae]]] clade has 100% support in three- and four-gene trees (Chase et al. 2000a; Fay et al. 2000; Bogler et al. 2006).
There is a fair amount of resolution of relationships around Agave and Yucca (see also Pollination & Seed Dispersal for Yucca). Agave includes Manfreda, Polianthes, etc., and [Beschorneria + Furcraea] are sister to Agave s.l., e.g. Bogler and Simpson (1995), Bogler et al. (2006) and Rocha et al. (2006). Jiménez-Barron et al. (2020: 83 spp., ca 1/3; 4 chloroplast markers, ITS) looked at relationships in Agave s.l., and found that A. bracteosa and A. ellemeetiana were successively sister to the rest of the genus, although in some analyses the latter was part of the striatae group; overall, here there was rather little resolution of relationships. The position of Yucca is unclear, but it may well be sister to that combined clade (Bogler et al. 2006; Archibald et al. 2015; McKain et al. 2016c); for relationships in Yucca, see also Ayala-Hernández et al. (2025: 46 spp., nuclear ITS and 3 plastid genes), although the plastid genes had really rather little to say about relationships, Hesperocallis undulata may be sister to the whole clade (Bogler et al. 2006), although it has also been placed sister to a clade that includes many ex-Chlorogaloideae (Halpin & Fishbein 2013; see also Archibald et al. 2014, esp. 2015), i.e. [Hesperocallis [paraphyletic Chlorogalum [Camassia + Hastingsia]]], there is a well-supported [Hesperoyucca [Hesperoaloe + Schoenolirion]] clade, although exactly where it might go is unclear (see also McKain et al. 2016: sister to the immediately preceding clade), and Hosta is sister to the rest of this whole clade. Within Camassia relationships show a fair bit of resolution (Fishbein et al. 2010; Halpin & Fishbein 2013; Archibald et al. 2015). For other phylogenetic work on this group, see Eguiarte et al. (1994), Bogler and Simpson (1996: molecular) and Sandoval (1995: morphological).
Classification. The circumscription of group 4b above, Agave, etc. + Hesperocallis, or the ABK clade, corresponds to that of Agavaceae s.l. in Bogler et al. (2006). The broad concept of Agavoideae adopted here may not seem very satisfactory, but none of the alternative solutions is any better.
Agave itself should probably include Polianthes, Manfreda, etc.; Thiede et al. (2019) provide an infrageneric classification. However, Vázquez-García et al. (2024: esp. pp.) placed the basal clades in Agave s.l. (see Jiménez-Barron et al. 2020) in separate genera based on "genetic, morphological [data], and estimated divergence times"; more names in this area in the offing? An infrageneric classificaion for Hosta, still rather unclear, is provided by M.-J. Yoo et al. 2021), while that in Yucca was not supported by the ITS relationships recovered by Ayala-Hernández et al. (2025).
Previous Relationships. Paradisea (ex Asphodelaceae/Xanthorrhoeaceae-Asphodeloideae) belongs to the Anthericeae above (e.g. Chase et al. 2000b). Behnia (Behnieae) was included in Luzuriagaceae (Liliales here) by Taktajan (1997), but it has also been placed in other lilialean and asparagalean families (Bogler et al. 2006); Camassia, etc. (Agaveae), used to be in Liliaceae (Cronquist 1981) or Hyacinthaceae-Chlorogaloideae. Traub (1982) noted that Hesperocallis undulata (Agaveae) smells of onions, and he even associated it with his Alliales. The genus was geographically odd in Hostaceae s. str., which is where other workers had placed it (c.f. Kubitzki 1998b), but not in Agavoideae as here circumscribed; now it is Hosta that is a little odd from the geographical point of view. Patil (2015) included four unrelated groups in his Agavaceae.
Botanical Trivia. The inflorescences of Furcraea might be the longest of those of any plant (Mauricio Bonifacino pers. comm. - horse for scale), although in terms of mass and flower number, Corypha (Arecaceae) is the largest.
[Brodiaeoideae + Scilloideae]: leaves spiral; inflorescence scapose, pedicels bracteate; raphides in carpel wall; ovules anatropous; endosperm (nuclear); cotyledon not photosynthetic.
Age. The age of this node is estimated to be (56-)45, 40(-15) Ma by Bell et al. (2010), (58-)48(-40), around 40.6 Ma by S. Chen et al. (2013) and (63.9-)48.3(-20.1) Ma by Ji et al. 2022).
Evolution: Divergence & Distribution. For some other characters of this pair, see Fay and Chase (1996); laticifer-like structures may occur in both.
3. Brodiaeoideae Traub - Back to Asparagales —— Synonymy: Themidaceae Salisbury
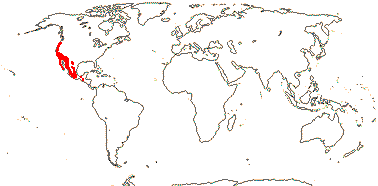
Plant monopodial, cormose, storing starch; root pith 0; laticifers +; mucilage cells?; leaves (unifacial - Brodiaea), sheath closed, fibrous; inflorescence umbellate, cymose, inflorescence bracts several, scarious, also internal bracts; pedicels often articulated; (T free; corona +); A (3), connate and/or adnate to T, (filaments flattened); (ovary (long) stipitate, adnate to T by flanges opposite the outer tepals (completely adnate to T)), stigma capitate to trifid, dry (wet - Bloomeria); ovule with outer integument 3-4 (5-7 - Dichelostemma) cells across, (inner integument 3+ cells across), parietal tissue 3-4 cells across, (nucellar cap +); seeds angular, cells of tegmen much enlarged (not - Triteleia); "embryo short"; n = 5-12+; hypocotyl?, primary root persistent.
12[list]/62: Brodiaea (14). S.W. North America, to British Columbia and Guatemala. Map: see H. E. Moore (1953) and Fl. N. Am. vol. 26: 2002. Photo: Flower, Flower, Fruit.
Age. Divergence within Brodiaeoideae began around 25.1 or (26-)20(-24) Ma (S. Chen et al. 2013).
Chemistry, Morphology, etc.. Little is known about the chemistry of Brodiaeoideae.
For inflorescence morphology and development, see Martínez-Gómez et al. (2022); although umbels of sorts characterize the group, only those of Dichelostemma congestum are derived from racemose inflorescences. Vogel (1998b) discusses septal nectaries and where nectar ends up in the flower. Quite commonly there are nectar tubes in the flower, and in species like Milla biflora these tubes are up to 13 cm long. They run most of the length of what is apparently the pedicel and convey nectar from the top of the ovary, where it emeges from the nectaries, to the bottom of the "pedicel", i.e. the floral tube; pollination by hawkmoths is likely. These "tubes" are shown as semi-enclosed channels by Vogel (1998b) but were not mentioned by H. E. Moore (1953); there are often three broad tubes in this part of the flower formed by the adnation of the antesepalous portion of the stipe to the perianth tube (Vogel 1998b: Figs 2K, 3C-F). Embryologically Brodiaeoideae are quite variable. The inner integument is massive or not, ditto the base of the nucellus, endosperm development varies, etc. (Berg 1978, 2003 for a summary).
Some information is taken from Rahn (1998: general) and H. E. Moore (1953: morphology).
Phylogeny. There are two major clades, [Muilla, Triteleia] and [Dipterostemon, Dichelostemma, Brodiaea], albeit with only moderate support. The first clade has a long tepalline tube and the second has appendages on the bases of the filaments that form a nectar cup; both characters arise in parallel in the two clades (Pires & Sytsma 2002; c.f. Seberg et al. 2012). See also Pires et al. (2001) for phylogeny and morphological evolution.
Previous Relationships. Themidaceae/Brodiaeoideae have often been included in Alliaceae/Amaryllidaceae-Allioideae because of their superficially similar umbellate inflorescence and rather undistinguished monocot flowers (e.g. Takhtajan 1997).
4. Scilloideae Burnett - Back to Asparagales
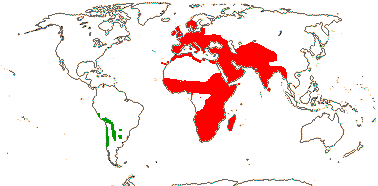
Plant bulbous, geophytic, roots often contractile; endomycorrhizae 0; polyhydroxyalkaloids, homoisoflavones, flavone C-glycosides +; root contractile, exodermis 1-layered; leaf with little (well developed) sclerenchyma, mucilage cells +; (leaf waxes with parallel platelets); bulb leaf sheaths closed or not; inflorescence scapose [?all], (branched), (spike), pedicels not articulated, bracteole 0; (corona +); stigma capitate to punctate and papillate; ovules 1-many/carpel, outer integument 2-4 cells across, parietal tissue 1-4 cells across, (nucellar epidermis radially elongated), nucellar cap +/0, hypostase +, raphides +, obturator +; seeds black; testa multi-layered; chromosomes 1.2-18 µm long; nucleus with protein crystals; (hypocotyl 0; collar rhizoids +); x ?= 10, asymmetric.
37/996 [list, to tribes] - seven groupings below. Predominantly Old World in Mediterranean climates, esp. S. Africa and the Mediterranean, to Central Asia and Japan; a few in South America. Map: both colours.
Age. Oziroë diverged from the rest of the clade in the Oligocene ca 28 Ma (Ali et al. 2012) while the age of the subfamily is estimated to be (50.9-)35.2(-20.1) Ma by Ji et al. (2022: Milla, Oz, Alb.).
4A. Oziroëeae M. W. Chase, Reveal & M. F. Fay - Oziroëe Rafinesque
Flowers (1-)2(-3)/bract; A basally connate and adnate to C; stigma punctate; seeds rounded, surface rugose; embryo long; n = 15, 17; cotyledon?
1/5. Western South America. Map: see above, green, from Guaglianone and Arroyo-Leuenberger (2002).
[Ornithogaleae + Urgineeae + Hyacintheae]: fructan sugars accumulated; root stele narrow, not medullated, with a central vascular element; rhexigenetic lacunae +; also styloids +; (pollen mixed with raphides); (antipodal cells polyploid).
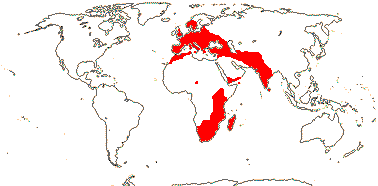
Age. The beginning of divergence within this clade can be dated to (47-)37(-29) or around 25.2 Ma (S. Chen et al. 2013).
4B. Ornithogaleae Rouy - Ornithogalum L. —— Synonymy: Ornithogalaceae Salisbury
Cardenolides +; (root stele with several central vascular elements); inflorescence (umbellate); bracteoles usu. 0; T multinerved, basally connate, (slightly dimorphic, outer T with caudate appendages); A (3), adnate to base (usu.) of T, (filaments flat, with appendages); tapetal cells 2-5-nucleate; stigma 3-lobed/capitate; ovules few-many/carpel; antipodal cells persist in pouch of embryo sac; seeds flattened/angled, black; cotyledon photosynthetic or not; n = 2-10+, (karyotype bimodal), nucleus with protein crystals.
1/312 (Dipcadi - if separate - 44). The Mediterranean, Africa, especially the south, to W. Asia (Afghanistan, India and Sri Lanka).
4C. Urgineeae Rouy / Hyacintheaceae-Urgineoideae
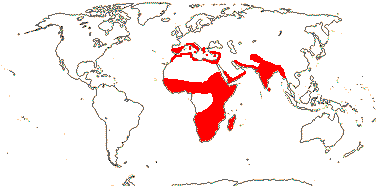
(Plant climbing); bufadienolides +; root stele with several central vascular elements; bracts spurred-peltate [as small leaves in Bowiea], bracteoles 0; flowers usu. short-lived; T basally (usu.) connate, (free - Bowiea), outer whorl with 5 traces, inner whorl with 3; A adnate to base of T, with two bundles in filaments [Drimia], variously connivent with the style, anthers (porose or slits <1/2 the anther length); (stylar canals 3 - Boweia), stigma ± capitate/lobed; ovules several/carpel; capsule ± abruptly narrowed at apex, (T persistent - Bowiea); seeds angled/flattened-winged; testa brittle/delicate, not tightly adherent to endosperm; n = (6-)10 [x = 10], karyotype bimodal, nucleus without protein crystals.
3/112: Drimia (110), or 30/220: Austronea (22), Drimia (19), Rhodocodon (19), Urginavia (16). Mainly southern Africa, also Madagascar and the Mediterranean to India. Map: from Pfosser and Speta (2001). Photos: Boweia Collection.
4D. Hyacintheae Dumortier
Homoisoflavanones +; root (stele with several central vascular elements/medullated); (leaves with pustules or coloured spots); T with a single trace; stigma feathery; parietal tissue +; embryo sac variable, e.g. bisporic (the chalazal dyad) [Allium-type]/8-celled bisporic (the micropylar dyad) [Endymion-type]/monosporic, 8-celled [Polygonum type]; seeds (brown to yellow), usu. rounded, elaiosomes common.
32/566.
Age. Crown Hyacintheae are ca 18.8 Ma (Ali et al. 2012).
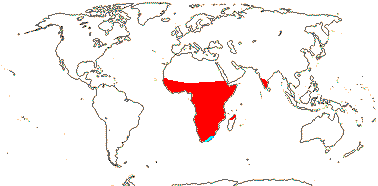
4Da. Pseudoprosperinae J. C. Manning & Goldblatt - Pseudoprospero firmifolium (Baker) Speta
Root stele?; (inflorescence branched); bracteole +, ± lateral; 2 ovules/carpel; seeds 1/loculus, subglobose; testa black; n = 9; cotyledon not photosynthetic.
1/1. Southeast South Africa. Map: blue-green.
[Massoniinae + Hyacinthinae]: ?
Age. The age for this node is ca 17.9 Ma (Ali et al. 2012).
4Db. Massoniinae Bentham & J. D. Hooker —— Synonymy: Eucomidaceae Salisbury, Lachenaliaceae Salisbury
Leaves (pseudopetiolate); bracteole filiform; T one- or multinerved; (flowers monosymmetric); A basally connate; ovary and style sulcate, style with 3 canals; ovules 2-many/carpel); (suprachalazal tissue long, with central column of cells - Drimiopsis [= Ledebouria]); testa (ruguse/puberulous/echinulate), (elaiosomes +); n = 5-16, 18, many 20< [x = 10]; cotyledon not photosynthetic (photosynthetic).
10/270: Lachenalia (142), Ledebouria (70). Africa S. of the Sahara, esp. South Africa, Ledebouria 1-2 spp India and Madagascar. Map: above, from Venter (2008), red, including blue-green area.
Age. Crown Massoniinae are ca 16.3 Ma (Ali et al. 2012).
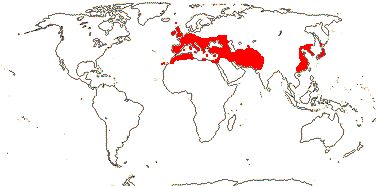
4Dc. Hyacinthineae Parlatore —— Synonymy: Hyacinthaceae Borkhausen, Scillaceae Vest
Endomycorrhizae +; (bracts 0), bracteoles quite common; perianth tube 0, corona 0; stylar canal papillate; ovules 2-8(-many)/carpel, (outer integument 4-5 cells across); (antipodal cells large, polyploid - some Scilla); seed (papillate), (elaiosome/partial sarcotesta +), phytomelan +/0; embryo suspensor filamentous; n = 4-8+ [x = 9]; cotyledon photosynthetic or not.
21/295: Muscari (80), Bellevalia (50), Scilla (30), Prospero (25, ?= Scilla). Europe (not the northeast), the Mediterranean, the Mid East, North Africa, Barnardia [= Scilla] japonica in the Far East. Map: see Meusel et al. (1965). [Photo: Scilla Collection.]
Age. Crown Hyacinthinae are ca 15.3 Ma (Ali et al. 2012).
Evolution: Divergence & Distribution. Hyacintheae may have originated in sub-Saharan Africa and dispersed north and also east, but details depend on the analytic method used (Ali et al. 2012). There are about 300 species of Scilloideae in the Cape flora alone (Procheŝ et al. 2006), about 400 species in southern Africa (Johnson 2010). Lachenalia, with some 142 species, is a major component of the flora in this area, and Duncan et al. (2022: Figs 4, 5) optimize the evolution of a number of characters in this genus - notice the variation in leaf morphology. The Scilla-Puskinia-Hyacinthella group probably evolved ca 36 Ma in the Mediterranean area (Özüdogru et al. 2022).
Martínez-Azorín et al. (2022) looked at the variation in some 40 "discrete" morphological characters in Urginieae; if discrete, the limits of the states quite often seemed arbitrary. Features like anthers porose or with slits less than half the length of the anther may be plesiomorphic/an apomorphy for the tribe; the distinctive, much-branched but more or less leafless vine Bowiea volubilis appears to be sister to the rest of the clade.
Ecology & Physiology. Many species in the foggy deserts of Namaqualand, South Africa, have water-catching leaves with very distinctive morphologies (Vogel & Müller-Doblies 2011; Duncan et al. 2022).
Pollination Biology & Seed Dispersal. Lachenalia has monosymmetric flowers in which the median member of the outer whorl is in the adaxial position. The same is true of the remarkable monosymmetric flowers of Massonia (Daubneya) aurea that are on the outside of the inflorescence. These flowers have the three abaxial tepals greatly enlarged, while the inner flowers are polysymmetric, the tepals forming a simple, lobed tube: The result is an inflorescence looking like a flower. Pollination in Albuca is noteworthy in that the pollen is deposited by leaf-cutter bees on the tips of the inner tepals, but pollination is not completed until two to three days later when the flower withers, the tepals then press against the stigma, and the pollen finally germinates (Johnson et al. 2009b, 2012). In southern Afica, particularly the winter rainfall region of the Cape, nine or more genera of hopliine scarab beetles are known to pollinate around 55 species (in eleven genera or so) of Iridaceae, although the beetles are not necessarily exclusive pollinators and more than one species may visit the same species of flower (Goldblatt et al. 1998b; Goldblatt & Manning 2011b); most of the species in genera like Romulea, with some 90 species, may be pollinated by these beetles. Scarab-pollinated flowers produce little nectar, and scent seems not to be an attractant, but the distinctive colours (red is quite common) and patterning of the flowers (black marks) is important (Goldblatt et al. 1998b; Goldblatt & Manning 2011b), and these patches may be iridescent (Whitney et al. 2011: e.g. variation in cell type).
Species with myrmecochorous seeds are scattered throughout the subfamily (Lengyel et al. 2010).
Vegetative Variation. Some species of Scilloideae have terete, unifacial leaves, as in Ornithogalum, where they develop from the upper part of the leaf (Kaplan 1973). Even the bulb scales of some species of Rhadamanthus (= Drimia) are terete. Urgineeae sometimes have a backwardly-directed process at the base of the leaves and/or bracts (c.f. Asparagus-Asparagoideae). Vegetative variation - in both leaf and bulb - is also considerable in Ledebouria (Venter 2007). However, there is less anatomical variation.
Genes & Genomes. High C values in Scilla are associated with long cell cycles, as has been noticed elsewhere (Francis et al. 2008). For the cytology of some sub-Saharan members of the subfamily, see Goldblatt and Manning (2011a). Goldblatt et al. (2012) suggest base numbers for tribes, etc. - karyotypes may be bi- or even trimodal. Nath et al. (2023) summarize the literature on chromosome numbers, genome size, etc..
Chemistry, Morphology, etc.. Bufadienolides are cardiac glycosides. Although mucilage cells are particlarly common in Scilloideae, they also occur elsewhere (Lynch et al. 2006). The vascular bundles in the scape of Drimia are endarch (Carpenter 1938),
Martínez-Gómez et al. (2022) describe the umbel-like inflorescences of Ornithogalum umbellatum. Remizowa (2022) described the interlocular septal nectaries of Ledebouria. Some Scilloideae have a filament tube. Wunderlich (1937) described the endosperm as being both helobial and nuclear in Hyacinthineae. The embryo sac of Scilla can be mono-, bi- or tetrasporic, and there is variation in the development of the antipodal cells - an antipodal embryo sac may develop (Haig 2020). The leaves of seedlings are two-ranked.
Information is taken from Speta (1998b: general, 2001: subfamilial characters), Pfosser and Speta (1999), Manning et al. (2004) and Manning and Goldblatt (2018) African taxa, van Jaarsveld and Eggli (2020b: Hyacinthaceae) and Martínez-Azorín et al. (2023: Urgineeae); for chemistry, see Kite et al. (2000), Pfosser and Speta (2001) and Koorbanally et al. (2008), for anatomy, see Lynch et al. (2001: leaf) and Sobotik and Speta (1997: root), for floral morphology in Ledebouriinae, see Lebatha and Buys (2006), for floral vasculature, see Carpenter (1938) and Deroin (2014), for embryology, very variable, see Sundar Rao (1940), Eunus (1950a), von Guttenberg and Jakuszeit (1957: Galtonia, polyembryonic), Berg (1962), Chennaveeraiah and Mahabale (1962), Svoma and Greihuber (1988, 1989), Ebert and Greilhuber (2005) and Dane (2006) and for seed morphology, also variable, see Brudermann et al. (2018, 2019).
Phylogeny. The topology [Oziroëeae [Ornithogaleae [Urgineeae + Hyacintheae]]] has moderate support in Manning et al. (2004), the latter including a tritomy (Pseudoprosperinae, Massoninae, Hyacinthinae above), the monophyly of the last two not having very good support. Oziroë and Albuca (Ornithogaleae) were successively sisters to the rest at the base of Scilloideae in Seberg et al (2012), the position of the latter genus having only moderate support. There is little well-supported structure along the backbone of Hyacintheae and again within Hyacinthineae in the trnL-F spacer analysis of Wetschnig et al. (2002); the positions of Ornithogaleae and Urgineeae were also unclear. See also Pfosser et al. (2003, 2012), the latter dealing with relationships of the Malagasy taxa. Martínez-Azorín et al. (2022) (4 plastid, 1 nuclear markers, sampling good) took a look at Urgineeae/Urgineoideae and recovered some well-supported relationships, [Bowiea [Rhadamanthus [Mucinaea ...]]] being sister to the remainder of the clade. Özüdogru et al. (2022: 3 plastid markers, almost 50 spp) found extensive polyphyly in Scilla. Howard et al. (2022: Angiosperms353 genes) looked at relationships in the Ledebouria/Drimiopsis/Resnova area (Massoniinae) and found four main clades, two made up of species ofLedebouria, and there was both incomplete lineage sorting and probable hybridization between an ancestor of D. botryoides and a member or members of one of the clades of Ledebouria. Hybridization may also have occurred in Muscari s.l., the nuclear (double digest RAD sequencing) and chloroplast (three plastid regions) analyses differing somewhat in the topologies recovered (Böhnert et al. 2023; for Muscari, see also Speta (1982). For relationships in Lachenalia, see Duncan et al. (2022) - the tree is very pectinate, and for those in Indian species of Dipcadi (Ornithogalum s.l.), see Shelke et al. (2024: 3 plastid and 1 nuclear markers); the species examined made a monophyletic genus, to which Pseudogaltonia was sister.
Classification. For a classification, see Speta (1998a: as Hyacinthaceae, = Scilloideae). However, there is considerable disagreement over generic limits and the ranking of groups in Scilloideae; are there 15 or 45 genera in sub-Saharan Africa? (e.g. Stedje 2001a, b; Pfosser & Speta 2001; Lebatha et al. 2006; Martínez-Azorín et al. 2015, 2019, esp. 2023: Hyacinthaceae-Urgineoideae, 30 genera; Crouch et al. 2018). Speta (1998a) dismembered Scilla and Martínez-Azorín et al. (2011) later broke up Ornithogaleae - 19 genera, of which 11 replace Ornithogalum; recognizability of taxa is not the issue (Speta 1998a recognized 13 genera). Manning et al. (2004) provided a generic synopsis of Hyacintheaceae in sub-Saharan Africa that integrates some morphology with relationships; like them, I take a generally broad view of genera. However, there are unresolved issues that include sampling, whether or not floral syndromes distort ideas of relationships (and so what effect characters taken from these syndromes have in combined analyses), the consequences of maintaining well-known generic names like Albuca and Galtonia as our knowledge of phylogeny becomes clearer, and the role cytological data should play in generic delimitations. Albuca is recognized in the reclassification of Ornithogaloideae by Manning et al. (2009).
Manning and Goldblatt (2018; see also Manning 2019) take a broad view of Drimia (followed here), but provide an infrageneric classification - 19 sections for the 70 southern African species, a majority of the genus, and another one for taxa from Madagascar. Manning (2020) revised the subtribal classification of Massonieae (Massoniinae above) within which Duncan et al. (2022) recognized 10 sections in Lachenalia, one of which had 13 subsections - all were monophyletic. Böhnert et al. (2023) reasonably expanded the limits of Muscari somewhat and recognized five subgenera there.
Previous Relationships. Chlorogaloideae, until quite recently included in Hyacinthaceae/Scilloideae (e.g. Pfosser & Speta 1999), are here included in Agavoideae.
[Lomandroideae [Asparagoideae + Convallarioideae]]: pedicels articulated; fruit a capsule; endosperm thick-walled, pitted, hemicellulosic.
Age. For the age of this node, estimated at (59-)49, 45(-35) Ma, see Bell et al. (2010); (60-)50(-42) or around 32.7 Ma are the estimates in S. Chen et al. (2013), (70.3-)56.6(-45.3) Ma those in Gunn et al. (2020) and (69.4-)53.4(-39.8) Ma in Ji et al. (2022).
Evolution: Genes & Genomes. The LOLOα duplication, ca 68.3 Ma, can be placed at this node (Landis et al. 2018: Lom. Asp.).
5. Lomandroideae Thorne & Reveal - Back to Asparagales
(Naphthoquinones +); (vessel elements in leaves); (T connate basally), nucellar epidermal cells enlarged, supra-chalazal zone long, with central elongated cells; antipodal cells large; T persistent in fruit; seeds rounded to angular; cotyledon photosynthetic or not, (coleoptile +; first leaves reduced).
12 [list, to tribes]/186 (+ 15 to be named) = four groups below. Madagascar, India, South East Asia to the Pacific, and South America, predominantly Australian. Map: see Schlittler (1951).
Age. The crown group age of this clade is (57-)47(-39) or around 32.7 Ma (S. Chen et al. 2013) or (60.6-)52.7(-43.7) Ma (Gunn et al. 2020).
[Lomandreae + Sowerbaea group]: flowers long-lived; T whorls at most slightly differentiated; infra-locular septal nectaries +.
Age. This clade has a crown-group age of (52.6-)41.4(-43.7) Ma (Gunn et al. 2020).
5A. Lomandreae Engler —— Synonymy: Lomandraceae Lotsy
Plant ± rhizomatous; root tubers 0; (monocot secondary thickening +); leaf blade with sclerenchymatous ribs extending from the inner sheath of the vascular bundle to the surface, outer bundle sheath with enlarged cells; leaves two-ranked, flat or curved, (margins with spines), (base auriculate); (plant dioecious, flowers with staminodes or pistillodes - Lomandra); pedicels articulated; inflorescence units cymose [?all]; flowers long-lived; (pollen grains spiraperturate/irregularly syncolpate); stigma wet; ovules 1-2/carpel, outer integument 6 or more cells across, Zuleitungsbahn well developed, surrounding nucellar cells enlarged, nucellar cap +; testa lacking phytomelan, thin, tegmen brown, collapsed, cellular; endosperm hemicellulosic, chalazal haustorium +; n = 7-10, chromosomes 2-7 µm long.
4/77: Lomandra (60). Australia, New Guinea, New Caledonia. Map: from Fl. Australia vol. 46. (1986) and Australia's Virtual Herbarium (consulted xi.2014). [Photo - Inflorescence © K. Stüber.]
Age. Crown group Lomandreae are (34.5-)24.0(-15.0) Ma (Gunn et al. 2020).
5B. Sowerbaea group —— Synonymy: Laxmanniaceae Bubani
Plant caespitose or rhizomatous, (stilt-rooted); (roots tuberous); leaves terete to triquetrous; inflorescence scapose (sessile), capitate to umbellate; (A 3, opposite inner T; staminodes 3 or 0 - Sowerbaea), (inner A adnate to T); stigma dilated or punctate; ovules 1-8/carpel; seeds dull brown to black, no aril, etc.; n = 4.
2/18: Laxmannia (13). Southern Australia.
Age. The crown group age of this clade is (40.1-)29.1(-19.3 Ma (Gunn et al. 2020).
[Arthropodium group + Cordylineae]: flowers open one day [check]; nectary 0; testa with phytomelan.
Age. The crown group age of this clade is (55.2-)47.4(-40.0) Ma (Gunn et al. 2020) and (55.3-)37.4(-18.3) Ma in Ji et al. (2022).
5C. Arthropodium group —— Synonymy: Eustrephaceae Chupov
Plant (annual), (rhizomatous), (climbing); (ecto)/vesicular-arbuscular mycorrhizae; roots often tuberous; (monocot secondary thickening + - Thysanotus); mucilage +; leaves spiral, vernation supervolute or conduplicate, (petiolate), (ligulate); flowers single or in groups, pedicel articulated or not; P whorls well differentiated, inner T long fimbriate/with hairy margins (not); (A 3), anthers dehiscing by pores (not - Trichopetalum)), (filaments with dense tufts of hairs [if inner T are not barbate]); stigma wet; seeds often arillate (strophiolate); exotesta often papillate, rest of testa cellular, tegmen thin; endosperm thin-walled; n = 9-11, chromosomes 0.5-2 µm long.
6/74: Thysanotus (50), Arthropodium (20). South East Asia to Australia, New Zealand and the Pacific, Madagascar, C. Chile, Neuquén Province, Argentina. (Map: Fl. Australia vol. 45. 1987; vol. 46. 1986; Schlittler 1951; Fl. China vol. 24. 2000; Australia's Virtual Herbarium xi.2014).
Age. The crown group age here is (46.3-)36.6(-26.7) Ma (Gunn et al. 2020).
5D. Cordylineae Nakai - Cordyline R. Brown
Rosette herbs to trees; storage roots +; fructan sugars accumulated; monocot secondary thickening +; mucilage +; stomata paracytic, subsidiary cells with oblique divisions, amphistomatouis; leaves spiral, vernation supervolute or conduplicate, (pseudopetiolate); flowers single along axis, pedicel articulated, short; fruit a berry; testa anatomy?; endosperm ?; n = 19, chromosomes 0.5-2.4 µm long.
1/17. Mascarenes, northeast Australia, New Guinea to the West Pacific and New Zealand, southeast South America. (Map: Fl. Australia vol. 45. 1987, vol. 46. 1986.) [Photo - Habit, Flower.]
Age. The crown group age of Cordylineae is (25.4-)22.0(-20.0) Ma (Gunn et al. 2020).
An Eocene fossil, Paracordyline aureonemoralis, from Adelaide is rather like members of the extant Cordyline stricta/C. fruticosa complex, and that genus is also known from somewhat younger (Oligocene) deposits on Kerguélen Island (Conran & Christophel 1998).
Evolution: Divergence & Distribution. Gunn et al. (2020, 2023) suggested that polyploid taxa in Lomandra, quite common, tended to have rather broad ecological distributions.
Plant-Bacterial/Fungal Associations. See Brundrett (2017a) and Tedersoo and Brundrett (2017) for the distinctive mycorrhizal association formed by the Australian Thysanotus. Fungi are associated with the subepidermal layer of cells (McGee 1988).
Chemistry, Morphology, etc.. There are reports of cell wall ferulates from Xerolirion (Rudall & Caddick 1994), which, if confirmed, would make it about the only non-commelinid genus with them.
The trunks of Cordyline develop massive aerial roots after injury, rather like those of Dracaena (Krawczyszyn & Krawczyszyn 2014). Eustrephus has vessels in its leaves. The leaves of Lomandra and its relatives have sclerenchymatous ribs extending from the inner sheath of the vascular bundles (c.f. also Cordyline?: Rudall & Chase 1996).
Xerolirion has solitary, terminal carpelate flowers, while its staminate flowers are in cymes. The pollen of Lomandra is very variable, sometimes being spiraperturate (c.f. Aphyllanthes). There is considerable variation in seedling morphology, even within individual groups (Conran 1998).
Additional information is taken from Chase et al. (1996), Conran (1998, as Lomandraceae), general, Schlittler (1951: Eustrephus), Ahmad et al. (2008: floral development of Lomandra longifolia), Chanda and Ghosh (1976: pollen, as Xanthorrhoeaceae) and Rudall (1994b, 2000: ovule, etc.).
Phylogeny. McLay and Bayly (2016) found two well supported clades in Lomandroideae. One included the [Chamaexeros + Lomandra] clade, with [Sowerbaea + Laxmannia] as its sister, the other was the [Trichopetalum, Arthropodium, Murchisonia] clade, and here Cordyline may be sister to the rest. Relationships found by S-C. Chen et al. (2013) are similar, as are those suggested by Gunn et al. (2020: sampling good). The latter found Trichopetalum to be well supported as sister to the rest of the Arthropodium clade (hence anther dehiscence is not likely to be an apomorphy for it), and Arthropodium itself was paraphyletic. A paraphyletic Lomandra included Xerolirion, was sister to/embedded in other Lomandreae (Gunn et al. 2020, 2023). Gunn et al. (2023: 45 taxa included) found relationships within Lomandra to be quite well resolved.
For the Cordyline group, see Chase et al. (1996), and for relationships in Thysanotus, see Sirisena (2010).
Classification. I have tentatively recognised four groups above, based partly on morphology and partly on molecular data.
Previous Relationships. Chamaescilla has been moved from Lomandroideae to Asphodelaceae-Hemerocallidoideae (McLay & Bayly 2016).
The leaves of Lomandra, etc., with their sclerenchymatous ribs extending from the inner sheaths of the vascular bundles, differ from those of Dasypogonaceae (Arecales), with which Lomandra and co. were previously associated, where this sheath is absent, and Xanthorrhoea (Asphodelaceae, see above), where the sheath develops from the mesophyll. However, the leaves of all three are xeromorphic and superficially similar (Rudall & Chase 1996).
[Asparagoideae + Convallarioideae]: steroidal saponins +; fructan sugars accumulated; (velamen +); flowers rather small[!]; T with a single trace; (fruit a berry).
Age. The age of this node is estimated at (55-)44, 42(-34) Ma by Bell et al. (2010) and at (57-)47(-37), about 27.8 Ma by S. Chen et al. (2013), (63.9-)47.7(-33.0) Ma by Ji et al. (2022) and 44.9-32.4 Ma (Bentz et al. 2024a: stem Asparagoideae).
Chemistry, Morphology, etc.. For ovule development, see Rudall (1994b).
Baccate fruits containing seeds that lack phytomelan are common here, but I do not know where they might be apomorphic. The capsular Hemiphylacus and Eriospermum are respectively sister to other Asparagoideae and Convallarioideae, and baccate fruits are probably derived several times (c.f. Judd et al. 2007) - or vice versa.
6. Asparagoideae Burmeister - Back to Asparagales
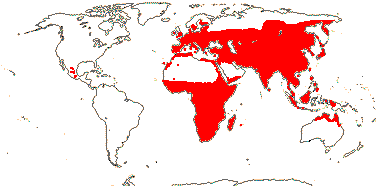
Rhizome +, horizontal or vertical; flavonols, saponins +; root (velamen +); leaves spiral; pedicels articulated; nucellar epidermal cells enlarged, persistent; embryo sac curved; seed rounded to ± angled, black, with phytomelan; embryo long, slightly curved.
2[list]/>220. Old World, also Mexico.
Age. Divergence within crown-group Asparagoideae began (25-)16(-9) or some 9.6 Ma (S. Chen et al. 2013), 39.6-12.7 Ma (McKain et al. 2016) or (15.0-)12(-9.4) Ma (Bentz et al. 2024a).
6A. Asparagus L.
Herbaceous to shrubby, often climbers, rhizome +, horizontal or vertical, root tubers +/0; saponins, flavonols +; root (velamen +), exodermis multilayered; vessel elements in roots often with simple perforation plates, vessels also in stem; cuticular wax rodlets parallel; leaves reduced, (scarious), (spiny), cladodes +/phylloclades, flattened and foliaceous or terete; plant (mon- or dioecious); inflorescence ± fasciculate/(flowers single); T free/basally connate; A free/(basally adnate to T)/(connate); tapetal cells 4-nucleate; G (slightly stipitate), stigma wet or dry; ovules 1-12/carpel; ovule with outer integument 3-6 cells across; embryo sac asymmetric, caecum basal, no nuclei, antipodal cells lateral, dividing; fruit a berry; testa multiplicative, collapsing, exotesta massive, (exfoliating), tegmen inconspicuous; endosperm cells thick-walled, pitted, hemicellulosic; n = 10, chromosomes 1-3 µm long.
1/>215. Old World, but only N. Australia (one species) in the Antipodes. Map: see above, from Hernandez S. (1995), Hultén and Fries (1986), FloraBase (2007), Seberg (2007), Kubota et al. (2011), B. Ford (pers. comm. 2011), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012), and see also Bentz et al. (2024a: Fig. 2, ?New Guinea). [Photo - Flower, Fruit.]
Age. Ji et al. (2022) estimate that the species of Asparagus that they sampled began diverging (17.3-)10.6(-5.7) Ma while the estimate in Bentz et al. (2024a, see also 2024b) is ca 5.1 Ma.
6B. Hemiphylacus S. Watson —— Synonymy: Hemiphylacaceae Doweld
Rosette-forming, (contractile roots +); leaves linear; inflorescence scapose, branched; (A 3, opposite inner T, + 3 staminodes), filaments in anther pocket; G stipitate, opposite inner T; ovules 3-6/carpel, outer integument ca 6 cells across, funicular obturator +; fruit a loculicidal capsule; testa multiplicative, collapsing, exotesta massive, tegmen inconspicuous; endosperm cells thick-walled, pitted, hemicellulosic; n = 56, chromosomes 0.7-1.7 µm long.
1/5: Hemiphylacus. Mexico. Map: see above.
Evolution: Divergence & Distribution. Fukuda et al. (2005a) and Norup et al. (2015) discuss diversification in Asparagus; this seems to have been rapid and to have started in southern Africa with several subsequent independent dispersal events to the north. Bentz et al. (2024b) confirm that southern Africa is the probable center of origin for all 12 major clades in the genus, and there have been eight subsequent range shifts from there, two of which are associated with long-distance dispersal events. Asparagus is quite often found in drier/sandy habitats. Dioecious and hermaphroditic taxa are found in a southeast-trending band from North Africa/France to West Malesia, dioecious taxa alone to the north and most diverse in the China area (Bentz et al. 2024a); dioecy has evolved twice, both times in the Asparagus clade, in the Mediterranean and East Asian areas respectively (see also below, Bentz et al. 2024b).
Pollination. Hybridization between species is quite common between members of the dioecious subgenus Asparagus (Kubota et al. 2011); dioecy seems to have evolved once or more likely twice in Asparagus (Norup et al. 2015; Bentz et al. 2024a, 2024b), but any connections with genome duplication events are unclear (Harkess et al. 2017). Species of Asparagus with flattened cladodes have perfect flowers (Kubota et al. 2011).
Vegetative Variation. There has been much discussion as to what the more or less leaf-like structures in the axils of the leaves in Asparagus "are" - stem or leaf or something else? (see e.g. Arber 1924b - prophyllar, axial; Cooney-Sovetts & Sattler 1986 - homeotic). Nakayama et al. (2012, see also Nakayama et al. 2010) looked at the development of both leaf-like and terete cladodes in Asparagus. The former (A. asparagoides) had an inverted (= prophyllar) orientation, while the latter (A. officinalis) were ventralised (c.f. Allium). They noted that both "leaf" and "stem" genes were expressed in the cladodes/phylloclades; the gene regulatory network for leaf development had been coopted by the axillary shoot (Nakayama et al. 2013). The lower stem leaves and the leaves subtending the main branches are themselves odd enough - peltate, and/or spiny, etc..
Economic Importance. Widely cultivated, Asparagus officinalis responds positively to inoculation by arbuscular mycorrhizal Glomus species (Klironomos 2003; Yeasmin et al. 2019).
Genes & Genomes. Harkess et al. (2017, 2018/20 and references; see also Henry et al. 2018) discuss the recent evolution of a two-gene dioecy system in Asparagus officinalis. This is an XY system, but the chromosomes are indistinguishable; the Y chromosomes have a closely linked pair of genes, one promoting the male function and the other suppressing the female function.
There may have been a genome duplication around here (Harkess et al. 2017; Zwaenepoel & Van de Peer 2020).
Chemistry, Morphology, etc.. Methyl mercaptans are known from Asparagus. Mucilage polysaccharides in the roots may have a storage role.
The prophylls ("bracts") at the bases of the pedicels in Hemiphylacus are described as being lateral (Hernandez S. 1995). For floral development in Asparagus, see Park et al. 2(003, 2004); b-class genes are not expressed in the outer tepal whorl.
Some information is taken from Malcomber and Demissew (1993: Asparagus) and Kubitzki and Rudall (1998), both general, Venkateswarlu and Raju (1958) and Rudall et al. (1998b), both embryology, and Robbins and Borthwick (1925: ovule and seed) .
Phylogeny. For phylogenetic relationships in Asparagus, see Fukuda et al. (2005b), Kubota et al. (2011) and Saha et al. 2016: (subgenus Protasparagus); subgenus Myrsiphyllum, at least, is probably paraphyletic. Norup et al. (2015) found six main clades within Asparagus as well as two small clades, but relationships between these groups were not well supported. Support was stronger in the plastome analysis of Bentz et al. (2024a: 39 spp. + many outgroups) while Bentz et al. (2024b: 158 spp., >1300 orthologous loci) recovered 10 well supported African clades (relationships between these forming a basal tetratomy) and the [Asparagus + Exuviali] clade.
Previous Relationships. Hemiphylacus used to be placed in Asphodelaceae-Asphodeloideae.
7. Convallarioideae Herbert (used to be Nolinoideae Burnett) - Back to Asparagales
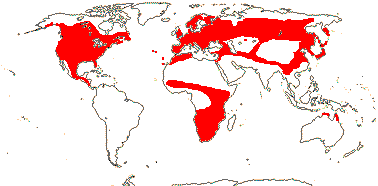
Flavonols, azetidine-2-carboxylic acid [non-protein amino acid]), (indolizidine alkaloids), saponins +; (velamen +); (vessel elements in roots with simple perforation plates; (vascular bundles amphivasal); cuticular wax rodlets parallel; pedicels articulated; nucellar cap 0; seed phytomelan 0; chromosomes 0.5-19 µm long; radicle well developed.
26[list: to tribes]/605 - 7 tribes below, some genera not included yet. Northern hemisphere, esp. South East Asia, Africa, esp. south of the Sahara. Map: from Meusel et al. (1965), Hultén and Fries (1986) and Perry (1994) - incomplete.
Age. Divergence within Convallarioideae began (53-)41(-31) or ca 35 Ma (Meng et al. 2021) or ca 23.6 Ma (S. Chen et al. 2013).
7A. Eriospermeae Reveal - Eriospermum Willdenow —— Synonymy: Eriospermaceae d'Orbigny
Plant tuberous (rhizomes/stolons); ?chemistry; root medulla 0; raphides ?0; leaves amphistomatous, no structural sclerenchyma; leaves single, hysteranthous [= appearing after flowering] (leaves few, synanthous [= appearing at flowering]), spiral, with sheath/petiolate, blade linear to cordate or peltate, (margin undulate), (enations + [stellate/long-penicillate/much branched (lamina proper ± 0)/etc.]; inflorescence scapose, (1 basal leaf-like inflorescence bract); bracteoles ?0; T basally connate; A adnate to base of T, anthers usu. dorsifixed, versatile; microsporogenesis successive; septal nectaries opening towards base of G, stigma punctate/slightly lobed; ovules 3-6/carpel, parietal tissue ca 1 layer thick, hypostase +, placental obturator +; fruit a loculicidal capsule; exotestal cells long-hairy, meso/endo testa with phlobaphene/empty, tegmen ± collapsed, endotegmen initially tanniniferous; nuclear endosperm +, 0, perisperm + [at the radicular end], embryo very large [the length of the seed]; n = (5-)7(9, 10, 12, etc.); cotyledon unifacial, photosynthetic, ring of hairs, radicle unbranched, tuber hypocotylar.
1/100. Africa S. of the Sahara (i.e. Eriospermum abyssinium Senegal to Ethiopia, then south), not the Congo forest, etc.), esp. S.W. Africa and the Cape. Photograph: flower, © M. Elvin.]
[Nolineae + the rest]: root medulla with xylem and/or phloem, or neither; raphides +; outer integument 2-8 cells across, parietal tissue (0-)1-3(-4) cells across, nucellar cap 0 (+), (chalazal vascular bundle branched); (embryo sac bisporic, 8 nucleate [Allium type]; tetrasporic, 16-nucleate [Drusa type]), (antipodal cells numerous, persistent); radicle branched or not.
Age. Diversification within this clade has been within the last ca 20 Ma (Meng et al. 2021) or (31.1-)19.0(-11.7) Ma (Ji et al. 2022).
[Nolineae, Polygonateae, Convallarieae, Ophiopogoneae]: ?
Age. The age of this clade may be (19.9-)14.0(-9.6) Ma (Ji et al. 2022).
7B. Nolineae S. Watson —— Synonymy: Nolinaceae Nakai
Plant usu. little-branched and ± tree-like, (base swollen/no trunk, rosette plants), (leaves persistent); (monocot secondary thickening +); vessel elements also with simple perforation plates [lf. root, not stem]; raphides?; cuticular wax with platelets, stomata in grooves, para-/tetracytic; leaves spiral, linear, isobilateral, margins usu. serrate; inflorescence branched; plant dioecious/polygamo-dioecious; ovary 3-locular, style ±0/+/styluli +; ovules 2/carpel, basal, parietal tissue 0, embryology unclear; (embryo sac haustorium - Dasylirion); fruit indehiscent, dry, ± 3-winged (terete), 1-/3-seeded; n = (18) 19.
4/52: Nolina (23). Mexico, also Central America and (S)/S.W. U.S.A..
Age. The age of crown-group Nolineae is some (13.2-)7.7(-3.2) Ma (Ji et al. 2022) .
7C. Polygonateae Bentham & J. D. Hooker —— Synonymy: Polygonataceae Salisbury
Plant rhizomatous (epiphytic) herbs; (cardenolides + - Polygonatum); stem leafy, leaves 2-ranked, (opposite, whorled), blade broad, base not sheathing; inflorescences axillary, bracts +/0; T (imbricate - Disporopsis), connate; A adnate to T, (corona at base of filament - Disporopsis); septal nectaries +, stigma capitate to lobed; ovules (2-)4-6(-12)/carpel; fruit a berry; n = 9-16, 20.
3/95: Polygonatum (75). N. Temperate, esp. China and adjacent areas.
Age. Crown-group Polygonateae are (19.2-)13.5(-9.3) Ma (Ji et al. 2022).
7. Convallarieae Dumortier —— Synonymy: Aspidistraceae Hasskarl, Convallariaceae Horaninow, Tupistraceae Schnizlein
Rhizomatous (erect) herbs; plant monopodial; (cardiac glycosides/cardenolides - Convallaria, Speirantha); root (velamen +), (cuboidal styloids +); leaves 2-ranked or spiral, 1-several per innovation, (petiolate); inflorescences axillary, scapose, (densely) spicate (racemose), (single flower - Aspidistra [A.]); (bracteole lateral - R.); flowers (2-13 merous - esp. A.); T often fleshy, ± connate, (tooth-like appendage at base of lobes - A.), (corona/annulus below A +); A adnate to base-middle of T, anthers as long as-5 times longer than wide-much, filaments 0-short (long exserted); septal nectaries 0, G (1), small [?all], style long-slender/massive/0, stigma punctate/3-(bi)lobed/massively peltate-fungiform (esp. A.); ovules (1-)2-4(-many)/carpel; fruit a berry (drupe); seeds (fleshy); n = 18, 19.
6/293: Aspidistra (240), Rohdea (30), Tupistra (20). Largely South East Asia to Sumatra, Convallaria majalis s.l. also S.E. U.S.A and Europe.
Age. Ji et al. (2022) estimated crown-group Convallarieae to be some (15.3-)10.6(-7.0) Ma.
7. Ophiopogoneae Voigt —— Synonymy: Ophiopogonaceae Meissner, Peliosanthaceae Salisbury
Steroidal saponins +; rhizomatous herbs; (cuboidal styloids +); leaves spiral, blade narrow, (blade, petiole +); T free to connate 50%; A (connate as corona - Peliosanthes), free to adnate to T, filaments short to long; G (subinferior), ?septal nectaries, (placentation basal), style (short to) long, stigma ± capitate; ovules 1-6/carpel; fruit a berry; (seed exposed early in development, testa fleshy - Liriope s. str.); n = 18, 2C = 8.62-24.65 pg.
3/145: Ophiopogon (67), Peliosanthes (60). Mostly (warm) temperate South East Asia, the Philippines, esp. China.
Age. Ophiopogoneae are perhaps (15.2-)10.2(-6.5) Ma (Ji et al. 2022).
[Dracaeneae + Rusceae]: plant woody; x = 20.
Age. The age of this clade is estimated to be (28.4-)17.0(-10.2) Ma (Ji et al. 2022).
7. Dracaeneae Dumortier - Dracaena L. —— Synonymy: Dracaenaceae Salisbury, Sansevieriaceae Nakai
Trees to rhizomatous/stoloniferous herbs; steroidal saponins +; roots often orange, velamen +; (monocot secondary thickening +, both root and stem, asymmetric in root); vascular bundles amphivasal [xylem surrounds phloem, ?level]; vessels in stem 0; (resin +, red, from wounds); plant glabrous; leaves unifacial, [amphistomatic, phloem always faces outwards], 2-ranked/spiral, ±flattened/pseudopetiolate/fleshy, terete, water storage cells central, dead; inflorescence racemose, (branched), (many-flowered clusters); (bracts 0), bracteoles 0 (+); T ± connate; A adnate to T tube; filaments (flattened/inflated); stigma capitate to 3-lobed; ovule 1/carpel, parietal tissue 0; fruit baccate, endocarp persistent, sclerotised, enclosing seed; seeds [really drupelets] often 1-2; testa obsolete; endosperm bony, ?embryo; n = 19-21.
1/170. Largely Old World, a few species in Hawai'i, Cuba and Central America.
Age. A possible age for Dracaeneae is (16.2-)10.7(-6.7) Ma (Ji et al. 2022).
7. Rusceae Dumortier —— Synonymy: Ruscaceae M. Roemer, nom. cons.
Shrubs to climbers; chrysophanol + [anthroquinone, in the roots]; root velamen +, cuboidal styloids +; (vessels in stem 0); plant glabrous; leaves spiral, scarious, phylloclades +; inflorescences axillary; flowers (imperfect): T free to connate; filaments connate, (T connate, A adnate to T); staminate flowers: A (?3), anthers extrorse; (pollen inaperturate); pistillode +; carpelate flowers: staminodes +; placentation axile to parietal; ovules 2/carpel or 1-4/ovary, hypostase +; fruit a berry; testa disintegrates, tegmen thick-walled, exotegmen cells longitudinally and endotegmen transversely elongated; endosperm thick-walled, hemicellulosic, embryo short to medium; n = 20.
3/8: Ruscus (6). Madeira and the Canary Islands to the Caspian Sea, scattered. Photograph: Ruscus flower.
Evolution: Divergence & Distribution. Diversification within the bulk of Convallarioideae (i.e. excluding Eriospermeae) has been within the last ca 20 ma (Meng et al. 2021; Ji et al. 2022), i.e. there was a phylogenetic fuse of ca 15 Ma after the divergence of Eriospermum (Meng et al. 2021). Much generic divergence has been since the mid-Miocene, a time of global climate cooling (Ji et al. 2022).
Biogeographical relationships in the Dracaena group are of considerable interest. It has been suggested that Pleomele (= Chrysodracon) from Hawai'i is sister to the rest of Dracaeneae, i.e. Dracaena s. str. (e.g. Lu & Morden 2010, 2013, 2014), which raises all sorts of biogeographical questions such as, is Chrysodracon an old inhabitant of the islands, like Hillebrandia (Begoniaceae) (see also Price & Wagner 2018)? In turn Central American species of Dracaena are sister to the remainder. However, Takawira-Nyenya et al. (2018) recently recovered a rather different set of relationships, for instance, Chrysodracon was embedded in Dracaena (the American species were not sampled), which suggests a rather different story. Cristini (2022) notes that the African-Macaronesian distribution of Aeonium (Saxifragaceae) is rather similar to that of Dracaena draco and its immediate relatives. Lu and Morden (2014) also noted several independent acquisitions of the arborescent habit (perhaps four times) and of cylindrical leaves (ca seven times, all in the erstwhile Sansevieria).
For several shifts between East Asia and America in Maianthemum, see Meng et al. (2021).
The flowers of Aspidistra (Convallarieae), sometimes borne beneath the litter (hence "Aspidistra plants have inconspicuous flowers" - J. Huang et al. 2023b: p. 1), show a positively bewildering amount of variation, e.g. see the descriptions of six new species by C.-R. Lin et al. (2024) and the images in L. Zou et al. 2025: Cover, Figs 1-3). Flowers of some species look rather those of Aristolochiaceae or Burmanniaceae, while others are more conventional and sub-rotate with the stamens and stigma/style grouped in the centre. They often have a relatively huge, fungiform stigma occluding the perianth tube, the anthers being hidden below it (Endress 1995b; Vislobokov 2017), or they have a short corona at the apex of a perianth tube that is massively longitudinally-ridged, or they have a balloon-like perianth with a little opening at the apex, or... There may be anything from two to a dozen or more tepal lobes (see also Hou et al. 2009; G.-Z. Li 2004; Vislobokov et al. 2014a; Vislobokov 2017). Any relationship between pollinator type and floral morphology is unclear (Tillich 2023). Aspidistra is often found in karst country, and is most diverse in S.W. China and northern North Vietnam - thus some 59 species are endemic to Guangxi Province, China, alone (Zou et al. 2025). All told, a remarkable genus in which new species are being described at quite a rate, a mere 16 species being known in the 1970s.
It is possible that the non-protein amino acid azetidine-2-carboxylic acid is an apomorphy for Convallarioideae (as Nolinoideae in Gibson et al. 2025), being notably common here while being unknown from Asparagoideae, however, Gibson et al. (2025) noted that it had i.a. been recorded from Hosta (Agavoideae) and Bowiea (Scilloideae) just for starters, so any bigger picture is unclear. For more on NPAAs, see Fabaceae.
Ecology & Physiology. Jura-Moraviec and Marcinkiewicz (2020) discuss water uptake and storage by the leaves of Dracaena draco. The vascular bundles in the stems of Dracaena anastomose (Fahn & Schori 1967), presumably affecting water transport, etc.; ?details.
Pollination Biology & Seed Dispersal. Easy access to the inside of the flower/anthers of Aspidistra is usually blocked by the massive stigma, there is apparently no nectar, not often any appreciable (to humans) scent, no thermogenesis, and no distinctive UV colour patterning, at least in the few species examined (Vislobokov 2017). It has been suggested that Aspidistra flowers are pollinated by amphipods (Conran & Bradbury 2007 and references: perhaps least likely), fungus gnats (Suetsugu & Sueyoshi 2017), the phorid fly Megaselia (Vislobokov et al. 2013), drosophilids, or non-galling cecidomyiid midges (Cecidomyiidae, undetermined genus) which also lay eggs in the anthers, the larvae eating the pollen (Vislobokov et al. 2014b; Vislobokov 2017).
Vegetative Variation. Vegetative variation is particularly impressive. Dracaena is the only monocot known with a monocot cambium in its roots as well as its stems (Carlquist 2012a; see also below). Thickening of the massive "trunk" of Dracaena draco, which can reach some 8 m d.b.h., is in substantial part the result of the fusion of the aerial roots (interestingly, the non-vascular cells of these roots, like those of the roots of orchids, etc., have chloroplasts) with the trunk proper (Krawczyszyn & Krawczyszyn 2014) - they are a kind of corticating root. Thickening of the stem-born roots is excentric, being greater on the adaxial side, and the diameters of the root tracheids is much greater than those of the stem (Marcinkiewicz & Jura-Morawiec 2024). Nolina also has secondary growth in the stem and is tree-like, and Beaucarnea, also tree-like, has a much swollen stem base. The initiation of the vascular system in the rhizome of Ophiopogon is similar to that in palm stems (Pizzolato 2009).
The leaves of Dracaena draco have hypodermal bands of fibres and variously-oriented collateral bundles scattered through the blade (Jura-Moraviec & Marcinkiewicz 2020). Extensive studies of foliar anatomy of Sansevieria (Koller & Rost 1988a, b: 49 spp.) suggest that all species have unifacial leaves, with no differences between ad- and abaxial surfaces, stomatal densities being the same, there is a Vorläuferspitze, the phloem in the vascular bundles always faces outwards, etc.. The central tissue of the leaf consists of a network of living cells and dead water-storage cells, the latter single or in groups and with their walls variously thickened - or not. Golenberg et al. (2023) looked at the development of the leaves of three species of Sansevieria, S. subspicata, S. trifasciata (both ± flat leaves), and S. cylindrica (leaves cylindrical).
Eriospermum includes perhaps the most remarkable foliar variation of any genus of vascular plants. The leaf blades can have enations on the upper surface, and these include fungiform protrusions on the small, crisped, ovate and fleshy blade (E. titanopsoides), or be a much-branched structure to 12 x 7.5 cm on a much smaller blade (E. ramosum), or a bundle of enations with stellate hairs (E. dregei), or paired enations that look as if they should grace the helmets of the Valkyries (E. alcicorne), while in E. aphyllon the plant when at the flowering stage appears to lack leaves, photosynthesis being carried out by the persistent inflorescence axis and pedicels (see Perry 1994 for more details). At least some of these variants may be adaptations for catching water from fog in the arid coastal regions of southwest Africa where the plants grow (Vogel & Müller-Doblies 2011).
Classical morphology suggests that the fleshy leaf of Sansevieria (= Dracaena) develops from the leaf base, the apical portion of the leaf being represented by a Vorlaüferspitze (e.g. Kaplan 1997, vol. 2: chap. 16); depending on the species, the leaf can be developed predominantly from the base (and is flattened) or from the apex (and is terete: Kaplan 1973).
Ruscus and its immediate relatives have phylloclades, the flowers being born in the middle of a tough, more or less elliptical pseudopetiolate leaf-like structure that has developed from an axillary bud. The prophylls are lateral or in some interpretations completely adnate to the axillary shoot. Together they form an expanded phylloclade - sometimes called a cladode, but that is a more or less flattened, indeterminate stem (see Arber 1924a, 1930; also Cooney-Sovetts & Sattler 1987; esp. Dörken et al. 2024) which may indeed be the main photosynthetic organ of the plant that bears it. In any event, the leaves proper in Ruscus, etc., are small and scarious and subtend the phylloclades (see also Asparagus above).
Genes & Genomes. See Yamashita and Tamura (2004) for chromosomes in Convallarieae and G.-Y. Wang et al. (2013) for those in Ophiopogoneae.
Some mitochondrial genes have moved to the plastome in Convallaria (references in J. Huang et al. 2023b).
In Ruscus and immediate relatives a mitochondral cox2 intron is missing (Kudla et al. 2002).
Economic Importance. For the fabled dragon's blood, red resinous exudate produced after damage by some eleven or so not all immediately related species of Dracaena that are scattered from the Cape Verde islands to Vietnam, see Madera et al. (2020) and Durán et al. (2020). The species include D. draco, from Cape Verde islands, etc., and D. cinnabari, from Socotra. Dracaena draco may have (been) moved quite recently from the Canary Islands to Madeira and Morocco.
Chemistry, Morphology, etc.. Vanícková et al. (2019) analysed the resin of dragon's blood (Dracaena spp.), i.a. monoterpenes appeared to be species-specific.
Although Madera et al. (2020) mention that Dracaena trees branch dichotomously, the inflorescences are terminal and branching is pseudodichotomous.
The absence of septal nectaries in some Convallarioideae may be connected with the presence of prominent ovary wall obturators; the latter are possibly derived from the former. In Liriope, etc. (Ophiopogoneae: Liriope ?= Ophiopogon), the seeds, with their fleshy testa, are exposed early in development, so they are semi-gymnospermous.
Additional information can be found in Bos (1998: Dracaenaceae) and Madera et al. (2020: dragon trees), Conran and Tamura (1998: Convallariaceae), Bogler (1998: Nolinaceae), Duthie (1940), Dahlgren (in Dahlgren & Van Wyk 1988), Perry (1994) and van Jaarsveld and Eggli (2020a), all Eriospermum/Eriospermaceae, Yeo (1998), Judd et al. (2002), Judd (2003) and Eggli (2020b), all Ruscaceae in some sense, and Tillich (2023: Aspidistra, key, bibliography), all general, Rudall & Campbell (1999: floral morphology), van der Ham (1994: Peliosanthes distinctive) and L. Wang et al. (2017: Polygonatae s.l.), both pollen, Stenar (1934, 1953), Wunderlich (1950), Eunus (1950b), Björnstad (1970), A.-m. Lu (1985: Eriospermum), Ebert and Greilhuber (2006: references) and Song et al. (2018: Polygonatum), all embryology, and Tillich (1995: seed, etc.).
Phylogeny Note the following - Maianthemum: Plant rhizomatous; blade broad, base barely sheathing; inflorescences terminal, racemose; flowers (2-merous); A free; n = 18; Smilacina - T with three traces. Maianthemum (35). N. Temperate, esp. China and Cantral America. Perhaps close to Theropogon and together sister to all other Polygonateae. Theropogon: A free, extrafloral nectaries +, 1 sp. - Himalayan region. Another problem: Comospermum - A free; 2 tenuinucellate apotropous ovules/carpel, micropyle endostomal, o.i. developing long apical biseriate hairs, hypostase +, placental obturator +; septal nectaries opening towards apex; n = 20. 1 sp. S. Japan. Close to Polygonatum, Danae. These genera to go where?
Eriospermum (for which, see Perry 1994) is placed as sister to the rest of Convallarioideae and with quite strong support (Seberg et el. 2012, but c.f. ML analyses, where it links quite strongly with Asparagoideae!). It might be thought that it and and the very distinct Comospermum are likely to be sister to the rest of the subfamily since both have capsules and hairy seeds. However, the hairs on the seeds of the two genera develop in different ways, etc., and these and other characters suggest that the two are unrelated (Rudall 1999); they do not come out close in studies such as those of Seberg et al. (2012) and Meng et al. (2021). The poorly understood Peliosanthes may then be sister to the rest of the family (molecular data alone, e.g. Jang & Pfosser 2002). However, in several analyses it groups with Ophiopogoneae (e.g. Seberg et al. 2012; G.-Y. Wang et al. 2014; Floden & Schilling 2018; Meng et al. 2021: the last two with strong support). Relationships within other Convallarioideae are poorly resolved, although major clades largely correspond with tribes (see Conran & Tamura 1998). Convallarieae seemed to be paraphyletic with Aspidistreae and Ruscus and relatives embedded (Yamashita & Tamura 2000: Eriospermum not included; Rudall et al. 2000b; Seberg et al. 2012). Convallaria was also included in Aspidistreae by Floden and Schilling (2018: support strong, Ruscus not sampled), and the two are to be included in Convallarieae (see also Ji et al. 2022). For relationships of ex-Nolinaceae, -Dracaenaceae, etc., see also Bogler and Simpson (1996); Dracaeaneae may be sister to Ruscus and relatives. These last relationships were also recovered by Ji et al. (2022: plastome analyses, 18/23 genera included) - only 1 species of Rusceae was sampled, and at least two of the missing genera are important... In the plastome analysis of J. Huang et al. (2023b: focus on Aspidistra) recovered the relationships [[Dracaeneae + Rusceae] [Convallarieae [Polygonateae [Ophiopogoneae + Nolineae]]]]. In another plastome analysis of some of the subfamily, X. Chen et al. (2024) found that the tribes included were all well supported, but relationships between them were not, apart from a [Rusceae + Dracaeneae] clade perhaps sister to the rest, Theropogon was unplaced and Eriospermum (and other genera) was not included.
The sampling in the Seed Plant Tree of Life (Angiosperms353 i.2023 version) was too poor to show much - a very pectinate tree - but that in the v.2023 version was improved, and relationships were [Eriospermeae [Dracaeneae [Rusceae [Nolaneae...]]]], furthermore, Comospermum was moderately well supported as sister to Theropogon and there were Ophiopogoneae and [Rohdea + Reineckia] clades - although relationships between all these were unclear - and a well-supported [Convallarieae + Polygonateae] clade.
Within Dracaeneae, P.-L. Lu and Morden (2010, 2013, 2014: chloroplast data) found that Dracaena and Pleomele alternated up a highly pectinate backbone of the tree (many of the branches had moderate support), Pleomele from Hawai'i (= Chrysodracon) was sister to all other Dracaeneae, and American taxa sister to the remainder; Sansevieria was deeply embedded and polyphyletic, S. sambiranensis being in a separate clade (but Baldwin & Webb 2016 recognize the genus). However, recently Takawira-Nyenya et al. (2018: chloroplast and nuclear trees rather different) recovered a rather different set of relationships. Although Sansevieria was still polyphyletic and embedded in Dracaena and Pleomele, so was Chrysodracon (floral differences = bird pollination syndrome), but unfortunately American species were not sampled. Van Kleinwee et al. (2022: esp. plastome data, S. sambiranensis not included) also looked at relationships within the old Sansevieria. For relationships in Macaronesian taxa of Dracaena, see Durán et al. (2020). Nolineae. Rojas-Piña et al. (2014) evaluated relationships around Beaucarnea (inc. Calibanus) and Nolina; they found three morphologically distinctive clades of tree-like plants, although support for the monophyly of Nolina was not strong. Ophiopogoneae. Liriope kansuensis was well embedded in those Ophiopogon examined (Meng et al. 2021). Polygonateae. Meng et al. (2014) discussed relationships within/between Polygonatum and its relatives. Some evidence suggested that this tribe probably did not include Maianthemum, which may be best placed with Ophiopogoneae (support weak), otherwise relationships are [Disporopsis [Polygonatum + Heteropolygonatum]] (Floden & Schilling 2018: plastomes). However, this Polygonatum clade was sister to Maianthemum in Meng et al. (2021), the combined clade being well embedded in Convallarioideae, Ophiopogoneae not being close. Meng et al. (2021) found rather weak support along the backbone of Maianthemum, and subgenera and sections were largely intermingled although section Maianthemum was monophyletic. In plastome analyses, Ji et al. (2022: note sampling) recovered the tribes here as monophyletic (Polygonateae were perhaps sister to/included [Maianthemum + Theropogon]), but aside from a [Rusceae + Dracaeneae] clade, relationships between the tribes were not well supported, and again there was evidence of plastome - nuclear genome conflict.
Classification. The tribal classification above does not pretend to be exhaustive, and some of the tribes may not be monophyletic.
Maianthemum includes Smilacina, and the combined clade is well supported as being monophyletic (Kim & Lee 2007; Meng et al. 2008, 2021); a broad circumscription is also appropriate because there is little support for groupings within it. Genera in the Dracena area pose problems (e.g. Jankalski 2015); of course there is a journal Sansvieria, whether or not that name is applied to a genus or some group within Dracaena... Takawira-Nyenya et al. (2018) found that practically no supraspecific taxa in previous classifications of Dracaena were monophyletic; for combinations in Dracaena s.l., see Christenhusz et al. (2018).
Jessop (1976) suggested that Peliosanthes teta might be the only species in the genus, the ovary varying from superior to inferior. However, it is currently thought that Peliosanthes includes some 60 species or more, and with new species being described (e.g. Tanaka 2018). For the 200+ species of Aspidistra, see Tillich (2023).
Previous Relationships. There has been extensive confusion between Dracaena (Convallarioideae-Dracaeneae) and Cordyline (Lomandroideae-Cordylineae).