EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost. (Some synapomorphies - almost whatever the immediate sister taxa to monocots might be - are in bold.)
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [[LILIALES + ASPARAGALES] COMMELINIDS]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [[LILIALES + ASPARAGALES] COMMELINIDS]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [[LILIALES + ASPARAGALES] COMMELINIDS]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders]; trans-splicing of mitochondrial group II intron nad1i728.
Age. The age of this node was estimated to be (127-)118, 105(-94) Ma (Bell et al. 2010), around 120 or 114.5 Ma by S. Chen et al. (2013), ca 119.8 Ma by Magallón et al. (2015; see also Givnish et al. 2016b), and ca 128 Ma by Foster et al. (2016a: q.v. for details), but only about 83.6 or 83.1 Ma by Xue et al. (2012) and 101.7-58.6 Ma by J. Li et al. (2020: Paris + Dioscorea).
Evolution: Divergence & Distribution. This is the core monocot clade of Coiffard et al. (2019), and they described Cratolirion bognerianum from rocks ca 113 Ma from the Crato formation in northeast Brazil which they thought could be placed around here - it was often associated with this clade in morphological analyses, perhaps particularly in the Dioscoreales/Pandanales area. The fossil implied that Liliales could already have diverged (Coiffard et al. 2019) - Acaciaephyllum, an early monocot fossil, had been associated with Liliales by Doyle et al. (2008b).
I largely follow Tobe et al. (2018) in the evolution of gynoecial position in monocots, in part to help think about different paths the evolution of this feature may have taken. Thus Endress (2011a) suggested that an inferior ovary was a separate key innovation for Zingiberales, Asparagales and Dioscoreales. There is evidence that inferior ovaries can become secondarily superior (e.g. Simpson 1998a; Remizova et al. 2008a; Tobe et al. 2018).
Thadeo et al. (2015) found that the anatomy of fleshy fruits that are scattered in this clade was quite similar, and they entertained notions of a long-conserved developmental pathway for these fruits, or perhaps the differences between capsular and baccate fruits were so slight that transitions between the two might be relatively simple.
The optimisation of nuclear endosperm to this node of the tree (Tobe & Kadokawa 2010) may well not hold up; variation in the patterns of endosperm development is great in many orders. For the distribution of trans-splicing of the mitochondrial group II intron nad1i728, see Qiu and Palmer (2004).
Plant-Animal Interactions. Larvae, and sometimes also adults, of the Chrysomelidae-Criocerinae are scattered as herbivores on plants throughout this clade, being perhaps especially common on commelinids (e.g. Schmitt 1988; Gómez-Zurita et al. 2007).
Genes & Genomes. The ORSAγ duplication event (Landis et al. 2018), ca 137.5 Ma, is to be placed here; c.f. another(?) duplication event placed two nodes up. Indeed, T. Shi et al. (2022) placed the τ/tau genome duplication event immediately below Dioscoreales (and above Alismatales) on the tree; they suggested that x= 5 there.
[DIOSCOREALES + PANDANALES]: root hairs from unmodified rhizodermal cells, exodermal cells not dimorphic; outer integument 2(-3) cells across; genome size 10> pg [1 C].
Age. The divergence of these two orders is dated to about 131.4 Ma by Tank et al. (2015: Table S2: stem age of Dioscoreaceae) and similar in Alcantara et al. (2018: see spread), ca 134.4 and 119.6 Ma by Magallón and Castillo (2009); estimates were ca 124 Ma in Givnish et al. (2018a), (130-)121(-119) Ma in Merckx et al. (2008a), ca 110.5 Ma in Magallón et al. (2015), (129-)119, 116(-110) Ma in Hertweck et al. (2015), (131.4-)117(-102.4) Ma in Eguchi and Tamura (2016), and (124.5-)104(-78) Ma in Givnish et al. (2016b). 123-96 Ma is the spread in Mennes et al. (2013, 2015), and (117-)106, 84(-70) Ma by Soto Gomez et al. (2020). Although Janssen and Bremer (2004) showed Dioscoreales and Pandanales as successive branches in the tree, they gave stem ages for both of ca 124 Ma.
Evolution: Genes & Genomes. This clade is characterized by the DIVIβ duplication event, some 131 Ma (Landis et al. 2018).
Leitch and Leitch (2013) record small genomes in this clade, but sampling is poor.
Phylogeny. For discussion on the relationships of Dioscoreales and Pandanales, see the Petrosaviales page.
DIOSCOREALES Martius - Main Tree.
Steroidal saponins +; vascular bundles in rings; vessels also in stem and leaf; flowers or inflorescence with glandular hairs; styles free early in ontogeny, branches well developed, adaxially grooved; T persistent in fruit; ovules many/carpel; endotegmen tanniniferous; embryo at most short; genome size usu. 0.4-6.8 pg [1C]. - 2-6 families, 21 genera, 900 species.
Includes (perhaps) Afrothismiaceae, Burmanniaceae, Dioscoreaceae s.l., i.e. including Avetra, Stenomeris, Tacca and Trichopus, Dioscoreaceae s. str. = Dioscorea alone, Nartheciaceae, Thismiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown group Dioscoreales are dated to ca 123 Ma (Janssen & Bremer 2004); estimates were ca 119 Ma in Givnish et al. (2018a), (126-)116(-113) Ma in Merckx et al. (2008a), (120-)116(-111) Ma in Merckx et al. (2010a; see also Viruel et al. 2015), 116-85 Ma in Mennes et al. (2013, 2015), (123-)115, 110(-104) Ma in Hertweck et al. (2015) and (120.2-)95.2(-61.4) Ma in Eguchi and Tamura (2016), about 95.2 Ma in Magallón et al. (2015) and (115-)76(-35) Ma in Givnish et al. 2016b).
Evolution: Divergence & Distribution. Remember, the basic phylogenetic structure of Dioscoreales, perhaps even its very composition, is unclear, so things like ages for clades in the literature need to be read in the context of the phylogenies on which they were based. Furthermore, the basic patterns of variation in e.g. vegetative anatomy, pollen morphology and seed coat anatomy, all quite extensive, will have to be rethought when relationships settle down.
Chemistry, Morphology, etc.. Nuraliev et al. (2020b) suggest that the bracteoles here are lateral, and such bracteoles do occur in the order, but I am unclear how common this feature is; bracteoles may also be absent/adaxial/more than one. Nakagawa et al. (2020) describe the flowers of Diocoreales as a whole as being inverted, i.e. as having the odd member of the outer whorl in the adaxial position.
For morphology and anatomy, see Ayensu (1972), for seed coat, see Bouman (1995) and Oganezova (2000b), for pollen morphology and development see Caddick et al. (1998) and Schols et al. (2005a: Nartheciaceae and Dioscoreaceae). For much information on morphology in the order, see Caddick et al. (2000a: floral morphology and development, 2000b: general); prolongations of the anther connective are homoplasious.
Phylogeny. Nartheciaceae are rather consistently placed sister to all other Dioscoreales, albeit sometimes with only moderate support (e.g. Chase et al. 2000a; Caddick et al. 2002a; Tamura et al. 2004a: 97% bootstrap, Nartheciaceae well sampled, but otherwise only Dioscorea, Tacca and three Pandanales; Janssen & Bremer 2004: one gene, very good sampling; Chase et al. 2006; Givnish et al. 2006: see also Goldblatt 1995). However, J. I. Davis et al. (2004) found Nartheciaceae to associate with Pandanales (note: Pandanales and Dioscoreales are not sister taxa there), although support was weak (<70%) and they lack the 6bp atpA deletion of many members of that clade, while G. Petersen et al. (2006b) found that in in chondrome analyses Burmannia was sister to [Pandanales + Narthecium] yet Thismia was in Dioscoreales.
In Thismia and its relatives, Dioscoreales include some of the most remarkable holomycoheterotrophic plants known, but the holomycoheterotrophic members of Dioscoreales seem not to come close to forming a clade. Merckx et al. (2006: good sampling, no outgroup to Dioscoreales), using both mitochondrial and nuclear genes, found substantially different relationships within Dioscoreales from those depicted in the tree given by Caddick et al. (2002a: see also /APweb/ version 6 [November] and earlier, classification as in Caddick et al. 2002b). However, as Merckx et al. (2006) noted, the relationships found by Caddick et al. (2002a) were dominated by chloroplast data, and since Burmanniaceae s.l. are largely mycoheterotrophic they have much diverged plastid sequences. Indeed, Geomitra, apparently Thismiaceae without any doubt, nevertheless came out with Burmanniaceae in some analyses (Caddick et al. 2002a). However, there may be problems with some of the sequences in Caddick et al. (2000, 2002 - see Mark Chase in Lam et al. 2016), Lam et al. (2016) noting that they had not seen circular plastomes in Burmanniaceae. Yokoyama et al. (2007: support slight) found the relationships [Burmanniaceae [Dioscoreaceae [Taccaceae + Thismiaceae]]]. The situation is yet more complex, since Merckx and Bidartondo (2008) and Merckx et al. (2009a) suggested that Thismiaceae s. str. may be paraphyletic, Afrothismia being sister to [Taccaceae + rest of Thismiaceae] (but c.f. Yokoyama et al. 2007). Merckx et al. (2010a; see also Merckx & Smets 2014) confirmed the paraphyly of Thismiaceae, and also suggested that Trichopus could be sister to [Taccaceae + Thismiaceae]; Stenomeris was well embedded in Dioscorea. However, Hertweck et al. (2015) found Burmannia and Thismia to be sister taxa, albeit with very long branches, but sampling was poor since this was not the main point of their paper. In other studies where broader relationships were also not the point, Viruel et al. (2015) and Z.-D. Chen et al. (2016) found the relationships [Burmanniaceae [Taccaceae + Dioscoreaceae s.l.]]. In a three-plastid gene analysis, Lam et al. (2016) found that Thismiaceae tended to wander in gene and codon position unpartitioned analyses, being sister to Petrosaviaceae, but in other analyses they were included in Dioscoreales, while when photosynthetic Dioscoreales alone were included, relationships were [Burmanniaceae [Nartheciaceae [Dioscoreaceae [Taccaceae + Trichopus]]]]. Relationships found by Lam et al. (2018) are [Nartheciaceae [Burmanniaceae [Dioscoreaceae [Taccaceae + Thismiaceae]]]], although sampling was slight and only the accD gene could be used for Thismia, and similar relationships were also recovered by Givnish et al. (2018b) although support for the position of Thismia (one species sampled) was rather weak and again Afrothismia was not included.
Turning to more recent analyses, Shepeleva et al. (2020: 2 nuclear and 1 mitochondrial markers) found the relationships [Dioscorea, [Burmannia + Afrothismia], [Tacca + Thismiaceae]], i.e. not much structure, while in a 347 nuclear gene analysis (W. J. Baker et al. 2021a: Fig. S6, Thismia not included), Dioscoreaceae s.l. were paraphyletic, and the position of Burmanniaceae (sampling quite extensive) was poorly supported; see also Seed Plant Tree of Life (Jan. 2022 version) - [Nartheciaceae [[Dioscorea [Trichopus + Tacca]] [Burmannia + other Burmanniaceae (inc. B. championii)]]], but again no Thismiaceae s.l. were included. There were only five Dioscoreales included in the nuclear study of Timilsena et al. (2022a, b), but relationships were compatible with those just mentioned. In an extensive analysis of mitochondrial genomes Q. Lin et al. (2022b) recovered the relationships [Nartheciaceae [Burmanniaceae [Dioscorea* [Trichopus* [Afrothismia [Tacca* + Thismiaceae]]]]]] (* = 1 species examined; most nodes well supported; branches in Afrothismia very long, Thismiaceae long, Burmanniaceae quite long, but not in comparison with branch lengths in plastome analyses). Garrett et al. (2023: plastome analyses, general pattern of branch lengths as above) recovered the relationships [Nartheciaceae, Burmanniaceae [Dioscoreaceae, Trichopodaceae [Taccaceae + Thismiaceae]]], but if they included the burmanniaceous Apteria aphylla and Gymnosiphon longistylus, then Thismia was included in Burmanniaceae, Haplothismia alone being sister to Taccaceae. There is some further discussion elsewhere, and it is going to be interesting to see what well-sampled nuclear genome analyses look like. In the Seed Plant Tree as of ii.2025 Nartheciaceae are sister to a Dioscoreaceae s.l., i.e. [Stenomeris [Trichopus [Tacca + Dioscorea]]], the combined clade being sister to Burmanniaceae (13 spp., 7 genera, Burmannia polyphyletic), but with little support - no Thismiaceae s.l. were included. Zuntini et al. (2024) had recovered the relationships [Nartheciaceae [Burmanniaceae + Dioscoreaceae], the latter with the same circumscription as just mentioned; Thismia rodwayi, alone of Thismiaceae s.l. was included, and it was weakly supported as being sister to [Dioscoreales + Pandanales]. A recent plastome study with a focus on Tacca found that genus to be well embedded in Dioscorea, but apart from those two genera Zephyranthes (Amaryllidaceae, outgroup) was the only other genus included (Y. Y. Ma et al. 2024). Clearly, good sampling of the nuclear genome is needed before one can really think about evolution and classification here.
Classification. The classification here initially followed that suggested by Merckx et al. (2006), although for relationships within Dioscoreaceae, see Caddick et al. (2002a, b). However, if the tree suggested by Merckx et al. (2010a) holds up, Afrothismia and Trichopodaceae will have to be split off, the former as in Cheek et al. (2023a, following Q. Lin et al. 2022b), or... Trias-Blasi et al. (2015) recognised a Burmanniaceae that includes Thismiaceae and a Dioscoreaceae that includes Taccaceae, but, as they noted, phylogenetic relationships needed attention. Nothing has changed in this respect, however, there is a Dioscoreaceae s.l. below, and whatever the ultimate circumscription of Burmanniaceae, relationships there are very interesting.
Synonymy: Burmanniales Martius, Nartheciales Reveal & Zomlefer, Taccales Dumortier, Tamales Dumortier
NARTHECIACEAE Bjurzon - Back to Dioscoreales —— Synonymy: Lophiolaceae Nakai
(Plants Al accumulators - Aletris); chelidonic acid +, flavonols 0; air spaces in root cortex; fibers intermixed in phloem; (sieve tube plastids with large polygonal crystal - Narthecium); endodermal cells evenly thickened [distribution?]; raphides 0, usu. druses +, but prismatic crystals in bundle sheath; cuticular wax with parallel platelets; leaves spiral, (two-ranked, ventralized isobifacial [oriented edge on to the stem]); inflorescence raceme/thyrsoid; bracteole 0 (+); (odd T of outer whorl adaxial); T free to connate; A ± free to adnate to C; pollen orbicules with a circular perforation; septal nectaries +/0; G to half inferior, partly ascidiate, fusion congenital to postgenital, compitum +/0, (stylulus long), (with 3 canals); ovules ana-campylotropous, integuments lack cuticle [check], integumentary obturator + [Aletris]; antipodal cells not multinucleate; seeds obliquely stacked, (with appendages), shape various; tegmen flattened, persistent, or testa cells flattened, (esp. outer anticlinal walls of exotegmen thickened - Lophiola); endosperm helobial, [how much?, walls?]; embryo size?; n = (12) 13 (21, 22), x =14 (?13, ?7), chromosomes 0.7-1.4 µm long, nuclear genome [1 C] (0.0.31-)1.164(-43.279) pg; cotyledon bifacial or not, ?collar rhizoids.
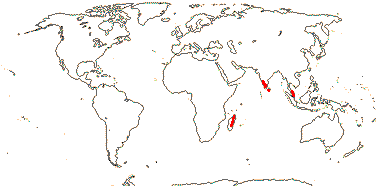
4-5[list]/41: Aletris (30). Interrupted N. Temperate, Venezuela and Guiana and scattered in W. Malesia. Map: from Hultén and Fries (1986), Jessop (1979), Fl. China v. 24 (2000) and Fl. N. Am. v. 26 (2002). Photo: Inflorescence.
Age. Crown-group Nartheciaceae are dated to ca 76 Ma (Janssen & Bremer 2004) and (92-)41(-10)Ma by Merckx et al. (2010a).
Evolution: Divergence & Distribution. For such a small family, variation in floral morphology and development is very considerable, and Tobe et al. (2018; see also Remizova et al. 2008a) have put it in a phylogenetic context. Although genera like Lophiola and Narthecium have two-ranked and isobifacial leaves, such leaves are unlikely to be an apomorphy for the family.
Chemistry, Morphology, etc.. Lophiola does not have phenylphenalenones – c.f. Commelinales-Haemodoraceae (J. M. Edwards et al. 1970).
The aerenchyma of Lophiola, at least, is more or less lysigenous (Simpson & Dickison 1981). Metanarthecium may have an abaxial prophyll, in Lophiola the prophyll can be lateral, while in Narthecium it is adaxial.
The ovary may be secondarily superior in Metanarthecium and Narthecium (Remizowa et al. 2008a; Fuse et al. 2012; Tobe et al. 2018). There are reports that the ovules of Narthecium are unitegmic (Remizowa et al. 2006a).
See Zomlefer (1997c) and Tamura (1998) for general information, and for endodermal thickening, see Zomlefer (1997a), for leaf anatomy, see Luque Arias et al. (2006), for pedicel anatomy, see Gatin (1920), for inflorescence and flower development, see Remizowa et al. (2006a, b), for pollen morphology, see Merckx et al. (2008b) and Simpson (1983: Lophiola), for embryology, see Ono (1929) and Cave (1968), and for seed coat, see Takhtajan (1985: as Hypoxidaceae).
Phylogeny. Aletris, with its spiral and bifacial leaves, was sister to the three other genera examined (Tamura et al. 2004a), with Metanarthecium having weak support as sister to the rest of the family in Fuse et al. (2012). Relationships in Merckx et al. (2008b: 3 chloroplast and 1 nuclear markers) are [Aletris (inc. Metanarthecium) [Lophiola [Narthecium + Nietneria]]], with strong support. In the chondrome analysis of Q. Lin et al. (2022b), [Aletris + Metanarthecium] were weakly supported as sister to the rest of the family. Although Isidrogalvia (= Alismatales-Tofieldiaceae!) sometimes ends up around here, this is because of contamination or misidentification (Tamura et al. 2004b; c.f. Azuma & Tobe 2011; Luo et al. 2016).
Previous Relationships. The relationships of Nartheciaceae, with their rather ordinary-looking monocot flowers, have long been problematic. Cronquist (1981) did not even mention them, but they would probably have been included in his highly heterogeneous Liliaceae, Dahlgren et al. (1985; see also Takhtajan 1997) placed them - along with representatives of Tofieldiaceae (here Alismatales) and Petrosaviaceae (Petrosaviales) - in Melianthaceae (Liliales), while Tamura (1998) recognized a Nartheciaceae, along with genera here included in Tofieldiaceae. Lophiola has been included in Commelinales-Haemodoraceae, for example in the Flora of North America, although with some hesitation.
[[Thismiaceae + Afrothismiaceae] [Burmanniaceae + Dioscoreaceae s.l.]]: plants geophytes, (holomycoheterotrophic); stem with endodermis; T tube well developed, broad, [?vasculature]; A incurved [± pendulous, anthers ending up extrorse], inserted towards the mid/upper part of the tepal tube, usu. close to stigma; ovary inferior, style short; exo- and endotesta tanniniferous.
Age. Divergence times at this node are (118-)109(-98) Ma (Merckx et al. 2010a: Burmanniaceae sister to rest); other estimates are (112-)96, 93(-87) Ma (Wikström et al. 2001), ca 115 and 127 Ma (Magallón & Castillo 2009) and (111-)92, 83(-68) Ma (Bell et al. 2010). The node is dated to ca 116 Ma by Janssen and Bremer (2004), (122.5-)104(-86.4)Ma by Couto et al. (2018) and ca 79.9 Ma by Magallón et al. (2015). The age of a clade including Afrothismia, Thismiaceae, and Trichopodaceae is some (109-)95(-79) Ma (Merckx et al. 2010a). Ages should be carefully checked here, because sampling in several studies is poor and relationships unclear.
Evolution: Divergence & Distribution. The early evolution of mycoheterotrophism here (see below) raises the issue of when more or less closed tropical rainforest developed (Merckx et al. 2008a, 2010a) - see also the parasitic Rafflesiaceae
Endress (2011a) thought that the inferior ovary in Dioscoreales might be a key innovation.
Ecology & Physiology. Mycoheterotrophy has evolved at least three, perhaps six or ten times in this clade (Merckx et al. 2006, 2008a, 2009, 2010a), the exact number of course depending on relationships within the group. As with many other holomycoheterotrophic taxa (but not Orchidaceae or Ericaceae), glomeromycota are the fungi involved (Franke et al. 2006). The holomycoheterotrophic habit may have evolved some time before the beginning of the Palaeocene (83-)63(-45) Ma when Afrothismia gesnerioides diverged from the rest of the Taccaceae-Thismiaceae clade (Merckx et al. 2010a).
Pollination Biology. Flowers with incurved stamens, more or less expanded stigma and sometimes also features of the corolla can combine to form complex flowers with the anthers very much in the interior in small chambers and opening towards the corolla tube - such flowers are found in Tacca, Thismia and some Dioscoreaceae other than Dioscorea itself which is dioecious, somtimes monoecious. Recent findings on pollination in Tacca (see below) suggest how the system might function.
Chemistry, Morphology, etc.. Cell walls in the endosperm are often thick, but are usually not pitted. The embryos of Taccaceae and Diocoreaceae are similar in their more or less lateral cotyledon (Solms Laubach 1878).
Phylogeny. Remember, relationships around here are unclear.
[Taccaceae + Thismiaceae + Afrothismiaceae]: septal nectaries 0; placentation parietal, stigmas broad.
Age. The age of this node (if it exists) is some (92-)79(-68) Ma (Merckx et al. 2010a) or ca 11.5 Ma (Givnish et al. 2018b), neither including Afrothismia.
THISMIACEAE J. Agardh nom. cons. - Back to Dioscoreales
Plants echlorophyllous, holomycoheterotrophic, associated with glomeromycotes; roots coralloid, vermiform [stems from along their length] or tuberous; saponins?; stele not medullated [?all]; root hairs 0; stem endogenous; stomata 0; leaves reduced to scales, immediately below the flower and/or along the stem; flowers single, or inflorescences +, (branched), ?drepanium/bostryx/(cincinnus)?; flowers with 0-1(-4) "bracts", (monosymmetric), odd member of outer whorl ad/abaxial, (to ca 5 cm across - Tiputinia), both T whorls with three traces, (T spreading), whorls not differentiated (Oxygyne, Haplothismia, etc.), or inner T whorl (apex) long, linear/connate and the whole like a mitre, (apex prolonged - "pillar"), outer T lobes small, 1 (0) traces or peltate and fungiform, tube well developed and expanded; corona as annulus or ring of short projections at mouth [?= congenitally fused bases of filaments], (0 - Haplothismia); A attached at apex of tube, (3, opposite outer T, Oxygyne), (abaxial/adaxial dimorphism - T. paradisiaca), incurved, connective and filaments broad (barely expanded, free - Relictithismia), with various projections and glands, often with paired apical ["wing-like appendages"] and basal projections, postgenitally connate and forming a tube [T.] or not, free/distant from G, (touching G - R.), (thecae 2 - R.) (interstaminal glands/projections +); tapetal cells uninucleate; endothecial cells with U thickenings (not); pollen grains (tricellular), monoporate, aperture equatorial, (monosulcate, 3-4(-7)-porate/3-colpate - some New World taxa), surface smooth/nano-/microperforate; G plicate, placentae 3, apical-parietal, often separating from wall, then appearing as separate columns, style short, branches slightly dorsi-ventrally flattened, (undivided), stigmas capitate to elongated (with round appendages - Oxygyne), pyramidal (lobes spreading, cristate - R.); (placentae 3, columnar, attached at base of loculus); ovules lacking parietal tissue, funicles long [?all]; (embryo sac bisporic); fruit pyxidium/irregularly dehiscent [apex withers, T abscised], ± cup-like, stem/pedicel elongating considerably; seeds dust-like, (with long, filiform projections - T. malayana); testal cells ± spiral, elongated [= the length of seed], tegmic cells compressed; endosperm helobial, basal chamber bicellular, thick-walled, with starch when young, not persisting; embryo undifferentiated; n = 6-9, 11-14, x = ?, chromosomes 1-4 µm long; (plastome I.R. 0); ?seedling.
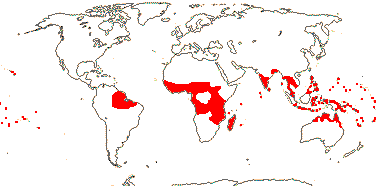
5 [list]/131: Thismia (116). Widely scattered, mostly (sub)tropical (temperate). Map: from Jonker (1938), van Steenis and van Balgooy (1966), Maas et al. (1986), Larsen and Averyanov (2007), Dauby et al. (2008), Ho et al. (2010: n.b. Thismiaceae s. str. in Africa only from Cameroon and Central African Republic, as Oxygyne), Cheek et al. (2018b), Aguilar-Cano et al. 2023) and Guzmán-Guzmán and Plato-Torres (2023: Colombia). [Photo - Thismia.]
Age. The age of crown-group Thismiaceae may be some (85-)68(-49) Ma (Merckx et al. 2010a: excl. Afrothismia, see also Yudina et al. 2021a) or (95.4-)75.8(-42.8) Ma (Ya et al. 2024).
The Late Barremian to Aptian Canrightia resinifera has been associated with Thismia (López-Martínez et al. 2023a: see also Chloranthaceae and Stemonaceae).
Evolution: Divergence & Distribution. Shepeleva et al. (2020) looked at the distribution of a number of floral features on their tree of Old World Thismia and found extensive homoplasy, thus zygomorphy has originated three times here alone. However, understanding the evolution of floral morphology is hampered by the fact that aspects of this morphology are simply bizarre, what little is known about development (but see Nuraliev et al. 2020b) is mostly from Old World taxa, and the relationships of the family are unclear. Haplothismia is different in a number of respects from the other genera. Hence the designation of characters as apomorphies in the characterization above should be considered as very tentative.
Thismiaceae are notably diverse in submontane forests around Rio de Janeiro (Sainge et al. 2017), and a continuous stream of new species is being described from the South-East Asia-West Malesian area in particular.
The immediate relatives of Thismia americana, collected near Chicago just before W.W. 1, but not seen after 1916 and now apparently extinct, were thought to be Antipodean, a remarkable disjunction (Thorne 1972, 1992). However, the recently-described Thismia huangi, from Taiwan, is also morphologically similar to these Antipodean species, although in molecular analyses they are not so close (Merckx & Smets 2014); either way, biogeographic scenarios need to be rethought. Indeed, T. kobensis, also recently described and from Japan, may also be close to T. americana, these three species (= sect. Glaziocharis) lacking connective appendages and interstaminal glands; their biogeographic connection perhaps is via Beringia; section Rodwaya is not immediately related biogeographically or morphologically (Suetsugu et al. 2023b). Oxygyne is known from Japan and Cameroon, a rather odd distribution (e.g. Cheek et al. 2018b).
Pollination Biology & Seed Dispersal. Most information here comes from Old World taxa - just to emphasize, floral morphology is usually exceedingly complex. Self-pollination or apomixis may be common (e.g. Maas et al. 1986; Severova et al. 2021 for literature), although (sapro)myophily is a likely means of cross pollination, fungus gnats, phorid flies and parasitic wasps all possibly being involved (Woodward et al. 2007; Mar & Saunders 2015; Yudina et al. 2021b; Guzmán-Guzmán & Plato-Torres 2023; for pollination by fungus gnats, see Mochizuki & Kawakita 2017). It is unclear if there are nectaries; Caddick et al. (2000) suggest there are none, Mar and Saunders (2015 and references) suggest that they are present. Certainly, taxa like Thismia malayana have glands in the flowers, although their function is unknown. Severova et al. (2021) noted that the morphology of the pollen grain did not suggest entomophily, while the small size of the grains (see below) did not suggest anemophily. Yudina et al. (2021b) suggested a bit of everything in Vietnam: A mix of autogamy, xenogamy, and root suckering. Indeed, if self-pollination or apomixis is widespread, the great floral variation in Thismiaceae becomes less easy to understand. For pollination, see also Waterman et al. (2013).
Splash-cup seed dispersal is likely in at least some taxa (e.g. Mar & Saunders 2015), and the seeds of Thismia malayane appear to have long, twisted, filiform appendages (Siti-Munirah et al. 2024).
Plant-Bacterial/Fungal Associations. Glomeromycote fungi form quite specific associations with species of Thismia (Merckx et al. 2012), however, the fungi (usu. Rhizophagus spp.) are quite widely distributed (Merckx et al. 2017; Suetsugu et al. 2022a) and the often rather localized distributions of the plant seem not to reflect any restrictions caused by fungal distributions. In the closely related Oxygyne there also tends to be an association with related fungi (Suetsugu et al. 2022a). In a study of Antipodean Thismia, Gomes et al. (2016) found that the specificity of their associations with local glomeromycotes was greater than that of AM plants from the same localities, but even the latter were associated with only a subset of the fungi. The mycorrhizae are of the Paris type (Imhof et al. 2013). Feller et al. (2022) studied the anatomy of the roots of 8 species of Thismia, noticing variation in gross root morphology - globose roots, with fine radiating roots (New World taxa), coralloid roots, and vermiform roots in which there were stem buds associated with root branches. Details of root anatomy, and cell layers in which fungal hyphae were to be found - in some they were being digested - also varied, as did the morphology of hyphae inside the cells. Hyphae might grow straight through the cells, or form closs and complex coils in the cell (the hyphae were thin), or form remarkable figure-of-eight coils within the cell (the hyphae were stouter) (Feller et al. 2022). For other information, see Johow (1889) and Merckx et al. (2013a), and for more on holomycoheterotrophy, see elsewhere.
Genes & Genomes. See Tsukaya et al. (2007) for chromosome number and size.
The plastome of Thismia tentaculata is very much reduced (Barrett & Kennedy 2018), as might be expected of a holomycoheterotroph (see also Yudina et al. 2021a). Interestingly, its IR is not syntenic with that of the two other Dioscoreales examined, suggesting that the original IR may have been lost and then reassembled de novo (Lim et al. 2016: see also Erodium-Geraniaceae), however, the plastomes of Thismia species in general are syntenic with each other (Yudina et al. 2021a). Garrett et al. (2023) found that the plastome of Haplothismia exannulata had lost 82 genes, and those of the species of Thismia examined at least 93 genes; the inverted repeat had been lost, apparently independently, in Haplothismia and T. panamensis. Interestingly, all Thismiaceae examined (except T. panamensis) have the cis‐spliced intron in rpl2 despite lacking matK; this intron is also to be found in other holomycoheterotrophs (Garrett et al. 2023).
Chemistry, Morphology, etc.. The stems may be endogenous in origin (Pfeiffer 1914). The underground parts of Tiputinia are quite massive, and the roots in Thismiaceae in general are thick.
The inflorescence morphology of species of Thismia described by Larsen and Averyanov (2007) is not easy to understand. Nuraliev et al. (2020b) suggest that the number of prophylls varies from 0-3, and floral orientation and inflorescence morphology also vary - all related features. Flowers in the axils of leaves along the stem lack associated involucral bracts and the odd member of the outer T whorl is abaxial. Details of floral morphology vary considerably, and it is difficult to make generalisations. Although the androecium is incurved in all species, details of how this is achieved differ considerably (Cheek et al. 2018b) and the morphology of the stamens, with variously expanded and ornamented connectives, sometimes with glands, varies considerably (Sochor et al. 2018; Nuraliev et al. 2020b). Cheek et al. (2018b) specifically note that the three stamens of Oxygyne are opposite the outer tepals, that is, they differ in position from the three stamens in Burmanniaceae, but from Maas-van de Kamer (1998) one would assume thay they were opposite the inner tepals; Jonker (1938), etc., do not, or do not clearly, state their position. Shepeleva et al. (2020: character 9) noted that in those species of Thismia they examined that had only three stamens, those stamens were opposite the inner tepals. The anther thecae of Relictithismia are drawn as if they are locellate (Suetsugu et al. 2024a). Pollen grains of S.E. Asian Thismia are very small, the average size of the few species measured being 7.1-17.1 X 12.1-26.7 μm (Severova et al. 2021).
Additional information is taken from Dahlgren et al. (1985), Rübsamen (1986), Maas et al. (1986), Maas-van der Kamer (1999), Merckx et al. (2013a) and Cheek et al. (2023), all general, also Woodward et al. (2007: Tiputinia), Cheek et al. (2018b) and Thorogood (2019), both Oxygyne, Severova et al. (2021: pollen) and A. N. Rao (1969: floral anatomy).
Phylogeny. For the delimitation of Thismiaceae, see above. Relationships within Thismiaceae s. str. are unclear, although Thismia itself may be paraphyletic. Although Shepeleva et al. (2020: 41 Thismia species, 2 nuclear and 1 mitochondrial genes) found that Old World Thismia was monophyletic, the one New World species they sampled was in a clade [Haplothismia [Thismia panamensis + Tiputina]] that was sister to the Old World species (see also Q. Lin et al. 2022b: chondrome analyses). There were five well supported (1.00 p.p.) clades and six unplaced species within the Old World species; the sections recognized by Kumar et al. (2017) were largely para- or polyphyletic. Yudina et al. (2021a) recovered the same relationships whether nuclear, plastid or mitochondrial genes were analyzed. Garrett et al. (2023: plastome data, Afrothismia not included) recovered the relationships [Haplothismia [T. panamensis + Old World Thismia]], T. panamensis being isolated and on a very long branch - but as mentioned under Phylogeny for Dioscoreales as a whole, recovering these relationships depends on the species of Burmannia included in the analysis. Relationships in Suetsugu et al. (2024a) are [Oxygyne [O.W. Thismia [[N. W. Thismia + Tiputina] [Haplothismia + Relictithismia]]]].
Oxygyne (as the Japanese species, O. shinzatoi) may be sister to the rest of the family (Yokoyama et al. 2007: 18S rDNA, parsimony; Merckx et al. 2009a; Merckx & Smets 2014; Shepeleva et al. 2020; Suetsugu et al. 2024a) or - less likely - be embedded in the family, as was Afrothismia (Yokoyama et al. 2007: maximum likelihood).
Classification. Kumar et al. (2017) provide an infrageneric classification for Thismia, but this has not held up well. Shepeleva et al. (2020) suggest how species groupings - with associated characters - may develop in Thismia, and note that generic limits in New World taxa need attention.
Most Thismiaceae are small to minute plants with small flowers (although they are quite spectacular in close-ups) and are not often found in flower. Judging by the continuous stream of new species that are being described they are very poorly known even at an alpha taxonomic level (see Franke 2007: comments on distributions); about half are known from only a single locality, and a third from a single collection (Dancáket al. 2020).
Botanical Trivia. Over the last decade or so, Thismia must have had the fastest relative growth rate in terms of numbers of new species appearing of any genus with more than 30 species.
AFROTHISMIACEAE Cheek & Soto Gomez - Afrothismia Schlechter - Back to Dioscoreales
Plants echlorophyllous, holomycoheterotrophic, associated with glomeromycotes, usu. epiterrestrial, (rooted in ground), ± rhizomatous, with aggregations of small basally swollen roots ["tubers"] each with a terminal rootlet; flowers single (inflorescence cymose, a cincinnus); bracts (1-)3; flowers monosymmetric, plane of symmetry oblique; P whorls not differentiated, T linear (triangular), (with lateral spreading or basal retrorse projections), corona +, annular, tube bent (straight), divided into two chambers by tube inpushing/narrowing, (annulus +) (tube globose, undivided); A free, inserted towards the base of lower chamber, incurved ± 180o, connective expanded, triangular at apex, touching / adnate to the stigma; pollen ulcerate/monoporate, surface coarsely reticulate; style short, stigma capitate or funneliform, (with flattened lobes), placentation free central (parietal, basal), placenta swollen, stalked; (funicle long); capsule circumscissile; seeds presented on elongated placental column/not, dust-like [to 1.5 mm long]; (?elaiosomes at both ends); n = ?; chondrome lacking atpB, rpl5; seedling with radicle, developing tuberous roots.
1[list]/16(?23). West and East Africa, most in the Cameroons, not in the Congo area. Map above: Africa; see Sainge et al. 2017).
Age. The age of crown-group Afrothismia is estimated to be (83-)63(-45) Ma (Merckx et al. 2010a).
Evolution: Divergence & Distribution. Merckx and Bidartondo (2008) described what they called delayed co-speciation (the delay is around 65-170 My!) of a group of Afrothismia on/with their Glomus fungal symbiont, which seems a little odd (see also Winkler & Mitter 2008; Cheek et al. 2023a).
Sainge et al. (2017) discuss the habitat preferences of Afrothismia and suggest that it could be quite widespread in wet, calcium poor, closed submontane rainforests in Africa.
Plant-Bacterial/Fungal Associations. For details of the complex fungal colonization pattern in the genus, see Imhof (1999b) and Imhof et al. (2013). The morphology of the fungus depends on the tissues in which it is growing, and there is also interspecific variation. Thus there are starch deposits in the cells of taxa like Afrothismia gesnerioides, while in A. saingei, for example, coiled and inflated hyphae seem to be the storage organs (Imhof et al. 2020). Mycoheterotrophy in general is discussed elsewhere.
Genes & Genomes. The possible loss of some mitochondrial genes in Afrothismia (see above) should be confirmed (Q. Lin et al. 2022b).
Chemistry, Morphology, etc.. Two adjacent tepals may be much longer than the other four; see also the floral diagram in Maas-van de Kamer (2003). There seems to be some confusion over/variation in placental morphology. Thus Cheek et al. (2023a) describe the ovary as follows: "placentation axile, placenta globose sometimes 3-lobed, massive, attached at base and apex by cylindrical stalks" and "unilocular with a single stalked, globose placenta". While Sainge and Franke (2005) show the placentation of Afrothismia hydra as being axile/free-central (ibid. Fig. 1B), they describe it as being "unilocular with basal placentation" (p. 301), yet the transverse section of the ovary of this species shown in Cheek et al. (2023a: Fig. 3A) suggests that the placenta is lateral. An illustration of A. baerae in Cheek et al. (2023a: Fig. 4E) clearly shows the placenta as being apically but not basally attached. In Sainge et al. (2005) A. korupensis seems to have free central placentation, although the authors note "the sterile central column not distended" (ibid. p. 289, see also Fig. 1G). Sainge et al. (2013) described two new species as both having basal placentation, the large placenta being lifted up by a "placenta column" (ibid. p. 592) in fruit - this interpretation is followed above. Finally, A. gesnerioides (see below) was described as having an ovary that was "1-celled, with 3 parietal placentas [shown as such in Fig. 1e] which are fused into one column at the base and free above"; no elongating column is mentioned in the fruit, but in Fig. 1f there are what appear to be radiating placentae rather similar to those that are shown in A. korupensis (Maas-van der Kamer 2003: p. 477). The seedling is not easy to describe (Imhof & Sainge 2008).
For general information, see especially Cheek et al. (2023a), also Cheek (2003b), Maas-van de Kamer (2003), Sainge et al. (2013) and Merckx et al. (2013a), also Imhof et al. (2013: esp. roots, mycorrhizae), and Waterman et al. (2013: pollination).
Phylogeny. Afrothismia gesnerioides is separated by a very long branch from the three other species of the genus examined by Q. Lin et al. (2022b; see also Merckx et al. 2009a).
[Burmanniaceae + Dioscoreaceae]: fruit winged, dehiscing laterally.
Age. Note that Janssen and Bremer (2004) included Geomitra (Thismiaceae) in their Burmanniaceae, so ages are compromised; ca 72.8 Ma is the age of this node in Magallón et al. (2015).
BURMANNIACEAE Blume, nom. cons. - Back to Dioscoreales
Plants echlorophyllous, holomycoheterotrophic, associated with glomeromycotes (fungus associated with scale leaves alone - Dictyostega orobanchoides) / mixotrophic / autotrophic; ?saponins; plant rhizomatous, roots thin, or stem ± 0, roots thick, fleshy, radiating, (root tubers +), (cortex disintegrating), (root hairs 0); root stele di- to pentarch, (not medullated); (stem with vascular bundles in a single ring); raphides 0; (stomata 0), (cuticular waxes as platelets transversely arranged in parallel series); leaves (two-ranked), usu. reduced to scales, (scales peltate); inflorescence (1-3-flowered), cymose; bracteole lateral (adaxial - Burmannia); (flowers monosymmetric); T (moderately large), outer larger (hardly), enclosing the inner, (3-lobed), inner (0), (trace 0), (3-lobed), (with marginal wings), T tube well developed (expanded below attachment of A); A 3, opposite inner T, filament ± 0 [?all], connective broad, (postgenitally adnate to style [gynostegium] - Burmannia), often with up to 4 short apical and basal appendages, thecae widely separate, lateral, transversely dehiscent; endothecial cells thickened/not; pollen often tricellular, monoporate with aperture equatorial, sulcate or inaperturate, surface smooth [psilate]; anthers ± associated with style; (nectary on top of ovary); G opposite outer T, nectaries 6, in pairs at apex of loculus [associated with septae], placentation ± parietal, (axile), style long, stigmas ± capitate, (with paired short to long and ± filiform apical processes); ovules many/carpel, (outer integument growing far beyond micropyle after fertilization, funicle long), parietal tissue none, chalazal tissue quite conspicuous, persistent; (antipodal cells persist - Gymnosiphon); fruit transversely (Burmannia) or septicidally and/or loculicidally dehiscent (indehiscent, or fruit horizontal, dehiscent down upper side only), T persistent, (circumscissile at the middle of the T tube); seeds dust-like; testal cells ± spiral, ± elongated, tegmic cells compressed, (tanniniferous); endosperm usu. helobial, basal chamber uni--multinucleate (multicellular - Hexapteralla), thick-walled, starch + when young, ± 0 when mature, chalazal endosperm semi-haustorial?; embryo undifferentiated; n = 6 (7) 8, 12, 16, ... 88, much and high polyploidy, x = 16, chromosomes 0.7-5.9 µm long, nuclear genome [1 C] (0.025-)1.098(-48.078) pg; ?seedling.
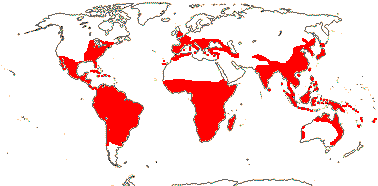
9 [list]/97: Burmannia (63), Gymnosiphon (36). Tropical, esp. America and the Guianan area, subtropical in both hemispheres. Map: from Jonker (1938) and Maas et al. (1986). [Photo - Flower, Campylosiphon, Hexapterella.]
Age. Crown-group Burmanniaceae are dated to ca 93 Ma (Janssen & Bremer 2004: three genera sampled); dates in Merckx et al. (2008a), at 96.4 Ma, are similar, while Merckx et al. (2010a) suggests somewhat younger ages of (99-)75(-52) Ma.
Evolution: Divergence & Distribution. The rather wide geographical range of the family is thought to have been achieved largely by migration. Diversification rates were notably high in the Cretaceous and again in the Eocene (Merckx et al. 2008a).
Ecology & Physiology. There have been perhaps eight losses of chlorophyll in Burmanniaceae, assuming that loss is irreversible - if Burmannia, the only genus which also has some partly mycoheterotrophic and autotrophic species, is indeed well embedded in the family (Merckx et al. 2006, 2008a, but c.f. Merckx et al. 2010a; see also Lam et al. 2016). At least some of the chlorophyllous species of Burmannia can grow well under high light conditions without any association with arbuscular mycorrhizal glomeromycotes (Merckx et al. 2010b) while others like B. coelestis are mixotrophic/partly mycoheterotrophic (Bolin et al. 2016); however, the echlorophyllous B. tenella, for example, is holomycoheterotrophic (Imhof 1999c). All species of Gymnosiphon are holomycoheterotrophic. Imhof (2001) described fungal growth in the scale leaves of Dictyostega orobanchoides. Timilsena et al. (2022b) compared the genome of the photoautrotrophic Burmannia biflora with that of the holomycoheterotroph Gymnosiphon panamensis along with two other comparable monocot examples, they - and mycoheterotrophy in general - is discussed in some detail elsewhere.
Plant-Bacterial/Fungal Associations. Glomeromycote fungi are involved in mycoheterotrophy, the mycorrhizae being of the vesicular-arbuscular Paris type (Imhof 1999c; Hynson & Bruns 2010; Imhof et al. 2013).
Genes & Genomes. See Aoyama et al. (2014 and references) for chromosome numbers.
The plastome of the holomycoheterotrophic Burmannia itoana has undergone extensive gene loss, being a little more than a quarter of the size of that of B. disticha, and there have also been four inversions and two translocations; the sequence of loss of some of the genes is a little unusual (X. Li et al. 2019).
Chemistry, Morphology, etc.. Although roots of Burmanniaceae are often described as lacking root hairs (e.g. Maas-van der Kamer 1998), as might befit their close association with fungi, root hairs have been shown in Burmannia (von Guttenberg 1968). In echlorophyllous taxa vessels may be restricted to the roots, in others there are vessels in the leaves.
Rübsamen (in Maas et al. 1986) emphasizes the great variety of nectaries in the family. In a floral diagram (Eichler 1874) the stigmas are shown as being commissural; the long stigmatic lobes in taxa like Gymnosiphon tend in this direction (Cheek & Traclet 2020). Yudina et al. (2022) provide much information on floral morphology and anatomy of Burmannia - information on variation in inflorescence morphology, the positions of prophylls and floral parts in general, inner perianth whorl development and vasculature, etc., and with some more general comments.
Information is taken from Jonker (1938), Dahlgren et al. (1985), Rübsamen (1986), Maas et al. (1986), Maas-van der Kamer (1998) and Merckx et al. (2013a), all general, Imhof et al. (2013: roots, mycorrhizae), Waterman et al. (2013: pollination), A. N. Rao (1969: floral anatomy) and Johow (1889 and references: anatomy, embryology).
Phylogeny. Merckx et al. (2008a) provide a detailed phylogeny of the family that has quite good support. Relationships are [[Campylosiphon + Burmannia reducta (holomycoheterotrophic)] [Dictyostega [[Hexapterella + Gymnosiphon] [Aptera + Burmannia]]]]. However, in a subsequent analysis Burmannia and [Campylosiphon + Burmannia reducta] were successively sister to the rest of the clade. In the mitochondrial genome analysis of Q. Lin et al. (2022b) relationships were [[Campylosiphon + Burmannia (3 spp., inc. B. itoana, holomycoheterotrophic] [Apteria + Gymnosiphon]], with overall rather strong support. X. Li et al. (2023: some disagreement between analyses) expanded the limits of Campylosiphon. Zuntini et al. (2024) recovered the relationships [Burmannia madagascariensis (heterotrophic)] [Dictyostega [Hexapterella + Gymnosiphon]]].
DIOSCOREACEAE R. Brown, nom. cons. - Back to Dioscoreales
Plant rhizomatous; lianes or vines, climbing by twining; (saponins 0), norditerpenes, flavonols +; in the stem small common bundles [proceeding to the leaf] and larger cauline bundles alternating, vascular bundles in two rings, outer ring ± V-shaped, inner ± elliptic, with 1-5 separate phloem units, phloem internal to metaxylem, xylem and phloem glomeruli at nodes joining vascular bundles and interrupting general course of vascular tissue; vessel elements with scalariform perforation plates in petiole but not blade; sieve tubes large, plates with very oblique end walls and several sieve fields; epicuticular wax crystalloids +, not oriented, stomatal ontogeny irregular; hairs glandular; (prophylls lateral); leaves ?spiral, with petiole and lamina, lamina cordate, venation palmate, fine venation reticulate, (vein endings free), vernation conduplicate, petiole pulvinate at both ends, leaf base not sheathing; inflorescences axillary; microsporogenesis simultaneous [tetrads tetrahedral]; carpels plicate, compitum +, stigma wet; ovules with bi(endo-)stomal micropyle, (outer integument ³3 cells across); seeds winged; endotestal cells elongated, thick-walled, with crystals, exotegmen thickened; embryo broad, plumule terminal, cotyledon ± lateral [?level]; x = 8 (?7, ?14), nuclear genome [1 C] = (0.022-)0.093(-38.176) pg.
4[list]/870. Largely tropical. Three groups below.
Age. Crown-group Dioscoreaceae are dated to ca 80 Ma (Janssen & Bremer 2004), some (54-)26(-9) Ma (Merckx et al. 2010a: c.f. topology), or (91.4-)77.2(-63.7) Ma (Viruel et al. 2015: Tri Ste Dio).
1. Stenomeris Planchon —— Synonymy: Stenomeridaceae J. Agardh

Stem annual, left twining, underground stem thickened; ?chemistry; tanniniferous cells 0; petiole bundles in arc; hairs with two-celled gland heads; ?petiole pulvini?; inflorescence ?type; T tube well developed and expanded below attachment of A [= torus], lobes (very) long; anther connective broad, long-prolonged apically, adnate to stigmatic head, filaments flattened; microsporocytes markedly elongated; tectum (undulate) perforate; G half inferior, style bowling-pin shaped, stigmas 3, bifid; ovules many/carpel; fruit elongated, dehiscence loculicidal; seeds distally asymmetrically winged; seed coat with phlobaphene, endotesta not crystalliferous, tegmen collapsed; endosperm walls thin; n = ?; seedling?
1/2. Northern West Malesia, not Java and islands east (Map: from Caddick & Wilkin 1998).
Age. The age of the [Stenomeris + Dioscorea] clade is (85.9-)71.4(-57.7) Ma (Viruel et al. 2015).
[[Trichopus + Avetra] [Tacca + Dioscorea]]: anthers ± erect, adnate to base of the tepal tube; pollen with orbicules; ovules (1) 2/carpel, superposed, hypostase +; endosperm walls thickened.
Stems long-lived; endodermoid layer fibrous; petiole with bundles in arc; anther connective (very) broad, ridged adaxially between thecae, apical prolongation +, connivent over stigma; microsporocytes large, somewhat elongated; pollen exine spinulate; nectary 0; fruit ± indehiscent; seeds not winged; endotesta not thickened, crystals ?0, exotegmic cells elongated, with reticulate thickenings [mechanical layer]; endosperm irregularly ruminate, walls thick, not pitted, embryo minute; n = 14, chromosomes 1.5-2.7 µm long; seedling?
2/2. Madagascar, Peninsula India, Sri Lanka, Peninsula Malaysia.
2A. Trichopus zeylanicus Gaertner —— Synonymy: Trichopodaceae Hutchinson, nom. cons.
Shortly rhizomatous, scaly, stems erect, not twining, with 1(-2) leaves; flavones +; cauline vascular bundles in one ring, xylem and phloem glomeruli 0; large sieve tubes 0, plastids lacking starch grains; glandular hairs with transversely elongated cells in series; petiole pulvini 0, lamina base cordate to truncate, cuneate; inflorescence axis 0, bracts several, scaly; flowers 1-6 together; pedicels very long [2-4 cm], bracteole 0; pollen sulcate; stigmas lobes 3, erect, longitudinally bilobed; parietal tissue ?0, nucellar cap 0, lateral nucellar cells +, hypostase +, obturator +; fruit keeled, slightly fleshy, opening irregularly; seeds 1-6, shortly winged; exotesta minutely hairy, exotegmen tanniniferous.
1/1. Southern Peninsula India, Sri Lanka, Thailand to Singapore.
2B. Avetra sempervirens H. Perrier —— Synonymy: Avetraceae Takhtajan
Stem right twining; glandular hairs 0; petiole with basal pulvinus; flowers 1-4 together; T "long" [1.5-3 cm]; microsporogenesis successive; pollen 4-5-pantoporate; stigma lobes 6, spreading-recurved; ?embryology; fruit winged; seed 1.
1/1. Madagascar.
[Tacca + Dioscorea]: pollen mother cells with simultaneous division.
3. Tacca J. R. & G. Forster —— Taccaceae Dumortier, nom. cons.
Plant with root tubers or rhizomes; (velamen +); secondary thickening +[?]; vessels 0; hairs with multicellular stalk row, a head, and then another cell row; petiole bundles in ring; leaves basal, blade ± deeply pinnately or palmately decompound-lobed to entire, fine venation reticulate, petiole +, base somewhat sheathing; inflorescence scapose, umbellate, of groups of cincinni, inflorescence bracts petal-like, floral bracts long, filiform; flowers medium in size; T with median member of outer whorl adaxial, whorls weakly to moderately differentiated, tube short; A adnate to P at base, connective broad, not prolonged, forming a hood enclosing anther; middle layer of anther wall from outer secondary parietal cells [dicot type]; pollen sticky; septal nectaries 0; G opposite C/inner T, stylar canal with secretion, stigma ± petal- or mushroom-like, (lobes bilobed), placentation parietal; nucellar cells laterally anticlinally expanded, nucellar cap 0; megaspore mother cells several; fruit baccate (loculicidal capsule); seed longitudinally ridged, (corky); (endotesta crystalliferous), exotegmen ± thick-walled and elongated, esp. radially; embryo short to minute, cotyledon ± lateral; n = 15, x = ?; cotyledon ?bifacial, sheath lobed.
1[list]/20. Pantropical, esp. Malesian-Pacific. Map: from Drenth (1976) and Australia's Virtual Herbarium (consulted i.2015). Photo: Collection.
Age. Crown-group Tacca is estimated to be (60-)35(-15) Ma (Merckx et al. 2010a).
For the fossil record of Tacca, see Ran (2017); seeds are the oldest fossils and are known from Europe at the Eocene-Oligocene boundary ca 33.9 Ma.
3. Dioscorea L.
Stem annual, left twining; spirostanol steroidal saponins +, chelidonic acid, (mannans) +; (root thorns +), (root tubers +); (velamen +); (monocot secondary thickening +); sieve tube plastids with protein crystals and starch grains; nodes 3:3, mucilage cells +, tanniniferous cells +; petiole bundles in ring (not D. hemicrypta); (stem tubers +); stomata oriented randomly, (morphology odd/actinocytic); gland heads many-celled; leaves two-ranked, (palmately lobed/compound), fine venation reticulate, (midrib +), vernation flat to curved or conduplicate, (apex glandular), petiole pulvinate at both ends, bifacial [?all], (base with paired evascular processes); serial axillary buds common; plant dioecious, (monoecious), inflorescences (two or more together), (branched) racemose to spicate, (bracteole lateral); flowers small, (sessile), white; T free or connate, spreading, with a single trace; staminate flowers: A (1)/(3 [opposite outer T] (+ 3 staminodes)), (3, 6 connate); pollen sexine perforate (to perforate-reticulate), (striate), (rugulate); pistillode +; carpelate flowers: staminodes +; loculi filled with secretion [?level], stylar canal with secretion, stigma bilobed or not; ovules 2/carpel, outer integument 4-5 cells across, nucellar cap ca 3 cells across, supra-chalazalal tissue ± massive, hypostase +; capsule longer than wide (wider than long) seeds 1-6, wing all around/basal; testa (multi-layered), endotesta with much phlobaphene, crystalliferous, exotegmen sclerotic [with branched protrusions of the cell walls], (not thick-walled if seeds proximally winged), endotegmen tanniniferous; endosperm usu. (very) thick-walled, embryo small to medium; chromosomes 0.3-2.9 µm long; cotyledon flattened and photosynthetic or not, second leaf a scale leaf.
1/649: 2 subgenera below. Largely tropical, also warm temperate, esp. seasonal. Map: see Meusel et al. (1965), Fl. N. Am. vol. 26 (2002) and FloraBase (2004). [Photo - Inflorescence, Flower, Fruits.]
Age. Crown-group Dioscorea is dated to (49.1-)48.3(-47.6) Ma, in the Eocene (Viruel et al. 2015) or rather earlier, (77-)63.7(-52.6) Ma (Couto et al. 2018).
The fossil Dioscorea eocenicus was recently described from Early Eocene deposits 57-54 m.y.o. from northwest India (Mehrotra & Shukla 2018) - at that time the plant would have been growing at the Equator. Fossils of fruits of two species of Dioscorea ca 52 Ma have also recently been described from Wyoming (Herrera & Manchester 2024).
4A. Subg. Dioscorea (section Stenophora)
Plant rhizomatous; staminate flowers: A (3 + 3 staminodes), (thecae separate); pollen monosulcate; tapetal cells 2-4-nucleate; carpelate flowers: (styles free), stigmas ± strongly bilobed; micropyle bi-/endostomal, parietal tissue 0; (embryo sac Drusa type, tetrasporic, 16-celled); x = 10, diploid.
35 spp.. Temperate east Asia to Malesia, the Caucasus, D. villosa North America.
4B. Subg. Helmia (Kunth) Bentham & J. D. Hooker —— Synonymy: Tamaceae Berchtold & J. Presl, Tamnaceae J. Kickx f..
Plant with ± hypocotylar/other tubers, (stem straight/not twining); leaves (opposite); pollen disulcate (monosulcate - sect. Borderea); fruit (baccate - Tamus s. str./samaroid - Rajania s. str.); seed wings also apical/basal + apical/0; x ?= 9, n = 6, 7, 9, 10 and polyploid.
Ca 595 spp.. Tropical, also warm temperate, esp. seasonal.
Evolution: Divergence & Distribution. For an evaluation of the fossil record of Dioscorea, see Iles (2015), Viruel et al. (2015) and Raz (2017); the latter thought that the oldest fossil attributable to the genus was from deposits in the Paris basin ca 47.8 Ma, early Eocene in age.
Viruel et al. (2015: many dates) discuss the biogeographic history of Dioscorea; they thought that most diversification was Oligocene or later and that Madagascar was colonized from Asia. It has been suggested that there have been four movements into the Neotropics (Couto et al. 2018). Herrera and Manchester (2024) thought that the genus may have originated in North America given the early Eocene fruits that they described from there.
Schols et al. (2005b) outline pollen evolution; there is considerable variation within Dioscorea (Alzer et al. 2024). The pollen of Avetra s. str. is pantoporate; for microsporogenesis there, see Caddick et al. (1998, 2000b). There can be infraspecific variation in pollen apertures; pollen grains may have one or two sulci, and this variation may even occur between grains from the one anther (Schols et al. 2003). For more on pollen, see van der Ham (1994: not Dioscorea) and da Luz et al. (2020). For the pollen of Tacca, see Tarasevich (2019: Convolvulaceae = Convallariaceae?).
The inflorescence of Tacca can form a single monosymmetric unit - indeed, it has been described as a pseudanthium (Baczynski & Claßen-Bockhoff 2023). Thus in T. integrifolia there are two large and conspicuous white inflorescence bracts that are held above the dark purple flowers, and there is a less conspicuous lower inflorescence bract and whitish-purplish, long-linear dangling floral bracts. In each flower the broad stamens are incurved and held horizontally in the mouth and they abut the stigma, more or less enclosing the centre of the flower; in T. cristata, at least, the anthers are incurved 180o or more, forming a small chamber and ultimately facing upwards (Lim & Raguso 2017). For inflorescence morphology and development, see Martínez-Gómez et al. (2022).
Ecology & Physiology. Dioscoreaceae are a major clade of mostly rather herbaceous twining climbers that have some sort of rhizome/"tuber"/underground rootstock; some taxa are left-handed twiners (Burnham et al. 2019); Dioscorea itself is among the ten largest genera of seed-plant climbers (Sperotto et al. 2023).
Pollination Biology & Seed Dispersal. The dark purple flowers of most species of Tacca and the dangling, long, filiform, purplish bracts suggest some sort of fly pollination. Although a variety of insects may visit the flower, pollination is likely to be carried out by female biting midges (Ceratopogonidae: Forcipomyia, Culicoides: Lim & Raguso 2017; Chua et al. 2020). The midges visit the flowers on the first day of anthesis when the flowers are held vertically; on the second day they become pendulous. Pollination by fungus gnats is also a possibility (Suetsugu et al. 2022b).
Plant-Animal Interactions. Extrafloral nectaries are common in Dioscorea (Weber & Keeler 2013).
Plant-Bacterial/Fungal Associations. Orrella dioscoreae (Burkholderiales) is the recently-described inhabitant of the leaf tip glands of Dioscorea sansibarensis, the glands running the length of the acumen (I. M. Miller & Reporter 1987; De Meyer et al. 2019). Indeed, the leaf/its lobes have notably long-acuminate apices, hence the plant's name, "stiff-tipped air potato". The bacteria live in the apoplast (Parniske 2018; Pinto-Carbó et al. 2018); for more information, see I. M. Miller (1990) and C.-J. Yang and Hu (2018).
Vegetative Variation. The cork in the tubers of Dioscorea is subepidermal and there may be secondary thickening. The exact morphological nature of the tuber is in some dispute. There may be a swollen hypocotyl in Dioscorea; in New World species new shoots arise from the dorsal surface of the tuber, roots from the ventral surface, and there is a transitory periderm (Martin & Ortiz 1963: Dioscorea floribunda, D. spiculiflora). In D. cayenensis/D. rotundata, at least, tthe tuber develops in the hypocotyl/cotyledondary node area; the second leaf of the seedling is a scale leaf which has a number of axillary buds (Trouslot et al. 1994).
The vascular bundles in the stem may be arranged in one or two rings (e.g. Ayensu 1972; Tenorio et al. 2017: much other detail), and are either cauline or common bundles, the former running up the stem and the latter proceeding to the leaves. If there are two rings, the cauline bundles are in the inner ring, and if there is but a single ring, the two kinds of bundles tend to alternate. Nägeli (1858), Brouwer (1953), Ayensu (1969) and others discuss nodal anatomy in Dioscorea; as Ayensu (1972) emphasized, the phloem and xylem glomeruli at the nodes are unique in angiosperms. Trichopus zeylanicus lacks the distinctive vascular bundles of Avetra and the xylem and phloem glomeruli in the stem found in other Dioscoreaceae (Ayensu 1966, 1972), but Trichopus is not a climber and its stems have but a single leaf. For midrib anatomy, see Edeoga and Ikem (2001); three vascular bundles may enter the leaf (Brouwer 1953; Periasamy & Muruganathan 1985) although there are often more bundles in the petiole (e.g. Ayensu 1972). There are very distinctive raphides in Dioscorea polystachya: The individual raphides are longitudinally lamellate, each is surrounded by a membrane, and they are individually and as a group embedded in mucilage (Raman et al. 2014). Leaflets of compound leaves are initiated in basipetal pairs and may be represent localised activity in the marginal blastozone (Periasamy & Muruganathan 1985: apparently not; Gunawardena & Dengler 2006), and the very apex of the leaflets differentiates early; there is a late-developing adaxial petiolar meristem, as in palms, Acorus and Araceae. Prophylls are at least sometimes lateral. Tacca lacks the distinctive vasculature of other Dioscoreaceae. It is unclear if its midrib is distinct or multistranded (Inamadar et al. 1983).
Genes & Genomes. Both XY and ZW sex-determination systems are known in Dioscorea (Girma et al. 2019). Martin and Ortiz (1963) discuss aspects of chromosome evolution; for chromosome numbers, etc., see also Viruel et al. (2008). Noda et al. (2020a) suggest that the base chromosome number for Dioscorea is x = 10. The chromosomes of Dioscorea/Epipetrum are relatively large, being 1.9-2.9 µm long.
The plastomes show nothing much out of the ordinary (Zhao et al. 2018).
Economic Importance. In addition to being important sources of starch, tubers of Dioscorea spp. can contain very large amounts of steroidal saponins that provide the precursors of drugs like testosterone, progesterone, estrone, cortisone, and the like, although these are now synthesized artificially.
Chemistry, Morphology, etc.. Dioscorea batatas has storage mannans in its vegetative tissues (Meier & Reid 1982).
The position of the inflorescence in Trichopus is unclear. The flowers of Dioscorea are shown with the median member of the outer whorl in the adaxial position (Spichiger et al. 2004); Eichler (1874) shows variation in the arrangement of the parts of the flower. The flowers of Tacca are drawn with the odd member of the outer whorl in the adaxial position by Ronse de Craene (2010), and they are described as being "monosymmetric in aestivation" by Cheek et al. (2023a). The pollen is undistinguished, being monosulcate and with a finely reticulate-striate surface; its shape varies considerably (Borokini & Ayodele 2012). The thickness of the parietal layer of the ovule of Dioscorea is taken from Torshilova et al. (2003); Nagaraja Rao (1953) described that of D. oppositifolia as being "massive", and although he (Nagaraja Rao 1955) described the ovule of Trichopus as being crassinucellate, it is unclear from the description and illustrations whether or not there is a parietal layer; Huber (1998: Trichopodaceae) described the ovule of the latter as being tenuinucellate. Seed coat anatomy would repay attention (Huber 1998). Huber (1998) noted that the mechanical layer of the seed coat was the exotegmen. However, Nagaraja Rao (1953) drew the endotesta of D. oppositifolia as being made up of small, heavily U-thickened and crystal-bearing cells, the testa itself was 4-5 cells across, and the endotegmen (and some exotestal cells) were tanniniferous. See also Huber (1998) for variation in testa anatomy.
For additional information, see Burkill (1960), Conran and Clifford (1985), Al-Shehbaz and Schubert (1989), Sivarajan et al. (1990), Huber (1998: narrow view of family, sections of Dioscorea ≡ genera; also Trichopodaceae), Viruel et al. (2010), and Limpricht (1928) and Kubitzki (1998b), both as Taccaceae, all general, and Rowley and Eggli (2020: succulents), for steroidal saponins, see Sautour et al. (2007) and references, for vegetative anatomy, Queva (1894: Tacca, including that of the young plant, Dioscoreaceae) and Ayensu (1972) and Behnke (1990b: nodal anastomoses), and for xylem, see Carlquist (2012a), for inflorescence and flower development, see Remizowa et al. (2010c: Dioscorea tokoro with A-T common primordia), for pollen of sect. Stenophora, see Torshilova (2024), for ovules, see Igersheim et al. (2001), for ovules and seeds of D. nipponica, see Torshilova and Titova (2010) and for seedlings, see Tillich (1985).
Phylogeny. Much detailed information is provided by Caddick et al. (2002b), although the positions of Stenomeris and Trichopus remained unclear (Viruel et al. 2015 and references); most recently Noda et al. (2020a) placed Stenomeris as sister to Dioscorea.
For the circumscription of Dioscorea and relationships within the genus, see Bharathan et al. (2001), Caddick et al. (2002a), Wilkin et al. (2005) and especially Viruel et al. (2015) and Couto et al. (2018: 2 plastid genes). Analysis of variation in the nuclear Xdh gene yielded a topology largely similar to that using variation in four plastid genes (Viruel et al. 2018); see also Noda et al. (2020a) and Soto Gomez et al. (2020: target enrichment) for relationships. The largely North Temperate section Stenophora (= subgenus Dioscorea) is sister to the rest of the genus, which has implications for generic apomorphies since subgenus Dioscorea appears to be the only clade in the genus with tricolpate pollen, rhizomes, etc. (Viruel et al. 2015, 2018; Noda et al. 2020a, b: Table 1). M. Chen et al. (2022: 43 spp., 5 plastid and 2 mitochondrial markers) looked at relationships in Chinese species of Dioscorea, optimizing the evolution of four characters on the tree; section Shannicorea was polyphyletic.
S. Y. Wong and Chua (2019: ITS, matK) found that the clade [Tacca leontopetaloides + T. maculata] was sister to the rest of the genus.
Classification. Dioscorea, Stenomeris, Trichopus and Avetra have usually been placed in separate (albeit more or less closely related) families, although Ayensu (1972) was inclined to think that Trichopus s. str. (see above for its anatomy) was rather separate from the other three genera, all of which he included in Dioscoreaceae. Indeed, Trichopus and Avetra are often put in a single genus, but here they are kept separate.
Dioscorea s. str. was divided into 22 sections by Huber (1998), and all were fully described, but, even if their monophyly held up, having 22 genera would not seem a desirable option. Noda et al. (2020a, esp. b) provide a new infrageneric classification, with two subgenera, i.e. Dioscorea (= the old section Stenophora) and Helmia, the latter with 19 sections.