EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of pore], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[LILIALES [ASPARAGALES + COMMELINIDS]]: (inflorescence branches cymose); protandry common.
[ASPARAGALES + COMMELINIDS]: style long; whole nuclear genome duplication [τ/tau event].
Cell wall wih ferulic acid (up to 4%) and ρ-coumaric acid (up to 3%) [hydroxycinnamates], with >3.5 mg g-1 ferulate [e.g. ester-linked to non-cellulosic glucuronoarabinoxylans, fluorescing blue under UV, green with NH3], cell wall with cellulose fibers encased in glucuronoarabinoxylans, high levels of hydroxycinnamates, very little pectin and structural proteins [Type II cell walls], pcoumarate acylates lignin [mostly on syringyl units], flavonolignins + [resinols ± 0]; exodermal cells monomorphic; (vessels in stem and leaves); SiO2 bodies +, in leaf bundle sheaths; stomata para- or tetracytic, (cuticular waxes as laterally aggregated rodlets [looking like a scallop of butter]); inflorescence branches determinate, peduncle bracteate; P = K + C [stamens adnate to/inside corolla/inner whorl only]; pollen starchy; ovary superior; endosperm reserves starchy [?Arecales], embryo short, broad. 5 orders, 30 families, ca 23,500 species.
Includes Arecales, Commelinales, Dasypogonales, Poales and Zingiberales.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Estimates of the time when divergence began within commelinids are ca 135 Ma (Z. Wu et al. 2014), ca 124 Ma (Tang et al. 2016), ca 122 Ma (Tank et al. 2015: Table S2), ca 120 Ma (Janssen & Bremer 2004), (128.7-)118.7(-109.1) Ma (Eguchi & Tamura 2016), (122-)116(-94) Ma (Merckx et al. 2008a), ca 116 Ma (Bremer 2000b), around 112 Ma (Foster et al. 2016a: q.v. for details), about 108.2 Ma (Magallón et al. 2015), (113-)103, 96(-86) Ma (Bell et al. 2010), (104-)99, 91(-86) Ma (Wikström et al. 2001), ca 83.4 Ma (Magallón et al. 2013), or only ca 67.1 or 64.5 Ma (Xue et al. 2012: note topology). Magallón and Castillo (2009: relaxed and constrained penalized likelihood datings) estimate ages of ca 128 and 115 Ma, and ages are (127-)118, 110(-104) Ma in Hertweck et al. (2015), 93-90 Ma or 109-97 Ma in Mennes et al. (2013, 2015 respectively), around 106 or 83 Ma in S. Chen et al. (2013) or 131-116 Ma (Jiao & Wang 2022). Christin et al. (2014) suggest a variety of ages for stem-group Arecaceae - ?topology.
Evolution: Divergence & Distribution. Magallón et al. (2018) suggested that there had been an increase in the diversification rate around here - but in a clade that included commelinids minus Poales and dated to (106.7-)102.1(-98.2) Ma... The rate of molecular evolution in commelinids other than Arecales is generally high, ca 0.003 substitutions/site/Ma (Smith & Donoghue 2008). However, in genes like ndhF, at least, the rate is similar to that in Asparagales (Orchidaceae), etc. (Givnish et al. 2006). Although genes in all three genomic compartments apparently evolve slowly in Arecaceae (Wilson et al. 1990; W. J. Baker et al. 2000a, 2000b), the rate is not that dissimilar to that in these other monocots (S. W. Graham et al. 2006; Barrett et al. 2015b). Was there an increase in the rate of molecular evolution within commelinids, some Poales being spectacular examples?
Ecology & Physiology. Silica (SiO2), an apomorphy for the commelinids, is an important plant defence, causing mechanical damage to mouthparts of would-be grazers and also having a number of physiological effects. For discussion on the effects of silica and silica bodies on herbivores, see Poaceae; most of the experimental work on this subject has been carried out on grasses. For phytoliths, silica bodies dispersed after the plant rots, see Piperno (1991). The element silicon is important ecologically in other ways. There is a negative correlation between leaf longevity and silicon concentration in plant tissues (Cooke & Leishman 2011b). Hardly surprisingly, silicon concentrations in tissues of commelinids are generally high, although less so in those commelinids that lack silica bodies (Ma & Takahashi 2002; Hudson et al. 2005). For more on silica bodies, see Benvenuto et al. (2015).
Franks and Farquahar (2006) found that in both Tradescantia and Triticum there were major changes in K+ ions in the guard and subsidiary cells that greatly affected stomatal opening, making the stomatal pore larger, but a lycopod and fern were the only other plants in their comparison.
Pancaldi et al. (2023) compared details of cell wall composition and development of grasses (both C3 and C4 species) with those of other angiosperms and found very considerable differences. (Note, however, that Pancaldi et al. (2023; see Fig. 1C) included four non-Poaceae commelinid monocots in their study, i.e. two Arecaceae spp., Musa acuminata and Ananas comosus, although they were scored as being non-commelinid monocots; they were more or less intermediate between grasses and other angiosperms, including the other monocots and the one mesangiosperm they examined, however, one member of Brassicaceae did group with the four non-grass commelinids.) Thus in grasses (Type II walls) xylans were common, but there was little in the way of xyloglucans and glucomannans, phenylpropanoids were common, as were mixed-linkage glucans, i.e. (1,3; 1,4)-β-glucans. Eudicots and non-commelinid monocots had Type I walls, and here there were xyloglucans and glucomannans, pectin and structural proteins, but little in the way of phenylpropanoids - the differences between these Types I and II walls have been described as "profound" (Pancaldi et al. 2023: e.g. p. 274). Vogel (2008) noted that an unusual feature of both primary and secondary cell walls of grases was the presence of significant quantities of the hydroxycinnamates ferulic acid and ρ-coumaric acid. These hydroxycinnamates exist as unbound acids or ester- and ether-linked to the arabinosyl units of glucuronoarabinoxylan and to various positions in lignin. The ferulate residues could dimerize through ester and ether linkages and cross link adjacent glucuronoarabinoxylan molecules, and so on (Vogel 2008). Note that it is unclear in the literaure what is meant by "grasses" = Poaceae, (part of) Poales, commelinids...? There was considerable variation in gene copy number (e.g. high copy number of genes involved in phenylpropanoid and ferulic acid production in grasses), gene presence and absence, presence of tandem arrays, and so on. It is interesting that these mixed-linkage glucans have been recorded from other members of Poales and other commelinids, although sometimes in very small amounts. In Poales, at least, the gene families, CslF, is responsible for making these mixed-linkage glucans (Vogel 2008). In Poaceae the glucans are found in endosperm cell walls as a storage carbohydrate, but also in various vegetative tissues, both elongating cells and mature tissues (Vega-Sánchez et al. 2013; S.-J. Kim & Brandizzi 2021).
Ferulates in high concentrations in the primary cells walls are restricted to commelinids and to core Caryophyllales (P. J. Harris & Hartley 1980; Rudall & Caddick 1994; Harris & Trethewey 2009). Thus ferulic acid was detected in alkaline hydrolysis products in all palms examined except Sabal palmetto (Pearl et al. 1959), indeed, their wall composition is similar to that of eudicots and with relatively little in the way of xyloglucans, however, they do have some feruloylated glucuronoarabinoxylan (Carnachan & Harris 2000; Harris & Trethewy 2009). Ferulic acid is transferred to cell wall arabinoxylans via a sucrose derivative, 3,6-O-diferuloyl sucrose, at least in grasses (D. Yang et al. 2024). Ferulate polysaccharide esters can be incorporated into lignins (Ralph et al. 1995: see also Scheller & Ulvskov 2010; Busse-Wicher et al. 2016). In other angiosperms resinols, β-β-linked units produced by the dimerization of the monolignol sinapyl alcohol, can nucleate lignin chains, whereas here tricin, a flavone, and ferulate serve as nucleating agents, hence flavonolignins (Lan et al. 2015, 2016). For the synthesis of lignins from hydroxycinnamyl alcohols, which can act as the lignin monomers p-coumarate, ferulate and sinapate units, see Ralph (2009; also Seigler 1998). For a very useful comparative study on monocot lignins and their acylation, R1-C=O is the unit involved, see Karlen et al. (2018). For primary cell wall composition, see literature in P. J. Harris (2005); Arecaceae sampled are somewhat intermediate between the [Poales [Commelinales + Zingiberales]] clade and other monocots; Pearl (1959) and Karlen et al. (2017) discuss palm lignins.
The primary cell wall hemicellulose and pectin polysaccharides of grasses are very different from those of other seed plants, both in overall composition and in the particularities of the composition of the xyloglucans (O'Neill & York 2003), and the polysaccharides are less branched than those elsewhere - but overall sampling is very poor. Harrington et al. (2012) outline the molecular biology of lignification, noting that H (p-hydroxyphenyl) unit concentrations are low, yet in "dicotyledons" they are still lower, while those of p-hydroxycinnamic acids are high, especially in C4 grasses; ferulate esters seem to be initiation sites for lignin deposition in grasses, but not in "dicotyledons". In grasses, at least, ferulic acid is transferred to cell wall arabinoxylans via a sucrose derivative, 3,6-O-diferuloyl sucrose (D. Yang et al. 2024). Hatfield et al. (2009) discuss acylation of lignin in grasses, and Boerjan et al. (2003) note that grasses in particular have a variety of minor lignin monomer units; p-coumarate units are usually terminal pendant units in grasses, and there are also high levels of esterified coumaric acid in the cell walls (Ralph 2009; see also Petrik et al. 2014) - note that H-lignin, with p-coumaryl units, has been found in Juncaceae, for example, but not in Lilium (Gross 1981). Barros et al. (2016) discuss the synthesis of the distinctive syringyl-rich lignins of grasses in the PACMAD/BOP clade (Brachypodium was the particular grass studied), and found that the BdPTAL1 gene, involved in the first step of phenylpropanoid pathway and ultimately lignin synthesis, can deal with both phenylalanine (the PAL pathway) and tyrosine (the TAL pathway); it is unusual for tyrosine to be a lignin precursor. Tricin is a lignin monomer that is quite/very common in grasses, but, unusually, it is not produced by the monolignol biosynthetic pathway; it is also known from Cyperaceae, for instance, and a few core eudicots like Medicago, and from lycophytes (Lam et al. 2021). For lignins, see also Erickson et al. (1973b). For more on cell walls in grasses and their relatives, see Vogel (2008). There is further discussion on monocot cell walls, etc., under Acorales - but integration is much needed...
Plant-Animal Interactions. Larval food plants of Satyrinae (Nymphalidae) butterflies, "browns", are widely distributed in the commelinids (Ehrlich & Raven 1964; Peña & Wahlberg 2008). Basal clades in Satyrinae feed on BLAs, while larvae of other clades eat monocots, especially commelinids, and within commelinids, especially Poaceae; Amathusiini seem to be the only satyrines that use a variety of non-commelinid monocot hosts (Peña & Wahlberg 2008; see also Peña et al. 2011; Nylin et al. 2013). Unfortunately, the sister group of Satyrinae is unclear (Heikkilä et al. 2011).
Beetles of the Chrysomelidae-Hispinae+Cassidinae group are also common on commelinids (Jolivet 1988; Schmitt 1988; Vencl & Morton 1999; Chaboo 2007). 14/39 tribes of Hispinae+Cassidinae for which there is at least some larval host plant data are found on commelinids, 26/39 on some monocot or other (Chaboo 2007); the beetle larvae eat between the veins. Among the core eudicots larvae are mostly found on Boraginaceae, Solanaceae, Convolvulaceae and Asteraceae - asterids are particularly favoured. However, it is unclear if Criocerinae are in a clade immediately related to Donaciinae/Hispinae and related monocot-eating beetles, or not; the latter situation seems more likely (check Chaboo 2007; c.f. Wilf et al. 2000; Gómez-Zurita et al. 2007); Gómez-Zurita et al. (2007) found that hispines, including cassidines, were widely separated from Donaciinae, the latter being part of the [Criocerinae [Bruchinae + Donaciinae]] clade. Furthermore, feeding patterns in fossil material that look as if they were the result of the activities of hispines cannot easily be ascribed to their activities, as García-Robledo and Staines (2008) noted.
Sap-eating chinch bugs of the Hemiptera-Lygaeidae-Blissinae have been recorded from taxa throughout this clade (?Arecales), although they occur on Poales and especially Poaceae most frequently (Slater 1976).
Genes & Genomes. The τ (tau) duplication event is common to all commelinids examined, but it may be properly pegged to monocots as a whole (Jiao et al. 2014), or at least to the [Asparagales + commelinids] clade (Deng et al. 2015; McKain et al. 2016); it is not known from Alismatales (H. T. Lee et al. 2016; see also Gao et al. 2018; c.f. Olsen et al. 2016). Zwaenepoel and Van de Peer (2020: no Dasypogonaceae) suggested that the σ duplication event, often placed at the Poales node, would be better placed here.
The GC content of those commelinid genomes known, especially those of grasses, is quite high (Serres-Giardi et al. 2012).
The rate of molecular evolution in the plastome is high (with some notable exceptions) in this whole clade (Barrett et al. 2015).
Chemistry, Morphology, etc.. Endo et al. (2021) note that there are basipetal vascular bundles that end blindly at the base of the stem that are at least scattered in commelinids - something to do with the aquatic ancestry of monocots, perhaps, here is no need for water to be transmitted, but photosynthesates can move basally? Although vessels in both stem and leaves are common (Wagner 1977), this does not appear to be a synapomorphy. For distinctive sieve tubes, see Botha (2013). For the chemistry of the distinctive scallop-shaped epicuticular waxes that are scattered in this area of the monocots (Barthlott & Fröhlich 1983), see Meusel et al. (1994). For stomatal development, see Tomlinson (1974) and Rudall (2000); that in Dasypogonaceae is apparently unknown.
The perianth may be quite sharply differentiated into a calyx and corolla, or both whorls may be petal-like, but in either case the inner whorl usually completely surrounds the floral apex and the stamens are borne inside it (for floral development, see Endress 1995b in part). In those few commelinids where floral developmental genes have been studied in detail, expression of the B-class gene orthologue of PISTILLATA seems to be restricted to the stamens/staminode and petals (Adam et al. 2007); this may be connected with the more fully bicyclic nature of the perianth here. Starch-containing pollen is common, but has not been found in Hanguanaceae or Dasypogonaceae (only one species of the latter examined) and in some species of Haemodoraceae, Bromeliaceae, etc.. Broad embryos may be a synapomorphy at about this level.
Some morphological information about the commelinids is summarized by Givnish et al. (1999).
Phylogeny. The commelinid clade is well supported in molecular studies (e.g. S. W. Graham et al. 2006 and references; Barrett et al. 2015b) and it has morphological support as well, but support for relationships between the main groups within it has quite often been weak (Chase et al. 2000a; Soltis et al. 2007a). Overall, evidence has suggested that Arecaceae were sister to the rest of the clade (but see below), and the clade [Commelinales + Zingiberales] is more or less well supported (Chase et al. 2006; Givnish et al. 2006, 2010b; Soltis et al. 2007a), and, with Poales sister to this clade, also by Qiu et al. (2010: mitochondrial genes; Davis et al. 2013; S. Chen et al. 2013; Ruhfel et al. 2014: chloroplast genomes; Magallón et al. 2015; Hertweck et al. 2015; Barrett et al. 2015b). Hilu et al. (2003: matK) suggested that Poales might be sister to other commelinids, as did Soltis et al. (2011: Kingia, etc. not included), but in both cases support was weak, as was that for the position of Arecales as sister to remaining commelinids in the latter study. Arecales were weakly supported as sister to Poales in some analyses (e.g. S. W. Graham et al. 2006; Givnish et al. 2010b: maximum parsimony).
Support for the [Commelinales + Zingiberales] clade was sometimes rather weak (e.g. Chase et al. 2000a; Davis et al. 2004: Givnish et al. 2006b, one gene), although it has 100% support in the multi-gene analysis of S. W. Graham et al. (2006) and Chase et al. (2006), good support in the multigene (but poor sampling) study of Soltis et al. (2011) and the plastome analysis of Barrett et al. (2015a, b), and it also appears in Hertweck et al. (2015). In some individual analyses in Davis et al. (2004) Commelinales were paraphyletic and included Zingiberales, while Lam et al. (2015) found a moderately supported [Zingiberales + Poales] clade. Overall, however, this [Commelinales + Zingiberales] has been rather consistently recovered (see also H.-T. Li et al. 2021; Zuntini et al. 2024).
The position of Dasypogonaceae has been unclear. Neyland (2002b: 26s rDNA) found Dasypogonaceae to be strongly associated with Restionaceae and other families (Poales), but this particular relationship has not been recovered in other studies. Some multi-gene analyses did not link Dasypogonaceae with Arecales, even if where they ended up had no strong support (see Givnish et al. 2006 and Chase et al. 2006: near Poales; S. W. Graham et al. 2006: near [Commelinales + Zingiberales]; Magallón et al. 2015 and Foster et al. 2016a: sister to all other commelinids). Givnish et al. (2011b, 2016b) and Davis et al. (2011: structural mutations; see also Barrett & Davis 2011; Barrett et al. 2012a, esp. b, 2013, 2015a, esp. b; Ruhfel et al. 2014; Lam et al. 2015) have found some support in various analyses of plastomes for a position of Dasypogonaceae as sister to Arecaceae, consistent with what morphological data there is, although in some analyses they were placed sister to [Commelinales + Zingiberales], if with little support. In a tree based on the work of Givnish et al., Carlquist (2012a) depicted the relationships [[Arecales [Commelinales + Zingiberales]] [Dasyopogonales + Poales]]. Rudall and Conran (2012) were inclined to think Dasypogonaceae might be included in Poales, partly because both have epidermal silica bodies; within Poales Dasypogonaceae might be close to Rapateaceae in particular. Indeed, alternative topologies remained possible in some analyses in Barrett et al. (2013), and these included completely novel relationships. Overall, however, analyses of full plastomes suggested the relationships [[Dasypogonaceae + Arecaceae] [Poales [Commelinales + Zingiberales]]] (e.g. Barrett et al. 2012a, esp. b, 2013, 2015b; Givnish et al. 2018b; H.-T. Li et al. 2021), and also in some nuclear analyses (Timilsena et al. 2022a). These relationships were followed here until vii.2024.
That being said, W. J. Baker et al. (2021a: Fig. S6: first version of the Seed Plant Tree) found that Pontederia sp. was sister to all other commelinids, Poaceae were sister to the remainder, and [Dasypogonaceae [Arecaceae ...]] continued the relationships. In the second version (i.2022) there was a very weakly supported clade [[Zingiberales + Commelinales], well, more or less, and [Dasypogonaceae + Arecaceae]]; in the v.2023 version Poales remained sister to all other commelinids where relationships were [[Zingiberales + Commelinales] [Dasypogonaceae + Arecaceae]], this topology had strong support; in the ix.2024 version (see Seed Plant Tree) things were much the same, although in Poales Kamphochloa (Poaceae-Chloridoideae!) are sister to the whole of the rest of the order. Relationships suggested by Y.-L. Qiu et al. (2024) are [Arecales [Poales [Dasypogonales [Zingiberales + Commelinales]]]]. However, in the recent Angiosperms353 probe set analysis by Zuntini et al. (2024) the relationships [Poales [Dasypogonales [Arecales [Zingiberales + Commelinales]]]] were recovered, although the position of Dasypogonales had rather less support. These relationships are recognized here.
For other discussions of relationships within monocots, see the Poales, Petrosaviales and Acorales pages.
Classification. Givnish et al. (1999) erected a classification of four superorders and 10 orders for the commelinids based on a rbcL phylogeny. A.P.G. III (2009) recognised four orders; these were well supported clades with stable contents, Dasypogonaceae being the only family of uncertain position. Given the predominance of evidence that suggested a [Dasypogonaceae + Arecaceae] clade, an expanded Arecales seemed reasonable (see A.P.G. IV 2016), although H.-T. Li et al. (2021) obtained only weak support for this clade and suggest that the two should be separated as they are here, following more recent work. Using a rather different line of argument, Givnish et al. (2018b) argued for two orders, suggesting inter alia that the Dasypogonaceae-Arecaceae split was older than that of any other two monocot families or orders, although taking ca 125 Ma as a cutoff would surely necessitate another half dozen or so orders... Some worms are best left in cans.
Previous Relationships. Engler (1892) recognised a group, Farinosae, distinguished by its mealy (versus fleshy or oily) endosperm, which included many of the taxa in Poales and Commelinales. Engler thought that Farinosae were close to his Liliflorae, perhaps partly because he included Juncaceae (Poales here) in the latter. Hamann (esp. 1961, 1962c) provided a comprehensive evaluation of the variation pattern of the taxa included in the Farinosae.
Mycorrhizae absent; vessel elements in roots often with simple perforation plates, vessels also in stem and leaf, also with simple perforation plates; SiO2 epidermal; cuticular waxes not as laterally aggregated rodlets; raphides 0; inflorescence indeterminate; style well developed, stigmas small, dry; micropyle bistomal, both integuments ca 2 cells across; tegmen with thick cuticle, tanniniferous [esp. endotegmen]; embryo size?; whole nuclear genome duplication [σ/sigma event], 2 C genome (0.6-)1.02(-1.73) pg; mitochondrial sdh3 [succinate dehydrogenase 3] gene lost; primary root degenerates early, cotyledon phanomer, haustorial [?level]. - 14 families, 997 genera, 18,875 species.
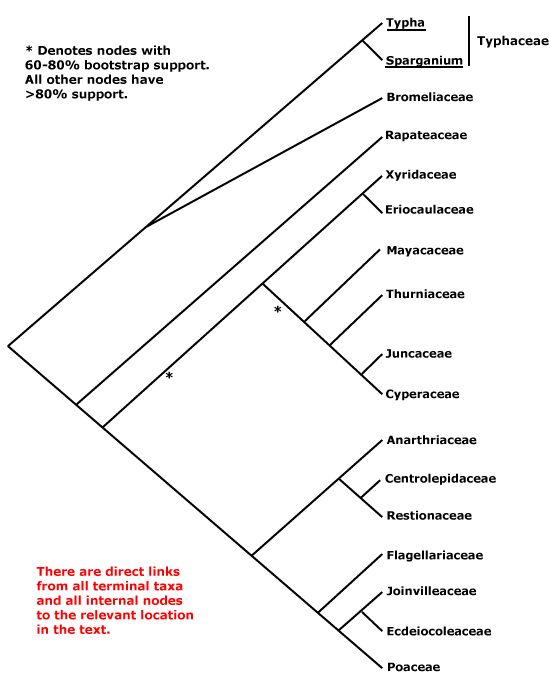
Includes Bromeliaceae, Cyperaceae, Ecdeiocoleaceae, Eriocaulaceae, Flagellariaceae, Joinvilleaceae, Juncaceae, Mayacaceae, Poaceae, Rapateaceae, Restionaceae, Thurniaceae, Typhaceae, Xyridaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Ages for crown-group Poales are notably variable. Leebens-Mack et al. (2005) suggested an age of 109-106 Ma, Magallón and Castillo (2009) ages of ca 109 and 99.2 Ma, Bell et al. (2010) ages of (103-)93, 85(-73) Ma, Wikström et al. (2001) an age of 72-69 My; estimates are ca 120 Ma in Givnish et al. 2018b), (124-)117(-108) Ma in Givnish et al. (2016b), (116-)106(-88) Ma in Merckx et al. (2008a), (115.3-)104.8(-93.6) Ma in Eguchi and Tamura (2016), ca 101 Ma in Magallón et al. (2015), ca 83 Ma in Janssen and Bremer (2004: c.f. topology), only ca 52.3 or 51.6 Ma in Xue et al. (2012) but 98-78 Ma and 104-82 Ma in Mennes et al. (2013, 2015 respectively), while ages in Hertweck et al. (2015) are (122-)113(-109) or (105-)99(-94) Ma, in D.-F. Xie et al. (2020) they are (69.3-)54.2(-34.6) Ma, in Can et al. (2020: Brom Cyp) they are ca 114.3 Ma, while 121-107 Ma is a similar estimate (Jiao & Wang 2022).
However, there are problems. Poinar (2004, 2011) proposed that Programinis laminatus, found fossil in deposits from the Early Cretaceous of Myanmar some 110-100 Ma, can be placed in Poaceae-Poöideae, i.e. a clade rather highly embedded in Poaceae. More recently Poinar et al. (2015) described a spikelet assigned to Poaceae (parts spirally arranged) that was infected with a Claviceps-like fungus, and they noted “With the discovery of a Claviceps-like fossil infecting a floret of an Early–mid Cretaceous Asian grass, we propose that the progenitor of Claviceps evolved in Asia among early grasses sometime in the mid- to Late Jurassic.” (ibid., p. 17). Similarly, if the identity of the putative stem Bromeliaceae Protoananas lucenae, 114-112 Ma old and from Brazil (Leme & Brown 2011), is confirmed (Kessous et al. 2021 were not sure), once again older dates for the clade are suggested.
Evolution: Divergence & Distribution. Magallón and Castillo (2009) suggested that Poales, which include about 1/3 of all monocots, have the highest diversification rates in the monocots and about the same as in Asparagales, but in both the rate is only little over half that of Lamiales.
Linder and Rudall (2005) provide a detailed discussion of morphological evolution and diversification in Poales. Subsequently, Bouchenak-Khelladi et al. (2014: tree rather distinctive) looked at correlations of various ecological parameters and diversification; they thought that much diversification in major lineages was Late Cretaceous, even if the origins of those clades was substantially earlier, while Bromeliaceae were a particularly good example of a much more recent radiation with an extremely long phylogenetic fuse, i.e. with a very old stem group. In Linder et al. (2017b: Fig. 5) largely ecophysiological features are displayed on a tree of the order. Males (2016) suggested that a propensity (sic) for aerenchyma formation was plesiomorphic in Poales.
Ecology & Physiology. Cornwell et al. (2008) found that litter decomposition of forbs was faster than that of graminoids - presumably mostly grasses and sedges, indeed, litter decomposition of monocots (sic) was slower that that of other angiosperms; in fact, the main difference is core eudicots (higher on average) versus other angiosperms + gymnosperms (LeRoy et al. 2019). Bamboo wood is particularly slow to decompose (G. Liu et al. 2015). Such differences affect the rate of nutrient cycling in the environment.
Recently it has been emphasized that within commelinid monocots, and especially Poales, some sieve tubes lack companion cells, are notably thick-walled, and are close to the tracheary elements; they seem to be involved in short distance transport of not very concentrated sugars. Interestingly, aphids do not probe such cells, rather, they prefer the thinner walled "normal" sieve tubes which have more concentrated sugars (Botha 2013). The distribution of these distinctive sieve tubes is unclear.
Pollination Biology & Seed Dispersal. For the repeated evolution of wind pollination in Poales, see Givnish et al. (2010a, b).
Plant-Animal Interactions. Host-plants of the ca 165 species of reed beetles, Chrysomelidae-Donaciinae, noted for the larvae being able to live under water, are scattered in Poales in particular, especially in Typhaceae, Juncaceae and Cyperaceae (Kölsch & Pedersen 2008: much discussion on the age and evolution of the group). They are mono- or oligophagous and show close co-cladogenesis with endosymbiotic bacteria (γ proteobacteria-Enterobacteriaceae, near Burkholderia) which produce the material that makes up the cocoon that characterises this beetle clade. The bacteria live in blind sacs in the foregut of the larvae and in the Malpighian tubules of the female adult in particular; transmission is vertical and external (Kleinschmidt & Kölsch 2011).
Plant-Bacterial/Fungal Associations. Mycorrhizae are absent in many Poales (Brundrett 2017b), although Poaceae commonly have arbuscular mycorrhizae.
Genes & Genomes. The σ/sigma nuclear genome duplication event is placed at this node (Ming et al. 2015; McKain et al. 2016a; H. Wang et al. 2024), however, Zwaenepoel and Van de Peer (2020) place this event at the commelinid node... Although WGDs are common in Poales (Wang et al. 2024), given the sampling of this study, only one other WGD, at the level of Poaceae, was linked to clades of any size. Ca 90.2 Ma is the age of the ORSAβ event (Landis et al. 2018: no Typhaceae). Ming et al. (2015), working on pineapple, think that the sequence of changes in chromosome numbers was x = 7 → x = 14 (tetraploidy: τ/tau genome duplication event]) → x = 12 → x = 24 (tetraploidy: σ/sigma genome duplication event) → x = 16 ("extant chromosomes"), although they also mention a sequence x = 7 × 2 × 2 = 28, i.e. no chromosomal rearrangements. On the other hand, the proposed sequence in Murat et al. (2017) is x = 5 → x = 10 (tetraploidy: τ/tau genome duplication event) → x = 9 → x = 27 (hexaploidy: σ/sigma genome duplication event). For these events, see also Qiao et al. (2019).
Puttick et al. (2015) estimate the genome size of Poales - it is small.
Lam et al. (2016) found very long branches for Cyperus and Poaceae (somewhat shorter in Flagellaria, short/ordinary in Typhaceae and Bromeliaceae) that they examined in a three-gene analysis (plastome), and these branch lengths were similar to those of some holomycoheterotrophic taxa.
For the general pattern of movement of genes from the mitochondrion to the nucleus, see Adams and Palmer (2003). J. J. Doyle et al. (1991) discussed chloroplast inversions. Ong and Palmer (2006) examined the rps14 nuclear gene/mitochondrial pseudogene system.
Chemistry, Morphology, etc.. Eriocaulaceae, Poaceae, Cyperaceae and Juncaceae at least have lateral roots originating opposite the phloem of the vascular tissue, in Restionaceae and Bromeliaceae they originate opposite the xylem (ref.?). Note that at least some members of the Poaceae and Cyperaceae groups have distinctive cellulose orientation in the outer epidermal walls of their roots, but that some Typhaceae and Bromeliaceae do not (Kerstens & Verbelen 2002); one wonders what improved sampling will show.
For details of variation in gynoecium initiation/development, see Oriani and Scatena (2019); de Oliveira et al. (2020) summarize obturator variation in Poales. Von Guttenberg (1960) drew attention to embryological similarities between Typhaceae, Bromeliaceae, Cyperaceae and Juncaceae.
See Prychid et al. (2004) for SiO2 bodies (phytoliths), Tiemann (1985) for embryology, L. R. de O. Silva and Oriani (2022) for seed and seedling, de Carvalho et al. (2023: seed anatomy) and Tillich (2007) and Silva et al. (2023) for seedling morphology and evolution.
Phylogeny. Poales do not always have very strong support, but c.f. e.g. Givnish et al. (2010b) and Barrett et al. (2012b). The topology of the tree in early versions of this site was based on the work of K. Bremer (2002) in particular, and also that of Harborne et al. (2000), but Janssen and Bremer (2004) suggested a rather different set of relationships, albeit some groups had little support, and relationships in Givnish et al. (2010b) were somewhat different again. It will become clear from the discussion below that the topology of the tree above may be incorrect in detail; the topologies of nuclear and plastid phylogenies tend to differ in detail (McKain et al. 2016a). However, nuclear phylogenies seem to be clarifying relationships.
The general pattern of movement of genes from the mitochondrion to the nucleus suggests that Bromeliaceae and Typhaceae (of the taxa sampled) were sister to other Poales (Adams & Palmer 2003), and of course Bromeliaceae, along with the near-basal Rapateaceae, alone have septal nectaries in this clade. A three-nucleotide deletion in the atpA gene was found to characterise Typhaceae and Bromeliaceae (J. I. Davis et al. 2004), although there was little bootstrap support for this group (but see also Givnish et al. 2005, 2007; c.f. Givnish et al. 2006b). Typhaceae were placed sister to Bromeliaceae with weak jacknife support but strong Bayesian posterior probabilities (Bremer 2002; see also Eguchi & Tamura 2016). Other work also suggests that Typhaceae and Bromeliaceae form a clade sister to other Poales, and Rapateaceae are in turn consistently sister to the remainder (Givnish et al. 2005; Chase et al. 2006; also Rudall & Linder 2005; Givnish et al. 2008a: ?rooting; Graham et al. 2006: see Rapateaceae; Soltis et al. 2011: strong support, but sampling; Ruhfel et al. 2014; Bouchenak-Khelladi et al. 2014b), although these relationships are not always obtained (Givnish et al. 2010a). Support for this family pair was also quite strong in the Seed Plant Tree of Life i.2022 version and the position of Rapateaceae was strongly supported. However, Bromeliaceae and Typhaceae are also often placed as successive basal branches with respect to other Poales (Givnish et al. 2005, 2008a, 2010b: quite strong support, 2016b; also Graham et al. 2006; H. Wu et al. 2022: some analyses), while Typhaceae are sister to all other Poales in the phylogenomic analyses of McKain et al. (2016a) and Timilsena et al. (2022a: only moderate support). Other than these two families, Rapateaceae appear to be sister to remaining Poales in many analyses (e.g. J. I. Davis et al. 2004; Timilsena et al. 2022), albeit sometimes with little support (see also Barrett & Davis 2011; Barrett et al. 2013; J. I. Davis et al. 2013).
Away from the base of the tree, in the complete plastome analysis of Ruhfel et al. (2014), for example, a clade [[Eriocaulaceae + Mayacaceae] [Xyridaceae + Poaceae, Restionaceae, etc.]] was obtained - only a single species of each of the first three families was included - while Bouchenak-Khelladi et al. (2014b) recovered the clades [[Rapateaceae + Mayacaeae] [Thurniaceae [Juncaceae + Cyperaceae]]] and [[Eriocaulaceae + Xyridaceae] [Restionaceae et al. + Poaceae et al.]]. There is support for these groups forming a larger clade (e.g. Givnish et al. 2005, 2010b; Chase et al. 2006), perhaps compatible with the distribution of deletions in the chloroplast inverted repeat ORF 2280 region and absence of a full accD gene (Hahn et al. 1995; Katayama & Ogihara 1996).
Thinking of Xyridales of Kubitzki (1998c), which included Mayacaceae, Xyridaceae, Eriocaulaceae and Rapateaceae, there was some evidence for a group made up of the first three families alone, and perhaps, but not very probably, also including Rapateaceae (see above). Bremer (2002) noted that Mayacaceae (and Hydatellaceae - see below) might be weakly associated with Xyridaceae or Eriocaulaceae, depending on what taxa were included in the analysis, but there were a number of long branches in this area and he excluded the first two families from his final analysis (Chase et al. 2006 also found the position of Hydatellaceae to be problematic; for the association of Mayacaceae with Eriocaulaceae and Xyridaceae, see also Campbell et al. 2001). J. I. Davis et al. (2004) found a more complex set of relationships, although with very little support. Members of this group of families were in adjacent branches along the spine of the tree, with one including Flagellariaceae, the Juncaceae group, some Xyridaceae, Mayacaceae, and perhaps Hydatellaceae. Both Xyridaceae and Mayacaceae have more or less clawed petals and anthers with an exothecium. Finally, as mentioned some Mayacaceae have been linked with Rapateaceae (e.g. Bouchenak-Khelladi et al. 2014b), and both have poricidal anthers. The three members of the old Xyridales that remain here may form a grade as follows: [Mayacaceae [[Xyridaceae + Eriocaulaceae] [Thurniaceae [Juncaceae etc.]]]] (Givnish et al. 2006b; Chase et al. 2006); the topology of the tree in Graham et al. (2006) although with poor sampling, is consistent with such relationships. Although there are several distinctive characters in this group of families, relationships remain unclear. Givnish et al. (2010b) found that in maximum likelihood analyses the general relationships were [[Juncaceae, etc.] [[Xyridaceae etc.] [Abolboda [Poaceae, etc.]]]] and later (Givnish et al. 2018b) a variant of this [[Juncaceae, etc.] [Mayacaceae [[Xyridaceae + Eriocaulaceae] [[Poaceae, etc.]]]]. Quite similar relationships were found by Barrett and Davis (2011), while J. I. Davis et al. (2013) recovered the grouping [Eriocaulaceae/Mayacaceae [Xyridaceae + Restionaceae/Poaceae area]], but there Abolboda stayed with other Xyridaceae. Givnish et al. (2010b) found that Abolboda, the only member of Xyridaceae examined, did not link with the Eriocaulaceae-Mayacaceae clade in maximum likelihood analyses, while in maximum parsimony analyses there was some support for a clade [Juncaceae etc. + Xyridaceae etc.] (the latter grouping was also recovered by Davis et al. 2013).
Poaceae and their immediate relatives consistently form a clade, although some details of relationships within it are unclear. Note that in versions 6 and earlier of this site, Eriocaulaceae and their relatives were tentatively associated with the Poaceae group, and analyses of the nuclear PhyB gene recovered a clade [Poaceae [Mayacaceae [Typhaceae + Eriocaulaceae]]] (Hochbach et al. 2018).
Part of the problem with the early analyses of Poales may have been caused by the inclusion of Hydatellaceae. However, Saarela et al. (2006, esp. 2007) showed that Hydatellaceae were completely misplaced in the monocots, rather belonging to Nymphaeales where they are sister to other members of that clade, and this position has very strong molecular and morphological support.
There is indeed still some way to go. Bouchenak-Khelladi et al. (2014b: rbcL, ndhF) recovered the relationships, not all well supported, [[Typhaceae + Bromeliaceae] [[[Eriocaulaceae + Xyridaceae] [Restionaceae etc.]] [Mayacaceae [Rapateaceae [Thurniaceae etc.]]]]]. There was weak support for an [Eriocaulaceae + Xyridaceae] clade in the phylogenomic analysis of McKain et al. (2016a). A more recent comparison of relationships suggested by two nuclear genes and the plastid workhorse, matK, showed several differences, although many were weakly supported. For example, Mayacaceae, Xyridaceae, and Eriocaulaceae were not immediately related in trees that used the two nuclear markers while the position of the old Centrolepidaceae within Restionaceae varied according to the analysis (Hochbach et al. 2018). Darshetkar et al. (2019: plastomes) recovered the relationships [Typhaceae [Bromeliaceae [Rapateaceae [[Thurniaceae, etc.] [[Mayacaceae + Eriocaulaceae] [Xyridaceae [Restionaceae etc.]]]]]]], but H.-T. Li et al. (2021: * = nodes with weak support), in a very much more comprehensive study, found the relationships [Bromeliaceae *[Typhaceae [Rapateaceae [[Thurniaceae, etc.] *[Mayacaceae [*[Eriocaulaceae + Xyridaceae] [Restionaceae etc.]]]]]]]. H. Wu et al. (2022: 69-91 taxa, plastome and chondrome data) in their organelle analyses found about equal support for the three topologies at the base of the poalean tree involving Bromeliaceae and Typhaceae. As Wu et al. (2022) concluded in their analyses of conflict in the Poalean tree, "Many factors, such as the missing data of mitochondrion, insufficient nuclear sampling, rapid radiation, heterogeneity of molecular evolution rate, and allopolyploidy by hybridization are potentially involved in generating these conflicts in the Poales." Hmmm. Many relationships around here are in a state of flux, nodes may have little support, and one can only wait for analyses of nuclear genomes - although of course the water may be muddied still further.
The first Angiosperms353 analysis (see W. J. Baker et al. 2021a) unfortunately had poor sampling in critical areas - no Eriocaulaceae or Rapateaceae - but other relationships were [[Typhaceae + Bromeliaceae] *[Mayacaceae [[Restionaceae including Xyris] [Flagellariaceae, etc.]] [Thurniaceae, etc.]]]. However, the i.2022 data release shows the relationships ...[[Thurniaceae, etc.] [Mayacaceae [[Eriocaulaceae + Xyridaceae] [Restionaceae etc.]]]] (see the Seed Plant Tree), although the [Eriocaulaceae + Xyridaceae] clade had little support (most other relationships were fairly conventional, and Typhaceae were sister to Bromeliaceae with quite strong support). A similar topology [...[[Juncaceae, etc.] [Mayacaceae [Eriocaulaceae [Xyridaceae [Restionaceae etc.]]]]]] was often recovered by Wu et al. (2022), although they found the topology [...[[Eriocaulaceae + Mayacaceae] [[Juncaceae, etc.] [Xyridaceae [Restionaceae, etc.]]]]] in some of their analyses. As Wu et al. (2022) concluded: "Many factors, such as the missing data of mitochondrion, insufficient nuclear sampling, rapid radiation, heterogeneity of molecular evolution rate, and allopolyploidy by hybridization are potentially involved in generating these conflicts in the Poales." Hmmm. In the recent nuclear phylogenomic analyses of Timilsena et al. (2022a: sampling pretty minimal) relationships, all strongly supported, are [[Thurniaceae et al.] [Mayacaceae [[Xyridaceae + Eriocaulaceae] [etc.]]], while H. Wang et al.(2024: transcriptome analyses, 75 genera, all 14 families) found the relationships - some poorly supported - [[Typhaceae + Bromeliaceae] [Rapateaceae [[Thurniaceae et al.] [Mayacaceae [[Xyridaceae + Eriocaulaceae] [Restionaceae to Poaceae]]]]]]. Again, a variety of factors affected the analyses, but it does seem that the old Xyridales are turning out to be paraphyletic. Indeed, in the Seed Plant Tree version of ix.2024, apart from the fact that Kamphochloa (monotypic, Poaceae) was sister to the rest of the order, relationships were [[Typhaceae + Bromeliaceae] [[Thurniaceae [Juncaceae + Cyperaceae]] [Rapateaceae [Mayacaceae [[Eriocaulaceae + Xyridaceae] [Aratityopea (Xyridaceae) [Restionaceae, etc.]]]]]]]. Y.-L. Qiu et al. (2024) found that Stegolepis, the only member of Rapateaceae they studied, was sister to the other Poales. See also Zuntini et al. (2024) for relationships in Poales, largely similar to other recent analyses, they did find a ...[Mayacaceae [Eriocaulaceae [Xyridaceae [... grade...
Synonymy: Eriocaulineae Thorne & Reveal, Xyridineae Thorne & Reveal - Avenales Bromhead, Bromeliales Link, Centrolepidales Takhtajan, Cyperales Berchtold & J. Presl, Eriocaulales Nakai, ciliariales Reveal & Doweld, Juncales Berchtold & J. Presl, Mayacales Nakai, Rapateales Reveal & Doweld, Restionales Berchtold & J. Presl, Typhales Berchtold & J. Presl, Xyridales Lindley
[Typhaceae + Bromeliaceae]: stomatal subsidiary cells with oblique divisions; leaf without distinct sheath; endosperm helobial, cell wall formation in small chalazal chamber before that in large micropylar chamber; embro linear, differentiated; three-nucleotide deletion in the atpA gene.
Age. The divergence date of these two families is about 75.9 Ma (Magallón et al. 2015), about 85.5. Ma (Tank et al. 2015: Table S2), ca 98.6 Ma (B. Zhou et al. 2018), around 100 Ma (Givnish et al. 2011a: divergence of Bromeliaceae from rest of Poales; Sulman et al. 2013) or ca 96.7 Ma (Vera-Paz et al. 2024).
TYPHACEAE Jussieu, nom. cons. - Back to Poales
Marsh or aquatic plants; flavonoids +; SiO2 bodies 0; starch grains pteridophyte-type, amylophilic; leaves two-ranked; plant monoecious; inflorescences dense, carpelate units below the staminate; flowers very small [3> mm across], monosymmetric by reduction; P chaffy; nectary 0; staminate flowers: A 1-8; tapetum amoeboid, 8 nuclei/cell; pollen grains trinucleate, monoulcerate, intine thickening at the apertures [= oncus]; carpelate flowers: G 1 [?pseudomonomerous], style single, stigma rather elongated, on one side; ovule 1/carpel, pendulous, apotropous, nucellar cap ca 2 cells across, obturator +; antipodal cells multiply after fertilization [to 150 cells or so]; fruit indehiscent; seed coat ± obliterated; endosperm +, thin, perisperm +, thin; x = 15 (?16, ?14), nuclear genome [1 C] (0.012-)0.529(-23.75) pg; ORF 2280 deletion; seedling with phanomer, hypocotyl and collar hairs.
2[list]/ca 25. More or less world-wide.
Age. The two genera separated ca 89 Ma (Janssen & Bremer 2004), (76-)72(-70) Ma (Sulman et al. 2013), (87-)80.9(-74) Ma (Bouchenak-Khelladi et al. 2014b) or ca 71.3 Ma (B. Zhou et al. 2018).
For the rich fossil record of the family - although Cretaceous occurrences need re-evaluating - see S. Y. Smith et al. (2010) and Iles et al. (2015). Collinson and van Bergen (2004) found similar chemical signatures in fruits of extant and fossil representatives of both genera.
1. Sparganium L. —— Synonymy: Sparganiaceae Hanin, nom. cons.
Emergent (floating) aquatic; myricetin + [?here]; stomatal subsidiary cells with intersecting oblique divisions; inflorescence units globose heads, well separated; P 1-6, when 3, median member adaxial; staminate flowers: anthers extrorse-latrorse; pollen mixed with raphides; carpelate flowers: bracteoles 0; G (-3), stigma papillate; micropyle endostomal, parietal tissue ca 1 cell across; fruit a spongy drupe, with micropylar plug, P persistent; testa membranous; initially with large central lumen containing developing endosperm cells, antipodal cells multiplicative, to 150, perisperm with oil; phanomer + [unifacial, ± assimilating], hypophyll quite well developed.
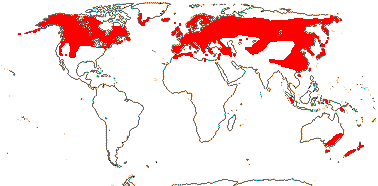
1/14. Temperate and Arctic, few in S. hemisphere, but to New Zealand. Map: see Hultén (1958, 1962), Meusel et al. (1965) and Hultén and Fries (1986).
Age. Crown-group Sparganium has been dated to (17-)13(-8.9) Ma (Sulman et al. 2013) and (36.7-)18(-5.8) Ma (B. Zhou et al. 2018).
2. Typha L.
Stems lacking vessels; (styloids/calcium oxalate crystals +); cuticular waxes as aggregated rodlets; (foliar fibre strands +); leaf with distinct sheath; inflorescence units densely spicate, staminate and carpelate units adjacent or not; flowers with whorls separated by internodes [check]; P 0; staminate flowers: A connate; (pollen in tetrads, tetrads acalymmate, cohesion simple); carpelate flowers: (bracteoles 0); pedicels with long hairs; G stipitate, stigma filiform/spathulate; fruit an achene with a small operculum; endotesta with stalactite-type thickenings; endosperm also with oil.
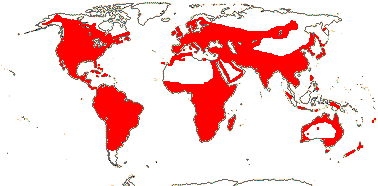
1/8-13. Temperate and tropical regions worldwide. Map: see Hultén (1962), Meusel et al. (1965), Hultén and Fries (1986), Flora Base (consulted 2005 - somewhat notional); the map in Knobloch and Mai (1986) differs very considerably from its source, Meusel et al. (1965). [Photos - Collection.]
Age. Crown-group Typha may be (10-)7.1(-4.1) Ma (Sulman et al. 2013: see sampling) or (57.6-)39(-22.6) Ma (B. Zhou et al. 2018).
Fossils of Typha are known from the Late Cretaceous onwards (B. Zhou et al. 2018 and references).
Evolution: Divergence & Distribution. Sulman et al. (2013) discussed the biogeography and evolution of Sparganium, not easy to work out partly because of the very long evolutionary "fuse" of the genus, perhaps 80 Ma or more.
Pollination Biology & Seed Dispersal. Sparganium is ambophilous, being pollinated both by insects (beetles, hoverflies) and by wind (Gottsberger 2020).
Typha has a sort of hyperextragynoecial compitum; the carpelate flowers are very close and are organized in groups of four, and in species where the pollen grains are in tetrads, pollen tubes from the four grains may reach the stigmas of four different flowers (Krattinger 1975; Nicholls & Cook 1986: Kubitzki 1998d: Fig. 113b, c; Carvalho & Mariath 2019).
Plant-Bacterial/Fungal Associations. Similar rusts are shared by the two genera (Savile 1979).
Genes & Genomes. The TYPHα genome duplication, which happened around 43 Ma, characterises the family (Landis et al. 2018).
Guisinger et al. (2010) looked at the plastid genome of Typha latifolia, comparing it with that of some grasses.
Chemistry, Morphology, etc.. Corrêa et al. (2023) looked at foliar development in Typha domingensis, finding that most leaf tissues developed basipetally (the intercalary meristem producing these tissues was near the base), except for the xylem and phloem, as is common in monocots. Witzum and Wayne (2016) discuss the unlignified foliar fibre cables of Typha that run parallel to the leaf surfaces and at right angles to the diaphragms that divide the air spaces.
Some flowers of Sparganium may have a second, empty loculus, or there may even be three fertile loculi (Dahlgren et al. 1985). Indeed, since fossil Sparganium may have up to 7-locular fruits (Cook & Nicholls 1986), it seems likely that the pseudomonomerous gynoecium in the two genera evolved independently and therefore should not be a family-level apomorphy.
Much information is taken from Kubitzki (1998d: general); see also D. Müller-Doblies (1970: inflorescence and flower), Dietz (1887: development of flower and fruit) and Grayum (1992) and Albert et al. (2011), both pollen - the two genera are palynologically almost identical. For general information on Typha, see Thieret and Luken (1996: southeast U.S.A.). As to Sparganium, see Cook and Nicholls (1986, 1987) for a monograph, Carlquist (2012a) for vessels, U. Müller-Doblies (1970) for flower and embryology, Campbell (1901) for ovules/developing seed and Tillich (1985: seedling of Sparganium).
Phylogeny. For phylogenetic relationships in Typha, see C. Kim and Choi (2011) and especially B. Zhou et al. (2018); the latter suggested that [T. elephantina + T. minima] were sister to the rest of the genus.
Classification. Sulman et al. (2013) provide an infrageneric classification for Sparganium.
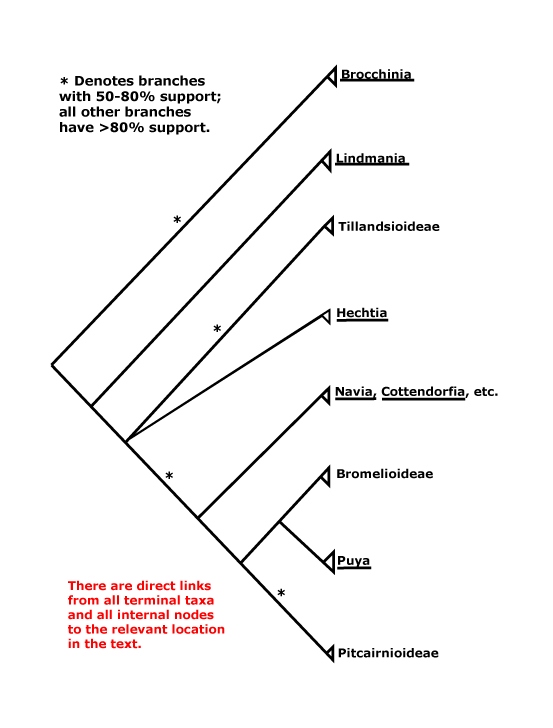
BROMELIACEAE Jussieu, nom. cons. - Back to Poales
Plants rosette-forming; (C-glycosylated/6-oxygenated) flavones, flavonols +; vessel elements with scalariform perforation plates; mucilage +; cuticular waxes as aggregated rodlets; water storage tissue in mesophyll, fibrous bundle sheaths + [?higher level]; indumentum lepidote; epidermal SiO2 bodies +; leaves spiral, blade vernation curved, thick, horny, base dilated; (A basally connate), (adnate to C); septal nectaries +, infralocular; style +, long, apically ± 3-branched, branches conduplicate-spiral, stigmas also wet; micropyle bistomal, parietal tissue 1-3 cells across, nucellar cap +, nucellar epidermal cells anticlinally elongated, chalazal appendage +, micropylar appendage + [appendages epidermal and subdermal]; fruit a septicidal capsule, K persistent; seeds caudate, testal-tegmic, testa 3-7 cells across, cells variously thickened and lignified, tegmen ca 2 cells across, (exotegmen thickened), endotegmen tanniniferous; embryo often lateral; hypocotyl and hypophyll common; x = 25, chromosomes 3> µm long, nuclear genome [1 C] (0.051-)0.561(-6.111) pg.
82 [list]/3,802 [see Gouda et al. 2023] - eight groups below. Throughout (sub)tropical America; 1 sp. W. tropical Africa. Photo - Flower.
Age. From estimates in Janssen and Bremer (2004) the age of crown-group Bromeliaceae must be in excess of 112-96 Ma. However, other estimates are much younger, thus Givnish et al. (2004a, 2008) suggest a crown age of a mere 24.9-18.4 Ma, Givnish et al. (2011a) an age of ca 19.1 Ma, Givnish et al. (2014a) an age of ca 22.7 Ma, Bouchenak-Khelladi et al. (2014b) an age of (32.3-)19.5(-17.4) Ma, while ca 20.7 Ma is the estimate in B. Zhou et al. (2018) and ca 28.7 Ma that in Vera-Paz et al. (2023). Möbus et al. (2021: T. 3) has stem and crown ages for various subfamilies.
Protoananas lucenae, from the Crato limestone of Brazil and some 114-112 Ma old, is considered to be a "putative ancestral stem-lineage of Bromeliaceae" (Leme & Brown 2011: p. 217) and in the discussion it is also referred to as if it were in a separate family, Protoananaceae. Its age is greater than some estimates of the age of the whole order. It appears to have an inferior ovary, presumably of independent origin from that found in other Bromeliaceae (but see below — superior ovaries in some Bromeliaceae are thought by some to be secondarily so). However, Kessous et al. (2021) were unsure of the identity of the plant, and the oldest fossil that they thought could be reliably placed in Bromeliaceae was Karatophyllum bromelioides, initially believed to be from the upper Eocene but reassigned to the Pleistocene...
1. Brocchinioideae Givnish - Brocchinia J. H. Schultes
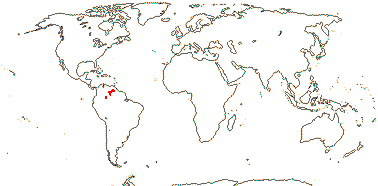
(Plant carnivorous), (tank epiphytes), (stem erect, with intracauline roots); leaves with stellate chlorenchyma; (leaf blade deciduous); (K ± = C); G ± inferior, septal nectary above the insertion of the ovules; ovules 2 (3+?)/carpel, parietal tissue?, nucellar cap ca 2 cells across; (seeds with basal tuft of hairs); n = ?9, 23.
1/20. South America, the Guyana Highlands. Map: from L. B. Smith and Downs (1974), see also Zizka et al. (2019: Fig. 2a).
Age. Divergence within Brocchinia may have begun some 14 Ma (Givnish et al. 2004a, 2008) or 14.3-13.1 Ma (Givnish et al. 2011a).
[Lindmanioideae [Tillandsioideae [Hechtioideae [Navioideae [Pitcairnioideae [Puyoideae + Bromelioideae]]]]]]: cap cells of trichomes dead; leaf margin serrate/with spines [?level, ?apomorphy]; septal nectaries below the insertion of the ovules; parietal tissue ca 1 cell across.
Map: from Givnish et al. (2004a).
Age. The age for this node was estimated to be between 112 Ma and 96 Ma (Janssen & Bremer 2004), ca 15.6 Ma is the age suggested by Givnish et al. (2011a) and ca 18.1 Ma that by Givnish et al. (2014b).
2. Lindmanioideae Givnish
Stellate chlorenchyma 0; leaf margin (entire); K contorted; stigmas straight; ?ovule number; cotyledonary hypophyll blade-like; n = ?
1-2/43: Lindmania (39). South America, the Guyana Highlands. Map: see Zizka et al. (2020: Fig. 3d).
Age. Crown-group Lindmanioideae can be dated to 16.3-8.9 Ma (Givnish et al. 2011a) or ca 5.8 Ma (Givnish et al. 2014a: ?sampling).
[Tillandsioideae [Hechtioideae [Navioideae [Pitcairnioideae [Puyoideae + Bromelioideae]]]]]: (inflorescence axis, bracts, floral bracts coloured); C (with paired subbasal ligules and/or longitudinal callosities); ovules 6-many/carpel, micropyle often endostomal, (placental obturator +, palisade tissue); x = 25.
Age. The age for this node has been estimated as ca 96 Ma (Janssen & Bremer 2004), 15.4-14 Ma (Givnish et al. 2011a), ca 16.9 Ma (Givnish et al. 2014a) and ca 16.6 Ma by Möbus et al. (2021).
3. Tillandsioideae Burnett —— Synonymy: Tillandsiaceae Wilbread
Air epiphytes, leaves succulent, roots often for attachment only (0), CAM photosynthesis +, or tank-forming, leaves thin, C3 photosynthesis +; (cauline intracortical roots +); scales radially symmetric; (flowers in inflorescence 2-ranked); K (abaxial extranuptial nectaries), C corona/ligule +/0; (filaments with 2 vascular bundles); (pollen with raphides); G (± inferior); (micropyle also bistomal), (outer integument to 5 cells across), (inner integument ca 3 cells across), parietal tissue (2-)3-4 cells across, (nucellar cap ca 2 cells across), hypostase +, ovules with long chalazal appendage (several, 0 - Guzmania); seeds with apical and/or basal appendages, ditto tufts of hair, hairs usu. cells of the outer integument longitudinally split, (multiple chalazal appendages - Catopsis); embryo short to long; (n = 16, 18, 20-22, 24 [quite common]), karyotype bimodal; seedling primary root none or soon aborting, cotyledonary sheath medium, hypocotyl poorly developed.
22/1,557: Racinaea (79). Almost the range of the family in America (southern U.S.A. to Argentina). Map: see Zizka et al. (2019: Fig. 4b). [Photo - Flower.]
Age. Divergence within Tillandsioideae may have begun some 14.2-11.8 Ma (Givnish et al. 2011a), ca 15.2 Ma (Givnish et al. 2014a), ca 13.0 Ma (Loiseau et al. 2020) or (19.3-0)17.7(13.7) Ma (Vera-Paz et al. 2023).
[Catopsideae + Glomeropitcairnieae]: ?
3A. Catopsideae Harms - Catopsis Grisebach
(Plant ?carnivorous - C. berteroniana
1/17. Mexico to Brazil, Florida, West Indies.
Age. The age of crown-group Catopsideae is (8.7-)5.4(-2.7) Ma (Vera-Paz et al. 2023).
Werauhia (92).3B. Glomeropitcairnieae Harms - Glomeropitcairnia Mez
?
1/2. Lesser Antilles and northeastern Venezuela.
[Tillandsieae + Vrieseeae]: petal appendages +, ± basal, (0).
Age. This clade is estimated to be (15.0-)11.4(-7.7) Ma (Vera-Paz et al. 2023).
3C. Tillandsieae Dumortier
seed coma double/single umbrella; (endosperm ± 0; embryo well developed - T. recurvata).
Tillandsia (794), Guzmania (220).
Age. Crown-group Tillandsieae are around (12.0-)8.8(-5.9) Ma (Vera-Paz et al. 2023).
3D. Vrieseeae W. Till & Barfuss
Vriesea (2i4), Alcantarea (47) - 2 subtribes. Largely Brazilian Atlantic Forest.
Age. The age of crown-group Vrieseeae is ca 7.7 Ma (Loiseau et al. 2020) or (9.0-)6.3(-3.8) Ma (Vera-Paz et al. 2023).
[Hechtioideae [Navioideae [Pitcairnioideae [Puyoideae + Bromelioideae]]]]: seeds not caudate.
Age. This node can be dated to 15.2-14 Ma (Givnish et al. 2011a), ca 16.3 Ma (Givnish et al. 2014a) or ca 14.2 Ma (Möbus et al. 2021).
4. Hechtioideae Givnish - Hechtia Klotzsch
Plant terrestrial, (monopodial), xeromorphic; stellate sclerenchyma 0; CAM photosynthesis +; hypodermal sclerenchyma +, central water storage tissue +, chlorenchyma undifferentiated; trichomes in parallel rows; leaf margin (entire); plants dioecious; flowers (sessile); (G ± inferior), style short, stigmas erect/recurved; seeds fusiform, wings terminal, small/not; cotyledonary hypophyll blade-like.
1/78. Extreme southwest Texas, Mexico (most) to northern Nicaragua. Map: see Ramírez-Morillo et al. (2018) and Zizka et al. (2020: Fig. 3C).
Age. The age of crown-group Hechtioideae is 12.1-10.3 Ma (Givnish et al. 2011) or ca 9.9 Ma (Givnish et al. 2014a).
[Navioideae [Pitcairnioideae [Puyoideae + Bromelioideae]]]: ?
Age. This node is some 12.1-10.3 Ma (Givnish et al. 2011a) or ca 15.9 Ma (Givnish et al. 2014a).
5. Navioideae Harms
Plant xeromorphic; peripheral water storage tissue +, stellate chlorenchyma 0; leaf margin often entire; C minute; seeds circumferentially winged or not.
5/107: Navia (94). Guyana Highlands, N.E. Brazil. Map: see Zizka et al. (2020: Fig. 4A).
Age. Crown-group Navioideae are 10.4-9.4 Ma (Givnish et al. 2011a) or ca 10.5 Ma (Givnish et al. 2014a).
[Pitcairnioideae [Puyoideae + Bromelioideae]]: CAM photosynthesis common (0).
Age. This node has been dated to ca 15, 13.4-13.3, or 14.1 Ma (Givnish et al. 2004a, 2011a, 2014a respectively) and ca 12.3 Ma (Möbus et al. 2021).
6. Pitcairnioideae Harms
(Cauline intracortical roots +); scales ± divided, or hairs stellate; leaves (pseudopetiolate), (margins entire); (flowers monosymmetric); K (abaxial extranuptial nectaries quite common), (C with basal ligule/s); filaments with 2-3 vascular bundles; G to inferior, style (also with 2 lateral vascular bundles), stigma (spiral-conduplicate); ovules (outer integument ca 5 cells across - Dyckia), (parietal tissue several cell layers across); antipodal cells multiply after fertilization [Dyckia], (micropylar appendage 0); seeds tailed, body cells differing from tails, (winged), (appendages 0); embryo lateral or not; (n = 24), (karyotype bimodal), 2C genome size (0.6-)1.21, 1.24, 1.29(-2.9) p.g.; hypocotyl quite long, cotyledonary hypophyll blade-like, primary root well developed, (collar rhizoids - Pitcairnia).
5/642: Pitcairnia (409), Dyckia (175), Forsterella (34). Mexico to Chile, Pitcairnia feliciana W. Africa. Map: see Neves et al. (2020).
Age. Crown-group Pitcairnioideae are around 11.8-9.4 Ma (Givnish et al. 2011a) or ca 11.9 Ma (Givnish et al. 2014a).
[Puyoideae + Bromelioideae]: seed micropylar appendage 0.
Age. This node is around 10 Ma (Givnish et al. 2011a), ca 10.7 Ma (Givnish et al. 2014a) or (15.6-)11.9(-8.2) Ma (Silvestro et al. 2013).
7. Puyoideae Givnish - Puya Molina
Plant rather xeromorphic; hypodermal sclerenchyma +, internal water storage tissue +, chlorenchyma undifferentiated; trichomes in parallel rows, foliar trichomes ± well developed scales; flowers monosymmetric, K contorted, C clawed, in a tight spiral after anthesis; parietal tissue several cell layers across [?all]; dead C persistent in fruit; seeds circumferentially winged; (n = 24); hypocotyl quite long, cotyledonary hypophyll blade-like, primary root well developed.
1/225. Especially mountains, Costa Rica and Guyana to Chile and Argentina. Map: see Dykia et al. (2020: Fig. 4C). [Photos - Puya Flower, Puya Habit, Puya Habit.]
Age. Divergence within Puyoideae may have begun some 10-8.7 Ma or ca 9.4 Ma (Givnish et al. 2011a and 2014a respectively).
8. Bromelioideae Burnett
Tanks forming at bases of leaves, with roots, or central tank epiphytes, roots often for attachment only; leaves succulent; scales irregularly peltate, (colleters +); leaf (petiolate), (blade margin entire); (plant andromonoecious - Cryptanthus subg. Cry.); inflorescence branched or not, (axis barely developed, flowers initiate under water in tank - nidularoids); (flower dorsiventrally compressed); (perianth tube/hypanthium +), K (asymmetric), (abaxial extranuptial nectaries +), C contorted [all?], (with adaxial subbasal appendages); A (elongated cells between sporangia [= interlocular zone]), (connective raised between thecae) (neither P T), (filaments with 2 or so vascular bundles); pollen (2-polyporate/inaperturate), (with raphides); septal nectaries interlocular; G inferior, placentation (apical-)axile, stigma usu. spiral conduplicate; ovules (10/loculus), (micropyle bistomal), (outer integument to 4 cells across), (nucellar epidermal cells not elongated anticlinally), nucellar cap ca 2 cells across (0), parietal tissue 1-4 cells across, obturator secretory; fruit baccate; chalazal appendages 0-long, sarcotesta [gelatinous] + [core bromelioids], or seed ridged, tegmic-endotestal, endotesta secondarily thickened, also testa ± thin-walled, exotegmen thick-walled, (thickenings U-shaped), endotegmen with phenolics, thick- or thin-walled; embryo lateral, medium to long; (n = 16, 17 [quite common], 21, 22); (cotyledon not photosynthetic), short hypocotyl present; seedling primary root moderately developed, collar rhizoids +, cotyledonary sheath short, hypocotyl poorly developed to none.
41/995: Aechmea (244), Neoregelia (123), Bromelia (70), Billbergia (63), Orthophytum (67), Cryptanthus (66), Hohenbergia (49), Nidularium (47). Mexico and the West Indies to Chile and Argentina, esp. Brazil, the Atlantic Forest. Map: see Zizka et al. (2020: Fig. 3b). Photo: Flower, Fruit, Flower, Flower.
Age. Crown-group Bromelioideae are estimated to be 9.5-8.9 Ma (Givnish et al. 2011a), ca 9.4 Ma (Givnish et al. 2014a) or (14.6-)10.9(-7.6) Ma (Silvestro et al. 2013).
Evolution: Divergence & Distribution. Givnish et al. (2011a, b) suggested that there was ca 80 million years between the origins of stem and crown Bromeliaceae (stem - sister to all other Poales - ca 100 Ma, crown ca 19 My), a very long evolutionary fuse, although there must of course have been at least some extinction during this period. Furthermore, where proto-Bromeliaceae were hanging out during this time is unclear, although Givnish et al. (2004a, 2008a) thought that Bromeliaceae had radiated from an ancestral home on the Guyana Shield (see also Givnish et al. 1997, 2011a: much detail, b). Given the size of the family and its recent diversification, establishing a phylogenetic framework to think about diversification has been tricky. Thus within clades like Bromelioideae there may have been a combination of incomplete lineage sorting and some hybridization - radiation has been rapid radiation, and there are about 1,000 species here, yet the clade is a mere ca 10 Ma (Givnish et al. 2011a, 2014a; Silvestro et al. 2013; Bratzel et al. 2022). Hybridization is also implicated in the rapid diversification of clades like Vriesea (Tillandsioideae) where diversification is mostly Pleistocene; here ca 11% of the species are hybrids, and there are also hybrids with Tillandsieae (Loiseau et al. 2020 for discussion and references).
Centres of overall diversity in Bromeliaceae are the Atlantic Forest in Brazil, the central and the northern Andes, while endemism is particularly high in the Atlantic Forest, the central and southern Andes, southern Mexico and parts of Venezuela (Zizka et al. 2020). Most diversification within Bromeliaceae is indeed very recent. Divergence within the Guianan Brocchinia (Brocchinioideae, sister to the rest of the family) may have begun a mere 14 Ma (Givnish et al. (2004a, 2008a), Givnish et al. (2014a) thought that 99% of the 1,300+ species of Tillandsioideae originated in the last ca 9.6 Ma, 95% of the >100 species of Navioideae in the last ca 6.7 Ma, and so on. Much of the diversity (at least in terms of species numbers) in the family is in the Andean core tillandsioids, which some workers estimate began to radiate ca 14.2 Ma (see also Granados Mendoza et al. 2017), and the Brazilian Shield bromelioids, which started speciating only around 9.1 Ma (Givnish et al. 2011a). Within Tillandsia in particular there are two major clades that diversified in North/Central America and Andes/Chile respectively, the latter with migration events to the Brazilian Shield (Granados Mendoza et al. 2017). Vera-Paz et al. (2023, q.v. for further details) suggested that Tillandsia colonized North and Central America by long-distance dispersal from South America. Diversification of the Andean Puya seems to have begun in Peru (along with other early branches of Bromelioideae), and then following the Andean orogeny north (Schmidt Jabaily & Sytsma 2010, esp. 2013; Madriñán et al. 2013); it has occurred within the last ten Ma or so (Givnish et al. 2010). The speciose clade made up of Aechmea and relatives (Bromelioideae) seems to have diverged from Ananas about seven Ma and diversified within the last four Ma (Givnish et al. 2004a; Sass & Specht 2010). For diversification rates in the family, particularly high in tank epiphytes, see also Givnish et al. (2011b).
There is a fair bit of geographic structure in the tree of the Ronnbergia alliance (Bromelioideae: Aguirre-Santoro et al. 2016) and of Dyckia (Pitcairnioideae: Pinangé et al. 2017; see also morphology). For diversification in Tillandsia, see Granados Mendoza et al. (2017), there a North and Central American clade is sister to a cenral South American clade in which there had been several shifts from the Andes to the Brazilian shield. The ancestor of Pitcairnia feliciana probably moved to Africa by long distance dispersal perhaps 12-9.3 Ma (Givnish et al. 2008a, 2011; see also Porembski & Barthlott 1999), and it is basal in the genus (plastid markers)/basal in one of the two major clades in the genus (nuclear markers) (Schütz et al. 2016).
Givnish et al. (2008a, 2013, 2014a) discuss the evolution of Crassulacean Acid Metabolism (CAM, see also Ecology and Physiology below), bird pollination, epiphytism, periodically water-limited sites and xeromorphic traits in the family, and how all these features interact (see also J. A. C. Smith et al. 2005; Nyffeler & Eggli 2010b; Quezada & Gianoli 2011; Palma-Silva et al. 2016; Holtum 2023); geography and other factors can be added to the mix and localised to the appropriate part of the tree (Bouchenak-Khelladi et al. 2015; Donoghue & Sanderson 2015). Quezada and Gianoli (2011) considered that the acquisition of CAM photosynthesis consisted of a series of key innovations; in five sister group comparisons the CAM clade was significantly more diverse than the non-CAM clade. About 13.6% of bromeliads are CAM plants, and its evolution may have initially occurred as the family moved to dry/arid terrestrial habitats, rather than as an adaptation facilitating epiphytism (Quezada & Gianoli 2011), while C. E. Martin (1994) had suggested that some 69% of bromeliads were CAM plants, either epiphytes or growing in arid habitats. Note, however, that Zotz et al. (2023) question the nature of any general connection between CAM and the epiphytic habit. In any event, Bromeliaceae are certainly a major CAM group and they are second most diverse family of Neotropical epiphytes (Bratzel et al. 2022). Silvestro et al. (2013) noticed an increase in speciation rates in CAM Bromelioideae in particular (CAM treated as a single character), although extinction rates also increased sharply (c.f. Orchidaceae). They suggested that there had been multiple origins of CAM there (Silvestro et al. 2013: Fig. 1, Table 3) given a highly asymmetric model of trait evolution, but this may need to be rethought as ideas of relationships in the family change. Thus Bratzel et al. (2022) found that the C4 Deinacanthon urbanianum was sister to [Fernseea [[Ananas group + Cryptanthoid complex] [core Bromelioideae]]], while in Silvestro et al. (2013) it was sister to a clade that was sister to the rest of the subfamily; unfortunately, Bratzel et al. (2022) did not sample widely in basal Bromelioideae. Givnish et al. (2013, 2014a) thought that extensive speciation depended on the evolution of ecological traits such as the adoption of the epiphytic habitat (ca 60% of the family are epiphytes), the evolution of CAM in arid environments (terrestrial CAM species are ca 13.6%), or adaptations to understory life in forests (ca 12.3%), with subsequent geographic spread and radiation into different areas and environments. However, Givnish et al. (2014a) did not see CAM photosynthesis as being a major driver of speciation here; Males (2016) also thought that CAM was unlikely to be an explanation for diversity in the family as a whole. Air and tank bromeliads may have had different centres of origin/diversification (see below). Within Bromelioideae, the evolution of tanks may be a key innovation, diversification rates increasing because extinction rates had decreased in the epiphytic habitat as compared to the harsh, dry and open conditions of more ancestral Bromelioideae (Silvestro et al. 2013). For more on tank and CAM bromeliads, see Ecology & Evolution below.
Perhaps 1,060-1,800 species of Bromeliaceae, around half the family, are pollinated by hummingbirds, and these species are commonest at higher altitudes in the Andes, but less common in drier and in lowland forest habitats (Snow & Snow 1980; Stiles 1981; Benzing et al. 2000a; Kessler & Krömer 2000; Krömer et al. 2006; Givnish et al. 2008a; Kessler et al. 2019). However, insect pollination may be ancestral in the family (Aguilar-Rodríguez et al. 2019). Kessler et al. (2019) suggested that there had been 4-12 shifts to hummingbird pollination and about three times as many shifts away from bird pollination, but overall they thought that there were several factors that could lead to increased diversification rates, but that little was understood about them. Certainly simple adaptation of bromeliads to hummingbirds with different bill lengths did not fit the bill here (Kessler et al. 2019). Bird pollination in the [Pitcairnioideae [Puyoideae + Bromelioideae]] clade (there was one origin of ornithophily here) can be dated to ca 15, 13.4-13.3, or 14.1 Ma (Givnish et al. 2004a, 2011a, 2014a respectively), and bird pollination is also very common in Tillandsioideae (another origin), with a crown group age of ca 15.2 Ma, 99% of the species originating in the last ca 9.6 Ma (Givnish et al. 2014a). Nevertheless, Givnish et al. (2014a) did not see bird pollination as being a major driver of speciation in the family. Neves et al. (2020) focused on bird and bat pollination in Vriesea from the Atlantic Forest in Brazil, suggesting that pollinators were driving ecological isolation there, although there seemed to be little molecular separation of the species. Bird pollination (plants epiphytes, habitats shaded) may sometimes have been derived from bat pollination (plants epilithic, habitats open) (Neves et al. 2023). (For other major bird-pollinated clades in Central and (Andean) South America, see Lamiaceae-Nepetoideae-Salviinae, Gesneriaceae-Gesnerioideae, Heliconiaceae and Ericaceae-Vaccinieae, and for hummingbird pollination in general, see elsewhere.)
A number of epiphytic Tillandsioideae are quite small plants, and heterochrony (neoteny, paedomorphosis) has been invoked to explain their evolution (Benzing et al. 2000b; see Orchidaceae-Epidendroideae-Oncidiinae for a comparable example). Donadío et al. (2022) also invoked heterochronic processes, both paedomorphosis and peramorphosis, to explain the evolution of features throughout the plant in Tillandsia subgenus Diaphoranthema.
Kuhn et al. (2020) summarized information on embryological variation in the family, putting it in the context of a phylogenetic tree; overall there was relatively little variation. Aguirre-Santoro et al. (2016), focussing on the Ronnbergia alliance, but also taking in some other Bromelioideae, optimize the evolution of a number of characters, while de Oliveira et al. (2017) looked at anatomical variation in the context of the phylogeny of the nidularoid complex - 11/ca 150 species were examined.
Brocchinia, although a small genus of about 21 species restricted to the Roraima region, is remarkable in that it includes different growth forms - both tank plants and true terrestrials - and plants take up nitrogen (N) in different ways. Of the tank plants, B. reducta may acquire N through carnivory (see below), while the terrestrial B. acuminata is an ant plant. Givnish et al. (1997) discussed the radiation of the genus, which seems to have begun only ca 14 Ma, although, compared with other younger but far more speciose bromeliad clades this is hardly a very diverse=species-rich clade (Givnish et al. 2004a, 2008a, 2011a; Givnish 2015b), and overall these other clades encompass a considerable amount of morphological diversity; they are, of course, all in a clade sister to Brocchinia (c.f. Givnish 2016).
Ecology & Physiology. The ecophysiology of Bromeliaceae has attracted much attention; for an early summary see C. E. Martin (1994) and for a nice discussion of water relationships in Bromeliaceae, central to understanding the ecophysiological evolution of the family, see Males (2016). The ecological preferences of basal Bromeliaceae (and of Typhaceae) are for wet and nutrient-poor conditions, and in Bromeliaceae this would include conditions like those that occur on the tepuis and surrounding areas of the Guyana shield, perhaps where the family originated (Givnish et al. e.g. 2011a; Crayn et al. 2015).
All told, about 1,913 species of bromeliads are epiphytes, the family being 2nd in the list of angiosperm epiphytic families (Svahnström et al. 2025; also Zotz et al. 2021b, checklist of all vascular epiphytes, hemiepiphytes, etc.). Epiphytes here make up rather over half the family and ca 7% of all epiphytes. Svahnström et al. (2025) also discuss how common epiphytic taxa are relative to non-epiphytic taxa, indeed, in Bromeliaceae terrestrial species tended to have small ranges, those of epiphytic species being relatively larger. See also Kress (1989), Holtum et al. (2007), etc.; epiphytic bromeliads are especially common in montane habitats (Benzing 1990 for much discussion; Luther & Norton 2008: epilithic species not included). Associated with the adoption of the epiphytic habit in bromeliads are the evolution of CAM photosynthesis and various novel forms of water uptake, and these are discussed below. Along with orchids and ferns, bromeliads are one of the three main epiphytic groups, but despite their smaller genomes they have larger and fewer stomata than orchids, and thicker leaves, lower nutrient concentrations, and higher water content and water use efficiency than ferns (Hietz et al. 2021); see also Benzing (1990) and Zotz et al. (2021a) for the ecophysiological features of epiphytes in general - they are on the slow end of the Leaf Economic Spectrum. See also Piperaceae, while Melastomataceae, Ericaceae and Gesneriaceae are other big epiphytic families in the eudicots, Bromeliaceae and Orchidaceae in the monocots, and also ferns.
The diversity of growth forms in Bromeliaceae is well known (e.g. Pittendrigh 1948; Benzing 2000; Males 2016). Bromeliads can be grouped in five ecophysiological types that depend on how the plant grows, how it absorbs water, and on its photosynthetic pathway (see also Males & Griffiths 2017b; Eggli & Gouda 2020). Type I. Terrestrial, with a well-developed absorbtive root system, C3 or CAM photosynthesis. Type II. Terrestrial, tanks forming at bases of leaves, absorbtive roots, also absorbtive trichomes at leaf base, CAM photosynthesis. Type III. Terrestrial (saxicolous)/epiphytic, central tank present, with water etc. absorbing scales, thick leaves, roots for support, most CAM photosynthesis. Type IV. Mostly epiphytic, plants with tank, with water etc. absorbing scales at leaf base, thin leaves, roots for support, C3 photosynthesis. Type V. Epiphytes (saxicolous), air/atmospheric epiphytes with water etc.-absorbing scales over whole leaf, thick leaves, CAM photosynthesis, roots for support, or none (for germination/seedling characteristics in Bromeliaceae related to these five ecophysiological types, see Kowalski et al. (2021). Note that leaves of members of Bromelioideae and Tillandsioideae (types III, IV, V) have very low stomata:trichome ratios of around 3.2-0.5:1 while in the rest of the family ratios are ca 13.6:1 (Tomlinson 1969; Males 2016). This great diversity in physiology and morphology has arisen well within the last 18 Ma (see above), thus Givnish (2014a) estimated the crown-group age of a clade of ca 630 species of tank Bromelioideae to be only some 5.5 Ma.
As just mentioned, Bromeliaceae include a major concentration of CAM plants, including the most such species of any group, along with Crassulaceae and probably Orchidaceae-Epidendroideae. Some half to two thirds of Bromelioideae have some form of CAM metabolism and ca 44% have strong CAM, CAM photosynthesis having evolved some five times or more; however, there is phylogenetic uncertainty in Puyoideae and Bromelioideae and details of CAM evolution remain somewhat unclear (Crayn et al. 2000, 2004, 2015; Reinert et al. 2003; Keeley & Rundel 2003; Schulte et al. 2005; Ming et al. 2015: parallelism at the molecular level); Aguirre-Santoro et al. (2016) noted that in the Ronnbergia alliance (Bromelioideae) there had been reversals from CAM to C3 photosynthesis. In pineapple, at least, the CAM pathway results from the reconfiguration of C3 pathways via regulatory neofunctionalization of preexisting genes, not through gene duplication (Ming et al. 2015). The number of CAM species decreases with altitude although that of C3 species reaches a maximum at around 2000 m, however, some CAM Puya grow at over 4000 m (Crayn et al. 2015). Malate in tissue of CAM plants during the night reduced the osmotic potential of the tissue, and water uptake increased (C. E. Martin 1994). Bromeliaceae, like other CAM plants, tend to have less in the way of intercellular spaces than C3 plants, presumably enabling greater storage of CO2 as malic acid in the cell vacuoles during the day, and the extreme form of CAM, succulence plus much compacted cells (Earles et al. 2018), was the result of anatomical changes perhaps not easy to accomplish that happened late in the whole process (see E. J. Edwards 2019; c.f. C4 photosynthesis). J. A. C. Smith et al. (1986) and Martin (1994) discussed the comparative ecophysiology of CAM and C3 bromeliads and Hermida-Carrera et al. (2020) the molecular evolution of RuBisCO (ribulose-1,5-bisphosphate carboxylase-oxygenase), an enyzme centrally involved in C fixation. For more on the details of CAM evolution, its geography, etc., see Holtum (2023) and Gilman et al. (2023); Zotz et al. (2023) question the nature of particular connections between CAM photosynthesis and the epiphytic habit.
There is considerable plasticity in the development of the CAM syndrome within genera like Puya (Schulte et al. 2011) and seedlings of Ananas, at least, CAM when adult, are C3 plants (J. Zhang et al. 2014). Menezes et al. (2020) discuss how seedlings of the CAM Acanthostachys strobilacea deal with drying conditions, interestingly, many tank Bromelioideae carry out CAM photosynthesis (Silvestro et al. 2013). There can be specialization in function within a single leaf, thus in Guzmania monostachia the bottom part of the leaf stores water as a reserve for when the tank dries out, while CAM photosynthesis intensified in the upper part of the leaf (Freschi et al. 2009); Males (2016; see also Petit et al. 2014) gives several other examples of such flexibility in photosynthesis.
As bromeliad plants grow, their morphology may change quite dramatically, and along wih this there may be major changes in the ecophysiology of the plant, also, note that the distinction between the various features used to characterise these types may not be that sharp. Bromeliaceae can be heteroblastic, and Meisner et al. (2013; see also Males 2016) discuss the physiological changes involved. For example, young plants of tank epiphytes in shaded conditions have narrow, leaves densely covered by scales and are air epiphytes, type 5 above, but as they get bigger and have more numerous leaves there is self-shading by the leaves, and under such circumstances the adult form of the plant, a tank bromeliad with fewer, broader leaves that self-shade less, is at an advantage (Beyschlag & Zotz 2017). Heteroblasty also occurs in tank bromeliads growing in more exposed conditions, the ecophysiology of the plant changingf as it grows (Schmidt & Zotz 2001; Petit et al. 2014). Reinert and Meirelles (1993) looked at the development of Vriesea geniculata, a plant of the inselburgs of the Atlantic Forest. The young plant was an atmospheric type IV plant, with succulent narrow leaves, scales covering ca 83% of the adaxial and ca 68% of the abaxial leaf surfaces. The adult plant was a tank type 3 plant, the scales covering only around 15% of the leaf. Similarly, young plants of Alcantarea are succulent rosette plants, but when mature they are tank bromeliads (Versieux et al. 2010). Stryker et al. (2024) followed the change from rosette to tank life form in Tillandsia utriculata. In the rosette plant N uptake was in the form of ammonium, NH4+, bacterial N-fixers being involved - overall, the bacterial composition of the phyllosphere was not very diverse. With the transition to the tank form, not a paricularly simple event, the bacterial community diversified considerably, nitrate reducion increasing, ammonium, urea and nirates being the main forms in which N was taken up. Detritus in the tank was broken down, but ureolysis, N fixation and methanotrophy, reduced - alhough there was an uptick here at the time of the rosette→tank transition (Stryker et al. 2024). Although Meisner et al. (2013) had suggested that heteroblastic changes in leaf shape were quite often not accompanied by any obvious changes in physiology or anatomy and were not associated with the change from atmospheric to tank form, in a comparison of various aspects of the leaf - its morphology, venation density, hydraulic capacity, etc. - in Pitcairnia and some terrestrial Bromelioideae along with environmental factors like precipitation, Males (2017a) found suggestive correlations between foliar and environmental features. Indeed, there is quite extensive variation in leaf morphology within genera like Pitcairnia and Cryptanthus (Males 2017a). Roots may change, too, switching from being absorbtive to being involved in attachment alone, as in V. geniculata, where younger plants might have root hairs; in both young and older plants the root system was sclerified (Reinert & Meirelles 1993).
Indumentum in Bromeliaceae is very variable (Ehler 1977) and multicellular hairs on the leaf surface are integral to the ecophysiology of both tank plants and air epiphytes; details of how they take up water and nutrients are discussed in C. A. Takahashi et al. (2022). As mentioned above, how much the scales cover the leaf may vary during development - and even proceeding from the base to the apex of the leaf (e.g. Reinert & Meirelles 1993). Tillandsioideae have elegant multicellular peltate trichomes that may completely cover the plant and through which the plant takes in water and nutrients - water moves in as liquid by wicking, but can only be lost by evaporation (see Mez 1904 for detailed early studies; Ehler 1977; Papini et al. 2010 for scale development). In Tillandsia there are regularly-arranged central shield cells, four in the centre, eight surrounding those four, then sixteen; these shield cells have much thickened outer cellulosic walls, and they are surrounded by radially arranged cells. Underneath the shield cells is a central dome cell. These cells are all usually dead - although in some cases they may be alive (Takahashi et al. 2022). Beneath the dome cell are two living foot cells with outer walls surrounded by cuticle (Raux et al. 2020). The scales flex as they dry and so pull away from the leaf surface, but when there is fog or it rains they readily take up water, coming to lie flat on the surface (Benzing 1976; Pierce et al. 2001). Ha et al. (2021) noted that in T. usneoides this flattening of the scale wings was sequential, a film of water between scale and leaf propagating itself across the leaf. Lian et al. (2025) described water movement along the leaf surface in T. capitata; there it can be bidirectional, and also move from one leaf to another. Droplets of water slowly develop at the leaf tip in mist but then abruptly disappear, the water moving along the adaxial surface of the rest of the leaf very quickly (Lian et al. 2025). The subsequent absorbtion of water and nutrients by the scales is a two-step process. For example, in the rosette-forming T. ionantha water moves into the cells of the scales by capillary action in the fluid phase, and this happens within minutes of wetting, and then over the next three hours or so it moves into the body of the leaf by the aid of aquaporins, proteins in plant cell walls (Ohrui et al. 2007). Indeed, water moves through the thick cellulose walls of the scale surface and into the lumina of the scale cells with almost no impediment, the plasma membrance of the foot cell being the main resistor. However, when the scale dries, there is no reverse wicking effect. Rather, movement of water is in the gas phase, the dead cells drying out, water moving into their empty lumina only by evaporation, and then water vapour diffusing with difficulty through the thick walls of the shield cells; the cuticle surrounding the foot cells further impedes water loss (Raux et al. 2020). It has also been suggested that the dry, white scales of some species of Tillandsia may reflect light and so provide photoprotection (Pierce 2008); see also Orchidaceae for UV light and epiphytism.
Epiphytic bromeliads tend to be found on trees with rough bark, although species of Tillandsia on the Pacific coast of Mexico preferred large trees with compound, pulvinate leaves, the leaflets moving during the day and affecting the light that the plants received (Reyes-García et al. (2007). In general, air epiphytes can maintain photosynthetic activity when leaf relative water content is low, their leaves tend to be more succulent, and they obtain water from rain showers (Cháves-Sahhagún et al. 2019); as mentioned above, succulence is common in the leaves of group III and V bromeliads, and Eggli and Gouda (2020) discuss succulence in Bromeliaceae; the water storing tissue there is parenchymatous mesophyll. Reyes-García et al. (2011) compared water uptake in CAM tank, succulent air and non-succulent air epiphytes in Tillandsia; the non-succulent air epiphytes like T. usneoides, etc., caught water from fog while the tank epiphytes relied on rain water stored in their tanks, their stomata closing when it was used up. Species of Tillandsia on the Yucatan Peninsula of Mexico varied in their ability to take up water from dew; a tank and an air epiphyte could do this, two other air epiphytes could not, although the effect of drought on the plant depended on far more than this (Cháves-Sahhagún et al. 2019). However, in Tillandsia growing on the Pacific coast, air epiphytes taking up water from dew, etc., had their maximum photosynthetic activity when there was no rain, while in tank epiphytes photosynthesis was practically limited to the rainy season (Reyes-García et al. (2007). Different species of Tillandsia from the Ilha Grande, southeast Brazil, grew in different places in the tree canopies, T. usneoides with its long, dense scales happily growing in the most exposed sites (Miranda et al. 2020).
Perhaps somewhat paradoxically, adaxial leaf surfaces in Bromelioideae and some other subfamilies are hydrophilic while abaxial surfaces are hydrophobic (Reuter & Brown 2009; see also Fagaceae). Dense scales or a powdery epicuticular wax make the abaxial leaf surface water-repellent so the stomata there remain functional even in wet conditions, and it has even been suggested that repelling water was an ancestral function of bromeliaceous scales (Pierce et al. 2001). The less dense scales on the adaxial surfaces of the leaves are involved in nutrient uptake (Benzing et al. 1985; Pierce et al. 2001) here, too. Thus phosphate is taken up very efficiently by the hairs on the adaxial surfaces of the leaves in tank epiphytes like Aechmea fasciculata and either moved elsewhere in the plant or stored as phosphorus-containing phytin (Winkler & Zotz 2009; Gonsiska & Givnish 2009). For further details of xylem anatomy, etc., and water transport, see Males (2016 and references). Leaves of Bromeliaceae also do not seem to lose metabolites easily (Benzing & Burt 1970; McWilliams 1974).
Air epiphytes are common in Tillandsioideae, in some species roots being for attachment only while other species have no roots at all (for seedlings, see Gatin 1911; Kowalski et al. 2021). Thus adult plants of Tillandsia usneoides (Spanish moss) lack roots, the plants growing readily on any available support - branches, telegraph wires, etc. (Wester & Zotz 2010 and references); the leaves take over the nutritional function of roots, however, the plant lacks photoprotection (Reyes García & Griffiths 2009). Perhaps somewhat paradoxically the dense trichomes may lead to a reduction in CO2 uptake when the plant is wetted, the trichomes plastering the surface of the leaf and making CO2 uptake more difficult (C. E. Martin 1994).
Tank epiphytes occur in a major clade in Bromelioideae, some Brocchinia, a few Tillandsioideae, etc., the tanks being formed by the closely appressed overlapping bases of the leaves (in some Bromelioideae every leaf forms a small basal tank). Tanks may hold up to around 6.5 l of water, more commonly half a litre or less, although some old records suggest that tanks of Alcantarea imperialis, for example, might hold up to 45 l; the water content of the hydrated plant varies little, although much of that may effectivly be unavailable, depending on the species (Zotz et al. 2020). Zotz and Thomas (1999) followed the drying out of tanks; they found that by ca 30 days the tank of Guzmania monostachia (= Tillandsia) had dried out and the plant had lost ca 50% of the water in its tissues, while in similarly-sized plants of T. fasciculata it took 40-50 days for this to happen. In such situations, the hydraulic conductance of the leaf is affected by changes both in the xylem, e.g. the formation of embolisms, and in extraxylary tissues, e.g. changes in intercellular spaces, aquaporins (membrane proteins involved in water transport), etc., indeed, changes in the latter seem to most affect hydraulic resilience in G. monostachia (North et al. 2019). Intercellular spaces in Vriesea and other Vrieseeae are area of stellate parenchyma between the vascular bundles (Versieux et al. 2010). Roots of tank bromeliads may grow into the tank where they absorb water and the products of the rotted detritus in the tank; Pittendrigh (1948) noted that such roots were mycorrhizal, while roots growing into the soil were not obviously mycorrhizal. Many tank Bromelioideae also carry out CAM photosynthesis (Silvestro et al. 2013: see above). Aguirre-Santoro et al. (2016) note that in the Ronnbergia alliance (Bromelioideae) there have been reversals to C3 photosynthesis and the terrestrial habitat, and also the loss of tanks.
In some tank bromeliads the apical meristem remains submerged and at the bottom of the tank. Nogueira et al. (2021) focussed on nidularoids (Bromelioideae), and there the inflorescence is congested, the flower buds developing under water and opening above; after flowering, the perianth rots, and the fleshy fruits initially develop under water but later rise above the surface as their pedicels elongate. The walls of the tank are made up of large, conspicuously-coloured inflorescence bracts, although Canistropsis has water-containing bracts at the end of a somewhat elongated peduncle. Taxa with this behaviour appear not to form a monophyletic group; genera tend to be small and restricted to the Atlantic Forest, but the large genus Neoregelia is widespread (Nogueira et al. 2021). The whole system is perhaps functionally analogous to a water calyx.
Bromeliaceae in general have extremely low leaf N concentrations. Takahashi et al (2022) suggested considerable complexity in details of N uptake, for example, the scales of air epiphytes may be modified depending on the source of N (urea, nitrate, etc.), and the N source might affect the amount of water-storage tissue in the leaf. N metabolism in epiphytic tank Bromeliaceae can be complex, with N moving into the plant via frass and general detritus produced by insectivores living in close association with the plants, sometimes aided by the activities of associated microbiota. In a study of 22 individuals of Aechmea nudicaulis (Bromelioideae), Louca et al. (2016) found that the various operations involved in breaking down the contents of the tanks - ureolysis, cellulolysis, methanogenesis, etc. - went on in each, but different organisms were involved from plant to plant; I have not seen species comparisons. The pH of the water in the tank affects the microorganisms that grow there (Goffredi et al. 2011). Inselbacher et al. (2007) found that micro-organisms in the tank of Vriesea gigantea increased the mineralization of organic compounds to NH4+, the form in which N was taken up, and chitinases may have been produced by the plant (as they are in carnivorous plants - e.g. Jopcik et al. 2017 and references). A variety of βproteobacteria isolated from the tanks of Werauhia gladioliflora could utilize N-acetyl-D-glucosamine, the building block of chitin; they also broke down a variety of plant-derived C sources (Klann et al. 2016). The presence of chitinases and nitrogenases emphasized the importance of tanks in both C and N cycling (Goffredi et al. 2011). That being said, in a study of Mexican tank epiphytes growing at various altitudes the amount of litter that they captured was small relative to the total amount of litter produced and it had overall little effect on the C and nutrient cycles (Aguilar-Cruz et al. 2021).
However, detritivores themselves may not be directly involved in facilitating N uptake by the plant. They may indeed break down debris in the tanks, but Ngai and Srivastava (2006) suggested that carnivorous damsel fly larvae might be more important: They ate the detritivore larvae, and it was N from the damsel fly faecal pellets that was important for the plant, and when the detritivore adults flew away, the N that they contained was lost to the plant. Leroy et al. (2013) proposed that associations of tank bromeliads in particular with mutualistic ants enhances N uptake of the former, N moving from ants and their debris into the plant via its roots. Carnivorous ants may show this effect most, thus in an ant-association of the terrestrial Quesnelia arvensis, which grows on poor soils in the Atlantic Forest in Brazil, it was the predatory ant Odontomachus hastatus that had the greatest effect on the plant, the largely vegetarian Camponotus crassus the least, although the latter is also a very small ant... (Gonçalves et al. 2016a). Bromeliads can also take up N from the excreta of spiders living on the plants, the amount depending on the season, the disposition of the N inside the plant, and on the growth form of the plant (Gonçalves et al. 2011), thus frass, etc., from the jumping spider Psecas chapoda improved the N balance of the terrestrial Bromelia balansae, bacteria perhaps being a way station in the movement of N to the plant; note that B. balansae does not form tanks (Romero et al. 2006; Gonçalves et al. 2014). Nyman et al. (1987) found that a variety of amino acids moved from liquid placed on the leaves of Tillandsia pauciflora into the plant, arginine and lysine moving in fastest, about four times as fast as neutral amino acids, which in turn moved in about three times faster than acidic amino acids. Blue-green algae may fix N in some bromeliads, although in such cases there are perhaps more algae in seepage areas from the tank than in the tank itself (Bermudes & Benzing 1991). Vergne et al. (2021) noted that a high proportion of anoxygenic phototrophic bacteria (APG) in particular - they range from aerobic photoheterotrophs to anaerobic photoautotrophs - were found in the phytotelmata of the bromeliads from French Guiana that they sampled. Indeed, some 41-79 of the sequences used to enumerate the bacterial taxa in these phytotelmata were associated with APG groups that had a N-fixing gene toolkit, although N fixation had yet to be demonstrated. Furthermore, the bacteria involved varied depending of the identity of the bromeliad (Vergne et al. 2021).
Phosphorus may be another important nutrient for the plant, as in other epiphytes (Zotz & Asshoff 2010).
There are important physiological differences among epiphytes that do not seem to respect the tank/air distinction as much as they reflect phylogeny. L.-L. B. Müller et al. (2017b) found that the young Tillandsioideae they studied, which included both tank and air epiphytes, grew much more slowly and had a much lower relative growth rate than the Bromelioideae, all tank epiphytes, whose relative growth rate was much more normal, the differences being due to differences in the net assimilation rate. Interestingly, Tillandsioideae showed relatively high total amino acid/asparagine accumulation when fed anuran faeces compared with Bromelioideae, perhaps allowing for faster growth in the latter, greater stress-resistance in the former (Gonçalves et al. 2016b). Some aspects of germination behaviour, for example, lower thermal limits and lower thermal optima, distinguish germination/young seedlings of Tillandsioideae from those of Bromelioideae, although overall thermal niche breadth did not differ much (L.-L. B. Müller et al. 2017b; see also Whigham et al. 2008 for germination, seedlings, etc.).
As mentioned above, the air and tank groups possibly have different centres of origin/diversification (the Andes versus the Brazilian Shield). However, they often grow together, and in any particular locality, or even on a single tree, tank and air bromeliads may partition the environment in distinctive ways. Thus Reyes García et al. (2007) observed that in Chamela (the west coast of Mexico) almost 40% of the bromeliads were growing in about 5% of the trees, and they were most frequent in trees with compound leaves, perhaps because the light could penetrate the canopy more easily. Different species grew in different parts of the canopy, i.a. depending how the plant could take up water; perhaps paradoxically, the air epiphyte Tillandsia usneoides prefered shady conditions, but it lacks photoprotection - and it also seemed to prefer plants with simple leaves (Reyes García & Griffiths 2009).
C. E. Martin (1994) suggested that epiphytic bromeliads were in general resistant to drought, their water potentials remaing high; juvenile atmospheric Tillandsia deppeana was more drought-tolerant than the adult plants, which were tank epiphytes. Transpiration rates of epiphytic bromeliads were low, and those of C3 plants were rather lower than those of CAM plants. The plants could be very desiccation tolerant, detached shoots taking months before their water content was reduced 50%. Most epiphytic bromeliads had the photosynthetic responses of shade plants, although many could/did grow in full sunlight. Epiphytic species had (4-)29-104(-411) stomata/mm2, while the figures for terrestrial bromeliads were (10-)78-110(-446)/mm2
For ideas about the importance of the evolution of features like CAM photosynthesis and the tank habit in the diversification of the family, see Divergence & Distribution above. See also Piperaceae, while Melastomataceae, Ericaceae and Gesneriaceae are other big epiphytic families in the eudicots, Araceae and Orchidaceae in the monocots, and also ferns.
Virzo de Santo et al. (1976) also discuss the uptake of water from the atmosphere, in this case by ground-dwelling species of Tillandsia growing in the hyper-arid Atacama Desert - they have no roots and are basically air epiphytes; they obtain both water and nutrients from fog (A. L. González et al. 2011, see also papers cited by Koch et al. 2022 - "Living at its dry limits: Tillandsiales in the Atacama desert."). In the hyperarid lomas of coastal Chile and Peru, bromeliads, especially Tillandsia, can dominate, thus T. landbeckii is found by itself growing in bands on the otherwise bare ground over some 1,500 km2, the plant - it has CAM photosynthesis - absorbing water from the coastal fogs (Koch et al. 2019); T. landbeckii also seems to hybridize with other species of the genus (Möbus et al. 2021). Such communities have the highest biomass of any bromeliad community in the world, and practically no other angiosperms can stand conditions here (Rundel & Dillon 1998). The rootless T. latifolia and T. purpurea are quite happy lying around in the full sun in Peruvian deserts (McWilliams 1974).
Carnivory has evolved more than once in Bromeliaceae; see Givnish (2015a), the papers in Ellison and Adamec (2018), and the Carnivorous Plants Database. The tillandsioid Catopsis berteroniana traps terrestrial arthropods and is possibly carnivorous; interestingly, it harbours larvae of the mosquito Wyeomyia, which also live in the pitchers of Sarracenia (Frank & O'Meara 1984; Gonsiska & Givnish 2009). For possible carnivory in Brocchinia reducta, see Givnish et al. (1984) and Plachno and Jankun (2005); the breakdown of organic matter in the trap is probably handled by the organisms living in the trap, everything from bacteria on up (Leroy et al. 2016). Brocchinia reducta does not produce nectar, only a sweet scent, but lives in the same area as the rather similar-looking and nectar-producing Heliamphora (Sarraceniaceae), and the former may even be a Müllerian mimic of the latter (Joel 1988). Brocchinia hechtioides may also be carnivorous (Fruend et al. 2022).
Finally, quite a number of Bromeliaceae have variously patterned and coloured leaves (Zhang et al. 2020), increasing their horticultural potential.
Pollination Biology & Seed Dispersal. Perhaps as many as 1,500 species of Bromeliaceae are pollinated by hummingbirds (see Divergence & Distribution above). Given that there are only some 365 species of hummingbirds in total, one hardly expects to find much in the way of mutual coevolution=cospeciation here. Thus only a few species of hummingbirds are the major bromeliad pollinators in southeastern Brazil, a centre of diversity of the family (Snow & Snow 1986; Sazima et al. 1996; Placentini & Varassin 2007). For example, in three Atlantic Forest sites examined Bromeliaceae made up around 1/3 of the bird-pollinated species - 15 species of hummingbirds pollinating 86 species of plants of which 31 were bromeliads (Buzato et al. 2000). It is odd that there appears to be no prezygotic reproductive isolation between sympatric bromeliads there; the flowers are not notably different morphologically and flowering times overlap extensively, yet hybrids are very uncommon (Wendt et al. 2008) as they are in the family as a whole (see also Kessler et al. 2019). There are pseudanthia in some Bromelioideae like Wittrockia and Canistrum (Baczynski & Claßen-Bockhoff 2023). Benzing et al. (2000a) summarize many aspects of the reproductive biology of Bromeliaceae.
Many Hechtioideae have scented flowers, unusual in the family (Ramírez-Morillo et al. 2018). Stigmatodon (Tillandioideae-Vrieseeae) includes 35 night-flowering species (Leme et al. 2025). Bat pollination is known from about 42 - perhaps as many as 123 - species, mostly in Tillandsioideae; 19 species from 11 genera of phyllostomid bats have been recorded as visitors (Aguilar-Rodríguez et al. 2019). Cascante-Marín and Núñez-Hidalgo (2023) review breeding systems in the family.
Dispersal is primarily by ingestion of fruits by animals (Bromelioideae) or movement of the seeds by wind. Friar birds (Euphoniinae, near Fringillidae), are mistletoe specialists that also eat the fruits of epiphytic Bromelioideae (Snow 1981; Restrepo 1987). K. R. Silva et al. (2020) noted that in some species of Aechmea, at least, the birds may consume the mucilaginous appendages on the seeds. Indeed, seeds of Bromelioideae commonly have appendages at the micropylar and/or chalazal ends, and these can be up to 4 cm long; it has been suggested that such seeds may stick to animals, trees, etc. (Benzing 2000; Aguirre-Santoro et al. 2016; Leme 2021). In Billbergia bats may disperse the fruits, initially being attracted by their shiny surfaces or the scales on the fruits (and the smell of the fruits), and here the mucilaginous surface of the seed may facilitate its movement through the gut of the animal. Silva et al. (2020) discuss seed dispersal by both bats and birds in Bromelioideae. Ronnbergia is reported to have explosive berries (Aguirre-Santoro et al. 2016).
There are seed hairs in Tillandsioideae and they develop in a variety of ways, including the almost complete separation of series of exotestal cells from the rest of the seed, and they are involved in wind dispersal (Rohweder 1956; Palací et al. 2004; Barfuss et al. 2005; Magalhães & Mariath 2012). The seeds of Tillandsia fall only slowly, although comparison should be made with other epiphytic taxa (Chilpa-Galván et al. 2018). The coma on the seeds of Catopsis (Tillandsioideae) may also assist materially in germination and seedling establishment by taking up water which can be used by the plantlet; this could be critical in allowing its establishment in the epiphytic habitat where it grows and where water may be at a premium (Wester & Zotz 2011).
Plant-Animal Interactions. Caterpillars of Nymphalinae-Riodininae eat Bromeliaceae (and Orchidaceae: Hall 2003 and references). However, bromelain, a cysteine protease from the stem of pineapple, has a negative effect on the growth of insect larvae (Konno et al. 2004: Agrawal et al. 2008 and Mason et al. 2019 for more on how such proteases may affect the herbivore).
Water in the tanks (= phytotelmata), so conspicuous in many bromeliads, provide habitats for many organisms, including tadpoles, while a few carnivorous (and viviparous!) Utricularia (Lentibulariaceae) also live there (Thienemann 1934; Benzing 1990; Kitching 2000; Greeney 2001: bibliography). Indeed, the evolution of a group of specialised diving beetles that live in the tanks (Dystiscidae) may be almost contemporaneous with the appearance of the tank habitat itself (Balke et al. 2008). Eggli and Gouda (2020) discuss species of malaria-transmitting Anopheles whose larvae grow in bromeliad tanks; known from Brazil and Trinidad, early work on this system was carried out in Trinidad and represented important contributions to bromeliad ecology (e.g. Pittendrigh 1948). There is considerable diversity in microhabitats within a single tank (Klann et al. 2016). The rainfall pattern determines the kinds of organisms in the tanks. Thus Romero et al. (2020) found that with an increasing amount of rain, predator abundance declined, but with infrequent rains large predators in particular increased, while detritovore biomass was higher when the rainfall was stable. Overall resilience in the functional structure of assemblages can be high, and water loss in understory habitats being slower than that in plants growing in more exposed conditions (Dézerald et al. 2015). For further details of the rich biota living inside the tanks, which includes mites, flagellates, ciliates as well as bacteria, see Frank (1983; references in Grothjan & Young 2019).
Some epiphytic Bromeliaceae are more or less closely associated with ants, more than one species being found in different individuals of the tank epiphyte Aechmea mertensii where the association is obligate and the ants build gardens (Petit et al. 2013; see also Benzing 1990; Orivel & Leroy 2011). Perhaps paradoxically, ants associated with Tillandsia caput-medusae remove seeds of the plant from its phorophyte host so negatively affecting its establishment... (Vergara-Torres et al. 2021).
Peromyscus in Mexico has been found to comprehensively predate seeds of epiphytic Tillandsia (Chilpa-Galván et al. 2017) - apparently they are tasty, if rather small... Ants were also seed predators.
Plant-Bacterial/Fungal Associations. Bacteria of various kinds are important components of bromeliad phytotelmata (see above). Fine endophytes, with their distinctive fan-like arbuscules, are known from epiphytic bromeliads; these endophytes are probably mucoromycetes (Orchard et al. 2016 and references).
Genes & Genomes. The rate of molecular evolution in Bromeliaceae is very low, ca 0.00059 substitutions/site/Ma, even lower than in palms; although the family is not particularly woody, its members have a long generation time, which may be connected with this low rate (S. Y. Smith & Donoghue 2008). However, the rate of evolution in the plastome of Typhaceae is also rather low (Bennett et al. 2015), so this may be a feature of the whole clade (assuming it exists).
Correlations between genome size and relative growth rate in Bromelioideae (low-high respectively) and Tillandsioideae (high-low) seem to break down in analyses within the subfamilies (L.-L. B. Müller et al. 2019).
Brown and Gilmartin (1989) suggested that the base chromosome number in the family was the result of sequential polyploidy: n = 8 x n = 9 → n = 17 x n = 8 → n = x = 25. Gitaí et al. (2014, see also 2005) agreed that the base chromosome number is 25 (that number is not known from Brocchinia) and they also gave details about genome size (for these two for the family, see also Carta et al. 2020) and chromosome length, thus Tillandsioideae tended to have longer chromosomes that do other subfamilies. Bromeliaceae, at n = 32, have the second highest minimum chromosome number in seed plants - Bignoniaceae have the highest (Elliott et al. 2022b: Fig. S6). For variation in genome size in Pitcairnioideae and further suggestions for a base chromosome number for the family, see Moura et al. (2018), and for base chromsome numbers, e.g. for Ananas, see Q. Xu et al. (2021). There is variation in diploid chromosome numbers in the vegetative parts of the plants in some Bromeliaceae (G. K. Brown & Gilmartin 1986).
Chemistry, Morphology, etc.. The roots of Bromeliaceae have a uniseriate epidermis, not a velamen as has sometimes been reported (Kowalkski et al. 2018). Intracortical roots are known from the family, and in Aechmea castelnavii a periderm develops immediately under/in the exodermis (da Silva & Scatena 2017). In at least some species that have roots, the radicle of the embryo aborts (Fiordi Cecchi et al. 1996; Magalhães & Mariath 2012). Idioblasts in the roots containing bundles of raphides may break down and generate aerenchyma (Kowalski et al. 2021).
Colleter-like structures have been reported from Aechmea, they have a hand-like shape and are epidermal in origin (Ballego-Campos & Paiva 2018 and references). Peltate trichomes on the abaxial surface of the bracts in several Tillandsioideae produce mucilaginous secretions, sometimes with lipids (especially species growing on rocks), and there is mucilaginous tissue in the bracts in a number of Vrieseinae - protection against desiccation, excessive radiation and herbivory may be involved (Ballego-Campos et al. 2023).
Extranuptial nectaries have been reported from the family, especially from the abaxial surface of the calyx in Pitcairnioideae; although nectar is produced, there is nothing much in the way of a distinctive structure involved (Ballego-Campos et al. 2022). De Oliveira et al. (2020) looked at a number of variables - some potentially systematically interesting - in the androecial and gynoecial anatomy of species of Bromelioideae, Pitcairnioideae and Tillandsioideae. The flowers of Tillandsia are shown as being inverted, but those of Bilbergia have the normal position with an abaxial median sepal (Spichiger et al. 2004); the former position is incorrect (W. Till, pers. comm.). Paired appendages varying in shape are quite common at the bases on the inner tepals and they enclose the bases of the stamens associated with the tepals - do they affect nectar movement? (G. K. Brown & Terry 1992; Santa-Rosa et al. 2020; Leme et al. 2021: Bromelioideae, 2025: Tillandsioideae). Fot stamens in Bromelioideae, often free, see Leme et al. (2025). Tapetum development is "intermediate", the cells initially being secretory, but tending to become invasive later (Sajo et al. 2005), The pollen of T. leiboldiana is described as having a proximal sulcus (Albert et al. 2010); for pollen in Tillansioideae, see Leme et al. (2025). The superior ovary of Bromeliaceae such as Tillandsioideae may be secondarily so (Böhme 1988; Sajo et al. 2004b), although if so, I find it difficult to understand why the vascular traces to the various floral organs should then often depart independently in these taxa; they are fused when the ovary is clearly inferior. See also de Oliveira et al. (2020) for variation in androecial and gynoecial anatomy. Labyrinthiform nectaries are common in Bromeliaceae (e.g. de Oliveira et al. 2020), Santa-Rosa et al. (2020) suggested that in some species of Aechmea nectar from the septal nectaries might move up the stylar canal to the stigma. Variation in stigma morphology in Tillandsioideae, Bromelioideae, etc., is great (G. K. Brown & Gilmartin 1989; Leme et al. 2021), 18 character states being recognized in the former subfamily (Barfuss et al. 2016), or 19 in the family as a whole (Siqueira et al. 2023). The stigmatic hairs secrete mucilage, and mucilage is also secreted by the epidermis of the glabrous stigmatic margin; a variety of other exudates are also produced by the stigmas (Siqueira et al. 2023). Fot stigmas, see also Leme et al. (2025: Tillandsioideae), usu. strongly 3-lobed and "hairy", "hairs" glistening, sometimes stigma only slightly lobed, the apex containing liquid.
Variation in ovule and seed morphology is also extreme (e.g. Gross 1988a). Fagundes and Mariath (2014) and Kuhn et al. (2020) note extensive variation in the chalazal appendages - in position, number, and extent of development; see also the illustrations and discussion on seed morphology and anatomy in Leme et al. (2021). Ovule number in Brocchinioideae is unclear; L. B. Smith (in Smith & Downs 1974) did not record this, but illustrations suggest there can be at least three ovules per carpel. De Carvalho et al. (2023) detail extensive variation in the seed coat of Bromelioideae - they looked at 33 characters in 24/41 genera here,the characters having up to seven states, i.a. they found that the chalazal appendage in Acanthostachys strobilacea was up to several centimetres long. The cotyledon has but a single vascular bundles, the eophylls have three (Kowalski et al. 2021).
L. B. Smith and Downs (1974), Varadarajan and Gilmartin (1988b), Rauh (1990: cultivated bromeliads), L. B. Smith and Till (1998), Benzing (2000), Eggli and Gouda (2020: succulent taxa) and especially Gouda et al. (2023 onwards) provide much general information while G. K. Brown (2017) summarized work on the family; see Schubert (2017) for a monograph of Pitcairnia. For more on general anatomy, see Robinson (1969) and Tomlinson (1969), for phytoliths, see Piperno (2006), on rhizome and root anatomy, see Proença and Sajo (2008) and da Silva and Scatena (2011), on leaf anatomy see Versieux et al. (2010) and Santos-Silva et al. (2013), on androecial morphology, see de Oliveira et al. (2020), on microsporogenesis, esp. callose deposition, see Albert et al. (2014), on pollen, see Halbritter (1992), Albert et al. (2011: Hohenbergia tetraporate) and dos Santos-Teixeira et al. (2020: Aechmea), on gynoecial anatomy, see de Oliveira et al. (2020), on stigma morphology, see Brown and Gilmartin (1989), on ovules, Sajo et al. (2004a), Fagundes and Mariath (2014: summary table), Noguiera et al. (2015: Bromelioideae) and Kuhn et al. (2016: Tillandsioideae), on stigmas, fruits, and seeds of Bromelioideae, see Leme et al. (2021), on just fruits and seeds, see K. R. Silva et al. (2020) and Leme et al. (2022, also pollen, etc.), on fruit anatomy, see Fagundes and Mariath (2010), on seed anatomy, Szidat (1922), Rohweder (1956), Gross (1988a), and Varadarajan and Gilmartin (1988a), and for germination and seedlings, see Gatin (1911), Gross (1988b), Tillich (2007) and Kowalski et al. (2021).
Phylogeny. Here I largely follow Givnish et al. (2008a: 1 gene, good generic sampling, few species, but note rooting of Fig. 1, also 2009b, 2011a, b) for the phylogeny of the family; see also Schulte et al. (2005: focus on Bromelioideae) and Escobedo-Sarti et al. (2013). In earlier studies, Bromelioideae were monophyletic, even when Pitcairnioideae were included (Crayn et al. 2004: matK + rps16), however, in some studies (Horres et al. 2000) Puya did not link with them (see also Jabaily & Sytsma 2010). Hechtia and Navia were of uncertain position in the study by Horres et al. (2000: trnL), but were weakly linked with Tillandsioideae in Crayn et al (2004); in that study Navia was polyphyletic. Support for Pitcairnioideae in early studies was weak (55% - Terry et al. 1997: ndhF), and the subfamily was not apparent in Horres et al. (2000), although a group of Pitcairnioideae genera was apparent, albeit with <50% bootstrap. See also Crayn et al. (2004) for phylogenetic problems with Pitcairnioideae; it has of course turned out to be eminently paraphyletic (see e.g. Givnish et al. 2007, 2011a). Givnish et al. (2011a) found strong support for most elements of the topology used here, support for the monophyly of Pitcairnioideae s. str. and for the position of Navioideae improving over earlier studies, but the monophyly of Puyoideae was still not well supported. Indeed, there is conflict between chloroplast and nuclear data, the former suggesting that Puyoideae and Pitcarnioideae are monophlyetic, the latter that Puya is embedded in Pitcairnioideae (Schütz et al. 2016). For other phylogenetic studies, see Crayn et al. (2000), and Givnish et al. (2004b: ndhF). In general, the very low rate of evolution in the markers used coupled with the relatively recent divergence times in the family often makes disentangling relationships difficult. Indeed, given the poor support for many relationships in molecular studies, morphological and/or combined analyses are being attempted. For general references, see Palma-Silva et al. (2016).
For the association of Ayensua with Brocchinia and the phylogeny of Brocchinioideae, see Givnish et al. (1997) and Horres et al. (2000).
For relationships within Bromelioideae, see Horres et al. (2008), Schulte and Zizka (2008) and especially Schulte et al. (2009); Bromelia serra alone may be sister to the rest of the subfamily, although support for this position is weak. Indeed, Evans et al. (2015) emphasize the variety of topologies obtained in earlier work for the basal branchings in this clade. They find some support for the topology [Ochagavia et al. [Bromelia + The Rest]]; within The Rest there is some support for a Bilbergia-nidularoid clade and rather more for a Hohenbergia-Orthophytum clade, but overall relationships are still very much pectinate, with Aechmea popping up everywhere, even in the two clades just mentioned. In a study focussing on the Aechmea alliance, it, Canistrum and Portea were all polyphyletic (Heller et al. 2015), while Faria et al. (2010) also discussed relationships in Aechmea. Core Bromelioideae may be made up of two clades within which one, the Ronnbergia alliance, a fair bit of structure is becoming apparent (Aguirre-Santoro et al. 2015, 2016), Aechmea (of course), Hohenbergia and Ronnbergia iself being polyphyletic. In Angiosperms353/plastome/chondrome analyses of 86 species and some 34 genera that had a focus on core Bromelioideae, Bratzel et al. (2022) confirm that generic limits in this subfamily remain a disaster area. They recovered 26 clades (two with one species), noting that four had long been recognized as genera (but two of these were not recovered in the plastome analyses). There was indeed a fair bit of resolution along the spine of the phylogeny and there was geographic signal in some clades recovered, but overall polyphyly/paraphyly was pervasive, e.g. Aechmea appeared in eleven or so clades, Quesnelia in four, and so on (Bratzel et al. 2022), even given the limited sampling of the study. Relationships are/may be [Deinacanthon [Fernseea [[Ananas group + Cryptanthoid complex] [Acanthostachys [Neoglaziovia + the rest]]]] - note that Acanthostachys and Neoglaziovia were polyphyletic in plastome analyses and [Ananas group + Cryptanthoid complex] did not form a clade; Ochagavia et al., early diverging Bromelioideae, were not included.
Rex et al. (2009 and references) discussed relationships within Forsterella. Cruz et al. (2016) found support for some species groups in the Brazial endemic Cryptanthus, although relationships between these groups were largely unsupported; subgenus Cryptanthus, which did have support, is andromonoecious. However, Leme et al. (2017) largely clarified the main patterns of relationships there. A large group including Bromelia serra are all tank epiphytes; Bromelioideae with tanks also often have flowers with asymmetric sepals and porate pollen (Schulte & Zizka 2008; Schulte et al. 2009). For relationships in the Neoregelia area, see Santos-Silva et al. (2017: 101 morphological characters). Leme et al. (2021: 5 nuclear and chloroplast markers) concentrated on relationships in the nidularoid complex, Neoregelia and the subgenera ofAechmea as they clarified the position of N. subgenus Hylaeacium. Leme et al. (2022) examined relationships in the nidularoid complex carrying out both molecular and moecular+morphological analyses; Krenakanthus, described by them as a new genus, was in fact embedded in Orthophytum in the molecular analysis (weak support), but it was sister to that genus, although again with weak support, only in the joint analysis.
Hechtioideae. There is a fair bit of structure along the spine in a combined (morphology, chloroplast, nucleus) analysis of Hechtia, with the H. tillandsioides complex being sister to the rest of the genus (Ramírez-Morillo et al. 2018).
Weising et al. (2011) outline phylogenetic relationships within Pitcairnioideae. Dyckia was monophyletic, but its internal relationships showed little resolution, and Encholirium was paraphyletic at its base (Krapp et al. 2014; see also Pinangé et al. 2017: still no support along the backbone). Schütz et al. (2016) found that Deuterocohnia did not always appear to be monophyletic, the particular results obtained depending on the markers analysed. A morphological analysis of Pitcairnia yielded little resolution of relationships (Saraiva et al. 2015). The Encholirium-Dyckia-Deuterocohnia complex showed varying, albeit poorly-supported, relationships in morphological, molecular and combined analyses (Moura et al. 2019), and Gomes-da-Silva et al. (2019) returned to this problem.
Puyoideae: Puya, the only genus in the subfamily, has little well-supported phylogenetic structure along the backbone of (Jabaily & Sytsma 2010: morphological study of Puya subgenus Puya; Hornung-Leoni & Sosa 2008; Schütz et al. 2016: somewhat different relationships; Schulte et al. 2011).
For phylogenetic relationships in Tillandsioideae, see Barfuss et al. (2004, 2005, 2016: last two extensive discussion about morphology), Granados Mendoza et al. (2017) and Gomes-da-Silva and Souza-Chies (2018); [Catopsis + Glomeropitcairnia] are sister to the rest of the subfamily - [Vriesieae + Tillandsieae] - and support is strong. Relationships within the Tillandsia group are being reorganized (e.g. Barfuss et al. 2011; Granados Mendoza et al. 2017), and Donadío et al. (2015: morphology, 2022) looked at some relationships within Tillandsia itself. Vera-Paz et al. (2023) focused on relationships in the large subgenus Tillandsia (103/270 species sampled; plastome analyses) and obtaining a fair bit of resolution; Goudaea, Lutheria, etc., are not monophyletic. Alcantarea is close to or embedded within Vriesea (Versieux et al. 2012), a sister group relationship being found by Gomes-da-Silva and Souza-Chies (2018: good sampling, inc. 99 morphological characters), but using morphological characters, Gomes-da-Silva et al. (2012) suggested that Vriesea was not monophyletic. Kessous et al. (2019) also looked at relationships around Vriesea. Loiseau et al. (2020: 108/231 species, genome skimming) noted conflict between plastome, chondrome and nuclear compartments here, perhaps hybridization; V. drepanocarpa was sister to the rest of the genus, but branch lengths tended to be short.
Classification. The family was monographed quite recently by L. B. Smith and Downs (1974, 1977, 1979) although the supraspecific groups that they recognized are now very much dated. The subfamilial classification of Givnish et al. (2008a) is followed here; see also the World Checklist of Monocots and Gouda et al. (2023 onwards). Barfuss et al. (2005) provided a tribal classification of Tillandsioideae, but that now needs re-evaluation.
Generic limits need much attention. Escobedo-Sarti et al. (2013) estimated that only 14/58 of the genera with two or more species that they included in their analysis were monophyletic. Genera like Puya (Givnish et al. 2011a), Tillandsia (Granados Mendoza et al. 2017; Gomes-da-Silva & Souza-Chies 2018), etc., are para/polyphyletic. There is a generic classification of Tillandsioideae in Barfuss et al. (2016, but c.f. Gomes-da-Silva & Souza-Chies 2018, esp. Vriesea). Gomes-da-Silva et al. (2019) expanded the limits of Dyckia (Pitcairnioideae). Leme et al. (2017, esp. 2022) looked at generic limits around Bromelioideae-Cryptanthus - it will be interesting to see how the newly-described genera (?premature, ?unnecessary) fare. Indeed, as noted above, Aechmea in particular is hopelessly poly/paraphyletic (Schulte et al. 2009; Sass & Specht 2010; Evans et al. 2015; Aguirre-Santoro et al. 2016) and generic limits in Bromelioideae are in general unclear (Horres et al. 2008; Evans et al. 2015; Bratzel et al. 2022), however, Aguirre-Santoro (2017) adjusted the limits of Ronnbergia and Wittmackia. This is another area where it will be best to have a much more detailed (nuclear) phylogeny before describing more genera/naming clades.
Botanical Trivia. Tank bromeliads may contain as much as 50,000 litres of liquid/hectare (Fish 1983), and there may be up to 45 litres of water in a single plant of Vriesea imperialis (Frank 1983).
[Rapateaceae [[Thurniaceae [Juncaceae + Cyperaceae]] [Mayacaceae [[Eriocaulaceae + Xyridaceae] [Restionaceae [Flagellariaceae [[Joinvilleaceae + Ecdeiocoleaceae] Poaceae]]]]]]]: little oxalate accumulation; endosperm nuclear, embryo broad, ± undifferentiated.
Age. Givnish et al. (2004a) suggested that the age of this node is ca 87 My; the age in Givnish et al. (2000) was ca 62 Ma, in Tank et al. (2015: Table S2) around 84.2 Ma, and in Magallón et al. (2015) ca 97.6 Ma.
Chemistry, Morphology, etc.. For oxalate accumulation, see Zindler-Frank (1976); I do not know about oxalate accumulation in Xyridaceae and Eriocaulaceae (the latter has calcium oxalate crystals, at least), the small families in the Poacaeae clade and some of the infrafamilial groupings in Restionaceae.
The embryo is usually small, little differentiated and rather broad (but c.f. Juncaceae, Cyperaceae, Poaceae). Thus Malcomber et al. (2006) described the embryo of Joinvilleaceae and Ecdeicoleaceae as being undifferentiated, embryos of Restionaceae-Centrolepidoideae also seem to be undifferentiated (Hamann 1975), those of other Restionaceae, largely undifferentiated (Linder et al. 1998), of Mayacaceae, undifferentiated (Stevenson 1998), and those of Eriocaulaceae, "poorly differentiated", or with "no exomorphological differentiation" (Stützel 1998); see also C. C. Baskin and Baskin (2018).
RAPATEACEAE Dumortier, nom. cons. - Back to Poales
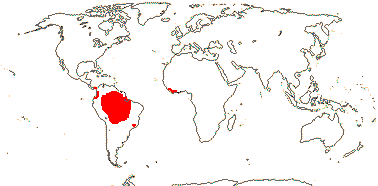
Plants monopodial, rosette-forming; plants Al-accumulators; root endodermis much thickened; vessels in leaf?; aerenchyma +; (culm vascular bundles amphivasal); cuticular wax with globules or wax 0, stomatal guard cells dumbbell shaped; epidermal SiO2 bodies +; leaves (spirally) two-ranked, sheath open, asymmetric and conduplicate, axillary uniseriate hairs + [slime-secreting]; inflorescence scapose, axillary ["subterminal"], capitate, subtended by ± spathaceous bracts longer than head, units cymose, with several basal "bracteoles", sessile, flowers single; K ± rigid, C basally connate, clawed, fugaceous; A basally connate or not, adnate to C or not, wall with three layers [epidermis, endothecium, tapetum - Reduced type], anthers dehiscing by pores/short slits, endothecium in upper half of anther only, cells with spiral thickenings (0); microsporogenesis simultaneous [tetrads tetrahedral]; placentation often apically parietal, style +, stigma capitate/punctate; ovules apotropous, micropyle bistomal, suprachalazal area ± massive, placental obturator +, with hairs; chalazal appendage +, exo- (and endo)testa with SiO2, endotestal cells with U-shaped thickenings, cuticular layer between testa and tegmen, tegmen tanniniferous; suspensor 0; x = ?; hypophyll with median sheath lobe, no collar or rhizoids, primary root at most short/0.
16[list - to tribes]/94. Tropical South America, esp. the Guianas, West Africa: three subfamilies below (map: from Givnish 2004a).
Age. Crown-group Rapateaceae are dated to ca 79 Ma (Janssen & Bremer 2004: note topology); ages in Givnish et al. (2000) and Givnish et al. (2004a) are substantially different, being ca 32 Ma and (44.6-)40.8(-33.8) Ma respectively; ages in Bouchenak-Khelladi et al. (2014b), at (55.7-)36.3(-28) Ma, are more in line with the latter.
1. Rapateoideae Maguire
Epidermal SiO2 bodies +; supernumerary axillary buds +; leaf blade (ventralized isobifacial [oriented edge on to the stem]); (inflorescence bract single, adnate to inflorescence - Spathanthus); K contorted, C ± open; anthers apically ± narrowed (and curved [= "cochleariform appendage"]), pore/cleft 1(2); middle layer of anther wall persistent; pollen grains with encircling aperture/bisulcate; septal nectaries 0; ovule 1/carpel, ± basal, (only 1G fertile - Spathanthus), outer integument 5-12 cells across; hilar scar long; seeds ovoid-oblongoid, (with papillate appendage/wing), longitudinally striate or not; (endotegmen massively suboliquqely palisade - Cephalostemon); n = 26, chromosomes very small.
3/21: Rapatea (9). The Guianas to Bolivia and the Matto Grosso.
Age. Crown-group Rapateoideae can be dated to ca 38 Ma (Givnish et al. 2004a).
[Monotremoideae + Saxofridericioideae]: (inflorescence 2 or more/axil); middle layer of anther wall ephemeral.
Age. The age of this node is around 29 Ma (Givnish et al. 2004a).
2. Monotremoideae Givnish & P. E. Berry
Vessels with simple perforation plates; inflorescence (± sessile - Maschalocephalus), bracts at most as long as head, spikelets ("pedicellate" - Windsorina); anther apex narrowed, sterile; pollen grains monosulcate; ovule 1/carpel, ± basal, outer integument 4-6 cells across, nucellar epidermis vertically elongated, (with anticlinal divisions [= nucellar cap]); antipodal cells several; seeds ovoid-oblongoid, white-granulate [muriculate], with flattened appendage, striate; testa multi-latered; n = 11.
4/8: Monotrema (5). Guiana, upper Rio Negro in Colombia and Venezuela, Maschalocephalus dinklagei West Africa, from Guinea to the Ivory Coast.
Age. Crown Monotremoideae are only some 7.3 Ma old (Givnish et al. 2004a).
3. Saxofridericioideae Maguire
Inflorescence bracts (± connate), enclose infloresecence; septal nectaries 0, intra-ovarian trichomes +; ovules 2-many/carpel.
9/54. N. South America, Panama.
Age. The age of crown-group Saxofridericioideae is ca 15 Ma (Givnish et al. 2004a).
3A. Saxofridericieae Maguire
(Leaf with pseudopetioles, ligules/auricles); inflorescence (1-flowered), bracts (pierced by the flowers as they develop), (0); A free, basal part of anthers somewhat concertina-like, pores/slits 1-2; pollen grains monosulcate; outer integument 2-4 cells across, (micropyle zig-zag), nucellar epidermis vertically elongated, (with anticlinal divisions [= nucellar cap]), suprachalazal area not massive [Stegolepis]; seed wing 0, hilar scar not long; n = ?
6/46: Stegolepis (30+). The Guyana Highlands, Colombia, Panama. Photo: Epidryos Habit © A. Gentry, Stegolepis Flower © G. Davidse.
3B. Schoenocephalieae Maguire
C not longer than K (longer - Kunhardtia); anther (2-locular), surface muriculate, pores 2-4; ?pollen grains; ?embryology; ?seed; n = ?
3/8: Schoenocephalium (5). Guyana Highlands and surrounds, Colombia.
Evolution: Divergence & Distribution. The ancestor of Maschalocephalus dinklagei, the only African representative of the family, probably arrived there by long distance dispersal 7.6-6.9 Ma (Givnish et al. 2004a: much information on diversification).
Ecology & Physiology. Rapateaceae often grow on poor/waterlogged soils, white sand, or similar habitats (Stevenson et al. 1998a). G. H. H. Tate noted of some Stegolepis on Mt Duida that their leaves "reflect the blue of the sky like water does" (in Gleason 1931: p. 333).
For dumbbell-shaped stomata here and in other monocots and the speed of guard-cell movement, see Franks and Farquhar (2006).
Photonic structures, reflecting helicoid structures in the cell wall, are known from some taxa in Saxofridericioideae-Saxofridericieae; they produce a distinctive blue iridescence of the leaves of plants with them (Lundquist et al. 2024).
Pollination Biology & Seed Dispersal. Septal nectaries may occur only in Monotremoideae, but there are also reports of hummingbird pollination in other genera (Stevenson et al. 1998a); Vogel (1981) was not sure if there were nectaries in the family and Tiemann (1985) did not mention them.
Chemistry, Morphology, etc.. In Saxofridericia the flowers appear to be resupinate (Ferrari & Oriani 2016: Fig. 1A). For the exudate produced by the abundant colleters in the flowers, see Ferrari and Oriani (2016). There is considerable variation in pollen grain size and shape (e.g. Tiemann 1985), but it seems not to be correlated with phylogeny. The ovules are described as being crassinucellate (e.g. Rudall 1997), but in some illustrations (e.g. Tiemann 1985) the nucellar epidermis alone seems to cover the embryo sac. Chalazal appendages are found here, and are formed by periclinal divisions of the nucellar epidermis (Venturelli & Bouman 1988; see also Kuhn et al. 2020).
Some general information is taken from Maguire (1958, 1965) and Stevenson et al. (1998a); for anatomy, see Carlquist (1966a), Ferrari et al. (2014: leaf and peduncle) and Fernández-Lucero et al. (2016: Saxofridericoideae-Schoenocephalieae), for some floral morphology, see Oriani and Scatena (2013), for pollen, see Carlquist (1961), and for embryology, see Stenar (1925).
Phylogeny. Subfamilies and tribes are all well supported at a level of ≥95% bootstrap, although the [Monotremoideae + Saxofridericioideae] clade has only 74% bootstrap support (Givnish et al. 2004a).
Classification. See Givnish et al. (2004a) for an infrafamilial classification; see also the World Checklist of Monocots.
[[Thurniaceae [Juncaceae + Cyperaceae]] [Mayacaceae [[Eriocaulaceae + Xyridaceae] [Restionaceae [Flagellariaceae [Joinvilleaceae + Ecdeiocoleaceae] Poaceae]]]]]]: (isoflavonoids +); cellulose fibrils in the outer epidermal walls of root elongation zone oriented parallel to root axis; (trichoblast cells in vertical files, distal cell smaller); rhizodermal cells dimorphic; pollen grains tricellular; septal nectary 0; ovules lacking parietal tissue.
Age. This node has been dated to (96-)87, 79(-68) Ma (Bell et al. 2010), ca 93.8 Ma by Magallón et al. (2015) and ca 97.6 Ma by Can et al. (2020: Cyp. Po.).
Evolution: Divergence & Distribution. Aside from the inherent interest in understanding the evolution of the very speciose Cyperaceae and Poaceae, there is much variation in ovule morphology, etc., here (e.g. see Oriani & Scatena 2016), but until phylogenetic relationships are sorted out this variation cannot be placed in its proper context. Furthermore, the sampling of the more cryptic characters is - as per normal - poor.
Ecology & Physiology. This clade, along with core Caryophyllales, make up the two major foci of the evolution of C4 photosynthesis (Ehleringer et al. 1997), although whether the origins in Poaceae and Cyperaceae are "really" independent is not known (c.f. the N-Fixing Clade). An extimated 24/62 of the origins of this syndrome in angiosperms - and nearly all the origins in monocots - occur here (Sage et al. 2011).
Genes & Genomes. Graham et al. (2006) found an accelerated rate of change in the chloroplast genes they sequenced in the Poales - but not in the representatives of Bromeliaceae and Typhaceae examined (other genes also show accelerated evolution, see G. Petersen et al. 2006b); S. A. Smith and Donoghue (2008) found a similar pattern. Givnish et al. (2010b) confirmed that the rate of evolution of the whole plastome has markedly increased in this part of the tree compared to that of most other monocots, although Joinvillea and in particular Flagellaria seem to be exceptions (rate slow-down?).
Chemistry, Morphology, etc.. For the orientation of cellulose fibrils in the root, see Kerstens and Verbelen (2002), sampling still poorer. Sampling for the distribution of trichoblast position is also poor, only the larger families here having been sampled, and in other Poales, only Typhaceae are known to have proximal trichoblasts, but only one species of Bromeliaceae (trichoblasts absent) and no Rapateaceae have been sampled... (Clowes 2000).
[[Mayacaceae [Eriocaulaceae + Xyridaceae]] [[Thurniaceae [Juncaceae + Cyperaceae]]]]: flavonoids +; leaves spiral; A basifixed; fruit with persistent K/T; embryo broad; deletions in ORF 2280 region, chloroplast accD and mitochondrial sdh4 genes lost.
Age. The age of this clade is around 86.2 Ma (Magallón et al. 2015).
Evolution: Genes & Genomes. Branch lengths of the ndhF and other genes are notably longer in this part of the monocot tree than anywhere else (e.g. Givnish et al. 2005, 2006b; Saarela et al. 2006).
The distribution of the sdh4 gene deletion (see Adams et al. 2002b) is consistent with the topology of the tree presented by Bremer (2002); for the accD gene and ORF 2280 region, etc., see especially Hahn et al. (1995), Katayama and Ogihara (1996) and M. E. Harris et al. (2013).
[Thurniaceae [Juncaceae + Cyperaceae]] / cyperids: 3-desoxyanthocyanins [1 + 2], luteolin 5-methyln ether +; starch grains pteridophyte-type, amylophilic; air canals + [?= septate aerenchyna], stem solid [?J]; leaves 3-ranked, inflorescence racemose; flowers small [<1 cm across]; P = T, scarious; microsporogenesis simultaneous [tetrads tetrahedral], pollen in tetrads, grains with distal pore [= ulcerate]; style short, branches/stigmatic surface long; ovules anatropous, (outer integument ³3 cells across), parietal tissue +, hypostase +, obturator +; seeds testal-tegmic; 3-nucleotide deletion in chloroplast atpA gene; seedling with phanomer [photosynthetic unifacial cotyledonary hyperphyll], hypocotyl +, radicle +, seedling collar inconspicuous, with rhizoids.
Age. The age for this node is estimated at ca 98 Ma (Janssen & Bremer 2004; Besnard et al. 2009b), or ca 101.4 Ma (Escudero & Hipp 2013).
Evolution: Divergence & Distribution. Elliott et al. (2022b) noted that cyperids with smaller chromosomes tended to have more extensive geographical distributions.
Pollination Biology & Seed Dispersal. Whether or not the families here and to the end of the Poales page form a clade, they represent a major aggregation of wind-pollinated taxa, if with some derived insect-pollinated species (e.g. Ackerman 2000),
Plant-Bacterial/Fungal Associations. Mycorrhizae seem to be infrequent (but c.f. some Cyperaceae), although cluster roots are common, at least in Juncaceae and Cyperaceae. Soudzilovskaia et al. (2020) noted that "multiple families" in "Cyperales" were either non-mycorrhizal or were arbuscular mycorrhizal—non-mycorrhizal.
Genes & Genomes. Smarda et al. (2014) noted that the genome of this group had a very low GC content as well as being very small, at least as compared with Xyris (Xyridaceae) and Poaceae. Indeed, this clade has about the smallest mean chromosome sizes (measured in Mbp) in seed plants, and this includes very appreciably larger sizes in many Schoeneae, Rhynchosporeae, Eleocharideae and in some Juncaceae; mean holoploid genome size is also very small, only those of Brassicaceae, Crassulaceae and Rosaceae being smaller - again, Schoeneae and Eleocharideae have large genomes (Elliott et al. 2022b: see also Figs S2, 3, 11). Mean chromosome size and number are inversely correlated here (Elliott et al. 2022b).
For the plastid atpA gene, see J. I. Davis et al. (2004).
Chemistry, Morphology, etc.. Both Thurniaceae and Juncaceae have basically racemose (polytelic) inflorescence units (Köbele & Tillich 2001); for possible variation in Cyperaceae, see that family. The inflorescences themselves are often more or less scapose. There is variation in the way pollen is arranged in the anther loculi in the whole Cyperaceae—Poaceae area. The plesiomorphous condition is likely to be central, i.e., there are many pollen grains in an anther sporangium and they are often not in contact with the tapetum, but in some taxa around here it is peripheral, and there is a central uniseriate cylinder of grains all of which are in contact with the tapetum (Kirpes et al. 1996) - however, sampling is very poor, hence the sporadic mention of this feature. Tiemann (1985) discussed the embryology of the group, suggesting that it was quite similar to that of Rapateaceae.
Phylogeny. The relationships [Thurniaceae [Juncaceae + Cyperaceae]] have strong support (Givnish et al. 1999; Bremer 2002; J. I. Davis et al. 2004), although Oxychloë was not included. However, Brozová et al. (2022: 3 markers) did not always recover this clade - c.f. their Figs 1 and 3A.
THURNIACEAE Engler, nom. cons. - Back to Poales
Stem ["rootstock"] upright, trunk-forming or not; flavone C-glycosides +; vessel elements with scalariform perforation plates; stem angled, fibre strands +; cuticular waxes as aggregated rodlets; leaf blade margin serrate; T basally connate; endothecial cells with spiral thickening, tapetal cells?; exine granular; styles ± separate, stigmas long; ovules ascending, micropyle bistomal/zig-zag, outer integument ca 3 cells across, inner integument ca 2 cells across, parietal tissue ca 1 cell across, funicular obturator +; seeds arillate; testa of sclerenchymatous fibres and unthickened cells, exotegmen also tanniniferous; endosperm helobial; embryo linear, undifferentiated; n/x = ?
2[list]/4. North east South America, South Africa. Map: inaccurate, see Munro et al. (2001).
Age. Crown-group Thurniaceae are some 33 Ma old (Janssen & Bremer 2004) or (52.9-)26.1(-15.8) Ma (Bouchenak-Khelladi et al. 2014b).
1. Thurnia J. D. Hooker
Roots with large air canals; stem vascular bundles?; some foliar vascular bundles in pairs, abaxial member inverted, fibers/fiber bundles +; inflorescence capitate and involucrate; ventral bundles basally opposite loculi, distally divide and branches fuse with bundles of adjacent carpels; ovule 1/carpel, basal, with a chalazal projection; exotesta with short hairs.
1/3. Colombia, Venezuela, the Guyana region, northern Amazonia. Photo - Thurnia Habit, Inflorescence.
2. Prionium serratum E. Meyer —— Synonymy: Prioniaceae S. L. Munro & H. P. Linder
Stem vascular bundles amphivasal; leaf sheath closed; inflorescence much branched; placentation apically parietal; ovules few/carpel; n = 23, chromosomes ca 1 µm long, genome size [1 C] ca 335 Mbp.
1/1. South Africa. Photo - Prionium - Inflorescence.
Evolution: Genes & Genomes. For the cytology of Prionium serratum, see Baez, Kuo et al. (2020); the chromosomes are monocentric.
Chemistry, Morphology, etc.. Tillich (1994) described the seedlings as being similar to those of Juncaceae.
For more information, see Kubitzki (1998d: general) and Givnish et al. (1999), C. A. Williams and Harborne (1975) for chemistry, Zimmermann and Tomlinson (1968) for stem anatomy, Cutler (1963) for leaf anatomy of Thurnia, A. de L. Silva et al. (2020) for floral morphology/anatomy of Thurnia and Munro and Linder (1999) for that of Prionium.
Thurniaceae are poorly known.
Classification. See the World Checklist of Monocots.
[Juncaceae + Cyperaceae]: luteolin +, SiO2 accumulation common; mycorrhizae 0; flowers protogynous; embryo capitate, relatively poorly differentiated; ?chromosomes holocentric?; chloroplast rpl23 gene absent.
Age. The age for this node is estimated to be ca 88 or ca 100 Ma (Janssen & Bremer 2004; Besnard et al. 2009b); other ages are about 55.2 Ma (Magallón et al. 2015), (74-)61, 55(-43) Ma (Bell et al. 2010), around 86.2 Ma (Escudero et al. 2012b), ca 90.6 Ma (Escudero & Hipp 2013), (100-)96(-88) Ma (Spalink et al. 2016), or an unlikely 39-28 Ma (Wikström et al. 2001, 2004).
Evolution: Divergence & Distribution. The clade [Juncaceae + Cyperaceae] is notably species-rich (Magallón & Sanderson 2001: p. 1773) and it shows nested increases in diversification rates. Juncaceae + Cyperaceae at (77.1-)62.7(-55.2) Ma and in Cyperaceae at the end of the Eocene (55.1-)37.3(-29.9) Ma (Magallón et al. 2018; see also Sanderson & Donoghue 1994). The clade including both sedges and grasses is described as being perhaps seven times more speciose than its animal-pollinated sister clade, the bromeliads (sic) (Kay et al. 2006b: supplement not accessable xii.2012; Kay & Sargent 2009), but comparisons need to be made more locally.
Rocha et al. (2018) drew attention to the similarity in microsporogenesis between members of Juncaceae and Cyperaceae, i.a. noting that the walls of the individual grains of the tetrads had an intine made up of cellulose and polysaccharides - they did not examine pollen of Thurniaceae. Taxa with holocentric chromosomes are common in these families, perhaps especially in Cyperaceae where monocentric chromosomes have not been reliably recorded at all (Elliott et al. 2022b); whether or not they are synapomorphic is unclear. Prior to xi.2020 holocentric chromosomes (= diffuse centromeres) were considered to be an apomorphy for the whole cyperid clade, however, it has recently been found that chromosomes of Prionium serratum (Thurniaceae) are monocentric (Baez, Kuo et al. 2020). Zedek et al. (2020) looked at some species of Cyperaceae, Juncacaceae and Poaceae across an altitudinal transect to see if any correlation between altitude and the development of endopolyploidy caused by UV-B radiation when comparing taxa with holocentric chromosomes (Juncaceae, Cyperaceae) and those with monocentric chromosomes. There was: Endopolyploidy increased with altitude more rapidly in the species with monocentric chromosomes than in those with holocentric chromosomes - in the latter fragmented chromsomes were more likely to behave normally during cell division.
Ecology & Physiology. Juncaceae in particular, but also Cyperaceae, have quite a number of halophytic species (22 and 121 respectively). As in Poaceae, the ability to tolerate salt has arisen several times in both families, ca 8 and 52 times respectively (Moray et al. 2015 - see also Saslis-Lagoudakis et al. 2016).
Juncaceae and Cyperaceae, along with Poaceae, are well known for accumulation large amounts of silica, SiO2, however, the role that Si plays in the plant is unclear (de Tombeur et al. 2022).
Kempe et al. (2013) discuss the role of the leaf sheaths in supporting the stem, particularly the region with the intercalary meristem which has low structural rigidity in contrast to the rest of the internode above; like grasses, these plants have quite slender stems. Sheaths in Luzula showed lower contributions to rigidity than did those in Carex (but one species - Kempe et al. 2013).
Plant-Animal Interactions. Food plants of bugs of the Hemiptera-Lygaeidae-Cyminae and -Pachygronthinae are concentrated here (Slater 1976). Also, most host records (although there aren't that many) of caterpillars of Yponomeutoidea-Ypsolophidae-Glyphipteriginae (on which be peace), a subfamily of leaf mining or stem boring moths with almost 400 species, are from this clade (Sohn et al. 2013).
Plant-Bacterial/Fungal Associations. The distributions of parasitic fungi suggest that Cyperaceae and Juncaceae are close (Savile 1979b); for fungal records on the two families, see A. Tang et al. (2007).
Clavicipitaceous endophytes have been recorded from some genera, they are also found on Cyperaceae, but they are not as common as they are on Poaceae (Clay 1986, 1990; Pazoutová 2003); see also the distribution of the parasitic Claviceps itself (Píchová et al. 2018).
Entorrhiza forms root galls on Juncaceae and Cyperaceae world-wide; it has recently been described as a new fungal phylum perhaps sister to dikarya or basidiomycetes (Bauer et al. 2015).
Chemistry, Morphology, etc.. Karlen et al. (2018) found that Cyperus papyrus and Juncus inflexus had substantially more p-hydroxyphenyl units (H) in their lignin than the other commelinids that they examined. For SiO2 accumulation, see Trembath-Reichert et al. (2015).
Endress (1995b) provides some details of floral morphology.
Phylogeny. Muasya et al. (1998) suggested that Oxychloë (Juncaceae), a cushion plant from Chile, was sister to Cyperaceae, with moderate support, other Juncaceae were paraphyletic, but with poor support, while Prionium was sister to the whole clade and with good support (see also Muasya et al. 2000: sampling in Juncaceae poor). A study by Plunkett et al. (1995) placed Oxychloë within Cyperaceae and its relationships remained unclear (Záveská Drábková et al. 2003), although a position in Juncaceae, near Distichia, also a cushion plant, seems likely (Simpson 1995: morphological data; Roalson 2005; see especially Záveská Drábková & Vlcek 2007). Part of the problem seems to have been caused by the misidentification of the material from which early molecular samples of Oxychloë were obtained (Kristiansen et al. 2005).
JUNCACEAE Jussieu, nom. cons. - Back to Poales
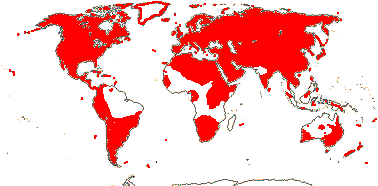
Plant glabrous (hairs + - Luzula); endodermoid layer +; culm bundles in rings; (vessel elements with scalariform perforation plates); stem rounded; SiO2 bodies 0 (SiO2 sand - Juncus); leaves ([spirally] two-ranked), terete-unifacial or isobifacial, margins usu. entire, (sheath closed - Luzula), (auricles lacerate-fimbriate - Oreojuncus), (ligule +); (flowers single); (flowers 2-merous; imperfect); (T large - Marsippospermum), outer T with 1[Luzula]/3[Juncus] VBs, inner T with 1 VB; (A 3); tapetal cells uninucleate; pollen grains central in loculus, aperture obscure; G shortly stipitate, (placentation ± parietal), (styles separate), (branches spirally twisted); ovules 1 basal to many central/carpel, micropyle often bistomal, (outer integument 4 cells across), placental obturator [hairs] and hypostase +/0; seed (?arillate), with (mucilaginous) exotesta, endotesta, cuticular layer; endosperm helobial; n = 3, 6[esp. Luzula]-20[esp. Juncus], etc., x = 5, (chromosomes holocentric - Luzula), genome size [1 C] (0.047-)0.714(-0.786) (4.25 pg - L. elegans) pg; phanomer (0), apical meristems of shoot and root developing late [Juncus], primary root long, well developed.
8[list]/442: Juncus (300: paraphyletic - Agathryon (107), Alpinojuncus (74), Verojuncus (37), Juncinella (30)), Luzula (115). Worldwide, esp. Andes (3 endemic genera), S. South America-New Zealand (2 genera). Map: see Vester (1940), Hultén (1961), Frankenberg & Klaus (1980), Balsev (1996), Australia's Virtual Herbarium (consulted xii.2012) and FloraBase (consulted xii.2012). [Photo - Juncus Inflorescence, Luzula Flower.]
Age. Crown group Juncaceae are some ca 74 Ma (Janssen & Bremer 2004) or (87-)71.8(-51) Ma (Bouchenak-Khelladi et al. 2014b).
Evolution: Pollination Biology & Seed Dispersal. For the insect-pollinated flowers of Juncus allioides, see Barrett (2013) and Huang et al. (2013).
The seeds of Luzula are often myrmecochorous (Lengyel et al. 2010), and myxospermy is also reported both here and in Juncus (Western 2012).
Ecology & Physiology. Substantial amounts of phosphorus moved from mucoromycote fine root endophytes associated with Juncus bulbosus to the plant, while movement of phosphorus was relatively less important (Hoysted et al. 2019).
Roots of saltmarsh Juncus, along with those of Spartina (= Sporobolus sect. Spartina), are associated with Celerinatantimonas diazotrophica, the closest relative of Candidatus C. neptuna, known to fix N in the sea-grass Posidonia (Mohr et al. 2021).
Plant-Bacterial/Fungal Associations. The ergot alkaloid-synthesising fungus Claviceps can grow on Juncaceae (Píchová et al. 2018).
Vegetative Variation. Yamaguchi et al. (2010) show how the terete and laterally flattened (= ventralized isobifacial) leaves in Juncus are fundamentally similar, being abaxialized and unifacial in both cases, the laterally-flattened leaves also expressing the DL gene that is responsible for "midrib" formation in normal bifacial monocot leaves. Indeed, species with terete and those with laterally-flattened leaves can cross. However, in J. prismatocarpus, at least, gene expression patterns suggest that DL was not involved in the flattening in that species, rather, there was a diffuse 'thickening meristem' that was most active adaxially (Yin & Tsukaya 2018). Nukazuka et al. (2021) noted differences in where auxin was concentrated when comparing J. wallichianus (terete) and J. prismatocarpus. For unifacial leaves, which may be laterally flattened, see also Yamaguchi and Tsukaya (2010) and Nakayama et al. (2013, 2022). Within Iridaceae in particular there is also a variety of leaf morphologies that are variants of ventralized isobifacial.
Genes & Genomes. Záveská Drábková (2013) summarized what was known about chromosome numbers here, their evolution, etc.; diffuse centromeres are restricted to Luzula. For holocentric chromosomes/diffuse centromeres in Juncaceae, certainly not detected in Juncus, see also Guerra et al. (2019). In Luzula, at least, events in meiosis are reversed, homologous non-sister chromatids initially being held together by chromatic threads and separating only in anaphase II (Heckmann et al. 2014; Guerra et al. 2019).
There is a negative correlation between chromosome numbers and the amount of nuclear DNA (Záveská Drábková 2013). For a possible genome duplication in Juncus, see McKain et al. (2016a).
Chemistry, Morphology, etc.. In Luzula stamens are opposite individual tepals (Payer 1857), the median tepal in the outer whorl may be adaxial, and a variety of bract structures are associated with the flower (Eichler 1874). Indeed, inflorescence morphology may repay investigation, for example, Záveská Drábková (2010) suggested that that both cymose and racemose inflorescences and flowers with two and no bracteoles were to be found in Juncus.
Some information is taken from Balslev (1998: general); for anatomy, see Cutler (1969), for some chemistry, see C. A. Williams and Harborne (1975), and for floral morphology and anatomy, see Oriani et al. (2012) and Shamrov et al. (2012), for embryology, etc., of some Juncus and Luzula, see Laurent (1904), and for seedlings, see Tillich (1985) and Silva and Oriani (2022: J. tenuis, also seed).
Phylogeny. For a phylogeny, with Juncus perhaps being paraphyletic, see Záveská Drábková et al. (2003), Roalson (2005) and especially Záveská Drábková (2010). Záveská Drábková and Vlcek (2009) found that Juncus trifidus and J. monanthos were separate from other Juncus and sister to the rest of the family (recognized as Oreojuncus - Záveská Drábková & Kirschner 2013); these authors also noted that five genera were embedded in Juncus from the southern Hemisphere. Brozová et al. (2022: I follow Fig. 1 below, other topolgies may be somewhat different) looked at the variation in two plastid (rbcL and trnL-trnF) and the nuclear ITS genes, although there was much homoplasy in the latter. [Juncinella + Oreojuncus], the former including J. capitatus and perhaps J. dregeana - ITS only, may be sister to the rest of the family. Luzula elegans was sister to the rest of the genus, within which classical infrageneric groupings were a mess. Most previous infrageneric groupings within Juncus did not hold up, but a number of clades were evident - including one made up of Rostkowia, Oxychloë, etc., the Southern Hemisphere Clade, kept separate in the classification - see below. Further analyses of relationships in Juncaceae would semm reasonable; Elliott et al. (2022a) also discuss this paper.
Classification. For a family monograph, see Kirschner et al. (2002a-c); see also the World Checklist of Monocots. However, generic limits - bar those of Luzula - seemed to be in a mess (Záveská Drábková 2010). To clear this up, Brozová et al. (2022) divided Juncus into seven genera - and placed the whole lot into supragenus Juncus; this was in part because the Southern Hemisphere Clade, including genera like Marsippospermum and Oxychloe, were embedded in Juncus old style, but since Brozová et al. (2022) thought that they should be kept distinct, they were not included in supragenus Juncus. Procków and Záveská Drábková (2023) described six more new genera in the Juncus area, but acceptance of changes surely depends on further analyses...
CYPERACEAE Jussieu, nom. cons. - Back to Poales
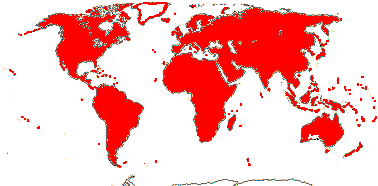
Aurones, flavonoid sulphates, flavone C-glycosides, tricin, fructan sugars accumulated as kestose and isokestose oligosaccharides [levans and inulins]; (monocot secondary thickening +); (also thick-walled sieve elements); stems angled; chlorenchyma cells lobed; hairs/prickles unicellular; stomatal guard cells dumbbell shaped, cuticular waxes as aggregated rodlets; leaves (two-ranked, tetrastichous, spiral), sheath closed, (margins serrate); inflorescence units spikelets or heads, axis terminating in a spikelet; flowers usu. monosymmetric by reduction; T variously reduced, with 1 trace; A (connate); endothecial cells with spiral thickenings; tapetal cells bi-multinucleate; microsporogenesis simultaneous; pollen as pseudomonads [?all: disintegration of three nuclei after meiosis], grains (2-celled), pyriform, thin-walled, (pontoperculate); G initiated as an annular primordium, (gynophore +); ovule one/flower, basal, parietal tissue to 4 cells across, micropylar/funicular obturator +, with hairs; fruit an achene, (with bristles, etc.); testa and tegmen thin, not mucilaginous, ± coalescent, exotesta with SiO2 bodies, tanniniferous [?level], other testal layers fibrous, endotegmen not tanniniferous; endosperm with micropylar and chalazal haustoria, embryo (mid-sized); x = 5, chromosomes holocentric [?level], nuclear genome [1 C] (0.057-)757(-10.06) pg; 3 bp 5.8S nrDNA insertion, rps14 gene to nucleus, pseudogene remaining in mitochondrion; seedling (mesocotyl +), coleoptile +, appendix at apex [median lobe of sheath] (0), primary root develops late, soon degenerates.
95[list, to tribes]/5,690. World-wide. Map: see Hultén (1961), Vester (1940) and Australia's Virtual Herbarium (consulted xii. 2012). [Photo - Carex Carpellate Inflorescence, Eleocharis Spikes.]
Age. Crown-group Cyperaceae have been dated to ca 76 Ma or ca 52 Ma (Janssen & Bremer 2004; Besnard et al. 2009b). Escudero et al. (2012b) suggest a crown age of (87.6-)83.7(-78.5) Ma, while (85.6-)82.6(-75.9) Ma is the age in Escudero and Hipp (2013), (87.6-)82.3(-73.7) Ma in Bouchenak-Khelladi et al. (2014b), (89-)85(-77) Ma in Spalink et al. (2016), (95-)85(-74) Ma in Léveillé-Bourret et al. (2017a) and (115.3-)87.8(-65.3) Ma in Larridon et al. (2018).
The fossil Volkeria messelensis, some 47 Ma, has been placed as stem group Mapanioideae (Iles et al. 2015) and so would provide a minimum age for the family.
Includes Abildgaardieae, Bisboeckelereae, Bolboschoeneae, Calliscirpeae, Cariceae, Carpheae, Chrysitricheae, Cladieae, Cryptangieae, Cypereae, Cyperoideae, Dulicheae, Eleocharideae, Fuirineae, Hypolytreae, Khaosokieae, Mapanioideae, Pseudoschoeneae, Rhynchosporeae, Schoeneae, Schoenoplecteae, Scirpeae, Sclerieae, Sumatroscirpeae, Trichophoreae.
1. Mapanioideae C. B. Clarke
Rhizomes with amphivasal vascular bundles; SiO2 bodies wedge- or bridge-shaped, also conical?; stomata often tetracytic; leaves with pseudopetiole + blade, transverse veins often prominent, ligule 0; inflorescence units spicoids +, subtended by glume-like bracts and aggregated to spikelet-like structures, with paired lateral keeled bracts, and vertical bracts; A 2, lateral, (additional bracts subtending A/interior to A); pollen monoaperturate, sexine thick; carpelate flowers: G [2]; pericarp 10-15 cells across, SiO2 deposition in inner layers, as grains or amorphous.
11/185. Largely tropical.
Age. Crown-group Mapanioideae have been dated to a mere ca 33 Ma (Escudero et al. 2012b), while other estimates are ca 49 Ma (Spalink et al. 2016), (67-)49.2(-33.5) Ma (Escudero & Hipp 2013) and (ca 60-)45.9(-28.3) Ma (Larridon et al. 2018).
1A. Chrysitricheae Nees
(Leaves reduced to sheath, sheath ± open); floral development centrifugal; spicoid with lateral scales keeled (yes, no); lateral A 2 (0, 2); inner scales 2 (7-many); inner A 1-6 (7-many); microsporocytes peripheral in loculus, pollen with distal ulcus, (4 lateral apertures), sexine (rugulate-)granulate; G [2 (2, 3)].
6/14: Chorizandra (6). Tropical, mostly southern hemisphere, but N. to southern China.
1B. Hypolytreae Wight & Arnott —— Synonymy: Mapaniaceae Shipunov
Rhizomes with collateral vascular bundles; (petiole + - Mapania-type); floral development centripetal; spicoid with lateral scales keeled (yes, sometimes no; connate); lateral A 2 (1 [rare], 2); inner scales 0, 1, 4 (1-9); inner A 0-1 (0-1, 7-9; anthers lacking phenolic idioblasts, microsporocytes central/random in loculus, they and pseudomonads ± central; pollen spheroidal, covered in lipids, thick-walled, sexine usu. microreticulate; G [2, 3 - Paramapania (2, 3)]; micropyle bistomal, zig-zag, outer integument 3 calls across [Hypolytrum].
5/171: Hypolytrum (65), Mapania (65). Tropical, inc. oceanic islands.
2. Cyperoideae Beilschmied
Fine roots dauciform; (velamen +); SiO2 bodies smooth, conical, with pointed apices, attached to cell walls; ligule +/0; inflorescence branched, with spikelets; (flowers monosymmetric by reduction); P/T (0-)3-6(-many) [= scales, bristles], vascularized or not, (connate;=), (lateral outer P/T opposite), (inner P/T clawed); A usu. 3, opposite outer P/T whorl; anthers with phenolic idioblasts, microsporocytes peripheral in loculus, pollen grains with 2 or more lateral apertures; (G [2], superposed, ?less often collateral); (style base persistent in fruit), pericarp 4-8(-20) cells across, SiO2 bodies cone-shaped, in exocarp cells, endocarp fibrous; suspensor 1-few-celled, uniseriate; embryo well differentiated; n = 3 and up.
85/5,500. Worldwide, but esp. N. Temperate.
Age. Diversification within Cyperoideae is estimated to have begun ca 77 Ma (Escudero et al. 2012b), (85.6-)78.4(-70.9) Ma (Escudero & Hipp 2013), (105.7-)81.4(-62.7) Mys (Larridon et al. 2018), or ca 83 Ma (Spalink et al. 2016).
2A. Trilepideae Goetghebeur
Perennial, tufted/mat-forming (rhizomatous) or ± shrubby [stem covered by leaf sheaths and roots]; (stomata tetracytic); leaves (2-ranked, sheath open - Coleochloa), blade deciduous (not), ligule +, fimbriate (0), contraligule +; plant monoecious; spikelets very small, glumes 2-ranked; P/T 3 fimbriate scales; staminate flowers: A (1-)3; pollen grains (spheroidal, monoporate - Coleochloa); embryo Trilepis-type.
4/16: Coleochloa (8). Guyana, southeast Brazil, Africa and Madagascar.
2B. Cladieae Nees - Cladium P. Browne
Perennial, rhizomatous/stoloniferous; leaves with inverted vascular bundles; ligule 0; plant andromonoecious; glumes spiral; P/T 0; A 2; pollen ?spherical, monoporate; fruit almost drupe-like, with thick corky beak, scar basal, broadly disciform [= how the fruit sat on the flower]; embryo Juncus-type [embryo small, poorly differentiated, obovate, root cap basal, poorly developed, leaf primordia 0].
1/3. ± Cosmopolitan.
[Bisboeckelereae + Sclerieae]: "hypogynium" + [= disciform, fimbriate, structure below flower, with three large rounded lobes, etc.].
Age. The age of this clade is some (60.0-)55.3(-50.4) Ma (Larridon et al. 2020b).
Annuals, tufted/rhizomatous/stoloniferous perennials; foliar air cavities; (petiole + - Mapania-type), ligule 0, (contraligule +); plant monoecious; inflorescence often scapose; spikelets (neuter), (pseudo)terminal, 2-(3-)ranked, glumes 1-10; staminate flowers: glume 1; A 1; carpellate flowers: enveloped by (connate) glumes; hypogynium trilobed (inconspicuous); embryo Carex/Schoenus/Fimbristylis-type.
4/28: Diplacrum (10), Calyptrocarya (8). Central and South America, Diplacrum pantropical.
2D. Sclerieae Wight & Arnott - Scleria P. Bergius —— Synonymy: Scleriaceae Berchtold & J. Presl
Annuals to large tufted (rhizomatous/scrambling) perennials; leaf sheath (winged on angles), with marginal wings with inverted vascular bundles, (ligule + (hairy)), contraligule + (0); plant usually monoecious; spikelets 2-ranked; staminate flowers: A3; fruit: cupule +, 3-lobed, usu. detaching from fruit, hypogynium various, often 3-lobed; achenes/nutlets bony, white or discoloured; embryo Fimbristylis-type.
1/258. Tropical to warm Temperate, not northern Africa, Arabia, Europe. Distribution: Larridon et al. (2020b: Fig. 4).
Age. Crown-group Sclerieae are some (54.1-)43.4(-33.9) Ma (Larridon et al. 2020b).
[Carpheae [Cryptangieae [Schoeneae [Rhynchosporeae [Dulichieae etc. + Eleocharideae etc.]]]]]: ?
2E. Carpheae Semmouri & Larridon
Annual [Trianoptiles], perennial, caespitose; (foliar air cavities - Carpha); (petiole + - Oreobolus-type), ligule 0; inflorescence ± scapose or not; glunes 2-ranked; flowers surrounded by wings of apical glume[?], usu. perfect; P/T bristles plumose or not; anthers greenish yellow/greyish, apiculate; (G. stipitate); embryo Carpha-type, rhomboid to top-shaped, scutellum tapered, notch below root cap.
3/18: Asterochaete (11). Scattered: S.W. Cape, African mountains, Madagascar and the Mascarenes, S.E. Australia, New Zealand, New Guinea, Chile - not Japan.
[Cryptangieae [Schoeneae [Rhynchosporeae [Dulichieae etc. + Eleocharideae etc.]]]]: ?
2F. Cryptangieae Bentham
Perennial, rhizomatous (± shrubby); ligule +, (contraligule +); plant mon-/dioecious; spikelets unisexual, glumes usu. ± spira;; P/T 3 fimbriate scales (0); staminate: spikelets several-flowered; flowers: A 1-9; carpelate: usu. 1-flowered; style and stigma reddish/pinkish; achenes triangular, hypogynous scales persistent, fimbriate, reduced, on sides of achenes (0 - Exochogyne); embryo Juncus/Carex-types.
6/47: Cephalocarpus (18), Lagenocarpus (15). Cuba, Central and South America.
[Schoeneae [Rhynchosporeae [Dulichieae etc. + Eleocharideae etc.]]]: ?
2G. Schoeneae Dumortier - plus 8 subtribes.Annuals, tufted/rhizomatous/stoloniferous perennials; (stomata tetracytic); leaves (2 ranked, sheath open - Oreobolus), petiole + - Oreobolus-type\, ?ligule; (concaulescence of flower and spikelet, so apparently cymose - Schoenus), glumes spiral (2-ranked); flowers perfect, surrounded by wings of glume above; P/T bristles 0-6, (scales); anthers yellow to red; embryo Carex/Schoenus(Helothrix)-types; 1 C genome 205.5-10,381 Mbp.
Age. The age of this clade is (74.9-)57.3(-42.8) Ma (Larridon et al. 2018).
25/420 (8 subtribes): Schoenus (149), Lepidosperma (>80, ca 200 undescribed), Machaerina (55), Gahnia (>41), Tetraria (39), Pleurostachys (30). ± Worldwide, but mostly southern, esp. Old World, Malesia to the Antipodes.
[Rhynchosporeae [Dulichieae etc. + Eleocharideae etc.]]: ?
2H. Rhynchosporeae Wight & Arnott - Rhynchospora Vahl
Annuals, tufted/rhizomatous/stoloniferous perennials;(C4 photosynthesis); ligule 0; flowers perfect/plant andromonoecious; P/T bristles, 0-6(-10); A (1-03(-12), anthers with phenolic idioblasts; style 2-fid, base enlarged, persistent in fruit [= tubercule]; exotesta tanniniferous; embryo Carex-type, top-shaped, root cap (sub-)basal, leaf primordium lateral.
1/399. Cosmopolitan, esp. the Americas.
Age. Crown-group Rhynchosporeae are estimated to be (69-)56(-40) Ma (Buddenhagen et al. 2017).
[Dulichieae etc. + Eleocharideae etc.]: flowers perfect; plastome with six genes missing [see C. Lee et al. 2023 - ?level].
[Dulichieae [Khaosokieae [Calliscirpeae [Scirpeae [Trichophoreae [Sumatroscirpeae + Cariceae]]]] / SDC clade / Dulichieae etc.: plant perennial; stem rounded/obtusely angled; leaves ligulate; spikelets spiral on rhachis, glumes spiral, all bracts/glumes fertile; P/T bristles with ?direction barbs; anthers yellow to red; anticlinal walls of epicarp ± straight; embryo Carex-type, plumule lateral, root cap basal.
Age. The age of this clade is (57-)50(-43) Ma or ca 50.7 Ma (Léveillé-Bourret et al. 2017a and 2019 respectively).
2I. Dulichieae W. Schultze-Motel
Plant long-rhizomatous; spikelets 2-ranked, laterally compressed, 3-7-flowered; glumes 2-ranked; prophylls fertile; P/T bristles (1-)3-8(+); G apiculum longer than wide; spikelets break up into 1-flowered units [Dulichium]; fruit with long, narrow beak.
3/5: Blysmus (3). North Temperate-Boreal.
[Khaosokieae [Calliscirpeae [Scirpeae [Trichophoreae [Sumatroscirpeae + Cariceae]]]]: spikelets usu. with ≥10 flowers, flowers spiral, prophylls sterile; P/T bristles with antrorse barbs.
2J. Khaosokieae Léveillé-Bourret & J. R. Starr - Khaosokia caricoides D. A. Simpson et al.
Rhizomatous perennial; stem triquetrous/crescentic; plant monoecious; inflorescence scapose; basal glume smaller than others, basal glumes sterile, glumes lacking nerves: P/T bristles 7-9(-12); staminate flowers: A 3; ?embryo.
1/1. South Thailand.
[Calliscirpeae [Scirpeae [Trichophoreae [Sumatroscirpeae + Cariceae]]]: spikelets spiral; glumes spiral.
2K. Calliscirpeae Léveillé-Bourret & J. R. Starr - Calliscirpus C. N. Gilmour, J. R. Starr & Naczi
Plant long-rhizomatous; ligule ciliate; inflorescence capitate; glumes spiral, all fertile; P/T bristles 6(-12); A 3, white; bristles elongating in fruit, forming cottony mass.
1/2. California and adjacent Oregon.
[Scirpeae [Trichophoreae [Sumatroscirpeae + Cariceae]]]: ?
2L. Scirpeae Dumortier —— Synonymy: Scirpaceae Borkhausen
Tufted/rhizomatous perennial; ligule (ciliate), (0); inflorescence scapose or not; (basal glumes sterile); P/T bristles very reduced-6 (10-many - Eriophorum); anthers yellow; (bristles elongating in fruit - Eriophorum); anticlinal walls of epicarp sinuous; embryo Fimbristylus/Schoenus-type, with (sub)basal plumule and sublateral root cap.
6/73: Scirpus (47), Eriophorum (18). ± Cosmopolitan, scattered, South America only Andean, Malesia to Australia.
[Trichophoreae [Sumatroscirpeae + Cariceae]]: (Ustilaginales-Anthracoidea parasitic); ligule +; inflorescence scapose.
Age. The crown-group age of this clade is (41.9-)38.8(-36.1) Ma (Léveillé-Bourret et al. 2019)
2M. Trichophoreae Léveillé-Bourret & J. R. Starr - Trichophorum Persoon
Tufted/rhizomatous perennials; leaf blade shorter than oe equal to sheath (reduced to a mucro), to 1(-7.5) mm across; inflorescence with 1 spikelet (few to many - compound corymb); basal glumes sterile; flower number variable; P/T bristles (1-)6(-14), (scales - Oreobolopsis), (0), barbs near apex, blunt; A 1-3, anthers yellow.
1/19. Esp. North Temperate to Arctic, also Andean South America to Argentina.
Age. Crown-group Trichophoreae are (17.5-)14.3(-11.3) Ma (Léveillé-Bourret et al. 2019)
[Sumatroscirpeae + Cariceae]: prophyll subtending flower, margins fused [= perigynium].
Age. (41-)36(-34) Ma is the age of this clade estimated by Léveillé-Bourret et al. (2017a).
2N. Sumatroscirpeae Léveillé Bourret & J. R. Starr - Sumatroscirpus Oteng-Yeboah
Tufted/rhizomatous perennials; stem often triquetrous; foliar air cavities +; (inflorescence not scapose); lateral spikelets mostly "pedicellate" [pedicel longer than subtending bract], ± beaked; P/T bristles, (antrorse barbs 0); style base swollen or not, persistent; embryo type?; plastid transmission biparental.
1/4. Southwest China to Sumatra, montane.
2O. Cariceae Dumortier - Carex L. —— Synonymy: Kobresiaceae Gilly
Tufted/rhizomatous/stoloniferous perennials, (ectomycorrhizal associations +); stem often triquetrous; (petiole + - Mapania-type), foliar air cavities +; plant monoecious; (inflorescence not scapose); lateral spikelets truncate/much reduced, ± beaked; P/T 0; (anthers white); style base swollen or not, (persistent); n = 5-66, plastid transmission biparental.
1/2,328 - 6 subgenera, ?100+ sections. Worldwide, but especially North Temperate to Arctic.
2O1. Carex subg. Siderosticta Waterway
polyploidy common.
30 spp. East, and esp. Southeast, Asia.
[Carex [Euthyceras [Psyllophorae [Uncinia + Vignea]]]]: Chromosomes holocentric.
?
1,374 spp. Cosmopolitan.
[Euthyceras [Psyllophorae [Uncinia + Vignea]]]: ?
2O3. Carex subg. Euthyceras Petermann
?
124 spp. N. Hemisphere, also South America, New Zealand.
[Psyllophorae [Uncinia + Vignea]]: ?
2O4. Carex subg. Psyllophorae (Degland) Petermann
?
53 spp. W. Palaearctic, Southern Memisphere, esp. Patagonia and the Cape.
2O5. Carex subg. Uncinia (Persoon) Petermann
Rhachilla often +, hooked at apex.
99 spp. New World, Malesia, the Pacific, esp. South America, New Zealand.
2O6. Carex subg. Vignea (G. T. Lestiboudois) Heer
?
330 spp. Cosmopolitan, esp. North America.
Age. A "root age" of ca 32 Ma was estimated by Hoffmann et al. (2017 and references), while for Martín-Bravo et al. (2019) the crown-group age was ca 37.2 Ma (see also Márquez-Corro et al. 2021), and they listed other dates that had been suggested that ranged from 42.2 to 31.3 Ma.
[[Eleocharideae + Abildgaardieae] [Bolboschoeneae [Fuireneae [Schoenoplecteae [Pseudoschoeneae + Cypereae]]]]] / the FAEC clade / Eleocharideae group: ?
[Eleocharideae + Abildgaardieae]: style base thickened/not, stigmatic papillae annulate/moniliform; achene with style base persistent [apomorphy around here of this or related feature].
2P. Eleocharideae Goetghebeur - Eleocharis R. Brown
(Annuals), tufted/rhizomatous/stoloniferous perennials, often ± submerged; (C4 photosynthesis +); culms with dense peripheral bundles of fibres; leaf sheath only, blade 0 (small mucro +); inflorescence of 1 spikelet; glumes (2-ranked); flower type?; P/T bristles (0-)3-6(-11); achenes apically tuberculate [= persistent swollen style base]; cotyledon broadened; embryo Eleocharis-type.
1/ca 302. Cosmopolitan, esp. (sub)tropical America.
Annuals, tufted (rhizomatous) perennials; C4 photosynthesis +; leaves (reduced to mucro), (ligule +); inflorescence usu. scapose; glumes 2-ranked (spiral); P/T 0; A 1-3; style base swollen (not); achenes with style base persistent/not; embryo various.
10/571: Fimbristylis (320), Bulbostylis (227). Pantropical (warm temperate).
[Bolboschoeneae [Fuireneae [Schoenoplecteae [Pseudoschoeneae + Cypereae]]]]: ?
2R. Bolboschoeneae (Tatanov) J. R. Starr - Bolboschoenus (Ascherson) Palla
Long-rhizomatous, often tubers at apices, perennial; P/T 3-6; A 3; style base persistent; pericarp with 3 distinct layers; embryo Bolboschoenus-type, fungiform, with 3 leaves, notch below the root cap; 2 whole genome duplications.
1/15. Temperate to tropical.
[Fuireneae [Schoenoplecteae [Pseudoschoeneae + Cypereae]]]: ?
2S. Fuireneae Fenzl - Fuirena Rottbøll
Annuals, rhizomatous/stoloniferous perennials; ligule +, tubular, membranous, (leaves reduced to a sheath); glumes spiral/(5-ranked), each sunbtending a flower; P = 0-6 bristles, (scale-like, 3); A 1-3; style base persistent; embryo Fuirena-type, turbinate/somewhat fungiform, cotyledon broadened, first leaf not strongly developed, second leaf at most poorly developed.
1/55. Tropical to warm Temperate, esp. America and Africa.
[Schoenoplecteae [Pseudoschoeneae + Cypereae]]: ?
Long-rhizomatous perennials (tubers at tips); leaves usu. reduced to sheath; glumes spiral, each subtending a flower; P/T (5)6, bristle/plumose; A 2-3; style base persistent; embryo Schoenoplectus I-type, embryo fungiform, cotyledon turbinate to rhomboid, root cap lateral, embryonic leaves basal, 2, first well-developed.
2/18: Schoenoplectus (17). India and China to Australia (Queensland).
[Pseudoschoeneae + Cypereae]: ?
2U. Pseudoschoeneae J. R. Starr
Annuals to perennials; leaves usu. reduced to mucronate sheath, (ligule +); glumes spiral, each subtending a flower; (plant polygamo-dioecious); P/T 0-10; A 2-3; style base persistent; (plant amphicarpous, basal flowers with large nutlets, style long, lignified); embryo Schoenoplectus II-type, embryo fungiform, cotyledon umbonate or pileate, root cap lateral, embryonic leaves basal, 2, first well-developed.
2/63: Schoenoplectiella (62). Temperate to tropical, worldwide.
2V. Cypereae Dumortier —— Synonymy: Papyraceae Burnett
(Annuals), tufted/rhizomatous/stoloniferous perennials; (C4 photosynthesis +); leaves (reduced), (petiole + - Oreobolus/Mapania-type), ligule usu. 0; inflorescence often scapose, with a whorl of involucral bracts; glumes 2-3-ranked, bracts papery; (plant dioecious); P/T very reduced/scales/bristles; G ([2]), (gynophore +, 3-lobed or cup-shaped at apex); dispersal as nutlets or spikelet, nutlet orientation usu. dorsiventral (lateral); embryo Cyperus/Ficinia [derived from C]-type; n = 5-112.
8/1132 - 2 subtribes: Cyperus (961), Ficinia (87), Isolepis (69). Cosmopolitan.
Where?: Rhynchocladium (1).
Evolution: Divergence & Distribution. Mapanioid sedges are common as fossils (Volkeria messelensis, Caricoidea) in the Eurasian Eocene, where they perhaps grew in wet tropical forests and swamps as do the extant members of this group (S. Y. Smith et al. 2009a, b; see also Friis et al. 2011 for references), while Rhychospora is reported from 47-34 Ma Baltic amber (Sadowski et al. 2016b). About 1/3 of all monocot Caenozoic macrofossils have been referred to Cyperaceae (Xing et al. 2016); Jiménez-Mejías et al. (2016) review the copious fossil record of Carex, the oldest fossil they place with certainty in the genus is from the Priabonian (late Eocene) of the Isle of Wight.
Cyperaceae may have originated in Late-Cretaceous South America, perhaps spreading in the southern hemisphere via Antarctica (Spalink et al. 2016; Martín-Bravo et al. 2019); the circum-Pacific distribution of Oreobolus may be the result of dispersal (Sanmartín & Ronquist 2004). For diversification rates in the family, quite strongly correlated with clade age, see Escudero and Hipp (2013: c.f. topology, lots of dates), Bouchenak-Khelladi et al. (2015) found an increase in diversification rate in Cyperoideae and again in Cypereae, the latter, they thought, being triggered by the appearance of C4 photosynthesis, while Spalink et al. (2016) noted increases within Carex (temperate) and Cyperus (tropical). Márquez-Corro et al. (2019, see also 2018) found that changes in the mode of karyological evolution seemed to be associated with most rate shifts within Cyperoideae. Maherali et al. (2016) suggest that high diversification rates here (Magallón & Sanderson 2001 - "Cyperales" with ca 11,000 species?) are associated with the non-mycorrhizal and herbaceous habit. Cyperaceae, in particular Cyperoideae, are somewhat unusual in that they are more diverse in temperate than in tropical areas (Spalink et al. 2015, 2016; c.f. normal latitudinal trends of biodiversity, q.v.).
Carex. There has been much recent discussion about the evolution of the around 2,000 species of Carex. Escudero et al. (2012b) suggest that crown group Carex is about (54.9-)42.2(-29.7) Ma, but this is much older than ages for the tribe in Hoffmann et al. (2017: see above). Martín-Bravo et al. (2019) suggest a crown-group age of ca 37.2 Ma in the late Eocene and an origination in East Asia, major diversification in the genus beginning 8-20 Ma later (Márquez-Corro et al. 2021), and this may be linked to decreasing temperatures which were especially marked ca 34 Ma at the end-Eocene (see also Escudero et al. 2012b), and perhaps also chromosomal evolution (see below). However, given the age of the clade, overall diversification rates have really not been particularly high (Escudero et al. 2012b; see also Tietje et al. 2022). Martín-Bravo et al. (2019, see also Table 2 for these and alternative dates) suggest that the crown-group ages of the six subgenera were close, between 25.2-22.9 Ma, and the ancestral areas of four of the subgenera were East Asian. General variation in the taxa of the basal pectinations of Carex is very considerable, some being insect-pollinated, or with branch prophylls causing the inflorescence branches to spread (c.f. grass lodicules), or with broad, pseudopetiolate leaf blades, etc. (Starr et al. 2015).
Carex has a number of wide intrasectional biogeographical disjunctions and includes six or seven bipolar species, 1/5 of all such species (29% in Villaverde et al. 2017); long distance dispersal best explains such disjunctions, "the genus having a striking capacity for long distance dispersal" - but see below (Martín-Bravo 2019: p. 696, see also Villaverde et al. 2017; Moore & Chater 1970; Escudero et al. 2009, 2010, 2012b; Míguez et al. 2017: dating an issue). Villaverde et al. (2017) discuss the bipolar species of Carex in some detail - Cyperaceae as a whole include 15 (6%) of American amphitropical disjuncts (Simpson et al. 2017a). Martín-Bravo and Escudero (2012) noted numerous wide disjunctions in Carex section Spirostachyae. There are ca 63 species of Carex on tropical African mountains, and these mountains have been colonised from the north 9-14 times, 4-5 times from North America alone, and that suggests as many one-way trips of ca 10,000 km (Gehrke & Linder 2009); see also Ranunculus, Dianthus. A recent comprehensive analysis of relationships and biogeography in Carex (Martín-Bravo et al. 2019: Fig. 2A Table 3) emphasized the number of dispersal events that have yielded the current distribution patterns in the genus, indeed, there may be just about every distribution pattern known in angiosperms here. These dispersal events have been particularly common around the Northern Hemisphere (well over 200), but there have been 5-10 from North Africa-Europe to Australia, and so on - and all this in a genus in which most fruits show litlle in the way of obvious dispersal mechanisms. Carex is quite well represented on African mountains in Afroalpine habitats following several dispersal events from the north (Brochmann et al. 2021). There have been a number of diversification rate increases (much diversification is quite recent, late Miocene-Pliocene) and Martín-Bravo et al. (2019: Table 4) also emphasized notable radiations especially in the North America-East Asia-New Zealand area (the latter includes that of subgenus and section Uncinia (see also below), where speciation has been quite rapid within the last 10 Ma, but it is usually difficult to link these mini-radiation to the evolution of any particular morphological feature.
Having just said that Carex in general shows few obvious adaptations for dispersal, an exception is subgenus Uncinia, whose phylogeny has recently been clarified by García-Moro et al. (2022). The basal condition here is to lack or have a poorly developed rhachilla, but in most species the rhachilla is long, exserted from the utricle, and hooked. The origin of this subgenus was probably in North America, whence section Uncinia in particular moved to South America and probably twice to the Antipodes; its centre of diversity is now in New Zealand (it is the only "southern" subgenus), and it has also moved on to islands in the SubAntarctic and the Pacific; possibly this hooked rhachilla, which has evolved just once here (García-Moro et al. 2022: Fig. 5) but is found in most of the species, helped facilitate epizoochoric dispersal.
Morphology in Carex is rather labile and an uncertain predictor of relationships, section Racemosae being a case in point (Massatti et al. 2016); apparent vicariance/long distance dispersal patterns there in part reflect homoplasy (Gebauer et al. 2015). Evolution is not necessarily complex → simple (see also the Global Carex Group 2015), thus conventional wisdom suggesting that a highly compound inflorescence is the plesiomorphic condition for Carex, taxa with simple spicate branches being derived, perhaps several times, seems the exact opposite of what actually happened (Ford et al. 2006; see also Léveillé-Bourret et al. 2017a). Similarly, species of Carex believed to be intermediate between that genus and Uncinia, with its hooked inflorescence axis protruding through the apex of the perigynium (prophyll), seem not to be (Starr et al. 2008b: support not strong) - indeed, Uncinia is now a synonym of Carex.
Overall, partitioning of ecological and perhaps particularly geographical space is associated with diversification in Carex (Spalink et al. 2016). Waterway et al. (2009) discussed ecological diversification in Carex, where there are widespread wetland species, and their sister species might be on separate continents, however, the forest taxa are often more geographically restricted. Species relationships in North American Cyperaceae are in general not correlated with the geography of the species involved, although this is not true for species growing at high altitudes and latitudes (Spalink et al. 2015), however, Waterway et al. (2016) observed that closely related species of Carex growing in subarctic fens were more unlikely to grow together than chance would suggest, those species that were growing together tending to be immediately unrelated and to show niche differentiation. All this being said, the discussion in Márquez-Corro et al. (2021) suggests that there is a way to go before we understand the big picture of diversification here - or perhaps there is no big picture. These authors used chromosome number (the genome of all bar one subgenera is made up of holocentric chromosomes) as a proxy for diversification, and they suggested that overall chromosome evolution seemed to support lineage diversification, but climate seemed not to characterise individual major lineages.
On an unrelated issue, clones of Carex curvula may persist for 2,000 years or so in the Alps despite climatic fluctuations (Steinger et al. 1996: growth rate changes probably not important).
Elliott et al. (2022b: Fig. S5) note the extensive chromosome number variation in Cyperaceae - ca 55-fold; Poaceae show more, ca 66-fold, but other families have less than 30-fold variation. Both Poaceae and Cyperaceae have minimum diploid numbers of 4, the equal lowest in land plants (Elliott et al. 2022b: Fig. S6); of course, Cyperaceae, but not Poaceae, have holocentric chromosomes (see below). The numerous fission and fusion events associated with chromosome change in Carex in particular have been implicated in its diversification (Márquez-Corro et al. 2019), although exactly how is unclear (see also below). High chromosome numbers may promote recombination, and there is some correlation between high chromosome number and stable habitats, perhaps facilitating evolutionary innovation, and there are complex patterns of hybrid dysfunction and non-Mendelian segregation; chromosome number changes may facilitate reproductive isolation (Escudero et al. 2012a, 2016a and references; Escudero 2015). The Siderostictae clade, sister to all other Carex, lacks the wholesale chromosomal rearrangements that characterize the rest of the genus and has only a few species (Escudero et al. 2012a), and it may be that diversification in the non-Siderostictae clade has increased (e.g. Escudero et al. 2012b; Spalink et al. 2016). For more on chromosome numbers and evolution in Carex, see also Hipp (2007) and Hipp et al. (2009).
Cyperaceae are diverse on Madagascar, and with 310 species they are in the ten most diverse families there (Muasya et al. 2011; Larridon et al. 2020a). Most genera there are small, exceptions being Cyperus s.l., with about 143 species, Carex (ca 30 species), Scleria (25), Bulbostylis (23), Eleocharis (12) and Costularia (11 species). Overall endemicity is low, only some 38%, although that of Costularia, Bulbostylis and Carex is higher (100%, 71% and 88% respectively), and other large Madagascan families have 80-99% endemicity - except Poaceae and Asteraceae (Muasya et al. 2011; Larridon et al. 2020a: ?numbers). Cyperaceae are uncommon in l.t.r.f. in Madagascar and only some 36% are C4 species; endemics are uncommon in the savanna grasslands, consistent with the secondary origins of the latter (Joseph & Seymour 2020; c.f. Bond et al. 2008), commoner in forests and at higher altitudes (Larridon et al. 2020a).
Taxa in Cyperaceae other than Carex may also show extensive dispersal. Thus there have been numerous dispersal events in the much smaller genus Scleria (ca 250 spp.), perhaps 12 from Africa to Madagascar and 7 from Africa to South America alone; it is impossible to estimate ancestral areas for basal nodes in the tree, and the rate of dispersal is over twice that of Carex, even although that genus is temperate and Scleria is tropical (Larridon et al. 2020b). Larridon et al. (2020b) also looked at connections between diversification rate shifts and niche shifts (none) and bioclimatic differences (perhaps). Viljoen et al. (2013) examined biogeographical relationships in Schoeneae s. str., where there was much trans-oceanic dispersal - no fewer than 29 transoceanic movements since the Oligocene...
Miscellanous: Diversification rate changes may have occurred in the [SDC + FAEC] and the FAEC clades as they moved into the northern hemisphere, and diversification may also have increased in C4 Cyperus (Escudero et al. 2012b; Spalink et al. 2016). The FAEC clade, barring C4 Cyperus, tends to have stable chromosomes, but the C4 clade shows much more variation, however, causes for such correlations are unclear (Márquez-Corro et al. 2019). Rhynchospora originated on the Brazilian Shield (Buddenhagen et al. 2017). Divergence within Eleocharis has occurred within the last ca 20 Ma (Besnard et al. 2009b), while nearly all the ca 60 species of Ficinia and many of those of Tetraria are restricted to the Cape Floristic Region (Linder 2003).
Bruhl (1991) discussed the extensive literature on the possible pseudanthial nature of flowers in Mapanioideae. He noted that the tepal-type structures in the Cyperoideae he studied were outside the stamens, either in one or two whorls (there was infrageneric variation in Eleocharis), and they probably did represent perianth parts. However, in Mapanioideae things seem rather different (see e.g. Prychid & Bruhl 2013: figs 2, 3 for diagrams). Quite commonly the flowers of Mapanioideae have a pair of lateral "T", inside which are a pair of vertically oriented "T", and then there is a pair of anthers opposite the lateral "T". These lateral "T" develop quite precociously, and they have been described as bracteoles, perhaps a modified adaxial bicarinate bracteole (the lateral stamens apparently immediately opposite the two parts would be a little odd - see also Prychid & Bruhl 2013). In taxa like Scirpodendron (= Mapania s.l.) the stamens were in a spiral, each stamen being subtended by a perianth-type structure (and perhaps there is a whorl of such structures between the stamens and gynoecium - c.f. Goetghebeur 1998 and Prychid & Bruhl 2013), while other species of Mapania varied in this; in the past Scirpodendron was thought to have primitive "flowers", those of other Hypolytreae, at least, evolving via reduction (Monteiro et al. 2021 for references). Monteiro et al. (2020) also draw Lepironia as having numerous stamens each subtended by a perianth-type structure, again, such structures form a whorl interior to the androecium. Recent studies suggest that mapanioid flowers are pseudanthial (Prychid & Bruhl 2013; see also Vrijdaghs et al. 2004a; Richards et al. 2005, esp. 2006: Exocarya scleroides), although Monteiro et al. (2020) thought that the reproductive units, which they called spicoids, might be modified flowers, modified inflorescences, or something quite novel and sui generis - the jury was still out. Of course, the interpretation of mapanioid flowers affects ideas of floral evolution in the family as a whole. Thus if these spicoids are modified inflorescences, bisexual flowers in Cyperaceae would be derived, rather unusual; the numbers of flowers per inflorescence have become reduced, while there has been an increase in loss events of stamens per flower in Cyperoideae, both unexpected in anemophilous species (Chaves et al. 2024).
However, the broadly comparative study of the spicoids of Mapanioideae by Monteiro et al. (2021) helps clarify the situation. We can see that the basal genera in the two tribes (Paramapania and Hypolytrum in Hypolytreae; Diplasia and Exocarya in Chrysitricheae - see Phylogeny below) have a rather similar basic floral morphology, as is evident in the tribal characterizations above (which are based on these taxa; characters in parentheses are from the other genera), and there are also some similarities with Trilepis (in Trilepideae, sister to all Cyperoideae); Scirpodendron is derived, and is embedded in Mapania; and overall floral evolution seems to have proceeded by elaboration, not simplification in Mapanioideae. Of course, one now needs to know more about Trilepideae and about the basal Juncaceae, Juncaceae being sister to Cyperaceae. In Juncaceae the widespread Oreojuncus is sister to the rest of the family, but it does not seem to be particularly morphologically remarkable, apart from its lacerate-fimbriate auricles (Záveská Drábková & Kirschner 2013).
As Vrijdaghs et al. (2011) note, it is difficult to talk about carpels in Cyperoideae since the gynoecium develops from an annular primordium - on top of which there is a two- or three-, sometimes even four-, branched style, and altough there is no obvious fusion of carpels (Reynders & Vrijdaghs et al. 2012; Lucero et al. 2014) the number of style branches is used as a proxy for carpel number. I have placed "gynoecium initiated as an annular primordium" as characterizing the whole family (for Mapanioideae, see Prychid & Bruhl 2013), but Reynders and Vrijdaghs et al. (2012) note that Luzula and many Poaceae have a similar gynoecium, so where this feature will end up on the tree is unclear.
Naczi (2009) discussed the use of morphological characters in phylogenetic analyses of Carex in particular; this is tricky because the highly derived/reduced nature of the flowers makes character coding difficult, although adding "micro" and anatomical characters improved the resolution of the analyses. However, Léveillé-Bourret and Starr (2019) optimised a number of characters (some states divisions of continua) on their tree of the scirpo-caricoid clade, and their work has been incorporated into the tribal characterizations as far as possible. The evolution of the variable cyperaceous embryos was treated by Semmouri et al. (2019) in some detail, and they put embryo types on the tree, but, importantly, they also thought about the evolution of the individual characters/variables that make up these types (see Supplemental material).
Ecology & Physiology. Despite their diversity and their abundance in many ecosystems, not too much is known about the eco-physiology of the family (Barrett 2013). Cyperaceae are common in phosphorus-poor habitats (Shane et al. 2006 for some references). They are often particularly common in wet tundra habitats (ca 8% of the earth's land surface), and moist sedge tundra is quite productive, carbon cycling faster there than in adjacent dry tundra (Kade et al. 2012; Parker et al. 2015). Eriophorum and Carex - possibly lacking mycorrhizae, or some with ectomycorrhizae (ECM) - are two of the seven major contributors to tundra biomass (Chapin & Körner 1995), the other five being core eudicots with ECM or ericoid mycorrhizae. ECM Cyperaceae may also dominate in alpine and other extreme habitats. Thus there are some 450,000 (or 1.5 x 106 - Qiu 2016) km2 between 3,000 and 5960 m altitude on the Tibetan plateau dominated by the ECM Kobresia pygmaea (= Carex parvula: Kobresia is in the Core Unispicate Clade of Carex - Global Carex Group 2015) (Miehe et al. 2008). This community may be of quite recent origin, reaching its current extent since the spread of the Tibetan empire in the seventh century CE, although this and other sedges first became dominant ca 8,000 years ago (Miehe et al. 2008, 2014, see also X. M. Zhou 2001). There is around 18.1 x 1016 g of carbon in these "grasslands" (Qiu 2016). Other species of Carex that were in the old Kobresia are to be found in Europe, Greenland and other high latitude areas, and they, too, are ECM plants and may dominate the vegetation (e.g. Gardes & Dahlberg 1996; Muhlmann & Peintner 2008; Newsham et al. 2009 and references; Gao & Yang 2010).
Carex is the most speciose genus in the Arctic with some 136 species there alone (Hoffmann et al. 2017), although it is not so prominent in the very far north (Elven et al. 2011), and other cyperaceous genera are also major components of the vegetation in Arctic habitats. Cyperaceae-dominated communities were notably extensive during the last glacial maximum north of 550 N (Bigelow et al. 2003). Similarly, about 16% of all species growing in Quebec and Labrador north of 54o N are Cyperaceae (Poaceae are next at 11%), 13% belonging to Carex alone, and they they can be major components of plant cover especially in wetter habitats such as rich fens (Cayouette 2008; Escudero et al. 2012b). The parallel adoption of the Arctic habitat in Carex sections Phacocystis and Vesicaria is discussed by Gebauer et al. (2014), while Hoffmann et al. (2017) found that the 131 species of Arctic Carex they examined (out of 136 total) could be placed in 48 separate clades. The ancestors of these clades grew mostly in swamps and bogs, whether in the lowlands or high mountains (Hoffmann et al. 2017).
Although the primary productivity of such communities can be relatively high, they may sequester less carbon than Sphagnum-dominated communities in poor fens (Flanagan 2014). The roots of cyperaceous plants can penetrate into the mineral soil below the shallow layer of soil dominated by the roots of the ericoid and ECM members of the community (Read 1993; Iversen et al. 2014 for references), although in general the soil is often quite shallow. Eriophorum roots are notably short-lived - 1-2 years, versus ≥5 years for other plants (Iversen et al. 2014). Indeed, a number of Cyperoideae-Cariceae and -Rhynchosporeae (but not some - at least - -Scirpeae) have rather short-lived (ca 1 month) dauciform roots, roots which develop a dense covering of very long root hairs and look rather carrot-shaped; these may facilitate P uptake by the plant when growing in the P-poor soils which many Cyperaceae frequent (Lamont 1982; Shane et al. 2005: some Juncaceae also have such roots, 2006; Lambers et al. 2008; Playsted et al. 2006; V. A. S. jones & Dolan 2012, etc.). Carboxylates, especially citrate, move into the soil where they make mineral-bound P soluble (Shane et al. 2005; Playsted et al. 2006). Epidermal cells in dauciform roots are elongated at right angles to the long axis of the root (Shane et al. 2005, 2006). However, Tibetan Kobresia (= Carex) is both an ECM plant and has dauciform roots, and ECM fungi are reported to grow over the surface of such roots (Gao & Yang 2010: not shown in Fig. 1C). Newsham et al. (2009, but c.f. Iversen et al. 2014) noted the frequency of arbuscular mycorrhizae in Cyperaceae growing in polar regions.
Many, but not all, tundra-dwelling Cyperaceae, whatever their mycorrhizal status, take up nitrogen predominantly in an organic form, which may suggest ECM associations (Raab et al. 1996, 1999), although Carex seems to prefer NO3-, and that genus has high rates of P uptake, as do other Cyperaceae, although to a lesser extent (Iversen et al. 2014).
About 1,325 species of Cyperaceae carry out C4 photosynthesis. This has perhaps six origins in the family, and there have also been some reversals to the C3 pathway (Soros & Bruhl 2000; Besnard et al. 2009b; Bruhl & Wilson 2008; Roalson 2011; Larridon et al. 2011a; Sage et al. 2012; Buddenhagen et al. 2017). Besnard et al. (2008b, 2009b) suggested that evolution of C4 photosynthesis had occurred within the last (19.6-)14.9(-10.2) Ma, first appearing in Bulbostylis; genetic changes in the important enzymes phosphoenolpyruvate carboxylase and rbcl may have occurred in parallel. For the complexity of possible patterns of the evolution and loss of the C4 pathway and that of intermediate pathways within Eleocharis, see Roalson et al. (2010). In Eleocharis C3/C4 or C4 photosynthesis may occur in emergent leaves, C3 photosynthesis in submerged leaves (G. E. Edwards et al. 2004), while in E. acicularis there is single-celled C4 photosynthesis (Bowes 2010). There is only a single major C4 clade in Cyperus, but it has some 760 species (Reid 2011; Larridon et al. 2013; Sage 2016; Reid et al. 2017). S. Martins and Scatena (2011) looked at the diversity of Kranz-type morphologies in the family from a developmental point of view. C4 Cyperaceae quite often grow in wet, fertile conditions, rather unlike many other C4 plants (Christin & Osborne 2014), and in some Cyperaceae with submerged leaves C4 photosynthesis may help increase nitrogen use efficiency (Besnard et al. 2009b; Bruhl & Wilson 2008; Roalson 2011).
A few taxa like Rhynchospora anomala are desiccation-tolerant and arborescent; their roots, which make up the "trunk" of the plant along with the persistent leaf bases through which the roots run, have a well-developed velamen. Indeed, Cyperaceae are quite important components of the vegetation of tropical inselbergs where soils are shallow and dry out fast (Porembski & Barthlott 2000; Porembski 2006; Porembski et al. 2021). All told, some seven genera include species that show extreme desiccation tolerance, i.e., they are resurrection plants (Gaff & Oliver 2013); for Late Embryogenesis Abundant (LEA) genes and geen families, involved in desiccation tolerance, in angiosperms in general, see Artur et al. (2018).
A few Cyperaceae have leaves with (psudo)petioles; Alves dos Santos et al. (2025) divide such leaves into two types, the Oreobolus and Mapania types based on the nature of the basal constriction of the blade, e.g. gradual or not. Taxa with Mapania-type basal constrictions tend to grow in forested habitats.
Van Mazijk et al. (2024) compared Cape species of Schoenus, with their very large genomes, with Tetraria, with much smaller genomes (both are Schoeneae). The very large genomes of the former were associated with fewer, large stomata, decreased stomatal conductance, increased water use efficiency, lower C:N ratios, etc..
Photonic structures, reflecting helicoid structures in the cell wall are known from a number of Cyperaceae, including Mapanioideae-Hypolytreae and Cyperoideae-Cypereae and -Cariceae. These structures are perhaps basically involved in providing strength and support for the plant, the distinctive blue iridescence of the leaves of such plants then being secondary (Lundquist et al. 2024).
Miscellaneous. Dark septate hyphae are known from Cyperaceae, and the hyphae are likely to be dark because they have fungal melanin, so this could have implications for carbon cycling (c.f. Clemmensen et al. 2014). Some sieve tubes in the leaves that are adjacent to the xylem lack companion cells, are notably thick-walled, and seem to be involved in short distance transport of not very concentrated sugars (Botha 2013). For dumbbell-shaped stomata and stomatal pore movement, see Franks and Farqhar (2007); all the literature is about grasses...
Pollination Biology & Seed Dispersal. Cyperaceae are normally pollinated by wind, and Ennos (1993) discussed how the triangular stems of the lowland sedge Carex acutiformis twisted readily in a light wind, thereby reducing drag and the possibility of selfing, but at higher altitudes the sedges had more rounded stems and tended to twist less, however, selfing was unlikely because of the windier conditions (for bending and twisting in C. pendula, see Speck et al. 2020).
There are a few cases of insect pollination in Cyperaceae, and genera like Ficinia and Rhynchospora have pseudanthia (Baczynski & Claßen-Bockhoff 2023). Lorougnon (1973) noticed that species of Mapania and Hypolytrum (Mapanioideae) from the forest undergrowth in the Ivory Coast were visited by slugs (Vaginula - especially when conditions were wet), beetles and hymenoptera; the inflorescences of the plants were white and the inflorescence bracts and glumes were sometimes red (see also Mesterházy et al. 2022). In Cyperoideae like Eleocharis and Rhynchospora (inc. Dichromena) spikelet bracts, inflorescence bracts and/or anthers can be white or yellow, they may reflect UV light, scent may be produced, pollenkitt developed, etc., and entomophily and ambophily occur along with anemophily (Wragg & Johnson 2011; da Costa et al. 2017, 2021). There have been some 6 or so origins of showy inflorescences in Rhynchospora alone (Buddenhagen et al. 2017).
Fruit dispersal mechanisms are very varied, including water, wind (e.g. the bristles surrounding the fruits of Eriophorum and the remarkable persistent filaments of Androtrichum (= Cyperus) that elongate to over 1 cm long, the persistent stigma-style also being long), and animals (both epi- and endozoochory), including ants attracted by elaiosomes with a variety of morphologies (Allessio Leck & Schütz 2005: also seed dormancy and germination requirements; Barrett 2013; Léveillé-Bourret et al. 2017a; Pereira-Silva et al. 2020). However, Villaverde et al. (2017) did not notice any dramatic modifications in the utricles of the bipolar species of Carex that might enable long distance dispersal, and Martín-Bravo et al. (2019) also emphasized the near absence of obvious dispersal mechanisms in the genus - see also Boraginaceae-Amsinckiinae).
Plant-Animal Interactions. R. L. Barrett (2013) noted a number of close associations between species of Lepidosperma and moths like the small grass-miner moth Elachista and the sun-moth Synemon; caterpillars of butterflies like Satyrini (Hamm & Fordyce 2015) and the [Heteropterinae-Trapezetinae-Hesperiinae] clade of skippers (Warren et al. 2009) feed on Cyperaceae - and also Poaceae, of course.
A high population density of herbivorous Microtus voles eating Carex appropinquata led to the induction of silica first in the rhizomes and a year later in the leaves (Wieczorek et al. 2014).
Plant-Bacterial/Fungal Associations. Cyperaceae, like other plants in the tundra habitat, often lack mycorrhizae (see above). However, ectomycorrhizae are common in Kobresia (= Carex, see above: Gardes & Dahlberg 1996; Miller at al. 1999; Muthukumar et al. 2004; Muhlmann & Peintner 2008; Gao & Yang 2010; references in Tedersoo 2017a). Dauciform roots (see above), dark septate hyphae and/or ECM may all be found in the one species, whether from the same or different localities (Michelsen et al. 1998; Gao & Yang 2010). Largely ascomycetous fine endophytes are also commonly found in Cyperaceae from tundra habitats (Higgins et al. 2007), and are more prevalent than arbuscular mycorrhizal fungi. Some are members of Clavicipitaceae, elsewhere especially prominent on Poaceae (Pazoutová 2003; Schardl 2010; Píchová et al. 2018), and taxa like Claviceps section Claviceps (the ancestral host for this may have been Cyperaceae - see Píchová et al. 2018) and Balansia are involved. Claviceps is of course the ergot fungus, and species growing on Cyperaceae synthesise ergot alkaloids (Píchová et al. 2018).
Smuts (Ustilaginales, basidiomycetes) are very diverse on Cyperaceae (Kukkonen & Timonen 1979; Savile 1979b). Species of Anthracoidea are parasitic on Carex, but by no means all, and also on Trichophorum - and since Trichophoreae and Cariceae are close, this distribution is consistent with the phylogeny (c.f. Kukkonen & Timonen 1979 in part). Escudero (2015) found that diversification of Anthracoidea on Carex was largely the result of speciation after shifting hosts, these shifts often being to species that were quite closely related to the original hosts.
Genes & Genomes. For a possible genome duplication in Cyperus, see McKain et al. (2016a), and for a nrDNA insertion, see Starr et al. (2008a).
At n = 2-112, Cyperaceae show extreme variation in chromosome base numbers, and this is probably connected with the holocentric chromosomes that are common here (Roalson 2008; Roalson et al. 2008a; Márquez-Corro et al. 2019: Elliott et al. 2022b). Carex in particular has great variation in chromosome numbers - n = 5-66 - because of extensive chromosome fissions, fusions and translocations that are facilitated by these holocentric/holokinetic chromosomes (Hipp et al. 2011); comparable variation in Eleocharis is n = 3-98 (Bureš et al. 2013). (Such chromosomes are often described as having diffuse centromeres.) However, subgenus Siderostictae, sister to all other Carex, lacks these rearrangements, and has large and few chromosomes - and includes only a few species (e.g. Escudero et al. 2012a). When chromosomes are holocentric, genome size is little affected by chromosome number change, indeed, the two may be negatively correlated in diploid taxa, and although polyploids tend to have larger genomes than related diploids, they do not have absolutely large genomes (Chung et al. 2012; Lipnerová et al. 2013), thus López et al. (2017) noted that in Bulbostylis n = 15-51, but the size of the nuclear genome (1 C) at 0.93-1.2 pg varied less. Polyploidy, albeit not true polyploidy, has caused major changes in chromosome number in the holocentric genus Rhynchospora, and again, chromosome number and genome size are poorly correlated (Burchardt et al. 2020 and references). However, in southern African Schoenus there is a positive relationship between genome size and chromosome number, and polyploidy seems to be quite common (Elliott et al. 2022c; van Mazijk et al. 2024 - q.v. for environmental/physiological correlations). Roa and Guerra (2012) found that 45S rDNA sites were always terminal on the chromosomes when these were holocentric. Holocentric chromosomes have also been recorded from Cyperus (references in Záveská Drábková 2013), but localized centromeres have been reported from (?some) Scirpus (Nijalingappa 1974). Finally, Neumann et al. (2023) found that details of how chromosomes function during cell division in species of Cuscuta subgenus Cuscuta, another group with holocentric chromosomes, differed substantially from those in Rhynchospora pubera.
Roalson (2008) and Roalson et al. (2008a) surveyed chromosome numbers in the family. López et al. (2017) and Carla et al. (2020) suggested that the base number for the family is x = 5. Shafir et al. (2023) noted that in all simulations rate homogeneity - assumed in earlier discussions of base number here (and commonly elsewhere) - was rejected, and they found that the rates of chromosome number change were notable in most of Carex subgenus Uncinia, Cyperus including the C4 species, and and the non-Siderostictae Carex clade. For the cytology of Kobresia, see Seeber et al. (2014).
Variation in both plastome and chondrome is notably high in Cyperaceae (C. Lee et al. 2023). There are similarities in chondrome gene content and loss between those few Cyperaceae and Poaceae known (C. Lee et al. 2023).
Plastome variation in Eleocharis is extensive (C. Lee et al. 2020), but little is known about plastomes elsewhere in the family or even Poales (apart from Poaceae). Lee et al. (2020) noted that there was heteroplasy in Eleocharis, and they found at least four different plastome structural types there. There are also quite extensive gene duplications and losses, the genic DNA content is low, the intergenic spacers are large as is the inverted repeat; Hypolytrum is intermediate between Eleocharis and two more basal Poales (Lee et al. 2020: Table 2).
The high variability of the chondrome in Eleocharis/Cyperaceae may be associated with the loss of most of the cis-spliced chondrome introns (there are only six) and the very few RNA editing sites in the 24 core mitochondrial genes - perhaps genes involved in DNA replication, recombination and repair are more or less dysfunctional (C. Lee et al. 2023). There has been a rapid acceleration in mitochondrial DNA substitution rates here and in several genera from other families (Zwonitzer et al. 2024: see also Characters).
Economic Importance. Cyperaceae include more serious and widespread weeds than would be expected (Daehler 1997).
Chemistry, Morphology, etc.. Betekhtina et al. (2023) noted that the Cyperaceae roots they examined in the Middle Urals were thin (but the cortex was relatively thick), much-branched, and had long root hairs - c.f. Iridaceae, Orchidaceae, Poaceae. Some species of non-mycorrhizal Carex have distinctive, bulbous-based root hairs (Miller et al. 1999). Vegetative variation is quite extensive, and leaves with a variety of more or less unifacial morphologies are to be found in the family (Metcalfe 1969; c.f. in part Fisher 1971). The contraligule is to be found on the top of the leaf sheath on the opposite side of the blade yo the ligule (see Goetghebeur 1998: p. 143). Yashiro and Endo (2021) described the morphology and anatomy of the culms and bladeless leaves of Eleocharis, optimizing the distributions of the characters they observed on a phylogeny. Rodrigues and Estelita (2009) described secondary thickening in the stems of Cyperaceae they examined.
For inflorescence morphology, see Reutemann et al. (2012: individual variables listed); branching can be from the axils of prophylls, and/or from collateral or superposed meristems, phyllotaxis may be spiral and distichous in the same species depending on the flower types in the spikelet, and so on. X. Zhang et al. (2004) suggested that spikelets in Schoeneae, at least, were sympodial, however, those of Cyperoideae as a whole are indeterminate (Vrijdaghs et al. 2005c, 2010 [esp. Cyperoideae]). Indeed, there is concaulescence of flower and spikelet in Schoenus and so the inflorescence there appears to be cymose (Vrijdaghs et al. 2008). Studies by Guarise et al. (2012) emphasized the diversity in developmental pathways that produced superficially similar capitate inflorescence in Cyperus; relatively few developmental changes could produce substantial diversity in mature inflorescence morphology. Inflorescence morphology in Cyperaceae is also discussed by Bender et al. (2016), in Cypereae in particular by Uberti et al. (2016), and in Carex et al. by D. L. Smith and Faulkner (1976).
For "floral" morphology in Mapanioideae, see Diversity & Distribution above, also illustrations in Clarke (1909). Scirpus sylvaticus has a relatively conventional monocot flower - its three stamens and the carpels are opposite the outer perianth members - to which the more derived morphologies in Cyperoideae can perhaps be related (Vrijdaghs et al. 2005a, 2009). In general, the stamens are shown as being opposite the outer perianth whorl (Bruhl 1991), the angles of the gynoecium (Goetghebeur 1998) or the style-stigma branches (Larridon et al. 2011b), all consistent with a flower in which members of the whorls alternate and the absence of the inner stamen whorl is of no consequence. The distinctive hairs of Eriophorum (Cyperoideae) arise centripetally on a perianth ring-primordium (Vrijdaghs et al. 2004b). Pollen apertures in Carex have a very thin underlying intine while that in interapertural areas is much thickened, i.e., the reverse of the normal condition (Halbritter et al. 2010). What exactly the "hypogynium", a structure below the flower that may be disciform, fimbriate, with three large, rounded lobes, etc., in Scleria - and there is also something similar in the related Bisboeckelereae - might be is unclear (Bauters et al. 2016). Pseudomonads develop in Cyperaceae (see also Ericaceae-Styphelieae), and although they were thought not to occur in some Mapanioideae (e.g. R. C. Brown & Lemmon 2000; Simpson et al. 2003), Ike Coan et al. (2010) record thema from throughout the family. The median carpel in Carex is shown as being adaxial (Eichler 1875), i.e. in the inverted position (see also Spichiger et al. 2004, but c.f. Reynders & Vrijdaghs et al. 2012, see also above). Parietal tissue in some Bulbostylis ovules may be only a single cell across (Maria & López 2010). Lye (2016 and references) discussed pericarp anatomy; that of Cyperoideae-Schoeneae is notably variable.
There is also a great deal of variation in embryo morphology (e.g. van der Veken 1965: hundreds of illustrations), but to understand the evolution in embryo morphology the embryo types need to be decomposed into the individual variables that characterise them, and I have done little of this. Tribes tend to vary little in embryo morphology (Goetghebeur 1998; Semmouri et al. 2019). For more details, see van Tieghem (1897a: grass and sedge embryos quite different), Schneider (1932: also germination), Pankow and von Guttenberg (1957), von Guttenberg and Semlow (1957), Shah (1965), Verbelen (1970), Vanhecke (1974), Semmouri (2016) and Silva et al. (2023).
For a vast amount of additional information, see Bruhl (1995); for general information see also Goetghebeur (1998), Naczi and Ford (2008), papers in Bot. Review 75(1) (2009), Léveillé-Bourret and Starr (2019: inc. key to all tribes) and Costa et al. (2021: Cryptangieae). For anatomy, see Cutler (1969 and references), Piperno (2006), Stevenanto et al. (2019) and Murungi and Bamford (2020), all phytoliths, Rodrigues and Estrelita (2009) and A. de L. Silva et al. (2018), mostly stem anatomy, Hoffmann (2019: root anatomy of Carex), for winged leaves in Scleria, see Hoss et al. (2015), for the prophyll, see Blaser (1944), for inflorescence morphology, see Eiten (1976) and Nijalingappa and Goetghebeur (1989: Ascopholis, bristle = axis), for inflorescences in Carex, see the Global Carex Group (2015), for floral morphology, see e.g. Schultze-Motel (1959), Vrijdaghs et al. (2006), Bauters et al. (2014: Lipocarpha/Cyperus) and Monteiro et al. (2016), for pollen, see van Wichelen et al. (1999), Nagels et al. (2009), Ike Coan et al. (2010) and Furness and Rudall (2011), for the gynophore, etc., see Vrijdaghs et al. (2005b) and for ovule and seed development, see Nijalingappa and Devaki (1978) and Ike Coan et al. (2008).
Phylogeny. Mapanioideae and Cyperoideae are monophyletic (e.g. Simpson et al. 2003, esp. 2008; Hinchcliff & Roalson 2013. For a detailed phylogeny of the family, see Semmouri et al. (2019: 7 genes, concatenated) and in particular the recent study by Larridon et al. (2021a) who carried out targeted sequencing on 311 taxa (the Angiosperms353 probe kit, see also W. J. Baker et al. 2021a). Larridon et al. (2021a) should be consulted for details of relationships within and between the tribes. However, a recent study by Brozová et al. (2022) using only ITS plus two chloroplast genes, but quite extensive sampling, produced a tree with apparently quite extensive para-/polyphyly (Hypolytrum, Rhynchospora, Schoenus-Tetraria-Oreobolus, Trichophorum, Scirpus, Carex, Ficinia, Cyperus, etc.). There was variation in tree topology in the analyses using different data sets, and in the case of Carex, for example, apparent paraphyly was largely because the nomenclature used was old style - c.f. the Global Carex Group (2016) and Villaverde et al. (2020a), although I don't know what Amphiscirpus is doing there (Brozová et al. 2022). Elliott et al. (2022a) discuss some problems with this paper.
Mapanioideae. Capitularia (Escudero & Hipp 2013) or Diplasia (Spalink et al. 2016) may be sister to other Mapanioideae. However, Larridon et al. (2021a) found that Diplasia was moderately supported as sister to all other Chrysitricheae [Diplazia [Exocarya ...]] are the relationships used by Monteiro et al. (2021), while Capitularia is more deeply embedded in that tribe. Support for the monophyly of Hypolytreae is somewhat underwhelming (Larridon et al. 2021a), and [Paramapania [Hypolytrum ...] are the relationships in Monteiro et al. (2021), furthermore, Scirpodendron is embedded in Mapania, while Mesterházy et al. (2022: Sanger sequencing) found that Principina was embedded in and to be synonymized under Hypolytrum, that clade and Mapania being monophyletic, but only African species examined.
Within Cyperoideae, relationships have been rather unclear; see Z.-D. Chen et al. (2016) for relationships among Chinese taxa and Larridon et al. (2021a) for general relationships. Trilepideae are sister to the remaining genera (Muasya et al. 2009a), while in a supermatrix analysis these other taxa were placed in a well-supported set of relationships [[Sclerieae + Bisboeckelereae] [Schoeneae [Rhynchospora [clade including Carex and Scirpus + clade including Eleocharis, Isolepis and Cyperus]]]] (Hinchcliff & Roalson 2013) - the first three clades are somewhat different to those in Muasya et al. (2009a). In another study, Schoeneae were in three separate basal pectinations and Cryptangieae were in a fourth, and then came [Rhynchospora + The Rest] (Escudero & Hipp 2013). Viljoen et al. (2013; see also Spalink et al. 2016; Musili et al. 2016: polyphyly of Schoenus) focussed on schoenoid sedges and the relationships they found were [Cladium (ex-Schoeneae) [[Sclerieae + Bisboeckelereae] [[Cryptangieae + Schoeneae] [Rhynchospora + The Rest]]]], although some nodes had little support, Costularia was paraphyletic and Schoenus and Tetraria polyphyletic. In a fairly comprehensive analysis of the Cyperoideae, Cryptangieae were consistently recovered as a clade somewhere near the base of the subfamily (S. M. Costa et al. 2018), and Larridon et al. (2021a) found that Koyamea was sister to the rest of that tribe. Jung and Choi (2013) emphasized relationships in Korean taxa and found relationships were [Trilepideae [[Sclerieae + Bisboeckelereae] [Schoeneae [Cryptangieae, Schoeneae [Schoeneae [Rhynchospora + The Rest]]]]]], i.e. Schoeneae are very paraphyletic, but again there was little support for the basal nodes. Larridon et al. (2018) help clarify relationships here - Carpha and Trianoptiles are not to be included, and genera like Costularia are very polyphyletic. Fuireneae were also strongly paraphyletic, although most could be included in Cypereae, while Fuirena and/or Bolboschoenus were at the base of the phylogeny of the combined tribes (Monfils et al. 2014; Spalink et al. 2016; Glon et al. 2017). Abildgaardieae: Ghamkar et al. (2003) found some differences in the phylogeny depending on whether nuclear or chloroplast data were used, and Fimbristylis was paraphyletic. However, Larridon et al. (2021b: Angiosperms353 enrichment panel) have clarified relationships in the tribe (two monotypic genera could not be included).
Cryptangieae. Backbone relationships in S. M. Costa et al. (2021: 2 chloroplast, 3 nuclear ribosomal genes) were not very well supported, but the genera were - the clade [Didymyandrium + Krenakia] was sister to the rest of the tribe.
Cypereae. The old Cyperus was massively paraphyletic (e.g. Muasya et al. 2002; Larridon et al. 2011a, b; Hinchcliff & Roalson 2013), but over the years its circumscription has been expanded. Larridon et al. (2013; see also Bauters et al. 2014) suggested that C4 members of the clade were monophyletic and embedded in a paraphyletic C3 group, although relationships along the spine of the C4 clade were for the most part little supported (as in Reid et al. 2017: good support for [C. andinus + C. seslerioides] as sister to the C4 clade). Relationships in the rest of the tribe were clarified by Muasya and Larridon (2021), who found that these differed somewhat in nuclear and plastome analyses; Ficinia and Isolepis are sister, although their mutual limits needed slight adjustment, and below them were five small genera whose relationships varied - they were totally pectinate with nuclear data.
W. W. Thomas et al. (2009) discussed relationships within Rhynchosporeae. For relationships around and in Eleocharis, see Roalson and Friar (2000), Hinchcliff et al. (2010) and Roalson et al. (2010). Burchardt et al. (2020) found that Kükenthal's sections in Rhynchospora held up fairly well.
For the relationships of Gahnia and other Schoeneae, see X. Zhang et al. (2007), Viljoen et al. (2013) and Larridon et al. (2018: extensive para/polyphyly around Costularia). Van Mazijk et al. (2024: 3 chloroplast and 1 nuclear genes) looked at relationships among species of Schoenus and Tetraria from the Cape.
Within Scirpeae, Eriophorum is embedded in Scirpus (Léveillé-Bourret et al. 2014). The limits of Scirpus were adjusted by Léveillé-Bourret et al. (2015; see also Gilmour et al. 2013; Starr et al. 2019), although Eriophorum remained embedded in Scirpus s. str..
Sclerieae. Scleria is embedded in or sister to Bisboeckelereae (see literature in Bauters et al. 2016), and there is strong support for backbone relationships in that large genus (Bauters et al. 2016). The phylogeny of Scleria was revisited by Larridon et al. (2020b), and the small subgenus Brownieae, scattered from Malesia to the Pacific, was found to be sister to the rest of the genus.
Trichophoreae. Although RAD-seq data did not resolve many relationships along the spine of Trichophoreae, the tribe is clearly monophyletic and the expansion of the limits of Trichophorum is well supported - there are extensive parallelisms (Léveillé-Bourret et al. 2019).
The Scirpo-Caricoid clade: Léveillé-Bourret et al. (2014, 2015) found a quite well supported but paraphyletic Scirpeae that included Cariceae, although internal relationships were not well supported. Dulichieae may be sister to this clade, and the odd Khaosokia is also somewhere around here; the poorly-known Sumatroscirpus has turned out to be sister to Carex (Léveillé-Bourret et al. 2017a, 2018), and the two are both in monogeneric tribes. Léveillé-Bourret and Starr (2019) carried out a comprehensive study of the scirpo-caricoid clade and found six clades along the backbone below Carex, and a number of relationships between these clades were well supported, again, Scirpus (Scirpeae) is paraphyletic, as are Trichophorum and Oreobolopsis (both Trichophoreae).
Relationships are being resolved within the large and complex Cariceae which include over one third of the species in the whole family (e.g. Reznicek 1990 and associated papers; Yen et al. 2000; Roalson et al. 2001; Roalson & Friar 2004; Starr et al. 2006, 2015). The old Carex turned out to be paraphyletic, as has been shown by several studies (see also Yen & Olmstead 2000; Starr et al. 1999, 2004, 2015; Waterway & Starr 2008; King & Roalson 2008: use of nrDNA problematic; Starr & Ford 2009; Escudero & Luceño 2009). Starr et al. (2008b) suggested that there are four major clades in the Carex area - [Uncinia, Kobresia, Cymophylla, some Carex] [Schoenoxiphium, some Carex] [Carex subgenus Vignea], and [Carex subgenus Carex, etc.], and Carex has been expanded to cope. However, details of the relationships within Carex s.l. were uncertain (Hinchcliff & Roalson 2013), although as more taxa from east and southeast Asia were added, things became clearer (Starr et al. 2015). Classical sectional limits were often severely awry, as in Carex section Racemosae (Gebauer et al. 2015; see especially the Global Carex Group 2016). Relationships found by Starr et al. (2015) were [[sections Hypolytroides + Siderostictae] [Schoenoxiphium [core unispicate clade (inc. Kobresia) [Vignea clade [C. dissitiflora clade + core Carex]]]]]. In the massive analysis by the Global Carex Group (2016) of almost 1,000 species there was a surprising amount of resolution despite only three gene regions being used, two nuclear and one chloroplast, although some relationships along the spine (see also Gehrke et al. 2010) and between many of the species were poorly supported. Villaverde et al. (2020a) looked at 594 low-copy nuclear genes in 94 species of Carex and with a variety of analyses; they were able to identify six main clades, the Siderostictae clade being sister to the rest. Some of the relationships they recovered were rather unexpected, and there were some short branches deep in the phylogeny. Villaverde et al. (2020b: RAD-seq) disentangled relationships in section Schoenoxiphium, centred in southeast Africa (to Arabia and Madagascar); support was in general strong. García-Moro et al. (2022) has clarified relationships in subgenus Uncinia.
Classification. Goetghebeur (1998) provided a useful classification that has been modified in the course of the extensive molecular work that has since been carried out on the family. Léveillé-Bourret and Starr (2019) recognized a number of tribes, three new, in the scirpo-caricoid clade. For a general evaluation of generic limits in Cypereae, see Muasya et al. (2009b); Cyperus is to include about thirteen genera (see also Muasya et al. 2002; Hinchcliff et al. 2010; Huygh et al. 2010; Reynders et al. 2011; Larridon et al. 2011a, b, c, 2013, 2014; Reid 2011; Bauters et al. 2014); Eleocharis is also to be slightly expanded (Hinchcliff et al. 2010). Understanding the limits of Scirpus is difficult (Léveillé-Bourret et al. 2015). For the development of generic concepts in Schoeneae, see Larridon et al. (2018), while for pre-lapsarian nomenclature, etc., see Goetghebeur (1985); the World Checklist of Monocots (Govaerts et al. 2007) is a printed version of this. Larridon et al. (2021a, see also Larridon 2022 for a summary) have recently provided a revised classification for the whole family, and they recognise 2 subfamilies, 24 tribes and 10 subtribes (the latter are not included here). Bruhl et al. (2024) separate Asterochaete from Carpha, the two being easily distinguished by fruit characters, etc..
Carex in the old sense was paraphyletic (see above) including genera like Kobresia, Cymophyllus, Uncinia and Schoenoxiphium; the Global Carex Group (2015) formalized the extension of Carex to include these genera, although sectional limits were unclear. The Global Carex Group (2016) and Villaverde et al. (2020a) provide an infrageneric classification, assigning all species to the six subgenera that now make up the genus (however, note that subg. Psyllophorae is very much paraphyletic in Márquez-Corro et al. 2021: Fig. 1). For sections in subgenus Uncinia, see García-Moro et al. (2022). T. M. Jones provides a Carex interactive identification key; there is also a visual version. For comments on Carex and its classification, see also Muñoz-Rodríguez et al. (2023). Scleria has a new subgeneric/sectional classification (Bauters et al. 2016).
MAYACACEAE Kunth - Mayaca Aublet - Back to Poales
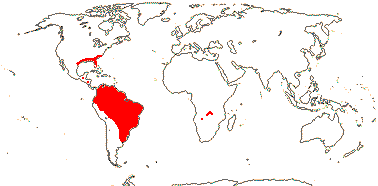
Marsh plants; plant ?monopodial; lateral roots originate opposite the phloem; vessels in leaves 0; stem with endodermis, vascular bundles arranged in a ring, often 3; stomatal subsidiary cells with intersecting oblique divisions; leaves scattered along the stem, amphistomatic, apically bidentate, univeined, lacking a distinct sheath, uniseriate colleters +; K with a single trace, C with a single trace, ± clawed, epidermal cells rounded, papillose; A 3, opposite K, dehiscing by pores/apical flap, (sporangia 2), wall with three layers [epidermis, endothecium, tapetum - Reduced type], exothecium +, endothecium lacking thickenings; tapetal cells uninucleate; pollen surface reticulate; nectary O; G initiated as an annular primordium, opposite K, placentation parietal, style impressed, with dorsal [?fused] (and ventral) carpellary bundles, stigma simple, lobes small/O; ovules 2-30/carpel, outer integument ca 2 cells across, inner integument ca 2 cells across, micropyle open, obturator + at apex of loculus; exotestal cells with U-shaped lignifications; n = 8, x = ?; primary root 0, cotyledon haustorial, cotyledonary hypophyllar sheath 0.
1 [list]/4-10. Mostly tropical and American, centre in Guyana, also S.E. U.S.A., 1 sp. from Africa. Map: from Hamann (1961), Distr. Pl. Afric. Vol. 3 (1971), Fl. N. Am. vol. 22. (2000) and Trop. Afr. Fl. Pl. Ecol. Distr. Vol. 7. (2012).
Age. An estimate of the age of crown-group Mayacaceae is (109-)82.4(-68.8) Ma (Bouchenak-Khelladi et al. 2014b).
Chemistry, Morphology, etc.. Mayacaceae are vegetatively rather different from many other Poales. There are no vessels in stems and leaves, perhaps associated with the aquatic habitat of the plant (Carlquist 2012a). The vascular bundles outside the endodermal ring are well separated from it (c.f. Eriocaulaceae: Malmanche 1919).
Tomlinson (1969) described the flowers as being terminal, although evicted into an exillary position by the growth of an axillary shoot, but the flowers seem to be axillary and associated with a broad, adaxial prophyll-like structure. Given the possible association of Mayacaceae with families that have scapose inflorescences with involucral bracts, the inflorescence of Mayacaceae bears re-examination. Sajo (in Sajo & Rudall 2012) noted that all perianth members had a single trace, but that to the petals divided into six. Anthers in some species are monothecal, and the stamens may be basically extrorse (Silveira de Carvalho et al. 2009). Venturelli and Bouman (1986) thought that the seeds lacked an operculum, despite some reports to the contrary, and they drew developing seeds with large-celled nucellar tissue towards the base.
Some information is taken from Thieret (1975), Stevenson (1998) and de Carvalho and Machado (2015), all general, Tomlinson (1969, 1974: anatomy), Oriani and Scatena (2012: esp floral morphology), and Endress (2008c: ovule).
The family is poorly known.
Classification. See the World Checklist of Monocots.
[Eriocaulaceae + Xyridaceae]: Rosette plants; root not medullated, vascular tissue with xylem and phloem mixed, or with single central vessel, cortex with air spaces; vessel elements with simple perforation plates; leaves also two-ranked; inflorescence capitate, with involucral bracts, terminal (axillary), scapose; flowers (monosymmetric), (2-merous); C clawed, epidermal cells elongated, walls straight; (common A-C primordia [only antepetalous A]); if A = 3, opposite C; anther wall development of the monocot type, endothecial cells with U-shaped band-like thickenings; pollen more or less spiny; styluli + stigmas commissural, not vascularized; ovules straight, obturator 0; seed surface ± ridged; seed endotestal, cuticular layer between testa and tegmen.
Age. Eriocaulaceae and Xyridaceae may have diverged ca 105 Ma (Janssen & Bremer 2004) or about 77.1 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. For anatomical similarities between the two families, see de Oliveira et al. (2015). The pattern of floral evolution in this clade is unclear. Some Eriocaulaceae and Xyridaceae have very similar carinal nectariferous appendages at the base of or along the style, and unvascularised commissural stylar branches with stigmas, a very unusual and distinctive arrangement; these have been placed as synapomorphies for the larger clade, with subsequent loss in some pre 2021 versions of the site, although A. de L. Silva et al. (2021: the two families treated as sister taxa) suggest that independent origins are more likely. Similarly, an androecium consisting of three antepetalous stamens may have evolved independently; it is treated below as apomorphy in both Eriocaulaceae-Paepalanthoideae and Xyridaceae. See Oriani and Scatena (2012) and Sokoloff et al. (2020) for comparisons of reproductive features of the two families.
Oriani and Scatena (2012, 2014) evaluated variation in many floral characters in the context of a clade [Mayacaceae [Eriocaulaceae + Xyridaceae]]; see also Nardi et al. (2015) and Sajo et al. (2017). For syringyl lignin, see Gibbs (1958); the aquatic habitat tends to be linked with low amounts of S-lignin in the plant. Characters are hard to place on the tree: is the absence of a leaf sheath an apomorphy for the clade, with a reversal, or two independent aquisitions, ditto colleter presence, etc.? Getting the phylogeny straight is also crucial...
Pollination Biology. Nectar is produced by the stylar appendages (Sajo et al. 2017).
Chemistry, Morphology, etc.. The scape of Eriocaulaceae lacks inflorescence bracts, i.e., it is a "true" scape, while that of Xyridaceae may have inflorescence bracts half way up. The various layers in the seed are differently thickened (see below).
ERIOCAULACEAE Martinov, nom. cons. - Back to Poales
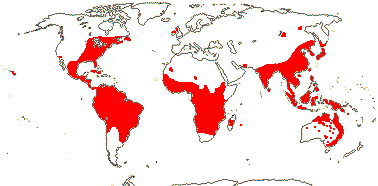
Root cortex aerenchymatous, pericycle ?not lignified, (vessel elements with scalariform perforation plates); bundles arranged in a ring; calcium oxalate crystals +; hairs various, on vegetative parts with foot cell and bulbous persistent usually dark colored basal cell; cuticle waxes as aggregated rodlets, stomata variable; in leaves aerenchyma alternates with lignified strands, photosynthetic tissue in separate packets in t.s., bundle sheath cells large, without chloroplasts, palisade tissue 0; leaf sheath not distinct; plant monoecious (dioecious, flowers perfect); scape spirally twisted, basal inflorescence bract with closed sheath; receptacle ± flat; flowers with median K adaxial, abaxial K lateral [?all - E. yes], flowers small [6> mm across], (dimerous); K basally connate, with 1 trace, lacking stomata, aestivation open, C with 1 trace, scarious, aestivation open, nectar glands +; staminate flowers: anthophore + [= elongated internode between K and rest of flower]; A adnate to C, anthers (dorsifixed); endothecial cells with complete base plate; microsporocytes in a single row in each loculus; pollen grains spherical, spiraperturate; pistillode +, nectariferous; carpelate flowers: C connate in the middle, fenestrate at base with schizogenous slits, staminodes +; G opposite K, (2, collateral), floral centre massive, convex, G develop around it, placentation axile, style branched; ovule 1/carpel, pendulous; antipodal cyst + [formed by fusion of antipodal cells]; K/C persistent in fruit; seed operculum tegmic; exotesta disintegrates, but areas of thickening on walls persist, endotesta thickened on (anticlinal and) inner periclinal walls; n = 9, 15, 20, 25; x = 7 or 6 (?8), nuclear genome (1 C) (0.076-)1.279(-21.67) pg; plastid (ORF 2280 +); primary root 0 [area covered by operculum].
7 [list: to subfamilies]/1,090 - two subfamilies below. Pantropical (to temperate), but esp. Guyana Highlands and S.E. Brasil. Map: from Hamann (1961), Giulietti and Hensold (1990), Fl. N. Am. vol. 22 (2000), Australia's Virtual Herbarium (consulted i.2014) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). 2 groups below.
Age. Crown-group Eriocaulaceae are ca 57 Ma (Larridon et al. 2019), ca 58 Ma (Janssen & Bremer 2004) or (78-)64(-55.2) Ma (Bouchenak-Khelladi et al. 2014b).
1. Eriocauloideae Burnett
Plants usu. of aquatic habitats; roots and leaves with aerenchyma; (inflorescence bracts petal-like); plant dioecious; K 2 cell layers thick, (with 3 traces - Mesanthemum), (monosymmetric, connate, not abaxially, forming spathe-like structure), C free or connate, with short-palisade epidermis, black/brown nectar glands apical/subapical/adaxial/0; staminate flowers: A 4, 6, inner whorl ± adnate to C; carpelate flowers: C free [Eriocaulon]; staminodes inconspicuous; stylar appendages 0 (+, very small); ventral bundles basally opposite loculi, divide distally, branches fuse with bundles of adjacent carpels [Mesanthemum]; exotestal cells transversely (horizontally) elongated, anticlinal and ?inner periclinal walls very variously thickened.
2/486: Eriocaulon (470). Pantropical (to Temperate).
Age. Diversification in Eriocauloideae began ca 36.5 Ma (Larridon et al. 2019).
2. Paepalanthoideae Ruhland
Plants usu. terrestrial, (stem +, decumbent/rigidly erect); (monocot secondary thickening +); (aerenchyma +); (hairs T-shaped); (apex of leaf bifid); (synflorescences +, branched or not); (flowers perfect); K = to or longer than C, (C 0); staminate flowers: C basally fused, eglandular, (fleshy), (reduced to long trichomes); A (2)3, opposite C, (bisporangiate, dithecal/monothecal); (styluli +); carpelate flowers: K (valvate), C (free), (0); G primordium initially 3-lobed, ovule exposed, (styles branched ± to the base), stylar appendages +, carinal, vascularized by carinal bundle [= nectariferous branches], stigmas commissural [= carpel margins], not vascularized [= stigmatic branches]; fruit (indehiscent, 1-seeded); seeds exo-(endo-)testal, the anticlinal exotestal walls with prominent rib-type/columnar thickenings and longituinal walls often with grapnel-type projections, the outer periclinal wall breaking down and exposing fuzzy-looking thickenings.
5/605: Paepalanthus (392), Syngonanthus (120), Leiothrix (47), Comanthera (40), Giuliettia (30). Most New World, esp. E. Brazil and Andean South America (both Paepalanthus), few Africa.
Age. Crown-group Paepalanthoideae are ca 37 Ma (Larridon et al. 2019).
Evolution: Divergence & Distribution. Eriocaulaceae are the Asteraceae of the monocots with their capitate inflorescences and tiny flowers that nevertheless show a great deal of variation.
There is much local diversification in Neotropical Paepalanthoideae, and early-divergent taxa are found in the Pantepui region, the Venezuelan-Guyanan higlands (Trovó et al. 2013); the large genus Paepalanthus is centred on the northwestern Andes and East Brazil (Andrino et al. 2020) and characters are displayed on the tree (Andrino et al. 2022). Diversification within Eriocaulon began (28.4-)21.7(-15.9) Ma, but most has occurred within the last 10 Ma or so (Larridon et al. 2019).
A. de L. Silva et al. (2021b: e.g. Fig. 15) discuss the evolution of a number of floral characters in Eriocaulaceae. For inflorescence morphology and evolution in Paepalanthus, see Trovó and Stützel (2019).
Ecology & Physiology. In some submerged species of Eriocaulon, CO2 is taken up from the mud in which they grow by way of their very well developed root systems (Raven et al. 1998).
Water may be trapped in the rosettes of Paeapalanthus bromelioides growing in poor soil over quartzite. The plant may acquire nitrogen indirectly from the excreta of spiders that form webs on the plant or from termites in the termite mounds on which it often grows (Nishi et al. 2013), but whether or not there is carnivory here is unclear (Freund et al. 2022).
There are quite a few cushion plants in the family (Boucher et al. 2016b).
Photonic structures, helicoid structures in the cell wall, are known from Paepalanthus stegolepoides; they produce a distinctive blue iridescence of the leaves of the plant (Lundquist et al. 2024).
Pollination Biology & Seed Dispersal. Inflorescences can be very long-lived (up to a year), and the opening of the flowers on the one head is synchronized; in some cases all open flowers are staminate or carpelate (Stützel 1998 for a summary) or a single whorl of staminate and of carpellate flowers open together (Martins Junior et al. 2022). Pseudanthia are quite common, being reported from members of both subfamilies (Baczynski & Claßen-Bockhoff 2023) Although the flowers are individually rather small and inconspicuous, pollination seems to be by insects, in some Paepalanthus at least ants being effective pollinators (Martins Junior et al. 2022). The dark-colored glands on the petals of Eriocaulon may produce nectar (Stützel 1985b; Hensold 1988). Rosa and Scatena (2003) suggest that in at least some Paepalanthoideae the pistillode (in staminate flowers) and carinal appendages on the gynoecium (carpelate flowers) are nectariferous (see also Rosa & Scatena 2007; c.f. Ramos et al. 2005); the nectary in both cases is made up of much-elongated epidermal cells (Oriani et al. 2009).
For diaspore dispersal in the family, see Trovó and Stützel (2011). In some species of Paepalanthus the fruit is an achene that is explosively dispersed as the sepals abruptly recurve, while other species are described as having loculicidal capsules (Trovó et al. 2018). The ?exotestal appendages of Eriocaulon are sometimes mucilaginous (Wantongsuk et al. 2024).
Genes & Genome. There has been major movement of protein genes involved in the large and small subunits of mitochondrial ribosomes and of succinate dehydrogenase genes from the mitochondrion to the nucleus in Lachnocaulon (= Paepalanthus), at least (K. L. Adams et al. 2002b; Adams & Palmer 2003).
Chemistry, Morphology, etc.. In an anatomical survey of Brazilian Eriocaulaceae, secondary thickening was reported from species of Paepalanthus and Syngonanthus (Scatena et al. 2005; see also Rapateaceae). Malmanche (1919) draws phloem and xylem elements intermixed in the root stele of Paepalanthus weddellianus, but not in other taxa; this feature is common in Xyridaceae (Carlquist 1966a).
Inflorescence morphology shows considerable variation, even if the variation is on a single theme (Stützel 1982; Stützel & Trovó 2013: lovely shots of closed bract = branch prophyll associated with the young inflorescences). In Tonina (= Paepalanthus) the scape is short and not twisted; at the base is a sheathing adaxial prophyll that is shortly fused abaxially. Trovó et al. (2010) discussed inflorescence morphology and anatomy, and in the scape in particular there was a sinuous band of sclerenchyma snaking around the single ring of bundles in such a way that it was alternately outside and inside the bundles, and bands of chlorenchyma in the cortex were opposite the latter bundles (elsewhere in the reproductive/inflorescence axis the vascular bundles were scattered in the pith, although there might be none at the very centre).
In dimerous Paepalanthus the outer tepals are in the horizontal plane and the inner tepals are vertical (Nakagawa et al. 2020). For floral morphology in Eriocaulon in particular, see A. de L. Silva et al. (2021b). Staminate flowers, at least, commonly have an anthophore, a prolongation between K and the rest of the flower, and other floral whorls may be separated by short internodes, for instance, there may be a short gynophore (Sokoloff et al. 2020). The distinction between staminate and carpelate flowers becomes apparent only late in development (e.g. Sokoloff et al. 2020), and Wurdackia (= Rondonanthus) flabelliformis and a few other species have perfect flowers (Stützel 1985b; Watanabe et al. 2015). The flowers are tiny, yet show a great deal of variation in merosity, tepal texture, connation of the two whorls, sometimes with differences between staminate and carpelate flowers of the same species (the tepals may be connate at the base and the apex, or apex only, and then there are three apertures on the sides), the presence of perianth glands, etc. (e.g. Giulietti & Hensold 1990). Stützel (1985a) found that the morphologically apical glands on the petals of Eriocaulon were displaced by an abaxial outgrowth of tissue and became adaxial-subapical. The tetrasporangate anthers are described as being extrorse (Ike Coan et al. 2012), but they seem to be more or less latrorse; the two sporangia of bisporangiate anthers usually represent a single theca, but there is considerable variation as to which theca develops, even within a single flower (Stützel 1985). Rosa and Scatena (2007) describe staminodial scales opposite to the ovary septae or adnate to the base of the petals in Paepalanthoideae. When the style is commissural, as in Paepalanthoideae, it is unvascularized; the ovarian appendages of Syngonanthus, etc., are in the position of the style branches of Eriocaulon, and both are vascularized (Ike Coan & Scatena 2004; Rosa & Scatena 2007). The seed coat of Eriocaulaceae is described as being endotestal, but if this is the case in Mesanthemum (see Liang et al. 2019) and Eriocaulon, at least, the exotesta would seem to have completely disappeared; the diversity of thickenings of the ?exotesta in the latter genus has to be seen to be believed (Wantongsuk et al. 2024).
For general information, see Stützel (1998), Unwin (2004) and Liang et al. (2019: Mesanthemum), for anatomy, see Malmanche (1919), Tomlinson (1969), Stützel (1988), Alves et al. (2013), Mascarenhas et al. (2019: Leiothrix leaf, scape) and Khoshnaw et al. (2022: Eriocaulon leaf), for inflorescence and flower, Stützel (1984) and Andrino et al. (2023), for floral morphology, see Stützel (1990), Stützel and Gansser (1995) and A. de L. Silva et al. (2021a: Mesanthemum, 2021b: Eriocaulon), for floral anatomy, Sajo et al. (1997: petals compound structures) and Rosa and Scatena (2003), for pollen morphology, de Borges et al. (2009), for embryology and seed development, Arekal and Ramaswamy (1980), Ramaswamy and Arekal (1982 and references) and Scatena and Bouman (2001), for seed morphology, see Giulietti et al. (1984), Barreto et al. (2013) and Andrino et al. (2023), and for seeds and seedlings of Paepalanthus, see Kraus et al. (1996) and for those of Leiothrix (and comments on variation in the whole family), see Mascarenhas and Scatena (2021).
Phylogeny. Unwin (2004: three genes) found good support for the monophyly of Eriocauloideae and Paepalanthoideae sampled, while the more detailed study by Gomes de Andrade et al. (2010: also three genes) provided considerable additional phylogenetic resolution; the basic phylogenetic structure in the family is [[Mesanthemum + Eriocaulon] [Comanthera, Syngonanthus, The Rest]]. Within Paepalanthoideae, Giulietti et al. (2012) found the relationships [Syngonanthus [Comanthera [[Rondonanthus + Leiothrix] [The Rest]]]], although not with particularly strong support. In the extensive study of Echternacht et al. (2014) there was good support for Rondonanthus being sister to the all other Paepalanthoideae, [Paepalanthus [Leiothrix [Syngonanthus + Comanthera]]] was the topology in the rest of the subfamily. Trovó et al. (2013) also discussed the phylogeny of Paepalanthoideae. Sections in Syngonanthus are not holding up (Echternacht et al. 2014; Watanabe et al. 2015). Andrino et al. (2020) looked at Paepalanthus in some detail (197 species, 3 chloroplast and 2 nuclear markers) and confirmed the paraphyly of the genus and of subgenus Paepalanthus, the former including three other genera and the latter including the four other subgenera, albeit the latter were largely monophyletic. Furthermore, only seven of the 37 sections that had more than one species were recovered as being monophyletic (Andrino et al. 2020). Similarly, within Eriocauloideae, Larridon et al. (2019), with a focus on Old World taxa, found little support for earlier morphology-based infrageneric groupings in their study of the phylogeny of Eriocaulon. See Darshetkar et al. (2021b) for relationships in Indian Eriocaulon; Liang et al. (2019) discuss some relationships in Mesanthemum.
Classification. Generic limits in Paepalanthoideae have been unclear, and it seemed as if Syngonanthus needed to be divided (Parra et al. 2010; Gomes de Andrade et al. 2010) and Paepalanthus, already large, expanded somewhat (Gomes de Andrade et al. 2010); see also Trovó et al. (2013). Andrino et al. (2020) discussed the classificatory options that became evident in their study of Paepalanthus, perhaps inclining towards adopting a broad cirumscription of the genus, which would seem reasonable, although Andrino et al. (2021) adjusted the limits of Actinocephalus, a potential segregate of Paepalanthus. In any case the infrageneric taxonomy of Paepalanthus, over 100 years old (it goes back to Wilhelm Ruhland in Das Pflanzenreich in 1903 - and parts are earlier) needed a major overhaul (Andrino et al. 2020). Andrino et al. (2023) carried out a comprehensive revision of Paepalanthoideae; they recognized 12 new and resuscitated genera, although Paepalanthus still had some 257 species; Stützel et al. (2024) presented a critique of this new classification, and it does seem that a broad circumscription of Paepalanthus makes sense. Larridon et al. (2019) reasonably decided to wait until after looking at New World Eriocaulon before reworking its infrageneric classification. The World Checklist of Monocots provide a listing of species.
XYRIDACEAE C. Agardh, nom. cons. - Back to Poales
Anthraquinones +; root with stellate cortical cells, vascular tissue scattered in pith; inflorescence axis/culm vascular bundles amphivasal, cortical bundles +, sclerenchymatous ring around central cylinder; cuticle with insoluble [organic solvent] secretion; mucilage-producing multicellular hairs +; foliar vascular bundles with inner thick-walled and outer thin-walled sheaths; flowers perfect; K monosymmetric, keeled, (2 keeled), the median [abaxial] membranous, smaller, (deciduous), C apex fimbriate, more or less clawed, fugacious, connate or not, cuticle ornamented; A 3, opposite C, anthers basifixed, extrorse or latrorse, (free), (sporangia connate); anther wall with three layer's [epidermis, endothecium, tapetum - Reduced type: ?level]; placentation axile, stigma complex, branched/lobed/infundibular; ovules many/carpel; 2 lateral K persistent in fruit; operculum +, testal and tegmic; endosperm helobial [chalazal chamber smaller, nuclear divisions only]; deletions in ORF 2280 region [?whole family]; seedling radicle +.
5/275 (399) [list] - two subfamilies below (classification/relationships rather notional). Pantropical to warm temperate, esp. South American.
Age. Crown group Xyridaceae are ca 87 Ma old (Janssen & Bremer 2004) or rather younger, (84.5-)63(-53.5) Ma (Bouchenak-Khelladi et al. 2014b).
1. Xyridoideae Arnott - Xyris L.
Plant rhizomatous/bulbous, (monopodial), ± tufted/clustered; vessel elements usually simple; stem vascular bundles in a single ring; leaves 2-ranked, ventralized isobifacial [oriented edge on to the stem], (terete), ligulate or not, Vorläuferspitze +; K lacking stomata, with 1 trace, C with 3 traces, (enclosing stamen + two half-staminodes from adjacent staminodes); A (endothecium lacking thickenings), staminodia opposite K, branched, moniliform hairs on branch ends; pollen often binucleate, elliptic (subspherical), (sulcus U-shaped), (bisulcate), surface reticulate and punctate or foveolate, 32-70µm; long; placentation also (intrusive) parietal/basal/free central, stylar appendages 0, (style branched); funicle long, hypostase 0; (embryo sac bisporic (= the chalazal dyad), 8-celled [Allium type]); seeds with apical exotestal scales or fimbriae; endosperm (?nuclear), starch grains compound; n = ?8, 9, 13, 14, 16, etc., extensive polyploidy; cotyledon part haustorial, hypophyll blade-like and photosynthetic, hypocotyl and collar rhizoids +.
1/225-300. Pantropical to warm temperate, 150 spp. in Brazil. Map: from Hamann (1960), FloraBase (consulted 2004) and Trop. Afr. Fl. Pl. Ecol. Distr. 7 (2012). Photos: Xyris Flower, Infructescence © H. Wilson.
2. Abolbodoideae Reveal
Plant ± tufted; vessel elements scalariform or simple; cauline vascular bundles also scattered in centre; leaves spiral; K (polysymmetric), (abaxial K with 1 or 0 traces), C with 5-9 traces; A (introrse), (dorsifixed), staminodes 0; tapetum multilayered, plasmodial, microsporocytes as single row; pollen binucleate, spherical, inaperturate, surface with large and small clavate projections [baculae]/spiny, 49-250 µm long; placentation intrusive parietal, etc., stylar appendages +, carinal, vascularized by branches of the dorsal carpel bundle, stigma (capitate), (plumose); ovules anatropous (slightly campylotropous), (outer integument 4-5 cells across, suprachalazal tissue massive, hypostase +; exotesta thick-walled, anticlinally elongated, endotesta enlarged, exotegmen thick-walled; (endosperm cellular); n = 8-10, 13, 17.
4/26. Northern South America, Guyana Highlands in particular. Map: from Campbell (2004).
2A. Achlyphila disticha Maguire & Wurdack
Leaves 2-ranked, isobifacial; inflorescence branched, with 1 or more pairs of opposite bracts along the scape; C with 3 traces; pollen 22-34 µm long, clypeate; stylar appendages 0; n = .
1/1. Southern Venezuela, Guyana Highlands.
2B. Abolboda Humboldt —— Synonymy: Abolbodaceae Nakai
Stomata also tetracytic; inflorescence (branched), (with 1 or more pairs of opposite bracts along the scape); (abaxial K 0), C [3]; staminodia (+, opposite K, filiform); anther tapetum amoeboid; style branches solid, with conducting tissue, 1 appendage reduced; endotestal cells large, alternating with projecting exotegmic cells; n = ?
1/22. Central and tropical South America, Trinidad, few Andean.
[Orectanthe + Aratitiyopea]: plant rosette-forming; leaf blade broad.
2C. Orectanthe Maguire
pollen ca 185µm across; style branches solid, with conducting tissue, stigma/style wth secretory ducts; outer integument ? cells across; embryo sac with ears; exotesta of several layers, forming wing; n = ?; seedling cotyledon haustorial.
1/2. Venezuela, on tepuis.
2D. Aratitiyopea lopezii (L. B. Smith) Steyermark & L. Berry
Roots (with radially elongated air canals); stem trailing; inflorescence sessile; C contorted, apex entire; style branches?; embryo sac tetrasporic, 16-celled, 11 cells congregating at the chalazal end [Drusa type]; n = ?
1/1. Northern South America.
Evolution: Divergence & Distribution. If Achlyphila is sister to other Abolbodoideae, apomorphies for the latter will need to be adjusted.
Pollination Biology. Pollen grains may collect among the hairs on the bifid staminodes of Xyris, perhaps a form of secondary pollen presentation (Remizowa et al. 2012a).
Plant-Bacterial/Fungal Associations. The family apparently lacks mycorrhizae.
Laraba er al. (2021) found that Xyris setigera and X. surinamensis on Guayana savannas were infected by Fusarium xyrophilum; the plants did not produce their normal, short-lived flowers, but relatively long-lived fungal look- (and smell-)alike structures that were visited by bees that dispersed the fungal conidiospores (the fungus may be heterothallic).
Genes & Genomes. Benko-Iseppson and Wanderley (2002) discuss some aspects of the cytology of the family.
Chemistry, Morphology, etc.. Cury et al. (2012) describe primary thickening in the rhizome of Xyris. Mucilage is secreted by hairs in the leaf axils (c.f. Mayacaceae?).
The scape of Xyris is sometimes spirally twisted (c.f. Eriocaulaceae). Placentation is very variable in Xyris in particular, where axile placentation seems to be the plesiomorphic state (Sajo et al. 2017; Nardi et al. 2021), but that of the whole family may be basically parietal. Sajo et al. (1997) show small swellings on the style of Xyris paradisiaca just below the stylar branches. Seedling collar rhizoids are not drawn in Tillich (1994).
Additional information is taken from Carlquist (1960), Kral (1988: Xyris, 1992: most Aboboldieae, 1998), Judd et al. (2002), Campbell (2004, especially 2008, all general. See also Malmanche (1918), Tomlinson (1969), Sajo and Rudall (1999: development of ensiform Xyris leaf), Scatena et al. (2011: Abolboda), de Oliveira (2015), and Cury et al. (2017: Xyris), all anatomy, Kral (1988: Xyris, 1992: other than Xyris), Rudall and Sajo (1999: flower and seed), Scatena and Bouman (2001: seed operculum), Campbell (2012: pollen, Achlyphila perhaps multiaperturate), while for embryology, floral morphology, etc., see Tiemann (1985: Abolboda), Stützel (1990), Campbell and Stevenson (2008: esp. Aratitiyopea), Winzieher (1914), Govindappa (1955), especially Nardi et al. (2015), Remizowa et al. (2012a), all Xyris, and Oriani and Scatena (2011, 2012, 2015, 2016), all Abolbodoideae.
Phylogeny. In some analyses Xyridaceae is not recovered as being monophyletic (Michelangeli et al. 2003; J. I. Davis et al. 2004), thus Abolboda was separate from Xyris (Givnish et al. 2010b), but sampling there could be improved. Only Aratitiyopea and Xyris were included in the Angiosperms353 tree as of 6.ix.2024; Xyris was sister to the Eriocaulaceae included, Aratitiyopea was on an adjacent but very poorly-supported branch. Campbell (2004: q.v. for more information) carried out a detailed phylogenetic analysis of morphological variation.
Classification. For the above subfamilial classification, see Campbell (2004), also the World Checklist of Monocots.
[Restionaceae [Flagellariaceae [[Joinvilleaceae + Ecdeiocoleaceae] Poaceae]]] / graminid clade: flavones +, tannin-like substances +; root trichoblast-atrichoblast pair with the former furthest from the apex; sieve tube plastids with cuneate and other less densely packed crystals; (chlorenchyma with peg cells [c.f. arm cells of some Poaceae?]); leaves two-ranked, with sheath; inflorescence branches spiral [?at higher level]; spikelets +; bracteoles 0; flowers small [<1 cm across]; P = T 3 + 3, membranous/scarious; endothecial cells with ± helical/girdle-like thickenings; pollen monoporate, margin annulate [= ulcerate], wall scrobiculate [minute pores penetrating tectum and foot layer]; style branches long, stigmas plumose, receptive cells on multicellular branches; ovule 1/carpel, straight, apical, pendulous; fruit a capsule; seedling with collar rhizoids; CslF gene.
Age. The age of this node may be ca 108 Ma (Janssen & Bremer 2004: c.f. topology), but the age in Wikström et al. (2001: again c.f. topology) is only (52-)49, 45(-42) Ma, Bell et al. (2010) suggested an age of (76-)65, 58(-45) Ma, while around 80.8 Ma is the age in Magallón et al. (2015).
Evolution: Divergence & Distribution. Magallón and Sanderson (2001) thought that this clade, the graminid clade, with about 11,000 species (sic), was notably speciose, although the diversification rate was lower than that in the Cyperaceae area. However, there is considerable asymmetry in clade size within the clade. Most species belong to Poaceae, with ca 11,400 species, the second most species-rich family (Restionaceae) having only some 545 species, fewer than 1/20th the species in Poaceae (Chase 2004; c.f. Linder & Rudall 2005 for diversification), and within both Poaceae and Restionaceae there are also considerable asymmetries of clade sizes. This is discussed further below under both families.
Linder (1987) suggested some possible apomorphies here, altough some are now thought to be plesiomorphies.
Genes & Genomes. Information on the ORF 2280 region is taken from Hahn et al. (1995) and Katayama and Ogihara (1996: Ecdeiocoleaceae not included). For the loss of the chloroplast PEP' subunit β rpoC1 gene, see Morris and Duvall (2010). The pattern of gain and and loss of the 28 kb chloroplast gene inversion is complex: Either a minimum of two gains, and probably subsequent loss, or one gain and at least two subsequent losses (see also Michelangeli et al. 2003).
Chemistry, Morphology, etc.. Tannin-like substances are reported for many members of this clade (e.g. Cutler 1969; Ellis 1990), and are likely to be plesiomorphic, although I do not know their exact distribution, for instance, in the basal clades of Poaceae. For the flavonoids of Restionaceae and Ecdeiocoleaceae, see C. A. Williams et al. (1997a); the variation is complex and needs to be re-evaluated in light of the current position of the last family. Mixed-linkage glucans (MLGs) occur here (Trethewey et al. 2005), but work by Little et al. (2018) suggests that they are found in all monocots. However, of the genes of the Cellulose Synthase gene superfamily that are involved in this synthesis, two (CslH and CslJ) are found in all/most monocots, while one (CslF) can be pinned to this node (Little et al. 2018).
For inflorescences, see Kellogg et al. (2013); Sokoloff et al. (2019a) suggest that posession of spikelets has been "conserved across the restiid and graminid clades". Ladd (1977: no Poaceae) and Linder and Ferguson (1985) discuss variation in pollen morphology. Flagellariaceae have "multicellular papillae" on their stigmas (Appel & Bayer 1998), but whether these are receptive in the same way as the multicellular branches of, say, Poaceae, needs clarification. Determining that the pollen is operculate can be tricky. In Poaceae, although the style is hollow, the pollen tubes grow between elongated transmitting cells of the multicellular stigmatic branches (Lersten 2004). The ovule is scored as lacking any parietal tissue (and so being tenuinucellate) and the pollen as being tricellular for the whole group by Givnish et al. (1999), but c.f. Appel and Bayer (1998) for these characters. Fomichev et al. (2019) describe the Anarthria group as having straight ovules, but differing in position and orientation, that of Anarthria itself, apical and pendulous, being like that of other members of this whole clade. They note that in Poaceae the ovule changes its position during development, and even if it starts off as being basal, the micropyle ends up facing the base of the gynoecium, in Anomochlooideae the ovule becoming anatropous (Fomichev et al. 2019; see also Batygina 1987).
Phylogeny. An analysis of 26S rDNA suggested that Dasypogonaceae might be part of this clade, being very closely linked with Ecdeiocoleaceae, Anarthriaceae and Centrolepidaceae (the latter two now in Restionaceae) (Neyland 2002b), slightly less so with the one member of Restionaceae included. However, data from atpB, rbcL, 18S, etc., have not confirmed this grouping (see e.g. Givnish et al. 2010b), and Dasypogonaceae are sister to Arecaceae in Arecales (q.v.). J. I. Davis et al. (2004: very weak support) found that Flagellaria grouped with Mayacaceae, etc., rather than with the Poales near Poaceae.
If the composition of the graminid clade seems settled, relationships within it are in part still somewhat unclear. In an early morphological analysis, Linder (1987) suggested the relationships [Poaceae, Ecdeicoleaceae [the rest]]. Bremer (2002) found a sister group relationship between Ecdeiocoleaceae and Poaceae (see also Harborne et al. 2000). A combined morphological and molecular (mitochondrial and chloroplast genes) analysis placed Flagellariaceae, Ecdeiocoleaceae and Poaceae in an unresolved tritomy (Michelangeli et al. 2002, esp. 2003), a not dissimilar result to that obtained by J. I. Davis et al. (2004). Graham et al. (2005) obtained a set of relationships [Flagellariaceae [Restionaceae [Ecdeiocoleaceae + Poaceae]]], perhaps a branch length or sampling problem. Using two chloroplast genes, Marchant and Briggs (2007) found strong support for a sister group relationship between Joinvilleaceae and Ecdeiocoleaceae (both genera of the latter were included), and these relationships were also found by Saarela and Graham (2010), but only in Bayesian analyses (see also McKain et al. 2016a: phyogenomic analysis; W. J. Baker et al. 2021a: see also Seed Plant Tree, inc. version of ix.2024; Timilsena et al. 2022a; H. Wang et al. 2024: transcriptome analyses; Zuntini et al. 2024). However, Givnish et al. (2010b: plastome sequences; see also Barrett et al. 2015b; H.-T. Li et al. 2019, see also 2021) found good support for an [Ecdeiocoleaceae + Poaceae] clade, while H. Wu et al. (2022: fig. 3) found about equal support for the two topologies. The [Joinvilleaceae + Ecdeiocoleaceae] clade is recognised below.
Classification. This clade is the old Poales s. str..
RESTIONACEAE R. Brown, nom. cons. - Back to Poales
Sand-binding roots + [capillaroid roots], root hairs usu. persistent, lignified, originating from any epidermal cells; culm with fibre cylinder [outer and sometimes inner cauline vascular bundles embedded in it, no vascular bundles outside it], parenchymatous sheath +, chlorenchymatous tissue palisade, with peg cells; leaf blade much reduced, ± unifacial [adaxial surface reduced]; plant dioecious; culm branched or not; T ± scarious; staminate flowers: A 3, opposite inner T, anthers dorsifixed, bisporangiate; pistillode 0/+; carpelate flowers: staminodes 0/+; floral centre convex, G develop around it, opposite outer P; fruit a loculicidal capsule; ; x = ?7, ?6, nuclear genome [1 C] (0.062-)1.333(-28.86) pg; plastome loss of PEP subunit β' rpoC1 gene; seedling with phanomer [photosynthetic unifacial cotyledonary hyperphyll].
64 [list: to tribes]/545 - five subfamilies below. Rather scattered, mostly in (warm) temperate Southern Hemisphere, especially Australia, southern Africa (map: from Good 1974; Trop. Afr. Fl. Pl. Ecol. Distr. 7. 2012).
Age. The age of this node is ca 96 or 97 Ma, depending on relationships (Janssen & Bremer 2004), ca 80 Ma (Litsios et al. 2014: "root" age for Restionaceae, Centrolepis, etc., not included), or about 75.9 Ma (Magallón et al. 2015).
The 27.7 Ma fossil Restiocarpum latericum was assigned to this node (Iles et al. 2015).
1. Anarthrioideae [name informal]
(Flavonol glycosides +); SiO2 0; rhombic calcium oxalate crystals in stem; culm branched or not [add below]; plant dioecious; staminate flowers: anthers dehiscing by two slits; pollen operculate, operculum small, raised-annular, [exine inside annulus], pistillode +; carpelate flowers: hypostase +; (pollen grains in embryo sac); seed coat?; endosperm type?, embryo?; chromosomes 1.7-7µm long; ORF 2280 +, 28 kb chloroplast genome inversion 0, trnL gene with 3bp deletion and 5bp insertion; ?collar rhizoids.
3/12. S.W. Australia. Map: from FloraBase (consulted 2004). Photo: Anarthria: Staminate & carpelate inflorescences © D. Woodland.
Age. The age of crown-group Anarthrioideae is ca 55 Ma (Janssen & Bremer 2004) or much younger, (60-)25.6(-17.2) Ma (Bouchenak-Khelladi et al. 2014b).
1a. Anarthria R. Brown —— Synonymy: Anarthriaceae D. F. Cutler & Airy Shaw
Culm lacking sclerenchymatous cylinder, vascular bundles in one/two rings, inner embedded in sclerenchymatous sheath; leaves two-ranked, blade +, equitant; spathe +, caducous; outer T basally connate; carpelate flowers: staminodes +; micropylar epidermal cells isodiametric; embryo sac lacking starch grains, chalazal part massive; endotegmen tanniniferous; n = 11.
1/7. S.W. Australia.
[Hopkinsia + Lyginia]: vesicular arbuscular mycorrhizae +; A bilobed; pollen pore small, 6>µm, margin raised [foot layer much thickened], microverrucate; ovule facing abaxially/dorsally, spherical.
1b. Hopkinsia W. Fitzgerald —— Synonymy: Hopkinsiaceae B. G. Briggs & L. A. S. Johnson
Chlorenchyma pillar cells +, peg cells 0; leaf sheath with shoulder; carpelate flowers: staminodes +; G 1, stigma plumose; embryo sac starch grains?; fruit a nut/drupe, pedicel fleshy, style deciduous; n = 9; cotyledon apparently not photosynthetic.
1/2. S. W. Australia.
1c. Lyginia R. Brown —— Synonymy: Lyginiaceae B. G. Briggs & L. A. S. Johnson
Arbuscular mycorrhizae +; fructans [in rhizome], allose oligosaccharides +; stomata at angle to epidermal cells; culm unbranched; A with filaments connate; carpelate flowers: staminodes 0; embryo sac with starch grains; seeds minutely spiny, with a central hyaline flange; n = 6.
1/3. S.W. Australia.
[Restionoideae [Sporadanthoideae [Centrolepidoideae + Leptocarpoideae]]]: rhizome with endodermoid sheath [?level]; lignified chlorenchymatous cells lining substomatal cavities [protective cells; ?level]; plant ± glabrous; SiO2 not in epidermis, but in parenchymatous bundle sheath; (foliar epidermis with long and short cells); (leaf sheath closed); (plant monoecious), (flowers perfect - rare); outer T hooded [?how common], (T 0), staminate flowers: anthers monothecal, dehiscing by a single slit; tapetal cells 1-4-nucleate, microspore mother cells central in loculus; pollen (binucleate), not annulate, with coarse granules [exine fragments] on pore; carpelate flowers: P variable; common style short or 0, stigmatic receptive cells on multicellular branches; ovule with cells of nucellar epidermis anticlinally elongated, suprachalazal zone ± massive, hypostase +; embryo sac with compound starch grains [esp. surrounding polar nuclei], antipodal cells ± proliferating, persistent; exotesta persistent, ± thick-walled, tegmen tanniniferous; chromosomes 0.7-2.4 µm long; 28 kb chloroplast genome inversion +; (cotyledon not photosynthetic), hypocotyl and collar at most small, collar rhizoids +, first seedling leaf with blade.
61/535. Africa (inc. Madagascar), Southeast Asia to Australia, New Zealand, Chile. Map: from Good (1974) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). [Photos - Collection. Dovea tectorum is properly Chondropetalum tectorum.]
Age. This node has been dated to ca 74 Ma (Janssen & Bremer 2004) or (72.6-)71.2(-64) Ma (Bouchenak-Khelladi et al. 2014b).
Fossil pollen from South Africa dated to ca 65 Ma has been used to calibrate the age of this clade; the pollen is assignable to Restionoideae in particular (Linder et al. 2003 and references).
2. Restionoideae Bartling
Flavonols, myricetin derivatives +, flavones less diverse; root aerenchyma +, pericycle 4+ cells across, (pith with scattered xylem elements/central xylem element); protective cells around substomatal cavities; (spikelets 0); pollen grains with pores 4-10 µm across, margin raised; G ([2]); (n = ?20), nuclear genome [1C] (0.47-)1.39(-7.66) pg.
11-16/350: Africa south of the Sahara, Madagascar.
2a. Restioneae Bartling —— Synonymy: Elegiaceae Rafinesque
SiO2 bodies often in parenchyma sheath only; microsporocytes central in loculus; pollen pore (with foot layer much thickened); (G 1), styles 1-3, often widely separate; fruit (a nut); n = (10), 16; (28 kb chloroplast genome inversion 0 - Elegia); germination phanerocotylar.
3-8/300: Restio (95-[?167, Tang et al. 2016]), Ischyrolepis (48), Elegia (50), Thamnochortus (35). Madagascar, Africa south of the Sahara, especially the Cape Region. [Photo - Elegia, Habit.]
2b. Willdenowieae Masters
Fibre cylinder with ridges extending ± all through chlorenchyma ["ribs"], alternating with vascular bundles (0), SiO2 bodies usu. in cylinder only, chlorenchyma cells often radially short and squat (lignified chlorenchyma cells extending from ridges); G unilocular, style branches 2, (basally connate); ovule (micropyle endostomal - Willdenowia), (parietal tissue ca 1 cell across - ?Hypodiscus); proliferating antipodals?; fruit a nut, often with elaiosome [= fleshy pedicel]; young seed coat not tanniniferous; germination cryptocoylar.
8/50: Anthochortus (15). The Cape region of South Africa.
[Sporadanthoideae [Centrolepidoideae + Leptocarpoideae]]: flavonols rare, except quercetin, non-hydrolysable tannins rare, flavones diverse, sulphated flavonoids +; (sand binding roots 0) [apo here?]; pollen pore not annulate, 8-25 µm across, margins irregular, thickened foot layer 0; n = 6, 7, 9, 11, 12; cotyledon not photosynthetic [ca half the genera], seedling culm internodes elongated, leaves terete.
3. Sporadanthoideae Briggs & Linder
Myricetin +; sand-binding roots 0; protective cells around substomatal cavities; flowers solitary and with bracteoles, (spikelets +), (flowers perfect); fruit (a nut).
3/31: Lepyrodia (22). Australia and New Zealand.
[Centrolepidoideae + Leptocarpoideae]: (parietal tissue 1 cell across).
4. Centrolepidoideae Burnett —— Synonymy: Centrolepidaceae Endlicher, nom. cons.
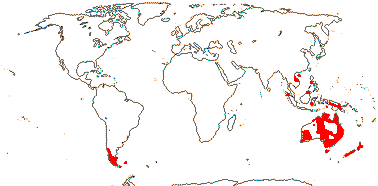
Plant ± caespitose, (annual); sand-binding roots 0; root hairs lateral to epidermal cells; vascular bundles in culm on either side of thickened cylinder (not Gaimardia), palisade tissue 0; SiO2 ?0; epidermis with hairs and papillae; (plane of distichy of vegetative branches transverse to the main axis; prophyll lateral); leaf blades well developed, rounded-unifacial, (ligulate); inflorescence scapose, capitate and with inflorescence bracts, or spicate, branches 2-ranked; plant monoecious; (bracts 0); (T 0-3, lacking vascular traces); staminate flowers: A (1-2); pollen pore margin irregular, surface scrobiculate, punctate, granular, endexine 0; carpellate flower: G [1-14(-45)], styles separate, adaxially channeled; ovule pendulous, straight, micropyle bitegmic, nucellar cap 0; antipodal cells usu. binucleate; fruit (indehiscent); endotegmen alone persistent; embryo conoid; n = 10; (phanomer 0), chlorenchymatous cells isodiametric or palisade.
3/35. Southeast Asia and Malesia (rather scattered) to New Zealand, S. South America (Gaimardia) (map: from Ding Hou 1957; Hamann 1960; van Balgooy 1984; Australia's Virtual Herbarium (consulted 1.2014). Photo: Gaimardia Habit and Close-up, Centrolepis Habit.
Age. Estimates of the stem-group age of this clade are ca 97 or ca 45 Ma depending on where it is placed in the tree - within Restionaceae or not (Janssen & Bremer 2004); (40.8-)31.2(-22.8) Ma is the age in Bouchenak-Khelladi et al. (2014b).
5. Leptocarpoideae Briggs & Linder
(Arbuscular mycorrhizae +); flavones, flavonoids [hypolaetin], sulphated flavonoids, (8-hydroxyflavonoids, e.g. gossypetin) +; (sand binding roots 0); chlorenchyma interrupted by pillar cells [radiating ± lignified cells of chlorenchyma, extending from epidermis to sclerenchymatous sheath] (0), (sclerenchymatous bundle girders opposite outer vascular bundles +); substomatal protective cells 0, (elongated, thick walled epidermal cells +); leaves also dorsiventral; (flowers 2-merous); staminate flowers: anthers (tetrasporangiate - Harperia); (G 1), (style strongly recurved [hair-pin style]); carpelate flowers: ovule (micropyle endostomal - some Leptocarpus); fruit (a nut); tegmen tanniniferous?; n = ?; (28 kb chloroplast genome inversion 0 - Desmocladus); germination usu. phanerocotylar.
28/117: Chordifex (20). Hainan and Vietnam to Australia, New Zealand, Chile (Apodasmia).
Evolution: Divergence & Distribution. Restionaceous pollen is reported from Late Cretaceous deposits in southern Africa (Scholz 1985: Namaqualand). There are a number of records of pollen of Restionaceae (including Centrolepidoideae) from the northern hemisphere from sites in both the Old and New Worlds (Muller 1984), but the identity of these grains has been questioned (Linder 1987). Pollen has also been found on the Ninety East ridge in the Indian Ocean, and elsewhere (Linder et al. 2003 and references).
There are ca 350 spp. of Restionaceae-Restionoideae in the Cape region, diversification beginning in the late Eocene-early Oligocene some 43-28 Ma, the rate increasing during the early Miocene and remaining high (Linder 2003; Linder et al. 2003; Hardy et al. 2004a; Linder & Hardy 2004; Galley et al. 2007; Hardy et al. 2008). For ploidy level and genome size in African Cape Restionoideae and their possible correlation with altitude, geography, fire, etc., see Linder et al. (2017a). The less speciose Australian Restionaceae began diversifying earlier, and the rate has remained steady, but lower (extinction rates may also be lower); there are no obvious major morphological differences between the Restionaceae in the two areas, so no key innovations are implicated, but there is greater environmental heterogeneity in southern Africa (Linder et al. 2003; Linder & Verboom 2015). Some diversification in Australian Restio may be associated with the aridification of the Nullarbor Plain some 14-13 Ma separating what became eastern and western clades (Crisp & Cook 2007).
Dispersal rather than vicariance has been invoked to explain the distribution of the family (Sanmartín & Ronquist 2004).
Centrolepidoideae may be paedomorphic, the plant being reproductively mature while in a juvenile stage, i.e. progenetic (see also Briggs et al. 2014 and references). Sokoloff et al. (2015a) place the extensive vegetative and floral variation in this bizarre little clade in a phylogenetic context; Centrolepis racemosa in particular is highly derived.
Briggs et al. (2014) suggest apomorphies of clades throughout Restionaceae.
Ecology & Physiology. Many Restionaceae flourish on nutrient-poor (oligotrophic) conditions, whether wet or dry, especially in southern Africa or in Australia, thus Restionaceae replace Poaceae in the graminoid layer in the nutrient-poor soils of the fynbos vegetation of the Cape Floristic region (Bell et al. 2000). Ehmig and Linder (2020) looked at the roots of Restionaceae-Restionoideae from the Cape flora (the focus was on older roots) and were able to place then in five syndromes varying in features like dimorphism and presence/absence of aerenchyma that reflected differences in soil, rainfall, drainage, etc.. Genera tended to have the same root type, thus species of Thamnochortus lacked aerenchyma, present in its sister taxon and more generalyy in the plants examined (Ehmig & :inder 2020). The rootlets are sometimes described as being capillaroid, with dense, exceptionally long, and sometimes lignified root hairs that cause the roots to have a sheath of sand attached to them; initially, at least, these roots facilitate the uptake of water and nutrients, and even when dead the persistent sheath helps ensure the functioning of the vascular tissue (Shane et al. 2011). Empodisma minus (Leptocarpoideae) is a major component of ombrotrophic (rain-fed) peat/mire habitats in New Zealand, where its dense, negatively-geotropic capillaroid roots form dense mats that make up a major component of the peat there (Agnew et al 1993). Other dominants in similar conditions in New Zealand include Sporadanthus (Sporadanthoideae) and Apodasmia (more coastal conditions: Leptocarpoideae). There are other distinctive root morphologies like cluster/proteoid roots in this clade (Meney & Pete 1999b; Lambers et al. 2006). Cyperaceae and Proteaceae growing in similar phosphorus-poor environments develop analagous root structures that are believed to facilitate phosphorus uptake. T. L. Bell et al. (2000) suggested that the total root length of the grasses tested was considerably greater than that of Restionaceae, although the dense root hairs of the latter were not taken into account. At least some Restionaceae seem unable to benefit from transient nitrogen availability (Meney & Pate 1999b; Briggs et al. 2014).
The habitats Restionaceae prefer are often subject to seasonal fires, and some species, sprouters, accumulate starch in their rhizomes from which they grow after fire, while others, seeders, reproduce by seeds (c.f. Ericaceae). Overall diversification of seeders in southern Africa is greater than that in Australia, perhaps in response to the greater climatic heterogeneity of the former, however, there are numerous sprouter species because of the frequency of the seeder → sprouter transition (Litsios et al. 2014). However, Lamont et al. (2018a, b and references) noted that fire-(= smoke/karrikinin)stimulated germination probably occurred throughout the family (= Restionaceae-Anarthriaceae), even though the diaspores are not hard, and the first resprouting could be dated to 91 Ma in the family; resprouting was probably more common in Australia because the fires were more intense there. Fire-stimulated germination is common in Restionaceae and may even be the ancestral condition for the family, or just for its African members, and thus the origin of this condition is at least 70 Ma, so the Cape flora must have been subject to fire for a very long time... (T. He et al. 2016b; c.f. Bond et al. 2003b; Midgley & Bond 2011).
African Restionaceae are heteroblastic, that is, the young plants are branched and have thin stems (see also Linder & Caddick 2001), but flowering stems are notably thicker, taller and unbranched; the stems persist, photosynthesizing, for a few years after flowering (Ehmig et al. 2018). Some species, usually members of moister, less seasonal habitats, have axillary branch complexes that repeat the growth pattern of the juvenile plant (Ehmig et al. 2018: neoteny).
Restionaceae-Centrolepidoideae include a number of species that are cushion plants, often adapted to dry and cold conditions (Boucher et al. 2016b).
Pollination Biology & Seed Dispersal. Linder (2020) looked at flowering time in Restionoideae, all wind-pollinated. Flowering times of local communities was linked with environmental conditions - elevation, temperature and rainfall.
Restionaceae probably have ballistic dispersal as their ancestral condition, but there are clades with winged propagules, while myrmecochory is common in the South African (Cape) Restionoideae-Willdenowieae, the nutlets having fleshy pedicels that attract ponerine ants (Briggs & Linder 2009; Linder et al. 2017a and references). The large (ca 1 cm long), hard nuts of the Cape Ceratocaryum argenteum look and smell like dung and are dispersed by dung beetles (Midgley et al. 2015).
In most Australian Restionaceae (= Leptocarpoideae) at least 10-12 months elapse between flowering and fruiting (Meney & Pate 1999a); Alexgeorgea fruits underground, although its stigmas appear above the surface (Carlquist 1976c).
Vegetative Variation. I have not attempted to digest the considerable variation in root anatomy; vessels are scattered throughout the pith in a number of taxa (Meney et al. 1999). Linder (2018) placed the variation in root anatomy in African Restionoideae in the context of the phylogeny of the subfamily (see also Ehmig & Linder 2020).
For heteroblasty and neoteny in African Restionaceae, see above. Unifacial leaves are common in Restionaceae, and are provisionally placed as a family apomorphy. Anarthria, one of the few Restionaceae with well-developed leaves, has more or less terete leaves with a very small adaxial surface, although the scale leaves of the related Hopkinsia appear to be bifacial (Briggs et al. 2010). Adult plants of Centrolepidoideae may have terete leaves, and young seedlings of Restionaceae in which Centrolepidoideae are embedded also have terete leaves. However, Sokoloff et al. (2015a) question the interpretation of all centrolepidoid leaves as being unifacial. Furthermore, they equate the blade of these leaves with the hyperphyll, suggesting that it is developed from a different part of the leaf than the blade of a grass leaf, for example, which is a part of the hypophyll (the hyperphyll in monocots is often represented by the ± terete Vorläuferspitze).
Plant-Bacterial/Fungal Associations. Meney et al. (1993) mention a few examples of arbuscular mycorrhizal associations.
Genes & Genomes. McKain et al. (2016a) suggest that there have been a number of genome duplications in this area: for the family as a whole (a "restiid" event), for the Centrolepidoideae ("Centrolepidaceae"), and perhaps the whole family bar Anarthrioideae ("Restionaceae"). Ca 1/3 of the species of African Cape Restionoideae show infraspecific variation in ploidy level (Linder et al. 2017a).
Chemistry, Morphology, etc.. The culm has subepidermal chlorenchyma separated from the cortex by parenchymatous or sclerenchymatous rings; these may not be strictly comparable in different taxa (Cutler 1969) and so may not be an apomorphy for the family (c.f. above). See Malmanche (1919) for vegetative anatomy; the stomata he described would now be called brachyparacytic.
The tepals are often scarious. How the flowers are aggregated can be difficult to see in Anarthrioideae in particular, so too much should not be made of whether or not the inflorescence is described as being spicate. The distinctive morphology of carpellate flowers with the carpels developing around the floral apex, etc., are mentioned by A. de L. Silva et al. (2021b and references). For the extensive variation in the surface of the seed, see e.g. Briggs and Johnson (1999).
Information is taken from Kircher (1986; guard cells not dumbbell-shaped), Linder et al. (1998), Meney and Pate (1999a), all general, Linder (1984: African members of the family), Meney et al. (1999) morphology and anatomy of Australian Restionaceae, C. A. Williams et al. (1998) and Harborne et al. (2000), flavonoid patterns, Pate and Delfs (1999), Pate and Meney (1999), and Briggs and Johnson (2010), all anatomy, Linder (1992a: African taxa, b: Australian taxa) and Sokoloff et al. (2019a: anarthrioids), all female flowers, Ronse Decraene et al. (2001a, 2002b) and Fomichev et al. (2019: Anarthria group), all floral development, Borwein et al. (1949) and Krupko (1962), both embryology, Newton et al. (2002: seeds), and Linder and Caddick (2001: esp. seedlings). For Peter Linder's "Intkey thingy" on African Restionaceae - a mere 2,000 pictures - see http://www.systbot.unizh.ch/datenbanken/restionaceae/.
For Anarthrioideae, general information is taken from Cutler and Airy Shaw (1964), Linder et al. (1998) and Briggs and Johnson (2000), also Linder and Rudall (1993: esp. Anarthria). All in all, little is known about embryology and seed development here.
Cutler (1969: as Centrolepidaceae) emphasized the fact that the root hairs in Centrolepidoideae arose from one side of the epidermal cell and that the root lacked a pericycle. He suggested that the peg cells of Centrolepidoideae and other Restionaceae might be rather different, peg cells s.s. perhaps being absent in the former. Whether or not Centrolepidoideae have SiO2 bodies needs confirmation.
There has been some discussion as to whether Centrolepidoideae have a flower (e.g. Sokoloff et al. 2009b) or pseudanthium (e.g. Prakash 1970). Sokoloff et al. (2010; see also Hamann 1962a; Remizowa et al. 2011a) interpret the inflorescence of Centrolepis as being a racemose, not cymose, spikelet. Hou (1957) described the anthers as being 1- or 2-celled. The carpels are more or less fused, while in Centrolepis itself, although the gynoecium is definitely syncarpous, the carpels appear to be more or less one on top of each other because of developmental gymnastics resulting in the greater development of one side of the receptacle (Sokoloff et al. 2009b). The ovule is described by Hamann (1975) and Cooke (1998) as being weakly crassinucellate and also as having a megasporocyte that lacks a parietal cell, while illustrations in Prakash (1970) show both types of ovules; although cells in the nucellar epidermis may have divided (thus = nucellar cap), this seems unlikely from the illustrations in Hamann (1962a).
Phylogeny. For general phylogenetic relationships in Restionaceae, see Briggs et al. (2010, esp. 2014). The position of the old Centrolepidaceae - morphologically quite a distinctive group - with respect to Restionaceae was uncertain for some time (e.g. Linder et al. 2000), most studies concentrating on either the Australian or African Restionaceae. A position sister to Restionaceae was possible (Linder & Caddick 2001) as well as one within the family (Bremer 2002: support weak). In more recent studies Centrolepidaceae and Restionaceae were sister taxa in parsimony analyses of trnK and trnL-F, while in Bayesian analyses, and also in rbcL analyses, the relationships [Restionoideae [Sporadanthoideae [Leptocarpoideae + Centrolepidoideae]]] were recovered (Briggs & Linder 2009; Briggs et al. 2010). Briggs et al. (2014) obtained the topology [Restionoideae [Leptocarpoideae [Centrolepidoideae + Sporadanthoideae]]] in most analyses (and followed here), but the topology [Centrolepidoideae [Restionoideae [Sporadanthoideae + Leptocarpoideae]]] was also obtained; very long branches were associated with Centrolepidoideae. Note that the pollen apertures of Australian Restionaceae in particular are like those of Centrolepidoideae, a largely Malesian-Australian group (Chanda 1966). Linder et al. (2003: Centrolepidoideae not included) retrieved Australian and African clades even in morphological analyses, although bootstrap support in the latter was restricted to a few small clades with at most four members; they recovered the other major clades.
Within Restionioideae, there is a Willdenowia and a Restio clade (Linder 2018). For relationships within the latter, see e.g. Moline and Linder (2005) and Linder and Hardy (2010) and references; Soroveta is sister to the rest of the group. In Sporadanthoideae, Sporadanthus is sister to the rest, and in Centrolepidoideae, relationships are [Gaimarda [Aphelia + Centrolepis]]. Within the large group Leptocarpoideae, the monotypic Eurychorda is well supported as sister to all other genera. Anarthrioideae were sometimes paraphyletic at the base of Restionaceae (Briggs & Johnson 2000: morphological analyses), but their monophyly and the relationships [Anarthria [Hopkinsia + Lyginia]] seem well established (Briggs & Johnson 2000; Briggs et al. 2000, 2014).
Classification. The old Centrolepidaceae should be included in Restionaceae as a subfamily (c.f. Trias-Blasi et al. 2015). Putting the three genera of Anarthriaceae in three separate families seemed a bit much, no hierarchical information being conveyed by this move, although the three are morphologically distinct (see above). However, Linder et al. (2000) suggested that these genera belonged to Restionaceae, perhaps sister to the rest. Although Anarthria lacks the distinctive sclerified steath in the culm, the two other genera have it, and Lyginia, at least, has starch in the embryo sac, like Restionaceae. Overall, we are returning to the pre-1965 circumscription of Restionaceae, albeit with the addition of Centrolepidaceae and removal of Ecdeiocoleaceae; a single family is recognized by A.P.G. IV (2016).
For the classification of old-style Restionaceae (excluding Centrolepidoideae) and characterization of the subfamilies, see Briggs and Linder (2009); Leptocarpoideae have been pulverized. Linder and Hardy (2010, see also Linder 1984, 1985) provide generic characterisations and an enumeration of the species of Restionaceae-Restioneae in southern Africa.
[Flagellariaceae [[Joinvilleaceae + Ecdeiocoleaceae] Poaceae]]: leaf blade with ligule; inflorescence branches spiral [?level], with adaxial basal swellings; endothecial cells with complete base plate thickenings; ovule nucellar cap +, suprachalazal zone massive; fruit indehiscent, fleshy; cotyledon not photosynthetic.
Age. This node has been dated to (76-)65, 58(-42) Ma (Bell et al. 2010) or about 74.7 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. Bouchenak-Khelladi et al. (2015) suggested that there was a slow-down in diversification rates at this node.
Ecology & Physiology. The linked features of net venation, animal-dispersed propagules, tolerance of shady habitats, and a preference for well drained and fertile substrates are found in some members of this group (Givnish et al. 2005, 2010b).
Chemistry, Morphology, etc.. For the occurrence of mixed-linkage glucans, in versions prior to vii.2018 placed at this node (or the next one down), see the discussion under monocots and also above.
For the cross (= transverse) veins in the leaf, see Soreng and Davis (1998). For alternating long and short cells, see Stevenson in Michelangeli et al. (2003) and Briggs in Givnish et al. (2010b). In the interpretation of floral morphology of Ecdeiocoleaceae I follow Rudall et al. (2005a) and especially Whipple and Schmidt (2006); see also the discussion after Poaceae. For the patterns of thickening of the endothecial cell walls, see Manning and Linder (1990); these are difficult to categorize in Joinvilleaceae and Ecdeicoleaceae.
FLAGELLARIACEAE Dumortier, nom. cons. - Flagellaria L. - Back to Poales
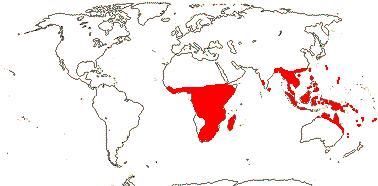
Climber, leaves with terminal tendril; stem apices dichotomise; flavonols +; endodermal cells radially elongated; culm solid; SiO2 associated with vascular bundles only; leaves amphistomatous, neighbouring cells of stomata with oblique divisions; axillary buds 0; prophylls lateral; leaves auriculate, sheath closed; T members with single trace [?level], whitish, soft; microsporocytes ?peripheral in loculus; microsporogenesis simultaneous; style solid, with transmitting tissue; micropyle endostomal, conspicuously oblique, outer integument ca 4 cells across, parietal tissue +, 1 cell across; embryo sac bisporic, chalazal dyad, eight-celled [Allium-type], antipodal cells numerous; fruit a drupe, inner mesocarp sclereidal, endocarp also lignified, seed coat adnate to pericarp; outer periclinal wall of exotesta persisting; n = 19, x = 6, 7, nuclear genome [1 C] (0.284-)0.972(-3.323) pg; ORF 2280 present?; seedling coleoptile at most short.
1 [list]/5. Palaeotropics, to the Pacific Islands. Map: see van Steenis and van Balgooy (1966) and Heywood (1978). [Photo - Flower.]
Age. Estimates of the age of crown-group Flagellariaceae are (102.5-)95.3(-85.8) Ma (Bouchenak-Khelladi et al. 2014b).
Evolution: Ecology & Physiology. Hesse et al. (2016) studied the biomechanics of climbing in Flagellaria, noting i.a. that the basal part of the stem lacked tendrils and became quite rigid, structural Young's modulus increasing during development, rather as in free-standing trees and shrubs, but unusual in vines/lianes; flexibility was greater towards the tip of the stem and tendrils developed on the leaves there. The actual process of establishment of a tendril attachment is very slow indeed, some 4-5 weeks (it is often a matter of days or even less in other plants with tendrils), curvature as well as thickening is adaxial (Rjosk et al. 2017).
Genes & Genomes. The rate of plastome molecular evolution is low (Barrett et al. 2015). There is disagreement as to whether or not the ORF 2280 gene is present (c.f. Hahn et al. 1995 and Katayama & Ogihara 1996).
Chemistry, Morphology, etc.. Flagellaria indica is reported to have a dichotomising stem apex, the vegetative leaves of aerial shoots lacking axillary buds (Tomlinson & Posluszny 1977). However, Kircher (1986) interprets the dichotomy as being a stem apex plus modified axillary branch; either way, the particular configuration in Flagellaria is an apomorphy.
Since the seed coat is adnate to the fruit wall, I suppose the fruit is a caryopsis.... Is the coleoptile a part of the cotyledon, as in Poaceae (Takacs et al. 2012), or the first foliage leaf?
Some information is taken from Appel and Bayer (1998), Tillich and Sill (1999), both general, Wepfer and Linder (2014: revision), Sajo and Rudall (2012: floral morphology), Sajo et al. (2007: style), Subramanyam and Narayana (1972) and Rudall and Linder (1988), both embryology, Thadeo et al. (2015: fruit), and Tillich (1996b: seed and seedling).
[[Joinvilleaceae + Ecdeiocoleaceae] Poaceae]: epidermis with microhairs; foliar epidermis with long + short cell alternation [latter SiO2-containing] throughout, SiO2 bodies cubic; stomatal guard cells dumbbell shaped; culm hollow [level?]; fusoid cells + [thin-walled mesophyll cells flanking vascular bundles]; endothecial cells with girdle thickenings; 28 and 6.4 kb chloroplast genome inversion; first seedling leaf lacking blade [possible].
Age. This age of this node may be ca 90 Ma (Janssen & Bremer 2004) or ca 58.6 Ma (Magallón et al. 2015: note topology).
Evolution: Ecology & Physiology. For dumbbell shaped stomatal guard cells, relatively faster pore opening, and large stomatal apertures (in grasses at least stomatal opening/closing is facilitated by movement of K+ ions back and forth between guard and subsidiary cells), see Franks and Farquhar (2006) and Haworth et al. (2011) and references; see also Woolfenden et al. (2018) for stomatal opening. The stomata also have an enhanced response to blue light, perhaps advantageous in understorey conditions - Hetherington and Woodward (2003) discussed this in the context of the selective advantage of such stomata in the early evolution of grasses, but note that dumbbell-shaped stomata are also found in Rapateaceae and Cyperaceae, at least.
Characters like long and short epidermal cells alternating could equally well be autapomorphies for Joinvilleaceae and Poaceae. Similarly, Leandro et al. (2018) report the development of large, colourless cells lateral to the vascular bundle in Joinvillea ascendens, and they are similar to the fusoid cells of Poaceae, although there is no breakdown of such cells as is common in that family (and there are no such cells in Flagellaria indica). Nevertheless, Leandro et al. (2018) suggest that presence of fusoid cells is an apomorphy at this level - Ecdeiocoleaceae barely have leaves, so fusoid cells are not expected there. In Poaceae the pattern of loss and regaining of this character is very complex.
Genes & Genomes. For chloroplast genome inversions, see Doyle et al. (1992); the 6.4 kb inversion has been reported in Ecdeiocoleaceae (Michelangeli et al. 2002, 2003; Marchant & Briggs 2007). For a trnT inversion, see Morris and Duvall (2010).
Chemistry, Morphology, etc.. See Endress (1995b) for some details of floral morphology; for endothecial thickening, see Sajo and Rudall (2012).
[Joinvilleaceae + Ecdeiocoleaceae]: ?
JOINVILLEACEAE Tomlinson & A. C. Smith - Joinvillea Brongniart & Gris - Back to Poales
Microhairs multicellular; starch grains compound; colourless cells in mesophyll; leaf vernation plicate, auricles or ligules +; T green-brown, rather dry, outer T ]hooded; microsporogenesis ?simultaneous; pollen grains peripheral in loculus; carpels with lateral bundles; ovule with integuments ca 2 cells across; antipodal cells binucleate; fruit a drupe, epidermis and middle mesocarp lignified, T persistent; seeds 1-3; testa fleshy, tegmen hard, endotegmen tanniniferous; coleoptile +; n = 18, x = 6, nuclear genome [1 C] (0.059-)1.289(-28.317) pg; rps14 gene to nucleus, pseudogene remaining in mitochondrion.
1 [list]/2. Malay Peninsula to the Pacific. Map: from van Steenis and van Balgooy (1966) and Newell (1969). Photo: Habit, Flower.
Age. Crown-group Joinvilleaceae are estimated to be some (79.7-)76.7(-43.5) Ma (Bouchenak-Khelladi et al. 2014b).
Evolution: Divergence & Distribution. Joinvilleaceae are reported fossil from late Miocene deposits in New Zealand, although they do not grow there now (D. E. Lee et al. 2001).
Genes & Genomes. The rate of plastome molecular evolution is low (Barrett et al. 2015).
Chemistry, Morphology, etc.. The outer tepals may have only a single trace (Newell 1969), although Sajo and Rudall (2012) describe both whorls of tepals as being supplied by three vascular traces.
Some information is taken from Bayer and Appel (1998: general), Campbell and Kellogg (1996: embryology) and Thadeo et al. (2015: fruits).
Joinvilleaceae are little known.
ECDEIOCOLEACEAE D. F. Cutler & Airy Shaw - Back to Poales
(Roots with metaxylem scattered through pith); vessels?; stomata in grooves down culm; ?microhairs; chlorenchya with peg cells; epidermal long + short cell alternation 0, SiO2 phytoliths 0, sand +; fusoid cells 0; leaf blade much reduced, sheath closed, auricles +; culm branched; inflorescence branch swellings?, with spikes (head-like); 2 outer T ± conduplicate and keeled, 4 T flat; staminate flowers: (A 4, lateral members suppressed Ecdeicolea); carpelate flowers: G [3] (1-2 aborted) or [2], with lateral bundles; ovule antipodal cells 0, area of enlarged cells at chalazal end of embryo sac, chalazal zone massive; embryo sac tetrasporic, 16-celled [Drusa type]; (fruit loculicidal capsule - Georgeantha); exotestal cells large, U-thickened, unlignified, or anticlinal walls lignified; n = 19 [other tentative numbers], x = 6, nuclear genome [1 C] (0.059-)1.289(-28.317) pg; seedling?
2 [list]/3: Ecdeiocolea (2). S.W. Australia. Map: from FloraBase (consulted 2004).
Age. Crown-group Ecdeiocoleaceae are dated to ca 73 Ma (Janssen & Bremer 2004), or rather younger, (48.6-)36.7(-16.3) Ma (Bouchenak-Khelladi et al. 2014b).
Pollination Biology & Seed Dispersal. Briggs and Tinker (2014) described synchronous/serial monoecy in the family. Zones of staminate and carpelate flowers alternated up the spike, and all inflorescences on the one plant would be in the staminate or carpelate phase at any one time.
Chemistry, Morphology, etc.. The illustration in Linder et al. (1988) shows leaves on the inflorescence axis with quite well developed blades. There is no evidence of differentiated long/short epidermal cells (B. G. Briggs, in Givnish et al. 2010b).
In Georgeantha the two adaxial outer T members (= "K") are keeled, the other two are flat, while in Ecdeiocolea the differentiation is somewhat less pronounced. The flowers of Ecdeicolea are monosymmetric; the four stamens probably represent the three stamens of the outer whorl plus the adaxial stamen of the inner whorl (Rudall et al. 2005a). The exotesta is very differently thickened in the two genera, and the fruits are quite different. Details of ovule morphology, etc., are taken from Ecdeiocolea (Rudall 1990a), who thought embryo sac development was probably of the Drusa type, although many of the cells had disappeared by the time that the ovule was mature; Oriani and Scatena (2016) score the embryo sac as being the Allium type.
Some information is taken from Briggs and Johnson (1998) and Linder et al. (1998), both general, Cutler and Airy Shaw (1965: anatomy Anarthria), and especially Rudall et al. (2005a: floral development, fruits).
POACEAE Barnhart, nom. cons. / GRAMINEAE Jussieu, nom. cons. et nom. alt. - Back to Poales
(Aerial branching + [?level]); arbuscular mycorrhizae +; 3 desoxyanthocyanins, flavone 5- and C-glycosides, tricin, flavonoid sulphates, (cyanogenic glycosides) +; pectin 10³%, xyloglucans lacking fucose; lateral roots initiating from pericycle and endodermis together [?level]; some sieve elements with thick walls, sieve tube plastids also with rod-shaped protein bodies, P-proteins 0; mesophyll cells with invaginated walls, fusoid cells often collapsing; short cell pairs, SiO2 bodies over veins; cuticle waxes as aggregated rodlets; stomatal subsidiary cells conical to dome-shaped; microhairs bicellular; plane of distichy of vegetative branches transverse to the main axis [but prophyll adaxial]; leaf with pseudopetiole and blade, ligulate [ligule fringe of hairs], vernation supervolute(-plicate), midrib +, complex; [see below for spikelets]; P with two adaxial outer members distinct, abaxial smaller; tapetal cells binucleate; pollen wall with intraexinous channels; gynoecium initially annular, (carpels open during development), stigmas three; ovule one/flower, lateral, hemicampylotropous, funicle ± absent [ovule broadly attached to ovule wall], micropyle endostomal, conspicuously oblique; abscission zone of fruit immediately above the glumes; seed coat closely adherent to pericarp [= caryopsis]; testa not persistent, hilum long; peripheral layer of endosperm meristematic, endosperm hard, peripheral layers meristematic [?]; embryo [?size], lateral, well differentiated, cotyledon lateral, = scutellum + coleoptile, collar [= epiblast, the ligule of the cotyledon] conspicuous, plumule terminal, coleoptile +, enclosing plumule, embryonic leaf margins overlapping; coleorhiza enclosing radicle, deep cleft between scutellum and coleorhiza [= scutellar cleft], radicle 0/persisting for a few months; x = 6, nuclear genome [1 C] (0.113-)1.449(-18.552) pg, whole nuclear genome duplication [ρ/rho event; duplication of AP1/FUL genes, = FUL1 and FUL2], etc.; plastome expansion of the inverted repeat [level?], with [third] trnT inversion in the single-copy region, only 17 introns [that in clpP absent], loss of accD, ycf, ycf2 genes [?level], PEP subunit β" rpoC2 gene insert, SteYCF1 gene lost, intergenomic translocation of rpl23 gene, ADP-glucose pyrophosphorylase also in cytosol, mitochondrial rps14 gene to nucleus, pseudogene remaining.
793/11,783 - [list]. Twelve subfamilies below. Worldwide. Map: from Vester (1940) and Hultén (1961).
Includes Andropogoneae, Anomochloöideae, Aristidoideae, Arundinarieae, Arundineae, Arundinelleae, Arundinoideae, Bambuseae, Bambusoideae, BOP clade, Brachyelytreae, Brachypodieae, Bromeae, Centotheceae, Centropodieae, Chasmantheae, Chloridoideae, Crinipedeae, Cynodonteae, Cyperochloeae, Danthonioideae, Diarrheneae, Duthieeae, Eragrostideae, Eriachneae, Gynerieae, Isachneae, Jansenelleae, Lecomtelleae, Meliceae, Micraireae, Micrairoideae, Molinieae, Nardeae, Olyreae, Oryzoideae, PACMAD clade, Paniceae, Panicoideae, Paspaleae, Phaenospermateae, Pharoideae, Poeae, Poöideae, Puelioideae, Steyermarkochloeae, Stipeae, Streptogyneae, Thysanolaeneae, Triraphideae, Tristachyideae, Triticeae, Zeugiteae, Zoysieae.
Age. Crown group Poaceae are estimated to be ca 83 Ma by Janssen and Bremer (2004; see also Bremer 2002). Bouchenak-Khelladi et al. (2009, 2010a) suggest an age of (97-)76(-43) Ma, and in Bouchenak-Khelladi et al. (2014b) the age is (74.4-)68.9(-65) Ma. Estimates are getting older, thus Jones et al. (2014) suggested ages of around (248.1-)137.3, 113.6(-62.7) Ma and also others very much older, Prasad et al. (2011) an age of (159.8-)128.9(-99.5) Ma and Schubert et al. (2019) a fossil-calibrated age of (112.4-)105.1(-101.0) Ma. P.-F. Ma et al. (2021) suggest an age of at least 90 Ma, Orton et al. (2021) an age of ca 118 Ma, W. Huang et al. (2022) an age of ca 101 Ma and Gallaher et al. (2022) an age of (103.5-)98.5(-92.9) Ma. See Genes & Genomes below for estimates of the age of the genome duplication that characterizes the family.
Poinar (2004) had proposed that Programinis burmitis, found fossil in deposits from the Early Cretaceous of Myanmar some 110-100 Ma, represented a bambusoid grass. To others, it seemed to have some vegetative features that are common in Poaceae, but not the distinctive features of the family and so was unlikely to be included in it (S. Y. Smith et al. 2010). Nevertheless, in a recent more detailed analysis of P. laminatus, Poinar (2011) affirmed that the silica bodies, etc., did support a placement in Poaceae, and particularly in Poöideae, so suggesting an age for that subfamily about twice that of most previous estimates (see also below). Although the age of these amber deposits has been revised slightly downwards to no earlier than Early Cenomanian, (99.4-)98.8(-98.2) Ma (Shi et al. 2012), this is still very much inconsistent with nearly all other age estimates for grasses. More recently, Poinar et al. (2015) described a spikelet from this amber that they assigned to Poaceae (parts spirally arranged); it was infected with a Claviceps-like fungus, and they proposed that early grasses were mid- to Late Jurassic in age, i.e., at least 145 Ma. However, the divergence of Epichloë and the Claviceps group has since been dated to the Upper Cretaceous 101-73.4 Ma, divergence in the Claviceps group itself not beginning until about 14 Ma afterwards (Píchová et al. 2018: note topology).
The age of grasses, not to mention the animals, both vertebrates and insects, associated with them, and the ages of other monocot groups, seems also to be called into question by the discovery of well-preserved phytoliths of types currently found in members of the PACMAD and BOP clades in coprolites of sauropod dinosaurs from Late Cretaceous deposits of the Deccan Traps 67-65 Ma from central India (Prasad et al. 2005). By extrapolation, this would date the origination of the PACMAD-BOP clade to some 85-80 Ma - X. Wang et al. (2015: see also Prasad et al. 2011; Burke et al. 2016b) suggest ca 70 Ma. These phytoliths have been identified as Oryzoideae-Oryzeae (Prasad et al. 2011; see also Iles et al. 2015), but it is difficult to know what to make of this, since Prasad et al. (2011) show the BOP clade as being paraphyletic, stem Oryzeae were estimated to be (72.5-)62.6(-52.8) Ma, stem Bambusoideae (99.7-)86.2(-69.4) Ma, while the whole of the PACMAD clade was only (72.9-)61.1(-50.4) Ma. Various grass phytoliths have also been recovered from other Intertrappean deposits of about the same age, suggesting that grasses were quite diverse then, although their ecology is as yet unknown (Strömberg et al. 2014). An analysis of some ages in Poaceae comparing those obtained using or not using these fossils and comparing chloroplast and nuclear data, etc. (Christin et al. 2014a; see also Jones et al. 2014: fossil dates not included), while Gallaher et al. (2019) found very different ages for nodes inside and immediately outside Poaceae depending on the calibrations used (these included phytoliths), and they inclined towards older dates. All this underscores the importance of confirming the identity of these fossils.
There are further complications. The fossil pollen genus Graminidites occurs widely (but not in Australia) in the Late Cretaceous (Srivastava 2011), even if at least locally not in association with dinosaurs, similarly, "bamboo cane" is reported from the Western Ghats at the K/P boundary (Cripps et al. 2005). Y. Wu et al. (2017) found epidermal fragments with phytoliths and long and short cells, the latter in pairs, that were associated with dinosaur teeth (these fragments were taken from a "special structure") of a hadrosauroid duck-billed dinosaur Equijubus normani and dated to 113-101 Ma (Albian); they thought that "grasses and other phytolith-bearing plants were likely a part of its diet" (ibid. p. 726). Although the enigmatic Late Cretaceous mammalian sudamericid gondwanatherians had hypsodont teeth - not necessarily associated with grazing grasses - and there is a record of a Cretaceous hadrosaurian dinosaur with carbon isotope ratios that suggests that it might have been eating C4 plants (Prasad et al. 2005; Bocherens et al. 1994), the origin of C4 grasses and most other C4 plants is usually thought to be in the middle of the Caenozoic (see below for discussion of these issues).
There are other approaches to dating grasses. Thus Heads (2018c), using strict vicariance arguments, suggested that the stem-group age of Simplicia (Poöae), a genus endemic to New Zealand, was late Jurassic-Cretaceous, i.e. around 140 Ma, and the crown-group age, the divergence of the extant species, was mid-Cretaceous, ca 105 Ma, and this would suggest that crown-group Poaceae started diversifying way back when. Somewhat less problematically, Aguiar et al. (2019) discussed the possibility of there being Palaeocene cerrado-like savannas in South America; although Malpighiaceae/oil bees were their focus, one wonders what else grew in this vegetation.
To summarize: Dates determined using different assumptions/lines of evidence are irreconcilably in conflict (Vicentini et al. 2008); some of the older ages for the origin of grasses are now making their way into more general literature (e.g. Ladd 2018).
1. Anomochloöideae Potzdal
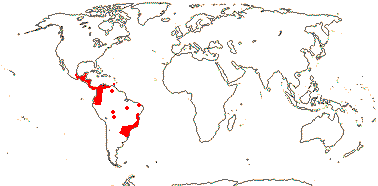
Microhairs 75-150 µm long [i.e., huge], basal cells constricted part way up; (SiO2 bodies transverse-unlobed); pseudopetiole with an apical pulvinus, midrib apparent on both surfaces; flowers perfect, protogynous; A centrifixed, basally connate, anthers ± latrorse; microspore mother cells in locule central; style solid, with transmitting tissue; stigma not plumose; ovule endostomal, suprachalazal nucellar tissue massive; embryo small, (scutellar cleft +), (embryonic leaf margins not overlapping); first seedling leaf lacking blade.
2/4. Tropical America, scattered, forests. Map: from Judziewicz et al. (1999).
Age. Divergence within Anomochloöideae is estimated to have occurred (101.5-)69.7, 68.7(-28.2) Ma (Burke et al. 2016b), (86-)68(-53) Ma (Bouchenak-Khelladi et al. 2010c) or (167.3-)77.8, 65.2(-14.4) Ma, or still older, (90.3-)*0.2(-67.5) Ma (Gallaher et al. 2022). See also Jones et al. (2014).
1A. Anomochloa marantoidea Brongniart —— Synonymy: Anomochloaceae Nakai
Leaves basal, long-pseudopetiolate; ligule fringed, pulvinus also basal; inflorescence branches two-ranked; two "bracts" along each branch unit, two more "bracts" below each flower, one immediately below flower with sheath and blade; ring of fimbriate structures; A 4[inc. 3 members of the inner whorl], microsporocytes central in loculus,; style single; ovary with one strong + two weak traces; testa lignified, persistent; n = 18.
1/1. Brazil, Bahia.
1B. Streptochaeta Nees —— Synonymy: Streptochaetaceae Nakai
Short cell pairs 0, SiO2 bodies transversely elongated; leaves spiral; several spiral "bracts" below each flower, one with a long awn, T = 3 + 3 [inner controlled by "lodicule genes"], coriaceous; anther wall lacking cell layer between endothecium and tapetum [= Reduced type], endothecium lacking thickenings; microsporogenesis simultaneous, microsporocytes ?peripheral in loculus; style long; ovary with three traces; epiblast 0; n = 11.
1/3. Central and South America, in Brazil only Espirito Santo.
[Pharoideae [Puelioideae [PACMAD + BOP clades]]] / The Spikelet Clade: ligule a ciliolate membrane; inflorescence bracts 0, spikelets + [but see below], racemose, pedunculate, laterally compressed, with two basal glumes [sterile bracts = spikelet bract + prophyll], flowers two-ranked, plane of symmetry of flower relative to spikelet horizontal; flower protandrous, with lemma and palea [= bract and 2 adaxial connate outer-whorl T], lodicules +, 3 [= inner whorl T], esp. in staminate flowers, vascularized; A dangling; embryo long [?here]; x = 12; 1 bp deletion in the 3' end of the chloroplast mat K gene, intron loss in PEP subunit β and rpoC1 gene, rpl5 retroprocessing, rp123 pseudogenization, 1,700 bp deletion.
Age. The spikelet clade may have originated in the Late Cretaceous (107.5-)101.1, 98.9(-88) Ma (Burke et al. 2016b), (95-)74(-73) Ma or (83-)67(-55) Ma (Bouchenak-Khelladi et al. 2009, also 2010a, c.f. 2010c), (197.4-) 114.7, 95.2(-55.6) Ma or substantially yet older (Jones et al. 2014), (102.8-)93.9(-86.0) Ma (Schubert et al. 2019), (112.9-)89.9(-70.9) Ma (P.-F. Ma et al. 2021), 109.9-105.8 Ma (Orton et al. 2021) or (100.3-)95.7(-90.8) Ma (Gallaher et al. 2022).
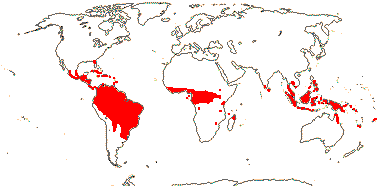
2. Pharoideae L. G. Clark & Judziewicz —— Synonymy: Pharaceae Herter
Inner bundle sheath multi-layered; intercostal epidermis with files of fibres alternating with files of normal long cells, (short cell pairs 0); microhairs 0; leaves resupinate, lateral veins running obliquely from midrib to margin; plants monoecious (?polygamous); inflorescence and spikelets with hooked [= uncinate] microhairs; spikelets dorsiventrally compressed, 1-flowered; staminate flowers: lodicules 1-3, minute, (0); A (4-)6, anthers basifixed, latrorse; anther wall lacking cell layer between endothecium and tapetum [= Reduced type], endothecium lacking thickenings, microsporocytes centrral in loculus; carpelate flowers: lodicules 0; ovule with micropylar beak, micropyle bistomal; (scutellar cleft +), epiblast +; coleoptile [= sheathing base of cotyledon] with blade; chloroplast rps19 gene insertion, etc.. [Microscopic details in the characterization are nearly all from Pharus alone].
3/12: Pharus (7). Pantropical, in forests. Map: from Judziewicz (1987) and Judziewicz et al. (1999). Photo Flower.
Age. Leptaspis and Pharus diverged perhaps (84-)40.4, 37.6(-15.8) Ma (Burke et al. 2016b); (38.2-)25.0(-15.0) Ma is the age suggested by Schubert et al. (2019) and (17.6-)15.9(-15.2) Ma by Gallaher et al. (2022). Both - ?content.
[Puelioideae [[PACMAD + BOP clades]] / The Bistigmatic Clade: SiO2 bodies saddle-shaped [transversely elongated]; spikelets several-flowered, disarticulating above the glumes; lodicules 2; anthers versatile; pollen grains in single layer next to tapetum, pore facing tapetum [?here]; stigmas 2; ovule from carpel without a stigma; chloroplast accD gene 0, 15bp ndhF insertion.
Age. The age of this node may be (76.8-)58(-57.6) Ma or ca 65 Ma (Bouchenak-Khelladi et al. 2010a, c.f. 2010c), (103.6-)90.9, 90.3(-81.7) Ma (Burke et al. 2016b), (107.1-)74.7, 61.8(-46) Ma or older (Jones et al. 2014), (93.0-)84.8(-76.8) Ma (Schubert et al. 2019) or (89.6-)84.7(-79.5) Ma (Gallaher et al. 2022).
3. Puelioideae L. G. Clark, M. Kobayashi, S. Mathews, Spangler & E. A. Kellogg
Culm hollow; culm leaves +, (dorsal ligule + - Puelia); (minute bracts subtending inflorescence branches); spikelet ?compression, basal flowers staminate or sterile, apical pistillate or perfect; ?lodicules; ?anther wall, microsporocytes ?in loculus;; (stigmas 3); embryo small, otherwise unknown; seedling leaf unknown.
2/11: Guadelia (6). Tropical Africa. Map: from Emmet Judziewicz (pers. comm.).
Age. Crown-group Puelioideae may be (85.7-)51.6(-16.5) Ma (Schubert et al. 2019) or (88.8-)76.8(-71.0) (Gallaher et al. 2022) - ?content.
[PACMAD + BOP clades] / Crown Grasses: fructan levels low, (benzoxazinoids [e.g. DIMBOA], ergot alkaloids [latter synthesized by endophytes] +), lignins acylated with p-coumarates or acetate; SiO2 bodies often axially elongated; mesophyll cells with invaginated walls 0; leaf blade linear, transverse veins 0; pseudopetiole 0; spikelets laterally compressed; C/lodicules 2; A 3, opposite K/outer whorl of T; microsporocytes central in loculus; G 2, styles separate; antipodal cells proliferating (not), basal, (polyploid) [?here]; scutellar cleft + [?here], epiblast +; radicle long-persistent in dry conditions, no/few roots from the collar; 15 bp insertion in ndhF gene, Helminthosporium carbonum [HC]-toxin reductase gene [Hm1 gene].
Age. Molecular evidence suggests that the [PACMAD + BOP] clade may have begun to diversify (53.8-)51.9(-49) Ma (Wu & Ge 2011). Other estimates including Vicentini et al. (2008: (60-)52(-44) Ma), Bouchenak-Khelladi et al. (2010c: (55-)52(-50) Ma) are similar, some are a little older - C. Kim et al. (2009: MAD members not included), 67.8-50 Ma, Bouchenak-Khelladi et al. (2010a), (75-)57(-51) Ma, Naumann et al. (2013) about 47.7 or 32.3 Ma, and Z. Peng et al. (2013), 64.5-53.9 Ma (see also Christin et al. 2008a: ca 54.9 Ma), while Bell et al. (2010) and Teischer et al. (2017), at (42-)31, 28(-17) and (47-)33(-28) Ma respectively, provide rather younger ages. Estimates in Jones et al. (2014) are within these limits, except for those based on fossils; X. Wang et al. (2015) date "the major grass radiation" to about 70 Ma, while (81-)73(-65) Ma are the ages in Murat et al. (2017), (88.4-)80.2(-72.9) Ma those in Schubert et al. (2019), (93.3-)73.6(-58.6) Ma those in P.-F. Ma et al. (2021), ca 101 Ma in Orton et al. (2020), ca 81.4 Ma in W. Huang et al. (2022) and (84.6-)80.5(-75.4) Ma in Gallaher et al. (2022).
Fossil spikelets assignable to this clade are known from the Palaeocene-Eocene boundary, about 55 Ma (Crepet & Feldman 1991).
[Panicoideae, Aristidoideae [[Arundinoideae + Micrairoideae] [Danthonioideae + Chloridoideae]]] / The PACMAD Clade: C4 photosynthesis prevalent; fusoid cells 0; SiO2 bodies dumbbell shaped; ligule often hairy; lemma awned; starch grains compound; mesocotyl internode elongated, epiblast 0, embryonic leaf margins meeting; x = 6; extension of ndhF gene from the short single copy region into the inverted repeat, rpl5 from mitochondrion to nucleus.
Age. This node may be approximately 45-37 Ma old, rather younger than the crown-group BOP clade (see Bouchenak-Khelladi et al. 2010a); Bouchenak-Khelladi et al. (2010c) suggest an age of only (34-)28(-22) Ma, while (50.6-)32.7, 32.4(-11.9) Ma are the estimates in Cotton et al. (2015). Schubert et al. (2019) suggest an age (68.8-)55.1(-41.6) Ma, Orton et al. (2021) an age of (95.1-)63.7(-32.2) Ma, W. Huang et al. (2022) an age of ca 66.3 Ma and Gallaher et al. (2022) an age of (69.8-)64.4(-58.0) Ma; these latter ages, although substantially older than the others, are still notably younger than the crown-group age suggested for the BOP clade.
Phylogeny. 6 bp insertion in the 3' end of the mat K gene - to go where?
4. Aristidoideae Caro
(Plants annual); (spikelets not compressed [cylindrical]), with one flower; lemma awn trifid and with basal column, or 3 (1); callus pubescent; germination flap +, (scutellar cleft 0); n = 11, 12 [x = 11].
3/367: Aristida (304), Stipagrostis (56). Warm temperate, few in Europe.
Age. Stem Aristidoideae date from (38-)29(-9) Ma (sister group?), crown Aristidoideae date from (25.5-)20.3(-15.9) Ma (Bouchenak-Khelladi et al. 2010a; Cerros-Tlatilpa et al. 2011), ca 47.5 Ma (W. Huang et al. 2022) or (21.1-)17.0(-16.3) Ma (Gallaher et al. 2022); the stem-group age in Cotton et al. (2015: sister to the CMAD clade) is (46.6-)31.2, 20.5(-10.5) Ma.
5. Panicoideae A. Braun
(Plant annual), (forest dwelling); (culms branched); microhairs often with slender, elongated thin-walled apical cells [panicoid type]; (mesophyll differentiated into palisade and spongy tissues), (chlorenchyma cells lobed [c.f. arm cells]), (fusoid cells +); culms usually solid; leaf (blade narrowly ovate, pseudopetiole +), (midrib complex); (inflorescence bracts +); plant often monoecious; (spikelets dorsiventrally compressed, 2-flowered, development basipetal), lower flower staminate or sterile [gynoecial cell death caused by Tasselseed2], rachilla with appendages/0; plane of symmetry of flower relative to spikelet vertical; glumes, palea, (awn twisted, geniculate), (lemma not awned); style +); (spikelet disarticulation below the glumes); caryopsis hilum punctate; embryo scutellar bundle and coleoptile separated by distinct elongation, epiblast 0, margins of first leaf overlapping ["panicoid"]; (starch grains simple); 5 bp insertion in the rpl16 intron; n = (5, 7) 9 [Paniceae], 10 (11, 12, 14), x = ?11, ?12; (epiblast +), germination flap +; rps14 pseudogene lost.
242/3,325: Digitaria (227), Urochloa (135), Dicanthelium (120), Setaria (115), Axonopus (104), Ischaemum (87), Dimeria (59), Chrysopogon (48). Tropics to temperate.
Age. Crown-group Panicoideae may be (36.8-)23.6, 20.2(-7.9) Ma (Cotton et al. 2015: [Thysanolaena + Centotheca] sister to the rest), ca 54.2 Ma (W. Huang et al. 2022) or (67.6-)61.8(-56.4) Ma (Gallaher et al. 2022).
[Alloeochaete + Dichaetaria]: ?
2/7: Alloeochaete (6). Africa, Sri Lanka, Tamil Nadu. Sister to the rest of the subfamily?: ?C fixation
Age. The divergence of this genus pair has been dated to (53.5-)39.4(-22.3) Ma (Gallaher et al. 2022).
[Tristachyideae [Centotheceae [Thysanolaeneae + Cyperochloeae]]]: ?
Tristachyideae Sánchez-Ken & L. G. Clark
?photosynthesis.
8/84: Loudetia (25), Tristachya (20). Africa (most), South America.
Age. Crown-group Tristachyideae are (28.5-)23.4(-18.4) Ma (Gallaher et al. 2022).
[Centotheceae [Thysanolaeneae + Cyperochloeae]]: ?
Centotheceae Ridley
scutellar bundle and coleoptile separated by distinct elongation, epiblast +, distinct cleft between coleoptile and coleorhiza, margins of first leaf overlap ["centothecoid"].
2/5: Centotheca (3). Africa to China, Malesia, the Pacific Islands.
Age. The age of this clade is (21.4-)17.5(-13.0) Ma (Gallaher et al. 2022).
[Thysanolaeneae + Cyperochloeae]: ?
Thysanolaeneae C. E. Hubbard - Thysanolaena latifolia (Hornemann) Honda
Spikelets 2(3-4)-flowered, (lower and uppermost florets neuter), fertile lemma membranous.
1/1. Sri Lanka and India to S.E. Asia and Malesia.
Cyperochloeae Sánchez-Ken & L. G. Clark
.
2/2. S.W. Australia.
Age. Crown-group Cyperochloeae have been dated to (23.3-)15.0(-7.0) Ma (Gallaher et al. 2022).
Chasmanthieae Sánchez-Ken & L. G. Clark
embryo Centotheca type.
2/7: Chasmanthium (6). U.S.A., Mexico, Central Africa.
Age. This clade has been aged at (12.2-)6.2(-1.9) Ma (Gallaher et al. 2022).
Zeugiteae Sánchez-Ken & L. G. Clark
embryo Centotheca type
4/15: Zeugites (10). Mexico (most) to Bolivia, Africa, India, Sri Lanka and Korea to Queensland.
Age. Crown-group Zeugiteae are (26.8-)19.1(-11.8) Ma (Gallaher et al. 2022).
Steyermarkochloeae Davidse & R. P. Ellis
.
2/2. Brazil, Colombia, Venezuela.
Age. Steyermarkochloeae are (11.5-)4.9(-0.5) Ma (Gallaher et al. 2022).
Gynerieae Sánchez-Ken & L. G. Clark - Gynerium sagittatum (Aublet) Palisot de Beauvois
1/1. Neotropics.
Lecomtelleae Potzdal - Lecomtella madagascariensis A. Camus
1/1. Madagascar>
[Paniceae + Paspaleae, etc.]: plant C4 (C3).Age. (Gallaher et al. 2022).
Paniceae R. Brown —— Synonymy: Cenchraceae Link, Panicaceae Berchtold & J. Presl - PanPasAnd (48.7-)44.9(-40.7)
x = 9.
84/1500 (7 subtribes): Panicum (272 - Moonlight et al. 2024), Cenchrus (121: inc. Pennisetum).
Age. Crown-group Paniceae are (34.3-)30.0(-26.8) Ma (Gallaher et al. 2022).
[Paspaleae [Jansenelleae [Andropogoneae + Arundinelleae]]] / Paspaleae, etc.: ?
Paspaleae J. Presl —— Synonymy: Paspalaceae Link
x = 10.
39/680 (3 subtribes): Paspalum (330). New World.
Age. The age of crown-group Paspaleae is (41.2-)36.0(-33.8) Ma (Gallaher et al. 2022).
[Jansenelleae [Andropogoneae + Arundinelleae]]: ?
Jansenelleae Vorontsova
2/3. India, Sri Lanka, Myanmar.
Age. The age of Jansenelleae is estimated to be (23.0)-17.1(-11.7) Ma (Gallaher et al. 2022).
[Andropogoneae + Arundinelleae] The age for this clade is (21.1-)17.5(-13.9) Ma (Welker et al. 2020).
Andropogoneae Dumortier —— Synonymy: Andropogonaceae Martinov, Ophiuraceae Link, Saccharaceae Berchtold & J. Presl, Zeaceae A. Kerner
spikelets paired [fertile sessile + sterile/staminate stalked (lemma awned)]
91/1200 (12 subtribes): Andropogon (86: polyphyletic), Schizachyrium (64), Cymbopogon (59), Anatherum (45), Sorghum (25). Tropical, subtropical.
Age. The age of crown-group Andropogoneae is (18.0-)13.9(-9.9) Ma (Welker et al. 2020) or (21.1-)18.9(-16.3) Ma (Gallaher et al. 2022).
Arundinelleae Stapf —— Synonymy: Arundinellaceae Herter
2/76: Arundinella (47), Garnotia (29). Tropical and subtropical.
Age. This clade is (Gallaher et al. 2022).
[[Arundinoideae + Micrairoideae] [Danthonioideae + Chloridoideae]]: ?
Age. Ages for this clade are (38.2-)25.8, 19.1(-9.5 Ma (Cotton et al. 2015), (65.8-)60.7(-56.1) Ma (Gallaher et al. 2022) and (65.8-)60.7(-56.1) Ma (Gallaher et al. 2022).
[Arundinoideae + Micrairoideae]: C3 photosynthesis; (hilum short).
Age. Suggested ages of this clade are (34.3-)22.6, 17.3(-7.3) Ma (Cotton et al. 2015), 26-10 Ma (Teischer et al. 2017) and (60.6-)55.1(-45.1) Ma (Gallaher et al. 2022).
6. Arundinoideae Beilschmied —— Synonymy: Arundinaceae Döll
Plants C3; Microhairs with elongated, slender, thin-walled apical cells [panicoid type]; (awn twisted, geniculate), callus pubescent; embryo (margins of first leaf overlapping) ["arundinoid-danthonioid"]; n = 6, 9, 12.
16/36: Arundo (6). Temperate to tropical, hydrophytic to xerophytic.
Age. Crown Arundinoideae are estimated to be ca 57.4 Ma (W. Huang et al. 2022) or (57.4-)49.9(-40.5) Ma (Gallaher et al. 2022).
6A. Arundineae Dumortier
4/18: Amphipogon (9), Arundo (6). Old World.
Age. This clade is (44.2-)39.0(-31.7) Ma (Gallaher et al. 2022).
6B. Crinipeae Hardion
4/8: Styppeiochloa (3). Old World tropics.
Age. Crown-group Crinipeae are some (42.1-)37.9(-33.5) Ma (Gallaher et al. 2022: as Crinipineae).
6C. Molinieae Jirásek
4/8: Phragmites (4). ±Worldwide, not Arctic.
Age. Crown-group Molinieae are (10.2-)7.1(-4.2) Ma (Gallaher et al. 2022: as Moliniinae).
7. Micrairoideae Pilger
culms solid or hollow; (lemma awn 0); starch grains simple, embryo small; n = 10; germination flap +.
9/192. Tropics.
Age. Crown-group Micrairoideae are some 33.1 Ma (W. Huang et al. 2022) or (46.7-)41.2(-34.5) Ma (Gallaher et al. 2022).
7A. Micraireae Pilg - Micraira F. Mueller
Leaves spirally arranged.
1/15. Australia.
Age. Micraireae are (31.5-)19.9(-11.1) Ma (Gallaher et al. 2022).
7B. Eriachneae Eck-Borsboom
C4 photosynthesis.
2/50: Eriachne (48). Australia, to S.E. Asia, Micronesia.
Age. Crown-group Eriachneae are (11.2-)7.7(-4.9) Ma (Gallaher et al. 2022).
7C. Isachneae Bentham
(Annuals), (aquatics); leaf blade broad.
6/127: Isachne (103), Coelachne (12). Tropical and subtropical, inc. oceanic islands.
Age. This clade is some (26.9-)21.7(-14.4) Ma (Gallaher et al. 2022).
[Danthonioideae + Chloridoideae]: lemma bilobed, awned from the sinus; hilum punctate; scutellar cleft +.
Age. The age of this clade is (61.7-)57.8(-53.6) Ma (Gallaher et al. 2022).
8. Danthonioideae Barker & H. P. Linder
Usually temperate habitats; (plants annual), with C3 photosynthetic pathway; (stomata with parallel-sided subsidiary cells); ligule a fringe of hairs; awn twisted, geniculate/trifid/3 awns; lodicules with microhairs; style bases well apart; micropyle not oblique, outer integument ± reduced to a collar around base of ovule, nucellar cap +, cells enlarged; embryo sac with haustorial synergid cells [interrupting nucellar epidermis], often with large starch grains; embryo "arundinoid-danthonioid"; n = (7, 9).
18/292: Pentaschistis (83), Rytidosperma (73). Widespread, esp. Southern Hemisphere, few Southeast Asia-Malesian.
Age. Crown-group Danthonioideae are ca 35.8 Ma (W. Huang et al. 2022) or (57.0-)51.2(-45.7) Ma (Gallaher et al. 2022).
9. Chloridoideae Beilschmied
Plants tolerate drought, high salinity; C4 PCK subtype (phosphoenolpyruvate carboxykinase) +; (culm solid); microhairs with ± hemispherical and thick-walled apical cells the same thickness as the long base cell, latter with internal membranes and secretory [chloridoid type]; SiO2 bodies cross-shaped; plant often monoecious; ovule nucellar cap 0; seeds endo/tegmic, (coat free from pericarp); embryo epiblast +, margins of first leaf do not overlap ["chloridoid-eragrostoid]"; 4 bp insertion in the rpl16 intron; n = (6-8) 9, 10.
131/1,603. Tropical to warm temperate, more or less dry environments especially in Africa and Australia.
Age. Chloridoideae are some 57.8 Ma (W. Huang et al. 2022) or (58.1-)54.7(-52.1) Ma (Gallaher et al. 2022).
9A. Centropodieae P. M. Peterson, N. P. Barker & H. P. Linder
(C3 photosynthesis - Ellisochloa)
2/6: Centropodia (4). Asia, Africa.
Age. Crown-group Centropodieae are (38.6-)30.3(-20.3) Ma (Gallaher et al. 2022).
[Triraphideae [Eragrostideae [Cynodonteae + Zoysieae]]]: ?
9B. Triraphideae P. M. Peterson
Plant (annual); microhairs long, narrow basal and terminal cells [= panicoid]; ligule a ring of hairs/membranous, marginally ciliate; inflorescence paniculate; lemma 3-veined, marginally ciliate, awned, apically bifid, (trifid, 3-awned - Triraphis), lateral veins usually hairy.
4/14: Triraphis (8). Africa, some Australia to tropical and temperate Asia, 1 sp. South America.
Age. Crown-group Triraphideae are (13.2-)10.6(-7.8) Ma (Gallaher et al. 2022).
[Eragrostideae [Cynodonteae + Zoysieae]]: ?
9C. Eragrostideae Stapf —— Synonymy: Eragrostidaceae Herter
(embryo Centotheca type).
Eragrostis (444)
Age. Crown-group Eragrostideae are (37.2-)31.2(-22.9) Ma (Gallaher et al. 2022).
Age. The age of this clade is some 36.8 Ma (Gallaher et al. 2022).
9D. Cynodonteae Dumortier —— Synonymy: Chloridaceae Berchtold & J. Presl, Cynodontaceae Link, Lepturaceae Herter, Pappophoraceae Herter
Leaves amphistomatous (epistomatous, also producing resin).
25 subtribes. Muhlenbergia (183), Triodia (110), Chloris (55), Bouteloua (50).
Age. Crown-group Cynodonteae are some (35.0-)32.7(-29.9) Ma (Gallaher et al. 2022).
9E. Zoysieae Bentham —— Synonymy: Spartinaceae Link, Sporobolaceae Herter, nom. inval., Zoysiaceae Link
.
4/233 (2 subtribes): Sporobolus (220: inc. Spartina).
Age. Crown-group Zoysieae are estimated to be (25.2-)19.3(-14.0) Ma (Gallaher et al. 2022).
[Oryzoideae [Bambusoideae + Poöideae]] / The BOP Clade: SiO2 bodies transverse- or axial bilobate; ligule often membranous; endosperm softness gene +, ?embryo short; first seedling leaf lacking blade.
Age. Bouchenak-Khelladi et al. (2009, 2010a, c) suggested that the BOP clade began to diversify at the end of the Palaeocene (66-)53(-49) Ma while Magallón et al. (2013) gave a much younger age of around 38.5 Ma. X.-Z. Zhang et al. (2015) suggested an age of around 68 Ma, P.-F. Ma et al. (2021) an age of (86.5-)67.8(-54.3) Ma, X. Wang et al. (2015: P, O) an age of ca 64 Ma, Wu and Ge (2011) offer an age of (53.8-)51.9(-50) Ma, Z. Peng et al (2013) an age of ca 48.6 Ma and Murat et al. (2013) an age of about 46 My; (16-)15, 12(-11) Ma is the age in Wikström et al. (2001). Schubert et al. (2019) suggest an age (85.3-)77.8(-70.3) Ma, Orton et al. (2021) an age of (100.1-)95.0(-89.8) Ma, W. Huang et al. (2022) an age of ca 77.9 Ma and Gallaher et al. (2022) an age of (81.1-)77.1(-72.8) Ma, all much older than the crown-group ages provided for the PACMAD clade abova.
10. Oryzoideae Beilschmied (= Ehrhartoideae in pre-2016 literature)
(Some SiO2 bodies elongated transverse to the long axis of the leaf); (fusoid cells +); (longitudinal walls of epidermal cell straight); (microhairs 0); leaf blade often narrowly ovate, (ligule a ring of hairs); (plant monoecious); spikelet maturation basipetalb>, glumes 0, 2, spikelets 1-flowered [= apical floret alone fertile, two basal florets sterile]; A (1-)6, style branches separate almost from the very base; ovule nucellar cap ?0; embryo scutellar bundle and coleoptile not separated by distinct elongation, epiblast +, distinct cleft between coleoptile and coleorhiza, margins of first leaf overlap ["bambusoid"]; ; n = (10, 15).
18/119. Widespread, esp. S. hemisphere.
Suddia Renvoize (1 sp.) - Africa. Leaves hastate. Goes where?
Age. These other Oryzoideae are somewhat under 93 Ma (Orton et al. 2021), ca 66 Ma (W. Huang et al. 2022) or (78.2-)74.3(-70.7) Ma (Gallaher et al. 2022).
Fossils accepted as belonging to stem-node Oryzeae are ca 66 Ma (Iles et al. 2015; see also Prasad et al. 2011), in some conflict with the molecular ages.
10A. Streptogyneae Calderón & Soderstrom - Streptogyna P. BeauvoisOuter ligule +; fusoid cells in leaf mesophyll; spikelets with two glumes, two to four fertile florets, rachilla extension with rudimentary florets; style branches and stigmas persistent; rachilla internodes develop into hooks.
1/2. Southern Mexico to Brazil and Peru, Trinidad. - perhaps sister to the complete BOP clade, or sister to all other Oryzoideae (as here).
[Ehrharteae [Oryzeae + Phyllorachideae]]: ?
Age. These other Oryzoideae are ca 68.3 Ma (Gallaher et al. 2022).
Fossils accepted as belonging to stem-node Oryzeae are ca 66 Ma (Iles et al. 2015; see also Prasad et al. 2011), in some conflict with the molecular ages.
10B. Ehrharteae Nevski —— Synonymy: Ehrhartaceae Link
Leaves spirally arranged; roots at scutellar node.
4/
Age. Ehrharteae are around (36.9-)25.7(-15.2) Ma (Gallaher et al. 2022).
[Oryzeae + Phyllorachideae]: ?
10C. Oryzeae Eck-Borsboom —— Synonymy: Oryzaceae Berchtold & J. Presl
Arm cells +.
Oryza (20), Leersia (20).
Age. Crown-group Oryzeae are (35.9-)32.6(-30.0) Ma (Gallaher et al. 2022).
10D. Phyllorachideae C. E. Hubbard
.
2/.
Age. Phyllorachideae are some (26.1-)19.5(-14.2) Ma (Gallaher et al. 2022).
Age. Wu and Ge (2011) suggested that this node was some (51.6-)47(-40.8) Ma, in Z. Peng et al (2013) the age was ca 47.8-46.9 Ma, in X.-Z. Zhang et al. (2015) about 65 Ma, while in P.-F. Ma et al. (2021: youngest date at which subfamilies "arose") it was around (79.1-)62.0(-49.7) Ma; somewhat over 83 Ma is the age in Orton et al. (2021) and (77.0-)70.6(-64.1) Ma is the suggested age in Gallaher et al. (2022).
11. Bambusoideae Luersson
Epidermal cells papillate, arm cells + [= strongly asymmetrically infolded mesophyll cells], fusoid cells +; leaf blade narrowly ovate; mass flowering common; (inflorescence bracts +); lodicules 3, strongly vascularized; A (2-)6(-140), (basally connate), (endothecial cells with ± U-shaped thickenings); (stigmas 1-3); (ovules ategmic, unitegmic); (fruit a berry), (testa free from pericarp), embryo scutellar bundle and coleoptile not separated by distinct elongation, epiblast +, distinct cleft between coleoptile and coleorhiza, margins of first leaf overlap ["bambusoid"]; x = ?12.
115/1,642 (the numbers of genera suggested in this subfamily are rather notional). Tropical to temperate, often in forests. Map: see Judziewicz et al. (1999) and Sungkaew et al. (2009).
Age. Crown-group Bambusoideae diversified some (48-)29(-26) Ma in the middle of the Oligocene (Bouchenak-Khelladi et al. (2009, 2010a: c.f. topology, c), (58.3-)47.3, 43.2(-34.5) Ma (X.-Z. Zhang et al. 2015) or (59.0-)54.4(-50.2) Ma (Gallaher et al. 2022). Wu and Ge (2011) dated the separation of Phyllostachys and Bambusa to (35.6-)22.5(-9) Ma; stem Olyreae were estimated to be 38.2-26.9 Ma (Burke et al. 2014) or ca 42 Ma (Z.-H. Guo et al. 2019). A Bambuseae-Olyreae split was dated to (52.9-)40.8, 38(-28.7) Ma (X.-Z. Zhang et al. 2015), ca 66.9 Ma (W. Huang et al. 2022) or (52.7-)49.0(-45.8) Ma (Gallaher et al. 2022).
The age of this clade might seem to be more towards the upper end of the molecular estimates, since some of the earliest fossils ascribed to the subfamily, the small-leaved Chusquea oxyphylla, are Eocene (Frenguelli & Parodi 1941; see also L. Wang et al. 2013) - however, that species is a fossil Podocarpaceae (Wilf 2020). On the other hand, "bamboo cane" has been reported from the Western Ghats, India, at the K/P boundary ca 66 Ma (Cripps et al. 2005), and if its identity is confirmed this will upend the molecular age estimates.
11A. Olyreae Spenner / herbaceous bamboos. —— Synonymy: Olyraceae Berchtold & J. Presl
Plant ± herbaceous, ± rhizomatous; culm development uniphasic, branching slight; epidermal SiO2 bodies usu. variously cross-shaped in the costal zone, crenate [olyroid] in the intercostal zone (Buergersiochloinae saddle-shaped); blade not articulated, culm leaves similar to the others, dorsal ligule 0, adaxial midrib raised; flowering annual (synchronized, plants monocarpic); plant monoecious; spikelets often dorsiventrally compressed, unisexual, dimorphic, one-flowered, rachilla extension 0; staminate flowers: (lodicules 0); n = 7, 9, 10, 11, (12) [x = 11], genome = H, 1.37-8.32 pg; rpl5 from mitochondrion to nucleus.
24/124 (2 subtribes): Pariana (35). Central and South America and Africa, also New Guinea (Buergersiochloa).
Age. Crown-group Olyreae are (54.9-)36.5(-35.8) Ma (Ruiz-Sanchez et al. 2019) or (46.3-)42.6(-39.4) Ma (Gallaher et al. 2022). (42.2-)30.8, 28.3(-18.1) Ma are the ages in X.-Z. Zhang et al. (2015: Pariana sister to the rest), the age of this latter group being ca 34.8 Ma in Ruiz-Sanchez et al. (2019).
[Arundinarieae + Bambuseae]: plant woody, with aerial branching, rhizomes massive [= pachymorph]; culm usu. hollow, development often biphasic, lignification and branch development in 2nd phase; tannin-like substances 0; SiO2 bodies saddle-shaped; microhairs with elongated, slender, thin-walled cap cells [panicoid type]; leaves petiolate [?here], blade deciduous, articulated, dorsal ligule +, culm leaves different from the others, largely sheaths, outer ligule +/0; flowering synchronized (intermittent), plants monocarpic; flowers perfect; polyploidy common.
Age. This node is estimated to be (51.6-)30.1(20.2) Ma (C. Guo et al. 2020).
If relationships are [Arundinarieae [Olyreae + Bambuseae]], most of the above will be subfamilial characters...
11B. Arundinarieae Ascherson & Graebner / temperate woody bamboos.
(Rhizomes slender [= leptomorph]); culms hollow, branch development basipetal, (1-)3-many branches/node; midrib complex; (pseudospikelets +); genome = CC DD, (2.55-)4.38(-6.32) pg, n = 23-24, x = 11, 12; 500 bp deletion in the chloroplast rps16-trnQ intergenic spacer.
33/550 (5 subtribes): Fargesia (60/?87), Sasa (40-60), Phyllostachys (55), Arundinaria (50). More or less temperate E. U.S.A., eastern Asia (Sri Lamka, S India eastwards), also Africa, Madagascar, scattered, ± montane.
Age. Estimates of the age of crown-group Arundinarieae are (24.3-)14.3, 12.7(-6.9) Ma (X.-Z. Zhang et al. 2015), (30.0-)18.7(-11.5) Ma (C. Guo et al. 2020) and (12.6=)9.7(-7.4) Ma (Gallaher et al. 2022).
11C. Bambuseae Dumortier / tropical woody bamboos —— Synonymy: Bambusaceae Berchtold & J. Presl, Parianaceae Nakai
Rhizomes often stout; branch development acropetal or bidirectional, ?branches/node; midrib complex; (style hollow - Melocanninae, or 0); n = 20-24, genome = BB CC, 2.04-4.58 pg [Neotropical taxa] and n = 35-36, genome = AA BB CC, 2.63-3.47(-4.25) pg [Palaeotropical taxa], x = 10, 12.
63/784 (11 subtribes): Chusquea (200, + 50 undescribed), Bambusa (120), Merostachys (50), Schizostachyum (50), Guadua (26). Tropical to often warm temperate.
Age. The crown-group age of this clade is estimated to be (39-)28.2, 25.9(-16.9) Ma (X.-Z. Zhang et al. 2015) or (29.6-)24.8(-19.3) Ma (Gallaher et al. 2022).
Note that Chusquea oxyphylla, a ca 52.2 Ma fossil from Chubut, Argentina, is in fact the podocarpaceous Retrophyllum (see Wilf 2020)...
12. Poöideae Bentham
Temperate habitats, perennials; Epichloë endophytes pervasive; 1-aminopyrrolizidine [loliine] and indole alkaloids +, fructan sugars accumulated as kestose oligosaccharides [levans] in stem; (fusoid cells + - uncommon); trichoblasts in vertical files, distal cell smaller, hypodermal cells lacking Casparian strips; longitudinal anticlinal walls of epidermal cells straight [?level]; culms hollow, branching at most rare; auricles 0; flowering requires vernalization; lemma usually with 5 nerves, (awned); lodicules at most slightly vascularized, (0); styluli +; ovule nucellar cap usu. 0, (postament +); antipodal cells lateral; (endosperm with some non-starch soluble storage polysaccharides); embryo small, epiblast +, festucoid [internode between coleoptile and scutellum traces 0, no deep cleft between scutellum and coleorhiza, margins of first leaf do not overlap ["festucoid"]; n = (2, 4-13); duplication of the ß-amylase gene.
219/4,126. Largely North Temperate.
Age. The crown-group age of Poöideae is estimated to be (77-)69(-61) Ma by Schubert et al. (2019), (93.2-)84.8(-76.3) Ma by Orton et al. (2021), (59.4-)59.0(-58.6) Ma by L. Zhang et al. (2022), ca 6x7.0 Ma by W. Huang et al. (2022) or (65.6-)62.1(-57.9) Ma by Gallaher et al. (2022).
12A. Brachyelytreae Ohwi - Brachyelytrum P. Beauvois
Stomata subsidiary cells with parallel sides; microhairs 0; spikelets terete to dorsiventrally compressed; n = 11, 12.
1/3. Eastern Asia, E. North America.
N.B.: the tribal sequence below deviates somewhat from that in Soreng et al. (2022); see Gallaher et al. (2022) for more ages, but c.f. topology. Also, it was unclear where most of the ages for supertribes, etc., in Orton et al. (2021) were to be placed on the tree - Table 2 and Fig. 3 seem somewhat at odds.
[Nardeae [Duthieeae [[Diarrheneae [Ampelodesmeae + Stipeae]] [[Phaenospermateae [Brylkinieae + Meliceae]] [Neomolinia [Brachypodieae + Core Poöideae]]]]]: microhairs 0; primary inflorescence branches 2-ranked [primary branches from two orthostichies]; spikelets laterally compressed; lodicules not vascularized; embryo lacking scutellar cleft, embryonic leaf margins non-overlapping.
Age. This node can be dated at (57-)44(-40) Ma (Bouchenak-Khelladi et al. 2010), (73.5-)65.5(-57.8) Ma (Schubert et al. 2019) or (55.1-)54.7(-54.3) Ma (L. Zhang et al. 2022).
12B. Nardeae W. D. J. Koch (inc. Lygeeae - two tribes for two species, or even a supertribe, Nardodae?) —— Synonymy: Nardaceae Martynov
Rhachilla extension +; lodicules 0; style and stigma 1; n = (10) 13.
2/2. Europe.
Age. The crown-group age of Nardeae is estimated to be (47.8-)32.1(-16.4) Ma (Schubert et al. 2019), (68.8-)42.4(-16.0) ma by Orton et al. (2021), (33.9-)33.4(-32.9) Ma by L. Zhang et al. (2022), or (40.6-)34.4(-27.2) Ma by Gallaher et al. (2022: as Nardodae).
Age. The age of this node may be (48.7-)48.4(-48.1) Ma (L. Zhang et al. 2022) or (49.3-)44.8(-40.1) Ma (Gallaher et al. 2022: as Core Poöideae).
12C. Duthieeae Röser & Jul. Schneider
Lemma with bifid apex, (lobes awn-like), awn twisted, from sinus; (lodicules 3); (styles 3); 21 bp insertion in rpl32-trnL; n = (7) 12 (?14).
8/16. Central to East Asia, also S.E. Australia, Mexico, Balkans, Caucasus; scattered.
Age. The crown-group age of Duthieae is estimated to be (47.8-)30.1(-16.7) Ma (Schubert et al. 2019: ?sampling), or (38.4-)37.8(-37.5) Ma (L. Zhang et al. 2022).
Age. This node is estimated to be (65.6-)57.9(-50.8) Ma (Schubert et al. 2019) or (47.7-)47.5(-47.2) Ma (L. Zhang et al. 2022).
[Phaenospermateae [Brylkinieae + Meliceae]: ?
Age. The age of this node is some (55.4-)45.6(-35.8) Ma (Schubert et al. 2019: ?taxa) or (44.9-)44.5(-44.3) Ma (L. Zhang et al. 2022).
12E. Phaenospermateae Renvoize & Clayton - Phaenosperma globosum Bentham
Leaves pseudopetiolate, blade resupinate; spikelets disarticulating below the glumes; lemma rounded at the apex; style persistent on fruit; 21 bp insertion in rpl32-trnL; n = 12.
1/1. Assam to China.
Age. This node is estimated to be (33.0-)32.6(-32.3) Ma (L. Zhang et al. 2022) or (44.8-)38.9(-33.2) Ma (Gallaher et al. 2022).
12F. Brylkinieae Tateoka - Brylkinia caudata (A. Gray) F. Schmidt
.
1/1. East Asia.
12F. Meliceae Endlicher —— Synonymy: Glyceriaceae Link, Melicaceae Martynov(Annuals), (corms/bulbs +); leaf sheath closed; veins of lemma not converging; lodicules thick, truncate, usu. connate; n = 9, 10.
5/135: Melica (90), Glyceria (35). Global, esp. North Temperate.
Age. Meliceae may be (27.0-)26.8(-26.5) Ma (L. Zhang et al. 2022).
[[Diarrheneae [Ampelodesmeae + Stipeae]] [Neomolinia [Brachypodieae + Core Poöideae]]]: microhairs 0 (+ - some Stipeae); distichous inflorescence phyllotaxis; style +; rpl5 from mitochondrion to nucleus.
Age. This node is thought to be (67-)59.6(-52.1) Ma (Schubert et al. 2019) or (45.8-)45.5(-45.2) Ma (L. Zhang et al. 2022).
.[[Diarrheneae [Ampelodesmeae + Stipeae]]: ?
Age. This clade is some (37.2-)37.0(-36.8) Ma (L. Zhang et al. 2022).
12G. Diarrheneae C. S. Campbell - Diarrhena P. Beauvois
Non-distichous 2-ranked inflorescence phyllotaxy; n = ?10.
1/2. Central U.S.A. (inc. Neomolinia in Gallaher et al. (2022), and with crown-group age!).
[[Ampelodesmeae + Stipeae]]: ?
Age. The age of this clade (as Stipodae) is estimated to be (38.7-)30.4(-26.0) Ma (Gallaher et al. 2022).
Age. Stipa florissanti, from the late Eocene Florissant Beds in Colorado 36.7-34.1 Ma (MacGinitie 1953), has been assigned to stem-node (Iles et al. 2014) or crown (Schubert et al. 2019) Stipeae.
12D. Ampelodesmeae Tutin - Ampelodesmos mauritanicus (Vahl) Link
1/1. Mediterranean (not the east), inc. North Africa.
12D. Stipeae Dumortier —— Synonymy: Stipaceae Berchtold & J. Presl
Spikelets not compressed; lemma usu. not deeply bifid; (lodicules 3); n = 9-12, chromosomes <2.0 µm long.
Ca 28/527: Stipa (300). North Temperate, North Africa.
Age. The crown-group age of Stipeae is around (47.3-)38.6(-30.6) Ma (Schubert et al. 2019) or (30.2-)30.0(-29.8) Ma (L. Zhang et al. 2022).
[Neomolinia [Brachypodieae + Core Poöideae]]: ?
Age. The age of this clde is (42.8-)42.5(-42.3) Ma (L. Zhang et al. 2022).
[Brachypodieae + Core Poöideae]: stomata subsidiary cells with parallel sides; rpl5 to the nucleus.
Age. The age of this node is around 39-32 Ma (International Brachypodium Initiative 2010), ca 51 Ma (X. Wang et al. 2015), ca 31 Ma (Murat et al. 2013), (58.6-)51.8(-44.8) Ma (Schubert et al. 2019) or (40.4-)40.1(-39.9) Ma (L. Zhang et al. 2022).
12H. Brachypodieae Harz - Brachypodium P. Beauvois
?
1/18. Temperate, tropical montane, in America only Mexico to the Andes, not the Antipodes.
Age. Crown-group Brachypodieae may be (28.5-)18.9(-10.1) Ma (Schubert et al. 2019), (9.3-)9.2(-9.0) Ma (L. Zhang et al. 2022) or ca 11.5 Ma (Hasterok et al. 2022).
[Poeae [Littledaleeae [Bromeae + Triticeae]]] / [Poödae + Triticodae] / Core Poöideae: fructan concentration often high; (stomata subsidiary cells with parallel sides); primary inflorescence branches usu. 2-ranked; x = 7, chromosomes "large".
Age. This node is dated at Early Oligocene, just under 35 Ma (Bouchenak-Khelladi et al. 2010a) or rather older, some (55.9-)49.0(-42.3) Ma (Schubert et al. 2019), (35.9-)35.7(-35.5) Ma (L. Zhang et al. 2022) or (45.3-)40.1(-36.4) Ma (Gallaher et al. 2022 - what is the core Pooideae 49.3/44.8/40.1?)
130/3,275.
12I. Poëae R. Brown / Poödae L. Liu —— Synonymy: Agrostidaceae Berchtold & J. Presl, Alopecuraceae Martynov, Anthoxanthaceae Link, Avenaceae Martynov, Chaeturaceae Link, Cynosuraceae Link, Echinariaceae Link, Festucaceae Sprengel, Holcaceae Link, Laguraceae Link, Loliaceae Link, Miliaceae Link, Phalaridaceae Link, Phleaceae Link, Sesleriaceae Döll
Predominantly β-2,6- fructans with terminal or internal glucose; foliar endodermis + ["Festuceae"]; awns dorsal.
/2,895:Festuca (686: inc. Lolium), Poa (617), Agrostis (220), Calamagrostis (130), Anthoxanthum (50), Sesleria (40: ?here). Some 38 subtribes are recognised in Soreng et al. (2022), but the clade is rather large
Age. The age of this node is some (50.8-)44.3(-38.1)) Ma (Schubert et al. 2019; ?content), [Anthoxanthum + Aveneae/Poeae] 22.6-12.3 (plastid) or 30-21 (nuclear) Ma (Pimentel et al. 2013), (29.1-)28.8(-28.7) Ma (L. Zhang et al. 2022) or (40.6-)36.2(-32.0) Ma (Gallaher et al. 2022).
[Littledaleeae [Bromeae + Triticeae]] / Triticodae: ; apex of ovary pubescent.
Age. This clade is some (22.8-)22.6(-22.4) Ma (L. Zhang et al. 2022) or (26.7-)23.0(-20.0) Ma (Gallaher et al. 2022).
14. Littledaleae Soreng & J. I. Davis - Littledalea Hemsley
sheath open.
1/4. Central Asia to W. China.
[Bromeae + Triticeae]]: mixed β-2,1- and -2,6- branched fructans with terminal glucose.
Age. The age of this node is around (45.9-)37.0(-29.4) Ma (Schubert et al. 2019), (21.5-)21.3(-21.1) Ma (L. Zhang et al. 2022) or (25.4-)20.6(-18.0) Ma (Gallaher et al. 2022).
13. Bromeae Dumortier —— Synonymy: Bromaceae Berchtold & J. Presl
Leaf sheath closed; awns subapical; ovary with hairy appendages.
1/165: Bromus. Temperate regions worldwide.
14. Triticeae Dumortier - inc. Hordeeae —— Synonymy: Aegilopaceae Martynov, Hordeaceae Berchtold & J. Presl, Triticaceae Link
Inflorescence spicate.
/501. Elymus (150), Roegneria (130), Nassella (122), Hordeum (30), Leymus (30). Largely North Temperate.
Age. The age of this node is around 33.5-26 Ma (Sandve & Fjelheim 2010) or (16.3-)16.1(-15.15.9) Ma (L. Zhang et al. 2022: ?taxa included).
Synonymy: Coeleanthaceae Pfeiffer??
Note: in older literature the PACMAD (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae, Danthonioideae) and BOP (Bambusoideae, Oryzoideae, Poöideae) clades were variously called the PACCMAD (extra C = Centothecoideae), PACCAD or PACC and BEP (E/O = Ehrhartoideae/Oryzoideae) clades.
Evolution: Divergence & Distribution. The Poaceae group of families has been described as being notably speciose (Magallón & Sanderson 2001), but there is considerable asymmetry in clade size within this larger clade. The great majority of species belong to Poaceae alone, perhaps seven times more speciose than their animal-pollinated sister clade (Kay & Sargent 2009: comparison wrong - 000s of times more speciose than its sister clade). In any event, within Poaceae there are three (perhaps four - e.g. W. Huang et al. 2022) species-poor clades that are successively immediately sister below the [PACMAD + BOP] clade (to add to the two to three similarly small clades successively sister below Poaceae as a whole). A shift in the diversification rate around the origin of the [PACMAD + BOP] clade is estimated to have occurred (58.5-)45.3(-39.7) Ma (Magallón et al. 2018; see also Givnish et al. 2018b), and Puttick et al. (2015) note that the rates of both genome size evolution and speciation are very high. Moreover, foci of diversification are likely to be found within the PACMAD and BOP clades (c.f. Linder & Rudall 2005; S. A. Smith et al. 2011; and especially Bouchenak-Khelladi et al. 2010c), but since estimates of diversification rates depend on clade ages, and there are major problems here (e.g. Christin et al. 2014a; Spriggs et al. 2014: see above), until ages stabilize, it is not worth worrying too much about details of diversification (c.f. the discussion immediately below, see also the numerous ages in Huang et al. 2022: Supplemental Table 7, Fig. 9) - and even with stabilized ages, yet further problems await (Louca & Pennell 2020). Species numbers aside, Burleigh et al. (2006) suggest that by some measures Poaceae show a notable increase in morphological complexity.
The family may have originated in Africa (Bouchenak-Khelladi et al. 2010c), South America (Bremer 2002) or the Neotropics plus the Afrotropics (Gallaher et al. 2022), in any case on Gondwanan continents (e.g. Visser et al. 2013). Some Gondwana rafting may have occurred, but early diversification of the family seems to have been on Africa (Gallaher et al. 2022). Gallaher et al. (2022) discuss the distributions of all the named species groups in the family from the subtribes on up in the context of their relationships (chloroplast data), ages (see Table 1), summary morphology and ecology, and distributions (see Fig. 2 for ancestral areas); these authors suggest that the ages that they mention are more likely to be underestimates than overestimates. Soreng et al. (2021) carry out a similarly detailed study on the PPAM clade of Poeae, i.e. Coleanthinae (syn. Puccinelliinae), Poinae, the Alopecurinae superclade and Miliinae. They date this clade to (24.3-)21.8(-19.1) Ma, suggesting that it originated in alpine/subalpine habitats in S.W. Asia (Soreng et al. 2021, q.v. for many more dates).
Elliott et al. (2022b: Fig. S5) note the extensive chromosome number variation in Poaceae, ca 66-fold, Cyperaceae are next at ca 55-fold, and all other families show less than 30-fold variation - Poaceae have the equal lowest diploid number, 4 (ibid. Fig. S6).
Of the three species-poor basal clades, Pharus has a number of features in common with Anomochloöideae including broad and more or less pseudopetiolate leaves, perhaps because they are all plants of the forest floor (Sajo et al. 2007, 2012: Gallaher et al. 2016), and there are also similarities in spikelets (P.-F. Ma et al. 2021 and references). Although Pharus itself has numerous distinctive features, whether other members of Pharoideae have these features is unknown; Pharoideae are described as being monoecious, but P.-F. Ma et al. (2021) described P. latifolius as if it were polygamous. Puelioideae, the third clade, are also forest grasses that are very poorly known (see also Judziewicz & Clark 2008; Kellogg 2013b). Interestingly, Micrairoideae-Isachneae, C3 grasses in a C4 part of the clade, have moved to more shaded habitats and also have rather broad leaf blades (Teischer et al. 2019).
Hodkinson et al. (2008) discuss increases of diversification rates in Poaceae in the context of a supertree; there seems to have been one increase when true spikelets developed, at the Puelioideae etc. node, the evolution of spikelets themselves, open vegetation and dry environments being the triggers (see also Bouchenak-Khelladi et al. 2010c, 2015: the spikelet clade). The herbaceous habit and annual life cycle appear to be correlated with species richness (Salamin & Davies 2004; S. A. Smith & Donoghue 2008), however, the speciose Bambusoideae are woody. D. Soltis et al. (2009) suggested that diversification in Poaceae might be connected with the ρ/rho genome duplication, pegged at the level of the family here, however, diversification of the groups including the C3 cereals seem to have have occurred ca 20 Ma or more later (Paterson et al. 2004; c.f. International Brachypodium Initiative 2010), while Zwaenepoel and Van de Peer (2020) question the existence of this event. P.-F. Ma et al. (2021: p. 5) suggests that "radiation of the core lineages" occurred after a ca 16 Ma lag; eee also P. Soltis et al. (2019) for the PACMAD/BOP clade and diversification. The age of the PACMAD/BOP node is broadly in line with that of the age of this duplication that is estimated to have happened 70-50 Ma (Blanc & Wolfe 2004; Schlueter et al. 2004; Paterson et al. 2004; C. Kim et al. 2009). Schranz et al. (2012) thought that there was a lag time between the duplication and subsequent diversification increases, although the two might be linked.
Ecology and diversification have been examined at finer levels and in various groups, and the evolution of the PACMAD and BOP clades are associated with other shifts in diversification rates (Bouchenak-Khelladi et al. 2010c). Thus episodes of diversification may be associated with some, but not all, C4 clades during the Miocene expansion of grasslands within the last 15 Ma (Spriggs et al. 2014). C4 photosynthesis (see below) has arisen many times in the PACMAD clade, and clades with C4 photosynthesis tend to be more speciose than their sister clades with C3 photosynthesis; Chloridoideae tolerate drought and saline conditions particularly well; and so it goes. Rather unusually, the C3 Isachneae (Micrairoideae) are more speciose (and morphologically diverse, etc.) than their C4 sister clade, Eriachneae (Teisher et al. 2019). Grasses are quite diverse on Madagascar, although like Cyperaceae their overall endemicity is low (Larridon et al. 2020a); C4 grasses had twice as many lineage origins as C3 grasses but their endemic species:lineage ratios were much lower, two C3 forest clades in Bambusoideae and Panicoideae being particularly diverse (Hackel et al. 2018).
The adaptive radiation of grasses may well be linked to the number of whole genome duplications in the family, perhaps more than in other comparable monocot clades. These duplications lead to chromosomal rearrangements, karyotype diversification and rapid substitution rates (see also McKain et al. 2016a). As T. Shi et al. (2022) suggested, a rapid substitution rate may be a signature of adaptation, and neofunctionalization may occur in rapidly evolving genes. Interestingly, it has been estimated that 75-80% of all grasses are recent polyploids (P.-F. Ma et al. 2021).
Thinking about biogeography and diversification in a rather loose sense, we find
— Much diversification in Aristidoideae is considerably more recent than the (25.5-)20.3(-15.9) Ma crown age (Bouchenak-Khelladi et al. 2010a; Cerros-Tlatilpa et al. 2011, q.v. for other estimates).
— The very diverse Old World members of Arundinarieae (Bambusoideae) are a mere 15 Ma, while tropical Old and New World bamboos may have diverged 40.2-24.8 Ma (Burke et al. 2012, c.f. other ages there; Z.-H. Guo et al. 2019). Arundinarieae may have originated in East Asia, the African and Indian members of the tribe being derived (X.-Z. Zhang et al. 2015). W. Wang et al. (2020a) suggested that bamboos provided an example of tropical plants evolving faster than their temperate relatives, noting that the rate of evolution of the plastome in Olyreae, largely tropical, was about three times that in Arundinarieae, more or less temperate; generation times in the former were also shorter (no synchronized periodic flowering), which ceteris paribus would lead to faster evolution. However, here at least speed of evolution seems to have little to do with species numbers, since temperate woody Arundinarieae with their slowly-evolving plastomes include four times as many species as the tropical herbaceous Olyreeae with more quickly-evolving plastomes, while the tropical woody Bambuseae with somewhat intermediate plastome evolutionary rates (Wang et al. 2020a) have over six times as many species. Furthermore the stem group age of Olyreae is about twice the ages of the polyploidy events that characterise the woody groups (e.g. Guo et al. 2019). However, the relation between polyploidy and woodiness is unclear, and there may be linkages with climatic events (Guo et al. 2019). Ye et al. (2019) looked at the alpine bamboos of southeast Asia, around 200 species in 8 genera, and found very rapid diversification starting less than 4 Ma and persisting for around 1.5 M years and especially noticeable at high altitudes in the Hengduan Mountains, S.W. China - although diversification rates along the Andes tend to be appreciably higher. For more on the ecology and evolution of Bambusoideae, see below.
—In Chloridoideae-Cynodonteae, all six major clades of Muhlenbergia, a genus of some 183 species, seem to have originated in the Sierra Madre of Mexico (Peterson et al. 2021b, 2023: also dates, etc.). The genus may be ca 9.3 Ma (Peterson et al. 2021b) or ca 15 Ma (Peterson et al. 2010b). Peterson et al. (2010b) noted around 8 amphitropical disjunction events here (12-13 - Guilliams et al. 2017).
— Linder et al. (2013) discussed the distribution of the largely austral Danthonioideae; they thought that the main variables explaining it were the distance between suitable areas and the extent of those areas, but not wind direction, extent of water gaps, etc.; dispersal rather than vicariance has been invoked to explain distributions in the Danthonia area in particular (Sanmartín & Ronquist 2004). Linder and Barker (2014) found that polyploids were more successful at long distance dispersal (the ancestral area for the subfamily is perhaps Africa, southern Africa in particular - see Linder & Bouchenak-Khelladi 2017; Linder et al. 2017b) into new areas/habitats, thus facilitating diversification. Linder et al. (2014; see also Visser et al. 2013) emphasized topographic activity/heterogeneity as drivers of radiation, although the particular factors that might drive diversification are difficult to disentangle (Linder & Bouchenak-Khelladi 2017). Within Danthonioideae, the speciose Pentaschistis probably originated in the Cape region, while immediately unrelated clades of Danthonioideae grow in colder conditions (Humphreys & Linder 2013); overall, elevation may have had rather little to do with clade species richness here (Visser et al. 2013).
— Welker, McKain, et al. (2020) suggested that there had been some 246-373 dispersal events (the number depends on the analysis) in Panicoideae-Andropogoneae alone, vicariance events being about one tenth those numbers. A summary is not attempted here; the origin of the tribe may be in East Asia. Allopolyploidy is very common in Andropogoneae, but few allopolyploidization events have been followed by anything much in the way of speciation, although the [Zea + Tripsacum] clade is one modest exception (Estep et al. 2014).
— Poöideae are largely temperate, although they can live in very harsh (frigid, arid) conditions; they may have originated in Eurasia (Schubert et al. 2019). Das et al. (2021) suggested that their distributions were affected by their tolerance to aridity and cool conditions, but that stomatal behaviour had little effect. Their diversification, particularly high in the Oligocene-Miocene, occurred against a background of a constant haploid chromosome number (x = 7: Pimentel et al. 2017). Inda et al. (2008a) discussed the biogeography of Loliinae, which seems to have involved multiple dispersal events from a centre in the Mediterranean region over the last ca 13 Ma. Peterson et al. (2020c) suggested that Calamagrostis originated in North America. For diversification of the large Poa alliance (Hoffmann et al. 2013; Birch et al. 2014), see below; much is Pleistocene or younger, Poa being 17.6-9.9 Ma and probably originating in Eurasia (Giussani et al. 2016). There is more on cold tolerance in Poöideae below.
Both Festuca and Anthoxanthum are notable components of the Afroalpine flora; Festuca may have moved to Africa from northern South America (Brochmann et al. 2021; see also Kandziora et al. 2022). Endopolyploidy, a measure of UV-B stress, increased with altitude more rapidly in Poaceae with their monocentric chromosomes than in Cyperaceae-Juncaceae with their holocentric chromosomes - in the latter fragmented chromosomes were more likely to behave normally during cell division (Zedek et al. 2020).
There are some 51 (21% of the total) American amphitropical disjuncts in the family, by far the most in any family (Simpson et al. 2017a; see also Raven 1963b), although Poaceae are rather speciose... Indeed, in terms of disjunctions/clade size and age, the story is somewhat different. Relatively more such events have occurred in Boraginaceae-Cynoglosseae-Amsinckiinae, which has 19 American amphitropical disjunctions in a clade of a mere 334 species that is ca 21.9 Ma (Guilliams et al. 2017; see also L. A. Johnson & Porter 2017: Polemoniaceae), although in Muhlenbergia s.l. (= Chloridoideae-Cynodonteae), a clade of some 183 species that may be ca 9.3 Ma (Peterson et al. 2021b; ca 15 Ma, Peterson et al. 2010b), Peterson et al. (2010b) noted around 8 such events (12-13 - Guilliams et al. 2017; see also Amarilla et al. 2015: another chloridoid). All seven bipolar species in Poaceae, 29% of all such species (Carex has a similar number), are in Poöideae-Aveneae (Villaverde et al. 2017).
One result of these various diversification events seems to be that in grasses the overall north-south diversity gradient is much flatter than might be expected (Visser et al. 2013; see also elsewhere). Thus the diversification of Poöideae, which make up about one third of all grasses, is largely temperate, many members of the PACMAD clade are specialists in more or less arid conditions, and grasses have diversified in topographically heterogeneous mountainous regions. A paradox perhaps is that grasses are not necessarily that notably diverse in grasslands (Visser et al. 2013), only a few species tending to dominate there (e.g. E. J. Edwards et al. 2010; Yu et al. 2015; Kellogg 2015).
Perhaps paradoxically, although Poaceae are practically ubiquitous on oceanic islands, being found there more frequently than any other family, they have the fewest insular endemic species of any major family (Lenzner et al. 2017). On Madagascar, too, their endemicity, ca 40%, is low, and is about half that of other major plant groups there (but not Cyperaceae and Asteraceae), although for grasses 40% endemicity in such an area is a pretty high figure (Hackel et al. 2018). There is debate over whether Madagascan grasses and grasslands in the central highlands are native. Endemic grasses are largely absent from these grasslands, and it has been suggested that they are anthropogenic, endemic grasses in Madagascar being found more in woodlands and forests (Joseph & Seymour 2020; but c.f. Bond et al. 2008; Solofondranohatra et al. 2020: c.f. in part Crowley et al. 2020; also Lehmann et al. 2021), indeed, two papers in a recent issue of Plants, People, Planet (6(1) 2024) tended towards opposite sides of this argument. The absence of endemics in lineages of sedges that might be expected in savanna grasslands (Muasya et al. 2011) is consistent with the idea that the grasslands are not native.
The results of the analysis of the Streptochaeta angustifolia (Anomochloöideae) genome are consistent with the idea that the evolution of spikelets, structures that are integral to understanding the diversity of the family, may indeed be an apomorphy for the family as a whole. The odd flowers/inflorescences of Anomochloöideae themselves then would not be in any way "intermediate" between those of other Poales and of the spikelet clade, but rather represent a loss (Seetharam et al. 2021). Looking at the genome of Streptochaeta, these authors thought that "many of the genes known to control the structure of the grass spikelet were found in an ancestor of both Streptochaeta and the spikelet clade, but have then been lost in Streptochaeta" (ibid., p. 14). The apomorphy scheme above follows the conventional interpretation of spikelet evolution; sequencing the genome of Anomochloa might help clarify the issue. For more on the evolution of spikelets, see also Y. Wang et al. (2022).
Linder et al. (2017b) emphasized that grass diaspores, the spikelet with its innumerable modifications, were extremely effective in dispersal, and could perhaps be thought of as a key innovation for the family. Indeed, diaspores and dispersal mechanisms in Poaceae are very varied (e.g. Werker 1997, see also Pollination Biology & Seed Dispersal below). The caryopsis is a variant of an achene in which the testa and pericarp are fused; it is often described as being the distinctive fruit type of Poaceae, but it develops in quite a variety of ways in the family (Nakamura et al. 2009 and references). There is little phylogenetic signal (subfamily, tribe) in the anatomy and histology of the abscission zone the activity of which causes the spikelet and mother plant to separate (Yu et al. 2020) and the part of the plant that is dispersed along with the seed s. str. is correspondingly various; that the abscission zone is above the glumes is the ancestral condition (Y. Yu et al. 2019; McSteen & Kellogg 2022: Fig. 3). In clades like Poodae/Poeae s.l. fruiting characters show high homoplasy (and there has also been extensive hybridization in this group), however, the great diversity in spikelet morphology and resultant dispersal mechanisms may help explain the diversity of this clade, particularly throughout the north temperate and Arctic zones (Tkach et al. 2020). Overall, there is a tendency for clades with awns to have more species than those that do not (Humphreys et al. 2010b). However, despite the apparent advantages in having an awn, it has been lost at least 25 times in Danthonioideae alone, perhaps in association with the adoption of the annual habit where passive burial of seeds suffices (Humphreys et al. 2010b); seeds that lack awns tend to become buried and may have delayed germination (Peart 1984).
Leandro et al. (2019) looked at the variation of leaf micromorphology in Bambusoideae in the context of its phylogeny; much variation there is more or less at the subtribal level.
In a study of the variation of grass pollen in South America since the early Miocene ca 23 Ma, C. Wei et al. (2024) noted that pollen ornamentation had become less dense over this period - functional morphology changing then? a response to the decrease in atmospheric CO2?
J. Ma et al. (2022) suggested that horizontal gene transfer was rather uncommon in seed plants. However, they thought that Poaceae were something of an exception, genes from bacteria, viruses and fungi being incorporated into grass genomes. For example, genes from the fungal endophyte Epichloë are to be found in the genomes of Pooideae like barley, wheat and rye (Shinozuka et al. 2020; H. Wang et al. 2020). This is discussed further below.
There are similarities in chondrome gene content and loss between those few Cyperaceae and Poaceae known (C. Lee et al. 2023) - ?significance.
Ecology & Physiology. The global extent of contemporary grassland, including savanna with its C4 grasses, is 52.5x106 km2, or somewhere between 41-56x106 km2, that is, 31-43% of the total land surface area (Gibson 2009: excluding Greenland and Antarctica; Beer et al. 2010); other estimates are ca 20% of the earth's surface (Hall et al. 2000; Sabelli & Larkins 2009). Of course, these figures depend on the definitions of grassland, savanna and forest (see also Dixon et al. 2014; Bond 2016a, c.f. DeWitt et al. 2016 and Bond 2016b). Grasses in the Great Plains alone cover slightly over 3x106 km2, the Campos Cerrado ca 2x106 km2 - see also the map, where rather virulent green = more or less pure grassland, and olive green = communities with trees and shrubs as well as substantial grass (the map here is based on those on the endpapers in Coupland 1992, 1993; esp. White et al. 2000, Map 1; see also Lehmann et al. 2019, Fig. 1A, S1; Griffith et al. 2020, Fig. 1); for more details; see also Clade Asymmetries.
Lehmann et al. (2019) characterise grassy biomes as having 50% or more of the ground layer made up of grasses, some 1,154 species of grasses in all being involved, and these biomes cover around 41% of the earth's land surface. Three groups predominate: The BOP clade, mostly Po¨ideae, but also some Bambusoideae, dominate in 38% of these biomes, Andropogoneae in 37% and Chloridoideae, absent from South America and much of Eurasia, in about 14%. The 1,154 species just mentioned make up only ca 10% of the family and the family as a whole, at somewhat over 11,300 species, makes up slightly more that 3% of all angiosperms, yet it is responsible for around 28% gross primary productivity/25% of terrestrial photosynthesis (Beer et al. 2010; Lehmann et al. 2019; see also Griffith et al. 2020). For summaries of the ecology of grasses and grasslands, see e.g. Coupland (1992, 1993), White et al. (2000), Gibson (2009), Lehmann et al. (2019) and others, while Linder et al. (2017b: esp. Fig. 5) focus more particularly on the aspects of grass ecophysiology that may have facilitated their success.
The discussion below is divided up as follows:
1. Grasses and C4 photosynthesis.1. Grasses and C4 photosynthesis.
Although there have been suggestions that there have been plants with C4 photosynthesis throughout the Mesozoic (Keeley & Rundel 2003 for literature), the appearance os C4 photosynthesis in clades of extant angiosperms is a Caenozoic phenomenon, and the dominance of grasses largely developed only during the late Miocene (10-)7-4 Ma (see the next section). Estimates vary, but around 8,145 species of flowering plants have the C4 photosynthetic syndrome, about 3% of all flowering plants, and of these about 5,044 species, around 60%, are grasses, where they make up most of the PACMAD clade (Sage et al. 1999, 2012; Sage 2004, 2016; Grass Phylogeny Working Group II 2011; see Osborne et al. 2014 for a global database of C4 grasses). For summaries and discussion Of C4 photosynthesis, see e.g. Sage et al. (2012), Kellogg (e.g. 1999a, 2013a), Sage (2016), Sedelnikova et al. (2018), Schlüter and Weber (2020), etc..
As mentioned before, there has been massive parallelism in the origin of C4 photosynthesis in angiosperms - overall, some 62 times in 19 families (Sage 2016). There are some 20-24 origins in Poaceae alone, or more specifically in the PACMAD clade, and ca 61% of all C4 species are to be found there (e.g. Kellogg 2000; Roalson 2011: 12-19 transitions; Christin et al. 2008a, 2009b; Vicentini et al. 2008; Cerros-Tlatilpa & Columbus 2009 and Christin & Besnard 2009 [both Aristidoideae]; Edwards et al. 2010; Grass Phylogeny Working Group II 2011; Sage et al. 2011, 2012; Morrone et al. 2012; Christin et al. 2015; Sage 2016; Gallaher et al. 2019). Both origins of and reversals from C4 photosynthesis may be phylogenetically clustered, although reversals are not very common (Vicentini et al. 2008; for reversals, see also Ibrahim et al. 2009), indeed, Bräutigam et al. (2017) suggest that they are very unlikely, and Dunning et al. (2017) discuss an example of a reversal in Alloteropsis that wasn't. Lundgren et al. (2018) emphasized that just a single change, specifically, an increase in venation density, might be all that was needed for the origination of C4 anatomy in the photosynthetically variable Alloteropsis semialata (Paniceae-Boivinellinae), a fascinating case of a species that includes C4, C3 and intermediate photosynthetic types and is found quite widely in the Old World (Bianconi et al. 2020). Christin et al. (2012a, b) and Phansopa et al. (2020) noted the complexities of C4 evolution in Alloteropsis (Panicoideae-Paniceae), which i.a. involves maybe three cases of horizontal transfer (and two separate origins of C4 photosynthesis) of the important C4 gene phosphoenolpyruvate carboxylase (PEPC) from taxa that diverged from Alloteropsis 20 Ma or more. W. Huang et al. (2022) discuss the complex evolution of the ppc gene family in the PACMAD clade in the context of gene duplications and independent origins of C4 photosynthesis. Photosynthesis type varies quite extensively on a fine scale, thus C3, C3-C4 intermediates, and C4 grasses are all found among the 35 species of Paspaleae-Otachyriinae (Acosta et al. 2019), and there is similar variation in Paniceae-Neurachninae, another case where there has been horizontal transfer (Christin et al. 2012b; Khoshravesh et al. 2019; Lauterbach et al. 2024). Alvarenga et al. (2024) discuss the evolution of photosynthesis type in the small genus Homolepis (Panicoideae-Arthropogoninae) with C2, C3 and intermediate types, the latter being the result of intrageneric hybridization.
The adoption of C4 photosynthesis is associated with anatomical changes such as closer spacing of the veins, the development of a sheath of chloroplast-rich cells around the vascular bundles, etc., i.e., changes in the various components that make up the Kranz anatomical syndrome (see W. V. Brown 1999 for the diversity of morphologies here), although details of the control of this are still poorly understood (Kellogg 2013a; see Pengelly et al. 2011: vein spacing; Sack & Scoffoni 2013: vein length/leaf area and potentiation of the repeated evolution of C4 grasses?; Kumar & Kellogg 2018; Q. Liu et al. 2023). In taxa like Arundinella hirta, a C4 grass, there are rather distant veins about seven mesophyll cells apart, however, there are files of bundle sheath-type cells only one or two mesophyll cells apart, and they are intermittently connected by obliquely-running cross veins (Crookston & Moss 1973). E. J. Edwards (2019) suggested that anatomical changes, perhaps involving the bundle sheath, are the "rate limiting step" in the evolution of C4 photosynthesis. C4 grasses have 5-9 times more plasmodesmata per mesophyll:bundle sheath cell wall contact area than C 3 grasses, perhaps facilitating the movement of C4 acids between the two, although similar differences were seen in mesophyll:mesophyll cell contacts (Danila et al. 2016). In Cleome s.l., at least, a delay in differentiation of the mesophyll cells is connected with increased venation density of C4 plants (Külahoglu et al. 2014). Genes involved in root endodermal development have been coopted in bundle sheath development, suberin in the bundle sheath cells probably helping to maintain the concentration of CO2 in the bundle (Slewinski et al. 2012; P. Wang et al. 2013; Slewinski 2013; Mertz & Bruntnell 2014; see also Huang et al. 2016 and Duvall et al. 2017 for C4 anatomy - more variation than expected). For the bundle sheath in C3 grasses, see Leegood (2008) and Ueno and Hatakeyama (2018).
W. Huang et al. (2022) suggested that all the subfamilies of the PACMAD clade initially had C3 photosynthesis, except perhaps Chloridoideae and Aristidoideae. Lyu et al. (2024) looked at the early steps in the evolution of C4 photosynthesis. They suggested that there was just one origin in Chloridoideae, perhaps 32-25 Ma, giving rise to the ca 1,500 C4 species there; C4 photosynthesis arose somewhat later in other groups, and the mechanisms of C4 photosynthesis and the anatomies associated with it are particularly various in Paniceae - in Panicoideae alone C4 photosynthesis may have evolved between four and eight times and the C3 pathway is likely to be plesiomorphic (Kellogg 2000; Giussani et al. 2001; Christin et al. 2007a, 2009a; Washburn et al. 2015; W. Huang et al. 2022; Lyu et al. 2024). However, to understand what is going on here a resolution of the phylogeny of the tribe is needed and chloroplast and nuclear genes do not always tell the same story (e.g. Washburn et al. 2015), hybridization has been extensive (see below), etc.. Bianconi et al. (2019) suggested that there was an initial burst of changes associated with the adoption of C4 photosynthesis, but changes continued, the result being a variety of C4 subtypes. The relatively uncommon C4 PEPCK subtype (phosphoenolpyruvate carboxykinase) may be basal in Chloridoideae, being subsequently lost and reacquired (Christin et al. 2009b, 2010a: reversals; but c.f. Ingram et al. 2011b); Y. Wang et al. (2014) suggested that the pure PEPCK subtype does not really exist... In the chloridoid Orcuttia there is single-celled C4 photosynthesis (Keeley 1998b; Bowes 2010; von Caemmerer et al. 2014). Although anatomy has been used to characterize subtypes of C4 photosynthesis, the correlation of anatomy with photosynthetic pathway may not be that good (Ingram 2010) and, very importantly, the typology needs to be revisited (Y. Yang et al. 2014; Washburn et al. 2015) - indeed, breaking down C4 photosynthesis into the independent variables that make it up, e.g., location, amount of RuBisCO (ribulose-1,5-bisphosphate carboxylase-oxygenase) activity, etc., may be the way to go (Dunning et al. 2017). There are a few C3-C4 intermediates that carry out C2 photosynthesis, Paniceae-Neurachninae being well studied from this point of view (Monson & Rawsthorne 2000; Bauwe 2011; Clayton et al. 2017; Khoshravesh et al. 2019). In this small group of ca 18 species mostly from Australia (and also the Philippines), C3 Neurachne annularis is sister to the rest of the genus and the outgroup is a C3 plant, so the proto-Kranz state is probably the ancestral condition within Neurachne; there have been two origins of C4 photosynthesis and three of C3-C4 intermediacy here alone (Christin et al. 2012b; Khoshravesh et al. 2019; Lauterbach et al. 2024). Genes involved in C4 photosynthesis seem to have independently evolved from common precursors, interestingly, there were parallel changes in 14 sites in C4 PEPCs in the PACMAD species examined by Lyu et al. (2024) and there were similar changes in non-C4 PEPC genes, e.g. alanine-to-serine and other substitutions in Chloridoideae but not Panicoideae; there were no such changes in non-C4 PEPC genes in the few BOP species examined.
All in all the gradual evolution of C4 photosynthesis can be followed in considerable detail in Poaceae, and with the bundle sheath accumulating organelles and the mitochondrial glycine decarboxylase, and interveinal distances decreasing, the changes are similar to those in Asteraceae and Boraginaceae (Khoshravesh et al. 2019, see also 2016; c.f. B. P. Williams et al. 2013). Heckmann et al. (2013) discussed C4evolution in Asteraceae (Flaveria in particular has been much studied, but Panicum and even some Brassicaceae seem to be similar), likening the basic evolutionary landscape to Mt Fuji, and they noted that "the fitness gain achieved by each individual change remained comparable along evolutionary trajectories" (ibid. p. 1585). There was, however, only weak selection on the initial mutations, furthermore, conditions of drought, high light and high temperature that favour C4 photosynthesis are not universally favoured (see Heckmann et al. 2013).
The ecophysiology of C3 and C4 grasses has frequently been compared, as by S. H. Taylor et al. (2010, 2011), Ripley et al. (2010, 2015), Christin and Osborne (2014), and others. C4 plants use less nitrogen (N) because C4 is more efficent than C3 photosynthesis, and so less RuBisCO is needed; that enzyme alone may account for around 30% of leaf N in C3 species (Long 1999; Linder et al. 2017b). In a comparative study, Pinto et al. (2014) found that C4 grasses used both N and water more efficiently than C3 or C3-C4 intermediates at low CO2 concentrations (such as occurred during glacial periods). C3 grasses are sometimes more sensitive to drought and recover more slowly from it (see also H. Liu & Osborne 2014 for drought response; Hanslin et al. 2019 for response of young seedlings to drought), although it is just when in the year there is drought that matters (Knapp et al. 2020, also see next section). When stomata close in plants with C4 photosynthesis transpiration losses are reduced, so mitigating the effect of higher temperatures and water stress, however, carbon fixation is not necessarily reduced and damaging photorespiration (a "design flaw" of RuBisCO most evident at higher temperatures and low CO2 concentrations) is avoided because of the high concentration of CO2 released e.g. from C4 malate in the bundle sheath cells (Morgan et al. 2011; R. Sage et al. 2012; Bena et al. 2017 and references). Although both C3 and C4 grasses have very small stomata, the stomatal density of the latter is substantially less (to half) although because they use CO2 more efficiently so they need to take up less of it (Franks & Beerling 2009: below/above 400 stomata/mm2 tends to separate the two groups; Taylor et al. 2011); within C4 grasses, Chloridoideae have smaller and denser stomata than do Panicoideae (H. Liu & Osborne et al. 2014). Indeed, Cano et al. (2019) noted that all C4 subtypes had similar high leaf-level water use efficiency (WUE, see also Asteraceae), while Ozeki et al. (2022) in their study of some C3 and C4 crops found that the latter had both denser and smaller stomata that closed faster on shading (and opened faster when light was increased), and as a result their overall WUE was notably higher. Variation in stomatal size and number will affect stomatal conductivity, and although C4 and C3 grasses photosynthesize at the same rate (e.g. Long 1999), the stomatal conductance of the former is lower because they have fewer stomata (Taylor et al. 2011). Normally, species with denser venation (i.e. C4 grasses in this case) would be expected to have denser stomata (Fiorin et al. 2016; see also Théroux-Rancourt, Roddy et al. 2021). For more on grass stomata and their evolution, see Z.-H. Chen et al. (2017: c.f. Fig. 1) and McKown and Bergmann (2020). Note that some C4 taxa like Miscanthus x giganteus photosynthesize under decidedly cooler conditions than is common (D. Wang et al. 2008), and this is associated with the ability of the plant to maintain concentrations of pyruvate phosphate dikinase protein, central in C4 photosynthesis. Furthermore, C3 grasses like Lolium perenne can also tolerate water stress (Holloway-Phillips & Brodribb 2010, see below), so there is blurring of the ecophysiological boundaries of the photosynthesis types. To summarize: C4 grasses can tolerate quite warm and dry conditions and need less N, less CO2 and less water, the distinctive features of C4 photosynthesis; they can produce much - up to twice as much - more biomass annually than C3 grasses (Snaydon 1991: not a feature of C4 plants in general; Sage & Zhu 2011).
Aside from comparisons between C3 and C4 grasses in which the immediate concerns are with photosynthesis per se, there may be substantial differences in other features like the early growth of these grasses. One comprehensive study (Atkinson et al. 2016) involved 382 species growing in "resource-rich, tropical conditions" (ibid.) in a greenhouse. C4 grasses were found to grow relatively more quickly (sister group comparisons), especially at later stages of this experiment, i.e., the plants were larger, and it was noted that their net assimilation rate increased considerably. Interestingly, C4 grasses had a higher specific leaf area, i.e., they had relatively less leaf mass per unit area despite their higher assimilation rates, and they also allocated over 50% more biomass to their roots (Atkinson et al. 2016). However, the simple C3/C4 distinction by no means captures all variation that is of ecological/physiological interest, even in grasses growing in the one area; dividing up grasses on their phylogenetic history captures groups with distinctive variation in other structural and physiological traits (Kellogg 1999b; Donnelly et al. 2023). Thus variation in SLA might indeed be best represented by plant functional type (i.e. photosynthetic type), but variation in the leaf C:N ratio is best captured by phylogeny (Donnelly et al. 2023). The seedlings of perennial pooid grasses studied by Hanslin et al. (2019) adjusted to water stress by changes in their primary root systems, the main root elongating, but there was no growth of the secondary roots. It would be interesting to know how mycorrhizal associations (see below) might affect such findings.
Christin et al. (2013a) thought that particular anatomical changes facilitated the transition to C4 photosynthesis in grasses. As mentioned, veins - or at least bundle sheaths - became closer in the common ancestor of the PACMAD + BOP clade, and a high proportion of vascular bundle sheath tissue, involved in C4 photosynthesis and integral for its functioning, facilitated this transition. For venation densities, ca 5.1 vs 10.6 mm mm-2 in C3 vs C4 plants, see Ueno et al. (2006), however, little is known about the control of venation density here (Kumar & Kellogg 2018; c.f. in part Q. Liu et al. 2023). Baird et al. (2021) also looked at venation density in the context of leaf development and final leaf size. They too found that C4 grasses tended to have denser venation (some panicoids - alone in the grasses they studied - developed quaternary veins), leaves became shorter and narrower in cooler and drier conditions, the dense venation of C4 grasses being a probable advantage if temperatures increased and precipitation became more seasonal (Baird et al. 2021). C4 photosynthesis did not develop in the BOP clade, and/because the outer bundle sheath cells subsequently became smaller, but it did in the PACMAD clade because they became larger (the outer sheath cells were sometimes lost there, but then the inner sheath cells became dramatically larger). Finally, mesophyll cells have sometimes been lost in the PACMAD clade (Christin et al. 2013a). In a less elaborate analysis, Griffiths et al. (2012) suggested that bundle sheath proliferation had begun before there were any changes in vein densities. Associated with the origin of the expanded bundle sheath are changes in the physical organization of the photosynthetic machinery, with chloroplasts in C4 plants developing particular functions and being found in different places (Majeran & van Wyk 2009; Solymosi & Kereztes 2012).
The numerous acquisitions of C4 photosynthesis within the PACMAD clade may reflect an initial change that faciltated subsequent "independent" acquisitions of the pathway (Grass Phylogeny Working Group II 2011: gene duplication not involved; B. P. Williams et al. 2012; see Marazzi et al. 2012). However, Monson (2003) and others have suggested that the changes needed to establish the enzymatic machinery of C4 photosynthesis after a gene duplication may not be that great, enzymes, etc., being coopted from other areas of the plant's metabolism, including C3 photosynthesis itself. X. Wang et al. (2009) suggested that the evolution of C4 photosynthesis occurred perhaps 30-40 Ma after a palaeopolyploidization event ca 70 Ma, and the genes coopted for the new pathway came both from duplicated genes that were produced during this event and by more recent single gene duplications. Of the three main kinds of C4 photosynthesis in grasses, both NAD-ME and NADP-ME types evolved in parallel, while PCK/PEPCK evolved in parallel from both these types (the PCK pathway may occur in the other two types and not exist as an independent pathway - H. Liu & Osborne 2014; Y. Wang et al. 2014). Parallelisms may even be at the level of particular amino acids being substituted, similar changes occurring independently in the phosphoenolpyruvate carboxykinase gene in grasses (Christin et al. 2007a, esp. b, 2009a). John et al. (2014) also discuss parallelisms in C4 grasses, while Emms et al. (2016) found very extensive parallelisms between independent origins of C4 photosynthesis in Andropogoneae and Paniceae, with some 21 genes being duplicated and retained independently in the two and preferentially expressed in the bundle sheath; genome duplications are likely to have been involved (Emms et al. 2016). Indeed, a mutation to serine at position 780 seems to have occurred in all plants with C4 photosynthesis (Bläsing et al. 2000; see also the Grass Phylogeny Working Group II 2011), and functionally important parallelisms are also found in rbcL (Christin et al. 2008b). N. J. Brown et al. (2011, p. 1438) noted that "functionally equivalent mechanisms that control the accumulation of proteins important for C4 photosynthesis" had evolved in parallel in Cleome gynandra and in maize, root endodermal cells seem to have been coopted in bundle sheath development in both (Külahoglu et al. 2014), while there is a set of transcription factors expressed along with C4 photosynthesis genes in the two (Aubry et al. 2014).
Comparing three origins of C4 photosynthesis in Panicoideae, Christin et al. (2017) found that in five of the seven enzymes examined the same gene lineage had been recruited - the probability of that happening by chance was rather low... Gene/genome duplication, horizontal gene transfer and diurnal regulation of genes are also part of this story, for instance, the latter was found in C4 but not C3 members of Alloteropsis (Paniceae). In some populations of A. semialata an important C4 gene has come from a member of Andropogoneae and has become integrated with the other C4 genes there - the C4 pathway in A. semialata was only recently acquired (Christin et al. 2012a; Dunning et al. 2017, 2019; Dunning & Christin 2020) - horizontal transfer has been very common here (see also Wickell & Lee 2019). Parallelisms can be far-reaching. In the C4 Neurachne munroi carbonic anyhydrase had become localized to the mesophyll cytosol whereas in the other species it was in the chloroplasts; an isoform of the enzyme catalyzes the first step of the C4 pathway, converting H2 + CO2 → HCO3-, and changes in the CA1a gene which involved the loss of transit peptides paralleled comparable changes in Flaveria (Asteraceae), although they were not so extensive (Clayton et al. 2017). At another level, in a broad survey of C4 plants Lundgren et al. (2014) noted that concentration of chloroplasts in the area in which the Calvin cycle went on was about the only feature common to all of them, while at still another level horizontal transfer of genes within grasses following wide hybridizations may have facilitated putting together C4 pathways, and this latter is discussed further under below.
For variation in hydraulic conductance, see below.
2. Grasslands, Water, Fire and Forests.
The original habitat of grasses was probably shaded conditions in the understory of humid tropical forests (e.g. Osborne & Freckleton 2009; Bouchenak-Khelladi et al. 2010a, c: Puelioideae not included; Givnish et al. 2010b; Cotton et al. 2015). Grasses growing in such conditions tend to have broader leaf blades, although seed size is similar to that of grasses growing in the open (Cayssials & Rodríguez 2013: Uruguay, no Anomochloöideae), and members of the basal pectinations in the family that grow in forest habitats do have broad leaf blades, etc.. The ancestors of the PACMAD and BOP clades also favoured forest margins, and grasses of those clades growing in such habitats often have more or less narrowly elliptic ("lanceolate") blades, even if the great majority of those clades (other than Bambusoideae, many Oryzoideae and some Panicoideae in particular) have linear blades (Bouchenak-Khelladi et al. 2010a; Gallaher et al. 2019). Extant grasses are typically associated with open habitats and represent separate radiations in tropical and temperate areas, but there have been shifts back to more or less shaded forest conditions, especially in Panicoideae.
It is generally thought that the great ecological importance of grasses, especially those that carry out C4 photosynthesis, and their dominance over considerable areas of the surface of the globe, has developed only within the last (10-)7-4 Ma (e.g. Jacobs et al. 1999; Keeley & Rundel 2003; Jacobs 2004; Edwards et al. 2010). Indeed, some cases of the expansion of C4 vegetation may be yet more recent, e.g. ca 3.5 Ma in N.W. Australia (Andrea et al. 2018), and not much older in South America (Palazzesi & Barreda 2012). The origin of the C4 trait may indeed go back 20 Ma or more; by the late Eocene ca 33 Ma grass pollen was 30-40% of the total in the Niger Delta region, and there was evidence of fires (Maley 1996 and references), but the conditions favouring the spread of C4 grasses seem to have been different from those at the origination of this trait (T. J. Davies et al. 2020). Tropical and subtropical savannas include the widespread Cerrado vegetation of Brazil (for which, see Simon et al. 2009; Simon & Pennington 2012); for Old World savannas, see Denk et al. (2018) and Fortelius et al. (2019) and discussion. C4 Triodia (Chloridoideae-Cynodonteae-Triodiinae: some 110 species) dominates in the understory of maybe 30% of Australia, particularly in the savanna and arid biomes (Toon et al. 2015).
Both tropical and temperate grasslands have spread very widely, and although dominated by relatively few species of grasses, as mentioned they have come to play a major role in the terrestrial biosphere (Lehmann et al. 2019). Some of the most important effects of grasslands on ecosystems and biomes are mediated by fire, the fires being stimulated by grasses, perhaps Andropogoneae in particular, and grasses and other angiosperms respond to this fire in various ways. The discussion here includes both C4 and C3 grasslands; details of the evolution of cold tolerance, most notable in C3 grasses and grasslands, are dealt with later.
The three species-poor and largely forest-dwelling basal clades of the family (see also above) had probably diverged by the end of the Cretaceous; diversification of the PACMAD/BOP clade, largely consisting of plants growing in more open habitats (in this context, bamboos are sui generis), is probably entirely Caenozoic in age. Jones et al. (2014) suggest that forest-dwelling grasses were around for about 50 Ma, grasses moving into more open habitats only as forest retreated with increasing temperatures and dryness 56.5-53 Ma around the Palaeocene-Eocene thermal maximum (PETM); the dates in Cotton et al. (2015) were substantially younger (ca. 32.6 Ma), and the latter suggested that the rapid radiation of the PACMAD clade was at the Eocene-Oligocene transition, with temperatures falling at around that time (c.f. discussion on dates in Poaceae above). Small amounts of grass pollen have been found in West Africa in the Palaeocene to Middle Eocene and rather more abundantly in the Eocene from Egypt, although it disappears at least in some areas as the climate became wetter (Salard-Chaboldaeff 1981; Jacobs 2004). Germeraad et al. (1968) had proposed that that there was a pantropical Monoporites annulatus zone in the Early to Mid Eocene. In Africa, woodland vegetation in which genera like Acacia s.l. were to be found is known from 45.8 Ma, savanna is known from the Miocene, by 16 Ma grass pollen was increasing to 10%, along with charred grass cuticle, and grasses were a component of mammalian diet ca 15 Ma, becoming exclusive 8.5-6.5 Ma (Jacobs 2004). Indeed, Peppe et al. (2023) recently reported a rather more complicated story in East Africa from the early Miocene. In that area there was considerable habitat/vegetational heterogeneity, and although there was no true grassland or rainforest, C4 grasses were to be found locally by as early as ca 21 Ma; in other parts of the World C4 grasses seem to have replaced C3-dominated grassy mosaics or other dry, open vegetation, and rather later in time (Peppe et al. 2023).
Understanding the ecological relationship between grasslands and woodlands over time is important since the two differ greatly in associated flora and fauna and in their effects on the biosphere. Of course, the initial move of grasses from woodlands to open habitats, the evolution of C4 photosynthesis, and the rise to dominance of C3 and C4 grasses in more temperate and more tropical environments respectively, may all have quite different facilitating/precipitating causes which in turn differed in different parts of the globe. Climates and local conditions have been changing in different ways in different parts of the world (e.g. Andrae et al. 2018).
In particular, the ecological/environmental factors that favoured the initial development of grasslands, caused the clustering of origins and losses of different photosynthetic mechanisms, and were involved in the great spread and rise to dominance of C4 grasslands in the late Miocene, remain unclear (see also Tipple & Pagani 2007; Jacobs et al. 1999; Sage & Kubien 2003; Fox & Koch 2004; Osborne & Beerling 2006; Bond 2008; Westhoff & Gowick 2010; Christin & Osborne 2014) and will be still less clear if some of the older clade ages (see above) are confirmed (Christin et al. 2014a). Some combination of higher temperatures, decreasing/low atmospheric CO2 concentrations, the prevalence of fire, and water stress is now emphasized (e.g. Ehleringer et al. 1997; Bond et al. 2003a, b; Beerling & Osborne 2006; E. J. Edwards & Still 2008; Vicentini et al. 2008; Edwards 2009; Strömberg & McInerney 2011; Christin et al. 2011b; Kellogg 2013a; Forrestel et al. 2014; Bond 2015). Thus there was a decline - perhaps quite rapid - in atmospheric CO2 concentration ca 30 Ma in the Oligocene (Pagani et al. 2005; Zachos et al. 2008; Gerhart and Ward 2010; Arakaki et al. 2011), a decline perhaps caused by the activities of ectomycorrhizal plants (L. L. Taylor et al. 2009; see above). Decreasing atmospheric CO2, linked with decreasing temperatures, increasing aridity, etc., might have given C4 grasses an advantage photosynthetically (e.g. Ehleringer et al. 1997; E. J. Edwards et al. 2010; Y. Wu et al. 2023), and it might also have reduced the growth rate of woody vegetation, so hindering its recovery from fires (Bond et al. 2003a, b). However, the effect of changes in atmospheric CO2 concentration is unclear, indeed, recent work suggests that increasing CO2 may result in increasing grass biomass in C4 savannas in South Africa, at least, i.a. because their water use efficiency via CO2 fertilization has increased (del Toro et al. 2024).
NAD-ME species (Chloridoideae) favour drier habitats, NADP-ME species (especially Panicoideae) comparatively wetter habitats; within Chloridoideae NAD-ME species may be more drought-avoidance plants and PCK species more drought-tolerant plants (H. Liu & Osborne 2014) while within Paniceae C3 species tolerate greater tree cover (Arthan et al. 2017 and references). The initial development of C4 photosynthesis may have occurred in humid climates, with subsequent expansion into drier and more arid but not necessarily warmer habitats (Osborne & Freckleton 2009), although in panicoids C4 plants moved into warmer climates, and Paniceae, but not Paspaleae, also into drier areas (Aagesen et al. 2012, 2016). In the PACMAD clade C4 grasses seem to be able to tolerate drier conditions than the C3 grasses (Christin & Osborne 2014), but different groups of C4 grasses do not all behave the same. Forrestel et al. (2017) found that Cynodonteae in North America and Aristidoideae occupying a similar "precipitation niche" nevertheless differed substantially in several important functional traits such as height, specific leaf area, stomatal density, some of these traits being associated with resistance to/tolerance of grazing. Here a focus on single variables would yield only part of the story about how different clades evolve functional syndromes reflecting particular environmental conditions (see also Griffith et al. 2020 and the ecological decomposition of monolithic "grasses"). Many C4 origins seem to be correlated with a reduction in annual rainfall, and aridity may favour grass expansion (Sage 2004). Seasonality is important, too. The adoption of C4 photosynthesis in grasses is associated with the ability to live at higher temperatures and increased WUE. Interestingly, C3 grasses were favoured in the droughts associated with the formation of the Dustbowl in the USA in the 1930s (Knapp et al. 2020), but this period was associated with an increase in the relative amount of rain falling during the winter months which is when C3 grasses grow, so they became relatively more common. C4 grasses, especially Andropogoneae, are larger plants often favoured when high temperatures are associated with high rainfall - they are much more "water spenders" - and their dead dried ("cured") leaves that remain at the end of the growing season promote fires. Chloridoideae, on the other hand, tend to be smaller plants with seeds of lower mass, low water conductance and high embolism resistance and they can grow in drier conditions (Lehmann et al. 2019; Knapp et al. 2020; Griffith et al. 2020). It should also be noted that increased biomass produced during the wet season then burns during the dry season, clearing forests and favouring grasslands (Karp et al. 2018). However, although one thinks of C4 grasses as being plants of warmer climates, it has recently been suggested that they are really as good as or better than C3 grasses at colonizing cold climates, they just haven't had time and opportunity (Watcharamongkol et al. 2018: somewhat problematic), so give them time, and they will surely show you...
Different C4 clades respond differently to these factors, with phylogeny (especially linked with structural [stomatal] traits), C4 photosynthetic subtype (linked with physiological traits) and habitat interacting. Thus members of four C4 subfamilies sorted out along gradients of disturbance (grazing), moisture, temperature and fire frequency (Visser et al. 2011). Scheiter et al. (2012) see an interaction between increased temperatures, favouring C4 grasses, relatively low atmospheric CO2, favouring the invasion of C3 grassland by C4 grasses, and fire, allowing the expansion of grassland and also the development of savanna, with its shade intolerant and fire-resistant trees and abundant C4 Andropogoneae (see also Lehmann et al. 2011; Forrestel et al. 2014; Estep et al. 2014). C4 grasses, grasslands and savanna may be favoured in environments with some combination of high temperatures and low CO2 concentrations. Although it has been suggested that the current increasing atmospheric CO2 concentrations may favour the spread of woody vegetation (e.g. Harrison & Prentice 2003; Y. Wen et al. 2023) and could even lead to all C4 grasses becoming extinct by the end of the century (Palazzesi et al. 2022a), this latter forecast depends on the particular aspects of C4 photosynthesis emphasized (Forrestel & Edwards 2019). Bai et al. (2025) studied the effect of increased temperatures over a 10-year period in Chinese temperate desert steppe. There the two C3 grasses studied responded more to increased temperatures than did C4 plants (including one grass), but flowering time length decreased, and overall dominance of the C3 species decreased - addition of N tended to increase these effects, perhaps via its effects on water availability. Monson et al. (2025) show that it is difficult to envision a simple response of grasses with a particular photosynthetic type to future climate changes.
The change of forest to savanna is a major transition and depends on events such as suppressed saplings becoming trees and the timing of successive fires (Hoffmann et al. 2012). Evidence from palaeosols suggests that grasses replaced woodland. Grassland soils are notably moister than corresponding woodland soils, yet somewhat paradoxically grasslands support a drier climate, transpiration being relatively low; woodlands have a lower albedo and transpire more, so their soil is drier (Retallack 2001, 2013a). Declining CO2 concentrations may also have made trees less competitive (Pagani et al. 2009), while at higher temperatures photorespiration becomes more likely, so favouring C4 plants (Christin & Osborne 2014 for a summary). In the Siwaliks of Pakistan fires increased ca 10 Ma, initially leading to the replacement of conifers growing there (retene, derived from abietic acid, a diterpene in conifer resin, decreased) by C3 grasses, or in Nepal by deciduous angiosperms, and only later, around 8-6 Ma, by C4 grassland (Karp et al. 2018; see also Badgley et al. 2008). In East Africa by ca 5 Ma large herbivores had consumed C3 plants, from trees to herbs, and about 4 Ma C4 grasses became much more abundant, perhaps hastening the demise of several species of the afore-mentioned megaherbivores and the animals that preyed on them (Faith et al. 2018; c.f. Peppe et al. 2023: at least some C4 grasses around by 21 Ma), while in the Siwaliks 15/17 lineages that depended on fruit and browse disappeared (Badgley et al. 2008). New species in lineages of mixed feeders and grazers, but not of frugivores and browsers, adopted C4 plants at around 7.5 Ma (Badgley et al. 2008). Note that Faith et al. (2018) emphasize how difficult it is to understand what causes such changes, and in the case of C4 grasses in particular, what caused their continued spread even when (one might have thought) conditions became conducive to the spread of trees. Shifts between ecosystems like grassland and woodland may have been catastrophic (see Scheffer et al. 2001).
Once established, the dense - and sometimes quite deep - root masses of grasses make the invasion of grassland by woody vegetation difficult (D'Antonio & Vitousek 1992), these dense roots not allowing seedlings of woody plants to establish, even if there is otherwise enough water (Wakeling et al. 2015); the seedling/young plant stage is critical (Bond & Midgley 2000). Increasing temperature, open habitats, and perhaps especially decreasing precipitation all increase water stress, but many, but by no means all, C4 grasses are drought tolerant (e.g. Edwards & Still 2007, esp. 2008; Edwards et al. 2007; Edwards 2009; Pau et al. 2013; S. H. Taylor et al. 2014). K. Yu and D'Odorico (2015) noted that woody plants can obtain water from deeper layers of the soil and during the night, and some water returns to shallow layers of the soil (the phenomenon of hydraulic lift) where it is scavenged by the shallower-rooting grasses (see also Holdo & Nippert 2015 for the rooting pattern: 40 vs 5 cm); overall, savannas are favoured in marginal conditions that might otherwise support woodlands. Indeed, trees like the African Entandrophragma cylindricum (Meliaceae) have roots descending to 6 m or more (perhaps to 10 m) depending on the rock/soil type (Freycon et al. 2015 and references). Overall, C4 savanna grasses shower higher water uptake, more transpiration and higher biomass production irrespective of water availability, at least in shallower soils (Belovich et al. 2023). Furthermore, it was found in North American tallgrass prairies when moisture was high that at least some C4 grasses showed pronounced nocturnal transpiration (other herbs and shrubs were compared), although the significance of this is unclear (O'Keefe & Nippert 2018). There is variation in N cycling depending on the dominant tree in the savanna, thus Vachellia and Senegalia (Fabaceae-Caesalpinioideae) fix N, Eucalyptus (Myrtaceae) tends to be ectomycorrhizal.
Savannas in different parts of the world may look very different, depending on the architecture of the dominant trees. The distinctive look of African savannas is because they are dominated by Vachellia and Senegalia which have rather low, spreading crowns, while Australian savanna is dominated by Eucalyptus s.l., taller trees and with narrower crowns (Moncrieff et al. 2014).
A number of grasses grow in more or less freshwater aquatic habitats, and also in salt marshes and sand dunes by the sea; Soreng et al. (2021) discuss the evolution of tolerance to saline and alkaline conditions in Poeae-Coelanthinae, a group where such proclivities were notably common. Rabei et al. (2021) compared the growth rates of some C3 and C4 grasses in aquatic and terrestrial habitats. They found that C4 grasses had a higher photosynthetic efficiency than the C3 species and that, somewhat surprisingly, this increased in the aquatic environment.
Some vegetation simulations show circum-Arctic grasslands early in the Caenozoic (Shellito & Sloan 2006), although this is unlikely. Palaeosol and other evidence suggests that grassland grasses began diversifying in the Eocene (e.g. Bouchenak-Khelladi & Hodkinson 2011), the grasses involved being mostly caespitose bunch grasses (Retallack 2013a). Open-habitat grasses, probably C3, appear in the Middle Eocene ca 42 Ma, and may have become locally dominant (Strömberg 2011; see also Soreng et al. 2021); they diversified taxonomically in North America in the early Oligocene ca 34 Ma (Strömberg 2005). Evidence from palaeosols suggest that there may have been grasslands in the Great Plains in the late Oligocene ca 24 Ma, and some Argentinian grasslands may be older, Eocene in age (Retallack 1997b; E. J. Edwards et al. 2010). High diversification rates in both Asteraceae and grasses, in terms of species numbers both major components of grasslands although the former not ecologically so important, have been dated to 20-15 Ma, and again 13-10 Ma, or under some scenarios for grasses, 35-30 Ma ago (Palazzesi et al. 2022a). From the examination of phytolith assemblages, grass-dominated open habitats in Patagonia did not develop before ca 18.5 Ma, and again it was open-habitat C3 poöid grasses that were prominent (Strömberg et al. 2011). Of the ruminants, basal bovids were mixed (grazing + browsing) feeders and their crown-group age is mid-Oligocene, basal cervids were browsers, but in both mixed feeding ultimately predominated, with shifts both to grazing (some before the C4 grassland expansion) and to browsing (Cantalapiedra et al. 2014). Horses began diversifying in North America somewhat before 20 Ma, genera like Parahippus eating mostly grasses, so, rather than equid radiation reflecting changing diet and habitat, the early Miocene radiation of equid tribes, particularly before 15 Ma, probably took place without there being rapid accompanying ecomorphological shifts (Cantalapiedra et al. 2017 and references).
C4 grasses may have first appeared in the Oligocene ca 33 Ma, and C4 photosynthesis is known from grasses from the Early to Middle Miocene in both the Great Plains and Africa, some 25-12.5 Ma (e.g. Ehleringer 1997 and references; Christin et al. 2008a, 2011b; Peppe et al. 2023). Fossils in ca 14 Ma Mid-Miocene deposits from Kenya have been interpreted as evidence for everything from C4 savanna to moist highland forest, although isotope analyses of tooth enamel of the animals in these deposits provided no evidence for the existence of C4 grasses (Cerling et al. 1997a; c.f. Peppe et al. 2023). Indeed, there are suggestions that the initial expansion of grasslands/savannas in Africa, at least, was the result of interactions between medium-sized social herbivores, bovids that were mixed grazers and browsers, and trees and grasses that began perhaps 20 Ma, with woody plants developing thorns/spines, now characteristic of woody plants in open environments, as a protection against these animals ca 18 Ma, bovid diversification beginning a little later, ca 16 Ma (Charles-Dominique et al. 2016; see Wardle et al. 2002 for the interaction between nutrients, rate of litter decomposition, damage to vegetation by browsers, etc.). The animals grazed nutrient-rich savannas during the wet season, but these dried out in the dry season and the animals then browsed woody vegetation. Currently wet savannas on nutrient-poor soils are seasonally humid, and the growth of grasses for part of the year generates the litter that burns during the dry season (see also Maurin et al. 2014).
Grasslands, both C3- and C4-dominated, spread in North America during the mid-Miocene Climatic Optimum of 17-14.5 Ma (E. B. Harris et al. 2014) - MAT then was ca 3o warmer, CO2, at 900-1100 p.p.m., quite high, but temperatures in the late Miocene decreased (Arakaki et al. 2011). Fire activity associated with C4-type savannas is dated rather later to ca 11 Ma and the evolution of fire-resistant "underground trees" is still later, beginning ca 5.3 Ma (Charles-Dominique et al. 2016). Indeed, C4 grasses seem to have first made a major contribution to overall vegetation biomass only in the late Miocene 9-8 Ma, C4 grassland becoming widespread only as recently as the late Pliocene 3-2 Ma (Bouchenak-Khelladi et al. 2009, 2014a; E. J. Edwards et al. 2010; Strömberg & McInerney 2011; McInerney et al. 2011 for North America; Strömberg et al. 2011: South America; Arakaki et al. 2011; R. Sage et al. 2012). C4 grasses became common in NW Australia ca 3.5 Ma as atmospheric circulation patterns changed with the development of the East Asian winter monsoon (Andrae et al 2018).
Overall, there is a pronounced lag at both global and local scales between the initial evolution and diversification of C4 grasses and their ecological expansion (e.g. Strömberg & McInerney 2011) - a lag of over 20 Ma. Interestingly, allopolyploidy has been extremely common in C4 Andropogoneae, and many allopolyploid events date to the period of grassland expansion, even if subsequent diversification of the newly polyploid clades has been nothing to write home about (Estep et al. 2014).
The amount and persistence of litter in grasslands may be another important factor in their success. Grasslands, perhaps especially those dominated by Andropogoneae, accumulate litter very easily, and there is also a negative correlation between silicon concentration - especially high in annual grasses - and rate of leaf decomposition (Cook & Leishman 2011b). The relatively low N content in grass litter, especially that of C4 grasses, also means that it decomposes slowly and accumulates (Knapp & Seastedt 1986; D'Antonio & Vitousek 1992;; Wedin 1995; Pérez-Harguindeguy et al. 2000: Bromeliaceae could be similar; Wardle et al. 2002; Cornelissen et al. 2001: decomposition fast; Chapin & Körner 1995: comparison with mosses). Leaves of poöid monocots in general decompose more slowly than do those of core eudicot-type angiosperms - sedges may also be included in the slow-decomposers (Cornwell et al. 2008; LeRoy et al. 2019: Ranunculales not included). Grasslands are particularly flammable because of this litter accumulation (Scheiter et al. 2012; Sage et al. 2012), and charcoal from fires has become abundant since the Late Miocene about 10 Ma, the time during which grasslands have spread (e.g. Bond & Midgley 2000; Keeley & Rundel 2005; Bond & Scott 2010; Bond 2015). In monsoon-like climates biomass produced during the growing season later dries out and burns, and this is consistent with the record of fossil charcoal (Keeley & Rundel 2005: check). Thus the high flammability of dry grasses, disturbance by grazers, and windiness are among the factors, more or less related, that would lead to the increase of fires and further spread of grasslands (Retallack 2001; Woodward et al. 2004; Bond & Scott 2010). Since grasses have their perennating parts underground, fire-adapted species recover quite quickly and with little harm from fires (Linder et al. 2017b and references: see also below). Grasses resprouting after fires are generally perennials that have buds more or less underground, as in rhizomatous grasses, although if fires become frequent it is seeders, often annuals, that are favoured (Simpson et al. 2020). Grassland fires are generally of the "fast flammable" type, i.e. the fires are of low intensity and do not last long, and as a result they cause only moderate damage to living plants (Pausas et al. 2017; Simpson et al. 2020). N of course tends to be volatilized in fires, and interestingly, seeders, which tend to be found in areas with more frequent fires, tend to have higher leaf N and a lower C:N ratio than resprouters, the former growing faster, the latter perhaps showing high N use efficiency, at the same time litter accumulates so increasing their flammability (Simpson et al. 2020). In Madagascar, at least, now extinct meagafaunal grazers may have encouraged mat-forming grasses with wider leaves, etc., resprouters, while grasslands maintained by fire favour tall, caespitose species with narrow leaves that are altogether more fluffy, that is, they have low bulk density (Solofondranohatra et al. 2020). The geoxylic shrubs and herbs that make up the "underground forests" in the Kalahari region of Africa are similarly protected (waterlogging and low nutrients may be of importance here - White 1976), and there are parallel adaptations in the plants of South American savannas (Bond 2016a); burning suppresses woodland by killing fire-susceptible trees, again, N is volatilized and lost (e.g. Knapp & Seastedt 1986; Lehmann et al. 2019; Tierney et al. 2019; Simpson et al. 2020). Both would favour grasses: The habitat was opened, and C4 grasses in particular have a reduced requirement for photosynthetic enzymes because C4 is more efficent than C3photosynthesis and so less N is expended in the synthesis of enzymes and so a lower N requirement - less N is expended in the synthesis of enzymes (Wedin 1995; Forrestel et al. 2014). The productivity of tall-grass prairies is negatively affected by the accumulation of litter, but fires improve productivity (Knapp & Seastedt 1986). Interestingly, C4 plants tend to be less attractive to herbivorous animals because of the greater amount of fibrous tissue in their leaves - they have more sclerenchyma because their veins are closer, characteristic of Kranz anatomy (Caswell et al. 1973; Schoonhoven et al. 2005 for references), and they have a lower N concentration. Thus in the "sour" grasslands of South Africa the dominating C4 Arundinelleae and Andropogoneae contain tannin-like substances that affected N cycling in the dystrophic soils in which they grew. As mentioned, when the grass burns volatilized N will be lost to the ecosystem, the soils becoming poorer. However, although panicoid grasses recover well after fires, C4 grasses do not always perform better in this respect than C3 grasses (Ripley et al. 2011; Forrestel et al. 2014). Forrestel et al. (2014) specifically noted that the reponse of Andropogoneae to fire was overall independent of whether or not there was grazing, although that might not be true for individual species. The local diversity of grasses is also affected by the particular fire regime, although not in any simple way (Forrestel et al. 2014). Indeed, different clades of grasses respond to burning differently, and this was connected with the amount of living tissue lost to fire rather than photosynthetic type, recovery after fire being quicker in C4 Andropogoneae and C3 Paniceae than in C4 Aristida and C3 Danthonioideae (Ripley et al. 2015). Similarly, the flammability of grasses from the Eastern Cape, South Africa, was found to vary quite substantially at the species level (there was also a phylogenetic component to the variation), and was higher than that of woody vegetation; high flammability coupled with the ability to resprout might be an adaptation for life in frequently-burned habitats (Simpson et al. 2016).
Bond et al. (2005) estimated that if there were no fires, about 52% of C4 grassland and 41% of C3 grassland would revert to forest; of the latter, over half would be dominated by gymnosperms. A further wrinkle is that fires may have increased over the last 50,000 years because of climate changes and/or the widespread extinction of megaherbivores by humans (Lorenzen et al. 2011; Gill 2013; Karp et al. 2021). The extensive Brazilian Cerrado and African savanna vegetation with abund+ant flammable C4 grasses and woody plants that have become adapted to a fire regime have also developed only within the last (10-)5 Ma, and they replaced woodland (Pennington et al. 2006b; Simon et al. 2009; Simon & Pennington 2012; Hoffmann et al. 2012; Maurin et al. 2014; Pennington & Hughes 2014). In both Cerrado and savanna there has been widespread evolution of geoxylic suffrutices, "underground trees" with massive subterranean fire-protected perennating woody axes (White 1976), that flourish under a regime of frequent fires and moderate rainfall. In the Cerrado, clades including 50 or so fire-resistant species evolved, although comparable clades in Africa are smaller (Pennington & Hughes 2014). By and large, however, any phylogenetic signal in the woody plants adapted to environments in the South American Cerrado vegetation and the underground forests of Africa is small (Simon & Pennington 2012).
McInerney et al. (2011) suggested that in North America, at least, the late Neogene expansion of C4 grasses was at the expense of C3 grasses rather than of woody vegetation. With decreasing temperatures the survival of tree seedlings in the forest-grassland transition increased (Will et al. 2013).
Grasslands and savannas remain dynamic entities, and grasslands may be very sensitive to changing climates, some reconstructions showing the current increase in atmospheric CO2 being accompanied by quite extensive spread of C3 grasslands (e.g. Collatz et al. 1998; Hall et al. 2000; Knapp & Smith 2001). (Long term experiments may seem to tell a different story, as Reich et al. 2018a suggest when examining the biomass enhancement in C3 and C4 grasses over the course of an experiment that lasted 20 years - but c.f. Wolf & Ziska 2018; Reich et al. 2018b; Nie et al. 2018; Reich et al. 2018c.) More generally, simulations suggest that with increasing atmospheric CO2 concentrations the possibility for the coexistence of several stable biomes within Africa, at least, will be lost (Moncrieff et al. 2103). Seedlings of forest trees show decreased survivorship as temperatures rise (Will et al. 2013). How increasing temperature affects above-ground woody biomass in savannas also depends on the continent, thus projections suggest that above-ground woody biomass will tend to increase in Africa and decrease in Australia and South America (Lehmann et al. 2014), and dominance relationships brtween grasslands herbs may also change as climate changes (Yu et al. 2015). Changing rainfall patterns will also affect the balance between savanna and grassland. Thus grasses are more susceptible to water stress than savanna trees when conditions are dry, but when conditions are wetter they have a much higher relative growth rate, so at least initially they will tend to spread at the expense of trees (Xu et al. 2015).
The total carbon sequestration of grasslands is greater than that of the forests that they in many cases seem to have replaced, with a shift in the sequestration pattern from above-ground parts to the soil (McGuire et al. 1992; Retallack 2001), although of course large amounts of CO2 can be produced by grassland fires (see Archibald & Hempson 2016: see also next section). Some Oligocene palaeosols approach C-rich mollisols, a soil type known only from the Caenozoic and uniquely associated with grasslands (Retallack 1997b). Short sod grassland (the grasses are mostly rhizomatous) with shallow soils may have appeared in the early Miocene ca 20 Ma in relatively warmer and wetter (>400 mm/yconditions (Retallack 2001, 2013a). Tall sod grasslands made up mostly of C4 grasses and with deeper soils appeared in the late Miocene ca 7-5 Ma in areas with up to 750 mm annual precipitation, and it was these grasslands that had true mollisols. In their fullest development, mollisols are dark and deep (the carbon-rich layer may be 1 m or so); carbon is mixed with rounded clods of clay 2-3 mm across, they are nutrient-rich, with carbonate and easily-weathered minerals, and are densely permeated by grass roots (Retallack 1997b). Grassland soils, being relatively moist (see above), will support increased rock weathering (Retallack 2001, 2013a).
3. Grasses, Grasslands, Grazing and the Silicon Cycle.
It has been suggested that C4 grasses in particular, grasslands in general, and grazing are connected (e.g. Retallack 2013a; Katz 2015). Thus Bouchenak-Khelladi and Hodkinson (2011) thought that there were "adaptive co-evolutionary processes" (unspecified) going on between grass and grazer, and Bouchenak-Khelladi et al. (2009) had earlier noted that the density of silica bodies in the leaf epidermis seems to have increased in a number of grass groups, perhaps as a defence against herbivory (but c.f. Strömberg et al. 2016). In Madagascar now-extinct meagafaunal grazers (?tortoises, ?elephant birds) may have encouraged resprouting, mat-forming grasses with wider leaves, etc., while in grasslands maintained by fire it is tall, caespitose species with narrow leaves that are common (Solofondranohatra et al. 2020; Lehmann et al. 2021; c.f. Crowley et al. 2020: mosaic landscape, biome limits fluctuating). Archibald and Hempson (2016: Table 1) compare the particular grassland conditions under which fires and grazing are favoured, also noting i.a. the larger amounts of methane and lower amounts of CO2 produced by the latter activity. Different kinds of grasslands may be more or less subject to fires and/or grazing, and the two may interact, for example, after fires there may be flushes of young vegetation favoured by grazers, however, grazing animals can move from patch to patch of grassland in a way that fires generally cannot (Archibald & Hempson 2016). Sometimes the result of the activities of the animals are grazing meadows, areas where the vegetation is very short because of the attentions of the grazers, but the plants there actively regenerate and produce dense short vegetation, which attracts the attention of the grazers... (Launchbaugh 2020). Grazing of grasses by mammals may lead to an increase in the light reaching the understory of the grasslands, and this may result in an increase in the diversity of such grasslands (Eskelinen et al. 2022). (Interestingly, marine green turtles eat seagrasses with similar results, although their grazing meadows are not really grasses, and they may last for years, rather than months - Gulick et al. 2022!)
Diversification of grazing mammals began in the Oligocene ca 35 Ma and there was a Miocene radiation of grazing mammals (Thomasson & Voorhies 1990; MacFadden 1997; Retallack 2001; Keeley & Rundel 2003) that has been linked to the spread of prairie and savanna grasses (see also Cerling et al. 1997a, b; Bouchenak-Khelladi et al. 2009, 2010a: considerable detail and many dates; Mihlbachler et al. 2011). Macropodinae (kangaroos, etc.), especially Macropodini, in Australia radiated ca 5 Ma and later in response to mid-Pliocene C4 grassland expansion (ca 3.5 Ma, Andrae et al. 2018), and there was an increase in the crown height of their teeth at the Miocene-Pliocene transition; they may have eaten dicot leaves as well (Couzens & Prideaux 2018). Crown hight change seems not to be a simple response to aridity, interestingly, the browsing Sthenurinae also radiated somewhat (Couzens & Prideaux 2018). Most extant grazers eat C4 plants, but C3 grazers, now uncommon, were found in a diversity of ecosystems before 7 Ma (e.g. MacFadden 1997: see below). There was also a Miocene radiation of scarab beetles into the new habitat of the plentiful dung left in large part by these grazers (Ahrens et al. 2014). However, Bouchenak-Khelladi and Hodkinson (2011) observed that hypsodonty (the teeth have high crowns, enamel extends below the gum lines, and the roots are short) had been gradually increasing for 20 Ma and the spread of grasslands was not contemporaneous with this increase; silica-containing grasses evolved well before the mammals that ate them, so here there would seem not to be any simple co-evolution (Strömberg et al. 2016; see also Kergoat et al. 2018 for conflicts in this area). The dead leaves of most grasses persist and this may also decrease their palatability to grazers - but of course cows, etc., have been known to eat hay; the blades of danthonioid grasses in New Zealand abscise, apparently reflecting the lower herbivore pressure there (Antonelli et al. 2010).
Grazing mammals in different parts of the world independently evolved hypsodont or hypselodont (the teeth are ever-growing) dentition, and both kinds of teeth can apparently deal with the wear caused by eating abrasive grasses with their complex silica bodies (phytoliths). Tooth enamel is indeed harder than the silica in grasses (Sanson et al. 2007), but surprisingly little is known about the mechanics of tooth action (Sanson 2006). Sanson and Heraud (2010) suggested that the silica in silica bodies might not be in crystalline form and so would not cause wear on the enamel of mammalian teeth (but c.f. references in Hartley & DeGabriel 2016). Indeed, dust particles, likely to be more abundant in food eaten by a grazer than in that by a browser, may be the most abrasive element in the food ingested (e.g. Kay and Covert 1983). Hypsodont and hypselodont teeth then evolved to deal with silica in the dust covering the foliage in environments that were becoming drier and more open, hypselodont lagomorphs in particular cropping their food very close to the ground; this is the "grit not grass" hypothesis (Jardine et al. 2012). In a comprehensive review, it was suggested that incidental consumption of sand or soil, not phytoliths or grasses per se, may be the most important element in the development of hypsodonty (Damuth & Janis 2011, see also 2014; Kaiser et al. 2016: very fine particles and tooth wear; Lopresti et al. 2017). In southern South America the evolution of hypsodonty may be associated with the presence of abrasive volcanic ash from eruptions in the Andes (Pascual & Ortiz Jaureguizer 1990; see also Palazzesi & Barreda 2012), However, a long-term study involving elderly French ewes and different kinds of forage, including forage to which realistic amounts of dust had been added, suggested that most of the wear on the teeth was caused by the forage, not the dust (Merceron et al. 2016). As a final complication, note that the silica content of forest and wetland Bambusoideae and Oryzoideae is substantially higher than that of open habitat grasses, and that large mammals seem unable to differentiate between silica-rich and -poor grasses (Strömberg et al. 2016).
Prairie grasses expanded in Nebraska in the Early Miocene ca 23 Ma, moderately hypsodont ungulates and rodents were already around by then, but some evolved later, while the major radiation of horses in North America was some 18-15 Ma (MacFadden et al. 1996; Strömberg 2004, 2006; Mihlbacher et al. 2011; Jardine et al. 2012); hypselodont lagomorphs (hares, etc.) date from the Eocene-Oligocene boundary around 34 Ma (Jardine et al. 2012). Major diversification of North American ungulates is largely a Miocene phenomenon, Bovidae and Cervidae starting to diversify at least 26 Ma (Bouchenak-Khelladi et al. 2009), and herbivores that are now specialists on C4 grasses seem to have evolved before those grasses came to dominate ecosystems (E. J. Edwards et al. 2010). Grazers in South America appeared earlier than in North America, ca 50 Ma, and were "pervasive" there by the Oligocene ca 35 Ma (MacFadden 1997, esp. p. 185), probably eating C3 plants. In Patagonia hypsodont teeth became common in the now-extinct notoungulates in the late Eocene-Oligocene 39-21 Ma, a time before grasses were a major component of the vegetation (Strömberg et al. 2013b; Dunn et al. 2015). Indeed, the pollen record suggests that the vegetation in southern South America around 20 Ma was dominated by Podocarpus and Nothofagus, and it was not until around 10.5 Ma that more open vegetation with Atriplex, Asteraceae, C4 grasses, etc., appeared (Palozzesi & Barreda 2012). In southern South America hypsodonty can perhaps be linked to the evolution of C3 grasses, and there are suggestions that equid diversity declined as C4 grasses became more common (Cerling et val. 1998; Passey et al. 2002; Keeley & Rundel 2003). Finally, the teeth of mammals in the widespread savannas that were thought to extend from Greece to Inner Mongolia to Kenya, the Pikermian palaeobiome of 12-5 Ma, suggest a grazing diet. The vegetation in a substantial part of this area was at least initially temperate broadleaf and mixed and evergreen needleleaf forests, questioning any simple connection between teeth and diet, rather, the Pikermian chronofauna inhabited a variety of biomes (Denk et al. 2018; c.f. Fortelius et al. 2019). The recent description of local C4 communities in East Africa as early as ca 21 Ma may necessitate some recalibration of mammalian evolution (Peppe et al. 2023). Around 2.37 Ma there was a shift in diet in the hominim Paranthropus aethiopicus in the Omo Valley, Ethiopia, from mostly C3 to mostly C4 foods, as also happened in other herbivores around 2.69-2.02 Ma, but only sometimes (and not in P. aethiopicus) did the teeth suggest that there had been such a shift (Wynn et al. 2020).
The relationship between C4 and other grasses, herbivory, silica, and the protection of plant tissues is by no means straightforward and it differs in different parts of the world (Kelley & Rundel 2003). The simple formula, high SiO2 in grasses = hypsodonty, may not fully capture the interconnections between the rise to dominance of grasses in some ecosystems and the evolution of grazing animals. In the more distant past hypsodonty has evolved in yet other ecological contexts, e.g. in Triassic non-mammaliform stem-mammals (Meio et al. 2019). And what exactly Si does in grasses is unclear, although it is perhaps involved in some sort of of trade-off between growth and defence (de Tombeur et al. 2022).
Largish mammals are not the only animals that eat grass, and silica bodies do affect the feeding of at least some smaller herbivores, both mammals and insects (for rabbits, see Cotterill et al. 2007; for caterpillars, aphids, etc., see S. N. Johnson et al. 2021). In Poaceae there is a positive correlation between the annual habit and high silicon concentration (Cooke & Leishman 2011b), and more silica in grasses decreases the amount of N taken up by both armyworm (Spodoptera exempta) larvae and voles (Microtus), and in the former in particular there are long-term negative effects on the growth of the caterpillar, perhaps because of damage to its midgut (Johnson et al. 2021). The chitin of caterpillar mandibles is not as hard as enamel, and the mandibles of armyworm larvae are worn down by silica; grass tissues produced after attack by a herbivore have increased silica and are less attractive to animals, interestingly, this does not happen after comparable purely mechanical damage (see Schoonhoven et al. 2005; Massey & Hartley 2006, 2009; Massey et al. 2007b; Cook & Leishman 2011; Katz et al. 2014; Lopresti et al. 2017). Kergoat et al. (2018) followed diversification in C4 Paniceae and Noctuidae-Sesamiinae stem-borer moths, the latter with some 200 species that eat such grasses and largely live in Africa. Although about the same age, 25.4-21.0 and 20.7 Ma respectively being the estimates of medians, with the cooling and drying climate of the late Miocene the diversification of Paniceae increased and that of the moths decreased; with drier climates the grasses became smaller and had higher concentrations of silicon, neither conducive to the growth of these moths (Kergoat et al. 2018).
Of course, silica is not the only defence that grasses have against grazing (Massey et al. 2007a), for instance, they vary in toughness (C4 grasses are notably tough) and may contain noxious metabolites, sometimes because of their association with endophytes (see below). Interestingly, C4 plants tend to be less attractive to herbivorous animals because of the greater amount of fibrous tissue in their leaves (Caswell et al. 1973; Schoonhoven et al. 2005 for references), and they they have a lower N concentration because C4 is more efficient than C3photosynthesis and so less N is expended in the synthesis of enzymes. Although Taylor et al. (2010) suggested that the N content of C3 and C4 grasses was similar, in forage science C4 grasses are known as "sour" grasses (R. Sage 2016), while "sweet" C3 grasses, on the other hand, do not have Kranz anatomy with its associated fibrous tissue (Sage 2016). In any event, grassland s.l. grasses are hemicryptophytes, and they readily recover after being grazed or burned (Linder et al. 2017b).
There is a final and rather different ecological dimension to silica and grazing. Grasses contain up to 7% silica and it becomes mobilized during digestion, more particularly in ruminants because grasses stay in their guts for a long time. The result is there is a massive mobilization of silica in grazed grasslands. The spread of grasses in the Miocene and the increased activity of herbivores may have increased the flux of this mobilized silica into rivers, so sparking the Early Miocene increase of diatomite (diatomaceous earth, kieselguhr) deposits and diatom diversity in fresh waters; the cell walls (frustules) of diatoms are made up of silica (Kidder & Gierlowski-Kordesch 2005; Vandevenne et al. 2013). Marine diatom diversity peaked at the Eocene/Oligocene boundary well before the spread of grasslands but at the time when the Himalayan orogeny was beginning, and peaked again in the later Miocene onwards (Rabosky & Sorhannus 2009; Cermeño et al. 2015). The mobilization of silica, in part associated with the spread of grasslands in the later Miocene (in the sea Si is available as orthosilicic acid - H4SiO4), contributed to this later peak in diatom diversity; interestingly, the thickness of radiolarian tests decreased over this whole period, in part because of competition with diatoms for Si (Cermeño et al. 2015). The calcite compensation depth (the depth below which calcite dissolves) considerably increased in post-Eocene times, with calcium carbonate nanofossil ooze replacing siliceous radiolarian ooze (Coxall et al. 2005; Tripati et al. 2005) in connection with the developing ice caps.
It will have become clear that the effect of grazing on grasses/grasslands has changed over time. The presence of megaherbivores in grasslands in general leads to an increase in diversity of the native plants in these grasslands, no matter whether the megaherbivores are native or introduced (Lundgren et al. 2024). The recent extinction of many megaherbivores leads both to a decrease in grazing/diversity and to an associated increase in fires (see elsewhere), and the increase in the numbers of humans, leading directly to the conversion of grasslands to other uses and indirectly to the increase in cattle and sheep populations, has had a major effect on grasslands. There are some parallels here with seagrass communities, although seagrasses are not Poaceae; for further discussion see elsewhere.
4. Grasses, especially Poöideae, and Endophytes.
The endophyte-grass relationship exemplified by the Poöideae-Neotyphodium/Epichloë (ascomycetes, Clavicipitaceae-Balansieae) association is usually described as a mutualism, although this may sometimes, at least, not be so (see Saikkonen et al. 1998; Gundel et al. 2006, 2016; Ren & Clay 2009). Some 30 species of Epichloë have been described, most asexual and restricted to a particular grass genus or group of closely-related genera (Card et al. 2024). Some of these endophytic fungi synthesize a diversity of secondary metabolites (Spatafora et al. 2007), Epichloë and Claviceps in particular both synthesising a variety of "grass" alkaloids including indole diterpenes, lolines (1-aminopyrrolizidines), peramine, and the ergot (ergoline) alkaloids (Schiff 2006; Fleetwood et al. 2007; Schardl et al. 2007, 2012, 2013; Simpson et al. 2014; Píchová et al. 2018). Lolines are primarily active against insects whether as toxins or feeding deterrents, ergot alkaloids are toxic to mammals, and so on (Schardl et al. 2007; D.-X. Zhang et al. 2009; Simpson et al. 2014; Realini et al. 2024). The alkaloid loci in Epichloë include numerous large complex and unstable blocks of repeats of various origins, perhaps facilitating alkaloid diversification (Schardl et al. 2013). Interestingly, the gene makes caterpillars floppy-like expressed in Epichloë is a bacterial insect toxin gene that moved to the ancestor of Epichloë via horizontal gene transfer (HGT: Ambrose et al. 2014); for more on HGT see Pereira et al. (2022) and elsewhere. The asexual stage of the fungus (Neotyphodium) may increase the competitive ability of the host grass under stressful conditions (Saari & Faeth 2012; see also Dupont et al. 2015), although haploid Epichloë severely reduces sexual reproduction in the grass, causing choke disease (Oberhofer et al. 2013: greenhouse experiments; Realini et al. 2024). Endophytes improve the resistance of Lolium perenne and Bromus laevipes, for example, to the effects of water stress (Hahn et al. 2007; Afkhami et al. 2014). In the latter case the habitable range was increased, but when conditions were more mesic, the plant was less likely to have the endophyte (Afkhami et al. 2014). Similarly, Poa leptocoma, with endophytes, grew in wetter soil (and was also more colonized by endomycorrhizae) than did P. reflexa, lacking endophytes (Kazenel et al. 2015). Protection of the plant may be indirect, thus recent work suggests that a gene that confers resistance to Fusarium head blight in wheat moved from Epichloë, where it is found in a number of species, to Thinopyrum ca 5 Ma (H. Wang et al. 2020).
Indeed, the more that is found out about such relationships between grass and fungus, the more complex they appear to be. For instance, the presence of endophytes affects both the palatability of grasses for herbivores and of their seeds for granivorous birds (Madej & Clay 1991), animals eating endophyte-infected material sometimes not thriving at all. However, in the absence of herbivory the grass genotype may be more important in determining the outcome of of intraspecific competition than presence of an endophyte (Cheplick et al. 2014). Endophytes that are insect pathogens may also be antagonistic to plant pathogens (Vega et al. 2009 and references). Fungal endophytes may also affect root growth and root hair production (Sasan & Bidochka 2012). In Elymus virginicus the presence of Epichloë resulted in the plant diverting more of its resouces to producing seeds, through which the fungus is transmitted, and less to producing pollen (Gorischek et al. 2013); it is unclear if this was to the benefit of the plant. Epichloë can suppress colonization of the plant by arbuscular mycorrhizal fungi, and although direct effects on the endophyte community in Lolium perenne were unclear, those due to season and locality were evident (König et al. 2018) Epichloë may also protect against nutrient deficiencies (Card et al. 2024). Clay and Holah (1999) found that endophyte-infected Festuca arundinacea suppressed diversity when compared with endophyte-free plants, even although overall plot productivity/plant biomass was unaffected; Marks and Clay (2007) discuss the growth rates of endophyte-infected and -free plants under various conditions. Ammophila breviligulata is described as an ecosystem engineer, and there association with Epichloë is linked with a reduced incidence of non-epichloid fungi in the seeds (?protection against pathogens), fewer inflorescences produced, increased seed viability, overall reduction in plant fecundity, increased root production, a reduction in root-associated bacteria and arbuscular mycorrhizal fungi, and so on; the outcome of such interactions can be affected by rainfall, and so on (Emery et al. 2017; Bell-Dereske et al. 2017; David et al. 2019). For the effect that Claviceps may have on its hosts, see Píchová et al. (2018), overall, how much of what pathogen is produced depends on the species of grass and the part of that grass being examined (Realini et al. 2024). Some of these endophyte-mediated effects are being used in the development of improved strains of forage grasses (Gundel et al. 2013; esp. Simpson et al. 2014 and Card et al. 2024).
Márquez et al. (2007) noted that only when the fungus Curvularia protuberata (Pleosporaceae), endophytic in Dicanthelium lanuginosum, was infected with a double-stranded RNA virus was the grass able to grow in volcanically-heated soils in Yellowstone at ca 65o C, suggesting the complexity of such relationships. Franken (2012: Piriformospora [= Serendipita]) and Weiß et al. (2016) review the various effects that endophytic Sebacinales-Serendipitaceae may have on Poaceae. Ghimire and Craven (2011 and references) looked at how Serendipita enhanced germination, increased biomass production, etc., in switchgrass, Panicum virgatum. In general, endophyte-grass associations appear to be less common in areas with Mediterranean climates, being found in less than 10% of grasses in California, for example (Afkhami 2012). See also below for mycorrhizae and roots, also, Convolvulaceae-Ipomoea and Fabaceae-Faboideae for production of alkaloids by fungi associated with the plant.
The level of aphid infestations and that of their parasites and parasitoids is also affected by such endophytes (Omacini et al. 2001), as is the infestation of the plant by nematodes, insect herbivory (Tanaka et al. 2005), and even the pattern and rate of decomposition of dead grass (Lemons et al. 2005; see also Popay & Rowan 1994; Schardl 2010). Indeed, in Epichloë symbioses, the jasmonic acid pathway, involved in defence against microorganisms that kill plant tissue and then getting nutrients from the dead tissue, and also defence against chewing insects, is upregulated. Anatagonism between this pathway and the salicylic acid pathway causes the down-regulation of the latter, perhaps so making the plant more vulnerable to attacks by microorganisms that utilize living tissues and by sap-sucking insects (Bastias et al. 2017; see also Harley & Grange 2008; Thaler et al. 2012). From this point of view, alkaloids, etc., produced by the endophyte may provide yet more defence against chewing insects (Bastias et al. 2017). There may also be interactions with larger herbivores. Thus the saliva of large grazers like moose may reduce endophyte growth and the production of toxic endophyte alkaloids (Tanentzap et al. 2014), but this depended on the provenance of the plant (Festuca rubra), and how it might affect herbivory is unclear given that the growth reduction of the fungus took some time. Population crashes of Soay sheeep feeding on Festuca rubra have been studied in detail; endophytes peaked at about the time of the crash, and although alkaloid concentrations seemed to increase in response to defoliation, the amount of defoliation seemed to have little effect (Vicari et al. 2018).
Of course, why any individual grass has endophytes is a separable question. Thus the composition of the foliar endophyte community in Panicum virgatum was found to depend on the local environment rather than the switchgrass genotype, although a number of endophytes were to be found in the leaves of the grass throughout its range (Whitaker et al. 2018).
Some root-associated endophytic fungi (class 4) are also coprophilic (Herrera et al. 2009), perhaps aiding in their dispersal in prairie conditions where dung from larger vertebrates can be quite prominent. In some grasses defence against herbivores is mediated by the production of volatile mono- and sesquiterpenes which can, for example, attract nematodes, to attack Diabrotica (the chrysomelid corn root-worm) larvae, or parasitic wasps to attack caterpillars (Degenhardt 2009).
Other aspects of the eco-physiology of Poaceae to be taken into account:
Woody bamboos, some 1,300 species, can grow to 30 m tall or more, and may live for 100 years before flowering; palms and bamboos are the two major woody monocot clades. Given that there is no secondary thickening in bamboos, how the vascular tissue of such plants remains functional is unclear. However, Cao et al. (2012 and references) found that in the bamboos they studied root pressure was sufficient to drive water up the entire height of the plant; root pressure and plant height were strongly correlated. This would help in the repair of embolisms in the xylem, although Knipfer et al. (2016) found that living tissues control embolism formation/removal in grapes, at least.
Evans and Ortega (2019) provide some information about xylem conductivity in a small sample of C3 and C4 grasses, the former tending to have wider vessels in their culm bundles but the latter had almost double the number of bundles, and foliar bundles were also more numerous in the latter (see also above). The overall result was that the xylem conductivies of the culms and especially the leaves were higher in the C4 than the C3 grasses examined, differences that Evans and Ortega (2019) attributed largely to the differences in the numbers of bundles, not diameters or numbers of vessels.
In the poöid Lolium perenne, leaf hydraulic conductance may decrease during the day, cavitation presumably occurring, yet photosynthetic rates may stay high, the stomata remaining open. The plant was able to recover from quite extreme daytime hydraulic dysfunction, although here root pressure seems not to be involved (Holloway-Phillips & Brodribb 2010). It will be interesting to see how widespead such behaviour is in the family. Indeed, Harrison Day et al. (2024) found that cavitation in the roots of wheat growing in dry conditions was irreversible, unlike that in the roots of maize. Although such diferences are of considerable ecological/physiological importance, little is known about such variation, possible connections with carbon fixation, etc..
6. Cold Tolerance (and the Annual Habit).
The ecological success of Poaceae is not just because many taxa in the PACMAD clade have adopted C4 photosynthesis. Cooler temperate grasslands in the northern hemisphere are dominated by Poöideae, with some 3,900 species; this is the largest subfamily and all its members are C3 grasses. Thus although about 16% of all species growing in Quebec and Labrador north of 54o N are Cyperaceae, it is Poaceae that are next at 11%, and all are Poöideae (Escudero et al. 2012). Early diverging clades here tend to be plants of closed habitats (L. Zhang et al. 2022), and taxa in Poöideae have complex relationships between freezing tolerance, day length, vernalization, and flowering (e.g. E. J. Edwards 2009; Edwards & Smith 2010; Dhillon et al. 2010). Note, however, that scenarios for the evolution of cold tolerance in Poöideae depend on their age, and at ca 73.7/68.7 Ma for stem and crown ages and ca 50 Ma for the beginning of diversification in core Poöideae, Schubert et al. (2019: p. 1178) invoke somewhat notional "cold microhabitats of such nascent Eurasian mountains" (they are talking about the Alpine Orogeny), and for these Poöideae perhaps to be largely restricted in their distributions for some 20 Ma. Schubert et al. (2019) noted that Poöideae that could tolerate strong seasonality and colder winters had evolved in more than one clade, while Baird et al. (2021) linked the evolution of shorter and narrower leaves (i.a.) with tolerance of cold (and) dry conditions. There are also C3 grasslands in the Argentina-Brazil-Uruguay area, and Streit et al. (2024) looked at grasslands spanning 26-38o S - depending on the grasslands, Panicoideae-Paniceae (largely C4, or Pooideae-Stipeae, C3, were the major grass groups.
On the other hand, Zhang et al. (2022) date the core Poöideae to around 35.7 Ma, not long before the Eocene-Oligocene transition. Indeed, the evolution of core Poöideae may be linked with the cooling at the beginning of the Oligocene ca 33-27 Ma (Strömberg 2005, 2011; Sandve et al. 2008; Zachos et al. 2008; Soreng et al. 2021; c.f. Christin et al. 2014a), gene families implicated in low temperature-induced stress response expanding prior to the diversification of Poöideae (Sandve & Fjellheim 2010). These genes seem to have been under positive selection (Vigeland et al. 2013: clades downstream from Brachypodium not examined). Proteins that inhibit ice recrystallization - IRI, Ice Recrystallization Inhibition-like genes - are known from the group, but not from Oryzoideae (Sidebottom et al. 2000; Tremblay et al. 2005; Sandve et al. 2008, 2011). Fructans are probably also involved in cold tolerance. Low levels of fructans - specifically levans - occur in many Poaceae, however, notably high levels are found only in Poöideae, although not in taxa of the basal pectinations like Nardus, Stipa and also Poeae-Phalaridinae (Smouter & Simpson 1989; Hendry 1993; Pollard & Cairns 1991; Bonnett et al. 1997). Fructans may enable Poöideae that accumulate them to survive drought or frost better, and they have been implicated in stabilizing cell membranes at low temperatures (Livingston et al. 2009; Sandve & Fjellheim 2010; Sandve et al. 2011). Interestingly, polyploidy is commoner in plants growing at higher latitudes, and there is a correlation of polyploidy with latitude in Poaceae (Hagen et al. 2024). For more on the general problem of adaptations of plants to cold conditions, see Preston and Sandve (2013).
The establishment of vernalization requirements may also have contributed to the diversification of Poöideae (Preston & Kellogg 2008), although how widely they occur outside the subfamily is unclear; vernalization responses probably evolved early in the subfamily (McKeown et al. 2016; see also L. Zhang et al. 2022). Finally, the development of the Epichloë/Poöideae relationship may have been involved in the spread of Poöideae from shady to sunny open habitats in the predominantly cool-season climates that they favor (Kellogg 2001), the mutualism aiding the plant's defences against herbivores, drought and cold (Schardl et al. 2008; Schardl 2010; L. Zhou et al. 2021). Thus Epichloë down-regulates the genes involved in responding to heat stress and up-regulates those responding to cold stress (Dupont et al. 2015). For more on this association, see above.
Although the evolution of Poöideae may initially be linked with the cooling that occurred at the onset of the Oligocene, much diversification is later and associated with Pleistocene cooling. Thus the Poa alliance, whose 775 species are about 1/5th of all Poöideae, may have begun diversifying in the Miocene ca 15 Ma, but most diversification is Pleistocene and younger, occurring within the last 4 Ma (Hoffmann et al. 2013). Diversification rates are very high, up to 3.93 species/million years, although this depends on dating (Hoffmann et al. 2013; see also Birch et al. 2014: ages slightly older).
The evolution of the annual habit may also be important. Hjertaas et al. (2023) noted that somewhat fewer than 10% of angiosperms were annuals (they included biennials in the total) of which maybe around 1,700 were to be found in Poaceae (ca 17% of the family according to their figures), especially in core Pooideae, ca 1,700 in Asteraceae (7%) and 1,000 in Fabaceae (5%); winter annuals are notable in Scrophulariaceae and Brassicaceae. They suggested that evolution of a precursor trait, a shift in the allocation of resources from below to above-ground parts, might have facilitated the evolution of this feature, which is not easily reversed (Lindberg et al. 2020; Hjertaas et al 2023: Fig. 6).
Other grasses including the more northerly temperate bamboos (Bambusoideae-Arundinarieae) and in particular Danthonioideae, largely austral in distribution, and some chloridoids, also tolerate cooler conditions. In the danthonioids, evolution of cold tolerance is estimated to have begun ca 25 Ma during the late Oligocene in Africa, and, as in Poöideae, cold tolerance evolved in separate clades in the group; there are no vernalization requirements (Humphreys & Linder 2013; see also Linder et al. 2013). Wharton et al. (2010) looked at two species of Chionochloa (Danthonioideae), and they seemed to tolerate cold conditions by controlling ice nucleation.
There has been discussion about the possibility of evolutionary connections between stress responses - to drought, cold - in grasses, and in particular whether drought tolerance was a precursor to cold tolerance (Schat et al. 2025 for literature). Drought tolerance is especially notable in panicoids, aristidoids and chloridoids, not notably in Poöideae, cold tolerance - there are various degrees of this - occurs in Danthonioideae and some Chloridoideae and of course most notably in Poöideae. Although adaptations to drier conditions seem to have evolved before those to colder conditions in the PACCMAD clade, this is certainly not so in Poöideae, and in the latter evolutionary connections beteen drought and cold are not evident (Schat et al. 2025), indeed, Pal Stolsmo et al. (2024) found that species of Poöideae with the highest levels of frost tolerance had ancestors with the lowest level of drought tolerance.
7. Stomatal Opening.
The dumbbell-shaped stomata of grasses have a large stomium when fully open and the rate of opening/closing is very fast indeed, up to an order of magnitude faster than in those few species with kidney-shaped stomata that have been examined (Franks & Farquhar 2006: two non-angiosperm species included; see also Haworth et al. 2011; Woolfenden et al. 2018: the general problem); Triticum aestivum was compared with Tradescantia virginiana, and in both there is movement of K+ ions back and forth between guard and subsidiary cells. Note, however, that the stomata of quite a number of other Poales are morphologically similar (see above) and it is unclear if the dumbbell shape per se played a major role in the ability of grasses to spread as climates became drier at the end of the Eocene (Hetherington & Woodward 2003). See also Z.-H. Chen et al. (2017), McKown and Bergmann (2020) and Cheng and Raissig (2023) for grass stomata, how they work and their evolution.
Grass leaves often roll up quite readily, affecting both water and gas transpoty. In maize the cuticle of the bulliform cells is thicker than that elsewhere, but its composition is such that these cells are in fact more permeable to water than other epidermal cells, and bulliform cells are involved in leaf rolling (Matschi et al. 2020). However, not all grasses have bulliform cells (Shields 1951).
8. Roots: Mycorrhizae, Bacteria, Etc..
Freschet et al. (2017), Linder (2017b) and others have discussed the interaction of a number of ecologically significant traits of graminoid (i.e. including Cyperaceae, etc.) roots. The primary root system of grasses is usually short lived, being replaced by lateral roots developing from the crown (that part of the stem with very short internodes immediately above the radicle), and, in perennial grasses, from nodes along the rhizomes or stolons (= "adventitious" roots). Interestingly, in drought conditions crown root development in annuals is suppressed, or only a few crown roots develop, and the main root system develops from the primary root; when the moisture regime improves, crown roots develop (Sebastian et al. 2016: both panicoids and pooids).
Prominent rhizosheaths made up of mucilage from root cap cells, soil particles, bacteria, etc., all anchored to root hairs occur in many Poaceae (McCulley 1995), especially those growing in drier conditions; they can be up to several metres long and are impermeable to water (Kellogg 2015). The general distribution of such roots is poorly known, but they have been found in some other Poales but are apparently rare in broad-leaved angiosperms. Some crops, including maize and wheat, show a substantial uptake of N that ultimately comes from bacteria that are either in the rhizosphere or are endophytic (Santi et al. 2013). An example is a landrace of maize growing on N-poor soils in Mexico that ultimately obtained 29-82% of its N from the diazotrophic microbial biota associated with the carbohydrate-rich mucilage produced by its aerial prop roots (Van Deynze et al. 2018). Along the same lines, ammonia and nitrous oxide were scavenged from the air by bacteria associated with the roots of Leptochloa fusca (Chloridoideae-Cynodonteae: Hurek et al. 1988). There glasshouse experiments suggested that the bacteria were transmitted via the caryopses and affected root growth (roots were positively geotropic) and the development of root hairs; grasses produced reactive oxygen species during the day that denatured bacterial proteins that were broken down at night by root and microbial proteases, organic N then being taken up by the plant (White et al. 2015). Note that plant roots of at least some eudicots also produce proteases (Paungfoo-Lonhienne et al. 2008; Adamczyk 2010), and what, if anything, is unique in such aspects of grass root physiology is unclear. Rhizobium and related bacteria that fix N when in legume hosts have been found to thrive in a variety of crops (both C3 and C4), gaining entrance through cracks where lateral roots emerge and then actively spreading through the plant up into the leaves; there can be extensive positive effects on just about all aspects of the plant's physiology (Chi et al. 2005). Finally, Poaceae, apparently alone in flowering plants (Römheld 1987), acquire iron through chelation of ferric ions with non-protein amino acid siderophores which are then taken up by the roots; iron (and zinc) are commonly limiting trace elements in alkaline soils (Schmidt 2003; Kraemer et al. 2006). Interestingly, ectomycorrhizal plants, also noted for dominating the communities in which they occur, also produce siderophores.
There are interactions between endomycorrhizal/arbuscular mycorrhizal (AM) associations and photosynthesis type, phosphorus (P) availability, germination, the effect of burning on flowering, and even parasitism. Although these factors are treated independently below, they clearly interact. Phosphorus. C4 grasses have roots with long root hairs yet may respond positively in terms of P uptake when forming AM associations - long root hairs and AM associations tend to be thought of as alternative ways of securing a supply of P, etc. (Schweiger et al. 1995 and references; see also above for P, AM, and C3 and C4 photosynthesis). Grman (2012) found that the two C4 grasses she examined responded positively to AM fungi at low P concentrations, negatively at higher concentrations; under these latter conditions plants that lacked AM grew larger, while in plants that did have AM fungi colonizing the roots, this was only somewhat reduced; the plants were supporting the fungus, but to no obvious benefit to themselves, so were effectively being parasitized by the fungi. However, C3 grasses showed little AM-mediated response to P at all, and at high P concentrations AM colonization of roots was very greatly reduced (Grman 2012). One wonders exactly how AM fungi and perennial C3 grasses might be interacting. However, there are suggestions that AM fungi increase P availabilty in C3 ryegrass, but only over a particular range of P availability; thus at low availability, citrate produced by the plant displaces P from the soil (references in Lambers et al. 2015c).
Drought. AM fungi improve plant growth during drought, i.a. improving P uptake by the plant (Augé 2001) and also increasing stomatal conductance. An increase in biomass was associated with AM presence in C3 but not C4 grasses, and the more severe the drought, the greater the effect of the fungi, interestingly, clavicipitaceous endophytes had little effect on Core Poöideae examined, although there was a positive effect on Elymus (Worchel et al. 2013; see also Larimer et al. 2012; Augé et al. 2015).
Herbivory. Jürgens and collaborators (2022) and Jürgens and Gröngröft (2023) suggested that vegetation rings, called fairy rings/circles, in grasslands in Namibia to South Africa were caused mostly by sand termites, Psammotermes allocerus, eating the roots of grasses like Stipagrostis - however, Getzin et al. (2022) and Getzin and Yizhaq (2024) preferred an explanation based on the drying out of the soil. The erratic rains there trigger the development of the circles, Jürgens (2022) noting that in years with much rain termite activity in the rings may be absent. The bare circles are surrounded by a "perennial belt" - perennial grasses that are notably lush and do not readily die after the rains, unlike other grasses further away from the bare patch (in some cases there is a "halo" of perennial grasses immediately beyond the perennial belt). Similar vegetation patterns are to be found in West Australian vegetation, where the activities of Drepanotermes have been implicated.
Fungi. G. W. T. Wilson and Hartnett (1998, see also Hartnett et al. 1994) showed that the growth of perennial C4 grasses in a tall-grass prairie responded positively to AM fungi (10/16 species highly significant positive response), as did that of perennial forbs, but the growth of perennial C3 grasses was much less affected (3/14 species weakly significant positive response). Similarly, in a study of the effects of AM on herbaceous plants the eight grasses included (all C3 species) showed a mixture of negative and positive interactions with AM fungi (Horton & van der Heijden 2007). Indeed, looking at mycorrhizal responsiveness across angiosperms, Poöideae, along with annual grasses and forbs, were notable in the absence of response (Reinhart et al. 2012; for the latter, see also Trappe 1987; Jayne & Quigley 2014). Although the glomeromycote Funneliformis tended to have relatively lower beneficial effects on its AM associates than other taxa, this was not true of Poaceae, but there Gigaspora had particularly low beneficial effects, and this did not seem to be associated with photosynthesis type (Hoeksema et al. 2019). S. Wang et al. (2020) recently found that NO3- uptake was increased in mycorrhizal associations with Rhizophagus in rice (some C4 grasses were also responsive), and amino acids and small peptides may also be involved; Fabaceae-Faboideae, at least, may show a similar response. P availability may control these AM symbioses (Wang et al. 2020). Sui et al. (2014) noted that Pedicularis kansuensis growing on Elymus nutans that had an association with the AM fungus Glomus mosseae grew significantly worse that when growing on grasses without this association, although taking into account the other players in the associations in which grasses are involved may make life less simple (e.g. Sui et al. 2018). It has recently become apparent that associations between embryophytes and mucoromycote fine root endophytes (FREs) seem to be notably common in Poaceae. FREs may enhance the uptake of P in low P conditions, and may be quite common on Poaceae growing in the Arctic (Orchard et al. 2017 and references). Poöideae-Neotyphodium/Epichloë endophyte associations are discussed above.
Some Poaceae show allelopathic reactions with other plants, Sorghum roots producing an allelopathic quinone (an oxygen-substituted aromatic compound) and Festuca roots meta-tyrosine, a non-protein amino acid (Bertin et al. 2007). Benzoxazinoids like DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one), cyclic hydroxamic acids, are largely restricted to Poaceae, and are found in both Panicoideae and Poöideae. They confer resistance to fungi, insects (volatilized, they attract wasps parasitic on insect herbivores of grasses), and even herbicides, and are also allelopathic, but less so to other grasses than to other plants (Frey et al. 1997, 2009; Gierl & Frey 2001; Sicker et al. 2000: ecological role; Dick et al. 2012; Pentzold et al. 2014; Schullehner et al. 2008: non-grasses; Nützmann & Osbourne 2014: clustering of gene involved in their synthesis, clusters can be split; Boycheva et al. 2014). Their breakdown products, e.g. 6-methoxy-benzoxazolin-2-one, achieve these effects, and in maize some of their effects are on the next generation by altering the soil microbiota associated with the root (L. Hu et al. 2018a). DIMBOA also chelates iron, which may be good for the maize plant, but the chelated complex also attracts the noxious western corn rootworm, the galerucine beetle Diabrotica (L. Hu et al. 2018b).
10. "Salt" Tolerance.
Salt tolerance is quite widespread within Poaceae, and two thirds of the 350+ species involved are also C4 plants (Flowers & Colmer 2008; Bromham & Bennett 2014). Weak salt tolerance - tolerance of salinity up to ca 80mM NaCl - has evolved some 76 times (Bennett et al. 2013), possibly being preceded by the acquisition of C4 photosynthesis (Bromham 2015), and euhalophytes, tolerating at least 200mM NaCl, about half the salinity of sea water, have evolved some 43 times, and in both cases the clades involved are young and small (Moray et al. 2015 suggest that halophytes have evolved perhaps 127 times; see also Saslis-Lagoudakis et al. 2016). Overall, there is a high rate of gain and even higher rate of loss, or perhaps raised extinction rates, of salt tolerance (Bromham et al. 2015b) - macroevolutionary self-destruction? Poaceae such as Spartina (= Sporobolus sect. Spartina: Chloridoideae) and Puccinellia (salt grass, alkali grass: Poöideae) are major elements of salt marshes, and it is interesting that roots of the former, along with those of Juncus, are associated with Celerinatantimonas diazotrophica, the closest relative of Candidatus C. neptuna that is known to fix N in the sea-grass Posidonia, Posidoniaceae (Mohr et al. 2021). The C4 Sporobolus sect. Spartina is a particularly prominent component of temperate salt marshes where it dominates large areas; there has been past hybridisation here, and also hybridization between introduced and native species, some of the species that result (like S. anglica) being very invasive (Strong & Ayres 2013). Functioning salt glands, which can look like modified bicellular hairs (Liphschitz & Waisel 1982) are known only from Chloridoideae (Céccoli et al. 2015; see also Liphschitz & Waisel 1982). A number of grasses in different subfamilies accumulate glycine betaines and other compounds commonly associated with tolerance of saline conditions (Rhodes & Hanson 1993). On the other hand, Bambusoideae and Danthonioideae are notable for lacking species that are even weak halophytes, although the former do grow in cooler and often wetter conditions where salt tolerance would be less expected (Bennett et al. 2013; Bromham 2015). This connection between salt tolerance and the adoption of C4 photosynthesis has been noted elsewhere, as in Amaranthaceae-Chenopodioideae (Sage & Monson 1999; Jacobs 2001; Sage 2002; Flowers & Colmer 2008; Kadereit et al. 2012; Bromham & Bennett 2014), and there is also a connection with heavy metal tolerance (Lutts & Lefève 2015).
Although I have been talking vaguely about "salt tolerance" above, this comes in two ecologically different flavours. Much work on salt tolerance has been carried out on plants growing on salinized soils that tolerate sodium chloride and sodium sulphate. However, the area of land with alkalized soils is greater, and here tolerance of sodium carbonate and sodium bicarbonate is important. Sahu and Liu (2023) discuss recent work in which salt-alkali tolerance has been found in sorghum and other grains with potential major implications for global grain production.
Herbaceous bamboos, Olyreae, are sister to the woody bamboos, Bambuseae + Arundinarieae (see Phylogeny below). The woody bamboos tend to colonize forest gaps and edges and can dominate in the canopy and understory of both temperate and tropical forests, particularly in mountainous regions. Thus in many parts of the world (although not in Europe or much of temperate North America) oaks, beech or southern beech (Nothofagus) are major components of oak/bamboo forests, the bamboo making up the understory (Kappelle 2006b). In south western Brazilian Amazonia around 160,000 km2 or more of forests is dominated by two species of Guadua (Bambuseae), possibly because of the activities of the geoglyph builders who became active around 4,000 years ago (McMichael et al. 2014). Even herbaceous bamboos (Olyreae) may dominate understory vegetation (Bamboo Phylogeny Group 2012b for details and references). However, although bamboos are the second most important woody monocot clade (after palms), they do not appear in the very top ranks as having any of the important ecological traits studied by Cornwell et al. (2014). However, they do grow very fast, for example, Phyllostachys edulis (Arundinarieae) can grow over a metre a day, and G. Jin et al. (2021) suggest that it is a mixture of new genes, some the result of the divergence of duplicated genes, as well as whole-genome duplications where gene copies have evolved divergent expression patterns, that together are involved in various aspects of cell wall construction that enable this fast growth (see also Z.-H. Guo et al. 2019 for the evolution of such genes in woody bamboos). Large quantities of CO2 come from the culms (but overall net emitters of CO2? - see Zacharias et al. 2016). Their overall construction is very efficient, the plants being taller and their leaf area being larger than that of trees with the same biomass - their culms are hollow, yet strong, and all the vascular tissue is functional, not just the sapwood (Fujinuma et al. 2018). Furthermore, they have flexibility in that they can quickly produce new shoots whose metabolic cost is partly paid for by mobilization of the carbohydrates in the older shoots that are being replaced (Fujinuma et al. 2018). Bamboo wood, very dense, decomposes more slowly than that of other angiosperms (G. Liu et al. 2015).
Woody bamboos play a very important role in the dynamics of the forests in which they live, both because of their dominance and because of their synchronised monocarpic flowering (see also below), which can be thought of as an extreme form of masting. Broad-leaved angiosperms can still grow in areas where bamboos dominate if they can establish themselves in the period immediately after the bamboos die after fruiting. However, as Keeley and Bond (1999) noted, flowering was often imperfectly synchronized, and there were "regions of flowering (103-105 ha) that spread wave-like" from year to year across the landscape (ibid, p. 384). This form of reproduction coupled with semelparity and clonal growth generate huge amounts of fuel locally, and catastrophic fires burn into the canopy producing open conditions for the seedlings. The original habitat for bamboos may have been the ecotone between forest and savanna; rainforest bamboos do not show the same phenological behaviour, and there is no masting/frugivore satiation there (Keeley & Bond 1999). Bamboos are not known to be ectomycorrhizal plants, as is common in other woody masting taxa, at least. Z.-H. Guo et al. (2019) discuss the evolution of genes that may be involved in the control of this remarkable behaviour, but how this reproductive behaviour is controlled is largely unknown, although it may well have originated by successive small multiplications of the initial synchronization intervals (Veller et al. 2015) - but why is this behaviour overall so uncommon in plants? See Fagaceae for masting in a rather different system, Pesendorfer et al. (2021a) and other papers in "The ecology and evolution of synchronous seed production in plants", Phil. Trans. Royal Soc. B, 376(1869). 2021 for mast fruiting in general, and Botanical Trivia below.
Indeed, Zheng and Pacala (2024) suggest that the distinctive aspects of the bamboo life style - and note that this includes a fair amount of variation, for instance, bamboos may be herbaceous, woody and arching (sympodial or monopodial), semi-erect, erect, scandent, twining, or erect-hanging (Zheng & Pacala 2024: Fig. S10) - can be incorporated in what these authors call the arrested succession hypothesis. Bamboo germinates from sexually produced seeds and starts competing with tree seedlings as seedlings. Both bamboos and trees follow power-law allometries. If bamboo seedling density is sufficiently high, the seedlings will collectively close the canopy, continuing to grow larger (if for some reason they become members of the understory they will die), until they reach their maximum size. At maximum size net carbon gain does not result in further growth in height, but the plant saves carbon that is ultimately used to construct clones, which they develop as soon as they have accumulated enough carbon to make shoots of the maximum culm size along with the associated rhizome. Thus the vegetative daughter culm replaces surviving seedlings at the end of their life spans, the bamboo switching from its sexually-derived seedling phase to its vegetative propagation phase - instead of the population consisting of seedlings, it is now a group of genets, each clone of culms ultimately coming from a single seedling. Although the bamboo canopy is often lower than that of the surrounding forest, the dense shade that it casts suppresses the growth of young plants of that forest; the bamboo-dominated forest persists. When the entire bamboo population eventually flowers, all culms and associated rhizomes convert their mass into seeds and begin this cycle again. The life cycle of bamboo thus consists of a power-law growth (seedling) phase followed by a clonal integration (genet) phase (this paragraph has largely been taken from Zheng and Pacala 2024: S1). Note that tropical bamboos are taller than temperate bamboos, they have shorter rhizomes, and time to mass flowering is shorter - thus species that form close-canopy monospecific stands in temperate Asia are only about 5–10 m tall (K.-F. Cao et al. 2012), they have running rhizomes that can spread beyond 10 m (e.g. L. Xiao et al. 2021), and long, synchronized, flowering intervals greater than 30 years (Triplett et al. 2014).
A final feature of bamboos is the development of positive nocturnal xylem pressure in the stem or rhizome, but not in the roots (Michaud et al. 2024). This has been studied in detail only in Bambusa oldhamii and is very poorly undersood - does this occur in other bamboos (probably) or in grasses other than bamboos (probably not, certainly not in Zea)?
12. Resurrection Plants.
Poaceae include more resurrection plants than any other family, nine genera including species that show extreme desiccation tolerance and are often poikilochlorophyllous, that is, their chloroplasts ± break down as the plant dries (Gaff & Oliver 2013); some species of Micraira (Micrairoideae) are to be included here (Sanchez-Ken et al. 2007). Most are chloridoids, which tend to be rather small plants that can grow in dry, open, often rocky environments, salt glands are common, they have small, dense stomata, and C4 grasses adapted to arid environments often have amphistomatous leaves (Pardo & VanBuren 2021). Extreme desiccation-tolerant chloridoids include Tripogon loliiformis; this is homoiochlorophyllous; here the photosynthetic apparatus is retained, the disaccharide trehalose (two glucoses) accumulates and apparently encourages autophagy (autophagosomes develop and scavenge toxins produced as the cell dries out), and nutrient recycling may also be involved (B. Williams et al. 2015). In desiccation tolerance in another chloridoid, Oropetium thomaeum, also homoiochlorophyllous, at least some genes involved are the result of gene duplications associated with the family-level ρ/rho event, although overall the genome of this species, at ca 245 Mb, is the smallest in grasses and is only twice the size of that of Arabidopsis (VanBuren et al. 2015). Late Embryogenesis Abundant (LEA) genes and gene families are involved in desiccation tolerance in Oropetium (VanBuren et al. 2017) as in angiosperms in general (Artur et al. 2018), and Pardo et al. (2019/2020) noted that they were involved in the poikilochlorophyllous chloridoid Eragrostis nindensis, but also in desiccation sensitive grasses. Artur et al. (2018) found that PACMAD/BOP grasses had distinctive synteny groups of LEA_1 genes when compared with those of other angiosperms (pineapple was the only other member of Poales examined). As in other resurrection plants, there are large tandem arrays of early light induced proteins that help to protect the plant against photooxidative damage (Pardo et al. 2019/2020). Marks et al. (2024) looked at the evolution of desiccation tolerance in three species (in different genera) of Chloridoideae, parallelism, convergence, and the evolution of species-specific features all being involved. For drought resistance and chloridoids, see also Pau et al. (2013) and S. H. Taylor et al. (2014); Porembski et al. (2021) discusses desiccation-tolerant mat-forming grasses growing on tropical inselbergs.
13. Phloem/Fine Vein Transport.
Some sieve tubes adjacent to the xylem, especially in the cross-veins in the leaves, lack companion cells, have notably thick walls, and are symplastically isolated from "normal" sieve tubes. They seem to be involved in short distance transport of not very concentrated sugars (Botha 2013). They are quite common in grasses, and probably evolved well before 7-5 Ma (c.f. Botha 2013); although their distribution in other monocots is unclear, they are also reported from Cyperaceae. Indeed, the small transverse veins seem not to be directly involved in water transport to the mesophyll (Altus & Canny 1985; Fiorin et al. 2015).
For mineral movement at nodes, with different types of vascular bundles and different patterns of movement between between xylem and phloem depending on the mineral, see Yamaji and Ma (2014 and references); again, there is little comparative knowledge.
The relationships between C3 and C4 grasses, temperature, trees, moisture, atmospheric CO2 concentration and fire are complex and dynamic, although it has even been suggested that the current climatic restrictions of grasses with the two kinds of photosynthesis has less to do with physiology, more with simple time and opportunity that would allow expansion of clades into climatic regions different from those they currently inhabit (Watcharamongkol et al. 2018). Be that as it may (unlikely), grasslands and savanna, vegetation where grasses occupy at least 50% of the ground layer, covered (before agriculture) about 40% of the land surface of the globe, about half that area being inside the tropics (Gibson 2009; Lehmann et al. 2019), yet grasses include a mere 3% of vascular plant species (Lehmann et al. 2019: dominating grasses far fewer, see next paragraph). The global distribution of C4 vegetation, which of course includes more than just grasslands, has been estimated at ca 18.8 x 106 km2, somewhat over 15% of the total vegetation coverage (Still et al. 2003). All told, C4 photosynthesis accounts for about 23-28% of terrestrial gross primary productivity, although the biomass of C4 plants is only ca 5% of the global total and fewer than 3% of all species are C4 plants (Still et al. 2003: GPP = 35.3 vs 114.7 Pg C yr-1, simulated biomass, leaf, wood, root = 18.6 vs 389.3 Pg C; Ito & Oikawa 2004; see also Lloyd & Farquhar 1994; Ehleringer et al. 1997; Gillon & Yakir 2001; Retallack 2001). Beerling et al. (2012) suggested that at 200 ppm CO2 concentration C4 grasses had a higher net primary productivity than did C3 grasses, and also a biomass that was somewhat over twice that of the latter group; this disparity increased dramatically as [CO2] increased up to 400 ppm. Other estimates suggest that grasslands in general - both C3 and C4 species - currently account for 11-19% of net primary productivity on land (Hall et al. 2000), more than 25% of terrestrial photosynthesis (Lehmann et al. 2019), or even 33% global primary productivity (Linder et al. 2017b). Grasslands are made up of rather small plants, but estimates of the proportion of below-ground biomass in grasslands is as high as 80-95% (Dormaar 1992) and this accounts for 10-30% of global soil carbon storage (Hall et al. 2000); Gibson (2009) estimated as much as ca 33% total C storage - 650-810 Gt, broadly in line with the estimates in Retallack (2013a). More general figures for tropical savannas and grasslands together (the latter are of course likely to be C4 grasses) in Carvalhais et al. (2014: Tables S1 + S2) are ca 338 Pg total C, a carbon density of ca 17.7 kgC m-2 and a mean turnover time of (12.2-)16(-22.1) years, while comparable figures for temperate grasslands and shrublands are ca 187 Pg total C, and a carbon density of ca 16.7 kgC m-2 with a mean turnover time of (32.8-)41.3(-54.6) years; the residence time for the carbon in temperate grasslands is much longer, largely because of the cooler temperatures. Overall, grasslands function as a long-term carbon and water sink, and one consequence of their activities is long-term global cooling (Retallack 2001, 2013a).
What makes grasslands and savannas particularly distinctive ecologically is not just the abundance of grasses and their caespitose or rhizomatous growth habit, but that relatively few species of grasses are ecologically dominant in any one place, and most of these seem to be C4 grasses (for dominance in grasslands, see Yu et al. 2015). Of the some 11,300 species of grasses, E. J. Edwards et al. (2010) estimated that only some 600 species, most C4 photosynthesizers of the PACMAD clade, dominate ecologically in grasslands and savanna, while Kellogg (2015) suggested that fewer than 10% of grass species made up most of the biomass in major grasslands, just four species dominating in tall grass prairie in North America, for example. Andropogoneae, with some 1,200 species and 90 genera, are notable in their positive response to annual burning (Forrestel et al. 2014 and references), and in African, Australian and North American grasslands and savannas in particular members of the ASH clade ("Andropogon", Schizachyrum, Hyparrhenia), with some 244 species, are prominent among the dominants (Estep et al. 2014; DASH clade - Diheteropogon added, 250 spp., see McKain et al. 2016b for its composition). As Lehmann et al. (2019) emphasized, 88% of the area occupied by grassy vegetation is dominated by members of three clades. Thus 37% of grassy vegetation/14.8% vegetation globally is dominated by tall (maximum plant height ca 1.5 m - but what about big bluestem?) C4 Andropogoneae, a group found mostly in warmer, wetter areas where disturbances such as grazing and in particular fire maintain the open nature of the vegetation, 14/5.6%/ca 0.6 m by C4 Chloridoideae, which favour more semi-arid vegetation, and 38/15.2%/ca 0.6 m by C3 members of the BEP clade, mostly Poöideae, which grow in cool, rather dry areas that are subject to only short droughts (Lehman et al. 2019, q.v. for details). See above for the complexity of responses within grasses to particular ecological stimuli.
It is still more remarkable that this ecological dominance seems to be quite recent. Although C4 grasses may have first appeared in the Oligocene ca 33 Ma and begun to diversify soon after, they seem to have made a major contribution to overall vegetation biomass only in the late Miocene 9-8 Ma - or perhaps a little before - and subsequently (Jacobs 2004; Spriggs et al. 2014; Linder et al. 2017b). It was then that grasslands and savannas began to spread rapidly, the process being completed as recently as the late Pliocene 3-2 Ma (e.g. Keeley & Rudel 2003; Bouchenak-Khelladi et al. 2009, 2014a; E. J. Edwards et al. 2010; Strömberg & McInerney 2011; McInerney et al. 2011; Strömberg et al. 2011; Arakaki et al. 2011; Sage et al. 2012; Godfrey & Crowley 2016: Madagascar). Indeed, Linder et al. (2017b) observe that a number of grasses are currently notable invasives, and suggest that features that make them such successful invasives are largely those that have made grasses successful since the Miocene. Most of these are discussed in more detail elsewhere, and include tolerating disturbance, whether from fire or grazing, efficient propagule dispersal, and changing the environment to their own advantage. The Neogene has rightly been called the age of grasses (c.f. Palaeos).
Pollination Biology & Seed Dispersal. Despite the small size of the flowers that are nearly all wind pollinated, there is quite considerable variation in floral morphology in the family (see e.g. Le Roux & Kellogg 1999a; Chuck 2010 for the development of and Schrager-Lavelle et al. 2017 for the literature on unisexual flowers; also Rudall & Bateman 2004; Ronse de Craene 2010; Kellogg 2015). Preston and Fjellheim (2020) discuss variation in flowering time in grasses.
Lodicules, apparently modified members of the inner tepal whorl, help in the opening of grass flowers, however, they may be absent from carpelate flowers (e.g. Sajo et al. 2007; Reinheimer & Kellogg 2009; Schrager-Lavelle et al. 2017; see also Appleton & Kramer 2024). Poaceae are predominantly wind-pollinated and usually have protandrous flowers with dangling anthers. Given this pollination mode and also that a number of grasses live in quite dry conditions, it is perhaps surprising that grass pollen is partly hydrated (>30% water content) and sensitive to desiccation, i.e., practically all grasses have recalcitrant pollen (see Ackerman 2000). Pollen longevity in angiosperms in general is low, thus wind-pollinated Fagales, for instance, have a mix of species with orthodox (not hydrated and rather longer-lived) and recalcitrant pollen (Franchi et al. 2002, 2011; Smarda et al. 2014). McComb and Ackerman (2018) looked at details of wind pollination in Phleum pratense, noting that the efficiency of pollen capture increased if the inflorescences moved transversely to the direction of pollen movement - true of both wind and water pollination. Niklas (1985) looked at the aerodynamics of wind pollination in some grasses.
Some forest-dwelling grasses, especially Bambusoideae-Olyreae, small plants of the forest floor in the New World, are pollinated by insects (Soderstrom & Calderón 1971: Parinari, Olyra) or are visited by pollen-collecting insects that trigger the release of pollen, the actual pollination being by wind (Ruiz-Sanchez et al. 2016). Similar pollination systems are reported from a few Chloridoideae and Panicoideae by Schulze-Albuquerque et al. (2019: references to 24 other grass pollination papers) who also noted odours being produced by the plants that might attract pollinators - ambophily. Streptochaeta may also be animal pollinated, since it lacks a plumose stigma and its anthers do not dangle; the flowers are protogynous (Sajo et al. 2008). What pollinates the remarkable pseudanthium-type inflorescences of the Madagascan bambusoid Sokinochloa is unknown (see S. Dransfield 2016; Baczynski & Claßen-Bockhoff 2023).
Floral reduction here has been achieved in a variety of ways and a number of breeding systems are common (for which, see Connor 1981 and references; Giussani et al. 2016: Poa). Grasses have a gametophytic self incompatibility mechanism, but rather surprisingly it also has a dry stigma, etc. (Allen & Hiscock 2008); for a summary of self incompatibility in Poaceae, see Langridge and Baumann (2008). Pollen germination is controlled by the maternal parentt, and it seems almost as if pollen from just about any plant can germinate on a grass stigma (Lausser et al. 2010; Kellog et al. 2015); for possible implications for hybridization, etc., see below. Apomixis is quite common in Poaceae, especially in Panicoideae (Asker & Jerling 1992; Hörandl et al. 2007; Hojsgaard et al. 2014; Majeský et al. 2017 and literature); cleistogamy is very common, occurring in over 300 species (Campbell et al. 1983; Kellogg 2015). A final wrinkle is that a phenomenon known as heterofertilization can occur. This happens when gametes from different pollen grains fuse with the female gamete and the diploid primary endosperm nucleus respectively, and it has been recorded from Zea mays (C. C. Wu et al. 2013 and references).
The caryopsis is a variant of an achene in which the testa and pericarp are fused, that is, it is a fruit proper, and it characterises Poaceae. However, the caryopsis is rarely the dispersal unit, taxa like the fleshy-fruited Alvimia (Bambusoideae) being exceptions. It is the spikelet as a whole that is so important in dispersal, and it may include the glumes, the lemma, any awn on it and its indumentum, as well as the callus at the base of the spikelet, etc., and all these in some combination make up the functional unit in dispersal (e.g. Linder et al. 2017b; Cavanagh et al. 2019 for the diversity of awns). The abscission zone that allows the separation of the diaspore from the plant (= shattering) varies in position (it may be absent), and depending on its position, the composition of the diaspore varies; the plesiomorphic condition is in the rhachilla above the glumes. The genes involved in shattering may be quite different in unrelated species, the overall commonality in different taxa being about zero, and the morphology and cell wall composition (e.g. lignified or not) of the abscission zone varies greatly, in some cases this zone not being evident even on detailed inspection although abscission does occur; there is little phylogenetic signal in the variation (Y. Yu & Kellogg 2018, see also Yu et al. 2019, 2020).
Overall, diaspore morphology and dispersal mechanisms in Poaceae are very varied (e.g. Werker 1997). In the Brazilian bamboos Raddia and Sucrea the glumes are turgid and inroll on stimulation, ejecting the fruit (its stigma is plumose - any function here?) - ballistochory (Sendulsky 1993). The spikelet as a whole may be involved in dispersal. The abscission zone that separates the diaspore from the plant varies in position, the plesiomorphic condition being in the rhachilla; it may be absent. This abscission zone also varies in anatomy, and even if the genes expressed during its formation are similar in different grasses, the patterns of expression of these genes and gene networks vary, probably reflecting changes in transcriptional regulation (Y. Yu & Kellogg 2018; Yu et al. 2019), and that will affect the nature of the diaspore.
Dispersal is quite often by animals, attracted by structures like elaiosomes (Davidse 1987), and myrmecochory in particular is known in grasses with distinctive scars at the base of the glume and lemma (C. Silva et al. 2019 and references). A variety of hooks and spikes attach other grass diaspores to passing animals (Centotheca is a good example). The fleshy fruits ("baccoid caryopses") mentioned above in Bambusoideae evolved at least seven there during the Late Miocene when the climate was notably warm and wet; these fruits are viviparous, or germination begins as soon at they fall to the ground (Ruiz-Sanchez & Sosa 2015). Wind-dispersal is quite common, for example, "Andropogon" and Hesperostipa have long hairs on their awns, while Spinifex and a few other genera are tumbleweeds. Awns come in a variety of sizes - up to 10 cm long in Hyparrhenia anemopaegma and ca 22 cm long in H. neomexicana, shapes and numbers, they are borne on different parts of the spikelet (e.g. Cavanagh et al. 2019), and they can aid in both wind and animal dispersal, they may photosynthesize and contribute to the developing seeds, and they may have a variety of other functions (Petersen & Kellogg 2022 for a review). The surface microstructure on awns, minute retrose bristles, the twisted nature of the awns, the stiff hairs on the callus and sometimes elsewhere on the diaspore, all may help the fruit proper to become "planted" in the ground, providing the soil is the right consistency and/or there are small objects on the surface or cracks in the ground the facilitate the act of burying (Peart 1979; Peart & Clifford 1987 and references; Elbaum et al. 2007; Humphreys et al. 2010b; McAllister et al. 2018; Cavanagh et al. 2021). Diaspores with awns become embedded in cracks in the ground or under bits of earth, and in taxa with long geniculate awns the perversion, the point at which the direction in which the awn is coiled changes, is at the bend or geniculum itself (by no means all awns have such a perversion), and the awn helps in getting into these cracks, burying the diaspore (Cavangh et al. 2021). In other cases the diaspore may move along the surface (Kulic et al. 2009; see also Davidse 1987); this movement is by a ratchet principle similar to that which operates when you put an inflorescence of Hordeum up your sleeve - the inflorescence migrates up your arm and then sometimes down your back as you walk along. In taxa like Schizachyrium fragile the diaspores jump as the two awns rapidly slip over each other (Peart 1979). For additional literature on awns, see Schrager-Lavelle et al. (2017).
There is a fascinating relationship between cordgrass (Spartina, = Sporobolus) and the polychaete worm Hediste diversicolor. The worm takes caryopses of the grass (which it cannot eat) into its burrow, but when they germinate the seedlings provide the worm with high quality food - H. diversicolor has been called a gardener worm (Z. Zhu et al. 2016). If not eaten, the seedlings readily establish themselves.
Panicoideae-Andropogoneae have distinctive paired spikelets, one being sterile/staminate. Interestingly, in cultivated sorghum ca 9% of the yield comes from photosynthate produced by this sterile spikelet, and in wild Andropogoneae there is similar movement of the photosynthate from the sterile spikelet to the seed (AuBuchon-Elder 2020). In the latter taxa the awn on the fertile spikelet does not contribute to the grain, although such awns in wheat, barley and rice, for example, do photosynthesize and their photosythesate does contribute to the development of the grain (references in AuBuchon-Elder 2020; see also K. B. Petersen & Kellogg 2022).
Woody bamboos are well known for their tendency to dominate the vegetation and their synchronized flowering and fruiting, evident even when transported thousands of miles from their native habitat (this latter story is qualified slightly by Keeley & Bond 1999). They are monocarpic, flowering may occur only every 120 years or so, and after a rather protracted period of reproduction all the plants of the species die. This has profound effects both on the local vegetation and all organisms dependent on bamboos for food and shelter. The fruits are used as food by humans and they also attract animals like birds and rats in very large numbers (Janzen 1976; Judziewicz et al. 1999). This behaviour is also known from some herbaceous bamboos (Olyreae), but it is perhaps unlikely to be an apomorpy for Bambusoideae as a whole; it has been thought of as an extreme form of masting (Curran & Leighton 2000 and references). See also above for more on bamboo ecology.
Plant-Animal Interactions. Despite the fibrous texture of many grasses and the silica bodies mentioned above, as well as alkaloids and other defences, caterpillars of nymphalid butterflies, Nymphalidae, and skippers, Hesperiidae, are common herbivores of Poaceae (Kawahara et al. 2023), and caterpillars of a number of clades of moths also feed on the family. In general, host specificity of these lepidoptera is low, largely because defences are rather general and widespread (Sahoo et al. 2017).
Within Nymphalidae, the brush-footed butterflies, it is the browns, subfamily Satyrinae, with around 2,400 species, that are common on Poaceae. Satyrinae include Satyrini, graylings, ringlets and the like, and related tribes like Morphini, Melantini, etc.. Estimates are that Satyrinae diverged from other Nymphalidae some 80-85 Ma (or perhaps at the K/P boundary: Heikkilä et al. 2011), stem Satyrini may be about 65-55 Ma old, and the crown group is later Eocene, some (47.8-)41.8, 36.6(-31.5) Ma (Peña & Wahlberg 2008; Wahlberg et al. 2009; Peña et al. 2006, 2011: age depends on calibration points, position of Satyrini varies). Other tribes of Satyrinae may eat grasses or other commelinid monocots, including palms, but other than Elymniini (which is sometimes broken up) none has more than 110 species; the 2,400 species of Satyrinae represent about 1/8 of all butterflies and about half of all Nymphalidae.
Although diversification rates have increased (perhaps through reduced extinction rates) in Satyrini, this group is in a larger clade that has Poaceae as a major food plant, so rate increase is not simply linked to a host-plant shift (Hamm & Fordyce 2015; also Peña & Espeland 2015); the drier and cooler conditions of the Oligocene may be associated with their diversification (see also Heikkilä et al. 2011). The crown group age of Satyrini, dated to the later Eocene, is perhaps contemporaneous with the initial spread of grasses (Peña et al. 2006, 2011; Peña & Wahlberg 2008). It has been suggested that it was the move of satyrine butterflies from forests to more open environments, rather than grass feeding per se, that may have helped spur their diversification (Peña et al. 2011), although Satyrini of more forested habitats have also diversified, Satyrini in general diversifiying 33-26 Ma (Peña & Wahlberg 2008). Caterpillars of the largely western South American satyrine subtribe Pronophilina, with well over 400 named species (?600 species total: Fisher et al. 2014 for a phylogeny) eat largely bamboos like Chusquea. They are most diverse in the Andes just below the cloud forest-páramo transition at ca 3050-3250 m altitude, but some species are found in the páramo itself, where Swallenochloa and some other bamboos grow (Pyrcz et al. 2009; Casner & Pyrcz 2010; Sklenár et al. 2011). However, there are few Pronophilina in high-altitude forests in east Brazil and Central America.
Skippers (Hesperiidae) make up another major clade of butterflies the caterpillars of which eat mostly Poaceae - these make up the [Heteropterinae [Trapezetinae + Hesperiinae]] clade with around 2,250 species, of which over 2,000 are the grass skippers, Hesperiinae (Warren et al. 2009). Skippers are common in the New World, and they, too, are found on bamboos in the Andes, the genus Dalla, with perhaps 100 species, being an example. Warren et al. (2009) suggest an origin of skippers as a whole in the mid-Cretaceous, another estimate is (78.1-)65.2(-55.8) Ma (Chazot et al. 2019: other dates there; see also Espeland et al. 2018 - diversification began ca 43 Ma). Sahoo et al. (2017) suggest that diversification of Hesperiidae began ca 82 Ma, but note that extant members of basal skipper clades eat mostly eudicots. The monocot-eating [Heteropterinae [Trapezetinae + Hesperiinae]] clade, around one half of all skippers, started diversifying ca 65 Ma around the K/P boundary, and there are two notable rate increases in diversification, both in Hesperiinae, dated to around 50 and 40 Ma and in the Oriental-Afrotropical and Neotropical regions respectively. Notably, however, there were no obvious upticks in diversification as grasses became abundant and grasslands/savannas became widespread in the Miocene and afterwards (Sahoo et al. 2017).
Moth larvae are quite common on Poaceae. Thus there are perhaps 725 species of gelechioid leaf- ot stem-mining moths - Elachistidae s. str. - whose main hosts are members of Poaceae, but the larvae also eat Cyperaceae, especially of the Palaearctic (Kaila 1999), and they are also to be found on Commelinaceae (but all these are commelinids) in the tropics (Menken et al. 2009). Snout moths, pyralids, may include Crambinae, the grass rollers, or Crambidae as a family are kept separate from Pyralidae; in any event, Crambidae are a large group of moths (over 10,000 species in over 1,000 genera) that are often found on Poaceae; the caterpillars are stem borers and a number of species are pests. Crambideae also include some 500 species or so of Crambus and Herpetogramma whose larvae are the sod webworms, they live underground during the day and feed on the plant at night (Solis 2007, see also Wikipedia xi.2020). Noctuidae-Sesamiinae are stem-borer moths with some 200 species that eat C4 Paniceae and live largely in Africa; with the cooling and drying climate since the late Miocene the diversification of Paniceae has increased and that of the moths decreased (Kergoat et al. 2018: see above). Finally, the ca 120 species of the tortricid leaf roller Bactrini are found on Poaceae, and their radiation at ca 19.4 Ma has been linked to the expansion of grasslands (Fagua et al. 2017: age from two species of Bactra).
Recent work on Zea mays suggests how chitinases and proteases, gut bacteria and physical plant defences interact. They cause the protective peritrophic matrix in the gut of insect herbivores like the fall armyworm (Spodoptera frugiperda) to become disrupted, and immune responses are upregulated, etc., all this depending in part on the genotype of the plant (Mason et al. 2019; see also Agrawal et al. 2008).
Galling dipterans, especially Cecidomyiidae, are common in grasses (Labandeira 2005). Cecidomyiid gall midges, notably Mayatiola (M. destructor is the Hessian fly), grow on Poöideae in North America (Gagné 1989). Shoot flies (Diptera-Chloropidae) form galls on monocots, especially grasses, but they are also simple herbivores and have other life styles (de Bruyn 2005). Chinch bugs (Hemiptera-Lygaeidae-Blissinae) have been most commonly observed on members of the PACMAD clade, less commonly on those of the BOP clade; the lygaeid Teracrini are also concentrated on Poaceae (Slater 1976). Poaceae provide food for both adults (as pollen) and larvae (as roots) of the corn rootworms, Chrysomelidae-Galerucinae-Luperini-Diabrotica beetles (Jolivet & Hawkeswood 1995).
See also: 1. Pectin polysaccharides are uncommon in grasses, and Kirsch et al. (2025) found that some chrysomelid leaf beetles found on grasses had lost their ability to break them down, although they had retained other plant cell wall degrading enzymes.
2. Kaufmann et al. (02001) discuss the ant gardens that develop on Gigantochloa scortechinii, from Peninsula Malaysia; Crematogaster (Myrmicinae) was commonly involved; there are other bamboo—ant associations.
3. For the association between cordgrass (Spartina, = Sporobolus) and the polychaete worm Hediste diversicolor, see above.
4. Water often congregates in the hollow stems of bamboos, and a distinctive fauna lives there (Kurihara 1983; Kitching 2000).
5. For grasses and grazing mammals, see above.
Plant-Bacterial/Fungal Associations. The diversity of the endophyte community - 100-200 or more species per species of grass - and the infection patterns of individual endophytes within a plant (e.g. Sánchez-Márquez et al. 2007; König et al. 2018), almost beggars description. Thus over 600 endophyte OTUs were found in Agrostis stolonifera, but over 98% of these did not occur in all individuals; of those that did, basidiomycetes tended to be related while ascomycetes and glomeromycotes were not (Lê Van et al. 2017: fungi of the endosphere). Weiß et al. (2016) thought that there were perhaps 500 species of Serendipitaceae, common on grasses, but only three of them had been described. A. M. C. Tang et al. (2007) summarized records of the very numerous and diverse fungi to be found on grasses; at least 1,933 species of fungi are known from bamboos alone.
Ascomycete endophytes, most notably Epichloë (Clavicipitaceae), a systemic endophyte restricted to Poöideae, are widely distributed (Clay 1990: review; Leuchtmann 1992: distribution and host specificity; Schardl 2010; Card et al. 2014: esp. Triticeae; Rodriguez et al. 2009: endophytes in general). There is both horizontal and in particular vertical transmission of the fungus, sometimes in the same plant (Schardl 2010). Its immediate relatives include the convolvulaceous endophyte Periglandula ipomoeae, Balansia, etc. (Ambrose et al. 2014); members of this clade are also found on Cyperaceae and Juncaceae. Neotyphodium is the asexual stage of Epichloë (in some literature Acremonium is recorded from Poöideae - see Leuchtmann 1992), and Rudgers et al. (2009) discussed the patterns of infection of the two forms.Epichloë species may hybridize (Roberts et al. 2005; Moon et al. 2005). Leuchtmann (1992) thought that 20-30% Poaceae might be involved in these associations (4% were known to be infected). There may be a fair degree of host specificity of the fungus (Simpson et al. 2014 for literature), and Schirrmann et al. (2018) noted that the host plant effectively constituted a postzygotic barrier for the fungus by determining what hybrid fungi it allowed to grow. Balansieae have also been found on other Poaceae, especially C4 grasses (Leuchtmann 1992). For details of the phylogeny and evolution of these endophyte associations see Schardl (1996, 2002, 2010), Craven et al. (2001), Clay and Schardl (2002), Jackson (2004, possible codivergence), Gentile et al. (2005) and Sieber and Grünig (2013: general).
Note that Clavicipitaceae-Balansieae (Clay 1986; White et al. 2003: review) are now included in Hypocreales, the old Clavicipitaceae having been split. Hypocreales include many insect pathogens, plant parasites, and especially parasites of other fungi, but also yeast-like obligate symbionts (of leaf hoppers). There has been widespread cross-kingdom host switching (e.g. Vega et al. 2009; Kepler et al 2012). Hypocreales may ancestrally have been plant parasites, although the immediate ancestor of the grass endophytes may have been an insect pathogen (e.g. Spatafora et al. 2007; Vega et al. 2009), while clavicipitaceous fungi like Metarhizium robertsii can be both endophyte and insect pathogen (e.g. Sasan & Bidochka 2012). Interestingly, Epichloë is rather like a parasite in the extent of its effect on the gene expression of the host, ca 38% of the genes being affected, most down-regulated, far more than in endomycorrhizal associations (Dupont et al. 2015). Schirrmann et al. (2018 and references) noted that in the sexual phase of the fungus a limited number of fungal enzymes that can degrade cells walls were active, apparebntly facilitating the development of the fungal fruiting bodies or stomata.
The relationships between the plant and fungus are complex. Epichloë develops inside the young grass inflorescence, disrupting its development ("choke disease") and producing stromata with male gametes, spermatia, and receptive female hyphae. The larvae of Phorbia (or Botanophila) flies develop on the fertilized stromata, and the adult flies transmit the fungal spermatia to new stromata in a fashion analogous to insect pollination of flowers; ascospores are produced, and they infect other plants (Bultman 1995; Schardl et al. 2004; Schirrmann et al. 2018). Indeed, Epichloë synthesizes unique compounds that specifically attract the flies (Steinebrunner et al. 2008) and which may also be toxic to other fungi that secondarily invade the fungal stromata (Schiestl et al. 2006), however, the equilibrium of such relationships can easily be disturbed (Eaton et al. 2010). Claviceps, the ergot fungus, produces spores in slimy masses (an apomorphy for the genus!) and insect-attracting exudates (Píchová et al. 2018). Many species of Claviceps are found on Panicoideae/the PACMAD clade, section Claviceps alone being found pretty much throughout the family as well as on Cyperaceae (Pazoutová 2003; Píchová et al. 2018).
The age of the association, or rather, the age of the common ancestor of Epichloë and Claviceps, could be ca 40 Ma (Schardl et al. 2004), ca 58.8 Ma (Ambrose et al. 2014) or 101-73.4 Ma (Píchová et al. 2018), however, the crown-group age of Epichloë is estimated to be only ca 28.2 Ma (Píchová et al. 2018). The immediate relatives of Epichloë include the convolvulaceous endophyte Periglandula ipomoeae, Balansia, etc., and this larger clade has been estimated to be ca 81 Ma (Ambrose et al. 2014). Poinar et al. (2015) described a spikelet in amber from Myanmar ca 99 Ma that they assigned to Poaceae that was infected with a Claviceps-like fungus. The divergence of Epichloë and the Claviceps group has recently been dated to the Upper Cretaceous 101-73.4 Ma, the Claviceps group itself beginning to diversify ca 14 Ma later (Píchová et al. 2018: note topology). Píchová et al. (2018) suggest that Claviceps originated in South America, howveer, only two of the species they examined seem to be originally from there and the situation in the two immediate outgroups is similar (see also Pazoutová 2003).
The main endophyte in three species of Poöideae growing on dunes in Oregon was a dark septate endophyte, the ascomycete Microdochium bolleyi (David et al. 2017), which in some circumstances, at least, is a pathogen, however, a dark septate endophyte, the ascomycete Chaetomium cupreum, makes the panicoid Miscanthus sinensis, its host, more tolerant of heavy metals (Haruma et al. 2017: other examples in references). Many species of apparently symptomless endophytes (= class 3 endophytes: Rodriguez et al. 2009) are also found in Poaceae, but little is known about these host-endophyte interactions. Fine endophytes with distinctive fan-like arbuscules are common in grasses; these endophytes are probably mucoromycotes, not Glomus, as was thought (Orchard et al. 2016 and references). Little is known about these associations, either. For Epichloë and horizontal gene transfer, see Diversity & Distribution above.
Endomycorrhizae/arbuscular mycorrhizae are discussed briefly above. In Distichlis spicata they preferentially infect female plants (Reuss-Schmidt et al. 2015).
Bacteria, too, may be endophytic in grasses, and several bacterial endophytes are implicated in fixing one third to one fifth of the N (N) needed by sugarcane in Brazil - the bacteria include Gluconacetobacter (α-Proteobacteria), and Herbaspirillum and Burkholderia (ß-Proteobacteria) (de Carvalho et al. 2011; de Bruijn 2015: Chapter 94; more cases in Van Deynze et al. 2018), for Burkholderia, see also Fabaceae, Primulaceae-Myrsinoideae and Rubiaceae. Atmospheric N fixed by diazotrophic bacteria growing in the mucilage covering the apices of prop roots of a race of maixe growing in Oaxaca, Mexico, meets up to 82% of the N needs of the plant (Van Deynze et al. 2018: teosinte may also do this). A wide variety of bacteria has been isolated from the roots of Chrysopogon zizanioides (vetiveria grass) where they are implicated in the synthesis of terpenoids, etc., that are found in the prized essential oils found in the plant (del Guidice et al. 2008). Interestingly, the composition of the bacterial community growing in the rhizosphere of Phragmites australis is determined by the particular lineage of Phragmites involved rather than local soil/environmental conditions (Bowen et al. 2017).
Parasitic rusts and smuts are common on Poaceae, and those on Bambusoideae and Poöideae are particularly distinctive (Savile 1979b); two thirds of Ustilaginales (smuts, basidiomycetes) - close to 600 species - are found on Poaceae (Kukkonen & Timonen 1979; Stoll et al. 2003), and of the remainder, a number live on Cyperaceae. Some seventy species of Berberis are alternate hosts (the aecial stage) for the basidiomycete Puccinia graminis, the black stem rust of wheat and other grain crops in Poöideae; this species (or complex) infects some 77 genera of mostly poöid grasses (Abbasi et al. 2005 and references). Rusts and leaf spot growing on leaves of dominant grasses like Leymus chinensis may affect community composition by their indirect effect on grazers, in this case cattle (T. Li et al. 2023). The fungi increased alkaloid and reduced nitrogen levels in the leaf tissue of their host, although overall they seemed to have rather little affect on the grass, but the cattle disliked the infected grass and grazing on it was reduced so Leymus remained dominant; in the absence of the fungi cattle preferentially grazed Leymus, suppressing it, and overall community diversity increased (T. Li et al. 2023) - c.f. Orobanchaceae.
Sindhu et al. (2008) suggested that the whole PACMAD/BOP clade could be characterized by a gene that protects the plant against toxins produced by the ascomycete Cochliobolus (anamorph Helminthosporium) carbonum; they described the gene as "a guardian of the grass family" (ibid.: p. 1766). This gene could be an apomorphy of the PACMAD/BOP clade, but its wider distribution in monocots is unknown. Schmelz et al. (2014) review terpenoid phytoalexins and their antimicrobial activities in Poaceae, particulatly in the economically important taxa. The pattern of evolution of the Rp1 disease resistance gene family in the PACMAD/BOP clade is complex (Luo et al. 2010). Cyclic hydroxamic acids are widely distributed in the family and confer resistance against a variety of fungal and insect pathogens (Frey et al. 1997).
Vegetative Variation. Betekhtina et al. (2023) noted that the grass roots they examined in the Middle Urals were thin, relatively poorly branched and had short root hairs - c.f. Cyperaceae, Iridaceae, Orchidaceae. Burns (1946) observed that corms/bulbs were to be found particularly in Pooiodeae from the Mediterranean region, but also in Ehrharta (Oryzoideae) and one species of Panicum…
Fusoid "cells", often quite large cavities in the leaf caused by the collapse of small groups of cells, i.e. the initial fusoid cell and its descendants are known from some Poaceae. There are similar cells, the so-called colourless cells, in Joinvilleaceae (e.g. Leandro et al. 2018), but any such cells are lacking in Ecdeicoleaceae, but of course that family lacks much in the way of leaf blades, period. Leandro et al. (2018) suggest that fusoid cells are quite possibly a synapomorphy for the whole [Joinvilleaceae [Ecdeicoleaceae + Poaceae]] clade (and they are so scored above), fusoid cells would have been lost several times in Poaceae, but probably regained, also perhaps several times; present in the APP grade, they are absent from most of the PACMAD clade, common in Bambusoideae, but absent from most of the other members of the BOP clade. Their function is unclear, although it may have something to do with starch metabolism (Leandro et al. 2018 summarise the various functions that have been suggested).
Grasses have hollow stems, but the nodes are more or less solid and there is a nodal vascular plexus (Arber 1919; Hitch & Sharman 1971; Pizzolato 2000); variation here has not been placed in a comparative context. Similar stems are also to be found in Equisetum and Apiaceae and they seem to provide maximum protection against buckling of the stem when it is bent for a minimum investment in tissues (Spatz & Speck 1994; Speck et al. 2003). Thinking of buckling in the stems of Arundo donax, the properties of the stereome and underlying lignified parenchyma were found to form a continuum from a biomechanical point of view and hence both were load-bearing tissues (Spatz & Speck 1994; Speck et al. 1997); leaf sheaths were involved in the support of the young stem. However, overall rather little is known about how grass stems bend (Spatz et al. 1997; Robertson et al. 2015). There has been some study of the role of the leaf sheath in supporting the stem, particularly the region of the latter with the intercalary meristem (Kempe et al. 2013 and references). However, the stem of Zea mays may snap at nodes close to ground level (or sometimes higher up, depending on stem development; this is known as "green snap" or "brittle snap" and was widespread in storms in the mid-west of the U.S.A. in 2020.
Woody bamboos, for example Chusquea, may have a hundred or so branches at a node, all borne in a fan-like arrangement. They are produced by a combination of multiple buds and axillary shoots with very short internodes, all nodes producing branches (see e.g. McClure 1973; Judziewicz et al. 1999; Tyrrell et al. 2012: Fisher et al. 2014 for a phylogeny of Chusquea). The anatomy of bamboos has ; there is no secondary thickening here at all.
Ligules in the ventral or adaxial position of the leaf at the blade/sheath junction are very common in Poaceae. Variation in such ligules is extensive, and quite frequently it is a ring of hairs, especially in the PACMAD clade, or it is membranous (see Edson-Chaves et al. 2023 for ligule "types"), although taxa like Streptochaeta lack a ligule (Judziewicz & Clark 2008). The ligule and auricle together act as a kind of hinge, so the leaf blade is held at an angle to the stem; developmentally, a number of the genes involved in ligule development in maize are expressed elsewhere in angiosperms (bases of leaf, branch) where they mark developmental boundaries (Zhu et al. 2013; Johnston et al. 2014). Some taxa, ezpecially Bambusoideae, have a dorsal ligule (sometimes called a contraligule or external ligule), which is found on the abaxial side of the leaf, the ligule being on the adaxial side (Edson-Chaves et al. 2023); a contraligule s. str. is found in some Cyperaceae, q.v.. Edson-Chaves et al. (2023) looked at the distribution of dorsal and ventral ligules, and the variants of the latter, in the context of the phylogeny of the family.
Gallaher et al. (2016, esp. 2019) discuss leaf morphology and its variation in connection with the degree of shading of the leaf.
Successful grafts have been established between C3 and C4 grasses of the PACMAD and BOP clades, providing that the graft junction is in the mesocotylar area (Reeves et al. 2022).
Genes & Genomes. For the ancestral grass karyotype of seven protochromosomes, see Murat et al. (2017). Genomes in Poaceae are very plastic and extensive changes have been documented (Salse 2016 for literature). As an example, in Oryza, a small genus of about 20 species, Dai et al. (2022) found that the genome size varied from 279-1203 Mb/1 C, the diploid species varying three fold, the polyploid species two fold, but there was no sharp distinction between diploid and polyploid genome sizes; LTR retrotransposons were responsible for much of the variation. Indeed, nuclear genome evolution in Poaceae has been particularly active. Comparisons of expressed sequence tags, etc., suggested that the genomes of Poaceae differ more from the genome of Allium (Asparagales-Asparagaceae-Allioideae) than the genome of Allium does from that of Arabidopsis (Brassicales-Brassicaceae: Kuhl et al. 2004). Overall, rates of genome size evolution and of speciation are very high (Puttick et al. 2015). For an entry into the extensive cytological work that has been carried out on the family, see Kellogg (2015) and also Roodt and Spies (2003), Winterfeld (2006) and Z.-S. Zhang et al. (2018: chromosome numbers for subbasal Poöideae).
As in many other groups, genome duplication is thought to have played a major role in the evolution of the family. A genome duplication, the ρ/rho event, that characterises this clade (P.-F. Ma et al. 2021; Seetharam et al. 2021: Streptochaeta), has been variously dated to ca 70/70-66/70-50/73-56/50-40/ca 19[!!] Ma (Goff et al. 2002; Paterson et al. 2004, 2009; X. Wang et al. 2005; Schlueter et al. 2004; International Brachypodium Initiative 2010; Vanneste et al. 2014a; Landis et al. 2018: the ORSAα event). Indeed this event may best be placed at the level of the whole family (McKain et al. 2016a, see also Gao et al. 2018; J. W. Clark &anp; Donoghue 2018; Seetharam et al. 2021) and thus must be at least as old as the family; Ma et al. (2021) dated the duplication at (115.7-)98.2(-82.7) Ma. However, Zwaenepoel and Van de Peer (2020) questioned the existence of this duplication. Yockteng et al. (2013) date duplication of SEPALLATA genes in Poaceae to around 82-58.2 Ma - and of course ages are being bandied about, and so we have to deal with the issue of what the age of the family might be - see above. Interestingly, there are duplicates of the PISTILLATA (PI) gene pretty much throughout the family (including in Streptochaeta and Pharus), and in one of the PI clades (PI-1) there is predominantly heterodimerization, in the other (PI-2), homodimerisation , and Bartlett et al. (2016) noted that hetero- and homodimerization seems to flip back and forth in the few other Poales examined. Indeed, thinking about any relationship between genome duplication, morphological change and diversification gets complicated (Ma et al. 2021). Although Vandepoele et al. (2003) thought that the duplication may rather be an aneuploidy event, duplication of the whole genome is the generally favoured hypothesis.
There are three genome duplications associated with Oryza sative (Zwaenepoel & Van de Peer 2019; Qiao et al. 2019, etc.). The evolution of a cytosolic ADPglucose phosphorylase, unique to Poaceae and a major component of the starch biosynthesis pathway, has been associated with a deep genomic duplication; this enzyme is normally to be found in the plastid (Comparot-Moss & Denyer 2009; McKain et al. 2016a). Malcomber and Kellogg (2005) suggest that there has been duplication of LOFSEP genes within Poaceae, while the duplication of AP1/FUL gene, apparently in stem-group Poaceae, may be involved in the evolution of the spikelet (Preston & Kellogg 2006; McKain et al. 2016a). Indeed, developmental gene duplication and subsequent functional divergence may have played a major role in facilitating the development of the baroque diversity of inflorescences in the family (Malcomber et al. 2006; Zanis 2007; Y. Wang et al. 2022; see also Doust & Kellogg 2002). L. Zhang et al. (2022) looked at gene clustering and whole genome duplication in Poöideae throughout the Cenozoic, and both seem to have occurred there, the latter in particular some five times in the mid- to late-Miocene. X. Wang et al. (2009) looked at the evolution of genes, variously duplicated, that became involved in C4 photosynthesis in grasses. See also the post-duplication evolution of the NADP-malate dehydrogenase gene (Rondeau et al. 2005), of the API, AG and SEP families, but not genes of the AP3 lineage (Zahn et al. 2005a), DNA-dependent RNA polymerase (Trujillo et al. 2018), and other examples (e.g. Saski et al. 2007). Other small-scale duplications are common, most genes involved retaining their functions, however, a sizeable proportion acquire new functions; the genes tend to have male-biased expression patterns (Jiang & Assis 2019). For the general evolution of genes following genome duplication, see Jiang et al. (2013
Z. Peng et al. (2013) suggested that there had been a genome duplication in the ancestor of the giant bamboo Phyllostachys heterocycla (= P. edulis) dating it to (15-)11.5(-7.7) Ma and Vanneste et al. (2014b) dated what is probably the same duplication to (21.0-)19.7(-18.7) Ma. Z.-H. Guo et al. (2019) discuss the origin of woody bamboos in terms that entail three independent allopolyploid events involving four extinct diploid ancestors (Triplett et al. 2016 suggested that there had been six ancestral gene donors). G. Jin et al. (2021) looked at genome duplication and fast stem growth in bamboos.
Hilu (2004) thought that the base chromosome number for Poaceae might be x = 11, and x = 12 occurs in rice (Oryza), for example, and x = 10 in Panicoideae. Suggested changes in base chromosome numbers here are x = 5 → x = 10 (polyploidy) → x = 12 (two interchromosomal translocations and fusions), or x = 7 → x = 14 (polyploidy) → x = 12 (two interchromosomal translocations and fusions) in the ancestor of the PACMAD/BOP clade, with much subsequent rearrangement, chromosome number reduction, etc. (Bennetzen 2007; Salse et al. 2008, 2009a, b, 2016: c.f. Fig. 1; Bolot et al. 2009; Abrouk et al. 2010; Murat et al. 2010, 2013, 2015b, 2017; X. Wang et al. 2015; Devos et al. 2016; P.-F. Ma et al. 2021: p. 7; Q. Xu et al. 2021). In this scenario, rice has the most slowly-evolving genome in Poaceae (X. Wang et al. 2015), while in Panicoideae there was more genome loss (resulting in x = 10), and then genome duplication (x = 20) and extensive subsequent chromosome loss, inversions, and translocations to return to the x = 10 of extant Panicoideae (summarized in Salse 2016: see also for changes in Poöideae). Ma et al. (2021) suggested that the genome of Pharus, an understory plant, x = 7, had remained largely stable for as much as 42 Ma following the divergence of Pharoideae from other grasses. However, Carta et al. (2020) have recently proposed that x = 6 was the most likely base number for the family, but whether x = 5, 6 or 7, there has been a considerable reduction in chromosome numbers from the x = ≥20 that resulted from the σ/sigma genome duplication event (Ming et al. 2015; Murat et al. 2015). Muffato et al. (2022) noted how the various parts of the 19 or so contiguous ancestral regions of the grass genome that they identified in grasses were disposed in the 12 chromosomes of Oryza. Chiavegatto et al. (2020) discuss the evoution of chromosome numbers in Chloridoideae-Eleusineae.
In Sorghum evidence of fractionation bias and genome dominance is likely to be associated with a duplication, probably rather old, as also in Zea, and in both cases alloploid events are suggested (Garsmeur et al. 2013). The overall genomic multiple within Saccharum is as high as 128 (Wendel 2015), however, after several rounds of genome duplications, there have also been many independent reductions in chromosome numbers (e.g. Schnable et al. 2009; Abrouk et al. 2010; Murat et al. 2013 and references; C. Kim et al. 2014). Within Poöideae there have been independent reductions in chromosome number from n = 12, for example, Brachypodium has n = 5 (International Brachypodium Initiative 2010; Murat et al. 2010). Winterfeld (2006) discussed cytogenetic evolution, mainly in Aveneae (= Poöeae). All Bambusoideae-Arundinarieae appear to be descended from an allotetraploid ancestor (x= 12), the duplication being ca 22 Ma, and there are other polyploidy events in Bambusoideae (Triplett et al. 2011; Z.-H. Guo et al. 2019; C. Guo et al. 2020). Woody bamboos as a whole are all polyploids, but there has been subsequent biased fractionation and from genome size estimates there have also been trends toward diploidization. Interestingly, from the genome sizes given, it is difficult to see obvious correlations between the polyploidy of some of the major bambusoid clades and their genome sizes, indeed, the largest genome is from a member of the predominantly diploid Olyreae, where it perhaps represents a recent polyploidy event (Chalopin et al. 2021). This rather complicates evolutionary scenarios for the group - thus Chalopin et al. (2021) suggest there may have been an early hybridization event between Olyreae and an ancestor of the New World woody bamboos, transposable elements in Olyreae then moving to the New World woody bamboo genome, the Olyreae genome otherwise being largely lost (Chalopin et al. 2021 suggest that Olyreae were also involved in the ancestry of Old World woody bamboos). There are many other duplication events in Poaceae, some quite recent, and most of them clearly involve hybridization. Indeed, hybridization is common in much of the family, perhaps particularly in Bambusoideae and Poöideae, which will be clear when looking at the Phylogeny section below; it has also been involved in the evolution of a number of grain crops in the core Poöideae, as is mentioned under Economic Importance below.
The grass genome has the highest GC content of that of any land plant, as is particularly evident in grasses growing in seasonally stressed grassland biomes, but not in those growing in forests or in wetlands (Smarda et al. 2014; see also X. Wang et al. 2009). For genic GC content in monocots as a whole, perhaps basally bimodal, and not simply in Poaceae, see Clément et al. (2014). T. Zhao and Schranz (2019), looking at genome order across angiosperms, found that overall there was a low proportion of syntenic genes, although many Poaceae had a notably higher proportion.
Many Triticeae have massive genomes in part because of changes in base chromosome number (Jakob et al. 2004). In general, nuclear genome size varies considerably and at least in part independently of chromosome number, both increasing and decreasing (Caetano-Anollés 2005; Smarda et al. 2008; Schnable et al. 2009). Polyploidy in Sesleria (Poöideae) has been followed by reduction in genome size (Lazarevic et al. 2015). Interestingly, Poaceae are somewhat unusual (as a family, but not in the context of all angiosperms) in that they show marked variation in genome size, smaller genomes being more tropical, larger genomes more temperate (Levin & Funderberg 1979; Paterson et al. 2009). Furthermore, the GC content of the genome is notably higher, and heterogeneous within the genome, in Poaceae in particular - there is GC-biased gene conversion (Serres-Giardi et al. 2012).
Recent work suggests that there may be quite extensive horizontal (lateral) gene transfer (HGT) in at least some panicoid grasses - quite large fragments of DNA can be involved - (Dunning et al. 2019). Tettelin et al. (2005) introduced the concept of the pan-genome, with core genes, present in all individuals of a species, and accessory genes, present in only some individuals, and this concept has been extended to gene movement between genomes within grasses (e.g. Tao et al. 2021: Sorghum; Grass Phylogeny Working Group 2024). In general, HGT is quite common in Poaceae, and here reproductive contamination through illegitimate pollination seems a possible cause (e.g. Phansopa et al. 2020; Hibdige et al. 2020/2021; Raimondeau et al. 2023), and as a result there is sometimes quite extensive movement of genes within the family. Thus overall 168 loci in 82 genomic blocks (45 loci were on their own) were found in the five genomes of Alloteropsis examined, there being 2.66-15.74 lateral gene transfers per Ma, of which 11-16(-24)%/Ma were lost (the rate of loss of native genes was 0.04-0.25%/Ma). Raimondeau et al. (2023) ruled out various causes for HGT in grasses, including hybridization (because of the coexistence and lack of synteny between the original and laterally acquired genes in the recipient genome), incomplete lineage sorting, etc.; the mechanism seems likely to be specific to Poaceae, reproductive contamination through illegitimate pollination being the suggested mechanism, both hybridization and HGT being facilitated. Despite the extensive HGT and the prevalence of hybridization here (Grass Phylogeny Working Group 2024: Fig. 2) - 45-80% of grasses are polyploid - this group noted that phylogeny still seemed to be best represented as a tree-like structure, albeit somewhat modified.
Indeed, hybridization and HGT may be facilitated by the ability of just about any pollen grain - even grains of Arabidopsis - to at least germinate on grass stigmas, and zygotes may be formed in crosses between e.g. Poöideae and Panicoideae (Lausser et al. 2010; Kellogg 2015 and references). Kynast et al. (2001) note a number of very wide crosses within the family, some involving Poöideae and Panicoideae as parents, i.e. between plants that have been separated for around (80-)65-45(-30) Ma. This is all very difficult to get one's head around, but the ability of just about any random pollen grain to at least germinate on a grass stigma may contribute... Although the panicoid chromosomes in such wide crosses were usually quickly eliminated, Kynast et al. (2001) were able to produce hybrids between Zea mays and Avena sativa in which chromosomes from Zea were individually incorporated into the genome of Avena, in most cases as disomics, although the plants involved often looked rather unhappy. Note, however, that heterospecific pollen transfer, subsequent growth of the pollen tube down the style of an immediately unrelated species (these are core eudicots that may have been separated fror around 100 My), the pollen tube even entering the ovule of the unrelated plant, i.e. ovule usurpation, has been reported from self-compatible plants in the species-rich Alpine habitats of North America (Cohen et al. 2025) - genera like Linum, Lupinus and Pedicularis were involved. To the extent that there may be links between diversification and hybridization, the general prevalence of hybridization in Poaceae should be noted; for a possible link between genus size, life form and polyploidy, see Hilu (2007).
Hibdige et al. (2020/2021) looked specifically at horizontal gene transfer and found examples in most of the grasses that they examined; it tended to be more frequent in taxa with the rhizomatous habit (see also Dunning et al. 2019; Wickell & Li 2020). Transposable elements, Mutator-like elements (MULEs), seem to have moved fairly recently by horizontal transfer between rice, East Asian bamboos, and a number of Panicoideae-Andropogoneae (Diao et al. 2006), while Stowaway Miniature Inverted repeat Transposable Elements (MITEs) are common in the BOP clade, especially in Poöideae, but not in the PACMAD clade; multiple acquisitions, losses and horizontal transfer events may be responsible for their current distribution (Minaya et al. 2013). Nuclear ribosomal genes from five genera of Panicoideae have been found in Poöideae, especially in Hordeum section Stenostachys. These transfers have happened within the last 5 Ma, but none of the genes are functional; how this happened, and the overall extent of such events in angiosperms, are unclear (Mahelka et al. 2017). M. Park et al. (2021) compared the genomes of 19 previously uncharacterised panicoids with those of 115 species of plants looking for the horizontal transfer of transposable elements such as LTR retrotransposons, and found it between these panicoids and Oryza; some 48 events involved Echinochloa, on which they focused, and O. punctata in particular, and they suggested that aphids, root grafting or other mechanisms might mediate in the transfer.
The sequential transfer of genes over a period of millions of years from quite unrelated grasses, perhaps via movement of genes from pollen of a grass that lands on the stigma of a plant that it cannot pollinate, may explain the nature of the C4 pathway in Alloteropsis (Panicoideae). Initially no other genes seemed to be involved, and a taxon embedded in the clade has ordinary C3 photosynthesis (Christin et al. 2012a). However recent work on A. semialata suggests that there has been the transfer of some 26 genomic fragments containing 59 genes and involving a minimum of nine donors; all but one of these donors were other Panicoideae, although not immediately related, and the other one was a chloridoid (Dunning et al. 2019; Wickell & Li 2020). Transferred genes were functional, and were involved in photosynthesis, disease resistance, etc.; functional transfers were also found in three other panicoid and one chloridoid genera (Dunning et al. 2019; see also Wickell & Lei 2019). Thus in A. semialata the C4 photosynthetic pathway included mostly "native" genes, but also an important photosynthetic gene that had been acquired from another panicoid (see also Dunning et al. 2019; Dunning & Christin 2020). Phansopa et al. (2020) suggested that HGT of core C4 genes in Alloteropsis on occasion resulted in the replacement of the original C3 versions (for HGT here, see also Raimondeau et al. 2023). Bianconi et al. (2020) describe recent work on this plant; the ploidy level ranges from diploid to duodecaploid. For more on HGT in Poaceae, see Pereira et al. (2022).
There has been substantial evolution in the plastome of Poaceae (Leseberg & Duvall 2009; Guisinger et al. 2010; Burke et al. 2016b for literature), although details as to where on the tree (and so when) particular changes occurred await more extensive sampling both in Poales and in "basal" Poaceae; the rate of plastome evolution may have now slowed down. These rate changes are placed at the level of Poaceae as a whole above, although they might more correctly be put at the PACMAD/BOP node... There seem to be higher substitution rates in the plastome in herbaceous Olyreae compared with other Bambusoideae, which are predominantly woody (Wysocki et al. 2015), although Y.-J. Zhang et al. (2011) found rather little variation and slow evolution of the plastomes in the Bambusoideae they examined. More recently, W. Wang et al. (2020a) confirmed the substantially higher plastome evolutionary rates in Olyreae, somewhat intermediate rates in Bambuseae, and the lowest rates in Arundinarieae (?significance - see Divergence & Distribution above). Morris and Duvall (2010) discuss aspects of plastome evolution with a focus on Anomochloa, and Duvall et al. (2020) look at the influence of alignment gaps in the plastome on the topology of the PACMAD clade. For a series of inversions in the single copy region and expansion of the inverted repeat at the single copy-inverted repeat boundary, see Hiratsuka et al. (1989) and Saski et al. (2007). For the plastome accD gene loss, see Kinoshi et al. (1996) and Katayama and Ogihara (1996), for deletions, etc., in the 3' end of the mat K gene, see Hilu and Alice (1999), for loss of introns in the plastome, see Daniell et al. (2008) for references, and for subrepeat size in the PEP subunit β" rpoC2 insert region, see Jones et al. (2014). The highly conserved streptophyte version of the ycf1 and 2 genes have been lost in Poaceae, although the exact extent of this loss is unclear (certainly in the PACMAD/BOP clade, but deeper?), as is the very function of this gene (Jansen et al. 2007; Guisinger et al. 2010; de Vries et al. 2017a; see also Nakai 2015).
The coxII.i3 intron of the chondrome has developed a moveable element-like sequence (Albrizio et al. 1994), but there is a fair bit of variation in other monocots, too. There appears to have been horizontal gene transfer from the chondrome to the plastome in Bambusoideae-Olyreae (Wysocki et al. 2015; Gandini & Sanchez-Puerta 2017), while the rpl5 gene has moved from the chondrome to the nucleus three times, the movement being facilitated by an earlier retroprocessing event in which all but one of the RNA editing sites in this gene were lost (Z. Wu et al. 2017). The rps16 and rps14 genes have been lost from several members of the PACMAD clade (Lei et al. 2013), but I am unsure of the extent of these losses.
Y. Chen et al. (2023) looked at the overall movement of genes from organelles to the nucleus in the family (67 genomes from 15 species examined) and found that genes from the chondrome were more likely to move than those from the plastome. Most of the movement was recent, and after the move genes became more likely to be lost or mutate; nuclear—organellar gene movement was highest in Triticeae.
Economic Importance. Glémin and Bataillon (2009) and Fuller (2009) take a comparative viewpoint and look at how grasses in general have evolved under domestication; see also papers in Front. Plant Sci. anno 2021 - "Grass genome evolution and domestication". Sang (2008) noted that single genes are involved in a number of major morphological transitions in the domestication of grains, such as the development of non-shattering rhachises, important when harvesting (see above). Hua et al. (2014) discuss the molecular basis of the loss of barbs on the awns and the shortening of the awns in the context of domestication of Oryza. The initial transition from wild plants to domesticates may be a gradual and quite slow process, as with other crop plants (Purugganan & Fuller 2011), and in barley at least there is a complex pattern of movement of genes from local wild populations into landraces (Poets et al. 2015). An important plant for the study of C3 cereals is Brachypodium (Girin et al. 2014; Catalán & Vogel 2020 and papers in New Phytol. 227(6). 2020; Hasterok et al. 2022), although its exact relationships within Poöideae are unclear.
Many cereals are C3 plants. Among the C3 domesticates, several are Poöideae-Triticeae, which include important grain genera such as Triticum (wheat), Hordeum (barley) and Secale (rye). Wheat (mostly Triticum aestivum) provides around one fifth of the calories eaten by humans and it began to be domesticated ca 10,000 years ago; see Israel J. Plant Sci. 55(3-4). 2007, for an entry into the literature on domestication, also Fuller (2007), Baum et al. (2009) and Mason-Gamer and White (2024), both haploid genomes, and Carver (2009) and J. Syst. Evol. 52(6). (2014), both general. Most domesticated forms are polyploid and are old hybrids (for polyploidy in wheat, see Gornicki et al. 2014 and references, and for hybridization, see Glémin et al. 2019), and genome plasticity in connection with this polyploidy has been implicated in the success of wheat in cultivation (Dubcovsky & Dvorak 2007). Edet et al. (2018) worked out genomes of diploids and polyploids in the Triticum-Aegilops area using genotyping-by-sequencing methods. For the domestication of barley, Hordeum vulgare, see Fuller (2007), Pourkheirrandish and Komatsuda (2007) and Azhaguvel and Komatsuda (2007), for hybridization, etc., see Brassac et al. (2012), and for a series of papers on Triticeae, see J. Syst. Evol. 52(6). 2014. Naming of such hybrids is not simple, in part because the generic attributions of a number of the parental species involved is unclear, indeed "There seems to be only two rational treatment[s] for such a PPC [polploid pillar complex]: one is to lump all involved genera into one, which will evidently ruin the current nomenclature; the other is moderately splitting taxa containing different genomes, treating the polyploid genera as “hybridogenous” ones." (Z.-H. Feng et al. 2024: p. 17). Taking the latter course of action, the addition of the following nothogeneric names seemed to be reasonable (Z.-H. Feng et al. 2024): × Agrothinopyrum Su Liu & Bing Liu = Agropyron Gaertner × Thinopyrum Á. Löve, × Campeiordeum Z. H. Feng & Su Liu = Campeiostachys Drobow × Hordeum L., × Dasyticum Su Liu & Bing Liu = Dasypyrum (Coss. & Durieu) T. Durand × Triticum L., × Elyleymus B. R. Baum = Elymus L. × Leymus Hochst., × Leydeum Barkworth = Hordeum L. × Leymus Hochst., × Psammopyrordeum Su Liu = Hordeum L. × Psammopyrum Á. Löve, × Pseudelymus Barkworth & Dewey = Elymus L. × Pseudoroegneria (Nevski) Á. Lüve, × Sibirokoeleria Su Liu & Z. H. Feng = Koeleria Persoon × Sibirotrisetum Barberáett al., × Thinoleymus Su Liu & Bing Liu = Leymus Hochst. × Thinopyrum Á. Löve, × Triticosecale A. Camus = Secale L. × Triticum L., and × Tritipyrum Curwen-McAdams et al. = Thinopyrum Á. Löve × Triticum L. ... (Hybridization and genome evolution in grasses in general are discussed in both the Genes & Genomes and in the Phylogeny sections above.)
Another major C3 grain is rice (Oryza spp.). For information on the complex history of domestication of rice, which occurred in both Africa (perhaps northern Mali), Asia and South America, see Sweeney and McCouch (2007), Fuller (2007), Cubry et al. (2018), Iriarte et al. (2020) and references. Shenton et al. (2020) discuss the different genomes to be found in the genus, especially the C genome, while W. He et al. (2021) analysed 1,445 plastomes, including 1,135 from O. sativa and 295 from O. rufipogon. They suggested that introgression from japonica and aromatica to indica and aus rice cultivars had been common; there seems to have been a single origin of japonica but several of indica rice.
Sorghum and Zea (Panicoideae) are the most important C4 grain genera. A genome duplication in a clade that includes Zea is dated to (21-)20.4(-19.7) Ma (Vanneste et al. 2014a). Another genome duplication/hybridization in the ancestor of Zea occurred ca 4.8 Ma (Swigonová et al. 2004), and the clades involved may have diverged ca 11.9 (Swigonová et al. 2004) or ca 20.5 Ma (Gaut & Doebley 1997). The domestication of maize seems to have occurred in seasonal tropical forests in southwestern Mexico, perhaps the Balsas valley, some 8,700 years ago (Piperno et al. 2009; Ranere et al. 2009: summarized in Hastorf 2009; Grimaldo wet al. 2018); for a detailed summary of all aspects of maize biology, see Bennetzen and Hake (2009) and for important genes involved in the shift from wild plant to domesticate, including major changes in branching pattern and expression of sexuality, see Doebely (2004) and Dong et al. (2017 and references). Interestingly, yield per plant has changed little over the course of domestication - there are lots of small ears in teosinte (Zea mays ssp. parviglumis), just a few large ears on maize itself (Yang et al. 2019: q.v. for comparison of the genetic architecture of the two), however, maize plants grow closer together in cultivation than teosinte does in the wild, and that has lead to an increase in yield.
Sorghum bicolor and Saccharum officinarum can be hybridized (e.g. Nair 1999). For the domestication of sorghum (Sorghum spp., inc. durra), see Dillon et al. (2007). For the domestication of sugarcane (Sacc. officinarum) in New Guinea, see Dillon et al. (2007). Welker et al. (2019), Vorontsova et al. (2019) and others have looked at the immediate relatives of Saccharum. In an analysis of 31 characters taken from external morphology Vasquez et al. (2022) recovered three groups - Saccharum s. str., and Tripidium, Miscanthus, etc., both including Old World taxa, and Erianthus, New World taxa. Vorontsova et al. (2019) found that Miscanthus is close to Saccharum, while Lasiorhachis, which used to be included in Saccharum, is sister to Sorghum section Sorghum in several analyses, and Sarga, which used to be in Sorghum, was very much polyphyletic in the Sorghum part of the tree, in particular in the analysis using three plastid regions, but close to [Sorghum + Lasiorhachis] in a tree based on non-coding plastome data.
Fuller (2007) discussed the domestication of pearl millet (Pennisetum glaucum). The ecologically-important Themeda and Heteropogon are phylogenetically entwined, while Cymbopogon and H. triticeus are sister taxa in plastome analyses (Arthan et al. 2021). For Setaria, which includes foxtail millet, see Doust and Diao (2016).
In these grain crops, as in other Poaceae, the outer layer or layers of the endosperm (the exact number depends on the species, where in the seed one is looking, plant genotype, etc.) make up the aleurone. These cells are distinct from the other endosperm cells, both in morphology - the calls tend to be larger, and in physiology - relatively less starch is stored in aleurone cells than in other endosperm cells, but they include more proteins (in "aleurone grains"), lipids and minerals, the fibre content is higher, and the cells are involved in seed dormancy, etc. (Liang et al. 2025). Planting aleurone-less wheat alone might yield some additional 50 million tons of carbohydrates, however, it is clear that other dietary benefits would be lost (Liang et al. 2025). This is an area of active research.
Given the major importance of grain crops, knowing about their parasites and their control is essential. Thus wheat rusts, e.g. Puccinia triticina, can cause major problems, see The Wheat Rust Fungi, a series of papers from 2017-2020 published by the British Society for Plant Pathology. One way of controlling P. triticina rusts is by getting rid of the alternate host, Berberis vulgaris (e.g. Barnes et al. 2020). Magnaporthe grisea, M. oryzae s. str. (rice blast fungus, rice rotten neck, rice seedling blight, blast of rice, oval leaf spot of graminea, pitting disease, ryegrass blast, Johnson spot, neck blast, wheat blast and Imochi - from Wikipedia iv.2025), causes very serious blast diseases in a number of grain crops, as do Colletotrichum (= Glomeralla, the anamorph/asexual stage) graminicola and other species of the genus, all sordariomycetes. I.a. they interfere with phosphate signaling in the host grass and exacerbate disease symptoms (McCombe et al. 2025).
Sugar and monocot grain crops from West Africa to Southeast Asia can become severely parasitized by Striga - S. hermonthica and S. aspera in Africa and S. asiatica to the east (e.g. Bellis et al. 2020, 2021; see also Orobanchaceae). Indeed, all but one of the ca 30 species of Striga parasitize monocots, and in Africa an area of cereal crops the size of Spain was infested with Striga spp. - only 5 species were commonly involved (Spallek et al. 2013). Rice is attacked i.a. by the plant hopper Nilaparvata lugens and caterpillars of the moth Chilo suppressalis. Interestingly, the two cooperate, and they prefer to attack plants that the other is already attacking since they both affect the plant's defences to the benefit of the other (Q. Liu et al. 2021). On the other hand, genes from Epichloë, a common endophyte in Pooideae (see above for details), have sometimes moved on to Pooideae like barley, wheat and rye via horizontal gene transfer (e.g. Shinozuka et al. 2020). Thus Fusarium causes head blight in wheat (and also barley and oats), but the trichothecanes produced by Fusarium are detoxified by Fhb7, a gene originally from Epichloë and obtained from the wheat relative Thinopyrum elongatum (H. Wang et al. 2020).
Another factor affecting grain production is the inability of the plant to flourish on saline soils of various types - such soils occupy large areas of the globe and are not generally conducive to crop growth. This is discussed above.
Finally, Poaceae are more common than would be expected among clades that are invading natural areas (see also Pysek et al. 2017), being "perhaps the most spectacularly over-represented among both serious and widespread weeds" (Daehler 1997: p. 171), and they include around 23% of the 200 most widely naturalized plants (Pysek et al. 2017). As D'Antonio and Vitousek (1992) observe, introduced grasses affect everything from local community interactions to ecosystem processes. They are effective competitors for light and water, so i.a. affecting the growth of tree seeedlings and the woodland-grassland boundary, they also greatly increase the frequency of fires, and this, coupled with the low N content of many grasses, especially C4 grasses, can effect the N cycle, reducing the availability of N for native planta (see also Cornwall 2022). And with the invasion of exotic grasses there are also often major effects on the local fauna (D'Antonio & Vitousek 1992). Cornwall (2022) drew attention to six particularly important invasive grasses from Europe and Africa that were having severe effects on the communities in many areas of the world into which they had moved. The grasses were Pooideae (Bromus tectorum and Ventenata dubia) and Panicoideae (Andropogon gayanus, Cenchrus ciliaris, Imperata cylindrica and Melinia minutifloa) (see also Fusco et al. 2019: U.S.).
Chemistry, Morphology, etc.. There have been several comprehensive surveys of many aspects of grass morphology, anatomy, cytology, etc., over the years. Although by no means all of these really useful early surveys are cited below, they are easily traceable in the literature. For more on cell walls in grasses and their relatives, see Vogel (2008), also under commelinids (i.e. above) and monocots as a whole (see Acorales).
ADP-glucose pyrophosphorylase, involved in starch synthesis, is present largely in the cytosol, not in the plastids, in the endosperm of members of the PACMAD/BOP clade. However, it occurs in plastids in the starch-storing organs of other seed plants, probably even including the starchy endosperm of other commelinid monocots, but the sampling here, too, is poor (Beckles et al. 2001; Comparot-Moss & Denyer 2009). Finally, Ellis (1999) carried out an extensive survey of tannin-like substances (TLS) found in grass leaves and with a focus on taxa from southern Africa. TLS are particularly common in Andropogonoideae-Andropogoneae and -Arundinelleae, particularly uncommon/absent in Poöideae and Bambusoideae (Oryzoideae were not sampled). Although absence of TLS is perhaps likely to be the derived condition (c.f. Linder et al. 2017b), sampling is currently too poor to understand the overall pattern of variation, not to mention that one would like to know more about their actual identity. For variation in fructan type in Poöideae, which may be correlated with clades there, see Bonnett et al. (1997). R. Dick et al. (20102) discuss the synthesis of benzoxazinoids, common in much/?all of the family, and scattered in broad-leaved angiosperms. However, convergence seems to be involved, the genes being homologous but not orthologous (gene duplication?). The enzymes involved are notably promiscuous, and benzoxazinoids are apparently protective and alleloptahic (Dick et al. 2012; Moghe & Last 2015).
Brachypodium has a metaxylem element in the very centre of the pith in the root stele which is otherwise unremarkable for a monocot, having about eight vascular bundles in a ring, etc. (Pacheco-Villalobos & Hardtke 2018: other taxa?). Vanneste and Beeckman (2020: p. 728) noted that lateral roots were initiated in the periucycle "in front of phloem poles or between the phloen and xylem poles", not at the xylem pole because the pericycle was interrupted. The division resulting in the trichoblast/atrichoblast pair in roots may be asymmetric (Poöideae) or not, and if it is symmetric, the subsequent development of the two cells may vary (C. M. Kim & Dolan 2011). The sampling is poor, with no species from the basal pectinations and only one species each in Oryzoideae and Bambusoideae examined (Row & Reeder 1957: exceptions are no longer so; Kim & Dolan 2011); see also D. W. Kim et al. (2006 for root hair development). Microhair variation in the family is extensive and of some use in delimiting major groups (Liphschitz & Waisel 1974; Amarasinghe & Watson 1988, 1990; Liu et al. 2010; Oli et al. 2012). Stomatal development in the leaf blade (mcKown & Bergmann 2020) is basipetal and chloroplasts can move between the guard cells (Cullen & Rudall 2016: ?level). The leaf blades of Neurolepis (Bambusoideae) may be up to 4 m long. Tao et al. (2019) explore the possibilities of identifying woody bamboos by their phytoliths; the prospects seem rather dubious if the identification is blind and fossils are included. Bahadur et al. (2019b) discuss various aspect of chirality in the family, including the handedness of the first seedling leaf.
For inflorescence morphology and development, see Kellogg et al. (2004, 2013), Kellogg (2017), Y. Wang et al. (2022), etc.. Developmental gene duplication and subsequent functional divergence may have played a major role in facilitating the evolution of inflorescence diversity in the family (Malcomber et al. 2006; Zanis 2007; see above, also Doust & Kellogg 2002; Reinheimer & Vegetti 2008; D. Zhang & Yuan 2014; Muchut et al. 2018); Whipple (2017: p. 368) mentions the importance of "interacting signaling centres that coordinate determinancy of adjacent meristems". Reinheimer et al. (2013) discuss the evolution of inflorescences in Panicoideae and Pilatti et al. (2018) that in Chloridoideae-Cynodonteae. Spikelets of closely related species in Andropogoneae may vary in features of both early and late development (Hodge & Kellogg 2014). Where and when abscission/shattering occurs in grass spikelets varies considerably, and the development of non-shattering spikelets is of course important in the domestication process (e.g. Doust et al. 2014; Y. Yu & Kellogg 2018). The duplication of AP1/FUL gene, apparently in stem-group Poaceae, may be involved in the evolution of the spikelet (Preston & Kellogg 2006; McKain et al. 2016a).
Ciaffi et al. (2011) and Kellogg (2015) summarize floral development. Unfortunately, the immediate relatives of Poaceae are somewhat unclear, and within Poaceae, the flowers of Anomochloa are sui generis, and those of Streptochaeta are not that simple (see also Judziewicz & Soderstrom 1989). Flowers of Streptochaeta can be interpreted as having an outer perianth whorl of two (adaxial) members - c.f. the single, bicarinate palea (there are sometimes three members in this outer whorl), and an inner perianth whorl of three members - c.f. the lodicules. The three stamens common in grass flowers would then be those opposite the three members of the outer perianth whorl (see Whipple & Schmidt 2006; Sajo et al. 2008: c.f. diagrams; Preston et al. 2009; Reinheimer & Kellogg 2009 for further details); Seetharam et al. (2021) suggested that the morphology of Streptochaeta and Anomochloa may in fact be derived from a more conventional grass flower. Although Sajo et al. (2011, esp. 2012) tentatively described the flowers of Anomochloa as having paleas and lemmas (bracteoles and prophylls respectively), lodicules, etc., working out similarities between its flowers and those of other monocots around here is difficult. The flowers of Ecdeicolea in particular are notably monosymmetric, with the two adaxial tepals of the outer whorl larger and keeled, and comparable differentiation in the outer perianth whorl occurs in Xyridaceae (q.v.), although these are likely to be parallelisms; there the lateral stamens are suppressed.
The grass palea, which is often bicarinate, has often been interpreted as being prophyllar/bracteolar in nature, monocots commonly having bicarinate prophylls. However, in this scenario bracteoles would probably have had to be regained since the immediate outgroups to Poaceae lack them. In early studies of gene expression, the palea and perhaps even lemma appeared to be calycine in nature while the lodicules were corolline (Ambrose et al. 2000); A-type genes are expressed in both the palea and lemma (Whipple & Schmidt 2006). General comparative morphology suggests that the lemma is a bract and the palea represents two connate tepals of the outer whorl, and, consistent with this interpretation, the lemma and palea are inserted at different levels in the flower (Schrager-Lavelle et al. 2017 and literature; Patterson et al. 2023); add the fact that there is only a single functional carpel, the flowers of Poaceae are thus monosymmetric (see e.g. Bartlett et al. 2015). However, this interpretation of the palea and lemma in the preceding sentence is not always accepted (e.g. Hirano et al. 2014: rice). Although the tepaloid nature of the lodicules is relatively uncontroversial (see Sajo et al. 2007; Reinheimer & Kellogg 2009; Yoshida 2012; Glover et al. 2015), they can be surprisingly (for a non-agrostologist) variable (Wölk & Röser 2014). In thei review of the recent literature on the identity of th outer parts of a grass flower, Patterson et al. (2023) note that they may be intermediate-type structures, the lemma being part bract, part sepal
It is difficult to understand the arrangement of the pollen grains in the small anthers of Streptochaeta; they may be peripheral, at least initially (Kirpes et al. 1996; Sajo et al. 2009). Pacini and Franchi (1991) noted that the anthers of Poaceae were narrow, all the grains directly abutting the tapetum - peripheral as defined here.
Most Poaceae have two stigmas, but the single ovule represents the third (= abaxial) carpel (Kircher 1986). The gynoecium is often annular in early development, although in Pharus it is quite strongly 3-lobed (Sajo et al. 2007). Ovules both with and without parietal tissue are reported for grasses, but reports of the former (e.g. Guignard 1882) need confirmation. Indeed, although parietal tissue is likely to be absent, a nucellar cap is quite commonly developed (de Triquell 1987; Bhanwra 1988; Rudall et al. 2005a; see also Nakamura et al. 2009). Various aspects of ovule morphology, e.g. the extent of development of the outer integument - for instance, it is little developed in Panicoideae, well developed in Poöideae and Chloridoideae, and details of just how the ovule is bent, etc., seem to have some phylogenetic signal (de Triquell 1987; Bhanwra 1988). The caryopsis, in which the seed coat and pericarp are fused, is often described as being a distinctive fruit type of the Poaceae; one can perhaps think of it as being a variant of an achene. However, as Nakamura et al. (2009 and references) note, grass fruits develop in a variety of ways (see also Pollination and Seed Dispersal). Guérin (1899) early suggested that the persistent part of the seed coat was tegmic, and he sometimes showed the exotegmen in particular as having quite large cells, or quite thick walls.
All Poaceae have a well-differentiated lateral embryo with a scutellum, coleoptile, coleorhiza, and often mesocotyl (Pankow & von Guttenberg 1957; Tillich 2007). For embryo morphology, variation and evolution, see e.g. van Tieghem (1897a: grass embryos quite different from those of other monocots), Yakolev (1950), Reeder (1957) and C. C. Baskin and Baskin (2021). Reeder (1957) emphasized the course of the vascular system, epiblast presence, fusion of scutellum with coleorhiza and appearance of the first leaf in t.s. when recognizing six different kinds of embryo in the family; these embryo types were subsequently adjusted (Reeder 1962), one being merged and a new type recognized. The scutellum is the distinctively-shaped haustorial part of the single cotyledon of other monocots (= the haustorial cotyledonary hyperphyll if one wants to be technical), and the coleoptile is the sheathing base of the cotyledon, thus the scutellum and coleoptile originate on the same side of the embryo (Kaplan 1997: 1 ch. 5; Takacs et al. 2012). The mesocotyl could be an elongating nodal region or a structure that represents (part of) the hypocotylar region to which the cotyledonary stalk is adnate, while the coleorhiza may be part of the hypocotylar region. Kaplan (1997: 1 ch. 4, 5) thought that the "radicle" was endogenous in origin and was really a lateral root; Poaceae thus lacked a radicle proper, and so were homorhizic. Alpha prolamin genes, involved in the synthesis of seed storage proteins, evolved in Panicoideae-Andropogoneae 26-21 Ma (Xu & Messing 2008).
Kellogg (2015) provided a comprehensive account of the family, while Arber (1934) remains a classic treatment; see also A. Chase (1964) for an introduction, Bell and Bryant (2008) for a good general treatment of grass morphology; McClure (1966) gives an account of bamboos, and see also Clark (1997), Clark (1997), Judziewicz et al. (1999), Judziewicz & Clark (2008), the Bamboo Phylogeny Group (2012b), Clark et al. 2015), Liese and Kohl (2015), Wong et al. (2017: Malesian and S.W. Pacific Bambuseae), and Sánchez-Ken et al. (2007: Micrairoideae).
For the occurrence of ergot alkaloids, see Gröger and Floss (1998), for pyrrolizidine alkaloids, see Nurhayati et al. (2009), for cell wall composition; see Fincher (2009), for non-starch soluble storage polysaccharides in the seed and fructans in vegetative parts, see MacLeod and McCorquodale (1958), Meier and Reid (1982) and Shen et al. (2023), for anatomy, see Metcalfe (1960), Cox (2017: functional root anatomy), Schweingruber and Berger (2017: culm anatomy, ca 300 Central European species), Lima et al. (2019: Olyrinae), Leandro et al. (2019: what to look out for in Bambusoideae), also Piperno and Pearsall (1998), Piperno and Sues (2005), Piperno (2006), Rudall et al. (2014) and Lima et al. (2019), all phytoliths, SiO2 bodies, and their distribution, complex typology, and H.-Q. Yang et al. (2008a: foliar epidermis). For inflorescence morphology and development, see Vegetti and Anton (1996), Vegetti and Weberling (1996 and references: classical approach), Perreta et al. (2009), B. E. Thompson and Hake (2009) and Pilatti et al. (2019: esp. Cynodonteae), for floral/spikelet evolution, see Yuan et al. (2009) and Thompson et al. (2009), for aerial branching, Malahy and Doust (2009), for pollen in Chloridoideae, see Liu et al. (2004) and for a quantitative analysis of grass pollen, see Mander et al. (2013), for the style of Triticum, see B. L. Li and You (1991), for ovules, see e.g. Bhanwra and Sharma (1991, 2001) and Verboom et al. (1994: Danthonioideae), for proliferating antipodal cells, Anton and Cocucci (1984 and references) and C.-C. Wu et al. (2011), for the development, etc. of cereal grains, see Batygina (1987b), for endosperm and its development, see Olsen (2007) and Sabelli and Larkins (2009) and for the morphology of starch grains in the endosperm, see Shapter et al. (2008).
Phylogeny. General. For overviews of the phylogeny of the family, see Kellogg (2000a, 2015) and the Grass Phylogeny Working Group (2001, 2011, 2024). Duvall et al. (2010) provide a preliminary tree based on whole chloroplast genomes, Ruhfel et al. (2014) looked at the genomes of some 35 taxa, the general relationships they found being those that are discussed below, Z.-D. Chen et al. (2016) looked at the relationships of ca 450 Chinese species of the family, and J.-H. Lee et al. (2022) those of 145 accessions of Korean taxa using chloroplast genes. In a multi-gene study, Bouchenak-Khelladi et al. (2008) did not find strong evidence for the monophyly of Anomochloöideae, Streptochaeta possibly being sister to all other Poaceae. Much work on the family has involved analyses of chloroplast genes, but extensive data from nuclear genomes is now being examined (e.g. W. Huang et al. 2022;), and by and large conflict with the plastome topology is not great (Grass Phylogeny Working gtoup 2024). In a number of groups, perhaps particularly in Bambusoideae and Pooideae-Poodae, there has been very extensive hybridization (see Grass Phylogeny Working Group 2024: Fig. 2 for the impressive extent of nuclear gene reticulation).
Relationships of the major clades within the PACCMAD and BEP clades (as they used to be called) were initially largely uncertain. The relationships of Poöideae (Hodkinson et al. 2007; Duvall et al. 2008a) and Oryzoideae (as Ehrhartoideae in earlier literature) have been unclear in some analyses (e.g. Cahoon et al. 2010; Saarela & Graham 2010; c.f. J. I. Davis & Soreng 2008), and Christin et al. (2008a) even found that the BEP clade was paraphyletic with the PACCMAD clade embedded in it. Relationships within the PACCMAD clade have remained particularly difficult (Saarela & Graham 2010: sampling). The recent 250-plastome phylogeny by Saarela et al. (2018) - data carefully examined, and see their Table 5 for conflicts in topology arising from analyses of different data partitions - recovered most of the strongly supported relationships found in previous plastid analyses. However, there is the recurrent problem of disagreement between nuclear and chloroplast trees to bear in mind. Indeed, in the analysis of W. Huang et al. (2022: >1200 low copy nuclear genes, 231 genera) the bigeneric Puelioideae were recovered as paraphyletic, i.e. [Anomochooideae [Pharoideae [Guaduella [Puelia [PACMAD + BOP]]]]] - at the base of the [PACMAD + BOP] clade; support was good. Although the Grass Phylogeny Working Group (2024: 331 nuclear genes, 1,133 spp., 79 genera; plastome data from 893 spp., 408 genera) found largely similar topologies using these different data, only 8 taxa having problematic placements at the tribal/subfamilial level - the problems included this possible paraphyly of Puelioideae, paraphyly of Arundinoideae-Micrairoideae (sister taxa with plastome data), and polyphyly of Pooideae-Diarrheneae (monophyly using plastome data).
The PACMAD Clade.
The Grass Phylogeny Working Group II (2011 and references) found strong support for many of the relationships in the PACMAD (as it is now called) clade, i.e. for the topology [Aristidoideae [Panicoideae [[Arundinoideae + Micrairoideae] [Danthonioideae + Chloridoideae]]]], although support for the first two branches was weak (see also Spriggs et al. 2014). Indeed, the positions of the first two subfamilies above were reversed in the complete plastome analysis of Cotton et al. (2015), being [Panicoideae [Aristidoideae ...]], although other relationships were the same and support was mostly strong, however, relationships were scrambled in mitochondrial analyses. Burke et al. (2016a) also found support for this reversed position, as did Duvall et al. (2016) and Teischer et al. (2017), although the latter noted that the position of Aristidoideae was sensitive to the inclusion of gaps; see also Saarela et al. (2018). Thus in a plastome analysis Duvall et al. (2019) found that without alignment gaps Panicoideae was placed with moderate support as sister to all other subfamilies, but as gaps were sequentially added, Aristidoideae finally became sister - although with poor support. Givnish et al. (2018b) recovered a weakly supported [Aristidoideae + Panicoideae] clade in some analyses. There was support for the [Danthonioideae + Chloridoideae] clade in Bouchenak-Khelladi et al. (2008; see also Pirie et al. 2008), and the [[Arundinoideae + Micrairoideae] [Danthonioideae + Chloridoideae]] clade is often quite well supported (e.g. Saarela et al. 2018). It is largely the positions of Aristidoideae and Panicoideae that are at issue, although W. Huang et al. (2022) noted that basal relationships might be [Aristidoideae [[Micrairoideae + Panicoideae] ...]] or [Aristidoideae [Panicoideae [Micrairoideae ...]]]. In the transcriptome analysis of H. Wang et al. (2024) the relationships suggested were [Aristidoideae [[Panicoideae + Micrairoideae] [Arundinoideae [Danthonioideae + Chloridoideae]]]], although support tended to be weak and sampling was exiguous.
Panicoideae. For discussions of the relationships - close and becoming ever more entwined - between Panicoideae and the old Centothecoideae, see Duvall et al. (2008a) and especially Sánchez-Ken and Clark (2008); the two subfamilies are now combined (e.g. Sánchez-Ken & Clark 2010; Teerawatananon et al. 2011), hence PACCMAD → PACMAD - C. Silva et al. (2015) provide a summary phylogeny. Burke et al. (2016a) found a rather weakly supported clade including Centotheca and Chasmanthium to be sister to the rest of the subfamily, and then sister to the remainder was the C3 Lecomtella, however, the latter appears to be an old hybrid and its position varies in different analyses; as Besnard et al. (2013: p. 1062) noted, it has "morphological features of no known functional signficance" (see also Besnard et al. 2018). [Alleochaete + Dichaetaria], the latter from Arundinoideae, were sister to those Panicoideae that were included in the analysis (Teischer et al. 2017). Other relationships include [Paspaleae [Arundinelleae + Andropogoneae]] (e.g. Washburn et al. 2015). For more information on relationships within Panicoideae, including those of some of its constituent genera, see papers in Aliso 23: 503-562. 2008). The C3 [Jansenella + Chandrasekhariana] clade (= Jansenelleae) is sister to Andropogoneae (Hackel et al. 2018; Besnard et al. 2018) or to [Andropogoneae + Arundinelleae] (Welker, McKain et al. 2020).
For relationships within Paniceae, see Zuloaga et al. (2000), Gómez-Martínez and Culham (2000) and Morrone et al. (2010, esp. 2012). Core subtribes include [Panicinae [Melinidinae + Cenchrinae]] (Washburn et al. 2015: some conflict between the genomic compartments). For the bristle clade of Paniceae, see Doust et al. (2007), and for relationships within Panicum itself, see Aliscioni et al. (2003) and Sede et al. (2008); see also Sede et al. (2009) and Zuloaga et al. (2015) for new genera, etc.. Paniceae-Cenchrineae include Pennisetum, in which Cenchrus is embedded (see Donadío et al. 2009; Chemisquy et al. 2010), and also Setaria, which is paraphyletic or worse (see Kellogg et al. 2009; Kellogg 2016b). Salariato et al. (2010) examined relationships within Paniceae-Melinidinae, particularly from the point of view of inflorescence evolution. E. J. Thompson and Fabillo (2021) discuss the composition of Paniceae-Neurachninae. For the phylogeny of Andropogoneae, see Kellogg (2000c), Mathews et al. (2002) and Welker, McKain et al. (2020); "Andropogon" and Schizachyrium remain (as of ii.2021) scattered throughout the tree, taxa like A. burmanicus being particularly isolated (Arthan et al. 2017; McAllister et al. 2018). For Saccharinae and Sorghinae, perhaps to be combined as Saccharinae, see Kellogg (2013b: summary) and Welker et al. (2019: immediate relatives of Saccharum). Vorontsova et al. (2019) examined relationships around here, and found that Miscanthus was close to Saccharum, and Lasiorhachis, which has quite recently been included in Saccharum, was often sister to Sorghum section Sorghum in nuclear analyses, while Sarga, which used to be included in Sorghum, was hopelessly polyphyletic in an analysis of four chloroplast genes and not notably close to Sorghum in the nuclear analyses. Ng'uni et al. (2010) and Hawkins et al. (2015) had looked at relationships within Sorghum; Sarga there was sister to Saccharum and Miscanthus. [Lasiurus + Thelepogon], with three species widely distributed in the Old World tropics, may be sister to the rest of the tribe (Welker, McKain et al. 2020). There is extensive hybridization in the ecologically dominant DASH clade (Diheteropogon, Andropogon, Schizachyrum, Hyparrhenia), which has some 250 species (McKain et al. 2016b), and also in Otachyriinae (Acosta et al. 2019); overall, "at least a third of all speciation events in the tribe have resulted from allopolyploidy" (Welker, McKain et al. 2020, p. 1004, see Estep et al. 2014). In Paspaleae, for the relationships of Paspalum, basically monophyletic, see Rua et al. (2010) and Scataglini et al. (2014), López and Morrone adjusted the limits of Axonopus, for two new genera, see C. Silva et al. (2015), finally, there seems to have been quite a bit of allopolyploidy in Otachyriinae (Acosta et al. 2019) and hybridization in Hildaea (C. Silva et al. 2019: nice example).
Chloridoideae. Duvall et al. (2016: 10 taxa/genera in study) used plastome variation to look at relationships in the subfamily. Peterson et al. (2009, 2010a, 2011a, b) suggest that relationships are something like [Centropodieae [[Triraphidae - Neyraudia (panicoid microhairs) + Triraphis] [Eragrostideae [Zoysieae + Cynodonteae (the bulk of the group)]]]], and the small Centropodieae include both C4 and the only C3 taxa in the subfamily (see also Fisher et al. 2016: 122 nuclear loci, also the Grass Phylogeny Working Group II 2011). [Neyraudia + Nematopoa], the latter from Arundinoideae, were found to be sister (Teischer et al. 2017).
Chloridoideae (Eragrostoideae). Eragrostis and Sporobolus may be polyphyletic, although the bulk of the latter genus - with the inclusion of a few genera like Spartina - forms a well-supported clade, and internal structure there is considerable (Peterson et al. 2014b, 2017). R. L. Barrett et al. (2019) found that Australian Eragrostis was paraphyletic, with a radiation in the Andes and another in the monsoon tropics. Muhlenbergia was paraphyletic, but it was expanded to include nine genera and includes a number of well supported (and by morphology, too) clades (Peterson et al. 2010b, 2021b: ITS plus 6 chloroplast markers, 6 clades; Columbus et al. 2010). Leptochloa is polyphyletic (Peterson et al. 2012). Peterson et al. (2015a, b) provide a phylogeny for Cynodonteae-Eleusininae and -Boutelouinae respectively; for more on relationships in Cynodonteae, see Peterson et al. (2014a, 2016a, esp. 2016b) and Fisher et al. (2016), the latter in particular emphasizing the prevalence of hybridization in this tribe, with widespread incongruence between relationships suggested by nuclear and chloroplast data. Triodiinae include three genera, but Triodia is paraphyletic and should include the other two genera (Toon et al. 2015: 66/69 species, chloroplast and nuclear markers; M. D. Barrett et al. 2025 - addition of 40 species). Triraphidae also include 3 genera, and Peterson et al. (2022a) discuss relationships there. For a morphological phylogenetic analysis of the subfamily, see Liu et al. (2005), for other relationships, see papers in Aliso 23: 565-614. 2008.
Danthonioideae. For general relationships in the subfamily, see Barker et al. (2007a), Pirie et al. (2008), Linder et al. (2010), and Cerros-Tlatilpa et al. (2011); Merxmuellera is very para/polyphyletic. Some relationships within Danthonioideae are reticulating (Pirie et al. 2008, 2009), indeed, these complex relationships may be linked to extensive past hybridizations (Pirie et al. 2008, 2009). For a phylogeny of the Pentaschistis group, also character evolution there, see Galley & Linder (2007).
Arundinoideae. Although monophyletic, the whole clade was subtended by a rather short branch (Duvall et al. 2017). Hardion et al. (2017) and Teischer et al. (2017) are close to having excluded all the extraneous elements in Arundinoideae and moved them to their appropriate subfamilies; the subfamily is now considerably slimmed down.
Micrairoideae. In Bouchenak-Khelladi et al. (2008) it appeared that Micrairoideae might not be monophyletic, Isachne not having a fixed position, indeed, Isachneae were found to be paraphyletic by Duvall et al. (2017); see also Grass Phylogeny Working Group (2024). For the addition of a genus that used to be in Arundinoideae, see Hardion et al. (2017), while Teisher et al. (2019) focus on relationships among the C4 Eriachneae.
The BOP Clade.
Duvall et al. (2007) had found strong support for the BOP clade, albeit the taxon sampling was slight (see also Grass Phylogeny Working Group 2001); relationships between the three subfamilies were initially uncertain (e.g. Hisamoto et al. 2008). Peng et al. (2010: 43 genes, only 10 taxa) found strong support for the relationships [O [B + P]] (ML and Bayesian analyses) and even stronger support for the relationships [B [O + P]] (neighbour joining). However, the analyses of Wu and Ge (2011: 76 genes, 22 taxa; see also Bouchenak-Khelladi et al. 2008; Grass Phylogeny Working Group II 2011; Y.-J. Zhang et al. 2011; Kelchner & the Bamboo Phylogeny Group 2013; Z.-D. Chen et al. 2016; H. Wang et al. 2024) supported the former set of relationships, and these are followed here. Note that Blaner et al. (2014) found that Brachelytrum moved outside Poöideae in analyses using nuclear rather than chloroplast data, while a floral transcriptome analysis focussing on four Bambusoideae favoured the relationships [P [O + B]] (Wysocki et al. 2016, see also Pimentel et al. 2017: chloroplast markers).
The position of Streptogyna is uncertain, and it may even be sister to the whole PACCMAD clade - it lacks the possible synapomorphies of that clade (Bouchenak-Khelladi et al. 2008; see also Bouchenak-Khelladi et al. 2009; Hisamoto et al. 2008; Saarela et al. 2018), but it may also be close to Oryzoideae or sister to the BOP clade.
Bambusoideae. W. Zhang and Clark (2000) restricted Bambusoideae to the limits accepted here; most taxa in what is now the basal grade of Poaceae have been included in the bamboos at one time or another, as have some genera in Poöideae, etc.; see the Bamboo Phylogeny Group (2012b) for a summary of phylogenetic work on the subfamily. Disentangling relationships in bamboos is difficult, and tree topologies often differ depending on whether the genes analysed are from the chloroplast - a [Bambuseae + Olyreae] clade is usually recovered, or nucleus - a [Bambuseae + Arundinarieae] clade is found (Wysocki et al. 2014). The over 500 species of temperate bamboos (Arundinarieae) form a clade that appears to have descended from an allotetraploid ancestor (Triplett et al. 2011), while Triplett et al. (2014) suggested several hybridization events involving three main genomes and two minor genomes that link Bambuseae and Arundinarieae; diploids with those genomes are extinct, while Olyreae have yet another genome that has doubled by autopolyploidy. Z.-H. Guo et al. (2019) revisited this story, suggesting that there had been three hybridization events involving four different genomes; Olyreae were not involved. There are mentions of hybridization and different relationships suggested by chloroplast and nuclear markers throughout the next couple of paragraphs.
Burke et al. (2012) and Y.-J. Zhang et al. (2011) looked at relationships based on analyses of whole plastid genomes. Clark and Triplett (2006) discussed relationships within the subfamily, previously divided into the woody Bambuseae and the herbaceous Olyreae. However, the woody temperate Arundinarieae may be sister to the rest of the subfamily, thus Sungkaew et al. (2009; five plastid genes; Kelchner & the Bamboo Phylogeny Group 2013) retrieved the relationships [Arundinarieae [Olyreae [Neotropical (strictly) Bambuseae + Paleotropical & Austral Bambuseae]]] and mapped the distributions of each of these groups (see also Ruiz-Sanchez & Sosa 2015); for Neotropical Bambusoideae, see also Burke et al. (2014). However, Kelchner and the Bamboo Phylogeny Group (2013) noted that the position of Olyreae in particular was not secure, and they might be sister to the rest of the subfamily. Indeed, as elsewhere, relationships may differ depending on whether the genes analysed are from the chloroplast (= [Bambuseae + Olyreae]) or nucleus (= [Bambuseae + Arundinarieae]) (Wysocki et al. 2014, esp. 2015, 2016: floral transcriptome analysis; Triplett et al. 2014: single-copy nuclear markers; X.-Z. Zhang et al. 2015: plastid genes; Attigala et al. 2016: plastome analyses; Saarela et al. 2018; Chalopin et al. 2021); the latter topology is followed above. Within Olyreae there may also be comparable differences between chloroplast and nuclear analyses (see Lima et al. 2019). Triplett et al. (2014) suggested that there were several hybridization events that involved three main genomes and two minor genomes that link Bambuseae and Arundinarieae; diploids with those genomes are extinct, while Olyreae have yet another genome that has doubled by autopolyploidy. Indeed, hybridization seems to pervade this clade. Note that most of the studies in the next paragraph have focussed on genes from a single genome, mostly from the chloroplast... For a number of other papers discussing other relationships in Bambusoideae, hybridization there, new genera, etc., see Bot. J. Linnean Soc. 192(1). 2020.
For the phylogeny of Neotropical woody bamboos, see Clark et al. (2008) and Fisher et al. (2009), and for hybridization here, see Goh et al. (2013). For relationships in palaeotropical woody bamboos, see H.-Q. Yang et al. (2008b: resolution o.k., baccate fruit arose in parallel) and M.-Y. Zhou et al. (2017, Palaeotropical woody bamboos, 2020 and 2022, Melocanninae - all complete plastomes). Zhou et al. (2022) found both para- and polyphyly and some conflict in the topologies obtained using various subsets of the plastome data. Bambuseae. For Bambusa and its relatives, see J. B. Yang et al. (2010) and Goh et al. (2010), for Dendrocalamus, see Sungkaew et al. (2010), and for Arthrostylidiinae, see Tyrrell et al. (2009, 2012), de Jesus-Costa et al. (2018: Arthrostylidium polyphyletic) and Vinícius-Silva et al. (2020: Merostachys clade, recent radiation, ILS but not hybridization). Rakontonasolo et al. (2023) looked at relationships in Hickeliinae using plastid genomes finding in general quite well-supported relationships; the ever-shrinking Nastus was still polyphyletic. Disentangling relationships in Arundinarieae, the temperate woody bamboos, is difficult (see Peng et al. 2008). Thus Zeng et al. (2010) found rather little resolution despite sequencing ca 9,000 base pairs. The extent of the problem has been confirmed: A Phyllostachys clade that was recovered in plastome analyses was pulverised into 24 bits in nucleome analyses; hybridization is involved (Y.-X. Zhang et al. 2012; see also H.-M. Yang et al. 2013), indeed, all Arundinarieae may be descended from an allotetraploid ancestor (x= 12) (Triplett et al. 2011; C. Guo et al. 2020); see L.-N. Zhang et al. (2019) for a careful analysis of the Phyllostachys problem. P.-F. Ma et al. (2014) recovered Ampelocalamus calcareus as sister to the rest of the tribe (see also Attigala et al. 2016: strong support; Saarela et al. 2018). There are 12 main clades within Arundinarieae, as has been confirmed by several studies (Triplett & Clark 2010; Ma et al. 2014: substantial resolution, chloroplast phylogenomics; Y.-X. Zhang et al. 2017); Y.-X. Zhang et al. (2020) provide a phylogenomic analysis (ddRAD) for members of Arundinarieae, the outline tree being based on work by C. Guo (2019: not seen). Even with the rather restricted sampling of Ma et al. (2014), Indocalamus and Arundinaria were found to be polyphyletic, as also by X.-Z. Zhang et al. (2015) with more extended sampling - Ampelocalamus was also polyphyletic. Y.-Q. Zhang et al. (2019) found that Fargesia was polyphyletic while Y. Zhou et al. (2020) and Ye et al. (2021) also found extensive hybridisatioin around here, with intergenomic/intergeneric hybridization, hybrid species, etc. (see also Ye et al. 2022 for inflorescence morphology - Fargesia s.l. preferable). Tong et al. (2020) used variation in the nuclear GBSS1 to look at the relationships of a bamboo discovered in Vietnam since that gene was likely to give more resolution than chloroplast genes; their bamboo, which they described as a new genus, was part of a 22-tomy... Malagasy species of Arundinaria have been removed to Oldeania, not immediately related to Arundinaria (Y.-X. Zhang et al. 2017). C. Guo et al. (2019) found plastid and nuclear topologies to conflict in Shibataea, and a later ddRAD analysis of 213 taxa (Guo et al. 2020) showed the extent of the problem. There seemed to be some well supported clades in the ddRAD analyses, Hsuehochloa being sister to the rest of the tribe in some analyses, but plastome phylogenies were another story. Relationships within the large Neotropical Chusquea are discussed by Fisher et al. (2014). Triplett and Clark (2021) used amplified fragment length polymorphism data to explore relationships in the tribe, and they suggested that the combination of recent rapid diversification, long generation times and hybridization helped to explain the recurring problems in Arundinarieae, and they finished their paper with the question "And importantly, are any bamboo lineages unaffected by hybridization?" (ibid.: p. 64). And recently a new genus in Arundinarieae has been described because of the polyphyly of Sasa (Qin et al. 2021, plastome genes only). Within Olyreae, the herbaceous clade, the monotypic Buergersiochloa, from New Guinea, may be sister to the rest of the tribe, which are plants of the New World (e.g. Kellogg & Watson 1993; W. Zhang & Clark 2000; Bouchenak-Khelladi 2008; Ruiz-Sanchez et al. 2019: introduced into Africa), and this position is particularly evident in analyses using platid/plastome data (Lima et al. 2019). However, recent work has rather surprisingly aligned the monotypic Cuban Piresiella with Buergersiochloa, and morphological data suggest that two more monotypic Cuban genera, Ekmanochloa and Mniochloa, also belong with it (de Carvalho et al. 2021; Lima et al. 2021); the first genus, at least, also lacks crenate (olyroid) SiO2 bodies (Lima et al. 2019). However, much remains uncertain about relationships here in combined analyses, with 17/32 nodes collapsing in a strict consensus (Oliveira et al. 2014), and while genera like Pariana, Olyra and Sucrea were found not to be monophyletic by Ruiz-Sanchez et al. (2019: 2 chloroplast, 1 nuclear markers). However, R. P. Oliveira et al. (2019) and I. L. C. Oliveira (2019) have begun to rectify such problems.
Oryzoideae. The relationships of Oryzeae have been much studied (Y.-L. Guo & Ge 2005; L. Tang et al. 2010 and references); for diversification within Zizaniinae, see L. Tang et al. (2015) and within Oryza itself, see Zou et al. (2008).
Poöideae. Hybridization is notably common in core Poöideae (e.g. Orton et al. 2021), and is involved in the origin of many grain crops. Triticeae (= the old Hordeeae) are notorious for the extent and complexity of the reticulating relationships that they show (Jakob & Blattner 2006; G. Petersen et al. 2006a; Mason-Gamer 2008; Meimberg et al. 2009; G. Sun & Komatsuda 2010; Fan et al. 2013: Elymus s.l. and the Y genome; Martis et al. 2013: rye; Sha et al. 2014: Leymus; Middleton et al. 2014: chloroplast genome and ages; G. Sun 2014: Elymus; R. R. Wang & Lu 2014: perennial species, also other references in J. Syst. Evol. 52(6). (2014); Dong et al. 2015: Elymus s.l. and the St genome; Soltis et al. 2016b; Lei et al. 2017, 2018: esp. Roegneria/Elymus; Gillespie et al. 2022), with hybridization (evident in diploid species), polyploidy, genome re-arrangements and introgression making for a very complex picture (see especially Mason-Gamer & White 2024: focus on diploid taxa). Mason-Gamer and White (2024) also noted major conflict between nuclear and chloroplast trees, between nuclear trees that focused on different chromosome arms, and so on (see also Economic Importance above). Furthermore, Triticeae plastomes are paraphyletic with respect to those of Bromeae (Bernhardt et al. 2017). The Sesleria area seems to be the result of hybridization between an ancestor with an "Aveneae" nucleus and a "Poeae" plastid (Kuzmanovic et al. 2017); both are included in Poeae here. Indeed, although Tkach et al. (2020) noted that relationships found using plastid or nuclear genes generally agreed, there is no relief in sight, because as they went on to say "severe conflict between the [nuclear and plastid] trees, however, occurs in sometimes larger stretches of the trees" (ibid. p. 265). Thus they found that hybridization within and between their subtribes was common, and perhaps seven of these subtribes were the result of hybridization between their Aveneae and Festuceae (Tkach et al. 2020). In Stipeae genomes from extinct clades persist in their hybrid descendents (Romaschenko et al. 2014).
There are several papers on Poöideae in Aliso 23: 335-471. 2008; see also Soreng and Davis (2000), Schneider et al. (2009), Blaner et al. (2015), Hochbach et al. (2015: inc. nuclear genees), Pimentel et al. (2017) and Saarela et al. (2018) for relationships within the subfamily. Some of the more basal clades used to be in Bambusoideae and there is still uncertainty about some relationships along the spine. For the ndhF gene, structural features of chloroplast and nuclear genomes, etc., and the phylogeny of Poöideae, see J. I. Davis and Soreng (2008). It is not certain the duplication of the ß-amylase gene is an apomorphy here; one of the gene copies breaks down starch into fermentable sugars in the endosperm, while the other is more broadly expressed in the plant, as it is in other Poaceae (Mason-Gamer 2005; see also Minaya et al. 2015). For relationships and morphology in Phaenospermateae (inc. Duthieae), see Schneider et al. (2011), but c.f. in part Blaner et al. (2015) and Hochbach et al. (2015); Phaenosperma itself is a very distinct plant previously included in Bambusoideae. Relationships in the Phaenospermateae-Stipeae-Diarrheneae area remain unclear. Saarela et al. (2015; see Romaschenko et al. 2014) suggested that Ampelodesmeae are hybrids between Stipeae (the maternal parent, hence the placement of the former within the latter in chloroplast analyses) and Phaenospermateae, hence some of the phylogenetic conflict in this area. An analysis of 45 plastomes yielded the relationships [Brachyelytreae [Phaenospermateae [Meliceae [Stipeae [Diarrheneae [Brachypodieae [Bromeae, Poeae, Triticeae]]]]]] (Saarela et al. 2015), but Nardeae, Duthieae, Brylkineae, Lygeae, and genera like Littledalea were not included. The single species of Brachypodium included, an annual, was on a very long branch, and Saarela et al. (2015, see also 2018) discuss uncertainties as to the exact position of Brachypodieae - Brachypodium is very important plant for research on C3 cereals (Hasterok et al. 2022; see also Economic Importance above). Briza is polyphyletic, its relationships being disentangled by Persson and Rydin (2016). Most of the work on the subfamily prior to 2020 used plastome data, although Hochbach et al. (2015), for example, compared relationships obtained using plastid and nuclear data. The most complex plastome analyses are those in Orton et al. (2021) which involve 164 Pooideae, only Calothecinae and Duthieeae not being included, and this paper should be consulted for details (Orton et al. also suggest removing all gaps from the alignment columns), while nuclear analyses are to be found in the Grass Phylogeny Working Group (2024).
Recently, L. Zhang et al. (2022) looked at 1236 nuclear genes (and subsets of these) from 157 taxa of Pooideae (including nearly all the subtribes), and most of the relationships they recovered had strong support (they noted that 21 genera were not monophyletic); these relationships differed somewhat from those obtained earlier. I have largely followed this study above; given the issue of hybridization, it will be of interest to see more comments on paralogy, gene tree support, etc..
Indeed, Zhang et al. (2022; see also Hochbach et al. 2017; esp. W. Huang et al. 2022: family-level study), noting that Diarrheneae were the only tribe/subtribe that they did not recover as being monophyletic (the two genera, Diarrhena and Neomolinia, were well separated), discussed the possibility that this was the result of hybridization, which seems reasonable (see also the Grass Phylogeny Working Group 2024). A number of taxa show complex reticulating patterns of relationships (see also above, Genes and Genomes). Genera like Ampelodesmos and Stephanachne may end up in Stipeae or Duthieae, depending on the analyses, perhaps suggesting ancient hybridization (Blaner et al. 2015). For the Anthoxanthum/Hierochloë (Phalarideae) problem, the resolution of which depends on understanding the patterns of hybridization there, see Pimentel et al. (2013), in Stipeae, Romaschenko et al. (2014) disentangled relationships in which an old hybridization was involved. Saarela et al. (2018) discuss where Ampelodesmeae might be placed, Ampelodesma representing an intertribal hybrid (see also Saarela et al. 2018). The distribution of topoisomerase 6 copies in the African Trisetopsis (Helictotrichon area, Aveninae) shows some species with a copy currently known only from New World grasses (Wölk & Röser 2014, see also 2017).
Meliceae. For relationships in the potential model grass, Melica, see Khodaverdi et al. (2023: 25 spp., ITS and ndhF). Three main clades correlated with geography and morphology seemed to be developing.
For a phylogeny of Poeae s.l./Poodae (including Poeae s.str., Aveneae, Festuceae), which should now include Aveneae, see Grebenstein et al. (1998), Quintinar et al. (2007, also Döring et al. 2007; Soreng et al. 2007; Saarela et al. 2010, 2011, 2015; Gillespie & Soreng 2011; Tkach et al. 2020). Saarela et al. (2015) found that the Poeae they examined fell into two well-supported clades, both with numerous indels. Two plastid groups were flagged by Soreng et al. (2017: PCG 1, PCG 2). Saarela et al. (2017: incongruence defined, but not tested for) focussed on Poeae plastome group 1 (inc. Aveneae s. str), a group that was not recovered in ITS and ITS + ES analyses, furthermore, support for relationships along the backbone was not strong. (L. Zhang et al. (2022) found that PCG 2 was para-/polyphyletic in their nuclear gene analyses; Poeae divided into two groups, which they recognized informally as PNG 1 and PNG 2.) Orton et al. (2019) emphasized plastome relationships and rare genomic changes that could be found there, and obtained good support for relationships in both the plastome groups 1 and 2 (= Poeae s. str.). Kuzmanovic et al. (2017) looked at relationships between taxa in the Sesleria area unplaced into tribes. For relationships within Poa itself, see Gillespie and Soreng (2005), Gillespie et al. (2009), Soreng et al. (2010, 2011), Hoffmann et al. (2013), Birch et al. (2014: Australasian species), and Giussani et al. (2016: New World supersection Homalopoa). See also Gillespie et al. (2008, 2010) for relationships in Poinae, Quintanar et al. (2010) for those in Koeleriinae, Essi et al. (2008) for relationships around Briza, and Consaul et al. (2010) for polyploid speciation in Puccinellia. Aveninae have been much studied (Wölk & Röser 2017 and references). Peterson et al. (2021c: ITS plus three plastid markers) looked at relationships around Calamagrostis, in which they found seven major clades, and they also placed Dichelachne into Pentapogon. For a phylogeny of Stipeae, see Romashchenko et al. (2008, 2010, 2011, 2012, 2014; Jacobs et al. 2008; Barkworth et al. 2008): Macrochloa may be sister to the rest of the tribe and there are parallel diversifications in the Old and New Worlds, so characters traditionally thought to be phylogenetically important appear not to be so. For New World Stipeae, see Ciadella et al. (2010: sampling) and for relationships within Nassella, to include Amelichloa, see Ciadella et al. (2014).
Classification. The basic classification of the family has been outlined by the Grass Phylogeny Working Group (2001: a few small taxa remained unplaced, 2011); there have since been changes in detail, but the subfamilial groups now seem clear and are consistently applied - holdouts like Streptogyne are very few. Watson and Dallwitz (1992b onwards) includes generic treatments, etc., and a more current account is to be found in Kellogg (2015), while Soreng et al. (2000 onwards) is a phylogenetic classification, albeit a classification that is becoming a bit splitty and is at times hierarchically redundant, of the family that is being kept current (see Soreng et al. 2015, 2017, 2022 for static versions); a few genera are yet to be assigned to such lower-level groups. Hilu (2007) had noted the high proportion (ca 1/3) of monotypic genera in the family, while 78% had ≤10 species per genus, and there were a few very large genera; he attempted to find a biological explanation for this pattern.
Vorontsova and Simon (2012) estimated that 10-20% of all species names in Poaceae would be changed by the time all the phylogenetic rearrangements going on here were complete. The temptation is to chip off small monophyletic taxa from a paraphyletic residue; the temptation should be firmly resisted1 The challenge is to integrate information from different sources/acquired with different goals in mind and using differing classifications into a single resource (Vorontsova et al. 2015). As will have become clear from the preceding section, hybridization is common within several quite extensive clades, and how the chloroplast DNA data that have mostly been used to evaluate relationships and determine generic limits will mesh with nuclear data is unclear (as of 2022). Classificatory principles that can be followed in the numerous cases of conflict that are becoming evident remain to be articulated; alack, monophyly is a difficult principle to apply to classification here. Several papers appeared in the Journal of Systematics and Evolution alone in 2021 that contained several new genera and taxa between the supertribe and genus (but there were no papers on Bambusoideae!), and it is clear that stability in our understanding of relationships and hence of the classifications, becoming ever more complex (see also below), that depend on them remains an elusive goal; it is, unfortunately, premature to attempt a synthesis.
Peterson et al. (2010, 2017) provide a detailed suprageneric classification of Chloridoideae. See also Columbus et al. 2010 and Peterson et al. 2010a, 2021b, 2022b, 2023: all Muhlenbergia, with infrageneric classification; Peterson et al. 2012: Leptochloa and relatives, 2014a, 2016b [sutribes], 2021a, 2022c: all Cynodonteae, 2014b, 2015b: infrageneric classification of Bouteloua. The focus of Peterson et al. (2016a) was on Sporobolinae, and they included an infrageneric classification of Sporobolus where Spartina was a section; this has occasioned some angst among ecologists - see Bortolus et al. (2019). R. L. Barrett et al. (2020) expand the limits of Eragrostis in Australia; M. D. Barrett et al. (2025) provided a subgeneric classification for Triodia.
Sánchez-Ken and Clark (2010) outlined a tribal classification for Panicoideae s.l. (including Centothecoideae), Morrone et al. (2012) provide a comprehensive classification of Paniceae and their immediate relatives. There Setaria will have to be dismembered (Kellogg et al. 2009). Panicum itself is getting pulverized (Lizarazu et al. 2014 and references); Panicum s.l. had about 500 species, s. str. fewer than 100 species (Zuloaga et al. 2007), and Andropogon is also getting dismembered (Vorontsova et al. 2023). Cenchrus is to include Pennisetum (Chemisquy et al. 2010). Welker, McKain et al. (2020) provide a subtribal classification of Andropogoneae.
Linder et al. (2010) offer a generic classification of Danthonioideae; generic limits are difficult there and there has been some confusing hybridization (Pirie et al. 2009; Humphreys et al. 2010a).
For a suprageneric classification of Bambusoideae, see the Bamboo Phylogeny Group (2012a, b); generic limits are proving especially problematic here. For generic delimitation in Arundinarieae, the temperate bamboos, see Peng et al. (2008), Zeng et al. (2010), Triplett and Clark (2021), and so on; the whole clade is descended from an allotetraploid ancestor, and, complicating the issue, there has been hybridization since (Triplett & Clark 2010, 2021; Triplett et al. 2011). Y.-X. Zhang et al. (2020) provide a subtribal classification of Arundinarieae - 5 subtribes, 3 new. There are also generic problems in Bambusoideae-Bambuseae (Wong et al. 2017), and -Bambuseae-Arthrostylidiinae (Tyrrell et al. 2009, 2012); Chusquea must include Neurolepis (Fisher et al. 2009). For a subtribal classification of Olyreae, see Carvalho et al. (2021). Vorontsova et al. (2017) provide a checklist of bamboos of the World; one is sometimes tempted to put the whole lot - or most of them - into a single genus and then go out for a walk. Certainly, describing new genera around here is currently a hazardous and often ill-advised enterprise.
Schneider et al. (2009) outlined tribal limits within Poöideae, but they remained unclear (e.g. see Saarela et al. 2015 for two different classifications). Tkach et al. (2020) recognize a Poodae that includes Poeae, Aveneae and Festuceae, but there is extensive inter- and intratribal hybridization, genera remain unplaced, etc., so the supertibe is hardly a satisfactory solution. For a catalogue of New World Poöideae, see Soreng et al. (2003). Genera are certainly not monophyletic in Triticeae, but are based on different genome combinations that are (hopefully) correlated with morphological variation (Dewey 1984; Löve 1984); Barkworth (2000) summarised the history of the classification of this group (see also Goncharov 2011 for taxonomic confusion in Triticum; Fan et al. 2013 for Elymus s.l.); for Aveninae, see Wölk and Röser (2017). However, the main result of trying to understand information - such as hosts of the important endophyte Epichloë - in the context of these differing classifications is simply a headache (Card et al. 2014). For the adjustment of some generic limits in Poeae, see Saarela et al. (2017); Poa is not monophyletic, so its limits will have to be extended or the genus split (Hoffmann et al. 2013); the latter is occuring. For generic limits around Piptatherum, see Romaschenko et al. (2011). Peterson et al. (2020) hacked at Agrostis, but the final picture there is still murky, while Gillespie et al. (2022) described new subtribes in hybridizing members of the supersubtribe Poodinae. In their summary classification of Poaceae, Soreng et al. (2022) recognized some 38 subtribes (also some supersubtribes) in Poeae, and almost half of these are mono- or digeneric - add the extensive hybridization, and it makes for difficult reading there. For more on subtribes in Poeae, see Peterson et al. (2021c).
Thorne and Reveal (2007) thought that the earliest name for Chloridoideae was Chondrosoideae Link, a sort of resurrection name - Googling it (as of 3.vii.2007) returned only references to Thorne and Reveal themselves, apparently the only people to have used it for some time, and about 42,100 returns for Chloridoideae. However, the name Chloridoideae was later used by Reveal himself (2012). Chondrosoideae was something of a false alarm, but priority is very unhelpful in such situations even if the literature has been correctly interpreted (see also Welker et al. 2014).
Given all the ongoing work in the family, web-based lists are much to be desired, so see Soreng et al. (2000 onwards: Catalogue of New World Grasses), and at the same address "A worldwide phylogenetic classification of Poaceae (Gramineae): cao, capim, çayir, çimen, darbha, ghaas, ghas, gish, gramas, graminius, gräser, grasses, gyokh, he-ben-ke, hullu, kasa, kusa, nyasi, pastos, pillu, pullu, zlaki, etc." (http://legacy.tropicos.org/projectwebportal.aspx?pagename=ClassificationNWG&projectid=10) which includes all recognized suprageneric taxa - the most recent version is Soreng et al. 2022. GrassBase (see also lists dependent on it like the World Checklist of Monocots) provides DELTA-based descriptions of all grasses. There are also other resources like GrassWorld (Simon 2007), although this was likely to have become static after 2020.
Botanical Trivia. A typical sheep consumes more than 10 kg of silica phytoliths per year (G. Baker et al. 1959), yet this may affect its teeth very little (Sanson et al. 2007).
Pollen tubes grow down the styles of Zea at a rate of ca 12 mm/hour-1 (Lausser et al. 2010).
There is apparently just a single clone of Phyllostachys edulis in Japan (where the species was introduced in 1736) and much of China, and Isagi et al. (2016) estimate its weight to be 6.6 x 1011 kg; "exceptionally large" would seem to be an understatement if one is thinking of an individual...
Thanks. I am very grateful to E. A. Kellogg for continuing discussions about this family.