EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [MTOC = microtubule organizing centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia adaxial, columella 0; tapetum glandular; ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome size [1C] = 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [roots with positive geotropic response]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
EXTANT GYMNOSPERMS / PINOPHYTA / ACROGYMNOSPERMAE
Biflavonoids +; ferulic acid ester-linked to primary unlignified cell walls, silica usu. low; root apical meristem organization?, protophloem not producing sieve tubes, with secretory cells, sieve area of sieve tube with small pores generally less than 0.8 µm across that have cytoplasm and E.R., joining to form a median cavity in the region of the middle lamella, Strasburger/albuminous cells associated with sieve tubes [the two not derived from the same immediate mother cell], phloem fibres +; sclereids +, ± tracheidal transfusion tissue +, rays uniseriate [?here]; stomatal poles raised above pore, no outer stomatal ledges or vestibule, epidermis lignified; cuticle waxes as tubules, nonacosan-10-ol predominates, n-alkyl lipids scanty; buds perulate/with cataphylls; leaves simple, lamina 1-veined, development marginal; plants dioecious [?here]; parts of strobili spirally arranged; microsporangia abaxial, dehiscing by the action of the epidermis [= exothecium]; pollen saccate, tectate, endexine lamellate at maturity all around grain, esp. intine with callose; ovules aggregated into compound strobili, erect, pollen chamber formed by breakdown of nucellar cells, nucellus massive; ovules increasing considerably in size between pollination and fertilization, but aborting unless pollination occurs; ovule with pollination droplet, catches pollen; male gametophyte: two prothallial cells + tube cell + antheridial cell, the latter → sterile cell + 2 gametes; pollen germinates on ovule, usu. takes two or more days, tube with wall of pectose + cellulose microfibrils, branched, growing at up to 10(-20) µm/h-1, haustorial, breaks down sporophytic cells; male gametes released by breakdown of pollen grain wall, with >1000 cilia, basal body 800-900 nm long, spline hundreds of tubules wide, chromatin not condensed; fertilization 7 days to 12 months or more after pollination, to ca 2 mm from ovule surface to egg; seeds "large" [ca 8 mm3], but not much bigger than ovule, with morphological dormancy; testa mainly of coloured sarcoexotesta, scleromesotesta, and ± degenerating endotesta; first zygotic nuclear division with chromosomes of male and female gametes lining up on separate but parallel spindles, embryogenesis initially nuclear, embryo ± chlorophyllous; gametophyte persists in seed; plastid and mitochondrial transmission paternal; genome size [1C] 10< pg [1 pg = 109 base pairs]/(2201-)17947(-35208) Mb; two copies of LEAFY gene [LEAFY, NEEDLY] and three of the PHY gene, [PHYP [PHYN + PHYO]]; plastome IR expanded, with duplicated ribosomal RNA operons; chondrome with second intron in the rps3 gene [group II, rps3i2].
[CUPRESSALES [GNETALES + PINALES]] / PINOPHYTA / CONIFEROPHYTA / CONIFERAE / PINOPSIDA
Tree, branched, evergreen; βaryl ether concentration in lignin lower, biphenyls higher; compression wood + [reaction wood - much-thickened/lignified fibres on abaxial side of branch-stem junction]; wood pycnoxylic, torus:margo pits + [tracheid side walls]; phloem with polyphenol-containing parenchyma (PP) cells, resin ducts/cells in phloem and/or xylem +/0; lignins with guaiacyl units (G-lignin) [lacking syringaldehyde, Mäule reaction negative]; cork cambium ± deep seated; bordered pits on tracheids round, opposite; nodes 1:1; axillary buds + (0); leaves with single vein, fasciculate or not, needle-like or flattened; plants monoecious; microsporangiophore/filament simple, hyposporangiate; dehiscing by the action of the hypodermis [endothecium]; pollen saccate, exine thick [³2 µm thick], granular; ovulate strobilus compound, erect, ovuliferous scales flattened, ± united with bract scales [= ovules borne on predeveloped axillary structures]; ovules with pollination drop, lacking pollen chamber, inverted [micropyle facing cone axis at pollination and seed dispersal]; pollen buoyant, not wettable, pollen tube unbranched, not haustorial, growing towards ovule, development intercellular, wall with cellulose and arabinogalactan proteins; gametes non-motile, lacking walls, siphonogamy [released from distal end of tube directly to the egg cell]; female gametophyte lacking chlorophyll; seed cone components sclerified, seed dispersed with part of supporting structure, seed coat dry, not vascularized; embryo initially with 2 to 4 free-nuclear divisions, elongated suspensor cells, embryonal cells basal, polyembryony +; one duplication in the PHYP gene line; germination phanerocotylar, epigeal, (seedlings green in the dark).
[GNETALES + PINALES]: root cortical cell walls lacking phi [φ] thickenings; phloem fibres 0; microsporangiophore/filament simple with terminal microsporangia; microsporangia abaxial, dehiscing by the action of the hypodermis [endothecium]; lhcb 3 and 6 genes not functional/lost [light harvesting genes]; chloroplast transmission maternal, chloroplast ndh genes lost/pseudogenized, rpl16 gene lost.
Age. Davies et al. (2011) suggested an age for this clade of (259-)219(-174) Ma; Magallón et al. (2013) thought that it was about 312 Ma, while (259-)219(-174) Ma is the age suggested by Clarke et al. (2011); an age of 181-140.1 Ma is estimated by Naumann et al. (2013), around (339-)330(-320) Ma by Y. Yang et al. (2017), and about 174.1 Ma by Gil and Kim (2018).
Evolution: Divergence & Distribution, etc.. For the morphology of Gnetales as it relates to that of other gymnosperms and of angiosperms, and for the relationships of Gnetales, both complex subjects, see discussions elsewhere. On balance, the evidence from nuclear data is pointing towarda a GnePine grouping, and that is followed here.
Lignin with syringyl units [G- + S-type lignins, positive Mäule reaction]; roots diarch; bark with sclereids; ?root hairs; resin canals 0; shoot apex duplex-type [with initials in several layers, tunica/corpus construction], reaction wood on upper side of stem; tracheids with margo-torus pits, vessels + [from circular bordered pits], also in metaxylem, fibre tracheids +, nucleated, tracheids +, xylem parenchyma slight; phloem fibres 0, phloem parenchyma cells 0; cellulosic fibres +; vascular traces leaving stele one internode below their move into a petiole, ?nodal anatomy; internodes striate; minute intracellular calcium oxalate crystals +; foliar venation linear, two primary veins in leaves [and cone bracts]; resin canals 0, mucilage cells +; stomata paracytic, orientation various; leaves opposite, joined at the base, overall growth ± diffuse/marginal/from basal plate, axillary buds serial, collateral; plant dioecious; strobili compound, reproductive units decussating; microsporangiate strobili: associated with sterile ovules; microsporangiate cones of two fused microsporophylls, microsporangia in synangia, surrounded by a tubular "bract", dehiscence apical; pollen not saccate, very strongly oblate, inaperturate, strongly transversely ridged [= at right angles to the polar axis: plicate; pseudosulcate], tectate; ovulate cone: cone scale 0, ovules terminal, surrounded by a vascularized connate structure ["outer integument"/seed envelope], papillae on the inner surface around the micropyle; integument not vascularized, separating from ovule ca 1/2 way, parietal tissue massive, nucellar cap well developed, with much-elongated beak, micropylar tube with inner epidermis lignified, pollination droplets +; exine shed during germination; sperm cell binucleate, both nuclei fuse with gametophytic cells ["double fertilization"]; first zygotic nuclear division with one spindle, tiered proembryo 0, free nuclear stage in which each nucleus forms an embryo, secondary suspensor developing from upper embryonal tier, no primary suspensor; nuclear genome C value 1.4-3.5 pg, chloroplast accD, rpl23, chl B, L, and N genes all 0, ndh genes lost/pseudogenized, PHY0 gene, mitochondrial coxII.i3 intron 0; cotyledons with connate bases. - 3 families, 3 genera, 93 species.
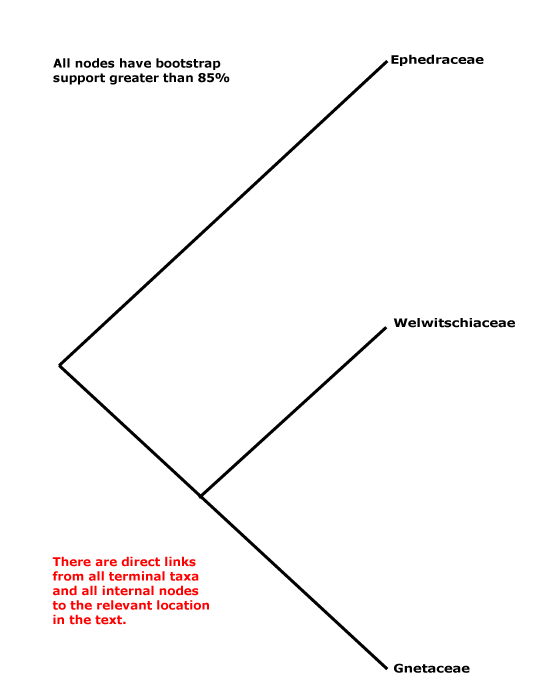
Includes Ephedraceae, Gnetaceae, Welwitschiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. Note that the particular node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Estimates of the age of crown-group Gnetales are (202-)155(-104) Ma (Smith et al. 2010: see also Table S3). Magallón et al. (2013) suggested an age of 140-120 Ma, Evkaikina et al. (2017) an age of ca 139 Ma, Won and Renner (2006) ages of (196-)159(-132) Ma, Ickert-Bond et al. (2010) ages of (192.3-)166.6(-90.6) Ma and - rather older - ages in Laenen et al. (2014) are around 261.8 Ma and in Y. Yang et al. (2017) they are (269-)230, 223(-174) Ma, while around 146.1 Ma is the estimate in Y. Lu et al. (2014), about 154 Ma that in Ran et al. (2018a), and it is 237-113 Ma in Rydin et al. (2021), the estimate in the latter greatly affected by the prior used.
Ephedra and Welwitschia had diverged by 110 Ma or more, the welwitschioid seedling, Cratonia, from Brazil, being of this vintage (Rydin et al. 2003 - and several other papers by Rydin et al. on gnetalean topics), while pollen and seeds attributed to a welwitschioid plant are known from the Lower Cretaceous both in Portugal and eastern North America (Friis et al. 2014). Indeed, both Ephedra and Welwitschia have distinctive ridged pollen grains, and such grains have a fossil record of ³250 m.y., being common from the Late Triassic onwards. Dilcher et al. (2005) noted that Gnetalean-like (striate/ribbed) pollen was common in both N. and S. Hemispheres; in the former, records are from the Upper Triassic onwards, in the latter, especially in the early Cretaceous from the northern half of South America. The pollen found by Z.-Q. Wang (2004) associated with his fossil, Palaeognetaleana auspicia, from the Permian some 250-270 Ma, is also of this general kind. However, the ovule ofb that fossil was radiospermic and had two complete integuments, a possible third integument being represented by scales, and the arrangement of parts in the cone was spiral, so what it represents is unclear (see also below).
Evolution: Divergence & Distribution. Gnetales s.l., i.e., stem-group Gnetales and including the fossil groups below, show considerably more variation than perhaps might have been expected given the small size of the extant clade. Both they and extant Gnetales have chlamydospermous seeds, i.e. consisting of an ovule with a long integument, the nucellus being at least half fused to the integument and the whole being surrounded by an often 4-ridged sclerenchymatous seed envelope (its morphology varies considerably, and there may be two envelopes) developing from a sheath outside the integument; the integument pokes through the apex of the envelope (e.g. Friis et al. 2011: chapter 5; 2019c; Y. Yang et al. 2020). There are some 16 genera and 28 species of plants with such seeds that have been described from the Early Cretaceous alone, but they soon became extinct (Friis et al. 2019c; the number of genera is increasing, see Yang et al. 2020). Confirming this early diversity, Ephedripites (11 species), Gnetaceaepollenites (7 species) and Welwitchiapites (1 species) and other taxa are known from the Lower Cretaceous Crato Formation, Brazil, dated at ca 115 Ma or a little older (Hofmann et al. 2021b). Genera like Ephedrispermum have ephedroid pollen in the micropyle, while Drewria potomacensis, from Early Cretaceous deposits in Virginia (Crane & Upchurch 1987), and some other fossils seem to have come from quite small plants of open habitats in flood plains - a possible habitat of early angiosperms, too (Friis et al. 2019c). For more on Gnetalean fossils, see, and for fossils of Ephedra in particular, see below.
See Y. Yang et al (2017) for ancestral features of Gnetales, which appear to be much more Ephedra- than Gnetum-like. Additional variation such as pollen size (e.g. Ickert-Bond & Renner 2016) can be optimized on the tree.
In Gnetales xylans are more common than glucomannans, as also in flowering plants (Zhong et al. 2019 and references). They have glucoronosyl units every 6 or 8 or so xylosyl residues, and they are acetylated every other xylosyl residue, but there are no α-arabinosyl units. Overall, they are more similar to xylans of angiosperms, perhaps more particularly to those of magnoliids than of eudicots, but less similar to those of other gymnosperms, which are galactoglucomannan-rich (Busse-Wicher et al. 2016; Zhong et al. 2019).
Ecology & Physiology. Gnetales have syringyl lignin (e.g. Gross 1981), like angiosperms. When stems of Gnetum are bent, more tissue develops on the upper side, but without tension wood fibres (Shirai et al. 2015: microfibril angle may be important). However, Nawawi et al. (2016) described a typical gymnosperm distribution of reaction wood, with more wood tissue developing on the lower side, but it has angiosperm lignin composition (syringyl/guaiacyl lignin: see elsewhere for discussion). For gelatinous fibres (G-fibres) in the bark in which the innermost layer of the secondary cell wall is rich in cellulose and poor in lignin, see Montes et al. (2012). In Ephedra, at least, the presence of such fibres had nothing to do with bending and they are not associated with wood tissues, so they are not reaction/tension wood (c.f. angiosperms; c.f. Tomlinson et al. 2014). although G-fibres in general are involved in plant movement. Carlquist (1997, 2012b) discussed wood anatomy of Gnetales from a functional point of view.,
Pollination Biology & Seed Dispersal. Ovules in all three extant genera, in Ephedra, including the sterile ovules found in male inflorescences in E. foemina (perhaps sister to the rest of the genus), many Gnetum and Welwitschia (see Jörgensen & Rydin 2015 for homologies), secrete pollination droplets, and these may be notably sucrose-rich (Ziegler et al. 1959; Frohlich 2001; Nepi et al. 2009: c.f. pollination droplet of Welwitschia, fructose-rich). Plants are visited by diptera, lepidoptera (moths), and other pollinators (see Kato & Inoue 1994; Labandeira 2005; Bolinder et al. 2015; Rydin & Bolinder 2015). Insect pollination may well be the ancestral condition of the whole clade (Hofmann et al. 2021b and references), while wind pollination (especially common in Ephedra) is derived (Bolinder et al. 2015; Jörgensen & Rydin 2015; Rydin & Bolinder 2015; Bolinder et al. 2016). For further details, see below.
For details of the time elapsing between pollination and fertilization, quite short compared with that in many gymnosperms, see e.g. J. H. Williams (2008 and references) and Fernando et al. (2009), however, the rate of pollen tube growth is slower than that of the vast majority of angiosperms (Fernando et al. 2009; Reese & Williams 2019). The time between pollination and fertilization in Ephedra, 10-36 hours, is the shortest in extant gymnosperms; this is because of a combination of relatively fast germination (two hours or less) and a short pollen tube pathway (J. H. Williams 2008; Fernando et al. 2010).
Plant-Bacterial/Fungal Associations. For mycorrhizae, see Jacobson et al. (1993); Brundrett (2008, seen viii.2012) summarizes information on the mycorrhizal status of members of Gnetales (see also Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, etc.).
Genes & Genomes. The nuclear genome is small, 1C values being 1.4-3.5 picograms (Leitch et al. 2001, 2005; see also Leitch & Leitch 2013; Lomax et al. 2014). The extensive polyploidy in Ephedra is unusual among conifers (Scott et al. 2016), Juniperus being the other major exception. However, there appears not to be an ancient whole genome duplication in stem Gnetales, although any evidence for such an event may have been lost because of the high rate of genome evolution here (Ran et al. 2018a; Wan et al. 2018; Zwaenepoel ∧ Van de Peer 2019).
Using several estimates of evolutionary rates, in appears that the rate of molecular evolution is much higher in Gnetales than in other gymnosperms, and is more like that of angiosperms - see Ran et al. (2018a) for possible reasons, also de la Torre et al. (2017).
W. Wang et al. (2018) emphasized the variability in ribosomal DNA organization, including copy and locus number, in Gnetales. There is colocalization/linking of the 5S and 35S rRNA sites in Ephedra, while in Gnetum and Welwitschia they are separate.
Plastid transmission appears to be maternal, at least in Ephedra distachya (Moussel 1978). All three genera have very reduced plastomes, Welwitschia rather less so than the others, and it has been suggested that this is because they grow in resource-poor environments; although genome sizes in Pinus, for example, may not be that much bigger, that genus, too, can grow in similar environments (see also C.-S. Wu et al. 2007, 2009 and references); what about other seed plants growing in similar environments? Up to 18 genes have been lost from the plastome here (McCoy et al. 2008; C.-S. Wu et al. 2009; Jansen & Ruhlman 2012 and references). There are expansions at both ends of the inverted repeat in all Gnetales, some similar to changes in all other gymnosperms, but others are unique to Gnetales or to some of them (Mower & Vickrey 2018). Gnetales have high substitution rates in the protein-coding sequences of their plastomes compared to those in other gymnosperms, and their dN/dS ratio is lower (B. Wang et al. 2015; C.-S. Wu & Chaw 2015). Compared with other seed plants, the non-coding content in these genomes is also low, less than 35% - it is normally above 40%, several cupressophytes being the main exception (C.-S. Wu & Chaw 2016). Variation in the nad1 intron 2 needs clarification; it is absent in Welwitschia, present in Gnetum, and what is going on in Ephedra is not entirely clear (Gugerli et al. 2001).
Mitochondria have lost some 12 genes in Gnetum gnemon and Welwitschia, and 4 introns in the former and 16 in the latter (W. Guo et al. 2020).
Morphology, Anatomy, etc.. Species of Gnetum and Ephedra, but not Welwitschia, may contain silica (Trembath-Reichert et el. 2015). Although vessels in Gnetum, for example, are commonly described as being derived from circular pits, this has been questioned (e.g. Rodin 1969; Muhammad & Sattler 1982). Rodin (1969) suggested that Gnetales lack pits with a margo-torus construction, but they are clearly shown for Ephedra, but not Gnetum, by Eicke (1957), and in Welwitschia they are poorly developed (Carlquist 2012b: see also helical thickenings on the vessel walls, growth rings in the xylem). These tori do not stain for lignin, unlike those of angiosperms (Dute et al. 2014), and overall they can look like those of conifers, rather than angiosperms. There are nodal girdles of tissue very like transfusion tissue, at least in Ephedra (Beck et al. 1982). Although Gnetales may be thought of as having many of the wood adaptations of angiosperms and conifers, their wood anatomy has to be thought of in terms of their distinctive life styles - lianes, xeromorphic shrubs - while Welwitschia is pretty much sui generis. Romanzova et al. (2023a) discuss aspects of stem and leaf construction here.
For the numbers of veins entering the leaves, see Rydin and Friis (2010). Boyce and Knoll (2002), Nardmann and Werr (2013), and others discuss leaf development; the scale leaves found in some species of Ephedra are reductions. Rudall and Rice (2019) describe epidermal/stomatal development, noting that stomatal development in Ephedra is more similar to that in other extant gymnosperms than to angiosperms.
The plants are functionally dioecious. Interpretations of the parts of both the microsporangium- and megasporangium-bearing structures vary substantially in the literature (e.g. Gifford & Foster 1989; Hufford 1997a; Mundry & Stützel 2004). In microsporangiate plants of all three extant genera both stamens and non-functional ovules (pollination droplets may still be produced) are closely associated, although this perhaps least marked in Ephedra (see also Flores-Rentería et al. 2011), and the microsporangiate cones can be interpreted as being compound (Mundry & Stützel 2004), rather like the megasporangiate cones of Pinales (note that no attempt has been made to integrate the work on conifer cone morphology by Herting et al. 2020 here). For a careful discussion about pollen grain morphology, and the counter-intuitive orientation of the pollen grains of Ephedra and Welwitschia, see El-Ghazaly et al. (1997). Gnetum ula is reported to have two sperm cells (Singh 1978). Rudall (2021) notes how the position of the integument relative to the rest of the ovule changes over development, the ovule ending up being more or less pachychalazal (but c.f. Table 2 and p. 951). The megaspore membrane is thin, but is definitely present (Doyle 2006). For seedling morphology, particularly for discussion about the distribution of the "feeder" and the haustorial (or otherwise) nature of the cotyledon, see Sokoloff et al. (2015b).
Martens (1971) provides an extensive and very useful treatment of Gnetales as a whole (see also Gifford & Foster 1989; for a survey of recent work on the group, see Ickert-Bond & Renner 2016). For the morphology of Gnetales in the context of that of fossil gymnosperms, see e.g. Doyle and Donoghue (1986a, b) and especially Doyle (2006, 2008b), for some wood anatomy, see Esteban et al. (2023), for aspects of leaf anatomy and development, see Romanova et al. (2023a), for nodal anatomy, see Rodin and Paliwal (1971: summary), for stomata, see Rudall and Rice (2019) and also Herrera et al. (2020), for microsporangium arrangement, see Hufford (1997a) and for pollen, see Osborn (2000: comparison with gymnospermous "anthophytes"), Yao et al. (2004: comparison with pollen of Nymphaea colorata), Rydin and Friis (2005: pollen germination) and Tekleva and Krassilov (2009), Tekleva (2015) and articles in Grana 55(1). 2016, for pollen morphology, including that of fossils. Friedman (1992), Carmichael and Friedman (1996) and Friedman and Carmichael (1997, and references) discuss double fertilization and Friedman (2015) that and much more, Takaso (1985 and references) described integument morphology, Endress (1997) and Rudall (2021) details of megasporangiate structures and Mathews and Tremonte (2012) greening of seedlings in the dark.
Phylogeny. Within Gnetales the relationships [Ephedra [Gnetum + Welwitschia]] have consistently been recovered (e.g. Price 1996).
Classification. Y. Yang et al. (2022) place the three genera in their Gnetidae in separate orders, families and genera; rather surprisingly, although they think that Gnetidae and Pinidae are sister taxa, there is nothing in their classification that might suggest this... But the classification below, separate families for the three genera, is also nonsensical from a strictly classificatory point of view.
For further information on the other major seed plant groups, see angiosperms, Cupressales, Cycadales, Ginkgoales, and Pinales, and for discussion about their relationships, see also Angiosperm History I, conifers in general, and extant seed plants in general.
Synonymy: Ephedrales Dumortier, Tumboales Wettstein, Welwitschiales Reveal - Ephedridae Reveal, Gnetidae Pax, Welwitschiidae Reveal - Ephedropsida Reveal, Gnetopsida Thomé, Welwitschiopsida B. Boivin - Gnetophytina Reveal - Gnetophyta Bessey
EPHEDRACEAE Dumortier - Ephedra L. - Back to Gnetales
Xeromorphic shrubs (small trees, climbers); cyclopropyl amino acids +; vessel elements with scalariform perforation plates; nodes 1:2; stem green, photosynthetic; stomatal parallel to organ axis, development perigenous; leaves (whorled), venation open, reduced, scale-like, palisade mesophyll 0/(long, linear, terete); microsporangiate units: strobili of 2-8 synangia, each with 2(-4) sporangia, dehiscence porose, sterile ovules usu. 0; pollen smooth, pseudosulci unbranched (with short branches at right angles), (sterile ovules 0); megasporangiate units: cone bracts 2-5 pairs (3-6 whorls of three), each bract with two longitudinal bundles; ovules axillary to cone bracts, 1-2(-3) ovules/cone, seed envelope elliptic, triangular or square in t.s., with 2-4 bundles, integument ca 2 cells across, micropylar closure by mucilaginous secretion, parietal tissue ca 20 cells across, develops from epidermal and subepidermal tissue; male gametophyte: pollen germinates in 1-2 hours, 6-nucleate [prothallial cell/prothallial nucleus/tube cell/sterile nucleus/gametes], tube reaches nucellus in 10-16 hours, grows ca 14 µm h-1, pollination-to-fertilization 10-36 hours, one gamete fuses with nucleus of ventral canal cell to produce supernumerary zygote; female gametophyte: monosporic, archegonia exposed at base of deep pollen chamber, archegonial neck very long; eight separate proembryos formed; cone bracts with mucilaginous cells, becoming fleshy, or dry, winged or not; seeds to ca 2 cm long, papillate on the inner side of the outer covering; embryo spathulate; n = 7 (polyploidy - to 8n); nuclear genome [1 C] (8.1-)9.5(-38.3) pg; loss of two more group II mitochondrial introns.
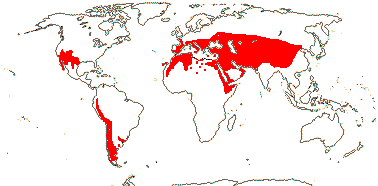
1/54. North (warm) temperate, W. South America, S.E. Brazil; drier habitats. Map: see Frankenberg and Klaus (1980), Caveney et al. (2001) and Ickert-Bond and Renner (2016: Fig. 2). [Photos - Ripe seed, Megasporangia, Habit, Microsporangia, Dwarf plant.]
Age. Ickert-Bond et al. (2009; see also Rydin & Ickert-Bond 2010; Rydin et al. 2010) estimate that divergence within Ephedra occurred quite recently, perhaps only (73.5-)30.4(-20.55) Ma (see also Huang & Price 2003: 32-8 Ma; Y. Yang et al 2017: (28-)19, 18(-12) Ma), but c.f. the fossil record below. On the other hand, estimates in Laenen et al. (2014) are ca 221.8 Ma, (130-)120(-110) Ma are those in Rydin and Hoorn (2016), around the later Palaeocene, somewhat under 60 Ma, in Loera et al. (2015) and ca 29.3 Ma (Ickert-Bond et al. 2020). At 74-2 Ma the estimates in Rydin et al. (2021) allow you to say pretty much anything you like - which is really the point.
A variety of fossils assignable to Ephedraceae, even Ephedra itself, are reported from the Lower Cretaceous in China (Zhou et al. 2003; Y. Yang & Ferguson 2015 and references). Rothwell and Stockey (2009) report a fossil from the Lower Cretaceous that has purportedly ancestral characters for Ephedra - two ovules together, and absence of a tubular micropyle and of a structure surrounding the ovule (seed envelope above), but this is unlikely to be assignable to crown group Gnetales (other Ephedra-type fossils from this period may have three ovules - Yang & Wang 2013). The distinctive pollen of the E. foemina type has been found inside fossil seeds that are morphologically also Ephedra-like in late Aptian to Early Albian (early Cretaceous) deposits from Portugal, suggesting that diversification in the genus occurred some 127-110 Ma (Rydin et al. 2004). Indeed, Early Cretaceous fossils of Ephedra have a "modern" morphology, E. paleorhytidosperma having distinctive seeds very like those of the extant E. rhytidosperma (Yang et al. 2005) - the basic morphology of Ephedra seems to have been stable for a long time. However, Doyle (2016) suggested that early fossil seeds cannot be assigned to crown Ephedra. Pollen with a derived morphology, i.e. with laterally-branched pseudosulci, the E. distachya type, is known from around 95 Ma in the Cretaceous-Raritan (Bolinder et al. 2015b), although if such pollen is derived within extant Ephedra there is some conflict over dates/the evolution of this pollen type (see also Norbäck Ivarsson 2013; Rydin et al. 2021 for ages).
To summarize: Rydin et al. (2021) noted that molecular estimates of the age of Ephedra of 4-3 and 74-13 Ma were both still on the table; fossil pollen ca 100 Ma (e.g. Bolinder et al. 2015b) might indeed be that of crown Ephedraceae, while seeds from the early Cretaceous (Rydin et al. 2004, 2006) also showed remarkable similarity to those of crown Ephedra (but c.f. Rydin et al. 2021 for such Cretaceous fossils). This is another group where there is much sorting out to do.
Evolution: Divergence & Distribution. The history of Ephedra has a number of surprises, not least that we still currently cannot be at all sure of both details of relationships and ages of clades in the genus (Rydin et al. 2021), which means that we cannot really say anything much about evolution here at all, and there may b e quite a long evolutionary fuse. A variety of stem-group Ephedraceae are known fossil especially from the Jurassic-Cretaceous in northern China (Y. Yang et al. 2020 and references). Ephedroid pollen was notably common 125-85 Ma in northern Gondwana, and derived pollen morphologies (pollen with pseudosulci) are known from the Late Cretaceous (Crane & Lidgard 1980; see also Friis et al. 2014; Bolinder et al. 2016). Ephedra (or ephedroid) seeds with a variety of morphologies are known from both South America and East Asia, and include fleshy dispersal units found in 125-120 Ma deposits (Y. Yang & Wang 2013).
Seeds of Ephedraceae are similar to those of fossil Erdmanithecales (Rydin et al. 2006) [Develop]. Within Ephedra, there has been parallel evolution in micromorphological details of the seed envelope (Ickert-Bond & Rydin 2011).
Ephedra seems to have gone into a severe decline at the end of the Cretaceous, and subsequent diversification in the Palaeogene may be linked to the adoption of wind pollination (Bolinder et al. 2012, 2016). Furthermore, how far the different seed types of Ephedra are dispersed may affect diversification rates (Loera et al. 2015), who also discuss diversification times, evolution of niche breadth, etc., in South American taxa. Qin et al. (2013) discuss Miocene movement of Ephedra on to the Qinghai-Tibet plateau and its diversification there.
Ephedra perhaps moved from the Old to the New World in the Oligocene (41.5-)29.6(-8.8) Ma and to South America in the Miocene - estimates are (41.5-)24.8(-8.8) Ma (Ickert-Bond et al. 2009). Ephedra had the oldest amphitropical disjunction, temperate or desert, mentioned by Simpson et al. (2017a) in his summary of such disjuncts.
The vascular pit membranes of Ephedra are quite like those of other gymnosperms, not angiosperms (Dute et al. 2014).
For allopolyploidy and speciation in Ephedra, see H. Wu et al. (2016), also discussion in Ickert-Bond and Renner (2016). Hybridization, introgression and/or polyploidy seem to have been involved in the evolution of the genus (Rydin et al. 2021); see also below under Genes & Genomes.
Pollination Biology & Seed Dispersal. Pollination in extant species of Ephedra is mostly by wind (Niklas 2015). However, E. foemina is pollinated by insects and i.a. has pollen with a faster settling velocity than that of wind-pollinated taxa with a thin tectum and a low density of granules in the infratectum. Indeed, some fossil ephedroid pollen also has characteristics of insect pollination with a thick tectum and dense infratectal layer, and insect pollination may be the plesiomorphic condition for the genus (Bolinder et al. 2015a, b; Hofmann et al. 2021b; c.f. Hall & Walter 2011 in part). Insect-pollinated taxa like E. foemina also have pollen with some 12 or so unbranched pseudosulci, compared with maybe 6 or so branched pseudosulci in wind-pollinated taxa (see also Steeves & Barghoorn 1959 for pollen variation). Pollination in E. foemina is probably by nocturnal moths, pollen being released around the time of the full moon, and as in other species, both the sterile ovules in male strobili and the ovules in female strobili produce pollination droplets (Rydin & Bolinder 2015) - but is this mechanism basal for the genus (see topology in Rydin et al. 2021)? Interestingly, the gross morphology of the pollen of the wind-pollinated E. trifurca is more like that of E. foemina, but its settling velocity is slower and more like those of other wind-pollinated taxa (Bolinder et al. 2015a; see also Bino et al. 1984). Niklas and Buchmann (1987) carried out experiments on two species of Ephedra (one was E. trifurca) and found that under at least some airspeeds pollination of ovules by conspecific pollen was more likely. Pollination in E. fragilis, from the Balearics, is ambophilous, with both wind pollination and lizards on occasion lapping up the pollination droplets and pollinating the plants (Fuster & Traveset 2019). But whatever the method of pollination, pollination droplets rich in sucrose are produced by the ovules, and in wind-pollinated taxa they aid in pollen tube formation and development (von Aderkas et al. 2014). There are protein and peptide fragments (= degradome) in the pollination drops, probably formed as cells break down as the pollen chamber develops (von Aderkas et al. 2014), although some proteins may have defensive functions, etc. (c.f. Wagner et al. 2007). Of course, work on wind-dispersed spores in all groups is relevant to what goes on here - so see spore dispersal in bryophytes, lycophytes, pines) etc., leptosporangiate ferns and even wind-pollinated angiosperms under Pollination & Seed Dispersal/Fertilization & Spore Dispersal.
Because the pollen exine of Ephedra is shed on germination (shed exines can be seen in some fossils - Bolinder et al. 2015b), the male gametophyte is naked. El Ghazaly et al. (1998) qualify the apparent absence of germination apertures in the pollen grains. Fertilization occurs 10-15 hours after pollination (Williams 2012b and references).
As the seeds ripen, the bracts surrounding the ovule may become fleshy and brightly coloured, and in the case of Ephedra fragilis, from the Balearics, the lizards that pollinate the plants also eat the seeds and associated bracts, dispersing the seeds (Fuster & Traveset 2019).These bracts may dry and become membranous, enclosing winged seeds, so wind-dispersed, or the seeds may be faintly nondescript, being individually dispersed by scatter-hoarding rodents (Hollander & Vander Wall 2009; Loera et al. 2015).
Plant-Animal Interactions. Ceutorhynch seed weevils, specifically, Oxyonychini, have radiated on Ephedra; there are about as many species of the weevil as there are of Ephedra, and there has been a single movement on to the genus but no movements on to other hosts (Letsch et al. 2018).
Genes & Genomes. Ickert-Bond et al. (2020) found that around 80% of the species of Ephedra for which they had data (41/49) were polyploids, 22 of these species having mixed ploidy levels, some up to four; all the species from Asia examined were polyploid while there were most diploid taxav in the New World. Overall the genome size of the monoploids varied little - 1 Cx = 8.1-10.4 pg, and genome size increasing in proportion to the ploidy level of the plant - there was no genomic downsizing (Ickert-Bond et al. 2020).
There has been a great increase in the rate of synonymous substitutions in the mitochondrial genome, and plastome and nuclear sequences are also divergent compared with those of other seed plants (Mower et al. 2007 and references).
The nuclear genome is variable in size, and can be quite large (see above: Ickert-Bond et al. 2014b, 2015a, 2020). This is connected with extensive variability in chromosome numbers, which in turn is linked with polyploidy, unusual for gymnosperms (see also Rastogi & Ohri 2020). However, diploidization in Ephedra is slow, there has been little subsequent genome downsizing and evolution of the subgenomes is unbiased, unlike the common situation in angiosperms (Ickert-Bond et al. 2015a, 2020; see also Scott et al. 2016; H. Wu et al. 2020).
Chemistry, Morphology, etc.. S-lignin was produced by Ephedra viridis, the S:G ratio there being similar to that of the angiosperms examined (Gómez Ros et al. 2007b). Species of Ephedra are pharmacologically very active and contain a number of distinctive secondary metabolites (Caveney et al. 2001).
Biswas and Johri (1997) mention the "deep origin of the periderm", although from the literature summarized by Martens (1971) exactly where it originates seems to be variable. Early Cretaceous fossils are described as having a dichasial branching pattern and linear leaves with two parallel veins (Y. Yang & Wang 2013). For leaf and nodal anatomy of extant species of Ephedra with well developed, long, linear leaves, as in E. altissima, see Dörken (2014) and Deshpande and Keswani (1963); the xylem of the two vascular bundles that run the length of the leaf faces each other, the leaf having been ventralised.
San Martin et al. (2022) discuss the development of the foliar stuctures surrounding the seed proper and their maturation; heterochrony is involved, and they become either fleshy or dry and scarious. There is only a single integument (see also above). The seed envelope surrounding the ovule proper is first apparent as an arcuate structure adaxial to the ovule, but it is thought to represent connate foliar structures; it is both dermal and subdermal in origin, compared with the dermal origin of the integument (Takaso 1985; Rydin et al. 2010); Y. Yang (2004 and references) also examined the ontogeny of the outer covering of the ovules. Martens (1971) lists some 13 hypotheses concerning the nature of the bits and pieces surrounding the ovule... Each of the eight cells formed in the initial cellularization of the young embryo produces a separate proembryo (Rudall & Bateman 2019b).
For general information, see Rydin et al. (2004) and the Gymnosperm Database, and for nodal anatomy, see Marsden and Steeves (1955) and Singh and Maheshwari (1962).
Phylogeny. There is little strong phylogenetic structure along the backbone of a 7 plastid gene-2 compartment analysis of extant species of Ephedra, indeed, there is overall rather little molecular divergence within the genus (Rydin & Korall 2009; Rydin et al. 2010). Mediterranean species may form a grade at the base of the tree, and in the monophyletic New World clade, a South American clade is embedded in a paraphyletic North American group, however, in some analyses the Mediterranean species form a clade (e.g. Rydin & Korall 2009; Ickert-Bond & Renner 2016 and references). The insect-pollinated E. foemina (see above) may be sister to the rest of the genus (e.g. Ickert-Bond et al. 2020 - Rydin et al. 2021 did not recover this position) - overall relationships there are [W. Asia to Mediterranean [Asia [Asia + the New World]]]. Indeed, Rydin et al. (2021) noted that nuclear ITS data had largely determined previous ideas of relationships, but they sequenced plastomes and the nuclear ribosomal cistron, and obtained very different results from the analyses of the two sets of data. Although an American clade, and also Mediterranean and Asian clades, were recovered, in the first E. pedunculata was migratory, and in the latter two a number of species moved around, some out of the clades entirely; E. foemina was never sister to the rest of the genus (Rydin et al. 2021).
Classification. For a classification of Ephedraceae, including fossil members, see Y. Yang (2014).
>[Gnetaceae + Welwitschiaceae]: cyclopropenoid fatty acids in seed oil, polysaccharide gums +; (successive cambia in roots); nodes multilacunar, three [or more] primary veins proceeding to the leaves; branched sclereids +; stomatal development syndetocheilic, mesogenous; cataphylls 0; leaves with closed second order venation; microsporagia in groups, with abortive apical ovules; pollen endexine lamellate [?Ephedra]; male gametophyte with one ephemeral prothallial cell, sterile cell absent; ovules with additional pair of bracts, integument 5-7 cells across, apex lobed, micropyle blocked by tissue from expanded integument [by periclinal cell divisions, also radial cell expansion], pollen chamber small; male gametophyte: 4-nucleate [prothallial cell/tube cell/gametes]; female gametophyte tetrasporic, chalazal portion densely cytoplasmic, nuclei free, scattered, cell walls enclosing groups of nuclei that later fuse, archegonia per se 0; embryonic initial free-nuclear phase basically 0, some cells of embryonal mass elongate, (cleavage polyembryony +), lateral protrusion of the hypocotylar axis + ["feeder"]; germination hypogeal.
Age. Ickert-Bond et al. (2010) suggest ages of (127-)111.3(-87.2) Ma for this node, Won and Renner (2006) ages of (175-)138(-112) Ma, Y. Yang et al. (2017) ages of (189-)151, 119(-91) Ma and Rydin et al. (2021) an age of 123-113 Ma. Ages around 87.7 Ma in Tank et al. (2015: Table S2), ca 94.4 Ma in Laenen et al. (2014) and about 81.9 Ma in Magallón et al. (2013) are all rather younger; on the other hand, the age in Magallón et al. (2015: note topology) was around 239 Ma.
Siphonospermum, a fossil in deposits 125-120 Ma from the Lower Cretaceous from Northeast China, may be assignable to this part of the tree; although its ovules are single, the micropylar tube is very long (Rydin & Friis 2010). See below for fossils like Cratonia placed in Welwitschiaceae which, if correctly identified, would rule out the younger ages above.
Evolution: Divergence & Distribution. The absence of dicer-like 2, which makes sRNAs, from Gnetum and Welwitschia (L. Ma et al. 2015) may be an apomorphy somewhere around here.
Chemistry, Morphology, etc.. For cyclopropenoid acids, similar to those in Malvales, see Aitzetmüller and Vosmann (1998). Rodin (1968) suggested that the reticulate venation of Gnetum, at least, was a modified dichotomizing system. For the tetrasporic female gametophyte that is an apomorphy here, see Haig (2020).
GNETACEAE Blume - Gnetum L. - Back to Gnetales —— Synonymy: Thoaceae Kuntze
Usually lianes, often twining (trees); ectomycorrhizae +; (Si02 accumulation - G. gnemon]); roots diarch; shoots with reaction wood on ?upper side, (successive/ectopic cambia developing in inner cortex - lianes); vessel elements with vestured pits, (tracheids lacking margo), xylem parenchyma +; sieve tubes with companion cells [not derived from tube cells]; nodes multilacunar; laticifers +; intracellular calcium oxalate crystals 0; petiole with 4-11 bundles arranged in an arc; stomatal orientation various; leaves petiolate, lamina +, midrib +, secondary veins pinnate, fine venation reticulate [altogether more than two orders of branching], with internally directed veinlets, development dispersed, veins (4.4-)5.7(-7.4) mm/mm2, palisade mesophyll +, subepidermal system of hydrophilic intrusive cellulosic fibres (G. g.)/other kinds of (branched) fibres/stellate sclereids; (plant monoecious), (mega- and microsporangia together); microsporangiate cones: microsporangiophore with (1-)2(-4) sporangia; pollen spherical, not ridged, surface spinose; (sterile ovules 0); megasporangiate cones: ovules, etc., whorled, with two envelopes, outermost envelope + [formed by connate bracts], envelopes and integument vascularized, integument developing a flange that grows between inner and outer envelopes (?0), parietal tissue ca 8-?20 cells across, nucellar cap ca ?20 cells across; female gametophyte with 256->1500 free nuclei; pollen tube reaches nucellus in up to 7 days, pollination-to-fertilization 6-8 days, both gametes fuse with nuclei in the syncytium; polyembryony common, only one embryo develops; seeds large, ca 7 cm long, outer envelope fleshy, middle envelope ± sclerotic, inner envelope ± crushed; embryo with elongated, branched suspensor tubes, also several embryonal tubes, nucleus at end divides forming an embryonal mass; n = 22 (24); nuclear genome [1 C] 2.15-4.1 pg [2 C 4401-7785 Mbp], one copy of the LEAFY gene.
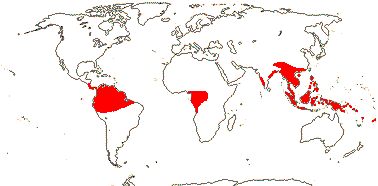
1/38. Tropical, rather disjunct. Map: see Renner (2005b) and Ickert-Bond and Renner (2016: Fig. 2). [Photos - Collection]
Age. Crown-group Gnetaceae are (77-)44-26(-13) Ma, or using a strict clock, as little as 6 Ma (Won & Renner 2006); (98-)81(-64) Ma is the age suggested by C. Hou et al. (2015) and (95-)71, 62(-43) Ma that in Y. Yang et al. (2017); for other dates, see Tedersoo and Brundrett (2017). The spread of ages in Rydin et al. (2021) is 57-9 Ma.
Protognetum jurassicum, with whorled ovules and rather Ephedra-like foliage, was described by Y. Yang et al. (2017) amd they thought that it was stem-group Gnetaceae.
Evolution: Divergence & Distribution. For biogeographical relationships in the genus, basically a story of post-Eocene diversification and dispersal, see Renner (2005b) and Won and Renner (2006).
Gnetum gnemon (?the whole genus) is the only non-angiosperm seed plant in which production of provisions for the embryo - of course, it is a gymnosperm, and these come from the female gametophyte - is largely a post-fertilization phenomenon (Friedman & Carmichael 1996); the embryo itself largely develops after the seed falls (see also Ginkgo, etc.). However, the ovule does increase appreciably in size between pollination and fertilization (Leslie & Boyce 2012).
Ecology & Physiology. The reaction wood in Gnetum consists of gelatinous extra-xylary (reaction) G-fibres in the adaxial position (Tomlinson 2001b, 2003; see also Höster & Liese 1966); it is not typical tension wood. Such G-fibres may be involved in the twining movements of the stems of the climbers (Chery et al. 2021). The leaves of Gnetum have increased venation density compared with that of other gymnosperms and are more like angiosperms, although details of leaf anatomy, etc., differ and physiologically New Guinean species of Gnetum are rather like understory angiosperms (Feild & Balun 2007; Boyce et al. 2009).
B and C-class genes alone are involved in the development of the microsporangia and ovules of Gnetum, and in a generally similar way as those two classes of genes are involved in stamen and carpel development in angiosperms (Ruelens et al. 2017 and references).
Pollination Biology & Seed Dispersal. Both fertile and sterile ovules have pollination droplets coming from the micropyle, and entomophily has been reported from Malesian species of Gnetum (e.g. Kato & Inoue 1994). Jörgensen and Rydin (2015) float the possibility that African species of Gnetum, which lack sterile ovules in the male cones and so have no obvious attractants for insects there, may be wind pollinated.
Plant-Bacterial/Fungal Associations. The ECM fungal community associated with Gnetum is not very diverse; Scleroderma (Boletales) is reported to be associated with Gnetum in both Africa and Papua New Guinea (references in Corrales et al. 2018).
Genes & Genomes. For repetitive sequences in long terminal repeat retrotransposons, common in other gymnosperms and in Gnetum montanum, but not in angiosperms (Amborella is an exception), see Ran et al. (2018a) - G. montanum and Amborella are somewhat similar in intron size. Gnetum montanum has many fewer pseudogenes than the two Pinaceae with which it was compared.
Horizontal gene transfer of the mitochondrial nad1 intron 2 from flowering plants (an asterid) to an Asian clade of Gnetum seems to have occurred within the last 5 Ma (Won & Renner 2003).
Chemistry, Morphology, etc.. Gnetum gnemon contains fair amounts of silica (Trembath-Reichert et el. 2015).
The stomata may be in quartets, paired stomata formed almost simultaneously and oriented at right angles to one another; the lateral subsidiary cells quite often divide (Rudall & Rice 2019). Not surprisingly, the wood of the lianoid taxa is distinctive, with serial cambia being formed. Boyce and Knoll (2002) discuss leaf development in megaphyllous vascular plants, including Gnetum. See also Martens (1971) and Rodin (1968) for the vascularization of the leaves; both talk about the venation at least initially dichotomizing, but c.f. Tomlinson and Fisher (2005).
There is vascular tissue in the two outer envelopes covering the ovule, but vascular bundles barely enter the base of the integument, although this latter is very short (Takaso & Bouman 1986; see also Rodin & Kapil 1969). The outer envelope is definitely bilobed early in development, the lobes alternating with the bract, but the middle envelope is only weakly bilobed, the lobes decussating with those of the outer envelope (Takaso & Bouman 1986). Both the nucellar cap and the nucellus s.str. in Gnetum gnemon are massive affairs, although their respective limits are hard to determine. Ovules from mixed-sex strobili in G. gnemon have only a single envelope (plus integument), while those from ovulate strobili have two; the former ovules abort (Zumajo-Cardona & Ambrose 2021). These authors looked at the expression of four genes involved in the development of the integuments in Arabidopsis during ovule development in Gnetum. In Arabidopsis two of these genes, AINTEGUMENTA (ANT) and BELL1 (BEL1), were expressed in both integuments, and two, KANADIs and UNICORN, were expressed in the outer part of the outer integument only. In Gnetum, however, things were rather different. Expression in the integument was localised or even (BEL1) absent, expression in all or part of the nucellus was often quite strong and occurred in all four genes, and genes like ANT might also be expressed in the microsporangia. Expression in the integument tended to be at the apex (Zumajo-Cardona & Ambrose 2021; see also Zumajo-Cardona et al. 2021a: fig. 7). For a comparable study of ovule development in Ginkgo, see Zumajo-Cardona et al. (2021a), and for ovule evolution in general, see elsewhere.
For general information, see Maheshwari and Vasil (1961), Martens (1971) and the Gymnosperm Database, for mycorrhizae, see Onguene and Kuyper (2001), for nodal anatomy, see Rodin and Paliwal (1971), for pollen, see Gillespie and Nowicke (1994) and Tekleva (2015), for reproductive morphology and development, see Sanwal (1962), for embryology and seed coat, see Vasil (1959).
Phylogeny. Relationships within Gnetum are strongly correlated with geography, i.e. [South America [Africa + S.E. Asia-Malesia]] (Won & Renner 2006), somewhat elaborated as [South America [Africa [S. Asia - 2 arborescent spp. + The Rest]]] (see also C. Hou et al. 2015).
WELWITSCHIACEAE Caruel - Welwitschia mirabilis J. D. Hooker - Back to Gnetales
Cyclopropenoid fatty acids +; main stem apex aborts, tunica-corpus construction unclear; fibre tracheids 0, vessel elements with simple perforation plates, tracheids lacking margo; successive/ectopic cambia + developing in phelloderm; mucilage ducts +; sclereids with crystals in wall; nodes 1:1, ?departure level; plant with three pairs of leaves, second pair of leaves persisting for the life of the plant, with basal cambium, veins numerous, isobilateral [palisade tissue on both surfaces], amphistomatic, stomata parallel to the long axis of the leaf, brachyparacytic (tetracytic), 3rd leaf whorl scales, laterally flattened, strobili of 6 synangia each with three sporangia, dehiscence radial; pollen monosulcate, plicae + [?= psuedosulci], numerous, surface otherwise smooth, exine not shed during germination; sterile ovule +; micropylar closure by secretion; additional bracts free; female gametophyte lacking central vacuole, cell wall formation throughout, micropylar cells with separate nuclei, growing upwards through nucellus forming prothallial tubes; only one sperm nucleus functional, fertilization in prothallial tube; seed with envelope forming broad membranous wing; proembryo pushed back down gametophytic tube by elongating embryonal suspensor; n = 21, all telocentric; nuclear genome [1 C] ca 7.2 pg [2 C 14083 Mbp], genome duplication +; 2nd intron in mitochondrial nad1 gene lost; seedling with collar.
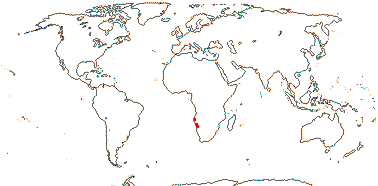
1/1. S.W. Africa, desert close to the Atlantic ocean. Photos: Collection.
Evolution: Divergence & Distribution. Cratonia cotyledon is a fossil seedling with distinctive vasculature of the cotyledons very like that of the leaves of Welwitschia, the secondary veins leaving from the primary veins fuse to form an inverted "Y" (Rydin et al. 2003). Cratonia was found in N.E. Brazil and is late Aptian or early Albian in age, perhaps 114-112 Ma; other fossils of welwitschiaceous or more generally gnetalean affinity have been found in the same area (Dilcher et al. 2005; Löwe et al. 2013; see also Kunzmann et al. 2024). If indeed Cratonia is close to Welwitschia, continental drift might explain their distributions.
For some time it was thought that Welwitschia mirabilis was neotenous, but Martens (1977a) showed that there were no juvenile characters in the adult. As he noted (ibid.: p. 916), the plant "has "lost its head" at an early stage".
Ecology & Physiology. Welwitschia mirabilis grows in the Namib desert close to the ocean. Although there is little rain there, although fogs are frequent - but not where Welwitschia grows (von Willert 1985). The plant is very deep-rooted. For aspects of vascular anatomy that may be associated with this habitat, see Carlquist (2012b). Welwitschia may be a CAM plant, but at very low levels, and malic/citric acids tend to concentrate towards the apex of the leaf, perhaps enhancing water flow at night (von Willert et al. 2005). There is quite a thick layer of calcium oxalate crystals immediately below the foliar cuticle, perhaps protecting the mesophyll beneath, the cell walls of the guard and subsidiary cells of the stomata have thin areas, facilitating their rapid movement as conditions improve or deteriorate, and new root hairs develop beneath the dry, dead old hairs within 50 hours of watering (Krüger et al. 2017, q.v. for much on anatomy and physiology).
Plants can live for hundreds of years, possibly up to 1,500 years or so, the same two leaves growing all this time at the base and fraying at the apex, yet remaining functional even at the apex (Herre 1961; Krüger et al. 2017). This prolonged basal growth of the leaves involves the expression of KNOX1 genes, so maintaining an undifferentiated state there (Pham & Sinha 2003). Wan et al. (2021) discuss various aspects of the growth of these leaves, i.a. noting that there was some similarity in the genes involved with those in Streptocarpus (Gesneriaceae), another plant with continuously-growing and long-lived (but not compared with Welwitschia) leaves.
Pollination Biology & Seed Dispersal. Pollination appears to be by diptera (Wetschnig & Depisch 1999; Bolinder et al. 2015 and references).
The seeds, although winged, are heavy and it is unclear that there is much in the way of dispersal (Ickert-Bond & Renner 2016).
Plant-Animal Interactions. Populations of the hemipteran Probergrothius angolensis - it and its relatives eat seeds of Malvaceae - have moved on to Welwitschia mirabilis, and the microbiome of the former has changed both qualitatively and even quantitatively; interestingly, larvae of the Malvaceae-eating ancestor die when fed seeds of Welwitschia (A. J. Martinez et al. 2019).
Genes & Genomes. Z. Li (2015; see also Li & Barker 2019/2020; Cui et al. 2006) thought that the genome of Welwitschia had been duplicated, as is perhaps suggested by its high chromosome numbers (this is not a duplication for Gnetales as a whole). Wan et al. (2021) dated the duplication at (96-)86(-78) Ma, and noted that there had been extensive genome shuffling since parts of the genome from a single chromosome of Gnetum were widely scattered in the genome of Welwitschia - and note that the two have similar chromosome numbers. Interestingly, all the chromosomes of Welwitschia are telocentric (see Rastogi & Ohri 2020).
The chloroplast genome of Welwitschia mirabilis is the smallest plastid genome of all non-parasitic land plants that have inverted repeats (McCoy et al. 2008). ndh genes have been lost here, as in other plants with various distinctive life styles that might affect the photosynthetic process and result in the loss of such genes (Mower et al. 2021; Sabater 2021).
The large mitochondrial genome of Welwitschia has lost genes and introns and is otherwise remarkable (W. Guo et al. 2016a, see also 2016b), however, practically nothing is known about the mitochondrial genome of other Gnetales.
Chemistry, Morphology, etc.. Because of the abundant, branched sclereids in the plant, "One might as well try to cut sections of a thick Scotch plaid blanket as to try and cut a stem of Welwitschia without imbedding." (Chamberlain 1935: pp. 388-389). There is a single vascular trace associated with the scaly body (Martens 1977b). The foliar mesophyll has vertical stacks of subhypodermal fibres (Krüger et al. 2017).
Kaplan (1997, vol. 1:6) described the seedling as having a haustorial collet (collar). Serial axillary buds are added throughout the course of the long life of the plant, the youngest such buds being in the centre of the axil - a branch-like organization? See Martens (1971) for the vascularisation of the bracts of the megasporangia and the complex organisation of the axis of the megasporangiate strobilus.
For general information, see papers by Martens and collaborators (Martens 1971, 1977b) and the Gymnosperm Database, for pollen, see Bolinder et al. (2015a), and for female gametophyte development and fertilization, see Friedman (2014, esp. 2015: remarkable).
Botanical Trivia. Since the age of the two main leaves is basically that of the plant as a whole, Welwitschia has the longest-lived leaves of any plant.
Synonymy: Tumboaceae Wettstein