EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[[LILIALES + ASPARAGALES] COMMELINIDS]: (inflorescence branches cymose); protandry common.
Age. Magallón and Castillo (2009) suggested ages for this clade of 135.7 and 120.1 Ma, and they are (137.4-)126.4(-116) Ma in Eguchi and Tamura (2016), ca 126 Ma in Givnish et al. (2018b), ca 125 Ma in Foster et al. (2016a: q.v. for details), ca 124 Ma in Janssen and Bremer (2004), rather older than the estimates in Bremer (2000), (131-)124(-116) Ma in Givnish et al. (2016b, see also 2014b), (131-)122(-109) Ma in Merckx et al. (2008a), (125-)117, 116(-111) Ma in Hertweck et al. (2015: c.f. ages for [Asparagales + commelinids]), 116.9 Ma in Magallón et al. (2015), (116-)111, 102(-97) Ma in Wikström et al. (2001), and around 96.1 Ma in Magallón et al. (2013). Estimates are 121-97 Ma in Mennes et al. (2013, see also 2015), around 106 or 95 Ma in S. Chen et al. (2013), 108-91 Ma in Foster and Ho (2017).
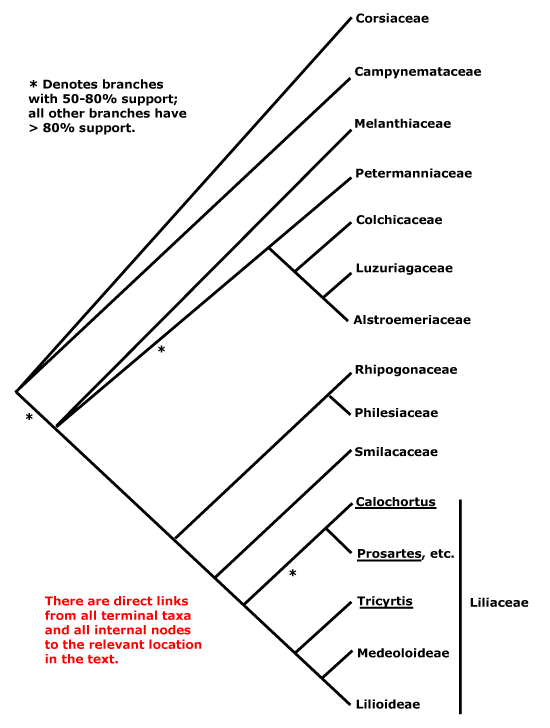
Phylogeny. For discussion about the relationships of Liliales, see Petrosaviales.
(Plants geophytes); storage fructans, chelidonic acid, steroidal saponins +; root hairs from unmodified rhizodermal cells, (velamen +); (cuticular waxes as platelets transversely arranged in parallel series); leaf blades elliptical, (main veins seven or fewer); inflorescence terminal; T large (small), free, (spotted), tepal nectaries +; anthers extrorse; style often long, stigma capitate; ovules many/carpel, parietal tissue none, nucellar cap +; P deciduous; tegmen with cellular structure; endosperm with thick pitted walls, hemicellulosic; mitochondrial sdh3 gene lost. - 10 families, 67 genera, 1,580 species.
Includes Alstroemeriaceae, Campynemataceae, Colchicaceae, Corsiaceae, Liliaceae, Melanthiaceae, Petermanniaceae, Philesiaceae, Ripogonaceae, Smilacaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown group Liliales were dated to ca 117 Ma by Janssen and Bremer (2004), rather older than the estimates in Bremer (2000: Campynemataceae sister to the rest); estimates are (131-)118(-78) Ma in Merckx et al. (2008a), (125-)113(-100) Ma in Givnish et al. (2016b - ca 100 Ma in 2014b, ca 111 Ma in 2018b), (131-)121(-117) or (112-)105(-98) Ma in Hertweck et al. (2015), 105-43 and 104-67 Ma in Mennes et al. (2013, 2015 respectively) and a mere 69.4 Ma in Tang et al. (2016). Other estimates are around 97.4 Ma (Magallón et al. 2015) and 112.9 Ma (Tank et al. 2015: Table S2, Melanthiaceae).
See also Mennes et al. (2015) for ages in the order - note topology.
Evolution: Divergence & Distribution. Furness et al. (2015) optimise some pollen characters on a rather poorly resolved and sampled tree.
Liliales may have arisen in Australia at a time when the southern continents were more or less connected, but latterly a fair amount of long distance dispersal is needed to explain current distributions (Givnish et al. 2016b).
Ecology & Physiology. Grime and Mowforth (1982) had early noted links between large genomes and Liliales in the taxa they examined (focus on the British flora), and large genomes are notable in this clade, the geophytic habit, and fast growth by cell expansion of large cells (link to large genomes) under cooler conditions in the spring all being associated.
Tribble et al. (2022a) examined possible connections between the different kinds of geophytes that are common in Liliales and the local climate, but found that the climatic niches occupied by bulbs, corms, etc., were all largely similar, differing no more than expected by chance. The exception was plants with root tubers, as in Alstroemeriaceae-Alstroemerieae, which grew in conditions with lower temperature seasonality (the difference was significant). Tribble et al. (2020/2021a) had earlier found that completely unrelated geophytes nevertheless had some active gene groups in common (the focus of their work was Bomarea multiflora and its root tubers), although the activity of other gene groups that had been implicated in geophyte development in other taxa seemed notably similar, as in those found in both root tubers and fibrous roots Bomarea multiflora. Nevertheless, they suggested that overall "repeated morphological convergence may be matched by independent evolutions of similar molecular mechanisms" (ibid. p. 170).
Plant-Bacterial/Fungal Associations. Mycorrhizal associations in Liliales are commonly of the Paris-type where the hyphae are intercellular and form coiled structures between the cells (see F. A. Smith & Smith 1997). Recently it has been shown (Giesemann et al. 2019, 2021) that carbon may move to the plant from the fungus, so this association may be of considerable ecological importance.
Genes & Genomes. Liliales have by far the largest nuclear genomes in monocots, and also the greatest absolute spread in values - 1.5-152.2 pg (1C: Leitch & Leitch 2013). For chromosome size, see Vijayavalli and Mathew (1990 - as Liliaceae) and Tamura (1995).
Chemistry, Morphology, etc.. For the accumulation of fructan sugars, see Pollard (1982).
Inflorescence morphology in Liliales needs attention. Liliaceae and Alstroemeriaceae, at least, have distinctive radially-elongated endothecial cells (Manning & Goldblatt 1990). Inaperturate pollen is described as having apertures with some kind of operculum (Furness et al. 2015). Glucomannan seed reserves are reported from some species of Liliaceae-Lilioideae and Colchicaceae-Colchiceae (Jakimow-Barras 1973).
There is much information in Rudall et al. (2000); see also Conran et al. (2009b) for leaf and stomata, Schlittler (1953a) for inflorescences, Endress (1995b) for some floral development, Handa et al. (2001) for pollen of Japanese representatives and Rudall et al. (2000) and Furness et al. (2015) for general surveys of pollen morphology, El-Hamidi (1952) for gynoecium, Oganezova (2000b) for details of ovule morphology, etc., and Fukuhara and Shinwari (1994) for seed coat anatomy.
Phylogeny. The topology of relationships within Liliales was uncertain for some time, but it now seems to be settling down. J. S. Kim et al. (2012, esp. 2013) had found a weakly-supported [Melanthiaceae + Petermanniaceae] clade forming a trichotomy with Colchicaceae et al. and Liliaceae et al., and although the position of Campynemataceae as sister to the rest of the order had no bootstrap support, it has commonly been found (e.g. Givnish et al. 2006; Chase et al. 2006). Corsiaceae were included in none of these studies. Petermanniaceae are variously associated with Colchicaceae, Melanthiaceae and Alstroemeriaceae (see also J. Davis in Vinnersten & Reeves 2003; Graham et al. 2005). Although some work (Rudall et al. 2000) suggested that Petermannia should be included in Colchicaceae, the sample had been misidentified (see Chase et al. 2006). Indeed, Colchicaceae and Alstroemeriaceae (and Luzuriagaceae, here included in the latter family) are commonly linked (Vinnersten & Bremer 2001; Tamura et al. 2004a; Davis et al. 2004; Janssen & Bremer 2004; Givnish et al. 2014b; Chacón & Renner 2014). See also Chase et al. (1995a), Patterson and Givnish (2002), Chen et al. (2007: Bayesian analysis, support for many branches weak) and Givnish et al. (2016b: Melanthiaceae on a notably short branch). Campynemataceae have also turned up as sister to a [Smilaceae, Philesiaceae, etc.] clade (Chacón & Renner 2014), but Colchicaceae were the focus of that study. An odd clade, [Petermanniaceae + Liliaceae and relatives], was recovered by Tang et al. (2016).
Neyland (2002a), analysing variation in 26S ribosomal DNA, thought that Corsiaceae were sister to other Liliales (Neyland in Rudall & Eastman 2002 also suggested that Corsia was sister to Campynema), and similar relationships were recovered by Fay et al. (2006c) and Hertweck et al. (2015: [Campynema + Arachnitis], the latter with an extremely long branch). Although this position in Neyland (2002a) had only weak support, it is largely consistent with morphological evidence. Davis et al. (2004) and Petersen et al. (2012) also found Corsiaceae to be associated with Liliales. Analysis of 26S rDNA sequences suggested that Corsiaceae were polyphyletic; Arachnitis perhaps being sister to Thismia and/or Burmannia (Burmanniaceae-Dioscoreales: Neyland & Hennigan 2003; G. Petersen et al. 2006b: combined analysis), while Arachnitis was found to be well outside Liliales in the analyses of Kim et al. (2013), however, this position has not been confirmed (see also Givnish et al. 2016b; Lam et al. 2018).
In the whole plastome analyses of Givnish et al. (2014b: Campynemataceae and Corsiaceae not included) the family relationships below all have strong support. Mennes et al. (2015) found good support both for a monophyletic Corsiaceae and for a [Campynemataceae + Corsiaceae] clade, which may be sister to other Liliales, although this position was not always recovered and relationships within other Liliales were somewhat different from those suggested by Givnish et al. (2014b); analysis of plastid genomes found Arachnitis to be on a very long branch indeed (Mennes et al. 2015). This same long branch was also evident in Lam et al. (2016, 2018), and there was again some support for a [Corsiaceae + Campynemataceae] clade. Givnish et al. (2016b: 75 plastid genes from 133 taxa, 2 genes from 146 more taxa) is an elaborated version of the preliminary results in Givnish et al. (2014b), and the overall family-level topology is confirmed. Liliaceae, Smilacaceae, Ripogonaceae and Philesiaceae always form a clade, but relationships in it are unclear, either [Liliaceae [Smilacaceae [Ripogonaceae + Philesiaceae]]] (J. S. Kim et al. 2013; Givnish et al. 2014b), [[Ripogonaceae + Philesiaceae] [Smilacaceae + Liliaceae]] (Givnish et al. 2006; Chase et al. 2006; Fay et al. 2006c; Chacón & Renner 2014; Lam et al. 2018; H.-T. Li et al. 2019: support strong), or [Smilacaceae [Liliaceae [Philesiaceae + Ripogonaceae]]] (Givnish et al. 2018b); support varies. Support for the position of Smilacaceae has been underwhelming (see also Lam et al. 2018), hence it was placed in a tritomy with Liliaceae and [Ripogonaceae + Philesiaceae], however, the grouping [[Ripogonaceae + Philesiaceae] [Smilacaceae + Liliaceae]] is now followed (see H.-T. Li et al. 2019, 2021) and Li et al. also recovered Melanthiacaeae as sister to this clade. However, W. J. Baker et al. (2021: Seed Plant Tree, see also Version 2 Jan 2022) recovered the relationships [Campynemataceae [Melanthiaceae [Petermanniaceae [[Colchicaceae + Alstroemeriaceae] [Liliaceae [Smilacaceae [Ripogonaceae + Philesiaceae]]]]], while relationships in Timilsena et al. (2022a) are similar, although in some analyses a clade [Liliaceae + Smilacaceae] was recovered. Corsiaceae were not included in either of these last two studies, but sampling in Version 2 of the Seed Plant Tree was otherwise quite extensive. Relationships in Zuntini et al. (2024) show a [[Corsiaceae + Campynemataceae] [Melanthiaceae ...]] grouping that is basal to the rest of the family and there is a poorly-supported [Smilacaceae + Liliaceae] clade. Xie et al. (2024: Corsiaceae not included, focus on Lilieae) carried out a variety of analyses using transcriptome, Angiosperms353, chloroplast and mitochondrial data, recovering the topology below with the exception of there being a [Smilacaceae [Philesiaceae + Ripogonaceae]] clade. Relationships were similar in the ix.2024 version of the Seed Plant Tree, support for a [Smilacaceae [Philesiaceae + Ripogonaceae]] clade was weak and for the position of Petermanniaceae practically non-existent.
Classification. Rudall et al. (2000) suggested that Philesiaceae could be included in Smilacaceae, characters like pollen, endosperm storage, disintegrating testa, and absence of stem fructans agreeing, while combining Smilacaceae and Ripogonaceae was an option in A.P.G. II (2003). However, if either option were followed, the families should really be part of a broadly-circumscribed Liliaceae. Petermanniaceae have not been moved, pending better support for a changed posiition.
Previous Relationships. Cronquist (1981) circumscribed Liliales very broadly, and members of his Liliales now make up the bulk of both Liliales and Asparagales and other things besides.
Synonymy: Campynematinae Reveal, Smilacineae Reveal - Alstroemeriales Hutchinson, Campynematales Doweld, Colchicales Dumortier, Liriales K. Koch, Melanthiales Link, Paridales Link, Smilacales Link, Trilliales Takhtajan, Veratrales Dumortier
[Corsiaceae + Campynemataceae]: (pollen inaperturate); styluli +.
Age. the age of this clade is (102-)74(-42) Ma (Givnish et al. 2016b), (93-)70(-47) Ma (Mennes et al. 2015) or (98.7-)81.3(-67.6) Ma (D.-F. Xie et al. 2020).
CORSIACEAE Beccari, nom. cons. - Back to Liliales
Plant echlorophyllous, mycoheterotrophic, associated with glomeromycote fungi; rhizomatous or almost cormose; chemistry?; roots stout, endodermis not obvious; vessels?; epicuticular wax platelets parallel, stomata ?type; leaves spiral to two-ranked, sheathing, sheath closed, venation parallel; (plant ?dioecious); flowers single, terminal; monosymmetric; T large, median T of outer whorl adaxial, standard-like [= labellum]; ?nectary [= callus on labellum - Corsia?]; A (basally adnate to style [= gynostemium] - C.); (A 5 + staminode opposite "labellum" - C. dispar); (pollen monoporate - Corsia); (placentation parietal), styluli/style +, (short/none), (± unbranched), stigmas 3, subcapitate; ovules many/carpel, integuments two cells across, parietal tissue 1 cell across, nucellar cap?, funicle long; fruit dehiscing laterally from base with three valves, separating from placentae at apex; seeds dust-like, testal; (endosperm also with starch), embryo undifferentiated; n = 9, x = ?; seedling?
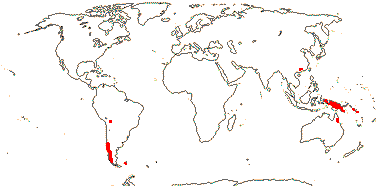
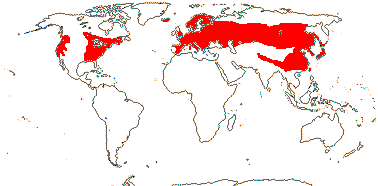
3 [list]/30. Scattered: western South America, Falkland Islands, S. China, Papuasia-Australia (map: from van Royen 1972; Ibisch et al. 1996; Ochoa et al. 2019). Photo: Flower.
Age. Crown-group Corsiaceae are (76-)53(-30) Ma (Mennes et al. 2015) or about 56 Ma (Givnish et al. 2016b: see discussion).
Evolution: Divergence & Distribution. The age of Corsiaceae - and of their fungal associates (ca 94 Ma - see Renny et al. 2017) - is compatible with a Gondwana break-up explaining the current distribution of the family, but other explanations are possible, too (Mennes et al. 2015; see also Givnish et al. 2016b).
Ecology & Physiology. Mycoheterotrophic Arachnitis is associated with glomeromycote fungi (e.g. Bidartondo et al. 2002; Winther & Friedman 2008). For more on mycoheterotrophy, see elsewhere.
Pollination Biology. The flowers of most species of Corsia are presented inverted and the labellum is erect or hangs down, the stamens, etc., being underneath it, however, in C. dispar the extreme curvature of the ovary just beneath the flower results in the labellum being abaxial (Jones & Gray 2008). The megaspore mother cell seems to have two cell layers above it; is this a nucellar cap? (Rübsamen 1986).
Plant-Bacterial/Fungal Associations. Glomus is involved in the mycoheterotrophic association of Arachnites and is in the same immediate clade as "species" involved in similar associations with Botrychium (Ophioglossaceae) and Lycopodiaceae (Winther & Friedman 2007, 2008), together part of the Glomus A group (Schußler et al. 2001). For the glomeromycote fungi, all notably similar, associated with Arachnitis, see also Bidartondo et al. (2002) and especially Renny et al. (2017), who dates a clade of fungi involved to (158.1-)94(-48.6) Mya, and for root morphology and mycoheterotrophy in the whole family, about which rather little is knowm, see Imhof et al. (2013).
Genes & Genomes. Not surprisingly, branch lengths in Corsiaceae are very long (Givnish et al. 2016b).
Chemistry, Morphology, etc.. For information, see Rübsamen (1986), Neinhuis and Ibisch (1998) and Merckx et al. (2013a), all general, and Rudall and Eastman (2002: morphology).
CAMPYNEMATACEAE Dumortier - Back to Liliales
Rhizome short, vertical, or 0; vessels 0; fibrous leaf bases persistent; chemistry?; stomata type?; leaf base sheathing [?type]; inflorescence morphology?, axis bracteate; flowers medium-sized, T green, not spotted; A adnate to base of T, tapetum 2- or multinucleate; (pollen inaperturate - Campynemanthe); style branches erect; ovules 3-many/carpel, crassinucellate; fruit a capsule or indehiscent, T enlarging, persistent; seeds angled, exotestal and endotegmic, with phlobaphene. or flattened; embryo minute; n = 11, x = 7 (?8, ?6), chromosomes to 3µm long; seedling?
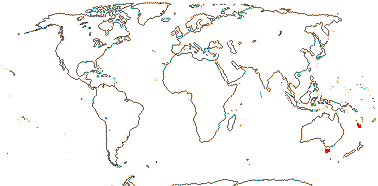
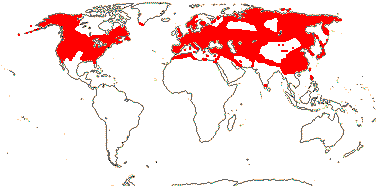
2 [list]/4: Campynemanthe (3). New Caledonia and Tasmania. Map: from van Balgooy (1984).
Age. The age of crown-group Campynemataceae has been estimated at ca 73 Ma (Janssen & Bremer 2004), (71-)39(-15) Ma (Givnish et al. 2016b), ca 36.5 Ma (Chacón et al. 2012b) or 70-16 Ma (Mennes et al. 2015).
Chemistry, Morphology, etc.. Campynemanthe has a subumbellate inflorescence, introrse anthers (see illustration in Kubitzki 1998b), a partly superior ovary and a dentate leaf apex; Campynema has extrorse anthers, multinuclear tapetal cells and an inferior ovary.
Additional information is taken from Kubitzki (1998b: general), Dahlgren and Lu (1985: Campynemanthe) and Lowry et al. (1987: cytology and embryology).
[[Petermanniaceae [Colchicaceae + Alstroemeriaceae]] [Melanthiaceae [[Liliaceae + Smilacaceae] [Philesiaceae + Ripogonaceae]]]]: root hypodermal cells dimorphic or not; rhizome +, ± woody; leaf blade venation reticulate, base not sheathing.
Age. The age for this node is estimated to be (123-)114, 86(-72) Ma (Bell et al. 2010), (117-)98(-82) Ma (Ginish et al. 2016b), ca 98.5 or 96 Ma (Conran et al. 2014), or (92.5-)82(-71.5) Ma (Vinnersten & Bremer 2001: Petermannia in Colchicaceae!). Other estimates (Petermanniaceae not in analyses) are (112-)96, 87(-81) Ma (Wikström et al. 2001), ca 125.5 and 114.4 Ma (Magallón & Castillo 2009), ca 79.5 or 54.8 Ma (S. Chen et al. 2013), ca 87.9 Ma (Magallón et al. 2015: note topology) or (115.4-)96.4(-77.3) Ma (Eguchi & Tamura 2016).
Chemistry, Morphology, etc.. Gatin (1920) looked at pedicel anatomy at taxa scattered through this clade; it was generally quite complex, although not in Smilax.
Genes & Genomes. Very large genomes with a C value of 35 picograms or more are found in some Melanthiaceae, Liliaceae and Alstroemeriaceae (Leitch et al. 2005).
[Petermanniaceae [Colchicaceae + Alstroemeriaceae]]: primary root of seedling well developed.
Age. This node is dated at (117.6-)105.6(-89.4) Ma (Chacón & Renner 2014).
PETERMANNIACEAE Hutchinson, nom. cons. - Petermannia cirrosa F. Mueller - Back to Liliales
Plant climbing, with leaf-opposed stem tendrils [≡ inflorescence]; saponins 0; velamen +; root cortex sloughs off, endodermis becomes superficial, vessels in root 0nly, sieve tubes not associated with companion cells; stem with prickles; leaves spiral, petiole +, short, blade with midrib and 2-4 suprabasal pairs of main veins, fine venation reticulate; inflorescence cymose; pedicels not articulated; T 1-veined, medium-sized; tapetum amoeboid; (pollen inaperturate); placentation parietal, stigma wet; ovules many/carpel, outer integument 3-4 cells across, parietal tissue 2-3 cells across, nucellar cap ?2-layered; fruit a berry; exo- and endotesta thickened, mesotesta "several-layered", a cuticle, tegmen crushed; n = 5, x = ?; first leaves cataphylls.
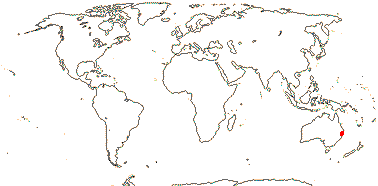
1 [list] /1. Central part of the E. coast of Australia, rare. Map: from Fl. Austral. vol. 46 (1986).
Age. Fossil Petermanniopsis is reported from the early Eocene of Australia (Conran & Christophel 1999); the fossil has paracytic stomata - probably plesiomorphic.
Evolution: Divergence & Distribution. Diversification rates in the Petermanniaceae clade have slowed down (Hertweck et al. 2015).
Ecology & Physiology. The inflorescences and tendrils (equivalent structures) are terminal, but they become leaf-opposed when they are evicted by the strong growth of an axillary shoot (but c.f. Tomlinson & Ayensu 1969; Sousa-Baena et al. 2018b).
Chemistry, Morphology, etc.. Additional information is taken from Conran and Clifford (1998: general), Prychid and Rudall (1999: crystals), Tomlinson & Ayensu (1967: anatomy), and Björnstad (1970) and Conran (1988), both embryology.
[Colchicaceae + Alstroemeriaceae]: ovary superior [one place to put this...].
Age. The age of this node is around (71.3-)59, 58(-51.2) Ma (Wikström et al. 2001), (71.3-)59, 58(-51.2) Ma (Vinnersten & Bremer 2001: problem), ca 59.1 Ma (Magallón et al. 2015), (83-)62, 59(-40) Ma (Bell et al. 2010), ca 76 Ma (Janssen & Bremer 2004), (102-)79(-60) Ma (Givnish et al. 2016b), (101-)86.5(-70.8) Ma (Chacón & Renner 2014), (116.7-)96.5, 93.4(-73.4) Ma (Chacón et al. 2012b), 98.5, 96.1 Ma (Conran et al. 2010) or (65.1-)46.5(-39.4) Ma (D.-F. Xue et al. 2020).
Evolution: Genes & Genomes. The GC content of the genome shows a notable increase in this clade (Smarda et al. 2014).
Chemistry, Morphology, etc.. For fructose oligosaccharide accumulation (only one record for Alstroemeriaceae s.l.), see Pollard (1982).
COLCHICACEAE de Candolle, nom. cons. - Back to Liliales
Colchicine alkaloids +, flavones +, steroidal saponins 0; raphides 0; cuticular wax with parallel platelets; leaves conduplicate, blade with midrib (0), base sheathing; inflorescence various, flowers axillary; T towards base U-shaped and folded around each stamen in bud, connate or not; A (latrorse, introrse); pollen (operculate), sexine thick; (G [2, 4]), styluli +/style ± branched, stigma punctate/decurrent/with recurved lobes, wet or dry; ovules 2-many/carpel, ± ascending, orientation various, (unitegmic), micropyle bistomal; antipodal cells multinucleate; capsule septicidal; seeds rounded, strophiole, sarcotesta or aril +; phlobaphene +; endosperm (with starch), embryo small; x = 7 (?8)y, nuclear genome [1 C] (0.426-)7.234(-22.877) pg, chromosomes 1-16 µm long; cotyledon photosynthetic or not, bifacial (ligulate).
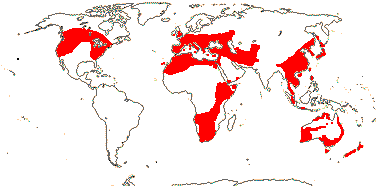
15 [list]/255 - six tribes below. Temperate to tropical, but not in South America. Map: see Meusel et al. (1965), Fl. Austral. 46. (1986), Hong (1993), Nordal et al. (2001) and del Hoyo and Pedrola-Monfort (2006, 2008).
Age. Crown-group Colchicaceae are estimated to be (82.1-)67.3(-54) Ma (Chacón & Renner 2014), ca 64 Ma (Chacón et al. 2012b), (84-)58.5(-40) Ma (Givnish et al. 2016b), ca 47 Ma (Conran et al. 2014), ca 44 Ma (Janssen & Bremer 2004) or (41.1-)34, 33(-24.9) Ma (Vinnersten & Bremer 2001).
1. Uvularioideae A. Gray —— Synonymy: Uvulariaceae Kunth, nom. cons.
Rhizomes +; fructan sugars accumulated; leaves 2-ranked, (petiole +); inflorescence umbellate or flowers single, axillary; flowers campanulate, pendulous; pollen monoporate or disulcate; nucellar cap massive; fruit septicidal, or a berry; endotesta enlarged; n = 7, 8, chromosomes 5-16 µm long.
2/15: Disporum (10). W. and E. North America, East Asia to W. Malesia.
Age. Crown-group Uvularioideae have been dated to (33.7-)26, 20(-15.1) Ma (Vinnersten & Bremer 2001) or (45.5-)28.8(-14.5) Ma (Chacón & Renner 2014; see also Chacón et al. 2012b).
2. Burchardioideae [?published] —— Synonymy: Burchardiaceae Takhtajan
Flowers with spreading or funnel-shaped tepals.
Age. This clade has been dated to around (37.1-)29(-20.9) Ma by Vinnersten and Bremer (2001).
2A. Burchardieae J. C. Manning & Vinnersten - Burchardia R. Brown
Corm with papery scales; leaves spiral; inflorescence umbellate, axis with leaves; capsule septicidal; seeds ± angular; n = 24.
1/5. Australia, Western Australia and the E. Coast, inc. Tasmania.
Age. Crown-group Burchardia is (36.6-)22.9(-11.6) Ma (Chacón & Renner 2014).
2B. Tripladenieae Vinnersten & J. C. Manning
Rhizomes +; (stem lignescent); leaves 2-ranked, (petiole +); inflorescence umbellate, or flowers single; (nectaries paired, stalked); n = 7, 18.
Age. Crown-group Tripladenieae are (36.8-)19.6(-6.2) Ma (Chacón & Renner 2014).
3/5. Australia and New Guinea.
3. Colchicoideae Burmeister (= Liliaceae-Wurmb(a)eoideae Buxbaum in older literature)
Tunicated corm +; alkaloids with a 7-C tropolone ring +; (vessels in stem); leaves spiral; inflorescence racemose, or flowers single; nectary on filament bases, (paired toothed ridges up T); G deeply trisulcate; outer integument 4-5 cells across, nucellar cap beak-like [= "cylindrical protuberance" - Sandersonia], obturator +; (antipodal cells persistent); suspensort multicellular, basal cell large.
9/210. Old World.
Age. This node is ca 40.1 (Chacón et al. 2012b) or (59.4-)48(-37.7) (Chacón & Renner 2014) Ma.
3A. Colchiceae Reichenbach —— Synonymy: Bulbocodiaceae R. A. Salisbury, Merenderaceae Mirbel
(Plants climbers, by leaf tendrils - Gloriosa), (rhizomatous); fructan sugars accumulated [Colchicum]; (vessels in stem - Sandersonia), (leaves whorled), (petiole short, base not sheathing); (inflorescence bracts coloured); flowers (monosymmetric by style position - Gloriosa); T (connate), (clawed); nectaries median on tepal or on stamen base (0); (pollen di- or polyporate); (ovary initially subterranean - Colchicum); ovule (micropyle to 1½ times longer than nucellus); antipodal cells divide; (capsule septicidal); seeds with (galacto)glucomannans [Colchicum]; (exotestal cell walls not so thick - Colchicum); n = 7, 9-12, ... 108, chromosomes 2.8-14.3 µm long.
5/170: Colchicum (150). Africa, Europe, Central to tropical South East Asia.
Age. The age of crown-group Colchiceae is (20.6-)16, 15(-9.1) Ma (Vinnersten & Bremer 2001) or (54.2-)43.3(-32.9) Ma (Chacón & Renner 2014).
[Iphigenieae + Anguillarieae]: ?
Age. This node has been dated to ca 29 Ma (Chacón et al. 2012b) or (53-)42,9(-32.6) Ma (Chacón & Renner 2014).
3B. Iphigenieae Hutchinson
Flowers single; nectaries 0; n = 11.
2/10: Iphigenia (9). Old World Tropics, South Africa.
Age. Iphigenieae started diversifying (35.4-)22.4(-10.6) Ma (Chacón & Renner 2014).
3C. Anguillarieae D. Don - Wurmbea Thunberg
Inflorescence racemose or spicate, bracts 0; (T connate); G free to connate only at base; ovules (unitegmic); capsule septicidal; n = 7, 10, 11.
1/48. Africa, Australia, 1 sp. New Zealand.
Age. Crown-group Anguillarieae are (42.2-)32.7(-23.7) Ma (Chacón & Renner 2014).
Evolution: Divergence & Distribution.
Age. Chacón and Renner (2014, q.v. for details) discuss the biogeography and diversification of the family, albeit in the context of a tree with a somewhat different topology towards the base than that used here. They were uncertain as to its place of origin, uncertainty that is probably likely whatever the topology, but most diversification within the family (i.e. Colchicoideae above) is African, with some excursions to Australia, Europe, etc. (Chacón & Renner 2014). Colchicum, with over half the species in the family, may have diverged from other Colchiceae 13.4±1.5 Ma, probably in southwestern Africa (del Hoyo et al. 2009), or around 32.3 Ma and with diversification beginning ca 25.5 Ma (Chacón & Renner 2014).
Ecology & Physiology. Colchicaceae are well represented in the taxa that have water-catching leaves with very distinctive morphologies that are particularly prominent in the foggy deserts of Namaqualand, South Africa (Vogel & Müller-Doblies 2011).
The tendrils of Gloriosa are described by Rjosk et al. (2017); curvature is abaxial, the thickening involved is adaxial (interestingly, the vascular bundles lack associated fibres).
The corms of Colchicum (inc. Androcymbium) may be quite deep in the ground, but they reach the depth they do in two quite different ways. In C. autumnale the corm may be 15-25 cm deep, and it reaches this depth over the course of 15-20 years by the successive growth of short, positively geotropic, vertical stolons each year. However, in species like C. stevenii the corm reaches 6-8 cm below the surface in a single year, the cortex of the primary root disintegrating and the base of the above-ground leaf, along with the associated plumule, etc., growing down the space formed by the disintegrated part of the root; the corm of C. ritchii may descend 20 cm in a single year in the same way (Galil 1968: some species of Iris and Oxalis establish themselves in the same way).
Pollination & Seed Dispersal. Colchicum in southern Africa may be pollinated by rodents (Kleizen et al. 2008); flowers in Colchicum s.l. can be closely aggregated, visually the most attractive organs being the inflorescence bracts - they are pseudanthia (Baczynski & Claßen-Bockhoff 2023). The South African Wurmbea elatior produces skatole, etc., and is pollinated by flies (Johnson et al. 2020). For variation in sexual expression in Australian, but not African, Wurmbea, see Case et al. (2008: infraspecific variation).
Myrmecochory predominates in Colchicaceae (Lengyel et al. 2009, 2010).
Genes & Genomes. A genome duplication found throughout the family, the GLSUα event, happened ca ca 59 Ma (Landis et al. 2018). For chromosome evolution in the family, see Chacón et al. (2014).
Chemistry, Morphology, etc.. The tropolone alkaloids, with their remarkable seven-carbon rings, have given plant chemists headaches for a century or more (colchicine - phenethylisoquinoline alkaloid). The protoalkaloid colchicine has been reported from some Melanthiaceae, probably also Liliaceae, as well as one or two other monocots not immediately related to Liliales like Tofieldiaceae and Hyacinthaceae [= Asparagaceae-Scilloideae] (Gibbs 1954), although a recent survey suggests that it is restricted to Colchicaceae (Vinnersten & Larsson 2010: sampling good; see also Larsson & Rønsted 2014).
Androcymbium [= Colchicum] longipes has tepals ca 4.5 cm long, each with a basal claw ca 3.5 cm long representing the part of the tepal adnate to the filament. There is considerable variation in nectary morphology and position within the family, but details of nectary evolution are unclear. Cave (1968) described Androcymbium as having a nucellar cap, although from the illustration if looks as if there is parietal tissue 2-3 cells across, similarly, illustrations in Vesque (1878) suggest that parietal tissue in Uvularia is ca 4 cells across.
Additional information is taken from Nordenstam (1998) an Grey-Wilson et al. (2020: esp. Colchicum s. str.), both general; for the floral morphology of Kunthera, see Endress (1995b), for pollen, see L. Wang et al. (2017: ex Polygonatae), for the gynoecium, see Sterling (1975) and references, for embryology, see Ono (1929), Cave (1968 and references) and Zou (2001), and for bulbs, etc., see Buxbaum (1936) and Tillich (1998).
Phylogeny. Molecular studies suggest that there is considerable phylogenetic structure within the family. It initially appeared that "Uvularieae" might be paraphyletic and basal. [Uvularia + Disporum] (N. Temperate) and [Schelhammera + Tripladenia], = Tripladenieae (Australian), were successively the first two branches of Colchicaceae, and Drymophila (Alstroemeriaceae) was also around here (e.g. Rudall et al. 2000; Fuse & Tamura 2000; Vinnersten & Reeves 2003). These genera, and some others, have rhizomes, flavonols, and their nucellar epidermal cells are enlarged. Although Colchicaceae are noted for their alkaloids, such secondary metabolites were thought to be absent from these basal clades (e.g. Kite et al. 2000, but c.f. Vinnersten & Larsson 2010). However, there was uncertainty over the relative positions of Uvularia and Burchardia (Fay et al. 2006c for a summary), and Vinnersten and Manning (2007) even thought that Burchardia, sister to the rest of the family, might be paraphyletic. J. S. Kim et al. (2013) placed Uvularieae basal while the three species of Burchardia they examined were in the next clade up. This latter result was confirmed in a more detailed study by Nguyen et al. (2013), however, Chacón et al. (2014) found Wurmbea to be sister to the rest of the family; although they sampled all species, this position had little support. Chacón and Renner (2014) recovered the relationships [Burchardia {Disporum, etc. [Schelhammera, etc. + The Rest]]], while Givnish et al. (2016b) found the relationships [[Burchardia + Disporum, etc. (very short branch)] [Tripladenia [Schelhammera, etc. + The Rest]]], i.e., rather unexpectedly, Tripladenieae are paraphyletic.
For relationships around Disporum, see Shinwari et al. (1994a, b) and Uvularia, etc., see Hayashi et al. (1998). Relationships in the rest of the family found by J. S. Kim et al. (2013) are consistent with those in the phylogeny above. Androcymbium, Colchicum, Merendera and Bulbocodium form a well supported clade with (currently) little internal resolution, but the whole clade may be characterisable (Vinnersten & Reeves 2003; del Hoyo & Pedrola-Monfort 2006). For the limits of Colchicum, see Persson (2007), Androcymbium was sometimes not included (see also del Hoyo & Pedrola-Monfort 2008; del Hoyo et al. 2009; Persson et al. 2011; Nguyen et al. 2015: loss of chloroplast ycf15 gene). Within Wurmbea the Antipodean and African members may both be monophylertic, although sampling of the latter was poor (Case et al. 2008); Onixotis, a small Cape genus, may be paraphyletic
Classification. The tribal classification above is that of Vinnersten and Manning (2007); see also Nguyen et al. (2013).
Generic limits in general have needed attention (Fay et al. 2006c; Vinnersten & Manning 2007). See Manning et al. (2007) for the combination of Colchicum and Androcymbium; the recognition of Colchicum may make Androcymbium paraphyletic (see del Hoyo et al. 2009; Persson et al. 2011), however, Grey-Wilson et al. (2020) prefer to recognize a narrowly delimited Colchicum. The sections of Androcymbium s. str. are often not monophyletic (del Hoyo & Pedrola-Monfort 2008).
Previous Relationships. Uvularia and Disporum used to be part of Convallariaceae (= Ruscaceae s. str., = Asparagaceae-Nolinoideae) and indeed are superficially like genera of that group such as Polygonatum.
Botanical Trivia. The style in Colchicum is up to 30 cm long (Grey-Wilson et al. 2020).
ALSTROEMERIACEAE Dumortier, nom. cons. - Back to Liliales
Leaves resupinate, stomata on morphologically adaxial surface; inflorescence ± cymose; ovules lacking parietal tissue; testa and tegmen thin-walled; endosperm walls thick, pitted; x = 10 (?9, ?7), karyotype bimodal, nuclear genome [1 C] (0.03-)8.182(-223.191) pg.
4 [list: to tribes]/190 (254). Central and South America, the Antipodes.
Age. Crown group Alstroemeriaceae may be (62.6-)55, 48(-37.5) Ma (Vinnersten & Bremer 2001), (85-)61.5(-40) Ma (Givnish et al. 2016b), (86.8-)64.2, 57.5(-37.8) Ma (Chacón et al. 2012b), ca 76 Ma (Janssen & Bremer 2004), ca 81.7 or 78.1 Ma (Conran et al. 2014), or (113.2-)73.5(-32.5) Ma (Tribble et al. 2022b/2023: "divergence of Bomarea and Alstroemeria", i.e., from Luzuriageae).
1. Alstroemerieae Bernhardi
Plant (climber - Bomarea), (annual), root tubers +; flavonols, tuliposides + [α-methylene-γ-butyrolactone - glucose esters]; (velamen +); cuticular wax with parallel platelets; leaves spiral, (not resupinate, stomata on both surfaces); inflorescence subumbellate (flowers axillary), bracteoles lateral; (flowers monosymmetric); T clawed, differentiated, inner whorl spotted, median member of the outer whorl adaxial, inner whorl often with nectariferous claw; A latrorse, basi/centrifixed; pollen wall "thick", surface striate to variously reticulate; ovary inferior, placentation parietal, stigma wet; ovules many/carpel, outer integument ca 4 cells across, nucellar cap 0 (2) cells across, obturator +; fruit dehiscing laterally, loculicidal, (indehiscent); seed tuberculate, brown, testa also ± thick-walled, (sarcotesta + - Bomarea), tegmen collapses; embryo short to medium; n = 8, 9, chromosomes 6-19 µm long; cotyledon not photosynthetic (photosynthetic - annual A. graminea]).
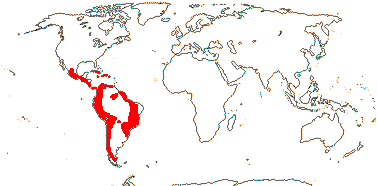
2/185: Bomarea (105), Alstroemeria (80). Tropical and temperate Central and South America (map: from Aker & Healy 1990; Hofreiter 2006 - the cultivated Bomarea edulis is particularly widely distributed). [Photo - Flower, Fruit.]
Age. Crown group Alstroemerieae may be (27.1-)18, 17(-10.5) Ma (Vinnersten & Bremer 2001), (47.8-)31.9, 29.0(-18.2) Ma old (Chacón et al. 2012b), ca 30 Ma (Janssen & Bremer 2004), ca 31.4 or 36.8 Ma (Conran et al. 2014) or (28.9-)21.8(-15.3) Ma (Tribble et al. 2022b/2023).
2. Luzuriageae Bentham & Hooker —— Synonymy: Luzuriagaceae Lotsy
Plant shrubby, ± climbing, stems perennial, usu. branched; chelidonic acid, fructans?; root pith 0; leaves two-ranked, petiole +/0, blade conduplicate or supervolute, sheath +, open; flowers solitary, (inflorescence a cincinnus); (pedicels articulated - Luzuriaga); (T rather small); (A introrse); tapetum amoeboid, 2 nucleate; pollen wall "thin", finely granulate; style short, deeply branched [Drymophila], stigma dry; ovules few to many/carpel, ?morphology; fruit a berry; seeds rounded, pale yellow; (exotesta shed); endosperm development?; n = 10; cotyledon ?not photosynthetic, ?primary root.
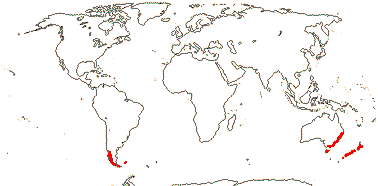
2/5: Luzuriaga (3). Peru to Tierra del Fuego, Falkland Islands, New Zealand and S.E. Australia, inc. Tasmania. Map: from Fl. Austral. vol. 46 (1986). [Photos - Collection, Luzuriaga polyphylla, Luzuriaga radicans, Luzuriaga Flower.]
Age. Crown group Luzuriageae are dated to (40.5-)32, 23(-17.3) Ma (Vinnersten & Bremer 2001), ca 56 Ma (Janssen & Bremer 2004), and (55.5-)35.9(-19.5) Ma, ca 23 Ma (Chacón et al. 2012b), ca 54 or 47 Ma (Conran et al. 2014) or (41.4-)28.4(-23.2) Ma (Tribble et al. 2022b/2023). [Check: The stem node of Luzuriaga is dated to 23.2 Ma (Conran et al. 2014; Iles et al. 2015).]
Evolution: Divergence & Distribution. See Hofreiter (2007) and especially Chacón et al. (2012b) for the biogeography and ecology of the whole clade; note there are some rather long stem groups here, the family probably originating in South America and/or Australia (Tribble et al. 2022b/2023, q.v. for caveats about ages). Givnish et al. (2016b) floated the possibility that Luzuriaga might be an old element of the New Zealand flora, persisting despite the near-submergence of the islands, although Conran et al. (2014), describing fossils of the genus ca 23 Ma from the South Island thought that long distance dispersal best explained its presence there.
Tribble et al. (2022b/2023) discussed the evolution and biogeography of Alstroemeriaceae in general and of Bomarea in particular; the latter genus they think evolved in south South America where the four species they recovered as a basal pectination in the genus tree grow, and then it moved South to North up the Andes (see also Puya-Bromeliaceae, Chuquiraga-Asteraceae and Gunnera-Gunneraceae) and with repeated and late movements into Central America, some species moved back south, etc.. The bulk of the genus is ultimately of Andean origin and is only some (6.1-)4.4(-2.3) Ma, diversifying at a high rate of some 7.8 events/lineage/million years (Tribble et al. 2022b/2023).
Conran et al. (2014) suggest a number of apomorphies for the two tribes.
Genes & Genomes. Chacón et al. (2012a) found that the position of the rDNA sites on the chromosomes of Alstroemeria varied, perhaps suggesting extensive genome rearrangements despite invariance in chromosome number.
Chemistry, Morphology, etc.. The leaves of Luzuriaga psittacina, at least, always twist in the same direction, regardless of the direction of the genetic spiral of the plant, and Chitwood et al. (2012a) discuss how this twisting interacts with asymmetries of the leaf resulting from auxin asymmetries and the effects of the spiral being clockwise or counter-clockwise.
Hirai et al. (2007) looked at the expression patterns of the duplicated B genes on the two perianth whorls of Alstroemeria, suggesting that these genes might be involved in the development of the distinctions between the tepal whorls.
Some information on Alstroemerieae is taken from E. Bayer (1998: general), Hofreiter and Lyshede (2006 and references: leaf anatomy), Sarwar et al. (2010: pollen), Stenar (1925: embryology) and Sanso and Hunziker (1998: cytology). Information on Luzuriageae is taken from Conran and Clifford (1985 [e.g. stigma], 1998).
Phylogeny. See Rudall et al. (2000a), Sanso and Xifreda (2001) and Aagesen and Sanso (2003). Tribble et al. (2022b/2023: 108 spp., 221 nuclear loci) recovered a basal pectination in Bomarea of [B. salsilla [B. ovallei [B. obovata [B. edulis [ ... ]]]], although there was some conflict between different analyses. Although support along the spine of Bomarea and within B. edulis tended to be strong, that within core Bomarea was the opposite.
Classification. For generic limits in Alstroemerieae, see Sanso and Xifreda (2001).
[Melanthiaceae [[Liliaceae + Smilacaceae] [Philesiaceae + Ripogonaceae]]]: (spirostanol steroidal saponins +); ovary superior.
Age. The age of this clade may be (72.8-)67, 63(-53.6) Myo (Vinnersten & Bremer 2001), (108-)96, 83(-68) Ma (Bell et al. 2010), (119.5-)104(-89) Ma (Givnish et al. 2016b), or 105-43 Ma (Mennes et al. 2013).
Chemistry, Morphology, etc.. R. Luo et al. (2017) noted that there were a number of spirostanol steroidal saponins from Smilax, and suggested that such saponins were a feature of "Liliaceae", including Paris, and also Anemarrhena (Asparagacaeae-Agavoideae), and a simple search suggests also Dioscoreaceae and Amaryllidaceae (Fang et al. 2015), for example, so perhaps a marker for Liliaceae in the old sense?
MELANTHIACEAE Borkhausen, nom. cons. - Back to Liliales
Leaves often evergreen; flavones, flavonols or flavonoids +; cuticle wax with parallel platelets; (leaf margins toothed), base sheathing; inflorescence paniculate (raceme); bracteole 0; (T 3, 4); A (latrorse; adnate to base of T); G (1) [3], placentation axile to parietal, styluli +, stigma dry (wet); ovules many/carpel, position variable, campylotropous, parietal tissue ca 1 cell across; T persistent in fruit, ± green; seed coat?, (phlobaphene +); endosperm helobial, embryo short (long); chromosomes 1-6 µm long; cotyledon bifacial or not, hypocotyl at most short; x = 9, x = 7 (?8. ?6), nuclear genome [1Cx] 1-5.5 pg, [1 C] (0.184-)4.175(-94.909) pg.
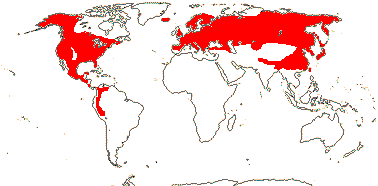
17 [list: to tribes]/172 - five groups below. N. temperate, esp. East Asia and E. North America, to Peru. Map: see Meusel et al. (1965), Frame (1990), Fl. N. Am. vol. 26 (2002) and Seberg (2007). [Photos - Collection 1.]
Age. The age of crown-group Melanthiaceae is estimated to be (62-)54, 42(-34) Ma (Vinnersten & Bremer 2001: note topology), (107-)85(-62) Ma (Givnish et al. 2016b), ca 97 Ma (Janssen & Bremer 2004), ca 89.2 Ma (L. Yang et al. 2019) or (86.7-)84.8(-82.9) Ma (Ji et al. 2019).
1. Melanthieae Grisebach —— Synonymy: Veratraceae Salisbury
(Plant ± bulbous); highly oxygenated esterified C-nor-D homosteroidal alkaloids, (steroidal saponins +); fructan sugars accumulated; (monocot secondary thickening +); vessels in roots only; styloids also +; (leaves curved-plicate, sheath closed [Veratrum]); anthers kidney bean-shaped, thecae confluent, dehiscing by valves; (G semi-inferior), style +, hollow; embryo sac bisporic [chalazal dyad], eight-celled [Allium-type], (antipodal cells divide / multinicleate), (persistent); capsule septicidal [ventricidal]; (seeds flat, winged); n = 8 (10, 11, 16), polyploidy common, chromosomes 1.3-4 µm long.
7/100: Veratrum (50), Schoenocaulon (25). North Temperate, Schoenocaulon to Peru.
[[Helionadeae + Chionographideae] [Xerophylleae + Parideae]]: calcium oxalate crystals cuboidal.
Age. The age of this node may be some (76-)70, 66(-60) Ma (Wikström et al. 2001), (85-)67, 59(-43) Ma (Bell et al. 2010), ca 76.8 Ma (L. Yang et al. 2019), (75.8-)73.9(-72) Ma (Ji et al. 2019) or ca 48 Ma (J. Li et al. 2020: Paridae, Xer., Heloniopsis).
[Helionadeae + Chionographideae]: vessels in roots only; inflorescence racemose, bracts 0; anther thecae ± confluent; pollen intectate.
Age. The age of this node is estimated to be ca 49.8 Ma (L. Yang et al. 2019).
2. Helionadeae Fries —— Synonymy: Heloniadaceae J. Agardh
Rhizome ± vertical; (steroidal saponins [Ypsil.]); raphides 0; (bracts + - Helonias)(anthers hippocrepiform, thecae confluent); pollen spinulate; style +, depressed, stigma capitate, ± 3-lobed, papillate; capsule with spreading lobes, loculicidal; seeds linear, ± long-caudate at both ends; n = 17, chromosomes 1.8-6 µm long.
3/12. East North America, Himalayas, East Asia.
Age. Crown-group Helionadeae may be ca 13.7 Ma (L. Yang et al. 2019: Hel, Yp).
3. Chionographideae Nakai - Chamaelirium Willdenow —— Synonymy: Chionographidaceae Takhtajan
Steroidal saponins +; inflorescence also spicate, axis, etc., usu. white; flowers (a-/monosymmetric), often imperfect, (plant dioecious); T 3-6, with 1 vein; nectaries 0; (staminate flower - pistillode 0; carpellate flower - staminodes 6); pollen inaperturate, or 4-porate, with clavate processes; styluli +, ± recurved; capsule septicidal; seeds winged (at one end); n = 12 (21, 22), chromosomes (holocentric), 1-2(-3.2) µm long.
1/12. E. North America, southern China to Vietnam and Japan.
[Xerophylleae + Parideae]: anther thecae distinct.
Age. Bell et al. (2010) estimated the age of this node at some (67-)49, 43(-27) Ma, Wikström et al. (2001) suggested an age of (59-)54, 50(-45) Ma and L. Yang et al. (2019) an age of (73-)59.2(-49.1) Ma; (54.3-)52.4(-50.5) Ma is the age in Ji et al. (2019).
4. Xerophylleae S. Watson - Xerophyllum Michaux —— Synonymy: Xerophyllaceae Takhtajan
Plant ± bulbous; pericycle 2-3 cells thick; calcium oxalate crystales as raphides and styloids; leaf long-linear, xeromorphic; inflorescence corymbose; T nectaries 0, septal clefts enclosed; ovules 2-4/carpel, campylotropous; n = 15, chromosomes 2.5-4.8 µm long.
1/2. North America.
5. Parideae Bartling —— Synonymy: Paridaceae Dumortier, Trilliaceae Chevallier, nom. cons.
Rhizome monopodial; steroidal saponins, flavonols +; raphides 0, cuboidal crystals +; stomata tetracytic; leaves whorled [3-15 leaves/whorl], (petiole +), broad blade +. midrib +, venation reticulate; flowers single, terminal, (sessile), quite large, (to 15-merous - Paris); P = K + C, K (0) 3-10, C (0) 3-6(-15), (long, linear); A 6-30, (-6 whorled), introrse to extrorse; (pollen inaperturate - Trillium); G [3(-10)], (septal nectaries +), placentation axile to parietal, (style +, unbranched / very short, branched), stigma dry; ovules many/carpel, position variable, parietal tissue 1-2 cells across, nucellar cap 2-4 cells across, suprachalazal zone short; embryo sac bisporic [chalazal dyad], eight-celled [Allium-type]; fruit a berry/(septicidal and) loculicidal capsule, A also persistent in fruit; seeds rounded, aril or sarcotesta +/-; endosperm also helobial [Tr.], starchy, embryo minute, undifferentiated; x = 5, (polyploidy), chromosomes heteromorphic, 6-40+µm long, nuclear genome [1 Cx] 31.2-56.6 pg, [1 C] -152.2 pg/148.9 Gb; chloroplast cemA gene pseudogenized; seed (with aril, sarcotesta), (to 7.5 cm across); cotyledon (with petiole and small blade - P. [?level]).
3/70: Trillium (43), Paris (26). North Temperate. Map: see Farmer (2006). [Photos: collection.]
Age. The age for this node is estimated to be (23.3-)16, 9(-3.9) Ma (Vinnersten & Bremer 2001: note topology), ca 38.2 Ma (L. Yang et al. 2019) or (37.8-)33.9(-29.7) Ma (Ji et al. 2019).
Evolution: Divergence & Distribution. Additional dates for divergence within Trillium can be found in L. Yang et al. (2019) and especially Ji et al. (2019).
Melanthiaceae show ca 230-fold genome size (Mbp) differences, the highest value in seed plants; bar Ranunculaceae, all other families are well below 150-fold (Elliott et al. 2022b: Fig. S10, see also below). For the evolution of some characters in Melanthiaceae, see S.-C. Kim et al. (2016). Parideae are quite a young clade, but show a notable amount of both floral and vegetative divergence from other members of the family, indeed, in Paris the large genomes seem not to have discouraged diversification, rather, hybridization may even have encouraged it (N. Zhou et al. 2024). Interestingly, sections with possible hybridization in their ancestry all have fleshy, probably bird-dispersed seeds (ibid., Fig. 6); note that Paris is shown as being paraphyletic in the reconstructions of hybridization, this hybridization involving the ancestor of the [Trillium + Paris] clade (ibid. Fig. 4).
Ecology & Physiology. Giesemann et al. (2019) found that almost 50% of the carbon in the leaves of Paris quadrifolia came from its associated fungus.
Pollination Biology & Seed Dispersal. Wasps (Vespula) disperse the seeds of Trillium in North America (Zettler et al. 2001), while the elaiosomes of T. grandiflorum, although being effective in seed dispersal, had a negative effect on the colonies of the ants that dispersed them and ate the elaiosomes (Turner & Frederickson 2013).
Plant-Bacterial/Fungal Associations. Melanthieae are susceptible to infection by rust fungi (Holm 1982).
Genes & Genomes. Melanthiaceae have more species with B chromosomes than any other family, over 40% of the family having them, Commelinaceae being next at ca 30% (Weiss-Schneeweiss & Schneeweiss 2013). For chromosome numbers in Melanthieae, see Zomlefer et al. (2014), and in Parideae, see Pellicer et al. (2013); holocentric chromosomes have been reported from Chionographis japonica (Tanaka & Tanaka 1977).
A genome duplication perhaps involving everything except Melanthieae, the HEBUα event, ca 50.4 Ma, is reported (Landis et al. 2018: Xer Hel only).
There has been a massive increase in genome size in Parideae in particular (Pellicer et al. 2013), the lowest genome size known in the clade being from Pseudotrillium [= Trillium] rivale. The range of chromosome sizes in the family as a whole is at least 10-fold and that of C-values is over 200-fold (Pellicer et al. 2010a, 2013, 2014; Leitch & Leitch 2013); T. hagae has a genome size of 2C = 264.9 pg (Zonneveld 2010) and Paris japonica a 1C value of 152.2 pg/148.8 Gb, the second largest known (Hidalgo et al. 2017c: see Psilotales-Psilotaceae for numbers one, three and four on the list, Fernández et al. 2024); these large genomes are likely to have evolved independently (L. Yang et al. 2019). Yang et al. (2019; see also Pellicer et al. 2013) suggest that there was an increase in the size of the genome in stem-group Parideae over a period of perhaps 20 Ma beginning up to ca 59.2 Ma, and the age of P. japonica, with the largest genome, is ca 20.3 Ma, or (22.4-)16(-7) Ma in Ji et al. (2019 - for relationships in Parideae, see also Ji et al. 2006; S.-C. Kim et al. 2016). High C values in Trillium are associated with long cell cycles (Francis et al. 2008). J. Li et al. (2020) suggested that the large size of the genome of P. polyphylla var. yunnanensis, developed over the last 20 million years or so, is due to the serial prolferation of transposable elements, and in general increases and decreases in such non-coding sequences drive extreme genome sie changes, although ultimately why there should be such changes is unclear (Hidalgo et al. 2017). Similarly, Zeng et al. (2025) recorded the genome of P. polyphylla var. yunnanensis as being 54.58 Gb/1C - individual chromosomes to 14.14 Gb - and that of Veratrum dahuricum as being a mere 3.93 Gb/1C, although no genome duplication seems to have been involved. For genome size and its ancestral reconstruction, see also Pellicer et al. (2013).
L. Yang et al. (2019) discuss the evolution of the plastome, with a focus on Parideae, and they note that the pseudogenization of the cemA gene there was unusual for an autotrophic clade.
Chemistry, Morphology, etc.. The alkaloids of Veratrum and its relatives are very complex and distinctive (Kupchan et al. 1961 and references; Kite et al. 2000). There is no fructose oligosaccharide accumulation in Trillium, at least, and this absence is also reported from Melanthieae (Pollard 1982).
Xerophyllum is particularly distinctive in its vegetative anatomy (Ambrose 1975). Cataphylls in Paris may have closed sheaths (Narita & Takahashi 2008).
For the development of asymmetry/zygomorphy in the flowers ofChamaelirium japonicum, a complex affair, see Remizowa et al. (2023) - i.a. one of the carpels has only a single ovule and does not develop a stylulus. In apetalous Trillium, but not in apetalous Paris, the carpels are opposite the sepals, and Narita and Takahashi (2008, see also Takahashi 1994) think that the petals are derived from stamens, although no other Liliales have the three whorls of stamens that this hypothesis would entail (see also Ronse de Craene 2010). Except for Helionadeae, syncarpy in Melanthiaceae tends to be rather weak. In a number of taxa, including Veratrum and Paris, the tepals become greener and persist in fruit (e.g. Weberling 1989); other taxa, including Trillium, have persistent sepals. There are raphides in the ovule, but nowhere else. Although the embryo of Trillium is minute when the seed is dispersed, it grows to about the length of the seed before germination.
General information is taken from Ambrose (1975, 1980), Tamura (1998: Melanthiaceae, Trilliaceae, 2019: Helonieae, Helonias s.l.), Zomlefer (1996: Trilliaceae, 1997a [nice table], esp. 2001: Melanthiaceae), Zomlefer et al. (2006) and Ji (2021: Paris); see also Arber (1925). For secondary thickening, see Cheadle (1937), for floral morphology, see Endress (1995b), for the gynoecium, see Sterling (1982 and references), and for embryological information, see Ono (1929), Eunus (1951), Berg (1962), Cave (1968) and Ren et al. (2020; Veratrum).
Phylogeny. Possible relationships are [Veratrum [Trillium and relatives + the rest] (Tamura et al. 2004a; see also Fuse & Tamura 2000), however, Xerophyllum was not included and support for the basal dichotomy was weak. Consistent with this earlier work, the topology [Melanthieae [[Heloniadeae + Chionographideae] [ Xerophylleae + Parideae]]] has been recovered (Zomlefer et al. 2006; J. S. Kim et al. 2013; L. Yang et al. 2019; esp. S.-C. Kim et al. 2016).
Parideae. Farmer (2006) discussed the relationships of the Trillium group (as Trilliaceae); the backbone of the phylogeny was distinctly poorly supported (see Kim et al. 2016; also Kazempour Osaloo & Kawano 1999). Ji et al. (2006, 2019; see also Ji 2021) had noted that chloroplast and nuclear trees in the genus had rather different topologies, perhaps because of past hybridizations, the latter observing that Paris was paraphyletic in nuclear ribosomal DNA analyses, with Trillium embedded in Paris. Using chloroplast phylogenomic analyses, L. Yang et al. (2019) looked at relationships in Paris in particular. N. Zhou et al. (2024b) confirmed the conflict between different datasets, e.g. the trees based on nuclear r-DNA differered from those based on plastome data, and in some analyses, Paris and Trillium were not reciprocally monophyletic. Thus ribosomal data suggested that Paris was paraphyletic, the three species of Trillium examined being embedded in it, and depending on the analysis, support from the gene trees might be very low. There seem to have been five hybridization events in Paris (ibid. Fig. 4); ILS caused fewer problems in these analyses. Concatenation analyses in particular provided support for the sections of Ji (2021). As of vii.2025, the Kew Tree of Life suggested that Trillium included both Paris and Pseudotrillium, but sampling was exiguous, indeed, it will be interesting to see nuclear trees with good sampling of both Paris and Trillium.
Classification. See S.-C. Kim et al. (2016) for tribal and generic delimitations. Parideae have often been recognised as one or two separate families, Trilliaceae, and sometimes also Paridaceae; generic limits there have been uncertain, but seem to be settling down to three genera.
For a sectional classification of Paris (five sections), see Ji et al. (2006, esp. 2019: relationships based on plastomes; N. Zhou et al. 2024b), and for a subgeneric classification of Trillium, see Lampley et al. (2022: four subgenera).
Botanical Trivia. The genome of Paris japonica, at over 150 picograms, is the largest known of any organism (Pellicer et al. 2010a).
Previous relationships. Veratrum in particular looks superficially like Maianthemum (inc. Smilacina), a member of Ruscaceae s. str., Asparagaceae s.l.; these and most other Melanthiaceae were all included in Cronquist's (1981) Liliaceae. Even some circumscriptions of Melanthiaceae were not much of an improvement from that point of view... (see e.g. pollen in Kosenko 1987).
[[Liliaceae + Smilacaceae] [Philesiaceae + Ripogonaceae]]: leaf venation reticulate, base not sheathing.
Age. The age of this node may be about 58 Ma (Givnish et al. 2014b), or some (60.9-)54, 52(-42.3) Ma (Vinnersten & Bremer 2001), ca 55.6 Ma (Magallón et al. 2015), ca 64.7 Ma (Chacón et al. 2012b), about 80.3 Ma (Tank et al. 2015: Table S2), ca 85 Ma (Givnish et al. 2016b), ca 90±16 Ma (Janssen & Bremer 2004) or (86.4-)67.9(-55.4) Ma (Z.-C. Qi et al. 2023) - as mentioned, internal topologies differ.
Evolution: Divergence & Distribution. See Patterson and Givnish (2002) for characters of this group. It may be that characters for the erstwhile [Smilacaceae [Philesiaceae + Ripogonaceae]] clade - stem fructans 0; vessel elements in stems, with scalariform perforation plates; leaf with petiole and blade, blade with midrib; endothecium with spiral thickenings; testa disintegrates; endosperm with thick, pitted walls - will be best moved to this node.
Age. The age of this node may be (73-)68, 63(-58) Ma (Wikström et al. 2001), ca 64.7 Ma (Chacón et al. 2012b), (104-)92, 63(-51)) Ma (Bell et al. 2010) or (103-)80(-59.5) Ma (Givnish et al. 2016b).
LILIACEAE Jussieu, nom. cons. - Back to Liliales
Geophytes; flavonols +, chelidonic acid 0; raphides 0; bracteole lateral; flowers often large; T often spotted; A (latrose), anthers often centrifixed; (pollen operculate); (placentation parietal), stigma dry (wet); ovules 2-many/carpel, ± pendulous, (outer integument 3-6 cells across), nucellar cap (0-)1-3 cells across, (podium short), (funicular obturator +); antipodal cells not multinucleate; testal cells all ± thickened, some with brown contents; (embryo short); x = 7 (?8, ?6), nuclear genome [1 Cx] ca 6.7 pg/[1 C] 101.4 Gb/[1 C] (0.0209-)1.986(-18.907) pg; chloroplast infA gene a pseudogene; cotyledon ± photosynthetic, bifacial, hypocotyl 0.
15 [list, to tribes]/620 (705): five groups below. North Temperate, especially East Asia and North America (map: see Meusel et al. 1965; Fl. N. Am. 26: 2002).
Age. The crown-group age for Liliaceae is estimated at (57-)53, 48(-44) Ma (Wikström et al. 2001), (78-)53, 52(-40) Ma (Bell et al. 2010), ca 36 Ma (Carta & Peruzzi 2015), ca 44 Ma (Karimi et al. 2024: [[Prosartes + Scoliopus] Calochortus], Fig. S2) or as much as (92.5-)67(-48.5) Ma (Givnish et al. 2016b), (98-)73(-53) Ma (J. Huang et al. 2018), or even (91.1-)85.1(-64.7) Ma (J. S. Kim & Kim 2018).
1. Tricyrtidoideae Thorne & Reveal —— Synonymy: Compsoaceae Horaninow, nom. illeg., Scoliopaceae Takhtajan, Tricyrtidaceae Takhtajan, nom. cons.
Rhizomatous; (vessels in stem); leaves sessile, blade elliptic to ovate; venation also parallel and transverse, base sheathing or not?; (bracteoles 2, lateral - Tricyrtis); T (whorls very different - Scoliopus), (bearded), (nectary in short spur - Tricyrtis); A (3 - Scoliopus); (pollen disulcate - Streptopus); (placentation parietal), placental epidermis papillate, stigma capitate/trifid (branches bifid - Tricyrtis); ovular suprachalazal portion well developed, very long, thin/not; embryo sac bisporic [chalazal dyad], eight-celled [Allium-type] - Streptopus; fruit a loculicidal capsule/berry; seeds ± rounded (flattened), striate, with phlobaphene; endotesta cells thickened on anticlinal and inner periclinal walls; n = 8, (9), 13, chromosomes 1.1-5.6(-13.2 - Scoliopus) µm long, mean nuclear genome [1Cx] 3.4-9.2 pg/4-15(-30) pg.
4/35: Tricyrtis (20). N. (cool) temperate, esp. Eastern Asia and E. and W. North America. Map: from Fl. N. Am. vol. 26 (2002).
Age. The crown age for Streptopoideae is (83.5-)54.8(-36.6) Ma (J. S. Kim & Kim 2018).
[Calochortoideae [Medeoloideae + Lilioideae]]: leaves with parallel venation, no cross veins/reticulum.
Age. The age for this node is (90.4-)82.9(-61.7) Ma (J. S. Kim & Kim 2018).
2. Calochortoideae Dumortier - Calochortus Pursh —— Synonymy: Calochortaceae Dumortier
Bulbous; (γ-methyleneglutamic acid +); leaves linear, sheathing; inflorescence scapose; outer T smaller/± calycine, (patterned), inner T usu. ± bearded, patterned, (margins obscurely fringed); (style funneliform); placentation (parietal), epidermis papillate; parietal tissue 0, ovular suprachalazal portion well developed; fruit a loculicidal capsule; seeds ± flattened; seed coat thin walled; suspensor persistent; x = 9, n = 7-10(-11-)12, chromosomes 1.1-5.5(-13) µm long, mean nuclear genome [1 Cx] ca 5.4 pg/4-15(-30) pg.
1/74. West North America, esp. California, Mexico.
Age. The crown-group age for Calochortus is estimated to be (33-)23(-13.9) Ma (J. S. Kim & Kim 2018) or ca 10.3 Ma (Kamimi et al. 2024).
[Medeoloideae + Lilioideae]: (steroidal saponins +); ovular suprachalazal portion not well developed; embryo sac tetrasporic, three chalazal megaspores fuse, divide twice [Fritillaria-type]; (elaiosomes +); exotesta palisade or lignified [level?]; endosperm pentaploid; chromosomes long [see below]; mean nuclear genome [1 Cx] large [14.2< pg].
11/595. (Cold) temperate, esp. North America, East Asia.
Age. The age for this node is estimated at (33-)30, 27(-24) Ma (Wikström et al. 2001), (36.9-)30, 29(-22.5) Ma (Vinnersten & Bremer 2001: note topology), (45-)31, 28(-18) Ma (Bell et al. 2010), or ca 27 Ma (Carta & Peruzzi 2015) - or (81.4-)66.4(-49.5) Ma (J. S. Kim & Kim 2018).
3. Medeoloideae —— Synonymy: Medeolaceae Takhtajan
Rhizomatous; Clintonia type VAM; leaves (whorled), blade ± elliptic, midrib +; pollen inaperturate [Clintonia]; styluli +, long, stigmatic their entire lenth [Medeola]/style +, stigma ± capitate; suprachalazal nucellus prominent; fruit a berry; seeds rounded, elaiosome 0; exotesta palisade, outer wall thickened; embryo minute; n = 7, 14, 16, chromosomes heteromorphic, 7.7-20.1 µm long, mean nuclear genome [1 Cx] 14.2, 18.9 pg.
2/6: Clintonia (5). North America, East Asia. [Photos - Collection.]
Age. The age for crown Medeoloideae is estimated to be (29.8-)21, 16(10.7) Ma (Vinnersten & Bremer 2001: note topology), about 18.5 Ma (Carta & Peruzzi 2015) or (58.9-)46.1(-22.5) Ma (J. S. Kim & Kim 2018).
4. Lilioideae Eaton
Bulbous, with contractile roots; tuliposides [α-methylene-γ-butyrolactone - glucose esters], γ-methyleneglutamic acid, di- and triferulic acid sucrose esters, fructan sugars accumulated; A versatile; stigma crested/shortly lobed, wet; nucellar cap?; capsule loculicidal; exotesta palisade ?or lignified, tegmen also persisting; endosperm with (galacto)glucomannans; (embryo minute); n = (9, 11-)12(-13), chromosomes (hetermorphic), (1.8 [Gagea]-)5-27 µm long, nuclear genome [1C] 6.6-100+ pg; (cotyledon unifacial).
9/595. (Cold) temperate, esp. North America, East Asia. [Photos - Collection, Nectaries.]
Age. The age for this node is estimated to be (34.5-)28, 24(-17.2) Ma (Vinnersten & Bremer 2001: note topology), about 20 Ma (Carta & Peruzzi 2015), (60.6-)44.5(-33.3) Ma (J. Huang et al. 2018), or (74.6-)61.8(-44.8) Ma (J. S. Kim & Kim 2018).
4A. Lilieae Lamarck & de Candolle —— Synonymy: Fritillariaceae R. A. Salisbury, Liriaceae Borkhausen
Bulb with 2-many scales, (scales articulated), (also rhizomatous); (midrib +, secondary veins palmate, fine venation reticulate, petiole + - Cardiocrinum); inflorescence (umbellate); anthers dorsifixed; ?embryo sac; seeds usually winged, ± stongly curved; n = (9) 12, chromosomes "large", ? µm long, mean nuclear genome [1 Cx] 35.3-50.9 pg/(15-)30-65(-130) pg; (cotyledon not photosynthetic [germination "hypogeal" - some Lilium]).
4/258: Fritillaria (130), Lilium (120). North Temperate, to Mexico, the Philippines.
Age. The age of this node is estimated to be ca 16.1 Ma (Gao et al. 2015),(50.2-)42.9(-24.5) Ma (J. S. Kim & Kim 2018), (30.1-)28.2(-26.2) Ma (J. Huang et al. 2018), ca 27 Ma (Givnish et al. 2020) or (25.9-)24.8(-22.5) Ma (N. Zhou et al. 2024a).
4B. Tulipeae Duby —— Synonymy: Erythroniaceae Martynov, Tulipaceae Borkhausen
Bulb with single scale; leaf blade elliptic (±linear - Gagea), (venation reticulate - Erythronium), (base sheathing - some Tulipa); anthers pseudobasifixed; (pollen tricolpate - some Tulipa); (style 0); ?embryo sac; seeds not winged, ± straight; endosperm pentaploid, thick-walled, not pitted [Erythronium]; n = 9-12, chromosomes (1-)2-11 µm long, mean nuclear genome [1 Cx] 14.2-32.8 pg/(3-)4-25(-70) pg; chloroplast infA gene 0.
4/220: Tulipa (106 [90-150]), Gagea ([70-]90[-300]), Erythronium (25). North America, Eurasia, North Africa.
Age. Crown-group Tulipeae are estimated to be (66.9-)57.6(-39.5) Ma (J. S. Kim & Kim 2018).
[[[[Also possible:
[Calochortoideae + Streptopoideae]: (flowers 3-merous); ovules with nucellar cap; placental epidermis papillate; chromosomes short [see below].
Age. The age for this node is around (49-)44, 37(-32) Ma (Wikström et al. 2001) or (65-)46, 42(-27) Ma (Bell et al. 2010).
1. Calochortoideae Dumortier —— Synonymy: Calochortaceae Dumortier, Compsoaceae Horaninow, nom. illeg., Tricyrtidaceae Takhtajan, nom. cons.
Bulbous and rhizomatous; (γ-methyleneglutamic acid +); (vessels in stem); (leaves sheathing), (with parallel venation, reticulum not developed); (bracetoles 2, lateral - Tricyrtis); (outer T ± calycine / smaller), tepals usu. ± bearded; nectaries ± saccate; (style funneliform), (stigmas bifid - Tricyrtis); (placentation parietal); capsule septicidal, seeds ± flattened; seed coat thin walled; n = 7-10, (11), 12, chromosomes 1.1-5.5 µm long.
2/94: Calochortus (74), Tricyrtis (20). Temperate East Asia and E. North America (map: from Fl. N. Am. 26: 2002). [Photo - Flower.]
2. Streptopoideae Reveal —— Synonymy: Scoliopaceae Takhtajan
Rhizomatous; leaf base sheathing or not?; (P whorls very different - Scoliopus); (A 3 - Scoliopus); (pollen disulcate - Streptopus); nucellar podium well developed, very long, thin; embryo sac bisporic [chalazal dyad], eight-celled [Allium-type - S.]; seeds ± rounded, striate, with phlobaphene; endotesta cells thickened on anticlinal and inner periclinal walls; n = 8 (9), chromosomes 1.1-5.6(-13.2 - Scoliopus) µm long.
3/15. N. (cool) temperate, esp. East Asia and E. and W. North America. Map: from Fl. N. Am. vol. 26 (2002).
Age. The crown age for Streptopoideae is around 9.5 Ma (Carta & Peruzzi 2015).
]]]]Evolution: Divergence & Distribution. For more ages in Liliaceae, see J. S. Kim and Kim (2018), and for those in Lilieae, see J. Huang et al. (2018) and N. Zhou et al. (2024a).
J. Huang et al. (2018) examined biogeographical relationships in Lilieae, especially in Lilium, noting that in some analyses the New World Fritillaria subgenus Liliorhiza (its distribution was colour-coded as Northern Asian) was sister to Lilium and the rest of Fritillaria, very largely from the Old World; Huang et al. (2020) found that the same subgenus (as F. maximowiczii) was sister to the rest of Fritillaria, but with weak support. Gao et al. (2015) noted that Nomocharis, along with some species of Lilium, was nested in Lilium s.l., and that mountain building provided novel habitats for the group as well as enabling hybridization. Givnish et al. (2020) thought that Lilieae as a whole originated in the Qinghai-Tibet-Himalayas-Hengduan Mountains region, and they discussed the biogeography of the North American species of Lilium in some detail. N. Zhou et al. (2024a) suggested that the common ancestor of Lilium might have been widely distributed in the northern hemisphere, rather than S.W. China and the Qinghai-Tibet plateau region, subsequent diversification being in response to climatic and geological changes and being constantly high over the last 10 million years or so.
Patterson and Givnish (2002) emphasized the similarities among the large-flowered heliophilous Liliaceae, with their bulbs, capsules, and linear leaves with parallel venation, and those among the broad-leaved, reticulate-veined, smaller-flowered, rhizomatous, baccate, woodland Liliaceae (e.g. Prosartes, Tricyrtis) respectively, and they suggested that the latter morphology was plesiomorphous in this part of Liliales ("concerted convergence" and "concerted plesiomorphy": see also Givnish 2003; esp. Givnish et al. 2004b, 2005, 2006b). The focus in Karimi et al. (2024) was on Calochortus with ca 74 species, although where it originated seemed elusive (c.f. Patterson & Givnish 2002); diversification there was connected with topographic and edaphic complexity (some 40% of the genus was associated with serpentines in some way), chromosome number evolution and migration. There was some geographic signal in the phylogeny, thus all and only Mexican species (apart from those found in Baja California) formed a single clade (Karimi et al. 2024).
Ecology & Physiology. Species with large genomes have larger cells, the plants are larger and they have larger flowers; Liliaceae are geophytes, so quick growth associated with these larger cells may be an advantage, although not in more extreme conditions where the growth period is short (Carta & Peruzzi 2016). Pan et al. (2023) discussed growth after the breaking of dormancy in Lilium bulbs; this speeded up after the opening of plasmodesmata via epigenetic repression of callose synthesis genes, the plasmodesmata opened and intercellular communication was established.
Some species of Fritillaria are climbers with leaf-tip tendrils (Sousa-Baena et al. 2018b).
Pollination Biology & Seed Dispersal. For pollination in some North American Lilium see Givnish et al. (2020) and in some species from China, see Duan et al. (2025). Bennett (1972 and references) noted that in at least some Lilieae with large genomes it took 3-8 days for fertilization to occur after the pollen tube penetrated the ovule. The Japanese Streptopus streptopoides is pollinated by fungus gnats (Mochizuki & Kawakita 2017).
Plant-Bacterial/Fungal Associations. For fungi on Liliaceae s.l., see Savile (1961).
Genes & Genomes. For genome size, chromosome length and much else, see Peruzzi et al. (2009) and also Pellicer et al. (2013), for genome size in Tulipa, see Zonneveld (2009), and for the evolution of genome size, see Leitch et al. (2007), Pellicer et al. (2018), etc.. Lilium and relatives have very large genomes, those of Tulipa and relatives rather smaller (and the 1 C value of Gagea may be only some 6.6 pg); the genome of Medeola is also quite large, so large genomes may be a feature of [Medeoloideae + Lilioideae] (Pellicer et al. 2013). Indeed, 1 C values in Fritillaria range from 190-540 times the size of the Arabidopsis genome, being around 30-85 Gb in size, and substantial variation can occur between closely related clades (Ambrozovâ et al. 2011; Day et al. 2014). Here the large genomes are made up of many different and quite heterogeneous repeat families, these not being removed but rather steadily accumulating over time (Kelly et al. 2015; Pellicer et al. 2018); c.f. Passiflora. Carta and Peruzzi (2015) found that genome size in Liliaceae correlated with features such as plant size, humid climates (positive) and precipitation seasonality (negative); see also Carta and Peruzzi (2016). Du et al. (2017) found a correlation of genome size with phylogeny in Lilium, and small genomes (range of 2C values - 45.2-168 pg) were likely to be basal, and they were also found in species growing at high elevations and subject to cold stress.
In Lilium and relatives, the largest chromosomes are 14-22.9 µm long and the smallest are 7.3-12 µm, while in Tulipa and relatives the comparable figures are 5.5-12.3 µm and 1.8-5.2 µm respectively. This emphasizes the differences between the two groups within Lilieae (see Lilieae and Tulipeae above) - and the taxa being compared all had n = 12 (Peruzzi et al. 2009). Depending of the method of analysis, there may be confusion in some 37 taxa of Gagea as to whether thay are diploid or polyploid (Halabi et al. 2023). For more on chromosomes, karyotype, etc., in Lilieae, see Gao et al. (2012) and Yin et al. (2014), and for Lilioideae, see Xie et al. (1992).
R.-S. Lu et al. (2021) compare plastome variation in the 15 genera whose plastomes had been sequenced; there was not that much variation (see also J. Huang et al. 2020). The infA gene may be a pseudogene, but it is lost in some species of Fritillaria and in Tulipeae.
At least some mitochondrial genes show an accelerated rate of change in this clade (G. Petersen et al. 2006).
Chemistry, Morphology, etc.. For the distribution of tuliposide and the possibly biosynthetically related γ-methyleneglutamic acid, the latter reported also from Haworthia (Asparagales-Asphodelaceae-Asphodeloideae, see Fowden and Steward (1957) and Slob et al. (1975). Lilium, at least, has storage mannans in the vegetative tissues (Meier & Reid 1982).
Martínez-Gómez et al. (2022) discuss the development of umbellate inflorescences in some species of Fritillaria. In Streptopus (Streptopoideae) the pedicel is adnate to the stem. The flowers of Lilium are shown with the median member of the outer whorl in the adaxial position (Spichiger et al. 2004; see also Eichler 1874). For floral development, see Tzeng and Yang (2001: Lilium) - B-class genes also expressed in out perianth whorl, but proteins only produced in the inner whorl, and Otani et al. (2016: Tricyrtis) - B-class genes are expressed in the outer whorl of tepals, as is common in monocots with petaloid tepals (Dodsworth 2016, c.f. Asparagaceae-Asparagoideae-Asparagus). The pollen grains of the family are relatively large (e.g. 74-139 µm long - Handa et al. 2001), and the sulcus of Lilium grains can be seen even under a dissecting microscope. Medeola has been described as having crassinucellate ovules and lacking a nucellar cap, but illustrations suggest that it has an ovule rather like that of other Liliaceae (Berg 1962 and references). In Clintonia the chalazal megaspores degenerate and the endosperm is diploid (Lord 2009); for the Fritillaria embryo sac and its development, see Haig (2020). There are a variety of seed dormancy mechanisms in the family, and the embryo may grow extensively after dispersal but before germination - Cardiocrinum is an example (Kondo et al. 2006).
Some general information is taken from Schnarf (1929, 1948) and Tamura (1998: Calochortaceae, Liliaceae); for (galacto)glucomannans, see Jakimow-Barras (1973), for rootstock, growth, etc., see Buxbaum (1938, 1958), Tillich (1998), and Levichev (2013), for some chemistry, see L. Chen et al. (2009), for floral anatomy of Fritillaria, see Novikoff and Kazemirska (2012), and for pollen of ex-Polygonatae, see L. Wang et al. (2017).
Phylogeny. The limits of the family adopted here agree with a phylogeny presented by Hayashi and Kawano (2000), although the sampling there was poor, however relationships within the family have been somewhat in flux. The clade [Clintonia + Medeola] may be sister to the rest of Lilioideae (e.g. Patterson & Givnish 2002; Fay et al. 2006c; Givnish et al. 2016b; J. S. Kim & Kim 2018), from which it differs somewhat morphologically. Calochortus and relatives are not monophyletic in Rudall et al. (2000), but their paraphyly is not clear, either. However, support in general is stronger, and there is a [Calochortoideae + Streptopoideae] clade, if only with weak support, in Patterson and Givnish (2002: esp. ndhF and combined trees) and Rønsted et al. (2005). In Fay et al. (2006c: two genes), the positions of neither Calochortus and Tricyrtis had any support. In the summary tree in Peruzzi et al. (2009), Lilioideae and Streptopoideae are well supported, as is some structure within the former, Calochortus, but not Tricyrtis, linked with the latter. Relationships in Z.-D. Chen et al. (2016) are [Streptopus [Tricyrtis [Clintonia + The Rest]]]. Indeed, the monophyly of Calochortoideae is questionable. Tricyrtis linked with Streptopoideae in J. S. Kim et al. (2013, 2018), and also in the plastome analysis of J. Li et al. (2021), in which the topology is that followed here. Relationships in Givnish et al. (2016b) were [[Streptopoideae + Calochortus] [Tricyrtis + Lilioideae s.l.]], but the latter clade was subtended by a very short branch. Relationships in the nuclear genome tree to be found in W. J. Baker et al. (2021: see Seed Plant Tree) are pectinate, even Gagea and Tulipa do not form a clade. The toplogy in a plastome analysis by R.-S. Lu et al. (2021) is that above; support values were strong.
Calochortoideae. Patterson and Givnish (2002: 64 spp., 3 plastid genes) recovered seven clades in Calochortus . In a later comprehensive study of the genus, Karimi et al. (2024: 294 nuclear loci) recovered six main clades (two from the previous analysis were combined), and there were also differences in details of the relationships they found.
Lilioideae-Lilieae. For a phylogeny of Fritillaria and Lilium, see Rønsted et al. (2005a) and especially Day et al. (2014). The monophyly of Fritillaria was not yet established, with Fritillaria subgenus Liliorhiza being of uncertain position (?sister to Lilium and the rest of Fritillaria; see also some analyses in J. Huang et al. 2018, c.f. also Huang et al. 2020, plastome analyses). For more detailed phylogenies of Lilium, see C. S. Lee et al. (2011: Korean species), Gao et al. (2013: esp. chromosomes), and Du et al. (2014: Chinese species, see also 2017). The focus in Du et al. (2014) was on high-altitude East Asian species, and they found that different genes gave different topologies, one more consistent with morphological relationships, the other with geography. Huang et al. (2018) carried out a particularly detailed analysis of relationships of Lilium and Lilieae, relationships at the base of the tribe being [Notholirion [Cardiocrinum ...]] (see also J. Li et al. (2021). Givnish et al. (2020) looked at whole plastomes (69 spp.), and they also used 440 single-copy nuclear loci (67 spp.); earlier classifications were not supported and there was some conflict in the topologies that resulted from analyses of the two sets of data. Watanabe et al. (2021: 64 spp.), using ten cpDNA and nuclear ITS + ETS regions found substantial agreement between chloroplast and nuclear trees in terms of the 12 major clades apparent (in turn divided into two well supported clades), although the relationships between those 12 clades differed somewhat: ancient hybridization again? Y. Li et al. (2022) found that the sections did not hold up in an analysis of protein-coding plastome genes, sections Leucolirion and Sinomartagnon, for example, being polyphyletic. Similarly, in analyses by D.-F. Xie et al. (2024) that included 54 species of Lilium all sections with more than a single species included were para- or polyphyletic. These problems were also evident in the plastome analysis of N. Zhou et al. (2024a); the two main clades of Lilium that they recovered included East Asian plus North American and Eurasian species respectively.
Tricyrtidoideae. Streptopus, Scoliopus and Prosartes are included in Streptopoideae (e.g. Shinwari et al. 1994a, b; S.-C. Chen et al. 2007: = Tricyrtidoideae), even appearing linked in morphological analyses (Patterson & Givnish 2002). Fay et al. (2006c) found the strongly supported relationships [Streptopus [Scoliopus + Prosartes]], c.f. the character optimizations in Patterson and Givnish (2002). Tricyrtis is sister to those genera (e.g. J. S. Kim & Kim 2018).
Lilioideae-Tulipeae. Gagea (one species) is sister to the other Tulipeae in the plastome analysis of J. Li et al. (2021). For the relationships of Gagea and Lloydia, the latter para/polyphyletic, see A. Peterson et al. (2008) and Zarrei et al. (2009); hybridization is important in speciation in Gagea (Peterson et al. 2009). Christenhusz et al. (2013: 24 spp., 5 plastid markers) looked at relationships in Tulipa; relationships in Eker et al. (2024: 45 spp., ITS) were somewhat ambiguous in subgenus Tulipa.
Classification. For a classification of Liliaceae, including generic keys, etc., see Peruzzi (2015); he recognized six tribes in the family. However, the classification of J. S. Kim and Kim (2018) with four subfamilies, and two tribes in one of them, Lilioideae, is followed here. The hierarchy above is very much an interim effort.
Lloydia is to be included in Gagea (Peterson et al. 2008; Zarrei et al. 2009). For a classification of Fritillaria, see Rix (2001), for that of Tulipa, see Christenhusz et al. (2013: Table 1) and Eker et al. (2024: 4 subgenera, one divided into two sections) and for that of Lilium - 12 sections - see Watanabe et al. (2022).
Previous Relationships. Cronquist (1981) and many earlier authors circumscribed Liliaceae very broadly, for instance, Cronquist included some 280 genera and 4,000 species in the family. Ex-Liliaceae are now scattered widely through Liliales and Asparagales in particular, also Petrosaviales, Dioscoreales, etc..
Streptopus and Scoliopus (Streptopoideae) have been included in Uvulariaceae, but the other members of that family are in Colchicaceae above, while Prosartes (Streptopoideae) even used to be included in Disporum, also now in Colchicaceae (Shinwari et al. 1994a, b). Streptopoideae were included in Calochortaceae by Tamura (1998).
SMILACACEAE Ventenat, nom. cons. - Smilax L. - Back to Liliales
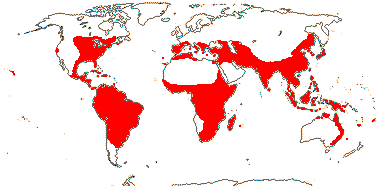
Plant climbing by tendrils, with sometimes ferocious spines, (herbs); rhizomes short, woody; stems monopodial (sympodial by apical abortion),; steroidal saponins, flavonols +; (root cortex sloughs, endodermis becomes superficial); (primary thickening meristem +); vessel elements (in stem 0) and leaves, with scalariform perforation plates; stem bundles in a ring [?]; mucilage cells +; cuticle ± with parallel platelets, stomatal type various, often anomocytic, un- or transversely oriented; leaves two-ranked, blade broad, formed from the primordial leaf apex, with 4-6 basal main veins, (vein endings free), vernation conduplicate, involute or supervolute-involute, (magin serrate), base cuneate to cordate, petiole +, with paired lateral tendrils [= "stipules"], spines +/0; plant dioecious; inflorescence umbellate; flowers small, 8> mm across; T with single trace, outer whorl valvate, median member adaxial, (± connate); staminate flowers: A 3-12, (± connate - Heterosmilax s. str.), latrorse to introrse, bisporangiate, monothecal; nectariferous trichomes on A; anther wall primary parietal layer gives rise to two secondary parietal layers, the outer producing the endothecium and middle layer, the inner producing the tapetum only [dicotyledonous type], (exothecium also with thickenings); pollen inaperturate, ± spherical, ± spinulose, ektexine thin, endexine thick; ?pistillode; carpelate flowers: staminodes +; style short/0, branches stigmatiferous, long, stigma dry; ovule 1(-2)/carpel, ± apical, pendulous, (straight), apotropous, outer integument 6-10 cells across, inner integument ca 2 cells across, parietal tissue 3-5 cells across, hypostase +; fruit a berry; seeds rounded (subangled), testa ± elastic, tegmen persistent, exo- and endotegmen with cuticle; endosperm with aleurone and fatty oils; embryo minute to small; n = 13-17, x = 16, nuclear genome [1 C] (0.75-)4.074(-22.131) pg, chromosomes 1.25-5.4(-9.7) µm long; cotyledon not photosynthetic, primary root well developed, epicotyl elongated, ligule +.
1 [list]/260. Pantropical to temperate. Map: from Fl. Austral. vol. 46 (1986); Fl. N. Am. vol. 26 (2002), Australia's Virtual Herbarium (consulted xii.2013), Seberg (2007) and Qi et al. (2013). [Photo - Flower, Fruit.]
Age. Divergence within this clade may have begun at the end-Eocene ca 40 Ma (Z. Qi et al. 2012) or (37.3-)35.5(-33.6) Ma (Z.-C. Qi et al. 2023).
Denk et al. (2015: esp. Table 1) summarize information on fossils of Smilax; these may go back to the Late Cretaceous and are notably abundant in Europe, and new taxa continue to be described (e.g. J.-L. Dong et al. 2020).
Evolution: Divergence & Distribution. The stem of Smilax seems to be around 32 Ma (Z.-C. Qi et al. 2023), and within extant members of the genus S. aspera (East Africa, Mediterranean, Indian continent) may be sister to the rest of the genus, within which there are two clades, largely New World and largely Old World (Cameron & Fu 2006; Z. Qi et al. 2012, 2013, 2023). Smilax may initially have been a member of the Boreotropical flora, and there seems to have been a substantial emount of diversification in the Old World, Asia in particular, associated with Late Miocene aridification, the environment becoming heterogeneous (Qi et al. 2023 - see the Messinian Salinity Crisis). C. Chen et al. (2014) looked at the biogeography of S. aspera in particular, but their fossil constraints were questioned by Denk et al. (2015). Within the New World clade, there may have been three shifts to the Old World, for example, the fossil S. miohavanensis, quite widely distributed in Miocene Europe, is related to Caribbean taxa (Denk et al. 2015).
Ecology & Physiology. For tendril climbing in Smilax, see Sperotto et al. (2023) - it is one of the 10 most speciose genera of climbers.
Vegetative Variation. Note that there is extensive infra- and inter-specific variation in Smilax leaf morphology, for example, fossils properly to be included in it have been placed in Mahonia (= Berberis), Quercus and Ilex (Denk et al. 2015)... Vessels in the stem of Smilax are up to 700 μm across (Ewers et al. 2015); the nodes tend to be trilacunar. There has been some debate as to whether the paired tendrils are stipules or not (Colomb 1887; Sinnott & Bailey 1914; Ye & Ronse De Craene 2024), but they certainly show precocious development (Martin & Tucker 1985); Sousa-Baena et al. (2018b) prefered the idea that they were duplications of the petiole (see also Arber 1920b: origin by chorisis/dédoublement). The leaf blade develops from the upper part of the leaf primordium (see Arber 1920b: blade is an expansion of the upper part of the petiole; Martin & Tucker 1985: blade develops from the "leaf apex"; Bharathan 1996; Rudall & Buzgo 2002). The leaf apex may sometimes be almost tendrillar, on the other hand, tendrils may be entirely absent, or absent from the reproductive parts of the plant. Both stomatal orientation and morphology vary here (J. M. Silva et al. 2012). There is considerable variation in the leaf base, which may be more or less expanded and sheathing; the prophyll is drawn with a closed sheath by Andreata (1997). For leaf anatomy, with a focus on species from Thailand, see Kladwong et al. (2025), the species groups that they found were not correlated either with taxonomic or phylogenetic groupings.
Genes & Genomes. Kong et al. (2007) discuss phylogeny and karyotype evolution; for chromosome numbers, etc., see also Peruzzi et al. (2009).
Chemistry, Morphology, etc.. There are suggestions that the umbel is basically cymose in construction, and that plant growth may be sympodial (Andreata 1997; see also Martin & Tucker 1985). Nakagawa et al. (2020) mention floral orientation.
Some information is taken from Arber (1925), but see especially Conran and Clifford (1985: vernation, seedling, etc.) and Conran (1998: general); for pollen, see S.-C. Chen et al. (2006b) and for ovules, etc., see Martins et al. (2012) and Ao (2013).
Phylogeny. Molecular analyses suggest that the Old and New World species of the genus form largely separate clades, a result not found in morphological analyses (c.f. Cameron & Fu 2006 and S.-C. Chen et al. 2006a). In the former study, Smilax aspera is sister to the rest of the family, although S. vitiensis was not included (see also Qi et al. 2012, esp. 2013), in the latter, S. vitiensis is in this position and S. aspera is apparently well embedded in the genus, although with little support. 135 species of Smilax were included in the study by Qi et al. (2023: 5 chloroplast genes, nuclear ITS, 2 low-copy nuclear genes) and with similar results; S. aspera was basal, and S. vitiensis, also included, was well embedded in the genus.
Classification. The morphology-based infrageneric classification is not supported by molecular studies (Qi et al. 2013).
Previous Relationships. Smilacaceae of Cronquist were more broadly circumscribed; the twelve genera he included are now scattered throughout Liliales and some are in Asparagales (see Asparagaceae-Lomandroideae).
[Philesiaceae + Ripogonaceae]: climbers by stem twining; cuticular wax with parallel platelets; stomata transversely oriented; blade vernation conduplicate-flat or curved; pollen ± echinate; fruit a berry; chromosomes heteromorphic.
Age. The age of this node is estimated to be (55.4-)47, 33(-25.4) Ma (Vinnersten & Bremer 2001: note topology), ca 50.7 Ma (Chacón et al. 2012b) or (58-)51(-50.5) Ma (Givnish et al. 2016b).
The stem node of Ripogonaceae is dated at 52-51 Ma based on fossils from Tasmania (Isles et al. 2015).
Evolution: Divergence & Distribution. Diversification in this clade has slowed down (Hertweck et al. 2015: c.f. topology).
Classification. Kim et al. (2013) suggested that the two families could be merged.
PHILESIACEAE Dumortier, nom. cons. - Back to Liliales
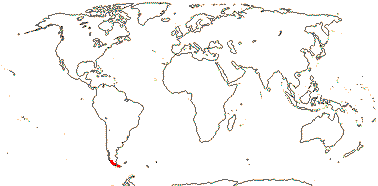
Plant rhizomatous, stem branching; chelidonic acid?; velamen +; tannin and mucilage cells 0; vessels 0 [Carlquist]; leaves two-ranked or spiral, vernation conduplicate [?level]; nectar in pocket formed by T and very base of A; A ?endothecial thickening; pollen inaperturate, surface spiny; placentation intrusive parietal; ovules many/carpel, parietal tissue ca 1 cell across; seeds pale yellow [= colour of tegmen], exotesta disintegrates, exo- and endotegmic; endosperm development?, with aleurone layer and fatty oils; x = 8 (?16, ?7), chromosomes 2.5-12 µm long; cotyledon not photosynthetic, primary root well developed.
2 [list]/2. S. Chile. [Lapageria Flower.]
Age. Crown-group Philesiaceae are (36.5-)14(-2.3) Ma (Givnish et al. 2016b) or ca 20 Ma (Givnish et al. 2018b), although the latter age may be meant to refer to the Philesiaceae-Ripogonaceae split (c.f. ibid.: Fig 3).
1. Philesia magellanica J. F. Gmelin
Plant erect shrub; blade 3-nerved; outer T ± calycine; A basally connate, extrorse; stigma dry; n = 19.
1/1. Southern Chile.
2. Lapageria rosea Ruíz & Pavón —— Synonymy: Lapageriaceae Kunth
Plant climbing by twining; stomata transverse to the long axis of the leaf, mesophyll undifferentiated; blade (3-)5-nerved; A free, introrse; stigma wet; nucellar cap +; n = 15.
1/1. Southern Chile, not the Straits of Magellan.
Chemistry, Morphology, etc.. Some information is taken from Conran and Clifford (1985, 1998); see Carlquist (2012a) for xylem anatomy.
Previous Relationships. Both the content and the proposed relationships of Philesiaceae have varied greatly in the past (Conran & Clifford 1998).
RIPOGONACEAE Conran & Clifford - Ripogonum J. R. Forster & G. Forster - Back to Liliales
Stems with prickles; flavonols +; mucilaginous cells +; stomata unoriented; leaves opposite, petiolate, blade with 4 near-basal lateral veins; inflorescence various, but ± racemose; flowers rather small [8 mm across]; A latrorse to introrse; tapetum amoeboid [?]; pollen prolate, surface reticulate; style short, unbranched, stigma lobed, wet; ovules 2/carpel, ?morphology; seeds rounded (subangled); exo- and endotegmen with cuticle; endosperm with starch, ?embryo; n = 15, x = ?; seedling?, ligule 0.
1 [list]/6. New Zealand to New Guinea. Map: from Fl. Austral. vol. 46 (1986), fossil localities (see below) in green.
Age. Fossil Ripogonum is reported from the Eocene of Tasmania (Conran et al. 2009b), from around 52.2 Ma in Patagonian deposits (Carpenter et al. 2014) and from 56-52 Ma deposits in New Zealand (Conran et al. 2018).
Evolution: Divergence & Distribution. Ripogonum used to have a broad distribution in the Southern Hemisphere, despite its current rather restricted distributuion (Carpenter et al. 2014; Wilf & Escapa 2014). This distribution/temporal pattern is known from a number of other taxa (see elsewhere for more details).
Chemistry, Morphology, etc.. Some information is taken from Arber (1925), but see especially Conran and Clifford (1985: vernation, seedling, etc.; 1986: general), and Conran (1998: general, under Smilacaceae); for pollen, see S.-C. Chen et al. (2006b).