EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0; fruit dry [very labile].
Age. Bell et al. (2010: BEAST exponential and lognormal respectively) suggested ages of (168-)156(-146) or (142-)136(-130) Ma and Tank and Olmstead (2017) ages of (178.4-)160.7(-142.6) Ma for this node. Wikström et al. (2001) suggested ages of (162-)155, 140(-133) Ma, Soltis et al. (2008: a variety of estimates), 160-123 Ma, Magallón and Castillo (2009) ages of ca 201.4 and 128 Ma for relaxed and constrained penalized likelihood datings respectively, Moore et al. (2010: 95% HPD), (140-)132(-125) Ma, Xue et al. (2012) an age of around 137.3 Ma, Salomo et al. (2017) an age of (193-)164(-135) Ma, Foster et al. (2016a) an age of ca 145 Ma, Naumann et al. (2013) an age of ca 138.1 Ma, Tank et al. (2015: Table S1, S2, but stem eudicots 136.9 My) ages of around 191.7-185.6 Ma, at the other extreme Magallón et al. (2013, 2015) suggested ages of about 132.5 Ma and 134.4 Ma respectively, while at ca 154.9 Ma X. Guo et al. (2021) were somewhere around the middle.
Bell et al. (2010: monocots sister to Chloranthaceae, magnoliids, etc.) suggested ages for stem monocots[??] of (156-)146(-139) or (138-)130(-123) Ma depending on the method used, Chaw et al. (2004: 61 chloroplast genes, sampling poor) an age of 150-140 Ma, Davies et al. (2011: 95% credibility intervals) ages of (161-)137(-124) Ma, and Y. Yang et al. (2020: Suppl. Fig. 22) an age of around 144 Ma.
An early fossil-based estimate for the age of this clade was ca 100 Ma (Crepet et al. 2004). For several fossils assigned around here, see Doyle and Upchurch (2014). Thus Donlesia from earliest late Albian North American deposits dated to ca 105 Ma (Dilcher & Wang 2009; Wang & Dilcher 2018; Kvacek et al. (2012) linked Pseudoasterophyllites, vegetatively quite like Ceratophyllum and perhaps growing in somewhat saline conditions, with Tucanopollis, an abundant palynomorph from Africa-South America over 125 Ma. Furthermore,Montsechia (= Montsechiaceae† Gomez, Daviero-Gomez, Coiffard, Martín-Closas & Dilcher), a rather odd fossil found in deposits from the Iberian Peninsula and also from Italy that are aged at 130-105 Ma (Lower Barremian to Upper Albian) may be in this area (Gomez et al. 2015, 2021; Bueno-Cebollada et al. 2023). See also Doyle et al. (2015), the mesangiosperm node, and below.
Evolution: Divergence & Distribution. The question is, how are characters in this area to be optimized? Are some of them to be placed below the node [Ceratophyllales + eudicots]? Did the common ancestor of this clade have tricolpate pollen? Had it already lost ethereal oils? These are going to be very difficult questions to answer unless, e.g., detailed studies of the development of the distinctive inaperturate Ceratophyllum pollen gives clues as to its derivation - and of course the relationships of Ceratophyllaceae have to b e sorted out. Being a very old and aquatic lineage, Ceratophyllaceae have a very derived morphology, and any relationships suggested by morphology are likely to be ambiguous. However, somewhat less radically modified fossils like Montsechia that appear to be strongly associated with Ceratophyllaceae may help - thus Montsechia has anomocytic stomata (Gomez et al. 2015, but not always - Bueno-Cebollada et al. 2023), here pegged to the eudicot node.
- If Ceratophyllaceae are sister to Chloranthaceae, a relationship quite often suggested (e.g. Doyle et al. 2015; Kvacek et al. 2016), then features common to the combined clade might include: Leaves opposite, margin toothed; flowers small, [<4 mm across], sessile; anthers embedded in broad filament, G 1, ovule 1, pendent, straight, nucellar cap +; fruit indehiscent (see in part also Duvall et al. 2006; Endress & Doyle 2009, 2015: similarities greater if the male flower is treated as an inflorescence). The distinctive Canrightia resinifera from around 125-110 Ma in Portugal with its 4-merous flowers, hypanthium, syncarpous unilocular gynoecium that has apical placentation ovules with an endothelium, etc., may be associated with this clade, although there may also be connections with the magnoliids (Friis & Pedersen 2011; Friis et al. 2011), and Montsechia may also belong in this area, if not assignable to Ceratophyllales in particular (Gomez et al. 2015) - but Gomez et al. (2020) later found that it consistently grouped with Ceratophyllaceae in constrained analyses, along with a couple of other fossils in the analysis. Further immediate relationships in this situation involve magnoliids.
- If Ceratophyllaceae are sister to monocots, features for that combined clade might include: Plant herbaceous; primary root at best weak; vascular bundles in stem closed [no interfascicular cambium developing]; vessels in stem and leaves 0; (lamina margin spiny toothed); microsporogenesis successive. However, if Ceratophyllaceae are sister to eudicots, as now seems more likely (Jansen et al. 2007; Saarela et al. 2007; Moore et al. 2007), any similarity between Ceratophyllaceae and monocots that could be linked with a more or less aquatic habitat are likely to be parallelisms. There are no morphological characters in particular linking Ceratophyllaceae with eudicots - one might almost expect a submerged aquatic plant to lack ethereal oils.
- And if Ceratophyllaceae are sister to mesangiosperms in their entirety (Zuntini et al. 2024: support for this position could be stronger), or sister to eudicots (H.-T. Li et al. 2021) what then? (Note that I have followed the suggestion made by Zuntini et al. 2024 here.)
Genes & Genomes. Martín and Sabater (2010) note a change from cytosine to thymidine at certain editing sites of some chloroplast ndh genes (it is also possible that there are interesting changes within the ANA grade).
Phylogeny. Relationships between the lineages immediately above the basal pectinations in the main tree, the ANA grade (Amborellales, Nymphaeales and Austrobaileyales here), are only slowly being clarified. For further information, see especially the discussion at the Mesangiospermae node. Chloranthales, magnoliids, and monocots are the other clades immediately basal to the eudicots and whose positions are still rather uncertain.
CERATOPHYLLALES Link - Main Tree.
Just the one extant family, 1 genus, 1-5 species.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Includes Ceratophyllaceae, Montsechiaceae†.
Synonymy: Ceratophyllanae Reveal & Doweld
CERATOPHYLLACEAE Gray, nom. cons. - Ceratophyllum L. - Back to Ceratophyllales
Herbaceous, submerged aquatic; mycorrhizae 0; delphinidin +, alkaloids 0; roots 0; vascular cambium 0; vessels/tracheids 0; vascular bundles lacking associated sclerenchyma; nodes?; stomata 0; cuticle wax crystalloids 0; colleters +; leaves opposite, dichotomously divided, venation then dichotomous, or not; vegetative bud one (two) per node; lamina margins spiny-toothed; plant monoecious; inflorescences extra-axillary, alternating with leaves; staminate inflorescence capitate; staminate flowers: inflorescence bracts whorled, glandular at the apex, basally ± connate; bracts 0; perianth 0; A ?1/?3-many, anthers extrorse; ± sessile, connective with ephemeral gland at apex, also two pointed projections, staminodes + [central]; tapetum amoeboid, cells uninucleate; microsporogenesis ?successive; pollen inaperturate, ectexine 0, tetrads lacking callose wall; pollen tubes branched; pollen tri(bi-)cellular; pistillode 0; carpelate inflorescence: flower single; carpelate flowers: staminodes 0; G 1, ascidiate; compitum necessarily 0, style quite long, stigma small, at base of lateral groove; ovule 1/carpel, straight, apical, pendulous, unitegmic, integument 2-5 cells across, nucellar cap + [ca 2 cells across], parietal tissue 8-10 cells across, postament, podium, hypostase +; fruit achenial, spiny; seed coat ± obliterated; endosperm 0, embryo long, chlorophyllous, plumule well developed; suspensor 0; n = 12, x = 12 (?13, ?6), nuclear genome [1 C] (0.246-)1.622(-10.686) pg.
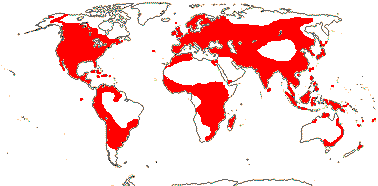
1[list]/ca 6. Map: see Vester (1940), Hegi (1965), Hultén (1971), Les (1989) and Wilson (2007). World-wide. Photo: Habit © from D. Les website, Fruit © H. Wilson.
Age Silvestro et al. (2020) estimate the time-of-origin of Ceratophyllaceae to be a mere ca 68.4 Ma.
Evolution: Divergence & Distribution. The distinctive fruits (with associated leaves) of Ceratophyllum are known from the Aptian and Albian onwards and are widely distributed in the Caenozoic (see Dilcher & Wang 2009; Friis et al. 2011 for references).
Ceratophyllaceae are a very ancient clade of aquatics (Gomez et al. 2015) which once may have been quite diverse. Dilcher and Wang (2009) described Donlesia from deposits in Kansas of end-Albian age some 100 Ma. This is a plant that they think may be sister to Ceratophyllum; note that it has a basal ovule, not an apical ovule as in extant Ceratophyllum. The 125 Ma or more old Portugese fossil Montsechia vidalii, placed in Montsechiaceae, has been associated with Ceratophyllum. Its carpels are borne two together (?two flowers), there is no style, there is an hole shaped liked a cat's eye similar to the pore in the carpel of Ceratophyllum (?underwater pollination), and the ovule is pendent on a funicle that runs the length of the carpellary loculus, the placenta being basal (Gomez et al. 2015, 2020).
Triterpenoids produced by CYP716 enzymes are found neither here nor in monocots - they are also absent from Nymphaeales, well, from Nuphar, at least (Miettinen et al. 2017).
For the ages of some intercontinental disjunctions within Ceratophyllaceae, see Les et al. (2003).
Ecology & Physiology. Absorbotrophic mixotrophy, in which the plants obtain some of their carbohydrates (for example) from the water, occurs here (see Firmin et al. 2022 for references).
Pollination Biology. Pollination is hypohydrophilous, i.e., it happens below the surface of the water (Gottsberger 2016a and references).
Genes & Genomes. Three polyploidization events have been suggested for Ceratophyllum demersum that have been dated to 177-157, 143-127 and 15-13 Ma respectively (Y. Yang et al. 2020); the first event could even be placed in the ANA grade.
Chemistry, Morphology, etc.. It is unclear whether or not Ceratophyllum has a fully-developed cauline endodermis, however, a starch sheath has sometimes been recorded (Seago 2020). There is a ring of air canals in the stem outside the pericycle, and also a central air canal, although the vascular cylinder is otherwise solid, lacking pith (Rutishauser 1999). Although the leaves are whorled, there is usually a single vegetative bud (sometimes two) and a single floral bud (up to four) per node; for leaf morphology, see Rutishauser and Sattler (1987). The flowers are borne on the same orthostichy as the vegetative buds; the latter alternate their positions at each node, hence the floral buds will be lateral to the leaves (Rutishauser 1999). Iwamoto et al. (2015) suggest that the leaves are opposite (hence the up to two buds per node) and the flowers come from accessory (prophyllar) buds (hence they are up to four in number per node).
For floral morphology, see Endress (1994d) and Endress and Doyle (2015) in particular. The "flower" with 3 to many stamens may be better treated as an inflorescence made up of flowers that consist of a single stamen and nothing more (see also Tekleva et al. 2021); the identity of things called bracts, petals, etc. (Iwamoto et al. 2003, 2015) also become suspect - so treat the familial/ordinal floral apomorphies carefully... Shamrov (2009) described the gynoecium as being two-carpelate and syncarpous (c.f. Iwamoto et al. 2015 and references; Endress & Doyle 2015); see also Shamrov (2022b: Fig. 12A) for the morphology of the ovule. The latter depicts a massive vascular supply to the ovule, while Les (1993) suggests that two fused ventral bundles supply the ovule, terminating at its insertion.
Some information is taken from Les (1993: general); see Iwamoto et al. (2003) for floral morphology, Takahashi (1995) for pollen development, Batygina et al. (1982) for embryology and Floyd and Friedman (2000) for endosperm development.
Phylogeny. See Les (1989 and references) and Szalontai et al. (2018) for species limits (unclear) and sectional groupings. Mavrodiev et al. (2021) reanalysed the data in Szalontai et al. using various ways to polarize the data (including a notional all-primitive outgroup) - not easy, since the relationships of Ceratophyllum are so unclear - and found that C. echinatum was sister to the rest of the genus.
Classification. It seems unneccessary to place Ceratophyllum echinatum in a separate genus (c.f. Mavrodiev et al. 2021).