EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene; PHYE +. - Back to Main Tree
Age. Wikström et al. (2001) suggested an age of (172-)165, 147(-140) Ma for this node, while ages in Bell et al. (2010: BEAST exponential and lognormal respectively) are (187-)173(-160) or (150-)144(-138) Ma; Soltis et al. (2008) give dates of some 174-127 Ma, Moore et al. (2010) ages of (151-)144(-138) Ma, and Iles et al. (2014) ages of (145.6-)140.2(-135.5) Ma, while ca 137.7 Ma is the age in Magallón et al. (2015) and (148.1-)144.6(-140.6) Ma in Lutzoni et al. (2018). Magallón and Castillo (2009) offer very divergent estimates - ca 235.5 and 129.7 Ma for relaxed and constrained penalized likelihood datings respectively. Foster et al. (2016a: q.v. for details) suggest an age of cs 195 Ma, Clarke et al. (2011: q.v. for other estimates) ages of (208-)177(-152) Ma, N. Zhang et al. (2012; Xue et al. 2012 are very similar) ages of (185-)161(-146) Ma, and Magallón et al. (2013) an age of around 170.4 Ma. At (250, 215-)211, 180(-156), ca 237, and (267-)228(-192) Ma, the estimates by Zeng et al. (2014), Z. Wu et al. (2014) and Salomo et al. (2017) respectively are towards the upper end of the spectrum.
A fossil-based estimate for the age of this clade is ca 113 Ma (Crepet et al. 2004). Indeed, there are a number of fossils perhaps to be assigned to the Nymphaeales-Austrobaileyales area from the Early Cretaceous (see above for more discussion). However, pollen data suggest to Zavialova and Tekleva (2021: Fig. 11) an age somewhat in excess of 165 Ma in the Jurassic.
Chemistry, Morphology, etc.. See Hegnauer (1990) for a discussion of the chemistry of the Polycarpicae, which also includes the magnoliids and Ranunculales. The sampling for the presence/absence of tension wood is poor, for instance, only one member of Austrobaileyales is mentioned in Höster and Liese (1966), and its position at this node is only provisional. However, in a number of taxa that appear to lack a G layer, it becomes lignified, and so its absence is only apparent (Roussel & Clair 2015). Cuticular striations are known to occur only at and above this node (Upchurch 2013).
Columellar infratectal structure of pollen grains may be best optimised here; the plesiomorphic condition is granular, also found in the pollen of several Magnoliales, Monimiaceae, etc., as reversals (Doyle et al. 1990b). For the 12BP deletion, see S. Kim et al. (2003, 2004b) and Aoki et al. (2004).
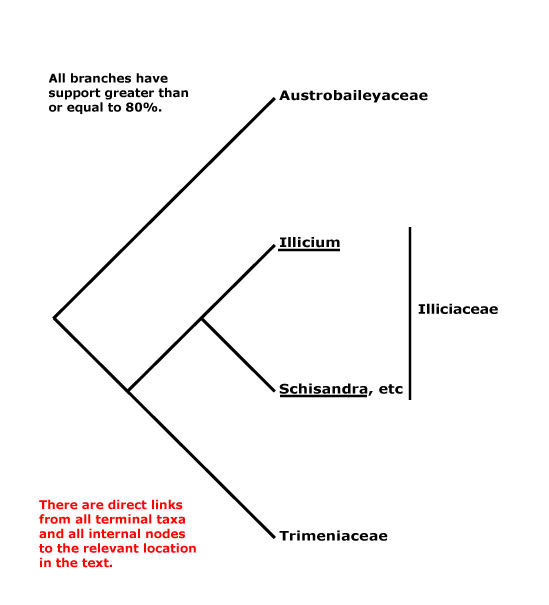
AUSTROBAILEYALES Reveal - Main Tree.
Tiglic acid +; vessels solitary; stomata paracytic/laterocytic, often mesoperigenous; nodes 1:2; petiole bundle(s) arcuate; leaves opposite, lamina margin?; P spiral; A spiral; G spiral, extragynoecial compitum +; ovules apotropous, outer integument 5-7 cells thick; fruit a berrylet/drupelet, P deciduous; mesotestal cells ± sclerotic, tegmen collapsed. - 3 families, 5 genera, 100 species.
Includes Austrobaileyaceae, Schisandraceae, Trimeniaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Tank et al. (2015: Table S2) thought that this node might be ca 179 Ma, Wikström et al. (2001) suggested an age of (155-)148, 133(-126) Ma for crown Austrobaileyales, Schneider et al. (2004) ages of 168 and 85 Ma, Magallón and Castillo (2009) ages of ca 203 and 125 Ma, Salomo et al. (2017) ages of (166-)131(-106) Ma, Bell et al. (2010) ages of (145-)122(-99) (see also Magallón et al. 2015) or (130-)114(-110) Ma, while Iles et al. (2014) suggested an age of (130.8-)118.3(-109.9) Ma and L. Yang et al. (2020) ca 200 Ma. Rather younger estimates include those by Magallón et al. (2013) who thought that this node might be some 103.4 Ma old, while ca 91.5 Ma is the estimate in Naumann et al. (2013).
Evolution: Divergence & Distribution. For some Early Cretaceous seeds that may be in this part of the tree, see Friis et al. (2018b), and these include seeds from Portugal (Serialis, Riaserialis) may belong around here (Friis et al. 2019a); see also Magnoliales below. Similar relationships have been proposed for some apocarpous flowers, three genera of which were recently described (Friis et al. 2020b) - these are also known from North America, and also some fossils discussed by Doyle and Endress (2023); again, relationships with magnoliids are also possible.
Endress and Doyle (2015) discuss some possible floral apomorphies and Romanov et al. (2023) the same for fuit and seed; it can be difficult to interpret the descriptions included in the latter in terms of conventional fruit and testal types.
Ecology & Physiology. For the evolution of the liane habit, common here, in the context of plants with rather unspecialized vascular systems, see Feild and Isnard (2013) and Isnard and Feild (2015).
Pollination Biology & Seed Dispersal. Thien et al. (2009) survey what is known about pollination in the clade. For stigmatic incompatability reactions in at least some members of this order, see Allen and Hiscock (2008).
Chemistry, Morphology, etc.. The wood has paratracheal parenchyma (Carlquist & Schneider 2001). Laterocytic stomata are common in the order, and the cuticle surface is radiate-striate around the secretory cells on the lower surface of the leaf blade in Austrobaileyaceae and Schisandraceae, at least (Baranova 2004b for discussion; Carpenter 2006); see also Rudall and Knowles (2013), mesoperigenous stomatal development is recorded here.
For vegetative anatomy, see Metcalfe (1987), for some developmental morphology of ovules and seeds, inc. details of lobing, etc., at micropyle, see Yamada et al. (2003a) and for embryo sac and endosperm development, see Floyd and Friedman (2001), Friedman et al. (2003), J. H. Williams and Friedman (2004) and Tobe et al. (2007).
Phylogeny. Soltis et al. (1997) and Källersjö et al. (1999) initially circumscribed the order; Trimeniaceae are also to be included (e.g. Qiu et al. 1999). Austrobaileyaceae have beeen found to be sister to the other members of the order in several studies, but W. J. Baker et al. (2021: see Seed Plant Tree) found Trimenia moorei to be in that position.
Synonymy: Illicineae J. Presl - Illiciales Cronquist, Schisandrales Martius, Trimeniales Doweld - Schisandrineae Shipunov - Austrobaileyanae Chase & Reveal, Illicianae Doweld, Trimenianae Doweld - Illiciidae C. Y. Wu
AUSTROBAILEYACEAE Croizat, nom. cons. - Austrobaileya - Back to Austrobaileyales

Liane, climbing by twining; alkaloids 0, flavonols?; primary stem with separate bundles; wood with broad rays; sieve elements with non-dispersive protein bodies; nodes 1:1; calcium oxalate as crystal sand; (stomata anomocytic), giant or D-type stomata + (?both spp.?); lamina vernation conduplicate, margins entire, petiole short; flowers axillary, large [ca 5 cm across],cortical vascular system?; P 12-24, with two traces; A 6-11, laminar, vascular bundle(s) branching, anthers embedded in connective, staminodes internal, 6-16; G (4-)6-9(-14), stigma bilobed; ovules (4-)6-8(-14)/carpel, outer integument to 10 cells across, chalazal end of ovule massive, vascular bundle in antiraphe; fruits long-stipitate; seeds ruminate, large [>2 cm long]; perichalazal; testa multiplicative, vascularized, sarcotesta +, outer mesotesta lignified; perisperm transient, starchy, endosperm starchy, embryo developing after seed is ripe; n = 22, ?23, x = 7 (?6), nuclear genome [1 C] (0.066-)2.522(-96.262) pg; germination epigeal, plumule develops ca 4 years from pollination [see below].
1 [list]/2. N.E. Australia. Map: from Heywood (1978). [Photo - Flower.]
Evolution: Ecology & Physiology. For the ecophysiology of Austrobaileya scandens, a liane that can tolerate shade, see Feild et al. (2003b). The development of the embryo/germination is a very protracted affair. The fruitlets take about a year to develop on the plant, and then it takes over another year for the embryo to develop, with a month or so between the emergence of the radicle and the appearance of the cotyledons; the plumule starts developing about four years after pollination without there baing any particular resting state all this time (Losada et al. 2017). Despite the relatively large size of the embryo when the fruit is mature, it is small relative to the size of the seed, and in this it is much like the seeds of other members of the ANITA grade. The very slow development/initial growth of Austrobaileya may have something to do with the low-light conditions in which the plant initially grows (Losada et al. 2017).
Pollination Biology & Seed Dispersal. The flowers smell unpleasant; for pollination - possibly sapromyophily - see Gottsberger (2016a). The stigmas are postgenitally united by secretion (Losada et al. 2017).
Chemistry, Morphology, etc.. There is some discussion as to whether the highly inclined end walls of the sieve tube have sieve plates, or not (Evert 2006: 393 for literature); in any event, other details of the sieve connections are typically angiospermous. The stylar canal is filled with secretion.
See Bailey and Swamy (1949) and Endress (1993) for general information, Carlquist (2001) for wood anatomy, Behnke (1986) for phloem anatomy, pores are very narrow, Endress (1980a, 1984) for floral morphology and Zavada (1984) for pollen (c.f. Furness 2014).
[Schisandraceae + Trimeniaceae]: mucilage cells +; pollen other than mono[ana]sulcate; stigma dry; megaspore mother cells 2-many; exotesta ± palisade.
Age. Ages for this node of (107.9-)102.1(-98.8.) Ma (Iles et al. 2014), ca 109.7 Ma (Magallón et al. 2015), (116-)106(-101) Ma (Salomo et al. 2017) or as much as around 158.6 Ma (Tank et al. 2015: Table S2) have been suggested.
Anacostia, fossils with graded-reticulate monosulcate to trichotomosulcate pollen and exotestal seeds from Cretaceous (Barremian-Aptian) deposits some 130-115 Ma old of E. North America and Portugal (Friis et al. 1997b), may be sister to Schisandraceae (Doyle & Endress 2010, 2014; Doyle & Upchurch 2014), not really compatible with the estimate in the preceding paragraph. However, Friis et al. (2011) found the fossils hard to place.
SCHISANDRACEAE Blume, nom. cons. - Back to Austrobaileyales
Tetracyclic triterpenes [cycloartanes], flavonols +, flavones 0, ellagic acid +; (phloem fibres +); true tracheids +; astrosclereids +, with crystals in their walls; mucilage cells in phloem elongated; (leaf epidermis silicified); leaves spiral, lamina vernation supervolute; A 4-many, latrorse to introrse, pollen tricolpate, syncolpate at the poles [= trichotomocolpate or syntricolpate], semitectate-reticulate, muri tall; infra-stylar extra-gynoecial compitum/pollen tube growth; exotesta with sinuous anticlinal cell walls, ?lignified mesotesta; n = 13, 14, x = 7 (?8), nuclear genome [1 C] (0.132-)6.555(-325.467[!]) pg.
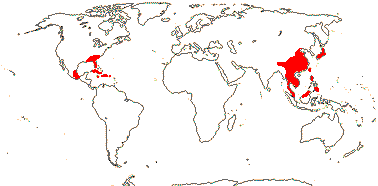
3 [list]/83 - two tribes below. Sri Lanka and South East Asia to W. Malesia, S.E. U.S.A., E. Mexico, Greater Antilles.
Age. Magallón et al. (2013) suggested an age for crown-group Schisandraceae of around 42.2 Ma and Naumann et al. (2013) an age of ca 40 Ma, and estimates are slightly under 50 Ma in N. Zhang et al. (2012). Ages in Luo et al. (2018) and Morris et al. (2007) are very different, the ages being around 135.8 Ma and 131.7 Ma respectively, while those in Wikström et al. (2001) at (119-)108, 93(-82) Ma are somewhat intermediate, as are those in Bell et al. (2010), at 91-89 Ma. The first pair of ages in particular are likely to be severe underestimates if the identity of the trimeniaceous fossil seed (see below) is correct.
1. Illicieae de Candolle - Illicium L. —— Synonymy: Illiciaceae Berchtold & Presl nom. cons.
Shrubs or trees; plants Al accumulators; (pits vestured); nodes 1:1; stomata with cuticular intercellular inclusions capping guard cells; petiole and midrib bundles arcuate; branching on previous innovation; leaves pseudoverticillate, lamina margins entire; P (7-)12-many, with nectaries; A (7-10); pollen isopolar; G (5-)7-15(-21), pseudo-whorled, basi-laterally connate, residual axis quite massive; G occlusion also by postgenital fusion; ovule 1/carpel, near basal, micropyle various, parietal tissue 6-8 cells across; fruit an explosive follicle, endocarp palisade, lignified; seed with a circular cap; testa multiplicative; endosperm first cell wall oblique, develops mostly from chalazal cell, starch 0; n = .
1/42. South East Asia to W. Malesia, S.E. U.S.A., E. Mexico, Greater Antilles. Map: from Wood (1972). Photo: Flower, Fruit.
Age. Morris et al. (2007) suggested that crown group Illicium was (91.5-)89.4, 76.3(-72.2) Ma, Salomo et al. (2017) suggested an age of (93-)64(-34) Ma, while ca 45.1 Ma is the age in Luo et al. (2018).
Yoo et al. (2005) thought that the ca 90 Ma fossil Microvictoria was stem group Nymphaeales, although other studies had suggested it was crown-group Nymphaeaceae (Gandolfo et al. 2004 [its initial description]; Endress 2006; Friis et al. 2011). In a recent general phylogenetic analysis it ended up in a number of positions in the ANA grade, also in Laurales-Calycanthaceae, although not in the [Cabombaceae + Nymphaeaceae] clade itself (Schönenberger et al. 2020). Doweld (2022) placed it in its own family which he thought was in his Illiciales.
2. Schisandreae de Candolle —— Synonymy: Kadsuraceae Radogizky
Lianes, climbing by twining; (silicon concentration high), distinctive neolignans, myricetin +; vessel elements with simple perforation plates, (torus-margo pits + - S.); nodes 1:3; petiole bundle interrupted arcuate, midrib bundle incurved-arcuate; fibres/sclereids (branched)>, with crystals in the walls; stomata also laterocytic; leaves (two-ranked), lamina (vernation involute), teeth with clear persistent swollen cap into which proceed higher order veins as well as secondaries or tertiaries (margins entire); plant monoecious or dioecious; P 5-15; staminate flowers: (4-7), ± connate, endothecium biseriate; pollen grains heteropolar, not syncolpate at proximal pole, (6 colpate - 3 long, meeting at one pole, 3 short); pistillodes 0, (residual apex + - S.); carpelate flowers: staminodes 0; G 12-many, stigma papillate; ovules 2-5(-11)/carpel, ?type, parietal tissue 2-4 cells across, nucellar cap ca 2 cells across; (more than one embryo sac developing); fruit berrylets, usu. 2-seeded, false septum +, (inner hypodermal layer of pericarp with anticlinally-elongated cells receptacle enlarging greatly; endotesta also lignified; cotyledons convolute; n = 7, .
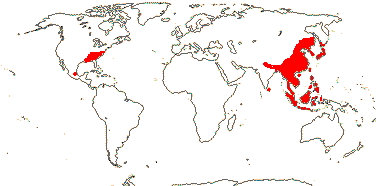
2/41: Schisandra (25), Kadsura (16). Sri Lanka, East Asia to W. Malesia. Map: from Saunders (1998, 2000).[Photo - Infructescence.]
Age. The crown-group age of this clade is ca 66.2 Ma (Luo et al. 2018).
Evolution: Divergence & Distribution. For the fossil record of Schisandraceae, which dates back only to the Late Cretaceous, see Friis et al. (2011). However, Friis et al. (2020, 2021a) note that trichotomocolpate pollen, a feature of Schisandraceae, is relatively common in the early angiosperm record. Furthermore, Friis et al. (2018b) described Nitaspermum, based on seeds perhaps 112-107 Ma from the Albian of the S.E. U.S.A., that was similar in some respects to Illicium.
Z. Liu et al. (2006) discuss character evolution in Schisandra.
Pollination Biology & Seed Dispersal. Illicium, Schisandra glabra (Schisandra s. str.) and Kadsura longipedunculata all have thermogenic flowers (Seymour 2001; Liu et al. 2006; Yuan et al. 2008; Luo et al. 2010). Pollen may be a floral reward for the pollinator here, e.g. the cecidomyid Megommata, and the pollinator may also oviposit, and/or deceit pollination may be involved (see also Gottsberger 2016a); reports of nectar/nectaries need to be substantiated (Erbar 2014; see Liao et al. 2025). Details of pollination, with small diptera being the commonest pollinators, have recently been clarified (see especially Luo et al. 2010, 2017b, 2018). Luo et al. found that pollination in the whole family was predominantly by nocturnal resin-feeding Resseliella gall midges, beetle pollination being quite uncommon and a derived condition. In Kadsura midges laid eggs in the flowers, usually in the androecium or gynoecium, but in K. coccinea, sister to the rest of the genus, eggs are laid on inner members of the perianth. Luo et al. (2017b) described how the midge larvae ate resinous substances produced by the plant (the sesquiterpene caryophyllene was a major component) when its tissues were damaged by the female midge when it was laying eggs - the larvae ate such floral exudates, but not pollen or ovules. Only female midges visited the flowers - three species of midge were reported to visit K. longipedunculata (Hao et al. 2024), while Luo et al. (2017b) found that the species of Kadsura, for example, that they examined were all visited by different species of midge. These Resseliella midges have tactile and chemosensory setae up to 20 mm long on their cerci (Hao et al. 2025). In this midge-Schisandraceae system, larval development can be quite a protracted affair (Luo et al. 2010, 2017b).
Luo et al. (2018) suggested that plant-pollinator relationships in Kadsura, for example, were an example of Jansenian co-evolution: "interacting guilds of plants and arthropods coevolve" (ibid.: p. 7), although without strictly coinciding divergence times being expected. Dates are indeed interesting. Ignoring error bars, divergence in Schisandraceae, in all three genera in which the midge Resseliella is currently the major pollinator, began ca 135.8 Ma, however, the midge itself was still a mere idea in the mind of god since divergence in these midges began only ca 11.2 Ma (the species in the first-diverging midge clade does not lay its eggs in the flower, but the species of Kadsura it pollinates cannot be thought of as being "primitive" - Luo et al. 2018). Luo et al. (2017b) suggest a crown age for Kadsura in particular of about 40 Ma, and for its pollinating Resseliella of ca 11.2 Ma, the ranges not even overlapping. Although hardly co-evolution, Luo et al. (2018) suggest that extinct midges might have been pollinators here - perhaps a not unreasonable suggestion, but hardly satisfying, since such midges must have been involved for 110 Ma or so. Luo et al. (2018) also mention the small size of Schisandraceae flowers, which is interesting, given the small size of many early angiosperm fossil flowers... For more on pollination and possible coevolution in the family, see Hao et al. (esp. 2024, also 2025).
For the growth of the pollen tube through mucilage on the surface of the epidermis rather than between cells, i.e. the presence of an extragynoecial compitum, and the nature of the stigma surface, see Lyew et al. (2007), Du and Wang (2012), and Luo et al. (2017b) and references. E. G. Williams et al. (1993: Illicium) and X.-F. Wang et al. (2011: Schisandra) described an infrastylar extragynoecial compitum where the pollen tubes move out of the (unsealed) base of the carpel into an adjacent carpel.
Romanov et al. (2013) discuss the the explosive dehiscence of the follicle of Illicium and its distinctive anatomy.
Genes & Genomes. A genome duplication event has been placed at this level and dated to ca 105.4 Ma (Landis et al. 2018: App. S1).
Chemistry, Morphology, etc.. The vessel member endings of Illicium may also be reticulate. The prophylls of Schisandra are reported to be adaxial (Keller 1996), but those I have seen are lateral.
In Schisandra, at least, the parts of the perianth that are exposed in bud are sepal-like on their exteriors (Warner et al. 2009). The anther connective is especially well developed. The pollen is modified monosulcate (via tritomosulcate). X. Zhang et al. (2019) discuss carpel/ovule development in detail interpreting the carpel as being a leaf subtending an ovule-bearing branch; micropyle morphology seems variable. The endotegmen may persist (Corner 1977).
Some general information is taken from Keng (1993), and Saunders (1997, 2000); Sy et al. (1997) discuss phytochemical relationships, for wood anatomy, see Z.-R. Yang and Lin (2007), for general vegetative anatomy, see Bailey and Nast (1948), for floral development, see Robertson and Tucker (1979), Tucker (1984), Tucker and Bourland (1994) and Dong et al. (2012), for pollen morphology, see Praglowski (1976) and Wang et al. (2010) and for embryology, etc., see Kapil and Jalan (1964: 4-nucleate embryo sac); Floyd and Friedman (2001) outline endosperm development, while Swamy (1964a: embryo sac unlike any others he knew), Friedman et al. (2003a, esp. b) and Friedman and Williams (2004) provide information on the female gametophyte.
Phylogeny. Kadsura may be paraphyletic, based on the analysis of both trnL-F and ITS sequences, although paraphyly is not found in morphological analyses (Hao et al. 2001; Denk & Oh 2006; Liu et al. 2006). Molecular and morphological studies also suggest rather different relationships within Illicium (Hao et al. 2000; Oh et al. 2003). Within Kadsura s. str., the distinctive K. coccinea, in which the inner perianth members are much swollen, is sister to the rest of the genus (Luo et al. 2017b).
Classification. There is the option in A.P.G. II (2003) of placing the two parts of this quite well characterised clade in separate families, but combination seems in order (A.P.G. III 2009).
TRIMENIACEAE Gibbs, nom. cons. - Trimenia Seemann - Back to Austrobaileyales
Trees or lianes; 5-O-methyl flavonols, flavones +, alkaloids?; primary stem with separate bundles; (vessels in radial multiples); rays 6-9-seriate; (secondary phloem with broad rays); (nodes 1:4); lamina margins entire or toothed; inflorescence axillary (terminal), branched; plants monoecious or flowers perfect; receptacle small; P 2-many, deciduous, not attractive, outer members in pairs [bracteoles, etc.?]; A 6-25, anthers latrorse to extrorse (± introrse), connective somewhat prolonged; pollen disulcate, pantoporate or inaperturate, monads or tetrads, endexine lamellate; G 1 (2), style 0, stigma ± penicillate; compitum necessarily 0; ovule 1/carpel, pendulous, ?type, micropyle bistomal, parietal tissue 9-30+ cells across, nucellar cap ca 6 cells across, hypostase +; megaspore mother cells at base of nucellus, embryo sac much elongated (few), reaching micropyle; seed with a circular cap; testa vascularized, almost all cell walls thick, outer layers lignified, palisade, (mesotesta not lignified), tegmen persistent; thin layer of nucellus persistent, embryo suspensor multicellular, multiseriate; n = 9, x = 7 (?6, ?8).
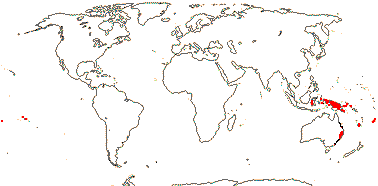
1 [list]/6. New Guinea and S.E. Australia to Fiji. Map: from van Balgooy (1975) and Philipson (1986b).
Age. Pollen of Trimeniaceae is reported from Brazil in deposits ca 100 Ma, and from West Africa and southeast Australia somewhat later in the Cretaceous (Dettmann & Jarzen 1990 and references). Distinctive trimeniaceous seeds, albeit without the vascularized testa of extant taxa, have been found in Late Albian deposits some 118 Ma in Hokkaido, Japan (Yamada et al. 2008).
Evolution: Pollination Biology & Seed Dispersal. For pollination, see Bernhardt et al. (2003) and Luo et al. (2018); stigmatic self-incompatibility occurs here, and both wind and insects may be involved in pollination (hence ambophily). In Trimenia moorei, for example, the spreading stamens with their white filaments are quite attractive.
Chemistry, Morphology, etc.. Rather remarkably, some plants of Trimenia papuana have inaperturate pollen, while others have polyporate grains (Sampson & Endress 1984; Sampson 2007); the endexine is lamellate. The nucellus sometimes elongates greatly after meiosis of the megaspore mother cells, but the origin of the cells making up the massive nucellus (Bachelier & Friedman 2011; Friedman & Bachelier 2013) and of the megaspore mother cells themselves is unclear; are the latter hypodermal when they are first evident? The embryo sac grows through the central, starch-rich cells of the nucellus. Friedman and Bachelier (2013: much useful information) observed a thin, persistent layer of nucellar tissue surrounding the endosperm in the seed, but it did not contain food reserves.
See also Money et al. (1950: general), Endress and Sampson (1983: floral development) and Philipson (1986b, 1993) for more information.