EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
[DILLENIALES [SAXIFRAGALES + ROSIDS]]: stipules + [usually apparently inserted on the stem].
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID I / FABIDAE / [ZYGOPHYLLALES [the COM clade + the N-fixing clade]]: endosperm scanty.
[the COM clade + the N-fixing clade]: ?
[FABALES [ROSALES [CUCURBITALES + FAGALES]]] / the N-fixing clade / fabids: (N-fixing by associated root-dwelling bacteria); tension wood +; seed exotestal.
[ROSALES [CUCURBITALES + FAGALES]]: (actinomycete Frankia infection +); styles separate; ovules 1-2/carpel, apical.
[CUCURBITALES + FAGALES]: P parts similar; ovary inferior; fruit 1-seeded, indehiscent.
Plant ectomycorrhizal; (flavonols), dihydroflavonols, ellagic acid +; vessel elements also with scalariform perforation plates, aggregate rays + (0); sieve tubes with non-dispersive P-protein bodies; (cork cambium outer cortical); buds perulate; lamina margins toothed, secondary veins proceeding straight to teeth [venation craspedodromous], teeth not glandular, with higher-order veins convergent on them [= urticoid]; plants monoecious, inflorescence with compact cymose clusters of flowers, flowers very small, (± monosymmetric by reduction), sessile; P +, uniseriate; nectary 0; staminate flowers: inflorescence a catkin; A arrangement?; pollen oblate, thickened sexine arches going from pore to pore, visible as bands [arci], cavity between inner and outer pores [vestibulum], tectum ± spinulate, infratectum granular; carpelate flowers: style undivided, ± 0, stigma ± decurrent, linear, dry; ovule 2/carpel, pendulous, epitropous, unitegmic, suprachalazal tissue massive; megaspore mother cells several; ovules poorly developed at pollination, fertilization delayed; fruit ± a nut; testa vascularized, not mechanical, exotesta often enlarged and persisting; cotyledons large. - 7 families, 33 genera, 1,175 species.
Includes Betulaceae, Casuarinaceae, Fagaceae, Juglandaceae, Myricaceae, Nothofagaceae, Ticodendraceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Ages for crown-group Fagales suggested by Cook and Crisp (2005) are 95-75 Ma and by X.-G. Xiang et al. (2014a) rather older, (107.3-)105.2(-101.3) Ma, while Sauquet et al. (2011) give a range of dates from (124.8-)120.2-67.3(-48.9) Ma and Xing et al. (2014) suggest ages of 125-100(-91.8) Ma; at around 92 Ma the dates in Larson-Johnson (2015) fit with most of these. Ages of (106.5-)104.9, 104.1(-99.9) Ma in H.-L. Li et al. (2015) show little spread, while the youngest dates, at around 68.6-62.2 Ma, are those of Xue et al. (2012), and of ca 61 Ma, that of Quirk et al. (2012). Other dates: ca 82.3 Ma (Tank et al. 2015: Table S2). See also dates in Tedersoo and Brundrett (2017).
For fossils in the Nothofagus area, so crown Fagales, that are up to ca 84 Ma, see Dettmann et al. (1990), Dettmann (1994) and Barreda et al. (2019); Silvestro et al. (2021) estimated that the time-of-origin of Nothofagaceae was ca 88.2 Ma (see also below).
Evolution: Divergence & Distribution. From the rich fossil record of Fagales, Friis et al. (2006a) suggested that all major fagalean lineages were present at the latest by the Cenomanian (early Late Cretaceous), ca 97 Ma, and fagalean leaf fossils of this age are reported from Queensland, Australia - at that time, Australia had barely separated from Antarctica (e.g. McLoughlin et al. 1995). Normapolles pollen is discussed below.
X.-G. Xiang et al. (2014a) discuss diversification of the order in considerable detail, emphasising the distinction between wind- (most species of Fagus were included here!) and animal-dispersed fruits, and also the closed habitats preferred by species in the former group and the open habitats preferred by species in the latter group; they also detected a number of shifts in diversification rates. Larson-Johnson (2015) integrated fossils with fruits in a study of fruit evolution and diversification patterns in the order, suggesting that clades with biotically dispersed propagules had both larger ranges and often higher diversification rates than did clades with abiotically dispersed propagules, in the former, scatter-hoarding by jays and small rodents was most commonly involved. Wind dispersal is perhaps the plesiomorphic condition in Fagales (Larson-Johnson 2015: ?outgroups), while within the order there have been around seven transitions to biotic dispersal and four to abiotic dispersal, three of the latter being reversals.
The morphologies of flowers of some Cretaceous fossils placed in or near Fagales are substantially different from those of extant members. Thus there are suggestions of nectary lobes between the stamens in the staminate and perfect flowers of Antiquacupula and the inner walls of the fruit loculi are glabrous; the flowers have a floral formula of T 3 + 3; A 12, dorsifixed; nectaries +, G [3], inferior (Sims et al. 1998; Herendeen et al. 1999). Unnamed fossils from Massachusetts have a character combination of flowers perfect, P 6, A 12, adnate to P, nectaries, inferior ovary with ovules mature at time of pollination, and single-seeded and very small fruits that are just possibly dehiscent (Taylor et al. 2012). If they belong to stem Fagales, single-seeded fruits may have evolved before wind pollination; the very small size of the seeds/fruits (<2 mm long) is consistent with the age of the fossils, ca 75 Ma in the Campanian, since angiosperm flowers then tended to be small (Taylor et al. 2012). Taylor et al. (2012) suggested that the members of the uniseriate perianth might be connate via hairs, but note that the perianth bases are very small, in this respect being rather unlike sepals in the rosids. For fossils, see especially Friis et al. (2011), while Larson-Johnson (2015) placed a number of fossils with fruits on a phylogenetic tree of the order (see the supplementary material for a critical evaluation of the fossil material, ages of fossils assigned to extant genera, data matrix, etc.). The recently described 94-90 Ma Soepadmoa from New Jersey has some features reminiscent of Nothofagus, as well as unique (for Fagales) characteristics such as tepals that become enlarged and large, strongly-toothed primary bracts. Archaefagacea has a tricarpelate gynoecium, two ovules per carpel and sometimes three-seeded fruits (Takahashi et al. 2008a); Gandolfo et al. (2018) suggest this is unlikely to be crown-group Fagales.
Wheeler et al. (2021) looked at wood anatomy of extant and fossil members of the order in the context of a phylogeny; the phylogenetic signal was not strong, and it was interesting to see that Fagus tended to differ from other Fagaceae. Where change in infratectum structure is to be placed on the tree seems unclear when treated at the family level; either one gain, an ordinal apomorphy, and one reversal (Fagaceae), or two gains (see also Doyle 2009). For the evolution of the foot layer, see Denk and Tekleva (2014); the ancestral state for Fagaceae - foot layer thick, regular - should be pegged to this node, but I do not know variation in the foot layer above Fagaceae. However, Jiang et al. (2019) discuss the evolution of pollen morphology for the whole order.
Fruits of extant taxa throughout the order are single-seeded, as is very common in wind-pollinated plants, whatever the number of ovules in the young ovary - note, however, this feature may be an apomorphy for [Fagales + Cucurbitales], if this clade exists. See Tiffney (1986a) and especially Larson-Johnson (2015) for the evolution of fruits in Fagales; optimising fruit charaters like cupule presence, with valves, can be tricky.
Schönenberger et al. (2001b) and Friis et al. (2003a) give useful tables comparing the morphology of extant and fossil members of Fagales; see also Crepet et al. (2008), Takahashi et al. (2008a) and Taylor et al. (2012: some outgroups). The roots of ECM Fagales tend to be narrow in diameter, but the same is also true of roots of Rosales, so it could be a plesiomorphic feature for Fagales (Valverde-Barrantes et al. 2017).
Ecology & Physiology. Fagales are the largest clade of extant seed-plants in which ectomycorrhizal (ECM) associations are pervasive. Interestingly, it has recently been found that coarse roots (to 10 mm in diameter) in deciduous western European taxa like Fagus sylvatica and Betula pendula and their saplings, and also saplings of Quercus robur and Populus tremula (the last Malpighiales-Salicaceae), all probably ECM plants, continue growth throughout the winter, even when soil temperatures were below 3o for extended periods (Marchand et al. 2025). Note, however, that these authors cite work by Hugo von Mohl from 1862 suggesting similar behaviour in two more species - not Fagales - that are probably AM trees; this work should be confirmed and extended. Nevertheless, although ECM Fagaless are hardly particularly speciose (e.g. Magallón & Sanderson 2001), other than Quercus, perhaps, they can dominate the forests in which they grow, and this is discussed further under individual families, especially Fagaceae (and Pinaceae, another major ECM group), and also under Carbon Sequestration, while more details of ECM - and other fungal - associations can also be found elsewhere. The order is now particularly well represented in forests in arctic to temperate and in tropical montane regions and the climatic preferences of the immediate ancestor of the order may have been for more or less warm and aseasonal temperate conditions.
ECM Agaricales are common on Fagales, and the crown group origins of the 10 or so clades involved are split almost equally between Late Cretaceous and Eocene (Ryberg & Matheny 2012). However, the relative timing of diversification of the ECM fungi and of Fagales is unclear. In Boletales, at least, ECM fungi may have evolved from brown rot fungi in the ecological context of soils low in nitrogen (N) (and high in organic material) formed by the activities of these fungi. Dates of origin of ECM associations here are also late Cretaceous and younger (Eastwood et al. 2011); there are maybe five origins of the ECM habit here, although the diverse Boletaceae are the main group on Fagales (Sato & Toju 2019). See also Brundrett (2017a), Tedersoo (2017b) and Tedersoo and Brundrett (2017) for literature, dates, etc., and for additional information, see papers in Martin (2017).
AM associations are not very common in Fagales, although they do seem to be scattered in the order (Teste et al. 2019: Table S2). It is however, interesting that they occur - maybe as dual mycorrhizal associations - in groups that are known to fix nitrogen (Rose 1980). N-fixation in Ticodendron is unknown, and although the plant forms mycorrhizal associations, whether they are AM, ECM or both is not totally clear (Corrales et al. 2018). For N fixation by Frankia, the bacterium involved in N fixation in this clade, and N fixation in general, see also elsewhere.
Pollination Biology & Seed Dispersal. Wind pollination and monoecy pervade the order, although taxa like Lithocarpus are pollinated by insects, and insect pollination may have occurred in some fossil taxa, too. Delayed fertilization, intermittent pollen tube growth (e.g. Sogo & Tobe 2005, 2006a, b, d [the last for a summary]), and chalazogamy (see e.g. Benson 1894; Nawashin 1899; Swamy 1948b and references) are also common, however, chalazogamy is not an apomorphy for the order (fertilization of Nothofagaceae is unknown, while Fagaceae, Myricaceae and some Juglandaceae are porogamous). Delayed fertilization, which also occurs in Fagaceae, is associated with the immaturity of the ovules at pollination and competition between the ovules and sometimes between the several embryo sacs that develop within the one ovule (see Sogo & Tobe 2006d). The immaturity of the ovules can be extreme, thus in Corylus avellana the ovules do not even begin to develop until after pollination (Germain 1994).
All Fagales have single-seeded fruits, as is very common in wind-pollinated plants. Animal dispersal is often by scatter-hoarding, which is notably common here compared with other angiosperm groups (Vander Wall & Beck 2012), and there is also an association of scatter-hoarding with masting behaviour; see also below. Various kinds of wind dispersal of fruits are also quite common.
Pollen and seeds here may be dessication-tolerant or -sensitive, sometimes in different species within the one genus (e.g. Dickie & Pritchard 2002; Franci et al. 2011).
Plant-Animal Interactions. Interestingly, larvae of the two basal clades in the lepidopteran Angiospermivora, Heterobathmoidea (the adults have jaws) and Eriocranoidea (belong to Glossata: most lepidoptera, with a proboscis, but not jaws), are found as leaf miners on Fagales, on Nothofagaceae and on Fagaceae and Betulaceae respectively, and also on Rosaceae (Shields 1988; Imada et al. 2011; Regier et al. 2015); stem-group Heterobathmoidea are (276.7-)257.7(-234.5) Ma (Kawahara et al. 2019). Phyllonorycter leaf-mining moths, members of the fairly basal glossatan microlepidopteran Gracillariidae-Phyllocnistinae, are also quite common here, with about half the known host records being on members of Fagales (Lopez-Vaamonde et al. 2003; see also Regier et al. 2013; Kawahara et al. 2016). Diversification of the moth clade seems to have occurred about 50.8-27.3 Ma, well after that of Fagales (see above), although the origination of the clade was some 76.3-50.3 Ma (Lopez-Vaamonde et al. 2006). Lycaenidae caterpillars are also quite commonly to be found on members of Fagales (see Fiedler 1991). Overall Fagales are very important host plants for lepidopteran larvae in the contiguous U.S.A. - Quercus is the major host, but other Fagaceae, Betulaceae and Juglandaceae are on the list (5/20 top genera: Narango et al. 2020). Interestingly, R. M. Anderson et al. (2024) found that in their study system, Q. alba in Connecticut, some 19% of caterpillars were parasitized when there were no phloem-feeding insects like coccids and membracids, on the other hand, when such phloem feeders were around, no caterpillars were parasitized. Anderson et al. (2024) suggested that this was because phloem-feeding herbivores typically induce the salicylic acid signaling pathway with its distinctive volatiles, and this can inhibit the jasmonic acid signaling pathway normally induced by leaf-chewing herbivores such as caterpillars; the result was that there was probably a reduction in the emission of the volatile organic compounds produced by the latter pathway that the parasitoids would otherwise have sensed.
The speciose aphid [Phyllaphinae + Calaphidinae] clade probably initially evolved on Fagales-Fagaceae, but perhaps Betulaceae, in the Late Cretaceous - Juglandaceae are also hosts; these insects later moved on to Ulmaceae, Poales (no heteroecy/seasonal host switching there), etc. (Y. Lee et al. 2021).
Looking at fossil evidence of arthropod feeders in the Cenozoic fossil record, Fagales seem to have more than their fair share of margin feeders (Albrecht et al. 2023).
Plant-Bacterial/Fungal Associations. Associations with N-fixing Frankia bacteria are sporadic; for the strains of Frankia associated with N-fixing Fagales, see elsewhere. For stem-group ages of N-fixing clades, Late Cretaceous, see H.-L. Li et al. (2015). See also above under Ecology & Physiology and links there.
Apart from Agaricales, Sebacinales-Sebacinaceae are other ECM fungi commonly associated with Fagales (Weiß et al. 2016). Some ECM fungi form tuberculate structures on the roots of Quercus (Smith & Pfister 2009). For additional information, see papers in Martin (2017).
Rusts on Fagales are predominantly members of Pucciniastraceae, a group also to be found on ferns (Savile 1979).
Genes & Genomes. Plant haplotypes are widely shared between taxa, sometimes apparently quite distant according to relationships suggested by the nucleome, not only in Fagaceae but also in Nothofagaceae and Juglandaceae (Simeone et al. 2016).
Y.-Y. Yang et al. (2021: plastome data) found that there was evidence of extensive conflict in relationships in Fagales, and there seems to have been chloroplast capture and ancient gene flow here.
Chemistry, Morphology, etc.. Although the perianth parts in Fagales are small, they may have three traces, and petals/inner tepals with three traces are also found in some fossil taxa. Carpel orientation and integument number are variable. Ovule structure is unclear in part because of the great development of megaspore mother cell tissue in some taxa and the relation of these mother cells to the cells of the mature embryo sac. Taylor et al. (2012) suggested that an ovule that is straight when young, becoming curved only later, might be a distinctive feature of the order. Germination is often both hypogeal and epigeal in the one family.
For chemistry, see Giannasi (1986); for cork cambium initiation, see Weiss (1890); vegetative morphology, see Hickey and Taylor (1991); flower and inflorescence morphology, see Abbe (1974); pollen, see Zavada and Dilcher (1986), Feuer (1991) and especially Friis et al. (2006a); embryology, Benson (1894) and Xing et al. (1998); and for fruit wall anatomy, see Soepadmo (1967b).
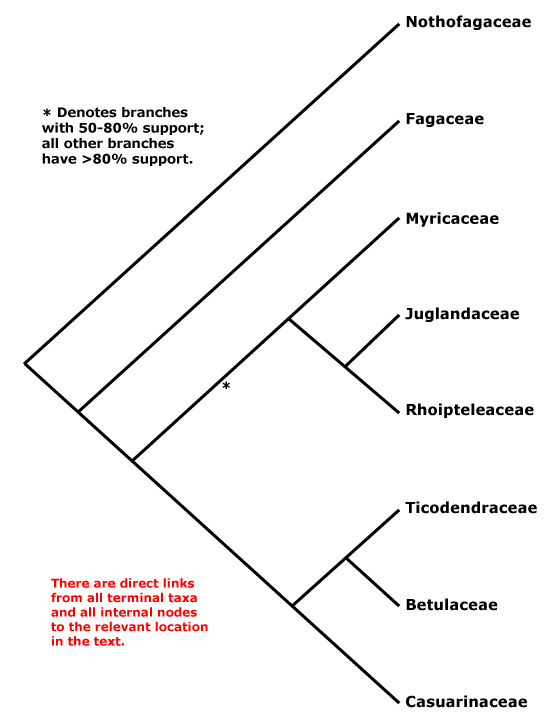
Phylogeny. Relationships within Fagales are fairly well resolved, although the position of Myricaceae remains somewhat uncertain. Manos and Steele (1997) placed Myrica as sister to [Casuarinaceae [Ticodendraceae + Betulaceae]] in a matK and combined matK + rbcL analysis, although support was weak (see also Sauquet et al. 2012: 67% bootstrap support), but sister to all Fagales except Nothofagaceae and Fagaceae in a rbcL analysis, the latter set of relationships also being found by R.-Q. Li et al. (2002: only 61% bootstrap support) using trnL-F sequence data. Cook and Crisp (2005) found the relationships [Juglandaceae [Myricaceae [Casuarinaceae + Betulaceae]]]. However, in R.-Q. Li et al. (2004: six genes, all three genomes) Myricaceae was sister to Juglandaceae, although still support was not strong, as did Herbert et al. (2006: three genes, little support for the position of Myricaceae); support was, however, good in the Bayesian analysis of Soltis et al. (2007a: sampling poor; see also Larson-Johnson 2015: support not strong; H.-L. Li et al. 2016; Karumuna et al. 2019: plastome data; Y.-Y. Yang et al. 2021: analyses of 256 species, mostly transcriptome-generated plastome data, strong support). A comprehensive five-locus chloroplast phylogeny of Fagales did not change the situation, and although there was some support for a [Juglandaceae [Myricaceae + The Rest]] clade, it was not strong (X.-G. Xiang et al. 2014a). The topology below was also recovered by Xing et al. (2014), H.-L. Li et al. (2015), M. Sun et al. (2016) and Z.-D. Chen et al. (2016), and the last two included details of infrafamilial relationships. Note thsat relationships in Vento et al. (2022: focus on Nothofagaceae) are [Fagaceae [Betulaceae + Nothofagaceae]] and ages in their tree are appropriate for this topology ([F. [B. + N.]] = ca 92 Ma, [B. + N.] = ca 86 Ma), however, in the Kew Tree of Life (as of vii.2025) using Angiosperms353 data relationships are as suggested below.
Previous Relationships. Fagales are the core of the old "Englerian" Amentiferae which have since been comprehensively demolished; within a somewhat larger group, Juliflorae, were to be found all broad-leaved angiosperms with very reduced flowers, so including Platanaceae, Lacistemaceae, Chloranthaceae, Piperaceae, etc. (e.g. Eichler 1878). Its members have since found resting places among many otherwise entirely unrelated groups within eudicots such as Malpighiales (Salicaceae), Proteales (Platanaceae), and Rosales (Ulmaceae and relatives: e.g. Qiu et al. 1998), and in or near the magnoliids (Piperaceae, Chloranthaceae respectively). They also included Eupteleaceae (Ranunculales), Myrothamnaceae (core eudicot), and Eucommiaceae (Garryales, a lamiid). In the late nineteenth and early twentieth centuries in particular, a number of botanists thought that Amentiferae were primitive, and the chalazogamy common in the order was thought to be intermediate between fertilization in some gymosperms and the porogamy of most angiosperms (e.g. Nawaschin 1895). Hamamelid taxa such as Altingiaceae and Hamamelidaceae seemed to be intermediate between Amentiferae and more conventional broad-leaved angiosperms, but they, too, are not related to Fagales (see Saxifragales here).
Fagales comprise the Faganae and two and a half more superorders in Takhtajan (1997: check).
Synonymy: Juglandineae Thorne & Reveal, Myricineae Thorne & Reveal - Betulales Martius, Carpinales Döll, Casuarinales Berchtold & J. Presl, Corylales Dumortier, Juglandales Berchtold & J. Presl, Myricales Martius, Nothofagales Doweld, Quercales Burnett, Rhoipteleales Reveal - Casuarinanae Reveal & Doweld, Faganae Takhtajan, Juglandanae Reveal
NOTHOFAGACEAE Kuprianova - Nothofagus Blume - Back to Fagales
Tree (shrub), (deciduous); flavanonols, flavanones, stilbenes [in wood]; sclereid nests?; peltate glandular hairs +; (giant/D-type stomata on veins); perulae decussate; leaves two-ranked (spiral), lamina (punctate), vernation various, (margins entire), stipules peltate/not, enclosing colleters; staminate flowers: dichasia 1-3(-5)-flowered dichasia; P connate; A 10-15(-many), basifixed, connective usu. produced; pollen 3-10 colpate, colpi short, margins raised/not [brassii type], surface short-spiny, imperforate, tectate-granular; pistillode 0; carpelate flowers: dichasia 1-3(-5)-flowered, central flower developed, 2-merous; P -≤4 small scales; (staminodes +); G [2-3], median member abaxial, stigma-style ± strap-shaped; ovule unitegmic, integument 4-7 cells across, parietal tissue 1-2 cells across, nucellar cap 0, supra-chalazal tissue massive, postament +, flat-topped; megaspore mother cells?; fertilization?; cupule +, single, 2-(3-)4-valved, lamellate, morphology various; nuts (1-)3(-7)/cupule, angled/winged [lenticular (central - 2-carpelate)/triangular (lateral - 3-carpelate)], wall with transversely elongated sclereids in middle; testa?; endosperm 0, suspensor ?0, cotyledons folded, reserve fats; n = x = 13; germination epigeal.
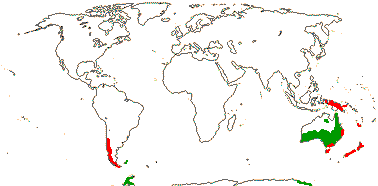
1/35: [list]. New Guinea and New Caledonia to South America. Map: from Good (1974, slightly modified); green, Cretaceous to Mid-Caenozoic fossils, also in Australia, New Zealand and South America where the genus is currently found, from Dettmann et al. (1990). [Photo - Branch.]
Age. Cook and Crisp (2005) estimated the age of crown group Nothofagaceae at only (66-)58-ca. 43(-ca 35[?-21]) Ma, i.e., decidedly after the first fossil records (see below), while Heads (2006), accepting the identity of those fossils, saw a crown-group ag]e of around 72 Ma at least. Sauquet et al. (2012) used a variety of calibrations and obtained ages ranged from (113.6-)100.3-23.5(-13.3) Ma, while (77-)76.7(-75.2) Ma is the estimate in X.-G. Xiang et al. (2014a). A crown age of ca 36 Ma was suggested by Quirk et al. (2012), while ca 65 Ma is the crown-group age in Premoli et al. (2011); Vento and Agraín (2018: Fig. 2) had suggested an age of ca 62 Ma, while the age in Vento et al. (2022: Fig. 1) is ca 81 Ma.
Fossils identified as belonging to all four subgenera of Nothofagus are known from the Late Campanian 73-71 Ma in Antarctica, the family becoming dominant, at least locally, then (Dettmann et al. 1990; Swenson et al. 2001; Cantrill & Poole 2005; Cantrill 2018) and Nothofagus-like pollen (Nothofagidites) characterized southern temperate Gondwanan areas during the later Cretaceous (Nichols & Johnson 2008). Given such dates and non-fossil estimates of ages of groups within Nothofagus, the pollen types must have evolved in parallel (Cook & Crisp 2005), or the pollen has been misinterpreted, or some of the age estimates are (very) incorrect.
Evolution: Divergence & Distribution. One does not generally think of Fagales as being a southern group, other than Nothofagaceae, prominent in this section, and maybe Casuarinaceae. However, there were preliminary reports of fossils of Fagaceae from Early Eocene deposits in Argentinian Patagonia (M.-Q. Liu & Zhou 2006; Hermsen & Gandolfo 2016). These reports have been confirmed: Ca 52 Ma fruiting fossils named as Castanopsis rothwellii, to be included in crown-group Castanopsis, have recently been described from Palaeocene deposits in Argentina, and leaves, probably of this species, were originally described as Tetracera, in Dilleniaceae (Wilf et al. 2019a). This recent description of Castanopsis has occasioned some interest. However, fossils like Castanopsis rothwellii are known from Palaeocene-Eocene deposits in Tennessee, and there they are called Castanopsoidea columbiana (Crepet & Nixon 1989). However, Denk et al. (2019) thought that the exact phylogenetic position of the Argentinian Castanopsis was unclear, futhermore, there is no pollen of "Castaneoideae" in the southern part of the world; to Denk et al. this southern route of Castanopsis - movement from North to South America, thence to Australia, and finally to (Indo-)Malesia - seemed unlikely, but c.f. Wilf et al. (2019b), etc.. Indeed, although fossils of Fagaceae are overwhelmingly from the northern hemisphere, and the area of origin of the family amy well be there, details of migration/evolution here seem to be decidedly more complex than advertised. Furthermore, fossils described as Alatonucula ignis, Juglandaceae-Engelhardioideae, have been described from Eocene deposits in Chubut, Patagonia (Hermsen & Gandolfo); fossils of Juglandaceae are widespread in the Northern Hemisphere, but these seem to be the only fossils described from the Southern Hemisphere.
Importantly, a number of other genera of vascular plants in particular that have been found in Argentinian deposits are now known mainly from forests in the Malesia/Australasia (S.E. Asia) area, suggesting perhaps that Castanopsis might indeed have moved to (Indo-)Malesia from South America via the southern route just mentioned. Other genera also known fossil from these Patagonian deposits include Agathis, Araucaria sect. Eutacta (both Araucariaceae: Diversity & Distribution; Rossetto‐Harris et al. 2020), Ceratopetalum and a number of Cunonieae (Matel et al. 2022; Fajardo-Gutiérrez et al. 2025) and other Cunoniaceae, Dobinea (Wilf et al. 2024), Eucalyptus (Gandolfo et al. 2011), Gymnostoma (e.g. Carpenter & McLoughlin 2025), Lomatia (Milner et al. 2015) and other Proteaceae, Macaranga/Mallotus (Wilf et al. 2023), Nothofagus, Osmoxylon (Wilf 2025), Papuacedrus (see Cupressaceae: Divergence & Distribution), Podocarpus (Quiroga et al. 2016; L. Chen et al. 2021) and other Podocarpaceae, Ripogonum (Carpenter et al. 2014; Wilf & Escapa 2014), etc., the fern Todea, and so on. Similarly, Proteaceaefolia araucoensis, perhaps a member of Grevilleoideae, was described from Palaeocene-Thanetian deposits perhaps ca 55 Ma from the Lota–Coronel flora from central Chile (Carpenter & McLoughlin 2025), who questioned a number of vegetative fossils from this flora supposedly of Neotropical affinities, suggesting rather that the flora had southern/Palaeotropical connections. A number of the genera mentioned in these papers have since become extinct in South America, but relatives are now to be found in Malesia/Australasia; these are the palaeo-Antarctic rainforest lineages (Wilf et al. 2019a: biome conservatism, 2023). Kooyman et al. (2014, 2019, 2022), Wilf and Kooyman (2023), Andruchow-Colombo et al. (2023) and others discuss such southern elements in the Malesian flora. Of course, genera like the podocarp Retrophyllum, also found in Patagonian deposits (Wilf et al. 2017b), still grow in both areas while a genus like Weinmannia (Cunoniaceae-Cunonieae) is predominantly American, but there are two species in the Mascarenes... (Fajardo-Gutiérrez et al. 2025).
There is another interesting issue that is connected with this migration of taxa from South America/Antarctica. Wilf and Kooyman (2023) suggest that several of these genera like Dacrydium, Dacrycarpus, Agathis, etc., now large trees in Malesian forests, have been important in large-scale reduction in global atmospheric CO2 over the last ca 15 million years. They make up important elements in the forests that grow over the mafic and ultramafic rocks of these areas, their roots ultimately facilitating combination of HCO3- with Ca++ and Mg++ ions in marine deposits, so removing C from circulation; pH values in these soils are notably low, and this has been facilitated by litter decay, etc. (Wilf & Kooyman 2023 for details).
The biogeography of Nothofagus in particular has been much discussed: Does vicariance or long-distance dispersal (LDD) best explain the current distribution of the genus? Is Nothofagus a long-time denizen of New Zealand, or has it moved there only recently? Is the current distribution of the genus a reflection of continental drift, with the massings of species seen today reflecting ancestral massings (Heads 2006)? In this case global vicariance is involved, Nothofagaceae being the southernmost representatives of Fagales that initially were distributed more or less world-wide, especially in extra-tropical areas. Or perhaps LDD has shaped the distribution of the family (e.g. Swenson et al. 2001; in particular Sauquet et al. 2011). Sanmartín and Ronquist (2004) favoured a dispersalist distribution, but they noted that the incorporation of fossil data more suggested vicariance; Vento et al. (2022) suggested that a mixture of the two was likely.
Dettmann and Jarzen (1990, see also Dettmann et al. 1990) suggested that Nothofagus had evolved by the early Campanian (ca 80 Ma) in the high southern latitudes - it is a founding member of the group of paleo-Antarctic rainforest lineages just mentioned; the age in Vento et al. (2022: Fig. 1) is similar, ca 81 Ma. The late Cenomanian/Early Turonian (ca 93.5 Ma) southernmost pollen province is characterized by Nothofagidites pollen (e.g. Kedves 1989; Friis et al. 2011). Although Podocarpaceae were common in Eocene Patagonia, at the end of the Eocene to early Oligocene Nothofagus came to dominate in these southernmost areas (Pujana & Ruiz 2017; see Pujana et al. 2020 for a summary of the fossil record of the genus in South America). Nothofagoxylon is the predominant angiosperm wood in Antarctica by the mid-Campanian ca 78 Ma, and in the Palaeocene and Eocene it further increased in proportional abundance, although podocarp-type conifers may have been replacing non-Nothofagus angiosperms and Nothofagus may not have been very common in Patagonia then (Cantrill & Poole 2005, see also 2012 for fossils of the genus on Antarctica). As Cantrill and Poole (2005, 2012) emphasized, different groups of Nothofagus were involved over time - first section Nothofagus predominated, then sect. Lophozonia, while sect. Fuscospora also became quite common in the Eocene and later (see also Kooyman et al. 2014). The ancestral niche for the genus may be mesothermal - or rather cooler, depending on how niches are reconstructed (Hinojosa et al. 2016). Nothofagus is the commonest angiosperm fossil in Antarctica (de la Estrella et al. 2019b: Podocarpaceae and Araucariaceae are the commonest gymnosperm fossils). Antarctica is also of central importance in the evolutionary scenario suggested by Vento et al. (2022); they thought that the Antarctic peninsula was a likely place of origin of the genus. movement subsequently being affected by climatic cooling, the opening of Drake's passage, etc.. Even as the climate in Antarctica deteriorated, N. fusca-type plants in particular persisted until the Pliocene ca 3 Ma if only in tundra-type vegetation, although exactly when woody vegetation finally disappeared is unclear (Cantrill & Poole 2012 for detailed discussion; Rees-Owen et al. 2018).
Three of the four pollen types commonly recognized in Nothofagus are found in the same locality in Tasmania in Oligocene deposits and the fourth was found nearby (Hill 2001 and references). Dettmann and Jarzen (1990) and others had recognized three pollen types, fusca, menziesii, brassi, the latter known only from subsection Bipartitae (= subgenus Brassospora) sspecies of which grow in east New Guinea and New Caledonia. However, it was even then evident that the fusca type could be divided, hence the four current fossil groups which are called by the names of the subgenera they characterize: brassii, = subgenus Brassospora; fusca a, = subgenus Fuscospora; fusca b, = subgenus Nothofagus, menziesii = sugenus Lophozonia (e.g. Dettmann et al. 1990; Hill 2001). Since the distribution of extant Nothofagus spans latitudes from almost 0-50+o S, and the subgenera have latitudinal preferences, either the climatic preferences of the clades have changed, or there was a very distinctive climate in Tasmania in the Oligocene, or... (Hill & Brodribb 2006). Perhaps this is a late example of the general intermingling of taxa with now very different climatic preferences similar to that which occurred earlier in the equable early Caenozoic (Palaeocene-Eocene) climates. Similarly, Nothofagus in Miocene Patagonian South America was represented by three pollen types, subgenus Brassospora not being known from South America (Palazzesi & Barreda 2007; Pujana et al. 2020), and all four subgenera are known from Antarctica in the later Cretaceous (see above). Note, however, that this conflicts with the subgeneric relationships suggested by Vento et al. (2022), i.e. [Lophozonia [Fuscocarpa [Brassospora + Nothofagus]]], with the last two subgenera diverging ca 58 Ma (ibid., Fig. 1), i.e. in the late Paleocene.
The general pattern of diversification in the genus is the "broom and handle" type (Cook & Crisp 2005); either persistence of a not very speciose lineage for some time, or diversification followed by near-extinction and then diversification again. Fossil evidence points strongly towards a southern distribution in the Cretaceous. There has been extensive discussion about the occurrence of Nothofagus on New Zealand. Knapp et al. (2005) and Cook and Crisp (2005) suggested that Nothofagus reached New Zealand, at least, by LDD only ca 30 Ma, and Brassospora-type plants have since become extinct there (see also Pole 2003; Waters & Craw 2006; Wallis & Trewick 2009); McCulloch and Waters (2019) discuss how to interpret the ages of fossils found in places like New Zealand with controversial geological histories. Kooyman et al. (2014) noted that the Neogene recolonization of New Zealand resulted in rainforests different from those in Australia, etc., while Y. Sun et al. (2014) argued strongly for the continued presence of an emergent New Zealand landmass, their focus group being the liverwort Schistochilaceae, common in Nothofagus forests. Although de Queiroz (2014 and references) thought that New Zealand had been submerged perhaps 25-23 Ma, Wallis and Jorge (2018; see also He et al. 2016) argue strongly for the persistence of an emergent New Zealand (see also Oliveros et al. 2019 and references: birds); c.f. however McCarthy et al. (2020). As to Nothofagus on New Caledonia, there are similar arguments (e.g. Cruaud et al. 2012c; Nattier et al. 2017; Giribet & Baker 2019; Heads 2023). Further complicating the issue, there are also reports, albeit questionable, of Caenozoic Nothofagus pollen from the Gulf Coast of the USA as well as in the Pacific North West (Elsik 1974).
Bouchenak-Khelladi et al. (2015) suggested that there has been a slow-down in diversification rate in Nothofagaceae.
For characters and clades in Nothofagus s.l., see Heenan and Smissen (2013). Fernández et al. (2016) discuss the ancestral pollen morphology of Nothofagus.
Ecology & Physiology. As will have become clear, Nothofagaceae have played a major role in more temperate southern hemisphere forests from the later Cretaceous onwards. Extant Nothofagus forms forests with bamboo, the latter making up the understory, in both South America and Papua (Kappelle 2006b: see also Fagaceae). In Chile, ignoring the emergent Nothofagus dombeyi, the other trees in forests both with and without the plant had similar total carbon sequestration levels, that of N. dombeyi, when present, essentially being an add-on and almost doubling the total (Parada et al. 2018); see also Dipterocarpaceae.
The loranth Desmaria mutabilis parasitizes Nothofagus alone, Tristerix corymbosus a considerable variety of trees, but not Nothofagus - however, the latter can be a hyperparasite on D. mutabilis... (Fontúrbel et al. 2022). Loranthaceae parasitize the genus throughout its range, except in Australia-Tasmania; in South America Misodendrum (Misodendraceae) is also to be found on Nothofagus, and it is restricted to that genus.
Pollination Biology & Seed Dispersal. Poole (1952) did not observe actual fertilization, but noted thst 9-10 weeks elapsed between pollination and fertilization.
Plant-Animal Interactions. The ten species of Heterobathmia (Heterobathmiidae), a jawed basal moth, make their homes exclusively on Nothofagus, both as adults - they eat pollen, and as caterpillars - they are leaf miners (see also Futuyma & Mitter 1996). Heterobathmiidae may be sister to Agathiphagidae (food plant Agathis), the two diverging perhaps ca 158.5 Ma (Wahlberg et al. 2013; see also Heikkilä et al. 2015), although the latter are more probably one clade down the lepidopteran tree and sister to Micropterigidae (Regier et al. 2015; Kristiansen et al. 2015). Ages of around 125 Ma for Heterobathmidae have also been suggested. Mitter et al. (2016) suggest that Heterobathmia is sister to all other Angiospermivora, in this case they would be three branches up the lepidopteran tree.
The cynipid clade Paraulacini are all parasitoids or inquilines in chalcidoid (both groups are wasps) galls on Nothofagus in southern Chile; diversification may have begun here ca 25 Ma, although Paraulacini are sister to all other Cynipoidea, and the clade is around 190 Ma (Blaimer et al. 2020).
Humphries et al. (1986) suggested the possibility of some co-evolution of Nothofagus with Eriococcus scale insects that grow on it.
Hille Ris Lambers (1979) drew attention to similarities between aphids like Neuquenaphis living on Chilean Nothofagus with those from elsewhere in the southern hemisphere - and also known from Baltic Amber.
Plant-Bacterial/Fungal Associations. Humphries et al. (1986: c.f. more recent ideas of relationships) discuss the parasites and associates of Nothofagus. The inaperturate discomycete Cyttaria grows on Nothofagus in both the Antipodes and South America, but neither in New Guinea nor in New Caledonia. Only subgenera Fuscospora and Lophozonia, basal in the genus, are affected, and the pattern of association of host and parasite is not simple; the split between Australian and New Zealand species of Cyttaria is estimated at ca 44.6-28.5 Ma (K. Peterson et al. 2010), overlapping with Crisp and Cook's estimates above. Van Galen et al. (2023) found that different species of Nothofagus in New Zealand had preferences for different species of fungi, particularly evident in species of Nothofagus from different subgenera, with the phylogenies of the host and symbiont showing similarities, and there may also be specialisation of fungi on different hosts - correlated evolution.
Tedersoo et al. (2008) examined ECM fungi on Tasmanian Nothofagus), and other than basidiomycetes, Tuberaceae and associated clades (ascomycetes) are also found on the genus (Bonito et al. 2013) - and of course truffles are found on Fagaceae (Fagus, Quercus) and Betulaceae, as well as other unrelated angiosperms and gymnosperms. ECM fungi found on Nothofagaceae and Myrtaceae, both southern groups, are similar, perhaps because the fungi moved from the former onto the latter as the climate became drier (Tedersoo et al. 2008, 2014a). For more on ectomycorrhizal associations, see elsewhere.
The rust parasites of Nothofagaceae are rather different from those of other Fagales (Savile 1979).
Chemistry, Morphology, etc.. Nothofagus obliqua may have unilacunar nodes; does it have stipules?
Unlike Fagaceae, there is no obvious relationship between the number of fruits and the number of valves of the cupule. The central flower of the cupule often has two carpels, lateral flowers have three. Staminate flowers that apparently have many stamens are interpreted as being the result of the fusion of separate flowers of a dichasium (see also Betulaceae). Embryology is poorly known; Poole (1952) suggested that it is the chalazal megaspore that develops, and the cotyledons are at the micropylar end of the seed and the radicle at the antipodal end...
Further information (sometimes under Fagaceae) may be found in Hill and Jordan (1993), Kubitzki (1993b) and Steed-Mundin (2024, and the other articles in Curtis's Bot. Mag. 41(4), species of subgenus Brassospora not included), all general, also Hegnauer (1989: chemistry), Lersten and Horner (2008b: leaf crystals, etc., Fagaceae are similar), Philipson and Philipson (1979: leaf vernation), Langdon (1947) and Poole (1950), inflorescence and cupule, Rozefelds (1998) and Rozefelds and Drinnan (1998), stamens and staminate flowers, and Hanks and Fairbrother (1976). Praglowski (1982) Crepet and Daghlian (1980) and Fernández et al. (2016), all pollen. There is also a website, Nothofagus, les hêtres du bout du monde..., nothofagus.free.fr, that will be of interest to francophone visitors.
Phylogeny. Manos (1997), Knapp et al. (2005 and references) and Heenan and Smissen (2013) discuss relationships within Nothofagus, which are subgenera [Lophozonia [Fuscospora [Brassospora + Nothofagus]]]; see also X.-G. Xiang et al. (2014a). For relationships suggested by plastomes, rather different, see Premoli et al. (2012); there is a strong geographical signal. The phylogenetic study in Vento et al. (2022) is based on 10 characters from leaf morphology that included a number of fossils, including from the Antarctic Peninsula, and 36 other characters taken from extant taxa; there is also a rich fossil record of Nothofaguds pollen.
Classification. For a now dated checklist and bibliography, see Govaerts and Frodin (1998: in Fagaceae). Four subgenera were recognized by Hill and Read (1991), their classification replacing i.a. that of Philipson and Philipson (1988). However, Heenan and Smissen (2013) then replaced these subgenera with genera - taxon limits were not changed as Hill et al. (2015) noted as they argued for a nomenclatural status quo, and this is the genus/subgenus classification that is currently used.
Previous Pelationships. Nothofagus has often been included in Fagaceae, as by Cronquist (1981) and Kubitzki (1993b).
[Fagaceae [[Myricaceae + Juglandaceae] [Casuarinaceae [Ticodendraceae + Betulaceae]]]: bud scales spiral; leaves spiral; anthers dorsifixed; endocarp parenchymatous.
Age. Estimates of the age of this node are (65-)61(-57) Ma (Wikström et al. (2001), 62.7 Ma (Tank et al. 2015: Table S1, S2), and (93-)90, 88(-84) Ma (H. Wang et al. 2009: Bayesian relaxed clock estimates up to 100 Ma). Magallón and Castillo (2009) estimated an age of ca 93.5 Ma, Bell et al. (2010) ages of (68-)55, 55(-40) Ma, and X.-G. Xiang et al. (2014a) an age of (101.2-)99.3(-95.5) Ma. Naumann et al. (2013) offered an age of ca 52.8 Ma but the age of around 110.3 Ma in Sauquet et al. (2011) is almost double this, while (99.3-)97.3(-87.3) Ma is the age in X.-Y. Yang et al. (2018), ca 87.0 Ma in H. Zhou et al. (2021) and ca 98.4 Ma in T.-R. Wang et al. (2024).
Evolution: Plant-Animal Interactions. Some larvae of Eriocranidae, a small group of near-basal lepidoptera, are found as leaf miners on this clade (Powell et al. 1998).
Chemistry, Morphology, etc.. For the parenchymatous endocarp, see Manchester (2011), ?Nothofagaceae.
FAGACEAE Dumortier, nom. cons. - Back to Fagales
(Periderm very long-lived); sclereid nests in bark, cells with large rhomboidal crystals; airs often stellate/branched; veins often associated with crystals only; lamina vernation conduplicate-plicate; inflorescence branched or not; flowers often trimerous; P biseriate [thus = T] or not, 4-6(-9), (connate); staminate flowers: bract; A (4-)12(-20) (connective produced); pollen tectate-columellate, not spiny; pistillode +; carpelate flowers: cupules dichasial, subtended by valvate structure; G [3], alternating with P, or median member abaxial; ovules bitegmic (unitegmic), funicle evident, central strand in supra-chalazal tissue; embryo sac with chalazal caecum; cupule +, with valves [one more than fruit number], ± spiny; nut trigonous, wall with lignified tissue to the outside, endocarp hairy inside; seeds pachychalazal; (endosperm cellular), 0; n = 12 (13, 21), x = 12, nuclear genome [1 C] (0.282-)0.979(-3.392) pg.
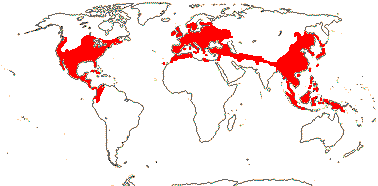
8 [list]/1,047: 2 subfamilies below. North Temperate to N. South America, North Africa and Malesia. Map: from Soepadmo (1972), Fl. N. Am. vol. 3. (1997) and M. V. Castro Campos in Nixon (2006).
Age. Estimates for the crown age of the family are 37-34 Ma (Wikström et al. 2001), 77-67 Ma (Cook & Crisp 2005), (45-)31, 28(-16) Ma (Bell et al. 2010), or (103.6-)84.7, 82.3(-64.2) Ma (Sauquet et al. 2012); see Grímsson et al. (2016: Table 2) for other dates. B.-F. Zhou et al. (2022) suggested an age of (82.0-)81.6(-81.1) Ma and T.-R. Wang et al. (2024) an age of ca 82.6 Ma.
The oldest Fagaceae fossils are some 90 Ma old (Crepet et al. 2004 for references), while Grímsson et al. (2016) discuss fossils from Wyoming assignable to stem Quercoideae and Fagoideae, as well as to an extinct clade of Fagaceae, in deposits that are dated to 81-80 Ma.
1. Fagoideae K. Koch - Fagus L.
Plant deciduous; ellagic acid 0; stomata also cyclocytic; leaves two-ranked; staminate flowers: male inflorescence capitate, many-flowered; pollen subprolate-spheroidal, tectum with ± flat-lying and fused rod-like elements; pistillode 0; carpelate flowers: female dichasia 2-flowered, lacking central flower; staminodes 0; styles long, recurved, stigma decurrent; ovule ?straight, micropyle bistomal, elongate, inner integument thinner than outer, parietal tissue 5< layers across, ?nucellar cap ca 13 cells across; pollen tube at ovule at dormancy; nuts 2/cupule; cotyledons folded, reserve fats; genome size [1C] 0.56-0.63 pg; germination epigeal.
1/10. Temperate N. hemisphere. [Photo - Fruiting Branch, © M. Brand.]
Age. Estimates of the crown-group age of Fagoideae are (24-)16.2, 15.7(-8.4) Ma (Xing et al. 2014), (24.2-)17.2(-7.9) Ma (X.-G. Xiang et al. 2014a), (62-)53(-43) Ma (Renner et al. 2016)t, 51-39 Ma (Grímsson et al. 2016), ca 50 Ma (L. Jiang et al. 2020) and ca 32.7 Ma (S. Jiang et al. 2021).
2. Quercoideae Örsted —— Synonymy: Castaneaceae Brenner, Quercaceae Martinov
(Plant deciduous); leaves (two ranked/whorled), (margins entire/lobed/biserrate); inflorescence spike or catkin, staminate and carpelate flowers on separate inflorescences or not; staminate flowers: sessile; pollen prolate, exine (micro)verrucate/scabrate, ± striate; pistillode +, (secreting nectar); carpelate flowers: (flowers single, lateral flowers 0); staminodes +; G (-9 - Castanea), (style relatively long, occupying most of the gynoecium), stigmas capitate(-decurrent)/punctiform and with a terminal pore; ovules (to 18/flower - Castanea), integuments the same length [micropyle endostomal?], parietal tissue one cell across, nucellar cap ca 2 cells across, (tracheids +); pollen (tubes branched), in style at dormancy; fruits taking over a year to develop [?here], subtended by valveless cupule [esp. Quercus, Notholithocarpus, perhaps Lithocarpus, ?Chrysolepis], with scales/lamellae, (largely enclosed by woody receptacle); nuts 1-3(-15[Trigonobalanus - T.])/cupule, (rounded); (endosperm with basi-lateral caecum), cotyledons (connate), reserves starchy; genome size [1C] 0.5-1.3 pg; rpl22 gene transferred from chloroplast to nucleus; germination hypogeal (epigeal - T.).
7/857: Quercus (627, + ca 180 named hybrids...), Lithocarpus (250), Castanopsis (150). N. temperate, at higher elevations in the tropics, to N.W. South America (Quercus humboldtii, Trigonobalanus excelsa in Colombia) and Malesia, Lithocarpus alone in N.E. Australia. [Photo - Fruit]
Quercus L.
Subgenus Quercus: pollen rugulae obscured by sporopollenin.
Section Lobatae Loudon, the red oaks - 4 subsections, ca 120 spp., North America, especially the east, Mexico, Central America, N.W. South America (Q. humboldtii). Plant (sclerophyllous, shrubby), deciduous (subevergreen); styles long, perigon at base.
[Sects [Protobalanus [Ponticae [Virentes + Quercus]]]]: ?
Section Protobalanus (Trelease) O. Schwarz, the golden-cup oaks - 5 spp., California and Mexico. Plant evergreen; stamens apiculate; pollen verrucae small; abortive ovules usu. lateral.
[Sects [Ponticae [Virentes + Quercus]]]: abortive ovules basal; fruit maturation within a year; endocarp glabrous.
Section Ponticae Stefanovic - 2 spp., Western North America, the Caucasus. Plant shrubby, rhizomatous; (leaves deciduous); leaf margins serrate, stipules large.
[Sects [Virentes + Quercus]]: ?
Section Virentes Loudon - 7 spp., Southern live oaks. The Gulf of Mexico, Cuba, Baja California. Abaxial lamina with fused stellate trichomes; (leaves ± deciduous); seeds with fused cotyledons; seedlings with cotyledonary tube.
Section Quercus - ca 150 spp., white oaks. North Temperate, ca 30 spp. Eurasia. Plant (rhizomatous), (sclerophyllous, shrubby), deciduous; lamina without bristles or spines tipping teeth.
Subgenus Cerris Oersted: pollen rugulae visible.
Section Cyclobalanopsis (Oersted) Bentham & J. D. Hooker - ca 90 spp., cycle-cup oaks. Eastern Himalayas to Japan. Acorns often densely clustered, cupules with rings of concrescent scales.
[Sects Ilex + Cerris]: lamina with bristles at ends of lobes/teeth.
Section Cerris Dumortier - ca 13 spp. Western Eurasia. Acorns glabrous inside.
Section Ilex Loudon - ca 40 spp. Eurasia, North Africa.
Age. Estimates of the crown-group age of Quercoideae are around (58.1-)48.7(-43.8) Ma (Sauquet et al. 2012), (66.1-)56.4(-50.6) Ma (X.-G. Xiang et al. 2014a), (72.6-)69.8(-66.9 Ma (B.-F. Zhou et al. 2022: Trig. + rest) and ca 63.0 Ma (T.-R. Wang et al. 2024); see Grímsson et al. (2016) for more suggestions.
Pollen ca 56 Ma and identified as Quercus is known from Austria (Hipp et al. 2020). Rather unexpectedly, fossils of Castanopsis, ca 52 Ma and to be placed in the crown group of the genus, are known from Argentina (Wilf et al. 2019). Such ages make some of those in the paragraph above somewhat problematical.
The Hypogeous Seed clade, everything except Trigonobalanus, has been dated to (67.0-)64.5(-62.1) Ma (B.-F. Zhou et al. 2022)
Evolution: Divergence & Distribution. There are two main issues here. One concerns the possible origin of some Fagaceae in the general Argentinian area of South America and the subsequent movement of the plants involved to the Australian-Malesian area; for further details, see Nothofagaceae.
The other issue concerns the history of Fagaceae in the Northern Hemisphere. For more ages, see Xing et al. (2014), X.-G. Xiang et al. (2014a) and M. Deng et al. (2017: Quercus). The later Cretaceous Protofagacea, with 6 tepals and 12 stamens (Herendeen et al. 1995) and early Eocene Fagus scofieldii (Mindell et al. 2009) have fruits with a hairy endocarp, like most extant Fagaceae. For Cretaceous fossil leaves perhaps to be assigned to Fagaceae, see Gnilovskaja and Golovneva (2016). Grímsson et al. (2016) provide images of pollen and leaves and an evaluation of the fossil record. Indeed, the diversity of the family in the earlier Caenozoic was considerable with numerous records from throughout the northern hemisphere (e.g. Manchester & Crane 1983: fruits very small, ?wind dispersed; Herendeen et al. 1995; Manchester & Dillhoff 2004: Fagus 50 Ma from British Columbia; Mindell et al. 2007; Kvacek 2008; Denk et al. 2012; Bouchal et al. 2014; Grímsson et al. 2015a: Eocene of W. Greenland). The detailed account by Sadowski et al. (2020) of the numerous Fagaceae known from the Late Eocene Baltic amber 38-34 Ma is noteworthy; Fagaceae are the most diverse family found in that flora, and they include 11 named species of Quercus, perhaps two species of Castanea, one species of Trigonobalanus, and three of Eotrigonobalanus, all very largely based on remains of staminate catkins (Sadowski et al. 2020).
Jiang et al. (2020) suggested that Fagus could have originated in the North Pacific region in late early Eocene, but, as noted elsewhere, past distributions may differ from those of today... Thus fossils of Lithocarpus, now basically South East Asian-Malesian, are widespread in the northern hemisphere. Fossils identified as the New World Quercus sections Lobatae and Protobalanus and also Trigonobalanus (now known from South America, east Asia and W. Malesia) have been recorded from the Late Eocene Baltic amber (Sadowski et al. 2020). Understanding the evolution of tropical forests dominated by Fagaceae in Thailand is not straightforward. They seem to date back to the Late Oligocene, variation in the composition of the forests then not reflecting replacement of North Temperate-type forests, but simply different ecological settings that were inhabited by a diversity of forest types (Malaikanok et al. 2023).
Overall, members of Fagaceae are major components of mesic temperate and subtropical forests, especially in the Northern Hemisphere. Predominantly wind pollinated, Castanea in particular is one of the most important entomophilous genera in such forests (Petit & Larue 2022), fruits of the family are a major source of food for vertebrates, not to mention the caterpillars that eat the leaves, galling insects, and their like. For more information on the evolution and biogeographic relationships of Fagaceae, see e.g. Manos and Stanford (2001), Manos et al. (2002) and Grímsson et al. (2016) and for those of Fagus in particular see Denk and Grimm (2009a). Larson-Johnson (2015: fig. 10) discuss increases in diversification rates in the family, while Bouchenak-Khelladi et al. (2015) suggested that possible factors triggering similar increases within Quercoideae include moving into areas with Mediterranean-type climates.
Renner et al. (2016) discussed diversification within Fagus where, as in Quercus, there is a major phylogenetic split that is correlated with geography, if complicated by fossil distributions. Speciation here has been slow (or there has been much extinction), for instance, F. grandifolia is the only extant member of a ca 45 Ma lineage.
Oh and Manos (2006, 2008) thought that the scaly or spiny cup-shaped cupule that encloses a single, rounded fruit, the acorn, has evolved more than once within Quercoideae, while Cannon and Manos (2001) looked at the evolution of the fruits of a few species of Malesian Lithocarpus in some species of which a woody receptacle (sic) almost entirely surrounds the fruit (enclosed receptacle(ER)-type fruits - somewhat like a cactus fig), rather than being a circular structure at the base, as in acorn-type fruits (AC). X. Chen et al. (2023) noted that the latter were surrounded by a thin pericarp (sic; the fruit is of course derived from an inferior ovary), while the former are latgely surrounded by a massive receptacle, the pericarp being on top, and they may also be almost entirely surrounded by the cupule, although variation here was not discussed. Scatter-hoarding rodents may disperse the AC fruits, but nothing seems to be known about dispersers or pre-dispersal predators of ER-type fruits; the variation these authors observed was disscussed in the context of a chloroplast phylogeny of the genus and also the climatic variation encountered by the species (Chen et al. 2023). (Note that the fossil Cascadeicarpa has a "doubly inferior ovary" similar to that of ER-type fruits - Mindell et al. 2007: G [2].) X. Chen et al. (2012) found that ER fruits tended to have more structural carbohydrates than AC-type fruits, which had more indigestible (to a small mammal) fibres, although the sample size was small and how such differences actually worked out in the field was unclear. Later Chen et al. (2020) suggested that the differences between these AC- and ER-type fruits in Lithocarpus might be the result of heterochrony, the receptacle and the wall of the inferior ovary developing at different rates in the two fruit types. In any event, the conflict between nuclear and plastome relationships has to be sorted out before the evolution of these fascinating fruits can be understood.
Quercus. Axelrod (1983; see also Bouchal et al. 2014) discussed the evolution and the distributional history of Quercus, and this has been the focus of much recent work; Cavender-Bares (2018) provides an extensive discussion of the ecology and evolution of American oaks which is of considerable general interest. Indeed, a great deal of work on Quercus has been carried out in the last decade or so (e.g. see New Phytol. 226(4). 2020). Fossil acorns of Quercus in particular are known from ca 44 Ma deposits in Oregon (Manchester 1994), but there are probably older fossils (fossil pollen to ca 56 Ma) that can be placed here - see in particular Hofmann et al. (2011b), Barrón et al (2017), also Mensing (2014: California oaks).
The basic diversification patterns in Quercus seem to be first, associated with geography, and second, associated with ecology (Hipp et al. 2019). Quercus is made up of an Old World/mid-latitude and a New World/high latitude clade, with divergence between the two beginning in the later Eocene (Hubert et al. 2014; M. Deng et al. 2017: crown-group age (39.2-)35.9(-34.1) Ma). By the end of the Eocene Quercus with sclerophyllous foliage had evolved in western North America, where conditions were more or less seasonal, and it grew then in vegetation similar to modern chaparral and nemoral forest; ecologically similar Quercus evolved somewhat later and independently in Europe where Mediterranean conditions developed only in the Pleistocene (Bouchal et al. 2014). Picking up on the geography/ecology theme just mentioned, Skelton et al. (2021: p. 7) noted that "Habitat filtering combined with trait conservatism suggest that closely related Quercus species occupy similar climatic niches in western North America", closely related species showing similar vulnerability to embolism, for example, and this was linked to the habit of the plant. However, in the east ecological convergence was more common (see also Cavender-Bares et al. 2004). The greatest diversity of Quercus in the New World is in southern Mexico, Mexico as a whole having around 154 species; within subgenus Quercus, clades of white (section Quercus) and red (section Lobatae) oaks have diversified rapidly there within the last 23 Ma, indeed, their rates of diversification are comparable with the highest estimates in any other plants (Hipp et al. 2017, 2020; for white oaks, see also Fitz-Gibbon et al. 2017; McVay et al. 2017b) - and note that species numbers in both Mexican and Central American oaks may be underestimates (Hipp et al. 2019). Rodríguez-Correa et al. (2015) studied niche divergence in species from Mexico and Central America, suggesting that it was affected by various barriers to dispersal. In a comprehensive study of New World taxa, Hipp et al. (2017, see also 2020) linked speciation to parallel sympatric diversification of the two major North American sections, the red and white oaks, both responding to moisture gradients, etc., and resulting i.a. in the concentration of species in Mexico and Central America: Red and white oaks are commonly sympatric, but allopatry is common at the level of closely-related species. Fallon and Cavender-Bares (2018) looked at species growing at different elevations in the Sky Islands of S.E. Arizona, finding that species growing at lower elevations responded to drought by dropping their leaves, but species at higher elevations were more drought-tolerant, recovering from daily dessication because their leaves stored more water - and such niche partitioning occured between species of the one section (for parallel evolution of leaf form, see Tucker 1974). There have been 23 or more origins of the deciduous habit, "niche" convergence par excellence, and both red and white oaks have deciduous species with lobed leaves in small basal clades (Hauser et al. 2017; McVay et al. 2017b). In the Old World, species of Quercus subg. Cerris sect. Cyclobalanopsis in particular are common dominants of drier sites in warm temperate to tropical lower montane forests in East Asia (some 60 species in southwest China and northern Indo-China - M. Deng et al. 2017) and in West Malesia (Ashton 2014). Divergence here began around 26.3 Ma, being shaped by the tectonic/climatic changes in Southeastern Asia (Deng et al. 2017). Simeone et al. (2018) discuss the diversification of oaks of section Cerris in western Eurasia. Section Ponticae (subgenus Quercus) includes only two species, Q. sadleriana, from west North America, and Q. pontica, from N.E. Turkey-Georgia. Overall, there are four main increases in diversification rates: The Eurasian white oaks of the Roburoid clade of section Quercus, the sympatric diversification of red and white oaks in North America, and the Eurasian section Cyclobalanopsis (Hipp et al. 2019).
However, there is no simple story about oak diversification. Kremer and Hipp (2019: p. 987) emphasize four main themes when trying to understand the evolutionary success of oaks: "accumulation of large reservoirs of diversity within populations and species; ability for rapid migration contributing to ecological priority effects on lineage diversification; high rates of evolutionary divergence within clades combined with convergent solutions to ecological problems across clades; and propensity for hybridization, contributing to adaptive introgression and facilitating migration." As they note, these themes play out if different ways in different parts of the range of the genus. Thus the rate of post-glacial migration of Quercus in Europe has been 400-500(-1000) m/year, in addition to longer distance dispersal events; this is much faster that in at least parts of North America and it leads to genetic diversity being conserved (Kremer & Hipp 2019). Simeone et al. (2018) note that in species of section Cerris from western Eurasia there has been/is currently extensive hybridization, especially evident in the chloroplast genome, including in this case with species from section Ilex, and the latter group has chloroplasts from five different sources (also Simeone et al. 2016; for hybridization elsewhere in the genus, see e.g. McVay et al. 2017a). There has been some ancient hybridization between Californian tree and scrub oaks (B. Y. Kim et al. 2018) and between oaks in eastern Europe (Curtu et al. 2007), although without much effect on species identity. Recent plastome analyses even suggest that sections of Quercus form clades with other co-occuring genera of Fagaceae (Y.-Y. Yang et al. 2021), perhaps suggesting very early hybridization. S.-Y. Liu et al. (2023) found evidence for gene flow between all genera of Quercoideae and between and within the sections of Quercus itself. But hybridization at shallower levels, although common, is often less involved in speciation per se than in introgression. The bottom line is that Quercus forms a syngameon, a group made up of morphologically distinct species that are nevertheless interconnected by hybridization and gene exchange (Cannon & Petit 2019 and references; Manos 2023; Backs 2025); as Canon and Petit (2019: p. 979) note, "the network of species, interacting over time and space and responding to periods of enduring stasis and rapid change, may create a synergistic evolutionary process that generates resilience and flexibility beyond the ability of any of its constituent parts". Hybridization may not "simply" result in gene flow, but it may be how some species migrate; thus in Europe Q. petraea may hybridize with Q. robur, and further crossing of the hybrid with Q. petraea may lead to the replacement of Q. robur by Q. petraea (Petit et al. 2003; there are similar goings-on in Eucalyptus). As might be expected, nuclear and chloroplast genes commonly suggest different relationships (Liu et al. 2023: Fig. 2); importantly, Hipp et al. (2019: pp. 1208-1209) noted that particular genes or regions of the genome that might help recover the phylogeny of Quercus as a whole were not to be found, "rather, the phylogenetic history of oaks is defined by different genes in different lineages, making [the] evolutionary history of oaks a phylogenetic and genomic mosaic" ... "the phylogeny we unravel will neither be unitary nor told by a small subset of the genome, as the regions of the genome capturing the divergence history for one clade are not the regions capturing the divergence history of another" (see also Hipp 2019; B.-F. Zhou et al. 2022: e.g. Fig. 2, section Quercus in particular). Parallel evolution is common, as in Mexico, in the Cerris and Ilex clades in Europe. In general, within any particular area, species in divergent lineages have evolved convergent ecologies (Kremer & Hipp 2019). For more on various aspects of the evolution of Quercoideae/Quercus, see articles in New Phytologist 226(4). 2020, Hipp et al. (2019), Plomion and Martin (2020), T.-R. Wang et al. (2024), etc., and for more on hybridization and evolution in Quercoideae, see Stull et al. (2023).
Ecology & Physiology. Fagaceae are often very common in north temperate areas and they are also common and notably diverse on hills and mountains in Mexico, Central America and Malesia. The family frequently dominates the vegetation in which it grows, taxa like Castanopsis acuminatissima being conspicuous in montane vegetation as far east as the island of Papua. Quercus has the greatest biomass of any genus in the forests of the whole North American-Mexican region (Hipp et al. 2020). Along with Magnoliaceae, Theaceae and Lauraceae (Tang 2015; Yu et al. 2017; Hai et al. 2022), Fagaceae, particularly species of Quercus, are a notably prominent component of the temperate and subtropical evergreen broad-leaved forests (EBLFs) of East Asia, particularly in the period 23-4 Ma. Interestingly, Fagales-dominated ECM forests can prevent the upward movement of forests dominated by arbuscular mycorrhizal plants; the ECM forests develop a thick litter layer which tends to dry out easily, and Helotiales like Rhizoscyphus ericae that are found in such forests seem to behave like ECM fungi; overall, soils with ECM (and Ericoid mycorrhizae) have some 70% more C per unit nitrogen than soils with AM plants, to the detriment of the growth of the latter (Dalling & Willey 2025).
The effect of such Fagaceae on the community is increased by the sometimes rather sporadic episodes of heavy fruiting that can be more or less synchronized across some 1,500 km; this is mast fruiting (see Pollination Biology & Seed Dispersal below). Fagaceae are/were an important element of the diet of many animals, including the now-extinct passenger pigeon (Ectopistes migratorius) in North America (Halliday 1980). The demise of the passenger pigeon, which had hundreds of millions of individuals, if not more, and once made up 25-40% of the total North American avian biomass, may have been hastened in part by the destruction of its food sources as settlers cleared woodlands for agriculture. Fagaceae - oaks, beech, chestnuts - were a major element in its diet (Curran & Leighton 2000). How the pigeons, along with the other birds eating these plants, handled the nuts, is of great interest; the passenger pigeon is likely to have destroyed the nuts it consumed, but it may have regurgitated some, so allowing their germination (and this would also happen if the pigeon died). A number of Fagaceae have nuts too large for the birds to eat (and in the case of chestnuts, they may have been deterred by the spines; note also that the chestnut did not mast), but here infraspecific variation in nut size is important (Novak et al. 2018) - and nut size may be affected by the demise of the bird that dispersed it... (Galetti et al. 2013).
White oak (Quercus alba - subgenus and section Quercus) alone represents (12-)19-26(-49)% of witness trees - i.e. trees that were probably present before Europeans arrived - in the oak-dominated forests of eastern North America (81% in some southern Illinois forests). In such forests, (36-)50-80(-± 100)% of all trees are white oak, along with with up to three more ectomycorrhizal (ECM) species, two of which are usually other Fagaceae (Abrams 2003; see also Faison & Foster 2014). Six of the 30 species of Quercus growing in these forests are notable dominants (Abrams 1996). Fagaceae, again mostly Quercus, are abundant in western North America, and in California the black oak, Quercus kelloggii (a red oake, subgenus Quercus, section Lobatae, is particularly widespread and has the greatest timber volume of any oak (Waddell & Barrett 2005). Papers in Gil Pelegrín et al. (2017) discuss the ecophysiology of oaks.
The composition of forests dominated by ECM trees may show a fair amount of dynamism while still remaining ECM forests. Many oaks like high light and moderately dry conditions, and fires are quite common; oak can replace pine in such situations (Adams 1992; Mensing 2014). There are suggestions that more recently Fagaceae may be replacing Pinaceae in some temperate forests (Alfaro Reyna et al. 2018). Here climate stress and successional dynamics may be involved, but changes are less obvious in eastern North America and in cases where there have been natural disturbances, and in any event there appear not to have been significant changes in dominance (Alfaro Reyna et al. 2018). In Mexican oak-pine forests oak may also be replacing pine (Alfaro Reyna et al. 2019). The issue is complex. Changes may be happening in part because Pinus in particular, along with ECM Populus, are early successional plants at higher latitudes, but they are replaced by other genera of Pinaceae; with increasing temperatures and associated fires this replacement may be retarded (Searle & Chen 2017). At least in some situations Pinus may become more common, and at the expense of other conifers (see also Baltzer et al. 2021).
The recent history of the ECM American chestnut, Castanea dentata, is part of the same story. It has been suggested that the chestnut was until quite recently a dominant large tree covering some 800,000 km2 in forests in eastern North America, and it was an important timber tree and source of food for humans and other animals, but it now persists largely as suckers after its devastation by chestnut blight in the first half of the last century beginning around 1904 (Thompson 2012). However, Faison and Foster (2014) suggested that literature reports of the abundance of chestnut in pre-contact forests should perhaps be qualified, i.a. noting that some of the dominance of chestnut was quite recent, being accentuated by coppicing after being cut down by early Europeans (see also Russell 1987). In any event, both the fallen leaves and excorticated bark are likely to have been very flammable, and over a considerable part of the original range of the plant the mean fire interval was 12 years or less (Kane et al. 2020). For whatever the reason, much of the chestnut forest has since been replaced by Quercus rubra-dominated, mixed oak or oak-hickory forests - red oaks take over (Abrams 1996; Novak et al. 2018); see e.g. van der Gevel et al. (2012) for the future. The forests thus remain dominated by ECM trees, in some cases at least associated with the same species of ECM fungi as were associated with the chestnut (Horton & van der Heijden 2007). However, the forests replacing the chestnut are probably more mesophytic (Kane et al. 2020), furthermore, the American chestnut seems not to have been a masting plant, the oaks are, and this, the sizes of the acorns, etc., have to be taken in to account when thinking of the dispersal of these plants (Novak et al. 2018).
Oaks or beech are major components of oak/bamboo forests in many parts of the world, the bamboo making up the understory, although such forests are not to be found in Europe and much of temperate North America (Kappelle 2006b). Fagus-dominated forest may have very little in the way of understory vegetation.
In a study of Fagus sylvatica from two localities in Germany, Khokon et al. (2023) found that 53 fungal taxa were associated with the beech, and they suggested that it was the diversity of the associations that was important for nitrogen acquisition by the plant as much as the presence of any one particular species. Furthermore, they noted that NH4+ was taken up more that NO3-, indeed, 5/19 fungal genera had no nitrate-transporter genes at all (Khokon et al. 2023).
Fagaceae are hosts to 3 separate clades and some 10 genera of angiosperm holoparasites (Hatt et al. 2024c).
In holm oak, Quercus ilex, the adaxial surface of the leaf is wettable and can absorb water, the lower side is densely covered in trichomes and is not wettable, so preserving stomatal functioning in wet conditions (Fernández et al. 2014) - see also Bromeliaceae.
An individual of Quercus petraea, an estimated 934 ± 65 years old and from the Aspromonte forests in southern Italy, is thought to be the oldest temperate angiosperm tree - or angiosperm tree, period - in the world (Piovesan et al. 2020; Piovesan & Bondi 2021), however, Wikipedia should be consuted over such matters - see Wikipedia, "List of Oldest Trees". American chestnut trees, Castanea sativa, could be over 10 m in circumference, and it is estimated that they were 400->600 years old, but such figures pale in comparison with the ages of a number of gymnosperms, Pinaceae and Cupressaceae in particular (Piovesan & Bondi 2021), and still more the ages of plant clones. In an examination of the genome of Q. robur in the context of trying to understand the longevity of oak trees, the accumulation of somatic mutations, the expansion of R[esistance] genes involved in immune defence, and the diversification of defence genes were all noted, and much local and recent gene duplication was also observed (Plomion et al. 2018). Similar connections are to be expected in long-lived trees in general (Tobias & Guest 2014), but note that there may be many fewer cell generations - and hence mutations - in the buds that produce inflorescences than the age of the plant or the number of cell generations in the tissues in the stem that bear the bud might suggest (Burian et al. 2016, see also Y. Ren et al. 2021: Salix).
For intergeneric and intersubfamilial graft hybrids, see Herrmann (1951).
To link: The dead foliage of white oaks is richer in nutrients than that of red oaks, members of different sections of subgenus Quercus (Hipp et al. 2020).
Pollination Biology & Seed Dispersal. Quercus and Fagus are wind pollinated, otherwise, insect pollination dominates in the family (Cannon 2019). Larue et al. (2021) describe pollination in some cultivated Castanea, noting two bursts of pollen production - the first is caused by flowering of the staminate catkins, the second by that of staminate flowers in bisexual catkins. Beetles are the main pollinators, and with about 4,000 staminate flowers to one carpelate flower, the pollen:ovule ratio is very high (Larue et al. 2021). Petit and Larue (2022) confirmed entomophily here, noting the nectariferous flowers with a powerful smell, pollen with pollenkitt and the small stigma.
Fertilization in the family is porogamous, according to Johri et al. (1992), and it can be much delayed (Sogo & Tobe 2006d and references; M. Deng et al. 2008; Larue et al. 2021: ovules take about six weeks to develop). In Lithocarpus dealbatus K. Yao et al. (2023) found that the pollen tube reached the base of the style in January of the year after pollination; it then stopped growth for about four months, and on finally reaching the micropyle there was another month's hiatus... X.-R. Yang et al. (2017) discuss variation patterns in various floral/reproductive features in the family; they suggest that the pollen tube normally is dormant for a while in the style, but in the funicle and integument or micropyle in Fagus (see also M. Deng et al. 2022; for Fagus, see Sogo & Tobe 2006d - there is a fair amount of variation in the whole process of fertilization in the family). Castanea has a post-zygotic incompatibility system (ref.). Although Yao et al. (2023) suggested that beetle pollination was plesiomorphic in Fagaceae, this would seem to be unlikely - for instance, both Fagus and Nothofagus are wind-pollinated.
Individuals in a number of species of Fagaceae simultaneously, if somewhat erratically, produce large numbers of fruits over large areas, the phenomenon of masting (for general accounts of this distinctive fruiting behaviour, see Janzen 1978; Silvertown 1980; Kelly 1994; Koenig & Knops 2000, 2005, Koenig et al. 2017; Pesendorfer et al. 2021a and other papers in "The ecology and evolution of synchronous seed production in plants", Phil. Trans. Royal Soc. B, 376(1869). 2021 - to a certain extent the discussion below is about masting in general). Fagus sylvatica is an example of masting in northwest Europe where the species fruits more or less synchronously across ca 1,500 km in response to climate cues, however, masting further south is at a very local scale (Bogdziewicz et al. 2021). Weather-sensing on a continent-wide scale (some 2,000 km) in F. sylvatica begins at the summer solstice – a continent-wide cue - helping to ensure synchrony of subsequent masting events the immediate stimuli of which are climate events (Journé et al. 2024). Particular phases of broad-scale regional to subglobal climatic events such as the North American Monsoon, the El Niñ Southern Oscillation (ENSO - tropical/southern hemisphere in its effects) and the North Atlantic Oscillation (NAO - northern hemisphere) may trigger masting events (e.g. Ascoli et al. 2021; Wion et al. 2021). The complexity of the Malay archipelago - dipterocarp masting - may result in masting events happening at different times in different parts of the archipelago, although the events are more widespread if the ENSO event is strong (see also Ascoli et al. 2021). The series of events associated with masting may also include facilitation of germination and seedling establishment. As suggested, the trigger of a masting event is often an environmental cue, and in at least some species studied replenishment of reserves after a masting period is unnecessary (Bogdziewicz et al. 2019). Fire and weather together may affect seed production in rhizomatous Florida scrub oaks, and as in other cases the behaviour of animals that eat or disperse the seeds is also affected (Pesendorfer et al. 2021b). For fruit dispersal and habitat preferences, see also X.-G. Xiang et al. (2014a: Fagus with winged fruits; c.f. Mindell et al. 2009; Larson-Johnson 2015).
Bogdziewicz et al. (2020) examine factors that might contribute to variability or synchrony in reproduction, and it is certainly no simple story; environmental disturbances can affect seeding, and in some cases particular kinds of disturbance events and masting become associated (Vacciano et al. 2021). Satake and Kelly (2021) found a relationship between delayed fertilization and flowering time diversity in Fagaceae, particularly in those groups with insect pollination: Competition for pollinators, seeds taking a long time to mature and an unfavourable season for flowering and/or fruiting all contributed to this, and predator satiation was also involved in the evolution of delayed fertilization. Thus diversity of flowering times was particularly great in Lithocarpus and somewhat less so in Castanopsis and still less in the species of the other four genera examined; one or two species in the first two genera flowered twice each year; fruiting was predominantly from August to November in all genera, most of the species in the first two genera taking over a year to produce ripe fruits (Satake & Kelley 2021: esp. Fig. 1).
It is also well known that fruits of red oaks (subgenus Quercus, section Lobatae) take one and a half years to mature ("2-year species"), and masting is independently synchronized within red and white oaks (Mohler 1990). Immature fruits containing very small embryos may overwinter, or fertilization may not occur until the spring after flowering/pollination (Stairs 1964; Sork et al. 1993; Borgart & Nixon 2003). Interestingly, a number of species of red oaks in Mexico and Central America can mature acorns in under a year - evolutionary reversal (Nixon 2006), while up to four years between pollination and seed maturity may elapse in species of section Ilex (M. Deng, in Denk et al. 2017), and it can be difficult to recognize biennial maturation patterns (Denk et al. 2017). Most 2-year species produce new shoots every year, and so have acorns each year (consecutive-bearing species), while others, especially in Quercus subgenus Cyclobalanopsis, are alternate-bearing species that produce shoots, and so acorns, only every other year (Hirayama et al. 2019: see below for possible connections with a weevil seed predator).
The effect of masting on animals that eat the fruits of masting plants is considerable (Koening & Knops 2005). As with the mast-fruiting dipterocarps, q.v., some of the seed predators like passenger pigeons (Ectopistes migratorius: see above) in eastern North America and wild pigs in Europe are (or were) migratory, moving to areas where masting is occurring. Scatter-hoarding occurs in animals like mice and birds that disperse the fruits of masting plants, and given sufficient time between masting events, the populations of these animals fluctuate accordingly, and this then affects the recruitment of the plants (Zwolak et al. 2021). Jays are often particularly important dispersers of Quercus. In Europe Quercus robur is dispersed by Garrulus glandularius, each jay hoarding several thousand acorns, and their nestlings were fed caterpillars eating oak leaves (Bossema 1979). In Virginia jays (Cyanocitta cristata) disperse the seeds of Q. palustris, other oaks with smallish acorns, and Fagus, some 133,000 acorns being removed from a single stand of Q. palustris and moved (100 m-)1.1(-1.9 km); the jays ate about half the acorns they did not cache while weevils ate most of the acorns that simply fell from the trees (Darley-Hill & Johnson 1981). Koenig et al. (2017, see also 2014) include a remarkable photo of the 000's of acorns that acorn woodpeckers, Melanerpes formicovorous, had cached in the bark of an oak tree.
Indeed, much has been written about the behaviour of animals that eat and disperse the acorns of red/black oaks (subgenus Quercus section Lobatae/Erythrobalanua) and white oaks (section Quercus/Leucobalanua). Acorns of red oaks mature in the year after flowering and germinate in the following spring, i.e., the whole process takes about two years total, and they are high in tannins and lipids, however, acorns of white oaks mature in about six months, germinate almost immediately, and their seeds are usually less rich in tannins and lipids (e.g. Steele et al. 1993). Interestingly, blue jays, grackles and squirrels tend to eat the basal portion of the seed, the cup end, which is lower in tannins than the apical portion where the plumule/radicle are, furthermore, despite the loss of part of the cotyledons the seeds germinate quite well (Steele et al. 1993). (Note that the phylogenetic signal of tannins in Quercus is weak (Pease & Hipp 2009), as is common with plant protective compounds (Cacho et al. 2015).) Gray squirrels in general prefer to eat acorns of white oaks in the fall and to cache those of red oaks, but if they cache the acorns of white oaks they excise the embryo first (Smallwood et al. 2001; Wood 2005 for literature), although in non-masting years there may be no difference in their behaviour (López-Barrera & Manson 2006). In general, it is the state of germination of the seed that affects the behaviour of squirrels, so old/germinating red oaks are treated in the same way as white oaks, while the fruits of species of red oaks that mature acorns in one year are treated in the same way as those of white oaks (Smallwood et al. 2001). Kang et al. (2023) discuss how seeds, i.e. the cotyledons and embryonic axis, might dry out. Seedlings of white and red oaks differ, those of the former being largely subterranean in the first autumn of their existence, most of the seed reserves being quickly transferred into their large, woody and so not terribly palatable taproot, the plumule appearing in the next year (Fox 1982; Mohler 1990), while red oak seedlings germinate in a more conventional way. All these differences potentially translate into differing rates of spread of species of the two groups, the acorns of red oaks being more widely dispersed than those of white oaks, and perhaps there are different reactions to soil fungi, etc. (Steele et al. 1993; Steele & Smallwood 1994; Smallwood et al. 2001). Given the extent of the differences between red and white oaks, it is not surprising that they commonly co-occur (Mohler 1990). For the growth of oak seedlings with and without their cotyledons and their response to damage/herbivory, see Gelviz-Gelvez et al. (2017).
Schermer et al. (2020) noted that flowering was earlier in temperate than in Mediterranean oaks; with early flowering, the amount of pollen produced, and hence, later, the number of acorns that resulted, might increase, although freezing or other untoward climatic events might decrease pollen and seed propduction (note that flowering is directly linked to leafing and photosynthesis; the one shoot first produces flowers, then leaves). This uncertainty in acorn production would help control the dynamics of the populations of seed consumers, masting years being unpredictable, climate, etc., having introduced a stochastic element into the equation (in Mediterranean oaks, drought might play the same role (Schermer et al. 2020, see also Bogdziewicz et al. 2019). Of course, seed consumers like weevils do nothing but kill the seeds, while those like jays and pigs are also involved in seed dispersal. In a study of pig populations in northeastern France, Touzot et al. (2022) emphasized apects of the negative temporal autocorrelation of acorn production, that is, high yields one year tended to be followed by lower yields the following year. If conditions ameliorated, then autocorrelation became stronger, the result being a regular two year cycle of fruiting-no fruiting. This would fit the reproductive cycle of pigs, which start reproducing in their second year, and pig populations - and their effect on oaks - would increase. But even among mammals, effects vary. Thus female dormice produced more young in masting years, but they tended to die as a result (Touzot et al. 2022). In general, masting is a complex affair, and there has been recent discussion as to what to measure and how to analyse the data when dealing with masting (Qiu et al. 202j3; Bogdziewicz et al. 2023). It had been thought that environmental conditions and masting were more extreme towards the peripheries of the ranges of masting species, but recent work on a variety of angiosperms and gymnosperms - but no dipterocarps - suggests that masting events in such habitats are less frequent, but they are also less extreme, and overall annual seed production shows less variation (Foest et al. 2025).
Finally, and linking on to the next section, there is a connection between acorn production and cicada eclosion in the eastern half of the United States. Every 13 or 17 years adult cicadas (Magicicada spp.) appear, mate, lay eggs and die - the numbers are huge, maybe 3,000 kg ha-1 of dead bodies start rotting, leading to a flush of nutrients, but the growth of the trees had been negatively impacted by the egg-laying and feeding activities of the adults when they were alive. Koenig et al. (2023) found that acorn production of white oaks was somewhat reduced in the year after cicada emergence, red oaks in the year of emergence but also increasing two years afterwards.
Plant-Animal Interactions. Fruits of Fagaceae are a major source of food for a diversity of animals (see also above). These include birds like the acorn woodpecker, also grackles, blue jays, corvids, etc., as well as squirrels, mice, pigs, and so on, although the relationships of the parties involved are complex and rarely obligate.
Oaks support the highest diversity of insect herbivores of all temperate holarctic forest trees. Theclines (Lycaenini) caterpillars are common on Fagaceae (Ehrlich & Raven 1964), while leaf-mining larvae of Eriocraniidae, probably the subbasal clade in the lepidopteran Angiospermivora, eat Fagaceae, and also Betulaceae (Regier et al. 2015 and references). Indeed, Quercus is the most important host plant for lepidopteran larvae in the contiguous United States of America, hosting around 23% of the species (Narango et al. 2020); Castanea ia also quite important, and see also Betulaceae. Pearse and Hipp (2012) found that leaf defences in Quercus were highest at lower latitudes (little temperature fluctuation, mild winters, low minimum precipitation). Herbivores here are an important food source for birds in particular, but once every 13 or 17 years caterpillars and other herbivores on oaks in the eastern U.S.A. get a bye year, as the attention of birds turns to the millions of emerging cicadas (Koenig et al. 2023).
Some evergreen species of Quercus subgenus Cyclobalanopsis are alternate-bearing species that produce shoots, and so acorns, only every other year (Hirayama et al. 2019, see also above for masting). This odd behaviour is apparently connected with the attention paid to the plant by the weevil Mechoris ursulus which lays its eggs in mature acorns (it chooses the largest acorns) and then cuts off the twigs on which the acorns are growing; this part of the whole process taking about a week. The eggs hatch, and the mature larvae leave the fallen acorn that same year but then overwinter underground - so the weevil is also a two-year species, unusual for a weevil (Koo et al. 2002; Hirayama et al. 2019). These Cyclobalanopsis oaks lose only one year's growth/one crop of acorns. However, this is not the whole story, for instance, the weevil also lays aggs on other two-year species which are consecutive-bearing, bearing shoots and acorns every year, and here the weevil cuts off two years' growth and the plant loses two seasons of acorns; these species are deciduous, and so they would have lost their leaves in any case... (Hirayama et al. 2019).
And on to galling. By some estimates half of all galls in the north temperate region are found on Fagaceae, especially on oaks, and perhaps especially in North America, where Quercus is particularly diverse (Mani 1964; Abrahamson et al. 1998; Oyama et al. 2006: galls in Mexico). Indeed, it is estimated that there are some 700 species of cynipid gall wasps inducing galls on over 150 species of oaks in the Nearctic alone (Ward et al. 2023); Price (2005) thought that there were some 1,000 species of Cynipidae in 41 genera. Galls produced by Cynipidae-Cynipini in particular are common, and they differ in morphology/location on the plant depending on whether they are produced by sexual or agamic individuals, Cynipini having two generations a year - sexual in the spring and asexual in the autumn (Ronquist et al. 2015). Leaves, twigs (twig galls in particular can damage the plant), fruit, even roots may all be galled, although the wasps generally lay their eggs in the buds; shifts by gallers between different organs are quite common, and the resultant morphological diversity of galls is remarkable (Stone & Cook 1998; Stone & Schönrogge 2003; Price 2005: some 300 spp. of Andricus involved; Redfern 2011; Hearn et al. 2019). The gall chambers are lined by nutritive cells formed by tha plant (Price 2005), and as with galls in general, the galls represent the extended phenotypes of the gallers – that is, the galler largely controls the morphology of the gall, the host much less so, and the galling insect can even induce galls of similar morphologies on hosts belonging to different taxa. Price (2005) noted that there was substantial genetic variation between the wasps, and heteroecious gallers formed galls on different species of Quercus, even on species from different sections.
Betancourt et al. (2020) described gene expression in a cynipid gall by the plant during the course of the development of the gall, while Markel et al. (2024) looked at galls on Quercus lobata induced by two different cynipids and followed the development of novel vascular tissue in the galls (this is a common but not universal phenomenon), i.a. deposition of lignins and xylans were affected. Overall, there can be quite extensive change in the expression of the host genome, but details of the galling process are still poorly understood, although changes in the host may relate to some sort of somatic embryogenesis-like process being involved; other organisms (fungi, bacteria) seem not to be part of the association in cynipid galls (Blaimer et al. 2020), although they may be elsewhere. Markel et al. (2024) found that the mechanics of gall induction varied between the two wasps they studied, with different changes to phytohormones, different levels of abscisic acid, hexose phosphate, trehalose, and so on, in the different galls. At least initially the gall larvae here live in fluid-filled chambers, absorbing photosynthate and nutrients presumably brought there by the vasculature developed in the gall. Markel et al. (2024: p. 10) note that “the phylogenetic distribution of the galling habit within cynipid wasps suggests it is ancestral, and therefore at least some of the core mechanics are likely to be conserved.” Ronquist et al. (2015), Gatjens-Boniche (2019) and others can also be consulted with profit; although the emphasis here in Fagaceae is on cynipid wasps, remember that a variety of immediately unrelated organisms, including rhizobial bacteria, induce galls or gall-like structures.
Host plant conservatism by cynipid oak gall wasps has persisted over the last ca 20 Ma at least, and major clades of these gall wasps are usually associated with major clades of oaks, sometimes other genera of Fagaceae (J. M. Cook et al. 2002; G. N. Stone et al. 2009; Leckey & Smith 2015). Crown-group Cynipini are dated to 76-71 Ma (Blaimer et al. 2020), and their some 1,000 species make up an appreciable part of the whole Cynipidae. Diplolepidini, found on Rosa alone (Y. M. Zhang et al. 2020), used to be closely associated with Cynipini, but Blaimer et al. (2020) find four other tribes to be successively sister to Cynipini; Diplolepidini are no longer part of Cynipidae.
The overall diversity of the oak-herbivore-galler set of relationships is considerable. Furthermore, quite a number of species of Cynipini, especially members of Synerginae, are inquilines, that is, they lay eggs in galls made by other species (Csóka et al. 2005; Stone et al. 2009; Blaimer et al. 2020 for the vagaries of ancestral state reconstruction). Interestingly, Synergus, has members that are mostly inquilines, however, S. itoensis forms galls inside the acorns of
Ants. Staab et al. (2017: planted forests) noted that ants were notably common visitors to sap exuding from wounds on leaves of Fagaceae in subtropical S.E. China, the species involved largely being the same as those visiting extrafloral nectaries. Interestingly, extrafloral nectaries are uncommon in Fagaceae, and one might think that it was not surprising that the frequency of ant visits increased with the severity of leaf damage - but this was not true of other species of plants visited by the ants (Staab et al. 2017). For wound-type extrafloral nectaries, see also Solanaceae-Solanum.
Galls and ants. Tiny galls containing the cynipid Kokkocynips rileyi fall from the oak tree (the wasp makes galls on red oaks) and they are then picked up by ants that are attracted by an elaiosome-like appendage on the gall (Warren et al. 2022). The ant treats the gall just like a seed with an elaiosome, and the cynipid emerges from the gall some time in the next year. Such galls are sometimes produced in very large numbers and have even been used to fatten hogs, turkeys, etc. (Fagan 1918: as Callirhytis sp.; Warren et al. 2022). Oak galls made by other cynipids (K. decidua is an example) produce nectar and are visited by ants which deter galler parasitoids and inquilines (Nicholls et al. 2016; Warren et al. 2022).
Plant-Bacterial/Fungal Associations. Ectomycorrhizal associations are dealt with under Ecology & Physiology above.
There are a number of major fungal diseases that attack Fagaceae. 1. The chestnut (Castanea dentata) forests in eastern North America have been utterly devastated by the introduced ascomycete fungus Cryphonectria parasitica (Endothia parasitica in older literature) over a period of less than forty years, 1904-1940 (Hepting 1974; H. Thompson 2012; see also Molina et al. 1992). Although chestnut plants persist in the understory for many years after initial infection because they sucker from collars of the otherwise dead old trees or from stumps, the suckers practically never reach reproductive age before being reinfected by the fungus (Schlarbaum et al. 1997). 2. Sudden oak death, caused by the oomycete Phytophthora ramorum, has become a major pathogen on some Fagaceae in coastal California and in Oregon over the last 20 years or so, and the infestation is out of control (Cunnliffe et al. 2016). Quercus agrifolia and Notholithocarpus densiflorus have been particularly badly affected, but a variety of other Fagaceae and other plants may also be attacked. Interestingly, sporulation occurs particularly on Umbellularia californica, bay laurel (Lauraceae), in years when rainfall is higher than average - altogether, a rather complex system (Kozanitas et al. 2022). 3. Fungal oak decline is widespread, and fungi like Ceratocystis fagacearum and Diplodia (both ascomycetes, unrelated) are involved in oak wilt diseases in particular (Rodríguez-Calcerrada et al. 2017), and these authors make it clear that in such diseases many factors interact - human activities in forests, herbivory by insects and other animals, climate, decreasing rainfall, the activities of microbes - and symbionts can become parasites. 4. Acute oak decline in England is bacterial, and is caused by interactions between the buprestid jewel beetle Agrilus biguttatus, which excavates galleries in the phloem and sapwood, and bacteria such as Gibbsiella quercinecans and especially Brenniera goodwinii - or all three may simply take advantage of oak trees that have sub-par health (Doonan et al. 2020). The two bacteria may interact positively, as also Agrilus and Brenniera, etc. - again, complex relationships. There are also other unpleasant oak pathogens like the ascomycete Raffaela quercivora that is associated with ambrosia beetles (weevils) which obligately cultivate the fungi (Huler & Stelinski 2016; see also Jordal & Cognato 2012, Pinaceae, and papers in Vega & Hofstetter 2015).
Kühdorf et al. (2015: p. 110) noted that the arbutoid "C[omarostaphylis] arbutoides is a refuge plant for ectomycorrhizal fungi as it shares these fungi with ectomycorrhizal tropical trees such as Quercus costaricensis". Interestingly, in temperate North America Quercus has been reported to be both an ECM and an arbuscular mycorrhizal plant (Bennett et al. 2017: Supplementary Material). These joint associations can be simultaneous or successive; generally the adult plant has ECM associations alone (Egerton-Warburton & Allen 2001).
Fort et al. (2021; see also Lespiaucq et al. 2021) found that there was vertical transmission of fungal associates, ascomycetes predominating, in Quercus petraea, and when the acorns fell additional fungi moved into the embryo, sometimes replacing the fungi that were already there.
Genes & Genomes. For genome sizes in the family, see Chen et al. (2014). Although it was suggested that tropical species generally had larger genomes than temperate species, the smallest genomes were found in temperate species (Fagus, some species of Quercus: S.-C. Chen et al. 2014).
T.-R. Wang et al. (2024: focus on Quercus gilva) found considerable genome stability in Quercus. Larson et al. (2025) looked at genomes of Quercus alba, comparing it with what was known of other genomes in the genus. They found that the number and position of gene clusters was constant, although the number of genes within a cluster varied. Some 12,000 or more structural variants were found in two haplotypes of Q. alba - interestingly, perhaps up to 57.7% of of the variable sites within the species were found within other white oaks (section Quercus); after correcting for this ancestral variation, estimates of divergence times were 8-10 My younger than previously suggested (Larson et al. 2025).
A mitochondrial gene has moved from the parasite Mitrastemon (Ericales) to its host, Quercus (Sytsma et al. 2009).
Economic Importance. Woods of Fagaceae, including Fagus and various species of Quercus, are valuable hardwoods (Luppold 2019.
The cork of wine bottles is produced by Quercus suber, a species that is unusual in that its cork cambium or phellogen is long-lived, so producing large amounts of phellem, or cork; this is harvested by stripping the bark (Teixeira 2022).
Oaks and beechs (and other Fagales) are hosts of the extremely pricey truffle fungi, an ascomycete (Pezizales, Tuber - various other subterranean and edible fungi are also called truffles).
Chemistry, Morphology, etc.. Syllepsis is very uncommon in the family (Keller 1994). For leaf vernation, see Couturier et al. (2009, 2011). M. Deng et al. (2013) discuss stomata and hairs in Lithocarpus in particular.
For general inflorescence morphology in Fagaceae, see Kaul and Abbe (1984). There has been much discussion about the morphological nature of the small protrusions surrounding the ovary, and the whole complex is often interpreted as a modified cymose inflorescence (e.g. Forman 1966a; Sims et al. 1998; Manos et al. 2001a; Pigg et al. 2001; Oh & Manos 2008 for references; c.f. Jenkins 1993: modifications of the perianth). When the cupule has valves, probably the plesiomorphic condition, there is one valve more than the number of fruits; the valves represent modified cymose part inflorescences (Fey & Endress 1983; for inflorescence morpohology, see also Brett 1964; Cannon & Manos 2001).
Endress (1979a) suggested that the flowers of Fagaceae were hexamerous. For the orientation of the carpels, see Endress (1977a) who shows the median member of outer T whorl in both staminate and carpelate flowers as being abaxial (c.f. Sims et al. 1998). Denk and Grimm (2009b) describe pollen morphology with a focus on Quercus; I use their terms above (see also Praglowski 1982, 1984); Denk and Tekleva (2014) think about foot layer variation (see also Grímsson et al. 2016: thick foot layer plesiomorphic in the family).
Variation in ovule and fruit is extensive, and is conveniently tabulated by M. Deng et al. (2008); the polarity of some of this variation is unclear. X.-R. Yang et al. (2017) suggested that the endocarp of Fagus lacks trichomes, Kubitzki (1993b) that it has them. J. Li et al. (2022) found that the plumule-radicle part of the enbryo was near the base of the fruit in Castanopsis, but apical in Quercus, at least (and c.f. Fagus?). Hjelmqvist (1953) discussed the basi-lateral embryo sac/endosperm caecum he noted in some Quercoideae.
See also Nixon (1989, 2006), Kubitzki (1993b) and Manois and Hipp (2021: North American Quercus, all general and Denk et al. (2024: Fagus), Hegnauer (1966, 1989: chemistry), Loreto et al. (2009: monoterpenes, isoprenoids in Quercus), Huang et al. (2011: ellagitannins with triterpene alcohol cores), Clowes (1951: anatomy of mycorrhizae), Whitmore (1963: bark), Lersten and Horner (2008b: leaf crystals, etc.), Liu et al. (2009: stomata, hairs), Crepet and Daghlian (1980: pollen), Du et al. (2021: ovule number in Castanea) and Langdon (1939: ovules, etc.) for additional information.
Phylogeny. Fagus is sister to all other Fagaceae (Manos et al. 1993; Sauquet et al. 2012); Quercoideae s. str. are paraphyletic, Trigonobalanus being sister to the rest of the clade (see also Karumuna et al. 2019: plastome data, very long branch, rest of family on much shorter branches). For phylogeny see also Manos and Stanford (2001), Manos et al. (2002) and X.-G. Xiang et al. (2014a). Li (1996) did not find Fagaceae to be monophyletic in his morphological analysis.
Fagoideae. Renner et al. (2016) discuss relationships in Fagus. Jiang et al. (2020: 28 nuclear single/low-copy loci) looked at all species bar one; resolution was quite good, but there were substantal conflicts within the genus. The study by Denk et al. (2024) used nuclear isozyme, simple sequence repeat and 5S intergenic spacer data and clarified the limits of the eastern European species of the genus in particular.
Quercoideae. Trigonobalanus had long been known from Fraser's Hill in Peninsula Malaya, but it was first described some 55 years ago from Mt Kinabalu in Borneo (Forman 1964), then a previously described (as Quercus doichangensis) species that grows from Yunnan to northern Thailand was included, it was then found in South America 35 years ago, and then fossil in North America, and then... (Nixon & Crepet 1989 for some information). However, support for the position of Trigonobalanus was not that strong and Quercus even appeared to be paraphyletic - or at least its monophyly was unclear (X.-G. Xiang et al. 2014a), and similar relationships are still sometimes found in plastome analyses (X. li et al. 2018; Xuan et al. 2018; Ye et al. 2019; S.-Y. Liu et al. 2023), however, as Simeone et al. (2016) and others have pointed out, such confused relationships may well be caused by hybridization and plastid introgression. Oh and Manos (2006, 2008) suggested that Lithocarpus, which has fruits like those of Quercus, is polyphyletic, the South East Asian members grouping with Chrysolepis. Indeed, the single species from the Californian floristic province of West North America, L. densiflorus (now = Notholithocarpus), is in a clade with Quercus, Castanopsis, and Castanea. For relationships in Vietnamese oaks, Quercus and Lithocarpus, see Strijk and Hinsinger (2019: phylogenomics), the sections in the latter in particular are more or less wildly polyphyletic. Castanea is currently widely distributed in the Northern Hemisphere and with a rich fossil record, although relationships within the genus still need work, and in nuclear analyses it was unclear if C. sativa was sister to the other East North American member(s) of the genus or to the East Asian taxa (see Xing et al. 2016; Q. Zhang et al. 2021; Spriggs & Fertakos 2021; W. Zhou et al. 2022). Relationships in Liu et al. (2023, see also Manos 2023) are [Trigonobalanus [[Castanea + Castanopsis] [[Lithocarpus + Chrysolepis] [Notholithocarpus + Quercus]]]] (only Trigonobalanus has epigeal germination), but this is accompanied by extensive generic-level hybridization, introgression, etc., and the plastome data suggest quite different relationships, pretty much [subgenus Quercus + all other Quercoideae] (see Manos 2023: Fig. 3). treeoflife.kew.org (consulted vii.2025) showed similar relationships, although sampling was notably poorer and Neolithocarpus was embedded in Quercus.
There seems to have been substantial hybridization/gene flow in Castanopsis (Eocene diversification), and there is extensive gene- (but not species-) tree incongruence (S. Tang et al. 2025: 39 spp., Rad-seq).
For early work on phylogenetic relationships within Quercus, see Manos et al. (1999), Oh and Manos (2008) and especially Hubert et al. (2014). However, detailed relationships may be difficult to disentangle if the various positions that different accessions of species like Q. ilex, Q. suber and Q. robur appear on the one tree is any indication (Simeone et al. 2013); relationships were also affected by the outgroup chosen (Hubert et al. 2014). Plastome analyses by Y.-Y. Yang et al. (2021) suggest chloroplast capture in Quercus, even of plastomes of other Fagaceae, and this both in the Old and New Worlds, while McVay et al. (2017a, b) discuss the influence of hybridization/introgression on our understanding of relationships in the speciose section Quercus. For relationships in North American oaks, see Manos et al. (1999), Pearse and Hipp (2009) and Hipp et al. (2014); Hipp et al. (2017) have produced a well-sampled and well-resolved phylogeny for North American oaks, sections Protobalanus and Pontica (see above) are basal in subgenus Quercus, and general relationships in the genus are becoming resolved (Hipp et al. 2019). For relationships in the speciose Southeast Asian Quercus section Cyclobalanopsis (subgenus Cerris) see M. Deng et al. (2013a, especially 2017). Overall relationshipos at the sectional level are [[Cyclobalanopsis [Cerris + Ilex]] [Lobatae (red oaks) [Protobalanus [Ponticae [Virentes (live oaks) + Quercus (white oaks)]]]]], the first three sections being included in subgenus Cerris, the rest in subgenus Quercus (Hipp et al. 2020).
X. Chen et al. (2023: 5 plastid markers and nrITS) looked at relationships between 76 species of Lithocarpus. L. Yang et al. (2024) looked at relationships based on analyses of plastomes variation in 33 species; trees based on variation of plastome data were rather different from those based on analyses of nuclear data (see also C. K. Yang et al. 2018, for example). S.-Y. Liu et al. (2024) had also noticed this incongruence, although this was all within the genus, unlike that in other members of Quercoideae.
Classification. The three extant species of Trigonobalanus have been placed in three genera, but they form a single clade; one genus for the three species is fine. For an infrageneric - two subgenera, eight sections - classification of Quercus with mention of lots of distinguishing characters, see Denk et al. (2017: Fig. 2); this classification is holding up; Vázquez Pardo et al. (2023) discuss infrageneric nomenclature here. For the infrageneric classification of Fagus, see Jiang et al. (2020) and in particular Denk et al. (2024); two subgenera are recognized.
[[Myricaceae + Juglandaceae] [Casuarinaceae [Ticodendraceae + Betulaceae]]] / the Normapolles group: myricetin +; (N-fixing Frankia +), (cluster roots +); pollen tripororate, oblate, exine of two layers separated by an alveolar zone and obviously expanded around the apertures, exine infratectum granular, intine thickening at the apertures [= oncus] [?level]; G [2]; funicle not evident; fertilization chalazogamous.
Age. This node has been dated to 47-46 Ma (Wikström et al. 2001), (50-)41, 37(-28) Ma (Bell et al. 2010), earlier, at ca 79.6 Ma by H. Zhou et al. (2021), and as early as (96.9-)93.4(-88.2) Ma (X.-G. Xiang et al. 2014a, but c.f. topology) and, similarly, ca 93.6 Ma in X.-Y. Yang et al. (2018) and ca 91.3 Ma in T.-R. Wang et al. (2024).
Evolution: Divergence & Distribution. For variation patterns in various floral/reproductive features, see X.-R. Yang et al. (2017).
The pollen of Betulaceae and Juglandaceae in particular is rather like that of the fossil Normapolles type: They are all usually triporate and with elaborate pore architecture, the walls are thick anf often multi-layered, the exine ornamentation is undistinguished, and they have a granular exine infratectum (e.g. Doyle 2009; Daly & Jolley 2015); see Christopher (1979) and Batten (1980) for the classification of these grains, also papers in Rev. Palaeobot. Palynol. 35(2-3). 1981. Batten (1989; see also Clarke et al. 2011) was cautious about the organismal connections of these grains, not all of which may have come from members of Fagales, and the grains did not always seem to him to belong to wind-pollinated plants (Batten 1981). As he observed, "In due course it may be reasonable to refine the Normapolles as a distinct pre-juglandalean/myricalean order for plants which produced pollen grains of the Trudipollis-Oculipollis type and morphologically comparable forms" (Batten 1989: p. 19), and he even thought that Betulaceae, for instance, might not have Normapolles-producing ancestors. Polette and Batten (2017) have begun the task of clearing up the synonymy (13 genera were synonymized) by trying to identify the names, noting that "for many [species] this required interpretations of obscure descriptions often full of unnecessarily complicated terminology describing features that are commonly unrecognizable in the accompanying figures" (ibid.: p. 89). Further confusing the issue, Larson-Johnson (2015) placed a number of fossils purportedly of Normapolles-producing plants outside crown-group Fagales. Odd character combinations in pollen of late Campanian/early Maastrichtian age ca 70.6 Ma have even been explained as the result of hybridization (Hofmann et al. 2011b). Normapolles-type grains have also been compared with those of Urticaceae and relatives and Proteaceae (Batten 1981), while Feuer (1991) did not find much similarity between the pollen of many extant members of this clade. Normapolles pollen is to be found in early Palaeocene sediments from eastern Europe (Daly& Jolley 2015).
There is much variation in Normapolles-producing fossil flowers (see Friis et al. 2006a: Fig. 13, morphological and palynological features of Normapolles-producing fossils and their distributions in extant Fagales, also 2011: e.g. Chap. 14.6.6, Fig. 20.6, etc.), and new genera are still being described; the pollen is also very diverse (Friis et al. 2006a). The flowers are usually perfect, with a simple, undifferentiated, uniseriate perianth and an inferior ovary. Some variation: Zlivifructus has a 2-carpelate gynoecium with a hypanthium, four perianth members in pairs and four stamens, while the fruit has a single, erect, straight seed (Hermanová et al. 2017). Caryanthus has a four-parted perianth with six stamens in two groups of three and a bicarpellate gynoecium (Sims et al. 1999: Table 2). Normanthus, from the late Cretaceous of Portugal, also bicarpellate, has five quincuncial perianth members that alternate with the stamens, and there are two collateral carpels with separate and quite long styles; the placentation is described as being parietal, with one ovule/carpel (Schönenberger et al. 2001b; ovary drawn bilocular in Friis et al. 2006a). Endressianthus has imperfect flowers, with four stamens alternating with the perianth members (Friis et al. 2003a); again, there are two carpels. Other taxa have three carpels and stamens opposite the perianth members. These include Dahlgrenianthus which, unusually, has a superior ovary with short, more or less separate styles (Friis et al. 2006a). Other taxa like Antiquocarya are similar, but have an inferior ovary. Finally, in Bedellia staminate flowers only are known, and these have two five-parted perianth whorls and pollen with circular exoapertures. As with other fossil Fagales (see above), relating these fossils to extant families is a challenge.
That being said, Normapolles pollen was abundant in the Turonian-Campanian of the Cretaceous, some 94-80 Ma, peaking in the Coniacian-Santonian ca 88 Ma, and occurring in much of the Northern Hemisphere in the area 20-45oN (Cretaceous palaeolatitudes) from west Siberia to east North America in particular (Kedves & Diniz 1983; Kedves 1989; Sims et al. 1999; Friis et al. 2003a, esp. 2006a, 2011: Figs 20.6, 7 for summaries; Daly & Jolley 2015); Aquilapollenites, variously linked with Santalales (e.g. Jarzen 1977), Apiaceae, and Caprifoliaceae-Morinoideae, is found in between this area and it is also circumboreal (Vakhrameev 1991; Farabee 1993). Trees that perhaps produced Normapolles pollen may have dominated open late Cretaceous (Campanian) woodland in Texas (Lehman & Wheeler 2001), but this needs confirmation. Normapolles disappeared from the fossil record at the Eocene-Oligocene boundary, although the pollen of Rhoiptelea (Juglandaceae) is very Normapolles-like (Traverse 1988).
Details of wood anatomy, including the presence of chambered crystals in the axial parenchyma, that are common in this group (Carlquist 2002c) may properly be features of Fagales as a whole.
Ecology & Physiology. Taxa that have cluster roots, usually associated with an enhanced ability to take up phosphorus, and that are also associated with N-fixing bacteria, are scattered through this clade (Myricaceae, Casuarinaceae, Betulaceae-Alnus: Dinkelaker et al. 1995; Lambers et al. 2008).
In a number of taxa, including Casuarina spp., Betula alba and Myrica gale, the stomata were found to be blocked by wax deposits, perhaps allowing some photosynthesis while reducing transpiration more (Wulff 1898).
Chemistry, Morphology, etc.. Normapolles pollen is oblate in shape and triaperturate with protruding, elaborate and strongly thickened apertural regions, the apertures themselves often being formed from expansions of the granular infratectal layer (see also Feuer 1991); the result is that the pollen is more or less triangular in transverse section. The apertures have internal pores and externally short colpi or pores. The wall is usually tectate-granular, but there is sometimes an atectate polar zone, the surface being almost smooth to finely spinulate to rugulate. The infratectum is granular (details from Friis et al. 2003a).
[Myricaceae + Juglandaceae]: chains of crystal-containing cells in the wood; sieve tube P-protein bodies 0; peltate glandular hairs +; stipules 0; 1 flower/bract; stigma lamellular/laciniate; ovule single [per flower], straight, erect, integument entirely free from nucellus; x = 8, longest chromosome with (sub)median secondary constriction.
Age. This node has been dated to ca 47.1 Ma by Tank et al. (2015: Table S2), and as early as 38-36 Ma by Wikström et al. (2001) or (43-)32, 29(-11) Ma by Bell et al. (2010), or considerably older, ca (80.5-)78.6(-76.6) Ma by H. Zhou et al. (2021) and ca 85.3 Ma by T.-R. Wang et al. (2024), while the age of a node [Myricaceae + The Rest] is estimated at (94.6-)90.4(-85.0) Ma (X.-G. Xiang et al. 2014a).
Evolution: Divergence & Distribution. The evolution of features of inflorescence and ovule is particularly difficult to understand; they could be synapomorphies of this clade as a whole (as above), or be independent apomorphies of Myricaceae and Juglandaceae-Juglandoideae. Herbert et al. (2006) discuss possible synapomorphies around here.
Chemistry, Morphology, etc.. The leaf teeth in Myricaceae in particular are intermediate in "type" and venation characters do not distinguish sharply between Cunoniaceae and Fagales (see Hickey & Taylor 1991).
MYRICACEAE Kunth, nom. cons. - Back to Fagales
Plants dioecious or monoecious; staminate flowers: A (opposite P); carpelate flowers: G [(3)], ?superior to inferior [clearly axial]; ovule basal, straight; fruit a drupe; seed ?pachychalazal, testa ± thickened; endosperm slight or 0; n = 8, 12, x = 8, nuclear genopme [1 C] (0.042-)0.611(-f8.842) pg.
4 [list]/57: two groups below. ± Cosmopolitan, scattered (map: Hultén 1958; van Balgooy 1974; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010).
Age. An age of (81.7-)69.7(-60.4) Ma for crown-group Myricaceae is suggested by X.-G. Xiang et al. (2014a).
Fossil pollen attributed to the family is Cretaceous-Cenomanian (97.5-91 Ma) in age (H.-L. Li et al. 2015).
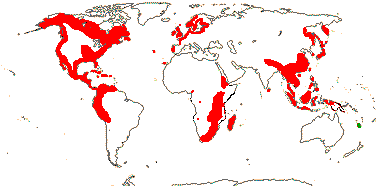
1. Canacomyrica monticola Guillaumin —— Synonymy: Canacomyricaceae Doweld
?Nodes; P connate, 6-lobed; staminate flowers: A 6; pistillode +; carpelate flowers: staminodes +; ovule ?bitegmic, micropyle much elongated; ?fertilization; in fruit micropyle becoming still more elongated, recurved.
1/1. New Caledonia. Map: green.
(Plant deciduous); cluster roots +, N-fixing Frankia +; nodes also 1:1; lamina (pinnatifid), vernation conduplicate to curved or revolute, (?stipules foliaceous, lobed - Comptonia); P 0; staminate flowers: A 2-8(-20); carpelate flowers: staminodes 0; ovule quite well developed at pollination, unitegmic, integument vascularized, 3-7 cells across, parietal tissue 4-9 cells across, nucellus apex exposed/not, central strand in supra-chalazal tissue; megaspore mother cell single; fertilization porogamous.
3/55: Morella (46). ± Cosmopolitan but scattered, not in the southern half of South America nor south and east of New Guinea (map: above, red). [Photos - Collection.]
Age. The crown-group age of this clade is estimated at around 24.9 Ma (Sauquet et al. 2012), (58.6-)54.3(-49.6) Ma (X.-G. Xiang et al. 2014a), or (95.7-)71, 69.8(-55.1) Ma (H.-L. Li et al. 2015).
Evolution: Divergence & Distribution. Comptonia, now restricted to eastern North America, was widespread in the Northern Hemisphere (including Greenland) in the Caenozoic, with some records dating from the Late Cretaceous; most of the records are of the distinctive foliage which has sometimes been identified as a fern (Liang et al. 2010; Sadowski et al. 2019). Canacomyrica, now endemic to New Caledonia, has been reported from New Zealand as recently as 17-11 Ma (D. E. Lee et al. 2001) while fossil myricaceous pollen is reported from S.W. South Africa in deposits ca 15 Ma (Coetzee & Praglowski 1984).
Ecology & Physiology. Myricaceae - but not Canacomyrica - are associated with N-fixing Frankia and cluster roots are also developed; mycorrhizae appear to be absent (Hurd & Schwintzer 1997). Carboxylate exudation may help in phosphorus acquisition (Lambers et al. 2012b).
Pollination Biology & Seed Dispersal. Although fertilization is porogamous, it is somewhat delayed, rather as in other Fagales. The pollen tubes pause in their growth on the nucellar surface, which is at least initially exposed (according to some accounts), and this pause has been compared with the pause at the chalaza that occurs in other members of this clade; this method of fertilization has been called pseudoporogamy and may be derived (Sogo & Tobe 2006a, b: ?two characters - micropyle +/0, pollen tube enters the ovule through the micropyle). The flowers of plants of some species differ in sex from year to year (Jurzyk 2005 and references).
Plant-Bacterial/Fungal Associations. Both AM and/or ECM associations have been reported from Myricaceae (e.g. Rose 1980) - do the fungi come from nearby ECM plants?
Chemistry, Morphology, etc.. Hickey and Taylor (1991) showed a leaf of Canacomyrica in which two adjacent teeth were cunonioid and rosid respectively.
Bracteoles may be present or not. Although the ovary appears to be superior, as in Comptonia, it is often so highly reduced that any traces of its inferior construction would be lost, however, in Canacomyrica, from New Caledonia, staminodes are borne on top of the ovary and there is a six-lobed perianth. In some species of Myrica the ovary is invested by tissue from a meristem developing below the flower, even below the bracteoles, which are then borne on the flower (Kershaw 1909). The flowers of Canacomyrica have three "bracts" (Herbert et al. 2006) - do these represent a floral bract plus two bracteoles/prophylls? Kubitzki (1993b) draws the ovule of Canacomyrica as being basal, straight, and with a much elongated integument forming an apical tube (see also Macdonald 1989); Herbert et al. (2006) simply describe the ovules as being bitegmic.
For general information, see Kubitzki (1993b), for chemistry, see Hegnauer (1969, 1990), for wood anatomy, see Carlquist (2002c), for floral morphology, very tricky to interpret, see Macdonald & Sattler (1973) and Macdonald (1978, 1979 and references) and Leroy (1957: Canacomyrica), for pollen, see Coetzee and Praglowski (1984), for ovules and fertilization, see Kershaw (1909).
Phylogeny. Relationships within Myricaceae are likely to be [Canacomyrica [Comptonia + The Rest]] (Herbert et al. 2006; X.-G. Xiang et al. 2014a: support strong; M. Sun et al. 2016), which, depending on the phylogeny of the order, could have interesting implications for character evolution. Huguet et al. (2004) found the relationships [[Myrica + Comptonia] other Myrica], the two clades being colonized by different strains of Frankia. Conflicting generic relationships were found in analyses of plastome data by Y.-Y. Yang et al. (2021).
Classification. If the topology of Huguet et al. (2004) holds, then the limits of Myrica will have to be either expanded or contracted.
JUGLANDACEAE Perleb, nom. cons. - Back to Fagales
Xylem with gelatinous fibres; buds lacking scales; leaves compound, odd-pinnate; P 4; pollen scabrate; carpels median, apically 1-locular, stigmas decurrent, ± recurved; endosperm 0; x = 16 (?8), nuclear genome [1 C] (0.029-)0.699(-16.844) pg.
9 [list, to tribes]/51, three groups below. North temperate, also Malesia and the Andes.
Age. Crown-group Juglandaceae are estimated to be around 85.8 Ma (Sauquet et al. 2012), (96.4-)79.9-(71.2) Ma (X.-G. Xiang et al. 2014a; see also J.-B. Zhang et al. 2013), ca 81.4 Ma (Mu et al. 2020), ca 68.6 Ma (H. Zhou et al. (2021) or (129.5-)105.1(-90.5) Ma (Q. Zhang et al. 2021, p. 247: "origin of Juglandaceae").
1. Rhoipteleoideae Reveal - Rhoiptelea chiliantha Diels & Handel-Mazzetti —— Synonymy: Rhoipteleaceae Handel-Mazzetti, nom. cons.
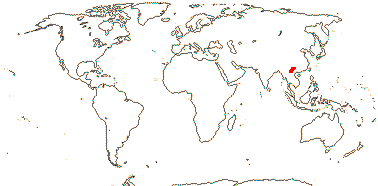
Plant deciduous; chemistry?; cork?; sieve tube protein bodies?; resinous glands +; leaves two-ranked, leaflets alternate, stipulate, asymmetrically caudate; inflorescence branched-catkinate; flowers in triads, central flower perfect; A 6, with small glands; pollen 3-colporate, ectoapertures elliptic, exine folded [plicate], vestibulum 0, endexine lamellate, thick at the apertures; G [2], adaxial member fertile, stigmas commissural, flattened; ovule campylotropous, bitegmic, micropyle endostomal, outer integument ca 4 cells across, inner integument 4-5 cells across, parietal tissue 2-5 cells across, ?suprachalazal tissue; ?fertilization; fruit a samaroid nut, wings lateral; P persistent; testa?; n = 8; ?seedling.
1/1. Southwest China, northern Vietnam. Map: from Fu (1992).
Age. Cretaceous fossil flowers like Dahlgrenanthus and Caryanthus have been compared with Rhoiptelea (Friis et al. 2006a). Fossil pollen of the monotypic Rhoiptelea, now known from southwestern China and adjacent Vietnam, has been reported from eastern North America (Fu 1992).
[Engelhardioideae + Juglandoideae]: flavones, naphthoquinones [inc. plumbagin], raffinose and stachyose [phloem exudate] +; xylem with banded parenchyma; nodes also 5:5; leaves spiral, leaflet (margins entire); (inflorescence branched); (P 0-3); staminate flowers: A 4-many; pollen 2-20 porate, apertures usu. elongate, endexine homogeneous, not at the apertures; pistillode +; carpelate flowers: ?staminodes; [(G 3, 4)], when 3, median member adaxial, loculi ± divided [false septum], (stigmas not decurrent), (commissural); ovule borne at the top of the septum, straight, integument 6-10 cells across, (lobed), parietal tissue 3-11 cells across; megaspore mother cells nume/rous; (fertilization porogamous); fruit samaroid, winged by persistent bract/bracteoles, (drupaceous, nut), pericarp intrusive; seeds (large [>1.5 cm long]), pachychalazal; endosperm very slight or 0, cotyledons much folded; genome duplication, x = 16, second longest chromosome with (sub)median secondary constriction.
Ca 8/50. North Temperate, S. to Argentina and Malesia. Photo: Collection.
Age. The crown group age of this clade is around 77.1 Ma (J.-B. Zhang et al. 2013), 72.6 Ma (Sauquet et al. 2012), (75.9-)70.6(-64.1) Ma (X.-G. Xiang et al. 2014a), ca 79.2 Ma (Mu et al. 2020) or ca 60.7 Ma (H. Zhou et al. 2021).
The oldest fossils that have been placed in Juglandoideae are some 98-83 (Budvaricarpus: see Hermanová et al. 2011) or 88-73 (Crepet et al. 2004 for references) or ca 78 (Manos et al. 2007: the age of Caryanthus) Ma. However, both genera mentioned are placed outside crown-group Fagales in the combined molecular-morphological study of Larson-Johnson (2015).
2. Engelhardioideae Iljinskaya —— Synonymy: Engelhardiaceae Reveal & Doweld
Plant (deciduous); vessel elements with simple and scalariform perforation plates; leaf parenchyma (with druses); leaves (opposite), even-pinnate, leaflets usu. entire, (vernation involute or ± conduplicate - Alfaroa); carpelate flowers: inflorescence catkinate; bracts 3-lobed, bracteoles 0-2, ± abaxial, adnate to lower half of ovary; nuts with fibrous cells in wall.
3-4/14. Himalayas and southern China to Malesia, Mexico to Colombia (?Bolivia). Map: see Meusel et al. (1965), Manchester (1987) and H. Zhou et al. (2021: Fig. 1).
Age. The crown-group age of this clade is around 36.2 Ma (Sauquet et al. 2012); estimates in J.-B. Zhang et al. (2013) are ca 49.5 Ma, those in Manos et al. (2007) are around 44 Ma, in X.-G. Xiang et al. (2014a) are (49.4-)37.7(-28) Ma and in Q. Zhang et al. (2021) are (99.3-)89.2(-69.5) Ma (see also below).
3. Juglandoideae Eaton
Plant deciduous; vessel elements with simple perforation plates alone; (pith septate); plant heterodichogamous; staminate flowers: bracts 1-lobed; pollen usu. at least 26 µm across [17-26 µm is the plesiomorphic condition], (>4 porate); carpelate flowers: bracts unlobed, bracteoles [if present] usu. lateral, adnate at least to half the ovary; (G collateral); integument vascularized [?level], (nucellar cap ca 2 cells across); nuts with sclereids (fibres) in wall; n = (11, 12, 14, 15), polyploidy common.
5/35. Temperate N. Hemisphere (only 1 sp. in Europe), Central America and down the Andes (Souza & Lorenzi 2012), c.f. versions prior to vii.2014. Map: see Meusel et al. (1965), Manchester (1987) and H. Zhou et al. (2021: Fig. 1).
Age. Crown-group Juglandoideae have been dated to around 65 Ma (Sauquet et al. 2012), while Manos et al. (2007) gave a range of estimates between about 52 to ca 67 My; there is an estimate of ca 66.6 Ma in J.-B. Zhang et al. (2013), (69.7-)66.6(-62.6) Ma in X.-G. Xiang et al. (2014a), (76.6-)72.0(-67.7) Ma in Mu et al. (2020), ca 57.9 Ma in H. Zhou et al. (2021), of (106.9-)101.3(-77.9) Ma in Q. Zhang et al. (2021) and of ca 58.6 Ma (T.-R. Wang et al. 2024: Platycarya + the rest). On the other hand, Bell et al. (2010) had suggested that Carya and Juglans (on the two main branches of Juglandoideae) diverged only (8-)4(-1) Ma and Wikström et al. (2001) estimated a mere 7-6 Ma.
Polyptera manningii, a well-understood fossil common in what is now S.W. Wyoming in deposits as much as 65 Ma, is assignable to Juglandoideae (Manchester & Dilcher 1997; Donovan et al. 2016) and fossils of Cyclocarya of a similar age (Pigg et al. 2023).
3A. Platycaryeae Nakai - Platycarya Siebold & Zuccarini —— Synonymy: Platycaryaceae Doweld
(Leaves simple); inflorescences erect; staminate flowers: bracteoles 0; K 0; carpellate flowers: K 2, adnate to bracteoles; style 0, stigmas carinal; infructescence cone-like; fruit 3-6 mm long, narrowly 2-winged; germination epigeal.
1/2. China, Vietnam, Korea, Japan.
Age. Pollen of the fossil Platycarya platycaryoides is known from deposits ca 56 Ma from the Bighorn Basin, Wyoming - there were range shifts and local extinctions then - and slightly earlier from late Palaeocene fossils in Europe (Korasidis et al. 2022).
3B. Juglandeae Reichenbach
3B1. Juglandineae D. E. Stone & Manos
Pith chambered; pollen pantopororate; (fruit unwinged).
3/21: Juglans (21). S.E. Europe to Japan, S.E. Canada to California and in W. South America south to Argentina, not in Brazil.
Age. This clade is estimated to be (70.3-)65.4(-61.4) Ma (Mu et al. 2020).
3B2. Caryineae D. E. Stone & Manos - Carya Nuttall
(Buds naked); staminate flowers: K usu. 0; carpellate flowers: bracteoles 3; K 0; style 0; stigmas commissural, stigmatic disc +, 4-lobed; fruiting spike erect; fruit a drupe-like nut; germination hypogeal.
1/18. Assam to Vietnam and China, also Mexico north to Canada.
Age. Crown-group Carya is estimated to be (53.8-)31.0(-10.9) Ma (Mu et al. 2020) or ca 25 Ma (H. Zhou et al. 2021).
Evolution: Divergence & Distribution. A number of extinct genera, some showing very interesting combinations of characters, are known from early Caenozoic deposits in North America, and the family was very diverse there (e.g. Manchester & Dilcher 1982; Manchester 1991; Elliott et al. 2006; Q. Zhang et al. 2021). Pollen of Platycarya, a genus now known only from eastern Asia, has been found in deposits from the Palaeocene-Eocene transition in both North America and Europe (Korasidis et al. 2022).
J.-B. Zhang et al. (2013) found that all the fossil genera ended up in the crown groups of Juglandoideae or Engelhardioideae. Several extant genera found fossil in North America and especially Europe no longer grow there (Manchester 1987); for the early Caenozoic fossil history of what are now East Asian endemics, see Manchester et al. (1987, 2009) and Q. Zhang et al. (2021). There are also reports of Engelhardioideae (as Alatonucula, also pollen records) from the early Eocene of Patagonian Argentina, far to the south of the current distribution of the subfamily, in deposits at least 47.8 Ma (Hermsen & Gandolfo 2016). Cyclocarya, now endemic to China, is known pretty much throughout the Northern Hemisphere, the oldest fossils being from Palaeocene deposits in western North America (J.-Y. Wu et al. 2017).
Details of diversification in Juglandaceae are somewhat unclear, and there is great variation in the age estimates for Juglandoideae in particular (Manos et al. 2007 - see above). Taxa with biotic dispersal of their propagules may have evolved in the early Caenozoic from wind-dispersed taxa (Tiffney 1986a; Larson-Johnson 2015). Lyson et al. (2019) found an increase in the diversity of juglandaceous Momites pollen in Colorado very soon (ca 300,000 years) after the arrival of the Chicxulub bolide (the pollen is known from the latest Cretaceous onwards), possibly reflecting the evolution of large-fruited taxa that were eaten by Palaeocene periptychid mammals with appropriate dentition. However, information in Larson-Johnson (2015: Table S6) suggests that wind-dispersed taxa like Cyclocarya and Polyptera are known from the Palaeocene 61.6-55.8 Ma and 63.3-56.8 Ma respectively, while animal-dispersed taxa like Beardia, Carya and Juglans did not appear until rather later 48.6-33.9 or ca 43.8 Ma, so there is no obvious connection with the bolide event. Diversification in the Rhoiptelea clade has decreased (!: Larson-Johnson 2015). For more on the distribution of the family, see H. Zhou et al. (2021).
Given the diversity of the family in southeast Asia, a - or perhaps the - question is, where did the family originate? Do extant taxa + relationships and the copious fossil record fit the Arcto-Tertiary, the boreo-tropical, or the out-of-Asia hypothesis (see Q. Zhang et al. 2021)? Y.-G. Song et al. (2019: fossil-based node constraints) looked at diversity of the family over time in the context of changing climates, etc., proposing that the family originated in Europe-North America, although the main generic-level diversity is now in China and surrounds, species diversity centres on the U.S.A., taxa with tropical proclivities are perhaps most notable in the New World, while there are practically no Juglandaceae left in Europe. In another study integrating the fossil record - especially copious for Juglandoideae - with phylogeny (4 plastid genes and nuclear ITS), Zhang et al. (2021) addressed this issue in edatil. They suggest that the crown-group ancestors of both Juglandoideae and Engelhardioideae were North American, and the subsequent distributional history entailed an increase in temperature tolerances in the former and higher precipitation tolerances in the latter. There were also a large number of emigration and immigration events that were aided by land bridges across the Atlantic and Pacific (see e.g. Tiffney 1985b; Tiffney & Manchester 2001; etc.), and also extinctions; the boreo-tropical hypothesis seemed to be the best fit. Overall, there were ca 43 emigration events, 33 immigration events, and ca 50 extinctions involving North America (= 33 immigrations→ N. America →43 emigrations, 50 r.i.p.), see also 44 immig.→ Europe →40 emig., 59 r.i.p. and 37 immig.→ East Asia →19 emig., 32 r.i.p., major interchanges involving first North America, then from the early Oligocene to early Miocene, Europe, and after that East Asia. And all this in one quite small family!
Manos and Stone (2001) put morphology in the context of phylogeny. Friis et al. (2011) noted that some fossils that are very similar to Rhoiptelea chiliantha have a half-inferior ovary; the ovary of Rhoiptelea is presumably secondarily superior.
Ecology & Physiology. Oreomunnea may dominate forests in Panama (references in Peay 2016). Interestingly, it seems that despite strong negative plant-soil feedback at the seedling stage, monodominant stands of Oreomunnea mexicana formed because the ectomyccorhizal fungal associated with the plant could obtain N directly from organic matter (Corrales et al. 2016).
Pollination Biology & Seed Dispersal. There is a form of heterodichogamy in several [Engelhardioideae + Juglandoideae] (Renner 2001 for references; Fukuhara & Tokumaru 2014). Platycarya (Engelhardioideae) is distinctive in having sticky pollen and strongly scented flowers, which suggests insect, perhaps thrip, pollination (Li et al. 2005 and references; Fukuhara & Tokumaru 2014).
For fertilization, see e.g. Schanderl (1964), Sartorius et al. (1984), Luza and Polito (1991) and Sogo and Tobe (2008) and references; chalazogamy is not constant in Juglandoideae, and fertilization in Rhoipteleoideae is unknown.
Plant-Animal Interactions. Carya is an important host plant for lepidopteran larvae in the contiguous United States of America, being included in the top 10 such plants (Narango et al. 2020).
Plant-Bacterial/Fungal Associations. Juglans, at least, is not ectomycorrhizal (Tedersoo & Brundrett 2017), although Alfaroa costaricensis does seem to have ECM associations (Corrales et al. 2018).
Genes & Genomes. A duplication of the whole genome can be placed at the [Engelhardioideae + Juglandoideae] node (Luo et al. 2014). Juglans regia is an hybrid between the American and the Asian lineages of the genus and is some 3.4 Ma in age; the native range of the species is from the Caucasus to Kashmir, so the range of at least one of its parents has changed... In J. cinerea there has been introgression from an Asian butternut into an American black walnut; this probably happened in North America. For karyomorphology, see Oginuma (1999).
Economic Importance. Juglans regia, the walnut, is widely cultivated, being noted both for its nut-like fruits and the quality of its wood.
Chemistry, Morphology, etc.. The seedlings of genera with opposite leaves (Alfaroa, Oreomunnea) have spiral leaves.
Triads of flowers are found as abnormalities in the [Engelhardioideae + Juglandoideae] clade (Manning 1940) and of course they are the normal condition in Rhoiptelea (see above), although only the central flower is fertile. The fossil Budvaricarpus has similar triads that are surrounded by a common bract (Hermanová et al. 2011). Perfect flowers are also known from Platycarya, although normally the cone-like inflorescences have staminate and carpelate flowers mixed; here the bracts are not part of the fruit (Li et al. 2005). For discussion on the nature of the bract/perianth-type structures associated with the flowers, especially the carpelate flowers, see Lin et al. (2016); in Juglans regia, for example, there is a more or less annular rim encircling the carpelate flower, and a bract develops from this in the abaxial position and a single bracteole in a (sub)adaxial position - rather odd.
The staminate flowers of Juglans regia and Engelhardia spicata are monosymmetric (Lin et al. 2016). The pollen surface is almost microspinulose (Stone & Broome 1975). The stigma may be commissural or not and the orientation of the carpels varies (Manchester 1987; Manos & Stone 2001 - summaries). All three gynoecia of a Budvaricarpus triad are shown as having carpels with the same orientation, although this is supposed to be a cymose unit (Hermanová et al. 2011). Although the ovule of J. regia normally has a single integument, a second integument, probably the outer integument, may also develop (Schanderl 1964).
For general information, see Manchester (1987), Wu and Kubitzki (1993), Stone (1993), Schaarschmidt (2014) and Kozlowski et al. (2018), for chemistry, see Hegnauer (1966, 1989), for pollen, see Stone and Broom (1975), for embryology, see Nicoloff (1904) and Boesewinkel and Bouman (1967). For Rhoiptelea in particular: chemistry, see Hegnauer (1990), pollen, see Stone and Broome (1971) and Friis et al. (2006a), embryology, see Z.-Y. Zhang et al. (1994: micropyle described as bistomal, but c.f. micrograph), breeding system, Sun et al. (2006).
Phylogeny. For relationships in the family, see Gunter et al. (1994) and especially Manos and Stone (2001); Engelhardia is paraphyletic, but the monophyly of Engelhardioideae and Juglandoideae is well supported. Platycarya is often sister to other Juglandoideae, but its position there was not clear, nor was the monophyly of Juglans (Manos et al. 2007; see also Sauquet et al. 2012; Larson-Johnson 2015). Mu et al. (2020) recovered the relationships [Hicorieae [Platycaryeae + Juglandeae]]; plastid- and nuclear-based topologies tended to differ within tribes, but otherwise were similar and strongly supported. Engelhardia s. str. is sister to the rest of Engelhardioideae (Manos & Stone 2001; Manos et al. 2007). Y.-Y. Yang et al. (2021) found that plastome data suggested conflicting generic relationships in Engelhardioideae, as did H. Zhou et al. (2021) - nuclear data suggested tribal relationships in Juglandoideae were [Platycaryeae [Caryeae + Juglandeae]] (see above), while chloroplast data reversed the position of the first two and relationships differed yet again when using mitochondrial data; only Alfaropsis of Juglandoideae was included in the study, so its position there had no bearing on its generic status (c.f. Zhou et al. 2021). Y. Liu et al. (2024) sampled much of the family in their comparison of barcoding methods; they used plastid DNA and found that Oreomunnea was embedded in Engelhardia.
Classification. Including Rhoipteleaceae in Juglandaceae s.l. was optional in A.P.G. II (2003); the two were merged in A.P.G. III (2009).
Manos and Stone (2001) provide a revised classification of [Engelhardioideae + Juglandoideae], and there have since been some adjustments of generic limits.
[Casuarinaceae [Ticodendraceae + Betulaceae]]: dihydroflavonols +[?]; pollen tubes branched; stigmas elongate.
Age. Distributions of ages of this node are bimodal and non-overlapping - some 40.7 Ma in Tank et al. (2015: Table S2), 36-35 Ma (Wikström et al. 2001) and (36-)29, 27(-18) Ma (Bell et al. 2010), or up to 81-71 Ma (Cook & Crisp 2005), ca 87.2 Ma (Sauquet et al. 2012), (88.6-)82.8(-74.7) Ma (X.-G. Xiang et al. 2014a), (97-)92.5, 90(-84.3) Ma (H.-L. Li et al. 2015) and ca 74.2 Ma (T.-R. Wang et al. 2024).
The age in Cook and Crisp (2005) was driven in part by the assignment of the fossil Endressianthus, ca 71 Ma, to the stem of this clade.
Evolution: Pollination Biology & Seed Dispersal. Sogo and Tobe (2008) suggest that the chalazogamous fertilization that occurs in all families of this clade is similar down to the details of where the pollen tube growth is temporarily delayed. Pollen tubes may branch when they have "lost their way" (Sogo & Tobe 2008: p. 624).
CASUARINACEAE R. Brown, nom. cons. - Back to Fagales
Cluster roots, fungus-associated root nodules +, N-fixing Frankia; flavonols, biflavonoids +, flavones, myricetin 0; banded apotracheal parenchyma +, rays broad, compound (0); cauline endodermis +; cork cambium both superficial (sulci) and apparently deep-seated on the one stem; nodes 1:1, leaving central stele two internodes before departure to leaf; stomata [hidden] usu. tetracytic, transversely oriented; leaves 4-whorled (-20-whorled), adnate to and entirely covering stem [= phyllichnia], margins entire, stipules 0; plant monoecious or dioecious, inflorescences capitate-spicate, one flower/bract, bracts and bracteoles ± well-developed; staminate flowers: P 2 ["inner bracteoles"], median; A 1, filament incurved in bud, anthers ± longer than connective, thecae separate or not; tapetal cells uni(bi-)nucleate; pollen infratectum not granular; pistillode 0; carpelate flowers: bracteoles large; P 0; staminodes 0; abaxial G alone fertile, stigma wet; ovules bitegmic, micropyle endostomal or nucellus apex exposed, outer integument 3-4 cells across, inner integument 2-3 cells across, parietal tissue 5-8 cells across, central tracheids in supra-chalazal tissue, vascular bundle branched in chalaza; megaspore mother cells numerous, embryo sacs several, chalazal caecum +, (reaching the funicle); fruit a samara; seed coat adnate to pericarp; endosperm 0; n = 8[Gymnostoma]-14, x = 8 (?9), nuclear genome [1 C] (0.064-)0.645(-6.503) pg; germination epigeal, phanerocotylar.
4 [list]/95. South East Asia and Malesia to the S.W. Pacific, esp. Australia. Map: from Coetzee and Muller (1984) and Fl. Austral. vol. 3 (1989); fossils blue, see references below. [Photo - Collection.]
Age. The beginning of divergence within Casuarinaceae has been dated to (65.7-)56.2(-45.3) Ma (X.-G. Xiang et al. 2014a).
Material from the Patagonian Eocene of ca 52.2 Ma has been placed in Gymnostoma (Zamaloa et al. 2006) and pollen of Casuarinaceae, presumably Gymnostoma (as Halogoacidites harrisii, initially placed in Haloragaceae) is known from Palaeocene-Eocene South America, and is sometimes very abundant (Carpenter & McLoughlin 2025).
Evolution: Divergence & Distribution. Despite the currently quite restricted distribution of Casuarinaceae, fossils of the family are known from Caenozoic deposits in South Africa, the Ninety East Ridge in the Indian Ocean, Ross Bay, Antarctica, Chile and Argentina (Coetzee & Muller 1984; Coetzee & Praglowski 1984; Hill & Brodribb 2001). Casuarinaceae were especially prominent in the lower Middle Miocene of New Zealand (Lee et al. 2001; Pole 2003; Vanner 2019). All in all, a very wide distribution in the southern hemisphere compared to that of today (see also see above).
The diversification rate increased in Allocasuarina (Bouchenak-Khelladi et al. 2015), and this may be associated with the aridification of the Nullarbor Plain some 14-13 Ma that separated eastern and western clades (Crisp & Cook 2007). This increase happened within the last 10 Ma (Xing et al. 2014).
Gymnostoma seems to belong to a group of plants with a south South American - Australian/Malesian connecton (see elsewhere for more details).
Ecology & Physiology. A number of Casuarinaceae are halophytes (Moray et al. 2015). Casuarina is a fire-adapted dominant tree in some parts of Australia (references in Lawes & Neumann 2022). There was a switch from scleromorphy (Gymnostoma) to xeromorphy, perhaps some time in the Miocene (Hill & Brodribb 2001). R. S. Hill et al. (2020) suggested that initially both light and soil nutrients, especially phosphorus, were low in places where Casuarinaceae grew, but water supply was not an issue, later water became scarce and the plants grew in high-light conditions - hence scleromorphy→xereomorphy. Dörken et al. (2018) and Hill et al. (2020) discuss the adnation of the leaves to the stem (such leaves = phyllichnia), only the tips being free; the stomata become restricted to the channels between the leaves, and their orientation is at least sometimes transverse. It is these adnate leaves that provide the photosynthetic tissue for the plant, as also in Callitris (Cupressaceae), although at first sight one would say that the stem was green. Stems are commonly green in plants with reduced leaves, but in such cases the photosynthetic tissue is indeed of cauline origin.
Nitrogen-fixing is known from the family, and Casuarina oligodon plays an important role in agriculture in parts of montane New Guinea, both in providing firewood and in fixing N for the sweet potato crop (Golson & Garner 1990). Cluster roots, probably enhancing phosphorus uptake, are also common; cluster roots, gram-positive N-fixing actinomycetes, and ECM make a very unusual combination (Shane et al. 2006; Lambers et al. 2008).
Pollination Biology. Riley (2020) suggests that the samara is extruded from the infructescence as the much-accrescent bracteoles incurve as they dry.
For the evolution of breeding systems in Allocasuarina, see Goldberg et al. (2017).
Plant-Bacterial/Fungal Associations. The receptor-like kinase gene SymRK involved in the common signaling symbiotic pathway is an integral part both of arbuscular mycorrhizal associations and Frankia nodulation in Casuarina glauca (Gherbi et al. 2008). The N-fixing Frankia strains of Casuarinaceae live in a close to obligate association with their hosts and have not been found in the soil outside the native range of the family (Norman et al. 2006). See Subbarao and Rodríguez-Barranco (1995) for microbes associated with Casuarina.
In New Caledonian Gymnostoma, at least, there are root nodules, modified roots, that are formed in association with ECM fungi (Duhoux et al. 2001), however, species like C. glauca may have AM associates (Duponnois et al. 2003; see also Rose 1980) - note that these nodules differ from those in Podocarpaceae, etc. (Russell et al. 2022). Tedersoo and Brundrett (2017) suggest that the ECM habit is commonest in Allocasuarina, but a number of species of both Allocausarina and Casuaria are dual mycorrhizal plants (Teste et al. 2019: Table S2).
Chemistry, Morphology, etc.. Do flavananols occur in Casuarinaceae? The starch grains are distinctive (Czaja 1978). Crystals have been observed in the cuticle (Flores & Moseley 1982). For cork cambium initiation, see de Cordemoy (1923 and references). For nodal anatomy, with the leaf traces traversing the cortex for the internode before exiting to a leaf, see Balfour and Philipson (1962). Note that what is called a leaf in the characterization is the free part of the lamina remaining after the adnation of the rest of the leaf to the stem, much of the course of the "free" vascular bundle in the stem in fact being in the adnate portion of the leaf (see also R. S. Hill et al. 2020); the characterization above should be interpreted accodingly. There are no intermediates between the cotyledons and scale leaves, and there is a whorl of buds at the cotyledonary node (Hwang & Conran 2000).
The texture of what some have called the outer and inner bracteoles of the staminate flowers is very different; the latter are called the perianth here, however, inflorescence development is clearly very complex (Flores & Moseley 1982). In a floral diagram, Swamy (1948b) illustrated collateral carpels, but he talked about the carpels as if they were superposed (as also in Swamy 1955). The ovules are described as being orthotropous by Johnson and Wilson (1993), but they are drawn as anatropous by Treub (1891), Swamy (1948b) and Flores and Moseley (1982). Although Swamy (1944, 1948b) mentioned quite well developed parietal tissue in the ovule, in the first article a well-developed nucellar cap is illustrated. The nucellus of Casuarina montana (= C. junghuhniana) protrudes broadly between the integuments, so the apex of the nucellus is exposed and there is a sort of nucellar beak; this is because of the great development of the megaspore mother cells in the lower part of the nucellus (Swamy 1948).
See also de Cordemoy (1923), Rogers (1982a), Dilcher et al. (1990) and Johnson and Wilson (1993) for general information; also Hegnauer (1964, 1989: chemistry), Poisson (1874: stem anatomy), Moseley (1948: wood anatomy), Coetzee and Praglowski (1984: pollen) and Sogo et al. (2001: fertilization).
Phylogeny. Steane et al. (2003) provide a phylogeny of the family. Gymnostoma is sister to the rest (see also X.-G. Xiang et al. 2014a; M. Sun et al. 2016) and it also has many plesiomorphous features. Both its carpels are fertile (although this feature is likely to be an apomorphy, given the situation in the rest of the order), with 2 ovules/carpel, its stem stomata are not hidden, and perhaps n = 8.
Classification. Although the monophyly of Causarina s.l. has never been in doubt, it has been split into four genera, themselves monophyletic.
[Ticodendraceae + Betulaceae]: sclereid nests in bark, cells with large rhomboidal crystals; mucilage cells +; leaves two-ranked; anther thecae ± separate.
Age. The age of this node is about 302-211 Ma (Forest et al. 2005: huge confidence intervals), around 76.7 Ma (Sauquet et al. 2012), about 143 or 88 Ma (Grimm & Renner 2013), or (80.3-)74.0(-66.9) Ma (X.-G. Xiang et al. 2014a).
TICODENDRACEAE Gómez-Laurito & L. D. Gómez P. - Ticodendron incognitum Gómez-Laurito & L. D. Gómez P. - Back to Fagales
Buds not perulate?; foliar hypodermal idioblasts +; hairs T-shaped, unicellular, not glandular; lamina margin serrate, stipules encircling the stem; plant (polygamo-)dioecious; staminate flowers: inflorescence erect; P 0; A 8-10+, filaments = or longer than anthers; carpelate flowers: flower single; ?P minute, connate; (staminodes +); G ?tangentially arranged, with deeply lobed loculi; ovules hemitropous, integument 20-30 cells across, parietal tissue ca 6 cells across, nucellar cap 0, ?supra-chalazal tissue; fruit large, drupaceous, stone longitudinally ridged; integument well vascularized, exotestal cells initially radially elongated, all cells ± thick-walled and tanniniferous; endosperm development?; n = 13, x = 7 (?8).
1/1. Central America. Map: from Hammel and Burger (1991), fossil locations from Manchester (2011): blue). [Photo - Fruit.]
Evolution: Divergence & Distribution. Ferrignocarpus fruits assignable to Ticodendraceae have been found in Middle Eocene deposits from Oregon and Early Eocene deposits in the London Clay; they are up to ca 50 Ma (Manchester & Renner 2005; Manchester 2011). Chambers and Poinar (2014) report female flowers from Tertiary Dominican amber.
Plant-Bacterial/Fungal Associations. A variety of fungi, including ECM fungi, formed associations with Ticodendron in Panama, but on balance the genus seemed to be an AM plant (Corrales et al. 2018). On the other hand, however, Põlme (2018), working in Costa Rica, thought that it had ECM associations, although they were not very common. Corrales et al. (2018) found the sitaution here to be unclear.
Chemistry, Morphology, etc.. The nodes are trilacunar, judging from the condition in the outer cortex. Lersten and Horner (2008c) describe hypodermal idioblasts (they also occur below the midrib); these sometimes contain druses, and the authors suggest that they may be an apomorphy for the family. Almost all the leaf teeth are vascularized directly by secondary veins, unlike Betulaceae.
The bracteoles of the carpelate flowers have groups of vascularized scales in their axils, suggesting that the carpelate inflorescence has a fundamentally cymose construction. Interestingly, Feuer (1991) did not find much similarity between the pollen of Ticodendron and that of Juglandaceae, Fagaceae, Nothofagaceae, or even Betulaceae. The embryology is poorly known.
For information, see Kubitzki (1993b: general), Carlquist (1991a: wood), Tobe (1991: floral morphology), and Sogo and Tobe (2008: fertilization).
BETULACEAE Gray, nom. cons. - Back to Fagales

Flavones +; stratified phloem +; sieve tube P-protein usu. 0; lamina vernation usu. laterally or vertically conduplicate, margin doubly serrate, colleters +; plant monoecious or dioecious, catkinate, (bracts peltate); staminate flowers: cymule with <3 flowers, bracts 2/cymule; filaments ± divided), anthers longer than connective; pollen starchy; pistillode 0; carpelate flower: inflorescence catkinate; staminodes 0; G [(3)], collateral, septae incomplete, (short style +); ovules (1-4/carpel), collateral (superposed), lower part of integument vascularized; (megaspore mother cell 1); endosperm slight; n = 8, 11, 14, x = 7, nuclear genome [1 C] (0.032-)0.69(-14.781) pg; horizontal transfer of rps11 gene; sporophytic incompatibility system present; germination epigeal.
6[list]/145 - 2 groups below. North Temperate, to Andes and Sumatra. Map: from Meusel et al. (1965) and Hultén (1971). [Photo - Flower.]
Age. Crown-group Betulaceae may be around 131-115 Ma (Forest et al. 2005: large confidence intervals), about 64 Ma (Sauquet et al. 2012), 63-43 Ma (Grimm & Renner 2013: preferred age, one estimate twice this), (72.5-)64.4(-59.4) Ma (X.-G. Xiang et al. 2014a), (88.6-)85.9(-83) Ma (H.-L. Li et al. 2015: Alnus sister to the rest of the family) or (74.3-)70.5(-66.6) Ma (Z. Yang et al. 2019). A crown age of a mere 25 Ma was suggested by Quirk et al. (2012: stem - ?joins what - 36 My), ca 61.5 Ma (H. Zhou et al. 2021), (74.9-)69.5(-63.7) Ma by X.-Y. Yang et al. (2018), (93.2-)84.8, 60.2(-59.8) Ma by Helmstetter et al. (2019) and ca 54.0 Ma (T.-R. Wang et al. 2024).
The oldest fossils possibly assignable to the family are from 94-83 Ma (see Crepet et al. 2004 and Forest et al. 2005 for references); Bedellia pusilla, the plant involved, was described from east North America (Sims et al. 1999; not included in Larson-Johnson 2015). Normanthus and Endressianthus (see above), from the late Cretaceous of Portugal, may also be close to the root of the Betulaceae clade (Friis et al. 2003a, 2005, see esp. 2011), but the former is described as having perfect flowers with five perianth members alternating with the stamens and an ovary with parietal placentation (Schönenberger et al. 2001b). Endressianthus, a Normapolles-producing (c.f. Interporopollenites) angiosperm from the Late Cretaceous (Turonian) of Portugal, consists of fossils with imperfect flowers, the staminate flower apparently superior and the carpellate flower with an inferior bicarpellate ovary, and is perhaps root Betulaceae (Friis et al. 2003a, 2006a). Both are placed outside crown-group Fagales by Larson-Johnson (2015).
1. Betuloideae Arnott
Plant deciduous; (cluster roots + - Alnus), (N-fixing Frankia +), (ectomycorrhizal); spirally-thickened vessel elements 0, tracheids +; peltate glandular hairs +; (prophyll adaxial - A.), (buds naked - some A.); (leaves spiral - A.); bracts (4/cymule [inc. 2 tertiary bracts] - A.); staminate flowers: P 4; pollen grains (4< porate), endexine lamellate; carpelate flowers: (inflorescence cone-like - A.), flowers 2-3/cymule, abaxial realignment of second order bracts - Betula); P 0 (2); pollen pororate [A.]; parietal tissue 1-2 cells across, nucellar cap ca 2 cells across, suprachalazal tissue?; infructescence with woody or scaly bracts separate from fruit, nut small [<3 mm long], ± flattened, samaroid; genome size [1 C] 0.2-0.75 pg.
2/95: Betula (60/?97). N. hemisphere, and Alnus also to about 28o S. along the Andes in South America.
Age. The two genera diverged 25-19 Ma (Wikström et al. 2001) or (28-)20, 18(-10) Ma (Bell et al. 2010). Other estimates are older: 109-92 Ma (Forest et al. 2005: large confidence intervals), about 60-38 Ma (Grimm & Renner 2013: preferred age, some estimates almost double), (66.0-)59.6(-46.8) Ma (X.-G. Xiang et al. 2014a), (64.3-)61.1(-58.7) Ma (X.-Y. Yang et al. 2018) with the crown group ages of the two genera either about half or less than a third to a fifth of this respectively, (71-)61.8(-49.8) Ma (Z. Yang et al. 2019) or ca 50.2 Ma (T.-R. Wang et al. 2024).
The earliest records of the distinctive infructescences of Alnus are Palaeocene in age (X. Liu et al. 2014). Particularly complete material of Betula leopoldae was collected from deposits ca 52.1 Ma in British Columbia (Crane & Stockey 1987).
2. Coryloideae J. D. Hooker —— Synonymy: Carpinaceae Vest, Corylaceae Mirbel
Plant usu. deciduous; spirally-thickened vessel elements +, tracheids 0; lamina vernation conduplicate-plicate [Corylus]; staminate flowers: individuality ± obscure; bracts usu. 0 (+ - Corylus); P 0/+; A hairy; carpelate flowers: (inflorescence 1-5-flowered, ± erect - Corylus), usu. 2 flowers/cymule, bracts ± encircling flower; P primordium ± annular; integument 6-11 cells across, parietal tissue 4-8 cells across, nucellar cap 0-2 cells across, (suprachalazal tracheids +); embryo sac with chalazal caecum; pollen tube penetrates the chalazal/lateral pole of the embryo sac; fruit with accrescent leafy bracteoles [from one or two orders of branching]; nuts large to medium, not or little flattened; germination (hypogeal - Corylus).
4/50: Corylus (20). N. Temperate, South East Asia, Central America.
Age. The crown-group age of Coryloideae is estimated to be 87.5-70 Ma (Forest et al. 2005), about 39-22 Ma (Grimm & Renner 2013), (56.4-)54(-50.6) Ma (X.-G. Xiang et al. 2014a), (53.9-)36.8(-18.6) Ma (X.-Y. Yang et al. 2018), (48.9-)47.9(-46.9) Ma (Z. Yang et al. 2019), (78.7-)53.9(-36.2 Ma) (T. Zhao et al. 2019: C. + Ostryopsis), (63.2-)55.7, 49.5(-49.0) Ma (Helmstetter et al. 2019) or ca 28.3 Ma (T.-R. Wang et al. 2024: C. + Carpinus). See also Correa-Narvaez and Manchester (2022).
Evolution: Divergence & Distribution. Correa-Narvaez and Manchester (2022) examined the diversity of fruits in the some dozen of so species of Palaeocarpinus, a genus widely distributed in the Northern Hemisphere from the Palaeocene onwards. The fruits show some similarity to those of Corylus, somewhat more to those of Carpinus. Greenwood et al. (2016 and references) discuss a number of Betulaceae around 50 Ma collected in British Columbia.
For other clade ages in the family, see X.-Y. Yang et al. (2018), and within Corylus, see T. Zhao et al. (2019).
There may have been an increase in the diversification rate in Betulaceae, perhaps triggered by the acquisition of the deciduous habit (Bouchenak-Khelladi et al. 2015).
Xing et al. (2014) suggested that diversification of Betula, prominent in Boreal/cold temperate regions, could be dated to a mere 18.9-7.5 Ma (they thought the genus had 97 species), and diversification of Carpinus happened at about the same time. T. Zhao et al. (2019) discussed diversification and biogeographic relationships within Corylus, a genus that may have originated in southwestern China. There have been repeated shifts (long distance dispersal) between Asia and Europe, and between Asia and the New World and the tree form has evolved 4-5 times; ages for clades vary considerably depending on the marker and the assumptions of the methods (Helmstetter et al. 2019).
Z.-D. Chen et al. (1999) outline the phylogeny and evolution of Betulaceae. The ITS phylogeny of Betula provided by N. Wang et al. (2016) suggests that there has been quite extensive hybridization, even between species now placed in separate subgenera (see also Tarieiev et al. 2021). Adding data from ITS1 and ITS2 secondary structure to the analyses resulted in only a little improvement in our understanding of relationships (Tarieiev et al. 2021). RAD-seq data clarified the parentage of a number of hybrid species, although support for relationships towards the base of the tree was rather modest (N. Wang et al. 2021). There has been extensive chloroplast capture in Corylus, analyses of chloroplast and nuclear genomes giving quite different topologies (T. Zhao et al. 2019; Helmstetter et al. 2019; Z. Wang et al. 2025). Karumuna et al. (2019: plastome analyses) found Ostrya and Carpinus to be somwhat muddled up, while Z. Wang et al. (2022) suggested that Carpinus sect. Distegocarpus, currently with three species, was the result of homoploid hybrid speciation that occured between parental species in Carpinus and Ostrya, this happened around 33-23 Ma (divergence of the two genera) and 26-17 Ma (divergence of the two sections of Carpinus).
Zhu et al. (2018: Nothofagus outgroup) provide a convenient summary of variation in the family, especially that of floral development, and place it in a phylogenetic context.
Ecology & Physiology. Betula grows with other ECM trees in the boreal forest, and it can be a major component of the biomass in tundra vegetation (Chapin & Körner 1995).
Alnus is common in some Neotropical montane forests, A. acuminata dominating in montane cloud forests of the Yungas at 1500-3000 m altitude to about 28o S. (Peay 2016; Wicaksono et al. 2016). Alnus is well known to be a N-fixing plant, hence its use in land remediation. The co-occurrence of N fixation and the ECM habit is rather unusual (but see Fabaceae, Casuarinaceae), and the ECM fungi may also help enhance phosphorus acquisition by the plant (Walker et al. 2013). Cluster roots have been reported from the genus (Skene 1998; Shane & Lambers 2005), although they are rather sporadic in their occurrence and do not seem to be very abundant (Hurd & Schwintzer 1996). Roots of Alnus rubra caused a substantial increase in bedrock weathering, and hence in the access of the plant to the nutrients like phosphorus this would provide (Perakis & Pett-Ridge 2019a); this increase was perhaps caused by nitric acid, itself converted by soil microbes from excess N fixed by Frankia. Lambers et al. (2019, but c.f. Perakis & Pett-Ridge 2019b) think carboxylate exudation by cluster roots may help in phosphorus acquisition here (see also Lambers et al. 2012b). There is also an association between enhanced P acquisition, lower pH and increased rock weathering in N-fixing Fabaceae.
Pollination Biology & Seed Dispersal. Heterodichogamy is reported from Corylus (Renner 2001 for references). In Corylus avellana in particular there may be three to five months between pollination and fertilization. Ovules start to develop about half way through this period and the developing nuts are already 7-10 mm across at the time of fertilization. If pollination does not occur, the stigma may remain receptive for up to three months (Germain 1994; see also Nawaschin 1899 for pollination). There is intermittent pollen tube growth in Alnus (Sogo & Tobe 2005). See also Benson et al. (1906), for pollination, etc., of Carpinus and Dahl and Fredrikson (1996), fertilization of Betula, for more information.
Falling fruits of Carpinus develop a vortex at the leading edge of the rotating wing that generates lift, so facilitating wind dispersal (Lentink et al. 2009). However, Larson-Johnson (2023) found no correlation between the distance of dispersal of Carpinus fruits with descent rate and wing loading because of factors like the variability of wind speed and turbulence.
Plant-Animal Interactions. Both Betula and Alnus are very important host plants for lepidopteran larvae in the contiguous United States of America, being included in the top 15 such plants (Narango et al. 2020: Quercus is #1). Leaf-mining caterpillars of Eriocraniidae, probably the subbasal clade in the lepidopteran Angiospermivora, are to be found on Betulaceae (and Fagaceae: Regier et al. 2015 and references).
Plant-Bacterial/Fungal Associations. Põlme et al. (2013) reported quite an assemblage of ECM fungi on Alnus, even if compared with some other ECM plants there are not that many species, however, they are unrelated and are rather species-specific (Bruns et al. 2002; Walker et al. 2013; Wicaksono et al. 2017). This specificity may be connected with the tendency of Alnus to be a pioneer, plant-fungus specificity tending to be higher in such situations (Bruns et al. 2002); Tedersoo et al. (2009) found that all but one of the ca 40 species of basidiomycetes they found on Alnus in their study were restricted to that genus (the exception was also a mycoheterotroph on Orchidaceae) and 4/6 ascomycetes were similarly restricted. Kennedy et al. (2011), noting the patterns of similarity of ECM fungi associated with particular species of Alnus in different parts of the world, suggested that the plants and their fungi might co-migrate. Both AM and ECM associations have been recorded here (e.g. Rose 1980), and Alnus may also lack ECM (Michelsen et al. 1998). 5-15% of fixed 15N2 moved from Alnus glutinosa to Pinus contorta via a common ECM associate (Ekblad & Huss-Danell 1995), although the ecological significance of this is unclear. For more on ECM associations, see elsewhere; species of both Alnus and Betula are dual mycorrhizal plants (Teste et al. 2019: Table S2).
Chemistry, Morphology, etc.. Staminate flowers in Coryloideae are sometimes reported as being single (e.g. Mabberley 1997), however, as Abbe (1935) noted, there are usually three together. The staminate "flower" of Ostrya, with some 15 pairs of half stamens, is apparently pseudanthial in nature, being derived from three flowers (Abbe 1935, 1974; Macdonald in Sattler 1973: see also Nothofagaceae). Betula has three carpelate flowers per bract. In staminate flowers of Corylus the perianth is reduced to a ridge, and pollen of C. avellana has spongy endexine at the pore (Weber & Ulrich 2010). Correa-Narvaez and Manchester (2022) discuss the morphology of the fuits with their "bracts" in Corylus, etc.. The orientation of stamens or carpels in the flower may change during development in Betula and other taxa (Lin et al. 2010; Zhe et al. 2018), while Endress (2008c) discussed the structural lability of carpelate flowers of Carpinus betulus. The ovary of Corylus is not always obviously inferior.
See Crane (1989) and Kubitzki (1993b), both general, Ashburner and McAllister (2013: Betula), Endress (1967: general, comparison with Hamamelidaceae, Corylopsis is the link), Hegnauer (1964, 1989: chemistry), Horne (1914), Heller (1935) and Abbe (1935, 1974), all flowers, inflorescences, Z.-Y. Zhang et al. (1994: embryology of Carpinus), and Manchester and Chen (1998: fossils).
Phylogeny. R.-Q. Li et al. (2004) suggested that Betuloideae were paraphyletic, with Alnus and Betula being successively sister to the rest of the family (see also H.-L. Li et al. 2015, 2016; M. Sun et al. 2016; Z.-D. Chen et al. 2016: support very weak). However, Forest et al. (2005), analysing variation in ITS and the 5S spacer, recovered the two subfamilies above as monophyletic (see also Grimm & Renner 2013; X.-G. Xiang et al. 2014a; Ma et al. 2015: phylotranscriptomic analyses; X.-Y. Yang et al. 2018; Z. Yang et al. 2019). Corylus is sister to other Coryloideae in both molecular (e.g. Grimm & Renner 2013; Y.-Y. Yang et al. 2021, but conflict in the data) and morphological (Zhe et al. 2018) analyses; indeed, a number of morphological features suggest these relationships (Y.-Y. Yang et al. 2021). The monophyly of Ostrya and Carpinus is unclear, the latter genus perhaps being paraphyletic (see Yoo & Wen 2002, 2007, 2008; Xiang et al. 2014a; Y.-Y. Yang et al. 2021). In a plastome analysis of X.-Y. Yang et al. (2018) the two were monophyletic, but in their ITS tree Carpinus was paraphyletic. Ostryopsis was sister to [Ostrya + Carpinus] in another complete plastome analyses, but sister to Corylus in intergenic spacer region analyses (Z. Yang et al. 2019), although it was in the former position in all analyses in X.-Y. Yang et al. (2018).
Classification. The two subfamilies have sometimes been recognised as families, as by Brummitt (1992).
For a checklist and bibliography, see Govaerts and Frodin (1998: as Corylaceae and Betulaceae). For an infrageneric classification of Betula, see Ashburner and McAllister (2013), but c.f. N. Wang et al. (2016); N. Wang et al. (2021) recognized just two subgenera with different seed wing morphologies which included members of all four of Ashburner and McAllister's subgenera. T. Zhao et al. (2019) recognized four sections in Corylus.