EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
TRACHEOPHYTA†
Vascular tissue + [tracheids, walls with bars of secondary thickening]; stomata numerous, involved in gas exchange.
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[DILLENIALES [SAXIFRAGALES + ROSIDS]]: stipules + [usually apparently inserted on the stem].
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE - Back to Main Tree.
Anthers ± dorsifixed, transition to filament narrow, connective thin.
Age. Using penalized likelihood, Hengcheng Wang et al. (2009) suggested that the Vitaceae/rest of rosids split occurred (115-)111(-109) or (96-)92(-88) Ma; two Bayesian relaxed clock estimates were between 119 and 113 Ma Ages of (132-)125(-118) or (105-)101(-97) Ma are offered by Bell et al. (2010). Wikström et al. (2001) suggested an age of (121-)117, 108(-104) Ma, Magallón and Castillo (2009) estimated ages of ca 112.6 to 113.2 Ma, Argout et al. (2011) gave a date of ca 123 Ma, and Magallón et al. (2013) an age of around 108.7 My; 100.6-97.6 Ma is the estimate in Xue et al. (2012), around 111-105.6 Ma in Naumann et al. (2013), about 123.5 Ma in Hohmann et al. (2015), ca 117.5 Ma in Tank et al. (2015: Table S2), 160-117 Ma (Barba-Montoya et al. 2018) or ca 110.2 Ma (Fan et al. 2019).
Evolution: Divergence & Distribution. Diversification may have increased at this node, dated to (122.4-)122(-121.3) Ma (Magallón et al. 2018).
Ecology & Physiology. This node is notable for its relatively high ratio of leaf mass per area (SLA) (Cornwell et al. 2014).
Plant-Animal Interactions. Overall herbivory in this clade is relatively high (Turcotte et al. 2014: see caveats).
Genes & Genomes. For the possible palaeohexaploidy of Vitales, see Jaillon, Aury et al. (2007). However, it now seems that this genome triplication is widespread in eudicots and occured in the common ancestor of the [rosid + asterid] clade, indeed, in the immediate ancestor of the core eudicots as a whole; for further information, see discussion elsewhere. For genome synteny, transposition, etc., in Vitales, see Woodhouse et al. (2011).
Zheng et al. (2013) suggested that that the ancestral chromosome number of this clade, x = 18.
The orf56 pseudogene is widespread here, but there is an intact open reading frame in Vitis and all 11 taxa of the two families of Myrtales examined (Su et al. 2014).
Chemistry, Morphology, etc.. For exudates, see Lambert et al. (2013), and for disc nectaries, see Endress (2010c).
Phylogeny. See the Dilleniales
[SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: ?
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node and for relationships of Vitales in particular, see Saxifragales pages.
Just the 1 family, 17 genera, 1000 species.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Synonymy: Leeales de Candolle - Vitanae Reveal
VITACEAE Jussieu, nom. cons. - Back to Vitales
Ellagic acid, myricetin +; tension wood 0; cambium storied; vessel elements with simple perforation plates, rays heterocellular, broad; sieve tube plastids with protein crystalloids and starch; (cork cambium deep-seated); nodes multilacunar [3-7:3-7], often swollen; raphide bundles +, raphides twinned; pearl glands + [food bodies]; petiole with ring of bundles; stomata variable; branching from previous flush; leaves palmately compound or -veined, lamina vernation conduplicate, teeth glandular; inflorescences leaf-opposed [terminal, but usu. evicted], branched, ultimate units cymose, dichasial; flowers small [≤1 cm across]; C valvate, protective in bud; A = and opposite C, from a common primordium; tapetal cells 3-4-nucleate; pollen 3-celled; nectary at base of G; G initially a ring primordium, at least apically unilocular [± strongly intrusive parietal], style short, stigma ± capitate or -fid, dry; ovules 2/carpel, apotropous, ?micropyle, nucellar cap +, hypostase +, placental obturator + or 0; fruit a berry, K deciduous; seeds perichalazal, ± surrounded by vascular bundle; testa multiplicative, exotesta fleshy, mesotesta 2-17 layered, endotesta 2-5-layered, lignified, ?all palisade, crystalliferous, exotegmen (crossed) tracheidal, endotegmen tanniniferous, ± mucilaginous; endosperm ruminate, with paired longitudinal raphal inpushings [± T-shaped in t.s.], embryo minute; x = 13 (?10), nuclear genome [1 C] (0.028-)0.591(-12.441) pg; loss of RPB2 d copy.
17/975: [list, to tribes] - 2 subfamilies, 5 tribes below. Pantropical and (warm) temperate.
Age. Wikström et al. (2001) suggested a crown group age for Vitaceae of (97-)92, 78(-73) Ma, Magallón and Castillo (2009) estimated ages of ca 90.75 Ma, while ca 95 Ma was the age in Wen et al. (2013) and ca 72 Ma in N. Adams et al. (2016).
1. Leeoideae Burmeister - Leea L. —— Synonymy: Leeaceae Dumortier, nom. cons.
Herbs to trees; raphides barbed; leaves spiral, to twice compound, (simple - some L. macrophylla), teeth with small glandular apex, one lateral vein continues its course above the tooth, stipules borne along the length of the petiole margin-basal, sheathing; flowers 4-5-merous; K becoming basally connate [intercalary ring meristem develops], C basally connate; stamens basally adnate to C, extrorse, incurved over nectary [so appearing introrse], anthers becoming connivent; nectary of very large lobes alternating with A, becoming recurved; G [2-3(-4)], (semi-inferior - see Chemistry, Morphology, etc. below), odd member abaxial, secondary septae +; micropyle bistomal, outer integument 4-5 cells across, inner integument ca 2 cells across, parietal tissue ca 6 cells across, nucellar cap ca 2 cells across; seed-coat with inverted T-shaped ingrowth at chalaza, also two, linear, lateral, rarely raphides in the seed coat, palisade ca 1-4-layered, mesotegmic cells divide once, expand, then collapse; n = (10-)12, nuclear genome [1 Cx] = ca 1.13 pg.
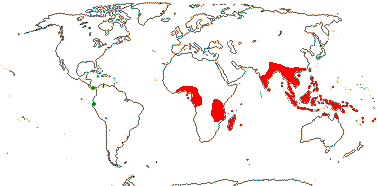
1/34. Most Indo-Malesian, few Africa and Madagascar. Map: from Ridsdale (1975), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 5. (2010), Australia's Herbarium (consulted i.2013), while information on fossils [green] is taken from Manchester et al. (2012b). Photo: Flower.]
Age. Moline et al. (2013: HPD) estimate the age of crown group Leeoideae to be (86.2-)72.1(-65.0) m.y, while ca 17.3 Ma is the age in N. Adams et al. (2016: sampling).
Leeoxylon multiseriatum, an arborescent plant from the easternmost Deccan Traps (India) in deposits from Mahurzari laid down a little before the K/C boundary (Prakash & Dayal 1964), may be best placed here, but it may also be associated with fruits from the same site that are placed in Vitoideae (see below: S. Y. Smith et al. 2015; Wheeler et al. 2017).
2. Vitoideae Eaton
Lianes, climbing by leaf-opposed stem tendrils, tendrils with 2 branches; (vessels with two diameter classes), (rays heterocellular/homocellular-procumbent); vascular traces arising 3 internodes or so before entering leaf; raphides smooth; (cuticle waxes as tubular rodlets); (more than one bud/node); leaves two-ranked, lamina palmately compound/simple, (fleshy), teeth with gland broadening distally and with foramen, (stipules adaxially connate), (not vascularized); K (lacking vascular traces), C (connate by papillae and calyptrate); (tapetum amoeboid); (pollen binucleate); nectary raised annular/cupular; G [2], collateral or superposed, (placentation parietal), (style long), (hollow); micropyle endo(bi)stomal, outer integument 4-7 cells across, inner integument 2-3(-4) cells across, parietal tissue 3-20 cells across, in radial rows, nucellar cap 2-10 cells across; seeds 1-4/fruit; seed coat ingrowths at raphe [seed ± T-shaped in transverse section], with raphides, endotesta palisade ["seed coat with columnar cells"], 1-2 layered; x = n = 20, nuclear genome [1 Cx] = (0.49-)0.68(-0.87) pg.
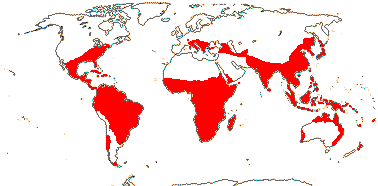
16/940. Pantropical and (warm) temperate. Map: from Wickens (1976), Meusel et al. (1978), Morley and Toelken (1983), Lombardi (2000), Fl. China vol. 12 (2007), Trop. Afr. Flow. Pl. Ecol. Distr. vol. 5 (2010), Australia's Virtual Herbarium (consulted xii.2012) and Fl. Pakistan. Photo: Flower.
Age. The age of crown-group Vitoideae is (98.6-)97.2(-95.9) Ma (Hearn et al. 2018a), ca 91 Ma (Wen et al. 2013), ca 87 Ma (X.-Q. Liu et al. 2015) or ca 66 Ma (N. Adams et al. 2016: Appendix S8).
Fruits that can be placed in crown-group Vitaceae, probably in Vitoideae (Indovitis chitaleyae), have recently been found in the Deccan Traps and dated to somewhere around/a little before the K/C boundary ca 66 Ma (Manchester et al. 2013); they have up to 6 seeds/fruit.
2A. Ampelopsideae J. Wen & Z. L. Nie —— Synonymy: Ampelopsidaceae Kosteletzky
Leaves (bicompound, pinnately compound), domatia common; inflorescence dichasial cymes (compound); flowers 4 (5-7) merous; K initially free [?all]; disc thin; berries variously coloured; nuclear genome [1 Cx] = (0.18-)0.27(-0.35) pg.
4/47: Ampelopsis (18). Tropical and subtropical.
Age. Ca 60 Ma is the approximate age of this clade (X.-Q. Liu et al. 2015).
[[Cisseae + Cayratieae] [Parthenocisseae + Viteae]]: (phloem stratified), (rays dilated distally); K as ring, initially as ring primordium; berries dark blue to black.
Age. The approximate age of this clade is 83 Ma (X.-Q. Liu et al. 2015).
[Cisseae + Cayratieae]: (CAM photosynthesis +); flowers usu. 4-merous.
Age. This clade is around 79 Ma (X.-Q. Liu et al. 2015).
2B. Cisseae Reichenbach - Cissus L. —— Synonymy: Cissaceae Drejer
(Stout trees, herbs; stem/rootstock swollen, former angled or not), (tendril branches 0-6); leaves (pinnately compound), (spiral), domatia (+); inflorescence (terminal), (on compressed axillary shoot, monochasial), dichasial cyme, often compound; disc thick, ± adnate to and often burying G; endotesta cells isodiametric; n = (12-14, 16, 24, 25, etc.), nuclear genome [1 Cx] = (0.34-)0.39(-0.45) pg.
1/300. Tropical and warm temperate.
2C. Cayratieae J. Wen & L. M. Lu
(Stem succulents, trees, herbs); (successive cambia - Tetrastigma); stomata cyclocytic (T.); tendrils (0), with (no - many Cayratia)2-3(-5) branches; leaves various, often 3-9 foliolate, imparipinnate or palmate, (succulent); inflorescences (on compressed axillary shoot), (corymbose or paniculate); (C constricted in middle); disc thick, adnate to G, (lobes separate); stigma (4-lobed); endosperm (regularly ruminate); (endotesta cells isodiametric); n = (13), nuclear genome [1 Cx] = (0.35-)1.73(2.89) pg.
7/405: Cyphostemma (200), Tetrastigma (137), Causonis (30). Palaeotropical, particularly Southern Hemisphere.
Age. This crown-group age of Cayratieae is around 79 Ma (Hearn et al. 2018a) or (79.8-)68.4(-56.9) Ma (Rabarijaona et al. 2020).
[Parthenocisseae + Viteae]: rays homocellular; flowers 5-merous; disc not free from G.
2D. Parthenocisseae J. Wen & Z. D. Chen
Tendrils (on long shoots), often with 3-12 branches, usu. with adhesive tips; stipules (falcate); inflorescences (on short shoots, axillary branches), corymb- to panicle-like; C often cucullate; disc indistinct/lobed/rudimentary; G (initially as separate bumps, then ring); nuclear genome [1 Cx] = (0.21-)0.30(-0.46) pg.
2/16: Parthenocissus (14). Temperate Asia, North America.
2E. Viteae Dumortier —— Synonymy: Pterisanthaceae J. Agardh
Ribbon-like trichomes +; tendrils (0), (un- or 3-branched); domatia (+); (plant dioecious); inflorescence a thyrse, (axis flattened); flowers (3-9-merous); disc ±ring-shaped at base of and adnate to G, (nectar 0); (inner integument 1 cell across - Vitis); n = (19), nuclear genome [1 Cx] = (0.16-)0.26(-0.31) pg.
2/190: Ampelocissus (110), Vitis (80). Old World, also North and Central (South) America.
Age. The crown-group age of this clade is thought to be (82-)70.5(-57.9) Ma (X.-Q. Liu et al. 2015); see also Nie et al. (2023).
Evolution: Divergence & Distribution. Fossil leaves assigned to Vitoideae are reported from the Late Cretaceous (see R. Burham's Fossil Record of Climbers for references), although wood and the distinctive seeds of the subfamily are usually found somewhat later (Wheeler & LaPasha 1994; Smith et al. 2012; Manchester et al. 2013: Deccan Traps; Rozefelds & Pace 2018; southern hemisphere). Seeds of Vitoideae with their distinctive mmorphology are quite common in Caenozoic deposits of North and South America and Europe, although there are more than twelve seeds per berry in some of these fossils, which suggests a gynoecium unlike that of extant Vitoideae (Manchester & Chen 2009). Fossil woods also have unexpected character combinations, perhaps to be expected of lianas (see below: Wheeler & LaPasha 1994).
Ampelocissus has been found in younger Oligocene deposits 30-28.5 Ma old from north coastal Peru; the nearest current localities of the genus are in Central America (Manchester et al. 2012a, b - for some discussion on the limits of Ampelocissus, see Nie et al. 2023). Even more notably, fossils of seeds from the Late Eocene of Panama (perhaps 40-37 My) have been identified as c.f. Leea (Herrera et al. 2012: identity confirmed - Manchester et al. 2013), L. macmillanae being described fromLate Eocene deposits ca 34 Ma from Panama (Herrera et al. 2024), and Leea has also been found in younger Oligocene deposits 30-28.5 Ma from north coastal Peru (Manchester et al. 2012a, b); Leea is currently Old World in its distribution. Lithouva susmanii, stem Viteae from Cundinamarca, Colorado in deposits 60-58 Ma, is notable because Viteae are not currently known from South America (Herrera et al. 2024). Saxuva draculoidea, described from Central America, belongs to Cayratieae, also not currently known from the New World - all told, there is a remarkable record of Vitaceae extinctions from the New World (Herrera et al. 2024).
Peng et al. (2021: ages for a number of clades) outline the diversification of Tetrastigma, the genus probably originating in continental Asia and with much subsequent movement to the Sunda shelf, and some to the Sahul shelf and Australia.
X.-Q. Liu et al. (2012) suggest ages for various clades within Cissus s.l. (the three clades mentioned below) as does N. Adams et al. (2016: Appendix S8, S9; ages throughout the family, but especially African Cissus, while L. Lu et al. (2013) gives ages for clades in the Cayratia area; the transcriptome phylogeny of Wen et al. (2013) and the Vitis-centred tree of X.-Q. Liu et al. (2015) are also dated.
Species of Cyphostemma grow in a wide range of habitats and have a great variety of growth forms - species range from an almost baobab-like tree (C. mappia) to herbs, stem succulents, some (especially southern African succulents) lacking tendrils, etc.. Moving into novel environments seems to have been associated with the evolution of novel adaptations, but not with increased diversification (this seems to be quite common in the evolution of succulence), and it should be noted that although Cyphostemma has around 200 species, it is also thought to be quite old, some (55.1-)43.3(-32) Ma (Hearn et al. 2018a). For evolution in Tetrastigma, see Habib et al. (2017: seed morphology, 2018).
Vitis may have originated in mid-latitude North America (Nie et al. 2023); there are suggestions of early introgression between Vitis and the New World Ampelocissus and subsequently between species of Vitis itself. A member of the old Boreotropical flora (for which, see Morley 2000, 2003, 2007), Vitis initially diversified in North America under cooler temperatures (but with movement north and south as temperatures changes subsequent to the Eocene), this diversification being accompanied/caused by extensive hybridization and introgression (see also Zecca et al. 2020, etc.), and the genus also moved to Eurasia (Nie et al. 2023).
Z.-Y. Ma et al. (2020) plot the phylogenetic distribution of a number of characters in Vitoideae.
The recently-described Pseudocayratia, from China and Japan, may be a hybrid between members of the Cayratia s.l. and Tetrastigma clades (Wen et al. 2018b).
Ecology & Physiology. Vitaceae-Vitoideae are a major clade of vines/lianas - perhaps 800 species of them - and Cissus is one of the ten largest genera of climbers (Sperotto et al. 2023). All are tendrillate and some have adhesive pads on the ends of the tendrils (Sousa-Baena et al. 2018b). Steinbrecher et al. (2011) describe how these pads may function, and also the possible role of small hooks on the shoots as mechanoreceptors in Parthenocissus, and there also seem to be spirally coiled hairs; for further details of the biomechanics, etc., of climbing here, see see Rowe and Speck (2015; additional references in Schnitzer et al. 2015). Fukano (2017) suggests that vine tendrils in Cayratia japonica use contact chemoreception - specifically, sensing oxalate, and since Vitaceae tissues tend to have large amounts of calcium oxalate, the plant can avoid coiling around the leaves of conspecifics. Vitoideae lianas may develop quite high root pressures (Fisher et al. 1997) in the spring that may help repair embolisms in the xylem, and this may facilitate the ability of genera like Vitis to grow in more temperate climates where lianas otherwise tend to be uncommon (Tibbetts & Ewers 2000; Schnitzer 2005). However, recent work suggests that embolism formation and refilling may be controlled by the activity of living cells around the vessels (Knipfer et al. 2016) and/or lipid surfactants in the xylem that i.a. coat nanobubbles, so preventing the formation of full-fledged embolisms (Schenk et al. 2017). Species of Vitis as it were drain their pipes during the winter, the huge vessels - up to 0.5 mm across and 8 m long - being air-filled then, but they fill with water during the spring (Sperry et al. 1987). Being lianas, the stem anatomy of Vitaceae is distinctive, having the liana syndrome (references in Chery et al. 2020). Among other distinctions, vessels tend to be dimorphic, and Bouda et al. (2019) found that in young stems of Vitis vinifera some 15% of the water flow in the wide vessels was diverted into narrow vessels, a transverse pressure gradient developing in the stem, end-wall resistance having a greater effect in wider than narrower vessels (Bouda et al. 2019).
In drier parts of Africa there are a number of non-climbing species of Cyphostemma with rather grotequely swollen stems (but with the flaky bark of the family); crassulacean acid metabolism (CAM) has been reported from such species (Virzo do Santo & Bartoli 1996; see also Gilman et al. 2023).
Tetrastigma in West Malesia is the only host of the giant parasite Rafflesia (Rafflesiaceae, Malpighiales), and at least some other Rafflesiaceae have this genus as their host. Dating estimates are somewhat in conflict. P. Chen et al. (2011b: HPD) suggest ages of (65.3-)50.6(-36.4) Ma for stem Tetrastigma, (49.3-)36.9(-25.7) Ma for the crown group, while ages in Lu et al. (2013) are somewhat older, at (67.7-)57.4(-47.4) and (59.4-)47.6(-36.4) Ma respectively, both sets of estimates being well before the origin of crown group Rafflesia (for which, see Bendiksby et al. 2010). Peng et al. (2021) estimated that stem-group Tetrastigma was only ca 49.4 Ma. However, if crown-group Rafflesiaceae are (95.9-)81.7(-69.5) Ma old (Bendiksby et al. 2010) and the family is always and only associated with Tetrastigma...
The association of host and parasite is particularly close. Some genes of Tetrastigma are expressed in Rafflesia, and codon usage properties of many other Rafflesia genes are like those of its host, a degree of integration of two genomes unknown in any other host-parasite association (Xi et al. 2012a). Further details can be found under Rafflesiaceae.
Pollination & Seed Dispersal. Massonnett et al. (2020) discuss the evolution of perfect flowers from dioecy in Vitis vinifera.
Plant-Animal Interactions. Caterpillars of the noctuid Agaristinae, often day-fliers, are often found on Vitaceae (Sihvonen et al. 2021), and Forbes (1956) noted that some lepidopteran larvae were found there and on Onagraceae alone - and both families contain raphides. Agaristinae are a large group, but a cursory survey of their hosts suggests they eat a variety of families, at least some of which are likely to contain raphides.
For the interaction of the hemipteran parasite phylloxera (= Daktulosphaira vitifolia) on its American host, Vitis riparia, see Schultz et al. (2018); a number of genes involved in carpel development are coopted by the insect larvae in the production of leaf galls.
Vegetative Variation. There has long been discussion about the morphology of tendrils in Vitoideae (e.g. Eichler 1878). These are stem structures, and some are replaced by inflorescences in fertile shoots, and structures that are part tendril-part inflorescences are not uncommon (Calonje et al. 2002; Wen et al. 2018a; Carmona et al. 2008 for development). Floral meristem genes studied are expressed in inflorescences and tendrils, or inflorescences alone, but not or hardly in leaves (N. Zhang et al. 2015a). A major question is whether the inflorescence/tendril is an evicted terminal shoot or develops from an axillary bud, i.e. whether the plant is basically monopodial or sympodial (Wilson et al. 2002 and references), thus Shah and Dave (1970) thought that the tendril was an extra-axillary lateral branch. The inflorescence/tendril arises from the flank of the terminal meristem opposite a leaf primordium (Gerrath & Posluszny 2007) as an uncommitted primordium, and Gerrath et al. (2017: p. 556) note that "the terminal inflorescence of the kind that characterizes [Leeoideae] may have morphed into a unique structure, the uncommitted primordium of [Vitoideae]". In an excellent summary of how Vitaceae grow, Gerrath et al. (2017) note that tendrils may be simple or branched, inflorescences and tendrils may have intermediates, and inflorescences may be borne on long shoots, distinct branch orders, or even on short shoots; for more on the molecular control of tendril development, see Sousa-Baena et al. (2018a, b).
Pterisanthes, embedded in Ampelocissus, has remarkable flattened and coloured inflorescence axes on which the flowers are borne; these axes represent inflorescences with two orders of racemose branching, as in other Ampelocissus, but with lamellate outgrowths on which the flowers are borne - most flowers are more or less sessile, but some on the margins are long-pedicellate (Ickert-Bond et al. 2015c). In some species not all leaves have obvious axillary buds, and in Vitoideae the basic construction of the stem consists of a three-node repeating unit (e.g. Gerrath et al. 1998, 2001; Gerrath & Posluszny 1994 and references), perhaps an apomorphy for the subfamily. For more on shoot architecture, which should be integrated with the recent reclassification of the family, see also Gerrath and Posluszny (2007 and references), Murata and Maeda (2022) and Murata (2025); a deeper understanding of tendrils will also be important.
The vascular supply to the leaf is complex, partly because it starts well down the stem below the leaf it innervates, and Gerrath et al. (2001) noted an asymmetry in the vascular traces supplying the leaf, the midvein always coming from the ventral/abaxial vascular sympodium - remember, the leaves are usually two-ranked - the two sympodia each supplying the same number of lateral traces. However, depending on the species, this rather oversimplifies the situation. Given that a likely apomorphy of Vitaceae is compound leaves, it is not surprising that simple leaves of Vitis have a cryptic compound developmental program (KNOX1 is reactivated) early in their development (Bharathan et al. 2002; Nakayama et al. 2022).
Genes & Genomes. Murat et al. (2015b) suggest the ancestral chromosome number for Vitis - and perhaps a larger clade - is x = 19 (see also Zheng et al. 2013); x = 20 seems to be the likely base number for Vitoideae. Chu et al. (2018) looked at genome size evolution in Vitoideae; the genome of Cayratieae is often relatively large, but that in the species of Leea included is also relatively quite large, see also estimations of size for Vitoideae...
For a palaeohexaploidy event that seemed to link Vitales with rosids (Jaillon, Aury et al. 2007), see the core eudicot node. P.-L. Liu et al. (2020) talk about the possibility of an independent genome duplication in the Vitis vinifera lineage.
Vitis, at least, has a massive mitochondrial genome largely because of the expansion of intergenic spacers; some DNA from this genome may have migrated to the nucleus - rather unusual (Goremykin et al. 2009a).
Economic Importance. See Terral et al. (2010) and especially Y. Dong et al. (2023) for the complex early history of the domestication of the grape. Dong et al. (2023) thought that grapes had been domesticated twice, the results being table and wine grapes, although timing here is somewhat unclear (Allaby 2023). H. Xiao et al. (2023) suggested that there had been a single domestication event, but with subsequent introgression from wild to wine grapes in Europe. Wild species of Vitis are dioecious, while cultivated plants have perfect flowers (Carmona et al. 2008). Thephylloxera (Daktulosphaira vitifoliae Fitch.) and other pests in Europe (Millardet, 1880; Töpfer et al., 2011). Vineyards throughout Europe were decimated by the activities of phylloxera in the late 1800s; phylloxera, Daktulosphaira vitifoliae, is a hemipteran related to aphids with a very complex life history, and when it infects grape roots, the plant dies. Most grapes now grown are grafted onto stock of American origin, American Vitis being more or less resistant to phylloxera, and hybridization has also been attempted (Töpfer et al. 2011). Some countries like Chile and most of Australia remain free of phylloxera, and even in Europe some soils do not favour the growth of the insect.
Chemistry, Morphology, etc.. In temperate Vitaceae there is pronounced vessel dimorphism while in tropical members of the family there are often distinctive cambial structures and associated secondary thickening patterns (e.g. Pace 2018). The raphides of Vitaceae are twinned, square in transverse section, and like an arrow in longitudinal section (Cody & Horner 1983; Horner & Wagner 1995). Food bodies, often called pearl glands, are common on the surface of the plant. They are multicellular, with a multiseriate stalk, sometimes with a stoma on the swollen head, and the central parenchymatic cells accumulate oils and sugars (Pavia et al. 2009). For domatium development in Vitis, see Chomicki et al. (2024).
Prophylls are sometimes shown as being adaxial, or both lateral and adaxial within a single bud (Gerrath & Posluszny 2007).
Inflorescence morphology is variable, being variously congested, if basically cymose. Ridsdale (1974) suggests that the inflorescence of Leea was cymose, while Z.-Y. Ma et al. (2020) score Viteae alone as having a thyrse. The Malesian Pterisanthes (= Ampelocissus) has remarkable flattened red to purplish inflorescence axes on which the flowers and fruits are scattered. Flowers in Vitaceae usually have a common stamen-petal primordium (but not in Rhoicissus, Gerrath et al. 2004). There is considerable variation in nectary morphology, from enveloping the ovary and forming little projections on top to being absent. Although Leea lacks an obvious nectary like that of Vitis, etc., developmental data show that the lobes alternating with the stamens are comparable with the gynoecial nectary of Vitoideae, and it is vascularized from the gynoecium (Gerrath et al. 1990, 2001). Since the part of the nectary alternating with the stamens in Leea was supplied by several traces, Nair (1968) thought that it might represent a number of stamens, however, it is best thought of s a gynoecial nectary. Early development in this part of the flower can be a little complicated in Leea. Thus Gerrath et al. (1990) described the androecium and gynoecial walls as being uplifted by a common primordium, and there was a depression in the centre (phrased somewhat differently, this was considered to be an apomorphy for the genus by Gerrath et al. 2004), and they also mentioned that the corolla, androecium and disc were joined at the base by intercalary growth. (The gynoecium is sometimes described as being partly inferior, presumably connected with this pattern of development.) The ovary has been described as being "anatomically parietal" (Brizicky 1965, and references); in fact, the ovary is at least apically unilocular, with fully parietal placentation in some Cyphostemma (Ickert-Bond et al. 2014a, see also 2014c). The egg apparatus of Cissus is reported to lie outside the ovule (Nair 1970 for references). For the distinctively ruminate seeds of the family, see Periasamy (1962a).
Some information is taken from Ridsdale (1974: Leea), Lombardi (2000), Timmons (2006), Wen (2006), Gerrath et al. (2015) and Descoings et al. (2023: succulents), all general; for vascular anatomy, see Wheeler and LaPasha (1994) and Pace et al. (2018b), for nodal anatomy and stipules, see Shah (1959), for leaf teeth, Hickey and Wolfe (1975), for floral development, see Gerrath and Posluszny (1989 and references - Vitoideae), Timmons et al. (2007: some Vitoideae, useful table), and Gerrath et al. (1990: Leeoideae, 2004: Rhoicissus), for nectaries, see Erbar (2014), for embryology, etc., see Nair (1970), Nair and Nambisan (1957), and Nair and Bajaj (1966), for ovules and seeds, see Berlese (1892), and for seed anatomy in extant and fossil taxa, see Chen and Manchester (2007, 2011).
Phylogeny. Ingrouille et al. (2002) in a study of rbcL phylogeny considered in the context of morphological variation found little strong support for clades within Vitaceae. Subsequent studies using more genes (e.g. Soejima & Wen 2006) find somewhat more resolution, although support values and relationships of the clades other than the Cyphostemma-Cayratia-Tetrastigma clade were often still rather uncertain (Rossetto et al. 2002; Wen et al. 2007; Wen 2008; Chen et al. 2011; Trias-Blasi et al. 2012: Leea placed sister to Dillenia without comment, but see rooting; X.-Q. Liu et al. 2012). Ren et al. (2011) found two main clades in Vitoideae, one included species with 4-merous flowers (e.g. Cayratia, Cissus, Tetrastigma), the other, species with 5-merous flowers; within these clades, especially the former, there is a fair bit of well-supported phylogenetic structure that is correlated with names in current use. However, poly/paraphyly is also obvious, e.g. Cissus, Tetrastigma/Cayratia (Rossetto et al. 2007). Liu et al. (2012) found six well supported clades, one the Cyphostemma-Tetrastigma clade again, three clades including species of Cissus (two entirely so), and the final two, which may form a single clade, include species of Vitis et al. and Parthenocissus et al.. In a nuclear transcriptome study, relationships were [[Ampelocissus + Rhoicissus] [[Vitis + Parthenocissus] [Cissus [Cyphostemma-Tetrastigma]]]] (Wen et al. 2013). This basic structure, [[Ampelocissus + Rhoicissus] [[[Vitis + Ampelocissus] [Parthenocissus + Yua]] [[Cissus [Cyphostemma-Tetrastigma]]], is more or less holding up, although support along the backbone within e.g. the [Vitis + Ampelocissus] clade could be strengthened (N. Zhang et al. 2015b: all three compartments give largely the same topology; Ickert-Bond et al. 2015c; N. Adams et al. 2016; esp. X.-Q. Liu et al. 2015; Yu et al. 2017; B.-B. Liu et al. 2021a: not always strong support for tribal relationships). A number of fossils were incorporated into a phylogeny of the family using quantitative morphometric data by Parkins-Fukuchi (2018), but the topology is very odd - partly a rooting problem? Z.-Y. Ma et al. (2020) recovered the same (tribal level)/largely similar (generic level) relationships in their comprehensive phylogenomic studies, although they noted some differences when comparing nuclear and plastome analyses (see also B.-B. Liu et al. 2021a: e.g. North American Vitis was paraphyletic when using nuclear but not plastome data).
Within Cayratieae, there are five major clades; Cayratia itself has three main clades and is paraphyletic, and Tetrastigma may be sister to one of the Cayratia clades (Lu et al. 2013; see also Rossetto et al. 2007). For the phylogeny of Tetrastigma itself, host of Rafflesia, see P. Chen et al. (2011a) and especially Habib et al. (2017: 10 chloroplast genes); major clades in the latter only partly agree with those in the former, and a small clade of two undescribed species from S.E. China is sister to the rest of the genus. Similarly, different stories resulted from the analyses of different genome compartments by Rabarijaona et al. (2020), Cyphostemma, Afrocayratia and Cayratia switching positions.
Cisseae. See Rodrigues et al. 2014 for South American Cissus and Adams et al. (2016: inc. early Miocene fossils) for African Cissus in particular.
Viteae. Pterisanthes turns out to be polyphyletic and embedded within Ampelocissus (X.-Q. Liu et al. 2015). Vitis itself is monophyletic, and some species hybridize (Tröndle et al. 2010); the small subgenus Muscadinia, from the New World, is sister to the other species which are from both the Old and New Worlds (X.-Q. Liu et al. 2015). Nie et al. (2023: 318 low-copy nuclear genes, ±complete plastomes, 40 spp., 23 New World spp., often several collections) looked at relationships in Vitis with a focus on North American taxa; there was discordance between the topologies resulting from coalescent and concatenation analyses and very extensive conflict between the nuclear concatenation and plastome trees (ibid. Fig. 4), and so on - hybridization and introgression seem to have been widespread.
Classification. Although Vitis and relatives and Leea are morphologically distinguishable, there are numerous features that unite the two and they are sister taxa in all phylogenetic studies; the wood anatomy of Leea and Rhoicissus in particular is very similar. Inclusion in a single family seems reasonable.
Generic limits in Vitoideae need attention, for example, species of Cissus occur all over the tree, while Tetrastigma, although monophyletic, is embedded in Cayratia (e.g. P. Chen et al. 2011b). This is now being taken care of, and the classification above largely follows that in Wen et al. (2018a); note that five neotropical species of Ampelocissus seem best included in Vitis (Nie et al. 2023).
Previous Relationships. The affinities of Vitaceae were for long uncertain. They were often associated with Rhamnaceae, since both have stamens opposite the petals, and Takhtajan (1997) placed them near Proteanae, in his Rosidae; Proteanae also have stamens opposite their tepals, but are otherwise very different.