EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II] // CORE ASTERIDS // EUASTERIDS // GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; ="apo">seed single; duplication of the PI gene.
ASTERID I / LAMIIDAE: ?
[METTENIUSALES [GARRYALES [GENTIANALES, VAHLIALES, SOLANALES, BORAGINALES, LAMIALES]]]: ?
[GARRYALES [GENTIANALES, VAHLIALES, SOLANALES, BORAGINALES, LAMIALES]]: G [2], superposed; loss of introns 18-23 in RPB2 gene d copy [?level].
[GARRYALES, AQUIFOLIALES [ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]]: G [2], superposed; loss of introns 18-23 in RPB2 d copy. - check
[ICACINALES [[GENTIANALES + BORAGINALES], VAHLIALES, SOLANALES, LAMIALES]]: vessel elements with with simple perforation plates; nodes 1:1.
[[GENTIANALES + BORAGINALES] VAHLIALES, SOLANALES, LAMIALES] / CORE LAMIIDS: herbaceous habit widespread; (8-ring deoxyflavonols +); C forming a distinct tube, initiation late [sampling!]; A epipetalous; (vascularized) nectary at base of G; style long; several ovules/carpel; fruit a septicidal capsule, K persistent.
Evolution: Divergence & Distribution. For the complex patterns of variation in a number of characters in this part of the tree, see the Gentianales page.
BORAGINALES Berchtold & J. Presl, nom. cons. - Main Tree.
Plant ± herbaceous; (pyrrolizidine alkaloids + [esp. lycopsamines, also triangularines]), rosmarinic acid + [caffeic acid ester], iridoids 0; root lacking hypodermis [2 records]; (nuclear crystalloids in sieve tubes); (cork mid-cortical to pericyclic); (silicon concentration high [?level; see hairs]); axial parenchyma (±) 0; sieve tubes with nuclear non-dispersive protein bodies; petiole bundle(s) arcuate; plant ± roughly hairy, hairs with a basal cystolith or cystolith-like body, walls calcified or silicified, secretory trichomes +, heads uni-/multicellular; leaves spiral, lamina margins entire; inflorescences terminal, cyme scorpioid, axis straightening in fruit, (bracteoles 0); K ± free, valvate, C tube formation late; anther placentoid 0; (pollen with pseudocolpi) [= heterocolpate]; nectary +, not vascularized; placentation intrusive parietal, stigma dry; ovules many/carpel; fruit a loculicidal capsule, K ± accrescent; testa with single layer of transfer cells [cell walls have labyrinthine ingrowths]; endosperm copious, chalazal haustorium +, embryo small, straight, cotyledons accumbent[?]. - 8 families, 150 genera, 3,120 species. [Photos - Collection.]
Includes Boraginaceae, Codonaceae, Cordiaceae, Coldeniaceae, Ehretiaceae, Heliotropiaceae, Hoplestigmataceae, Hydrophyllaceae, Wellstediaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) estimate crown-group Boraginales as being (63-)59, 56(-52) My; the age is (73-)57, 54(-39) Ma in Bell et al. (2010) and rather older, (101-)91(-82) Ma in Luebert et al. (2016b), (92.8-)76.4(-62) Ma in Tank and Olmstead (pers. comm.), (87.7-)72.6(-54.3) Ma, in Nazaire et al. (2014) and (88.4-)67.9(-46.4) Ma in Naumann et al. (2013). There is also discussion of stem ages for the order in Luebert et al. (2016b), but since its relationships are unknown...
Evolution: Divergence & Distribution. The diversification of primarily woody boraginalean taxa may have taken place in the mid-Cretaceous, some 90 Ma, in South America (Gottschling et al. 2004), however, many ages estimates there seem overly high. M. J. Moore and Jansen (2006) suggest rather later dates, with the very end Cretaceous at 67-63Ma being a date for the diversification of the woody taxa. Luebert et al. (2016b) think that Boraginales were initially African-South American in distribution, with the two main clades initially diversifying on a single continent after a West Gondwanan vicariance event, subsequently there were some 25 intercontinental dispersal events in the order.
The removal of Codon from Hydrophyllaceae makes biogeographical sense; it was the only genus endemic to Africa in that family under its old circumscription. Wellstedia, the next branch of that part of the tree where Codon now resides (but see below), is also African, perhaps suggesting the origin of Boraginaceae in Africa.
Overall variation in gynoecial morphology and fruit type in Boraginales is considerable, and I have not really got my brain around this. Vasile et al. (2025: Fig/Box 1, see also Fig. 6) mention ten fruit types that are either drupes, schizocarps or eremocarps, and then there is the very considerable variation in gynoecial morphology in Hydrophyllaceae (ibid. Fig. 7, also variation in the order as a whole), and this family often has capsular fruits. For the evolution of pollen morphology in Boraginales, see L.-E Yang et al. (2020), but c.f. topology, etc.. Boraginaceae and Hydrophyllaceae have meroterpenoids, rusts and very variable endosperm development in common - all plesiomorphies??
Ecology & Physiology. Namaceae and Ehretiaceae include some taxa that can live on gypsum (calcium sulphate)-rich soils (C. T. Muller et al. 2017).
Pollination Biology. Pseudocolpi and other features of the pollen like triangular polar poroid areas, as in Myosotis, appear to be involved in harmomegathic movements of the walls of the pollen grains (Volkova et al. 2013, 2017).
Plant-Animal Interactions. Boraginales are not often eaten by caterpillars (Ehrlich & Raven 1964). However, some Boraginaceae and Heliotropiaceae are visited by adult moths, especially tiger moths (Arctiidae), that use the pyrrolizidine alkaloids they contain as a basis for their pheromones (see Heliotropiaceae), and these alkaloids may be an apomorphy for the order (see Reimann et al. 2004; Opitz & Müller 2009: literature). Arctiid catepillars also eat the plants (Hartmann 2009; other articles in Conner et al. 2009), and caterpillars of Arctiids like Grammia incorrupta self-medicate on pyrrolizidine alkaloid-containing plants, prefering food with more of these alkaloids when they themselves are heavily infected by endoparasites, their survival thereby being enhanced (Singer et al. 2009). Pyrrolizidine alkaloids and pentacyclic triterpene saponins variously sequestered and modified are also found in the secretions of the defensive glands of some Chrysolina and Platyophora beetles, both very speciose chrysomelids (Pasteels et al. 2001; Termonia et al. 2002; T. Hartmann et al. 2003). See Livschulz et al. (2018a) for molecular-level parallelisms in plant groups that produce these alkaloids (inc. Apocynaceae, Convolvulaceae, Fabaceae, Asteraceae, etc.).
Plant-Bacterial/Fungal Associations. Holm (1979) noted that some Boraginaceae and Hydrophyllaceae have similar rusts.
Genes & Genomes. The MEPAβ genome duplication event (dated at ca 55.8 Ma) would be placed at the Boraginales node given the list of taxa that have it (Landis et al. 2018).
Chemistry, Morphology, etc.. The homospermidine synthase gene, the first gene in the pathway that leads to pyrrolizidine alkaloids, has diverged considerably from the deoxyhypusine gene, from which it is derived, and this suggests an ancient separation of the two (Nurhayati et al. 2009; see also Langel et al. 2010). All Boraginales sampled (three families) have rosmarinic acid, no other lamiids do except Lamiaceae themselves, although the related chlorogenic acid (both are esters of caffeic acid) is also reported from Boraginales and is scattered in lamiids (Petersen et al. 2009). A recent study on trichome mineralization - silica, calcium carbonate and calcium phosphate wete the focus - suggests correlations with phylogeny (Mustafa et al. 2018a). Although sampling is poor and what the other lamiid families are doing is largely unknown, where silica might have been incorporated into the hair walls is tentatively indicated on the tree; cystoliths are scattered through the order (Fernández Honaine et al. 2023).
Taxa with root trichoblasts in radial files are quite common in Boraginales. Rabaey et al. (2010) discuss the distribution of vestured pits in the order.
Floral evolution shows much of interest in this group, although lacking both a well-supported phylogeny as well as developmental studies of the more distinctive flowers, details remain poorly known. Three unrelated clades have flowers that are not the normal 5-merous asterid flower. These include Codon (10-12-merous), Hoplestigma, and Lennoa and relatives (see also discussion under the euasterids). The first two of these have many more stamens and petals than normal, but a simple bicarpelate gynoecium; Lennoa and relatives also show an increase in carpel number. Both the petals and stamens of Hoplestigma are described as being in several series (Goldberg 1986), and its floral morphology and development would clearly repay investigation.
There are usually many veins diverging in the corolla lobes of Boraginales, but not in Wellstedia. Boraginaceae are well known to have a gynobasic style, and it has been suggested that the terminal style of Heliotropiaceae, at least, may be derived from the gynobasic condition: In the latter the pollen transmitting tissue proceeds to the base of the gynoecium (e.g. Hanf 1935).The style in at least some Boraginaceae and Hydrophyllaceae apears to be hollow (Guéguen 1901). This group has a variety of septal structures in the ovary (see Gottschling 2004). The considerable variation in ovule and endosperm development in Boraginales is conveniently summarized by Khaleel (1985). The extent of development of parietal tissue in the ovules is unclear - Boraginaceae, for example are tenuinucellate, while Ehretiaceae and Heliotropiaceae show development of parietal tissue, Hydrophyllaceae s. str. may have both conditions (e.g. Di Fulvio 1981, 1987; c.f. Gottschling 2004; Berg 2009). Suspensor size in the embryo varies considerably, and it may be haustorial (e.g. Lloyd 1899, 1902).
Since Boraginaceae often included Heliotropiaceae, etc., and Hydrophyllaceae included an even greater variety of taxa, much literature on Boraginaceae and Hydrophyllaceae in particular refers to two or more of the families as circumscribed below. For additional literature, see Al-Shehbaz (1991) and Gürke (1891), both general, Aniszewski (2007: alkaloids), Hartmann and Witte (1995) and Reimann et al. (2004), both pyrrolizidine alkaloids, Gunstone (1992) and Velasco and Goffman (1999), fatty acids, esp. gamma linolenic acid, but not restricted to Boraginoideae, Fisher et al. (1989), sieve tubes with massive nuclear ?protein bodies, not always present in families other than Boraginaceae, Buys and Hilger (2003: inflorescence morphology), Scheel et al. (1996: pollen), Prósperi and Cocucci (1979: callose in pollen tube variable in Hydrophyllaceae s.s., sampling needs to be extended), Diane et al. (2002b: transfer cells in seeds), Guignard (1893) and Svensson (1925), both embryology, and di Fulvio (1991: protein inclusions in nucleus). For Hydrophyllaceae s.l., see Brand (1913) and K. A. Wilson (1960), both general, di Fulvio 1997 (floral vasculature), Constance and Chuang (1982: pollen), and Cave and Constance (1959 and references) and Constance (1963) for chromosome numbers.
Phylogeny. For the relationships of Boraginales, see discussion under Gentianales.
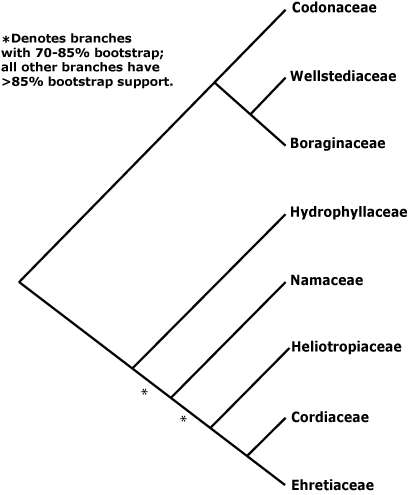
Within the Boraginales I clade, Wellstedia and Codon (ex Hydrophyllaceae), both African, are successive sister clades to Boraginaceae. Thus Codon linked with Boraginaceae in M. J. Moore and Jansen (2006) and both linked with Boraginoideae in Ferguson (1999), Luebert and Wen (2008), and in the comprehensive studes of Weigend et al. (2013a, see also 2013b) and Nazaire et al. (2014: Suppl. Fig. 4B).
Two groups also made up of ex-Hydrophyllaceae may be successive sister taxa and basal in the Boraginales II clade. It has been suggested that Nameae in particular, which include the only woody and tropical members of the old Hydrophyllaceae, may be sister to the [Cordioideae + Heliotropoideae + Ehretioideae] clade (Olmstead & Ferguson 2001; M. J. Moore & Jansen 2006: low bootstrap but high p.p. values; Luebert & Wen 2008). The relationships of Hydrophyllaceae were unclear in Weigend et al. (2013a), although this was not the focus of their study; there was weak support for their polyphyly in Weigend et al. (2013b). Gottschling et al. (2001), looking at the secondary structure of the ITS1 transcript, found Hydrophyllaceae s.l. to be monophyletic (within these, Nameae were paraphyletic) and sister to a clade [Heliotropiaceae [Cordiaceae + Ehretiaceae]], all with some support. Nazaire and Hufford (2012) sampled the whole Boraginales in the course of placing Mertensia, and relationships were [[Boraginaceae + Codonaceae] [Hydrophyllaceae mono- or paraphyletic + the rest]], but support was not strong (see also Nazaire et al. 2014: Suppl. Fig. 4A). Within a monophyletic Hydrophyllaceae s.l., again Nameae might be paraphyletic. Heliotropoideae were sometimes polyphyletic (see also Weigend et al. 2013b), while the grouping [Cordiaceae + Ehretiaceae] had some support in Weigend et al. (2013b). Refulio-Rodriguez and Olmstead (2014) found moderate support for the relationships [Hydrophyllaceae [Nameae [Heliotropiaceae [Cordiaceae + Ehretiaceae]]]] (see also Cohen 2014: sampling slight, general relationships not the focus, Nazaire et al. 2014: Suppl. Fig. 4A; Luebert et al. 2016b; Hasenstab-Lehman 2017). Z.-D. Chen et al. (2016) found the relationships [Cordiaceae [Heliotropiaceae + Ehretiaceae]] with quite good support but poor sampling, but no Hydrophyllaceae s.l. were included. See also Ferguson (1999) for relationships of Hydrophyllaceae s.l.. Details of relationships in this area in Vasile et al. (2020) are unclear, particularly where the Boraginaceae, etc., go. Note that although sampling was sparse, initial results from Angiosperm353 nuclear genome analyses suggested that Hydrophyllaceae s.l. might be monophyletic, Wigandia and Phacelia being sister taxa (Baker et al. 2021), and monophyly of Hydrophyllaceae was also recovered in Version 2 (Jan. 2022), where relationships were [Nama [[Wigandia, etc.], [Phacelia ...]]], although there was little support for the basal branch. Plastome relationships in this area are [Hydrophyllaceae [Namaceae [Ehretiaceae [Heliotropiaceae + Cordiaceae]]]] (H.-T. Li et al. 2021), while Zuntini et al. (2024) recognised a Boraginaceae s.l., as also in the Seed Plant Tree of Life ix.2024 version - note, neither Wellstedia nor Hoplestigma were included.
Two recent studies are helping to clarify relationships here. These are by Cohen (2025: 531 nuclear regions from lineage-specific and Angiosperms353 loci, 46 spp. of Boraginaceae, also Angiosperms353 loci from 115 accessions of Boraginales et al.) and Vasile et al. (2025) who added 161 new samples of the Angiosperms353 probe set for a total of 251 samples from 111 genera and including over 80% of the genera of Boraginales. Vasile et al. (2025) found that the position of Codon was somewhat ambiguous, and it might be sister to the Boraginales II clade, and both Cohen (2025) and Vasile et al. (2025) suggested that Hydroleaceae should include Namaceae and that Ehretiaceae should include Lennoaceae (see below), while family status for the distinctive Hoplestigmataceae (not included by Cohen 2025) and Coldeniaceae could be supported, although these latter two families include but two and one species respectively.
The holoparasitic Pholismateae/Lennoaceae have often been associated with Boraginaceae and/or Hydrophyllaceae (Cronquist 1981; Takhtajan 1997; esp. Yatskievych et al. 1986), and this general position is holding up in Angiosperms353 nuclear genome analyses (W. J. Baker et al. 2021a: see also Seed Plant Tree of Life). There are links with Ehretiaceae in particular. Thus both Ehretia and Lennoaceae have a shared intron in the mitochondrial gene cox1, and Tiquilia in particular has been placed as sister to Pholisma by Smith and dePamphilis (1998; see also Smith et al. 2000; Olmstead & Ferguson 2001; Hardy & Cook 2012; Nazaire & Hufford 2012: some analyses; Weigend et al. 2013b; Luebert et al. 2016b; Cohen 2025: Bourreria sister), although support values are often rather low, and a position sister to the rest of the family is possible (see also Nazaire et al. 2014: Suppl. Fig. 4A; Hasenstab-Lehman 2017: extremely long branch). As mentioned, even as early as the Seed Plant Tree of Life i.2022 version, Lennoa and Pholisma were embedded in Ehretiaceae, although support values in that area tended to be weak, c.f. later versions.
Classification. Given the uncertain position of Boraginales, sister to Lamiales (Refulio-Rodriguez & Olmstead 2014) is just one, although this has been confirmed by Zuntini et al. (2024), and the quite distinctive morphology of the group, ordinal status is appropriate (see A.P.G. IV 2016: included families not listed). However, family names have been given to clades in this part of the tree (e.g. Weigend & Hilger 2010), and Weigend et al. (2013b) and others provide a phylogeny on which to hang them (but see above); Cohen (2014) discusses the need to recognize Codonaceae and Wellstediaceae if Boraginaceae are to have features characterising them. A classification suggested by Luebert et al. (2016a) was largely followed until 2025, and that in Vasile et al. (2025) is now being followed - see also above.
Previous Relationships. Boraginaceae/Boraginales have sometimes been associated with Lamiaceae (Lamiales) because both often have gynobasic styles and fruits with four separate nutlets, but the latter have iridoids (but lack alkannin and pyrrolizidine alkaloids), opposite leaves, square (not rounded) stems, monosymmetric flowers usually with 4 stamens, endosperm with haustoria and embryo with a long suspensor - the two are not close. Furthermore, the radicle in Boraginaceae points upwards in fruit, while in Lamiaceae it points downwards. In previous classifications the woody Verbenaceae with a terminal style were separated from a more or less herbaceous Lamiaceae with a gynobasic stye, almost a parallel between a herbaceous Boraginaceae s. str. with a gynobasic style and the rest, more often woody and with terminal styles.
Pteleocarpa, with vestured pits, has often been included in Boraginaceae s.l., but it is here included in Gelsemiaceae (Gentianales). Hydrolea, ex Hydrophyllaceae but with axile placentation, is here placed in Solanales (as Hydroleaceae).
Although members of this group were previously thought to be related, the main division within them was drawn between a Hydrophyllaceae s.l., with parietal placentation and capsular fruits, and a Boraginaceae s.l., with ovaries that usually had four ovules and fruits that were usually indehiscent.
Thanks. I am grateful to Mark Gottschling for discussion.
Synonymy: Cordiales Martius, Echiales Lindley, Ehretiales Martius, Hydrophyllales Martius - Boraginanae Doweld
[Codonaceae [Wellstediaceae + Boraginaceae]] / Boraginales I: C with basal scales or similar structures +.
Age. The crown-group age of this clade is estimated to be (91-)81(-72) Ma by Luebert et al. (2016b) or (100.6-)88.9(-78.3) Ma by Chacón et al. (2017).
Evolution: Divergence & Distribution. Jeiter et al. (2020) note that there are structures at the base of the corolla apparently enclosing nectar and/or nectar sources throughout this clade; they suggest that such structures are homologous, hence their position above.
Ecology & Physiology. Members of the first three pectinations live in semi desert/desert areas (see also Bittrich & Kadereit 2016).
CODONACEAE Weigend & Hilger - Codon L. - Back to Boraginales
Perennial shrub-like herb; ?chemistry; plant spiny-hairy, unicellular hairs with cystolithic epidermal/foot cells; inflorescence weakly scorpioid; flowers (10-)12(-20)-merous; K deeply linear-lobed, open, broad and narrow lobes alternating; C broadly campanulate; filament bases with septae joining to C, these form lateral walls of nectar compartments [= lateral filament scales]; pollen tricolp(or)ate, pseudocolpi 0; placentae T-shaped, style deeply bilobed, stigmas papillate, punctate; integument ca 12 cella across; testa reticulate-papillate; endosperm copious; n = 17, x = ?
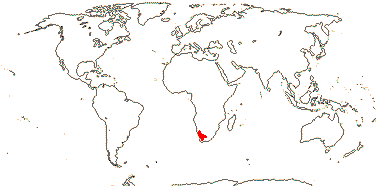
1[list]/2. S.W. Africa. Map: see Retief et al. (2005).
Age. Crown-group Codonaceae are (13-)7(-3) Ma (Luebert et al. 2016b).
Chemistry, Morphology, etc.. Codon is unique in Boraginales in having a kind of revolver flower, with basal nectar chambers, variously protected, alternating with the stamens (Jeiter et al. 2016).
For additional information, see Retief and van Wyk (2005), and Weigend and Hilger (2010, 2016).
[Wellstediaceae + Boraginaceae]: hairs with silica; C faucal invaginations + [= fornices, faucal scales], (0); ovules 2/carpel, apical, pendulous; testa transfer cells 0; endosperm 0.
Age. This node is around (77-)68(-60) Ma (Luebert et al. 2016b) or (89.2-)78.8(-68.4) Ma (Chacón et al. 2017).
WELLSTEDIACEAE (Pilger) Novák - Wellstedia Balfour f. - Back to Boraginales
Small shrubs; ?chemistry; indumentum ± scabrid, glands multicellular, peltate; leaves 2-ranked; inflorescences along the branches; flowers four-merous; C lobes three-veined, tube eight veined, (faucal scales 0); A free from C; pollen 12-25 x 8-15 µm, exine perforate to reticulate, tricolporate, pseudocolpi +, mesocolpium coarse-reticulate; nectary ?0; style (slightly) bifid, wet; ovule one/carpel, apical, epitropous, chalazal end pointed; fruit flattened, septae may separate from the walls; seed with fringe of hairs near the apex, slightly curved; embryo curved, cotyledons accumbent; n = x = ?
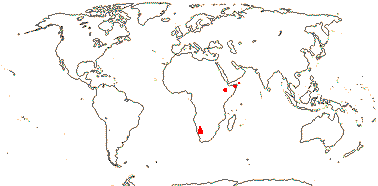
1[list]/5. S.W. and N.E. Africa. Map: see Thulin and Johansson (1996) and Reteif and van Wyk (2008).
Age. Crown-group Wellstediaceae are (49-)33.5(-21) Ma (Luebert et al. 2016b).
Evolution: Pollination Biology & Seed Dispersal. Wellstedia is a tumbleweed (?all species), and its capsules open only when wetted (Thulin & Johansson 1996).
Chemistry, Morphology, etc.. The field notes of Goldblatt & Manning 8763 describe the flowers as being "filled with nectar", however, Hunt (1969) and Retief and van Wyk (2008) noted that there was no disc; where is the nectar secreted? Thulin and Johansson (1996) described the capsule of Wellstedia as being septifragal, Reteif and van Wyk (2008) as being loculicidal and Hunt (1969) described it as being both, but in different places in the text. The ovule has a distinctive shape apparently caused by a chalazal projection (e.g. Reteif & van Wyk 2008).
See Hilger and Weigend (2016) for general information.
It would be interesting to know details of embryology and seed development.
Previous Relationships. Wellstedia was often associated with Coldenia (now Cordiaceae), largely, it seems, because both have 4-merous flowers.
BORAGINACEAE Jussieu, nom. cons. - Back to Boraginales
Annual or perennial herbs to shrubs; cork cambium deep seated [?sampling]; lamina with midvein alone obvious; (K open), corolla often funneliform, lobes spreading; anther connective produced or not; tapetal cells multinucleate, or nucleus polyploid; pollen grains tricellular, prolate or dumbbell-shaped; pollen tube with callose; G median, ovary very deeply lobed almost ab initio, with false septae, style gynobasic, hollow; ovules basal, integument 7-20 cells across, vascularized, (nucellar cap +), placental obturator +, (hypostase +); fruits nutlets, with central column/gynobase, nutlets 4, exocarp sclerified; testa (vascularized), exotestal cells with outer walls thickened and lignified (other patterns of thickening, or unthickened), most other cells disappear; endosperm (nuclear), at most slight, haustoria 0, oily [oils omega 6 gammalinoleic acid GLA, omega 3 stearidonic acid SDA], suspensor short [1-2 cells] or 0; x = 9 (?8), (protein inclusions in nucleus), nuclear genome [1 C] (0.062-)1.546(-38.51) pg.
Ca 94 [list]/1,793 - eleven groups below. Largely (north (warm) temperate, some on mountains in the tropics (map: see Wickens 1976; Meusel et al. 1978; Hultén & Fries 1986; Böhle et al. 1996; Långström & Chase 2002; FloraBase 2005; Wiegend et al. 2010: still incomplete).
Age. The age of this node is estimated at some 43 Ma (56-30 My) by Weigend et al. (2009), substantially older, around (76.9-)63.5(-46.5) Ma, by Nazaire et al. (2014) or (71.7-)62.6(-54.6)Ma by Chacón et al. (2017), or intermediate, (59.5-)53(-46.5)Ma by Luebert et al. (2016b).

1. Echiochiloideae Weigend
(Annual) herbs, subshrubs; ?chemistry; (vestured pits +); indumentum ± sericeous; leaves ± opposite; (flowers monosymmetric); corolla throat densely hairy or with cilia, (with ± obscure invaginations); pollen 2-3 colpate/colporate, not heterocolpate, (oblate-square in equatorial view); stigma punctate, (subterminal, tip sterile, bilobed to notched); gynobase flat or shortly pyramidal, nutlets (laterally compressed), (with non-basal attachment), (scar triangular, with a rim), thickened pericarp several-layered, ± ovoid, surface verrucose; n = ?
3/28: Echiochilon (16). Mexico, S. Brazil to N.E Argentina, Canary Isles, North and N.E. Africa to W. India (Map: from Lönn 1999; Långström & Chase 2002).
Age. Crown-group Echiochiloideae are estimated to be (50.1-)45(-41.5) Ma (Chacón et al. 2017).
Fossil nutlets of Boraginocarpus from northwest Africa that were confidently placed in Echiochileae (= Echiochiloideae: ?crown-group) are dated to 56-41 Ma (Hammouda et al. 2015).
[Boraginoideae + Cynoglossoideae]: rosmarinic (and lithospermic) acid + [caffeic acid esters], (pyrrolizidine alkaloids), (meroterpenoids) +, D-bornesitol + [cyclitol], isokestose oligosaccharides [= fructans], (with starch), (none in annuals); indumentum often ± hispid/scabrid, mineralized with Ca or Si, hairs with basal tubercule [usu. whitish], (cystoliths +); (flowers heterostylous); corolla salverform; pollen grains 2-5-colporate; nutlet scar lacking a rim.
Age. The age of this node is estimated to be ca 59.8 Ma (Chacón et al. 2017) or (65.3-)49.4(-37.9) Ma (Otero et al. 2019a).
2. Boraginoideae Arnott
Perennial (annual) herbs; flowers (monosymmetric), also with basal scales; gynobase flat, nutlets with basal attachment scar.
42/610. Mostly Mediterranean, East Asia, also Africa, South America.
Age. Crown-group Boraginoideae are estimated to be (64.4-)55.5(-46.8) Ma (Chacón et al. 2017).
2A. Boragineae Reichenbach —— Synonymy: Anchusaceae Vest
Basal leaves + (0); (floral bracts 0); pollen 4-6-aperturate (to 15-zonocolporate); (style hollow); embryo sac with lateral outgrowth towards the funicle; nutlets also 1, with thickened pericarp, usu. laterally compressed, ventrally keeled, surface tessellate and papillate, elaiosome + [= gynobase tissue] (0); n = (6-)7-9(-11<).
17/146: Anchusa (35), Symphytum (35). Mediterranean, also South America
Age. Crown-group Boragineae are some (40.9-)33(-25) Ma (Chacón et al. 2017).
2b. Lithospermeae Dumortier —— Synonymy: Buglossaceae Hoffmannsegg & Link nom. illeg., Cerinthaceae Berchtold & Presl, Echiaceae Rafinesque, Onosmaceae Martynov
(Annual) perennial herbs to (little-branched) shrubs; (naphthoquinones + [e.g. alkannin, reddish or purple dye]); vestured pits +; flowers (monosymmetric), (heterostylous); corolla often urceolate or funnelform, (faucal appendages 0, but pubescent/glandular patches +), tube commonly with abaxial trichomes, ("glands" inside); pollen (porate), (heteropolar [apertures closer to one pole]), (pollen basket + - threads cellular elements from modified septal regions [Echium]); style with/without sterile tips, stigma lobes (2), 4; nutlet with several-layered thickened pericarp, walls encrusted with calcium carbonate, surface often smooth, shiny; n = (4-)6, 7 (8<).
(20-)25/460: Onosma (150/?160), Lithospermum (80), Echium (60/?95), Arnebia (30). Mainly N. Africa and Eurasia, America (esp. Lithospermum), not tropical or Antipodean.
Age. Crown-group Lithospermeae are estimated to be (39.7-)31.9(-24.7) Ma or (51.5-)42.5(-35.3) Ma (Chacón et al. 2017, 2019 respectively).
3. Cynoglossoideae Weigend
(Basal leaves +); lamina with evident secondary venation; floral bracts 0 (+); flowers polysymmetric; tapetal cells uninucleate; pollen grains (6-8 porate); (G lacking secondary/false septum); gynobase broadly pyramidal to subulate (flat), nutlets often dorsiventrally compressed, dorsally concave, ventrally attached, (with large, triangular scar), glochidiate (marginal wings/other ornamentation/smooth), thickened pericarp single-layered.
Ca 48/1,070. ±World-wide.
Age. Crown-group Cynoglossoideae are estimated to be (38.3-)31.1(-23.2) Ma (Nazaire et al. 2014), (60.2-)52.3(-45.2) Ma (Chacón et al. 2017) or (64.1-)46(-33.4) Ma (Otero et al. 2019a).
first two: inflorescence bracteate; stigma capitate.Age. This node is (53.1-)42.3(-30.8) Ma (Chacón et al. 2017) or (53.9-)34.6(-16.5) Ma (Otero et al. 2019a).
3A. Trichodesmeae Riedl
Biennial herbs with rosette to (tall) shrubs; indumentum hispid to sericeous (0); leaves (opposite); K large, (winged); anthers exserted, forming a cone [?all]; pollen tricolporate, pseudocolpi 0; (K accrescent, bladdery), nutlets 1(-4), to ca 10 mm long, usu. laterally winged, glochidiate or not; n = 7, 11, 12.
2/ca 50: Trichodesma (45). Tropical to warm temperate Old World, inc. Australia.
Age. Crown-group Trichodesmeae are around (35.7-)22.9(-11.5) Ma (Chacón et al. 2017).
[Lasiocaryeae [Omphalodeae [Asperugeae [Rochelieae [Craniospermeae [Myosotideae + Cynoglosseae]]]]]]: pollen with pseudocolpi [heterocolpate].
3B. Lasiocaryeae Weigend
Small, annual to perennial herbs; indumentum sericeous; anthers included; nutlets very small, erect, ovoid to subcylindrical, often hairy; n = ?
3/5: Lasiocaryum (3). Himalayas to S. Central China.
Age. Crown-group Lasiocaryeae are estimated to be (27.9-)19(-11.1) Ma (Chacón et al. 2017).
[Omphalodeae [Asperugeae [Rochelieae [Craniospermeae [Myosotideae + Cynoglosseae]]]]]: ?
Age. This node is some (54.1-)46.8(-40.1) Ma (Chacón et al. 2017).
3C. Omphalodeae Weigend
Annual to perennial herbs; pollen dumbbell-shaped; style short, stigma capitate; nutlet compression?, glochidiate or not, (attachment median); n = 10, 11, 12..., also 2n = 19.
7/35: Omphalodes (25). Northern Hemispere, but one sp. on Juan Fernandez and another on the Chatham Islands.
Age. The age of crown-group Omphalodeae is (24.9-)17.5(-11.2) Ma (Chacón et al. 2017), (25.5-)16.6(-8.6) Ma (Otero et al. 2019a) or (36.6-)28.6(-20.4) Ma (Otero et al. 2019b).
[Asperugeae [Rochelieae [Craniospermeae [Myosotideae + Cynoglosseae]]]]: ?
3D. Asperugeae Ovczinnikova
Annual to perennial herbs; indumentum often 0, leaves glaucous; (faucal invaginations 0); anther epidermis with fibrous thickening [Mertensia]; style capitate [?all]; nutlets not glochidiate, smooth to rugose; n = 12.
4/50: Mertensia (40). North temperate.
Age. Crown-group Asperugeae are some (46.8-)36(-25.3) Ma (Chacón et al. 2017) or (37.6-)25.2(-13.7) Ma (Otero et al. 2019a).
[Rochelieae [Craniospermeae [Myosotideae + Cynoglosseae]]]: ?
3E. Rochelieae A. de Candolle
Annual to perennial herbs; indumentum often hispid; anthers included; pollen grains dumbbell-shaped, (syncolporate - Pseudolappula); stigma capitate; nutlets ?compression, (not glochidiate), gynobase subulate; n = 10, 11, 12...
7/210: Lappula (55, ?70), Eritrichium (50), Hackelia (45). Esp. Eurasia, also America, Australia, North Africa.
Age. Crown-group Rochelieae are (36.4-)27.8(-18.9) Ma (Chacón et al. 2017) or (37.6-)21.6(-13.7) Ma (Otero et al. 2019a).
[Craniospermeae [Myosotideae + Cynoglosseae]]: inflorescence usu. at least apically ebracteate.
Age. This node is around (47.5-)41(-34.1) Ma (Chacón et al. 2017).
3F. Craniospermeae Meisner - Craniospermum Lehmann
Biennial to perennial herbs; indumentum hirsute to floccose; faucal invaginations 0, (scales between C lobes); anthers exserted; stigma depressed capitate to disciform; nutlets erect, ovoid, rugose, dentate-winged; n = ?
1/6. Central and Central-East Asia.
Age. Crown-group Craniospermeae are (7.8-)4.1(-1.2) Ma (Chacón et al. 2017).
[Myosotideae + Cynoglosseae]: ?
Age. This clade is (53.2-)38.1(-27) Ma (Otero et al. 2019a).
3G. Myosotideae Reichenbach f.
Annual to perennial herbs; stem with endodermis [Myosotis]; indumentum various; (bracts +); (C contorted); pollen grains (4-6 (col)porate, pseudocolpi forming polar cap); stigma subcapitate (obscurely bilobed); nutlets (→10), hemispherical to tetrahedral, not much dorsally compressed, with lateral (ventral) keels, not glochidiate, smooth to papillate/?lenticillate, (carpopodium +); n = 6≤.
5/160: Myosotis (80-100), Trigonotis (60). ± Cosmopolitan.
Age. The age of crown-group Myosotideae is some (41.7-)35(-28.2) Ma (Chacón et al. 2017) or (42.2-)29.7(-20) Ma (Otero et al. 2019a).
3H. Cynoglosseae W. D. J. Koch —— Synonymy: Cynoglossaceae Döll
Annual to perennial herbs; (naphthoquinones + [e.g. alkannin, reddish or purple dye]); indumentum sericeous to hispid; (inflorescence bracteate); (heterostyly +); corolla with basal scales; (style deeply divided), stigma capitate [?all]; nutlets glochidiate (surface granular); n = (6, 7, 10, 11) 12...
Ca 20/553: Cynoglossum (180-200), Cryptantha (160), Plagiobothrys (70). America (Amsinckiinae), Eurasia, Australia (few).
Age. Crown-group Cynoglosseae are (39.4-)33.5(-27.9) Ma (Chacón et al. 2017).
Evolution: Divergence & Distribution. For lots of dates within Boraginaceae and discussion of the fossil record, see Chacón et al. (2017) and for dates within Cynoglosseae, see Otero et al. (2019a), in Omphalodeae, Otero et al. (2019b) and for those in Lithospermeae, see Chacón et al. (2019).
Weigend et al. (2013a) and Hammouda et al. (2015) suggested that Boraginaceae are likely to have originated in the Africa(-West/Central Asia) area, where its immediate outgroups and Echiochiloideae, sister to the rest of the family, are to be found; they all grow in rather arid conditions. As Weigend et al. (2013a) emphasized, extant Boraginoideae are predominantly Asian(-European), including eastern Asian; taxa elsewhere are clearly derived. Thus the South American Moritzia and Thaumatocaryon, the only Boragineae in the New World, are sister taxa (= Moritziinae), and are in turn sister to all other Boragineae, which are largely Eurasian; however, there are fossils from North America of late Miocene age that are assignable to this clade, so filling the distributional gap (Weigend et al. 2010; Chacón et al. 2017). Indeed, long distance dispersal (LDD) is common in Boraginaceae (Chacón et al. 2017: Fig. 2). All told, there may have been 11 intercontinental dispersal events in Boraginaceae (Luebert et al. 2016b), but this is likely to be a considerable underestimate given the suggestions in the next paragraph.
Thinking about American amphitropical disjunctions in particular, there are some 19 in Boraginaceae alone, and this is perhaps 8% of all such disjunctions (Simpson et al. 2017a), but the focus can be considerably narrowed. That is because most of these disjunctions are in Cynoglossoideae-Cynoglosseae-Amsinckiinae, a clade of a mere 334 species that is ca 21 Ma (Hasenstab-Lehman & Simpson 2012; Mabry & Simpson 2018; esp. Guilliams et al. 2017; also Otero et al. 2019b: Omphalodeae). (There are other notable clusters of such events in Poaceae - Peterson et al. 2010 - and Polemoniaceae - L. A. Johnson & Porter 2017; see also the Hydrophyllaceae—Namaceae area below.) Nearly all these disjunctions have happened within the last 6 Ma or so, although one is as old as ca 17.1 Ma, old for taxa showing this pattern (Simpson et al. 2017a) and the first part of another, in Omphalodeae (Otero et al. 2019b) may have been around 23.1-11.2 Ma, and all but one are from North to South America. The lopsided directionality of such events seems to be connected with the behaviour of the migrating birds that are likely to have dispersed the fruits (Guilliams et al. 2017; see also Guilliams & Baldwin 2011; Guilliams et al. 2016). Amsinckiinae are not notably associated with arid conditions, as with other taxa showing this N→S movement (see also Simpson 2017a), and many are selfers (see also Raven 1963b). There are no dramatic modifications to the nutlets of the disjuncts that might seem to enable long distance dispersal (Guilliams et al. 2017: having smaller, rougher nutlets may help; Chacón et al. 2017: endozoochory in the widely-distributed Lithospermum a preadaptation to LDD), but in Omphalodeae the fruits have a variety of wings, hooks, spines, etc. (Otero et al. 2019b; also Schenk & Saunders 2017); see also Cyperaceae-Carex. Although Amsinckiinae are largely restricted to the Americas, a few species of Plagiobothrys are scattered in the southern half of Australia - another major disjunction.
Although many Cynoglossoideae have glochidiate nutlets, it is unclear that such nutlets of themselves have had anything to do with diversification rates in the subfamily. Rochelieae and Cynoglossinae both have high diversification rates and glochidiate nutlets, but other clades with such nutlets had lower diversification rates, interestingly, no particular range expansions are associated with the two clades just mentioned, although they have occurred elsewhere in the subfamily (Otero et al. 2019a).
Echium has diversified on Macaronesia where there are a number of more or less woody species, and there also seem to have been two reversions to the herbaceous habit. Divergence within the genus may have begun some 20.6 Ma, but the Macaronesian diversification - the majority of the species are on the Canary Islands - can be dated to a mere (8.0-)4.2, 3.9(-1.5) Ma (Böhle et al. 1996; S. C. Kim et al. 2008; García-Maroto et al. 2009; Stöcklin 2011; Nürk et al. 2019: see Mansion et al. 2009 for more Mediterranean insular endemic Boraginaceae and Knope et al. 2012 for other rapid radiations). Here both disparification (in this case plant height), ≡ Simpsonian adaptive radiation, and diversification (species number increase) have been rapid, the latter despite an increase in generation time (Nürk et al. 2019): see also Hypericum, Hawaiian Lobelioideae, Lupinus, and Asteraceae-silverswords for similar radiations on (sky) islands.
Nazaire et al. (2014, q.v. for dates) discussed biogeographical relationships within the Asian-North America Mertensia. The 40-plus species of Myosotis in New Zealand represent a notable diversification for the islands. The species are morphologically very diverse - caespitose, rosette-forming, etc., flowers yellow, white, or blue, stamens exserted or not, with quite variable pollen grains - yet there is very little resolution of relationships in molecular studies (Meudt et al. 2015a; Meudt 2016; see also Winkworth et al. 2002; Chacón et al. 2017). Cohen (2021) looked at the biogography and aspects of floral evolution in Lithospermum, suggesting that the genus moved from the Old to the New Words via landbridges, with a subsequent increase in the diversification rate in the New World. But Cohen (2021) made it clear how much more there was still to do, including clarifying the outgroup, deciding on the position of fossils, checking methods of analyses, increasing sampling, and so on.
Weigend et al. (2013a) give further details of the evolution and diversification of Boraginaceae, especially Cynoglosseae s.l., while Cohen (2014) discussed the evolution of numerous morphological characters in the context of the phylogeny of the family. Noroozi et al. (2021) looked at various features of pollen morphology, especially size, shape and aperture number and type, in the context of the family phylogeny; links between these features and diversification were difficult to establish. At 5.60 x 1.68 µm, the pollen grains of PLagiobothrys (Cynoglosseae) are the snallest in the family (and the subfamily as a whole, minus Trichodesmeae, has the smallest pollen grains of the three subfamilies), while Boragineae have the largest grains - Boraginoideae as a whole also tend to hve large genomes (Noroozi et al. 2021).
Ecology & Physiology. Lithospermeae, which include a number of more or less shrubby and sclerophyllous species, are plants of open, rather dry habitats and prefer mineral soils (Chácon et al. 2019). Furthermore, they can tolerate the low calcium - high magnesium concentrations of soils derived from ultramafic rocks (Cecchi et al. 2011: Onosma), and they also grow on calcareous rocks, sandstones, etc. (Chácon et al. 2019 and references).
Echium is a good example of "island woodiness", where woody, more or less tree-like (up to 3 m tall) and sometimes monocarpic species have evolved on Macaronesia from herbaceous ancestors (Stöcklin 2011). The monotypic Myosotidium, from the Chatham Islands so somewhat east of New Zealand, is also quite a large plant.
Pollination Biology & Seed Dispersal. Pollination is predominantly by insects. North temperate megachilid osmiine bees like Hoplitis species of the Annonosmia-Hoplitis group collect pollen from concealed-pollen flowers of this family and/or members of Fabaceae-Faboideae. This odd pairing is perhaps because both groups of plants have pyrrolizidine alkaloids and/or nutrients that are essential for the larval development of the bees (Sedivy et al. 2013). Interestingly, bumble bees, but not honey bees, seem to avoid collecting pollen from Boraginaceae in the U.K., at least (Goulson 2010). Bumble bee larvae eat pollen and nectar, hence potentially being exposed to the deleterious effects of pyrrolizidine alkaloids. However, the larvae of honey bees that have been collecting pollen of Echium vulgare eat bee jelly in which the levels of alkaloids in this pollen have been much reduced; bee jelly is produced by nurse bees (Lucchetti et al. 2018). Basal Halictidae pollinate Boraginaceae (and Hydrophyllaceae: Patiny et al. 2008). In a number of taxa like Trichodesma and Onosma the anthers form a cone and the petals are more or less reflexed (the former genus), and buzz pollination occurs/is likely, and nectar may also be produced by these flowers, which is somewhat unusual (Teppner 2011, 2018; Chácon et al. 2019: Onosma) - see also Ericaceae. Bird pollination is uncommon in Boraginaceae, most reports being from Lithospermeae (Chácon et al. 2019). Finally, in Echium there is secondary pollen presentation, the pollen being held in anther hairs that develop from tissue between the sporangia (Hesse et al. 2000).
In Boraginaceae the corolla often changes colour as it ages, pink to blue, yellow to pink to blue, yellow to white, and so on; p.H. changes in the protoplasm are involved in the shift of pink to blue, and perhaps cell turgor changes in the shift of the colour of the "eye" of the flower from yellow to white (Weiss 1995; Nuttman & Wilmer 2008). Such changes seem to affect pollinator behaviour (Casper & La Pine 1984; Nuttman & Wilmer 2008 and references). Heterostyly occurs in some Boraginaceae and has evolved at least twelve times here (Cohen et al. 2012; Cohen 2014; see also Barrett & Shore 2008, Cohen 2019: general). Lithospermum, one of the genera with some heterostylous species, has undergone large increases and decreases in corolla tube length, which varies from about 1 to 120 mm long, some species being pollinated by hummingbirds (Cohen 2012, 2021). In Amsinckia there is also heterostyly and homostyly, as well as selfing, heterochrony being involved in the development of homostylous flowers that self, but the extent of paedomorphosis, the nature of the ontogenetic trajectories, etc., differ in the different selfing clades (P. Li & Johnston 2001, 2010).
Trunz et al. (2020 and references) describe three flower types in Boraginaceae: In the Pulmonaria type, the corolla tube is narrow and erect and the anthers are hidden, and nectar is the primary reward (see also Myosotis), in the Borago type, the flowers are pendulous and the anthers are enlarged and are joined medially to form a cone, pollen and nectar are the rewards, and in the Echium type, the corolla is monosymmetric, the flower being held more or less horizontally and the anthers and style being exposed, and nectar is the reward. Interestingly, all three flower types might be visited for pollen or nectar, depending on the species of bee, and solitary bees visited species with all three floral types (12 species of specialized solitary bees visited Echium alone, over twice the number of any other species studied); pyrrolizidine alkaloids were the main toxic compound in the pollen, and depending on the bee species, larvae might not develop or the pupae might develop, but not hatch; concentrations of the alkaloids in the pollen grains were often more or less lower than in the anthers (Trunz et al. 2020) - note, nectar was not a focus here. Altogeher, however, this is a complicated story.
The fruits of Boraginaceae may be called eremocarps, the nutlets separating from the central axis, rather than from each other as in a schizocarp s. str.; in Boraginaceae the lobes of the ovary, each with a single ovule, are separate from each other almost from the very beginning (Luebert et al. 2016a). The nutlets of Boragineae may have elaiosomes and are then dispersed by ants, while in taxa like Cynoglossum the nutlets have glochids and are dispersed in the fur of larger animals (Selvi et al. 2011). Some species of the large genus Cryptantha, especially diverse in western North and South America, have inflorescences with basal, cleistogamous flowers and non-dispersing fruits (Grau 1983; Weigend et al. 2016), and heterocarpic species are also known from Pentocarya.
Plant-Animal Interactions. Ceutorhynch seed weevils are quite commonly found on Boraginaceae; weevils moved on to the family perhaps only once, but there have been movements on to other hosts in other ceutorhynchs (Letsch et al. 2018).
Genes & Genomes. For variation in genome size in Boraginaceae, quite extensive for a lamiid clade, and possible correlations, see Kobrlová and Hrones (2019: focus on Czech Republic) and also Noroozi et al. (2021: whole family).
Chemistry, Morphology, etc.. Fructans are absent in annual species but also in the perennial Alkanna; starch may sometimes also be present (Bourdu 1957; Meier & Reid 1982). Carlquist (2017b) described vestured pits in Echium. Ma et al. (2018) noted that Boraginaceae, despite their herbaceous habit, had rather thick first-order roots.
Echium has obliquely monosymmetric flowers, while the flowers of some species of Nonea may be vertically monosymmetric, the abaxial stamen being much longer than the others (the flowers of Echiochilon are also monosymmetric - I would guess obliquely so); the flowers of Cerinthe have an oblique plane of symmetry (Selvi et al. 2009), although the flowers are functionally polysymmetric. Jeiter et al. (2020) discuss faucal scales and basal scales in Boraginaceae. The former are oppositipetalous invaginations of the corolla tube at its mouth that are borne more or less above the insertion of the stamens, and they can be quite large and finger-like and with a variable indumentum. They are probably involved in aspects of the pollination process. Basal scales vary from small swellings to complete rings to small to large (more or less enclosing the nectary or whole gynoecium) structures; there are often ten of them borne at the bottom of the corolla tube and with variable indumentum. Jeiter et al. (2020) were unable to suggest particular patterns of gains and losses for the faucal scales, but suggested that basal scales might be an apomorphy for the whole Boraginales I clade. ,/P.
Shamrove and Anisimova (2023) describe fruit development in Symphytum asperum; they describe a "placental obturator" from this species, although it seems to be developing from the carpel wall (ibid. Fig. 4: 17). Amsinckia has very strongly bilobed cotyledons.
For general observations, ee Weigend et al. (2016), Lönn (1999: Echiochileae),and D.-H. Liu et al. (2021: Rochelieae), also Carke (1977), Bigazzi and Selvi (1998: Boragineae), Hargrove and Simpson (2003: Cryptantha), Weigend et al. (2009) and Attar et al. (2018: Cynoglosseae) for pollen morphology, Y. Heslop-Harrison (1981) and Bigazzi and Selvi (2000) for the morphology of stigma papillae, Millsaps (1940) and Khaleel (1977a) for embryology, and Hilger (1985, 2014: Cynoglosseae), Seibert (1978: Lithospermeae), Ovczinnikova (2007 and references), Simpson and Hasenstab (2009: Cryptantha, see the cover photograph!) and Pourghorban et al. (2023: Cynoglossinae) for fruit morphology and development.
Phylogeny. Långström and Chase (2002) discussed tribal relationships within Boraginaceae. Nazaire and Hufford (2012; see also Cohen 2011a; Nazaire et al. 2014: Suppl. Fig. 4B) found quite good support for the relationships [Echiochileae [Cynoglossseae [Boragineae + Lithospermeae]]], the position of Echiochileae having the least support. In the detailed study of Weigend et al. (2013a) and especially Cohen (2014) and Chacón et al. (2016) the same general relationships, including the position of Echiochileae, were recovered. Boraginoideae were paraphyletic in the study of Chinese taxa by Z.-D. Chen et al. (2016), and with quite good support.
Support for the monophyly of Boraginoideae is quite good, but relationships within the two tribes it contains, Boragineae and Lithospermeae, are not well supported (Chacón et al. 2016: sampling rather poor); for the limits of the tribes, see also Saadati et al. (2011). Within Boragineae, Trigonotidae are to be completely dismembered, its two South American genera being sister to the Eurasian Boragineae (Weigend et al. 2010). Within Lithospermeae, the limits of Lithospermum are perhaps best extended (Weigend et al. 2009; Cohen & Davis 2009a, esp. b, 2012), however, the tree was poorly supported and the addition of relatively few (22) morphological characters had a major effect on support values and some on topology (Cohen & Davis 2011). Vegetative and floral features are highly homoplastic when optimised on the tree (Cohen & Davis 2011). Cohen (2021: 34 species, 298 nuclear genomic regions) found that the New and Old World species were monophyletic and sisters; there were differerences with the chloroplast gene-based topology. Chacón et al. (2019), sampling about half the species in Lithospermeae, clarified relationships there, although the monophyly of Echium and Onosma was unclear. Thomas et al. (2008) found that Lithodora is polyphyletic; for the phylogeny of insular Echium, see García-Maroto et al. (2009).
The monophyly of Cynoglossoideae, which in the circumscription above include representatives of just about all the tribes of Boraginaceae that have ever been described (Nazaire & Hufford 2012), is well supported, and its tribes also have good support (Chacón et al. 2016; Otero et al. 2019a); although Chacón et al. (2016) found relationships between the tribes to be unclear, resolution in Otero et al. (2019a) was somewhat better. Weigend et al. (2013a, see also Selvi et al. 2011; Nazaire & Hufford 2012) noted that Omphalodes and in particular Cynoglossum were highly para/polyphyletic, clades with species currently included in both Cynoglossum and Paracynoglossum may have arisen independently while Omphalodes is now in several pieces (Holstein et al. 2016a, b) - see also the trees and discussion in Cohen (2025) and Vasile et al. (2025). Cohen (2025) found that Echiochiloideae might be sister to Cynoglossoideae, but it depended on the analysis. Within Cynoglossoideae, and both Cohen (2025: q.v. for detailed discussions over several alternative placements in Boraginaceae in the context of different data sets) and Vasile et al. (2025) found that Trichodesmeae and Lasiocaryeae were successively sister to the rest of the subfamily rather than forming a clade together.
Asperugeae. Nazaire et al. (2014), focussing on Mertensia, found a largely Asian and a largely North American clade; the Beringian M. rivularis was sister to the latter.
Cynoglosseae. The subtribes are well supported (see also Otero et al. 2019a), but relationships within them for the most part need more support (see Chacón et al. 2016). Thus support along the spine of Cynoglossinae was weak, and Cynoglossum and other genera were para- or polyphyletic (Pourghorban et al. 2020). Hasenstab-Lehman and Simpson (2012) and Mabry and Simpson (2018) looked at relationships within the large North American genus Cryptantha, either highly para- or polyphyletic. Relationships in this group, Amsinckiinae, continue to be disentangled, and the small clade [Andersonoglossum + Adelinia] may be sister to the rest, or perhaps Andersonoglossum alone (Simpson et al. 2017b: the first good support with mtDNA only, the second obtained in other analyses, but low support) - overall support along the backbone of the phylogeny and for major groups went like this - cpDNA > mtDNA > nrDNA. The relationships of groups like Microulinae in particular are discussed by Cohen (2025, "tribes"; Vasile et al. 2025).
Myosotidae. Within Myosotis, the Antipodean species are derived, and although morphologically and palynologically very diverse they show little divergence at the molecular level (Winkworth et al. 2002: sections not monophyletic), while the focus in Meudt et al. (2015a, 2025: Angiosperms353 baits, genome skimming of whole plastomes nrDNA) was on New Zealand species, species limits, movement fron New Zealand to adjacent areas, etc.. X.-M. Xu et al. (2023a: 29 taxa, plastomes) looked at relationships of Trigonotis in China, and found good support for two main clades that correlated with nutlet type, bracteole presence, etc..
In Omphalodeae, the monotypic Gyrocaryum is sister to the rest of the tribe (Otero et al. 2019b; see also Holstein et al. 2016b).
Rochelieae. Huang et al. (2013) discussed relationships within Lappula; it and Eritrichum are polyphyletic, and Khoshsokhan-Mozaffar et al. (2018 and references) began disentangling relationships here. Recently D-H. Liu et al. (2025) carried out extensive analyses (single copy nuclear genes, plastomes, etc.) that had a focus on Asian Lappula (38 taxa., relatives 11 spp.); Lepechiniella and some Rochelia were embedded in Lappula, while La. mogoltavica might not belong, being sister to Pseudolappula sinaica. There was extensive gene-tree discordance and cytonuclear conflict, and with hybridization-driven gene flow (hybridization especially between polyploids) and ILS.
Classification. See Chacón et al. (2016) for the classification used above; they also recognize subtribes.
As will already be clear, generic limits need much attention - see Weigend (in Kadereit et al. 2016) for generic limits in European taxa. Hasenstab-Lehman and Simpson (2012) adjusted the limits of Cryptantha, but broader limits were adopted by Chacón et al. (2016); genera like Lithospermum, Trigonotis, Rochelia and Anchusa are polyphyletic (e.g. Nazaire & Hufford 2012; D.-H. Liu et al. 2025). The limits of Lithospermum have been adjusted, but the relationships of names to clades around there is still unclear (Cohen & Davis 2009b; Cohen 2011b). Cohen (2014) found that genera like Myosotis, Anchusa and Cynoglossum were not monophyletic, indeed, the findings of Weigend et al. (2013a) point out the need for a generic reclassification of Cynoglossoideae s.l. (see also Chacón et al. 2016). Holstein et al. (2016a, b) summarize changes in generic limits around the old Omphalodes. The limits of Echium and Onosma (Lithospermeae) need adjusting (Chacón et al. 2019).
Botanical Trivia. At ca 4 x 2 μm, the pollen of Cryptantha clevelandii (= C. hispidissima - M. Simpson iii.2019) is about the smallest of that of any angiosperm (Hargrove & Simpson 2003).
[Hydrophyllaceae [Ehretiaceae [Heliotropiaceae [Hoplestigmataceae [Coldeniaceae + Cordiaceae]]]]] / Boraginales II: (plant smells unpleasantly); tapetal cell nucleus number?; endosperm with both chalazal and micropylar haustoria; suspensor long.
Age. The crown-group age of Boraginales II is some (81.5-)72(-64) Ma (Luebert et al. 2016b).
HYDROPHYLLACEAE R. Brown, nom. cons. - Back to Boraginales —— Synonymy: Ellisiaceae Berchtold & J. S. Presl, Eutocaceae Horaninow, Namaceae Molinari
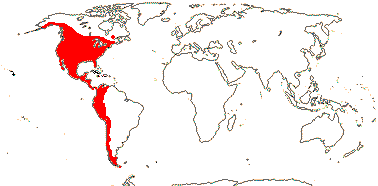
Plant (smelly ["scented"]); (meroterpenoids), inulin?, alkaloids?; (mycorrhizae 0); (wood rayless); ?cork; (vascular bundles separate); (stinging hairs + - Phacelia); leaves (opposite), (secondary veins palmate), (bipinnately compound), lamina margins (toothed, lobed); (inflorescences leafy); (bracts and bracteoles 0); (flowers 4-6-merous); ; (K free), C (lobes serrate); A elaborations at each side of the filament base; pollen tricolporate, (pseudocolpi +), tubes with callose (0); placentation variously axile or (stalked) parietal, placentae swollen, stigma punctate (capitate-funneliform); ovules (2)-many, epi-/apo-/pleurotropous, integument ca 6(-12) cells across; embryo sac (bisporic [the chalazal dyad], 8-nucleate [Allium type]); capsule loculicidal (+ septicidal), connate part of K postflorally adnate to fruit, persistent; seeds ruminate by inpushings of the exotestal cells/endothelium, exotestal cells thickened on inner and radial walls, endotestal cells persistent, walls esp. the inner periclinal ± thickened; endosperm various, copious to scanty, chalazal and micropyar haustoria +, with large lateral projections, (reserve hemicelluloses), embryo (short), chlorophyllous or not; x = ?, (protein inclusions in nucleus).
16[list]/320: Phacelia (210); Nama (53). Western North and South America, esp. drier areas of southwestern North America, Antilles, Hawai'i. Map: see Brummitt (2007) and Heckard (1963). Photo - Undetermined Flower.
Age. Crown-group Hydrophyllaceae are estimated to be (52.5-)40.5(-29.5) Ma (Luebert et al. 2016b) or substantially older, some (83.1-)66.1(-51.7) Ma (Vasile et al. 2020).
Some hierarchy to integrate - Nameae: Annuals to (rhizomatous) perennials to small trees; nectary 0; G (seminferior), styuli separate ± to base; n = 7, 13, 14, 17, 19. Romanzoffieae: (C scales +/reduced); (A unequal in size); nectary disciform; n = (5-)8(-14), etc.. Hydrophylleae: annuals to rhizomatous perennials; hairs (with silica); C contorted, (scales +/reduced); nectary as glands; placentae enlarging after ovule initiation; (ovules with some parietal tissue + - Nemophila), embryo sac (with chalazal haustorium - N.), (with chalazal haustorium - N.); (seeds with elaiosomes - N.), (exotesta largely disappearing - N.); n = (6, 7)9(10-12).
Evolution: Divergence & Distribution. Hydrophyllaceae probably originated in North America, and there appear to have been three separate long distance dispersal events to South America within the last ca 8.6 Ma (Vasile et al. 2020), Wigandia perhaps getting to South America via stepping-stone dispersal events.
Vasile et al. (2021, 2022: ndhF topology) looked at the evolution of ovule number and gynoecial morphology in Hydrophyllaceae s. str.; 4 ovules/gynoecium would appear to derived in the family, and more ovules, perhaps with axile placentation, plesiomorphic.
Pollination Biology & Seed Dispersal. There are paired structures at the bases of the filaments that range from being barely perceptible to quite elaborate - tubular and longer than the corolla tube itself; nectarostomata on the lobed nectaries are usually restricted to the lobes that are in the antepetalous position (Jeiter & Weigend 2018).
Myxospermy is reported from Hydrophyllaceae (Grubert 1974), while elaiosomes are reported from Nemophila. Pyrophytes are quite common in Hydrophyllaceae, germination occurring only after exposure of the seeds to smoke (Hofmann et al. 2016).
Chemistry, Morphology, etc.. Acicular protein bodies are found in the nuclear remnants in the sieve tubes of Hydrophyllaceae s. str.. For the morphology and mineral composition of the stinging hairs of Wigandia, see Mustafa et al. (2018b).
The mesocolpium of Hydrophyllaceae s. str. is coarse-reticulate, as in some Boraginaceae (Wagenitz 1992). The various structures so common in the corolla tube at about the level of stamen insertion presumably have something to do with guiding the pollinator to a nectar source. Cronquist (1981) suggested that Hydrophyllaceae lacked nectaries, but nectaries that are either raised and annular or are swellings at the base of the ovary wall are often described (di Fulvio 1997; Hofmann 1999; di Fulvio et al. 1999). There are alsp nectaries at the base of the ovary in the ovary wall of erstwhile Namaceae, and the number (1, 3) of vascular bundles varies (di Fulvio et al. 1997). Di Fulvio (1989a) described the ovules of Nama as being crassinucellate, but there can only be a single cell layer below the nucellar epidermis (for the nucellus, see also Berg 2009.
For general information, see Hofmann et al. (2016), for wood anatomy, see Carlquist and Eckhart (1984), for floral morphology, see Hilger (1987), for embryology, see di Fulvio (1989a, 1993), for seed morphology, see Chance and Bacon (1984), Bacon et al. (1986), and Chuang and Constance (1992), and for chromosomes in Phacelia, see Walden et al. (2014).
Phylogeny. Erstwhile Namaceae are basal and paraphyletic, and Hydrophyllaceae s. str. are embedded in them; Nama itself is paraphyletic (Vasile et al. 2025). Within the old Hydrophyllaceae, Phacelia is sister to other Hydrophyllaceae examined (Luebert et al. 2016b); for its phylogeny, see also Gilbert et al. (2005), Hansen et al. (2009) and Walden et al. (2014: sampling quite good). Romanzoffia was sister to Phacelia, and within the latter subg. Pulchellae sensu restricto was sister to the rest, albeit with poor support. Relationships obtained by Vasile et al. (2020) depended on whether ITS or ndhF sequences were examined. The topology of the latter analysis, [[Draperia [Tricardia + Hesperochiron]] [[Emmenanthe [Eucrypta [Hydrophyllum [Nemophila + Pholistoma]]]] [Romanzoffia + Phacelia]]], has mostly rather moderate support; this is the tree used to understand biogeography and on which characters have been optimized (Vasile et al. 2021, 2022).
Age. The crown-group age of this clade is estimated to be (91-)81(-72)Ma by Luebert et al. (2016b).
[Ehretiaceae [Heliotropiaceae [Hoplestigmataceae [Coldeniaceae + Cordiaceae]]]] / Primarily Woody Boraginaceae: trees or shrubs; bark oxidises; hairs with silica; pollen tubes lack callose; ovary with false [secondary] septae; ovules 2/carpel, epitropous [check], parietal tissue 1(2) cells across, (nucellar cap ca 2 cells across); transfer cells in funicle and placenta also; fruit a schizocarp, or drupaceous, endocarp multilayered; endosperm cellular.
Age. The age of this clade is ca 62 (Chomicki & Renner 2015: fig. S5) or (74.5-)66.5(-59.5) Ma (Luebert et al. 2016b).
EHRETIACEAE Martius, nom. cons. - Back to Boraginales
?Smell; perennial herbs to trees, (twining - Keraunea); inulins?; (vestured pits + - e.g. Ehretia, Rochefortia); petiole bundle arcuate; bracteoles?; (plant dioecious); (inflorescence laxly cymose / few-flowered corymb [K.]); flowers (adnate to foliaceous "bract" - K.), (4-)5-merous; K quincuncial, (imbricate), C ± rotate, quincuncial, (apert/imbricate); (tapetal cells uninucleate; polyploid); pollen (3-porate), pseudocolpi common, exine very various; G (loculi divided), placentation apical to axile, stylar compitum 0, stigmatic and gynoecial compitum +, style (impressed), bifid (twice-, unbranched), stigmas capitate or elongate; ovules (1 fertile/carpel), apotropous, integument 6-12 cells across, epidermal cells anticlinally elongated or not, placental obturator +/0, (hypostase +); (embryo sac bisporic, [chalazal dyad], 8-nucleate [Allium type]), (-3 megaspores functional); fruit drupaceous (schizocarp), stones 1-2-seeded, or 4 nutlets, K accrescent or not; endosperm copious (to 0), micropylar haustorium esp. variable, embryo curved, (cotyledons plicate - Cordia); n = 5, 7-11, 13, 16, etc., x = ?
10[list]/155: (7/160) Ehretia (ca 50), Bourreria (48), Tiquilia (28). Mostly tropical, S.W. U.S.A..
Age. Gottschling et al. (2004: plausible ages) estimated the age for the [Ehretia + Bourreria] clade at (120-)107, 98(-86) Ma, while ca 51.9 Ma is the estimate in Chomicki and Renner (2015: fig. S5) and (63.5-)57.5(-53) Ma in Luebert et al. (2016b).
Endocarps of ca 47.8 Ma Ehretia clausentia have been found in the London Clay, and seeds in them have coats with transfer cells clearly visible (Gottschling & Hilger 2003; see also Chacón et al. 2017).
[Bourreria + Pholismateae]: K long, ± envelops C in bud; stigma ± capitate-lobed, wet, extragynoecial compitum +.
Bourreria P. Browne
G truncate, style bifid or undivided, stigma papillate; ovules with parietal cells; fruit a drupe; n = 19.
1/50. Central and N. South America, the Antilles, E. Africa to Madagascar and the Mascarenes
Pholismateae Horaninow - Synonymy: Lennoaceae Solms-Laubach, nom. cons.
Plants echlorophyllous, root parasites, annual to perennial herbs; mycorrhizae 0; stele with 7-25 separate bundles, fibres 0 [imperforate tracheary elements], rays 0, vessel elements with simple perforation plates much narrower than lumen, pits not vestured; cortical bundles + [?leaf traces]; hairs glandular, uniseriate, leaves = scales; inflorescence ± capitate, (involucrate); flowers (4-)5-9(-10)-merous; K linear to flat, connate to free, C induplicate-valvate/imbricate, (margin toothed/lobulate); A (in two series - Lennoa), largely adnate to C; tapetal cells 1-2-nucleate; pollen 3-4-colporate, smooth or reticulate; nectary 0; G [5-16], loculi divided, placentation parietal [?L.], ovary truncate/style slightly impressed in G, stout, (hollow), stigma crateriform to slightly 5-9-lobate, wet; ovules epitropous, (sub)campylotropous, integument ca 2 cells across, parietal tissue 0; fruit with irregularly circumscissile outer pericarp, K and C persistent; seeds surrounded by endocarp, minute [≤ 1 mm long]; exotesta with fine reticulate thickenings; endosperm copious, starchy, with chalazal haustorium, embryo undifferentiated; n/x = 9.
2/4: Pholisma (3). California and Arizona to Guatemala, N. South America. Map: green area above, see Yatskievych and Mason (1986) and Parasitic Plants..
Age. Stem-group ages are (88-)68(-46) Ma, crown-group ages, (66-)41(-16) Ma (Naumann et al. 2013: Pholisma, Lennoa respectively). Around 55 Ma is the stem-group age in Luebert et al. (2016b).
Evolution: Divergence & Distribution. Initial diversification in the family may well have been in the New World (Gottschling et al. 2014b). M. J. Moore and Jansen (2006) and Moore et al. (2006) outline speciation and distribution in the amphitropical disjunct desert genus, Tiquilia, in some detail; it may have originated in the Palaeocene ca 58 Ma but began to diversify only rather later, some 33-38 Ma, and with repeated dispersals from W. North America to W. South America. Species of Tiquilia are mostly plants of American deserts and are unusual within Ehretiaceae in being herbs, or shrubs that flower very quickly, and their inflorescences may be crowded. Their fruits are dry, and secondary veins in the leaves go to the sinuses (Richardson 1977). Tiquilia nuttallii is a wide disjunct that grows in the western U.S.A. and also western Argentina.
Recent work using nrITS and matK suggests that paratype specimens of both Keraunea brasilensis and K. capixaba (Keraunea was only recently described, but in Convolvulaceae - Cheek & Simão-Bianchini 2013) were to be placed in Ehretiaceae, perhaps close to Ehretia itself (Muñoz-Rodríguez et al. 2022). Recent work (Moonlight & Cardoso 2023; Cheek et al. 2023b) confirms this position.
More work is needed to clarify the evolution of the holoparasitic Ehretiaceae (= Pholismateae above). However, Jeiter et al. (2022) note distictive features of these plants like the absence of a stylar compitum and the presence of a false septum in the gynoecium, the fruitlets ultimately being pyrenes (also sometimes called endomericarpids, endocarpids), which link Pholismateae closely to other Ehretiaceae like Bourreria succulenta in particular.
Ecology & Physiology. Pholismateae may parasitize their close relatives in Ehretiaceae (Smith et al. 2000), but their hosts include various other plants.
Genes & Genomes. For plastome evolution in Lennoa and Pholisma, see A. C. Schneider et al. (2018b: comparison with Tiquilia). There is normal (for a parasite) reduction in size (both the large and small single copy regions are getting on to two thirds smaller than in Tiquilia) and extensive (almost half) gene loss.
Chemistry, Morphology, etc.. Ehretiaceae vary in their wood anatomy. Lepidocordia has vessels in radial groups, apotracheal parenchyma, fibre tracheids with bordered pits, etc., and Gottwald (1982) kept it here largely because there was already so much variation that it did not seem out of place.
Gottschling et al. (2014a) describe a variety of septae in the gynoecium of Tiquilia. In embryology Ehretiaceae are perhaps closest to Heliotropiaceae (Diane et al. 2002a); the embryology of Lennoa is largely unknown.
For general information, see Gottschling et al. (2016) and for Lennoa et al., Suessenguth (1927), Yatskievych and Mason (1986), Bittrich (2016), Nickrent (2020) and The Parasitic Plant Collection, Carlquist and Guilliams (2017: wood anatomy, Lennoa etc.), Delgado-Pérez et al. (2024: Pholisma floral morphology, etc.), J.-X. Liu et al. (2003: pollen), Johri and Vasil (1956), Nagaraj and Fathima (1967), Khaleel (1977b) and Hanumantha Rao and Prakana Rao (1984), all embryology, and Baskin and Baskin (2021: seeds Lennoa, etc.).
Phylogeny. Gottschling and Hilger (2001), Gottschling et al. (2014a, b) and Luebert et al. (2016b) have all discussed relationships in Ehretiaceae. Although the exact position of the old Lennoaceae (= Pholismateae) was unclear as basal nodes in the family were not strongly supported, possible relationships in Luebert et al. (2016b) are [Pholisma [Tiquilia [Bourreria + Rochefortia]]]. The two genera of Pholismateae end up sister to Bourreria in the Seed Plant Tree of Life Jan. 2023 version, albeit support could be stronger and sampling better. However, relationships coming from analyses of the mitochondrial atp1 gene are [Pholismateae [other Boraginales [Gentianles [Boraginales [Lamiales + Solanales]]]]] (Q. Lin et al. 2022a), although that gene is not a reliable indicator of relationships. Recent work that focused on where Keraunea might be placed aligned that genus with Ehretia, Cortesia and Halgania, while Bourreria, Lepidocordia, Rochefortia, and sometimes Tiquilia, formed another clade (Moonlight & Cardoso 2023; Cheek et al. 2023b) - Pholismateae not included.
Classification. For genera in Ehretiaceae, see Gottschling et al. (2014b, 2016). It seems that Lennoaceae are yet to be incorporated into a suprageneric hierarchy here - I have included them in a Pholismateae above.
[Heliotropiaceae [Hoplestigmataceae [Coldeniaceae + Cordiaceae]]]: ?
HELIOTROPIACEAE Schrader, nom. cons. - Back to Boraginales
(Annual to perennial herbs to trees (lianes); fructan sugars accumulated as isokestose oligosaccharides [inulin: Heliotropium]; cambium not storied; pericyclic sheath 0 [?always]; pits not vestured (+); (cystoliths +); petiole bundles arcuate; (tetrahedral crystals +); hairs branched, eglandular; leaves usu. conduplicate [Tournefortia], (margins serrate); bracts 0 (+); C imbricate, with involute margins, contorted, etc., (mouth with scales); anthers basifixed, usu. connate at apex, connective produced or not; tapetal cells binucleate; pollen (4 colpate), (porate), (heterocolpate); ovary becoming deeply lobed, style (deeply) impressed, much swollen apically (0), stigma receptive only basi-laterally, discoid, then conical and ± bilobed at sterile apex, or hemispherical, with a ring of hairs, stigma wet; ovules anatropous and hemitropous [same ovary], integument (?3-)8 cells across, parietal tissue 0, nucellar cap +, (obturator +); fruit a schizocarp, stones 1-(2-)seeded, (drupe with 4 1-seeded stones); seed exotestal; endosperm 0 (slight) at maturity, ?haustoria, embryo curved or straight, cotyledons large, suspensor long; n = 5, 7-9, 11-14, etc., x = ?
4[list]/450: Heliotropium (241), Euploca (164). Tropical to warm temperate. Map: see Frankenberg and Klaus (1980), Flora of China vol. 16 (1995), Gottschling et al. (2004) and Flora Base (consulted 2005). [Photo - Flower.]
Age. Most of the ages for this clade were over 122 Ma (Gottschling et al. 2004), but its age in Chomicki and Renner (2015: Fig. S5) is ca 42.4 Ma and there is an estimate of (62.5-)54(-46.5) Ma in Luebert et al. (2016b).
Evolution: Divergence & Distribution. Luebert et al. (2011a) suggest that Heliotropiaceae diversified in the Palaeocene or earlier, and within Heliotropium (inc. Tournefortia) diversification began about 45 Ma, stem node age is ca 60.7 Ma in the Middle Eocene (see Luebert et al. 2011b for many more details). For the evolution of habit, etc., in Neotropical Heliotropium, see Luebert et al. (2011b). Heliotropium sect. Cochranea is a notable endemic group of the Atacama Desert (Luebert & Wen 2008).
Frohlich et al. (2022) note extensive homoplasy in Euploca, including in the C4 photosynthetic pathway. Also, species that are called "inflorescence plants" have evolved more than once; these species, annuals or perennials, produce only a few leaves after germination and then the whole plant body switches and produces what can be thought of as modified cincinni, and nothing else. The bracts are large and foliaceous, opposite the flowers, and they may subtend branches with the same morphology - perhaps a tendency to evolve... (Frohlich et al. 2022)?
Ecology & Physiology. The old Heliotropium section Orthostachys etc. (= Euploca) contains ca 100 species with C4 photosynthesis, as well as about 40 species with proto-Kranz, C2 and C3 pathways (Vogan et al. 2007; Sage et al. 2011, 2014; Muhaidat et al. 2011; Frohlich et al. 2022); C4 photosynthesis may have evolved four times here. Overall, development of C4 photosynthesis here, with the bundle sheath accumulating organelles and the mitochondrial glycine decarboxylase and with interveinal distances decreasing, is similar to that in Asteraceae and Poaceae (Khoshravesh et al. 2019).
Plant-Animal Interactions. Wilting plants and/or flowers of Boraginaceae and Heliotropiaceae, especially the latter, are visted by adult butterflies (Danainae, Ithomiinae) and moths (Ctenuchidae, Arctiidae). The pyrrolizidine alkaloids the plants contain are used in their pheromones of these insects or deter predation by other animals (see also Crotalaria, Apocynaceae and Asteraceae-Asteroideae: e.g. Edgar 1984; Edgar et al. 1974; Ackery & Vane-Wright 1984: detail for the danaines; Boppré 1986; Brown 1987; Opitz & Müller 2009). Singer et al. (2009; see other articles in Conner et al. 2009; Zaspel et al. 2014) discuss self-medication (pharmacophagy) and its evolution by caterpillars of Arctiinae on food containing high concentrations of pyrrolizine alkaloids. Cordia (Cordiaceae) is also sometimes visited, although it is not known to contain these alkaloids. Caterpillars of arctiid moths also eat alkaloid-containing members of these families (Hartmann 2009; other articles in Conner et al. 2009).
Chemistry, Morphology, etc.. Tournefortia astrotricha lacks inulin. Fernández Honaine et al. (2023) notes some occurrences of cystoliths here.
Although the style in species of Heliotropium section Heliothamnus appears to be gynobasic, as in Boraginaceae, it is attached to the gynoecium, and several details of fruit morphology and development distinguish the fruits of the two groups (Jeiter et al. 2018). Thus in Boraginaceae there are four separate mericarpids that are distinct from the very earliest stages of their development while in Heliotropiaceae the fruits are schizocarps.
For general information, see Förther (1998) and Diane et al. (2016), for comments on the pollen morphology of Heliotropium, see Scheel et al. (1996), and for details of embryology, see DiFulvio (1978).
Phylogeny. Hilger and Diane (2003; see also Luebert et al. 2016b) found a clade [Ixorhea [Myriopus + Euploca]] to be sister to Heliotropium, but relationships of the first group were not very well supported. For relationships in Neotropical Heliotropium, see Luebert et al. (2011b), while in Baker et al. (2021: see also Seed Plant Tree of Life) Heliotropium texanum, the only species of the genus examined, was firmly embedded in the nine species of Euploca included. Indeed, Frohlich et al. (2022: rbcL, matK, ITS), recovered the relationships [[Heliotropium sect. Orthostachys (= Euploca) + Myriopus] Heliotropium s. str. (inc. Tournefortia)].
Classification. Generic limits in Heliotropiaceae need attention (Diane et al. 2002a; Hilger & Diane 2003; Luebert et al. 2011a, b); Craven (2005) suggested that everything was best placed in Heliotropium s.l. - a suggestion not without merit. Certainly genera like Tournefortia and Ceballosia are to be synonymised under Heliotropium (e.g. see phylogeny in Luebert et al. 2016b) and part of Heliotropium split off as Euploca (see above; Frohlich et al. 2020).
[Hoplestigmataceae [Coldeniaceae + Cordiaceae]]: ?
HOPLESTIGMATACEAE Gilg - Hoplestigma Pierre - Back to Boraginales
Deciduous trees; ?nodes [3 traces at the base of the petiole]; buds perulate; bracts 0; calyx connate, irregularly splitting, C 11-15, connate, opening irregularly; stamens 20-35, in 3 irregular series, adnate to very base of C; pollen with pseudocolpi; ?nectary; G with intrusive parietal placentation, style once branched, branches long, bent outwards, then inwards, with U-shaped, expanded stigmas; ovules apical; fruit drupaceous, endocarp undivided, 4-seeded, with 2 sterile chambers; n = ?
1[list]/2. Western Africa from Cameroon to Liberia.
[Coldeniaceae + Cordiaceae]: ?
COLDENIACEAE Weigend & Hilger - Coldenia procumbens L. - Back to Boraginales.
Procumbent annual, adventitious roots +; lamina margins crenate-serrate; inflorescence leafy; flowers 4-merous; corolla urceolate; style branches short, stigma capitate; fruit deeply lobed, schizocarp, with 4 nutlets, K much accrescent; n = ?
1[list]/1. Tropical and subtropical Old World.
Classification. Gottschling et al. (2005) equivocated as to whether Coldenia should be included in Cordiaceae - if it is, that family has few apomorphies (if separate, the former has adventitious roots and teramerous flowerrs as apomorphies, the latter, four stigmatic branches, an undivided endocarp, and plicate cotyledons (Luebert et al. 2016a). Luebert and Wen (2008) suggest a narrow circumscription for Cordiaceae; Hoplestigmataceae then have to be recognized as well.
Previous Relationships. Hoplestigmataceae were included in the Violales by Cronquist (1981), perhaps because of their parietal placentation.
CORDIACEAE Dumortier, nom. cons. - Back to Boraginales —— Synonymy: Sebestenaceae Ventenat, nom. illeg.

Shrubs to trees, (lianes); terpenoid-based quinones +; secondary phloem stratified; nodes 3:3 [Cordia, see below]; petiole bundles (invaginated) annular/arcuate, (cortical and) 0, 2, 4 wing bundles; crystal sand and prismatic or columnar crystals +; (lamina venation subpalmate); bracteoles usu. 0; (cymes subdichasial); (flowers heterostylous), (4-8-merous); (C contorted); pollen often spiny/with clavate projections, (substriate, rugulose), pseudocolpi 0 (+), (3-porate - Varronia); styles twice divided, stigmas punctate to capitate; ovules straight, integument 6-10 cells across, (parietal tissue 0), (obturator +); fruit drupaceous, endocarp undivided, K often much accrescent [an anthocarp!]; testa vascularized [Cordia], 3-4 layers of transfer cells; endosperm haustoria?, 0, cotyledons plicate, margins toothed; n = 7-9, x = ?
3[list]/350: Cordia (200+), Varronia (125). Tropical, especially South America. Map: see Gottschling et al. (2004), Flora Base (consulted 2005, green), and Hoplestigma, from Brummitt (2007). Photo: Flower, Fruit.
Age. Gottschling et al. (2004: plausible ages) estimated the age for Cordia alone at (107-)95, 92(-80) Ma, but ca 50.8 Ma for the family seems more likely (see Chomicki & Renner 2015: fig. S5; Cordia s.l., ca 38.6 Ma), similarly, (60-)50(-38.5) Ma is the estimate in Luebert et al. (2016b).
Evolution: Pollination Biology & Seed Dispersal. Heterostyly occurs in Cordia (Cohen 2019 and references).
The calyx can be much accrescent in Cordia, either fleshy (the usual condition, = sect. Cordia) or dry and inflated and completely enveloping the fruit, or developing as five wings like propeller blades (Gottschling & Miller 2006); myxospermy is also reported from the genus (Grubert 1974).
Plant-Animal Interactions. Cordia nodosa is a well-known myrmecophyte. An ant associated with it, Myrmelachista schumanni, forms so-called "devil's gardens" by poisoning the surrounding vegetation with formic acid, C. nodosa alone persisting; the gardens are known from western Amazonia and French Guiana (Davidson & McKey 1993; Salas-Lopez et al. 2016 and references). Scale insects are part of this association, and Pringle and Moreau (2017) studied the bacteria and fungi associated with ants and scale insects. Allomerus octoarticulatus also protects C. nodosa against herbivory, at the same time sterilizing its host (Frederickson et al. 2012 and references; Malé et al. 2017). For the food bodies of Cordia, see Solano et al. (2005), for a connection between indumentum presence and the size of the ants associated with the plant, see Davidson and McKey (1993), and for the evolution of myrmecophytism see Chomicki and Renner (2015); see also Ruiz-González et al. (2011).
Vegetative Variation. The growth pattern of some species of Cordia, including the myrmecophilous species, is distinctive: the apex of the stem aborts, some branches are plagiotropic, while one becomes orthotropic and forms the renewal shoot. The inflorescence can also be oddly placed.
Chemistry, Morphology, etc.. The central foliar trace of Cordia is at least sometimes a striking inverted "C" when it joins the central stele, but I do not know how widespread this character is (see Neubauer 1977b for nodal anatomy of Cordia myxa - a similarly modified 1:3 node).
For information, see Gottschling et al. (2016: general), for the leaf anatomy of Varronia, see T. S. Silva et al. (2023), and see also Heubl et al. (1990: esp. Cordia), Nowicke and Miller (1990) and Scheel et al. (1996), both pollen, and Khaleel (1975, 1982: embryology).
Phylogeny. For the phylogeny of Cordiaceae, see Gottschling et al. (2003); Coldenia is there sister to the rest of the family, but Hoplestigma places outside Coldenia in later analyses (e.g. Luebert et al. 2016b). For relationships within Cordia s. str. and its immediate relatives, see Miller and Gottschling (2007) and Weeks et al. (2010).
Classification. For generic limits in Cordiaceae see Gottschling et al. (2003), Gottschling and Miller (2014) and Luebert et al. (2016a). Miller and Gottschling (2007) segregated Varronia from Cordia; the latter has a rather lengthy synonymy.