leaves 1-3-compound, pulvinate along petiole/base of petiolules; T free; pollen with ring‐like apertures, smooth, perforate.
2/6: Gonatopus (5). East and southeast Africa.
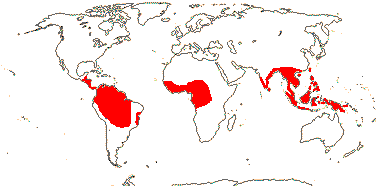
Habit various, (plant epiphytic); (plant monopodial); (glucomannans +); laticifers +, articulated, (anastomosing), (0); collenchyma in cortical bands or bundle-associated strands (0); fibres variously associated with bundles; biforine raphides + [H-shaped in T.S., wall thick, except for papillae at the two ends, unlignified, cell contents mucilaginous] (0); leaves very variable, (blade developing basipetally from hyperphyll/hypophyll junction), (parallel veins of different orders), (vein endings free); (inflorescences several together), (spadix with sterile zones); P 0; staminate flowers: A connate, connectives thick, (anthers introrse); pollen (mixed with raphides), atectate, (with polysaccharide spines), (in tetrads), ectexine thin [pollen not resistant to acetolysis], sporopollenin 0 or +, wall with polysaccharides, endexine bilayed, (thick, spongy), intine massive; pistillode +; carpelate flowers: staminodes +, (G [2-4(-47)], placentation parietal, apical, basal, (styles connate), stigma various; ovules 1-many/carpel, (straight), parietal tissue 0-3 cells across; (endosperm chlorophyllous), (with starch), chalazal haustorium +, unicellular, (storage cotyledon +); n = 7+, 13, 14, 17 common; (cotyledon sheath leafy); (collar rhizoids +); long-chain ω-phenylalkanoic and ω-phenylalkenoic acids in seed lipids.
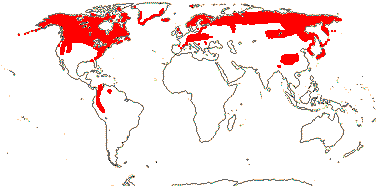
75/2,580. Tropical (warm temperate - Arum and relatives; cool temperate - Calla). Map: from Mayo et al. (1997). Photo Flowers.
Age. Crown-group Aroideae are estimated to be some (92-)82(-73) Ma (Nauheimer et al. 2012b) or (83.5-)73, 60.9(-55.9)/89.7-)79.2(-65.4) Ma (Canal et al. 2018, 2019: sampling).
Macrofossils apparently of Araceae-Aroideae, although with a loosely reticulate tectum, have been discovered in deposits 120-110 Ma in Portugal (Friis et al. 2010, 2011). Afrocasia (Araceae) from deposits in Egypt ca 73 Ma has been placed in crown-group Aroideae (Coiffard & Mohr 2016, 2018).
[Anubiadeae + Calleae]: plant aquatic, rhizomatous; laticifers simple, articulated; leaf venation striate; spadix appendix 0.
Anubiadeae Engler - Anubias Schott
A connate, connectives thickened; G usu. 2–3‐locular, placentation axile.
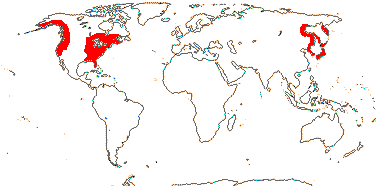
1/8. Tropical Central and West Africa.
Calleae Bartling - Calla palustris L. —— Synonymy: Callaceae Bartling
Leaves 2-ranked, ligulate; spathe open; flowers perfect, (apical flowers staminate); P 0; A 6(-12), free, connectives slender; pollen grains disulcate/aperture ring-like, surface psilate to perforate, exine tectate-columellate; G 1-locular, placentation basal; ovules 6-9; testa thick; endosperm copious; n = 18.
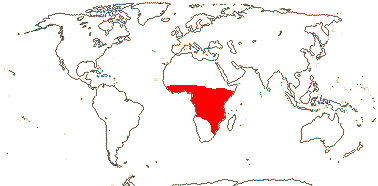
1/1. North cool Temperate. Map: see Aroideae, from Mayo et al. (1997), area in green.
Montrichardia Crüger
Swamps; plant caulescent; spadix appendix 0; pollen smooth, ectexine thin, intine massive, grain explodes on contact with water, intine tube develops.
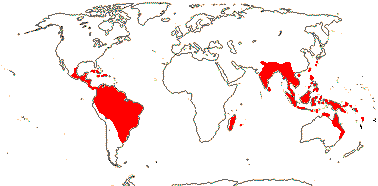
1/2. Central and ± northern South America, the Antilles.
Schismatoglottidodae Ezedin / [Philonotieae [Schismatoglottideae + Cryptocoryneae]]: ?
Philonotieae S. Y. Wong & P. C. Boyce - Philonotion Schott
Lamina apex long-caudate, often tubular; peduncles longer than petioles; spadix basally adnate to spathe, spathe kettle and connate-margined tube 0, not twisted; P 0; anther dehiscence porose; placentae 2, parietal; ovules 1-2/carpel; spathe limb abscising; seeds costate.
1/3. Northeast South America, white sand soils.
[Schismatoglottideae + Cryptocoryneae]: ?
Schismatoglottideae Nakai
Spathe kettle 0, tube not twisted, edges not connate; anther thecae (with horn-like apex), porose; pollen smooth, with crystals or druses, (echinate - Apoballis); placentae 3, parietal, ovules many/carpel, (>4, placentation basal); spathe limb abscising; infructescence a splash-cup.
Schismatoglottis (120), Apoballis (14). South China to Vanuatu, esp. Borneo
Cryptocoryneae Wydler —— Cryptocorynaceae J. Agardh
±Aquatic, rhizomatous; plant rosette-forming; vernation involute/convolute; spathe with conspicuous lower kettle and long straight tube with connate margins [Cryp.] / shortly tubular, twisted; spadix with long sterile zone between staminate and carpellate flowers, terminal appendix short, apex of spadix postgenitally fused to spathe [Cryp.]; staminate flowers: A 1, anthers porose; ovules several/many carpel; fruit a dehiscent syncarp / pseudo-apocarp; spathe limb deliquescing.
2/85: Cryptocoryne (70), Lagenandra (15). India and southern China to Malesia.
Philodendrodae Ezedin / [Aglaonemateae + Culcasieae + Nephthytideae + Philodendreae + Spathicarpeae + Zantedeschieae]: ?
Spathicarpeae Schott
±Plant (geophytic, stem tuberous / rhizomatous); leaves entire to bipinate / pedate; spathe ± adnate to spadix or free, spadis with flowers variously arranged; carpellate flowers: staminodes +/-, connate or not; G 1-8-locular, stigma capitate to variously lobed; ovules 1-6/carpel, orthotropous / anatropous; n = 17.
14/96: Dieffenbachia (57), Asterostigma (10) - 7 genera monotypic. South Mexico to Uruguay (c.f. Hentz Júnior et al. 2026: Fig. 1).
Culcasieae Engler
.
.
Aglaonemateae Engler
(supra-annually deciduous - Boycea); venation striate/reticulate; spadix appendix 0; anthers porose [B.]; pollen grains smooth/verrucate, verrucae easily dislodged [B.]; female zone dorsally adnate to spathe [B.]; placentation basal; ovule 1/ovary, funicle very short; testa thin; endosperm 0, embryo chlorophyllous [?all].
2/22: Aglaonema (21). Western India and S. China to East Malesia.
Nephthytideae - Nephthytis Schott + Callopsis Engler
Plant rhizomatous (rhizome deep); spadix appendix 0; pollen covered with deciduous verrucae; ovule 1/gynoecium, basal, outer integument massive, nucellar cap +, parietal tissue 0; fruit a berry; seed coat 0; endosperm 0, embryo chlorophyllous; n = 18, 20
2/6: Nephthytis (5). Tropical Africa.
Zantedeschieae Engler - Zantedeschia K. Sprengel
spadix appendix 0; anther with apical pores, connective thickened apicallu; G 1-5-locular, placentation apical to subaxile; ovules (1-)4(-8)/loculus, anatropous; plastid transmission biparental.
1/8. Angola to South Africa.
Philodendreae Schott —— Synonymy: Philodendraceae Vines
(Epiphytic); plant aromatic; foliar extrafloral nectaries [Philodendron, etc.]; staminate flowers: A free; carpelate flowers: staminodes +, stylar canals as many as carpels; funicle long; cotyledonary hypophyll blade-like, photosynthetic [P.].
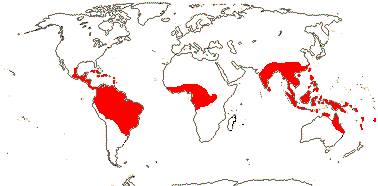
3/725: Philodendron (569 [?700-1,000]), Homalomena (140). South Mexico to tropical South America, the Antilles, South China to Malesia.
Arodae Ezedin / [Areae + Arisaemateae + Arophyteae + Peltandreae + Protareae + Pistieae + Thomsonieae + Caladieae + Arisareae + Colocasieae + Typhonodoreae] / Core Aroideae: ?
Areae Duby
Seasonally dormant; stem tuberous; spadix appendix +; G 1-locular; ovules straight, base broad, massive [Therio.]; suspensor with large terminal cell [Arum]; seed strophiolate; n = 7-13, etc..
8/106: Typhonium (40), Arum (26), Biarum (22). Esp. Mediterraneann, Macaronesia, Europe to southern China, Malesia, and E. and N.E. Australia.
Age. Crown-group Areae are thought to be (73.7-)59.2(-42.9) Ma (Renner & Zhang 2004).
Arisaemateae Nakai
Stem tuberous (rhizome); blade in bud with one (more, none) leaflets erect, vernation involute (convolute); sequential hermaphroditism [A.]; spadix appendix + (long, filiform, pendulous); G 1-locular; ovules 1-7, basal, straight; fruit a berry; testa not multiplicative - A..
2/192: Arisaema (185). N. Mexico to S.E. U.S.A., E. Africa to the Arabian Peninsula, E. Asia to W. Malesia, ± temperate, montane in the tropics.
Arophyteae Bogner
Spadix with female-male zonation; A filaments connate; pollen globose, exine spinose; n = 14, 19.
3/12: Arophyton (7). Madagascar, esp. the north.
Peltandreae Engler
(Large water plant; pseudostem +; viviparous - Thphonodorum) pistilate flowers: staminodes +; endosperm sparse-0, embryo relatively large.
2/3: Peltandra (2). Madagascar (introduced into Zanzibar, the Mascarenes, etc. - Renner & Zhang 2004), Quebec and eastern U.S.A., Washington, California, Cuba.
Age. The crown-group age of this clade is thought to be (79.5-)65.7(-60.2) Ma (Renner & Zhang 2004).
Fossil Peltandra is known in late Palaeocene-Eocene deposits from the U.S.A., Bohemia and Kazakhstan (Wilde et al. 2005).
Protareae Engler - Protarum sechellarum Engler
?To Colocasieae?.
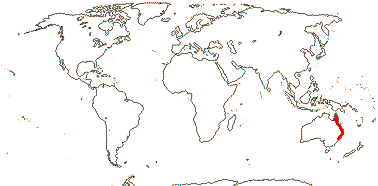
1/1. The Seychelles (the only member of the family found there).
Age. Protarum is part of a polytomy (it includes a paraphyletic Colocasieae) dated at (84.5-)80.3(-70.0) Ma (Renner & Zhang 2004).
Pistieae Lecoq & Juillet - Pistia stratiotes L. —— Synonymy: Pistiaceae C. Agardh
Floating aquatic, plant rhizomatous; leaves rosette-forming, ligulate [?= "stipules"], subsessile, main veins parallel; spadix with staminate flowers 2-8, single whorl; carpellate flowers single, adnate to spathe basally and also at tip of spadix; nectar-secreting collar above and scale beneath spathe constriction [≡ sterile flowers?]; spadix appendix 0; A 2, connate, thecae dehiscing apically; carpellate flowers: G 1, placentation basal, loculus with hairs, filled with mucilage; ovules many, straight, parietal tissue 0; testa thick; n = 14..
1/1. ?Africa, South America, widely introduced throughout tropics and subtropics.
Age. Pistia is part of a polytomy (it includes Colocasieae, Areae, etc.) dated at (84.5-)80.3(-70.0) Ma (Renner & Zhang 2004).
Seeds of Pistia have been found in deposits from the late Oligocene to mid Miocene in Europe and Russia (Renner & Zhang 2005).
Thomsonieae Blume - Amorphophallus Decaisne
Stem tuberous; annual dormancy; one (few) leaf/season, vernation involute; floral thermogenesis +; spadix appendix +; G 1-4 locular; ovules 1/loculus, anatropous; embryo large, endosperm 0; fruit a berry; n = 13, 14.
1/237. Old World tropics to Malesia and Australia (Queensland).
Caladieae Schott —— Synonymy: Caladiaceae Salisbury
Climber, (submerged aquatic); exudate white, with sterols/sterol esters on terpenoid particles; (pollen in tetrads); ovule 1, basal; cotyledon sheath photosynthetic, bifacial [X.].
7/339: Xanthosoma (204), Chlorospatha (68). Neotropical, South China to West Malesia (Hapaline).
Colocasieae Brongniart —— Synonymy: Colocasiaceae Vines
Exudate clear to cloudy, terpenoid particles 0; cotyledonary hypophyll blade-like, photosynthetic [Col.].
8/125: Alocasia (97), Colocasia (11). Tibet and the Indian subcontinent to China and Taiwan, thence to North and northeast Australia, 1 sp. Africa and the southern Arabian peninsula, the Seychelles.
Arisareae Dumortier - Arisarum Miller —— Synonymy: Arisaraceae Rafinesque
(apex of spathe very long-attenuate); spadix appendix +; staminate flowers scattered; A 1, thecae apically confluent and dehiscing by a single slit; carpellate zone adnate to spathe, carpellate flowers few; G 1-locular, placentation basal; ovules straight; seeds strophiolate.
1/3. Mediterranean, to the Caucasus and the Atlantic Islands.
- Araceae are herbs with leaves that are usually divided into a petiole and expanded blade. They have a distinctive inflorescence of a more or less petal-like spathe plus a spadix made up of densely-packed, sessile, ebracteate flowers - in many taxa the spadix has a large, terminal, sterile part, and the individual flowers may lack a perianth. The fruit is usually a berry.
Evolution: Divergence & Distribution. Mayoa pollen grains that look very like those of Monsteroideae have been found in Early Cretaceous deposits of the late Barremian-early Aptian of some 120-110 Ma in Portugal (Friis et al. 2004; see also Hesse & Zetter 2007). Although the identity of some of these grains has been questioned (Hoffmann & Zetter 2010), macrofossils apparently of basal Araceae have been found in early Cretaceous deposits ca 105 Ma from Portugal (= Turolospadix bogneri - inflorescence, Orontiophyllum ferreri - leaves: Sender et al. 2019), while at the other extreme fossils from Vancouver Island, British Columbia (Appianospadix bogneri) and aged ca 50 Ma (?Early Eocene) have also been placed with these basal Araceae (Stockey et al. 2023). Similarly, Stockey et al. (2021) placed leaf fossils from Alberta ca 59 Ma in Orontiophyllum grandifolium (Orontioideae), while spadices named Bognerospadix spiersiae and from the same locality were grouped with Gymnostachydoideae. Indeed, Stockey et al. (2023) discussed the evolution of Araceae in the context of the relationships [Lemnoideae [Gymnostachys, Symplocarpus, the fossils just mentioned [Calla [Pothoidium, etc.]]]]. On the other hand, staminate flowers thought to belong to Aroideae (a decidedly non-basal clade) have been discovered in deposits ca 112 Ma from Portugal (Friis et al. 2010: c.f. pollen; Friis et al. 2011; Iles et al. 2015). Ages suggested for the subfamilies by L. Zhao et al. (2022) are confusing, and p. 204 and Fig. 2 in part do not correspond, although there were clearly problems in working out subfamilial relationships; I have ignored some of these ages. These conflicts aside, the fossil record would suggest that all eight subfamilies of Araceae might have diverged in the Cretaceous. See also Wilde at al. (2005), Bogner et al. (2007), Herrera et al. (2008: leaf fossils) and Stockey et al. (2021: evaluation of early fossils of reproductive structures).
Molecular estimates of diversification are rather younger than those based on these Portugese fossils, but even they suggest separation of the subfamilies before the K/P boundary, early evolution in the family possibly occuring in Laurasia (Nauheimer et al. 2012b: table S4, esp. S5 with 140+ dated nodes and further discussion of diversification in the family). If Limnobiophyllum scutatum is stem group Lemnoideae (Iles et al. 2015 think that it definitely goes there), much diversification in Araceae could be Palaeogene since the fossil is early Caenozoic, only some 66 Ma - but not "would be Palaeogene", since of course the age of a stem group may have little to do with that of the crown group associated with it.
Givnish et al. (2018b) and P. Soltis et al. (2019) suggested that there has been an acceleration of speciation in the [[Pothoideae + Monsteroideae] [Lasioideae [Zamioculcadoideae + Aroideae]]] clade, the second group proposing that fleshy fruit (a family-level apomorphy) and the epiphytic habit (common in this clade) limited dispersal and promoted diversification...
Aquatic Araceae have a rich fossil history. Although Limnobiophyllum seems morphologically "intermediate" between Lemnoideae and Pistia, in Aroideae (Stockey et al. 1997), those two groups are not at all close in molecular phylogenies. The palynomorph Pandaniidites, spiny and monoporate, is associated with flowers of Limnobiophyllum known from North American rocks up to 70 Ma that span the Cretaceous-Caenozoic boundary (Hotton et al. 1994; see Stockey et al. 1997; Stockey 2006), and this is quite like the pollen of Lemnoideae (Bogner 2009). The pollen of Pistia is dramatically different, being inaperturate, lacking sporopollenin, and having a plicate-undulate surface (Bogner 2009). There are yet other unrelated fossil floating aquatics in the family (Stockey et al. 2007). The recently-described Aquaephyllum auriculatum from rocks ca 67 Ma of the Crato formation in Argentina is sister to Lemnoideae in morphological phylogenetic analyses, Pistia and some other fossil aquatic Araceae are in turn sister to that clade, and the whole lot are embedded in Aroideae (Gallego et al. 2014; some analyses in Stockey et al. 2016b). Aquaephyllum has a petiolate peltate lamina with a crenate margin and veins proceeding to the margin and it is difficult to know what to make of it; it is placed apart from all other aquatic Araceae in some analyses in Stockey et al. (2016b). Interestingly, the palynomorph Pandaniidites is known from these Crato sediments. In general, all the aquatic taxa tend to group together in morphological analyses, parallel evolution of adaptations to the aquatic habitat overwhelming any signals of relationships (Stockey et al. 2016b). Both Lemnoideae and Pistia are quite old, the latter being perhaps 90-76 Ma (Renner & Zhang 2004) - older than some estimates for the age of Lemnoideae (see above).
For the ages of some intercontinental disjunctions within Lemnoideae, see Les et al. (2003). It has been estimated that Alocasia, centred in Borneo, diversified ca 13.5 or 19.3 Ma (stem group ages are ca 10 Ma more), and there were many subsequent dispersal events through the whole Southeast Asian/Malesian region (Nauheimer et al. 2012a: m.l. trees with little support). The speciose Philodendron subgenus Philodendron, largely epiphytic, started major diversification ca 12 Ma, perhaps associated with the uplift of the Andes, the other two clades in the genus, terrestrial and vining respectively, are similar in ages but much less speciose (Canal et al. 2018), however, Caddick et al. (2019) suggest rather earlier start of diversification at around (32.1-)24.7(-17.8) My; both give several other ages for clades within the genus.
The large genus Arisaema (Aroideae-Arisaemateae) is of considerable interest since it is one of the few angiosperms that is a sequential hermaphrodite, an apomorphy for the genus. Large plants produce pistilate, and sometimes also staminate, flowers (they cannot self), while smaller plants produce staminate flowers alone - sex expression ultimately depends on the vigour of the plant (Kinoshita 1986, see also Schlessman 1985). Interestingly, of the three subspecies of A. flavum only one shows this phenomenon - and it is diploid. The other two subspecies can self fertilize, the only taxa in the genus that can do this, and they produce seeds consistently - and they are polyploid (Murata 1990). These polyploids are from 13-8 My old (Renner et al. 2004). Arisaema is widely distributed, and there are some taxa that have Beringian disjunctions, one species being in the Siberia area, its sister taxon is in North America, and there are also Africa-Nepal disjunctions, etc. (Renner et al. 2004).
L. Zhao et al. (2022) suggested that the ψ duplication, associated with the [Zamioculcadoideae + Aroideae] clade, might be linked with an increase of diversification rates. Zhao et al. (2022) also noted a number of gene families that expanded following this diversification, which they dated to 110.5-100.7 Ma. Gene/genome evolution in Lemnoideae is also discussed in Genes & Genomes below.
Araceae are the fifth most speciose family in Amazonian forests (Cardoso et al. 2017).
For distinctive characters/apomorphies for the first three subfamilies, see Tippery et al. (2021). Hesse (2006b) discussed the evolution of the pollen that characterises most Aroideae - there the pollen, commonly more or less smooth, is often extruded from the anthers in little balls or toothpaste-like threads, the individual grains being held together by pollenkitt, and the anthers of several genera open by pores.
Ecology & Physiology. Croat (1990, 1991) discussed life forms and ecology in Araceae, while Schuyler (1984) focused on classifications of growth forms of aquatic species, but Cook (1996) thought that this latter was largely unnecessary. The original habitat for the family is likely to have been more or less marshy (Nauheimer et al. 2012b).
Climbers/lianes and epiphytes, not mutually exclusive categories here as elsewhere, are common (see also Apocynaceae, Ericaceae). Climbers are notably common in Pothoidaeae and Monsteroideae, particularly in the former. All told, about 570 species of Araceae are climbers (Gentry 1991), with ca 400 species in the New World in particular, where Araceae are one of the major groups of climbers (see also Benzing 1990; Sperotto et al. 2023), and they are root climbers. Skototropism, the movement of the seedling to dark areas, i.e. where there might be a tree trunk to climb, rather than the reverse, phototropism, movement from dark to light and generally common in seedlings, is known from Araceae (Strong & Ray 1975). The attachment of Syngonium podophyllum (Aroideae-Caladieae) to its support is largely mediated by root hairs which initially produce a mucilaginous substance which hardens and attaches the root to the surface, later the walls of the root hairs break down into spirals (= helical crack root hairs) that are probably effective energy-dissipating units like tendrils - and as with tendrils, the direction of the spiral may reverse (X. Yang & Deng 2016: see also Orchidaceae, Posidoniaceae). Climbing Araceae are often strongly heteroblastic, in this case the leaves of a plant in the climbing phase are notably smaller and sometimes simpler than when it is reproductive. Indeed, the shingle-leaf syndrome in which the climbing form of the plant is characterised by more or less sessile leaves closely adpressed to the trunk/branch of the host plant/phorophyte is common in geners of climbing Araceae like Monstera, Pothos and Rhaphidophora (Zona 2020). Frequently the plant is heterophyllous, the climbing form changing into a reproductive phase with more conventional leaves (heteroblasty, Zona 2020), although in some taxa the plant is homophyllous, the shingle-leaf form persisting during reproduction, perhaps reflecting neoteny (Boyce & Bogner 2000). Hemiepiphytic Araceae quite commonly have positive root pressures, and this is also a feature of climbers, perhaps reducing the dangers of irreversible cavitation (Fisher et al. 1997), however, embolism formation and refilling may controlled by the activity of living cells around the vessels (Knipfer et al. 2016) and/or lipid surfactants in the xylem that i.a. coat nanobubbles, so preventing the formation of fully-fledged embolisms (Schenk et al. 2017).
Epiphytes in Araceae are to be found largely in the Neotropics where there are around 716 species of such plants, the 4th highest number in angiosperm families, see e.g. Svahnström et al. (2025), who also discussed the commoness (e.g. Extent Of Occupancy) of epiphytic compared with related non-epiphytic taxa - in Araceae, the former had greater EOOs. Epiphytes are common in Anthurium (Pothoideae) (ca 2/3 of its species), see also Kress (1989), Holtum et al. (2007), Zotz (2013), Reimuth and Zotz (2020), and they are also common in Philodendron subgenus Philodendron (Aroideae: Canal et al. 2018); see Zotz et al. (2021b) for a list. In the Neotropics, Carmona-Higuita et al. (2025) found that most "endemic" Araceae grew in the Paramo (20.1%), Guatuso-Talamanca (18.9%), Cauca (15.7%) and Chocó-Darién (15.7%) biogeographic provinces; Anthurium included 71 of these endemic species. Hietz et al. (2021) and Zotz et al. (2021a) discuss the ecophysiological characteristics of epiphytes in general. Interestingly, in Anthurium endemism is highest in the central Andes, and Reimuth and Zotz (2020) note that the median range size of the epiphytic species is more than eight times that of the terrestrial species, although the altitudinal ranges of the two are similar (similar range disparities are common, but not universal, in epiphytes). Svahnström et al. 2025, q.v. for commoness of epiphytic relative to non-epiphytic taxaA number of these epiphytes, perhaps especially in Anthurium (e.g. sectionPachyneurium), trap litter (Zona & Christenhusz 2015) - the so-called "trash-basket epiphytes", see Ortega-Soils et al. (2021). See also Piperaceae, while Melastomataceae, Ericaceae and Gesneriaceae are other big epiphytic families in the eudicots, Bromeliaceae and Orchidaceae in the monocots, and also ferns.
In groups like Orchidaceae and Bromeliaceae crassulacean acid metabolism (CAM) is associated with the epiphytic habit, but not in Araceae, which tend to live in moister habitats than members of those families. The only known CAM species in the family (as of vii.2025) is Zamioculcas zamiifolia, a ground-dwelling plant of drier habitats (Holtum et al. 2007). Like Orchidaceae in particular, roots of epiphyte Araceae may have a velamen.
Araceous rheophytes, often narrow-leaved plants growing in or by rivers (in waterfalls, on river banks) and periodically subject to immersion by the fast-flowing waters (Boyce & Wong 2019; see also van Steenis 1981, 1987 and references for a general treatment) are commonly members of Schismatoglottidae (Aroideae) and are notably diverse in Borneo. Boyce and Wong (2019) noted that some 149 described species of this subtribe were rheophytes, overall, perhaps one third of the aroid flora of Borneo; some 20 genera of Schismatoglottidae (well, it depends on generic concepts...) are restricted to the island, although most have only few species, however, Bucephalandra has 32 species (Neo 2023); some rheophytic species are restricted to a single stream (Wong 2013). The rheophytic habit in Schismatoglottidae has evolved several times, and Wong (2013) discussed features that might be adaptations to this habitat, including the ability of the shoot to break off from the root system when the water flow becomes too strong; roots develop both from the rootstock and from the shoot, wherever it ends up downstream. Rheophytic Araceae also have a distinctive ligule and seeds, and the spathe may break off in a remarkable fashion (see "Pollination Biology & Seed Dispersal" below).
Many Araceae are plants of shaded conditions, and net-veined leaves and fleshy fruits are associated with this habitat (Givnish et al. 2005). Variegation, whether white and varuous shades of green, or purple-reddish/green, is also common here, as in other plants which grow in such habitats. However, little work has been carried out on this variegation (but see La Rocca et al. 2011: Arum italicum). Soltau et al. (2009) presented evidence that variegation (absence of chloroplasts) in the leaves of Caladium steudneriifolium might mimic the activities of leaf-mining microlepidopteran larvae, whose effect on the plant was not so much the damage they caused, but that caused by subsequent fungal attack, etc.; the activities of these caterpillars was less on white-painted leaves than on wholly green leaves. For variegation, etc., in leaves of plants growing in shaded conditions, see e.g. J.-H. Zhang et al. (2020: classification of variegation types), Begoniaceae, etc..
It has been suggested (e.g. Hejnowicz & Bartlott 2005; esp. Claudel et al. 2019) that the mottling and sculpturing of the surfaces of the petioles that is quite common in Amorphophallus, especially in Malesian species with larger leaves, may be protective mimicry. This mottling, etc., makes the petioles look as if they are covered with lichens and the like, and so they appear to be tree trunks. As a result the petioles are avoided by animals; without such defences, animals might crash into them (Claudel et al. 2019), and the petioles are not that strong (Hejnowicz & Bartlott 2005, see also under Vegetative Variation). This is not easy to test, alack. The leaflets often have drip-tips like the young seedling trees that may be growing in the same area - perhaps also protective (see Claudel et al. 2017)?
Lemnoideae are widespread in freshwater aquatic ecosystems, and they are an important food source for many organisms while at the same time preventing others fromn growing as they compete successfully for light and nutrients (Acosta et al. 2021 and references). Spirodela in particular efficently takes up nitrogen, including in the form of ammonia, from polluted systems - aside from the plant being 15-45% protein dry weight, comparable with soybeans and alfafa, it can produce up to about 30% dry weight of starch, depending on the conditions, and amounts of starch in the turions may be very high (e.g. Cheng & Stomp 2005; Appenroth et al. 2017). Ziegler et al. (2014, 2023), K. A. Robinson et al. (2025) and others discuss the speed of plant growth in Lemnoideae, the fastest in embryophytes, with a doubling time of as little at 1.34 days (as frond number or biomass, and varying at the strain rather than species level) under standardized culture conditions, and it is estimated that one frond of Wolffia microscopica, if given access to unlimited nutrients and CO2, could give rise to 1030 fronds in 4 months, and these would occupy a volume roughly equivalent to that of the Earth (see also Sree et al. 2015b; Michael et al. 2020; Lam & Michael 2022). Roots in those Lemnoideae that have them function more like sea anchors than absorbtive organs; they neither branch nor have root hairs, so perhaps helping to stabilize the plant in the water (An et al. 2019). The sticky roots may also help in attaching the plant to birds and so aiding in vegetative long distance dispersal (Cross 2017). Nutrient uptake is via the lower surface of the frond (An et al. 2019 and references), indeed, in a number of Lemnoideae there is absorbotrophic mixotrophy - that is, the plants obtain some of their carbohydrates (for example) from the water (see Firmin et al. 2022; Ziegler et al. 2023).
Scheffer et al. (2003) discuss the conditions - generally high nutrient levels and fairly protected water bodies - that allow small floating aquatic plants like Lemnoideae to dominate; this is at the expense of algae and bottom-dwelling plants. In more tropical environments it is larger floating aquatics like Pistia stratiotes (Aroideae) that may dominate - they can handle more exposed conditions. Indeed, Pistia also has exceptional growth capabilities, it can sequester noxious compounds, etc., and it may be dominant in lakes and slowly-flowing waters and is then an important aquatic weed.
An et al. (2019) note that the number of disease-resistance genes increases by tandem duplication in Spirodela - potentially noxious microorganisms may be all around the plant in the aquatic habitat (for disease-resistance genes in Wolffia, see Michael et al. 2020) - and, as in Zostera, there has been the loss of a number of gene families involved in life on land. Michael et al. (2020) discuss genome change in W. australiana in some detail, and here in particular relatively few genes are expressed at a particular time of day, and although there is a core set of cycling genes associated with energy acquisition and utilization, growth is continuous, all told, only ca 13% of the transcriptome is under circadian control - the figure is usually over 40% in other plants, and X. Xu et al. (2022) describe the complexity of circadian control in Arabidopsis, where ca 89% of the transcriptome is expressed at a particular time of day. Root development and light signalling genes, for example, have been lost in Wolffia; overall, much of the functioning genome seems independant of the environment (Michael et al. 2022). Overall, only some 70% of Benchmarking Universal Single-Copy Orthologs, near-universal single-copy orthologues, were detected here, rather similar to the situation in groups like Cuscuta (Convolvulaceae) and Utricularia (Lentibulariaceae), also lacking much in the way of conventional leaves and roots (Michael et al. 2020). See also Acosta et al. (2021), Ziegler et al. 2023: general ecophysiology; articles in Plants 12(11). 2023, etc., for more on Lemnoideae.
Pollination Biology & Seed Dispersal.
Pollination Biology.
Flowers in Araceae are often in pseudanthia made up of a spathe and spadix - the spathe is more or less petal-like, and the flowers are densely aggregated and individually inconspicuous (Baczynski & Claßen-Bockhoff 2023). Gibernau (2003, 2011, 2016) and Gottsberger (2016a) summarize information on pollinators, the focus in S. D. Johnson and Schiestl (2016) is deceit pollination, predodminant in Araceae, while Chouteau et al. (2008) looked at possible connections between pollination devices and life form and habitat in the family. Important elements in pollination mechanisms in Araceae are the morphology of the infloresecence, the nature of the scent it produces (for osmophores, see Gonçalves-Sousa et al. 2017), and the occurrence of thermogenesis, and these three elements interact in pollination systems that generally involve insects of one sort or another (see also Barabé & Gibernau 2015: pollination in French Guyana). Diáz Jiménez et al. (2019) recently reviewed pollination mechanisms in Araceae with perfect flowers; the inflorescences tended to remain open longer than in the monoecious Aroideae. However, as with other Araceae, beetles, flies and bees (the latter less so) were the main pollinators - Gottsberger (2016a) estimated that together the two former groups might pollinate ca 1,500 species of Araceae. Beetle pollination may be the plesiomorphic condition here (Sannier et al. 2009; c.f. Bröderbauer et al. 2012; Chartier et al. 2014a), and there are oviposition/pollination mutualisms. For a discussion on the evolution of the distinctive pollen that characterises most Aroideae, see Hesse (2006b).
The spathe of Aroideae is usually more or less differentiated into tubular and blade-like portions, although it may be undifferentiated and is then more or less spreading. Sterile female (?sometimes male - see Low et al. 2016) flowers may be at the very bottom of the spadix, then female flowers, then sterile male flowers, then male flowers, and then there is a sometimes much elongated sterile portion. In other subfamilies all the flowers may be perfect. The fertile flowers may be more or less enclosed by the sometimes inflated tubular portion of the spathe (= kettle), mostly obviously in Aroideae, but there is considerable variation in the number and arrangement of the flowers, for instance, there may be only a single carpelate flower, as in some species of Arisarum (Prime 1960). Pollinators of Aroideae are mostly flies and beetles, attracted by the color of the blade of the spathe, the smell, the heat that is emitted, and/or even the dangling apical portion of the spadix. The pollinators may be temporarily (male stage inflorescences) or permanently (female-stage inflorescences: Suetsugu et al. 2021) trapped inside the tubular basal portion of the spathe by hairs, radiating sterile flowers, etc.. The insects are released when the staminate flowers open and they get covered with pollen as they leave the inflorescence. Enlarged sterile staminate flowers at the base of the staminate portion of the inflorescence may serve as food bodies (Etl et al. 2022). However, in many situations the pollinators are not rewarded and it is while they are trapped in female-stage inflorescences that they deposit pollen (Chartier et al. 2014a). There are a variety of traps in the family, and they have evolved at least ten times (Bröderbauer et al. 2012; see also Delpino 1873).
Hoe and Wong (2016) looked at the pollination of Schismatoglottis baangongensis and chronicled all the events as the inflorescence matured; pollinators, drosophilid flies and hydrophilid beetles, were trapped soon after the inflorescence opened, the female phase, but they were freed when the spathe abscised transversely near the base (the male phase), and they then could pick up pollen.
Thermogenesis has been detected in the inflorescences of a number of Araceae (e.g. Prime 1960; Meeuse & Raskin 1988; Skubatz 2014; Gottsberger 2016a; Milet-Pinheiro et al. 2017; Diáz Jiménez et al. 2019). It is caused both by proteins that uncouple the components of the glycolytic pathway and by the mediation of an alternative oxidase, the net result being that glycolysis results in heat, not energy in the form of adenosine triphosphate, and rates of respiration can be very high. The spadix may become warm at particular times of the day, photoperiodicity being involved, too, and the trigger may be in the staminate flowers (Meeuse & Raskin 1988; Gibernau et al. 2005; Watling et al. 2006; Onda et al. 2008; Barthlott et al. 2008 and references; Chouteau et al. 2009; Seymour 2010). There is biphasic thermogenesis in Schismatoglottis calyptrata marking the opening of the staminate and carpelate flowers and it is normally the sterile spadix that becomes hot (Hoe et al. 2018). The heat may volatilize compounds that attract pollinators, and/or provide a warm roost for them inside the spathe. Thus temperatures reach 200 above ambient in some species of Xanthosoma, a large genus that is thought to be predominantly pollinated by these beetles (Milet-Pinheiro et al. 2017 and references), and over 30oC above ambient in Xanthosoma robustum when the plant is kept at 10oC (Meeuse & Raskin 1988). In at least some species of Philodendron the staminate flowers are the source of the heat, although not all species in the genus are thermogenic (Gonçalves-Souza et al. 2017; Barbosa et al. 2018) - commonly, staminate flowers, staminodes and/or the appendix are the source of the heat (Claudel et al. 2023), although in a few species like Schismatoglottis calyptrata the pistillate zone of the inflorescence may also be involved, in this case the spadix lacking a sterile zone/appendix (Hoe et al. 2020). In Amorphophallus the heating ranges from 0-21.7oC (the latter in A. longituberosus) and may last for up to two weeks or so (A. schmidtiae); heating is likely to be plesiomorphous in the genus (Claudel et al. 2023). The temperature of the spadix in the skunk cabbage, Symplocarpus renifolium (it grows in East Asia), may be ca 23oC, yet ambient temperatures drop below 0o C. Here hydrogen sulfide is produced, and this inhibits cytochrome c oxidase-mediated mitochondrial respiration, but alternative oxidase-mediated respiration is unaffected - thermoregulation takes place at the low temperatures at which this aroid flowers, the higher temperatures that result facilitating pollen germination and pollen tube elongation (Tanimoto et al. 2024).
As mentioned, smell is important, indeed, Diáz Jiménez et al. (2019) consider variation in scent bouquets to be key to understanding pollination mechanisms, at least in Araceae with perfect flowers. More or less unpleasant (to us) smells are frequent in araceous inflorescences, as is evident from common names like skunk cabbage (Symplocarpus foetidus) and dead horse arum (Helicodiceros [Dracunculus] muscivorus) - the latter might be more accurately called the dead gull arum (Jürgens et al 2006; Stensmyr et al. 2002 for the volatiles). Oligosulphides are reported from S. renifolius, as from some other unrelated dung/carrion mimics. Schiestl and Dötterl (2012; see also Schiestl & Johnson 2013) argue that the ability to detect particular volatile compounds in scarab beetles developed in the Jurassic, while similar compounds such as the indole skatole evolved in the plants that they now pollinate in the Cretaceous/Palaeocene about 100 Ma later; this is an example of what appears to be a quite common disconnect between the evolution of the pollinator and that of the plant it pollinates. Interestingly, 4-methyl-5-vinylthiazole (heterocyclic, C, N, S in a 5-membered ring) plays an important role in attracting cyclocephaline scarabs both in Caladium and in Annona (Maia et al. 2012; also M. R. Moore & Jameson 2013; Moore et al. 2015 and Gibernau et al. 2023 for cyclocephaline pollinators - they also pollinate some Nymphaeaceae anf Magnoliaceae); the beetles seem to have no particular preferences for compounds that are apparently unique to the plant (Schiestl & Dötterl 2012), nevertheless, different species of Araceae seem to use different combinations of attractants to attract the beetles (Gibernau et al. 2023). The scent emitted by some species of Xanthosoma has been analysed, and it was suggested that the distinctiveness of a scent was the result of the predominance of "unique dominant compounds", but whether visitors are unique to a species and/or are elements in barriers separating sympatric species is currently unknown (Milet-Pinheiro et al. 2017). However, Maia et al. (2018) noted that different species of scarabs were attracted to the inflorescences of the three species of Araceae whose scents they were analysing; see also Philodendron, Montrichardia, Xanthosoma, Caladium, etc. (Gibernau et al. 2023).
The volatiles produced by Arum attract insects in search of brood sites, thus the smell of A. palaestinum is that of rotting fruit (yeasty, as if there is fermentation); here a single inflorescence can attract hundreds of Drosophila flies, and the odorant receptors are conserved across the genus, the Drosophila species attracted not being at all closely related (Stökl et al. 2010; Linz et al. 2010; see also Kite et al. 1998: pollinators mostly dipterans; Gibernau et al. 2004; Urru et al. 2011). Brood site mimicry is quite widespread, as with fruit flies and Alocasia (Nauheimer et al. 2012a), fruit flies and Colocasia (Bröderbauer et al. 2014), and flies or coleoptera visiting two species of Typhonium that differed in morphology and scent emitted (Sayers et al. 2021). Yeasts are a common food of Drosophila larvae, the adults tending to be attracted to different rotting fruits (Stökl et al. 2010). Punekar and Kumaran (2010) described the pollination of some Indian species of Amorphophallus (see also Kite et al. 1998: odours; Claudel et al. 2017 and Wong et al. 2022 for pollination: inc. mimicry of mammalian dung). The genus produces a variety of odours, including dimethyl sulphides that are "gaseous" or smell like rotting meat, phenylethanol derivatives that smell of fruit or anise, trimethylamine that smells like fish, and isocaproic acid that smells (I think) like cheese or goats. There was some correlation of the production of specific odours both with phylogeny and with ecology/geography, thus some odours seemed to have evolved only once, others several times, interestingly, Amorphophallus species that smelled like fish were not all immediately related but were found only in northern Borneo, while sister species that can be sympatric have inflorescences that smell of dung and corpses respectively... (Kite & Hetterscheid 2017; see also Stökl et al. 2010). The wall of the pollen grain of Amorphophallus is sometimes shed before the pollen tube develops (Ulrich et al. 2017). Zulfiqar et al. (2024; see also Raman et al. 2024) recently described thermogenesis and scent production in the iconic Amorphophallus titanum; here the odours dimethyl disulfide and dimethyl trisulfide, derived from methionine, and also, somewhat surprisingly, putrescine, derived from arginine, predominated.
Some Neotropical Araceae, including species of both Anthurium and Spathiphyllum, are pollinated by euglossine bees (orchid bees) which show fair visitor specificity despite the apparently unspecialised flowers - this is because the scent bouquets of the attractants are different (N. Williams & Dressler 1976; Schwerdtfeger et al. 2002; Roubik & Hanson 2004; Hentrich et al. 2010b; Schiestl 2012). Schwerdtfeger et al. (2002; see also Croat 1980) found that rather limited data showed that euglossine bees were common pollinators of Anthurium, although curculioinid weevils (for which, see Franz 2007b; Haran et al. 2023), cecidomyid midges and drosophilid flies were also visitors. A nectar-like but sometimes foul-tasting exudate may be produced by stigmatic hairs, etc., as in Anthurium (Daumann 1931; see also Fahn 1979), and this, too, attracts pollinators. Duckweeds produce sucrose-containing drops of liquid at the stigmatic apex, and pollination is probably by small flies (Landolt 1986).
A number of Aroideae are pollinated by cyclocephaline dynastine scarab beetles that use the inflorescences as mating sites (Chartier et al. 2014a; Gibernau 2015; Milet-Pinheiro et al. 2017), thus Caladium is a member of a clade characterised by being pollinated by these beetles (Mayo & Bogner 1988; Maia & Schlindwein 2006). In Philodendron each species attracts usually but a single species of scarab (see also Maia et al. 2010), and Gottsberger (2016a: Fig. 2) shows a marvellous scrum of Erioscelis emarginata beetles at the bottom of an inflorescence of P. selloum. The inflorescences are often highly thermogenic (c.f. Barbosa et al. 2018)`, in P. adamantinum the male flowers being the source of the heat, and terpenoids, etc., are produced by the osmophores (Gonçalves-Souza et al. 2017). True resins are produced on the inside of the spathe and covers the smooth body of the beetle so enabling pollen to stick to it (Gonçalves-Souza et al. 2018) Xanthosoma is another large genus in which there is thermogenesis and which is thought to be predominantly pollinated by these beetles (Milet-Pinheiro et al. 2017 and references).
Arisaema is another member of Aroideae in which pollination has been studied in some detail. This genus is serially monoecious, i.e. in any one year a plant is either staminate or carpelate, but it can change "sex" from year to year, apparently connected with the size of the plants - female plants are larger (see also Araliaceae-Panax, Schlessman 1991), interestingly, two tetraploid subspecies can self (Renner et al. 2004, see also "Divergence & Distribution" above). Dipteran fungus gnats are the pollinators, thus Mycetophaga occurs in Arisaema thunbergii ssp. urashima (Suetsugu et al. 2022b); in two sympatric species of Arisaema different species of fungus gnat seem to be involved (Suetsugu et al. 2021). The fungus gnats can escape from any male inflorescence they visit via a hole at the base of the spathe. Visiting female inflorescences is another story. Covered in pollen, they slide down the slippery slope inside the spathe and pollinate the female flowers - but there is no basal exit hole, they cannot climb out of the inflorescence, and so they die (Vogel & Martens 2000). The gnats are attracted by the smell produced by osmophores which are usually at the tip of the spadix, and the spadix itself can be up to 80 cm - some reports suggest 1.5 m - long, and dangles from the mouth of the spathe (see also Suetsugu et al. 2022b), and the spathe itself may have a very long, dangling apex. In some species the spadix may reach the ground (as also in Amorphophallus pendulus), and the insects then may climb up the spadix into the spathe from the ground (Vogel & Martens 2000); perhaps rather surprisingly, the relationship between particular species of fungus gnat and of Arisaema may be close, the two covarying along an altitudinal gradient, isolation being enhanced by phenological variation between the five species of Arisaema in the study.
There are over 250 species of Schismatoglottideae (Aroideae), over 150 being known from Borneo alone. Low et al. (2016 and references, see also Low & Wong 2024) describe the diversity of the pollination mechanisms of these species, where complex movements of the spathe, or its splitting in various ways, sometimes very irregularly, even its abscission (e.g. Boyce & Wong 2007) are part of the whole process; see also Boyce and Wong (2007) for the mechanics of spathe senescence in Schismatoglottidae, also Uhl et al. (2013) and Neo (2023: Figs 80, 93), the latter showing species in which there is a long, spiral-annular dehiscence zone in the top part of the spathe, and as it breaks away it exposes the staminate flowers. See also Boyce and Wong (2007) for the mechanics of spathe senescence in Schismatoglottidae, also Uhl et al. (2013) and Neo (2023: Figs 80, 93), the latter showing species in which there is a long, spiral(-annular) dehiscence zone in the top part of the spadix, and as the spathe breaks away it exposes the staminate flowers. In some species the pollen is then extruded as droplets or threads from the needle-like projections at the apex of the stamens (Neo 2023). The drosophilid Colocasiomyia is one of the pollinators. There is thermogenesis (see above), and a mixed fly-beetle pollination system may be ancestral here, with the beetles eating the interpistillar staminodes; the staminodes are fewer in fly-pollinated species (Hoe et al. 2018). After the female flowers have opened in Bucephalandra, flattened staminodes reflex so sealing the female flowers off as the staminate flowers open and thus preventing selfing (Wong & Boyce 2014). Various scents are produced, and these seem to be more evident in staminate flowers (Low & Wong 2024).
How some of the variables mentioned above interact is evident in the evolution of the Neotropical mirid-pollinated Syngonium hastiferum (Etl et al. 2022). Syngonium is usually pollinated by scarab beetles, mirids being florivores. However, S. hastiferum (Araceae) is pollinated by the plant bug Neella sp. nov. (Miridae-Heteroptera), and it differs in several floral traits from its beetle-pollinated relatives: Scent emission and thermogenesis occur in the morning, the scent being a compound previously totally unknown in plants, not the evening, a time for the scarabs, and the pollen is spiny, not smooth but with pollen kitt, as it commonly is in scarab-pollinated plants. There are also no enlarged sterile flowers, common rewards in beetle-pollinated flowers (Etl et al. 2022). For a general treatment of chemical mimicry of different kinds, including mimicry of carrion and of oviposition sites, kinds of deceptive pollination, see also Jürgens et al. (2013). Note that in general in Araceae, and in other situations where there is deceptive pollination, there is no evidence that the plant and pollinator are evolving togther, rather, the plant is tapping in to the sensory biases of the pollinator.
A discriminant analysis of thirteen putatively pollinator-related characters of the pollen (but not the pollen surface) of aroids identified bee- and also less sharply differentiated beetle- and fly-pollinated morphologies (Gibernau et al. 2010; Gibernau 2003 for references), although how such analyses would fare if pollinator groups were not assumed is unclear. Grayum (1986) had earlier looked at features of the pollen surface and how they might correlate with pollinators; smooth and sometimes rather larger pollen and beetle pollination were linked, as were spiny pollen and fly pollination, and so on. Sannier et al. (2009: but see their caveats, Lemnoideae and Gymnostachys not included, only two Orontioideae, etc.) looked at pollinator and pollen across the whole family, and also suggested there was some correlation between pollen morphology and pollinator, as will have been evident from the discussion above. The arborescent South American Montrichardia has "explosive" pollen; on contact with water the massively thick intine swells and forms an elongated structure ca 400 µm long within a few seconds, perhaps aiding the attachment of the pollen to the visiting hairless beetle pollinator (Weber & Halbritter 2007).
Raphides, prismatic calcium oxalate crystals, and other crystal types from the walls of the anther may become mixed with the pollen (Barabé et al. 2004b; Barabé & Lacroix 2008b, Coté 2010; Coté & Gibernau 2012; Low et al. 2016); their exact function is unclear. There can be a variety of crystalline forms in different cells and tissues of the one plant - see Coté 2009). A variety of crystals is also found in the ovary (Coté & Gibernau 2012). In Monsteroideae there are numerous trichosclereids in the stylar tissue and the spathe is deciduous; the trichosclereids may protect the exposed ovary against insects.
Seed Dispersal.
The fruits of understory Araceae in the Neotropics are a particularly important source of food for bats (Lobova et al. 2009). Monstereae are frequently epiphytic and often have seeds embedded in pulp, whether from the testa, trichomes, or the inner pericarp, perhaps to help them stick to branches (Mayo et al. 1998); in South America birds like the mistletoe-specialist friar birds (Euphoniinae, near Fringillidae) may disperse the seeds of epiphytic taxa like Anthurium (Snow 1981; Restrepo 1987; Reid 1991). In some Araceae the basal part of the spathe remains fleshy and encloses the fruits; when the latter are mature it may split irregularly; the seeds are exposed to the dispersing animals. See also Boyce and Wong (2007) for the mechanics of spathe senescence in Schismatoglottidae, also Uhl et al. (2013) and Neo (2023). A number of these small Malesian Aroideae-Schismatoglottidae growing on the forest floor or on rocks have splash-cup dispersal mechanisms (see especially Neo 2023), indeed, it looks almost as if somebody had come along and cut off the top of the spathe with a razor. The persistent cup-shaped part of the spathe directs the dispersal of the seeds/fruits (described as caryopses by Neo 2023), or the fruits may be corky and water-dispersed, or the seeds of rheophytic taxa may have distinctive micropylar appendages that act as grapnels for anchoring the seeds as they germinate (Wong 2013; Boyce & Wong 2015 for references). The aquatic Cryptocoryne is the only Araceae to have dehiscent fruits (clearly derived - andsee also Neo 2023 and the dehiscent fruits of Bakoella). Here the female part of the spadix is described as consisting of gynoecia (Mayo et al. 1998) or female flowers (Wongso et al. 2017) and the fruit as being a syncarp (Wongso et al. 2017); images of dehiscing fruits in the latter look like a septicidal capsule, although they are in fact a whorl of follicles with basally-attached seeds. The seeds float and are dispersed by water (Bown 2025).
In Lemnoideae the individual plantlets may be dispersed by e.g. aquatic birds (externally) as may the turions (internally) (Acosta et al. 2021).
Plant-Animal Interactions. Araceae are little eaten by butterfly caterpillars (Ehrlich & Raven 1964). A number of species of galerucine beetles (Aplosonyx) have been found feeding on laticiferous Aroideae from South East Asia where they make circular trenches in the leaves to interrupt the latex flow and then eat out the portion of the leaf so isolated - the resultant holes in the blade look as if they were made by a paper punch (Darling 2007); galerucines are known from other monocots and beetle herbivory in Araceae may be geographically more widespread.
Arastichus gallicola (Hymenoptera, Eulophidae) is an unusual case of a wasp that galls ovaries (but c.f. non-pollinating fig wasps), laying eggs with remarkable long filiform pedicels on the funicles of the ovules of Thaumatophyllum bipinnatifidum(Philodendreae). The wasp does not pollinate the plant, but relies on normal pollination by scarab beetles to provide the pollination stimulus that prevents abscission of the inflorescence, so allowing the larvae to complete their development (Jansen-González et al. 2024).
Philodendron (Aroideae), species of which are scandent or hemiepiphytic, is unusual in that it has foliar extrafloral nectaries. Ants are common on the plants, perhaps affording them a measure of protection against herbivores (Gonçalves-Souza et al. 2016). In the New World in particular species of Anthurium and Philodendron may be inhabitants of ant gardens (Orivel & Leroy 2011).
Vegetative Variation. Ferreira et al. (2020) found a fair amount of variation in root anatomy in Philodendron: The stele might be lobed or not, medullated or not, resin ducts might have parenchymatous or sclerified sheaths, there were phloem islands scattered through the protostele, and so on. Some of this variation, i.e. the lobing of the stele, seemed to have taxonomic importance (Ferreira et al. 2020: cork superficial!).
Although Araceae may appear to be monopodial, the whole plant is usually a complex sympodium built up of regularly repeating units, and this is also true of the floating rosettes of Pistia (Buzgó 1994). Each unit/module is made up of an expanded leaf, a reduced leaf, a prophyll, and a terminal inflorescence (e.g. Engler 1877, translated by Ray & Renner 1990; Ray 1987, 1988). These stems often have conspicuous annular scars surrounding the stem and C-shaped scars on one side of the stem; the former represent leaf scars, the latter bract scars around the stem apex/inflorescence, the termination of each unit of the sympodium. Growth continues by the development of a bud in the axil of the prophyll that evicts the stem apex/inflorescence; details of branching patterns seem to correlate with major groups within the family (see Mayo et al. 1998 and references). Ray (1987b) questioned whether all taxa have axillary buds; axillary buds may be superposed, and the upper (developing) bud may be displaced some way from the axil of the leaf that subtends it. For growth patterns, see also Barabé and Gibernau (2015).
Leaves and their development vary considerably in Araceae. Those of a number of taxa, including Monstera, the swiss cheese plant, and relatives, are fenestrate (see Melville and Wrigley 1969) because of localised cell death (Kaplan 1984; Gunawardena & Dengler 2006), while in Anchomanes localised cell death results in what is an initially simple leaf blade with entire margins separating into a complex structure with numerous "leaflets" (c.f. palm leaves). The leaves of Zamioculcas appear to be truly compound, with the blastozone, the marginal leaf meristem, becoming discontinuous and producing the individual leaflets (Kaplan 1984; Gunawardena & Dengler 2006); the related Gonatopus is similar. The related Gonatopus also has dissected leaves (?development) and remarkable pulvini in the middle of its long petioles (hence its name, "giraffe's knees"), and they also occur along the petiole of Zamioculcas. Pulvini are also quite commonly found at the top of the petiole, as in Monstera and Rhaphidophora. Aroid leaves can be huge, in Dracontium gigas and Amorphophallus titanum the dissected foliar part, which can reach up to 4 m in diameter, being born on a massive erect petiole up to ca 5 m or so tall (Bown 2025). Hejnowicz and Barthlott (2005) examined the structure and function of the petiole of such leaves, noting that the parenchymatous shell of the petiole in which there are vascular bunndles and also collenchyma bundles occupied 10% of its volume and ca 35% of its mass, there was no lignification, and air spaces occupied almost 80% of its volume, also, flimsy though the aerenchyma might appear to be, it can still offer some support, and in the humid environment in which the plants grow, cells were likely to have high turgor. The leaves of Anthurium are notably variable, being entire to deeply lobed or apparently compound.
The leaves of Scindapsus develop in a "typical" monocot fashion, i.e. from the leaf base (Troll & Meyer 1955; Bharathan 1996; Doyle 1998b), while in taxa like Arisaema, Orontium, and Zamioculcas the blade develops from the upper part of the leaf primordium, i.e., they are similar in this to broad-leaved angiosperms (Troll 1955; Periasamy & Muruganathan 1986: Arisaema; Bharathan 1996) - however, see elsewhere for monocot leaf development. Kaplan (1973) thought that the blade in Zantedeschia aethiopica developed from the lower part of the leaf, although he also noted that it developed acropetally, and in this was like other Araceae (unspecified) and broad-leaved angiosperms. Ertl (1932) suggested that leaves with more normal monocot venation may be common in taxa now placed in the basal pectinations of Araceae, but blades with highly reticulate venation are common in the family (Mayo et al. 1997; Coiffard & Mohr 2016).
The highly reduced vegetative body of Lemnoideae is variously called a thallus, frond or plantlet with branches (F. Li et al. 2023). It is usually interpreted as being some combination of leaf and shoot; some taxa also have what are clearly roots, albeit lacking root hairs, others have no roots at all. Wolffia and Wolffiella entirely lack roots and also veins/vascular tissue in the thallus, although W. microscopica has a root-like structure of uncertain function (Landolt 1986); despite the absence of roots in Wolffia australis it has retained just about all the root genes in its genome (Li et al. 2023). The thallus of Wolffia may be less than 1 mm across; it is the smallest flowering plant known (see Lemon & Posluszny 2000b; Sree et al. 2015a). The reproductive parts have been interpreted as either a reduced but perfect flower or a very highly reduced inflorescence; at least some species are interpreted as having a staminate flower with a single stamen and a perfect flower (Bogner 2009; Gramzow & Theißen 2020); clarification of what the single stamen and single ovule of Wolffia represent will be of considerable interest if W. australis becomes some kind of model organism (Li et al. 2023). (The flowers of Balanophora (Santalales) are apparently smaller.) W. Wang et al. (2014), in a genome analysis of Spirodela, found that genes promoting the juvenile phase of growth and inhibiting adult features like flowering were preferentially enhanced, suggesting that the adult body is paedomorphic, retaining juvenile traits, but whether by progenesis, speeding up sexual development, or neoteny, slowing down vegetative development, is unclear, although perhaps the former is the better fit; as might be expected, some genes involved in cell wall biosynthesis have been lost or their copy numbers reduced. The aquatic Pistia (Aroideae) has a much less unconventional plant body, and its inflorescence, although reduced, is basically similar to that of other Aroideae; however, its vegetative shoots are monopodial (Lemon & Posluszny 2000a). Both Lemnoideae and Pistia have supernumerary axillary buds which increases the complexity of their branching patterns, however, the two are not immediately related (c.f. Lemon & Posluszny 2000b, see also above). Prime (1960) noted similarities between the embryos of Lemnoideae and those of other Araceae. For more on growth and flowering in Lemnoideae, see Acosta et al. (2021).
Genes & Genomes. A genome duplication in Lemnoideae and the clades above it, the SPPOα event, is dated at 83 Ma (Landis et al. 2018). Earlier, W. Wang et al. (2014; see also An et al. 2019; Zwaenepoel & Van de Peer 2020; L.-Y. Chen et al. 2022) had suggested that there was evidence of two whole genome duplications in Spirodela polyrhiza, the αSP and βSO events, and dated these to around 95 Ma, i.e., the duplication may have happened in stem Araceae. L. Zhao et al. (2022) noted that a duplication, the ψ [psi] duplication, was associated with the true Araceae, i.e. everything above Zamioculcadoideae on the tree, and they dated it to 110.5-100.7 Ma; see also L.-Y. Chen et al. (2022).
For chromosome number changes, descending dysploidy being common, see Sousa and Renner (2015), although even species with chromosome numbers as high as 2n = 60 showed no evidence of polyploidy (Sousa & Renner 2015), which is remarkable if there has been a duplication. Chromosome number is especially variable in e.g. Cryptocoryne, haploid numbers of 5, 7, 10, 11, 13, 14, 15, 17, 18 being recorded in Wongso et al. (2017); for chromosome numbers, etc., see Urbanska-Worytkiewicz (1980) and Cao and Vu (2020), both Lemnoideae, Bogner and Petersen (2007) and E. V. Vasconcelos et al. (2018: Philodendron and 35S rDNA sites). For a possible base chromosome number of x = 16 for the family, see Cusimano et al. (2012 and references); Urbanska-Worytkiewicz (1980) had suggested that x = 10 in Lemnoideae, commonly n = 20 there (Hoang et al. 2019); for some other base numbers, see Q. Xu et al. (2021). L.-Y. Chen et al. (2022) mentioned a possible hybridization event that had involved Aroideae (Zantedeschia, etc.), but not Lemnoideae and Monsteroideae, the other Araceae in their analysis.
Bliss and Suzuki (2012) found substantial variation in genome size in Anthurium, but this showed little correlation with anything. The nuclear genome of Spirodela is quite small, around 158 Mb, but other Lemnoideae can be around 12 times larger (Acosta et al. 2021: Fig. 3 for a summary) and genome size seems to have increased within the subfamily; there is comparable variation in chromosome size (Cao & Yu 2020). Although Hoang et al. (2019) noted a correlation between genome size and the size of e.g. guard cells here, there was no correlation with chromosome number. In Lemnoideae several (several hundred in Wolffia australiana - Michael et al. 2020) genes have been lost, concentrated blocks of heterochromatin are uncommon, and there is no evidence of retrotransposons, however, there are many microsatellite tandem repeats, ca 1 Mb in total (W. Wang et al. 2014; Harkess et al. 2020). In Spirodela in particular several methylation genes are non-functional, however, this may not affect the plant much because of its very rapid vegetative reproduction, and maintenance methylation persists (Harkess et al. 2020), Lemnoideae also seem to have lost genes involved in e.g. lignin biosynthesis, cell growth and flowering, but there has been diversification in some genes, e.g. in those involved in nitrogen metabolism; genome size variation in Lemnoideae is largely due to variation in repetitive sequences (An et al. 2018). Gramzow and Theißen (2020) discussed the extensive loss of MADS-Box genes in duckweeds, while L. Zhao et al. (2022) and the papers in Cao et al. (2020) provide more information on duckweed genomes and their evolution.
Plastome DNA substitution rates are particularly high in the free-floating Lemnoideae, Pistia, and Aroideae-Cryptocoryneae, all more or less aquatic, and perhaps along the stem [Pothoideae ... + Aroideae] (Nauheimer et al. 2012b). For the highly autapomorphic plastome of Zantedeschia and to a lesser extent that of Anchomanes (both Aroideae, close to each other?), see Henriquez et al. (2014). The organization of the plastome in Lemna is also distinctive (Mardanov et al. 2008), although overall, plastomes show little variation (J. Tang et al. 2016; Abdullah et al. 2020b).
In crosses within Zantedeschia, there may be incompatability between chloroplasts from one parent and the hybrid genome (plastome-genome incompatability - PGI) resulting in the death of the chloroplasts and thus to variegation or complete albinism (Snijder et al. 2007 and references).
The mitochondrial genome of Spirodela has been substantially rearranged, and it shows no synteny with other mitochondrial genomes (W. Wang et al. 2012; see also G. Petersen et al. 2017).
Economic Importance. Lemnoideae are nutritious, having a high protein content, and can be eaten by humans and other animals, they are potential sources of biofuelsm they grow well on municipal and animal waste and efficiently take up nutrients from wastewater, and so on. They have a very high growth rate, up to some 20 tons/acre/year (Acosta et al. 2020; K. A. Robinson et al. 2025 - see also Ecology & Physiology and Vegetative Variation above
Chemistry, Morphology, etc.. Raphides in those taxa that have been studied are twinned calcium oxalate crystals, H-shaped in transverse section, and often with lateral barbs (Sakai & Hanson 1974; Cody & Horner 1983); Lemna also has such raphides [check]. Raphides appear earlier in development than druses, at least in Amorphophallus, and may help protect young tissue, as well as helping to regulate calcium (Prychid et al. 2008). Looking at Araceae as a whole, raphides may be found in just about any tissue (Lawrie et al. 2023).
In the medulla of roots of Monstera, Heteropsis and Philodendron, at least, xylem and phloem are mixed (Huggett & Tomlinson 2010), and knowing the broader distribution of this distinctive feature would be interesting. For stem anatomy in the family, see surveys by French and Tomlinson (1981a: Calloideae, Lasioideae, 1981b: Philodendroideae, 1981c: Pothoideae, 1981d: Colocasioideae, Aroideae, Pistioideae, 1984: Philodendron), for that of Philodendron, see Tenorio et al. (2012: superficial storied cork), and for compound vascular bundles, see French and Tomlinson (1984, 1986) and Tenorio et al. (2012). Gonçalves et al. (2004) noted that some taxa with perfect flowers may have collenchyma at the apex or base of the petiole; their comparative data is of collenchyma presence at the middle of the petiole. This may perhaps explain the apparent conflict in the literature. Thus although Keating (2000b) recorded collenchyma for a few members of Lasioideae and Pothoideae, Gonçalves et al. (2004) failed to find it for some of the same taxa.
In addition to Gymnostachys, I remember seeing one taxon (unnamed, from Thailand) with a leaf blade that had softly dentate/spinulate margins.
There have been suggestions that the reproductive stuctures in Araceae are neither flower nor inflorescence, rather, they have properties of both (e.g. all flowers on a spadix open together, behaving more like a single unit) and thus should be reinterpreted accordingly (S. Y. Wong et al. 2020 and references). Buzgo (2001) suggested that Orontium was more like core Araceae in floral development than was Lysichiton or Symplocarpus. Orontieae have a long internode (not always obvious) between the base of the spike and the subtending leaf or spathe and there may be common A-C primordia (Buzgo 2001). The "flowers" of Lemnoideae may have up to three stamens, but because they mature at different times, Bogner (2009) suggested that they came from different flowers. The sterile flowers that are often found between the staminate and carpelate zones of the inflorescences of many Aroideae develop in a variety of ways, but whether this implies that there are correspondingly different evolutionary pathways is unclear (c.f. Barabé et al. 2004a). When flowers are 2-merous, the outer pair of tepals are lateral. Some taxa have binucleate tapetal cells (Wunderlich 1954), but they are usually uninucleate. Pseudomonomery has been documented for the family (Eckardt 1937; see Buzgo 2001). See Buzgo (2001) for discussion on the gyneocial construction of [Gymnostachydoideae + Orontioideae]; the gynoecium often has a single loculus.
Variation in ovule morphology is considerable but confusing. The ovules of Pothoidaeae and Monsteroideae are described as frequently being ana-campylotropous (Seubert 1997a). Gymnostachys has no micropyle since the integuments do not cover the nucellus, Pistia is exostomal, and other taxa are bistomal; the ovules of Pothos macrophyllus are shown by Buzgo (2001) to be anatropous and apotropous, although Pothos is described in the same paper as having straight ovules (the former is correct); in a number of taxa the ovules are reported to be tenuinucellate while the nucellus of Pistia is "very well developed" and the cells appear to be in radial files (Mercado-Noriel & Mercado 1978); both a nucellar cap and an endothelium may be present; and so on. Parameswaran (1959) described Theriophonum minutum as being tenuinucellate, but he drew a complete layer of cells below the nucellar epidermis, while Jüssen (1929) noted that Spathiphyllum had a doubled epidermal layer, but that is not evident from the illustrations given, and it is in general difficult to match statements of nucellus type with the illustrations there. Taxa in which the megaspore that germinates is micropylar are scattered in the family, especially in the basal clades (Grayum 1991).
Maheshwari and Khanna (1957) and Tobe and Kadokawa (2010) describe the endosperm as being cellular, but it could be interpreted as being an extreme form of helobial (see also Acorales). According to Mercado-Noriel and Mercado (1978) the seeds of Pistia have large amounts of perisperm as well as some endosperm. Variation in seedling morphology is great; in some taxa the roots are green, and in others they are always white (Tillich 1985, 2003b; Leck & Outred 2008). The cataphylls of the seedlings of Orontium are relatively long, linear structures (Tillich 2003b).
Information is taken from Bogner (1972), Grayum (1990), Mayo et al. (1997: particularly useful, 1998), Cusimano et al. (2011) and Bown (2025), all general, Prime (1960: esp. Arum), Buzgó (1994: Pothos), Buzgo and Endress (1999: Gymnostachys), Hetterscheid and Ittenbach (1996: Amorphophallus), Boyce and Wong (2015: Malesian genera), Croat et al. (2017 and references: Xanthosoma, 2018: Caladieae), Croat and Ortiz (2020: distribution, life forms), Hay (2022: Aglaonemateae) and Hentz Júnior et al. (2026: Spathicarpeae). See also Gilman et al. (2023: CAM photosynthesis), Dring et al. (1995: chemistry), Meija and Soukup (2024: long-chain seed fatty acids), Behnke (1995a: sieve tube plastids), French (1987b, 1988: laticifers, 1998: stem anatomy very variable), Keating (2000b: collenchyma, 2003a: general anatomy, b: leaf anatomy, 2004a: classification, b: raphides), Gonçalves et al. (2004: collenchyma), Carlquist and Schneider (2013: vessels), Murata (2021: leaf vernation). For floral morphology, see Mayo (1989: Philodendron), Barabé and Lacroix (2008a) and Poli et al. (2012, 2015), all Anthurium, Barabé and Lacroix (2008b: Anaphyllopsis), Barabé et al. (2012: Syngonium), Fukai (2004: Arisaema) and Barabé (2011: floral merosity of Lasioideae, 2013: general, esp. Lasioideae), for pollen, see Grayum (1991, 1992: much variation), Weber et al. (1999), Hesse (2002: Lasioideae), Jayalakshmi (2004: phylogenetic framework inadequate), van der Ham et al. (2005: Amorphophallus and relatives), Hesse (2006a, b: summary, phylogenetic framework reasonable), Ulrich et al. (2012: Schismatoglottideae, 2013: Calla, 2017: Amorphophallus) and French (1985 and references: endothecial thickenings), for ovary loculus hairs, see French (1987a), for floral anatomy, see Eyde et al. (1967), also Campbell (1900, 1903, 1905), Gow (1908, 1913), von Guttenberg (1960: Arum), Maheshwari and Khanna (1956), Swamy and Krishnamurthy (1971), and Tobe and Kadokawa (2008: good summary, 2010: endosperm development), all embryology, French (1986), ovular vasculature, Smith and Stockey (2013), seeds of Lasioideae, Gatin (1921: unfortunately Gatin died before he could make more than this "première contribution") and Tillich (1985, 2003b, 2014) all seedlings, Seubert (1993: starch grains, seeds and seedlings, most of family; 1997a: Lasioideae).
As to Lemnoideae, for general information see den Hartog and van der Plas (1970), Plant Biol. 17 (2015: special issue), Cao et al. (2020), Acosta et al. (2021: as Lemnaceae) and F. Li et al. (2023: esp. Wolffia), for a monograph and much else, see Landolt (1980, 1986) and Landolt and Kandeler (1987) and for general morphology, see Landolt (1998) and especially Bogner (2009), for the morphology of Wolffia, see Sree et al. (2015a) and Lam and Michael (2022), for chemistry, etc., see Landolt and Kandeler (1987) and for embryology, see Maheshwari (1954).
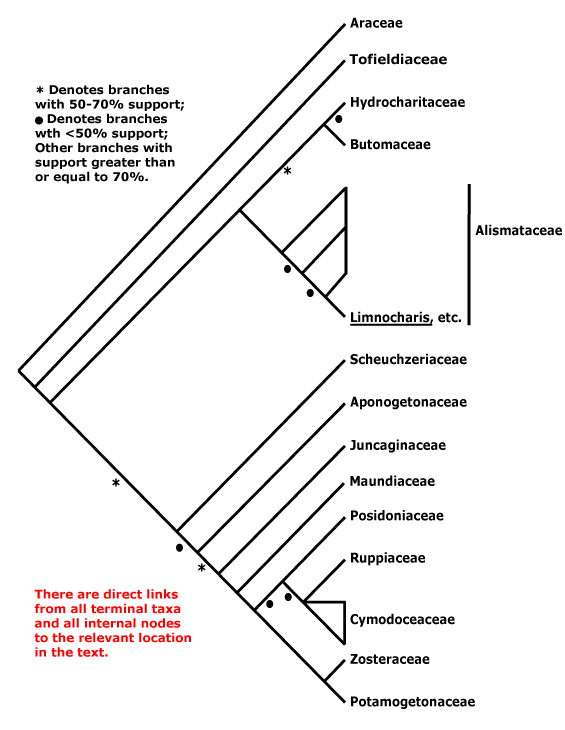
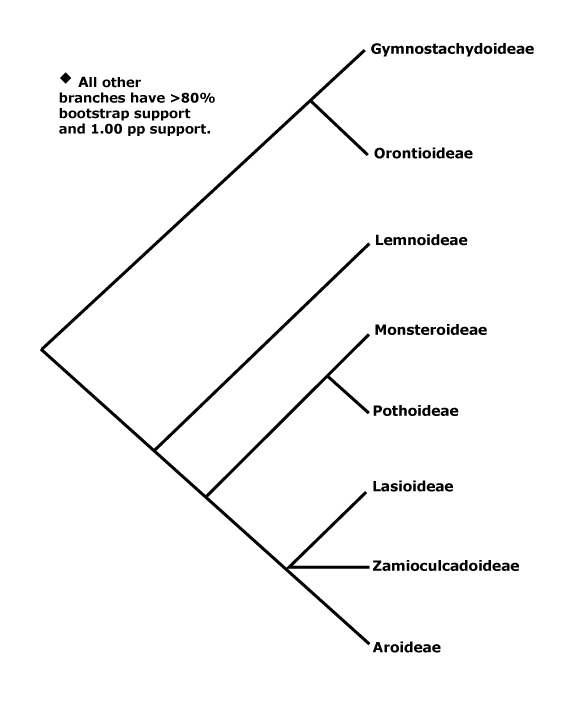
Phylogeny. Mayo et al. (2013) summarize phylogenetic work on the family. Early hypotheses of phylogeny based on restriction site analysis (French et al. 1995) suggested rather pectinate relationships in the family, while a consensus tree of morphological characters (Mayo et al. 1997) showed somewhat less resolution. Indeed, relationships have turned out to be pectinate, although of course that is in part because of what we have decided to call subfamilies. In most analyses the clade [Gymostachydoideae + Orontioideae] is sister to the rest of the family, and Lemnoideae are strongly supported as sister to the remainder, although Iles et al. (2013) recovered a topology [Gymostachydoideae [Orontioideae + the rest]], although support was weak and sampling poor. Barabé et al. (2004a: little support) found that Lasioideae were not clearly separated from Aroideae. Although a trnL-trnF phylogeny (Rothwell et al. 2004) placed Callopsis and Asterostigma (both Aroideae) outside a clade with 100% jackknife support that included other Aroideae, Lemnoideae and Pothoideae, Tam et al. (2004: trnL-F sequences, Calla not examined) again suggest the phylogeny is rather pectinate, as do Cabrera et al. (2008: five chloroplast genes). The topology that Cabrera et al. (2008) present, quite well supported, is used here (see also Nauheimer et al. 2012b; Henriquez et al. 2014: chloroplast sequences, note that analyses of mitochondrial sequences suggest rather different relationships). Abdullah et al. (2020b: plastome analysis) recovered the relationships [Orontioideae [Lemnoideae [Lasioideae [Zamioculcadoideae + Aroideae]]]], all with strong support; Calla was in Aroideae, somewhere fairly basal, although support for its position was not strong. Note. hopwever, that Calla is sometimes placed outside Aroideae (e.g. Stockey et al. 2023).
A good number of genera are included in the Angiosperms353 tree - see the Seed Plant Tree of Life (version ii.2022), but after the first three subfamilies mentioned above that had conventional positions (but Wolffia was well distant from other Lemnoideae), Gonatopus (Zamioculcadoideae) was placed sister to all remaining taxa, quite separated from Zamioculcas itself, and a number of major internal nodes had very poor support, although the enigmatic Calla was placed in a small, well supported clade along with Pinella, Spathicarpa, Philodendron, etc.. Note that there are a number of differences when compared with the v.2023 version of the Seed Plant Tree. Indeed, Haigh et al. (2022: 128 species, 111/143 genera) analysed an Angiosperms353 probe set, and this sets things up nicely. The position of Lasioideae is somewhat unclear as is that of Zamioculcadoideae (Haigh et al. 2022 suggest including the latter in Aroideae) but Calla does seem to belong in Aroideae, where its position may be near basal (but see above). Over 1/3 of the likely polytomies (13/32) were in Schismatoglottideae. There were substantial differences between the nuclear and chloroplast trees (the trees come from an analysis of 95 species from 79 genera), and the latter was somewhat better supported (Haigh et al. 2022). L. Zhao et al. (2022: 57 genera, 1081 orthologous genes) found that relationships at the bottom of the aroid tree were [Gymnostachys [Orontioideae [Lemnoideae [Lasioideae + the rest]]], although support for this last grouping was poor and there were problems with establishing relationships between the subfamilies.
Orontioideae: Orontium is sister to the rest of the subfamily (e.g. Nauheimer et al. 2012b) although in the Seed Plant Tree of Life (consulted ii.2022) Gymnostachys occupies that position.
For a phylogeny of Lemnoideae see Les et al. (2002), Rothwell et al. (2004), Wang and Messing (2011), Nauheimer et al. (2012b), Borisjuk et al. (2014), Tippery et al. (2015, 2020), and Ding et al. (2017) - relationships are [Spirodela [[Landoltia + Lemna] [Wolffia + Wolffiella]]], however, Tippery and Les (2020) suggested the relationships [Spirodela [Landoltia [Lemna [Wolffia + Wolffiella]]]], with Wolffia perhaps being paraphyletic (see also . For speciation in Lemnoideae, see Crawford et al. (2006).
Monsteroideae: Spathiphyllum may be sister to the rest of the subfamily (Henriquez et al. 2014; see also Tam et al. 2004).
Pothoideae: Carlsen and Croat (2013, 2019) have begun to disentangle relationships in Anthurium, the classical sections there are a poor guide to relationships (see also Tam et al. 2004). Weng et al. (2020 and references) examine the circumscription of Pothos.
Lasioideae: Urospatha is sister to the rest of the subfamily (Nauheimer et al. 2102b).
The exact position of Zamioculcadoideae needs confirmation, but they can reasonably be excluded from Aroideae. Although there is no strong molecular support for a clade [Zamioculcadoideae + Aroideae] in some analyses (e.g. Nauheimer et al. 2012b), it was well supported in the plastid analysis of Henriquez et al. (2014); this clade has several morphological features in common (see above). Bogner and Hesse (2005) raised the group [Zamioculcas + Gonatopus] to subfamilial status as Zamioculcadoideae. Stylochaeton has the same pollen as most Aroideae and simple leaves (Hesse et al. 2001), and since it is sister to other Zamioculcadoideae (see also Nauheimer et al. 2012b; c.f. some analyses in Chartier et al. 2014a), its inclusion in that subfamily (see Cabrera et al. 2008) means that the distinctive morphological features of [Zamioculcas + Gonatopus] are apomorphies at that level. The phylogeny of Cusimano et al. (2011) is largely similar to that of Cabrera et al. (2008); the former recognise a Zamioculcadoideae s. str.. It may be of interest that the pollen of Lasioideae, at least, has a lamellate endexine rather like that of Zamioculcadoideae.
Aroideae. Cabrera et al. (2008) offer a number of suggestions about tribal relationships here; for Chinese taxa, see Z.-D. Chen et al. (2016). Nauheimer et al. (2012b) found little support for relationships between many of the tribes; Callopsis was sister to the rest of the subfamily, but support was weak. In a plastome analysis, Hein et al. (2025) found that Areae were embedded in Arisaemateae and Colocasieae, both paraphyletic.
Cusimano et al. (2010) and Ohi-Toma et al. (2010) discuss relationships within Areae, particularly Typhonium and related genera - Renner and Zhang (2004) had found that Typhonium was paraphyletic - while Espíndola et al. (2010) looked at Arum itself; see also Hein et al. (2025).
Arisaemateae. Renner et al. (2004: support varied from slight to strong depending on the analysis) found that Arisaema tortuosa was sister to the rest of the genus, a position not recovered by Ohi-Toma et al. (2016) who found that relationships along the backbone of Arisaema were for the most part poorly supported.
Colacasieae. Nauheimer et al. (2012a) looked at relationships in Alocasia.
Philodendreae. For a phylogeny of Philodendron, see Gauthier et al. (2008); Homalomena may be part of the same clade. S. Y. Wong et al. (2013) focussed on Southeast Asian Homalomena and found that H. cochinchinense was sister to all other species, while the New World species formed a quite separate clade that was sister to a clade of Philodendron, within which they are embedded (see also S. Y. Wong et al. 2016). New World species were found probably to be sister to Philodendron (as Adelonema: Loss-Oliveira et al. 2016; Canal et al. 2018, 2019; S. Vasconcelos et al. 2018). Canal et al. (2018, 2019) also examined relationships within Philodendron; sections within subgenus Philodendron, which makes up the bulk of the genus, were largely non-monophyletic (geography works better than morphology here).
For relationships in the speciose Schismatoglottideae, see S. Y. Wong et al. (2010, 2018) and Wong (2013); relationships around Schismattoglottis were unclear, although a reaction looking at Wong et al. (2010) might be to put everything in Schismatoglottis...
Gonçalves et al. (2007) discuss the phylogeny of the Andean Spathicarpeae, a clade in which the spathe is adnate to the spadix; many of the species grow in very dry conditions and/or high altitudes, rather atypical for the family.
Thomsonieae. In an early matK and trnL study, Grob et al. (2002) found that Amorphophallus was paraphyletic and included Pseudodracontium; there were a number of clades between which relationships were unresolved and one of these clades contained all the African species in the study. For other work on the genus, see Sedayu et al. (2010) and in particular Claudel et al. (2017), the latter, who used nuclear ITS1) and plastid (rbcL and matK) obtained a fair amount of structure in their phylogeny, i.a. a monophyletic subgenus Afrothallus. More recently, Wong et al. (2022) have focussed on relationships of the Bornean species, all of which are to be included in subgenus Amorphophallus.
The position of Calla palustris, in early (prior to ed. 7) versions of this site placed in a separate subfamily, needs confirmation, although it seems to be best included in Aroideae for now. Barabé et al. (2004a) found that it was embedded in Aroideae, although without strong support (see also Nauheimer et al. 2012b: well embedded, but again with poor support). Calla came out in a clade of Aroideae along with other rooted aquatics/marsh plants in some molecular analyses (Cabrera et al. 2008), but its position was unclear in both morphological and restriction site analyses. Chartier et al. (2014a) found a clade [Anubias [Calla + Montrichardia]] sister to other Aroideae, but support was low. Calla and Schismatoglottis ("Calla and the rheophytes") formed a clade, albeit with weak support, that was sister to one of the two major clades within Aroideae and there were some morphological features for that clade (Henriquez et al. 2014). Just looking at Chinese taxa, Calla was in a separate clade off the backbone of the tree (Z.-D. Chen et al. 2016), while as mentioned above the genus is sometimes placed well outside Aroideae (Stockey et al. 2023).
What does morphology have to say about all this? Calla and Aroideae both have laticifers, but the former has bisulcate pollen with sporopollenin and a tectate-columellate ektexine, perfect flowers, etc. (Ulrich et al. 2013) - and it has a much more northerly distribution (see the Map above), overlapping with that of other Aroideae only in Western Europe and N.E. North America. The ovules have parietal tissue, but so do those of some other Aroideae (Ariopsis, Arum). Its perfect flowers and many pollen features would represent reversals, its acropetal flowering is unique (c.f. Cusimano et al. 2011; Ulrich et al. 2013; Barabé 2013: floral morphology), although some other Aroideae do have perfect flowers, albeit atypical. Pollen data suggested to Ulrich et al. (2013) similarities with Stylochaeton (Zamioculcadoideae) and Aroideae on the one hand and with Lasioideae on the other. The phylogenetic position of Calla needs to be clarified in order to understand the evolution of its morphology.
Classification. See Mayo and Bogner (2013) for Adolf Engler's classification of Araceae, which lasted for about a hundred years. For a tribal classification, see Cabrera et al. (2008) and especially Cusimano et al. (2011), however, a few genera immediately basal to their Aroideae s. str. and Zamioculcadoideae s. str. are unplaced, and a broad circumscription of both subfamilies is adopted here. See also Mayo et al. (2013) for the classification of Araceae. The recent study by Haigh et al. (2022) suggests modifications of parts of the classification, for instance, they include Zamioculcadoideae in Aroideae, but pending less ambiguous phylogenies, I have changed little above. For a checklist and bibliography, see Govaerts and Frodin (2002), the World Checklist of Monocots, and for several keys and much more, see CATE-Araceae.
The recognition of Lemnaceae, which entails the recognition of Orontiaceae, has its attractions for some (e.g. Acosta et al. 2021), especially those who work on these plants, potentially very important in agriculture, etc.. Although the title of Tippery et al. (2021), "Lemnaceae and Orontiaceae are phylogenetically and morphologically distinct from Araceae" seems convincing, exactly the same title, other than the names of the plants, could be used to justify dozens of additional families. Note that although the taxon pairs Lemneae + Wolffieae and Lemnoideae + Wolffioideae are often recognized in non-systematic literature, as in Borisjuk et al. (2014) and Michael et al. (2020), the first member of each pair is paraphyletic (and Wolffia as recognized there is not monophyletic). That aside, there is an active community that uses the name Lemnaceae for their plants...
Low et al. (2018) delimit genera in Aroideae-Schismatoglottideae, but the result is perhaps rather splitty, indeed, there are many monotypic genera in Araceae. Sakaragui et al. (2018) raise a long-recognized group in Philodendron (Aroideae) - actually, its relationships are somewhat unclear - to generic rank, which seems a little odd (c.f. Canal et al. 2019). Ohi-Toma et al. (2016) provide a sectional classification for Arisaema (15 sections) and Claudel et al. (2017) a subgeneric classification for Amorphophallus.
Croat and Ortiz (2020) suggested that there may be a very large number of species in Anthurium, and most of them were undescribed...
Botanical Trivia. The nucleus in the chalazal endosperm haustorium of Arum maculatum is reported to be 24,576 n (Werker 1997).
Wolffia is perhaps the fastest growing embryophyte: "if Wolffia had access to unlimited nutrients and CO2, it could give rise to 1030 plants in 4 months, a volume roughly equivalent to Earth" (Lam & Michael 2022: p. 431).
The unpleasant smell of skunk cabbages (Symplocarpus) is evident only when the plant is damaged, not when it is in flower.
Thanks. I am grateful to Monica Carlsen and Richard Keating for discussions about Araceae and to Simon Mayo for comments.
[[Alismataceae [Butomaceae + Hydrocharitaceae]] [Aponogetonaceae [Scheuchzeriaceae [Juncaginaceae [Maundiaceae [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]]]]]]] / Helobiae / Fluviales / Core Alismatids - and including seagrasses: mycorrhizae uncommon; plant rooted aquatic (with floating stems), leaves often emergent; roots often not medullated [pith slight to none], rhizodermal cells dimorphic, root hairs from short cells; (protoxylem lacunae +); stem with lacunae; little oxalate accumulation; raphides and druses 0, (prismatic crystals +); bulliform cells 0 [?this level]; plant glabrous; pollen grains tricellular; carpel fusion via the central floral axis, partial at the carpel periphery; endosperm 0, suspensor unicellular, cell large, endopolyploid [128-ploid - ?all]; whole genome triplication, mitochondrial rpl5, rps2, -4, -13 genes 0, rpl16 0/pseudogenized; seedling collar and collar rhizoids +.
Age. The divergence of the two main clades above is dated at 91-81 Ma by Wikström et al. (2001: note topology), ca 107 Ma (Janssen & Bremer 2004), or a little younger, (115-)96, 83(-66) Ma (Bell et al. 2010: note topology), ca 96.6 Ma (Magallón et al. 2015), (119-)105(-92 Ma (Waycott et al. 2018) or (89.9-)87.0(-79.8) Ma (X. Ma et al. (2023/2024) - but c.f their Fig. 2, where the same MRCA of the clade that includes Potamogetonaceae, Zosteraceae, Posidoniaceae, Cymodoceaceae and Hydrocharitaceae is around 110-105 Ma.
Evolution: Divergence & Distribution. L.-Y. Chen et al. (2013: disregard ages in Table 1) thought that the most recent common ancestor of this clade inhabited Eurasia.
J.-M. Chen et al. (2004a) suggested that free carpels had evolved twice, there had been reversions to syncarpy, and the unicarpelate condition also showed a complex pattern of evolution. An early sudy by Boutard et al. (1973) suggested that flavonoid variaton placed the species of this group studied into two groups, everything up to Aponogetonaceae and Juncaginaceae below, and the oher taxa. Posluszny and Charlton (1993) looked at floral evolution here, especially the perianth-androecium association in taxa like Juncaginaceae, Potamogetonaceae, etc., and they grappled with the relationships between flowers and inflorescences, but not invoking pseudanthia. Sokoloff et al. (2013) suggest an apomorpohy scheme for the group; the positions of Aponogetonaceae and Scheuchzeriaceae in their Fig. 17 are reversed compared with the topology below.
There are a number of cases in this clade of apparently widespread species in which species limits have to be examined (Ito et al. 2020).
Ecology & Physiology. Nearly all members of this clade, the old Helobiae, grow in more or less marshy or aquatic environments, and these may be more or less saline. The group is common in all aquatic environments in which angiosperms grow except fast-flowing rivers, and they can be locally abundant. J.-M. Chen et al. (2004b) discuss the evolution of various life forms in Helobiae - parallelisms are common.
This clade is particularly notable for the number of taxa it contains that can tolerate salt concentrations of 200 mM (Flowers et al. 2010; see also Saslis-Lagoudakis et al. 2016), and it includes all fully marine angiosperms that grow submerged in the sea where salt concentrations are ca 35 ppt (Waycott et al. 2018). Before going further, I will briefly mention 1), some other angiosperms that live in more or less marine habitats, and 2), some other angiosperms more or less adapted to the aquatic habitat.
1) Other Angiosperms in Marine Habitats. Angiosperms growing in more or less marine habitats are found in three main vegetation types - seagrasses, mangroves, and tidal saltmarshes, the blue carbon ecosystems. Looking at carbon (C) sequestration in such wetland habitats from a global perspective, although C density [C/area] is highest in peatlands and mangroves (900≤ Mg C ha-1; see also peatlands and mangroves), salt marshes (400 Mg C ha-1) and seagrass meadows (330 Mg C ha-1) both sequester and trap large amounts of C, more than other vegetation types (see Mcleod et al. 2011; Chmura 2011; Lovelock et al. 2013; Temmink et al. 2022). Indeed, saltmarshes trap allochthonous C at a rate of ca 25 mm year -1, faster than in other wetlands, although they may also have lost around 42% of their area in recent centuries; comparable figures for seagrass communities are 10 mm year-1 and 29% (Temmink et al. 2022). Note that monocot clades like Poales, so Juncaceae in particular, but also Cyperaceae, are prominent in estuarine conditions and they include quite a number of halophytic species (22 and 121 respectively). As in Poaceae, the ability to tolerate salt has arisen several times in both families, ca 8 and 52 times respectively (Moray et al. 2015 - see also Saslis-Lagoudakis et al. 2016). True grasses like Spartina (= Sporobolus sect. Spartina) and Puccinellia can dominate in estuaries, but they, too, are not thought of as being true seagrasses (for the evolution of salt tolerance in Poaceae, see above). Interestingly, roots of both saltmarsh Juncus and Sporobolus sect. Spartina are associated with Celerinatantimonas diazotrophica, the closest relative of Candidatus C. neptuna, known to fix N in Posidonia (see below, Mohr et al. 2021). Caryophyllales like Plumbaginaceae, Amaranthaceae-Amaranthoideae and especially the old Chenopodiaceae are also notable herbaceous components of such habitats. There are also a few woody taxa, i.e. the mangroves, that grow in more or less marine habitats.
2) Other Angiosperms in Aquatic, Non-Marine Habitats. Araceae-Lemnoideae are also much modified in connection with life in the aquatic habitat (see also above), albeit in freshwater, and, like the marine Zostera, they have lost a number of gene families involved in life on land; however, unlike Zostera, which are submerged rooting plants, Lemnoideae float and so have to deal with problems caused by high UV radiation, etc., and of course they live in freshwater (An et al. 2019; see also Aroideae-Pistia), as does Ceratophyllum, another very highly modified fresh-water aquatic angiosperm. Scheffer et al. (2003) examine the conditions that enable aquatic communities to be dominated by submerged and rooting plants, algae (phytoplankton), or small or large free-floating plants. Important variables are how exposed to the environment (large lake compared with small, tree-surrounded pond) the particular habitat is, the nutrient content of the water, and so on (Scheffer et al. 2003). Indeed, absorbotrophic mixotrophy - the plants obtaining some of their carbohydrates (for example) from the water - is possible here, as in Araceae-Lemnoideae (see Firmin et al. 2022). Note that the vegetative anatomy and aspects of the physiology of angiosperms that grow submerged in freshwater is similar in several respects to that of seagrasses.
SEAGRASSES: Evolution: Divergence & Distribution. The focus here is on seagrasses, an ecological grouping, and along with mangroves and tidal salt marshes (group 1 immediately above), they make up the so-called "blue carbon ecosystems"; for blue carbon ecosystems in the Gulf of Mexico, see Thorhaug et al. (2018). Such ecosystems all have very high C burial rates, well over 100 g C M2 y-1 (Mcleod et al. 2011; Chmura 2011; Lovelock et al. 2013). True seagrasses include some 65-72 species placed in Posidoniaceae, Cymodoceaceae (perhaps to include Ruppiaceae), Zosteraceae and Halophileae, a small clade of Hydrocharitaceae; the exact figures vary, and they depend on whether or not taxa like Althenia (Potamogetonaceae-Zannicellieae) are included (e.g. Short et al. 2011; Larkum et al. 2018b). Such plants are not at all close to true grasses, Poaceae. This extreme halophytic habit has evolved probably two or three times and only in this part of Alismatales - once in Hydrocharitaceae and again in the [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]] clade (the evolution of the halophytic habit may be separate in Zosteraceae and Potamogetonaceae-Lepilaena). Hartog and Kuo (2006) estimate that there are some 48 species of extreme halophytes in this latter clade and another 18 such species in Hydrocharitaceae, while Waycott et al. (2018) estimate that there are some 84 species of halophytic plants in this part of the tree, 70 of which are in Hydrocharitaceae, Posidoniaceae, Ruppiaceae, Cymodoceaceae and the like. There are reversals from the extreme halophytic habit in Posidoniaceae, etc., if extreme halophily there evolved only once (Les et al. 1997b; Les & Tippery 2013), some species of Ruppiaceae, Zosteraceae and Potamogetonaceae in particular tolerating a range of salinities, even growing in freshwater habitats (Barbour 1970; Hartog & Kuo 2006). Furthermore, Maundiaceae and many Juncaginaceae happily grow in salt marshes. Note, however, that da Silva et al. (2021) suggest that there have been four origins of marine angiosperms - within Hydrocharitaceae, and Zosteraceae, Posidoniaceae and Cymodoceaeae s.l. (including Ruppiaceae) - but since essentially only marine angiosperms were included in their analysis, it is difficult to make much of such ideas.
What about the age of seagrasses? Uncertain. Pseudoasterophyllites, ca 97 Ma from the European Cenomanian, is possibly the earliest halophyte, and it was described as growing in supratidal salt marshes, however, morphologically it tends to link Chloranthaceae and Ceratophyllum (Kvacek et al. 2016), so if this is confirmed it would not be immediately related to Alismatales or any other of the extant halophytic groups under discussion. Fossils of Thalassocharis bosquetii, ca 72 Ma from the early Maastrichtian of western Europe, have been identified as those of a seagrass, although to what clade they should be assigned is unclear - they seem to lack intravaginal squamules. The stem anatomy of this plant is rather complex: There is a well-developed fibrous layer in which the vascular bundles are embedded and the bundles going to the leaves are constricted just before they depart the stem (van der Ham et al. 2017).
Within Alismatales, the crown-group age of the seagrass clade in Hydrocharitaceae-Hydrilloideae is estimated to be only (41.3-)19.4(-15.9) Ma (Iles et al. 2015), although other estimates are far older - thus L.-Y. Chen et al. (2022) suggest an age for this clade of around 62.8 Ma. However, there are possibly seagrasses in Late Cretaceous Campanian deposits from Colorado (Ivany et al. 1990 for references), while fossils from the late Middle Eocene perhaps as young as 35-33 Ma in Florida have been identified as Thalassodendron and Cymodocea (Cymodoceaceae), but neither genus now lives in the New World (Lumbert et al. 1984). Thalassia testudinum (Hydrocharitaceae) is also recorded from these deposits, and it is currently found in Florida (Lumbert et al. 1984). These and other records from the Eocene in the Old World suggest both a considerable age for the seagrass community and also that Tethyan seagrasses once extended to the New World which then had a richer seagrass community, and New World fossils like those of the sirenian Protosiren and cheloniid sea turtles help confirm this (Lumbert et al. 1984; Ivany et al. 1990). Although ages for crown-group Zosteraceae are much younger, around 33-17 Ma, the stem-group age of Zosteraceae is estimated to be 100-47 Ma (see below) - understanding the evolution of the marine habit is not straightforward. Da Silva et al. (2021: p. 2) note "The ancestral haploid chromosome number of marine angiosperms is n = 10, which diverged about 55 mya.", but it is unclear to what clade they are refering. Ages of the whole Helobieae clade (see above) are also rather up in the air. To summarize: Sea grasses may have originated in the eastern Tethys in the Late Cretaceous, and some taxa recorded from the New World in the Eocene are now known only from the Old World. Like mangroves, seagrasses are now most diverse in the area from the western Pacific to east Africa, less so in the Americas and west Africa (e.g. Tomlinson 1986), even if earlier in the Tertiary this difference may have been less marked. See also Marbà et al. (2015) and Clade Asymmetries for additional information.
Lam et al. (2016) in a three-gene analysis (plastome) of monocots found that Thalassia hemprichii (Hydrocharitaceae) was on a very long branch, similar to those of some holomycoheterotrophic taxa that were the focus of their study. Other Alismatales, including Lemnoideae, also had fairly long branches; Tofieldiaceae and Araceae-Gymnostachys had normal short branches.
Ecology & Physiology. Seagrasses often grow in monodominant stands made up of clones that may be very extensive indeed (see Cymodoceaceae below). Seagrass meadows, marine prairies, occupy some 3.45 × 105 km2 which is about 0.1–0.2% of the total area of the oceans (Munné-Bosch et al. 2022) or 1.604-2.666 × 105 km2 (Unsworth et al. 2022), which means either that we don't know much and/or there is no fixed definition of what a seagrass community is. Other estimates of the area occupied by seagrass communities range from 22.8 x 106 (Waycott et al. 2009) or 30 x 106 to ca 40 x 106 ha, and although this is still less than 0.2% of the area of the oceans (Duarte et al. 2005), the gross primary productivity of seagrass communities is high, around 1903 g C m2 y-1 (like that of mangroves) and global primary productivity is 628 Tg C y-1, while their net ecosystem production (1211 g Cm2 y-1 and globally 400 Tg C y-1) is substantially higher than that of mangroves because of a relatively low respiration rate. Seagrasses are responsible for 1.13% of all marine primary productivity; 27-44 Tg C y-1 produced by seagrasses is buried, some 10-18% of the total C storage in the marine ecosystem (Duarte 2011: macroalgae excluded, area occupied = 0.3 x 1012 m2 [30 x 106 ha]; see also X. Ma et al. 2023/2024). Indeed, this estimate of the amount of C buried may be only one half the actual amount (Fourqueran et al. 2012). Although the amount of C in seagrass plants themselves may be small - but note that they contain lignin, Posidonia in particular (see above) and may be very long lived (see elsewhere), the C stored in the soil/trapped sediment in seagrass communities, which can be up to 11 m thick in the Mediterranean, is greater than that in the soils of most forests and comparable with that in terrestrial peats and in mangroves. Other estimates of global C storage by seagrasses range from 4.2-8.4 or 9.8-19.8 Pg C, depending on the assumptions made, somewhat over 0.5% the global total (Fourqueran et al. 2012), and this C may be sequestered for maybe 12,000 years or so in the anoxic seagrass peats (Orem et al. 1999; Mateo et al. 2006; K. A. Moore & Short 2006; Serrano et al. 2011, 2013). As seagrasses decay, long-lived refractory C may be produced, and/or conditions may be more or less anoxic, which allows the persistence of other forms of C (Mateo et al. 2006). Posidonia oceanica in particular may form massive deposits some 5 m thick containing refractory C (Mateo et al. 2006; Gobert et al. 2006). Indeed, seagrasses trap not only sediment but allochthonous C, too (e.g. Marbá et al. 2006), and when thinking about seagrass communities as being C sinks, then an estimate of 169-186 g C m-2 yr-1 seems reasonable - net community production of ca 120 g plus 41-66 g of allochthonous C (Kennedy et al. 2010: higher areal estimate above).
The gross primary productivity of seagrasses has been estimated at 3595 g C m2 y-1 and global primary productivity is 1438 Tg C y-1, while net ecosystem production is 1585 g C m2y-1 and globally 634 Tg C y-1, substantially higher than either mangrove or seagrasses. Estimates of C burial are 60.4-70.0 Tg C y-1 (Duarte et al. 2005). Furthermore, a substantial amount of seagrass C moves into other marine ecosystems, including those in the deep sea (Suchanek et al. 1985). Mcleod et al. (2011) suggest a C burial rate of (100-)138(-176) g C m-2 y-1 (range 45-190), total C burial of 48-112 Tg C y-1), in a seagrass area of 17.7-60.0 x 106 ha. Looking at the same issue in another way, Vermaat (2009) noted that seagrass detritus was recalcitrant, and although seagrass communities produced about 1% of the C fixed in the oceans, they stored about 12%.
As already suggested, at one level the anatomy and physiology of submerged marine angiosperms are very similar to those of fully submerged freshwater angiosperms. In both groups there is a diffusive boundary layer immediately surrounding the leaves, i.e., because of the physiological activities of the leaf, the chemical and physical environment in this boundary layer is different from that of the surrounding seawater, and this can affect the physiology of the leaf, a sort of vicious cycle - thus photosynthesis can proceed at its maximum rate only if the water is moving, so reducing the thickness of this layer. Stomata are absent, and chloroplasts are concentrated in the epidermal layers in particular (if there are any chloroplasts in the epidermis, they are normally restricted to the stomatal guard cells). The thickness of the leaf is correspondingly reduced, although rather less so in marine aquatics - but the thin-leaved Halophila is an exception in that group. Aerenchyma is present. Finally, some kind of carbon-concentrating mechanism (CCM) is likely to be developed (Larkum et al. 2006b, 2018c). Seagrasses also have a thin cuticle, perhaps allowing HCO3- to diffuse across, and they have little in the way of water-conducting tissues (Kuo & den Hartog 2006; Larkum et al. 2018c). Another feature of the environment in which submerged aquatics live is light in which red and far red wavelengths quickly decrease with depth (Soong et al. 2013; Abdelrhman 2016: figs 9-11). Recently Sogin et al. (2022) noted that sucrose was exuded from seagrass roots, as were phenolics; the deeper layers of the mud were anoxic and the sucrose was not broken down, the microbial community being not very diverse and unable to break down sucrose under such conditions - this then may have affected C cycling. (It is possible that this is a feature of aquatic habitats in general.) Another interesting feature of seagrass beds is the possibility of sulphide poisoning. In the decomposition of organic matter under the more or less anaerobic conditions of these beds sulphate gets converted to sulphide, and this reaches levels that are toxic. However, van der Heide et al. (2012) found that genera of locinid bivalves like Loripes were widespread in seagrass beds, sometimes at densities of over 1000/m2. The molluscs hosted bacteria in special cells in their gills, and these bacteria oxidized the sulphur; productivity of the seagrasses increased and their poisoning was averted. Locinids are associated with all seagrasses except Phyllospadix, which grows on rocks, and appear to have diversified when the seagrasses did; they are ubiquitous in the tropics, but are found in only ca 1/2 the seagrass beds in more temperate regions (van der Heide et al. 2012). Interestingly, bacteria associated with Loripes can fix nitrogen (J. M. Petersen et al. 2016), although I do not know if this happens in seagrass beds.
Recently Capó-Bauçà et al. (2022) showed that there had been convergent evolution in CCMs in seagrasses, noting that they were biophysical in nature and were associated with a reduction in the affinity of Rubisco for CO2, etc.; the changes were also similar to changes in the CCMs of marine eukaryotic algae, hence the convergence. They looked at Posidonia, Cymodocea and Zostera among the seagrasses, also at a number of other Alismatales, but of course it is not easy to work out the evolution of the seagrass habit in these taxa. Gibbs (1958) found that the members of Helobieae in general that he sampled tended to have very low concentrations of syringyl lignin (although not some Alismataceae, at least), but from his observations it seems that this may be more a general feature of angiosperms that grow in aquatic habitats; Erickson et al. (1973) were unsure if Zostera marina had lignin at all. However, Mateo et al. (2006) noted that seagrasses were unusual among aquatics in containing substantial amounts of lignin; there was not much in the xylem, but it was to be found in cauline epidermal and outer cortical tissues, for example (Klap et al. 2020: Zostera, Posidonia), and this affects C cycling in the seagrass ecosystems (see below, also Lewis & Yamamoto 1990; Kuo & den Hartog 2006). Looking at marine angiosperms in general, Posidonia seems to have appreciably more lignin than the other taxa sampled (Pfeifer & Classen 2020). However, as those authors note, our knowledge of cell wall composition in seagrasses is limited.
Touchette (2007; see also Marbà et al. 2006; Mateo et al. 2006 and other papers in Larkum et al. 2006a) and in particular X. Ma et al. (2023/2024) discuss the physiological problems faced by marine angiosperms, particularly problems with too much/little Na, Cl, and P. Romero et al. (2006) noted that seagrasses were adapted to nutrient poor conditions. N metabolism and N cycling in the seagrass community is of great interest, if poorly understood; it affects the C fixation just discussed. Cramer et al. (2011) described Celerinatantimonas diazotrophica, a N-fixing gammaproteobacterium found associated with the estuarine saltmarsh plants Spartina alterniflora and Juncus roemerianus. N-fixing Celerinatantimonadaceae are known from mud, associated with roots, in microbial mats, from permafrost, etc. (Mohr et al. 2021). More recently Candidatus Celerinatantimonas neptuna, a strongly N-fixing endophyte, has been described from the seagrass Posidonia oceanica in the Mediterranean (Mohr et al. 2021), which itself fixes substantial amounts of C. The foliar microbiome may be involved in mineralizing organic N and facilitating its uptake by the plant (Tarquinio et al. 2018). Healthy seagrass communities also emit relatively small amounts of the greenhouse gases NOx and CH4 compared with when they are disturbed and eutrophified, and as mentioned elsehwere they also trap considerable amounts of small particles, including fragments of organic material, bacteria and viruses, so removing them from the water column (Unsworth et al. 2022). Overall, however, the microbiome of seagrass communities is poorly understood.
L.-Y. Chen et al. (2022) looked at the physiological adaptations of Alismatales to aquatic environments. Invers et al. (1999) examined carbon acquisition via dissolved bicarbonates. For the effect of the oxygen concentration of the water on submerged angiosperms in general, see Caraco et al. (2006). Sulphated phenolic compounds and flavones are common in seagrasses (McMillan et al. 1980), and there are distinct seagrass pectins; such substances probably arose in parallel in different seagrass clades, and although their function is sometimes unclear, it is perhaps connected with the problems of living in saline environments (McMillan et al. 1980 and references; Marbà et al. 2006: sulphates/sulphites; Olsen et al. 2016: sulphated polysaccharides in Zosteraceae, involved in osmotic balance; H. T. Lee et al. 2018: conservation of genes involved in cell osmoregulation, etc.). Wissler et al. (2011) compare gene expression in Posidonia and Zostera with that in grasses and broad-leaved angiosperms, and H. T. Lee et al. (2018) carry out similar comparisons involving Zosteraceae and Hydrocharitaceae. In such cases adaptation to the marine habitat has also involved the loss of a number of genes such as those involved in hormone response, the synthesis of volatile compounds, stomatal development (often loss), photosynthesis and cell wall composition (Lee et al. 2016, 2018; Olsen et al. 2016; see also Roodt et al. 2019; L.-Y. Chen et al. 2022), furthermore, since dessication is a problem that is unlikely to be faced much by marine angiosperms, Z. marina, at least, has fewer Late Embryogenesis Abundant gene families (and a lower absolute number of such genes, which are expressed in response to stress, dessication in particular) than most other angiosperms, although not surprisingly Spirodela polyrhiza comes close (but more surprisingly Vitis vinifera is also close, although the latter in particular has at least has all the eight gene families normally found in angiosperms: Olsen et al. 2016; Artur et al. 2018). Ma et al. (2023/2024) suggested that overall the adaptation of these plants to the marine habitat had involved much tweaking of the genome, but little massive change of key traits; additional genes may have been integrated into the genome following the whole genome triplication. Changes that occurred include the production of volatile metabolites, hypolignification, etc.; interestingly, relatively little seems to have happened to the salt tolerance of the plant, alhtough some genes here were lost (Ma et al. 2023/2024).
Marine fairy rings have been observed in seagrasses like Zostera marina and Posidonia aquatica at least (Borum et al. 2014; Ruiz-Reynés et al. 2023). In the latter case the sediments are rich in organic matter but poor in oxygen, and sulphates get converted to toxic hydrogen sulphide (H2S). H2S concentration is particularly high on the inside of the spreading rings, and when those rings merge, H2S moves through the plant to the rhizome apex, causing its death and the disappearance of the rings (Ruiz-Ryenés et al. 2023). H2S is also involved in the break-up of the rings in Z. marina, although its presence there may be because of the calcareous nature of the substrate (Borum et al. 2014).
Although the focus above is on seagrasses themselves, they are part of a community that includes a diversity of other organisms. Importantly, a variety of epiphytes, often including calcareous red algae, grow on seagrasses, and Borowitzka et al. (2006) cite studies where the total primary production of epiphytes in seagrass ecosystems ranged from (2-)19-60≤%. For discussion on seagrass herbivory, see Plant/Animal Interactions below.
Estimates of the value of the ecosystem services provided by seagrasses are about $20,000/ha y-1, twice or more those for mangroves, saltmarshes, and coral reefs (Orth et al. 2006a). However, seagrass ecosystems are under considerable pressure from humans and occasional pandemic diseases (Orth et al. 2006a).
Overall, the ecological importance of seagrasses is very great (see e.g. Marbà et al. 2006; Mateo et al. 2006, papers in Larkum et al. 2006a, 2018a; Pirog 2011, etc.). The seagrass ecosystem is very productive, supports a considerable amount of diversity, especially in associated animals, it captures much sediment, and it stores much carbon and perhaps also nitrogen (e.g. Marbà et al. 2006; Orth et al. 2006a; Vermaat 2009; Kennedy et al. 2010; also Marbà et al. 2015; Mohr et al. 2021: nitrogen). For comparisons with the other major peat-producing ecosystems, see Sphagnum bogs, mangroves and the somewhat less extensive dipterocarp forests.
The rate of C accumulation and longer-term sequestration in coastal wetlands, including seagrass habitats, is likely to have been affected by recent changes in the sea level. Under stable sea levels, C accumulation in tidal marshes in particular is low, as it rises, accumulation increases, however, some C may be buried by fluvial and coastal processes and so sequestered, however, if sea levels fall, new areas become available for the establishment of such communities (Rogers et al. 2019; Treat et al. 2019). Seagrass communities may also have been similarly affected by such changes in sea levels. Human activities have also had a major effect leading to CO2 emissions from such ecosystems (Lovelock et al. 2017).
Pollination Biology & Seed Dispersal. This part of Alismatales includes most water-pollinated angiosperms, whether epihydrophilous (pollen is transported along the surface of the water) or hypohydrophilous (pollen travels through the water) (e.g. Ackerman 2000: q.v. for floral syndromes). The remarkable pollination devices of water-pollinated Alismatales, both marine and freshwater, have been much discussed (e.g. Ducker et al. 1978; Cox 1988: review; Cox et al. 1991: computer simulation of underwater pollination; Les et al. 1997b: phylogeny and hydrophily; see also Pettit et al. 1980; McConchie & Knox 1989; Les et al. 2006; Ackerman 2006; Remizowa et al. 2012b; Du & Wang 2014). These include staminate flowers that detach from the parent plant and rise to the surface, the floating flowers themselves transporting pollen to the stigma; pollen variously aggregated and forming masses especially on the water surface; and underwater pollination (hypohydrophily) where the pollen grains are sometimes very much elongated, aggregated to form elongated strands or germinate precociously, all increasing the chances of pollination - and parallel evolution is widespread, for example, elongation of the pollen occuring at different times of the meiotic process (Ackerman 2000). The title of a paper by Cox and Knox (1989), "Two-dimensional pollination in hydrophilous plants: Convergent evolution in the genera Halodule (Cymodoceaceae), Halophila (Hydrocharitaceae), Ruppia (Ruppiaceae) and Lepilaena (Zannicelliaceae [= Potamogetonaceae-Althenia below])" emphasized the explosive dehiscence of anthers as they reached the water surface and epihydrophily – and taxa like Hydrilla (Hydrocharitaceae) should join the list (see Q. Zhang et al. 2019). There is a connection between linear vs spherical to reniform pollen and non-papillate vs papillate stigmas (Pettitt 1980). Monoecy or dioecy is common, the carpelate flowers often have a single ovule per carpel and the fruits have but a single seed, and so on - such features parallel those of wind-pollinated angiosperms. Interestingly, it has recently been suggested that marine arthropods may carry out night-time pollination of Thalassia testudinum, a species in which mucilage aggregates the pollen into strands (van Tussenbroek et al. 2016), supposedly an adaptation to hypohydrophily. Pollen exine is often absent (Ackerman 2006), and the genes involved in exine development may have been lost (Olsen et al. 2016). The progamic phase, the time between pollination and fertilization, is notably short in most of this clade, as in at least some other aquatic angiosperms (see Williams et al. 2010: I have not tried to optimize this on the tree). These adaptations are so striking that the flowers and inflorescences in particular, but also the vegetative bodies, of the plants appear very different both from one another and from other Alismatales with more conventional life styles.
Pollen grains here may be produced in very large numbers – 0.16 x 106/flower in Thalassia testudinum (Hydrocharitaceae-Halophileae) to 1.14 x 106/flower in Syringodium filiforme (Cymodoceaceae), however, details of pollination of these plants in the Mexican Caribbean were unclear (Van Tussenbroek & Muhlia-Montero 2013). A good proportion of the exposed flower buds with their protective foliar structures removed is eaten by fish and the mucilage in the anthers is digestable. However, the lengthening of the bud and release of pollen happen within a few hours, hence the time in the wild during which they can be eaten is limited. Pollination of female flowers even 30 cm from male flowers can be appreciably reduced in Thalassia testudinum (Van Tussenbroek & Muhlia-Montero 2013).
Sea grasses in particular can disperse very long distances, perhaps hundreds of kilometres, whether as separate fruits, or as fruits on plant fragments (Kendrick et al. 2012). Individual seeds generally do not disperse so far. The seed coat is sometimes photosynthetic (Celdran 2016), and the surface of the testa can be quite elaborate (e.g. Birch 1981), and all in all the surface of the disseminule shows a considerable amount of variation (e.g. Kuo & den Hartog 2006; Orth et al. 2006).
Plant/Animal Interactions. The morpho-ecological characterisation of seagrasses by Valentine and Duffy (2006) parallels that of Poaceae, and both groups tolerate grazing by large vertebrates (for grazing of grasses, see elsehwere). However, seagrass stands are now usually relatively little grazed by marine mammals (e.g. Smith 1981; Waycott et al. 2009; Arnaud-Haond et al. 2012), and Valentine and Duffy (2006: p. 465) note that "one of the few existing paradigms of marine ecology is that little of the production of seagrasses is grazed by marine herbivores" - however, they question this paradigm, although we shall see that grazing of seagrasses today in at least some areas of the world is indeed much less than it has been in the past.
The Caribbean is the center of diversification of the sirenians, sea cows, large herbivorous marine vertebrates, where there is a 50 My history of the beasts (Domning 2001: most of this paragraph is taken from this review). Grazing of seagrasses by dugongids in particular was moderate to intense through much of the Tertiary. There has been extensive dugongid evolution (they make a paraphyletic group), and from their skull morphology - e.g. rostral inflection (≡ downwards bending of the front part of the head), size of tusks - different co-occuring species could probably tackle seagrass rhizomes of different thicknesses (all dugongids could eat the leaves). The tusks, modified incisors, of dugongids would probably have helped in uprooting rhizomes. There seem to have been only a few species of marine angiosperms in these seagrass beds, and they evolved quite early in the Tertiary (Ivany et al. 1990), with relatively little change since. Dugongids became much less diverse in the later Pliocene ca 3.1 Ma when the isthmus of Panama developed, the last dugongid from this area being known from the late Pliocene, and there were major changes in the marine ecosystems in the whole Caribbean area then. Manatees, trichechids, are known from the middle Miocene onwards (Potamosiren is the first fossil placed in this clade). They were initially freshwater and evolved distinctive supernumerary molars, perhaps to deal with lignin in the true grasses and/or the silt in the freshwater habitat in which they initially lived, and they moved over to marine habitats as the dugongids in the tropical Americas died out.
More recently direct and indirect human pressure has had substantial effects on dugongid populations, and Steller's sea cow, a seaweed-eating dugongid that lived in more temperate climates became extinct only recently, perhaps in 1768 (Turvey & Risley 2005). Now only the dugong, Dugong dugon, from East Africa to New Caledonia, and the West Indian manatee Trichechus manatus, from the West Atlantic, remain important seagrass herbivores, while two other species of manatees are also found in rivers in South America and Africa where they eat other aquatic angiosperms. Dugongs, with a colon up to 25 m long, 8≤ times the length of the animal, are very efficient hind-gut fermenters and fibrous material is converted by bacteria to volatile fatty acids (Marsh et al. 2018).
Seagrass herbivores also include green turtles, and their populations are recovering (Gulick et al. 2022). They are also hindgut fermenters, and they graze on Thalassia testudinum. Their gut microflora digests ca 90% of the cellulose, converting it to short-chain fatty acids. Interestingly, these turtles maintain grazing meadows for two years or more (see Launchbaugh 2020 terrestrial grazing meadows); in these meadows the Thalassia is cropped by the turtles, but it regenerates well, and the turtles prefer eating the dense short young blades of seagrass that result (Gulick et al. 2022); terrestrial grazers show similar behaviour. There are other herbivores like birds, sea urchins, etc., and small invertebrates grazing on the epiphytic seagrass algae are also important (Hemminga & Duarte 2000; Valentine & Duffy 2006; see also Duarte et al. 2006).
Thus those ecological relationships in seagrass beds that show dominance by mostly small herbivores, some grazing the algae on seagrasses rather than the plants themselves, and detritivores, may be fairly recent. Domning (2001) notes that sirenians, large marine herbivores from less than 100 to more than 1,000 kg in size, are well known as fossils in the New World from the Eocene onwards, with three or more species being sympatric; sirenians extant today do not reflect past diversity (for recent seagrass declines, see Hemminga & Duarte 2000). The different sizes of the tusks of these animals, and the degree of rostral inflection (how much the front of the jaw is bent downwards) allows speculation about their feeding habits, which likely ranged from eating seagrass leaves to grubbing up the sometimes quite large rhizomes (to 10 mm across) of genera like Thalassodendron. However, in the later Pliocene the whole system may have broken down (see also above), with some sirenians and seagrasses going extinct. Thus general ecological relationships in the whole Caribbean region showed extensive changes at this time, whether because of geological changes or cooling of the climate or both (Ivany et al. 1990; Domning 2001 and references). Extant seagrass communities serve as nurseries for the young of a variety of animals, and this relationship is also evident in fossil seagrass communities (Ivany et al. 1990).
The complexity of the relationships beteen herbivores and seagrasses, in this case the green sea turtle, Chelonia mydas and the seagrass Halodule uninervis is clear in the study by Christianen et al. (2014) carried out in marine preservation areas in Indonesia. Here the carnivores that might eat the sea turtles had been reduced and the population of turtles exploded, their feeding habits changed, and the seagrass populations were in danger of being wiped out. Although indeed a complex story, the two factors perhaps immediately precipitating the extinction was the adoption by the turtles of novel feeding strategies - they ate both leaves and below-ground roots and rhizomes - and the disinclination of the turtles to move outside the preservation areas even when their food source was disappearing (Christianen et al. 2014).
Seagrasses reduce the relative abundance of bacteria that are potential pathogens of fish and invertebrates living in seagrass communities, although how they do this is unknown (Lamb et al. 2017).
Plant-Bacterial/Fungal Associations. Seagrass stands are susceptible to attack by pathogens such as Labyrinthula, a heterokont protist that causes eel-grass wasting disease (Rasmussen 1998; K. A. Moore & Short 2006). For associations with bacteria both in the rhizosphere and as endophytes - some of these may fix nitrogen - see Cramer et al. (2011), Mohr et al. (2021), and also above.
Vegetative Variation. Tomlinson (1974b) described the vegetative morphology of seagrasses. He noted that in some taxa branching patterns were remarkably precise; roots were initiated within apical meristems, and were themselves unbranched, although a few taxa did have branched roots. The growth of seagrasses is highly modular, and members show an allometric scaling of their parts, the sizes of leaves, fruits, shoots and rhizomes all being correlated: Species with thin rhizomes grow fast and have short-lived leaves; plants with thicker rhizomes grow more slowly, but have longer-lived leaves and more inter-module integration (Tomlinson 1974b; Duarte 1991). Resting buds are produced sporadically throughout this group.
Non-medullated roots are quite common, occurring in e.g. Butomaceae, Alismataceae (inc. Limnocharitaceae) (Stant 1964, 1967), Aponogeton, Triglochin, Potamogeton, and many of the core seagrass clade (Kuo & den Hartog 2006), although roots of e.g. Posidonia are somewhat medullated (von Guttenberg 1968, but c.f. Mohr et al. 2021). Maundia (the roots are sometimes branched here) has triarch roots that lack pith (Platonova et al. 2016), and so from this point of view are more like broad-leaved angiosperms, while other taxa have 3-5-arch roots with a central vessel or space (Schenck 1886). Stems of a number of taxa have central vascular tissue (Schenck 1886). Arber (1921) noted that inverted vascular bundles were to be found in the leaf blades of some Hydrocharitaceae, Butomaceae, Alismataceae, Cymodoceaceae and Potamogetonaceae, while other taxa have unifacial leaves. Chloroplasts are often to be found in epidermal cells, as in other aquatics, hardly surpringly, stomata are absent, aerenchymya is common, occuring practically throughout the plant, etc. (Sculthorpe 1967; Han et al. 2020). Plants that produce some kind of exudate are scattered in this group; I know rather little about the morphology of the secretory canals and the composition of the exudate, so any mention of laticifers should not be interpreted literally.
Genes & Genomes. A genome duplication for taxa in this clade, the SALAα event, has been dated to ca 96.5 Ma (Landis et al. 2018; see also L.-Y. Chen et al. 2022); X. Ma et al. (2023/2024) mention a genome triplication. The cmt2 allele, chromomethylase, a methylation gene, is not functional in Spirodela and Zostera, and perhaps not in aquatic angiosperms in general (Harkess et al. 2020); see above for parallel evolution in the genomes of seagrasses.
For extensive cytological studies, see e.g. Harada (1956), Uchiyama (1989), Sharma and Chatterjee (1967) and Costa and Forni-Martins (2003). Da Silva et al. (2021) suggested that the base number of all seagrasses was x = 10, and although they discussed subsequent changes, what was going on in their non-marine relatives was hardly incorporated into their account.
There are a number of reports of sex chromosomes around here, e.g. in Phyllospadix (Harada 1956).
X. Ma et al. (2023/2024) noted that in both Thalasssia testudinaris and Posidonia oceanica over 85% of the genome was occupied by long terminal repeat retrotransposons, while in taxa like Zostera marina and Cymodocea nodosa the figure was ca 75%, and inPotamogeton acutifolium ca 40% - any correlation with ecology and phylogeny was unclear.
At least some genes from the mitochondrion show an accelerated rate of change in aquatic Alismatales (G. Petersen et al. 2006). Indeed, there is extensive variation here in this part of Alismatales, both in marine and freshwater taxa, with the unique loss/pseudogenization of several genes at this node in particular; elsewhere in the clade such changes are also common, but they occur in parallel (Petersen et al. 2017: Figs 2, 3).
Chemistry, Morphology, etc.. For the distribution of sulphated compounds, see especially McMillan et al. (1980). Thickened (nacreous) walls occur in the sieve tubes of a variety of seagrasses (Kuo 1983), and Platonova et al. (2016) discuss foliar vasculature in detail.
Remizowa et al. (2010b) discuss carpel fusion in the clade; when carpels are adnate to the central floral axis, they are often free laterally (see also Nymphaeaceae) and there is no compitum. The large single-celled suspensor is the micropylar cell produced by the first division of the zygote, and Hasitschka-Jenscke (1959) noted that in both Alisma and Potamogeton that cell was 128-ploid.
Den Hartog (1970), Tomlinson (1982: esp. anatomy), Kuo and McComb (1989), Hemminga and Duarte (2000: ecology), Green and Short (2003: inc. distribution maps, etc.), den Hartog and Kuo (2006, in Larkum et al. 2006a: generally very useful, 2018a: Australian seagrasses), Pirog (2011: esp. ecology), Wissler et al. (2011) and Hogarth (2015) provide general accounts of seagrasses; see Zidorn (2016) for a summary of secondary metabolites, Zindler-Frank (1976) for oxalate accumulation, Kuo et al. (2018) for the anatomy of Australian taxa, Wilder (1975) for vegetative branching, inflorescence morphology, etc., Eber (1934) and Eckardt (1957) for gynoecium and placentation, and Sokoloff et al. (2013c and references) for the rather scattered distribution of taxa with air canals in the testa.
Phylogeny. Les and Tippery (2013: main tree 167 taxa, rbcL) provide a phylogeny of the whole group as well as detailed studies of most of the families within it. However, recent studies using 347 nuclear genes are not giving a clear picture of relationships in this area (c.f. Baker et al. 2021: see Seed Plant Tree).
[Alismataceae [Butomaceae + Hydrocharitaceae]] : apical meristems of vegetative axes bifurcating; C-glycosyl flavones +; stomata paracytic, (adaxial peripheral foliar vascular bundles +, inverted); Vorläuferspitze on blades of emergent leaves 0; inflorescence branches determinate; bracteole +; P = K + C, members of both whorls with many traces; (androecium with trunk bundles), A pairs +; compitum 0, placentation ± laminar; (ovules many/carpel); seeds testal; chromosomes (0.8-)2-13.6 µm long; (seedlings with collar rhizoids).
Age. The age of this clade is mid-Cretaceous, some (127≤-)103.6(-74) Ma (L.-Y. Chen et al. 2012a), ca 95 Ma (Janssen & Bremer 2004), or around 83.5 Ma (Tank et al. 2015: Table S2); ca 72.1 Ma is the estimate in Magallón et al. (2015) and ca 66.2 Ma is the estimate in Z.-Z. Li et al. (2020: But-Alis ca 46.3 Ma) and ca 80.0 Ma in Z.-Z. Chen et al. (2021).
Evolution: Divergence & Distribution. L.-Y. Chen et al. (2013) thought that the ancestor of this clade inhabited Eurasia.
I have tentatively put a character of leaf development at this node, but sampling is exiguous in the extreme (see Bharathan 1996). Floral morphology is very variable, particularly in Hydrocharitaceae (some species have very reduced flowers) and Alismataceae. And even in the monotypic Butomaceae, Sattler and Singh (1978), emphasising the timing of primordium initiation, describe paired stamens being associated with the inner T whorl, while Iwamoto et al. (2018), emphasising position, describe the flowers as having paired stamens opposite the outer T whorl. A glance at the literature shows disagreements over morphological interpretations are common. Sokoloff et al. (2013) thought that a perianth differentiated into calyx and corolla might be an apomorphy here - it might, or there might be parallel evolution in Hydrocharitaceae and Alismataceae, as suggested below.
Ecology & Physiology. Crassulacean acid metabolism (CAM) is known from both Hydrocharitaceae and Alismataceae (Keeley 1998a).
Pollination Biology & Seed Dispersal. In carpels that are incompletely sealed at anthesis (see Kaul 1976), pollen can get inside the carpels and even germinate there (Johri & Bhatnagar 1958).
Genes & Genome. Many protein genes involved in the large and small subunits of mitochondrial ribosomal proteins have been lost in this clade (Adams et al. 2002b). For cytogenetics in this clade, see Feitoza et al. (2009).
Chemistry, Morphology, etc.. The prophylls of Limnocharis (Alismataceae) and Vallisneria (Hydrocharitaceae) may not be in the normal adaxial position (Wilder 1975).
Whether or not and where staminal pairs develop and other aspects of androecial development seems to depend on available space in the meristem, itself determined by how (relatively) fast the members of the two perianth whorls grow (Iwamoto et al. 2018; see also Posluszny et al. 2000). Islam (1950) described both Alismataceae and Hydrocharitaceae as having tenuinucellate ovules.
For floral development, on which much work has been carried out, see e.g. Charlton and Ahmed (1973) and Charlton (2004), for tepal vasculature, see Glück (1919).
ALISMATACEAE Ventenat, nom. cons. - Back to Alismatales
Plant (cormose, stoloniferous), with exudate; flavone and phenolic sulphates, tannins + (0); rhizome with endodermis; (vessels 0); hairs (unicellular or stellate); stomatal subsidiary cells with parallel divisions; leaves two-ranked or spiral, emersed leaves with petiole and blade, blade narrowly elliptic to sagittate or suborbicular, base cordate, vernation involute, apical subepidermal pore +, primary veins merging with each other/not, (vascular bundles inverted), petiole terete; plant (mon- or dioecious);inflorescence scapose, branches/flowers whorled; C more or less crumpled in bud, thin, fugaceous, initiation delayed; A initially in pairs opposite C, when >2 whorled or many, initiation centrifugal, endothecium with base-plate thickenings; pollen pantoporate, grains 3-celled; nectary on carpel flanks; G 2-many, ± free, with residual floral apex, partly ascidiate, (style ventral); ovules one/carpel, epitropous, (parietal tissue none); embryo sac bisporic [chalazal dyad], 6-nucleate [variant of Allium-type], (monosporic, 4-nucleate [Oenothera-type]); fruit an achene; exotesta with outer wall thickened, (thin-walled, cells with upturned ends [Limnocharis]), (with glandular hairs), tegmen ± obliterated or walls ± thickened; endosperm nuclear [Alisma etc.]; embryo strongly curved; n = (5-)7-8(-13), x = 7, chromosomes 2.4-14.4 µm long, nuclear genome [1 C] (0.777-)10.007(-128.895) pg; radicle +, ephemeral.
15 [list, to tribes]/88 (115). Pantropical, also temperate. Map: see den Hartog (1957), Hultèn (1961), Meusel et al. (1965), Haynes and Holm-Nielsen (1997), Fl. Austral. vol. 39 (2011) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012).
Age. L.-Y. Chen et al. (2012a) estimated the age of crown group Alismataceae to be Upper Cretaceous and some (109.2-)79.4(-68.6) Ma, the estimate in Z.-Z. Li et al. (2020) is a mere ca 32.7 Ma but in Li et al. (2021) it is (81.0-)71.5(-64.9) Ma.
1. Alismateae Dumortier / Clade C —— Synonymy: Damasoniaceae Nakai
(C margins serrate); A 6 [Alisma]; pollen surface granular [Alisma etc.]; (G connate basally - Damasonium); ovules (>2/carpel - Damasonium); fruit (a follicle - Damasonium); n = .
5/20: Alisma (9). More or less world-wide. Photo: Hydrocleys flower.
Age. This crown age of Alismateae may be (73.4-)55.2(-34.0) Ma (Z.-Z. Li et al. 2021).
[Limnochariteae + Sagittarieae]: ?
Age. The age of this clade is around (71.5-)67.1(-64.1) Ma (Z.-Z. Li et al. 2021).
Riley and Stockey (2004) described leaf fossils from Alberta around 72 Ma as Cardstonia tolmanii, which they placed in Limnocharitaceae.
2. Limnochariteae Piton / Clade B —— Synonymy: Limnocharitaceae Cronquist
Lamina suborbicular, base cordate to elliptic, apical gland +, fine venation reticulating; inflorescence umbellate/flowers single; A initially 3 opposite C [Hydrocleis]/3 opposite K [Limnocharis], (latrorse), (outer A staminodial); G 3-many, in single whorl, connate laterally, open at anthesis, placentation laminar; ovules many/carpel, endostomal; endosperm helobial; fruit a follicle; n = 7, 8, 10.
3/4: Limnocharis (2). Mexico to South America, the Antilles, India to Southern China, Thailand, Java, northern Australia. Photos: Limnocharis flower, vegetative.
Age. Crown-group Limnochariteae are estimated to be (65.1-)46.3(-29.7) Ma (Z.-Z. Li et al. 2021).
3. Sagittarieae Dumortier / Clade A
(CAM photosynthesis +); (nectary at base of C, A - Echinodorus); pollen (0-3 porate - Caldesia), surface spinose; stylulus with narrow canal filled with secretion [Sagittaria]; ovules 1-15/carpel, (apotropous - Luronium), (nucellar cap ca 2 cells across - e.g. Sagittaria); fruits (achenes), pericarp (with air canals); n = .
7/69: Sagittaria (ca 40), Echinodorus (30). Photos: Echinodorus Flower, Fruit, Sagittaria Flower.
Age. This clade is estimated to be (66.3-)56.7(-44.3) Ma (Z.-Z. Li et al. (2021).
Fossils from Early to Middle Campanian deposits in Egypt perhaps 79 Ma have been placed in Echinodorus (Coiffard & Mohr 2018) - something of a conflict with the age above, furthermore, the age of stem Echinodorus is estimated to be only around 43.3 Ma by Z.-Z. Li et al. (2021).
- Alismataceae are aquatic herbs, sometimes with white exudate. Their leaves, whether floating or aerial, are petiolate and have a prominent midrib that is paralleled by veins running from the base that are linked by fine transverse veins. Their petals are crumpled in bud and their staminate flowers usually have many extrorse anthers while their carpelate flowers have many free carpels. It is obvious from looking at the seeds that the embryo is strongly bent.
Evolution: Divergence & Distribution. For the fossil record of Alismataceae, see Friis et al. (2011); fossils are common in the Europe-Northern Asia area. Ages for clades within the family are given by L.-Y. Chen et al. (2012a) and Z.-Z. Li et al. (2021). Haggard and Tiffney (1997) recorded fruits of Caldesia from the early Miocene Brandon lignite deposits in Vermont that are dated to some 20 Ma (the genus is also recorded from Idaho); Caldesia is currently known only from the Old World.
There are also biogeographical reconstructions for Alismataceae in L.-Y. Chen et al. (2012a) and Z.-Z. Li et al. (2021). The former (better species sampling) thought that the ancestral area for the family was in Eurasia, the latter (better generic sampling) thought that it was in the Neotropics + West Palearctic + Afrotropics. Both suggest that the current distribution of the family is a mixture of vicariance and long-distance-dispersal events, the latter occurring in Sagittaria and Butomopsis, and, as Li et al. (2021) note, the mixture was common in other clades of aquatics.
Basal diversification within Sagittaria may have been in South America (Ito et al. 2020).
Ecology & Physiology.There is single-celled C4 and also CAM photosynthesis in Sagittaria (Bowes 2010; Silvera et al. 2010b).
Pollination Biology & Seed Dispersal. For a summary of pollination in Alismataceae, see Gottsberger (2016a). Danforth et al. (2019) draw attention to the unusual reciprocally obligate relationship between plant and pollinator in Hydrocleys martii and Protodiscelis palpalis (a colletid bee). In at least some species of Ranalisma and Sagittaria the pollen tubes grow down the style, out through an opening at the base of the carpel, into the floral axis and thence into adjacent carpels (X.-F. Wang et al. 2002, 2006, 2011; L.-J. Huang et al. 2014) - such an extragynoecial compitum might be quite a high-level apomorphy in the family. Interestingly, in S. potamogetifolia the pollen grains germinate inside the anther and grow through the tissues of that plant to the ovules (Wang et al. 2002) - c.f. Lacandonia-Triuridaceae.
The individual fruitlets of Limnocharis separate from the axis and float, while in Baldellia ranunculoides Sculthorpe (1967) noted that the fruits sank but the seedlings floated.
Genes & Genomes. Hybridization is reported from Alismataceae (L.-Y. Chen e al. 2022). For cytogenetics here, see Feitoza et al. (2010).
The plastome of Sagittaria lichuanensis, at a hair over 179,000 bp, is the second largest known (Y. Luo et al. 2016), after that of Pelargonium hortorum.
Chemistry, Morphology, etc.. Leme et al. (2021b) looked at the morphology and development of the branched secretory canals in several Alismataceae; they are lined by an epithelium, the exudate contains lipids, alkaloids, proteins and polysaccharides, and is probably involved in wound sealimng and defence. The blade of Sagittaria develops from the upper part of the leaf; there is a Vorläuferspitze on cataphylls, but not on the blades of emergent leaves, and the petiole is evident in these leaves early in development (Bloedel & Hirsch 1979). Both leaf form and flower type (staminate, carpelate) are extremely plastic in taxa like Sagittaria latifolia (Dorken & Barrett 2004).
Although there are often many carpels and stamens, organ initiation is basically whorled. The inner tepals of Alisma are initiated after the stamens (Rudall 2010). Stamen initiation in the family may be centrifugal or centripetal; there are common stamen primordia (Sattler & Singh 1977). The pollen often contains starch, and the pores of the pollen grains have very irregular margins. The carpels in the antesepalous position may initiate first, sometimes on gynoecial bulges, or the carpels may be in many whorls (looking spiral!) and completely covering the axis (Singh & Sattler 1972, 1973, 1977a; Charlton 2004 and references; Rudall 2008). Alternatively, there may be a single whorl of carpels with a large, residual floral axis in the center, as in members of the old Limnocharitaceae (e.g. Leins & Stadler 1973); there the carpels are connate laterally, there are many ovules per carpel, and the placentation is laminar. Hasitschka-Jenscke (1959) noted that the basal/chalazal cell of the endosperm of Alisma lanceolatum divided several times, and only one cell was endopolyploid - hexaploid (c.f. Potamogeton).
General information is taken from Haynes et al. (1998b: as Alismataceae and Limnocharitaceae) and Hooper and Symoens (1982: as Limnocharitaceae); for general morphology, see Charlton and Ahmed (1973), for vegetative anatomy, see Stant (1964, 1967), for floral development, see Leins and Stadler (1973), Charlton and Ahmed (1973), Charlton (1991 and references), Wang and Chen (1997) and K.-M. Liu et al. (2002), for the endothecium, see Manning and Goldblatt (1990), for pollen, see Chanda et al. (1988), for embryology, etc., see Dahlgren (1928b, 1934b), Johri (1936 and references) and von Guttenberg and Jakuszeit (1957: Alisma), and for fruits, see Haggard and Tiffney (1997).
Phylogeny. Details of the relationships between and within Alismataceae s. str. and Limnocharitaceae have been rather unclear (Soros & Les 2002; Y. Kato et al. 2003; J.-M. Chen et al. 2004a, b; L.-Y. Chen et al. 2012a; von Mering & Kadereit 2010; Lehtonen 2009 for a summary). Echinodorus is polyphyletic (Soros & Les 2002; see also Lehtonen & Myllys 2008). Morphological analyses yield poorly supported basal pectinations with Butomopsis, Hydrocleys, and Limnocharis successively sister to the remainder of the clade; Alismataceae in the old sense then form a well supported clade (Lehtonen 2009: several characters show continuous variation). In an analysis with comprehensive sampling and using ITS plus three chloroplast genes a well-supported clade [Luronium, Damasonium, Baldellia, Alisma] was sister to the rest of the family (see also J.-M. Chen et al. 2004a), but there was only moderate to weak support along the basal part of the backbone. Furthermore, this clade was not recovered in a smaller study using additional chloroplast genes, or was in a different position when mitochondrial genes were used (L.-Y. Chen et al. 2012a). It was again well supported in the plastid phylogenomic study of Ross et al. (2015), but Du and Wang (2014) and Du et al. (2016) found that Ranalisma and Burnatia were successively sister to the rest of the family; basal relationships were unclear in Lehtonen (2017). Z.-Z. Li et al. (2021: plastome analyses) recovered the three clades above as [Clade C [Clade B + Clade A]], and with strong support, although they noted that the positions of some genera within those clades was uncertain. In the pectinate Clade A, relationships were [Ranalisma [Albidella [Echinodorus [Caldesia [Helanthium [Sagittaria ...]]]]]]. in Clade B (= the old Limnocharitaceae, they were [Limnocharis [Butomopsis + Hydrocleys]], and in Clade C they were [Burnatia [Luronium [Damasonium ...]]] (Li et al. 2021). L.-Y. Chen et al. (2022) found that Burnatia seemed to have been involved in a rather deep hybridization event, which explains some of the confusion over phylogenies here.
For relationships in Sagittaria, see Ito et al. (2020).
Classification. Alismataceae include the "old" Limnocharitaceae (first recognized by Cronquist only in 1954), and the two certainly have much in common. Z.-Z. Li et al. (2021) did not give names to the three main clades in the family that they found; I have simply added the earliest tribal names for each from Reveal's list. Li et al. (2021) suggested that Wiesneria and Astonia might be best merged into Limnophytum.
[Butomaceae + Hydrocharitaceae]: C/inner T whorl development not delayed; ovary loculi with secretions.
Age. The divergence of these two families is dated to ca 88 Ma (Janssen & Bremer 2004), around 21.8 Ma (L.-Y. Chen et al. 2012a) or about 39.7 Ma (Z.-Z. Chen et al. 2021).
BUTOMACEAE Mirbel, nom. cons. - Butomus umbellatus L. - Back to Alismatales
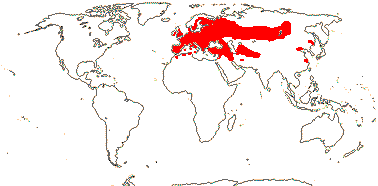
Plant rhizomatous; monopodial; flavonols?; stomata variable; leaves ± two-ranked, blade triangular; inflorescence scapose, umbellate, with inflorescence bracts, (floral bracts 0), prophylls 2, lateral; flowers protandrous; K ± C-like; A 9, 6 in pairs opposite outer T + 3 opposite inner T, some latrorse; pollen monosulcate; nectar from carpel flanks; G 6, fusion postgenital, placentation laminar, stigma ± decurrent; ovules many/carpel, chalazal cells ± hypertrophied, surounding nucellar cells radially arranged; testa with air canals, exotestal cells with outer walls thickened and with encrustations, tegmen persistent; embryo straight, ?colour; n = 7, 8, 10, 11, 12, etc., x = ?8, ?7, chromosomes 3.7-8.3 µm long, nuclear genome [1C] (0.174-)4.581(-120.373) pg; ?seedlings.
1[list]/1. Temperate Eurasia. Map: from Hultén and Fries (1986). Photo: Habit © D. Woodland, Inflorescence © E. Parnis.
- Butomaceae are aquatic monocots that may be recognised by their long, three-angled leaves and axillary, scapose, umbellate, bracteate inflorescence. The flowers are perfect and there are clearly two distinct perianth whorls although both are petal-like; the stigmas are yellow when they are receptive, and then they look very like the pollen-filled anthers immediately after they have dehisced. The fruit is a follicle.
Age. The age of crown-group Butomaceae is estimated at (50-)30(-15) or (10-)9 Ma (Hertweck et al. 2015).
Evolution: Divergence & Distribution. Diversification rates in this clade are reduced (Hertweck et al. 2015).
Pollination Biology. The staminate and carpelate phases of the flowers in an umbel, possibly in an entire ramet, but not in different ramets, are synchronized (Bhardwaj & Eckert 2001).
Genes & Genomes. The mitochondrial genome of Butomus umbellatus is 450,826 bp, relatively large for Alismatales, yet it has a rather low percentage of chloroplast inserts (G. Petersen et al. 2017).
Chemistry, Morphology, etc.. Cook (1998) hesitantly suggested that the rhizome was monopodial (see also Bhardwaj & Eckert 2001); this should be checked. Stant (1967) reported crystals "in the form of small rods" (styloids) in the diaphragm cells surrounding the air spaces in the stem; she also suggested that the leaf of Butomus was equivalent to the petiole of Alismataceae (Limnocharitaceae).
Martínez-Gómez et al. (2022) discuss the umbellate inflorescence of Butomus which appears to be derived from a cymose inflorescence. For the position of the prophyll, see Eichler (1875). There appear to be C-A primordia, with a pair of stamens differentiating first, and then a single stamen adaxial to that pair (Singh & Sattler 1974). The basal cell of the endosperm may remain undivided but become hypertrophied, or there may be some free nuclear divisions (Fernando & Cass 1996).
Much information is taken from Cook (1998); see also Charlton and Ahmed (1973) for morphology, Iwamoto et al. (2018) for floral morphology, Chanda et al. (1988) for pollen and Roper (1952) and Fernando and Cass (1996) for embryology.
HYDROCHARITACEAE Jussieu, nom. cons. - Back to Alismatales
Stem ± stoloniferous, leaves in groups; branching?; flavone and phenolic sulphates +; vessels 0; endodermis obscure or thick-walled; stomata ?type; (prophyll lateral); leaf blade ± linear; inflorescence subtended by 2 often connate bracts; androecium with trunk bundles?, (A introrse); pollen inaperturate, exine thin to none [pollen not resistant to acetolysis]; nectaries 3, staminodial (0); G inferior, carpels laterally ± free, closure by secretion only, placentae much intruded [= carpel walls], style +, short, stigmas branched, papillate adaxially; ovules with outer integument often >3 cells across, micropyle bistomal, parietal tissue 1-2(-more?) layers across, nucellar cap 2-3 cells across (0); fruit often ± fleshy, dehiscence irregular; seeds with short hairs/papillae/0, mesotesta of stone cells, 2-3 layers across, endotegmic cells with variously tuberculate inner walls; chalazal endosperm haustorium +; x = 8 (?7, ?6), nuclear genome [1 C] (0.285-)4.446(-40.263) pg; extensive loss of mitochondrial genes; cotyledon bifacial.
18[list]/116 (135) - four groups below. World-wide. Map: blue, marine Hydrocharitaceae; red, freshwater members - see Hultén (1961), Hultén and Fries (1986), Fl. N. Am. vol. 22 (2000), FloraBase (consulted 2005), Fl. Austral. vol. 39 2011; Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012), and van Steenis & van Balgooy (1966); but commonly introduced and original distributions unclear; see den Hartog (1970) for marine taxa.
Age. Crown group Hydrocharitaceae are thought to be ca 75 Ma (Janssen & Bremer 2004), while L.-Y. Chen et al. (2012b) suggest a rather younger crown-group age of (72.6-)65.5(-54.7) Ma, Z.-Z. Li et al. (2020) an age of ca 61.0 Ma and Ulrich et al. (2024) an age of 67-56 Ma.
The oldest fossils of Hydrocharitaceae are from around 56 Ma (Ulrich et al. 2024).
Roots branched; foliar vascular bundles inverted; stomata paracytic; leaves spiral or spirally 2-ranked, with petiole and blade, blade vernation involute or convolute, ligule +, basal, adaxial (paired, lateral) [totally enclosing young leaves], ?sheathing base; plants monoecious; staminate flowers: A (3-)6-12(-18), extrorse, (connate in pairs - different whorls; innermost whorl staminodial Hydrocharis), (pistillode +); pollen tectate-columellate, with (minute) spines; nectary + [?also staminodial]; carpelate flowers: (C 0); (staminodes +); G [3-9], (carpels basally ascidiate - Limnobium), nectary at abaxial base of style opposite C [Hydrocharis]; fruit loculi filled with mucilage [H]; ovules straight; exotesta with 1-3-celled papillae/glochidiae, cells with annular thickenings [[Hydrocharis]; n = 7-11, 13-15: seedling with collar rhizoids, radicle.
2/5: Hydrocharis (3). Temperate and subtropical.
Age. Crown group Hydrocharitoideae are estimated to be ca 16.2 Ma by Z.-Z. Li et al. (2020: huge error bars, ca 41-2 Ma).
[Stratiotoideae [Anacharidoideae + Hydrilloideae]]: roots unbranched; leaf blade ± linear, base not sheathing; plant dioecious (flowers perfect); anthers latrorse [?all]; (ovules straight).
Age. This clade is about 23.7 Ma (Z.-Z. Li et al. 2021).
2. Stratiotoideae Luersson - Stratiotes aloides L. —— Synonymy: Stratiotaceae Schultz Schultzenstein
Plant floating, vegetative growth monopodial; stem endodermis 0; leaves spirally 3-ranked, margins strongly spiny; staminate flowers: A many, adaxial 5-17 fertile, staminodes of three kinds, two nectariferous, ± abaxial; pollen reticulate, echinate suprasculpture, spines longitudinally furrowed, ± twisted, numerous baculae/nanoclavate and nanogemmate within the lumina, tectum incomplete; pistillode 0; carpelate flowers: staminodes +; G 3, 6 in 2 whorls, outer opposite K, (basally ascidiate); ovules 4-6/carpel; fruit loculi filled with mucilage; testa multiplicative, mesotestal sclereids 9-11 cells across; n = 10+; ?seedlings.
1/1. Eurasia.
Age. The age of the stem node of Stratiotes is estimated to be 55.9 Ma (Iles et al. 2015). Stratiotes has a rich fossil record (as seeds) from the middle of the Eocene onwards (Cook & Urmi-König 1983), and the very distinctive pollen is known fossil from the early Eocene in Germany, and not much later from Kenya (Ulrich et al. 2024).
[Anacharidoideae + Hydrilloideae]: plant monopodial; submerged; (with C4 or CAM photosynthesis); roots lacking root hairs [?all]; (leaves scattered along stem), (margins spiny); inflorescences axillary, emersed or not; P = T 3 + 3; placentae usu. not much intruded; ovules usu. few/carpel; fruit fleshy, capsular/dehiscence irregular/indehiscent; radicle + [O, V.].
3. Anacharidoideae Thomé —— Synonymy: Blyxaceae Nakai, Elodeaceae Dumortier, Otteliaceae Chatin, nom. illeg.
(Plant sub-cormose); root trichoblasts 0 [Blyxa]; leaves whorled, spiral, two ranked, (scales +, usually opposite), (petiole + blade - some Ottelia); (flowers perfect - Apalanthe, Blyxa, Ottelia); staminate flowers: released, usually as buds, (not); P (3); A 2 + 1 staminode [Maidenia], 3 (+ 3 staminodes)-12, (dorsifixed); pollen (bicellular - Ottelia), (with discontinuous exine, little or no sculpturing), (surface spiny); carpelate flowers: hypanthium +, usu. long; P 3 + 3; staminodes +; G [3(-20+)]; (carpel walls much intruded); (ovules many/carpel), (micropyle bistomal); antipodal cells persist; seeds usually <30; n = ?6, 8-12, 14, etc., heteromorphic [Maidenia]; plastid transmission biparental [Ottelia]; seedling with collar rhizoids [O.].
7/38: Ottelia (23). Tropical to temperate, esp. America. Photo: Elodea habit © D. Woodland, Blyxa habit, Elodea flower © M. Clayton, and Egeria.
Age. The age of the clade [Apalanthe + Lagarosiphon] is ca 40.5 Ma (Les et al. 2003) or ca 32.8 Ma (Z.-Z. Li et al. 2020).
4. Hydrilloideae Luersson —— Synonymy: Hydrillaceae Prantl, Najadaceae Jussieu, nom. cons., Vallisneriaceae Dumortier
(Marine); (plant rhizomatous), (annual); (leaves with spines - some Naias); root trichoblasts 0 [Vallisneria]; leaves (spiro)two-ranked or whorled, linear, (base expanded, rounded to 2-lobed - Naias); plant dioecious (monoecious - Najas); P (3 + 1), (3), (0); staminate flowers: (released as buds); A 1-9, (1 staminode); pollen (surface smooth, mucilaginous, germination precocious - Naias); pistillode 0; carpelate flowers: staminodes (0-)3; hypanthium +; G [2-9], (stigmas commissural), (filiform, smooth); (micropyle bistomal), (obturator +); (seeds smooth), (with starch); testa (papillae with fenestrate thickenings - V), (with air canals), (exotegmic tuberculae +); n = 6-8, 10, 12, 15; plastome ndh genes lost [Naias].
8/61: Najas (40), Vallisneria (12). Tropical and subtropical, especially Old World; Najas subcosmopolitan. Photo: Hydrilla, © H. Wilson.
Age. Z.-Z. Li et al. (2020: Naias sister to the rest) estimated an age of ca 46.1 Ma for this clade, although L.-Y. Chen et al. (2022) suggested an age for the seagrass clade alone of around 62.8 Ma.
4A. Halophileae Ascherson / The Seagrass Clade —— Synonymy: Enhalaceae Nakai, Halophilaceae J. Agardh, Thalassiaceae Nakai
Marine, (annual), rhizomatous (stoloniferous); sulphated flavones and phenolic acids + [H.], sulphated phenolic acids 0 [E.]; roots unbranched, root hairs long (short); leaves opposite, pseudoverticllate or spiral, linear, quite thick / blade elliptic, thin, midrib and spreading laterals +, (margin serrulate), petiole +, sheath 0 [H.] / veins in two series, adaxial inverted [E.]; plant (mono-)dioecious; flowers submerged / staminate flowers abscise and float, carpelate flowers at surface [E.]; P uniseriate (biseriate - E.); A 3(-13); pollen spherical to ellipsoid, (in mucilaginous strands - T.), exine 0; (styles 6 - H.); testa cells with small, peg-like projections; fruit a capsule; (seeds minute - H.); testa (photosynthetic - T.); radicle 0; n = 6, 9, chromosomes 0.39-13.2 µm long, mitochondrial genome with only 6 cis-spliced introns; seedlings with collar rhizoids/hypocotylar hairs.
3/22: Halophila (19). Tropical, but v. little coasts of America apart from the Caribbean, none W. Africa or the Mediteranean. Map: see above, blue. Photos: Halophila, Enhalus, flower, © [from D. Les website], Thalassia, fruit, © [from D. Les website.
Age. The crown-group age of this clade (as [H [T + E]]) is estimated to be around ca 19.4 Ma (L.-Y. Chen et al. 2012b - note that they place the split between marine and non-marine Hydrocharitaceae at (70-)50.8(-45) Ma), while (43-)31(-20) Ma is the estimate in Waycott et al. (2018) - and they place the split between marine and non-marine (Vallisneria) Hydrocharitaceae at (75-)59(-42) Ma. The stem-group age of this clade has also been estimated to be 47.8-38 Ma (Iles et al. 2015) and ca 40.7 Ma (Z.-Z. Li et al. 2020); the latter estimated the age of the crown group to be ca 18.7 Ma, while the crown-group age in Ulrich et al. (2024) is 27-11 Ma (the stem-group age is somewhere around the Eocene-Ypresian).
- Hydrocharitaceae are aquatic plants with petiolate or (usually) undifferentiated leaves. The inflorescence often has two fused (sometimes free) bracts at the base and the ovary is inferior, often with laminar or more or less strongly intruded parietal placentation, and the stigma lobes are bifid.
Evolution: Divergence & Distribution. Y. Kato et al. (2003) proposed ages for Hydrilloideae of (130-)119(-108) Ma, while He et al. (1991) proposed a Cretaceous age and Gondwana origin for Ottelia (note that the stem group age for the genus is ca 16.7 Ma and the crown-group age is (20.1-)13.1(-7.1) Ma in Z.-Z. Li et al. 2020). Basal nodes in the family are mostly in Europe (Ulrich et al. 2024: note topology), although L.-Y. Chen et al. (2012b) suggested an Asian origin for it.
As will be obvious below, although the four subfamilies are well enough supported, their relationships are unclear. Ulrich et al. (2024) suggested that "Stratiotes pollen is primitive within the family", the genus being sister to the rest of the family - it may have given rise to Lagarosiphon. Z.-Z. Li et al. (2020) discuss diversification in Ottelia.
Ecology & Physiology. Hydrocharitaceae such as Ottelia, Hydrilla and Egeria have C4 photosynthesis with metabolic compartmentalisation within a single cell - PEPC is in the cytosol, RuBisCO in the chloroplast; it is not terribly efficient (Bowes et al. 2002; Bowes 2010; von Caemmerer et al. 2014). When CO2 concentrations were low, Ottelia alismoides also carried out CAM photosynthesis (Han et al. 2020). C4 photosynthesis probably evolved independantly in the genera mentioned (see also Keeley & Rundel 2003).
For more about the small clade of seagrasses in Hydrocharitaceae, see Halophileae, especially above.
Pollination Biology & Seed Dispersal. Pollination mechanisms in Hydrocharitaceae include entomophily, anemophily, epi- and hypohydrophily and zoobenthophily, not to mention selfing. Parallelism is pervasive, and sex expression of the flowers/plants is very labile, whatever the tree (e.g. see L.-Y. Chen et al. 2012b). For details of pollination mechanisms, see Cook (1982, 1994-1995, 1996), these correlate with pollen morphology and phylogeny (N. Tanaka et al. 2004). Hypohydrophily has evolved at least twice (e.g. Najas, marine genera), and staminate flowers that detach from the plant and rise to the surface of the water perhaps five times (Les et al. 2006 and references). Pollination in those taxa where the staminate flowers are released may be epi- or hypohydrophilous. In epihydrophily, small detached staminate flowers floating on the surface of the water on reflexed sepals are caught by the carpelate flowers; the staminate flowers may have two stamens (Maidenia [?= Vallisneria], each anther 3-locular, 8 pollen grains/loculus, = 48 grains/flower, also Nechamandra), three stamens and three erect staminodes that act as little sails (Lagarosiphon), or six stamens (Appertiella). Hydrilla is wind pollinated, the pollen being released explosively by the anthers as they reach the surface in little gas bubbles produced by the submerged staminate flower, while in Elodea the pollen, similarly produced from submerged flowers, floats. (Note, howver, that Q. Zhang et al. 2019 discussed the mechanics of explosive pollination in Hydrilla verticillata in the context of epihydrophilous pollination.) In a number of species the hypanthium elongates greatly, but in others the carpelate flowers have long pedicels, e.g. Maidenia and the marine Enhalus; in either case the flowers open onto the surface of the water. In the marine Halophila the pollen is released embedded in strands of mucilage, and pollination is by collision of the search vehicles that the pollen-mucilage forms with the long, linear stigmas at the surface of the water (Cox & Knox 1989). Halophila lacks exine; it is present in two the other marine genera, and all three genera have more or less spherical pollen and papillate stigmas (Pettitt 1980). Thalassia testudinum may even be pollinated by small marine arthropods; here the anthers open at night when the arthropods are active (van Tussenbroek et al. 2016). The insect-pollinated Blyxa has secondary pollen presentation, while Cook and Lüönd (1982) suggested that in Hydrocharis the staminate flowers, which lack nectaries, mimic the carpelate flowers, which have nectariferous antepetalous staminodes. For more details, see e.g. Ernst-Schwarzenbach (1945) and N. Tanaka et al. (2013: details of pollen and stigma morphology). The pollen of Naias germinates underwater and so the globose-ellipsoid pollen grain becomes functionally elongated, being 2 mm long or so, and is captured by the filiform stigmas (S.-Q. Huang et al. 2001).
The fruit may be follicular or achenial, or in Hydrocharis opening because of the mucilage developing inside it (for other examples, see Kaul 1978) - and in that genus, the embryo, escaping from the seed, floats (Scriabalo ∧ Posluszny 1984b). Although a number of taxa from all four subfamilies have tuberculate seed coats, whether or not the complex anatomy of the exotesta of Hydrocharis morsus-ranae occurs more widely is unclear (but see Montesantos 1913 - Limnobium); it was not mentioned by Shaffer-Fehre (1991a, b). Floating fruits of Enhalus and Thalassia may be transported for 63.5 and 73.5 km by tides and wind (seeds by themselves do not travel nearly so far), not to mention what might happen during a typhoon (Lacap et al. 2002; see also Orin et al. 2006b); van Dijk et al. (2009) suggested transport distances of T. testitudinum of up to 360 km.
Plant-Aninal Interactions. A cyanobacterium, Aetokthonos hydrillicola is associated with the introduced Hydrilla verticillata in North America. The bacterium produces a pentabrominated biindole alkaloid (there is bromine in some of the weedkillers used to control the Hydrilla, which - ironically - is where the bromine may come from), and this causes vacuolar myelinopathy in animals along the food chain. These include coots, which eat the plant, and the apex predator the bald eagle; in the latter it has caused a number of hitherto inexplicable deaths (Breinlinger 2021).
Plant-Bacterial/Fungal Associations. Thalassia is associated with the N-fixing gammaproteobacterium Celerinatantimonas (Mohr et al. 2021), but details are lacking.
Vegetative Variation. Marine taxa are rhizomatous, with leaf-bearing short shoots (see Tomlinson 1974b for a summary). Taxa like Elodea have leaves borne all along the stem, while others have whorled leaves, Stratiotes aloides forms floating rosettes in the summer, but the plant sinks to the bottom of the water in winter, rising again in the next year. Leaf shape and margin also vary a great deal.
Indeed, growth and branching in Hydrocharitaceae needs more study. In Enhalus and Stratiotes the first leaves produced after the cotyledon are at right angles to it, whereas in most other taxa these leaves are borne in the same plane as the cotyledon (Haccius 1952a). Axillary buds along the stems are commonly precocious (Wilder 1975), and pseudodichotomous branching, often interpreted as being the result of this precocious axillary branching, is also quite common (Tomlinson 1974b, 1982). Posluszny and Charlton (1999) described the extremely complex branching in Hydrocharis morsus-rana, suggesting that it has components of flower/inflorescence morphology. They thought that the sheathing bracts, separated by a short internode, might be comparable to the first two leaves on a branch. N. Tanaka et al. (1997) noted that flowers and axillary branches frequently arose from the same leaf axil, and there is also rather odd variation in bracteole number and position (lateral, paired, etc.: Eichler 1875).
Root anatomy seems to be variable, and Montesantos (1913) described that of Ottelia as having a single central vessel, the phloem perhaps being in five groups.
Genes & Genomes. Hybridization is reported from Hydrocharitaceae (L.-Y. Chen e al. 2022). Vanitha et al. (2016) discuss the cytology of Halophileae.
The chloroplast ndh genes are all lost or are pseudogenes in Naias, but not in Elodea (King et al. 2017); I know not what happens in other Hydrilloideae. Blyxa has notably short chromosomes (Uchiyama 1989); for some cytology, see Vanitha et al. (2016)
The mitochondrial nad1 intron 2 is absent in two representatives of this family (Gugerli et al. 2001); the extent of this loss is unclear.
Chemistry, Morphology, etc.. The plants may be tanniniferous. Hydrocharis, apparently alone in the group, has a root epidermis that comes from the inner epidermis of the root cap (ref.?).
There may be paired bracteoles in flowers of Hydrocharitaceae (Nuraliev et al. 2020b). Pistillate flowers of species of Naias like N. minor seem to lack any trace of a perianth (or androecium) whatsoever (Kajita & Tanaka 2018). The anthers sometimes lack an endothecium (Ernst-Schwarzenbach 1945). Elodea is shown as having its carpels opposite the inner perianth whorl (Eichler 1875). The staminodes of Vallisneria are opposite the petals/inner P and V. spiralis, at least, has commissural stigmas (Les et al. 2008). Kaul (1976) looked at the fusion of the carpels at anthesis; carpels are best interpreted as being more or less free from each other laterally but adnate to the receptacular wall abaxially (Weberling 1989 for references, esp. Kaul 1968). The ovary in at least some taxa is filled with mucilage, but it is unclear if there are intra-ovarian trichomes (Rudall et al. 1998c, c.f. Oriani & Scatena 2012). The variation in testa morphology in Halophila is considerable (e.g. Birch 1981) and the germination of the curious bell-shaped seeds of Thalassia hemprichii - the upper part is white, the lower part is chlorophyllous and has starch grains, the plumule seems not to be terminal - is very odd (see Soong et al. 2013). Vijayarahavan and Kapoor (1985) described what they called "intraseminal germination" in Naias marina - in the seed, in addition to the cotyledon, the first leaves of the plumule were also evident (c.f. Poaceae!); they also mentioned the enlarged and polyploid basal cell.
General information is taken from Kuo and McComb (1980), Tomlinson (1980: esp. anatomy, marine taxa), Cook (1998: he and collaborators have revised almost the entire family, e.g. see Cook & Urmi-König 1985 and earlier papers), Haynes et al. (1998a), Haynes and Holm-Nielsen (2001) and van Tussenbroek et al. (2006: Thalassia); for morphology and anatomy, see Ancibor (1979), for floral anatomy, etc., see Singh (1966 and references), for floral development, see McConchie (1983: Maidenia) and Scribailo and Posluszny (1985a: Hydrocharis, 1985b: seed, etc.), for flowers and fruits, see S. Singh et al. (2020: Halophila), and for ovules, etc., see Kausik (1940a), Islam (1950) and Govindappa and Naidu (1956).
Phylogeny. N. Tanaka et al. (1997: two genes) found a series of quite well-supported nodes in Hydrocharitaceae; the ultimate groupings recognised there are similar to those of Les et al. (1997b). Les et al. (2006) in a four-gene analysis of all genera bar one, and including morphological characters, again found largely similar relationships: Hydrocharitoideae were sister to the rest of the family (support unclear, only a single outgroup used), and then Stratiotoideae (support barely moderate, 72% bootstrap, all characters), and then the clade [Anacharidoideae + Hydrilloideae] (52% support); however, the monophyly of all four subfamilies was strongly supported. However, analyses of an eight-gene supermatrix by L.-Y. Chen et al. (2012b) yielded an appreciably different tree. Basic relationships were [Stratiotoideae [Anacharidoideae [Hydrocharitoideae + Hydrilloideae]]]. The position of Stratiotoideae was largely driven by mitochondrial data, otherwise support for the backbone of the tree was strong, but that for the particular position of Hydrilla poor (L.-Y. Chen et al. 2012b). Ross et al. (2015) also recovered this topology, again with strong support for all the subfamilies, but the position of Stratiotoideae was unclear. Les and Tippery (2013) noted the variety of topologies that had been found for the four subfamilies; they themselves found the relationships [Hydrocharitoideae [Anacharidoideae [Stratitoideae + Hydrilloideae]]], althought support was weak, as it was for the monophylyy of Anacharidoideae and Hydrilloideae. Du et al. (2016) found two main clades in the family, one [Hydrocharitoideae + Anacharidoideae] and the other [Stratiotoideae + Hydrilloideae], while in Bernardini and Lucchese (2017: 51 spp, ITS + 5 plastid genes) Stratiotoideae grouped with Anacharidoideae and Hydrocharitoideae with Hydrilloideae, although support could have been stronger. For relationships between Chinese taxa, see Z.-D. Chen et al. (2016). However, in some analyses using just a few genes Waycott et al. (2018) recovered a heptatomy in the family. Recently Z.-Z. Li et al. (2020) in an analysis of 78 protein-coding plastome genes recovered the relationships [Stratiotoideae [Anacharidoideae + [Hydrocharidoideae + Hydrilloideae]]] - support values were "high", while Chen et al. (2022) discuss possible hybridization events in the family. One awaits results from analyses of the nuclear genomes - indeed. In the most recent Angiosperms353 tree as of ix.2024 (but c.f. Zuntini et al. 2024) relationships are [Hydrilloideae [Stratiotoideae [Hydrocharitoideae + Anacharidoideae]]] with support for the last pair only weak, so one isn't much the wiser.
Within Hydrilloideae, Najas was strongly supported (98%) as sister to Hydrilla in a combined molecular analysis, although not in all individual analyses, yet the two are notably distant in the tree in morphological analyses (Les et al. 2006). Despite the obvious morphological differences between Najas and other Hydrocharitaceae, Posluszny and Charlton (1999 and references) noted that both branching and seed anatomy link them, however, Liu and Li (2010) found Najas to be sister to the other Hydrocharitaceae that they examined, while L.-Y. Chen et al. (2012b) found the relationships [seagrasses [Najas [Hydrilla + the rest]]]. Najas flexilis was on a very long branch in the chloroplast gene analysis of Y. Luo et al. (2016), and had lost eleven ndh genes. , and for that of Naias see Ito et al. (2017a). For a phylogeny of Vallisneria, see Les et al. (2008).
There is a marine/seagrass clade, Halophileae, i.e. [Enhalus [Halophila + Thalassia]], here; the latter genus was on a very long branch in the plastid gene analysis of Lam et al. (2016), while the relationships [Halophila [Enhalus + Thalassia]] were recovered by Nguyen et al. (2018). The latter relationships were also found e.g. by e.g. Waycott et al. (2018) and da Silva et al. (2021) - Blyxa was their sister in the latter study. Che Alias et al. (2024) looked at the relationships between 13 species of Halophila, the majority of the genus; they seem to be consistent with the sectional classification in Kuo (2020).
- Najas: Leaves with "auricle" at very base, margins spiny; staminate flowers: two envelopes; A 1, secondary parietal layers of the anther wall do not divide; carpelate flowers: single floral envelope; carpel 1, ascidiate, style one- or two-branched; ovule one, basal; fruit ?achenial; exotegmen +; endosperm nuclear, embryo chlorophyllous, cotyledon unifacial. The root system is well-developed, the roots are unbranched but they have many root hairs. Variation in chromosome length (1.6-11.7 µm) is considerable. Miki (1937) suggested that the ovary of Najas, apparently superior, might really be inferior; floral development would repay re-examination. Even in a morphologically very variable group, Najas is a distinctive genus.
- Ottelia is distinctive in Anacharidoideae having several apparently plesiomorphic features; it has a petiole, broad leaf blade, and reticulate fine veins; its petals are large and there are up to 15 stamens; the fruits dehisce quite regularly, and the seeds have some endosperm.
Classification. There are eleven family names available for the eighteen genera in Hydrocharitaceae, reflecting the variety of pollination mechanisms and vegetative adaptations to the aquatic life style. I follow the subfamilial circumscriptions suggested by Les et al. (2006), although relationships in the family were clearly up in the air.
Kuo (2020) divided Halophila into eight sections; section Halophila included 13 species, most of the genus.
[Aponogetonaceae [Scheuchzeriaceae [Juncaginaceae [Maundiaceae [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]]]]]]: primary root poorly developed; calcium oxalate crystals of any sort absent; floral bracts 0; P members with a single trace; pollen surface reticulate; parietal tissue >2 cells across; chromosomes 0.5-2.3(-4.5) µm long.
Age. The stem node of Aponogetonaceae is estimated to be ca 98 Ma (Janssen & Bremer 2004) while the age is around 81.1 Ma in Iles et al. (2015); it is estimated to be some 100 Ma in L.-Y. Chen et al. (2014b).
Silvestro et al. (2020) estimate the time-of-origin of Aponogetonaceae to be ca 115.7 Ma, based on fossil pollen, and this is over twice that based on macrofossils; Gríimsson et al. (2014) had earlier suggested that the oldest pollen was 82-81 Ma.
Evolution: Divergence & Distribution. L.-Y. Chen et al. (2013) thought that this clade began to diverge in Eurasia.
Volkova et al. (2016) suggest that pollen apertures have been lost and then regained (in Aponogetonaceae, Potamogetonaceae, Ruppiaceae) in this clade.
Chemistry, Morphology, etc.. A number of taxa around here have a radicle that is more or less lateral in origin (von Guttenberg 1960; Yamashita 1970, 1972, 1976).
The nature of the small, tepal-like structures closely associated with the stamens that are found in many taxa in this part of the tree has occasioned much discussion. Sattler (1962) and Singh (1965) considered the perianth and androecium of Potamogetonaceae to be distinguishable although there was but a single trace to each P/A pair. This tepal-like structure is called a retinaculum by some, and then considered to be some kind of enation, not a tepal; indeed, von Mering and Kadereit (2010) suggest that the clade [Maundiaceae + the rest] may be characterized by a flower that lacks a perianth (several taxa here do entirely lack perianth-type structures). Rudall (2003b, see also references) suggested that the flowers of all or many of the taxa were some kind of pseudanthia. However, flowers in which a tepal/perianth member seems to come from the back of a stamen are here considered to be an extreme form of the tepal-stamen association that is common in monocots.
For a summary of much information about the families below, see Sokoloff et al. (2013c). Markgraf (1936) described general floral studies in the "simplest Helobiae", and Eber (1934) described carpel morphology and ovule morphology and position.
Classification. There are many small families in this clade reflecting the very distinctive floral and vegetative morphologies that have evolved in connection with the aquatic habitat its members favour. Maundiaceae are recognised below, further increasing the number.
APONOGETONACEAE Planchon, nom. cons. - Aponogeton L. fil. - Back to Alismatales
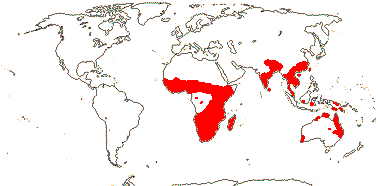
Plant with a short rhizome or corm, apical meristems of vegetative axes bifurcating [?all]; vessels 0; laticifers +, articulated; peripheral vascular bundles in the leaf various; leaves ± amphistomatous, spiral, with petiole and blade, blade vernation involute, primary veins merge with each other, tertiary veins few, apex of old leaves with pore; plants mon(di-)oecious; inflorescence spicate; plant monoecious (dioecious; flowers perfect); flowers sessile, (monosymmetric), (bracts +); P (1-4); staminate flowers: A (8-16), (stamen pairs +), (anthers introrse); microsporogenesis also simultaneous; pollen monosulcate, reticulum uniform, muri broad, (micro)echinate; pistillode ?; carpelate flowers: (P 0); staminodes +; G 2-9, free, alt. P, septal nectaries + (0), placentation basal; ovules 1-12/carpel, (unitegmic - integument 5-6 cells across), nucellar cap ca 2 cells across; fruit ?dehiscence, T etc. persistent or not; seed coat mucilaginous, testa (multiplicative), with air canals, exotesta protective or not, endotegmen tanniniferous, or undifferentiated and translucent; embryo chlorophyllous or not, radicle sublateral (0 - Aponogeton crispus), (with numerous spirally-arranged subtriangular appendages); n = 8, ?12, 13, 15, 16, 19, etc., x = 12 (?13), chromosomes 0.5-2.5 µm long, nuclear genome [1C] (0.254-)2.1(-17.381) pg; cotyledon bifacial.
1[list]/50. Old World, esp. South Africa, largely tropical and warm temperate, suspected of being introduced in parts of Southeast Asia-Malesia - localities not on map. Map: from van Bruggen (1985, 1990). [Photo - Aponogeton Flower © H. Wilson, Habit © R. Kowal.]
- Aponogetonaceae are water plants with petiolate leaves; the sometimes fenestrate blades have a midrib, rather distant parallel veins coming from the base, and strong transverse veins. The often densely spicate inflorescence has a long scape and the rather small flowers that nevertheless may have more or less conspicuous tepals; the whole inflorescence is often coloured.
Age. Crown-group Aponogetonaceae may be ca 23.3 Ma (Les et al. 2003) or (48-)39.8(-32.2) Ma (L.-Y. Chen et al. 2014b).
The distinctive pollen of Aponogeton has recently been reported from western Greenland and North America in deposits around 82-81 Ma (Grímsson et al. 2013); if confirmed, this will be another example of current and past distributions being rather different.
Evolution: Divergence & Distribution. Given the age of the pollen identified as Aponogetonaceae from the northern hemisphere (Grímsson et al. 2013) and the strongly supported sister-group relationship of the Australian Aponogeton hexatepalus to the rest of the genus, the place of origin and diversification of the clade is difficult to ascertain (L.-Y. Chen et al. 2014b: c.f. Fig 3 and abstract). There is a long lag time - perhaps 60 Ma - between the origin and diversification of this clade.
Pollination Biology. Aponogeton distachyos is described as having a pseudanthium (Baczynski & Claßen-Bockhoff 2023). Madagascan and Indian species of Aponogeton can be hybridized (Yadav 1995; see also Grímsson et al. 2013).
Chemistry, Morphology, etc.. Epidermal cells may have small chloroplasts (P. Baas, in van Bruggen 1985). For cell death and the development of the fenestrate leaves of Aponogeton madagascariensis, see Gunawardena and Dengler (2006), Wright et al. (2009), Dauphinee et al. (2017) and Rantong and Gunawardena (2018).
The bract may form an hybrid organ with a tepal, so making the flower slightly monosymmetric; separate bracts were not seen (Buzgo 2001). More pronounced monosymmetry occurs in flowers in which only two perianth members develop; these appear to be the abaxial pair, and in a monocot flower with "normal" orientation these would be members of the inner perianth whorl; the median member of the outer whorl of stamens is abaxial (see Singh & Sattler 1976b); a parallelism with Maundia (Sokoloff et al. 2013c). Septal nectaries are absent, according to Tobe et al. (2018). How/if the fruit dehisces is unclear (van Bruggen 1998).
Some information is taken from van Bruggen (1985, 1990, 1998); for floral morphology, see Remizowa et al. (2010b), for embryology, see Sâné (1939), and for embryo development, see Yamashita (1976).
Phylogeny. For the phylogeny of Aponogeton, see Les et al. (2005), Les and Tippery (2013) and especially L.-Y. Chen et al. (2014b). The Australian A. hexatepalus, with six tepals and quite distinctive pollen, is sister to the rest of the genus.
[Scheuchzeriaceae [Juncaginaceae [Maundiaceae [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]]]]]: floral bracts 0.
SCHEUCHZERIACEAE F. Rudolphi, nom. cons. - Back to Alismatales
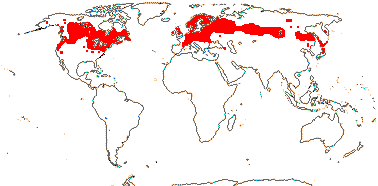
Plant irregularly sympodial, leafy shoot above substrate; "tannins" +, cyanogenic glucoside triglochinin +, flavonoids 0e; adaxial peripheral foliar vascular bundles inverted; stomata tetracytic; leaves two-ranked, linear, ligulate, with apical pore, intravaginal squamules ≡ hairs; inflorescence botroid [≡ raceme with terminal flower]; inflorescence bracts +, floral bracts +, ± foliaceous; A 6, ?introrse; pollen in dyads, inaperturate, exine forming a common covering [= calymmate]; septal nectary 0; G 3(-6), opposite outer T, basally connate [fusion usually congenital], stylulus 0, stigma abaxially subdecurrent, long-papillate; ovules (1-)2/carpel, subbasal, endostomal, outer integument ca 4 cells across, inner integument ca 2 cells across, parietal tissue 4-5 cells across, (nucellar 5cap 2 cells across); T persistent in fruit; testa smooth, mesotesta many layered, cells with thick walls; embryo chlorophyllous, radicle terminal; n = 11, x = ?7. ?6, ?8, chromosomes 0.8-2 µm long; cotyledon not photosynthetic, nuclear genome [1 C] (0.065-)1.628(-40.566) pg.
1[list]/1: Scheuchzeria palustris. N. Temperate to Arctic. Map: see Hultén (1961) and Fl. N. Am. vol. 22 (2000). Photo: Habit.
- Scheuchzeriaceae are small herbs of marshy places with 2-ranked leaves; the open sheaths have auricles at the top and there is a little pore at the tip of the blade. The racemose inflorescence has large, leafy bracts and all the parts of the flowers (except the bases of the carpels) are free from one another.
Chemistry, Morphology, etc.. Although Scheuchzeriaceae are chemically like Juncaginaceae, they are not otherwise particularly similar.
The inflorescence is described as being a closed raceme; there is a terminal flower. The numbers of the various parts of the flower vary, and, on balance, there would seem not to be a compitum (Volkova et al. 2016).
Some information is taken from Haynes et al. (1998b: general); see Volkova et al. (2016) for pollen and Stenar (1935) for embryology.
[Juncaginaceae [Maundiaceae [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]]]]: leucanthocyanins, flavones 0; stem/rhizome endodermis +; fibre bundles in leaf; leaf ± linear, ligulate, base with auricles; flowers rather small, closely aggregated, inconspicuous; T-A pair with single vascular trace [T often adnate to A]; filaments shorter than the anthers [anthers ± sessile]; pollen inaperturate; septal nectary 0, G free, ascidiate [sampling!]; ovule 1/carpel; carpels the dispersal unit; endosperm nuclear/coenocytic.
Age. The age of this node is estimated at ca 82 Ma (Janssen & Bremer 2004; see also L.-Y. Chen et al. 2014b), about 77.4 Ma (Tank et al. 2015: Table S2) or ca 68.8 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. The character "ovule one/carpel" is placed at this node by Remizowa et al. (2012b), q.v. for the evolution of a number of characters in this part of the tree. For the evolution of syncarpy around here, see Sokoloff et al. (2013d).
Chemistry, Morphology, etc.. See Thadeo et al. (2015) for fruitlet anatomy of some taxa in this clade.
Phylogeny. Iles et al. (2009, 2013) and von Mering and Kadereit (2010) suggested that Juncaginaceae are paraphyletic; the separation of Maundia from the rest of the family in fact clarifies gynoecial variation within Juncaginaceae s.l.. However, von Mering and Kadereit (2010) were not sure of the exact position of Maundiaceae.
JUNCAGINACEAE Richard, nom. cons. - Back to Alismatales
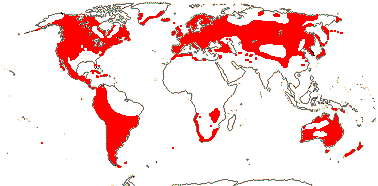
Rosette herbs; apical meristems of vegetative axes bifurcating [?all]; O- and C-glycosyl flavones, cyanogenic glucoside triglochinin; (stem endodermis 0); (adaxial peripheral foliar vascular bundles inverted); stomata also tetracytic, subsidiary cells with parallel divisions; leaves ± unifacial; (flowers polygamous); inflorescence ± spicate, flowers ± sessile; flowers 1-4-merous, (monosymmetric), P 0-4, 6; A (1-)3-8; (pollen bicellular); G 1 [3-10], weakly (more strongly) connate, fertile G opposite inner P, (alternating with an outer whorl of sterile carpels), stigma penicillate; ovules 1-few/carpel, basal, outer integument ³3 cells across, parietal tissue (?2-)4-6 cells across; fruit schizocarpic/drupaceous/achenial/(hooked, winged), T persistent or not; exotesta and endotegmen with cuticle, otherwise crushed; (endosperm +), embryo ?colour, horizontal, with short thick hypocotyl, primary root lateral; n = 6, 8, 15, etc., x = ?12, ?8, ?11, chromosomes 0.6-1.1 µm long, nuclear genome [1 C] (0.063-)1.441(-33.115) pg.
3[list]/30. Cosmopolitan, but largely coastal. Map: see Hultèn (1961), Meusel et al. (1965), Hultén and Fries (1986), Fl. N. Am. Vol. 22 (2000), FloraBase (2004) and Köcke et al. (2010). Photo: Habit, Fruit.
Age. Crown-group ages for Juncaginaceae are ca 51.7 Ma (Les et al. 2003) and (80.4-)70.2, 44.1(-27.4) Ma (von Mering & Kadereit 2014).
1. Tetroncium magellanicum Willdenow
Leaves 2-ranked, ventralized-isobifacial [oriented edge on to the stem]; plant dioecious; flowers 2/4-merous; fertile G opposite P, styluli long; ovules 1/carpel; seed with endosperm.
1/1. Chile and Argentina, ca 38o S. to Tierra del Fuego, Falkland Islandsc, Gough Island. Map: von Mering (2013: Figs 4, 5).
[Cycnogeton + Triglochin]: leaves spiral
2. Cycnogeton Endlicher
G ± free.
1/8. Australia, S. New Guinea.
3. Triglochin L. —— Synonymy: Heterostylaceae Hutchinson, Lilaeaceae Dumortier, Triglochinaceae Berchtold & J. Presl
(Laticifers - Lilaea s. str.); ligules +; leaves flattened; (flowers imperfect); A 1-6; styluli long (some L.).
1/15.
- Juncaginaceae are herbs often growing in saline marshy places. They have more or less unifacial leaves and a scapose racemose or spicate inflorescence; all parts of the flower appear to be free.
Evolution: Divergence & Distribution. Movement of the family in the southern hemisphere may have been facilitated by the proximity of ex-Gondwana fragments; there is a fair amount of habitat-linked diversification in Triglochin (von Mering & Kadereit 2014).
Chemistry, Morphology, etc.. Laticifers have been reported from Triglochin (references in Leme et al. 2021b).
Imperfect flowers may lack a perianth (carpelate flowers of Lilaea) and have a single stamen and carpel, or there may be a single perianth member; to a certain extent the number of parts in the flower is connected with flower size (Buzgo et al. 2006). The abaxial median tepal is somewhat bract-like (Buzgo 2001; Buzgo et al. 2006; Remizowa et al. 2010b). Stamens of the outer tepal-stamen unit may be outside tepals of the inner tepal-stamen unit (Dahlgren et al. 1985; Endress 1995b). Tor alternative interpretations of the gynoecium, see Igersheim et al. (2001). There is no evidence of pseudanthia; terminal flowers are close to being peloric (Buzgo et al. 2006). Seedlings of Triglochin have two-ranked leaves.
Some information is taken from Arber (1925) and Haynes et al. (1998b), both general, Campbell (1898: Lilaea flower, embryo), Agrawal (1952: embryology), and von Mering (2013) and Sokoloff et al. (2015c), both Tetroncium.
Phylogeny. Relationships are [Tetroncium [Cycnogeton + Triglochin]]; Lilaea is embedded in Triglochin (von Mering & Kadereit 2008, 2014).
Classification. For a classification of the family, see von Mering and Kadereit (2008). Trias-Blasi et al. (2015) still include Maundia (see Maundiaceae here).
[Maundiaceae [[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]]]: vessels 0; peripheral ring of sclerenchyma in peduncle 0 [M, Pot, R]; ovule apical, pendulous, straight.
Age. Janssen and Bremer (2004) suggest that the age of this node is ca 75 Ma.
Phylogeny. For discussion of the position of Maundiaceae, see Les and Tippery (2013).
MAUNDIACEAE Nakai - Maundia triglochinoides F. Mueller - Back to Alismatales
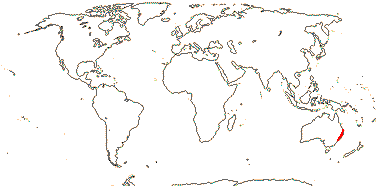
Rosette herb; roots triarch, not medullated; rhizome endodermis +, outer vascular bundles of scape inverted; abaxial peripheral foliar vascular bundles inverted, no fibre bundles; leaves 2-ranked, 2 expanded leaves/module, ± triangular, isobifacial, with apical pore, ligule 0, base narrow [ca 1/2 surrounding the stem], cataphylls +, sheath closed, branching extravaginal; inflorescence spicate, scapose, flowers sessile; flowers monosymmetric by reduction; bracteoles also 0; P 2, tranverse-abaxial, (-4 - terminal flowers), clawed, (with three traces); A (4-)6, with separated thecae; G [(3-)4], laterally ± free, cruciform, styluli marginal, ± recurved; outer integument 3-4 cell layers across, with air canals, inner integument ca 2 cells across, parietal tissue 4(?+) cells across, nucellus coenocytic [cell walls of tissue immediately distal to vascular bundle break down]; fruit a schizocarp, T persistent; testa obliterated; n/x = ?
1[list]/1. East Australia. Map: from Australia's Virtual Herbarium (consulted viii.2009).
Evolution: Vegetative Variation. Platonova et al. (2016) drew attention to the remarkable similarity in anatomy of the inflorescence axis and leaves, also describing the distinctive vegetative construction of the plant in detail.
Chemistry, Morphology, etc.. There appear to be twice as many stamens as tepals, but this is because the anther thecae are separated. Von Mering and Kadereit (2010) discuss the interpretation of the androecium; as they note, similar stamens are found in Posidoniaceae and Zosteraceae. Sokoloff et al. (2013c) described the morphology of Maundia in detail, noting i.a. variation in floral diagrams.
[[Posidoniaceae [Ruppiaceae + Cymodoceaceae]] [Zosteraceae + Potamogetonaceae]] / submerged marine aquatics: leafy shoot above substrate; rhizome with central vascular tissue; peripheral foliar vascular bundles 0 [= subepidermal ad-/abaxial bundles 0]; epidermis chlorophyllous, stomata 0; hydrophily + [water pollination]; flowers imperfect; carpelate flowers: G free; fruit a drupe; embryo with massive elongated hypocotyl; x = 10; seedling hypocotyl prominent, base [= collar] much enlarged.
Age. Janssen and Bremer (2004: c.f. topology; see also L.-Y. Chen et al. 2014b) suggest that the first split within this clade can be dated to ca 73 Ma, and they also give other divergence dates within it; see also Coyer et al. (2013). (88-)67(-56) Ma is the age of this node in Waycott et al. (2018) while L.-Y. Chen (2022) suggest an age of (78.4-)67.3(-56.8) Ma, while from Fig. 2 in X. Ma et al. (2023/2024) the age is around 76 Ma.
Evolution: Divergence & Distribution. Whether vessels and stomata are lost in parallel several times, and/or are lost and then regained, is unclear (see Zosteraceae for more discussion). Thus genes involved in stomatal differentiation have been lost in Zostera (Olsen et al. 2016; H. T. Lee et al. 2016), while the stomata of Potamogetonaceae have a rather odd development, perhaps suggesting that there they have been reacquired - although this would seem unlikely.
Ecology & Physiology. Les et al. (1997b) observed that halophily might have evolved at this node, and was subsequently lost, or it evolved twice within this clade, depending on how optimisation was carried out; Australia figures prominently in scenarios for the evolution of halophily (see also L.-Y. Chen et al. 2013). For the ecology of seagrasses, see the discussion above.
Pollination Biology & Seed Dispersal. Underwater pollination, hypohydrophily, is particularly common in this clade and pollination mechanisms in general throughout the group show a considerable amount of diversityt - they have been much studied (e.g. Cook 1982, 1998; Pettit et al. 1980; Cox 1988; Cox et al. 1991; Ackerman 2006; Remizowa et al. 2012b; Du & Wang 2014; see also above).
Chemistry, Morphology, etc.. There are many questions about plant growth, for which, see Tomlinson (1974b); information about the sympodial/monopodial growth habit is taken from Hartog and Kuo (2006). For anatomy, see Sauvageau (1891, 1892), and for details of morphology, anatomy, etc., although not of the non-marine members, see papers in Larkum et al. (2006a); stomata are absent even on the carpels (Sokoloff et al. 2013c).
Inflorescence and flower morphology in this clade can also be difficult to interpret. Iurmanov et al. (2021) discuss fruit anatomy here, and note that the fruit is a drupe s. str., the endocarp alone being ligified, in all families except Zosteraceae.
Phylogeny. This clade is only poorly supported in some molecular studies (Les et al. 1997b), but the relationships above were e.g. found by Les and Tippery (2013), Ross et al. (2015: strong support for all families and their relationships), Du and Wang (2014) and Du et al. (2016). However, Cymodoceaceae was found to be sister to the other families of this group by Nauheimer et al. (2012b). L.-Y. Chen et al. 2013) did not recover a [Posidoniaceae [Ruppiaceae + Cymodoceaceae] clade, rather, Cymodoceaceae were either very paraphyletic or polyphyletic, depending on one's interpretation of the tree, and they are paraphyletic in Iles et al. (2013) and unresolved in J.-M. Chen et al. (2004a); see also da Silva et al. (2021). Janssen and Bremer (2004) found a clade [Ruppiaceae [Zosteraceae + Potamogetonaceae]. Ruppiaceae are sister to [Posidioniaceae + Cymodoceaceae] in a rbcL analysis of Y. Kato et al. (2003), in the analysis of Petersen et al. (2015a), dominated by mitochondrial genes, but relationships in this area were generally unclear in the studies of Liu and Li (2010) and Les and Tippery (2013). A [Zosteraceae + Potamogetonaceae] clade was recovered by J.-M. Chen et al. (2004a). Da Silva et al. (2021) recovered the relationships in Cymodoceaceae s.l. of [[Phyllospadix + Zostera] [Posidonia [Ruppia [Halodule [Syringodium [Cymodocea [Thalassodendron + Amphibolis]]]]]]].
For phylogenies of several of the genera included here, see Waycott et al. (2006) and Les and Tippery (2013).
[Posidoniaceae [Ruppiaceae + Cymodoceaceae]]: plant monopodial; sulphated phenolic acids +; leaves two-ranked; P 0; anthers with apical development of the connective; pollen much elongated.
Age. For a possible date for this node of ca 27 Ma, see Coyer et al. (2013: c.f. topology); Waycott et al. (2018) suggest an age of (68-)49(-35) Ma.
POSIDONIACEAE Vines, nom. cons. - Posidonia Koenig - Back to Alismatales
Rhizome cortex with fibre strands, strands from leaf sheath persistent; inflorescence branched, branches with bracts, flowers sessile [ultimate units spicate]; flowers usu. perfect, bracts +, unvascularized; A 3, thecae more or less separate, deciduous, connective broad, shield-like [T-like after thecae fall]; pollen filiform, smooth, exine 0 [pollen not resistant to acetolysis], germination precocious; G 1, stylulus 0, stigma complex, not papillate; compitum necessarily 0; ovule sessile, campylotropous, with an outgrowth of fused integuments opposite the micropyle, outer integument ca 6 cells across, inner integument ca 4 cells across, parietal tissue ca 10+ cells across; embryo sac monosporic, 4-nucleate [egg, 2 synergids, polar nucleus - Oenothera type]; fruit a fleshy follicle; seed coat photosynthetic; first cleavage of zygote vertical; n = 10, x = 10, chromosomes dimorphic, nuclear genome [1 C] (0.07-)1.435(-29.345) pg; chloroplast ndh genes lost/subfunctionalized; seedling viviparous, primary root 0/+, root hairs few.
1[list]/9. Mediterranean, temperate Australia. Map: see den Hartog (1970).
- Posidoniaceae are seagrasses that are readily recognisable by the fibrous strands, remains of the leaf sheaths, that persist along the monopodial rhizome. These fibres form grape-fruit size balls by wave action and can be found along the beach. The inflorescence is branched.
Evolution: Ecology & Physiology. Clones of Posidonia oceanica in the Mediterranean may be up to 15 km across and thousands to tens of thousands or more years old - estimates were up to 200,000 years (Arnaud-Haon et al. 2012: dissemination by fragments of plants taken into consideration; see also Centenaro et al. 2023 for ages of clonal plants - the oldest ca 13,000 years, but Lomatia tasmanica (Proteaceae) up to 43,600 years old, see Lynch et al. 1998, also discussion on ageing). For ring-formation in seagrass meadows by the growth of P. oceanica, see above. Carbon in dense Posidonia oceanica meadows in the Mediterranean may be more than 3,000 years old, and plant deposits, whether as rhizomes in sediment or leaves washed up along the shore, may be massive (Mateo et al. 2006; Gobert et al. 2006). Although the water in the Mediterranean Sea is nitrogen-poor, Mohr et al. (2021) recently found that P. oceanica growing there is associated with the N-fixing gammaproteobacterium Candidatus Celerinatantimonas neptuna that lives in its roots and fixes substantial amounts of N during the summer months, the main growing period of the plant. There may be movement of gamma aminobutyrate (GABA) and carbohydrates from the plant to the bacterium and of various forms of N from the bacterium to the plant (Mohr et al. 2021). For oxygen supply, gas movement, etc., in P. oceanica, see Borum et al. (2006)
In Posidonia oceanica, at least, the thickening on the walls of the root hairs is in spirals, and the root hairs break down into spirals (= helical crack root hairs) that are probably effective energy-dissipating units, furthermore, the apices of the hairs are irregularly expanded, the combination probably helping in the attachment of the plant (Kolátková & Vohník 2019: see also Orchidaceae and Araceae).
Seed Dispersal. For vivipary, see Sinclair et al. (2016). The fleshy fruits can float (Ackerman 2006).
Plant-Bacterial/Fungal Associations. For the association between Posidonia and the bacterium Celerinatantimonas, see Ecology & Physiology above.
Chemistry, Morphology, etc.. The morphology of the ovules is distinctive and basically uncategorizable (see also Remizowa et al. 2012b); the ovule is sessile, and from the integuments it would seem to be curved, but the embryo sac is straight. G. Ma et al. (2012) described embryo sac development; it is monosporic and 4-nucleate; two of the nuclei may perhaps fuse (synergid and central nucleus?) and form a diploid polar nucleus. This should be confirmed, as should the plane of division of the zygote, which was described as being vertical but looks almost oblique (Ma et al. 2012).
Additional information is taken from Tomlinson (1982: esp. anatomy), Kuo and McComb (1998), Gobert et al. (2006) and den Hartog and Kuo (2006), all general; for chemistry, see Heglmeier and Zidorn (2010), for germination, see Kuo and Kirkman (1997).
[Ruppiaceae + Cymodoceaceae]: stem with central stele, cortical bundles +, endodermis indistinct, xylem lacunae + [ruptured xylem tissue, xylem ± 0]; leaves serrulate; A 2; compitum 0.
RUPPIACEAE Horaninow, nom. cons. - Ruppia Horaninow - Back to Alismatales
Flowering plant sympodial; sulphates?; roots unbranched; rhizome cortical bundles 2; leaves 1-veined, sheath not ligulate, ± auriculate [= "stipule"]; inflorescence densely spicate, peduncle long [to 1 m] (not); plant monoecious; anther thecae open violently; pollen elongate-arcuate, triaperturate; G 2-15(-many), stylulus 0, stigma ± peltate-funneliform, lobed; ovules also lateral, campylotropous, micropyle bistomal, parietal tissue ca 7 cells across; fruit achene/drupelet, long-stipitate, stone operculate; testa 2-layered, exotegmen cells large with branched protuberances from the walls, all becoming crushed; endosperm helobial, primary root lateral; n = 8-12, 15, x = 10, chromosomes dimorphic, 0.7-4.4 µm long, nuclear genome [1 C] (0.067-)1.413(-29.85) pg.
1[list]/1-10. More or less world-wide, apparently quite frequently growing well away from the sea in all continents (map: see Hultén 1961; Fl. N. Am. 22: 2000; Heywood 1978 [for some of the southern hemisphere]; Ito et al. 2010; Fl. Austral. vol. 39: 2011; Trop. Afr. Fl. Pl. Ecol. Distr. 7. 2012).
- Ruppiaceae are submerged aquatic plants growing in saline to fresh water that may be recognised by their two-ranked, serrulate leaves with an obvious midrib and an open, sheathing, auticulate base; the internodes are well developed.
Evolution: Ecology & Physiology. Ruppia quite often grows in brackish or fresh water, and as with other aquatics, the epidermis is the main photosynthetic tissue in the plant (Haynes et al. 1998a).
Pollination & Seed Dispersal. Anther thecae break free from the flower and open violently when they reach the surface of the water and the pollen grains form rafts; selfing may also occur (Gamerro 1964; Cox & Knox 1989; M. L. Taylor et al. 2020).
The fruits are eaten by water birds; long distance dispersal is likely (Ito et al. 2010).
Chemistry, Morphology, etc.. For pollen development, see M. L. Taylor et al. (2018). Although Haynes (1978; see also Haynes et al. 1998a) described the ovules as being campylotropous, they are shown as being straight by Gamerro (1968) and Posluszny and Sattler (1974). Although I follow the latter for the interpretation of stamen morphology, they also mention that the parietal tissue of the ovule is about 1 cell across - but c.f. Haynes et al. (1998a). Ovule morphology needs to be cleared up.
General information is to be found in Tomlinson (1982: esp. anatomy) and Haynes et al. (1998).
Phylogeny. Ito et al. (2010) looked at the biosystematics of this difficult group in which cytological variation is considerable and hybridisation is likely; the focus in Ito et al. (2015) was on southern African taxa.
Classification. Ruppiaceae are only doubtfully distinct from Cymodoceaceae (Les et al. 1997b); see also the Phylogeny section of the Posidoniaceae to Potamogetonaceae clade above.
CYMODOCEACEAE Vines, nom. cons. - Back to Alismatales
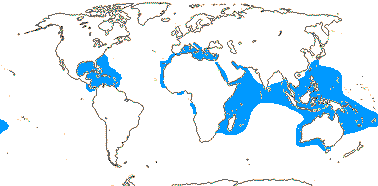
(Plant sympodial - Th.), stems erect; (C4 photosynthesis + - Cy.); inositols like l-chiro inositol; roots (branched), (hairs 0 - Am.), pith 0; rhizome with two or a ring of vascular bundles, pith 0; (sieve tubes with thick nacreous walls - Ha.); (adaxial peripheral foliar vascular bundles +, inverted, abaxial bundles normal - Sy.), leaves two ranked, (blade terete - Sy.), (apex serrulate); plant dioecious (monoecious); flowers in cymose groups enclosed by bracts [?kind], or solitary, terminal; staminate flowers: A (2), ± connate, introrse [H. - check], (apical appendages 0 - Sy.), (filaments +); (microsporogenesis simultaneous - Th.); pollen filiform, coiled, (smooth), tapered/forked at the ends, mucilaginous, exine 0/?+; carpelate flowers: G 2, style unbranched/stylulus +, stigmatic branches 2, 3, long-linear, not papillate; fruit achene or drupelet, pericarp/endocarp stony, 1 (2) seeded; testa 0, (seed coat has flattened cells with annular thickenings - H.); n = 7, 8, 10, 12, 14-16, x = 10 (?9), chromosomes 0.22-16.3 µm long, nuclear genome [1 C] (0.067-)1.413(-29.85) pg; (chloroplast ndh genes lost/subfunctionalized - Am.); seedlings (viviparous), with tuft of root hairs.
5[list]/16. More or less tropical (to warm temperate), Australia in particular. Map: see den Hartog (1970), van Baloogy (1975) and Australia's Virtual Herbarium (consulted xii.2013). [Photo - Habit.]
Evolution: Divergence & Distribution. Both Thalassodendron and Cymodocea are known fossil from the oldest rocks in Florida, late Middle Eocene deposits maybe 35-33 Ma, although the current distribution of these genera is entirely Old World (Lumbert et al. 1984; Ivany et al. 1990).
Thalassodendron and Amphibolis, sister taxa (see below), have erect. leafy, woody, sympodial stems (those of other taxa are monopodial and herbaceous), and they are viviparous (Kuo & McComb 1998).
Ecology & Physiology. During the decomposition of the remains of Posidonia oceanica large amounts of refractory carbon may be produced, conditions may be anoxic, and massive long-lived peat-type deposits some 5 m or more thick develop (Mateo et al. 2006). Munné-Bosch et al. (2022) discuss aspects of the ecophysiology of Cymodocea nodosa, particularly the role that vitamin E played, noting i.a. that low temperatures induced dessication in its leaves, but not rhizomes. Inositols (cylohexane-1,2,3,4,5,6-hexol) like l-chiro- and muco-inositol are well known from Cymodoceaceae, where they may accumulate in quite large amounts, although their function seems unclear (Larkum et al. 2006b: note, myo-inositol is widespread).
Cymodocea nodosa may be a C
Pollination and Seed Dispersal. For pollen morphology and development and pollination, see Ducker et al. (1978), Cox and Knox (1989) and Cox and Humphries (1993).
Amphibolis and Thalassodendron are viviparous (Ackerman 2006).
Genes & Genomes. For chromosomes, see Kuo (2013: ?Thalassodendron) and Vanitha et al. (2016). There may have been a genome duplication in Halodule (L.-Y. Chen et al. 2022) and/or Cymodocea (X. Ma et al. 2023/2024).
Chemistry, Morphology, etc.. For Remizowa et al. (2011) the flowers of Cymodoceaceae represented racemose partial inflorescences. Tomlinson (1982) described Thalassodendron as having a basal, anatropous ovule, while Takhtajan (1985) and Tomlinson and Posluszny (1978) described the ovules of Syringodium as being apical and straight. Cymodocea is viviparous, and the cotyledon is at most small (e.g. Arber 1925: C. antarctica).
Additional information is taken from Kuo and McComb (1989, 1998: general); for cyclitols, see Drew (1983) and for floral morphology see Kay (1971) and McConchie et al. (1982).
This family needs work.
Phylogeny. For relationships in Cymodoceaeae, see Ross et al. (2014); Halodule is well supported as sister to the rest of the clade (see also Petersen et al. 2015a) and Cymodocea is paraphyletic.
Classification. Trias-Blasi et al. (2015), da Silva et al. (2021), and others have included Ruppiaceae in Cymodoceaceae.
Botanical Trivia. Pollen of Amphibolis is up to 5 mm long.
[Zosteraceae + Potamogetonaceae]: roots unbranched; leaf with apical pore, (sheath closed); plant mono- or dioecious; compitum 0.
Age. Estimates of the time these two clades diverged range from ca 100 Ma (Y. Kato et al. 2003; Sullivan & Short 2023), (70-)52(-38) Ma (Waycott et al. 2018) and (60.7-)51.7(-43.0) Ma (L.-Y. Chen et al. 2022) to ca 47 Ma (Janssen & Bremer 2004). Wilf and Escapa (2014) date the Patagonian Babiancarpus, stem Potamogetonaceae, to 56-42 Ma while the mean stem age of Zosteraceae is estimated to be ca 53.5 Ma (Hertweck et al. 2015, see Chen et al. 2022).
Evolution: Divergence & Distribution. Both families seem to have rather long phylogenetic fuses.
ZOSTERACEAE Dumortier, nom. cons. - Back to Alismatales
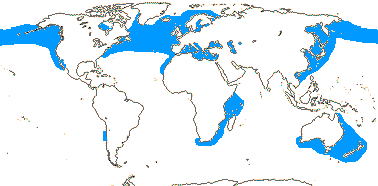
Plant monopodial (sympodial - some Z.); sulphated phenolic acids and flavonoids + (0), fructan sugars accumulated; roots in two groups/rows, unbranched, root hairs many, long; rhizome cortical bundles 2 (several - some Z.), unlignified fibrous strands (0 - Ph.), (pericycle 0 - Z.), endodermis +; sieve tubes with thick nacreous walls; leaf with fibre bundles, vascular bundles with xylem and phloem separated, single wide lacuna≡xylem, ingrowths of walls of phloem parenchyma cells, stomata 0; leaves two-ranked, ligule +, (sheath closed); plant (mon-)/dioecious; inflorescence leaf opposed, branched, with spathe and spadix, spadix axis flattened, flowers in two ranks, alternating on adaxial surface; flowers monosymmetric by reduction; staminate flowers: P 1/0; A 1, retinaculum +/0, anther thecae separate, joined by connective, filament 0; pollen filiform, smooth, mucilaginous, exine 0 [pollen not resistant to acetolysis]; pistillode 0; carpelate flowers: staminode +, G 1, ± asymmetrical, stylulus +, stigmatic branches 2, long, not papillate (± fimbriate - P.), abscise; ovule with outer integument to 7 cells across, parietal tissue none, 2 nucellar layers laterally, supra-chalazal area massive, postament +; fruit not a drupe [tissue not lignified - Z., dorsally = abaxially dehiscing follicle/mesocarp and endocarp lignified - Ph.]; exotestal cells ± anticlinally and periclinally elongate, other cells persist, ± thickened or not, tegmen degenerates; embryo horizontal, radicle ?normal; n = 6, 9, x = 6, chromosomes 0.9-1.6 µm long, nuclear genome ca 202.3 Mb [Z.]; hypocotylar hairs +, (primary root 0, leaves, hypocotyl emerge well before roots - Z., P.).
4[list]/18: Phyllospadix (5), Zostera (5). Temperate to subtropical. Map: see den Hartog (1970) and van Balgooy (1975).
Age. Divergence within Zosteraceae may have started ca 33 Ma (Y. Kato et al. 2003), ca 17 Ma (Janssen & Bremer 2004), or (60-)23.3(-7) Ma (Coyer et al. 2013 - also other ages within the family); the crown-group age of the family in Waycott et al. (2018) is (38-)24(-11) Ma and in Sullivan and Short (2023) ca 23.3 Ma.
- Zosteraceae are seagrasses with leaf-opposed branches and flattened inflorescence axes (spadices) with flowers on the adaxial surface; the inflorescences are enclosed by a spathe. Most taxa have a small perianth member opposite the stamen.
Evolution: Ecology & Physiology. The genome of Zostera marina shows many changes that can be linked with adaptations to problems of living the submerged life - genes for u.v. protection, exine production, terpenoid synthesis and stomatal differentiation have all been lost, while the cell wall pectin has a distinctive composition and there are sulphated polysaccharides (galactans) involved in osmotic balance (Olsen et al. 2016; H. T. Lee et al. 2016; Roodt et al. 2019). The ability to synthesize ethylene, an important plant hormone, seems to have been lost (see also Golicz et al. 2015; L.-Y. Chen et al. 2022) and the jasmonate and gibberellin pathways have also been affected. However, it is unclear to what extent these features might be restricted to Zosteraceae, to the immediate group of largely marine Alismatales, or to seagrasses in general, indeed, some of these features may be associated with the loss of secondary thickening in monocots as a whole (Roodt et al. 2019).
There appears to be significant nitrogen fixation by bacteria on the root surfaces in Zostera marina (Marbà et al. 2006).
The age of a clone of Zostera marina in the Baltic was estimated to be more than 1,000 years (Reusch et al. 1999). For growth rings in such clones, see above
Pollination Biology & Seed Dispersal. De Cock (1980) described pollination in Zostera marina in detail, noting i.a. that germination of the filiform pollen grains occurs anywhere along their lengths (see also Ackerman 1993 for pollen germination).
The ripe fruit of Zostera marina splits down its abaxial side and the seed falls out - a sort of follicle (De Cock 1980; c.f. Kuo & McComb 1998). Harwell and Orth (2002; see also Orth et al. 2006b) suggested that seeds of Z. marina enclosed in detached reproductive shoots might travel ca 23 km in a single tidal cycle (combination of wind and tide), but they estimated figures of ca 108 km given extant distributions of the species around the Chesapeake Bay, and thought that maximum dispersal events might even be 103 km or more.
Plant-Bacterial/Fungal Associations. The heterokont Labrinthula zosterae causes the wasting disease that has severely affected Zostera marina and may affect other species of the genus (K. A. Moore & Short 2006).
Fungi grow in the intercellular spaces in the leaves of Zostera muelleri (Kuo et al. 2018).
Genes & Genomes. Olsen et al. (2016) discuss the genome of Zostera marina; there may have been a genome duplication around here (see also Zwaenepoel & Van de Peer 2020) while Sullivan and Short (2023) date a WGD in common between Nanozostera and Zostera at 72-64 Ma, which of itself makes little sense, the two genera being estimated to split ca 14 Ma. J. Zhang et al. (2020) note the extensive loss of gene families in Z. marina; it would be good to compare this with other marine Alismatales (Genislea aurea also showed extensive losses...) - indeed, Z. marina has fewer genes in its genome than some Lemnoideae (F. Li et al. 2023).
The mitochondrial genome of Zostera marina is very small, only 191,481 bp, of which 20.5% is made up of chloroplast inserts and ca 20% is made up of repeats; a number of mitochdrial genes have been lost, of which some are to be found in the nucleus (G. Petersen et al. 2017).
Chemistry, Morphology, etc.. All leaves on a plant are similar in morphology. There are cell wall ingrowths in the foliar phloem parenchyma of Zostera (Kuo et al. 2018).
Tomlinson (1982) suggested that the staminate flowers had two bisporangiate/monothecal anthers. It is unclear whether the pollen grains of Zostera marina are bicellular or tricellular (e.g. Ackerman 1993). The course of endosperm development is unclear. Iurmanov et al. (2021) describe fruit anatomy in Zosteraceae; it differs from that in the other members of the marine clade. In the germination of Zostera and Phyllospadix, at least, the hypocotyl and leaves emerge long before the roots appear (A. Taylor 1957) - there may be apomorphies associated with germination.
General information is taken from Kuo and McComb (1998), Tomlinson and Posluszny (2001), Sullivan and Short (2023) and K. A. Moore and Short (2006: Zostera) and reproductive morphology from Soros-Pottruff and Posluszny (1994: Phyllospadix).
Phylogeny. For relationships in Zosteraceae see Y. Kato et al. (2003), Les et al. (2001) and in particular Coyer et al. (2013); Phyllospadix is sister to the rest of the family (see also J.-M. Chen et al. 2004a).
Classification. For generic limits, see Les et al. (2001); Sullivan and Short (2023) recently suggested that there should be four genera in the family.
POTAMOGETONACEAE Berchtold & J. Presl, nom. cons. - Back to Alismatales
Plants of fresh (± saline) water; root bundles 2/several; rhizome (with cortical bundle system); xylem mostly as xylem lacunae, vessels +;; leaf with ligule, basal, (sheath closed - Zostera), auricles 0; (inflorescence bracts +, = subtending spathe); (plant monoecious); (floral bracts +, unvascularized); flowers (perfect), (2-)4-merous; staminal retinaculum +/0, (pollen vestigial sulcate?); G (1-)4(-8), alternating with P, ± stipitate, partly ascidiate, stigma ± expanded; ovule becoming campylotropous, parietal tissue 4-6 cells across, (nucellar cap 2-5 cells across), (hypostase +), obturator 0; fruit a drupelet, T persistent; seed exotestal; embryo strongly curved, chlorophyll 0; n = 12-18, x = 13 (?14), chromosomes 0.5-2.3 µm long, nuclear genome [1 C] (0.067-)1.481(-32.972) pg.
4[list]/111. Worldwide, esp. temperate. Map: see Hultén (1961), Meusel et al. (1965), Haynes and Holm-Nielsen (2003), Kaplan (2008) and Trop. Afr. Fl. Pl. Ecol. Distr. 7 (2012). [Photo - Habit, Potamogeton Inflorescence.]
Age. Divergence within Potamogetonaceae may have begun ca 25 Ma (Janssen & Bremer 2004); (37-)23(-12) Ma is the estimate in Waycott et al. (2018).
1. Potamogetoneae Dumortier - Potamogeton L.
Leaf blade often floating; rhodoxanthin + [red colour]; stomata + (0), development odd; leaves spiral, 2-ranked, subopposite pair just below the inflorescence [Potomageton], (whorled), often with petiole and blade, blade vernation involute, primary veins merge with each other, ligule long, adnate to petiole/not, free or connate/0; inflorescence aerial, capitate to spicate, or flowers 2; flowers sessile, 4-merous; P clawed, adnate to A; fruit (1-seeded berry - Groenlandia); seed (coat crushed); n = 13 (14).
1(-3)/100: Potamogeton. ± Cosmopolitan.
2. Zannichellieae Dumortier —— Synonymy: Zannichelliaceae Chevallier, nom. cons.
Plants submerged, (annuals); flavone sulphates +; apical meristems of vegetative axes bifurcating; leaves 2-ranked/pseudowhorled, ± linear, (margin serrulate), sheath/ligule adnate to leaf base, free ligule (bifid); plant monoecious (dioecious), inflorescence sympodial, ± fasciculate; flowers long-pedicillate (sessile); staminate flowers terminal: P 0 or 3; A 1, 2-12-sporangiate, connective bluntly triangular; pollen grains in mucilaginous matrix/not; carpelate flowers: P tubular, or 3-4; G 1-8, when 3, opposite P, when 4, diagonal or cruciform, stipitate, stylulus +, stigma enlarged, peltate or infundibular with ± feathery margin; embryo sac bisporic (the chalazal dyad) and 8-celled [Allium-type; seed coat crushed; n = 6.
3-4/13. Europe and North Africa, South Africa, the Antipodes, Zannichellia palustris almost cosmopolitan.
Age. Divergence within Zannichellieae may have begun ca 38.3 Ma (Les et al. 2003).
- Potamogetonaceae-Potamogetoneae are aquatic plants which have quite broad and more or less petiolate leaves with midrib and cross veins; there is usually a conspicuous basal ligule. The inflorescence is densely spicate and the flowers have small tepals appearing to be borne on the backs of the stamens. The carpels are stipitate, as is particularly clear in Zannichellia, although that genus does not have a spicate inflorescence.
Evolution: Divergence & Distribution. For character evolution in Zannichellieae, see Ito et al. (2016); there has been some pretty serious long distance dispersal in this clade.
Ecology & Physiology. Iida et al. (2016) looked at stomatal development in Potamogeton amplexicaule, which grows submersed, and P. wrightii, which has floating leaves; the former could be induced to produce stomata, and interestingly, it could tolerate salt/brackish water. The marine is sometimes included with seagrasses. Stomata are found at the apices of the leaves of some species of Zannichellia (Haynes et al. 1998).
Pollination Biology & Seed Dispersal. Cross-pollination is by wind in Potamogeton (Haynes et al. 1998b). Pereira Nunes et al. (2012) suggested that Potomageton illinoensis might have a hyperstigma. Anthers of Lepilaena (= Althenia) separate and rise to the surface where they explode; the pollen grains, apparently embedded in a gelatinous matrix, clump together in search vehicles and pollinate the carpelate flowers which reach the surface on very long pedicels (Cox & Knox 1989).
Potamogeton in particular is a very important source of food for ducks in North America; the fruits float and are photosynthetic (Haynes et al. 1998b).
Genes & Genomes. Quite wide hybridization is reported from wihin Potomagetonaceae (L.-Y. Chen e al. 2022). There seems to have been a chromosome duplication in Potamogeton acutifolius (X. Ma et al. 2023/2024) - extent?
Chemistry, Morphology, etc.. Potamogeton tends to have trilacunar nodes; the central conducting tissue (vascular bundles surrounded by endodermis, and there are generally lacunae) is narrow, and in some species there are also small vascular bundles scattered in the cortex (Schweingruber et al. 2020). There is great variation in the leaf base, including the ligules (often called stipules), and in leaf blade shape; this occurs both within and between species, some taxa of Potamogeton being heterophyllous, with submerged and floating leaves differing greatly in form. There has been some debate as to whether the ligule is "really" a stipule (Colomb 1887; Sinnott & Bailey 1914).
Remizowa et al. (2011) thought that the flowers of Zannichellia might represent racemose partial inflorescences. Posluszny and Tomlinson (1977) suggested that the staminate flowers of Zannichellia had a single anther with up to 12 sporangia, although other interpretations seem possible; the anthers are sessile. There has also been debate as to the nature of the ovule, which is often more or less campylotropous, sometimes because of an ingrowth of the carpel wall (Takaso & Bouman 1984; Pereira Nunes et al. 2010). Hasitschka-Jenske (1959) described endosperm development and in particular the high endopolyploidy (192-ploid) of the single basal/chalazal nucleus - c.f. Potamogeton.
Much general information on Alismataceae s. str. is taken from from Haynes (1978) and Haynes et al. (1998b: also Zannichelliaceae) and Haynes and Holm-Nielsen (2003); see also Posluszny (1981) and Charlton and Posluszny (1991) for floral morphology, Pereira Nunes et al. (2009) for pollen development (esp. tetrad shape) and Kaplan et al. (2013) for chromosome numbers (not Zannichellia).
Phylogeny. The morphologically very distinctive Zannichellia and relatives, which alone in the family commonly have flavone sulphates, is rather weakly embedded within Potamogetonaceae (Les et al. 1997) or sister to the rest of the family (Les & Tippery 2013), however, the old Zannichelliaceae are quite well supported as sister to Groenlandia in the plastid phylogenomic study of Ross et al. (2015) while in Du and Wang (2014) and Du et al. (2016) Groenlandia was sister to the rest of the family. For relationships around Zannichellia, see Ito et al. (2016). Potamogeton itself is para- or polyphyletic (Les & Haynes 1995); for the phylogeny and evolution of Potamogeton in particular, see Lindqvist et al. (2006). For hybridization, especially in Potamogeton, see L.-Y. Chen et al. (2022).
Classification. Althenia was expanded to include Lepilaena (Ito et al. 2016),