EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[DIOSCOREALES + PANDANALES]: root hairs from unmodified rhizodermal cells, exodermal cells not dimorphic; outer integument 2(-3) cells across; genome size 10> pg [1 C].
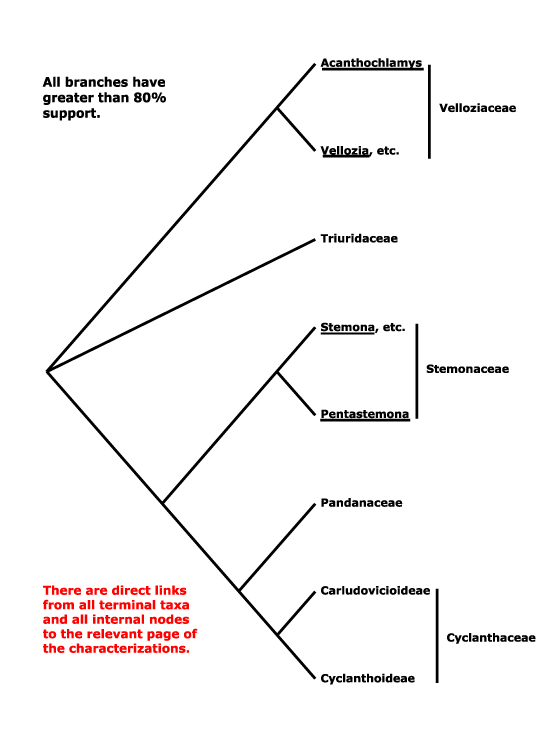
PANDANALES Berchtold & J. Presl - Main Tree.
Anthers basifixed; nucellar cap +; endosperm type?, (reserves starch), embryo minute; genome size 0.4-1.5 pg [1C]. - 5 families, 36 genera, 1345 species.
Includes Cyclanthaceae, Pandanaceae, Stemonaceae, Triuridaceae, Velloziaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown group Pandanales are estimated to be (130-)117(-116) Ma (Merckx et al. 2008a), ca 114 Ma (Janssen & Bremer 2004), (111-)103(-95) or (118-)101.2(-91) Ma (Alcantara et al. 2018), ca 93 Ma (Givnish et al. 2018b), (98-)91(-89) Ma (Hertweck et al. 2015), (96-)90, 84(-78) Ma (Wikström et al. 2001), (117-)82, 72(-65) Ma (Bell et al. 2010), (101-)74(-53) Ma (Givnish et al. 2016b), ca 72.3 Ma (Magallón et al. 2015) or only ca 50 Ma (Bremer 2000b). Ages are 110-69 Ma in Mennes et al. (2013, 2015) and ca 90.9 Ma (Leal et al. 2022). Ages based on chondrome data in Soto Gomez et al. (2020) are nearly always older than those based on plastome data and are (104-)93, 71(-58) Ma, but the disparity is less for nodes within the order.
Evolution: Divergence & Distribution. Stevenson et al. (2000) suggest possible characters for the order and the groups of families within it. These include a 6 bp deletion in atpA - absent in Talbotia (Velloziaceae), whether or nor it occurs in Barbaceniopsis is unclear (c.f. Davis et al. 2004) - and a distinctively connate androecium; tenuinucellate ovules may be another synapomorphy, and starchy endosperm is common here.
Floral variation in this really quite small clade is remarkable and is conveniently summarized by Rudall (2017), indeed, even in Velloziaceae stamen number is often other than six. However, confirmation of the position of Triuridaceae in particular is needed to understand morphological evolution (see Garay-Arroyo et al. 2012), and the flowers in some families are so highly modified that it is unclear exactly at what one is looking (e.g. Rudall 2017) - so it gets tricky talking about evolution. There is also some pretty odd vegetative variation - especially in Acanthochlamys and Sararanga.
Genes & Genomes. Soto Gomez et al. (2020) discuss variation in the rates of evolution of the plastome in particular, the position of the junction of the inverted repeat and large single copy region, etc..
Chemistry, Morphology, etc.. Rudall et al. (2005b) note that floral merosity, usually not very variable in the monocots, varies considerably in Pandanales; general floral construction is rather labile here.
Some information on pollen morphology is taken from Grayum (1992); for pollen and tapetum, etc., see Furness and Rudall (2006a).
Phylogeny. For discussion on the relationships of Pandanales, see the Petrosaviales page.
The composition of Pandanales is somewhat unexpected, including as it does some of the most delicate (Triuridaceae), most woody and robust (Pandanaceae) and most drought-tolerant (Velloziaceae) monocots. The phylogeny that was on this site for some time, [Velloziaceae [Stemonaceae [Triuridaceae [Cyclanthaceae + Pandanaceae]]]], was taken from Behnke et al. (2000, 2013), and especially Caddick et al. (2002a), Davis et al. (2004: Fig. 1) and Mennes et al. (2013), although even in the last study, quite comprehensive, the position of Stemonaceae was not very strongly supported. Indeed, the composition of the order seems stable, even if relatiosnhips within it have been somewhaat unclear. Soto Gomez et al. (2020) found that Sciaphila was sister to Pentastoma in parsimony analyses of plastid data, although Janssen and Bremer (2003: Triuris minus) had suggested a somewhat different set of relationships. In the plastome analysis of Wanga et al. (2021: good support, poor sampling) relationships were [Stemonaceae [Cyclanthaceae + Pandanaceae]]. The [Pandanaceae + Cyclanthaceae] clade is found consistently and it also has strong morphological support.
Triuridaceae often group with Pandanales in molecular analyses (e.g. Chase et al. 2000a: 18S rDNA; Graham et al. 2005), but their relationships within the order have varied. Those in Hertweck et al. (2015) are [Velloziaceae [Triuridaceae [Stemonaceae...]]]. Some morphological analyses even suggested that Triuridaceae were nested within Stemonaceae (Rudall & Bateman 2006), but without strong bootstrap support (50³%, hardly the robust placement claimed); characters that supported this position included thick filaments, free carpels, and pollen morphology. However, subsequent analyses using plastomes, including the very small plastome of Sciaphila, yielded the rather well supported relationships in the tree below; only in parsimony analyses was a clade [Sciaphila + Xerophyta] obtained, and it had low support (Lam et al. 2015); sampling may be a worry, only 10 Pandanales were included in the study (see also Lam et al. 2016: weak support, some sequences on occasion placed with Dioscoreales-Nartheciaceae, 2018: support quite good; Givnish et al. 2018b; H.-T. Li et al. 2019: quite strong support; Soto Gomez et al. 2020: chloroplast and mitochondria). H.-T. Li et al. (2021) recovered a [Triuridaceae + Stemonaceae] clade.
Sciaphila has recently been included in the Angiosperms353 nuclear genome project (W. J. Baker et al. 2021a: see the Seed Plant Tree of Life, version ix.2024, and also Zuntini et al. (2024); relationships are [Velloziaceae [Triuridaceae [Stemonaceae [Pandanaceae + Cyclanthaceae]]]], but the position of Sciaphila/Triuridaceae is sometimes poorly supported. However, recently T. Shi and He (2025: 12 plastomes, 17 taxa, 9 genera, 2,668 single-copy nuclear genes, see also Angiosperms353 data, 29 spp., 19 genera) also recovered these relationships in concatenation analyses; the positions of Triuridaceae and Stemonaceae were switched in both the coalescent and plastome analyses. However, Shi and He (2025) noted that Stemonaceae contributed ca 79% of the genetic material to the [Cyclanthaceae + Pandanaceae] clade, Triuridaceae only ca 21% (19% of the Triuridaceae genome originated from Velloziaceae), hence they preferred the concatenation topology, and that is followed below.
Although Triuridaceae were found to be sister to Zingiberaceae in some analyses by Davis et al. (2004), this is an unlikely position, if morphology means anything. Nartheciaceae have sometimes tended to associate with Pandanales, rather than Dioscoreales (q.v. for further details), the clade in which they are included here.
Synonymy: Cyclanthales Martius, Roxburghiales Martius, Stemonales Doweld, Triuridales J. D. Hooker, Velloziales Reveal
VELLOZIACEAE J. Agardh, nom. cons. - Back to Pandanales
Xeromorphic; roots with vessel elements with simple perforation plates, band of fibres in cortex; vessels in stem and leaf; phloem in at least smaller bundles in two abaxial-lateral strands; sieve tube plastids in the stem 1³ µm across; stem cortex in three zones, divided by bundles of fibres or fibrous sheath; stomata brachyparacytic; leaves persistent; inflorescences axillary; flowers large; A borne in mouth of "hypanthium"; pollen grains bi- or tricellular; placentae bifid, stalked-capitate, style long; ovules many/carpel, parietal tissue none; fruit dehiscing laterally, loculicidal; exotesta thick walled, endotegmen thin-walled, with phenolics/tanniniferous; aleurone cells with thick walls, endosperm with starch; x = 16 (?17), nuclear genome [1C] (0.087-)0.502(-2.917) pg; plastome (rps16 gene 0).
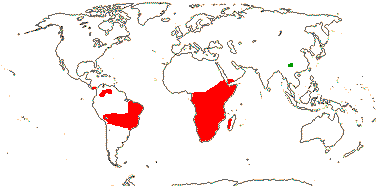
9 [list]/266 (306). South America and Africa-Madagascar to Arabia, China.
Age. The crown age of this family is estimated to be 115 Ma or so (Mello-Silva et al. 2011), ca 81.8 Ma (Gao et al. 2021), (97-)74.3(-52) Ma (Alcantara et al. 2018), (58-)41, 34(-27) Ma in Soto Gomez et al. (2020: Table 1 for other estimates) and ca 47.8 Ma (Leal et al. 2022) - another case where the estimates are not immediately helpful.
1. Acanthochlamydoideae P. C. Kao - Acanthochlamys bracteata P. C. Kao —— Synonymy: Acanthochlamydaceae P. C. Kao
Caespitose rhizomatous herb; steroidal saponins +?; (velamen +), root stele tri- or tetrarch, not medullated; lateral roots arise opposite xylem poles; sieve tube plastids lacking crystals, "small"; cauline vascular tissue with a central tetrach vascular cylinder, phloem dispersed within the xylem, scape with 5-6 central collateral vascular bundles; raphides and tannin cells 0; foliar palisade tissue 0, endodermis surrounding two back-to-back vascular strands [i.e xylem confluent]; leaves spiral, ligule basal, sheathing, large, mostly open; inflorescence scapose, compound-capitate; T ca 1/2 connate; anthers short, thecae unisporangiate, filaments short; septal nectaries 0; nucellar cap 0; exotestal cells evenly thickened, ± elongated; endosperm nuclear, embryo large/medium; n = 19, chromosomes 1> μm long; seedling?
1/1. S.E. Tibet and S.W. China. Map: from Ying et al. (1993 - green). [Photo - Flower, Fruit.]
2. Vellozioideae Rendle —— Synonymy: Barbaceniaceae Arnott
Herbaceous, tussock- or rosette-forming, to small trees; plants showing extreme dessication tolerance, roots often growing down through persistent leaf bases; biflavonoids + [Xerophyta]; (velamen +); stem tracheids main conducting tissues; sieve tube plastids with angular crystals and other loosely-packed crystals; (vessels in leaves absent - Talbotia); transfusion tracheids in leaf bundles; (raphides/styloids +); stomata in grooves, (on both surfaces), (cuticular waxes as aggregated rodlets), leaves with marginal bundles and transfusion tissue, Water storage tissue various/0; indumentum various; leaves (spirally)3-ranked, (blade abscising), vernation conduplicate-flat (plicate), midrib +, margins usu. spiny; inflorescences often single-flowered, bracteoles lateral; flowers with uniseriate or massive multiseriate protrusions/glands on the outside: hypanthium +/0; T often violet, median T in outer whorl adaxial [e.g. Barbacenia], largely free; (corona +, fimbriate, usu. adnate to adaxial A); A not adnate to T, 6(-many, 3 or 6 fasciculate [A in fascicle connate], development within fascicles ± centripetal - Vellozia), filaments cylindrical, anthers long, (dorsifixed); tapetal cells uninucleate; (pollen in tetrads); late-acting self incompatibility [Vellozia]; septal nectaries +; stigmas large, erect or spreading, capitate; micropyle bi/endostomal, outer integument 2 cells across, inner integument 2 cells across, nucellar cap ?+/0, endothelium +, funicular obturator +, suprachalazal tissue massive; archesporial cells several; fruit (apically or basally loculicidal/with intercostal apertures/dehiscence ± irregular); exotesta with spiral/reticulate (0) thickenings, endotegmen with outer periclinal and anticlinal walls thickened (not); endosperm helobial, cells elongated, wall formation first in small chalazal chamber then in large micropylar chamber, (aleurone cell walls unthickened), embryo medium to short; n = 7, 8, 17, 24 [x = 6?]; collar rhizoids +.
8/280: Vellozia (135), Barbacenia (100). South America, mainly Brazil (esp. Minas Gerais), and Africa-Madagascar (to Arabia). Map: see Ayensu (1973b) - red. Photo: Habit, Flower.
Age. Crown-group Vellozioideae have been dated to a mere ca 14 Ma (Janssen & Bremer 2004: note sampling), or some 100 Ma or so (Mello-Silva et al. 2011). Cabral et al. (2021) dated the clade that contained the Brazilian species to (40.4-)30.4(-22.2) Ma.
Evolution: Divergence & Distribution. Mello-Silva et al. (2011) interpreted the split of Acanthochlamys from the rest of the family and other generic disjunctions in the family in terms of drift-induced vicariant events.
Velloziaceae are predominantly New World, and are most diverse in eastern Brazil, especially in Minas Gerais/Southern Espinaço "particularly in areas such as the Grão-Mogol region, the Diamantina Plateau, and the Serra do Cipó mountain chains" (Magri et al. 2024: p. 124); they are an important component of the flora growing in the extreme habitats of the campos rupestris and also the Atlantic Forest inselbergs (see also below). In Barbacenia there are separate clades largely restricted to those vegetation types both of which started diversifying around 13-12 Ma (Cabral et al. 2021; rather old for such clades - Vasconcelos et al. 2020), the crown group age of the genus being ca 15.3 Ma. Interestingly, the campos rupestris clade is far more speciose and morphologically diverse, especially in floral characters, than the clade from Atlantic Forest inselbergs (Cabral et al. 2021). The crown age of the bulk of Vellozia is estimated to be (29.1-)22.5(-16.8) Ma (Magri et al. 2024, the small V. plicata clade not included), and the ancestral area of the genus included both the Andes and the Brazilian Atlantic rain forest areas. Temperatures at the beginning of this period were relatively high, but then they cooled, and this seems to have stimulated the diversification of the genus (Magri et al. 2024). Larocca et al. (2021) looked at the evolution of the diversity of growth forms and ways in which the plant survived the dry season in Barbacenia and Vellozia in particular; the evolution of growth forms seems to have preceded dry-season functional divergence. (Poikilohydry, and folding of the leaves on drying in particular, may be the plesiomorphic condition for the family as a whole - see also Porembski et al. 2021.) Much morpho-functional divergence in the two genera just mentioned may have occurred in the last 6-5 Ma or so, indeed, the ability to live in the harsh conditions of the rock outcrops of the campos rupestres are perhaps best seen as exaptions (Larocca et al. 2021).
Ecology & Physiology. African Velloziaceae include many dessication-tolerant taxa common on inselbergs and also a number of arborescent taxa (Porembski & Barthlott 2000; Farrant 2000: Xerophyta; Naidoo et al. 2009: Xerophyta viscosa, analysis of substances secreted on adaxial surface of the leaf). Indeed, Vellozioideae as a whole show extreme dessication tolerance, being poikilochlorophyllous (their chloroplasts ± break down) resurrection plants (Gaff & Oliver 2013). The physiology of these plants has been studied in detail using the octoploid African Xerophyta viscosa as as example (Farrant et al. 2015; M.-C. D, Costa et al. 2017), and this species i.a. has several clusters of dessication-associated genes, including late embryogenesis-abundant (LEA) genes, genes that are also involved in the expression of dessication tolerance in seeds (Costa et al. 2017; see also Oliver et al. 2005; Gaff & Oliver 2013; Artur et al. 2018; Pardo et al. 2019). For the dessication tolerance of X. humilis, see also Lyall et al. (2019). The dry leaves of Xerophyta may remain viable for more than two years; interestingly, the plant briefly has no dessication tolerance when it germinates (Costa et al. 2017). For more on dessication tolerace, see Porembski et al. (2021: mat forming species) and Larocca et al. (2022).
Alcantara et al. (2018) noted the importance of Velloziaceae in the origin of campos rupestris vegetation in Brazil in particular which has very phosphorus-poor soil over quartzite rocks; Vellozioideae may make up about one third of the cover in this vegetation. Vellozia, most speciose there, has aerial stems. Barbacenia develops water-holding parenchyma in the leaf; Teodoro et al. (2019) describe the vellozioid roots of Barbacenia with their very dense and long root hairs that are immediately behind the root apex - indeed, they look rather like dauciform roots. Carboxylates are produced, especially malates and citrates, and the roots can break down the quartzite, the plant taking up both phosphorus and manganese, the latter accumulating in the leaves (Teodoro et al. 2019).
Martins and Paiva (2016) looked at resin secretion and composition in Vellozia variabilis (see also Saldala-Castilho et al. 2016), noting that the resin had both flavonoid and terpenoid components and, perhaps paradoxically, protected the plant from serious fire damage by burning easily and quickly. The true stem is narrow and soon rots away, the "trunk" consisting of roots, each initially with a velamen, and persistent leaf bases (Porembski 2006).
Acanthochlamys bracteata is both a resurrection plant and also cold-tolerant; it lives in high valleys at 2,700–3,500 m altitude that are very warm and dry during the day and cold at night (Z.-Y. Gao et al. 2021). There were a substantial number of late Embryogenesis Abundant proteins in both Acanthochlamys and Xerophyta viscosa (for the latter, see above), and these are involved in responses to abiotic stress (Gao et al. 2021).
Plant-Animal Interactions. Meliponine bees (Trigonia, Tetragonisca) remove resin from the glands on the outside of the flowers of Barbacenia and Vellozia, although this seems to be of little importance for the plant (Sadala-Castilho et al. 2016).
Plant-Bacterial/Fungal Associations. There are endophytic bacteria in the glands on the outside of the flowers of Barbacenia and Vellozia (Sadala-Castilho et al. 2016).
Genes & Genomes. There appear to be Paleogene-Neogene genome duplications in stem Acanthochlamys and Xerophyta (T. Shi & He 2025). African Velloziaceae are all polyploid, the base number for the family perhaps being x = 6 (de Melo et al. 1997; M.-C. D. Costa et al. 2017). For the genome of Acanthochlamys bracteata see Z.-Y. Gao et al. (2021).
Vegetative Variation. The vegetative morphology and anatomy of Acanthochlamys are distinctive and rather difficult to understand. Its roots are tetrarch or triarch and lack pith, and so are unlike the roots of most other monocots (Cattai 2007), some Alismatales excepting, Triuridaceae, some Burmanniaceae, etc., also having non-medullated roots. The anatomy of the stem/rhizome of Acanthochlamys is unclear; it seems to consist of perhaps four vascular bundles in the centre, almost touching, and apparently with a small amount of non-vascular tissue in the middle (c.f. de Menezes et al. 2013). The anatomy of the scape has been described as "similar to that of a leaf ensheathing a rhizome" (Kao & Kubitzki 1998: p. 56; see also Gao 2017), although the central part of the stem is more like a root, not a rhizome and exactly how the stem bundles relate to the stele and to an inflorescence bract that they supply is unknown. Large amounts of sclereidal/fibrous tissue ("secondary phloem", "sieve cell": Gao 2017) underlie the epidermis and surround the central stele. There are apparently two pairs of leaves (Z.-D. Chen et al. 2016), and these leaves have a rather large basal adaxial ligule ensheathing the ?stem and lack axillary buds (de Menezes et al. 2013); the other genera have more simply sheathing bases. However, leaves are not shown as sheathing the rhizome by Gao (2017: fig. 1J[= 1L]), so what the ligule is ensheathing is unclear. There appear to be two U-shaped collateral vascular bundles surrounded by an endodermis in the centre of the leaf (de Menezes et al. 2013; Gao 2017).
Phloem in the "midrib" bundles of the leaves is sometimes arcuate in Vellozioideae, but in smaller bundles in particular it is broken up into two abaxial-lateral strands (L. B. Smith & Ayensu 1976). Amaral and Mello-Silva (2005, esp. 2008) suggest that the tetracytic stomata of Vellozioideae are better thought of as paracytic.
Chemistry, Morphology, etc.. The single flowers/inflorescences are apparently always terminal (Kubitzki 1998b), although this can be hard to make out; work on branching patterns would be useful. What is described here as the hypanthium in Velloziaceae may have the same indumentum as that on the pedicel; assuming the traditional definition of a hypanthium, the perianth members here would usually be more or less free. The stamens may be adnate to the tepals for most of their length, as in Barbaceniopsis. The corona develops late, and although partly associated with the bases of the filaments, its vasculature is in part tepal-like (Sajo et al. 2010: q.v. also for androecial development). In Acanthochlamys the anthers opposite the inner tepal members are inserted higher up on the tube than the anthers opposite the outer members. Sousa-Baena and de Menezes (2019) describe gynoecial and fruit morphology og Brazilian Velloziaceae. For the possibility of a chalazal haustorium in the embryo that develops from the antipodal cells or from chalazal nucellar tissue, see Sajo et al. (2013).
Additional information is taken from Ayensu (1973a), Kubitzki (1998b), and Behnke et al. (2000), all general, Williams et al. (1991: chemistry), Smith and Ayensu (1976: monograph - with anatomy - of New World taxa), Behnke et al. (20013: anatomy), de Menezes (1980: androecial evolution), Stenar (1925: embryology of Vellozia elegans, both layers of cells of inner integument palisade), Strassburg and de Menezes (2001: fruit and seed) and Sousa-Baena and de Menezes (2014: seed coat anatomy); for Acanthochlamys in particular, see also Kao and Kubitzki (1998: much detail), Kao (1989), de Menezes et al. (2013) and Gao (2017 and references) - Kao and Gao are one and the same.
Phylogeny. Talbotia (African) is mesophytic and its filaments are not adnate to the tepals; it is perhaps sister to the other Vellozioideae (but c.f. Behnke et al. 2000), however, basal relationships are unresolved. Barbacenia is paraphyletic, but Barbacenioideae are monophyletic (corona +; n = 7). Vellozioideae sensu Menezes are paraphyletic (Salatino et al. 2001), however, there is considerable disagreement between the topologies implicit in different morphology-based classifications of the family (Mello-Silva 2005), and that in Mello-Silva is only partly congruent with that of the molecular tree of Behnke et al. (2000, see also 2013). In a joint molecular and morphological study, Mello-Silva et al. (2011: see data matrix) found the relationships [Acanthochlamys [Xerophyta (inc. Talbotia) + the rest (support not strong)]] (see also Behnke et al. 2013: support much higher in Bayesian than ML analyses), and they suggested a number of apomorphies for the main clades, although optimisation of some of them is difficult. Relationships within the family were also not clearly resolved in the plastome and chondrome analyses of Soto Gomez et al. (2020), while those in Magri et al. (2024: 3 plastid markers, nuclear ITS) were [Barbacenia [Xerophyta [Barbaceniopsis + Vellozia]]]. Nevertheless, Acanthochlamys is clearly sister to the rest of the family (Behnke et al. 2000, 2013; Mello-Silva 2005; Wanga et al. 2021: plastome analysis).
For relationships within Xerophyta, see Behnke et al. (2013).
Classification. Although Acanthochlamys, sister to the rest of the family, is morphologically and anatomically distinct, subfamilial status is appropriate (Behnke et al. 2000; Mello-Silva 2005; c.f. Gao 2012, 2017). If a family, it would be monotypic, yet Velloziaceae s.l. are well characterized.
For suggestions about generic limits in the family, see Mello-Silva et al. (2011). The list above will have to be emended.
[Triuridaceae [Stemonaceae [Pandanaceae + Cyclanthaceae]]]: vessels also in stem, with scalariform perforation plates; (styloids +); flowers other than P3 + 3 A6 G3; G superior, septal nectaries 0; placentation parietal, stigmas/stigmatic branches ± separate.
Age. The age of this node is estimated to be ca 101.7 Ma (Magallón & Castillo (2009), (109.6-)99.3(-91.9) Ma (Eguchi & Tamura 2016), about 80.8 Ma (Tank et al. 2015: Table S2), (84-)76, 66(-50) Ma (Bell et al. 2010), (90-)81, 64(-52) Ma (Soto Gomez et al. 2020: Table 1 for other estimates), ca 77.5 Ma (Leal et al. 2022) or only ca 53.7 Ma (Magallón et al. 2015) or (71.1-)53.8(-39.7) Ma (D.-F. Xie et al. 2020).
The stem node of Triuridaceae has been dated to 86.3 Ma based on the Turonian New Jersey fossil Mabelia connatifila (Iles et al. 2015), however, this fossil may be best placed in Salicaceae - see López-Martínez et al. (2023a). Silvestro et al. (2021), also basing their ideas on fossils attributed to Triuridaceae, suggested that the time-of-origin of Triuridaceae was much earlier in the late Jurassic, ca 147.2 Ma.
TRIURIDACEAE Gardner, nom. cons. - Back to Pandanales
Plant mycoheterotrophic, echlorophyllous; chemistry?; roots in pairs from the axils of scale leaves; vessels 0; root stele not medullated; stem vascular bundles in a single ring, associated with sclerenchymatous ring; (endodermis +); crystals 0; plant glabrous; cuticular waxes as parallel series of platelets, within a series transversely arranged; stomata 0; leaves reduced, scale-like, (base sheathing), (closed); bracteole 0; T valvate, ?trace number; A connate or not, filaments stout; tapetal cells uninucleate; pollen grains tricellular, 15-25 µm across, inaperturate, surface gemmate,
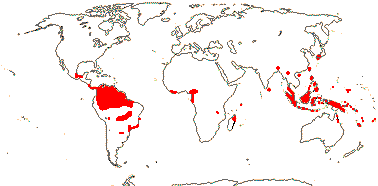
11 [list - in tribes]/50. Pantropical. Map: from van de Meerendonk (1984), Maas and Rübsamen (1986), Rübsamen-Weustenfeld (1991), Rudall et al. (2016: Triurideae in South America) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012).
Age. The age of crown-group Triuridaceae has been estimated to 90-50 Ma (Mennes et al. 2013).
Despite the delicate nature of plants in this family, fossil flowers of Triuridaceae (Mabelia, Nuhliantha) were reported from ca 90 Ma rocks in New Jersey (Gandolfo et al. 1998a, 2002). They were then the earliest monocot fossils known, however, that honour has now been taken by Araceae (see Friis et al. 2004), and anyhow their identification has been questioned on the grounds of pollen differences, the presence of an apical connective extension, etc. (Rudall 2003b; Friis et al. 2011; Nuraliev et al. 2020a; López-Martínez et al. 2023a).
1. Kupeaeae Cheek
Roots stout, radiating, root hairs 0; inflorescence a spike; plant dioecious; flower monosymmetric; T 4, (basally connate), with a single trace [Kupea], apex acute, (bifid); staminate flowers: (bract adnate to flower - Kupea); A 4, anther dehiscence transverse; pollen ca 25 µm across; carpellate flower: staminodes 0; style (sub)terminal, stigmatic zone indistinct; ovules 2/carpel, hemitropous to campyltotropous; fruit an achene, 2-seeded; ?endosperm.
2/3. Africa: Cameroon and Tanzania.
[Sciaphileae + Triurideae]: plant rhizomatous; staminate flowers: pistillodes usu. 0; carpellate flowers: staminodes 0; style ± lateral to basal; ovule 1/carpel.
2. Sciaphileae Miers
Rhizome slender, roots 1/2 from axils of scales, internodes long or rhizome stout, tuberous, with scales, roots ± radiating; roots diarch, not medullated, root hairs 0; stem a dictyostele; leaf trace single; inflorescence racemose/branched; plant monoecious, (flowers perfect), (bracteole 0); T 4, 6, 8, (basally connate), apex acute, with a tuft of hairs or not, (paired adaxial nectaries +); staminate flowers: A 2, 3 (+ 3 staminodes), 4, 6, anther dehiscence diagonal-transverse (vertical), (3-locular), (filament much shorter than the anther), (adaxial-basal staminal appendages +); tapetum amoeboid; pollen (monosulcate - Sciaphila), 24-40 µm across, (pistillode 0); carpellate flowers: (staminodes +); carpel development centripetal, spiral/whorled, style gynobasic; fruit a follicle (achene); seed with testal cells on chalazal side collapsed, or small, thickish walled, over rest of seed endotestal cells large and radially elongated, wall thickenings radial; plastome ca 12.8 kb, inverted repeat 0, ndh genes 0.
4/40: Sciaphila (30). Mostly Old World, esp. Indo Malesia and the Pacific, few New World. [Photo - Sciaphila].
3. Triurideae Miers —— Synonymy: Lacandoniaceae E. Martínes & Ramos
(Roots thin, radiating)/(moderately thick), root hairs few to none; plant usually dioecious; T 3, 6, (connnate basally), apex with caudate appendage (short); staminate flowers: A 3 (6, unithecal), (androphore +, anthers sessile), anther dehiscence longitudinal (horizontal); pollen 15-21 µm across; central appendage +; carpellate flowers: carpel development centrifugal, ± fasciculate, (style (sub)terminal); embryo sac tetrasporic, the three chalazal megaspores fuse, divide twice, 7-celled and 8-nucleate, the antipodals triploid [Fritillaria-type]; fruit an achene; endotestal cells with wall thickenings parallel to long axis of seed; endosperm pentaploid, initially with starch.
4/9. American Tropics, esp. the Guyanan area, scattered. [Photo - Triuris.]
Evolution: Divergence & Distribution. Mennes et al. (2013) found long branch lengths within Triuridaceae, and noted that these, along with the age of the family and its mycoheterotrophic life style, made its morphological distinctiveness comprehensible.
Rübsamen-Weustenfeld (1991) lists other characters, especially of endosperm, embryo size and seed anatomy, that separate Sciaphileae and Triurideae; some may turn out to be apomorphies, but Kupeaeae are poorly known.
The Mexican Lacandonia schismatica (a very similar species, L. brasiliana, was subsequently found in Brazil) has three stamens and these are borne inside the numerous carpels which are in turn borne on ridges in radial double rows (≡ fascicles), a situation almost unique in angiosperms - Hydatellaceae-Trithuria is the only other angiosperm with inside-out flowers (Rudall 2024). Although it has been suggested that these flowers may be pseudanthia (Rudall 2003b, see also 2010), heterotopy/homeosis may be a more likely explanation (Ambrose et al. 2006; esp. Álvarez-Buylla et al. 2010; Rudall et al. 2016; Baczynski & Claßen-Bockhoff 2023 for possible pseudanthia in the family). Interestingly, the carpels still develop after the stamens, despite their reversed positions (Garay-Arroyo et al. 2012), and Endress (2014) offered an ingenious suggestion that the relative position of the two is normal, only appearing to be abnormal because of the distortion at the floral apex caused by the development of numerous carpels (also see e.g. Araliaceae - Nuraliev et al. 2019). Indeed, the carpels of Peltophyllum luteum develop on ridges snaking up and down around the apex of the flower (Rudall et al. 2016; see also Sokoloff et al. 2007b). Interestingly, the recognition of Lacandonia may make Triuris paraphyletic (Vergara-Silva et al. 2003); if this relationship were to be confirmed (it was not by Mennes et al. 2013), the morphological context for an explanation of the distinctive floral construction of Lacandonia becomes very specific indeed.
Pollination Biology. Lacandonia can self pollinate: Pollen grains germinate while still in the anther and the tubes grow through the tissue of the flower to the ovules (Márquez-Guzmán et al. 1989; X.-F. Wang et al. 2011; Rudall et al. 2016). Pollination by small flies is likely in other members of the family (Rudall & Bateman 2006); see also Waterman et al. (2013).
Plant-Bacterial/Fungal Associations. Endomycorrhizae are of the Paris type (Imhoff 1998). Glomeromycote fungi are involved in mycoheterotrophy, and there can be very complex patterns of fungal colonization in the one plant (Hynson & Bruns 2010; Imhof et al. 2013); Sciaphila ledermannii in particular somewhat unusually associates with a variety of fungi including Acaulosporaceae, Gigasporaceae and Glomeraceae (Merckx et al. 2012). Imhof (2004) discussed the extensive variation in underground parts in the family. For other information, see Johow (1889) and Merckx et al. (2013a); mycoheterotrophy in general is discussed elsewhere.
Genes & Genomes. Timilsena et al. (2022b) looked at the nuclear genomes of Lacandonia schismatica and Triuris brevistyla in the course of their study of genome evolution in holomycoheterotrophs - for which, see elsewhere.
Lam et al. (2015 described the very small plastome of Sciaphila densiflora which has only 28 functional genes, however, it is largely colinear with that of Carludovica, over seven times its size. The plastome of S. thaidanica has a mere 20 genes and more than half of these have overlapping reading frames; both species lack a copy of the inverted repeat and have one or more novel inversions themselves (Petersen et al. 2018). For more on plastomes, see e.g. Lin et al. (2017: loss of ndh genes) and Soto Gomez et al. (2020).
Chemistry, Morphology, etc..The roots lack pith (von Guttenberg 1968) and the root stele is monarch or diarch - the entire stele from the endodermis inwards may be lignified (Johow 1889 and references).
Nuraliev et al. (2020a) described floral development in some Vietnamese Sciaphila in considerable detail, noting i.a. that both inflorescence morphology (branched/not; bracteole +/0) and floral orientation were variable, the outer tepals had late congenital fusion, the inner tepals early congenital fusion, and so on. The tiny staminate flowers of Kupea and Kihansia are quite strongly monosymmetric (Cheek 2003a; Rudall et al. 2007b), and Nuraliev et al. (2020a) noted that the flowers of Sciaphila could be quite monosymmetric during development. Endress (1995b) illustrated paired tepal nectaries in Seychelleria (= Sciaphila). Inter- and intraspecific variation in the number of parts of the flower is considerable (see Rübsamen-Weustenfeld 1991; Maas-van der Kamer 1995; Rudall et al. 2007b). In the perfect flowers of Lacandonia and carpellate flowers of Triuris individual carpel primordia develop from compound primordia, and in the former stamen and carpel primordia develop from a common precursor. Rudall (2008) suggested that the basic construction of the gynoecium in some Triuridaceae is fasciculate, the fascicles being radially elongated and developing centrifugally. There is considerable variation in gynoecial arrangement (whorled/spiral/intermediate, opposite the tepals or not - Nuraliev et al. 2020a) and much discussion as to the nature (plicate/ascidiate) of the carpel, and whether the ovules are attached to the receptacle or to the carpel (see also Rübsamen-Weustenfeld 1991; Nuraliev et al. 2020a and references). Hooker (1859) noted that the ovules were anatropous and unitegmic. Although some Triuridae have tetrasporic embryio sacs of the Fritillaria-type, ordinary monosporic Polygonum-type embryo sacs have also been reported here (Espinosa-Matiás et al. 2012). The strong asymmetry of the seeds, apparently restricted to some Sciaphileae (e.g. Wirz 1910, c.f. Batygina et al. 1990: interpretation difficult, plane of sections?), is reflected in their being more or less winged on one side.
For general information, see Tomlinson (1982: esp. anatomy), Rübsamen-Weustenfeld (1991), Maas-van der Kamer and Weustenfeld (1998), Franke et al. (2000: Triurideae), and Merckx et al. (2013a), also Imhof et al. (2013) for roots, mycorrhizae; for pollen, etc., see Furness et al. (2002a), for ovule and seed, see Johow (1889), and for carpels, etc., see Igersheim et al. (2001).
Phylogeny. In morphological analyses Kupea was sister to the rest of the family and Sciaphileae were paraphyletic (Rudall & Bateman 2006: Kihansia not included). The three tribes above were recovered as monophyletic in the four-gene study by Mennes et al. (2013), but inclusion of additional taxa with fewer than three genes suggested that Sciaphileae, and in particular Sciaphila, were paraphyletic, Sciaphileae and Triurideae together being sister to Kupeaeae (see also Mennes et al. 2015).
Classification. For tribes, I follow Rübsamen-Weustenfeld (1991) and Cheek (2003a).
Previous Relationships. Triuridaceae have often been associated with Alismataceae and relatives, all having separate carpels and so assumed to be related.
[Stemonaceae [Pandanaceae + Cyclanthaceae]]: ?
STEMONACEAE Caruel, nom. cons. - Back to Pandanales —— Synonymy: Croomiaceae Nakai, Pentastemonaceae Duyfjes, Roxburghiaceae Wallich
Plant monopodial, rhizomatous/root tubers/(juicy herb - Pentastemona [= P.]), stem (twining); (unspecified saponins - Stemona), dehydrotocopherols [Stemona, = S.], alkaloids with pyrrolo- or pyrido(1,2,-α)-azepine nucleus, panadanamine alkaloids +; root with cortical fibres; stem vascular bundles in 1 or 2 rings, those of inner or only ring amphivasal, (rhizome vascular tissue solid - Croomia); petiole bundles in arc; (styloids +); plant glabrous; stomata oriented transverse to long axis of leaf [S.]; leaves two-ranked/spiral/opposite, petiolate, base sheathing or not, petiole bifacial, midrib simple, +, (not distinct), cataphylls +, sheathing; inflorescences (epiphyllous), axillary, cymose/racemose/flowers single; pedicel articulated [?all], (flower monosymmetric - Croomia heterosepala); T 4(-5 - P.), (halfway connate - P.); A adnate to base of T, (thecae short, ± curved/horizontal over apex of filament, with dorsal gland), (connective apically expanded, petal-like); pollen (inaperturate), reticulate to perforate, intectate, (hyper-)scabrate); ?nectary; G [2] ([3]) to inferior, 1-celled, placentation apical/basal/parietal, style short, stigma punctate (broad, lobed); ovules 2 to many/carpel, outer integument to 5 cells across, inner integument to 3 cells across, parietal tissue (0?-2) cells across; fruit a capsule [?type]; funicle long/short, pseudofunicle +/short; seeds large [>5 mm long], longitudinally ridged, aril of uniseriate or vesicular hairs from hilum, raphe or micropyle (seeds many, small [1> mm long], not ridged, no aril - P.); testa multiplicative, several-layered, ridges many cells high, tanniniferous, inner 4 layers thick-walled, (testa ca 2 layers across, radial wall of exotesta thickened, forming the ridge - Pentastemona), (tegmen persists, cell walls thickened); endosperm copious, starchy, walls not pitted; n = 7, 9, 12, x = 8 (?7, ?9), nuclear genome [1C] (0.037-)0.791(-6.825) pg; seedling with non-photosynthetic cotyledon, primary root well developed.
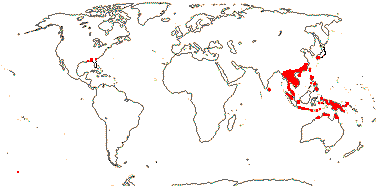
4 [list]/37. China and Japan to Australia, also S.E. U.S.A.(Croomia ): Stemona (23). Map: from Duyfjes (1993) and Fl. N. Am. vol. 26 (2002). Photo Croomia - Habit.
Age. Crown group Stemonaceae are dated to ca 84 Ma (Janssen & Bremer 2004) or ca 48.4 Ma (Leal et al. 2022).
The Late Barremian to Aptian Canrightia resinifera has been associated with Stemonaceae (López-Martínez et al. 2023a., but see also Chloranthaceae and Thismiaceae).
Evolution: Divergence & Distribution. The distinctive alkaloids found in Stemonaceae with their pyrrolo- or pyrido(1,2,-α)-azepine nucleus - there are over 200 of them (Pilli & Ferreira de Oliveira 2000; Greger 2019) - are unique to the family. Pandanamine alkaloids are known from both Stemonaceae (Stichoneuron calcicola) and Pandanaceae and may be intermediary in the synthesis of these Stemonaceae alkaloids (Greger et al. 2009).
Pollination Biology & Seed Dispersal. At least some Stemonaceae are pollen flowers (Vogel 1981). G. Chen et al. (2017b) looked at pollination in six Chinese Stemona species, of which five were ± purplish in colour and produced a variety of foetid-smelling compounds (there was infraspecific variation in both these features). A variety of flies were the main visitors; interestingly, earwigs ate pollen and stamens of S. tuberosa, consuming ca 20% of the flowers (Chen et al. 2017b).
Myrmecochory is common in the family (Lengyel et al. 2010). The seeds of Stemona tuberosa are reported to be first dispersed by hornets that are attracted by the arils, and then by ants after they are dropped by the hornets (G. Chen et al. 2017a).
Genes & Genomes. A genome duplication in Stemonaceae, the STTUα event, is dated to 56.3 Ma (Landis et al. 2018; see also T. Shi & He 2025: just before the K/P boundary). Aspects of the genome of Croomia pauflora were examined by Timilsena et al. (2022) in the course of a study of the evolution of mycoheterotrophy - see elsewhere.
Chemistry, Morphology, etc.. Duyfjes (1992) inadvertently suggested that Stemonaceae s. str. - i.e. not including Pentastemona - lacked cataphylls, however, these are well documented on the underground parts (e.g. Tomlinson & Ayensu 1968), while van Steenis (1982) noted that they were sheathing and compared this feature to the sheathing photosynthetic leaves of Pentastemona, suggesting an equivalence. Stemona, etc., do not have sheathing leaves.
In Stichoneuron and Pentastemona at least there seem to be other than hermaphroditic flowers (e.g. Najumdar & Datta 2013). Stemona phyllantha and Stichoneuron membranaceum both have epiphyllous inflorescences. The anatomy of the seed ridges varies considerably, while the length of the funicle and pseudofunicle (is what?) in the seed varies considerably (Inthachub et al. 2009).
Additional information is taken from Duyfjes (1991), Kubitzki (1998b) and Inthachub et al. (2009: Stichoneuron), all general, Brem et al. (2004: tocopherols), Kongkiatpaiboon et al. (2011) and Greger (2019), Stemona alkaloids, Ayensu (1972: anatomy), Swamy (1964b) and Bouman and Devente (1992), both ovules and seeds, Endress (1995b: flowers of Pentastemona), van der Ham ((1994) and Furness and Rudall (2000b), both pollen, and Rudall et al. (2005b: floral morphology).
The morphology of the inflorescence and the nature of the breeding system of Pentastemona need more study - also its anatomy, chemistry, etc..
Phylogeny. Pentastemona appears to be well embedded within the family (e.g. Jiang et al. 2006: molecular data, c.f. Rudall & Bateman 2006: morphological analysis), R. Lu et al. (2018: transcriptome analyses) recovering the relationships [[Stemona + Pentastemona] [Croomia + Stichoneuron]] (see also Q. Lu et al. 2018; Soto Gomez et al. 2020: plastome data). The distinctive features of Pentastemona are likely to be derived. However, Lam et al. (2015: plastome analyses) found the relationships [Pentastemona [Stemona [Croomia + Stichoneuron]]] (see also Soto Gomez et al. 2020: chondrome data; Leal et al. 2022: plastome and nuclear data).
Pentastemona: Succulent monopodial herb; cataphylls 0; roots fibrous; hairs uniseriate; stomata para- or tetracytic; leaves spiral, with compound midrib, sheath closed; inflorescences (branched) racemose; flowers 5-merous, perianth tube long to short; A 5, connate into a fleshy ring, basally adnate to T and connectives apically adnate to stigmas; pollen inaperturate, atectate; [G 3], inferior, placentation parietal, many ovules/carpel, stigmatic lobes well developed; fruit a berry; funicle short; seed arillate; sarcoexotesta +, endotesta with massive U-shaped thickenings, producing ridges; seedling?
[Pandanaceae + Cyclanthaceae]: stem vascular bundles compound; styloids +; stomata tetracytic, subsidiary cells with oblique cell divisions; leaves spiral; inflorescence racemose, a spike or head [a spadix], inflorescence bracts conspicuous, coloured or not; flowers dense, sessile, in spirals, small, imperfect; staminate flowers: stamens usu. several-many; pollen porate; pistillode +; carpellate flowers: staminodes +; style 0; ovules apotropous, with radiating subepidermal nucellar/chalazal cells; fruit an indehiscent syncarp [baccate or drupaceous]; endotesta well developed, internally to that are two persistent cuticular layers; cotyledon not photosynthetic, seedling with all internodes ± elongated.
Age. The divergence between the two families has been dated to (74-)68, 66(-60) Ma (Wikström et al. 2001), ca 98 Ma (Janssen & Bremer 2004), (71-)52, 47(-31) Ma (Bell et al. 2010), (139.3-)86, 81(-49) Ma (Gallaher et al. 2014), (71-)61, 56(-49) Ma (Soto Gomez et al. 2020: Table 1 for other estimates) and (79.3-)62.8(-50.4) Ma (Leal et al. 2022).
Evolution: Genes & Genomes. Soto Gomez et al. (2020) discuss differences in the rates of evolution of the three genomes; there is substantial variation in rate in the chondrome within both families.
Chemistry, Morphology, etc.. The compound nature of the stem vascular bundles is sometimes visible under a hand lens; the vascular bundles have two or more groups of vessels at opposite ends or the periphery of the bundles with smaller cells in between. See French and Tomlinson (1986) for these compound bundles; they differ somewhat in the two families. See also Carlquist (2012a) for xylem anatomy.
Previous relationships. Spadiciflorae, a group that has been recognised in the past, included those taxa with a spadix and often also a spathe, i.e. Pandanaceae, Cyclanthaceae, Araceae and Arecaceae. These families are here placed in three orders, Pandanales (the first two families), Alismatales and Arecales. Engler (1892) linked Pandanaceae, Arecaceae and Cyclanthaceae.
PANDANACEAE R. Brown, nom. cons. - Back to Pandanales —— Synonymy: Freycinetiaceae Le Maout & Decaisne
Plant woody, trees, shrubs, climbers with roots from leaf axils [Freycinetia - F.], (epiphytes), rhizomes 0 (+); pyrrolidine, indolizidine, piperidine alkaloids + {Pandanus - P.]; stilt roots common; vascular bundles compound, individual bundles separating and re-associating, vessel elements also in leaf; sieve tube plastids also with peripheral fibres; (cuticular waxes as aggregated rodlets), (hairs stellate); leaves (amphistomatic), spirally three-ranked, (4-ranked, ?decussate), vernation conduplicate-flat, ± V or M-shaped when mature, margins spiny, (sheaths closed; base auriculate - F.); plant dioecious (monoecious); inflorescence (branched, paniculate, flowers (?single - Sararanga-S.); (pedicel + - S.); T 0 (connate as cupule - S./6 primordia - F.); staminate flowers: A 1-many, variously free to connate or aggregated [on underside of peltate structure], filaments papillate or not; pistillode 0; pollen exine three-layered [level?]; (pistillode 0); carpellate flowers: inflorescence capitate, (staminodes 0); G 1-many, free / in fascicles [= polydrupes], (connate, placentation parietal - F.), intra-ovarian trichomes +, stigmas separate, = loculus number; ovule 1/carpel (many - F.), apotropous, (micropyle bistomal), parietal tissue 1-5 cells across, (nucellar cap ca 2 cells across), obturator and hypostase +; (embryo sac incorporating nucellar cells at chalazal end - P.), (bisporic - P.); fruit baccate or drupaceous, differentiated into proximal [= locular, basal] and distal parts; seeds inseparable from endocarp {P.], free [F.], seed coat thin, (exotesta sclereidal, some cells spiny, groups of sclereidal cells at both ends - S.); (endosperm starchy); n = 25, 28, 30; x = 30 (?16, ?18), nuclear genome [1C] (0.043-)0.725(-12.361) pg; hypocotyl long [F.], primary root branched [P.].
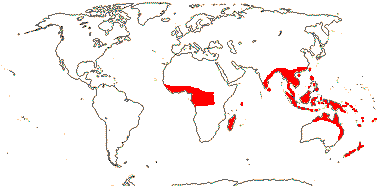
5 [list]/(700) 840 (982): Pandanus (450/605), Freycinetia (180), Benstonea (60). W. Africa to the Pacific, inc. Hawai'i. Map: see Heywood (1978: Africa) and Callmander et al. (2003). [Photos - Staminate Flower, Carpellate Flower.]
Age. Crown group Pandanaceae began to diverge ca 49 Ma (Buerki et al. 2017), ca 51 Ma (Janssen & Bremer 2004), (101-)65, 58(-38.3) Ma (Gallaher et al. 2014), 95 Ma or more (Callmander et al. 2003), (51-)35, 26(-20) Ma (Soto Gomez et al. 2020: Table 1 for other estimates), ca 31.4 Ma (Leal et al. 2022) and (59.7-)48.5(-41.9) Ma (Wojahn et al. 2025).
The pollen genus Pandaniidites is known from North America where it spans the Cretaceous-Caenozoic boundary in rocks up to 70 Ma (Hotton et al. 1994). However, such pollen has been found associated with flowers of Limnobiophyllum and it is to be assigned to Araceae-Lemnoideae (Stockey et al. 1997; Stockey 2006), not Pandanaceae. Fossil leaves, Pandanites can perhaps be assigned to the family, they may be as much as 83.6 Ma in Europe, somewhat younger, but still Cretaceous, in North America (e.g. see Kvacek & Herman 2004; Rozefelds et al. 2022); fruits, Gruenbachia, have been associated with the European fossils (Herman & Kvacek 2010). Gallaher et al. (2014) are sceptical of the identity of most other fossils attributed to the family. Recently, Rozefelds et al. (2020) described Pandanus estellae in volcanic deposits from Queensland perhaps 32-28 Ma; these fossils, rather small for the genus, are moulds and completely lack internal detail.
Evolution: Divergence & Distribution. Callmander et al. (2003) discussed the biogeography of the family in the context of the break-up of Gondwana, which sets up rather discordant estimates for the age of the family (see above). However, Gallaher et al. (2014) revised age estimates in Pandanaceae and suggested that dispersal was more likely to explain what appear to be the Gondwanan elements of current distributions here, indeed, given the Laurasian fossils, they thought that Pandanaceae might even have originated there. Rozefelds et al. (2022) also talk about "a clear Gondwanan history" (ibid., p. 327) for Pandanus based on the fossils they found (see above). Confirmation of the identity of these fossils is needed.
Bobrov et al. (2024) looked at variation of fruit morphogenesis in Pandanaceae with a focus on Freycinetia, although whether berry-type fruits evolved into druopaceous-type fruits or vice versa was, they thought, unclear. The development of staminate and carpellate flowers seems to be quite labile in Freycinetia, and sex changes of plants have been recorded (Huynh 1992 and literature; Cox 1990 for breeding systems).
Diversification within the speciose Pandanus itself started a mere (20.2-)12.9, 11.5(-5.4) Ma, and water dispersal in particular is discussed as facilitating its current wide distribution, with the P. tectorius complex achieving the range of almost the entire family within the last 6 Ma (Gallaher et al. 2014). Buerki et al. (2017) discuss the evolution of Benstonea which may have begun on Borneo.
Ecology & Physiology. Freycinetia makes up a sizeable clade of Old World woody root-climbers. A number of species of Pandanus are trees, sometimes moderate in size, and they have massive unbranched "adventitious" prop roots. In P. forsteri, from Lord Howe Island, it has been sugested that the gutter-like leaves and grooves on the roots channel water to the tips of the growing roots before they reach the ground; there the water is absorbed by what has been called a velamen radicum, swollen tissue at the very apex of the root (Biddick et al. 2018). Similar tissue, perhaps representing proliferating/exfoliating root cap, is found in other species of the genus, so this method of taking up water may be more widespread. Members of the family like Benstonea may collect litter in their leaf rosettes, and the litter decomposes, the plant then taking up nutrients from it, indeed, the habit of many members of the family is effectively a branched Schopfbaum (Zona & Christenhusz 2015).
Pollination Biology & Seed Dispersal. Bat pollination may be quite common in Pandanaceae (Fleming et al. 2009), and there is also pollination by birds and other animals like squirrels, bats and birds feeding on the inflorescence bracts of Freycinetia (Marshall 1985; Cox 1990; Baczynski & Claßen-Bockhoff 2023 for pseudanthia here). At least some species of Pandanus are wind pollinated (and apomictic) (Cox 1990). There is pollination by beetles of the Microporum group (Meligethinae--Nitidulidae), the larvae of which eat pollen, especially in the western Indian Ocean islands (M. Liu et al. 2025). Note, however, that flowering may be an occasional event, furthermore, in Peneinsular Malaysia plants with staminate inflorescences have been collected far less often (16%!) than those with carpellate inflorescences (Beentje & Callmander 2023).
For diaspore dispersal in Pandanaceae, predominantly by water or animals, see Gallaher et al. (2014).
Plant-Animal Interactions. Phasmids (stick insects) like Megacrania seem to be associated with Pandanus spp. growing close to the sea, and even have eggs that may be dispersed by the sea (O'Hanlon et al. 2020 and references).
Genes & Genomes. T. Shi and He (2025) suggest that that there was a genome duplication here just before the K/P boundary.
Chemistry, Morphology, etc.. For alkaloids in Pandanaceae, including pandanamine, also to be found in Stemonaceae, see Tan and Takayama (2019 and references). Although the leaves of Sararanga in branches developing after the first inflorescence has been produced are described as being two ranked, both North and Willis (1971) and Cox (1990) illustrate them as being decussate, Cox (1990: p. 821) noting "each successive node produc[es] a pair of opposite leaves".
Since there is usually no perianth, the position of the ovary is difficult to ascertain, but it is clearly superior in some species of Freycinetia (e.g. Huynh 1991, 1992) at least. Cox (1990) shows staminate flowers of F. arborea as having bracts and six perianth primordia, while North and Willis (1971) described both staminate and carpellate flowers of Sararanga as having an entire perianth or cupule (Cox 1990 is less sanguine about its morphological nature). The carpels vary from free to variously connate and fasciate, and they may face ad- or abaxially (c.f. Cercidiphyllum), sometimes within a single floral unit (Stone 1968, see also Bobrov et al. 2025). Tobe et al. (2018) suggest that Pandanaceae have septal nectaries. The stigmas of Sararanga are sessile and in two sinuous lines, and North and Willis (1971) described the fruits as being polypyrenoid drupes, and they also talked about flattened seeds with a one-layered sclereidal testa. Zdravchev et al. (2023) thought that the floral construction of Pandanus was unclear, various interpretations being possible, and this does seem to be a reasonable summary of our current knowledge. Bobrov et al. (2025) suggested that the phalanges of P. utilis might be made up of monomerous apocarpous fruits, while P. odorifer might have an inflorescence with spirally-arranged monocarpellate flowers or flowers with a coenocarpous spiral gynoecium - so the phalanges in the one genus would be interpreted as being of quite different origins...
Fagerlind (1940a) suggested that Pandanus did not always have a bisporic embryo sac. The embryo sac of Pandanus appears to have extra antipodal cells, however, these come from the nucellus (see Maheshwari 1955 for discussion).
Some information is taken from Dahlgren et al. (1985), Stone et al. (1998) and Beentje and Callmander (2023), all general, see also Zimmermann et al. (1974: vascular organization in the stem), Strömberg (1956) and Cheah and Stone (1975), both embryology, Hotton et al. (1994: pollen), and Huynh (2001: Sararanga).
Phylogeny. For early studies on the phylogeny of Pandanaceae, see Stevenson and Loconte (1995) and Cox et al. (1995). Callmander et al. (2003) did not show the full tree they obtained, albeit it was apparently poorly resolved. In a tree with more limited taxonomic but greater gene sampling, relationships were [Pandanus [Martellidendron, Freycinetia, Sararanga]] (Callmander et al. 2003). Buerki et al. (2012b: sampling extensive) found the well-supported set of relationships [Sararanga [Freycinetia [Acrostigma clade (later = Benstonea), core Pandanus, Martellidendron]], and these were similar in Gallaher et al. (2014), although there was little support for Benstonea as sister to Pandanus in the chloroplast data alone; monophyletic subgenera were not recovered. Buerki et al. (2017) found the relationships [Saraanga [Freycinetia [Martellidendron [Benstonea + Pandanus]]]], with moderate to good support. However, Nadaf and Zanan (2012: Indian species the focus) found the relationships [[Benstonea + Pandanus] [Sararanga [Freycinetia + Martellidendron]]]. Wojahn et al. (2025: plastomes of 50 spp.) looked at relationships in Pandanus; just one (of the seven) subgenera was monophyletic (see also Buerki et al. 2012b). However, Wojahn et al. (2025) found a number of features correlating with the clades that they were picking up, and these included extrinsic features such as geography, climate and soil type, also intrinsic features such as androecial variation (anther shape, nature of the androphore (= stemenophore)) and presence of hypertrophied hydrenchyma (water storage tissue).
Classification. Stone (1974) recognized 8 genera and 61 sections in Pandanus. Callmander et al. (2003, 2012) split the genus somewhat, and about 7 subgenera and 81 sections are recognized in Pandanus as of iv.2025 (see Wojahn et al. 2025); the sections in particular can often be identified only with fertile material, sometimes pistillate alone.
Botanical Trivia. Gill and Tomlinson (1975) note that the apices of aerial roots of Pandanus - no secondary thickening - may be up to 11 cm in diameter.
CYCLANTHACEAE A. Richard, nom. cons. - Back to Pandanales
Large herbs; vascular tracheids or vessels [?type] in root and leaf; petiole bundles scattered; mucilage cells +; leaves spirally two-ranked, petiolate, blade vernation plicate or variants, bi-or tricostate, veins extend to the end of the leaf, Vorläuferspitze 0 [in elaborated leaves], petiole +. ± terete, sheath open; plant monoecious; inflorescence (branched), spadiciform; flowers with mucilage canals; staminate flowers: A connate basally; pollen lacking endexine lamellae; carpellate flowers: ovules many/carpel, (epitropous), micropyle bistomal, outer integument 3-5 cells across, parietal tissue none, nucellar epidermal cells radially elongated, hypostase + (0), (postament +); endosperm helobial, embryo short; x = 9 (?8), nuclear genome [1C] (0.054-)0.93(-15.967) pg; seedling with collar rhizoids.
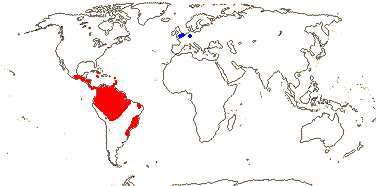
12 [list]/230: Two subfamilies below. Central and tropical South America (map: from Harling 1958; fossil Cyclanthus [blue] from S. Y. Smith et al. 2008).
Age. Crown group Cyclanthaceae are dated to (55-)49, 45(-39) Ma (Wikström et al. 2001), ca 77 Ma (Janssen & Bremer 2004), (50-)33, 30(-14) Ma (Bell et al. 2010), (72.7-)45, 42.3(-22.9) Ma (Gallaher et al. 2014), (55-)49, 49(-47) Ma (Soto Gomez et al. 2020: Table 1 for other estimates) and (59.0-)50.6(-47.0) Ma (Leal et al. 2022).
Fossils identified as Cyclanthaceae (as Cyclanthodendron) have been found in deposits in the Deccan Traps aged around 67-65 Ma (Kapgate 2013; see also S. Y. Smith et al. 2008).
1. Cyclanthoideae Burnett - Cyclanthus bipartitus A. Richard
(Growth monopodial); root with multiseriate circum-cortical sclerenchymatous tissue; subepidermal sclereids +; druses +, mucilage cells in inflorescence only, tanniniferous cells 0, non-articulated laticifers +; lysigenous air spaces with transverse septae +; leaf blade (very) deeply bifid [by tearing of tissues], otherwise entire, bicostate, vernation modified plicate [plicate across principal veins]; inflorescence with whorls of staminate and carpellate "flowers", flowers coalescent; staminate flowers: P 0; A in 4 rows per whorl; carpellate flowers: P +, small; staminodes +; G, ovary cavity with closely-set placentae; outer integument ca 2 cells across, inner integument ca 3 cells across, nucellar cap 0, funicles long [longer than embryo sac]; embryo sac with chalazal prolongation, fruit dry, syncarpous, carpellary annulus sliding off the inflorescence axis and splitting down the middle; seeds ridged, embedded in mucilage; endotestal cells palisade, inner periclinal walls granular; n = 9.
1/1(?2). Central and N. South America, the Lesser Antilles. [Photo - Flower.]
Age. There are fossils of Cyclanthus from Eocene sediments ca 47 My in Europe (Germany - type; also southern England); the seeds had previously been misidentified as Scirpus (S. Y. Smith et al. 2008; see also Collinson et al. 2012; Smith 2013; Iles et al. 2015). If correctly identified, this questions some of the ages that have been suggested for the family.
2. Carludovicoideae Harling
Also epiphytes, and/or lianes and vines; root (with multiseriate circum-cortical sclerenchymatous tissue - Schultesiophytum), (not medullated, centre portion occupied by intermixed xylem and phloem); (stem cork subepidermal or outer cortical); (styloids +); mucilage and tanniniferous cells +; leaves (spiral), blade ± bilolobed or 3-partite, 2-3-costate [lateral costae short], apex usu. deeply dissected, (blade entire, 1-costate), (hastula +); inflorescences (axillary), pistillate flowers surrounded by four staminate flowers; staminate flowers: (monosymmetric); P (0-)10-many, small [?= staminodia], glandular abaxially; A ca 10-many, filaments swollen basally; carpellate flowers: connate basally, 4(-8)-merous; staminodes long-filiform, opposite P; G 4, ± inferior, alternating with P, (placentae apical); outer integument ca 3 cells across, inner integument ca 2 cells across, nucellar cap 2-3 cells across; fruit baccate, syncarpous or not, apically circumscissile or with other unusual methods of opening; (seeds exotegmic); (endosperm starchy); n = 9, 15, 16.
11/229: Asplundia (100), Dicranopygium (50), Sphaeradenia (50). Central and tropical South America, the Greater Antilles. [Photo - Flower, Fruit.]
Age. An estimate for the age of crown-group Carludovicoideae is (46.5-)35.9(-25.0) Ma (Leal et al. 2022).
Evolution: Divergence & Distribution. The discovery of Cyclanthus in the European Eocene (S. Y. Smith et al. 2008; see also Collinson et al. 2012; S. Y. Smith 2013) changes how one thinks of the evolution and distribution of the family. Cyclanthus is common there and may even be a diagnostic element of the vegetation - along with Nypa - neither now growing within thousands of miles of the fossil sites. Leal et al. (2022) suggested that Cyclanthaceae may have moved from the Old to the New Worlds by a boreo-tropical migration route and then moved to South America ca 40 Ma, and Leal et al. look at other biogeographic possibilities in the family. Fossil Cyclanthaceae are also reported from the Deccan Traps, India (see above), so present and past distributions seem to have little to do with each other.
Cyclanthus bipartitus is one of the five angiosperms with the greatest floral eccentricities (divergence from the average: López-Martínez et al. 2023b).
Pollination Biology & Seed Dispersal. Carludovicia is associated with derelomine flower weevils the adults of which pollinate the flowers while the larvae eat the developing seeds - a brood-site pollination mutualism (Franz 2004). Indeed, weevils are commonly found on inflorescences of Carludovicioideae, different species of the latter having differet major scent compounds, and weevil larvae may eat the staminate flowers, as in Evodianthus (Teichert et al. 2018; Haran et al. 2023). Thermogenesis is reported from flowers of Carludovicioideae (common in weevil-pollinated flowers), and the long staminodes produce scent (Eriksson 1994a). Cyclanthus is pollinated by cyclocephaline scarabeid beetles (Eriksson 1994a; Moore & Jameson 2013).
The fruits of Carludovicioideae are baccate, syncarpous or not, and are apically circumscissile or have other unusual methods of opening. For example, the subfleshy infructescences of genera like Carludovica open irregularly from the apex as the outer part of the infructescence (the apical parts of the connate gynoecia) recurves and pulls away, exposing the brightly-coloured interior. Dispersal is by animals.
Chemistry, Morphology, etc.. Leaf development is rather like that of broad-leaved angiosperms, a Vorläuferspitze was evident only in leaves of Carludovica palmata developing early in the ontogenetic sequence (Wilder 1976, 1981a). Leaf insertion can change from spiral to two-ranked or two-ranked to spiral during the growth of the plant (Wilder 1981a). Further information on the morphology and vernation of leaves is given by Wilder (1981b) and on growth habit by Wilder (1992b).
In staminate flowers, both perianth and stamen number may increase. There are varying interpretations of the staminate flowers of P in one (two) whorls, 6+ (-30), with abaxial glands, or P 0, staminodia abaxial, glandular (Sajo et al. 2014). The gynoecium/carpellate flowers of Cyclanthus are shown as having the gynoecium immediately surrounded by staminodes and then by perianth lobes; stamens/staminate flowers alternate with the gynoecium (Sajo et al. 2014). There is considerable variation in pollen morphology and seed morphology and anatomy; Furness and Rudall (2006) suggest that the pollen grains of Cyclanthaceae, alone in the order, lack endexine lamellae.
For much information on just about everything, see Harling (1958) and Harling et al. (1998), for anatomy, see French et al. (1983: stem), Tomlinson and Wilder (1984: general) and Wilder (1986: leaves, 1992a: roots and references), and for embryology, see Harling (1946).
Phylogeny. Cyclanthus is always recovered as sister to the rest of the family. For a detailed morphological phylogeny of Cyclanthaceae, see Eriksson (1994b). Soto Gomez et al. (2020), analyzing both chloroplast and mitochondrial data in a variety of ways, found a certain amount of variation in relationships within Carludovicioideae, but usually [Carludovicia + Schultesiophytum] were sister to the rest of the subfamily. Leal et al. (2022: 99 spp., all genera, 2 nuclear and 5 chloroplast markers) recovered the relationships [Carludovicia [[Schultesiophytum [Dicranoptygium + Asplundia]] [rest of he family]]] in some analyses, rather different relationships in a concatenated maximum likelihood analysis, but with little support; infrageneric relationships also need examination.