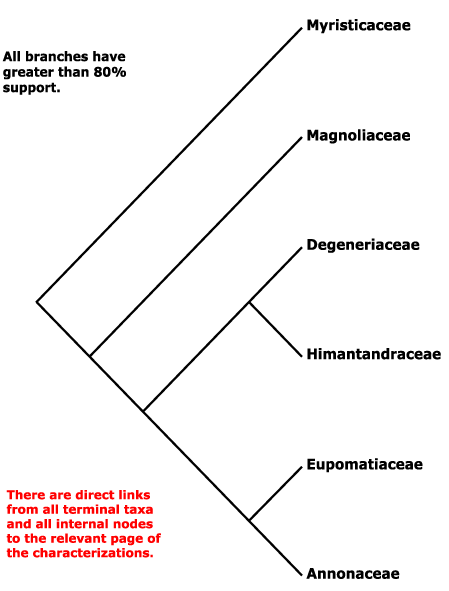
EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[MONOCOTS [MAGNOLIIDS + EUDICOTS]]: Age: (210.9-)176.1(-154.4) Ma (P.-L. Liu et al. 2020).
[MAGNOLIIDS + EUDICOTS]: Age: (185.8-)160.6(-143.9) Ma (P.-L. Liu et al. 2020).
Mag, Mono, Eudicot: 189-142 Ma (Jiao &am; Wang 2022)
[CHLORANTHALES [[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]]]: sesquiterpenes +; (microsporogenesis also simultaneous); seed endotestal.
[[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]] / MAGNOLIIDS / MAGNOLIANAE Takhtajan: (neolignans +); root cap meristem open; vessels solitary and in radial multiples, (with simple perforation plates in primary xylem); (sieve tube plastids with polygonal protein crystals); stomata paracytic; lamina margins entire; A many, spiral [possible position here], extrorse; ovules with hypostase, nucellar cap +, raphal bundle branches at the chalaza; antipodal cells soon die. - Back to Main Tree
4 orders, 20 families, 9,900 species.
Age. Bell et al. (2010: note topology) suggested ages of (138-)122(-108) or (130-)125(-121) Ma for this clade depending on the method used. Magallón and Castillo (2009) variously estimated crown group divergence at ca 201.7-198.2 and 128-127.7 Ma, while ca 149 Ma is the estimate in Foster et al. (2016a, q.v. for details). Other estimates range from around 133.1 Ma (Naumann et al. 2013), (144-)116(-89) Ma (N. Zhang et al. 2012), (152.7-)117.3(-39.1) Ma (B. Xue et al. 2012), around 146 Ma (Tank et al. 2015: Table S1), or (155-)149, 137(-131) Ma (Wikstöm et al. 2001). Z. Wu et al. (2014) estimated an age of about 186 Ma, while (178.8-)174.6, 127.1(-126.8) and 150-119 Ma are the ranges of ages in Massoni et al. (2015a) and Morris et al. (2018) respectively, 171-115 Ma in Foster and Ho (2017), and (208-)181(-156) Ma in Salomo et al. (2017). H.-T. Li et al. (2019) estimate an age of (155-)144(-136) Ma (ages in Moore et al. 2007; S. A. Smith et al. 2010; Magallón et al. 2013, 2015; Zanne et al. 2014; Zeng et al. 2014; Beaulieu et al. 2015; Foster et al. 2017; Barba-Montoya et al. 2018 - see H.-T. Li et al. 2019: table 2 - are (195-)122(-108) Ma). Other recent estimates are 187-175 Ma (L. Hu et al. 2019), 170.7-147.1 Ma (L. Yang et al. 2020), about 117.0 Ma (X. Guo et al. 2021), 134-118 Ma (Jiao & Wang 2022) and (211.1-)207.9(-203.3) Ma (Y. Xiang et al. 2024).
An earlier fossil-based estimate for both stem and crown divergence is ca 98 Ma (Crepet et al. 2004: magnoliids sister to monocots). For summaries of the fossil history of the group, especially prominent in the mid Cretaceous and later, see Friis et al. (1997, 2006, 2011).
Evolution: Divergence & Distribution. Lesqueria, about 101 Ma, may belong somewhere around the magnoliids; it has similar fruits (Crane & Dilcher 1984), although the carpels are in some ways like those of Austrobaileya; there is a similar pattern of relationships inEarly Cretaceous apocarpous flowers from E. North America and Portugal (Friis et al. 2020b). The fruits of Protomonimia, in Turonian deposits from Japan ca 91 Ma, have several carpels borne in spirals on a concave axis; there is a stigmatic crest. The young seeds have a thick testa, the exotesta being palisade and with sinuous anticlinal cell walls (Nishida & Nishida 1988); they may belong to a taxon somewhere near Magnoliaceae, although i.a. the shape of the receptacle is very different (see also Laurales). Santaniella, from the Crato Formation in northeast Brazil and initially identified as a ranunculid (Gobo et al. 2022), may rather be a magnoliid, but attempts to identify it more precisely suggest the problems involved (Pessoa et al. 2023). For more discussion/fossils, see Friis et al. (2011) and Doyle (2014b).
For details of diversification within the whole clade, see Massoni et al. (2015a), the five main shifts in diversification, two up and three down, are mentioned under Magnoliales, Laurales and Piperales), although what might have caused these shifts is unclear.
For stomatal morphology and evolution in magnoliids, see Rudall (2023a). Sauquet et al. (2017) discuss the nature of the ancestral magnoliid flower; it is similar to that of the angiosperms as a whole other than in anther dehiscence (see also above).
Ecology & Physiology. This clade has distinctively large leaves (Cornwell et al. 2014), and magnoliids are generally plants of well watered, warm, and equable conditions. Venation density is at least 1 dm/mm2 (Brodribb & Feild 2010; Crifò et al. 2014), however, given the uncertainty of relationships around here, it is unclear where to put changes in this character on the tree. Carlucci et al. (2016) suggest that phylogenetic overdispersion in western Amazonian tree communities near the Andes was partly because magnoliids with conserved habitat preferences - they thought that these were for upland, shady and wet habitats (see also Feild & Arens 2007) - grew there, along with monocots and recently-diversifying eudicots. Of course Myristicaceae and many Annonaceae are plants of lowland rainforests.
Many extant magnoliids are large trees, interestingly, their litter decomposability and associated nutrient turnover tends to be at the slow end of the spectrum, Piperaceae being a notable exception (G. Liu et al. 2014).
Plant-Animal Interactions. Herbivory in magnoliids is relatively high (Turcotte et al. 2014: see caveats, Chloranthaceae not included). Caterpillars of Papilionidae-Papilioninae butterflies are notably common (almost 33% of the records) on members of this group; they are, however, not known on Myristicaceae, for exanple, in Laurales they predominate on Lauraceae, and in Piperales two clades in particular are found on Aristolochiaceae (see Scriber et al. 1995 for references; Zakharov et al. 2004; Simonsen et al. 2011; Condamine et al. 2011). Crown-group Papilioninae (paraphyletic, including Parnassinae) stated diversifying at the K/P boundary ca 66 Ma (Espeland et al. 2018). For more on swallowtails in general, see Aristolochiaceae.
Chemistry, Morphology, etc.. Hegnauer (1990) discussed the chemistry of the Polycarpicae, which in addition to this clade also includes Austrobaileyales and Ranunculales; similar isoflavonoids are found in Magnoliaceae, Lauraceae and Chloranthaceae.
For a convenient summary of a number of features of wood anatomy, see Herendeen et al. (1999b). Magnoliid roots are scattered through the clade, being recorded from a number of woody members of Laurales and Magnoliales. These roots are stout, often ³3 mm across, lack root hairs (?always), and are endomycorrhizal - associated conditions (see Baylis 1975; St John 1980). Other magnoliids, members of the ANA grade and Pinales other than Pinaceae also have rather stout roots, and of course nearly all are woody, and magnoliid roots also have high nitrogen concentrations, etc. (Valverde-Barrantes et al. 2017). Rudall (2023a) discussed stomatal morphology and development in the magnoliids. Taxa with broadly lobed leaf blades are scattered throughout this clade (they are absent in Canellales, although this is a small order).
See Erbar (1983, inc. Illicium) for carpel morphology and Ronse Decraene and Smets (1992b) for androecial morphology.
Phylogeny. The sister group relationship of Piperales with Canellales in particular is at first sight unexpected, but the composition of the magnoliid clade and the relationships within the group are turning out to be quite robust (Massoni et al. 2014: good generic sampling, 12 markers from three compartments). There was not much morphological support for this grouping (Doyle & Endress 2000), features like tectum structure, etc., showing considerable variation (J. A. Doyle 2005), and Piperales in particular tended to link with other groups. Hilu et al. (2003: matK analysis) found the poorly supported relationships [Piperales [Canellales [Magnoliales + Laurales]]]. However, molecular support for the topology below is usually stronger in more recent studies (e.g. Qiu et al. 1999, 2000, 2005: support levels depend on the analysis, the node sometimes collapses], 2006b, 2010; Zanis et al. 2002; Jansen et al. 2006b; Zhengqiu et al. 2006; Cai et al. 2006; Müller et al. 2006: support for [Canellales + Piperales] poor; Jansen et al. 2007: little maximum parsimony support; M. J. Moore et al. 2007: group not evident in all analyses; Soltis et al. 2007a; Soltis et al. 2011; Foster et al. 2016a: q.v. for details, support rather poor). Support includes the possession of unique indels (Löhne & Borsch 2005). Soltis et al. (2007a) found weak support for a grouping [Magnoliales + Canellales]; relationships in Bell et al. (2010) are [Piperales [Laurales [Canellales + Magnoliales]]], although they, too, have little support. For relationships in this clade, [[Piperales + Canellales] [Magnoliales + Laurales]], see the Angiosperms353 probe set analysis by Helmstetter et al. (2024: 199 genera, ca 3/4 the total); there were topological differences between trees derived from coalescent analyses and those derived from concatenation analyses.
Classification. Helmstetter et al. (2024) provide a classification of the entire Magnoliidae which is largely followed below except that Aristolochiaceae here include both Lactoridaceae and Asaraceae – see also comments under Aristolochiaceae - things do seem a bit fluis around here. Notable novelties compared with earlier versions of this site are a Hydnoraceae that are sister to rest of Piperales, a tribal classification for Myristicaceae and some tribal adjustments in Annonaceae.
Chloranthales, Ceratophyllales and monocots are the other clades immediately basal to the eudicots and all have somewhat uncertain positions. For a discussion about the relationships of these groups, see Mesangiospermae below.
[MAGNOLIALES + LAURALES]: epicuticular waxes as annularly-ridged rodlets, palmitol the main wax; A whorled; pollen 1-2 nexine foliations, outer member massive, lamellate endexine; (supra-stylar extra-gynoecial compitum/pollen tube growth); carpel cross-zone initiated late; ovules 1(-2)/carpel, basal, erect, apotropous; fruitlets 1-seeded; palaeopolyploidization event.
Age. Magallón and Castillo (2009) suggest that the two clades diverged ca 198.2 and 127.7 Ma - relaxed and constrained penalized likelihood datings, while the age in Foster et al. (2016a, q.v. for details) is around 138 Ma, that in Magallón et al. (2013) ca 137.2 Ma and in Naumann et al. (2013) ca 126.4 Ma, similar to the ca 127.7 Ma suggested by Magallón et al. (2015). B. Xue et al. (2012) estimated an age of only 104.5 Ma, the lowest estimate so far, ca 126.9 Ma is the estimate in Tank et al. (2015: Table S1), (176.8-)171.9, 124.3(-121.3) Ma is the spread of ages in Massoni et al. (2015a), (186-)157(-129) Ma in Salomo et al. (2017) and (156.5-)122.1(-79.4) Ma that in P.-L. Liu et al. (2020). Y. Liu et al. (2021) estimated that this node was ca 138.6 Ma, Lv et al. (2020) ca 142 Ma, and the estimate in Jiao and Wang (2022) is 123-109 Ma.
Well preserved fossils of magnoliids - their identity is unclear - are known from the Early Cretaceous of Portugal and North America (Friis et al. 2020 and references); Catanthus, one of these fossils, has extrorse anthers and it may have trichotomonocolpate pollen.
Evolution: Divergence & Distribution. Cecilanthus, from early Cenomanian Maryland ca 100 Ma or slightly younger, has a floral formula of * P many; A many; G many, with a well-developed receptacle and probably one ovule/carpel, that while perhaps Magnolialean might also be assignable to Laurales, Nymphaeales, etc. (Herendeen et al. 2016). Doweld (2022) placed this in its own family, which he thought might belong in Magnoliales. He described the flower parts as being whorled, the stamens havd relatively long anthers, the anthers and filaments were not much differentiated in width, etc..
Both Laurales and Magnoliales have relatively high diversification rates, and extinction rates may have decreased in Laurales (Massoni et al. 2015a). Tank et al. (2015) note an increase in net diversification that can possibly be linked with a genome duplication associated with this node (see below).
One of the results of the λ/lambda duplication may have been retention of heat shock transcription factors (HSF) involved in the regulation of the heat stress response pathway (L. Zhang et al. 2020), and 120-100 Ma was a period of some aridity (Heimhofer et al. 2005). As Zhang et al. (2020: p. 2852) noted of this and other possible physiological and morphological changes here, and of more or less contemporaneous duplications in Nymphaeales, Chloranthales, the core eudicots, and in the monocots, perhaps at the [Asparagales + commelinds] node, "many duplicated stress-related genes were retained ..., as well as key regulators of morphological and physiological innovations, which facilitated the adaptive radiation and environmental adaptation of the major clades in flowering plants".
Ecology & Physiology. Magnoliids have notably thick rootlets, and this may be connected with more extensive mycorrhizal associations (Kong et al. 2014 and references; B. Liu et al. 2015). However, little is known about root thickness overall and the significance of its variation.
A major increase in seed mass, and to a lesser extent an increase in plant height and in leaf mass per area (SLA) can be pinned to this node (Cornelissen et al. (2014; for seed size, see also Moles et al. 2005a; Sims 2012).
Plant-Animal Interactions. Larvae of a good number of swallowtails eat members of this clade - Lauraceae and Hernandiaceae in Laurales and Magnoliaceae (few) and Annonaceae in Magnoliales in particular - see papers in Scriber et al. (1995).
Genes & Genomes. Cui et al. (2006) suggested that there had been a palaeopolyploidization event at this node (see also Soltis & Soltis 1990; Landis et al. 2018: the SASSβ event, ca 120.7 Ma; Chaw et al. 2018; J. Chen et al. 2018: ca 116 Ma), and these dates "fit" with several of the others included above. On the other hand, L. Qin et al. (2021) suggested that there was a duplication shared by Liriodendron chinense, Persea americana and Cinnamomum kanehirae, the LCT event, and they dated this to around 83.8-74.1 Ma. This is the λ/lambda duplication, which presumably happened before the divergence of Magnoliales and Laurales - L. Zhang (2020) date it to 100, perhaps 120, Ma or more, Lv et al. (2020) date an event around here (but not in monocots) to ca 142 Ma, and X. Guo et al. (2021) to 103.0-98.2 Ma; see also Y.-C. Chen et al. 2020).
Lv et al. (2020: Fig. 2a-c) thought that genes in the members of this clade (four species examined), also in Amborella, were notably long, >20 kb, most of this length being introns, but this may be a sampling issue.
Chemistry, Morphology, etc.. There is considerable variation as to how the androecium is initiated; it would not take much for "flowers with inner staminodes" to be an apomorphy at this level. For the supra-stylar compitum, see Wang et al. (2011) and for fruit wall anatomy, see Bobrov et al. (2017a). Lauraceae, Degeneriaceae and Magnoliaceae, at least, develop a massive, multiseriate suspensor during embryogenesis (Wardlaw 1955).
MAGNOLIALES Bromhead - Main Tree.
(Si02 accumulation +); vessels in multiples; secondary phloem stratified; pith septate [with sclerenchymatous diaphragms]; nodes 3:3; petiole vasculature an arc with an adaxial plate; growth monopodial, branching from the current innovation, leaves two-ranked, lamina vernation conduplicate; "bract" sheathing; P whorled; G occluded by fusion and secretion; ovule with outer integument 5-10 cells across, obturator +; seeds medium-sized, testa vascularized, multiplicative; endosperm ?type, ruminate, ruminations irregular. - 6 families, 128 genera, 3,140 species.
Includes Annonaceae, Degeneriaceae, Eupomatiaceae, Himantandraceae, Magnoliaceae, Myristicaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Magallón and Castillo (2009) suggest possible ages for the crown group of around 171.5 and 116.6 Ma, Bell et al. (2010) ages of (96-)76, 69(-50) Ma; other estimates are (119-)113, 108(-102) Ma (Wikstöm et al. 2001), (164.1-)156.8, 117.3(-116) Ma (Massoni et al. 2015a) and (136-)121(-113) Ma (Salomo et al. 2017).
Evolution: Divergence & Distribution. Early Cretaceous seeds from Portugal recently placed in Serialis and Riaserialis may well belong around here (Friis et al. 2019a), although near Austrobaileyaceae is another possibility. The seeds are tiny (the fruits are usually less than 2 mm long), they tend to remain together, and they have a one-(two-)layered sclereidal mesotesta, an endotesta several cell layers across the cells of which have sclerified endoreticulate infillings, and a vascular bundle in the antiraphe, but there are no ruminations (Friis et al. 2019a). For Cecilanthus, from early Cenomanian rocks in Maryland ca 100 Ma, see Herendeen et al. (2016) and above.
Three of the five angiosperms (i.e. two species of Eupomatia, also Galbulimima belgraveana) with the greatest floral eccentricities, i.e. divergence from the average, are found here (López-Martínez et al. 2023b). Indeed, intra- and interfamilial variation of morphological characters that are frequently used to reconstruct phylogenies is considerable. There is extensive discussion on character evolution in Sauquet et al. (2003), and some of the character hierarchy here is based on this paper. However, where characters like extrorse/introrse anther dehiscence and ruminate/non-ruminate testa are placed on the tree depends on how the characters are optimised, or even defined (see e.g. ruminate endosperm in Himantandraceae). There is some conflict with the positions of characters as they are optimised on a more extensive tree for basal angiosperms, although less detailed for Magnoliales (c.f. Ronse De Craene et al. 2003, also Judd et al. 2003). Doyle and Endress (2000) and Soltis et al. (2005) suggest additional characters for the clade like reduced fibre pit borders, palisade parenchyma, foliar astrosclereids, and so on. Doyle (2009) and Doyle and le Thomas (2012, see also 1997) outline pollen evolution, variation in the nature of the exine infratectum being considerable, while Furness et al. (2002) emphasize the variability of microsporogenesis in the order. Characters may well need to be added/moved in the apomorphy scheme for the order.
Doyle and Endress (2023) have recently returned to the issue of the potential placement of a number of fossils within Magnoliales, and their results are followed here. They scored 66 extant taxa (ranging from species to families) for 154 characters, and the relationships of these taxa were constrained by those in molecular trees; the positions of nine fossils were then evaluated. Some of the fossils they discussed could not be placed, some perhaps best being associated with Austrobaileyales. This work replaces an earlier study that used fewer taxa, both fossil and living, fewer characters, etc. (see Doyle & Endress 2010).
Ecology & Physiology. Magnoliaceae, along with Theaceae, Lauraceae and Fagaceae (Tang 2015), are a notably prominent component of the subtropical evergreen broad-leaved forests of East Asia.
Genes & Genomes. Morawetz (e.g. 1986b, 1988, see also Doyle & Le Thomas 1997) describe distinctive patterns of chromosome condensation in the order. See S. Kim et al. (2003, 2004, 2005a) for the AP3 and P1 genes, Myristicaceae were not sampled.
Chemistry, Morphology, etc.. The "bracts" with sheathing bases that have been reported for a number of Magnoliales (Endress & Armstrong 2011: condition in Degeneriaceae?), are a little unexpected since the leaf bases in the clade tend to be quite narrow - except for the stipulate Magnoliaceae. Deroin (2010) noted a tendency for the vasculature of the perianth and/or androecium in some Annonaceae and Magnoliaceae to be pentamerous.
For additional information, see Taylor and Hickey (1995: general), Benzing (1967), Kavathekár and Pillai (1976) and Sugiyama (1976a, b, 1979), all nodal anatomy, considerable variation esp. of the cotyledonary node, Metcalfe (1987: general anatomy), Hiepko (1964b: perianth vasculature), Endress (1977b, 1986a, 1994a: floral morphology), Erbar and Leins (1983: floral development), Ronse Decraene and Smets (1996a: androecium), van Heel (1981, 1983: carpel development), and Kimoto and Tobe (2001: embryology).
Phylogeny. Molecular data suggested that Myristicaceae are sister to the rest of the order, but support was only moderate (D. Soltis et al. 2000); the addition of morphological data strengthened that position, and also placed Magnoliaceae as sister to the remaining taxa (Doyle & Endress 2000), although the latter position had only moderate support (c.f. P. Soltis et al. 2000; see also Sauquet et al. 2001; H.-T. Li et al. 2019: support strong. ?sampling). The family pairs [Annonaceae + Eupomatiaceae] and [Degeneriaceae + Himantandraceae] are both well supported (D. Soltis et al. 2000; P. Soltis et al. 2000; Doyle & Endress 2000). Other studies confirm these general relationships (Sauquet et al. 2003; Müller et al. 2006; Z.-D. Chen et al. 2016), and they are followed here.
However, the position of Magnoliaceae in particular remained somewhat unclear. Some genes seem to have particularly disconcerting effects, thus the 26S rDNA gene caused the association of Degeneriaceae with Myristicaceae, and this was not because of a mislabelled sequence (Massoni et al. 2014). Qiu et al. (2010: mitochondrial genes) also recovered a somewhat different topology, Degeneriaceae being sister to the rest of the order minus Myristicaceae, although support was weak. Soltis et al. (2011) found that Magnoliaceae were sister to the rest of the order, the clade [Degeneriaceae + Myristicaceae] had good support although otherwise support was weak; this unexpected topology was ascribed to signal from rDNA sequences. Morton (2011: nuclear gene Xdh) found a set of relationships [Himantandraceae [Myristicaceae [Degeneriaceae [Magnoliaceae [Eupomatiaceae + Annonaceae]]]]], relationships in Magallón et al. (2015) are [[Mag [Deg + Myrist]] [Himant [Eupom + Ann]]], those in Thomas et al. (2017: Appendix S4), not the focus of the study, are [Myrist [Deg [Mag + Himant]] [Eupom + Ann]], those in Salomo et al. (2017) and H.-T. Li et al. (2021: plastomes) are [Myrist [[Deg + Himant] [Mag [Eupom + Ann]]]], but in the latter there are some very low support values. Relationships in W. J. Baker et al. (2021a: Angiosperms353 data, see also Seed Plant Tree version ix.2024) are [Myrist [[Mag [Himant + Deg]] [Eupom + Ann]]], all main nodes being well supported. Phylogenies in Helmstetter et al. (2024) and Zuntini et al. (2024) are based on a more extensive generic-level sampling, and they found the same relationships, which are followed here.
Synonymy: Annonales Berchtold & Presl, Degeneriales C. Y Wu et al., Eupomatiales Reveal, Himantandrales Doweld & Shevyryova, Myristicales Berchtold & Presl - Magnoliineae, Myristicineae Chatrou - Annonanae Doweld, Magnolianae Takhtajan - Magnoliidae Takhtajan - Magnolipsida Brongniart - Magnoliid I group (Nandi et al. 1998).
MYRISTICACEAE R. Brown - Back to Magnoliales
Tree, branches plagiotropic, pseudowhorled; exudate +, usu. (becoming) red; isoflavonoids, flavonoids diverse [flavones +], phenylpropanoids, also indole alkaloids [tryptamine derivatives, β-carbolines] +, isoquinoline alkaloids 0; cork also in outer cortex; primary stem ± with continuous vascular cylinder; vessel elements with simple or scalariform(-reticulate) perforations; (septate pith 0); tannin-containing tubes in the xylem; sieve tubes with non-dispersive protein bodies, (plastids with protein crystalloids and starch); petiole bundles bicollateral; (branched) sclereids or fibres +; (acicular) crystals +; hairs branched [arbuscular to stellate/(T-shaped)]; cuticle waxes as platelets; (leaves spiral), with dots or not; plants dioecious; flowers small (1> cm across), polysymmetric, receptacle small; P (2-)3(-5), uniseriate, connate; staminate flowers: A (spiral), 2-40, anthers fused to filemant column / connectives connate and forming a central column; pollen boat-shaped, sulcate; pistillode 0; carpelate flowers: staminodes 0; G 1, plicate, stigma sessile, 2-lobed to peltate, wet, (stylulus ± long), compitum necessarily 0; ovule 1/carpel, subbasal, inner integument (3-)7-10 cells across; fruit a follicle, dehiscing abaxially as well, (indehiscent), pericarp not distinctive; seed large [³1 cm across], pachychalazal, aril + (small), micropylar-funicular, vascularized; exotesta thick-walled, endotesta palisade, lignified, crystalliferous, tegmen multiplicative or not, exotegmen with fibres/sclereidal/tracheidal cells, chalaza massive, with a lignified counter-palisade; endosperm nuclear, with oil, embryo with hypocotyl not developed; n = 20, 22, 25, 26, x = ?20, ?21, nuclear genome [1 C] (0.044-)1.422(-46.252) pg; germination epigeal, phanerocotylar, or hypogeal.
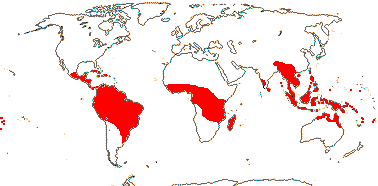
20 [list, to tribes]/485 (520). Pantropical, few mainland Africa. Map: from de Wilde (2000 - Indo-Malesia), Heywood (2007), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). Photo: Carpellate flower, Fruit.
1. Mauloutchieae Ezedin & Sauquet
(Lianes +); leaf vernation convolute; monoecy common; synandrium various; (anthers free, basifixed), pollen (globose), (ulcerate), exine sculpturing continuous to granulately verrucate, rugulate (minutely reticulate with smooth spines - Pycnanthus); (aril 0 - Maloutchia); (endosperm ruminate - Pycnanthus)
6/20: Mauloutchia (9). Brochoneura, Cephalosphaera, Doyleanthus, Pycnanthus, Staudtia
2. Scyphocephalieae Ezedin & Sauquet - Scyphocephalium Warburg
Leaf vernation convolute; pollen ulcerate, exine sculpturing reticulate with muri subunits triangular, crotonoid; fruit indehiscent.
1/2. West Africa.
[Myristiceae [Horsfieldieae + Viroleae]]]: ?
3. Myristiceae Ezedin & Sauquet
Leaf vernation conduplicate; inflorescences lacking basal cataphyll scars (cataphyll scars +, bracteoles 0 - Paramyristica); anthers not septate; pollen exine sculpturing reticulate to rugulate; G lacking dorsal bundles; seeds also with starch.
3/245: Myristica (175), Knema (69).
[Horsfieldieae + Viroleae]]]: ?
4. Horsfieldieae Ezedin & Sauquet
Leaf vernation conduplicate; P connate basally; anthers septate, connectives free to connate; pollen exine sculpturing reticulate (rugulate - Gymnacranthera); G with 2 dorsal bundles.
3/117: Horsfieldia (106), Endocomia, Gymnacranthera.
5. Viroleae S. M. Oliveira & Sauquet
Leaf vernation convolute (conduplicate - Otoba;(anthers basiiifixed, free), pollen (globose spherical), exine sculpturing reticulate (smooth - Otoba); cotyledons often vestigial, divergent, becoming haustorial during germination.
5/124: Virola (71).
Bicuiba, Compsoneura, Iryanthera, Osteophloeum, OtobaUnplaced - Coelocaryon, Haematodendron.
Age. Crown-group Myristicaceae are estimated to be a mere 21-15 Ma (J. A. Doyle et al. 2004) or (51.5-)32.6, 13.8(-10.2) Ma (Massoni et al. 2015a).
The discovery of fossil seeds apparently of Myristicaceae from the Eocene (London Clay) does not bear on the crown-group age because they cannot be placed precisely on the tree of the family (J. A. Doyle et al. 2008a).
Floral formula: * P [3]; A [2-40]: * P [3]; G 1.
Evolution: Divergence & Distribution. Crown-group diversification in Myristicaceae, estimated to occur 21-15 Ma or somewhat more, is very recent; indeed, given possible stem ages of the family that are mostly (considerabaly) over 100 Ma (see crown-group ages for the order above), its distribution throughout the humid tropics, and its apparently low dispersability (J. A. Doyle et al. 2004). Sauquet et al. (2003) also found a long branch leading to crown Myristicaceae and little molecular divergence between its extant members, which also suggests recent diversification. In this respect Myristicaceae can be compared with Annonaceae, also pantropical but a little younger, however, there diversification may have begun in mid-Cretaceous times or slightly later (Doyle et al. 2004; Scharaschkin & Doyle 2005; esp. Couvreur et al. 2011a; Doyle et al. 2012).
Buerki et al. (2013; see Sauquet et al. 2003, support for relationships hardly very strong) suggested that the closest relatives of the large-fruited Malagasy Haematodendron were to be found in the New World, long distance dispersal explaining the disjunction.
See Sauquet et al. (2003) and Chatrou (2003) for possible apomorphies; Sauquet (2003) suggested that monoecy had evolved at least four times in the family.
Ecology & Physiology. Myristicaceae are disproportionally well represented (11 of the 57 species of the family recorded) of the 227 common trees (d.b.h. at least 10 cm) of the Amazonian forests (ter Steege et al. 2013).
Pollination Biology & Seed Dispersal. Pollination of Myristicaceae in the Indo-Malesian region, at least, is by small beetles (Corlett 2004; Gottsberger 2016a for a summary). Kishna and Somanathan (2018) suggest that the rewardless female flowers of Myristica fatua, and probably other species in the genus, are pollinated by deception, being visited by pollen-collecting bees.
Seed dispersal is by primates and toucans in the New World and hornbills, pigeons and birds of paradise in the Old World; the aril may be quite thin but it is nutritious (McKey 1975; Fleming & Kress 2013).
Genes & Genomes. Isozyme duplication (in Myristica) suggests ancient polyploidy (Soltis & Soltis 1990). There are reports of holocentric chromosomes from Myristica fragrans (Escudero et al. 2016b and references). Oginuma et al. (2012) list chromosome numbers, those of only a few South East Asian-Malesian taxa are known.
Chemistry, Morphology, etc.. Barman et al. (2024) review the various hallucinogens known from Myristicaceae, they include phenylpropanoids in particular, also indole alkaloids, both tryptamine derivatives and β-carbolines; Barman et al. (2024) discuss the synthetic pathways that may be involved. There are free phloem strands in the centre of the midrib bundle. The wood rays are not notably broad.
Inflorescence morphology is a little confusing. Ronse de Craene (2010; see also Armstrong & Tucker 1986) shows a large, abaxial "bracteole" in addition to paired lateral prophyllar/bracteolar-like structures that may (staminate inflorescence) or may not subtend buds. The "bract" immediately below the staminate flower of Myristica fragrans has a very broad base.
The perianth lobes each have three traces (Siddiqui & Wilson 1976). The synandrium of Myristica has a sterile apex, probably axial (Armstrong & Tucker 1986), and judging from the vasculature, the unithecal anthers found in some species of Myristica represent half of a divided bithecal anther (Wilson & Maculans 1967; Armstrong & Tucker 1986); anthers in Knema are tetrasporangiate (Xu & Ronse de Craene 2010a). However, the androecial vasculature varies as does the nature of the central sterile column of the staminate flower (G.-F. Yang & Xu 2016). For the complex vascularisation of the carpel in Myristica, see Wilson and Maculans (1967), and for the vascularization of the ovule in Myristica fragrans, see Dickison (2000). A small structure developing between the two integuments is reported by Nair (1972). Corner (1976) commented on the complexity of the seed coat in Myristicaceae which is as elaborate as that of any other angiosperm. Not all taxa have a multiplicative testa, and some have a multiplicative tegmen; the ruminations may develop best in the pachychalazal part of the seed.
For more information, see Kühn and Kubitzki (1993: general), de Wilde (2000: Malesian Myristicaceae), Hegnauer (1969, 1990: chemistry), Kerster and Baas (1981: anatomy), Cremers (1973) and Jiménez-Rojas et al. (2002), both growth patterns, Sauquet (2003: androecium), Sauquet and Le Thomas (2003: pollen), and Mauritzon (1939a), Endress (1973) and Mohana Rao (1975b), all seeds.
Phylogeny. Relationships within the family are unclear. The African and Madagascan taxa may form a clade, possibly sister to the New World Compsoneura (but perhaps long branch attraction), overall, geography and relationships may be summarized as [[Asia + America] [Madagascar and Africa]]. Within Asian/Malesian Myristicaceae, Knema and Myristica may be sister taxa. Sauquet et al. (2001, 2003), Sauquet (2003) and Sauquet and Le Thomas (2003) suggest that the free stamens (in some species they are numerous and apparently spirally inserted) and small aril of Mauloutchia, apparently plesiomorphic features, are in fact more likely to be derived. Helmstetter et al. (2024) returned to the problem in the context of their general study of the magnoliids, obtaining a fair amount of resolution, although some support values were low.
Classification. For the tribal classification, see Helmstetter et al. (2024); two genera are unplaced.
[[Magnoliaceae [Degeneriaceae + Himantandraceae]] [Eupomatiaceae + Annonaceae]]: primary stem with distinct bundles [eustele]; wood with broad rays; flowers solitary, large [usu. >1.5 cm across], (with cortical vascular system); P = K + C; A many, spiral [possible position here], filaments with three veins, anther thecae separate, embedded in the broad connective, connective prolonged [one position]; tectum imperforate; G spiral; ovule with funicular obturator, vascular bundle in antiraphe; often ³2 seeds/fruitlet; 10-aa deletion in PI-derived motif in AP3 gene.
Age. This node can be dated to 120-100 Ma (Doyle et al. 2004), also (108-)101, 97(-90) Ma (Wikström et al. 2001), (106-)102(-98) Ma (Su & Saunders 2009), ca 114.8 Ma (Naumann et al. 2013), (119.9-)115.7(-112.6) Ma (P.-S. Li et al. 2017), (116-)106(-101) Ma (Salomo et al. 2017) or as recently as ca 71.1 Ma (Magallón et al. 2013).
Chemistry, Morphology, etc.. There is more than one kind of cortical vascular system in the flower in this clade, and what parts of the flower are supplied by which system varies (Ronse De Craene et al. 2003, see also Deroin 1991, 1999a).
Phylogeny. For a discussion of the relationships in this part of the tree, see above under the order.
Note that prior to x.2022, a clade [[Degeneriaceae + Himantandraceae] [Eupomatiaceae + Annonaceae]] was recognised, and its members share a number of features. The discussion associated with that clade is included immediately below; of course the apomorphies for this clade could still be apomorphies for a clade that included Magnoliaceae, their absence in Magnoliaceae representing apomorphies for that family.
Here are the main features of this [[Degeneriaceae + Himantandraceae] [Eupomatiaceae + Annonaceae]] clade: flower haplomorphic; anthers valvate [H-dehiscence], inner staminodes +; pollen smooth, exine thin, infratectum ± granular; [0.2-1.3 µm across], nexine foliations?; style with differentiated transmission tissue; mesotesta fibrous (all this from APweb then).
Age. Ages around here are ca 92.5 Ma (Tank et al. 2015: Table S1, S2, c.f. topology).
For Endressinia brasiliana, see Magnoliaceae.
Evolution: Divergence & Distribution. There may have been a slow-down in diversification at this node (Massoni et al. 2015a).
Pollination Biology & Seed Dispersal. For the inner staminodes that are often so conspicuous in the flowers, and their function in pollination - food for pollinators, attractants - see Endress (1984). The staminodes produce a secretion, not nectar, presumably involved in pollination (Erbar 2014; Gottsberger 2016a).
Chemistry, Morphology, etc.. There is an adaxial plate of vascular tissue in the midrib, including that of Anaxagorea, but not in all Annonaceae (Gottsberger 2016b). Doyle (2007) noted that the venation here is often poorly differentiated and so of low rank (but c.f. many Annonaceae, albeit not "basal" members). I have not placed these characters on the tree.
Doyle (2009) noted that the pollen exine of Degeneria, Galbulimima and Eupomatia was more or less homogeneous, although basically granular.
[[Magnoliaceae [Degeneriaceae + Himantandraceae]]: ?
MAGNOLIACEAE Jussieu - Back to Magnoliales
(Silicon concentration high), sesquiterpene lactones +; vessel elements with simple and scalariform perforation plates; wood fluorescing; (secondary phloem ± stratified, rays broad); nodes ≥6:≥6; resin cells +; hairs simple; growth sympodial, lamina vernation also laterally conduplicate (supervolute-curved), stipules +, paired, lateral, connate, sheathing stem, open opposite petiole, bud scales +, stipuliform (0); flowers terminal, ± polysymmetric; receptacle much elongated; K and C ± distinguishable, ± whorled; (endothecium biseriate), (filament vein single); tapetal cells multinucleate; pollen grains boat-shaped; G many, fusion complete, but secretory canal +, (postgenitally connate), stigma (terminal), elongate (not); micropyle bistomal (zig-zag, exostomal), inner integument 2-3(-4) cells across, parietal tissue 2-8 cells across; exotesta forms a tube around chalaza [= heteropyle], pore marking passage of vascular bundle, endotesta containing crystals, tegmen crushed; endosperm not ruminate; n = 19, x = ?20, ?19, nuclear genome [1C] (0.042-)1.415(-47.43) pg.
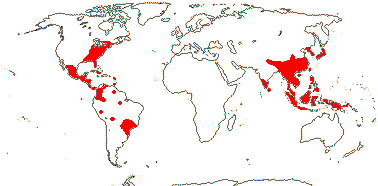
2 [list]/227 (294): Magnolia (ca 225). The Americas (but not W. North America), and South East Asia to Malesia. Map: from Good (1974) and Lozana-Contreras (1994). Photo: Collection.
Age. Molecular estimates of the crown group age are (54-)36, 33(-17) Ma (Bell et al. 2010: note topology), (86-)79, 70(-63) Ma (Wikström et al. 2001), (115.1-)94, 33.8(-5.7) Ma (!!: Massoni et al. 2015a), (98-)84.1(-59) Ma (Veltjen et al. (2021) or ca 85.7 (plastome analyses) or ca 87.5 Ma (nuclear) (S.S. Dong et al. 2022).
Archaeanthus linnenbergii, an Albian to mid-Cenomanian fossil of some 96.5 Ma from Kansas, U.S.A., may be assignable to Magnoliaceae (Dilcher & Crane 1984); it is placed sister to the family in the constrained morphological analysis of Doyle and Endress (2010), or somewhere in the ANA grade/magnoliid area (see also Friis et al. 2011; Massoni et al. 2015b: detailed discussion of its placement; López-Martínez et al. 2023a). It has follicular fruits that fall off the axis and each follicle has 10-18 winged seeds. The genus has in the past also been associated with Liriodendron in particular (see below), and Doyle and Endress (2024) placed it somewhere around here, but its position could not be fixed.
Endressinia brasiliana, from the Brazilian Crato Formation of some 113 Ma, has i.a. distinctive glandular staminodia. It has been placed sister to [[Degeneriaceae + Himantandraceae] [Eupomatiaceae + Annonaceae]] clade by Doyle and Endress (2010; see also Massoni et al. 2015b; see Magnoliaceae here), largely confirming the position suggested by Mohr and Bernardes-de-Oliviera (2004). However, Mohr et al. (2013) linked it with Schenkeriphyllum glanduliferum, which they also described from the Crato Formation, and put the two in a clade sister to Magnoliaceae (see also Doyle 2014b; Doyle & Endress 2023)...
1. Magnolia L.
(Deciduous); sieve tube plastids with polygonal protein crystalloids and starch - Magnolia; petiole also with medullary bundles; veinlet endings with dead enlarged cells with wall adjacent to tracheary element thickened [= tracheoidal element]; growth (monopodial - Michelia), sylleptic (proleptic); leaves also spiral, (lamina emarginate), stipules two lobed, (not encircling stem); (plant dioecious); P 2, 3 + 3, or 3 + many, (3 outer members small, K-like); anthers introrse/latrorse; (exine infratectum granular); G 1-many, carpels conduplicate (open), nectar secretion by epidermis; ovules (1-)2-12(-16)/carpel, funicular obturator ?0; fruit dehiscent ad/abaxially [so fruit is not follicle s. str.]/(circumscissile)/(fleshy, indehiscent), endocarp with fibre-like sclereids; sarcotesta +, scleroendotesta with lignified fibrils in the cells, endotesta with sclerified endoreticulate infillings, (tegmen ± tanniniferous); endosperm copious, (nuclear - M. septentrionalis).
1/225. The Americas (but not W. North America), and South East Asia to Malesia.
Age. Crown group Magnolia is estimated to be ca 54.6 Ma (Nie et al. 2008), (46-)44.7(-44) Ma (Veltjen et al. 2021) or (44.9-)39.2(-33.6) Ma (plastome analyses) or ca 54.9 Ma (nuclear analyses) (S.S. Dong et al. 2022).
2. Liriodendron L. —— Synonymy: Liriodendraceae F. Barkley
Deciduous; wood vestured; petiole bundle strictly annular; leaves spiral, lobed, with palmate secondary veins; P 3 + 3, [3 + 3], (with colour blotches), nectaries on P; ovules (1)2/carpel; fruit a samara, seed 1; sarcotesta 0, mesotesta more or less sclerotic, endotesta palisade, lignified, with fibrous lignifications; endosperm slight, not ruminat; genome ca 1.75 Gb.
1/2. East Asia, east North America.
Age. The two species may have diverged around 22 Ma (Veltjen et al. (2021) or ca 26.6 (plastome analyses) or ca 20.6 Ma (nuclear) (S.S. Dong et al. 2022).
The ca 96.5 Ma old Archaeanthus has also been associated with the ca 94 Ma Liriodendroidea, known from its winged seeds (Frumin & Friis 1996), as sister to Liriodendron, which would call into question the accuracy of the molecular age estimates for the family given above.
Evolution: Divergence & Distribution. For some other dates in Magnoliaceae, see Azuma et al. (2001), Nie et al. (2008) and Veltjen et al. (2021). The family has a rich fossil record (Friis et al. 2011 for references), Liriodendron, for example, being widely distributed in the Northern Hemisphere in the early Caenozoic (Ferguson et al. 1997; J. Chen et al. 2018).
Gottsberger et al. (2012) suggested that the predominance of large flowers in the family could be linked with the prevalence of beetle pollination.
Magnolia may have originated in the Asian-North America area and was a member of the boreotropical flora, and S.S. Dong et al. (2022) suggest details of its subsequent history, often linked with climate change through the Tertiary. About three quarters of all Magnolia grow in China, and H. Liu et al. (2016) found substantial phylogenetic conservatism of many of the plant traits that they studied. Veltjen et al. (2021) suggested that Magnolia had moved onto the Antillean islands four times between 16 and 4 Ma.
W.-Z. Liu et al. (2014) and X. Zhang et al. (2017) and references discuss the possibility that the ovular bundle in Magnolia is amphicribral (the phloem surrounds the xylem) and cauline in origin; this bundle develops from the cortical system of the flower while the other carpellary bundles (collateral) develop from the stelar system.
Ecology & Physiology. Magnolia is an important component of temperate evergreen broad-leaved forests (EBLFs) in Southeast Asia (Tang 2015; Yu et al. 2017).
Pollination Biology & Seed Dispersal. Beetles are common pollinators, but insects like flies and bees are other pollinators. The flowers of species like Magnolia ovata, pollinated by dynastid cyclocephaline scarab beetles, are thermogenic (Seymour 2001; M. R. Moore & Jameson 2013; Gottsberger et al. 2012; Gottsberger 2016a: summary). Floral scents of the family have been extensively studied (Azuma et al. 1999; Gottsberger et al. 2012); for nectar secretion by the carpels, see Erbar (2014 and references).
The red/orange seeds of Magnolia can be quite conspicuous as they dangle from the open fruitlet attached by extended annular thickenings of the protoxylem vessels. The seeds commonly become exposed as individual carpels open, either adaxially or abaxially or both, or the whole outer part of the inflorescence may fall off, as in section Talauma, or occasionally the fruits are indehiscent (e.g. Romanov & Dilcher 2013; Y.-B. Wang et al. 2020).
Plant-Animal Interactions. K. S. Brown et al. (1995) noted that most swallowtail larvae could be reared on leaves of Magnoliaceae, the family, they thought, that might be the ancestral host plant for Papilioninae (unlikely); only larvae of the two species of Teinopalpus, sister to other Troidini, are found on Magnoliaceae, and Troidini otherwise eat Aristolochiaceae (Allio et al. 2020/2021). For more on swallowtails and Magnoliaceae, see papers in Scriber et al. (1995), and for swallowtails in general, see Aristolochiaceae.
Genes & Genomes. For the genome of Liriodendron chinense, see J. Chen et al. (2018). Both isozyme duplication and stomatal size increase over time, so suggesting ancient polyploidy (Soltis & Soltis 1990; Masterson 1994).
The chondrome of Liriodendron has 41 protein-coding genes, the most of any angiosperm (equal to Amborella, see C. Lee et al. 2023).
Chemistry, Morphology, etc.. Sclerenchymatous diaphragms in the pith of the stem are particularly conspicuous in the Michelia group of Magnolia. Terminal tracheids are commonly silicified, forming distinctive phytoliths (Piperno 2006) and veinlets often terminate in tracheoidal elements (see above) and a variety of other distinctive cell types (Tucker 1964).
Flowers of Magnolia may be closely surrounded by a leaf in which thepetiole and blade are at most rudimentary; flowers that appear to be axillary terminate short shoots, and growth in taxa with such flowers is monopodial, but otherwise it is sympodial (Praglowski 1974).
For floral development in some species of Magnolia and ABC gene expression, see Wróblewska et al. (2016). The endexine is lamellate and the columellae fused-granular (Xu & Kirchoff 2008). Nectar is secreted from the exposed surfaces of the carpels in some species of Magnolia. The micropyle is exostomal in the Michelia group of Magnolia. There is a simple or tubular pore in the seed coat of Magnolia that marks the passage of the vascular bundle through the sclerotesta (Xu 2003). Friis and Pedersen (2011) discuss variation in the thickness of the crystal-bearing tissue in the testa.
For additional information, see Praglowski (1974) and Nooteboom (1993), both general, Hegnauer (1969, 1990: chemistry), Yoshizawa et al. (2000: ?absence of tension wood, but see Roussel & Clair 2015), Figlar (2000: branching patterns), Charlton (1994) and Liao and Xia (2007), phyllotaxis and vernation, Postek and Tucker (1982), leaf development, F.-X. Xu (2006) and Xu and Rudall (2006), floral development, Praglowski (1974), Xu and Kirchoff (2008) and Gabarayeva and Grigorjeva (2012), all pollen morphology, Kaeiser and Boyce (1962) and Bouman (1977), ovules and seeds of Liriodendron, Yamada et al. (2003b: ovules), and Pan et al. (2003) and especially Fu et al. (2012 and references), embryology
Phylogeny. Magnolia s. str. is pretty wildly paraphyletic, and section Talauma is sister to the rest of Magnolia (Qiu et al. 1995; S. Kim et al. 2001a, b; Nie et al. 2008; Z.-D. Chen et al. 2016). Support along the backbone of the Magnolia phylogeny is poor, although there are 11 or more well-supported clades along it (Azuma et al. 2000, 2001, 2011; Nie et al. 2008; S. Kim & Suh 2013). See also Ueda et al. (2000) and Y.-L. Wang et al. (2006) for molecular phylogenies. Y.-B. Wang et al. (2020) in a plastome skimming operation found that the clade (these are sections) [Gwillimia [Splendentes + Talauma]] was sister to the rest of the genus, but the authors suggested that there might be differences between plastome and nuclear genomes, the position of section Gwilliamia being a case in point. Veltjen et al. (2021) also suggested genome problems; they used five chloroplast and six nuclear markers. These problems recurred in S.S. Dong et al. (2022: 48 spp., 13/15 sections, plastome and SNP nuclear data) who recovered all 13 sections in both plastome and nuclear analyses, but the relationships of the sections differed, as did some of those within the sections.
Classification. It seems best to recognize a broad Magnolia that encompasses the whole of the current Magnolieae/Magnolioideae; Magnoliaceae thus includes only two genera (there are 15 sections in Magnolia), and the tribes and subfamilies are superfluous (see Nooteboom 2000; Nooteboom in Xia et al. 2008; Kim & Suh 2013; Y.-B. Wang et al. 2020: 15 sections; Callahan & Png 2020 and literature). However, Romanov and Dilcher (2013) needed a separate order for the family, while Xia (2012) and Sima and Lu (2012) provided a reclassification of Magnolia in which it was divided into 16 genera placed in two tribes; these treatments are not being followed.
[Degeneriaceae + Himantandraceae]: isoquinoline alkaloids 0; flowers axillary; staminodes adaxial to stamens; nexine foliations absent; outer integument with annular opening; x = 12.
Age. The age of this node is around (59-)42, 38(-22) Ma (Bell et al. 2010), (80-)73, 63(-56) Ma (Wikström et al. 2001), c.f. topology, or (146.2-)134.2, 59.9(-17.1) Ma (Massoni et al. 2015a).
Evolution: Divergence & Distribution. Diversification rates in this clade show a notable slow-down (Massoni et al. 2015a).
DEGENERIACEAE I. W. Bailey & A. C. Smith - Degeneria I. W. Bailey & A. C. Smith - Back to Magnoliales
Cork ?; vessel elements with scalariform perforation plates; sieve tube plastids with polygonal protein crystalloids and starch; nodes 5:5; petiole also with medullary bundles; secretory cells 0; cuticle waxes as platelets; leaves spiral; bracts?; K 3, C many, whorled, trilacunar; pollen boat-shaped; G 1, basally ascidiate, occluded by fusion only, sclereids numerous, stigmatic crest much elongated, ?surface, compitum necessarily 0; ovules many/carpel, inner integument ca 3 cells across, funicle long; fruit a follicle, endocarp undistinguised; exotestal cells palisade, thin-walled, sarcomesotesta +, endotesta with sclerified endoreticulate infillings, with crystals; suspensor massive, embryo with 3 (4) cotyledons; x = 7, (?6).
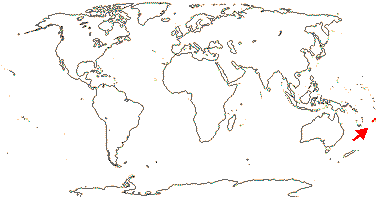
1 [list]/2. Fiji, Viti Levu. Map: original!
Chemistry, Morphology, etc.. Some information is taken from Hegnauer (1973, 1990, as Winteraceae: chemistry) and Kubitzki (1993b: general).
HIMANTANDRACEAE Diels - Galbulimima Bailey - Back to Magnoliales
Polyketide alkaloids +; vessel elements with simple (and scalariform) perforations; sieve tubes with non-dispersive protein bodies; trichomes peltate, stomata surrounding their bases; cuticle wax crystalloids 0; branching?; bracts tubular, 2, enclosing the flower, caducous; outer staminodes petal-like, 3-23, inner staminodes with glands; pollen scabrate; G 6-30, sclereids numerous; ovules (1) 2/carpel, pendulous; stigma wet, suprastylar extragynoecial compitum +; fruit ± syncarpous, drupaceous, with several stones, endocarp with fibre-like sclereids; seeds laterally flattened, ?ruminations; testa not multiplicative, endotesta aerenchymatous; endosperm development?; x = 7 (?6, ?5); seedling?
1 [list]/2. The Celebes, New Guinea and N.E. Australia. Map: from Hoogland (1972) and Endress (1983). [Photo - Flower, Fruit, Buds, Habit]
Chemistry, Morphology, etc.. Foliar stomata surround the bases of the scales, their axes being tangentially oriented (Rudall 2023a).
The perianth may be staminodial in origin. According to Johri et al. (1992) the outer integument is not vascularized; for the testa, see Doweld and Shevyryova (1997). Endosperm ruminations are at best obscure.
Some other information is taken from Hegnauer (1966, 1989: chemistry) and Endress (1993: general).
[Eupomatiaceae + Annonaceae]: sieve tube plastids also with polygonal protein crystalloids; rays 8-15-seriate; petiole bundles arcuate; prophyll single, adaxial; inflorescence +; nexine foliations undifferentiated; G many; fruit ± berry-like.
Age. Estimates of the time of divergence of these two families vary considerably: Around (69-)55, 50(-35) Ma (Bell et al. 2010), ca 64.75 Ma (Naumann et al. 2013), (97-)91, 82(-76) Ma (Wikström et al. 2001), and (110.4-)106.3(-102.0) Ma (Couvreur et al. 2011a: HPD estimates). Other suggested ages are (110.4-)101.7(-99.4) Ma (Surveswaran et al. 2010: HPD estimates), (101.5-)98.0(-94.9) Ma (Su & Saunders 2009), or somewhere between 84.7 and 62.6 Ma (Erkens et al. 2009: a variety of estimates); see also the estimate of 109-100 Ma in Pirie and Doyle (2012), ca 94.2 Ma in Magallón et al. (2015), (148.4-)138.5, 102.5(-86) Ma in Massoni et al. (2015a), and ca 81.2 Ma in Tank et al. (2015: Table S1).
Evolution: Divergence & Distribution. Optimisation of prophyll position on the tree is uncertain. Adaxial prophylls are common in Annonaceae, including Anaxagorea, although some taxa have paired, lateral prophylls (Fries 1911).
EUPOMATIACEAE Orban, nom. cons. - Eupomatia R. Brown - Back to Magnoliales
Also rhizomatous, with xylopodium or root tubers; vessel elements with scalariform perforation plates; secondary phloem stratification?; pith not septate; sieve tubes with non-dispersive protein bodies [check], plastids also with protein rods; nodes (5-)7(-11):(5-)7(-11); secretory cells +; (stomata anomocytic); inflorescence fasciculate; "pedicels" with 2-3 2-ranked bracts; receptacle concave; calyptra +, thick, deciduous [= modified bract], with sclereids; P 0; A latrorse-introrse, staminodes 15+, petal-like, connate basally, with many glands; pollen with encircling equatorial sulcus; G ± connate, ascidiate, occluded by fusion only, placentation sublaminar, stigma flat, papillate, suprastylar extragynoecial compitum + [at least inner carpels]; ovules 2-11/carpel, outer integument 3-4 cells across, inner integument 2-3 cells across, hypostase +, vascular bundle ends in the chalaza; fruit baccate, ?endocarp cells palisade, unlignified; testa not vascularized, exotestal cells with thickened unlignified walls, mesotestal cells fibres, endotesta unlignified, exotegmic cells cuboid, slightly lignified, endotegmic cells enlarged, crushed; n = 10, x = 7 (?6, ?5), nuclear genome [1 C] (0.027-)1.287(-62141) pg; germination epigeal/phanerocotylar, leaves initially spiral.
1 [list]/3. New Guinea and E. Australia. Map: from Hoogland (1972) and Endress (1983). [Photos - Flower, Flower.]
Evolution: Pollination Biology & Seed Dispersal. Looking at the flowers from above, all one can see are numerous spreading petals. In fact these are staminodes, which both produce an odour that attracts weevils (Elleschodes) and a sticky exudate that attaches the pollen to them; the stamens are much smaller and are outside the staminodes, becoming reflexed. The weevils both mate and oviposit on the flowers, the eggs being laid at the bases of the staminodes and inner stamens, the eggs/larvae all falling to the ground when the androecium falls off (Armstrong & Irvine 1990; Bergström et al. 1991; Gottsberger 2016a).
Genes & Genomes. There is no evidence of isozyme duplications here (Soltis & Soltis 1990).
Chemistry, Morphology, etc.. The main axes are mixed, initially being orthotropic and bearing spirally-arranged leaves, later becoming more or less plagiotropic and with 2-ranked leaves.
There are about three two-ranked bracts on the pedicel. The calyptra itself is often interpreted as being a modified, amplexicaul bract, although there is no evidence of a midrib (c.f. Galbulimima) (Endress 2003b), but as S. Kim et al. (2005b) noted, the B- and E-class genes expressed in the calyptra were also expressed in the leaves, and also in the leaves and bracts of Magnolia, but not in the perianth. Bobrov et al. (2017a) described the fruit of Eupomatia as developing an unlignified palisade endocarp, perhaps like the palisade tissues of Laurales (see also Mohana Rao 1983); note that the epicarp here is "derivative of the receptacle tissues" (Bobrov et al. 2017a: p. 133, see also 132).
Some information is taken from Hegnauer (1966, 1989: chemistry), Endress (1983, 1993: general) and Rix and Endress (2007: an easy account).
ANNONACEAE Jussieu - Back to Magnoliales
Vessel elements with simple perforation plates; parenchyma in bands joining tall multiseriate rays [wood cobweb-like in t.s.]; nodes 3:5, lateral traces leaving stele well before central trace, latter trifurcates; sieve tube plastids also with protein filaments; epidermal cells with single crystals [?level]; inflorescence terminal; pedicels articulated at base; flowers 3-merous, polysymmetric; P very thick, = "K" 3 (2), valvate, "C" 3 + 3, (2 + 2; 2), aestivation open to valvate (imbricate); A whorled, outer alternating with C, filaments with a single vein; endintine thick below/near aperture, extruding through aperture; G plicate; (stigma wet, suprastylar extragynoecial compitum - ?level); ovules perichalazal, outer integument 4-10 cells across, funicle short; fruit stipitate, monocarp abscission at the base of stipe; endotestal plug +, mesotesta with several layers of externally longitudinal and internally transverse fibres, aerenchyma internal; vascular bundle in antiraphe; endosperm with thickened cells walls (not); x = 8 (?9, ?7), nuclear genome [1 C] (0.045-)1.027(-23.603) pg.
108 [list: to tribes]/2,503 - four subfamilies below. Largely tropical.
Age. Crown group diversification may have occurred (90.4-)89.5(-89) Ma (Su & Saunders 2009: calibration on Futhabanthus), 82-57 Ma (J. A. Doyle et al. 2004), ca 84 Ma (Scharaschkin & Doyle 2005; check), 98-89 Ma (Pirie & Doyle 2012), (104.9-)99.3(-94.0) Ma (P.-S. Li et al. 2017), (126.7-)114.1, 85.3(-72.4) Ma (Massoni et al. 2015a) or (97.7-)91.9(-89.0) Ma (Brée et al. 2020); see Couvreur et al. (2011a) and Ortiz-Rodriguez et al. (2018) for other estimates in the same area.
Futhabanthus, based on fossil flowers from the late Cretaceous (Early Coniacian, ca 89 Ma) of Japan, can perhaps be assigned to crown-group Annonaceae (Takahashi et al. 2008b; Friis et al. 2011; Doyle & Endress 2023). The family seems to have been widely distributed by the end of the Cretaceous (Friis et al. 2011; Wheeler et al. 2017).
Includes Ambavioideae, Anaxagoreoideae, Annickieae, Annoneae, Annonoideae, Bocageeae, Canangeae, Dendrokingstonieae, Duguetieae, Fenerivieae, Guatterieae, Maasieae, Malmeeae, Malmeoideae, Meiocarpidieae, Miliuseae, Monocarpeae, Monodoreae, Phoenicantheae, Piptostigmateae, Tetramerantheae, Uvarieae, Xylopieae.
1. Anaxagoreoideae Chatrou, Pirie, Erkens & Couvreur - Anaxagorea A. St-Hilaire
Ray cells with druses; uniseriate hairs terminating in a rounded cell, other hairs ± stellate; trunk leaves 2-ranked; (petiole vascular tissue ± annular; with adaxial bicollateral plate; small arcuate bundles variously arranged); stomata allelocytic; flowers (single), pedicels basally articulated, bracteoles ["bracts"] 2; C whorls similar; anther connective pointed or rounded, inner staminode secretions 0; pollen surface smooth, microperforate; G (3<), receptacular vascular system 0, stigma sessile; ovules 2/carpel, parietal tissue 7-11 cells across; fruit explosively dehiscent follicle, (also shortly dehiscing abaxially), endocarp with fibre-like sclereids; seeds 2/follicle, asymmetric; endotesta aerenchymatous, tegmen alone involved in ruminations, with oil globules; n = 8; germination epigeal, phanerocotylar.
1/25. Tropical America, Sri Lanka to West Malesia, also Halamahera and Ceram. Map: from Maas & Westra (1984, 1985). [Photo - Flower, Fruit, Fruit.]
Age. Divergence within Anaxagorea may have begun ca 44 Ma (Scharaschkin & Doyle 2005) or 57-16 Ma (Pirie & Doyle 2012).
[Ambavioideae [Malmeoideae + Annonoideae]]: (acetogenins + [C-32/C34 polyketide fatty acids]); ray cells lacking druses; inner staminodes 0; ovules with outer integument 4-6 cells across, inner integument 2-4 cells across, nucellar cap to 5 cells across; fruit berrylets, (monocarp abscission at the apex of the stipe), endocarp undistinguished [?all]; seed symmetric, with perichalazal ring; ?endosperm cell walls with xyloglucans. Map: from Aubréville (1974a) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
Age. This node has been dated to (84.4-)74.7(-63.6) Ma (Su & Saunders 2009), (76.6-)68, 64.5(-53.0) Ma (Erkens et al. 2009), 70-65 Ma (Couvreur et al. 2011a), (97.2-)88.1, 81.7(-75.4) Ma (Ortiz-Rodriguez et al. 2018), 90-73 Ma (Pirie & Doyle 2012) and (99.2-)93.6(-88.3) Ma (P.-S. Li et al. 2017). To be checked - Meiocarpidium included?
2. Ambavioideae Chatrou, Pirie, Erkens & Couvreur
; anther connective truncate, dilated; endintine not extruding through aperture; carpels 3-8, stipe articulated; ovules with middle integument +, 4-6 cells across, parietal tissue 2-3 cells across; (megaspore mother cells 2); monocarp abscission basal or apical.
9/56. Tropical.
Age. Crown-group Ambavioideae have been dated to (80.9-)57.0(-52.3) Ma (Surveswaran et al. 2010), (78-)69.4(-60.2) Ma (Couvreur et al. 2011a), 59-27 Ma (Pirie & Doyle 2012), and a little over 75 Ma (Thomas et al. 2015).
2A. Meiocarpideae Chatrou, Couvreur & Erkens - Meiocarpidium oliverianum (Baillon) D. M. Johnson & N. A. Murray mdash;mdash; Meiocarpidioideae Chaowasku
Indumentum lepidote; all leaves 2-ranked; inflorescences 1-few-flowered, opposite leaves; K/C valvate, C whorls similar; anther connective truncate, dilated; pollen sulcate-ulcerate, exine perforate, basal layer massive; G 3-5; ovules biseriate, many/carpel, middle integument +; monocarps subsessile, abscission basal; endosperm ruminations lamelliform;; n = ?
1/1. Cameroon, the Central African and Congo Republics, Gabon.
[Tetramerantheae + Canangeae]: ?
2B. Tetramerantheae Reveal
Trunk leaves (2-ranked - Cleistopholis), (branch leaves spiral - Tetrameranthus); infloresecences axillary, 1-several-flowered, umbel-like; (C imbricate - T.); anther (connective tongue-shaped or ± conical); pollen monads 45> µm across, (intine thick opposite from aperture - Ambavia); ovules 2(-3)/carpel; endosperm ruminations irregular, stout; n = 7.
4/19: Tetrameranthus (8). Tropical, inc. Madagascar, West Malesia to the Moluccas.
2C. Canangeae Chaowasku
Trunk leaves spiral; inflorescences 1-many-flowered, axillary or terminal; anther (connective apiculate); pollen monads 45->90 µm across, (as tetrads/polyads, basal layer massive - Cananga); (extragynoecial compitum + - Cananga); ovules 2-many/carpel; (bilobed raphal aril +); endosperm ruminations ± irregular to ± flattened peg-like, (stout); n = 8.
4/36: Drepananthus (26). East Africa, Southeast Asia to E. Australia.
[Annonoideae + Malmeoideae]: (polyacetylenes +); neolignans 0?; petioles short; inflorescence rhiphidium; bracts + [?level]; anther connective expanded, peltate-truncate [= connective shield]; pollen tectum retiperforate, (exine infratectum columellate), outer foliation of nexine massive; stigma capitate; parietal tissue 2-6 cells across; (endosperm starchy).
Age. This node has been dated to (69-)66.7-56.6(-54.3) Ma (Richardson et al. 2004), (78.1-)67.3(-55.2) Ma (Su & Saunders 2009), 86-71 Ma (Pirie & Doyle 2012), (97.5-)92(-86.7) Ma (P.-S. Li et al. 2017) and (70.9-)57.4, 38.8(-28.3) Ma (Thomas et al. 2015).
3. Annonoideae Rafinesque / The Long Branch Clade
[Acetogenins mostly non-acetylenic, C35 to C39]; (branch leaves spiral); (anthers polysporangiate [septae often tapetal]); pollen orbicules usu. 0, (exine foliations numerous), endintine not extruding through aperture; ovules 1-many/carpel; (antipodal cells persisting); endosperm ?soft, ruminations lamelliform; x = 7-9.
48/1,526 - eight tribes below. Predominantly lowland tropics, rarely temperate.
Age. Crown-group Annonoideae may be (62.5-)60.2-51.1(-48.7) Ma (Richardson et al. 2004), (70.5-)59.6(-48.1) Ma (Su & Saunders 2009), (94.8-)89.3(-84) Ma (P.-S. Li et al. 2017), (87.4-)77.2, 69.9(-61.3) Ma (Ortiz-Rodriguez et al. 2018) or 80-64 Ma old (Pirie & Doyle 2012, also other estimates; see also Thomas et al. 2015).
3A. Bocageeae Endlicher —— Synonymy: Hornschuchiaceae J. Agardh
Flowers solitary, pedicel internodal, ebracteate; (K connate); (A 6), anthers polysporangiate; pollen in 8(or more)-grained polyads (tetrads); (G 2), ovules lateral; (follicles +, dehiscence ad- or abaxial); (micropylar caruncle +), (bilobed vascularized aril +); n = 9.
8/65: Cymbopetalum (27), Hornschuchia (12). 3 subribes. Mexico to tropical South America, coastal East Africa (Mkilua fragrans).
Age. Crown-group Bocageeae are some (74.5-)52.5(-35.5) or perhaps ca 34.6 Ma (Lopes et al. 2023).
[Guatterieae [Xylopieae [Duguetieae [Annoneae [Monodoreae [Uvarieae + Ophrypetaleae]]]]]]: ?
3B. Guatterieae J. D. Hooker & Thomson - Guatteria Ruíz & Pavón
Pollen sulculate; ovules basal; (endosperm ruminations spiniform).
1/183. Tropical America.
[Xylopieae [Duguetieae [Annoneae [Monodoreae [Uvarieae + Ophrypetaleae]]]]]: ?
3C. Xylopieae Endlicher - Xylopia L.
Trees or shrubs (scandent), (deciduous); acetogenins +; hairs simple; inflorescences axillary; K variously connate, C 6, free; anthers polysporangiate, (filaments connate basally [= staminal cone/hypanthium]), (outer and/or inner staminodes +); pollen ?in tetrads, (sulcate); G 1-many; ovules several/carpel, lateral; monocarps abaxially dehiscent, stipitate (sessile); (endosperm ruminations spiniform); raphal aril + / sarcotesta +.
1/191. Pantropical.
Age. The age of crown-group Xylopieae is (41.0-)31.8(-22.6) Ma (D. M. Johnson et al. 2025).
[Duguetieae [Annoneae [Monodoreae [Uvarieae + Ophrypetaleae]]]]:
3D. Duguetieae Chatrou & R. M. K. Saunders
Shrubs to trees, lianas wih stem hooks [Artabotrys]; hairs stellate/lepidote/simple; inflorescences axillar/terminal; K (connate, tearing at anthesis), C 6 (connate); (outer A staminodial - Fusaea); (anthers polysporangiate); pollen as tetrads/polyads, (sulculate), (spiny); ovules 1-2/carpel, basal, (middle integument + - An); carpels connate in fruit [pseudosyncarpous]/not, usu. stipitate [Art.], indehiscent sessile; n = 8.
6/213; 2 subtribes: Artabotrys (110), Duguetia (96). Tropics.
[Annoneae [Monodoreae [Uvarieae + Ophrypetaleae]]]: stem leaves 2-ranked; anthers (locellate [septae usu. parenchymatous]); pollen with foliated basal layer.
Age. This node may be (76.1-)71(-66.2) Ma (P.-S. Li et al. 2017) or (74.8-)64, 55.3(-47.7) Ma (Thomas et al. 2017).
3E. Annoneae Endlicher
(Plant deciduous); (hairs stellate); (petiole enclosing the axillary bud); (trunk leaves 2-ranked); (K 2, C connate - Disepalum); pollen in tetrads (polyads); (extragynoecial compitum +); ovules basal or lateral, outer integument ca 4 cells across [Neostenanthera]; (G connate in fruit); (bilobed micropylar aril +), (endosperm ruminations spiniform or peg-like).
7/342: Annona (172), Goniothalamus (137); 2 subtribes. Tropics, ± subtropical North America and Southeast Asia.
Age. Crown-group Annoneae are estimated to be (71.3-)66.5(-61.6) Ma (P.-S. Li et al. 2017) or (67-)56.6, 48.7(-41.1) Ma (Thomas et al. 2017).
[Monodoreae [Uvarieae + Ophrypetaleae]]: ?
3F. Monodoreae Baillon —— Synonymy: Monodoraceae J. Agardh
(Plant monoecious); K 2/3 (connate, lobes ± 0); C 3/4/6, (free); (connective expansion ± 0); pollen in tetrads/polyads (monads); (G connate, placentation parietal); ovules lateral; (monocarps sessile).
12/90; 3 subtribes: Isolona (20), Uvariodendron (18), Uvariopsis (17). Africa, Isolona is also known from Madagascar.
Age. Crown-group Monodoreae are thought to be ca 25 Ma (Dagallier et al. 2023b).
3G. Uvarieae J. D. Hooker & Thomson
Lianes (trees or shrubs); hairs stellate (simple); leaves (spiral [?]), (glands at base); C imbricate; (pollen sulculate), (spiny); G 1-many; ovules basal or lateral; (stigma sessile - Uvaria); monocarps (moniliform), stipitate, (1-seeded); (aril +, rudimentary).
13/472; 4 subtribes: Uvaria (166), Monanthotaxis (93), Fissistigma (55), Friesodielsia (47), Desmos (19). Old World tropics.
3H. Ophrypetaleae Dagallier & Couvreur
Shrubs or trees; hairs simple; C 2 x 3, free, inner stipitate/6, basally connate; G 1-many; monocarps sessile to shortly stipitate, elongate (esp. Sanrafaelia, at least 5 cm long).
2/2. East Africa.
4. Malmeoideae Chatrou, Pirie, Erkens & Couvreur / The Short Branch Clade
(Outer C sepal-like); pollen (globose), monosulcate, (surface ± spiny); ovules 1-2/carpel, ± basal, (parietal tissue -9 cells across); (tegmen with oil globules); endosperm glass-like, ruminations spiniform; x = 8, 9.
49/896 - 9 tribes below. Lowland tropics.
Age. The age of crown-group Malmeoideae has been dated to (66.1-)62.5-53.1(-49.5) Ma (Richardson et al. 2004), (55.1-)39.8(-26.7) Ma (Su & Saunders 2009), 70-62 or 54-34 Ma (Pirie & Doyle 2012, q.v. for several other estimates), (70.9-)57.4, 38.8(-28.3) Ma (Thomas et al. 2015), (62.3-)48.3, 44.8(-33) Ma (Ortiz-Rodriguez et al. 2018) or ca 42 Ma (Brée et al. 2020).
[Malmeeae, Maasieae [Fenerivieae [Phoenicantheae [Dendrokingstonieae [Monocarpieae + Miliuseae]]]]]]: ?
4A. Annickieae Couvreur - Annickia Setten & Maas
Hairs ± branched; flower single, terminal → extra-axillary; "C" 0 + 3; pollen with columellar infratectum; G >30; monocarps stipitate; endosperm ruminations flattened and peg-like/spiniform.
1/8. Tropical Africa, Sierra Leone to Tanzania.
Age. Crown group Annickieae are (6.2-)5.4(-4.6) Ma (Brée et al. 2020).
4B. Piptostigmateae Chatrou & R. M. K. Saunders
Flowers 2 or more together; pollen with columellar infratectum; G 20>; ovules several-many/carpel, usu. lateral; monocarps sessile/stipitate, (abscission at apex).
6/32: Piptostigma (13), Polyceratocarpus (9). Tropical Africa.
Age. Crown group Piptostigmateae may be (37.1-)33.4(-30.0) Ma (Brée et al. 2020 - ca 36 Ma, p. 11).
[Malmeeae [Maasieae [Fenerivieae [Phoenicantheae [Dendrokingstonieae [Monocarpieae + Miliuseae]]]]]: ?
4C. Malmeeae Chatrou & R. M. K. Saunders
(Plants androdioecious); C imbricate (valvate); anther connective tongue-like (truncate); ovules basal (lateral); endosperm (soft), (ruminations irregular pegs or lamelliform).
13/184; 3 subtribes: Unonopsis (48), Crematosperma (34), Oxandra (26), Pseudoxandra (24). Neotropical.
[Maasieae [Fenerivieae [Phoenicantheae [Dendrokingstonieae [Monocarpieae + Miliuseae]]]]]: ?
4D. Maasieae Chatrou & R. M. K. Saunders - Maasia Mols, Kessler & Rogstad
Lamina abaxially papillate; pollen with columellate infratectum; ovules 1-2/carpel, basal.
1/6. Malesia.
[Fenerivieae [Phoenicantheae [Dendrokingstonieae [Monocarpieae + Miliuseae]]]]: ?
4E. Fenerivieae Chatrou & R. M. K. Saunders - Fenerivia Diels
Flower single, axillary; K reduced to narrow rim, outer C K-like, inner C and 3 outer A narrow petal-like; pollen with columellate infratectum; ovules basal.
1/10. Madagascar.
[Phoenicantheae [Dendrokingstonieae [Monocarpieae + Miliuseae]]]: ?
4F. Phoenicantheae X. Guo & R. M. K. Saunders - Phoenicanthus Alston
Tertiary venation percurrent; A 6 or 9, connectives not extending over thecae, apex obtuse; G 1–3; ovules 1–2/carpel, ?position; monocarps sessile.
1/2. Sri Lanka.
[Monocarpieae [Dendrokingstonieae + Miliuseae]]: lamina venation eucamptodromous.
4H. Monocarpieae Chatrou & R. M. K. Saunders
Tertiary venation percurrent; pollen with columellate infratectum; G 1-?, stigma enlarged, lobed; ovules lateral; monocarp large, walls thick, hard; endosperm ruminations spiniform.
2/5: Monocarpia (4). Vietnam, Thailand, W. Malesia.
[Dendrokingstonieae + Miliuseae]: ?
4G. Dendrokingstonieae Chatrou & R. M. K. Saunders - Dendrokingstonia Rauschert
(Hairs stellate); tertiary venation percurrent; (flowers single, axillary); (K connate); pollen with columellate infratectum; G 1-2, ovules lateral, stigma enlarged; monocarp large, walls thick, hard; endosperm soft, ruminations lamelliform.
1/3. S. Thailand to Sumatra.
4I. Miliuseae J. D. Hooker & Thomson
Acetogenins +; (petiole enclosing the axillary bud); (venation brochidodromous); (plant androdioecious); connective does not extend over the thecae; pollen grains (with pollen-connecting threads), a- or cryptoaperturate/disulcate, with foliated basal layer; ovules basal or lateral; (mesotesta with transverse fibres only - Polyalthia); endosperm (?sort), (ruminations irregular pegs or lamelliform); inversion in the large chloroplast single copy region.
24/556; 8 subtribes: Polyalthia (89), Monoon (76), Miliusa (61), Pseuduvaria (59), Orophea (58), Mitrephora (53), Desmopsis (38), Meiogyne (36), Alphonsea (35),Huberantha (34). Pantropical, but mostly South East Asian-Malesian.
Age. This clade has been dated to (24.5-)18.9, 17.3(-12.9) Ma (Ortiz-Rodriguez et al. 2018).
Characters to be assigned to the above two subfamilies - terminal cell of uniseriate hairs pointed; (primary stem with continuous cylinder); cambium storied; (sieve tube plastids also with protein fibres); petiole bundle also annular; branched sclereids or fibres common; A (latrorse, introrse), tapetal cells amoeboid, multinucleate; (microsporogenesis successive); (pollen grains tricellular); stigma wet; endotesta crystalliferous, (with thin walled, longitudinal fibres); germination epigeal/crypto- or phanerocotylar.
Floral formula - Anaxagoreoideae: * K 3; C 3 + 3; A many, + several Ao; G 3-many.
Just about everything else: * K 3; C 3 + 3; A many; G 1-many.
Evolution: Divergence & Distribution. Su and Saunders (2009) offer dates for additional splits in Anonaceae, focussing on Pseuduvaria, Thomas et al. (2012) and Pirie and Doyle (2012) give many dates for divergences within both main subfamilies, as do Ortiz-Rodriguez et al. (2018). Thomas et al. (2017: Appendix S4) has a time tree for the family from which ages for tribes, etc., can be estimated, and they are largely similar to the ages above, while Thomas et al. (2017) also give estimates for ages of nodes within Annoneae (see also P.-S. Li et al. 2017; B. Xue et al. 2019: whole family). Note that although stem age estimates of the subfamilies are in the 80-70 Ma range, crown ages vary widely, the ages in Couvreur et al. (2011a) being the youngest, and those of Pirie and Doyle (2012, q.v. for other estimates) the oldest.
The biogeography and diversification of the family has been much studied (see also Doyle et al. 2004; Richardson et al. 2004; Su & Saunders 2009; Surveswaran et al. 2010; P.-S. Li et al. 2017). Much diversification happened after the break-up of the continents and is Caenozoic in age, although rafting of the ancestors of Malmeoideae north on India is a possibility (D. C. Thomas et al. 2015), while Q. Li et al. (2019; see also Srivastava & Mehrotra 2013: Alphonsea) suggest that Anonaspermum orientalis seed fossils in late Oligocene deposits from Nanning, southern China, arrived there via India - there is a seed of Artabotrys from the same locality, so same history? There are some 15 or more intercontinental tropical disjuctions - species in sister clades occur on different continents - in the family (P.-S. Li et al. 2017 and references; Thomas et al. 2017). In this context, Lopes et al. (2023) suggested that Annonaceae originated in the African portion of West Gondwana, and in Annonoideae-Bocageeae, sister to the rest of the subfamily, follicles/follicle-type fruits and mammal dispersal seem to be plesiomorphic, the tribe moving from Africa to South America via the North Atlantic land bridge, the ancestral area may have included proto-Amazonia and 65/66 species are now New World. In the New World, there was extensive dispersal by birds and mammals, latterly including bats (Lopes et al. 2023). Couvreur et al. (2011a) contrast speciation in Annonoideae and Malmeoideae. The former began to diversify ca 66 Ma and the latter much later, ca 33 Ma; the former has about twice as many species as the latter, even if rather paradoxically the latter may have a higher diversification rate - once it got going (see Couvreur et al. 2011a; Thomas et al. 2012, older, but similar disparity: both give other ages; Erkens et al. 2012). Dagallier et al. (2023b) looked at diversification in the African rainforest. Here there seems to have been an extinction in the late Miocene 10-6.5 Ma caused by increased aridification and associated with range fragmentation and then speciation as the climate improved, and also speciation associated with the East African orogeny - there was an increase in speciation some 6.5-4.25 Ma (Dagallier et al. 2023b). Malmeoideae-Miliuseae represent a major increase in diversification within the magnoliids as a whole (Massoni et al. 2015a); Ortiz-Rodriguez et al. (2018) make suggestions about the diversification of the New World taxa in this clade.
Erkens et al. (2012b) found little in the way of changes in diversification rates in the family, speciation behaving as if it were a random branching process, and so there was little need to invoke key innovations, and Couvreur et al. (2011a) also found rather steady diversification rates. However, B. Xue et al. (2019) suggested that there had been a number of radiations in the family that accounted for over 4/5ths of the diversity there. They thought that features like circadian pollinator trapping, lomentoid fruits in which the one-seeded units matured sequentially, the liane habit and androdioecy were all associated with increased diversification rates (Xue et al. 2019).
<>In Annonoideae, divergence in the Old World Goniothalamus (ca 134 species) began ca 22 Ma (it also has a stem of ca 26 My), and diversification there was not that recent and rapid (Thomas et al. 2017). Goniothalamus has quite often moved on to islandsThe large New World genus Guatteria (ca 177 species) has undergone much speciation in (Amazonian) South America perhaps only within the last 8.8-4.9 Ma, having moved to South America from Central America, and ultimately from Africa via Europe (Erkens 2007; Erkens et al. 2007a, b, 2009). Rather like other rainforest trees in South America, relationships in Guatteria show little geographic structuring, apart from those of species in the Atlantic rainforest in south east Brazil (Dexter et al. 2017).
The Old World Uvaria, ca 100 species, lianes, may have originated (38.4-)31.6(-25.1) Ma in Africa, whence it dispersed to other parts of the Old World (L. Zhou et al. 2012: HPD). Indeed, Thomas et al. (2014) suggest that there have been around 11 dispersals from Africa to Asia, and only one from Asia to Africa; Late Palaeocene to late Miocene are the suggested times.
Xylopia, with some 191 species, is the only pantropical genus in Annonaceae. It probably originated in Eocene boreotropical forest, Africa certainly being important in its early history (see e.g. two of the three basal sections - Nge et al. 2025b), then moving to the Asia-Pacific region and also to the Neotropics, the latter movement probably being over water (D. M. Johnson et al. 2025, see also Nge et al. 2025b). A few species grow in inundated habitsts, and may even have stilt roots. Interestingly, the genus is quite well represented in Africa, 42/59 of the species there growing in subhumid habitats (Johnson et al. 2025). It is found on some 51 tropical islands, most species there being single-island endemics, and all told there are 59 island species; 18 species of Xylopia grow on ultramafics, and all occur on islands. Although long distance dispersal seems to have been important here, it hhas rarely involved inter-continental movements; Johnson et al. (2025) focus on bird dispersal, noting that the seeds are fleshy (aril or sarcotesta) and quite moderate in size.
For pollen morphology and evolution, see Doyle (2009), Doyle et al. (2000) and especially Doyle and Le Thomas (1994), and for this and other aspects of floral morphology, see Doyle and Le Thomas (1996, 1997) and especially Saunders (2020). Mols et al. (2004b) explored character evolution within Malmeoideae, while Chaowasku et al. (2014) optimised the distributions of a number of characters in Miliuseae. Lopes et al. (2017) optimised characters on a tree of Malmeeae; are anthers with tongue-like connectives ancestral for the family, with truncate connectives evolving seven times, or is the ancestral condition truncate, the tongue-like connective evolving three times and reversing to truncate three times (ibid.: c.f. Figs 3 and 5C...)? Additional data used for the hierarchy was taken from Mols et al. (2008), Saunders et al. (2011), Chaowasku et al. (2012), Guo et al. (2017b) and especially Chatrou et al. (2012). For the evolution of syncarpy, etc., in African Annonaceae, see Couvreur et al. (2008a).
Ecology & Physiology. Annonaceae are important lianes of the tropical South East Asian forests in particular (Gentry 1991; Appanah et al. 1993), and there have been several origins of this habit within Annonoideae (Meinke et al. 2011); all told, ca 500 species of annonaceous lianes are known from the palaeotropics (Couvreur et al. 2015). Artabotrys (?Xylopieae), one of these lianes, has curved hooks on the ultimate branches that are modified inflorescences, while the orthotropic stems may have stout, paired and rather vicious straight thorns (Xylopia itself may have two axillary branches per node - D. M. Johnson & Murray 2018). Other lianes may lack hooks or thorns, as in the major liane clade Monanthotaxis, in Uvarieae (Guo et al. 2017a; see also Sousa-Baena et al. 2018).
Annonaceae are notably common in terms of both numbers of species (third in the list) and individuals in the Amazonian tree flora, but they include only 4 of the 227 species that make up half the stems 10 cm d.b.h. or more (ter Steege et al. 2013). Guatteria, often a smallish tree, is diverse there, and like similar Amazonian taxa it has a rather short generation time (T. R. Baker et al. 2014).
Xylopia rubescens (Annonoideae-Xylopieae) is one of the four common species mentioned growing in the ca 145,500 km2 of peat in the Cuvette Centrale in the Congo (Dargie et al. 2017).
Pollination Biology & Seed Dispersal. Saunders (2010: good photographs, and in particular 2020) and Gottsberger (2012, 2016a) summarize variation in floral morphology in Annonaceae in the context of variation in pollination mechanisms; deceptive pollination is also well known here (S. D. Johnson & Schiestl 2016). Flowers in which the stamens are enclosed in some sort of bowl-shaped structure formed by the inner perianth whorl are common, and the androecium can become completely enclosed by the movements of the tepals, so trapping the beetle visitors (e.g. B. Xue et al. 2019; Mei & Xu 2020: pollination chamber). There are often floral rewards, thus nectar is quite often produced by epithelial nectaries, whether or not there are nectarostomata, on the inner surfaces of the inner perianth whorl (Erbar 2014; B. Xue et al. 2017; Paiva et al. 2020), and there may be elaiophores, producing substances like lipids that help the pollen to stick to the pollinator (Paiva et al. 2020). Nectar production is scattered throughout the family, although it is not yet recorded from Anaxagoreoideae (Xue et al. 2017). A number of Annonaceae-Annonoideae have so-called circadian trap flowers, a specialization of the open pollen chamber otherwise ubiquitous in the family. The petals open and close in time with the bimodal circadian rhythms of their insect visitors (Lau et al. 2017b). Anthesis is shorter here - overall, about a day - than in many other non-trapping Annonaceae, where it is about two days; furthermore, there may be an association with floral synchrony, the pistillate and staminate phases of anthesis not occurring together on the one individual (Lau et al. 2012b). There are also trap flowers in the species-rich part of Artabotrys (J. Chan et al. 2020 - relationships here uncertain).
The flowers smell, sometimes quite strongly, and the odours produced are diverse and include those of decaying animal tissues (Goodrich 2012; Jürgens et al. 2013: different odour bouquets; Gottsberger 2016b), interestingly, 4-methy-5-vinylthiazole plays an important role in attracting cyclocephaline scarab beetles in Annona as well as in Caladium (Araceae) (Maia et al. 2012) and there are other similarities in the odours that the two families produce. Flowers of many Annonaceae are also thermogenetic (Seymour 2001; Gottsberger 2016b). Some kind of extracarpellary compitum - some taxa have a suprastylar extragynoecial compitum, others perhaps an infrastylar extragynoecial compitum - is common (Gottsberger 2016a), although I do not know details of their distribution - certainly Annoneae for the former (Deroin 1991; Igersheim & Endress 1997; Lau et al. 2017a; B. Xue et al. 2019; Saunders 2020), indeed, stigmatic exudates from the one species may function both as a compitum, a food reward for the pollinator, and provide a suitable medium for the germination of the pollen (Lau et al. 2017a). The tough, apically expanded connective that is commonly found in stamens of Annonoideae and Malmeoideae may protect the pollen from the depradations of unwanted visitors (Gottsberger 1999, 2012; Silberbauer-Gottsberger et al. 2003).
Pollination of the flowers is predominantly - and perhaps ancestrally - by beetles. Thus Neotropical members of Anaxagorea are pollinated by Colopterus, small nitidulid (sap) beetles, but other families like scarabs - some of which are quite large - and weevils are involved (Gottsberger 2016a, b). Other groups of insects, including flies, thrips, bees, and cockroaches, are also pollinators (Corlett 2004; Su et al. 2008; Saunders 2012; Gottsberger 1970, 2012, 2016a; Paiva et al. 2020; Haran et al. 2023; Dagallier et al. 2023a; M-F. Liu et al. 2024, etc.). Interestingly, the nitidulid-pollinated Anaxagorea prinoides is heterodichogamous (see also Laurales), and the insects move from old flowers at the male stage to young flowers on another plant at the female stage, being attracted by the scent of the latter (Teichert et al. 2011). Large-flowered species of Neotropical genera like Annona are pollinated by larger dynastid scarab beetles, Scarabaeidae, e.g. Cyclocephala, although again nitidulids may also be important pollinators (Nadel & Peña 1994; M. R. Moore & Jameson 2013; Gottsberger 2016a, b). Dagallier et al. (2023a and references) noted that Scarabaeidae and Curculionidae beetles both ate and laid eggs in the fleshy petals of Uvariodendron (Monodoreae), for example, and there was also thermogenesis of sorts. There is often development of corrugations, etc., on the inner petals, and these are frequently eaten by beetles, drosophilids, etc., affording a reward - indeed, the flowers are commonly fruit mimics in Annonaceae (M.-F. Liu et al. 2025). Recent work suggests an interesting form of deceit pollination in Meiogyne heteropetala; here the corolla is a dark maroon colour (the inner petals are covered with excrescences - function?), and the plant produces volatile terpenoids similar to those produced by decaying litter (Liu et al. 2024). The flower is visited by Loberus sharpii, an erotylid beetle that normally lays eggs in decaying litter in trees; here it may lay eggs in the flowers, and the eggs hatch, although the larvae eventually die. As elsewhere in the family, there is deceptive floral mimicry of brood sites.
Nearly all Annonaceae have perfect flowers, and protogyny or protogyny will not prevent movement of pollen from one flower to another on the same plant (geitonogamy). However, all the flowers on an individual plant may be at either the pistillate or the staminate phase, so preventing selfing; this condition is known as duodichogamy and is quite widely distributed within the family, including Anaxagorea (Pang & Saunders 2014, 2015). Since not all the flowers on a single tree may be at the same phase, or the synchronization of the between-individual switch between phases may not be precise (Gottsberger 2016b), there is a bit of slop in the system. The internal staminodes of Anaxagorea cover the gynoecium in the staminate floral phase, apparently preventing selfing (Gottsberger 2012; Saunders 2012).
Dispersal of fruit is largely by mammals and birds. The lianes of tropical South East Asia are notably important food resources for frugivores because they fruit more or less continually (Leighton & Leighton 1983). In species with lomentum fruits maturation is acropetal, birds removing individual units of the lomentum as they become ripe (B. Xue et al. 2019, q.v. for other fruit types). The colour combinations in species with arillate seeds can be somewhat startling - see the red aril contrasting with a green endocarp in Xylopia (Stull et al. 2017; also D. M. Johnson & Murray 2018).
Plant-Animal Interactions. Annonaceae are important food plants for swallowtail caterpillars, e.g. Graphium, Eurytides (Nazari et al. 2007). Over 550 kinds of acetogenins, C-35/37 metabolites derived from C-32/34 fatty acids in the polyketide pathway are known from Annonaceae, epecially from Annonoideae, and these are variously antimicrobial, feeding deterrents, etc., and they even inhibit corrosion of mild steel (Cavé et al. 1997; Liaw et al. 2016; Neske et al. 2020). Acetogenins from pawpaw, Asimina spp., are sequestered by larvae of the zebra swallowtail, Protographium marcellus, while larvae of the wasp Bephratelloides cubensis live inside the seeds of Annona macroprophyllata, tolerating the toxic levels of acetogenins there (Durán Ruiz et al 2019). Interestingly, the swallowtails that eat Annonaceae belong to Leptocircini, a tribe of around 140 species that may not be immediately related to other Papilioninae, in which they have often been included (Espeland et al. 2018: [Baroniinae [Leptocircini [Parnassinae + other Papilioninae]]]). Leptocircini diverged from other Annonaceae ca 66 Ma at the K/P boundary, and their diversification rate seems to have temporarily increased as they moved on to Annonaceae (Allio et al. 2020/2021). For more on Annonaceae and swallowtails, see papers in Scriber et al. (1995), and for swallowtails in general, see Aristolochiaceae.
Vegetative Variation. The main axes of Annonaceae are often mixed (?Troll's model), initially being orthotropic and with spirally-arranged leaves, later being more or less plagiotropic with two-ranked leaves (see also Eupomatia). Taxa such as Asimina and Annona have orthotropic axes with 2-ranked leaves, the very apex being bent over (D. M. Johnson 2003), while Johnson and Stull (2014) outline vegetative variation in the family. The optimisation above is based largely on their work, especially that of Johnson (2003); how Meiocarpidium (near basal, Meiocarpidioideae) grows is unknown. In general, axillary branches depart from the stem slightly to one side of the axil. Branches are often plagiotropic, and their leave are two ranked, and in some species of Xylopia, etc., the plagiotropic branch systems are frondose and very beautiful - lateral branches may not branch and they may fall off as units (Johnson 2003) - cladoptosis. Tetrameranthus sometimes seems to have opposite leaves, and according to George Schatz (pers. comm.) it lacks plagiotropic branches and all branches have spirally-arranged leaves, while Chatrou et al. (2012) suggest that species in Annonoideae-Bocageeae, -Xylopieae and -Duguetieae may also have have branches with spiral leaves. Fries (1959) noted that a number of taxa, including Anaxagorea, had vegetative shoots with adaxial prophylls, i.e. the monocot position, but prophylls were lateral in some Annonoideae, and these latter genera are all reported to have main axes with 2-ranked leaves. A detailed survey of vegetative/floral prophylls (for the latter, see below) and leaf position would be useful.
Genes & Genomes. Substitution rates in chloroplast DNA have long been known to be higher in Annonoideae than in Malmeoideae, about three times higher being the estimate in Chatrou et al. (2014), hence their informal names, the long and short branch clades. This difference is also evident in nuclear rDNA (Hoekstra et al. 2016) and recently it has been found in nuclear DNA, too (Chatrou et al. 2019).
Okada and Ueda (1984) and Morawaetz (1986, 1988) provide some cytological information. Morawetz (e.g. 1986b, 1988, see also Doyle & Le Thomas 1997) describe distinctive patterns of chromosome condensation in Annonaceae, which may well have some phylogenetic signal.
The plastome of Annonaceae is notably large, over 170,000 bp (Y. Yu et al. 2019b). For a chloroplast inversion in Miliuseae, see Arias et al. (2014b), and for the PEP subunit α rpoA gene, see Blazier et al. (2016a).
Chemistry, Morphology, etc.. Protoberberidine alkaloids are reported from Annonaceae (Wink 2008). Hundreds of different 35- or 37-C polyketide acetogenins, unique to the family, are known from Annonaceae, especially from Annona itself, but from no other plants (Cavé et al. 1997; Alali et al. 1999; Liaw et al. 2016). Based on the list of genera in which they have been found in Liaw et al. (2016), their occurrence is noted at four places in the hierarchy above, but there seems to have been no systematic survey for their occurrence throughout the family, or even in Anaxagorea. Ultimately they will need to be placed as an apomorphy at some level.
Cork in the roots of Goniothalamus may be superficial (Blunden & Kyi 1974). The wood occasionally fluoresces. Terminal tracheids are fairly commonly silicified (Piperno 2006). For the petiolar anatomy of Anaxagorea, see Jovet-Ast (1942) and Maas and Westra (1984); it is unlikely that there are annular petiolar bundles, although the midrib anatomy can be complex.
Adaxial prophylls seem to be consistently present in the inflorescences of Annonaceae (Friis 1911, 1919, 1959, see also Kessler 1989). The inflorescence is terminal, but when the plant is in flower or fruit it is often apparently axillary or leaf-opposed, but this is due to complex patterns of recaulescence, eviction, etc.; along the same lines, single axillary flowers are also only apparently axillary. There is an articulation at the base of the pedicel, and whether there are bracts/bracteoles above and/or below this articulation, or none at all, varies (Fries 1919, 1959). Rutishauser (2016b) discussed the irregular phyllotactic patterns in the flowers of Cananga and other angiosperms.
The anthers of Annonoideae are sometimes polysporangiate and they then produce pollen tetrads/polyads that can be very large and with a massive exine (Tsou & Johnson 2003 and references). The septae of these polysporangiate anthers may be tapetal or parenchymatous, the latter perhaps especially in the [Annoneae [Monodoreae + Uvarieae]] clade, although both kinds occur in Annoneae (Tsou & Johnson 2003). There are reports that the pollen grains of both Annona and Asimina may germinate via the proximal pole (Hesse et al. 2009a), although in the former (and some other genera) individual grains of the tetrad may rotate during development (Tsou & Fu 2002, 2006; Lora et al. 2009b). Hesse et al. (1985) described the pollen of Anaxagorea, noting that the endintine near and under the colpus was massive, and became extruded through the colpus as a hemispherical blob (it "swell[s] up in an exorbitatnt manner" - ibid., p. 273), sometimes even before the anthers dehisced (see also Le Thomas 1980). The exact distribution of this feature in the family is unclear, although it is found in members of at least five tribes in Malmeoideae (Chaowasku 2021). Pollen work on Annonaceae has been quite extensive - see Walker (1971), Le Thomas (1980, 1981: major survey), Couvreur et al. (2008b: Monodora group), Chaowasku et al. (2008: Miliusa et al.), Doyle (2014a: summary) and and Shao and Xu (2018: Thai taxa).
Reports that the micropyle is formed by both integuments need to be confirmed (c.f. Svoma 1998b). Monodora (derived) has a rather thin outer integument, and the anatomy of the seed coat of Ambavioideae with a third integument ("Zwischenintegument") can be complex (Christmann 1986; see also Perisamy & Swamy 1961). Cymbopetalum is reported to have a vascularized aril, while the aril of Xylopia is at the micropylar end of the seed (see also Corner 1976), perhaps because of the extension of the raphe to the micropyle (Stull et al. 2017); other genera may also have reduced arils (Svoma 1997). In Bogageeae, at least, what otherwise appear to be follicular fruits may open on their abaxial surface (Lopes et al. 2023).
For an introduction - and much more - to Annonaceae, see the papers in Bot. J. Linnean Soc. 169(1). 2012, including Erkens et al. (2012a, also 2017) for a comprehensive bibliography and Couvreur et al. (2012) for keys to the genera; see also Fries (1959), Kessler (1993), D. M. Johnson and Murray (1995: Bocageeae, 2018: African Xylopia), Chartrou and He (1999: Fusaea), Bakker (2000a, b), Maas et al. (2003: Duguetia, 2015: Guatteria) and Versteegh and Sosef (2007: Annickia) for general information. Also Hegnauer (1964, 1989: chemistry), dos Santos et al. (2017: alkaloids) and Neske et al. (2020: 115 new acetogenins in the last 15 years), also Jovet-Ast (1942: indumentum and anatomy), Junikka and Koek-Noorman (2007: bark anatomy), Koek-Noorman and Westra (2012: wood anatomy), Kavathekár and Pillai (1976: nodes), van Setten and Koek-Noorman (1986: leaf anatomy), Sun et al. (2008: epidermal anatomy), van der Wyck and Canright (1956: anatomy), Fries (1919: inflorescence), G. H. Smith (1928: floral anatomy), Deroin (1991b: stigmatic surface and carpel), Steinecke (1993: esp. flower and fruit), Rudall and Furness (1997: tapetum), Endress (2011b: ovules), Deroin (1999a: receptacular vascular system), Lora et al. (2009: number of pollen nuclei varies within Annona cherimola), also Corner (1949a, 1976), Svoma (1998a), van Setten and Koek-Noorman (1992), Corona Velásquez et al. (2016), Galastri and Oliveira (2016) and Gan and Xu (2017: Cananga), all ovules, fruits and seeds, and for seed xyloglucans, see Kooiman (1960, 1971). For floral (developmental) morphology, see van Heusden (1992), Ronse Decraene and Smets (1990b), Leins and Erbar (1980, 1982, 1996, 2010), Xu and Ronse De Craene (2010b), Endress (1975: carpel and stamen development), Endress and Armstrong (2011: Anaxagorea) and Ronse de Craene (2018).
Phylogeny. See Doyle and Le Thomas (1994, 1996) for morphological phylogenies; as Doyle and Le Thomas (1997) emphasized, the inclusion of pollen characters yielded a topology very different from when they were excluded, Anaxagorea and Ambavioideae being successively basal in the tree in the first case and deeply embedded in the tree in the second. Interestingly, the first topology largely agrees with that of molecular analyses. Two major clades make up the rest of the family (see also Richardson 2004). Although this topology is generally recovered (see also X. Guo et al. 2017b), Chatrou et al. (2014) found a clade [Annonoideae + Ambavioideae]. See Z.-D. Chen et al. (2016) for relationships among Chinese taxa. The position of Unonopsis on the tree has been somewhat erratic, perhaps because of ancient paralogy (Pirie et al. 2007); in Couvreur et al. (2018: nuclear phylogenomic analysis) it was placed in Malmeeae as sister to Onychopetalum, and with strong support; there seems to have been a certain amount of ancient hybridization in the family (Guo et al. 2014, 2018: Dasymaschalon × Friesodielsia; Couvreur et al. 2018), however, Couvreur et al. (2018) did not expect mejor differences between analyses based on plastome and nuclear DNA... Thomas et al. (2017: Appendix S4) found relationships in both Malmeoideae and Annonoideae that differ in detail from those below, although support was commonly rather poor. Nge et al. (2024) examined relationships between all 108 genera using a 373 locus Annonaceae-specific nuclear bait kit.
Anaxagoreoideae. Scharaschkin and Doyle (2005, 2006) provide a molecular phylogeny of Anaxagorea that is integrated with morphological variation; the South American A. prinoides is sister to the rest of the genus.
For relationships in Ambavioideae, see Surveswaran et al. (2010); the basic phylogenetic structure seems to be [Meiocarpidium [Tetrameranthus et al. + Cananga et al.]] - see also Chatrou et al. (2014) and B. Xue et al. (2018). However, support for the position of Meiocarpidium was not always strong, and Couvreur et al. (2018: nuclear genomes) found that this position had weak support, or the genus might be sister to [Malmeoideae + Annonoideae]. In an analysis using plastid genes Chaowasku (2020: up to 8 chloroplast markers depending on analysis, focus on Ambavioideae) found that Meiocarpidium did not group with the rest of Ambavioideae, rather, relationships were [Meiocarpidium [Ambavioideae [Malmeoideae + Annonoideae]]], with good suport. He suggested subfamilial status for Meiocarpidium, and dividing Ambavioideae into Ambaveae and Canangeae - relationships there were [[Tetrameranthus [Mezettia [Ambavia + Cleistopholis]]] [Lettowianthus [Cananga, Drepananthus, Cyathocalyx]]] - support generally good. However, Helmstetter et al. (2024) found that Meiocarpidium was sister to other Ambavioideae and so placed it in that subfamily as a tribe rather than as a separate subfamily, ad did Nge et al. (2024: Fig. 2G, support could be stronger...).
Malmeoideae have relatively short molecular branches, hence their early name, the short-branch clade, and they are not very speciose, mostly lacking large genera aside from Polyalthia (Mols et al. 2008; B. Xue et al. 2011; Thomas et al. 2015). There was some support for the pectinate structure of the classification above in X. Guo et al. (2017b; see also B. Xue et al. 2018), however, support for the monophyly of Malmeeae was not very strong. Couvreur et al. (2018) found that within Piptostigmateae Annickia was either sister to the rest of the tribe, but with rather poor aupport (concatenated analyses), or sister to rest of the subfamily and with stronger support (gene tree analysis). Within Malmeeae, relationships around Oxandra and Klarobelia are particularly unclear (Guo et al. 2017b), and the first genus turned up in three places in the comprehensive analysis of Lopes et al. (2017) and the second genus in two places. Miliuseae: Chaowasku et al. (2014) clarified some relationships here, although support for the branches along the spine was still somewhat ambiguous; see also Xue et al. (2018) and Couvreur et al. (2018), the latter group finding that support for some relationships here was still problematic in their phylogenomic analyses. Polyalthia was found to be polyphyletic, but it is still a sizeable genus (Richardson et al. 2004; Xue et al. 2011, 2012). New World members of this clade form a monophyletic group (Pirie et al. 2006), and see also Ortiz-Rodriguez et al. (2016). For Pseuduvaria, see Su and Saunders (2006), Su et al. (2008, 2010) and Guo et al. (2017).
Annonoideae show more internal molecular divergence (hence their name, the long branch clade: see also J. A. Doyle et al. 2004; Pirie et al. 2005; Thomas et al. 2015). For the likely position of Guatterieae as sister to the rest of the subfamily except Bocageeae, see Couvreur et al. (2018). Interestingly, these authors found that although Xylopieae and Duguetieae were sister taxa, as in other studies, the large genus Artabotrys moved from the former to the latter, and with good support (Couvreur et al. 2018), while in the nuclear analysis of Helmstetter et al. (2024) there was some support for the relationships [Xylopia [Artabotrys + Duguetieae]] - Artabotrys is included in Duguetieae above, Xylopia-Xylopieae are the next branch down (Nge et al. 2024: Figs 2H, I). Although chloroplast data suggested that [Ophrypetalum + Sanrafaelia] were sister to all other Monodoreae, they moved - and with strong support - to sister to Uvarieae, and were then placed in their own tribe (Dagallier et al. 2023a, see also e.g. Couvreur et al. 2019).
Annoneae. Tang et al. (2015: character optimisations; see also Nakkuntod et al. 2009) focussed on Goniothalamus, where there is quite extensive variation in the fruit, while Larranaga et al. (2018) looked at relationships around the cultivated species of Annona.
Bocageeae. Lopes et al. (2023: 47/66 spp., 116 nuclear markers) found general similarity in the topologies obtained in their RaXML and ASTRAL analyses, although Porcelia was migratory and there were differences in species relationships (these were not their focus); the African Mkilua fragrans was sister to the rest of the tribe and Bocagea was embedded in Trigynaea.
Guatterieae. For Guatteria, see Erkens (2007), Erkens et al. (2007a, b); G. heteropetala and G. anomala are successively sister to the rest of the genus.
Monodoreae. In the comprehensive study by Dagallier et al (2023a: 334 nuclear loci, 90 spp., 207 specimens; see also Dagallier et al. 2023b: 32 most clock-like nuclear markers) most of the genera had strong support, but within several genera nodes were weakly supported and there was phylogenetic conflict.
Piptostigmateae. Brée et al. (2020: 30 spp., 32 nuclear sequences) clarified relationships here (and in the tiny Annickieae).
Uvarieae. Uvaria is polyphyletic (Mols et al. 2004a), and L. Zhou et al. (2009a, esp. b, 2010) suggested that its limits be extended (see also Meade & Parnell 2018: Asian species); geography and phylogeny correlate quite well. The topologies of molecular and morphological trees of Uvaria (Meade & Parnell 2018) have rather little to do with each other, other than both having branches. The limits of genera like Dasymaschalon and Friesodielsia were initially unclear (Wang et al. 2012: independent loss and subsequent parallel evolution in taxa that have lost their inner petals), but there is probably intergeneric hybridization, some species of the former grouping with the latter in chloroplast analyses, although Dasymaschalon is monophyletic in nuclear analyses (X. Guo et al. 2014, see also 2018).
Xylopieae. For relationships in Xylopia, see Stull et al. (2017: plastid data and seed morphologu). The genus was also examined by Nge et al. (2025b: 168/191 spp., Annonaceae-specific bait set, 272/267 loci); basaal sectional relationships are [Rugosperma [Neoxylopia [Ancistropetala + the rest]]], the first three sections being very small, a mere 11 species total. There is little difference between the topollogies of the coalescent and concatenation analyses.
Classification. A useful classification was outlined by Chatrou et al. (2012; see also X. Guo et al. 2017b); Chatrou et al. (2012) explain clearly their classificatory principles (those of Haston et al. 2007) while Chatrou et al. (2018) provide a linear generic sequence for the family. The limits of genera in Annonoideae seem particularly uncertain, but for genera throughout the family and keys to identify them, see Couvreur et al. (2012) and Nge et al. (2024). L. Zhou et al. (2010) clarify the limits of Uvaria and B. Xue et al. (2011, 2012) those of Polyalthia, Surveswaran et al. (2010) discuss generic limits in the ambavioid clade and Couvreur et al. (2018) propose a few modifications to previous classifications and suggest places that relationships are problematic. Stull et al. (2017) provide an infrageneric classification for Xylopia; there are five sections, one of which is divided into six groups, another into four (see also Nge et al. 2025b: note that section Verdcourtia of Stull et al. has become one of the six groups of sect. Stenoxylopia). The classification above follows that proposed by Nge et al. (2024), q.v. for discussion; they also recognized 25 subtribes. For a checklist of the New World species of the genus, now somewhat dated, see Maas et al. (2011); Annonbase should also be consulted.
Thanks. To Joshua Grosse, for information about acetogenins.