EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID II / MALVIDAE / [[GERANIALES + MYRTALES] [CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]]: ?
[CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]: ?
[PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]: ovules 2/carpel, apical.
[SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]: flavonols +; vessel elements with simple perforation plates; (cambium storied); petiole bundle(s) annular; style +; inner integument thicker than outer; endosperm at most scanty.
[HUERTEALES [MALVALES + BRASSICALES]]: ?
Age. The age of this node was estimated to be (104-)96, 86(-75) Ma (Bell et al. 2010) or about 104.1 Ma (Tank et al. 2015: Table S1).
Phylogeny. For discussion see the Sapindales page.
HUERTEALES Doweld - Main Tree.
Vessel elements with scalariform perforation plates; mucilage cells +; leaves spiral, lamina margins toothed, stipules cauline; inflorescence cymose; pedicel articulated; flowers "small" [≤8 mm across], hypanthium +, short; nectary inside A; G [2], ovary unilocular; fruit a berry; exotegmen rather massive, of more or less strongly laterally-compressed fibres; endosperm +, embryo at most medium. - 4 families, 6 genera, 29 species.
Includes Dipentodontaceae, Gerrardinaceae, Petenaeaceae, Tapisciaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Evolution: Divergence & Distribution. For some comments on the biogeography of Huerteales, see Christenhusz and Chase (2013).
Overall, the flowers of Huerteales seem to be rather similar, although there is variation in androecial position in particular. The mechanical (lignified) layer of the seed may differ in its origin within the order, but I have tentatively suggested that exotegmic seeds are a synapomorphy for the clade, however, developmental studies of seeds of taxa like Gerrardina and Petenaea are much needed.
Chemistry, Morphology, etc.. Worberg et al. (2009) summarize what is known about this group, and information in their summary is slightly emended below; see also Yang et al. (2009) for chromosome numbers.
The morphology, anatomy, and secondary chemistry of the whole group are badly in need of detailed investigation.
Phylogeny. For the association of the Tapisciaceae and Dipentodontaceae, see Peng et al. (2003). Perrottetia was previously in Celastraceae, if only rather uneasily so; molecular data suggested that it was to be placed near Tapiscia (M. Simmons, in Matthews & Endress 2005b), a placement with which its morphology is in general agreement. Gerrardina eylesiana shows a possibly superficial but still striking similarity to Perrottetia in particular, and there may be a similarity in seed coat anatomy between the two, yet they do not appear to be sister taxa (Worberg et al. 2009). Petenaea has been found to be rather weakly (bootstrap) associated with Gerrardina, and in general bootstrap values for relationships are low, even if posterior probabilities are higher (Christenhusz et al. 2010). It has been suggested that relationships within the order are [[Gerrardinaceae + Petenaeaceae] [Tapisciaceae + Dipentodontaceae]] (e.g. M. Sun et al. 2016; see also Worberg et al. 2009), while in the nuclear analysis of W. J. Baker et al. (2021: see Seed Plant Tree and other versions) and also in the plastome analyses of H.-T. Li et al. (2021) relationships are well supported as being [Gerrardinaceae [Petenaeaceae [Tapisciaceae + Dipentodontaceae]]], and these relationships are followed here.
Classification. Like Crossosomatales, Huerteales have accumulated several small families; when the clade is better known, rationalization (= reduction in number) may be in order - we currently hardly know enough so say whether or not the families are similar other than in gross phenetic terms, and none is of any size.
Synonymy: Dipentodontales C. Y. Wu et al. - Huerteanae Thorne & Reveal
GERRARDINACEAE M. H. Alford - Gerrardina Oliver - Back to Main Tree
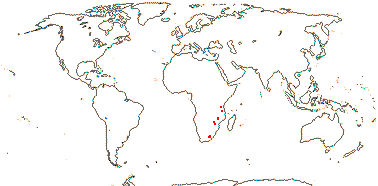
Petiole bundle arcuate, or three in an arc; mucilage cells in epidermis; lamina with vein proceeding to glandular tooth and with a branch to the vein above; ?pedicel articulated; C clawed, thin, small; A = and opposite C; nectary cupular; style, stigma?; ovules?; seed with a fleshy outer layer, lignified laterally-compressed cells inside; endosperm ?type, embryo minute; n = ?
1 [list]/2. Eastern and southern Africa. Map: data from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and GBIF (consulted vii.2009).
Chemistry, Morphology, etc.. Gerrardina foliosa appears to have clawed petals and three sepals larger than the others (ToL, vii.2009).
See Alford (2006) for what little is known about the family; the description of the venation is modified from that source. Christenhusz et al. (2010) describe the wood anatomy of young stems. In addition, see G. eylesiana: stem anatomy, J. D. & E. G. Chapman 9242; leaf anatomy, Brass 16641; seed anatomy, Iversen et al. 85748; G. foliosa: stem and seed anatomy, Strey 11052; leaf anatomy, Hilliard & Burtt 6751.
Previous Relationships. Gerrardina has previously been included in Flacourtiaceae.
[Petenaeaceae [Tapisciaceae + Dipentodontaceae]]: ?
PETENAEACEAE Christenhusz, M. F. Fay & M. W. Chase - Petenaea cordata Lundell - Back to Main Tree
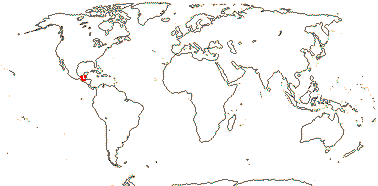
Vessels in radial multiples; lamina venation palmate, stipules ?minute; P +, uniseriate, valvate, with adaxial moniliform hairs and basal glands; A 8-12; G [5], placentae pendent, style long, stigma punctate; ovules many; berry lobed, with a persistent style; n = ?
1 [list]/1. Central America. Map: from Christenhusz et al. (2010).
Chemistry, Morphology, etc.. For the wood anatomy of Petenaea, see also Kukachka (1962). The genus is described as having minute stipules (Bayer 2002).
For some general information, see Christenhusz et al. (2010).
Previous Relationships. Petenaea has previously been linked with Tiliaceae and Elaeaocarpaceae (Mabberley 2009), Bayer et al. (1999, but sampling) had suggested on molecular data that it might be associated with Malvales, being tentatively associated with Muntingiaceae (see also A.P.G. 2003).
[Tapisciaceae + Dipentodontaceae]: wood parenchyma 0; A = C, opposite K/outer T.
Age. The age of this clade is around 89.8 Ma (Tank et al. 2015: Table S2).
TAPISCIACEAE Takhtajan - Back to Main Tree —— Synonymy: Huerteaceae Doweld
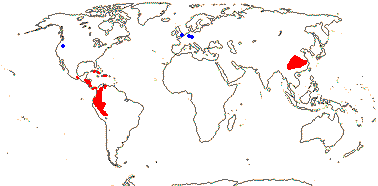
Wood fluorescing; fibres with simple pits, axial parenchyma 0 [Tapiscia]; nodes also 4:4, 5:5; petiole bundle annular, fibrous sheath 0; stomata ± paracytic; leaves compound, odd-pinnately or trifoliolate, glands or stipels at articulations, (stipules obscure); (plant androdioecious - Tapiscia); K connate or not; (anthers extrorse, inflexed - Huertea); pollen 10-20 μm long, colpate; nectary not vascularized (0); G septate or not, style hollow, apically branched or styles ± separate, stigma?; ovule 1/carpel, basal, apotropous, outer integument 2-3 cells across, inner integument 2-3 cells across, parietal tissue 4-6 cells across, hypostase +; (fruit a drupe); testa thin-walled, (mesotesta sclerotic), chalaza/hypostase ballooning into endosperm; endosperm ?type, embryo medium to small, coyledons flat; n = 13, 15, x = ?
2 [list]/5. China, West Indies and N.W. South America. Map: from Ying et al. (1993) and GBIF and TROPICOS (consulted vii.2009); fossils [blue] from Manchester (1988). [Photo - Fruit]
Evolution: Divergence & Distribution. The distinctive fruits of Tapiscia are known from the Eocene onwards in Europe, and somewhat later in North America (Manchester 1988; Manchester et al. 2009 and references).
Pollination Biology & Seed Dispersal. In Tapiscia sinensis the fruit takes about eighteen months to develop, and the receptacle also becomes inflated and suberised (Liu et al. 2008; Teng & Liu 2009).
Chemistry, Morphology, etc.. Dickison (1987b) noted that small accessory traces from the central bundle also proceeded into the leaves.
There is quite a prominent nectariferous disc in Huertea, but it is not vascularized (Dickison 1986a; Danilova 1996). In ripe fruit of Tapiscia sinensis, at least, the seed is born in the middle of the loculus, perhaps partly due to the strong post-fertilization development of the hypostase (Liu & Ni 2013).
For general information, see Kubitzki (2002d), also Carlquist and Hoekman (1985: wood anatomy), G.-L. Xin et al. (2019: floral development Tapiscia, Dickison (1987a) and Wei et al. (2002), both pollen, and Corner (1976: seed anatomy).
Previous Relationships. The two genera that make up the family were long included in Staphyleaceae (but see Corner 1976 for differences in seed coat, Jin and Wei 2002 for the pollen of Tapisciaceae, smaller than that of Staphyleaceae, and Dickison 1987b and Simmons (2002) for a table of differences separating them from the rest of Staphyleaceae) and placed in Sapindales by Cronquist (1981), while a segregated Tapisciaceae were still included in Sapindales by Takhtajan (1997).
DIPENTODONTACEAE Merrill, nom. cons. - Back to Main Tree
Trees; ?vessel elements; leaves two-ranked, secondary veins pinnate, stipules +; T +, outer T ± valvate; ovules 2/carpel, erect; T persistent in fruit; endosperm ?type; x = ?
2 [list]/21. Southeast Asia to Malesia, Mexico to Peru.
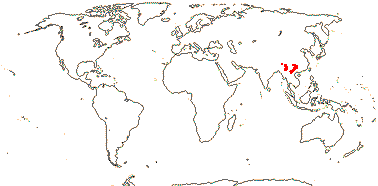
1. Dipentodon sinicus Dunn
Petiole bundle arcuate; ?stomata; hairs uniseriate; stipules lobed; inflorescence umbellate; ?pedicel articulation; flowers (4-)5(-7)-merous, hypanthium spreading; outer T basally connate, inner T valvate; nectaries opposite C [?staminodia]; G [3], placentation axile basally, with a free-central prolongation, ovules borne on top of placenta, stigma punctate; ovule ?morphology; fruit laterally compressed, ?septicidally dehiscent from the base upwards, funicle ?much enlarged; seed single; coat with obliquely-lying lignified ribbon-like cells, collapsed polygonal cells underneath; embryo ?very short; n = 17.
1/1. S. China and adjacent Burma, India and Vietnam. Map: from Yuan et al. (2008).
2. Perrottetia Kunth
(Vessel elements simple); paratracheal parenchyma +; sclereids +; petiole bundle depressed annular with an adaxial inverted bundle and wing bundles; stomata (anisocytic); buds naked; leaves (spiral), vernation involute; plant dioecious [?always]; inflorescence thyrsoid, ultimate units ± cymose; (flowers 4-8-merous); inner T valvate; ovary septate, style short; ovules basal, epi-apotropous, micropyle endostomal; testa thin-walled, ± fleshy, inner tangential walls of endotesta with rod-like structures, exotegmen ridged; embryo medium; n = 10.
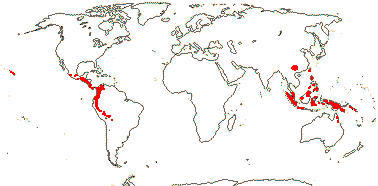
1/20. China, Taiwan and Myanmar to Malesia and N.E. Australia, Hawai'i (2 spp.), Mexico to Bolivia. Map: from Ding Hou (1962), Thorne (1972), and FoC vol. 11 (2008). New World rather approximate.
Chemistry, Morphology, etc.. Ding Hou (1962) described and illustrated the nectary of Perrottetia as being disc-like and entire (c.f. Bachelier & Merran 2014). The genus has idioblasts in the sepals with thickened inner tangential walls, and the seeds lack an aril (Corner 1976; c.f. Ding Hou 1962; Yang et al. 2009).
Mabberley (1997) described the leaves of Dipentodon as being spiral. In both of the two flowers that I examined I could find only a single well-developed ovule.
For general information on Perrottetia, see Ding Hou (1962), for floral morphology, etc., see Matthews and Endress (2005b: also mucilage cells!), for mostly stomatal morphology, see den Hartog and Baas (1978), for vegetative anatomy, see P. sessiliflora - Cogollo et al. 7294. For general information on Dipentodon, see Merrill (1941), for pollen, see Lobreau-Callen (1982), and for floral morphology see Liu and Cheng (1991: comparison with putatively related taxa) and Bachelier and Matthews (2014).
Previous relationships. Dipentodontaceae were included in Santalales by Cronquist (1981) because of apparent similarities in gynoecial structure, but the toothed lamina margin and large, lobed stipules suggested that this was incorrect. They were placed by Takhtajan (1997) in his Violales (= Malpighiales), while Wu et al. (2002, 2003) recognised a Dipentodontales C. Y. Wu et al. in Dilleniidae, where it was placed between Passiflorales (which included Caricaceae) and Violales (Cucurbitales were next). Perrottetia has often been included in Celastraceae (e.g. Ding Hou 1962).
But not only has Perrottetia been difficult to place, it must be one of the most misidentified genera in herbaria. Thus Bartholomew et al. (2020) noted that all the specimens they placed in P. taronensis sp. nov. had been misidentified - as Celastrus (Celastraceae), Gaultheria (Ericaceae), Ilex (Aquifoliaceae), Maesa (Primulaceae) and Rhamnus (Rhamnaceae). That pretty much covers the waterfront.