EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; septal nectaries + [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[[LILIALES + ASPARAGALES] COMMELINIDS]: (inflorescence branches cymose); protandry common.
COMMELINIDS: unlignified cell walls with >3.5 mg g-1 ferulate [ester-linked to non-cellulosic glucuronoarabinoxylans], these walls fluorescing blue under UV, green with NH3, pcoumarate acylates lignin [mostly on syringyl units], also glucuronoarabinoxylans; exodermal cells monomorphic; (vessels in stem and leaves); SiO2 bodies +, in leaf bundle sheaths; stomata para- or tetracytic, (cuticular waxes as laterally aggregated rodlets [looking like a scallop of butter]); inflorescence branches determinate, peduncle bracteate; P = K + C [stamens adnate to/inside corolla/inner whorl only]; pollen starchy; ovary superior; embryo short, broad.
[POALES [COMMELINALES + ZINGIBERALES]]: primary and secondary cell walls mostly with (glucurono)arabinoxylans; stomata subsidiary cells with parallel cell divisions; endosperm reserves starchy.
[COMMELINALES + ZINGIBERALES]: rhizodermal cells dimorphic; inflorescences with cincinnal branches [helicoid cymes]; P = T 3 + 3; A opposite T; tapetum syncytial; pollen orbicules 0 [?sampling]. - Back to Main Tree
Age. Divergence of the two clades dates to ca 114 Ma (Janssen & Bremer 2004; Givnish et al. 2018b), while the figures in Wikström et al. (2001) are (85-)81, 73(-69) Ma, and in Bremer (2000b) ca 84 My; Magallón and Castillo (2009) estimated ca 109.7 and 99.9 Ma while Bell et al. (2010) offer the figures of (101-)88, 86 Ma. However, Kress and Specht (2005) thought stem-group Zingiberales might be 158 Ma (but ages of 124-122 Ma are given by Kress & Specht 2006), while estimates are (107-)101, 99(-94) Ma in Hertweck et al. (2015), (112-)97.5(-82) Ma in Givnish et al. (2016b), (114-)92(-83) Ma in Merckx et al. (2008a), 85-83 Ma and 82-72 Ma in Mennes et al. (2013, 2015 respectively), about 79.8 Ma in Magallón et al. (2015), and a mere 52.4 Ma in Naumann et al. (2013).
Evolution: Divergence & Distribution. Särkinen et al. (2018) suggest that the ca 50 Ma Cantisolanum daturoides, ex Solanaceae, may well be the seed of a commelinid monocot; there are also Cretaceous fossils that are identified as Zingiberales (q.v.).
Monosymmetric flowers can perhaps be optimised to this node, as by Endress (2011a), who suggested that they might be a key innovation here. However, monosymmetric flowers in this clade have a variety of very different morphologies; for different types of monosymmetry, see Rudall and Bateman (2004). Interestingly, monosymmetry in Commelinaceae, at least, is associated with the abaxial/ventral expression of the CYC gene (Bartlett & Specht 2011; Preston & Hileman 2012; Hileman 2014), i.e. a position inverse to that in Lamiales in particular, and the floral orientation of monocots in general is inverted. However, many monosymmetric monocot flowers are upside down (for a monocot)... A change from P = K + C to P = T 3 + 3 and with the stamens individually opposite each tepal, may be placed at this node (see above), and it then reverses, or a tepaloid perianth evolved half a dozen times around here... Understanding floral evolution has been made difficult given uncertainties in the reconstruction of relationships in Zingiberales, although these are at last being alleviated (see Carlsen et al. 2018; Givnish et al. 2018), as are those in Commelinales (see H.-T. Li et al. 2019), which may help.
Chemistry, Morphology, etc.. The tapetum is variously described as being invasive, amoeboid or plasmodial (basically the same thing); tapetal variation in those few Zingiberales studied is extensive (Prakash et al. 2000; Furness & Rudall 2001; Simão et al. 2007).
Phylogeny. For further discussion of the relationships of the [Commelinales + Zingiberales] clade, see the Arecales page.
COMMELINALES Dumortier - Main Tree.
Mycorrhizae absent; (phenyphenalenones +); vessel elements with scalariform perforation plates; cuticle waxes not as aggregated rodlets; seed coat testal and tegmic; endosperm abundant, helobial [cell wall formation in small chalazal chamber precedes that in large micropylar chamber]; collar rhizoids +. - 5 families, 68 genera, 812 species.
Includes Commelinaceae, Haemodoraceae, Hanguanaceae, Philydraceae, Pontederiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Crown-group Commelinales are dated to ca 110 Ma (Janssen & Bremer 2004: c.f. topology; Givnish et al. 2018b), about 101 Ma by Tank et al. (2015: Table S2), (108.4-)95.9(-83.2) Ma by Eguchi and Tamura (2016), ca 90.2 Ma by Tang et al. (2016), (103.5-)80.5(-57.5) Ma by Givnish et al. (2016b), (104-)75(-50) Ma by Merckx et al. (2008a), (75-)71, 66(-62) Ma by Wikström et al. (2001), about 68.5 Ma by Magallón et al. (2015), while Bell et al. (2010) suggest an age of (91-)76, 70(-55) Ma, Hertweck et al. (2015) (101-)95, 90(-87) Ma, and Mennes et al. (2013, see also 2015) 84-47 Ma.
Evolution: Plant-Bacterial/Fungal Associations. Commelinales commonly lack mycorrhizae (Brundrett 2017b).
Genes & Genomes. There has been a reduction in the GC content of the genome in this clade (Smarda et al. 2014).
There is a five base pair insertion in the chloroplast matK gene in Hanguanaceae and Pontederiaceae sampled (Tamura et al. 2004a) but not in Haemodoraceae, but no other taxa were examined; probably two independent events.
Chemistry, Morphology, etc.. For phenyphenalenones, see Otálvaro et al. (2002).
Monosymmetry here is discussed by Rudall and Bateman (2004) and enantiostyly by de Almeida and de Castro (2019). Tapetal raphides are known from Commelinaceae, Philydraceae and Haemodoraceae, but their general distribution is unclear (Hardy & Stevenson 2000; Prychid et al. 2003a). There may be systematically interesting variation in the pattern of endothecial thickenings (Manning 1996).
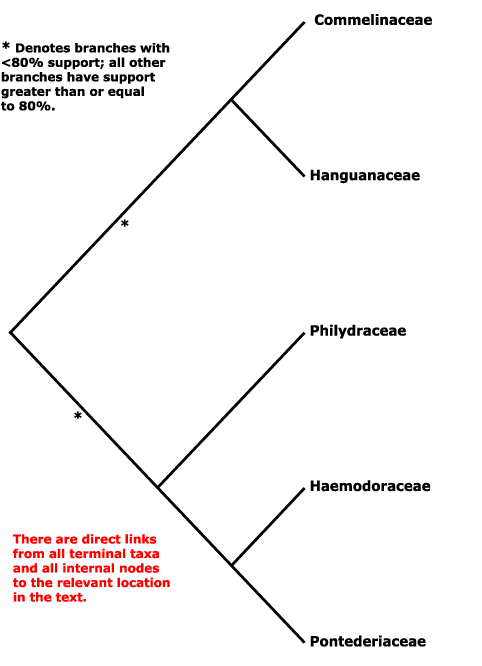
Phylogeny. For relationships, see Hopper et al. (1999), S. W. Graham et al. (2006), Chase et al. (2006), Saarela et al. (2008). There has been much discussion over the phylogenetic position of Hanguanaceae in particular, which in morphological analyses tends to cluster - sometimes quite strongly (Rudall et al. 1999) - with Zingiberales, although it lacks the inferior ovary of members of that order. However, molecular analyses suggested the inclusion of Hanguanaceae in Commelinales, and Givnish et al. (1999: rbcL analysis) early suggested that Hanguanaceae were sister to Commelinaceae in particular (see also Tillich 1997), while S. W. Graham (in Graham et al. 2002) noted that there was very strong support for the grouping [Haemodoraceae + Pontederiaceae]; these two family pairings have stuck. Janssen and Bremer (2004) suggested a rather different - and perhaps unlikely - set of relationships, [Philydraceae [Hanguanaceae [Haemodoraceae [Commelinaceae + Pontederiaceae]]]]; Givnish et al. (2006b: ndhF gene only) also found Philydraceae to be sister to other Commelinales, but the position had little support (see also Hertweck et al. 2015), while Davis et al. (2004; see also Chase et al. 2000) found a clade [Hanguanaceae [Commelinaceae + Pontederiaceae]]. Relationships in Z.-D. Chen et al. (2016) are [Philydraceae [Commelinaceae + Pontederiaceae]]. Givnish et al. (2018b) suggested the relationships [[Hanguanaceae + Commelinaceae] [Philydraceae [Haemodoraceae + Pontederiaceae]]]. H.-T. Li et al. (2019, see also 2021) found strong support in the chloroplast genome for the relationships [[Philydraceae [Hanguanaceae + Commelinaceae]] [Haemodoraceae + Pontederiaceae]], and that topology is followed here - but see below.
However, W. J. Baker et al. (2021) in the initial Angiosperms353 analysis had found not only that Pontederia sp. was sister to all other commelinids, but that Pon. cordata was sister all other Commelinales, and that relationships between the families - [[Commelinaceae + Hanguanaceae] [Philydraceae [Pontederiaceae (= Pon. diversifolia) + Haemodoraceae]]] - differed from those below. In an Angiosperms353 analysis that focused on Commelinales, Zuntini et al. (2021) recovered a topology [[[Commelinaceae + Hanguanaceae] Philydraceae] [Pontederiaceae (inc. Pon. diversifolia) + Haemodoraceae]], although the position of Philydraceae was not that strongly supported; Pon. cordata was not included, although it was in two plastome analyses, where it behaved itself. In the i.2022 version of the Seed Plant Tree Pon. cordata remained sister to the commelinids while Heteranthera, the only other member of Pontederiaceae included, was sister to Zingiberales. Within/around Commelinales, relationships were [Philydraceae [[Haemodoraceae [Hanguanaceae + Commelinaceae]] [Pontederiaceae + Zingiberales]]. Timilsena et al. (2022a: sampling minimal) recovered the relationships [[Philydraceae [Hanguanaceae + Commelinaceae]] [Haemodoraceae + Pontederiaceae]] in their ASTRAL analysis, but using RAxML there was quite strong support for Philydraceae being sister to the rest of the order, and these relationships, [Philydraceae [[Hanguanaceae + Commelinaceae] [Haemodoraceae + Pontederiaceae]]] are those in the v.2023 version of the Seed Plant Tree, although support for the sister-group relationship of the two pairs of families was not very strong.
Previous Relationships. Commelinales had been thought to include Eriocaulaceae, Mayacaceae, Xyridaceae and Rapateaceae, here all in Poales, and these relationships were indeed recovered in a morphological phylogenetic analysis by Stevenson and Loconte (1995).
Synonymy: Haemodorales Martius, Hanguanales Reveal, Philydrales Dumortier, Pontederiales Martius
[Philydraceae [Pontederiaceae + Haemodoraceae]] - ca 104.8 Ma; [Pontederiaceae + Haemodoraceae] - ca 88 Ma (Jung et al. 2025).
PHILYDRACEAE Link, nom. cons. - Back to Commelinales
Proanthocyanins +; stem vascular tissue various; SiO2 bodies 0; styloids +; (stomata tetracytic); hairs often wooly; leaves two-ranked, ventralized isobifacial [oriented edge on to the stem]/terete - Philydrella, no cross veins; inflorescence spicate, flowers single or in groups [?arrangement] in axils of spathe-like bracts; flowers open for one day, enantiostylous, ± bilaterally symmetrical; T whorls differentiated, but both C-like, with tannin cells, 2 adaxial outer T plus adaxial inner T connnate [upper lip], abaxial outer T large, free [lower lip], inner lateral T small, adnate to A (not - Helmholtzia); A 1 [median member of outer whorl], versatile, (anther curved or coiled), (filament winged); tapetum glandular, cells 2-4-nucleate; pollen with raphides, (in tetrads - Philydrum); septal nectaries 0, sclereids in placentae; (style impressed), stigma large, capitate; ovules many/carpel, outer integument 1-3 cells across, inner integument 2-3 cells across, (parietal tissue 2 cells across), ± postament, hypostase, funicular obturator +; fruit , T persistent; seeds carunculate [radially enlarged testal cells], (chalazal processes); exotestal cells with thick cellulose walls, elongated, (spiralling around seed), endotegmen tanniniferous, operculum +, tegmic; endosperm also with oil and crystalline aleurone bodies, chalazal haustorium +, chalazal chamber cellular ab initio, embryo long, suspensor hardly developed; n = 8, 16, 17, x = 8, nuclear genome [1 C] (0.062-)1.287(-26.774) pg; cotyledon linear, bifacial.
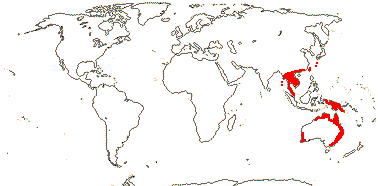
3/6: [list]: Helmholtzia (3). Australia (all genera) to Southeast Asia. Map: from Fl. Austral. vol. 25 (1987) and Hamann (1998b).
Age. Crown group Philydraceae are dated to (37-)33(-29) Ma by Wikström et al. (2001) and ca 47 Ma by Janssen and Bremer (2004).
Cantisolanum daturoides, from London Clay deposits ca 50 Ma and previously thought to be the oldest fossil identifiable as Solanaceae, may in fact be Philydraceae (Särkinen et al. 2013).
Evolution: Divergence & Distribution. Philydrum languinosum is found throughout most of the range of the family; all other species have local distributions in New Guinea and Australia. If Cantisolanum daturoides (see above) belongs here, it does something to the historical biogeography of Philydraceae.
Ecology & Physiology. The corms of Philydrella pygmaea are dessication tolerant, being able to perennate when in a state of extreme dessication (Gaff & Oliver 2013).
Chemistry, Morphology, etc.. Information is taken from Hamann (1966: much detail, 1998) and Adams (1987), both general, Malmanche (1919: anatomy), Kapil and Walia (1965: embryology of Philydrum), Hamann (1962b: endosperm), and Tillich (1994: seedlings).
Phylogeny. For phylogenetic relationships within Philydraceae, see Saarela et al. (2008); Philydrella is sister to the rest of the family. However, in the plastid analyses carried out by Zuntini et al. (2021) neither of the hypotheses, Philydrella basal or Helmholtzia basal, could be preferred; they did not have nuclear genomes for all three genera.
[Hanguanaceae + Commelinaceae]: stem above ground; embryo small; cotyledon not photosynthetic.
Age. The age of this node is estimated to be (113.0-)104.8(-94.2) Ma (Jung et al. 2025).
HANGUANACEAE Airy Shaw - Hanguana Blume - Back to Commelinales
Robust perennial herbs, (stoloniferous); vessels in roots only; mucilage canals +, silica bodies in bundle sheath; hairs multicellular, branched; epicuticular waxes 0, stomata tetracytic; leaves spiral, with petiole and blade; plant dioecious; inflorescence terminal, branched-spicate, flowers sessile, bracts and bracteoles 0; T small; staminate flowers: filaments broadened and connate at very base; ?tapetum; pollen grains inaperturate, exine spinulose; pistillode +; carpelate flowers: staminodes +, base of inner staminodes ± surrounded with scale-like structure [esp. adaxial; ?nectar-secreting]; G with intra-ovarian trichomes, mucilage-producing, style ± 0, stigmas 3; ovule 1/carpel, basal, straight, micropyle endostomal, parietal tissue ?1 cell layer across, epidermal cells anticlinally elongated, suprachalazal zone massive; fruit a berry, seed 1(-3); seed bowl-shaped [placenta inside the bowl!]; testa ca 5 cells across, endotesta with inner periclinal walls thickened, tegmen with two layers of crossing fibres; endosperm ?type, ?embryo; n = ca 20-24 ... 72, x = 20/22/24, chromosomes 1> µm long, nuclear genome [2 C] = 1.276-3.561 pg, two rps 19 genes; primary root well developed.
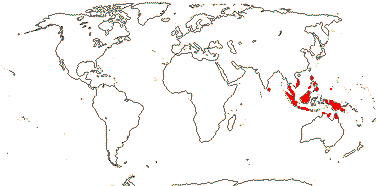
1 [list]/22, perhaps >50. Sri Lanka, South East Asia to Palau and N. Australia. Map: see Hewson (1986). [Photo - Fruit]
Evolution: Pollination and Seed Dispersal. Six taxa of Hanguana from Singapore are apomictic, and in only one species, the diploid H. nitens, does there appear to be sexual reproduction and recombination (Niissalo et al. 2020). Apomixis is probably common throughout the genus.
Genes & Genomes. Niissalo et al. (2020) looked at chromosome numbers (not easy to count), geneme size and evolutiom, etc. in the genus.
Chemistry, Morphology, etc.. Raphides may occur, but they are rare (Prychid & Rudall 1999). In Takhtajan (1985) there are illustrations of a several-layered testa and a massively-thickened tegmen with crossing fibres. However, Tillich (1996b) described the seed coat as being testal - but his outer layer seems comparable to Takhtajan's endotesta and his inner layer to one of the tegmic layers.
Additional information is taken from Bayer et al. (1998b), Tillich and Sill (1999), and Rudall et al. (1999), all general; also Leong-Skornicková and Boyce (2015: carpelate flowers) and Tillich (1996b: seedling).
Previous Relationships. Hanguana was often included in Flagellariaceae (now in Poales), Cronquist (1981) included them in his broadly-circumscribed Liliales, while more recent morphological studies have linked them with Zingiberales (see above).
COMMELINACEAE Mirbel, nom. cons. - Back to Commelinales
Perennial herbs; 6-hydroxyflavonoids +; silica bodies in epidermis [?whole family?]; hairs uniseriate; stem with swollen nodes; (prophylls lateral); leaves spiral, midrib prominent, sheath closed, Vorläuferspitze 0 [?level]; andromonoecy common; flowers open one day, enantiostyly common; septal nectaries 0; micropyle (exo)/endostomal/nucellus exposed, outer integument 3-7(-10) cells across, inner integument ca 2 cells across, parietal tissue 0-2 cells across, nucellar cap 0-2 cells across, hypostase +; fruit a loculicidal capsule, endocarp with fibres in vertical series, transversely elongated, variously thickened; seeds (uniseriate), operculate [= "embryotega"]; outer part of testa sloughed off, endotesta silicified, (walls thickened), exotegmen silicified, (with silica bodies), (endotegmen tanniniferous); chalazal haustorium +, suspensor 0; coleoptile +; x = 9 (?10, ?7), nuclear genome [1 C] (0.297-)4.731(-75.289) pg.
40 [list, to tribes]/652 (810): three subfamilies below. Mostly tropical, some temperate.
Age. The age of crown-group Commelinaceae is (96.1-)81.4(-68.5) My (Jong et al. 2025) - but note the uncertainty of basal relationships in particular here.
[Cartonematoideae + Triceratelloideae] - if a clade: stem collenchyma 0; glandular microhairs 0; leaves not succulent; P = T 3 + 3, yellow; embryo undifferentiated [embryotega without a micropylar collar].
1. Cartonematoideae (Pichon) G. C. Tucker - Cartonema R. Brown —— Synonymy: Cartonemataceae Pichon, nom. cons.
Cormose perennial; vessels in roots only; chlorenchya around foliar bundles radiating, raphide canals 0; stomata with 2-4 subsidiary cells; large glandular hairs +, on internodes and/or leaves; inflorescence leaf opposed, with 1-flowered cincinni [= pseudoraceme]; flowers enantiostylous, ± sessile; T (white); ?tapetum; ovules many/carpel, biseriate; stigma capitate; T persistent, enclosing capsule; seed ridged, hilum punctiform, embryostega dorsal, prominent, lacking micropylar collar; mesotestal cells enlarged; n = 12, intact accD, rpo A, one rps 19 genes; seedling collar short, mesocotyl +, primary root well developed.
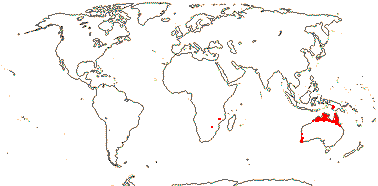
1/11. Tropical (and southwest) Australia, south western New Guinea. Map: FloraBase (consulted ix.2010).
2. Triceratelloideae (Faden & D. Hunt) Zuntini & Frankel - Triceratella drummondii Brenan
Annual; plant glandular pubescent; raphide canals +, next to veins, mesopphyll cells lobed, stomata with 2 subsidiary cells; leaf transverse veins ± 0; inflorescence leaf opposed, with 1-flowered cincinni, cymose; flowers enantiostylous, ± sessile; median K adaxial[?], K longer than C; ?tapetum; ovules many/carpel, biseriate; stigma capitate; K persistent, enclosing capsule; seed ridged, hilum punctiform, embryostega dorsal, prominent, operculum lacking micropylar collar; mesotestal cells enlarged; n = ?
1/1. Zimbabwe. Map: see above - Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012).
3. Commelinoideae Bruckner —— Synonymy: Ephemeraceae Batsch, nom. rej.
Herbs, (annual); (mycorrhizae 0); cyanidin 3,7,3'-triglucoside +; roots (tuberous), (velamen +); stem collenchyma +; vessels also in stem and root, vessel elements with simple perforation plates [?-level]; stem vascular bundles connect only at nodes [nodal vascular plexus], endodermis-like sheath surrounding bundles, cortex narrow; raphide canals between veins; leaf-opposite line of uniseriate hairs, 3-celled glandular microhairs on leaves; leaves rather soft and fleshy, blade liear to broad-elliptic, (vernation involute), sheath closed; (boat-like infloresence bracts conspicuous); (prophyll lateral); flowers with the median sepal adaxial, (obliquely monosymmetric), (enantiostylous); T = K + C, 1- or 3- trace, blue, pink or white (yellow), C deliquescent, (connate, tubular); A 1-6, if 3, opposite K, (adnate to C), heteranthy common, anthers poricidal, with 2+ fibrous middle layers, (connective [much] expanded), (staminodes 1-4, often attractive); tapetal cells with druses; pollen with raphides; nectary 0; (G [2]), stigma shortly lobed to capitate, wet (dry), papillate; ovules >1/carpel, straight to campylotropous; (embryo sac bi- or tetrasporic, 8[haploid]-nucleate), polar nuclei fuse early; (fruit a berry); seed operculum with a micropylar collar [testal in initiation], (outer part of testa fragile), endotestal, (also exotegmic); starch grains complex, embryo differentiated, cotyledon ± lateral; n = 4³, often "large"; collar rhizoids +.
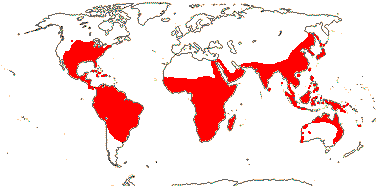
36/640. Tropical, also temperate, not Europe. Map: see Heywood 1978; modified from Fl. N. Am. vol. 22 (2000), FloraBase (2004), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 7 (2012). [Photos - Collection, Flower, another Flower.]
Age. Crown-group Commelinoideae are dated to ca 62 Ma (Janssen & Bremer 2004) or (68.9-)59.0(-50.1) Ma (Jong et al. 2025).
3A. Palisoteae (Faden & D. Hunt) Zuntini & Frankel - Palisota Endlicher
Stout herbs, (procumbent), (liane); hairs verrucose, irregularly branched or not; stomata with 4 subsidiary cells, palisade cells elaborately lobed; leaves with petiole and blade, blade (broadly) elliptic; inflorescences (axillary), (as flagelliform rooting shoot); flowers weakly monosymmetric; P = T 3 + 3; A heteranthous, A 1 [longer] + 2 [shorter, pollen infertile], opposite inner T, staminodes 3, with moniliform hairs, anthers 0; stigma various; fruit a berry; hilum punctate, embryostega dorsal; n = 20; chromosomes ca 2-10 µm long.
1/32. Tropical subSaharan Africa, esp. Gabon.
[Commelineae + Tradescantieae]: ?
3B. Commelineae Meisner
Epidermal silica bodies in lumen or cell wall), stomata with 6 subsidiary cells [terminal pair smaller than outer lateral pair]; (flowers strongly monosymmetric); (abaxial C much reduced); filament hairs 0 (+, not moniliform), staminodes (all posterior/opposite C); pollen spiny, tectum peforate; n = 6-13, etc.; chromosomes small, ca 1-5 µm long.
11/348:Commelina (170), Aneilema (68), Murdannia (50-60).
Age. The age of crown-group Commelineae is (56.3-)47.1(-37.3) Ma (Jong et al. 2025).
3C. Tradescantieae Meisner —— Synonymy: Tradescantiaceae Salisbury
(CAM photosynthesis +); stomata with (2)4(6, = or bigger than outer lateral pair) subsidiary cells; flowers usually polysymmetric; (P = T 3 + 3); (C with fringed margins; A 3 posterior, staminodes anterior = Cochliostemon); filament hairs +, moniliform (not - Tripogandra [T.]), (staminodes opposite C); pollen spines 0, tectum cereband (not - T.); (antipodal cells ± persisting - Tinantia); (endocarp with cross-shaped fibres - Amischotolype); n = 5-13, chromosomes large, ca 2-10 µm long.
21/272: Tradescantia (70), Dichorisandra (54), Cyanotis (50).
Age. Crown-group Tradescantieae are some (60.1-)50.4(-41.2) Ma (Jong et al. 2025).
3C1. Streptoliriinae Faden & D. Hunt
Climbers (shortly rhizomatous herb); leaves with petiole and blade; plant andromonoecious, perfect flowers in basal cincinni; inflorescence leaf opposed, cincinni separate, at least lower with large foliaceous bracts; (flower inverted - Aëtheolirion/A.); P = T 3 + 3 (C +); A 5, 3 with moniliform hairs (3 - A.); ovules 2-8/carpel, biseriate; fruit (long-linear - A.); seeds (winged - A.), verruculose [not winged]/reticulate, hilum linear, embryostega dorsal; n = 5, 6, 10.
3/9: Spatholirion (7). E. Himalayas, southern China to Thailand.
Evolution: Divergence & Distribution. Jong et al. (2025) suggested that Commeliaceae originated some 104.8 Ma in the East Gondwanan (Australian) area, and there were perhaps four intercontinental dispersal events 81-32 Ma.
Tomlinson (1966: esp. anatomy) and Faden and Hunt (1991) put a number of characters into an evolutionay/phylogenetic context, while C.-K. Lee et al. (2024) looked at the evolution of monosymmetry in Murdannia.
Pollination & Seed Dispersal. Heteranthy is common, and the stamens can be of very different sizes and the anthers - in blue-coloured flowers, for example - may be blue and apparently invisible to the pollinator or bright yellow; the staminodes (yellow anthers) may be prominent and apparently attractive, but their pollen may be less/inviable, functioning more as a reward to the pollinator (e.g. Hrycan & Davis 2005). The filaments in Tradescantieae and Palisoteae in particular have copious long, moniliform hairs which completely fill the mouth of the flower, although their function is unclear (Faden 1992). Indeed, surprisingly little is known about pollination in the family, but the flowers are not nectariferous and buzz pollination is likely to be common (Faden 1992; Sigrist & Sazima 2015; Rubin 2015 and references). For example, Cochliostemon (Tradescantieae) has asymmetric flowers, and here the filaments of two stamens form a tube enclosing the three, helically-twisted, longitudinally-dehiscent anthers (there are also three staminodes); pollen coming out of the apex of the tube is directed onto the pollinator's body (Hardy & Stevenson 2000a; Amorim et al. 2019). For more on buzz pollination, see elsewhere.
Hertweck and Pires (2004) discuss the evolution of breeding systems in Tradescantieae.
For a summary of animal dispersal mechanisms - quite varied - see Pellegrini and Faden (2017). Pollia has remarkable hard, dry, indehiscent fruits (P. condensata is called the marble berry) that are intense pointillist blue in colour because of the nature of the layered structure of the cell walls of the outer pericarp and the effect thay have on light - there is no pigment (intense blue colours are also produced by entirely structural means in the fruits of Elaeocarpus and a few species of Viburnum, for example). These fruits mimic berries and/or are perhaps collected by birds for display (Vignolini et al. 2012b). Seeds of other taxa have arils which may be dispersed by birds or ants, depending on the habit of the plant (Pellegrini & Faden 2017). Indehiscent geocarpic fruits are produced by Commelina bengalensis and Tapheocarpa.
Genes & Genomes. Over 1/4 of Commelinaceae examined have B chromosomes (Melianthaceae have still more, see Weiss-Schneeweiss & Schneeweiss 2013). For cytology, see Jones and Jopling (1972).
Jung et al. (2021) looked at variation in the plastome of the family, and structural variation was not very extensive; they found that accD, rpoA and ycf15 were psuedogenes in all the taxa that they examined.
Chemistry, Morphology, etc.. Raphides may be found in just about any tissue in one member of the family or another (Lawrie et al. 2023). Seedling leaves and often those at the base of axillary shoots May be two-ranked even in those taxa that have spiral leaves predominating in the vegetative plant. Vita et al. (2019) described the complex nodal vasculature plexus in some 14 species of the family.
Remizowa et al. (2011) suggested that the apparently cymose part-inflorescences of Tradescantia were in fact racemose, but c.f. e.g. Panigo et al. (2011). On the other hand, the apparently racemose inflorescences of Cartonema have been described as having one flower/cincinnus (Brenan 1966). Leaf-opposed (= terminal) inflorescences and axillary inflorescences that perforate the leaf sheath as they emerge (as in Buforestia) are known from the family (e.g. Forman 1962; Brenan 1966).
Floral symmetry and construction are variable; for a discussion that focuses on Tradescantia and its relatives, see Pellegrini (2017b), for floral diagrams. Flowers may be monosymmetric primarily by the corolla, one petal being much reduced, or primarily by the androecium, or by some combination of the two, and the expression of monosymmetry may change during the development of the flower (Hardy et al. 2004). In Dichorisandra the bracteole is more or less lateral and the plane of symmetry of the flower is transverse, while in other taxa it is oblique (e.g. Eichler 1875). Thus Preston and Hileman (2012) show most flowers of Commelinoideae as having an inverted orientation (see also Pellegrini & Faden 2017; Bidault & van der Burg 2019), and this is compatible with Eichler's suggestion. There is very considerable variation in androecial morphology and development in Commelinaceae. For instance, either individual whorls of stamens or the stamens on particular sides of the flower (anterior, posterior) may be staminodial (e.g. Faden 1998). Although all supraspecific taxa recognized by C.-K. Lee et al. (2021: see Fig. 2, all flowers drawn inverted, variation in K/C not shown) apart from Palisoteae include at least some taxa with three adaxial staminodes and three abaxial stamens, overall variation in stamen/staminode presence/absence/position is considerable. In some taxa androecial development is reported to be centrifugal, perhaps a variant of obdiplostemony (Hardy & Stevenson 2000b; Hardy & Ryndock 2012, but see Endress 2010d). Interestingly, TB1-like genes, involved in the development of monosymmetry elsewhere in monocots and eudicots, are not expresssed in the sepal-like abaxial petal of Commelina communis, although they are in the other petals, the stamens and the staminodes; there is a similar pattern of expression of B-class DEF-like genes that are not expressed in the abaxial member of the petal whorl either (Preston & Hileman 2012) or in the sepals (Ochiai et al. 2004). The flowers of some species have tepals, the stamens then being opposite each tepal member (or the fertile stamens are opposite the inner tepals); more commonly, there is a calyx and corolla. The inner tepal/corolla members may have only a single trace. A floral tube is formed by connation of the petals, as in Weldenia, or by adnation of the corolla and the filaments of the alternating antisepalous stamens (Rohweder 1979b).
The embryo is isolated in the apical part of the seed by an annular inpushing of the seed coat, and this relationship is evident even at the ovular stage (Chikkannaiah 1962). Mabberley (1987) suggested that some taxa may have a small second cotyledon, but to what structure this might refer is unclear. Tillich (1996a [check]) described the cotyledon as being of the dropper type.
Some information is taken from Brückner (1926), Rohweder (1963, 1970b), Brenan (1966), Tucker (1989), Faden and Hunt (1991), Faden (1998) and Hunt (2020), all general, Brenan (1960: Triceratella), Forman (1962: Aätheolirion), Bidault and van der Burg (2019:Palisota), see also Gilman et al. (2023: CAM), Calderón et al. (2009: ecdysteroids), Stirton and Harborne (1980: anthocyanins - see cyanidin 3,7,3'-triglucoside distribution, Cartonematoideae not sampled), Martínez and Swain (1985: flavonoids), Tomlinson (1964: Triceratella, 1969: anatomy), Hofreiter and Tillich (2002: root anatomy, quite a bit of variation), Burns et al. (2008: vegetative morphology), Evans et al. (2000: stomatal development), Choob and Mavrodiev (2001: prophylls, etc), Hardy et al. (2000b, 2004: floral development), Tischler (1915: tapetal development), Poole and Hunt (1980: pollen), Owens and Kimmins (1981: stigma), and McCollum (1939), Maheshwari and Baldev (1959), Chikkannaiah (1962, 1963), Grootjen and Bouman (1981a) and Grootjen (1983b), all embryology.
Phylogeny. A morphological phylogeny of Commelinaceae provided little resolution, although anatomical characters gave significantly more support for a rbcL phylogeny than did other kinds of characters (Evans et al. 2000, c.f. Evans et al. 2003). In morphological studies, most taxa with strongly monosymmetric flowers form a clade; Triceratella is widely separated from Cartonema, but the latter is sister to the rest of the family, a position also recovered in the rbcL analysis of Evans et al. (2003). Givnish (2003, summary tree only, no support values) emphasized that morphological data did not retrieve a monophyletic Commelineae and Tradescantieae largely because of high homoplasy of androecial characters, while molecular (rbcL) data did find these tribes to be monophyletic (with the exception of Floscopa, see below; Calisia [Tradescantieae] had a similarly isolated position in the morphological analyses). Evans et al. (2003: rbcL phylogeny, Triceratella not studied) also note conflict between morphology and molecules. However, a morphological phylogeny of Tradescantia and its relatives recovered the same major groupings in the genus as did the molecular phylogeny, although the position of the Tradescantia clade differed (Pellegrini 2017b). W. J. Baker et al. (2021: see also Seed Plant Tree) found the relationships [Aetheolirion [Cartonema ...]] at the base of the family, and Aetheolirion occupied a similar position in the Angiosperms353 analyses of Zuntini et al. (2021). However, this seems to be because of the similarity in five(!) nuclear genes between Aetheolirion and Cartonema; in plastid/chloroplast gene analyses Aetheolirion grouped with Streptolirion, etc., as morphological studies had suggested 30 years ago (Faden & Hunt 1991), and this grouping, sister to all other Tradescantieae, is obtained quite consistently, and it is recognised as a subtribe above. Cartonema is otherwise separate from other Commelinaceae, as is Triceratella in plastome analyses (Zuntini et al. 2021: Triceratella was not included in Angiosperms353 analyseds). C.-K. Lee et al. (2021: 3 plastid genes, 33 genera) found that Cartonema was sister to the rest of the family, and while Palisota and [Streptolirion + Spatholirion] were sisters to the two clades that made up the rest of the family, but their positions, especially that of the latter pair, could have had stronger support; five genera, including Pollia, Aneilema and Callisia were para- or polyphyletic. In their analysis of the family (49 taxa) and its immediate relatives using 79 plastid protein-coding genes, Jong et al. (2025) found that Cartomena and Triceratella were successively sister to the rest of the family.
Within Commelinoideae, Commelineae and Tradescantieae are both largely monophyletic, but with Floscopa (chromosomes 3³ µm long), previously included in Tradescantieae, as sister to both in some analyses (Givnish 2003). Wade et al. (2006) carried out a two-gene analysis of Tradescantieae; the position of Palisota was unresolved. Burns et al. (2011), in an extensive analysis of the subfamily, found Commelineae and Tradescantieae to be moderately supported, while Palisota and Spatholirion were successive sister taxa to Commelineae with moderate support. W. J. Baker et al. (2021: see also Seed Plant Tree) recovered Palisota as sister to Commelineae, but with weak support - this was only a preliminary study. However, looking at chloroplast genomes, Jung et al. (2021) again found Palisota to be sister to Commelineae, the combined clade being sister to the other 11 Tradescantieae in the analysis, where Streptolirion was sister to the rest; support was strong, although there was no support in maximum parsimony for the position of Palisota. In the analyses of nuclear genomes by Zuntini et al. (2021) Palisota was again separate from both tribes.
Callisia (Tradescantieae) was found to be paraphyletic, Gibasis polyphyletic by Hertweck and Pires (2004; see also Pellegrini 2017b). Commelineae. Spalink et al. (2009) outlined relationships in Aneilema.
Classification. Although I tentatively follow Zuntini et al. (2021) in recognizing three subfamilies and then three tribes within Commelinoideae, what to do with Triceratelloideae is in fact unclear (Jung et al. 2025). Zuntini et al. (2021) found a clade [Aetheolirion + Cartonema] - Triceratella was not included - in an Angiosperms353 analysis, but in most studies Aetheolirion has been placed in Commelinoideae, its normal position. Zuntini et al. (2021: plastome analyses) found the relationships [Cartonema [Triceratella + the rest]] at the base of the tree, although those two genera formed a clade using and extended plastid data set - altogether rather confusing. Wade et al. (2006) provide a subtribal classification of Tradescantieae; some subtribes are paraphyletic, and generic limits will have to be adjusted (e.g. Hertweck & Pires 2004). C.-K. lee et al. (2021) suggested a classification for the family in whichtwo subfamilies, six tribes and seven subtribes were recognized; the genera were reduced to 37. Pellegrini (2017b) suggested a sectional classification for Tradescantia.
[Philydraceae [Haemodoraceae + Pontederiaceae]] - if this clade exists: SiO2 bodies 0; styloids +; leaves two-ranked, ventralized isobifacial [oriented edge on to the stem]; T with tannin cells; sclereids in placentae; T persistent in fruit.
Age. The age of this clade is about 62.3 Ma (Magallón et al. 2015).
Evolution: Divergence & Distribution. Optimisation of the extensive foliar variation in these three families is difficult; the above is just one way of doing it.
[Haemodoraceae + Pontederiaceae]: phenylphenalenones +; SiO2 bodies 0; styloids +; leaves two-ranked, ventralized isobifacial [oriented edge on to the stem]; T with tannin cells; endothecial cells with base-plates; pollen exine baculate, 2-layered (1-layered); enantiostyly +; sclereids in placentae; T persistent in fruit.
Age. Magallón et al. (2015) suggested that this clade is around 44.4 Ma old, although that conflicts with reports of rather older Pontederiaceae fossils (see below).
Evolution: Divergence & Distribution. For possible characters, including some apomorphies, of this clade, see Pellegrini et al. (2018).
Chemistry, Morphology, etc.. For phenylphenalenones, see Otálvaro et al. (2002); these are phytoalexins with the formula (phenylphenalenone) of C19 H12 O, three six-C rings fused plus a benzene.
For the base plates of endothecial cells, see Manning (1996: not in Commelinaceae, other families unknown, sampling poor), for pollen, see Simpson (1987).
HAEMODORACEAE R. Brown, nom. cons. - Back to Commelinales
(Plant cormose); fructans, chelidonic acid, flavones +; vessel elements in roots often with simple perforation plates, (vessels also in stem and leaf); vascular bundles surrounded by fibrous tissue; leaf (margins ± spiny); cyme bifurcated (not); prophyll ± lateral; flowers (large), (monosymmetric), plane of symmetry transverse to oblique; T connate to free; A (connective appendages +); pollen with raphides; exine (1-)2(-3)-layered [no foot layer]; placentae swollen; ovules 1-many/carpel, micropyle (exo)/endostomal, parietal tissue to 3 cells across; fruit a loculicidal capsule, cells of testa (and tegmen) variously elongated, ± thin-walled; embryo small/minute; x = 7 (?9, ?8), nuclear genome [1 C] (0.053-)0.972(-17.807) pg; ?collar rhizoids, first seedling leaf isobifacial [?level].
13/119: [list: to subfamilies] - two subfamilies below. Tropics and warm temperate regions.
Age. Divergence within crown-group Haemodoraceae began ca 81 Ma (Janssen & Bremer 2004; He et al. 2016b).
1. Haemodoroideae Arnott —— Synonymy: Dilatridaceae M. Roemer, Wachendorfiaceae Herbert, Xiphidiaceae Dumortier

Plant rhizomatous (cormose/corms + stolons); roots red; tannin containing cells 0; root cortical cells radially aligned, (4-)8-12-layered; (bulliform cells +); (stem vessels with scalariform perforation plants - L.); (silica bodies +) hairs with distinctive basal cells; flowers (inverted - odd outer T adaxial), enantiostylous, tannin cells 0; A 3, opposite inner P, (unequal lengths - Cubanicola, etc./1 + 2 staminodes - Pyrorrhiza), anthers (poricidal); (septal nectary 0); placental sclereids 0; ovular nucellar cap +/0; seeds often flattened or marginally winged, tuberculate/pubescent or not; chalazal endosperm haustorium +/0; n = 12, 15, 19-2; cotyledon not photosynthetic, hypocotyl at most short.
8/39: Haemodorum (20). Tropics and warm temperate regions, largely southern, rather scattered, only Lachnanthes caroliniana in North America. Map: from Heywood (1978: Africa), Australia's Virtual Herbarium (consulted i.2014), Maas and Maas-van der Kamer (1993), Fl. N. Am. vol. 26 (2002) and Pellegrini et al. (2020). Photo: Flower, Fruit, Flower.
Age. The start of divergence within Haemodoroideae has been put at the early Eocene ca 47.9 Ma (Hopper et al. 2009).
2. Conostylidoideae Lindley —— Synonymy: Conostylidaceae Takhtajan
Root (stele 2-arch - Tribonanthes), vessels scattered throughout the pith, cortical cells 10-20-layered, endodermal cells usu. rectangular/radially elongated, outer tangential wall relatively thin; tannin-containing cells +; SiO2 sand +; (epidermal walls thickened), hairs branched; (inflorescence capitate); flowers ± pubescent outside (and inside), (monosymmetric); T (valvate); (A adnate to T), (with ± petal-like dorsal appendage); tapetal cells not binucleate, pollen 2-8 porate; ovary at least partly inferior, placental sclereids +; fruit (dehiscence valvular - Macropidia/schizocarp/nut - Phlebocaryum); (seeds ridged, tegmen massive - Anigozanthus); n = 4-8, 11; cotyledon photosynthetic, hypocotyl +, primary root well developed.
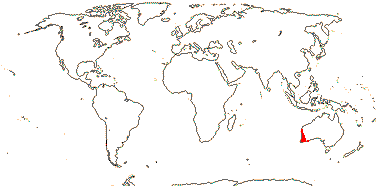
5/80: Conostylis (50). S.W. Australia. Map: from Fl. Austral. vol. 45. (1987).
Age. Divergence within Conostyloideae began in the Eocene ca 42 Ma (Hopper et al. 2009).
Evolution: Divergence & Distribution. Haemodoraceae may have originated in S.W. Australia (see also He et al. 2016b), and the restriction of Conostylidoideae to this area is remarkable given that it is not exactly a young clade. Species distributions/relationships of Haemodorum within Australia largely fit the peripheral vicariance pattern, being centred on the periphery of the continent after the drying out of the centre, a process that began in the Eocene (Nge et al. 2021c). However, the overall distribution of the family, primarily in the Southern Hemisphere, may be the result of dispersal rather than vicariance (Sanmartín & Ronquist 2004). Hopper et al. (2006, 2009) discuss the diversification of Conostylidoideae, and in particular that of Conostylis itself.
Aerne-Hains and Simpson (2016) put variation in anatomical features in the family in a phylogenetic context (see also Simpson et al. 2006). The ovary in Haemodoroideae may be secondarily superior (Simpson 1998a), the septal nectaries being found below the point of insertion of perianth (see especially Simpson 1993; for more on nectaries and nectar movement in the flower, see Vogel 1998a). Note, however, that Pontederiaceae, sister to Haemodoraceae, and other Commelinales have superior ovaries...
Ecology & Physiology. Fire stimulated flowering may be the ancestral condition for Haemodoraceae as a whole (He et al. 2016b), indeed, resprouting after fire has been dated to 89.5 Ma here, the family being the oldest in which this is known, although the trait has subsequently been lost (Lamont et al. 2018b). Smoke-stimulated germination - things like karrikinin may be involved - is also known from Haemodoraceae (Lamont & Hey 2017; Lamont et al. 2018b). See also Proteaceae and Pinaceae for fire and its adaptations.
Pollination Biology & Seed Dispersal. In Anigozanthus flavidus sugars in the nectar come directly from the phloem (Solhaug et al. 2019 and references).
Plant-Animal Interactions. Bugs of the Hemiptera-Lygaeidae-Blissinae eat seeds of Haemodoroideae from South Africa; most other bugs of this clade are sap-eaters (Slater 1976).
Chemistry, Morphology, etc.. There is much variation in basic floral organization in this small family. The median petal is abaxial in monosymmetric flowers of Haemodoroideae such as Wachendorfia, i.e., the flowers are inverted (this genus has only two septal nectaries - Vogel 1998b), while in Anigozanthus (Conostyloideae) zygomorphy is evident as a slit down one side of a tube formed by the six connate tepals, so here the plane of symmetry must necessarily be slightly oblique. As Eichler (1880) noted, if flowers are examined early in development, Wachendorfia has flowers with transverse symmetry, while in Anigozanthus they are oblique. Taxa like Anigozanthus have the six stamens individually opposite the six perianth lobes, while Xiphidium, also Conostyloideae, has a more or less differentiated perianth with the members of each whorl fully encircling the apex, the three stamens being borne opposite the petals/inner tepals (see illustrations in Simpson 1990). Steinecke and Hamann (1989) noted that the prominently hook-shaped synergid cells with a filiform apparatus of the Conostegioideae were not to be found in Haemodoroideae. The outer periclinal wall of the testa is thick.
Some general information is taken from Maas & Maas-van de Kamer (1993) and Pellegrini et al. (2020), both Neotropical Haemodoroideae, and Simpson (1990, 1998b); for an extensive anatomical survey of the family, see Aerne-Hains and Simpson (2016), for pollen, see Simpson (1983) and Pierce and Simpson (2009: Conostylis) and for embryology, see Simpson (1988: Lachnanthes caroliniana).
Phylogeny. The phylogeny of Haemodoraceae has been quite extensively studied (e.g. Hopper et al. 1999). Within Conostyloideae, Tribonanthes is sister to the rest - and it is morphologically quite distinct (Hopper et al. 2009); other relationships are [[Anigoxanthus + Macropidia] [Phlebocarya [Conostylis + Blancoa]]] (Zuntini et al. 2021). In Haemodoroideae there was some conflict between nuclear and plastome data, the position of Xiphidium varying between the two; [Haemodorum + Lachnanthes] were sister to the rest of the subfamily, with the addition of Dilatris in a 3-chloroplast gene tree; that genus was included neither in the early Angiosperms353 nor in the plastome analyses (Zuntini et al. 2021).
Previous Relationships. For Lophiola, previously included in Haemodoraceae, see Dioscoreales-Nartheciaceae.
PONTEDERIACEAE Kunth, nom. cons. —— Synonymy: Heterantheraceae J. Agardh - Back to Commelinales
Water or marsh plants, (annuals); (vessels also in stems); styloids or prismatic crystals 0 (+); vegetative stems indeterminate; stomatal subsidiary cells with oblique divisions; leaves spiral or spirally two-ranked, (whorled), bifacial, blade broad, initially surrounding petiole of older leaf, (linear), petiolate (sessile), 2ndary veins transverse, sheath open or closed, often long-ligulate, "stipules" acicular to linear/0, colleters +; flowering module = leaf, bract/spathe, terminal inflorescence/flower; flowers open for one day, tristyly/enantiostyly common, mono-/polysymmetric, sessile; T (4, 3), mostly blue or yellow, ± connate, (tube to 11 cm long), adaxial inner T with markings; A adnate to T, of different lengths, (1, 3, 4), (staminodes 2), filaments hairy, (winged); (tapetum glandular); pollen 2- or 3-sulcate; (septal nectaries 0); (1 carpel fertile), (placentation parietal), stigma small, dry; ovule (1/carpel, apical [1 G fertile]), micropyle bistomal, parietal tissue none (single layer - Monochoria), (epidermal cells ± radially elongated), postament +; (fruit an achene surrounded by P base); (seed ridged); exotestal cells box-like, endotestal cells narrow, tranversely elongated, multinucleate, inner periclinal wall thickened; micropylar endosperm haustorium +, embryo long, suspensor hardly developed; n = (7-)8(-13), x = 8 (?7), nuclear genome [1 C] (0.145-)1.015(-7.093) pg; cotyledon linear, bifacial.
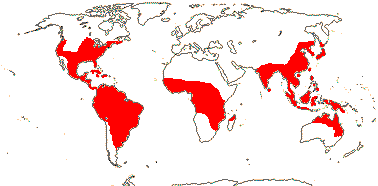
2/37: [list]: Pontederia (25). Tropics, also temperate, esp. New World. Map: Fl. N. Am. vol. 26 (2002). [Photos - Collection.]
Age. Divergence within the crown-group Pontederiaceae is estimated to have begun around 39 Ma (Janssen & Bremer 2004).
Fossils from deposits in Egypt perhaps 79 Ma have been placed in Pontederia and Eichornia (= Pontederia) (Coiffard & Mohr 2018), while the genus is also reported from the later Intertrappean flora in India at the K-P boundary 70.6-65.5 Ma (Kapgate 2013), or perhaps younger, in the Eocene.
Evolution: Divergence & Distribution. Simpson and Burton (2006) discussed the evolution of features of floral anatomy in the family while De Sousa et al. (2015) focussed on the evolution of vegetative and anatomical features. See also Pellegrini et al. (2018) for a number of possible apomorphies here; they note that one might be "leaf-blades with xylem and phloem alternate in the central portion of the blade and xylem abaxial and phloem adaxial at the margins" (Pellegrini et al. 2018: pp. 30-31), along with five other foliar characters, including non-equitant leaves, a reversal.
Some species of Pontederia not only have single, apical ovules in the single fertile carpel and an achene surrounded by the perianth, but they are also the only members of the family to have styloids or prismatic crystals. Other features Simpson and Burton (2006) studied also correlate with the clades evident in the phylogenetic analyses of e.g. Barrett and Graham (1997); Pellegrini et al. (2018) also discuss variation within the family.
Graham and Barrett (1995) discussed the evolution of the breeding system, while Barrett and Graham (1997) outlined the phylogeny and diversification of the family and Kohn et al. (1996) the evolution of reproductive features (see also Barrett & Shore 2008). However, there have been problems with rooting the tree (see below, also Ness et al. 2011 and references).
Pollination Biology & Seed Dispersal. Considerable work has been carried out on the floral biology of Pontederiaceae, where tristyly, enantiomorphy and monosymmetry are all well known. Thus Pontederia cordata is obliquely monosymmetric, P. crassipes is not, the flowers being presented "normally" for a monocot; Puentes et al. (2013) discuss tristyly in P. obovata; enantiostyly is known from Monochoria (= Pontederia). In Monochoria (= Pontederia) there are blue tepals, five smaller stamens with yellow anthers and one larger stamen with a blue anther; the former served as pollinator attractants, but the pollen of both kinds of stamens was equally effective in pollination (G. Wang et al. 1995; L.-L. Tang & Huang 2007 for pollination). Lunau (2006) suggested that the yellowish spot on the median-adaxial tepal member of some other Pontederiaceae mimicked a stamen. The paired monosymmetric flowers of Heteranthera gardneri form a polysymmetric pseudanthium (Baczynski & Claßen-Bockhoff 2023).
Economic Importance. Pontederiaceae include a number of notably serious and widespread weeds (Daehler 1997), water hyacinth, Eichhornia (= Pontederia) crassipes perhaps being the most notable (Pysek et al. 2017). Scheffer et al. (2003) discuss the conditions that enable such dominance to occur.
Chemistry, Morphology, etc.. Amounts of syringyl alcohol were found to be very low here (Gibbs 1958), perhaps connected with the aquatic habitat of the group.
The blade of the first leaf of the axillary shoot completely encircles the main stem in bud, and in general the blade of the young leaf completely encircles the petiole of the next oldest leaf - this may be a unique arrangement (see also Eichler 1880). Although Hydrothrix [= Heteranthera] gardneri, the only species that grows submerged, appears to have whorled, linear leaves, in fact there is a leaf with a single short, sheathing ligule at each node, the other leaves having narrower bases and being borne inside the sheath (Rutishauser 1999); there is only one bud and one vascular trace per node (Pellegrini 2017a).
The endothecial walls of Eichhornia have distinctive base-plate thickenings (Manning & Goldblatt 1990).
Additional information is taken from Horn (1987) and Cook (1998), both general, Tomlinson (1982: colleters), Pellegrini and Horn (2017: inflorescences), Endress (1995b: flowers), Strange et al. (2004: floral anatomy), Simpson (1987: pollen), Ono (1928: ovule development), Coker (1907: seed anatomy - multinucleate endotestal cells), and Tillich (1994: seedlings).
Phylogeny. For some time now, it has been clear that Eichornia is hopelessly para/polyphyletic, Pontederia and Monochoria being embedded in it (Graham et al. 1998; see also Ness et al. 2011; de Sousa et al. 2015) - Pontederia now includes the two other genera. There have been persistent problems in rooting the tree (also Graham et al. 1998, esp. 2002), and one study suggested that Eichornia meyeri was sister to the rest of the family - although there were still doubts (Ness et al. 2011). Du and Wang (2014) and Du et al. (2016) recovered Pontederia as sister to the other members of the family (Chinese) that they studied. Eichornia paniculata-E. paradoxa were often sister to the rest of the genus in the analyses of Pellegrini et al. (2018), although there was a basal tritomy in parsimony analyses of the combined molecular-morphological data and in analyses of morphological data alone quite another group was sister to the rest of the genus. Even with the genera reduced to two, Zuntini et al. (2021) found that Heteranthera made Pontederia paraphyletic in both plastome and nuclear analyses, but not in a three plastome-gene tree with quite good sampling... See also Zuntini et al. (2024; the Angiosperms353 Seed Plant Tree version ix.2024).
Classification. Pellegrini (2017a) and Pellgrini et al. (2018) provide a classification of the family - two genera are recognized (see elsewhere).