EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae node.
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID I / FABIDAE / [ZYGOPHYLLALES [the COM clade + the N-fixing clade]]: endosperm scanty.
[the COM clade + the N-fixing clade]: ?
Age. The age of this node is estimated to be 82-78.1 Ma by Xue et al. (2012), ca 121 Ma by Foster et al. (2016a: q.v. for details) and about 142 Ma by Z. Wu et al. (2014). If there is a clade [Malvids [the COM clade + the nitrogen-fixing clade]], i.e., no Zygophyllales, its age has been estimated at (131-)124(-118) Ma (Foster et al. 2016a).
[OXALIDALES [CELASTRALES + MALPIGHIALES]] / the COM clade: seed exotegmic, cells fibrous.
Age. Ages for this clade in Magallón and Castillo (2009) were ca 102 Ma and in Bell et al. (2010) were (109-)102, 98(-94) Ma.
Evolution: Divergence & Distribution. Endress (2011a) suggested that a key innovation around here might be incompletely tenuinucellate ovules.
Endress and Matthews (2006a) noted that an inner integument that is thicker than the outer is common in the COM clade, although this may not be so in Celastrales; this character is not optimised to this part of the tree here, but see within each order. Endress and Matthews (2006a) also note that members of the COM clade commonly have a relatively thin nucellus and arillate seeds.
Chemistry, Morphology, etc.. Similarity in seed coat anatomy had suggested relationships between families now placed in Malpighiales, Celastrales and Oxalidales (and also Zygophyllales) to authors like Corner (1976), Dahlgren (1991) and Boesewinkel (1994). Indeed, a number of Malpighiales, including Linaceae, have a fibrous exotegmen similar to that of Oxalidales. In Celastrales, a similar exotegmen is found in Lepidobotryaceae, but not Celastraceae, given the departure of Perrottetia to Huerteales and Bhesa to Malpighiales (Zhang & Simmons 2006).
Phylogeny. This clade of three orders has often been retrieved, e.g. P. Soltis et al. (1999: weak support), Zhang and Simmons (2006), Zhu et al. (2007: mitochondrial matR gene, but appreciable support only when chloroplast genes added), H. Wang et al. (2009), Qiu et al. (2010), and M. Sun et al (2016). Oxalidales (including Huaceae) are sister to the other two orders in some analyses (e.g. Zhu et al. 2007, but support very weak); support weakened when chloroplast genes were added (see also Soltis et al. 2007a; Bell et al. 2010; Xia et al. 2022). Relationships are unclear in Moore et al. (2011). However, H.-T. Li et al. (2019) found quite strong support (85% bootstrap for a [Celastrales + Malpighiales] clade), and this clade was also recovered by Valencia-D et al. (2020), so strictly speaking the larger clade is now the OCM clade...
The COM clade may be the result of an ancient hybridization between the fabids and malvids; for further discussion, see the Zygophyllales page.
Zhang and Simmons (2006: see also Soltis et al. 2007a) found that Huaceae were sister to the Oxalidales that they examined, with quite strong support (jacknife values over 80%); they suggest that Huaceae should be included here. Zhu et al. (2007) also found quite strong bootstrap support for this position when the mitochondrial matR gene was examined, but support was lost when two chloroplast genes were added. Support was only weak in the analysis of Wang et al. (2009) and Qiu et al. (2010), but moderate to strong in the multiple gene analysis of Soltis et al. (2011). All in all, however, Huaceae seemed to be finding a more fixed place on the tree (see also Wurdack & Davis 2009); they were not sampled by H.-T. Li et al. (2019), but were sister to the rest of the oredr in Y.-L. Qiu et al. (2024). Nevertheless, they lack even the rather unimpressive morphological features that characterize other Oxalidales.
Furthermore, relationships in the initial Angiosperms353 genome analysis (W. J. Baker et al. 2021a) were rather different; Huaceae (both genera were sampled) linked with Celastraceae and the combined clade was sister to Malpighiales, but all other Oxalidales decamped and were in the Malvales—Brassicales area; relationships in version 3 (v.2023) had not changed - Rafflesia was also around here, but support for both its position and that of Huaceae was weak (see the Seed Plant Tree of Life, also Zuntini et al. 2024). H.-T. Li et al. (2021: comprehensive plastome analyses) also found similar immediate relationships for Huaceae, if with very weak support, while the rest of Oxalidales were sister to a [Huaceae [Celastraceae + Malpighiales]] clade; the findings of Simmons et al. (2023: focus on Celastraceae) were i.a. consistent with Huaceae being a member of Celastrales. Zuntini et al. (2024) recovered the relationships [[Celastrales [Apodanthaceae (ex Cucurbitales) [Huaceae + Malpighiales]]] [Oxalidales + 4 other orders]]. Thus the big picture of relationships around here is unclear, but Huaceae will probably need an order by themselves (see also Li et al. 2021), and so it is treated separately here:
Just the one family, 2 genera, 4 species.
HUACEAE A. Chevalier
Evergreen, woody (lianes); smelling of garlic; tannins 0; vessel elements with simple (scalariform) perforations, libriform fibres +, parenchyma confluent; phloem with broad rays; nodes 3:3; cork cambium ± superficial; mucilage cells 0, cristarque cells +; petiole vasculature complex, ± interrupted-annular, medullary budles +/0; stomata paracytic; leaves two-ranked, lamina margins entire, venation closely ± closed-reticulate, glands +, basal on margin or on abaxial surface; inflorescence axillary, cymose, fasciculate; (flowers 4-merous); K with adaxial glands; pollen ± triangular, anguloaperturate, porate, pores operculate, 3 folds towards both poles/0; nectary?; G [5], opposite K, unilocular, placentation basal, style +, stigma punctate; ovule micropyle, etc., ?; fruit pericarp with brachysclereids towards outside, otherwise parenchymatous; seed 1; testa parenchymatous, ?several layers thick, with amphicribral vascular bundles, exotegmen of lignified palisade cells, tegmen otherwise parenchymatous; endosperm copious, with starch grains and oil droplets, ?development, embryo long, straight, cotyledons large, flattened; n/x = ?
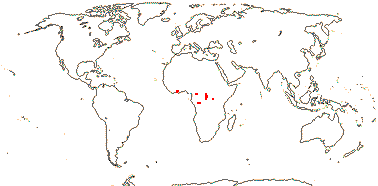
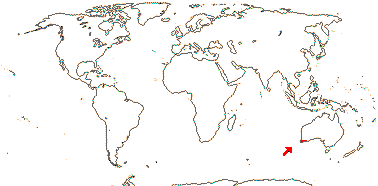
2 [list]/4. Tropical Africa. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003).
Age. Ca 109.8 Ma is the age of stem-group Huaceae (Tank et al. 2015: Table S1, S2).
1. Hua gabonii de Wildeman
Hairs unicellular, submesifixed; K valvate; C long-clawed, blade peltate; A minute, anthers open apically; ovule 1/gynoecium; fruit a 5-6 valved ?septicidal capsule; testa with unicellular hairs.
1/1. Gabon.
2. Afrostyrax G. Perkins & Gilg
Hairs stellate-peltate; K connate; C strongly obovate, abaxially with peltate/stellate hairs; anther connective aristate, dehiscing from the apex; ovules (4-)6, apotropous; fruit a drupe.
1/3. West Africa.
Chemistry, Morphology, etc.. For additional information, see Bayer (2006) and Heywood et al. (2007) both general, Baas (1972: anatomy) and Hegnauer (1989: a little chemistry).
The family is poorly known, especially its chemistry and floral development/embryology.
Previous Relationships. As mentioned, the position of Huaceae has been very uncertain, being included in Malvales (e.g. Baas 1972: anatomical similarities, but position there tentative; Takhtajan 1997), in the more heterogeneous Violales (Cronquist 1981) and hardly unsurprisingly less out of place there. Baas (1972) also suggested that Euphorbiaceae and Irvingiaceae (both Malpighiales) showed a number of "striking" similarities in wood anatomy with Huaceae, although they did not translate to close relationships phylogenetically. Huaceae were left unplaced in the rosids by A.P.G. I and II (1999, 2003).
OXALIDALES Berchtold & J. Presl - Main Tree.
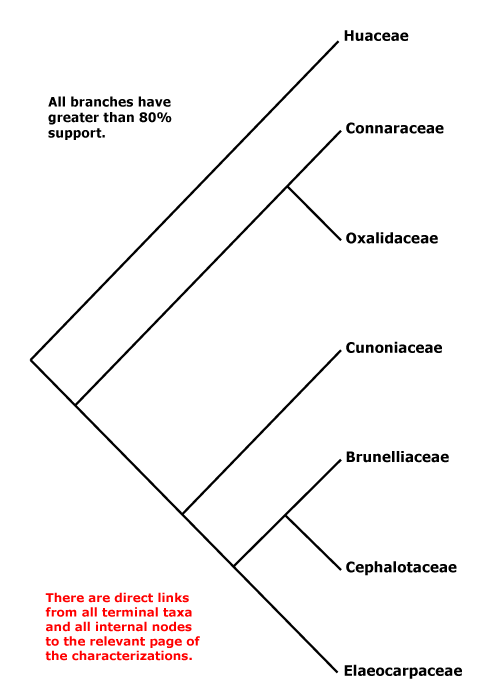
Vessel element type?; mucilage cells +; stomata ?; leaves compound, odd-pinnate; nectary extrastaminal; styluli +, stigma secretory; micropyle bistomal; testa multiplicative, endotesta crystalliferous and palisade, exotegmen also tracheidal. - 6 families, 58 genera, 1,845 species.
Includes Brunelliaceae, Connaraceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Elaeocarpineae, Oxalidaceae, Oxalidineae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age of crown group Oxalidales was estimated as (105-)93, 89(-78)Ma by Bell et al. (2010; note topology).
Age. The age of crown-group Oxalidales was estimated to be (74-)69, 62-(57) Ma (two penalized likelihood dates: H. Wang et al. 2009). Wikström et al. (2001) suggested an age of some (80-)77, 72(-69) Ma, Magallón and Castillo (2009) an age of ca 90.5 Ma, Bell et al. (2010) ages of (78-)64, 59(-44) Ma, and Tank et al. (2015: Table S1, S2) ages of around 63.8 to 58.9 Ma.
Evolution: Divergence & Distribution. Tao et al. (2018) discuss pollen evolution in the order.
The flowers of Anisophyllea (Anisophylleaceae-Cucurbitales) are very similar to those of Ceratopetalum (Matthews et al. 2001; see also Matthews & Endress 2004, 2006b), and Matthews and Endress (2002b) note the filaments are longer than the anthers in bud in Oxalidales (but not in Elaeocarpaceae) and in Anisophylleaceae. There are perhaps comparable similarities in the fossil Platydiscus peltatus (Schönenberger et al. 2001a; Schönenberger & von Balthazar 2006), also identified as Cunoniaceae, see below. However, none of these similarities is likely to reflect a close relationships between the two; Ceratopetalum is embedded in Cunoniaceae and the clades that the two families are in are not at all closely related.
Ecology & Physiology. Lamont et al. (2018b) draw attention to a number of features that suggest a connection between fire and this part of Oxalidales - even the germination of seeds of the bog-dwelling Cephalotus is stimulated by fire.
Chemistry, Morphology, etc.. Leaf development in this clade would repay attention. Oxalis regnelli has peltately-palmate compound leaves, leaves of Cephalotus are epiascidiate (Kim et al. 2013; Franck 1976).
Simple diplostemony may not occur in Oxalidales. The androecium of Cunoniaceae is obdiplostemonous, according to Huber (1963), and so agrees with that of Oxalidaceae (and Brunelliaceae [Orozco 2002] and Connaraceae); see Ronse De Craene and Bull-Hereñu (2016) for literature. Connaraceae and Brunelliaceae have ovaries with adaxial furrows (c.f. the ventral slit: Matthews & Endress 2002b). Is the distribution of taxa that have carpels with five vascular traces of any interest?
Some information is taken from Nandi et al. (1998). There is much information on floral morphology and development for the whole order in Matthews and Endress (2002b, summarized in 2006b).
Phylogeny. Molecular data have for some time recovered a not unexpected clade [Oxalidaceae + Connaraceae] (Price & Palmer 1993; Williams et al. 1994; Fernando et al. 1995, etc.), and this also has strong morphological support. The other families are in a clade sister to this pair (e.g. Zhang & Simmons 2006: Cephalotaceae not included; Soltis et al. 2011), but relationships there vary. For the relationships of Brunelliaceae, see Bradford and Barnes (2001) and Murillo-A et al. (2022), although morphological analyses (Miranda-Esquivel 2001; Orozco 2001a; Orozco Pardo 2002) variously mixed Brunelliaceae and Cunoniaceae. The family pair [Brunelliaceae + Cephalotaceae] was retrieved by Davis et al. (2004), Crayn et al. (2006) and M. Sun et al. (2016: support weak); Matthews and Endress (2006b) suggest characters possibly linking these two families. However, Heibl and Renner (2012: focus Oxalis) suggest the relationships [Cunoniaceae [Brunelliaceae [Cephalotaceae + Elaeocarpaceae]]], which implies a rather different scenario of character evolution. Relationships in H.-T. Li et al. (2019) are [Cephalotaceae [Cunoniaceae + Elaeocarpaceae]], with strong support, but Brunelliaceae were not included and only Elaeocarpus and Sloanea of Elaeocarpaceae (but they are in the two clades that make up the family); in Li et al. (2021) relationships - not overly strong - are [[Cephalotaceae + Brunelliaceae] [Cunoniaceae + Elaeocarpaceae]] and the latter relationships were also recovered by X. Li et al. (2021: one taxon/family) while Xia et al. (2022: no Cunoniaceae) found the relationships [Bruniaceae [Cephalotaceae + Elaeocarpaceae]] - these last four are all some version of plastome studies. W. J. Baker et al. (2021a: Seed Plant Tree=) recovered the relationships [Cephalotaceae [Cunoniaceae [Brunelliaceae + Elaeocarpaceae]]], yet another arrangement of these families, and these relationships were also found in the analyses of Murillo-A et al. (2022), the Seed Plant Tree version of ix.2024 and Zuntini et al. (2024) - see also below. However, relationships were [Cunoniaceae [Cephalotaceae [Brunelliaceae + Elaeocarpaceae]]] in the Angiosperms353 analysis of Pillon et al. (2021) although support for the position of Cephalotaceae could have been stronger. Y. Wang et al. (2023: 20 spp., focus on Sloanea, plastome data) suggested the following rather unexpected relationships - [Connarus [Averrhoa [Oxalis [Brunellia [Cephalotus [Crinodendron [Elaeocarpus [Aristotelia [Vallea + Sloanea]]]]]]]]] - and dates were associated with many of the nodes, including a crown-group age of ca 0.4 Ma for Sloanea.
Classsification. Pillon et al. (2024) provide a classification of Oxalidales s. str. down to tribes and with included genera; this classification is followed below.
Synonymy: Bauerales Martius, Cephalotales Martius, Connarales Link, Cunoniales Martius, Elaeocarpales Berchtold & J. Presl, Tremandrales Martius
Oxalidineae Pillon, Crayn, Streiff & J. M. de Vos
Plant construction sympodial; benzoquinone rapanone +, ellagic acid 0; roots diarch [lateral roots 4-ranked]; vessel elements with simple perforations, wood rays largely uniseriate; sieve tube plastids with protein crystalloids; calcium oxalate druses 0; petiole bundle(s) annular (with medullary bundles), cuticle wax platelets as rosettes; leaves (simple), trifoliolate, imparipinnate, leaflets articulated, pulvinate, margins entire, secondary veins pinnate to palmate, stipules 0; flowers di- and tristylous, pedicels articulated; C postgenitally subbasally united, with uniseriate glandular hairs; A obdiplostemonous, in two whorls of different lengths, connate basally, (5, with antepetalous A staminodial), with uniseriate glandular hairs; (pollen colpate); G opposite C; (stigma with rounded multicellular ornamentations); ovules with endothelium; K persistent in fruit; exotesta ± fleshy.
Includes Connaraceae, Oxalidaceae.
Evolution: Pollination & Seed Dispersal. Heterostyly is very common here (Simó-Porcar et al. 2023).
Chemistry, Morphology, etc.. Sieve tube plastids may have protein crystalloids + starch [Connaraceae], crystalloids + fibres + starch [both families], or crystalloids alone [Oxalis].
For the androecium, see Ronse De Craene and Bull-Hereñu (2016).
CONNARACEAE R. Brown, nom. cons. —— Synonymy: Cnestidaceae Rafinesque - Back to Oxalidales
Shrubs or lianes, scrambling or twining, (trees); wood commonly siliceous or with SiO2 grains; (rays herringbone-patterned, associated with narrow tubules); phloem stratified; (nodes 5:5, 7:7); hairs uniseriate, submesifixed or not; stomata anomo-/para-/(-aniso-)cytic; leaves two-ranked or spiral, (unifoliolate); (plants dioecious); (flowers 4-merous), ( (heterostylous); (K connate); (nectary 0); A connate or not; tapetal cells binucleate; G separate, with adaxial furrow, (stipitate), placentation near-basal, stigma capitate, ?type; ovules 2/carpel, collateral, apotropous, funicle 0, (micropyle exostomal), outer integument 5-15 cells across, inner integument 3-5 cells across, parietal tissue 1-3 cells across; postament ± +; fruit a follicle (also dehiscing abaxially; drupe), wall expanding early, often only one carpel developing, K ± indurated; seed 1 (2), large; testa vascularized, black, sarcoexotesta ± developed, exotesta various, inc. palisade (lignified), tegmen multiplicative; endosperm 0 to abundant, oily; chloroplast rpl22 gene to nucleus.
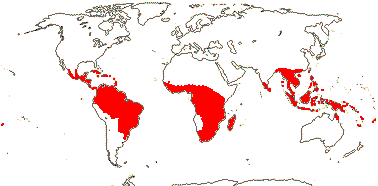
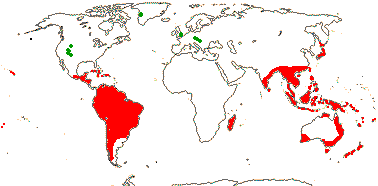
11(?14) [list, to tribes]/205. Pantropical, especially Africa and Old World. Map: from Leenhouts (1958), Forero (1983) and Trop. Afr. Fl. Pl. Ecol. Distr. Vol. 6 (2011). Photo: Flower, Fruit.
1. Manotoideae J. M. de Vos & Streiff - Manotes Planchon
Fibre tracheids and rather abundant parenchyma in long tangential bands, annual growth rings at most indistinct; leaves imparipinnate, highest order veins very fine, parallel; androgynophore +; G 5, style solid, transmitting tissue 0; seed with elongated fleshy thread-like arilloid, pendulous; n = 13; seedling with radicle aborting, "adventitious" roots +.
1/5. West Tropical Africa, Guinea to Angola.
2. Connaroideae Gilg
Leaves simple to pinnate; n = 14; seed with basal or all-enveloping arilloid.
2A. Connareae de Candolle
(Phloem not stratified - Conn.); (hairs dendroid); (P punctate - Conn.); (pollen tetracolpate - Jollyodora); G 1, stigma often bilobed; (seeds 2/carpel - Jo.), endosperm usu. 0.
6/95: Connarus (82). Pantropical.
2B. Cnestideae Planchon
G 5.
5(?9)/104: Rourea (78: polyphyletic). Pantropical.
Age. Fossil wood ca 65 Ma in which there are distinctive radial tubules which together with rays converging on them form a herringbone pattern has been collected in the Deccan Traps - around the K-P boundary ca 66 Ma - and named Connaroxylon dimorphum (Baas et al. 2017).
Evolution: Divergence & Distribution. The oldest fossil wood attributed to climbing Connaraceae is ca 19 Ma, from Panama (Jud & Nelson 2017), but see the much older woods from the family described by Baas et al. (2017) mentioned above...
Although various forms of heterostyly, including tristyly, are known from Connaraceae, details of the evolution of the variants is largely unknown, other than that parallelisms, including reversals, are common, and tristyly may be the ancestral condition (de Vos et al. 2024).
Seed Dispersal. For myxospermy, reported from three genera, see Grubert (1974).
Chemistry, Morphology, etc.. The plants are often poisonous. The cuticle waxes of Connaraceae are similar to those of Fabaceae-Fabales (Ditsch & Barthlott 1994). Growth is rarely sylleptic (Keller 1994).
There are quite often two or three vascular bundles in the foliar pulvini, although a single vascular bundle is the norm in the petiole (e.g. Forero 1976).
There are often five traces to each carpel. The ovules may be straight or anatropous. Number of nuclei in pollen?
There is much useful information in Schellenberg (1910), Jongkind and Lemmens (1989) and Lemmens et al. (2004); see Dickison (1971a: carpel anatomy, 1973a: stem anatomy), Ronse De Craene and Bull-Hereñu (2016: androecium), Mauritzon (1939a) ovule morphology. However, the family is not well known.
Phylogeny. Z.-D. Chen et al. (2016) found that the clade [Connarus + Rourea glabra] was sister to the rest of the family; Rourea was polyphyletic. The sampling in the v.2023 version of the Seed Plant Tree of Life is quite good; there Manotes is sister to the rest of the family, but again Rourea is polyphyletic, as was confirmed in the Angiosperms353 analysis of de Vos et al. (2024).
Classification. The clasification above is that proposed by de Vos et al. (2024); note that generic limits in Cnestideae are unclear, given the unresolved polyphyly of Rourea (de Vos et al. were unable to include the type).
OXALIDACEAE R. Brown, nom. cons. - Back to Oxalidales
Tannins +; mucilage cells?; juice acrid [soluble calcium oxalate accumulation]; leaves spiral (two-ranked), (stipules +, small); inflorescence cymose; plant heterostylous; C contorted [direction variable], often clawed; nectaries often glands opposite C; A (5 + 5 staminodes), anthers extrorse; androgynophore +; G [(3-)5], styluli +, stigmas spathulate/capitate; ovules (1-)2-many/carpel, (micropyle zig-zag; exostomal), outer integument 3-5 cells across, inner integument 3-5(-6) cells across, parietal tissue often 0; (megaspore mother cells several), (embryo sac bisporic, 8-celled), antipodals degenerate; fruit a ± ribbed/angled loculicidal capsule or berry; seed (arillate, explosive), (subruminate); testa (not multiplicative), often mucilaginous, (endotesta walls thickened; not palisade), (exotegmen 2-layered), testa and tegmen less differentiated when fruit a berry; endosperm +, starchy, embryo chlorophyllous or not; n = (5-)7(-12), x = 11, nuclear genome (1C) (0.016-)0.609(-22.644) pg.
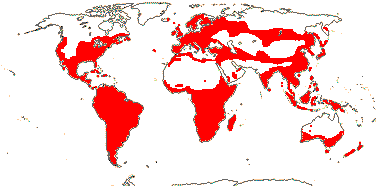
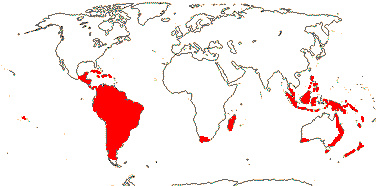
6/627: [list]. Usu. tropical or subtropical. Map: from Hultén (1958, 1971) Hultén and Fries (1986), Lourteig (2000 and references), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), GBIF Biophytum (vii.2008) and FloraBase (consulted vii.2008). Photo Flower.
Age. Crown-group Oxalidaceae have been dated to (56-)52, 43(-39) Ma (Wikström et al. 2001). A rather older age for stem Oxalis (i.e. crown Oxalidaceae) of (62.3-)56(-49.2) Ma was suggested by by Heibl and Renner (2012), and the estimate in Bell et al. (2010) was (52-)37, 34(-22) Ma.
1. Oxalidoideae Arnott - Oxalis L.
Plants herbs (annuals), (vines, shrubs), (bulbs, root/stem tubers +); leaves trifoliolate, palmate (simple), colleters +; tegmen (not lignified); (endosperm ±0).
1/557. Tropical and temperate, esp. tropical Brazil, Mexico, and South Africa, species like Oxalis corniculata very weedy and widespread.
2. Averrhoideae Pillon —— Synonymy: Averrhoaceae Hutchinson
Trees to herbs; leaves pinnate, (sensitive); tegmen (0 - Bio.).
4/75: Biophytum (50). Tropics and subtropics.
Age.
Evolution: Divergence & Distribution. It is estimated that divergence within Oxalis began (55.2-)48.6(42.3) Ma, possibly in South America (Heibl & Renner 2012, q.v. for other dates within the genus); African Oxalis has its origin in South America (see also Oberlander et al. 2011).
Germination (see Ecology & Physiology below), vegetative reproduction and perennation in Oxalis is often distinctive, and the genus shows more vegetative than floral variation (Oberlander et al. 2009); Oxalis also includes shrubs and vines. Oxalis is particularly diverse in drier parts of southern South America, with some 54 species known from Chile alone where it seems to have moved into drier habitats over a period of years. There have been 6-8 invasions of the Andean alpine habitat alone, but with no obvious major radiations there or correlation of the origins of those clades with the uplift of the Andes; one clade is predominantly to be found in the hyperarid Atacacama desert (Heibl & Renner 2012). However, the O. tuberosa alliance seems to have radiated along the Andes (Emshwiller & Doyle 2002), perhaps as little as 10-6 Ma (Heibl & Renner 2012). Oxalis in the Cape region is also very diverse and is a major element of the geophytic flora (Procheŝ et al. 2006); of the some 210-230 species in southern Africa, about 180 grow in the Greater Cape floristic region (see also Jooste et al. 2019b). Diversification of Oxalis in the Fynbos began about (31-)15.75 Ma, that in the Succulent Karoo some (20-)10 Ma (Verboom et al. 2009).
Ecology & Physiology. New World species of Oxalis that are bulbous have scaly (tunicate) bulbs, or fleshy scales are borne along a rhizome, and these scales may be equivalent to stipules or to whole leaves (Emshwiller et al. 2009); there are few other non-monocot bulbous taxa (Oberlander et al. 2009). Other species have stem and/or root tubers. Species of Oxalis in the Cape flora have recalcitrant seeds, that is, they germinate immediately on being shed from the plant; these seeds i.a. lack endosperm and a lignified tegmen and cannot stand dessication, and they are commoner in the winter rainfall area in the southern part of the Cape. More orthodox species with endosperm, a lignified tegmen, and that germinate more slowly are common in the summer rainfall areas to the north (Jooste et al. 2019a); this variation is not much correlated with phylogeny. Moreover, seedlings of recalcitrant taxa initially lack much in the way of a radicle or roots, and they produce much mucilage when they germinate (Jooste et al. 2019b). A close association with vertically-transmitted Bacillus species (see below) is also part of the equation.
A number of these Old World species germinate and become established in a most remarkable way. Dewey (1946) looked at Oxalis rubella/O. hirta, a species with recalcitrant seeds. The seedlings seem to be phanerocotylar and pretty ordinary, but what goes on underground is not. First of all, the radicle is able to grow straight through hard clay, and a swelling forms at the base (the base of the petiole proper?) just above where lateral roots later develop. The cortex of the root becomes disorganised, so freeing the central stele from the rest of the root. At the end of the first year the plumule ends up 4-12 cm underground - but how? The central stele and immediately-associated tissues contract and end up as a concertina-like structure (the stele is still functional, and is surrounded by the epidermis of the root that looks quite normal) immediately above the basal swelling, which also becomes disorganised, while simultaneously the base of the first leaf, with associated plumule, grows down the space vacated in the central part of the root, and the first-year bulb develops from the base of the plumule replacing the basal swelling and deeply "planted" in the soil. It is unclear if the base of the first leaf exerts pressure and helps cause the concertina-like structure just mentioned (Dewey 1946: see also Galil 1968 - some species of Iris and Colchicum establish themselves in a similar way).
The leaves of Oxalidaceae often fold at night; those of some species of Averrhoa and Biophytum are sensitive to touch.
Pollination Biology & Seed Dispersal. Tristyly, rather uncommon in angiosperms, is known from Averrhoa, Oxalis and Biophytum; see Shaw (2023) for a summary. There has been parallel evolution of distyly from tristyly within the New World bulbous species of Oxalis, and distyly is particularly common in a North American clade (Gardner et al. 2012; Barrett & Shore 2008: general). For heterostyly, see also Cohen (2019).
The mucilaginous testa is often mistaken for an aril; the turgor pressure that builds up in it forces the rest of the seed out explosively, rather like squeezing a grape pip (for myxospermy, see Grubert 1974).
Plant-Bacterial/Fungal Associations. Jooste et al. (2019b) found that species of Oxalis from southern Africa (with both methods of germination - see above), and apparently also from South America, are associated with a variety of endophytic bacteria that can be found throughout the plant and also often in the soil and in the seed mucilage; at least some of these bacteria are vertically transmitted. They noted that some of the Bacillus species involved could metabolize oxalic acid (oxalotrophy) and were able to fix nitrogen; in the Cape, at least, species of Oxalis grow in soil with extremely low nitrogen and phosphorus concentrations (Jooste et al. 2019b).
Genes & Genomes. For the genome of Averrhoa carambola, see Fan et al. (2019: some dates - Populus ca 94.5 Ma). A genome duplication event ca 56.5 Ma, the OXALα event, seems to involve the whole family (Landis et al. 2018). Diploid plants with x = 5 had far larger genomes than a polyploid clade with x = 6 in a group of Oxalis (Vaio et al. 2013).
X. Li et al. (2021) discuss plastome variation in Oxalidaceae - not that much.
Economic Importance. For Oxalis tuberosa (oca) and its relatives, see Emswhiller (2002).
Chemistry, Morphology, etc.. Leaves of Oxalis pes-caprae, at least, have small cells at their bases where they break when pulled; the rest of the plant is unaffected (Shtein et al. 2019).
The pollen of Oxalidaceae often contains starch. Link (1992a) describes the nectary glands as being opposite the calyx, but they are opposite the corolla at the bases of the filaments (e.g. Rama Devi 1991).
Some information is taken from Robertson (1975), Cocucci (2004) and Shaw (2023: succulents), and Govindappa and Boriah (1956), Herr and Dowd (1968), L. L. Narayana (1970), and Rosenfeldt and Galati (2012), all embryology, etc.. See Lourteig (2000 and references) for extensive monographic work on Oxalis.
Phylogeny. Heibl and Renner (2012) found the relationships [Oxalis [Biophytum [Dapania [Averrhoa + Sarcotheca]]]] (see also the ix.2024 version of the Seed Plant Tree of Life - sampling minimal), M. Sun et al. (2016) [Oxalis [Sarcotheca [Dapania + Averrhoa]]] and X. Li et al. (2021: whole plastomes) the relationships [Oxalis [Biophytum + Averrhoa]].
Oberlander et al. (2004, esp. 2011) discuss relationships within some southern African species of Oxalis. The ca 210 African species of the genus, all bulbous and largely from the Cape region, form a clade with small, basal pectinations and then two major subclades (Oberlander et al. 2011). For a preliminary phylogeny of the genus as a whole, see Emshwiller et al. (2009). Cabral et al. (2023) looked at relationships in Oxalis with a focus on subgenus Thamnoxys (34+/77+ spp., nrITS + 2 plastid markers); the two subgenera were largely monophyletic, the limits of the sections needed some adjustment.
Previous Relationships. Cronquist (1981) placed Oxalidaceae in Geraniales, and he included Hypseocharis (here Geraniaceae-Geraniales), Lepidobotrys (Lepidobotryaceae-Celastrales) and Dirachma (Dirachmaceae-Rosales) in the family.
Elaeocarpineae Engler
Flowers rather small [1> cm across]; P uniseriate, valvate; A 2 x P; G free; ovules 1-2/carpel, epitropous, inner integument 3-5 cells across.
Age. Estimates for the age of this node are (70-)66, 64(-60) Ma (Wikström et al. 2001) or (64-)50, 46(-34) Ma (Bell et al. 2010). Ages for all family nodes range between 81.8 and 76.4 Ma in Tank et al. (2015: Table S1, S2), but the topology differs from that here - and the MRCA of Elaeocarpaceae and [Cephalotaceae + Cunoniaceae] is dated at around 38.2 Ma (Table S1)...
Platydiscus peltatus (Schönenberger et al. 2001a; Schönenberger & von Balthazar 2006, see below), perhaps a member of Cunoniaceae, has been used to date the crown age of this clade (i.e. as stem Cunoniaceae), (84.2-)83.5(-82.8) Ma (see Heibl & Renner 2012). However, a position somewhere in Myrtales is suggested by López-Martínez et al. (2023a). It was not sampled by Zuntini et al. (2024) nor by the Seed Plant Tree version of ix.2024.
Includes Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae
[Cephalotaceae [Brunelliaceae + Elaeocarpaceae]]: ?
CEPHALOTACEAE Dumortier, nom. cons. - Cephalotus follicularis Labillardière - Back to Oxalidales
Herbs, rhizomatous, carnivorous [insectivorous]; flavanols and ellagic acid +, tannin 0; vessel elements with scalariform perforations; true tracheids +; vascular bundles initially separate; nodes ?1:1; petiole bundles annular; lamina amphistomatous, stomata brachyparacytic; leaves spiral, simple, margins entire, also hypoascidiate pitchers [opening towards the base of the leaf, lid at apex of petiole], stipules 0; inflorescence more or less scapose, branches scorpioid cymes; flowers 6-merous, hypanthium +, broad; P apex incurved-cucullate; longest A alternating with P, anthers ± incurved, anther connective swollen-abaxial, glandular; pollen grains triangular from polar view; nectary with fimbriate-glandular projections, esp. alternating with P; G 6, alternating with P, carpels plicate, loculi filled with secretion, styles initially straight; ovules 1(2)/carpel, micropyle bi/endostomal, inner integument 3-5 cells across, endothelium +; fruit long-hairy, ?follicle, hypanthium accrescent; seed coat mostly collapsed, exotesta papillate; endosperm development?, embryo short, cotyledons accumbent; n = 10; x = 7 (8), nuclear genome (1 C), (0.011-)0.569(-30.238) pg /ca 1983.6 Mbp; seedling phanerocotylar, hypocotyl with non-vascularized lateral extension, roots poorly developed.
1 [list]/1. S.W. Australia. Map: from FloraBase (consulted viii.2012). [Photo - Habit, Plant © H. Schneider.]
Evolution: Ecology & Physiology. Like many other carnivorous plants, Cephalotus is a plant of moist, acid habitats; in this case, it prefers sandy and peaty soils. There are nectar glands in the mouth of the pitcher which may facilitate the capture of insects (Bauer et al. 2008); Cephalotus produces enzymes in the pitcher (Peroutka et al. 2008b; Adlassnig et al. 2011). The digestive enzymes involved seem to be coopted stress-responsive and pathogenesis-related proteins, as is the case in other carnivorous plants, whether with pitchers (e.g. Sarraceniaceae) or gland-headed hairs (Droseraceae) (Fukushima et al. 2017 and references; see also Wheeler & Carstens 2018 for changes in gene expression categories). Interestingly, a change in the ambient temperature resulted in a switch between pitcher leaves and non-carnivorous flat leaves (Fukushima et al. 2017). Schulze et al. (1997) found that some 26±5.9% of the nitrogen in mature Cephalotus plants came from insects, on the low side for carnivorous plants in general. For more on carnivory, see Hatcher et al. (2020), Adamec et al. (2021), and references.
Plant-Animal Interactions. Larvae of the flightless dipteran micropezid fly, Badisis ambulans (the genus is monotypic), have been found only inside the pitchers.
Vegetative Variation. Arber (1941) noted that the vascular bundles in the simple leaves were not uniseriate, as in typical foliage leaves. The pitchers of Cephalotus are upside down compared with those of Nepenthes and Sarracenia, both of which open towards the apex of the leaf. For leaf development, see Franck (1976) and Froebe and Baur (1988: the trap as a modified pinnate leaf). Indeed, Froebe and Baur (1988; see also Brittnacher 2020) described the pitcher of Cephalotus as representing the "modified rhachis of a bijugal acroplast pinnate leaf" (ibid.: p.3); the lid of the picher is equivalent to the basal pair of leaflets. The ordinary leaves of Cephalotus may have fairly deep and irregular teeth.
Genes & Genomes. Veleba et al. (2020) discuss the size of the nuclear genome (Mbp above); some reports seem to be erroneous. Long-terminal repeat retrotransposons make up three quarters of the nuclear genome (Fukushima et al. 2017).
Chemistry, Morphology, etc.. The stomata of Cephalotus are brachyparacytic (Cross et al. 2019). Plants can be propagated from leaf cuttings, but it is unclear whether roots, etc., develop from stem tissue at the base of the petiole or from the leaf proper.
Cross et al. (2019) described the fruits as being variously achenes or (indehiscent) follicles; the embryo is described as being small, the seed having much endosperm.
Some information is taken from Diels (1930a), Conran (2004), the Carnivorous Plants Database, also Lloyd (1942), Juniper et al. (1989), McPherson (2010), Lowrie (2013: vol. 1), papers in Ellison and Adamec (2018) and Cross et al. (2019), all general; see also Jay and Lebreton (1973: chemistry), Carlquist (1981c: related to Saxifragaceae) and Gregory (1998), both anatomy, Arber (1941), vegetative, Ronse De Craene and Bull-Hereñu (2016: androecium), Danilova (1996: seeds) and Just et al. (2019: germination).
Previous Relationships. Cephalotaceae were included in a heterogeneous Rosales by Cronquist (1981) where they were surrounded by families now included in Saxifragales; Cephalotales immediately followed Saxifragales in the system of Takhtajan (1997).
[Brunelliaceae + Elaeocarpaceae]]: ?
BRUNELLIACEAE Engler, nom. cons. - Brunellia Ruiz & Pavón - Back to Oxalidales
Woody; chemistry?; cork?; vessel elements with simple and scalariform perforations; (nodes 5:5); petiole bundles annular or D-shaped, wing bundles +/0; stomata actinocyclic (anomocytic); hairs unicellular; leaves opposite, (simple), stipellate (stipels 0), leaflet vernation conduplicate, 2ndaries prominent, proceeding to the (doubly toothed) margin, stipules cauline, ± linear; breeding system various; flowers 4-8-merous; (C2, fugaceous); A (-3 x P); pollen reticulate(-rugulate) to punctate; nectary +; G 2-8, carpels also alternating with C, stigma long-decurrent; ovules 2/carpel, epitropous, ?endothelium, inner integument ca 4 cells across, obturator +; adaxial side of carpels much developed, fruitlets spreading, follicular, endocarp separating from the rest, U-shaped/urceolate/navicular, K persistent; seeds shiny, raphe ± aril-like; testa with subepidermal sclerenchymatous layer and palisade innermost layer; endosperm mealy, cotyledons ?incumbent; n = 14.
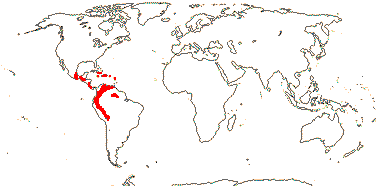
1 [list]/70. Especially Andean, also Central and South America and the Antilles. Map: from Cuatrecasas (1970); note that Orozco Pardo (2002) does not include the easterly locations in South America. Photo: Flower, Fruit.
Evolution: Divergence & Distribution. Orozco Pardo (2002) commented on the biogeography of Brunellia; species relationships came from a morphology-based phylogeny. However, Murillo-A. et al. (2022) in the course of their analyses of relationships in the family (see below), optimised seven characters on their "tree"; all were highly homoplastic.
Chemistry, Morphology, etc.. The nodes are described as being unilacunar (Orozco Pardo 2002; Orozco & Coba 2002), but there may be confusion here; some nodes illustrated by Orozco Pardo (2002) certainly do not look unilacunar.
Orozco et al. (2017) describe Brunellia ephemeropetala as sometimes having two fugaceous petals; these are illustrated as being opposite the petals (ibid. Fig.2D3), and the number of parts in the tiny flowers are variable, for instance, the 8-10 stamens varying in their development, so what these petals "are" is unclear. The inner androecial whorl may have twice as many stamens as perianth members. There are pistillodes in staminate flowers and staminodes in carpelate flowers. The ovules are epitropous, unlike those of most Cunoniaceae. Pollen morphology is uninformative (Orozco 2001b). There are often five traces to each carpel. Orozco Pardo (2002) described the seeds as being arillate.
For general information, see Cuatrecasas (1970, 1985), Orozco Pardo (2002) and Kubitzki (2004b), for anatomy, Gregory (1998) and Orozco and Coba (2002), and for seed coat (which needs more study), Naranho and Huber (1971) and Danilova (1996).
Phylogeny. Orozco Pardo (2002) provides a morphology-based species level phylogeny of Brunelliaceae. In various analyses - quite good sampling - based on an Angiopserms353 data set, Murillo-A. et al. (2022) indeed found quite high bootstrap and local posterior probability support in some species-level analyses. Two main clades were always recovered; in Clade I (6 subclades) clade IA was always sister to the rest, and in Clade II (4 subclades, 3 isolated species) Brunellia acostae. But otherwise, relationships between the groups/species varied, and there was a great deal of discordance in the data, as the DensiTree plot and network analyses in particular clearly show (Murillo-A et al. (2022: esp. Figs 5A, 6); hybridization and incomplete lineage sorting is involved.
Classification. Earlier infrageneric classifications, including that of Orozco (2001) who recognized 5 sections and 4 subsections, do not mesh well with the relationships found by Murillo-A. et al. (2022).
ELAEOCARPACEAE Jussieu, nom. cons. - Back to Oxalidales
Trees to shrubs; pyrrolizidine and tropane alkaloids, etc., ellagic acid +; growth rings common; vessel elements in radial multiples and with simple (scalariform) perforations; fibres often septate; petiole bundle annular, often with medullary (and wing) bundles; (epidermis mucilaginous), stomata anomo-, para-, actino- or cyclocytic; leaves spiral or opposite (two-ranked), simple, lamina vernation variable, margins toothed (entire), secondary veins pinnate or palmate, stipules lateral (0), (colleters +); inflorescence racemose or cymose or flowers axillary; flowers pendant, (4-merous), pedicels articulated (not); K (4-9), (connate), (± petal-like), C (3-6), aestivation (induplicate-)valvate (cochlear), margins fringed/toothed/lobed/(entire), with three traces; nectary large, androgynophore + (0); A ([1] 2 x K) / many, centrifugal, (± in groups opposite K), anthers basifixed, tubular-porose or with short apical slits, (connective prolonged), with lignified hairs, filaments shorter than anthers; G [2-9], placenta various, lignified hairs in the loculi, style single, stigma ± punctate; ovules 1-many/carpel, (epitropous), ± hairy, micropyle zig-zag, outer integument 2-6 cells across, inner integument 3-7 cells across, parietal tissue 3-4 cells across, nucellar cap 0, endothelium + (?0), (hypostase +), (long supra-chalazal zone), (curved chalazal appendage +); (megaspore mother cells several); fruit a loculicidal capsule (loculicidal + septicidal), or drupe (berry); when capsules, seeds with chalazal, raphal or "integumentary aril", or apical chalazal strophiole, or sarcotesta; testal cells ± elongated, thickened and lignified, (endotesta not crystalliferous), tegmen with vascular bundles, (multiplicative), (endotegmen lignified); endosperm ± copious, oily, embryo chlorophyllous [1 record]; x = 7 (8), nuclear genome (0.011-)0.569(-30.238) pg.
12/770 [list]. Tropical, not mainland Africa, also southern (warm) temperate, Map: from Vester (1940) and van Balgooy (1993); for early Caenozoic fosssils [green], see Manchester and Kvacek (2009).
Age. The age of this node has been estimated to be (63-)59, 56(-52) Ma by Wikström et al. (2001). Other estimates are (55-)42, 38(-27) Ma (Bell et al. 2010) or much older, (126-)118(-110) Ma (Crayn et al. 2006).
The family may occur in North America in the Late Cretaceous (Manchester & Kvacek 2009).
1. Sloaneoideae Pillon & Crayn
Stomata cyclocytic (anomocytic, actinocytic); (C 0); anthers with apical pores/slits to the base; G [2-5], style ± branched or not; capsule spiny; n = 14; (seedling leaves compound/deeply lobed).
3/170. Indo-Malesia to Australasia, Madagsacar, Tropical America.
Age. Crown-group Sloaneoideae are estimated to be (95-)89(-83) Ma (Crayn et al. 2006).
1A. Sloaneeae Endlicher - Sloanea L.
(C 0 - most American spp.)
1/150. Indo-Malesia to Australasia, Madagsacar, Tropical (temperate South) America. Photo: Fruit.
Age. Fruits and leaves identified as Sloanea are known quite widely from the early Palaeocene (late Danian, ca 62 Ma) onwards - North America and Greenland from the Palaeocene, Europe from the Oligocene, although they are quite often identified asCarpolithus spinosus or even Castanea (Kvacek et al. 2001b).
1B. Aristotelieae K. Schumann —— Synonymy: Aristoteliaceae Dumortier
.
2/20: Aristotelia (18). The Antipodes and South America, Ecuador southwards.
2. Elaeocarpoideae Arnott
([Ericoid] shrublets); (nodes 1:1); (indumentum stellate); K valvate(-induplicate); (style branched); 1-2 ovules carpel, epitropous, (inner integument -17 cells across - Tremandra et al.); (fruit a drupe); seeds (with chalazal elaiosome); endosperm initially starchy [Tremandra, etc.], embryo (short); n = 12, 14, 15, 21.
9/600. China, Southeast Asia to the Pacific, few South America. Photo: Flower.
Age. Crown-group Elaeocarpoideae are estimated to be (110-)103(-96) Ma (Crayn et al. 2006).
2A. Tricuspidarieae Endlicher
Shrubs to trees..
2/6: Crinodendron (4). South America, N.E. Australia.
[Dubouzetieae [Tremandreae + Elaeocarpeae]]: ?
2B. Dubouzetieae Pillon & Crayn - Dubouzetia Brongniart & Grisebach
Shrubs to trees; C (minutely serrulate), 2-pocketed at base; A many; G [3–5]; ovules 4–12/loculus; seeds 1–3 per loculus, with apical spiral waxy strophiole (0); (testa with fine spreading hairs).
1/11. New Caledonia, Northern Australia, Papuasia, the Moluccas.
[Tremandreae + Elaeocarpeae]: .
2C. Tremandreae Reichenbach —— Synonymy: Tetrathecaceae R. Brown, Tremandraceae de Candolle, nom. cons.
testa hairy - esp. Tremandra, etc.
3/56: Tremandra (50). Australia, esp. the S.W..
2D. Elaeocarpeae Bartling —— Synonymy: Aristoteliaceae Dumortier
C often ± lobed; (endothecium of stone cells - E.); embryo (curved - S., some E.).
3/526: Elaeocarpus (488), Aceratium (20), Sericolea (18). China to Malesia and the western Pacific Islands.
Age. Wood thought to be close to that of Elaeocarpus is known from the Deccan Traps at the K-P boundary (Prakash & Dayal 1964).
Evolution: Divergence & Distribution. For numerous dates in the family, see Crayn et al. (2006).
Pillon et al. (2021) suggested that Elaeocarpaceae were American in origin; they did not include Sloanea in their study. Crayn et al. (2006) discussed the biogeography of the family, finding substantial dispersal in its history. Dispersal was also invoked by Sanmartín and Ronquist (2004) to explain the distribution of the southern Aristotelia and Vallea, sister taxa in Sloaneae that diverged (60-)56(-52) Ma (Coode 1985; Crayn et al. 2006); these taxa are perhaps American in origin (Pillon et al. 2021). Divergence of the distinctive xeromorphic Australian Tremandra et al. clade occurred some (42-)29, 26(-15) Ma (Bell et al. 2010) or 64 Ma, diversification within it beginning some 37 Ma (Crayn et al. 2006); again, the effects of the Eocene-Oligocene transition. Crayn et al. (2006) emphasized this diversification in the context of the drier habitats to which Tremandra et al. became adapted (see also Donoghue & Edwards 2014), note, however, its rain-forest sister taxon, which includes Elaeocarpus et al., contains about two thirds of the species in the family...
Pollination Biology & Seed Dispersal. The more or less porose anthers and pendulous flowers common in the family suggest buzz pollination, but nectar is sometimes produced; although buzz pollination is indeed likely, observations are few (Coode 2004; Ladd et al. 2019: Tetratheca).
The blue colour of the fruits of Elaeocarpus are not caused by pigments, but by the structure of the epidermis (D. W. Lee 1991; other purely structural mechanisms cause intense blue fruit colours in Pollia in Commelinaceae, Viburnum).
Genes & Genomes. Although there seems to have been elevated molecular divergence in the Tremandra et al. clade, it is distinctly less speciose than its sister clade, which includes Elaeocarpus, the largest genus in the family (Crayn et al. 2006).
Y. Wang et al. (2023) discuss plastome morphology in Sloanea.
Chemistry, Morphology, etc.. The corolla is more or less (induplicate-)valvate, at least near its insertion, each petal enclosing a group of stamens, and the corolla is larger than the calyx in advanced bud (it is usually smaller in rosids). There are lignified hairs on the insides of the ovary loculi. These and many other similarities strongly link the old Tremandraceae and Elaeocarpaceae (Matthews & Endress 2002a).
Leaf teeth have a single vein running to an opaque (non)glandular deciduous apex. Juvenile leaves of Sloanea may be pinnately compound. The sepals may be more or less petaloid and the petals can vary considerably in width within the same flower; in some species they are connate. The androecium is very variable, and sometimes, when there are many stamens, they are clearly fasciculate; Venkata Rao (1953a) suggested that in Elaeocarpus there were antesepalous groups of stamens and single antepetalous stamens. Seeds and fruits are also very variable. Some species of Elaeocarpus have curved embryos.
See Coode (2004) for a general account, for information on wood anatomy, see Gasson (1996), embryology, see Mauritzon (1934f), Venkata Rao (1953b) and Biddle and Christophel (1979), and for seed anatomy, etc., see Boeswinkel (1999: as Tremandraceae, similarity to Linaceae noted).
Phylogeny. In Bradford and Barnes (2001) monophyly of Elaeocarpaceae was not established, but sampling in that part of Oxalidales was poor. However, monophyly is strongly supported in the more detailed analysis of Crayn et al. (2006: 88% bootstrap, 99% p.p.). The well-supported clade [Sloanea [Vallea + Aristotelia]] was sister to the rest of the family (see also Miissalo in Pennington & Wise 2017), [Crinodendron + Peripentadenia] and Dubouzetia perhaps being successively sister to [old Tremandraceae + [Sericolea, Aceratium, Elaeocarpus]], but there was little resolution of this latter group, nor was Elaeocarpus clearly monophyletic (see also M. Sun et al. 2016). The relationships suggested by Y. Wang et al. (2023, plastome data - see under the order) are a little odd.
Within Sloanea, Old World and New World clades may be sister taxa, but support for the monophyly of the former and for groupings in the latter was not strong (Miissalo in Pennington & Wise 2017).
Previous Relationships. Elaeocarpaceae were previously usually placed either in (Cronquist 1981) or adjacent to (Takhtajan 1997) Malvales, but there are numerous differences (e.g. absence of mucilage, indumentum type). Tremandraceae have long been of very uncertain position, for example, they were placed in Rosidae-Vochysiales (Takhtajan 1997) or Pittosporales (Cronquist 1981).
CUNONIACEAE R. Brown, nom. cons. - Back to Oxalidales
Woody, branching from the current flush; plants often Al-accumulators; ellagic acid +; wood with crystals; vessel elements with (simple to) mixed or scalariform perforation plates; sieve tubes with non-dispersive protein bodies; young stem with vascular cylinder; nodes 3:3; petiole bundles (arcuate) annular (adaxial or medullary bundles +); stomata various; leaves opposite, margins gland-toothed, 2ndaries proceeding to the teeth or not, stipules single, interpetiolar, colleters +; A 2 x K, longer than P/C/K, filaments incurved in bud; tapetal cells binucleate; G opposite C [?always]; micropyle various, inc. zig-zag, outer integument 2-3 cells across, inner integument 4-5 cells across, parietal tissue 1-5 cells across, (nucellar cap +, 2 cells across), chalaza ± columnar, massive, obturator +, postament ± +; archesporium multicellular; exotesta tanniniferous, cells crystalliferous, exotegmen fibrous or sclerotic, endotegmen tanniniferous; endosperm +, starchy; x = ?16.
28 [list]/280 (330). Largely temperate and tropical S. hemisphere, a few from South Africa and Madagascar. Map: from Good (1974), see also Pillon et al. (2021: Fig. 4), Matel et al. (2022: Fig. 1). Photo: Flower, Flower.
Age. Stem ages: 90.9 to 66.55 Ma (Magallònet al. 2015; Ramírez-Barahona et al. 2020). Crown ages of Cunoniaceae range from 83.32 to 63.49 Ma (Heibl & Renner 2012; Ramírez-Barahona et al. 2020), at least 80 Ma (K. K. Tang et al. 2022) or (88.9-)87.1(-80.5) Ma (Fajardo-Gutiérrez et al. 2025).
1. Spiraeanthemoideae Reveal - Spiraeanthemum A. Gray —— Synonymy: Spiraeanthemaceae Doweld
Trees or shrubs; leaves simple, (margins entire); (plant dioecious); flowers in inflorescence opening simultaneously; A 4-5, alternating with K; G 2-6, ± free; ovules 2/carpel; fruit follicular; seeds winged; n = ?
1/20. New Guinea, N.E. Australia to New Caledonia, Fiji and Samoa.
[Hooglandioideae [Aistopetaloideae [Baueroideae [[Davidsonioideae + Schizomerioideae] Cunonioideae]]]]]: flowers in inflorescence opening basipetally; G connate, styluli free.
2. Hooglandioideae Pillon - Hooglandia ignambiensis McPherson & Lowry
Trees; stipules small, (paired); plant dioecious; P 4-merous, imbricate; G [?2]; ovules 1-2/carpel; fruit a 1-seeded drupe; n = ?
1/1. N.E. New Caledonia.
[Aistopetaloideae [Baueroideae [[Davidsonioideae + Schizomerioideae] Cunonioideae]]]]: ?
3. Aistopetaloideae Pillon - Aistopetalum Schlechter
Trees; interpetiolar stipules barely connate; P 4-6-merous; disc annular; G [4-6]; one pendulous ovule/carpel; fruit drupaceous, 1 seed/carpel; testa ca 7 and tegmen 7-9 cells across, sclerotic layer +; n = ?
1/2. New Guinea.
[Baueroideae [[Davidsonioideae + Schizomerioideae] Cunonioideae]]]]: P = K + C; fruit a septicidal + ventricidal capsule.
4. Baueroideae Burnett - Bauera Andrews —— Synonymy: Baueraceae Lindley
Scrambling shrubs; ellagic acid 0; nodes 1:3; leaves unifoliolate, ± sessile, stipules leaf-like, paired [plant apparently with 6-whorled leaves]; flowers solitary, axillary, large [1.5-2 cm across], 6-8-merous; C (?biseriate), much larger than K; A 12-many, much shorter than C; tapetal cells uninucleate; ?pollen; nectary 0; ovules /carpel, micropyle zig-zag, parietal tissue ca 4 cells across, (nucellar cap ca 2 cells across); seeds ellipsoid, tegmen multiplicative, 5-6 cells across, exotesta and endotgmen tanniniferous, sclerotic layer 0; n = 16.
1/4. E. and S.E. Australia, Tasmania.
[[Davidsonioideae + Schizomerioideae] Cunonioideae]]]]: ?
[Davidsonioideae + Schizomerioideae]: ?
Age. This age of this node is thought to be ca 83.8 Ma (Fajardo-Gutiérrez et al. 2025).
5. Davidsonioideae Thorne & Reveal - Davidsonia F. Mueller —— Synonymy: Davidsoniaceae Bange
Myricetin +; urticating hairs +; leaves spiral, stipules paired, large; K half connate, C 0; A barely exserted; ?pollen; nectariferous lobes alternaring with A; G [2], placentation apical-axile, G with septal bundles; ovules 5-7/carpel, pendulous, epitropous; fruit schizocarpic, "hairs" = sclereids/fibres from decaying mesocarp; seeds 2-4, pachychalazal [bottom half], vascularized, testa 5-6 cells across, vascularized, tegmen 5-6(-11) cells across; endosperm 0; n = ?
1/3. E. Australia.
6. Schizomerioideae Pillon
C ± deeply bifid (0); (pollen 2-colpate); disc annular; G (half-inferior); (ovules 4/carpel, epitropous); Fruit (indehiscent, K enlarged, woody - Cer.); testa and/or tegmen usu. >5 cells across, (mesotestal cells crystalliferous - ?Schi.), tegmen sclerotic layer +/0; n = 16.
4/21: Schizomeria (10), Ceratopetalum (9). E. Australia, Tasmania, Moluccas to the Solomon Islands, Platylophus trifoliatus South Africa.
Age. Well-preserved fossils described as Ceratopetalum suciensis have been collected in Campanian (82-80 Ma) deposits from Sucia Island, Washington, U.S.A. (K. K. Tang 2022). Lacinipetalum spectabilum, from Palaeocene deposits in Argentina in the Salamanca Formation 62.5-62.2 Ma, is perhaps to be placed in Schizomerieae (Jud et al. 2018c), while fruits described as C. edgardoromeroi have been found in the remarkable ca 52 Ma deposits (early Eocene) of the Laguna del Hunco, Argentinian Patagonia (Merkhofer et al. 2015; Gandolfo & Hermsen 2017).
7. Cunonioideae Beilschmied
?
Age. The age of this node is ca 78.9 Ma (Fajardo-Gutiérrez et al. 2025).
7A. Eucryphieae G. Don - Eucryphia Cavanilles —— Synonymy: Eucryphiaceae Gay, nom. cons.
Shrubs to trees (deciduous); petiole bundle aruate to annular, with wing bundles; leaves (simple), abaxial surface with peltate cuticular protrusions (0 - E. glutinosa); flowers solitary, "multibracteate", axillary, large [>2 cm across]; C much larger than K, 4, imbricate; A many, much shorter than C, fasciculate, with trunk bundles, surrounded by hairy scales; pollen 2-colpate; G [4-many]; G separating in fruit, ventricidal; seeds winged; n = 15.
1/9. Eastern Australia, Tasmania, Chile.
[[Gillbeeae + Geissoieae] [[Acrophylleae + Caldcluvieae] [Codieae + Cunonieae]]]: flowers in inflorescence opening ± simultaneously.
Age. Ramírez-Barahona et al. (2025) estimate the age of this node to be around 77.4 Ma.
7B. Gillbeeae Pillon
Trees; hairs stellate, on inflorescence; stipules paired; C bifid, with apical glands; pollen syncolpate; nectary annular, lobed; G [3]; ovules 2(3)4/carpel; fruit indehiscent, G developing wings; tegmen lacking sclerotic layer; n = ?
1/3. N.E. Australia, New Guinea.
7C. Geissoieae Meisner —— Synonymy: Belangeraceae J. Agardh
(Nodes to 17:17); (stipules paired, connate, intrapetiolar), (stipels +); inflorescence racemose; K (large), C 0; A (8-)many; (pollen 2-colp/or/ate); nectary annular, adnate to G; seeds winged; (testa ca 4 cells, tegmen 7-8 cells across - Pseudoweinmannia); n = 16.
3/25: Geissois (18). S.E. South America, E. Australia, Fiji and E. Solomon Islands to New Caledonia.
[Acrophylleae + Caldcluvieae] [Codieae + Cunonieae]]: ?
Age. Around 74.1 Ma is the age of this node (Fajardo-Gutiérrez et al. 2025).
[Acrophylleae + Caldcluvieae]: ?
7D. Acrophylleae Pillon - Acrophyllum australe (A. Cunningham) Hoogland
Shrubs; leaves whorled, subsessile, simple, deeply serrate, base cordate, with peltate hairs; inflorescences from successive axils, stem resuming vegetative growth; ?pollen; nectary annular, small, at base of G; ovules 9-11/carpel, epitropous; seeds small, ellipsoid; n = ?
1/1. Blue Mountains, New South Wales, Australia.
7E. Caldcluvieae Bradford & Barnes
Leaves (with stellate hairs), tuft domatia on the underside, (stipels +), (stipules paired); (nectary annular (segmented); seeds winged (hairy [Caldcluvia]); n = 16.
3/12: Ackama (10). New Guinea, Solomon Islands, E. Australia, New Zealand, southern Chile and adjacent Argentina.
Age. Cupaniocarpa stylosa, from ca 52 Ma deposits iin Laguna del Hunco, Argentina, may belong to Caldcluvieae (Matel et al. 2022).
[Codieae + Cunonieae]: K imbricate (valvate).
Age. This age of this node may be (75.3-)68.4(-63.3) Ma (Fajardo-Gutiérrez et al. 2025).
7F. Codieae G. Don —— Synonymy: Callicomaceae J. Agardh
Leaves unifoliolate; inflorescence capitate; C 0 (+, slender); (pollen 2-colpate); nectary 0 / 5 segments; G inferior to superior; fruit indehiscent, hairy / enlarged persistent K / capsule; (tegmen sclerotic layer 0 - Codia); n = ?
3/16: Codia (13). Eastern Australia, New Guinea to Fiji.
7G. Cunonieae Schrank & Martius —— Synonymy: Ornithrophaceae Martynov
(Plant hemiepiphyte - W.); inflorescence racemose (capitate); K (one trace - Pancheria); pollen tricolporate; nectary adnate to G / annular / segmented; capsule with vertical replum; seeds winged/hairy; n = 12, 15, 16; trnL-trnF spacer with deletion.
5/214: Weinmannia (92), Pterophylla (68), Pancheria (27), Cunonia (24). Malesia to E. Australia and the Southeast Pacific, esp. New Caledonia, tropical America, Madagascar and the Mascarenes, 1 sp. South Africa.
Age. The crown-group age of this tribe is estimated to be (61.2-)56.0(-52.0) Ma (Fajardo-Gutiérrez et al. 2025).
Wood from the Coniacian of the Antarctic Peninsula 88.6-85.8 Ma has been placed in Weinmannioxylon (Poole et al. 2000b; Cantrill & Poole 2012). Fossil fruits from the Laguna dek Hunco area in Argentina and described as Racemofructus fasciculatus probably belong to Cunonieae (Matel et al. 2022).
Evolution: Divergence & Distribution. Fossil flowers of Platydiscus peltatus found in Late Cretaceous rocks from Sweden ca 83 Ma seemed assignable to Cunoniaceae (Schönenberger et al. 2001a; Friis et al. 2011). The stamens are obdiplostemonous and no longer than the petals, which are elliptic, concave, with narrow bases and peltate hairs on the outside; the placentation looks very intrusive parietal, with the placentae shaped like arrow-heads. Tropidogyne, of which four species have been described from Burmese amber some 110-97 Ma old (Poinar et al. 2021 and references) have a floral formula of K 5, spreading, C 0, A 10, G [2/3], inferior, the styluli arching and the stigmas decurrent or the styluli erect and stout and the stigmas capitate; the flowers are rather like those of Ceratopetalum (Chambers et al. 2010) or Cunonia. However, neither of these fossil genera grouped with crown or stem Cunoniaceae or even Oxalidales in phyloscan analyses (Platydiscus grouped with Saxifragales, Tropidogyne far more broadly), while analyses in López-Martínez et al. (2023a) suggested the possibility of a resting place in Myrtales. Interestingly, Ceratopetalum suciensis (fossil fruits) did not initially group with Cunoniaceae in the phyloscan analyses, but with Saxifragales, and linked with Cunoniaceae only when sampling there was improved (basic matrix from Schönenberger et al. 2020, see K. K. Tang et al. 2022); this is an interesting fossil, not only because at 82-80 Ma it is nevertheless assigned to an extant genus, but also because it was found in Washington State, U.S.A., extant members of the genus being known only from Australia and New Guinea.
In addition to these fossils, there are others, such as Cunoniantha bicarpellata, from Palaeocene deposits in Argentina 62.5–62.2 Ma (Jud & Gandolfo 2020), that attest to the early diversity of Cunoniaceae in southern South America. The ca 52 Ma Ceratopetalum edgardoromeroi from Argentinian Patagonia (Merkhofer et al. 2015; Gandolfo & Hermsen 2017) is notable in that it, too, was placed in crown-group Ceratopetalum, although the genus is currently known only from New Guinea-Australia; other fossils from this locality are placed in Cunonieae and perhaps also Caldcluvieae (Matel et al. 2022). However, more remarkable is the 82-80 Ma C. suciensis from Washington, U.S.A. (see above: K. K. Tang et al. 2022); Pillon et al. (2021) and Matel et al. (2022) discuss other early fossil records of Cunoniaceae. For some other "southern" fossils, palaeo-Antarctic rainforest lineages with links to Malesia, etc., see Kooyman et al. (2014), Merkhofer et al. (2015), Wilf et al. (2019a) and especially Nothofagaceae.
There is a fair bit of variation in ages for nodes in the family, and López-Martínez et al. (2025) noted that the ages they recovered tended to be older than those in Pillon et al. (2021), but noted that their sampling was much more extensive, tghey relied on platid rather than nuclear data, etc..
The ancestral area for Cunoniaceae may be the Australia/New Guinea/New Caledonia region and, perhaps minus New Caledonia, that area also seems to be ancestral for much of the rest of the family. Both extant members and fossils of the family are very largely from the Southern Hemisphere, and Cunoniaceae are fourth among angiosperms in numbers of fossils from Antarctica (de la Estrella 2019b). Segovia et al. (2020) looked at diversification in the family, where there seem to have been frequent shifts between temperate (where there is more phylogenetic diversity, if fewer species) and tropical (where species richness is concentrated) areas; a preponderance of movement from extratropical to tropical regions is unexpected. Sanmartín and Ronquist (2004) invoked dispersal to explain the distribution of the family, and that of Cunonieae in particular, and cunoniaceous fossils and their distributions are largely compatible with such scenarios. Bradford (2002) also discussed evolution in Cunonieae, and Matel et al. (2022) looked at relationships in Cunonieae in some detail as did Fajardo-Gutiérrez et al. (2025); see also below.
Ceratopetalum suciensis, from the western U.S.A., rather complicates the issue. Ceratopetalum itself is quite well embedded in the family, and the fossil suggests that the evolution and biogeography of Cunoniaceae may need rethinking. The early history of the family is perhaps somewhat up in the air: Did it have a global distribution in the Cretaceous? Is the family of northern or southern origin? (K. K. Tang et al. 2022). Indeed, López-Martínez et al. (2025) noted that early fossils were really quite widespread, but after the K/P boundary event, fossils from the Northern Hemisphere seemed to be absent. Latterly, of course, movement of the family between southern South America, Antarctica and Australia, and with extinction of the family in Antarctica, and of genera like Ceratopetalum itself in other parts of this area, have shaped its current distribution; Cunoniaceae are predominantly a southern family.
López-Martínez et al. (2025) drew attention to a number of interesting distributions in Cunonieae, although a better-supported phylogeny is as always very much a desideratum. ITS data suggested that Cunonia was paraphyletic, relationships being [C. capensis (the only African species in the genus) [other species of Cunonia + Pancheria]]; Pillon et al. (2021) had looked at only two species of the genus, but included C. capensis. Turning now to Weinmannia, analysis of chloroplast data suggested that W. tinctoria, one of the two species of the genus from the Mascarenes, was sister to the rest of the genus (the other species from the Mascarenes, plastids unsampled, is W. mauritiana); Weinmannia s. str. is known largely from Andean South America to the Antilles, etc., and evolution has largely been south to north (López-Martínez et al. 2025). Note, however, that ITS data placed W. mauritiana within the Northern Andean clade of Weinmannia, while it was W. tinctoria that was sister to the rest of the genus (López-Martínez et al. 2025). Bradford (2002) had found that these two species from the Mascarenes were in a small clade of Weinmannia which in turn was a member of a decatomy made up of other species of Weinmannia, also species of Cunonia and of species of Weinmannia now included in Pterophylla - all rather complicated. Segovia et al. (2024) found that a small clade sister to the rest of the genus and made up of W. trichosperma and W. boliviensis, from south central Peru and central Bolivia respectively, were the southernmost members of the genus examined, members of the next two clades (bar the Mascarene taxa, see below) were mostly from central Bolivia and south Peru, while last clade, the main clade in the genus, included species mostly from Northern Peru to Columbia. Movement south to north was confirmed, the species to the north growing at somewhat higher altitudes than the others - overall, there was climatic niche conservatism, that of an extratropical climatic niche (Segovia et al. 2024). And the species from the Mascarenes? These two species form a clade immediately above the {W. trichosperma + W. boliviensis] clade and the Mascarene clade is considerably older than the Mascarenes themselves; how those species got where they have is a mystery (Segovia et al. 2024).
Ecology & Physiology. Gei et al. (2020) record a number of nickel-accumulating Cunoniaceae from New Caledonia.
Some species of Weinmannia are hemiepiphytes. Thus W. tinctoria, from Réunion, preferentially germinates on the trunks of Cyathea (= Alsophila) species, generally on sizeable individuals, where it finds the well-lit conditions it needs for germination; the adult is a quite sizeable free-standing tree (Derroire et al. 2007).
Chemistry, Morphology, etc.. There are numerous lignified cells in the bark of Cunoniaceae. Eucryphia and other Cunoniaceae have very small sieve tube plastids, those of the former have protein inclusions only and are about the smallest known (Behnke 1988b). Bauera has been described as having unilacunar nodes, sessile, trifoliolate leaves and no stipules (Dickison 1980) and 1:3 nodes and sessile, large-stipulate leaves (Dickison & Rutishauser 1990); there seems to be a whorl of six leaves at each node (the latter interpretation is followed above). The leaf teeth of Cunoniaceae have a glandular apex, and the lower branch of the main vein goes into the tooth, the other proceeds above it. Nodal anatomy is variable (Dickinson 1980b), as is stipule development; single interpetiolar stipules may be paired as primordia (Rutishauser & Dickison 1989). Dickison (1978) illustrates the remarkable (?unique) peltate cuticular structures on the abaxial surface of the leaf blade in Eucryphia.
As with vegetative variation, floral variation in Cunoniaceae is extensive. The flowers in an inflorescence often open almost simultaneously (Bradford & Barnes 2001) and sometimes centrifugally/basipetally. The nectary is basically intrastaminal. The pollen grains are typically very small. In Eucryphia carpel number (i.a.), very variable in the family, depends on the size of the floral apex, the carpels differentiating in a ring around the periphery of the apex (Bull-Hereñu et al. 2018). Cunonia has two oblique carpels (Engler 1930b). Although Hooglandia was described as having a single carpel, Bradford et al. (2004) suggested a bump on the ovary might represent a second carpel. There are often five traces to each carpel. In th descriptions of seed coats of the family by Dickison (1984), all genera of Schizomerieae are noted as having a testa and/or tegmen thicker than normal (= 2-3 cells across), and/or lacking a sclerotic layer. The endosperm is described as being oily by Cronquist (1981) and Mabberley (1997), but starchy by Hopkins and Hoogland (2002) and Bradford et al. (2004). It is not clear how common pachychalazal seeds are (for Davidsonia, see Doweld 1998a).
Hoogland (1960), Dickison (1989b) and especially Bradford et al. (2004) provide general information; see also Jay (1968b) for chemistry, Gregory (1998) and Wilkinson (1998) for general anatomy, Dickison (1978, 1980a) for wood anatomy, Dickison (1975a) and Bensel and Palser (1975d) for floral anatomy of Bauera and Dickison (1975b, 1978) for that of other Cunoniaceae, Mathews et al. (2001), Schönenberger et al. (2001a), Moody and Hufford (2000b: Davidsonia) and Rozefelds and Barnes (2002: Ceratopetalum) for floral morphology, etc., Hideux and Ferguson (1976) and Hideux and Abadie (1986) for pollen, Mauritzon (1939a) for ovules, Prakash and McAlister (1977: Bauera) for embryology, etc., and Dickison (1984: much detail) for fruits and seeds.
Phylogeny. Morphological phylogenetic analyses of Cunoniaceae in the old sense, i.e. including Aphanopetalum (now Saxifragales), do not signal the latter out as being anything particularly distinctive (Hufford & Dickison 1992; Orozco Pardo 2002). Morgan and Soltis (1993) early associated Baueraceae with Cunoniaceae. Sweeney et al. (2004) placed the distinctive and then recently-discovered Hooglandia firmly within the family, the clade [Acsmithia + Spiraeanthemum] (for which, see Pillon et al. 2009, they are synonyms and the latter is the name to use) was sister to the rest of the family (see also Bradford & Barnes 2001; Hopkins et al. 2013: tree rooted by the latter genus). These Spiraeanthemoideae have follicular fruits and vessel elements with scalariform perforation plates, but both features occur elsewhere in the family. Bauera, Davidsonia and Hooglandia are probably all close to the base of the tree (Hopkins et al. 2013), although exactly which of these genera were involved, their immediate relationships, and support values depended on the method of analysis (here they are placed in three separate subfamilies). W. J. Baker et al. (2021: see Seed Plant Tree) recovered most of the same suspects as basal, but the order was [Spiraeanthemum [Anodopetalum (but polyphyletic) [Hooglandia [Davidsonia [[small clade perhaps including Bauera] ...]]]]]. The basal pectinations are [Spiraeanthemum [Hooglandia [Aistopetalum [Bauera ...]]]] in the Angiosperms353 analysis of Pillon et al. (2021). Within the rest of the family, the pectinations can be represented by tribes for the most part - [Schizomerioideae [Cunonioideae = Eucryphieae [Geissoieae [Caldcluvieae [Codieae + Cunonieae]]]]], with unattached - and rather distinctive - genera sister to the first three tribes; quartet support varies, that for the position of the extended Schizomerieae and the monophyly of both the extended Schizomerieae and extended Caldcluvieae being weakest, while there are suggestions that Ackama (Caldcluvieae) might be paraphyletic and Weinmannia (Cunonieae) polyphyletic (Pillon et al. 2021); hybridization in the latter is extensive. The focus in Fajardo-Gutiérrez et al. (2025) was on Cunonieae (81/214 species included), and three plastid genes along with nuclear ITS were sampled; the Australian Vesselowskya was sister to the rest of the tribe. It was, however, unclear if Cunonia was monophyletic, while the topology of Weinmannia, monophyletic, varied according to the data (nuclear/plastid) analyzed.
Classification. I followed the tribal classification in Bradford et al. (2004; see also Pillon et al. 2021), although I have added a few tribal names that were already available where the phylogeny in the Pillon et al. (2021) suggests that they may be needed; the limits of Schizomerieae, Geissoieae and Caldcluvieae can each be extended to include their hitherto unattached sister genera... Relationships in the family are very pectinate.