EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
MONOCOTYLEDONS / MONOCOTYLEDONEAE / LILIANAE Takhtajan
Plant herbaceous, perennial, rhizomatous, growth sympodial; non-hydrolyzable tannins [(ent-)epicatechin-4] +, neolignans 0, CYP716 triterpenoid enzymes 0, benzylisoquinoline alkaloids 0, hemicelluloses as xylan, cell wall also with (1->3),(1->4)-ß-D-MLGs [Mixed-Linkage Glucans]; root epidermis developed from outer layer of cortex; endodermal cells with U-shaped thickenings; cork cambium [uncommon] superficial; stele oligo- to polyarch, medullated [with prominent pith], lateral roots arise opposite phloem poles; stem primary thickening meristem +; vascular development bidirectional, bundles scattered, (amphivasal), vascular cambium 0 [bundles closed]; tension wood 0; vessel elements in roots with scalariform and/or simple perforations; tracheids only in stems and leaves; sieve tube plastids with cuneate protein crystals alone; ?nodal anatomy; stomata oriented parallel to the long axis of the leaf, in lines; prophyll single, adaxial; leaf blade linear, main venation parallel, of two or more size classes, the veins joining successively from the outside at the apex and forming a fimbrial vein, transverse veinlets +, unbranched [leaf blade characters: ?level], vein/veinlet endings not free, margins entire, Vorläuferspitze +, base broad, ensheathing the stem, sheath open, petiole 0; inflorescence terminal, racemose; flowers 3-merous [6-radiate to the pollinator], polysymmetric, pentacyclic; P = T = 3 + 3, all with three traces, median T of outer whorl abaxial, aestivation open, members of whorls alternating, [pseudomonocyclic, each T member forming a sector of any tube]; stamens = and opposite each T member [A/T primordia often associated, and/or A vascularized from T trace], anther and filament more or less sharply distinguished, anthers subbasifixed, wall with two secondary parietal cell layers, inner producing the middle layer [monocot type]; pollen reticulations coarse in the middle, finer at ends of grain, infratectal layer granular; G [3], with congenital intercarpellary fusion, opposite outer tepals [thus median member abaxial], placentation axile; compitum +; ovule with outer integument often largely dermal in origin, parietal tissue 1 cell across; antipodal cells persistent, proliferating; seed small to medium sized [mean = 1.5 mg], testal; embryo long, cylindrical, cotyledon 1, apparently terminal [i.e. bend in embryo axis], with a closed sheath, unifacial [hyperphyllar], both assimilating and haustorial, plumule apparently lateral; primary root unbranched, not very well developed, stem-borne roots numerous [= homorhizic], hypocotyl short, (collar rhizoids +); no dark reversion Pfr → Pr; nuclear genome [2C] (0.7-)1.29(-2.35) pg, duplication producing monocot LOFSEP and FUL3 genes [latter duplication of AP1/FUL gene], PHYE gene lost.
[ALISMATALES [PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]]: ethereal oils 0; (trichoblasts in vertical files, proximal cell smaller); raphides + (druses 0); leaf blade vernation supervolute-curved or variants, (margins with teeth, teeth spiny); endothecium develops directly from undivided outer secondary parietal cells; tectum reticulate with finer sculpture at the ends of the grain, endexine 0; (septal nectaries +) [intercarpellary fusion postgenital].
[PETROSAVIALES [[DIOSCOREALES + PANDANALES] [LILIALES [ASPARAGALES + COMMELINIDS]]]]: cyanogenic glycosides uncommon; starch grains simple, amylophobic; leaf blade developing basipetally from hyperphyll/hypophyll junction; epidermis with bulliform cells [?level]; stomata anomocytic, (cuticular waxes as parallel platelets); colleters 0.
[[DIOSCOREALES + PANDANALES] [[LILIALES + ASPARAGALES] COMMELINIDS]]]: nucellar cap 0; ovary inferior; endosperm nuclear [but variation in most orders].
[[LILIALES + ASPARAGALES] COMMELINIDS]]: (inflorescence branches cymose); protandry common.
COMMELINIDS - Back to Main Tree
Unlignified cell walls with >3.5 mg g-1 ferulate [ester-linked to non-cellulosic glucuronoarabinoxylans; unlignified cell walls fluorescing blue under UV, green with NH3],pcoumarate acylates lignin [mostly on syringyl units], also glucouronarabinoxylans; exodermal cells monomorphic; (vessels in stem and leaves); SiO2 bodies +, in leaf bundle sheaths; stomata para- or tetracytic, (cuticular waxes as laterally aggregated rodlets [looking like a scallop of butter]); inflorescence branches determinate, peduncle bracteate; P = K + C [stamens adnate to/inside corolla/inner whorl only]; pollen starchy; ovary superior; embryo short, broad, straight.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Estimates of the time when divergence began within commelinids are ca 135 Ma (Z. Wu et al. 2014), ca 124 Ma (Tang et al. 2016), ca 122 Ma (Tank et al. 2015: Table S2), ca 120 Ma (Janssen & Bremer 2004), (128.7-)118.7(-109.1) Ma (Eguchi & Tamura 2016), (122-)116(-94) Ma (Merckx et al. 2008a), ca 116 Ma (Bremer 2000b), around 112 Ma (Foster et al. 2016a: q.v. for details), about 108.2 Ma (Magallón et al. 2015), (113-)103, 96(-86) Ma (Bell et al. 2010), (104-)99, 91(-86) Ma (Wikström et al. 2001), ca 83.4 Ma (Magallón et al. 2013), or only ca 67.1 or 64.5 Ma (Xue et al. 2012: note topology). Magallón and Castillo (2009: relaxed and constrained penalized likelihood datings) estimate ages of ca 128 and 115 Ma, and ages are (127-)118, 110(-104) Ma in Hertweck et al. (2015), 93-90 Ma or 109-97 Ma in Mennes et al. (2013, 2015 respectively), around 106 or 83 Ma in S. Chen et al. (2013) or 131-116 Ma (Jiao & Wang 2022). Christin et al. (2014) suggest a variety of ages for stem-group Arecaceae - ?topology.
Evolution: Divergence & Distribution. Magallón et al. (2018) suggested that there had been an increase in the diversification rate around here - but in a clade that included commelinids minus Poales and dated to (106.7-)102.1(-98.2) Ma... The rate of molecular evolution in commelinids other than Arecales is generally high, ca 0.003 substitutions/site/Ma (Smith & Donoghue 2008). However, in genes like ndhF, at least, the rate is similar to that in Asparagales (Orchidaceae), etc. (Givnish et al. 2006). Although genes in all three genomic compartments apparently evolve slowly in Arecaceae (Wilson et al. 1990; W. J. Baker et al. 2000a, 2000b), the rate is not that dissimilar to that in these other monocots (S. W. Graham et al. 2006; Barrett et al. 2015b). Was there an increase in the rate of molecular evolution within commelinids, some Poales being spectacular examples?
Ecology & Physiology. Silica (SiO2), an apomorphy for the commelinids, is an important plant defence, causing mechanical damage to mouthparts of would-be grazers and also having a number of physiological effects. For discussion on the effects of silica and silica bodies on herbivores, see Poaceae; most of the experimental work on this subject has been carried out on grasses. For phytoliths, silica bodies dispersed after the plant rots, see Piperno (1991). The element silicon is important ecologically in other ways. There is a negative correlation between leaf longevity and silicon concentration in plant tissues (Cooke & Leishman 2011b). Hardly surprisingly, silicon concentrations in tissues of commelinids are generally high, although less so in those commelinids that lack silica bodies (Ma & Takahashi 2002; Hudson et al. 2005). For more on silica bodies, see Benvenuto et al. (2015).
Franks and Farquahar (2006) found that in both Tradescantia and Triticum there were major changes in K+ ions in the guard and subsidiary cells that greatly affected stomatal opening, making the stomatal pore larger, but a lycopod and fern were the only other plants they compared.
Plant-Animal Interactions. Larval food plants of Satyrinae (Nymphalidae) butterflies, "browns", are widely distributed in the commelinids (Ehrlich & Raven 1964; Peña & Wahlberg 2008). Basal clades in Satyrinae feed on BLAs, while larvae of other clades eat monocots, especially commelinids, and within commelinids, especially Poaceae; Amathusiini seem to be the only satyrines that use a variety of non-commelinid monocot hosts (Peña & Wahlberg 2008; see also Peña et al. 2011; Nylin et al. 2013). Unfortunately, the sister group of Satyrinae is unclear (Heikkilä et al. 2011).
Beetles of the Chrysomelidae-Hispinae+Cassidinae group are also common on commelinids (Jolivet 1988; Schmitt 1988; Vencl & Morton 1999; Chaboo 2007). 14/39 tribes of Hispinae+Cassidinae for which there is at least some larval host plant data are found on commelinids, 26/39 on some monocot or other (Chaboo 2007); the beetle larvae eat between the veins. Among the core eudicots larvae are mostly found on Boraginaceae, Solanaceae, Convolvulaceae and Asteraceae - asterids are particularly favoured. However, it is unclear if Criocerinae are in a clade immediately related to Donaciinae/Hispinae and related monocot-eating beetles, or not; the latter situation seems more likely (check Chaboo 2007; c.f. Wilf et al. 2000; Gómez-Zurita et al. 2007); Gómez-Zurita et al. (2007) found that hispines, including cassidines, were widely separated from Donaciinae, the latter being part of the [Criocerinae [Bruchinae + Donaciinae]] clade. Furthermore, feeding patterns in fossil material that look as if they were the result of the activities of hispines cannot easily be ascribed to their activities, as García-Robledo and Staines (2008) noted.
Genes & Genomes. The τ (tau) duplication event is common to all commelinids examined, but it may be properly pegged to monocots as a whole (Jiao et al. 2014), or at least to the [Asparagales + commelinids] clade (Deng et al. 2015; McKain et al. 2016); it is not known from Alismatales (H. T. Lee et al. 2016; see also Gao et al. 2018; c.f. Olsen et al. 2016). Zwaenepoel and Van de Peer (2020: no Dasypogonaceae) suggested that the σ duplication event, often placed at the Poales node, would be better placed here.
The GC content of those commelinid genomes known, especially those of grasses, is quite high (Serres-Giardi et al. 2012).
Chemistry, Morphology, etc.. Ferulates in high concentrations in the primary cells walls are restricted to this clade and to core Caryophyllales (P. J. Harris & Hartley 1980; Rudall & Caddick 1994; Harris & Trethewey 2009). Thus ferulic acid was detected in alkaline hydrolysis products in all palms examined except Sabal palmetto (Pearl et al. 1959), indeed, their wall composition is similar to that of eudicots and with relatively little in the way of xyloglucans, however, they do have some feruloylated glucuronoarabinoxylan (Carnachan & Harris 2000; Harris & Trethewy 2009). Ferulic acid is transferred to cell wall arabinoxylans via a sucrose derivative, 3,6-O-diferuloyl sucrose, at least in grasses (D. Yang et al. 2024). Ferulate polysaccharide esters can be incorporated into lignins (Ralph et al. 1995: see also Scheller & Ulvskov 2010; Busse-Wicher et al. 2016). In other angiosperms resinols, β-β-linked units produced by the dimerization of the monolignol sinapyl alcohol, can nucleate lignin chains, whereas here tricin, a flavone, and ferulate serve as nucleating agents, hence flavonolignins (Lan et al. 2015, 2016). For the synthesis of lignins from hydroxycinnamyl alcohols, which can act as the lignin monomers p-coumarate, ferulate and sinapate units, see Ralph (2009; also Seigler 1998). For a very useful comparative study on monocot lignins and their acylation, R1-C=O is the unit involved, see Karlen et al. (2018). For primary cell wall composition, see literature in P. J. Harris (2005); Arecaceae sampled are somewhat intermediate between the [Poales [Commelinales + Zingiberales]] clade and other monocots; Pearl (1959) and Karlen et al. (2017) discuss palm lignins.
Endo et al. (2021) note that there are basipetal vascular bundles that end blindly at the base of the stem that are at least scatttered in commelinids - something to do with the aquatic ancestry of monocots, perhaps, no need for water to be transmitted, but photosynthesate can move basally? Although vessels in both stem and leaves are common (Wagner 1977), this does not appear to be a synapomorphy. For distinctive sieve tubes, see Botha (2013). For the chemistry of the distinctive scallop-shaped epicuticular waxes that are scattered in this area of the monocots (Barthlott & Fröhlich 1983), see Meusel et al. (1994). For stomatal development, see Tomlinson (1974) and Rudall (2000); that in Dasypogonaceae is apparently unknown.
The perianth may be quite sharply differentiated into a calyx and corolla, or both whorls may be petal-like, but in either case the inner whorl usually completely surrounds the floral apex and the stamens are borne inside it (for floral development, see Endress 1995b in part). In those few commelinids where floral developmental genes have been studied in detail, expression of the B-class gene orthologue of PISTILLATA seems to be restricted to the stamens/staminode and petals (Adam et al. 2007); this may be connected with the more fully bicyclic nature of the perianth here. Starch-containing pollen is common, but has not been found in Hanguanaceae or Dasypogonaceae (only one species of the latter examined) and in some species of Haemodoraceae, Bromeliaceae, etc.. Broad embryos may be a synapomorphy at about this level.
Some morphological information about the commelinids is summarized by Givnish et al. (1999).
Phylogeny. The commelinid clade is well supported in molecular studies (e.g. S. W. Graham et al. 2006 and references; Barrett et al. 2015b) and it has morphological support as well, but support for relationships between the main groups within it has quite often been weak (Chase et al. 2000a; Soltis et al. 2007a). Overall, evidence has suggested that Arecaceae were sister to the rest of the clade (but see below), and the clade [Commelinales + Zingiberales] is more or less well supported (Chase et al. 2006; Givnish et al. 2006, 2010b; Soltis et al. 2007a), and, with Poales sister to this clade, also by Qiu et al. (2010: mitochondrial genes; Davis et al. 2013; S. Chen et al. 2013; Ruhfel et al. 2014: chloroplast genomes; Magallón et al. 2015; Hertweck et al. 2015; Barrett et al. 2015b). Hilu et al. (2003: matK) suggested that Poales might be sister to other commelinids, as did Soltis et al. (2011: Kingia, etc. not included), but in both cases support was weak, as was that for the position of Arecales as sister to remaining commelinids in the latter study. Arecales were weakly supported as sister to Poales in some analyses (e.g. S. W. Graham et al. 2006; Givnish et al. 2010b: maximum parsimony).
Support for the [Commelinales + Zingiberales] clade was sometimes rather weak (e.g. Chase et al. 2000a; Davis et al. 2004: Givnish et al. 2006b, one gene), although it has 100% support in the multi-gene analysis of S. W. Graham et al. (2006) and Chase et al. (2006), good support in the multigene (but poor sampling) study of Soltis et al. (2011) and the plastome analysis of Barrett et al. (2015a, b), and it also appears in Hertweck et al. (2015). In some individual analyses in Davis et al. (2004) Commelinales were paraphyletic and included Zingiberales, while Lam et al. (2015) found a moderately supported [Zingiberales + Poales] clade. Overall, however, this [Commelinales + Zingiberales] has been rather consistently recovered (see also H.-T. Li et al. 2021; Zuntini et al. 2024).
The position of Dasypogonaceae has been unclear. Neyland (2002b: 26s rDNA) found Dasypogonaceae to be strongly associated with Restionaceae and other families (Poales), but this particular relationship has not been recovered in other studies. Some multi-gene analyses did not link Dasypogonaceae with Arecales, even if where they ended up had no strong support (see Givnish et al. 2006 and Chase et al. 2006: near Poales; S. W. Graham et al. 2006: near [Commelinales + Zingiberales]; Magallón et al. 2015 and Foster et al. 2016a: sister to all other commelinids). Givnish et al. (2011b, 2016b) and Davis et al. (2011: structural mutations; see also Barrett & Davis 2011; Barrett et al. 2012a, esp. b, 2013, 2015a, esp. b; Ruhfel et al. 2014; Lam et al. 2015) have found some support in various analyses of plastomes for a position of Dasypogonaceae as sister to Arecaceae, consistent with what morphological data there is, although in some analyses they were placed sister to [Commelinales + Zingiberales], if with little support. In a tree based on the work of Givnish et al., Carlquist (2012a) depicted the relationships [[Arecales [Commelinales + Zingiberales]] [Dasyopogonales + Poales]]. Rudall and Conran (2012) were inclined to think Dasypogonaceae might be included in Poales, partly because both have epidermal silica bodies; within Poales Dasypogonaceae might be close to Rapateaceae in particular. Indeed, alternative topologies remained possible in some analyses in Barrett et al. (2013), and these included completely novel relationships. Overall, however, analyses of full plastid genomes suggest the relationships [[Dasypogonaceae + Arecaceae] [Poales [Commelinales + Zingiberales]]] (e.g. Barrett et al. 2012a, esp. b, 2013, 2015b; Givnish et al. 2018b; H.-T. Li et al. 2021), and also in some nuclear analyses (Timilsena et al. 2022a). These relationships have long been followed here.
That being said, W. J. Baker et al. (2021a: Fig. S6: first version of the Seed Plant Tree) found that Pontederia sp. was sister to all other commelinids, Poaceae were sister to the remainder, and [Dasypogonaceae [Arecaceae ...]] continued the relationships. In the second version (i.2022) there was a very weakly supported clade [[Zingiberales + Commelinales], well, more or less, and [Dasypogonaceae + Arecaceae]]; in the v.2023 version Poales remained sister to all other commelinids where relationships were [[Zingiberales + Commelinales] [Dasypogonaceae + Arecaceae]], this topology had strong support; in the ix.2024 version (see Seed Plant Tree) things were much the same, although in Poales Kamphochloa (Poaceae-Chloridoideae!) are sister to the rest of the order. However, in the recent Angiosperms353 probe set analysis by Zuntini et al. (2024) the relationships [Poales [Dasypogonales [Arecales [Zingiberales + Commelinales]]]] were recovered, and for the most part they had strong support, although the position of Dasypogonales rather less so. These relationships are followed here.
[Arecales [Zingiberales + Commelinales]]: ?
ARECALES Bromhead - Main Tree.
Just the one family, 188 genera, 2,457 species.
Includes Arecaceae.
Synonymy: Cocosales Nakai
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Evolution: Divergence & Distribution. The rate of molecular evolution in Arecaceae is rather low, as might be expected from a woody plant with a relatively long generation time, and is ca 0.0014 substitutions/site/My; this is interpreted as a reduction in the rate of molecular evolution (S. A. Smith & Donoghue 2008). Dasypogonaceae are also not exactly fast when it comes to plastome evolution (see also Gaut et al. 1996: comparison between Poaceae and Arecaceae; Comer et al. 2015a: chloroplast genome; Barrett et al. 2015a, esp. b). It has been suggested that the very different sizes of the Arecaceae and Dasypogonaceae may be connected with the presence of a genome duplication in the former but not in the latter (Barrett et al. 2019).
ARECACEAE Berchtold & J. Presl, nom. cons. // PALMAE Jussieu, nom. cons. et nom. alt. - Back to Arecales
Growth monopodial, plant unbranched, erect, stem well developed, woody; flavonoid sulphates abundant; roots lacking real elongation zone, with radially elongated air spaces, root hairs from unmodified rhizodermal cells; vascular bundles collateral [not amphivasal]; vessels also in stem and leaf; sieve tubes with simple sieve plates, plastids lacking P-protein; sustained growth of the ground parenchyma; 1 (2) vessels/fibrovascular bundle, centrifugal differentiation of fibrous phloem cap; endodermal cells with O-shaped thickenings; cellulose fibrils in the outer epidermal walls randomly oriented; SiO2 bodies in bundle sheath, spherical, often spiny-verrucate; stomata tetracytic, subsidiary cells with oblique cell divisions, leaf (cuticle with substantial wax crust), waxes as aggregated rodlets, epidermal cells rectangular, hypodermal cells rectangular, longitudinally elongate; fibre strands +, both free in mesophyll and attached to the epidermes, sheaths of transverse veins fibrous, centrifugal differentiation of fibrous phloem cap of fibrovascular bundle; leaves spiral, massive, with petiole and blade, blade developing from the upper part of the leaf, vernation reduplicate-plicate, pinnately pseudocompound ["leaflets" = blade segments], sheath closed; plant monoecious; inflorescence with basal bicarinate prophyll, inflorescence units cincinni [condensed helical cymes], prophylls lateral; flowers ± sessile, ± small; staminate flowers: A basically trimerous, basifixed; tapetum binucleate; pistillode ± +, nectariferous; carpelate flowers: staminodes +; G (1-4), [2-10]), carpels initially free; ovule 1/carpel, basal, straight to campylotropous, apotropous, ± sessile, attachment broad, micropyle bistomal, outer integument (4-)6+ cells across, inner integument 2-3 cells across, parietal tissue (0)1-5(-6) cells across, (postament +), suprachalazal area ± massive; micropylar embryo sac haustorium +; fruit drupaceous, usually ≥2 cm across; seeds large [1≤ cm long], 1(-10)/fruit, ± rounded; testa usually with two outer layers thickened, (basal portion at least often vascularized); micropylar endosperm haustorium +, endosperm copious, thick-walled, with mannans; x = 9, nuclear genome [1 C] (0.185-)2.426(-31.865) pg; cotyledon not photosynthetic, collar short (with roots), primary root strong, branched.
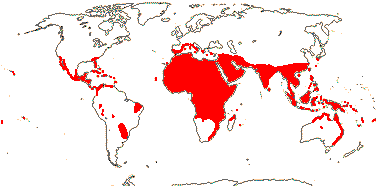
188[list - PALMweb]/2,457 - five main groups below. Humid tropics and subtropics (warm temperate), Africa relatively depauperate. [Photo - Flowers, Fruits.]
Age. Divergence within crown-group Arecaceae is estimated to have begun ca 110 Ma (Janssen & Bremer 2004; see also Onstein et al. 2018), ca 85 Ma (Givnish et al. 2018b), (103-)83(-75: check) Mya (Givnish et al. 2016b) or (78-)73, 63(-58) Ma (Wikström et al. 2001). Bell et al. (2010) suggest the remarkably young - and surely incorrect - age of (38-)33, 31(-21) Ma for the crown group, while there are the very different estimates of (90-)81(-72) and (39-)37(-35) Ma in Hertweck et al. (2015), also (108.8-)100.1(-92.2) Ma in W. J. Baker and Couvreur (2013a) and 71-14 or 90-84 Ma in Mennes et al. (2013, 2015 respectively). Crown-group Arecales/Arecaceae are (114-)109, 102(-98) Ma (Hertweck et al. 2015), (122-)112.5(-100) Ma (Givnish et al. 2016), ca 119 Ma (Givnish et al. 2018b) (check these three), or ca 233/(123-)119(-116) Ma (Bellot et al. 2024 - note that the second ages here and elsewhere below, the younger ages (the ages after the slash), use the age of the oldest monocot fossils as the oldest possible age for the family, other ages reflecting this, while the first ages given, the older ages, would accomodate the possibility that palms may be much older than their oldest reliably dated fossils, and used instead a normal root age prior distribution with a mean of 149.1 Ma and a standard deviation of 28 My, so this would take older (molecular) ages for palms that have been proposed into account (Bellot et al. 2024: see Fig. S4). The age in G. Yao et al. (2025) is (109.7-)108.3(-107.0) Ma.
Fossil Arecaceae date to ca 93 Ma or maybe a little younger (Pan et al. 2006; Harley 2006; Matsunaga & Smith 2021); Iles et al. (2015) suggest the family can be calibrated on the Santonian Sabalites carolinense (stem-group Coryphoideae) that is 86.3-83.6 Ma. However, Greenwood et al. (2022) suggest that this fossil is late Campanian (around 76-72.1 Ma) and that the Coniacian to Campanian pinnate-entire species Phoenicites imperialis may be the oldest palm leaf fossil at 88-80 Ma. Pollen of palms is reported from the Turonian ca 92 Ma in Utah (Jud et al. 2018b).
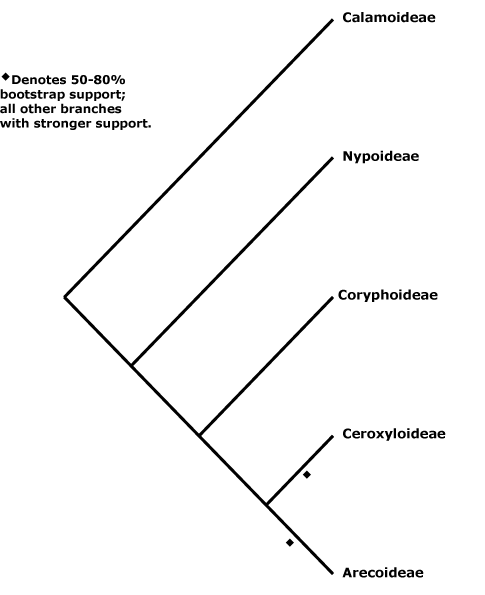
Includes Areceae, Arecoideae, Borasseae, Calameae, Calamoideae, Caryoteae, Ceroxyleae, Ceroxyloideae, Chamaedoreae, Chuniophoeniceae, Cocoseae, Corypheae, Coryphoideae, Cryosophileae, Cyclospatheae, Eugeissoneae, Euterpeae, Geonomateae, Iriarteeae, Leopoldineae, Lepidocaryeae, Manicarieae, Nypoideae, Oranaieae, Pelagodoxeae, Phoeniceae, Phytelepheae, Podococceae, Reinhardtieae, Roystoneae, Sabaleae, Sclerospermeae, Trachycarpeae, Truongsonieae.
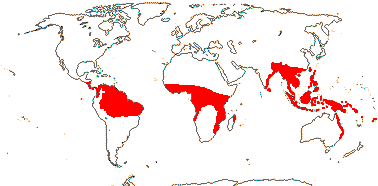
Plant lianes, climbing by ± recurved spines; root periderm 0; (sustained growth of the ground parenchyma 0/slight); fibrovascular bundles with 1-2 wide vessel elements; petiole central v.bs with two phloem strands; epidermal cells rectangular, anticlinal walls sinuous; adaxial subepidermal fibre bundles +; parenchyma cells near protoxylem inflated (not), longitudinal veins bridging to adaxial epidermis via vertically-elongated sclereids, lateral [cross] veins adaxial to longitudinal veins, adaxial non-vascular fibres subepidermal; internodes well-developed; (plant monocarpic); (inflorescence axes adnate to the internode above, or to sheath of the leaf of the next node); plant often dioecious (flowers perfect); C valvate, basally connate, (free); A ≤12; (style branched); funicle twisted [but ovule basically apotropous]; fruit covered by reflexed scales, endocarp thin/0; seeds 1-3, sarcotesta +, usually thick, (testa thickened on one side); n = 13, 14.
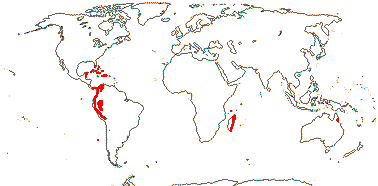
21/655. Tropical, but esp. Sri Lanka to West Samoa and Fiji. Map: from Uhl and Dransfield (1987) - for maps of genera and tribes, etc., see Dransfield et al. (2008).
Age. The age of crown-group Calamoideae is (99.3-)80.2(-70.3) Ma (W. J. Baker & Couvreur 2013a), ca 139/(112-)107(-103) Ma (Bellot et al. 2024) or (232-)126(-122) Ma (Kuhnhäuser et al. 2025) - see also immediately below.
1A. Lepidocaryeae Dumortier —— Synonymy: Lepidocaryaceae Martius
(Tree-like), plant spiny or not; leaves (2-ranked), (palmate - Mauritiinae); pollen (monosulcate/monoulcerate), (ektexinal sculptural elements +, impressed); (fruit a berry); (sarcotesta 0); endosperm (ruminate); n = 14, 15.
7/46 (three subtribes): Raphia (20). Africa, Madagascar, northern half of South America, 1 sp. Central America.
Age. The crwon-group age of this clade is ca 101/73 Ma (Bellot et al. 2024).
The age of Lepidocaryeae-Mauritiinae is estimated to be 94-83 Ma (Bogotá-Ángel et al. 2021).
Age. The age of this clade is estimated to be ca 131/104 Ma (Bellot et al. 2024) or (214-)123(-118) Ma (Kuhnhäuser et al. 2025).
1B. Eugeissoneae W. J. Barker & J. Dransfield - Eugeissona Griffith
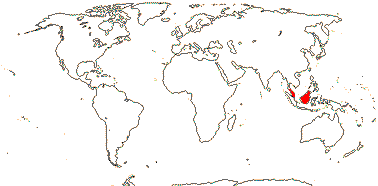
Not lianescent, growth sympodial; endodermal cell walls barely thickened; SiO2 bodies minute, disciform; inflorescence terminal, inflorescence units surrounded by cupule of 7-11 overlapping bracts, monopodial, dyads of staminate and perfect flowers; flowers large [to 9 cm long]; K connate, C woody; A 20-70, development centrifugal; nectar also produced from ventral slits of the carpels; outer integument 50-60 cells across, inner integument ca 10 cells across; fruit scales small, many longitudinal fibro-vascular bundles, mesocarp forming lignified stony layer, ridged, endocarp s. str. massive, becoming crushed, germination pore basal; seeds longitudinally ruminate; testa ?thin, dry; n = ?; plastid ndhF gene 0.
1/6. S. Thailand to Borneo. Map: from Dransfield et al. (2008b).
Age. The age of this node is some 48-40 Ma (Wikström et al. 2001).
1C. Calameae Kunth —— Synonymy: Calamaceae Perleb, Sagaceae Schultz-Schultzenstein
Plant (acaulescent), (tree-like), spiny; leaf (sheath open), (cirrus [extension of leaf rachis] +), (flagellum [sterile inflorescence] +); (plant dioecious), pollen equatorially disulcate (diporate/zonosulcate/inaperturate); seed with ventral depression, raphal bundles 2; sarcotesta tanniniferous (tannins 0, sarcotesta slimy); endosperm (ruminate); n = 13, 14, 16.
13/598 (six subtribes): Calamus (414), Daemonorops (101), Korthalsia (26). West Africa to Sri Lanka, India and Southeast Asia, and Malesia to eastern Australia, Samoa and Fiji.
Age. The age of Calameae is ca 104/97 Ma (Bellot et al. 2024).
Two kinds of fossi pollen from New Guinea have been described as species of Dicolpopollis; they are dated to 100.5-93.9 and 93.9-89.8 Ma and are similar to the pollen of Calaminae and Plectocomiinae (Kuhnhäuser et al. 2025).
[Nypoideae [Coryphoideae [Ceroxyloideae + Arecoideae]]]: sustained primary growth +; root periderm +; 2 vessels/fibrovascular bundle; anticlinal epidermal walls ± straight, adaxial subepidermal fibre bundles 0.
Age. This node is estimated to be 71-14 Ma by Mennes et al. (2013), which allows the imagination plenty of freedom, (100.7-)93.5(-87.5) Ma by W. J. Baker and Couvreur (2013a), ca 75 Ma by Z. He et al. (2022) and ca 193/(116-)112(-106) Ma (Bellot et al. 2024).
2. Nypoideae Griffith - Nypa fruticans Wurmb —— Synonymy: Nypaceae Le Maout & Decaisne
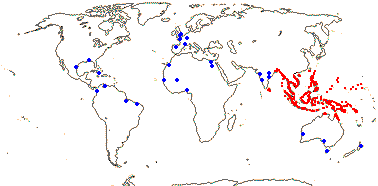
Plant rhizomatous, stem dichotomously branched; endodermal cell walls barely thickened; fibrovascular bundles diffuse, no centrifugal differentiation of fibrous phloem cap, vessels 2, narrow; vessel elements with scalariform perforation plates only; petiole central v.bs with single phloem strand; epidermis with hydathodes, epidermal cells hexagonal to spindle-shaped, guard cells with several ledges [in t.s.], SiO2 bodies small, hat-shaped; hypodermal cells several layered, lignified, hexagonal, transversely elongate, veins bridging to epidermis via vertically-elongated sclereids, sheaths of transverse veins sclereidal; blade veins sinuous, irregular; plant monoecious; inflorescence racemose, staminate inflorescence a spike, carpelate inflorescence a head, axis adnate to the internode above; P free, ± undifferentiated; staminate flowers: A 3, opposite outer P, connate, synangial stalk solid, anthers extrorse; microsporogenesis successive; pollen with encircling sulcus [meridionosulcate], surface spiny; pistillode 0; carpelate flowers: staminodes 0; G 3 (4), margins conduplicate, placentation laminar to submarginal; ovule [position?], outer integument ca 10 cells across; fruit with apical umbo [remains of stigma], pericarp with many longitudinal bundles, woody layer derived from mesocarp, with longitudinal ridge protruding into seed, germination pore basal, round; endosperm ruminate or not; n = 17; nuclear genome [1C] ca 1149 Mb; seeds ± viviparous.
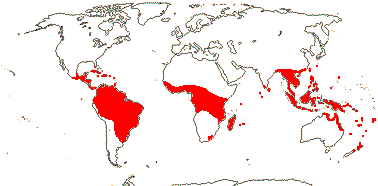
1/1. Bengal to Queensland. Map: current distribution in red, from Uhl and Dransfield (1987) and Spalding et al. (2010); fossil records from places outside this area are in blue and are mostly taken from Plaziat et al. (2001).
Age. Nypa has a rich fossil record in both Hemispheres. Nypa-like pollen is known from ca 75 Ma, e.g. from the Late Cretaceous in Patagonia (Gee 2001; Barreda et al. 2012); see also references in Rull et al. (2023b) for the Caribbean area. Pyritized fruits, Nypa burtini, from Eocene London Clay deposits, were placed sister to N. fruticans with moderate to strong support (Matsunaga & Smith 2021).
[Coryphoideae [Ceroxyloideae + Arecoideae]]: pbenzoate acylates lignin [?level]; sieve tube with compound sieve plates; (sustained growth of the ground parenchyma 0); endodermal cell walls with U-shaped thickenings (thickened all around); (leaf veins bridging to epidermis by fibres); (A numerous, development centripetal); microsporogenesis simultaneous; ovule position and type various.
Age. The age of this node may be 68-61 Ma (Wikström et al. 2001), while estimates are (98-)51(-15) Ma in Merckx et al. (2008a), 86.3-83.6 Ma in Iles et al. (2015: fossils, stem node age of Coryphoideae), (100.1-)90.7(-85) Ma in Eguchi and Tamura (2016: c.f. comparisons) and ca 175/107 Ma (Bellot et al. 2024).
Root hairs +; septate fibres +; 1, 2 or more vessels/fibrovascular bundle, no centrifugal differentiation of fibrous phloem cap; no fibre bundles free in mesophyll, longitudinal veins with ad/abaxially elongated bridging sclereids, transverse veins with broad sheath of fibres, petiole central v.bs with single phloem strand; adaxial vein rib with 5 or more independent vascular bundles; leaves palmate or costapalmate, with several major divisions, vernation induplicate-plicate; inflorescence various, (terminal; adnate to the internode above; flowers (perfect), solitary or in cincinni (triads); C often valvate, connate (free); anther tapetal cells binucleate; microsporocyte with callose ring; G (1), free, style well developed, (stylar canals separate, compitum 0); x = 18.
46/448. Pantropical (to warm temperate), fewer in South America, quite frequently outside tropical rain forest. Map: from Uhl and Dransfield (1987) - for maps of genera and tribes, etc., see Dransfield et al. (2008).
Age. The age of crown-group Coryphoideae is estimated to be (80.1-)66.0(-51.4) Ma (W. J. Baker & Couvreur 2013a), (110.7-)95.1(-83.9) Ma (Kadam et al. 2022) or ca 156/(105-)100(-93) Ma (Bellot et al. 2024).
Sabalites carolinense (stem-group Coryphoideae) is probably from the middle Campanian ca 78 Ma, rather than Santonian (Iles et al. 2015; c.f. Greenwood et al. 2022). Similarly, Breña-Ochoa and Cevallos-Ferriz (2022) found that the fossils Sabalites lanceocuspis (leaf) and Coryfloramus reinhardii (inflorescence) from Early Maastrichtian (or slightly earlier) deposits in northern Mexico ca 72 Ma were both Coryphoideae, although exactly where they might go in the subfamily was unclear in the morphological analyses carried out.
[Chuniophoeniceae [Caryoteae [Borasseae + Corypheae]]] / Syncarpous Clade: G syncarpous; fruit mesocarp fleshy, longitudinal vascular bundles ± 0.
Age. The age of this clade is ca 110 Ma (Bellot et al. 2020) or ca 127/89 Ma (Bellot et al. 2024).
3A. Chuniophoeniceae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. E. Lewis
(Plant monocarpic; branching dichotomous); rachilla bracts tubular; C base ± pedicilliform; stylar canals separate; (endosperm ruminate); n = ?; plastome inverted repeat ± 0 [Tahira].
4/7: Chuniophoenix (3). Madagascar; E. Arabian Peninsula and adjacent Afghanistan, etc.; Hainan, S. China and North Korea, Peninsula Thailand. Map: see Dransfield et al. (2008: p. 287) and Bellot et al. (2020: Fig. 1A).
Age. Crown-group Chuniophoeniceae are ca 74 Ma (Bellot et al. 2020), (59.5-)41.3(-25.7) Ma (Kadam et al. 2022) or ca 75/51 Ma Bellot et al. 2024).
[Caryoteae [Borasseae + Corypheae]]: ?
,Age. The age of this clade is ca 121/85 Ma (Bellot et al. 2024).
'style='margin-top:6.0pt;margin-right:0in;margin-bottom:0in; margin-left:.25in;margin-bottom:.0001pt;line-height:1.2'>(Branching, stems with basipetal hapaxanthy [Caryota]); centrifugal differentiation of fibrous phloem cap; leaves (two-ranked - Wallichia), pinnate/bipinnate [Caryota, Arenga], fibres in leaf sheath finely net-like; (plant hapaxanthis), flowers in triads [lateral flowers staminate, central flower carpelate]; microsporocyte lacking callose ring; pollen intectate, spines/clavae +, (interlocking over sulcus); style 0; endocarp s. str. +; n = 16.
3/42: Arenga (20). India and South China to north Australia and the south Pacific (just). Map: see Bellot et al. (2020: Fig. 1A).
Age. Crown-group Caryoteae are some (56.7-)43.1(-31.2) Ma (Kadam et al. 2022) or ca 63/43 Ma (Bellot et al. 2024); G. Yao et al. (2025) estimate that the crown-group age of Caryota alone is ca 56.6 Ma.
The Indian Arengapollenites, with the distinctive pollen of the tribe, is 56-47.8 Ma (Iles et al. 2015).
Age. The age of this clade is ca 92 Ma (Bellot et al. 2020), (69.6-)54.1(-40.2) Ma (Kadam et al. 2022) or ca 105/78 Ma (Bellot et al. 2024).
3C. Borasseae Martius —— Synonymy: Borassaceae Schultz-Schultzenstein
Trunk (± dichotomously branched - Hyphaene); plant dioecious, staminate (and carpelate) flowers sunken in pits, axis elongating between T whorls; flowers large [Lodoicea]; anther wall 4-8 cells across [H.]; microsporocyte (lacking callose ring - Bismarckia); ovule straight [?H.]; fruits large, fibrous, woody layer mesocarpial, thick, germination pore +, apical; n = (14, 17).
8/23 (two subtribes): Hyphaene (8). Old World tropics, to New Guinea, scattered. Map: see Bellot et al. (2020: Fig. 1A).
Age. The age of crown-group Borasseae is estimated to be (48.7-)36.0(-25.4) Ma (Kadam et al. 2022) or ca 82/70 Ma (Bellot et al. 2024).
Hyphaene kappelmanii, from Ethiopia, is ca 27.2 Ma (Iles et al. 2015). However, Hyphaeneocarpon indicum, from Indian Interappean beds ca 67-64 Ma, has been placed in Borasseae-Hyphaeninae (immediate relationships there are [Hyphaeneocarpon [Satranala + Bismarckia]], the other two genera are from Madagascar) (Matsunaga et al. 2019), while Matsunaga and Smith (2021) placed them in the same general area, but as sister to Bismarckia.
3D. Corypheae Martius - Corypha L. —— Synonymy: Coryphaceae Schultz-Schultzenstein
Plant monocarpic; inflorescence terminal.
1/6. Sri Lanka and adjacent S. India, Bay of Bengal to West Malesia, northeast Australia and adjacent New Guinea. Map: see Bellot et al. (2020: Fig. 1A).
[Trachycarpeae [Phoeniceae [Cryosophileae + Sabaleae]]] / CSPT Clade: G apocarpous but synstylous; fruit fleshy, woody layer mesocarpial, thin, postament +.
Age. The age of this clade, as [[Phoenicieae + Trachycarpeae] [Cryosophileae + Sabaleae]] (stem [Cryosophileae + Sabaleae]) is ca 87 Ma (Cano et al. 2018), while the estimate in Bellot et al. (2024: (Trachycarpeae sister to the rest, see below) is ca 143/91 Ma, and the age of a [Phoeniceae + Trachycarpeae] clade is given as (89.7-)76.8(-57.4) Ma (Kadam et al. 2022).
3E. Trachycarpeae Satake (inc. Livistoneae)
Petiole central v.bs with single phloem strand; fibres in leaf sheath finely net-like; (flowers solitary); n = (14, 17).
17/272 (two subtribes): Licuala (135), Livistona (35), Pritchardia (27). Tropics to subtropics, especially IndoMalesia, few Africa.
Age. This tribe has been dated to 47.2-23.0 Ma by W. J. Baker and Couvreur (2013), (73.0-)55.8(-40.1) Ma by Kadam et al. (2025), ca 111/73 Ma by Bellot et al. (2024) and ca 63.7 Ma (G. Yao et al. 2025).
Palmoxylon trachycarpeaeense has recently been described from fossils win the Deccan Intertrappean Beds ca 66-65 Ma from Central India (S. Kumar et al. 2025b, q.v. for fossil record of Trachycarpeae). Note that fruits of Coryphoides poulsenii from deposits in western Greenland dated at 64-62 Ma (Koch 1972) were placed in Trachycarpeae total group in total evidence analyses, and with a backbone constraint the fossils were strongly supported as crown-group Livistoninae (Matsunaga & Smith 2021).
[Phoeniceae [Cryosophileae + Sabaleae]]: ?
Age. The age of this clade is ca 138/87 Ma (Bellot et al. 2024).
3F. Phoenicieae Horaninov - Phoenix L. —— Synonymy: Phoenicaceae Burnett
Fibrous bundles 0; leaves pinnate, basal pinnae spines [acathophylls], others with spinose apex; plant dioecious, flowers solitary; seed with longitudinal furrow/raphe; n = (16).
1/14. Cape Verde and Canary Islands, Crete, Africa and Madagascar to southern China and westernmost Malesia.
[Cryosophileae + Sabaleae]: flowers solitary.
Age. The age of this clade is ca 77 Ma (Cano et al. 2018), (80.8-)78.5(-77.1) Ma (Kadam et al. 2021) or ca 115/73 Ma (Bellot et al. 2024).
3G. Cryosophileae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. E. Lewis
(Root spines +); large petiolar vascular bundles with single phloem strand; fibres in leaf sheath various, inc. spiny; (blade with single major split [medial] - Sabinaria); (rachis bracts well developed - S.), flowers perfect, (plant monoecious - S.); P uni-/biseriate, (K adnate to C in one place - S.), C if present usu. connate; A 5-25, (much longer than C - Trithinax), free to half-connate; G 1-3, stigma cup-like or oblique, papillate; endosperm deeply lobed/not.
11/67: Coccothrinax (49). Mostly Mexico and Central America and the Caribbean, scattered in South America, U.S.A. (S. Florida, 2 spp.). Map: see R. Thomas and de Franceschi (2012).
Age. Crown-group Cryosophileae are thought to be (56-)45(-35) Ma (Cano et al. 2018), ca 74/50 Ma (Bellot et al. 2024) or ca 62.1 Ma (G. Yao et al. 2025).
Fossil stems, roots and leaves described as Uhlia allenbyensis and ca 48.7 Ma are known from British Columbia - c.f. Rhaphidophyllum and Brahea (Erwin & Stockey 1994; Greenwood et al. 2016). Wood of Palmoxylon vestitum from Oligocene and Miocene deposits in France is anatomically similar to that of Coccothrinax (R. Thomas & de Franceschi 2012), while somewhat more conventionally (from a distributional point of view) Poinar (2002) identified flowers of about the same age in Dominican amber as Trithinax dominicana.
3H. Sabaleae Dumortier - Sabal Adanson —— Synonymy: Sabalaceae Schultz-Schultzenstein
A adnate to C; G [3], stylar canal single.
1/17. Southeast U.S.A., Mexico to northern South America.
Age. Crown-group Sabaleae are estimated to be 19-10 Ma (Cano et al. 2018).
Fossils in Cretaceous-Campanian rocks around 77 Ma from Texas have been identified as Sabal (S. bigbendense, see Manchester et al. 2010a).
[Ceroxyloideae + Arecoideae]: petiole bundles arranged in one or more Vs (scattered); sheaths of transverse veins sclereidal, epidermal cells hexagonal to spindle-shaped; lamina veins sinuous, irregular; endocarp s.str. of palisade sclereids/0, silica bodies +.
Age. The age of this node is (85.3-)78.3(-70.7) Ma (W. J. Baker & Couvreur 2013a), around 78 Ma (Iles et al. 2015: check) or ca 161/101 Ma (Bellot et al. 2014).
2-4≤ vessels/fibrovascular bundle, abrupt decrease of fibrous part internally, ground parenchyma very lacunose; plant usu. dioecious, (flowers perfect); inflorescence racemose, spicate; flowers single/in monopodial groups; (K and C elongate), (free); G [3-10], receptacle elongated; fruit (also layers of sclerenchyma from adjacent mesocarp), germination pore 0.
8/45. Mostly Central and W. South America, Florida and the Antilles, also N.E. Australia, Madagascar. Map: from Uhl and Dransfield (1987) - for maps of genera and tribes, etc., see Dransfield et al. (2008).
Age. This subfamily is estimated to be (74.2-)52.2(-30.0) Ma (W. J. Baker & Couvreur 2013a, ?63.4-)40.6(-21.0) Ma - W. J. Baker & Couvreur 2013a), ca 67-66 Ma (Escobar et al. 2021).(79.7-)68.8(-60) Ma (Pichardo-Marcano et al. 2018) or ca 126/(94-)78(-61) Ma (Bellot et al. 2024).
Stems named Palmoxylon ceroxyloides, fossils 66-65 Ma from the Deccan Traps, have been confidently assigned to Ceroxyloideae (Khan et al. 2020b).
4A. Ceroxyleae Satake —— Synonymy: Ceroxylaceae Vines
Root hairs +; 3-≥4 vessels/fibrovascular bundle; petiole central v.bs with single phloem strand; plant dioecious; (A development centripetal - Ceroxylon); (pollen monoporate); inner integument to 7 cells across [Ceroxylon]; pericarp fleshy, no fibrovascular bundles; seed 1; n = 15, 16, 18.
4/33: Ravenea (20), Ceroxylon (11). Andean South America, inc. Juan Fernandez islands, Madagascar and the Comoros, Australia (Queensland).
Age. Crown-group Ceroxyleae are (29.2-)17.2(-7.1) Ma (W. J. Baker & Couvreur (2013a) or ca 46/30 Ma (Bellot et al. 2024).
[Cyclospatheae + Phytelepheae]: ?
Age. This clade is estimated to be ca 118/73 Ma (Bellot et al. 2024).
4B. Cyclospatheae O. F. Cook - Pseudophoenix Sargent —— Synonymy: Pseudophoenicaceae O. F. Cook
Crownshaft +; 2 vessels/fibrovascular bundle; petiole central v.bs with two phloem strands; fruit deeply lobed, pericarp fleshy, hilar seam + [= endocarp not continous where seed attaches - ?elsewhere], no fibrovascular bundles; seeds to 3; silica bodies 0; n = 17.
1/4. Florida, the northern Caribbean, and the Yucatan Peninsula.
4C. Phytelepheae —— Synonymy: Phytelephaceae Perleb
3, 4 or ≥4 vessels/fibrovascular bundle; petiole central v.bs with two phloem strands; plant dioecious; (flowers in clusters monopodial - some Phytelephas); flowers large; staminate flowers: 4-merous; A very many, with trunk bundles, development centrifugal; pollen large [70-90 µm]; G 4-10; fruits multilocular, pericarp dry, with blunt corky protrusions formed by clusters of large radial fiber bundles; seeds -10; n = 18.
3/8: Phytelephas (6). Panama, trans- and cis-Andean, western Amazonia.
Age. The age of crown-group Phytelepheae is estimated to be (24.2-)14.3(-13.2) Ma (W. J. Baker & Couvreur 2013a: ?numbers muddled), (20.6-)19.3(-17.0) Ma (Escobar et al. 2021) or ca 42/28 Ma (Bellot et al. 2024).
Palmoxylon phytelephatoides, represented by fossils of stems with roots and collected from the Deccan Traps, India, has recently been described (Chate et al. 2019).
Root hairs 0; 1 vessel/fibrovascular bundle (2), highly lacunose gground tissue, massive fibrous part of vascular bundles; petiole central v.bs with two phloem strands; (SiO2 bodies hat-shaped); hypodermal cells hexagonal, transversely elongate; plant monoecious; flowers in triads [central (upper) flower carpelate, lateral flowers staminate] or in two vertical rows, (inflorescences spicate); (flowers protandrous); (C valvate); (A 3); (1 G fertile), style branches separate, (style single, short to long); endocarp opercula + (0), (germination pores, no opercula, +); x = ?16.
107/1,390. Pantropical, the most diverse subfamily in South America. Map: from Uhl and Dransfield (1987) - for maps of genera and tribes, etc., see Dransfield et al. (2008).
Age. The age of crown-group Arecoideae is estimated to be (81.4-)73.6(-66.2) Ma (Baker & Couvreur 2013a) or ca 161/(107-)101(-94) Ma (Bellot et al. 2024).
Relationships and characters below particularly tentative.
5A. Iriarteeae Drude —— Synonymy: Iriarteaceae O. F. Cook & Doyle
Establishment growth 0, trunk obconical, crownshafat +; stilt roots + (spiny); petiole central v.bs with single phloem strand; leaflets praemorse; peduncular bracts 3-12; (pollen spiny/gemmate); ovules 1/carpel, /gynoecium; n = 15, 18.
5/32: Wettinia (21). Central and tropical South America.
Age. Crown-group Iriarteeae may be some (36.3-)30(-24.1) Ma (Pichardo-Marcano et al. 2018: Ir-Wett) or ca 74/50 Ma (Bellot et al. 2024).
Age. This clade is estimated to be 150/95 Ma (Bellot et al. 2024).
5B. Hyophorbeae Luerssen (= Chamaedoreeae Drude) —— Synonymy: Chamaedoraceae O. F. Cook, Moreniaceae O. F. Cook, Synechanthaceae O. F. Cook
Crownshaft +; root hairs +; (crownshaft 0); vessel elements with scalariform perforation plates only; ?petiole central v.bs; (plant dioecious); inflorescence of largely ebracteate cincinni [= acervulus, Wenlandiella], peduncular bracts (1-)2-several; nectary 0; ovules 1/carpel; n 13, 14, 16.
5/123: Chamaedorea (110). Central and South America, Mexico, the Antilles, also the Mascarenes (Hyophorbe).
Age. Crown-group Hyophorbeae are thought to be (57.2-)47.7(-39.8) Ma (Pichardo-Marcano et al. 2018: Cham-Hyoph) or ca 92/60 Ma (Bellot et al. 2024).
Age. This clade is some 140/91 Ma (Bellot et al. 2024).
[Oranieae + Sclerospermeae] [Podococceae + Truongsonieae]]] / POST clade: crownshaft 0; leaflet margins praemorse; endosperm homogeneous.
Age. This node is around 108/68 Ma (Bellot et al. 2024).
][Oranieae + Sclerospermeae]: ?
Age. The age of this clade is some 103/63 Ma (Bellot et al. 2024).
5C. Oranieae Beccari - Orania Zippelius
]Leaves (2-ranked); 1 ovule/carpel; n = ?
1/25. E. Madagascar, S. Thailand and throughout Malesia.
Age. The age of crown-group Oranieae is estimated to be ca 53.2 Ma (G. Yao et al. 2025).
5D. Sclerospermeae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis - Sclerosperma G. Mann & H. Wendland
Plant acaulescent; staminate flowers: many stamens; pollen triporate, pores apical, operculate; carpellode 0; carpellate flowers: staminodes 6; 1 ovule/gynoecium; n = ?
1/3. Equatorial Guinea, Cameroon, Nigeria, Central Africa.
[Podococceae + Truongsonieae]: staminate flowers: K imbricate, C valvate; 1 ovule/carpel.
Age. The age of this node is around 65/39 Ma (Bellot et al. 2024).
5E. Podococceae Dransfield & Uhl - Podococcus G. Mann & H. Wendelbo
Plant (acaulescent), crownshaft ?; leaf sheath becoming split, blade pinnate; flowers in deep pits; receptacle between K and C elongates; fruit 1[stigma ± basal]-3-seeded; n = ?
1/2. Nigeria to Gabon, never far inland.
5F. Truongsonieae W. J. Baket, S. Bellot, Dransfield & Eiserhart - Truongsonia lecongkietii N. S. Lý, W. J. Baket & A. J. Henderson
Plant acaulescent, with saxophone growth; leaf shtath open [?when], blade entire-bifid; flowers in triads; pistillate flowers: P imbricate; fruit 1-seeded [stigma ± basal]; n = ?
1/1. Vietnam, Quang Ngai Province.
Age. This clade is some 134/87 Ma (Bellot et al. 2024).
[Roystoneae [Reinhardtieae + Cocoseae]] / RRC clade: ?
Age. The age of the RRC clade is around 129/84 Ma (Bellot et al. 2024).
5G. Roystoneae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis - Roystonea O. F. Cook
Crownshaft +; inflorescences below leaves; carpellate flowers: staminodes connate, adnate to inner T; ovule 1/gynoecium; n = 16.
1/10. Southern Florida, Antilles and adjacent American mainland
[Reinhardtieae + Cocoseae]: crownshaft 0; ovules 1/carpel.
Age. This clade is some 110/72 Ma (Bellot et al. 2024).
5H. Reinhardtieae - Reinhardtia Liebmann —— Synonymy: Malortieaceae O. F. Cook
Leaflets at least bifid, (with basal "windows"); carpellate flowers: staminodes connate, adnate to inner T; endosperm ruminate; n = ?
1/6. Mexico (the Yucatan), Central America, Dominican Republic.
Age. An estimate for this clade is ca 53.5 Ma (G. Yao et al. 2025).
5I. Cocoseae Dumortier —— Synonymy: Cocosaceae Schultz-Schultzenstein
(Lianes), (plant prickly - Astrocaryum); fibres in leaf sheath finely net-like; peduncular bract long, often very thick, woody; carpellate flowers: staminodes usu. connate; inner integument ca 7 cells across [Cocos, Elaeis]; pericarp with thick woody layer derived from fibres of inner mesocarp, (if seeds 2-3, fruit multilocular, common endocarp surrounding seeds), germination pores 3≤, often with opercula, position variable; endosperm (ruminate); n = 15, 16, 18, ca 90, 248≤.
18/324 (three subtribes): Bactris (77(-257)), Attalea (69), Desmoncus (61), Acrocomia (34), Syagrus (31), Astrocaryum (36). Pantropical, mostly New World.
Age. Crown-group Cocoseae are estimated to be (64-)62.6(-61.9) Ma (Pichardo-Marcano et al. 2018: Coc-Bact) or ca 96/70 Ma (Bellot et al. 2024).
Age. Crown-group Attaleinae were reckoned to be 49.8-23.2 Ma (W. J. Baker & Couvreur 2013a).
Fossil fruits, Palmocarpum drypetiodes, from the Indian Intertrappean beds 67-64 Ma (Manchester et al. 2016) were placed in Attaleinae total or crown group, support varying from moderate to strong depending on the analysis (Matsunaga & Smith 2021).
[Leopoldinieae [[Geonomateae [Manicarieae + Pelagodoxeae]] [Areceae + Euterpeae]]] / Core arecoids: germination pores with opercula basal.
Age. This node is some (54.7-)45.3(-39) Ma (Pichardo-Marcano et al. 2018) or ca 111/73 Ma (Bellot et al. 2024).
5J. Leopoldinieae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis - Leopoldinia Martius
Crownshaft 0 leaflets entire; ?1 ovule/gynoecium; endosperm homogeneous; n = ?
1/3. Western Brazil.
[[Geonomateae [Manicarieae + Pelagodoxeae]] [Areceae + Euterpeae]]: ?
Age. This clade is around 107/71 Ma (Bellot et al. 2024).
[Geonomateae [Manicarieae + Pelagodoxeae]]: ?
Age. The age of this node is 105/69 Ma (Bellot et al. 2024).
5K. Geonomateae Luersson —— Synonymy: Geonomataceae O. F. Cook
(Plant acaulescent); crownshaft 0; flowers in pits in the rhachillae; staminate flowers: A(3) 6-many, anthers sagittate, or thecae separate, terminal on erect/recurved connective processes; carpellate flowers: staminodes basally connate (± completely connate;, apex truncate/slightly crenulate); styles long, slender, (gynobasic, 1 G develops); 1 ovule/carpel; massive longitudinal bundles in pericarp; endosperm homogeneous; n = 14.
6/104: Geonoma (68), Calyptrogyne (21). Yucatan, etc., to tropical South America, the Antilles.
Age. Geonomateae may have started to diversify (39.2-)33(-26.7) Ma (Pichardo-Marcano et al. 2018: Pholido-Geonom), 79/52 Ma (Bellot et al. 2024) or ca 64.5 Ma (G. Yao et al. 2025).
[Manicarieae + Pelagodoxeae]: crownshaft 0.
Age. This node is estimated to be 98/63 Ma (Bellot et al. 2024).
5L. Manicarieae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis - Manicaria saccifera Gaertner
± acaulescent; leaflets praemorse; prophyll and peduncular bract enclosing inflorescence, net-like; flowers somewhat sunken; 1 ovule/carpel; fruit warty; endosperm homogeneous; n = ?
1/1. Costa Rica to W. Colombia, Trinidad, the Orinoco and Amazon.
5M. Pelagodoxeae Dransfield, Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis
1 ovule/gynoecium; fruit with corky warts; endosperm homogeneous; n = 16, 17.
2/3: Sommieria (2). Western half of New Guinea, Marquesas Islands.
Age. The age of this clade is eatimated to be 36/24 Ma (Bellot et al. 2024) or ca 63.8 Ma (G. Yao et al. 2025).
[Areceae + Euterpeae]: 1 ovule/gynoecium.
Age. This clade is around 102/67 Ma (Bellot et al. 2024).
5N. Areceae Dumortier —— Synonymy: Manicariaceae O. F. Cook
Crownshaft + [formed by elongated leaf sheaths]; outer integument ca 8 cells across, inner integument ca 4 cells across [Dypsis]; antipodal cells polyploid; fruit with massive longitudinal bundles in pericarp, (innermost pericarp sclereidal), operculum, hilar seam +; seeds (attached apically); endosperm (ruminate); n = 16 (17).
61/665 (16 subtribes; 10 unplaced genera): Pinanga (145<), Dypsis (106), Chrysalidocarpus (54), Areca (47), Hydriastele (47), Heterospathe (40). Madagascar, Tanzania (Pemba), S. India and Sri Lanka, South East Asia (inc. the Ryukyu Islands) to Malesia and the West Pacific, N. and N. E. Australia and the North Island of New Zealand.
Age. Crown-group Areceae are some (42.1-)33.3(-26.5) Ma (Pichardo-Marcano et al. 2018: Oncos/p-Dyp), 70/47 Ma (Bellot et al. 2024) or ca 56.9 Ma (G. Yao et al. 2025).
Fossil fruits, Friedmannia messelensis, are known from oil shale deposits ca 47 Ma in Germany, and they have been assigned to crown-group Areceae, specifically to the Western Pacific clade (Matsunaga & Smith 2021).
5O. Euterpeae J. Dransfield, N. W. Uhl, Asmussen, W. J. Baker, M. M. Harley & C. Lewis —— Synonymy: Acristaceae O. F. Cook
Crownshaft usu. +; leaf sheath with extension [= ochrea]; inflorescences usu. below leaves, once-brached/simple, spicate; fruit purple-black, smooth, stigmas persisting; ?endocarp crustaceous; endosperm (ruminate); n = 18.
5/33: Prestoea (10). S. Yucatan, Central America, the Caribbean, lowland tropical America, inc. S.E. Brazil.
Age. Crown-group Euterpeae are around (48.4-)39.2(-32.2) Ma (Pichardo-Marcano et al. 2018), 84/54 Ma (Bellot et al. 2024) or ca 68.2 Ma (G. Yao et al. 2025).
Evolution: Divergence & Distribution. Baker and Couvreur (2013a) give stem and crown ages for palm subfamilies and tribes; only some have been entered above.
There is extensive discussion of fossils identified as Arecaceae in Bellot et al. (2024). Fossils of palm leaves, pollen and wood are quite common and widely distributed in the later Cretaceous when global temperatures were warmer (Srivastava 2011; Burnham & Johnson 2004; Nichols & Johnson 2008: pollen; Friis et al. 2011), including in both Africa and India where palms are not very diverse today. Bellot et al. (2024) assembled data from some 156 fossils of Arecaceae, with an additional 38 possibly to be placed here, and also some 103 records of fossil pollen, plus 1 possible (as of 2024). For palms in Late Cretaceous Africa, see Jacobs (2004) and in drier but warm parts of South Ameerica, see Burnham and Johnson (2004); there was an Upper Cretaceous northern Gondwanan palm province (Herngreen et al. 1996). Palms were also widely distributed in the earlier Caenozoic (e.g. Greenwoood & West 2016), palm pollen being recorded from palaeo 85o N sediments dated to 53.5 Ma in the Eocene on the Lomonosov Ridge (Sluijs et al. 2009) and from palaeo 70o S sediments dated to ca 51.9 Ma in the Eocene off Wilkes Land in the Antarctic (Pross et al. 2012). Fossils (leaf, stem, fruit) from Cretaceous-Campanian rocks in Texas around 77 Ma have been identified as the modern genus Sabal, and young dinosaurs might perhaps have eaten their fruits (Manchester et al. 2010a; see Greenwood et al. 2022); dinosaurs aside, if the identification is correct, some ages for the family and for Coryphoideae in particular, to which Sabal belongs, are incorrect - indeed, Cretaceous fossils placed in the Cryosophileae-Sabaleae area are quite widespread in the Northern Hemisphere (see also Caro et al. 2018: Fig. 1). Iles et al. (2015; c.f. Greenwood et al. 2022: discussion over identification and dating of early palm leaves from North America) provide dates for well-attested palm fossils, while Matsunaga and Smith (2021) look at the rich fossil record of palm fruits. They suggest that only well supported placements of fossils are worth much, and carried out a series of "pseudofossilization" experiments in which living taxa included in the molecular analyses were removed and replaced by just the fruit characters that they had been able to observe for those taxa in an analysis of morphological characters in the context of a constraint tree - generally, the taxa were correctly placed (Matsunaga & Smith 2021). S. Kumar et al. (2022a) found that a number of the older Indian fossils - often leaves, etc. - were difficult to identify, but of the identifiable fossils, Coryphoideae were rather abundant, moving from India when it docked with Asia (see also S. Kumar et al. 2022b; Kumar & Khan 2024). Bogotá-Ángel et al. (2021) found that distinctive pollen that could reliably be placed in Calamoideae-Lepidocaryeae-Mauritiinae (sister to Raphidinae), currently tropical American in distribution, had a much wider distribution in the past. They estimated the age of the group to be 94-83 Ma, and suggested that it originated in Cretaceous equatorial Africa and then moved to South America, and to India in the Palaeocene, movements probably aided by continental drift and extinctions (the group is not now known from the Old World) and perhaps driven by climate changes (Bogotá-Ángel et al. 2021). Parmar et al. (2023), also using pollen data, suggested that there had been extensive movement of palms from Africa to India via the Kohistan—Ladakh island arc at the end of the Cretaceous and beginning of the Palaeocene. This was followed by diversification in India - Parmar et al. (2023) placed the pollen that they recovered in all subfamilies except Lepidocaryoideae, the pollen coming from Palaeocene/early Eocene deposits from N.W. India (especially the Akli Formation 63-54 Ma); most of the pollen (ca 92%) was that of Spinozonocolpites, probably to be equated with Nypa. However, when the climate deteriorated at the end of the Eocene, some species went extinct, others moved to tropical Southeast Asia, relatively few persisting in India (Parmar et al. 2023), but as they note, details of their evolutionary scenario differ somewhat from those of earlier proposals where Laurasia played a more prominent role. See also Ctenolophonaceae, Dipterocarpaceae, Ebenaceae and Euphorbiaceae-Crotonoideae.
As the previous paragraph suggests, some past and present distributions of palms can be difficult to reconcile. Other examples: 1. The monotypic Lodoicea (= L. maldivica) is currently restricted to the Seychelles, and although its fruits are widely distributed by the sea, they are not salt-water tolerant. Ocean crust separating India and the Seychelles dates to ca 63.4 Ma (Collier et al. 2008), so either Lodoicea is that old, or it had somehow moved onto these islands, and since Bellot et al. (2020) estimated that the stem-group age of Lodoicea was ca 58 Ma the latter is a possibility. Bellot et al. (2020) suggested that the ancestor of Lodoicea, probably from mainland Asia, may have had fruits to 82 or 100 mm long that were dispersed by megafauna. 2. Nypa seems to have had a more or less world-wide distribution in the early Caenozoic, fossils being known from Tasmania, England (the London Clay flora), both Americas and the Caribbean, etc. (Plaziat et al. 2001; Rull 2023b: see map above). 3. Pollen of some species of the West Malesian Eugeissona is distinctive, being thick-walled and extended-monosulcate; such pollen has been found fossil throughout the tropics (Dransfield et al. 2008b: records need checking). 4. Wood of Coryphoideae-Cryosophileae, a New World group, has been found in Lower Oligocene to Upper Miocene deposits in France, indeed, [Cryosophileae + Sabaleae] are New World, mostly Central American, but their extensive fossil record in the Northern Hemisphere dating back to the Cretaceous is consistent with a Laurasian ancestry (R. Thomas & de Franceschi 2012; Caro et al. 2018 and refererences - but see below). 5. Fossil fruits, Friedmannia messelensis, from deposits ca 47 Ma in Germany have been assigned to crown-group Areceae, specifically to the Western Pacific clade (Matsunaga & Smith 2021). 6. Hyophorbe, known only from the Mascarenes, is older than those islands and probably diversified elsewhere - Africa, submerged islands in the Indian Ocean (Cuenca et al. 2008)? - see also Asteliaceae, Monimiaceae, Rousseaceae. 7. Finally, calamoid palm leaves and fruits are found in Late Eocene rocks from the very southern part of New Zealand (Hartwich et al. 2010); the closest Calamoideae now grow in eastern Australia, a rather less dramatic range difference than for Nypa, but still noteworthy.
As for dating nodes, Bellot et al. (2024) provide two ages, separated by a slash in the account above. The first set of ages, the older ages, accomodates the possibility that palms may be much older than their oldest reliably dated fossils, and here Bellot et al. (2024) used a normal root age prior distribution with a mean of 149.1 Ma and a standard deviation of 28 My. This allows older (molecular) ages for palms that have been proposed to be taken into account. The second set of ages given, the younger ages (the ages after the slash), use the age of the oldest monocot fossils as the oldest possible age for the family, other ages reflecting this. The medians of the older ages are on average ca 21 My older than the medians of the younger ages (Bellot et al. 2024: see esp. Fig. S4). These authors also divided the area inhabited by palms into seven or ten parts, used fossils to infer ancestral ranges or not, looked at the distribution of entire genera or sample species - all in all, thinking about biogeography gets complicated.
G. Yao et al. (2025) suggested that the subfamilies of Arecaceae all originated in the Cretaceous (see also Bellot et al. 2024), and also the tribes had all originated well before (ca 10 My before) the K/P boundary; 127/185 genera had originated before the Oligocene. There were early several (10/11 shifts) diversification rate increases, but extinction rates have tended to increase continuously since the early Oligocene; global climate changes have been important in overall speciesd diversity accumulation Yao et ala. 2025).
For details of the diversification of palms worldwide, see Kissling et al. (2012a, b), Bacon et al. (2012), W. J. Baker and Couvreur (2012, 2013a, b), Couvreur et al. (2015), etc.. It has been suggested that Arecaceae have diversified at a constant rate since their origin in the Cretaceous ca 100 Ma or more ago (perhaps in Laurasia) until ca 24 Ma, the K/C boundary passing unmarked (Couvreur et al. 2011b, esp. c; see also Faurby et al. 2016). Palms might serve as markers for tropical rain forest or a rain forest-like biome (Couvreur et al. 2011b). This being said, the early evolution of the rain forest biome is still not well understood (see elsewhere). R. Thomas and Boura (2015 and references) suggested that palms that had fibro-vascular bundles with two narrower-diameter vessels per bundle, e.g. Coryphoideae, could tolerate a dry period, while palms with one-vessel bundles, the vessels being wider, were common in rain forest-dwelling Arecoideae, for example. Interestingly, stems of palm fossils of Cretaceous age commonly had the first sort of vascular bundle, and it predominates in palm stems from the Deccan Traps 67-65 Ma (S. Y. Smith et al. 2015). On the other hand, Coiffard and Gomez (2009) thought that early palms might have been swamp dwellers, as were living basal Arecaceae (they mentioned Calamus, Nypa, and Mauritia, members of the two basal subfamilies). Low and mid latitude pollen assemblages in the Palaeogene were characterized by abundant palm or palm-like pollen (H. Huang et al. 2020). By the Oligocene extra-tropical climates were becoming more seasonal and so less favourable for palms, and palm diversity was also decreasing (Maley 1996; Burnham & Johnson 2004; Epihov et al. 2017).
As with several other angiosperm groups, palms are not very diverse on mainland Africa. They became less common there at the beginning of the Caenozoic and again at the end of the Eocene ca 34 Ma (Pan et al. 2006; Harley 2006: summary of Arecaceae fossil record; Kissling et al 2012a). Thus Faye et al. (2016) thought that there may have been an extinction event at the Eocene-Oligocene boundary in the African Calamoideae that they were studying. Overall, the relative paucity of palms on the continent can perhaps be explained by the increase in diversification rates elsewhere in the tropics (Bacon et al. 2012) and also because African palm diversity is the historical legacy of past climatic events (esp. since the Late Miocene, ca 10 Ma), including a drying climate, that is evident in their present restricted distributions (Blanch-Overgaard et al. 2013). Interestingly, specialized frugivorous birds that can handle fruits the size of those of many palms, i.e. rather large, are uncommon in Africa (Lauraceae, with similar fruits, are uncommon there - see Snow 1971). Indeed, fruits of African palms are about twice the size of those of palms elsewhere, associated i.a. with a lower canopy, and a number of these palms grow in dry, open savannas; large fruits may be linked with the continued presence of the African megafauna, unlike the New World tropics in particular (Wölke et al. 2023, q.v. for details; Blach-Overgaard et al. 2013 for climate and African palms). However, palms are richer and more abundant in Madagascar (Snow 1981; Muscarella, Emilio et al. 2020). The radiation of Dypsis and the other Dypsidinae (Arecoideae-Areceae) in Madagascar - some 178 species (4 not endemic) - is particularly spectacular, species having a great diversity of growth form and with entire, bifid and pinnate leaves, etc. (Eiserhardt et al. 2022). Madagascan palms lack spines, etc., unlike their mainland relatives, perhaps because there are no large herbivores on the island (Dransfield & Rakotoarinivo 2011). Torres Jiménez et al. (2021) noted that the majority of species in Calamoideae-Lepidocaryeae (42 of 51 species) were to be found on mainland Africa, one on Madagascar; the nine American species, all Mauritiinae, include Mauritia flexuosa, with an estimated 1.5 billion individuals (see Bogotá-Ángel et al. 2021), a notably abundant plant in wetter areas of the Amazon.
The diversity and diversification of palms in the New World and the Indo-Malesian area has been linked to the persistence of the relatively warm and wet areas that the plants favour and also the environmental heterogeneity of those areas (Svenning et al. 2008; Kissling et al. 2012b; Bacon et al. 2012). Arecoideae-Bactridineae diverged in America at the end of the Eocene (Eiserhardt et al. 2011a). Bjorholm et al. (2006) discussed patterns of diversity in Neotropical subfamilies, the Antilles excluded. Roncal et al. (2008) explored the biogeography of Antillean palms. Roncal et al. (2010) examined the biogeography of the mostly understory Geonomateae-Arecoideae (see also Roncal et al. 2012 for the evolution of Geonoma; "characters" here are curious constructs). Crown group divergence began in the Oligocene and species are older than Quaternary refugium theory would predict; again, there seems to have been considerable dispersal. Olivares et al. (2023), Webster et al. (2025) and others have looked in detail at the molecular variation in the Geonoma undata complex; this includes perhaps three morphological species, but these authors detected some 14 molecular clades there alone, both symaptric and allopatric - there is active hyper-cryptic radiation here, some genes involved apparently responding to temperature and light, as evidenced in variation in various aspects of phenology. The Coryphoideae [Cryosophileae + Sabaleae] clade, now Antillean-New World, was probably originally Laurasian (e.g. Dransfield et al. 2008) and may have been adversely affected by climate changes at the end-Eocene; it seems to have moved on to the Antilles by one (Cryosophileae) or more (Sabaleae) dispersal events - the Greater Antilles-Aves Ridge (GAARlandia) was probably not involved (Caro et al. 2018). However, Kumar and Khan (2024) record palm stems from the Deccan Intertrappean Beds of 65-63 Ma that they identify as Cryosophiloxylon indicum, suggesting that this is evidence for the Gondwanan origin of Cryosophileae; after India docked with Asia, Cryosophileae moved to Europe and to the New World, the latter via the Bering land bridge. For diversification in Euterpeae, see Pichardo-Marcano et al. (2018). Kristiansen et al. (2012) looked at palm communities in western Amazonia in the context of the dispersal abilities of the species and their ecological preferences - ± canopy, understory. For more on diversification in S.E. Asian/Malesian palms, see W. J. Baker and Couvreur (2012) and Bacon et al. (2013).
In the Old World, Couvreur et al. (2011b) suggested that the stem age of the Malesian-centred Calamus, with some 400 or more species, was only 24 Ma, however, this age is clearly far too young if the recent study by Kuhnhäuser et al. (2025) is taken into account. The climbing habit in calamoid palms (rattans), which originated more than once, is associated with increased diversification rates - interestingly, some less diverse clades are hapaxanthic, perhaps an odd feature to find in lianas (Couvreur et al. 2015). Marazzi et al. (2012) discuss the evolution of the climbing habit here in terms of tendencies, given the several origins of the climbing habit around here. Kuhnhäuser et al. (2025) looked at the biogeographical patterns in the 499 species of Asian rattans (360 in their analysis; Asia = Sri Lanka to Australia). They noted that Borneo was a major centre for the diversification of these palms, with both high speciation and high emigration rates, while areas like New Guinea, Southeast Asia and the Celebes were incubators, with substantial speciation alone, corridors like the Thai-Malay Peninsula, the Moluccas and Sumatra havd low speciation rates but high emigration rates, while accumulators like N.E. Australia, the Philippines and Sri Lanka and environs had low rates of both speciation and emigration. It is thought that there were no fewer than 262 dispersal events in the Asian tropics (Kuhnhäuser et al. 2025).
Despite the size of many palms and of their fruits, dispersal rather than vicariance is increasingly frequently being invoked to explain many aspects of present generic, etc., distributions in the family (Bacon et al. 2012; W. J. Baker & Couvreur 2012). There seem to have been 3-4 movements of palms on to New Caledonia (three times for Areceae, once for the single species of Coryphoideae) and then some five dispersal events, mostly to the Pacific; speciation on the island began around (27-)19, 17 (-13) Ma and has resulted in 39 species of Areceae (Pérez-Calle et al. 2024). The scattered and apparently ancient Gondwanan distribution of Ceroxyloideae is probably best explained by several mid-Caenozoic trans-oceanic dispersal events (Trénel et al. 2007), although Khan et al. (2020b) suggest that the ancestor of the subfamily may have drifted north on the Indian plate, with subsequent movement both via land bridges and long distance dispersal. For diversification within Arecoideae, see Comer et al. (2015b). Species of Hyophorbe (Arecoideae-Hyophorbeae) are disjunct on the Mascarenes, and the genus may have radiated in that area on islands that are now submerged, hopping from island to island (Cuenca et al. 2007: Myrtaceae, Begoniaceae, and Sapotaceae may also be island-hoppers). Coryphoideaea-Borasseae are hardly a large clade - some 8 genera and 22 species - but Bellot et al. (2020) estimate that there had been 10 or so over-sea dispersal events there alone; there was just a single over-sea event in the three other tribes immediately related to it.
Bacon et al. (2018b) discuss diversification in Iriarteeae and how it was affected by the rise of the Andes. Diversification in the New World Bactris (Arecoideae-Cocoseae) seems to have occurred at a more or less constant rate since the mid-Miocene (Hoorn et al. 2023).
Seed size increased considerably in stem Arecaceae (Moles et al. 2005a; Linkies et al. 2010: Sims 2012; Cornwell et al. 2014). This may be associated with the woody habit of the family, but seeds of Arecaceae are absolutely large when compared with those of all other angiosperms, including those in Dasypogonaceae, their erstwhile sister clade (some of the latter are also woody). Their dispersal is primarily by animals, although the coconut, Cocos nucifera, may be dispersed by sea currents or wind for some considerable distance - currents and wind may conflict, one must also factor in how long the seeds remain viable (Ward & Brookfield 1992) - is a notable exception (Zona & Henderson 1989; Henderson 2002). Indeed, fruit size may affect palm distributions. Thus dispersal of some Malagasy palms may now be compromised because of the recent extinctions of all the largest frugivorous lemurs and the elephant bird and its relatives on the island (Federman et al. 2016), while palms with megafauna-sized fruits, at least 4 cm across and with one or a few large seeds (perhaps the ancestral condition for the family), seem to have fared differently in the Old and New Worlds during the Quaternary. In the New World extinction was relatively common, while in the Old World such palms tended to evolve smaller fruits, perhaps in the Malesian area (Onstein et al. 2018; Wölke et al. 2023). The distinctively large fruits of palms on the African mainland in particular may be associated with the persistence of the megafauna there, and evolutionary change was relatively rapid (Wölke et al. 2023, see also above). However, even in the New World there can be rapid evolutionary change in fruit size - almost one thirds reduction, really rather remarkable - in places where large frugivores have been lost only within the last 200 years; an example here is Euterpe edulis in the Atlantic Forest of Brazil (Galetti et al. 2013: see also Moraceae-Maclura and Fabaceae for frugivores and fruit size).
There is much debate as to where and when Cocos (Arecoideae) originated. Crown-group Cocoseae are estimated to be around 63.8 My; nothing much happened divergence-wise for the next ca 25 Ma (Meerow et al. 2014). Gunn (2004) suggested that Cocos might be sister to the New World Parajubaea (see also W. J. Baker et al. 2009; Faurby et al. 2016) and be at least 22 Ma, while Meerow et al. (2009a, esp. b) suggested a sister relationship with the New World Syagrus, the two separating (39.5-)34.9(-20.7) Ma and crown-group divergence in Cocos beginning ca 11 Ma (Meerow et al. 2009b: 95% HPD limits), but general relationships in this part of the tree are unclear (c.f. Baker et al. 2009 and Faury et al. 2016). Furthermore, fruits in Late Cretaceous Deccan Intertrappean Beds 65.5-61.7 Ma are reported to be those of Cocos (Srivastava & Srivastava 2014), Gómez-Navarro et al. (2009) found fruits that they compared with Cocos from northern Colombia that are only a little younger - about 58 Myo (see also Giraldo et al. 2022: evidence of infestation of such seeds by Bruchinae-Pachymerina beetles), while Palaeocene fruits ca 62 Ma from Argentina have been identified as Arecoideae-Cocoseae-Attaleinae (Futey et al. 2012). Something is very wrong somewhere.
Horn et al. (2008, 2010a, esp. 2009b) and especially Tomlinson et al. (2011) looked at the evolution of various aspects of lamina anatomy and R. Thomas and Boura (2015) at some stem anatomy in the context of the phylogeny of the family. Their work has in part been integrated above, but there is widespread homoplasy in the characters. Nadot et al. (2011) mapped androecial evolution on a phylogenetic tree; developmental details of polyandrous flowers vary considerably, but apart from Phytelepheae (Uhl & Moore 1977a), the androecium is basically trimerous, the stamens are supplied by separate bundles, and in a few genera (Socratea, Wettinia) there are antesepalous stamen pairs (see also Alapetite et al 2014: Areceae-Ptychospermatinae; Uhl 1976 and Uhl & Moore 1980: androecial development). Carpels in all palms studied are initially free (Dransfield et al. 2008b), and perhaps connected with this, apocarpy has probably evolved some four times here (Rudall et al. 2011b). Loo et al. (2006) thought that the evolution of protogyny in Arecinae might be correlated with a radiation in Pinanga and diversification in pollen morphology and genome size.
Henderson (2022) reviewed the literature on hybridization between palms in the wild; there are not really that many records, and just over half are known from Trachycarpeae and Cocoseae, not particularly close.
Perhaps more than in some other families, a number of the issues mentioned above involve ecology. Kissling et al. (2019) note the development of Palm Traits 1.0, a database of functional traits for palms that will be integral in attempts to answer such issues, so deepening our knowledge of the evolution of Arecaceae.
Palms seem quintessentially megatherm plants, for the most part not tolerating cold (freezing) conditions (Reichgelt et al. 2018), and palm fossils are used as climatic markers by palaeontologists (e.g. Greenwood & Wing 1995; Sluijs et al. 2009) based on the climatic proclivities of present-day palms. Extant palms are very susceptible to frost, most having only a single vegetative meristem and being unable to produce a replacement if it is killed, hence they are usually found in places where the mean annual temperature is more than 10° C, mean temperatures in the coldest month are more than 5°, and the coldest temperature does not dip below -10° C (Larcher & Winter 1981; Greenwood & Wing 1995; Tomlinson 2006; Couvreur et al. 2011c; Kissling et al. 2012b). In their analysis of major functional traits in vascular plants, Cornwell et al. (2014) noted that Arecaceae were both relatively large plants and had relatively large seeds compared with other monocots, and that this was associated with a strong preference for humid and warm conditions. Eiserhardt et al. (2011b) thought about the distribution of palms world-wide in the context of ecological determinants both of geography and diversity. The suspects are very much as one might expect, with climatic limitations having a major effect on broad-scale patterns while dispersal affects patterns at all scales. Interestingly, over two thirds of the palm genera that grow outside tropical rainforest are members of Coryphoideae, which has about 1/4 of the genera in the family (Couvreur et al. 2011c; Thomas & de Franceschi 2012). Reichgelt et al. (2018) also suggest that it is unusual for palms to grow in conditions where the mean temperature for the coldest month is ≤5oC, i.e. they grow where there is no frost. Although some tribes like Trachycarpeae (Coryphoideae) are able to tolerate colder conditions (Areceae are also rather cold tolerant), this ability is likely to have developed since the Oligocene.
Nypa fruticans dominates some mangrove habitats in the Southeast Asian-Malesian area. It prefers less saline conditions than many other mangrove plants and is found along rivers up to the limit of tidal influences (for the evolution of the mangrove habitat, see Rhizophoraceae; Tomlinson 1986, 2017; see also Clade Asymmetries). Chomicki et al. (2014a) discussed how air reaches the submerged roots of Nypa; they found that lenticellar tissue developed on the emergent leaf base after the frond rhachis fell away, and air then moved down canals into the roots. Peat formed in Palaeocene Nypa-dominated mangrove habitats in Guyana (Leidelmeyer 1966). Indeed, palms are notable components of swamps throughout the tropics, dominating large areas there, sequestering large amounts of carbon, etc. (Dargie et al. 2017; Muscarella, Emilio et al. 2020). For example, Raphia laurentii (Lepidocaryeae) is one of the four common species mentioned as growing on the ca 145,500 km2 of peat in the Cuvette Centrale in the Congo (Dargie et al. 2017).
Arecaceae are the largest clade of woody monocots, bamboos, at somewhat over half their size, are the only other major woody monocot clade. They have the oldest functioning xylem elements and sieve tubes in vascular plants, but how the conducting system remains functional is largely unknown. Since the oldest living palms are hundreds of years old and it is commonly thought that there is no secondary thickening, the vascular tissue at the base of the stem must remain active for the whole life of the plant; physiologically functional cells must be very old (Tomlinson & Huggett 2012) - note, however, that sapwood in angiosperm trees can be quite old (e.g. K. C. Yang & Hazenberg 1991), and medullary vascular bundles (and stomata) in some Cactoideae (q.v.) may remain funcional for the life of the plant, some 150 years or so. However, old palm stems are not inert. Renninger and Phillips (2012, 2013; c.f. Tomlinson & Quinn 2013) found that the stems of Iriartea, at least, lengthened appreciably over time, and this was accompanied by a straightening of the spiral course of the vascular bundles; lengthening has also been noticed in other palms (Waterhouse & Quinn 1978). The walls of vascular fibres continue to thicken, so increasing stem stiffness as the tree grows bigger (Tomlinson et al. 2011). Ground tissues in both stem and root may remain undifferentiated for some time, with limited mitosis and/or cell expansion and/or formation of schizogenous lacunae and the trunk markedly thickening and lengthening (diffuse secondary thickening - Tomlinson 1961c; sustained primary growth - Waterhouse & Quinn 1978; sustained growth of the ground parenchyma - R. Thomas & Boura 2015; see also Tomlinson et al. 2009, 2011). This ability of cells to remain metabolically active may help explain the functional longevity of palm vascular tissue. There are also persistent reports that at least some palms have monocot-type secondary thickening (Angyalossy et al. 2008; Botánico & Angyalossy 2013). Waterhouse and Quinn (1978) suggested that in some palms there was initially sustained primary growth and this was followed by growth in which the trunk did not increase in width and was strictly columnar. Problems of vascular tissue in particular, but also other plant tissues, that involve age, are also mentioned elsewhere; in some respects, palms are similar to fossil tree lycopods, q.v., and the latter are also likely to have had a period of establishment growth (see Vegetative Variation below).
R. Thomas and Boura (2015) examined various aspects of stem anatomy of palms in the context of their ecology. They found the fibro-vascular bundles with two vessels per bundle were common in palms that lived in places with a dry period, and these palms include half of all Coryphoideae. Such two-vessel bundles, but not the one-vessel bundles that are common in extant rain forest-dwelling Arecoideae, for example, are known from palm fossils of Cretaceous age (Thomas & Boura 2015 and references).
Calamoid palms are a very important group of lianes in the South East Asian rain forests (Gentry 1991; see also Kuhnhäuser et al. 2025); elsewhere, palm lianes are uncommon, although Desmoncus (Arecoideae-Cocoseae) is a New World liane. There are about 535 species of climbing palms all told, and Calamus is one of the ten largest genera of climbing plants (Sperotto et al. 2023); it would be interesting to establish exactly where "plant a climber" is an apomorphy. When the climbing habit evolved is unclear, perhaps early Eocene to Miocene (Couvreur et al. 2015). Various features of these palms might have made them successful: All have thin woody stems and climbing is effected by spines or hooks, often recurved and either on leaves and/or modified leaf segments or inflorescences, the last only in Calamoideae (Couvreur et al. 2015). These are very efficient grapnels that work on the ratchet principle - if they become disengaged, larger hooks or spines lower down the rhachis/axis take over (Isnard & Rowe 2008b, 2009). The leaf sheath can add significantly to the rigidity/flexural stiffness of the younger stem, but when the sheaths fall off there is a decrease in the structural Young's modulus, and hence flexibility (Isnard & Rowe 2008a), although sheaths may persist in some species of Desmoncos (Isnard et al. 2005). When rattans grow through the canopy, or their sheaths with associated cirrus fall off, they may slip until the spines re-engage; older rattans may form heaps of coiled stems on the forest floor, but they still may grow back through the canopy (Putz 1990). Rowe and Speck (2015) discuss further biomechanical aspects and Isnard and Feild (2015) morphological-functional aspects of being a climbing palm.
Vessels in Rhapis excelsa are quite resistant to embolism, but what goes on in the vessels of some calamoid palms - they can be over 0.5 mm wide, almost 4 m long (remember, such vessels are made up of a whole series of vessel elements), and span (8-)13(-18) internodes - is unclear (Sperry 1986; Fisher et al. 2002; Tomlinson et al. 2001; Tomlinson 2006b). Such vessels would appear to be susceptible to embolisms/cavitations, but Davis (1961) noted that root pressures in several palms he studied in India were very high, sometimes exceeding the height of the plant, and this could help remove embolisms (see also Cao et al. 2012, but c.f. Knipfer et al. 2016: living tissues control embolism formation in grapes; Schenk et al. 2017: lipid surfactants in xylem help prevent embolisms forming). Tomlinson et al. (2001) were perplexed as to how rattan stems might function since none of the main vascular elements seemed to connect with any of the others - the long cauline vascular bundles were not in direct connection, nor were the foliar bundles connected to the cauline bundles. Indeed, differentiation of vascular tissue in Calamus is very distinctive when compared to palms like Areca and extremely complex (Tomlinson & Spangler 2002). When thinking about how xylem functions in 100 m long rattans, it should not be forgotten that the plant rarely branches, nor does it form adventitious roots (Putz 1990).
Palms, perhaps particularly species of Licuala, collect humus (Zona & Christenhusz 2015), the palm habit being close to the Schopfbaum habit of other humus-collecting angiosperms. These authors note that Calaamus and other Calamoideae, covered in spines, may also trap litter, and ants may nest in the litter, nutrients then moving from the ant nests to the plant.
Arecoid palms in particular are an important food resource for specialized frugivorous birds in the New World (Snow 1981; Staggemeier et al. 2017).
Arecaceae include many examples of the eco-morphological combination of shaded conditions, net-veined leaves and fleshy fruits that has evolved several times in monocots (Givnish et al. 2005, 2006b).
Pollination Biology & Seed Dispersal. Pollination is predominantly by insects, whether beetles, mainly Nitidulae, cyclocephaline scarabs and Curculionidae-Derelomini weevils, "palm-flower weevils", the latter commonly nocturnal, and pollinating taxa in at least 23 genera (Bernhardt 2000; Straarup et al. 2018; Haran et al. 2022, 2023a: weevils), bees, especially Halictidae (sweat bees) and Meliponini (stingless bees), and flies, which may visit understory palms in particular (Henderson 1986, 2002; Silberbauer-Gottsberger 1990; Knudsen et al. 2001; Barfod et al. 2011; M. R. Moore & Jameson 2013; Moore et al. 2015). Indeed, although the ancestral associates of derelomine weevils may have been palms, at a crown age of ca 40 Ma for the weevils, they obviously evolved long after the palms did; closely related species of the weevil may be found on the one species of palm, and from palms, the weevils moved on to a variety of other angiosperms (Haran et al. 2021, 2022; for ages, see above). Mystrops, a nitidulid, is a common pollinator of palms, and shows quite a degree of host specificity, at least locally (Restrepo Correa et al. 2016), as do cyclocaphaline scarabs (M. R. Moore et al. 2015). It is volatiles produced by the leaves of Chamaerops humilis that attract its weevil pollinator (Dufaÿ et al. 2003), while thermogenesis has been detected in the flowers of some palms, including the beetle-pollinated Ceroxyloideae-Phytelepheae, mostly in flowers lacking nectar, and also in Nypa (Silberbauer-Gottsberger 1990; Seymour 2001; Straarup et al. 2018); for floral scent in geonomoid palms, see Knudsen (1999) and in Neotropical palms, Knudsen et al. (2001). Beetles may lay eggs in staminate inflorescences (Silberbauer-Gottsberger 1990), weevils, for example, being involved in brood-site pollination mutualisms (Haran et al. 2023a), and there are several records of cyclocephaline scarabs visiting palm flowers (Moore & Jameson 2013). Bakerian mimicry by female flowers of male flowers, only the latter offering rewards, is reported (Olesen & Balslev 1990; Knudsen et al. 2001; Stauffer et al. 2002: Geonoma). The calamoid Eugeissona tristis produces considerable amounts of fermented nectar (to ca 3.8% alcohol) in its very large, robustly-constructed and long-lived flowers (Stauffer et al. 2016), and small pen-tailed tree shrews, its pollinators, and other animals quaff the nectar without any apparent damage to themselves or the plant; the beginning of this association has been dated to some 55 Ma (Wiens et al. 2008). What pollinates the subterranean flowers of Pinanga subterranea (Areceae) is unclear; pigs may disperse the fruits (Randi et al. 2023). For wind pollination, not very common, see Stauffer et al. (2019); the wind-pollinated Wendlandiella lacks a nectary, the carpelate flower is single, and only one carpel is fertile. There is no clear correlation of pollen morphology with pollinator (Sannier et al. 2009) nor of floral scent with taxonomy (Moore & Jameson 2013).
Seeds in Arecaceae are frequently bird-dispersed, specialist frugivores being involved (Snow 1981), although water and other animals including fish are also dispersal agents (Zona & Henderson 1989). For water dispersal, see e.g. the coconut palm (Ward & Brookfield 1992). In Colombia the seeds of Oenocarpus bataua at 35 x 18 mm were among the largest dispersed by the oilbird Steatornis caripensis which moved them up to 42.3 km in the one trip (Stevenson et al. 2021).
There is a great variety of breeding systems in the family, although particular systems tend to predominate in each subfamily; protandry is common, or, in monoecious taxa, the male flowers open first (Nadot et al. 2016).
The speciose New World Hyophorbeae (Arecoideae, the old Chamaedoreae) all have raphides in their flowers and in Chamaedorea these are particularly common in the perianth and gynoecium; for these and other potentially protective structures in floral tissues, see Askgaard et al. (2008). Indeed, Arecaceae are noted for one species or another having raphides in any part of the plant (Lawrie et al. 2023).
Plant-Animal Interactions. Satyrinae-Morphini butterfly larvae may be found on Arecaceae, along with larvae of Hispinae-Cassidinae beetles; these latter can be serious pests of oil palms and other commercial or ornamental palms (Chaboo 2007). The move of satyrines on to palms was important in the diversification of the former, groups like Elymnini, Amathusiinae and Morphini being well represented here (Peña et al. 2011; Nylin et al. 2014). Caterpillars of Hesperinae (skippers) are quite common on palms from the Guanacaste area, Costa Rica (Quicke et al. 2025; see also Poaceae). Stem Bruchinae - the highly speciose seed beetles, now found mostly on Fabaceae - are about 85-82.6 Ma old (age spread far greater); that is, they evolved long before Fabaceae, and their original diet may have been seeds of Arecaceae (Kergoat et al. 2005b, 2011). A male of the (now) palm-eating Pachymerini was found in Canadian amber dated at ca 79 Ma (Poinar 2005). For other palm-insect associations, see papers in Trucchi et al. (2003).
Myrmecophily in the family has been little studied, but some 50 species of rattans (Calamoideae) are myrmecophilous (Dransfield 2003), myrmecophily having evolved four times or so. Ants live in domatia formed by the ochreae/ocreae (= ligules) which either ensheath the stem, ants living between the stem and ligule, or stand at an angle to it with incurved margins and a hole at the apex and base (e.g. Davidson & McKey 1993: importance of spines on the ochreae; Mattes et al. 1998; Merklinger et al. 2014: ochrea development). Domatia may also be formed by whorls of spines along the stem that form the scaffolding for ant nests, the ants adding plant materials, domatia/nests of adjacent whorls being connected by carton-covered runways (Rickson & Rickson 1986; Dransfield 2003: carton made up of rattan scales and hairs and ant saliva), or the ants may live surrounded by leaf segments that are held close to the stem, inside imbricate inflorescence bracts, etc. (e.g. Dransfield 2003; Sunderland 2004: African rattans). Plant debris accumulates in the leaves of the rattan Daemonorops in Pasoh, the Malay Peninsular, and the leaves rot, nutrients being washed down the stem and then perhaps being absorbed by the carton of ant nests, from which nutrients move into the palm (Rickson & Rickson 1986; c.f. Dransfield 2003: secondary consequence?). In some cases the ant (Camponotus spp.) exists on honeydew produced by the hormaphid Cerataphis which the ant actively tends, for instance moving the aphids as the colony expands; this aphid is also to be found on palms other than rattans and also inside galls on its primary host, Styrax benzoin (Mattes et al. 1998; see also Chan et al. 2012). Camponotus - depending on the species - appears to protect the rattan Korthalsia against herbivory and also remove epiphylls (Edwards et al. 2019; Miler et al. 2016: no mention of aphids in either case), however, bacterium-eating nematodes are not associated with the rubbish dumps inside the domatia here, as they are in some other myrmecophytes (Maschwitz et al. 2016). The specificity of these ant-rattan associations is unclear (see Rickson & Rickson 1986; Mattes et al. 1998; Sunderland 2004; Chan et al. 2012), and since there was no "sanctioning" by the plant of ants that were of no benefit to it, Edwards et al. (2010) suggested that cheaters could occupy the domatia of K. furtadoana, at least - an idea to be checked.
Plant-Bacterial/Fungal Associations. Differences in AM taxa associated with two species of the palm, Howea (Areceae), growing on different soil types on Lord Howe Island, off Australia, may be connected with the evolution and coexistence of these very closely related species (Osborne et al. 2017 and reference).
Vegetative Variation. Seedling morphology is very variable within Arecaceae (Henderson 2002, 2006; Tillich 2007). Seedlings of palms with aerial stems usually undergo a period - up to 50 years - of establishment growth (Henderson 2002 and references). During this time the apical meristem becomes gradually larger, as does the width of the axis it produces, and only when it has reached adult size does elongation growth of the trunk occur. With some exceptions, the size of the apical meristem and hence the width of the trunk remains constant for rest of the life of the palm since there is no secondary thickening of any kind (see below formonocot secondary thickening). Iriarteeae (Arecoideae) are one of those exceptions. Here there is no underground establishment growth, and the trunk becomes gradually wider as it grows, being narrowly obconical overall. A plant of any size with such a stem would obviously be highly unstable, but massive prop roots develop from the lower part of the trunk and stabilise the plant. The base of the stem rots away, and the older plant then depends entirely on its prop roots for support, water, etc..
As mentioned, vegetative branching in trees like palms that lack secondary growth is unlikely. However, dichotomous branching of one sort or another is scattered in the family, having first been recorded by Schoute in 1909 (Fisher 1974 and references; see also Fisher & Maidman 1999) in Hyphaene thebaica with its distinctively-branched aerial trunks. There are other forms of vegetative branching. Of course axillary branching that produces inflorescences is the norm, and in some rattans (Calameae) the axillary bud is displaced onto the sheath of the next youngest leaf, where it is evident as an inflorescence or cilium (Fisher & Maidman 1999); the old inflorescence branches fall off. Corypha, with its massive terminal inflorescence, is monocarpic. The above-ground stems of Caryota die after an extended period of flowering, axillary inflorescences developing basipetally down the trunk, however, the plant persists because axillary vegetative shoots are formed at or immediately below ground level and these develop roots from the stems. Edelman and Richards (2019) provide a comprehensive survey of non-reproductive branching in the family and found that it was most common and diverse in Calamoideae.
Despite appearances, the leaves of all palms are simple. The deep lobes in simple palm leaves and the "leaflets" (= segments, Pinedo & Gomes 2022) in apparently compound leaves are commonly thought of as being the result of cell death. However, a detailed study of species of Chamaedorea showed that the process is more like abscission (Nowak et al. 2007, 2008), although nothing (apart, sometimes, for the lamina margin - see below) falls off. There is differential growth in the blade, and the parts that will separate first become thin, then split; tissue at the zone of separation becomes lignified, suberised, or covered by a cuticle, so protecting the rest of the blade. Details of the timing of this process may differ even between closely-related species (Nowak et al. 2007, 2008). The leaf blade early becomes strongly plicate, and blade separation occurs in various places with respect to these plications (see also Kaplan et al. 1982: good review of the earlier literature; Gunawardena & Dengler 2006). In the leaves of Rhapis excelsa, at least, so-celled hinge cells on the upper sides of the veins are larger and more elongated than the others, and water loss through these cells causes the leaf to fold adaxially, there being differential cell shrinkage comparing these and other cells of the folds – interestingly, such cells alternate between the adaxial and abaxial sides of the vascular bundles, as might be expected for cells with such a function (see K. Guo et al. 2024 for details). In a number of palms thin "reins" hang down from the sides of the leaves; these are the margins of the originally simple blade (Eames 1953), and fibrous filaments sometimes to be found between the leaf segments provide other evidence for the originally simple nature of the leaf. Given the distinctive nature of these palm leaves, it is not surprising to find that KNOX genes are not involved in their development, although they are in the compound leaves of broad-leaved angiosperms (Nowak et al. 2011). However, the blade of a palm leaf may develop from the upper part of the leaf, as in broad-leaved angiosperms (Gunawardena & Dengler 2006). Pinedo and Gomes (2022) discuss leaf development in Euterpe oleracea emphasizing the difference between a palm leaf and the compound leaf of a broad-leaved angiosperm.
Fibers at the bases of the leaves are produced by the decay of the sheaths, while the spines found on so many palms are produced in various ways. They may be modified leaf segments (Phoenix), roots (Cryosophila, but this is relatively uncommon) or branches of roots (Uhl & Moore 1973), partly detaching outer parts of the stem that become erect, etc. (Tomlinson 1962b; Tomlinson et al. 2011).
Many Calamoideae (the rattans) and some Arecoideae (e.g. Desmoncus), are climbers, all told around 535 species (Couvreur et al. 2015), and climbing is either by a long, hook-bearing apical portion of the leaf, the cirrus, and also, in Calamus and relatives, a very much modified long and very thin inflorescence axis with recurved spines, the cilium, that is basally adnate to the sheath of the leaf of the node immediately above. These climbing aids are remarkably effective, indeed, Calamus is sometimes called the lawyer vine, because once you are caught by the cilium, it can be very difficult to get free. However, they eventually fall off with the leaves, and the stems, which can reach lengths of up to some 200 m, then tend to sag; Isnard and Rowe (2008 and references) give details of the biomechanics of climbing of these palms. Some ant-associated rattans (see above) have an ochrea that develops at the junction of the leaf sheath and petiole - it is basically a souped-up ligule as long as 1.5 m that functions as an ant domatium (see Merklinger et al. 2014 for its development).
Genes & Genomes. A genome duplication in Phoenix dated to (57.8-)53.7(-48.5) Ma (Vanneste et al. 2014a) could involve quite a lot of the family (see also D'Hont et al. 2012; McKain et al. 2016; Zwaenepoel & Van de Peer 2020), the NYFRα event dated at 110.5 Ma involved Nypa and the clades above it, i.e., most of the family (Landis et al. 2018). A genome duplication involving the whole family, but not Dasypogonaceae, is confirmed by Barrett et al. (2019). For the genome size of coconuts and their relatives, see Gunn et al. (2015).
Röser (1994) suggested that the basic chromosome number for the family was x = 18, subsequently there was much descending dysploidy. However, Barrett et al. (2019) suggested that the ancestral chromosome number was x = 15, although x = 16 was the number that came from another model used; subsequent changes in chromosome number were largely by ascending dysploidy. The base chromosome number of Elaeis may be 16 (Q. Xu et al. (2021). In Voaniola (Arecoideae-Cocoseae) n = around 303 (Röser 1994).
For plastome evolution in Arecaceae, see Barrett et al. (2015a, esp. b). They noted that the plastome inverted repeat (IRb) had been almost completely lost in Tahira spectabilis; it would be interesting to know about the plastome in other Coryphoideae-Chuniophoeniceae, to which Tahira belongs.
Economic Importance. Domestication and cultivation of the coconut, Cocos nucifera (Arecoideae-Cocoseae), seems to have occured independently in southern India and the Malesian archipelago (Gunn et al. 2011). Interestingly, C. nucifera from Palmyra Atoll, in the centre of the Pacific, although a rather small tree, has large, lobed (especially basally) fruits (Griffith et al. 2025); it is something of an enigma. The coconut hispine beetle Brontispina longissima - its relatives eat other commelinids - is a serious pest. Elaeis guineensis (also Cocoseae), the oil palm, is a major tropical plantation crop; its cultivation is responsible for particularly massive and ever-increasing deforestation. For oils from the coconut and the oil palm, see papers in Vollmann and Rajcan (2009). The sago palm is Metroxylon sagu and relatives (Ehara et al. 2018), and sago is a very pure starch extracted from the stem (starch from Cycas revoluta and Manihot esculenta is also sometimes called sago). For the heart of palm, Euterpe, and for oils from other Euterpeae, see Pichardo-Marcano et al. (2018).
Chemistry, Morphology, etc.. For pbenzoate/phydroxybenzoic acid (alkaline hydrolysis product) and lignin, see Pearl et al. (1959: 11.5-72%, Nypoideae and Calamoideae not examined) and Karlen et al. (2018); palms can have very highly decorated lignins (monolignol conjugates - Karlen et al. 2017). Unfortunately, what goes on in Dasypogonaceae appears to be unknown. Parthasarathy (1974) described phloem development. According to Arber (1925), the vascular bundles are not amphivasal. "Leaflets" of induplicate leaves are V-shaped in cross section, those of reduplicate leaves are inverted V-shaped (see also Kaplan 1997, vol. 2: chap. 17 for leaf morphology).
Caryoteae quite often have basipetal hapaxanthy, a feature that has been used to suggest that the group should be recognised as a separate subfamily (Dransfield & Mogea 1984). In Arecaceae, the cincinnus seems to be the basal inflorescence units from which others have been derived (see also Castaño et al. 2014; Comer et al. 2015b). For variation in details of the inflorescence units in Arecoideae-Hyophorbeae, which have acervuli, that is, modified largely ebracteate cincinni, see Uhl and Moore (1978), Cuenca et al. (2009), and Ortega-Chávez & Stauffer 2011), The flowers in general (e.g. Rudall et al. 2003b; Uhl & Moore 1971) and pollen in particular (see Harley 1999b; Harley & Baker 2001; Dransfield et al. 2008b) show a considerable amount of variation. For floral development in monoecious and dioecious taxa in Arecoideae-Hyophorbeae, see Castaño et al. (2016). The number of vascular traces to the sepals and petals is very variable (Uhl & Moore 1978). The stamens are never adnate to the corolla/inner tepals in staminate flowers, although in carpelate flowers of Roystonea the corolla has a broad staminal cup at the base, and in carpelate flowers of Geonomeae (Arecoideae) both the calyx and corolla are connate and the stamens are adnate to the latter (Stauffer & Endress 2003), and the style there is more or less gynobasic. Microsporogenesis is simultaneous in some Coryphoideae and Arecoideae (Harley 1999a; Sannier et al. 2006). Cocos and Hyphaene have bisporic 8-nucleate embryo sacs and in the former the ovules lack parietal tissue (Robertson 1976; Mahabalé & Chennaveeraiah 1958). Uhl and Moore (1973) discuss the various ways that pollen and ovules are protected in palms.
Variation in gynoecial development and fruit morphology/anatomy is summarized by Matsunaga and Smith (2021), on which the account above leans heavily. Variation in the gynoecium is especially pronounced in Coryphoideae where there are all intermediates between syncarpous and apocarpous gynoecia (there is sometimes only a single carpel); quite a number of taxa have flowers with only a single fertile carpel, and then the style is gynobasic (Stauffer et al. 2002 and references). Furthermore, in this subfamily the stylar canals may be separate, so there is no compitum, or they may join somewhere up the style (Dransfield et al. 2008), For details on the development of the pericarp, see also Murray (1973), Bobrov et al. (2012a, b), Thadeo et al. (2015), etc.; Dransfield et al. (2008b) noted that the stony layer of Eugeissona differed from that of other Arecaceae. Indeed, although Matsunaga and Smith (2021) and others call the fruits of Arecaceae "drupes", Matsunaga and Smith are careful to note that they are often not drupes in the strict sense, rather, it is mesocarpial tissues that produce the woody/stony layer, not the endocarp. Nevertheless, an endocarp s. str. develops in quite a number of palms (e.g. Murray 1973; Matsunaga & Smith 2021). Mannans, reserve celluloses, are common in (?throughout) Arecaceae where they are to be found in the endosperm, while immature seeds have legume-like galactomannans (Kooiman 1971; Reid 1985).
We are fortunate in having a series of major works written over the years that are devoted to palms. These include von Martius's magnificent Historia naturalis palmarum (1823-1850 - see also Martius 2010), surely one of the greatest of all botanical publications; his collaborators included Hugo von Mohl, whose anatomical studies are still worth consulting. Other publications include those of Corner (1966), H. E. Moore (1973), Uhl and Dransfield (1987), Tomlinson (1990), Henderson (2002), Dransfield et al. (2005, 2008b) and Tomlinson et al. (2011), and there is also the valuable resource Palmweb - Palms of the World Online.
Additional information may be found in Uhl (1972: Nypa), Zona (1997: esp. south east U.S.A.), Dransfield and Uhl (1998), Tomlinson (2006a), Galeano and Bernal (2013: esp. Sabinaria), Henderson (2020: Calamus, 2023: Caribbean Cryosophileae) and Lý et al. (2023: Truongsonieae), all general, Tomlinson (1970: vascular organization in the stem), Tomlinson (1974: stomatal development), Seubert (1996a, b, 1997b, 1998a, b: root anatomy, considerable variation), R. Thomas and di Franceschi (2013: stem anatomy), Gunawardena and Dengler (2006: leaf development), Prychid et al. (2004) and Piperno (2006), both SiO2 bodies/phytoliths, Punt and Wessels Boer (1966: Geonomateae), Uhl and Dranfield (1984), Balhara et al. (2013: Ceroxylon), Castaño et al. 2014: Gaussia) and Stauffer et al. (2016: Eugeissona), all floral morphology/development, Stauffer et al. (2009: labyrinthine nectaries), Harley (1990: pollen), Harley and Dransfield (2003: triporate pollen), Sannier et al. (2007: microsporogenesis evolution, ?Ceroxyloideae), Gabarayeva and Grigorjeva (2010: Caryota pollen self-assembly), all pollen, Kajale and Rande (1953) and Krishna Kumar (2021, 2023), embryology, Uhl and Moore (1971) and van Heel (1977b), both gynoecium, Essig (2008), Romanov et al. (2011) and Reis et al. (2017), all fruit anatomy, Zona (2004: embryo raphides), and Hill (1937) and Henderson (2006: observations not integrated with phylogeny), both germination.
Phylogeny. [[Nypoideae + Calamoideae] (strong support for that clade) + the rest of the family (moderate support)] formed a tritomy in an early three-gene study by Asmussen et al. (2000); other characters supported these general relationships. Soltis et al. (2007a) also recovered a well-supported clade [Nypoideae + Calamoideae], but support was weak in Comer et al. (2015b). However, Calamoideae have been found to be sister to all other Arecaceae in other studies, if some morphological groupings were not supported by molecular data (Hahn 2002a, b; see also W. J. Baker et al. 1999a; Asmussen & Chase 2001; Lewis & Doyle 2001). Henderson and Stevenson (2006), after a analysis of morphological and anatomical features for selected genera, discussed groupings, relationships and character evolution. They found that Phoenix and Thrinax appeared as successively sister to the rest of the family, which even in 2006 might have seemed rather unlikely. However, Asmussen et al. (2006: good generic-level sampling, four genes) helped clarify this confusion and presented a quite well supported set of relationships; those parts less well supported (the monophyly of Ceroxyloideae and Arecoideae) were strongly supported by low copy number nuclear DNA data (W. J. Baker, unpubl. data, in Asmussen et al. 2006). These relationships were confirmed in the comprehensive analysis of Baker et al. (2009), although the position of Nypoideae was less well supported than that of the other subfamilies, and more recently by Barrett et al. (2015a, esp. b: support for monophyly and position of all subfamilies strong) and Faurby et al. (2016: again Nypa is a little perambulatory...).
Recent work suggests further resolution of relationships. The tree in Bellot et al. (2024) is based on the analysis of 1033 nuclear genes and includes all 184 genera; the topology in this paper is followed below. G. Yao et al. (2025) looked at the plastomes of 181(/184) genera and 604 species, and the topology of the tree they obtained is similar to that of Bellot et al. (2024) although positions of five tribes differ, also ca six genera are polyphyletic and the monophyly of Geonomateae was uncertain.
Arecoideae. W. J. Baker et al. (2011, see also 2006, 2009) examined phylogenetic relationships within Arecoideae; the tribes were monophyletic (see also Lewis & Doyle 2002; especially Faurby et al. 2016). Relationships within the subfamily in a plastome analysis by Comer et al. (2015a) are [Chamaedorea [Iriartea + The Rest]], and there was a fair bit of resolution; looking at the whole chloroplast genome got over the rather small signal in individual chloroplast genes, the chloroplast genome evolving rather slowly. Comer et al. (2015b) found that the position of the two genera immediately above reversed in a nuclear phylogenomic study, but branch lengths there are short (but see also Faurby et al. 2016: as tribes; Bellot et al. 2024; G. Yao et al. 2025), while the two were sister taxa in Pichardo-Marcano et al. (2018). G. Yao et al. (2025) recovered a [Leopoldinieae + Pelagodoxeae] clade, but the two were separated in Bellot et al. (2024: see above).
Relationships within the large Areceae have been unclear. Norup et al. (2006) discussed generic limits here. Loo et al. (2006) examined relationships in Arecinae (betel nut and relatives), while Alapetite et al. (2014) discussed relationships within the East Malesian to Pacific Ptychospermatinae in the context of variation in stamen number. Eiserhardt et al. (2002: target sequence capture, 161 loci, 157 species) looked at relationships in Dypsidinae, and obtained a fair bit of resolution, although Dypsis, Chrysalidocarpus and Marojejya basically formed a tritomy. Cuenca and Asmussen-Lange (2007) and Cuenca et al. (2007, 2008) looked at the phylogeny and biogeography of the largely New World understory Hyophorbeae (Chamaedoreae); for the phylogeny of Chamaedorea, which includes the bulk of the tribe, see Thomas et al. (2006).
Gunn (2004) provided a phylogeny of Cocoseae as did Noblick et al. (2013: morphological analysis); the latter found a paraphyletic Syagrus that included Cocos, but bootstrap values were nearly all below 50%. Cocos was sister to Attalea in most analyses of variation in the WRKY gene family (Meerow et al. 2014; see also Pichardo-Marcano et al. 2018). Both Baker et al. (2009) and Faurby et al. (2016) found that Cocos and Parajubaea were sister taxa, although they disagreed over relationships immediately beyond this. Eiserhardt et al. (2011a) discussed phylogenetic relationships in the spiny Neotropical Bactridinae, which includes the climbing Desmoncus.
Within Euterpeae Hyospathe is sister to the rest of the tribe (Pichardo-Marcano et al. 2018).
Geonomateae are an important clade of New World palms, mostly understory taxa. For Geonoma, within which it was difficult to get much resolution, and other members of the tribe, see Roncal et al. (2012 and references). Loiseau et al. (2019: 82 spp., 3988 genomic regions, 795 genes) recobvered all genera as monophyletic, except Calypronoma. There were 14 clades, some monotypic, within Geonoma itself, and these largely agreed with those apparent in earlier morphological work, however, matches with infraspecific variation were less apparent; gene trees within species complexes tended to be incongruent (Loiseau et al. 2019).
For a phylogeny of the South American Iriarteeae, see Bacon et al. (2016a); the distinctive clustered inflorescences of some species of Wettinia have evolved several times in parallel.
Calamoideae. For morphology, phylogeny and classification, see W. J. Baker et al. (1999b, 2000a, b, c); there are three main clades, and Eugeissona seemed to be sister to the rest of the subfamily (see also G. Yao et al. 2025). However, this position was not recovered by Baker et al. (2009), while in the species-level supertree of Faurby et al. (2016) Eugeissona was sister to a [Korthalsia + Retispatha, etc.] clade. More recently, Kuhnhäuser et al. (2021: 75 taxa - all genera, etc. - and 971 nuclear markers) recovered the well-supported relationships [Lepidocaryeae [Eugeissoneae + Calameae]] (see also Bellot et al. 2024); the positions of the tribes and most of the subtribes were well supported (see ibid., Fig. 4 for some of the problem areas). Relationships in the largest tribe, Calameae, are [Korthalsia [[Eleiodoxa + Salacca] [Metroxylum [Pigafetta ...]]]] (Kuhnhäuser et al. 2021). Kuhnhäuser et al. (2025: 360/499 species, 354 single-copy nuclear genes) looked at relationships in the rattan palms growing from Sri Lanka to Northeast Australia; all the subtribes of Calameae were recovered as being monophyletic. For relationships in African rattans, see Faye et al. (2016). Torres Jiménez et al. (2021) looked at relationships in Lepidocaryeae and found some incongruences between the various analyses, but there was some support, including morphological, for the relationships [Mauritiinae [Ancistrophyllinae + Raphiinae]] (see also Kuhnhäuser et al. 2021).
Ceroxyloideae. This subfamily includes the erstwhile Phytelephoideae (Dransfield et al. 2005); for relationships, see Faurby et al. (2016), Bellot et al. (2024) and G. Yao et al. (2025). Phytelepheae. Escobar et al. (2021) looked at relationships here.
Coryphoideae. These include Caryoteae, previously placed in Arecoideae (Uhl & Dransfield 1987; see also Dransfield et al. 2008a for a phylogeny). A number of taxa have diversified on islands, and Bacon et al. (2012) discussed the relationships of several island genera in Trachycarpeae. Relationships in Faurby et al. (2016) are [[syncarpous clade] [Sabal [Cryosophileae [Trachymeneae + Phoenix]]]], however, Sabal and Phoenix had switched positions compared with those in W. J. Baker et al. (2009) - remember the Cretaceous fossil Sabal (see above); within the syncarpous clade Corypha moved around. However, Bellot et al. (2020: two nuclear and five plastid regions) found that the entire backbone of the tree, i.e. branches up to genera, and including Corypha, of the syncarpous clade - [Chuniophoeniceae [Caryoteae [Borasseae + Corypheae]]] - was pretty well supported (see also Bellot et al. 2024), but with the exception ofLodoicea and Latanea (Borasseae-Lataninae) - for similar data and relationships, see Kadam et al. (2022). Bellot et al. (2024) recovered vthe relationships [Trachycarpeae [Phoeniceae [Cryosophileae + Sabaleae]]], while Trachycarpeae and Phoeniceae are sister taxa in G. Yao et al. (2025).
Classification. Dransfield et al. (2008b, see also Dransfield et al. 2005) present the classification largely followed here. Arecoideae above are the Arecoideae of Uhl and Dransfield (1987), but minus Caryoteae, and they also include Ceroxyloideae (Hahn 2002b; also W. J. Baker et al. 1999a), basically, the arecoid line of H. E. Moore (1973: see Dransfield et al. 2005). There is a family checklist in Govaerts and Dransfield (2005), and see also the World Checklist of Monocots, Vorontsova et al. (2017) for a checklist of rattans, but the best - and ever improving - resource for the family is PALMweb.
For an expanded circumscription of Calamoideae-Calamus, see W. J. Baker (2015). Kuhnhäuser et al. (2021) provide a classification of the subfamily down to subtribes. Eiserhardt et al. (2022) offer a revised classification of Areceae-Dypsidinae in which Dypsis is divided into three genera.
Previous relationships. The old Spadiciflorae included those taxa with a spadix and often also a spathe. Families included, Pandanaceae, Cyclanthaceae, Araceae and Arecaceae, are now placed in three immediately unrelated orders, Pandanales, Alismatales and Arecales. Engler (1892) linked Pandanaceae, Arecaceae and Cyclanthaceae.
Botanical Trivia. Arecaceae include the tallest monocot, Ceroxylon quindiuense at ca 200 feet (61 m) tall; the plant with the longest unbranched stems, perhaps up to 200 m long in the climber Calamus manan; the plant with the largest leaf - Raphia sp. with blades to ca 25 x 3 m; the largest inflorescence - Corypha umbraculifera, ca 7.5 m long with some 10,000,000 flowers and 5280 m of flower-bearing axes (Tomlinson & Soderholm 1975, but c.f. Furcraea (Asparagaceae) in terms of inflorescence length); and Lodoicea maldavica, with seeds that are up to 50 cm long and weighing 15-30 kg, the largest angiosperm seeds, which in turn produce the longest cotyledon (strictly speaking, an apocole, the elongated, unifacial, non-photosynthetic part of the cotyledonary hyperphyll) which may be "several yards" or "twelve feet or more" long (Thiselton-Dyer 1910). Finally in the vascular system the xylem elements and sieve tubes may remain functional for hundreds of years (for possible secondary thickening, see above) and the xylem includes perhaps the longest vessels, to 3.96 m long in some calamoid palms (Tomlinson & Spangler 2002). They also have close to the oldest viable seeds; a seed of Phoenix dactylifera perhaps 2,000 years old has been germinated (Sallon et al. 2008; see also Kew Magazine, Winter 2008: 28-31).