EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CHLORANTHALES [[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]]]: sesquiterpenes +; (microsporogenesis also simultaneous); seed endotestal.
[[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]] / MAGNOLIIDS / MAGNOLIANAE Takhtajan: (neolignans +); root cap meristem open; vessels solitary and in radial multiples, (with simple perforation plates in primary xylem); (sieve tube plastids with polygonal protein crystals); lamina margins entire; A many, spiral [possible position here], extrorse; G connate by congenital intercarpellary fusion [possible position]; ovules with hypostase, nucellar cap +, raphal bundle branches at the chalaza; antipodal cells soon die.
[CANELLALES + PIPERALES]: flavonols, aporphine alkaloids +; nodes 3:3; G whorled.
Age. Magallón and Castillo (2009: note topology) offer estimates of ca 201 and 128 Ma for this node; ages suggested by Magallón et al. (2013, 2015) are around 137.9 Ma and 130.5 Ma respectively (see also Foster et al. 2016a, q.v. for details, for the first age) and ages in Naumann et al. (2013) are ca 120.3 Ma; at ca 101.4 Ma, the age in Xue et al. (2012) is the youngest, and see also 175.2-126.5(-67.4) Ma (Massoni et al. 2015a) and (196-)171(-146) Ma (Salomo et al. 2017).
Evolution: Divergence & Distribution. Sokoloff et al. (2013d) optimised congenital intercarpellary fusion to this node (ACCTRAN), which would make the apocarpy of many Winteraceae-Winteroideae derived.
Genes & Genomes. A genome duplication, the CANEα duplication, ca 147.1 Ma, is reported to have occurred here (Landis et al. 2018).
Chemistry, Morphology, etc.. Doyle (2007) noted that the venation of members of this group was poorly differentiated and of low rank.
CANELLALES Cronquist - Main Tree.
Neolignans?, drimane-type sesquiterpenes +; primary stem with continuous vascular cylinder; sieve tube plastids with starch and protein crystalloids and/or fibres; petiole bundle(s) arcuate; leaf cuticle waxes as tubules, nonacosan-10-ol the main wax; indumentum 0; foliar sclereids +, branched; branching from previous innovation; flowers of moderate size; K and C distinct; fruit indehiscent, fleshy. - 2 families, 10 genera, 125 species.
Includes Canellaceae, Winteraceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Magallón and Castillo (2009) offer estimates of around 128 and 125 Ma for crown group Canellales, Wikström et al. (2001) ages of (111-)105, 99(-93) Ma, Bell et al. (2010: note topology) ages of (111-)80, 77(-50) Ma, Xue et al. 2012) an age of ca 101.3 Ma, and Magallón et al. (2013, 2015) ages of about 122.8 Ma and 126 Ma respectively; ca 83.9 Ma is the estimate in Naumann et al. (2013), ca 128 Ma that in Thomas et al. (2014), (134-)128, 127(-125) Ma that in S. Müler et al. (2015), ca 111 Ma in Tank et al. (2015: Table S2) and (132-)127(-125) Ma in Salomo et al. (2017); the oldest estimates are (143.2-)132.4, 125.9 Ma (Massoni 2015) and (205-)153(-108) Ma (Lamb & Naidoo 2016).
Grains of the Walkeripollis type have been placed sister to Winteraceae in a constrained morphological analysis (Doyle & Endress 2010), and, dated to ca 126 Ma at the Barremian-Aptian bounday (Massoni et al. 2015b), they provide a minimum crown-group age for Canellales. Note that Silvestro et al. (2021a) estimated the stem ages of Canellaceae as being 59-4-16.5 Ma and of Winteraceae as 185.9-82.9 Ma (see also Sauquet et al. 2021/2022)...
Evolution: Divergence & Distribution. This is not a clade in which there has been much diversification (Tank et al. 2015: Table S1).
Chemistry, Morphology, etc.. Drimane-type sequiterpenoids are practically restricted to Canellales; they are quite diverse in Canellaceae but are relatively uncommon in Winteraceae (Bastos et al. 1999).
For nodal anatomy, see Sugiyama (1979), for general vegetative anatomy, see Metcalfe (1987), and for a comparison of general embryology and seed coat anatomy, see Tobe and Sampson (2000).
Phylogeny. For relationships in the order, see e.g. Massoni et al. (2014), Helmstetter et al. (2024).
Synonymy: Winterales Reveal - Winterineae Shipunov - Winteranae Doweld - Winteridae Doweld
CANELLACEAE Martius - Back to Canellales —— Synonymy: Winteranaceae Warburg
Vessel elements with scalariform or reticulate perforation plates; true tracheids +; sieve tube plastids with peripheral protein fibrils; leaves spiral or two-ranked; inflorescence variable; receptacular cortical vascular system +; flowers single, with several bracts, red; K 3, C (4-)5-12, free (connate); A 6-12(-many), connate, (connective little produced); nectar from filament tube; pollen (trichotomosulcate), ektexine microreticulate to psilate granular [Cinnamosma intectate]; G [2-6], also occluded by secretion, compitum +, placentation parietal, style short, stigma lobed; compitum +; ovules 2-many/carpel, campylotropous, micropyle bistomal, zig-zag, outer integument 4-8 cells across, parietal tissue?, nucellar cap 0; fruit a berry, K persistent, exotesta sclerosed, rest undistinguished, (?pachychalazal - Cinnamosma); endosperm ruminate or not, development?; n = 11, 13, 14, x = 7, nuclear genome [1 C] (0.143-)1.677(-19.628) pg.
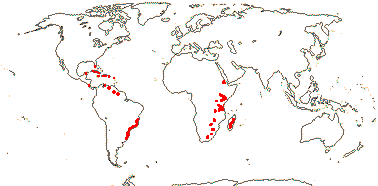
5[list]/23: Cinnamodendron (8). Tropics; U.S.A. (S. Florida), Antilles, South America, E. Africa, Madagascar (map: from Jackeline Salazar Lorenzo). [Photo - Flowers]
Age. Crown-group Canellaceae are (59-)49, 41(-23) Ma (S. M¨ller et al. 2015), (64.9-)45.5(-31.2) Ma (Lamb & Naidoo 2016), or (62-)36.2, 31.3(-11.3) Ma (Massoni et al. 2015a).
Evolution: Divergence & Distribution. 49 Ma Early Eocene wood from SW Wyoming has been identified as belonging to Canellaceae (Boonchai & Manchester 2012).
Dispersal has been invoked to explain the distribution of the family (Sanmartín & Ronquist 2004: age ca 121 Ma).
S. Müller et al. (2015, also dates) discussed the diversification of the family.
Ecology & Physiology. For vascular flow in Canellaceae, see Hudson et al. (2010) and Feild et al. (2011: Canella winterana is a little odd).
Pollination Biology. What little is known about pollination biology was summarized by Gottsberger (2016a).
Chemistry, Morphology, etc.. The floral formula of Warburgia is K3, C 5 + 5, A 5 + 5, G [5] - see Sokoloff et al. (2015a] - but are the sepals "really" bracts - perhaps unlikely? - and/or bracteoles (for the latter, see Hiepko 1964). There has been discussion on the insertion of the petals, whether spiral or whorled, and if whorled, the number of whorls; Wilson (1966) also noted that the sepals have two traces and the petals three, although inner petals may have only a single trace. A vestigial aril has been reported from some genera (Igersheim & Endress 1997).
Some general information is taken from Kubitzki (1993b) and Salazar et al. (2020); for chemistry, see Hegnauer 1964, 1989, 1990 - the last under Winteraceae), also Bastos et al. (1999: drimane-type sequiterpenoids) and Mandal (2014: variation in stomatal morphology).
Phylogeny. For a phylogeny of Canellaceae, see Salazar and Nixon (2008) and S. Müller et al. (2015); Cinnamodendron is polyphyletic and Canella is sister to the rest of the family.
WINTERACEAE Lindley - Back to Canellales
(Plant Al accumulator); vessel elements 0; rays 1-2 and 3-10+-seriate, heterocellular, with sclerotic nests, oil cells +; (petiole bundles complex); leaves spiral, lamina vernation supervolute; inflorescence cymose; "K" 2, connate and forming calyptra, calyptra splitting early, with 2+ traces, "C" with 1-3 traces, (outer members connate); A spiral, 3-many, (subintrorse), filaments stout, expanding during anthesis, (connective prolonged), both secondary parietal cell layers dividing, endothecium biseriate [fibrous outer layer of middle wall layer]; pollen in tetrads, acalymmate [each grain has separate exine], (monads), with distal pore [ulcerate], (trichotomocolpate), reticulate, muri with columellae; stigma bilobed, (not decurrent); ovules (1-)5-many/carpel, nucellar cap 2-3 cells across; endostome persistent, cells thick-walled, outermost enlarged; remains of calyptra under fruit; seed with palisade exotesta, (tegmen subfibrous); x = 13 (?12, ?9).
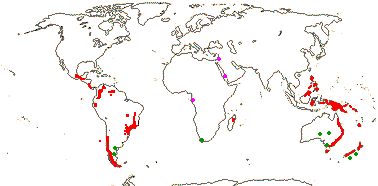
5[list]/105 - two groups below. Montane tropics, not mainland Africa. Map: from Vink 1993; Marquínez et al. 2009 [New World]: for fossil localities outside the range of extant members of the family, see Doyle (2000b) and Grímsson et al. (2018) - records in the former but not the latter have an asterisk - mauve = early Cretaceous, green = late Cretaceous to Caenozoic - if apparently in the sea, really in adjacent red areas...
Age. Some estimates of the divergence of Takhtajania from other Winteraceae are as low as (65.1-)47.4, 34.3(-12.1) Ma (Massoni et al. 2015a), (53-)46, 41(-34) Ma (Wikström et al. 2001), or even less, (27-)18.5(-11) Ma (Bell et al. 2010: note topology). However, Marquínez et al. (2009) suggested that Takhtajania diverged from the rest of the family ca 135 or 120 Ma, an age of (120.5-)91.5(-74.5) Ma is given by Thomas et al. (2014) and one of (91-)62, 49(-28) Ma by S. Müller et al. (2015).
Pollen tetrads about 122.5 Ma old from Gabon have been assigned to Winteraceae, while other slightly younger, common, and more widespread pollen types are rather more certainly associated with the family (e.g. Dettmann & Jarzen 1990: Fig. 9). Doyle et al. 1990a; Doyle 1999). The earlier Cretaceous records are of calymmate tetrads, that is, the ectexine forms a continuous covering over the tetrad, that have fine ornamentation, and grains of this type (e.g. Walkeripollis: Schrank 2013 and references) have been placed sister to Winteraceae in a constrained morphological analysis (Doyle & Endress 2010), and they are dated to ca 126 Ma at the Barremian-Aptian bounday (Massoni et al. 2015b; see also Grímsson et al. 2018).
1. Takhtajanioideae Leroy - Takhtajania perrieri (Capuron) M. Baranova & J. Leroy —— Synonymy: Takhtajaniaceae J.-F. Leroy
Lignification in stem diffuse; reaction wood 0; inflorescence terminal, axis well developed; flowers red; "K" with four more members; anther thecae transverse; tetrads 65-83 μm in diameter; G [2], collateral, placentation parietal, placentae subapical, oblique to horizontal, stigma large, cap-shaped; compitum +; outer integument 4-5 cells across, (inner integument 4 cells across), parietal tissue 5-6 cells across; fruit ?"septifragally" dehiscent; n = 18.
1/1. Madagascar.
2. Winteroideae Arnott —— Synonymy: Drymidaceae Baillon
Sesquiterpene dialdehyde cinnamates +; reaction wood abaxial, (tracheid walls vestured); sieve tubes with non-dispersive protein bodies; (petiole bundles several, arrangement complex); (hairs +); stomata anomocytic, apertures often occluded by wax-cutin plugs; inflorescence axillary (terminal), axis not developed; flowers ± white; "K" with 0-4 or more members, (enclosing the flower until anthesis), "C" 2-15; A (>7), (anther thecae transverse); tetrads 25-55 μm in diameter; post-zygotic incompatibility system [?all]; G (1-)5-10(-many), free (connate), with ± elongated paired stigmatic crests; outer integument 3-4 cells across, inner integument 2(-3) cells across, parietal tissue 2-5 cells across, hypostase +; fruit berrylet (follicle), (K deciduous); cotyledons convolute [Drimys]; n = 13 (19, 43).
4/105: Tasmannia (ca 50), Zygogynum (ca 50). New Guinea to New Zealand and New Caledonia, few Borneo, the Philippines and South America, inc. Juan Fernandez I., usu. not tropical lowlands. [Photo - Flower.]
Age. Crown-group Winteroideae have been dated to 92.4-77 Ma by Marquínez et al. (2009), (74.8-)69.9(-66.9) Ma by Thomas et al. (2014) and to ca 45 Ma by S. M¨ller et al. (2015).
Evolution: Divergence & Distribution. Fossils assigned to Winteraceae have a much wider distribution than that of the family today, being scattered in Africa and Israel (Dettmann & Jarzen 1990; Doyle 2000b; Friis et al. 2011; Grímsson et al. 2018: see map above). Poole and Francis (2000) identified ca 84 Ma wood from the Antarctic Peninsula as Winteroxylon, a genus also known from deposits ca 52.2 Ma from Chubut, Argentina, and the latter fossils are quite similar to the wood of Zygogynum, now known from New Caledonia, etc. (Brea et al. 2021). Winteroxylon has also been found in Upper Eocene deposits from Germany (see also Atherospermataceae). Fossil wood has also been reported from the end-Cretaceous Maastrichtian of California (Vink 1993 for literature); for early pollen records, see above, Bubbia-type pollen has been reported from southwest South African deposits only some 15 Ma (Coetzee & Muller 1984). Pollen identified as Pseudowinteropollis is known from end-Cretaceous sediments ca 67-65.5 Ma from Central Australia (Carpenter et al. 2015; not mentioned by Grímsson et al. 2018). For other ages in Winteraceae, see Marquínez et al. (2009: focus on Drimys and New Caledonian Zygogynum) and Thomas et al. (2014: focus on Australian species).
Thomas et al. (2014) suggested that Takhtajania showed a Gondwanan distribution, rather unusual in flowering plants; they thought that the genus originated on Madagascar as the Seychelles-Madagascar-India area separated from the rest of Gondwana (but c.f. Sanmartín & Ronquist 2004: dispersal involved; S. M¨ller et al. 2015 in part). Wallis and Jorge (2018) discuss Pseudowintera on New Zealand; its distribution there is quite ancient. However, Grímsson et al. (2018) note the wide distribution of the family on both hemispheres in the past (see map above) and not unreasonably suggest that a focus on Gondwana is too narrow.
Feild et al. (2012: Fig. S1) estimated divergence began within Tasmannia (T. lanceolata splitting from other Australian and New Guinean species) at 59.1-49.2 Ma, which conflicts with some of the ages above, although estimates in Thomas et al. (2014) are only ca 32.5 Ma. There has been a major radiation of the genus in New Guinea in particular (ca 30+ species), with morphologically and ecologically very distinctive species growing together and at most rarely hybridising, and there are yet more species in Borneo and Australia.
Ecology & Physiology. Most Winteraceae grow in moist, cool environments, over a wide range of altitudes and habitats, and they range from rhizomatous subshrubs to 30 cm tall to trees, they are sometimes epiphytes and even include the odd liane (Tasmannia cordata, no vessels - Feild et al. 2012). The occlusion of the stomatal apertures by stomatal plugs that is common in Winteroideae may keep the apertures open when humidity is high and the surface is otherwise wet; the rest of the lower surface has an elaborate waxy cuticle (Feild et al. 1998, 2000a). For vascular flow in Winteraceae in conditions where freezing may occur, see Feild et al. (2002); their tracheids are relatively immune to freezing-induced xylem embolism, particularly when compared to the vessel-containing wood of Canella.
Both compression and tension wood - and their complete absence - have been reported from various species of Winteraceae (Westing 1965; Timell 1986, vol. 1, chap. 8; Aiso et al. 2016 and references). Overall, it seems likely that there is reaction wood, generally (but not always) abaxial, but lacking the characteristics of compression or tension wood (Kucera & Philipson 1977, 1978; see also Nawawi et al. 2016). For a general discussion about reaction woods in seed plants, see elsewhere.
Pollination Biology & Seed Dispersal. The flowers of some Winteraceae are thermogenic (Seymour 2001). Nectar may be produced by the filaments, and pollination is by a diversity of small insects, including thrips, and a variety of dipterans and small beetles, and in at least some cases the animals eat the stigmatic exudate (e.g. Thien 1980; Lloyd & Wells 1992; Erbar 2014; Gottsberger 2016a and references). In New Caledonia adults of the primitive jawed moths, Sabatinca (Micropterigidae), a clade up to >210 Ma and sister to all other Lepidoptera [Wahlberg et al. 2013]), eat the pollen of Zygogynum; the tetrads are covered by much oily pollen-kitt which makes them stick to the moths. Sabatinca also uses the flowers as a place of assembly prior to mating (Thien et al. 1985: see also Nothofagaceae). Sabatinca on New Caledonia is older than the estimated time of final emergence of the island some (40-)37(-34) Ma, that of Zygogynum itself a little younger (Nattier et al. 2017 and references; c.f. Heads 2018 for a different take on the biogeography of the island). Adults of other jawed moths eat pollen and spores of a variety of other plants, including asterids and ferns (Imada et al. 2011 and references).
Tasmannia is usually dioecious.
Genes & Genomes. The substitution rate of ITS rDNA seems to have been notably slow here (Suh et al. 1993).
See Ehrendorfer and Lambrou (2000) for chromosome numbers.
Chemistry, Morphology, etc.. Takhtajan (1997) suggested there are no alkaloids; Cronquist (1981) claims that there are at least some. Sesquiterpene dialdehyde cinnamates are known only from Winteroideae, and there are prenylated flavanones in Pseudowintera (Larsen et al. 2007).
Keating (2000a) found that the leaf traces may sometimes have paired vascular bundles. The pericyclic sclerenchyma is of various origins (Metcalfe 1987). The petiole vasculature is often quite complex (Keating 2000a and references). For the stomatal morphology of Takhtajania, see Baranova (2004a), but c.f. Vink (1988) and Keating (2000a: brachyparacytic).
Deroin (2000) noted that there are no cortical bundles in the flower. Doust (2000) and Marquínez (2014) described the complexity of phyllotactic patterns in the perianth; the latter also described the sepals as being lateral in position; Doust (2000) did not mention bracteoles. Petals of Drimys s.l. and some other taxa have either one or three vascular bundles at the base (see also Canellaceae), as have the sepals, but only a single trace leaves the stele (Endress et al. 2000). The carpels of Tasmannia may be plicate. For the fruit anatomy of Takhtajania, see Deroin (2000).
See Vink (1985, 1988, 1993: general), papers in Ann. Missouri Botanical Gard. 87(3). 2000, all focussing on Takhtajania, also Bailey and Nast (1945: comparative studies), Hegnauer (1973, 1990: chemistry), Nast (1944) and Tucker (1959), both floral vasculature, Praglowski (1979), van der Ham and van Heuven (2002), Sampson (2007) and Grímsson et al. (2017d: see also supplementary material), all pollen, Svoma (1998b: ovules), Bhandari (1963) and Bhandari and Venkataraman (1968), both embryology, and Floyd and Friedman (2000: endosperm development), for further information.
Phylogeny. For the phylogeny of Winteraceae - Drimys s.l. is paraphyletic and the limits of Zygogynum are to be extended - see Suh et al. (1993), Karol et al. (2000), Doust (2003), Doust and Drinnan (2004) and Marquínez et al. (2009). Morphological analyses by Endress et al. (2000) suggested that Takhtajania, which they thought might be polyploid, was more or less associated with Pseudowintera within Winteraceae (c.f. Vink 1988: Takhtajania sister to the rest). More recent studies suggest the well-supported relationships within Winteroideae of [Tasmannia [Drimys s. str. [Pseudowintera + Zygogynum]]] (Thomas et al. 2014; see also Seed Plant Tree of Life, version i.2022).
Classification. Is Drimys piperita the only species in Malesia, with Drimys also in S. America, or should there be two genera - Drimys (flowers perfect: South America), and Tasmannia (plant dioecious: Australia-Malesia)? A generic distinction between the Old and New World taxa is strongly supported (see above).
Thanks. To Susanne Renner, for useful comments about fossil Winteraceae in particular.