EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID II / CAMPANULIDAE / [METTENIUSALES [BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]]]: myricetin 0; style shorter than the ovary; endosperm copious, embryo short/very short.
[BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]] / APIIDAE: tube initiation early; G inferior, [2], style long.
[ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]] / CORE CAMPANULIDS: C forming distinct tube; A epipetalous.
[APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]: ?
If the next two are clades...
[APIALES [PARACRYPHIALES + DIPSACALES]] / DIPSAPIIDAE: nodes 3:3.
[PARACRYPHIALES + DIPSACALES] / DIPSIDAE: true tracheids +; nodes 3:3; lamina margin serrate; inflorescence terminal.
Phylogeny. For discussion on the relationships of Dipsacales, see the asterid II/gentianid clade.
DIPSACALES Berchtold & J. Presl - Main Tree.
Route I secoiridoids +; vessel elements with scalariform perforation plates; nodes 3:3; petiole bundles arcuate; colleters 0; buds perulate; leaves opposite, margins gland-toothed, bases ± confluent, (amplexicaul); inflorescence with terminal flowers, branches cymose; A 5; pollen grains tricellular; G [3], at least partly inferior; ovules apotropous; fruit indehiscent, wall with fibrous layers perpendicular to each other and thick walled sclereids, containing crystals, K persistent; testa vascularized, exotestal cells enlarged, cuboid/rectangular, variously thickened and lignified, seed coat 1-21 μm across; endosperm without haustoria, cells thin-walled, not differentiated; x = 9. - 2 families, 46 genera, 1,090 species.
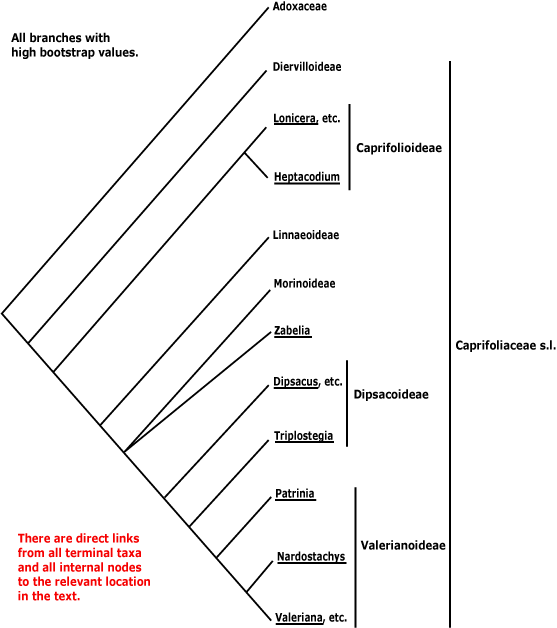
Includes Caprifoliaceae, Viburnaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The first split within crown-group Dipsacales may have occurred in the mid-Cretaceous, some 111-102 Ma, the oldest of the estimates (Bell & Donoghue 2005a and references; Lee et al. 2021); H.-X. Wang et al. (2019) estimate ca 103 Ma, but from the tree in their Fig. 6 it would have to be 110 Ma or a little more, while using nuclear data H.-X. Wang et al. (2021) estimate an age of 78.9 Ma. The age has also been estimated at (90-)62(-32) Ma in Lemaire et al. (2011b), who also unaccountably note "more recent stem node ages" of 31 Ma for Dipsacales, which in the context of their tree would be the age of the [Apiales [Paracryphiales + Dipsacales]] clade, and which they dated at (105-)84(-58) Ma. K. Bremer et al. (2004a) date the beginning of diversification here at 101 Ma, Wikström et al. (2001) at (87-)82, 78(-73) Ma, Beaulieu et al. (2013a: 95% HPD) at (94-)80(-87) Ma, Magallón et al. (2015) at around 70.9 Ma, Bell et al. (2010) at (74-)60, 57(-46) Ma, and N. Zhang et al. (2012), (82-)68(-45) My; ca 77.5 Ma is the age in Nicolas and Plunkett (2014; ?sampling), around 75.6-62.0 Ma (Nylinder et al. 2012: suppl.), (94-)80(-65) Ma in Wikström et al. (2015), ca 90.8 Ma in Tank et al. (2015: Table S2) and ca 99.3 Ma (C. Zhang et al. 2020).
Evolution: Divergence & Distribution. Magallón and Sanderson (2001) described this as a very species-rich clade (a combination of age and size). The diversification rate may have increased in Dipsacales at around (81.8-)75.8(-70.9) Ma (Magallón et al. 2018). The origin of Dipsacales was probably in the northern hemisphere (Beaulieu et al. 2013a). Major diversification within both Dipsacoideae and Valerianoideae - and hence a major part in terms of species numbers of diversification of the whole clade - is relatively recent, occurring within perhaps ca 10 Ma (Bell & Donoghue 2005a); diversification in Dipsacales as a whole has often seemed to increase as plants moved into new geographical areas, especially if these were mountainous (Moore & Donoghue 2007).
For a very detailed discussion of morphological evolution, see Donoghue et al. (2003); Jacobs et al. (2010b) discussed the evolution of characters of fruit anatomy, and Beaulieu and O'Meara (2016) looked at links between fruit type and diversification - the story is more complicated than in Beaulieu and Donoghue (2013) .Xu et al. (2011) optimised some pollen characters on the tree. Howarth and Donoghue (2004, esp. 2005, 2008, 2009) and Howarth et al. (2011) noted intriguing correlations between gene duplications with possible neofunctionalisation of e.g. Cycloidea-like genes and changes in floral form in the order, particularly in Caprifoliaceae, with concomitant restriction of expression of some genes in the petaline whorl and resultant changes in the symmetry of the flowers (see also Caprifoliaceae). Nevertheless, direct connections to diversification rates remain to be established.
Genes & Genomes. For cytological variation, see Benko-Iseppon and Morowaetz (2000a); the two families are rather different. There is considerable variation in the presence of the mitochondrial coxII.i3 intron. Rates of molecular evolution vary considerably in Dipsacales, and this is usually associated with changes in the life style, woody plants usually having lower rates of change than herbaceous (Smith & Donoghue 2008).
Chemistry, Morphology, etc.. Anthocyanin variation does not allow a sharp distinction between Caprifoliaceae and Viburnaceae (Jordheim et al. 2006).
The periderm of Sambucus is cortical (Rudall 2020). Involute vernation of the lamina is commoner in Viburnaceae than in Caprifoliaceae (Cullen 1978). For the morphology of the stipule-like structures and amplexicaul leaves in Caprifoliaceae and Viburnaceae see Weberling (1957); amplexicaul leaves, perhaps most common in the inflorescence, are scattered throughout the order. Split-lateral bundles and a girdling vascular trace occur in taxa with perfoliate leaves (e.g. Colomb 1887) as well as in the Morinoideae-Dipsacoideae area (see below). Glands at the base of the leaf in Sambucus and Viburnum may be vascularized (see also Colomb 1887).
Troll and Weberling (1966) and Weberling (1961, 1989) described the inflorescence structure of the woody Dipsacales in particular in considerable detail; inflorescence structure is notably complex in Caprifoliaceae, although Wagenitz and Laing (1984) suggest that most Caprifoliaceae s.l. lack terminal flowers. The morphology of the basic nectary type for the order is unclear. In Viburnum it is a disc-like structure on top of the inferior ovary, in Adoxa it consists of multicellular hairs on the corolla, and in Caprifoliaceae, unicellular hairs (Wagenitz & Laing 1984, Takhtajan 1997; Leins & Erbar 2010; Erbar 2014). For variation in floral anatomy, which clearly separates two groups of genera in the old Caprifoliaceae, see Wilkinson (1949 and references). Interestingly, some taxa in both families may have one or more ovary loculi infertile, the fertile loculus/i having but a single ovule, however, the reduced loculi may have two to six or so ovules. Particularly when there are only three carpels, their orientation is unclear (Sokoloff et al. 2018). For more on the inferior/superior ovary distinction in the asterid II clade, see e.g. the Asterales page.
Phylogeny. For the relationships of Dipsacales, see the asterid IIclade.
The order has strong support in D. Soltis et al. (2000: see also Wagenitz 1997; K. Bremer et al. 2001; c.f. A.P.G. 1998). The phylogeny here is based especially on work by Donoghue et al. (2001b, 2003), W.-H. Zhang et al. (2003), Davis et al. (2001), Bell (2003), Moore and Donoghue (2007), C.-L. Xiang et al. (2019: plastome analyses) and Lee et al. (2021: esp. nuclear analyses). There are some (not major) disagreements with the phylogeny in Pyck and Smets (2001), while Judd et al. (1994) provide an early morphological analysis which gives a similar topology.
Previous Relationships. This grouping has often been recognised. Takhtajan (1997) included Dipsacales, Adoxales and Viburnales in his Dipsacanae, while Cronquist 1981) recognised four families in his Dipsacales.
Thanks. I am very grateful to Volker Bittrich for catching a number of mistakes on this page.
Synonymy: Dipsacineae Shipunov - Adoxales Nakai, Caprifoliales Berchtold & J. Presl, Lonicerales T. Leibe, Sambucales Berchtold & J. Presl, Valerianales Berchtold & J. Presl, Viburnales Dumortier
VIBURNACEAE Rafinesque, nom. cons. - Back to Dipsacales
0-methylated flavonols only +; true tracheids?; pericyclic sheath poorly developed; (stomata paracytic); (petiole also with adaxial inverted bundles; closed C-shaped and with wing bundles); palisade mesophyll with arm cells; glandular hairs common; (buds not perulate); flowers small [<5 mm across]; K open, sepals with one trace, C rotate; pollen reticulate; G not entirely inferior, lateral and dorsal carpellary bundles free, ventral bundles move from axis to ovary septae, style short, stigma (long-)lobed, dry or wet; ovule 1/carpel, median; fruit drupaceous, stone compressed; seed single, antiraphe bundle +, exotestal cells palisade or not, not lignified; x = 9, chromosomes large, 2.6-10 µm long, centromeres subterminal, interphase nucleus (semi)reticulate, euchromatic granules coarse, chromocentres large, distribution irregular, mitotic condensing behaviour continuous, telomeric areas in particular of mitotic chromosomes condensed when col, nuclear genome [1 C] (0.109-)3.04(-85.14) pg; plastome ndhF gene inverted.
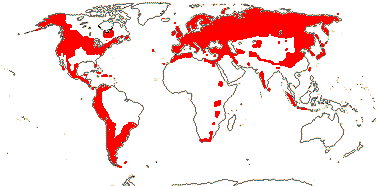
5 [list]/200 - two tribes below. North temperate, tropical montane, S.E. Australia, rare in Africa (map: from Hultén 1958, 1971; Meusel & Jäger 1992). 2 groups below. [Photos - Collection]
Age. Crown Viburnaceae are around (67-)60, 57(-50) Ma (Wikström et al. 2001), 85-70.5 Ma (Bell & Donoghue 2005a; see also Lens et al. 2016), or (54-)33, 31(-13) Ma (Bell et al. 2010).
1. Viburneae O. Berg - Viburnum L. —— Synonymy: Tinaceae Martynov
Shrubs (trees), deciduous or evergreen; hairs stellate and variants; lamina vernation involute or conduplicate (plicate), (aestivation convolute), secondary veins palmate to pinnate, (margin entire); (plant dioecious); G [3], odd member abaxial, 2 abort, nectary on top of G, epithelial; ovule with parietal tissue ca 1 cell across; (embryo sac bisporic [the chalazal dyad], 8-nucleate [Allium type]); endocarp with layers of fibres oriented parallel to each other, 1+ layers of sclereids; seed coat 21-100 µm across; endosperm ruminate, with crystals, cell walls ± thickened, embryo short, chlorophyllous; n = 9-11, 16, etc..
1/175. North temperate, tropical montane, but not in Africa. [Photo - Flower.]
Age. Crown-group Viburneae are ca 80 or ca 55 Ma, depending on calibration (Spriggs et al. 2015), some (63-)56(-50) Ma (Lens et al. 2016), ca 89.3 Ma (H.-X. Wang et al. 2019) or 58-42 Ma (Lee et al. 2021); see cautionary comments by Beaulieu and O'Meara (2018).
2. Adoxeae Dumortier —— Synonymy: Adoxaceae E. Meyen, nom. cons., Sambucaceae Borkhausen
Shrubs or herbs; (cambium storied - Sambucus); (vessel elements with simple perforation plates); stem with endodermis [Adoxa]; (calcium oxalate crystals 0 - Adoxeae); (nodes 1:1, 5:5), (+ split laterals - Sambucus); leaves compound (deeply lobed), (extrafloral nectaries +), (pseudostipules on leaf base); (flowers 3-4-merous); (lateral flowers of cymule with odd K abaxial); (K 2, 3 develop - Adoxa); anthers extrorse (8, monothecal); nectary on stigma, on C as multicellular hairs, or 0; G 1, [(4), 5] [opposite C - Sambucus], (all fertile), styles separate, stigmas capitate, dry [Ad]; integument ca 4 cells across, (ovules lacking parietal tissue - Adoxa); embryo sac tetrasporic, eight nucleate [Adoxa-type]; endocarp with three layers of cells, 2 layers of fibres, 1 layer of sclereids; (embryo long [Sambucus] or short); n = (8-)9.
4/23: Sambucus (to 25!). Mainly north temperate, esp. China, also tropical montane (few in Africa), S.E. Australia.
Age. Crown-group Adoxeae are estimated to be around 35.5-32.5 Ma (Bell & Donoghue 2005a) or (66-)58.5(-49) Ma (Lens et al. 2016).
Evolution: Divergence & Distribution. The evolution and diversification of Viburnum has been much studied over the last twenty years or so especially by M. J. Donoghue and associates. A number of deep branches within the genus are occupied by taxa that are currently tropical (they used to be in section Megalotinus - polyphyletic), and the dioecious Malesian V. clemensiae is sister to the rest of the genus (Clement & Donoghue 2011; Clement et al. 2014; Spriggs et al. 2015). For Spriggs et al. (2015), diversification in Viburnumwas driven by extrinsic traits such as the occupation of mountainous regions (in South America and the eastern Himalayas) and shifts between cooler and warmer temperate forests rather than by intrinsic traits. They were inclined to suggest a tropical origin for Viburnum (warm evergreen forests - Landis et al. 2020), while Lens et al. (2016) thought that cool temperate conditions with freezing were likely in its place of origin. There may have been several shifts from evergreen leaves with elliptical blades and entire margins to deciduous leaves with more rounded blades and serrate margins, and there is also very substantial size variation (Schmerler et al. 2012), and leaves with very different morphologies are produced during the course of a single season's growth (preformation vs. neoformation); this heteroblasy can be quite strongly developed. Clement et al. (2014: no out-group) discuss the evolution of these and other such traits, while Spriggs et al. (2017) show that a) there has been quite frequent evolution of wider leaves, whether broadly ovate or trilobed, and b) the evolutionarily novel leaf forms are interpolated at the beginning of the season's growth - i.e. this kind of recurrent ontogeny does not recapitulate phylogeny... Donoghue et al. (2022) noted that there had been parallel evolution of leaf morphology in 9/11 disjunct cloud forest areas from Mexico to Argentina in the Oreinotinus clade.
Park and Donoghue (2021) suggested that there had been four origins of sterile marginal flowers in Viburnum, all in some ways associated with cooler climates and making the infloresecnces in some ways more like flowers. Spriggs et al. (2015) found similar increases in diversification as did Beaulieu and O'Meara (2016) in their rexamination of links between fruit type and diversification. Although most Viburnum are dispersed by birds, there are neverthess distinct fruit syndromes within the genus differing in colour, nutritional content, etc.; the fruits in an inflorescence may also develop synchronously or sequentially (Sinnott-Armstrong et al. 2020). The evolution of one-seeded fruits in Viburnum seems to be connected with increased speciation rates (Moore & Donoghue 2009). Weber and Agrawal (2014) suggested that the evolution of extra-floral nectaries was associated with an increase in diversification rate. For more on possible apomorphies within the family, see Donoghue et al. (2001b, 2003), while B. Jacobs et al. (2008, 2010b) discuss fruit and seed characters.
Ecology & Physiology. Lens et al. (2016) looked at xylem evolution in the family with a focus on Viburnum. That genus has tracheids, however, both Sambucus, which has vessels and the xylem of which differs greatly in other respects from that of Viburnum, and Viburnum itself have similar climatic niches, at least given present-day distributions; perhaps these differences reflect past climates/events (Lens et al. 2016).
Pollination Biology & Seed Dispersal. The drupaceous fruits of woody members are dispersed by birds (for Viburnum, see Sinnott-Armstrong et al. (2020).
Plant-Animal Interactions. Extrafloral nectaries are notably common (Weber & Keeler 2013).
Genes & Genomes. Moegelein et al. (2020) looked as chromosome number and genome size in Viburnum where there has been quite frequent polyploidy - and also some reductions in chromosome number.
The duplication of the Cycloidea gene has occurred within Viburnaceae, e.g. in Viburnum plicatum (Howarth et al. 2011), a species with large, monosymmetric marginal flowers that serve as "petals" for the inflorescence.
There is little large-scale variation in the plastome (Ran et al. 2020).
Chemistry, Morphology, etc.. Tannin-secreting tubes in Sambucus are coenocytic and up to 32.8 cm long, they span the length of an entire internode, but do not extend into the nodal region (Zobel 1986). Viburnum may lack a fibrous pericyclic sheath and that of Sambucus is interrupted, however, that of Caprifoliaceae is better developed, and the cells in the latter are also longer, except those of Linnaea (Cooper 1939). Sambucus has crystal sand. In Adoxa the leaves at the bases of the rosettes and on the rhizomes are spiral and there are no extrafloral nectaries (c.f. Sambucus).
There is considerable floral variation in Sambucus and in particular Adoxa and its immediate relatives. Adoxa itself lacks any obvious corolline ring primordium and it has eight stamens with monothecate anthers, nectary on the petal lobes, weak endothelium, etc. (Erbar 1994). There are five semi-superior carpels with five styles/styluli (Leins 2000) and there are initially five stamen primordia. Reports of an amoeboid tapetum are incorrect (e.g. Wangenitz & Laing (1984). In the recently-described Sinadoxa the stamens appear to be divided into two half stamens, each with a separate filament and a monothecal anther; it is molecularly close to Adoxa (Liu et al. 2000). The flowers have three corolla lobes and a single carpel (Donoghue et al. (2003). In another curious genus, Tetradoxa, the half anthers are peltate, there are separate styles (= styluli), the ovary was described as being superior, and the sepals are persistent (Ying et al. 1993).
The ovules in Sambucus may be attached to the axis below the level of insertion of the sepals (Roels & Smets 1994), although they are also shown as being attached slightly above (Thomé 1889, pl. 555). In both Sambucus and Adoxa there are small groups of meristematic cells above the ovules which may represent aborted ovules (Roels & Smets 1994). Haig (2020) discusses the development of the Adoxa embryo sac in Adoxeae, the only place where this kind of embryo sac is known to occur (but only those two genera of the tribe have been examined). As Haig (ibid., pp. 7, 8) noted, "synergids are derived from a different megaspore nucleus than the egg nucleus and upper polar nucleus" and went on to suggest that "most cells of Adoxa and Sambucus embryo sacs are potential eggs and dedicated synergids are absent" - very odd.
For general information, see Hara (1983: Japanese taxa) and Backlund and Bittrich (2016), for some nodal anatomy, see Neubauer (1977a), for arm cells, see Wagenitz and Laing (1984), for sympetaly, see Reidt and Leins (1994), for floral anatomy and morphology, Wilkinson (1949 and references), for nectaries, see Vogel (1997), for some embryology, see Lagerberg (1909: Adoxa), Horne (1914) and Moissl (1941), and for fruits and seeds, see Jacobs et al. (2010b).
Phylogeny. For a phylogeny of Viburnum, see Winkworth and Donoghue (2004, 2005), Schmerler et al. (2012), Clement et al. (2014), Eaton et al. (2017) and C.-L. Xiang et al. (2019: plastome analyses). Ran et al. (2020) could not confirm the monophyly of sections Megalotinus and Odontotinus in their plastome analyses, although support values in that part of the tree could be higher, while relationships in the genus wre unstable in the analyses shown by Lee et al. (2021.
Classification. Wilson (2016) discusses the use of the name Viburnaceae, rather than Adoxaceae, for this clade.
CAPRIFOLIACEAE Jussieu, nom. cons. - Back to Dipsacales
Bark papery-flaky; O-methylated flavones, flavonols +; cork cambium deep-seated; wood often fluorescing; (pith diaphragms +); pericyclic fibres moderately developed; inflorescence axis racemose, (branched), with ultimate units cymes; K shortly connate, C 2 + 3, tubular, main petal veins laterally connected [transpetalar veins], nectary on C, of unicellular hairs, with irregularly rugose surface; anthers sagittate; tapetum amoeboid; pollen grains spheroid, echinate; ovary inferior, constricted below K, lateral and dorsal carpellary bundles 0, ventral bundles in axis, style usu. longer than C, stigma capitate, wet; embryo small; x = 9, chromosomes small, 0.6-4.2 µm long, centromeres median or submedian, no C-banding, interphase nucleus semireticulate, euchromatic granules fine, chromocentres small, regular or polarized, mitotic condensing behaviour (continuous to) proximal-early, no condensation of mitotic chromosomes when cold, nuclear genome [1 C] (0.093-)1.371(-20.12) pg; plastid transmission biparental; duplication of dipsCYC2 and dipsCYC3 genes, horizontal transfer of mitochondrial rps11 gene [?Morinoideae and upwards in the tree].
31 [list: as subfamilies]/890 - 7 groups below. Largely temperate/warm temperate in the northern hemisphere, some montane tropics (esp. Valerianoideae), but in neither the Antipodes nor the Pacific.
Age. An estimate of the crown-group age of Caprifoliaceae is 85.8±5.2 Ma (Bell & Donoghue 2005a); K. Bremer et al. (2004a: c.f. topology, Lonicera sister to rest) suggested an age of around 75 Ma, Bell et al. (2010: again, c.f. topology) an age of (40-)37, 36(-35) Ma, Wikström et al. (2015) an age of (76-)62(-49) Ma, while (82.3-)66.1(-50.8) Ma is the estimate in Tank and Olmstead (2017); on the other hand, H.-X. Wang et al. (2019) suggest an age of (119.5-)100.5(-67.4) Ma while estimates in H.-X. Wang et al. (2021) vary, some, based on nuclear data, being as low as (69.4-)66.7(-56.3) Ma, estimates here and elsewhere based om chloroplast data tending to be older.
1. Diervilloideae Rafinesque —— Synonymy: Diervillaceae Pyck
Deciduous shrubs; (successive periderms +); lamina vernation involute [Weigela]; (K monosymmetric); nectary at base of C; filaments hairy; pollen pororate, membrane granulose; G [2]; ovules many/carpel, ?marginal; fruit beaked, dehiscing laterally, septicidal; seeds many, (winged); exotesta with outer periclinal cell walls not lignified; n = 18.
1-2/16. East Asia, S.E. U.S.A.. Map: from Li (1952, approximate).[Photo - Flowers © M. Clayton]
Age. The age of crown-group Diervillioideae is 53.8±8.6 Ma (Bell & Donoghue 2005a).
For the early Caenozoic fossil history of Weigela, see Manchester et al. (2009).
Chemistry, Morphology, etc.. The large nectary at the base of the corolla in Diervilla (perhaps including Weigela) is a swelling covered by nectar-secreting hairs similar to those in Lonicera, etc.. Only one carpel may be fertile, and in some species there may be an "epicalyx" (see below) immediately below the ovary.
Phylogeny. For relationships in Diervilloideae, see Kim and Kim (1999). Weigela maximowiczii is of uncertain position at the base of the tree, while W. middendorffiana is weakly supported as sister to Diervilla.
[Caprifolioideae [Linnaeoideae [[Valerianoideae + Dipsacoideae] [Zabelioideae + Morinoideae]]]]: inflorescence lacking terminal flower; flowers rather weakly monosymmetric [K ± polysymmetric].
Age. The age of this node is 84.0±6.9 Ma (Bell & Donoghue 2005a); Wikström et al. (2001: c.f. scrambled topology) suggested an age of (62-)58, 54(-50) Ma, while age of ca 40.7 Ma is suggested by Naumann et al. (2013) and (61.5-)56.4-(47.8) Ma by H.-X. Wang et al. (202s1).
2. Caprifolioideae Eaton
Inflorescences with flowers in whorls of 6; rhexigenous cavities in fruit wall; endosperm starchy.
Age. The age of crown Caprifolioideae is 77±6.8 Ma (Bell & Donoghue 2005a).

2A. Heptacodieae Golubkova - Heptacodium miconioides Rehder
Deciduous shrub; cork mid-cortical; vessel elements with simple perforation plates; fibres in cortex; lamina tripli-nerved, vernation involute; ?"bracteoles"; C 2:3; G [3], 2 abort; fertile ovule 1/carpel; fruit cypsela [enlarged K lobes]; testa ± compressed-parenchymatous; embryo small; n = 14, centromeres submedian and (sub)terminal.
1/1. E. China, uncommon. Map: Flora of China vol. 19 (2011).
2B. Caprifolieae Dumortier —— Synonymy: Loniceraceae Vest
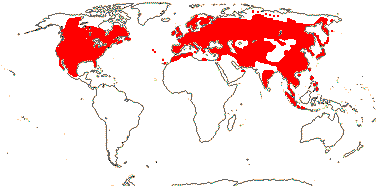
Evergreen or deciduous shrubs (trees, vines); (pith hollow); lamina vernation supervolute or conduplicate, margins entire, secondary veins pinnate to palmate; (bracteoles connate); K small; C 4/5 polysymmetric/4:1/2:3, (spurred); A (4-)5, filaments glabrous; (ovaries of adjacent flowers fused); G [(2-)3-5(-10)], (1 or 2 sterile), (G opposite C - Leycesteria); ovules 1-8/carpel; fruit baccate [Lonicera] or drupaceous [Symphoricarpus]; testa (multiplicative), antiraphe bundle, exotesta with U-shaped thickenings [outer periclinal wall not thickened], (coat crushed - Symphoricarpus); embryo small-medium [1/3-1/2 length of seed]; n = 9; deletion in the chloroplast gene clpP.
6/181: Lonicera (140). Mostly N. temperate, esp. East Asia and E. North America. Map: see Hultén (1971), Hultén and Fries (1986) and Meusel et al. (1992). Photo: Flower.
Age. The age of crown Caprifolieae is 46.5±4.6 Ma (Bell & Donoghue 2005a). Other estimates of their age are 51-36 Ma (Smith 2009), (66.4-)47.9(-35.9) Ma (Bell & Gonzalez 2019) or almost double that, (115.6-)93.9(-76.2) ma (H.-X. Wang et al. 2019) - check topologies.
Evolution: Divergence & Distribution. For additional ages in Caprifolioideae, see Bell and Donoghue (2005a) and Bell and Gonzalez (2019). Diversification in Caprifolioideae possibly began in Asia (Smith 2009), and Beaulieu and O'Meara (2016: hidden rates shift) suggest there may have been an increase in the diversification rate.
Chemistry, Morphology, etc.. There is no stem endodermis.
Landrein and Prenner (2016) suggest that flowers of Lonicera subgenus Periclymenium have some kind of nectar disc. Triosteum seems to lack dorsal carpellary bundles only and has apotropous ovules (Wilkinson 1949). The drupaceous fruits of Symphoricarpus have a thick outer layer of narrow, vertical fibres and a crossing inner layer of horizontal fibres (Jacobs et al. 2009); the seed coat itself is crushed, as is not totally unexpected in such a fruit.
[Linnaeoideae [[Valerianoideae + Dipsacoideae] [Zabelioideae + Morinoideae]]]: epicalyx + [from aborted flowers, etc.]; A 4, didynamous staminode 0; 1 carpel fertile, 2 carpels abort; ovule single (-7 in sterile loculi); fruit achenial; seed 1; seed coat flattened, exotesta not lignified.
Age. Magallón and Castillo (2009: note topology) offer an estimate of ca 59 Ma for the age of this node, Bell and Donoghue 2005) an age of 71.5-49.5 Ma, H.-X. Wang et al. (2019) an age of (99.2-)78.9(-60) Ma and H.-X. Wang et al. (2021) an age of (57.5-)51.2(-43.8) Ma - + Mor.Zab) (51.7-)48.3(-42.4) Ma.
3. Linnaeoideae Rafinesque —— Synonymy: Linnaeaceae Backlund
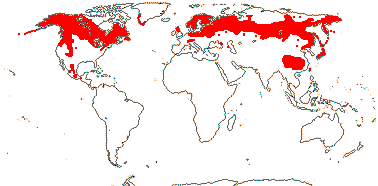
Deciduous (evergreen) shrubs (prostrate), (plant ± herbaceous); (stomata paracytic - Abelia); lamina vernation supervolute(-curved); epicalyx with 4 or 6 members, accrescent or not; (K foliaceous), C 2:3, nectariferous petal abaxial, bulging, (nectary as 1-3 lines of compact hairs between abaxial filaments); filaments hairy; (pollen 3-4-colpate, surface spiny - Abelia); G ([4]), style often shorter than C, stigma dry; ovule ?apotropous, (obturator + - Abelia); embryo sac (bisporic [the chalazal dyad], 8-nucleate [Allium type] - Abelia); K and/or bracts often modified, enlarged, in fruit, two empty carpels at maturity, (2 G fertile - Dipelta), fruits dorsiventrally compressed; exotesta, inc. outer periclinal wall, lignified; embryo ?length [small - Abelia]; n = 8, 9.
6/20. Circumboreal (Linnaea), Mexico (Abelia), China to Japan (five genera). Map: from Hultén and Fries (1986), Meusel and Jäger 1(992) and Villareal-Quintanilla (2013); see also H.-F. Wang et al. (2015). Photo - Habit, Flower.
Age. The crown-group age of Linnaeoideae has been estimated at 48.5-37.5 Ma (Bell & Donoghue 2005a), (60.2-)50.9(-42.8) Ma (H.-F. Wang et al. 2015), (65.3-)52.2(-40.6) ma (H.-X. Wang et al. 2019) and (51.7-)48.3(-42.4)/(57.8-)38.2(-32.7) Ma (H.-X. Wang et al. 2021).
[[Dipsacoideae + Valerianoideae] [Zabelioideae + Morinoideae]] - if this clade exists: vessel elements with simple perforation plates; petioles connate basally/leaves amplexicaul; nectary at base of C.
[Morinoideae [Valerianoideae + Dipsacoideae]] - if this clade exists: perennial rosette herbs with taproot; monoterpenoids, cathecolic tannins, alkaloids +; stele with vascular bundles separate [?here]; libriform fibres +; nodes 5:5; vascular flank-bridge in stem between lateral bundles; buds not perulate; filaments glabrous, anthers dorsifixed; pollen "large"; G with 1 fertile loculus, sterile loculi usu. much reduced; ovule apical, integument massive, endothelium prominent, with crystal layer; chalazal nuclei variously proliferating; exotesta not thickened; endosperm copious, embryo large; distinctive expansion of the chloroplast inverted repeat.
Age. The age of this node is around 69-48 Ma (Bell & Donoghue 2005a: Zabelia not included).
[Dipsacoideae + Valerianoideae] - if this clade exists: saccharose +, quercetin 0; stem with endodermis; (nodes 5 or more:5 or more); leaves simple to ± pinnate, lamina vernation conduplicate, (margins entire); flowers rather small; transpetalar vein single, strong; C and A develop almost together; A ± equal in length; tapetal cells 4 or more nucleate [?level]; pollen 3-celled, with prominent and branched exine columellae; stigma dry; embryo sac with 2-4-nucleate chalazal cells; K reduced or ± pappus-like; embryo chlorophyllous; centromeres metacentric to subtelocentric, no C bands.
Age. The age of this node is estimated to be ca 62.5-44.5 Ma (Bell & Donoghue 2005a), (41-)31(-22) Ma (Wikström et al. 2015), (92.4-)70.2(-51.2) Ma (H.-X. Wang et al. 2019), or (49.4-)41.8(-34.4) Ma (H.-X. Wang et al. 2021).
4. Dipsacoideae Eaton
Starch almost 0; (stomata anisocytic); lateral abaxial C lobes overlapping adaxial lobes; stigma entire or 2-lobed; ovule apotropous, micropyle very long, hypostase +; chalazal nuclei polyploid; embryo >3/4 length seed.
11/290. Eurasia, Africa, esp. Mediterranean region, to Malesia.
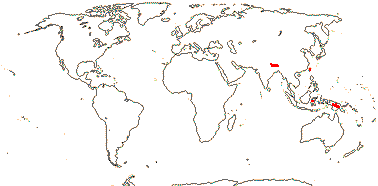
Age. Crown-group Dipsacoideae can be dated to ca 52.5-39.5 Ma (Bell & Donoghue 2005a) or ca 57 Ma (H.-X. Wang et al. 2019).
4A. Triplostegieae Höck - Triplostegia grandiflora Gagnepain —— Synonymy: Triplostegiaceae Airy Shaw
Perennial herb; valepotriate-type iridoids +; fusiform tap root, rhizomes +; glandular hairs +; outer epicalyx 4-lobed, inner epicalyx 8-ribbed; C ± polysymmetric, (4-)5-lobed; pollen ?cells, aperture with a halo, colpus granulose, surface spiny; stigma capitate; outer epicalyx capitate-glandular, persistent, the tips hooked; "achenes 8-ribbed"; endosperm slight; n = 9.
1/?1. Southeast Asia, Celebes, Papua New Guinea; scattered. Map: from Niu et al. (2019).
4B. Dipsaceae Reichenbach —— Synonymy: Dipsacaceae Jussieu, nom. cons., Scabiosaceae Martinov
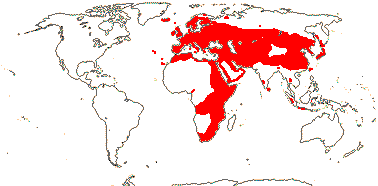
Perennial (annual) herbs, (shrubby); (vessel elements with scalariform perforation plates); (cork cambium subepidermal - Knautia); inflorescence a capitulum, (pseudanthia, ray flowers +), involucral bracts + (0); flowers (initially horizontally bisymmetric - Knautia), (polysymmetric); epicalyx single, 8-ribbed; C (4, -12), (± polysymmetric); A (2-3); pollen (3-porate, anguliaperturate - Lomelosia), surface echinate (gemmate)/microechinate; G [2], ?1 aborts; ovule with integument 10-15 cells across; fruit with calycine awns or bristles (K caducous); n = 5, 7-9(-10).
10/290: Cephalaria (80), Knautia (60), Lomelosia (>50), Scabiosa (>30). Europe inc. Iceland, esp. Mediterranean region, to Japan,, Africa, not Madagascar, few Sri Lanka West Malesia. Map: Photo - Habit, Inflorescence, Flower.
5. Valerianoideae Rafinesque —— Synonymy: Valerianaceae Batsch
(Annual herbs; climbers); foetid monoterpenoids and sesquiterpenoids, valepotriate-type iridoids + (0 - Patrinieae); root (swollen), (periderm superficial - Valeriana), pericyclic - Centranthus, etc.; (vascular cambia successive; 0); (vessel elements with scalariform perforation plates), (tracheids + - Patrinia), (parenchyma unlignified); stem often hollow; leaves (bijugate, 2-ranked)), (bases not sheathing); (plant dioecious); flowers small, corolla ± polysymmetric, (1:4), bracteoles +, epicalyx 0; K (0), C-A common primordium, C 4-5, abaxial C basally spurred or not; A (1-)3(4-5) [also 2 + 1 staminode], (anthers bisporangiate, bithecate - South American Valeriana subg. Phyllactis); pollen colp(oroid)ate, nexine adjacent to colpus disrupted; two empty carpels at maturity, (lacking sterile loculi), K usu. accrescent; testa 1-layered; endosperm 0 (+ Patrinia); n = (7-)8(9-13).
5/375: Valeriana (270), Valerianella (65). N. temperate, especially Mediterranean, and Andean South America (half the subfamily). Map: from Hultén (1958) and Meusel et al. (1978). [Photo - Habit, Flower, Flower.]
Age. Crown-group Valerianoideae may be 56-34.5 Ma (Bell & Donoghue 2005a), ca 50 Ma (Moore & Donoghue 2007), (54-)42(-33) Ma (Beaulieu et al. 2013a: 95% HPD) or ca 58 Ma (chloroplast data)/ca 50 Ma (nuclear data: Lee et al. 2021).
[Zabelioideae + Morinoideae] - if this clade exists: leaves connate at the base; pollen grains smooth, with endocinguli [endoaperture with extra thickened layer of pollen wall].
Age. This clade is (80.6-)48.2(-24.4) Ma (H.-X. Wang et al. 2019) or (39.9-)33.6(-32.1) Ma (H.-X. Wang et al. 2021).
6. Zabelioideae H.-X. Wang, Morales-Briones, M. J. Moore, J. Wen & H.-F. Wang - Zabelia (Rehder) Makino
Shrubs, deciduous; ?chemistry; wood ring-porous, aggregate rays +; twigs with six longitudinal grooves; lamina margin entire to dentate, petiole bases swollen, enclosing axillary bud; epicalyx with 6 or 14 members, not accrescent; flowers polysymmetric; nectary pouched abaxially, with four lines of compact hairs, bottom half of hairs smooth; stigma capitate, mucilaginous; ovule?; seed?; embryo small; n = 9 [most spp. polyploid].
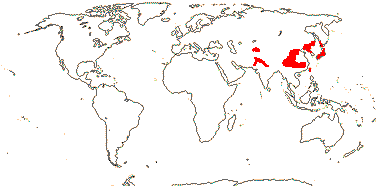
1/4-6 or so: Central Asia (Afghanistan, Tian Shan) to the Far East (Japan). Map: from Meusel and Jäger (1992) and FoC vol. 19. (2011).
7. Morinoideae Rafinesque —— Synonymy: Morinaceae Rafinesque
Iridoids 0; stems hollow; lamina vernation involute, margins spinose to pinnatifid (entire), secondary veins palmate; inflorescence of sessile cymes [verticels], epicalyx 12-ribbed; flowers strongly monosymmetric; K 4, monosymmetric; A pairs inserted at different heights on the C tube, (A 2, adaxial, + 2 staminodes); pollen binucleate, (turret-like triporate), exine columellae reduced, intine-covered tubular extrusions; ovule with integument 14-18 cells across, hypostase +; embryo sac with chalazal cells producing multicellular structures; K large, herbaceous, two empty carpels at maturity; integument multiplicative, not vascularized, outer layer only persisting; endosperm ruminate, embryo >3/4 length seed; n = 17, chromosomes small [0.7-1.5 μm long]; duplication of dipsCYC2 and dipsCYC3 genes.
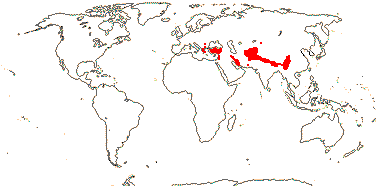
2-3/13. Balkans to China. Map: see Cannon and Cannon (1984). Photo: Morina Inflorescence, Acanthocalyx Inflorescence.
Age. Crown-group Morinoideae are estimated to be around 46-31 Ma (Bell & Donoghue 2005a).
Evolution: Divergence & Distribution. For distinctive late Eocene fruits (Diplodipelta, ca 37 My) assignable to Linnaeoideae and their implications for biogeography and evolution, see Manchester and Donoghue (1995); the genus may be sister to Dipelta. H.-F. Wang et al. (2015) offer a somewhat different interpretation, suggesting similarities between the fossil and Diabelia or perhaps Kolkwitzia, but these are also Linnaeoideae. Manchester et al. (2009) discussed the fossil distribution of Dipelta, which is known from Eocene/Oligocene rocks in southern England. Lee et al. (2021) offer a number of cautionary comments for ages suggested here, noting i.a. differences in ages suggested by chloroplast and nuclear data.
Endress (2011a) suggested that monosymmetric flowers might be a key innovation in Caprifoliaceae, but, as noted above, understanding diversification in Dipsacales as a whole is not simple. The work by H.-X. Wang et al. (2021) is clarifying what we know - and don't know - about relationships here, and they outline the distributions of a number of characters in the context of the phylogeny of Caprifoliaceae in particular; above I have treated Linnaeoideae, Zabelioideae, Morinoideae, Valerianoideae, Dipsacoideae largely separately (= Linnaeoideae et al., see also Phylogeny below). For some characters in the group, see Weberling (1957) and Verlaque (1984). For fruit characters perhaps apomorphic for [Linnaeoideae [Zabelioideae + Morinoideae] [Valerianoideae + Dipsacoideae]], see Jacobs et al. (2010b, 2011).
Beaulieu and O'Meara (2016: hidden rates shift) suggest that there may have been an increase in the rate of diversification of Dipsacoideae (excluding Triplostegia). For large-scale migration within Scabiosa, including north to south movement in Africa, see Carlson et al. (2012). Beaulieu and O'Meara (2016) also thought that the rate of diversification might have increased in the clade above Valeriana celtica (see below). There were around four migrations of Valerianoideae to South America about (20-)15.7(-12) Ma (Bell et al. 2012a), and diversification in the Andean paramo, where ca 1/7 of the subfamily grow, happened less than 5 Ma (Bell & Donoghue 2005b). Further diversification - ca 1/6 of the species - occurred south of 33oS (Kutschker & Morone 2012).
H.-F. Wang et al. (2015, q.v. for dates) discussed the biogeography and evolution of Linnaeoideae in detail, while Bell and Donoghue (2003) discussed biogeographic relationships within Morinoideae.
Howarth et al. (2011) looked at the evolution of monosymmetry in Dipsacales, in particular, the diversification of CYCLOIDEA expression; although the patterning of gene expression might be monosymmetric, that was not always reflected in the appearance of the corolla. Centranthus has monosymmetrical 1:4 flowers - very unusual given that the basic floral development in Dipsacales is that common in eudicots - it can also be thought of as being obliquely symmetrical - or asymmetrical (see Donoghue & Ree 2000). Valerianella locusta also has but four petals (Berger et al. 2020). The corolla tube of Centranthus is divided vertically into two by a septum. Naghiloo and Claßen-Bockhoff (2017) discussed floral development, expecially the timing of development of the floral whorls, in some detail; their focus was on part of Dipsacoideae. Heterochrony was involved, there were also changes in floral meristem size, with the increase in number of sepals (two different mechanisms), the timing of initiation of the calyx and corolla overlapped, indeed, as the size of the sepals was reduced, their initiation was delayed, sometimes occuring after that of both petals and stamens (Naghiloo & Claßen-Bockhoff 2017; Remizova 2019).
L. Zhang and Clement (2021) discuss the evolution of perfoliate leaves in a group of Lonicera, and they are also found in Triosteum and Dipsacus. Srivastav et al. (2023) also looked at the evolution of connation of the bracteoles and also of the gynoecia of flowers - this latter happened only when they were paired, the terminal flower of the cymule having been lost - in Lonicera in the context of a nuclear phylogeny; there has been substantial paralellism in the evolution of connation, but little reversal. Caputo et al. (2004a) examined the evolution of seed dispersal syndromes in Dipsacoideae, while for fruit and seed evolution in Valerianoideae, see Jacobs et al. (2010a: Nardostachys not included) - here the fruits are very variable in their morphology.
For relationships suggested by some morphological characters, see Peng et al. (1995). For a comparison of Morinoideae, Valerianoideae and Dipsacoideae, see Cannon and Cannon (1984), and for tables of differences between Morinoideae and Dipsacoideae, see Vijayaraghavan and Sarveshwari (1968), Cronquist (1981), Cannon and Cannon (1984) and Johri et al. (1992). The placement of Zabelia will affect character optimizations in the group [[Zabelioideae + Morinoideae] [Valerianoideae + Dipsacoideae]] (if it exists); above in the character hierarchy there is the option of following C.-L. Xiang et al. (2019). I have suggested some characters the whole group has in common; given that the petioles of Zabelia are basally connate, I would not be surprised to find that its nodal vascular architecture was like that of other members of this group. However, Zabelia is poorly known, and I have listed characters for both [Zabelia + Morinoideae] and [Morinoideae [Valerianoideae + Dipsacoideae]] separately.
Cruz et al. (2023) looked at variation in stem anatomy and growth form in Andean Valerianoideae, finding substantial variation in both stem anatomy - from no secondary thickening to successive cambia - and growth form - plants climbers, shrubby, perennating parts underground or annuals. As Pyck and Smets (2004) note, the clade [Triplostegia + Valerianoideae] has valepotriate-type iridoids, a pollen aperture with a halo, a granulose colpus, and endosperm reduction in common, and possibly even epicalyx/bracteole similarities. Avino et al. (2009) thought that Triplostegia was sister to [Valerianoideae + Dipsacoideae], and used this topology in their character state reconstruction; for more on Triplostegia, see Hofmann and Göttmann (1990). As with Zabelia, where Triplostegia ends up will substantially affect apomorphy positions, and the characters that Triplostegia and Valerianoideae have in common may be either parallelisms, or apomorphies for a larger clade, but with reversals. For relationships of Triplostegia suggested by some morphological characters, see Peng et al. (1995).
Ecology & Physiology. The ecological significance of the connate leaves of Dipsacus and their associated phytotelmata has occasioned speculation over the last 200 years or so (Vergne et al. 2023 for literature). There are glandular hairs on the inner surfaces of these connate leaves that have one or more drops of lipid- and terpene-containing exudates on their heads. Under the right conditions the drops elongate greatly and produce a thread which vibrate in a distinctive way - the threads are up to 1 mm long, and they spontaneously and rapidly retract (Vergne et al. 2023). There is no evidence that the plant obtains nitrogen from decaying animal material in the phytotelmata and any function of the threads is unclear. However, there may well be bacterial nitrogen fixation in Dipsacus (diazotrophs are 13.3±11.4% of the bacterial assembly) and the terpenes in the threads, being anti-oxidants, could protect oxygen-sensitive nitrogenases (Vergne et al. 2023, see associated video).
Pollination & Seed Dispersal. The rather small flowers of Dipsacoideae and Valerianoideae at first sight often look unremarkable, but interactions between these flowers and generalist/specialist pollinators can be complex (e.g. Larsson 2005). X.-Y. Wang et al. (2019) noted that bumble bees might be deterred from grooming pollen grains of species like Dipsacus chinensis (i.e. removing the grains from their bodies) because of the bitter-tasting saponins that they contained, so the grains were not moved to the insects' corbiculae - but perhaps paradoxically, there were then more grains on the insects' bodies that were available for pollination. Pseudanthia are known from Dipsaceae (Baczynski & Claßen-Bockhoff 2023).
The well-developed epicalyx of Dipsacoideae (for details, see Mayer 2016, also discussion below) suggests that the fruits are often wind-dispersed, but myrmecochory may occur in almost half of the subfamily, especially in the large genera Knautia and Scabiosa (Lengyel et al. 2009, 2010). For fruit and seed evolution in Valerianoideae, see Jacobs et al. (2010a: Nardostachys not included); the fruits are very variable in their morphology. A pappus is common in Valerianoideae, but a variety of different structures assist in wind dispersal, there also can be hooks, elaiosomes, or the fruit may even be explosive (Erikson 1989; Jacobs et al. 2010a; Weberling & Bittrich 2016). Wind-dispersed fruits predominate in Linnaeoideae (H.-F. Wang et al. 2015).
Genes & Genomes. For the cytology, etc., of Heptacodium see Z.-Y. Zhang et al. (2001).
Genome size increases towards the periphery of the range of Knautia, although why is unclear; the distribution of polyploidy, etc., was also studied (Frajman et al. 2015).
Biparental plastid transmission is reported for most of the major clades of Caprifoliaceae (Corriveaux & Coleman 1988; Q. Zhang et al. 2003). A more detailed study suggests strongly that the feature is an apomorphy here (Y. Hu et al. 2008; Q. Zhang & Sodmergen 2010).
Chemistry, Morphology, etc.. Valepotriates are triesters of route I secoiridoids; for their distribution other than Valerianoideae s. str. - they are found in Nardostachys, from which the aromatic spikenard comes, and Triplostegia (Backlund & Moritz 1998).
For nodal flank-bridges in a [[Zabelioideae + Morinoideae] [Valerianoideae + Dipsacoideae]] group, see Neubauer (1979); it is as if two bundles on each side form split laterals, and one branch from each bundle fuses, so forming the bridging bundle. Bundles innervating the petiole arise from the bridging bundle, except in Morina. The anatomy of some of the high-Andean Valerianoideae will repay further investigation (e.g. see Weberling & Uhlarz); they may have unilacunar nodes and distinctive secondary vascular tissue, lack girdling bundles, etc.. The cork of Knautia (Dipsacoideae) may be superficial (Metcalfe & Chalk 1950). Glandular leaf teeth in Caprifoliaceae have a main vein plus two accessory veins, or one accessory vein proceeding above the tooth.
For details of inflorescence morphology, especially paired flowers as inflorescence units in Linnaeoideae and Zabelia, see Landrein and Prenner (2013); Berger et al. (2020) described the inflorescence of Valerianoideae they examined as "bifurcating". The morphological nature of structures variously described as supernumerary bracts, bracteoles, epicalyx or involucel that are associated with flowers in the Linnaeoideae et al. clade has occasioned much discussion - see e.g. Troll and Weberling (1966), Weberling (1992), Roels and Smets (1996), Donoghue et al. (2003), Pyck and Smets (2004), Carlson et al. (2009), Landrein et al. (2012), Landrein and Prenner (2013) and H.-F. Wang et al. (2015) . The epicalyx is sometimes described as consisting of four fused bracts and having nothing to do with bracteoles (Hofmann & Göttmann 1990; Mayer 1998); the individual flower that it surrounds may then be the terminal flower of a partial thyrsoid inflorescence (esp. Roels & Smets 1996; see also Landrein et al. 2012, Landrein & Prenner 2013). Some of the bracts presumed to signify "things" like aborted flowers, missing inflorescence branches, etc., could also be developmentally duplicated structures... In the characterisations below, the particular condition of the epicalyx for each subfamily is described in general terms; what the epicalyx might "be" morphologically is a separate issue. Note that H.-X. Wang et al. (2021, fig. 9) recorded only 3 species (in Morinoideae and Dipsacus) of the 35 species of the whole group that they examined as having an epicalyx. The calyx often persists, characteristically remaining small and perched on top of the fruit, which may be narrowed towards the apex, or it may be absent, as in some Valerianoideae. In the latter there is a common ring primordium with corolla and androecium that appears first, and the stamen(s) develop very quickly, well before the gynoecium (Berger et al. 2020).
Transpetal veins, branch veins linking adjacent median veins, occur in the upper part of the corolla in at least Diervilla, Kolkwitzia and some Valerianoideae and Dipsacoideae (Gustafsson 1995).
The sequence of appearance of parts in the flower of Valeriana officinalis is A, C, K, G (Sattler 1973). The calyx in Patrinia and Nardostachys is polysymmetric and persistent but not much accrescent. Landrein and Prenner (2016) describe the floral nectaries of this group in detail. Where there are only two stamens, it is usually the adaxial pair that persists. Of the three carpels, it is an adaxial lateral carpel that remains fertile (Donoghue et al. 2003). If the flower has four carpels (e.g. Dipelta), two are fertile (Landrein et al. 2012). There may be several reduced ovules in the sterile carpels, as in Zabelia (H.-X. Wang et al. 2021). Evident sterile loculi, a well developed calyx, an androecium with either four or five stamens, presence of endosperm, etc., are likely to be plesiomorphic here. The ovule of Abelia tyaihyoni (Linnaeoideae) has a massive raphal region, a long micropyle, no endothelium, a multiplicative testa and a distinctive embryo sac (Ghimire et al. 2018a); not all these features are in the characterization above. The extra "bracteole" of Patrinia may be an interpolated structure that develops into a fruit wing (Hofmann & Göttmann 1990).
Remarkable intine-covered structures that look like pollen tubes are produced in all Morinoideae before pollen germination (Blackmore & Cannon 1983), and sometimes also in Dipsacoideae (Hesse et al. 2009b). For pollen size, see Xu et al. (2011), for endocinguli, see Jacobs et al. (2011) and Landrein et al. (2012). There seems to be confusion over the tapetum of Morina: Vijayaraghavan and Sarveshwari (1968) describe it as being polyploid, multinucleate and secretory, Kamelina (1983) as cellular, binucleate and glandular, and Johri et al. (1992) as being amoeboid (and that of Dipsacoideae as being glandular). The integument is 14-18 cells across when the embryo sac is mature, becoming ca 25 cells across later (Vijayaraghavan & Sarveshwari 1968).
Some general information is taken from Hara (1983: Japanese Caprifolioideae), Hofmann and Göttmann (1990), Backlund and Pyck (1998), Benko-Iseppon and Morawetz (2000a), Backlund and Donoghue (1996), Donoghue et al. (2001b), Bell et al. (2001), Jacobs et al. (2011), Hofmann and Bittrich (2016) and G.-Q. Wang et al. (2024: summary of recent work). See also Nilova (2001: bark anatomy), Magócsy-Dietz (1899: pith diaphragms, woody taxa only), Carlquist (1982) and Ogata (1988, 1991: wood anatomy), Backlund and Nilsson (1997) and Aksoy and Atasagun (2023: Dipsacus s.l.), both pollen, Johri et al. (1992; embryology of the whole group), and Hofmann and Göttmann (1990: e.g. Tab. 1) for a general comparison of Valerianaceae and Dipsacaceae.
For general information on Linnaeoideae, see Hara (1983), Landrein et al. (2012), Hofmann and Bittrich (2016) and Landrein and Farjon (2019), all general, also H.-F. Wang et al. (2015: generic characterizations) and Villarreal-Quintanilla et al. (2014: Abelia). For general information on Zabelia in particular, see Hara (1983), Landrein et al. (2012), Landrein and Prenner (2013) and Hofmann and Bittrich (2016).
Morinoideae - see Hofmann and Göttmann (1990: Morina), Cannon and Cannon (1984: monograph) and Hofmann and Bittrich (2016), all general, and for pollen, see Verlaque (1983).
For Valerianoideae, see Eriksen (1989) and Weberling and Bittrich (2016), both general, for anatomy, see Vidal (1903), and for ovule morphology (no vascular tissue?), see Guignard (1893).
For Dipsacoideae, see Verlaque (1984, 1985, 1986: Scabiosa), Niu et al. (2018, 2019: Triplostegia) and especially Mayer (2016), all general, for embryology, see Kamelina (1983), and for fruit, see Verlaque (1977).
Phylogeny. The position of Heptacodium was initially somewhat uncertain (Pyck & Smets 2000, 2001), although it perhaps could be included in Caprifolioideae (e.g. Donoghue et al. 2001a, 2003; Soltis et al. 2011 [support weak]; Landrein et al. 2012). It has also been suggested that it was a hybrid between members of Caprifolioideae and Linnaeoideae (Z.-Y. Zhang et al. 2002; Landrein & Prenner 2013), and it might be sister to Linnaeoideae and the other Caprifoliaceae (Jacobs et al. 2009). Although C.-L. Xiang et al. (2019) and H.-X. Wang et al. (2019), focusing on plastomes as they did, were unable to clarify the story, Lee et al. (2021) showed that it was very probably sister to other Caprifolioideae, characters that Heptacodium shares with some Linnaeoideae being parallelisms (relevant changes made vii.2021).
Recently renewed attention has been paid to the circumscription of Linnaeoideae, in particular where Zabelia (ex Abelia), like Heptacodium a phylogenetically migratory taxon, was to go. Jacobs et al. (2010c) found that Zabelia might be in the [Morinoideae [Dipsacoideae + Valerianoideae] clade, although support was only moderate (see also Soltis et al. 2011). Pollen morphology is perhaps consistent with this position (Jacobs et al. 2010d), while Landrein and Prenner (2013) suggested that Zabelia and Diabelia (Linnaeoideae) had similar "primitive" (their scare quotes) inflorescences. In some analyses Zabelia and Morinoideae formed a clade, although support for this was only moderate (Jacobs et al. 2011). Landrein et al. (2012: useful table of characters, topology of some trees difficult to interpret) suggested that Zabelia was sister to the whole [Morinoideae [Valerianoideae + Dipsacoideae]] clade; see also H.-F. Wang et al. (2015), while relationships in Z.-D. Chen et al. (2016: support weak) are [Morinoideae [Zabelia + The Rest]]. Morinoideae migrated basally in an analysis by Beaulieu et al. (2013a: only 4 genes), and they were sister to Linnaeoideae in Wikström et al. (2015: taxon sampling?). In the analyses of complete plastomes of 32 species by C.-L. Xiang et al. (2019), they recovered a clade [Zabelia + Morinoideae], and it was part of a tritomy that also involved Linnaeoideae and [Dipsacoideae + Valerianoideae]. Finally, in the plastome analysis of H.-X. Wang et al. (2019), strong support was found for the relationships [[Linnaeoideae [Morinoideae + Zabelia]] [Dipsacoideae + Valerianoideae]]. In the Angiosperms353 analyses of Lee et al. (2021) the topologies [Zabelia [Linnaeoideae [Morinoideae [Dipsacoideae + Valerianoideae]]]] (exon and flanking region) and [[Zabelia + Morinoideae] [Linnaeoideae [Dipsacoideae + Valerianoideae]]] (exons only; also plastome analyses) were recovered. Things remain up in the air, most studies being based on plastome data (G.-Q. Wang et al. 2024).
For relationships in Linnaeoideae, see Jacobs et al. (2010c, 2011), Landrein et al. (2012) and C.-L. Xiang et al. (2019); the basic phylogenetic structure may be [Linnaea [Vesalea + The Rest]], but H.-F. Wang et al. (2015) found quite good support for the relationships [[Linnaea + Vesalea] The Rest]. Lee et al. (2021) found that Diabelia and Abelia were separated in chloroplast analyses, but were sister taxa in nuclear analyses, so perhaps they should not be separated...
Relationships in Caprifolioideae - [Heptacodium [Triosteum *[Lonicera [Leycesteria + Symphoricarpos]]]] - are suggested by Theis et al. (2008); the node with the asterisk has a low posterior probability, but a good bootstrap value. Bell and Gonzalez (2019) looked at relationships in Symphoricarpus where the Asian S. sinensis was sister to the rest of the genus (North American); C.-L. Xiang et al. (2019) thought that the genus was poly/paraphyletic. Relationships within Lonicera varied according to the particular analysis carried out (Lee et al. 2021), and Srivastav et al. (2023) also noted conlict between nuclear and chloroplast trees. They obtained a fair bit of resolution in their RADseq tree, and the two classical subgenera (Rehder) were monophyletic, even if some of the sections and subsections were not (Srivastav et al. 2023: ca 63 spp., RADSeq).
Within Morinoideae, Acanthocalyx is sister to the rest of the subfamily, Morina may be paraphyletic (Bell & Donoghue 2003).
Triplostegia, which has a double epicalyx and valepotriates, seems best assigned to Dipsacoideae (e.g. 100% support in a 30 taxon-5 gene analysis - Davis et al. 2001; see also Bell & Donoghue 2000, 2005a; W.-H. Zhang et al. 2001; Bell 2004; Soltis et al. 2011; C.-L. Xiang et al. 2019; Lee et al. 2021). However, Pyck and Smets (2004) showed that although a two-gene analysis placed Triplostegia in this position, morphological data alone and when combined with the molecular data placed it sister to Valerianoideae. There is probably just a single species (Niu et al. 2018),
Dipsacoideae. Pterocephalodes, a recent segregate from Pterocephalus (Mayer & Ehrendorfer 2000), and Bassecoia together form a clade that is sister to the remainder of the subfamily (Avino et al. 2009; Carlson et al. 2009). For other phylogenetic studies, see Caputo et al. (2004a) and Carlson et al. (2012: Scabiosa). Resetnik et al. (2014) looked at relationships between diploid species of Knautia.
Valerianoideae. Soltis et al. (2007a) found that the position of Valerianoideae was unstable in some analyses; analyses of mitochondrial data being perhaps particularly challenging (Winkworth et al. 2008b). Patrinia may be sister to the rest of the subfamily (Pyck 2002), with Nardostachys sister to the remaining taxa (Bell 2004; Bell & Donoghue 2005a, b: both with very strong support), or the two together may form a well-supported clade sister to other Valerianoideae (Hidalgo et al. 2004); the former topology is more likely. Valeriana celtica, Valerianella and Centranthus may be successively sister to Valeriana (Bell & Donoghue 2005b, for which see for further details); Valeriana may be para- or polyphyletic (Hidalgo et al. 2004; H.-X. Wang et al. 2021). For other phylogenies, see Bell (2004) and Bell et al. (2015). Cruz et al. (2023: focus on Andean species) found relationships here to be [Patrinia [Nardostachys + very paraphyletc Valeriana]]. For Triplostegia, see above.
Classification. The circumscription and number of families in the area of the Caprifoliaceae and Dipsacaceae sensu APWeb versions 12 and before (see also A.P.G. II 2003) was the direct result of deciding to maintain the well known Dipsacaceae and Valerianaceae in their old circumscriptions - the small clades resulting from the break-up of the broadly-circumscribed and strongly paraphyletic Caprifoliaceae had to be accounted for as separate families (Backlund & Bremer 1997; Backlund & Pyck 1998; c.f. Stevens 1998). In fact, the whole lot can usefully be combined in a Caprifoliaceae s.l. (see A.P.G. III 2009; esp. H.-F. Wang et al. 2021), since similarities between the main clades are considerable and differences are mostly slight, however, C.-L. Xiang et al. (2019) elected to recognise separate families. Given what is known about relationships, aside from a [Valerianoideae, Dipsacoideae] clade, the [Zabelioideae, Morinoideae, Linnaeaoideae, Valerianoideae, Dipsacoideae] clade seems best treated as a polytomy for now. G.-Q. Wang et al. (2024: Table 1, Fig. 1) summarize relationships in the family in the context of the eight subfamilies that they recognize.
For generic limits and groupings in Linnaeoideae, see Landrein (2010) and Landrein et al. (2012). Mayer and Ehrendorfer (2013: much morphology) and Mayer (2016) provide a very detailed classification of Dipsacoideae (as Dipsacaceae). The limits of Valeriana are unclear (Hidalgo et al. 2004; discussion in Eriksen 1989: infrageneric classification; Weberling & Bittrich 2016).
Botanical Trivia. Some 218 botanical names are associated with Linnaea borealis (Landrein & Farjon 2019).