EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes. - Back to Main Tree
Age. Wikström et al. (2001) estimated that this node was (179-)171, 153(-145) Ma, in line with other early estimates (Magallón 2009); Soltis et al. (2008: a variety of estimates) give an age of 180-132 Ma. Magallón and Castillo (2009) offer an age of ca 229 Ma for relaxed and 127 Ma for constrained penalized likelihood datings, while Moore et al. (2010: 95% HPD) suggest an age of (162-)155(-145) Ma, Clarke et al. (2011: also other estimates) ages of (231-)197(-169) Ma, towards the upper end of the suggestions then, N. Zhang et al. (2012; see also Xue et al. 2012) ages of (211-)179(-158) Ma, and Magallón et al. (2013) an age of around 183.4 Ma. Ages around ca 139 Ma (Magallón et al. 2015) and 184.5-173 Ma are suggested in Naumann et al. (2013) and, the other extreme, (266, 233-)227, 195(-173)Ma by Zeng et al. (2014), ca 255 Ma by Z. Wu et al. (2014), and (297-)250(-207) Ma by Salomo et al. (2017). Y. Yang et al. (2020: Suppl. Fig. 22) suggested an age of around 189.6 Ma for this node, P.-L. Liu et al. (2020) an age of (251.5-)209.5(-177.1) Ma and X. Guo et al. (2021) an age of about 188.6 Ma, while and ca 194 Ma is the estimate in W. Song et al. (2024).
A fossil-based estimate for the age of this node is ca 113 Ma (Crepet et al. 2004), while the estimate in the pollen-based study of Zavialova and Tekleva (2021) is around 200 Ma.
Evolution: Divergence & Distribution. Magallón et al. (2018) suggested nested increases in diversification rates around here, one (139.4-)139.2(-139) Ma and another at the mesangiosperm node.
M. L. Taylor and Osborn (2006) and also Friis et al. (2009b) discuss as to where characters of pollen morphology and development are to be placed on the tree; the actual position depends partly on how the characters are defined and partly on the recent discovery of fossil Nymphaeales that do not have the pollen characteristics of extant members of the clade. For vessel evolution in angiosperms, including that in Nymphaeales, see the Amborellales page.
Genes & Genomes. For the possibility of a genome duplication at about this position, see Cui et al. (2006) and dePamphilis et al. (2009); some authors place an old duplication within Nymphaeales (see below).
Chemistry, Morphology, etc.. For cell lineages in the embryo sac see Huang and Russel (1992) and Friedman (2006); identification of the pattern described above apparently goes back to Porsch (1907), although it has been observed for relatively few plants. For embryo sac evolution, see Friedman and Ryerson (2009).
Phylogeny. For discussion of the relationships of Nymphaeales, see the Magnoliophyta node.
NYMPHAEALES Dumortier - Main Tree.
± aquatic, herbs, internodes short, ?sympodial construction; mycorrhizae 0; starch grains compound; radicle aborts; root apex with secondary dermatogen, etc., epidermis derived from outer layer of cortex [?Hydatellaceae]; trichoblasts in vertical files, proximal cell smaller; root stele monarch; diaphragms in root aerenchyma; primary stem with ± scattered vascular bundles; cauline endodermis + (0); protoxylem lacunae +; vascular cambium 0; nodes?; articulated laticifers +; vascular bundles lacking associated sclerenchyma; aerenchyma common; 4-celled uniseriate secretory trichomes with a large terminal cell [= hydropoten]; stomata anomocytic; leaves spiral, ?lamina margins, leaf base broad; bracts 0; pollen boat-shaped, tectum continuous, infratectal space large, columellae conspicuous; ovule with semi-annular [hood-shaped] outer integument; first division of endosperm transverse, chalazal cell undivided; P persistent; fruit maturation underwater; seed operculate, endotegmen below operculum, hilum outside; seed coat exotestal, palisade/?exotestal cells low, thin-walled; endosperm scanty, [develops from micropylar cell], chalazal endosperm haustorium +, single-celled, perisperm +, starchy, precocious, cells ± multinucleate, embryo broad, cotyledons connate; intergenic inversion in chloroplast inverted repeat; germination hypogeal. - 3 families, 6 genera, 80 species.
Includes Cabombaceae, Hydatellaceae, Nymphaeaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age of crown-group Nymphaeales is around 125 Ma (Magallón et al. (2015), (133.2-)126.7(-120.6) Ma (Iles et al. 2014), (160-)132(-114 Ma (Salomo et al. 2017), as little as (176.6-)97.7(-42.8) Ma (Zhou et al. 2014), or as much as ca 164 (Z. Wu et al. 2014), ca 188 Ma (Tank et al. 2015), ca 194 Ma (W. Song et al. 2024) or even ca 209 Ma (Foster et al. 2016a: q.v. for details).
The curious fossil Archaefructus, probably an aquatic plant and about 124 My old, has been linked with Hydatellaceae in morphological analyses (e.g. Doyle & Endress 2007, 2010; Doyle 2008b; Endress & Doyle 2009); see Sokoloff et al. (2011) for an evaluation. Although the two have very little in common in terms of overall appearance, Archaefructus may be another early aquatic angiosperm with very unconventional floral morphology.
Evolution: Divergence & Distribution. Cretaceous fossils assignable to Nymphaeaceae are quite common and these are discussed below under Cabombaceae and Nymphaeaceae. Indeed, it has been suggested that Nymphaeales were "the first globally diverse clade" (Borsch & Soltis 2008: p. 1051; see also Sender et al. 2010). Although looking only at extant taxa, their diversity may seem slight even if their morphology is difficult to interpret (see Crane & Friis 2020 for the way forward), Nymphaeales as a whole are aquatic herbs, again, the first such group represented by extant herbaceous hydrophytes, and some are very highly specialized.
Saarela et al. (2007) suggest a few additional possible synapomorphies for Nymphaeales, and Zeng et al. (2014) thought that having protoxylem lacunae and vascular bundles lacking associated sclerenchyma might be apomorphies. For the development of root hairs, see e.g. Clowes (2000) and Sokoloff et al. (2008a). The basic plant construction in Nymphaeales may be sympodial (see Kraehmer et al. 2023 and discussion). Borsch et al. (2007) outline the evolution of a number of floral characters within the order, M. L. Taylor et al. (2015) outline palynological variation in the context of its phylogeny, and Endress and Doyle (2015) look at floral morphology. Understanding where some of these features should be placed on the tree is complicated by the highly autapomorphic nature of Hydatella and some uncertainty over the position of Nuphar.
The development of perisperm (2n, maternal, c.f. endosperm, 2n, maternal/paternal) is an apomorphy for the order. Povilus et al. (2018 - read the details carefully) examined seed development in Nymphaea thermarum and interpreted perisperm development there as a way for the female parent to control resource allocation to the offspring. In general, the endosperm is slight if quite persistent and it probably functions as transfer tissue between embryo and perisperm (Friedman et al. 2012).
As might be expected, endomycorrhizae are at most uncommon in Nymphaeales (e.g. de Marins et al. 2009).
Chemistry, Morphology, etc.. It is possible that there are epidermal oil cells in Nymphaeaceae (Wilkinson 2006); do they contain ethereal oils? Hydrolysable tannins in this group (e.g. in Nuphar) are different to those found elsewhere (Gottlieb et al. 1993; Ishimatsu et al. 1989), although the chemistry of Hydatellaceae is poorly known.
A cauline endodermis is common in Nymphaeales although by no means universal; it may surround all or some of the individual vascular bundles (Seago 2020; C. Yang et al. 2019: Cabomba, all). Hydatellaceae (Trithuria filamentosa) are reported to have vessel elements with scalariform perforation plates throughout the plant (Cheadle & Kosokai 19), however, Carlquist and Schneider (2009) could not find them when they examined the original sections. For micromorphological details of tracheids, see e.g. Carlquist and Schneider (2009), E. L. Schneider et al. (2009), Schneider and Carlquist (2009a, b); details of the wall structure of tracheids, at least, in Cabombaceae and Nymphaeaceae are very distinctive. Roots of Cabombaceae and Nymphaeaceae may have vessels of a sort (Carlquist & Schneider 2009; Schneider & Carlquist 2009). The distinctive uniseriate trichomes found in all groups may secrete nectar or mucilage, or they may be involved in ion exchange (Vogel 1998a); Wilkinson (2006) calls the trichomes on the leaves, hydropotes.
The inner bracts found in some Hydatellaceae and the inner petals of Cabomba are notably slow in developing (Rudall et al. 2007). If the corolla represents sterilised stamens, as some think, having external staminodes will probably be another synapomorphy at least for [Nymphaeaceae + Cabombaceae]. For discussion about the presence of a granular infratectum in Nymphaeales, see M. L. Taylor et al. (2013, 2015, also 2014 for other characters); the infractectum is generally columellate, how obviously so depending on the thickness of the infratectal space. Some genera in all families have exotestal cells that are neither very tall nor much thickened (Hamann et al. 1979; Collinson 1980). Rudall et al. (2009b) discuss the distinctive single-celled chalazal endosperm haustorium, the presence of which is sometimes suggested by a space between the embryo plus endosperm and the perisperm. Baskin and Baskin (2018) discuss embryo morphology and seed germination.
Phylogeny. Hydatellaceae are sister to Xyridaceae in Stevenson et al. (2000; see also Stevenson & Loconte 1995); both have latrorse anthers and seeds with an operculum "stopper" that is tegmic in origin. Trithuria and Xyris appear as sister taxa (weak support) and in turn are sister to Mayaca (still weaker support), although other Xyridaceae are not immediately related in the phylogeny of Michelangeli et al. (2003). However, although Bremer (2002) noted that Mayacaceae and Hydatellaceae might be weakly associated with Xyridaceae or Eriocaulaceae, depending on what taxa were included in the analysis, there were a number of long branches in this area and he excluded the first two families from his final analysis, while Janssen and Bremer (2004) suggested that the association of Hydatellaceae with Mayacaceae was probably an artefact (see also Chase et al. 2006). Subsequent studies (Saarela et al. 2006, esp. 2007: several genes from two compartments, morphology; Friis & Crane 2007: commentary) placed Hydatellaceae firmly with Nymphaeales, and sister to [Cabombaceae + Nymphaeaceae]; the sequence that placed Hydatellaceae in Poales was a chimaeric pcr recombinant involving a grass and a moss.
Hydatellaceae aside, for a morphological phylogeny of [Cabombaceae + Nymphaeaceae], see D. W. Taylor (2008). Y.-L. Liu et al. (2005) provide an ITS phylogeny focussing on Nymphaeaceae, but with some at first sight rather surprising relationships - [Nuphar [Cabomba + Brasenia] [Nymphaea [Euryale + Victoria]]]. Nelumbo, which was included in the analysis, did at least stay outside this clade... However, this position of Nuphar is recovered in other analyses, too (e.g. Borsch et al. 2008; Löne et al. 2007; D. W. Taylor & Gee 2014: some analyses; Gruenstaeudl et al. 2017) and so its position below as sister to Nymphaea, etc., may be provisional. He et al. (2018: chloroplast data) found that Nuphar was sister to Nymphaea and its associates, and with quite strong support; interestingly, a UPGMA dendrogram of RNA editing sites linked Nuphar with Cabombaceae. Moreover, in a renalaysis of the data in He et al. (2018), Gruenstaeudl (2019) found that how the data were partitioned affected the results, and individual gene trees also gave different results, thus almost as many supported a relationship of Cabombaceae with Nuphar as supported an association of Nuphar with Nymphaea and friends. H.-T. Li et al. (2019) found a [Nuphar + Nymphaea] association.
Previous Relationships. Many of the morphological features of Hydatellaceae that made it so different from other monocots are consistent with a position in Nymphaeales. Hamann (1998) had even noted that the antipodal cells were absent or degenerated early, and absence of these cells would be expected if Hydatellaceae were to be placed here - and Friedman (2008a) and Rudall et al. (2008) found that Hydatellaceae had the distinctive 4-celled embryo sac of other Nymphaeales and of Austrobaileyales.
Classification. Doweld (2022) placed members of Nymphaeales - extant and fossil - in two subclasses, four orders and six families; 32 fossil genera are recognized (over two thirds are in Nymphaeaceae), and one order and one family are based on fossils alone.
Synonymy: Barclayales Doweld, Cabombales Richard, Euryalales H. L. Li, Hydatellales Reveal & Doweld, Hydropeltidales Spenner - Hydatellanae Reveal, Nymphaeanae Reveal - Hydatellidae Doweld, Nymphaeidae Takhtajan - Hydropeltopsida Bartling, Nymphaeopsida Horaninow
HYDATELLACEAE U. Hamann, nom. cons. - Trithuria J. D. Hooker - Back to Nymphaeales
(Plant annual), growth sympodial, rhizome short, erect; chemistry?; mycorrhizae 0; cuticular waxes 0; leaves linear, with a single vein, margins entire; plant monoecious; perennial taxa sympodially branched/inflorescence axillary, scapose (sessile), capitate, with involucral bracts; flowers very small, monosymmetric by reduction; P 0, nectary ?0; staminate flowers: A 1, filament long, slender, ± articulated with the anther; endothecium 0; tapetal cells?; pollen with spinules; carpelate flowers: pedicel articulated; G 1, three vascular bundles equidistant (2, 1), style 0, stigma penicillate [hairs as rows of plump cells]; extragynoecial compitum 0; ovule pendulous, anatropous, apotropous, micropyle bistomal, parietal tissue ?0, nucellar cap +/0, suprachalazal nucellar tissue massive; (two embryo sacs developing); fruit smooth to papillate, follicular and splitting into three valves, or ?achenial; testa smooth or reticulate, exotestal cells in files, (with sinuous anticlinal walls), other layers ± collapsed, tanniniferous; embryo barely differentiated, suspensor unicellular; n = 7, x = 7 (?8), (chromosomes holocentric), nuclear genome [1 C] (0.064-)1.686(-44.679) pg/1335-1350 Mbp; seedling - see below.
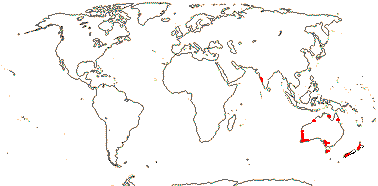
1 [list]/10. India, New Zealand and Australia. Map: from Cooke (1987) and FloraBase (consulted 2004).
Age. It has been estimated that crown-group Hydatellaceae are (23.4-)19.1, 17.6(-14.7) Ma (Iles et al. 2014) or as much as ca 76.8 Ma (W. Song et al. 2024).
Hydatellaceae may be represented in the pollen record in rocks from the Isle of Wight that are some 130 Ma (Hoffmann & Zetter 2010), while the pollen Monosulcites riparius in ca 75-70 Ma rocks from Eastern Siberia has been identified as that of Trithuria - and this of course disagrees with the age in Iles et al. (2004).
Evolution: Divergence & Distribution. For the evolution and biogeography of the family - crown Hydatellaceae are certainly not Gondwanan in age - see Iles et al. (2014). Trithuria konkanensis (India) and T. lanterna (N. Australia) diverged (1.3-)0.76(-0.24) Ma and evolution in the genus seems in general to be pretty active (e.g. Marques et al. 2016 and references).
The inflorescence is described as being cymose and capitate, although bractless and with highly reduced flowers, i.e., it is a sort of pseudanthium, although alternative interpretations are possible (Rudall et al. 2007a, 2009a; Rudall 2010; Rudall & Bateman 2010; Baczynski & Claßen-Bockhoff 2023); for the sympodial growth of perennial species, see Sokoloff et al. (2009a). The pedicels seem at least sometimes to be articulated. Early work suggested that the carpels might be initiated outside the stamens, and this has been confirmed (Rudall et al. 2007a); staminate flowers are the first to be initiated in the cymose inflorescence, and the result is that several "carpels", developing centrifugally, surround a few "stamens". How the reproductive structures are to be interpreted, whether flowers or inflorescences, remains unclear (see also Rudall 2024: flowers of the monocot Triuridaceae-Lacandonia are perhaps comparable), and Sauquet et al. (2017) reasonably elected not to include the family in their reconstruction of the morphology of the ancestral angiosperm flower. Trithuria sects Hydatella and Altofinia are considered by Romanov et al. (2023) to have berries of the Schisandra type, in which they also include the fruits of Cabombaceae.
Pollination Biology & Seed Dispersal. For reproductive ecology - wind-, self-, or hyphohydrous pollination - see M. L. Taylor et al. (2010); the pollen tubes grow down the multicellular stigmatic hairs under the cuticle (Prychid et al. 2011).
Genes & Genomes. For chromosome numbers, see Marques et al. (2016). The genome of Trithuria submersa shows signs of polyploidy (n = 28), and meiosis during microsporogenesis has a number of odd features, and it is possible that (some of) the chromosomes, perhaps belonging to one of the genomes, are holocentric (Kynast et el. 2014).
Chemistry, Morphology, etc.. The sieve tube plastids were reported as having triangular proteinaceous inclusions, but the inclusions appear to be of the starchy type as are more to be expected in this part of the tree (Tratt et al. 2009). There is some variation in the epidermis of the root and whether or not root hairs are produced (Sokoloff et al. 2008a). Hairs with possible apical secretory cells are known only from the inflorescences.
The inflorescence is described as being cymose and capitate, although bractless and with highly reduced flowers, i.e., it is a sort of pseudanthium, although alternative interpretations are possible (Rudall et al. 2007a, 2009a; Rudall 2010), indeed, how the reproductive structures are to be interpreted, whether flowers or inflorescences, is unclear. Early work suggested that the carpels might be initiated outside the stamens, and this has been confirmed (Rudall et al. 2007a); staminate flowers are the first to be initiated in the cymose inflorescence (see also Begoniaceae). Perennial Hydatellaceae have a cymose branching pattern (Sokoloff et al. 2009).
The fruit often opens along three lines as the three vascular bundles separate from the rest of the pericarp (see also Sokoloff et al. 2013a). Both integuments have two cell layers; the operculum is formed from enlarged cells of the inner integument. Starch deposition in tissues that will become perisperm begins before fertilization (Friedman 2008a).
There is some disagreement over the interpretation of the embryo. Tuckett et al. (2010: discussion of "ancestral" embryo type for angiosperms must include Amborellaceae, at least; see also Sokoloff et al. 2014) found that the embryo differentiated and the shoot and root appeared only after germination began. Tillich et al. (2007) compared seedling morphology with that of a monocot, describing collar rhizoids, a coleoptile, two cotyledonary sheath lobes, and a haustorium. Sokoloff et al. (2008a) suggested that the sheathing structure with its bilobed apex that is found in some species could be interpreted as two more or less completely connate cotyledons; the rest of the embryo is attached to the sheathing structure. In some taxa there is apparently no sheathing structure at all, only a haustorial lateral outgrowth (cotyledonary) that goes into the rest of the seed, the rest of the cotyledon being photosynthetic, so the seedlings are simultaneously both phanero- and cryptocotylar - c.f. some monocots (Sokoloff et al. 2013b), and Sokoloff et al. (2008a, 2014) suggested that Hydatellaceae showed how monocot-like embryos/seedlings might have originated. Some species are phanerocotylar (Sokoloff et al. 2013b). Both Tillich et al. (2007) and Sokoloff et al. (2008a) examined largely the exterior morphology of the embryo, neither looked in any detail at anatomy (c.f. Friedman et al. 2012: superb micrographs; Sokoloff et al. 2014); for a discussion on germination, see Baskin and Baskin (2018).
Additional information is taken from Hamann (1998: general), Sokoloff et al. (2008b: family monograph, 2011: important literature review, 2019b: species limits), Cutler (1969: vegetative anatomy), Remizowa et al. (2008b: pollen), Hamann (1975), Rudall (1997) and Rudall et al. (2008), both embryology and Hamann et al. (1979), Cook (1983) and Rudall et al. (2009b), all seeds and germination.
Previous Relationships. Hydatellaceae had long been thought to be monocots, largely because of their superficial similarity to Centrolepidaceae (= Restionaceae). Both groups are very reduced morphologically, and indeed Hydatellaceae have been misidentified as Centrolepidaceae. It was unclear if the gynoecium of Hydatellaceae was 1- or 3-carpelate, and since the fruits of Trithuria (= Hydatella) opened by three valves, they looked rather monocot-like. The combination of characters in Hydatellaceae was recognised as being unique to that group, and it made them very distinctive within monocots as a whole (e.g. Hamann et al. 1979; Dahlgren et al. 1985).
[Cabombaceae + Nymphaeaceae]: plant monopodial, rhizome/stolon elongated; hydroyzable [ellagi]tannins +; laticifers +; vessel elements in roots [of sorts: pit membranes in end walls lyse], tracheids with pit membranes of end walls with two layers of large fibrils; astrosclereids [stellate parenchyma cells] +, with minute rhombic crystals; guard mother cells produced directly from protodermal cell, no asymmetric division; lamina vernation involute, secondary veins palmate, actinodromous, festoon brochidodromous, margin toothed/crenate/entire, (hydathodes +); flowers single, extra-axillary; receptacle with cortical vascular system; P whorled, outer members enclosing the rest of the bud, nectaries on P; A whorled, (stamens in pairs opposite P members); tapetum (amoeboid), cells multinucleate; pollen tube growth moderately fast; carpel margins with postgenital fusion, placentation ± laminar; nucellar epidermal cells ± radially elongated, supra-chalazal tissue massive; mesocarp ± aerenchymatous; exotesta ± thin-walled and tabular, with sinuous anticlinal cell walls; endosperm bipolar, chalazal cell ± enlarged (and elongated), haustorial, embryo differentiated, suspensor often ± filamentous; seedling cryptocotylar, hypogeal, first leaf acicular.
Age. There has been much discussion over when diversification within the Cabombaceae-Nymphaeaceae clade began (e.g. Nixon 2008), however, the disagreement is not a simple morphology vs molecules dichotomy. Wikström et al. (2001) suggested that divergence of the two families occurred (152-)144, 111(-103) Ma and the figure in Salomo et al. (2017) is (117-)110(-106) Ma, while the age in Magallón and Castillo (2009) is ca 112 Ma (see also Tank et al. 2015: ca 112.5 My), that in Magallón et al. (2013) is around 122.7 Ma, and that in Iles et al. (2014) (107.1-)102.1(-98.8) Ma. However, Löhne et al. (2008: molecules) thought that divergence was only Palaeocene in age, (75-)56.4(-38) Ma, while Bell et al. (2010) offered a still younger date of (56-)42, 38(-25) Ma; Yoo et al. (2005) pegged the crown group age to 44.6 ± 7.9 Ma, while in Naumann et al. (2013), at around 94.75 Ma, the age was intermediate. Recent estimates, as high as they go, are 185-147 Ma (L. Zhang et al. 2019) and ca 163.2 Ma (W. Song et al. 2024).
Early fossil-based estimates for the age of this group were only ca 90 Ma (Crepet et al. 2004), but substantially earlier dates are likely (Friis et al. 2009b). There are fossils of stem-group Nymphaeaceae, Cabombaceae and/or [Nymphaeaceae + Cabombaceae] from several parts of the world in the Lower Cretaceous (e.g. D. W. Taylor et al. 2001, 2008; Friis et al. 2011; see Taylor & Gee 2014: ca 113 Ma). Indeed, a dozen or more genera based on fossil seeds have been described that belong around here, and exotestal seeds, e.g., having tall exotestal cells with sinuous antinclinal walls, were diverse in the Middle Albian-Middle Aptian some 115-105 Ma, and their limited occurrence now seems to reflect extensive extinction at around this time (Friis et al. 2017b, 2018b, c). Silvestro et al. (2020) estimate the time-of-origin of Cabombaceae to be ca 120 Ma and that of Nymphaeaceae to be ca 112.5 Ma.
Although other fossils possibly of this group (to a certain extent characters of the two families are combined) are known from the Barremian-Aptian deposits 125-113 Ma in Portugal (Friis et al. 2001) and (placed in Nitaspermum) from deposits 112-107 Ma from the S.E. U.S.A. (Friis et al. 2018b), they may also be from a member of Austrobaileyales (Gandolfo et al. 2004). See also von Balthazar et al. (2008) for another fossil perhaps assignable to this general [Nymphaeales-Austrobaileyales] area.
Pluricarpellatia, probably to be placed in or near Cabombaceae (Doyle & Endress 2014), is known from the Early Cretaceous (Mohr et al. 2008). The Early Cretaceous Monetianthus was embedded within crown Nymphaeaceae in morphological analyses (Friis et al. 2009b, but c.f. 2011; see also Doyle & Upchurch 2014/; Doyle 2016), and this may be true for the mid-Albian Carpestella, from Virginia (Doyle & Endress 2014); both these genera have very small flowers. The distinctive reticulate-perforate pollen of Monetianthus would then be independently derived within Nymphaeales, but other analyses also placed the genus at the node above Nymphaeales along the spine of the angiosperm tree (Friis et al. 2009b). Yoo et al. (2005) thought that the ca 90 Ma fossil Microvictoria svitkoana was stem-group Nymphaeales, although other studies had suggested it was crown-group Nymphaeaceae (Gandolfo et al. 2004 [its initial description]; Endress 2006; Friis et al. 2011; López-Martínez et al. 2023a: ANA grade and Magnoliidae). In another recent general phylogenetic analysis it ended up in a number of positions in the ANA grade, also in Laurales-Calycanthaceae, although not in the [Cabombaceae + Nymphaeaceae] clade itself... (Schönenberger et al. 2020), while Doweld (2022) placed it in its own family which he thought was in Illiciales (= Austrobaileyales here).
Evolution: Divergence & Distribution. D. W. Taylor et al. (2008) looked at vegetative evolution in [Cabombaceae + Nymphaeaceae]; the inclusion of different fossils in analyses of morphological variation affected the relationships they obtained, and hence evolutionary interpretations. Friis et al. (2011: fig. 20.2) discussed diversification here in some detail, also assigning many Palaeogene fossils to the two families. Carlquist and Schneider (2009) suggest a number of distinctive features of the xylem in this clade.
Carpenter (2005) described the stomata as being largely variants of the actino/stephanocytic types; only one member of Cabombaceae was studied. Rudall and Knowles (2013) looked at stomatal development in some detail, observing that it was perigenous, the subsidiary cells being members of different cell lineages than the guard cells (other members of the ANA grade are more or less mesogenous); there is a similarity here to other aquatic lineages such as Callitriche (Doll et al. 2021). Rudall and Knowles (2013, see also Rudall 2023b) noted that other taxa with anomocytic stomata had mesogeneous stomatal development; very unusually, in the two families here guard mother cells were produced directly by protodermal cells, there being no asymmetrical cell division immediately preceding their formation.
Titova and Batygina (1996) described the morphology of the embryo of Nelumbo, which they thought belonged to Nymphaeaceae, in some detail, noting that it was dorsiventral, and in that being similar to other Nymphaeaceae (both families below), Ceratophyllum and monocots.
Ecology & Physiology. Members of this clade, along with other angiosperms, may have come to dominate aquatic habitats in Europe by the Albian ca 105 Ma (Sender et al. 2010).
Pollination Biology & Seed Dispersal. Generalist pollination is common here (Gotttsberger 2016); see also Luo et al. (2018) for a summary and references.
Genes & Genomes. L. Zhang et al. (2019) note that there is a genome duplication around here, certainly in Nymphaeaceae, but perhaps also including Cabombaceae.
For the chloroplast inverted repeat, see Graham and Olmstead (2000).
Chemistry, Morphology, etc.. Warner et al. (2008, 2009) discuss perianth evolution, and in Warner et al. (2008) there is a useful summary of the literature on perianth morphology. Zini et al. (2016) described the distinctive nucellar epidermis of the ovules as being an epistase.
For information on anatomy, see Gwynne-Vaughan (1897), for root epidermis, see Voronkina (1974: ordinal characterisation above), for root anatomy, see Seago (2002), for laticifers, see Prado and Demarco (2018), for peduncular/floral anatomy, see Moseley et al. (1993), for perianth venation, Hiepko (1965b), for pollen morphology, Osborn et al. (1991), for embryo sac development, Orban and Bouharmont (1998), for endosperm evolution, Floyd and Friedman (2001), for ovule development, Yamada et al. (2001b), for pericarp anatomy, Yatzenko et al. (2012), for seed anatomy, see Collinson (1980) and Chen et al. (2004), for seedlings, see Ito (1982), and for general information, Raciborski (1894a, b), Les et al. (1999) and E. L. Schneider et al. (2003).
Phylogeny. See above.
CABOMBACEAE A. Richard - Back to Nymphaeales
Rhizomes monopodial; alkaloids 0; stem with two pairs of vascular bundles, protoxylem lacunae + [see below]; internodes long; petiole with one bundle pair; lamina peltate; inflorescences sympodial (in part); pedicel with 3 bundle pairs; flowers rather small, parts whorled, (2)3(4)-merous, polysymmetric [hexamerous]; P = two whorls of T, C-like, all members with single trace; filaments moderately slender; tapetum more or less amoeboid; pollen endexine lamellate when young, not when mature; pollen tube growth intra-gynoecial; when G 3 or more, inner whorl of three ± opposite inner P, three vascular bundles equidistant, stylulus +, short, stigma papillate; extragynoecial compitum at least initially 0; ovules 1-3(-5)/carpel, attached variously, outer integument semi-annular [hood-shaped], hypostase +; hilum and micropyle sharing same opening in center of operculum; micropylar endosperm cell with free-nuclear divisions [endosperm helobial]; x = 7 (?8, ?6), nuclear genome [1 C] (0.036-)1.989(-109.219) pg; seedling erect, internodes short [= short shoot of sorts].
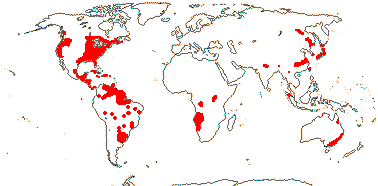
2 [list]/6. World-wide, rather scattered, Brasenia schreberi subfossil remains show it to be far more widespread in Europe than at present. Map: from Raymond and Dansereau (1949), Fassett (1953), Ørgaard et al. (1992), Hultén (1961), Fl. N. Am. vol. 3 (1997), Trop. Afr. Fl. Pl. Ecol. Distr. vols 1 (2003), 6 (2011) and Löhne et al. (2008). [Photo - Brasenia Habit] [Photo - Flower.]
Age. Bell et al. (2010) suggested that the two genera diverged (31-)21, 20(-10) Ma, but other estimates are considerably older, e.g. (109-)101(-93), (75-)67, 60(-52) Ma (Wikström et al. 2001) and ca 114.4 Ma (W. Song et al. 2024).
Pluricarpellatia, perhaps Cabombaceae, is known from rocks 115 Ma in northeast Brazil (Mohr et al. 2008); Doweld (2022) put the genus in its own order.
Rhizomes from erect short shoots or other rhizomes; vascular plexus at nodes, stomata [on floating leaves] parallel to the veins; short shoots with cataphylls, submerged leaves opposite or 3-whorled, lamina deeply palmately (subdichotomously) dissected, lobes linear; flowering shoots long shoots, from erect shoots or rhizomes, monopodial (becoming sympodial); flowers initially axillary; T ± in a single whorl, inner T somewhat delayed in development, nectaries +, trichomatous; A 3 [opposite inner T], 6 [alternating with T], extrorse; pollen trichotomocolpate, tectum continuous, striate; G 1-4(-7), (basally connate), style becoming hollow [lysigenous], stigma capitate; fruit follicular; seeds 2-3, spiny; n = 13.
1/5. New World; warmer areas.
Age. It is estimated that divergence within the genus began ca 57.8 Ma (W. Song et al. 2024: ?sampling).
2. Brasenia Schreber - Brasenia schreberi J. F. Gmelin —— Synonymy: Hydropeltidaceae Dumortier
Rhizomes from seedling-derived short shoots; root stele 6-8-arch; vascular bundles bicollateral with central protoxylem lacuna, each surrounded by an endodermis glandular hairs 3-celled; stems encased in thick layer of mucilage, paired, glandular patches at nodes; "lateral bud opposite to the leaf"; flowering on short shoots, from rhizomes, sympodial; pedicel often with 3 bundles; T whorls somewhat different in size; A many, outer ± alternating with T, inner with variable position; pollen scabrate; nectary 0; G 4-18, stigma elongate; fruit achenial, 1-2-seeded; testa cells tall, thick-walled; n = 40.
1/1. Scattered, tropical and (warm) temperate, also southeast Australia and southeast Siberia.
Evolution: Pollination Biology & Seed Dispersal. Brasenia is wind pollinated, while Cabomba has paired nectaries (trichome patches) on its inner tepals and is pollinated by flies. M. L. Taylor and Williams (2009) describe details of reproduction of Cabomba from pollination to fertilization, while E. L. Schneider and Jeter (1982) discuss its pollination. For more details, see Erbar (2014) and Gottsberger (2016)
Chemistry, Morphology, etc.. The root endodermis has a Casparian strip and suberin lamellae. The two bundles that share protoxylem lacunae are adjacent vascular bundles in Cabomba but members of opposite pairs in Brasenia (Moseley et al. 1984; C. Yang et al. 2019). The "vessels" in the roots and rhizomes of Cabomba have helical thickenings that are not developed away from the end plate (E. L. Schneider & Carlquist 1996). It is unclear how to interpret nodal anatomy. In Cambomba a trace leaves from each member of a vascular bundle pair which shortly thereafter fuse commissurally, creating a nodal plexus; the foliar traces fuse and then divide, providing two petiolar bundles (Moseley et al. 1984); Schneider et al. (2003) suggest that the axillary bud in Brasenia is on the opposite side of the stem from the leaf that subtends. The peltate leaves are spirally arranged, although in some taxa they are uncommon, while the more or less dichotomously-divided submerged leaves are opposite; for leaf morphology, see Rutishauser and Sattler (1987).
Schneider et al. (2003: p. S287) described the nodal organisation of Brasenia as follows: "a leaf and lateral shoot bud [are] in opposite positions, with the flower to one side at the same node", perhaps consistent was a basically sympodial organization. There are often five vascular bundles in the sepals and three vascular bundles in the petals of Cambomba, in both cases a single trace leaves the floral axis (Moseley et al. 1984). Stamens are sometimes physically close to each nectary and then they appear paired (Ørgaard et al. 1992). Pollen of Cabomba has striate exine; although the endexine of mature pollen of Brasenia schreberi is not lamellate, it is laid down in plates (M. L. Taylor & Osborn 2006).
The granular infratectum of the pollen of Podostemaceae has been compared with that of Cabombaceae; both are aquatics (Passarelli et al. 2002).
Some information is taken from Raciborski (1894a, b), Moseley et al. (1984: esp. tables 4, 5) and Williamson and Schneider (1993), both general, Richardson (1969: development of Brasenia flowers), Ito (1986a: floral morphology/anatomy), Erbar (2014) and Tölke et al. (2019), both nectaries, Khanna (1965) and Batygina et al. (1982), both embryology, Floyd and Friedman (2000: endosperm development), and M. L. Taylor et al. (2008: esp. pollen).
NYMPHAEACEAE Salisbury - Back to Nymphaeales
Perennials (annuals); myricetin, sesquiterpene [pseud]alkaloids; roots arise at the base of each leaf; root stele polyarch; stem bundles (in concentric rings), axial bundles collateral/concentric, pith with vascular tissue; peduncle bundles radially arranged; astrosclereids +; nodes 3 traces; flowers lateral, replace leaf in spiral/not, large [>3 cm across], haplomorphic; P members usu. with K- and C-like areas; A many, often whorled, laminar, the staminal bundle branched from near base in thecal region, (filaments stout), connective produced or not, staminodes +, next to G; tapetum both glandular and amoeboid; microsporogenesis simultaneous; pollen (exine 0), membranous granular layer + [innermost endexine]; G laterally connate, whorled, postgenital margin fusion complete, placentation laminar, residual receptacle apex + [?level], stylulus 0, stigma dry, radiate; ovules many/carpel (-3), not filling the locule, outer integument also cap-shaped [annular]; embryo sac ± 8-shaped [?level]; fruit baccate [sort of], dehiscence irregular; testa between micropyle and hilum; endosperm scanty, embryo chlorophyllous or not, broad and plug-like, (large); x = 7 (?8, ?6), nuclear genome [1 C] (0.053-)1.666(-52.735) pg; cotyledons, etc., 1-trace.
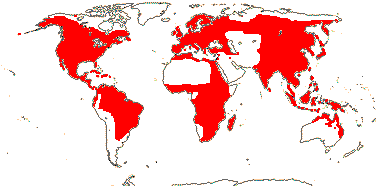
3 [list]/64 - two groups below. World-wide. Map: from Vester (1940), Wickens (1976), Hultén (1961), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), Heywood (2007), and Löhne et al. (2008). Photo: Leaf, Flower.
Age. E. L. Schneider et al. (2004) suggested a crown age for the family of ca 121 Ma, Magallón et al. (2013) an age of around 100.1 Ma, while Iles et al. (2014) suggested an age of (99.6-)95.5(-92.9) Ma while W. Song et al. (2024) suggested an age of as much as 131.7 Ma. However, estimates in Bell et al. (2010) are only (49-)32, 29(-15) Ma and those in Zhou et al. (2014) (60.6-)28.2(-3.8) Ma, while those in Naumann et al. (2013), at around 51.8 or 40.8 Ma, are somewhat intermediate.
The Late Aptian/Early Albian Cretaceous Monetianthus, from Portugal, is embedded in Nymphaeaceae in morphological analyses (Friis et al. 2009b). Microvictoria, a somewhat later fossil from the Turonian ca 90 Ma old and found in New Jersey, U.S.A., is very like Victoria (= Nymphaea). Victoria has paracarpels (= ) immediately surrounding the gynoecium, and these are also found in Microvictoria; indeed, flowers of this latter are like those of Victoria in almost all respects, although they are less than 1/10th their size (Gandolfo et al. 2004) - however, Doweld (2022) placed it in Illiciales (= Austrobaileyales). Jaguariba is perhaps assignable to crown group Nymphaeaceae; it is from the Aptian Crato flora of northeast Brazil and is some 115 Ma (Coiffard et al. 2013a); Doweld (2022) was unsure where it went in the order. For Cecilanthus, from rocks in early Cenomanian Maryland ca 100 Ma, see Herendeen et al. (2016) and above; Doweld (2022) placed it in a family by itself that he thought might best be included in Magnoliales.
1. Nupharoideae Ito - Nuphar Sibthorp & Smith —— Synonymy: Nupharaceae A. Kerner
Rhizomes stout, horizontal, creeping; indolizidine alkaloids +; roots with 10-18 xylem poles, pith broad; lamina deeply cordate, margin entire; peduncle at base with several vascular bundles, one outer satellite bundle, with subbasal abaxial leaf [= bract]; P dimorphic, first member initiated sublaterally, outer members 5-6(-14), spiral, with 3 traces, initially green, often becoming ± completely yellow, inner members many, smaller, C-like, with 1 trace, somewhat delayed in development; nectary on abaxial surface of inner members; no intermediates between A and C; anthers (longer than filament), valvate [H-dehiscence], connective strongly produced; pollen grains tricellular, spiny, tectum continuous, anasulcate, aperture operculate; G 5-23(-36); outer integument 4-6 cells across, parietal tissue ca 2 cells across, nucellar cap ca 4 cells across, hypostase, postament +; fruit emergent, outer P turn green, persistent; endocarp and funicle becoming aerenchymatous, surrounding seeds, individual carpel dispersal unit; seeds hairy, operculate, micropyle and hilum very close; exotestal cells with straight anticlinal walls, lignified, mesotesta ca 4 cells thick, slightly lignified; n = 17.
1/1-15 - ca 11?. North Temperate.
Age. A crown-group age for this genus is ca 21.6 Ma (W. Song et al. 2024: note sampling).
Friis et al. (2017b) were inclined to link Notonuphar, representing fossil seeds perhaps some 37 Ma found commonly in mid to upper Eocene deposits from Seymour Island, Antarctica, to those of Nuphar, which has low exotestal cells (but the two have a similar micropyle-hilum area), although they have tall, thick-walled exotestal cells like those of Brasenia (Cabombaceae).
2. Nymphaeoideae Arnott —— Synonymy: Barclayaceae H.-L. Li, Euryalaceae J. Agardh
(Mycorrhizae +); (rhizome +, short, erect); phloem loading active, cell walls smooth, no plasmodesmata; roots with 5-9 xylem poles, pith at most small; stomata parallel [?orientation]; vegetative buds not axillary; lamina peltate to cordate/hastate, (margin serrate), (prickles on lower surface, pedicel, etc.); stipules +, adaxial and bicarinate or lateral/0; growth ?sympodial, peduncle at base with one bundle, inner satellite bundle +; bract 0 (+ - Victoria); hypanthium ± developed; P members 3-veined, "K" 4-5, spiral/quincuncial, abaxial K first initiated, (apex hooded/"long aristate" - Barclaya [B.]), "C" (0 - some Ondinea - O.), (basally connate - B.), intermediates between A and "C"; A (15 - O), (latrorse - O.), (adnate to "C"), (outer staminodes +, showy, inner staminodes [= paracarpels] + - V.); (staminal bundle unbranched, esp. in smaller inner A); pollen (in tetrads), bi/tricellular, (globose), with encircling sulcus [zonasulcate], (inaperturate), surface various, inc. tectum continuous, infratectal space usu. small, columellae inconspicuous; G 3-many, ± inferior [A alone on top of G, also often K and C], with inter-carpellary septal slits, marginal appendages + [= pseudostigmas/carpellary styles]/(0), central pool of stigmatic fluid, floral axis projecting in the middle (not - B.), stigmatic surface continuous, (not - O); ovules (straight - B.), (micropyle bistomal), outer integument (2[B.]) 4-5, ca 20 cells across [Euryale], (nucellar epidermal cells palisade), (parietal tissue 3-4 cells across - V. ); embryo sac hour-glass shaped [N. thermarum]; fruit maturing submerged; seeds arillate (not, surface spiny - B.); exotesta mucilaginous [?level], (cells cuboid, anticlinal walls straight - E.); (embryo suspensor 0); n = 10, 12, 14-18, nuclear genome [1 C] 450-4557 Mbp; seedlings with hastate leaves.
2/54: Nymphaea (46). World-wide.
Age. L. T. Smith et al. (2022) suggest an age for [Nymphaea ampla + Victoria] of ca 75 Ma while another estimated age is ca 99.8 Ma (W. Song et al. 2024: includes Barclaya, Nymphaea s.l. is ca 69.5 Ma).
Evolution: Divergence & Distribution. The family is thought to have been much more diverse in earlier epochs, distinctive seeds with a micropylar and palisade exotesta with sinuous anticlinal walls that can be assigned here being common in the Cretaceous (Friis et al. 2009b, 2011, 2017b; see also above). Recently, fossils assigned to crown group Nymphaeaceae (as Jaguariba) have been found in the Aptian Crato flora, some 115 Ma in northeast Brazil (Coiffard et al. 2013a) and the first seeds from the Southern Hemisphere have been described in Eocene deposits from Seymour Island, Antarctica (Friis et al. 2017b).
Although the family is widespread and probably very old, individual clades within it are relatively localized, and it has even been suggested that crown group diversification may have occurred in the northern hemisphere in the early Caenozoic (Löhne et al. 2008; see also Friis et al. 2011).
Borsch et al. (2007) and Löhne et al. (2009) suggest possible apomorphies of/within the family.
The cauline vascular bundles are separate, although with much anastomosoing, and the basic stem structure is unlike that of monocotyledons (Weidlich 1976a, b, 1980 and references; Kraehmer et al. 2023). Indeed, it is very difficult to interpret the basic construction of the plant. There has been the loss of some genes involved in cambial activity in Nymphaea thermarum and changes in some genes involvedd in cambial regulation (see also Povilus et al. 2019/2020). Kraehmer et al. (2023) discuss the problem in some detail, and incline to think that Nymphaea alba, at least, is sympodially constructed, the flowers being terminal, hence the absence of the parenchymatous cushions associated with the leaves and the fact that the so-called "peduncle trace" moves to the centre of the stem where it is surrounded by 5-8 vascular bundle complexes.
The floral scents of Nymphaea colorata, at least, may have evolved in parallel with those in mesangiosperms (L. Zhang et al. 2019).
For possible ecophysiological and morphological changes associated with the π duplication, and in more or less contemporaneous duplications in [Magnoliales + Laurales], the core eudicots, and in the monocots, perhaps at the [Asparagales + commelinds] node, see elsewhere.
Ecology & Physiology. The leaves of Nymphaea amazonica are huge, the lamina reaching about 3 m across although it is only ca 1 mm thick. Their construction is interesting: The eight main veins that radiate from the petiole forking as they go to the margin and the cross veins that join them at right angles are relatively much thicker than the rest of the lamina. The distribution of the tissues is such that the blade is remarkably stiff compared with a circular blade of equal mass but also overall equal thickness, but at the same time it is very buoyant and it has flexibility, bending when a bird, for example, walks on it (Box et al. 2022).
Barclaya rotundifolia is a terrestrial plant of wet forests in western Malesia.
Pollination Biology & Seed Dispersal. Thermogenesis has been detected in the flowers of some Nymphaeaceae (Prance & Arias 1975: ca 9.5o in Victoria; Seymour 2001; Seymour & Matthews 2006). Beetles and a variety of other insects, including bees and flies, are pollinators (e.g. Gandolfo et al. 2004; Padgett 2007; Thien et al. 2009; Zini et al. 2019). Scarab beetles (Cyclocephalini) may have pollinated night-flowering water lilies for some 100 My; they pollinate species both in America, where the beetles are common, and in Africa, where the beetles are otherwise very uncommon (Ervik & Knudsen 2003; Moore & Jameson 2013); beetle pollination may have occurred even in the early small-flowered members of the family (M. L. Taylor et al. 2013 and references). The marginal carpellary appendages of the flowers in Nymphaeaoideae function as osmophores in both day- and night-flowering taxa (in Nymphaea s. str. and Victoria, e.g. L. T. Smith et al. 2022) that are pollinated by deceit - beetles and bees (also visual cues in the latter) are involved (Zini et al. 2019); Prance and Arias (1975) suggested that in Victoria amazonica the beetles ate the carbohydrate-rich marginal appendages. The distinctive flowers of Ondinea (= Nymphaea ondinea), wind pollinated (evidence for this?), are derived from Nymphaea-type flowers (Löhne et al. 2009). E. L. Schneider (1979) summarized information about the pollination biology of the family; see also Erbar (2014), Gottsberger (2016: Table 2, much detail), Luo et al. (2018) and Coiro and Barone Lumaga (2018: floral epidermal micromorphology).
The progamic phase, the time between pollination and fertilization, is notably short, up to a mere 8 hours, as in at least some other aquatic angiosperms (including Nelumbo: see Williams et al. 2010).
The fruit of Nymphaeoideae splits open as the mucilage inside (from arils? - L. T. Smith et al. 2022) swells, whereupon the wall splits irregularly; as Jacobsen et al. (2022: p. 14) described the process for Barclaya (which has spiny seeds) "At full maturity the pericarp ruptures, and the spongy substance surrounding the seeds quickly swells, enabling the embedded seeds to spread on the water surface". The newly-released seeds in Victoria s. str. at least initially float (Smith et al. 2022). Romanov et al. (2023) described the individual carpels of Nuphar as being the initial disseminule unit, however, since the outer part of the pericarp fell off irregularly, the fruits could not be called a schizocarp.
Plant-Animal Interactions. Nymphaeaceae are host plants of reed beetles, Chrysomelidae-Donaciinae (see also Poales: Kölsch & Pedersen 2008: much discussion on the age and evolution of the group). Enterobacteriaceae near Buchnera are thought to produce the material that makes up the cocoon that characterises Donaciinae, a group that is also noted for the ability of the larvae to grow under water (Kölsch & Pedersen 2010).
Genes & Genomes. A genome duplication in Nuphar is estimated to have occurred (76.8-)72.8(-67.9) Ma (Vanneste et al. 2014b) and one in Euryale ferox at 106-94 Ma (Y. Yang et al. 2020); it is pegged at the level of [Nymphaea + Nuphar] and dated to around 106.3 Ma (Landis et al. 2018: App. S1), so its extent depends on confirmation of the position of Nuphar... See also L. Zhang et al. (2019) for this/another duplication, while L. Zhang et al. (2020) date what is called the π/pi duplication to (120-100(-98) Ma and X. Guo et al. (2021) date it to 119.8-85.8 Ma.
For chloroplast organisation, etc., see He et al. (2018). Interestingly, Nuphar has the highest number of short tandem repeats (>50), followed by Barclaya (>30), and Nymphaea as few as 19 (He et al. 2018).
Economic Importance. Nymphaeaceae include a disproportionally high number of serious and widespread weeds (Daehler 1997).
Chemistry, Morphology, etc.. The root endodermis has a Casparian strip. There are sometimes sclerenchymatous diaphragms in the pith. The vasculature of the stem is exceedingly complex, especially at the node, with the peduncular complex and a root trace forming from the same axial bundles that supply the leaf (Weidlich 1976, 1980 and references); the vascular bundles were mesarch, the central xylem being mixed with phloem or not and there may be vascular tissue in the pith. E. L. Schneider et al. (2008, 2009) and Schneider and Carlquist (2009) discuss stem tracheids and root vessels, emphasizing the rather arbitrary distinction between vessels and tracheids; Carlquist (2012c) suggested that there were no vessels. The astrosclereids of Nuphar and Nymphaea, at least, have calcium oxalate crystals in the walls (Fink 1991).
In both Nuphar and Nymphaea flowers and even branches may replace leaves in the genetic spiral, and they are certainly not simply axillary (e.g. Cutter 1957a, b; Groß et al. 2006). Cutter (1957b) noted that the leaf apparently subtending the flower of Nuphar was in fact borne on the flower stalk; if a prophyll, it should be noted that it is abaxial in position; for more discussion as to whether or not Nuphar has bracts, see E. L. Schneider et al. (2003).
Flower parts are generally whorled (see Endress & Doyle 2015 and references). Yoo et al. (2010) discuss the evolutionary/developmental relationship between sepals and petals; see also Doyle and Endress (2011), who suggest that Nuphar has both tepals and petals, the latter being the smaller, inner, petal-like structures. E. L. Schneider (1976) and Moseley and Uhl (1985) note that the vascular supply to perianth and androecial members consists of two radially associated bundles; Williamson and Schneider (1994) describe the stamens of Barclaya as having a single trace, and there seem to be intermediates between structures described as petals and stamens (see also Tamura 1982). Zini et al. (2017) looked at the epidermal micromorphology in particular of these tepal whorls, noting that the outer "series" were sepal-like, the innermost corolla-like, and the middle either a mixture of traits or corolla-like. In Euryale the filaments are quite slender and are basally adnate to the staminodes (Floyd & Friedman 2001. Weberling (1989) suggested that in at least some Nymphaeaceae the individual carpels were free laterally but adnate to the central axis inside and to hypanthial tissue outside (see also Moseley 1965; von Balthazar et al. 2008). The ovules of Victoria and Euryale are massive affairs (Zini et al. 2016). Zhou and Fu (2008) found that at anthesis, but not before or after, the micropyle of Nuphar was bistomal, not endostomal (for ovules, see also Shamrov 2022b: Fig. 11). Weberling (1989) described how in Nuphar axial tissue separates from the gynoecium when the fruits are ripe, so exposing the basically free carpels. However, Moseley (1965) seems more accurate, and he described the fruit as eventually being irregularly circumscissile, the seeds being in groups surrounded by aerenchyma that developed from the endocarp and funicle; Padgett (2007) described dehiscence as being along lines in the septal and ovarian walls where aerenchymatous tissue had developed. It is unclear if Euryale has free nuclear endosperm (Floyd & Friedman 2001 - see also Kanna 1964, 1967 for endosperm development in the family). For embryo development and germination in Nymphaea, see Baskin and Baskin (2007b), the embryo may be fully developed before germination begins. The seedling axis in some species of Nymphaea has a lateral projection.
Some information is taken from Raciborski (1894a, b), E. L. Schneider and Williamson (1993) and Padgett (2007: Nuphar), all general; see Schneider and Carlquist (1995) for root xylem of Barclaya, Moseley (1958) and Hufford (1996b) for stamen morphology, Yao et al. (2004), M. L. Taylor et al. (2012, 2013) and Coiro and Barone Lumaga (2013), all pollen morphology, Zini et al. (2014b) and Shamrov (1998: Nuphar) for ovules, Takhtajan (1988) and Losada et al. (2014) ovules and seeds, Romanov et al. (2023: fruit and seed), Cook (1909), Khanna (1964, 1967: 8-nucleate embryo sacs...), Floyd and Friedman (2001) and Povilus et al. (2015, 2018), all embryology, Valtzeva and Savich (1965), embryo development, and Wheeler Haines and Lye (1975: evolutionary stories) and Tillich (1990), both seedling morphology.
Phylogeny. Nymphaea s. str. was monophyletic in a phylogenetic analysis of vegetative characters, but without much support (D. W. Taylor 2008); see also Borsch et al. (2007, 2012) - the genus definitely includes the wind-pollinated and usually apetalous Ondinea, but some relationships lacked much support, e.g. the position of [Euryale + Victoria] as sister to Nymphaea s.l. (see also Gruenstaeudl et al. 2017: support also not that strong). An increasing number of studies are finding that the spiny Victoria and Euryale ("spines" = prickles) are more or less well embedded in Nymphaea s.l. (Löhne et al. 2007, 2009; Borsch et al. 2008; He et al. 2018; Laczkó et al. 2019; c.f. Friis et al. 2011). However, there is no concensus exactly where they should go. Dkher et al. (2011: nuclear ITS only) found them to be sister to the rest of the genus (maximum parsimony, moderate support), or sister to subgenus Nymphaea and in turn sister to the rest of the genus (Bayesian analyses, good support), or "within Nymphaea". The analyses of L. Zhang et al. (2019) placed them as sister to subgenera Lotos and Hydrocallis and in turn sister to the rest of the genus, C. Sun et al. (2021), Roestel et al. (2024) and W. Song et al. (2024), plastome analyses, retrieved the same (or similar) immediate relationships, but the combined clade was embedded in the genus. Sampling in in the Seed Plant Tree of Life i.2022 release was quite good, and there Victoria and Euryale were placed sister to Nymphaea, and with strong support. See also D. W. Taylor and Gee (2014) and Coiro and Barone Lumaga (2018) for relationships, the former integrating data from fossils and the latter floral epidermal micromorphology in their analyses. Padgett et al. (1999) discussed relationships/species numbers in Nuphar.
Classification. Despite a certain amount of confusion, it seems reasonable to include Nuphar in Nymphaeaceae (see above ). It could be argued that a broad circumscription for Nymphaea, certainly including Ondinea, but also the Victoria/Euryale genus pair, is best; note that Brasenia is recovered as the immediate sister group to Nymphaea s.l., although there is not often comment about its position (e.g. Roestel et al. 2024; W. Song et al. 2024), so its separation is a matter of taste - hence the generic numbers above.
Botanical Trivia. The prominent ribs on the uderside of the lamina of Victoria amazonica (as it was then called) were in part the inspiration for William Paxton's design of of the Crystal Palace, essentially a huge greenhouse, that was built for the Great Exhibition in London in 1851 (Box et al. 2022; see above).