EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID II / MALVIDAE / [[GERANIALES + MYRTALES] [CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]]: ?
[CROSSOSOMATALES [PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]]: ?
[PICRAMNIALES [SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]]: ovules 2/carpel, apical.
[SAPINDALES [HUERTEALES [MALVALES + BRASSICALES]]]: flavonols +; vessel elements with simple perforation plates; (cambium storied); petiole bundle(s) annular; style +; inner integument thicker than outer; endosperm at most scanty.
[HUERTEALES [MALVALES + BRASSICALES]]: ?
[MALVALES + BRASSICALES]: ?
BRASSICALES Bromhead - Main Tree.
Glucosinolates + aliphatic, from phenylalanine and branched-chain amino acids [Branched Chain Amino Acids - valine/isoleucine/leucine]; myrosin cells +, little oxalate accumulation; endoplasmic reticulum with dilated cisternae; myricetin, other methylated flavonols, tannins 0; vasicentric axial parenchyma +; tension wood?; mucilage cells in leaf 0; leaves spiral, stipules small; inflorescence racemose; (petals clawed); G [3], ovules in one or two rows; seed coat?; embryo often green, oils erucic and eicosenoic acids. - 19 families, 405 genera, 5,035 species.
Includes Akaniaceae, Bataceae, Brassicaceae, Capparaceae, Caricaceae, Cleomaceae, Emblingiaceae, Gyrostemonaceae, Koeberliniaceae, Limnanthaceae, Moringaceae, Pentadiplandraceae, Resedaceae, Salvadoraceae, Setchellanthaceae, Tiganophytaceae, Tovariaceae, Tropaeolaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) dated crown-group Brassicales to 79-71 Ma. Other estimates are (76-)73(-70) and (63-)69(-57) Ma (H. Wang et al. 2009, two penalized likelihood dates), while Magallón and Castillo (2009) estimated ages of ca 65.9 Ma, Bell et al. (2010) ages of (94-)83, 82(-69) Ma, while (133-)92(-50) Ma is the estimate in Edger et al. (2015: note topology) and (112.6-)102.8(-94.4) Ma is that in Cardinal-McTeague et al. (2016).
Dressiantha, the earliest fossil placed by some in this clade, is from the Turonian, ca 89.5 Ma (Gandolfo et al. 1998c), but c.f. Couvreur et al. (2010) and also below.
Evolution: Divergence & Distribution. For ages in this clade, see Walden et al. (2020b: plastome analyses).
Brassicales contain ca 2.2% eudicot diversity (Magallón et al. 1999) and show quite high diversification rates (Magallón & Sanderson 2001; Magallón & Castillo 2009), perhaps also linked to the absence of mycorrhizae (see below) and the herbaceous habit (see Maherali et al. 2016), both very common in the order.
Cardinal-McTeague et al. (2016) provide ages for nodes throughout Brassicales, and the dates below are taken from their Fig. S1 and Table S5 which are based on all four calibrations that they used; these calibrations are discussed in their Table S3. Interestingly, if they exclude the fossil Dressiantha, as would seem to be reasonable (see below), they obtain somewhat older ages for the deeper nodes in particular - and ages in Fig. S1 are already older than most others proposed for the order and for branches within it. Cardinal-McTeague et al. (2016) go on to discuss the biogeography of the order.
There is a close association between pierine butterflies ("cabbage whites") and Brassicales in general, and with Brassicaceae in particular. Pieridae started diversifying (108-)87(-67) Ma (Espeland et al. 2018). Two clades of Pierinae, Pierini-Pierina and Anthocharidini (Edger et al. 2015; Wahlberg et al. 2014 for phylogeny and classification), may have moved on to Brassicales from an original host in Fabaceae (Braby & Trueman 2006; Braga et al. 2021) some (90-)85(-60) Ma; Brassicales as a whole seem to be able to synthesize glucosinolates (GSLs). Note that Fabaceae tend to be both rich in N and myrmecophilous, while pierid butterflies, although non-myrmecophilous, also prefer N-rich plants (Pellissier et al. 2012). In thinking about the diversity of hosts of Pieridae and the particular pierid groups involved, combining elements of the escape-and-radiate (Ehrlich & Raven 1964) and the oscillation (Janz & Nylin 2008) hypotheses seems the right approach (Braga et al. 2021); Santalales are also part of the story.
Edger et al. (2015) discuss GSL evolution in the order in the context of genome duplications, linking this evolution to both brassicalean and pierine diversification; their dates for the two major genome duplications in Brassicales are followed here, but note the difference in the topology of their tree and the one immediately below. Edger et al. (2015) suggested that within 10 Ma or so of the origin of Brassicales (133-)92(-50) Ma Pierinae had evolved GSL detoxification in the form of nitrile-specifier protein (NSP) genes, and so they could colonize Brassicales (the two clades mentioned above have independent NSP lineages), and this seems to have been accompanied by bursts in diversification of the butterfly. Espeland et al. (2018) suggest somewhat different dynamics, with Pierinae starting to diversify ca 60 Ma, well after the origin of GSL-containing Brassicales that was dated to 92-80 Ma. Tryptophan-derived indole glucosides, representing a notable increase in the brassicalean GSL armamentarium and perhaps characterising the clade [Limnanthaceae + the rest] or the next node up, are associated with the At-β WGD event (112-)77.5(-42) Ma (but see McKibben et al. 2024); however, from the pierid's point of view these are now important cues for host recognition and oviposition (Edger et al. 2015). (Other suggestions are that the crown-group age of the Pieridae may be (63.9-)51.7(-40.4) Ma (Kawahara et al. 2019) or (92.4-)76.9(-63.1) Ma (Chazon et al. 2019: also other estimates).) Interestingly, subfamily Coliadinae are sister to Pierinae, but their caterpillars are not to be found on Brassicales and with ca 300 species the group is rather less diverse than Pierinae with its ca 700 species (Wheat et al. 2007; Fordyce 2010). For more on GSLs, see below.
Rodman et al. (1996) proposed some apomorphies forBrassicales (and for some nodes within it), but where some are to be placed on the tree is currently unclear; Tobe (2015b) also makes suggestions about the evolution of a number of characters. Characters like ovules in two ranks, parietal placentation and clawed petals are notably common in this clade. Ronse de Craene and Haston (2006) examined morphological evolution in Brassicales in the context of a combined molecular and morphological analysis.
Ecology & Physiology. Nearly all GSL-producing flowering plants are in this clade (c.f. Kjær 1974; Dahlgren 1975); Putranjivaceae (Malpighiales) are confirmed as being unrelated GSL-containing plants, however, families like Phytolaccaceae (Caryophyllales) and Pittosporaceae (Apiales) that were once thought to have GSLs are unlikely to contain them (Fahey et al. 2001 for a summary), while Oceanopapaver, a genus once of uncertain affinities but now firmly placed in Malvaceae (= Corchorus: Whitlock et al. 2003), was also once thought to have myrosin cells. There are recent reports of GSLs from Rinorea, in Violaceae (Montaut et al. 2016), and Luvunga, Rutaceae (Sirinut et al. 2017), which should be confirmed.
The ecophysiological interactions involved in the GSL pathway and their evolution make a complex story, and some of the physiological interactions are shown by Nakano et al. (2016: esp. Fig. 6); see also Moghe and Last (2015), Mérillon and Ramawat (2017), Chhajed et al. (2020), etc., for much information on GSLs. Most work on GSLs has been carried out in Brassicaceae, q.v., and within Brassicaceae on Arabidopsis, Brassica and other cultivated plants like Eruca sativa. GSLs are mustard oil glycosides, and mustard oils are esters of isothiocyanic acid with a R—N=C=S group (organic thiocyanates have a -S-C≡N group; glucosinolates = glucose-S-(C-R)=N-C-SO3-); they have a pungent smell and sharp taste. Specialised, protein-rich myrosin cells with rough endoplasmic reticulum contain enzymes like thioglucoside glucohydrolase (a ß-thioglucohydrolase, or myrosinase) that break down GSLs - of themselves not of particular interest - into glucose and aglucones when plant tissue is damaged, as by a herbivore, and enzyme and GSL substrate are brought into contact; the two parts of the defence system are physically separate before the damage (Nakano et al. 2016). The aglucones then automatically rearrange and form toxic isothiocyanates, or are converted into thiocyanates (mustard oils), nitriles (not necessarily toxic), and a variety of other compounds (e.g. Andréasson et al. 2001). As Chhajed et al. (2020: p. 9) summarize the situation, "contact of glucosinolates with myrosinases activates the rapid generation of an unstable aglycone—a thiohydroximate-O-sulfate intermediate which undergoes elimination of the sulfate group, leading to the formation of biologically active chemicals, including nitriles, epithionitriles, thiocyanates, oxazolidine-2-thiones, and/or isothiocyanates". Eisenschmidt=Bönn et al. (2019) discuss how specifier proteins, Fe++-dependent lyases (they can form new double bonds or new rings in their substrate) are involved in the production of compounds other than isothiocyanates (see also Wittstock et al. 2016 for a review). Together such reactions make up the "Senfölbombe" or "mustard oil bomb", most details of which, as mentioned, have been worked out in Brassicaceae, especially Brassica itself (see Rask et al. 2000, Wittstock et al. 2003; Grubb & Abel 2006; Halkier & Gershenzon 2006; Agerbirk et al. 2008, 2010; Burow et al. 2009; Textor & Gershenzon 2009 and other papers in Phytochem. Review 8(1). 2009; Pentzold et al. 2014; Kakizaki 2017; Blazevic et al. 2017, 2019; Chhajed et al. 2020; Okamura et al. 2022; Abdel-Massih et al. 2023). The ß-glucosidase is similar to those that break down cyanogenic glycosides, and GSL synthesis has evolved from the pathway by which cyanogenic compounds are synthesized (Halkier & Gershenzon 2006; Bak et al. 2006; Morant et al. 2008), ancestral cyanogenic glucoside transporters evolving an affinity for specific GSLs by gene duplication and subfunctionalization (Jeon & Kim 2024). Although GSL products may be protective (but see below), their metabolic cost is high (Bekaert et al. 2012). For more on the breakdown of GSLs, see e.g. Wittstock et al. (2016).
It has been suggested that there are well over 200 GSLs (Fahey et al. 2001; Agerbirk & Olsen 2012; Leite & Castilho 2017; Kakizaki 2017); Arabidopsis thaliana alone can synthesize over 40 different kinds (Olsen et al. 2016). However, a recent critical survey listed only 88 GSLs that were well documented, or maybe up to 137 if poorly-documented structures were included (Blazevic et al. 2019). Interspecific variability in the genes sythesizing GSLs is notably high (Kliebenstein 2006). GSLs are synthesised from a variety of amino acids, including methionine, alanine, valine/isoleucine and/or leucine (= BCAA - Branched Chain Amino Acids) in several families of Brassicales, i.e. they are aliphatic GSLs, however, there is much more diversity in families in core Brassicales, although some families there are poorly known (Rodman 1991a; esp. Mithen et al. 2010 for data). It has often been thought that in addition to BCAA glucosinolates there are also indolic (tryptophan is the amino acid) and aromatic/benzenic (from phenylalanine or tyrosine) GSLs. However, the best way to classify GSLs is unclear. Thus Blazevic et al. (2019) note that indeed indolic GSLs are formed from amino acids like tryptophan, aromatic GSLs from phenylalanine, etc., and aliphatic GSLs from methionine, etc., however, they suggest that a classification based on GSL degradation products, i.e., stable isothiocyanates, isothiocyanate ions, or OAT (oxazolidine-2-thiones), might be better, or perhaps classifications should be purpose-driven (see also Agerbirk et al. 2021b). Furthermore, although Agerbirk et al. (2021b: e.g. Fig. 5) distinguished between recent GSLs, largely restricted to Brassicaceae, those of intermediate age/recently-evolved, known from Brassicaceae, Cleomaceae and Capparaceae, and ancient GSLs, which are found more basally in the tree, they noted that the distributions of some of the GSLs derived from different amino acids needed checking. Comments on GSL evolution here should be treated accordingly.
Note that not all taxa producing mustard oils have idoblastic myrosin cells, and myrosinases may also be found in guard cells (perhaps their original location - Shirakawa & Hara-Nishimura 2018; Chhajed et al. 2020), aleurone-type cells, etc., overall, GSLs and myrosinases show a variety of degrees of colocalization, details of where all the various components of this whole system are found in the plant being poorly understood (Chhajed et al. 2019). Furthermore, there are typical (classical) and atypical myrosinases, and they differ in their need for ascorbate as a cofactor, whether or not they can also use O-glucosides as substrates, etc. (e.g. Chhajed et al. 2020). There is no evidence that the myrosinases in the two systems have a common origin; the myrosinases in myrosin cells are "typical" myrosinases, but those in aphids, bacteria, etc. are atypical (Nakano et al. 2016; Chhajed et al. 2020); for further details, see below. Even in Brassicaceae, which do have myrosin cells, their distribution varies with the age and species of the plant concerned (Maile 1980; Andréasson et al. 2001). In many Brassicales with stomatal myrosin cells, myrosinases occur in large quantities (Jørgensen 1995), but they also occur in large amounts (thioglucoside glucohydrolase 1 - TGG1) in the guard cells of Arabidopsis thaliana, at least, even although Brassicaceae lack stomatal myrosin cells. Here myrosinases may have become involved in the signaling mechanisms of stomatal opening and closure, or the products of hydrolysed GSLs may evaporate through the stomatal pores, deterring herbivores and/or attracting their parasites (Zhao et al. 2008). For more, see Schranz et al. (2011, 2012), Jeon and Kim (2024), etc..
Attempts are being made to engineer GSLs into non-GSL-containing plants such as tobacco (Geu-Flores et al. 2009); this has now succeeded in Nicotiana benthamiana (Sønderby et al. 2010), so anybody for cabbage-tasting tobacco?
Plant-Animal Interactions. GSL-plant-herbivore interactions have been much studied, e.g. see Schoonhoven et al. (2005) and Textor and Gershenzon (2009) for literature. The activity of the various products of GSL breakdown depends in part on the structure of the side chain (Hopkins et al. 2009; Sønderby et al. 2010; see also above). Furthermore, the plant may respond to herbivory by increasing its production of GSLs, and/or by priming its response to subsequent herbivores (Chhajed et al. 2020). GSL products may protect against herbivory by generalist herbivores, and Müller et al. (2010) discuss the effect of different classes of GSLs on different caterpillar species. However, as in other cases where plants produce notably noxious metabolites (e.g. Apocynaceae), groups of herbivores have specialized to eat Brassicales. Thus caterpillars of the 780-840 species of Pieridae-Pierinae (food plants of ca 360 species in 33+ genera have been recorded) are commonly found on brassicalean plants (Fraenkel 1959; Tempère 1969; Ehrlich & Raven 1964; Braby & Trueman 2006; Braby et al. 2006; Beilstein et al. 2010), including Bretschneidera, although they are abundant only in the [Capparaceae [Cleomaceae + Brassicaceae]] clade, a clade that neverthless includes around 90% of the species in the order. Caterpillars of the pierid Appias subgenus Catophaga (albatrosses) are found more or less indiscriminately on Drypetes (Malpighiales-Putranjivaceae) and Capparaceae (Yata et al. 2010; see also Quicke et al. 2025), and of course both groups contain GSLs. Pierinae-Aporiina are found on on santalalean hosts (Braby & Trueman 2006), but few details of the interactions between the two seem to be known and GSLs have not been recorded from Santalales. Some members of subtribe Pierina have moved from Brassicaceae back to Fabaceae, their original hosts (Braga et al. 2021).
Two clades of pierids have moved on to Brassicales, and GSLs stimulate the adults to oviposit and the larvae to feed (Müller et al. 2010: indole and aliphatic GSLs with additive effect; Okamura et al. 2022). The herbivores that have evolved to be able to handle the GSL defence do so in a variety of ways, including avoiding breaking down the cells (so the different parts of the breakdown pathway do not come together), rapidly absorbing the GSLs (which has much the same effect), converting GSLs to desulfoGSLs that cannot be broken down by myrosinase, producing less toxic nitriles, eating parts of the plant with less GSLs, and so on (e.g. Bones & Rossiter 2006; Agerbirk et al. 2010; Winde & Wittstock 2011; Pentzold et al. 2014; Chhajed et al. 2020). The ability of pierine caterpillars to live on Brassicales (in general, there is little host specificity of such insects within the order) is associated with the evolution of a novel GSL detoxifying mechanism in which nitriles rather than toxic isothiocyanates are produced on the hydrolysis of the GSLs (the nitrile-specifier protein - NSP - gene: e.g. Fischer et al. 2008; Edger et al. 2015), and more basal pierine clades that lack this mechanism are less diverse (Wheat et al. 2007). These nitriles are then excreted unchanged (aliphatic nitriles) or further metabolized (aromatic nitriles), and for pierids, the evolution of NSP may have been a key innovation (Winde & Wittstock 2011) allowing them to radiate in GSL-containing Brassicales. Okamura et al. (2022) looked at the interaction of NSPs and MAs, major allergens, that together facilitate detoxification, finding that they worked in a complementary fashion and in response to particular GSL profiles of natural host plants, NSPs more on aliphatic GSLs and MAs more on benzyl GSLs. There are normally three domains in the Brassicaceae GSL-detoxifying NSP genes, but a 2-domain NSP major allergen gene has recently been found in Leptosia nina - it also has a three-domain NSP - which is a member of a small, monogeneric pierid tribe, Leptosiaini, the caterpillars of which eat Capparaceae (Okamura & Vogel 2024). It is interesting that a single carbon atom can be removed from aromatic nitriles in the form of hydrogen cyanide, and the mustard oil bomb changes into a cyanide bomb, but the larvae of Pieris rapae, at least, detoxify the HCN in their guts (Stauber et al. 2012). Benzyl GSLs that are detoxified in this way are found in Tropaeolaceae, Caricaceae, etc. (see below; Stauber et al. 2012). In the [Capparaceae [Cleomaceae + Brassicaceae]] clade GSLs are synthesized primarily from methionine and caterpillars of about four times as many pierines are to be found here as are found on Brassicales with indoline-derived GSLs (Edger et al. 2015).
Relationships between herbivores and plants - again, nearly all information comes from Brassicaceae - are complex. Both specialised herbivores and their hymenopteran parasites may be attracted by isothiocyanates (Hopkins et al. 2009 and references), even if the isothiocyanates may deter predators of the herbivores, the latter being aphids in this case. Although it has been suggested that the slender stylets of aphids may miss the myrosin cells (e. g. Kos et al. 2012a), such phloem-feeding insects are in fact exposed to glucosinolates. However, Malka et al. (2020) found that the whitefly, Bemisia tabaci (Sternorrhynca), was able to detoxify glucosinolates with short aliphatic side chains by serial α-glucosylation - the glucosinolates could then not be hydrolytically activated, and so were impotent. Other phloem-feeding arthropod herbivores dealt with glucosinolates in a similar fashion, but chewing herbivores did not (Malka et al. 2020). While the growth rates even of specialized herbivores may be reduced by GSLs (but that of generalized herbivores is reduced more), that of parasitoids may increase as the growth rate of their host caterpillar decreases (Kos et al. 2012b).
Some chrysomelid beetles also favour Brassicales, for example, the 180 species of the flea beetle Phyllotreta (Alticinae - see Jolivet & Hawkeswood 1995). Phyllotreta striolata produces its own myrosinase to break down the GSLs it acquired from the plant - aliphatic GSLs suit the insect best (Beran et al. 2014). A number of other insects can also sequester GSLs intact, and at least some aphids also have their own myrosinases (Beran et al. 2014 for references) - although sometimes the plant seems to ignore aphids, perhaps because the latter attack single cells (Textor & Gershenzon 2009, but see above). The dipteran leaf miner Liriomyza brassicae is found on Resedaceae, Cleomaceae, Tropaeolaceae and Brassicaceae (Spencer 1990).
Plant-Bacterial/Fungal Associations. Chhajed et al. (2020) summarize work on the interactions between GSLs and potential bacterial and fungal pathogens; less studied than insect-plant interactions, they are still of importance in plant-microbe interactions.
Genes & Genomes. For ca 800 genes (including those lost in Brassicaceae) unique to Brassicales - or at least [Caricaceae + The Rest], see Bhide et al. (2014). Lysak (2018) discusses genome duplications, the evolution of chromosome numbers (base number - x = 9 or 14?), etc., in the order.
Chemistry, Morphology, etc.. A report of ellagic acid in "Capparidaceae" (Bate-Smith 1962) needs to be confirmed. Zindler-Frank (1976) lists seven families scattered throughout the order as having little oxalate accumulation.
Many taxa seem to have diarch roots, although not some Cleomaceae; sampling in the basal pectinations is poor. Carlquist (2016) carried out a comprehensive anatomical survey of the order; as he noted, features like storying of the vascular tissue might be hard to see in a group with many herbs, or plants in which woodiness is not well developed. Most families have "stipules" of some sort or another, although they are often small; 1:1 nodes are common in the order (Ezelarab & Dormer 1966), and nodal and stipule anatomy and morphology would repay further study - are some stipules modified colleters? Strongly developed and fused ventral carpellary bundles may be another synapomorphy for the order (Ronse de Craene & Haston 2006).
For general information, see Mehta and Moseley (1981) and Kubitzki (2002a, b: as Capparales), for chemistry, see Leite and Castilho (2017) and Fay and Christenhusz (2010), for anatomy, see Carlquist (1985a); also Tobe and Raven (2012: aspects of seed evolution).
Phylogeny. For early phylogenetic work on Brassicales, see Rodman (1991a, b) and Rodman et al. (1993, 1994). Relationships within Brassicales show a fair bit of structure, as Rodman et al. (1997, 1998), Carol et al. (1999), Chandler and Bayer (2000), Kubitzki (2002a), Olson (2002a), Hall et al. (2004) and Cardinal-McTeague et al. (2016) have found. A fairly early transcriptome analysis yielded a clade [[Moringaceae + Caricaceae] [Tropaeolaceae + Akaniaceae]] sister to a clade made up of the rest of the order (Edger et al. 2015: sampling only moderate), W. J. Baker et al. (2021a: see Seed Plant Tree, support seems good; also version i.2022, Tropaeolum tuberosum has wandered into Brassicaceae), H.-T. Li et al. (2021: plastome analyses) and Hendriks et al. (2022/2023: nuclear data, sampling poor, general relationships not the focus of the study). These relationships are followed below, although Walden et al. (2020b: plastome data) recovered the basal relationships [[Carica [Akania + Tropaeolum]] [Moringa ...]], although, again, these relationships were not the focus of this study.
Proceeding up the tree. Setchellanthus has often come out just basal to Limnanthaceae in molecular phylogenies (Karol et al. 1999: support weak, see also Rodman et al. 1997; Chandler & Bayer 2000; Karol et al. 1999: strong support; Edger et al. 2018) although the position of the two was reversed in M. Sun et al. (2016) and the position of Limnanthaceae had very little support in H.-T. Li et al. (2021). [Koeberliniaceae [Bataceae + Salvadoraceae]] is an odd little group, but it has often formed a clade, however, Sun et al. (2016) suggest that Salvadoraceae are paraphyletic, Azima tetracantha in particular being sister to Batis. Edger et al. (2018; Edger et al. 2015 is compatible with this topology, see also Lysak 2018) found the well supported relationships [... [[Bataceae + Salvadoraceae] [Koeberliniaceae ...]]]; the position of Koeberliniaceae is poorly supported in Li et al. (2021). Relationships in this area are indeed unclear. Swaenepoel et al. (2020) used molecular data to place the remarkable Tiganophyton in Brassicales, and in an analysis of one mitochondrial and three plastid markers they suggested that it was in the Bataceae area. However, the relationships they found there were ...[Limnanthaceae [[Koeberliniaceae [Tiganophytaceae [Salvadoraceae with Bataceae embedded]]]..., and these last three families are placed in a tritomy below for the time being. Relationships in Baker et al. (2021a) were more pectinate, but sampling was insufficient to work out what was happening. In the Seed Plant Tree ii.2022 version, relationships in this area are ... [[Limnanthaceae + Setchellanthaceae] [[Tiganophytaceae [Salvadoraceae + Batidaceae]] [Koeberliaceae ..., support was generally strong apart for that for the position of Koeberliniaceae, while there was no support for the [Limnanthaceae + Setchellanthaceae] clade. So what to do?
There are details of relationships in the Core Brassicales below.
An analysis of morphological data alone yielded only one clade ([Polanisia + Cleome]!) in Brassicales that had even weak support, and Bretschneidera and Akania were associated with Sapindaceae (Ronse de Craene & Haston 2006). Morphology was also combined with molecular data (four genes, some taxa lacked up to three of them) in this study; there are differences in detail of the topology of the tree there and that used here (c.f. Ronse de Craene & Haston 2006).
Analysis of nuclear phylotranscriptomic data, albeit with sparse samping, confirmed some of the relationships already suggested along the spine of Brassicales (Mabry et al. 2020); this work should be extended, it is to be hoped it will help resolve the problem areas just mentioned.
Classification. For the history of the classification of the group, see Fay and Christenhusz (2010). The limits of families in the core Brassicales have been rather labile in the past, but it is to be hoped that they can now settle down (see A.P.G. IV 2016).
Previous Relationships. Some Brassicales, particularly Brassicaceae and immediately related families, have always been placed together based on morphological similarity and chemistry (smell!), but until quite recently the others have been widely separated. Many brassicalean families are included in Violanae (Dilleniidae) by Takhtajan (1997), but Gyrostemonales are in Gyrostemonanae (Caryophyllidae) and Limnanthales in Solananae (Lamiidae). Cronquist (1981) placed families here included in Brassicales in his Violales (Caricaceae), Capparales (several families), Batales (Gyrostemonaceae, Bataceae), orders scattered through Dilleniidae, as well as in his Geraniales (Limnanthaceae) and Sapindales (Akaniaceae), both in his Rosidae, etc.. Rolf Dahlgren began the process of pulling the order together by emphasizing the distinctive chemistry (e.g. R. Dahlgren 1975a; G. Dahlgren 1989, and references; summary in Jørgensen 1995).
Synonymy: Brassicineae Shipunov, Resedineae Engler - Akaniales Doweld, Batales Engler, Capparales Berchtold & J. Presl, Caricales L. D. Benson, Gyrostemonales Takhtajan, Limnanthales Martius, Moringales Martius, Resedales Link, Salvadorales Reveal, Tovariales Nakai, Tropaeolales Martius - Capparanae Reveal, Gyrostemonanae Takhtajan,
[[Akaniaceae + Tropaeolaceae] [Moringaceae + Caricaceae]]: testa multiplicative.
[Akaniaceae + Tropaeolaceae]: young stem with separate bundles; vessel elements with scalariform perforation plates; axial parenchyma sparse, adjacent to vessels; bracteoles 0; flowers quite large [ca 1.5≤ cm across], obliquely monosymmetric [median C adaxial]; K + C forming a tube, C clawed; A 8; pollen colpate; placentation apical-axile, style long; ovules 1-2/carpel, epitropous; K deciduous; testa vascularized.
Age. The crown age of this clade is estimated at 61-54 (Wikström et al. 2001), (56-)35, 34(-18) Ma (Bell et al. 2010), (54-)36(-19) Ma (Edger et al. 2015: note topology), or (86.6-)75(-66.1) Ma (Cardinal-McTeague et al. 2016).
Chemistry, Morphology, etc.. Carlquist and Donald (1996) give additional characters of wood anatomy that may unite these two families. Tropaeolaceae may have basically pinnate leaves (Endress 2003c), but this can perhaps be cleared up by developmental studies - another synapomorphy for the clade?
Although both families have a nectary, it is extrastaminal in Tropaeolaceae and intrastaminal in Akaniaceae. Furthermore, exactly which stamens are reduced and details of the plane of asymmetry of the flowers differ between Tropaeolum and Bretschneidera; the former is obliquely asymmetric only in bud (Ronse Decraene et al. 2002a; Ronse Decraene & Smets 2001a). A "hypanthium" was described as "lifting sepal lobes and petals high above the stamen insertion" by Ronse Decraene et al. (2002a: p. 44), this "hypanthium" is a calyx + corolla tube in the strict sense; there is also a true hypanthium in Bretschneidera, but not elsewhere (Stapf 1912; Ronse Decraene & Smets 2001a).
AKANIACEAE Stapf, nom. cons. - Back to Brassicales
Tannins?; cork subepidermal; (vessel elements with simple perforation plates); no bordered pits in imperforate tracheary elements; ?nodes; petiole bundle?; cuticle waxes 0, strong cuticular cracks; stomata associated with papillae; leaves odd-pinnate, leaflets opposite, vernation supervolute-curved, venation pinnate, brochidodromous; pollen tricolpate; G [3-5]; stigma small, 3-lobed; micropyle bistomal; capsule loculicidal; exotestal cells palisade, thick-walled, mesotesta ± thick, cells thick-walled, endotesta thickened; embryo color?; x = 9.
2/2 [list]. Southeast Asia, E. Australia.
Age. Bell et al. (2010) suggested that the two genera diverged (12-)6(-2) Ma, the age in Wikström et al. (2001) is 31-23 Ma, and that in Cardinal-McTeague et al. (2016) is (66.3-)64.1(-62.4) Ma - note that Cardinal-McTeague et al. (2016) included fossils of both Akania (see below) and Dressiantha (also see below) among their calibration points.
Fossils attributed to Akania are known from Patagonia in deposits as old as the Palaeocene 61.7 Ma (Iglesias et al. 2007; Gandolfo et al. 2011; Wilf et al. 2011).
1. Akania bidwillii (R. Hogg) Mabberley
Evergreen tree; stomata in clusters; leaflet margins spinulose; inflorescence branched, ?morphology; flowers polysymmetric, ca 10 mm across; C barely clawed; nectary 0; A 10; stigma small, 3-lobed; ovules superposed, parietal tissue 5-7 cells across; endosperm copious; n = ?
1/1. E. Australia. Map: see above, Australia's Virtual Herbarium (consulted i.2014); also Conran et al. (2019 - fossils), Bannister and Conran (2019: Fig. 1).
2. Bretschneidera sinensis Hemsley —— Synonymy: Bretschneideraceae Engler & Gilg, nom. cons.
Deciduous tree; leaflet margins entire, vernation supervolute-curved, petiolules swollen or articulated; flowers weakly monosymmetric; K ± connate; nectary +; A 8 [?position]; G ([4-5]), stigma small; ovules campylotropous, parietal tissue 7-8 cells across; embryo sac bisporic (the spores chalazal) and 8-celled [Allium type]; n = 9.
1/1. S.W. China, adjacent Vietnam and Thailand, Taiwan. Map: see above. Photo: Collection.
Evolution: Divergence & Distribution. Fossils of Akania are known from deposits in Argentinian Patagonia as young as 18-16 Ma (Brea et al. 2017 and references) and from Miocene deposits ca 29 Ma in New Zealand (Conran et al. 2019), so if the identifications are confirmed, its distribution in the fairly recent past was very different from that now. However, Bannister and Conran (2019) note problems with the interpretation of the fossil record, e.g. the wood of the two genera is very similar, the cuticle of Akania is thin, perhaps explaining why its distinctive stomata have never been found, and so on.
Chemistry, Morphology, etc.. In Bretschneidera, GSLs are also produced from valine/isoleucine and/or leucine.
Stapf (1912) described the pollen of Akania as being 4-porate, c.f. Conran et al. (2019). Bretschneidera has clearly strongly obliquely monosymmetric flowers, the stamens are curved and held under one petal, there is a disc. The seeds of Akania have endosperm and the plant may lack myrosin cells, but wood of the two genera is almost identical. Seedlings of Akania initially produce at least five simple leaves with pinnate venation.
For general information, see Bayer and Appel (2002), for the epidermis, see Bannister and Conran (2019), for the embryology of Bretschneiders, see Tobe and Peng (1990), for ovules, see Mauritzon (1936), for fruit and seed, see Doweld (1996a, b).
Classification. Separating Bretschneideraceae from Akaniaceae was considered optional in A.P.G. II (2003), however, there is nothing lost in combining them (see A.P.G. III 2009).
Previous Relationships. A relationship with Sapindales was often suggested in the past (e.g. Carlquist 1997a), and a phylogenetic analysis of morphological data also suggested this position (Ronse de Craene & Haston 2006). Bretschneidera does look rather sapindaceous, and with its eight stamens and often three carpels...
TROPAEOLACEAE Candolle, nom. cons. - Tropaeolum de Candolle - Back to Brassicales
Fleshy vines, petioles twining, (caespitose herbs), often with tuberous roots; GSLs also from valine/isoleucine and/or leucine, benzyl and 4-methoxybenzyl types, stomatal myrosin cells 0, erucic acid [fatty acid] +; cork cambium deep seated? to more superficial; nodes 3:3; petiole bundles annular, pericyclic fibres 0; cuticle waxes tubular, nonacosan-10-ol prdeominates; leaves flat in bud, lamina peltate, palmately-lobed or -compound, margins toothed to entire, stipules small and in seedling only, to fringed, subfoliaceous and throughout the plant; flowers often axillary, (bracteoles +), ± strongly monosymmetric; K petal-like (not), imbricate or valvate, connate, C 2 (5), (margins ± deeply lobed/laciniate); adaxial spur + [≡ K + 2 C, at the base of the median K], (ca ≤1 mm/0), nectary in spur; A 8 [5 + 3]; pollen grains tricellular, with filaments [= pollen kitt]/0; median G adaxial, style impressed, stigma trifid, dry; ovules ± sessile, outer integument 6-9 cells across, polyploidy in cells, inner integument 3-6 cells across, parietal tissue 0, chalazal zone massive; fruit a schizocarp, mericarps drupaceous or nut-like, (1 G fertile, fruit a samara), K deciduous; seed pachychalazal, integuments digested (part of mesotesta suberized), coat undistinguished; embryo suspensor multiseriate, penetrates micropyle, two much elongated haustoria develop at micropyle [one grows in space around ovule and then into the carpel wall, the other grows ± in opposite direction through the placenta into the funicular vascular bundle], cotyledon cell walls with xyloglucans [thick, pitted - amyloid]; n = 12-15, x = 7 (?8), haploid genome [1 C] (0.037-)0.816(-18.245) pg; germination hypogeal, epicotyl +.
1 [list]/105. New World, esp. Andean. Map: see Sparre and Andersson (1991). [Photos - Tropaeolum Flower, ditto.]
Evolution: Divergence & Distribution. Distinctive reproductive morphologies - section Magallana, with winged fruits, and Trophaeastrum, which lacks a nectary spur - are derived from within the general Tropaeolum morphology of spurred, nectariferous flowers and simple schizocarpic fruits (see also Hershkovitz et al. 2005).
Plant-Animal Interactions. An association between pierine butterflies and Tropaeolaceae may be very recent (Holocene) or much older (c.f. Edger et al. 2015; Braga et al. 2021).
Genes & Genomes. A whole genome duplication may have preceded diversification in Tropaeolaceae (Lysak 2018 and references).
Economic Importance. Tropaeolum tuberosum has starchy tubers and is commonly grown as a food for humans and pigs in the high Andes (Ecuador, Peru, Bolivia) (Eggli 2023).
Chemistry, Morphology, etc.. Carlquist and Donald (1996) report vague storying of the secondary phloem of the root. Kumar (1977) described the nodes as being trilacunar. For the development of the peltate leaf of Tropaeolum majus - the petiole lacks even a basal bifacial zone and development of the lamina is basipetal - see Hagemann and Gleissberg (1996) and Gleissberg et al. (2005).
For an interpretation of the axillary flowers common in Tropaeolum, see Bayer and Appel (2002). Kopka and Weberling (1984) described the spur as developing from the floral axis, although there have been a variety of other theories as to the nature of the spur (see Ronse Decraene & Smets 2001a). The nectary is described as being hypanthial (Troll 1957), but it develops from the K/C spur and is outside the staminal ring which is inserted at the base of the gynoecium (Ronse Decraene et al. 2002a), and thus is not hypanthial in the strict sense. Martínez-Salazar et al. (2021) discuss the genes involved in spur formation, and they include STM homologs in T. longifolium, although there it is expressed only in that part of the floral tube that lacks a spur (it is involved in spur formation in at least some asterids, but it, and other 'asterid' genes, are not involved in spur formation in Aquilegia at all). Martínez-Salazar et al. (2023) note that a copy of the TlTCP4L2 gene is involved in spur/nectary formation in T. longifolium, and the same gene is independently duplicated in Akaniaceae - but of course there is no spur there. The androecium is described as being 4 + 4 in IntKey while Martínez-Salazar et al. (2021) describe it as being a made up of a complete antesepalous whorl and three stamens opposite the petals (T. longifolium: see also Ronse Decraene et al. 2002); there is a fair amount of variation in its development. The median carpel is actually slightly off the median (Eichler 1878; Ronse Decraene & Smets 2001a). There are initially two ovules per carpel, but one does not develop very much. Cells of the integuments become endopolyploid (Nagl 1962). The developing embryo has a very elaborate suspensor haustorial system: The multiseriate suspensor pushes the embryo into the chalazal region of the embryo sac while a cell mass develops at the distal end of the suspensor which is near the apex of the micropyle; from there two filiform haustoria develop which penetrate various ovular and ovarian tissues (Walker 1947; Nagl 1976; Yeung & Meinke 1993 and references).
For general information, see Bayer and Appel (2002) and Eggli (2023: succulents), for an account of the southerly and temperate Tropaeolum section Chilensia, see Watson and Flores (2010a, b), for monosymmetry, see Mair (1977), and for embryology, see Tiwari et al. (1978).
Phylogeny. For phylogenetic relationships in the family, see Andersson and Andersson (2000), and for those in section Chilensia, see Hershkovitz et al. (2006: some hybridization).
Classification. Only Tropaeolum can be recognized; maintaining Magallana and Trophaeastrum would make it paraphyletic and entail the recognition of more genera (Andersson & Andersson 2000). References in Hind et al. (2022) get you to an infrageneric classification.
[Moringaceae + Caricaceae]: woody, stems stout [pachycaul or cauduciform]; endoplasmic reticulum-dependent vacuoles +; xylem ± storied; nodes 3≤/3≤; cuticle wax platelets as rosettes; colleters +, on petiole/lamina, stipules as glands; inflorescences thyrses; flowers whitish; G opposite K, ovary longitudinally sulcate, placentation parietal, placental strands opposite the ventral bundles, style hollow; ovules many/carpel, micropyle bistomal, outer integument 4-6 cells across; fruit angled/strongly ridged; mesotesta ± lignified; embryo white.
Age. The two families diverged some 61-58 Ma (Wikström et al. 2001), (86-)67, 64(-45) Ma (Bell et al. 2010), (93-)64(-34) Ma (Edger et al. 2015: note topology), (94.1-)73.2(-46.4) Ma (Cardinal-McTeague et al. 2016) or ca 64.9 Ma (Rockinger et al. 2016).
Chemistry, Morphology, etc.. Ronse de Craene and Haston (2006) suggested that nodes were unilacunar in this clade; they are tri- or multilacunar.
The sulci in the ovary are in the interplacental position. Whether or not the thickened mesotesta of the two families is comparable needs to be confirmed, but there is certainly substantial anatomical variation in the seed coat (Olson 2002a).
MORINGACEAE Martynov, nom. cons. - Moringa Lamarck - Back to Brassicales —— Synonymy: Hyperantheraceae Link
Deciduous trees (pachycaul), or shrubs with tubers; GSLs also from valine/isoleucine and/or leucine; (vestured pits +), hairs unicellular; schizogenous gum canals +; leaves 1-3-compound, odd pinnate, leaflets opposite, margins entire, stipules 0, but glands at the articulations and at the leaf base; flowers monosymmetric, oblique; hypanthium +, short (long), lined with nectary, K petal-like, imbricate, C imbricate, median [abaxial] C usu. larger than others; A 5, opposite C, declinate, monothecal/(bithecal), staminodes +, opposite K; gynophore +; G ([2-4]), style slender, stigma truncate-porate; micropyle zig-zag, outer integument vascularized, inner integument ca 3 cells across, parietal tissue ca 3 cells across, endothelium +; (archesporium -3-celled); fruit 3-angled, explosively dehiscent, loculicidal; seeds 3-angled, winged (not); mesotesta thick, outer and inner parts with helical thickenings, middle part massive, tegmen thin, (weakly multiplicative), one layer persists; endosperm 0, embryo green, suspensor ?"massive"/0; n = 11, 14, x = 7 (?8, ?6); seedlings with simple or palmate leaves, thickened roots and/or root tubers develop early.
1/12 [list]. India to Africa, Madagascar, Moringa oleifera is quite widely cultivated. Map: from Olson (2001). [Photo - Flower, Collection]
Age. Although Eggli (2023) suggests that the crown age of Moringaceae is ca 14.7 Ma, this age is taken from Carvalho and Renner (2012), who examined only Moringa oleifera, M. peregrina and M. ovalifolia, not the basal taxa (c.f. Olson 2000b, see below). Rockinger et al. (2016) included seven species in their study, including the basal pachycauls Morinda drouhardii and M. hildebrantii (they were not basal here); the age of the clade was estimated to be a mere 11 Ma. Clearly, phylogenetic relationships in the family need to be revisited.
Evolution: Vegetative Variation. Olson and Rosell (2006) suggest that heterochrony is involved in the evolution of the various life forms in Moringaceae; the bottle-tree growth form is probably plesiomorphic, the tuberous shrub growth form is probably derived (see also Olson 2006 for wood anatomy). All species have rather fleshy roots/rootstock and usually grow in more or less arid habitats.
Chemistry, Morphology, etc.. For wood anatomy, see Olson and Carlquist (2001); there are reports of vestured pits from the family (Jansen et al. 2001b).
Flowers of all species are slightly monosymmetric early in development (Olson 2002b), but at anthesis they range from polysymmetric to strongly monosymmetric. When flowers are monosymmetric, they are borne with the median petal adaxial, and when they are polysymmetric the median petal is in the normal abaxial position (Olson 2003). Carpel orientation is in the plane of symmetry of the flower (Ronse Decraene et al. 1998a). Corner (1976) suggests that the micropyle is exostomal. Seeds are borne along the middle of the valves which means that dehiscence is effectively loculical given that the placentation is parietal. The seedlings have either palmately compound leaves or simple leaves with palmate venation (M. Olson, pers. comm.).
Some general information is taken from Ernst (1963), who described the ovules as being apotropous, and Kubitzki (2002d), the Moringa website (Olson 1999) and Eggli (2023) summarize information on the family; see Puri (1941, 1942) for embryology and floral anatomy and Muhl et al. (2016) for embryo and seed development.
Phylogeny. For relationships, see Olson (2000b); three pachycaul bottle trees, Morinda drouhardii, M. hildebrantii and M. stenopetala, the first two from Madagascar, are successively sister to the rest of the genus.
Classification. Given the pectinate base of the tree and the small size of the family, Eggli (2023) prefered not to recognise formal infrageneric taxa.
CARICACEAE Dumortier, nom. cons. - Back to Brassicales
(Tuberous herbs), (pachycaulous) small to medium trees, (viny and with stout tuber), usu. prickly, (stipular spines +); ?cyanogenic glycosides, cysteine proteases +, benzylglucosinolates +, idioblastic myrosin cells 0; laticifers +, articulated, anastomosing; (hairs stinging - Horo.); lamina palmately-lobed or -compound, vernation flat-curved to involute, margins entire or serrate, glands on adaxial surface at base, stipules +; plants di(mon-, tri-)oecious; inflorescences axillary, cymose; staminate flower: K connate, small, C connate, contorted or valvate; A adnate to corolla, 10, of two lengths, whorled, the longer opposite K, [shorter A unithecate - Jarilla], or = and opposite K, connective often developed; nectary on pistillode; carpelate flowers: as above, but C often free; A 0; nectary 0; G [5], (placentation axile), style short/± 0, style branches ± separate, stigmas flabellate or almost petal-like (capitate), dry; inner integument 4-6 cells across; fruit a berry, usu. ± strongly ridged (terete - Carica); sarcotesta +, mucilaginous, mesotesta with lignified ribs, tanniniferous, endotesta crystalliferous (lignified), exotegmen fibrous [?sclereidal]; endosperm +, embryo white; n = 7-9, x = 7 (?6, ?8) or 9, nuclear genome [1 C] (0.023-)0.666(-19.632) pg.
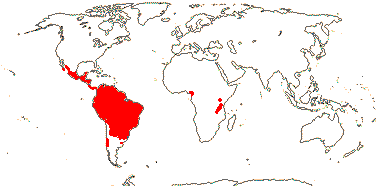
6/34 [list]: Vasconcellea (21). Mostly tropical (Andean) America (three genera in Mexico); Africa (Cylicomorpha). Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Carvalho (2013). Photo: Plant, Flower, Fruit.
Age. Crown-group Caricaceae have been dated to (52.6-)43.1-35.5(-28.1) Ma (Carvalho & Renner 2012) and ca 39.4 Ma (Rockinger et al. 2016).
Floral formula: * [♂] [♀]; K [5]; C 5; A 10; N 0; G 0< - [♂]. K 5; C 5; A 0; N 0; G [5] - [♀].
Evolution: Divergence & Distribution. Caricaceae seem to have originated in Africa, moving to the New World (Central America) by long distance dispersal ca 35 Ma (Carvalho & Renner 2012, q.v. for various dates within the family).
Pollination Biology & Seed Dispersal. Hawkmoths seem to be the pollinators. Note, however, that in Jacaratia nectar is produced only in the staminate flowers (Bawa 1980), as in other members of the family, the nectarless carpelate flowers with their white, spreading stigmas perhaps mimicking the androecium of staminate flowers. Carica (and Vasconcellea?) have a similar floral syndrome, and mimicry is probably general (see also Baker 1976; Eggli 2023b).
Myrmecochory is reported from Carica (Lengyel et al. 2010) despite (perhaps) its fleshy fruits.
Plant-Animal Interactions. Proteases - papain - in the latex of Carica papaya, at least, are involved in defence against herbivorous insects by breaking down the chitin in the peritrophic membrane that surrounds the food bolus, and this is to the detriment of the health of the insect (Konno et al. 2004; see Agrawal et al. 2008 and Mason et al. 2019 for more on such defences) - they are not just meat tenderizers.
Genes & Genomes. The basic chromosome number for the family may be 9 (Murat et al. 2015b). There is no polyploidy here, and unexpectedly the genomes of species with the lowest chromosome numbers are twice the size of those of their closest relatives - transposon amplification (Rockinger et al. 2016)?
The evolution of the X chromosome is likely to have happened more than once (three times or more?) in Caricaceae (e.g. in both Carica and Vasconcellea), although dioecy may be ancestral in the family as a whole (X. Wu et al. 2010). Sex chromosome evolution in Carica papaya is dated to only 0.5-2.5 Ma (Q. Yu et al. 2008 and references) or (9.5-)7(-1.9) Ma (Gschwend et al. 2012; J. Wang et al. 2012). There are inversions, etc., in the hermaphrodite-specific region of the Y chromosome (Yu et al. 2008).
Chemistry, Morphology, etc.. Reports of cyclopentenoid cyanogenic glycosides in the family have been questioned (Spencer & Seigler 1984; c.f. Jørgenson 1995).
For the morphology and mineral composition of the stinging hairs of Horovitzia, see Mustafa et al. (2018b).
Some general information is taken from Badillo (1971), Miller (1982), Kubitzki (2002d) and Eggli (2023: esp. succulents), and see the e-monograph by Carvalho (2013; also Carvalho et al. 2015); for latex coagulation, see references in Bauer et al. (2014), for wood anatomy, see Carlquist (1998c), for floral development, see Ronse Decraene and Smets (1998b), for pollen, see Zini et al. (2017) and for embryology, see Singh (1970).
Phylogeny. For phylogenetic relationships, see Kyndt et al. (2005), Carvalho and Renner (2012), Carvalho (2013) and M. Sun et al. (2016). The African Cylicomorpha is sister to the rest of the family, which are all American - [Cylicomorpha [[Jacaratia + Vasconcellea] [Carica papaya [Horovitzia + Jarilla]]]], generally good support.
Previous Relationships. Cronquist (1981) included Caricaceae in his Violales sandwiched between Achariaceae in the restricted sense (more or less herbaceous, South African) and the North American Fouquieraceae; Takhtajan (1997) placed his monofamilial Caricales in a generally similar position, i.e. along with other families that have parietal placentation.
[Setchellanthaceae [Limnanthaceae [[Tiganophytaceae [Bataceae + Salvadoraceae]] [Koeberliniaceae + Core Brassicales]]]]: nodes 1:1; C clawed; (A splitting) [= chorisis]; ovules several [>6]/carpel; extended 3' terminus of rbcL gene.
Age. The age of this clade is around 60-54 Ma (Wikström et al. 2001), (85-)73, 71(-61) Ma (Bell et al. 2010: c.f. relationships) or (97.9-)88.8(-80.8) Ma (Cardinal-McTeague et al. 2016).
Genes & Genomes. For the extended 3' terminus of rbcL gene and changes in stop codons, etc., see Karol et al. (1999).
Chemistry, Morphology, etc.. Chorisis, i.e. splitting, of the stamen primordia, of which dédoublement is a special case, is scattered in this clade (see Tobe 2015b); Tobe (2015b) noted that in such cases centrifugal development of the stamens is common.
SETCHELLANTHACEAE Iltis - Setchellanthus caeruleus Brandegee - Back to Brassicales
Shrub; myrosin cells 0; young stem with vascular cylinder; vestured pits +; fibres 0; rays uniseriate, one cell high; hairs T-shaped, unicellular, on multicellular podium; blade amphistomatal; lamina secondary veins subbasal, margins entire, stipules 0; flowers axillary, large [ca 4 cm across], 6-merous (5, 7); K connate, splitting irregularly; androgynophore +; A many, centrifugal, in 5-7 groups opposite K, on elongated axis; pollen tricolpate, surface striate-rugulate; nectary 0; gynophore +, short; placentation axile, style short, branches short, stigmas subcapitate; ovules 10-14/carpel, in two ranks; fruit a septifragal capsule, central column persistent; testa soft, multiplicative; endosperm development?, scanty; n/x = ?; seedling epigeal, phanerocotylar, cotyledons cordate.
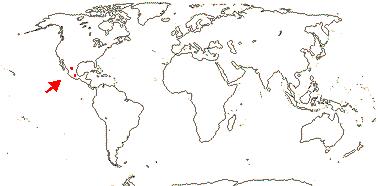
1 [list]/1. Mexico. Map: see Iltis (1999). [Photos - Flower, Flower, Fruit]
Chemistry, Morphology, etc.. The fusion of the marginal ventral carpellary bundles is commissural.
Some information is taken from Carlquist and Miller (1999: anatomy), Iltis (1999: general), Tobe et al. (1999: flowers), Tomb (1999: pollen), and Kubitzki (2002d: general).
Previous Relationships. Setchellanthus used to be included in Capparaceae.
[Limnanthaceae [Tiganophytaceae [Bataceae + Salvadoraceae]] [Koeberliniaceae [Core Brassicales]]]]: indole glucosinolates +; endomycorrhizae 0; root hair cells in vertical files alternating with files of cells lacking hairs; ARTH/At-β genome duplication [?next node down?].
Age. The age for this node is estimated to be 54-52 Ma (Wikström et al. 2001: c.f. topology), (112-)77.5(-42)/(123-)85(-46) Ma (Edger et al. 2015: genome duplication, 2018: note topology), (96-)87.1(-78.7) Ma (Cardinal-McTeague et al. 2016) or around 124 Ma (Kagale et al. 2014); see also below for suggested ages of the genome duplication placed at this node.
Evolution: Divergence & Distribution. For an increase in net diversification possibly associated with the At-β genome duplication, see below. Van den Bergh et al. (2016) suggest that this genome duplication is associated with the evolution of indole GSLs derived from tryptophan (see also Edger et al. 2015, 2018), but Agerbirk et al. (2021b) think that this may have happened more basally in the order. The Atβ genome duplication, with which the evolution of indole GSLs has been linked, is to be placed somewhere around here (Edger et al. 2015; McKibben et al. 2024). Indolic GSLs may be present in the plant after experimental herbivory treatments in Carica and Tropaeolum (Textor & Gershenzon 2009), thus suggesting a change in the position of the feature on the tree, i.e. placing it here may be incorrect (see also Schranz et al. 2011). However, relationships around here are somewhat unclear.
Tropaeolaceae are the phylogenetically closest family that is known to lack "striped" root hair development (Dolan & Costa 2001).
Plant-Bacterial/Fungal Associations. Loss of the ability to form endomycorrhizae (arbuscular mycorrhizae, = AM/AMF) has been placed at this node by Delaux et al. (2014), and Brassicaceae in particular are noted for being non-mycorrhizal, and its members often grow in habitats where soil phosphorus is abundant (Lambers 2015c) - AMF help in P uptake. The two Brassicales - Reseda and Arabis - included in a study of the effects of AMF on herbaceous plants showed negative interactions with AM fungi (Horton & van der Heijden 2007: n = 28). AMF are at best uncommon in Brassicales as a whole, and this is linked with the loss of the RAM2 - Reduced Arbuscular Mycorrhization - locus; RAM2 is involved in the production of cutin monomers recognized by both glomeromycotes during the establishment of endomycorrhizal associations and oomycetes during the initiation of parasitism (E. Wang et al. 2012; Geurts & Vleeshouwers 2012). Plaszkó et al. (2021, 2022) discuss the variety of breakdown products derived from glucosinolates, quite a number of which like nitriles and epecially isothiocyanates are phytoalexins and show antifungal activity. Indeed, plant-derived isothocyanates are being used in biofumigation whereby fungal pathogenesis in crops is reduced. However, like other chemical defences in plants, some fungi can break down the defence, while others even actively synthesize it (Plaszkó et al. 2021, 2022). Note that the loss of mycorrhizae in Brassicales has been dated to ca 30 Ma (Werner et al. 2018), i.e. well within Brassicaceae according to most dates (e.g. see below), so something is wrong here.
The establishment of AM associations has a considerable effect on plant defences, upregulating some and downregulating others (e.g. Jung et al. 2012). There seems to be a trade-off here: The loss of the ability to form AM associations is linked to resistance to oomycete infestations (see also Kamel et al. 2016). However, the oomycete Albugo s. str., a white blister rust, is a common parasite in this clade, A. candida growing on members of Brasicaceae, Capparaceae and Cleomaceae, for example (Choi et al. 2009; Thines & Voglmayr 2009; Ploch et al. 2010a). The effect of Albugo on the plant is complicated, and in some cases it facilitates infection by Phytophthora infestans, another oomycete (Prince et al. 2017). For other possible connections between AM and oomycete infections, see Estrada-Navarrete et al. (2016).
Chemistry, Morphology, etc.. Roots of non-mycorrhizal Brassicales are significantly thinner than those of AM rosids (Valverde-Barrantes et al. 2017). The distinctive vertical files of root hairs are known from Limnanthaceae and some members of core Brassicales; they have not been found in Tropaeolaceae, but other Brassicales basal to Limnanthaceae have not been studied (Dolan & Costa 2001).
Phylogeny. Edger et al. (2015) found the relationships [Limnanthaceae [Bataceae [Koeberliniacaeae + core Brassicales]]], but Setchellanthaceae, Salvadoraceae and Emblingiaceae were not included, and only one species in each included family was examined. However, Edger et al. (2018: plastome analyses, sampling somewhat better) recovered a variation on the same theme - [Setchellanthaceae (poor support) [Limnanthaceae [[Bataceae + Salvadoraceae] [Koeberliniacaeae [Emblingiaceae + other core Brassicales]]]].
Genes & Genomes. The At-β/ARTHβ genome duplication has been dated to 124.6±2.6 Ma (Kagale et al. 2014: exact position not specified), while other estimates are rather different, ca 50 Ma (Woodhouse et al. 2011), 69-53 Ma (Murat et al. 2015b) or ca 77 Ma (Landis et al. 2018); see also clade age estimates above, also Hohmann et al. (2015) for discussion. Either some ages of this duplication are very wrong indeed, and/or it is placed at the wrong node, indeed, the next node down may be a possibility (see also Schranz et al. 2011; Lysak 2018; Bayat et al. 2018; McKibben et al. 2024).
Thanks. To Jean-Michel Ané, for help with the literature on oomycete infection and plant-fungal signalling.
LIMNANTHACEAE R. Brown, nom. cons. - Back to Brassicales
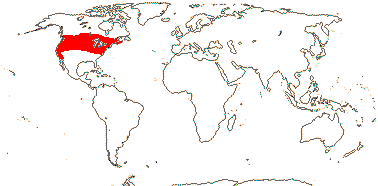
Annual herbs; erucic acid, ellagic acid, myricetin, non-hydrolysable tannins +, fructan sugars accumulated as isokestose oligosaccharides [inulins], stomatal myrosin cells 0; complete vascular cylinder not forming; cork?; leaf pinnate, or lamina pinnately lobed, vernation conduplicate, margins with teeth, stipules 0; flowers (single, axillary), bracteoles 0; flowers 3-5-merous; K valvate, with one trace [Limnanthes], C contorted (open); nectaries on abaxial bases of antesepalous A; A 2x K, of two lengths, largest opposite sepals; tapetal cells binucleate; pollen grains elongated bun-shaped, heteropolar, zonosulcate; G [2-5], opposite sepals, when 3 median member abaxial, no vascular bundles in carpel wall, placentation basal-parietal, style gynobasic, hollow, branches ± well developed, stigma punctate to minutely capitate, dry; ovule 1/carpel, apotropous, unitegmic, integument 14-20 cells across, parietal tissue 1 cell across; megaspore mother cells several, embryo sac tetrasporic, 6-7-nucleate; fruit a schizocarp, mericarps muriculate, K persistent, green; seed coat pachychalazal, thick, testa with vascular bundles, otherwise undistinguished; endosperm haustorium + [Floerkea], embryo color?, cotyledons with backwardly-directed lobes, cell walls with xyloglucans [thick, pitted - amyloid]; n = x = 5, genome 1.5-2.1 Gb; germination hypogeal or epigeal, radicle soon dies [Limnanthes].
2 [list]/8. Temperate North America. Map: from Culham (2007). [Photo - Flower] [Photo - Flower (close-up)]
Age. The two genera in Limnanthaceae separated ca 17-9 Ma (Wikström et al. 2001), (22-)13, 12(-5) Ma (Bell et al. 2010), or (22.1-)12.1(-4) Ma (Cardinal-McTeague et al. 2016).
Chemistry, Morphology, etc.. Hofmann and Ludewig (1985) showed the stem-born roots of Limnanthes departing from the sides of the leaf gaps.
There are very different interpretations of the pollen morphology of Limnanthes, Buchner et al. (1990) are followed here. What is going on in Floerkea is unclear (Maheshwari & Johri 1956), although the pollen does appear to be heteropolar. According to van Tieghem (1898), the ovules are epitropous, but they are shown as being apotropous by Maheshwari and Johri (1956). Maheshwari and Johri (1956) and Johri (1970) described an endosperm pouch or haustorium on the funicular side of the micropylar region in Floerkea.
Some general information is taken from Bayer and Appel (2002); for wood anatomy, see Carlquist and Donald (1996), for variation in floral nectaries, see Link (1992a), and for embryology, see Fagerlind (1939b) and Mathur (1956).
Although Limnanthaceae are a small clade, it is unclear if some of the bolded characters above really are apomorphies for the whole family...
Phylogeny. See Zuo et al. (2022) for relationships in the family; Floerkia is sister to Limnanthes.
Previous Relationships. Eckert (1966) compared floral morphology of Limnanthaceae with that of other families believed to be related, as did Hofmann and Ludewig (1985: they included other features; possible relatives were very various. Limnanthaceae were often included in Geraniales (e.g. Cronquist 1981), but were placed in Solananae by Takhtajan (1997).
[[Tiganophytaceae [Bataceae + Salvadoraceae]] [Koeberliniaceae [Core Brassicales]]]: GSL N-methoxylation; style short to absent; ovules campylotropous; seeds curved, exotegmic, exotegmen fibrous; embryo strongly curved.
Age. The age of this node is estimated to be around 72-68 Ma (Wikström et al. 2001), (112-)77.5(-42) Ma (Edger et al. 2015, 2018), or (88.6-)80.2(-72.5) Ma (Cardinal-McTeague et al. 2016) - note varying topologies, but the content is effectively the same.
The fossil Dressiantha, from some 90 Ma in the Cretaceous-Turonian of East North America, may be assignable to a node somewhere around here. Gandolfo et al. (1998c) in a morphological analysis placed it in a clade that included Koeberliniaceae, Bataceae, Brassicaceae, etc., although excluding Gyrostemonaceae. However, with a floral formula of K4, C5, A5 + 5 staminodes, G [2], calyx decussate (not always evident in the images presented), androecium obdiplostemonous* (diplostemonous in the text), stamens epipetalous*, antepetalous*, staminodes +; ?nectariferous disc internal to the androecium*, anthers monothecate* and with prolonged connectives*, gynoecium stipitate, carpels ?obliquely-oriented*. Asterisks denote some distinctive characters not included in the phenetic analysis. The relationships of this remarkable fossil are unclear to me, and nothing suggests a position around here. Its use in calibration would seem ill-advised and it is best excluded from Brassicales. Interestingly, its exclusion as a calibration point in Cardinal-McTeague et al. (2016: Table S5) resulted in ages of deeper nodes in particular in Brassicales clade becoming somewhat older.
Chemistry, Morphology, etc.. Amino acids like isoleucine with branched chains may have more carbons added along the chain; the occurrence of such chain-elongated branched-chain amino acids is to be pegged here on the tree (J. E. Rodman, pers. comm.). There is extensive variation of floral merosity in the Emblingiaceae-Brassicaceae group in particular, but 4-merous flowers could be another apomorphy at this level - but with plenty of reversals. A fibrous, if unlignified, exotegmen has been reported from Koeberliniaceae, Salvadoraceae, Resedaceae, Cleomaceae, etc. As Tobe and Raven (2008) suggest, optimisation of this and other embryological features on the tree is unclear; if ovule and seed characters are placed at this node, they reverse in the [Bataceae + Salvadoraceae] clade.
[Tiganophytaceae [Bataceae + Salvadoraceae]]: ?
Evolution: Divergence & Distribution. Although Tiganophytaceae go in this area, exactly where is unclear, and they are little known (other than their gross morphology).
For variation in and possible synapomorophies of this group, see Ronse Decraene and Wanntorp (2009) and Swaenepoel et al. (2020: Table 1).
TIGANOPHYTACEAE Swaenepoel, F. Forest & A. E. van Wyk - Tiganophyton karasense Swanepoel, F. Forest & A. E. van Wyk
Shrub, little branched; ?anatomy, ?myrosin cells; branches developing from apices of short shoots; leaves spiral, stipules 0; long shoots: internodes short, leaves deltate, with basal lobe/spines, sessile; short shoots: leaves ± obovate; flowers in axils of leaves, somewhat compressed laterally, 4-merous; K connate, C free, not clawed; A 4, opposite K; ?disc +; gynophore +, S-shaped, long, style gynobasic; 2 ovules/carpel, ?embryology; fruit a nutlet, 1-seeded; ?testa; ?embryo, etc.; n/x = ?
1/1. Southeast Namibia. Map: Swaenepoel et al. (2020: Fig. 4).
Chemistry, Morphology, etc.. For what is known about Tiganophyton, see Swaenepoel et al. (2020).
[Bataceae + Salvadoraceae]: perforation plates not bordered; rays wide, multiseriate; nodes 1:2; stomata paracytic; leaves opposite, lamina with secondary veins ± palmate, ascending from at or near base; bracts with colleters on their tips; A = C; ovules 2/carpel, basal; exotegmen not fibrous; endosperm 0, embryo ± straight, color?
Evolution: Divergence & Distribution. Bataceae and Salvadoraceae are really quite similar in details of morphology (Rodman et al. 1996) and anatomy (Carlquist 2002a), although quite unlike at first sight. Ronse de Craene and Haston (2006) and Ronse de Craene and Wanntorp (2009) listed a number of other features that the two share, including flowers that are slightly disymmetric and horizontally oriented relative to the inflorescence axis, a sepal tube, etc. The flowers of neither family are easy to interpret, so although they both have five stamens, whether they are in the same position is unclear (c.f. Tobe 2015b).
BATACEAE Perleb, nom. cons. - Batis Perleb - Back to Brassicales
Fleshy shrublets; (hydroxy)proline betaines +, tannins?; cork pericyclic; perforation plate borders vestigial; leaves fleshy, blade terete, amphistomatal, stipules intrapetiolar or cauline, unvascularized; plant monoecious or dioecious, inflorescences densely spicate, usually axillary; flowers small, bracteoles 0, nectary 0; staminate flowers: P median, enveloping flower, or P 4, connate; A = and alternate with P; pollen surface smooth, ektexine spongy, undifferentiated; pistillode -/0; carpelate flowers: P 0; staminodes 0; G with carpels divided [= "4-locular ovary"], gynophore 0; style 0, stigmas capitate-penicillate; ovules collateral, epitropous, micropyle ± zig-zag, nucellar cap +; fruit multiple, or a drupe with four pyrenes; seed coat membranous; ?cotyledons; x = 12 (?11).
1 [list]/2. N. Australia and S. New Guinea, tropical America, and the Galapagos. Map: from van Steenis and van Balgooy (1966), Heywood (1978) and Fl. Austral. vol. 8 (1982); introduced into the Hawaiian Is. [Photo - Flowers.]
Chemistry, Morphology, etc.. For the nodal anatomy of Batis maritima, with what is presumably the foliar trace disappearing as it approaches the node, the leaf being supplied by two bundles from the angles of the stem, see van Tieghem (1893), but c.f. Johnson (1935) and R. A. Howard (pers. comm.). The stipules need study: van Tieghem (1893) did not see them, Johnson (1935) thought that they were between the broad leaf base and the stem, Rogers (1982b) that they were cauline, while Ronse De Craene (2005) in a floral study describes the fairly massive structures in this position in the flowers as being colleters - these descriptions are not all mutually exclusive.
The morphological nature of the structure enveloping the staminate flowers is most obvious in B. argillicola, but there is controversy over the nature of this structure, too. Ronse De Craene (2005, see also 2018) described it as being derived from four sepals in Batis maritima, although he noted that it had only a single vascular trace; some of the lobing of the tubular structure may be caused by pressure from other parts of the developing flower rather than reflecting an inherently four-merous tube. Cronquist (1981) described the placentation as being parietal-basal, Tomlinson (2016) as being apical.
Batygina et al. (1985) provide information on the ovules, for pollen, see Tobe and Takahashi (1995), and for general information, see Bayer and Appel (2002).
SALVADORACEAE Lindley, nom. cons. - Back to Brassicales —— Synonymy: Azimaceae Wight & Gardner
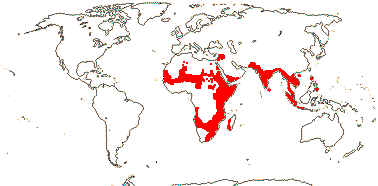
Woody, (paired ?thorns - Azima); tannins 0; cisternae of endoplasmic reticulum dilated; myrosin cells 0; cork superficial; interxylary phloem +; druses 0; cuticle waxes with platelets; leaves opposite, vernation flat-curved [Salvadora], margin entire; plant dioecious or polygamous, or flowers bisexual; (bracteoles 0); floral orientation oblique; K 2-4(-5), (one-trace), contorted, basally connate, C (5), contorted or imbricate, (connate); (C/A tube +, short [Salvadora]); nectar glands alternating with or abaxial to A, unvascularized, or 0; A = and opposite K, free, basally connate, or adnate to C; pollen triporate, surface reticulate; G (gynophore +), 1-2-4-locular [see below], orientation oblique, (style short), stigma punctate to at most slightly lobed; ovules (1/carpel), apotropous, micropyle exo-, endo- or bistomal, outer integument 10-15 cells across, (with a vascular bundle - Azima), inner integument 3-5 cells across, parietal tissue ?ca 3 cells across, (obturator +); fruit a berry or drupe; testa multiplicative, exotestal cells palisade, slightly thickened, inner walls mucilaginous, crystalliferous, tegmen becoming crushed, exotegmic cells fibrous, not lignified; cotyledons thick, accumbent; n also = 12; x = 12 (?11), nuclear genome [1 C] (0.027-)0.667(-16.988) pg.
3 [list]/11: Salvadora (5). Africa (inc. Madagascar) to South East Asia and West Malesia, often in drier regions (map: from Aubréville 1974; Frankenberg & Klaus 1980; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010; Malesian distribution rather optimistic, perhaps only in Java). [Photo - Habit, Fruits]
Chemistry, Morphology, etc.. The stipules of Salvadora persica are described as being colleter-tipped (Ronse de Craene & Wanntorp 2009). The ?thorns of Azima are paired; I do not know if they are modified stipules (so = spines) or develop from prophyllar buds (= thorns). Azima has two trace-one gap nodes to the bracts and bracteoles, Salvadora has bracts with 1:1 nodes (Kshetrapal 1970). R. A. Howard (pers. comm.) reported 1:2 nodes from both genera.
The flowers may be slightly monosymmetric and with a poorly developed petal-stamen cup. The gynoecium is probably originally bicarpelate and has parietal placentation, and if there appear to be two loculi it is because of the development of a structure perhaps comparable to the false septum of Batis (Ronse de Craene & Wanntorp 2009). Gynoecial morphology needs more study.
For general information, see Kubitzki (2002d), for wood anatomy, see Carlquist (2002a) and Saxena and Gupta (2011), for floral vascularization, see Kshetrapal (1970), for pollen, see Lobreau-Callen (1977) and Perveen and Qaiser (1996), for some embryology, see Maheswari Devi (1972), and for seed morphology, see Tobe and Raven (2012).
[Koeberliniaceae [Core Brassicales]]: ?
KOEBERLINIACEAE Engler, nom. cons. - Back to Brassicales
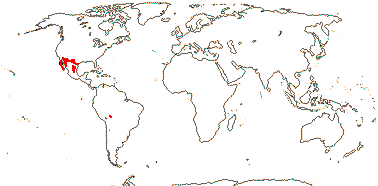
Woody, thorny; ellagic acid?, tannins?; glucosinolates absent; cork pericyclic; perforation plates bordered; intercellular canals +; druses 0; leaves minute, fugacious, stipules 0; inflorescences axillary; flowers 4-(5-)merous; A 8(10); tapetal cells multinucleate; nectaries at the adaxial base of A; G orientation oblique, gynophore +, short, placentation axile, style +, stigma ± minutely expanded; ovules ca 10/carpel, apotropous and epitropous, micropyle zig-zag, outer integument 2 cells across, parietal tissue 0, nucellar epidermal cells radially enlarged; fruit a berry; seed ?arillate; exotesta with massive cuticle, then tanniniferous cells, exotegmen fibrous, walls very thick, lignified, cells moderately elongated; embryo chlorophyllous, endosperm type?, moderate, cotyledons incumbent; x = 7 (?12, ?6).
1 [list]/2. Central and S.W. North America, Bolivia. Map: from Holmes et al. (2009). Photo: Habit, Flower.
Chemistry, Morphology, etc.. Nodal anatomy is taken from that of the bracts (Mehta & Moseley 1981). The ovules look as if they may be campylotropous (see also Tobe & Raven 2008).
For general information, see Kubitzki (2002d), for vegetative anatomy, see Gibson (1979), for floral anatomy, see Mehta and Moseley (1981), and for embryology, see Tobe and Raven (2008): von Schrenk, Aug. 8, Texas - seed anatomy.
Classification. See Holmes et al. (2009) for a monograph.
Previous Relationships. Koeberlinia itself has been included in Capparaceae (Cronquist 1981). Canotia is sometimes associated with Koeberlinia (e.g. Hutchinson 1973) and included in Celastraceae; although both are thorny shrubs, that is the main extent of their similarity.
Core Brassicales / [Emblingiaceae [[Pentadiplandraceae [Gyrostemonaceae + Resedaceae]], Tovariaceae, [Cleomaceae [Capparaceae + Brassicaceae]]]] : glucosinolates also from tryptophane [?here]; cisternae of endoplasmic reticulum dilated and vacuole-like; cuticle wax crystalloids 0; inflorescence terminal, bracteoles 0; floral development open; nectary outside A; endotesta crystalliferous; 3' rbcL extension.
Age. Core Brassicales have been dated to ca 66.1 Ma (Edger et al. 2018), (75.6-)68.5(-62) Ma (Hall et al. 2015) and (80.6-)72.9(-65.5) Ma (Cardinal-McTeague et al. 2016).
Evolution: Divergence & Distribution. Ronse de Craene and Haston (2006) suggest a number of other characters that may be synapomorphies for this clade, including floral symmetry and embryo development. Nectary morphology and absence/presence/position are very variable in Brassicales outside this core group.
Genes & Genomes. It has been suggested that the At-ß genome duplication is to be placed at this node; it is absent from Carica (Barker et al. 2009: sampling); see below for recent ideas as to where this should go.
Chemistry, Morphology, etc.. Whether or not Emblingiaceae have indole GSLs is unknown; only small quantities have been reported from Pentadiplandra (references in de Nicola et al. 2012). For quaternary ammonium compounds, see McLean et al. (1996).
Phylogeny. It seemed that relationships within this clade might be beginning to be resolved. Emblingia was often recovered as sister to the other members (e.g. Cardinal-McTeague et al. 2016; Edger et al. 2018), and although M. Sun et al. (2016) recovered a [Tovariaceae + Embligiaceae] clade, it had little support. Of the sampled Stixideae (ex Capparaceae), the Asian Tirania was close to Gyrostemonaceae and the New World Forchhammeria perhaps closer to Resedaceae (Hall & Sytsma 2000, 2002; Hall et al. 2002), or both may be associated with Resedaceae (Hall et al. 2004: relationships depend on the gene sequenced). Stixis, Borthwickia, and Neothorelia are the other genera involved. All had been excluded from Capparaceae (Kers 2002), but where they were to be placed was unclear; see Resedaceae-Stixideae below. Su et al. (2012) not only found some (60% bootstrap, 0.99 p.p.) support for Pentadiplandraceae as sister to the [Gyrostemonaceae + Resedaceae] clade, but there was strong support for [Borthwickia [Gyrostemonaceae + Resedaceae], the Resedaceae area including a well-supported [Tirania + Stixis] clade and Forchammeria; Neothorelia was not sampled. However, the position of Tovariaceae is unclear, being either sister to Pentadiplandraceae, etc. (Marín-Bravo et al. 2009; Cardinal-McTeague et al. 2016) or to Capparaceae et al. (Su et al. 2012), although in both cases with little support. Indeed, the relationships recovered by Cardinal-McTeague et al. (2016) are [[Tovariaceae [Pentadiplandraceae [Borthwickia [[Stixis + Tirania] [Forchhammeria + Reseda, etc.]]]]] [Capparaceae [Cleomaceae + Brassicaceae]]].
Edger et al. (2018) recovered the phylogeny followed below, but substantial modifications may well be needed. Phylogenetic relationships in core Brassicales were partly resolved in a three-gene study by Hall et al. (2004) and a four-gene analysis by Su et al. (2012). Ronse de Craene and Haston (2006) found that Emblingiaceae moved outside this group in a combined morphological-molecular study, but many data were missing for Emblingia in particular and its floral morphology is odd and was then poorly understood (but see below); they thought that it might be sister to [Bataceae + Salvadoraceae], but noted that there was little support for this position. In the Seed Plant Tree ii.2022 version, Azima (Salvadoraceae) was embedded in Capparaceae, and some other relationships in this area are ...[Emblingiaceae [[[Tovariaceae + Pentadiplandraceae] [Gyrostemonaceae, Capparaceae (some), Resedaceae (most)]] [Resedaceae [Resedaceae [Capparaceae (most) [Cleomaceae + Brassicaceae]]]]]]]. Recent suggestions based on an Angiosperms353 nuclear data >7000 genus data set are somewhat more conventioal, being [Emblingiaceae [Stixaceae (ex Resedaceae) *[[[Tovariaceae + Pentadiplandraceae] [Gyrostemonaceae + Resedaceae]] [Capparaceae [Cleomaceae + Brassicaceae]]]]].
Classification. Small families were added in this area because some fugitives from the old Capparaceae seemed to be finding homes, but they were combined (well, for the time being) in an expanded Resedaceae in A.P.G. IV (2016).
EMBLINGIACEAE Airy Shaw - Back to Brassicales
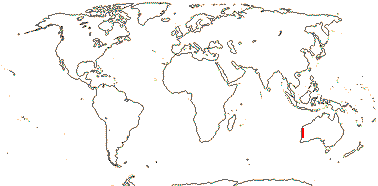
Subshrub; plant hispid; mustard oils?; cauline vascular tissue forming a closed cylinder, cork cambium deep-seated; cambium storying?; branched sclereids +; leaves ± opposite, lamina ± cartilaginous, margins ± entire, stipules +; hairs unicellular, minutely warty; flowers axillary, monosymmetric; K connate, lobed, deeply divided abaxially, C 2, adaxial, connate by epidermis, slipper-shaped, not clawed; nectary adaxial, partly enclosed by walls from the petals [= calceolus]; androgynophore +, curved abaxially; A 5, opposite C, doubling in number [= dédoublement], fertile A 4, adaxial, filaments shorter than anthers, staminodes +, 3-6, abaxial, forming a torus/hood; pollen with short colpi with rounded ends and bulging apertures, adjacent exine thickened; G [(2-)3], odd member abaxial, placentation axile, stigma shortly lobed; ovule 1/carpel, ?morphology; fruit indehiscent, pericarp thin; seed arillate, testa thick; endosperm ?type, scanty, embryo ?color, hypocotyl short; n/x = ?
1 [list]/1: Emblingia calceoliflora. W. Australia (map: from FloraBase 2004).
Chemistry, Morphology, etc.. The plant dries yellowish.
There has been considerable disagreement over the floral structure of Emblingia. Is the flower resupinate or not? Is the hood petal-like or not? Are there one or three carpels? I initially largely followed Melville's interpretation (in Erdtman et al. 1969), see also Mueller (1860). Recent studies by Tobe (2015b) have clarified what is going on, in particular, how the flower is oriented. Although the flower is axillary, it reorients during development so that it is presented to the pollinator as being inverted. In the characters allowing one to recognise the family (and in the family characterization pre-2015) the "pollinator's" orientation is emphasized, in the family characterization it is the "morphologist's" orientation. Tobe (2015b) also suggested that the androecium was haplostemonous and oppositipetalous; simple déduplication of the stamens had occured.
For general information, see Erdtman et al. (1969), Kubitzki (2002d) and Ronse de Craene and Wanntorp (2009: stipules present, reduced).
Previous Relationships. Suggestions of the relationships of Emblingia by the four contributors to Erdtman at al. (1969) - near Polygalaceae (Erdtman), allied to Sapindaceae (Leins), an affinity with Goodeniaceae (Melville and Metcalfe), or perhaps it should be placed in its own family. Emblingia was included in Polygalaceae by Cronquist (1981) and Polygalales by Takhtajan (1997) based on apparent floral similarities. Savolainen et al. (2000b) placed Emblingiaceae in Gentianales in a molecular study.
[[Pentadiplandraceae [Gyrostemonaceae + Resedaceae]], Tovariaceae, [Cleomaceae [Capparaceae + Brassicaceae]]]: (glucosinolates chain-elongated BCAAs); "stipules" +, minute; gynophore +, short; ovules in two ranks, outer integument 2(-3) cells across; exotegmen fibrous.
Age. The age for this node is estimated to be 42-33 Ma (Wikström et al. 2001), (68-)57, 54(-45) Ma (Hall et al. 2010), (89-)61(-33) Ma (Edger et al. 2015, 2018) or (75.6-)68.5(-62)/(70.1-)61.7(-54.0) Ma (Cardinal-McTeague et al. 2016: note Tovaria is sister to this clade, resultant crown group age of whole clade, first set of numbers...).
Evolution: Ecology & Physiology. Dilated cisternae, protein–containing vacuoles derived from the endoplasmic reticulum (ER), alias protein–rich ER–dependent vacuoles, have been described from members of all families of this clade except Pentadiplandraceae (Jørgensen 1981). have a common origin
Brassicaceae, Resedaceae and Capparidaceae, at least, have a number of species that are gypsophiles, growing in soils rich in hydrous calcium sulphate, and some accumulate sulphur i.a. in organic compounds as well as in gypsum crystals (Escudero et al. 2014; C. T. Muller et al. 2017). Brassicales are often not associated with arbuscular mycorrhizal fungi (AF), and such fungi in gypsum-derived soils may be quite distinctive (Torrecillas et al. 2014). There is perhaps a parallel here with Caryophyllales, quite commonly growing in gypsum-rich soils and also often not AM plants.
Animal-Plant Interactions. The larvae of Chrysomelidae-Alticinae beetles are quite commonly to be found on members of this clade (Jolivet 1988).
Plant-Bacterial/Fungal Associations. An oomycetous white blister rust, Albugo, grows on Brassicaceae, Capparaceae, Cleomaceae, and Resedaceae - as well as on Fabaceae (Onobrychis) (Choi et al. 2011).
Chemistry, Morphology, etc.. For details on GSL variation - quite extensive - within this clade, see Mithen et al. (2010). The wood anatomy of Brassicaceae and Resedaceae is rather similar (Schweingruber 2006). For stipules, see Weberling (2006).
For bracteoles, see Ronse Decraene (1992). Erbar (2010) surveyed the quite extensive variation in floral development in the clade. In Pentadiplandraceae, Brassicaceae and Tovariaceae the lateral sepals are initiated before the median sepal (Ronse Decraene 2002). For ovule type and the different mechanisms by which the ovule becomes campylotropous, see Boesewinkel (1990) and Bouman and Boesewinkel (1991). Nucellar tracheids have been reported in Capparaceae and Resedaceae, at least (Werker 1997).
[Pentadiplandraceae [Gyrostemonaceae + Resedaceae]]: placentation axile, stigma lobed.
Age. This node is estimated to be (80-)55(-29.5) Ma (Edger et al. 2015, 2018) or (70.1-)61.7(-54) Ma (Cardinal-McTeague et al. 2016).
PENTADIPLANDRACEAE Hutchinson & Dalziel - Back to Brassicales

Shrubs or tuberous lianes; benzyl and 4-methoxybenzyl glucosinolates +, ellagic acid?, tannins?; ?cork; vestured pits +; nodes 3:3; mucilage cells +; lamina margin entire; inflorescence axillary, subcorymbose; flowers polygamous, 5-merous; K imbricate, C base enlarged, ± concave, limb flat, ± spreading; andogynophore +, short, nectariferous; staminate flowers: A 9-13, connective shortly produced; pistillode +; carpelate flowers: staminodes +; G [3-5], opposite sepals, style long; ovules 3-10/carpel, type?; fruit a berry; 1 seed/loculus, coat with layer of white, wooly, elongated cells towards outside ["seed pubescent"], exotegmen not fibrous; embryo white; n/x = ?
1 [list]/?1: Pentadiplandra brazzeana. Tropical W. Africa (map: from Hall et al. 2004; Trop. Afr. Fl. Pl. Ecol. Distr. 5. 2010).
Evolution: Divergence & Distribution. Ronse de Craene and Haston (2006) suggest that its floral morphology is close to the ancestral form of the core Brassicales.
Plant-Animal Interactions. Fruits contains the sweet-tasting (to most primates and many other monkeys) protein, brazzein - they are also described as having the flavour of a horseradish - however, this is false advertisement, since they are not a good source of energy. However, gorillas have a mutation in a taste gene, do not find the fruits sweet, and so are not deceived (Guevara et al. 2016).
Chemistry, Morphology, etc.. The plant does not dry dark. Are there supernumerary buds? There are bordered pits in wood fibres and mucilage cells in the leaf epidermis (Boodle, K, ms.). Ronse Decraene (2002) described its floral anatomy; there are no marginal or placental strands in the ovary.
For general information, see Villers (1973) and Bayer and Appel (2002), for GSLs, see de Nicola et al. (2012), and for embryo colour, Martin Cheek (pers. comm.).
Embryologically - and in many other respects - Pentadiplandra is poorly known.
Previous Relationships. Cronquist (1981) included Pentadiplandra in his rather broadly circumscribed Capparaceae, Takhtajan (1997) segregated it as a family.
[Gyrostemonaceae + Resedaceae]: idioblastic myrosin cells 0; hairs unicellular; styluli +; seeds arillate.
Age. The age of this node is estimated to be (67-)46(-22.5) Ma (Edger et al. 2015, 2018) and (62.8-)53.5(-44.3) Ma (Cardinal-McTeague et al. 2016: Gyr + rest).
Evolution: Divergence & Distribution. There may have been an increase in diversification rates at this node (Edger et al. 2018).
Pollination Biology & Seed Dispersal. Posession of myrmecochorous seeds may be an apomorphy at this level.
GYROSTEMONACEAE A. Jussieu, nom. cons. - Back to Brassicales
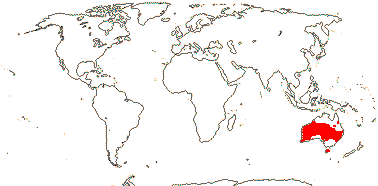
Trees to shrubs; stomatal myrosin cells 0, tannins?; cork subepidermal; wood storied; multiseriate rays +; petiole bundle arcuate; leaf vernation flat, (blade amphistomatal), (stipules 0); plants usu. dioecious, inflorescence various; flowers small; P +, uniseriate, connate, 4-8-lobed or not; nectary 0; axis flattened, disc-like; staminate flowers: A 6-many, in 1 or more whorls around axis, centripetal, anthers ± sessile; pollen tricolpate, ektexine spongy, undifferentiated; pistillode 0; carpelate flowers: staminodes 0; gynophore 0, G (1-)many, borne around axis in 1 (2) whorls, connate or not, when G 2, transverse, (ovary roof +, styluli ± marginal), stigmas decurrent, large and spreading or not; ovule 1/carpel, apical, apotropous; fruit a dry or succulent schizocarp (achene; syncarp), K persistent; endosperm copious, embryo ?color; n = 14, x = ?, nuclear genome [1C] ca 0.65 pg.
5 [list]/18+: Gyrostemon (12). Australia, not in the north (map: see Fl. Austral. 8. 1982).
Age. Crown-group Gyrostemonaceae - [Gyrostemon + Tersonia] - are about (9.9-)5.7(-2.3) Ma (Cardinal-McTeague et al. 2016).
Evolution: Divergence & Distribution. Fossils of Gyrostemonaceae have been reported from the early Miocene ca 20 Ma from New Zealand (D. E. Lee et al. 2001).
Pollination Biology & Seed Dispersal. Gyrostemonaceae are wind-pollinated, hence the absence of a nectary; the seeds are myrmecochorous (Lengyel et al. 2010).
Genes & Genomes. A genome duplication, the GYRAα event, associated with this family has been dated to ca 31.9 Ma (Landis et al. 2018).
Chemistry, Morphology, etc.. The perianth is uniseriate.
For general information, see Goldblatt et al. (1976) and George (2002d); for gynoecial orientation, see Friedrich (1956), for floral development, see Hufford (1996), for pollen, see Tobe and Takahashi (1995).
Phylogeny. Gyrostemon is paraphyletic in the study of Cardinal-McTeague et al. (2016); three species were examined.
RESEDACEAE Martinov, nom. cons. - Back to Brassicales
± Woody; K valvate; A many; x = ?
8 [list]/96 - three groups below. Mostly Northern hemisphere, a few southern Africa.
Age. Crown-group Resedaceae are estimated to be (57-)47.8(-37.8) Ma (Cardinal-McTeague et al. 2016).
1. Borthwickia trifoliata W. W. Smith —— Synonymy: Borthwickiaceae Su, Wang, Zhang & Chen
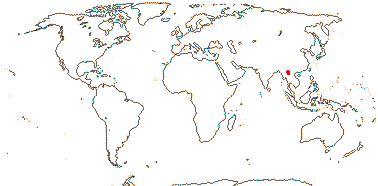
Small tree; ?hair type; leaves opposite, trifoliate, lamina margins entire, stipules 0; flowers 5-8-merous, ?monosymmetric; K connate; C with proximal and distal parts differentiated, not clawed; androgynophore +, short; filaments long; pollen exine perforate; gynophore quite long, G [4-6], ribbed, stigma undivided; ovules many/carpel, ?morphology; capsule ?septifragal; ?aril; n = ?
1/1. China (southwest Yunnan) and adjacent Myanmar.
[Stixideae + Resedeae]: x = 14, 13, nuclear genome [1 C] (0.091-)0.594(-3.886) pg [here?].
Age. This clade is dated to (46.7-)38(-29.3) Ma (Cardinal-McTeague et al. 2016: Stixis + rest; see also Hall et al. 2015).
Evolution: Divergence & Distribution. Cardinal-McTeague et al. (2016) noted an increase in species diversification at this node.
2. Stixideae Hallier —— Synonymy: Stixidaceae Doweld
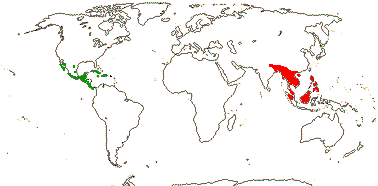
Shrubs or climbers; successive cambia +; vestured pits +; tracheids +; multiseriate rays +; conjunctive tissue with sclereids containing crystals; cortical sclereids +; leaves simple (trifolioliate), secondary veins ascending, (spines in the stipular position); (flowers axillary - Neothorelia); K 4-8, C 0, 6; pollen exine reticulate; G [2-4], placentation axile, (style short), stigma lobed; ovules (1) 2-several/carpel; fruits fleshy, ?drupes; ?aril; ?seed coat; cotyledons incumbent, (strongly anisocotylous); (germination hypogeal); n = ?
4/20: Forchhammeria (10), Stixis (7). Southeast Asia, Central America. (map: from Jacobs 1960, Forchhammeria green, from Hansen 1977).
3. Resedeae Reichenbach —— Synonymy: Astrocarpaceae A. Kerner
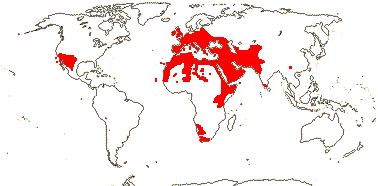
Usu. herbs; stomatal myrosin cells 0, BCAA glucosinolates 0, tannins 0; cork?; multiseriate rays +; no bordered pits in imperforate tracheary elements; lamina margins entire to pinnatifid, (stipules 0); flowers monosymmetric, hypanthium short or 0; K (4-)6(-8), C valvate, (0, 2, 4-)6(-8), unequal, the adaxial largest, ligule at junction of claw and limb, limb ± fringed or not; nectary esp. pronounced adaxially, (bipartite), disciform to almost petal-like; A 3-many, from ring primordium and centrifugal, basally connate or not; exine reticulate; (gynophore 0), G [(2-)3-6(-8)] (± free), opposite sepals or when 3, median member often adaxial, often open apical-adaxially, placentation often parietal, styluli often ± marginal, at most short; ovules (1-)several/carpel, to 3-seriate, (bistomal), inner integument 3-4 cells across, (parietal tissue none), hypostase +; fruit with apical opening within the styles, (follicle; berry), calyx persistent; (aril 0), endotestal cells cuboid, ± thickened, unlignified, with crystals, exotegmic cells elongated, lignified [palisade in t.s.], (thickening U-shaped; overgrown), endotegmen (with thick cellulose walls), crystaliferous; n = 5-15.
3/75: Reseda (68). Warm temperate and dry subtropical, esp. Mediterranean-Middle East-North African, also Oligomeris in southern Africa, S.W. North America and S.W. China (map: see Meusel et al. 1965; Frankenberg & Klaus 1980; Hultén & Fries 1986; Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003; Martín-Bravo et al. 2009). [Photo - Flower.]
Age. Crown-group Resedeae have been dated to (13.5-)12.6, 10.5(-8.7) Ma (Martin-Bravo et al. 2009).
Evolution: Divergence & Distribution. Martín-Bravo et al. (2007) discuss the phylogeny and biogeography of Resedeae. Within Oligomeris, native to both the Old and New Worlds (western), there are considerable disjunctions (Martín-Bravo et al. 2009), O. linifolia showing a recent disjunction between the Old and New Worlds (Martín-Bravo & Escudero 2012). There has been notably fast diversification within the Mediterranean Reseda sect. Phyteuma (Escudero et al. 2018; see also Martín-Hernanz et al. 2019: Table 4
Pollination Biology & Seed Dispersal. The seeds of Reseda are myrmecochorous (Lengyel et al. 2010), however the seed ecology of Stixideae in particular is little known.
Genes & Genomes. For a possible genome duplication involving at least the Reseda area (including Ochradenus, = Reseda s.l.), see OTPTI (2019) and Mabry et al. (2020).
Chemistry, Morphology, etc.. Forchhammeria contains methyl GSLs like those of Capparaceae and Cleomaceae (Mithen et al. 2010). The reaction wood contains gelatinous fibres, so if perhaps "normal" (Schweingruber 2006); c.f. Brassicaceae.
Resedeae are florally very variable. Thus Reseda alba seems to lacks a gynophore, Caylusea has a gynophore, Sesamoides has an androgynophore, while in R. luteola the stamens are inserted immediately below the carpels on a receptacular protrusion (Sobick 1983). The androecium of Reseda luteola may be in 3-4 whorls (for references, see Abdallah 1978), while Sobick (1983) shows the stamens as being opposite the petals and developing centrifugally, while in other taxa in Resedeae they develop from a ringwall. Ochradenus has C 0, A many, [G 3], the carpels are ultimately closed, and the fruit is berry-like - c.f. Gyrostemonaceae (Hufford 1996). Within Stixideae, Tirania has six sepals and petals, while Forchhammeria has two carpels, as well as an irregular number of sepals (again, see Gyrostemonaceae), no petals, one ovule/carpel, and only one ovule/fruit usually develops.
The seeds of Borthwickia are described as being 4-6 per capsule and the embryo as being scarcely differentiated by Zhang and Tucker (2008), but this must be incorrect. The appendages on the seeds in some Resedeae are also described as being caruncles. From illustrations in Hennig (1929) it is unclear whether the seeds are endotestal or exotegmic.
For general information on Resedeae, see Abdallah (1967), Abdallah and de Wit (1979) and Kubitzki (2002d), for glucosinolates, see Agerbirk et al. (2021a), for wood anatomy, see Carlquist (1998a) and Schweingruber (2006), for stipules, see Weberling (1968), for floral development of Reseda lutea, see Leins and Sobick (1977), and for some seed/ovule anatomy, see Guignard (1893) and Singh and Gupta (1967: comparison with Violaceae). For additional information on Stixideae, see Carlquist (1988b), Hansen (1977: Forchhammeria), and Carlquist et al. (2014: much anatomical detail), also Su et al. (2012: table comparing the genera), and also Kers (2002: general, he was clearly not happy having these genera in Capparaceae. For some information on Borthwickia, see Su et al. (2012).
Borthwickia in particular, but also most Stixideae, are poorly known.
Phylogeny. The above relationships between the three tribes were strongly supported in Z.-D. Chen et al. (2016). From the topology of the tree presented by Martín-Bravo et al. (2007), there are three main clades in Resedeae, Caylusea, [Sesamoides + Reseda]], and Reseda including both Ochradenus and Oligomeris.
Classification. The expanded limits of Resedaceae are those suggested by A.P.G. IV (2016).
TOVARIACEAE Pax, nom. cons. - Tovaria Ruiz & Pavón - Back to Brassicales
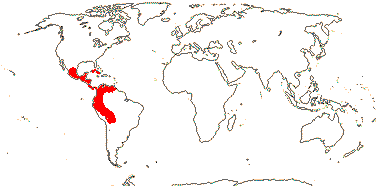
Herbs to shrubs; glucosinolates not from phenylanaline or tyrosine, tannins?; cork?; no bordered pits in imperforate tracheary elements; leaves trifoliolate, margins entire, stipules cauline or on leaf base; flowers (6-)8(-9)-merous; stamens (6-)8(-9), opposite K; G [(5-)6(-8)], alternating with K, placentation ± axile, style short, stigmas lobed, spreading; ovules many/carpel, in several ranks, micropyle zig-zag, inner integument ca 3 cells across, parietal tissue ca 2 cells across, funicle long; fruit a berry; exotestal cells ± enlarged, tanniniferous, walls thickened, endotestal cells small, exotegmic cells fibrous, walls reticulately thickened; endosperm thin, embryo colour?; n = 14, x = 7.
1 [list]/2. Tropical America, Map: see Hall et al. (2004). Photo Flower, Fruit.
Chemistry, Morphology, etc.. The ovules are ± anatropous, but become campylotropous by the post-fertilization development of the exotegmen, and nucellar tissue to the side of the embryo sac is less than in Capparaceae, etc. (Mauritzon 1934g; Boesewinkel 1990).
For general information, see Appel and Bayer (2002) and for floral morphology, see Fisch and Weberling (1990).
[Capparaceae [Cleomaceae + Brassicaceae]]: sinapine [alkaloidal amine], methyl glucosinolates, (aliphatic glucosinolates + - from methionine), erucic acid [fatty acid] +; stomatal myrosin cells 0, cisternae of endoplasmic reticulum as vacuoles and utricles [organelle-like]; cork also cortical; (wood ± storied); vestured pits +; nodes also 3≤: 3≤ [as in Brassiceae]; eglandular hairs simple, unicellular [?level]; leaves simple to palmately compound, blades usu. conduplicate, margins pinnately lobed to entire; flowers 4-merous, (monosymmetric); K 4, C 4; A 6, from 4 primordia, centrifugal, longer than the petals, filaments articulated; gynophore long, carpels 2 (more), placentation parietal, placental strands well developed, stigma lobed or subcapitate; ovules many/carpel, micropyle zig-zag (bi-, endostomal), (parietal tissue 0[?]); K deciduous; testa 2-layered, exotesta palisade or not, tanniniferous, endotesta with inner walls ± thickened, tegmen multiplicative, endotegmen tanniniferous, lignified (or not); endosperm ³2 cells across, cotyledons accumbent or incumbent, radicle in pocket formed by testa.
Age. The divergence between Capparaceae and [Cleomaceae + Brassicaceae] has been dated to as little as 31-24 Ma (Wikström et al. 2001), but other estimates are older, e.g. ca 41 Ma (Schranz & Mitchell-Olds 2006, see also Walden et al. 2020b), around 41-34.7 Ma (Tank et al. 2015: Table S1, S2), ca 39.6 Ma (Magallón et al. 2013), at (55-)43, 41(-30) Ma (Bell et al. 2010) and at ca 58 Ma (Hall et al. 2015). Beilstein et al. (2010) suggest a still older age of (83.2-)71.3(-59.7) Ma, so clarification here, as elsewhere, is in order. The age for this node is estimated to be (71.5-)49(-26.5)Ma by Edger et al. (2015, 2018), (60.8-)56.3(-52.1) Ma by Cardinal-McTeague et al. (2016), (54.2-)48.0(-44.2) Ma, substantially younger but still Eocene, by Magallón et al. (2018), (45.1-)43.2(–41.1) Ma by Hendriks et al. (2022/2023) and (61.2-)55.8(-51.2) Ma by Maurya et al. (2023).
Evolution: Divergence & Distribution. Tank et al. (2015) note a pronounced increase of net diversification at this node (see also Cardinal-McTeague et al. 2016) as do Magallón et al. (2018) and Edger et al. (2018) - for ages, see above. This may be associated with the At-β genome duplication, which, however, happened much earlier (see above).
Rodman et al. (1996) listed 11 possible apomorphies for the [Capparaceae [Cleomaceae + Brassicaceae]] node; Iltis et al. (2011) also suggested apomorphies for around here.
Ecology & Physiology. PYK10 β-glucosidase, which has myrosinase activity, is to be found localized in ER bodies, rod-shaped structures ca 10 x 1 μm derived from endoplasmic reticulum (Yamada et al. 2006; Nakano et al. 2016; Z. Wang et al. 2019). These ER bodies have been found only in core Brassicales and are especially noticeable in roots and seedlings, but can be induced elsewhere by wounding (Z. Wang et al. 2019 and references). (These ER bodies have been equated by what have been called "dilated cisternae" in the literature, - for literature on their distribution, see Nakano et al. (2016), however, Jørgensen (1981) described two kinds of dilated cisternae from Brassicales, and ER bodies may perhaps be compared with her utricular cisternae.) PYK10 β-glucosidase is a member of a group of some 16 myrosinases and it acts on indole GSLs; it seems to be involved in interactions with soil-dwelling microbes (Nakano et al. 2016). For further discussion, see below.
Plant-Animal Interactions. Pierid caterpillars (Pieridae-Pierinae - the whites - there are ca 840 species) are notably common on members of this clade (see also Beilstein et al. 2010; Edger et al. 2015, also above). For details of the interactions of butterflies and plants, see Courtney (1986) and Chew (1988 and references), and for the age of the association, see elsewhere.
Caterpillars of ditrysian moths of the Yponomeutoidea-Plutellidae are also common on this group (Sohn et al. 2013).
Plant-Bacterial/Fungal Associations. Ca 1,000 species are susceptible to pseudoflower-forming rust fungi, Puccinia spp. (Roy 1993, 2001); see especially Brassicaceae, also above.
Chemistry, Morphology, etc.. The distributions of root hairs and of methyl GSLs, and variation patterns in seed coat anatomy are a little odd. Both Brassicaceae and Resedaceae have GSLs derived from elongated amino acid chains, as do Tovariaceae and Gyrostemonaceae (Kjær 1973; esp. Fahey et al. 2001; Mithen et al. 2010). Wasabia japonica in Brassicaceae has a GSL similar to those in Cleomaceae and Capparaceae, and an aromatic GSL of Cleomaceae and Capparaceae is also found in Resedaceae. Quaternary ammonium compounds, including betaines, are common in both Capparaceae and Cleomaceae, while Forchhammeria (Resedaceae-Stixideae) has methyl GSLs with a similar distribution; quaternary ammonium compounds have not been detected in Pentadiplandra or Emblingia - or Buhsia (Capparaceae: McLean et al. 1996). For additional discussion on chemistry, see Leite and Castilho (2017); they suggest that "allyl GSLs" are common here.
For a survey of floral morphology, see Endress (1992); Brassicaceae were compared with Cleomaceae and Capparaceae combined. True blue or red flowers are very rare in the whole group. Campylotropy is by the inpushing of the chalazal bundle. The ventral carpellary bundles are fused and weakly developed (Ronse de Craene & Haston 2006). Guignard (1893) provides details of ovule and especially seed anatomy.
Phylogeny. Relationships are [Capparaceae [Cleomaceae + Brassicaceae]], for further details, see Hall and Sytsma (2000) and Hall et al. (2002). Vaughan and Whitehouse (1971) suggested that Brassicaceae differ from Capparaceae (inc. Cleomaceae) in that the latter have a testa that is only two (not three) cell layers thick, a persistent tegmen (rare in Brassicaceae) and endosperm that is more than one cell layer thick. Judd et al. (1994) provide a morphological phylogeny for Brassicaceae and Capparaceae sensu latissimo.
Classification. Cruciferae/Brassicaceae s. str., cabbage and mustard, have always been considered as one of the most "natural" plant families, however, their recognition makes Capparaceae s.l. (= Capparaceae s. str. + Cleomaceae) paraphyletic, as in Kers (2002). So the alternatives are to have one family (Brassicaceae s.l.); three families; or two families, a Brassicaceae including Cleomoideae along with Capparaceae. The second option is followed here (see also A.P.G. 2009; Iltis et al. 2011).
CAPPARACEAE Jussieu, nom. cons. - Back to Brassicales
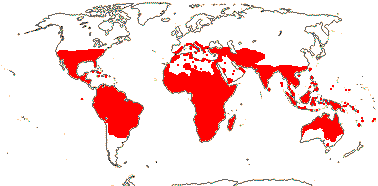
Trees and shrubs (soon deciduous, stems green) (herbs/twining lianes); root hairs 0; pyrrolidine alkaloids +; (successive cambia +); petiole bundle annular or arcuate; sclereids +; (inflorescence fasciculate); flowers often monosymmetric; (K + C tube +), (K connate), C (0); (nectary elongated - Cadaba); A (1-)4-8(-many and centrifugal); pollen (grains 3-celled), surface variously sculpted; post-zygotic incompatibility system [Capparis]; G [2-12], (when 2, superposed-oblique), (placentation axile), (secondary septae +), (style +); ovule (anatropous - Crataeva), inner integument 3-6 cells across, parietal tissue 2-7 cells across; fruit a berry (transversely schizocarpic/septicidal); seeds embedded in pulp/separated by a dry dissepiment, 5-30 mm long; testa ± multiplicative, ± lignified, (endotesta with crystals), tegmen to 10 layers across, outer layer(s) tangentially (radially) enlarged, sclerified, endotegmen (with anticlinal fibres); perisperm (+), slight, endosperm slight, suspensor uniseiate, radicle-hypocotyl medium-long, cotyledon accumbent/incumbent, ± circinate, etc.; n = (7-)10(-15+), x = 11 (?10); plastome 6 bp insertion in ndhF gene.
16 [list]/480 (324): Capparis (250), Maerua (100), Boscia (37), Cadaba (30). Largely tropical. Map: from Jacobs (1960), Frankenberg and Klaus (1980), Wickens (1976), Fl. Austral. vol. 8 (1982), Jalas and Suominen (1991), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Culham (2007 [New World]). [Photo - Flower, Fruit.]
Age. The age of crown-group Capparaceae is around (57.8-)49.5(-39.2) Ma (Cardinal-McTeague et al. 2016), ca 35.5 Ma (Walden et al. 2020b) or (55.2-)47.2(-37.9) Ma (Crataeva, etc., Maurya et al. 2023).
Evolution: Divergence & Distribution. For other ages in the family, see Cardinal-McTeague et al. (2016).
Capparaceae may have originated in Africa ca 47.2 Ma and Capparis in Peninsular India ca 29.3 Ma (Maurya et al. 2023); these authors also looked at the distribution/evolution of some floral/inflorescence characters in Capparis.
Ecology & Physiology. Capparaceae are notably prominent in seasonally dry tropical forests (Pennington et al. 2009). On the other hand, Quadrella traps litter in montane forests in Panama (Harms et al. 2020).
Genes & Genomes. There may be a whole genome duplication preceding diversification here, and variation is chromosome number is considerable (Lysak 2018 and references: x = 13?); Mabry et al. (2020) also discuss possible genome duplications here.
Chemistry, Morphology, etc.. New World Capparaceae have several distinctive and perhaps unique GSLs (Mithen et al. 2010).
Capparis retusa is reported to have stipules and 3:3 nodes, as well as as axillary nectaries (di Sapio et al. 2001). Crateva has glands (?colleters) at the base of the lamina. Some Capparaceae have supernumerary buds and dry yellowish.
There is discussion about the presence of three integuments here (and in Cleomaceae), and/or the development of some kind of tracheary tissue from the inner layer of the inner integument (Narayana 1965).
For general information, see Kers (2002), for some information on floral development, see Leins and Metzenauer (1979) and Ronse Decraene and Smets (1997a, b: esp androecium and gynoecium), and for embryology, see Mauritzon (1934g) and Narayana (1962b, 1965).
Phylogeny. A paraphyletic Crateva is strongly supported as being sister to the rest of the subfamily; Capparis is probably diphyletic (Hall et al. 2002; Hall 2008; Cardinal-McTeague et al. 2016; M. Sun et al. 2016). Maurya et al. (2023) recovered overall relationships in the family of [Crataeva[Buchholzia (Africa), New World Capparaceae, [African Capparaceae + Capparis]]. For relationships in Capparis itself, see Tamboli et al. (2017) and Maurya et al. (2023: 3 plastid genes, 55 Capparis).
Classification. Hall (2008) discusses generic limits in Capparaceae, which are in need of substantial work; for example, New World Capparis will need a new name. Maurya et al. (2023) suggested that there were five sections in Old World Capparis; they did not agree with the former sectional groupings.
Previous Relationships. This used to be a rather heterogeneous family, including Resedaceae-Stixideae, Setchellanthus (Setchellanthaceae), Pentadiplandra (Pentadiplandraceae) and Koeberlinia (Koeberliniaceae). At least all are still members of Brassicales!
[Cleomaceae + Brassicaceae]: annual or perennial herbs (shrubs); inflorescence ± corymbose, (bracts foliaceous); A 6; stigma not lobed; fruit septicidal, persistent placental strands + [replum] (0); seeds 0.5-4 mm long.
Age. Beilstein et al. (2010) suggested that this node is (76.5-)64.5(-54.4) Ma, while the estimate in Bell et al. (2010) is (43-)33, 31(-21) Ma and in Walden et al. (2020b) ca 36.6 Ma. Other estimates are 23-18 Ma (Wikström et al. 2001), some 41 Ma (Schranz & Mitchell-Olds 2006), (45-)19(-1) Ma (!: Franzke et al. 2009: 95% HPD), some 50 Ma (Al-Shehbaz et al. 2006) or (59.1-)50.5(-44.5) Ma (X. Guo et al. 2017), (62-)43(-23) Ma (Edger et al. 2015, 2018), ca 52.6 Ma (Kagale et al. 2014), and (60.7-)59.5, 55.5(-54.3) Ma (C.-H. Huang et al. 2015). The age of this node is (53.8-)50.8(-48.5) Ma in Cardinal-McTeague et al. (2016), ca 49.2 Ma in Schlüter et al. (2016), (40.5-)38.8(–36.9) Ma in Hendriks et al. (2022/2023), (52.7-)50.3(-48.4) Ma in Maurya et al. (2023) and ca 56.3 Ma in Saunders et al. (2024).
Evolution: Divergence & Distribution. The flowers of Cleomaceae are sometimes initially disymmetric, as in Brassicaceae, but the basal condition for Cleomaceae may be monosymmetry even early in development, with the abaxial sepal much enlarged and more or less covering the rest of the developing flower - almost cochleate aestivation (Patchell et al. 2010, esp. 2011). There are a variety of floral morphologies in Brassicaceae (what about Aethionema?) and although the phylogenetic structure at the base of Cleomaceae is being teased apart (Patchell et al. 2014; Saunders et al. 2024), where details of symmetry changes go on the tree remain to be firmly established; monosymmetric flowers are scattered throughout core Brassicales. How colours in flowers of at least some Cleomaceae and Brassicaceae change with age may be distinctive (Nozzolillo et al. 2010).
Ecology & Physiology. For a major reduction of plant height at this node, see Cornwell et al. (2014). About 1,050 species (ca 28%) of Brassicaceae are annuals (Hohmann et al. 2015), as are a number of Cleomaceae. The shrubby habit is also common and is often derived within Brassicaceae (Al Shehbaz 1984; Davin et al. 2016). However, Davin et al. (2016) looked at the molecular background of woodiness in an Arabidopsis thaliana mutant, noting that it was not that different from that of eudicot trees (Davin et al. 2016). Stress-related genes were involved in some features of wood development, in Brassicaceae, at least (Davin et al. 2016), a family that often grows in drier climates, as do other secondarily woody plants (Lens et al. 2013). In particular, Arabidopsis seemed a good model for insular woodiness in general, i.a. showing paedomorphic features like the absence of rays in its wood (Lens et al. 2012b).
Genes & Genomes. Bhide et al. (2014) studied gene and genome evolution, finding over 2,000 new genes in this clade (compared with Carica, including genes lost in one of the two members of Brassicaceae studied). Long non-coding RNAs found in the few species from this clade examined were conserved in position, often being near important genes, but they varied in their sequences - and in Arabidopsis thaliana, at least, they tended to be close to the telomeres (Mohammadin et al. 2015). The rate of molecular evolution of two herbaceous groups of this clade studied is notably higher than that of woody rosids (Barker et al. 2009). The gene that microRNA827 targeted had changed in the taxa in Brassicaceae and Cleomaceae examined, while microR827 itself was lost in Carica (Lin et al. 2017).
Chemistry, Morphology, etc.. Some Cleomaceae and Brassicaceae have similar acylated anthocyanins (Jordheim et al. 2009). For foliaceous bracts, see Eichler (1878) and Prenner et al. (2009).
The inflorescence of Cleomaceae may be a corymb and there are usually 6 stamens (e.g. Podandrogyne - also with orange flowers and 3-foliolate leaves), just like Brassicaceae, however, the stamens are rarely tetradynamous (but see Cleome africana). For floral development, see Leins (2000, and references).
CLEOMACEAE Berchtold & J. Presl - Back to Brassicales —— Synonymy: Oxystylidaceae Hutchinson
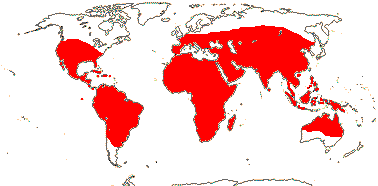
Herbs (annual) to shrubs (small trees); (C4 photosynthesis +); root hairs 0; hairs simple (plant glabrous), (multicellular glands); nodes (1:3, 5); petiole bundle(s) arcuate to forming a ring; leaves palmately compound (simple), stipules filiform to laciniate or spinose/0; bracts foliaceous (not); (plant monoecious - Podandrogyne); flowers monosymmetric (weakly so/polysymmetric); K with one trace [?all], adaxial member envelops the bud/all ± equal, C (toothed); nectary vascularized, (on C towards base)/receptacular, adaxial/(annular); (androgynophore +); A (1+staminodes-)6(-many), (staminodes +, 2-many), anthers coiled at dehiscence, linear, (filaments apophysate [± swollen towards the apex]); pollen surface variously sculpted, often spinulose; (andro-/gynophore +), (G 2, oblique [Oceanopapaver/[4], orthogonal), stigma capitate to punctate; inner integument 2-10 cells across, parietal tissue 2-5 cells across, (nucellar cap ca 2 cells across), (endothelium +); fruit (nut-like/winged - Dipterygium), longer than broad [= silique]; seeds (arillate), (winged), (variously ornamented); exotegmen cells radially enlarged, sclerified, endotegmen cells with lignified bands on periclinal walls; (suspensor massive, haustorial), radicle-hypocotyl long, cotyledons incumbent; Tr-α genome triplication; n = at least 9, x = 11 (?10).
27/270 (346) ([list]): Sieruela (40), Tarenaya (30), Cleome (27), Podandrogyne (26), Cleomella (25). Tropical and warm temperate, esp. America. Map: see Wickens (1976), Fl. Austral. vol. 8 (1982), Jalas and Suominen (1991), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and Culham (2007). [Photo - Inflorescence, Flower.]
Age. Crown-group Cleomaceae are (48.7-)43.2(-37.3) Ma (Cardinal-McTeague et al. 2016) or (53.5-)53.3 Ma (Saunders et al. 2024).
Evolution: Divergence & Distribution. The family has a surpringly good fossil record - entirely of seeds and from (then) boreotropical areas - for instance, fossils of the Cleomaceae stem lineage are all from the United Kingdom (Saunders et al. 2024). For additional ages for nodes in the family, see Feodorova et al. (2010), Cardinal-McTeague et al. (2016), Soares Neto et al. (2020) and Saunders et al. (2024); some ages are very different.
Biogeographic relationships in Cleomaceae are interesting. If Cleomella, a smallish North American clade, is sister to the rest of the family, it is surrounded by Old World clades both inside and outside (Brassicaceae-Aethionemeae) the family (see Patchell et al. 2014 in part). Soares Neto et al. (2020) looked at the biogeographical relationships within the New World clade of Cleomaceae in particular, a clade that probably arrived there around ca 35 Ma or a little after via long distance dispersal from Africa; there has also been movement back to Africa (Tarenaya afrospina). Saunders et al. (2024) noted that the family was boreotropical in origin, but with cooling beginning ca 34 Ma there had been extensive movement of the members of the family.
Soltis et al. (2009) suggested that diversification in Cleomaceae might be connected with the Thα genome triplication (see below); see also S. Cheng et al. (2013). The duplication may be linked with floral developmental in which the sepals are more or less equal in bud, termed the early disymmetric pathway (Patchell et al. 2011; Bayat et al. 2018), although mature flowers of the species with that pathway are frequently monosymmetric...
Ecology & Physiology. There are a few species of Cleome with C4 photosynthesis, e.g. C. gynandra (= Gynandropsis gynandra) (for its genome, see Hoang et al. 2023). Photosynthesis with carbon dioxide-concentrating mechanisms may have evolved four times in Cleome s.l., at least three of which are C4 types, and there are also C2 intermediates (Feodorova et al. 2010; see also Voznesenskaya et el. 2007 for details of the photosynthetic characterizations; Christin et al. 2011b for some dates; Patchell et al. 2014; Schlüter et al. 2016); some proto-Kranz/C2 species are also known here (R. Sage et al. 2014). In C. gynandra genes both from a whole-genome duplication and from single-gene duplications are involved in the evolution of C4 photosynthesis (Hoang et al. 2023). Koteyeva et al. (2011a, c) describe the C4 morphologies involved, which include radiating chlorenchyma surrounding both individual vascular bundles and groups of vascular bundles (see also Koteyeva et al. 2014 and references). Increased venation density in the C4 plants may be connected with a delay in differentiation of the mesophyll cells (Külahoglu et al. 2014; see also C.-F. Huang et al. 2016). Brown et al. (2011: p. 1438) noted that "functionally equivalent mechanisms that control the accumulation of proteins important for C4 photosynthesis" had evolved in parallel in Cleome gynandra and in maize. Thus root endodermal cells seem to have been co-opted into bundle sheath development in both (Külahoglu et al. 2014), and there is a similar set of transcription factors expressed along with C4 photosynthesis genes in the two species (Aubry et al. 2014). See also Bayat et al. (2018) for C4 photosynthesis, etc..
A number of taxa grow in quite arid environments.
Pollination Biology & Seed Dispersal. Despite their often monosymmetric flowers, there is little to report about pollination (Bayat et al. 2018).
Plant-Animal Interactions. For the evolution of the GSL pathway by duplicate retention in Tarenaya hassleriana following a genome duplication (tandem duplications, etc., are also involved), see van den Bergh et al. (2016), and they emphasized the importance of glucosinolates in the roots to protect against attack by bacteria, nematodes and their ilk. Van den Bergh et al. (2016) also noted that similar mechanisms were involved in Arabidopsis duplicate retentions, albeit events in the two were independent.
Genes & Genomes. A genome duplication/triplication, the Cs-α or Th-α event, or series of events, occurred ca 13.7 or 20 Ma (Schranz & Mitchell-Olds 2006: Cleome spinosa; M. S. Barker et al. 2009: C. spinosa; S. Cheng et al. 2013: Tarenaya hassleriana, also called the Th-α duplication; van den Bergh et al. 2014, 2016). After a careful analysis, Mabry et al. (2020) were still not sure where to put this event, although it was perhaps unlikely to characterize the whole family. Hoang et al. (2023) suggest that a Gg-α whole genome duplication, an allotetraploid event, may be common here, while Tarenaya hassleriana has a duplication (the Th-α event) that its relative, Gynandropsis gynandra, does not. Certainly, not all Cleomaceae genomes show evidence of the former event; for example, it is not found in the genome of Cleome violacea (Hoang et al. 2023). Clear subgenome dominance/gene fractionation is evident in the family (see also T. Feng et al. 2025, c.f. Asteraceae).
The genome of Cleome spinosa is about twice the size of that of Arabidopsis thaliana (Schranz & Mitchell-Olds 2006; c.f. Bhide et al. 2014).
Chemistry, Morphology, etc.. Tarenaya hassleriana has prickles more or less in the stipular position.
Ronse Decraene and Smets (1993b) suggested that the four petals of this clade were equivalent to four outer diagonally-inserted stamens. Zenchyzen et al. (2023a) described the development of the androgynophore in Gynandropsis gynandra, formed from elements made up of the receptacle and androecium and gynoecium; there appeared to be three vascular bundles in the filaments.
Saunders et al. (2024) discuss (and illustrate) variation in seed morphology in detail. Appreciable amounts of catechyl lignins have been found in the seed coat of Cleome (Tobimatsu et al. 2013).
For general information, see Kers (2002: in Capparaceae) and Bayat et al. (2018), for floral development, see Karrer (1991), for nectary development, see Carey et al. (2023: Cleome) and Zenchyzen et al. (2023b: family), for anther dehiscence, see Mitchell-Olds et al. (2005), for pollen and seed, see Sánchez-Acebo (2005 and references), and for embryology, see Mauritzon (1934g: Polanisia trachysperma has a huge suspensor cell) and Sachar (1956b).
Phylogeny. Relationships within Cleomaceae have had rather little support, but Cleome s.l. itself is widely scattered on the tree (Hall 2008; Bayat et al. 2018). An ITS study with quite broad sampling recovered a highly paraphyletic Cleome; rooting was somewhat of a problem and there was little support for the backbone, but guite good support for much of the finer detail (Feodorova et al. 2010). A 5-marker 3-genome analysis, the most comprehensive yet, found stronger support for clades in analyses of the chloroplast markers than from ITS: A group of North American Cleomaceae (= Cleomella) were sister to the rest of the family, and the C. droserifolia group (= Rorida) and Cleome s. str. (c.f. Feodorova et al. 2010) were successively sister to the remainder (Patchell et al. 2014: q.v. for further details); Cardinal-McTeague et al. (2016) also found Cleomella to be sister to the rest of the family. Although Barrett et al. (2017) found that Cleome s. str. was sister to the rest of the family, although support was weak, the pendulum swings, and Sauders et al. (2024) found good support for Cleomella to be in this position. The analysis by Saunders et al. (2024) used the Angiosperms353 probe set and included all but one of the genera and 236/270 species; eleven main clades were recovered (there were 15 primary clades in Bayat et al. 2018).
For phylogenies of parts of the family, see Sánchez-Acebo (2005), Catalan et al. (2007), Inda et al. (2008b), Riser et al. (2013) and Barrett et al. (2017). The small-flowered Dipterygium, placed in Capparaceae in a subfamily by itself by Kers (2002), is to be included here; it is well embedded in the family in a clade with some other Old World taxa (clade 6 of Patchell et al. 2014; see also Cardinal-McTeague et al. 2016; Barrett et al. 2017). Soares Neto et al. (2020) concentrated on Tarenaya and other New World taxa; genera like Cleome and Podandrogyne are still polyphyletic.
Classification. Generic limits were for some time very unsatisfactory and obviously needed attention (see e.g. Hall et al. 2008; Riser et al. 2013); Cleome was broadly circumscribed. Many of the clades did not correspond even to sections of Cleome, however, the wholesale dismemberment of Cleome that was under way then seemed poorly advised; piecemeal splitting is not a course to be advocated. However, Patchell et al. (2014) provided a careful outline and analysis of what has been done and what may still need to be done (see also Feodorova et al. 2010), and Roalson et al. (2015), Roalson and Hall (2017) and Soares Neto et al. (2020) and McGinty and Roalson (2020) (the two latter focus on New World taxa) have begun a re-evaluation of generic limits in the family as a whole; in Roalson and Hall (2017), five monotypic genera rather than one variable genus were recognized in part because the species came out in four (actually three) places in the key...
BRASSICACEAE Burnett, nom. cons. / CRUCIFERAE Jussieu, nom. cons. et nom. alt. - Back to Brassicales
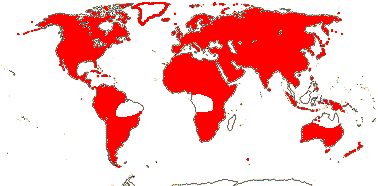
Nortropane alkaloids +, (camalexin - indole alkaloid - +), methyl glucosinolates 0, chain elongation of methionine-derived glucosinolates +; cork ?always deep-seated; stomata anisocytic; (leaves deeply pinnately lobed), stipules 0; floral development closed, flowers disymmetric; A about as long as C, the two outer shorter than the four inner [tetradynamous]; lateral nectary lobes outside inner A, etc.; tapetal cells binucleate; pollen grains tricellular, with tryphine, orbicules 0 [?level], surface often reticulate; gynophore short/0; ovary with commissural septum [= false septum], stigma commissural, dry; ovules slender, outer integument 2-4(-5) cells across, inner integument (2-)3-9(-15) cells across, endothelium +, body of ovule long, slender, parietal tissue 1 cell across (0 - Malcombia), hypostase +; (megaspore mother cells several); commissural septum persistent in fruit; testa 3-layered, exotestal cells reticulately thickened on radial walls, often mucilaginous, endotesta lignified, thickenings U-shaped or on anticlinal walls alone, (unthickened), (with crystals), tegmen (not multiplicative), cells usu. flattened; chalazal endosperm cyst +, endosperm 1-layered, embryo folded, (spiral), radicle-hypocotyl short to long, radicle not in testal pocket; x = 8; duplication of PHYB → PHYD gene, loss of CYP73 gene duplicate [class II]; sporophytic self-incompatibility system present; x = 7 (?8), At-α genome duplication, genome size [1C] (0.073-)0.61(-5.12) pg/[2 C] (157-)617(-4273) Mb.
349/4,140 spp. [list]: 58 tribes, 5 supertribes, 2 subfamilies. World-wide, esp. Tajikstan, Turkey and the North Temperate region, drier areas in general, but few Australia and southern Africa. Map: from Vester (1940), Hultén (1971) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photos - Collection].
Age. Crown group diversification, i.e. separation of Aethionemoideae from the rest, occurred perhaps ca 40 Ma (Al-Shehbaz et al. 2006, see also Koch 2011). Other estimates are a mere (35-)15(-1) Ma (Franzke et al. 2009), ca 29.9 Ma (Walden 2020b), (46-)32(-17) Ma (Edger et al. 2015, 2018, see also Hohmann et al. 2015), (42.5-)35.2(-30) Ma (X. Guo et al. 2017), (49.4-)37.6(-24.2) Ma (Couvreur et al. 2010: also summary), ca 40.1 Ma (Mandáková et al. 2017b), (46.6-)43.4(-40.3) Ma (Hall et al. 2015, see also Cardinal-McTeague et al. 2016), (42.7-)42, 37.1(-36.3) Ma (C.-H. Huang et al. 2015), (58.9-)48(-37.5) Ma (Mohammadin et al. 2017) and (33.2-)29.9(-26.8) Ma (X.-C. Huang et al. 2019). Beilstein et al. (2010) suggested an age of (64.2-)54.3(-45.2) Ma for crown group Brassicaceae, Hendriks et al. (2022/2023: see also Table 3) a late Oligocene age of (25.7-)24.5(–23.1) Ma. Franzke et al. (2016) discuss the various problems surrounding dating here.
Couvreur et al. (2010) reasonably thought that the fossil Dressiantha might not belong to core Brassicales, or even to Brassicales at all (see above).
Includes Aethionemoideae, Alysseae, Alyssopsideae, Anastaticeae, Anchonieae, Aphragmeae, Arabideae, Arabidopsideae, Arabodeae, Asperuginoideae, Biscutelleae, Boechereae, Brassiceae, Brassicodae, Brassicoideae, Buniadeae, Calepineae, Camelineae, Camelinodae, Cardamineae, Chamireae, Chorisporeae, Coluteocarpeae, Conringeae, Cremolobeae, Crucihimalayeae, Descuranieae, Dontostemoneae, Erysimeae, Euclidieae, Eudemeae, Eutremeae, Fourraeeae, Halimolobeae, Heliophileae, Heliophilodeae, Hemilophieae, Hesperideae, Hesperodae, Hillielleae, Isatideae, Kernereae, Lepidieae, Malcomieae, Megacarpaeeae, Microlepidieae, Notothlaspideae, Oreophytoneae, Physarieae, Plagiolobeae, Schizopetaleae, Schrenkielleae, ×Shehbazieae, Sisymbrieae, Smelowskieae, Stevenieae, Subularieae, Thelypodieae, Thlaspideae, Turritideae, Yinshanieae.
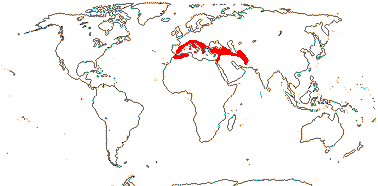
1. Aethionemoideae D. A. German, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & Al-Shehbaz / Clade F - Aethionema Aiton
Plants subshrubs, perennial (annual) herbs, (spiny); nortropane alkaloids +; plant glabrous; leaves articulated between petiole and blade, blade ± linear (to broadly obovate), often fleshy, margin entire; flowers ebracteate; 3 veins on petal claws [?all]; filaments usu. winged; nectaries 2, lateral; (1-)2-4(-8) ovules/ovary; fruit silicula, surrounded by wing, angustiseptate, bilocular, few-seeded, dehiscent, and/or unilocular, 1-seeded, indehiscent, samara (heterocarpy); testa (mucilaginous); cotyledons accumbent; n = 7, 8 (11, 12, 14...); chloroplast ycf15 gene 0.
1/58. The Mediterranean and Southern Europe to Afghanistan, esp. Turkey to Iran. Map: from Hedge (1976), see also Mohammadin et al. (2017). [Photo - Flowers.]
Age. Estimates for the age of crown-group Aethionemeae range from (28-)11(-1) Ma (Franzke et al. 2009), ca 11.3 Ma (Hohmann et al. 2015), ca 13.5 Ma (Mandáková et al. 2017b: note sampling; see also Walden et al. 2020b), (30.9-)20.6(-10.8) Ma (Cardinal-McTeague et al. 2016), (34.6-)24(14.6) Ma (Mohammadin et al. 2017), (20.7-)14.6(-9.5) Ma (X. Guo et al. 2017: as Clade F) and (54.3-)46.9(-39.4) Ma (Beilstein et al. 2010).
2. Brassicoideae Prantl / Clades [E [D [A [B + C]]]] / Lineages [III [IV [I [[various genera + V] II]]]] / core Brassicaceae
Structural elaborations of glucosinolates, (methionine-derived glucosinolates 0); (included phloem +); (C fringed or lobed); A (2, 4), (long); tapetosomes with T-oleosin; (pollen omniaperturate); (G stipitate), (style long); fruit angusti- or latiseptate, quadrangular; (seeds winged); cotyledons also variants of conduplicate-incumbent, etc.; Br-α].
Age. Koch (2012) suggested an age for this node of around (53.5-)49(-43) Ma, in line with the estimates of Beilstein et al. (2010); 38 Ma was the estimate in Hall et al. (2015), (35.6-)35.1, 29.7(-29.1) Ma in C.-H. Huang et al. (2015), (29.7-)25.3(-21.7) Ma in X. Guo et al. (2017), 29-24 Ma in Moghe et al. (2014, q.v. for other estimates), and ca 23.3 Ma that in Hohmann et al. (2015: note topology). Couvreur et al. (2010) suggested an age of ca 32.3 Ma, (28-)11(-1) Ma was suggested by Franzke et al. (2009), ca 25.3 Ma by Edger et al. (2018: Arab-Brass), ca 30.5 Ma by Mandáková et al. (2017b) and (22.9-)21.3(-19.6) Ma by X.-C. Huang et al. (2019).
2A. Hesperodae D. A. German, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & Al-Shehbaz / Lineage III or E
Hairs usu. +, simple/branched, capitate glands usu. +; lamina usu. little divided, base very rarely auriculate; x = 7.
[Chorisporeae, ×Shehbazieae, Dontostemoneae]: hairs simple.
Chorisporeae C. A. Meyer [parent of ×Shehbazieae]
hairs ?simple, glands + [multiseriate stalk and multicellular gland]; lamina margin various; style conical, stigma strongly 2-lobed, lobes connivent, (sub)decurrent; fruit moniliform, latiseptate, (indehiscent), (schizocarp, segments corky); seeds winged or not; cotyledons accumbent; n = 7 (9).
5/64: Parrya (ca 40). Central to Southwest Asia (Arctic, northeast Asia to northweat North America - some Parrya).
×Shehbazieae German [= Chorisporeae × Dontostemoneae - ×Shehbazia tibetica (Maximowicz) D. A. German
Biennial; lamina margin deeply pinnate; style conical, stigma ?minute, strongly 2-lobed, lobes connivent, subdecurrent; fruit ; seeds not winged; cotyledons accumbent. [From German and Friesen 2014 - intermediate between the two parents...]
1/1. Northeastern Tibet, China (Gansu).
Dontostemoneae Al-Shehbaz & Warwick [parent of ×Shehbazieae]
Capitate glands +; lamina margin entire (lobes +, distant); style cylindrical, stigma capitate, 2-lobed, lobes free, not or slightly decurrent; cotyledons various, usu. incumbent.
2/17: Dontostemon (11).
[Hesperideae [Euclidieae [Anchonieae + Buniadeae]]]: hairs branched; seeds not winged.
Age. The age of this clade is ca 20.7 Ma (Mandáková et al. 2017b; H. Chen et al. 2020) or (25.4-)20.5(-15.5)) Ma (Eslami-Farrouji et al. 2021).
Hesperideae Prantl - Hesperis L.
Biennial to perennial; hairs unicellular, simple and/or forked, also glandular [uniseriate stalk and unicellular gland]; cauline leaves petiolate to sessile, (base auriculate or amplexicaul); flowers bracteate/ebracteate, colour various; ovules 4-40/ovary; fruit siliqua, terete, 4-angled/slightly latiseptate; seeds uniseriate, coat not mucilaginous; cotyledons incumbent.
1-2: Hesperis (45). Europe to northern China.
Age. Crown-group Hesperis is (19.9-)13.6(-7.7) Ma (Eslami-Farrouji et al. 2021).
[Euclidieae [Anchonieae + Buniadeae]]: .
hairs branched (simple, 0); fruit silqua, silicula, terete/4angled; cotyledons incumbent; n = 7 (8); chloroplast ndh gene loss/pseudogenization, chloroplast ycf15 gene 0.
29/>150: Malcomia (32); Braya (17). Mainly Turkey and central to southwest Asia, few North America.
Age. This clade is dated to ca 17.4 Ma (Couvreur et al. 2010) or ca 18.2 Ma (H. Chen et al. 2020).
hairs branched, glands + [multiseriate stalk and multicellular gland] (0); n = (5-)7(-8); chloroplast ycf15 gene 0.
9-12/75-100: Matthiola (56). S.W. and C. Asia, Africa, some Europe, 4 spp. North Amerioc.
Buniadeae de Candolle - Bunias L.
1/2.
[Arabodeae [Camelinodae [Heliophilodae + Brassicodae]]]: ?
Hairs mainly branched (along with simple); lamina usu. un-/slightly divided, basally auriculate or not; x = 8.
Note: it is uncertain what tribes are to be included here (German et al. 2023).
[Arabideae + Stevenieae]: hairs branched; fruits often latiseptate; cotyledons usu. accumbent.
Arabideae de Candolle —— Synonymy: Arabidaceae Döll, Drabaceae Martynov
Plant (shrubby), often annuals; fruits (terete); n = 8; rps16 gene 0.
18/560: Draba (410), Arabis (100), Aubrieta (23). North Temperate to Arctic, N.W. Africa mountains in Central Africa, and Draba in particular also Andean to Patagonian South America.
Age. The age of this clade is (18.6-)14.6(-11.0) Ma (X. Guo et al. 2017) or ca 14.1 Ma (Mandáková et al. 2020).
Stevenieae Al-Shehbaz, D. A. German & M. Koch
(Plant annual); hairs branched [stellate to furcate]; cauline leaves +; bracts usu. 0; style slender; fruit siliqua or silicula; seeds uniseriate.
3/11: Stevenia (8). (Europe to) Siberia, eastern or central Asia.
[Alysseae + Asperuginoideae]: ?
Hairs stellate; filaments with appendages; pollen cuticle finely ridged (and wrinklked), epidermal cells rectangular to elongated; (stigma lobed, lobes not decurrent); fruit silicula, latiseptate to terete (angustiseptate); seeds "few"; n = (7) 8 (9, 11, 15), extensive polyploidy; rps16 gene 0.
24/282: Alyssum (114), Odontarrhena (91). Eurasia, North Africa, 1 sp. northwest North America.
Asperuginoideae Al-Shehbaz, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & D. A. German - Asperuginoides axillaris (Boissier & Hohenacker) Rauschert
Annuals; hairs ± stellate; cauline leaves petiolate, not auriculate; flowers bracteate, white; pollen 3-colpate; ovules 2, apical; fruits silicula, latiseptate or septum 0, hairs apically glochidiate; seeds broadly winged; cotyledons accumbent; n = 16.
1/1. E. Turkey to the Western Himalayas.
[Camelinodae [Heliophilodae + Brassicodae]]: ?
Camelinodae D. A. German, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & Al-Shehbaz - Lineages A / I
Hairs usu. +, simple and/or stellate; lamina entire to compound, basally auriculate or not; fruit siliqua; x = 6-8.
Age. The crown-group age of clade A is (24-)23.6(-23.1) Ma (C.-H. Huang et al. 2015), (24.2-)20.3(-16.9) Ma (X. Guo et al. 2017), 21-15 Ma (Fiebig et al. 2004) or ca 22.6 Ma (Mandáková et al. 2017b).
Stem with endodermis [Rorippa]; hairs simple/0; leaves usu. pinnately lobed/-compound; (A 4 - Cardamine hirsuta); fruit terete/latiseptate/(angustiseptate -Armoracia), siliqua; cotyledons accumbent; x = 8, many polyploids.
10-14/344:Cardamine (200), Rorippa (86), Barbarea (29). Widespread.
; Rorippa (85).
Age. Crown-group Cardamineae are (18.6-)14.2(-10.2) Ma (X. Guo et al. 2017).
Lepidieae de Candolle - Lepidium L.
leaves pinnately compound/not, sessile, base acute to perfoliate; flowers ebracteate, white (to yellow);; (A 4 - no laterals/2 median), (C 0; A 2); fruits silicula, ± winged or not, angustiseptate; 1 seed/carpel; embryo notorrhizal, cotyledons folded; extensive hybridization/high polyploidy.
1/268. Cosmopolitan, esp. Temperate.
[Descurainieae [Yinshanieae + Smelowskieae]]: ?
Descurainieae Al-Shehbaz, Beilstein & E. A. Kellogg
Annuals to perennials (woody - Canary Islands); hairs dendritic (forked, simple), unicellular glandular papillae +/0; cauline leaves to tripinnatisect, base not auriculate; flowers usu. ebracteate, yellow or white; ovules many/ovary; fruit siliqua/silicula; seeds uni- ot biseriate, mucilaginous; cotyledons incumbent; n = 7 (6).
6/48: Descurainia (50). Widespread, not S.E. Asia to the Antipodes.
Yinshanieae Al-Shehbaz, Warwick, Mummenhoff & M.A. Koch - Yinshania Y. C. Ma & Y. Z. Zhao
Annuals (perennials), tuberous, rhizomatous; hairs 0/simple/branched; cauline leaves various, petiolate, exauriculate; flowers ebracteate (bracteate), white (pinkish); ovules 1-many/ovary; fruit silicula, terete (latiseptate/angustiseptate); seeds uniseriate, mucilaginous (c.f. FoC!); cotyledons incumbent (accumbent).
1/13. China, 1 sp. to Taiwan and North Vietnam.
Smelowskieae Al-Shehbaz, Beilstein & E. A. Kellogg [parent of ×Microlepidieae] - Smelowskia C. A. Meyer
Perennials, (subshrubs), (annuals); hairs multiple-branched, (simple); cauline leaves pinnatisect, not auriculate; flowers bracteate or ebracteate, white to yellow (purple); ovules many/ovary; fruit siliqua/silicula, terete/latiseptate/angustiseptate; seeds uni-/biseriate; cotyledons accumbent/incumbent; x = 6.
1/25. C. and E. Asia, few N. and W. North America.
[[Erysimeae + Malcomieae] [Physarieae [Arabidopsideae [Oreophytonaceae etc. [Camelineae ...]]]]]: ?
Erysimeae Dumortier - Erysimum L. —— Synonymy: Erysimaceae Martynov
Annual to perennial herbs (shrubs); cardenolides +; hairs mesifixed/stellate; cauline leaves petiolate to sessile, (base auriculate); flowers bracteate or not, yellow; fruits siliqua, latiseptate; seeds usu. uniseriate; coyledons incumbent/accumbent.
1/225. Northern Hemisphere.
Malcomieae Al-Shehbaz & Warwick - Malcomia R. Brown
.
1/6. Eurasia.
hairs sessile, stellate (simple, branched, etc.); pollen 4≤ colpate (3-colpaate); ovules 2-many/ovary fruit silicula, inflated/angustiseptate), (siliqua); long-chain hydroxy fatty acids in seeds; x 8 [n = 4-11], WGD [hexa.].
7/133: Physaria (105: inc. Lesquerella). North America, few Agentina and Bolivia, 1 sp. circumpolar.
Arabidopsideae Al-Shehbaz, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & D. A. German - Arabidopsis (de Candolle) Heynhold
Annual; hairs simple/stalked forked; cauline leaves petiolate or subsessile, not auriculate; flowers ebracteate, white to purple; fruit siliqua; seeds uniseriate; cotyledons accumbent (incumbent).
1/18. North Temperate, North Africa.
[Oreophytoneae [Alyssopsideae + Turritideae]]: ?
Oreophytoneae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch
Perennials; hairs 0/simple/bravhed; cauline leaves sessile, pinnatifid, base auriculate/0; flowers solitary/ebracteate, white to reddish; ovules many/ovary; fruit siliqua, terete/latiseptate; seeds uni-/biseriate, (mucilaginous); cotyledons incumbent [?all].
2/8: Murbeckiella (7). S.W. Europe, Algeria, Caucasus, East Africa.
Age. The crown-group age of Oreophytoneae is estimated to be ca 0.2 Ma (Hendriks et al. 2022).
[Alyssopsideae + Turritideae]: ?
Alyssopsideae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch
.
4/9: Olimarabidopsis (3). (E. Mediterranean to) Central Asia (to W. China).
Turritideae Buchenau - Turritis L.
.
1/2. Eurasia.
[Camelineae [Hemilophieae etc. + Crucihimalayeae etc.]]: ?
Annuals to perennials; hairs various [eglandular]; cauline leaves sessile, auriculate or sagittate; flowers ebracteate, colour various; ovules 2–40/ovary; fruit siliqua/silicula, (indehiscent), latiseptate or angustiseptate; cotyledons incumbent (accumbent).
4/16: Camelina (8), Capsella (5). North Temperate, inc. Mexico.
Age. Crown-group Camelineae are perhaps (12.8-)9.9(-7.5) Ma (X. Guo et al. 2017).
[[×Microlepidieae + Hemilophieae] [Crucihimalayeae [Halimolobieae + Boechereae]]]: ?
[×Microlepidieae + Hemilophieae]: ?
×Microlepidieae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch [= Crucihimalayeae × Smelowskieae]
Annuals to perennials; hair simple to branched; cauline leaves sessile or not, exauariculate; flowers ebacteate, white; 4-many ovules/ovary; fruit silicula, angustiseptate, carinate; seeds mucilaginous; cotyledons incumbent; x = 15, n = 4, 6-8, 10 (24), WGD [tetra]/many polyploids. (Description when there were only 3 spp. included).
17/56: Pachycladon (11). Australia (most), New Zealand.
Hemilopheae Al-Shehbaz, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & D. A. German
Rhizomatous perennials; cauline leaves petiolate to sessile, not auriculate; flowers bracteate, white to purple; pollen 3-colpate; ovules 2, 4/ovary, apical; fruit silicula, terete or slightly angustiseptate, (septum 0); cotyledons accumbent.
2/7: Hemilophia (6). China (Sichuan and Yunnan).
[Crucihimalayeae [Halimolobieae + Boechereae]]: ?
Crucihimalayeae D. A. German & Al-Shehbaz [parent of Microlepidieae]
Annual to tussock-forming perennial; hairs simple to dendritic; cauline leaves ± sessile, base auriculate (cuneate), (0); flowers bracteate or ebracteate (single), white to purple; ovules 2-many/ovary; fruit siliqua to silicula, dehiscent, terete or latiseptate; seeds mucilaginous or not; cotyledons incumbent or accumbent.
3/15: Crucihimalaya (13). Central Asia, esp. China.
[Halimolobieae + Boechereae]: x = 8.
Age. The age of this clade is ca 11.1(+/- 0.9) Ma (Mandáková et al. 2017) or ca 4.0 Ma (Hendriks et al. 2022/2023).
Halimolobieae Al-Shehbaz, Beilstein & E. A. Kellogg
Annuals to perennials; hairs (simple) bracnhed; cauline leaves auriculate or not; flowers usu. ebracteate, white (purplish); ovules many/ovary; fruit silicula/siliqua, terete/angustiseptate; seeds uni-/biseriate, mucilaginous; cotyledons incumbent.
5/40: Mancoa (11), Pennellia (9). Esp. C. and N. Mexico, also S.W. U.S.A
Boechereae Al-Shehbaz, Beilstein & E. A. Kellogg
Annuals to suffructecent perennials; hairs simple to branched; cauline leaves often auriculate; flowers ebracteate, white to pink; 2-many ovules/carpel; fruit siliqua (silicula), terete/latiseptate; seeds uni-/biseriate; cotyledons accumbent/incumbent; x = 7, WGD + [tetra.].
9/110: Boechera (83, + 400≤ apomictic hybrids). North America, 1 sp. (Borodinia) Russian Far East, 1 sp. (Boechera) Greenland. Map: see Hay et al. (2023: Fig. 2).
Capitate glands (0); leaves simple, lamine gradually narrowing to petiole; filaments of median A united in pairs; stigma lobes decurrent (not lobed); fruit silicula; x = 7.
/Age. The age of this clade is (17.6-)17.2(-16.8) Ma (C.-H. Huang et al. 2015), (22-)17.3(-12.7) Ma (X. Guo et al. 2017) or ca 24.6 Ma (Mandáková et al. 2017b).
Conringeae D. A. German & Al-Shehbaz - Conringia Fabricius
Stigma entire; fruit 4-8-angled.
1/3. Eurasia.
[Heliophilodae + Brassicodae]: ?
Heliophilodae D. A. German, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & Al-Shehbaz / Lineage IV
Hairs 0/simple (branched); lamina not or slightly divided (compound), base usually not auriculate; x = various.
[Hillielleae, [Biscutelleae + Megacarpaeeae], [Iberideae + Anastaticeae]: ?
Hillielleae H. L. Chen, T. Deng, J. P. Yue, Al-Shehbaz & H. Sun - Hilliella (O. Schulz) Zhang & Li
1/11.
[Biscutelleae + Megacarpaeeae]: 2 ovules/ovary; fruit silicula, didymous, angustiseptate; cotyledons accumbent.
Biscutelleae Dumortier
Annual to perennial; hairs simple/0; cauline leaves sessile (0), (auriculate); flowers bracteate or not, white/yellow; WGD (allop.)
2/54: Biscutella (53). Especially Mediterranean, to the Far East.
Megacarpaeeae D. A. German - Megacarpaea de Candolle
Perennial; hairs simple; cauline leaves ± compound, base often auriculate or amplexicaul; flowers ebracteate, colour various; A (-24); fruit indehiscent, each carpel with prominent circumferential wing; seed not mucilaginous; chloroplast ycf15 gene 0.
1/9. Central Asia, Himalayas, China.
Age. Megcarpaeeae are some (19.6-)14.8(-10.1) Ma (X. Guo et al. 2017).
Iberideae Webb & Berthelot
Annuals to perennials, subshrubs; cucurbitacins +; hairs simple/0; cauline leaves ± sessile; flowers white to pink, monosymmetric [2 abaxial C larger than adaxial]; ovules (4/ovary); fruits winged, strongly angustiseptate; seeds often 2, (winged), (mucilaginous); WGD + [auto.].
2/30: Iberis (27). Europe, esp. Mediterranean, N.W. Africa to C. Asia.
n = 8-13, WGD + [auto.]; chloroplast ycf15 gene 0.
13/65. Although Anastaticeae have been included in Clade E, evidence suggests that they do not belong there (X. Guo et al. 2017).
[Notothlaspideae [Heliophileae + Chamireae]]: ?
Notothlaspideae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch - Notothlaspi J. D. Hooker
Perennial; hairs 0/simple; cauline leaves 0/petiolate, exauriculate; flowers ebracteate/basally bracteate, white; many ovules/ovary; fruit seeds biseriate; cotyledons incumbent n = >18.
1/3. New Zealand.
Heliophileae de Candolle - Heliophila L.
Annuals to shrubs, lianes; stem with endodermis; flower bracts?, white to purple; (filament appendages +); (1)2-many ovules/ovary; fruit siliqua/silicula, schizocarp, samara, terete/latiseptate/angustiseptate; seeds (winged); cotyledons twice-folded transversely [= diplecolobal] (spiral); WGD hexa., x = 8?, n = (8-)10, 11 (etc.).
1/90. Southern Africa, esp. the Cape.
Age. Crown-group Heliophila is estimated to be somewhere around (1.9)2.7-5.8(-7.4) Ma (Mandáková et al. 2012: T. 1).
Chamireae Sonder - Chamira circaeoides (L. f.) Zahlbruckner
Annuals; cauline leaves small/0, not auriculate; flowers ebracteate, white; pollen 3-colpate; ovules 2-8/ovary; fruit siliqua; cotyledons longitudinally folded, becoming much enlarged [the main photosynthetic organ].
1/1. Western Cape, South Africa.
[Subularieae [Asteae [Eudemeae [Schizopetaleae + Cremolobeae]]]: ?
Subularieae de Candolle
Annuals, ± aquatic or not; hairs 0; flowers from centre of rosette, or cauline leaves 0 [i.e. scapose]; flowers ebracteate, white; pollen 3-colpate; ovules 4-18/ovary; fruit silicula, latiseptate or slightly angustiseptate, seeds biseriate, broadly winged, cotyledons accumbent, or wing 0, cotyledons incumbent; n = 8, 14, 15.
2/3: Subularia (2). N. North America, temperate Eurasia, East African mountains.
Note that Teesdalia (Iberideae) comes out between Idahoa and Subularia in Hendriks et al. (2022/2023).
Age. Crown-group Subularieae are ca 18.1 Ma (Hendriks et al. 2022/2023).
[Asteae [Eudemeae [Schizopetaleae + Cremolobeae]]]: ?
Age. The age of this clade is ca 20 Ma (Salariato et al. 2016).
Asteae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch (inc. Scoliaxoneae)
Annuals, biennials.
2/2. Mexico.
[Eudemeae [Schizopetaleae + Cremolobeae]] / the CES clade: ?
Age. The age of this clade is ca 18.5 Ma (Salariato et al. 2016). [C + E 21.9-17.7-13.7].
Eudemeae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch
Perennials, rosette/cushion plants; hairs 0/simple/branched; cauline leaves 0; flowers single, or racemose, bracteate/ebracteate, white/yellow; nectaries 4; ovules 2-many/ovary; fruit siliqua/silicula, terete/latiseptate/angustiseptate, (indehiscent); seeds uni-/biseriate; cotyledons incumbent; n = 9.
11/43: Eudema (10), Onuris (5), Stenodraba (5), Xerodraba (5). Andes, rare in Colombia, otherwise Ecuador to Patagonian Chile and Argentina. Map: see Salariato et al. (2014: Fig. 1A; 2016: Fig. 1C).
Age. Crown-group Eudemeae are (16.6-)13.0(-9.3) Ma (Salariato et al. 2016) or ca 10.5 Ma (Al-Shehbaz et al. 2023).
[Schizopetaleae + Cremolobeae]]: ?
Schizopetaleae Barnéoud —— Synonymy: Schizopetalaceae A. Jussieu
(Annuals); n = 9.
2/16: Schizopetalum (10). Southern Peru, northern Chile (Salariato et al. 2016: Fig. 1D).
Age. The age of crown-group Schizopetaleae is (20.8-)15.2(-10.3) Ma - Atacama diverging, otherwise (7.3-)5.2(-3.3) Ma (Salariato et al. 2016).
Annual to perennial, (cauduciform); hairs simple (branched); cauline leaves petiolate/sessile, not auriculate, (0); flowers bracteate or not, white to yellow; ovules 2/ovary; fruits silicula, variously winged (wings 0), schizocarpic, angustiseptate; cotyledons accumbent/oblique/incumbent; n = 9, 10.
9/40 (or 3/32?): Menonvillea (24). Andes, southern Colombia southwards (Salariato et al. 2016: Fig. 1B).
Age. Crown-group Cremolobeae are some (21.9-)17.2(-13.3) Ma (Salariato et al. 2016).
[Clade B + C]]: (stigma variously lobed).
Age. The crown-group age of this clade is (27.1-)26.6(-26.1) Ma (C.-H. Huang et al. 2015) or ca 26 Ma (Mandáková et al. 2017b).
Brassicodeae V. E. Avetisyan / Lineage II or B or expanded Lineage II
Hairs 0/simple (branched); lamina un-/slightly (much) divided, often basally auriculate; fruit silicula?, (terete), relatively broad, (siliqua, often segmented [lomentum]); seeds usu. not winged; x = 7.
Age. This clade has been dated to (24.2-)23.7(-23.3) Ma (C.-H. Huang et al. 2015), (24.5-)20.6(-17.6) Ma (X. Guo et al. 2017: c.f. content) or ca 25.4 Ma (Mandáková et al. 2017b).
Aphragmeae D. A. German & Al-Shehbaz - Aphragma de Candolle
Annual to perennial; hairs minute, simple/branched; cauline leaves petiolate or sessile, not auriculate; inflorescence bracteate; fruit siliqua/silicula, terete or latiseptate; cotyledons incumbent.
1/11. Central Asia and the Himalayas, 1 sp. N.E. Asia and N.W. North America.
[Kernereae [Cochlearieae etc. + Calepineae etc.]]: ?
Kernereae Al-Shehbaz, Warwick, Mummenhoff & M. A. Koch
Perennial; hairs simple; cauline leaves sessile, not auriculate; flowers bracteate or not, white; 6-24 ovules/ovary; fruit silicula, terete or latiseptate; seeds biseriate; cotyledons accumbent; n = 7.
3/3. Central and eastern South and eastern Central Europe.
[Cochlearieae [Conringieae [Coluteocarpeae + Plagiolobeae]]]: ?
Cochlearieae Buchenau
hairs 0; cauline leaves often sessile; flowers ebracteate, white; fruit silicula, terete/angustiseptate; seeds biseriate; x = 6/7, WGD + (allo.).
2/30: Cochlearia (20). The western Mediterranean, maritime Western Europe, N.W. Africa, circum-Arctic. Map: from Hultén and Fries (1986) and Koch (2012).
Age. Cochlearieae are estimated to be (15.9-)11.6(-7.8) Ma (X. Guo et al. 2017).
[Conringieae [Coluteocarpeae + Plagiolobeae]]: ?
Conringeae D. A. German & Al-Shehbaz - Conringia Fabricius
Annuals to biennials; hairs 0/simple; cauline leaves sessile, cordate to sagittate; flowers ebracteate; fruit siliqua, 4- to 8-angled, latiseptate; seeds uniseriate; cotyledons incumbent (subconduplicate). CHECK
1/3. Eurasia.
[Coluteocarpeae + Plagiolobeae]: ?
Coluteocarpeae V. I. Dorof. (inc. Noccaeeae Al-Shehbaz, Beilstein & E. A. Kellogg) - Noccaea Moench
Perennials, annuals; hairs 0, (simple); cauline leaves sessile, entire, usu. auriculate/amplexicaul; flowers white to rose; style usu. shorter than apical notch of ovary; ovules 4/many ovary; fruit silicula, strongly compressed, winged/not, angustiseptate; seeds uniseriate.
1/130. Eurasia, few North Africa, North America, Mexico (1), Patagonia (1).
Plagiolobeae Khosravi & Eslami-Farouji - Plagioloba Reichenbach
Annuals to biennials; hairs 0/simple; cauline leaves sessile, cordate to sagittate; flowers ebracteate; stigma ± 2-lobed; fruit siliqua, terete, latiseptate; seeds uniseriate; cotyledons incumbent (subconduplicate). CHECK
1/5. E. Mediterranean, Iran, Arabian Peninsula.
[Calepineae [[Eutremeae + Thlaspideae] [Schrenkielleae [Fourraeaeae [Brassiceae, etc.]]]]]: ?
Calepineae Horaninow
Annual to biennial; hairs 0/simple; cauline leaves sessile, auriculate-amplexicaul, or petiolate; flowers bracteate or not, colour various; ovules 1-3/ovary; fruit indehiscent, woody, siliqua/silicula; cotyledons.
3/8: Goldbachia (6). Europe to temperate Asia.
[[Eutremeae + Thlaspideae] [Schrenkielleae [Fourraeaeae [Brassiceae, etc.]]]]]: ?
Eutremeae Al-Shehbaz, Beilstein & E. A. Kellogg - Eutrema R. Brown
Annual to perennial; hairs 0/simple; basal leaves palmately veined, long-petiolate; cauline leaves auriculate or not; flowers white (rose); fruit silicula/siliqua, terete/4-angled/compressed; cotyledons incumbent; n = 7.
1/25. Mostly Asian, few Europe, N. North America.
Age. The age of this clade is (14.3-)10.1(-6.5) Ma (X. Guo et al. 2017) or (13.7-)10.5(–7.2) Ma (Salariato et al. 2022).
Thlaspideae de Candolle —— Synonymy: Thlaspidaceae Martinov
.
13/39: Thlaspi (6)
[Schrenkielleae [Fourraeaeae [Brassiceae, etc.]]]: ?
Schrenckielleae Al-Shehbaz, Hendriks, M. Koch, F. Lens, Lysak, C. D. Bailey, Mummenhoff & D. A. German - Schrenckiella parvula (Schrenk) German & Al-Shehbaz
Annuals; hairs 0; cauline leaves ± petiolate; flowers ebracteate, white; C usu. 0; fruit siliqua, latiseptate; seeds biseriate, mucilaginous; cotyledons incumbent.
1/1. Turkey to China (Xinjiang) and Kazakhstan.
Bivonaea lutea (Brassiceae) may come out around here - see Hendriks et al. (2022/2023).
[Fourraeaeae [Brassiceae [Isatideae [Thelypodieae + Sisymbrieae]]]]: ?
Fourraeeae Al-Shehbaz, M. A. Koch, R. Karl & D. A. German
.
2/3: Hurkaea (2). Europe, Morocco.
[Brassiceae [Isatideae [Thelypodieae + Sisymbrieae]]]: ?
Brassiceae de Candolle (inc. Bivonaeeae) —— Synonymy: Raphanaceae Horaninow
Annuals to short-lived woody perennials; nortropane alkaloids +, (proto-Kranz/C2 photosynthesis +); hairs simple/0; nectaries 4; stigma papillate; fruit a siliqua, relatively broad, segmented [= heteroarthrocarpous]/not; cotyledons conduplicate/not; n = (7, 9, 11), 12, (13); brα WGD [hexa.].
53/243: Brassica (38), Diplotaxis (25). Photo: Raphanus flowers, fruit, Diplotaxis flowers. Mediterranean to S.W. Asia, to South Africa, North America.
Age. This clade is ca 28.3 Ma Schlüter et al. 2016: see sampling); 19-13 Ma Raph.Brass. (Moghe et al. 2014).
[Isatideae [Thelypodieae + Sisymbrieae]]: ?
Isatideae de Candolle —— Synonymy: Isatidaceae Döll
.
5/99: Isatis (80).
[Thelypodieae + Sisymbrieae]: ?
Age. Some estimates of the age of this clade include those of Walden et al. (2020) of ca. 6.7 Ma, Hendriks et al. (2023) of (5.7-3.8(-2.3) Ma (plastome data) and (10.4-)9.3(–8.2 Ma) (nuclear data) and of Daundasekara et al. (2025) of ca 6.4 Ma.
Thelypodieae Prantl —— Synonymy: Stanleyaceae Nuttall
(± woody prostrate stems - Pringlea [Pr.]); (flowers monosymmetric - Str.); (inflorescences axillary - Pr.); flowers ; A (to 24 - Pr.), (commissural septum 0 - Pr.); fruit (testa mucilaginous); extensive polyploidy, WGD + [tetrap.].
>29/>278: Streptanthus (58). New World, also Indian Ocean Subantarctic Islands (Pringlea).
Age. Crown-group Thelypodieae are (17.3-)11.8(-6.7) Ma (Bartisch et al. 2012) or ca 4.9 Ma (Daundasekara et al. 2025); WGD ca 7.4 Ma (Cacho et al. 2014; J. T. Davis et al. 2024: inc. Pringlea, whole tribe?).
Sisymbrieae de Candolle - Sisymbrium L. —— Synonymy: Sisymbriaceae Martynov
Cardenolides +; hairs simple; leaves often pinnately lobed, never clasping; fruit a siliqua; cotyledons incumbent (2-seeded silicula, cotyledons accumbent); n = 7 (8), little polyploidy.
1/48. Eurasia, northern and southern Africa, 1 sp. W. North America.
Evolution: Divergence & Distribution. Thlaspi primaevum, some 30.8-29.2 Ma old, is known from Oligocene deposits in Montana, and Manchester and O'Leary (2010) thought it was indeed a Thlaspi; there are even impressions of the distinctive seeds on the capsule wall (Beilstein et al. 2010). However, the attribution of this fossil to the genus, even to the family, has been questioned (Franzke et al. 2011), indeed, it is older than some estimates of the age of the family as a whole in C.-H. Huang et al. (2015: e.g. c.f. Fig. 5). Franzke et al. (2011) noted how age estimates for the family changed when the fossil was used for calibration - deeper nodes are ca 5 Ma older, and many prefer a crown-group age of 38-32 Ma (Esmailbegi et al. 2018). The very different ages suggested for clades in Brassicaceae (see also Moazzeni et al. 2014; esp. Franzke et al. 2016 for literature; Cardinal-McTeague et al. 2016; X. Guo et al. 2017, Mandáková et al. 2017b; Walden et al. 2020b: plastome phylogeny) are of course reflected in differences in ages of diversification within tribes, of those of ancient genome duplication/hybridization events, etc.; few of these dates are mentioned above. Couvreur et al. (2010: Table 3), Hohmann et al. (2015), C.-H. Huang et al. (2015), Walden et al. (2020b: plastome data) i.a. all give diversification times for various clades within the family; Hendriks et al. (2022/2023) noted that ages based on their plastome phylogeny were about 5 million years younger than those based on their nuclear phylogeny.
The diversity of Brassicaceae, particularly coupled with their economic importance and the fact that they include THE model plant, Arabidopsis thaliana (recently placed in its own tribe - see German et al. 2023), mean that various ideas have been floated to explain their diversification. Brassicaceae overall have high speciation rates, a small genome and a high rate of genome change, all correlated (Puttick et al. 2015; see also Mandáková et al. 2017a, b, c for the evolution of genome size). There has been much polyploidy in Brassicaceae, about half its extant members being recent polyploids, thus Jeon and Kim (2024) describe extensive palaeo-, meso- (at the tribal level, or within tribes) and neopolyploidy here (see also Mandáková et al. 2017c; for ancient polyploidy, somewhat different from palaeopolyploidy, see Stull et al. (2023). Overall, polyploidy has had a positive effect on diversification, despite the turnover rate of polyploids being slightly higher than that in diploids (Román-Palacios et al. 2019/2020 - the story is not simple). Tribes with diversification rate shifts and/or genome duplications may also show increased disparity, although there is not much in the way of evolutionary novelty in these tribes (Walden et al. 2020b).
Stem Brassicaceae may have been adapted to warm and humid conditions in the east Mediterranean area, later moving into more open and dry environments (Franzke et al. 2009). Alternatively, the family may have originated in a tropical environment, subsequently radiating with the onset of aridification and global cooling in the mid-Caenozoic (Couvreur et al. 2010). Most authors suggest that initial diversification was in the Old World, perhaps in the Irano-Turanian region from Turkey to Lake Balkhash where the family is very diverse now (Hedge 1976; Franzke et al. 2009, 2011 and references; Karl & Koch 2013; Eslami-Farouji et al. 2021; Medterranean, Saharo-Arabian, etc., taxa ofHesperis clearly derived). Aethionema itself, sister to the rest of the family, seems to have diversified along the Anatolian Diagonal, the area in Turkey between the N.E. Mediterranean and S.W. Black Sea (Mohammadin et al. 2017: Cleome droserifolia used in ancestral area reconstructions); note that in this reconstruction there is a fuse of ca 24 Ma before diversification in the genus begins. Arabideae may be the next clade to separate (Walden et al. 2020a); Mohammadin et al. (2017) cite several papers suggesting that inital diversification of everything from species (Arabis alpina) and genera to tribes (Arabideae) had been in this particular area or in the general Anatolian region. Radiation of the lineages that encompass most of the extant diversity of the family seems to have been rather rapid, in line with the poor support along the backbone of the phylogeny (Beilstein et al. 2006; Al-Shehbaz et al. 2006; Bailey et al. 2006a, b; Couvreur et al. 2010) - but did this happen around about the Eocene-Oligocene cooling or substantially later (C.-H. Huang et al. 2015)? Possible relationships between the herbaceous and woody habits - both common in the family - and the response of the plant to stress are discussed briefly above.
Soltis et al. (2009) and Franzke et al. (2011) thought that diversification in Brassicaceae might be connected with the At-α palaeopolyploidization (see also Genes & Genomes below); this duplication has been dated to ca 25 Ma (Woodhouse et al. 2011) or ca 47 Ma (Kagale et al. 2014) - for Couvreur et al. (2010), the genome duplication occurred after the divergence of Aethionemeae. Schranz et al. (2012) suggested that there was a lag time between the At-α event - they thought it characterized the whole family - and diversification there, although it is difficult to link any particular "key innovation" to such an event. Kagale et al. (2014) and Hohmann et al. (2015) surveyed chromosome number and genome size across the family, and they saw links between changes in these features and increasing diversification, and also to climatic changes in the Neogene (the last ca 23 million years) in particular; most diversification has taken place in this period. Along similar lines, Hendriks et al. (2022/2023) thought that the evolution of Brassicaceae was connected with changes beginning at the Eocene—Oligocene transition ca 34 Ma, a time of falling temperatures and the development of open vegetation and deserts, i.e. the sorts of conditions that many extant members of the family favour.
Edger et al. (2015, 2018) noted a notable increase in diversification in core Brassicaceae and the evolution of considerable diversity in GSLs following the At-α duplication event, interestingly, anthocharine and pierine Pierinae independently colonized the family and diversified there. Anthocharine speciation increased ca 22 Ma and that of the [Brassiceae + Sisymbrieae] clade increased ca 21 Ma (Cardinal-McTeague et al. 2016).
Biogeographical histories within the family can be complex; see Koch and Kiefer (2006) for a summary of earlier work. In Lepidium chloroplast and nuclear genomes of Californian and African ancestry are variously combined in polyploid Antipodean species of the genus, the initial hybridization occurring probably in Australia and within the last 1.3 Ma (Mummenhoff et al. 2001, 2004; Dierschke et al. 2009; Smissen et al. 2024). Lepidium s. str. commonly grows in extreme conditions, whether montane or Arctic. Kiefer et al. (2009) discuss the phylogeographic structure of the speciose largely North American Boechera (ex Arabis); divergence seems to be a Pleistocene phenomenon, and there is considerable apomixis and widespread hybridization (Majeský et al. 2017 and literature; Mau et al. 2021; Windham et al. 2022; Vinogradova et al. 2023). The speciose crown-group Arabideae are perhaps around 14 Ma, but much diversification has been within the last 5 Ma, perhaps associated with the evolution of the perennial life style, associated movement into high-altitude habitats, and extensive polyploidization (Karl & Koch 2013: perennial → annual → perennial). Draba is a young polyploid complex in which there is considerable geographical structure in the distribution of diploids and polyploids, species with high ploidy levels being common in the Arctic and also at high altitudes, as in Arabis (Jordon-Thaden & Koch 2008; see also Jordon-Thaden et al. 2010, 2013). Draba is diverse at high altitudes on the Andes and a number of distinctive shrubby species have evolved there, and particularly in the northern Andes species are often narrowly distributed (Koch & Al-Shehbaz 2002; Sklenár et al. 2011; Jordon-Thaden et al. 2013). Relationships within the speciose Erysimum clade also show considerable geographic structure, diversification beginning around 3.5 Ma (Moazzeni et al. 2014; Züst et al. 2019/2020). Species here produce a diversity of cardenolides, a novel defence, and related species tend to have similar cardenolides, but not glucosinolates;a greater variety of cardenolides than glucosinolates is produced, and the former are not inducible, the latter are (Züst et al. 2019/2020). The rate of diversification in Western European taxa has been notably high and is dated to ca 1.6 Ma (Moazzeni et al. 2014; see also Martín-Hernanz et al. 2019: Table 4). There have been a number of very long distance dispersal events in Cardamine (Carlsen et al. (2009); although immediate ballistic dispersal is effective only over short distances, many taxa have mucilaginous seed coats which will aid longer distance transport (see below). Interestingly, Pringlea antiscorbutica, from islands in the southern Indian Ocean, also has a mucilaginous testa which its relatives in South America lack; Pringlea diverged 7.7-2.6 Ma, but details of how it got to where it grows now are unclear (Bartisch et al. 2012). For dates and place (N.E. Africa?) of diversification within Brassiceae, see Arias et al. (2014a). Some distributions have been explained by vicariance. Thus the sister-group relationships between taxa of Descuraina from Macaronesia and the Mediterranean and those from North America has been attributed to vicariance events as the North Atlantic opened 120-100 Ma (Grehan 2017), which adds another if perhaps unlikely dimension to the whole discussion. For diversification in the Macaronesian Crambe section Dendrocrabe within the last ca 8.2-7.2 Ma, see S. C. Kim et al. (2008).
The CES clade - Cremolobeae, Eudemeae and Schizopetaleae - is the largest clade endemic to South America; the three tribes are found along the Andes (but only one record from Colombia) and down to the Patagonian steppe, so inhabiting some very dry habitats. Salariato et al. (2014, 2022; Al-Shehbaz et al. 2023 for genera) discussed evolution in the Eudemeae, which has northern and southern clades, with the cushion life form evolving independently in taxa growing in more extreme conditions in both these clades. Salariato et al. (2022) suggested that the tribe had moved into three areas with somewhat different climates, subsequent diversification occurring within these areas/in the context of these different climates. Cremolobeae also live in a wide variety of habitats along the Andes (Salariato et al. 2020). Salariato et al. (2016) noted that diversification in the CES clade had been rather constant, and compared with other Andean clades (mentioned there) it was quite old, diversification being well under way by 12 Ma
Aethionema, which has a more or less sessile gynoecium, is clearly sister to all other Brassicaceae. However, as Hendriks et al. (2022/2023) observed, some combination of incomplete lineage sorting, more or less ancient hybridization (sometimes deeper than tribes), introgression, whole-genome duplication and postpolyploid diploidization has made the recovery of other deeper (below the tribe) relationships in the family rather difficult (for hybridization, see e.g. Genes & Genomes below). Stanleya, etc., are well embedded in Brassicaceae (see Thelypodieae, Koch et al. 2012; not included by C.-H. Huang et al. 2015), and their similarities to Cleomaceae, e.g. long gynophore and stamens, earlier thought to indicate relationships, are parallelisms (Galloway et al. 1998).
Heliophila is a hideously variable genus, and Mummenhoff et al. (2005) and Mandáková et al. (2012), discuss various aspects of its evolution. Salariato et al. (2014) discussed the evolution of a number of features in the morphologically quite heterogeneous but not very speciose Eudemeae. Beilstein et al. (2006) discuss trichome evolution; this is put in a different phylogenetic context by C.-H. Huang et al. (2015).
Ecology & Physiology. There is a notable decrease in seed mass and increase in leaf mass per area (SLA) in Brassicaceae. Members of the family quite often live in temperate, disturbed areas that are rather dry yet the precipitation that does fall is not very seasonal (Franzke et al. 2010; Cornwell et al. 2014). Brassicaceae also include over 60 species of cushion plants, disproportionately many for the size of the family; such plants favour cold and dry conditions (Boucher et al. 2016b).
Some species of Brassicaceae are well known to be hyperaccumulators of unusual elements (Brooks 1998; Krämer 2010; Cappa & Pilon-Smits 2014). Most angiosperms that are both nickel and zinc hyperaccumulators are members of the family (see also Cappa et al. 2014b: hardly fair to compare Brassicaceae with Malpighiales or Asteraceae!; Gei et al. 2020). Brassicaceae are common on magnesium-rich dolomites and serpentines (Cecchi et al. 2010). Interestingly, the selenium-accumulating Stanleya pinnata (see below) is phylogenetically close to a number of serpentine endemics and serpentine-tolerant species of Streptanthus and relatives, although Streptanthus s. str. is not an accumulator (Cacho et al. 2014). Odontarrhena (Alysseae) is a large genus of around 95 species which are commonly found on ultramafic soils and accumulate nickel; although some species do not accumulate the metal, they may nevertheless still be able to grow on soils with the metal. Overall, more than 100 species in Brassicaceae are involved in unusual/heavy metal tolerance, and there have been ca five or well over a dozen origins of the ability to accumulate such elements here (Cecchi et al. 2010, 2014; Krämer 2010; J. T. Davis et al. 2024), although of course this number will depend on the topology of the tree. Daundakesara et al. (2025, see sampling, comparisons of topologies in nuclear and organelle phylogenies) looked at some species of Thelypodieae, a group in which a number of species tolerate more or less extreme edaphic conditions (there is a split between species that like cold and wet conditions and those that prefer warm and dry conditions in the tribe), and they identified a number of genes that might perhaps affect the ecological tolerances of these latter plants. Nickel and other heavy metals that Brassicaceae can accumulate may act as e.g. a feeding deterrent by themselves, or they may interact with other aspects of the plant's defence system (Baker & Brooks 1989; see Boyd 2007, Grennan 2009, and Anjum et al. 2012 for reviews; Australian J. Bot. 63(1-2). 2015). Interestingly, in the Streptanthus area it seems the adaptation to living in bare environments was a precursor to the ability to live on serpentine-derived soils, etc. (Cacho & Strauss 2014). Some species of Brassicaceae actively forage for such metals, the root density of these species increasing in areas of the soil where there is more metal (Qiu et al. 2012).
Selenium (Se), normally a sulphur antagonist, is accumulated by Stanleya pinnata and S. binnata (?species limits: White 2016 for a summary of the literature), but not by other species of the genus, however, a number of its immediate relatives, including Brassica napa, are also Se tolerant (Cappa et al. 2014b). Se - up to ca 1.5% dry weight, 30% elemental Se has been recorded - may protect the plant against herbivory by prairie dogs and arthropods alike (Galeas et al. 2007; Freeman et al. 2009; Cappa et al. 2014a; Schiavon & Pilon-Smits 2016). High Se accumulation in B. juncea is associated with reduced flower production, so there is a possible balance between accumulating enough Se to deter herbivores but not too much more, which would reduce flowering (Steven & Culver 2019), so hyperaccumulation of Se may reflect a particular kind of plant-insect association. Stanleya pinnata incorporates Se into the non-protein amino acid methylselenocysteine (with a C-Se-C motif) and so is unaffected by it. Endophytic bacteria are involved in Se uptake into the plant (Lindblom et al. 2013; Sura-deJong et al. 2015 and references). There are also complex interactions with non-Se-accumulating plants in the same habitat, these sometimes growing better if associated with Se-accumulating plants, perhaps because of reduced herbivory, or growing worse, perhaps because of Se allelopathy (El Mehdawi et al. 2012 and refs). For Se tolerance, see also Astragalus (Fabaceae: Ecology and Physiology).
Brassicaceae are quite well represented in the Arctic, 11 genera being known from the Canadian Arctic alone. They can tolerate the cold and often extremely dry conditions there, and Birkeland et al. (2020) compared three species in three genera and found convergence in the suite of adaptations to these conditions. One does not usually think of "Brassicaceae" and "island woodiness" in the same breath, but there have been some 12 transitions to island woodiness in the family that have resulted in some 46 species, mostly from the Canary Islands and Madiera (Zizka et al. 2022).
Thellungiella halophila reproduces even when the salt concentration of the water in which it is growing is that of seawater (Gong et al. 2005), while tolerance to salinity in Eutrema has been studied in detail (see articles in Ann. Bot. 115(3). 2015).
There are a number of proto-Kranz/C2 species species in Brassicaceae-Brassiceae, especially in Moricandia (R. Sage et al. 2014). Here one of the two copies of glycine decarboxylase is lost, while the retained copy is expressed in the bundle sheath cells alone (Schlüter et al. 2016, q.v. for details, also suggestions as to why C4 photosynthesis did not evolve).
Arabidopsis thaliana can take up organic nitrogen as amino acids (Hirner et al. 2006), although the significance of this is unclear. Furthermore, Paungfoo-Lonhienne et al. (2010) found that nitrogen from Escherichia coli and Saccharomyces cerevisiae that had entered intact roots of Arabidopsis was used by the plants as the protists were broken down, so Arabidopsis is technically carnivorous... (see also Selosse et al. 2017c).
Glucosinolate Diversity and Activity, Herbivory, and More. GSL diversity in the family is considerable, as is variation in content between different brassicaceous species in the same community and different genera in the same tribe, although GSLs are rarely unique to a particular species. Arabidopsis thaliana alone can synthesize over 40 different kinds of GSLs and a number of other taxa can synthesize around 20 or so, indeed, members of Brassicaceae-Cardamineae alone can synthesize around 60% of the known GSLs (Olsen et al. 2016; Agerbirk et al. 2021b). There is infraspecific variation in the type of GSL produced - as well as in triterpenoid saponins - in Barbarea vulgaris (Byrne et al. 2017). Species like A. thaliana have distinct chemotypes (six in this case) which may have distinct distributions within species, overall, herbivory is affected by the particular GSLs a plant may have (e.g. Bidart-Bouzat & Kliebenstein 2008). Both myrosin cells and GSLs are often localized along the veins in Brassicaceae, perhaps as a defence to prevent herbivores from damaging the vascular tissue, and GSLs may also be common towards the edges of the lamina and may help deter herbivores that eat from the edge inwards, as is quite common (Shroff et al. 2008; Shirakawa et al. 2014). However, Czerniawski et al. (2020) noted that even within Camelineae (this included Arabidopsis then), species varied as to where in the plant GSLs were to be found, thus in Camelina, for example, the concentration of GSLs in the leaves was very low.
Although GSLs are defences against many generalist herbivores, specialists like cabbage whites (Pieris spp.) may be keystone herbivores of Brassicaceae (Poelman & Kessler 2016), thus P. brassicae prefers plants with GSLs over those in which GSL-producing genes have been inactivated (Schweizer et al. 2013). Interestingly, the butterfly responds to the GSLs - it detects them by their odour - only after mating (Ikeura et al. 2010). Winde and Wittstock (2011) discuss the various ways in which insects can avoid the harmful effects of GSLs; caterpillars of cabbage white butterflies and the diamond-back moth (Plutella xylostella) are able to detoxify the isothiocyanates produced when they damage plant tissue. The hydrolysis of glucosinolates is normally catalyzed by myrosinase, instead, the process is redirected, and non-toxic substances such as nitriles are produced by the plant's nitrile-specifier protein (Wittstock et al. 2004; Ratzka et al. 2002; Nallu et al. 2018 and references); the whole process can be very complex (Agerbirk et al. 2010).
As mentioned, the presence of particular GSLs may induce oviposition by cabbage white butterflies, and this whether or not the crucifer with those GSLs is edible or kills the caterpillar (Chew 1979, see also 1988). Gene expression in both host and herbivore change most as eggs are laid, less in the plant as it is being eaten or in the caterpillar, indeed, about 50%, some 14,560, of the genes of Arabidopsis thaliana change their expression on oviposition by Pieris rapae, but when feeding began, relatively few more genes were expressed, and responses of the genome of the egg/caterpillar were rather similar (Nallu et al. 2018, see also Hilke & Fatouros 2015) - note, however, that the response of Arabidopsis to egg-laying by Pieris was remarkably strong. Interestingly, systemic acquired resistance (SAR, defence against pathogens) may result when P. brassicae lays eggs on Arabidopsis thaliana, and SAR may move to other plants via the roots; this development of SAR may ensure that the food supply for the caterpillars remains in good condition... (Orlovskis & Reymond 2020). The cost of GSL production is considerable, being estimated at ca 15% of the total energy needed to synthesise the contents of a leaf cell (Bekaert et al. 2012).
Cacho et al. (2015) discussed aspects of GSL diversity and amount and habitat preferences in Streptanthus. As is common in plant-herbivore interactions, there was only a weak phylogenetic signal in total GSL amount and diversity. Plants growing on serpentines showed little change in GSL production, although when the substrate was bare, GSL diversity increased. GSLs can depress vesicular-arbuscular mycorrhizal activity in other plants, and this may help Alliaria petiolata be invasive in parts of North America (Wolfe & Klironomos 2005; Horton & van der Heijden 2007: see also "Bacterial-Fungal Associations" below for fungal associations in Brassicaceae).
GSL genes are usually present in several copies, and this has been linked to the genome duplications common in the family (Kakizaki 2017 and references). See Windsor et al. (2005), Schranz et al. (2011), Grubb and Abel (2006), Wittstock et al. (2016), Eisenschmidt=Bönn et al. (2019) and Groen and Whiteman (2022: esp. detoxification) for more on the diversity of GSLs and the complexities of their metabolism, and see also above; further details of plant-insect associations are discussed in Plant-Animal Interactions below and the age of GSL production is discussed elsewhere.
There is, of course, far more to the story of herbivory here. For example, parasitoids of pierid caterpillars are attracted to where the latter are feeding, and interactions between the two insects become very intricate (Fatouros et al. 2008). Thus the effect of caterpillars on the plant can be manipulated by braconid parasitoids of the caterpillars - indeed, it was found that plant responses to herbivory depended more on the identity of the parasitoid than of the pierid caterpillar, and was ultimately affected by polydnaviruses associated with and specific to the parasitoid. The effect of the parasitoid on the plant was mediated by the composition of the fluid that the caterpillar regurgitated during eating, and this effect also involved changes in the volatiles the plant produced; these volatiles gave hyperparasites, in this case ichneumonid wasps, information about whether or not their prey, the braconid larvae, were present (Poelman et al. 2011; Zhu et al. 2018; see also Tan et al. 2018). Further details of the plant-insect association are discussed above.
elsewhereIn a number of taxa, expecially Brassiceae, hypersensitive response-like necrosis occurs in the plant tissues where pierid butterflies lay eggs - the cells die, and the eggs do, too (Grise et al. 2021).
Pollination Biology & Seed Dispersal. Floral variation in the family is quite extensive, despite the rather stereotypical floral formula - K4, C4, A6, [G 2] - that we are all taught. The flowers of Iberis amara are monosymmetric, although the whole corymbose inflorescence is functionally more like a single polysymmetric flower with numerous radiating petals - the two large petals of each outermost flower (Busch & Zachgo 2007: Busch et al. 2012); selection on monosymmetric flowers will thus be at the functional level of the inflorescence. Genera like Streptanthus have strongly monosymmetric flowers borne on a more elongated axis. Ornithocarpus and Schizopetalum, for example, have more or less fimbriate petals, while those of Draba are bilobed. Flowers quite commonly change colour as they age (Weiss 1995). In general, flowers are visited by quite a variety of insects, an example being Hormathophylla spinosa, plants of which which were visited by 70 or more species of insects in 19 families and 5 orders over the over four-year study period (Goméz & Zamora 1999). Goméz et al. (2014) describe variation in pollinators in 40 (35 in the figures) species of Erysimum in detail. 746 species of insects in 99 families and eight orders, assigned to 19 functional groups, were visitors; most species were visited by more than nine functional groups. Pollinators may have driven some of the variation in floral shape, but overall the connection between pollinator and floral morphology is slight (Gómez et al. 2015), although another way of looking at things is that despite a "very similar floral bauplan", there were connections between floral morphology and pollinators, especially when these latter were specialized (Gómez et al. 2016: p. 889). Beetle pollination - Brassicogethes (Nitidulinae) is involved, especially in the western Palaearctic; the beetle larvae eat the pollen and may destroy the flower (M. Liu et al. 2021). For sucrose, produced from starch in the nectary, for nectar, see Solhaug et al. (2019 and references) and for nectaries, pervasive but hideously variable (see below), see Erbar (2024).
The sporophytic incompatibility system common in Brassicaceae has been much studied (e.g. Dickinson et al. 1998; Sherman-Broyles & Nasrallah 2008; Watanabe et al. 2008; Tarutani et al. 2010; Nasrallah 2011; S. Cheng et al. 2013). Some of the components of the incompatibility system are contained in the distinctive tryphine covering the pollen grains, and some components are produced by the individual pollen grains, so although the incompatibility system is usually described as being sporophytic, it is at least in part gametophytic in its genetics (Doughty et al. 1998; Dickinson et al. 2000). The incompatibility of Arabidopsis grains placed on a Brassica stigma can be removed by the action of Brassica tryphine (Dickinson et al. 2000). Indeed, brassicaceous pollen tubes seem singularly easily (mis)led - expression of a single Arabidopsis peptide involved in pollen tube guidance in a Torenia (Lamiales-Linderniaceae!) synergid directed the pollen tube and allowed it to penetrate the Torenia embryo sac (Takeuchi & Higashiyama 2012).
Apomixis at both the diploid - rather unusual - and polyploid levels is known from Boechera, and pollen may be haploid or diploid - in apomicts, haploid pollen tended to be more deformed, less viable, etc. (Hörandl et al. 2007; Mau et al. 2021). Diploid and polyploid apomixis can be initiated in sexual Boechera in a single generation, overall, the diversity of the results of the various crosses carried out is mind-boggling (Mau et al. 2021 and references). Mandáková et al. (2020) also looked at apomixis in Boechereae.
Explosively dehiscent capsules are known in Cardamine, probably the result of a combination of square exocarp cells that contract in length but increase in volume when the fruit dehisces, and hypodermal cells of the endocarp with a distinctive hinged U- or V-shaped pattern of lignification that cannot contract; it is notable that dehiscence occurs when the fruit is turgid, not dry (Hofhuis et al. 2016; Hofhuis & Hay 2017; Cullen & Hay 2024) - see also Acanthaceae. (Interestingly, C. chenopodiifolia is amphicarpic, some fruits being subterranean.) In taxa like Arabidopsis fruit opening is not explosive and testa morphology differs somewhat (Neumann & Hay 2020). Situations where there are dimorphic diaspores (= heterodiaspory) on the one individual are scattered in Brassicaceae, and a particularly well studied example is from Aethionema arabicum (see Arshad et al. 2018). Here there are angustiseptate capsules that open and allow the seeds to fall out; on wetting, these seeds become mucilaginous, dormancy is slight, and dispersal is mostly local. Other fruits are single-seeded, indehiscent and dormancy is well developed; the fruits are winged, and dispersal is over longer distances. Even Capsella bursa-pastoris shows heterodiaspory. Here under humid conditions (the plant is ± ombrohydrochorous) the capsules open and most of the seeds fall out; the seeds are myxospermous. However, a single seed at the apex of each carpel often remains in the siliqua, and dispersal here may be long distance, by wind or water (Teppner 2003).
Heteroarthrocarpic fruits, a variant of dimorphic diaspores, are common in Brassiceae. They consist of an apical, indehiscent portion that does not differentiate into valve tissue, and a basal portion with typical valve-type tissue that may or may not be dehiscent (Snogerup et al. 1990; Hall et al. 2006, 2011); the two parts of the fruit may separate transversely and so the one plant can have very different dispersal mechanisms. These fruits may also be completely indehiscent, and they are in a clade along with taxa that do not have heteroarthrocarpic fruits, and these also may be dehiscent or not (Brock & Hall 2019: inheritance of copies of the FRUITFULL gene). Willis et al. (2014c) looked at dispersability and diversification in Brassiceae and found that the two are somewhat correlated. Although phylogenetic relationships in Brassiceae are uncertain, heteroarthrocarpy is likely to have evolved more than once (Hall et al. 2011). For the evolution of heteroarthrocarpy in Raphanus, see Avino et al. (2012 - there probably has been a gene triplication around here - Lysak et al. 2005.)
Seeds are commonly mucilaginous in Brassicaceae. For example, in Cardamine hirsuta mucilaginous pectins accumulate in the endocarpial area (Vaughan et al. 2011), while in other taxa it is the extotesta that is mucilaginous (e.g. d'Arbaumont 1890; Vaughan & Whitehouse 1971) - note, however, that Neumann and Hay (2020) suggest the exotestal layer in C. hirsuta is mucilaginous. Mucilage helps either in the establishment of the dispersed seed when it rains and/or in the further dispersal of the seed if it becomes attached to animals (Western 2011; X. Yang et al. 2012: review of seed coat mucilage, many examples from Brassicaceae).
Plant-Animal Interactions. For the diversification of Pierinae butterflies on Brassicaceae, see Ecology & Physiology above; caterpillars of Yponomeutoidea-Plutellidae moths are also common here (Sohn et al. 2013).
Other insects such as the cabbage aphid, Brevicoryne brassicae, and turnip sawfly larvae (a tenthredine, Athalia rosae), sequester GSLs, the aphid even producing its own myrosinases that break the GSLs down, so helping to deter potential predators, while the sawfly also sequesters iridoids and clerodanoids (for which, see also Lamiaceae), and i.a. gaining protection against the Japanese tree frog (Kazana et al. 2007; P. Singh et al. 2022). The level of polymorphism of defence gene alleles of Arabidopsis thaliana is related to the intensity of attack by specialist aphids (Züst et al. 2012).
There is a major radiation of Ceutorhynchinae seed weevils, specifically, Ceutorhynchus s.l., on Brassicaceae, with about 600 species known from the family. There was a single movement on to the family but there have been no movements on to other hosts (Letsch et al. 2018). The horseradish flea beetle Phyllotreta armoraciae is another obligate myrosinase-containing herbivore on Brassicaceae, and it sequesters some of the GSLs it ingests, also modifying/excreting some (Z.-L. Yang et al. 2020).
Plant-Bacterial/Fungal Associations. The association between the pseudoflower-forming Puccinia rust, a basidiomycete, and its brassicaceous host, Boechera stricta, has been much studied (Roy 1993 [particularly fine photograph], 2001; Ngugi & Scherm 2006; Cano et al. 2013). Insects come to the yellow pseudoflowers, which perhaps look like buttercups, attracted both by the colouration of the leaves (caused by yellow spermogonia) and the floral fragrances that the pseudoflower produces. These latter differ from those of both the crucifer host and the other plants in the general area, containing i.a. constitutively-released isothiocyanates, aldehydes, esters, and aromatic alcohols (Raguso & Roy 1998). The insects pick up the rich fructose nectar secreted by the fungus along with the fungal spermatia, fly to another "flower", deposit the spermatia and pick up more. On combination of spermatia of the appropriate mating types, diploid aecia are produced, the "flower" stops producing nectar, and the "petals", i.e. the leaves, become green. Ruxton and Schaefer (2011) discuss the evolution of such associations and Cano et al. (2013) the extensive reprogramming of the B. stricta transcriptome that Puccinia infection entails. Note that other Brassicaceae and a variety of species of Puccinia are involved in this association, which may have elements of a Mullerian mimicry system (Roy 1994).
Any arbuscular mycorrhizal (AM) associations in the roots of Brassicaceae are at most weak and facultative (Medve 1983). Thus although AM have been reported from Thlaspi, it is doubtful if an effective symbiosis has been generated (Regvar et al. 2003), and this is certainly true of Arabidopsis thaliana (Veiga et al. 2013). Cosme et al. (2018) develop this theme, noting that the role of AM associations in Brassicaceae was unclear, but at the same time, understanding the behaviour of those taxa that formed rudimentary arbuscular mycorrhizal (RAM) associations might help in understanding how AM in general function. Furthermore, how Brassicaceae might compensate for the absence of functional AM associations is beginning to be understood. For example, in Arabidopsis growing in low phosphate conditions, primary root growth is reduced, lateral root growth increases, phosphate tending to be found near the soil surface (Fusconi 2014). Ascomycete helotialean fungi common in the root cortex of non-mycorrhizal Arabis alpina are involved in the uptake of P in low-P soils with the consequent improved growth of the plant (Almario et al. 2017). Hiruma et al. (2016) looked at the association of another ascomycete endophyte, Colletotrichum tofieldiae, with Arabidopsis and found a similar relationship, which, they noted, was reminiscent of AM associations (see also Werner et al. 2018).
The oomycete Albugo, the white blister rust, parasitizes a number of Brassicaceae (Ploch et al. 2010a), although it quite commonly also persists as a symptomless endophyte (Ploch & Thines 2011); in some cases it facilitates infestation by Phytophthora infestans, another oomycete (Prince et al. 2017). However, the loss of the RAM2 locus compromises the establishment both of AM associations and of infections by a variety of oomycetes (Wang et al. 2012; Gobbato et al. 2013). Brassicaceae are also noted for having lost nucleotide-binding site-leucine-rich repeat (NBS-LRR) resistance genes of the CNL type (Y.-M. Zhang et al. 2016). See also above.
There are a number of studies suggesting that GSLs are involved in the defence of the plant against pathogenic fungi, although little in detail is known about this. In at least some cases mutants affecting these fungal defences also show changes in the plant cuticle (Chhajed et al. 2020 for literature).
cytochrome P450 (CYP) enzymes such as phytocassane and oryzalexin classes of diterpenoid phytoalexins in "monocots" (certainly C3 and C4 grasses), the CYP76M subfamily, the genes occuring in clusters and having considerable metabolic plasticity (Hansen et al. 2021) cytochrome P450 (CYP) enzymes, the CYP76AH/AK subfamilies, producing the labdane-type phenolic diterpenes carnosic acid carnosol in Lamiaceae (Hansen et al. 2021) of the cytochrome P450 (CYP) enzymes, the CYP76AD subfamily is involved in the synthesis of tyrosine-derived betalain pigments in Caryophyllales, Hansen et al. 2021 the cytochrome P450 (CYP) enzyme the subfamily CYP76C is specific to Brassicales cytochrome P450 (CYP) enzymes the subfamily CYP76AA is specific to gymnosperms. CYP765 family (clan 72) and CYP947 family (clan 85) lost in angiospermsGenes & Genomes. The immediate origin of the Brassiceae genome can perhaps be represented by 4x X 2x → 3x → 6x, where x = 7 (Cheng et al. 2013 and references; see also Mabry et al. 2020). Carta et al. (2020) also suggested that the base chromosome number of the family was 7, although x = 8 was also a possibility (also Schranz et al. 2006; Lysak et al. 2006, 2016; Mandáková & Lysak 2008; both Murat et al. 2015b and Mandáková et al. 2020a for ancestral genomes); Raven (1975) thought that x might be 12. Genome duplications are common here, x = 4 perhaps being the number of the pre-At-α duplication genome (Franzke et al. 2011 and references). This duplication of the whole genome, the Ata/At-α/ARTHα palaeopolyploidization, has been dated to 34-25 or 60-20/ca 25 Ma, either fairly soon after the split of Brassicaceae from Capparaceae (Vision et al. 2000; Blanc & Wolfe 2004a; M. S. Barker et al. 2009; Woodhouse et al. 2011), perhaps just before (Griese et al. 2021) or after the divergence of Aethionemeae (Blanc et al. 2003; de Bodt et al. 2005; Schranz & Mitchell-Olds 2006; Franzke et al. 2009; Galloway et al. 1998: pattern of duplication of the ADC [arginine decarboxylase] gene; Y. Yang et al. 2015; Edger et al. 2015; c.f. Schranz et al. 2012) or before it (Lysak 2018). Ca 14.1 Ma is the age suggested by Landis et al. (2018) for this event. Three duplication events were picked up by Vanneste et al. (2014a) and dated at (69.4-)61.2(-54.6) Ma (the ARTHβ event?), (52.3-)50.1, 48.7(-47.6) Ma, and (28.6-)26.8(-24.8) Ma, while Kagale et al. (2014) date the main duplication to 47±1 Ma - note that some of these ages predate some suggested ages for the split of Cleomaceae and Brassicaceae... Walden et al. (2020b) noted that there were eleven tribe-specific whole-genome duplications in addition to the At-α event, and these include theRaphanus/Brassica whole genome triplication and a WGD in the strepanthoid complex (Thelypodieae), the latter either an autopolyploid event or hybridization between close relatives (). No morphological features seem to be key innovations arising after such events, even if there are no decreases in net diversification or rate shifts. For the α and β events in the Arabidopsis thaliana lineage, see also Zwaenepoel and Van de Peer (2019) and Qiao et al. (2019).
Woodhouse et al. (2011) discussed gene transposition after the Ata/At-α/ARTH-α duplication event; see also Jiang et al. (2013) and Murat et al. (2015a) for what happened to duplicated genes and for the latter in particular for the fates of the centromeres. There has been extensive transposition of genes in the Arabidopsis genome - between 1/4 and 3/4 of the genes may have moved some time after the origin of Brassicales as a whole (comparison with Carica: Freeling et al. 2008; see also Schranz et al. 2007), and chromosome remodelling in Brassicaceae is also discussed by Lysak et al. (2016). Lysak et al. (2016) found that numbers of genome blocks (for which, see also e.g. Parkin et al. 2005), polyploidy events, and chromosome numbers were not necessarily connected. In Arabidopsis the extra copy of signal transduction and protein encoding genes tended to be kept and that of DNA repair and plant defence genes lost (Blanc & Wolfe 2004). There is evidence of fractionation bias or genome dominance associated with duplications in Arabidopsis and in particular in Brassica, suggesting alloploid events in both cases (Garsmeur et al. 2013).
Overall, there is very extensive hybridization along with associated genome duplication and downsizing, aneuploidy, etc., in Brassicaceae, and there is an equally extensive literature associated with it (Vision et al. 2000; Kellogg & Bennetzen 2004; Warwick et al. 2006a; Blanc & Wolfe 2004a; Marhold & Lihová 2006; Blanc et al. 2007; Lysak et al. 2007, 2016; Hohmann et al. 2015; Mándeková et al. 2017a, b; X. Guo et al. 2020; Mabry et al. 2020; Dogan et al. 2022). Franzke et al. (2011) discuss genome duplication events in detail, suggesting that several more remain to be discovered. Apparently diploid species like Brassica oleracea, with n = 9, are hypothesised to be ancestral hexaploids, the hexaploidy event occurring 19-5 Ma (the Br-α' event: Mitchell-Olds et al. 2005; Lysak et al. 2005; X. Wang et al. 2011; Tang et al. 2012; S. Liu et al. 2013: extensive changes to the B. oleracea genome after duplication; S. Cheng et al. 2013; Mabry et al. 2020) - and see also below. Moghe et al. (2014) dated this event to around 29-24 Ma. Jaillon and Eury et al. (2007) suggested that Arabidopsis has had two quite recent whole genome duplications, although there was some discussion as to the exact number. However, an At-α event has been dated to ca 23 Ma (Barker et al. 2009; Hao et al. 2018 and references), and appears to involve the whole family (Mabry et al. 2020). Hao et al. (2018) discuss the subsequent response to natural selection of the genes that have remained duplicated, and this reflects their pre-duplication selection regimes. Given the various duplications in the Brassicaceae genome and earlier duplications in angiosperms, the genome of extant Brassica represents all told around 288 multiples of the angiosperm ur-genome (Wendel 2015; see also Chalhoub et al. 2014; Qiao et al. 2019: the genome of B. napus has multiplied ca 72 times). Not surprisingly, in some Brassicaceae chromosomal reorganization has been very extensive, given that the base chromosome number for the family is 7 or perhaps 8 (Koch & Kiefer 2005). The La-α (the Leavenworthia alabamica triplication event) is about the same age as the Br-α event. Mandáková et al. (2012) discussed a probable genome triplication in Heliophila, and Mandáková et al. (2017c) noted a number of WGDs/hybridization events (mesopolyploidies, i.e. WGDs at the level of or within tribes) in the family. The origin of a trnF pseudogene has been associated with a duplication in the common ancestor of the Halimolobus + Boechera + Cardamine clade, some 21-16 Ma (Koch et al. 2005). Thelypodieae are very diverse, and WGD(s) may be involved here, too, one being dated to ca 7.4 Ma (Hawkins et al. 2017; J. T. Davis et al. 2025: Pringlea antiscorbutica also involved). X. Wang et al. (2011) noticed the extensive gene loss following genome triplication in Brassica, as in polyploidization events in general. For genome duplication and glucosinolate evolution, see above, also Kakizaki (2017 and references); see also Phylogeny below.
Details of karyotype evolution in the family are of considerable interest and are associated with the WGD/hybridization events just mentioned. Chromosome numbers range from n = 4-13 on upwards - thus the diploid number of Brassica napus is 38. One major clade (lineage I) has x = 8, as does lineage II, while another clade that includes lineage II has x = 7, but with subsequent increase (Mandáková & Lysak 2008; Franzke et al. 2011) [Check this]. Mandáková et al. (2019) discuss the extensive hybridization-associated karyotype changes in Camelina, close to Arabidopsis, where n = 6-20. Mandáková et al. (2020b) looked at genome evolution in the Boechereae area and they suggested that the assembly of four chromosomes with distinctive rearrangements were a "key evolutionary event" underpinning diversification in the tribe. For chromosome numbers, see also Warwick and Al-Shehbaz (2006: summary) and Spaniel et al (2015: Alysseae)
Genome evolution has been very rapid, especially in Brassica rapa, for example, when compared with that in Carica, and this may be connected with differences in life histories, annual versus perennial (X. Wang et al. 2011). Johnston et al. (2005) discussed the evolution in genome size in Brassicaceae; in that part on which they focussed (Caps. Lepid Cardam. Brass Draba) there tended to be an increase in size, although the ancestral size was estimated to be a mere 1C = 0.2 pg. Overall, genomes are quite small and not that variable when compared with those of other families (Johnston et al. 2005; Elliott et al. 2022b: minimum holoploid genome size the third smallest in those seed plants examined) and genome size was not particularly linked with chromosome number (Lysak et al. 2009, 2016). X.-C. Huang et al. (2019) found that genome size tended to decrease after mesopolypoidization events, diploidization occurring. In Lineage III/clade E, x = 7, there has been descending dysploidy, but also a considerable increase in genome size to ca 8 Gb, the largest in Brassicaceae - the smallest genomes in this clade are ca 265 Mb (Mandáková et al. 2017b) while the largest monoploid genome, in Hesperis sylvestris, is 4273 Mb (tetraploid H. matronalis - 8117 Mb: Hlousková et al. 2019); overall, chromosome number might be about the same but genome size varied some 16-fold. Genome size in Streptanthus and relatives was affected by biotic interactions (via glucosinolates, fraction of aliphatic glucosinolates up, genome size down) and seasonality (in seasonal continental climates, genome size up, in more equable coastal climates, genome size down), but not features such as life history or soil nutrients (Cacho et al. 2021). The small genome of Arabidopsis, ca 135 Mb, n = 5, is probably the result of illegitimate recombination (Devos et al. 2002).
T. Zhao and Schranz (2019), looking at genome order across angiosperms as a whole, found that overall there was a low proportion of syntenic genes, however, the proportion in Brassicaceae in particular was notably high, and they also had a number of multicopy and/or lineage-specific microsynteny clusters that were specific to them (Zhao & Schranz 2019). Interestingly, in some Arabideae (and other taxa) the centromere has moved within the chromosome (= an evolutionarily new centromere), colinearity of the genes being maintained (Mandáková et al. 2020a).
There has been a tandem duplication of the nuclear acc gene, and acc2 is plastid-targeted (Babichuk et al. 2011: Arab. Brass. Caps.). For the loss of the CYP73 class II duplicate gene involved in the phenolic network, see Renault et al. (2017); this duplication may have occurred in the common ancestor of extant seed plants or, perhaps more probably, that of flowering plants.
T. Sun et al. (2018) discuss the evolution of a set of transposable elements that was probably in place at the Core Brassicaceae node, at least, and the subsequent movement of these genes in Brassicaceae.
Solmslaubachia eurycarpa (Euclidieae), alone in the family, has lost most of its chloroplast ndh genes, indeed, ndh loss or pseudogenization is scattered through the Euclidieae, there being infraspecific variation in the extent of these losses within S. minor (H. Chen et al. 2020). The loss of the rps16 gene is scattered throughout the family while that of the ycf15 gene is rather more localized - Aethionemeae, [Anchonieae + Euclidieae] and scattered in [Camelineae + Alyssopsideae], and it is also lost in Cleome (X. Guo et al. 2017).
The mitochondrial orf164 gene is derived from part of the nuclear ARF17 gene, and unusually for such transpositions, it is expressed in the mitochondrion (Qiu et al. 2015). It has so far been found only in Lineage I members - e.g. in Arabidopsis, but not in Brassica.
Economic Importance. The Aegean endemic Brassica cretica is closest to B. oleracea (cabbage, Brussels sprouts, cauliflower) (Mabry et al. 2021: wild, domesticated and feral B. oleracea); Snogerup et al. (1990) discuss these species and their immediate relatives. For oils from oilseed rape (B. napus ssp. napus, inc. canola oil) and other Brassica species, between them accounting for ca 12% of the total edible oil production, see S. Liu et al. (2014). Oil is also obtained from false flax, Camelina sativa, even Lesquerella (= Physaria) (see papers in Vollmann &samp; Rajcan 2009), and from Camelina in particular (see Mandáková et al. 2019). Root crops like turnips and radishes are also obtained from members of Brassiceae; Arias and Pires (2012) discuss generic limits here.
Caterpillars of the diamondback moth, Plutella xylostella (Yponomeutoidea-Plutellidae), are a major pest of cruciferous crops, especially cabbages et al. (Brassica oleracea: Sohn et al. 2013). Weevils of the genus Ceutorhynchus are also serious pests of cabbage, rape seed, etc. (Alford et al. 2003).
Chemistry, Morphology, etc.. For tocopherols in Brassicaceae, see Goffman et al. (1999) and especially Brock et al. (2006) for nortropane alkaloids, which are found in Aethionema, Cochlearia, etc., Badami and Patil (1981) for seed fatty acids, and Harborne (1999, but sampling) for distinctive sulphur-containing phytoalexins.
Bowman (2006) put morphology in general here in the context of comparative developmental genetics. The border cells of the root cap dissociate in rows (Driouich et al. 2006); other Brassicales should be examined for this character. Aleamotu'a et al. (2018, see also Collings et al. 2020) discussed the development of phi (φ) cortical cell wall thickenings in Brassicaceae; they could be induced in some species, but not in others. In some, in addition to the conventional phi thickenings, a fine reticulum of such thickenings developed on the periclinal wall adjacent to the endodermis. The reaction wood is made up of thick-walled fibres (Schweingruber 2006); c.f. Resedaceae-Resedeae. Nodal anatomy appears to be quite variable; that of Lepidium latifolium is 5≤:5≤ and of Draba it is 1:5 (Ezelarab & Dormer 1966). Lepidium oleraceum showed KNOX1 expression and had lobed leaves in early leaf development like other species of the genus that had compound adult leaves, but in L. oleraceum the leaves ended up simple, but with apical teeth that were equivalent to the pinnules of the other species - simple leaves are derived here (Bharathan et al. 2002). Nikolov et al. (2019) also examined gene expression in the context of leaf morphology, simple vs complex. For more on teeth and lobing of the lamina, see elsewhere.
Hearn et al. (2018b: focus on proliferation of parenchyma, supplemental vascular bundles) found similarities at the gene expression level in the development of stem and hypocotyl/root tubers in Brassica (kohlrabi, turnips), and also in tubers of Solanaceae. Interestingly, they noted amphivasal vascular bundles here.
Ca 29 genera include species in which the inflorescence axis does not develop, leading to the so-called "rosette flowering"; here long-pedicellate flowers spring directly from the rosette, whether or not they are subtended by foliaceous bracts (Bosch et al. 2008). There are rarely more or less glandular "stipules" in the inflorescence and elsewhere (Weberling 2006 for a summary, also Bowman 2006; Bosch et al. 2008). Gookin and Assman (2021) describe what they call a cantil (a cantilever-like structure) in Arabidopsis thaliana, this is a somewhat swollen structure that is cauline in origin, more or less spreading to retrorse but adaxially curved, often articulated apically, sometimes with a spur, and bearing a flower; stem spikes, spur-like stuctures, can occur independently of a flower. Cantils occur at the base of the inflorescence and are most noticeable when flowering is delayed, although short days are also implicated (Gookin & Assmann 2021, q.v. for genetic control).
C. Y. Huang et al. (2013) discuss the evolution of a cluster of tandem oleosin genes known from several Brassicaceae but that are unknown from Cleomaceae. These genes produce lipids that end up on the pollen surface; the pollen tolerates dehydration quite well. Exactly where this character should go on the tree awaits better sampling. Brassicaceae have tryphine covering the pollen grains, not pollenkitt, as in other angiosperms; in tryphine some constituents of the disorganised tapetal cells are still visible (Pacini & Hesse 2005); details of the distribution of this feature are also unclear. These and other distinctive features of pollen development in Brassicaceae are discussed by Suzuki et al. (2013) and Gotelli et al. (2023).
There has been a long-standing controversy about the evolutionary origin of the six stamens in Brassicaceae: Did they arise by doubling/dédoublement or by reduction (Endress 1992; Ronse Decraene & Smets 1993b; Smyth 2018 for literature)? The former is perhaps more likely, and stamen number in Brassicaceae certainly sometimes increases, thus Megacarpaea polyandra can have 24 stamens. In Succowia the outer secondary parietal cells produces the endothecium and middle layer, the inner producing the tapetum only (Hakki 1974). Maybe a quarter of the family have omniaperturate pollen grains, that is, whatever the morphology of the grains, the pollen tubes can break through the pollen wall anywhere. However, in 8/9 apparently omniaperturate species examined, there was also normal pollen tube germination, and only in Matthiola incana was there 100% germination through the walls (Edlund et al. 2016). Another controversy concerns carpel number and morphology (e.g. Carlquist 1970 for references). The commissural stigmas of Brassicaceae have been supposed to be an indication that the gynoecium is basically 4-carpelate, but such stigmas are notably common in groups with parietal placentation. Similarly, normally-oriented bundles outside the inverted placental ventral carpellary bundle in Crataeva religiosa has been thought to indicate an original 4-carpelate condition with axile placentation (Dickison 2000, but c.f. Brückner 2000). However, flowers with four carpels are decidedly uncommon in Brassicales.
Nectary morphology in Brassicaceae is very variable indeed, Erbar (2024: see esp. Fig. 2) recognizing 30 types (40 in the abstract!). These vary from annular to up to eight quite separate structures, and in nearly all cases they are borne outside at least the two stamen pairs; when nectaries are associated with the two lateral stamens they are often linear structures surrounding those stamens individually, if open ad- or abaxially. Note that the variation responsible for the designation of these types by no means encompasses all the variation that Erbar saw. Despite the necessarily restricted sampling in this study, Erbar (2024) found that nectaries nearly always varied in morphology within a tribe, quite often within a genus or even within an individual, and there was no particular correlation beween phylogeny and the seven groups of nectary types that she asssembled.
The ovule of Succowia has a zig-zag micropyle (like Capsella) and something quite like a postament, endothelial cells may have up to 32 nuclei, and the suspensor is uniseriate and quite large (Hakki 1974). The chalazal endosperm cyst may be involved in the movement of metabolites into the developing seed, there being transfer cells around it (Brown et al. 2004). Guignard (1893) suggested that the seed coat of Lunaria was endotegmic; there was no lignification in the testa at all. Indeed, Ciftci et al. (2022; see also Prasad 1977) found variation of systematic interest that needs to be integrated into the tribal phylogeny above.
See Al-Shehbaz (1984), T. Zhou et al. (2001: Chinese taxa), Appel and Al-Shehbaz (2002), Koch et al. (2003), Hurka et al. (2005) and Mitchell-Olds et al. (2005) for general information, Moazzeni et al. (2018: Aethonema), also Koch et al. (2012, 2017) for BrassiBase, a very useful resource - check out "Morphology Tool", for instance, and it also includes an interactive key to the genera. See also Agerbirk et al. (2021a, b: esp. Cardamineae) for glucosinolates, P. J. Horn et al. (2016) for fatty acids in Physaria seeds, Schweingruber (2006) for phloem and xylem anatomy, Balfour and Philipson (1962) for nodal anatomy, Iberis, five sympodia), Erbar and Leins (1997a, b) and Leins and Erbar (2010) for floral development, Tantawy et al. (2021) for floral vasculature, Bernadello (2007) for nectary variation, Khalik et al. (2002) and Ranjbar and Karami (2023: Alysseae) for pollen morphology, Pammel (1897), Vaughan and Whitehouse (1971: much information), Prasad (1975), Bouman (1975), Khoul et al. (2002) and Vinogradova et al. (2023: Boechera) for ovules and seed coat, Abraham (1885) and Moïse et al. (2005) for seed coat structure and development, Brown et al. (2004) for endosperm cysts (Aethionema not sampled, cysts probably not in in Cleome, at least) and Mummenhoff et al. (2009) for fruit development.
Phylogeny. Progress is beinmg made in providing a phylogenetic framework for the family (e.g. Koch et al. 2001; Koch 2003; Beilstein et al. 2006; Warwick et al. 2010; Z.-D. Chen et al. 2016; Hendriks et al. 2022/2023) and tribes have been reconstructed and realigned accordingly. Aethionema, a variable genus with angustiseptate fruits, consistently appears as sister to the rest of the family (e.g. Zunk et al. 1996, 1999; Koch et al. 2001; Beilstein et al. 2006, 2010; Kagale et al 2014). Arabideae may be sister to the remainder of the family, and Arabideae and Aethionemeae, both with much rearranged genomes, have boundaries of genome blocks in common; in other Brassicaceae genome structure is largely conserved in diploid lineages (Walden et al. 2020a). The relationships between many of the other tribes - and even the circumscription of some - has been for some time unclear, although there have been recent improvements (Beilstein et al. 2006; Al-Shehbaz et al. 2006; Bailey et al. 2006a, b; Franzke et al. 2009; Warwick et al. 2010; Zhao et al. 2010; M. Sun et al. 2016; Walden et al. 2020b; Hendriks et al. 2022/2023).
As mentioned in Genes & Genomes above, hybridization is rampant in Brassicaceae. Nevertheless, tribes are for the most part well supported even if their relationships, i.e. relationships along the backbone of the tree, tend not to be (e.g. Hendriks et al. 2022/2023). Some examples of hybridization. The Antipodean ×Microlepidieae are thought to be the polyploid descendents of a Crucihimalayeae (n = 8) × Smelowskieae (n = 7) → n = 13 event that occured 10.6-7 Ma in the Northern Hemisphere, with several subsequent reduction series; n = 4 in some species (Joly et al. 2009; Mandáková et al. 2010, esp. 2017a, b). Along the same lines, the recently described monotypic ×Shehbazieae is a hybrid between members of Chorisporeae and Dontostemoneae (German & Friesen 2014). Arias et al. (2014a) noted that hybridization within Brassica involved members of clades that have been separate for around 18 Ma (20 Ma in the text), and intergeneric hybridization is also reported from Boechereae (Windham et al. 2014) and in the Subularieae area (Dogan et al. 2022). X. Guo et al. (2020) found that within Biscutelleae alone there had been four different whole genome duplications, in Biscutella itself, Heldreichia, Lunaria and Ricotia, auto- (Heldreichia) and allopolyploidy (the others) being involved (see also Mandáková et al. 2018; Hendriks et al. 2022/2023). There is extensive polyploidy and hybridization within Cardamine (Lihová & Marhold 2006), and such lower-level hybridization/polyploidization events are common in the family, including within Arabidopsis and Brassica (see Soltis et al. 2016b and references). Hardly surprisingly, there is extensive cytonuclear discordance in phylogenies in the family (see also Mabry et al. 2020), and some 25 tribes differ in position when chloroplast and nuclear trees are compared (Hendriks et al. 2022/2023: Fig. 3 - number an underestimate?); different plastome analyses may also not give the same topology (see also Walden et al. 2020b).
The discussion in the preceding paragraph helps explain why our understanding of the phylogeny of the family has proceeded in fits and starts, and why it is difficult to tell a coherent story about what we do know. However, the references may be useful. Three larger clades, Lineages I, II, and III, have been recognised (Beilstein et al. 2007), but relationships between these clades were uncertain in the mitochondrial gene analysis of Franzke et al. (2009) - Aethionema aside, there was a hexatomy that included Lineage I and Lineage III, but the latter was recovered only in parsimony, not Bayesian, analyses. Relationships may be [paraphyly [[Lineage I + Lineage III] [paraphyly + Lineage II]] (Beilstein et al. 2010). Kagale et al. (2014: transcriptome pyrosequencing) recovered the relationships [Lineage III [Lineage I + Lineage II]], but sampling was poor. However, the monophyly of Lineage II in particular was uncertain, and several well-known genera like Alliaria and Thlaspi were outside the three main clades (Beilstein et al. 2007, 2010; Franzke et al. 2009).
C.-H. Huang et al. (2015) recovered five main clades in a tree based on the analysis of nuclear genes of 29 of the 51 tribes then recognized; although neither clade C nor its sister-group relationship with clade B were particularly well supported, support for other relationships was generally strong. Clade E includes Lineage III taxa, clade D two Lineage II taxa, clade C, Lineage II and III and unassigned taxa, clade B nearly all Lineage II (plus one unassigned) taxa, and clade A nearly all Lineage I (plus one Lineage II) taxa (C.-H. Huang et al. (2015; see also X. Guo et al. 2017 for variations on this theme). In the plastome phylogeny of Guo et al. (2017: focus on basal clades) relationships, all well supported, were basically [Lineage I [Lineage II + Lineage III]]. However, in a more recent nuclear phylogenomic analysis including members of 50/52 tribes of the family, Nikolov et al. (2019) recovered the three lineages just mentioned and they delimited two more, fifteen genera remaining unplaced; there was a fair bit of support for the topology of their tree, which was [Lineage III [Lineage IV [Lineage I {Lineage II + IV]]], although there was less support along the spine of the tree in the [Lineage II + Lineage V] area and at the bases of those two lineages themselves. The basic relationships are the same in Mabry et al. (2020) - [Lineage III [Lineage II + Lineage I]] - suggesting conflict between the nuclear and chloroplast genomes that was "important to consider when using the phylogeny to assess character evolution and divergence dating" (ibid. p. 1156; see also Walden et al. 2020b). Indeed.
Many relationships in the family remained to be established (e.g. Koch et al. 2012; Moazzeni et al. 2014). Relationships along the spine of Lineage I are quite well supported (Salariato et al. 2014; see also Mohammadin et al. 2017), although groups like Erysimeae are not yet placed; Malcomia is wildly polyphyletic (Moazzeni et al. 2014) and has been dismembered (Al-Shehbaz et al. 2014). Salariato et al. (2017) focussed on the CES clade; other taxa immediately related are much as outlined above, however, Alysseae and Aphragmeae were also linked with this group. There are studies on Microlepidieae, somewhat expanded (Heenan et al. 2012) and on Physarieae (Fuentes-Soriano & Al-Shehbaz 2013: also morphological diversification).
For the limits of Aethionema, see Khosravi et al. (2008), and for relationships in the genus, see Mohammadin et al. (2017); [A. spinosum + A. lepidoides], spiny plants, are sister to the rest of the genus.
Within Erysimeae relationships within the speciose Erysimum itself have little morphological or even molecular support, and hybridization and polyploidy are widespread (Moazzeni et al. 2014). Züst et al. (2019/2020) looked at nuclear transcriptomes, and nodes in the tree, although often with quite high posterior probabilities, tended to be supported by (very) few gene trees.
For relationships in Lepidieae, see Zunk et al. (2011) and for those within Lepidium itself, see Mummenhoff et al. (2001, 2004).
Although Arabidopsis thaliana (now in Arabidopsideae) is the most important model vascular plant in biology, the limits of Arabidopsis itself are only now being established (see Clauss & Koch 2006 for a discussion of its immediate relatives; German et al. 2023).
For relationships in Cardamineae, see Olsen et al. (2016), and although these are largely unresolved along the spine of the tree, the South African Aplanodes is sister to the rest, while Cardamine itself is likely to be most horribly para- or polyphyletic (see also Carlsen et al. 2009; Agerbirk et al. 2021b).
Boechereae. For work on on Boechera and relatives, where there is major conflict between nuclear and plastid relationships, see Kiefer et al. (2009a, b), Alexander et al. (2013: seven low-copy nuclear loci) and Hay et al. (2023: Angiosperms353 + Brassicaceae bait set, 1114 loci in total, 81 spp., only diploids). In the latter study there was substantial support in general, although there was notable gene tree discordance along the backbone of the phylogeny within Boechera; this is in the context of the analysis of nuclear data. Within Boechera the clade [B. shevockii + B. davidsonii] was sister to the rest of the genus that was divided into two clades (good support) - all are from W. North America; the rest of the tribe, in which [Borodinia + Nevada] are sister to the rest, is sister to Boechera. There was substantial incongruence between nuclear- and plastid-based phylogenies (Hay et al. 2023).
Relationships between tribes and groups of tribes in Lineage II, itself often not well supported, are not yet soundly established, although the monophyly of most tribes there does have good support (see also Mohammadin et al. 2017). Studies on other Lineage II tribes include that on Schizopetaleae and Thelypodieae by Warwick et al. (2009).
Arabideae: For relationships in this large tribe, see Karl and Koch (2013), for the circumscription of Arabis itself, see Koch et al. (2010), and for a phylogeny of Draba, with three major clades, the species mostly perennial and a number Arctic-alpine, see Koch and Al-Shehbaz (2002) and Jordon-Thaden et al. (2010, 2013).
Brassiceae: Warwick and Sauder (2005) found that to make this tribe monophyletic little adjustment from its classical delimitation was needed, but "well-known" genera such as Brassica, Diplotaxis, Raphanus and Erucastrum were polyphyletic; as they noted, this should affect how breeders went about their business. Relationships in Brassiceae are still somewhat unclear (Hall et al. 2011), although there are eight well-supported clades (Arias & Pires 2012); ITS and cpDNA analyses give different topologies (Zipfer-Berger et al. 2015 and references); for Brassica section Brassica, see also Snogerup et al. (1990).
Coluteocarpeae. Ali et al. (2016) and especially Özüdogru et al. (2019) provide a phylogeny, the latter finding Microthlaspi s. str. to be sister to the rest of the tribe, although with rather poor support.
Sisymbrieae: Sisymbrium s. str. is largely restricted to the Old World, the New World taxa being unrelated and mixed in with Thelypodieae (Warwick et al. 2002, 2006a). Zerdoner Calasan et al. (2021: 5 nuclear, 3 chloroplast markers) found some conflict between nuclear and plastid relationships; S. afghanicum and S. aculeolatum were sister to the rest of the genus.
Thelypodieae were in the past thought to be close to Capparaceae because of the apparently plesiomorphic gynoecial, etc., morphology of some species, but they are now placed well within Brassicaceae near Brassiceae (Koch et al. 2012). Streptanthus and relatives include several serpentine endemics as well as the selenium-tolerant Stanleya pinnata, but genera did not reflect relationships, thus in Cacho et al. (2014, 2021) Stanleya, Streptanthella, and Caulanthus were all intermingled, while in Bartisch et al. (2012) Streptanthus and especially Mostacillastrum were polyphyletic. Daundasekara et al. (2025) looked at some relationships here - around 28 species in four genera using plastid and mitochondrial data and 18 species using SNPs for the nuclear analyses. Even with the rather sparse sampling, generic polyphyly was evident, and although the plastid and mitochondrial trees were not that different, the "nuclear phylogeny exhibited several stark discordances with the organellar phylogenies" (ibid. p. 9).
Thlaspideae. Relationships in the tribe, which has around 42 species, are discussed by Esmailbegi et al. (2018); the sometimes invasive Alliaria petiolata, with very different fruit morphology, is to be included here. Pseudoturritis turrita is sister to the rest of the tribe (Mandáková et al. 2020?a and references). Mummenhoff et al. (1997), Koch and Mummenhoff (2001) and Meyer (2003) discuss generic limits surrounding Thlaspi, a polyphyletic genus.
Salariato et al. (2020) outlined relationships in Cremolobeae, from South America.
Eudemeae. Salariato et al. (2015) looked at relationships here, not all genera were monophyletic, and in Salariato et al. (2022) nuclear and plastid trees told somewhat different stories. Monophyly was cleared up by Al-Shehbaz et al. (2023) with the description of three new genera: [Xerodraba [Alshehbazia + Onuris]] were sister to the rest of the tribe.For relationships in Isatideae, see Moazzeni et al. (2010), in Cochlearieae, Koch (2012), and in Chorisporeae-Dontostemoneae, see German et al. (2011).
For work on Matthiola (Anchonieae), see Jaén-Molina et al. (2009). Salariato et al. (2018) found that Stenodraba, whose previous assignment was morphology-based, was wrongly assigned, and of course it is polyphyletic.
Hesperideae. Eslami-Farouji et al. (2021) looked at relationships within Hesperis, recovering four main clades (but very few of the classical sections); hybridization seems to have been minimal, however, taxa like H. dvorakii moved around the tree depending on the method of analysis.
H. Chen et al. (2020: nuclear ribosomal DNA, various analyses of plastid protein-coding genes) looked at relationships within Euclidieae; there Leiospora was sister to the rest of the tribe and Solmslaubachia and Braya were para/polyphyletic in the chloroplast but not the nuclear phylogenies.
Lineage IV. Heliophileae. Mummenhoff et al. (2005: ITS) and Mandáková et al. (2012) have looked at variation and character evolution in the speciose and very variable Heliophila and relatives. Biscutelleae and some other tribes are unplaced. Alysseae are also outside the three main clades, and for a study of Alysseae and related tribes, see Warwick et al. (2008) and Spaniel et al. (2016). Resetnik et al. (2013) looked at Alysseae in detail finding four four major clades, although support along the backbone was not strong and genera were often not monophyletic; see also Spaniel et al. (2015). The focus in Y. Li et al. (2015) was Alyssum s.l., and they found that both the old sections and also the subsections within section Odontarrhena did not hold up well, and there also seemed to be a fair amount of species-level poly/paraphyly. See also Price et al. (1994), Galloway et al. (1998), Y.-W. Yang et al. (1999), and Warwick et al. (2007) for other phylogenetic studies. For work on some Asian taxa, see German et al. (2009: ITS), on Iranian Brassicaceae, see Khosravi et al. (2009), and on some Chinese Brassicaceae, see Liu et al. (2011).
Overall, the literature is rather confusing, and one needs a phylogeny with really good genus-level sampling and using both nuclear and chloroplast data to begin to make sense of evolution in the family. It is tribes/genera like Anastaticeae, Biscutelleae, Cochlearieae, Megacarpaeeae, Iberis, Idahoa, and Subularia that have caused particular problems in the past (e.g. Nikolov et al. 2019; Hendricks et al. 2022); these rogue taxa are middle-aged polyploids. However, there is now substantial progress in understanding relationships. L.-M. Liu et al. (2020) looked at plastomes and nrDNA cistrons from 73 genera and 61.5% of the tribes then described, but more tribes have recently been proposed (Hendricks et al. 2022). In the Seed Plant Tree (i.2022 version) a disconcerting number of nodes had no support; I have not tried to relate the topology suggested there with the discussion above. Hendriks et al. (2022/2023) used 297 nuclear genes from 319/345 genera and nearly all tribes, mostly one species per genus, in their nuclear analyses, while in their chloroplast analyses they looked at 60 genes from about 260 genera and all tribes; their nuclear tree is the basis for the summary classification and tribal sequence above.
Classification. Early classifications of the family have turned out to be rather much of a disaster. Both generic and tribal limits were often based on single characters like fruit "types" and embryo curvature and have proven to be very unsatisfactory (e.g. Mummenhoff et al. 1997; Al-Shehbaz et al. 2006; Moazzeni et al. 2010). Thus the combination of flattened fruits and accumbent cotyledons that characterized Lepidieae sensu Schultes has arisen perhaps 54 times in the family, and his Lepidieae are now placed in ca 13 tribes (I. Al-Shehbaz, pers. comm.), while the variation in such characters within quite small clades is impressive (e.g. Mummenhoff et al. 2005; Mummenhoff et al. 2005: Heliophila; Salariato et al. 2014). Mummenhoff et al. (2005) noted that recent work had shown that in Arabidopsis thaliana single genes controlled fruit dehiscence and relative length - these were important characters in earlier classifications of the family.
A tribal reclassification began when Al-Shehbaz et al. (2006) recognised 25 monophyletic tribes based on well-supported clades; these tribes included somewhat over three quarters (260 of the 338) genera of the family then recognised (see also Beilstein et al. 2008; German & Al-Shehbaz 2008). The number of tribes has been creeping up since then, and is now 59 (as of v.2023), having more than doubled (see also Al-Shehbaz 2012, Koch et al. 2012; H. Chen et al. 2020; Hendriks et al. 2022/2023), while German et al. (2023) added five supertibes in which the tribes of Brassicoideae are finding homes, and the classification above follows the names and relationships, these latter now largely being based on analyses of aspects of nuclear genomes, in Hendriks et al. (2022/2023) and German et al. (2023); see also BrassiBase. A number of tribes were described a long time ago, but their composition has changed greatly over the years, so be careful! Things are perhaps getting a bit splitty partly because previously unplaced genera that end up as sister to named tribes are put in new tribes, not accommodated in old tribes; the tribal classification above was begun when relationships were imperfectly known (Al-Shehbaz et al. 2006). Note that tribes like ×Shehbazieae and ×Microlepidieae are thought to have originated from hybrids between taxa now placed in different tribes... (e.g. German & Friesen 2014 and references).
At the generic level the story is rather similar, as the tale of the recent nomenclatural peregrinations of German Brassicaceae recounted by Koch (in Kadereit et al. 2016) makes clear. There has been parallel or convergent evolution of just about all the morphological features used to distinguish genera (e.g. Koch 2003; Al-Shehbaz et al. 2006; Bailey et al. 2006a, b; Beilstein et al. 2008; Franzke et al. 2011; Walden et al. 2020b), hence the major disagreements when comparing new and past generic limits. Some genera such Draba and Lepidium are indeed monophyletic or largely so on both morphological and molecular grounds, but many, including Brassica itself, are not (e.g. Mitchell-Olds et al. 2005 and references). For Isatideae, see Moazzeni et al. (2010). For the suggested placement of some of the more temperate annual species of Draba in separate genera, see Jordon-Thaden et al. (2010), for genera around Boechera, see Alexander et al. (2013), and for generic limits in Coluteocarpeae, c.f. Al-Shehbaz (2014) and Özüdogru et al. (2019), followed here, and Ali et al. (2016). For the genera of Alysseae, see Spaniel et al. (2016). As with tribes, things are tending to be a bit splitty.
Warwick et al. (2006b) provide a species checklist, while BrassiBase (see Koch et al. 2012, 2017; Kiefer et al. 2014) is a developing resource for the family.
Thanks. To I. Al-Shehbaz, for interesting discussions.