EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; reaction wood ?, associated gelatinous fibres [g-fibres] with innermost layer of secondary cell wall rich in cellulose and poor in lignin; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
[BERBERIDOPSIDALES + ASTERIDAE]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [8> mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID II / CAMPANULIDAE / [METTENIUSALES [BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]]]: myricetin 0; style shorter than the ovary; endosperm copious, embryo short/very short.
[BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]] / APIIDAE: tube initiation early; G inferior, [2], style long.
[ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]] / CORE CAMPANULIDS: C forming distinct tube; A epipetalous.
Age. The age of the clade [Asterales + Apiales] is ca 72 Ma (Iorizzo et al. 2016].
)Phylogeny. For the relationships of Asterales, see the asterid II/gentianid clade.
(Route I secoiridoids +), fructan sugars accumulated as isokestose oligosaccharides [inulins], starch generally 0; apotracheal parenchyma 0; leaves spiral; flower size?; C tubular, lobe apiculi inflexed; A (basifixed), free from C; pollen grains often tricellular; orbicules 0; nectary +; style long; ovules many/carpel, integument <7 cells across, endothelium +, hypostase 0; antipodal cells ephemeral; embryo suspensor filamentous, micropylar and chalazal endosperm haustoria +; x = 9; mitochondrial rpl2 gene lost. - 11 families, 1743 genera, 26,870 species.
Includes Alseuosmiaceae, Argophyllaceae, Asteraceae, Calyceraceae, Campanulaceae, Goodeniaceae, Menyanthaceae, Pentaphragmataceae, Phellinaceae, Rousseaceae, Stylidiaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) suggested an age of (95-)90, 83(-77) Ma for the crown group, not too different from the (94-)82(-71) Ma that Wikström et al. (2015), the (98.2-)84.5(-71.4) Ma that Tank and Olmstead (pers. comm.) and the ca 83.7 Ma that Tank et al. (2015: Table S1) were to suggest considerably later, K. Bremer et al. (2004a) suggested around 93 Ma, while estimates in Janssens et al. (2009) are 94±11.2 Ma old. Magallón and Castillo (2009) estimate an age of (84.7-)84.5, 84.3(-84.1) Ma, Magallón et al. (2015: note topology) an age of ca 83.2 Ma, and Beaulieu et al. (2013a: 95% HPD) thought that the crown clade was (101-)89(-79) Ma old. At (113.6-)106.6, 93.2(-87.9) Ma, ages in Barreda et al. (2015: table S2) tend to be a little older, although one at (83.2-)81.1(-78.9) Ma was something of an outlier. Ca 5.4 Ma is the age suggested by C. Zhang et al. (2020) and 134.7-130.4 Ma by Benítez-Villaseñor et al. (2023).
Divergence & Distribution. Asterales contain ca 13.6% eudicot diversity (Magallón et al. 1999). Diversification rate increases in the order are sometimes nested - 3/8 such events (Zuntini et al. 2023). The clade is characterised by having notably small seeds (Moles et al. 2005a; Sims 2012).
Movement of Asterales into the northern hemisphere may be linked with the origin of hyperdiverse clades like Asteraceae and Campanulaceae (Beaulieu et al. 2013a), although basal relationships within Campanulaceae are unclear and there are suggestions that diversification in Asteraceae began in South America (see that family).
Endress (2011a) thought that the character "monosymmetric flowers" in Asterales might be a key innovation, although where it is to be placed on the tree is unclear; a position near the speciose Campanulaceae-Lobelioideae may represent one acquisition of this feature, a position near Asteraceae another. Furthermore, although monosymmetric flowers may occur in most Asteraceae, the capitulum itself is polysymmetric or haplomorphic, and major pollinators behave accordingly (see below under Asteraceae). As Endress (1998) noted, monosymmetry here is expressed mainly in the corolla, the number of stamens being equal to the corolla lobes, while in core Lamiales in particular one (or three) stamen is commonly reduced or lost, and the corolla is monosymmetric. Endress (2011a) also suggested that a key innovation somewhere in Asterales was tenuinucellate ovules. Unfortunately, corolla and endosperm development, endothelium presence, not to mention chemistry (for a partial summary, see Grayer et al. 1999), and the like, are unknown in some critical families, so understanding character evolution is particularly difficult. For fructans/inulins, see e.g. Pollard and Amuti (1981), Meier and Reid (1982) and Shen et al. (2023); they are provisionally considered to be an apomorphy for the order, although whether or not several of the smaller families synthesize inulin is uncleare. Absence of apotracheal parenchyma and x = 9 may also be features of Asterales (Lundberg & Bremer 2001; K. Bremer et al. 2001). Variation in pollen morphology (pollen disparity/phylomorphospace) is discussed by Jardine et al. (2022). Members of Campanulaceae + Rousseaceae and core Asterales form one group, those of Asteraceae 1 and 2 form two more, while members of Asteraceae-Barnadesioideae (Asteraceae 1) are more or less between the three; Campanulaceae + Rousseaceae include the least disparity (Jardine et al. 2022: the first two principal coordinates account for ≤10% of the variance, 221 principal coordinates accounting for the rest).
Pollination Biology. Several families, notably Campanulaceae and the Asteraceae area, have various forms of secondary pollen presentation (Carolin 1960b; Erbar & Leins 1995a; Leins 2000; Leins & Erbar 1997, Erbar 2003b: Yeo 1993 and El Ottra et al. 2023 for a general summary). Leins (2000) and Leins and Erbar (2006, 2010) in particular discuss in considerable detail the evolution of these pollen presentation mechanisms.
Ecology & Physiology. Fructans may stabilize cell membranes under drought and/or freezing conditions (Livingston III et al. 2009).
Chemistry, Morphology, etc.. The corolla lobes quite often appear to consist of a central portion and marginal "wings" reflecting the induplicate-valvate corolla aestivation of such flowers. For a study of petal vasculature, which shows interesting variation, see Gustafsson (1995); this work needs to be extended. Monosymmetry is often associated with a slit the length of the corolla, i.e. is the 0:5 type. Variation of ovary position in Asterales is considerable.
Tobe and Morin (1996) summarize embryological knowledge of many members of the order. For some inflorescence morphology and development, see Philipson (1953) and Harris (1999), for integument thickness, see Inoue and Tobe (1999), and for pollen, see Polevova (2006). For general discussions of variation in the order, see J. Kadereit (2006) and Lundberg (2009).
Phylogeny. Extensive phylogenetic structure in Asterales, although often with rather weak support, was early apparent (Gustaffson & Bremer 1997; D. Soltis et al. 2000; see also Olmstead et al. 2000). Subsequent studies improved support for many clades, although there was still a basal polytomy (Kårehed et al. 2000; Lundberg 2001a, b; Kårehed 2002a; especially K. Bremer et al. 2001; Lundberg & Bremer 2001, 2003). Stylidiaceae have been somewhat peripatetic. Olmstead et al. (2000) and B. Bremer et al. (2002) suggested a sister group relationship between Campanulaceae and Stylidiaceae (but not Donatia), and the latter authors suggested that Pentaphragmataceae were also associated. Donatia itself is there very weakly linked with Alseuosmiaceae et al., and in some studies it is sister to Abrophyllum (Carpodetaceae: Gustafsson et al. 1997), not to Stylidiaceae. Stylidiaceae and Donatiaceae are weakly (D. Soltis et al. 2000) or quite strongly (Kårehed et al. 2000; Lundberg 2001; Tank & Donoghue 2010) supported as sister taxa. H.-T. Li et al. (2019) found that Stylidiaceae moved down the backbone of the tree, leaving immediately after Pentaphragmataceae, support being strong (although Alseuosmiaceae were not sampled).
There were suggestions that Rousseaceae, Pentaphragmataceae and Campanulaceae were together sister to the other Asterales (Lundberg & Bremer 2003), although the support was not very strong, while Soltis et al. (2007a) found Campanulaceae to be sister to rest of Asterales (1.0 p.p.). Rousseaceae s.l. are often sister to Campanulaceae alone (Kårehed 2002a; Tank et al. 2007; esp. Tank & Donoghue 2010), but in Bell et al. (2010) Roussea was sister to the rest of the order. Soltis et al. (2011) found that Pentaphragmataceae were sister to all other Asterales, but with little support - and perhaps because of the pull of some mitochondrial genes. Benítez-Villaseñor et al. (2023: 63 spp., 9/11 families, 403 low copy genes) carried out an Anchored Hybrid Enrichment study of the group; Rousseaceae were sister to the rest, and they recovered a [Campanulaceae [Argophyllaceae + Alseuosmiaceae]] clade in some analyses.
Relationships between Asteraceae and its immediate relatives also vary somewhat according to the gene studied (A.P.G. II 2003 for references). Menyanthaceae did not link with the other three basal families in the four-gene study of Albach et al. (2001b). Leins and Erbar (2003b) thought that Goodeniaceae were probably sister to Asteraceae, noting i.a. that Barnadesia polyacantha has a bulge beneath the style branches, perhaps homologous with the stylar cup of Goodeniaceae, while Soltis et al. (2007a) found the relationships [[Calyceraceae + Goodeniaceae] Asteraceae]. However, relationships along the spine of Asterales were quite well resolved in a ten plastome analysis of Tank and Donoghue (2010) and are followed below - but only faute de mieux; Soltis et al. (2011) found a largely similar topology, apart from the position of Pentaphragmataceae and a weakly supported [Phellinaceae [Alseuosmiaceae + Argophyllaceae]] clade.
Once again, recent work suggests substantial rearrangements will be needed, i.a. in two the studies mentioned below both Asteraceae and Stylidiaceae are para/polyphyletic. H.-T. Li et al. (2021) recovered the relationships [Escalloniaceae [[Rousseaceae + Campanulaceae] [Pentaphragmataceae [Stylidiaceae [[Alseuosmiaceae [Phellinaceae + Argophyllaceae]] [Menyanthaceae [Goodeniaceae [Calyceracaceae + Asteraceae]]]]]]]. Relationships in the Seed Plant Tree as of ix.2024 are [Pentaphragmataceae [[Rousseaceae + Campanulaceae] [[Stylidiaceae [Menyanthaceae [[Phellinaceae + Stylidiaceae-Donatia] [Argophyllaceae + Alseuosmiaceae]]]] [Goodeniaceae [Asteraceae [Calyceraceae + Asteraceae-Barnadesioideae]]]]]], while those in Zuntini et al. (2024) are [Pentaphragmataceae [Rousseaceae [Campanulaceae [Stylidiaceae [[Stylidiaceae-Donatia (this last not in the summary tree) [Phellinaceae [Argophyllaceae + Alseuosmiaceae]]] [Menyanthaceae [Goodeniaceae [Asteraceae [Calyceracaceae + Asteraceae-Barnadesioideae]]]]]]]]].
Previous Relationships. The Asterales here are basically Takhtajan's (1997) Asteridae, but with the addition of sundry Hydrangeales. Cronquist (1981) included some families below in the orders placed towards the end of his Asteridae, although some were also in his Cornales (Rosidae), etc..
Synonymy: Alseuosmiineae Reveal - Alseuosmiales Doweld, Ambrosiales Dumortier, Anthemidales Link, Boopidales Berchtold & J. Presl, Brunoniales Lindley, Calendulales Link, Calycerales Link, Campanulales Berchtold & J. Presl, Carduales Small, Cichoriales Link, Cynarales Rafinesque, Echinopales Link, Goodeniales Berchtold & J. Presl, Lobeliales Link, Menyanthales J. Presl, Pentaphragmatales Doweld, Phellinales Doweld, Rousseales Doweld, Scaevolales Martius, Stylidiales Berchtold & J. Presl
[Rousseaceae + Campanulaceae]: inflorescence terminal; C valvate; A free.
Age. Wikström et al. (2001) suggested an age of (86-)81, 76(-71) Ma for this node, Tank et al. (2015: Table S1, S2) ages of about 76.2 to 81.4 Ma, Magallón et al. (2015) an age of around 76 Ma and Crowl et al. (2016) (86-)76(-67) Ma, about the same. J. Lundberg (in Hansen & Müller 2009) estimated an age of anywhere from 100 to 20 Ma, while at (141.5-)115.7(-91.7) Ma the esimate in Maurin and Smissen (2021) is the oldest.
ROUSSEACEAE Candolle - Back to Asterales
Plant woody; young stem with separate vascular bundles; lamina margins gland-toothed; anthers basifixed; G [5], opposite C; integument 5-8 cells across; x = 9 (?8).
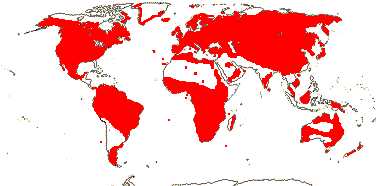
4 [list]/13 (6) - two subfamilies below. Mauritius, scattered from New Guinea to New Zealand.
1. Rousseoideae Horaninow - Roussea simplex Smith
Climber to small tree, evergreen; chemistry?, tannins 0; cork?; resin canals +; petiole bundle annular; buds perulate; hairs tufted-stellate and glandular-peltate; leaves opposite, leaf base broad; flowers single, large [2< cm long], (4-merous); K valvate, C connate; anthers attached their entire length to stout connective, sagittate, extrorse; pollen zono- 6- or 8-porate, tectum complete; G [(5-7)], style expanding apically, stigmatic lobes narrower, erect; ovule ?bitegmic; fruit a berry, K persistent; exotesta thick-walled, other cells crushed; micropylar haustorium +, embryo medium-long; n = ?
1/1. Mauritius. Map above: green. [Photo - Flower © D. Lorence]
2. Carpodetoideae J. Lundberg —— Synonymy: Abrophyllaceae Nakai, Carpodetaceae Fenzl
Trees; chemistry?; vessel elements with scalariform perforation plates; nodes 1:1, 3:3; petiole bundles arcuate or annular plus accessories; hairs unicellular, thick-walled, strongly curved, warty; inflorescence paniculate; flowers small [8> mm across/long], 4-7-merous; C free; A (adnate to base of C), (anthers dorsifixed - Cuttsia); (filaments very short - Abrophyllum [Ab.]); (pollen in tetrahedral tetrads - Carpodetus [Car.]); G (± inferior - Car.), style medium (0 - Ab.), stigma capitate (± divided - Cuttsia); fruit dry, baccate, or a loculicidal + septicidal capsule, K deciduous; seeds many, funicle elongated; exotestal cells massively thickened on anticlinal and inner periclinal walls (all around - Car.); endosperm hemicellulosic [Car.], ?haustoria; embryo small; n = 14, 15.
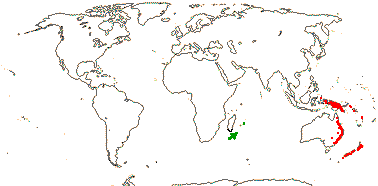
3/12. New Zealand, E. Australia, Halamahera, East Malesia to Vanuatu. Map: from Pillon et al. (2014) and Australia's Virtual Herbarium (consulted xii.2014) - see above, red. Photo: Inflorescence.
Age. Maurin and Smissen (2021) suggested that the age of this clade was (77.9-)51.7(-28.4) Ma and Benítez-Villaseñor et al. (2023) an age of 42.8-41.1 Ma.
Evolution: Divergence & Distribution. Mauritius is only some 8 Ma - what was the history of Roussea over the preceding ca 70 Ma (Lundberg 2001a; see also Asteliaceae, Monimiaceae, Arecaceae)?
Pollination Biology. Roussea is pollinated by the gecko Phelsuma. The pollen is embedded in a slimy substance and sticks to the gecko, which may also disperse its seeds, which are embedded in pulp (Hansen & Müller 2009).
Chemistry, Morphology, etc.. Roussea in particular is poorly known. It has an endodermis in its petiole, and its seed is drawn as if it were carunculate (Engler 1930a). Mauritzon (1933) suggested that it might have bitegmic ovules.
Abrophyllum and Cuttsia both have clusters of small, unlignified cells in the mesophyll that look like little white raphide bundles (Hils 1985). For a useful summary, see Gustafsson (2006).
For some general information, see Gustaffson and Bremer (1997) and Koontz et al. (2006: Roussea), for further details of vegetative anatomy of Carpodetoideae, see Gornall et al. (1998: as Escalloniaceae) and Carlquist (2012c), indumentum, see Al-Shammary and Gornall (1994), floral morphology, see Tobe and Raven (1999), for pollen, see Telleria et al. (2018), and seed anatomy, see Takhtajan (2000). For anatomy of Roussea, see Watari (1939), Ramamonjiarisoa (1980) and Gornall et al. (1998: as Escalloniaceae).
Phylogeny. [Carpodetus [Cuttsia + Abrophyllum]] is the strongly supported set of relationships within Carpodetoideae (Gustaffson & Bremer 1997; Lundberg 2001a; Pillon et al. 2014).
Classification. Species limits in Carpodetus need attention.
Previous Relationships. Rousseaceae were previously of uncertain position. Takhtajan (1997) placed Roussea (as Rousseaceae) in Rosidae-Celastranae-Brexiales, and Carpodetoideae have often been associated with Saxifragaceae s.l., i.e. the woody Saxifragaceae, thus Takhtajan's Carpodetaceae were members of his heterogeneous Hydrangeales (see also summary in Lundberg 2001a). No members of the family were mentioned by Cronquist (1981), which was perhaps wise.
CAMPANULACEAE Jussieu, nom. cons. - Back to Asterales
Herbs, whether annual or perennial, to shrubs and pachycaul rosette plants; iridoids and tannins 0, little oxalate accumulation; cork also inner cortical; vascular cylinder +; (medullary vascular bundles +); vessel elements with simple perforation plates; nodes 1:1; articulated laticifers +; crystals acicular; petiole bundles incurved-arcuate; leaves (opposite), lamina vernation variable, margins entire to toothed (lobed), hydathodes common; inflorescence racemose; flowers large, (3-)5(-10)-merous, monosymmetric, protandrous; median sepal abaxial, C with early tube formation, connate, venation reticulate; anthers dehisce in bud, connivent, introrse, at least initially close to stigma, 2ndary pollen presentation +, brush mechanism, simple deposition of pollen; pollen (bicellular), prolate, endexine throughout, not lamellate; G [2], sub/inferior, (placentation parietal), placentae intrusive, bilobed, style grows after anthesis, with hairs at tip, stigma lobed; integument ca 6 cells across; fruit a capsule, K persistent; seeds many, small [usu. 200> µg]; exotesta cells lignified, polygonal or elongated, (endotestal cells, esp. inner walls, thickened); endosperm (starchy), copious; x = 8, nuclear genome [1 C] (0.078-)1.686(-36.437) pg; plastid transmission biparental [?level], plastome expansion of inverted repeat into small single copy region, 5bp ndhF deletion, rpl23 duplication/transposition, chloroplast accD gene to the nucleus, infA gene 0 [but see Haberle et al. 2008a], mitochondrial coxII.i3 intron 0.
84 [list]/2,380 - five subfamilies below. World-wide.
Age. Bell et al. (2010) estimated a crown group age of (67-)56, 53(-41) Ma for the family, Wikström et al. (2001) suggested an age of (62-)59, 46(-43) Ma, Knox (2014) an age of around 60 Ma, while (72-)64(-56) Ma is the estimate in Crowl et al. (2016) and 65.9-64.5 Ma in Benítez-Villaseñor et al. (2023).
[Cyphioideae + Campanuloideae]: style ?hollow, hairs with bulbous bases; fruit a septicidal capsule.
1. Cyphioideae Schönland - Cyphia Bergius —— Synonymy: Cyphiaceae A. de Candolle
Perennial herbs (twining vines), shrubs, with tuberous roots; (flowers single); C 3:2, early sympetaly; filaments largely free or connate, (anthers slightly coherent); pollen smooth; G semi-inferior, style bends away from median K [adaxially], no growth after anthesis, stylar canal +[?], style hairs do not retract, pollen deposited in pollen box, base of box stigmatic head, [?2ndary pollen presentation], fluid-filled cavity at end of style with a lateral (terminal) pore; ?embryology; capsule septi- and loculicidal [valves bifid]; (seeds winged); n = 9.
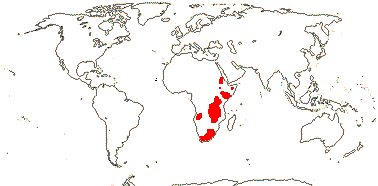
1/65. Especially South Africa, also east Africa, Cape Verde Islands. Map: from Thulin (1978).
Age. (33-)14(-22) Ma is the estimate for the age of crown-group Cyphioideae in Crowl et al. (2016).
2. Campanuloideae Burnett

Perennials (annuals), roots often thick; polyacetylenes + [14-C aliphatic tetrahydropyran derivatives - ?elsewhere], caffeic acid, p-coumaric acid +, latex rich in polysterols; (vessel elements with scalariform perforation plates); palisade mesophyll with arm cells; inflorescence often ± cymose/determinate; flowers polysymmetric; median K adaxial; stamens sprawling at bottom of corolla tube after anthers have dehisced, persistent bases conceal nectar; pollen spheroid to oblate-spheroid, verrucate or with spicules; G [(2-)3(4-10)], inferior, style long-hairy, especially in the upper half or so, hairs retract [2ndary pollen presentation brush-type, hairs later retract], stigma dry or wet; integument 7-11 cells across, vascularized, podium not persistent, (placental obturator + - Azorina); chalazal haustorium single-celled, (embryo medium); (fibrillar protein intranuclear inclusions); extensive rearrangements in the chloroplast inverted repeat.
50/1,050. More or less world-wide, especially Old World, few in the Antipodes and South America. Map: from Hultén (1971), Thulin (1975), Shulkina (1978) and FloraBase (consulted v.2011).
Age. Estimates of the age of this node are (48-)45, 33(-30) Ma (Wikström et al. 2001), (56-)43, 41(-28) Ma (Bell et al. 2010), and around 60 Ma (Knox 2014: Monopsis sister to rest); other ages are (41-)37.4, 23.5(-3.2) Ma (Roquet et al. 2009) and some 26.3-15.8 Ma (Wikström et al. 2001), or rather older, ca 50.55 (Crowl et al. 2014: Platycodon sister to the rest), (61-)53(-46) (Crowl et al. 2016), (62.3-)56.9(-51.6) Ma (K. E. Jones et al. 2017), (58.7-)53.1(-46.6) Ma (Yoo et al. 2018) or (102.4-)78.8(-57.3) Ma (Maurin & Smissen 2021). An age for stem Campanuloideae is ca 41 Ma (Wikström et al. 2001: sister to what?).
2A. Cyanantheae Meisner / Platycodoninae —— Synonymy: Cyananthaceae J. Agardh
Perennial herbs, often vines; (plant foetid); leaves often opposite; (A 3); pollen colpate/colporate; G [5], opposite C, (semi-superior); (fruit a berry); n = 7-9 (17).
10/60: Codonopsis (23), Cyananthus (23). Old World: Central Asia to Japan, West Malesia, few in Canaries and N. Africa, often (sub)tropical, not Europe or northern Asia..
Age. Diversification within Cyanantheae may have begun (36.9-)27.3(-18.7) Ma (K. E. Jones et al. 2017) or (45.3-)37.8(-29.7) Ma (Yoo et al. 2018).
[Wahlenbergieae + Campanuleae]: pollen porate, (flattened-triangular); G opposite K; epicotyl and hypocotyl usually not elongated.
2B. Wahlenbergieae Endlicher
(Leaves opposite); capsule apically dehiscent (circumscissile); n = 7-9, 17.
?: Wahlenbergia (260). Especially Africa and Australia.
Age. Diversification here is estimated at (44-)29.7(-17.1) Ma (K. E. Jones et al. 2017) or (42.6-)34.1(-26.4) Ma (Yoo et al. 2018).
2C. Campanuleae Dumortier —— Synonymy: Jasionaceae Dumortier
C (5-fenestrate); placentoids +, bilayered endothecium; fruit usu. dehiscing laterally by septifragal-hippocrepiform slits or pores [caused by activity of axicorn on drying], (indehiscent); n = 6-11, 13, 15, 17.
?: Campanula (420; Campanula s.l. ca 600), Adenophora (65). Especially N. temperate Old World, very few in the Antilles.
Age. Crown-group Campanuleae are at least (62.3-)56.9(-51.6) Ma (K. E. Jones et al. 2017) or (48.7-)43(36.9) Ma (Yoo et al. 2018).
Clade A: Cam 13–17, Feeria, Trachelium.
Pollen 3-porate; capsule usu. with basal pores.
Clade B: Cam 2-12, Homocodon, Peracarpa.
Pollen 4(5–15)-porate; capsule usu. with apical-central pores.
Also: Musschia, Jasione.
[Lobelioideae [Cyphocarpoideae + Nemacladoideae]]: ?
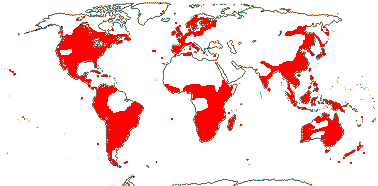
3. Lobelioideae Burnett —— Synonymy: Dortmannaceae Ruprecht, Lobeliaceae Jussieu, nom. cons.
(Annuals) to perennials, herbs to small trees (climbers); chelidonic acid, pyr[roliz]idine alkaloids +, p-coumaric acid, caffeic acid 0; stem with endodermis [Lobelia]; lamina vernation supervolute [Lobelia]; inflorescences terminal (axillary); flowers large to small, resupinate by pedicel torsion [so median C abaxial], (not); C (3:2), 2:3, 0:5 [split-monosymmetric], (spurred - esp. L. lobelioides), ((2-)5-fenestrate); (A 3 + 2 staminodes), filaments connate at least apically, (adnate to C); anthers connate, abaxial A at least with stiff hairs; pollen reticulate-striate; style elongating, bending away from median K, with brush hairs, hairs do not retract, stigma wet, pollen box straight to abaxially curved, 2ndary pollen presentation as pump mechanism [Nüdelspritze]; synergids hooked, (antipodal cells barely persistent); fruit dehiscing laterally, (capsule loculicidal), (circumscissile), (fruit fleshy); n = (6-)7(-13).
33[notional]/1,225: Lobelia (435), Siphocampylus (235), Centropogon (215), Burmeistera (125), Cyanea (85), Lysipomia (40 + ?10). Widespread, but not Arctic and absent from the Near East and central Asia, largely tropical, especially common in the New World, South America in particular (almost half the species). Map: see Wimmer (1943), Meusel and Jäger (1992) and FloraBase (consulted 2007). [Photo - Flower, Fruit.]
Age. Ages for diversification within Lobelioideae differ greatly - e.g. (59.6-)54.9(-50.9) Ma (penalized likelihood) versus (88.2-)72.7(-52.2) Ma (BEAST: see Antonelli 2009); another estimate is (65-)57(-49) Ma (Crowl et al. 2016).
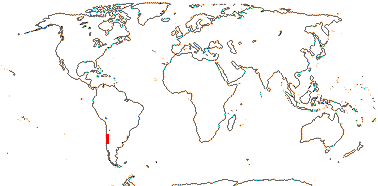
[Cyphocarpoideae + Nemacladoideae]: fibrillar protein intranuclear inclusions; n = 9.
4. Cyphocarpoideae Gustafsson - Cyphocarpus Miers —— Synonymy: Cyphocarpaceae Reveal & Hoogland
Annual to perennial herbs; leaf margins deeply lobed, spiny; bracts foliaceous; C induplicate-valvate, 1:4, adaxial C lobe sub-hooded, abaxial lobes with three ridges; A epipetalous, not connate, anthers connate, 2ndary pollen presentation ?pump mechanism?; ovary notably elongated, style ± straight, mostly glabrous, but hairy apically, hairs not retractile, apex with fluid-filled stigmatic cavity with a lateral pore; ?embryology; fruit dehiscing laterally, ?loc.; n = ?
1/3. Chile.
5. Nemacladoideae M. H. G. Gustafsson - Nemacladus Nuttall —— Synonymy: Nemacladaceae Nuttall
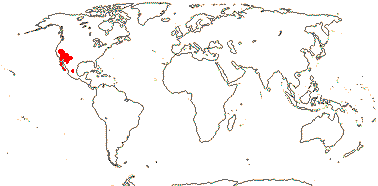
Tiny annuals (one sp. perennial, woody stem); leaves (subopposite); inflorescence a raceme, (bracteoles 0), flowers small [5> mm across], (not resupinate); C 3:2; A (adnate to C), filaments free basally or not, connate apically, anthers connivent, fimbriate/digitate groups of swollen, elongated cells at outside bases of adaxial filaments (0); pollen (6-colpate), spheroid to oblate-spheroid, verrucate or with spicules; G also [3], half inferior to superior, style hairs retractile, filament tube and style bending away from median K, pollen presented by simple deposition on closed stigmatic lobes; ?embryology; (fruit circumscissile - N. californicus); n = 9.
1-2/25: Nemacladus (24). S.W. U. S. A., esp. California, C. and N.W. Mexico (map: from Wimmer 1968, see also Morin & Ayers 2020).
Age. A suggested age for crown-group Nemacladoideae is (59-)44(-27) Ma (Crowl et al. 2016).
Evolution: Divergence & Distribution. For ages of various clades within Campanulaceae, see Roquet et al. (2009); ages for deeper nodes in different analyses varied considerably. Crowl et al. (2014) give dates for branching points within Campanuloideae, but note details of the topology there; see also Mansion et al. (2012) and K. E. Jones et al. (2017) for dates within Campanula.
The diversification rate may have increased in Campanulaceae around (76.0-)54.3(-45.6) Ma (Magallón et al. 2018).
The biogeographic history of Campanuloideae is complex and involves much movement. Yoo et al. (2018) suggested that Campanuloideae may have originated in Africa, but Platycodinae moved to South East Asia ca 53 Ma; all told there were some 14 dispersal events to South East Asia, mostly from Europe-West Asia, ten of these occurring within the last 10 Ma - there were only 6 dispersals from South East Asia. Nearctic Campanuloideae moved there from the Palaearctic (c.f. Lobelioideae: Crowl et al. 2016). The area from the Balkans to western Asia is particularly critical in the diversification of Campanula itself (Roquet et al. 2009 for details); there are over 100 species of Campanula in Turkey alone. Interestingly, Campanuloideae on Crete seem to be largely remnants of a flora that was on the island when it was originally isolated (Cellinese et al. 2009). In an extensive study of Campanula K. E. Jones et al. (2017) followed diversification in Campanula s.l., noting a major clade originating (39.7-)31.5(-24.2) Ma but diversifying only (14.2-)11.1(-8.2) Ma the species of which are mostly found in the area from the Alps to the Caucasus, while another uptick in diversification was cause by a small clade of ca 9 species from the Himalayas that diversified (3.8-)2.4(-1.4) Ma. Crown Wahlenbergia is (45.3-)29.6(-15.2) Ma old, stem Wahlenbergia is ca 32 Ma old (HPD: Prebble 2011); there was little diversification for ca 10 Ma, and W. krebsii, from the Cape, is sister to the other species of the genus sampled. There is quite a group of Campanuloideae endemic in the Cape region (Linder 2003). There are several remarkable disjunctions, although sampling needs to be improved; are Wahlenbergia linifolia, from St Helena, and W. berteroi, from Juan Fernandez, really sister taxa (Haberle et al. 2009)? Long-distance dispersal has also been implicated in the occurrence of Lobelia loochooensis in the Ryukus; it probably came from Australia ca 7,000 km distant (Kokubugata et al. 2012; see also L.-Y. Chen et al. 2016). However, the alpha taxonomy around here is poorly known (Z.-Z. Chen et al. 2018).
Knox et al. (2006, no Nemacladoideae or Cyphocarpoideae included) suggested that [Cyphia + Lobelioideae] originated in southern Africa, subsequently dispersing quite widely and with at least two returns to Africa (see also Knox & Li 2017, origin in the western Cape Province). Antonelli (2009) also suggested that Lobelioideae originated in Africa, and with much subsequent long distance dispersal of the tiny seeds, indeed, the whole family is likely to have spread from Africa following the K/P extinction events, with land bridges and island hopping rather than continental drift being the likely means of dispersal (Crowl et al. 2016). Thus Lobelioideae may have moved to the Neotropics from Africa several times, with some nine subsequent dispersals to the Nearctic and five in the reverse direction (Crowl et al. 2016). In the predominantly Antipodean Isotoma, I. hypocrateriformis, from Western Australia, is sister to the rest of the clade (which includes species from four separate "genera"), and there seem to have been three dispersals to New Zealand alone and two to East Asia (Kagame et al. 2021). Kagame et al. (2021) suggest places of origin for all lobelioid "genera".
To paraphrase Crowl et al. (2016: p. 242), polyploidy appears to have played a role in the evolution of island endemics, montane species, and insular woodiness (see immediately below), and there were polyploidy events more locally at the bases of the clades containing vernal pool specialists such as Downingia and the serpentine soil specialists Campanula exigua and C. griffithii. Well over half the species in Lobelioideae are found in the next two groups that represent radiations that have occurred within the last 15 Ma or so.
1. Andean South America. Note that generic names are used for convenience here; monophyly is not implied... There has been extensive diversification in the Siphocampylus-Burmeistera-Centropogon-Lysipomia clade in South America, particularly along the Andes - indeed, ca 1/2 Lobelioideae are South American. Here the Chilean Lobelia section Tupa is sister to the whole group, while Lysipomia includes ca 40 rosulate species with small to minute flowers (as little as ca 3 mm across, the size of the whole plant of L. mitsyae) that are sometimes almost polysymmetric, the plants growing in the páramo. The Siphocampylus-Burmeistera-Centropogon clade, the centropogonids, includes around 550 species (West & Ayers 2006; Sklenár et al. 2011; see also Knox et al. 2008; Antonelli 2008; Lagomarsino et al. 2014, 2017). Diversification of the whole clade began 18-15 Ma along with the elevation of the Andes, while the 550+ centropogonids are the result of a radiation that began ca 12 Ma, peaking at ca 5 Ma (Pennington et al. 2010; Lagomarsino et al. 2014, 2016); Gentry (1982) discussed the diversity of bird-pollinated taxa of Gondwanan origin in tropical and premontane parts of the northern Andes. Life form variation is considerable in these centropogonids (see Fig. 1 in Lagomarsino et al. 2016). Increases in diversification rates are perhaps associated with the elevation of the Andes, the evolution of fleshy fruits, and bat and various kinds of hummingbird pollination (the latter perhaps the original condition for the whole group - Lagomarsino et al. 2014), while cooling global temperatures may have affected extinction rates - all in all, a remarkable radiation (Lagomarsino et al. 2014, esp. 2016). Muchhala and Potts (2007) found that the dissimilarity in floral traits of co-occurring species of Burmeistera was greater than chance, perhaps because of local evolution (see also Rhinanthus and Stylidium).
2. Hawai'i. Lobelioideae make up the largest plant radiation on the Hawaiian archipelago, and probably the largest radiation on any such islands. The ancestor of Hawaiian lobelioids may have been a woody plant adapted to open habitats and with wind-dispersed seeds, the flowers being pollinated by birds (Givnish et al. 1995, 2006a, 2008b, 2009a; see also Givnish 2000; Lim & Marshall 2017; Schenk 2021; Buss et al. 2001 - seed morphology in particular). There are ca 114 species of Lobelioideae on Hawai'i (plus about a quarter more that have become extinct) that are perhaps to be included in five genera, including Lobelia itself, and they form a monophyletic group (nuclear data suggest that the Polynesian Sclerotheca may be part of this clade - Rose et al. 2025); they appear to have evolved only within the last 13 Ma or so (ages in Antonelli 2009 are slightly older, while Rose et al. 2025 suggest that the crown age based on nuclear data is only ca 9.88 Ma). Rose et al. (2025) suggest that initial diversification in the Hawai'ian Lobelia lineage was very quick, there being close to a basal tritomy in the clade, and this was accompanied by extreme ILS, and also hybridization and introgression, etc., in various parts of the tree. After this initial diversification, nothing much happened for the next 4-5 My, the Hawaiian species groups/genera all having long phylogenetic stems. These ages are older than that of the oldest island, but presumably there was movement from islands that subsequently have sunk (see Shaw & Gillespie 2016 for the progression rule - Rose et al. 2025 find that movements here do indeed largely follow this rule). Species vary in growth habit from trees, whether branched or unbranched, pachycaul or not, to small shrubs, and in leaf morphology from simple and linear to close to bipinnate (here there is heterophylly, the leaves on young plants being subentire). Givnish and Montgomery (2014 and references) discuss variation in photosynthetic responses to light in these lobelioids, and this is consistent with the growth of the group in a variety of habitats. Some species are spiny when young, and herbivory by the now-extinct flightless geese and the moa-nalo, a flightless duck as large as a small turkey, is suspected as having driven the evolution of the spines, etc., found in some taxa. In a number of species the sepals are petal-like, and fleshy fruits, which can be up to 4 cm across, have evolved more than once; there are a number of endemic Hawai'ian birds that are/were (some have recently become extinct) both pollinators and fruit dispersers (Carlquist 1970a; Givnish et al. 1994, 1995, 2009a). Hawaiian lobelioids are palaeotetraploids (Lammers 1988; Carr 1998). For other important Hawai'ian radiations, see the silversword alliance, etc., Cyrtandra, Myrtaceae, see Diversity and Distribution for Metrosideros, early stages, Schiedea, in Caryophyllaceae, and the Stachys area (Lamiaceae), etc..
The rosette/caulescent habit. The convergent evolution of the rosette/caulescent habit in giant lobelias is a classic example of its kind. Woodiness is derived in Lobelioideae, but at least 450 species are more or less woody (Givnish 2010). The immediate ancestors of the pachycaul giant lobelias are woody and probably of Asian origin (e.g. Knox et al. 1993; Givnish et al. 2009a; Kagame et al. 2021; see also Hedberg 1964; Carlquist 1970), and giant lobelias from widely separated parts of the globe (the Pacific, South America, Africa) may be in the same immediate clade (Antonelli 2009), indeed, it has been suggested that geographical relationships here are [Africa - paraphyletic [IndoMalaya [Oceania [Neotropics + Afrotropics]]]] (Crowl et al. 2016). Some South American taxa may even be derived from within the African giant lobelia clade (see also Kandziora et al. 2022 for the evolution of the Afroalpine flora), and giant lobelias in general, including those from Hawai'i and elsewhere, may have had an East Asian origin; the estimated dispersal distances involved here are mind-bending (Knox & Li 2017). The rosette habit in Lobelia is a good example of convergent evolution (Givnish 2010; Kagame et al. 2021; c.f. Antonelli 2009). Thus the giant lobelias in Bhutan and Hawai'i may well represent independent acquisitions of the woody habit (note, however, that the ancestor of Hawaiian Lobelioideae is likely to have been woody - Givnish et al. 2009a), and the Bhutanese giant lobelia, L. nubigena, is the only known member of a clade (15-)13.8(-12.6) Ma, rather older than the ages of the other giant lobelias which tend to be younger than the habitats in which they live (L.-Y. Chen et al. 2016). Of course, the extensive variation in habit, etc., of Lobelioideae on the Hawaiian archipelago just mentioned (see also Carlquist 1965, 1974) also involves variation in the nature of woodiness (there are no herbaceous taxa), and Nürk et al. (2019) discuss the radiation of other taxa on (sky) islands where variation in habit, including woodiness, is common, noting that both disparification (≡ Simpsonian adaptive radiation) and diversification (species number increase) have been rapid, the latter despite the increase in generation time that is associated with the evolution of the woody habit. See also Hypericum, Echium, Lupinus, and Asteraceae-silverswords for similar examples.
Ecology & Physiology. Fetene et al. (1998) discuss the physiology of caulescent Campanulaceae in the context of living in an afro-alpine environment, and suggest that the advantage of the caulescent habit is that the plants - at least, the growing part - inhabit a more favourable microclimate with less extreme temperatures than they would if they formed rosettes on the surface of the ground.
Canarina is a vine that is reported to have both twining petioles and twining pedicels (Sousa-Baena et al. 2018b).
It is possible that the seedlings of Australian species of Lobelia like L. gibbosa and L. dentata are mycoheterotrophic (see Fraser 1931).
Pollination Biology & Seed Dispersal. For the evolution of the secondary pollen presentation devices in the family, see Erbar and Leins (1988b) and Leins and Erbar (especially 2003a [Cyphia], 2003b, 2005 [Cyphia], 2006, 2010; El Ottra et al. 2023). However, pollination devices especially in Cyphocarpoideae and Nemacladoideae are poorly understood. Tracking the evolution of these mechanisms also awaits a better supported phylogeny, although progress along this front is being made, and the various features involved in the secondary pollination devices can then be individually placed on the tree (Crowl et al. 2016). The protandrous flowers are polysymmetric in bud and the introrse anthers are more or less connivent when they dehisce, pollen then being in a position suitable for secondary pollination, whether entangled with filament hairs or held immediately above the stigmatic head (e.g. Leins & Erbar 2003b, 2010).
Campanuloideae have a brush secondary pollination device. Here the pollen is caught in a brush of hairs on the style whence they are removed by the pollinator; in the female phase, the hairs retract so any grains present fall off and selfing is prevented. In the monotypic Petromarula the stigmatic head is swollen and hairs occur only there (Igersheim 1993a), but otherwise pollination is similar. Phyteuma has coherent corolla lobes although the corolla is open laterally; the style hairs are only partly retractile. The nectar of some Campanuloideae may be brightly colored and then the filament bases are not persistent; normally they are, and they enclose the nectar. Insect pollination is prevalent, but bird (and even lizard) pollination is also known, especially in taxa found on islands (Olesen et al. 2012).
In Lobelioideae the pollen is retained in a tube or box formed by the connate anthers; it is forced out - a pump pollination device - as the style elongates and the pollinator brushes the stiff hairs at the ends of the anthers (generally the abaxial anthers), the vibrations that result causing the pollen to fall out. The stigmatic lobes finally push through, then separate, recurve, and finally become receptive.
- There has been a major radiation of lobelioids in the Andes that is associated with the pollinators there. High-altitude species of Burmeistera (Lobelioideae) have both bird and bat pollination (Muchhala 2006; Lagomarsino et al. 2017); vertebrate pollination is commoner et higher elevations, as expected, although Lysipomia is an insect-pollinated centropogonid lobeliad that is found in the páramo at higher elevations than its insect-pollinated relatives (Dellinger et al. 2022).
- Bat-pollinated species show character displacement, sympatric taxa differing more in floral morphology than would be expected, so reducing the chances of pollen being deposited on the wrong stigma (Muchhala & Potts 2007). In Centropogon nigricans there seems to have been co-evolution with a remarkably long-tongued bat, Anoura fistulata (Muchhala & Thomson 2009: c.f. Angraecum - Orchidaceae). All told, some 110 species of Andean Lobelioideae may be bat pollinated (Dobat & Peikert-Holle 1985), although the figure in Fleming et al. (2009) is only 20, while L. Lagomarsino (pers. comm.) estimates that there are about 180 bat-pollinated species.
- Extrafloral nectaries are found in Andean Centropogon on the outside of the inferior ovary. These occur mostly in species growing at lower altitudes where ants are to be found, and generalist humming birds are usually the visitors to the flowers (there does not seem to be a connection between these nectaries and pollinators). In species growing at higher altitudes such nectaries are rare and pollination is often by the two species of the hermit sickle-bill humming birds, Eutoxeres (Heliconia-Heliconiaceae is the nectar resource for Eutoxeres at lower altitudes - Stein 1992; Abrahamczyk et al. 2017a). Indeed, some 50 species in Centropogon subgenus Centropogon are pollinated by the sickle-bills (Lagomarsino et al. 2017; Mansaray et al. 2025: subgenus Centropogon), their corollas being very strongly curved indeed; although this feature may be an apomorphy for the clade, there have been several reversions to pollination by ± straight-billed birds, and flowers with strongly-curved tubes have not re-evolved. The stem age of Eutoxeres is ca 21.5 Ma, but that of Centropogon is only 2-3 Ma (Abrahamczyk et al. 2017a). Indeed, the large clade of Andean centropogonid lobelioids is likely to be plesiomorphically pollinated by straight-billed hummingbirds, and bat-pollinated flowers are likely to have evolved ca 13 times in this clade, with ca 11 reversions back to straight-billed hummingbird-pollinated floral morphologies (Lagomarsino et al. 2017).
- Lobelioideae have also radiated extensively on Hawai'i, and the flowers of many species of Cyanea and Clermontia (which separated from each other ca 9.7 Ma) are conspicuously curved; pollination of around 125 species on the archipelago is/was by a few species of extinct and extant Drepanidae and extinct Mohoidae (Carlquist 1970a; Lammers & Freeman 1986; Givnish et al. 1995; Pender et al. 2014; T. J. Givnish pers. comm. x.2013). Some species of Clermontia have petaloid sepals, a feature that may have been lost twice (Givnish et al. 2013).
- In the erstwhile Heterotoma [= Lobelia], alone in Campanulaceae (the genus, from Mexico and Central America, has ca 10 species), there is a nectar spur, in H. lobelioides it is well-developed and is made up of part of the corolla and the apex of the inferior ovary; its immediate relatives have small, blue, insect-pollinated flowers and very small spurs (Koopman & Ayers 2005).
The floral biology on Nemocladoideae is unknown, including any function of the large, elongated cells attached to the filaments of Nemocladus (Morin & Ayers 2020).
Within Andean Lobelioideae there has been extensive switching betweem fleshy and dry fruits, the result being that Siphocampylus (previously defined as having capsules) and Centropogon (berries) have turned out to be wildly poly/paraphyletic (Lagomarsino et al. 2014; Mansaray et al. 2025).
Plant-Animal Interactions. For the trenching behaviour of herbivores on laticiferous Campanulaceae, see Dussourd (2016). In Lobelia trenching by moth cetarpillars initially reduced the alkaloid concentration in much of the leaf, but more persistently in the region distal to the trench (Oppel et al. 2009). Bauer et al. (2014) studied latex composition and coagulation in Campanula glomerata; coagulation was very fast, although details of the mechanism involved were unclear.
Givnish et al. (1994) noted that a number of species of Cyanaea from the Hawaiian archipelago were densely covered in prickles when young, and they suggested that this was to protect the plants against grazing by the (now extinct) flightless geese and goose-like ducks, the moa-nalos, that had diversified there, and some of which reached quite large sizes.
Plant-Bacterial/Fungal Associations. The tiny seeds of the ?annual Australian Lobelia gibbosa and L. dentata germinate quite deep in the soil and develop an extensive underground plant body, in the latter species up to 15 cm long, before forming above-ground stems; they have a close association with fungal rhizomorphs (Fraser 1931).
Genes & Genomes. The base chromosome number of Lobelioideae is probably 9 (Lammers 1993; Crowl et al. 2016; c.f. Stace & James 1996), in line with that for Asterales as a whole (K. Bremer et al. 2001). There have been a number of genome duplications within the family, but there seems to be no evidence for an ancestral duplication, the two genome duplications throughout the family being likely to be much deeper in the angiosperms, there being evidence for similar duplications in Phellinaceae (Crowl et al. 2016). Genome duplications cannot be linked to the changes in floral symmetry described above (Crowl et al. 2016); tetraploid chromosome numbers predominate in woody Lobelioideae in general (Lammers 1993; Crowl et al. 2016); the Hawaiian radiation of lobelioids is of polyploids (Crowl et al. 2016).
For the very extensive rearrangements in the plastome, including the inverted repeat, see Cosner et al. (1997, 2004), Knox and Palmer (1999: Cyphocarpus, Nemacladus, etc., not studied, Cyphia was), Haberle et al. (2008a), Knox (2014) and Barnard-Kubow et al. (2014: infraspecific divergence in plastomes). The chloroplast gene accD (= ORF512, zpfA) has been lost (Doyle et al. 1995 and references; see also Knox 2014; Rousseau-Gueutin et al. 2013). All told, over 125 inversions, most sizable, are known, and Knox (2014) has pieced together the sequence in which some of the larger inversions occurred. Along with these inversions, protein-coding genes, probably from the nucleus, have moved into the chloroplast (Knox 2014).
Biparental transmission of plastids has been recorded from both Campanuloideae and Lobelioideae (Corriveau & Coleman 1988; Q. Zhang et al. 2003); Barnard-Kubow et al. (2016) describe how this can rescue cases of cytonuclear incompatability in Campanulastrun americanum. In crosses, incompatability between chloroplasts from one parent and the hybrid genome (plastome-genome incompatability - PGI) may result in the death of those chloroplasts and thus to variegation (Ruhlman & Jansen 2018 and references).
Chemistry, Morphology, etc.. Details of the major variation patterns in secondary metabolites within the clade need to be established. Carlquist (1969b) examined the wood anatomy of some Lobelioideae, he noted the very long vessels (with scalariform pitting of the lateral walls; also other characters) of some taxa that grew in very wet habitats; he suggested that paedomorphosis was the cause. Schweingruber et al. (2014) found parallelism in characters of wood anatomy in Campanuloideae, tall species, and species growing in the Arctic, tending to have similar anatomical traits.
Since the pedicel of Lobelia and its relatives is twisted (resupinate), the flowers appears to have a "normal" orientation with the median petal abaxial, however, this does not usually occur in Lysiopoma (= pseudo-resupinate - Ayers 1997). Some species of Cyphia have a long corolla tube, but the two abaxial petals have slits at their bases; when the flowers are strongly bilabiate, the two abaxial petals are free (Thulin 1978). In Nemacladus (Nemacladoideae) there are sometimes groups of remarkable almost hand-like (in some S.E.M.s) ?nectaries at the bases of two adaxial filaments. It is not known if the style hairs there are retractile. Ostrowskia (Campanuloideae) has anthers with placentoids and the integument is massive (Kamelina & Zhinkina 1998). Gabarayeva et al. (2023) summarized pollen development ≡ self assembly in Campanula rapunculoides.
The ovary in species of Wahlenbergia and some taxa in Cyanantheae is almost superior ovary. Lammers (2006) mentions a hypanthium in the context of the inferior ovary of Lobelioideae. Fruit dehiscence, especially in Campanuloideae, is a complicated affair, depending in part on whether the fruit is pendant or upright (42 fruit types there alone - Kolakovsky 1995). There is the development of an axicorn, lignified tissue largely in the axis of the gynoecium, in Campanuleae (see also Kolakovsky 1985; Andreychuk & Odintsova 2019, 2021). Kausik and Subramanyam (1945, 1947), Rosén (1932, 1949) and Subramanyam (1949, 1970) discuss endosperm development, etc.; there may be taxonomically interesting variation in the number of cells that make up the chalazal endosperm haustorium.
Schönland (1889), Wimmer (1968), Shulkina et al. (2003) and Lammers (1998 and especially 2006) provide general information on the subfamilies, for stem-node anatomy of Campanuloideae, see Col (1904), for wood anatomy of Campanuloideae, see Schweingruber et al. (2014: esp. the root collar, the transition between the radicle/primary root and the hypocotyl/stem), for inflorescence morphology, see Bull-Hereñu and Claßen-Bockhoff (2011b), for flowers, etc., of Downingia, see Kaplan (1970 and references), for anthers, etc., of Campanula, see Zhinkina and Evdokimova (2023), for pollen see Dunbar (1975a, b), Eddie et al. (2010) and Hong and Pan (2012), also Erbar (2014) for nectaries, Vogel (1998c), Shamrov and Zhinkina (1994) and Shamrov (1998) for ovules, Kolakovsky (1985) for fruit morphology, and Murata (1995), Buss et al. (2001), Cupido et al. (2011 and references), Koutsovoulou et al. (2013) and Ahn et al. (2023: also ovule) for seeds, seed coat anatomy/morphology and germination; for the protein bodies in the nuclei, see Bigazzi (1986) and Haberle (1998), and for the remarkable Nemacladus, see Morin and Ayers (2011).
Phylogeny. The relationships of the five major groupings in Campanulaceae have been uncertain for quite some time. Both the monophyly and the relationships of the poorly known Cyphiaceae (there are three subgroups) have been unclear (Lammers 1992), although they are certainly all part of a monophyletic Campanulaceae s.l. (see Cosner et al. 1994; Gustafsson & Bremer 1995; Gustafsson 1996b; Gustafsson et al. 1996). ITS sequence data suggested that one of these subgroups, Cyphocarpus, was a member of Lobelioideae (Haberle 1998, Ayers & Haberle 1999; see also pollen - Dunbar 1975b), but if so, it has several characters in common (parallelisms?) with the Campanuloideae and Nemacladoideae (another subgroup of the old Cyphiaceae). The tree presented by Haberle (1998) suggests the groupings [[Nemacladoideae + Campanuloideae] [Cyphioideae + Lobelioideae]] (Cyphia is the third subgroup), which may imply that the polysymmetric flower of Campanuloideae with the median sepal adaxial (the "normal" condition) is a reversal from a monosymmetric flower with the median sepal abaxial (see also Crowl et al. 2016). Lundberg and Bremer (2003) found what was basically a trichotomy of Cyphia, Lobelioideae, and Campanuloideae. Tank and Donoghue (2010) suggested that Cyphia was sister to Campanuloideae and Pseudonemacladus to Lobelioideae; consistent with these relationships is the [[Cyphia + Campanuloideae] clade found by Knox (2014). Indeed, this latter clade seems quite well established, and it is sister to a [Nemacladoideae, Cyphocarpoideae, Lobelioideae] clade - relationships unclear here, although Cyphocarpoideae and Nemacladoideae may be sister taxa, at least in Bayesian, if not maximum likelihood, analyses in the extensive (13 loci, 921 taxa) study of Crowl et al. (2016). The various rearrangements of the plastome studied by Knox (2014 and references) need to be integrated with the tree.
General relationships within Campanuloideae are discussed by Eddie et al. (2002, 2003), Cosner et al. (2004) and Olesen et al. (2012). Platycodon, Codonopsis and Cyananthus form a clade and are strongly supported as being sister to all other Campanuloideae (e.g. Cosner et al. 2004; Crowl et al. 2014; Hong & Wang 2015). However, relationships in the rest of the subfamily are not so clear. Both Campanula and Wahlenbergia are seriously polyphyletic. Most of Wahlenbergia and some other genera form a clade sister to the rest of the subfamily, while within the latter, W. hederacea (now = Hesperocodon hederacea), linked with Jasione (Haberle et al. 2008b, 2009; Cellinese et al. 2009; Roquet et al. 2008, 2009; Borsch et al. 2009; Prebble et al. 2010; Cupido et al. 2013; Crowl et al. 2014). Other studies placed it with Wahlenbergia and other immediate relatives (Mansion et al. 2012; Hong & Wang 2015). The Campanula clade was divided into two main clades, one centred on Campanula s. str. and the other on Rapunculus, while successively basal to these (the relative order is unclear) are the Jasione (mentioned already) and Musschia clades, the latter also including some species of Campanula (e.g. Cupido et al. 2013; Crowl et al. 2014). Relationships were rather different in the four chloroplast gene analysis of Hong and Wang (2015), where Jasione and Musschia were successively sister at the base of the Campanula group. Although support was rather strong, sampling was good only in the Platycodon group, while in Crowl et al. (2014) sampling was extensive, but largely in the Campanula group. Mansion et al. (2012) carried out a particularly extensive study in Campanuleae analysing variation in the petD gene for 3/4 (310/420 species) of all Campanula, plus related taxa and some other Campanulaceae. Within Campanuleae, they found the relationships [[some Campanula + Musschia] [[Feeria + Jasione] [The Rest]]] - all told, there were 17 clades with species of Campanula, and in five of these they were mixed with representatives of other genera (Mansion et al. 2012). K. E. Jones et al. (2017) focussed on Campanula s. str., again, some some Campanula, Musschia and Jasione were in a clade sister to Campanula s. str., within which 14 small genera were embedded, eight of these being in one quite small clade in which they were still outnumbered by Campanula. Yoo et al. (2018: 5 plastid, 1 nuclear markers, 376 spp.) looked at relationships in East Asian Campanuloideae, indicating genera that were monophyletic and those that were para/polyphyletic. C. Xu and Hong (2020: 6 plastid genes and ITS, 333 spp. esp. Campanuleae, 27/28 genera) recovered 24 clades, 18 clades that included some to many species of Campanula (11 of these clades included only Campanula) and five small clades, including Peracarpa and Trachelium, that were made up only of members of particular genera. Furthermore, Musschia, along with some species of Campanula, was in a clade ("Cam 01") that may be sister to everything else and the position of Jasione was uncertain, but it was probably best excluded from Campanuleae (see account above). At least hybridization seems not to be a problem in Campanuleae. At the other extreme, Liveri et al. (2020) found that the chasmophytic Campanula section Quinqueloculares was polyphyletic (like just about all supraspecific taxa in the genus), largely dividing into a Greek and a Turkish-southeast Aegean clade.
Within Lobelioideae, molecular data show that Lobelia is wildly paraphyletic, all other genera of the subfamily being emmbedded within it (Knox & Muasya 2001; Antonelli 2008, 2009; Knox et al. 2008; Lagomarsino et al. 2014; L.-Y. Chen et al. 2016; Kagame et al. 2021) - reading up the tree is dizzying both in terms of the names and the geography of the clades. Kagame et al. (2021: 18 plastid genes plus nuclear ITS) found a small clade - only moderately supported - to be sister to the rest of the subfamily, it was made up of Pratia borneensis and species of three sections of Lobelia, two Australasian and one from Africa. Givnish et al. (1995: Cyanea, 2006a, esp. 2008b) examined relationships within Hawaiian Lobelioideae, a monophyletic group. Within South American lobelioids, Centropogon and Siphonocampylus, for example, are not monophyletic (e.g. Lagomarsino et al. 2011, 2014), but there is fair support for many relationships in the whole "Siphocampylus"-Burmeistera-"Centropogon"-Lysipomia clade. Within Burmeistera, very much a recent rapid radiation, classical sectional limits break down, and although resolution still leaves something to be desired, geographically- and morphologically-circumscribed clades are becoming evident (Uribe-Convers et al. 2016b). Examining the value of targeted sequence capture, Bagley et al. (2020: 50 species (36%) included) noted quite extensive movement of taxa in the tree depending on the analysis, and although the monophyly of Burmeistera was confirmed, it was supported by relatively few gene trees - 10>%; B. xerampelina was usually sister to the rest of the genus. Lagomarsino et al. (2022) looked at relationships in this centropogonoid clade, but with a focus on Siphonocampylus and emphasizing nuclear data, and they found that relationships between capsular taxa there (capsules are plesiomorphic) were rearranged; the focus of Mansaray et al. (2025) was on subgenus Centropogon - the subgenus and two of its sections were monophyletic. Overall, there are major issues here that have to be dealt with, including extensive gene tree discordance/incomplete lineage sorting and also considerable cytonuclear discordance/hybridization. Indeed, the conflict between the chloroplast and nuclear trees (Lagomarsino et al. 2022: Fig. 4) and the gene tree discordance in subgenus Centropogon (Mansaray et al. 2025) is quite remarkable, but at least burmeisterids were not involved.
Rose et al. (2025: 101/114 extant spp, 633 nuclear locii, plastomes) looked at relationships in Hawaiian lobeliods, and recovered the relationships [[Clermontia/Cyanea/Brighamia/Delissea - fleshy fruit, Hawai'i + Sclerotheca - capsular fruit, Polynesia] [Revolutella-L. [Trematolobelia + Galeatella-L.]] - capsular fruit, Hawai'i] using nuclear data, but support for the position of Sclerotheca was not strong. Overall, this is a monophyletic group, but obviously Lobelia species are part of a paraphyletic group, Cyanea in particular and Clermontia are also paraphyletic - with plastome data, Sclerotheca was sister to all Hawaiian taxa (Rose et al. 2025: "L." above = sections of Lobelia).
Classification. A.P.G. II (2003) suggested as an option keeping Lobeliaceae separate from Campanulaceae, but the two are best combined in view of their substantial similarities (see A.P.G. III 2009 et seq.). Kolakovsky (1985, 1987) developed a classification of Campanulaceae s. str. in which carpological variation was heavily emphasized: Prismatocarpoideae had circumscissile capsules, Canarinoideae, berry-type fruits, Wahlenbergioideae capsular fruits, the fruit walls being hard, and fruits of Campanuloideae had "soft" walls and an apical, sometimes median or basal axicorn - in the last subfamily there were also frequently small appendages in the calyx sinuses. For a world checklist and bibliography, see Lammers (2007). I follow Hong and Wang (2015) for a tribal classification of Campanuloideae, although such a classification is quite possibly premature; see also Andreychuk and Odintsova (2020) for comments on the classification of Campanuloideae in the context of variation in fruit types.
Indeed, generic limits in Campanulaceae need a great deal of attention - an understatement, limits here probably being the worst of those of any major family, Campanula, Lobelia and Wahlenbergia and their relatives (i.e., most of the family) all being particular problems (see also Kilian in Kadereit et al. 2016). Lammers (2007) recognized 28 genera in Campanuleae, although a much more broadly delimited Campanula might be a reasonable solution to its extensive paraphyly; its segregate genera have been based on floral features which are unreliable guides to broad relationships (Haberle et al. 2008b; Roquet et al. 2008). C. Xu and Hong (2020) paid most attention to Campanuleae, where they found 5 small genera interspersed with 18 clades of Campanula, some including species of other genera; all these were divided into two main clades, and outside these clades were a few more species of Campanula, Jasione, Wahlenbergieae and Cyanantheae... Xu and Hong (2020) suggest how things might go in the future - assuming that the topology of the tree that they provided was confirmed, it already seemed that some 16 or so clades would need names (some were already available). Cupido et al. (2013) outline possible taxonomic solutions to the developing patterns of relationships centred on Wahlenbergia, while Hong and Pan (2012) and Q. Wang et al. (2014) suggest the generic pulverization of the Codonopsis area. The current classification of Lobelioideae is of almost no use whatsoever, and considerable expansion of Lobelia may be a course to take. Thus the monophyletic Burmeistera is embedded in a clade where Siphocampylus and Centropogon are hopelessly intermingled (most of the infrageneric groups are also para/polyphyletic), the whole being a clade coming from within a paraphyletic Lobelia (Lagomarsino et al. 2014; L.-Y. Chen et al. 2016; Kagame et al. 2021). Fleshy fruits have been used as a generic character, but they have evolved more than once... The six genera of Lobelioideae on Hawaii - and Lobelia, with two sections, is also to be found there - represent but a single introduction (Givnish et al. 2009a). Of course the sectional classification of Lobelia (Lammers 2011) necessarily will itself need a complete overhaul. Overall, very substantial changes are likely, however, it is premature to attempt any reclassification until more nuclear phylogenies become available.
Previous Relationships. Takhtajan (1997) divided Campanulaceae s.l. into four families; these, plus Pentaphragmataceae and Sphenocleaceae (for the latter, see Solanales), made up his Campanulanae. Cronquist (1989) had taken the opposite tack, recognizing a broadly circumscribed Campanulaceae that included the four families just mentioned plus some other families that are elsewhere in Asterales in his Campanulales.
Botanical Trivia. At less than 5.5 mm tall, sometimes lacking true leaves and flowering from the axils of its cotyledons, Lysipomia mitsyae (Lobelioideae) is one of the smallest eudicots known (Sylvester et al. 2016).
Thanks. I am grateful to Tatyana Shulkina and Laura Lagomarsino for helpful discussions.
[Pentaphragmataceae [[Alseuosmiaceae [Phellinaceae + Argophyllaceae]] [Stylidiaceae [Menyanthaceae [Goodeniaceae [Calyceraceae + Asteraceae]]]]]] / Core Asterales: (corolla lobes with marginal wings) [could go here].
Age. Suggested ages for this node are (91-)78(-68) Ma (Wikström et al. 2015), ca 84 Ma (Tank et al. 2015: Table S1, S2) and (132.3-)110.7(-90.1) Ma (Maurin & Smissen 2021).
PENTAPHRAGMATACEAE J. Agardh, nom. cons. - Pentaphragma G. Don - Back to Asterales
Herbs, peremmial, rather fleshy, rooting at base of stem; chemistry?; cork?; wood rayless; nodes ?; hairs with uniseriate branches; leaves two-ranked, lamina usu. asymmetric, margins ± closely serrulate/crenulate (entire); inflorescences cymose, usu. scorpioid; hypanthium +; K large, (petal-like, 2 large + 3 small) C ± deeply lobed (free), with marginal wings, commissural veins +; stamens adnate to corolla, anthers extrorse, basifixed; pollen 2-celled, small [8-21 μm], oblate-peroblate, 3-lobed, apertures between lobes, smooth, endexine lamellate only by apertures; antesepalous septae connecting ovary with hypanthium, gynoecial nectaries in cavities so formed; G [2-3], stigma capitate; integument ca 3 cells across; embryo sac protruding from micropyle; fruit baccate, K and C persisting; seeds minute, exotestal cells cuboid, inner walls lignified; endosperm starchy, chalazal haustorium 0, embryo medium-short (1/3rd); n = 54-56, x = ?
1 [list]/30. South East Asia to Malesia, esp. W. Malesia (map: from Airy Shaw 1954). [Photo - Flower.]
Chemistry, Morphology, etc.. The family is very poorly known. The micropylar haustorium is single-celled (Kapil & Vijayaraghavan 1965).
For general information, see Lammers (2006), for wood anatomy, see Carlquist (1997b), for the nectary, see Vogel (1998c), and for pollen, see Dunbar (1978) and Telleria et al. (2018).
[[Alseuosmiaceae [Phellinaceae + Argophyllaceae]] [Stylidiaceae [Menyanthaceae [Goodeniaceae [Calyceraceae + Asteraceae]]]]]: C connate; ovary inferior [?level].
Age. Estimates of the age of this node (but note topology) are (79-)73, 67(-58) Ma (Bell et al. 2010), (88-)84, 76(-72) Ma (Wikström et al. 2001) and (84-)73(-62) Ma (Wikström et al. 2015).
[Alseuosmiaceae [Phellinaceae + Argophyllaceae]]: plant woody; lamina gland-toothed; x = 8.
Age. An estimate of the age of this node (note topologies again!) is (71-)61, 56(-44) Ma (Bell et al. 2010), (73-)69, 66(-62) Ma (Wikström et al. 2001), ca 71.9 Ma (Magallón et al. 2015) or ca 74 Ma (Tank et al. 2015: Table S1, S2).
Evolution: Divergence & Distribution. The distribution of this group of families is best explained by dispersal, not continental drift (Sanmartín & Ronquist 2004).
Phylogeny. There is a possible grouping [Alseuosmiaceae [Phellinaceae + Argophyllaceae]] or [Alseuosmiaceae [Stylidiaceae [Phellinaceae + Argophyllaceae]]] (e.g. Kårehed et al. 2000; Lundberg & Bremer 2001 respectively), and although jacknife support for the position of Alseuosmiaceae is not very strong, the posterior probability for the first grouping is 1.0 (Kårehed 2002a); Wikström et al. (2015) suggested a grouping [Argophyllaceae [Phellinaceae + Alseuosmiaceae]], but with little support.
ALSEUOSMIACEAE Airy Shaw - Back to Asterales —— Synonymy: Platyspermataceae Doweld
Small shrubs to small tree; condensed and ellagitannins +, inulin?, iridoids 0; young stem with separate bundles; true tracheids +; rays narrow to broad (0 - Alseuosmia); starch-storing living fibres +; axial parenchyma 0 (+); pericyclic fibres weakly developed; cauline and foliar endodermis +; petiole bundle(s) arcuate, (filiform foliar sclereids + - Crispiloba); hairs axillary, uniseriate; lamina vernation conduplicate, margins entire to serrate; flowers (4-)5(-7)-merous; (hypanthium present), K free, valvate, C margins with wings (hardly - Platyspermation), margins fringed, erose or entire; A adnate to C, anthers ± basifixed; pollen (in tetrads); G [2, 3], nectary +, stigma barely expanded to capitate; ovules 2 or more/carpel; fruit baccate, calyx usually persistent; exotesta little thickened, lignified, mesotesta persistent; ?haustoria; n = 9 [Alseuosmia], x = 9.
5[list]/10: Alseuosmia (5). New Guinea, E. Australia, New Zealand, New Caledonia (map: from van Balgooy 1993).
Age. Benítez-Villaseñor et al. (2023: Als. + Platy) suggested that the crown-group age of Alseuosmiaceae was 17.4-14.7 Ma.
Evolution: Divergence & Distribution. Woodiness here may also be derived from a herbaceous ancestry (Dickison 1989a).
Ecology & Physiology. In New Zealand Alseuosmia pusilla appears to mimic (Batesian mimicry) the leaves of Pseudowintera axillaris - the latter have sequiterpene dialdehydes etc. and taste rather nasty, as do other Winteraceae (Yager et al. 2016). Shepherd et al. (2020) note the variable leaf morphology of A. banksii which may be involved in mimicry with a variety of other taxa. See also Boquila trifoliata (Ranunculales-Lardizabalaceae) and Loranthaceae for other cases of possible mimicry.
Plant-Animal Interactions. Platyspermation and other Alseuosmiaceae on New Caledonia seem commonly have galled fruits or flowers.
Chemistry, Morphology, etc.. Ellagitannins are reported from Alseuosmia (Kårehed 2006 for references); this should be checked. Most Alseuosmiaceae have rayless wood, living mature fibres with stored starch (Dickison 1986b), and the stem has an endodermis. Uniseriate hairs in Platyspermation are not restricted to the leaf axils, although they are particularly dense there, rather, they cover the whole plant. Their persistent, reddish bases look rather like glands, hence, perhaps, the past inclusion of the genus in Rutaceae.
There are tanniniferous cells in the flower. The margins of the corolla lobes of Platyspermation have narrow flanges and papillae; the corolla is only shortly tubular, the lobes being rather spreading (buzz pollination?). The pollen of Alseuosmia linariifolia is described as being tricolpate, with an ectexine made up of a thick, tubercular tectum and massive, spherical columellae (Polevova 2006); whether this can be generalised to the family is unclear (see also Kårehed 2006).
Some details of vegetative anatomy are taken from Paliwal and Srivastava (1969), Dickison (1989a) and Gregory (1998), of pollen, from Telleria et al. (2018), and of testa anatomy from Nemirovich-Danchencko and Lobova (1998) and Takhtajan (2000). The embryology is poorly known. See Kårehed (2006) for general information.
Phylogeny. Platyspermation is strongly associated with other Alseuosmiaceae (Lundberg & Bremer 2001), and may be sister to the rest of the family (see also Tank & Donoghue 2010).
Classification. Generic limits are in some dispute (Tirel 1996).
Previous Relationships. Genera now included in Alseuosmiaceae have previously been placed in Caprifoliaceae, Rubiaceae, Rutaceae, Ericaceae, Epacridaceae, etc. (see e.g. Shepherd et al. 2010: Table 1). For Dickison (1989a), anatomical features also suggested relationships with genera in Rosaceae and Saxifragaceae. Although the family was recognized by Takhtajan (1997), it was included in his Hydrangeales, and of course previously it was thought that Hydrangeaceae and Saxifragaceae were close...
[Phellinaceae + Argophyllaceae]: cork subepidermal; pollen (spiny), with rugulose exine; style short; ovules apical, apotropous.
Age. Estimates of the age of separation of these two families are (68-)63, 62(-57) Ma (Wikström et al. 2001) and (124.7-)102.3(-81.4) Ma (Maurin & Smissen 2021).
PHELLINACEAE Takhtajan - Phelline Labillardière - - Back to Asterales
Trees (monocalous); hmoerythrina alkaloids + [a 7-C ring], iridoids?; true tracheids +; rays very broad [to 14 cells across, esp. monocaulous taxa]; sclerenchyma surrounding leaf veins; petiole bundles annular; cuticle waxes as platelets and rodlets; lamina margins serrate (entire); plant dioecious; bracteoles 0; flowers small, 4-6-merous; K connate basally, ± open, C free, apex incurved, ?commissural veins; nectary 0?; staminate flowers: filaments shorter to somewhat longer than the anthers>; pollen echinate; pistillode +; carpelate flowers: staminodes +; G superior, [2-5], style ± 0, stigmas large, lobed; ovule 1/carpel; fruit a drupe, stones separate; testa ?; endosperm haustoria?; n = 17, x = ?
1 [list]/10. New Caledonia.
Evolution: Divergence & Distribution. Phellinaceae are reported from Miocene deposits in New Zealand (Maurin & Smissen 2021).
Genes & Genomes. There is evidence of a genome duplication in Phelline (Crowl et al. 2016).
Chemistry, Morphology, etc.. The guard cells are huge, with inner and outer stomatal ledges (Baas 1975).
Telleria et al. (2018) suggest that the spines on the pollen grains are formed by the fusion of baculiform elements. The ovules are reported as being hemitropous to campylotropous, but Phelline is embryologically poorly known.
See also Kårehed et al. (2000) and Barriera et al. (2006) for much additional information, Baas (1975) for wood anatomy (it appeared to be extremely primitive) and Lobreau-Callen (1977) for pollen.
Previous Relationships. Takhtajan (1997) placed the family in his Icacinales, describing the leaves as being mostly estipulate, while Cronquist (1981) placed it in his Aquifoliaceae (adjacent to Icacinaceae), both were in his Celastrales.
ARGOPHYLLACEAE Takhtajan - Back to Asterales —— Synonymy: Corokiaceae Takhtajan
Shrubs, (divaricately-branched - Corokia); gallic acid +, inulin?; vessel element perforation plates with 10-20 bars, tracheids +/0, helical thickenings on tracheary elements +/0; axial parenchyma scarce, diffuse, multiseriate rays narrow, heterocellular; gum deposits +, crystals 0; (nodes 1:1, 5:5); petiole bundles arcuate; hairs T-shaped, multicellular, with slits over the stalk cell; lamina vernation supervolute-curved [Corokia macrocarpa], margins entire; flowers 4-5(-8)-merous; K valvate [always?], C basally connate, with adaxial fringed ligule, (and marginal wings); pollen grains 3-celled; G (1) [2, 3(-5)], (semisuperior), nectary + [Corokia], stigma punctate, lobed, clavate or capitate-lobed, wet; ovule 1 or several/carpel, integument 6-15 cells across, nucellus base massive; fruit a septicidal + septifragal capsule [Argophyllum] or drupe [s. str. - Corokia]; exotestal cells with inner walls massively thickened and lignified [Argophyllum] or all walls somewhat thickened [Corokia]; endosperm hemicellulosic, (embryo medium/?long, cotyledons very short), suspensor uniseriate; n = 9, x = 9.
2 [list]/24: Argophyllum (18). S.W. Pacific, including Rapa (Lautea, = Corokia). Map: from van Steenis and van Balgooy (1966). Photo: Corokia Flower © Gardenweek.org.]
Age. Crown-group Argophyllaceae are (46.0-)18.0(-11.3) Ma (Maurin & Smissen 2021: c.f. other estimates...) or 18.5-16.3 Ma (Benítez-Villaseñor et al. 2023).
Evolution: Divergence & Distribution. Biogeographic scenarios based on vicariance events end up with unrealistically old ages (Maurin & Smissen 2021). Argophyllum is reported from Miocene deposits in New Zealand (Maurin & Smissen 2021).
Argophyllaceae attained their present distribution in the Pacific by fairly recent long-distance dispersal. Thus Corokia originated in Australia, moving into the Pacific only within the last ca 3.5 Ma (Maurin & Smissen 2021); it is interesting that its distribution there is highly disjunct - ?birds.
Ecology & Physiology. There are a number of nickel-accumulating species of Argophyllum on New Caledonia.
Chemistry, Morphology, etc.. Septate fibres and vascular tracheids are present (Patel 1973; Noshiro & Baas 1998), but the significance of this is unclear. The guard cells in Argophyllaceae are raised above the epidermis (Kårehed et al. 2000).
There are tanniniferous cells in the flower, as in Alseuosmiaceae. The pollen is like that of Cornaceae, with complex H-shaped endoapertures (Ferguson 1977).
See also Eyde (1966) and Kårehed (2006) for general information, Gornall et al. (1998: as Escalloniaceae) for vegetative anatomy, Carlquist and Olson (2021) for vascular anatomy, Telleria et al. (2018) for pollen, Mauritzon (1933) for a little embryology and Bhatnagar and Pandey (2021) for the embryology, etc., of Corokia, and Lobova (1997) for testa.
Previous Relationships. Along with Cornaceae, Argophyllaceae were placed in Hydrangeales by Takhtajan (1997), while Cronquist (1981) included them in his very heterogeneous Grossulariaceae.
[Stylidiaceae [Menyanthaceae [Goodeniaceae [Calyceraceae + Asteraceae]]]]: herbs common.
Age. The age of this node is around 76.5 Ma (see Magallón et al. 2015), (82-)71(-61) Ma (Wikström et al. 2015) or ca 78 Ma (Tank et al. 2015: Table S1, S2).
Evolution: Divergence & Distribution. Diversification rates increased here around (77.1-)76.8(-76.5) Ma and twice more at nodes within this clade as recently as the middle Eocene ca 45 Ma (Magallón et al. 2018).
Taxa with (much) elongated synergid cells are scattered in some Stylidiaceaem, Goodeniaceae and Asteraceae.
STYLIDIACEAE R. Brown, nom. cons. - Back to Asterales
Herbs; young stem with separate bundles; nodes 1:1; lamina ± linear, margins entire, petiole 0; C imbricate; nectary +; A 2, anthers extrorse; pollen colpate; integument 4-6 cells across; synergid cells elongated; embryo "minute"; x = 9 (?8), genome duplication.
3 [list]/245 - 2 subfamilies below. Scattered in South East Asia to New Zealand, S. South America, but mostly Australia.
Age. Estimates of the age of crown-group Stylidiaceae are (80-)71, 65(-57) Ma (Bell et al. 2010) and (78-)73, 70(-65) Ma (Wikström et al. 2001).
1. Donatioideae B. Chandler - Donatia J. R. Forster & G. Forster —— Synonymy: Donatiaceae B. Chandler, nom. cons.
Dwarf cushion herbs; iridoids?, tanniniferous; cork cortical?; mucilage cells +; stomata also paracytic; hairs uniseriate, axillary; lamina terete; flowers solitary, terminal; K 3-7, free, C 5-10, free; A (3), free; nectary annular; pollen smooth, 3-colporate, nuclei?; G [2-3], styles separate, somewhat recurved, stigmas capitate; hypostase +; fruit indehiscent; seed coat?; embryo suspensor short; n = 24.
1/2. New Zealand, Tasmania, S. South America. Photo: Habit, Flower © Univ. of Tasmania.]
2. Stylidioideae Kittel
Herbs (annuals, climbers), cushion plants; cork also outer cortical; vascular bundles closed, scattered or in a single ring; cambium develops just inside the endodermis, storied, secondary tissue developing only towards the inside, interxylary phloem +; wood rayless [?always]; vessel elements with simple perforation plates; hairs glandular; leaves pseudoverticillate or in rosettes, lamina linear, bifacial/± terete, with axillary hairs; infloresecence a raceme [etc.], flowers resupinate [median sepal abaxial], (split-monosymmetric); K (6), connate, C connate, 4 in two pairs, or 4 + labellum, early tube formation, often with coronal appendages, (spurred - S. calcaratum), or 5-6(-9); nectary as paired ad-/abaxial lobes (1 lobe); A completely adnate to style [= gynostemium], anther thecae set end to end, (apically connate); pollen grains prolate [?level], (3 nucleate), 3-8-colpate; G [2] (adaxial much reduced), placentation free-central, stigma small, dry; fruit dahiscing laterally from the apex, septicidal (indehiscent); seed exo-endotestal, exotestal cells sclerosed; embryo often with single cotyledon; n = 5-16, protein bodies in nucleus.
2/240: Stylidium (220-300). Mostly Australia, especially Western Australia, also Sri Lanka to South East Asia, Malesia, New Zealand, and S. South America. Map: see Erickson (1958) and Australia's Virtual Herbarium (cpnsulted xi.2012). Photo: Flower.
Age. Diversification in Stylidioideae has been dated to ca 39 Ma (Wagstaff & Wege 2002) and 57.1-51.9 Ma (Benítez-Villaseñor et al. 2023: Forst. + Styl).
Evolution: Divergence & Distribution. Species distributions/relationships of Stylidium within Australia - there are over 200 species in Western Australia alone - seem to fit the peripheral vicariance pattern. The genus is now found on the periphery of the continent after the drying out of the centre, a process that began in the Eocene (Nge et al. 2021c). Armbruster et al. (1994) found that co-occurring species were more dissimilar than expected by chance (see also Rhinanthus and Burmeistera). Species in the same locality differed in both corolla tube and column lengths; even the pollen varied in colour. Since different species of Stylidium place the pollen in different locations on the pollinator, sympatry of these species is common (Armbruster et al. 1994; Armbruster 2014).
Ecology & Physiology. There are suggestions that Stylidium may be carnivorous. Insects are trapped by the mucilage-secreting glandular hairs on the flowers and inflorescences, and the hairs also show yeast-extract stimulated protease activity. The plants grow in acid, nutrient-poor soil like other carnivorous plants (Utricularia, Drosera), however, uptake of nutrients by the plant from the insects has yet to be demonstrated (Darnowski et al. 2006). Indeed, Nge and Lambers (2018) were unable to find evidence of carnivory in their comparison of δ15N signatures of Stylidium with those of co-occurring carnivorous and non-carnivorous plants.
Pollination Biology. In many Stylidioideae (Stylidium s.l.) the two stamens are adnate to the style, the extrorse anthers being borne near the stigma. The whole complex (a gynostemium, sometimes called a column), is sensitive to touch, moving rapidly when brushed by the pollinator, in some species the column being hinged; this movement can be repeated (Findlay & Findlay 1875, 1989 and references). As the column of Stylidium hits the pollinator, it adjusts to the body surface of the latter, so placing pollen in a place that allows the insect to be an effective pollinator (Armbruster et al. 1994). These authors found that species of Stylidium in the Perth area were pollinated mostly by solitary bees and bombyliid flies, and as Armbruster (2014: p. 7) noted, the flower is "phenotypically specialized to be an excellent ecological generalist" (see also Armbruster 2017). Ronse de Craene (2010) described the flower of S. graminifolium as being obliquely monosymmetric at maturity, the corolla and parts inside being illustrated as having rotated ca 60o relative to the calyx, and there is a single abaxial (adaxial in the text) nectary. In Levenhookia the gynostemium is held under tension in the hooded labellum, flipping only when the latter is disturbed; the gynostemium moves only once, not being able to reset. Furthermore, the stigmatic lobes are finger-like, and an upper finger-like lobe may develop/become apparent only after the style flips (Lowrie & Conran 2011; see also Wege 2020). Wege (2020) noted that the labellum of Levenhookia could be ventral, sometimes dorsal, or rarely lateral by the twisting of the pedicel; the abaxial position seems to be commonest.
Oreostylidium subulatum seemed to have rather "primitive" flowers - more or less polysymmetric, not sensitive to touch, etc. - and it came out basal in Stylidioideae in morphological analyses (Laurent et al. 1999). However, molecular analyses showed (with strong suppport) that it was well embedded within Stylidium. It is also the only member of Stylidioideae from New Zealand, and its white and radially symmetrical flowers are rather similar to those that have evolved in other families in that bee-poor island; the flowers of O. subulatum are an example of "reduction and extreme paedomorphosis" (Laurent et al. 1999: p. 302).
Genes & Genomes. A genome duplication event ca 72.5 Ma (STADα) is to be associated with the common ancestor of Stylidiaceae (Landis et al. 2018; Jardine et al. 2022).
Chemistry, Morphology, etc.. In Stylidioideae the cambium may develop beneath the endodermis; xylem, and sometimes also phloem, is produced towards the inside, and at most cork to the outside (Carlquist 1981a, 2013; see also Mullenders 1947). In older stems of Donatia the cortex is very thick, and the vascular tissue forms a narrow cylinder in the center (Chandler 1911). The leaves of Donatia are very small, and their venation is acrodromous. For anatomical differences between Donatioideae and Stylidioideae, see Repson (1953).
The floral morphology is a little complicated. The fertile stamens are the adaxial pair. Carolin (1960b) drew the anthers of Donatia as being introrse. See also Erbar (1992) for floral development, which needs more study in the family as a whole. The pollen of at least some Stylidiaceae has a very distinctive inner ectexine that lacks columellae but is permeated by numerous sinuous channels (Polevova 2006). Monocotyly is reported to be quite common in Stylidioideae (Carlquist 1981b).
The proembryo in Donatia is ovate, the suspensor is made up of short cells, but in Stylidioideae it is long, and is made up of cells that are longer than broad (Philipson & Philipson 1973), as in other Asterales (Tobe & Morin 1997).
For general information, see Carolin (2006), Carlquist (1969a), Carlquist and Lowrie (1989: Stylidioideae), Australian Plants 27(215). 2013, and Glenny (2009: Forstera); some anatomical details can be found in Thouvenin (1890), for embryology, see Rosén (1935) and Subramanyam (1950a, 1951a, 1970), for placentation, see Carolin (1960a), for protein bodies, see Thaler (1966), and for the testa anatomy of Stylidium, see Tobe and Morin (1996).
Phylogeny. Donatia is sister to the rest of the family, but there is some uncertainty over further relationships. Laurent et al. (1999) found that Forstera s.l. was sister to the remaining part of the family in combined molecular and morphological analyses, in a rbcL + ndhF analysis it was grouped with Levenhookia, but both positions had only weak support; Wagstaff and Wege retrieved the former topology in an analysis based on variation in ITS and rbcL. In gross floral morphology Donatia and Forstera are similar, both having basically radially symmetrical flowers that are whitish in colour.
Previous Relationships. Stylidiaceae have been treated as two families in Stylidiales (Takhtajan 1997) or merged in one family (Philipson & Philipson 1973). A.P.G. II suggested as an option keeping Donatiaceae and Stylidiaceae separate, although the two can reasonably be combined (e.g. Lundberg & Bremer 2003; A.P.G. III 2009, etc.).
[Menyanthaceae [Goodeniaceae [Calyceraceae + Asteraceae]]] / MGCA Clade: fructans, caffeic acid +; cauline stele with separate vascular bundles; vessel elements with simple perforation plates; inflorescence with a terminal flower, single flowers and then cymes below; C connate, early tube formation, with strong marginal [commissural] veins joining the median near the apex; A adnate to C; tapetal cells bi- or multinucleate; pollen grains bicellular; G [2], stylar bundles branched, stigma papillate; integument multiplicative, >10 cells thick, antiraphal vascular bundle proceeding to the micropyle, hypostase +; endosperm haustoria 0, embryo long; x = 9.
Age. Estimates of the age of this node vary - (71-)63, 58(-48) Ma (Bell et al. 2010), ca 68.3 Ma (Magallón et al. 2015), (73-)69, 65(-61) (Wikström et al. 2001), 72.2 or 73.7 Ma (Tank et al. 2015: Table S1, S2), (135-)82.5(-66) Ma (Jabaily et al. 2014, q.v. for other dates, some older) and (76-)65(-56) Ma (Wikström et al. 2015). Barreda et al. (2015) offer a series of estimates, at (103.3-)96.5, 69.1(-65.4) Ma they are mostly older, while (95.8-)84.5(-71.6) Ma is the estimate in Panero and Crozier (2016: Table S1), (117.7-)99.4(-82.7) Ma in Maurin and Smissen (2021) and ca 86.0 Ma in G. Zhang et al. (2024).
Evolution: Pollen of all families of this clade - and of some subfamilies of Asteraceae - had appeared by the Oligocene, and has been found in many places that are fragments of the Gondwanan continent (Barreda et al. 2010a).
The separate vascular bundles in the stem may be connected with the herbaceous habit that is so common here. For other characters common to this clade, see Anderberg et al. (2006, c.f. vessel perforation plates). Vegetatively and florally Menyanthaceae are rather different from many other Asterales, however, both Menyanthaceae and Goodeniaceae have corolla lobes with marginal wings, here placed as apomorphies for both groups (or they could be a synapomorphy for the whole clade, being lost later). Presence of sclereids and multi-nucleate tapetal cells may be additional synapomorphies (Lundberg 2009, q.v. there and Katinas et al. 2016 for more possible synapomorphies).
Chemistry, Morphology, etc.. The androecium is spirally initiated in some Menyanthaceae, Asteraceae and Goodeniaceae - and also Araliaceae (Erbar 1997). For inflorescence morphology and evolution, see Pozner et al. (2012).
MENYANTHACEAE Dumortier, nom. cons. - Back to Asterales
Aquatic or marsh herbs; flavonols only +, little oxalate accumulation, tannin 0; cork?; vascular cambium 0[?]; vascular bundles often scattered; stem with endodermis; nodes 3:3, 5:5; intercellular canals +; branched sclereids +; petiole bundles arcuate to annular or scattered; leaves (two-ranked), simple, lamina vernation involute, secondary veins palmate, margins usu. with hydathodal glands, colleters +, leaf base broad, petiole margins ± winged; flowers distylous (not); K basally connate, C lobes with marginal wings and/or with (marginal) fimbriae; (oppositipetalous fringed scales +); anthers sagittate; tapetal cells with many fused nuclei; pollen (syncolporate; isolated triangular polar areas), surface striate, etc.; G ± superior, placentation parietal, style (0), with 2-several vascular bundles, stigma bilobed-spathulate-flabellate, wet; ovules many/carpel; integument 3-11 cells across, vascularized; fruit a loculicidal capsule; seeds many, exotestal cells with outer walls thickened, (meso- and endotestal cells sclerotic); endosperm oily; n = 9 (17), x = 9, protein bodies in nuclei, nuclear genome [1 C] (0.054-)1.403(-36.485) pg; chloroplast rpl2 intron 0, ψrps19 pseudogene 0 [?level].
6 [list, to tribes]/58. World-wide. Map: from Heywood (1978), modified by Hultén (1971); see also Australia's Virtual Herbarium (consulted xii.2012). [Photo - Flower, Collection, Flower.]
Age. Estimates of the age of crown-group Menyanthaceae are (58-)54, 51(-47) Ma (Wikström et al. 2001), (57.8-)55.4(-53.0) Ma (Tippery et al. 2008), (60-)47, 44(-31) Ma (Bell et al. 2010), (88.6-)63.4(-36.1) Ma (Maurin & Smissen 2021) and 50.9-44.4 Ma (Benítez-Villaseñor et al. 2023).
For fossil pollen, see Barreda et al. (2010a).
1. Menyantheae Dumortier
Leaves erect, (trifoliolate), margin crenate; inflorescence several-flowered; pollen grains large, long-prolate, striate; integument becoming 8-10(-?15) cells across, with vascular bundles; antipodal cells not persisting; seeds smooth, exotestal cells palisade, narrowly elongated, mesotesta 6-7 cells across, with airspaces, endotesta palisade, sclereidal; endosperm cellular; embryo small.
2/2. Temperate North Hemisphere.
Age. Crown-group Menyantheae are around 36 Ma (Tippery et al. 2008).
2. Villarsieae Horaninow
(Photosynthetic root tubers - Nymphoides aquatica); leaves floating or erect, margins entire to dentate or subcrenate; plant (dioecious); inflorescence several-flowered to flowers single; pollen grains ± oblate, 3-celled [N.]; G (semi-inferior); ovules 1-many/carpel, ?hypostase; fruit (berry - Liparophyllum), (septi- and loculicidal capsule); seeds (carunculaate); exotestal cells polygonal/anticlinal walls sinuous or indistinct, smooth or with a variety of projections, hairs or groups of papillae, hair-like appendages in endothelium [N.].
4/56: Nymphoides (40). Aquatics, more or less world-wide.
Age. Crown-group Villarsieae are estimated to be ca 30 Ma (Tippery et al. 2008).
Evolution: Divergence & Distribution. Has the superior ovary of Menyanthaceae with its parietal placentation been derived from a more or less inferior ovary with axile placentation (c.f. Pittosporaceae - Apiales)?
Pollination Biology. For heterostyly in Nymphoides, see Barrett and Shore (2008). This seems to be a very labile character, with the ancestral condition perhaps being heterostyly, but with reversals to homostyly and then reversals back (Tippery & Les 2011).
Genes & Genomes. For the missing chloroplast rpl2 intron, see Downie et al. (1991b).
Chemistry, Morphology, etc.. Kasinathan and Kumari (2001) thought that the leaves of Nymphoides were opposite, indeed, plant architecture in that genus is complex (Richards et al. 2010).
Eichler (1878) drew the flower of Menyanthes with an oblique plane of symmetry. The vascular anatomy of the flower implies a basic monosymmetry - the lateral corolla traces are fused (Wood & Weaver 1982). There are sometimes fringed scales, "staminodes", on the corolla tube alternating with the stamens. Johri et al. (1992) suggested that the endosperm stores starch.
For general information, see G. Kadereit (2006), for heterostyly in Nymphoides (and seeds), see Lobato-de Magalhães and Martínez (2019), for floral development, see Erbar (1997), for pollen, see Nilsson (1973), for embryology, see Stolt (1921), Maheswari Devi (1963) and H.-r. Kim and Heo (2024: esp. Menyanthes), and for seed morphology, see Chuang and Ornduff (1992).
Phylogeny. Relationships within Menyanthaceae have been clarified by Tippery et al. (2006, 2008) and Tippery and Les (2008); [Menyanthes + Nephrophyllidium] are sister to the rest of the family (see also Du & Wang 2014; Du et al. 2016) while Villarsia is very much paraphyletic (for which, see also Njuguna et al. 2019). Many relationships within Nymphoides were initially poorly supported (Tippery & Les 2011), although improving in the study by Njuguna et al. (2019); the former noted extensive conflict between nuclear and plastome phylogenies, perhaps the result of hybridization.
Classification. Tippery and Les (2009) made the genera of the family agree with the phylogeny.
Previous Relationships. The iridoids of Menyanthaceae differ chemically from those of Gentianaceae, in which Menyanthaceae used to be included - placentation, etc., are similar. Menyanthaceae were placed in Solanales by Cronquist (1981). Branched sclereids and air canals are similarities between Menyanthaceae and Nymphaeaceae, but both are aquatics - and are hardly much related.
[Goodeniaceae [Calyceraceae + Asteraceae]]: acetylenes +; secondary pollen presentation + [flowers protandrous, anthers connivent at dehiscence, style elongates after pollen deposition]; pollen tectum spinulose, microperforate, columellae large, distally bifurcating; stigma dry, papillae all similar, involved in both pollen presentation and reception; integument not vascularized [?all]; K persistent in fruit.
Age. K.-J. Kim et al. (2005) date this node to (80-)64.5(-49) Ma, Tank et al. (2015: Table S1, S2) to around 59.8 or 56.1 Ma, Magallón et al. (2015) to about 57 Ma, Wikström et al. (2001: c.f. topology) to (43-)50, 44(-41) Ma, Naumann et al. (2013) to 52.4 Ma, Bell et al. (2010: also c.f. topology) to (50-)44, 40(-30) Ma, Xue et al. (2012) to only 39.7-39.0 Ma, and Wikström et al. (2015) to (63-)55(-49) Ma, although other recent estimates are much older, some (120-)78(-62) Ma to (130-)113(-66) Ma (Jabaily et al. 2014), ca 96.7 Ma (Denham et al. 2016), (86.9-)75.2(-63.6) Ma (Panero & Crozier 2016), (89.3-)78.9(-70.6) Ma (Maurin & Smissen 2021) and ca 77.7 Ma (G. Zhang et al. 2024: Fig. 4).
Evolution: Divergence & Distribution. DeVore et al. (2007) comparse aspects of pollen wall ultrastructure of the three families. Although secondary pollen presentation occurs throughout this clade, there is considerable variation in the details of how it is done (Leins & Erbar 2003b, 2006 and references; El Ottra et al. 2023), so it is perhaps debatable whether or not it should be an apomorphy (see also Katinas et al. 2016).
Study of early capitulum development in Arnaldoa macbrideana (Asteraceae-Barnadesioideae) suggests that the capitulum there is built up of partial inflorescences with cymose branching, so perhaps linking the racemose heads of Asteraceae with the apparently rather different inflorescences of many Calyceraceae and Goodeniaceae (Leins & Erbar 2003b, see their polytelic thyrses). Acicarpha is the only Calyceraceae with n = 8, and it is also the only member of that family with a possibly plesiomorphic condensed spicate inflorescence (DeVore 1994). For a comparison of the pollen of the three families, see DeVore et al. (2007), for chromosome numbers see Semple and Watanabe (2009).
GOODENIACEAE R. Brown, nom. cons. - Back to Asterales
Herbs (woody, arborescent); O-methyl flavonols only, alkaloids +; cork also cortical; (vessel elements with scalariform perforation plates), bordered pits +; (medullary vascular bundles +); nodes 1:1 (3:3, 5:5); branched sclereids +; indumentum variable, hairs often minutely warty; lamina margins entire to toothed; flowers split-monosymmetric; C induplicate-valvate, lobes with marginal wings; A basifixed; pollen (with intercolpar depressions); nectary usu. 0; G also [4], (placentation ± basal), style curved, vascular bundles 3-4, branching apical, branches divergent ["broccoli-like"], apical hairy pollen-collecting indusium and stylar cup, stigma initially included, bilobed; integument 6-20 cells across, hypostase +; synergids long, hooked, antipodal cells persistent; fruit dehiscing laterally, septicidal (and loculicidal); testa 7-14 cells thick, usu. with vascular bundle ± encircling seed, exotestal cells usu. palisade (crystalliferous), all walls (esp. inner) thickened, (hypodermal layers lignified); endosperm well developed, haustoria 0; filamentous suspensor 0; n = 9, x = 9, nuclear genome [1C] (0.069-)1.562(-35.202) pg; rpl16 intron missing.
7/471 [list] - three groups below. Very largely Australian, Scaevola alone pantropical. Map: esp. Leenhouts (1957b, excluding Scaevola).
Age. Crown-group Goodeniaceae are estimated to be (90-)67.3(-53) Ma (Jabaily et al. 2014) or 86.7-82.6 Ma (Benítez-Villaseñor et al. 2023).
1. Dampiera, etc. R. Brown
± Shrubby (rostte herbs); anthers connate; (pollen in planar tetrads, surface perforate-reticulate - Leschenaultia), (surface rugulate); stigma not protruding past the indusium; x = 9.
Age. The crown-group age of this clade is around (76-)59.2(-44) Ma (Jabaily et al. 2014).
3/97: Dampiera (66), Leschenaultia (20). Australia (S. New Guinea).
[Brunonieae + Goodenieae]: pollen microspinulose, spines acute, endapertures with distinct borders; stigma protruding past the indusium.
Age. The age of this node is (68-)52.1(-40) Ma (Jabaily et al. 2014).
2. Brunonieae G. Don - Brunonia australis R. Brown —— Synonymy: Brunoniaceae Dumortier, nom. cons.
Herbs; inflorescence capitate, involucrate; K with filamentous hairs, C ± polysymmetric, commissural veins 0, lobes lacking marginal wings; pollen with colpar ledges + [= exine ridges on inner margin of colpi]; G superior, stylar hairs forming brush; ovule single, basal, erect; fruit indehiscent, achene/nut; testa thin-walled, compressed; endosperm 0, cotyledons massive; x = ?
1/1. Throughout Australia.
3. Goodenieae Dumortier / core Goodeniaceae —— Synonymy: Scaevolaceae Lindley
Herbs, annual to perennial, to shrubs; (C spurred), (lobes lacking marginal wings); A (connate), adnate to base of C; pollen (with extexine bridges over pore), surface (striate/rugulate); (G ± superior); (fruit a drupe), (capsule - Velleia s. str.); (seeds winged); (anticlinal exotestal walls sinuous); n = 8 (7).
3/373: Goodenia (251), Scaevola (100). Throughout Australia, to New Zealand, Chile and China; Scaevola pantropical, with the coastal S. taccada in the E. and C. Pacific and Indian Oceans and S. plumieri in the W. Indian, E. Pacific and the Atlantic oceans (from van Balgooy 1975). [Photo - Flower.]
Age. The age of crown-group Goodenieae is estimated to be (48-)37.1(-27) Ma (Jabaily et al. 2014).
Evolution: Divergence & Distribution. For records of fossil pollen, see Barreda et al. (2010a); Goodeniaceae were quite widespread on Gondwana.
Jabaily et al. (2014) discuss ideas about the origin (place equivocal) and time (quite a spread of ages) for the origin of Goodeniaceae.
Currently the family is largely restricted to Australia, Scaevola being the only widley distributed genus. There seem to have been six movements of Scaevola out of Australia, three reaching Hawai'i, where the genus is represented by the widespread S. taccada (= S. sericea), the tetraploid S. glabra, the only member of that lineage, and a clade of eight diploid species that may be derived from the American S. plumieri. Some diversification in Australian Scaevola may be associated with the aridification of the Nullarbor Plain some 14-13 Ma separating eastern and western clades (Crisp & Cook 2007).
Pollination Biology & Seed Dispersal. For the diversity of pollen presentation devices in the family, see Erbar and Leins (1988) and Leins and Erbar (2003b, 2010). In nearly all species the pollen is initially enclosed by the indusium, and is pushed out by the elongating style; the pollen of Brunonia, with its capitate inflorescence and flowers with straight styles, is held in a brush formed by stylar hairs.
The seeds of many Goodenieae in particular are myrmecochorous (Lengyel et al. 2009, 2010), others have circumferential wings that are mucilaginous when wetted (Jabaily et al. 2012).
Plant-Bacterial/Fungal Associations. There is some discussion about mycorrhizal associations in Goodeniaceae which are apparently sometimes ectomycorrhizal (Brundrett 2017a; Tedersoo 2017b; Tedersoo & Brundrett 2017 for literature, etc.).
Genes & Genomes. Pascual-Díaz et al. (2021) suggewst that the plastome here is very variable, with many repeats the intergenic regions with low GC, etc..
The mitochondrial genes cox1, atp1 and matR showed massive divergence (Barkman et al. 2007: Scaevola only sampled).
Chemistry, Morphology, etc.. The cortical bundles in the stem sometimes reported for the family are leaf traces.
For a morphometric analysis of corolla shape in core Goodeniaceae, see Gardner et al. (2016); slit-monosymmetric fan flowers are rather distinct from other other corolla "types". The lateral veins of the corolla of Brunonia unite in the receptacle (Erbar 1997).
For a general account of most of the family, see Fl. Austral. vol. 35 (1992); see also Carolin (1978, 2006) for general variation and morphology, Gustaffson et al. (1997) for pollen morphology, Subramanyam (1950a) for embryology, Carolin (1966) for seeds and fruits, and Erbar and Leins (1988) and Cave et al. (2010) for the floral development of Brunonia.
Phylogeny. Relationships within Goodeniaceae are becoming fairly well understood (Gustafsson 1996a; Gustafsson et al. 1996; Jabaily et al. 2010, esp. 2012). There are two main clades, one includes Leschenaultia and allies, and the other Scaevola, a paraphyletic Goodenia and allies. Within the latter clade Brunonia is sister to the rest, and Gardner et al. (2015), using genome skimming (some conflict between relationships suggested by nuclear and those by chloroplast genes) found a strongly supported set of relationship in which Scaevola s.l. was sister to the rest, and again Goodenia was paraphyletic; some nodes in Goodenia have remained difficult to resolve (e.g. Jabaily et al. 2018: data from all three compartments; Shepherd et al. 2020), perhaps because of rapid radiation. Howarth et al. (2003) looked at relationships in Scaevola and found (rather low support) that the small section Enantiophyllum may be sister to the rest of the genus.
Classification. Generic limits around Goodenia are difficult and the limits of the genus may either have to be severely circumscribed (Jabaily et al. 2012; Gardner et al. 2015), or extended, as they have in the comprehensive study by Shepherd et al. (2020), q.v. for discussion. Most of the numerous features in which Brunonia differs from other Goodeniaceae are autapomorphies (see above), and some of these features might seem to suggest relationships with Asteraceae (Gustafsson 1996a), but clearly the exclusion of Brunonia from Goodeniaceae, as by Cronquist (1981), makes this family paraphyletic (Jabaily et al. 2012).
Shepherd et al. (2020) provide an infrageneric classification for Goodenia - 3 subgenera and 11 sections.
Thanks. I am grateful to Mats Gustafsson for comments.
[Calyceraceae + Asteraceae]: inflorescence capitate, involucrate, with cymose units; flowers sessile, rather small, polysymmetric; C tubular, deeply lobed, commissural veins connate; A adnate to C, anthers postgenitally ± connate, filament collar +; pollen prolate, shape of colpus end equivocal, spines blunt, short, columellae [in endosexine] evenly spaced, internal foraminae, caveae, internal tectum 0, foot layer continuous, pollenkitt +; stylar vascular bundles branching near apex, branches parallel, pollen presented on stigma; fruit a cypsela/achene, K persistent, modified, involved in dispersal; genome duplication [tetraploidy]; x = 8/9.
Age. These two clades diverged an estimated (49-)45.5(-42) Ma (K.-J. Kim et al. 2005), ca 48.8 Ma (Tank et al. 2015: Table S1, S2), ca 49.3 Ma (Magallón et al. 2015), ca 51 Ma (K. Bremer et al. 2004a), (56-)49(-47) Ma (Wikström et al. 2015), (61-)54.4(-49) Ma or older (Jabaily et al. 2014), or as much as (80.1-)69.5(-59) Ma (Panero & Crozier 2016), ca 76.5 Ma in Denham et al. (2016) and ca 74 Ma (C.-H. Huang et al. 2016) - or only ca 55 Ma in the latter. Other estimates: (90-)79(-72) Ma (Keeley et al. 2021), ca 83 Ma (C. Zhang et al. 2021), (39.0-)28.3(22.1) Ma (Brignone et al. 2023a: Fulcadea-Barnadesioideae outgroup - check) and ca 74.8 Ma (G. Zhang et al. 2024: Fig. 4).
Palazzesi et al. (2022) suggevst that the pollen Tubulifloridites lilliei type A, ca 72.1 Ma and from Antarctica, is stem group Asteraceae.
Evolution: Divergence & Distribution. The six basal clades (see [Barnadesioideae [Famatinanthoideae [[Stifftioideae + Mutisioideae] [[Wunderlichioideae + Gochnatioideae]...) in Asteraceae below and Calyceraceae are are all entirely or very largely South American, and this whole clade may have originated there in the Late Cretaceous. Although Asteraceae are very diverse, and so contrast with Calyceraceae (Brignone et al. 2023a), this diversity is scarcely evident in the eight basal clades of the former where only the [Stifftioideae + Mutisioideae] clade, at ca 660 species, is of any size.
Katinas et al. (2016) discussed possible stylar apomorphies in Calyceraceae and Asteraceae, particularly Barnadesioideae, in some detail, and many of their suggestions are adopted here. For other similarities or possible synapomorphies, see DeVore (1994) and Lundberg and Bremer (2001, 2003), these include libriform fibres with simple pits and vasicentric parenchyma. Pesacreta et al. (1994) suggest similarities in the micromorphology of the filaments and connective bases between at least some members of Calyceraceae and Asteraceae. Placement of characters like pollen with intercolpar depressions on the tree is difficult to ascertain (see also DeVore 1994; DeVore et al. 2000). Indeed, DeVore and Skvarla (2008) suggest that pollen characters thought to suggest a relationship between the two families are different in detail and are therefore not homologous, similarly, although both families have but a single ovule, the position and orientation of the ovule is such that the single ovule condition may well have been derived independently. Indeed, there are suggestions that the gynoecium of Calyceraceae is 5-carpellate (literature in Hellwig 2007), while that of Asteraceae is 2-carpellate, so I have placed the character "ovule single [per flower]" as an apomorphy for both families. I have also placed several pollen characters at this node to help in interpreting the changes in pollen morphology within Asteraceae as detailed by Blackmore et al. (2009). The inflorescences of the two families can be interpreted as being fundamentally similar, despite the largely indeterminate construction of the asteraceous capitulum; the inflorescence of Calyceraceae is more obviously made up of cymose units than the former. This is discussed in more detail at below.
Genes & Genomes. For a palaeotetraploidy (or palaeohexaploidy) event that can be placed at this node, see M. S. Barker et al. (2016a) and C.-H. Huang et al. (2016); this is described as being "at the base of the Asterids II clade" by Badouin et al. (2017: p. 148). For possible base chromosome numbers for this clade, see Denham et al. (2016).
Understanding more about the expression of CYC (Cycloidea) genes in this clade - and indeed in Asterales as a whole - is likely to be interesting. Chapman et al. (2012) note that Acicarpha (Calyceraceae) has only CYC2a, genes, Dasyphyllum (Barnadesioideae, flowers polysymmetric again) also has CYC2c genes, while the other Asteraceae studied all had CYC2b, d and e genes as well. Members of the CYC2c gene family are involved in the monosymmetric phenotype in which corolla formation on the adaxial side of the flower is more or less reduced (see also Garcês et al. 2016).
Chemistry, Morphology, etc.. Calyceraceae and Barnadesioideae have a similar simple flavonoid profile.
Previous Relationships. Both Cronquist (1981) and Takhtajan (1997) placed the two families in separate, if adjacent, orders.
CALYCERACEAE Richard, nom. cons. —— Synonymy: Boopidaceae Cassini - Back to Asterales
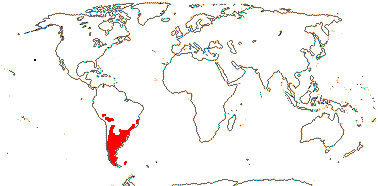
Herbs; ?flavonols 0; cork?; (bordered pits +); nodes ?; pericyclic fibres 0; plant ± glabrous; lamina margins entire; inflorescence entirely made up of cymose units, (not always obvious, but a terminal flower - Acicarpha), flowering ± centrifugal, heads usu. homogamous, involucral bracts connate at base (+ along margins) (0 - Moschopsis; all flowers bracteate, polysymmetric; K connate, aerenchymatous or spine-like, C midvein proceeding to apex beyond fusion with laterals; GAC zone + [above K but below C/A tube]; anther base tailed, etc., filaments ± connate, anther ?exodermis +; pollen grains spheroid-rhomboid to ellipsoid, (prolate), with intercolpar depressions/0, also extexine bridges over pores, also colpar ledges + [= exine ridges on inner margin of colpi]), (spines 0); nectaries alternating with filament bundles, at apex of C/A tube, opening by abaxial slits at base of A tube; G [5], opposite K, (embedded in inflorescence axis), apex of style/stigma clavate (slightly bilobed), papillate; ovule single [per flower], apical, apotropous; apex of fruit with a conical body [persistent base of C and style], K ((some lobes) accrescent), (apex spiny); seed coat rather undistinguished, chlorophyllous; endosperm +; n = 8, 12, 13, 15, 17, 18, 20-22, x = 8, chromosomes small, 1.6-2.7 µm long, centromeres (sub)metacentric, mostly centromeric C-bands, interphase nucleus reticulate, chromocentres sharply differentiated.
8/46: [list], Gamocarpha (13), Moschopsis (10), Boopsis (7). South America. Map: from Heywood (1978, S. part of range) and DeVore (1994). Photos - Acicarpha Habit, Calycera © H. Wilson., Undetermined Flowers.
Age. Acicarpha and Boopis [?species] diverged ca 51 Ma (K. Bremer et al. 2004a) or (40-)22(-7) Ma (Wikström et al. 2015); (57-)39.2(-24.9) Ma are the estimates in Panero and Crozier (2016), ca 27.4 Ma in Denham et al. (2016), ca 27 Ma in Pozner et al. (2021), 29.9-25.9 Ma in Benítez-Villaseñor et al. (2023) and (27.8-)23.6(-22.0) Ma in Brignone et al. (2023a).
Evolution: Divergence & Distribution. Brignone et al. (2023a) discuss diversification in the family. They think that the southern part of the Andes was its area of origin, and from there, there have been four dispersals to the Patagonian region beginning perhaps 14.6 Ma. Overall diversification rates have been stable, if low, and seem to be unaffected by climatic or oroogenic events.
Pollination Biology. There is secondary pollen presentation of the pump mechanism type; pollen is presented on top of the stigma, but there is neither indusium nor hairs, but when the stigma becomes receptive, it is covered in papillae (Erbar 1993; El Ottra et al. 2023).
Genes & Genomes. For some cytology, see Benko-Iseppon and Morawetz (2000b: one species).
Chemistry, Morphology, etc.. Cronquist (1981) described the flowers as being sometimes "slightly irregular"; they are commonly polysymmetric. There appear to be five carpels (e.g. Erbar 1993). The integument is described as being "thick" with the outer cell layers containing chloroplasts (Dahlgren 1915).
Some general information is taken from Hansen (1992), DeVore (1994), DeVore and Stuessy (1995), and Hellwig (2006), some details of morphology come from Pontiroli (1963), inflorescence development from Harris (1999: two species), floral development from Erbar (1993: Acicarpha tribuloides, very odd development), pollen morphology from DeVore et al. (2007), and embryology from Dahlgren (1915: one species).
Phylogeny. There are two main clades in the family, but details of relationships within those clades depend in part on whether nuclear ITS or chloroplast data are examined. Boopis in particular was hopelessly polyphyletic in the early analyses, and the only possibly monophyletic genus was Acicarpha (Denham et al. 2016; c.f. Beaulieu & O'Meara 2018: tip branches very long, artefactual?). Relationships in the total evidence analysis in Pozner et al. (2021; see also Brignone et al. 2023a) are [[Leucocera [Gamocarpha + Calycera]] [[Acicarpha [Boopis + Anachoretes]] [Moschopsis + Asynthema]]] - the position of the last genus is unclear, and see Pozner et al. (2024) for names...
Classification. Denham et al. (2019) and particularly Pozner et al. (2021, 2024) completely reworked the classification of the family.
ASTERACEAE Berchtold & J. Presl, nom. cons. / COMPOSITAE Giseke, nom. cons. et nom. alt. - Back to Asterales
Iso/chlorogenic acid, isoflavonoids, pentacyclic triterpene alcohols, terpenoid essential oils, (various alkaloids), a variety of fatty acids in the seeds, tannins, iridoids 0; (vascular tissue in a cylinder - woody taxa); (cork deep seated); (cortical or medullary vascular bundles +); cambium storied or not; (vessel elements with scalariform or reticulate perforations); nodes also 5:5; schizogenous secretory canals +; lamina vernation often conduplicate or revolute, margins various; capitulum with phyllaries in several series, lacking a terminal floret, cymose construction obvious in the area of the ray flowers, (reduced to a single flower, reaggregated); receptacle scrobiculate, bracts/bracteoles 0; (heads heterogamous, gynomonoecy common), (some florets [esp. ray florets] monosymmetric); K reduced, C midvein 0 (?+), lobes of disc floret corollas usu. longer than wide; anther apical connective conspicuous, entire, basally calcarate [sagittate], caudate [theca with basal appendage], (laciniate); endothecial cells elongated parallel to main axis of anther [?level]; tapetum amoeboid (glandular); pollen grains bicellular, 37-49.1 µm in diam., exine 6.1-6.7 µm across; nectary annular; style often lacking starch and druses, arms [branches, lobes] short [1.4> mm long], stigma dry, surface continuous, papillae with a single function [pollen presentation or reception]; ovule single [per floret], basal, epitropous, integument (4-)6-20 [Smallanthus] cells across; antipodal cells often persistent, proliferating and/or multinucleate; K pappose in fruit, pappus capillary/setose ; (testa not vascularized), (± obliterated), exotesta ± palisade, lignified/undistinguished; endosperm scanty to 0, (nuclear); embryo suspensor + [?level]; x = 9, protein bodies in nucleus, nuclear genome [1C] (0.146-)2.194(-32.88) pg/mean 2C size ca 6.50 pg.
1,620[list]/25,040 - sixteen subfamilies below. World-wide. Map: Vester (1940) and Hultén (1971). [Photo - Flowers, and more Flowers.]
Includes Anthemideae, Arctotideae, Astereae, Asteroideae, Athroismeae, Bahieae, Barnadesioideae, Calenduleae, Carduoideae, Chaenactideae, Cinchonoideae,Coreopsideae, Corymbioideae, Dicomoideae, Doroniceae, Eremothamneae, Eupatorieae, Famatinanthoideae, Gnaphalieae, Gochnatioideae, Gorterieae, Gymnarrhenoideae, Hecastocleidoideae, Helenieae, Heliantheae, Inuleae, Liabeae, Madieae, Millerieae, Moquineeae, Mutisieae, Mutisioideae, Nassauvieae, Neurolaneae, Oldenburgieae, Perityleae, Pertyoideae, Platycarpheae, Polymnieae, Senecioneae, Stifftioideae, Tageteeae, Tarchonantheae, Tarchonanthoideae, Vernonieae, Vernonioideae, Wunderlichioideae.
Age. Crown-group Asteraceae are dated to some 42-36 Ma (K. J. Kim et al. 2005), (52-)43, 40(-31) Ma (Bell et al. 2010), or (44-)41, 40(-37) Ma (Wikström et al. 2001); other suggested ages are similar (Funk et al. 2009c for a summary; see also Torices 2010). However, Beaulieu et al. (2013a: 95% HPD) estimated a somewhat older crown-group age of (52-)49(-48) Ma, ages in Funk et al. (2014) are 47.6-47.3 Ma, in Swenson et al. (2012) they range mostly from (71.1-)52.6, 47.4(-45.4) Ma, in Panero and Crozier (2016) they are as much as (74.4-)64.7(-55.1) Ma (see also Jabaily et al. 2014 for similar estimates), in C.-H. Huang et al. (2016) they are ca 72.1 Ma (or only (53-)52.5(-52) Ma), while ca 61.4 Ma is the age in Denham et al. (2016), (91-)83.5(-64) Ma in Mandel et al. (2019 - note, this age is misreported in Gostel et al. 2022), ca 81 Ma (C. Zhang et al. 2021) and (88-)75(-64) Ma (Keeley et al. 2021)... Other estimates are ca 49.6 Ma (Ackerfield et al. 2020) and 73.0 Ma (G. Zhang et al. 2024: Fig. 4). On the other hand, Heads (2012) suggested that the mostly Antipodean Abrotanella, basal Senecioneae, diverged from the rest of the tribe in the Jurassic or Early Cretaceous, which would imply an age for Asteraceae as a whole of around 500,000,000 to 1,500,000,000 years depending on which vicariance events you pick (Swenson et al. 2012).
Samant and Mohabey (2014) thought that the Late Cretaceous palynomorph Compositopollenites from India was evidence that the family was around there at this early date. Analysis of pollen from Antarctica dated 76-66 Ma suggests that this latter pollen was from a barnadesioid plant, and a crown age for Asteraceae of around (91.5-)85.9(-82.4) Ma was suggested (Barreda et al. 2015: fig. 5, suppl.; see also discussion in Proc. National Acad. Sci. 113: E411-E412. 2016), although other estimates, at (76.4-)67.9, 55.8(-53.6) Ma, are somewhat younger (Barreda et al. 2015: suppl.); Panero (2016) and Beaulieu and O'Meara (2018) question the placement of this fossil, which may rather be stem [Calyceraceae + Asteraceae].
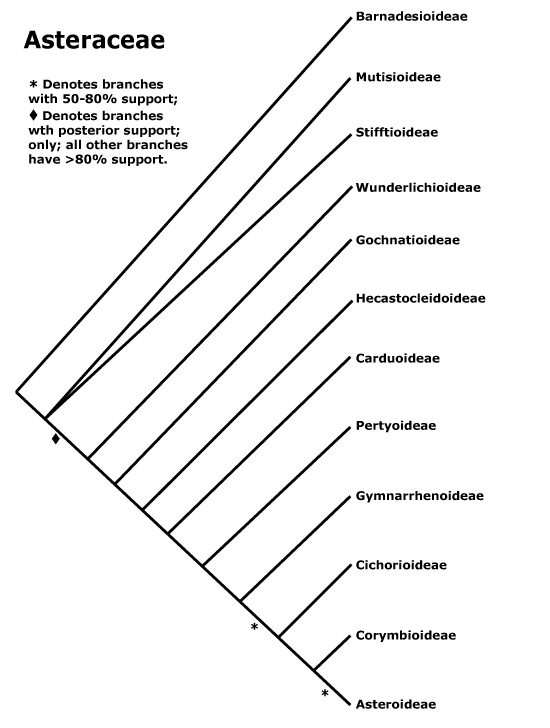
1. Barnadesioideae Bremer & Jansen
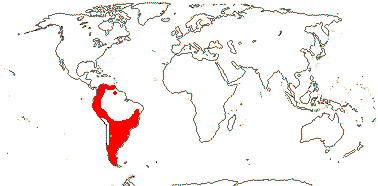
Plant (annual) perennial herbs to ± large trees (woody vines); notably poor in flavonoids, flavones 0; tracheids with bordered pits (= neotracheids; not annual spp.); hairs bicellular ["barnadesioid", short basal + long apical cells, often on florets and fruits], or 2-armed / (glandular) uniseriate; axillary spines [?= prophylls] + (0), (single) paired (fascicles); capitulum +, (phyllaries coloured); receptacle hairy (glabrous); florets polysymmetric-± monosymmetric; C hairy, (commissural veins not connate at apex of lobes); anther with apical connective rounded, (bifid), etc., base (not calcarate), (not caudate), filaments (connate), usu. glabrous; pollen (lophate), (with intercolpar depressions), (spines +, small), exine with spongy network [dist. in Ast.?], (± caveate), columellate-granulate [columellae sinuous], foot layer discontinuous; style-stigma types 4, unique, (style swollen - Fucaldea stuessyi), abaxially (minutely) rounded-papillate to smooth, branches short [ca 1:1 length:width ratio], apices rounded to cuneate, stigmatic surface continuous, vascular bundles at most entering base of arms; secondary pollen presentation pump/deposition/simple brush types; cypselas ?-ribbed, villous, foxy coloured, pappus hairs uniseriate, various; pericarp parenchymatous; exotesta short-palisade [Shlechtendalia]; endosperm cellular; n = 8, 12, 24-27..., x = ?9, mean 2 C size ca 8.50 pg.
10/86: Dasyphyllum (33), Chuquiraga (22), Barnadesia (19). South America, esp. Andean. Map: red, see Karis et al. (1992), Ezcurra (2002), Funk and Roque (2011) and Ferreira et al. (2021: Figs 1, 10). [Photo - Flower.]
Age. The age of crown-group Barnadesioideae is estimated to be about 39.4-19.4 Ma (Funk et al. 2014: Dussenia), (48.7-)33.7(-23.6) Ma (Panero & Crozier 2016: Table S1), (48.8-)37.4(-25.6) Ma (Abrahamczyk et al. 2017a), ca 64.3 Ma (Mandel et al. 2019: Schlecht.) or ca 59.1 Ma (G. Zhang et al. 2024: Fig. 4).
[Famatinanthoideae [[Stifftioideae + Mutisioideae] [[Wunderlichioideae + Gochnatioideae] [Hecastocleidoideae [Pertyoideae [Tarchonanthoideae] [Dicomoideae [Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]]]]]: anther base lignified; internal tectum +, homogeneous [?level]; pappus 2-5-seriate; ψrps19 pseudogene + [?level].
Age. The age of this node is around (66.5-)58.8(-51.4) Ma (Panero & Crozier 2016: Table S1), ca 62.3 Ma (Mandel et al. 2019) or ca 69.1 Ma (G. Zhang et al. 2024: Fig. 4).
Fossil pollen, Mutisiapollis sp., from deposits ca 56 Ma from southern South America, is placed at this node by Palazzesi et al. (2022).
2. Famatinanthoideae S. E. Freire, Ariza & Panero - Famatinanthus decussatus (Hieronymus) Ariza & S. E. Freire
Small shrub; cork deep-seated; stomata raised, with sub-stomatal cavity; leaves opposite, amphistomatal, petioles clasping; phyllaries 3-seriate; ray florets bilabiate [2:3], disc florets deeply lobed [ca 1/2]; anther with apical connective long, apiculate, sclerified; pollen surface microechinate/rugulate; style-stigma type unique, style abaxially with groups of large cells [cobblestone-like], branches short, apices cuneate, stigmatic surface continuous; secondary pollen presentation pump (deposition) type; ?embryology; pappus 2-3-seriate, of dimorphic barbellate bristles; ?testa; n = 27, mean 2 C size?
1/1. N.W. Argentina, Andean. Map: see above, green.
[[Stifftioideae + Mutisioideae] [[Wunderlichioideae + Gochnatioideae] [Hecastocleidoideae [Pertyoideae [Tarchonanthoideae [Dicomoideae [Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]]]]: often herbaceous; (benzofurans + [benzene + furan - C4H40 (heterocyclic) rings]); leaves usu. triplinerved; C lobing?, commissural vein fusion?; style arms long [>2 length:width rato]; cypsela with twin hairs [unicellular to uniseriate base, apical cell un/equally 2-armed], pappus capillary, developing late: genome duplicaion + [hexaploidy], 22.8 kb chloroplast DNA inversion, 3.3 kb inversion nested within it.
Age. The age of this node is about (62.7-)55.9(-49.2) Ma (Panero & Crozier 2016: Table S1), somewhere around 61.5 to 47.5 Ma (C.-H. Huang et al. 2016: three estimates), ca 56.4 Ma (Mandel et al. 2019) or ca 42.2 Ma (Ackerfield et al. 2020). Other estimates are 83.1-79.1 Ma (Benítez-Villaseñor et al. 2023) and ca 68.1 Ma (G. Zhang et al. 2024: Fig. 4).
Panero et al. (2014) suggested that the macrofossil, Raiguenrayun cura, a capitulum from the Middle Eocene of Patagonia ca 47.7 Ma, and its associated pollen (Barreda et al. 2010b, 2012a), is assignable to this node.
[Stifftioideae + Mutisioideae]: stigma apex ± rounded; secondary pollen presentation pump-type.
Age. The age of this clade is ca 54.8 Ma (Mandel et al. 2019) or ca 66.9 Ma (G. Zhang et al. 2024: Fig. 4).
3. Stifftioideae Panero - ?5 tribes.
(Shrubs and trees); marginal, central florets ± monosymmetric; (C lobes long, ± coiled); apex of anther appendage acute/apiculate; pollen spherical, surface echinate or not; style abaxially glabrous / with (clusters of) short hairs, stigma (with marginal ridges), surface continuous; secondary pollen presentation (deposition/brush-type); integument 15-20 cells across, obturator +; cypsela 10-ribbed, glabrous (villous), pericarp parenchymatous; testa cells elongated, anticlinal walls regularly thickened/beaded; endosperm cellular; n = ?, mean 2C size?
15/50: Gongylolepis (14). South America, esp. Venezuela-Guyana, (southeast Asia, inc. Himalayan foothills).
Age. Crown-group Stifftioideae - [Stifftia + The Rest] - can be dated to (52-)27.4(-7.9) Ma (Panero & Crozier 2016: Table S1), while an age of ca 47.9 Ma was suggested by Mandel et al. (2019) and ca 50.5 Ma (G. Zhang et al. 2024: Fig. 4).
4. Mutisioideae Lindley - 3 tribes.
(Plant ± shrubby); (leaves soon deciduous - Aphyllocladus); (capitulae variously aggregated); (receptacle hairy); (heads heterogamous), (central florets subligulate, etc.), (deeply lobed [1/3-1/2],) (lobing 4:1, 3:1); anther with apical connective rounded (acute), rarely calcarate; pollen ?shape, surface microechinate; style branches (short - e.g. Adenocaulinae), abaxially papillate only on branches, (sub)apical tuft of hairs, glabrous, stigma apex (truncate, or ± cuneate), (receptive zone marginal); secondary pollen presentation (brush-type); cypsela 5- or 10-ribbed, hairy to glabrous, (pappus uniseriate), (2-many-seriate, of dimorphic barbellate bristles; exotesta short-palisade (tetrahedral), pitted (3-layered; ± undifferentiated); endosperm nuclear; n = (6-)9(+), mean 2C size ca 6.04 pg, (genome duplication).
66/631. South America (North America, Africa to East Asia).
Age. The age of crown-group Mutisioideae is estimated to be (61.3-)52.5(-43.4) Ma (Panero & Crozier 2016: Table S1), ca 48.2 Ma (Mandel et al. 2019) or around 60.8 Ma (G. Zhang et al. 2024: Fig. 4).
4A. Onoserideae Panero & Funk
7/46: Onoseris (21), Lycoseris (11).
Age. The age of this clade is estimated to be ca 50.5 Ma (G. Zhang et al. 2024: Fig. 4).
4B. Nassauvieae Cassini —— Synonymy: Nassauviaceae Burmeister
Age. Huanilipollis cabrerae pollen, 52 Ma and from Southern South America, is placed in crown group Nassauvieae by Palazzesi et al. (2022).
26/330: Acourtia (81), Trixis (50), Leucheria (48), Nassauvia (40), Perezia (32), Jungia (29). Souch America (Central America, The Antilles.
4C. Mutisieae Cassini —— Synonymy: Mutisiaceae Burnett, Perdiciaceae Link, nom. inval.
Vine, leaf blades with terminal tendrils (Mut.); (achenes with phytomelan - Nasauviinae).
13 or 18/271: Chaptalia (69), Mutisia (58), Chaetanthera (47), Gerbera (40), Trichocline (24).
[[Wunderlichioideae + Gochnatioideae] [Hecastocleidoideae [Pertyoideae [Tarchonanthoideae] [Dicomoideae [Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]]]: pollen ± echinate; style arms ± rounded at the apex; x = ?
Age. This node can be dated to (69.1-)55.3(-48.6) Ma (Panero & Crozier 2016: Table S1) or ca 52.3 Ma (Mandel et al. 2019). Note, however, the topology and associated ages in G. Zhang et al. (2024: Fig. 4)
[Wunderlichioideae + Gochnatioideae]: style (arms short), apices rounded.
Age. This clade is ca 50.3 Ma (Mandel et al. 2019).
5. Wunderlichioideae Panero & Funk
Shrubs and trees; (receptacle alveolate), paleaceous; (marginal florets monosymmetric); apex of anther appendage acute to apiculate; pollen ?characters; obturator +; style arms and below with papillae in clusters, scale-like ["multiseriate"] or glabrous, stigma lobes ± adherent, otherwise surface continuous; cypsela 10-ribbed, hairy to glabrous, (pappus paleaceous), with phytomelan; testa cells longitudinally elongated, anticlinal walls with beaded thickenings; endosperm cellular; n = ?, mean 2C size?; 6 bp deletion in PEP subunit β rpoB gene.
4/35: Stenopadus (15). Venezuelan Guyana (E. South America, S.W. China - ).
Age. The age of crown-group Wunderlichioideae ([Wunderlichia + Hyalis]) is estimated to be (59.6-)46(-24.4) Ma (Panero & Crozier 2016: Table S1), while ca 47.5 Ma is the estimate in Mandel et al. (2019: 25 Ma without Cyclolepis - note that this genus was moved to Gochnatieae/Gochnatioideae by Gostel et al. 2022) and ca 36.2 Ma (G. Zhang et al. 2024: Fig. 4, also no Cyclolepis).
6. Gochnatioideae Panero & Funk
(Plant ± shrubby); (receptacle alveolate); (heads heterogamous); apex of anther appendage ± apiculate; pollen ?shape, anthemoid[!]; style thickened just below arms, arms short, round, abaxially glabrous, sclerenchyma prominent, stigmas with marginal ridges; secondary pollen presentation pump-type; cypsela 5-ribbed, hairy, pappus with 25-80(-90) bristles, uni--triseriate; testa parenchymatous, one large bud; n = 22, 23, 27, mean 2 C size ca 3.40 pg.
10/98: Anastraphia (33), Moquiniastrum (22), Richterago (16). South America, but not beyond 40oS, Central America, east Mexico, southern U.S.A. and the Antilles.
Age. The age of crown-group Gochnatioideae has been estimated to be 24.9-23 Ma (Funk et al. 2014), (54.5-)36.7(-14.2) Ma (Panero & Crozier 2016: Table S1), ca 18.8 Ma (Mandel et al. 2019), ca 53.2 Ma (Gostel et al. 2022) and ca 22.6 Ma (G. Zhang et al. 2024: Fig. 4).
Mutisiapollis viteauensis, 28 Ma pollen from Southern South America, has been placed in crown-group Gochnatieae (Palazzesi et al. 2022).
For these last two: perhaps [Gochnatieae [Cyclolepis + Wunderlichieae]], or the grade ... Cyclolepis [Wunderlichioideae [Gochnatioideae .... See Relationships below.
[Hecastocleidoideae [Pertyoideae [Tarchonanthoideae [Dicomoideae [Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]: pollen grains spherical, columellae sausage-like; stylar starch and druses common; deletion in PEP subunit β rpoB gene.
Age. The crown age of this clade is some 38-32 Ma (K.-J. Kim et al. 2005), (58.1-)51.7(-45.7) Ma (Panero & Crozier 2016: Table S1), ca 50.4 Ma (Mandel et al. 2019) or 66.2 Ma (G. Zhang et al. 2024: Fig. 4).
7. Hecastocleidoideae Panero & Funk - Hecastocleis shockleyi A. Gray
Shrubby; hairs multicellular, conical; leaves spiny; capitulae with 1 floret, grouped into synflorescence, synflorescence bracts attractive, but spiny; heads homogamous, all florets polysymmetric, corolla 5-lobed; apex of anther appendage rounded; pollen tricolpate; style thickened just below arms, arms short, abaxially glabrous, apices rounded, sclerenchyma prominent; secondary pollen presentation pump-type; cypsela 5-ribbed, glabrous, pappus of laciniate scales ["with scale-like corona"], uniseriate; n = 8, mean 2C size?; 15 bp deletion in rpoB gene.
1/1. Southwest U.S.A..
[Pertyoideae [Tarchonanthoideae [Dicomoideae [Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]: (heterocyclic sulphur compounds +) [thiophene/thiofuran - C4H4S - and oligomers]; leaf triplinerved?; capitulae variously aggregated, apical capitulum flowers first ["capitulescence cymose"]; pollen grains 3-nucleate [?always]; carpels of disc florets [at least] superposed, sweeping hairs +[?]; [?cypsela rib # and indumentum], x = 10; genome duplication [= palaeohexaploidy - ?here], deletion and insertion in PEP subunit β rpoB gene.
Age. This node is about (55.5-)49.8(-44) Ma (Panero & Crozier 2016: Table S1), ca 54-42.5 Ma (C.-H. Huang et al. 2016: three estimates), ca 44.5 Ma (Mandel et al. 2019) or ca 64.3 Ma (G. Zhang et al. 2024: Fig. 4).
8. Pertyoideae Panero & Funk
Herbs to shrubs; hairs conical, multicellular; florets not bilabiate, C irregularly divided; pollen (with solid spines), (tricolpate); style arms rather short, with abaxial hairs (below style arms), apices truncate, rounded to subacute, stigmatic zone confluent/inverted U-shaped; secondary pollen presentation pump/brushing-type; integument 12-29 cells across, obturator +; pappus usu. uniseriate, of (plumose) bristles; testa multiplicative, endosperm briefly nuclear; n = 12-15, mean 2C size ca 1.82 pg; 145 bp deletion in ndhI/ndhG intergenic spacer.
5-6/70: Ainsliaea (50). Afghanistan to East (and Southeast) Asia.
Age. The age of crown-group Pertyoideae is about (38.8-)18.8(-2.6) Ma (Panero & Crozier 2016: Table S1) or ca 49.9 Ma (G. Zhang et al. 2024: Fig. 4).
Age. This clade is ca 42.2 Ma (Mandel et al. 2019) or ca 35.6 Ma (Ackerfield et al. 2020).
Pert, Gochnat, Stiff. - ovule bundle proceeds to end of integument, but only to chalaza in Gaillarda, Eclipta, Launea...
9. Tarchonanthoideae S. Ortiz
Style swollen at apex, branches short, apices rounded; carpopodium 0; testa collapsed, or thickening U-shaped; mean 2C size?
2/20. Africa, inc. Madagascar, Arabia.
Age. This clade is ca ?47.1 Ma (Mandel et al. 2019), (40.1-)ca 35(-28.9) Ma (Kimball et al. 2023) or ca 31.5 Ma (G. Zhang et al. 2024: Fig. 4).
9A. Oldenburgieae S. Ortiz - Oldenburgia Lessing
Anthers tailed, apical appendage +; pollen echinate [remove?]; style branches abaxially ± papillate, stigma inverted U-shape, with marginal ridges; secondary pollen presentation pump-type.
1/4. South Africa, southern parts of the eastern and western Cape. (14.6-)ca. 9(-3.8) Ma, grandis paradoxa
9B. Tarchonantheae Kosteletzky
Shrubs to large trees, (deciduous); plant dioecious; male florets - hairs short, also on stylar shaft; pappus 0, fruits wooly, pappus 0/pappus of bristles.
1/16: Brachylaena (16). Saudi Arabia, Eastern Africa, esp. South Africa, Madagascar.
Age. Crown-group Tarchnonantheae began to diversify during the early Oligocene (37.6-)ca 32(-26.2) Ma (Kimball et al. 2023) or ca 25.4 Ma (G. Zhang et al. 2024: Fig. 4).
Age. The age of this clade is around 41.8 Ma (Mandel et al. 2019).
10. Dicomoideae S. Ortiz
Annual to perennial herbs or small trees; involucre pluri seriate, phyllaries coriaceous, pungent; receptacle alveolate or honeycombed/fimbriate between achenes; pollen echinate; style branches short to long, hairs abaxially, sweeping hairs at bases of branches, sclerenchyma prominent; stigmatic surface continuous, with marginal ridge; secondary pollen presentation pump/special pump type; carpopodium 0; n = 11, mean 2C size?
8/100: Pleiotaxis (35), Dicoma (30). Africa south of the Sahara, Madagascar.
Age. Crown-group Dicomoideae are ca 27.1 Ma (Mandel et al. 2019) or ca 44.0 Ma (G. Zhang et al. 2024: Fig. 4).
Pollen of crown-group Dicomoideae, 'Mutisiapollis viteauensis' from Africa, is about 41 Ma (Palazzesi et al. 2022).
[Carduoideae [Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]: genome duplication.
Age. This clade is ca 41.5 Ma (Mandel et al. 2019) or (49.3-)36.2(-23.9) Ma (Maurin & Smissen 2021).
11. Carduoideae Sweet
Plant often herbaceous, commonly biennial; alkaloids +; (laticifers +), (resin ducts +); leaves dissected; florets polysymmetric (monosymmetric); pollen (psilate), columellae "medium thickness", with internal tectum; style with ring of hairs/papillae below arms, stigmatic only on inner surfaces of branches; brush- and pump types secondary pollen presentation, (florets touch-sensitive); (cypsela lacking twin hairs), (pappus uniseriate); exotesta long-palisade (with linea lucida), (rather undistinguished); n = 9, 12, mean 2 C size ca 3.53 pg.
81/2,520. World-wide, but most N. hemisphere, esp. Eurasia/N. Africa. Map: from Hellwig (2004).
Age. Crown-group Carduoideae are ca 42.2 Ma (Mandel et al. 2019; see also Ackerfield et al. 2020), around (40-)34.1(-29.9) Ma (Herrando-Moraira et al. 2019) or ca 53.1 Ma (G. Zhang et al. 2024: Fig. 4).
11A. Cardueae (inc. Carlineae) —— Synonymy: Acarnaceae Link, nom. illeg., Carduaceae Berchtold & J. Presl, Carlinaceae Berchtold & J. Presl, Centaureaceae Berchtold & J. Presl, Cnicaceae Vest, Cynaraceae Burnett, Echinopaceae Berchtold & J. Presl, Serratulaceae Martynov, Xeranthemaceae Döll - 12 subtribes
Herbs, annual to perennial; (leaves/involucral bracts spiny); style swollen subbasally; pappus barbellate/plumose, basal pappus tissue a cylinder; achene with apical elaiosome, (phytpmelan +); n = 9, 16, 17.
72/ : Centaurea (793), Cousinia (709), Saussurea (400-520), Cirsium (450), Jurinea (>200), Echinops (120), Lophiolepis (129), Carduus (100), Serratula (70), Onopordum (60).
Age. The crown-group age of Cardueae is ca 26.0 Ma (Ackerfield et al. 2020) - Carl. + Card. ca 31.7 Ma (Ackerfield et al. 2020).
Fossils suggest that the age of this clade - or the whole subfamily? - is estimated to be ca 20 Ma (Palazzesi et al. 2022: Tricolporopollenites macroechinatus, from Central Europe).
[Gymnarrhenoideae [Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]]: herbaceous habit esp. common; pollen echinate or lophate [move up?].
Age. This node is some (52.1-)46.8(-41.7) Ma (Panero & Crozier 2016: Table S1), somewhere between ca 52 and 41 Ma (C.-H. Huang et al. 2016: three estimates), ca 40.6 Ma (Mandel et al. 2019) or ca 63.0 Ma (G. Zhang et al. 2024: Fig. 4).
12. Gymnarrhenoideae Panero & Funk
Leaves forming rosette, margin with (few) teeth; plant ± monoecious [∴ secondary pollen presentation unknown]; [[ecaudate or with short obtuse ± connate tails; endothecium ± absent;]] pollen tricolpate, spiny, [[caveate, infrategular baculae +]]; pappus with bristles.
2/2.
Age. Crown-group Gymnarrhenoideae are some 22.2 Ma (G. Zhang et al. 2024: Fig. 4).
12A. Gymnarrhena micrantha Desfontaines
Annual, stemless; lamina narrow, sessile; plant amphicarpic, subterranean heads: florets 1-3; self fertilized; C minute; pappus of ± scale-like bristles or 0; embryo with short hypocotyl + radicle; staminate florets: flowers 3-merous; anthers lacking apical appendages; carpellate florets: each surrounded by large bract; C filiform, at base much swollen [= corolla cupula]; style swollen subbasally, branches long; G with long twin hairs, rows of stiff hairs on the ribs; pappus scales long-acuminate and margins with hairs, pappus movement hygroscopic [thick walled cells adaxial]; pericarp parenchymatous; seed exotestal, anticlinal walls sinuous, thickened, other testal cells with calcium oxalate crystals; embryo with hypocotyl + radicle ca half its length; n = 9, 10.
1/1. North Africa to the Middle East.
12B. Cavea tanguensis (J. R. Drummond) W. W. Smith & J. Small
Perennial, woody-rhizomatous, stems stout, unbranched; lamine ± narrowly obovate, apex petiolate; plant monoecious; capitulae single; inflorescence bracts foliaceous; floral bracts 0; flowers 4-5-merous, vey slender; staminate flowers: C lobes and tube about the same length; anthers with triangular apical appendage; pappus uniseriate; carpellate flowers: C lobes relatively much shorter; stigma lobes rounded; cypsela with two whorls of ca 50 barbellate pappus hairs; n = ?
1/1. The Himalayas (Tibet, Sikkim, Szechuan).
[Vernonioideae [Cichorioideae [Corymbioideae + Asteroideae]]]: ?
Age. The age of this node is about (31-)28, 24(-21) Ma (Wikström et al. 2001), (37-)29, 27(-19) Ma (Bell et al. 2010), (51.1-)45.9(-40.9) Ma (Panero & Crozier 2016: Table S1), somewhere between 50.5 and 40 Ma (C.-H. Huang et al. 2016: three estimates), ca 40.2 Ma (Mandel et al. 2019) or ca 62.4 Ma (G. Zhang et al. 2024: Fig. 4).
13. Vernonioideae (Cassini) Lindley - ?8 tribes.
(both ray and disc florets); (fruits with phytomelan).
160/1,932.
Age. Crown-group Vernonioideae are ca 35.1 Ma (Mandel et al. 2019) or ca 58.4 Ma (G. Zhang et al. 2024: Fig. 4).
[Eremothamneae [Gorterieae [Platycarpheae + Arctotideae]]]: ?
Eremothamneae H. Robinson & Brettell
Shrub; leaves linear to sllipsoid, usu. spine-tipped..
2/3: Hoplophyllum (2). Namibia, South Africa, the Cape.
[Gorterieae [Platycarpheae + Arctotideae]]: ?
Gorterieae Lindley - Gorteria L.
.
1/8. Namibia, South Africa.
[Platycarpheae + Arctotideae]: ?
Platycarpheae Funk & H. Robinson
.
Arctotideae Cassini —— Arctotidaceae Berchtold & J. Presl
(Pappus coroniform).
15/218: Berkheya (79), Arctotis (65). Mostly Southern Africa
Age. Crown-group Arctotidae are ca 51.0 Ma (G. Zhang et al. 2024: Fig. 4).
[Liabeae [Distephaneae [Moquinieae + Vernonieae]]]: ?
Age. The age of this clade is (63-)53(-43) Ma (Keeley et al. 2021) or ca 52.7 Ma (G. Zhang et al. 2024: Fig. 4).
Annual herbs to small trees; leaves opposite; flowers yellow; pappus outer whorl scales, inner whorl bristles.
18/165: Munnozia (45), Liabium (37). Neotropics, inc. Greater Antilles, esp. North and Central Andes.
Age. The age of crown-group Liabeae is ca 40.8 Ma (G. Zhang et al. 2024: Fig. 4).
[Distephaneae [Moquinieae + Vernonieae]]: ?
Age. The age of this clade has been variously estimated as ca 30.5 Ma (Mandel et al. 2019), (63-)53(-43) Ma (Keeley et al. 2021) or (26.0-)22.7(-19.3) Ma (Gostel et al. 2024).
Distephaneae (S. C. Keeley & H. Robinson) H. Robison & V. A. Funk - Distephanus Cassini
Trees, shrubs, vines; elemanolides +; hairs often ± T-shaped; leaves triplinerved; capitulum phyllaries 3-8-seriate; florets yellow, polysymmetric; anthers with eglandular apical appendages; endothecial cells with separate sclerified shields; style branches with obtuse sweeping hairs, whole inner surface papillate; pappus various; n = 10.
1/43. SubSaharan Africa to Mauritius, also S.W. China, most spp. Madagascar.
Age. Crown-group Distephanus is ca 25 Ma (Mandel et al. 2019), (27.0-)15.1(-7.5) Ma (Keeley et al. 2021) or (22.9-)18.0(-13.2 Ma) Gostel et al. (2024).
Moquinieae H. Robinson
Vernonieae Cassini / "the evil tribe" - 20 subtribes —— Synonymy: Vernoniaceae Burmeister
(Pappus palaeaceous); x = 10, n = 7, 9-11, 13-20, etc..
Vernonia (723, but see section on Classification below). Africa, esp. the New World.
Age. The age of crown-group Vernonieae is (60-)51(-40) Ma (Keeley et al. 2021), ca 46 Ma (Angulo et al. 2022) or ca 43.0 Ma (G. Zhang et al. 2024: Fig. 4).
[Cichorioideae [Corymbioideae + Asteroideae]]]: 9 bp deletion in ndhF gene. [check: Kim, Jansen 1995]
Age. This clade is ca 38.6 Ma (Mandel et al. 2019) or ca 61.6 Ma (G. Zhang et al. 2024: Fig. 4).
14. Cichorioideae Chevallier - 14 subtribes / Cichorieae Lamarck & de Candolle , Lactuceae Cassini —— Synonymy: Aposeridaceae Rafinesque, Cichoriaceae Jussieu, nom. cons., Lactucaceae Drude, Picridaceae Martynov
Annual to perennial herbs to shrubs and rosette trees (vines); latex +; phyllaries ± various; heads homogamous, florets perfect; C ligulate, ligule 5-lobed (tubular); A (ring meristem - Taraxacum), anthers with elongate apical appendages, basal appendages calcarate/caudate; endothecium ± absent; tapetum non-syncytial invasive [Cich. intybus]; pollen echinolophate, columellae aggregated under spines, (echinate); style branches long, with sweeping hairs abaxially, also on shaft, stigmatic area continuous; secondary pollen presentation brush-type; carpels collateral, style arms usu. acute at apex, with long sweeping hairs; cypsela beaked or not; exotesta with fine porosity (not); n = (7-)9-10(-13), mean 2 C size ca 5.29 pg.
93/1,600 (+ >7,500 microspecies): Crepis (200), Scorzonera (175), Lactuca (73-130), Lepidaploa (115), Tragopogon (110), Lessinganthus (100), Hieracium (90 + 2,349 microspecies), Vernonanthura (65), Hypochaeris (60), Sonchus (60), also Pilosella (20-80), Taraxacum (60 + 2,387 microspecies). ± World-wide, but mostly mid-latitude north temperate, few tropical.
Age. Crown-group Cichorioideae are 35.3-25.7 or (37.9-)31.3, 25.8(-22.0) or (29.8-)25.8, 25.7(-22.0) Ma (various estimates in Tremewtsberger et al. 2013), ca 23.5 Ma (Mandel et al. 2019) or ca 58.4 Ma (G. Zhang et al. 2024: Fig. 4).
The age of "crown-group Cichorieae", represented by Cichoriaearumpollenites cf. gracilis from Central Europe, is estimated to be ca 28 Ma (Palazzesi et al. 2022 - see also Tremetsberger et al. 2013, Cichorium-type pollen not basal in Cichorieae).
[Corymbioideae + Asteroideae]: pollen ± lacking columellae in endosexine spanning space above foot layer [= fully caveate], with internal foramina, spines pointed.
Age. The age of this clade is about (49.4-)44.4(-39.8) Ma (Panero & Crozier 2016: Table S1), ca 38.2 Ma (Mandel et al. 2019) or ca 59.5 Ma (G. Zhang et al. 2024: Fig. 4).
15. Corymbioideae Panero & Funk - Corymbium L.

Plant perennial; sesquiterpene lactones 0, macrolides +; leaves linear, with parallel veins; heads with 1 floret, involucral bracts 2; C with broad, patent lobes; anthers black, apical appendage reduced, shortly sagittate, excaudate; style arms variable, with hairs abaxially to apex of shaft, apices truncate to tapered, stigmatic surfaces continuous; secondary pollen presentation brush-type; pappus of bristles or scales; n = 8, mean 2C size?
1/9. South Africa, the Cape. Map: from Weitz (1989).
Age. Crown-group Corymbioideae are around 5.1 Ma (G. Zhang et al. 2024: Fig. 4).
16. Asteroideae Lindley - 22 tribes.
(Plants shrubby), (annual); sesquiterpene lactones at biogenetic levels 3 and 4 [sic], (6,8-deoxygenation of flavonoids), benzopyrans, benzofurans, C10 acetylenes + [?level]; secretory canals + [associated with vascular bundles] (0); (florets bracteate); often radiate [ray florets ligulate [3 lobed], female/sterile], disc florets polysymmetric, perfect, C shallowly lobed; anthers (free), (black), (ecalcarate), ecaudate; pollen 25.0-34.3 µm in diam., colpus ends acute, (spines solid), (internal foramina 0), exine 2.3-4.2 µm across, with a double tectum; style arm hairs often rounded, only ± at the tip, stigmatic surface as two marginal bands; pump-type secondary pollen presentation common; (pappus paleaceous); n = (4-)9-10(-19 - Heliantheae, etc.), mean 2C size ca 8.54 pg; rbcL 6bp x 4 inversion, 15 bp deletion in rpoB gene; (5S and 35S ribosomal genes associated).
1,135/17,200. World-wide.
Age. Bergh and Linder (2009) suggested that diversification of Asteroideae began in the Eocene (56.6-)43.0(-29.6) Ma, as did Panero and Crozier (2016: Table S1) - (47.8-)43.2(-38.7) Ma, although most estimates are somewhat younger (Funk et al. 2009c), e.g. Pelser and Watson (2009), around 39-26 Ma, Mandel et al. (2019), ca 37.7 Ma and ca 59.5 Ma, G. Zhang et al. (2024: Fig. 4), while at around 46-36.5 Ma C.-H. Huang et al. (2016: three estimates) give one choices.
Crown-group Asteroideae (as Tubulifloridites cf. antipodica) are dated to ca 28 Ma (pollen from India), but the clade indicated seems to to be [Cichorioideae ... Asteroideae] (Palazzesi et al. 2022).
[Doroniceae [Calenduleae [Senecioneae [Anthemideae [Gnaphalieae + Astereae]]]]]: ?
Age. The age of this clade is ca 57.7 Ma (G. Zhang et al. 2024: Fig. 4).
Doronicum (29).
Age. The age of Doroniceae is ca 13.5 Ma (G. Zhang et al. 2024: Fig. 4).
[Calenduleae [Senecioneae [Anthemideae [Gnaphalieae + Astereae]]]]: ?
Age. The age here is around 56.6 Ma (G. Zhang et al. 2024: Fig. 4).
16B. Calenduleae Cassini —— Synonymy: Calendulaceae Berchtold & J. Presl
Pimarane diterpenes +; cyselae dimorphic, epappose; mean 2C size ca 3.27 pg.
8/118: Osteospermum (70), Dimorphotheca (21), Calendula (13). Mediterranean and tropical and especially southern Africa.
Age. Crown-group Calenduleae are some 37.5 Ma (G. Zhang et al. 2024: Fig. 4).
[Senecioneae [Anthemideae [Gnaphalieae + Astereae]]]: ?
Age. This clade is ca 55.6 Ma (G. Zhang et al. 2024: Fig. 4).
16C. Senecioneae Cassini —— Synonymy: Senecionaceae Berchtold & J. Presl, Tussilagaceae Berchtold & J. Presl
(Plants shrubby), (annual); (CAM photosynthesis +); pyrrolizidine alkaloids + [senecionines, also triangularines]; testa ca 10 cells across; mean 2C size ca 7.39 pg.
>150/>3,500: Senecio (1,681 - Moonlight et al. 2024, or ca 10), Pentacalia (162), Gynoxys (130), Ligularia (125), Gynoxys (117), Euryops (100), Cremanthodium (70), Packera (65), Dendrophorbium (60), Monticalia (60), Parasenecio (60). Cosmopolitan.
Age. The age of crown-group Senecioneae is ca 51.4 Ma (G. Zhang et al. 2024: Fig. 4).
[Anthemideae [Gnaphalieae + Astereae]]: ?
Age. The age of his clade is ca 54.1 Ma (G. Zhang et al. 2024: Fig. 4).
16D. Anthemideae Cassini —— Synonymy: Anthemidaceae Berchtold & J. Presl, Athanasiaceae Martynov, Artemisiaceae Martynov, Matricariaceae J. Voigt, Santolinaceae Martynov, Tanacetaceae Vest
Stem with endodermis [Artemisia]; pappus coroniform (0); mean 2C size ca 11.08 pg.
Ca 115/1,800: Artemisia (505, inc. Seriphidium), Anthemis (210), Tanacetum (160), Achillea (115), Cotula (58), Cota (43), Leucanthemum (43), Ursinia (43), Athanasia (39). Widespread, mostly Old World.
Age. Crown-group Anthemideae are ca 44.2 Ma (G. Zhang et al. 2024: Fig. 4).
The age of pollen of Anthemideae (as Artemisiapollenites sp.) from Asia is estimated to be ca 12 Ma (Palazzesi et al. 2022).
Age. An age suggested for this clade is ca 49.2 Ma (G. Zhang et al. 2024: Fig. 4).
16E. Gnaphalieae Lecoq & Juillet —— Synonymy: Gnaphaliaceae Rudolphi, Helichrysaceae Link, nom. inval.
mean 2C size ca 4.30 pg.
178/2,100: Helichrysum (580-600), Gnaphalium (150), Antennaria (70-several hundred), Pseudognaphalium (ca 137, but monophyly?), Anaphalis (110), Metalasia (60), Leontopodium (58), Rhodanthe (46), Ambrosia (40), Craspedia (20). More or less world-wide, esp. Australia; temperate.
Age. Nie et al. (2015) suggest that the crown-group Gnaphalieae are (35.3-)29.4(-24) Ma, while Bergh and Linder (2009) date it to (52.3-)34.5(-20.6) Ma and G. Zhang et al. (2024: Fig. 4) to ca 38.7 Ma
16F. Astereae Cassini —— Synonymy: Grindeliaceae A. Heller
(peltate trichomes +); mean 2C size ca 3.75 pg.
(?220)252/3,080 - 36 subtribes (for which, see Nesom 202a): Baccharis (440), Aster (180: F. N. A. vol. 20. 2006, c.f. Nesom 2020b), Erigeron (200), Solidago (138), Olearia (130), Emilia (120), Symphyotrichum (>100), Shawia (82), Diplostephium (70), Felicia (85), Pteronia (80), Brachy(s)come (70), Haplopappus (70), Celmisia (60), Psiadia (640, Grindelia (50-75), Conyza (60). Cosmopolitan.
Age. The age of crown-group Astereae is around 33.4 Ma (G. Zhang et al. 2024: Fig. 4).
Age. The age of this clade is ca 55.1 Ma (G. Zhang et al. 2024: Fig. 4).
16G. Inuleae Cassini —— Inulaceae Berchtold & J. Presl
mean 2C size ca 2.34 pg.
Blumea (100), Pluchea (80), Pulicaria (80).
Age. Crown-group Inuleae are estimated to be ca 40.2 Ma (G. Zhang et al. 2024: Fig. 4).
[Athroismeae [Helenieae [[Neurolaeneae [Heliantheae + Coreopsideae]] [Tageteae [[Polymnieae + Millerieae] [Madieae [[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]]]]]]]: cypsela with phytomelan.
Age. This clade is some 50.1 Ma (G. Zhang et al. 2024: Fig. 4).
16H. Athroismieae Panero / Asterodeae
10/80: Anisopappus (33), Blepharispermum (16). Africa and especially Madagascar to China, and Centipeda to Australia, New Zealand and south South America.
Age. The crown-group age of Athroismieae is ca 36.7 Ma (G. Zhang et al. 2024: Fig. 4).
Age. The age of the clade [Helianthus, Eupatorieae, Perityleae] is estimated to be (20.8-)15.8(-9.6) Ma (Lichter-Mark & Baldwin 2023).
Age. The crown-group of this clade is aged at ca 46.0 Ma (G. Zhang et al. 2024: Fig. 4).
16I. Helenieae Lindley
Viguiera (180),
Age. The crown group of Helenieae is aged at about 40.0 Ma (G. Zhang et al. 2024: Fig. 4).
[[Neurolaeneae [Heliantheae + Coreopsideae]] [Tageteae [[Polymnieae + Millerieae] [Madieae [[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]]]]] / Heliantheae alliance / Helianthodae: hydathodes +/0; genome duplication [6 X].
Age. The age of Helianthodeae is estimated to be ca 28 Ma, based on Echitricolporites mcneillyi pollen from Asia (Palazessi et al. 2022 - next clade, + Helenieae, Millerieae, Madieae, etc.).
[Neurolaeneae [Heliantheae + Coreopsideae]]: ?
Age. The age of this clade is ca 42.5 Ma (G. Zhang et al. 2024: Fig. 4).
16J. Neurolaeneae Rydberg - ?paraphyletic.
(phytomelan 0).
10/: Calea (80).
Age. Crown-group Neurolaeneae is around 37.6 Ma (G. Zhang et al. 2024: Fig. 4).
[Heliantheae + Coreopsideae]: pappus aristate.
Age. The age of this clade is some 40.8 Ma (G. Zhang et al. 2024: Fig. 4).
16K. Heliantheae Cassini —— Synonymy: Ambrosiaceae Berchtold & J. Presl, Helianthaceae Berchtold & J. Presl, Ivaceae Rafinesque, Partheniaceae Link, nom. inval., Xanthiaceae Vest
mean 2C size ca 9.74 pg.
Verbesina (300), Wedelia (100)
Age. Crown-group Heliantheae is about 39.1 Ma (G. Zhang et al. 2024: Fig. 4).
Feddeeae Pruski, P. Herrera, Anderberg & Francisco-Ortega - Feddea cubensis Urban - ?Here? ?To be kept separate?
Lianescent; florets all tubular; anther thecae with apical appendage, basally long caudate; base of style swollen, stigmatic bands 2, adaxial-lateral; cypsela epidermis with crystals, phytomelan 0, pappus uniseriate.
1/1: East Cuba.
16L. Coreopsideae Lindley —— Coreopsidaceae Link, nom. inval.(C4 photosynthesis + - Coreopsis); (pappus with two awns), (phytomelan 0 - Dicranocarpus); x = 12-17.
Bidens (235/?340), Coreopsis (75), Dahlia (42).
Age. The crown-group age of this clade is some 37.3 Ma (G. Zhang et al. 2024: Fig. 4).
[Tageteae [[Polymnieae + Millerieae] [Madieae [[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]]]]: (pappus palaeaceous).
Age. The age of this clade is around 40.1 Ma (G. Zhang et al. 2024: Fig. 4).
16M. Tageteae Cassini
Secretory cavities + [Tagetineae].
Pectis (100), Tagetes (50).
Age. Crown-group Tageteae is about 35.5 Ma (G. Zhang et al. 2024: Fig. 4).
[[Polymnieae + Millerieae] [Madieae [[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]]]: ?
Age. The age of this clade is ca 39.1 Ma (G. Zhang et al. 2024: Fig. 4) - but note the circumscriptions of clades around here.
16N. Polymnieae Panero
16O. Millerieae Lindley
mean 2C size ca 5.24 pg.Espeletia (140)
[Madieae [[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]]: ?
Age. The age of this clade is ca 39.8 Ma (G. Zhang et al. 2024: Fig. 4).
16P. Madieae Jepson
Age. The age of crown-group Madieae is ca 36.4 Ma (G. Zhang et al. 2024: Fig. 4).
[[Chaenactideae + Bahieae] [Perityleae + Eupatorieae]]: ?
Age. This clade is aged at ca 38.7 Ma (G. Zhang et al. 2024: Fig. 4).
Age. The crown-group age here is around 33.4 Ma (G. Zhang et al. 2024: Fig. 4).
16Q. Chaenactideae B. G. Baldwin
Age. The age of crown-group Chaenactideae is ca 23.1 Ma (G. Zhang et al. 2024: Fig. 4).
16R. Bahieae B. G. Baldwin
Age. The age of crown-group Bahieae is ca 28.1 Ma (G. Zhang et al. 2024: Fig. 4).
Age. The clade [Perityleae + Eupatorieae] is aged at ca 37 Ma (G. Zhang et al. 2024: Fig. 4).
16S. Perityleae B. G. Baldwin
Annuals to herbaceous/suffrutescent perennials/shrubs;
7/84: Laphamia (10). S.W. U.S.A. to Central America, Ecuador to Chile.
Age. Crown-group Perityleae are just over 15 Ma (Lichter-Mark & Baldwin 2023).
16T. Eupatorieae Cassini —— Synonymy: Eupatoriaceae Berchtold & J. Presl
(Plants shrubby), (stem twiner - Mikania), (annuals); pyrrolizidine alkaloids + [esp. lycopsamines]; ray florets 0; mean 2C size ca 3.33 pg.
Eupatorium (1200 [s.l.] or 40 [s.s.]), Mikania (430, 309), Ageratina (290), Stevia (235), Chromolaena (165), Brickellia (110), ?Koanophyllum (110), Fleischmannia (80).
Age. The age of crown-group Eupatorieae is ca 33.9 Ma (G. Zhang et al. 2024: Fig. 4).
Evolution: Divergence & Distribution. Papers in Funk et al. (2009a) summarize biogeography, clade ages, and much more for each tribe, while a number of ages are provided by Rivera et al. (2021) in which the focus is on Mexican taxa. For ages in Carduoideae, see Herrando-Moraira et al. (2019: note topology) and within Cardueae, see Ackerfield et al. (2020). Barreda et al. (2010a, esp. 2015, 2016) and also Panero (2016) discuss fossil pollen.
Palazzesi et al. (2022) summarize what is known about several aspects of composite evolution, including diversification, genome size and duplications, etc., and date the oldest Asteraceae fossils from ten nodes (see above) that could be used in calibration. This section ends with some brief notes on the capitulum that characterizes the family but whose nature is unclear, and on island woodiness that is common here.
Depending on the identification of the pollen grains, the subfamilies up to the Pertyoideae may all have diverged by the late Eocene about 34 Ma; other suggestions are of an (early) Oligocene radiation of the subfamilies (K.-J. Kim et al. 2005; Funk et al. 2005; M. S. Barker et al. 2008; Torices 2010). Alternatively, all of these subfamilies, apart from the Cretaceous Barnadesioideae, diverged rapidly, perhaps within 10 Ma, in the Palaeocene-early Eocene by around 50 Ma (Barreda et al. 2015; C.-H. Huang et al. 2016: Famatinanthoideae also diverged earlier); see also Funk et al. (2014) for dates. Using a rather different topology, Mandel et al. (2019) suggested that ca 20 Ma elapsed between the Cretaceous divergence of Barnadesioideae and the diversification of the rest of the family, which did not begin until the Palaeogene.
In any event, diversification of Asteraceae probably began in southern South America - the distributions of the basal clades are centred on South America (McDonald-Spicer et al. 2019). Subsequently there was movement to Africa, perhaps by way of islands on what are now the Rio Grande Rise and the Walvis Ridge; Asteraceae making this move may have evolved features common in island plants (Carlquist 1974) like more or less shrubby or tree-like growth forms (Katinas et al. 2013) and increased "seed" size (Kavanaugh & Burns 2014); a woody/shrubby habit is common in several of these early South American clades (Panero et al. 2104). With the earlier pollen-based date from the Antarctic suggested by Barreda et al. (2015: see above) movement around high southern latitudes is also plausible. Many clades then arose in the course of a subsequent radiation from Africa (Panero & Funk 2008; Funk 2009; Funk et al. 2009c; Mandel et al. 2019: ca 42 Ma; McDonald-Spicer et al. 2019; see also Kim & Jansen 1995). Panero and Crozier (2016: c.f. topology) suggest that the subfamilies from Carduoideae to Asteroideae on the tree (subfamilies 11-16 above) diverged within about 6.5 Ma of each other in the Middle Eocene in Africa, while Mandel et al. (2019) note that a somewhat different configuration of clades, but with similar included taxa (Tarchonanthoideae (9 above) to tribes in the Asteroideae-Senecioneae area in the phylogeny above) diverged, and they estimated about the same time, around 42.2-36.3 Ma. Thus both South America and Africa are central to our understanding of the early spread and diversification of the family, and Mandel et al. (2019) link this early diversification to climatic changes then. There were important increases in diversification during the Eocene period just mentioned, and also within the Senecioneae and especially the Heliantheae alliances (Mandel et al. 2019: see Fig. 3). However, there are other scenarios, for instance, Samant and Mohabey (2014) thought that Asteraceae originated in Late Cretaceous India.
It is hardly surprising, given the size, distinctiveness and ubiquity of the family, that there should be speculation about what has caused this, whether the development of the capitulum itself, functionally a single floret and often with very high seed set, the storage of carbohydrates as unbranched-chain fructans, the diversity of secondary metabolites produced (see Calabria et al. 2009, etc.), or some other reason (see also Funk et al. 2009c; Panero & Crozier 2016). For possible apomorphic characters for the family and its major clades, see e.g. Hansen (1991), Jansen et al. (1991), Bremer (1994), Leins and Erbar (2000), Erbar and Leins (2000), Funk et al. (2009c), Roque and Funk (2013), Panero et al. (2014) and Telleria et al. (2015: emphasis on Barnadesioideae). Burleigh et al. (2006) suggest that by some measures Asteraceae do show a notable shift (increase) in morphological complexity. There was a palaeopolyploidy event involving most or all of the family, and Schranz et al. (2012) thought that although there was a lag time between this duplication event and subsequent asteraceous diversification, the two might be causally linked (see also Tank et al. 2015). More attention should be paid to the significance of pollination of Asteraceae by oligolectic bees (see below).
However, there are perhaps parallels with Poaceae, which coincidentally also have large-scale genome duplications and store carbohydrates as fructans, also with Orchidaceae and even with angiosperms as a whole. Interestingly, both Asteraceae and Poaceae are very diverse in grassland vegetation (although in terms of biomass there is of course no contest), and Palazzesi et al. (2022a) suggested that major diversification in both families was associated with the development and spread of grassland perhaps 28-20 Ma that in turn could be linked to the decrease of atmospheric CO2 concentrations, decreasing precipitation, etc.. Note that Asteraceae in South Africa are notably common in the Renosterveld (de Preez et al. 2025b). However, diversification in Asteraceae, like that in many other groups, is perhaps best explained by focusing more on particular clades in the family rather than treating the family as a unit (Schranz et al. 2012; see also, perhaps, rate shifts in S. A. Smith et al. 2011; P. Soltis et al. 2019). Indeed, although Asteraceae contain about 8% of eudicot species, within Asteraceae, Asteroideae alone, the equal-youngest subfamily, include over 16,000 species, some two thirds of the family; only three to four of the fifteen other subfamilies have more than a (very) few species (see also Panero & Funk 2008; Panero et al. 2014; Panero & Crozier 2016) - Cichorioideae have ca 1,500 species, Vernonioideae ca 1,930 species and Carduoideae ca 2,520 species - and the [Stifftioideae + Mutisioideae] clade has ca 660 species (see also above). Thus the successive clades in the tree above have around 90, 1, 660, 125, 1, 70, 25, 100, 2,400, 2, 1,935, 1,100, 9, and 17,200 species (figures largely from Panero & Crozier 2016, emended in the context of Mandel et al. 2019), not surprisingly, evolutionary rates within the family are highly heterogeneous (S. A. Smith et al. 2011). Places where diversification rates seem to have changed are rather different in Panero and Crozier (2016, q.v. for more details), perhaps because of where they assign particular fossils and also because of their more detailed sampling; they thought that there were notable rate shifts at the [Cichorioideae [Corymbioideae + Asteroideae]] node and in a largely American clade in the Asteroideae-Heliantheae alliance whose fruits have phytomelanin (for which, see also Mesfin Tadesse & Crawford 2014; Mathur et al. 2024). C.-H. Huang et al. (2016) also discuss diversification in the family in the context of possible genome duplications. They suggest that there were increases in diversification rates in the [Carduoideae + The Rest] clade, within Asteroideae and in Barnadesioideae (the last only moderate - indeed), with decreases in diversification rates in Famatinanthoideae and Gymnarrhenoideae (Huang et al. 2016).
Note that the above set of stories have been made more or less compatible with the relationships suggested by Mandel et al. (2019). Turning now to individual groups
- Diversification in the initially eastern South American Gochnatioideae (sometimes Gochnatieae) has been discussed by Gostel et al. (2022).
- Carduoideae (= the old Cardueae) are now primarily Mediterranean, and Barres et al. (2013) discuss their history in some detail. Much of the diversification in the Mediterranean-Central Asian Centaureinae may be Plio-Pleistocene transition and younger (Hellwig 2004). Diversification of the high-altitude species of Saussurea (Saussureinae) may have occurred in the context of the uplift of the Qinghai-Tibetan plateau within the last 14 Ma (Y.-J. Wang et al. 2009); see also L.-S. Xu et al. (2019) but as with other studies of this genus topologies of chloroplast and nuclear analyses differ - crown-group ages range from 18.5-8.1 Ma. Herrando-Moraira et al. (2020) also outlined relationships here as did L. Xu et al. (2024: Fig. 4, hybridization extensive). Age estimates are within 2 Ma for the species from the Hengduan region in particular (Xing & Ree 2017). Xu et al. (2024: Fig. 6) looked at the variation of 6 morphological characters (18 states) in the context of their phylogenetic tree; parallel evolution was extensive. Jurinea (Cardueae) is an Irano-Turanian genus, and Herrando-Moraira et al. (2023) found a notable increase in diversification in Jurinea ca 3 Ma, a period of increasing aridity at the Plio-Pleistocene transition, with little happening between ca 10.7 Ma (crown age for the genus) and 6 Ma in particular. Here there are five main growth forms (one very uncommon): Plants can be annual, biennial, or perennial, rosette-forming, shrubby, etc., scapose or not. Species diversity is highest in the Central Asian region where there have been major orogenetic changes, lower in the climatically unstable Circumboreal (sic: not New World; speciation here allopatric) and lowest in the more humid East Asian region, the Jurinea clade here is the oldest. There have been other radiations of Asteraceae in this area (e.g. J. W. Zhang et al. 2011 and references). Cirsium, also Cardueae, arrived in North America ca 7-5 Ma, diversifying ca 2 Ma; the limited dispersability of its fruits, its liking for semi-arid conditions, and niche conservatism spurring diversification (Siniscalchi et al. 2023).
- Brachylaena (Tarchonanthoideae-Tarchonantheae) seems to have dispersed from Africa to Madagascar, but then wih a back dispersal to Africa (rather odd), or there may have been two dispersals to Madagascar (Kimball et al. 2023, q.v. for possible back-dispersal mechanisms).
- Bell et al. (2012b) suggested that there has been rapid diversification of Tragopogon et al. (Cichorioideae) in Eurasia, probably ca 2.6 Ma or at least within the last 5.4 Ma. Long distance dispersal of Hypochaeris from the Medierranean region to South America has been suggested (Tremetsberger et al. 2013), Similarly, dispersal of Arctotideae-Arctotidinae from South Africa to Australia probably happened within the last 14 Ma, most probably (4.4-)3(-1.7) Ma (McKenzie & Barker 2008).
- Africa may have been the centre of origin of the speciose Vernonioideae-Vernonieae, and they moved to South America (the Brazilian Shield), and then along volcanic chains to North America (see Ecology & Physiology below). Vernonieae in the Old World moved north throuh East Afrca, again over volcanic rock, and thence east to China and Australia and the northern Pacific, including Hawaii (Keeley et al. 2021). A variety of dispersal events of different kinds (all told, 400 biogeographic events plus) have been implicated in these extensive movements (Keeley et al. 2021).
- Asteroideae-Gnaphalieae include around 2,100 species, and Nie et al. (2015) found that the three basal clades of the tribe were from southern Africa (also Andrés-Sánchez et al. 2018), the other main clades also originating there, overall, an African origin for that tribe seemed likely although there has been extensive subsequent diversification in Australia (Schmidt-Lebuhn & Bovill 2021). Andrés-Sánchez et al. (2018) looked at diversification in this basal grade, emphasizing the association between life history and chromosome number: short life cycles (i.e., the annual habit) were correlated with low chromosome numbers (x = 4, 5, vs 7: Smissen et al. 2011). Bentley et al. (2017) focused on the Relhania clade of Nie et al. (2015), nearly all from Madagascar, Africa, and the Greater Mediterranean area and sister to the rest of the tribe; this is a confusing group some of the members of which have characters found elsewhere in Asteroideae. Australasia was the next area to be colonized, and most speciation in the tribe as a whole has been within the last 23 Ma, i.e. Miocene or younger (Nie et al. 2015, q.v. for much more detail; X.-M. Xue et al. 2024; etc.).
Bergh and Linder (2009) date the stem age of the some 550 species of the African part of Gnaphalieae to around (22.1-)15.6(-9.1) Ma and the crown age to (20.6-)14.6(-8.3) Ma. Blanco-Cavaldà et al. (2023) looked at relationships and distributions in the Helichrysum-Anaphalis-Pseudognaphalium (HAP) group (other genera are also involved), suggesting similar ages; 314 species are from Africa, of which ca 60 species are Afromontane, 12 species Afroalpine, 115 species are from Madagascar (some six colonizations), and so on; interestingly, although the pappus is deciduous, it seems that the small, light achenes are easily dispersed (Blanco-Cavaldà et al. 2023). With an origin in arid southern Africa, species of Helichrysum moved into the southern African grasslands, with subsequent dispersal north to the African mountains and elsewhere including at least four movements into the Afroalpine, adaptations to the extreme conditions there evolving independently. (Afroalpine taxa that came from the north seem already to have been adapted to conditions there - Blanco-Cavaldà et al. (2023).) (There is more on plants in this distinctive Afroalpine vegetation under Campanulaceae (the giant lobelias) and also below). X.-M. Xu et al. (2024, for which, see much detail) looked at relationships in the HAP clade - again, Africa, especially the Cape, was thought to be important in the origin of major groups here (see also Galbany-Casals et al. 2014), but there was also diversification in the general China-South East Asia area, etc.. Xu et al. (2024) provided a quite extensive discussion of the biogeography and relationships of the groups they obtained, and these authors noticed a correlation between the two groups of Anaphalis and ornamentation of the achene and presence of a decurrent leaf base (there were also connections between morphology and phylogeny in Leontopodium - Xu et al. 2023b). But things get complicated; as Xu et al. (2024: p. 15) noted, "Obviously, the ancestral region of Anaphalis was definitively in Southwestern China, ... but the dispersal patterns of the two Anaphalis lineages were markedly different ... The two Anaphalis lineages appeared to have originated in Africa, then spread to Western and Southern Asia,". Furthermore, there is extensive conflict between relationships suggested by plastid and nuclear data; these authors prefer the former, support being stronger (see Phylogenetic Relationships - Gnaphalieae below).
- For some divergence dates in Asteroideae, see C.-H. Huang et al. (2016: table S3). J.-Q. Liu et al. (2006) suggest that the uplift of the Qinghai-Tibetan plateau has been involved the diversification of the 200+ species of the Ligularia—Cremanthodium—Parasenecio clade (Asteroideae-Senecioneae), relationships within which are largely unresolved. Inuleae-Plucheinae are commonly plants of arid conditions that may have originated in southwest Africa, e.g. the Namib and Kalahari deserts (Nylinder et al. 2016). Riggins and Siegler (2012 and references; see also Pellicer et al. 2010c) discuss the biogeography of Artemisia (Anthemideae), Eurasian in origin, and they note that there seems to have been extensive migration here - A. magellanica is related to north temperate taxa in the small subgenus Pectinatae. Malik et al. (2017) suggest that the centre of the large subgenus Seriphidium was in Central Asia (see Jiao et al. 2025 for subgenera, etc.), and C. Guo et al. (2025) note that diversity in the some 114 Artemisia species growing in the Hengduan Mountain region correlates with topographic heterogeneity. Strijk et al. (2012) discuss the dispersal of a polyphyletic Psiadia (Astereae) to Madagascar and thence to the Mascarenes. Leptinella plumosa (Anthemideae) may have moved on to the isolated subantarctic Heard Island by long distance dispersal - it is an inhabitant of other such islands (Turner et al. 2006), indeed, Oberprieler et al. (2022) noted that the basal branches in Anthemideae were all made up of taxa from the southern hemisphere. Perityleae include a number of species found in desert habitats in North America and W. South America (the Atacama Desert). Diversification within the tribe began ca 12.6 Ma, well before the evolution of the desert habitat in which it grows which happened 7-5 Ma. However, the ability to grow in very dry habitats evolved in species that moved on to rock outcrops from tropical deciduous forest, growth on these outcrops being a preaption for growth in deserts (Lichter-Mark & Baldwin 2023).
Although Asteraceae are worldwide in distribution, around 14% of all Asteraceae, ca 3,300 species, are to be found in Mexico (3,113 species, 60% endemic, 417 genera: Rivera et al. 2021), Asteroideae-Eupatorieae alone having around 530 of its ca 2,000 species there (Schilling et al. 2015 and references); there is a fair bit of diversification in the pine-oak forests (Soejima et al. 2017). Asteraceae are also diverse in the Caribbean where there are some 41 endemic genera (Francisco-Ortega et al. 2008). Clades of Asteraceae have also diversified notably in the Cape flora (Linder 2003).
In a comprehensive survey, Martínez-Quezada et al. (2022: tree based on 11 chloroplast markers, nearly all tribes and subfamilies - as of 2021 - sampled) describe the variety of foliar secretory structures in the family, including hydathodes, glandular hairs and laticifers. The distribution of these features is examined in a phylogenetic context, and Martínez-Quezada et al. (2022) note that more basal clades tend not to have these structures.
In a study of pollen disparity, Jardine et al. (2022: 23 discrete characters, 113 taxa) found that there were two groups in Asteraceae, Asteraceae 1 (Barnadesioideae to Hecastocleioideae, ca 85 Ma) and 2 (Carduoideae + the rest, ca 40 Ma) - Pertyoideae, Tarchonanthoideae and Dicomoideae were not studied. Interestingly, members of Asteraceae-Barnadesioideae (= Asteraceae 1) are more or less intermediate between the three pollen groups (the third is made up of Campanulaceae-Rousseaceae plus core Asterales, and there was no disparity increase between the two Asteraceae groups (Jardine et al. 2022). Evolution of the considerable variation in pollen morphology in Asteraceae has often been understood using the distorting lens of pollen "types". However, Blackmore et al. (2009; see also Skvarla et al. 1977 for pollen terms) decompose these types into a number of individually-varying characters/states - note that some are still arbitrary, being divisions of continua - and then discuss their distribution on the Asteraceae tree; pollen characters provide apomorphies at various levels. Caveate pollen is found in Arctoteae and some Lactuceae (Cichoroideae), and may be more basal on the tree than it is placed here (as a synapomorphy for [Corymbioideae + Asteroideae]). Indeed, Blackmore et al. (1984) noted that caveae are evident early in development in Gerbera (Mutisieae), but not later, and suggested that pollen grains of Asteraceae might all be caveate - however, the very definition of caveate is unclear (Blackmore et al. 2009). Blackmore et al. (2010), in a very interesting review, discuss pollen development, showing how self-assembly of many pollen features is common, but that changes in the glycocalyx, the primexine matrix (made up of glycoproteins), and in pH, etc., affect details of this self assembly, and some of the more proximate variation is probably under genetic control.
Calabria et al. (2007) produced a phytochemical phylogeny at the tribal level. Svoma et al. (2019) looked at the evolution of staminal features in Barnadesioideae, of interest both because of the staminal variation in Calyceraceae (see Pozner et al. 2021 for recent ideas of relationships there) and also in the other Asteraceae. For morphological variation in Barnadesioideae, see also Ferreira et al. (2021). Stuessy and Urtubey (2006) had earlier looked at corolla morphology in the subfamily. Ezcurra (2002) suggested that diversification in Chuquiraga, a genus of southern South American origin and with around 23 species, could in part be linked with colonization of the northern Andes and adoption of hummingbird pollination (by Oreotrochilus) of a clade there and in part by adaptation to xeric climates in southern South America (a separate clade is involved) that are associated with a variety of foliar modifications and with herbivory by the camelid mammal, the guanaco. Character evolution in Inuleae (inc. Plucheeae) is discussed by Nylinder and Anderberg (2015).
The distinctive capitulum of Asteraceae, often with rays and disc florets of very different morphologies (see e.g. Harris 1995 for much on inflorescence and floret development, 1999), has no terminal floret and seems to be basically racemose, unlike that of its sister taxon, Calyceraceae. However, it can be derived from the inflorescence of the latter with its cymose lateral branches if these braches become reduced to a single floret (e.g. Pozner et al. 2012). Claβen-Bockhoff and Bull-Hereñu (2013) suggest that the capitulum develops centripetally by apical fractionation, although they note that in supercapitulae (capitulae of capitulae) the development of the whole inflorescence may be centrifugal - as may be the development of capitulae in ordinary inflorescences (Liatris (Ast.-Eupatorieae) is a good example) and that of the ray florets in particular. Thus in Gorteria (Vern.-Gorterieae) the outermost (ray) florets develop centrifugally and more slowly than the acropetally-developing more central (disc) florets (Philipson 1953, Harris 1995 and references; Leins & Erbar 2003b; Thomas et al. 2009), and there are several cases where the peripheral florets develop more or less centrifugally (literature summarized by Pozner et al. 2012). Interestingly, both those Calyceraceae which have an inflorescence most similar to that of Asteraceae, and those Asteraceae with some centrifugal development of florets that can perhaps be linked with the cymose part-inflorescences common in the outgroups to Asteraceae, are derived within their respective families (see also Pozner et al. 2012; Denham et al. 2016: phylogeny of Calyceraceae). However, the development of the capitulum of Gerbera, in the more basal Mutisioideae (Mutisieae), and the role that the Leafy (LFY) gene plays in both inflorescence and floral development is compatible with the interpretation that ray florets correspond to these lateral branches of the inflorescence of Calyceraceae (Zhao et al. 2016; see also Bello et al. 2013: Anacyclus). For the role that auxin plays in the patterning of the capitulum and regulating genes involved in the development of the florets, see Zoulias et al. (2019). Note that the floral bracts in some Asteroideae-Heliantheae (the tribe includes taxa that were in Eupatorieae) have been reacquired more than once and are certainly not plesiomorphic in the family as was once thought (see e.g. Harris 1995 for literature). In plants like Cirsium the involucre is well developed, the inflorescence bracts perhaps continuing the phyllotactic spiral of the stem, and the spiral phyllotactic lattice of the bracts developing into the spirals of the florets. However, in taxa like Gerbera with a scapose inflorescence there is no lattice, the bracts starting to initiate almost simultaneously, a lattice of florets arranged in parastichies then developing (see T. Zhang et al. 2021 for details).
Overall, the flower-inflorescence distinction has become blurred in Asteraceae, and at the functional and also the molecular developmental level there are surprisingly close parallels between inflorescences here and flowers (T. Zhang & Elomaa 2020). The capitulum is functionally very much like a single flower, with the ray florets the equivalent of petals, and in many Asteraceae it can be called a pseudanthium (Baczynski & Claßen-Bockhoff 2023: Table 1). Interestingly, just as the common petal numbers in angiosperms (3, 5) are sequential members of the Fibonacci series, so are the common ray floret numbers in Asteraceae (5, 8, 13). Not only is there often variation in the morphology of the florets in the one capitulum, but also in the sexes of those florets. The evolution of floral morphology and breeding systems in Gochnatioideae is discussed by Gostel et al. (2022: as Gochnatieae).
The whole capitulum can become reduced to a single floret, and such capitula usually aggregate and form a supercapitulum. A version of a supercapitulum in which each aggregated capitulum has a number of inflorescence bracts and more than a single floret is to be found in Pterocaulon (Ast.-Inuleae), and in Craspedia (Ast-Gnaphalieae), too, the supercapitular units each have a few flowers. Echinops (Card.) has a classic supercapitulum with a spherical head of numerous 1-flowered capitulae. The achenes in such supercapitulae generally lack a pappus, and the individual flowers of the reduced head surrounded by a keeled involucral bract may all be dispersed together (synaptospermy: Claßen-Bockhoff 1996b; Katinas et al. 2008a; Katinas & Forte 2020). There may also be yet another round of aggregation (Claßen-Bockhoff 1996b for details; Harris 1994 [Caenozoic capitulae], 1999; Leins & Gemmeke 1979; Katinas et al. 2008a). Supercapitulae have evolved at least three times in Vernonioideae, perhaps with reversion to normal capitulae (Loeuille et al. 2015a), and a couple of times in Inuleae s.l. (Nylinder & Anderberg 2015), in Fucaldea (Barn.), etc.. The presence of supercapitulae has been thought to be of taxonomic importance (Loeuille et al. 2015a).
Some genes whose expression is normally restricted to individual flowers may be more widely expressed in the asteracous capitulum as a whole, as well as in vegetative shoots (Ma et al. 2008). Disc florets are quite often polysymmetric and the ray florets monosymmetric, and this monosymmetry seems to be caused by the CYC2c gene family, different members of which have been independently co-opted by different taxa in Asteraceae (Chapman et al. 2010). Although the genes involved in the development of monosymmetric florets of Senecio vulgaris are homologous to those in Antirrhinum (Plantaginaceae) they are regulated and expressed differently, growth in the adaxial part of the corolla being reduced and that in the abaxial part increased, hence the ray phenotype (Garcês et al. 2016). Of course, the common ancestor of Plantaginaceae and Asteraceae is likely to have had polysymmetric flowers (see Euasterids below). Interestingly, Dasyphyllum (Barn.) lacks members of the CYC2b, d and e gene families common elsewhere in Asteraceae, while Acicarpha (Calyceraceae) also lacks CYC2c genes (Chapman et al. 2010).
Asteraceae, Islands, and Woodiness.
Asteraceae are prominent elements of the vegetation of both oceanic islands and sky islands (isolated high-altitude areas) and are noted for their high endemicity in such places (Lenzner et al. 2017; see Grossenbacher et al. 2017: high self-compatability; Cavanaugh & Burns 2014; Stuessy 2020). Thus Roeble et al. (2024) suggested that there were some 1,873 species of Asteraceae on oceanic islands of which around 52% were endemics; they are the result of at least 19 adaptive radiations, Asteraceae being the most diverse family on such islands (Roeble et al. 2024 also give figures for species on continental islands like New Guinea.) S. C. Kim et al. (2007) note that woody, island-dwelling forms have been independently derived within Cichorioideae-Sonchinae, and there is a radiation of woody species of Sonchus (Cichorioideae-Cichorieae) on the Mascarenes that can be dated to ca 8.9-8.5 Ma (Kim et al. 2008) and another of 11 species on the Juan Fernández Islands, where there have been three separate ca 181 km dispersal events from the mainland (Stuessy 2010, the plants used to be called Dendroseris). Argyranthemum (Asteroideae-Anthemideae) is another major radiation on Macaronesia, although less studied recently (Francisco-Ortega et al. 1997). Scalesia (Ast.-Heliantheae), from the Galapogas, includes ca 15 species that are shrubs to quite large trees.
The Hawai'ian clade that includes the remarkable silversword, Argyroxiphium sandwicense (Asteroideae-Madieae), is a classic example of both an island radiation and insular woodiness. It and other examples from Asteraceae make up just over one quarter of all the examples of adaptive radiations mentioned by Schenk (2021, see also Palazzesi et al. 2022); Zizka et al. (2022) suggest that Asteraceae make up ca 256/1,100 insular-woody species (overall, there have been 175 insular transitions to woodiness, 47 in Asteraceae alone). There are ca 204 insular-woody species, 37 transitions on the Canaries, ca 199 insular-woody species and 17 transitions on Hawaii (there is a monophyletic group of ca 126 woody lobelioids in five genera on Hawaii, but the ancestor of that clade was woody - e.g. Givnish et al. 2009a). It is descended from herbaceous tarweed-like ancestors of the Madia—Raillardopsis group from western North America. With some 33 species (currently placed in three genera, but forming a single clade) that diversified (6-)5.2(-4.4) Ma, it is a good example of hybridization between North American species (for which, see Barrier et al. 1999, also Stachys - Lindqvist & Albert 2002) followed by an insular adaptive radiation that has resulted in species that are trees, shrubs and cushion plants and grow in anything from quite xeric to bog habitats. Perhaps the most spectacular member is the silversword itself, a monocarpic rosette plant often found growing in desert-like conditions on the sides of the volcanos (see e.g. Baldwin 1997; Baldwin & Sanderson 1998; Baldwin & Wessa 2000; Carlquist et al. 2004; Lim & Marshall 2017; Baldwin et al. 2021; Schenk 2021, also the Madiinae Showcase). Ages in Landis et al. (2018) are ca 5.1 Ma for the stem group, ca 3.5 Ma for the crown group, so there is a distinct evolutionary fuse - but note that a mere (3.1-)1.1(-1) Ma is the crown-group age in Nürk et al. (2019). Kaua'i was probably the island colonized first, and there has been both continued diversification on that island and also movement on to the younger islands that largely follows the progression rule - the younger islands have been colonized later (Landis et al. 2018; Shaw & Gillespie 2016 for the progression rule). Ecologically, there has been movement from dry to wetter habitats and with several adoptions of the bog habitat (Baldwin et al. 2021). Variation in foliar functional traits like venation density here approaches that in angiosperms as a whole, but it shows little correlation with phylogeny (Blonder et al. 2016). Overall, regulatory genes showed an accelerated rate of evolution, but structural genes for the most part did not (Barrier et al. 2001). There is some chloroplast variation, and also intergeneric hybridization (Baldwin 1998). Overall, disparification (≡ Simpsonian adaptive radiation), plant height being emphasized, and diversification (= species number increase) has been rapid, the latter despite an increase in generation time in the woody taxa (Nürk et al. 2019). Woodiness in Asteraceae has also evolved in the Canaries, there are tarweeds on the California Islands and adjacaent mainland, etc. (Nürk et al. 2019; Zizka et al. 2022).
Bidens (Asteroideae-Coreopsideae) is also diverse on Hawai'i, where there are some 19 species with more variability in growth form, dispersal mode, etc., than in the whole of rest of the genus (in total there are some 230 species on five continents, including 25 species on the South Pacific islands). The crown-group age is estimated to be (2.6-)1.8(-1.1) Ma (Knope et al. 2020c). As with many other taxa on the islands, there is little in the way of molecular variation or of genetic barriers between the species; diversification rates are high here, too, but note that there is possible hybridization, etc. (Baldwin 1998; Ganders et al. 2000; Keeley & Funk 2011; Knope et al. 2012; Knope et al. 2020c). Knope et al. (2020a, b, c) discuss relationships among the Hawaiian species. Hawaiian species of Bidens are part of a radiation of about 41 species in Polynesia that has been dated to as recently as (2.7-)1.6(-0.7) Ma (Knope et al. 2020b, q.v. for more dates); the monophyly of the Hawaiian species with respect to the rest of the Polynesian species is somewhat unclear, as is the ancestry of the whole clade (Knope et al. 2020b, c: somewhere in America). The achenes of Bidens, which commonly have retrorsely-barbed awns, may lose these barbs, or the awns as a whole, sometimes then becoming winged, etc., the loss of the original dispersal mechanism being associated with movement into upland interior habitats (Carlquist 1966b), overall, a very interesting example of adaptive radiation (Knope et al. 2020b, c). Finally, Hesperomannia (Vernonioideae-Vernonieae) is another Hawaiian endemic, and its closest relatives (now placed in Gymnanthemum) are thought to be African, the two diverging (27-)26-17(-14) Ma, old for a Hawaiian endemic, so if this holds up there may have been more than the usual amount of intra-archipelago island hopping (H. G. Kim et al. 1998).
The iconic stout-stemmed (pachycaul) giant senecios (= Dendrosenecio, 5-7 spp.: Asteroideae-Senecioneae) live on sky islands, the East African mountains (e.g. Hedberg 1964). They form a closely-related clade members of which often grow in extreme conditions above 3,500 m; the species show extensive parallel adaptations, and there has also been movement between the volcanoes and subsequent hybridization (e.g. Knox & Palmer 1995a, b; Tusiime et al. 2020). Gizaw et al. (2022) found quite extensive differences between the topologies of nuclear and plastid phylogenies, and noted that the former showed better agreement with geography; there seems to have been both hybridization and infrequent long distance dispersal here (see also Brochmann et al. 2021; Kandziora et al. 2022 for the Afroalpine flora). Dendrosenecio is not immediately related to other Afro-alpine species of Senecio s. str., which are found in five separate clades and represent 4-14 independent movements into alpine habitats (Pelser et al. 2007 for the phylogeny of Senecio itself; Kandziora et al. 2016b). The initial adaptations to montane conditions probably took place in the Drakensberg, to the south (Kandziora et al. 2016b), and there were also subsequent colonizations of South America and the Palaeoarctic, probably twice, one clade including annuals (inc. S. vulgaris, iconic, perhaps, but in a different way) and the other, perennials of the mountains (Kandziora et al. 2016a). Within Senecio s. str. there has been intercontinental dispersal between areas with Mediterranean and desert climates (Coleman 2003). Helichrysum (Ast-Gnaphalieae) is also found in these Afroalpine habitats, but here taxa from the Drakensberg seem not to be the immediate antecedents of the species there (Blanco-Gavaldà et al. 2023); for further discussion, see below.
Espeletia (Ast.-Millerieae) is a characteristic genus of the Andean páramo, and the basic condition for the genus is a branched, woody, rosette-like growth form - interestingly, tree growth forms have evolved ca 3 times from rosette forms (the reverse change has never occurred) and grow at somewhat lower altitudes than the bulk of the genus (Pouchon et al. 2018). The around 145 species of Espeletiinae, frailejones (8 genera, = Espeletia s.l.) are endemic to the Andes and have diversified within the last (2.6-)2.3(-2.0) Ma; they represent a substantial and very rapid diversification, much occurring in the early Pleistocene, hybridization also being involved (Pouchon et al. 2018; Madriñan et al. 2013; Lagomarsino et al. 2016: see also Dianthus and Lupinus, Schley et al. 2022 for more examples). (Note that Heads (2019b) suggested that Espeletia arrived in the páramo via passive uplift, piggy-backing on the Andean orogeny to reach the altitudes at which it now grows, and this would make the genus rather older.) There have been more or less independent radiations in the Venezuelan and Colombian páramos and extensive parallel evolution of growth forms (Diazgranados & Barber 2017; Pouchon et al. 2018). Vargas-Martínez and Sanchez (2025) looked at variation in some foliar and root characters; the latter showed little variation, while groups of species which had vegetative variation in common clustered. Other Asteraceae like Diplostephium, made up of trees and shrubs, are to be found in the páramo - ca 70 species there (Sklenár et al. 2011 and references; Vargas et al. 2017). And as Small (1919: p. 142) noted of the ability of Asteraceae to colonize places like Krakatau, "The Compositae, indeed, seem to have been formed with the mountains by the mountains for the mountains".
For an account of island biogeography, see Whittaker et al. (2023, esp. Chapter 11). Cyrtandra (Gesneriaceae-Didymocarpoideae ), Cyanea and relatives (Campanulaceae-Lobelioideae), Myrtaceae, see Divergence & Distribution for Metrosideros, early stages, Schiedea (Caryophyllaceae), and the Stachys area (Lamiaceae), etc., include other major Hawaiian clades, and groups like Dracaena (Asparagaceae-Convallarioideae), Amaranthaceae and the Aeonium alliance (Crassulaceae) are also prominent on islands and have woody taxa - all told, it has been estimated that there have been some 175 transitions to woodiness on islands in general (i.e., including places like the Greater Antilles, etc.), some 1,100 species being the result (Zizka et al. 2022); see also Hypericum, Echium and Lupinus for similar radiations on (sky) islands.In general, species in clades of island plants, at least those in the S.W. Pacific (but also on sky islands), that were ancestrally large plants have tended to become smaller and those that were small plants have tended to become larger. Examples of increasing size/woodiness are particularly well known in Campanulaceae, Boraginaceae and here in Asteraceae (Biddick et al. 2019, see also Friedman 2020: c.f. also animals on islands). Woodiness has evolved many times in island plants and is associated with both species diversification and disparification there (Nürk et al. 2019). Woody Asteraceae are indeed common on ecological/actual islands, i.e. including mountains throughout the tropics, and there the evolution of woodiness and distinct dispersal modes is common (e.g. Carlquist 1974). Carlquist (1966c, see also 1974) discussed the loss of dispersability in Hawaiian taxa, a loss common in island taxa, while Burns (2018) suggests that this is not the whole story; overall, seed size has tended to increase in such island plants while obvious means of seed dispersal have been lost (Biddick et al. 2019), although there are some double mutualisms in which two interacting species benefit each other in two different ways, e.g. both pollination and seed dispersal. There is evidence for the operation of Baker's Law - self compatability is initially favoured, then dioecy (Whittaker et al. 2023), however, as Crawford et al. (2023) noted in their extensive study of compatability systems in island Asteraceae, dioecy is uncommon here. Freedman et al. (2024, see also Bowen & van Vuren 1997) compared shrubs on the Californian Channel Islands with their mainland representatives and carried out common garden experiments with the perennial herb Stachys bullata. They noted that defensive traits in the island plants were reduced, consistent with the historic absence of large herbivores there, however, they also observed that climatic conditions on these islands differ from those on the mainland, and this perhaps confounds our understanding of this variation (Freedman et al. 2024). Furthermore, a meta-analysis of insular woody species from the Balearic and Canary Islands did not suggest that they had lower anti-herbivore defences (Moreira et al. 2022). Interestingly, the proportion of AM plant species in the island floras was found to decrease as the isolation of islands increased (Delavaux et al. 2021). Zizka et al. (2022) discuss the reasons for the evolution of insular woodiness - factors like the aseasonal climate and drought play a role, but note that factors that promote woodiness on oceanic and continental islands may differ.
Ecology & Physiology. Growth form is notably labile in Asteraceae compared with that in most other campanulids, and many taxa are woody-herbaceous intermediates (Beaulieu et al. 2013b: the character there = woody vs herbaceous, but c.f. e.g. Carlquist 2013). As mentioned above, woody Asteraceae are common on ecological/actual islands, and more or less stout-stemmed (pachycaulous) trees have evolved a number of times on mountains. A number of species of Saussurea (Carduoideae) in the general Himalaya—Tibet—Hengduang Mountain region have evolved remarkable growth forms, including taxa like S. velutina, in which the young inflorescence is surrounded by large white leaves, apparently involved in keeping it warmer, also protection against ultraviolet light, etc. (Y. Yang & Sun 2009: c.f. Rheum-Polygonaceae), S. laniceps, where the capitula are individually surrounded by a ruff of bracts, long white hairs, etc., S medusa, in which the dense leaves along the young inflorescence axis are completely covered by long white hairs, the blade itself being terminated by a long, recurved, spine-like apex, and so on. Climbing Asteraceae are prominent in montane forests of South America, some 470 scandent species being reported from the New World (Gentry 1991), Mikania being one of the ten globally most species-rich genera of climbers (Sperotto et al. 2023), and a number of these have leaf tendrils (Sousa-Baena et al. 2018b).
The storage of carbohydrates as unbranched-chain fructans may contribute to the ability of Asteraceae to live in the rather dry conditions that many of them prefer (John 1996).
Siniscalchi et al. (2021b) summarize work on photosynthetic mechanisms in Asteraceae. Some species of Flaveria and Pectis (both Asteroidae-Tageteae, but not immediately related) have C4 photosynthesis, some have C3, and some are intermediate with C2 photosynthesis (see e.g. Schlüter & Weber 2020). Details of the metabolic changes involved are quite well understood, and there have been two or more (depending on the phylogeny) shifts to the C4 condition, and another shift in Coreopsideae (Bläsing et al. 2000; McKown & Dengler 2009; Ludwig 2011a and references, b, c; Gowik et al. 2011; Schulze et al. 2013; T. L. Sage et al. 2013; Christin & Osborne 2014; Aldous et al. 2014: PEPC protein kinases; R. Sage 2016). There are also proto-Kranz species (R. Sage et al. 2014). In C4 Flaveria carbonic anyhydrase (CA) becomes localized to the mesophyll cytosol, and changes in the CA gene that involved the loss of transit peptides paralleled comparable changes in the C4 Neurachne (Poaceae-Paniceae), although they were rather more extensive (Clayton et al. 2017). Overall, development of C4 photosynthesis here, with the bundle sheath accumulating organelles and the mitochondrial glycine decarboxylase and with interveinal distances decreasing, is quite similar to that in Boraginaceae and Poaceae (Khoshravesh et al. 2019; c.f. B. P. Williams 2013). Interestingly, the water use efficiency of C4 plants was found to be higher than in C3 and C3/C4 intermediate plants, as in Poaceae (Vogan & Sage 2011). Heckmann et al. (2013) discuss C4evolution here, likening the basic evolutionary landscape to Mt Fuji, and they noted that "the fitness gain achieved by each individual change remained comparable along evolutionary trajectories" (ibid. p. 1585). There was, however, only weak selection on the initial mutations, furthermore, conditions of drought, high light and high temperature are not universally favoured (see Heckmann et al. 2013). The initial steps in Christin et al. (2011b) suggest a number of dates for C4 origins, and all are less than 4 Ma; the repeated changes in photosynthetic mechanism may reflect an underlying "predisposition" (McKown et al. 2005), as in the evolution of C4 photosynthesis in other groups, but note that Lyu et al. (2015) using RNA-seq data found relationships that differed somewhat compared with those suggested in earlier phylogenetic work. CAM photosynthesis seems to be much less common, and 31/33 records are from Senecioneae. See also Mallman et al. (2014: photorespiration) and Bräutigam and Gowik (2016: general).
For selenium hyperaccumulation, see references in Schiavon and Pilon-Smits (2016). There are some nickel hyperaccumulators in the family, and Orlowska et al. (2013) found that inoculation of Berkheya coddii (Card.) with arbuscular mycorrhizal fungi led to an increase in the concentration of nickel in particular in the vascular tissue of lateral roots, and the uptake of other elements was also affected. Asteraceae as a whole quite commonly grow in soils developing over ultrmafic rock and in other extreme soil types. This is particularly pronounced in Vernonieae, i.a. found on iron-rich soils over volcanic rocks, hence ironweed, Vernonia itself, but also on other soils rich in iron, aluminium, calcium and/or magnesium, almost a signature of the tribe (Keeley et al. 2021).
Carlquist et al. (2021) discussed features of wood anatomy of Barnadesioideae in the context of the dry, cold and open conditions that some of its members prefer. Urtubey et al. (2023) focussed on the leaf morphology and anatomy of Schlechtendalia luzulifolia, sister to the rest of the subfamily; its linear leaves have parallel veins, often tetracytic stomata, uniseriate hairs (in addition to barnadesioid trichomes), etc..
Pollination Biology & Seed Dispersal. For the morphology and evolution of the capitulum, see Diversity & Distribution above.
Pollination Biology.
Whether a capitulum, supercapitulum, or something else, the whole inflorescence functions as a kind of polysymmetric/haplomorphic flower in terms of attracting pollinators, the ligulate ray florets, when present, being the "petals". Insect - especially bee - pollination occurs throughout the family; for bird and wind pollination, much less common, see below. Pollinators of Asteraceae might seem not to be very selective, since the frequent and diverse insect visitors so obvious on a capitulum of any size and trampling around on top appear to pollinate indiscriminately as they go, but this may not be quite true. Effective pollination is commonly carried out by a variety of broadly oligolectic small and often solitary bees belonging to Andrenidae (not in Australia) and Colletidae. These form complex and partly learned associations with individual species of Asteraceae; both these bees and Asteraceae are common in drier areas, but similar associations occur in more mesophytic Europe. In the western Palaearctic about one third of the megachilid anthidiine bees studied (26/72) specialized on Asteraceae - this was five times more than on the next family, Fabaceae - and all but two of these bees were specialists on a single tribe (Müller 1996). Moldenke (1979b) estimated that in North America about 525 bee species, well over one third of the total number of oligolectic bees, were restricted to Asteraceae (see also Fowler 2016). Thus some species of north temperate Colletes (plasterer bees) specialize on Asteroideae, other species rarely visiting them; specialization on Asteraceae has evolved three or four times there (Müller & Kuhlmann 2008). 84/85 species of Andrena subgenus Callandrena s.l. (genus polyphyletic, convergence because of food preferences? - Larkin et al. 2006) are found exclusively on Asteraceae, particularly Cichorioideae (the Lactuca area) and Asteroideae-Heliantheae, -Astereae, -Senecioneae, and -Helenieae. Larkin et al. (2008) discuss diet breadth, host switching, etc., and noticed that bees that were commonly to be found on Asteraceae tended to emerge in the autumn (see also Minckley et al. 1994: reproductive activities of bee and plant are synchronized), and there was also movement of the bees to the N.E. U.S.A.; autumn is a time when Asteraceae are commonly in flower there. Mason et al. (2017) noted that several species of oligolectic bees visited the one species of sunflower, 39 species of oligolectic bees (mostly Andrenidae and Anthophoridae, also Apidae, Halictidae) and 22 species of polylectic bees visiting 21 species of Helianthus regularly for pollen, 35 species of pollen specialists visited Helianthus in Missouri (Faupel 2023). (Note that some features of floral architecture here were affected by climate and soil (Mason et al. 2017) and that views of the open inflorescence under UV and visible light can be dramatically different (Moyers et al. 2017).) At most few of the bees were obligately associated with Helianthus, most also working other species of Asteraceae; all told, 284 species of bees visited sunflowers for pollen, 128 species for nectar (Hurd et al. 1980; also Minckley et al. 1994). Indeed, it is common for several species of oligolectic bees to visit the one composite species (Linsley 1958; Moldenke 1979b; Lane 1996; Minckley et al. 1999; Müller & Kuhlmann 2008; Praz et al. 2008; Kuhlmann & Eardley 2012; Vogel 2016), and Schemske (1983) noted that 11-20 species of bees, and over twice as many species of insects in general (in both cases, sometimes many more), commonly visited a single species of Asteraceae; Vásquez and Aizen (2004) discuss such asymmetries in plant-pollinator relationships. Similarly, in a study of the Cape flora Pauw and Stanway (2014) recorded at least 20 species of pollinators from three species of Asteraceae - but as the authors noted, such apparent promiscuity in pollinators in fact does not preclude such plants from being specialized in terms of pollinators. Overall, Asteraceae have nectar rich in hexose sugars and more or less shortly tubular flowers and pollinators are of the generalist type (Nicolson 2022). Cirsium palustre was one of the four major nectar-producing plants in the United Kingdom in 2007, the four together producing over 50% of the nectar (Baude et al. 2005).
However, the story is more complicated - and interesting. Pollen of Asteraceae-Asteroideae and -Cichorioideae, at least, may be unsuitable food for many bees. It may lack essential amino acids, have generally lower amino acid (e.g. arginine in Asteroideae) and protein concentrations than other pollen, and/or contain harmful secondary metabolites (Waser et al. 1996; Müller & Kuhlmann 2008; Praz et al. 2008; Goulson 2010; Sedivy et al. 2011). Consequently, some bees actively avoid collecting pollen from composites, and if bees that are not Asteraceae specialists are fed pollen of Asteraceae, their larvae may die (Sprear et al. 2016 for references). Thus female bumble bees may get covered in pollen as they collect nectar, yet they do not transfer that pollen to their corbiculae (Neff & Simpson 1990; Goulson 2010) - but note that spiny pollen in Malvaceae is not collected by corbiculate bees because of the spines/pollen kit (Lunau et al. 2015). This would not stop them being active in pollination (and might even enhance their effectiveness - dirty bees are more effective pollinators), and it may be connected with the fact that their larvae eat pollen and nectar, hence potentially being exposed to the deleterious effects of pyrrolizidine alkaloids (for example) - but see Plant-Bacterial/Fungal Associations below for bumblebee larvae, a parasite and Helianthus pollen, see also leaf-cutter bees. Note that honey bee larvae eat bee jelly produced by nurse bees in which the levels of these alkaloids (in Boraginaceae, at least) have been much reduced (Lucchetti et al. 2018). Brood parasitism (kleptoparasitism) is common is the solitary bees that are common on Asteraceae, yet nests of the megachilid Osmia mason bee feeding on asteraceous pollen (but not those feeding on pollen from other families of plants) were never parasitized by Sapygia wasps, and survival of the larvae of these wasps was reduced when they were fed pollen of Asteraceae (Spear et al. 2016).
Kemp et al. (2018) noted that details of the common colour patterning of the capitulae of Asteraceae - often there is a black centre - in different communities in the Succulent Karoo of South Africa were associated with the identity of the dominant pollinating fly. Asteraceae in the drier areas of southwestern Africa, including Namibia, are much visited by non-Apis bees, which also visit Aizoaceae there (Kuhlmann & Eardley 2011) - the two do have grossly similar flowers - and the bees are also important visitors on Zygophyllaceae and Fabaceae. The flowers of Gorteria diffusa (Vernonioideae-Gorterieae), from the winter rainfall area of the Cape, have distinctive black markings on the corollas of the ray flowers, and is pollinated by the bee-fly Megapalpus capensis, sexual deception often being involved (Ellis & Johnson 2009; Ellis et al. 2014), one of two species other than Orchidaceae with such pollination (Peakall 2023). This is a very complex set of interactions, some of the inflorescences entirely lacking these markings, and monkey scarabs (Hopliini) may also pollinate Asteraceae there (Goldblatt & Manning 2011b, see also Whitney et al. 2011).
Barreda et al. (2010b, 2012a) suggested that the florets of Raiguenrayun, known from the Middle Eocene of Patagonia ca 47.5 Ma, might be pollinated by birds, but hummingbirds, the iconic bird pollinators of the New World, are known only from Europe at that time (e.g. Mayr 2004), so bird pollination is unlikely or other birds were involved (see also Panero et al. 2014). Indeed, bird pollination is rather uncommon in Asteraceae (Cronk & Ojeda 2008; Erbar & Leins 2015), and although Vogel (2016) suggested that at least 60 species of the family were pollinated by birds and around 45 of these were pollinated by hummingbirds, that is very few in a family of this size. Abrahamczyk et al. (2017a) noted that a clade of the barnadesioid Chuquiraga was pollinated by Oreotrochilus, but although the stem ages of plant and bird were fairly similar, the crown ages were not, being ca 7.1 and 1.6 Ma respectively.
There is secondary pollination presentation throughout Asteraceae. The devices involved have been divided into three main types - the drag type, pollen grains attaching to microhairs on the style, the brush type, where hairs on the outside of the style/style arms sweep up the pollen, and the pump type, pollen accumulating in the hairs on the apices of the style arms as they move up the tube formed by the connate anthers; different types tend to predominate in individual subfamilies (Anderberg et al. 2007; Erbar & Leins 2015 and El Ottra et al. 2023: slightly different typologies). Leins and Erbar (2003b) suggest an evolutionary sequence for pollen presentation devices, and later they optimise features involved in such devices on a phylogenetic tree, the emphasis being on the basal pectinations of the family - as they note, variation is extensive there, perhaps especially so in Barnadesioideae (Erbar & Leins 2015). Within Barnadesioideae, sister to the rest of the family, secondary pollen presentation is of the drag type (as is found in at least some Calyceraceae) or by an unspecialised type of brush mechanism (e.g. Erbar & Leins 2000, 2015; Leins & Erbar 2006). However, variation in style morphology was found to be considerable and it did not fit easily into the three types mentioned, and so-called intermediate morphologies further confused the issue - function rather than phylogeny seemed to driving the variation (Torres & Galetto 2006). A number of Carduoideae, especially Centaurineae, have touch-sensitive stamens ("irritating stamens" - El Ottra et al. 2023: p. 23). Here the filaments contract when the stamens are touched by the pollinator, and the pollen is then forced out of the anther tube by the stigma, the stamens eventually returning to their normal positions; this pump-type pollination mechanism appears to have arisen more than once, and is associated with short and sticky, not long and dry, stigmas and smooth, not spiny, pollen (López-Vinyallonga et al. 2009). Stigmatic papillae either cover the inside of the style branches or they are organized into two marginal bands (Bremer 1994).
Herrera (2025) noted that at temperatures of 35-48oC the capitulae of species of Carduoideae-Cardueae from the southern Iberian Peninsula were 5-14oC cooler than the environment, a cooling that became evident at 25-35oC, depending on the species; evaporative cooling may be involved.
Note that the sunflower capitulum faces eastwards and its florets open in pseudowhorls early in the morning despite the continuous pattern of their initiation, the pseudowhorls developing because of entrainment by a circadian clock, and this seems to maximize pollination (Marshall et al. 2023). As in other Asteraceae, the stigmas become receptive the day after anthesis (Marshall et al. 2023).
Erbar and Leins (2020) have recently summarized stylar morphology in Asteraceae as a whole in the context of its function, particularly in secondary pollen presentation, and in the context of the phylogeny of the family.
In the >500 species of wind-pollinated Asteroideae the heads usually have either staminate or carpelate florets. In staminate heads the anthers are free and the capitulae are often pendulous, and the pollen grains have lost their spines. Since the carpelate heads may have only a single floret, the end result is a breeding system very much like that of other wind-pollinated plants like Fagales - monoecy, aggregated pollen-producing units, and female reproductive units that produce a single-seeded fruit. For the phylogeny, genome evolution, etc., of the wind-pollinated Artemisia (Anthemideae), with its multiple invasions of the Arctic (polyploidy is apparently not involved, but there has been extensive hybridization), see Vallès and Garnatje (2005), Sanz et al. (2008), Trach et al. (2008), Malik et al. (2017) and especially Jiao et al. 2025. Martin et al. (2017) and Tomasello et al. (2018) discuss relationships in the largely New World Ambrosia (Heliantheae) and Tomasello et al. (2018) in Ambrosiinae as a whole, many members of which have uncinate- or straight-spiny fruits.
Breeding systems in the family are very diverse (e.g. Burtt 1961, 1977a), and the evolution of different floret types in Inuleae (Asteroideae) has been examined by Torices and Anderberg (2009). Although protandry is very common, when there are different floret types, interfloral protogyny predominates (Bertin & Newman 1993). Dioecy has evolved from monoecy and back again in Leptinella (Cotula s.l.: Himmelreich et al. 2012). Apomixis is common in Asteraceae, especially in Cichorioideae, as in Taraxacum (see T. absurdum van Soest) where there is diplosporous agamospermy and there may be more than 2,800 "species" placed in around 60 sections (e.g. Kirschner et al. 2021; Štepánek & Kirschner 2022: literature on species concepts). Work on Taraxacum proceeds apace (e.g. Kirschner & Štepánek 2023a), Kirschner & Štepánek (2023b) noting in their study of the largely sexual sect. Macrocornuta that apomictic taxa had no or malformed pollen. Apomixis is also well known in other Cichorioideae like Hieracium and Crepis, and also in Asteroideae-Gnaphalieae, e.g. Antennaria, with 5 polyploid agamic complexes (Thapa et al. 2020a, b) and Gnaphalium itelf (e.g. Asker & Jerling 1992, Hojsgaard et al. 2014); see Hörandl et al. (2007) for general literature on apomixis. Mráz et al. (2020) describe a new diploid Hieracium from southern Europe - there are a few other such species there, and hybridization, polyploidy and apomixis then generates the diversity of extant hawkweeds. The study of Crepis by Babcock and Stebbins (1938) remains a classic account of an agamic complex (see also Sears & Whitton 2016). ?sporophytic incompatibility system?
Seed Dispersal.
Most diaspores, often called fruits or achenes (neither strictly correct), are crowned by a plumose pappus, a highly modified calyx, which may be uni- to pluriseriate and is sometimes associated with other pilose structures, and these fruits are cypselas; there may also be scales varying in morphology and position (Yu et al. 1999; Glover et al. 2015: confirmation at the level of gene expression; Small 1918; Mukherjee & Harris 1995; Mukherjee & Nordenstam 2008 and references for variation). The hairs that make the pappus up are themselves sometimes hairy, and wind dispersal is prevalent here. Tovar et al. (2023) looked at the relative investment in the pappus in Asteraceae growing in the high Andes, i.a. finding that almost 3/4 of the species there were Astereae, Gnaphalieae or Senecioneae and had relatively high investment in the pappus; the ancestors of those taxa were African, and there may be more general connections between dispersability and pappus investment here.
How the fruits fly has been analyzed by Andersen (1993) and how those of a dandelion in particular fly - a separate vortex ring develops - by Cummins et al. (2018); the results of the latter should be extended to pappuses with different morphologies. Seale et al. (2022) described how more humid conditions affected dandelion diaspore movement: The pappus hairs were held more erect, diaspores remained on the head even at quite high wind speeds, the rate of fall of the pappus was increased, etc.. Similar movement of the pappus hairs is very common in Asteraceae, but it does not occur in all species.
Fruits of a number of taxa are dispersed by animals, whether by retrorsely-barbed awns (most Bidens) or hooked inflorescence bracts (Arctium), or by myrmecochory, the fruits having some sort of elaiosome, as in Centaurea (Carduoideae) and Osteospermum (Asteroideae: Lengyel et al. 2009); for myxocarpy, which occurs in epappose taxa like Artemisia and relatives, see Kreitschitz and Vallès (2007), Western (2011) and Kreitschitz and Gorb (2017). Diaspore dimorphism (= heterodiaspory) is quite common in the family (Imbert 2002; Teppner 2003; Song & Wang 2015), and also in Amaranthaceae (Žerdoner Čalasan and Kadereit 2023), in Asteraceae different fruit types from the one capitulum may be dispersed in different ways and/or have different germination requirements (see also Kadereit et al. 2017 for discussion). Heterocarpy/heterodiaspory (and semelpary) seems to be the basic condition in Picris, homocarpy and iteroparity being derived (Slovák et al. 2017).
It is possible that the cypselas of Gymnarrehena can float and are water-dispersed; there is hygroscopic (hydrochasic) movement of the pappus here and in genera like Carduus and Cirsium (Kravtsova 2024).
Plant-Animal Interactions. The florets of some Senecioneae and Eupatorieae (Asteroideae) are visted by male Danainae and Ithomiinae (butterflies) and Arctiinae and Ctenuchidae (moths) and of larvae of Arctiinae because the pyrrolizidine alkaloids (PAs) they contain form the basis of their pheromones, or of compounds that other organisms find distasteful (see also Crotalaria, Apocynaceae, Boraginaceae and and Heliotropaceae: Edgar et al. 1974; Fiske 1975; Ackery & Vane-Wright 1984; Brown 1987; Weller et al. 1999; Anke et al. 2004; Opitz & Müller 2009). Singer et al. (2009) discuss self-medication by arctiid caterpillars on food containing high concentrations of PAs, while Zaspel et al. (2014) discuss the phylogeny of Arctiinae and the evolution of pharmacophagy there (see also Hartmann 2009; other articles in Conner et al. 2009). The two tribes of Asteroideae that synthesize PAs, Senecioneae and Eupatorieae, are not immediately related, their predominant PA types are different, and they synthesize PAs in different parts of the plant, so there has been independant evolution of these alkaloids within the subfamily, yet at the same time in both the critical homospermidine synthesis gene evolved by gene duplication and there are still more general parallelisms at the molecular level in PA synthesis (e.g. Reimann et al. 2004; Langel et al. 2010; Livschulz et al. 2018a). PAs and pentacyclic triterpene saponins obtained from Asteraceae and variously modified are also found in the secretions of the defensive glands of some Chrysolina and Platyophora beetles (Chrysomelidae) (Pasteels et al. 2001; Termonia et al. 2002; Hartmann et al. 2003). Sesquiterpene lactones from Asteraceae are also sequestered by insects (e.g. Pasteels et al. 2001). PAs protect the plants that have them against some herbivores, although individual alkaloids in Senecio section Jacobaea are readily and seemingly randomly gained and lost during evolution by the switching on or off of the genes involved in their synthesis, and there is also much variation in the amount of individual PAs (Pelser et al. 2005). There is literature on the effects of asteraceous metabolites on insects in Calabria et al. (2009).
The phytomelanin/phytomelan of the black cypselas in plants of the Heliantheae alliance is very distinctive and it may protect the fruits from dessication and also from the unwanted attentions of insect larvae (Pandey et al. 2014a; for phytomelanin, see also Graven et al. 1998; Mesfin Tadesse & Crawford 2014). Fruits with phytomelan may also be described as being carbonized; the phytomelan is laid down primarily between the hypodermis and fibre layer of the cypsela, and it is an exudate from the hypodermis into a schizogenous gap (Mathur et al. 2024). Mathur et al. (2024) noticed that if there were raphides or druses in the Heliantheae alliance, phytomelan was likely to be absent (but Dicranocarpus cypselas have neither phytomelan nor calcium oxalate crystals - Sánchez-Cháves et al. 2024); if there was phytomelan in the cypselas, it was unlikely to be in the vegetative parts. Phytomelan is also found in a few other taxa (Freitas et al. 2015; Bonifácio et al. 2019).
Caterpillars of Nymphalidae-Melitaeini butterflies are common on Asteraceae, as well as on Lamiales, from whence they probably moved less than 50 Ma (Wahlberg 2001; Nylin & Wahlberg 2008; Nylin et al. 2012), a move perhaps associated with an increase in their diversification rate (Fordyce 2010). Caterpillars in a clade of Nymphalidae-Heliconiinae-Acraeini utilise primarily Andean Asteraceae, probably switching from host plants in the Passifloraceae area (Silva-Brandão et al. 2008), but in this case without a change in diversification rates (Fordyce 2010). Most of the ca 1,000 species (Wikipedia xi.2017) of the tortricid leaf-rolling Cochylini are found on Asteraceae, and their divergence has been dated to ca 43 Ma, consistent with their adoption of Asteraceae as a food source (Fagua et al. 2017: relationships in this area of Tortricidae unclear).
Over 600 species of aphids, Aphididae, are known from Asteraceae, the most from any one family. These aphids include ca 200 species of Uroleucon, found on different tribes here, and they also moved on to Campanulaceae (Peccoud et al. 2010).
Within Carduoideae the stout root stocks and large inflorescences in particular are resources for the numerous herbivorous insects that specialize on this clade. More than fifty genera of specialized thistle insects, including representatives of Zygaenidae, Tortricidae, Pterolonchidae (all Lepidoptera), Curculionidae (Coleoptera), Tephritidae (Diptera), Tingitidae (Hemiptera) and Cynipidae (Hymenoptera), are found on Carduoideae of the west Palaearctic region, although their numbers are not great considering the diversity of Carduoideae there (Zwölfer 1988; Csoka et al. 2005; Brändle et al. 2005 and literature). All these herbivores are particularly abundant in the Mediterranean region, which is perhaps where Carduoideae evolved (Zwölfer 1988). Introduced insects including a weevil and the dipteran tephritid Urophora are often very effective biological control agents of introduced Carduoideae in North America and other parts of the world (Redfern 2011). Tephritid flies are particularly noteworthy on Carduoideae, either eating fruits, exudates they induce from the plant, or forming galls in the stem or inflorescence (Korneyev et al. 2005, esp. Urophora; Redfern 2011). They are also common on other species of the family pretty much world-wide (e.g. Prado & Lewinsohn 2004; Norrbom et al. 2010), and tephritid-induced (Eurosta) ball galls on the stems of Solidago (Asteroideae-Astereae) growing in the prairies of North America are conspicuous in the late summer (Abrahamson & Weis 1997; Helms et al. 2017: priming of plant defences by volatile emissions of males). Interestingly, Prado and Lewinsohn (2004) found that related species of Asteraceae in the Espinhaço mountains or Minais Gerais, Brazil, tended to support a similar tephritid fauna which, however, might not be made up of taxa that were immediately related.
Agromyzid dipteran leaf miners have diversified in north temperate Asteraceae; these insects prefer plants with noxious secondary metabolites (Winkler et al. 2009). There are an estimated 5,000 species, most undescribed, of the galling cecidomyiid Alycaulini that have diversified in the last 30 Ma, and most are to be found on Asteraceae (Dorchin et al. 2019; see also Stireman et al. 2010). Ceutorhynch seed weevils are quite commonly found on Asteraceae; the weevils have moved on to the family perhaps four times, and there have been movements on to other hosts (Letsch et al. 2018).
For the trenching behaviour (mitigates effects of plant defensive secretions) of herbivores eating Asteraceae, see Dussourd (2016).
A number of Asteraceae are quite densely and viscidly hairy, and insects may become trapped on the leaves and eaten by mirid bugs of subtribe Dicyphina in particular that are able to walk easily in such conditions (Wheeler & Krimmel 2015; LoPresti et al. 2015). Peucetia, lynx spiders, may also eat such trapped insects, so tiding them through harsh times (Romero et al. 2008). Nitrogen, mainly ultimately from the trapped insects, may be taken up by the leaf (Spomer 1999).
Plant-Bacterial/Fungal Associations. Ectomycorrhizae have been reported from a number of Australian Gnaphalieae (Warcup 1990; see also Tedersoo & Brundrett 2017), but there is no recent work on these plants. The oomycete Pustula, a white blister rust, is found quite widely on Asteraceae, with a few occurrences on Goodeniaceae, Araliaceae (Trachymene) and Gentianaceae (Ploch et al. 2010b).
It has quite recently been found that serious diseases of larval bees caused by the fungal microsporidian Nosema ceranae and the trypanosome Crithidia, especially C. bombi (the latter has been most studied), can be ameliorated if the bees eat the pollen of Asteraceae. Infection by Crithidia, which attaches to the hindgut of the bee by its flagellum, can cause quite serious effects on the young of Bombus impatiens, but if fed the spiny pollen of Helianthus or Solidago the parasite population is reduced and the bee larvae develop more or less normally; buckwheat pollen, which is not spiny, has no effect on the parasite (Giacomini et al. 2018; Malfi et al. 2023). Adult bees infacted by Crithidia are unable to distinguish between flowers that have nectar and those that do not and may die of starvation. Interestingly, not all species of Bombus respond to Helianthus pollen to the same extent or even at all (Fowler et al. 2022). For Calluna, Bombus and Crithidia, see Ericaceae.
Genes & Genomes. C.-H. Huang et al. (2016: Fig. 5) provide haploid numbers for some subfamilies and tribes, and these would suggest that x = 27 could be the basal number for the family as a whole. Watanabe et al. (1999) found that the range of chromosome numbers in Brachyscome (Ast-Astereae) is n = 2-18, B. dichromosomatica being n = 2. Chromosome number and total chromosome length have become smaller, while mean chromosome length, chromosome length heterogeneity and karyotypic asymmetry have increased as the habit has switched from perennial to annual and the plant grows in drier habitats (Watanabe et al. 1999); interestingly, Haplopappus (= Xanthisma) gracilis, another member of Astereae growing in drier habitats, also has n = 2, although obviously not all plants from such habitats have similarly low numbers. For the evolution of chromosome numbers in Helianthus (x = 17, polyploidy common), see Freyman and Höhna (2017), and for more on chromosome numbers, see Watanabe et al. (2007), Semple and Watanabe (2009) and Watanabe (2015).
M. S. Barker et al. (2008), Schranz et al. (2012), C.-H. Huang et al. (2016), Badouin et al. (2017), Z. Li and Barker (2019), Jardine et al. (2022: duplications in relationship to changes in pollen morphology) and others have discussed genome duplications in Asteraceae. It was initially thought that there was an early palaeopolyploidy event involving most or all of the family, and again near the base of Asteroideae and within Mutisioideae. Duplications have now been placed somewhat more precisely; one duplication (= a palaeotetraploidy) is at the [Calyceraceae + Asteraceae] node, but the position of another, which resulted in a palaeohexaploidy, was uncertain - somewhere above Barnadesioideae and below Carduoideae (see Barker et al. 2016a; Huang et al. 2016), the clade [Cichorioideae [Corymbioideae + Asteroideae]] (XASTβ, ca 34.9 Ma) being suggested by Landis et al. (2018). T. Feng et al. (2025) placed this event immediately after the divergence of Barnadesioideae and Famatinanthoideae. Helianthus experienced a duplication dated to ca 29 Ma (Badouin et al. 2017), this duplication involves all or most Heliantheae (Li & Barker 2019) or the entire Helianthodae (Jardine et al. 2022; Feng et al. 2025), while there are duplications in Calenduleae (20 Ma), Senecioneae (18 Ma) and Gnaphalieae (10 Ma: Huang et al. 2016; l. W. Watson et al. 2020) and hybridisation within Senecioneae and Anthemideae and also between Anthemideae and both Astereae and Gnaphalieae (L. E. Watson et al. 2020). The pattern of duplicate gene retention is distinctive - structural/cell organization genes, but fewer regulatory genes were retained (Barker et al. 2008). A genome duplication in Lactuca, perhaps the first such event mentioned above, has been dated to (60-)58.3(-55.6) Ma (Vanneste et al. 2014a). There have been three genome duplications fairly deep in Vernonieae with subsequent descending and some acending dysploidy (Angulo et al. 2022). Panero and Crozier (2016) discuss genome duplications and subsequent reductions in chromosome numbers in the family in some detail, with diploidization on occasion perhaps being linked with diversification.
Extreme dynamism in the evolution of the genome seems to be the order of the day rin Aseraceae. Thus the chromosomes of lettuce (n = 9) have been involved in at least 3 chromosomal fissions and 57 chromosomal fusions, those of artichoke (n = 17) in 14 fissions and 60 fusions, and those of the sunflower (n = 17) in 17 fissions and 126 fusions - in addition to the genome duplications in which they have recently (within the last 50 My) been involved (Badouin et al. 2017). For the reduction of chromosome numbers in Gnaphalieae, e.g. from n = 12 to n = 3 in Podolepis, see Smissen et al. (2011 and references), also Andrés-Sánchez et al. 2018: x = 7 → 5, 4, also reversals). For chromosome evolution within Vernonieae, see Angulo et al. (2022). Vallès et al (2012) discuss polyploidy and its connection with genome size, etc., and Vallès et al. (2013) summarize genome size variation in the family, although unfortunately little is known about this in the basal pectinations. Interestingly, in Cheirolophus (Carduoideae-Cardueae) there has been reduction in genome size but increase in the number of 35S rDNA loci, the latter increase normally being associated with genome duplications (Hidalgo et al. 2017a).
There is relatively common and wide (with respect to both taxonomy and current geography) hybridisation in Asteroideae in particular and this is evident in the frequent incongruence between topologies based on different genomes. Thus life for those involved in phylogeny reconstruction (and classification) is rather interesting (e.g. Fehrer et al. 2007; Pelser et al. 2008, 2010, 2012; Soejima et al. 2008; Morgan et al. 2009; Montes-Moreno et al. 2010; Schilling 2011; Smissen et al. 2011; Calvo et al. 2013; Galbany-Casals et al. 2014). Thus there is significant incongruence between relationships suggested by plastid and nuclear sequences in Senecioneae, probably due to ancient hybridisation rather than incomplete lineage sorting (Pelser et al. 2010). Smissen et al. (2011; see also Galbany-Casals et al. 2014) suggest that complex allopolyploidy may have been involved in the origin of at least four clades in Gnaphalieae, one of which is now globally distributed and that together encompass more than half the ca 1,240 species of the tribe. For some hybridization between members of different subtribes in Cichorioideae, see Y. Liu et al. (2013), between genera in -Lactucinae, see Kilian et al. (2017), in Vernonioideae-Vernonieae-Lychnophorinae, phytomelan +, see Loeuille et al. (2015b), in Asteroideae-Anthemideae (Oberprieler et al. 2019), intergeneric hybridization in general, Z.-H. Feng e al. (2024), and so on. And of course thinking about hybridization leads one back to genome duplications... For recent hybridization/speciation in Senecio and Tragopogon and its significance, see Soltis et al. (2016b and references). There is also widespread apomixis in a number of genera, particularly in Asteroideae and Cichorioideae, for which, see Majeský et al. (2017 and literature), and this is often connected with hybridization.
There has been considerable change in transposable element (TE) composition in Asteraceae and their immediate relatives, the outgroup (Nas[t]anthus patagonicus-Calyceraceae, = Moschopsis) and Fucaldea (Barnadesioideae-Asteraceae) each have different TEs, and the rest of Asteraceae differed yet again. In Helianthus and Heliantheae examined, Gypsy TEs in particular were notably abundant (Staton & Burke 2015).
For satellite DNA diversification in Carduoideae-Cardueae, see del Bosque et al. (2014). The 5S and 35S ribosomal genes have become associated in Asteroideae, but they are separate in the other subfamilies, as is the usual condition for flowering plants (Garcia et al. 2010b).
Garnatje et al. (2011) set up a site at which genome sizes could be collected, and 2C sizes are mentioned in the characterizations above. Vitales et al. (2019) looked at patterns of these sizes and found extensive variation in the Asteroideae in particular which included groups (reasonably sampled) with both the largest and smallest genomes. There was a correlation between 2C sizes and polyploid but not diploid chromosome numbers. Overall annuals had smaller genomes than perennials, but this was not so in Cichorioideae; invasives tended to have smaller genomes than their relatives (Vitales et al. 2019).
It was early realised that there was a large inversion in the large single copy region of the plastome in most of the family, but not in Barnadesioideae and other Asterales (Jansen & Palmer 1987; Bremer 1987; see also below), and the presence of the ψrps19 pseudogene shows a similar distribution (Pascual-Díaz et al. 2021). Other than that, the plastome shows rather little variation in size, nucleotides, etc., although 20kb and 3kb inversions in the LSC region are found ?throughout Asteraceae (Loeuille et al. 2021; Pascual-Díaz et al. 2021).
Jiang et al. (2023) found recombinations mediated by repeats, whether inverted or not, in the mitochondrial genome of Taraxacum mongolicum.
Economic Importance. Timme et al. (2007) provide a phylogeny of Helianthus (see also Lee-Yaw et al. 2018); Simpson (2009) summarised what is known of the otherwise rather slight economic importance of the family. For oils from sunflower and safflower, see papers in Vollmann and Rajcan (2009). Lebeda et al. (2025) discuss the domestication of lettuce in some detail; this may have initially occured in the western Caucasus region, and Lactuca seriuola seems to have been involved. Lactuca is quite a large genus - estimates are 73-130 species since 2000 - and some taxa are alloploid.
Chemistry, Morphology, etc.. For a general entry into the literature of Asteraceae, see papers in Funk et al. (2009a) - there is a helpful glossary; Anderberg et al. (2006) also summarize the variation in the family.
Asteraceae produce tens of thousands of secondary metabolites (Calabria et al. 2009 for a convenient summary; Barbero & Maffei 2017 for references), although nothing seems to be known about the secondary chemistry of Hecastocleioideae and Gymnarrhenoideae. See Seaman (1982) and Chadwick et al. (2013) for sesquiterpene lactones, Shulha and Zidorn (2019) for those of Cichorioideae, Seaman et al. (1990) for diterpenes, Aniszewski (2007) for alkaloids, and Bohm and Stuessy (2001: family) and Sareedenchai and Zidorn (2010: Cichorioideae) for flavonoid chemistry. For latex coagulation in Taraxacum, see references in Bauer et al. (2014).
Katinas (2025) attempts to standardize the termis used to describe trichomes, recognizing 17 types and 25 subtypes. The vascular tissue supply to the axillary bud is derived from several leaf gaps in genera like Petasites (Dormer 1950). Vegetative variation in the Kleinia-"Senecio" area is considerable, some species having very succulent terete or even almost spherical leaf blades, or the stems may be succulent and the leaves early deciduous. Although these terete leaves are abaxialized, there is always a narrow adaxial strip equivalent to the upper surface; the apex of the leaf is fully terete and has been described as a Vorläufespitze (Ozerova & Timonin 2009). Leaves of species like S. meuselii may be terete or laterally flattened and so appear to be equitant (see also Timonin et al. 2015). The leaf teeth may be hydathodal (Rios et al. 2020).
For capitulum development, see above. In general, the very different adult floral morphologies found in Asteraceae are quite similar early in development (Harris 1995). The pappus (modified calyx) is often not the first part of the floret to be initiated, and it may start to develop well after the corolla (see Mukherjee & Harris 1995, Nordenstam 2008), although otherwise the sequence of initiation of the parts of the floret is as might be expected. For theories as to the origin of the pappus as other than a simple modified calyx, see Harris (1995) and Bello et al. (2013). A corolla ring primordium may initate first, or petals may be initiated separately, i.e. there is variation between early and late corolla tube development, the former perhaps being derived (Harris 1995: Leins & Erbar 2000; Erbar & Leins 2000 for floral development). CYC-like genes appear to be expressed in the abaxial petals, rather than in the adaxial petals, as in other core eudicots (Citerne et al. 2010).
Floret morphology varies extensively in Asteraceae, and this variation needs to be put in the context of the tree, which I have barely begun to do. Koch (1930 and references) and Manilal (1971) discussed corolla venation; the corolla of the ray florets of some Asteroideae may even be unvascularized. Many Asteroideae have three-toothed ray florets that give the appearance of being slit-monosymmetric (0:5), but they may be a modified 2:3 bilabiate corolla in which the adaxial lobes have been suppressed (Weberling 1989; Gillies et al. 2002); true slit-monosymmetric florets are uncommon here. Some taxa, including some Barnadesioideae, have a midvein in the petal (see also Koch 1930a, b; Carlquist 1976; Gustafsson 1995); corolla variation in Barnesdeioideae alone is extensive (Stuessy & Urtubey 2006). The bicellular corolla hairs of Barnadesioideae are distinctive: The epidermal cell is undistinguished, the basal cell is short and thick-walled, and the other cell is longer and has thin walls (see e.g. papers in Funk et al. 2009). Osmophores are reported from the corollas of some Asteraceae, and in a study of Mutisioideae-Onoserideae, Katinas et al. (2020) recorded them from Plazia - and also from Famatinanthus, which has been removed to its own subfaily.
Details of the apex and base of the anthers have been much used in classification. Thus Senecio s. str. has balusteriform filament collars (Salomón et al. 2016) - one can think of the filaments as being abruptly swollen near the apex. Wortley et al. (2007b, 2012) used distinctive pollen characters to help place some genera whose relationships had previously been unclear. See also Roque and Silvestre-Capelato (2001: pollen of Gochnatioideae), Wortley et al. (2008: Arctotideae-Vernonioideae), Zhao et al. (2006: Mutisieae), Wortley et al. (2009, 2012: comprehensive bibliographies of palynological work), Tellería and Katinas (2009: Mutisia), Osman (2009: Carduoideae-Cardueae), H. Wang et al. (2009b: Cichorioideae-Cichorieae) and Gabarayeva et al. (2018: Echinops). There is considerable variation in tapetum "types" in the family (Pacini 1996).
The orientation of the gynoecium and style branches varies: carpels superposed, style branches arranged radially to the head surface; carpels collateral, style branches tangential to head surface: see Robinson 1984); details of the distribution of this feature are unknown. Buphthalmum has a hollow style (Leins 2000); I do not know how widespread such styles are in Asteraceae. Styles and style arms show much variation in indumentum/cell surface type, the distribution or receptive tissue, style arm length and apex and there is also variation in pollen presentation type. This is incompletely integrated with with the subfamilies above: see Erbar and Leins (2015: hair morphology, 2015b: styles and secondary pollen presentation) and Erbar (2015: multiseriate [= laterally connate] hairs on style, 2016: style morphology of basal asteraceous clades).
For embryo sac development, which I have not thought about, see e.g. Fagerlind (1939c): There are embryo sacs other than the common monosporic 8-nucleate type. The embryo sac may on occasion have synergid cells that are elogated and get in to the micropyle (see Johri & Agarwal 1965). Guignard (1893) suggested a), that the ovules may be vascularized, and b), that there is sometimes an antiraphe, as in Centaurea. The embryo of Syneilesis may lack cotyledons entirely (Teppner 2001).
The embryo is protected by the wall of the inferior ovary, and as is common in such situations, the testa is commonly poorly developed, e.g. as in most species discussed by Batista et al. (2015); the cells of the integument are described as commonly being some kind of "nutritive tissue" (Kolczyk et al. 2014). However, at times the exotesta is very well developed and lignified, the walls being variously thickened, especially the anticlinal walls, the thickening being evenly distributed or beaded/strongly pitted in tranverse section, and the cells may appear to be closely palisade, sometimes with a linea lucida, or less or not elongated vertically or horizontally (e.g. Guignard 1893; Lavaille 1912; Dittrich 1968, 1971; Grau 1980 - Mutisieae; Cabrera R. 2002; Boyko & Novozhilova 2018, 2022; Ozcan & Alinci 2019; Bonifácio et al. 2019) - a comprehensive survey of testa anatomy in Asteraceae would be useful. There may be calcium oxalate crystals of various kinds in the inner layer (Guignard 1893; Boyko 2014), and there seems to be a negative relationship between the presence of such crystals and of phytomelan (Mathur et al. 2024).
Frangiote-Pallone and de Souza (2014) discuss pappus and cypsela ontogeny, and Costas et al. (2024) outline pappus morphology in the family. The basic condition is setose, the hairs being branched or not, while aristate pappi have more rigid, needle-like hairs, sometimes with retrose spines, palaeaceous pappi have flattened hairs while coroniform pappi are low and rim-like and with a more or less irregular margin - and taxa may also be epappose. In Astereae, at least, there is extensive variation in the carpopodium, the basal abscission zone of the cypsela which has distinctive "pericarp" cells aiding in the detachment of the cypsela from the receptacle, however, this variation seems of little taxonomic value (Boyko et al. 2024: 81 spp. from 34 genera and 16 subtribes examined).
Nuclear endosperm is sometimes mentioned as being the only endosperm condition found in the family or as a synapomorphy for it (e.g. Tobe & Morin 1996; Inoue & Tobe 1999), but there is in fact considerable variation in endosperm development and it is difficult to clearly distinguish two "types" - sometimes cell walls do not form in the first division alone (e.g. Dahlgren 1920; Kapil & Sethi 1962; Johri et al. 1992) and you cannot be much less free-nuclear than that...
For general information on Asteraceae, see e.g. Carlquist (1976: variation in the context of a tribal classification), Heywood et al. (1977), Dittrich (1977: Cynareae), K. Bremer (1987, esp. 1994: classification rather different from that above, 1996: subfamilies), Hind et al. (1996), Katinas et al. (2008b: Mutisioideae), Freire et al. (2014: esp. Famatinanthus), Freire (2017: Pertya), Loeuille et al. (2019: Vernonioideae-Lychnophorinae), Katinas and Funk (2020: basal Asteraceae, all ex-Mutisieae), Ferreira et al. 2021: Barnadesioideae) and Gostel et al. (2024: Distephaneae). See also Gilman et al. (2023: CAM photosynthesis), Badami and Patil (1981) and Tsevegsüren (1999), fatty acids in seeds, Shen et al. (2023), inulin/fructan biosynthesis, terpenoids, Yasukawa (2013: bioactive compounds in florets), Carlquist et al. (2021: wood anatomy Barnadesioideae), Katz et al. (2014: phytoliths and their possible function, no effect on larger herbivores), Melo-de-Pinna (2016: leaf development in taxa with terete blades), Thomas et al. (2009: Gorteria) and Perez et al. (2019: Solidago), both floral development, Vogel (1998c), Wist and Davis (2006) and Erbar (2014), pollen, DeVore et al. (2007: esp. Barnardesioideae), Mendonça et al. (2010: Lessingianthus), Carrijo et al. (2013: Vernonieae); nectaries and nectar secretion, Hernández et al. (2015: stylar histochemistry, ?phylogenetic signal), Goldfluss (1898-9: antipodal cells), Small (1919: esp. variation in embryo sacs), Kapil and Sethi (1963: Anisliaea) and Bonifácio et al. (2019: esp. Stifftia, Wunderlichia, also more general comparisons), embryology, etc., Dahlgren (1924: endosperm development), Marques et al. (2020: cypsela in in some Vernonioideae-Vernonieae - phylogenetically not informative) and Tegel (2002: Cichorioideae-Lactuceae), seed anatomy.
Phylogeny. There has been much phylogenetic work on Asteraceae, and only a few of the older references are included. A large inversion in the chloroplast genome occurs in most of the family, but not Barnadesioideae and other Asterales, and its discovery in the early days of molecular systematics was very exciting, in part because it was consistent with a morphological phylogeny that came out at about the same time (see Jansen & Palmer 1987; Bremer 1987; see also Y.-D. Kim & Jansen 1995; Jansen & Kim 1996; Kim et al. 2005; Timme et al. 2005). Panero and Funk (2002, especially 2008; see also Funk et al. 2005, a supertree, 2009c, a metatree) and Panero et al. (2014) present a phylogeny many details of which are reflected in the classification above. There can be conflict between plastomee and nuclear data (Pascual-Díaz et al. 2021) Much, but not all, of the uncertainty in relationships around the old Mutisioideae seems to have been resolved, and it has been broken up, with distinctive gene deletions and insertions characterising a number of the clades (Panero & Funk 2008). Morphological data suggested to Roque and Funk (2013: c.f. character states) that Wunderlichioideae and Stifftioideae might form a clade, and there is also some molecular support for this clade (Funk et al. 2014). In the latter study, there was still weaker support for a [Mutisioideae + Stifftiodeae] clade and Pertyoideae were outside a [Cichorioidae + Carduoideae] clade, but the latter areas were not the focus of the study and so sampling was poor (Funk et al. 2014). Fu et al. (2016: focus on Chinese taxa) found that Stifftioideae, Wunderlichioideae and Gochnatioideae together formed a clade; and the supermatrix they examined included 512 genera all told, somewhat less than one third the total. See also Z.-D. Chen et al. (2016) for Chinese Asteraceae and K. E. Jones et al. (2019: hybrid capture). The distinctive Gymnarrhena was excluded from Asteroideae by Anderberg et al. (2005), it was described as a subfamily, and it has maintained its position since, although that subfamily now has two (morphologically rather different) genera.
With the realization that the recently-rediscovered Famatinanthus was rather different from other Mutisioideae-Onoserideae, Panero et al. (2014: 14 chloroplast loci) examined basal relationships in Asteraceae. Famatinanthus was strongly supported as sister to the rest of the family bar Barnadesioideae, and Mutisioideae was the next branch, also with strong support, although support for the monophyly of Mutisioideae themselves could have been stronger. Support for the positions of the next three clades up was also quite good (Panero et al. 2014; see also Panero & Crozier 2016), however, Gostel et al. (2022) thought that the relationships of Gochnatieae (sic) to other Asteraceae were unclear, although Wunderlichieae seemed to be close. Taking a rather different tack, Mandel et al. (2014, 2015) looked at a massive conserved orthologous set of nuclear genes, and found that Centrapalus pauciflorus had sprung outside the other Cichorioideae examined, however, sampling was poor. Later studies have shown a fair bit of support for branches along the spine of the tree, although this has depended in part on the method of analysis used, furthemore, there were questions over the monophyly of Cichorioideae and Carduoideae (Mandel et al. 2017).
The recent work by Mandel et al. (2019) in which data from ca 1000 nuclear genes from representatives of 207 genera, 45 tribes (only two small tribes of those then recognised were not included) and all of the subfamilies then recognised suggests an appreciable rearrangement of relationships. As of vii.2019 the tree structure in APweb was [Barnadesioideae [Famatinanthoideae [Mutisioideae [Stifftioideae [Wunderlichioideae [Gochnatioideae [Hecastocleidoideae [Carduoideae [Pertyoideae [Gymnarrhenoideae [Cichorioideae [Corymbioideae + Asteroideae]]]]]]]]]]]]. Subfamilies that Mandel et al. (2019) suggest translate (using names from this vii.2019 version) to something like [Barnadesioideae* [Famatinanthoideae* [[Stifftioideae* (inc. some Gochn.) + Mutisioideae*] [[Wunderlichioideae* + Gochnatioideae*] [Hecastocleidoideae* [Pertyoideae* [[Oldenburgieae* + Tarchonantheae (ex Card.)] [Dicomeae* (ex Card.) [Carduoideae [Gymnarrhenoideae* [Vernonioideae* (ex Cichorioideae) [Cichorioideae* [Corymbioideae + Asteroideae]]]]]]]]]]]]] (see also Katinas & Funk 2020) - the old Mutisieae have turned out to be very para-/polyphyletic, and the clades above with asterisks are most of those in which they are now to be found. The classification+/phylogeny above is based on the groups and topology in Mandel et al. (2019), with nomenclatural adjustments in Susanna et al. (2020). Note that there are a number of differences in the recent analyses of Rivera et al. (2021) that used 11 concatenated chloroplast markers; the focus there was on Mexican taxa. Relationships in C. Zhang et al. (2021: all 13 subfamilies, 41/45 tribes [both as of 2020], 243 spp.) were based on analyses of 1087 nuclear genes, and are largely similar to those above. Note, however, that there are topological differences in that paper such as ... [Wunderlichioideae [Gochnatioideae [Hecastocleioideae ..., ... [Tarconanthoideae + Carduoideae] [Dicomoideae ... (names used are those above), some variation in tribal relationships within Asteroideae. and so on. Relationships recovered in the various analyses carried out by G. Zhang et al. (2024: 706 spp., ca 2/3 of the subtribes, 1094 genes from genome skimming and nuclear transcriptomes) generally agreed wih those found in previous analyses, but there were problems with Carduoideae and Tarchonanthoideae (these two may be sister taxa), Wunderlichioideae and Gochnatioideae and Cyclolepis (perhaps a new subfamily), also non-monophyly of some tribes in Asteroideae, etc..
Asteroideae: Relationships within this very large clade were not well resolved by Bentley et al. (2015) using two nuclear and one plastome loci. Using plastome data, relationships between [Calenduleae [Gnaphalieae [Anthemideae + Astereae]]] seemed to becoming resolved as indicated (e.g. Panero & Funk 2016; Fu et al. 2016), however, in subsequent analyses using nuclear genomes and including Senecioneae, although the monophyly of the tribes was confirmed, their relationships were not stable (L. E. Watson et al. 2020). See also relationships suggested by Bengtson and Razafimandinbison (2024: 2 nuclear and 2 chloroplast markers, focus on Athroismeae). Some tribes in Asteroideae recovered by G. Zhang et al. (2024) were para- or polyphyletic, perhaps suggesting the need for additional tribes...
For a phylogeny of Anthemideae, see Oberprieler et al. (2007, 2009, 2019: some problem genera, 2022: ITS and 2 chloroplast genes); basal relationships (all southern hemisphere taxa) start off [Osmitopsidinae [Cotulinae [Ursiniinae [Athanasiinae ...]]]], although the position of the first is not totally clear. For Anthemideae in the southern hemisphere, see also Himmelreich et al. (2008 and references), Cotula is not monophyletic (Himmelreich et al. 2012) and will probably need to be expanded, and there is extreme polyploidy in Leptinella in particular (Himmelreich et al. 2014). For the delimitation of Anthemis itself, see Lo Presti et al. (2010); for circum-Mediterranean Anthemideae, also their biogeography, see Oberprieler (2005), for Chrysanthemum and other Anthemideae, see Zhao et al. (2010), and for Tanacetum, see Sonboli et al. (2012: little resolution). For relationships within and the evolution of Artemisia, see Vallès et al. (2003), Vallès and Garnatje (2005), Sanz et al. (2008), Pellicer et al. (2010b, c: genome size, etc., 2011), Garcia et al. (2011: North American taxa), Riggins and Siegler (2012: paraphyly, etc.), Malik et al. 2017: subgenus Seriphidium, species from Sardinia and the Canary Islands basal?) and Jiao et al. (2023: 205 spp., single nucleus polymorphisms). The latter group recovered eight well-supported clades, although relationships between some needed to be clarified; of the six subgenera, five were not recovered in this analysis. They examined six characters that were previously used to characterize the subfamilies in the context of their new tree, but with little satisfaction (Jiao et al. 2023). However, Jiao et al. (2025) returned to the problem, looking at 202 low-copy nuclear genes and 2 ribosomal DNA markers for 394 species. Thy found extensive conflict between nuclear and plastid trees, in the latter Artemisia was not monophyletic and 7/8 subgenera were also not monophyletic. They looked at 20 morphological characters, finding that features like capitulum and leaf type (11 states here!) and corolla shape and stylar morphology were of value for their supraspecific classification (Jiao et al. 2025).
North American Astereae are monophyletic and largely herbaceous (Noyes & Rieseberg 1999), however, Aster is extensively para/polyphyletic, and its limits are now restricted (e.g. Li et al. 2012; Nesom 2020b); for relationships in the American Grindelia, see A. J. Moore et al. (2012) and Schneider and Moore (2017), and for those in Emilia, whose limits have been somewhat extended, see Mapaya and Cron (2020, sections not holding up). The limits of Solidago have been very unclear, but Semple and Beck (2021) and in particular Semple et al. (2023) clarify its phylogeny, the latter group looking at 854 nuclear regions in 123/138 of the species; the tree recovered was quite well supported. Heiden et al. (2020: over 50% sampling) looked at relationships within Baccharis (c.f. Brouillet et al. 2009). For Machaerantherinae, see Morgan et al. (2009) and for some East Asian Tussilaginae, see C. Ren et al. (2017). For the Hinterhubera group, see Karaman-Castro and Urbatsch (2009: groupings geographic). Olearia is likely to be polyphyletic (Cross et al. 2002); Saldivia et al. (2020) has confirmed the assignment of the Australian ex-Olearia to Celmisiinae. Astereae (252/3080, 36 subtribes: Shawia (82). Nicol et al. (2024: 54 species, Angiosperms353 data set, 66 morphological characters) clarified relationships in Celmisiinae – i.a. species previously included in Olearia are to be placed in separate subtribes. Strijk et al. (2012) founnd that Psiadia (and Conyza) are also polyphyletic. Hybridization has been important in the evolution of the high-Andean Diplostephium (Vargas et al. 2017). Qu et al. (2025: 12 pollen traits, ITS, 21 spp.) attempted to resolve relationships in the tribe and better understand evolution by looking at characters of external pollen morphology and the nuclear ITS gene; the topologies obtained from analyses of the two kinds of data were different, but it is unclear where one should go from there.
Athroismeae: Relationships here were examined by Bengtson and Razafimandinbison (2024) who recovered a basal tetratomy (close to a pentatomy), although all four subtribes had strong support; Anisochaeta mikanioides was unplaced.
Relationships in Coreopsideae are poorly understood, the limits between the largest genera, the very variable Bidens (Ganders et al. 2000; Bringel et al. 2017) and Coreopsis being complex (Mesfin Tadesse et al. 1995; Mesfin Tadesse & Crawford 2014). Recent work suggests that Bidens is polyphyletic, a small group of species being part of a clade that includes Cosmos, Coreopsis, etc., all jumbled up, although the Bayesian posterior probabilities of that clade were low (Knope et al. 2020b, c, see 2020a for Hawaiian species of Bidens). There is probably hybridization in Bidens, and there is conflict between the topologies of the nuclear mitochondrial and chloroplast trees (Knope et al. 2020c). Mesfin Tadesse and Crawford (2023) address generic limits around Coreopsis, and Coreopsis itself is much reduced (see also below).
For Mexican Eupatorieae, especially Brickelia, see Schilling et al. (2015) and for the diversification and biogeography of Stevia, see Soejima et al. (2017).
The monotypic Feddeeae, from Cuba, was described in a paper by Cariaga et al. (2008). Although it is sister to Heliantheae (chloroplast ndhF data only), it is morphologically quite distinct; there has been no recent work on the genus.
There have been extensive studies in Gnaphalieae. Morphological variation was analyzed by Anderberg (1991a) in an early study, but relationshps there have been considerably modified since using molecular data, e.g. see Bayer et al. (2000), Smissen et al. (2011), Nie et al. (2015), Andrés-Sánchez et al. (2018) and others; see Smissen et al. (2020) for a summary tree. Nie et al. (2015) found the relationships [Relhania clade [Metalasia clade [Lasiopogon + The Rest]]]; Bentley et al. (2017) subsequently clarified relationships within the Relhania clade. Helichrysum is polyphyletic (Galbany-Casals et al. 2004, 2009, 2010, 2014; Bergh & Linder 2009); Ward et al. (2009), Montes-Moreno et al. (2010), Nie et al. (2013), Bengtson et al. (2015 and references: the South African Metalasia, also diversification), Schmidt-Lebuhn et al. (2015), Weber and Schmidt-Lebuhn (2015) and Schmidt-Lebuhn and Bovill (2021), all Australian taxa, Bentley et al. (2015), African Philyrophyllum, actually in Athroismeae, and Luebert et al. (2017), the South American Luculia group, comparing molecular relationships with achenial morphology and the presence of mucilage-producing cells, all further clarify relationships in this tribe; there has been hybridisation (see above). Genera like Achyrocline, Anaphalis and Pseudognaphalium are embedded in Helichrysum, indeed, the last-named is polyphyletic, and although species are being described there (e.g. Nesom 2023b), one has no idea of the shape of the final classification (see also Smissen et al. 2023 for relationshipss). The focus in Blanco-Gavaldà et al. (2023: 830 loci from the Compositae1061 probe set, paralogues cleaned up, 304 spp.) was the Helichrysum-Anaphalis-Pseudognaphalium (HAP) group; relationships on the whole quite well supported. Thapa et al. (2020a) worked on relationships within Antennaria, which has spawned numerous apomicts, and here nuclear and chloroplast trees differ strongly. Recent studies clarify some of the issues involved. X.-M. Xu et al. (2023b: 26 spp. plastomes + 4 outgroups, 34 spp ITS plus ETS, even more individual samples, outgroups) looked at relationships in Leontopodium - it was monophyletic - but they found substantial differences between the topologies produced by the analyses of the plastome and ITS + ETS data; they prefered the former because support was stronger, although it is a case of analyses based on whole plastomes versus those based on two nuclear genes. The focus in Xu et al. (2024) was the HAP group, and these authors found para- and polyphyly pervasive, Helichrysum appearing in five places. Again there were substantial differences between relationships suggested by the plastome and nuclear data (in the latter, Bayesian inference and maximum likelihood trees also differed), and again these authors prefered the topology they obtained in the plastome analyses. However, plastome analyses here have to be compared with extensive nuclear data, and the species-level sampling has to be thorough.
For the phylogeny of the helenioid Heliantheae, see Baldwin et al. (2002), and for relationships in Helianthus itself, see Mason et al. (2017) and Lee-Yaw et al. (2018: much chloroplast-nucleus conflict - cytoplasmic introgression). R. D. Edwards et al. (2018) examined relationships in the pantropical Melanthera alliance.
Anderberg et al. (2005), Englund et al. (2009) and especially Nylinder and Anderberg (2015) discuss the phylogeny of Inuleae (inc. Plucheeae); morphological relationships in Inuleae and Plucheeae were discussed by Anderberg (1991b, c). Nylinder et al. (2016) discuss the phylogeny of Plucheinae. The sections of Blumea (Inuleae) need overhaul, see Pornpongrungrueng et al. (2009).
Madieae. Relationships in the Hawaiian clade that includes Argyroxiphium and two other genera, related to North American Madieae, have been much studied (see Diversity and Distribution above). Baldwin et al. (2021) demonstrate the extent of chloroplast capure here.
Millerieae include Espeletia and relatives (= Espeletia s.l.), frailejones, relationships within which are discussed by Diazgranados and Barber (2017: a fair amount of hybridization) and in particular by Pouchon et al. (2018). Clades based on analyses of secondary metabolites (Padilla-González et al. 2018) do not agree with those found in molecular analyses.
Perityleae were studied by Lichter-Marck et al. (2020), who found conflict between relationships suggested by nuclear and chloroplast data, extensive hybridization, and an uncertain delimitation of the tribe (but most of it was made up of Perityle itself). Lichter-Marck and Baldwin (2022) managed to reorganize the tribe, recognizing that resolving the polyphyly of Perityle itself was the central problem; relationships in Lichter-Marck and Baldwin (2023) are [Galeana [Perityle (part) + the rest]]; there is a fuse of ca 2.5 Ma after the divergence of Galeana.
Relationships within Senecioneae, particularly the huge genus Senecio, are beginning to be disentangled (Pelser et al. 2006, esp. 2007, see also Pelser et al. 2010; Calvo et al. 2013; Liew et al. 2018; Kandziora et al. 2016a). J.-Q. Liu et al. (2006) discuss relationships in the large Ligularia–Cremanthodium–Parasenecio clade, a major part of the Senecioneae-Tussilagininae, Escobari et al. (2023: 50 spp., nrITS and ETS) examined relationships in the Gynoxys clade although they did not find much resolution (c.f. plastome analyses), while Quedensley et al. (2018) looked at relationships within Mexican members of this subtribe. For relationships within Euryops, see Devos et al. (2010). For Symphyotrichum and relatives, see Vaezi and Brouillet (2009).
For phylogenetic relationships within Barnadesioideae, see Urtubey and Stuessy (2001) and in particular Gustaffson et al. (2001), Gruenstaeudl et al. (2009) and Funk and Roque (2011). The position of Schlechtendalia was uncertain; [Huarpea + Barnadesia] may be sister to the rest of the subfamily. Ferreira et al. (2019) recovered the relationships [Schlechtendalia [Chuquiraga [Huarpea + Barnadesia] [The Rest]]] ], albeit with little support, and relationships within Dasyphyllum were re-evaluated; see also Mandel et al. (2019) for some comments on relationships. More recently, Ferreira et al. (2022) found that Schlechtendalia was sister to the rest of the subfamily, albeit not in all analyses, and support might be low, however, that seems to be its position; Chuquiraga was paraphyletic with respect to Doniophyton. Relationships in Chuquiraga had earlier been examined in a morphological phylogeny by Ezcurra (2002); the genus was found to be paraphyletic there, too.
For the phylogeny of Carduoideae (= Carduoideae-Cardueae here prior to 2019), see Garcia-Jacas et al. (2002), Susanna et al. (2006), Barres et al. (2013), Park and Potter (2013) and Herrando-Moraira et al. (2018). There has been much recent work on this complex. Thus Herrando-Moraira et al. (2019) looked at Cardueae in detail using both chloroplast and nuclear data and recovered twelve main clades, of which Carlininae, Cardiopatiinae and Echinopsinae are successively sister to the rest of the tribe and the paraphyletic Carduinae are comprehensively broken up. Ackerfield et al. (2020) looked at relationships around Carduus and Cirsium, both polyphyletic; see also del Guacchio et al. (2022) and Siniscalchi et al. (2023), the latter group looking at Cirsium in North America (64 taxa, 212 loci, extensive conflict between gene trees - ILS?). The focus in Moreyra et al. (2024: 159 spp., 177 plastid regions, 986 nuclear loci) and Moreyra et al. (2025: 251 taxa, 350 nuclear loci) was also on the Carduus-Cirsium group. In the first article three new genera were described, and the authors found some differences in the topologies of the trees recovered by coalescence and concatenation analyses of the nuclear data, while the tree resulting from the analysis of chloroplast data was noticeably rather more different, albeit several branches had rather weak support (Moreyra et al. 2024). In Moreyra et al. (2025) in particular, however, things can become more complicated - Bureš et al. (2024) suggested that Cirsium vulgare was an allotetraploid hybrid between carpelate Cirsium and staminate Lophiolepis parents, although the latter were included in Cirsium by Moreyra et al. (2025) - see also below. In their study of Jurinea, Herrando-Moraira et al. (2023: 502 nuclear loci, ca 187 spp.), J. mesopotamica was found to be sister to the rest of the genus, which was divided into three main clades plus a singleton species. For relationships in Echinops, see Garnatje et al. (2005: sectional classification) and Sánchez-Jimenéz et al. (2010: 2/3 the species, 2 markers; sections largely confirmed). Jurinea is largely monophyletic, but the sections are largely not monophyletic (Szukala et al. 2018). For relationships around Arctium see Susanna et al. (2003), and for those within Cousinia (biphyletic) and relatives, see López-Vinyallonga et al. (2009). For relationships in the large and monophyletic Centaureinae, see Garcia-Jacas et al. (2001), Hellwig (2004) and Herrando-Moraira et al. (2019). Chloroplast and nuclear data suggest rather different relationships within Saussurea and between it and its immediate relatives (Herrando-Moraira et al. 2018: nuclear NGS data, 2020: 558 nuclear loci; X. Zhang et al. 2019 and L.-S. Xu 2019: both plastome data; Kasana et al. 2020: ITS and morphology). This conflict was confirmed by L. Xu et al. (2024: see Fig. 4!) who looked at 103 spp from all sections using 475 conserved orthologous sequences; they found that coalescent and concatenation analyses gave the same topology.
Cichorioideae. In Arctotideae, the African Gorteriinae have been studied by Funk and Chan (2008) and Stångberg et al. (2018) and the Arctotidinae by McKenzie and Barker (2008); in the latter group, at least, genera are not monophyletic. Gundelia and Warionia were moved to the Cichorieae (sic), interestingly, both have simply echinate pollen grains (c.f. the echinolophate pollen grains in other genera), and tubular, not ligulate, corollas occur here (Lebeda et al. 2025). Indeed, Warionia may be sister to all other Cichorieae (Kilian et al. 2009); although no flavonoids have been reported from this tribe, they are diverse in the rest of the subfamily (Sareendenchai & Zidorn 2010 - see Zidorn 2008 for sesquiterpene lactones). Subtribes are monophyletic, although Faberia seems to be a hybrid between a member of Crepidinae and Lactucinae (it looks more like the latter), but relationships between them are only partly resolved (Y. Liu et al. 2013). For relationships, etc., in -Lactucinae, see Kilian et al. (2017) and Güzel et al. (2021) - in both papers there are major gene tree incongruences e.g. in the Melanoseris area. In -Sonchinae, Sonchus is para/polyphyletic (S.-C. Kim et al. 2007). Slovák et al. (2017) examine the phylogeny of Picris, morphologically diverse in Australia. For relationships within -Scorzonerinae, see Mavroidiev et al. (2004) and Zaika et al. (2020). For a phylogeny of Tragopogon and its relatives, see Mavrodiev et al. (2005) and Bell et al. (2012b). The classic studies by Babcock (e.g. 1947) on Crepis (Crepidinae) that assumed that evolution - in this case of the karyotype in particular - was unidirectional need re-evaluation (Enke & Gemeinholzer 2008). As mentioned above, species limits around here are difficult because of apomixis; Kirschner et al. (2015) looked at relationships within Taraxacum with a focus on sexually-reproducing taxa. Hieracinae: see Gottschlich (2009) for the complexities of variation in Hieracium in a smallish area of Italy.
Funk et al. (2014: as tribe) looked at relaionships in Gochnatioideae, as did Gostel et al. (2022: nuclear analyses, also as Gochnatieae) in some detail, and placed Cyclolepis as sister to the rest of the clade. See also G. Zhang et al. (2024), also Wunderlichioideae - problems around here.
For the limits of Gymnarrhenoideae, see Anderberg and Ohlson (2012). Kravtsova (2024) described the morphology of Gymnarrehna, a desert plant, in some detrail, and it is very distinctive indeed - although some its oddest features, like amphicarpy, are practically unkown in other Asteraceae (this particular feature occurs elsewhere only in Catananthe-Cichorieae) and would seem to be responses to habitat conditions, and have little/nothing to do with phylogeny. Indeed, features like pappus movement hygroscopic may seem to suggest relationships with taxa like Carduus or Cirsium - but note that in those taxa the thick-walled cells involved are abaxial, while in Gymnarrhena they are adaxial. Aspects of testa anatomy may be similar to some of those in Mutisieae s.l. (Grau 1980), the subbasal swelling of the style is perhaps similar to that in Cardueae, and the parenchymatous pericarp might be similar to that in Stifftioideae or Barnadesioideae, and on it goes (Kravtsova 2024). It is interesting to see that practically none of these features is to be found in Cavea (see Y. Chen & Anderberg), its sister taxon, which also grows in quite extreme conditions and at high altitudes.
Funk et al. (2016) added two genera, placed in a separate subtribe, to Mutisioideae, and Pasini et al. (2016) examined relationships around Gerbera.
Panero (2020) has clarified the circumscription of Stifftioideae.
Kimball et al. (2023: two nuclear ribosomal and one plastid markers) looked at relationships within Tarchonanthoideae-Tarchonantheae - Tarchonanthus was monophyletic, but nested within Brachylaena.
Vernonioideae [= Cichorioideae-Vernonieae prior to 2019]. It is a little difficult working out relationships here given that there are some 50 monotypic genera (Keeley & Robinson 2009). For a phylogeny of Vernonia (Vernonieae), a genus whose circumscription is problematic - either it is huge, or quite small - see Keeley et al. (2007). Loeuille et al. (2015a) found four main clades within American Vernonieae, although relationships between them were unclear. Within Lychnophorinae, one of these clades, relationships may be [Albertinia [[Blanchetia + Gorceixia] + The Rest]] (the first three genera are monotypic), and within the rest of the clade, which are mostly plants of the Cerrado, much of the spine was poorly resolved (Loeuille et al. 2015b). The analysis in Angulo et al. (2022: 136 spp., 2 plastid plus ITS markers) was focused on taxa whose cytology was known. The phylogenomic study by Siniscalchi et al. (2019) is further clarifying relationships. The [Distephanus + Moquineae] clade may be sister to Vernonieae (Siniscalchi et al. 2019, q.v. for comments on their morphologies; c.f. in part Mandel et al. 2019; Keeley et al. 2021). Relationships within Distephanus (= Distephaneae) are discussed by Gostel et al. (2024: 3 plastid and 1 nuclear markers). The position of the monotypic Stokesia is still unclear, but it is somewhere towards the base of the tribe; subtribes, particularly those in the Old World, are often not monophyletic, and taxa towards the base of the tree tend to have odd distributions (Siniscalchi et al. 2019; Keeley et al. 2021). For relationships in the largely Peruvian Liabeae, sister to Vernonieae, see Funk et al. (2012) and Gutiérrez et al. (2020).
Funk et al. (2014, as tribe) discuss relationships in Wunderlichioideae. Genera like Cyclolepis remained hard to place, but Mandel et al. (2019) put it sister to Wunderlichioideae (see also Gochnatioideae, G. Zhang et al. 2024), while Panero (2020) placed three genera from the Guyana highlands in that subfamily.
Classification. A classification by Cassini (1819) and its variants was for long dominant. Panero and Funk (2008) provided a subfamilial classification somewhat similar to that above (there are, of course, alternative classifications - e.g. Jeffrey 2004); it is similar in basic structure to that in Funk et al. (2009b), who recognised 43 tribes, for which, see the accounts there. Katinas and Funk (2020) summariza relationships in basal Asteraceae as they review where genera in Mutisieae as circumscribed by Cabrera in 1977 are now to be placed - in no fewer than 11 subfamilies - indeed Mutisieae and Eupatorieae in the old sense include many of the taxa whose relationships have recently changed. Susanna et al. (2020) outline a classification - subfamilies (14) and tribes (around 60) - for the family. (Note that applying the same principles for subfamilial recognition - numbers of subfamilies - here as used effectively in Orchidaceae (see Chase et al. 2015) would result in a large and heterogeneous Asteroideae of around 22,500 species.) Subtribes in the family are also listed by Susanna et al. (2020), although Keeley et al. (2021) noted that subtribes in Old World Vernonieae needed a lot of work, but that was also true of some New World subtribes. Recent work, e.g. C. Zhang et al. (2021) and G. Zhang et al. (2024), confirms that there are still a number of problems to be resolved.
Anderberg et al. (2006) enumerated the genera in the family, however, generic limits in many places are still (Jan. 2025) very much in a state of flux (see Kadereit et al. 2016 for European genera). Vernonia is a classic case - should it include 800-1000 species, or should these species be placed in 20 subtribes, of which two thirds of the genera are mono- or ditypic (Keeley et al. 2007 for a phylogeny; Robinson 2006 and Keeley & Robinson 2009 and references for genera)? Should there be lumping or splitting in the even larger genus Senecio (see Pelser et al. 2006, esp. 2007)? Moonlight et al. (2024) included both these genera in their list of big genera, genera with over 500 species - we shall see. Along similar lines, Nicol et al. (2024) suggested that species previously included in Olearia belonged in separate subtribes. And finally, hybridisation (see above) makes some genera and even subtribes non-monophyletic. The classification above is largely based on that in and consisent with that G. Zhang et al. (2024: some of his tribes are subfamilies).
- Herrando-Moraira et al. (2019) provided a subtribal classification for Carduoideae-Cardueae of 12 subtribes, seven of which are new. Although generic limits around Saussurea (Saussureinae) have been problematical (e.g. L.-S. Xu 2019 and references; see summary trees/diagrams in Herrando-Moraira et al. 2020: Fig 1 and Kasana et al. 2020: Fig. 1), Herrando-Moraira et al. (2020) proposed a solution to the problem in which three quite broadly delimited genera were recognised. However, before the ink on their paper was dry, Kasana et al. (2020: quite good sampling, but ITS and morphology alone) had come up with another solution to the problem in which one of the three genera recognized by Herrando-Moraira et al. (2020) was split into two... I follow Herrando-Moraira et al. (2020) here. L. Xu et al. (2024) suggest an infrageneric classification for the genus - 4 subgenera, basically agreeing with morphological variation (ibid. Fig. 3), and 13 sections - the composition of the latter, however, was unclear, species-level sampling not being up to the task. Generic limits around Carduus and Cirsium are difficult, and Ackerfield et al. (2020) discuss the merits and demerits of a Carduus s.l. and s.s.; pending more work that is needed on the group, they prefered the latter solution. Del Guacchio et al. (2022) split Cirsium... Recently Bureš et al. (2024) suggested that Cirsium vulgare was an hybrid beween carpelate Cirsium and staminate Lophiolepis parents (= × Ascalea), while Moreyra et al. (e.g. 2025) would consider this species to be a cross between members of different subgenera of Cirsium; see also Del Guacchio et al. (2024), Moreyra et al. (2023) and Moreyra and Susanna (2024) for more on nomenclatural disagreements around here, but as of early 2025 it would seem that the nomenclatural solution proposed by Moreyra et al. is more satisfactory. For sections in Echinops, see Garnatje et al. (2005) and Sánchez-Jimenéz et al. (2010).
- Substantial adjustments to generic limits will be needed in Asteroideae-Astereae (e.g. Li et al. 2012), and here Heiden et al. (2020) provide an infrageneric classification for a slightly-expanded Baccharis, some 440 species, that includes 7 subgenera and 47 sections. Neson (2020b) has begun the dismemberment of Australian Olearia, which had early (Cross et al. 2002, see above) been found to be polyphyletic and in some eight clades, many taxa from Australian and surrounds (now in Celmisiinae) not being at all close to Olearia s. str., while Saldivia et al. (2020) has confirmed the assignment of the Australian ex-Olearia to Celmisiinae (the type of Olearia does not belong to Celmisiinae at all), a species from the Pacific being placed in a new genus. For Ast.-Gnaphalieae, see Short (2017), where the suggestions for genera to be recognized in the area around Helichrysum sound like a slowly-unfolding disaster, but the alternative, Helichrysum s.l., seems little better (Galbany-Casals et al. 2014); nothing really can be done until the phylogeny is straightened out, but for some new genera, see Nesom (2024) and references. For Ast.-Inuleae-Inulinae, see Englund et al. (2009), for Ast.-Millerieae, Espeletia and relatives, see Pouchon et al. (2018) who reasonably suggest putting all Espeletiinae in Espeletia and for a reclassification of Perityleae, see Lichter-Marck and Baldwin (2022). Senecio (Ast.-Senecioneae) is polyphyletic, and its species are to be found scattered in eight clades or so, yet even so Senecio s. str., at ca 1,000 species, is paraphyletic (Pelser et al. 2007) - perhaps it includes only a tenth of that number, or even far fewer (see Pruski 2021 for complexities of relationships and nomenclature here). New genera based on species removed from Senecio like Curio (largely African succulents) and Jacobaea (phylogenetically quite separate, but morphologically very like Senecio) are being described (Schmidt-Lebuhn et al. 2020 and references). Semple and Beck (2021) and Semple et al. (2023) provide an infrageneric classification for Solidago - three subgenera and 15 sections (also subsections) - with extensive discussion. For the 17 tribes of Anthemideae, see Oberprieler et al. (2022). Here Jiao et al. (2023) adjusted the limits of Artemisia, recognizing eight subgenera of which two were new (see also Jiao et al. 2025, 24 sections, all but 3 of the 505 species were placed in their classification). Within Coreopsideae, Coreopsis itself has been dismembered, its species being placed in nine other genera in seven clades altogether (Mesfin Tadesse & Crawford 2023); these authors discuss the disposition of the sections of Coreopsis, and just three of the eleven sections that it used to have are retained - and now for Bidens? Sánchez-Chávez et al. (2024) discuss the morphology and relationships of the odd genus Dicranocarpus, perhaps Coreopsideae, where the outer "mesocarp" consisted of irregular rows of tracheoidal cells,, then there was aerenchyma; there was neither phytomelan nor calcium oxalate crystals.
- For Cichorioideae-Arctotidinae, see McKenzie and Barker (2008), while for C-Lactucinae see Kilian et al. (2017), etc. - there seems to have been hybridization here, but, as Güzel et al. (2021) noted, any nomenclatural adjustments will have to wait for a comprehensive phylogenetic analysis. The limits of Scorzonera (C-Cichorieae) have been adjusted and a classification of Scorzonerinae proposed; of the new genera, etc., not easily characterized, Zaika et al. (2020: p. 51) write "We assume, however, that practical taxonomic experience in the application of a phylogenetic classification will bring to light new means to distinguish the various entities" - one can but hopeSince 2000, the number of species has varied - ca 95 species (see Lebeda et al. 2025), ca 130 species (Mabberley 2008), ca 73 species (Lebeda et al. 2025); the latter group clearly find generic limits confusing around here, but a Lactuca s.l. would be one solution.
- Asteroideae-Eupatorieae provide a good example of how classifications have been changing. King and Robinson (1987) summarized their work on the tribe in which, emphasizing micromorphological characters, they dismembered the old Eupatorium, which had around species in 1960, describing over 100 new genera (which they placed in 18 subtribes) in over 200 papers (see also Robinson et al. 2009 for an update). The massive amount of work involved was carried out immediately before phylogenetic data became widely available, and problems early became evident (see Scott 1990 for a review). Grossi et al. (2020) carried out a careful analysis of reproductive characters in the tribe and found that members of a number of the subtribes and genera sensu King and Robinson did not cluster together, but the results of Grossi et al. tended to agree with available molecular data, which also show substantial polyphyly of these subtribes and genera, even if the focus of much molecular work has been fairly local (e.g. Rivera et al. 2016: eastern Brazil). As Grossi et al. (2020) emphasize, little can be said about the evolution of this large tribe until the basic morphology of characters that have been used at the generic level has been cleared up and both broad scale and more focused phylogenetic studies carried out; only then will we be able to talk about clades, characters, classification and evolution here.
Botanical Trivia. Arctium lappa (burdock) infructescences became attached to the dog of a Swiss engineer, George de Mestral, in 1945, and the result was the development of velcro (Wikipedia 2009). For the (self)assembly of the pollen exine of Echinops, billed as "the thickest plant cell wall", see Gabarayeva et al. (2018).
Thanks. I am grateful to Jose L. Panero for comments.