EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CHLORANTHALES [[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]]]: sesquiterpenes +; (microsporogenesis also simultaneous); seed endotestal.
[[MAGNOLIALES + LAURALES] [CANELLALES + PIPERALES]] / MAGNOLIIDS / MAGNOLIANAE Takhtajan: (neolignans +); root cap meristem open; vessels solitary and in radial multiples, (with simple perforation plates in primary xylem); (sieve tube plastids with polygonal protein crystals); lamina margins entire; A many, spiral [possible position here], extrorse; ovules with hypostase, nucellar cap +, raphal bundle branches at the chalaza; antipodal cells soon die.
[MAGNOLIALES + LAURALES]: cuticle waxes as annularly-ridged rodlets, palmitol the main wax; A whorled; pollen 1-2 nexine foliations, outer member massive, lamellate endexine; (supra-stylar extra-gynoecial compitum/pollen tube growth); carpel cross-zone initiated late; ovules 1(-2)/carpel, basal, erect, apotropous; fruitlets 1-seeded; palaeopolyploidization/WGD event.
LAURALES Berchtold & Presl - Main Tree.
(Cork subepidermal); sieve tube plastids with polygonal protein crystalloids and starch; petiole bundle(s) arcuate; calcium oxalate crystals small, as sand, etc.; leaves opposite; inflorescence ± cymose; receptacle ± concave, hypanthium +; P spiral; inner staminodia +; stylulus quite long, (0), extragynoecial compitum +; megaspore mother cells several; fruits indehiscent, hypanthium persistent/accrescent, mesocarp with oil cells, (inner cells lignified), endocarp cells palisade, lignified, or not; mesotesta crushed, seed endotestal, endotesta tracheidal, tegmen crushed; embryo long; duplication of the PI gene. - 7 families, 91 genera, 2858 species.
Includes Atherospermataceae, Calycanthaceae, Gomortegaceae, Hernandiaceae, Lauraceae, Monimiaceae, Siparunaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. Wikström et al. (2001) suggest an age of (121-)114, 108(-101) Ma for the beginning of divergence here, quite similar to the 108.8 Ma in Tank et al. (2015: Table S2), while Renner (2005a) thought that it began ca 130.2 Ma, Magallón and Castillo (2009: relaxed and constrained penalized likelihood datings) ages of ca 171.4 and 119.3 Ma, Bell et al. (2010) ages of (133-)119, 112(-107) Ma, Magallón et al. (2013) and Magallón et al. (2015) suggested ages of around 117.5 Ma and 114.9 Ma respectively, Massoni et al. (2015a) ages of (165.6-)158.9, 117.4(-112) Ma and Jiao and Wang (2022) an age of only 83-74 Ma. Silvestro et al. (2020) estimated the time-of-origin of Calycanthaceae to be ca 132.7 Ma.
The young fruits of Protomonimia, in Turonian deposits from Japan ca 91 Ma old, have several carpels borne in spirals on a concave axis; there is a stigmatic crest. The young seeds have a thick testa, the exotesta being palisade and with sinuous anticlinal cell walls (Nishida & Nishida 1988). If a member of Laurales, it would probably be assigned to stem Laurales if only because there are several ovules/carpel. For Cecilanthus, from early Cenomanian Maryland ca 100 Ma, see Herendeen et al. (2016) and above). Araripia florifera, from the Lower Cretaceous of Brasil around 113 Ma, has flowers that are very like those of Calycanthaceae, but its opposite leaves are lobed (Mohr & Ecklund 2003); nothing was known of the internal structure of the flower. More material from the Crato Formation has become available, and it has been placed in a separate family, Araripiaceae. This material has carpelate flowers, and the gynoecium is syncarpous or monomerous and stipitate, and there is no hypanthium; the leaves are "alternate", not opposite as originally thought (Pessoa et al. 2023b).
Evolution: Divergence & Distribution. See Renner (1999, 2005a) for some details of diversification within Laurales. Soltis et al. (2005b) summarize information on possible synapomorphies for the clade (see also Staedler et al. 2009); von Balthazar et al. (2011) map the distributions of many characters in the order while Doyle (2009) placed variation in exine infratectum morphology on the tree. An extragynoecial compitum is common in Laurales, and it could be considered an apomorphy for the order, and then it would have to be lost at least twice and regained once, or acquired independently several times (see e.g. Endress & Igersheim 2000; Endress 2001a; Erbar & Söte 2022). For the lignified palisade endocarp cells common in the order, see Bobrov et al. (2017a) and Romanov et al. (2018); this is unlikely to be a feature linking Laurales and Magnoliales, but it is also unclear if it is an apomorphy for Laurales since it is rather labile...
Pollination Biology & Seed Dispersal. Although at one level the order is uniform in gynoecial mophology, the carpel(s) usually being free, there is considerable diversity in the ways in which the pollen tube reaches the ovule (Endress & Igersheim 1997 and references), a diversity not captured even in Armbruster (2002). Similarly, there is extensive variation in the pattern of lignification of the pericarp (Romanov et al. 2018 and references). Endress and Igersheim (1997; see also Erbar & Söte 2022) note that an extragynoecial compitum is found in those Laurales that have separate carpels.
Genes & Genomes. Oginuma and Tobe (2006) suggest that the base chromosome number for Laurales is x = 11.
Chemistry, Morphology, etc.. Isorhamnetin occurs in Lauraceae, Gomortegaceae and "Monimiaceae" (Crawford et al. 1986).
Flowers that are white around the periphery and coloured in the centre are scattered throughout the clade (Calycanthaceae, Atherospermataceae, etc.).
The apex of the nucellus in some Atherospermataceae, and perhaps also in Siparunaceae and Calycanthaceae, is exposed (Endress & Igersheim 1997). Little is known of obturator presence in the order, and of many other embryological details, although Endress (1972a) suggests there is considerable variation, some of which may link nicely with phylogeny.
See also Eklund (1999) for general information, Benzing (1967a, b), for nodes, Metcalfe (1987) for vegetative anatomy, Endress (2011a) for the distribution of an extragynoecial compitum, Kimoto and Tobe (2001) for embryology and Bobrov et al. (2017a) for fruit anatomy.
Phylogeny. Calycanthaceae are sister to other members of the clade in just about all studies, if some other interfamily relationships remain unclear - but see immediately below for Calycanthaceae! [Siparunaceae [Gomortegaceae + Atherospermataceae]] form a moderately-supported clade, relationships between the last two being supported strongly in D. Soltis et al. (2000, 2011; see also Massoni et al. 2014), although Glossocalyx was weakly supported as sister to all Laurales bar Calycanthaceae in Massoni et al. (2015a: add. file 2). [Monimiaceae + Lauraceae + Hernandiaceae] form another clade. Although the clade [Lauraceae + Hernandiaceae] had no support in a molecular analysis (D. Soltis et al. 2000), in a morphological study it had strong support (Doyle & Endress 2000, see also Renner & Chanderbali 2000). The comprehensive molecular studies of Massoni et al. (2014, 2015a) also did find some support for this clade, however, Tank et al. (2015) found a [Hernandiaceae + Monimiaceae] clade, and that clade was strongly supported in analyses by H.-T. Li et al. (2019, 2021) and Y. Song et al. (2019), and this is the topology followed below. The [Monimiaceae + Lauraceae + Hernandiaceae] clade seemed to be one of the few cases where was persistent disagreement between relationships suggested by morphology and those suggested by molecules (Renner & Chanderbali 2000). Before v.2019 I followed morphology (see also Renner et al. 1997 and Renner 1998, 1999, esp. 2005a).
What has been suggested over the last four years? W. J. Baker et al. (2021a: nuclear genes) found that Hernandia nymphaeifolia was sister to other Laurales, while Gyrocarpus americanus, also Hernandiaceae, was sister to [Monimiaceae + Lauraceae], although the sampling was rather exiguous. For relationships in the order, see also the Angiosperms353 probe set analysis by Helmstetter et al. (2024/2025: 199 genera, ca 3/4 the total); Lauraceae were sister to [Monimiaceae + Hernandiaceae] and Calycanthaceae were paraphyletic, although the evidence was weak. Hernandiaceae do move around. The relevant relationships in Zuntini et al. (2024) are [Hernandiaceae [Lauraceae + Monimiaceae]] (the topology of their tree is that used above) while in the Seed Plant Tree (as of ix.2024) they are [Calycanthaceae [Siparunaceae [Hernandiaceae [Gomortegaceae + Atherospermaceae] [Monimiaceae + Lauraceae]]]]. However, relationships in Y.-L. Qiu et al. (2024) are [Calycanthaceae I [Hernandiaceae [Monimiaceae [Lauraceae [Siparunaceae [Gomortegaceae [Calycanthaceae II + Atherospermaceae]]]]]]]. Calycanthaceae seem to be causing some problems.
Classification. The contents of Cronquist's (1981) Laurales and Takhtajan's (1997) Lauranae are largely the same as Laurales as they are circumscribed here - although of course Amborella has moved.
Thanks. I am grateful to S. Renner for comments.
Synonymy: Atherospermatales Martius, Calycanthales Link, Gyrocarpales Dumortier, Hernandiales Martius, Illigerales Martius, Monimiales Berchtold & Presl - Lauranae Takhtajan - Calycanthidae C. Y. Wu, Lauridae C. Y. Wu - Lauropsida Horaninov
CALYCANTHACEAE Lindley - Back to Laurales
Deciduous or evergreen shrubs to trees; tryptamine [calycanth(id)ine] alkaloids +; primary stem ± with vascular cylinder; cortical bundles +, inverted; vessel elements with simple perforation plates; sieve tube plastids also with peripheral protein fibres; pericyclic fibres 0; nodes 1:2 [see below]; petiole bundle arcuate, with wing bundles; hairs unicellular; buds perulate, petiole enclosing the axillary bud; lamina vernation flat to curved; flowers large [>2.5 cm across], (terminal); receptacle with cortical vascular system; P many, spiral, with 3-5 traces; A with prolonged connective, nectariferous staminodes ³10; pollen grains (tricellular), disulcate; stigma dry, placentation lateral; ovules 2(-1)/carpel), vascular bundle dividing in chalaza, branches in base of outer integument, inner integument 4-5 cells across, hypostase +; embryo sac long; fruits ± achenes; testa multiplicative, exotestal cells cuboid, slightly lignified; endosperm diploid, development autonomous [lacking paternal contribution]; n = 11, x = 11 (?12), nuclear genome [1 C] (0.025-)0.998(-39.189) pg.
5 [list]/13 - two subfamilies below. East Asia, North America, N.E. Australia. Map: from Wu (1983), Endress (1983), Hong (1993), Fl. N. Am. vol. 3 (1997) and Qian and Ricklefs (2004).
Age. Crown group Calycanthaceae have been dated to as recently as the early Eocene (60-)52, 49(-41) Ma (Wikström et al. 2001) or back in the Campanian, ca 110 Ma (Zhou et al. 2006) or (100-)98(-97) Ma (Bell et al. 2010); Renner (2005a) suggested an age of 56-54 Ma while there are estimates of (119.7-)111.9, 97.9(-91.6) Ma in Massoni et al. (2015a). Lv et al. (2020) date a genome duplication in Chimonanthus salicifolius to ca 87 Ma, but given the evidence in that paper, it could be placed anywhere from stem Calycanthaceae on upwards in Laurales.
Fossils are interesting. Araripia florifera, from the Lower Cretaceous of Brasil around 113 Ma, has flowers that externally are very like those of Calycanthaceae, but its leaves are lobed (Mohr & Ecklund 2003), however, recent collections allow Araripia to be placed in a separate family, Araripiaceae (see above). The late Cretaceous Virginianthus calycanthoides (98-113 Ma, perhaps 108 Ma - Massoni et al. 2015b) has been placed in Calycanthaceae. It has small flowers, anthers dehiscing by lateral hinges, and reticulate pollen with a single sulcus (Friis et al. 1994). Its anasulcate pollen is like that of Idiospermum, plesiomorphic for the order. Assuming the lateral hinges on the anthers of Virginianthus are equivalent to the rather differently oriented hinges found in most other taxa (c.f. also those of Sinocalycanthus - parallelism?), hinged anthers may be a synapomorphy for Laurales, and anthers with slits a synapomorphy for crown-group Calycanthaceae. However, the phylogenetic position of Virginianthus has been questioned, and whether it is in Calycanthaceae, sister to Calycanthaceae (e.g. Doyle & Endress 2010), sister to all other Laurales, or perhaps not to be included in Laurales at all, is unclear (Eklund 1999; Crepet et al. 2005; Zhou et al. 2006; Doyle & Endress 2007; Doyle et al. 2008b), although the latter idea is least likely. Indeed, Schönenberger et al. (2020) found that all three maximum parsimony positions for this fossil were in at least stem-group Calycanthaceae, although there were other possible positions just one step longer. López-Martínez et al. (2023a: Table 3) placed it around here or in the ANA grade. See also Friis et al. (2011) for fossils.
The rather younger (Turonian, ca 85.8 Ma old - see Massoni et al. 2015b) Jerseyanthus is more certainly Calycanthaceae and may even be sister to Calycanthus; it has the same distinctive disulcate pollen (Crepet et al. 2004, 2005) - the same as Idiospermum. However, Jerseyanthus is remarkable in having flower parts in the sequence (outside) petal-like tepal - introrse staminode - extrorse stamen - abaxially curved "petal-like staminode" - pistillode (inside), an arrangement of parts unlike that of any other living angiosperm, although Staedler et al. (2007) interpret the outer staminode series as being inner tepals.
1. Idiospermoideae Thorne - Idiospermum australiense (Diels) S. T. Blake —— Synonymy: Idiospermaceae S. T. Blake
Evergreen tree; flavonols 0, but luteolin, etc. +; wood diffuse porous, vessels to 3 together, in radial multiples, vessel elements with scalariform perforation plates, parenchyma abundant, vasicentric/paratracheal; nodes 1:1; leaves spiral to whorled; A 10-20, almost P-like, thick, subsessile; G 1(-5), style 0, stigma stout, fleshy, extragynoecial compitum +; ovules 1(-2)/carpel, outer integument 12-15 cells thick, micropyle bistomal, nucellar beak +; fruit odd, rotting on the tree, endocarp not developed; seed large [³3 cm across], cotyledons (3-)4, massive, peltate.
1/1. Queensland, Australia.
2. Calycanthoideae Burnett —— Synonymy: Butneriaceae Barnhart, Chimonanthaceae Perleb
Evergreen/deciduous trees/shrubs; wood ring porous, vessels many, in radial multiples, small, parenchyma vasicentric, scanty; nodes 1:2; (terminal bud aborts); P (10³); A 5-20, filaments rather slender, anthers (valvate [H-dehiscence] - Sinocalycanthus), ± latrorse, (staminodes 0); pollen equatorially and vertically disulcate; G ³5, carpellary vascular supply recurrent [Calycanthus], stigma filiform, compitum by style coherence; ovules 2/carpel, lateral, apical ovule aborting, apotropous, outer integument 5-6(-8) cells thick, micropyle exostomal, parietal tissue ± 0, nucellar cap ca 7 cells across; (>1 embryo sac/ovule); mesocarp lacking oil cells, endocarp cells palisade, lignified; cotyledons spirally twisted; (n = 12 loss of one copy of rpl2 from IRb [whole of Laurales?]).
4/12: Daphnandra (6), Doryphora (4). China, Korea, Taiwan, North America. Photo - Flower © John Crellin.
Age. Renner (2005a) suggested an age of 33-24 Ma for crown-group Calycanthoideae.
Evolution: Divergence & Distribution. Massoni et al. (2015a) suggest that there has been a major slow-down of diversification in this clade.
For possible apomorphies of the two subfamilies in addition to those suggested above, see Staedler et al. (2009).
Pollination Biology & Seed Dispersal. Gottsberger (2016a) summarized pollination in the family. Nectar or food bodies are produced by the tepals and the latter sometimes by the stamens, although neither in Idiospermum. For a suprastylar extragynoecial compitum, see Endress and Igersheim (1997).
Genes & Genomes. Isozyme duplication in Calycanthus suggests that there has been ancient polyploidy here (Soltis & Soltis 1990). A genome duplication in Chimonanthus salicifolius has been dated to some 87 Ma (Lv et al. 2020).
For the chloroplast genome, etc., see Goremykin et al. (2003b).
Chemistry, Morphology, etc.. The two rings of vascular tissue in the stem are quite distinct from the seedling stage onwards. The leaf is usually innervated by paired traces from the inner vascular ring which very soon fuse and form the median petiole bundle, and also by two traces from the cortical bundles that form the lateral or wing bundles (Balfour & Philipson 1962; Benzing et al. 1967; Beck et al. 1982). Although I could not see central paired traces in Chimonanthus, these traces may fuse when in the stem (Fahn & Bailey 1957). Odd teeth are sometimes found on the lamina of Calycanthus virginianus, at least on the plant in my back garden when I was in St Louis.
Calycanthus occidentalis has inverted recurrent vascular bundles in the hypanthium (see above), perhaps evidence of receptacular epigyny (Tiagi 1963b; Dengler 1972). Staedler et al. (2007a) note that the numbers of floral parts, tepals, stamens, staminodes, etc., are more or less Fibonacci numbers (3, 5, 8, 13,....). The carpels in general are more or less plicate. The ovules of Calycanthus almost lack parietal tissue, but they do have a nucellar cap (Dahlgren 1927); Peter (1920) described them as lacking parietal tissue, but with a nucellar cap ca 7 cells across. The seeds are poisonous - as are those of Idiospermum, dead cattle prompting its rediscovery (Blake 1972) - and have characteristic alkaloids.
For the morphology, etc., of Idiospermum, see Blake (1972), q.v. for other possible differences between the two subfamilies, for chemistry, see Crawford et al. (1986). For general information on Calycanthaceae, see Nicely (1965) and Kubitzki (1993b), for floral anatomy, see G. H. Smith (1928), for floral development, see Rauh and Reznik (1951), for disulcate pollen of Calycanthus, see Albert et al. (2011), for gynoecial development, see Staedler et al. (2007b), and for fruit anatomy, see Romanov et al. (2018).
Phylogeny. Li et al. (2004) clarify phylogenetic relationships in the family; Idiospermum is sister to the rest - although, as noted above, Calycanthaceae are paraphyletic in some recent studies, including Helmstetter et al. (2024/2025).
Classification. Idiospermum is a very distinctive genus and has sometimes been recognised as a separate family (e.g. Cronquist 1981). However, it is monotypic and shares many features with the other Calycanthaceae, although the alkaloids and the distribution of xylem parenchyma differ in detail.
[[Siparunaceae [Gomortegaceae + Atherospermataceae]] [Lauraceae [Hernandiaceae + Monimiaceae]]] / core Laurales: evergreen trees; (plants Al accumulators); vessel elements with scalariform perforation plates; hippocrepiform sclereids in pericycle; mucilage cells + [?this level]; nodes 1:1; lamina with rather distant teeth, one vein entering opaque, persistent glandular cap; flowers rather small [usu. <1 cm across], inconspicuous; A whorled, stamens with paired nectaries/glands at base, anthers valvate, valves apically hinged; tapetum ?; pollen inaperturate, exine thin [pollen not resistant to acetolysis], infratectum intermediate, surface ± spinulose, intine thick, outer part with radial channels; placentation median; ovule 1/carpel; carpel development?; funicular vascular bundle not branching in chalaza; fruit variously fleshy; endosperm ± oily.
Age. This clade has been dated to (103-)96, 89(-82) Ma (Wikström et al. (2001) and fossil-based estimates are ca 91 Ma (Crepet et al. 2004) or ca 125 Ma (Friis et al. 2017c: see above); Renner (2005a) suggested an age of around 127 Ma, ca 107.9. Ma is the age in Magallón et al. (2015), while a very broad range of ages, (157.7-)147.6, 82(-38.5) Ma, was suggested by Massoni et al. (2015a).
Saportanthus was described from deposits in Portugal ca 125 Ma, and in morphological analyses it is either sister to Gomortega or to the whole [[Siparunaceae [Gomortegaceae + Atherospermataceae]] [Monimiaceae [Hernandiaceae + Lauraceae]]] clade; its 4-sporangiate anthers have laterally-hinged flaps, its pollen has a striate surface and is trichotomocolpate or dicolpate and its semi-inferior ovary has 1-3 carpels. Fossils of Lovellea wintonensis, from the upper Albian of Queensland, Australia, and perhaps 105 Ma have characters suggesting Laurales, although they do not suggest any particular family; morphological cladistic analyses place it somewhere around here (Dettmann et al. 2010). It has disulcate pollen (see Calycanthaceae-Calycanthoideae!), an inferior ovary, partly fused carpels with long styluli, the ovule has a hypostase, and the seed has two layers of cells, the inner being made up of transfer cells, each including a crystal; it lacks staminodes (Dettmann et al. 2010). Dettmann et al. (2010) compare Lovellea wintonensis with Gomortega (Gomortegaceae) and Tambourissa (Monimiaceae) in particular, i.e. with taxa from both branches of this clade (see also Massoni et al. 2015b: age ca 100 My).
Evolution: Pollination Biology & Seed Dispersal. Nectar is secreted by the paired glands at the base of the stamens/staminodes, although when the hypanthium is well developed the glands are lost (Erbar 2014 and references; Gottsberger 2016a).
Chemistry, Morphology, etc.. Leitão et al. (1999) summarize alkaloid distribution in Monimiaceae in the old sense, which includes Atherospermataceae, Monimiaceae s. str., Siparunaceae and sometimes even Hernandiaceae.
Staedler and Endress (2009) discuss floral phyllotaxis; there is considerable variation, with both whorled and spiral phyllotaxis in all families that are not monotypic, phyllotaxis even varying within a species. For variation in exine structure in this part of the tree, the pollen of many taxa having an infratectum that is more or less intermediate between granular and columellar, see Doyle (2009). For discussion as to what the nectary glands "are", whether stipules, staminodes, or structures arising de novo, see e.g. Sampson (1969b). How they are vascularized is quite variable (Kasapligil 1951; Sampson 1969b). The ovule is median, developing on on early-initiated cross-zone; Endress and Doyle (2015) note that carpel development in this clade in intermediate between plicate and ascidiate.
There is little information on tapetal development and most other embryological details and of seed anatomy; this is especially true of the first three families (however, see Kimoto & Tobe 2001, 2008a for summaries; Kimoto and Tobe 2008b).
For general information on Monimiaceae and the other families previously included in them, see Schodde (1970) and Philipson (1993), Doyle (2007) discusses leaf teeth, Hesse and Kubitzki (1983), Sampson (1993, 1997, 2007) and Foreman and Sampson (1987), pollen, and Romanov et al. (2007), fruit anatomy.
[Siparunaceae [Gomortegaceae + Atherospermataceae]]: acicular crystals +; hypanthium closed by roof; anthers bisporangiate, monothecal, pollen with columellar infratectum; embryo very small, endosperm copious.
Age. Renner (2005a) suggested an age of 124-118 Ma, Tank et al. (2015: table S1, S2) an age of ca 98.4/97 Ma, and Magallón et al. (2015) an age of ca 91.8 Ma for this node.
Crepet et al. (2016) assign Jamesrosea to stem [Gomortegaceae + Atherospermataceae]; found in Burmese amber ca 98 Ma it suggests a rather different biogeographic history than do the distributions of extant taxa, all southern/Gondwanan.
SIPARUNACEAE Schodde - Back to Laurales
Shrubs or lianes; plants Al accumulators; indumentum often stellate; vessel elements also with simple perforation plates; no hippocrepiform sclereids in pericycle; primary stem?; nodes 1:1; petiole bundles also flattened-annular, (medullar plate +); cuticle wax?; buds not perulate; lamina vernation curved-conduplicate, (margins entire); plants monoecious or dioecious, flowers imperfect; hypanthium well developed; (flowers monosymmetric - Glossocalyx); P 4-6(-7) or obscure, (lingulate), (connate); A (1-)2-many [e.g. 2 + 2 + 2], anthers often with one flap, paired glands 0, nectar 0; G 3-many, occluded by secretion as well, styluli short, postgenitally united in receptive zone; ovule basal, unitegmic, integument 3-5(-?8) cells across, hypostase +; megaspores elongating [Siparuna], embryo sac ≥2/ovule [?all], starch-rich; hypanthium thick, fleshy, splitting irregularly in fruit/not - Glossocalyx , (drupelet with a fleshy appendage - "stylar aril"), endocarp and inner mesocarp lignified; (seeds bilaterally flattened - Glossocalyx); testa parenchymatous, endotegmen with reticulate thickenings; n = 22, x = ?
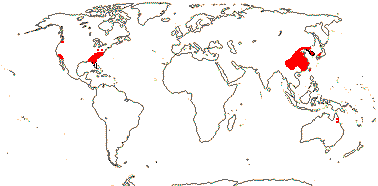
2 [list]/75: Siparuna (74). Tropical America (Siparuna), W. Africa (Glossocalyx). Map: S. Renner (pers. comm.). [Photo - Flower, Fruit.]
Age. Renner (2005a) suggested an age of around 90 Ma for crown-group Siparunaceae.
Evolution: Divergence & Distribution. Divergence within Siparuna may have begun ca 80 Ma (Renner 2005a).
Pollination Biology & Seed Dispersal. Pollination is by genera of cecidomyiid gall midges, which lay eggs mostly in staminate flowers and the larvae destroy the flower; however, if eggs are laid in the female flower, it aborts (Feil 1992; Renner et al. 1997; Gottsberger 2016a).
Dioecy seems to have evolved several times from monoecy in Siparunaceae (Renner & Won 2001).
The fruits of Siparuna may be a particulary valuable resource for frugivores (P. Jorgensen, pers. comm.). In most taxa the hypanthium splits irregularly when the fruits are ripe, the walls spreading widely and exposing the drupelets inside. The drupelets, and/or their fleshy appendages, differ strikingly in color from the inside of the fruit (see also Monimiaceae). These fleshy appendages are called arils by Renner and Hausner (1997) and are found only in dioecious taxa.
Chemistry, Morphology, etc.. The ovule is interpreted as having lost its outer integument by Kimoto and Tobe (2003).
General information is taken from Money et al. (1950) and Philipson (1993), details of floral morphology from Endress (1980c), of pollen development, etc., from Bello et al. (2002a) and embryology from Heilborn (1931: very strange female embryology) and Kimoto and Tobe (2003).
Phylogeny. For phylogenetic relationships in Siparunaceae, see Renner and Won (2001).
[Gomortegaceae + Atherospermataceae]: bud scales +; sieve tube plastids also with peripheral protein fibrils; outer A alone staminodial; microsporogenesis simultaneous.
Age. Estimates for the age of this node are (56-)51, 50(-45) and (44-)39(-34) Ma (Wikström et al. 2001), (44-)29, 25(-12) Ma (Bell et al. 2010), or (145.6-)130.5, 55(-24.4) Ma (Massoni et al. 2015a). Other ages for the clade (?= age of stem Atherospermataceae) from 244 to 140 Ma have been entertained (Renner et al. 2000), while Renner (2005a) suggested an age of around 116-112 Ma while >125 Ma is consistent wih some ideas of the relationships of Saportanthus (Friis et al. 2017c); ca 85.3 Ma is the age in Tank et al. (2015: table S2) and 78.4 Ma that in Magallón et al. (2015).
Evolution: Divergence & Distribution. Doweld (2001b) emphasized the similarities between Atherospermataceae and Gomortegaceae, especially the tracheoidal endotesta (perhaps plesiomorphic). Both have fruits (drupaceous, achenial) in which the seed coat would be expected to have lost its protective function.
Genes & Genomes. A duplication event that can be pegged to this node, the GOKEα event, has been dated at ca 97.7 Ma (Landis et al. 2018).
Chemistry, Morphology, etc.. Carlquist (2018c) gives an accrount of the wood anatomy of the two families.
GOMORTEGACEAE Reiche - Gomortega keule (Molina) I. M. Johnston - Back to Laurales
Alkaloids?, Al accumulation ?; primary stem with separate bundles; secondary phloem with flaring rays; nodes 1:2; cuticle wax?; lamina entire; flower parts between spiral and whorled, hypanthium 0; P 5-7(-9); A 7-13, filaments rather slender;G [2-3(-5)], inferior, style stout, branches erect, stigmatic; compitum +; ovules 1(2)/flower, apical, pendulous, hemianatropous [or straight?]; megaspore mother cell 1; fruit drupaceous, inner mesocarp and endocarp lignified, with layers of sclereids. fibres running down the long axis of the fruit internally; seed 1(-3); endotegmen tanniniferous; endosperm 2-layered, inner layer also with starch, embryo short; n = 21, x = 11 (?12, ?22)
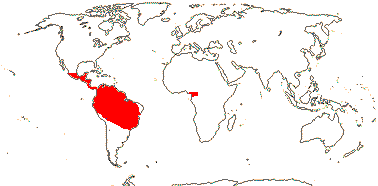
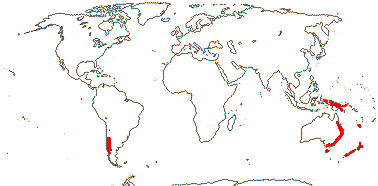
1 [list]/1. C. Chile, rare (map: from Donoso Z. 1994). [Photo - Flower.]
Evolution: Pollination Biology & Seed Dispersal. For pollination - probably by syrphid flies - see Gottsberger (2016a).
Chemistry, Morphology, etc.. Vessel elements in young wood may have simple perforation plates (Metcalfe & Chalk 1987). Stern (1955) thought that nodal anatomy was unclear; here I follow Howard (in Metcalfe & Chalk 1987).
The inflorescences, often described as being racemose, have a terminal flower. Although the body of the ovule is straight, its insertion on the funicle is oblique (c.f. Endress & Igersheim 1997). There is disagreement as to whether the seed in pachychalazal or not (Doweld 2001b).
Additional information is taken from Kubitzki (1993b: general), Muñoz-Concha et al. (2018: endosperm) and Heo et al. (2004: embryology).
ATHEROSPERMATACEAE R. Brown - Back to Laurales
Bisbenzylisoquinoline alkaloids +, Al accumulation 0; primary stem?; axial parenchyma ±0; nodes (1:1 - Atherosperma moschatum), (hairs T-shaped); stomata anomocytic; lamina vernation involute [Laurelia] or conduplicate; inflorescence racemose; (flowers medium sized - ca 2 cm across); P = 2-4 + 2-4, or K 2, C 8, or K 5 + 5, C 5 (+ 5); tapetum amoeboid, cells binucleate, "microsporogenesis modified simultaneous"; pollen grains polar di- or meridionally syncolpate, (tricellular), reticulate, exine infractectum columellar; G 4-many, occluded by secretion as well, stylulus lateral/gynobasic, (none), postgenitally united in receptive zone; outer integument 2-3 cells across, inner integument 2-4 cells across, (nucellus apex exposed), parietal tissue ?6-8 cells across; (megaspore mother cell 1); hypanthium woody, dehiscence irregular, fruit achenes, plumose [including style], or baccate, endocarp palisade; embryo also medium; n = 22, 57, x = 22 (?11, ?21).
6-7 [list]/16: Daphnandra (6). New Guinea to New Zealand and New Caledonia, Chile, scattered. Map: Philipson (1986a) and Andrew Ford (pers. comm.: Australia). [Photo - Fruit.]
Age. Divergence within Atherospermataceae may have begun ca 90 or ca 61 Ma (Renner 2005a) or (92.8-)79.4, 29.5(-12.6) Ma (Massoni et al. 2015a).
Atherospermataceae are known from forests on the Antarctic Peninsula of the late Cretaceous/early Caenozoic; the oldest fossils are ca 88 Ma (Poole & Francis 1999; Renner et al. 2000; Friis et al. 2011 for references).
Evolution: Divergence & Distribution. Fossil wood (?identification) of Atherospermataceae is recorded from the Upper Eocene of Germany and elsewhere in the northern hemisphere (Friis et al. 2011 for references), and distinctive pollen attributed to Laurelia is known from the lower Eocene in Europe (Hofmann et al. 2015) - see also Winteraceae.
Renner et al. (2000) and Renner (2005a) discuss the evolution and biogeography of the family; dispersal seems to have been involved (Sanmartín & Ronquist 2004). Atherospermophyllum, from the Eocene (52.2 Ma) in Patagonia, seems to be related to Australian members of the family, rather than to genera now found in South America (Knight & Wilf 2013).
Chemistry, Morphology, etc.. The plants do not accumulate aluminium (Webb 1954). Atherosperma has two sepals completely enclosing the bud, and then eight petals. The vasculature of the anther glands is independent of that of the anthers (Canright 1952).
Some information is taken from Money et al. (1950: general), Philipson (1993), Stanstrup et al. (2010: chemistry), Poole and Gottwald (2001: wood anatomy), Endress (1980c: flower) and Sampson (1969b, c: ovular morphology and embryology).
Phylogeny. Renner et al. (2000) suggested that the [Doryphora + Daphnandra] clade, from the Queensland-New South Wales area of Australia, was sister to the rest of the family.
[Lauraceae [Hernandiaceae + Monimiaceae]]: aporphine [isoquioline] alkaloids and variants +; vessel elements with simple perforation plates; (cork cambium outer cortical); crystals +, small; A whorled; pollen exine infratectum granular; extragynoecial compitum 0; ovule apical, pendent, (micropyle bistomal).
Age. Estimates for the age of a [Monimiaceae [Hernandiaceae + Lauraceae]] node are (67-)52, 45(-32) Ma (Bell et al. 2010), ca 105.1 Ma (Magallón et al. 2015), of a [Hernandiaceae [Monimiaceae + Lauraceae]] node ca 124 Ma (Renner 2005a), (146.2-)134.2, 59.9(-17.1) Ma (Massoni et al. 2015a), (134-)122(-110) Ma (Michalak et al. 2010) and (139.0-)125.3(-113.7) Ma (Y. Song et al. 2024: Monimiaceae not included), and of a [Lauraceae [Hernandiaceae + Monimiaceae]] node ca 93 Ma (Tank et al. 2015: Table S2).
The distinctive fossil Mauldinia mirabilis (see also below under Lauraceae) may best be placed around here, rather than within Lauraceae (Doyle & Endress 2007, 2010; see also Schönenberger et al. 2020; López-Martínez et al. 2023a); it was placed sister to a [Hernandiaceae + Lauraceae] clade in a constrained morphological analysis by Doyle and Endress (2010) and dated to around 95.5 Ma (Massoni et al. 2015b). Other fossils ca 108 Ma have also been placed at this node (Massoni et al. 2015b). Cohongarootonia (it has a hollow style) and perhaps Powhatania, both from Early-Middle Albian sediments 125-118 Ma old in Virginia, also probably belong in this general area (von Balthazar et al. 2011). Silvestro et al. (2020) estimate the time-of-origin of Hernandiaceae to be ca 29.3 Ma, that of Lauraceae ca 121.7 Ma, and that of Monimiaceae ca 122.9 Ma.
Evolution: Pollination Biology. Rohwer (2009) compared the heterodichogamous flowers (two morphs - in one the flowers discharge pollen first, in the other the stigma is receptive first) of Lauraceae with those of Hernandiaceae (for Hernandia, see Endress & Lorence 2004) and suggested that heterodichogamy may be common to the two families. He interpreted the floral morphology of Hernandiaceae from this point of view.
Chemistry, Morphology, etc.. Kimoto and Tobe (2008a) provide a comprehensive summary of the variation in embryology and seed for the whole group.
Phylogeny. See above for relationships in this area, which have been unclear, also, see Y. Song et al. (2019: plastomes) and H.-T. Li et al. (2019) for recent work. As mentioned above, there is quite extensive similarity between Hernandiaceae and Lauraceae.
Age. Ca 91.2 Ma is the age for this node in Magallón et al. (2015).
The most parsimonius position for Nothophylica piloburmensis, erstwhile Rhamnaceae from Burmese amber, is here, all the next most parsimonius positions, up to three steps longer, are also in Laurales (not including Calycanthaceae - see Beurel et al. 2025).
Evolution: Divergence & Distribution. For similarities in the wood of the two families, see Shutts (1960), and for additional possible synapomorphies, see Doyle and Endress (2000). A scenario for ovary evolution is that it became inferior in the common ancester of Hernandiaceae and Lauraceae, the inferior condition being lost (?more than once) in the latter family, but other scenarios are about equally parsimonious. Protrusion of the embryo sac from the nucellus may ultimately be placed at this node, too. For similarities in the pollen of these two families, see Kubitzki (1981). As more is understood about Hernandiaceae and basal Lauraceae one can but hope for clarification of what is going on around here.
LAURACEAE Jussieu - Back to Laurales
Flavones, 5-O-methyl flavonols, polyketides [acetogenins], (tryptamine alkaloids +), (plants Al-accumulators); xylem parenchyma ± aliform; wood often fluorescing; (secondary phloem stratified); nodes 1:2 (1:3); ?hippocrepiform pericyclic cells; also crystals and crystal sand +; (stomata anomocytic); buds perulate (naked); branching ± whorled, from current flush; lamina often glaucous below, vernation conduplicate or supervolute, secondary veins usually ± steeply ascending, higher-order areolation strong; plants heterodichogamous; inflorescence umbellate to thyrsoid; hypanthium often short, T 3 + 3, all members with three vascular bundles [odd member of the outer whorl adaxial, but #2]; A 9, = 3 [introrse] + 3 [introrse] + 3 [extrorse], with glands, three vascular bundles, sporangia widely separated on broad connective; tapetal cells 2(-4) nucleate, microspore mother cells in single layer, (microsporogenesis simultaneous); (pollen monosulcate); (stylulus 0), stigma also capitate, dry, compitum necessarily 0; ovule pachychalazal, outer integument 3-8(-20) cells across, inner integument 2-4 cells across, parietal tissue 5-7 cells across, hypostase 0; megaspore mother cell 1; fruit fleshy, pedicels (and tepals) often thickened and coloured, endocarp palisade, of columnar sclereids, anticlinal walls sinuous; (seed ruminate), testa vascularized or not, often multiplicative, (exotestal cells cubic), (palisade), endotestal cells longitudinally or transversely elongated, with helical thickenings; endosperm nuclear/coenocytic, 0; n = (11-)12, x = 12 (?6, ?7), chromosomes 1-5 µm long, nuclear genome [1 C] (0.101-)1.306(-16.825) pg, whole genome duplication [?here]; germination epigeal.
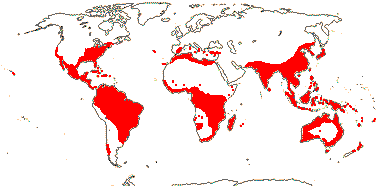
Ca 50/2,645 (2,850) [list - to tribes], nine groups below. Pantropical (temperate), lowland to montane. An appreciable part of the distributional area of the family, e.g. in most of West Australia, is attributable to Cassytha alone. Map: from Heywood (1978), modified as in Richter (1981), Fl. N. Am. vol. III (1997), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003) and FloraBase (consulted 2005); see also L. Li et al. (2025: Fig. 1).
Age. Chanderbali et al. (2001: c.f. calibration, 682±105 My!) suggested an age of 174±32 Ma for the family, but other estimates are as young as (93-)61(-26) Ma (Michalak et al. 2010); (146.6-)135.3, 66.7(-46) Ma is the range in Massoni et al. (2015a: note topology).
Potomacanthus lobatus, provisionally assigned to Lauraceae although with an unusual combination of floral characters - there are only two whorls of stamens and no nectaries/glands - has been described from deposits in Virginia some 112-105 Ma (von Balthazar et al. 2007). Tree trunks up to some 1.8 m in diameter from the Turonian ca 92 Ma in Utah were placed in Paraphyllanthoxylon c.f. alabamense and thought to be Lauraceae (Jud et al. 2018b); although perhaps a questionable identification, P. marylandense, early Cenomanian and so close to 100 Ma, was specifically compared with and identified as Lauraceae (Herendeen et al. 1991).
1. Hypodaphnideae Reveal - Hypodaphnis zenkeri (Engler) Stapf
Leaves spiral, lamina chartaceous; plant monoecious; staminate flowers: 1st and 2nd whorl A with 4 collateral pollen sacs, lateral pollen sacs latrorse, central pollen sacs introrse, 3rd whorl A often 2 pollen sacs, latrorse-introrse, nectar glands large, adaxial to whorl II [?interpretation]; tapetum amoeboid; pistillate flowers: G inferior; fruit a drupelet, enclosed by cupule; n = ?
1/1. Tropical West Africa.
[Cryptocaryeae [Cassytheae [Neocinnamomeae [Caryodaphnopsideae [Mezilaureae [Perseeae [Cinnamomeae + Laureae]]]]]]]: subsidiary cells of paracytic stomata envelop the guard cells both above and below, the guard cells having outer and inner cuticular ledges; lamina often coriaceous; tapetum glandular; pistillate flowers: staminodes + [= third whorl of stamens]; embryo sac elongated, protruding from nucellus; loss of rpl2 copy from IR copy A.
Age. Chanderbali et al. (2001) proposed an age of 158±31 Ma for this node, ca 82.2 Ma is the age in Renner (2005a), ca 98 Ma is the age in L. Li et al. (2016) and (129.5-)116.0(–110.1) Ma in Y. Song et al. (2024).
2. Cryptocaryeae Nees
Leaves (opposite), lamina (triplinerved); inflorescece thyrsoid-paniculate, flowers of ultimate cymes alternate; flowers ± sessile, (2-merous - Potameia); A (8, 6, 4, 3), (4th whorl A +, staminodial, with three vascular bundles - Crypt., Dahlg.), anthers bisporangiate (but longitudinal septum [so ≡ 4 sporangia] / sporangia 4, collateral); pollen also oblate to peroblate, perprolate, surface ± verrucate/ ± smooth / striate, striae alternating with prominent ± parallel ridges / etc.; hypanthium deep (shallow - Beil. group); cupule enveloping fruit (0 - Beil. group), innermost part of hypanthium bony, sclereidal, fruit proper a berry, but pericarp thin; seed pachychalazal, coat vascularized; (n = 15 - Eusid., Endi.); chloroplast IR copy B contracts, rpl2 and 23, trnl-CAV genes lost.
12/800: Cryptocarya (360), Beilschmiedia (270), Endiandra (130). Pantropical, some subtropical, to New Zealand.
Age. The age of a clade that includes the three genera above is estimated to be ca 90 Ma (Chanderbali et al. 2001), (68.9-)50.4(-35.9) Ma (H. Li et al. 2020) or (114.0-)94.4(–78.9) Ma (Y. Sun et al. 2024).
[Cassytheae [Neocinnamomeae [Caryodaphnopsideae [Mezilaureae [Perseeae [Cinnamomeae + Laureae]]]]]]: fruit a drupe; endotestal cells with helical bands of thickening; IR copy A contracts, rpl2, rpl23, etc. genes lost.
Age. The age of this node has been estimated at (118.4-)77.3(-33) Ma (Naumann et al. 2013) and (123.8-)102.0(–71.3) Ma (Y. Song et al. 2024).
3. Cassytheae Dumortier - Cassytha L. —— Synonymy: Cassythaceae Lindley, nom. cons.
Stem parasite, connection usu. via xylem only; perennial herbaceous vine; stomata orientation transverse; leaves much reduced, spiral; inflorescence raceme, spike, or head; bracteoles adnate to T; outer T small, with one trace; 4th whorl A +, staminodial, with three vascular bundles, anthers bisporangiate, ?thecae; pollen surface also ± strongly verrucate; micropyle bistomal, parietal tissue 3-6 cells across, nucellar cap 0, pachychalaza 0, lysigenous cavity developing in funicle opposite micropyle; embryo sac ≥2/ovule, elongating beyond nucellus, antipodal nuclei ephemeral; fruit surrounded by fleshy T, parenchymatous mesocarp ± collapsed, inner mesocarp palisade, lignified, endocarp s. str. layer of sclereids; seed with physical dormancy, coat ± collapsed; endosperm cellular; chromosomes to 7 µm long; plastome only one copy of IR, 6 ndh genes lost, 5 pseudogenes, etc., cox1 mitochondrial gene with intron; seedling initially independent.
1/19. Tropics, esp. Australia, inc. warm temperate regions there, Cassytha filiformis ± cosmopolitan. See The Parasitic Plant Collection, H. Zhang et al. (2022: Fig. 2). [Photo: Plant.]
Age. Naumann et al. (2013) suggest a crown-group age for Cassytha of (118-)77(-33) Ma.
[Neocinnamomeae [Caryodaphnopsideae [Mezilaureae [Perseeae [Cinnamomeae + Laureae]]]]]: anthers tetrasporangiate; ovary superior, hypanthium shallow; fruit ?type; testa usu. not multiplicative.
Age. Chanderbali et al. (2001) suggest an age of 142±24 Ma for this node and Y. Song et al. (2024) an age of (112.9-)84.9(-61.5) Ma.
4. Neocinnamomeae Yu Song, W. B. Yu & Y. H. Tan - Neocinnamomum H. Liu
(Plant deciduous); leaves spiral, lamina triplinerved; inflorescence condensed few-flowered thyrses; 4th whorl A +, staminodial; fruit sitting in cup, T accrescent; n = ?
1/7. Southeast Asia, western Malesia (Sumatra).
Age. The age of crown-group Neocinnamomum is (81.2–)14.2(-10.1) Ma (Y. Song et al. 2024).
[Caryodaphnopsideae [Mezilaureae [Perseeae [Cinnamomeae + Laureae]]]]: ?
Age. Caryodaphnopsis split from other Lauraceae ca 96.8 Ma according to L. Li et al. (2016), but note topology.
5. Caryodaphnopsideae Yu Song, W. B. Yu & Y. H. Tan - Caryodaphnopsis Airy Shaw
Leaves (sub)opposite, lamina triplinerved (venation pinnate); outer T notably smaller than inner; (anthers 2-celled); pedicel slightly thickened in fruit, T deciduous, cupule 0; n = ?
1/20. Central and South America, South East Asia to the Philippines and Borneo.
Age. Diversification in this clade began (78.7-)48(-25.7) Ma (L. Li et al. 2016) or (35.4-)10.8(–0.9) Ma (Y. Song et al. 2024: sampling).
Fossils possibly to be associated with Caryodaphnopsis are known from Europe and the southeast U.S.A. (Li et al. 2016).
[Mezilaureae [Perseeae [Cinnamomeae + Laureae]]] / Lauroideae Burnett / core Lauraceae: inflorescences thyrsoid, higher orders of branching with strictly opposite lateral flowers or cymes; (when anthers bisporangiate, reduction in number of sporangia); tapetum plasmodial; loss of ca 4500 bp in IR copy B.
6. Mezilaureae Rohwer
Leaves opposite or pseudoverticillate, venation pinnate; A 3-12(-20 - Chloroc.); anther sporangia as two ± superposed pairs/not [Mezi.]; paired anther glands 0.
6/23: Mezilaurus (18). Central (Costa Rica) to South America, Trinidad.
[Perseeae [Cinnamomeae + Laureae]]]: anther sporangia as two ± superposed pairs [?up one node].
Age. A series of estimates of the age of the Persea group, which includes Litsea and Alseodaphne, etc., range from (260.3-)166.8 ... 55.3(-41.4) Ma, depending on the calibration (L. Li et al. 2011) or ca 66.0 Ma (Y. Song et al. 2024), and that of the Persea-Cinnamomum clade is Cretaceous, being ca 76 Ma (Lv et al. 2020) or ca 59 Ma (J.-F. Huang et al. 2015). Lindera, Dehaasia and Machilus split ca 52 Ma (L. Li et al. 2016).
7. Perseeae Nees —— Synonymy: Perseaceae Horaninow
Leeaves penninerved; (plant dioecious - Alseodaphnopsis); inflorescence thyrsoid paniculate, ultimate cymes with opposite flowers; 4th whorl A staminodial, staminodes often with glandular head; fruit with P completely deciduous, cupule 0.
8/430: Machilus (100), Persea (100), Phoebe (100), Dehaasia (50), Alseodaphne (40), Nothaphoebe (40). Tropical America, India-Malesia to Australia and the Pacific, Macaronesia and Madeira, etc..
Age. The age of crown-group Perseeae is estimated to be ca 51.8 Ma (Y. Song et al. 2024).
Wood, Paraperseoxylon septatum, assigned to this tribe has been described from the Cretaceous Puerto Yeruá Formation of N.E. Argentina (Franco et al. 2015), and woods assignable to this clade and placed in Laurinoxylon have been found in Laguna del Hunco deposits ca 52 Ma (Pujana et al. 2024).
[Cinnamomeae + Laureae]]: P tube short.
Age. Renner (2005a: Cinn. + Lit.) estimated that the age of this clade was ca 29.2 Ma, while J.-F. Huang et al. (2015) thought that Cinnamomum and Laureae split (69.3-)56(-43.2) Ma and other estimates of the split of the two tribes are (54.6-)44.8(-34.0) Ma (T.-W. Xiao & Ge 2022) and ca 57.8 Ma (Y. Song et al. 2024).
8. Cinnamomeae Nees
(Plant deciduous); (cork pericyclic - Cinnamomum); (vessel elements with scalariform perforation plates); terminal bud perulate/not; leaves (opposite), venation pinnate/triplinerved; (plant dioecious); (infloresence psudoumbel, bracts ± linear) (T in three or more whorls); (anthers bisporangiate - Cinnamomum, a few Ocotea complex), (3rd whorl A introrse), (4th whorl A staminodial); tapetal cells 4 nucleate; ?pollen surface; micropyle exo- or bistomal, embryo sac not protruding.
23/1,315: Ocotea (545), Cinnamomum (polyphyletic: 250), Nectandra (225), Aiouea (80), Endlicheria (80), Aniba (50), Licaria (40), Rhodostemonodaphne (40). Pantropical (temperate - Sassafras). Photos - Flower, Fruit.
Age. The age of the node [Cinnamomum + Sassafras] or its equivalent is estimated to be (43-)37, 32(-26) Ma (Wikström et al. 2001), ca 90 Ma (Chanderbali et al. 2001), ca 60 Ma (L. Li et al. 2016), (46.1-)34,3(-23.4) Ma (T.-W. Xiao & Ge 2022) or ca 49.7 Ma (Y. Song et al. 2024). It is also estimated that Cinnamomum s.l. itself started to diversify (60.1-)46.5(-34.2) Ma (J.-F. Huang et al. 2015) or around 14.6 Ma (Song et al. 2024). Estimated ages here and at the next node up are rather variable (see Xiao & Ge 2022 for comments on this problem for ages based on plastome data; with use of data from complete plastid protein-coding genes, ages were more accurate - and younger).
9. Laureae Le Maout & Decaisne
(Plant deciduous); plant dioecious; inflorescence racemose-pseudoumbellate, sessile or not, with vegetative buds or not, involucral bracts +; 3rd whorl A usu. introrse.
10/700: Litsea (?200), Actinodaphne (100), Lindera (100), Neolitsea (100). The Americas, Macaronesia, esp. southeast Asia-Malesia and the Pacific Islands; tropical to subtropical, rarely temperate.
Age. The age of crown-group Laureae is around 48.6 Ma (Y. Song et al. 2024).
A staminate flower was found in amber ca 99 Ma from Myanmar and named Cascolaurus burmitis, and although somewhat similar to Litsea (Laureae) its precise relationships are rather unclear (Poinar 2016c). The fossil has has two whorls of tepals that are quite distinct, the innermost staminal whorl has extrorse anthers and large basal glands, and the two other staminal whorls lack glands and have introrse anthers (Poinar 2016c).
Evolution: Divergence & Distribution. Lauraceae have a very rich fossil record (Friis et al. 2011) and are prominent in Mid to Late Cretaceous floras, e.g. in deposits ca 89 Ma from Japan (Takahashi et al. 2001, 2014). Some taxa have odd character combinations (Takahashi et al. 2014: esp. fig. 5). Early Cenomanian Mauldinia mirabilis, ca 100 Ma (Drinnan et al. 1990; Viehofen et al. 2008), has remarkable inflorescences in which the lateral units are flattened and bilobed, bearing sessile flowers on their adaxial surfaces. The flowers are very like those of extant members of the family (see also Crepet et al. 2004; Friis et al. 2006b, 2011; López-Martínez et al. 2023a for references), but the 2-ranked bracts/prophylls of the inflorescence units are a distinctly unusual feature. Dispersed flowers of Perseanthus some 90 Ma, were compared with Perseeae, and although they lacked anthers, there was associated pollen (Herendeen et al. 1994). Atkinson et al. (2015) evaluate the flowers of fossils assigned to Lauraceae. Wood very similar to that of Sassafras and named S. gottwaldii is known from Late Cretaceous deposits ca 83 Ma at 60o S on the Antarctic Seymour Island (Poole et al. 2000a), but perhaps fortunately it is likely to be an example of convergence (Nie et al. 2007: stem Sassafras is ca 33 My - or it is ca 21.7 Ma in Y. Song et al. 2024). The Upper Cretaceous Marmarthia, identified as Lauraceae, has leaf blades with palmate venation; the margins are lobed or perhaps toothed (Peppe et al. 2007). Fossil Lauraceae are reported from Palaeocene deposits 50o S and 66o E on th Ninetyeast Ridge (Carpenter et al. 2010). Bandulskaia, from the Early Eocene of Tasmania and identified as Lauraceae based on several distinctive epidermal features, has huge leaf teeth 2000+ µm long that lack the glandular cap of teeth of other Laurales; if the fossil is correctly identified, independent origin of these teeth is likely (Carpenter et al. 2007). Labandeira et al. (2002b) assigned many fossil leaves from the earliest Caenozoic of North America to extant genera of Lauraceae.
Wood identified as c.f. Hyphodaphnys (sic) has been reported from Miocene deposits in S.E. Mexico (Castañeda-Posadas et al. 2009), while extant Hyphodaphnis is restricted to Gabon, Africa. However, the identification of the wood is questionable (E. A. Wheeler pers. comm. v. 2019).
Tank et al. (2015) suggested that an increase in net diversification somewhere around here might be linked to a genome duplication of the [Magnoliales + Laurales] node; stem Lauraceae were estimated to be some 93 Ma (Tank et al. 2015: Table S1, but c.f. topology). Diversification in the family may be as recent as (93-)61(-26) Ma (Michalak et al. 2010), although ages were rather uncertain there; Y. Song et al. (2024) suggested that net diversifcation rates - so extinction was taken into account - had been continuously increasing since around 73 Ma. Calibrations in Chanderbali et al. (2001) were based on continental drift events, effectively assuming what is to be proved (Gondwanan/vicariance shaping of initial divergences of the family); a crown group age for Lauraceae of (787-)682(-577) Ma (not followed here!) was based on a vicariance event in the Ocotea complex, another (dates above) assumed a Gondwanan origin for the Chlorocardium-Mezilaurus clade, the crown-group age of [[Chlorocardium-Mezilaurus] [Alseodaphne, etc.]] being around 90 Ma. These ages were used as calibration points by Nie et al. (2007: in part) and J.-F. Huang et al. (2015).
Overall biogeographic patterns in the family are unclear. Thus although intercontinental disjunctions seem likely, evidence for them is generally lacking (L. Li et al. 2025). The Macaronesian Persea indica is part of a New World clade, and sister (but with rather poor support) to this clade is another Macaronesian member of the family, Apollonias barbujana, also sister to New World Persea (Rohwer et al. 2009; see also L. Li et al. 2011). These distributions have been explained by vicariance as the North Atlantic opened 120-100 Ma (Grehan 2017). However, Palaeo-Macaronesia is likely to be 60 Ma or more old, the oldest currently emergent Canary Island dating to only around 21 Ma (Gelmacher et al. 2005; Fernández-Palacios et al. 2011), so at least some role for dispersal is likely. It is interesting that seven genera of Lauraceae were until quite recently (the Pliocene, ca 5.3-2.7 Ma) to be found on the Iberian peninsula/Balearic Islands - and they included Sassafras, now known only from East Asia and eastern North America, but also, as mentioned above, reported from Cretaceous Antarctica (Postigo-Mijarra et al. 2009; see also Fernández-Palacios et al. 2011). Given current sampling, Litsea calicaris, from the North Island of New Zealand, may have got there by LDD from New Caledonia (Munzinger et al. 2022), although Australian species need to be looked at.
H. Li et al. (2020) rejected the suggestion that the distribution of Cryptocaryeae was initially shaped by continental drift events (c.f. Chanderbali et al. 2001), rather, they thought that there had been a couple of dispersal events in the pantropical Beilschmiedia s.l. ca 25 and 18 Ma across the North Atlantic land bridge that resulted in E. Asia – America distributions (Li et al. 2020). Indeed, Cryptocaryeae may have originated in and diversified with the first angiosperm-dominated broad-leaved evergreen forests, and there has been subsequent extensive LDD, probably by birds (Y. Song et al. 2023). Of the estimated 27 LDD events here, 5 may in fact have utilized land bridges, there being relatively recent Central/South American movements all involving Beilschmiediinae.
Diversification in the speciose New World Ocotea is likely to have been rather recent (Renner 2005a), although our understanding of relationships in this area have until recently too poor to worry about this. However, Penagos Zuluaga et al. (2021) looked at relationships among 149 species using RAD-seq data and managed to provide a considerable amount of resolution of these relationships; species of Ocotea were in ten separate clades. They also looked at the evolution of breeding systems, anther loculus number, and numbers of staminal whorls. These features tended to be associated with particular parts of the tree, but variation in the latter two characters in particular was extensive (Penagos Zuluaga et al. 2021); overall, one is impressed/depressed because there seemed to be little variation that correlated with larger clades, although Andea, described since, includes 25 gynodioecious species.
However, a better understanding of the geography, diversification and evolution of Lauraceae depends on a more stable and better resolved phylogeny than we have at present - very extensive sampling, use of nuclear genomes, etc., are all very important (see also L. Li et al. 2025). Kimoto et al. (2006) noted that a minor change on the tree that they used would mean that a glandular anther tapetum and possibly also an embryo sac protruding from the nucellus might characterize a single clade, not reversing, however, they thought that such characters arose in parallel. Indeed, in the topology suggested by Rohwer and Rudolph (2005, not cited by Kimoto et al. 2006) these characters appear in all (glandular tapetum) or most (protruding embryo sacs) members that had been examined on three successive branches of the tree, but are not, or only very rarely, found in Mezilaureae and other core Lauraceae. Thus there may have been reversal/loss of these characters in other Lauraceae; protruding embryo sacs are also found in at least some Hernandiaceae-Gyrocarpoideae. I have optimised these and some other characters (see von Balthazar et al. 2007) in the context of the phylogeny of Lauraceae suggested by Rohwer and Rudolph (2005: see below). However, where many of these characters will end up on the tree is very uncertain; not only is the topology unclear, but our basic knowledge/sampling of the morphological variation is poor.
Aspidostemon, with some 31 species, is a notably large clade of Madagascan endemics (Buerki et al. 2013a).
Ecology & Physiology. Lauraceae are prominent components of the lowland tropical rainforests of South East Asia-Malesia and America, or, more generally, in temperate and tropical evergreen broad-leaved forests (EBLFs) (Tang 2015; Yu et al. 2017). They are much less common in Africa, in this being similar to groups like Arecaceae that are also dispersed by specialist frugivores (Snow 1981). In tropical montane forests in South America it may be the most speciose family (Gentry 1988) while it is the second most speciose family represented by plants with stems 10 cm or more d.b.h. in Amazonian forests in general, although it has only 4/227 of the common species there (ter Steege et al. 2013). Species of Nectandra growing together on Barro Colorado island show considerable differences in their foliar secondary chemistry, the differences being implicated in defence against herbivores, and in this they are like a number of other taxa that grow in swarms of ecologically similar species in LTRF (Sedio et al. 2017). Eusideroxylon zwageri dominates (or used to - it has been heavily logged) several thousand square kilometres of West Malesian forests (Hart et al. 1989), while Litsea is one of the five most diverse genera in West Malesian l.t.r.f. (Davies et al. 2005). Lauraceae, along with Magnoliaceae, Theaceae and Fagaceae (Tang 2015), are a notably prominent component of the subtropical evergreen broad-leaved forests of East Asia.
Eusideroxylon zwageri has rather odd reaction wood - it is in the gynmnosperm position, but it has angiosperm lignin composition (Nawawi et al. 2016); for further discussion, see elsewhere.
Cassytha is a largely tropical genus of parasites, and its leafless yellowish stems may cover even quite large trees. It is perennial and may flower the year round; its favoured hosts are woody plants in 1, Fabaceae, 2, Myrtaceae, and 3, Asteraceae (H. Zhang et al. 2022). Like other parasites, its chloroplast genome is extensively modified (Y. Song et al. 2017; C.-S. Wu et al. 2017: see below). The plant is able to photosynthesize and may have stomata, although the latter are apparently not developed after the parasitic connection is established (Heide-Jørgensen 2008). Haustoria of C. filiformis have been observed on a variety of galls growing on their host, the insect inside usually dying (Egan et al. 2018), although little is known about the nature of this association, whether the death of the insect is incidental, etc.. Cassytha can be a hyperparasite, parasitizing other parasites, forming haustoria on itself, and so on (Krasylenko et al. 2021). For stem parasites, see also Cuscuta and mistletoes, especially Santalaceae.
Pollination Biology & Seed Dispersal. Pollination, usually rather generalist, is summarized by Gottsberger (2016a). The basic mode of flowering in the family may be heterodichogamy or dianthesis. Here, plants are of two types, and one plant will be in the staminate phase while the other is in the carpelate phase, and vice versa. The staminodes produce nectar when the plant/flower is in the carpelate phase and the staminal glands of the third staminal whorl produce nectar when the plant/flower is in the staminate phase (Rohwer 2009, see also Chung et al. 2010 and above).
Dispersal of the seeds is by birds in particular, specialized frugivores for the most part, relatively large birds that can handle the often quite large fruits of Lauraceae (and Arecaceae, etc.) and in turn getting most of their nutrients from them. The plants are often trees in primary forests, their fruits mature quite slowly, their seeds are relatively large, and the pericarp, although thin, is nutritious (Snow 1971, 1981). Lauraceae are major components of such birds' diets in Central and South America and Southeast Asia-Malesia in particular (less so Africa - the birds aren't there, and nor are Lauraceae). In in some ways, Lauraceae are like figs, providing a supply of food throughout the year, but of higher nutritional quality than figs or Miconia, for example, and serving a rather different avian clientele (Snow 1971, 1981; Wheelwright 1986).
A final element of the fruit—disperser story also has to do with fruit size. Rossetto et al. (2015) noticed that genetic distances between populations, etc., of large, fleshy-fruited Lauraceae (the study plant was Endiandra globosa) were higher than than comparable measures in small-fruited Lauraceae (E. discolor), although the latter had higher overall diversity. Furthermore, smaller-fruited species more readily moved into areas where there had been recent contraction of the forests, they tended to have larger distributions (data base of 1,093 Australian species, the division between "large" and "small" was 30 mm across, and the proportion of smaller-fruited taxa increased with latitude (Rossetto et al. 2015: there are some similar correlations in Clusia - see Luján et al. 2023, and they probably occur more generally).
Plant-Animal Interactions. Lauraceae are important food plants for caterpillars of some species of the swallow tail butterflies Papilio (Papilionini) and Teinopalpini (e.g. papers in Scriber et al. 1995; Aubert et al. 1999; Condamine et al. 2012), and the diversification rate of the main clade of swallowtails on Lauraceae may have initially increased (Allio et al. 2020/2021). For swallowtails in general, see Aristolochiaceae.
Lauraceae are rich in secondary metabolites, and in genera like Ocotea closely related species that nevertheless differ in these metabolites and which are consequently eaten by different insects may grow together. For other similar tropical systems, see Eugenia, Inga, Passiflora, Piper, Protium and Bursera, Psychotria, and sundry Solanaceae the discussion about Inga is perhaps particularly detailed. For general patterns of diversity in seed plants, with the greatest diversity being at low latitudes, a pattern that may in part be driven by the factors just discussed, see elsewhere.
Plant-Bacterial/Fungal Associations. Raffaelea lauricola, an ophiostomatalean ambrosia fungus, causes laurel wilt, a serious disease of wild (especially redbay, Persea borbonica) and cultivated (avocado, P. americana) Lauraceae in the S.E. U.S.A.; its insect vector is the introduced scolytid ambrosia weevil Xyleborus glabratus (Fraedrich et al. 2008; Hulcr & Stelinski 2016; Vanderpool et al. 2017). For more on ambrosia beetles, see Pinaceae and Vega and Hofstetter (2015).
Genes & Genomes. For a summary, see L. Li et al. (2025). A genome duplication, the CASYα event which happened around 88.1 Ma, can be detected throughout the family, and this might be the same as the ca 76 Ma duplication event noted in the [Persea + Cinnamomum] clade by Lv et al. (2020: no other Laurales in the Siparunaceae, etc., clade examined). The SASSα event (Sass, Cinn, Lind, Pers.) is dated to ca 34.8 Ma (Landis et al. 2018). Chaw et al. (2018b), in their analysis of the genome of Cinnamomum kanehirae, thought that there was evidence of two whole genome duplications (WGDs), of which one might be in the family; Y.-C. Chen et al. (2020) suggested that there might have been four or so WGDs in various places in the family - or just one, while in the Litsea cubeba genome there seemed to be evidence of two old WGDs; see also Rendón-Anaya et al. (2019). L. Qin et al. (2021) proposed that there was a duplication shared by Persea americana and C. kanehirae, the CCT event, that they dated to around 52.4-46.3 Ma. Both isozyme duplication and stomatal size increase over time suggesting ancient polyploidy in this clade (Soltis & Soltis 1990; Masterson 1994). All a bit complicated. Is the base chromosome number 6?
There is a fair amount of variation in the plastome, and it seems to have a strong phylogenetic signal. Cassytha, in addition to losing one copy of the IR, has loss/pseudogenization of a number of genes of the NADPH-dehydrogenenase complex (Y. Song et al. 2017; C.-S. Wu et al. 2017). Furthermore, there have been shifts in the IR boundaries in the family such that Endiandra, for example, has lost the rpl2 copy in IR copy B, while Litsea, Cassytha, etc. have lost genes like rpl2 and rpl23 because of the contraction of IR copy A (Wu et al. 2017) and there are other phylogenetically informative losses, etc., like a ca 4500 bp loss in IR B (Song et al. 2017). On the other hand, T.-W. Kiao and Ge (2022) noted that the IR of Cinnamomum chartophyllum, but not other members of Cinnamomeae, had expanded by ca 5,000 bp. Trofimov et al. (2022) found notably less variation in the plastomes of the Ocotea in their study (six species from South America, one from Macaronesia) than in genera like Cinnamomum, Lindera and Litsea. However, variation in the plastome of those members of Laureae-Lauriineae examined (C. Liu et al. 2022) was found to be not that great, and it was unclear if three taxa with a 600 bp indel formed a monophyletic group.
Y. Song et al. (2024) found that Machilus yunnanensis had a chondrome that, at 735,392 bp, was larger than that of other magnoliids, and it also had a different gene order - 31/41 genes used in the phylogenetic analyses were rearranged compared with those in Magnoliales. Cassytha filiformis has two copies of the cox1 mitochondrial gene, the type II copy being pseudogenized, while other species of Cassytha have only a single copy. Both copies of cox1 in C. filiformis have an intron, as does the single copy in other species of the genus - captured from other species - but not that in other Lauraceae (c.f. Convolvulaceae, Calceolaria - C. Zhang et al. 2020).
Chemistry, Morphology, etc.. Lindera, at least, has distinctive C10, C12 and C14 mono-unsaturated fatty acids in its seeds (Badami & Patil 1981).
Lauraceae like Sassafras and Actinodaphne have reaction wood developing on the lower side of the stem (e.g. Kucera & Philipson 1978). Vestured pits are apparently absent; rays alone may be storied (Metcalfe 1987: P. van Rijckevorsel [pers. comm.] clarified reports of vestured pits and wood storying in the family). Bosela and Ewers (1997) describe root suckering in Sassafras. Distinctive paracytic stomata in which the subsidiary cells almost envelop the very small guard cells, the latter having outer and inner cuticular ledges, may be an apomorphy for all Lauraceae except Hyphodaphnis (Carpenter et al. 2007), indeed, from a surface view it may seem as if the stomata are anomocytic, the guard cells being totally obscured. However, Nishida and van der Werff (2007) found more conventional stomata in at least some Lauraceae other than Mezilaurus (for stomata, see also Hill 1986; Trofimov & Rohwer 2018). Trofimov and Rohwer (2018, see also 2020) outlined the quite extensive epidermal variation they found in New World species of the Ocotea complex. Zeng et al. (2014) described the distinctive papillate cells of the lower epidermis of Caryodaphnopsis; the apex of the papilla may be expanded and more or less fused with the apices of adjacent papillae and the stalk may be transversely septate; some Neocinnamomum and a few other Lauraceae also have papillate lower epidermides.
For the basic floral arrangement here, see e.g. Eichler (1875) and Walch and Blaise (2023); although the odd member of the outer perianth whorl is adaxial, as would be expected for a "dicot", it is the second member of that whorl to be initiated, not the first (see also Menispermum). It has been suggested that the perianth in Lauraceae represents modified bracts, "bracteotepals" (see Ronse Decraene 2008), or, largely on the basis of gene expression in Persea, modified stamens, "androtepals" (Chanderbali et al. 2004, 2006); see also Warner et al. (2009) for literature. Both the tepals and the stamens of Persea have three traces (Reece 1939: the ovule is anatropous!). However, other reports suggest that the stamens have single traces, even if both whorls of tepals have three traces (Laurus) or one trace in the inner whorl alone (Umbellularia: Kasapligil 1951). Sajo et al. (2016) conveniently summarize the literature - what "are" stamens with more than a single vascular trace? Y. Zhang et al. (2022: Table 1) summarize some androecial and other floral features of the family, but with a focus on the Chinese species of Sassafras. There are a few multistaminate Lauraceae, while others have fewer than nine stamens because stamen whorl(s) have been lost (Rohwer 1993a). Both extrorse and introrse anthers can occur in the same flower, although the extrorse condition seems to be developmentally derived (Buzgo et al. 2007). The spinules on the pollen surface found in many Lauraceae are often spirally ridged (Rai & van der Werff 1988; van der Merwe et al. 1990). The orientation of the single carpel varies (Ronse de Craene et al. 2010 and literature). Sastri (1963) and Kimoto et al. (2006) summarize embryological findings in Lauraceae; there is some discussion as to whether there is one or several megaspore mother cells, and the outer integument varies considerably in thickness (Doweld 2001b), and the testa is not always multiplicative. A remarkable lysigenous cavity develops in the ovule of Cassytha, and it involves the funicle opposite the micropyle and results in the apex of the embryo being directly connected to the ovarian cavity rather than bing blocked by the funicle, however, the pollen tube appears not to access the ovule by this cavity and the extent of its occurence in the genus is unclear (Sastri 1962). Variation in the anatomy of the pericarp, development of the cupule in fruit, etc., is summarized by Little et al. (2009). Kasapligil (1951) described a tracheidal endotegmen at the radicular end of the seed.
General information is taken from Kasapligil (1951), van der Merwe et al (1988: Dahlgrenodendron, Rohwer (1993a), Y. Song et al. (2019: tribal characters), L. Li et al. (2025) and H. Zhang et al. (2022: Cassytha); for alkaloids, see Silva Teles et al. (2019) and for wood anatomy, see Richter (1981) and van Rijckevorsel (2002); for leaf anatomy, see Vaz et al. (2019) and for epidermal features, see van der Merwe and van Wyk (1994: stomata), Christophel et al. (1996), Nishida and van der Werff (2011) and de Moraes et al. (2022), all cuticle, and Gomes-Bezerra et al. (2018: general), although how all this will help at higher levels in unclear (but see Gang et al. 2021: Cinnamomum s.l.); floral morphology and anatomy is taken from Sastri (1953, 1965), androecial development from Buzgo et al. (2007), pollen of the Cryptocarya group - and general references - from Rohwer (2018), some embryological details from Sastri (1958b), Heo et al. (1998) and Endress and Sampson (1983), Cassytha fruit and seed development from Mahadevan and Jayasuriya (2013), and fruit anatomy from Heo (199-).
Phylogeny. Rohwer (2000: matK) suggested that Hypodaphnis, with an inferior ovary, was sister to the rest of the family, then relationships were [Cassytha (but a long branch) [[Beilschmiedia + Cryptocarya + Endiandra] [Caryodaphnopsis [[Chlorocardium + Mezilaurus + Williamodendron] [all other Lauraceae]]]]]; for more details, see Chanderbali et al. (2001). There are a number of taxa with long branches, and complex analyses by Rohwer and Rudolph (2005) strongly suggest a slight modification of these relationships: [Hypodaphnis [[the Cryptocarya group] [Cassytha [[Caryodaphnopsis + Neocinnamomum] [[the Mezilaurus group] [The Rest]]]]]] - most of these clades have about 100% posterior probabilities. Although Han et al. (2014) obtained a topology in which Hypodaphnis was embedded in a clade largely made up of clades two and four above, this may be a rooting problem. Massoni et al. (2014) found a clade including Hypodaphnis, Cassytha and Eusideroxylon to be sister to the rest of the family, but these relationships were weakly supported, while L. Li et al. (2016) found that Caryodaphnopsis and Neocinnamomum were pectinations on either side of Cassytha, and although support was quite strong, sampling could have been better: i.a. no Hyphodaphnis. Cassytha was weakly linked with the Cryptocarya group, [Caryodaphnopsis + Neocinnamomum] were the next clade from the base, but support for the position of Cassytha was weak (Z.-D. Chen et al. 2016). Y. Song et al. (2017), using complete plastome sequences, recovered a well-supported topology similar to that of Rohwer and Rudolph (2005), although Hyphodaphnis was not included and Caryodaphnopsis and Neocinnamomum formed a grade, not a clade; Song et al. (2019), with greater sampling, recovered the same topology, and with good support; Song et al. (2024: 41 mitochondrial genes, 29 genera, 91 spp.) noted that topologies in mitochondrial, chloroplast and nuclear analyses varied, especially within Caryodaphnopsideae and Neocinnamomeae, however, bigger-picture relationships were largely similar in the three. Jo et al. (2019) recovered the relationships [Cryptocaryeae [Neocinnamomum [Caryodaphnopsis [Perseeae [Cinnamomeae + Laureae]]]]], and although there was little support for the position of the two genera mentioned there was strong support for the positions of the tribes. Relationships obtained by Y.-C. Chen et al. (2020: 275 single-copy genes, 22 species) were [Cassytha [[Cryptocarya + Beilschmiedia] [Caryodaphnopsis [Perseeae [Cinnamomeae + Laureae]]]]]. Overall, tribal relationships seem to have settled down (H. Li et al. 2025).
However, there are problems associated with the analyses of nuclear and chloroplast data. Thus Z.-F. Liu et al. (2021) noted that along the spine relationships were [... [Caryodaphnopsideae [Neocinnomomeae ...]]] in nuclear analyses, but the positions of those tribes was reversed in chloroplast analyses, and there was also extensive conflict between nuclear- and chloroplast-based relationships within Perseeae, Cinnamomeae and Laureae, although the relationships of these tribes was the same in both sets of analyses. Antonio et al. (2020), q.v. for details of the chemistry of the Ocotea complex in particular, noted that both [Litsea + Ocotea] and Lindera and Neolitsea had aporphine alkaloids, although the plants were not immediately related. Y. Tian et al. (2021) looked at relationships in this area, also the circumscription of Laureae, Cinnamomeae and Perseeae, with a focus on the differing relationships suggested by Kostermans (1957) and van der Werff and Richter (1996), and alternative interpretations of inflorescence morphology in the context of relationships suggested by variation in chloroplast genes. H. Li et al. (2025) also drew attention to cytonuclear discordance within the tribes, noting that the way forward was to analyse data from both nuclear and chloroplast genomes. Relationships within indiividual tribes are discussed below.
Caryodaphnopsidae. See L. Li et al. (2016, 2025) for relationships.
Cinnamomeae. In plastome analyses Cinnamomum was found to be para/polyphyletic (see also Y. Song et al. 2019; Jo et al. 2019: Sassafras embedded in Cinnamomum, but with little support). J.-F. Huang et al. (2015) found three main clades, one made up largely of Cinnamomum section Camphora that is sister to the other two which are largely made up of section Cinnamomum. Rohde et al. (2017) found that Old World sections Cinnamomum and Camphora, themselves both monophyletic, formed a weakly paraphyletic grade at the base of a clade that included New World Cinnamomum, largely grouping with Aiouea, the Ocotea complex, including taxa from several other genera, being its sister group in ITS analyses, and the two groups were also picked up in cpDNA analyses. Old World Cinnamomum was clearly not part of either clade, but details of the relationships between sections Cinnamomum and Camphora differed depending on the markers used (Rohde et al. 2017); Trofimov and Rohwer (2020) found some support for a [Sassafras + Cinnamomum section Camphora] clade and they found that an Old World clade of Ocotea (= Kuloa gen. nov.) was sister to section Cinnamomum. That the two sections of Cinnamomum are quite different was confirmed by Gang et al. (2021) who found a derived epidermal morphology in section Cinnamomum - the anticlinal cell walls were usually sinuous and the outer periclinal walls were reticulately thickened; in the plesiomorphic condition, found in taxa such as Sassafras, Cinnamomum section Camphora, etc., the anticlinal walls are straight and the outer periclinal walls are smooth, not reticulately thickened. Indeed, if one can get past the lobed leaves of Sassafras, it and section Camphora are quite similar in flower, fruit and leaf (Z.-F. Liu et al. 2021)... Z. Yang et al. (2021) compared nuclear ITS, psbA-trnH and plastome analyses and decided that the segregation of Camphora from Cinnamomum was justified, the two being separated by numerous features, included epidermal micromorphology (for which, see Gang et al. 2021); note that the basic topologies based on the first two sets of data were the same and differed from the third, although the authors could not obtain plastomes of Kuloa for that analysis. For relationships in the Nectandra (paraphyletic)/Ocotea (polyphyletic) area, see Trofimov et al. (2016, esp. 2019: 123/ca 400 species examined), in the latter study, relationships along the backbone of the tree remain poorly supported. Trofimov and Rohwer (2020) focussed on Old World taxa of this Ocotea complex, which are found from Macaronesia to the Mascarenes, and i.a. found weak support for a clade [Umbellularia (which has cellular endosperm) [Macaronesian + African and Madagascan Ocotea]], separate from Kuloa above which made up the other Old World Ocotea clade. There was a fair bit of support both along the spine of the tree and in shallower relationships in the RAD-seq analysis of 149 species carried out by Penagos Zuluaga et al. (2021), even if many of the genera (based on conventional characters such as anther loculus number and number of androecial whorls) turned out to be para- or polyphyletic. For more on relationships, see T.-W. Giao and Ge (2022: plastome analysis, ca 40 spp.) and L. Li et al. (2025).
Cryptocaryeae. Relationships are beginning to be resolved within the Cryptocarya clade (Rohwer et al. 2014). Beilschmiedia was paraphyletic in Song et al. (2019) Fig. 3, but not in Fig. 2 (nor in B. Liu et al. 2013), while it was hopelessly para/polyphyletic in the 25-plastome analysis of five genera in this group (B. Li et al. 2020), for instance, the Endiandra included were embedded in Australasian Beilschmiedia.
Laureae. Litsea is polyphyletic, although section Litsea is monophyletic, and Lindera is also polyphyletic (Fijridiyanto & Murakami 2009; Jo et al. 2019). The recognition of Neolitsea appears to make Actinodaphne paraphyletic (L. Li et al. 2007, esp. J. Li et al. 2004, 2008). Y. Song et al. (2019) also found genera like Litsea and Lindera to be polyphyletic (see also Jo et al. 2019; Munzinger et al. 2022; C. Liu et al. 2022). The latter group used whole plastomes and found a well supported clade made up of 16 Litsea + 2 Lindera, another with 7 Lindera, 5 Litsea and 2 Laurus, and a poorly supported clade with Lindera, Parasassafras and Iteadaphne. T.-W. Xiao et al. (2020: 47 plastomes, 12 new) found extensive polyphyly, again especially in Lindera and Litsea, in their analyses, and, depending on whether coding or non-codine data were used, the relationships of one of the three major groups changed, furthermore, some analyses had little support for nodes along the backbone of the tree. See also Munzinger et al. (2022) for Litsea on New Caledonia, and L. Li et al. (2025) for more on relationships. (Note that Parasassafras andSinosassafras have nothing immediately to do with Sassfras, for which, see Cinnamomeae).
Perseeae. For relationships in the Persea area, see Rohwer et al. (2009) and L. Li et al. (2011); Phoebe and Persea are para/polyphyletic, while Mo et al. (2017) found that Alseodaphne was polyphyletic.
Classification. A tribal classification that reflected current ideas of relationships was sketched out by Y. Song et al. (2019; see also Jo et al. 2019), and this is partly followed above; the four clades that Song et al. (2019) recovered in their Laureae are recognised here as separate tribes (for the time being, at least).
The classical genera are very difficult to recognise without flowers and are rather unsatisfactory even with them. They are often based on single character differences in the androecium, such as sporangium number and direction of sporangium opening. However, both extrorse and introrse anthers can occur in the same flower, thecae may be 2 or 4, their arrangement on the connective varies considerably, etc. (e.g. Kopp 1966; Rohwer et al. 1991; Rohwer 1993, 1994a; van der Werff & Richter 1997); substantial changes in generic limits are occurring. Trofimov et al. (2019) have begun what appears will be a piecemeal dismemberment of Ocotea; given the topology of their tree and the genera currently recognised, we may expect another half a dozen or so new genera there alone. However, Penagos Zuluaga et al. (2021) in their extensive analysis of this area sidestepped the manifest problems with para- and polyphyly and provided phylogenetic definitions for the clades in which they were particularly interested - given the size of the group, the Linnean hierarchy is likely to be unable to provide names for all the clades that are becoming apparent, so if one wants to name them, go elsewhere... Jo et al. (2019), referring to Lindera in particular, noted that para/polyphyly relationships here might reflect hybridization and plastome capture, or that the morphological characters used to recognize the genus evolved several times; analyses that included nuclear markers were needed - indeed, generic rearrangements that have no nuclear markers in the phylogeny on which they are based are premature (L. Li et al. 2025 - note that generic limits and relationships are difficult in all tribes that have more than a single genus...).
[Hernandiaceae + Monimiaceae]: ?
Age. This clade can be dated to ca 91.1 Ma (Tank et al. 2015: Table S2).
HERNANDIACEAE Blume - Back to Laurales
Nodes ?1:1; xylem parenchyma paratracheal; ?hippocrepiform pericyclic cells; petiole bundles vertically elliptic; (stomata anomocytic); branching from previous flush; lamina venation ± palmate; breeding systems very diverse; flowers (3-)4-5-merous; P 3-10; A 3-5(-7); tapetum amoeboid; ?nectaries; G inferior, usu. facing abaxially, stigma peltate, dry, compitum necessarily 0; ovule micropyle variable, endostome lobed, outer integument 9-23 cells across, inner integument 3-8 cells across, hypostase 0; fruit ?dry, ?drupe; outer integument multiplicative; endosperm 0; x = 7 (?6, ?12); germination epigeal.
5 [list, to subfamilies]/67 - two subfamilies below. Pantropical.
Age. Crown-group Hernandiaceae are about 120.2 or 96.0 Ma (Renner 2005a), (130-)112(-89) Ma (Michalak et al. 2010, c.f. abstract) or (137.7-)120.2, 64.4(-35.7) Ma (Massoni et al. 2015a).
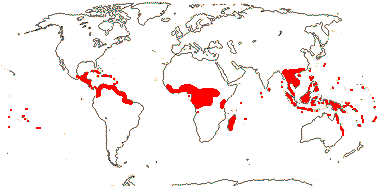
1. Hernandioideae Miquel —— Synonymy: Illigeraceae Blume
Trees (deciduous), (lianes climbing by twining petioles); Al accumulation?; petiole bundles horizontally [Valvanthera s. str.], glandular hairs in leaf epidermis; leaves (palmate); plant heterodichogamous; inflorescence thyrsoid, ultimate units cincinni; (bracteoles connate); flowers relatively large [>2 mm across]; anther valves laterally hinged; tapetal cells radially elongated, microspore mother cells in single layer; pollen grains 90-160 µm across; style grooved; parietal tissue 6-8 cells across, nucellar beak +/0, suprachalazal nucellus massive; fruit (2-4 lateral wings - Illigera), bracteoles accrescent [not Illigera]; (seeds ruminate - usu. Hernandia), testa vascularized, mesotesta massive [8-17 cells across], (spongy), tanniniferous, walls unthickened, mesotesta massive, 7-17 cells across; n = 18, 20.
3/51: Hernandia (32), Illigera (18). Tropical, esp. Madagascar and Indo-Malesia. Map: from Kubitzki (1969), van Balgooy (1975) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photo - Hernandia Flower, Fruit, Illigera Flower, Fruit.]
Age. The age of crown-group Hernandioideae is around 70.3 or 58.0 Ma (Renner 2005a) or about 76 Ma (Michalak et al. 2010).
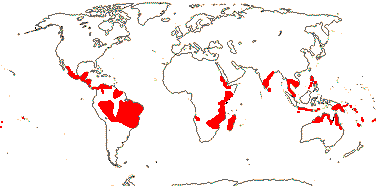
2. Gyrocarpoideae J. H. Balfour —— Synonymy: Gyrocarpaceae Dumortier
Trees, (deciduous) (climbers [recurved cauline hooks]); stomata anomocytic; cystoliths +; leaf blades (lobed), with strong veinlet areolation; inflorescence dichasial, ebracteate; flowers small [buds 1.5> mm across]; P uniseriate, 4-8; A 3-5, anther valves dorsally hinged; pollen grains 19-45 µm across; nectaries/glands 0/+; ovule with nucellar cap; embryo sac protruding from nucellus; fruit (2 long terminal wings - Gyrocarpus); 3-4 cell layers of inner testa anticlinally elongated, tegmen crushed; cotyledons contortuplicate [= much folded], bases cordate; n = 15.
2/16: Sparattanthelium (11). Pantropical, esp. America. Map: from Kubitzki (1969), van Balgooy (1975) and Trop. Afr. Fl. Pl. Ecol. Distr. 1. (2003).
Age. Crown-group Gyrocarpoideae are 80.5 or 57.0 Ma (Renner 2005a) or (102-)72(-43) Ma (Michalak et al. 2010).
Evolution: Divergence & Distribution. Although Illigera now is to be found only in the Old World, fossils are known from the Eocene to the Middle Miocene in North America (from the West and Southeast respectively), also from East Asia, perhaps suggesting an origin of the genus in the Northern Hemisphere (H. Zhu et al. 2025). Michalak et al. (2010) thought that the distribution of Hernandiaceae might initially have been affected by pre-drift continental arrangements, but that there had also been much subsequent dispersal.
Pollination & Seed Dispersal. Wind (Gyrocarpoideae) bees and flies may be the main pollinating agents; dispersal mechanisms are diverse (Michalak et al. 2010).
Plant-Animal Interactions. Larvae of swallowtails eat members of this family - see papers in Scriber et al. (1995), Allio et al. (2020/2021), and for swallowtails in general, see Aristolochiaceae.
Chemistry, Morphology, etc.. Some 128 alkaloids assignable to 17 different structural types have been found in the family; about half of them are aporphine alkaloids (Conserva et al. 2005; see also J.-J. Chen et al. 2011). Their distributions show no obvious correlations with phylogeny.
General information is taken from Kubitzki (1969, 1993b), wood anatomy from Shutts (1960), cystoliths from Fernández Honaine et al. (2023), floral morphology from Endress and Lorence (2004: Hernandia), embryology, etc. from Heo and Tobe (1995) and Kimoto and Tobe (2008a: nice summary) and fruit from Mohana Rao (1986: Gyrocarpus).
Phylogeny. Renner (2005a) discussed relationships here; within Hernandioideae they are [Hernandia [Illigera + Hazomalania]], American species of Hernandia being embedded in the genus.
Previous relationships. It is perhaps appropriate that Shutts (1960), with a focus on wood anatomy, thought that Gyrocarpaceae (sic) were closest to Lauraceae and Hernandiaceae closeat to Monimiaceae.
MONIMIACEAE Jussieu - Back to Laurales
(Plants Al accumulators); primary stem (with separate bundles); (vessel elements with scalariform perforation plates); wood with broad rays; sieve tubes with rosette-like non-dispersive protein bodies; nodes 1:2-7+; cuticle wax?; (stomata anomocytic); lamina vernation conduplicate, (margins entire); plants monoecious or dioecious; flowers medium-sized; A many, dehiscing longitudinally, (annular), ± sessile or filaments rather slender, connective produced; tapetal cells binucleate; (pollen grains with encircling aperture); G occluded by secretion as well, stylulus short-0, stigma broad, dry; outer integument 2-3 cells across, inner integument 2-4 cells across, parietal tissue ca 8 cells across, hypostase + [?level], funicle stout, obturator + (0); hypanthium fleshy or not, fruit drupelets; embryo short to quite long; x = 20 (?10, ?19).
22 [list, as subfamilies]/200 - three subfamilies below. Tropical-Southern Hemisphere, but esp. Australasia.
Age. Estimates for the age of crown-group Monimiaceae are (86-)78, 66(-58) Ma (Wikström et al. 2001); Renner (2005a) suggest an age of around 102.5 Ma and and Renner et al. (2010) ages of (93-)90(-87) Ma (there are ca 85 Ma old fossil woods); dates in Michalak et al. (2010) are (80-)73(-40) Ma, depending on the clock. The estimate in Bell et al. (2010) is (57-)41, 35(-21) Ma and that in Massoni et al. (2015a) is (143-)125.7, 62.8(-39.5) Ma.
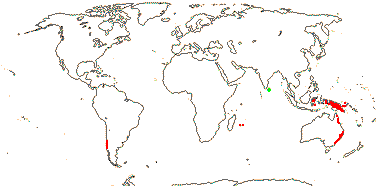
1. Monimioideae Rafinesque
Lianes [climbing by scrambling] to trees; septate fibres 0; sieve tube plastids with starch (not Peumus); hairs often stellate; (bud scales +); plants dioecious; T to 12, inner petaloid; staminate flowers: (staminate receptacle splitting); (anthers bisporangiate, ?thecae, dehiscing transversely - Monimia), staminodes +, (paired nectaries 0 - Palmeria); G 2-many, styles postgenitally united in receptive zone [?all]; hypanthium splitting irregularly in fruit, or circumscissile, endocarp massive [15+ cell layers thick]; (drupelets with fleshy appendages); n = 39, ca 46.
3/19: Palmeria (15). Scattered: Chile, the Mascarenes, east Australia, New Guinea. Map: red, in part from Fl. Australiense vol. 2 (2007).
Age. The crown age of Monimioideae is ca 76 Ma (Renner 2005a) or (75-)57, 52(-34) Ma (Renner et al. 2010).
[Hortonioideae + Mollinedioideae]: secondary phloem with flaring rays; septate fibres +; endocarp thin.
Age. Estimates of the age of this node are ca 100 Ma (Renner 2005a) or (84-)71(-57) Ma (Renner et al. 2010).
2. Hortonioideae Thorne & Reveal - Hortonia Wight —— Synonymy: Hortoniaceae A. C. Smith
Shrubs; hairs stellate; flowers perfect, ca 3 cm across; T many, spiral, inner petaloid; staminodes +; pollen with hollow, spiral sexinous strands, intine with tangential channels; G 6-14; suprastylar extragynoecial compitum; endocarp to 6 layers thick, sclereidal; n = 19.
1/3. Sri Lanka. Map above: green.
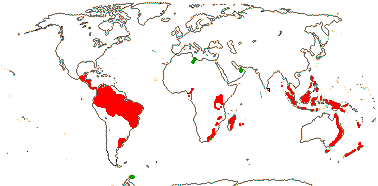
3. Mollinedioideae Thorne
Lianes [climbing by scrambling], shrubs or trees; (sieve tube plastids with starch - Tambourissa); plants monoecious; (hypanthium closed by roof), (splitting in flower, stamens borne on lobes), P not differentiated, often inconspicuous to 0; A (4-)many, paired nectaries 0; G (1-many, (connate, inferior - Tambourissa), styles postgenitally united in receptive zone or hyperstigma or suprastylar extragynoecial compitum; fruit a berry/hypanthium splitting irregularly/drupelets with fleshy appendages; endocarp cells lignified (not), with ± wavy anticlinal walls, 1-layered palisade and few-layered and subpalisade/unthickened; (cotyledons 4 - Kibaropsis); n = 19 [several], 22, 36, 39, 40-43.
18/180: Mollinedia (90 [?20 - S. Renner, pers. comm.]), Tambourissa (45), Kibara (45). Tropical-Southern Hemisphere, esp. Australasia. Map: from Renner et al. (2010, fossil localities in green) and Jordaan and Lötter (2012, Xymalos). [Photo: Fruit, Flower, Fruit, Levieria fruit, Wilkiea fruit.]
Age. The crown age of Mollinedioideae is about 51.3 Ma (Renner 2005a) or (58-)44.4(-31) Ma (Renner et al. 2010).
Evolution: Divergence & Distribution. The separation of Monimia from its sister genus, the East Malesian Palmeria, dates to (48-)32(-16) My; the former is endemic to the Mascarenes, which are a mere 15-8 Ma old (Renner et al. 2010), so Monimia must have dispersed on to the islands from elsewhere (?Malesia) - see also Asteliaceae, Arecaceae, Rhamnaceae, Rousseaceae, etc.. In general, there is little evidence of Gondwanan vicariance in the family (Renner et al. 2010). Interestingly, the affinities of the recently-described Monimiophyllum, from the Eocene of ca 52.2 Ma in Patagonia, are to the Australian-Malesian Wilkiea clade (Knight & Wilf 2013), the crown group age of which is (38-)26.1(-16) Ma (Renner et al. 2010: ?typos) and the stem group age is ca 27.6 Ma; another case of substantial conflict in ages involving fossils from south South Anerica. Bobrov et al. (2017b) make some suggestions as to the biogeographical history of the family.
Pollination Biology & Seed Dispersal. A variety of small insects pollinate the flowers. The floral parts may be more or less exposed or enclosed in a fig-like structure. If the flowers are enclosed, thrips (Thysanoptera) lay their eggs in young flowers, adults covered in pollen emerging when the flowers are mature (Gottsberger 1977, 2016a; Philipson 1986a).
There is an hyperstigma in Tambourissa and some other genera, a remarkable condition where the pollen germinates in a mucilaginous secretion at the entrance to the hypanthium/inferior ovary (Endress 1980c; Endress & Lorence 1983; Friis & Endress 1990). Other genera may have postgenital fusion in the stigmatic region or a suprastylar extragynoecial compitum (Endress 1980c; Endress & Igersheim 1997). The arrangement of the carpels in the female flowers of Tambourissa may be irregular (Rutishauser 2016b and references).
In genera like Palmeria, Tambourissa, etc., the drupelets are surrounded by a fleshy, accrescent hypanthium. This splits, the colour of the inside sometimes forming a striking contrast with that of the exposed drupelets.
Plant-Animal Relationships. Papilio dardanus caterpillars have been found on Xymalos (Jordaan & Lötter 2012); see also Lauraceae.
Chemistry, Morphology, etc.. The flowers of Decaryodendron have up to 1000 carpels, and those of Tambourissa up to 2000 carpels. Mollinedia has carpels that are initially unsealed, but are later occluded by secretion; it also has a whorled perianth. Since the ovules are initiated on the cross-zone, in their apical position they have effectively been inverted 180o.
See Money et al. (1950), Lorence (1985: Malagasy taxa) and Philipson (1993) for general information, for wood anatomy, see Poole and Gottwald (2001), for floral development, see Endress (1980b: Hortonia neither notably intermediate nor archaic, 1980c, 2019), for the embryology of Hedycarya, see Sampson (1969a), and for fruit anatomy, see Romanov et al. (2007).
Phylogeny. A [Peumus [Palmeria + Monimia]] clade is sister to the rest of the family (Renner 2002 and references, 2010), Hortonia is isolated (see also Massoni et al. 2014), but then relationships are unclear, although the monotypic Xymalos, the only mainland African member of Monimiaceae, and then a clade from Madagascar and surrounding islands are likely to be successive sister groups to the remaining Mollinedioideae. Renner et al. (2010) found that Mollinedia, Hedycarya, Steganthera and Wilkiea were all polyphyletic. Bobrov et al. (2017b: sampling poor) thought that Xymalos was sister to the rest of the family; Hedycarya was polyphyletic.
Classification. The three subfamilies recognised above help link with previous classifications of Monimiaceae s.l. (see below).
Previous Relationships. Only three of the subfamilies/major groups (Monimioideae, Hortonioideae and Mollinedioideae) of Monimiaceae as circumscribed by Money et al. (1950), Cronquist (1981), Philipson (1993) and Takhtajan (1997) are included here; for the rest of the family as they saw it, see Siparunaceae and Atherospermataceae.