EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1 C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1 C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
[DILLENIALES [SAXIFRAGALES + ROSIDS]]: stipules + [usually apparently inserted on the stem].
[SAXIFRAGALES + ROSIDS] / ROSANAE Takhtajan / SUPERROSIDAE: ??
ROSIDS / ROSIDAE: anthers ± dorsifixed, transition to filament narrow, connective thin.
[VITALES + ROSIDS]: ?
[ROSID I + ROSID II]: (mucilage cells with thickened inner periclinal walls and distinct cytoplasm); if nectary +, usu. receptacular; embryo long; chloroplast infA gene defunct, mitochondrial coxII.i3 intron 0.
ROSID I / FABIDAE / [ZYGOPHYLLALES [the COM clade + the N-fixing clade]]: endosperm scanty.
[the COM clade + the N-fixing clade]: ?
[FABALES [ROSALES [CUCURBITALES + FAGALES]]] / the N-fixing clade / fabids: (N-fixing by associated root-dwelling bacteria); tension wood +; seed exotestal.
[ROSALES [CUCURBITALES + FAGALES]]: (actinomycete Frankia infection +); styles separate; ovules 1-2/carpel, apical.
[CUCURBITALES + ROSALES]: ?
Age. This node is dated at ca 100.1 Ma (X. Guo et al. 2021).
Phylogeny. For relationships, see above.
CUCURBITALES Berchtold & J. Presl - Main Tree.
Ellagic acid ?; storied fusiform cambial initials; perforation plates not or minimally bordered; tension wood?; rays wide, multiseriate; cuticle wax crystalloids 0; leaves spiral; K or P valvate, stomata on K/P raised, the two whorls rather similar in texture; styluli +, (ovary with a roof, styluli often marginal), stigma unicellular-papillate, pollen tube transmitting tissue +; ovule with bistomal micropyle; antipodals ephemeral; codon changes [see Filipowicz & Renner 2010]. - 7 families, 107 genera, 2,985 species.
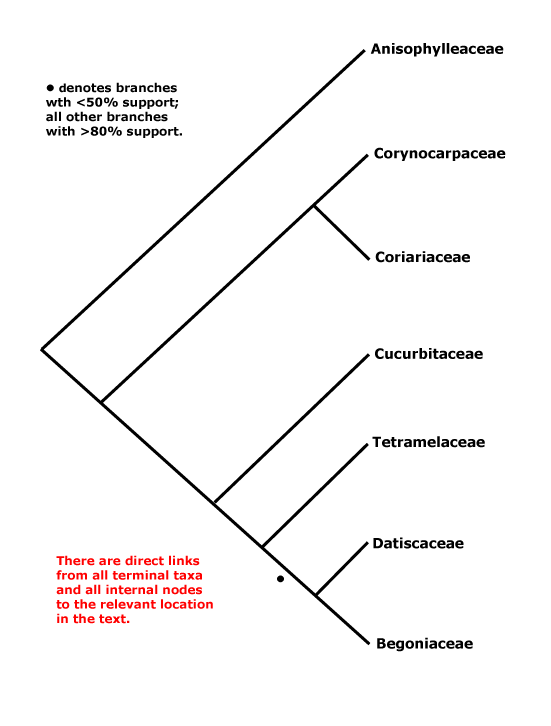
Includes Anisophylleaceae, Begoniaceae, Coriariaceae, Corynocarpaceae, Cucurbitaceae, Datiscaceae, Tetramelaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age suggested by H. Wang et al. (2009) for this node was (85-)80, 78(-73) Ma (two penalized likelihood dates), while Bayesian relaxed clock estimates were slightly older, to 90 Ma; (103.3-)86.9, 86.2(-71.3) Ma are the ages suggested by H.-L. Li et al. (2015).
Evolution: Divergence & Distribution. It is unclear where some of the features suggested by Filipowicz and Renner (2010) as being apomorphies of the order should be placed on the tree. Some, such as inferior ovary, are likely to characterize the [Cucurbitales + Fagales] clade, others, such as breeding system, are very labile and are perhaps more likely to characterize a clade within Cucurbitales. L.-B. Zhang et al. (2006) also discuss aspects of morphological evolution and evaluate the extensive variation in breeding system in the clade, while Jiang et al. (2019) discuss the evolution of pollen morphology.
M. Sun et al. (2019a/2020) critically examine the literature dealing with diversification rates in the order.
Ecology & Physiology. For nitrogen fixation in general, see elsewhere.
Pollination Biology & Seed Dispersal. Zhang and Renner (2003) suggested that the flowers of Cucurbitales are usually imperfect, but perfect flowers are known from Anisophylleaceae. Indeed, breeding systems vary considerably in the order. Schaefer and Renner (2010) suggested that within Momordica (Cucurbitaceae) there have been perhaps seven reversals from dioecy to monoecy in a clade that is very approximately 35 Ma, Bertin and Newman (1993) and Routley et al. (2004) note various kinds of dichogamy in the order.
Plant-Animal Interactions. Butterfly caterpillars appear to be relatively uncommon on members of the order.
Plant-Bacterial/Fungal Associations. For the strains of Frankia associated with N-fixing Cucurbitales, see elsewhere.
Genes & Genomes. J. Wang et al. (2017b, see also 2019t, 2022) date a genome duplication event, which they associate with Cucurbitaceae, to around 102-90 Ma, an age which suggests that it is perhaps better associated with a deeper node.
Chemistry, Morphology, etc.. Cuticle waxes are usually not well developed. Possession of libriform fibres and slightly oblique end walls of vessel elements may be synapomorphies (Wagstaff & Dawson 2000, see also data in Nandi et al. 1998), as may banded wood parenchyma (Baas et al. 2000), homogeneous rays, and the possession of bitter compounds. Stipules are not placed as a synapomorphy of the order, although the data presented by Matthews and Endress (2004) might suggest that this was an option. However, not only does the morphology of the stipules in Anisophylleaceae and Corynocarpaceae need clarification, but the presence of stipules may be a synapomorphy at a much higher level (see rosids et al. above). Polarity of the character of leaf venation is unclear, since Combretocarpus is sister to other Anisophylleaceae and Corynocarpaceae are sister to Coriariaceae; the first member of both pairs has pinnate venation, and the two clades are successively sister to the rest of the order.
More or less laciniate petals (and staminodes) are quite common in the order (Endress & Matthews 2006b). The thickness of the outer integument varies considerably. Matthews and Endress (2004, summarized in 2006b) provide an excellent survey of floral morphology of the group; see also Pandey et al. (2014b: summary of embryology and seeds, etc.).
Phylogeny. For the circumscription of the clade see e.g. Setoguchi et al. (1999) and Schwarzbach and Ricklefs (2000); there is now general agreement as to what it contains, apart from Apoodanthaceae (see below). Coriariaceae and Corynocarpaceae were often found to be sister taxa, and their very similar wood anatomy is consistent with such a position (Carlquist & Miller 2001). However, recently Baker et al. (2021) found the basal relationships [Corynocarpaceae [Aniosphylleaceae [Coriariaceae ...]]]. Other relationships were initially rather poorly understood (Brouillet 2001 for some comments), and although L.-B. Zhang and Renner (2003a), using a variety of both chloroplast and nuclear genes, suggested that Anisophylleaceae were sister to the rest of Cucurbitales, H.-L. Li et al. (2015) found the clade [Anisophylleaceae + Cucurbitaceae], but its position had little support (c.f. H.-L. Li et al. 2016). Zhang et al. (2006) in a nine-gene study (all three compartments) confirmed these relationships and also placed Cucurbitaceae as sister to a [Tetramelaceae + Datiscaceae + Begoniaceae] clade, although relationships within this latter group were not entirely clear. The clade [Datiscaceae + Begoniaceae] had at best only moderate support (Zhang et al. 2006; see also Schaefer et al. 2009; Schaefer & Renner 2011b), but this topology is followed here - c.f. Soltis et al. (2007a) and Bell et al. (2010). For possible relationships between Tetramelaceae and Datiscaceae, see Swensen et al. (1994, 1998) and H.-L. Li et al. (2015: support low); this family pair was also recovered by M. Sun et al. (2016), but most of the relationships there had little support. Baker et al. (2021) found the topology [Cucurbitaceae [Tetramelaceae [Octomelaceae + Begoniaceae]]] between the terminal members of the order, and things have still not settled dow, In the Seed Plant Tree, version ix.2024, relationships are [Corynocarpaceae [Anisophylleaceae [Cucurbitaceae [Coriariaceae [Begoniaceae [Tetramelaceae + Datiscaceae]]]]]] while in Zuntini et al. (2024) the positions of Cucurbitaceae and Coriariaceae are reversed; not all nodes have strong support. For a discussion about the relationships of Apodanthaceae, prior to x.2024 included here but now placed in Malpighiales-Rafflesiaceae as a subfamily, see Malpighiales.
Previous Relationships. Cucurbitales - Apodanthaceae aside - are another rather unexpected assemblage of families. Coriariaceae have often been placed with families that have separate carpels and so were thought to be "primitive". Rhizophoraceae and Anisophylleaceae were often associated in the twentieth century, e.g. being placed in separate but adjacent orders, as in Takhtajan (1997), or even in the same family. However, although both were placed in Rosidae by Cronquist (1981), they were not adjacent. Indeed, morphological differences between the two are marked (e.g. Juncosa and Tomlinson 1988; Tomlinson 1988), and Rhizophoraceae are now securely placed in Malpighiales where they are consistently placed as sister to Erythroxylaceae; the two have much in common. Cucurbitaceae, Begoniaceae and Tetramelaceae were placed adjacent in a group of families with parietal placentation in Cronquist (1981), but that group has since been fragmented.
Similarities in floral morphology between Anisophyllea and Ceratopetalum (Oxalidales-Cunoniaceae: see Matthews et al. 2001; Endress & Matthews 2006b), although striking, are unlikely to be evidence of immediate close relationships of the two. The fossil Platydiscus peltatus seems to suggest similar relationships (Schönenberger et al. 2001a; see also Schönenberger & von Balthazar 2006), and it was included in a study of Oxalidales by Heibl and Renner (2012).
Synonymy: Anisophylleales Reveal & Doweld, Begoniales Link, Coriariales Lindley, Corynocarpales Takhtajan, Datiscales Dumortier - Begonianae Doweld, Corynocarpanae Takhtajan, Cucurbitanae Reveal - Coriariopsida Parlatore, Cucurbitopsida Brongniart
ANISOPHYLLEACEAE Ridley - Back to Cucurbitales
Trees and shrubs; plants Al-accumulators; cork?; cambium storying?; nodes 1:1; cuticle waxes as platelets; stomata usu. paracytic; serial [superposed] axillary buds; branching from current growth, rythmic; lamina margins entire, (stipules 2-4, minute, at the very base of the petiole [= colleters?]); inflorescence branched, racemose or spicate; flowers small; K epidermis with mucilaginous inner walls, postgenitally coherent, C open (0), lobed or laciniate (entire), bundle number?, ± enclosing groups of A; A 2x K, obdiplostemonous, incurved in bud; nectary of separate lobes; G (stylulus hollow), compitum 0, stigma expanded or punctate, not papillate; ovules (unitegmic, integument 7-8 cells across), epitropous, nucellar cap +; fruit 1-seeded, K persistent; embryo fusiform, largely hypocotylar; n = 7, 8, x = ?; germination hypogeal.
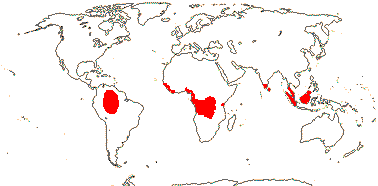
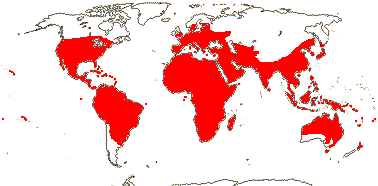
4 [list]/71: Anisophyllea (67). Pantropical (map: see Trop. Afr. Fl. Pl. Ecol. Distr. 1. 2003, 6. 2011; Clement et al. 2004). [Photo - Anisophyllea Fruit]
Age. Crown-group diversification in Anisophylleaceae may have begun (107-)85(-67) Ma (L.-B. Zhang et al. 2007).
1. Combretocarpus rotundus Bentham & J. D. Hooker
Indumentum lepidote [on flowers]; flowers perfect, 3(-4) merous; unitegmic, parietal tissue 1-2 cells across; embryo sac sac bisporic [chalazal dyad], eight-celled [Allium-type]; fruit 3(-4) longitudinally winged; cotyledons small.
1/1. Malesia: Malay Peninsula to Borneo.
2. Anisophylleae Bentham & Hooker —— Synonymy: Polygonanthaceae Croizat
(Cuticle waxes beaker-like - Polygonanthus); leaves (anisophyllous, appearing 2-ranked, alternate leaves very much reduced), lamina base asymmetric, (secondary veins palmate); plant monoecious (flowers perfect); flowers 4(-5) merous; nectary lobes also interstaminal; (roof over ovary, styluli marginal - Anisophyllea); (filaments very short); ovule 1/carpel, outer integument 4-5 cells across, (unitegmic), parietal tissue 3-5 cells across; fruit a drupe, (4-seeded), (K large, wing-like - Polygonanthus); testa multiplicative, 10-30 cells across, vascularized, (mesotesta also lignified); cotyledons minute to indistinct; n = ?
3/70: Anisophyllea (67). Pantropical, but not E. Malesia to the Pacific.
Age. The crown-group age of this clade is (63-)44(-30) Ma (L.-B. Zhang et al. 2007).
Chemistry, Morphology, etc.. Vincent and Tomlinson (1983) discussed the distinctive vegetative architecture of Anisophyllea, while Dengler et al. (1989) looked at the primary vascular organization of the orthotropic and plagiotropic shoots - quite different.
The staminate flowers of Anisophyllea disticha appear to have a semi-superior ovary with central styluli while the carpelate flowers have an inferior ovary, there is a roof on the ovary and the styluli are submarginal (Ruth Crevel, in Ding Hou 1858).
For further information, see Dahlgren (1988), Schwarzbach and Tomlinson (2011) and X. Chen et al. (2015), all general, Schönenberger et al. (2001a: fossils), Tobe and Raven (1987e, 1988a, c: floral morphology, embryology, fruit), and Matthews et al. (2004: floral development).
Phylogeny. Combretocarpus was found to be sister to the rest of the family (L.-B. Zhang et al. 2007), somewhat in conflict with morphology-based relationships (e.g. Tobe & Raven 1987c, 1988c), and this may have an important effect on the polarity of characters in the family or even the order as a whole; other relationships were [Polygonanthus [Poga + Anisophyllea]]. However, M. Sun et al. (2016) recovered the groupings [[Combretocarpus + Polygonanthus] [Poga + Anisophyllea]] so everything is a little confused.
Previous Relationships. Anisophylleaceae were often linked with or included in Rhizophoraceae (Malpighiales: see above). However, the inner integument is ca 2 cell layers thick, there are no laticifers, the petals are not aristate, and a sclerified exotegmen is absent; in these and many other characters Anisophylleaceae differ from Rhizophoraceae (see Juncosa & Tomlinson 1988a, b).
[[Corynocarpaceae + Coriariaceae] [Cucurbitaceae [Tetramelaceae [Datiscaceae + Begoniaceae]]]]: uniseriate rays 0; lamina with secondary veins palmate; filaments shorter than anthers in bud, anthers basifixed.
Age. Magallón and Castillo (2009) gave an age of ca 86.7 Ma for this node, Tank et al. (2015: Table S2) an age of around 71.8 Ma, and Bell et al. (2010) an age of (78-)67, 61(-48) Ma. Wikström et al. (2001: c.f. topology) estimated an age of (68-)66, 65(-63) Ma.
[Corynocarpaceae + Coriariaceae]: ellagic acid +; wood with broad rays; sieve tube plastids lacking both starch and protein inclusions; stomata paracytic; lamina margin entire; flowers small, K quincuncial; C thick, base broad; G superior, ascidiate; ovule 1/carpel, outer integument vascularized [?here]; cotyledons very large.
Age. Wikström et al. (2001) suggested that this node was (55-)52, 48(-45) Ma and Bell et al. (2010) that it was (64-)49, 43(-29) My; (79.5-)65.7, 41.4(-10.2) Ma is the rather broad spread in H.-L. Li et al. (2015), but they preferred the older ages, while Renner et al. (2020) estimated an age of 86.4 Ma.
Chemistry, Morphology, etc.. For sieve tube plastids, see Behnke (1981c), for compitum presemce/absence, see Armbruster et al. (2002).
CORYNOCARPACEAE Engler, nom. cons. - Corynocarpus Forster & Forster - Back to Cucurbitales
Trees; alkaloids +; young stem with separate bundles; petiole bundles in a line; lamina vernation conduplicate, secondary veins pinnate, stipule single, intrapetiolar; inflorescence paniculate; calyx and corolla distinct; stamens = opposite and basally adnate to C, incurved in bud, staminodes 5, fringed, petal-like, opposite sepals, nectary basal, adaxial; pollen heteropolar, dicolpate, ± smooth, exine infratectum granular; G [2], transverse?, only 1 fertile, stylulus short, usu. single, conduplicate, stigma capitate, dry, compitum 0; ovule with outer integument ca 11[?-30] layers across; fruit a drupe; seed one, large, stylulus excentric; seed coat ?pachychalazal, thick, becoming crushed; endosperm starchy; n = 22, 23, x = 22 (?23), nuclear genome [1 C] (0.025-)0.658(-17.324)[?] pg.
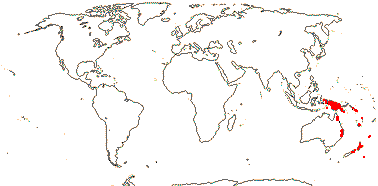
1 [list]/6. New Guinea to New Zealand, introduced on Hawai'i. Map: from van Steenis and van Balgooy (1966) and Fl. Austral. vol. 8. (1984). [Photo - Flower] [Photo - Fruit]
Chemistry, Morphology, etc.. The cork develops from the cell layer beneath the epidermis.
It is perhaps unclear whether the gynoecium is pseudomonomerous or unicarpelate. However, since some flowers have two styluli (e.g. Matthews & Endress 2004), the single, excentrically-placed structure at the apex of the gynoecium is called a stylulus and the gynoecium is pseudomonomerous {sic]. Tobe et al. (1992a) noted that fibres developing from the inner part of the mesocarp ran longitudinally in the fruit while those developed from the endocarp (the cells divided) ran circumferentially. The pulp is bitter, and the seeds are very poisonous, containing alkaloids.
Some information is taken from Hemsley (1903: general), Nowicke and Skvarla (1983a: pollen), Philipson (1987a: general), and Kubitzki (2011: general).
Previous Relationships. Corynocarpaceae were Celastralean in affinity according to Cronquist (1981), isolated, according to Takhtajan (1997), so here they are!
CORIARIACEAE Candolle, nom. cons. - Coriaria L. - Back to Cucurbitales
Usu. shrubs; roots with N-fixing Frankia; coriolic fatty acid [CH3(CH2)4CH(OH)CH=CHCH=CH(CH2)7COOH] in seed, sesquiterpenes, myricetin +; vessels in multiples, perforation plates bordered, true tracheids +, wood parenchyma (confluent) vasicentric; sieve tube plastids lacking both starch and protein inclusions; nodes 1:1; petiole bundle arcuate; buds usu. perulate; leaves opposite, lamina vernation ± flat, stipules small; plant polygamous or flowers perfect, inflorescences racemes; flowers often protogynous, (6-merous), bracteoles 0; K quincuncial, C open, fleshy, often keeled adaxially; A 10, connective thin, septum between sporangia of theca not developed; tapetal cells 2-4-nucleate; pollen (2-)3 brevicolpate, starchy, (3-nucleate); G [5], opposite K, [(10)], stylulus slender, deeply impressed, stigmatic all around, long-papillate, dry; ovule apotropous, micropyle endostomal, outer integument 3-4 cells across, inner integument 2-3 cells across, parietal tissue ca 8 cells across, nucellar cap ca 4 cells across; fruitlets achenes [nutlets], several, surrounded by fleshy accrescent C; exotesta of cuboid, "tanniniferous", thick-walled, lignified(?) cells, rest undistinguished; n = 10, 15, x = 20, nuclear genome [1 C] (0.025-)1.658(-17.324) pg[?].
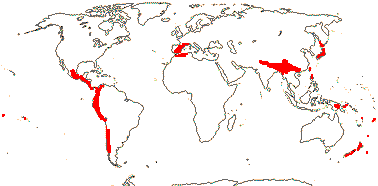
1 [list]/14-17. Circum S. Pacific to China and the Himalayas, W. Mediterranean. Map: from van Steenis and van Balgooy (1966), Good (1974) and Renner et al. (2020). [Photo - Inflorescence] [Photo - Fruit.]
Age. Molecular estimates of the age of crown-group Coriariaceae are around 69-63 Ma (Yokoyama et al. 2000) and (76-)57(-34) Ma (Renner et al. 2020).
Vegetative fossils of Coriaria are reported from about 33 Ma deposits in France (de Saporta 1865: pp. 211-217, pl. 12:1). However, pollen of Coriaripites was initially described from the Maastrichtian of Alberta, Canada, and it has also been found in rather older 87-82 Ma Campanian deposits from islands immediately to the N.E. of the Antarctic Peninsula (Renner et al. 2020).
Evolution: Divergence & Distribution. Biogeographic relationships within the family could be summarized as [Eurasia [[S. and W. Pacific + Central and N. South America]] (Yokoyama et al. 2000), and they are largely similar in Renner et al. (2020). This very disjunct distribution may have resulted from a south to northwest movement.
Chemistry, Morphology, etc.. Although the carpels seem to be separate, a compitum is developed (Guédès 1971; Matthews & Endress 2004). There is but a single seed per carpel, but several seeds are produced by each flower.
Information on nodal anatomy is taken from Sinnott (1914), on embryology from Sharma (1968a), and on wood anatomy from Yoda and Suzuki (1992). For gynoecial development, see Guédès (1971), and for general information, see Kubitzki et al. (2011).
Phylogeny. For phylogenetic relationships within Coriaraceae, see Yokoyama et al. (2000) and especially Renner et al. (2020).
Previous relationships. Coriariaceae were placed in Ranunculales by Cronquist (1981) and as a monotypic Coriariales in Rosidae (Takhtajan 1997), largely because of their apparently separate carpels.
[Cucurbitaceae [Tetramelaceae [Datiscaceae + Begoniaceae]]]: perennial herbs; cucurbitacins [unsaturated tetracyclic triterpenes] +, myricetin, ellagic acid 0; young stem with separate bundles; leaf teeth with medial vein ending in a pad of packed translucent cells [hydathode], lateral also entering (lateral dominant - Begonia) [= cucurbitoid teeth], stipules 0; flowers imperfect; nectaries 0 [?here]; G opposite sepals or median member adaxial, placentation parietal, ovary roof +, styluli ± marginal, stigmas large, elongated, bilobed; ovules many/carpel; cucurbit-common tetraploidy (CCT) event; seeds many/fruit.
Age. The age of this node has been estimated to be (74-)62, 56(-43) Ma (Bell et al. 2010), ca 71.1 or 69 Ma (Tank et al. 2015: Table S1, S2), (83-)81(-79) Ma (Schaefer et al. 2009), ca 57 Ma (Naumann et al. 2013) and 101-90 Ma (J. Wang et al. 2022).
Evolution: Divergence & Distribution. An apomorphy for this clade may be "plant dioecious" (L.-B. Zhang et al. 2006; Käfer et al. 2012) even if most members of the clade are monoecious, and in Cucurbitaceae in particular flower type is very labile (Käfer et al. 2017 for literature).
Genes & Genomes. J. Wang et al. (2017, see also 2019) date a genome duplication event, the cucurbit-common tetraploidy (CCT), an allotetraploidy event, to around 102-90 Ma, and suggest that it has been important in the evolution of the family. The CCT duplication event seems to involve both Begoniaceae and Cucurbitaceae, no other families with it being mentioned, hence its position here is provisional; it was dated to 105-93 Ma by J. Wang et al. (2020) who thought that "Cucurbitales", i.e. this particular clade, had 17 protochromosomes. No genome duplication in this area was noted by L. Li et al. (2022: Fig. 2c).
Chemistry, Morphology, etc.. For cucurbitacins, some of which affect microtubule development and mitosis, see X. Wang et al. (2017) and J. Wang et al. (2022). Davidson (1973: Datiscaceae), Hickey and Wolfe (1975), Brouillet et al. (1987: Begoniaceae) and in particular Gonçalves et al. (2020) discuss the distinctive cucurbitoid leaf teeth. Gonçalves et al. (2020) noted that in the Cucurbitaceae they examined the teeth were supplied by xylem only, there was no sugar in the exudate, and suggested that the activity of the hydathodes helps maintain the water balance of the plant.
The development of a roof over the ovary formed from tissue adaxial to the stylulus (Matthews & Endress 2004) is obvious when well developed; the styluli are then widely separate and borne towards the margin of the ovary. Matthews and Endress (2006) note details of ovule morphology that this group has in common.
Previous relationships. This group of families were members of the old Parietales, a group of families with parietal placentation (e.g. Cronquist 1981) or put in a group of small orders placed next to each other in Dilleniidae (Takhtajan 1997). .
CUCURBITACEAE Jussieu, nom. cons. - Back to Cucurbitales
Climbers, branch tendrils +, axillary-± lateral, bifid (unbranched), both branches and stem of tendril coiling [= zanonioid tendril], (0), (plant variously prickly/spiny), (stem base/roots variously swollen); (Si02 accumulation); alkaloids, bitter tetra- and pentacyclic triterpenoids, punicic acid [C18H30O2], non-protein amino acid citrullin [α-amino-delta-ureidopentanoic acid], saponins +, little oxalate accumulation, tannins 0; phloem loading via intermediary cells [specialized companion cells with numerous plasmodesmata, raffinose etc. involved]; root cork superficial; stem cork variable in position; cambium storying?; extra-fascicular phloem +; vascular bundles initially in two rings [outer opposite the angles of the stem]; rays multiseriate; outer collenchyma and sclerenchymatous sheath in cortex; petiole bundle arcuate, or ring of arcuate bundles; no pericyclic sheath; (cuticle waxes as platelets); indumentum rough hairy/prickly, walls calcified, cystoliths + (0), hairs often glandular; leaves often with extrafloral nectaries, (lamina margins entire); plants dioecious (monoecious), inflorescences axillary; interfloral protogyny, flowers ebracteolate or not, (3-)5(-7)-merous; hypanthium + (0; tube formed by K and C alone), short, K often connate, open, (0), C (induplicate-)valvate, connate; staminate flowers: nectary type?; A extrorse; pollen grains prolate, to 40 µm long, (micro)striate, starchy; tapetal cells usu. unicellular; pistillode 0; carpelate flowers: staminodes +; G 1 [(2) 3(-5)], inferior [axial/receptacular], (median member abaxial), placentae intrusive, stigmas dry or wet, (channelled; not bilobed); ovules pendulous or horizontal, micropyle endostomal/nucellus apex exposed, outer integument 6-15+ cells across, vascularized, apex of ovule pushing up micropyle, parietal tissue 5-11 cells across, suprachalazal region massive; seeds flattened (not), pitted or not, ± winged (not); testa multiplicative, exotesta inconspicuous, sclereidal layer +, inner aerenchymatous zone +, with lignified bands on walls, tegmen not persistent; endosperm 0, chalazal haustorium +, slender, (much) elongated, coenocytic, cotyledons large, flat; x = 11 (?12), genome duplication event, nuclear genome [1 C] (0.07-)1.055(-15.909) pg; germination epigeal, seedlings with a peg [= unilateral cortical outgrowth at hypocotyl-shoot transition].
98 [list, to tribes]/1,000 - 15 tribes below. Largely tropical and subtropical, especially drier parts of Africa. Map: from Heywood (1978 [N. part of range]), Saade (1998), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), vol. 6 (2011) and Florabase (consulted 2006). [Photos - Collection, Staminate flower, Carpellate flower, Fruit.]
Age. The crown-group age of Cucurbitaceae may be (66-)63(-60) Ma (Schaefer et al. 2009).
Fossil seeds identified as Cucurbitaceae have been found in London Clay deposits ca 65 Ma (Chandler 1964).
[Gomphogyneae [Triceratieae + Zanonieae]]: K and C ± distinct.
Age. The age of this node - if it exists - is some (62-)59(-56) Ma (Schaefer et al. 2009).
1. Gomphogyneae Bentham & J. D. Hooker
(Tendrils with adhesive pads); nectary of multicellular hairs on C; A 5, 1-thecal, or 3, 2-/2- and 1-thecal, inserted at base of C and connate to middle of C, thecae straight; (pollen perforate-rugulate - Alsomitra); (ovules ca 6/carpel); fruit a capsule [?type] (berry); seeds with broad, membranous wing (not); n = 11, 13.
6/56: Hemsleya (30). China and South East Asia to Australia and Fiji.
Age. Crown-group Gomphogyneae are (59-)65(-53) Ma (Schaefer et al. 2009).
Age. The age of this node - if it exists - is (61-)58(-55) Ma (Schaefer et al. 2009).
2. Triceratieae A. Richard (inc. Fevilleeae) —— Synonymy: Nhandirobaceae Lestibudois
(Plant glabrous); nectary of multicellular hairs on C; A 1-3, 5, variously 1 or 2-thecal, inserted at base of C, thecae straight or arcuate; (pollen larger, reticulate - Gerrardanthus - remove?); carpelate flowers: staminodia 5, (styluli central); 3 ovules/carpel; antipodal cells persistent; fruit circumscissile, with "longitudinal splits", or indehiscent 1(-3)-seeded samara, baccate; seeds narrowly winged or not; n = ?; germination phanerocotylar, hypogeal, cotyledon with palmate venation, base of epicotyl swollen, no obvious radicle [Fevillea].
5/24: Gerrardanthus (11). Mostly tropical, New World (E. and S. Africa and Madagascar - Cyclantheropsis).
Age. Crown-group Triceratieae are (51-)46(-41) Ma (Schaefer et al. 2009).
3. Zanonieae Bentham & J. D. Hooker —— Synonymy: Zanoniaceae Dumortier
(Flowers weakly monosymmetric); nectary of multicellular hairs on C[?]; A 5 (4), 1-thecal, inserted at base of C to middle of tube, thecae straight or reniform; (pollen reticulate); (flower single, central); fruit capsular; seeds with membranous (narrow) wing; n = ?
4/13: Gerrardanthus (6). Pantropical.
Age. Crown-group Zanonieae are (60-)56(-52) Ma (Schaefer et al. 2009).
[Actinostemmateae + Cucurbitoideae]: ?
4. Actinostemmateae H. Schaefer & S. S. Renner - Actinostemma Griffith
K and C similar, nectary of multicellular hairs on C; A inserted on base of tube, 5, or 2 pairs + 1, 1-thecal; style single, central; ovules 2-4, pendulous; fruit circumscissile; seeds winged or not; n = 8.
1/3. Central and East Asia.
5. Cucurbitoideae Eaton
Staminate flowers: hypanthium well developed (short); anthers 1-thecate [if 2-thecate, then A [2]]; pollen grains ± spherical, reticulate; fruit indehiscent; seeds not winged.
82/900. Tropical to warm temperate.
Age. The age of this node is around (56-)53(-50) Ma (Schaefer et al. 2009).
5A. Indofevilleeae H. Schaefer & S. S. Renner - Indofevillea Chatterjee
Woody liane; lamina margin entire; C with oil-secreting hairs; A inserted on base of C tube, 2 pairs + 1, thecae reniform, hairy, filaments short; pollen grains ca 49 x 53 µm long; carpelate flowers unknown; fruit dry, ?indehiscent, pericarp thick and woody; ?seed coat; n = ?
1/2. N.E. India, adjacent Tibet, Myanmar.
[Thladiantheae [Siraitieae [Momordiceae [Joliffieae [Bryonieae, Sicyoeae [Schizopeponeae [Coniandreae [Cucurbiteae + Benincaseae]]]]]]]: vines/lianas, tendrils with apical twining alone [?here]; additional non-protein amino acids +; vascular bundles bicollateral; extrafascicular phloem system linked with adaxial phloem of bundles; nectary +, parenchymatous, with stomata; pollen grains 40-70 µm long; carpelate flowers: style shortly branched or not, stylar canal +, filled with secretion; ovules horizontal (to erect), nucellar cap +/0; antipodals degenerate; fruit fleshy, indehiscent; exotesta enlarged, ± palisade or cubic, walls with (branched) radial lignified thickenings (none), mucilaginous, hypodermal tissue various, often ± sclereidal, sclereid layer +, sharply distinguished, single cell across, cells often much enlarged, elongated or not, walls much thickened, walls of aerenchyma usu. unthickened, innermost layers green, chlorenchymatous.
Age. This node is (54-)51(-48) Ma (Schaefer et al. 2009).
5B. Thladiantheae H. Schaefer & S. S. Renner
Plant with stem tubers (also rhizomes); leaves usu. simple; plant dioecious; anthers 2 pairs + 1, inserted near tube mouth; n = 9, 16.
2/35: Thladiantha (30). India and E. Russia to western Malesia.
5C. Siraitieae A. Richard - Siraitia Merrill
Plant tuberous; A 5/2 pairs + 1, inserted at base of tube, thecae to 3-plicate; fruit tomentose; seeds few, (with 2/3 corky wings); n = 14.
1/4. India and S. China to Malesia, 1 sp. Africa (S.E. Nigeria, Tanzania).
[Momordiceae [Joliffieae [Bryonieae, Sicyoeae [Schizopeponeae [Coniandreae [Cucurbiteae + Benincaseae]]]]]]: tendril twining?; plant dioecious or monoecious; x = 15.
Age. The age of this clade is ca 36.1 Ma (Xie et al. 2019).
5D. Momordiceae M. Roemer - Momordica L.
(Root tuberous/massive rootstock); foliar nectaries +/0; staminate flowers with orbicular bract/not; C rotate, not connate, 1-3 with basal scales; A 3, 2 2-thecate + 1 1-thecate/2, 1 3-thecate + 1 2-thecate, thecae to 3-plicate; fruit usu. spiny/tuberculate/winged, (with 3 valves); seeds often arillate; n = 11, 14; germination hypogeal.
1/60. Africa, India-Southeast Asia to Australia.
[Joliffieae [Bryonieae, Sicyoeae [Schizopeponeae [Coniandreae [Cucurbiteae + Benincaseae]]]]]: ?
5E. Joliffieae Schrader
Roots ± tuberous; A (5), to 3-plicate; n = 12.
3/10: Ampelosicyos (5). Tropical Africa, Madagascar.
[Bryonieae, Sicyoeae [Schizopeponeae [Coniandreae [Cucurbiteae + Benincaseae]]]]: ?
5F. Bryonieae Dumortier —— Synonymy: Bryoniaceae G. Meyer
Tendrils simple/0; lamina margins entire; A thecae to 3-plicate; pollen "medium to large"; (seeds ejected by contraction of fruit - Ecballium); inner integument to ca 6 cells across; n = 9, 10.
3/15: Bryonia (10). Europe and N. Africa to Central and S.W. Asia, Central and W. Australia.
5G. Sicyoeae Schrader
(Rootstock tuberous); tendrils to 8-fid; C (margins to long-fimbriate); A 2-5, thecae various, inc. 10-folded ring opening by continuous slit; pollen medium to large, (31-)61-158 x (30-)54-168 µm, 4-14 (col-)porate, surface various, rarely reticulate; nectary of multicellular hairs; ovule with nucellar beak [Echinocuystis]; fruit (dry) (prickly; (endocarp s.l. reticulate - Echinocystis), (explosively dehiscent/operculate), seeds 1-(very)many/fruit; inner integument to ca 6 cells across; n = 11-16, etc.; (chalazal endosperm haustorium massive, triangular - Sicyos).
12/265: Trichosanthes (to 100), Sicyos (75), Cyclanthera (40). Tropics and warm temperate, the Pacific.
[Schizopeponeae [Coniandreae [Cucurbiteae + Benincaseae]]]: (tendrils 3-(or more)fid/0)
5H. Schizopeponeae C. Jeffrey
A 3, thecae to 3-plicate; pollen medium to large, (3-porate, baculate); fruit (3-valved); n = 10, 11.
2/10: Schizopepon (7). Russia and India to Japan and Myanmar.
[Coniandreae [Cucurbiteae + Benincaseae]]: ?
5I. Coniandreae Endlicher
(Trees); A 2, 3, 5, thecae various; pollen (in tetrads); n = 12-14.
19/ca 150: Gurania (37), Kedrostis (28), Corallocarpus (17). S. U.S.A. to Argentina, Africa-Madagascar, few to Asia, India, Pakistan.
[Cucurbiteae + Benincaseae]: ?
Age. This clade is ca 26.4 Ma (Xie et al. 2019) or 31-27 Ma (J. Wang et al. 2022).
5J. Cucurbiteae Dumortier
Quite often annuals; (lamina dissected); A (2-)3(-4), thecae various; pollen grains large, 50->200 µm long, 3-10 zono- to pantoporate, surface echinate; fruit (dry, splitting); seeds (narrowly winged); n = 20.
11/105: Cayaponia (55). South U.S.A. to Argentina and Uruguay, the Antilles, C. africana W. and Central Africa, Madagascar.
5K. Benincaseae Seringe —— Synonymy: Cyclantheraceae Lilja
(Annuals), (shrubs); leaves (much reduced); A (2-)3(-5), thecae number and type various; pollen medium to large, (in tetrads), (surface striate); (chalazal endosperm haustoria 0 - Blastania, = Ctenolepis); n = (7, 11) 12.
24/210: Zehneria (60), Cucumis (55), Coccinia (30). (Sub)tropical Old World, inc. E. Mediterranean, the Pacific, few America (Melothria).
Age. Crown-group Benincaseae are ca 18.1 Ma (Xie et al. 2019) while another possible age for this clade is 25-22 Ma (J. Wang et al. 2022).
Evolution: Divergence & Distribution. For numerous dates within the family, see Schaefer et al. (2009).
Diversification rates may have increased in Cucurbitaceae - dates of (57.1-)42.6(-35.5) Ma (Magallón et al. 2018).
The current world-wide range of Cucurbitaceae is thought to be in large part the result of extensive dispersal, Madagascar being colonized an estimated thirteen times and Australia twelve times alone - yet only twelve genera and thirty species are found in the latter (Schaefer et al. 2009). The secondarily woody Socotran endemic Dendrosicyos is dated to (30-)22(-14) Ma, although Socotra itself is only about ten mya old, which suggests that the clade now represented by just the single species was once on the mainland and has since become extinct there (Schaefer et al. 2009, but c.f. Renner & Schaefer 2016). Sebastian et al. (2012) suggested that there had been four westwards trans-Pacific dispersal events in the New World Sicyos alone, while de Boer et al. (2014) also dated extensive dispersal in Trichosanthes. For more on dispersal in the family, see Duchen and Renner (2010).
Establishment of the topology within the basal polytomy of the family (see below) is important since it may well affect optimisation of characters on the tree, and unfortunately not that much is known about embryology, cytology, etc., of members of this basal complex. Leaf morphology, breeding system, inflorescence/nodal morphology, and androecium all vary greatly in Cucurbitaceae, and the character hierarchy above is even more provisional than normal.
Recently Z. Dong et al. (2025) found evidence suggesting that the KNOX1-type transcription factor KNOTTED-LIKE FROM ARABIDOPSIS THALIANA 2 (KNAT2-like1) plays a very important role in both gynoecial development and floral sex expression. In the development of female flowers of cucumber the receptacular tissue shows accelerated development and encloses the developing gynoecium, the latter becoming adnate to the receptacular tissue and rotating ca 180o (Dong et al. 2025) - the inferior ovary here is receptacular. More than that: If this gene is knocked out, the receptacular tissue does not show this accelerated development, the gynoecial tissue is not adnate to the receptacular tissue and does not rotate, the ovary is superior, and the flowers are generally hermaphroditic - all in all, they are rather like those of a tomato (Dong et al. 2025)! Indeed, if the flower is female and the ovary inferior, the medial region of the carpel is abaxial and the lateral region is adaxial, but these relationships reverse if the flower is perfect and the ovary is superior - the medial region is adaxial and the lateral region is abaxial. Altogether a fascinating story - other receptacle marker genes are not expressed, and of course we need to know more about the development of the inferior ovary in other Cucurbitaceae, not to mention that of the ovary in Begoniaceae and Tetrathecaceae, and so on.
Ecology & Physiology. Most Cucurbitaceae are climbing plants, whether lianes or vines, and are a particularly prominent component of this vegetation element in the New World (Gentry 1991). They climb by tendrils, whether branched or simple, and the spirals at the ends of the tendril (branches) are opposing, reversal occurring at what is called a perversion, the result being a very springy structure which dissipates any strain in the system (Gerbode et al. 2012). Many cucurbits are also more or less succulent plants, with variously swollen roots/and or stem bases, rarely stems (thus Seyrigia also has more or less swollen, leafless stems); some 35 genera are included in the treatment of succulents by Newton and Eggli (2023). Cucurbitaceous vines are notable components of the vegetation in the deserts of S.W. North America (Krings 2000).
The phloem system in Cucurbitaceae is very complex. Fischer (1884) early noted that not all Cucurbitaceae had bicollateral vascular bundles (they are an apomorphy of only part of the family - see above). Taxa that do have bicollateral vascular bundles also have extrafascicular phloem (EFP) strands in the cortex outside the sclerenchymatous ring, although their development there is weak in taxa like Thladiantha and Momordica (Fischer 1884). EFP strands link with the adaxial phloem cells of the vascular bundles (Schmitz et al. 1987), but only at the nodes (Fischer 1883); in taxa like Cucurbita EFP strands permeate the cortex, even occuring in the collenchyma, and commissural strands are common (Fischer 1884). The sieve tubes of the EFP system differ in morphology from those of the fascicular phloem (FP), although they may be similar to cells in the peripheral part of the latter (Crafts 1932).
When the plant is damaged, the copious phloem exudate comes largely from the EFP, any flow from the FP being blocked by callose almost immediately. The composition of the exudate from EFP and FP is very different: FP exudate is rich in sugars and unidentified proteins, etc., EFP exudate contains P-proteins, amino acids, protein synthesizing machinery, and various secondary metabolites, but little sugar (L.-B. Zhang et al. 2010). Zhang et al. (2012) found that phloem exudate came from different parts of the system in different species, although the completely extrafascicular sieve tubes were not involved. Not much of the exudate was from the FP itself, and they thought that one of the functions of the P-protein in EFP exudate might even be to block the flow of water from the cut xylem as it congealed, although initially the water helped make large droplets of noxious (the exudate contains cucurbitacins - see below) and sticky exudate (Gaupels & Ghirado 2013). There is substantial evidence (Gaupels et al. 2012; see also Turgeon & Oparka 2010) that the EFP system is involved more in plant defence, gumming up insects' mouthparts (e.g. McCloud et al. 1995), and Gaupels et al. (2012) even thought that ecologically EFP exudate was like latex (see also Dussourd in Tallamy 1985; Konno 2011; Gaupels & Ghirado 2013; see also below). Interestingly, aphids feed on the abaxial phloem, and autoradiograms of minor veins suggest that abaxial phloem cells here are the sole conduits for carbon export and import, indeed, in the very finest veins there is no adaxial phloem system at all, that is, the bundles there are collateral (Botha & Evert 1978; Schmitz et al. 1987; Hebeler 2000 and references). More needs to be done to understand this complex vascular system, especially that of the basal clades, and to work out further details of how the EFP is involved in plant defence, and how it links with the adaxial intrafascicular system. Note that much of the earlier work on phloem and phloem transport in Cucurbitaceae - because of the copious exudate, this had seemed to be an ideal system and has been much studied - actually has been carried out on the EFP (Zhang et al. 2010).
CAM photosynthesis occurs in the stems of Seyrigia humbertii (Cuc-Coniandreae) (references in Newton & Eggli 2023).
Pollination Biology & Seed Dispersal. There are a number of interesting pollinator-plant interactions in the family. In general, carpelate flowers produce no nectar and the style with its yellow, branching stigmas allows them to mimic nectar-producing staminate flowers (Renner & Schaefer 2016), although in taxa like Cucurbita pepo both kinds of flowers produce nectar (Nocentini et al. 2015). Heliconius butterflies, whose caterpillars eat Passiflora vines, are also closely associated with Psiguria (Coniandreae). Theae butterflies are practically unique in Lepidoptera for eating pollen; they are trapliners with good spatial memory (associated with the large mushroom bodfies they have in their brains) and pollinate the plant while at the same time obtaining nutrients from the pollen, probably by enzymatic activity of the saliva; the butterflies also visit some species of the closely related Gurania (e.g. Gilbert 1972, 1975; Boggs et al. 1981; Spencer 1988; Eberhard et al. 2009; F. J. Young & Montgomery 2020: see Steele 2010 for a summary and Steele et al. 2010 for a phylogeny of Psiguria). Interestingly, both Psiguria and Gurania have pollen grains in tetrads, a very uncommon feature in the family (Steele 2010). Psiguria in particular has very long-lived staminate inflorescences, the flowers being produced over a period of months or more - and Heliconius are also notably long-lived. Other Cucurbitaceae, notably Momordica (Momordiceae) and Thladiantha (Thladiantheae), but alsoSiraitia (Siraitieae) and Telfairea (Joliffieae), are oil flowers, the ca 19 extant species of Ctenoplectra bees being associated with Cucurbitaceae and collecting material from the multicellular oil-secreting hairs, as well as pollen and nectar (Buchmann 1987; Vogel 1981b, 1990, 1998c: variation in nectary morphology and secretion; Renner & Schaefer 2010; Possobom & Machado 2017a; Tölke et al. 2019 and references); stem ages of (55-)46(-40) Ma and crown ages of (34-)22(-12) Ma have been suggested (Renner & Schaefer 2010). These bees are an Old World group; they may have diverged (Eucerini sister, but within Ctenoplectrini Ctenoplectrina is a kleptoparasite) in the early Eocene ca 50 Ma and now probably pollinate over 100 species of the family - oil flowers seem to have evolved once and been lost several times, but the bees have not reverted (Schaefer & Renner 2008b; Renner & Schaefer 2010). Although Ctenoplectra bees visit Momordica, carpelate flowers in some species lack rewards even if in others they do produce oil (Schaefer & Renner 2010; Possobom & Machado 2017a and references), deceit pollination? (Ctenoplectra is probably sister to Tetrapedia-Xylocopinae (Bossert et al. 2018).) For pollination by solitary bees, see also Dötterl and Vereecken (2010).
Squash and gourd bees, some 20 species of the genera Peponapis and Xenoglossa, pollinate only species of Cucurbita. They feed early in the day, even flying in the dark pre-dawn hours (Hurd et al. 1971). They are attracted by particular floral volatiles, repelled by others which attract the cucumber beetles, while yet others seem to attract both herbivore and pollinator (Andrews et al. 2007). The bees, restricted to the Americas north of northern Peru, show some species-specific variation in pollen collecting devices (Hurd & Linsley 1964); Cucurbita can clearly be pollinated by a variety of bees since it is cultivated pretty much all over the world; for the production of sucrose in the nectar, coming in part directly from the phloem (note that stachyose is the main sugar there), see Solhaug et al. (2019: not integrated with phloem variation). A number of taxa in Sicyoeae have elaborately fringed margins of their corolla lobes, while Momordica anigosantha has quite strongly monosymmetric staminate flowers, both in form and especially colour patterning, yet the carpelate flowers are less remarkable (Zimmermann 1922).
Cayaponia is one of the few genera that is pollinated by bats in both the Old and New Worlds, and there have been shifts from bat to bee pollination, uncommon elsewhere in flowering plants (Duchen & Renner 2010). For other widely-distributed bat-pollinated plants, see Ceiba, also Mucuna (Moura et al. 2015, de Moura et al. 2016) and Parkia, both Fabaceae, and in the latter a small clade of Brazilian species has switched to being pollinated by bees and Thysanoptera (Conceição Oliveira et al. 2021b).
Breeding systems in Cucurbitaceae (Cucurbitoideae are discussed here) can be very labile, with multiple reversals from dioecy to monoecy likely in Momordica (Momordiceae) alone (Schaefer & Renner 2010; see also Kocyan et al. 2007; Volz & Renner 2008). Determination of the sex of the flower by environmental cues is known in plants of Gurania and Psiguria (Coniandreae). The plants, previously thought to be dioecious, were found to be male when young, but they switched to being female when they became large - but they switched back if conditions become unfavourable (Condon & Gilbert 1988; also Renner et al. 2007b; Newton & Eggli 2023). Sex expression in Momordica depends on a single factor (Schaefer & Renner 2010), while Coccinia grandis (Benincaseae) has XY chromosomal sex determination (references in Holstein 2015); Westergaard (1958) gives some other examples, and see also Henry et al. (2018) for sex determination in Cucumis (Benincaseae). Boualem et al. (2015) discuss the molecular control in the transition from monoecy to dioecy (see also Chuck 2010). Bertin and Newman (1993) considered the family to be interflorally protandrous, while Bentley et al. (2004) thought that it was protogynous; the latter are correct.
Fruits (and seeds) in Cucurbitaceae are very variable. Although the ovary is inferior, some taxa have truly capsular fruit, dehiscence being at the very apex (Schaefer & Renner 2011). In the squirting cucumber (Ecballium elaterium) the fruit becomes detached from its pedicel when ripe, the contents of the fruit, under pressure, squirt out of the hole where the pedicel was (Ingold 1939); see also Echinocystis (Shamrov and Anisimova (2023). Large mammals, relatively unaffected by the bitter cucurbitacins commonly found in the fruits (see below), have been implicated in the dispersal of cucurbitaceous seeds; the megafauna probably involved was in places (the New World) where they are now extinct (Kistler et al. 2015). Myxospermy (mucilaginous seeds) is reported from a number of Cucurbitoideae (Grubert 1974); a number of taxa in the other subfamilies have seeds with papery wings. Telfairea, with descent fruits, is described as having numerous seeds each with an endocarpic fibrous sheath (Schaefer & Renner 2011), which seems a little odd.
Plant-Animal Interactions. Low concentrations of the very bitter cucurbitacins, tetracyclic sterol-like triterpenes that are among the most bitter substances known to humans, elicit a compulsive feeding response from luperine beetles, including rootworm leaf beetles (Chrysomelidae-Galerucinae-Luperini: see Metcalf et al. 1980; Jolivet & Hawkeswood 1995). The ability of galerucine larvae to eat Cucurbitaceae may have evolved independently in Old and New World members, Aulacophorina and Diabroticina respectively (Gillespie et al. 2003); some chrysomelids eating Cucurbitaceae even sequester the cucurbitacins (Opitz & Müller 2009). Tallamy (1985) initially thought that feeding induced curcurbitacin production, trenching being defensive behaviour by the squash beetle, the coccinellid Epilachna borealis. However, at least some of these beetles will eat cucurbitacin crystals neat, so physical avoidance of the copious sap produced by Cucurbitaceae is a more likely explanation of this feeding behaviour. Indeed, the concentration of cucurbitacins inside and outside the trench is about the same, but the sap is very rich in P-protein and eventually gels and so would probably thoroughly gum up the mouth parts, etc., of the beetle larvae if they ate untrenched leaves (McCloud et al. 1995; see also below). Trenching by the insect depended on the turgor of the plant and probably was a defence against these phloem exudates (McCloud et al. 1995). Gillespie et al. (2003, 2008) outline the phylogeny of some cucurbitacin-tolerant root-eating chrysomelid beetles. 80% of the host plant records of the group are from Cucurbitaceae, and many species are pharmacophagous. That is, adults visit the flowers, feeding on pollen and sometimes other parts of the plant, and they sequester these bitter cucurbitacins (Eben 1999; Tallamy et al. 2005). The beetles are attracted by volatiles coming both from flowers and other parts of the plant (Andrews et al. 2007). Smaller mammals may find cucurbitaceous fruits to be toxic, but they also have more genes encoding bitter taste receptors to help avoid such fruits than do larger mammals (Kistler et al. 2015; X. Wang et al. 2017 and references for effects of cucurbitacins).
The glandular hairs of Cucurbitaceae are another element of the defence of the plant against herbivores, quickly-solidifying secretions being produced when the trichomes are touched by insects (Kellogg et al. 2002). Discharge of sap by these hairs may be explosive, Zimmermann (1922) mentioning what he called "Explosionshaare" in the family.
Larvae of a remarkable number of species of Blepharoneura (Diptera-Tephritidae fruit flies) are being discovered in flowers, fruits, seeds, etc. of Neotropical Cucurbitoideae, perhaps especially on Guraniinae-Gurania (Winkler et al. 2018). Some species are specific to staminate flowers, others to carpelate; all told some 52 species of flies were found on 24 species of cucurbits, and several species of fly may be found on the same part of the cucurbit species in the one locality (Condon et al. 2008; Steele 2010). It is estimated that there are around 200 species of Blepharoneura, all likely to be restricted to Cucurbitaceae, and often one particular part of the plant of one species, even distinguishing between male and female flowers (Norrbom & Condon 2010; Winkler et al. 2018) - perhaps the whole subfamily is. Thus 14 species of these flies, on which there were 18 species of parasitoid wasps, were found in one site in Peru; they depended on two species of Gurania (Condon et al. 2014; see also Ottens et al. 2017). Host shifts are uncommon, interestingly, the parasitoid braconid wasp Opius subgenus Bellopius seems yet more sensitive to plant identity and even sex of the flower than Blepharoneura - and each species of the wasp is restricted to a single species of Blepharoneura (Winkler et al. 2018). Parasite diversification in this system may have started ca 13 Ma (Winkler et al. 2018).
Butterfly caterpillars are not often to be found on members of this family (Ehrlich & Raven 1964).
Vegetative Variation. The tendrils of Cucurbitaceae, variously branched, represent a branch complex (Gerrath et al. 2008). The length of the unbranched part of the tendril varies considerably, and the tendril may be ad- or abaxially curved in bud (Zimmermann 1922). Often there is a sublateral tendril + bud + slightly lateral flower associated with each leaf, or a tendril + vegetative bud + carpelate flower + staminate inflorescence, all more or less collaterally arranged, or other variants. Eichler (1875) and Goebel (1932) suggested that the tendril branches were prophylls, and in Bryonia dioica there are paired tendrils on the pedicel of an axillary flower (see also Kumazawa 1964; Zitnak et al. 2010). Schaefer and Renner (2011a: Fig. 21) illustrated three main kinds of tendrils: Tendrils that twined below the branching point (the zanonioid tendril, found in more basal clades); un- or once-branched tendrils, but only the tip of the branch and that of the tip of the main axis twining; or 3- or more branched tendrils, and again, it is the tips alone of the tendrils that twine. Given the variation patterns described by Schaefer and Renner (2011), their latter two types are combined in the characterisations, where tendrils are either "tendrils with basal twining" or "tendrils with apical twining alone". Tendrils are not infrequently absent. They are sometimes paired spiny structures at the nodes, as in Acanthosicyos horridus - technically thorns - and there the leaves proper are very much reduced. In female-phase inflorescences of Gurania spinulosa the tendrils become reduced and non-functional (Condon & Gilbert 1988). Non-flowering Zanonioideae have tendrils that are branch structures more or less lateral to vegetative axillary buds. When flowering finally begins, tendrils are replaced by more or less axillary flowers. The axillary bud produces an inflorescence branch that has an internode below the prophyllar leaf that subtends the first flower. In one interpretation the inflorescence branch lacks a basal internode, so the first flower, often carpelate, arises in the leaf axil of the main branch and is subtended by a prophyll; the staminate inflorescence represents the development of this prophyllar bud (Lassnig 1997; axillary structures may be collateral in the vegetative part of the plant, sometimes superposed in the reproductive part). However, Gerrath et al. (2008) found that in Echinocystis lobata (Sicyoeae) the tendril, axillary bud, carpelate flower, and staminate inflorescence were all more or less independent in origin, although the latter two did arise from a common primordium (see also Zitnak et al. 2010). Most Cucurbitoideae lack an initial prolonged vegetative period. For the molecular control of tendril development, see Sousa-Baena et al. (2018a, b) and references; Joliffieae may be critical in understanding the evolution of the branch-tendril complex.
Genes & Genomes. For the cucurbit-common tetraploidy (CCT) event, see the Cucurbitaceae ... Begoniaceae] node above. There seems to have been another tetraploidy event (involving allotetrapoloidy), the Cucurbita specific tetraploidy (CST) event; this has been dated to 28-25 Ma (J. Wang et al. 2022). Wang et al. (2022) also suggested that the [Benincaseae + Cucurbiteae] clade had 15 protochromosomes.
D. Xie et al. (2019) emphasized the variability in genome size of the few Cucurbitaceae known, that of Benincasa hispida, at 913 Mb, being over twice the size of those of other species; this seems to be due to the massively increased number of copia and gypsy long terminal repeat retrotransposons the increase of which peaked at 8-9 ma, interestingly, the (smaller) increase of these LTRs in Cucumis is much more recent, even ongoing. Xie et al. (2019) also suggest a base chromosome number for within Cucurbitoideae.
In Cucumis, at least, mitochondria (but not chloroplasts) are transmitted paternally (Havey et al. 1998). The mitochondrial genome is very variable in size, being from ca 379,000-2,900,000 bp long, the shorter sequences, at least, having expanded by the acquisition of chloroplast sequences and the accumulation of numerous short repeats (Alverson et al. 2010). The genome in Cucumis is very large and is organised as a number of circular chromosomes (Alverson et al. 2011), its large size in C. melo being in part attained by the accumulation of repetitive sequences (ca 42%) and DNA of nuclear origin (47%) (Rodríquez-Moreno et al. 2011.
Economic Importance. Cucurbitaceae were particularly important in early agriculture in the Americas, being one of the triumvirate of foods - squash, corn and beans. For a discussion of various aspects of the history of cultivation of Lagenaria and Cucurbita in particular, see Teppner (2004). For the domestication of squash (Cucurbita spp., inc. C. moschata and C. agyrosperma) which began ca 10,000 years ago, see Dillehay et al. (2007), Piperno et al. (2009) and Ranere et al. (2009); Kates et al. (2017) provide the background to ideas about domestication by looking at relationships within the genus using 44 nuclear loci - instructive. Sebastian et al. (2010) suggest that the relatives of cucumber and melon (Cucumis) are Asian-Australian. Kistler et al. (2014) proposed that the pre-Columbian global distribution of the bottle gourd, Lagenaria siceraria, was in part caused by gourds drifting across the Atlantic from Africa to South America; seeds can remain viable for about a year in sea water, and simulations suggested that gourds could drift across the Atlantic between the equator and 20oS in less than this time. For the origin of watermelons (Citrullus), bedevilled by misidentifications in the past, see Chomicki and Renner (2014). See also Chomicki et al. (2019a) for a comprehensive summary and also papers in Grumet et al. (2016).
Grafting is often involved in the cultivation of the water melon, Citrullus lanatus (stock include Cucurbita spp., Lagenaria siceraria), melon, Cucumis melo, cucumber, Cucumis sativus, etc.; protection of the plant against soil-borne diseases in particular is a common goal (A. R. Davis et al. 2008; Kyriacos et al. 2017).
Chemistry, Morphology, etc.. Cucurbitaceae produce phytoalexins only with difficulty (Harborne 1999). Raffinose is the main transport carbohydrate (Turgeon & Ayre 2005). Distinctive long-chain fatty acids occur in the seed oils; eleostearic acid, an isomer of punicic acid, is restricted to Joliffieae (Hopkins 1990). There are crystalloid inclusions in the protein bodies found in embryos of this family, perhaps unusual for flowering plants (Lott 1981). Phytoliths are commonly produced by members of this family (Piperno 2006).
A number of African Cucurbitaceae have swollen stem bases/roots (see descriptions above). The ring of fibres in the stems of Cucurbita, at least, has nothing to do with the vascular bundles (Blyth 1958). Vascularisation of the leaf is complex, e.g. the leaf being supplied by one of the outer ring of bundles in its entirety and by branches from two other bundles of the outer ring, the bud being supplied by the inner ring (Sensarma 1955). Indeed, as the literature summarized by Sensarma (1955) suggests, there has been much speculation about the nature of the cauline vascular system and, related to this and based on how leaves are supplied from the cauline system, whether from the inner or inner + outer rings of bundles, about the presumed cauline versus foliar nature of the tendrils. For the interxylary cambia of Coccinia, see Pace et al. (2018). Xerosicyos is a woody, succulent-leaved vine.
Flowers in taxa in which the androecium has two pairs of stamens and a single stamen, four stamens and a staminode (Gerrardanthus), etc., are strictly speaking monosymmetric. The petals of Xerosicyos are free; those of Echinocystis and Lagenaria at least have several traces, in the former arising from branches from the single K bundle (Miller 1929). Miller (1929) thought that the androecium in Echinocystis consisted of A 2 fused, from different whorls, plus A 1; the morphology was clearer in the staminodes of the carpelate flower. When the stamens are connate 2 + 2 + 1, the vascular supply shows evidence of this, although there are differences over the interpretation of the apparently bithecal stamens (e.g. de Wilde & Duyfjes 1999); Schaefer and Renner (2011b; see also Renner & Schaefer 2016) suggest that the plesiomorphic condition of the family is to have five bithecal stamens. Several pollen tubes may arise from the one grain, i.e., the pollen is polysiphonous, and some Cucurbiteae have very large almost spherical grains up to 200 µm or so long and across. The carpelate flower may have two rings of what can be interpreted as rudimentary anthers, while in the staminate flowers a ring of processes may alternate with the stamens. The nucellar beak is very long and in some species it directly abuts placental tissue; depending on the species, the pollen-tube may swell up considerably there (Lizarazu & Pozner 2014). The chalazal endosperm haustorium of Sechium [= Sicyos] edule, at up to 19,000 µm long, is apparently the longest in the family, although others are also quite long; only some Loranthaceae have perhaps comparable structures in their embryos (Chopra & Seth 1990; Mikesell 1990; Johri et al. 1992). The seed coat is testal, and its anatomy is very complex; although D. S. Singh and Dathan (1973) describe the aerenchyma and chlorenchyma separating from the rest and forming the tegmen, this is incorrect (c.f. e.g. Singh & Dathan 1980). Seedlings commonly have a peg, a cortical outgrowth on the lower side of the seedling at the hypocotyl/radicle transition (e.g. Klebs 1884 for a list of taxa) and that appears to be a response to gravity (Takahashi & Scott 1994); this is not found in species in which germination is hypogeal (Zimmermann 1922).
For additional general information, see Zimmermann (1922), Jeffrey (1980), Bates et al. (1990), Jeffrey and de Wilde (2006), Pandey et al. (2014b: summary of embryology and seeds, etc.), Renner and Schaefer (2016) and especially Schaefer and Renner (2011a) and Schaefer (2020), and Newton and Eggli (2023: succulent taxa; for non-protein amino acids, see Fowden (1990), for cork cambium, see Dittmer and Roser (1963), for wood anatomy and secondary thickening, the latter odd, see Carlquist (1992c) and Patil et al. (2011), for floral morphology, etc., see Leins and Galle (1971) and Leins and Erbar (2010), for pollen, see Van der Ham et al. (2010: reticulate pollen derived?) and Barth et al. (2005: Cayaponia), for fruit of Echinocystis, see Shamrov and Anisimova (2023), for seed coat morphology and anatomy, Barber (1909), Kratzer (1918), B. Singh (1952, 1953), Dathan (1974), D. Singh and Dathan (1973, 1974, 1980), A. Singh and Dathan (1998, 2001), Teppner (2004), Gama-Arachchige et al. (2013: esp. water gap), Heneidak and Khalik (2015) and Kruk et al. (2022: protochlorophylls in seed coat) and for embryology, etc., Kirkwood (1905: some details about Fevillea), Warming (1913), Chopra and Agarwal (1958: endosperm haustoria), Johri and Roy Chowdhury (1957), D. Singh (1970), Dathan and Singh (1990) and Pozner (1994).
Phylogeny. Renner et al. (2002) suggested that Cucurbitoideae were probably monophyletic, with Thladiantha sister to the rest; tribes of Fevilleoideae (= Zanonioideae) formed an unresolved basal polytomy. This was largely confirmed by Kocyan et al. (2007); monophyly of Fevilleoideae was not well suppported, Alsomitra sometimes appearing as sister to Cucurbitoideae. Schaefer and Renner (2008a) also found that Fevilleoideae were paraphyletic and so did not recognise the subfamily. There may be a grade of four clades basal to a well-supported Cucurbitoideae, in which Indofevilleeae are well supported as being sister to the rest (Kocyan et al. 2007; Schaefer et al. 2009). This basal grade includes Gomphogyneae - only moderate support, Alsomitra sister to the rest of the tribe, a strongly supported Fevilleeae (= Triceratieae), Zanonieae, with 76% ML bootstrap, and a strongly supported Actinostemmateae, although relationships between these clades had no support (Schaefer et al. 2009; Schaefer & Renner 2011b). Schaefer et al. (2009) used a topology in which [Gomphogyneae [Triceratieae + Zanonieae]] were sister to the rest of the family, Actinostemma being placed within Zanonieae, in their reconstructions. H.-L. Li et al. (2015), M. Sun et al. (2016) and Z.-D. Chen et al. (2016) also provide extensive details of relationships within the family, the basal topology in the latter in particular being similar to that just discussed; see also Chomicki et al. (2019a) for relationships.
Within Cucurbitoideae, phylogenetic studies confirm that Jeffrey's tribes (Jeffrey 2005) are largely monophyletic, although his subtribes are not (Kocyan et al. 2007). Jobst et al. (1998: ITS; see also Heneidak & Khalik 2015 and references) found Benincaseae (Cucurbitoideae) to be polyphyletic; Chung et al. (2003) and Schaefer et al. (2008, esp. 2009: Alsomitra in Zanonieae) also looked at relationships within Cucurbitoideae. For smaller-scale studies within Cucurbitoideae, see Ghebretinsae et al. (2007), Wilde and Duyfjes (2006), Renner et al. (2007a), Schaefer and Renner (2010), Duchen and Renner (2010), Sebastian et al. (2010: Cucumis), Sebastian et al. (2012: Sicyos), de Boer et al. (2015: Benincaseae) and Dwivedi et al. (2018: Zehneria, but little resolution).
Classification. For the suprageneric classification above I follow Schaefer and Renner (2011b); the latter provide a comprehensive tribal classification which should be consulted for details (see also Renner & Schaefer 2016; c.f. Jeffrey 2005).
There are many small genera in Cucurbitaceae, and generic limits need attention, thus Ghebretinsae et al. (2007) had to adjust the limits of Cucumis; see also Wilde and Duyfjes (2006), Renner et al. (2007a) and Schaefer et al. (2009). Sebastian et al. (2012) included 13 of these small genera in Sicyos...
[Tetramelaceae [Datiscaceae + Begoniaceae]]: pollen spherical, stigmas elongated; fruit a septicidal capsule [dehiscing apically]; seeds with lid [operculate]; exotestal cells honeycomb, inner walls strongly thickened and lignified; cotyledons moderate in size.
Age. This node can be dated to (60-)57, 50(-47) Ma (Wilström et al. 2001) or (76-)73(-70) Ma (Schaefer et al. 2009); in both, c.f. topology, also around 62.7 or 59.7 Ma (Tank et al. 2015: Table S1, S2).
[Tetramelaceae + Begoniaceae]: The age of this clade - if it exists - is around (67-)63(-59) Ma (Schaefer et al. 2009). (85.8-)66.4, 42.7(-22.4) Ma is the date of divergence of Datiscaceae and Tetramelaceae (H.-L. Li et al. 2015: older ages prefered); (76-)73(-70) Ma is when Datiscaceae diverged from [Begoniaceae + Tetramelaceae] (Schaefer et al. 2009).
Chemistry, Morphology, etc.. Tebbitt (2005) suggests that the seeds of this group have a operculum or lid, but whether this is a synapomorphy or not is unclear. Seeds of Tetramelaceae are apparently unknown, and Boesewinkel (1984) found that the opercula of Datiscaceae and Begoniaceae were rather different.
See Mauritzon (1936b) for some details of the ovules, Clement et al. (2004) for testal morphology.
TETRAMELACEAE Airy Shaw - Back to Cucurbitales
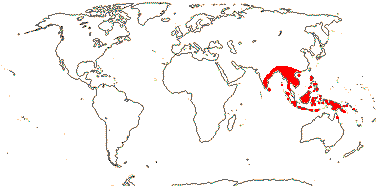
Trees, (deciduous); ?cucurbitacins, tannin 0; (wood fluorescing); (nodes with 2 traces from the lateral gaps); hairs glandular or lepidote; (lamina margins entire); plant dioecious; inflorescence spicate; K 4-8, postgenitally coherent; staminate flowers: C 0, or 6-8 [Octomeles], stamens = and opposite petals, incurved; carpelate flowers: C 0; G [3-8], (nectariferous disc on top), placentation axile, placentae bilobed [Octomeles], stigmas undivided, decurrent to clavate, multicellular papillate; fruit also opening down the sides; seed coat?; n = ca 23, x = 12 (?11).
2 [list]/2. Indo-Malesia (map: from van Steenis 1953). [Photo - Tree.]
Age. The two genera diverged (33-)26(-19) Ma (Schaefer et al. 2009).
Tetrameles wood is known fossil (as Tetramelioxylon prenudiflorum) from the Deccan Traps in India in rocks ca 70.6-65.5 Ma (L.-B. Zhang et al. 2007).
Evolution: Ecology & Physiology. The two genera are pioneer trees, and Tetrameles is deciduous, an odd combination of characters (see also Ashton 2014).
Chemistry, Morphology, etc.. Octomeles has sclereids; its capsular fruits split into two layers, the outer of which falls off.
For general information, see Swensen and Kubitzki (2011, in Datiscaceae).
[Datiscaceae + Begoniaceae]: herbs; outer integument ca 2 cells across, inner integument ca 2 cells across.
DATISCACEAE Dumortier, nom. cons. - Datisca L. - Back to Cucurbitales
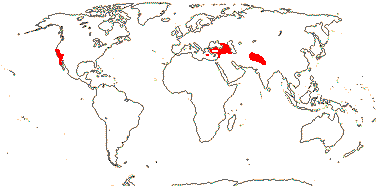
Shrub; roots with N-fixing Frankia; cambium not storied; medullary bundles +; tannin sacs +; nodes 1:3; leaves deeply divided to odd-pinnate, lamina vernation conduplicate, secondary veins ± pinnate; plant ± dioecious; inflorescence fascicles on racemose axis; flowers protogynous; P 4-10; staminate flowers: P valvate; A 6-25, outer members opposite P, filaments very short [anthers almost sessile]; pistillode 0; carpelate flowers: staminode 0; G [3-8], opposite P, styles elongated, stigma multicellular papillate; ovules with parietal tissue 3-5 cells across, cap 2-3 cells across; embryo sac bisporic, 8-nucleate [Allium type], antipodals persist; fruit septicidal?; exotegmic cells large, cuboid; endosperm slight; n = 11, x = 11, nuclear genome [1 C] (0.034-)0.736(-15.815) pg.
1 [list]/2. W. North America, Crete to India. Map: from Liston et al. (1989) and Clement et al. (2004). [Photo - Flower, Flowers.]
Age. Kapgate (2013) reported Tetrameloxylon prenudiflora from Deccan Intertrappean deposits 70.6-65.5 Ma; this should be confirmed.
Evolution: Ecology & Physiology. Nitrogen-fixation in this clade could be old, perhaps dating back to when Datiscaceae diverged from [Begoniaceae + Tetramelaceae] (Schaefer et al. 2009) - if those are the relationships.
Plant-Bacterial/Fungal Associations. Nod genes have been found in Datisca glomerate, but how they might be involved in the establishment of nitrogen fixation here is unclear (Mbengue et al. 2020).
Arbuscular mycorrrhizal associations have been reported from Datiscaceae (e.g. Rose 1980).
Chemistry, Morphology, etc.. The lid on the seeds of Datisca is not surrounded by a ring of collar cells (Boesewinkel 1984: c.f. Begoniaceae). The stamens show no particular positional relationships to the calyx (Takhtajan 1997).
Much general information is taken from Davidson (1973, 1976) and Swensen and Kubitzki (2011); see Leins and Bonnery-Brachtendorf (1977) for floral development.
BEGONIACEAE C. Agardh, nom. cons. - Back to Cucurbitales
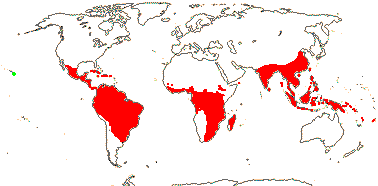
(Fleshy) herbs; quercetin 3-O-rutinoside +, plant tanniniferous, soluble oxalate accumulation; cork subepidermal; cortical (and medullary) bundles +; vessel elements also with scalariform perforation plates; nodes swollen; petiole bundles annular (central bundles +); no pericyclic sheath; sclereids and uncalcified cystoliths +; foliar epidermis with lamelloplasts [large plastids with regular lamellae] [adaxial surface] [leaves with blue iridescence] or minichloroplasts [abaxial surface], stomata anisocytic or with accessory cells in two rings [helicocytic]; hairs diverse, often prominent, flattened, pearl glands + [hairs spherical, multicellular, sessile]; leaves two-ranked, vernation laterally or vertically conduplicate/(supervolute-curved - prophylls), asymmetric, stipules +, large, cauline-extrapetiolar; inflorescence cymose, staminate flowers usu. produced first [= interfloral protandry]; K petal-like; staminate flowers: A many, centrifugal, connective enlarged, endothecial thickenings U-shaped; pollen colpate; carpelate flowers: placentation tending to be axile at base and parietal at apex, placentae large, deeply bilobed, stigmas twisted, (multicellular papillate); ovules with parietal tissue 1-2 cells across, micropyle zig-zag, endothelium +; seeds minute, with lid and surrounding collar cells; x = 13, nuclear genome [1 C] (0.126-)0.568(-2.556) pg, Begonia-common tetraploidy (BCT) event.
2 [list]/2,006. Largely tropical. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003), vol. 6. (2011) and Tebbitt (2005).
Age. The age of Hillebrandia, and hence crown-group Begoniaceae, has been estimated at 58.5-45 Ma (Clement et al. 2004, see Errata 2005), or rather younger, (35-)29(-23) (Schaefer et al. 2009) or (33.0-)24.6(-16.8) Ma (Moonlight et al. 2018); the age of [outgroup + Begonia] in L. Li et al. (2022: Fig. 1) is ca 30 Ma.
1. Hillebrandia sandwicensis Oliver
Plant ± tuberous; T 10, 2-whorled, inner [= "C"] very small; A often with branched vasculature; G [5], only partly inferior; capsule septicidal; n = 24; nuclear genome [1 C] ca 332.6 Mbp.
1/1. Hawai'i (green in the map above). [Photos - Collection, also Begonia.]
2. Begonia L.
Plant (rhizomatous/tuberous/scandent//other), (woody), (annual); flavonols and/or C-glycosylflavones +; interfascicular area parenchymatous [?level]; (lamelloplasts 0, minichloroplasts + [adaxial surface]), (water-storage hypodermis +; stomata in groups), (helicocytic); leaves (spiral/opposite), (compound/peltate/linear), (margins entire); (plant dioecious); (inflorescence racemose), (carpelate flowers first produced - sect Petermannia); staminate flowers: (flowers disymmetric), T in 2s, 2(3)4(-8); A 3-many, (basally connate), (porose), endothecium 0/with (perforate) base plates; carpelate flowers: (flowers ± di/monosymmetric); P (2-)5(-9), (connate); G [(1)2-3(-6)], placentation axile to parietal, (placentae not bilobed), styles central, (C-shaped/irregulary divided); (nucellar endothelium +); fruit a capsule, often ± strongly and often asymmetrically winged (with horns), dehiscing laterally by cracks/pore/slits in the loculi (and septicidally)/(apically) dehiscent, (baccate)/(dehiscent baccate); n = (8-)13, 14(-78), nuclear genome [1 C] 0.23-1.46 pg/245-2,497 Mbp.
1/2,144 [vi.2024 - 1,890 as of 11.v.2018, ca 30 spp. are described annually]. Largely tropical, but neither Hawai'i nor the Antipodes. Distribution: red in the map above. Photo: Flower, Fruit.
Age. Crown-group Begonia is estimated to be (29.4-)22.3(-14.9) Ma (Moonlight et al. 2015), and there are pretty similar ages, (30.4-)22.8(-16.1) Ma, in Moonlight et al. 2018) or 37.3-23.2 Ma (Clement et al. 2004, see Errata 2005), although other estimates put diversification of the genus as occurring some time between the Eocene and early Oligocene 45-25 Ma during a period of global cooling (Goodall-Copestake et al. 2009: sampling extensive). See also (31-)24(-18.2) Ma (95% HPD: Thomas et al. 2011b, focus on Malesia) or ca 25 Ma/24±3.57 Ma (L. Li et al. 2022) - an age of 23.7 Ma is also mentioned in the latter (ibid: Figs 1, 5d).
Evolution: Divergence & Distribution. The age of Hillebrandia, suggested as being anything from 58 Ma (Clement et al. 2004, errata 2005) to 24.6(-16.8) Ma (Moonlight et al. 2018), causes biogeographical problems. Clague et al. (1984), Tarduno et al. (2009), Wei et al. (2020) and others have suggested ages for the Hawaiian island chain and the adjacent Emperor sea mounts. The oldest Hawaiian island on which Hillebrandia is found is Kaua'i, which is perhaps 5 Ma old, while Niihua, the oldest, is only a little older than Kaua'i. The Emperor sea mounts trend more northerly, and its youngest members are ca 47 Ma, its oldest perhaps 80 Ma. Either Hillebrandia arrived in the Hawaiian area from some presumably continental region where it is now extinct, or it has been hopping from island to island in the Pacific for 20-50 Ma or so. The two scenarios could be combined, or Hillebrandia, and thus Begonia, may be far younger than previously thought (Renner 2005) - which would make the extensive diversification of the latter more remarkable - and if Begonia itself initially diversified in Africa (see below), as seems possible, the problems increase.
There is very strong geographical signal in the phylogeny of Begonia. The genus may have originated in Africa (e.g. Forrest & Hollingsworth 2003; Moonlight et al. 2015). Sister to the two South American and one Southeast Asian clades that include the bulk of the genus (whatever the reconstruction) are seasonally adapted African species with perennating organs (Goodall-Copestake et al. 2010). Moonlight et al. (2018) also found an African grade at the base of the genus and African sections are sister to both New World clades, however, the Socotran sect. Peltaugustia is embedded within the Asian clade of Begonia (see also Rajbhandary et al. 2011; L. Li et al. 2022) - although support for its position is not strong. Overall, diversity of Begonia in Africa is about a third of than in the American or the South-East Asia to Malesian regions; interestingly, all but one of the some 50 or so species from Madagascar (including the Comoros and Mascarenes), members of a single clade, are endemic to the islands (Plana 2003; Plana et al. 2004). Endemism in the cloud forests of the North Andean region is notably high (Hughes et al. 2016; Jara-Muñoz et al. 2019). Thomas et al. (2011b) found that there had been several invasions of Malesia by Begonia, including one that is represented by members placed in four sections, and subsequently generally west-to-east movement (see also Rajbhandary et al. 2011). The main clade of Malesian species includes members of four sections, including the speciose section Petermannia, with at least 270 species; the age of that whole clade was (16.5-)11.5(-6.6) Ma (95% HPD) (Thomas et al. 2011b). Sections in Begonia are generally limited to single continents, but the recently described B. afromigrata, from Thailand and Laos but in sect. Tetraphila, otherwise known only from Africa, is an exception (de Wilde et al. 2011). Chinese species of limestone-inhabiting Begonia form a single clade, despite a variety of sectional assignments, and they diversified (11.7-)8.4(-5.3) Ma, perhaps 2 Ma after the origin of the stem group of this clade (Chung et al. 2012). Moonlight et al. (2015) discuss the evolution of the Neotropical species, derived from two separate origins from Africa and much subsequent intracontinental movement.
Hughes and Hollingsworth (2008) suggested that the dearth of widespread species in Begonia is due in part to the low levels of gene flow (as evident in the few studies that have been carried out) and hence for the propensity of divergence in allopatry (see also Twyford et al. 2015a: strong reproductive barriers). De Wilde et al. (2011) drew attention to the broader distributions of those species of Begonia that had fleshy fruits compared to the narrower distributions of species with dry fruits, despite the fact that these latter species had minute seeds and perhaps might be supposed to be dispersed easily by wind. They noted that B. afromigrata seemed to have got to southeast Asia from Africa by long distance dispersal (de Wilde et al. 2011).
Hillebrandia has a number of perhaps plesiomorphic features, and some of the features we think of as being characteristic of Begoniaceae as a whole (style position, fruit dehiscence) may in fact be apomorphies for Begonia in particular. Note, however, that the interpretation of the flowers of Hillebrandia is not straightforward, there being extensive variation in the number, position and anatomy of the parts (J.-r. Wang & Ronse De Craene 2025). Begoniaceae are somewhat unusual among monoecious taxa with cymose inflorescences in that the first flowers produced in the inflorescence are staminate, carpelate flowers being produced only later. The Symbegonia group (= sect. Petermannia) has inflorescences with the reverse arrangement, the often single female flower being basal (de Wilde 2011; Gagul et al. 2018), as does section Petermannia as a whole. There are also similarities in perianth number between the two (staminate flowers P 2, carpelate flowers P 5), and in some Petermannia the corolla is partly fused (Forrest & Hollingsworth 2003) - such species are phylogenetically close to the erstwhile Symbegonia in Moonlight et al. (2018), anther filaments in the Symbegonia group are ± connate (Gagul et al. 2018) and the endothecium in Petermannia and one species of Symbegonia has perforated base plates (the other species of Symbegonia lack an endothecium: Tebbitt & MacIver 1999).
Subsequent to the genome duplication here (see Genes & Genomes below) there has been elaboration of the genes involved in anthocyanin production (L. Li et al. 2022), which is perhaps connected with some of the distinctive colouration patterns of the leaves in many taxa. Interestingly, however, there has been contraction of the gene families involved in pathogen resistance and defence. There is adaptation to shady conditions evident in both photoreceptor and light-harvesting genes, the genes concerned being involved in the whole genome duplication and also being produced by tandem duplications (Li et al. 2022).
Ecology & Physiology. Begonia is predominantly a plant of shady habitats, and the great diversity of colours, colour patterns and surface textures of the leaves that it shows are the marks of a group that grows in such places (Gould & Lee 1996; Jacobs et al. 2016 and references), and they are particularly striking here. Indeed, J.-H. Zhang et al. (2020, q.v. for classification of variegation types) estimated that there were around 220 species of Begonia with variegated leaves, more than in any other family of comparable size. Variegation of the blade is often caused by the distribution and nature of air spaces in the leaf, and this may well have no effect on photosynthesis (Sheue et al. 2012). Overall, there is lower total chlorophyll content and lower ratios of chlorophyll a to b, as is common in plants of shaded conditions (L. Li et al. 2022). A striking blue iridescence in some species is caused by the rearrangement of the thylakoids in chloroplasts (= iridoplasts, lamelloplasts) in the adaxial epidermis (but not in all species with such lamelloplasts). This allows them to capture light at green wavelengths, abundant in shady conditions, and also increases the quantum yield, overall substantially boosting photosynthetic efficiency (Jacobs et al. 2016; see Pao et al. 2018 for other hypotheses). These lamelloplasts, with thylakoids regularly arranged in the stroma, occur in the adaxial epidermal cells of some species (including Hillebrandia), while minichloroplasts, with normal grana-stroma structure, are to be found in the abaxial epidermis and in adaxial epidermal cells if there are no lamelloplasts (Pao et al. 2018, see also Gould & Lee 1996; Lee 1997 for more on iridescence and Beltrán et al. 2018 for other functional specializations of chloroplasts).
For the diversity of growth forms and their evolution in Begonia, see Kidner et al. (2015), secondary woodiness has evolved several times, probably in double figures. In Borneo, at least, the conditions in places where Begonia grows are probably rather stressful (montane; over ultramafic rocks); see also elsewhere.
Pollination Biology & Seed Dispersal. Rather little is known about pollination and seed dispersal in Begonia, despite its horticultural popularity. Staminate flowers of Begoniaceae produce pollen, carpelate flowers usually have no reward at all, but usually have yellow stigmas that look like anthers; deceit pollination is probably involved (e.g. Schemske et al. 1996; Le Corff et al. 1998; de Wilde 2001; Wyatt & Sazima 2011 and references). Pollen is collected from staminate flowers, and buzz pollination is often involved (Wyatt & Sazima 2011: Brazilian species; see also Russell et al. 2017). There are a few ornithophilous species with nectaries at the base of the styles in carpelate flowers only, others have no reward at all; various levels of deceit/Batesian mimicry are also involved, in such cases it is male flowers that perhaps mimick female flowers... (Vogel 1998c; Renner 2006; Jara-Muñoz et al. 2019). For the evolution of breeding systems in the genus, see Goldberg et al. (2017).
Tebbit et al. (2006) looked at the evolution of dispersal mechanisms in the speciose Southeast Asian Begonia; within a clade, taxa with animal or rain-ballist dispersal predominate, the latter mechanism being derived from wind dispersal (see also Rajbhandary et al. 2011). Splash-cup dispersal is also known (e.g. Jara-Muñoz et al. 2019). The spreading lobes of the fruit of B. tricuspidata open individually and it appears to be made up of three follicles. De Wilde (2011) discussed possible dispersal mechanisms in detail, and de Wilde et al. (2011) focussed on seed dispersal of fleshy-fruited members of the genus, which seem to have wider distributions than their dry-fruited congeners (see also de Lange & Bouman 1992 for seed morphology and disperal in African taxa).
Animal-Plant Interactions. Butterfly caterpillars are not often found on Begoniaceae (Ehrlich & Raven 1964).
Vegetative Variation. For the distinctive wood anatomy, species being secondarily woody, see Kidner et al. (2015).
Begonia is noted for the extent of variation in leaf morphology and the colours and colour patterns on its leaves (for the latter, see above), making it popular in horticulture. The lamina surface can be quite smooth, minutely bullate, or with almost mountainous up-pushings of the lower surface that convert to spines on the upper surface (B. ferox). Barabé et al. (1992) discuss the development of the distinctively asymmetrical laminas of the family; Martinez et al. (2016) noted that the leaf bases were mirror-asymmetrical, with auxin concentrations and leaf development as predicted by theory, rather than directionally asymmetrical, i.e. with all leaves asymmetrical in the same way, as in taxa with spiral phyllotaxis. There are various intermediates between trichomes, leaf teeth, and leaf-like appendages on the leaf, and some taxa have epiphyllous inflorescences (Dickinson 1978 for references). The leaf teeth may be associated with extrafloral nectaries (Rios et al. 2020) and they are supplied by several veins. For clustered stomata, see Hoover (1986), Peterson et al. (2010) and Rudall et al. (2017b: also helicocytic stomata), the stomatal clusters share a common substomatal cavity.
There are various forms of vegetative reproduction in Begonia. These include "gemmae" borne in groups in cup-shaped structures (splash cup dispersal) in B. gemmipara (sect. Putzeysia). These structures are themselves borne in groups and apparently develop in the axils of stipules. Horticulturalists can sometimes grow new plants by cutting the midrib/main veins tranversely and placing the leaves on damp soil, as in the B. rex group; plantlets develop quite quickly. In B. phyllomaniaca plants develop at the petiole-lamina junction or on the lamina itself; roots can develop from the leaf epidermis in Begonia (Rudall 2020).
Genes & Genomes. There may have been a genome duplication somewhere near the base of the Begonia clade some 22 Ma or more (Brennan et al. 2012); L. Li et al. (2022) dated this duplication to (43-)35(-27) Ma, and before the Hillebrandia-Begonia split, which makes a direct connection between this genome duplication and the subsequent diversification of Begonia a bit problematical. J. Wang et al. (2022) dated this duplication, the Begonia-common tetraploidy (BCT) event, to 43-38 Ma, and dates are 35±8.5 Ma in L. Li et al. (2022). Furthermore, Wang et al. (2022) thought that the ancestral karyotype for Begoniaceae was x = 16, which after the duplication and subsequent fusions, etc., became x = 21; Li et al. (2022) talk about 22 conserved ancestral regions.
Dewitte et al. (e.g. 2010a, b, esp. 2011) discuss cytological variation in Begonia, where there is extensive variation in chromosome number, size, structure, etc.. The base chromosome number may be 10-13, but these are perhaps polyploid numbers, or 6-7; polyploidy is associated with the production of unreduced gametes. Genome size varies by a factor of six to ten or so, and is not correlated with chromosome number (see also Campos-Domínguez 2020: also chromosome numbers, genome sizes, etc.); mean chromosome size varies at least 12-fold in the genus; prominent secondary constrictions in chromosomes are common; and so on. Genome size may link with transposable element insertion, and transposable element insertion in promoter regions may well be under selection (Li et al. 2022). For hybridization between species with very different leaf morphologies, etc., see Dewitte et al. (2011), and for that between African taxa, see Li et al. (2022). Horticultural hybridisation within Begonia has been extensive.
Chemistry, Morphology, etc.. There are five small orange inner perianth parts (= "petals") in Hillebrandia that are very different from the large white outer perianth members (= "sepals"); the stamens are also orange... (Gauthier & Arros 1963). It has been suggested that the perianth of Begonia is to be compared with the sepals of Hillebrandia (see Gauthier 1959), and also that the petals of Hillebrandia are staminodial (Ronse Decraene & Smets 1990a, comparison with Papaveraceae), which they are in colour but not in position. The plesiomorphic tepal number of Begonia may be four in staminate flowers (a single whorl, c.f. Garcinia, or two bimerous whorls?) and five in carpelate flowers (Forrest et al. 2005). Not surprisingly, symmetry types of flowers is various (e.g. Reyes et al. 2016). De Wilde (2011 and references) noted the variation in vascular supply to members of the perianth, while the vascular bundle to the stamen may be branched (Gauthier 1963). There is a variety of endothecial thickenings in the family, and some taxa with porose anthers have an endothecium (Tebbitt & MacIver 1999). The stigmas are described as being antisepalous (Davidson 1973); any style is at most short. For gynoecial morphology and anatomy, see Jin and Wang (1994).
For general information, see Sandt (1921), de Wilde (2011), and Smith et al. (1986), Golding and Wasshausen (2002), Tebbitt (2005: more horticultural) and Hughes et al. (2015 onwards: http://padme.rbge.org.uk/begonia/page/home), also Eggli (2023a: succulent taxa); for flavonols and C-glycosylflavones, see Iwashina et al. (2020: 127 species), for anatomy, see Lee (1974) and Cuerrier et al. (1991: leaf), for floral development, see Charpentier et al. (1989), for ovules, Boesewinkel and de Lange (1983) and Chandrasekhara Naidu (1985), for placentation, de Wilde and Arends (1989) and Jin and Wang (1994), and for seed morphology, see de Lange and Bouman (1999 and references).
Phylogeny. Hillebrandia, from Hawai'i, is sister to Begonia as a whole, including Symbegonia (Clement et al. 2001, esp. 2004; Swensen et al. 2001; etc.), and although H.-L. Li et al. (2015) found that Symbegonia sanguinea was sister to [Hillebrandia + B. poculifera], only these three species were included.
Early studies examining phylogenetic relationships in Begonia include Plana et al. (2004), Forrest and Hollingsworth (2003) and Forrest et al. (2005). Goodall-Copestake et al. (2010) found substantial incongruence when comparing chloroplast and mitchondrial trees, while Thomas et al. (2011a; see also Dewitte et al. 2011), with a focus on Asian Begonia, emphasized that several sections were para- or polyphyletic. In the extensive chloroplast analysis of Moonlight et al. (2018) there was good support for nodes along the backbone of the tree in particular, although relationships between (and within) sections were often less clear; there was still sectional paraphyly here. L. Li et al. (2022: nuclear data) noted that there was evidence of hybridization/introgression in the African taxa in particular in their analysis, but there was conflict between nuclear and chloroplast trees (ibid.: Fig. 5c), for instance. Jara-Muñoz et al. (2019) looked at relationships in section Casparya (including section Semibegoniella).
Classification. For a sectional classification, etc., of Begonia, see Doorenbos et al. (1998: descriptions) and Moonlight et al. (2018 and references: 70 sections, mostly no descriptions); sections in the latter may be para- or polyphyletic, so read the tree first. For comments on Begonia and its classification, see Muñoz-Rodríguez et al. (2023); as these authors note, not all species are included in the sections currently recognized.
Botanical Trivia. The 39 genera described by Klotzsch and all now synonymized under Begonia must be close to a record.