EMBRYOPSIDA Pirani & Prado
Gametophyte dominant, independent, multicellular, initially ±globular, not motile, branched; showing gravitropism; glycolate oxidase +, glycolate metabolism in leaf peroxisomes [glyoxysomes], acquisition of phenylalanine lysase* [PAL], flavonoid synthesis*, microbial terpene synthase-like genes +, triterpenoids produced by CYP716 enzymes, CYP73 and phenylpropanoid metabolism [development of phenolic network], xyloglucans in primary cell wall, side chains charged; plant poikilohydrous [protoplasm dessication tolerant], ectohydrous [free water outside plant physiologically important]; thalloid, leafy, with single-celled apical meristem, tissues little differentiated, rhizoids +, unicellular; chloroplasts several per cell, pyrenoids 0; centrioles/centrosomes in vegetative cells 0, microtubules with γ-tubulin along their lengths [?here], interphase microtubules form hoop-like system; metaphase spindle anastral, predictive preprophase band + [with microtubules and F-actin; where new cell wall will form], phragmoplast + [cell wall deposition centrifugal, from around the anaphase spindle], plasmodesmata +; antheridia and archegonia +, jacketed*, surficial; blepharoplast +, centrioles develop de novo, bicentriole pair coaxial, separate at midpoint, centrioles rotate, associated with basal bodies of cilia, multilayered structure + [4 layers: L1, L4, tubules; L2, L3, short vertical lamellae] (0), spline + [tubules from L1 encircling spermatid], basal body 200-250 nm long, associated with amorphous electron-dense material, microtubules in basal end lacking symmetry, stellate array of filaments in transition zone extended, axonemal cap 0 [microtubules disorganized at apex of cilium]; male gametes [spermatozoids] with a left-handed coil, cilia 2, lateral, asymmetrical; oogamy; sporophyte +*, multicellular, growth 3-dimensional*, cuticle +*, plane of first cell division transverse [with respect to long axis of archegonium/embryo sac], sporangium and upper part of seta developing from epibasal cell [towards the archegonial neck, exoscopic], with at least transient apical cell [?level], initially surrounded by and dependent on gametophyte, placental transfer cells +, in both sporophyte and gametophyte, wall ingrowths develop early; suspensor/foot +, cells at foot tip somewhat haustorial; sporangium +, single, terminal, dehiscence longitudinal; meiosis sporic, monoplastidic, MTOC [= MicroTubule Organizing Centre] associated with plastid, sporocytes 4-lobed, cytokinesis simultaneous, preceding nuclear division, quadripolar microtubule system +; wall development both centripetal and centrifugal, 1000 spores/sporangium, sporopollenin in the spore wall* laid down in association with trilamellar layers [white-line centred lamellae; tripartite lamellae]; plastid transmission maternal; nuclear genome [1C] <1.4 pg, main telomere sequence motif TTTAGGG, KNOX1 and KNOX2 [duplication] and LEAFY genes present, ethylene involved in cell elongation; chloroplast genome with close association between trnLUAA and trnFGAA genes [precursors for starch synthesis], tufA, minD, minE genes moved to nucleus; mitochondrial trnS(gcu) and trnN(guu) genes +.
Many of the bolded characters in the characterization above are apomorphies of more or less inclusive clades of streptophytes along the lineage leading to the embryophytes, not apomorphies of crown-group embryophytes per se.
All groups below are crown groups, nearly all are extant. Characters mentioned are those of the immediate common ancestor of the group, [] contains explanatory material, () features common in clade, exact status unclear.
POLYSPORANGIOPHYTA†
Sporophyte well developed, branched, branching dichotomous, potentially indeterminate; hydroids +; stomata on stem; sporangia several, terminal; spore walls not multilamellate [?here].
II. TRACHEOPHYTA / VASCULAR PLANTS
Sporophyte long lived, cells polyplastidic, photosynthetic red light response, stomata open in response to blue light; plant homoiohydrous [water content of protoplasm relatively stable]; control of leaf hydration passive; plant endohydrous [physiologically important free water inside plant]; PIN[auxin efflux facilitators]-mediated polar auxin transport; (condensed or nonhydrolyzable tannins/proanthocyanidins +); borate cross-linked rhamnogalactan II, xyloglucans with side chains uncharged [?level], in secondary walls of vascular and mechanical tissue; lignins +; roots +, often ≤1 mm across, root hairs and root cap +; stem apex multicellular [several apical initials, no tunica], with cytohistochemical zonation, plasmodesmata formation based on cell lineage; vascular development acropetal, tracheids +, in both protoxylem and metaxylem, G- and S-types; sieve cells + [nucleus degenerating]; endodermis +; stomata numerous, involved in gas exchange; leaves +, vascularized, spirally arranged, blades with mean venation density ca 1.8 mm/mm2 [to 5 mm/mm2], all epidermal cells with chloroplasts; sporangia in strobili, sporangia adaxial, columella 0; tapetum glandular; sporophyte-gametophyte junction lacking dead gametophytic cells, mucilage, ?position of transfer cells; MTOCs not associated with plastids, basal body 350-550 nm long, stellate array in transition region initially joining microtubule triplets; archegonia embedded/sunken [only neck protruding]; embryo suspensor +, shoot apex developing away from micropyle/archegonial neck [from hypobasal cell, endoscopic], root lateral with respect to the longitudinal axis of the embryo [plant homorhizic].
[MONILOPHYTA + LIGNOPHYTA]Sporophyte growth ± monopodial, branching spiral; roots endomycorrhizal [with Glomeromycota], lateral roots +, endogenous; G-type tracheids +, with scalariform-bordered pits; leaves with apical/marginal growth, venation development basipetal, growth determinate; sporangium dehiscence by a single longitudinal slit; cells polyplastidic, MTOCs diffuse, perinuclear, migratory; blepharoplasts +, paired, with electron-dense material, centrioles on periphery, male gametes multiciliate; nuclear genome [1C] 7.6-10 pg [mode]; chloroplast long single copy ca 30kb inversion [from psbM to ycf2]; mitochondrion with loss of 4 genes, absence of numerous group II introns; LITTLE ZIPPER proteins.
LIGNOPHYTA†
Sporophyte woody; stem branching axillary, buds exogenous; lateral root origin from the pericycle; cork cambium + [producing cork abaxially], vascular cambium bifacial [producing phloem abaxially and xylem adaxially].
SEED PLANTS† / SPERMATOPHYTA†
Growth of plant bipolar [plumule/stem and radicle/root independent, roots positively geotropic]; plants heterosporous; megasporangium surrounded by cupule [i.e. = unitegmic ovule, cupule = integument]; pollen lands on ovule; megaspore germination endosporic, female gametophyte initially retained on the plant, free-nuclear/syncytial to start with, walls then coming to surround the individual nuclei, process proceeding centripetally.
EXTANT SEED PLANTS
Plant evergreen; nicotinic acid metabolised to trigonelline, (cyanogenesis via tyrosine pathway); microbial terpene synthase-like genes 0; primary cell walls rich in xyloglucans and/or glucomannans, 25-30% pectin [Type I walls]; lignin chains started by monolignol dimerization [resinols common], particularly with guaiacyl and p-hydroxyphenyl [G + H] units [sinapyl units uncommon, no Maüle reaction]; roots often ≥1 mm across, stele diarch to pentarch, xylem and phloem originating on alternating radii, cork cambium deep seated, gravitropism response fast; stem apical meristem complex [with quiescent centre, etc.], plasmodesma density in SAM 1.6-6.2[mean]/μm2 [interface-specific plasmodesmatal network]; eustele +, protoxylem endarch, endodermis 0; wood homoxylous, tracheids and rays alone, tracheid/tracheid pits circular, bordered; mature sieve tube/cell lacking functioning nucleus, sieve tube plastids with starch grains; phloem fibres +; cork cambium superficial; leaf nodes 1:1, a single trace leaving the vascular sympodium; leaf vascular bundles amphicribral; guard cells the only epidermal cells with chloroplasts, stomatal pore with active opening in response to leaf hydration, control by abscisic acid, metabolic regulation of water use efficiency, etc.; branching by axillary buds, exogenous; prophylls two, lateral; leaves with petiole and lamina, development basipetal, lamina simple; sporangia borne on sporophylls; spores not dormant; microsporophylls aggregated in indeterminate cones/strobili; grains monosulcate, aperture in ana- position [distal], primexine + [involved in exine pattern formation with deposition of sporopollenin from tapetum there], exine and intine homogeneous, exine alveolar/honeycomb; ovules with parietal tissue [= crassinucellate], megaspore tetrad linear, functional megaspore single, chalazal, sporopollenin 0; gametophyte ± wholly dependent on sporophyte, development initially endosporic [apical cell 0, rhizoids 0, etc.]; male gametophyte with tube developing from distal end of grain, male gametes two, developing after pollination, with cell walls; embryo cellular ab initio, suspensor short-minute, embryonic axis straight [shoot and root at opposite ends], primary root/radicle produces taproot [= allorhizic], cotyledons 2; embryo ± dormant; chloroplast ycf2 gene in inverted repeat, trans splicing of five mitochondrial group II introns, rpl6 gene absent; ??whole nuclear genome duplication [ζ/zeta duplication event], 2C genome size (0.71-)1.99(-5.49) pg, two copies of LEAFY gene, PHY gene duplications [three - [BP [A/N + C/O]] - copies], 5.8S and 5S rDNA in separate clusters.
IID. ANGIOSPERMAE / MAGNOLIOPHYTA
Lignans, O-methyl flavonols, dihydroflavonols, triterpenoid oleanane, apigenin and/or luteolin scattered, [cyanogenesis in ANA grade?], lignin also with syringyl units common [G + S lignin, positive Maüle reaction - syringyl:guaiacyl ratio more than 2-2.5:1], hemicelluloses as xyloglucans; root cap meristem closed (open); pith relatively inconspicuous, lateral roots initiated immediately to the side of [when diarch] or opposite xylem poles; epidermis probably originating from inner layer of root cap, trichoblasts [differentiated root hair-forming cells] 0, hypodermis suberised and with Casparian strip [= exodermis]; shoot apex with tunica-corpus construction, tunica 2-layered; starch grains simple; primary cell wall mostly with pectic polysaccharides, poor in mannans; tracheid:tracheid [end wall] plates with scalariform pitting, multiseriate rays +, wood parenchyma +; sieve tubes enucleate, sieve plates with pores (0.1-)0.5-10< µm across, cytoplasm with P-proteins, not occluding pores of plate, companion cell and sieve tube from same mother cell; ?phloem loading/sugar transport; nodes 1:?; dark reversal Pfr → Pr; protoplasm dessication tolerant [plant poikilohydric]; stomata randomly oriented, brachyparacytic [ends of subsidiary cells ± level with ends of guard cells], outer stomatal ledges producing vestibule, reduction in stomatal conductance with increasing CO2 concentration; lamina formed from the primordial leaf apex, margins toothed, development of venation acropetal, overall growth ± diffuse, secondary veins pinnate, fine venation hierarchical-reticulate, (1.7-)4.1(-5.7) mm/mm2, vein endings free; flowers perfect, pedicellate, ± haplomorphic, protogynous; parts free, numbers variable, development centripetal; P = T, petal-like, each with a single trace, outer members not sharply differentiated from the others, not enclosing the floral bud; A many, filament not sharply distinguished from anther, stout, broad, with a single trace, anther introrse, tetrasporangiate, sporangia in two groups of two [dithecal], each theca dehiscing longitudinally by a common slit, ± embedded in the filament, walls with at least outer secondary parietal cells dividing, endothecium +, cells elongated at right angles to long axis of anther; tapetal cells binucleate; microspore mother cells in a block, microsporogenesis successive, walls developing by centripetal furrowing; pollen subspherical, tectum continuous or microperforate, ektexine columellate, endexine restricted to the apertural regions, thin, compact, intine in apertural areas thick, orbicules +, pollenkitt +; nectary 0; carpels present, superior, free, several, spiral, ascidiate [postgenital occlusion by secretion], stylulus at most short [shorter than ovary], hollow, cavity not lined by distinct epidermal layer, stigma ± decurrent, carinal, dry; suprastylar extragynoecial compitum +; ovules few [?1]/carpel, marginal, anatropous, bitegmic, micropyle endostomal, outer integument 2-3 cells across, often largely subdermal in origin, inner integument 2-3 cells across, often dermal in origin, parietal tissue 1-3 cells across, nucellar cap?; megasporocyte single, hypodermal, functional megaspore lacking cuticle; female gametophyte lacking chlorophyll, four-celled [one module, egg and polar nuclei sisters]; ovule not increasing in size between pollination and fertilization; pollen grains bicellular at dispersal, germinating in less than 3 hours, siphonogamy, pollen tube unbranched, growing towards the ovule, between cells, growth rate (ca 10-)80-20,000 µm h-1, tube apex of pectins, wall with callose, lumen with callose plugs, penetration of ovules via micropyle [porogamous], whole process takes ca 18 hours, distance to first ovule 1.1-2.1 mm; male gametophytes tricellular, gametes 2, lacking cell walls, ciliae 0, double fertilization +, ovules aborting unless fertilized; fruit indehiscent, P deciduous; mature seed much larger than fertilized ovule, small [<5 mm long], dry [no sarcotesta], exotestal; endosperm +, ?diploid [one polar nucleus + male gamete], cellular, development heteropolar [first division oblique, micropylar end initially with a single large cell, divisions uniseriate, chalazal cell smaller, divisions in several planes], copious, oily and/or proteinaceous, embryo short [<¼ length of seed]; plastid and mitochondrial transmission maternal; Arabidopsis-type telomeres [(TTTAGGG)n]; nuclear genome [2C] (0.57-)1.45(-3.71) [1 pg = 109 base pairs], ??whole nuclear genome duplication [ε/epsilon event]; ndhB gene 21 codons enlarged at the 5' end, single copy of LEAFY and RPB2 gene, knox genes extensively duplicated [A1-A4], AP1/FUL gene, palaeo AP3 and PI genes [paralogous B-class genes] +, with "DEAER" motif, SEP3/LOFSEP and three copies of the PHY gene, [PHYB [PHYA + PHYC]]; chloroplast IR expansions, chlB, -L, -N, trnP-GGG genes 0.
[NYMPHAEALES [AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]]: wood fibres +; axial parenchyma diffuse or diffuse-in-aggregates; pollen monosulcate [anasulcate], tectum reticulate-perforate [here?]; ?genome duplication; "DEAER" motif in AP3 and PI genes lost, gaps in these genes.
[AUSTROBAILEYALES [MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]]]: phloem loading passive, via symplast, plasmodesmata numerous; vessel elements with scalariform perforation plates in primary xylem; essential oils in specialized cells [lamina and P ± pellucid-punctate]; tension wood + [reaction wood: with gelatinous fibres, G-fibres, on adaxial side of branch/stem junction]; anther wall with outer secondary parietal cell layer dividing; tectum reticulate; nucellar cap + [character lost where in eudicots?]; 12BP [4 amino acids] deletion in P1 gene.
[MONOCOTS [[CHLORANTHALES + MAGNOLIIDS] [CERATOPHYLLALES + EUDICOTS]]] / MESANGIOSPERMAE: benzylisoquinoline alkaloids +; sesquiterpene synthase subfamily a [TPS-a] [?level], polyacetate derived anthraquinones + [?level]; outer epidermal walls of root elongation zone with cellulose fibrils oriented transverse to root axis; P more or less whorled, 3-merous [?here]; pollen tube growth intra-gynoecial; extragynoecial compitum 0; carpels plicate [?here]; embryo sac monosporic [spore chalazal], 8-celled, bipolar [Polygonum type], antipodal cells persisting; endosperm triploid.
[CERATOPHYLLALES + EUDICOTS]: ethereal oils 0 [or next node up]; fruit dry [very labile].
EUDICOTS: (Myricetin +), asarone 0 [unknown in some groups, + in some asterids]; root epidermis derived from root cap [?Buxaceae, etc.]; (vessel elements with simple perforation plates in primary xylem); nodes 3:3; stomata anomocytic; flowers (dimerous), cyclic; protandry common; K/outer P members with three traces, ("C" +, with a single trace); A ?, filaments fairly slender, anthers basifixed; microsporogenesis simultaneous, pollen tricolpate, apertures in pairs at six points of the young tetrad [Fischer's rule], cleavage centripetal, wall with endexine; G with complete postgenital fusion, stylulus/style solid [?here], short [<2 x length of ovary]; seed coat?; palaeotetraploidy event.
[PROTEALES [TROCHODENDRALES [BUXALES + CORE EUDICOTS]]]: (axial/receptacular nectary +).
[TROCHODENDRALES [BUXALES + CORE EUDICOTS]]: benzylisoquinoline alkaloids 0; euAP3 + TM6 genes [duplication of paleoAP3 gene: B class], mitochondrial rps2 gene lost.
[BUXALES + CORE EUDICOTS]: mitochondrial rps11 gene lost.
CORE EUDICOTS / GUNNERIDAE: (ellagic and gallic acids +); leaf margins serrate; compitum + [one position]; micropyle?; γ genome duplication [allopolyploidy, 4x x 2x], x = 3 x 7 = 21, 2C genome size (0.79-)1.05(-1.41) pg, PI-dB motif +; small deletion in the 18S ribosomal DNA common.
[ROSIDS ET AL. + ASTERIDS ET AL.] / PENTAPETALAE / [SANTALALES, CARYOPHYLLALES, SAXIFRAGALES, DILLENIALES, VITALES, ROSIDAE, [BERBERIDOPSIDALES + ASTERIDAE]: root apical meristem closed; (cyanogenesis also via [iso]leucine, valine and phenylalanine pathways); flowers rather stereotyped: 5-merous, parts whorled; P = K + C, K enclosing the flower in bud, with three or more traces, odd K adaxial, C with single trace; A = 2x K/C, in two whorls, alternating, (many, but then usually fasciculate and/or centrifugal); pollen tricolporate; G [(3, 4) 5], when 5 opposite K, whorled, placentation axile, style +, stigma not decurrent, compitum + [one position]; endosperm nuclear/coenocytic; fruit dry, dehiscent, loculicidal [when a capsule]; floral nectaries with CRABSCLAW expression, RNase-based gametophytic incompatibility system present.
Phylogeny. Prior to the seventh version of this site asterids were part of a major polytomy that included rosids, Berberidopsidales, Santalales, and Caryophyllales, but then the order of branching below the asterids seemed to be stabilizing, perhaps with a clade [Berberidopsidales [Santalales [Caryophyllales + Asterids]]] while rosid relationships seemed to be [Saxifragales [Vitales + Rosids]]]. However, recent work suggests a polytomy is indeed probably the best way to visualize relationships around here at present. So for further discussion of relationships at the base of asterids and rosids, see the Pentapetalae
[BERBERIDOPSIDALES + ASTERIDAE]]: ?
ASTERIDAE / ASTERANAE Takhtajan: nicotinic acid metabolised to its arabinosides; (iridoids +); tension wood decidedly uncommon; C enclosing A and G in bud, (connate [sometimes evident only early in development, petals then appearing to be free]); anthers dorsifixed?; if nectary +, gynoecial; G [2], style single, long; ovules unitegmic, integument thick [5-8 cells across], endothelium +, nucellar epidermis does not persist; exotestal [!: even when a single integument] cells lignified, esp. on anticlinal and/or inner periclinal walls; endosperm cellular.
[ONCOTHECALES [LAMIIDAE/ASTERID I + CAMPANULIDAE/ASTERID II]] / CORE ASTERIDS / EUASTERIDS / GENTIANIDAE: plants woody, evergreen; ellagic acid 0, non-hydrolysable tannins not common; vessel elements long, with scalariform perforation plates; sugar transport in phloem active; inflorescence usu. basically cymose; flowers rather small [<8 mm across]; C free or basally connate, valvate, often with median adaxial ridge and inflexed apex ["hooded"]; A = and opposite K/P, free to basally adnate to C; G [#?]; ovules 2/carpel, apical, pendulous; fruit a drupe, [stone ± flattened, surface ornamented]; seed single; duplication of the PI gene.
ASTERID II / CAMPANULIDAE / [METTENIUSALES [BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]]]: myricetin 0; style shorter than the ovary; endosperm copious, embryo short/very short.
[BRUNIALES [ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]]] / APIIDAE: tube initiation early; G inferior, [2], style long.
[ASTERALES [APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]] / CORE CAMPANULIDS: C forming distinct tube; A epipetalous.
[APIALES, DESFONTAINIALES, DIPSACALES, ESCALLONIALES, PARACRYPHIALES]: ?
And if the next is a clade...
[APIALES [PARACRYPHIALES + DIPSACALES]] / DIPSAPIIDAE: nodes 3:3.
Age. The age of this node is estimated at (105-)84(-58) Ma (Lemaire et al. 2011b); K. Bremer et al. (2004) suggested an age of ca 113 Ma, Foster et al. (2016a: q.v. for details) an age of about 95 Ma, Magallón and Castillo (2009: c.f. position of Paracryphiales) an age of ca 92 Ma, Magallón et al. (2015) an age of around 85.6 Ma, Naumann et al. (2013) an age of around 75.2 Ma, and Beaulieu et al. (2013a: 95% HPD) an age of (101-)91(-79) Ma; around 92 or 86.4 Ma are ages in Nylinder et al. (2012: suppl.), ca 124 Ma in Nicolas and Plunkett (2014), (106.5-)93(-79.7) Ma in Tank and Olmstead (2017) and (107-)96(-84) Ma in Wikström et al. (2015).
Divergence & Distribution. Initial diversification of this clade probably occurred in the southern hemisphere, but clades that it includes like Apiaceae "coincide" with more recent movements to the north (Beaulieu et al. 2013a). The clade that is sister to Apiales in Xie et al. (2022) includes species from almost throughout the campanulids.
Phylogeny. For the relationships of Apiales, see the asterid II/gentianid clade.
Woody; route II decarboxylated iridoids +; vessel elements solitary, with scalariform perforation plates, pits [both vessels and fibres] distinctly bordered, axial parenchyma diffuse-in-aggregate; nodes 3:3 [?level]; lamina venation pinnate, (margins toothed or lobed); inflorescence terminal, branched ["paniculate"]; plant dioecious; pedicels articulated; plant ± dioecious; flowers small, [<1.5 cm across], K small, C apparently free; A free; G [3], one carpel alone fertile, placentation apical; ovules 1-2/carpel, apical, pendulous, apotropous, nucellus type?, funicular obturator +; fruit a drupe, seed single; endosperm free-nuclear; x = ?6; mitochondrial rpl2 gene lost. - 7 families, 494 genera, 5,489 species.
Includes Apiaceae, Araliaceae, Griseliniaceae, Myodocarpaceae, Pennantiaceae, Pittosporaceae, Torricelliaceae.
Note: In all node characterizations, boldface denotes a possible apomorphy, (....) denotes a feature the exact status of which in the clade is uncertain, [....] includes explanatory material; other text lists features found pretty much throughout the clade. However, the precise node to which many characters, particularly the more cryptic ones, should be assigned is unclear. This is partly because homoplasy is very common, in addition, basic information for all too many characters is very incomplete, frequently coming from taxa well embedded in the clade of interest and so making the position of any putative apomorphy uncertain. Then there are the not-so-trivial issues of how character states are delimited and ancestral states are reconstructed (see above).
Age. The age of crown-group Apiales is estimated to be 90-85 Ma (Wikström et al. 2001), 87 ±14.1 MA (Janssens et al. 2009), ca 84 MA (Bremer et al. (2004), 75-74 MA (Magallón & Castillo 2009), 75–74 Mya, (130-)117(-103.7) Ma (Nicolas & Plunkett 2014), ca 91.4 Ma (Tank et al. 2015: Table S2), ca 80.8 Ma (Magallón et al. 2015), (100.9-)86.7(-73.3) Ma (Maurin 2020), ca 104.4 Ma (C. Zhang et al. 2020) and (109.9-)99(-88.4) Ma (Xie et al. 2022). For a summary of ages in the order, see Xie et al. (2022: Table 1).
For the fossil record of the order, see Martínez-Millán (2010) and Nicolas and Plunkett (2014).
Evolution: Divergence & Distribution. Apiales contain ca 2.4% eudicot diversity (Magallón et al. 1999). Although Magallón and Sanderson (2001) estimated that diversification in Apiales had increased, the order being "extraordinarily species-rich" (ibid., p. 1773), as Nicolas and Plunkett (2014) correctly point out, the bulk of the diversity in Apiales is in Apiaceae, and that is where any action must be.
Nicolas and Plunkett (2014: conclusions not affected by the position of Pennantiaceae) suggest that Pennantiaceae, Torricelliaceae, Griseliniaceae, and a clade representing the rest of the order are ancient (Cretaceous) and possibly of East Gondwanan origin, although East Asia is also involved. They thought that vicariance might be involved in some of the distributions, e.g. of the three genera of Torricelliaceae, etc..
Kårehed (2003: see also Chandler & Plunkett 2004; Bittrich & Kadereit 2018) provides a good summary of what is known of the main clades in Apiales (and where they have been placed in the past) However, thinking about morphological evolution is particularly difficult in Apiales because of our incomplete knowledge of and the extensive variation in the characters of interest. The basal pectinations are genera that have transseptal bundles in common, as well as some rays over 10 cells wide and with square or upright cells (Noshiro & Baas 1998; Baas et al. 2000). Pennantia, with a superior ovary, may also be placed here (see below). Unfortunately, corolla initiation, pollen cell number, and many other characters are unknown for some of these taxa, although some information for the ex-Cornalean genera can be found in Patel (1973), Philipson (1967) and Philipson and Stone (1980); Baczynski et al. (2021) surveyed the whole order for pollen characters, keyed out the major groups, etc.. Pennantia has similar (plesiomorphic) wood anatomy, rather like Griseliniaceae but unlike that of other other Apiales (Lens et al 2008a); this suite of characters is scored as having two origins in Apiales... Dioecy may be the ancestral condition for the order (Schlessmann 2010, q.v. for other characters; c.f. Schlessman et al. 2001), and if so, perfect flowers/monoecy have re-evolved, and they overwhelmingly predominate in the clade (Käfer et al. 2014, 2017). Griseliniaceae and Torricelliaceae both have the iridoid griselinoside, transseptal bundles in the ovary, and the abaxial carpel alone is fertile, the single ovule being apotropous. There is other interesting variation. Thus Pittosporaceae and Araliaceae have early corolla tube formation while the petals in at least some Apiaceae are separate even at their initiation (Erbar & Leins 2004c); these observations need to be extended. Xie et al. (2022) discuss the evolution of habit and fruit type in the order,
Pennantia has a superior ovary (and sometimes a thick, disciform, sessile stigma), and how its gynoecium is interpreted (summarized in Kårehed 2003a; see especially Karpunina et al. 2021) has many implications for character evolution. Here the gynoecium is interpreted as being tricarpelate, although it appears to be single ("pseudomonomerous"), and the carpel that is fertile is abaxial (see also Chandler & Plunkett 2004; Plunkett et al. 2004c for carpel number).
Erbar and Leins (1988a, 1996a, 2004c) suggested that the nectary "disc" of Apiaceae and Araliaceae is a carpellary flank nectary displaced by intercalary growth, and that the axile placentation and inferior ovary of these two families is easily related to the parietal placentation and superior ovary of Pittosporaceae which are florally apparently very dissimilar to all other members of the order, although probably a derived morphology (see below). The ovary of Pittosporaceae has a short basal zone with separate loculi, the ovules being borne above this zone - essentially the same position in which the ovules of Apiaceae and Araliaceae are found. For more on the gynoecium, and floral development in general, in Apiales, see Karpunina et al. (2021). Interestingly, clades with superior and inferior ovaries interdigitate in most other campanulid orders. Further complicating the issue, relationships around Apiales are not very well supported (C. Zhang et al. 2020).
There are some similarities between the [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]] clade and Asterales like Campanulaceae and Asteraceae (e.g. Erbar & Leins 2004; Leins & Erbar 2004b), thus 1-benzyltetrahydroisoquinoline alkaloids are found in both (Kubitzki et al. 2011). However, given the rather strongly supported set of relationships at the base of Apiales, the other clades that are in this part of the campanulids, and relationships within Asterales (Campanulaceae and Asteraceae are not immediately related), most of these similarities are parallelisms.
Genes & Genomes. Yi et al. (2004) suggest that the basic chromosome number in Apiales is x = 6 (see also Raven 1975), although the order then would have all polyploid/dysploid members, with several independent origins of polyploidy. The Dc-β genome duplication event probably occurred around here was probably an allotetraploidy and has been dated to 87-77 Ma (J. Wang et al. 2020; see/c.f. also Iorizzo et al. 2016).
Xie et al. (2022) examined the role of mutation in the adaptation and evolution of the plastid genome. There was negative selection in genes involved in photosynthesis, deleterious mutations being selected against.
Chemistry, Morphology, etc.. For wood anatomy, see Lens et al. (2008a).
Nuraliev et al. (2019) discuss the diversity of floral symmetry in the order; the orientation of the carpels in the basal clades with 3-carpelate gynoecia is unclear (Sokoloff et al. 2018). For nectary morphology, see Erbar and Leins (2010). Endress (2003c) summarizes the rather sparse literature on nucellus development in the clade.
Phylogeny. In earlier studies a grouping [Griselinia [Aralidium + Torricellia]] was rather weakly supported (Backlund & Bremer 1997; see also Chandler & Plunkett 2002). Plunkett (2001) and Lundberg (2001c) both suggested that Torricelliaceae and Griseliniaceae were successive clades near the base of Apiales (Kårehed 2002a: four genes, all genera sampled, strong Bayesian support), and this topology is followed here (see also Soltis et al. 2011; Tank & Donoghue 2010); the relationship is reversed in Chandler and Plunkett (2004), but with a p.p. of 0.93.
The ex-icacinaceous Pennantia is sister to all other Apiales, at least in chloroplast phylogenies (e.g. Kårehed 2003; Lens et al. 2008a: strong support; H.-T. Li et al. 2019 and Xie et al. 2022, both plastome analyses). However, analyses of nuclear markers may place it elsewhere in the campanulids, perhaps sister to Dipsacales (e.g. Chandler & Plunkett 2004), and Pennantia did not link with other Apiales in the nuclear analyses of Stull et al. (2020a) but using chloroplast data, it did. Its position must be confirmed, but the monophyly of the rest of the order seems not to be in doubt. Although in the analysis of the Jan. 2022 version of the Angiosperms353 dataset, q.v., Pennantiaceae were placed as sister to the rest of Apiales, and with strong support, as of ix.2024 suggested relationships for this family are [Pennantia [[Escalloniales + Paracryphiales] Dipsacales], support mostly quite strong, while sister to this clade, although with little support, are Berzelia spp., the only members of Bruniaceae included.
Pittosporaceae were found to be sister to the old Apiaceae and Araliaceae (e.g. Kårehed 2002c; Andersson et al. 2006: strong support, Myodocarpaceae not included), a position that was strongly supported by Tank and Donoghue (2010). However, earlier studies might find Pittosporaceae to be embedded in the clade, some Bayesian analyses giving strong posterior probabilities for a relationship between Myodocarpaceae and Pittosporaceae (Chandler & Plunkett 2004), or they provided only weak support for a sister group position (Nicolas & Plunkett 2009). Overall, the relationships [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]] for the core Apiales seem most likely (Tank et al. 2007; Tank & Donoghue 2010; Soltis et al. 2011; Nicolas & Plunkett 2014). Hardly surprisingly, perhaps, relationships in the plastome analyses of Xie et al. (2022: 271 species of Apiales, Myodocarpaceae not included, 72 genes) are very much those below, and support is generally strong.
Synonymy: Apiineae Plunkett & Lowry, Aralidiinae Thorne & Reveal - Ammiales Small, Araliales Berchtold & J. Presl, Aralidiales Reveal, Griseliniales Reveal & Doweld, Hederales Link, Pennantiales Doweld, Pittosporales Link, Torricelliales Reveal & Doweld
PENNANTIACEAE J. Agardh - Pennantia J. R. Forster & G.Forster - Back to Apiales
Trees or shrubs; iridoids ?; vessel elements 1080-1500(-2250) µm long, fibres 1790-2270(-2900) µm long; nodes 3:3; petiole bundle annular (with a medullary bundle) and with solid annular wing bundles; stomata paracytic; lamina ptyxis curved to conduplicate, (margins entire); inflorescence paniculate, bracts recaulescent, phyllomes 0-6, pedicels articulated; K collar-like, minute, C valvate, connate or not, apex inflexed; nectary 0; staminate flowers: A (epipetalous), anthers sagittate, dorsifixed, filament bent; pollen tricolporate; pistillode minute; carpelate flowers: staminodes +/0, pollen inaperturate; G superior, [3], also [2], (longitudinally ridged), 1-locular, styles short, stigmas punctate, or stigma sessile, broad, lobed; ovule 1(2), at most thinly crassinucellate, integument vascularized; fruit a drupe, endocarp sclereids isodiametric, fibrous lining of loculus well developed; seed coat thin; endosperm development?, embryo short/minute [to 1/3 the seed length]; n = 25, x = 25.
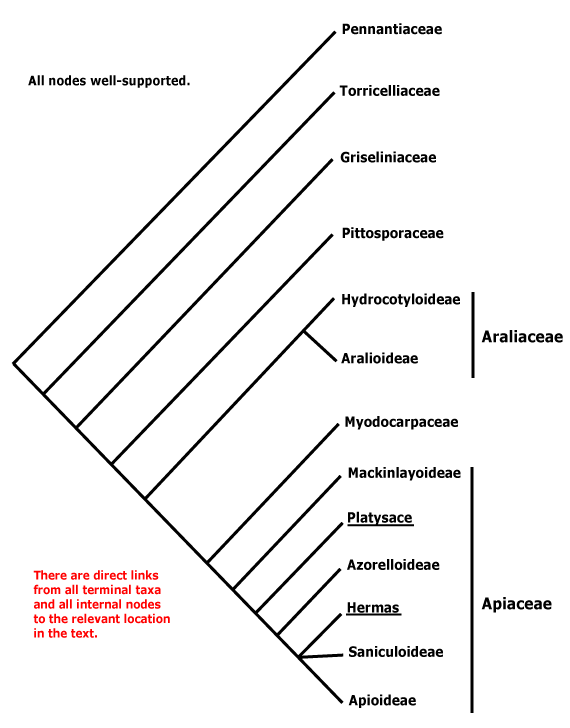
1 [list]/4. N.E. Australia, Three Kings Island, Norfolk Island, New Zealand. Map: from van Balgooy (1966) and Fl. Austral. vol. 4 (1984). [Photo - Habit.]
Age. Crown-group Pennantiaceae are around 6.6 Ma (Nicolas & Plunkett 2014) or (19.5-)9.5(-2.6) Ma (Maurin et al. 2020).
Chemistry, Morphology, etc.. Collenchyma is only poorly developed, and a pericyclic sheath is present; pits in general are bordered.
For the morphology of the stigma, see Kårehed (2002b). For information, which should be confirmed, on ovule morphology, see Mauritzon (1936c).
For other information, see Gardner and de Lange (2002), Sleumer (1942) and Potgeiter (2018), all general, also Bailey and Howard (1941a-d), Heintzelmann and Howard (1948), van Staveren and Baas (1973) and Baas (1973, 1974), all anatomy, Lobreau-Callen (1980: pollen), Miers (1852: ovule orientation) and Manchester et al. (2017: fruit).
Phylogeny. Pennantia cunninghamii is sister to the rest of the genus (Maurin 2020, see also Keeling et al. 2007).
Previous Relationships. Pennantia until a few years ago was in Icacinaceae, now in several separate orders in basal lamiids/campanulids, i.e. as Icacinaceae s. str. (Icacinales), Cardiopteridales and Metteniusales, while Oncothecales may be sister to all other gentianids. However, although Pennantia is the only genus that seems to have really wandered away from the old Icacinaceae, its exact position is unclear.
[Torricelliaceae [Griseliniaceae [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]]]]: polyacteylenes +; young stems with peripheral collechyma; pericyclic fibres 0 or few; nodes 5(+):5(+); leaf base broad [encircling 2/3 the stem or more]; C apparently free, imbricate; gynoecial nectary +; style branches/stigmas recurved; nectary on top of ovary.
Age. K. Bremer et al. (2004) dated this node to ca 84 Ma, Tank et al. (2015: Table S2) to ca 82.1 Ma, Tank and Olmstead (2017) to (89.5-)76.1(-63.6) Ma, Magallón et al. (2015) to ca 72.2 Ma, Wikström et al. (2001: note topology) to (74-)69, 63(-58) Ma, Wikström et al. (2015) to (83-)67(-56) Ma, Janssens et al. (2009) to 87±14.1 Ma, Magallón and Castillo (2009) offered an estimate of ca 74.9 Ma, Bell et al. (2010) one of (63-)53, 49(-39) Ma, while Beaulieu et al. (2013a: 95% HPD) thought that it was (95-)84(-71) Ma; (119.8-)106.9(-94) Ma is another estimate (Nicolas & Plunkett 2014) while almost at the other extreme, around 55 or 48 Ma are ages in Nylinder et al. (2012: suppl.). See also Maurin (2020) at (85.5-)73.1(-61.1) Ma and Xie et al. (2022) at (103.0-)92.3(-81.5) Ma.
Chemistry, Morphology, etc.. The polyacetylenes in the [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]] clade are aliphatic, while those of Torricellia angulata are C11 acids.
For the wood anatomy of this clade, see Noshiro and Baas (1998) and Lens et al. (2008a).
TORRICELLIACEAE H. H. Hu - Back to Apiales
Griselinoside/aralidioside [iridoid]; vessel elements clustered, septate fibres with minutely bordered pits, exclusively scanty paratracheal parenchyma; crystal sand +; mucilage cells +; glandular hairs +; petiole (with small adaxial flange [ligule]); flowers usu. imperfect; K imbricate or largely connate; staminate flowers: anthers sagittate; pollen (in tetrads), tectum reticulate; carpelate flowers: G [(2-4)]), transseptal bundles +, styluli/style branches distinct; ovule 1/carpel; fruit with two empty and one fertile loculi, sterile loculi with aperture in stone, fertile locule with germination valve, mesoocarp sclereids isodiametric, fibres lining loculus, 1-layer (0); x = 8.
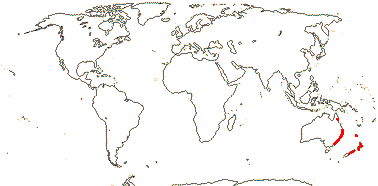
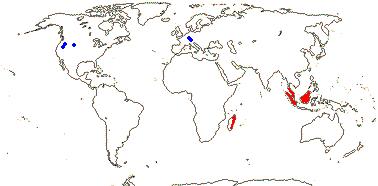
3 [list]/10. Madagascar, South East Asia and W. Malesia. Map: fossils of Torricellia [blue] from Meller (2006).
Age. The age of this node is estimated to be (81-)53(-27) Ma (Lemaire et al. 2011b), ca 57.2 Ma (Nicolas & Plunkett 2014), or a mere (41-)22(-8) Ma (Wikström et al. 2015).
Fossil Pantocarpon fruits known from ca 66 Ma Deccan intertrappean deposits have previously been placed in everything from monocots to Verbenaceae, but Manchester et al. (2020a) suggested that they may belong to Torricelliaceae. Like extant Torricelliaceae they have 3-carpellate drupaceous fruits with paired apertures over sterile carpels and valves in the single-seeded fertile carpels, but there are several differences. Pantocarpon has two fertile carpels, extant Torricelliaceae just one, three (versus two) longitudinal ?germination valves in each fertile carpel, notably smaller fruits and a distinctive testa made up of tracheoidal fibres with annular thickenings and of a kind unknown from any other Apiales (Manchester et al. 2020a).
1. Aralidium pinnatifidum (Jughuhn & de Vriese) Miquel —— Synonymy: Aralidiaceae Philipson & B. C. Stone
Tree, evergreen; alkaloids +; petiole bundles numerous, scattered; stomata anisocytic; lamina deeply pinnately lobed, petiole base with marginal flange; flower articulated; C imbricate; nectary vascularized; Carpelate flowers: staminodes +; integument "massive", parietal tissue ca 4 cells across, raphe dorsal; seed coat vascularized; endosperm ruminate; n = 20 ±2.
1/1. Southern Thailand, the Malay Peninsula, Sumatra and Borneo. Map: see above.
[Torricellia + Melanophylla]: vessel elements with simple perforation plates; stomata anomocytic; pedicels not articulated.
Age. This clade is estimated to be (61.2-)53.7(-48.0) Ma (Maurin et al. 2020).
2. Torricellia de Candolle
Shrub to tree, deciduous; polyacteylenes C11 acids with a chiral center [T. angulata], iridoids +; lamina (lobed), (margins serrate), venation palmate; K basally connate; staminate flowers: C induplicate-valvate, apex inflexed; pistillode +; carpelate flowers: C 0; staminodes 0; stigmas ± bifid; testa slightly sclerified; embryo thin, curved; n = 12.
1/2. Nepal to western China. Map: see above.
Age. Fossil endocarps of Torricellia are reported from Palaeocene deposits in North Dakota and from elsewhere in the U.S.A. and in Europe in the Eocene (Manchester et al. 2009).
3. Melanophylla Baker —— Synonymy: Melanophyllaceae Airy Shaw
Shrub to small tree, deciduous; lamina margin entire; partial inflorescence raceme, bracteoles 2, flowers perfect; plane of symmetry transverse; K open, basally connate, C contorted [direction variable]; anther connective broad; nectary 0; ?floral vasculature, style stout, short; exotesta scalariform-thickened, unlignified; n = ?
1/7. Madagascar. Map: see above. Photo: Melanophylla Habit.
Evolution: Divergence & Distribution. For the fossil history of the East Asian endemic Torricellia, see Meller (2006), Manchester et al. (2009, c.f. 2017) and Collinson et al. (2012); the genus was widespread in the northern hemisphere (western North America, Germany) by the late Palaeocene and the first records are dated to 48 Ma (see also Martínez-Millán 2010).
Chemistry, Morphology, etc.. Ellagic acid was mentioned (prior to 10.x.2017) as occurring here; this seems to have been a lapsus calami (it would have been, if I had been using one). The polyacteylenes of Torricellia angulata, quite recently described, are C11 acids that are unique in having a chiral center (Pan et al. 2006); iridoids are found in the same species (Liang et al. 2009).
The variabiity of the chirality of corolla aestivation is perhaps more to be expected in a rosid (Karpunina et al. 2019). Manchester et al. (2017) describe the distinctive anatomy of the fruit: In Torricellia and Melanophylla the two sterile loculi are larger than the fertile loculus, while in Aralidium the sterile loculi are quite large at the apex of the fruit but become much smaller at the base where they are entirely surrounded by the fertile loculus.
For general information, see Plunkett et al. (2018c) and Nuraliev (2019), for thata of Aralidium in particular, see Philipson and Stone (1980, and other papers in Taxon 29(4). 1980), for Melanophylla, see Schatz et al. (1998: revision) Sokoloff et al. (2018) and Karpunina et al. (2019), both floral development, Ferguson (1977: pollen) and Trifonova (1998: ovule and seed), and for Torricellia, see Lobreau-Callen (1977: pollen), Philipson (1977: ovule), and Takhtajan (2000: ovule and seed, etc.).
Phylogeny. For the circumscription of this clade, see also Plunkett et al. (2004c); relationships are [Aralidium [Melanophylla + Torricellia]] (see also Soltis et al. 2011).
Previous Relationships. Melanophylla was included in Hydrangeales and Torricellia and Aralidium were placed in separate monogeneric orders, both in Cornidae-Cornanae, by Takhtajan (1997); all have been placed in Cornaceae s.l. in the past.
[Griseliniaceae [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]]]: tannins 0; hairs rarely glandular; lamina vernation conduplicate; ovule with endothelium; seed with C18 petroselinic acid + [CH3-[CH2]10-CH=CH-[CH2]4-COOH, i.e. cis-6-octadecanoic acid].
Age. Naumann et al. (2013) suggested an age of a mere 33.9 Ma or so for this node; (115.8-)103.1(-90.2) Ma, three times as old, is the estimate in Nicolas and Plunkett (2014), while Magallón et al. (2015), at around 70.5 Ma, are close to splitting the difference; ca 80.8 Ma is the estimate in Tank et al. (2015: Table S2) and (100.2-)90.1(83.6) Ma in Xie et al. (2022).
GRISELINIACEAE A. Cunningham - Griselinia G. Forster - Back to Apiales
Trees or shrubs (climbing, (hemi)epiphytic); griselinoside [iridoid], petroselinic acid +, polyacteylenes?; vessel elements often >1,000 µm long; petiole bundles (incurved) arcuate; mucilage cells +; stomata cyclocytic; hairs unicellular; leaves often two-ranked, lamina margins with distant and strong spines to entire, petiole base (not encircling stem), adaxial flange +; hypaanthium +; K open, small; staminnate flowers: A dorsifixed; pollen tectum striate; pistilode obscure; carpelate flowers: (C 0); staminodes 0 (+); G [(4)], transseptal bundles +, (2 carpels fertile); ovule?, funicular obturator +; fruit ± baccate; testa ?many layered, outer two (and esp. third) layers with thickened walls; embryo long; n = 18, x = ?
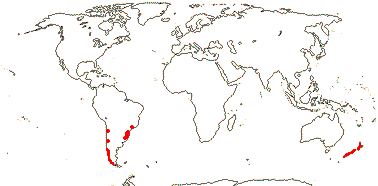
1 [list]/6. New Zealand and S. South America. Map: from Dillon and Muñoz-Schick (1993). Photo: Inflorescence, Leaves, Habit, Flower (scroll to end).
Age. Crown-group Griseliniaceae are around 12.1 Ma (Nicolas & Plunkett 2014; see also Dillon 2018).
Evolution: Divergence & Distribution. For the various interpretations of the disjunct austral distribution of the family, see Nicolas and Plunkett (2014) and Dillon (2018). Given the relative youth of the crown group, bird-assisted movement from Australia/New Zealand to South America seems most plausible, however, Wallis and Jorge (2018) emphasizing stem ages (perhaps over 100 My: see above) and categorizing its origin as "archaic", include Griselinia among the very oldest members of the New Zealand biota.
Chemistry, Morphology, etc..For fatty acids in the seeds, see Badami and Patil (1981). The wood has solitary vessels and apotracheal parenchyma (Baas et al. 2000).
For general information, see Philipson (1977), Dillon and Muñoz-Schick (1993) and Dillon (2018), for leaf insertion, c.f. Philipson (1967), and for leaf base, c.f. Takhtajan (1997); Takhtajan (2000) provides details of embryo and testa, for flowers, see Eyde (1964), for pollen, see Ferguson (1977), and for the ovule, see Warming (1913).
Previous Relationships. Eyde (1964) suggested relationships of Griselinia to Garrya (Garryales) and Cornaceae (Cornales); the genus was placed in a monogeneric order in Cornidae-Cornanae by Takhtajan (1997).
[Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]]: plant often aromatic, ethereal oils, acetate-derived anthroquinones, coumarins mainly aliphatic polyacetylenes + [C17 falcarinone, etc.] +, iridoids 0, flavonols 0; lateral roots originating from either side of the xylem poles [a secretory canal occupies the pole]; vessel elements clustered, (plates doubled), septate fibres with minutely bordered pits, exclusively scanty paratracheal parenchyma; schizogenous secretory canals + [in cortex, pericycle, secondary phloem - and then with specialized axial parenchyma surrounding the canals - and in groups with sieve tubes], fibres 0; (nodes 3:3); petiole bundles arcuate or annular; flowers often hermaphroditic, protandrous; C tube formation early; pollen grains tricellular, surface reticulate [?level]; G [2], both fertile, stigma wet; embryo (very) small; x = 12; RPB2 duplication.
Age. Suggested ages for this node are (110.2-)97.7(-85.1) Ma (Nicolas & Plunkett 2014), ca 76.4 Ma (Tank et al. 2015: Table S2), ca 63.4 Ma (Magallón et al. 2015), (52-)48, 46(-42) Ma (Wikström et al. 2001), (55-)42, 41(-33) Ma (Bell et al. 2010), (64-)49(-41) Ma (Wikström et al. 2015), (75.5-)63.4(-52.4) Ma (Maurin 2020) and (96.2-)86.0(-75.3) Ma (Xie et al. 2022).
Evolution: Divergence & Distribution. Diversification rates may have increased both here (70.5-)66.3(-63.4) Ma and also at the next node up ca 7 Ma later (Magallón et al. 2018).
Australasia seems the most likely place of origin of the whole clade, and of these four families in particular (Nicolas & Plunkett 2014).
There are a number of characters that may delimit clades of various sizes here. These include the presence of sesquiterpene lactones and benzylisoquinoline alkaloids and the presence of crystal sand; see Jay (1969) for suggestions based on plant chemistry that members of this group are related. Lateral roots originate from either side of the xylem poles because a resin canal runs down the stem at the apex of the pole as was noticed some time ago by van Tieghem and Douliot (1888).
Genes & Genomes. There may have been a genome duplication here ca 76.6 Ma (Lanis et al. 2018); for the complex history of the RPB2 gene (DNA-dependent RNA polymerase) duplications, see Nicolas and Plunkett (2013). Chromosome numbers and evolution are discussed by Yi et al. (2004).
Chemistry, Morphology, etc.. Triterpenoid ethereal oils produce the distinctive odour characteristic of many of these plants. There are polyacetylenes, mainly aliphatic and including the C17 acetylenes, falcarinone, etc., in the [Pittosporaceae [Araliaceae [Myodocarpaceae + Apiaceae]]] clade. Triterpenoid saponins like oleanene are found throughout the group, and also elsewhere (Wang et al. 2012). Stuhlfauth et al. (1985) discuss the distribution of various fatty acids in this clade (and in groups that used to be thought to be related to it).
Perforation plates in this area are sometimes paired (Rodríguez C. 1971). The vessel:ray pits are bordered (Baas et al. 2000). Leaf teeth have a broad glandular apex with a main and two accessory veins, or one vein proceeds above the tooth; a survey of tooth morphology might be interesting.
Some Pittosporaceae and a few Araliaceae have basally connate petals (Plunkett 2001) and early corolla tube formation while in the corolla of at least some Apiaceae are the petals are separate even at their initiation (Erbar & Leins 2004c). For information on ventral carpel bundles, see Philipson (1970) and Eyde and Tseng (1971); when the united ventral bundles are opposite the carpels, the two bundles come from the same carpel, and when they are in the septal radii, the two bundles come from adjacent carpels. For ovule morphology, see Jurica (1922) and van Tieghem (1898); according to the latter, Apiaceae have a thick integument (e.g. ca 7 cells - Gupta 1970), that of Hedera is very thick, although Philipson (1977) described the integument of Araliaceae as being "thin". Some Pittosporaceae have laterally compressed fruits (M. Liu et al. 2016), so where this character should be placed on the tree is unclear; it is currently at the next node up.
Previous relationships. Pittosporaceae were included in Rosales by Cronquist (1981) and Mabberley (1997). Takhtajan (1997) included Pittosporaceae as a separate order in his Aralianae (but along with Byblidales). However, evidence had been mounting for over 100 years that they were best associated with Apiaceae/Araliaceae (Hegnauer 1969b for a summary of some literature). Thus van Tieghem initially thought Pittosporaceae might be near Violaceae, but later (van Tieghem 1898b) he placed Pittosporaceae, Apiaceae and Araliaceae together in his Ombellinées-Ombellales, the three being united by their secretory canals (see also Evolution above). Rodríguez C. (1971) discussed relationships in this area from a pre-phylogenetic point of view in some detail.
PITTOSPORACEAE R. Brown, nom. cons. - Back to Apiales
Trees, shrubs, or twining vines/lianes, (± epiphytic); furanocoumarins, (hydroxycoumarins), non-hydrolysable tannins +, C18 [oleic/linoleic], C20, C22 fatty acids abundant; plant resiniferous; vessel elements with simple (scalariform) perforation plates; young stem with (± interrupted - Pittosporum) vascular cylinder; cork cambiun (mid cortical); nodes 1:3, 3:3; petiole bundles arcuate; stomata paracytic; hairs uniseriate, terminal cells distinct, (T-shaped hair; glandular); lamina vernation supervolute-curved, margins entire (lobed, serrate), secondary veins pinnate, leaf base narrow to sheathing; (plant dioecious); flowers medium-sized [ca 10 mm long/across], (monosymmetric by the androecium - Cheiranthera [C.]); K quite large, free (± connate), C often clawed, often slightly basally connate, 3-5-veined [?basal taxa], imbricate; anthers ± basifixed, (porose - C.), placentoid +; tapetal cells multinucleate; (pollen bicellular); nectary on flank of ovary, (0 -C.); G superior, [(-5)], placentation parietal (basal/axile)), style undivided, straight, continuous with ovary, (short), stigma punctate to capitate (lobed), wet; ovules many/carpel, anacampylotropous (apotropous), integument 8-20 cells across, (incompletely tenuinucellate), endothelium 0, obturator +, hairy, vascular bundle terminates in upper part of the funicle; fruit a loculicidal (+ septicidal) capsule/berry (woody, K deciduous; seeds with resin derived from secretions of placental hairs [?level], (?arillate - C.); testa (multiplicative), 3 or more layers persisting, exotestal cells ± thickened, little differentiated, unlignified; x = 12, nuclear genome [1C] (0.064-)1.322(-27.378) pg.
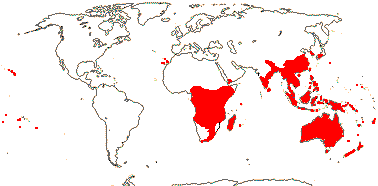
9 [list]/360: Pittosporum (ca 300). Old World, especially Australia, tropical and warm temperate, mainly Pittosporum outside the immediate Australian region. Map: from van Steenis and van Balgooy (1966), Good (1974), Coates Palgrave (2002) and Trop. Afr. Fl. Pl. Ecol. Distr. vol. 1 (2003). [Photo - Flower.]
Age. The appproximate age for this node ([Sollya + Pittosporum]) is (17-)14, 13(-10) Ma (Wikström et al. 2001), (20-)12, 11(-5) Ma (Bell et al. 2010), or around 19.2 Ma (Nicolas & Plunkett 2014: ?sampling).
Evolution: Divergence & Distribution. Many of the apparently plesiomorphic characters of Pittosporaceae, e.g. superior ovary with several ovules, etc., are likely to be derived (see above).
Pollination Biology & Seed Dispersal. Cheiranthera is likely to be buzz pollinated.
Chemistry, Morphology, etc.. Glucosinolates are reported from Bursaria spinosa, but this is probably a mistake (Fahey et al. 2001).
In Pittosporum the flowers are often functionally unisexual/the plant is dioecious. There is a tendency, especially evident in taxa like Cheiranthera, for the flowers to be obliquely monosymmetric, and this is largely because of the position of the stamens and gynoecium. In Pittosporum floribundum, ovules are epi- and apotropous within a single ovary (Narayana & Sundari 1983). Mauritzon (1939a) drew attention to the fact that the funicular bundle appears to expand and end in the upper part of the funicle, rather than in the chalazal region. This may be connected with how the embryo sac curves; I have not looked for this feature in other Apiales. Seedlings of Pittosporum have up to five cotyledons, and seedling leaves may be pinnatifid.
For general information, see Carolin and Bittrich (2018), Cayzer et al. (2023: Pittosporum), for seed oils, see Stuhlfauth et al. (1985), for vegetative anatomy, see Wilkinson (1992, 1998), for embryology, see Narayana and Sundari (1983) and references, for endothelium in particular, see Batygina et al. (1985) and for testa anatomy, see Takhtajan (2000).
Phylogeny. See L. W. Cayzer (references in Chandler et al. 2007) for phylogenetic analyses of parts of Pittosporaceae, and these are largely similar to a preliminary molecular study (Chandler et al., in Plunkett et al. 2004c) : [Pittosporum, [Auranticarpa, Bursaria, Rhytidispermum], the rest]. For relationships between species of Pittosporum found in the Pacific, see Gemmill et al. (2002).
Classification. Cayzer (references in Chandler et al. 2007) suggested a number of changes in taxon limits, largely confirmed by Chandler et al. (see Plunkett et al. 2004c). However, generic limits in the Billardiera-Sollya area are still unclear (Chandler et al. 2007), although Cayzer et al. (2004) had redrawn generic boundaries there.
[Araliaceae [Myodocarpaceae + Apiaceae]]: petroselinic acid +/0; axial parenchyma scanty, paratracheal; young stem with separate bundles; outer cortical collenchya +, forming a continuous layer; axillary bud vascular tissue derived from several leaf gaps [?level]; leaves compound (not), lamina margins toothed or otherwise incised; inflorescences terminal, ultimate units umbels; (plants andromonoecious), (interfloral protogyny); flowers often small [= 5> mm across]; K open, usu. much reduced, C valvate, inflexed, with apical thickening; A inflexed in bud; nectary continuous with ± swollen style base [stylopodium], divided, ventral carpel bundles are fused bundles of adjacent placentae; ovules two/carpel, one descending, the other ascending, aborting, epitropous; fruit often dry, schizocarpic, ± laterally flattened, (winged, wings with mesocarp and endocarp, vascular bundles at the margin of wing), carpophore +, undivided, rib oil ducts +, mesocarp vittae +, branched/anastomosing, calcium oxalate rhomboidal crystals +, endocarp sclerified; exotestal cells (tangentially elongated), tanniniferous [?level]; hemicellulosic seed reserves common; x = ?12; 92 bp deletion in rpl16 gene.
Age. The age of this node is estimated as (48-)38, 35(-25) Ma (Bell et al. 2010), while Wikström et al. (2001) thought that it was (49-)45, 41(-37) Ma and Wikström et al. (2015) (61-)43(-30) My; Xue et al. (2012) put it at (43.6-)41.1(-40.4) Ma, Magallón et al. (2015) at ca 60.2 Ma, Vandelook et al. (2012b) at ca 73.5 Ma, Tank et al. (2015: Table S1, S2) at about 76.5/74.9 Ma, Maurin (2020) at (68.6-)57.4(-47.2) Ma while (87.2-)68.9(-54.0) Ma was the age suggested by Wen et al. (2021). A much older age of (106.6-)94.6(-82.6) Ma was suggested by Nicolas and Plunkett (2014).
Evolution: Divergence & Distribution. Members of both Araliaceae and Apiaceae in clades that are sister to the rest of those families have simple leaves, as have some Myodocarpaceae (Plunkett 2001) and of course all Pittosporaceae, so compound leaves may have evolved more than once and further up in this clade. Of fatty acids in the seeds, C18 tariric acid is absent although petroselinic acid is common, and there are only small amounts of C20 and C22 fatty acids (Stuhlfauth et al. 1985); petroselinic acid is also known from Thunbergia (Acanthaceae), but its mode of synthesis there is different (Gan et al. 2022).
Pollination Biology & Seed Dispersal. For the evolution of andromonoecy in this clade, see Schlessmann et al. (2001; see also Schlessman 2010, 2011). It is rare elsewhere in flowering plants and its evolution here is perhaps connected with dichogamy, successively flowering umbels (all umbellules of a compound umbel may flower at the same time, or they may flower in waves), and umbels as functional units in pollination. Interfloral dichogamy is common in the clade; perfect flowers in Araliaceae are protandrous, as might be expected, and all flowers of a compound umbel can be at the same stage, indeed, the male and female phases may be synchronized across the whole plant. Nevertheless, about 40% of the records for Apiaceae are for protogyny (Bertin & Newman 1993; Schlessmann 2010).
Plant-Animal Interactions. Ehrlich and Raven (1964) noted that some butterfly larvae that do not seem to like Araliaceae, including Hydrocotyle (ex Apiaceae!), are nevertheless found on Apiaceae (?including Saniculoideae). Thus caterpillars of the Papilio machaon group are not found on Araliaceae, probably because that family lacks the furanocoumarins that they like (Berenbaum 1983).
Genes & Genomes. For a deletion in the rpl16 gene of the chondrome, see Downie et al. (2000a).
Chemistry, Morphology, etc.. Kleiman and Spencer (1982) surveyed Apiaceae and Araliaceae for the occurrence of petroselinic acid (see also Stuhlfauth et al. 1985). For similarities in wood anatomy between Apiaceae and Araliaceae, see Metcalfe and Chalk (1983); for bark anatomy, see Kotina et al. (2011: crystal types and distributions not integrated into the phylogeny); for wood and stem anatomy, see Rodriguez C. (1957) and Oskolski (2001), for young stem and petiole anatomy, see Mittal (1961).
Froebe (1971b) looked at the origin of the umbel and suggested that it was derived from a thyrse, not a raceme, although the latter idea is more common. For the sequence of initiation of the parts of the flower, see Erbar and Leins (1985: mostly Apiaceae, also Hydrocotyle); and for nectaries, see Erbar and Leins (2010).
Mention of the fruits being dorsi-ventrally or laterally compressed refers to the shape of the contents of individual seeds in transverse section of the mericarp. Individual mericarps can appear to be dorsi-ventrally flattened if they have lateral wings, while two non-flattened mericarps that have not separated can appear to be laterally flattened. For fruit wings and fruit anatomy, see M. Liu et al. (2006); for general fruit anatomy, with complex distribution patterns of characters, see Baumann (1946) and Liu et al. (2016), for the anatomy of the fruit wing, see Magee et al. (2010a). There is variation both in the kinds of calcium oxalate crystals that are found in the fruit wall, i.e., single rhomboidal crystals or druses, and also where they occur, e.g. in the endocarp or mesocarp, or all around the fruit or only in commissural area (Liu et al. 2006; see also Rompel 1895; Burtt 1991a).
For much still useful information on taxa in this area, see papers in Heywood (1971). For some wood anatomy, see Frankiewicz et al. (2021c and references).
Phylogeny. The old woody Araliaceae/herbaceous Apiaceae distinction is no longer tenable, and recent rearrangements have considerable implications for character evolution (c.f. Valcárcel et al. 2014 in part). Myodocarpaceae and some clades in Apiaceae as circumscribed here include genera that used to be in Araliaceae, although the precise relationships of Apiaceae-Myodocarpeae in particular have been uncertain - see Plunkett and Lowry (2001), Lowry et al. (2001), Plunkett (2001) and Chandler and Plunkett (2004) for more information. Some genera in Apiaceae-Mackinlayoideae below used to be in Araliaceae-Mackinlayeae, others in Apiaceae-Hydrocotyloideae, and ex-Araliaceous genera to be included here include Apiopetalum, Mackinlaya, Micropleura and Xanthosia. Melikian and Konstantinova (2006) thought that the gynoecial structure of Actinotus was so different from that of other Apiaceae that the genus deserved to be placed in its own family, which they duly did, however, it, too, is to be included in Mackinlayoideae (Nicolas & Plunkett 2009). There are indeed a number of morphological similarities between the Mackinlaya clade and Apiaceae s. str. and molecular data also link the two (Chandler & Plunkett 2002, 2004), so Mackinlayoideae are included in Apiaceae. Dickinsia (syn. Cotylonia, for which see Norman 1922) is an odd plant: annual; inflorescence scapose, inflorescence bracts large, foliaceous; C imbricate; G dorsally flattened (Norman says that the fruits of Hydrocotyle (and Micropleura) are laterally compressed), winged, vittae 0. It is included in Azorelloideae below. Nodes along the backbone of this basal part of the apiaceous tree have rather moderate support (e.g. Nicolas & Plunkett 2009), yet very few genera remain to be sequenced. It will be interesting to see what story nuclear genes will tell, although the basic phylogenetic structure followed here used here was indeed recovered in the Angiosperms353 study by Clarkson et al. (2021) - see also the Seed Plant Tree of Life, January 2022 version, agin, support for some nodes is rather weak.
The old Apiaceae-Hydrocotyloideae (see below), herbaceous and with simple leaves, are hopelessly polyphyletic, and their members now occur in several quite separate clades of which most at least remain in Apiaceae (Nicolas & Plunkett 2009). However, the large genus Hydrocotyle itself is in Araliaceae, a position that makes morphological sense, and although sampling of both it and the related Trachymene must be improved, this is unlikely to affect their position. Arctopus is now in Apiaceae-Saniculoideae; Azorella and a group of genera form a well supported Apiaceae-Azorelloideae, and Centella, Micropleura, Actinotus, etc., are in Apiaceae-Mackinlayoideae, although Centella in particular is superficially very similar to Hydrocotyle (e.g. Downie et al. 1998, Downie et al. 2000; Chandler & Plunkett 2003, 2004; Plunkett et al. 2004; Andersson et al. 2006; esp. Nicolas & Plunkett 2009).
For other work on phylogenetic relationships in the Apiaceae-Araliaceae area, see e.g. Rodrígez C. (1971), Judd et al. (1994), Oskolski et al. (1997), Oskolski & Lowry (2000), Plunkett (1998), Plunkett et al. (1996, 1997a, 1997b), and Kårehed (2002c).
ARALIACEAE Jussieu, nom. cons. - Back to Apiales
Hydroxycoumarins +, furanocoumarins 0; (vessel elements with scalariform perforation plates); fibres septate (not); rays heterocellular; vascular bundles in pith, usu. inverted; stomata para- or aniso-(anomo-)cytic; stipules +; sexine poorly differentiated into tectum and baculae [?level]; stigma punctate (dry); (nucellar cap +), (endothelium +), obturator +, as short unicellular funicular hairs; mesocarp thick-walled, lignified, mesocarp vittae branching/anastomosing [?extent]; endocarp sclereids transverse to long axis of fruit [?level]; testa ± collapsed, exotestal cell walls a little thickened; x = 8, nuclear genome [1 C] (0.057-)1.25(-27.505) pg.
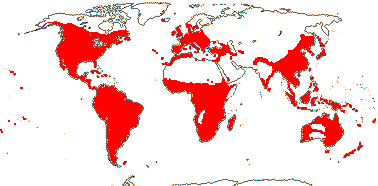
43 [list: subfamilies]/1,450 - three groups below. Largely tropical, few temperate (map: see Meusel et al. 1978; Hultén & Fries 1986; FloraBase 2006; Trop. Afr. Fl. Pl. Ecol. Distr. 6. 2011).
Age. The age of crown-group Araliaceae in Mitchell et al. (2012) refers to a subgroup of the family - see below; Beaulieu et al. (2013a: 95% HPD) suggested an age of (48-)44(-41) Ma and ages of (83.2-)65(-48.7) Ma (Nicolas & Plunkett 2014), (62.2-)39.7(-25.9) Ma have also been mentioned (Wen et al. 2021). See also (53.6-)41.3(-29.1) Ma (Xie et al. 2022) and (68.2-)44.8(-25.4) Ma (Kang et al. 2023).
1. Hydrocotyloideae Link —— Synonymy: Hydrocotylaceae Berchtold & J. Presl
± Herbaceous, perennial (annual); (aquatics); stem with endodermis; nodes 3:3 [Hydrocotyle]; (hairs glandular - Trachymene); lamina orbicular-peltate (± lobed)/deeply twice lobed palmately, margin crenate/serrate, stipules cauline or petiolar; (inflorescences axillary); (K 0 - Hydrocotyle); ovule with "short" funicle, integument ca 5 cells across, parietal tissue 0; mericarp 5-ribbed, carpophore +, undivided/0; n = ³6 [9, esp. 12 - Hydrocotyle, 11 - Trachymene].
4/180: Hydrocotyle (130), Trachymene (45). Tropical, including montane, also warm temperate.
Age. The age of crown Hydrocotyloideae has been estimated at ca 51.4 Ma (Nicolas & Plunkett 2014).
[Harmsiopanax + Aralioideae]: ?
Age. The age of this clade is around 57.7 Ma (Nicolas & Plunkett 2014) or some 90 Ma (R. Li & Wen 2016; Zuo et al. 2017).
2. Harmsiopanax Harms (if here)
(Plant monocarpic); (spiny); fruit schizocarpic, laterally compressed; ventral carpel bundles are fused bundles of the one placenta.
1/3. Malesia.
3. Aralioideae Eaton —— Synonymy: Hederaceae Giseke, Botryodendraceae J. Agardh
(Herbs), shrubs to trees (climbers by roots/stem twiners/grappling with prickles); nodes >7:>7; (palisade mesophyll with arm cells); (prickles + - origin various - Aralia spinosa = s.str.); petiole with ectophloic outer ring and usu endophloic inner ring of budles; (hairs stellate, dendroid, lepidote); leaves usu. pinnately to (peltately-)palmately compound, (vernation ± conduplicate-plicate), stipules connate, intrapetiolar, hooded/2, cauline/0; (inflorescences [sub]racemose); pedicels articulated or not; C (± connate to calyptrate), (imbricate - Aralia, Panax, etc.); (A many), (filaments with two traces); tapetal cells 1-nucleate [by fusion]-multinucleate, nectary not divided; G [>3] [(1-5-200+)], when 5, opposite C, when 3, median abaxial, (style with several vascular bundles); integument 10-19 cells across, parietal tissue (0) 1-4 cells across, funicular obturator +/0, hypostase +/0; fruit a drupe, as many pyrenes as carpels, (berry), (dry, schizocarpic - Astrotriche), (mesocarp sclereids +), (druses +); testa (multiplicative); (seed ruminate); x = ³11.
41/1,275: Heptapleurum* (317-ca ?517), Sciodaphyllum* (131-ca ?335), Polyscias (115), Oreopanax (80), Dendropanax (95), Aralia (68), Osmoxylon (60), Didymopanax* (37), Crepinella* (33) (* = ex Schefflera). Largely tropical, few temperate. [Photo - Flowers, Fruits.]
Age. An age of (100-)84(-70) Ma are suggested for the [Schefflera (= Neocussonia) longipedicellata + The Rest] clade (= "Crown Araliaceae": Mitchell et al. 2012, but note that Hydrocotyle, Harmsiopanax, etc., not included). Another estimate is perhaps 26-25 Ma (Kang et al. 2023: again, c.f. topology).
Leaves and fruits ca 40.4 Ma are attributed to this subfamily (Martínez-Millán 2010).
Age.
Greater Raukaua group
Shrubs to trees, (epiphytic) vines.
5/27: Schefflera s. str. (13), Cheirodendron (6), Raukaua (6, polyphyletic). E. Australia, Tasmania and New Zealand, Polynesia, inc. Hawaii, Fiji and New Caledonia, to the southern half of Chile.
Age. Mitchell et al. (2012) estimated that the age of this clade was (86-)70(-56) Ma (w/Cussonia ca 78 Ma).
[Osmoxylon + Aralia-Panax group] [Polyscias and Pseudopanax group + Asian Palmate Group]
Age. This age of this clade is perhaps 22.5 Ma (Kang et al. 2023).
[Osmoxylon + Aralia-Panax group]
Osmoxylon Miquel
Age. Recently Wilf (2025) described fossils, both leaf and inflorescence, from ca 52 Ma deposits in the Laguna del Hunco area in Argentinan Patagonia that were like Osmoxylon (the leaf fossils particularly so); he called them Caffapanax canessae and Davidsaralia christophae respectively.
1/60. Central Malesia to the West Pacific, Taiwan.
Aralia-Panax group]
Age. There is an estimate of ca 57.5 Ma for the age of clade [Aralia + Panax] (R. Li & Wen 2016).
[Polyscias and Pseudopanax group + Asian Palmate Group]
Age. For the clade [Gastonia (= Polyscias) + Hedera] an age of around 85 Ma or more can be inferred from Valcárcel et al. (2014).
Polyscias and Pseudopanax group
Asian Palmate Group
WGD +
Age. The age of the Asian Palmate group, with Oplopanax sister to the rest, is ca 60.2 Ma (R. Li & Wen 2016).
Evolution: Divergence & Distribution. Nicolas and Plunkett (2014) suggest some ages for nodes along the spine of Aralioideae.
Caffapanax canessae and Davidsaralia christophae described from southern South America by Wilf (2025) seem to be related to the largely Indo-Malesian Osmoxylon, and the extant distributions of other Araliaceae somewhat like the fossils are also South East Asian (Wilf 2025). There are no extant Araliaceae like these plants in South America, and this seems to be another example of the south South America→Australia/Malesia/South East Asia connection that developed during the Tertiary (see also elsewhere).
Major groupings within Aralioideae in particular show some geographical signal (see below), even if they do not map on to previous classifications. Mitchell et al. (2012, other Araliaceae, too; see also Wallis & Jorge 2018) give some dates in the Antipodean Greater Raukaua clade, which seems to be quite an ancient inhabitant of New Zealand; given the wide Pacific distribution of its few species, thinking of how it got to where it lives now is quite a challenge. Valcárcel et al. (2014) offer some dates in the Asian Palmate clade, although they depend on the marker used. Zuo et al. (2017) give ages for clades in Panax and discuss its biogeography.
Smith and Donoghue (2008) suggest some shifts in the rate of molecular evolution within Araliaceae that are correlated with changes in habit. The herbaceous Hydrocotyle is on a particularly long branch (Kang et al. 2023: both plastome and nuclear data).
Basal Araliaceae may well be bicarpelate (see also Wen et al. 2001) and have simple leaves, as in the herbaceous Hydrocotyloideae; more needs to be known about that subfamily and other relatively small and putatively basal clades to understand evolution in the family as a whole. In terms of diversification, given that Schefflera (in the old sense, see Frodin et al. 2010) contained ca 620 spp, but it is being divided into five or so genera with a total (including undescribed species) of around 1,600 spp, perhaps over half the family, it is difficult to say much. Thus Heptapleurum (from Asia) and Sciodaphyllum (from the Neotropics) are the largest genera, with 317 and 136 (respectively) currently recognized species (Lowry & Plunkett 2020b), yet each had an estimated 200+ undescribed species.
Ecology & Physiology. Melzer et al. (2012) and Rowe and Speck (2015) discuss the biomechanics of how Hedera helix attaches to trees and climbs. Hedera may have an interrupted fibrous pericyclic sheath; creeping forms have two-ranked leaves.
Pollination Biology & Seed Dispersal. Little seems to be known about pollination of the morphologically unspecialized flowers of Araliaceae. Ollerton et al. (2007) noted that flowers of Hedera helix, for example, were visited by a variety of insects, but they were in fact functionally quite specialized, Vespula wasps being the main pollinator. There is commonly quite a range of different visitors to the flowers (Plunkett et al. 2018b).
Although not much in is known about fruit dispersal in the family, in the New World the nutritious fruits are important food sources for frugivorous birds while in northern Europe Hedera fruits are fed to nestling birds that otherwise eat animal material (Snow 1981).
In Osmoxylon (inc. Boerlagiodendron) inflorescence development is somewhat protracted. The first heads to develop heads produce false (= infertile) fruits that attract frugivorous birds that also pollinate the flowers, and these birds later visit the infructescences, eat the true fruits that have been produced, and disperse the seeds (Roitman et al. 1997 for some references). Schlessman (1991) found that smaller plants of Panax trifolius produced staminate flowers, inflorescences with perfect flowers appearing when the plants became larger (see also Arisaema-Araceae). For andromonoecy, see above.
Genes & Genomes. Hydrocotyloideae, almost alone in the family, can be highly polyploid (Pimenov et al. 2003), although tetraploidy elsewhere is not uncommon, being found throughout the Asian Palmate Group (Gallego-Narbón et al. 2022). For chromosome numbers and chromosome evolution, see Yi et al. (2004); there are suggestions that x = 12 for Araliaceae (Raven 1975; see also K. Kim et al. 2017; Gallego-Narbón et al. 2022) - but c.f. Carta et al. (2020). There may have been a WGD at the base of the Asian Palmate Group (Gallego-Narbón et al. 2022).
Chemistry, Morphology, etc.. As to stipules: Fatsia (no stipules) x Hedera (no stipules) = XFatshedera (stipules), but some species of Hedera do have a hollowed leaf base with a flanged margin that encloses the axillary bud. Variation in basic leaf construction is considerable, and leaves may be peltate (Hydrocotyle) to peltately palmate (see Kim et al. 2003), to bundle compound, with leaflets arranged like flowers in an umbel, as in some species of Agalma and Heptapleurum, ex-Schefflera (Grushvitzky & Skvortsova 1970; Plunkett et al. 2020).
Araliaceae show considerable floral variation, and this is reflected in their floral vasculature. The calyx may be entirely absent (e.g. Hydrocotyle: Tseng 1967), not even reduced vascular traces suggesting that it was ever there, the petals may have three traces and may be slightly to completely connate (Osmoxylon has basally connate petals), and the stamens sometimes have two traces (Nuraliev et al. 2010, 2011). Both Aralioideae and Hydrocotyle show early corolla tube initiation (Leins & Erbar 1997; Erbar & Leins 2004). Tetraplasandra gymnocarpa and T. kavaiensis have secondarily more or less completely superior ovaries (Costello & Motley 2000, 2001, 2004 - see photograph on the cover of American J. Bot. 91(6). 2004).
Variation in merosity is particularly striking in Aralioideae, and flowers are up to 12-merous or more there (Viguier 1906). Some species have about as many stamens as carpels; separate members of the calyx cannot be distinguished (the calyx forms a low, entire rim (Nuraliev et al. 2014); the petals may be connate and form a calyptra; and although the symplicate zone of the gynoecium develops first, the carpels are largely synascidiate, and there is a large, flat remnant of the floral axis within the carpel whorl (Sokoloff et al. 2007b). Tupidanthus calyptratus (= Asian Schefflera) has up to 172 stamens and 132 carpels; the carpels are initiated in a single elongated and sometimes contorted whorl looking rather like a brain cactus (fasciation: Oskolski et al. 2005; Nuraliev et al. 2009, 2014; see also Eyde & Tseng 1971). Plerandra has up to 25 or more carpels and to 500 stamens, while in other species there are up to five series of stamens initiated centripetally, the vasculature of members of each whorl being connected radially (very unusual in the gentianids); carpel number is 14 or fewer (Philipson 1970; Oskolski et al. 2010c; Plunkett et al. 2020). A few species of Polyscias subg. Arthrophyllum have unicarpelate gynoecia (and five stamens), but there is no evidence that they represent reduced bi-(or more-)carpelate gynoecia, perhaps unlike the single carpels of some Apiaceae-Apioideae (Karpunina et al. 2016), and there is also extensive variation in carpel arrangement (Sokoloff et al. 2017 and references). Nuraliev et al. (2019) outline the variety of symmetry types in the family, largely the result of the variation just mentioned, and these are well illustrated. For further discussion, see the euasterid clade.
For general information, see Philipson (1970) and Plunkett et al. (2018b), also Wen (2011: Aralia) and Nuraliev et al. (2017a) (an extensive study of Asian Schefflera in the old sense); for general anatomy, see Viguier (1906) and Mittal (1981), for that of the leaf, see de Villiers et al. (2010), for bark, see Kotina & Oskolski (2010), for wood, see Oskolski (1996) and Kotina et al. 2013 and references, for gynoecial development in Seemannaralia, see Oskolski et al. (2010b), for embryology, see Ducamp (1902), Gopinath (1944) and Mohana Rao (1973b), and for fruit anatomy, see Konstaninova and Suchorukow (2010). For Hydrocotyle, see Leins and Erbar (2010), flowers, Cerceau-Larrival and Roland-Heydacker (1976: inc. Trachymene), Cerceau-Larrival (1971, 1980: Hydrocotyle) and Shu and She (2001: Chinese spp.), all pollen, and Håkansson (1923), ovules.
Phylogeny. Relationships between Apiaceae and Araliaceae, in particular the positions of Hydrocotyle, ex-Apiaceae, and Mackinlaya and relatives, ex-Araliaceae, are discussed above. In general, understanding relationships here have presented problems (S. A. Smith et al. 2013).
Hydrocotyloideae (ex Apiaceae) are sister to the rest of the family (Chandler & Plunkett 2004; Plunkett et al. 2004a; Nicolas & Plunkett 2009). The S.W. Australian genera Neosciadium and probably Homalosciadium also belong to Hydrocotyloideae (Andersson et al. 2006). Hydrocotyle has laterally flattened fruits with a sclerified (= woody) endocarp and stipules that are either cauline or are borne on the leaf base - and it also has trilacunar nodes (Sinnott & Bailey 1914). Trachymene, perhaps to include Uldinia, is also in Hydrocotyloideae; morphologically it is rather similar to the other genera, and although there is a carpophore in the fruit, it is undivided. For a phylogeny of Trachymene, see Henwood et al. (2010).
For more details about the phylogeny of Aralioideae in particular, see Henwood and Hart (2001) and especially Wen et al. (2001), Plunkett et al. (2004a, c), Lowry et al. (2004) and Z.-D. Chen et al. (2016: Chinese taxa). There are two sets of issues here, the first has to do with Schefflera, the second with general relationships within the family. Schefflera, with perhaps 1,600 species under 2/5 of which have been described (Frodin et al. 2010), is in fact highly polyphyletic, and includes five major clades - of which Schefflera s. str. is the smallest. These clades are circumscribed geographically and some also have morphological support: African plus Madagascan taxa form a clade, as do the some 250-300 Neotropical species (Sciodaphyllum, Crepinella, Didymopanax), the Asian species (the last two clades are close on the tree), and two groups of species restricted to the Pacific, the small Schefflera s. str. and the much larger Melanesian clades (Plunkett et al. 2005: general, 2009, 2010, 2020: extensive, inc. summary of some foliar and floral variation; Plunkett & Lowry 2012: Melanesian clade; Fiaschi & Plunkett 2011; Gostel et al. 2009: Africa-Madagascan; R. Li & Wen 2014: Asian; Z.-D. Chen et al. 2016: China). In the Neotropics, Sciodaphyllum has recently been monographed (Lowry et al. 2019a), Crepinella, most species of which are from the Guyana shield, belongs here (Lowry et al. 2019b), and Didymopanax, largely Brazilian, has recently been resurrected (Fiaschi et al. 2020; there is palynological support - Fiaschi et al. 2009), while completing the description of clades that used to be in Schefflera are two more small Neotropical genera (Plunkett et al. 2021). Shee et al. (2020) looked at the relationships of the some 200 species of Papuasia Schefflera s.l. using the Angiosperms353 probe set, suggesting that there was early movement from the Sunda region to the Woodlark plate, but is was unclear just what taxa of the fragmented Schefflera are involved. [To be fleshed out.]
The Schefflera problem aside, there are four major clades in Aralioideae, the largely South East Asian Palmate group and the Aralia-Panax groups (e.g. R. Li & Wen 2015, Zuo et al. 2017; K. Kim et al. 2017 for relationships), the Pacific and Indian Ocean basin Greater Raukaua group (Mitchell et al. 2012), and the Polyscias-Pseudopanax group (Wen et al. 2001; Mitchell & Wen 2004; Plunkett et al. 2004a; Valcárcel et al. 2014 for a summary). Astrotricha and Osmoxylon may be part of a polytomy at the node immediately above Hydrocotyloideae (see also Lee et al. 2008; Zuo et al. 2017), while the position of Harmsiopanax, which has fruits that are schizocarpic like those of Hydrocotyloideae, is also uncertain (Nicolas & Plunkett 2009), but it, too, is clearly basal within Aralioideae. Gallego-Narbón et al. (2022) should also be consulted. The major groupings recognized by Kang et al. (2023) are [1. Greater Raukana group [[2. Osmoxylon + 3. Aralia-Panax group] [4. Polyscias and Pseudopanax group + 5. Asian Palmate Group]]].
2. The position of Osmoxylon seems somewhat uncertain. The focus in Lee et al. (2008) was on relationships of Malesian Araliaceae; Osmoxylon was isolated.
3. Wen (2011: ITS plus 3 chloroplast markers) recovered a pentatomy in Aralia (and a hexatomy with Panax). J. Liu et al. (2023), with a focus on Aralia section Aralia, found extensive conflict between nuclear genes, but with rather little effect on the topology; there was also some cytonuclear discordance. For relationships in Panax, see Zuo et al. (2017: P. bipinnatifidus to be dismembered?).
5. Within the Asian Palmate Group, which includes Hedera, relationships are rather poorly resolved. Thus Kang et al. (2023) found quite extensive conflict between plastid and nuclear trees (the latter were based on an analysis of variation in 45S nuclear ribosomal DNA sequences). Overall, details of relationships depend on the markers used - polyploidy and ancient hybridization may be involved (Yi et al. 2004; Mitchell & Wen 2004; Valcárcel et al. 2014). The focus in Gallego-Narbón et al. (2022: 72 spp., 29 genera, plastomes and 936 targeted nuclear loci) was on hybridization and genome duplication, and they found the relationships at the base of this clade [Oplopanax [Tetrapanax [Heteropanax + Heptapleurum]]] (note that Kang et al. 2023 recovered similar relationships here, except that Heptapleurum = Schefflera, two of the epithets in the latter also being found in the former...). Gallego-Narbón et al. (2022) invoked ILS and intergeneric hybridization to explain conflict in the relationships that they found. Most (?all) Dendropanax are likely to be monophyletic, with two large clades restricted to the Old and New Worlds (Li & Wen 2013).
Classification. For a now dated checklist of the family, see Frodin and Govaerts (2003). It was early evident that generic limits in Aralioideae needed much attention; see Plunkett et al. (2004a) for genera, but many more changes were needed. In part the clades into which species of the old Schefflera have been placed map on to earlier infrageneric groupings recognised by David Frodin and have names at the generic level, which are being taken up (Plunkett et al. 2020 gives an outline). Thus the Pacific clade of Schefflera is to be called Plerandra (Lowry et al. 2013, q.v. for infrageneric classification), and for the Afro-Malagasy ex Schefflera, see Lowry et al. (2017: Astropanax, Neocussonia). Polyscias has been substantially enlarged (Lowry & Plunkett 2010). A suprageneric classification of Araliaceae is needed...
Lowry and Plunkett (2010) recognised subgenera within Polyscias, and Wen (2011) recognized 6 sections within Aralia.
[Myodocarpaceae + Apiaceae]: furanocoumarins +; perforation plates usu. not doubled; fruit 5-ribbed [or higher-level apomorphy?], carpophore +, mericarps separating at maturity.
Age. Ages for this node of (42-)32, 29(-20) Ma (Bell et al. 2010), (42-)38, 33(-29) Ma (Wikström et al. 2001), ca 58 Ma (Magallón et al. 2015), and much older, (103.2-)91.3(-78.6) Ma (Nicolas & Plunkett 2014), have been suggested. Tank et al. (2015: Table S1, S2) estimated it to be around 59.1 (?mistake) and 59.1 Ma respectively.
MYODOCARPACEAE Doweld - Back to Apiales
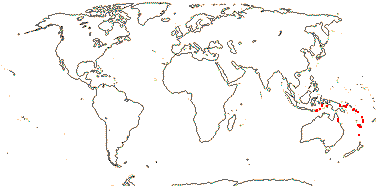
Trees; (sclereids in phelloderm); fibres non-septate; libriform fibres with very thick walls; axial parenchyma apotracheal (and paratracheal), diffuse and diffuse-in-aggregates; rays homogeneous; leaves pinnately compound, leaflet margin entire (serrate), venation brochidodromous, base with adaxial or lateral flange; pedicels articulated; K valvate, C imbricate; G ?arrangement; nucellus?; fruit ventral carpel bundles various, druses scattered in mesocarp, mesocarp vittae branching/anastomosing, secretory vesicles in inner pericarp; x = 12.
2 [list]/17. New Caledonia, E. Malesia, and Queensland, Australia, Map: from van Balgooy (1993). [Photo - Habit.]
Age. Crown-group Myodocarpaceae are estimated to be (47.4-)25.4(-8) Ma (Nicolas & Plunkett 2014).
1. Delarbrea Vieillard
Vessel elements with scalariform perforation plates, numerous thin bars; etiole with continuous ring of vascular tissue, secondary thickening +; C clawed, lacking inflexed apical thickenings; fruit drupaceous, endocarp thin, mesocarp sclereids +, rib oil ducts 0, pyrenes dorsally compressed, usu. a single ventral vascular bundle; testa thick, 2-layered.
1/7. Most New Caledonia, also E. Malesia and Queensland, Australia
2. Myodocarpus Bunge
Petiole with ring of vascular bundles and scattered medullary vascular bundles; leaves (simple); C connate apically, calyptrate; fruit dry, with median basal wing, carpophore +, undivided.
1/10. New Caledonia.
Evolution: Divergence & Distribution. The age of Myodocarpaceae suggests that their presence on New Caledonia is due to long distance dispersal rather than vicariance (Nattier et al. 2017).
For some apomorphies, see Raquet (2004) and M. Liu et al. (2010).
Chemistry, Morphology, etc.. In wood anatomy Myodocarpus is perhaps more like Cornaceae than any other members of the Apiaceae-Araliaceae complex, but in other features it is more like Apiaceae (Rodrigues C. 1957).
Myodocarpus has a number of distinctive features of the flower and in particular its schizocarp (Baumann 1946; Konstantinova & Yembaturova 2010 for morphology, etc.) - the mericarps are beautiful little laterally-flattened samaras that at first sight are similar to those of Serjania (Sapindaceae)!
General information is taken from Plunkett et al. (2004c) and Lowry and Plunkett (2018) and also Lowry (1986: Delarbrea); for wood anatomy, see Oskolski (1996) and Oskolski et al. (1997).
Previous Relationships. This group used to be included in Araliaceae as Myodocarpeae.
APIACEAE Lindley, nom. cons. / UMBELLIFERAE Jussieu, nom. cons. et nom. alt. - Back to Apiales
Herbs to shrubs; pyranocoumarins, myricetin, mannitol +, umbelliferose [raffinose (trisaccharide) isomer] the storage carbohydrate; hydroxycoumarins, flavones 0; vessel elements aggregated; (vascular bundles in cortex); stomata various; lamina vernation also supervolute; inflorescence compound umbels, K a ring of teeth, (obsolete), C free [no early C tube formation], vein single, unbranched [?all]; G superposed, stigma usu. capitate; ovule (with -2 lateral layers of nucellar tissue), funicle "short"; fruit ventral carpel bundles two, on opposite sides of the commissural plane, mesocarp lignified; exotestal cells thin-walled; endosperm oily, with starch; x = 8, nuclear genome [1C] (0106-)1.593(-23.888) pg.
446 [approx. list]/3,820 - four main groups below. World-wide, esp. N. temperate.
Age. Beaulieu et al. (2013a: 95% HPD) thought that crown-group diversification of Apiaceae began (66-)54(-43) Ma, however, (98.9-)87.4(-76) Ma, much older, is the age in Nicolas and Plunkett (2014), while at (71.2-)65.8(-60.9) Ma, Calviño et al. (2016) are in the middle.
Fruits (Carpites) from the late Cretaceous (Maastrichtian) of Wyoming and Montana, around 69 Ma, have been assigned to Apiaceae (Manchester & O'Leary 2010).
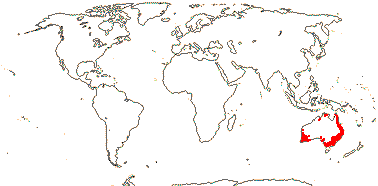
1. Platysace Bunge
Herbs to shrubs; ?vessel elements; (stem photosynthetic); leaves simple, (scale-like), blade linear and terete to orbicular, entire, (deeply lobed), base rather narrow; bracteoles usu. 3; K 0; carpophore undivided to bifid; ?ventral carpel bundles, ?endocarp, oil ducts in ribs obscure; n = 8; cotyledons rounded, toothed.
1(-2)/26. Australia, most in the southwest, not Tasmania. Map: from FloraBase (consulted viii.2009) and Australia's Virtual Herbarium (i.2013).
Age. Crown-group Platysace is around 18.4 Ma (Nicolas & Plunkett 2014).
[Mackinlayoideae [Klotzschia [Azorelloideae [Hermas [Saniculoideae + Apioideae]]]]]: (pseudanthia +).
Age. This node is estimated to be (89.7-)79.4(-70.1) Ma (Xie et al. 2022).
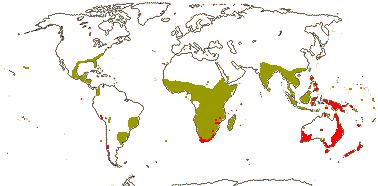
2. Mackinlayoideae Plunkett & Lowry —— Synonymy: Actinotaceae Konstantinova & Melikian, Mackinlayaceae Doweld
Herbs (annual) to shrubs; (centellose [oligosaccharide] - Centella); vessel elements (with scalariform perforation plates); axial parenchyma (also apotracheal, diffuse and diffuse-in-aggregates), (cork cambium subepidermal - C.); (vessel elements with scalariform perforation plates); parenchyma apotracheal (+ paratracheal); fibres non-septate; leaf ± pedately compound [Mackinlaya], ± palmate, to simple, (stipules +); (pedicel not articulated); (inflorescence bracts petal-like); (nectary not divided/on style - Actinotus/spherical-stipitate - Xanthosia); nucellus?; (fruit drupaceous), usu. compressed laterally, carpophore 0, outer mesocarp fibres transverse, inner fibres longitudinal [?= endocarp sclereids parallel to long axis of fruit], rhomboidal crystals in cells next to endocarp; n = 5, 7, 9-12, etc..
10/98: Centella (45-50), Xanthosia (20), Actinotus (18). Southern Hemisphere, scattered, Centella esp. South Africa, C. asiatica pantropical. Map: from Trop. Afr. Fl. Pl. Ecol. Distr. 6 (2011), Australia's Virtual Herbarium (consulted i.2013), GBIF (consulted i.2013) and van Wyk et al. (2013): green = mostly Centella asiatica).
Age. The age of crown-group Mackinlayoideae is estimated to be (83.6-)66.3(-50.2) Ma (Nicolas & Plunkett 2014) or (45.8-)36.8(-26.5) Ma (Calviño et al. 2016: genera examined?).
[Klotzschia [Azorelloideae [Hermas [Saniculoideae + Apioideae]]]]: vessel elements with simple perforation plates; .
3. Klotzschia Chamisso
Perennial herbs/suffrutescent, ?unbranched; lamina peltate, ± lobed, margin serrate, petiole base sheathing; inflorescence ± scapose or not, paniculate; plant monoecious; K well developed, ovate-oblong/toothed; sexine poorly differentiated into tectum and baculae [?level]; K persistent in fruit, mericarps 5-ridged, carpophore undivided; endocarp sclereids transverse to long axis of fruit; n = ?
1/3. Brazil - Bahia and Minas Gerais.
[Azorelloideae [Hermas [Saniculoideae + Apioideae]]]: fruits dorsally compressed.
Age. The age of this node may be (85.6-)75.8(-65.7) Ma (Nicolas & Plunkett 2014), (72.6-)63.6(-55.4) Ma (Calviño et al. 2016), (77.2-)62.7(-50.5) Ma (Wen et al. 2021) or (84.6-)74.5(-65.2) Ma (Xie et al. 2022).
4. Azorelloideae Plunkett & Lowry
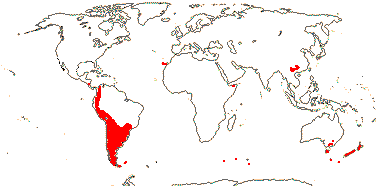
Herbs (annuals) to megaherbs or small shrubs, often hummock-forming; ?petroselinic acid; (cork cambium deep-seated - Mulinum); (hairs stellate); leaves simple, trifid to palmately lobed, (± compound), stipules (+); inflorescence (± 1-flowered), (scapose, the bracts foliaceous Dickinsia); C imbricate [D.], flat; nucellus "large", relatively persistent; megaspore mother cells 2-4, (embryo sac tetrasporic, 16-nucleate [Drusa "type"] - Azorella); fruit with wings/ribs [made up of the entire fruit wall, including a vascular bundle], lateral ribs/wings largest, carpophore (0), undivided, (apically cleft, bifid), ventral bundles 0, 1, 2, lateral [commissural], (opposite), (druses +, in outer mesocarp - Azorella), rhomboidal crystals in cells next to endocarp, (mesocarp vittae branching/anastomosing), (fruit fleshy); (endosperm tetraploid, pentaploid); n = 5, 8, x = 8.
17/154: Azorella (57). South American, esp. the Andes, to Australia/New Zealand, the Antarctic islands; Drusa glandulosa from the Canary Islands and Somalia, Dickinsia from China; probably not native in North America. Map: from Mathias & Constance (1965), Martinez (1993), Fl. China vol. 14 (2005) and Australia's Virtual Herbarium (consulted i.2013). [Photo - Habit.]
Age. The age of this node - [Bowlesia et al. + The Rest] - is around 65.5 Ma (Nicolas & Plunkett 2014) or (68.9-)58.4(-49.3) Ma (Calviño et al. 2016).
[Hermas [Saniculoideae + Apioideae]]: stipules 0.
Age. The age of this node is some (80-)70.6(-62) Ma (Nicolas & Plunkett 2014) or (72.2-)63.1(-54.9) Ma (Calviño et al. 2016).
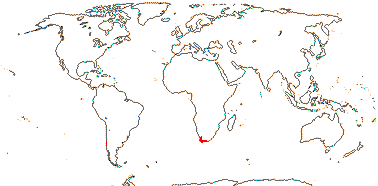
5. Hermas L.
Woody perennials or shrublets; plant densely hairy; leaf blade simple, margin variable; umbels compound, bracts quite large; K large, C-like, persistent, C filiform; fruit with large lateral wings, 2 ventral bundles, mesocarp vittae branching/anastomosing, rib oil ducts small, ?endocarp, carpophore +, entire; n = 7.
1/8. South Africa. Map: from van Wyk et al. (2013).
[Saniculoideae + Apioideae]: lamina with pinnate venation [?level]; ovules lacking parietal tissue [incompletely so], nucellar cap +, funicle "long"; fruit wings with mesocarp only, vascular bundles at the base, (secondary [i.e. lateral] ribs +), mesocarp cells lignified, endocarp single cell layer thick, parenchymatous, calcium oxalate as druses scattered in mesocarp and around commissure, rhomboidal crystals 0; RPB2 duplication +.
Age. The age of this node has been estimated as ca 64.3 Ma (Vandelook et al. 2012b: "crown group of Apiaceae" (sic)), (74.3-)65.8(-58.2) Ma (Nicolas & Plunkett 2014), (72.2-)63.1(-54.9) Ma (Calviño et al. 2016), (73.5-)56.6(-45.2) Ma (Wen et al. 2020), (66.4-)54.2(-45.5) Ma (Wen et al. 2021) or (81.2-)70.7(-61.6) Ma (Xie et al. 2022).
6. Saniculoideae Burnett
Kaurene-type diterpenoids +; (cork cambium outer cortical); umbels simple [?all]; development of K, C, and A sequential; (nectary outside A); nectary separated from styles by narrow groove [stylopodium 0]; exocarp often with outgrowths, outer sclereidal fibre-like cells longitudinal, innermost (if present) transverse, ribs with large oil ducts/cavities. mesocarp druses +; cotyledons rounded.
10/335 - three groups below. World-wide.
Age. Crown-group Saniculoideae are estimated to be around 60.7 Ma (Vandelook et al. 2012b: [Molopo [Stego....]]), 53.1 Ma (Nicolas & Plunkett 2014) or (76.1-)58(-46.8) Ma (Calviño et al. 2016).
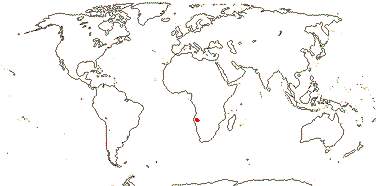
6A. Phlyctidocarpeae Magee, Calviño, Liu, et al. - Phlyctidocarpa flava Cannon & W. L. Theeobald
Annual herb; leaves tripinnately compound; ?inflorescence; bracts foliaceous; fruits compressed dorsally, with stipitate obconical processes, ribs bifurcate, with two vascular bundles, ventral bundles 2, lateral [vallecular and commissural], carpophore 0, vittae vallecular and commissural; n = ?
1/1. Namibia. Map: from van Wyk et al. (2013).
[Steganotaenieae + Saniculeae]: vittae irregular, anastomosing, (mesocarp druses 0).
6B. Steganotaenieae C. I. Calviño & S. R. Downie
Subshrubs to trees, (deciduous); phelloderm with chambered crystalliferous cells; dilation of secondary phloem by expansion of axial parenchyma [not ray cells]; leaves at ends of shoots, palmately compound, appearing after flowering; fruits dorsally or laterally compressed, heteromericarpic, 2-3 winged [wings exo- and mesocarp alone], rib oil ducts forming cavities, mesocarp vittae branching/anastomosing, druses scattered through mesocarp, ventral bundles 2, lateral [commissural], carpophore +, bifid; n = ?11, 12
2/5. Tropical, S.W. Africa. Map: from van Wyk et al. (2013).
6C. Saniculeae Burnett —— Synonymy: Eryngiaceae Berchtold & J. Presl, Saniculaceae Berchtold & J. Presl
Plant ± herbaceous, (annual); rosmarinic acid + [caffeic acid ester], (cardenolides - Eryngium); root lacking hypodermis [?level: Eryngium]; leaves simple, often palmately lobed (palmately compound), lamina often broad, (amphistomatous), teeth with hairy or spiny tips; umbels simple (a capitulum - Eryngium), (compound - Astrantia, Arctopus), with ± petal-like inflorescence bracts (green); (flowers blue); K quite large; carpelate flowers: ± sessile; sessile; fruit barely dorsally or laterally flattened, scaly or spiny, (± smooth), (ribs with two vascular bundles), carpophore 0 (+, entire - Alepidea), or ventral bundles 2, opposite [carinal], mesocarp vittae branching/anastomosing, (rib secretory ducts/cavities 0); n = 5-11 etc. [esp. variable Eryngium], x = ?8.
8/333: Eryngium (250), Sanicula (41), Alepidea (27). World-wide. Map: see Meusel et al. (1978), Hultén & Fries (1986), Trop. Afr. Fl. Pl. Ecol. Distr. 6 (2011), Wörz (2011) Australia's Virtual Herbarium (consulted i.2013) and van Wyk et al. (2013).
Age. The crown-group age of this clade (Arctopus and Sanicula) is (57.8-)41.7(-27.5) Ma or (Astrantia + Sanicula) (40.4-)27.3(-16.3) Ma (Calviño et al. 2016).
Flavones, methylated flavonoids, phenylpropenes +; cortical collenchyma often interrupted; leaves palmately compound; (outer flowers of umbel monosymmetric); tapetum multinucleate; integument 6-8 cells across, hypostase +, (postament +); ventral bundles 2, opposite [= carinal], seed reserves mannans [?level]; x = 11.
Ca 380/3,200 - six groups below. Worldwide, esp. N. Temperate.
Age. The age of crown-group Apioideae is some (71.6-)63.7(-56.1) Ma (Nicolas & Plunkett 2014) or (76.1-)58.4(-45.9) Ma (Calviño et al. 2016).
7A. Lichtensteinieae Magee, Calviño, Liu, et al. - inc. Marlothielleae, Choritaenieae
Annual to perennial herbs to compact shrublets, (basal leaves appearing well before flowering); leaflets often succulent, (margins serrate); K conspicuous or not; fruits dorsiventrally flattened or not, (heteromericarpic), (mericarps hairy, winged [not associated with ribs] Choritaenia), (ventral bundles 0, 1), carpophore +, bifid/short, hygroscopic - Choritaenia/0, (endocarp woody, druses 0 - Choritaenia), rib oil ducts +/0, two vascular strands in each rib, (huge oil ducts/cavities in wings), vittae 0; n = 11.
3/9: Lichtensteinia (7). South Africa, 1 sp. in the Namib desert.
Age. The age of crown-group Lichtensteinieae is about 39.6 Ma (Nicolas & Plunkett 2014).
[Annesorhizeae [Heteromorpheae + Euapioids]]: rib oil ducts 0 (small), carpophore free, bifid [mericarps attached at apex], vittae unbranched, vallecular; cotyledons (1), various.
Age. The age of this node is (65.3-)58.3(-52.2) Ma (Nicolas & Plunkett 2014) or (71.6-)54.5(-40.6) Ma (Calviño et al. 2016).
7B. Annesorhizeae Magee, Calviño, Liu, et al.
Herbs, roots ± fleshy; (plant woody); (fruits heteromericarpic), commissural vittae +, vascular bundles highly lignified; n = 11, 12.
6/41: Annesorhiza (22), Chamarea (11). Most South Africa, also Southern Europe (1 sp.) and North Africa, Canary Islands and Madeira (1 sp.) Map: from van Wyck et al. (2013).
Age. Crown-group Annesorhizeae are around 37.4 Ma (Nicolas & Plunkett 2014) or (78-)51.2(-42.8) Ma (Calviño et al. 2016).
[Heteromorpheae + Euapioids]: tanniniferous exotestal cells 0.
Age. The age of this node is about 63.4 Ma (Vandelook et al. 2012b), 58.3 Ma (Nicolas & Plunkett 2014) or (81.2-)70.7-(61.6) Ma (Xie et al. 2022).
7C. Heteromorpheae M. F. Watson & S. R. Downie
Plants often shrubby (scrambler); vessel (walls with helical thickenings), (perforation plates doubled); fibres septate; (periderm cortical - Anginon), chloroplasts in phelloderm cells [het. pol.]); (secretory canals in single ring in cortex); (leaves undivided); (involucral bracts dentate); (K well developed); fruits (heteromericarpic), not or slightly dorsiventrally or laterally compressed, (wing ducts + - Pseudocarum), (mesocarp vittae branching/anastomosing), (two vascular strands in each rib); n = 11, ?12.
12/27: Anginon (12). Africa (esp. the southwest) to the Yemen, Madagascar (Map: from Winter & van Wyk 1996; Allison & van Wyk 1997; Trop. Afr. Fl. Pl. Ecol. Distr. 6. 2011; van Wyk et al. 2013).
Age. Crown Heteromorpheae are around 60.7 Ma (Vandelook et al. 2012b), 35.5 Ma (Nicolas & Plunkett 2014) or ca 30 Ma (Calviño et al. 2016).
Euapioids: druses in fruit wall absent [check above] (present, on commissural side only).
Age. The age of this node is about (68-)51.4(-37.2) Ma (Calviño et al. 2016), (58.7-)49.9(-42.7) Ma (Wen et al. 2020) or (50.4-)43.4(-38.6) Ma (Wen et al. 2021).
7D. Chamaesieae J. Zhou & F. D. Pu - Chamaesium H. Wolff
Perennial herbs; leaves pinnate; stylopodium divided, very broad; fruit 9-ribbed.
1/8. Himalayas and west China, high altitudes.
[Bupleureae + The Rest]: (coumarins +); (cotyledon 1 - ?up the tree).
Age. The age of this node is estimated to be ca 59.3 Ma (Vandelook et al. 2012b), (51.6-)44.5(-39.1) Ma (Banasiak et al. 2013), ca 51.5 Ma (Nicolas & Plunkett 2014), (52.4-)44.9(-39.5) Ma (Wen et al. 2020), (48.0-)41.5(-36.9) Ma (Wen et al. 2021) or (65.4-)50.3(-39.2) Ma (Y.-x. Song et al. 2024).
7E. Bupleureae Sprengel - Bupleurum L. —— Synonymy: Bupleuraceae Berchtold & J. Presl
Vessel walls with helical thickenings; fibres septate; leaves simple, margins entire; umbels and umbellules (sessile), with well-developed leafy inflorescence/floral bracts, (bracts 0); K and C initiated simultaneously; pollen usu. rhomboidal; fruit not flattened, ?carpophore, ?vittae branching; cotyledons linear, single-veined, glabrous.
1/195. Europe and North Africa, to the Canary Islands and East Asia, N.W. North America and South Africa. Map: from Meusel et al. (1978), GBIF consulted iv.2010 and van Wyk et al. (2013).
Age. The age of crown-group Bupleureae is ca 37.4 Ma (Vandelook et al. 2012b: ?sampling), (63.3-)35.4(-13.0) Ma (R. Huang et al. 2021) or (52.5-)44.4(-37.4) Ma (Y.-x. Song et al. 2024).
7E1. Bupleurum subgenus Bupleurum
(Annual) to perennial (cushion) herbs, (shrubs); (upper leaves perfoliate); leaves with parallel venation; n = (6-)8.
190 spp.. Europe and North Africa, to the Canary Islands and East Asia, esp. the Mediterranean Basin and the Himalaya-Hengduan Mountains region, also N.W. North America (B. americanum) and South Africa (B. mundtii).
Age. Diversification in subgenus Bupleurum began (37.1-)27.9(-19.2) Ma (Y.-x. Song et al. 2024).
7E2. Bupleurum subgenus Penninervia Neves & M. F. Watson
Shrubs to perennial herbs; leaves with pinnate venation, (to 45 cm long, venation parallel, all veins conspicuous - B. rigidum); n = 7 (8).
5 spp.. Mediterranean, mostly the western part.
Age. This clade is estimated to be (83.4-)66.3(-52.7) Ma (Spalik et al. 2010).
7f. Pleurospermeae M. F. Watson & S. R. Downie
Age. Divergence within Pleurospermeae is estimated to have begun (53.8-)45.5(-38.1 Ma Spalik et al. 2010). This node is around (47.8-)41.2(-36.6) Ma (Wen et al. 2020). - Physospermopsis + [40.0-)33.19-27.4) Ma.
The Rest: (plant shrub or small little-branched tree); sugar alcohol sorbitol + [?level]; (stipules intrapetiolar, hooded); K, C, A initiated in groups/from common primordia, or K initiated after C; K + [e.g. Hohenackeria] or 0, C (imbricate); fruit not or dorsiventrally flattened, carpophore bifid; mesocarp fibres longitudinal, innermost fibres horizontal [?level], vallecular + commissural vittae [?level], (cotyledon 1); x = 11 [?level].
360/3045: Seseli (140), Heracleum (130), Peucedanum s.l. (110), Lomatium (85), Chaerophyllum (65), Arracacia (55), Ferulago (50), Thapsia (45). Map: from Meusel et al. (1978), Hultén and Fries (1986), Trop. Afr. Fl. Pl. Ecol. Distr. vol. 6 (2011) and van Wyk et al. (2013).
Age. This clade may be ca 42.9 Ma (Nicolas & Plunkett 2014).
Komarovieae J. Zhou & S. R. Downie
pollen grains subrhomboidal; fruits campylospermous, commissure narrow, heteromericarpy.
10/15: Pterocyclus (4). Central Asia, N.E. Iran to Eastern China.
Group A. Careae Baillon
Selineae Bentham & J. D. Hooker —— Angelicaceae Martynov, Selinaceae Berchtold & J. Presl
Angelica (90-120).
Coriandeae —— Coriandraceae Burnett
Pimpinelleae —— Pimpinellaceae Berchtold & J. Presl - "Pimpinella" (180).
marginal flowers petal-like.Age. Crwon-group Tordylieae are ca 14.9 Ma (Frankiewicz et al. 2021d).
Echinophoreae Bentham.
marginal flowers petal-like.
Pyramidoptereae BoissierAge. Crown-group Apieae are estimated to be (16.4-)13.8(-11.2) Ma (Frankiewicz et al. 2021c).
Group B. Scandiceae Sprengel —— Ferulaceae Saccardo -
Ferula (185): Scandicinae —— Scandicaceae Berchtold & J. Presl (marginal flowers/inflorescence bracts, etc., petal-like), Daucinae —— Daucaceae Martynov, Torilidinae, etc..
Age. Scandiceae are ca 20 Ma (Piwczynski et al. 2020).
Group C. Oenantheae Dumortier: Oenanthe (33).
Aciphylleae M. F. Watson & S. R. Downie, Careae Baillon, Smyrnieae Sprengel —— Smyrniaceae Burnett
Synonymy: Ammiaceae Berchtold & J. Presl, Caucaulidaceae Berchtold & J. Presl, Imperatoriaceae Martynov, Lagoeciaceae Berchtold & J. Presl, Pastinacaceae Martynov, Sileraceae Berchtold & J. Presl
Evolution: Divergence & Distribution. For some ages, see Wen et al. (2021: also table 4). Manchester and O'Leary (2010) thought that fruits of the Late Cretaceous Carpites ulmiformis, 69-66 Ma, might be of a member of Apiaceae (Dioscoreaceae [sic!] were less likely); these are not mentioned by Martínez-Millán (2010).
As relationships get sorted out, evolutionary and biogeographic studies become possible, but our understanding of the phylogeny of Apioideae in particular is still (2022) very imperfect. Nicolas and Plunkett (2014, q.v. for details) suggested an Australian origin for Apiaceae, with at least some scenarios suggesting an African origin for Apioideae and Saniculoideae. Calviño et al. (2016) also emphasized a southern connection for early branching in Apiaceae, movement being largely by long distance dispersal. Indeed, a glance at the maps of the clades from Hermas to core apioids shows a concentration of taxa in southern Africa in particular, the three basal clades of Apioideae and the two basal clades in Saniculoideae being small and made up very largely of southern African genera. 49/50 species of Centella (Mackinlayoideae) are restricted to the Cape Floristic Region, South Africa (Linder 2003). Euapioids, the bulk of the family species-wise and overwhelmingly Eurasian, represent a radiation from this southern African Apiaceae (see also Banasiak et al. 2013; Nicolas & Plunkett 2014; Calviño et al. 2016), and the features of these basal clades will affect character optimisations (Calviño et al. 2006; Magee et al. 2010a for details). The Dc-α; genome duplication event, dated at 52-46 Ma (J. Wang et al. 2020), may be associated with the euapioid node.
A number of taxa in the small clades of basal Apiaceae are woody and have simple or palmately-lobed leaves (see the characterisations above). Although these woody Apiaceae are quite common, and Myodocarpaceae, sister to Apiaceae, are also woody, the ancestral habit for Apioideae, at least, is likely to be herbaceous (Calviño et al. 2006). Woodiness in Bupleurum is derived and has evolved several times (H.-C. Wang et al. 2013); the genus may have evolved in archipelagic early Caenozoic Europe/the Mediterranean basin. Much subsequent diversification occurred in eastern Asia (Banasiak et al. 2013, q.v. for much more detail), with two main clades there, one sister to Mediterranean species (Y.-x. Song et al. 2024); B. mundtii, from southern Africa, is derived within the genus (Neves & Watson 2004), Song et al. (2024) noting that it belonged to a small clade sister to the rest of subgenus Bupleurum while B. americanum is embedded in an Asian clade - it probably got to America via the Bering Bridge. Myrrhidendron, from Central America and Colombia, and a few other euapioids are also woody. These include Daucus from the Cape Verde islands, Canaries, etc., woodiness having two origins here, the plants being monocarpic or polycarpic (Frankiewicz et al. 2021c, see also Banasiak et al. 2016). Two more woody genera, Monizia and Melanoselinum, the latter also known from fossils some 1.2 Ma, are endemic to the Madeira Islands and are derived from Daucus (Marques et al. 2020); island woodiness in general may be a response to the equable conditions common there, or a response drier conditions, as on the Canary Islands (Frankiewicz et al. 2021a). Frankiewicz et al. (2021a) looked at members of the Lefebvrea clade (Tordylieae) growing in the Cape Floristic Region and found that species living in drier conditions were quite often secondarily woody or annuals - alternative responses, both dealing with potential problems caused by cavitation, to the climate aridification perhaps (11.3-)8.9, 8.5(-6.2) Ma in the Late Miocene, although the taxa involved do not grow in particularly dry conditions now. Beaulieu et al. (2013b; see also Beaulieu & O'Meara 2018) found high rates of transition between the woody and herbaceous habits in Apiaceae. Apieae also include a number of woody taxa, but diversification rates there seem to have been affected by L.D.D. events, rather than changes in habit, etc. (Frankiewicz et al. 2021d).
Azorella has a circum-Antarctic-Andean distribution (Nicolas et al. 2025); was drift or dispersal via Antarctica involved? Crown-group Azorella is some (42.6-)31.7(-26.6) Ma, although looking at extant taxa nothing much seems to have happened in terms of further diversification for around 10 My. Nicolas et al. (2025: p. 17) suggested that the genus originated "in Antarctica during the time of the cooling and break-up of that region"; it is made up of two clades, one currently Austral-Antarctic and the other Andean, and it probably originated in the Subantarctic Islands-subantarctic South America. Dispersal seems likely.
Spalik et al. (2010) looked at wide disjunctions in Apioideae, providing dates for various nodes. Later Spalik et al. (2014) focused on biogeographic patterns in the largely aquatic Oenantheae where there seems to have been much dispersal from Eurasia to North America - wide distributions by small steps. Spalik et al. (2014) found that the Hawaiian "Peucedanum sandwicense" (a split from stem Oenanthe) may have arrived on Hawai'i around 17.2 Ma, so being another example of a clade older than the current islands. The aquatics Lilaeopsis brasiliensis and L. mauritiana, the latter from the Mascarenes, are very close, even though they are separated by the Atlantic Ocean and the African continent (Spalik et al. 2010). Chaerophyllum seems to have originated in North America, moved to Malesia (= Oreomyrrhis there), and thence on to New Zealand and southern South America, the Andes (from Australia) and then North America - one way of developing a circum-Pacific distribution (Spalik et al. 2010).
There have been around 36 origins of inflorescences with petal-like marginal structures, whether modified flowers or inflorescence bracts, in Apioideae and about 46 reversals (Baczynski et al. 2022: = pseudanthia; Baczynski & Claßen-Bockhoff 2023), but this seems to have had no major effect on diversification rates.
Around 60 species of Apioideae have seedlings with just one cotyledon, and a cotyledonary tube is also formed (Kljuykov et al. 2019). These features are likely to be adaptations to the geophilous life style, the adults of all such seedlings having tuber-like structures and growing in areas that have (or have had) Mediterranean climate. All told, monocotyly has evolved some seven times or so here (Kljuykov et al. 2019).
Ptácek et al. (2022) looked at embryo sac morphology and associated ploidy levels of the endosperm in Azorella and other Azorelloideae. The Penaea embryo sac and its associated pentaploid endosperm was characteristic of Azorella, the endosperm of Pozoa was normally tetraploid - apomixis perhaps - but triploid in P. coriacea, while Drusa and Bowlesia have a Drusa-type embryo sac (see also Tseng 1967). The polarity of all this variation is unclear.
M. Liu et al. (2016) mapped the distribution of a number of characters of hair and fruit morphology on to a phylogeny of Mackinlayoideae. Wojewódzka et al. (2019), looking at dispersal in Scandiceae where taxa have winged or spiny/hooked mericarps, could not recognize distinct dispersal syndromes; they also found that the homoplasy of the individual characters they examined was high and their systematic value was low. Carpophores, chromosome numbers other than 8, and inflorescences that are not condensed (all in African taxa) could be plesiomorphic in Saniculoideae; simple umbels are apomorphic for Saniculeae, etc. (Magee et al. 2010a, q.v. for numerous character optimisations; see also Calviño et al. 2008a; Kadereit et al. 2008).
Ecology & Physiology. Seed dormancy decreases with increasing relative embryo length, and fast-germinating seeds are likely to be found in short-lived plants of open and dry conditions (Vandelook et al. 2012b; see also Kadereit et al. 2017; Vandellok et al. 2021 and references - esp. Amaranthaceae). Vandelook et al. (2012b) noted that these relationships did not hold for seeds incubated at low (50C) temperatures, and species with seeds that did germinate at such temperatures did not show a positive correlation between temperature and germination; they had to cope with winter and so germination in the summer or autumn would not be desirable. For the evolution of anti-freeze proteins in Daucus carota, see references in Sandve et al. (2008).
The remarkable tussocks formed by species of Azorella growing along the Andes and on the Subantarctic Islands have occasioned much comment. On the latter, ages for tussocks of A. selago of some 80 years have ben estimated (le Roux & McGeoch 2004). However, those of A. compacta in the Central Andean region may live for centuries or more, although for a variety of reasons (indeed: what is a single plant?) ages are difficult to estimate; growth under some circumstances can be quite rapid (Kleier et al. 2015). Azorella compacta grows up to 5,250 m in altitude, and growth of the tussocks is oriented to maximize interception of solar radiation over the year - so the tussocks tend to face north (Kleier et al. 2015).
Apiaceae commonly have hollow stems but solid nodes, together a combination that saves on material costs and yet maintains rigidity (Speck et al. 2003), however, little is known about the biomechanics of umbel stems and details of the distribution of hollow stems in the family.
Pollination Biology & Seed Dispersal. All the flowers in an umbel open more or less simultaneously. In a number of Saniculoideae and a few Hydrocotyloideae and Mackinlayoideae in particular the inflorescence bracts function as petals, the rest of the inflorescence being much reduced and the whole looking more or less like a simple flower (Froebe & Ulbrich 1978) - Actinotus, of the latter subfamily, is a particularly striking example. In the umbels of a number of Apioideae, the marginal flowers are larger than the others, and their abaxial petals may be larger than the adaxial, so increasing the resemblance of the inflorescence to a polysymmetric flower, or the involucral or involucellar bracts may be petal-like (Baczynski et al. 2022). Interesting, inlorescences with enlarged marginal flowers tend to have flat, white umbels, while those with enlarged involucral/involucellar bracts have more spherical and yellow or purple umbels, the two occupying non-overlapping areas of the floral hyperspace examined (Baczynski et al. 2022). The dark flower in the centre of the umbel of taxa like Daucus carota may attract flies that pollinate the flowers (Westmoreland & Muntan 1996; also Baczynski et al. 2022 for possible functions). Claßen-Bockhoff et al. (2023) carried out a comprehensive study of several species with these flowers (their colour and morphology can vary very considerably, and they are polyphyletic in origin), finding that beetle pollination was common.
Although the flowers appear unspecialized, being potentially pollinated by a variety of pollinators, oligolectic pollinators may play a major role in their pollination - in the case examined, the bee Andrena ziziae as a pollinator of Thaspium and in particular Zizia in the southeast U.S.A. (Lindsey 1984; Lindsey & Bell 1985). However, as with other such flowers - Asteraceae are a case in point - other bees (and a syrphid fly) were also effective pollinators, and pollinators varied with geography, too. Ollerton et al. (2007) emphasized that some insects like large bees did not visit Apiaceae, so they showed at least a degree of ecological specialization, and Olesen et al. (2007) emphasized that many of the large numbers of flower visitors of temperate Apiaceae visited only species of Apiaceae.
Many Apiaceae have hooks (animals) or wings (wind) on their fruits, and Platysace and some Mackinlayoideae in particular have myrmecochorous fruits (Lengyel et al. 2010). However, Wojewódzka et al. (2019) could not recognize distinct dispersal syndromes in Scandiceae. Dispersal by water has been invoked for some Apiaceae (Calviño et al. 2016 and references).
Plant-Animal Interactions. For general insect-umbellifer relationships, see Berenbaum (1990) and Sperling and Feeny (1995). Microlepidopteran larvae of the Elachistidae-Depressariinae are also common on Apiaceae, which they have colonized twice, although initially they may have been associated with rosids and they have also colonised some Asteraceae (Fetz 1994: host plants; Berenbaum & Zangerl 1998: chemistry of the [co]evolution of resistance; Berenbaum & Passoa 1999: phylogeny). In particular, both this and the next group are most diverse on Apioideae with angular furanocoumarins, i.e. the axes of the two elements, the furan ring and coumarin elements, are not aligned (the furan ring is a five-membered aromatic ring with four carbons and one oxygen), and these are the most toxic form of furanocoumarins (Berenbaum 2001). Furthermore, genera of Apioideae with angular furanocoumarins are more diverse than those with other kinds of fumarocoumarins (Berenbaum 2001), although I do not know how this correlation is holding in the context of current ideas of relationships.
Caterpillars of Papilionidae-Papilioninae-Papilionini butterflies are quite common (ca 13% of all records) on Apiaceae, perhaps shifting here at least twice from Aristolochiaceae via Rutaceae (Dethier 1954; Fordyce 2010; see also Berenbaum et al. 1996; Berenbaum & Feeney 2008; Simonsen et al. 2011; Condamine et al. 2012; Allio et al. 2020/2021). Interestingly, they are found on neither Pittosporaceae nor Araliaceae; Rutaceae (q.v.), on which they are commonly found, also have furanocoumarins (Berenbaum 2001). The caterpillars will not eat Hydrocotyle (here Araliaceae), many of the larvae of Papilio ajax tested absolutely refusing to eat it (see also Dethier 1941; Fraenkel 1959; Ehrlich & Raven 1967). Linear furanocoumarins (the axes of the furan ring and coumarin elements are aligned) are often phototoxic but are tolerated by caterpillars that will not eat plants with the non-photoxic linear coumarins (Berenbaum & Feeney 1981). For more on swallowtails and Apiaceae, see papers in Scriber et al. (1995) and elsewhere in this site. For the trenching behaviour of herbivores on Apiaceae, see Dussourd (2016).
Agromyzid dipteran leaf miners have diversified on north temperate Apiaceae; they were previously on Ranunculaceae, also a group with noxious secondary metabolites (Winkler et al. 2009).
Perhaps 600 species of aphids (Aphididae), about 1/8 of the total, are known from Apiaceae, more than from any other angiosperm clade of comparable size (Peccoud et al. 2010).
Plant-Bacterial/Fungal Associations. Platysace is reported to be ectomycorrhizal, although this should be confirmed (Brundrett 2017a; Tedersoo & Brundrett 2017).
Vegetative Variation. Seeds with relatively longer embryos may have evolved in open habitats and in plants with an annual life cycle (Vandelook et al. 2012b). There is considerable variation in seedling morphology. A number of taxa have cryptogeal germination during which the plumule is as it were planted under ground; associated with this, monocotyly is also quite common (see Haccius 1952b; Cerceau-Larrival 1962; Haines & Lye 1979). This single cotyledon can look quite unlike any ordinary cotyledon (Cerceau-Larrival 1962; Plunkett et al. 2018a; Kljuykov et al. 2019), and monocotyledonous Apiaceae are all perennial herbs with perennating underground tubers (Kljuykov et al. 2019) - they are quite often called "pig-nuts".
Variation in leaf morphology, even within Apioideae, is considerable. Thus the small circum-Pacific genus Oreomyrrhis (= Chaerophyllum) includes species with ordinary-looking highly dissected leaves, leaves with a series of almost tooth-like leaflets on either side of the rachis, linear leaves, sometimes lobed at the apex, although the lobes are not articulated, and small, undivided leaves. In the last case the plant is tussock-forming and almost moss-like, and I found specimens identified as Centrolepidaceae (= Restionaceae), a monocot when I was at LAE! Species with all these leaf morphologies are found on the mountains of New Guinea. Although Oreomyrrhis is probably monophyletic (however, details of its relationships depended on the method of analysis in Spalik et al. 2010), it is well embedded in Chaerophyllum, a genus hitherto thought to be fairly well understood (Chung et al. 2005; Chung 2007; Sklenár et al. 2011: pâramo species), and with ordinary-looking compound leaves. Lilaeopsis occidentalis and Oxypolis greenmanii have linear, terete leaves that are marked by articulations at intervals. These leaves are comparable with the rhachis of compound leaves, hydathodes borne at the articulations apparently representing much reduced and modified pinnae (Kaplan 1970b, c.f. esp. Figs 3E and 6A; see Charlton 1992: L. brasiliensis ± dorsiventral). Such leaves appear to have evolved several times (Feist & Downie 2008; Feist et al. 2012). That simple leaves of Pimpinella have a cryptic compound developmental program (KNOX1 is reactivated) early in their development (Bharathan et al. 2002; Nakayama et al. 2022) is hardly surprising. Bupleurum has undivided leaves, those of species like B. rotundifolium being almost orbicular, entire, and perfoliate, hence its common name, thorow wax.
Azorelloideae show great variation in both habit and leaf form, as Plunkett and Nicolas (2017) emphasized. Thus the scale-like leaves of genera like Gymnophyton soon wither, and photosynthesis is carried out by the stems and fruits, the latter being bright green until just before they mature. Leaves are commonly more or less orbicular (up to the size of a large rhubarb leaf - Stilbocarpa) and palmately toothed or lobed, rarely very deeply lobed = compound (Domeykoa oppositifolia).
Genes & Genomes. For chromosome numbers in the family, see Bell and Constance (1957) and Pimenov et al. (2003). The Dc-α genome duplication event, probably an allotetraploidy, has been dated to ca 43 Ma (Iorizzo et al. 2016) or to 52-46 Ma (J. Wang et al. 2020). There is also a Dc-β genome triplication event that is dated at ca 70 Ma; this is different from any other Asterales-associated duplication event (Iorizzo et al. 2016), indeed, it has also been assoiated with Apiales as a whole.
For biparental inheritance of the plastome in Daucus, see Boblenz et al. (1990).
There is quite substantial variation in plastome size in Apiaceae, around 20%, and Wen et al. (2021) looked at plastome variation in general in the context of phylogenetic relationships. There has been quite extensive expansion and contraction of the chloroplast inverted repeat in Apioideae, for instance, around Aegopodium and Apium respectively (Plunkett & Downie 2000; Downie & Jansen 2015). For the occurrence of the coxl pseudogene (a mitochondrial gene) in the plastid in Daucus and relatives, see Straub et al. (2013); Downie and Jansen (2015; see also Gandini & Sanchez-Puerta 2017) discuss other examples of this in Apioideae, where it seems to be quite common.
Chemistry, Morphology, etc.. Gums and resins are scattered in the family. In Apiaceae the flavone apigenin is synthesized by an enzyme belonging to the oxoglutarate dependen dioxygenase family, not by a member of the cytochrome P450 family, as is usual in angiosperms (Pichersky & Lewinsohn 2011). For the distinctive rosmarinic acid glucoside found in Saniculoideae-Saniculeae, see Olivier et al. (2008), rosmarinic acid is an ester of caffeic acid and is uncommon elsewhere in Apiales (Petersen et al. 2009). Bowlesia lacks petroselinic acid, but it was apparently the only Azorelloideae studied (Kleiman & Spencer 1982). Oskolski et al. (2010a) describe wood and bark anatomy of Steganotaenieae in particular and Saniculoideae in general. Peripheral collenchyma in the stem is often especially well developed, and petiole anatomy is very variable and complex (e.g. Metcalfe 1950). Taxa such as Foeniculum have stipules of sorts.
The usually compact inflorescence units of Saniculoideae may be best interpreted as a group of reduced umbellules (Froebe 1964, 1971a), although they are called simple umbels above. There is quite a bit of variation in floral development (Ajani et al. 2016) which has tentatively been incorporated into the character hierarchy above. There is variation in the timing of organ initiation - is the initiation of organs within a whorl simultaneous or staggered?, is the initiation of whorls sequential?, are there common primordia? (Erbar & Leins 1985; Erbar 2010; Ajani et al. 2016). Centella (Mackinlayoideae) has a few branches from the petal bundle (Gustafsson 1995); petal vasculature may repay attention. For nectaries in other than Apiaceae-Apioideae in particular, see Magin (1983); nectaries are not only to be found at the base of the style. Leins and Erbar (1985a) discuss the development of the inferior ovary here. Spichiger et al. (2002) show the two carpels as being collateral. According to Eyde and Tseng (1971), whether the ventral carpel bundles are fused bundles of adjacent placentae or are from the same placenta varies within Apiaceae but without any particular systematic significance. Turgenia has relatively huge pollen grains, by far the largest in the order of those sampled by Baczynski et al. (2021). Van Tieghem (1898) noted that in Apiaceae the ascending ovule aborts and the pendulous ovule persists, however, other reports suggest that both ovules are pendulous (Philipson 1970).
Fruit anatomy and morphology vary greatly and have in the past been much used to separate genera; they have more recently been studied by M. Liu and co-workers and others. Some of this variation shows at least some correlation with clades (Kljuykov et al. 2020). For vittae, druses, etc. in the fruits, see Liu et al. (2007, 2012b), for fruit anatomy of Azorelloideae, see Liu et al. (2009), and for carpophores, see Liu et al. (2012a) - although Phlyctidocarpa (Saniculoideae) by one definition in this last paper would seem to lack a carpophore (group A), yet it is scored as having a bifid carpophore. Kljuykov et al. (2020) attempt to standardize the descriptions of anatomical characters of fruits by creating an illustrated list of 15 characters and 75 character states - the latter quite often divisions of apparently continuous variation.
There is much useful information in Chandler and Plunkett (2003, 2004), Kljuykov et al. (2004: terminology/taxonomically useful characters in the family), all four numbers of Plant Divers. Evol. 128. 2010, inc. Stepanova and Oskolski (2010: Bupleurum) and in Plunkett et al. (2018a); see also Burtt (1991a: southern African genera) and Van Wyk et al. (2013: African taxa) for information about Apiaceae in biogeographically/phylogenetically critical areas. For chemistry, see also Hegnauer (1971) and Olivier and van Wyk (2013: Saniculeae), for stomata, see Guyot 1971 and references - value slight?), for wood anatomy in Mackinlayoideae and Apioideae-Heteromorpheae, see Oskolski and van Wyk (2008, 2010; Long & Oskolski 2018), and in woody Saniculoideae, see Oskolski et al. (2010a), for stem anatomy in Apioideae, see Frankiewicz (2021c: no sharp distinction between woody and herbaceous taxa), for inflorescences of Saniculoideae and Hydrocotyloideae in particular, see Froebe (1964, 1971a, 1979), for those of Eryngium, see Harris (1999), for floral development, see Mair (1977), Leins and Erbar (2004b) and especially Erbar and Leins (1985) and Ajani et al. (2016), for pollen of Klotzschia, see Shoup and Tseng (1977), Platysace and Mackinlayoideae, Cerceau-Larrival (1980), European Apiaceae, Punt (1984), Cerceau-Larrival and Roland-Heydacker (1976: inc. PLatysace), Cerceau-Larrival (1971) and Shu and She (2001: Chinese spp.), for the nectary, see Erbar (2014), for the distribution of the hypostase, see Gupta (1970), for embryo sac morphology and fruit anatomy, esp. of genera in the old Hydrocotyloideae, see Tseng (1967), and for ovules, etc., see Håkansson (1923). See also general references for [Araliaceae [Myodocarpaceae + Apiaceae]].
Phylogeny. Relationships between Apiaceae and Araliaceae, and in particular the positions of Hydrocotyle, ex-Apiaceae, and Mackinlaya and relatives, ex-Araliaceae, are discussed above.
Within Apiaceae as here delimited, the Australian Platysace, perhaps to include Homalosciadium, is not a member of Mackinlayoideae, where it had been placed (e.g. Chandler & Plunkett 2004). It is sister to Apioideae in some analyses (Henwood & Hart 2001; Andersson et al. 2006), but a position rather lower in the tree sister to [Azorelloideae [Saniculoideae + Apioideae]] has been strongly supported (Nicolas & Plunkett 2009, 2014), while Soltis et al. (2011: but sampling) found that it was moderately supported as sister to Mackinlaya. The positions of Klotzschia ("distinctive fruits") and Hermas, both of which used to be in Hydrocotyloideae, are unclear (Andersson et al. 2006; Calviño et al. 2006, 2008). Klotzschia, herbs to subshrubs with peltate leaves from Brazil, may go with Azorelloideae, Apioideae, or join the back-bone immediately above Azorelloideae (Nicolas & Plunkett 2014). Hermas, a South African endemic, has some similarities with Saniculoideae, but it is unlikely to be placed within any currently recognized subfamily (Nicolas & Plunkett 2009); there is some support for a position as sister to [Saniculoideae + Apioideae] (e.g. Nicolas & Plunkett 2014; Calviño et al. 2016), and I have tentatively placed it there.
Clarkson et al. (2021) looked at relationships using the nuclear Angiosperms353 target capture probe set in a study that included half the genera (234/466) in the family. The relationships they obtained are somewhat different from those previously (as of vii.2021) recognized here, which were [Mackinlayoideae [Platysace [Azorelloideae [Hermas [Saniculoideae + Apioideae]]]]. In Clarkson et al. (2021) they are [Platysace [Mackinlayoideae [Klotzschia [Azorelloideae [Hermas [Saniculoideae + Apioideae]]]]]], and support was generally (quite) strong, except for various goings on in Saniculoideae including the placement of Phlyctidocarpa flava there. Although it would be nice if the backbone support at a couple of nodes were stronger, Clarkson et al. (2021) noted that the three genera just mentioned are very unlikely to find a home in a recognized subfamily, so, pending further work, the hierarchy above follows their sequence.
Mackinlayoideae. Within Mackinlayoideae, the clade of [Actinotus + Apiopetalum] is quite well supported as sister to the rest of the subfamily (Nicolas & Plunkett 2009; M. Liu et al. 2016; Clarkson et al. 2021: Apiopetalum not included).
Azorelloideae. This subfamily includes about half the genera that used to be in Hydrocotyloideae; for relationships here, see Downie et al. (1998, 2000a, 2001), Mitchell et al. (1999), Plunkett & Lowry (2001), Henwood and Hart (2001: the Bowlesia clade), Chandler and Plunkett (2004), Andersson et al. (2006), Nicolas and Plunkett (2009) and Fernández et al. (2017); Stilbocarpa used to be in Araliaceae, but it is probably sister to the Andean Huanaca in the old scheme of things. Klotzschia may also belong here, but its position is unstable (see above); this genus aside, the South American Diposis is sister to other Azorelloideae (Nicolas & Plunkett 2009). Azorella is para/polyphyletic with respect to Laretia, Mulinum, and three other genera; in particular, the type of Azorella is rather distant from most of the rest of the genus (Nicolas & Plunkett 2012) and there has been extensive hybridization (Fernández et al. 2017). The ultimate phylogenetic/taxonomic disposition of some of the taxa involved seemed unclear, but for a comprehensive phylogeny of the whole group and expansion of the limits of Azorella, see Plunkett and Nicolas (2017). Nicolas et al. (2025: 56/57 spp. of Azorella s.l., 9 plastid, 2 nuclear markers) confirmed extensive hybridization in the clade, some sections being of likely hybrid origin; the sections were all monophyletic, excet sect. Schizeilema in the nuclear analysis, although there was a fair bit of instability in the relationships both between and within the sections.
Saniculoideae. The monophyly of Saniculoideae (except Lagoecia and Lichtensteinia, both now in Apioideae) is upheld in all molecular analyses, and some details of relationships within it were suggested by Valiejo-Roman et al. (2002). The African ex-hydrocotyloid Arctopus (see Magin 1980 for floral development) was sometimes sister to other Saniculoideae, the woody Steganotaenia and Polemanniopsis, and perhaps also Lichtensteinia, being part of the same clade (Downie et al. 2001; van Wyck 2001; M. Liu et al. 2003a; Plunkett et al. 2004c; Magee et al. 2010a); at least some of the latter genera have a slightly lignified endocarp, apparently alone in both Saniculoideae and Apioideae (M. Liu et al. 2003b). Calviño et al. (2006, esp. 2007: good general discussion of variation), however, expressed reservations about this expanded - and characterless - Eryngioideae, but Calviño and Downie (2007, c.f. Magee et al. 2010a) found that the clade could be circumscribed satisfactorily so long as Lichtensteinia moved to Apioideae; they recognised two tribes, both well supported and with unique indels. A clade including Lichtensteinia and Choritaenia were weakly supported as being sister to other Saniculoideae (Nicolas & Plunkett 2009); although this set of relationships has not been upheld, the monotypic African Phlyctidocarpa does seem to be a member of Saniculoideae, although its exact position there is unclear (Magee et al. 2010a, see also Nicolas & Plunkett 2014; Clarkson et al. 2021). Steganotaenia and Polemanniopsis may be sister to the rest (Clarkson et al. 2021). The large genus Eryngium has separate New and Old World clades (Calviño et al. 2008b, 2010); a recent morphological analysis suggested that four unrelated species (unrelated in the system later used in the same paper) made up a series of basal pectinations, although there was no strong support for this topology (Wörz 2011).
Apioideae. Relationships at the base of Apioideae are strongly pectinate and are something like [Lichtensteinieae [Annesorhizeae [Heteromorpheae [Chamaesieae [Bupleureae + The Rest]]]]] (Downie & Katz-Downie 1999; Plunkett et al. 2004; Calviño et al. 2006, 2016; Magee et al. 2008, also a revision of Ezosciadium; Magee et al. 2010a; Downie et al. 2010; Nicolas & Plunkett 2014). Apart from the position of Lichtensteinia, in some analyses sister to [Saniculoideae + Apioideae], relationships in Nicolas and Plunkett (2009) are similar; immediately above Bupleurum on the tree was the Mexican Neogoezia. The Annesorhiza clade appeared to be allied with Heteromorpheae, while Bupleurum was strongly supported as sister to the remainder of the subfamily (Calviño et al. 2005, 2006). The position of Chamaesieae with respect to Bupleurum is unclear (J. Zhou et al. 2009), although Calviño et al. (2016) place them as sister to all remaining euapioids, i.e. branching off immediately below Bupleurum in the tree (see also Xie et al. 2022: sampling of Chamaesieae in particular very good). Similarly, in the single-copy-gene transcriptome analysis of Wen et al. (2020) relationships are [Chamaesium [Bupleurum [Pleurospermeae [Physospermopsis etc. + remaining Apioideae]]]], and similar relationships were recovered in a plastome analysis (Wen et al. 2021). Clarkson et al. (2021: nuclear phylogenomic analyses) found the relationships [Lichtensteinieae [Heteromorpheae [Annesorhizeae [Chamaesieae [Bupleureae [Pleurospermeae [Physospermopsis etc. [Oenantheae + The Rest]]]]]]]]; the individual tribes, etc., were in general well supported, as were the relationships between them, except for the position of Chamaesieae (again).
Bupleureae: For relationships within Bupleurum, in which the sole South African species is embedded in the genus and a Mediterranean clade of sometimes shrubby, often pinnately-veined taxa is sister to the rest of the genus, see Neves and Watson (2004), H.-C. Wang et al. (2014) and R. Huang et al. (2021) and Xie et al. (2022), the two latter plastome analyses. In the trees of Neves and Watson (2004) B. baldense was often sister to the rest of subgenus Bupleurum while the position of the distinctive B. rigidum within subgenus Penninervia was unclear. Y.-x. Song et al. (2024: 89 spp., 3 plastid genes and ITS) found one major Asian clade to be sister to a Mediterranean clade and another Asian clade sister to the combined clade; for more details see Diversity & Distribution above.
Within the remaining Apioideae, the monotypic Pleurospermopsis, from the eastern Himalayas, and included in an expanded Pleurospermeae (J. Zhou et al. 2009; Calviño et al. 2016), may be sister to the remainder. Apioideae also include Lagoecia, with its quite well developed calyx and 1-seeded fruits, which was previously associated with Saniculoideae (Magion 1980); it is now well embedded in Pyramidoptereae (e.g. Clarkson et al. 2021). For other relationships within Apioideae, see e.g. Downie et al. (2000a, b, 2001, 2002: Cymopterus, Lomatium about as polyphletic as you can get, other genera polyphyletic), Plunkett and Downie (2000: junction of chloroplast inverted repeat/large single copy shifts characterise clades in part of Apioideae), Spalik and Downie (2001a, b), Sun and Downie (2004, 2010a, b: perennial W. North American Apioideae, morphological and molecular analyses), Sun et al. (2004: Cymopterus, Lomatium again), Hardway et al. (2004: Oenantheae), Valiejo-Roman et al. 2006ab: Iranian taxa, Downie et al. (2008) and Spalik et al. (2009: Sium, 2014), all Oenantheae, J. Zhou et al. (2008: China), Ajani et al. (2008: Iranian taxa), Winter et al. (2008: Africa), Degtjareva et al. (2009: Bunium polyphyletic, monocotyledonous species separate), Magee et al. (2009: especially Cape genera), Logacheva et al. (2010: Tordylieae), Yu et al. (2011: Chinese Heracleum, genus polyphyletic), Valiejo-Roman et al. (2012: Pleurospermum - surprise, surprise, polyphyletic), Degtjareva et al. (2013: Asian geophilous genera), Terentieva et al. (2015: Coriandreae polyphyletic), and Dogru-Koca et al. (2020: tribes a mess, new genus described...). Komarovieae are a very small tribe to which monotypic genera have recently been added (Sultan et al. 2025). Lyskov et al. (2022) looked at relationships around the highly polyphyletic Seseli, finding that nuclear ITS and plastid data told somewhat different stories; they described a new genus. For relationships in Scandiceae (Group B above), particularly Ferula, somewhat paraphyletic, and relatives, see Kurzyna-Mlynik et al. (2008) and Panahi et al. (2015, 2018: again, conflict between nuclear ITS and plastid data). Piwczynski et al. (2020) confirmed the main patterns of relationships in the tribe - [Scandicinae [Daucinae + Torydylinae]], for which, see also Downie et al. (2000). Clarkson et al. (2021) found that relationships along the spine of Apioideae were strongly supported except in Group A Apioideae (see above); the monophyly of individual tribes, etc., was quite often well supported. There is quite extensive conflict between chloroplast and nuclear trees, perhaps especially in Group A Apioideae - hybridization-introgression may be involved, hybridization-polyploidy is less likely (Wen et al. 2021).
Turning to individual tribes. Apieae. Relationships here were examined by Frankiewicz et al. (2021d), with a focus on stem anatomy.
Pimpinelleae. Pimpinella is also turning out to be very much polyphyletic; Magee et al. (2010b) and Fernández Prieto et al. (2018) and Pimenov et al. (2022) discuss relationships here. Trachyspermum broke up into seven lineages (ITS was the molecular marker used), the type of the genus being separate from all other species, most clades being placed in Pimpinelleae, but also in two other tribes; although there was variation in carpological characters, fruit indumentum and hair microsculpture seemed to be quite useful characters (Pimenov et al. 2022).
The limits of Daucus and its relatives are discussed by Banasiak et al. (2016). Here Spooner et al. (2020) perhaps not surprisingly found that analyses of taxa within Daucus carota and between it and neighbouring species using chloroplast, mitochondrial and nuclear genes all suggested different relationships, however, this was true even in analyses of different aspects of the nuclear genome - SNPs, all chromosomes, 460726 parsimony informative characters, versus 94 nuclear orthologues, all chromosomes, 21011 characters.
Oreomyrrhis is embedded in Chaerophyllum (Chung et al. 2005; Chung 2007) and forms a largely Taiwanese-Australo-Papuan clade along with some species from Central America and two from the U.S.A. (Piwczynski et al. 2015).
Sun et al. (2008) and more recently J. Zhou et al. (2020) looked at relationships in Ligusticum, a genus centred on China, the Zhou et al. study including ca 46 of some 60 species placed in the genus in their ITS analysis. They included all Apioideae with ITS sequences, a wise move since species of Ligusticum appeared in six different clades and were associated with species from a variety of other genera, themselves sometimes also polyphyletic on other grounds. Sun et al. (2008) had carried out a morphological phylogenetic analysis of the Chinese species of Ligusticum, and there L. scoticum, quite separate in the molecular analyses of Zhou et al. (2020), was embedded in those species... Gou et al. (2021) looked at relationships in the Meeboldia area.
Selineae. 30 species of Arracacia (arracacha roots) and 62 species of its immediate relatives were studied by Danderson et al. (2017); 8 main clades were recognised, although some were poorly supported, and species of Arracacia were in six of these, five more of its species being unplaced as to a clade - "rampant polyphyly" seems an understatement. C.-Y. Liao et al. (2013) looked at Angelica and relatives, and later returned to the problem as they focused on North American species of the genus, all 26 of which they examined - topologies based on the three plastid markers they examined and on ITS differed (Liao et al. 2023). For European species of Angelica, see González-Toral et al. (2024) and for Turkish taxa, see Tuncay and Akim (2025). The focus of Feist et al. (2025, see also 2013) was on Tauschia, although they sampled quite widely in Selineae. They looked at 16/32 species of Tauschia using two nuclear and four chloroplast markers and found rampant polyphyly, the species of the genus examined coming out in ten or so clades (see also below) - but at least specimens from the same species came out together.
Classification. The subfamilial classification of Plunkett et al. (2004c, 2018a) and Downie et al. (2010) is largely followed here, but further changes may be needed given the positions taken by some ex-Hydrocotyloideae in the trees recovered by Nicolas and Plunkett (2009, 2014); see also Clarkson et al. (2021). For tribes in Saniculoideae and in the basal pectinations in Apioideae, see Magee et al. (2010a); five of the tribes in the latter were newly described (and all are small) and Marlothielleae and Choritaenieae, both monospecific, are combined with Lichtensteinieae above; the expanded Lichtensteinieae still muster a mere 9 species. Downie et al. (2010) placed the 41 major clades they obtained in Apioideae in 30 groups, recognising tribes and subtribes where possible and listing included genera; some groups of genera were unnamed; Clarkson (2021: Table 1) provides an updated list of tribal and subtribal names, and also some informal group names. Moussavi et al. (2020) assigned the majority of Iranian genera of Apioideae (about 1/4 of the genera grow in Iran) to tribes.
As should be obvious, generic and tribal limits in Apioideae have been a notable disaster area, perhaps as bad as in any other major angiosperm group (e.g. Spalik in Kadereit et al. 2016). The old tribal and generic classification was based primarily on gross fruit morphology; convergence of fruit architecture as adaptations to particular modes of fruit dispersal has resulted in many non-monophyletic groupings (see also Downie & Smith 2002; Piwczynski et al. 2015; Banasiak et al. 2016; etc.). For genera, old style, see Pimenov and Leonov (1993). It is not that variation of the fruits, hitherto the major source of generic characters, never correlate with redrawn generic boundaries (c.f. Feist et al. 2012: rhachis-leaved North American taxa; M. Liu et al. 2012), but they often mislead when used by themselves and/or without examination of their detailed anatomy.
Just about all the papers dealing with phylogenetic relationships in Apioideae (see above) have implications for generic limits. For instance, 13/18 genera for which two or more sequences were included in a study by Downie et al. (2000b) were found not to be monophyletic, while Weitzel et al. (2014) included five genera in Thapsia, "the deadly carrot", a genus of under 50 species. Downie et al. (2010) listed 18 genera that were wildly polyphyletic, i.e., had members in two or more of their 41 major clades; Pimpinella was no. 1 in the list, species being found in seven of these clades (see also above; Plunkett et al. 2018a; Lyskov et al. 2022: Seseli). Ligusticum, with some 60 species and previously in the list of larger genera in Apioideae, now includes just two species (J. Zhou et al. 2020) - c.f. Schefflera in Araliaceae. Feist et al. (2025) noted that the 32 species of Faustia (Scandiceae) had previously been placed in 16 genera, and that found the 16 spcies that they examined came out in ten or so clades... The problem is made worse by the disproportionately large number of mono- or di-typic genera - around 40% of the 466 genera listed by Plunkett et al. (2018a) are monotypic and over 75% had ten or fewer species (see also relationships suggested by Spalik et al. 2001: Scandiceae-Scandicinae; Valiejo-Roman et al. 2006a: Ligusticum area; Spalik & Downie 2007: Cryptotaenia; Frankiewicz et al. 2021d: Apieae). Thus many genera convey no information about relationships, and too many of those that apparently do convey such information, having a number of species, in fact convey misinformation.
It is clear from the work of e.g. Clarkson et al. (2021 and references) that there are a fair number of well-supported groupings even in Apioideae, and that a tribal reclassification there is becoming practicable. Indeed, Mousavi et al. (2021) provide a tribal classification for the Iranian genera, where there are over a quarter of the genera of Apioideae. For a classification of Apieae (and diagrams showing the plasticity of morphological characters used to classify the group), see Jiménez-Mejías and Vargas (2015) and for genera in Scandiceae-Daucinae, see Banasiak et al. (2016).
The extensive para- and polyphyly of the various genera in the Azorella group (Azorelloideae) seemed best solved by extending the limits of that genus and recognizing 10 sections in it (Plunkett & Nicolas 2017; sections confirmed in Nicolas et al. 2025). For the beginning of an infrageneric classification of Ferula, see Panahi et al. (2018).
Thanks. I am particularly indebted to Mark Watson for comments on Apiaceae s.l.; mistakes remain mine.